0000078003us-gaap:DefinedBenefitPlanEquitySecuritiesMemberus-gaap:QualifiedPlanMemberus-gaap:PensionPlansDefinedBenefitMembercountry:US2020-12-310000078003pfe:BiopharmaSegmentMemberpfe:AbrysvoMemberpfe:PrimaryCareMember2021-01-012021-12-31
| | |
| UNITED STATES SECURITIES AND EXCHANGE COMMISSION |
| Washington, D.C. 20549 |
|
|
FORM 10-K
| | | | | |
| (Mark One) | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 20202023
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 1-3619
PFIZER INC.
(Exact name of registrant as specified in its charter)
| | | | | |
| Delaware | 13-5315170 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
23566 Hudson Boulevard East, 42nd Street, New York, New York 1001710001-2192
(Address of principal executive offices) (zip code)
(212) 733-2323
(Registrant’s telephone number, including area code)
| | | | | | | | |
| Securities registered pursuant to Section 12(b) of the Act: |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Common Stock, $.05$0.05 par value | PFE | New York Stock Exchange |
0.250% Notes due 2022 | PFE22 | New York Stock Exchange |
| 1.000% Notes due 2027 | PFE27 | New York Stock Exchange |
| | |
Securities registered pursuant to Section 12(g) of the Act: None |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files.) Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large Accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Smaller reporting company ☐ Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting stock held by non-affiliates of the registrant, computed by reference to the closing price as of the last business day of the registrant’s most recently completed second fiscal quarter June 28, 2020, was approximately $169$207 billion. This excludes shares of common stock held by directors and executive officers at June 28, 2020.officers. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, directly or indirectly, to direct or cause the direction of the management or policies of the registrant, or that such person is controlled by or under common control with the registrant. The registrant has no non-voting common stock.
The number of shares outstanding of the registrant’s common stock as of February 23, 202115, 2024 was 5,577,629,4915,646,778,425 shares of common stock, all of one class.
| | | | | |
| DOCUMENTS INCORPORATED BY REFERENCE |
Portions of the Proxy Statement for the 20212024 Annual Meeting of Shareholders | Part III |
| | | | | |
| | Page |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| ITEM 1B. UNRESOLVED STAFF COMMENTS | N/A |
| |
| |
| |
| ITEM 4. MINE SAFETY DISCLOSURES | N/A |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
ITEM 9B. OTHER INFORMATION9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS | N/A |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| N/A = Not Applicable | |
Unless the context requires otherwise, references to “Pfizer,” “the Company,” “we,” “us” or “our” in this Form 10-K (defined below) refer to Pfizer Inc. and its subsidiaries. The financial information included in our consolidated financial statementsFor each year presented, Pfizer’s fiscal year-end for our subsidiaries operating outside the U.S. is as of and for the year ended November 30 for each year presented. Pfizer's fiscal year-endand for U.S. subsidiaries is as of and for the year ended December 31 for each year presented.31. References to “Notes” in this Form 10-K are to the Notes to the consolidated financial statements in Item 8. Financial Statements and Supplementary Data in this Form 10-K. We also have used several other terms in this Form 10-K, most of which are explained or defined below.below: | | | | | |
2018 Financial Report | Exhibit 13 to the Annual Report on Form 10-K for the fiscal year ended December 31, 2018 |
| Form 10-K | This Annual Report on Form 10-K for the fiscal year ended December 31, 20202023 |
| 2022 Form 10-K | Our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 |
| Proxy Statement | Proxy Statement for the 20212024 Annual Meeting of Shareholders, which will be filed no later than 120 days after December 31, 2020 |
AbbVie | AbbVie Inc.2023 |
| ABO | Accumulated benefit obligationobligation; represents the present value of the benefit obligation earned through the end of the year but does not factor in future compensation increases |
ACA (also referred to as U.S. Healthcare Legislation) | U.S. Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act |
| ACIP | Advisory Committee on Immunization Practices |
AkceaADC | Antibody-Drug Conjugate |
| Alexion | Akcea Therapeutics, Inc.Alexion Pharma International Operations Limited, a subsidiary of AstraZeneca PLC |
| ALK | anaplastic lymphoma kinase |
| Alliance revenues | Revenues from alliance agreements under which we co-promote products discovered or developed by other companies or us |
AllogeneArena | Allogene Therapeutics, Inc. |
AML | Acute Myeloid Leukemia |
Anacor | AnacorArena Pharmaceuticals, Inc. |
AOCI | Accumulated Other Comprehensive Income |
| Array | Array BioPharma Inc. |
| Arvinas | Arvinas, Inc. |
| Astellas | Astellas Pharma Inc., Astellas US LLC and Astellas Pharma US, Inc. |
| ATTR-CM | transthyretin amyloid cardiomyopathy |
Bain CapitalBeam | Bain Capital Private Equity and Bain Capital Life SciencesBeam Therapeutics Inc. |
BiogenBiohaven | Biogen Inc.Biohaven Pharmaceutical Holding Company Limited |
| BioNTech | BioNTech SE |
| Biopharma | PfizerGlobal Biopharmaceuticals GroupBusiness |
| Blackstone | Blackstone Life Sciences |
| BLA | Biologics License Application |
| BMS | Bristol-Myers Squibb Company |
BNT162b2 | Pfizer-BioNTech COVID-19 Vaccine |
| BOD | Board of Directors |
BRCA | BReast CAncer susceptibility gene |
CAR T | chimeric antigen receptor T cell |
| CDC | U.S. Centers for Disease Control and Prevention |
CellectiscGMP | Cellectis S.A. |
Cerevel | Cerevel Therapeutics, LLC |
cGMPs | current Good Manufacturing Practices |
| CGRP | calcitonin gene-related peptide |
CIASCMS | Centers for Medicare & Medicaid Services |
cognitive impairment associated with schizophreniaComirnaty* | Unless otherwise noted, refers to, as applicable, and as authorized or approved, the Pfizer-BioNTech COVID-19 Vaccine, the Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5), Comirnaty (COVID-19 Vaccine, mRNA, 2023-2024 Formula), the Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula), Comirnaty Original/Omicron BA.1, Comirnaty Original/Omicron BA.4/BA.5 and Comirnaty XBB.1.5. |
| Consumer Healthcare JV | GSK Consumer Healthcare JV |
| COVID-19 | novel coronavirus disease of 2019 |
CMA | conditional marketing authorization |
CStone | CStone Pharmaceuticals |
| DEA | U.S. Drug Enforcement Agency |
| Developed Europe | Includes the following markets: Western Europe, Scandinavian countries and Finland |
| Developed Markets | Includes the following markets: U.S., Developed Europe Japan, Canada, Australia, South Korea and New ZealandDeveloped Rest of World |
| Developed Rest of World | Includes the following markets: Japan, Canada, Australia, South Korea, Australia and New Zealand |
| EC | European Commission |
| EMA | European Medicines Agency |
| Emerging Markets | Includes, but is not limited to, the following markets: Asia (excluding Japan and South Korea), Latin America, Eastern Europe, Africa,Central Europe, the Middle East, Central EuropeAfrica and Turkey |
| EPS | earnings per share |
| ESG | Environmental, Social and Governance |
| ESOP | employee stock ownership plan |
| EU | European Union |
| EUA | emergency use authorization |
| Exchange Act | Securities Exchange Act of 1934, as amended |
| FASB | Financial Accounting Standards Board |
| FCPA | U.S. Foreign Corrupt Practices Act |
| FDA | U.S. Food and Drug Administration |
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | i |
| | | | | |
| FFDCA | U.S. Federal Food, Drug and Cosmetic Act |
| GAAP | Generally Accepted Accounting Principles |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | i |
| | | | | |
| GBT | Global Blood Therapeutics, Inc. |
| GDFV | grant-date fair value |
GISTGenmab | gastrointestinal stromal tumorsGenmab A/S |
| GPD | Global Product Development organization |
| GSK | GlaxoSmithKlineGSK plc |
| Haleon | Haleon plc |
| HHS | U.S. Department of Health and Human Services |
| HIPAA | Health Insurance Portability and Accountability Act of 1996 |
| Hospira | Hospira, Inc. |
Ionis | Ionis Pharmaceuticals, Inc. |
| IPR&D | in-process research and development |
| IRA | Inflation Reduction Act of 2022 |
| IRC | Internal Revenue Code |
| IRS | U.S. Internal Revenue Service |
IVIT | intravenous |
J&J | Johnson & Johnsoninformation technology |
| JV | joint venture |
| King | King Pharmaceuticals LLC (formerly King Pharmaceuticals, Inc.) |
LDL | low density lipoprotein |
| LIBOR | London Interbank Offered Rate |
Lilly | Eli Lilly & Company |
| LOE | loss of exclusivity |
| MCO | managed care organization |
| mCRC | metastatic colorectal cancer |
| mCRPC | metastatic castration-resistant prostate cancer |
| mCSPC | metastatic castration-sensitive prostate cancer |
mRNA | messenger ribonucleic acid |
| MD&A | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
| MDL | Multi-District Litigation |
| Medivation | Medivation LLC (formerly Medivation, Inc.) |
| Meridian | Meridian Medical Technologies, Inc. |
| Moody’s | Moody’s Investors Service |
| mRNA | messenger ribonucleic acid |
| MSA | Manufacturing Supply Agreement |
| Mylan | Mylan N.V. |
| Mylan-Japan collaboration | a pre-existing strategic collaboration between Pfizer and Mylan for generic drugs in Japan that terminated on December 21, 2020 |
Myovant | Myovant Sciences Ltd. |
| NAV | net asset value |
| NDA | new drug application |
| Nimbus | NimbusTherapeutics, LLC |
| nmCRPC | non-metastatic castration-resistant prostate cancer |
| nmCSPC | non-metastatic castration-sensitive prostate cancer |
NMPANSCLC | National Medical Product Administration in Chinanon-small cell lung cancer |
| NYSE | New York Stock Exchange |
| ODT | oral disintegrating tablet |
| Ono | Ono Pharmaceutical Co., Ltd. |
| OPKO | OPKO Health, Inc. |
| ORD | Oncology Research and Development |
| OTC | over-the-counter |
| Paxlovid* | an oral COVID-19 treatment (nirmatrelvir tablets and ritonavir tablets) |
| PBM | pharmacy benefit manager |
| PBO | Projected benefit obligation; represents the present value of the benefit obligation earned through the end of the year and factors in future compensation increases |
PCPPPC1 | Pfizer Consolidated Pension PlanCentreOne |
| PGS | Pfizer Global Supply |
| Pharmacia | Pharmacia CorporationLLC (formerly Pharmacia Corporation) |
| PIE | Pfizer Investment Enterprises Pte. Ltd. (a wholly-owned finance subsidiary of Pfizer) |
| PP&E | Property, plant and equipment |
| PRAC | Pharmacovigilance Risk Assessment Committee |
| PRD | Pfizer Research and Development |
PMDAPrevnar family | PharmaceuticalsIncludes Prevnar 20/Apexxnar (pediatric and Medical Device Agency in Japanadult) and Prevnar 13/Prevenar 13 (pediatric and adult) |
| PsA | psoriatic arthritis |
| QCE | quality consistency evaluation |
| RA | rheumatoid arthritis |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | ii |
| | | | | |
| RCC | renal cell carcinoma |
| R&D | research and development |
| ReViral | ReViral Ltd. |
| ROU | right of use |
SandozRSV | Sandoz, Inc., a division of Novartis AGrespiratory syncytial virus |
| S&P | Standard & Poor’s |
| Seagen | Seagen Inc. and its subsidiaries |
| SEC | U.S. Securities and Exchange Commission |
ServierSI&A | Les Laboratoires Servier SASselling, informational and administrative |
ShireSMPA | Shire International GmbHSumitomo Pharma America, Inc. |
| Takeda | Takeda Pharmaceutical Company Limited |
| Tax Cuts and Jobs Act or TCJA | Legislation commonly referred to as the U.S. Tax Cuts and Jobs Act of 2017 |
TevaTrillium | Trillium Therapeutics ULC (formerly Trillium Therapeutics Inc.) |
| TSAs | Teva Pharmaceuticals USA, Inc.transition service arrangements |
TherachonUC | Therachon Holding AGulcerative colitis |
| | | | | | | | |
Pfizer Inc.U.K. | 2020 Form 10-KUnited Kingdom | ii |
| | | | | |
| Upjohn Business | Pfizer’s former global, primarily off-patent branded and generics business, which includesincluded a portfolio of 20 globally recognized solid oral dose brands, including Lipitor, Lyrica, Norvasc, Celebrex and Viagra, as well as a U.S.-based generics platform, Greenstone, that was spun-off on November 16, 2020 and combined with Mylan to create Viatris |
UC | ulcerative colitis |
U.K. | United Kingdom |
| U.S. | United States |
VAI | Voluntary Action Indicated |
| Valneva | Valneva SE |
| VBP | volume-based procurement |
| Viatris | Viatris Inc. |
| ViiV | ViiV Healthcare Limited |
| Vyndaqel family | Includes Vyndaqel, Vyndamax and Vynmac |
| WRDM | Worldwide Research, Development and Medical |
| WTO | World Trade Organization |
| Wyeth | Wyeth LLC (formerly Wyeth) |
*The Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula) and certain uses of Paxlovid have not been approved or licensed by the FDA. The Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula) has been authorized for emergency use by the FDA under an EUA to prevent COVID-19 in individuals aged 6 months through 11 years of age. Paxlovid has been authorized for emergency use by the FDA under an EUA for the treatment of mild-to-moderate COVID-19 in pediatric patients (12 years of age and older weighing at least 40 kg) who are at high risk for progression to severe COVID-19, including hospitalization or death. The emergency uses are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical product during the COVID-19 pandemic under Section 564(b)(1) of the FFDCA, unless the declaration is terminated or authorization revoked sooner. Please see the EUA Fact Sheets at www.covid19oralrx.com and www.cvdvaccine-us.com.
This Form 10-K includes discussion of certain clinical studies relating to various in-line products and/or product candidates. These studies typically are part of a larger body of clinical data relating to such products or product candidates, and the discussion herein should be considered in the context of the larger body of data. In addition, clinical trial data are subject to differing interpretations, and, even when we view data as sufficient to support the safety and/or effectivenessefficacy of a product candidate or a new indication for an in-line product, regulatory authorities may not share our views and may require additional data or may deny approval altogether.
Some amounts in this Form 10-K may not add due to rounding. All percentages have been calculated using unrounded amounts. All trademarks mentioned are the property of their owners.
Our website is www.pfizer.com. This Form 10-K, our Quarterly Reports on Form 10-Q, our Current Reports on Form 8-K and our proxy statements, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, are, or will be, available (free of charge) on our website, in text format and, where applicable, in interactive data file format, as soon as reasonably practicable after we electronically file this material with, or furnish it to, the SEC.
Throughout this Form 10-K, we “incorporate by reference” certain information from other documents filed or to be filed with the SEC, including our Proxy Statement. Please refer to this information. This Form 10-K will be available on our website on or about February 22, 2024. Our Proxy Statement will be available on our website on or about March 14, 2024.
Our 2023 Impact Report, which provides enhanced ESG disclosures, will be available on our website on or about March 14, 2024. We also have a Pfizer Investor Insights website, which includes articles on the company, its products and its pipeline, located at insights.pfizer.com. Information in our 2023 Impact Report and on the Pfizer Investor Insights website are not incorporated by reference into this Form 10-K.
We may use our website as a means of disclosing material information and for complying with our disclosure obligations under Regulation Fair Disclosure promulgated by the SEC. These disclosures are included on our website in the “About—Investors” or “Newsroom” sections. Accordingly, investors should monitor these portions of our website, in addition to following our press releases, SEC filings, public conference calls and webcasts, as well as our social media channels (our Facebook page, Instagram account (@Pfizerinc), YouTube page, LinkedIn page, and X (formerly known as Twitter) accounts (@Pfizer and @Pfizer_News)). The information contained on our website, our Facebook, Instagram, YouTube and LinkedIn pages or our X accounts, or any third-party website, is not incorporated by reference into this Form 10-K.
Information relating to corporate governance at Pfizer, including our Corporate Governance Principles; Director Qualification Standards; Pfizer Policies on Business Conduct (for all of our employees, including our Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer); Code of Business Conduct and Ethics for Members of the Board of Directors; information concerning our Directors; ways to
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | iii |
communicate by e-mail with our Directors; information concerning our Board Committees; Committee Charters; Charter of the Lead Independent Director; and transactions in Pfizer securities by Directors and Officers are available on our website. We will provide any of the foregoing information without charge upon written request to our Corporate Secretary, Pfizer Inc., 66 Hudson Boulevard East, New York, NY 10001-2192. We will disclose any future amendments to, or waivers from, provisions of the Pfizer Policies on Business Conduct affecting our Chief Executive Officer, Chief Financial Officer, Principal Accounting Officer and executive officers on our website as promptly as practicable, as may be required under applicable SEC and NYSE rules. Information relating to shareholder services, including the Computershare Investment Program, book-entry share ownership and direct deposit of dividends, is also available on our website.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | iv |
FORWARD-LOOKING INFORMATION AND FACTORS THAT MAY AFFECT FUTURE RESULTS
This Form 10-K contains forward-looking statements. We also provide forward-looking statements in other materials we release to the public, as well as public oral statements. Given their forward-looking nature, these statements involve substantial risks, uncertainties and potentially inaccurate assumptions.
We have tried, wherever possible, to identify such statements by using words such as “will,” “may,” “could,” “likely,” “ongoing,” “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe,” “assume,” “target,” “forecast,” “guidance,” “goal,” “objective,” “aim,” “seek”“seek,” “potential,” “hope” and other words and terms of similar meaning or by using future dates.
We include forward-looking information in our discussion of the following, among other topics:
•our anticipated operating and financial performance, including financial guidance and projections;
•reorganizations, business plans, strategy, goals and prospects;
•expectations for our product pipeline, in-line products and product candidates, including anticipated regulatory submissions, data read-outs, study starts, approvals, post-approvallaunches, clinical trial results and other developing data that become available,data; revenue contribution and projections; potential pricing and reimbursement; potential market dynamics, including patient demand, market size and utilization rates; and growth, performance, timing of exclusivity and potential benefits;
•strategic reviews, capital allocation objectives, dividends and share repurchases;
•plans for and prospects of our acquisitions, dispositions and other business development activities, and our ability to successfully capitalize on these opportunities;growth opportunities and prospects;
•sales, expenses, interest rates, foreign exchange rates and the outcome of contingencies, such as legal proceedings;
•expectations forregarding the impact of or changes to existing or new government regulations or laws;
•our ability to anticipate and respond to and our expectations regarding the impact of macroeconomic, geopolitical, health and industry trends, pandemics, acts of war and other large-scale crises; and
•manufacturing and product supply.
In particular, forward-looking information in this Form 10-K includes statements relating to specific future actions, performance and effects, including, among others, the expected benefits of the organizational changes to our operations; our anticipated operating and financial performance; our ongoing efforts to respond to COVID-19, including our development of a vaccine to help prevent COVID-19,plans and expectations regarding Comirnaty and Paxlovid, and any potential future vaccines or treatments, including anticipated revenue and expectations for the forecasted revenue contribution of BNT162b2commercial market for Comirnaty and the potential number of doses that we and BioNTech believe can be delivered;Paxlovid; our expectations regarding the impact of COVID-19 on our business; expected patent terms; the expected impact of patent expiries and competition from generic manufacturers;and biosimilar competition; the expected pricing pressures on our products and the anticipated impact to our business; the availability of raw materials for 2021; the expected charges and/or costs in connection with the spin-off of the Upjohn Business and its combination with Mylan; the benefits expected from our business development transactions;transactions, including our December 2023 acquisition of Seagen; our anticipated liquidity position; the anticipated costs, savings and savingspotential benefits from certain of our initiatives, including our enterprise-wide Realigning our Cost Base program, which we launched in October 2023, and our Transforming to a More Focused Company program; our expectations regarding the impact from the 2023 tornado on our manufacturing facility in Rocky Mount, NC; our greenhouse gas emission reduction goals; our planned capital spending; the expectations forand our quarterly dividend payments; and the expected benefit payments and employer contributions for our benefit plans.capital allocation framework.
Given their nature, we cannot assure that any outcome expressed in these forward-looking statements will be realized in whole or in part. Actual outcomes may vary materially from past results and those anticipated, estimated, implied or projected. These forward-looking statements may be affected by underlying assumptions that may prove inaccurate or incomplete, or by known or unknown risks and uncertainties, including those described in this section, and in the Item 1A. Risk Factors section or in this Form 10-K.MD&A. Therefore, you are cautioned not to unduly rely on forward-looking statements, which speak only as of the date of this Form 10-K. We undertake no obligation to update forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable securities law. You are advised, however, to consult any further disclosures we make on related subjects.
Some of the factors that could cause actual results to differ are identified below, as well as those discussed in the Item 1A. Risk Factors section in this Form 10-K and within MD&A. We note these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. The occurrence of any of the risks identified below, or in the Item 1A. Risk Factors section, in this Form 10-K,or within MD&A, or other risks currently unknown, could have a material adverse effect on our business, financial condition or results of operations, or we may be required to increase our accruals for contingencies. It is not possible to predict or identify all such factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties:
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | iii |
Risks Related to Our Business, Industry and Operations, and Business Development:
•the outcome of R&D activities, including the ability to meet anticipated pre-clinical or clinical endpoints, commencement and/or completion dates for our pre-clinical or clinical trials, regulatory submission dates, and/or regulatory approval and/or launch dates, as well asdates; the possibility of unfavorable pre-clinical and clinical trial results, including the possibility of unfavorable new pre-clinical or clinical data and further analyses of existing pre-clinical or clinical data; risks associated with preliminary, early stage or interim data; the risk that pre-clinical and clinical trial data are subject to differing interpretations and assessments, including during the peer review/publication process, in the scientific community generally, and by regulatory authorities; and whether and when additional data from our pipeline programs will be published in scientific journal publications, and if so, when and with what modifications and interpretations;
•our ability to successfully address comments received from regulatory authorities such as the FDA or the EMA, or obtain approval for new products and indications from regulators on a timely basis or at all;
•regulatory decisions impacting labeling, including the scope of indicated patient populations, product dosage, manufacturing processes, safety and/or other matters;matters, including decisions relating to emerging developments regarding potential product impurities; uncertainties regarding the impactability to obtain, and the scope of, recommendations by technical or advisory committees;committees, and the timing of, and ability to obtain, pricing approvals and product launches;launches, all of which could impact the availability or commercial potential of our products and product candidates;
•claims and concerns that may arise regarding the safety or efficacy of in-line products and product candidates, including claims and concerns that may arise from the outcome of post-approval clinical trials, which could impact marketing approval, product labeling, and/or availability or commercial potential, including uncertainties regarding the commercial or other impact of the results of the Xeljanz ORAL Surveillance (A3921133) study or any potential actions by regulatory authorities based on analysis of ORAL Surveillance or other data;potential;
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 1 |
•the success and impact of external business development activities, such as the recent acquisition of Seagen, including the ability to identify and execute on potential business development opportunities; the ability to satisfy the conditions to closing of announced transactions in the anticipated time frame or at all; the ability to realize the anticipated benefits of any such transactions in the anticipated time frame or at all; the potential need for and impact of additional equity or debt financing to pursue these opportunities, which has in the past and could in the future result in increased leverage and/or a downgrade of our credit ratings;ratings and could limit our ability to obtain future financing; challenges integrating the businesses and operations; disruption to business and operations relationships; risks related to growing revenues for certain acquired or partnered products; significant transaction costs; and unknown liabilities;
•competition, including from new product entrants, in-line branded products, generic products, private label products, biosimilars and product candidates that treat or prevent diseases and conditions similar to those treated or intended to be prevented by our in-line drugsproducts and drugproduct candidates;
•the ability to successfully market both new and existing products, including biosimilars;
•difficulties or delays in manufacturing, sales or marketing; supply disruptions, shortages or stock-outs at our facilities;facilities or third-party facilities that we rely on; and legal or regulatory actions;
•the impact of public health outbreaks, epidemics or pandemics (such as the COVID-19 pandemic)COVID-19) on our business, operations and financial condition and results;results, including impacts on our employees, manufacturing, supply chain, sales and marketing, R&D and clinical trials;
•risks and uncertainties related to our efforts to continue to develop a vaccine to help preventand commercialize Comirnaty and Paxlovid or any potential future COVID-19 and potentialvaccines, treatments for COVID-19,or combinations, as well as challenges related to their manufacturing, supply and distribution;distribution, including, among others, the risk that as the market for COVID-19 products continues to become more endemic and seasonal, demand for our COVID-19 products has and may continue to be reduced or not meet expectations, or may no longer exist, which has and may continue to lead to reduced revenues, excess inventory on-hand and/or in the channel which, for Paxlovid and Comirnaty, resulted in significant inventory write-offs in 2023 and could continue to result in inventory write-offs, or other unanticipated charges; challenges related to the transition to the commercial market for our COVID-19 products; uncertainties related to the public’s adherence to vaccines, boosters, treatments or combinations; and risks related to our ability to accurately predict or achieve our revenue forecasts for Comirnaty and Paxlovid or any potential future COVID-19 vaccines or treatments;
•trends toward managed care and healthcare cost containment, and our ability to obtain or maintain timely or adequate pricing or favorable formulary placement for our products;
•interest rate and foreign currency exchange rate fluctuations, including the impact of possible currency devaluations and monetary policy actions in countries experiencing high inflation or deflation rates;
•any significant issues involving our largest wholesale distributors or government customers, which account for a substantial portion of our revenues;
•the impact of the increased presence of counterfeit medicines, vaccines or other products in the pharmaceutical supply chain;
•any significant issues related to the outsourcing of certain operational and staff functions to third parties; and
•any significant issues related to our JVs and other third-party business arrangements;arrangements, including modifications related to supply agreements or other contracts with customers including governments or other payors;
•uncertainties related to general economic, political, business, industry, regulatory and market conditions including, without limitation, uncertainties related to the impact on us, our customers, suppliers and lenders and counterparties to our foreign-exchange and interest-rate agreements of challenging global economic conditions, such as inflation or interest rate fluctuations, and recent and possible future changes in global financial markets;
•the exposure of our operations globally to possible capital and exchange controls, economic conditions, expropriation, sanctions and/or other restrictive government actions, changes in intellectual property legal protections and remedies, unstable governments and legal systems and inter-governmental disputes;
•the impact of disruptions related to climate change and natural disasters, including uncertainties related to the impact of the tornado at our manufacturing facility in Rocky Mount, NC in 2023;
•any changes in business, political and economic conditions due to actual or threatened terrorist activity, geopolitical instability, political or civil unrest or military action;action, including the ongoing conflicts between Russia and Ukraine and in the Middle East and the resulting economic or other consequences;
•the impact of product recalls, withdrawals and other unusual items;items, including uncertainties related to regulator-directed risk evaluations and assessments, including our ongoing evaluation of our product portfolio for the potential presence or formation of nitrosamines;
•trade buying patterns;
•the risk of an impairment charge related to our intangible assets, goodwill or equity-method investments;
•the impact of, and risks and uncertainties related to, restructurings and internal reorganizations, as well as any other corporate strategic initiatives and growth strategies, and cost-reduction and productivity initiatives, each of which requires upfront costs but may fail to yield anticipated benefits and may result in unexpected costs, organizational disruption, adverse effects on employee morale, retention issues or organizational disruption;other unintended consequences;
•the ability to successfully achieve our climate goals and progress our environmental sustainability and other ESG priorities;
Risks Related to Government Regulation and Legal Proceedings:
•the impact of any U.S. healthcare reform or legislation or any significant spending reductionsreduction or cost controlscontrol efforts affecting Medicare, Medicaid or other publicly funded or subsidized health programs, including the IRA, or changes in the tax treatment of employer-sponsored health insurance that may be implemented;
•U.S. federal or state legislation or regulatory action and/or policy efforts affecting, among other things, pharmaceutical product pricing, intellectual property, reimbursement or access or restrictions on U.S. direct-to-consumer advertising; limitations on
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 2 |
interactions with healthcare professionals and other industry stakeholders; as well as pricing pressures for our products as a result of highly competitive insurancebiopharmaceutical markets;
•legislation or regulatory action in markets outside of the U.S., such as China or Europe, including, China, affectingwithout limitation, laws related to pharmaceutical product pricing, intellectual property, medical regulation, environmental protections, reimbursement or access, including, in particular, continued government-mandated reductions in prices and access restrictions for certain biopharmaceutical products to control costs in those markets;
•the exposure of our operations outside of the U.S. to possible capital and exchange controls, economic conditions, expropriation and other restrictive government actions, changes in intellectual property legal protections and remedies, as well as political unrest, unstable governments and legal systems and inter-governmental disputes;
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | iv |
•legal defense costs, insurance expenses, settlement costs and contingencies, including without limitation, those related to actual or alleged environmental contamination;
•the risk and impact of an adverse decision or settlement and risk related to the adequacy of reserves related to legal proceedings;
•the risk and impact of tax related litigation;litigation and investigations;
•governmental laws and regulations affecting our operations, including, without limitation, the IRA, changes in laws and regulations or their interpretation, including, among others, changes in tax laws and regulations internationally and in the U.S., the adoption of global minimum taxation requirements;requirements outside the U.S. generally effective in most jurisdictions since January 1, 2024 and potential changes to existing tax law by the current U.S. Presidential administration and Congress, including the proposed “Tax Relief for American Families and Workers Act of 2024”;
Risks Related to Intellectual Property, Technology and Security:
•any significant breakdown infiltration or interruption of our information technologyIT systems and infrastructure;infrastructure (including cloud services);
•any business disruption, theft of confidential or proprietary information, security threats on facilities or infrastructure, extortion or integrity compromise resulting from a cyber-attack or other malfeasance by, but not limited to, nation states, employees, business partners or others;
•risks and challenges related to the use of artificial intelligence-based software;
•the risk that our currently pending or future patent applications may not be granted on a timely basis or at all, or any patent-term extensions that we seek may not be granted on a timely basis, if at all; and
•risks to our ability to protect ourproducts, patents and other intellectual property, including againstsuch as: (i) claims of invalidity that could result in LOE and in responseLOE; (ii) claims of patent infringement, including asserted and/or unasserted intellectual property claims; (iii) claims we may assert against intellectual property rights held by third parties; (iv) challenges faced by our collaboration or licensing partners to the validity of their patent rights; or (v) any pressure, or legal or regulatory action by, various stakeholders or governments that could potentially result in us not seeking intellectual property protection for or agreeing not to enforce or being restricted from enforcing intellectual property rights related to our products, including our vaccine to help prevent COVID-19Comirnaty and potential treatments for COVID-19.Paxlovid.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | v |
ABOUT PFIZER
Pfizer Inc. is a research-based, global biopharmaceutical company. We apply science and our global resources to bring therapies to people that extend and significantly improve their lives through the discovery, development, manufacture, marketing, salessale and distribution of biopharmaceutical products worldwide. We work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. We collaborate with healthcare providers, governments and local communities to support and expand access to reliable, affordable healthcare around the world. The Company was incorporated under the laws of the State of Delaware on June 2, 1942.
Most of our revenues come from the manufacture and sale of ourbiopharmaceutical products. We also sell products principally biopharmaceutical products,for the detection of certain illnesses and provide end-to-end R&D services to a lesser extent, from alliance agreements, under which we co-promote products discovered or developed by other companies or us.select innovative biotech companies. We believe that our medicines and vaccines provide significant value for healthcare providers and patients through improved treatment of diseases and improvements in health, wellness and productivity as well as by reducing other healthcare costs, such as emergency room visits or hospitalization.hospitalizations. We seek to enhance the value of our medicines and vaccines and actively engage in dialogues about how we can best work with patients, physicians and payerspayors to prevent and treat disease and improve outcomes. We seek to maximize patient access and evaluate our pricing arrangements and contracting methods with payerspayors to minimize adverse impact on our revenues within the current legal and pricing structures.
We are committed to fulfilling our purpose: Breakthroughs that change patients’ lives. By doing so,Our purpose fuels everything we expectdo and reflects both our passion for science and our commitment to create value for the patients we serve and for our colleagues and shareholders. Pfizer’s growth strategy is driven by five “Bold Moves” that help us deliver breakthroughs for patients and create value for shareholders and other stakeholders:
1.Unleash the power of our people;Trust is Everything
2.Deliver first-in-class science;Science Will Win
3.Transform our go-to-market model;Disruption Calls for Innovation
4.Win the digital race in pharma; andTime is Life
5.LeadExecution Makes the conversation.Difference.
We are committed to strategically capitalizing on growth opportunities, primarily by advancing our own product pipeline and maximizing the value of our existing products, as well asbut also through various business development activities. We view our business development activity as an enabler of our strategies and seek to generate growth by pursuing opportunities and transactions that have the potential to strengthen our business and our
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 3 |
capabilities. We assess our business, assets and scientific capabilities/portfolio as part of our regular, ongoing portfolio review process and also continue to consider business development activities that will help advance our business.business strategy.
Following (i)On December 14, 2023, we completed our acquisition of Seagen, a global biotechnology company that discovers, develops and commercializes transformative cancer medicines. With the recent spin-offaddition of Seagen’s pipeline and combinationits four in-line medicines (Padcev, Adcetris, Tukysa and Tivdak), Pfizer’s oncology portfolio spans multiple modalities, including ADCs, small molecules, bispecifics and other immunotherapies. In addition to the acquisition of the Upjohn Business (which wasSeagen, our global, primarily off-patent branded and generics business) with Mylan, which created a new global pharmaceutical company, Viatris, in November 2020 and (ii) the formation of the Consumer Healthcare JV in 2019, we saw the culmination of Pfizer’s transformation into a more focused, innovative science-based biopharmaceutical products business.
Our significant recent business development activities in 2020 include: (i) the April 2020 agreement with BioNTech to develop, manufacture and commercialize an mRNA-based coronavirus vaccine program, BNT162, aimed at preventing COVID-19, (ii) the June 2020 agreement to co-develop and commercialize Valneva’s Lyme disease vaccine candidate, VLA15, (iii)2023 include, among others, the September 2020 entry into a strategic collaboration with CStone2023 divestiture of our early-stage rare disease gene therapy portfolio to develop and commercialize a PD-L1 antibody, sugemalimab, and to bring additional oncology assets to China, (iv) the November 2020 spin-off and combination of the Upjohn Business with Mylan, and (v) the December 2020 entry into a collaboration with Myovant to jointly develop and commercialize relugolix in advanced prostate cancer and women’s health in the U.S. and Canada.Alexion. For a further discussion of our strategy and our business development initiatives, see theOverview of Our Performance, Operating Environment, Strategy and Outlook section within MD&A and Note 2.
In 2020, our business, operations and financial condition and results were impacted by the COVID-19 pandemic. To confront the public health challenge posed by the pandemic, we have made some important advances, including, among others, the development of a vaccine to help prevent COVID-19. For additional information, see the Overview of Our Performance, Operating Environment, Strategy and Outlook—COVID-19 Pandemic section within MD&A and the Item 1A. Risk Factors—Development, Regulatory Approval and Marketing of Products and —COVID-19 Pandemic sections in this Form 10-K.
COMMERCIAL OPERATIONS
In 2020,2023, we managed our commercial operations through a global structure consisting of two businesses—Biopharma, and, through November 16, 2020, Upjohn,operating segments, each led by a single manager.
On November 16, 2020, we completed the spin-offmanager: Biopharma, our innovative science-based biopharmaceutical business, and combination of the Upjohn Business with Mylan. Following the combination, we now operate as a focused innovative biopharmaceutical company engagedInnovation, an operating segment established in the discovery, development, manufacturing, marketing, sales and distribution of biopharmaceutical products worldwide. Beginning in the fourthfirst quarter of 2020,2023 that includes PC1, our contract development and manufacturing organization and a leading supplier of specialty active pharmaceutical ingredients, and Pfizer Ignite, an offering that provides strategic guidance and end-to-end R&D services to select innovative biotech companies that align with our R&D focus areas. In 2023, Biopharma was the financial resultsonly reportable segment. The commercial structure within Biopharma included three broad customer groups in 2023: Primary Care, Specialty Care and Oncology.
At the beginning of 2024, we made changes in our commercial organization to incorporate Seagen and improve focus, speed and execution. Specifically, within our Biopharma reportable segment we created the Upjohn Business are reflected asdiscontinued operations for all periods presented. Prior-period information has been restated to reflect our current organizational structure followingPfizer Oncology Division, the separation ofPfizer U.S. Commercial Division, and the Upjohn Business. In 2019, Consumer Healthcare, which was our OTC medicines business, was
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 1 |
combined with GSK’s consumer healthcare business to form a consumer healthcare JV in which we own a 32% equity stake. For additional information, see the Overview of Our Performance, Operating Environment, Strategy and Outlook section within MD&A and Notes 1A and 2C.
Our business includes the following therapeutic areas and key products:
| | | | | | | | |
Therapeutic AreaDivision | Description | Key Products |
| Internal Medicine | Includes innovative brands from two therapeutic areas, Cardiovascular Metabolic and Pain, as well as regional brands.Eliquis*, Chantix/Champix* and the Premarin family |
| Pfizer Oncology Division | Combines the U.S. Oncology commercial organizations, global Oncology marketing organizations and global and U.S. Oncology medical affairs from both Pfizer and Seagen. Includes innovative oncology brandsproduct portfolio of biologics,ADCs, small molecules, bispecifics and other immunotherapies and biosimilars acrossthat treat a wide range of cancers. | Ibrance*, Xtandi*, Sutent*, Inlyta, Retacrit, Lorbrenacancers including certain types of breast cancer, genitourinary cancer and Braftovi |
Hospital | Includes our global portfolio of sterile injectable and anti-infective medicines,hematologic malignancies, as well as Pfizer CentreOne, our contract manufacturingcertain types of melanoma, gastrointestinal, gynecological and active pharmaceutical ingredient sales operation. | Sulperazon, Medrol, Zithromax, Vfend and Panzygathoracic cancers, which includes lung cancer. |
VaccinesPfizer U.S. Commercial Division
| Includes innovative vaccinesthe U.S. Primary Care and U.S. Specialty Care customer groups, the Chief Marketing Office, the Global Chief Medical Affairs Office and Global Access & Value. |
| U.S. Primary Care includes: |
•Internal medicine product portfolio of brands in cardiovascular metabolic, bone graft for spinal fusion and women’s health, as well as post-LOE brands. |
•Migraine product portfolio. |
•Vaccines product portfolio across all ages—infants, adolescents and adults—in pneumococcal disease, meningococcal disease, tick-borne encephalitis and COVID-19,ages with a pipeline focus on infectious diseases with significant unmet medical need. | Prevnar 13/Prevenar 13 (pediatric/adult)*, Nimenrix, FSME/IMMUN-TicoVac, Trumenba and the Pfizer-BioNTech COVID-19 vaccineneed, including COVID-19. |
•Treatment for COVID-19. |
•Products for detection of COVID-19 and influenza. |
| U.S. Specialty Care includes: |
•Inflammation & Immunology | Includes innovativeimmunology product portfolio of brands and biosimilars for chronic immune and inflammatory diseases. | Xeljanz*, Enbrel (outside the U.S. and Canada)*, Inflectra and Eucrisa/Staquis |
•Rare Disease | Includes innovativedisease product portfolio of brands for a number of therapeutic areas with rare diseases, including amyloidosis, hemophilia, endocrine diseases and endocrine diseases.sickle cell disease. |
•Hospital product portfolio of sterile injectable and immunoglobulin medicines. |
| Pfizer International Commercial Division | Vyndaqel/Vyndamax*, BeneFIXIncludes the ex-U.S. commercial and Genotropinmedical affairs organizations covering Pfizer’s entire product portfolio in all international markets. |
Select products within Oncology, Primary Care and Specialty Care include:
*•EachOncology:Ibrance, Xtandi, Inlyta, Bosulif, Lorbrena, Braftovi, Mektovi, Padcev, Adcetris, Talzenna, Tukysa, Elrexfio and Tivdak
•Primary Care:
◦Internal medicine: Eliquis, the Premarin family and BMP2
◦Migraine: Nurtec ODT/Vydura and Zavzpret
◦Vaccines: Comirnaty, the Prevnar family, Abrysvo, FSME/IMMUN-TicoVac, Nimenrix and Trumenba
◦Treatment for COVID-19: Paxlovid
◦Detection of Prevnar 13/Prevenar 13, Ibrance, Eliquis,COVID-19 and influenza: Lucira by Pfizer
•Specialty Care:
◦Inflammation & immunology: Xeljanz, Enbrel (outside the U.S. and Enbrel recorded direct product and/or Alliance revenues of more than $1 billion in 2020, 2019Canada), Inflectra, Cibinqo, Litfulo and 2018. Each of XtandiVelsipity
◦Rare disease: the Vyndaqel family, Genotropin, BeneFIX, Oxbryta, Somavert and Vyndaqel/Vyndamax recorded direct product and/or Alliance revenues of more than $1 billion in 2020, Chantix/Champix recorded directNgenla
◦Hospital: Sulperazon, Zavicefta, Zithromax, Medrol and Panzyga
For additional information on our operating segments and products, including product revenues, of more than $1 billion in 2019 see Note 17, and 2018 and Sutent recorded direct product revenues of more than $1 billion in 2018. Eliquis includes Alliance revenues and direct sales.
COLLABORATION AND CO-PROMOTION
We use collaboration and/or co-promotion arrangements to enhance our development, R&D, sales and distribution of certain biopharmaceutical products, which include, among others, the following:
•Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) is an mRNA-based coronavirus vaccine to help prevent COVID-19 which is being jointly developed and commercialized with BioNTech. Pfizer and BioNTech will equally share the costs of development for the BNT162 program. BNT162b2 has now been granted a CMA, EUA or temporary authorization in more than 50 countries worldwide. We will also share gross profits equally from commercialization of BNT162b2 and are working jointly with BioNTech in our respective territories to commercialize the vaccine worldwide (excluding China, Hong Kong, Macau and Taiwan), subject to regulatory authorizations or approvals market by market. For discussion on BNT162b2, see the Overview of Our Performance, Operating Environment, Strategy and Outlook—COVID-19 Pandemic section within MD&A.
•Eliquis (apixaban) is part of the Novel Oral Anticoagulant market and was jointly developed and commercialized with BMS as an alternative treatment option to warfarin in appropriate patients. We fund between 50% and 60% of all development costs depending on the study, and profits and losses are shared equally except in certain countries where we commercialize Eliquis and pay a percentage of net sales to BMS. In certain smaller markets we have full commercialization rights and BMS supplies the product to us at cost plus a percentage of the net sales to end-customers.
•Xtandi (enzalutamide) is an androgen receptor inhibitor that blocks multiple steps in the androgen receptor signaling pathway within tumor cells that is being developed and commercialized in collaboration with Astellas. We share equally in the gross profits and losses related to U.S. net sales and also share equally all Xtandi commercialization costs attributable to the U.S. market, subject to certain exceptions. In addition, we share certain development and other collaboration expenses. For international net sales we receive royalties based on a tiered percentage.
•Bavencio (avelumab) is a human anti-programmed death ligand-1 (PD-L1) antibody that is being developed and commercialized in collaboration with Merck KGaA. We jointly fund the majority of development and commercialization costs and split profits equally related to net sales generated from any products containing avelumab.
•Orgovyx (relugolix) is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist approved by the FDA for the treatment of adult patients with advanced prostate cancer that is being developed and commercialized with Myovant. The companies are also collaborating on relugolix combination tablet (relugolix 40 mg, estradiol 1.0 mg, and norethindrone acetate 0.5 mg) in women’s health. The companies will equally share profits and allowable expenses in the U.S. and Canada for Orgovyx and the relugolix combination tablet, with Myovant bearing our share of allowable expenses up to a maximum of $100 million in 2021 and up to a maximum of $50 million in 2022. Myovant will remain responsible for regulatory interactions and drug supply and continue to lead clinical development for the relugolix combination tablet.
Revenues associated with these arrangements are included in Alliance revenues (except in certain markets where we have direct sales and except for the majority of revenues for BNT162b2, which are included as direct product revenues). In addition, we have collaboration arrangements for the development and commercialization of certain pipeline products that are in development stage, including, among others, with Lilly to jointly develop and globally commercialize tanezumab for the treatment of osteoarthritis pain and cancer pain, under which the companies share equally the ongoing development costs and, if successful, will co-commercialize and share equally in profits and certain expenses in the U.S., while Pfizer will be responsible for commercialization activities and costs outside the U.S., with Lilly having the right to
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 2 |
receive certain tiered royalties outside the U.S. For further discussion of collaboration and co-promotion agreements, see the Item 1A. Risk Factors—Collaborations and Other Relationships with Third Parties section in this Form 10-K and Notes 2 and 17.
RESEARCH AND DEVELOPMENT
R&D is at the heart of fulfilling our purpose to deliver breakthroughs that change patients’ lives as we work to translate advanced science and technologies into the therapiesmedicines and vaccines that may be the most impactful for patients. The discovery and development of drugs and biological products are time consuming, costly and unpredictable. In addition to discovering and developing new products, our R&D efforts seek to add value to our existing products by improving their effectivenesssafety, efficacy and ease of dosing and by discovering potential new indications.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 4 |
Our R&D Priorities and Strategy. Our R&D priorities include:
•delivering a pipeline of highly differentiated medicines and vaccines where we have a unique opportunity to bring the most important new therapies to patients in need;
•advancing our capabilities that can position us for long-term R&D leadership; and
•advancing new models for partnerships with creativity, flexibility and urgency to deliver innovation to patients as quickly as possible.
To that end, our R&D primarily focuses on our main therapeutic areas.
While a significant portion of our R&D is internal, we also seek promising chemical and biological lead molecules and innovative technologies developed by others to incorporate into our discovery and development processes or projects, as well as our product lines.portfolio. We do so by entering into collaboration, alliance and license agreements with universities, biotechnology companies and other firms as well as through acquisitions and investments. We also have arrangements with third parties that fund a portion of the development costs of one or more of our pipeline products in exchange for rights to receive future payments, such as milestone-based, revenue sharing, or profit-sharing payments or royalties. These collaboration, alliance license and fundinglicense agreements and investments allow us to share knowledge, risk and cost. They also enable us to access external scientific and technological expertise, as well as provide us the opportunity to advance our own products and in-licensed or acquired products. For information on certain of these collaborations, alliances license and fundinglicense arrangements and investments, see Note 2.
Our R&D Operations. In 2020,2023, we continued to strengthen our global R&D operations and pursue strategies to improve R&D productivity to achieve a sustainable pipeline that is positioned to deliver value in the near term and over time. Our R&D activity is conducted through various platform functions that operate in parallel withinsupport our global operations,operations. Beginning in July 2023, in consideration of planned future investments in oncology, including the following:
•WRDM. December 2023 acquisition of Seagen, we reorganized our R&D platform operations. Discovery to late-phase clinical development for oncology is performed by a new end-to-end Oncology Research and Development (ORD) organization and discovery to late-phase clinical development for all remaining therapeutic areas is consolidated into the end-to-end Pfizer Research and Development (PRD) organization. ORD and PRD replace our former WRDM and GPD organizations, where, prior to July 2023, research units arewithin WRDM were generally responsible for research and early-stage development assets for our business (assets that have not yet achieved proof-of-concept) and, are organized by therapeutic areaprior to enhance flexibility, cohesiveness and focus. We can rapidly redeploy resources within a research unit and between various projects to leverage, as necessary, common skills, expertise or focus.
•GPD. July 2023, GPD is a unified center for clinical development and regulatory activities that iswas generally responsible for the clinical development strategy and operational execution of clinical trials for both early-stageearly- and late-stage clinical assets in the WRDM portfolio as well as late-stage assets in our portfolio.
•Science-based platform-services organizations. These organizations provide technical expertise and other services to variousPfizer’s pipeline. In 2023, Biopharma received R&D projects, and are organized into science-based functions (which are part of our WRDM organization) such as Pharmaceutical Sciences and Medicine Design. These organizations allow us to react more quickly and effectively to evolving needs by sharing resources among projects, candidates and targets across therapeutic areas and phases of development. Another platform-service organization is the Worldwide Medical and Safety (WMS) group, which includes worldwide safety surveillance, medical informationservices from ORD, PRD and the Chief Medical Office. The WMS group provides patients, healthcare providers, pharmacists, payerspredecessor WRDM and health authorities with completeGPD organizations. These services included IPR&D projects for new investigational products and up-to-date information about the risks and benefits associated with Pfizer’s R&D programs and marketed products so they can make appropriate decisions on how and when to use ouradditional indications for in-line products.
We manage R&D operations on a total-company basis through our platform functionsPRD and ORD organizations described above. Specifically, the Portfolio Strategy & Investment committee, comprisedManagement Team, currently led by our Chairman and Chief Executive Officer and composed of other senior executives, is accountable for aligning resources among all of our WRDM, GPDacross PRD and R&D projectsORD, and for seekinghelping to ensure optimal capital allocation across the innovative R&D portfolio. We believe that this approach also serves to maximize accountability and flexibility.
We do not disaggregate total R&D expense by development phase or by therapeutic area since, as described above, we do not manage all of our R&D operations by development phase or by therapeutic area. Further, as we are able to adjust a significant portion of our spending quickly, we believe that any prior-period information about R&D expense by development phase or by therapeutic area would not necessarily be representative of future spending.
In 2020, the R&D organization within Upjohn supported the off-patent branded and generic established medicines and managed its resources separately from the WRDM and GPD organizations. Following the spin-off and combination of the Upjohn Business with Mylan to create Viatris, we have agreed to provide certain transition services to Viatris including support for R&D, pharmacovigilance and safety surveillance.
| | | | | | | | |
Pfizer Inc.. | 2020 Form 10-K | 3 |
Our R&D Pipeline. The process of drug and biological product discovery from initiation through development and to potential regulatory approval is lengthy and can take more than ten years. As of February 2, 2021,January 30, 2024, we had the following number of projects in various stages of R&D:
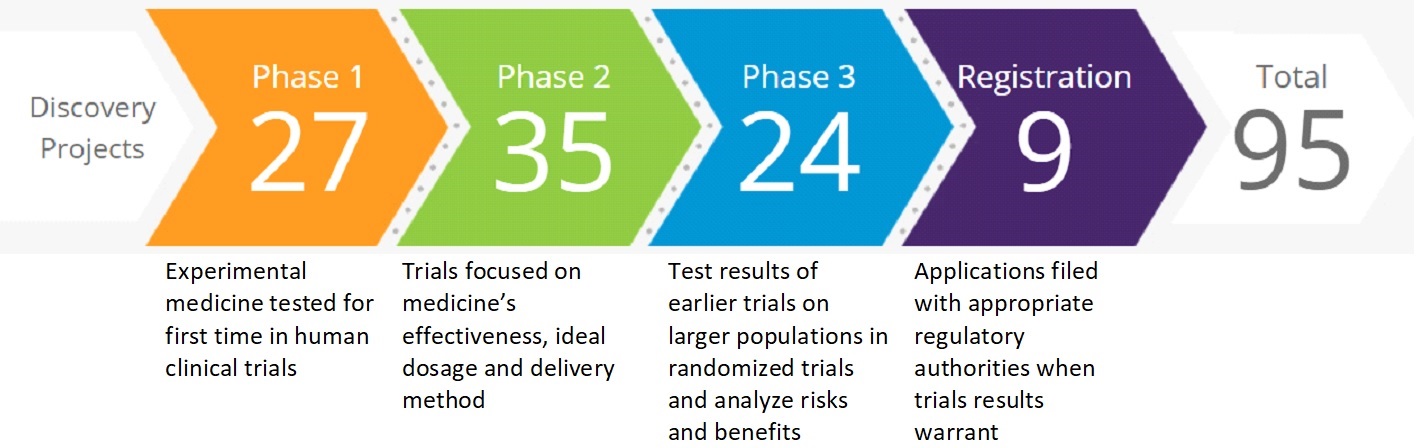

Development of a single compound is often pursued as part of multiple programs. While our drugproduct candidates may or may not receive regulatory approval, new candidates entering clinical development phases are the foundation for future products. Information concerning several of our drug and vaccine candidates in development, as well as supplemental filings for existing products, is set forth in the Analysis of the Consolidated Statements of Income—Product Developments section within MD&A. The discovery and development of drugs, vaccines and biological products are time consuming, costly and unpredictable. For information on the risks associated with R&D, see the Item 1A. Risk Factors—Research and Development section. COLLABORATION AND CO-PROMOTION AGREEMENTS
We use collaboration and/or co-promotion arrangements to enhance our development, R&D, sales and distribution of certain biopharmaceutical products, which include, among others, the following:
•Comirnaty is an mRNA-based coronavirus vaccine to help prevent COVID-19, which is being jointly developed and commercialized with BioNTech. Pfizer and BioNTech equally share the costs of development for the Comirnaty program. Comirnaty has been granted an approval or an authorization in many countries around the world in populations varying by country. We also share gross profits equally from commercialization of Comirnaty and are working jointly with BioNTech in our respective territories to commercialize the vaccine worldwide (excluding China, Hong Kong, Macau and Taiwan), subject to regulatory authorizations or approvals market by market. For discussion on Comirnaty, see the Overview of Our Performance, Operating Environment, Strategy and Outlook—COVID-19section within MD&A. •Eliquis (apixaban) is part of the Novel Oral Anticoagulant market and was jointly developed and commercialized with BMS as an alternative treatment option to warfarin in appropriate patients. We fund between 50% and 60% of all development costs depending on the study, and profits and losses are shared equally except in certain countries where we commercialize Eliquis and pay a percentage of net sales to BMS. In
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 5 |
certain smaller markets we have full commercialization rights and BMS supplies the product to us at cost plus a percentage of the net sales to end-customers.
•Xtandi (enzalutamide) is an androgen receptor inhibitor that blocks multiple steps in the androgen receptor signaling pathway within tumor cells that is being developed and commercialized in collaboration with Astellas. We share equally in the gross profits and losses related to U.S. net sales and also share equally all Xtandi commercialization costs attributable to the U.S. market, subject to certain exceptions. In addition, we share certain development and other collaboration expenses. For international net sales we receive royalties based on a tiered percentage.
•Orgovyx (relugolix) is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist for the treatment of adult patients with advanced prostate cancer that is being developed and commercialized with SMPA. The companies are also collaborating on Myfembree (relugolix 40 mg, estradiol 1.0 mg, and norethindrone acetate 0.5 mg) for heavy menstrual bleeding associated with uterine fibroids in premenopausal women and the management of moderate to severe pain associated with endometriosis in premenopausal women. The companies equally share profits and allowable expenses in the U.S. for Orgovyx, and in the U.S. and Canada for Myfembree. Pfizer does not have rights outside of these markets. SMPA remains responsible for regulatory interactions and drug supply and continues to lead clinical development for the relugolix combination tablet.
•Padcev (enfortumab vedotin-ejfv) is a first-in-class ADC that is directed to Nectin-4, a protein located on the surface of cells and highly expressed in bladder cancer, that is being co-developed and jointly commercialized with Astellas. In the U.S., Padcev has been approved for use with Keytruda (pembrolizumab) for adult patients with locally advanced or metastatic urothelial cancer. Other approvals and indications for Padcev vary by market. In the U.S., the companies jointly promote, and we record net sales and are responsible for all U.S. distribution activities for Padcev. The companies each bear the costs of their own sales organizations in the U.S., and equally share certain other costs associated with commercializing and any profits realized in the U.S. for Padcev. Outside the U.S., we have commercialization rights in all countries in North and South America, and Astellas has commercialization rights in the rest of the world. The agreement between us and Astellas provides that the companies will effectively equally share in profits realized in markets outside of the U.S. through: (i) a costs-incurred and profit-sharing mechanism based on product sales and costs of commercialization in certain markets and (ii) a royalty-payment mechanism intended to approximate an equal profit share for both parties in the remaining markets.
In addition, we have collaboration and/or co-promotion arrangements with respect to certain other biopharmaceutical products, including Adcetris and Tivdak as a result of our acquisition of Seagen.
INTERNATIONAL OPERATIONS
Our operations are conducted globally, and we sellsupply our products in over 125 countries.medicines and vaccines to approximately 200 countries and territories. Emerging markets are an important component of our strategy for global leadership, and our commercial structure recognizes that the demographics and rising economic power of the fastest-growing emerging markets are becoming more closely aligned with the profile found within developed markets. Urbanization and the rise of the middle class in emerging markets provide potential growth opportunities for our products.
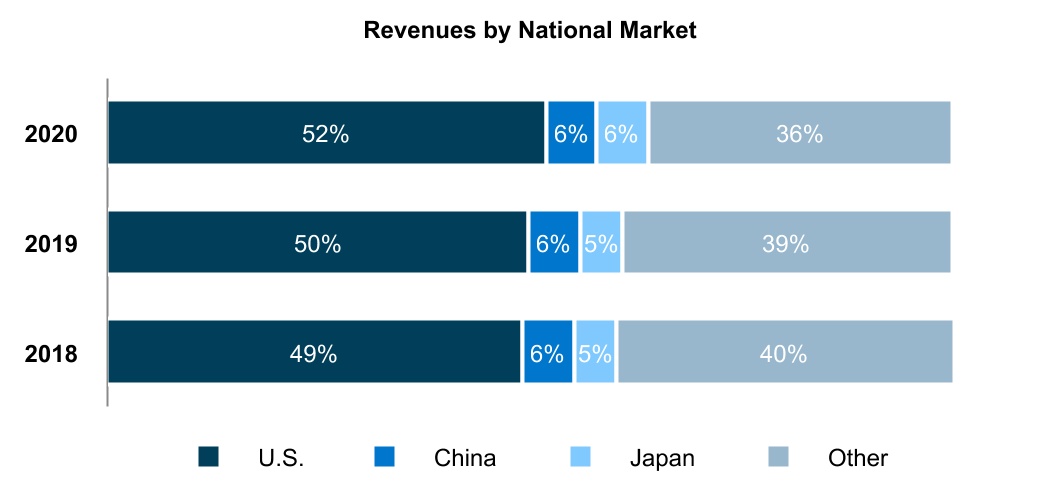
Revenues from operations outside the U.S. of $20.2$31.4 billion accounted for 48%54% of our totalTotal revenues in 2020.2023. Revenues exceeded $500 million in each of 8, 1014, 24 and 1021 countries outside the U.S. in 2020, 20192023, 2022 and 2018,2021, respectively. By totalThe decrease in the number of countries exceeding $500 million in revenues Chinafrom 2022 to 2023 was primarily driven by decreases in revenues related to Comirnaty and Japan arePaxlovid. As a percentage of Total revenues, our two largest national marketscountry outside the U.S. was Japan in 2023. For a geographic breakdown of Total revenues, see the Analysis of the Consolidated Statements of Income—Total Revenues by Geography section within MD&A and the table captioned Geographic Information in Note 17A17B. SALES AND MARKETING
Our prescription pharmaceuticalbiopharmaceutical products, with the exception of Paxlovid in 2022 and 2023, are sold principally to wholesalers, but we also sell directly to retailers, hospitals, clinics, government agencies and pharmacies. In 2022 and 2023, we principally sold Paxlovid globally to government agencies. Our vaccines in the U.S., we are primarily sell our vaccines productssold directly to the federal government CDC,(including the CDC), wholesalers, individual provider offices, retail pharmacies and integrated delivery networks. Outsidesystems. Our vaccines outside the U.S., we are primarily sell our vaccinessold to government and non-government institutions. A portionCertain of ourthese government contracts are subject to renegotiationmay be renegotiated or termination of contracts or subcontractsterminated at the discretion of a government entity. Our
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 6 |
contracts with government and supranational organizations for the sales of Comirnaty and Paxlovid, which are binding contracts, represented a significant amount of revenues in 2022 and 2023. Sales of Comirnaty and Paxlovid in the U.S. transitioned to commercial channels in the second half of 2023. For information on our October 2023 amended agreement with the U.S. government regarding Paxlovid, see Note 17C. We also seek to gain access for our products on healthcare authority and PBM formularies, which are lists of approved medicines available to members of thehealthcare programs or PBMs. PBMs use various benefit designs, such as tiered co-pays for formulary products, to drive utilization of products in preferred formulary positions. We may also work with payerspayors on disease management programs that help to develop tools and materials to educate patients and physicians on key disease areas. For information on our largest biopharmaceutical wholesalers,significant customers, see Note 17B17C.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 4 |
We promote our products to healthcare providers and patients.patients consistent with applicable laws. Through our marketing organizations, we explain the approved uses, benefits and risks of our products to healthcare providers;providers and patients; MCOs that provide insurance coverage, such as hospitals, Integrated Delivery Systems,integrated delivery systems, PBMs and health plans; and employers and government agencies who hire MCOs to provide health benefits to their employees. We alsoIn the U.S., we market directly to consumers in the U.S. through direct-to-consumer advertising that seeks to communicate the approved uses, benefits and risks of our products while motivating people to have meaningful conversations with their doctors. In addition, we sponsor general advertising to educate the public on disease awareness, prevention and wellness, important public health issues and our patient assistance programs.
As part of our commitment to engaging our customers in a manner they prefer, we take an omnichannel approach, including both virtual and in person interactions, and see generally positive customer response to both approaches.
PATENTS AND OTHER INTELLECTUAL PROPERTY RIGHTS
Patents. We own or have co-promotion and/or license rights related to a number of patents covering pharmaceutical and other products, their uses, formulations, and product manufacturing processes.
Patents for individual products extend for varying periods according to the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. The scope of protection afforded by a patent can vary from country to country and depends on the patent type, the scope of its patent claims and the availability of legal remedies. Patent term extensions (PTE) may be available in some countries to compensate for a loss of patent term due to delay in a product’s approval due to the regulatory requirements.requirements, while patent term adjustment may be available in some countries to compensate for administrative delays during prosecution of patents. One of the primary considerations in limiting our operations in some countries outside the U.S. is the lack of effective intellectual property protection for our products, although international and U.S. free trade agreements have included some improved global protection of intellectual property rights. For additional information, seeSee the Item 1. Business—Government Regulation and Price Constraintssection in this Form 10-K.
In various markets, a period of regulatory exclusivity may be provided for drugs or vaccines upon approval. The scope and term of such exclusivity will vary but, in general, the period will run concurrently with the term of any existing patent rights associated with the drug at the time of approval.
Based on current sales and other factors, and considering the competition with products sold by our competitors, the patent rights we consider most significant in relation to our business as a whole, together with the year in which the basic product patent expires, are as follows:
| | | | | | | | | | | | | | | | | | | | |
| Drug | | U.S. Basic Product Patent Expiration Year(1) | | Major Europe Basic Product Patent Expiration Year(1) | | Japan Basic Product Patent Expiration Year(1) |
| Chantix/Champix | | 2020(2) | | 2021 | | 2022 |
| Sutent | | 2021 | | 2022 | | 2024 |
| Inlyta | | 2025 | | 2025 | | 2025 |
| Xeljanz | | 2025 | | 2028 (3) | | 2025 |
| Prevnar 13/Prevenar 13 | | 2026 | | __(4) | | 2029 |
Eliquis(5) | | 2026 | | 2026 | | 2026 |
| Ibrance | | 2027 | | 2028 | | 2028 |
Xtandi(6) | | 2027 | | *(6) | | *(6) |
| Vyndaqel/Vyndamax | | 2024
(2028 pending PTE) | | 2026 | | 2026 |
| Xalkori | | 2029 | | 2027 | | 2028 |
| Besponsa | | 2030 | | 2028 | | 2028(7) |
Braftovi(8) | | 2031
(2031 pending PTE) | | *(8) | | *(8) |
Mektovi(8) | | 2031(9) | | *(8) | | *(8) |
Bavencio(10) | | 2033 | | 2032 | | 2033 |
| Lorbrena | | 2033 | | 2034 | | 2036 |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 7 |
| | | | | | | | | | | | | | | | | | | | |
| Product | | U.S. Basic Product Patent Expiration Year(1) | | Major Europe Basic Product Patent Expiration Year(1) | | Japan Basic Product Patent Expiration Year(1) |
| Inlyta | | 2025 | | 2025 | | 2025 |
| Xeljanz | | 2025 | | 2028(2) | | 2025 |
| Prevnar 13/Prevenar 13 | | 2026 | | (3) | | 2029 |
Eliquis | | 2026(4) | | 2026(5) | | 2026 |
| Ibrance | | 2027 | | 2028 | | 2028 |
Xtandi(6) | | 2027 | | (6) | | (6) |
| Vyndaqel/Vyndamax/Vynmac | | 2024(7) (2028 pending PTE) | | 2026 | | 2026/2029(8) |
Adcetris(9) | | 2024(10) | | (9) | | (9) |
| | | | | | |
| Nurtec ODT/Vydura | | 2030 (2034 pending PTE) | | 2035 | | 2030(11) |
Braftovi(12) | | 2030 (2031 pending PTE) | | (12) | | (12) |
Mektovi(12) | | 2031(13) | | (12) | | (12) |
| Talzenna | | 2029 (2032 pending PTE) | | 2034 | | 2029 |
| Oxbryta | | 2033 | | 2037 | | 2032(11) |
| Lorbrena | | 2033 | | 2034 | | 2036 |
Padcev(14) | | 2033(15) | | (14) | | (14) |
Tukysa(16) | | 2031 (2034 pending PTE) | | 2031 | | 2026(11) |
| Zavzpret | | 2031 (2034 pending PTE) | | 2031(11) | | 2031(11) |
| Velsipity | | 2029 (2034 pending PTE) | | 2029 | | 2029(11) |
| Prevnar 20/Apexxnar | | 2033 (2035 pending PTE) | | 2033 (2037 pending SPC) | | 2033(11) |
Ngenla(17) | | 2035(2) | | 2032(2) | | 2030(2) |
| Cibinqo | | 2034 (2036 pending PTE) | | 2036 | | 2038 |
Tivdak(18) | | 2033(19) | | 2031(11) | | (18) |
| Litfulo | | 2034 (2037 pending PTE) | | 2034 (2038 pending SPC) | | 2034 (2039 pending PTE) |
| Abrysvo | | 2036 (2037 pending PTE) | | (22) | | 2036 |
| Elrexfio | | 2036 (2037 pending PTE) | | 2036 | | 2036(11) |
| Penbraya | | 2038 | | 2038(11) | | 2038(11) |
| COVID-19 Products |
Pfizer-BioNTech COVID-19 Vaccine(20) | | 2041 | | (21)(23) | | (22) |
| Paxlovid | | 2041 | | 2041 | | 2041 |
Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5)/ Comirnaty Original/Omicron BA.1 Vaccine(20) | | (22) | | (22)(23) | | (22) |
XBB.1.5-Adapted Monovalent COVID-19 vaccine(20) | | (22) | | (22)(23) | | (22) |
(1)Unless otherwise indicated, the years pertain to the basic product patent expiration, including granted PTEs, supplementary protection certificates (SPC) or pediatric exclusivity periods. SPCs are included when granted in three out of five major European markets (France, Germany, Italy, Spain and the U.K.). Noted in parentheses is the projected year of expiry of the earliest pending patent term extension in the U.S. or Japan and/or SPC application in Europe, the term of which, if granted, may be shorter than originally requested due to a number of factors. In some instances, there are later-expiring patents relating to our products which may or may not protect our drugproduct from generic or biosimilar competition after the expiration of the basic patent.
(2)The basic product patent for Chantix in the U.S. expired in November 2020.
(3)Xeljanz Europe expiryExpiry is provided by regulatory exclusivity.exclusivity in this market.
(4)(3)The Europe patent that covers the combination of the 13 serotype conjugates of Prevenar 13 was revoked following an opposition and has now been withdrawn. There are other Europe patents and pending applications covering the formulation, various aspects of the manufacturing process, and the combination of serotype conjugates of Prevenar 13 that remain in force.
(5)(4)Eliquis was developed and is being commercialized in collaboration with BMS. For Eliquis inIn the U.S., two patents listedwe and BMS previously settled certain patent litigations with a number of generic companies permitting their launch of a generic version of Eliquis on April 1, 2028 (the settled generic companies). We continued to litigate against three
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 8 |
remaining generic companies and following the resolution of the litigation in our favor, the FDA Orange Book,three generic companies are not permitted to launch their products until the 2031 expiration date of the formulation patent. Both the composition of matter patent claiming apixaban specificallyexpiring in November 2026 and athe formulation patent were challenged by numerous generic companies and are theexpiring in 2031 may be subject of patent infringement litigation. Prior to the August 2020 ruling referenced in the following sentence, we and BMS settled with a number of these generic companies (settled generic companies) while continuing to litigate against three remaining generic companies (remaining generic companies). In August 2020, the U.S. District Court for the District of Delaware decided that the two challenged Eliquis patents are both valid and infringed by the remaining generic companies. The remaining generic companies have appealed the Delaware court decision and the final decision in this case could determine when generic versions of Eliquis will come on the market.
future challenges. While we cannot predict the outcome of this pendingany potential future litigation, thesethere are thecertain potential alternatives that might occur: (a) If the district court’s decision is upheld in the current appeal with respectoccur which could potentially permit generic launch prior to both patents, under the terms of previously executed settlement agreements with the settled generic companies, the permitted date of launch for the settled generic companies under these patents is April 1, 2028; (b)2028: (i) if the formulation patent is held invalid or not infringed in the currentfuture litigation, through appeal, the settled generic companies and the remaining generic companiesany successful future litigant would be permitted to launch on November 21, 2026; or (c)(ii) if both patents are held invalid or not infringed in the currentfuture litigation, through appeal, the settled generic companies and the remaining generic companiesany successful future litigant could launch products immediately upon such an adverse decision.
In addition, both patents may be subject to subsequent challenges by parties other than the remaining generic companies. If this were to occur, depending on the outcome of the subsequent challenge, the potential launch by generic companies, including challengers, if successful, could occur on timelines similar to those discussed above.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 5 |
(5)On October 31, 2023, the U.K. Supreme Court refused BMS’s permission to appeal in relation to the judgment having found the apixaban basic product patent and associated SPC invalid. Additional challenges are pending in other jurisdictions.
(6)Xtandi is being developed and commercialized in collaboration with Astellas, which has exclusive commercialization rights for Xtandi outside the U.S. Pfizer receives tiered royalties as a percentage of international Xtandi net sales.
(7)BesponsaJapanInterim patent term extension requests have been granted extending the expiry is provided by regulatory exclusivity.from December 2023 to December 2024 and Pfizer has filed applications for patent term extension to 2028.
(8)Vyndaqel (tafamidis meglumine) basic patent expiry in Japan is August 2026 for treatment of polyneuropathy. Vynmac (tafamidis) was approved in Japan for treatment of cardiomyopathy with regulatory exclusivity expiring in March 2029.
(9)Adcetris is being developed and commercialized in collaboration with Takeda. Pfizer has commercialization rights for Adcetris in the U.S. and its territories and in Canada. Takeda has commercialization rights in the rest of the world and pays Pfizer a royalty based on a percentage of Takeda's net sales of Adcetris in its licensed territories, based on annual net sales tiers.
(10)There are other U.S. patents covering related ADC uses, technology and manufacturing that remain in force beyond composition of matter expiry.
(11)Product not yet approved or authorized in this market.
(12)We have exclusive rights to Braftovi and Mektovi in the U.S., Canada and certain emerging markets. The Pierre Fabre Group has exclusive rights to commercialize both products in Europe and Ono Pharmaceutical Co., Ltd. has exclusive rights to commercialize both products in Japan. We receive royalties from The Pierre Fabre Group and Ono Pharmaceutical Co., Ltd. on sales of Braftovi and Mektovi in a majority of markets outside the U.S.
(9)(13)Mektovi U.S. expiry is provided by a method of use patent.
(10)(14)BavencioPadcev is being commercialized in collaboration with Astellas. Pfizer has co-promotion rights in the U.S. Outside the U.S., Pfizer has commercialization rights in all countries in North and South America, and Astellas has commercialization rights in the rest of the world, including Europe, Asia, Australia and Africa.
(15)There is a U.S. patent covering related ADC manufacturing that will remain in force beyond the composition of matter expiry.
(16)In September 2020, Seagen and Merck began a collaboration to commercialize Tukysa. As of December 31, 2023, this collaboration ended and all commercialization rights were returned to Seagen (Pfizer).
(17)Ngenla is being developed in collaboration with OPKO.
(18)Tivdak is developed and commercialized in collaboration with Merck KGaA.Genmab. Pfizer and Genmab have co-promotion rights in the U.S. Outside the U.S., Pfizer has commercialization rights in the rest of the world except for Japan, where Genmab has commercialization rights, and certain territories where Zai Lab Limited (Zai Lab) has commercialization rights (mainland China, Hong Kong, Macau, and Taiwan). Pfizer and Genmab equally share all costs and profits for Tivdak in the U.S., Europe, China (including the payments from Zai Lab described below) and Japan. In markets outside the U.S. other than Europe, China, and Japan, Pfizer will pay Genmab a royalty based on a percentage of aggregate net sales. Further, pursuant to the agreement with Zai Lab, Pfizer is entitled to receive potential development, regulatory and commercial milestone payments, and tiered royalties on net sales of Tivdak in the Zai Lab territories, which will be shared equally with Genmab.
(20)Product is being commercialized in collaboration with BioNTech.
(21)The basic product patent has been granted in the U.K. and expires in 2041. In the other major markets, a patent application has been filed. If granted, a full term is expected.
(22)The basic product patent application has been filed in this market. If granted, a full term is expected in this market.
(23)Pfizer does not have co-promotion rights for this product in Germany.
Loss of Intellectual Property Rights. The loss, expiration or invalidation of intellectual property rights, patent litigation settlements with manufacturersand judgments and the expiration of co-promotion and licensing rights can have a significantmaterial adverse effect on our revenues. Once patent protection has expired or has been lost prior to the expiration date as a result of a legal challenge, we typically lose exclusivity on these products, and generic and biosimilar pharmaceutical manufacturers generally produce identical or highly similar products and sell them for a lower price. The date at which generic or biosimilar competition commences may be different from the date that the patent or regulatory exclusivity expires. However, when generic or biosimilar competition does commence, the resulting price competition can substantially decrease our revenues for the impacted products, often in a very short period of time. Also, if one of our product-related patents is found to be invalid by judicial, court or regulatory or administrative proceedings, generic or biosimilar products could be introduced, resulting in the erosion of sales of our existing products. Additionally, we could be subject to claims that our intellectual property rights infringe third party patents.
Losses of Product Exclusivity. Certain of our products have experienced patent-based expirations or loss of regulatory exclusivity in certain markets in the last few years, and we expect certain products to face significantly increased generic competition over the next few years. The basic product patent for Chantix in the U.S. expired on November 10, 2020. Also, the basic product patent for Sutent in the U.S. will expire in August 2021. For additional information on the impact of LOEs on our revenues, see the Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion section within MD&A.
Trademarks. Our products are sold under brand-name and logo trademarks and trade dress. Registrations generally are for fixed, but renewable, terms and protection is provided in some countries for as long as the mark is used while in others, for as long as it is registered. Protecting our trademarks is of material importance to Pfizer.us.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 9 |
COMPETITION
Our business is conducted in intensely competitive and often highly regulated markets. Many of our products face competition in the form of branded or generic drugs or biosimilars that treat similar diseases or indications. The principal forms of competition include efficacy, safety, ease of use and cost. Though the means of competition vary among our products, demonstrating the value of our products is a critical factor for success.
We compete with other companies that manufacture and sell products that treat or prevent diseases or indications similar to those treated or prevented by our major products. These competitors include other worldwide research-based biopharmaceutical companies, smaller research companies with more limited therapeutic focus and generic drug and biosimilar drug manufacturers. Our competitors also may devote substantial funds and resources to R&D and their successful R&D could result in erosion of the sales of our existing products and potential sales of our products in development, as well as unanticipated product obsolescence. In addition, several of our competitors operate without large R&D expenses and make a regular practice of challenging our product patents before their expiration.
To help address competitive trends we continually emphasize innovation, which is underscored by our multi-billion-dollar investment in R&D, as well as our business development transactions, both designed to result in a strong and differentiated product pipeline. Our investment in research continues even after drug or vaccine approval as we seek to further demonstrate the value of our products for the conditions they treat or prevent, as well as investigating potential new applications. We educate patients, physicians, payerspayors and global health authorities on the benefits and risks of our medicines and vaccines, and seek to continually enhance the organizational effectiveness of our biopharmaceutical functions, including our efforts to accurately and ethicallyeffectively launch and market our products to our customers.
Operating conditions have also shifted as a result of increased global competitive pressures, industry regulation and cost containment. We continue to evaluate, adapt and improve our organization and business practices in an effort to better meet customer and public needs. We believe that we have taken an industry-leading role in evolving our ethical approaches to U.S. direct-to-consumer advertising, interactions with, and payments to, healthcare professionals and medical education grants. We also continue to sponsorsupport programs to address patient affordability and access barriers, as we strive to advance fundamental health system change through our support for better healthcare solutions.
For example, in May 2022, we launched
Our vaccines have and may continue to face competition, including from the introduction of alternative vaccines or “next-generation” vaccines prior to or after the expiration of their patents, which may adversely affect our future results.
Our biosimilars, which include biosimilars of certain inflammation & immunology and oncology biologic medicines, compete with branded products from competitors, as well as other generics and biosimilars manufacturers. We sell biosimilars of certain inflammation & immunology and oncology biologic medicines. We seek to maximize the opportunity to establish a “first-to-market” or early market position for our biosimilars to provide customers a lower-cost alternative immediately when available and also to potentially provide us with higher levels of sales and profitability until other competitors enter the market.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 6 |
Generic Products. Generic pharmaceutical manufacturers pose one of the biggest competitive challenges to our branded small molecule products because they can market a competing version of our product after the expiration or loss of our patent protection and often charge much less. Several competitors regularly challenge our product patents before their expiration. Generic competitors often operate without large R&D expenses, as well as without costs of conveying medical information about products to the medical community. In addition, the FDA approval process in the U.S. and in the EU exempts generics from costly and time-consuming clinical trials to demonstrate their safety and efficacy, allowing generic manufacturers to rely on the safety and efficacy data of the innovator product. In China, for example, given the expansion of the QCE process and continuation of the VBP program, we are expectedexpect to continue to face further intensified competition by certain generic manufacturers in 20212024 and beyond, which has and may continue to result in price cuts and volume loss of some of our products. In addition, generic versions of competitors’ branded products have and may alsocontinue to compete with our products.
MCOs that focus primarily on the immediate cost of drugs often favor generics over brand-name drugs. Many governments alsoCommercial and government payors typically encourage the use of generics as alternatives to brand-name drugs in their healthcare programs, including Medicaid in the U.S., and U.S. laws generally allow, and in some cases require, pharmacists to substitute generic drugs for brand-name drugs. In a small subset of states, prescribing physicians are able to expressly prevent such substitution.
Biosimilars. Certain of our biologic products, including Enbrel (we market Enbrel outside the U.S. and Canada), already face, or may face in the
future, competition from biosimilars (also referred to as follow-on biologics). Biosimilars are versions of biologic medicines that have been developed and proven to be highly similar to the original biologic in terms of safety and efficacy and that have no clinically meaningful differences in safety, purity or potency. Biosimilars have the potential to offer high-quality, lower-cost alternatives to innovative biologic medicines. In the U.S., biosimilars referencing innovative biologic products are approved by the FDA under the U.S. Public Health Service Act.Act, whereas in the EU the EMA is responsible for evaluating the majority of applications for biosimilars through the centralized procedure.
PRICING PRESSURES AND MANAGED CARE ORGANIZATIONS
Commercial Pricing Pressures. Pricing and access pressures in the commercial sector continue to be significant. Overall, there is increasing pressure on U.S. providers to deliver healthcare at a lower cost and to ensure that those expenditures deliver demonstrated value in terms of health outcomes. Many employers have adopted or make available high deductible health plans, which can increase out-of-pocket costs for medicines. This trend is likely to continue. Private third-party payers,payors, such as health plans, increasingly challenge pharmaceutical product pricing, which could result in lower prices, lower reimbursement rates for payors and a reduction in demand for our products.products, including denial of coverage of our products, if lower cost alternatives are available. Payors often require significant discounts, or rebates, from our prices in exchange for more favorable formulary placement. Pricing pressures also may occur as a result of highly competitive insurance markets.biopharmaceutical markets and increasing concentration of insurers and PBMs. Healthcare provider purchasers, directly or through group purchasing organizations, are seeking enhanced discounts or implementing more rigorous bidding or purchasing review processes.
Longer term, we foresee a shift in focus among payors and their PBMs away from fee-for-service paymentsreimbursement towards outcomes-based payments and risk-sharing arrangements that reward providers and pharmaceutical manufacturers for cost reductions and improved patient outcomes. These new payment models can, at times, lead to lower prices for, and restricted access to, new medicines. At the same time, these models can also promote utilization of drugs by encouraging physicians to screen and diagnose and consider drugs as a means of forestalling more costly medical interventions. Further, these models may also encourage payors and their PBMs to cover higher cost drugs where coverage is tied to patient outcomes and other quality incentives.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 10 |
The impact of large-scale healthcare disruptions, like the COVID-19 pandemic, on the pace of adoption of value-based payment models remains unclear. Both payors and providers may resist adopting such models or choose to adopt such models at a slower pace if the incentives available do not outweigh the financial risk involved. Adoption of such models, in particular models that involve downside risk, may depend on revenue predictability for hospitals and other institutional providers, many of which are still struggling to recover financially following the COVID-19 pandemic. Providers in more advanced value-based payment models, such as full capitation, a fixed amount paid in advance per-patient per-unit of time-period, generally found their revenues remained steady during the COVID-19 pandemic, which may ultimately encourage the growth of such models. Going forward, we expect continued focus on value-based payment models that support financial resiliency and advance healthcare equity by incorporating features intended to reduce disparities in healthcare quality and access experienced by underrepresented and underserved populations.
We believe medicines and vaccines are the most efficient and effective use of healthcare dollars based on the value they deliver to the overall healthcare system. We work with law makers and advocate for solutions that effectively improve patient health outcomes, lower costs to the healthcare system, and help ensure access to medicines and vaccines within an efficient and affordable healthcare system. This includes assessing our go-to market model to help address patient affordability challenges. We have engaged with major payors and the U.S. government to explore opportunities to improve access and reimbursement in an effort to drive pro-patient policies. In addition, in response to the evolving U.S. and global healthcare spending landscape, we work with health authorities, health technology assessment and quality measurement bodies and major U.S. payerspayors throughout the product-development process to better understand how these entities value our compounds and products. Further, we seekare developing stronger support designed to develop stronger internal capabilities focused on demonstratingdemonstrate the net value of the medicines and vaccines that we discover or develop, register and manufacture, by recognizing patterns of usage of our medicines and competitor medicines along with patterns of healthcare costs.
Managed Care Organizations. The evolution of managed care in the U.S. has been a major factor in the competitiveness of the healthcare marketplace. Approximately 299318 million people in the U.S. now have some form of health insurance coverage, and the marketing of prescription drugs and vaccines to both consumers and the entities that manage coverage in the U.S. continues to grow in importance. In particular, the influence of MCOs has increased in recent years due to the growing number of patients receiving coverage through MCOs. At the same time, consolidation in the MCO industry has resulted in fewer, even larger entities,MCOs, which enhances those MCOs’ ability to negotiate lower pricing and further increases their importance to our business. Since MCOs seek to contain and reduce healthcare expenditures, their growing influence has increased downward pressure on drug prices, as well as negatively impacted revenues.
MCOs and their PBMs typically negotiate prices with pharmaceutical providers by using formularies (which are lists of approved medicines available to MCO members), clinical protocols (which require prior authorization for a branded product if a generic product is available or require the patient to first fail on one or more generic products before permitting access to a branded medicine), volume purchasing, long-term contracts and their ability to influence volume and market share of prescription drugs. In addition, by placing branded medicines on higher-tier or non-preferred status in their formularies, MCOs transfer a portion of the cost to the patient resulting in significanthigher patient out-of-pocket expenses. This financial disincentive is a tool for MCOs to manage drug costs and channel patients to medicines preferred by the MCOs. The ACA has acceleratedWe expect payment reform by distributing risk acrossreforms for MCOs will continue to evolve with increased emphasis on expanded participation and other stakeholders in care delivery with the intent of improving quality while reducing costs, which creates pressure on MCOsremoving barriers to tie reimbursement to defined outcomes. We are closely monitoring these newer approaches and developing appropriate strategies to respond to them.
The breadth of the products covered by formularies can vary considerably from one MCO to another, and many formularies include alternative and competitive products for treatment of particular medical problems. MCOs also emphasize primary and preventive care, out-patient treatment and procedures performed at doctors’ offices and clinics as ways to manage costs. Hospitalization and surgery, typically the most expensive forms of treatment, are carefully managed, and drugs that can help in chronic care management and reduce the need for hospitalization, professional therapy or surgery may become favored first-line treatments for certain diseases.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 7 |
At the same time, MCOs may seek to exclude high-cost drugs from formularies in their efforts to manage and lower their costs.Exclusion of a product from a formulary or other MCO-implemented restrictions can significantly impact drug usage in the MCO patient population and beyond. Consequently, pharmaceutical companies compete to gain access to formularies for their products, typically on the basis of unique product features, such as greater efficacy, better patient ease of use, or fewer side effects, as well as the overall cost of the therapy. We have been generally, although not universally, successful in havingcontinue to seek to ensure that our major products are included on MCO formularies. However, increasingly our branded products are increasingly being placed on the higher tiers or in a non-preferred status. For additional information, seeContinuing efforts by managed care entities to contain or reduce costs of healthcare and/or impose price controls may adversely affect demand for our products and our financial performance. See the Item 1A. Risk Factors—Managed Care Trends section in this Form 10-K.section. RAW MATERIALS
We procure raw materials essential to our business from numerous suppliers worldwide. In general, these materials have been available in sufficient quantities to support our demand and in many cases are available from multiple suppliers. We have supplier management activities in place to monitor supply channels and to take action as needed to secure necessary volumes. No significant impact to our operations due to the availability of raw materials is currently anticipated in 2021.2024. However, we continue to see heightened demand in the industry for certain components and raw materials, which could potentially result in constraining available supply leading to a possible future impact on our business. We are continuing to monitor and implement mitigation strategies to reduce any potential risk or impact including active supplier management, qualification of additional suppliers and advanced purchasing to the extent possible.
GOVERNMENT REGULATION AND PRICE CONSTRAINTS
We are subject to extensive regulation by government authorities in the countries in which we do business. This includes laws and regulations governing pharmaceuticalthe operations of biopharmaceutical companies, such as the approval, manufacturing and marketing of products, pricing (including discounts and rebates) and healthprice reporting, interactions with healthcare professionals, institutions, and referral sources, reporting of remuneration provided to healthcare providers and academic medical centers, financial assistance provided to patients, clinical research, data privacy and information privacy,security, among others. These laws and regulations may require administrative guidance for implementation, and a failure to comply could subject us to legal andand/or administrative actions. Enforcement measures may include substantial fines and/or penalties, orders to stop non-compliant activities, criminal charges, warning letters, product recalls or seizures, delays in product approvals, exclusion from participation in government programs or contracts as well as limitations on conducting business in applicable jurisdictions, and could result in harm to our reputation and business. For additional information, seeSee Note 16A.16A. Compliance with these laws and regulations may be costly, and may require significant technical expertise and capital investment to ensure compliance. While capital expenditures or operating costs for compliance with government regulations
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 11 |
cannot be predicted with certainty, we do not currently anticipate they will have a material effect on our capital expenditures or competitive position.
In the United States
Drug and Biologic Regulation. The FDA, pursuant to the FFDCA, the Public Health Service Act and other federal statutes and regulations, extensively regulates pre- and post-marketing activities related to our biopharmaceutical products.products and devices. The regulations govern areas such as the safety and efficacy, of medicines, clinical trials, advertising and promotion, quality control, manufacturing, labeling, distribution, post-marketing safety surveillance and reporting, and record keeping. Other U.S. federal agencies, including the DEA, also regulate certain of our products and activities. Many of our activities are subject to the jurisdiction of the SEC.
For a biopharmaceutical company to market a drug or a biologic product, in the U.S.,including vaccines, the FDA must evaluate whether the product is safe and effective for its intended use. If the FDA determines that the drug or biologic is safe and effective, the FDA will approve the product’s NDA or Biologics License Application (BLA)BLA (or supplemental NDA or supplemental BLA), as appropriate.
A drug or biologic may be subject to postmarketing commitments, which are studies or clinical trials that the product sponsor agrees to conduct, or postmarketing requirements, which are studies or clinical trials that are required as a condition of approval. Once a drug or biologic is approved, the FDA must be notified of any product modifications and may require additional studies or clinical trials. In addition, we are also required to report adverse events and comply with cGMPs (the FDA regulations that govern all aspects of manufacturing quality for pharmaceuticals) and the Drug Supply Chain Security Act (the law that, among other things, sets forth requirements related to product tracing, product identifiers and verification for manufacturers, wholesale distributors, re-packagers and dispensers to facilitate the tracing of product through the pharmaceutical distribution supply chain), as well as advertising and promotion regulations. For additional information, seeSee the Item 1A. Risk Factors—Development, Regulatory Approval and Marketing of Productsand—Post-Authorization/Approval Data —Post-Approval Data section in this Form 10-K.
In the context of public health emergencies, like the COVID-19 pandemic, we may apply for EUA withto the FDA for an EUA which, whenif granted, allows for the distribution and use of our products during the term declared and extended by the government,emergency, in accordance with the conditions set forth in the EUA, unless the EUA is otherwise terminated atby the government’s discretion.government. Although the criteria offor an EUA differ from the criteria for approval of an NDA or BLA, EUAs nevertheless require the development and submission of data to satisfy the relevant FDA standards, and a number of ongoing compliance obligations. The FDA generally expects EUA holders to work toward submission of full applications, such as a BLA or an NDA, as soon as possible. For BNT162b2, we are working towards submitting a BLA for possible full regulatory approval.
Biosimilar Regulation. The FDA is responsible for approval of biosimilars. Innovator biologics, or reference products, are entitled to 12 years of market exclusivity by statute, andexclusivity. Applications for biosimilars applications may not be submitted until four years after the approval ofdate on which the reference innovator biologic.
Sales and Marketing Regulations. Our marketing practices are subject to federal and state laws as well as federal laws, such as the Anti-Kickback Statute (AKS), Civil Monetary Penalties Law and False Claims Act, intended to prevent fraud and abuse in the healthcare industry. The Anti-Kickback Statute generallyAKS prohibits soliciting, offering, receiving, or paying anything of value to generate business.business that may be paid for, in whole or in part, by a federal healthcare program. The Civil Monetary Penalties Law covers a variety of conduct, often violations under other laws, and includes penalties for AKS violations as well as causing the submission of false claims. The False Claims Act generally prohibits anyone from knowingly and willingly presenting, or causing to be presented, any claims for payment for goods or services, including to third-party payers (includinggovernment payors, such as Medicare and Medicaid)Medicaid, that are false or fraudulent and generally treat claims generated through kickbacks asincluding false or fraudulent.certifications of compliance with applicable law. The federal government and states also regulate sales and marketing activities and financial interactions between manufacturers and healthcare providers, requiring disclosure to government authorities and the public of such interactions, and the adoption of compliance standards or programs. State attorneys general have also taken action to regulate the marketing of prescription drugs under state consumer protection and false advertising laws.
Healthcare ReformPricing, Reimbursement and Access Regulations.. Pricing and reimbursement for our products depend in part on government regulation. Any significant efforts at the federal or state levels to reform the healthcare system by changing the way healthcare is provided or funded or to expand controls on drug pricing, government reimbursement, and access to medicines and vaccines on public and private insurance plans could have a material impact on us. This includes potential replacements for the ACA, if it is ultimately invalidated by the U.S. Supreme Court in California v. Texas, as well as efforts at the state level to develop additional public insurance options or implement a single payer healthcare system. We do not expect that invalidation of the ACA itself would have a material impact on our business given the modest revenues the health insurance exchanges and Medicaid expansion generate for us. However, a future replacement of the ACA or other healthcare reform efforts may adversely affect our business and financial results, particularly if such replacement or reform reduces incentives for employer-sponsored insurance coverage or dramatically increases industry taxes and fees.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 8 |
Pricing and Reimbursement. Pricing and reimbursement for our products depend in part on government regulation. In order to have our products covered by Medicaid, weWe must offer discounts or rebates on purchases of pharmaceutical products under various federalgovernment programs including Medicare, Medicaid, the Veterans Administration and state programs.the 340B Drug Pricing Program (340B Program). We also must report specific prices to government agencies. The calculations necessary to determine the prices reported are complex and the failure to do so accurately may expose us to enforcement measures. See the discussion regarding rebates in theAnalysis of the Consolidated Statements of Income—Product Revenue DeductionsRevenues by Geography section within MD&A and Note 1G.1G.The drug pricing provisions of the IRA, which was signed into law in August 2022, began to be implemented in 2022 and implementation will continue over the next several years. The IRA includes several provisions to lower prescription drug costs for Medicare patients and to reduce drug spending by the federal government. Among other things, the IRA enhances the Medicare Part D benefit by eliminating the coverage gap (“donut hole”) beginning in 2025, adds a maximum out-of-pocket cap for Medicare beneficiaries (set at $2,000 for 2025), and creates a new program that allows patients to pay their cost-sharing over time. The law also requires manufacturers to provide a 10% discount on branded prescriptions in the initial coverage phase and a 20% discount in the catastrophic phase, imposes rebates under Medicare Part B and Medicare Part D on drug price increases that outpace inflation, and directs HHS to set the prices of certain high-expenditure, single-source drugs and biologics covered under Medicare (known as the “Medicare Drug Price Negotiation Program”). In August 2023, the Biden Administration published the first ten medicines subject to the Medicare Drug Price Negotiation Program, which included Eliquis. As a selected drug, CMS will establish a “maximum fair price” for Eliquis and that price will be published by September 1, 2024. The price will be in effect in 2026. The maximum fair price established by CMS is required to be offered to all Medicare beneficiaries and to covered entities participating in the 340B Program if that maximum fair price is lower than the discounted price such entities are offered under the 340B Program ceiling price calculation. In addition, there will be a new Medicare manufacturer discount program agreement expected to be signed in March 2024 that will change our discounting obligations for all medicines in Medicare, with few exceptions, beginning in 2025. The Medicare Drug Price Negotiation Program is currently subject to legal challenges and therefore, the outcome of the Program remains uncertain. We continue to evaluate the impact of the IRA on our business, operations and financial condition and results as the full effect of the IRA on our business and the pharmaceutical industry remains uncertain.
Changes to the Medicaid Drug Rebate Program or the 340B Program could have a material impact on our business. For example, certain changes finalized by CMS in a December 2020 final rule, including which products qualify as so-called “line extension” drugs subject to increased rebate liability, may have a material impact on our business. Additionally, in May 2023, CMS proposed new rules that could, if finalized, have a material impact on our business. Those proposals include, for example, new rules regarding how manufacturers would be required to aggregate discounts for purposes of determining their Medicaid Best Price. Additionally, various potential changes to the 340B Program are undergoing
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 12 |
review or are the subject of current regulatory activity and/or litigation, and their status is unclear. In 2022, we implemented a policy that will help improve contract pharmacy integrity. The HHS Health Resources and Services Administration (HRSA), which administers the 340B Program, has sent letters to numerous manufacturers that have also implemented contract pharmacy policies and integrity initiatives; the letters express HRSA’s view that those manufacturers’ policies are in violation of the 340B statute. HRSA also has referred some of those other manufacturers to the HHS Office of Inspector General (OIG) for potential enforcement action. Pfizer has not received an enforcement letter from HRSA to date relating to our 340B Program integrity initiative. Several manufacturers have challenged HRSA’s enforcement letters in federal court and litigation is ongoing in those cases. We believe that our policy is consistent with the statute. In addition, some states have enacted laws seeking to restrict manufacturer policies related to contract pharmacy transactions in their states. At least one state has begun to pursue enforcement proceedings under its law. Several stakeholders have challenged such laws in certain states. Other states have considered and could enact similar laws going forward, although any such laws also may be subject to legal challenges. Additional legal or legislative developments at the federal or state level with respect to the 340B Program may have an adverse impact on our integrity initiative, and we may face enforcement action or penalties, depending upon such developments. The 340B Program continues to be a subject of regulatory activity, congressional scrutiny and inquiries, litigation, and other developments, any or all of which could affect the scope of the program and Pfizer’s obligation to offer discounts to 340B Program covered entities under the program. See the Item 1A. Risk Factors—Pricing and Reimbursement section. States seek to control healthcare costs related to Medicaid and other state regulated healthcare programs. A majority of states use preferred drug lists to manage access to pharmaceutical products under Medicaid, including some of our products. States may seek to negotiate supplemental rebate agreements that are larger than the minimum federal requirement for preferred formulary access. Preferred access to our products under the Medicaid managed care programs are often determined by the managed care health plans contracted by the state to administer benefits, which may also require supplemental rebates for preferred formulary access. We expect states will continue to seek cost cutting, which may focus on managed care capitation payments, supplemental rebates, and/or formulary management.
We expect to see continued focus by Congress and the Biden Administration on regulating pricing and access to medicine, in addition to actions already taken, which could result in legislative and regulatory changes. Government and private payerspayors routinely seek to manage utilization and control the costs of our products, and thereproducts. There is considerable public and government scrutiny of pharmaceutical pricing. Effortspricing and actions being taken at the state and federal level. Further efforts by states and the federal government to regulate prices or payment for pharmaceutical products, including proposed actions to facilitate drug importation, such as Florida’s drug importation program which was recently authorized by the FDA, limit reimbursement to lower international reference prices, require deep discounts, impose financial penalties related to pricing practices, and require manufacturers to report and make public price increases and sometimes a written justification for the increase, could adversely affect our business if implemented. In the Fall of 2020, the Trump Administration finalized an importation pathway from CanadaFurther, commercial payors often follow Medicare coverage and areimbursement policies when setting their own payment modelrates. Any reduction in cost or other containment measures may similarly be adopted by commercial plans. Payors may continue to tie Medicare Part B physician reimbursementpromote generic drugs and biosimilars more aggressively to international prices, though ultimate implementation of both is uncertain duegenerate savings and attempt to legal challenges. We expect to see continued focus on regulating pricing resulting instimulate additional legislation and regulation under the newly elected Congress and the Biden Administration. In addition, U.S. government action to reduce federal spending on entitlement programs including Medicare and Medicaid may affect payment for our products or services associated with the provision of our products. For additional information, see the Item 1A. Risk Factors—Pricing and Reimbursement section in this Form 10-K.
A majority of states use preferred drug lists to manage access to pharmaceutical products under Medicaid, including some of our products. For example, access to our products under the Medicaid and Medicare managed care programs typically is determined by the health plans with which state Medicaid agencies and Medicare contract to provide services to beneficiaries. States seek to control healthcare costs related to Medicaid and other state healthcare programs, including the implementation of supplemental rebate agreements under the Medicaid drug rebate program tied to patient outcomes.price competition. In addition, we expect that consolidation and integration among pharmacy chains, wholesalers and PBMs will increase pricing pressures in the industry. For additional information, seeSee the Item 1A. Risk Factors—Managed Care Trends section in this Form 10-K.section.
Anti-Corruption. The FCPA prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate to obtain or retain business abroad. The scope of the FCPA includes interactions with certain healthcare professionals in many countries. Other countries have enacted similar anti-corruption laws and/or regulations.
Data Privacy. The collection and usenumber of personal data by us as part of our business activities is subject to various federal and state privacy and data security laws and regulations including oversight by variousin the U.S. to which we are subject on the federal and state level continues to increase. We routinely collect and use sensitive personal information relating to digital health. The legislative, regulatory or other governmental bodies. Suchand litigation landscape for privacy and data protection requirements is rapidly evolving and changing. These requirements are not universal and can conflict between jurisdictions. Compliance with those laws and regulations haveis made more complex by the potentiallack of consistent standards, common definitions, or clear regulatory expectations. At the same time, enforcement of these laws and regulations is increasing and litigation is becoming more common. Any failure or perceived failure by us to affectcomply with applicable privacy and data protection laws and regulations, including cybersecurity breaches or incidents, could subject us to significant fines and penalties, litigation, and negatively impact our business materially, continue to evolve and are increasingly being enforced vigorously.reputation.
Outside the United States
We encounter similar regulatory and legislative issues in most countries outside the U.S.
New Drug Approvals.Approvals. In the EU, the EMA conducts the scientific evaluation, supervision and safety monitoring of our innovative medicinal products and employs athat are eligible for the centralized marketing authorization procedure. Through the centralized procedure, pharmaceutical companies may submit to the EMA a single application for approval fora marketing authorization valid in all the EU and the European Economic Area (EEA) countries. From January 1, 2021, asThe EC takes a consequencelegally binding decision based on the EMA's recommendation. For medicinal products that are not eligible for the centralized procedure, the mutual recognition procedure is based on the recognition of a pre-existing national marketing authorization by one or more EU member states, and the decentralized procedure allows the submission of a marketing authorization application simultaneously in several EU member states. In the U.K. leaving the EU (Brexit), the Medicines and Healthcare productsProducts Regulatory Agency is the sole regulatory authority for the U.K. In China, following significant regulatory reforms in recent years, the NMPA is the primary regulatory authority for approving and supervising medicines.authority. In Japan, the PMDAPharmaceuticals and Medical Device Agency is involved in a wide range of regulatory activities, including clinical studies, approvals, post-marketing reviews and pharmaceutical safety. In China, the National Medical Product Administration is the primary regulatory authority for approving and supervising medicines. Health authorities in many middle- and lower-income countries might require marketing approval or scientific opinions by a recognized regulatory authority (i.e.(e.g., the FDA or EMA) before they begin to conduct their application review process and/reviewing or issue their final approval.approving applications. By way of example, the EMA, in cooperation with the World Health Organization (WHO), can provide scientific opinions on high priority human medicines, including vaccines, for markets outside the EU.
Pharmacovigilance. In the EU/EEA,EU, the EMA’s Pharmacovigilance Risk Assessment CommitteePRAC is responsible for reviewing and making recommendations on product safety issues. Specifically, the PRAC focuses on detecting, assessing and communicating the risks associated with adverse reactions of medicinal products, while considering their therapeutic effects. It also evaluates post-authorization safety studies and conducts pharmacovigilance audits. Outside developed markets, pharmacovigilance requirements vary and are generally not as extensive, but there is a trend toward increasing regulation.
Pricing and Reimbursement. Certain governments, including in the different EU member states, the U.K., Japan, China, Japan, Canada and South Korea, provide healthcare at low-to-zero direct cost to consumers at the point of care and have significant power to regulate pharmaceutical
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 13 |
prices or patient reimbursement levels to control costs for the government-sponsored healthcare system, particularly under recent global financing pressures. Governments globally may use a variety of measures to control costs, including, proposing price reformamong others, legislative or legislation,regulatory pricing reforms, cross country collaboration and procurement, price cuts, mandatory rebates, health technology assessments, forced localization as a condition of market access, “international reference pricing” (i.e., the practice of a country linking its regulated medicine prices to those of other countries), QCE processes and VBP. In addition, the international patchwork of price regulation, differing economic conditions and incomplete value assessments across countries has led to varying access to quality medicines in many markets and some third-party trade in our products between countries. Several important multilateral organizations such as the World Health Organization and the Organization for Economic Cooperation and Development, are increasing scrutiny ofWHO scrutinize international pharmaceutical pricing through issuing reportspolicy recommendations and policy recommendations. On November 25, 2020,sponsorship of programs, such as “The Oslo Medicines Initiative” (OMI) which aims to ensure “affordability for high-priced medicines”. The OMI concluded its work in September 2022, and the European Commission published its new Pharmaceutical Strategy for WHO/Europe which envisions a broad range of new initiativesAccess to Novel Medicines Platform was established to enhance affordable and legislation including a significant focus on affordability andequitable access to medicines.
In China, pricing pressures have increased in recent years because of an overall focus on healthcare cost containment with the central government officials emphasizing improved health outcomes healthcare reform and decreased drug prices as key indicators of progress towards its healthcare reform. DrugState owned hospitals and the state insurance program account for the vast majority of all drug purchases. For patented innovative products, drug prices have decreased dramatically as a result of adding innovative drugs (including oncology medicines)medicines, medicines for children and orphan drugs) to the National Reimbursement Drug List (NRDL). In the off-patent space, numerous local generics have been officially deemed bioequivalent under a QCE process that required domestically-manufactured generic drugs to pass a test to assess their bioequivalence to a qualified reference drug (typically the originator drug).via access-price negotiation. A centralized VBP program has also been initiated and expanded nationwide, under whichwith a tendertendering process has been established where a certain portion of included molecule volumes are guaranteed to tender winners. The program is intendedaims to contain healthcare costs by driving utilization of generics that have passed QCE, whichQCE. This has resulted in dramaticfurther lowering the price cuts forof medicines, especially off-patent medicines. Furthermore, the Chinese government has discussed moving toward effortsmedicines; this trend is expected to unify the reimbursement price between QCE-approved generic medicinescontinue. China is increasing its use of Health Technology Assessment and the applicable original medicines, which the government currently plans to
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 9 |
implementis controlling mark-ups within the next few years. Wecountry using a two-invoice limited system, which is a government policy that regulates the pricing of pharmaceutical products and medical devices. Pfizer, along with most off-patent originators, have mostly not been successful in the VBP bidding process. The government has indicated that additional post-LOE drugs (including biological products) could be subjected to QCEVBP qualification in future rounds. Certain of our products, such as Sulperazon and Vfend injectables, were included as candidates in VBP rounds which could also be tied to volume-based procurement.in 2023, and Pfizer was not successful in the bidding process for such products. While certain details of future QCE expansion have been made available, we are unable to determine the impact on our business and financial condition untilof the initiation of these future rounds.
Healthcare Provider Transparency and Disclosures. Several countries have implemented laws requiring (or their industry trade associations have recommended) disclosure of transfers of value made by pharmaceutical companies to healthcare providers.
Intellectual Property. Reliable patent protection and enforcement around the world are among the key factors we consider for continued business and R&D investment. The World Trade OrganizationWTO Agreement on Trade Related Aspects of Intellectual Property Rights (WTO-TRIPS) requires participant countries to provide patent and other intellectual property-related protection for pharmaceutical products by law, with ana time-limited exemption provided for least-developed countries until 2033.countries. While some countries have made improvements, we still face patent grant, enforcement and other intellectual property challenges in many countries.
While the global intellectual property policy environment has generally improved following implementation of WTO-TRIPS and bilateral/multilateral trade agreements, our growth and ability to bring new product innovation to patients depends on maintaining those standards and further progress in intellectual property protection. In certain developed international markets, governments maintain relatively effective intellectual property policies. However, in the EU, following apursuant to the ongoing review of pharmaceutical intellectual property and regulatory incentives, proposals introduced in 2023 may reduce the basic period of regulatory data protection from eight to six years, subject to the outcome of the ongoing legislative change may result in the reduction of certain protections.procedure. In several emerging market countries, governments have used intellectual property policies as a tool to force innovators to accept less than fair value for medicines, as well as to advance industrial policy and localization goals.
Considerable political and economic pressure has weakened current intellectual property protection in some countries and has led to policies such as more restrictive standards for obtaining patents and more difficult procedures for patenting biopharmaceutical inventions, restrictions on patenting certain types of inventions, revocation of patents, laws or regulations that promote or provide broad discretion to issue a compulsory license, weak intellectual property enforcement and failure to implement effective regulatory data protection.
Our industry advocacy efforts focus on seeking a fair and transparent business environment for foreign manufacturers, underscoring the importance of strong intellectual property systems for localall innovative industries (both domestic and foreign) and helping improve patients’ access to innovative medicines.medicines and vaccines.
Data Privacy. Outside of the U.S., many countries haveWe are subject to extensive privacy and data securityprotection laws and regulations around the world concerning the collection, use and sharing of personal data. We routinely collect and use ofsensitive personal data, including the EU’s General Data Protection Regulations.information relating to digital health. The legislative, regulatory and regulatory frameworklitigation landscape for privacy and data protection issues worldwiderequirements is rapidly evolving as countries continueand changing. These requirements are not universal and can conflict between jurisdictions. Compliance with those laws and regulations is made more complex by the lack of consistent standards, common definitions, or clear regulatory expectations. At the same time, enforcement of these laws and regulations is increasing and fines and penalties are also increasing. Any failure or perceived failure by us to adoptcomply with applicable privacy and data security laws.protection laws and regulations, including cybersecurity breaches or incidents, could subject us to significant fines and penalties, litigation, and negatively impact our reputation.
ENVIRONMENTAL MATTERS
Our operations are affected by national, state and/or local environmental laws. We have made, and intend to continue to make, the expenditures necessary for compliance with applicable laws. We also are cleaning up environmental contamination from past industrial activity at certain sites. We incurred capital and operational expenditures in 20202023 for environmental compliance purposes and for the clean-up of certain past industrial activity as follows: $42$92 million in environment-related capital expenditures and $120$158 million in other environment-related expenses.
While capital expenditures or operating costs for environmental compliance cannot be predicted with certainty, we do not currently anticipate they will have a material effect on our capital expenditures or competitivefinancial position. See also Note 16A3. As a science guided organization, we take a proactive approach to our environmental sustainability initiatives. In 2022, we announced a new goal to further reduce greenhouse gas (GHG) emissions and achieve the Science Based Target Initiative’s voluntary Net-Zero Standard by 2040. As part of this goal, Pfizer aims to decrease its GHG emissions by 95% and its value chain emissions by 90% from 2019 levels by 2040. To support our goal, we are developing and implementing our emission reduction plan, which will include strategies to achieve reductions throughout our value chain including investing in new technologies and innovative climate solutions, and setting expectations for our suppliers to establish science-aligned GHG emission reduction goals. Our emission reduction plan-related expenses and capital spending incurred for 2023 were not
Climate change presents risks | | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 14 |
material to our operations, including the potential for additional regulatory requirementsconsolidated financial statements. While we expect to incur incremental capital and associated costs, and the potential for more frequent and severe weather events and water availability challenges that may impactoperational expenditures to meet our facilities and those of our suppliers. We cannot provide assurance that physical risks to our facilities or supply chain due to climate change will not occur in the future. We periodically review our vulnerability to potential weather-related risks and other natural disasters and update our assessments accordingly. Based on our reviews,goal, we do not believe these potentialcurrently anticipate they will have a material effect on our financial position in the near term. Longer term uncertainties such as the likelihood of commercially available technologies make it difficult to predict the financial impact of meeting the goal, and we will continue to assess and monitor the financial impact of the emission reduction plan.
HUMAN CAPITAL
Our purpose is clear:is: Breakthroughs that change patients’ lives. These breakthroughs are delivered through the relentless collaboration of our talented workforce. As of December 31, 2020,2023, including Seagen colleagues, we employed approximately 78,50088,000 people worldwide, with approximately 29,40035,000 based in the U.S. Women compose approximately 48%52% of our global workforce, and approximately 32%39% of our U.S.-based employees are individuals with ethnically diverse backgrounds.
Our continued success links directly to the commitment, engagement and performance of our employees. It is important that we not only attract and retain the best and brightest diverse talent, but also ensure they remain engaged and can thrive in an environment that is committed to helping them grow, succeed and contribute directly to achieving our purpose. As partAt Pfizer, prioritizing a positive colleague experience is of these efforts,utmost importance, particularly during times of business transformation. We were conscious of the impact that the challenges and opportunities facing our business throughout the year had on colleagues. Our goal is to prioritize the health and wellness of our colleagues, creating an environment where colleagues can excel in their work and advance our purpose. To achieve this, we strive forto cultivate an inclusive and empowering work environment, adopting practices to simplifyenvironment. This involves simplifying processes and remove needlesseliminating unnecessary complexity, rewardingrecognizing both performance and leadership skills, fostering career growth and offeringinternal mobility, and providing competitive compensation and benefits programs that encourage healthy work-life balance, so that all colleagues feel ready, equippedpromote mental and energizedphysical well-being.
Core Values.To fully realize Pfizer’s purpose we have established a clear set of goals regarding what we need to deliver innovative breakthroughs that extendachieve for patients and significantly improvehow we will go about achieving them. The “how” is represented by four simple, powerful company core values – Courage, Excellence, Equity and Joy.
Each value defines our company and our culture:
•Courage: Breakthroughs start by challenging convention – especially in the face of uncertainty or adversity. This happens when we think big, speak up and are decisive.
•Excellence: We can only change patients’ lives.lives when we perform at our best together. This happens when we focus on what matters, agree who does what and measure outcomes.
•Joy: We give ourselves to our work, and it also gives to us. We find joy when we take pride, recognize one another and have fun.
Diversity, Equity and Inclusion. At Pfizer, every person deserves to be seen, heard and cared for,for. We embed diversity, equity and inclusion in our workplace and our purpose of delivering breakthroughs that change patients’ lives. As we work to further this goal by bringingbring together people with different backgrounds, perspectives and experiences. Our new and expanded commitments to equity includeexperiences we take specific actions to help foster a morean inclusive environment within Pfizer and beyond, including, among others: (i) increasingbuilding a more inclusive colleague experience through representation and meaningful connections; (ii) advancing equitable health outcomes by evaluating our work through the representationlens of both women and underrepresented ethnic groups; (ii)the communities we serve, (iii) providing resources on allyship and the science behind inclusion to support managersall colleagues in having courageous conversations about equity, race and the avoidance of bias within their teams; (iii) revising our Political Action Committee (PAC) bylaws to help ensure that PAC recipients consistently demonstrate conduct that align with our core values; andbias; (iv) working to help transform society with external diversity, equity and inclusion partnerships, including deploying capital, engaging diverse suppliers and amplifying equity initiatives; and (v) working to help ensure recruitment demographics of all clinical trials correlate to those of the countries where trials are taking place.
Colleague Engagement. To attract, develop and inspire the brightest talent, we aim to support our colleagues by engaging and partnering with them to help ensure they feel they are part of a community. We understand the importance ofthat continuously listening and responding to colleague feedback is essential to fostering a healthy work environment particularly during times of change and uncertainty. We are passionate about creating safe spaces at work so our employees feel able and encouraged to provide the company with feedback. The Office of the Ombuds is a resource where all Pfizer colleagues at any level can come to get information and guidance to help them address and resolve work-related issues. We also host company-wide safe space calls and provide various other public, private and anonymous channels for employees to share feedback without fear of retaliation.
Our annual engagement survey, Pfizer Pulse, provides a forum for our colleagues to give structured feedback about their Pfizercolleague experience. Through this survey, we measure and track priority areas of the overall colleague experience and equipsequip leaders with
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 10 |
actionable insights for discussion and follow up. Regular topics in the survey includeinclude: (i) employee engagement, such as colleagues’ commitment to and advocacy for Pfizer, andPfizer; (ii) purpose, including how colleagues’ work connects with our purpose. Through thesepurpose; (iii) inclusion, such as having a climate in which diverse perspectives are valued; (iv) empowerment, such as colleagues feeling empowered and enabled to do their best work together; and (v) growth, including the ability for colleagues to gain new experiences that align with their individual career goals. In addition, we ask for feedback at various points in the employee lifecycle through surveys, focus groups and colleague forums. The information we receive helps enable us to adapt to the real-time needs of our employees and continuously improve our ways of working. While we have already made progress in reducing bureaucracy and streamlining processes, we recognize that there is still room for improvement, particularly in the effectiveness of our cross-functional teams.
Throughout 2023, we have developed and tested a new approach that aims to expedite decision-making, provide clarity in roles and responsibilities, enhance governance, redefine the role of a leader, and ultimately improve overall team productivity and performance. This new way of working signifies our commitment to becoming a more dynamic organization that thrives on collaboration and agility. By revolutionizing the way our teams operate, we believe we can measuredrive better business outcomes and, trackmost importantly, make a meaningful difference in the degreelives of people around the world.
Pfizer also prioritizes colleague recognition to whichdrive engagement, a sense of belonging, motivation, and productivity. Our global rewards and recognition program, Bravo, lets colleagues are proud to work atcelebrate and acknowledge each other for demonstrating Pfizer would recommend Pfizer asvalues in a great place to work to othersway that makes an impact on the company, a colleague, a team or a patient. In 2023, 84% of colleagues were recognized, and intend to stay with Pfizer.more than 650,000 recognitions were given.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 15 |
Performance Leadership and Growth.Leadership. We understand the significance of leadership and its crucial role in promoting growth and delivering breakthrough results. We believe that each of our colleagues has the potential to lead in a unique way and create a meaningful impact on a global scale. To support this belief, we have developed a new leadership profile for our colleagues that aligns with our company values of courage, excellence, equity and joy.
We believe this renewed focus on leadership applies to all colleagues, which may help us to foster transformational thinking and executional excellence. By pursuing these leadership qualities, we believe Pfizer can help ensure that its leaders and colleagues are aligned with the company’s values, behaviors and purpose, which may help lead to better outcomes and a positive impact on the lives they touch.
We are committed to helping our colleagues reach their full potential by rewarding both their performance and leadership skills and by providing opportunities for growth and development. Our performance management approach—called Performance and Leadership Insights—Insights—is based on six-month semesters during which our colleagues and their managers set goals, receive feedback and meet to discuss performance. These conversations are meant to help colleagues grow and develop by evaluating performance(what (what the colleague achieved, measured by outcomes), leadership(how (how they achieved it, taking into account Pfizer’s values of courage, excellence, equity and joy), and identifying areas of growth that help move colleagues towards fulfilling their career goals and their potential.
Growth and Development. By prioritizing the ongoing development of our employees, we not only support their individual success but also cultivate a resilient and adaptable workforce that can thrive in the face of change. As we navigate the evolving landscape of our industry, we recognize that providing our employees with opportunities for learning, skill-building and growth is essential to their engagement, productivity, and overall job satisfaction. In 2023, we continued to maintain low voluntary turnover rates relative to the pharmaceutical industry.
Our view of career growth is built on aspirations and empowers individuals to boldly own their growth journey. We strivedeepened our efforts to ensureredefine growth as a fluid process that promotes incremental in-role growth or mobility along horizontal, vertical or diagonal individualized pathways—what we are calling “zig-zag” growth. Our commitments to colleague development consist of specific actions to encourage non-linear “zig-zag” career growth paths for all colleagues, have an equal opportunityincluding (i) a common language around growth—along with a guiding framework—to help colleagues identify their next best growth experience, (ii) tools and resources to encourage growth conversations and offer transparency on the sources of growth available, and (iii) a variety of opportunities to grow through experiences, connections with others and offer a variety oflearning programs, including mentoring, job rotations, experiential project roles, skill basedprojects, skill-based volunteering and personalized learning programs focused on manypathways that address a variety of topics, including leadership and management skills and industry- and job-specific learning, as well as general business, manufacturing, finance and technology skills.
Health, Safety and Well-Being.Well-Being. Protecting the health, safety and well-being of colleagues and contingent workers, all of whom are essential to delivering our business objectives, is an integral part of how we operate. Our Global Environment, Health & Safety (EHS) Policy and supporting standards outline our approach to assessment, evaluation, elimination, and mitigation of EHS risks across our operations globally. We are committed to supporting and encouraging our colleagues’ well-being and use results from Pfizer Pulse and other employee feedback forums to inform the wellness services we offer, such as (i) a Wellness Day for every colleague, (ii) on-site health clinics for colleagues in select locations, with access to certain vaccinations, where allowed by law, (iii) digital accessibility cafés that provide employees with disabilities the tools and equipment to do their jobs effectively, (iv) mental health resources, including a manager/team toolkit designed to facilitate conversations, actively care for coworkers, and provide local resources for employees to access support, (v) programming through Employee Assistance Program (EAP) providers, including our mental health partner THRIVE, our fitness partner Exos, and healthcare partner Kepro, (vi) financial support, including short-term loans and natural disaster relief, and (vii) flexible work policies enabling employees to work from home and their local offices.
Pay Equity. Our commitment to pay equity for all colleagues is based in our value of Equity and our intention to continue to build a diverse, inclusive and highly motivated workforce. We are committed to the health, safety and well-being of our colleagues and continue to advance a comprehensive occupational injury and illness prevention program.
During 2020, our COVID-19 pandemic preparedness and response was a primary focus. Our comprehensive pandemic response plan incorporates guidance issued by external health authorities and is designed to keep onsite workersequitable pay practices at our manufacturing and research sites safe and healthy. A global employee assistance program provides stress management, mental health, emotional, resiliency and pandemic guidance and support to our colleagues.
Pay Equity. We are committed to pay equity,Pfizer for employees based on gender or race/ethnicity,role, education, experience, performance, and location and we conduct and report publicly on pay equity on an annual basis.
Additional information regarding our human capital programs and initiatives is available in the “Careers” section of Pfizer’s website.
Our website is located at www.pfizer.com. This Form 10-K, our Quarterly Reports on Form 10-Q, our Current Reports on Form 8-K and our proxy statements, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, are, or will be, available (free of charge) on our website, in text format and, where applicable, in interactive data file format, as soon as reasonably practicable after we electronically file this material with, or furnish it to, the SEC.
Throughout this Form 10-K, we “incorporate by reference” certain information from other documents filed or to be filed with the SEC, including our Proxy Statement. Please refer to this information. This Form 10-K will be available on our website on or about February 25, 2021. Our Proxy Statement will be available on our website on or about March 11, 2021.
Our 2020 Environmental, Social and Governance (ESG) report, which provides enhanced ESG disclosures, will be available on our website on or about March 11, 2021. Information in our ESG Report is not incorporated by reference into this Form 10-K.
We may use our website as a means of disclosing material information and for complying with our disclosure obligations under Regulation Fair Disclosure promulgated by the SEC. These disclosures are included on our website in the “Investors” or “News” sections. Accordingly, investors should monitor these portions of our website, in addition to following our press releases, SEC filings, public conference calls and webcasts, as well as our social media channels (our Facebook, YouTube and LinkedIn pages and Twitter accounts (@Pfizer and @Pfizer_News)). The information contained on our website, our Facebook, YouTube and LinkedIn pages or our Twitter accounts, or any third-party website, is not incorporated by reference into this Form 10-K.
Information relating to corporate governance at Pfizer, including our Corporate Governance Principles; Director Qualification Standards; Pfizer Policies on Business Conduct (for all of our employees, including our Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer); Code of Business Conduct and Ethics for Members of the Board of Directors; information concerning our Directors; ways to communicate by e-mail with our Directors; Board Committees; Committee Charters; Charter of the Lead Independent Director; and transactions in Pfizer securities by Directors and Officers are available on our website. We will provide any of the foregoing information without charge upon written request to our Corporate Secretary, Pfizer Inc., 235 East 42nd Street, New York, NY 10017. We will disclose any future amendments to, or waivers from, provisions of the Pfizer Policies on Business Conduct affecting our Chief Executive Officer, Chief Financial Officer and Controller on our website as promptly as practicable, as may be required under applicable SEC and NYSE rules. Information relating to shareholder services, including the Computershare Investment Program, book-entry share ownership and direct deposit of dividends, is also available on our website.
This section describes the material risks to our business, which should be considered carefully in addition to the other information in this report and our other filings with the SEC. Investors should be aware that it is not possible to predict or identify all such factors and that the following is not meant to be a complete discussion of all potential risks or uncertainties.
Additionally, our business is subject to general risks applicable to any company, such as economic conditions, geopolitical events, extreme weather and natural disasters. If known or unknown risks or uncertainties materialize, our business operations, financial condition, operating results (including components of our financial results), cash flows, prospects, reputation or credit ratings could be adversely affected now and in the future, potentially in a material way. The following discussion of risk factors contains forward-looking statements, as discussed in the
Forward-Looking Information and Factors that May Affect Future Results section in this Form 10-K.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 11 |
RISKS RELATED TO OUR BUSINESS, INDUSTRY AND OPERATIONS:
MANAGED CARE TRENDS
Private payers,payors, such as health plans, and other managed care entities, such as PBMs, continue to take action to manage the utilization and costs of drugs. Negotiatingdrugs in the U.S., the single largest market for biopharmaceutical products. The negotiating power of MCOs and other private third-party payerspayors has increased due to consolidation, and they, along with state and federal governments, increasingly employ formularies to control costs and encourage utilization of certain drugs, including through the use of formulary inclusiondeductibles, utilization management tools, cost sharing or favorable formulary placement. They may demand rebates from biopharmaceutical manufacturers for preferred placement on a drug formulary. The growing availability and use of innovative specialty pharmaceutical medicines that treat rare or life threatening conditions, typically with a relatively higher cost as compared to other types of pharmaceutical products, also has generated increased payor interest in development of cost-containment strategies. These initiatives have increased consumers’ interest in drug prices and input in medication choices, as they pay for a larger portion of their prescription costs and may cause them to favor lower-cost generic alternatives. We may fail to obtain or maintain timely or adequate pricing or formulary placement of our products, or fail to obtain such formulary placement at favorable pricing.
The growing availability and usepricing net of innovative specialty pharmaceutical medicines that treat rare or life-threatening conditions, which typically have smaller patient populations, combined with their relative higher cost as compared to other types of pharmaceutical products, also has generated increased payer interest in developing cost-containment strategies targeted to this sector.
Third-party payerspayors also use additional measures such as new-to-market blocks, exclusion lists, indication-based pricing and value-based pricing/contracting to improve their cost containment efforts and cost efficiency. Such payors are also increasingly imposing utilization management tools such as clinical protocols, requiring prior authorization for a branded product if a generic product is available or requiring the patient to first fail on one or more genericother products before permitting access to a particular branded medicine. As the U.S. private third-party payerpayor market consolidates further, and as more drugsthe IRA prices become publicly available, in generic form, we may face greater pricing pressure from private third-party payerspayors as they continue to drive more of their patients to use lower cost generic alternatives.alternatives
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 16 |
Also, business arrangements in this area are subject to a high degree of government scrutiny, and available safe harbors under applicable federal and state fraud and abuse laws are subject to change through legislative and regulatory action, as well as evolving judicial interpretations. Our approach to these arrangements may also be informed by such government and industry guidance.
COMPETITIVE PRODUCTS
Competitive product launches have and may erode future sales of our products, including our existing products and those currently under development, or result in unanticipated product obsolescence. Such launches have recently occurred,continue to occur, and potentially competitive products are in various stages of development. We cannot predict with accuracy the timing or impact of the introduction of competitive products that treat or prevent diseases and conditions like those treated or prevented by our in-line drugsproducts and drugproduct candidates.
In addition, competition from manufacturers of generic drugs, including from generic versions of competitors’ branded products that lose their market exclusivity, is a major challenge for our branded products. Certain of our products have experienced significant generic competition over the last few years. For example,We anticipate a more significant impact of reduced revenues from patent expiries in 2026 through 2030 as several of our in-line products experience patent-based expirations. See the basic product patent for Chantix in the U.S. expired in November 2020. While multi-source generic competition for Chantix has not yet begun, it could commence at anytime. Also, the basic product patent for Sutent in the U.S. will expire in August 2021.Item 1. Business—Patents and Other Intellectual Property Rightssection. In China, we expect to continue to face intense competition by certain generic manufacturers, which has resulted, and may result in the future, in price cuts and volume loss of some of our products.
In addition, our patented products may face generic or biosimilar competition before patent exclusivity expires, including upon thefrom “at-risk” launch (despite pending patent infringement litigation against the generic or biosimilar product) by a manufacturer of a generic or biosimilar version of one of our patented products. Generic and biosimilar manufacturers have filed or could file applications with the FDA seeking approval of product candidates that they claim do not infringe our or our collaboration and licensing partners’ patents or claim that our or our collaboration and licensing partners’ patents are not valid; these include candidates that would compete with, among other products, Eliquis, Ibrancevalid. We and Xeljanz. Ourour licensing and collaboration partners also face challenges in various jurisdictions by generic drug manufacturers to patents covering products for which we have patent rights, licenses or co-promotion rights.
We may become subject to competition from biosimilars referencing our biologic products if competitors are able to obtain marketing approval for such biosimilars.
We also commercialize biosimilar products that compete with products of others, including other biosimilar products. The entry to the market of competing biosimilars is expected to increase pricing pressures on our biosimilar products. Uptake of our biosimilars may be lower due to various factors, such as anti-competitive practices, access challenges where our product may not receive appropriate coverage/reimbursement access or remains in a disadvantaged position relative to an innovator product, physician reluctance to prescribe biosimilars for existing patients taking the innovativereference product, or misaligned financial incentives. For example, Inflectra has experienced access challenges among commercial payers. In September 2017, Pfizer filed suit in the U.S. District Courtincentives for the Eastern District of Pennsylvania against J&J alleging that J&J’s exclusionary contracts and other anticompetitive practices concerning Remicade® (infliximab) violate federal antitrust laws.
CONCENTRATION
We recorded direct product and/or allianceAlliance revenues of more than $1 billion for each of sevennine products that collectively accounted for 53%64% of our totalTotal revenues in 2020. For additional information, see2023. In particular, Comirnaty accounted for 19% of Total revenues in 2023. See Notes 1 and 17. If these products or any of our other major products were to, or continue to (if applicable), experience loss of patent protection (if applicable), changes in prescription or vaccination purchasing or growth rates, reduced product demand, material product liability litigation, unexpected side effects or safety concerns, regulatory proceedings or investigations, lower governmental and/or regulatory confidence, negative publicity affecting doctor or patient confidence, pressure from existing competitive products, changes in labeling, pricing and access pressures or supply shortages or if a new, more effective treatmentproduct should be introduced, the adverse impact on our revenues could be significant.significant and our revenue forecasts and expectations could prove to be inaccurate and we may fail to meet these expectations. In particular, certain of our products have experienced patent-based expirations or loss of regulatory exclusivity in certain markets in the last few years, andyears. We anticipate a more significant impact of reduced revenues from patent expiries in 2026 through 2030 as several of our in-line products experience patent-based expirations. In addition, patents covering a number of our best-selling products are, or have been, the subject of pending legal challenges. For additional information on our patents, see the Item 1. Business—Patents and otherOther Intellectual Property Rights section. For Comirnaty and Paxlovid, while we believe that these products have the potential to provide ongoing revenue streams for Pfizer for the foreseeable future, revenues of these products following the COVID-19 pandemic have decreased substantially, and our current expectations for total COVID-19 product revenues in 2024 are lower than the total 2023 revenues from COVID-19 products. For information on risks associated with Comirnaty and Paxlovid, see the COVID-19 section in this Form 10-K.below.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 12 |
In addition, we sellcertain of our prescription pharmaceutical products principally through wholesalers in the U.S. For additional information, see Note 17B.customers account for a significant portion of our revenues. If one of our significant biopharmaceutical wholesalerscustomers should encounter financial or other difficulties, it might decrease the amount of business the wholesalersuch customer does with us and/or we might be unable to timely collect all the amounts that the wholesalersuch customer owes us or at all, which could negatively impact our results of operations. In addition, we expect that consolidation and integration of pharmacy chains and wholesalers will increase competitive and pricing pressures on pharmaceutical manufacturers, including us. See
RESEARCH AND DEVELOPMENT
The discovery and development of new products, as well as the development of additional uses for existing products, are necessary for the continued strength of our business. Our product lines must be replenished over time to offset revenue losses when products lose exclusivity or market share or to respond to healthcare and innovation trends, as well as to provide for earnings growth, eitherprimarily through internal R&D or through collaborations, acquisitions, JVs, licensing or other arrangements. Growth depends in large part on our ability to identify and develop
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 17 |
new products or new indications for existing products that address unmet medical needs and receive reimbursement from payers.payors. However, balancing current growth, investment for future growth and the delivery of shareholder return remains a major challenge. The costs of product development continue to be high and are growing, as are regulatory requirements in many therapeutic areas, which may affect the complexity of drug trials, and the number of candidates we are able to fund as well as the sustainability of the R&D portfolio.
Decisions made early in the development process of a drug or vaccine candidate can have a substantial impact on the marketing strategy and payerpayor reimbursement possibilities if the candidate receives regulatory approval. We try to plan clinical trials prudently and to reasonably anticipate and address challenges, but there is no assurance that an optimal balance between trial conduct, speed and desired outcome will be achieved.
Additionally, our product candidates can fail at any stage of the R&D process, and may not receive regulatory approval even after many years of R&D. We may fail to correctly identify compounds or indications for which our science is promising or allocate R&D investment resources efficiently, and failure to invest in the right technology platforms, therapeutic areas, product classes, geographic markets and/or licensing opportunities could adversely impact the productivity of our pipeline. Further, even if we identify areas with the greatest commercial potential, the scientific approach may not succeed despite the significant investment required for R&D, and the product may not be as competitive as expected because of the highly dynamic regulatory and market environmentenvironments and the hurdles in terms of access, coverage and reimbursement.
GLOBAL OPERATIONS
We operate on a global scale and could be affected by currency fluctuations,and interest rate fluctuations; capital and exchange controls,controls; local and global economic conditions including inflation, recession, volatility and/or lack of liquidity in capital markets; expropriation and other restrictive government actions,actions; changes in intellectual propertyproperty; legal protections and remedies,remedies; trade regulationsregulations; tax laws and regulations; and procedures and actions affecting approval, production, pricing, and marketing of, reimbursement for and access to our products, as well as byimpacts of political or civil unrest or military action, including the ongoing conflicts between Russia and Ukraine and in the Middle East and their economic consequences, geopolitical instability, terrorist activity, unstable governments and legal systems, and inter-governmental disputes.
Some emerging market countries may be particularly vulnerable to periods of financial, economic or political instability or significant currency fluctuations or may have limited resources for healthcare spending. As a result of these and other factors, our strategy to grow in emerging markets may not be successful, and any growth rates in these markets may not be sustainable.
In addition, since a significant portion Additionally, local economic conditions may adversely affect the ability of our business is conducted in the EU,payors, as well as the U.K., the changes resulting from Brexit may pose certain implicationsour distributors, customers, suppliers and service providers, to pay for our research, commercialproducts, or otherwise to buy necessary inventory or raw materials, and general business operations in the U.K. and the EU.
Government financing and economic pressures can lead to negative pricing pressure in various markets where governments take an active role in setting prices, access criteria (e.g., through health technology assessments) or other means of cost control. For additional information on government pricing pressures, see the Item 1. Business—Business—Government Regulation and Price Constraints section in this Form 10-K.
We continue to monitor the global trade environment and potential trade conflicts and impediments that could impact our business. If trade restrictions or tariffs reduce global economic activity, potential impacts could include declining sales; increased costs; volatility in foreign exchange rates; a decline in the value of our financial assets and pension plan investments; required increases of our pension funding obligations; increased government cost control efforts; delays or failures in the performance of customers, suppliers and other third parties on whom we may depend for the performance of our business; and the risk that our allowance for doubtful accounts may not be adequate.
We operate in many countries and transact in over 100many different currencies. Changes in the value of those currencies relative to the U.S. dollar, or high inflation or deflation in thesethose countries, can impact our revenues, costs and expenses and our financial guidance. Significant portions of our revenues, costs and expenses, as well as our substantial international net assets, are exposed to exchange rate changes. 48%54% of our total 20202023 revenues were derived from international operations, including 23%24% from Europe and 17%20% from Japan, China Japan and the rest of Asia.the Asia Pacific region. Future changes in exchange rates or economic conditions and the impact they may have on our results of operations, financial condition or business are difficult to predict. For additional information about our exposure to foreign currency risk, see the Analysis of Financial Condition, Liquidity, Capital Resources and Market Risk—Selected Measures of Liquidity and Capital Resources Risksection within MD&A.
From time to time, we issue variable rate debt based on LIBOR, or undertake interest rate swaps that contain a variable element based on LIBOR. The U.K. Financial Conduct Authority announced in 2017 that it will no longer compel banks to submit rates that are currently used to calculate LIBOR after 2021. This deadline was extended until June 2023 for a number of key U.S. dollar benchmark maturities (including the 1-month and 3-month LIBOR rates). The U.S. Federal Reserve has selected the Secured Overnight Funding Rate (SOFR) as the preferred alternate rate and the transition away from LIBOR will continue despite the extended timeline. We are planning for this transition and will amend
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 13 |
any contracts to accommodate the SOFR rate where required. While our exposure to LIBOR is very low, market volatility related to the transition may adversely affect the trading market for securities linked to such benchmarks.
PRODUCT MANUFACTURING, SALES AND MARKETING RISKS
We could encounter difficulties, delays or delaysinefficiencies in our supply chain, product manufacturing and distribution networks, as well as sales or marketing, due to regulatory actions, shut-downs, work stoppages or strikes, approval delays, withdrawals, recalls, penalties, supply disruptions, shortages or stock-outs at our facilities or third-party facilities that we rely on, reputational harm, damagethe impact to our facilities due to health pandemics or natural or man-made disasters, including as a result of climate change, product liability or unanticipated costs. Examples of such difficulties or delays include the inability to increase or maintain production capacity commensurate with demand; challenges related to component materials to maintain supply and/or appropriate quality standards throughout our supply network and/or comply with applicable regulations; inability to supply certain products due to voluntary product recalls; and supply chain disruptions at our facilities or at a supplier or vendor.
Regulatory agencies periodically inspect our manufacturing facilities, as well as third-party facilities that we rely on, to evaluate compliance with cGMP or other applicable requirements. Failure to comply with these requirements may subject us to possible legal or regulatory actions, such as warning letters, suspension of manufacturing, seizure of product, injunctions, debarment, product recalls, delays or denials of product approvals, import bans or denials of import certifications. For example,
In 2021, Pfizer recalled all lots of Chantix in September 2017, our subsidiary, Meridian, receivedthe U.S. due to the presence of a warning letter fromnitrosamine, N-nitroso-varenicline, at or above the FDA assertinginterim acceptable intake limit. Regulatory authorities outside the FDA’s view that certain violationsU.S. have issued updated guidance on nitrosamine acceptable intake levels. With this recently issued guidance, which included an updated intake level for N-nitroso-varenicline, we expect to make regulatory submissions in 2024 to potentially enable Chantix to return to market outside the U.S., and our related discussions with FDA are ongoing.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 18 |
COLLABORATIONS AND OTHER RELATIONSHIPS WITH THIRD PARTIES
We depend on third-party collaborators, service providers, and others in the research, development, manufacturing and commercialization of our products and product candidates and also enter into JVs and other business development transactions. To achieve expected longer-term benefits, we may make substantial upfront payments as part of these transactions, which may negatively impact our reported earnings or cash flows. We rely heavily on these parties for multiple aspects of our drug development, manufacturing and commercialization activities, but we do not control many aspects of those activities. We also outsource certain services, including activities related to transaction processing, accounting, information technology,IT, manufacturing, clinical trial recruitment and execution, clinical lab services, non-clinical research, safety services, integrated facilities management and other areas. Failure by one or more of the third-party collaborators, service providers and others to complete activities on schedule or in accordance with our expectations or to meet their contractual or other obligations to us; failure of one or more of these parties to comply with applicable laws or regulations; disruptions in one or more of these parties’ businesses, including unexpected demand for or shortage of raw materials or components, cyber-attacks on supplier systems, labor disputes or shortage and inclement weather, as well as natural or man-made disasters or pandemics; or any disruption in the relationships between us and these parties have or could delay or prevent the development, approval, manufacturing or commercialization of our products and product candidates, expose us to suboptimal quality of service delivery or deliverables, result in repercussions such as missed deadlines or other timeliness issues, erroneous data and supply disruptions, and could also result in non-compliance with legal or regulatory requirements or industry standards or subject us to reputational harm, all with potential negative implications for our product pipeline and business. Further, our Alliance revenues will be adversely affected by the termination or expiration of collaboration and co-promotion agreements that we have entered into and that we may enter into from time to time.
COUNTERFEIT PRODUCTS
Our reputation, in-line and promising pipeline portfolios render our medicines and vaccines prime targets for counterfeiters. Counterfeit medicinesCounterfeits pose a significant risk to patient health and safety because of the conditions under which they are manufactured—manufactured—often in unregulated, unlicensed, uninspected, and unsanitary sites—sites—as well as the lack of regulation of their contents. Failure to mitigate this threat could adversely impact our business, by, among other things,Pfizer’s patients, potentially causing patient harm,them harm. This situation, in turn, may result in the loss of patient confidence in the Pfizer name and in the integrity of our medicines and vaccines, and potentially resulting inimpact our business through lost sales, product recalls, and an increased threat ofpossible litigation.
The prevalence of counterfeit medicines is an industry-wide issue due to a variety of factors, including the adoption of e-commerce, whiche-commerce. The increased adoption during the COVID-19 pandemic greatly enhancing consumers’ abilityfurther exposed consumers to obtain prescriptions and other medicalfake prescription treatments via the Internet in lieu ofinternet as access to traditional brick and mortar pharmacies.pharmacies or authorized full-service internet pharmacies that offer authentic treatments may have been hindered. The internet exposes patients to greater risk as it is a preferred vehicle for dangerous counterfeit offers and scams becausethat target unsuspecting consumers. Traffic to these generally deceptive pharmacy sites is largely driven by misplaced trust in sophisticated internet retailers and social media offers coupled with the convenience e-commerce affords consumers. Counterfeiters generally target any medicine or vaccine boasting strong demand and we have observed heightened counterfeit and fraud attempts to our internal medicine portfolio, as well as products utilized in the treatment of the anonymity it affords counterfeiters.
We consistently invest in an enterprise-wide strategy to aggressively combat counterfeit threats by educating patients and health carehealthcare providers about the risks, investing in innovative technologies to detect and disrupt sophisticated internet offers and scams, proactively monitoring and interdicting supply with the help of law enforcement;enforcement, and advising legislators and regulators. However, our efforts and those of others may not be entirely successful, and the presence of counterfeit medicines may continue to increase.
RISKS RELATED TO GOVERNMENT REGULATION AND LEGAL PROCEEDINGS:
PRICING AND REIMBURSEMENT
U.S. and international governmental regulations that mandate price controls or limitations on patient access to our products, create coverage criteria or establish prices paid by government entities or programs for our products impact our business, and our future results could be adversely affected by changes in such regulations or policies. TheIn addition to the recent expansion of price controls in the U.S. in the IRA, the adoption of restrictive coverage policies and price controls in new jurisdictions, more restrictive controls in existing jurisdictions or the failure to obtain or maintain timely or adequate coverage and pricing could also adversely impact revenue. We expect pricing pressures and other cost containment measures for drugs and vaccines will continue globally.
In the U.S., pharmaceutical product pricing is subject to government and public scrutiny and calls for reform, and many of our products are subject to increasing pricing pressures as a result. We expect to see continued focus by the U.S. Congress and the Biden Administration on regulating pricing and access to medicine. For example, in August 2022, the drug pricing provisions of the IRA were signed into law, which, among other things, require manufacturers of certain drugs, including Pfizer, to engage in price negotiations with Medicare which will permit the CMS to set a maximum fair price for selected drugs, impose rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation, and replace the Part D coverage gap discount program with a new discounting program. The drug pricing provisions of the IRA began to be implemented in 2022 and implementation efforts are expected to continue over the next several years. In August 2023, the Biden Administration unveiled the first round of medicines subject to the Medicare Drug Pricing Negotiation Program, which included Eliquis. CMS will establish a maximum fair price for Eliquis that will be in effect in 2026. That maximum fair price will be required to be offered to all Medicare beneficiaries and to covered entities participating in the 340B Program if lower than the 340B price. Health plans may also require rebates in addition to the maximum fair price for preferred placement on a Medicare plan formulary. The Medicare Drug Price Negotiation Program is currently subject to legal challenges and therefore, the outcome of the 340B Program remains uncertain. We continue to evaluate the impact of the IRA on our business, operations, financial condition and results as the full effect of the IRA on our business and the pharmaceutical industry remains uncertain.
Payors may promote generic drugs and biosimilars more aggressively to generate savings and attempt to stimulate additional price competition. In addition, we expect that consolidation and integration among pharmacy chains, wholesalers and PBMs will increase pricing pressures in the industry. Some states have implemented, and others are considering, price controls or patient access constraints or cost cutting under state regulated programs including the Medicaid program, and some are considering measures that would apply to broader segments of their populations that are not Medicaid-eligible.program. State legislatures also have recently focusedcontinued to focus on addressing drug costs, generally by increasing price transparency or limitingattempting to limit drug price increases.increases for state regulated insurance. Measures to regulate prices or payment for pharmaceutical products, including legislation on drug importation,
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 1419 |
products, including legislation on drug importation, such as Florida’s drug importation program which was recently approved by the FDA, could adversely affect our business. For additional information on U.S. pricing and reimbursement, see the Item 1. Business—Government Regulation and Price Constraintssection in this Form 10-K.
We encounter similar regulatory and legislative issues in most other countries in which we operate. In certain markets, such as in EU member states, the U.K., Japan, China, Japan, Canada and South Korea, governments have significant power as large single payerspayors to regulate prices, access criteria, or impose other means of cost control, particularly as a result of recent global financing pressures. For example, the QCE and VBP tender process in China has resulted in dramaticsignificant price cuts for off-patent medicines. Additionally, in the EU, the EC proposed the largest reform to drug pricing and access in 20 years, which if enacted would change regulatory exclusivity for our products. For additional information regarding these government initiatives, see the Item 1. Business—Government Regulation and Price Constraints section in this Form 10-K.section. We anticipate that these and similar initiatives will continue to increase pricing and access pressures in China and elsewhere in the future.globally. In addition, in many countries, with respect to our vaccines, we participate in a tender process for selection in national immunization programs. Failure to secure participation in national immunization programs or to obtain acceptable pricing in the tender process could adversely affect our business. We alsoPricing pressures have been, and we anticipate pricing pressures will continue to be, amplified by COVID-19 induced budget deficits and focus on pricing for new COVID-19 therapiestreatments and vaccines.
U.S. HEALTHCARE REFORM
The U.S. healthcare industry is highly regulated and subject to frequent and substantial changes. Any significant additional efforts at the U.S. federal or state levels to reform the healthcare system by changing the way healthcare is provided or funded could have a material impact on us. For additional information on U.S. healthcare reform,regulation, see the Item 1. Business––Government Regulation and Price Constraints section in this Form 10-K.
Other U.S. federal or state legislative or regulatory action and/or policy efforts could adversely affect our business, including, among others, general budget control actions, changes in patent laws, the importation of prescription drugs to the U.S. at prices that are regulated by foreign governments, revisions to reimbursement of biopharmaceuticals under government programs that could reference international prices or require new discounts, restrictions on U.S. direct-to-consumer advertising, limitations on interactions with healthcare professionals and other industry stakeholders, or the use of comparative effectiveness methodologies that could be implemented in a manner that focuses primarily on cost differences and minimizes the therapeutic differences among pharmaceutical products and restricts access to innovative medicines.
AAny additional reduction of U.S. federal spending on entitlement programs beyond the IRA, including Medicare and Medicaid, may affect payment for our products or services provided using our products. The Congressional Budget Office routinely releases options for reducing federal spending that could affect pharmaceutical utilization and pricing as does the Medicare Payment Advisory Commission. These and anyAny other significant spending reductions or cost controls affecting Medicare, Medicaid or other publicly funded or subsidized health programs that may be implemented could have an adverse impact on our results of operations. The IRA will be implemented largely through government guidance and as its effect on Medicare and commercial markets evolve, we will continue to evaluate the potential impacts to our business.
DEVELOPMENT, REGULATORY APPROVAL AND MARKETING OF PRODUCTS
The discovery and development of drugs, vaccines and biological products are time consuming, costly and unpredictable. The outcome is inherently uncertain and involves a high degree of risk due to the following factors, among others:
•The process from early discovery to design and adequate implementation of clinical trials to regulatory approval can take many years.years and have high costs.
•We may have difficulties recruiting and enrolling patients for clinical trials on a consistent basis.
•Product candidates can and do fail at any stage of the process, including as the result of unfavorable pre-clinical and clinical trial results, or unfavorable new pre-clinical or clinical data and further analyses of existing pre-clinical or clinical data, including results that may not support further clinical development of the product candidate or indication.
•We may need to amend our clinical trial protocols or conduct additional clinical trials under certain circumstances, for example, to further assess appropriate dosage or collect additional safety data.
•We may not be able to meet anticipated pre-clinical or clinical endpoints, commencement and/or completion dates for our pre-clinical or clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates.
•We may not be able to successfully address all the comments received from regulatory authorities such as the FDA and the EMA, or be able to obtain approval for new products and indications from regulators.
Regulatory approvals of our products depend on myriad factors, including a regulator making a determinationregulatory determinations as to whether a product is safethe product’s safety and efficacious.efficacy. In the context of public health emergencies like the COVID-19 pandemic, regulators evaluate various factors and criteria to potentially allow for marketing authorization on an emergency or conditional basis. Additionally, clinical trial and other product data are subject to differing interpretations and assessments by regulatory authorities. As a result of regulatory interpretations and assessments or other developments that may occur during the review process, andor even after a product is authorized or approved for marketing, a product’s commercial potential could be adversely affected by potential emerging concerns or regulatory decisions regarding or impacting the scope of indicated patient populations, labeling or marketing, manufacturing processes, safety issues and/or other matters.matters, including decisions relating to emerging developments regarding potential product impurities. Also, certain of our products have received and may in the future receive approvals under accelerated approval pathways where continued approval may be contingent upon confirmatory studies demonstrating the anticipated clinical benefit and/or safety profile.
We may not be able to receive or maintain favorable recommendations by technical or advisory committees, such as the ACIP thator an FDA Advisory Committee, which may impact the useavailability or commercial potential of our products.products and product candidates. Further, claims and concerns that may arise regarding the safety andand/or efficacy of in-line products and product candidates can negatively impact current or future product sales, as applicable, and potentially lead to product recalls or withdrawals, including regulator-directed risk evaluations and assessments, and/or consumer fraud, product liability and other litigation and claims. Further regulatory agencyRegulatory requirements may also result in a more challenging, expensive and lengthy regulatory approval process than anticipated due to requests for, among other things, additional or more extensive clinical trials prior
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 20 |
to granting approval, or increased post-approval requirements. For these and other reasons discussed in this Risk Factorssection, we may not obtain the approvals we expect within the timeframe we anticipate, or at all.
POST-APPROVALPOST-AUTHORIZATION/APPROVAL DATA
As a condition to granting marketing authorization or approval of a product, the FDA may require, or the sponsor may voluntarily agree to undertake, post-marketing commitments such as additional clinical trials or other studies. The results generated in these trials have in the past impacted certain of our products and could resultimpact our products in the future, such as by resulting in the loss of marketing approval, changes in labeling, and/or new or increased concerns about the side effects,safety and/or efficacy, or safety.including newly discovered adverse events. Regulatory agencies in countries outside the U.S. often have similar regulations and may impose comparable requirements.requirements, although there are differences between the U.S., the EU and other international regulatory requirements, which may contribute to inconsistency or uncertainty in the marketability of our products across different jurisdictions. Post-marketing studies and clinical trials, whether conducted by us or by others, whether mandated by regulatory agencies or conducted voluntarily, and other emerging data about products, such as adverse event reports, may also adversely affect the availability or commercial potential of our products. Further, if safety or efficacy concerns are raised about a product in the same class as one of our products, those concerns could implicate the entire class; and this, in turn, could have an adverse impact on the availability or commercial viability of our product(s) as well as
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 15 |
other products in the class. The potential regulatory and commercial implications of post-marketing study results for approved indications and potential new indications of an in-line product, typically cannot immediately be determined. For example, the potential impact of the co-primary endpoint results from a recently completed post-marketing required safety study of Xeljanz, ORAL Surveillance (A3921133), announced in January 2021, and related results, analyses and discussions with and reviews by regulators, remain uncertain. We are working with the FDA and other regulatory agencies to review the full results and analyses as they become available.
The terms of our EUA for the BNT162b2 vaccineComirnaty require that we conduct post-authorization observational studies. In addition,post-observational studies to evaluate the association between the Pfizer-BioNTech COVID-19 Vaccine (Original monovalent), Pfizer-BioNTech COVID-19 Vaccine, Bivalent, and the Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula), and a pre-specified list of adverse events of special interest, including myocarditis and pericarditis, along with deaths and hospitalizations, and severe COVID-19. The required study populations include individuals specified in our September 2023 authorization letter (reissued) as well as populations of interest, such as healthcare workers, pregnant women, immunocompromised individuals and subpopulations with specific comorbidities. Additionally, in relation to the FDA expectsapproval for Comirnaty, we are required to complete certain postmarketing study requirements and commitments through 2024 and beyond. In the FDA’s revision to the EUA holders to work towards submission of full application, suchfor Paxlovid, the FDA removed the post-authorization requirements as they were addressed as a BLA,post-marketing commitment associated with the approval of the Paxlovid NDA. The terms of our Paxlovid EUA had previously required monitoring of a genomic database(s) for the emergence of global viral variants of SARS-CoV-2 and providing reports to the FDA on a monthly basis summarizing any findings. Also, the FDA required Pfizer to assess the activity of the authorized Paxlovid against any global SARS-CoV-2 variant(s) of interest and complete certain other analyses and studies as soon as possible.identified in our October 2022 EUA.
LEGAL MATTERS
We are and may be involved in various legal proceedings, including patent litigation, product liability and other product-related litigation, including personal injury, consumer, off-label promotion, securities, antitrust and breach of contract claims, commercial and other asserted and unasserted matters, environmental, government and tax investigations, employment, tax litigation and other legal proceedings that arise from time to time in the ordinary course of our business. Litigation is inherently unpredictable, and excessive verdicts do occur. Although we believe that our claims and defenses in matters in which we are a defendant are substantial, we have in the past and could in the future incur judgments, enter into settlements or revise our expectations regarding the outcome of certain matters, and such developments could have a material adverse effect on our results of operations.
Claims against our patents include challenges to the coverage and/or validity of our patents on various products or processes. There can be no assurance as to the outcome of these matters, and a loss in any of these cases could result in a loss of patent protection for the product at issue, which could lead to a significant loss of sales of that product and could materially affect future results of operations.
We are also involved in government investigations that arise in the ordinary course of our business. There continues to be a significant volume of government investigations and litigation against companies operating in our industry, both in the U.S. and around the world. Government investigations and actions have and could result in substantial criminal and civil fines and/or criminal charges, and civil penalties, limitations on our ability to conduct business in applicable jurisdictions, corporate integrity or deferred prosecution agreements and other disciplinary actions, as well as reputational harm, including as a result of increased public interest in the matter. In addition, in a qui tam lawsuit in which the government declines to intervene, the relator may still pursue a suit for the recovery of civil damages and penalties on behalf of the government.
Our sales and marketing activities, and the pricing of our products and other aspects of our business are subject to extensive regulation under the FFDCA, the Medicaid Drug Rebate Program, the FCPA and other federal and state statutes, including those discussed elsewhere in this Form 10-K, as well as the Anti-Kickback Statute, anti-bribery laws, the False Claims Act, and similar laws in international jurisdictions. In addition to the potential for changes to relevant laws, the compliance and enforcement landscape is informed by government litigation, settlement precedent, advisory opinions, and special fraud alerts. Our approach to certain practices may evolve over time in light of these types of developments.
Requirements or industry standards in the U.S. and certain jurisdictions abroad require pharmaceutical manufacturers to track and disclose financial interactions with healthcare professionals and healthcare providers and can increase government and public scrutiny of such financial interactions. If an interaction is found to be improper, government enforcement actions and penalties could result. Like many companies in our industry, we have from time-to-time received, and may receive in the future, inquiries and subpoenas and other types of information demands from government authorities. In addition, we have been subject to claims and other actions related to our business activities, brought by governmental authorities, as well as consumers and private payers.payors. In some instances, we have incurred significant expense, civil payments, fines and other adverse consequences as a result of these claims, actions and inquiries. Such claims, actions and inquiries may relate to alleged non-compliance with laws and regulations associated with the dissemination of product (approved and unapproved) information, potentially resulting in government enforcement action and reputational damage. This risk may be heightened by digital marketing, including social media, mobile applications and blogger outreach.
In connection with the resolution of a U.S. government investigation concerning independent copay assistance organizations that provide financial assistance to Medicare patients, in 2018, we entered into a Corporate Integrity Agreement (CIA) with the Office of the Inspector General of the U.S. Department of HealthHHS (OIG), which expired in May 2023. Pfizer submitted its final annual report and Human Services, which is effective forawaiting a period of five years. Inresponse from the CIA, we agreed to implement and/or maintain certain compliance program elements to promote compliance with federal healthcare program requirements. Breaches of the CIA could result in severe sanctions against us.
We and certain of our subsidiaries are also subject to numerous contingencies arising in the ordinary course of business relating to legal claims and proceedings, including environmental contingencies. Amounts recorded for legal and environmental contingencies can result from a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions. While we have accrued for
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 21 |
worldwide legal liabilities, there is no guarantee exists that additional costs will not be incurred or additional payments will not be required beyond the amounts accrued.
For additional information, including information regarding certain legal proceedings in which we are involved in, see Note 16A.
RISKS RELATED TO INTELLECTUAL PROPERTY, TECHNOLOGY AND SECURITY:
INTELLECTUAL PROPERTY PROTECTION
Our success largely depends on our ability to market technologically competitive products. We rely and expect to continue to rely on a combination of intellectual property, including patent, trademark, trade dress, copyright, trade secret and domain name protection laws, as well as confidentiality and license agreements, to protect our intellectual property and proprietary rights. If we fail to obtain and maintain adequate intellectual property protection, we may not be able to prevent third parties from launching generic or biosimilar versions of our branded products, from using our proprietary technologies or from marketing products that are very similar or identical to ours. Our currently pending or future patent applications may not result in issued patents or be granted on a timely basis. Similarly, any term extensions that we seek may not be granted on a timely basis, if at all.all, and any term adjustments related to patent office delays in obtaining a patent may be reduced or eliminated entirely due to risks associated with changes in law relating to patent terms. In addition, our issued patents may not contain claims sufficiently broad to protect us against claims regarding validity, enforceability, scope and effective term made by parties with similar technologies or products or provide us with any competitive advantage, including exclusivity in a particular product area.
The scope of our patent claims also may vary between countries, as individual countries have distinct patent laws, and our ability to enforce our patents depends on the laws of each country, its enforcement practices, and the extent to which certain countries engage in policies or practices
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 16 |
that weaken a country’s intellectual property framework (e.g., laws or regulations that promote or provide broad discretion to issue a compulsory license). In countries that provide some form of regulatory exclusivity, mechanisms exist permitting some form of challenge to our patents by competitors or generic drug marketers prior to or immediately following the expiration of such regulatory exclusivity, and generic companies are employing aggressive strategies, such as “at risk”“at-risk” launches that challenge our patent rights. Most of the suits involve claims by generic drug manufacturers that patents covering our products, uses, processes or dosage forms are invalid and/or do not cover the product of the generic or biosimilar drug manufacturer. Independent actions have been filed alleging that our assertions of, or attempts to enforce, patent rights with respect to certain products constitute unfair competition and/or violations of antitrust laws. Such claims may also be brought as counterclaims to actions we bring to enforce our patents. We are also party to other patent damages suits in various jurisdictions pursuant to which generic drug manufacturers, payers,payors, governments or other parties are seeking damages from us for alleged delay of generic entry. We also are often involved in other proceedings, such as inter partes review, post-grant review, re-examination or opposition proceedings, before the U.S. Patent and Trademark Office, the European Patent Office, or other foreign counterparts relating to our intellectual property or the intellectual property rights of others. Also, if one of our patents or a competitors’ patents is found to be invalid in such proceedings, generic or biosimilar products could be introduced into the market resulting in the erosion of sales of our existing products. For additional information, including information regarding certain legal proceedings in which we are involved, see Note 16A1. Further, if we are unable to maintain our existing license agreements or other agreements pursuant to which third parties grant us rights to intellectual property, our operating results and financial condition could be adversely affected.
We currently hold trademark registrations and have trademark applications pending in many jurisdictions, any of which may be the subject of a governmental or third-party objection, which could prevent the maintenance or issuance of the trademark. As our products mature, our reliance on our trademarks and trade dress to differentiate us from our competitors increases and, as a result, our business could be adversely affected if we are unable to prevent third parties from adopting, registering or using trademarks and trade dress that infringe, dilute or otherwise violate our rights. We seek to protect our proprietary information, including our trade secrets and proprietary know-how, by requiring our employees, consultants, other advisors and other third parties to execute proprietary information and confidentiality agreements upon the commencement of their relationship with us. Despite these efforts and precautions, we may be unable to prevent a third partythird-party from copying or otherwise obtaining and using our trade secrets or our other intellectual property without authorization, and legal remedies may not adequately compensate us for the damages caused by such unauthorized use. Further, others may independently and lawfully develop substantially similar or identical products that circumvent our intellectual property by means of alternative designs or processes or otherwise.
THIRD PARTYTHIRD-PARTY INTELLECTUAL PROPERTY CLAIMS
A properly functioning intellectual property regime is essential to our business model. We are committed to respecting the valid intellectual property rights of other companies, but the patent granting process is imperfect. Accordingly, the pursuit of valid business opportunities may require us to challenge intellectual property rights held by others that we believe were improperly granted, including challenges through negotiation and litigation, and such challenges may not always be successful.
Part of our business depends upon identifying biosimilar opportunities and launching products to take advantage of those opportunities, which may involve litigation, associated costs and time delays, and may ultimately not be successful. These opportunities may arise in situations where patent protection of equivalent branded products has expired or been declared invalid, or where products do not infringe the patents of others. In some circumstances we may take action, such as litigation, asserting that our products do not infringe patents of existing products or that those patents are invalid or unenforceable in order to achieve a “first-to-market” or early market position for our products.
Third parties may claim that our products infringe one or more patents owned or controlled by them. Claims of intellectual property infringement can be costly and time-consuming to resolve, may delay or prevent product launches, and may result in significant damages.royalty payments or damages or potential licensing agreements. For example, our R&D in a therapeutic area may not be first and another company or entity may have obtained relevant patents before us. We are involved in patent-related disputes with third parties over our attempts to market generic pharmaceutical products, including related to Abrysvo, Comirnaty and biosimilars.Paxlovid. As we expand our mRNA portfolio, patent-related disputes may increase. Once we have final regulatory approval of the related generic products, or biosimilars, we may decide to commercially market these products even though associated legal proceedings (including any appeals) have not been resolved (i.e., “at-risk” launch). If one of our marketed products (or a product of our
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 22 |
collaboration/licensing partners to which we have licenses or co-promotion rights) is found to infringe valid patent rights of a third party, such third party may be awarded significant damages or royalty payments, or we may be prevented from further sales of that product. Such damages may be enhanced as much as three-fold if we or one of our subsidiaries is found to have willfully infringed valid patent rights of a third party.
INFORMATION TECHNOLOGY AND SECURITY
Significant disruptions of information technologyIT systems or breaches of information security could adversely affect our business. We extensively rely upon sophisticated information technologyIT systems (including cloud services) to operate our business. We produce, collect, process, store and transmit large amounts of confidential information (including personal information and intellectual property), and we deploy and operate an array of technical and procedural controls to maintain the confidentiality, integrity and integrityavailability of such confidential information. We develop and operate digital systems to engage patients, healthcare providers, governments, payors and supply chain partners to conduct business and deliver medicines, digital diagnostics, clinical trials and digital therapies. Such systems include mobile applications, wearable devices, internet websites and other digital technologies that may be targets of attack. We have outsourced significant elements of our operations, including significant elements of our information technologyIT infrastructure and, as a result, we manage relationships with many third-party vendorsproviders who may or could have access to our confidential information. We rely on technology developed, supplied and/or maintained by third-parties that may make us vulnerable to “supply chain” style cyber-attacks. Further, technology and security vulnerabilities of acquisitions, business partners or third-party providers may not be identified during due diligence or soon enough to mitigate exploitation. The size and complexity of our information technologyIT and information security systems, and those of our third-party vendorsproviders (and the large amounts of confidential information that is present on them), make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by, but not limited to, our employees, or vendors,contingent workers, service providers, business partners, customers or malicious attackers. Cyber-attacksAs a global pharmaceutical company, our systems and assets are the target of frequent cyber-attacks. Such cyber-attacks are of ever-increasing levels of sophistication, including the use of adversarial artificial intelligence techniques, and are made by groups and individuals with a wide range of motives (including, but not limited to, industrial espionage)espionage, extortion, property destruction and personal information theft) and expertise, including, but not limited to, organized criminal groups, “hacktivists,” nation states, employees, business partners and others. As a global pharmaceutical company, our systems are subject to frequent cyber-attacks. Due to the nature of some of these attacks, there is a risk that they may remain undetected for a period of time. While we have invested in the protection of data and information technology,IT and develop and maintain systems and controls, our efforts, like those of other similar companies, have not always and may not in the future prevent service interruptions, extortion, theft of confidential, personal or security breaches.proprietary information, compromise of data integrity or unauthorized information disclosure. Any suchtechnology service interruption or breach of our systems could adversely affect our business operations and/or result in the loss of critical or sensitivepersonal data, confidential information or intellectual property,property. Such incidents could require disclosure to government authorities and/or regulators and could require notification to impacted individuals and any incident could result in financial, legal, business and reputational harm to us. We maintain cyber liability insurance; however, this insurance may not be sufficient to cover the financial, legal, business or reputational losses that may result from an interruption or breach of our systems.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 17 |
GENERAL RISKS RELATED TO BUSINESS DEVELOPMENT:
BUSINESS DEVELOPMENT ACTIVITIES
We expecthave established significant growth goals, which we plan to enhanceachieve, in part, by not only advancing our in-lineown product pipelines and maximizing the value of our existing products, and product pipelinebut also through various forms of business development activities, which can include alliances, licenses, JVs, collaborations, equity- or debt-based investments, dispositions, divestments, mergers and acquisitions. Our recent acquisition of Seagen is part of that growth plan. We view our business development activity as an enabler of our strategies and seek to generate growth by pursuing opportunities and transactions that have the potential to strengthen our business and our capabilities. The success of theseour business development activities is dependent on the availability and accurate cost/benefit evaluation of appropriate opportunities, competition from others that are seeking similar opportunities and our ability to successfully identify, structure and execute transactions, including the ability to satisfy closing conditions in the anticipated timeframes or at all, and our ability to successfully integrate acquisitions.acquired businesses and develop and commercialize acquired products. Pursuing, executing and consummating these opportunitiestransactions may require substantial investment, which may require us to obtain additional equity or debt financing, which has in the past and could in the future result in increased leverage and/or a downgrade of our credit ratings. Where we acquire debt or equity securities as all or partratings and could limit our ability to obtain future financing. We have incurred substantial indebtedness to fund our recent acquisition of Seagen. We financed a portion of the consideration for business development activities,transaction with the valueproceeds from the $31 billion of those securities will fluctuate, and may depreciate. We may not control a companylong-term debt issued in whichMay 2023, plus $8 billion in additional short-term indebtedness issued prior to the acquisition. The amount of debt that we invest, and, as a result, we will have limited ability to determine its management, operational decisions and policies. Further, while we seek to mitigate risks and liabilities of such transactions through,incurred could have significant consequences including, among other things, due diligence, there may bereducing our operating or financial flexibility, requiring a portion of our cash flow from operations to make interest payments and reducing the cash flow available to fund capital expenditures and other corporate purposes and to grow our business. To the extent we incur additional indebtedness or interest rates increase, these risks and liabilities that such efforts fail to discover, that are not disclosed to us, or that we inadequately assess. could increase further.
The success of anyour business development transactions, including our recent acquisition of our acquisitions will depend, when applicable,Seagen, depends on our ability to realize the anticipated benefits of these transactions and is subject to numerous risks and uncertainties, many of which are outside of our control. Unsuccessful clinical trials, regulatory hurdles and commercialization challenges, among other factors, may adversely impact revenue and income contribution from integratingbusiness development transactions, including from acquired products and businesses. We may fail to generate expected revenue growth for our existing products, product pipeline and contribution from these transactions or from acquired products or businesses with us. We, for example,or we may fail to achieve anticipated cost savings, anticipatedsuch as those expected with certain of these acquisitions, or such cost savingsrespect to Seagen, within the expected time frame. frames or at all, which may impact our ability to meet our growth objectives. In certain transactions, we may agree to provide certain transition services for an extended period of time, which may divert our focus and resources that would otherwise be invested into maintaining or growing our business. Similarly, the accretive impact anticipated from certain of these acquisitionstransactions may not be realized or may be delayed. Integration of theseacquired products or businesses may result in the loss of key employees, the disruption of ongoing business, including third-party relationships, or inconsistencies in standards, controls, procedures and policies. We also may failFurther, while we seek to generate the expected revenue growth for the acquired business. Expected revenue from acquired productsmitigate risks and product candidates alsoliabilities through, among other things, due diligence, we may be constrained by developments outsideexposed to risks and liabilities as a result of business development transactions. There is no assurance that we will be able to acquire attractive businesses or enter into strategic business relationships on favorable terms ahead of our control. Unsuccessful clinical trials, regulatory hurdles and commercialization challenges may adversely impact revenue and income contribution from products and product candidates, including those acquired in these acquisitions.competitors, or that such acquisitions or strategic business development relationships will be accretive to earnings or improve our competitive position.
SPIN-OFF AND COMBINATION OF UPJOHN WITH MYLAN | | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 23 |
WeWhere we invest in or otherwise obtain debt or equity securities of third parties in connection with business development transactions, such as our ownership interest in Haleon, we may not realize somebe unable to direct or allinfluence the management, operational decisions and policies of such companies and the value of the expected benefits of the spin-off and combination (the Transactions) of the Upjohn Business with Mylan, which resulted in the creation of Viatris, due to many factors, including, among others, strategic adjustments required to reflect the nature of our business following the Transactions, increased risks resulting from us becoming a company that is a more focused, innovative science-based biopharmaceutical products business and the possibility that we may not achieve our strategic objectives. In addition, we have agreed to provide certain transition services to Viatris, generally for an initial period of 24 months following the completion of the Transactions (with certain possibilities for extension). These obligations under the transition services agreements may result in additional expensesacquired securities will fluctuate and may divert our focus and resources that would otherwise be invested into maintaininglose value. Any future distribution or growing our business.
CONSUMER HEALTHCARE JV WITH GSK
In 2019, we and GSK combined our respective consumer healthcare businesses into a JV that operates globally under the GSK Consumer Healthcare name. Although we have certain consent, board representation and other governance rights, we are a minority ownersale of the JV and do not control the JV, its management or its policies. As a result, our ability to realize the anticipated benefits of the transaction depend upon GSK’s operation and management of the JV. In addition, the JV is subject to risks that are different than the risks associated with our business. Many of these risks are outside GSK’s or the JV’s control and could materially impact the business, financial condition and results of operations of the JV.
GSK has indicated that it intends to separate the JV as an independent company listed on the U.K. equity market. Until July 31, 2024, GSK has the exclusive right to initiate a separation and listing transaction. We have the option to participate in a separation and listing transaction initiated by GSK. However, the separation and public listing transaction may not be initiated or completed within expected time periods or at all, and both the timing and success of any separation and public listing transaction, as well as the value generated for us or our shareholders in any such transaction,securities will be subject to prevailing market conditions and other factors, including the size of our ownership stake, at the time of such transaction. Any future distribution or sale and there is no assurance as to the price that such securities will ultimately be sold or that such securities will be sold at all.
PANDEMICS
Pandemics, such as the COVID-19 pandemic, have impacted and may in the future impact our business, operations and financial condition and results. Related risks and challenges for our business include, among others: uncertainty regarding the severity and duration of a pandemic; impacts to business operations; decreased demand for certain of our stakeproducts; increased costs of doing business; manufacturing disruptions and delays; supply chain disruptions and shortages, including challenges related to reliance on third-party suppliers resulting in reduced availability of materials or components used in the JV will similarly be subjectdevelopment, manufacturing, distribution or administration of our products; evolving macroeconomic factors and conditions, including general economic uncertainty, unemployment rates and recessionary pressures; changes in labor markets, including challenges related to prevailing market conditionsour human capital and talent development; unknown consequences on our business performance and initiatives stemming from the substantial investment of time and other resources to any potential pandemic response; increased difficulty and uncertainty regarding predicting or estimating future performance; pace of post-pandemic recovery, disruption and volatility within the financial or credit markets; and our financial performance in general.
COVID-19
The extent to which COVID-19 impacts our business going forward will depend on many factors, at the time of such transaction. Our ability to complete any such future distribution or sale may also be impacted by the sizeand we have made certain assumptions regarding COVID-19 for purposes of our retained stake at the time. The uncertainty relating to the separation and public listing transactions, their implementation, their timing and their yet to be determined effects on the JV’s business may subject us and the JV to risks and uncertainties that may adversely affect our businessoperational planning and financial results.
GENERAL RISKS:
projections, including assumptions regarding the global macroeconomic impact of COVID-19, PANDEMIC
Ouras well as the demand, revenues, supply, contracts, market share and commercial markets for our current or future COVID-19 products, which remain dynamic. Despite careful tracking and planning, we are unable to accurately predict the extent of the impact of COVID-19 or our COVID-19 products on our business, operations and financial condition and results have been anddue to the uncertainty of future developments. COVID-19 or our COVID-19 products may continue to be impacted by the COVID-19 pandemic to varying degrees. The pandemic has presented a number of risks and challenges foralso affect our business, including, among others, impacts due to travel limitationsoperations or financial condition and mobility restrictions; manufacturing disruptions and delays; supply chain interruptions, including challenges related to reliance on third-party suppliers; disruptions to pipeline development and clinical trials, including difficulties or delays in enrollment of certain clinical trials and in access to needed supplies; decreased product demand, due to reduced numbers of in-person meetings with prescribers, patient visits with physicians, vaccinations and elective surgeries, resulting in fewer new prescriptions or refills of existing prescriptions and reduced demand for products used in procedures; further reduced product demand as a result of increased unemployment; challenges presented by reallocating personnel and R&D, manufacturing and other resources to assist in responding to the pandemic; costs associated with the COVID-19 pandemic, including practices intended to reduce the risk of transmission, increased supply chain costs and additional R&D costs incurred in our efforts to develop a vaccine to help prevent COVID-19 and potential treatments for COVID-19; challenges related to our business development initiatives, including potential delays or disruptions related to regulatory approvals; interruptions or delays in the operations of regulatory authorities, which may delay potential approval of new products we are developing, potential label expansions for existing products and the launch of newly-approved products; challenges operatingresults in a virtual work environment; potential increased cyber incidents suchmanner that is not presently known to us or that we currently do not consider as phishing, social engineering and malware attacks; challenges related to our intellectual property, both domestically and internationally, including in response to any pressure or legal or regulatory action that could potentially result in us not seeking intellectual property protection for, licensing, or agreeing not to enforce,
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 18 |
intellectual property rights related to our products, including our vaccine to help prevent COVID-19 and potential treatments for COVID-19; challenges related to conducting oversight and monitoring of regulated activities in a remote or virtual environment; and other challenges presented by disruptions to our normal operations in response to the pandemic, as well as uncertainties regarding the duration and severity of the pandemic and its impacts, and government or regulatory actions to contain the virus or control the supply of medicines.
We also face risks and uncertainties related to our efforts to develop and commercialize a vaccine to help preventour COVID-19 and potential treatments for COVID-19,products, as well as challenges related to their manufacturing, supply and distribution, including, among others, others:
•the risk that as the market for COVID-19 products becomes more endemic and seasonal, demand for any of our COVID-19 products has and may continue to be reduced or not meet expectations, or may no longer exist, which has and may continue to lead to reduced revenues, excess inventory on-hand and/or in the channel which, for Paxlovid and Comirnaty, has resulted in significant inventory write-offs in 2023 and could continue to result in inventory write-offs or other unanticipated charges;
•challenges related to the transition to the commercial market for our COVID-19 products;
•uncertainties related to the public’s demand for vaccines, boosters and COVID-19 treatments;
•risks related to our ability to accurately forecast and achieve our revenue forecasts for Comirnaty and Paxlovid or any potential future COVID-19 vaccines or treatments;
•uncertainties inherent in R&D, including the ability to meet anticipated clinical endpoints, commencement and/or completion dates for clinical trials, regulatory submission dates, regulatory approval dates and/or launch dates, as well as risks associated with pre-clinical orand clinical data (including the in vitro and Phase 1/2/3 or Phase 4 data for Comirnaty or any vaccine candidate in the Pfizer-BioNTechBNT162 program or Paxlovid or any future COVID-19 vaccine (BNT162b2)),treatment) in any of our studies in pediatrics, adolescents or adults or real world evidence, including the possibility of unfavorable new pre-clinical, clinical or safety data and further analyses of existing pre-clinical, clinical or safety data; data or further information regarding the quality of pre-clinical, clinical or safety data, including by audit or inspection;
•the ability to produce comparable clinical or other results for Comirnaty, any vaccine candidate or other vaccines that may result from the BNT162 program, Paxlovid or any future COVID-19 treatment or any other COVID-19 program, including the rate of vaccine effectiveness andand/or efficacy, safety and tolerability profile observed to date, in additional analyses of the Phase 3 trial for any such products and additional studies, in real-world data studies or in larger, more diverse populations uponfollowing commercialization;
•the ability of BNT162b2Comirnaty or any future vaccine to prevent, or Paxlovid or any future COVID-19 treatment to be effective against, COVID-19 caused by emerging virus variants;
•the risk that more widespread use of the vaccineComirnaty or Paxlovid will lead to new information about efficacy, safety or other developments, including the risk of additional adverse reactions, some of which may be serious;
•the risk that pre-clinical and clinical trial data are subject to differing interpretations and assessments, including during the peer review/publication process, in the scientific community generally, and by regulatory authorities;
•whether and when additional data from the BNT162 mRNA vaccine program, Paxlovid or other COVID-19 programs will be published in scientific journal publications and, if so, when and with what modifications and interpretations;
•whether regulatory authorities will be satisfied with the design of and results from these and anyexisting or future pre-clinical and clinical studies;
•whether and when othersubmissions to request emergency use or conditional marketing authorizations for Comirnaty or any future vaccines in additional populations, for a potential booster dose for Comirnaty, or any potential future vaccine or vaccine candidates (including potential future annual boosters or re-vaccinations), and/or biologics license and/or EUA applications or amendments to any such applications may be filed in particular jurisdictions for BNT162b2Comirnaty or any other potential vaccines that may arise from the BNT162 program,vaccine or vaccine candidates, and if obtained, whether or when such EUA or licenses, or existing EUAs, will expire or terminate;
•whether and when submissions to request emergency use or conditional marketing authorizations for Paxlovid or any future COVID-19 treatment and/or any drug applications and/or EUA applications or amendments to any such applications for any indication for Paxlovid or any future COVID-19 treatment may be filed in particular jurisdictions, and if obtained, whether or when such EUA or licenses, or existing EUAs, will expire or terminate;
•whether and when any applicationsapplication that may be pending or filed for BNT162b2Comirnaty, any vaccine candidate or other vaccines that may result from the BNT162 program, Paxlovid or any future COVID-19 treatment or any other COVID-19 program may be approved by particular regulatory
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 24 |
authorities, which will depend on myriad factors, including making a determination as to whether the vaccine’s or drug’s benefits outweigh its known risks and determination of the vaccine’s or drug’s efficacy and, if approved, whether it will be commercially successful;
•decisions by regulatory decisionsauthorities impacting labeling or marketing, manufacturing processes, safety and/or other matters that could affect the availability or commercial potential of aany vaccine or drug, including developmentthe authorization or approval of products or therapies developed by other companies;
•disruptions in the relationships between us and our collaboration partners, clinical trial sites or third-party suppliers, including our relationship with BioNTech;
•the risk that other companies may produce superior or competitive products; the riskproducts that demand for any products may be reducedsuperior in terms of efficacy, safety, affordability, convenience, or no longer exist; a number of other competitive factors;
•risks related to the availability or cost of raw materials to manufacture or test any such products;
•challenges related to our vaccine’s ultra-low temperature formulation two-dose schedule and attendant storage, distribution and administration requirements, including risks related to storage and handling after delivery by us;
•challenges and risks related to medication errors such as prescribing or dispensing the wrong strength, improper dosing and self-administration errors;
•the risk that we may not be able to successfully develop other vaccine formulations; formulations, booster doses or potential future vaccines, potential combination respiratory vaccines or next generation COVID-19 treatments;
•the risk that we may not be able to recoup costs associated with our R&D and manufacturing efforts;
•risks associated with any changes in the way we approach or provide research funding for the BNT162 program, Paxlovid or potential treatment for COVID-19; any other COVID-19 program;
•challenges and risks associated with the pace of our development programs;
•the risk that we may not be able to maintain or scale up manufacturing capacity on a timely basis or maintain access to logistics or supply channels commensurate with global demand for our vaccine or any potential approved treatment,COVID-19 products, which would negatively impact our ability to supply the estimated numbers of doses of our vaccineCOVID-19 products within the projected time periods as previously indicated; periods;
•whether and when additional supply or purchase agreements will be reached; reached or existing agreements will be modified;
•uncertainties regarding the ability to obtain recommendations from vaccine or treatment advisory or technical committees and other public health authorities and uncertainties regarding the commercial impact of any such recommendations;
•pricing and access challenges for such products;
•challenges related to public vaccine confidence in, or awareness; awareness of Comirnaty, Paxlovid or any future COVID-19 product candidates, including challenges driven by misinformation or disinformation, access, concerns about clinical data integrity, or prescriber and pharmacy education;
•trade restrictions; and competitive developments.
Further, •the COVID-19 pandemic, and the volatile global economic conditions stemming from the pandemic, could precipitate or amplify the other risksrisk that we identifymay owe third-party royalties or other adverse outcomes from existing litigation related to Comirnaty and Paxlovid, or have additional other claims asserted related to Comirnaty or Paxlovid.
Certain of these risks and uncertainties also apply to our COVID-19 and influenza diagnostic tests.
CLIMATE CHANGE AND SUSTAINABILITY
Pfizer is subject to transitional and physical risks related to climate change. Transitional risks include, for example, a disorderly global transition away from fossil fuels that may result in this Risk Factors section,increased energy prices; customer preference for low or no-carbon products; stakeholder pressure to decarbonize assets; or new legal or regulatory requirements that result in new or expanded carbon pricing, taxes, restrictions on greenhouse gas emissions, and increased greenhouse gas disclosure and transparency. These risks could increase operating costs, including the cost of our electricity and energy use, or otherwise increase compliance costs. Physical risks to our operations include water stress and drought; flooding and storm surge; wildfires; extreme temperatures and storms, which could adversely affectimpact pharmaceutical production, increase costs, or disrupt supply chains of medicines for patients. For example, our business, operations and financial condition and results.
We are continuingmanufacturing facility in Rocky Mount, NC was damaged by a tornado in July 2023. While manufacturing has resumed, the supply of medicines impacted by the tornado is expected to
monitor the latest developments regarding the COVID-19 pandemic and its effectsbe affected through 2024. For additional details on
our business, operations and financial condition and results, and have made certain assumptions regarding the COVID-19 pandemic for purposes of our operational planning and financial projections, including assumptions regarding the duration, severity and the global macroeconomic impact of the pandemic, as well as COVID-19 vaccine supply and contracts, which remain dynamic. Despite careful tracking and planning, we are unable to accurately predict the extent of the impact of the
pandemictornado in Rocky Mount, NC, see the Overview of Our Performance, Operating Environment, Strategy and Outlook—Our Operating Environmentsection within MD&A. Our supply chain is subject to these same transitional and physical risks and would likely pass along any increased costs to us.In June 2022, Pfizer established our fourth consecutive greenhouse gas reduction goal with new near- and long-term targets to achieve the Science Based Target Initiative’s voluntary Net-Zero Standard by 2040. While we are working to develop and implement emission reduction plans to achieve our voluntary climate goals, various factors, including the long time horizons and commercial availability of new technologies to enable the emission reductions, in the time and scale needed, may present inherent risk in our ability to meet these goals. Additionally, success may depend on the actions of governments and third parties and may require, among other things, significant capital investment; R&D; and government policies and incentives to foster innovation and reduce costs of technologies that may not currently exist or be available at scale.
Governmental authorities, non-governmental organizations, customers, investors, employees, and other stakeholders are increasingly sensitive to ESG matters, such as equitable access to medicines and vaccines, product quality and safety, diversity, equity and inclusion, environmental stewardship, support for local communities, value chain environmental and social due diligence, corporate governance and transparency, and addressing human capital factors in our operations. In addition, governments and the public expect companies like us to report on our business operationspractices with respect to human rights, responsible sourcing and financial conditionenvironmental impact, as well as the actions of our third-party contractors and results duesuppliers around the world. This focus on ESG matters may lead to the uncertaintynew expectations or requirements that could result in increased costs associated with research, development, manufacture, or distribution of future developments. In particular,our products. Our ability to compete could also be affected by changing customer preferences and requirements, such as growing demand for companies to establish validated Net Zero targets or offer more sustainable products. While we believe the ultimate impactstrive to improve our ESG performance and meet our voluntary goals, if we do not meet, or are perceived not to meet, our goals or other stakeholder expectations in key ESG areas, we risk negative stakeholder reaction, including from proxy advisory services, as well as damage to our brand and reputation, reduced demand for our products or other negative impacts on our business operations and financial condition and resultsoperations. While we monitor a broad range of ESG matters, we cannot be certain that we will be affected by the speed and extent of the continued spread of the coronavirus globally, the emergence of additional virus variants, the duration of the pandemic, new information regarding the severity and incidence of COVID-19, the safety, efficacy and availability of vaccines and treatments for COVID-19, the rate at which the population becomes vaccinated against COVID-19, the global macroeconomic impact of the pandemic and governmental or regulatory actions to contain the virus or control supply of medicines. The pandemic may also affect our business, operations or financial condition and results in a manner that is not presently known to usmanage such matters successfully, or that we currently do not consider as presenting significant risks.will successfully meet the expectations of investors, employees, consumers, governments and other stakeholders.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 25 |
MARKET FLUCTUATIONS IN OUR EQUITY AND OTHER INVESTMENTS
COST AND EXPENSE CONTROL AND NONORDINARY EVENTS
Growth in costs and expenses, changes in product and geographic mix and the impact of acquisitions, divestitures, restructurings, internal reorganizations, product withdrawals, recalls and other unusual events that could result from evolving business strategies, evaluation of asset realization and organizational restructuring could adversely affect future results. Such risks and uncertainties include, in particular, our ability to realize the projected benefits of our cost-reduction and productivity initiatives, including our enterprise-wide cost realignment program, other corporate strategic initiatives and any acquisitions, divestitures or other initiatives, as well as potential disruption of ongoing business, such as potential impacts on our ability to deliver on our pipeline as planned. Additionally, as a result of these initiatives, we may experience a loss of continuity, loss of accumulated knowledge or intellectual property and/or inefficiency, adverse effects on employee morale, loss of key employees and/or other retention issues during transitional periods. Reorganizations and restructurings can require a significant amount of time and focus, which may divert attention from operating and growing our business.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 19 |
INTANGIBLE ASSETS, GOODWILL AND EQUITY-METHOD INVESTMENTS
Our consolidated balance sheet contains significant amounts of intangible assets, including IPR&D and goodwill. For IPR&D assets, the risk of failure is significant, and there can be no certainty that these assets ultimately will yield successful products. Our ability to realize value on these significant investments is often contingent upon, among other things, regulatory approvals and market acceptance. As such, we expect that many of these IPR&D assets willmay become impaired and/or be written off at some time in the future if the associated R&D effort is abandoned or is curtailed. See Note 4for a discussion of recent impairments of IPR&D assets. For goodwill, all reporting units can confront events and circumstances that can lead to a goodwill impairment charge such as, among other things, unanticipated competition, an adverse action or assessment by a regulator, a significant adverse change in legal matters or in the business climate and/or a failure to replace the contributions of products that lose exclusivity. Our other intangible assets, including developed technology rights and brands, face similar risks for impairment. Our equity-method investments may also be subject to impairment charges that may result from the occurrence of unexpected adverse events or management decisions that impact our estimates of expected cash flows to be generated from these investments. We may recognize impairment charges as a result of a weak economic environment, events related to particular customers or asset types, challenging market conditions or decisions by management. Any such impairment charge of our intangible assets, goodwill and equity-method investments may be significant. For additional details, see the SSignificant Accounting Policies and Application of Critical Accounting Estimates and Assumptions—Asset Impairments section within MD&A.
CHANGES IN LAWS AND ACCOUNTING STANDARDS
Our future results could be adversely affected by changes in laws and regulations or their interpretation, including, among others, changes in accounting standards, tax laws and regulations internationally and in the U.S. (including, among other things, the IRA, changes in laws and regulations or their interpretation, including, among others, the adoption of global minimum taxation requirements outside the U.S. generally effective in most jurisdictions since January 1, 2024 and potential changes to existing tax law by the current U.S. Presidential administration and Congress, including the proposed “Tax Relief for American Families and Workers Act of 2024”), competition laws, privacy laws and environmental laws in the U.S. and other countries. For additional information on changes in tax laws or rates or accounting standards, see the Provision/(Benefit) for Taxes on Income and New Accounting Standards sections within MD&A andNote 1B. Managing cybersecurity risk is a crucial part of our overall strategy for safely operating our business. We incorporate cybersecurity practices into our Enterprise Risk Management (ERM) approach, which is subject to oversight by our BOD. Our cybersecurity policies and practices are aligned with relevant industry standards.
Consistent with our overall ERM program and practices, our cybersecurity program includes:
•Vigilance: We maintain a global cybersecurity operation that endeavors to detect, prevent, contain, and respond to cybersecurity threats and incidents in a prompt and effective manner with the goal of minimizing business disruptions.
•External Collaboration: We collaborate with public and private entities, including intelligence and law enforcement agencies, industry groups and third-party service providers to identify, assess and mitigate cybersecurity risks.
•Systems Safeguards: We deploy technical safeguards that are designed to protect our information systems, products, operations and sensitive information from cybersecurity threats. These include firewalls, intrusion prevention and detection systems, disaster recovery capabilities, malware and ransomware prevention, access controls and data protection. We continuously conduct vulnerability assessments to identify new risks and periodically test the efficacy of our safeguards through both internal and external penetration tests.
•Education: We provide periodic training for all personnel regarding cybersecurity threats, with such training appropriate to the roles, responsibilities and access of the relevant Company personnel. Our policies require all workers to report any real or suspected cybersecurity events.
•Supplier Ecosystem Management: We extend our cybersecurity management control expectations to our supply chain ecosystem, as applicable. This includes identifying cybersecurity risks presented by third parties.
•Incident Response Planning: We have established, and maintain and periodically test, incident response plans that direct our response to cybersecurity events and incidents. Such plans include the protocol by which material incidents would be communicated to executive management, our BOD, external regulators and shareholders.
•Enterprise-Wide Coordination: We engage experts from across the Company to identify emerging risks and respond to cybersecurity threats. This cross-functional approach includes personnel from our R&D, manufacturing, commercial, technology, legal, compliance, internal audit and other business functions.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 26 |
•Governance: Our BOD’s oversight of cybersecurity risk management is led by the Audit Committee, which oversees our ERM program. Cybersecurity threats, risks and mitigation are periodically reviewed by the Audit Committee and such reviews include both internal and independent assessment of risks, controls and effectiveness.
Our risk assessment efforts have indicated that we are a target for theft of intellectual property, financial resources, personal information, and trade secrets from a wide range of actors including nation states, organized crime, malicious insiders and activists. The impacts of attacks, abuse and misuse of Pfizer’s systems and information include, without limitation, loss of assets, operational disruption and damage to Pfizer’s reputation.
A key element of managing cybersecurity risk is the ongoing assessment and testing of our processes and practices through auditing, assessments, drills and other exercises focused on evaluating the sufficiency and effectiveness of our risk mitigation. We regularly engage third parties to perform assessments of our cybersecurity measures, including information security maturity assessments and independent reviews of our information security control environment and operating effectiveness. Certain results of such assessments and reviews are reported to the Audit Committee and the BOD, as appropriate, and we make adjustments to our cybersecurity processes and practices as necessary based on the information provided by the third-party assessments and reviews.
The Audit Committee oversees cybersecurity risk management, including the policies, processes and practices that management implements to prevent, detect and address risks from cybersecurity threats. The Audit Committee receives regular briefings on cybersecurity risks and risk management practices, including, for example, recent developments in the external cybersecurity threat landscape, evolving standards, vulnerability assessments, third-party and independent reviews, technological trends and considerations arising from our supplier ecosystem. The Audit Committee may also promptly receive information regarding any material cybersecurity incident that may occur, including any ongoing updates regarding the same. The Audit Committee periodically discusses our approach to cybersecurity risk management with our Chief Information Security Officer (CISO).
Our CISO is a member of our management team who is principally responsible for overseeing our cybersecurity risk management program, in partnership with other business leaders across the Company. The CISO works in coordination with other members of the management team, including, among others, the Chief Digital Officer, the Chief Financial Officer, the Chief Compliance and Risk Officer and the General Counsel and their designees. We believe our business leaders have the appropriate expertise, background and depth of experience to manage risks arising from cybersecurity threats.
Our CISO, along with leaders from our privacy and corporate compliance functions, collaborate to implement a program designed to manage our exposure to cybersecurity risks and to promptly respond to cybersecurity incidents. Prompt response to incidents is delivered by multi-disciplinary teams in accordance with our incident response plan. Through ongoing communications with these teams during incidents, the CISO monitors the triage, mitigation and remediation of cybersecurity incidents, and reports such incidents to executive management, the Audit Committee and other Pfizer colleagues in accordance with our cybersecurity policies and procedures, as is appropriate.
As of the date of this Form 10-K, we are not aware of any cybersecurity incidents that have materially affected or are reasonably likely to materially affect the Company, including our business strategy, results of operations, or financial condition at this time. For further discussion of the risks associated with cybersecurity incidents, see the Item 1A. Risk Factors—Information Technology and Security section in this Form 10-K.
We own and lease space around the worldglobally for sales and marketing, customer service, regulatory compliance, R&D, manufacturing and distribution and corporate enabling functions. In many locations, our business and operations are co-located to achieve synergy and operational efficiencies. Our global headquarters are located in New York City. We continue to advance our global workplace strategy to provide workplaces that enable collaboration and foster innovation. As of December 31, 2020,2023, we had 363284 owned and leased properties (including properties acquired in the Seagen acquisition), amounting to approximately 4338 million square feet. The recent Seagen acquisition has increased our real estate portfolio by 14 sites totaling 1 million square feet.
In 2020, we reduced the number of properties in our portfolio by 90 sites and 4 million square feet, primarily due to the spin-off and combination of the Upjohn Business with Mylan to form Viatris.
We expect to relocate our global headquarters to the Spiral, an office building in the Hudson Yards neighborhood of New York City, with occupancy expected beginning in 2022. In April 2018, we entered into an agreement to lease space at this property. In July 2018, we completed the sale of our current headquarters in New York City. We remain in a lease-back arrangement with the buyer while we complete our relocation.
Our PGS platform function is headquartered in various locations, with leadership teams primarily in New York City and in Peapack, New Jersey. As of December 31, 2020,2023, of the 284 properties, PGS had responsibility for 4337 plants around the world, which manufacture products for our commercial divisions, including in Belgium, Germany, India, Ireland, Italy, Japan, Singapore and the U.S., which manufacture products The leadership team for our business. PGS expects to exit five of these sites over the next several years.is primarily located in New York City. PGS also operates multiple distribution facilities around the world.
In general, we believe that our properties, including the principal properties described above, are well-maintained, adequate and suitable for their current requirements and for our operations in the foreseeable future. See Note 9 for amounts invested in land, buildings and equipment. Certain legal proceedings in which we are involved are discussed in Note 16A.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 27 |
INFORMATION ABOUT OUR EXECUTIVE OFFICERS
The executive officers of the Company are set forth in this table. Each holds the office or offices indicated until his or her successor is chosen and qualified at the regular meeting of the BOD to be held on the date of the 20212024 Annual Meeting of Shareholders, or until his or her earlier death, resignation or removal. Each of the executive officers is a member of the Pfizer Executive Leadership Team.
| | | | | | | | | | | | | | |
| Name | | Age | | Position |
| | | | |
| Albert Bourla | | 5962 | | Chairman of the Board since January 2020 and Chief Executive Officer since January 2019. Chief Operating Officer from January 2018 until December 2018. Group President, Pfizer Innovative Health from June 2016 until December 2017. Group President, Global Innovative Pharma Business (responsible for Vaccines, Oncology and Consumer Healthcare since 2014) from February 2016 until June 2016. President and General Manager of Established Products Business Unit from December 2010 until December 2013. Our Director since February 2018. Board member of Pharmaceutical Research and Manufacturers of America (PhRMA). Board member of The Pfizer Foundation, which promotes access to quality healthcare. Director of the Partnership for New York City and Catalyst, a global non-profit organization accelerating progress for the advancement of women into leadership. |
| | | | |
William CarapezziChris Boshoff | | 6360 | | Chief Oncology Officer, Executive Vice President Global Business Servicessince December 2023. Chief Oncology Research and Transformation since June 2020. Senior Vice President of Global Business Operations from June 2013 until June 2020. Senior Vice President of Global Tax from 2008 until June 2013. |
| | | | |
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 20 |
| | | | | | | | | | | | | | |
Name | | Age | | Position |
| | | | |
Frank A. D’Amelio | | 63 | | Chief FinancialDevelopment Officer and Executive Vice President Global Supply since June 2020. from July 2023 until December 2023. Senior Vice President, Oncology, from 2017 until 2023. |
| David M. Denton | | 58 | | Chief Financial Officer, Executive Vice President Business Operations and Global Supplysince May 2022. Executive Vice President, Chief Financial Officer, Lowe’s Companies, Inc., from November 2018 until June 2020.April 2022; Executive Vice President, Business Operations and Chief Financial Officer from December 2010 until October 2018. Senior Vice President and Chief Financial Officer, CVS Health Corporation (a diversified health solutions company), from September 2007January 2010 until December 2010.November 2018. Director of ZoetisTapestry, Inc. and Humana Inc. and Chair of the Humana Inc. Board of Directors’ Audit Committee.from 2014 to 2023. Director of the Independent College Fund of New Jersey.Haleon plc. |
| Alexandre de Germay | | 56 | | | Chief International Commercial Officer, Executive Vice President since December 2023. Chief Executive Officer, Laboratoires Majorelle (a specialty pharma company based in France dedicated to women’s health and urology) from 2021 until January 2024 (assisting with transition matters after December 15, 2023). From 2020 until 2021 was Senior Vice President; Global Franchise Head of Cardiology, Transplant and Established Products, and from 2016 until 2020 was Head of Mature Markets General Medicines of Sanofi. Regional President of Asia-Pacific of Pfizer Inc. from 2013 until 2016. |
| Mikael Dolsten | | 6265 | | Chief Scientific Officer, President, Pfizer Research and Development since July 2023. Chief Scientific Officer and President, Worldwide Research, Development and Medical sincefrom January 2019.2019 until July 2023. President of Worldwide Research and Development from December 2010 until December 2018. Senior Vice President; President of Worldwide Research and Development from May 2010 until December 2010. Senior Vice President; President of Pfizer BioTherapeutics Research & Development Group from October 2009 until May 2010. He was Senior Vice President of Wyeth and President, Wyeth Research from June 2008 until October 2009. Director of Karyopharm Therapeutics Inc. Director of PhRMA FoundationAgilent Technologies, Inc, and Governor of New York Academy of Science (NYAS).Vimian Group AB. |
| | | | |
| Lidia Fonseca | | 5255 | | Chief Digital and Technology Officer, Executive Vice President since January 2019. Chief Information Officer and Senior Vice President of Quest Diagnostics Incorporated from 2014 to 2018. Senior Vice President of Laboratory Corporation of America Holdings from 2008 until March 2013. Director of Tegna, Inc. from 2014 to 2023. Director of Medtronic plc. |
| | | | |
Angela Hwang | | 55 | | Group President, Pfizer Biopharmaceuticals Group since January 2019. Group President, Pfizer Essential Health from January 2018 until December 2018. Global President, Pfizer Inflammation and Immunology from January 2016 until December 2017. Regional Head, U.S. Vaccines from January 2014 until December 2015. Vice President, Emerging Markets for the Primary Care therapeutic area from September 2011 until December 2013. Vice President, U.S. Brands commercial organization within Essential Health from October 2009 until August 2011. Director of United Parcel Service, Inc. |
| | | | |
| Rady A. Johnson | | 5962 | | Chief Compliance, Quality and Risk Officer, Executive Vice President since January 2019. Executive Vice President, Chief Compliance and Risk Officer from December 2013 until December 2018. Senior Vice President and Associate General Counsel from October 2006 until December 2013. |
| | | | |
| Douglas M. Lankler | | 5558 | | General Counsel, Executive Vice President since December 2013. Corporate Secretary from January 2014 until February 2014. Executive Vice President, Chief Compliance and Risk Officer from February 2011 until December 2013. Executive Vice President, Chief Compliance Officer from December 2010 until February 2011. Senior Vice President and Chief Compliance Officer from January 2010 until December 2010. Senior Vice President, Deputy General Counsel and Chief Compliance Officer from August 2009 until January 2010. |
| | | | |
A. Rod MacKenzieAamir Malik | | 6148 | | Chief DevelopmentU.S. Commercial Officer, Executive Vice President since June 2016. SeniorDecember 2023. Chief Business Innovation Officer, Executive Vice President from August 2021 until December 2023. Various U.S. geographic leadership roles with McKinsey & Company from 2019 to 2021; previously co-led McKinsey & Company’s Global Pharmaceuticals & Medical Products practice from 2015 to 2018. |
| | | | |
| Michael McDermott | | 58 | | Chief DevelopmentGlobal Supply Officer, from March 2016 until June 2016. Group SeniorExecutive Vice President and Head, Pharma Therapeutics Research and Developmentsince January 2022. President of Pfizer Global Supply from 20102018 until March 2016. Dr. MacKenzie represents2021. Vice President of Pfizer as a memberGlobal Supply from 2014 until 2018. Vice President of the Board of Directors of ViiV Healthcare Limited, TransCelerate Biopharma Inc. and the National Health Council.Biotechnology Unit from 2012 until 2014. |
| | | | |
| Payal Sahni | | 4649 | | Chief People Experience Officer, Executive Vice President since January 2022. Chief Human Resources Officer, Executive Vice President sincefrom June 2020.2020 to December 2021. From May 2016 until June 2020 served as Senior Vice President of Human Resources for multiple operating units. Vice President of Human Resources, Vaccines, Oncology & Consumer from 2015 until 2016. Ms. Sahni has served in a number of positions in the Human Resources organization with increasing responsibility since joining Pfizer in 1997. |
| | | | |
| Sally Susman | | 5962 | | Chief Corporate Affairs Officer, Executive Vice President since January 2019. Executive Vice President, Corporate Affairs (formerly Policy, External Affairs and Communications) from December 2010 until December 2018. Senior Vice President, Policy, External Affairs and Communications from December 2009 until December 2010. Director of WPP plc. |
| | | | |
John D. Young | | 56 | | Chief Business Officer, Group President since January 2019. Group President, Pfizer Innovative Health from January 2018 until December 2018. Group President, Pfizer Essential Health from June 2016 until December 2017. Group President, Global Established Pharma Business from January 2014 until June 2016. President and General Manager, Pfizer Primary Care from June 2012 until December 2013. Primary Care Business Unit’s Regional President for Europe and Canada from 2009 until June 2012. Director of Johnson Controls International plc. Mr. Young represents Pfizer as a member of the Board of Directors of the Consumer Healthcare JV. Director of Biotechnology Innovation Organization (BIO). |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 28 |
| | | | | |
| ITEM 5. | MARKET FOR THE COMPANY’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
The principal market for our common stock is the NYSE. Our common stock currently trades on the NYSE under the symbol “PFE”. As of February 23, 2021,15, 2024, there were 139,582123,387 holders of record of our common stock.
The following summarizes purchases of our common stock during the fourth quarter of 20202023(a):
| | | | | | | | | | | | | | | | | | | | | | | |
| Period | Total Number of Shares Purchased(b) | | Average Price Paid per Share(b) | | Total Number of
Shares Purchased as
Part of Publicly
Announced Plan | | Approximate Value of Shares that May Yet Be Purchased Under the Plan(a) |
| September 28 through October 25, 2020 | 26,921 | | $ | 36.99 | | | — | | | $ | 5,292,881,709 | |
| October 26 through November 30, 2020 | 84,279 | | $ | 37.48 | | | — | | | $ | 5,292,881,709 | |
| December 1 through December 31, 2020 | 69,317 | | $ | 37.39 | | | — | | | $ | 5,292,881,709 | |
| Total | 180,517 | | | $ | 37.37 | | | — | | | |
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 21 |
| | | | | | | | | | | | | | | | | | | | | | | |
| Period | Total Number of Shares Purchased(b) | | Average Price Paid per Share(b) | | Total Number of
Shares Purchased as
Part of Publicly
Announced Plan | | Approximate Value of Shares that May Yet Be Purchased Under the Plan(a) |
| October 2 through October 29, 2023 | 12,222 | | $ | 32.93 | | | — | | | $ | 3,292,882,444 | |
| October 30 through November 30, 2023 | 25,825 | | $ | 29.95 | | | — | | | $ | 3,292,882,444 | |
| December 1 through December 31, 2023 | 14,449 | | $ | 28.58 | | | — | | | $ | 3,292,882,444 | |
| Total | 52,496 | | | $ | 30.26 | | | — | | | |
(b)Represents (i) 174,55549,685 shares of common stock surrendered to the Company to satisfy tax withholding obligations in connection with the vesting of awards under our long-term incentive programs and (ii) the open market purchase by the trustee of 5,9622,811 shares of common stock in connection with the reinvestment of dividends paid on common stock held in trust for employees who deferred receipt of performance share awards.
PEER GROUP PERFORMANCE GRAPH
The following graph assumes a $100 investment on December 31, 2015,2018, and reinvestment of all dividends, in each of the Company’s Common Stock, the S&P 500 Index, and a composite peer group of the major U.S. and European-based pharmaceutical companies, which are: AbbVie Inc., Amgen Inc., AstraZeneca PLC, Bristol-Myers Squibb Company, Eli Lilly and Company, GlaxoSmithKlineGSK plc, Johnson & Johnson, Merck & Co., Inc., Novartis AG, Novo Nordisk, Roche Holding AG and Sanofi.Sanofi SA, the S&P 500 Index and the NYSE Arca Pharmaceutical Index (DRG index).
Five Year Performance
| | | 2015 | | 2016 | | 2017 | | 2018 | | 2019 | | 2020 |
| | 2018 | | | | 2018 | | 2019 | | 2020 | | 2021 | | 2022 | | 2023 |
| PFIZER | PFIZER | | $100.0 | | $104.5 | | $120.9 | | $151.0 | | $140.5 | | $145.4 | PFIZER | | $100.0 | | $93.1 | | $96.3 | | $160.5 | | $143.8 | | $84.5 |
| PEER GROUP | PEER GROUP | | $100.0 | | $100.8 | | $118.1 | | $127.8 | | $155.3 | | $161.7 | PEER GROUP | | $100.0 | | $122.0 | | $128.7 | | $154.4 | | $179.9 | | $207.8 |
| S&P 500 | S&P 500 | | $100.0 | | $112.0 | | $136.4 | | $130.4 | | $171.4 | | $203.0 | S&P 500 | | $100.0 | | $131.5 | | $155.6 | | $200.3 | | $164.0 | | $207.0 |
| DRG Index | | DRG Index | | $100.0 | | $118.4 | | $128.7 | | $158.8 | | $171.1 | | $184.3 |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 2229 |
| | | | | |
ITEM 6. | SELECTED FINANCIAL DATA |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended/As of December 31,(a) |
| (MILLIONS, EXCEPT PER COMMON SHARE DATA) | | 2020 | | 2019 | | 2018 | | 2017 | | 2016 |
| Revenues | | $ | 41,908 | | | $ | 41,172 | | | $ | 40,825 | | | $ | 38,757 | | | $ | 38,664 | |
| Income/(loss) from continuing operations | | 7,021 | | | 10,867 | | | 3,861 | | | 13,558 | | | (67) | |
| Total assets | | 154,229 | | | 167,594 | | | 159,588 | | | 172,064 | | | 171,912 | |
Long-term obligations(b) | | 64,835 | | | 66,844 | | | 63,972 | | | 69,981 | | | 80,957 | |
Earnings/(loss) per common share—basic(c) | | | | | | | | | | |
| Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | | $ | 1.26 | | | $ | 1.95 | | | $ | 0.65 | | | $ | 2.26 | | | $ | (0.02) | |
Income from discontinued operations––net of tax(a) | | 0.47 | | | 0.98 | | | 1.25 | | | 1.31 | | | 1.20 | |
| Net income attributable to Pfizer Inc. common shareholders | | $ | 1.73 | | | $ | 2.92 | | | $ | 1.90 | | | $ | 3.57 | | | $ | 1.18 | |
Earnings/(loss) per common share—diluted(c) | | | | | | | | | | |
| Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | | $ | 1.24 | | | $ | 1.91 | | | $ | 0.64 | | | $ | 2.23 | | | $ | (0.02) | |
Income from discontinued operations––net of tax(a) | | 0.47 | | | 0.96 | | | 1.23 | | | 1.29 | | | 1.19 | |
| Net income attributable to Pfizer Inc. common shareholders | | $ | 1.71 | | | $ | 2.87 | | | $ | 1.87 | | | $ | 3.52 | | | $ | 1.17 | |
| | | | | | | | | | |
| Cash dividends declared per common share | | $ | 1.53 | | | $ | 1.46 | | | $ | 1.38 | | | $ | 1.30 | | | $ | 1.22 | |
(a)Amounts reflect the Upjohn Business and the Mylan-Japan collaboration as discontinued operations in all periods presented following the November 16, 2020 spin-off and combination of the Upjohn Business with Mylan and the December 21, 2020 termination of the Mylan-Japan collaboration. Income from discontinued operations––net of tax, including per common basic and diluted share amounts, for the year ended December 31, 2020 include the operating results of the Upjohn Business through November 16, 2020, the date of the spin-off and combination with Mylan. See Notes 1A and 2B. In addition, other acquisitions and business development activities completed in 2020, 2019 and 2018, including the acquisitions of Array and Therachon, and the contribution of our Consumer Healthcare business to the Consumer Healthcare JV, impacted financial results in the periods presented. See Note 1A. 2017 reflects the acquisition of AstraZeneca’s small molecule anti-infectives business and the sale of Hospira Infusion Systems net assets. 2016 reflects the acquisitions of Medivation and Anacor.
(b)Defined as Long-term debt, Pension benefit obligations, Postretirement benefit obligations, Noncurrent deferred tax liabilities, Other taxes payable and Other noncurrent liabilities.
(c)All years presented, except for 2016, reflect the impact of the TCJA on the Provision/(benefit) for taxes on income. For additional information see Note 5A.
| | | | | |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
GENERAL
The following MD&A is intended to assist the reader in understanding our financial condition and results of operations, including an evaluation of the amounts and certainty of cash flows from operations and from outside sources, and is provided as a supplement to and should be read in conjunction with the consolidated financial statements and related notes in Item 8. Financial Statements and Supplementary Data in this Form 10-K. Discussions of 2021 items and year-to-year comparisons between 2022 and 2021 that are not included in this Form 10-K can be found within MD&A in our 2022 Form 10-K. OVERVIEW OF OUR PERFORMANCE, OPERATING ENVIRONMENT, STRATEGY AND OUTLOOK
Financial Highlights
The following is a summary of certain financial performance metrics (in billions, except per share data):
| | | | | |
20202023 Total Revenues––$41.958.5 billion | 20202023 Net Cash Flow from Operations––$14.48.7 billion |
An increase of 2% compared to 2019 | An increase of 14% compared to 2019 |
| | | | | |
2020 Reported Diluted EPS––$1.71 | 2020 Adjusted Diluted EPS (Non-GAAP)––$2.22* |
A decrease of 40%42% compared to 20192022 | An increaseA decrease of 16%70% compared to 20192022 |
| | | | | |
| 2023 Reported Diluted EPS––$0.37 | 2023 Adjusted Diluted EPS (Non-GAAP)––$1.84* |
| A decrease of 93% compared to 2022 | A decrease of 72% compared to 2022 |
*For additional information regarding Adjusted diluted EPS (which is a non-GAAP financial measure), including reconciliations of certain GAAP reportedReported to non-GAAP adjustedAdjusted information, see the Non-GAAP Financial Measure: Adjusted Income section within MD&A.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 23 |
References to operational variances pertain to period-over-period changes that exclude the impact of foreign exchange rates. Although foreign exchange rate changes are part of our business, they are not within our control and since they can mask positive or negative trends in the business, we believe presenting operational variances excluding these foreign exchange changes provides useful information to evaluate our results.
Our Business and Strategy
––Pfizer Inc. is a research-based, global biopharmaceutical company. We apply science and our global resources to bring therapies to people that extend and significantly improve their lives. See the Item 1. Business––About Pfizersection. As a science-driven global biopharmaceutical company, we remain focused on advancing our pipeline, supporting our marketed brands and deploying capital responsibly, with a focus on initiatives that can help contribute to our long-term revenue and future growth. Most of our revenues come from the manufacture and sale of biopharmaceutical products. WithWe believe that our medicines and vaccines provide significant value for healthcare providers and patients and continuously evaluate how we can best collaborate with patients, physicians and payors to support and expand patient access to reliable, affordable healthcare around the formationworld. In addition, we continually seek to expand and broaden our product portfolio offerings through prioritized development of our pipeline and business development opportunities targeted at critical unmet patient needs. As a result, our commercial organizational structure and R&D operations are critical to the Consumer Healthcare JV in 2019 successful execution of our business strategy. Our ability to fulfill our purpose, Breakthroughs that change patients’ lives, remains a core focus and underscores our commitment to addressing the needs of society to help sustain long-term value creation for all stakeholders. Our 2024 key priorities are: •Achieve world-class oncology leadership
•Deliver next wave of pipeline innovation
•Maximize performance of our new products
•Expand margins by realigning our cost base
•Allocate capital to enhance shareholder value
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 30 |
In 2023, we managed our commercial operations through a global structure consisting of two operating segments: Biopharma and Business Innovation. Biopharma was the only reportable segment. See Note 1Aand the completionItem 1. Business––Commercial Operationssection. In December 2023, we completed our acquisition of Seagen. At the spin-offbeginning of 2024, we made changes in our commercial organization that went into effect on January 1, 2024 to incorporate Seagen and combinationimprove focus, speed and execution. Specifically, within our Biopharma reportable segment we created:
•the Pfizer Oncology Division, which brings together U.S. oncology commercial operations from both Pfizer and Seagen and is led by the Chief Oncology Officer, Executive Vice President, who also leads Pfizer’s newly combined global oncology R&D operations;
•the Pfizer U.S. Commercial Division, which focuses on the commercialization of our Upjohn Business with Mylan in November 2020, Pfizer has transformed into a more focused, global leader in science-based innovative medicines and vaccines. We now operate as a single operating segment engagednon-oncology products in the discovery, development, manufacturing, marketing, salesU.S. and distributionis led by the Chief U.S. Commercial Officer, Executive Vice President; and
•the Pfizer International Commercial Division, which focuses on the commercialization of biopharmaceutical products worldwide. Beginning inPfizer’s entire product portfolio outside the U.S. and is led by the Chief International Commercial Officer, Executive Vice President.
In the fourth quarter of 2020, the financial results of the Upjohn Business and the Mylan-Japan collaboration are reflected as discontinued operations for all periods presented. Prior-period information has been restated to reflect2022, we began taking steps through our current organizational structure following the separation of the Upjohn Business. See Note 1A and Item 1. Business––Commercial Operations of this Form 10-K for additional information. We expect to incur costs of approximately $700 million in connection with separating Upjohn, of which, approximately 70% has been incurred since inception and through December 31, 2020. These charges include costs and expenses related to separation of legal entities and transaction costs.
Transforming to a More Focused Company: We have undertakenCompany restructuring program to optimize our end-to-end R&D operations to reduce costs and cycle times as well as to further prioritize our internal R&D portfolio in areas where our capabilities are differentiated while increasing external innovation efforts to ensureleverage an expanding and productive biotech sector. Beginning in July 2023, in consideration of planned future investments in oncology, including the acquisition of Seagen on December 14, 2023, we reorganized our R&D platform operations. See Note 17A. In the fourth quarter of 2023, we announced that we launched a multi-year, enterprise-wide cost base aligns appropriatelyrealignment program that aims to realign our costs with our longer-term revenue base. While certain direct costs transferredexpectations. See Note 3. For a description of savings related to the Consumer Healthcare JV and to the Upjohn Business in connection with the spin-off, there are indirect costs which did not transfer. In addition, we are taking steps to restructure our corporate enabling functions to appropriately support and drive the purpose of our focused innovative biopharmaceutical products business and R&D and PGS platform functions. Seethese programs, see the Costs and Expenses––Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives section of thiswithin MD&A.&A. R&D: We believe we have a strong pipeline and are well-positioned for future growth. R&D is at the heart of fulfilling our purpose to deliver breakthroughs that change patients’ lives as we work to translate advanced science and technologies into the therapiesmedicines and vaccines that may be the most impactful for patients. Innovation, drug discovery and development are critical to our success. In addition to discovering and developing new products, our R&D efforts seek to add value to our existing products by improving their effectiveness and ease of dosing and by discovering potential new indications. See the Item 1. Business—Research and Developmentsection of this Form 10-Ksection for our R&D priorities and strategy.
We seek to leverage a strong pipeline, organize around expected operational growth drivers and capitalize on trends creating long-term growth opportunities, including:
•an aging global population that is generating increased demand for innovative medicines and vaccines that address patients’ unmet needs; and
•advances in both biological science and digital technologyplatform technologies that are enhancing the delivery of breakthrough new medicines and vaccines; andvaccines.
•Our Business Development Initiativesthe increasingly significant role of hospitals in healthcare systems.––
We are committed to strategically capitalizing on growth opportunities, primarily by advancing our own product pipeline and maximizing the value of our existing products, as well asbut also through various business development activities. We view our business development activity as an enabler of our strategies and seek to generate growth by pursuing opportunities and transactions that have the potential to strengthen our business and our capabilities. We assess our business, assets and scientific capabilities/portfolio as part of our regular, ongoing portfolio review process and also continue to consider business development activities that will help advance our business.business strategy. For additional information, includinga discussion of recent significant business development activities, see Note 2. Our 20202023 Performance
Revenues
Total Revenues increased $736 million,––Total revenues decreased $41.8 billion, or 2%42%, to $41.9$58.5 billion in 20202023 from $41.2$100.3 billion in 2019,2022, reflecting an operational increasedecrease of $1.1$40.8 billion, or 3%41%, andas well as an unfavorable impact of foreign exchange of $331 million,$1.0 billion, or 1%.
The operational decrease was primarily driven by significant declines in revenues from Comirnaty and Paxlovid, including a $3.5 billion non-cash revenue reversal for Paxlovid recorded in the fourth quarter of 2023. Excluding contributions from Comirnaty and Paxlovid, Total revenues increased 7% operationally, reflecting an increase in revenues from Nurtec ODT/Vydura and Oxbryta; revenues from Abrysvo, primarily driven by the impactlaunch of the Consumer Healthcare transaction, revenues increased 8% operationally, reflecting strong growth in Vyndaqel/Vyndamax, Eliquis, Ibrance outside developed Europe, Inlyta, Xeljanz, Xtandi, Prevenar 13 outside the U.S., oncology biosimilars and certain productsolder adult indication in the Hospital therapeutic area inU.S.; as well as continued growth from the U.S.,Vyndaqel family and Eliquis; partially offset by Enbrel internationally and Prevnar 13 and Chantixa decline in the U.S. Revenues for 2020 included an estimated unfavorable impact of approximately $700 million, or 2%, due to COVID-19, primarily reflecting lower demand for certain products in China and unfavorable disruptions to wellness visits for patients in the U.S., which negatively impacted prescribing patterns for certain products, partially offset by increased U.S. demand for certain sterile injectable products and increased adult uptake for Prevenar 13 in certain international markets, resulting from greater vaccine awareness for respiratory illnesses, and U.S. revenues for BNT162b2.Ibrance.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 24 |
The following chart outlines the components of the net change in revenues:Total revenues:
For worldwide revenues,See the Total Revenues by Geographyand Total Revenues––Selected Product Discussion sections within MD&A for more information, including a discussion of key drivers of our revenue performance and revenues by geography, see the discussion in theperformance. See also Analysis of the Consolidated Statements of Income–The Global Economic Environment––Revenues––Selected Product DiscussionCOVID-19 and ––Revenues by Geography sections within MD&A.section below for information about our COVID-19 products. For additional information regarding the primary indications or class of certain products, see Note 17B.17C.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 31 |
While royalty income through December 31, 2023 has been recorded in Other Income/(Deductions)—net, we will begin reporting such royalty income in Total revenues beginning in 2024 and will restate prior periods for consistency with our 2024 presentation. Additionally, we will no longer record royalties from U.S. sales of Bavencio, as we have irrevocably chosen to donate the right to such royalties to the American Association for Cancer Research.
Income from Continuing Operations Before Provision/(Benefit) for Taxes on Income
––The following provides an analysis of the changedecrease in Income from continuing operations before provision/(benefit) for taxes on income for 2020:
| | | | | | | | |
(MILLIONS OF DOLLARS) | | |
Income from continuing operations before provision/(benefit) for taxes on income for the year ended December 31, 2019
| | $ | 11,485 | |
Favorable changeof $33.7 billion, to $1.1 billion in 2023 from $34.7 billion in 2022, was primarily attributable to (i) lower revenues, (ii) higher intangible asset impairment charges, and (iii) increases in revenues | | 736 | |
| | |
Favorable/(Unfavorable) changes: | | |
Non-recurrence of (Gain) on completion of Consumer Healthcare JV transaction
| | (8,080) | |
Higher Cost of sales(a)
| | (441) | |
Lower Selling, information and administrative expenses(a)
| | 1,136 | |
Higher Research and development expenses(a)
| | (1,010) | |
Lower Amortization of intangible assets(a)
| | 1,026 | |
| | |
Lower asset impairment charges(b)
| | 1,152 | |
Higher net periodic benefit credits other than service costs(b)
| | 308 | |
Lower business and legal entity alignment costs(b)
| | 300 | |
Higher Consumer Healthcare JV equity method income(b)
| | 281 | |
Lower charges for certain legal matters(b)
| | 264 | |
Higher income from collaborations, out-licensing arrangements and sales of compound/product rights(b)
| | 158 | |
Lower charges to separate our Consumer Healthcare business into a separate legal entity(b)
| | 152 | |
Lower interest expense(b)
| | 125 | |
Higher royalty-related income(b)
| | 124 | |
Lower net losses on early retirement of debt(b)
| | 101 | |
Higher net gains recognized during the period on equity securities(b)
| | 86 | |
Higher ViiV dividend income(b)
| | 58 | |
Higher net losses on asset disposals(b)
| | (268) | |
Lower interest income(b)
| | (153) | |
All other items, net | | (44) | |
Income from continuing operations before provision/(benefit) for taxes on income for the year ended December 31, 2020
| | $ | 7,497 | |
(a)See the Restructuring charges and certain acquisition-related costsCosts, Amortization of intangible assets, and Expenses section within MD&ASelling, informational and administrative expenses, partially offset by (iv) a decrease in Cost of sales and (v) net gains on equity securities in 2023 versus net losses on equity securities in 2022.
Our Operating Environment
Regulatory Environment––Pipeline Productivity
Our product lines must be replenished over time to offset revenue losses when products lose theirexclusivity or market exclusivity,share or to respond to healthcare and innovation trends, andas well as to provide for earnings growth.growth, primarily through internal R&D or through collaborations, acquisitions, JVs, licensing or other arrangements. As a result, we devote considerable resources to our R&D activities which, while essential to our growth, incorporate a high degree of risk and cost, including whether a particular product candidate or new indication for an in-line product will achieve the desired clinical endpoint or safety profile, will be approved by regulators or will be successful commercially. We conduct clinicalClinical trials are conducted to
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 25 |
provide data on safety determine, among other things, whether an investigational drug, vaccine or device is safe and efficacy to support the evaluation of a drug’s overall benefit-risk profileeffective for a particular patient population. In addition, afterAfter a product has been approved or authorized and launched, we continue to monitor its safety as long as it is available to patients. This includespatients, including conducting postmarketing trials, that may be conducted voluntarily or pursuant to a regulatory request to gain additional medical knowledge.request. For the entire life of the product, we collect safety data and report safety information to the FDA and other regulatory authorities.regulators. Regulatory authorities may evaluate potential safety concerns and take any regulatory actions in response, such asaction deemed necessary and appropriate. Such action(s) may include: updating a product’s labeling, restricting its use, communicating new safety information to the public, or, in rare cases, requiring usseeking to suspend or remove a product from the market. The commercial potential of in-line products may be negatively impacted by post-marketing developments.
Intellectual Property Rights and Collaboration/Licensing Rights
The loss, expiration or invalidation of intellectual property rights, patent litigation settlements with manufacturersand judgments, and the expiration of co-promotion and licensing rights can have a material adverse effect on our revenues. Certain of our products have experienced patent-based expirations or loss of regulatory exclusivity in certain markets in the last few years, and we expect certain products to face significantly increased generic competition over the next few years. For example, the basic product patent for Chantix in the U.S. expired in November 2020. Also, the basic product patent for Sutent in the U.S. will expire in August 2021. While additional patent expiries will continue, we expect a moderate impact of reduced revenues due to patent expiries from 20212024 through 2025. We anticipate a more significant impact of reduced revenues from patent expiries in 2026 through 2030 as several of our in-line products experience patent-based expirations. We continue to vigorously defend our patent rights against infringement, and we will continue to support efforts that strengthen worldwide recognition of patent rights while taking necessary steps to help ensure appropriate patient access.
section. For a discussion of recent developments with respect to patent litigation, see Note 16A1.16A1. Regulatory Environment/Pricing and Access––U.S. Healthcare Legislation
| | | | | | | | | | | | | | | | | | | | |
| In March 2010, the ACA was enacted in the U.S. We recorded the following amounts to reflect the impact of the ACA legislation: |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 |
Reduction to Revenues, related to the Medicare “coverage gap” discount provision | | $ | 1,175 | | | $ | 761 | | | $ | 418 | |
Selling, informational and administrative expenses, related to the fee payable to the federal government | | 195 | | | 210 | | | 134 | |
Regulatory Environment/Pricing and Access––Government and Other PayerPayor Group Pressures
The pricing of medicines and vaccines by pharmaceutical manufacturers and the cost of healthcare, which includes medicines, vaccines, medical services and hospital services, continues to be important to payers,payors, governments, patients, and other stakeholders. Federal and state governments and private third-party payerspayors in the U.S. continue to take action to manage the utilization of drugs and cost of drugs, including increasingly employing formularies to control costs by taking into account discounts in connection with decisions about formulary inclusionand encourage utilization of certain drugs, including through the use of deductibles, utilization management tools, cost sharing or favorable formulary placement. We consider a number of factors impacting the pricing of our medicines.medicines and vaccines. Within the U.S., we often engage with patients, doctors and healthcare plans. We also often provide significant discounts from the list price to insurers, including PBMs and MCOs. The price that patients pay in the U.S. for prescribed medicines and vaccines is ultimately set by healthcare providers and insurers. On average, insurers impose a higher out-of-pocket burden on patients for prescription medicines than for comparably priced medical services. Certain governments outsideGovernments globally, as well as private third-party payors in the U.S. provide healthcare at low-to-zero direct cost to consumers at the point of care and have significant power as large single payers to effectively regulate prices or patient reimbursement levels to control costs for the government-sponsored healthcare system. Governments, may use a variety of measures to control costs, including, proposingamong others, legislative or regulatory pricing reform or legislation,reforms, drug formularies (including tiering and utilization management tools), cross country collaboration and procurement, price cuts, mandatory rebates, health technology assessments, forced localization as a condition of market access, “international reference pricing” (i.e., the practice of a country linking its regulated medicine prices to those of other countries), QCE processes and VBP. For additional information,We anticipate that these and similar initiatives will continue to increase pricing and access pressures globally. In the U.S., we expect to see continued focus by Congress and the Biden Administration on regulating pricing. The drug pricing provisions of the IRA, which was signed into law in August 2022, began to be implemented in 2022 and implementation efforts will continue over the next several years. In August 2023, the Biden Administration unveiled the first ten medicines subject to the “Medicare Drug Price Negotiation Program,” which requires manufacturers of select drugs to engage in a process with the federal government to set new Medicare prices which would go into effect in 2026. Among the first ten medicines subject to the Program included Eliquis. We continue to evaluate the impact of the IRA on our business, operations and financial condition and results as the full effect of the IRA on our business and the pharmaceutical industry remains uncertain. In addition, changes to the Medicaid Drug Rebate program or the 340B Program, including legal or legislative developments at the federal or state level with respect to the 340B program, could have a material impact on our business. See the Item 1. Business––Pricing Pressures and Managed Care Organizationsand ––Government Regulation and Price Constraints sectionand the Item 1A. Risk Factors––Pricing and Reimbursementsections. Impact of the July 2023 Tornado in Rocky Mount, North Carolina (NC)––Our manufacturing facility in Rocky Mount, NC was damaged by a tornado in July 2023. The facility is a key producer of sterile injectables and is responsible for manufacturing nearly 25 percent of all our sterile injectables—including anesthesia, analgesia, and micronutrients—which is nearly eight percent of all the sterile injectables used in U.S. hospitals. While manufacturing has resumed, the supply of medicines impacted by the tornado is expected to be affected through 2024.
In 2023, we recorded $286 million to Cost of sales for inventory losses, overhead costs related to the period in which the facility could not operate, and incremental costs resulting from the tornado damage. Losses incurred in 2023 were partially offset by insurance recoveries received in the fourth quarter of 2023. We may record additional losses and/or costs and/or insurance recoveries in future periods, but we are unable to predict them with certainty at this time.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 32 |
Product Supply––We periodically encounter supply delays, disruptions and shortages, including due to voluntary product recalls and natural or man-made disasters. In 2021, Pfizer recalled all lots of Chantix in the U.S. due to the presence of a nitrosamine, N-nitroso-varenicline, at or above the FDA interim acceptable intake limit. Regulatory authorities outside the U.S. have issued updated guidance on nitrosamine acceptable intake levels. With this recently issued guidance, which included an updated intake level for N-nitroso-varenicline, we expect to make regulatory submissions in 2024 to potentially enable Chantix to return to market outside the U.S., and our related discussions with FDA are ongoing.
Except for the tornado in Rocky Mount, NC discussed above, we have not seen a significant disruption of our supply chain in 2023 and through the date of filing of this Form 10-K.10-K, and all of our manufacturing sites globally have continued to operate at or near normal levels; however, we continue to see heightened demand in the industry for certain components and raw materials, which could potentially result in constraining available supply leading to a possible future impact on our business. We continue to monitor and implement mitigation strategies in an effort to reduce any potential risk or impact including active supplier management, qualification of additional suppliers and advanced purchasing to the extent possible. For information on risks related to product manufacturing, see the Item 1A. Risk Factors––Product Manufacturing, Sales and Marketing Risks section. The Global Economic Environment
––In addition to the industry-specific factors discussed above, we, like other businesses of our size and global extent of activities, are exposed to the economic cycle.cycles. Certain factors in the global economic environment that may impact our global operations include, among other things, currency and interest rate fluctuations, capital and exchange controls, local and global economic conditions including inflation, recession, volatility and/or lack of liquidity in capital markets, expropriation and other restrictive government actions, changes in intellectual property, legal protections and remedies, trade regulations, tax laws and regulations and procedures and actions affecting approval, production, pricing, and marketing of, reimbursement for and access to our products, as well as impacts of political or civil unrest or military action, including the ongoing conflicts between Russia and Ukraine and in the Middle East and their economic consequences, geopolitical instability, terrorist activity, unstable governments and legal systems, inter-governmental disputes, and public health outbreaks, epidemics, and pandemics.pandemics, natural disasters or disruptions related to climate change. Government pressures can lead to negative pricing pressure in various markets where governments take an active role in setting prices, access criteria or other means of cost control.
COVID-19 Pandemic
The continuation of the COVID-19 pandemic has impacted our business, operations and financial condition and results. For additional information on the impact of COVID-19 onrisks related to our revenues, pleaseglobal operations, see theOverview of Our Performance, Operating Environment, Strategy and Outlook–Our 2020 Performance section of this MD&A.Item 1A. Risk Factors—Global Operationssection.
Our Response––In response to COVID-19,
We are committed to confronting the public health challenge posed by the pandemic by collaborating with industry partners we developed Paxlovid and academic institutions to develop potential approaches to prevent and treat COVID-19. In March 2020, we issued a five-point plan calling on the biopharmaceutical industry to join us in committing to unprecedented collaboration to combat COVID-19. Subsequently, we have made some important advances, including, among others:
•Entry into a global agreement (except for China, Hong Kong, Macau and Taiwan)collaborated with BioNTech to jointly develop Comirnaty, including an Omicron XBB.1.5-adapted monovalent vaccine. As part of our strategy for the development, manufacture and commercialization of an mRNA-based coronavirus vaccine, BNT162, to help prevent COVID-19. In November 2020, the companies announced that after conducting the final efficacy analysis in the Phase 3 study, BNT162b2 met both of the study’s primary efficacy endpoints.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 26 |
Analysis of the data indicated a vaccine efficacy rate against COVID-19, of 95% in participants without prior SARS-CoV-2 infection (first primary objective) and also in participants with and without prior SARS-CoV-2 infection (second primary objective), in each case measured from seven days after the second dose. The FDA authorized the distribution and use of BNT162b2 in the U.S. to help prevent COVID-19 for individuals 16 years of age and older under an EUA issued in December 2020. BNT162b2 has not been approved or licensed by the FDA. The EUA authorizes distribution and use of this product subject to the conditions set forth in the EUA, and only for the duration of the declaration by the Department of Health & Human Services that circumstances exist justifying authorization of emergency use of drugs and biological products (such as BNT162b2) during the COVID-19 pandemic under Section 564 of the FFDCA (the Declaration), or until revocation of the EUA by the FDA. The FDA has issued EUAs to certain other companies for products intended for the prevention or treatment of COVID-19 and may continue to do so during the duration of the Declaration. The FDA expects EUA holders to work towards submission of a BLA as soon as possible. BNT162b2 has now been granted a CMA, EUA or temporary authorization in more than 50 countries worldwide. The companies continue to study BNT162b2, including studies evaluating it in additional populations, booster doses and emerging variants. Based on the updated 6-dose labeling and subject to continuous process improvements, expansion at current facilities and adding new suppliers and contract manufacturers, the companies believe that they can potentially manufacture at least 2 billion doses in total by the end of 2021. The companies have entered into agreements to supply pre-specified doses of BNT162b2 with multiple developed and emerging nations around the world andwe are continuing to deliver dosesmake significant investments in breakthrough science and global manufacturing. This includes continuing to evaluate Comirnaty and Paxlovid, including against new variants of BNT162b2concern, developing variant adapted vaccine candidates and developing potential combination respiratory vaccines and potential next generation vaccines and therapies. We are also evaluating Paxlovid for additional populations. See the Product Developments section within MD&A.In 2023, we principally sold Comirnaty globally under government contracts. In September 2023, Comirnaty transitioned to governmentstraditional commercial market sales in the U.S., triggered by the expiration of current contracts and the COVID-19 vaccines from Pfizer and BioNTech purchased through them becoming either depleted or not used following the introduction of a new variant vaccine. Internationally, sales of Comirnaty in international developed markets were generally under such agreements. Asgovernment contracts in 2023, and in emerging markets, under a combination of February 2, 2021, based onprivate channels and government contracts; in both cases, we expect to start transitioning to commercial markets in 2024. Due to the dosescommercial market transition as well as the anticipated seasonal nature of COVID vaccination, we expect more than 80% of our 2024 global revenues for Comirnaty to be deliveredrecorded in 2021 primarily under agreements entered into asthe second half of February 2, 2021 (including, among others, agreementsthe year.
In 2023, we principally sold Paxlovid globally to government agencies. Internationally, for Paxlovid, we are continuing the transition to commercial markets and are expecting most revenue for Paxlovid to be generated through commercial channels in 2024. On October 13, 2023, we announced an amended agreement with the U.S. government, which facilitated the transition of Paxlovid to supply 200 million doses, the European Commission to supply 300 million doses, the Japanese government to supply 144 million doses and COVID-19 Vaccines Global Access (COVAX) for up to 40 million dosestraditional commercial markets in 2021, subject to the negotiation and executionNovember 2023, with minimal uptake of additional agreements under the COVAX Facility structure), we forecasted approximately $15 billion in revenues in 2021 from BNT162b2, with gross margin to be split evenly with BioNTech. This forecast was based on doses mostly covered under agreements entered into as of February 2, 2021 and did not include all of the doses we can potentially deliver by the end of 2021. The companies continue to enter into agreements with governments for additional doses, including, among others, the exercise by the U.S. government of an option for an additional 100 million doses and an agreement with the European Commission for an additional 200 million doses to be delivered in 2021. Accordingly, this forecast may change based, in part, on these and future additional agreements that may be signed and as circumstances warrant. NDA-labeled commercial product before January 1, 2024. See Note 17C. For additional information on risks associated with our COVID-19 vaccine development program, see Note 2 and the Item 1A. Risk Factors—COVID-19 Pandemic section in this Form 10-K.
•Initiation, in September 2020, of a Phase 1b clinical trial in hospitalized participants with COVID-19 to evaluate the safety, tolerability and pharmacokinetics of a novel investigational protease inhibitor for COVID-19, PF-07304814, which is a phosphate prodrug of a 3C-like (3CL) protease inhibitor, PF-00835231.
Despite our significant investments and efforts, any of our ongoing development programs related to COVID-19 may not be successful as the risk of failure is significant, and there can be no certainty these efforts will yield a successful product or that costs will ultimately be recouped.
Impact of COVID-19 on Our Business and Operations
The following discussion summarizes our current views of key business and operational areas impacted by the pandemic and its effects on our business, operations, and financial condition and results. As part of our on-going monitoring and assessment, we have madeproducts, including certain assumptions
regarding the COVID-19 pandemicmade for purposes of our operational planning and financial projections
including assumptions regarding the duration, severity and the
global macroeconomic impactuncertainty of
the pandemic,future developments, as well as COVID-19
vaccine supply intellectual property disputes, see theItem 1A. Risk Factors—COVID-19, —Intellectual Property Protectionand contracts, which remain dynamic. Despite careful tracking––Third-Party Intellectual Property Claims sections and planning, we are unable to accurately predict the extent of the impact of the pandemic on our business, operations and financial condition and results due to the uncertainty of future developments. In particular, we believe the ultimate impact on our business, operations and financial condition and results will be affected by the speed and extent of the continued spread of the coronavirus globally; the emergence of additional virus variants; the duration of the pandemic; new information regarding the severity and incidence of COVID-19; the safety, efficacy and availability of vaccines and treatments for COVID-19; the rate at which the population becomes vaccinated against COVID-19; the global macroeconomic impact of the pandemic and governmental or regulatory actions to contain the virus or control supply of medicines. We are focused on all aspects of our business and are implementing measures aimed at mitigating issues where possible, including by using digital technology to assist in operations for our commercial, manufacturing, R&D and enabling functions globally.Note 16A1.
––Our business andlocal operations have been impacted by the pandemicarmed conflict between Israel and Hamas that began on October 7, 2023. For the years ended December 31, 2023 and 2022, the business of our Israeli subsidiary represented less than 1% of our consolidated revenues and assets. We are closely monitoring developments in various ways. For example:
•this conflict, including evaluating potential impacts to our business, customers, suppliers, employees, and operations in Israel and elsewhere in the Middle East that may impact global operations. At this time, mostlonger term impacts to the Company are uncertain and subject to change.
Russia/Ukraine Conflict––Our local operations have been impacted by the armed conflict between Russia and Ukraine. For the years ended December 31, 2023 and 2022, the business of our colleagues who are able to perform their job functions outsideRussia and Ukraine subsidiaries represented less than 1% of our facilities continueconsolidated revenues and assets, and while we are monitoring the effects of the conflict between Russia and Ukraine, the situation continues to work remotely, while certain colleagues inevolve and the PGS and WRDM organizations continuelong-term implications, including the broader economic consequences of the conflict, are difficult to work onsite and are subject to strict protocols intended to reduce the riskpredict at this time. While as of transmission.
•While engagement with healthcare professionals has started to return to pre-pandemic levels due to our virtual engagement capabilities, our sales force colleagues continue to encounter mixed access as a result of ongoing restrictions on in-person meetings. We are actively reviewing and assessing epidemiological data and our colleagues remain ready to resume in-person engagements with healthcare professionals on a location-by-location basis as soon as it is safe to do so. During the pandemic,now, we have adapted our promotional platform by amplifying our existing digital capabilities to reach healthcare professionals and customers to provide critical education and information, including increasing the scale of our remote engagement.
•We have not seen a significant disruption to our supply chain to date, and all of our manufacturing sites globally have continued to operate at or near normal levels.
•After a brief pause to the recruitment portion of certain ongoing clinical studies and a delay to most new study starts, we restarted recruitment across the development portfolio (including new study starts) in late-April 2020.
•Our portfolio of products experienced varying impacts from the pandemic. Some of our products are medically necessary but also more reliant on maintenance therapy with continuing patients in addition to new patients, some of our products are more reliant on new patient starts and typically require doctor visits, including wellness visits, and some of our products are identified as medically necessary for treatment in the pandemic. A large proportion of our portfolio comprises oral or self-injected medicines that do not require a visit to an infusion center or a physician’s office for administration, but vaccines and physician-administered medicines, which do require office visits, were impacted in 2020 by COVID-19-related mobility restrictions or limitations and decline in patient visits to doctors. In addition, certain of our vaccines such as Prevnar 13/Prevenar 13 may be impacted by recommendations by certain health officials to not co-administer such vaccines alongside the COVID-19 vaccines. For additional detail on the impact of the COVID-19 pandemic on our products, see the Analysis of the Consolidated Statements of Income—Revenues—Selected Product Discussion section within MD&A.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 27 |
Notwithstanding the foregoing impact of the pandemic, given ouranticipate any significant operating cash flows, as well as our financial assets, access to capital markets and revolving credit agreements, we believe we have, and expect to maintain, the ability to meet liquidity needs for the foreseeable future. We will continue to pursue efforts to maintain the continuity of our operations while monitoring for new developments related to the pandemic. Future developments could result in additional favorable or unfavorablenegative impacts on our business,global operations from this conflict, continued regional instability, geopolitical shifts, potential additional sanctions and other restrictive measures against Russia, neighboring countries or allies of Russia, any retaliatory measures taken by Russia, neighboring countries or allies of Russia, and actions by our customers or suppliers, including financial conditioninstitutions, in response to such measures could adversely affect the global macroeconomic environment, our operations, currency exchange rates and results. If we experience significant disruptionfinancial markets, which could in our manufacturing or supply chains or significant disruptions in clinical trials or other operations, or if demand for our products is significantly reduced as a result of the COVID-19 pandemic, we could experience a material adverseturn adversely impact on our business operations and financial condition and results. See the Item 1A. Risk Factors—COVID-19 Pandemic sectionresults of this Form 10-K.operations.
SIGNIFICANT ACCOUNTING POLICIES AND APPLICATION OF CRITICAL ACCOUNTING ESTIMATES AND ASSUMPTIONS
Following is a discussion about the critical accounting estimates and assumptions impacting our consolidated financial statements. Also, see Note 1C.
For a description of our significant accounting policies, see Note 1. Of these policies, the following are considered critical to an understanding of our consolidated financial statements as they require the application of the most subjective and the most complex judgments: Acquisitions (Note 1D); Fair Value (Note 1E); Revenues (Note 1G); Asset Impairments (Note 1L1M); Tax Assets and Liabilities and Income Tax Contingencies (Note 1P1Q); Pension and Postretirement Benefit Plans (Note 1Q1R); and Legal and Environmental Contingencies (Note 1R1S). Acquisitions and Fair ValueFor a discussion of recently adopted accounting standards, see Note 1B.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 33 |
Acquisitions
We account for acquired businesses using the acquisition method of accounting, which requires, among other things, that most assets acquired and liabilities assumed be recognized at their estimated fair value as of the acquisition date. To estimate fair value, we utilize an exit price approach from the perspective of a market participant. For discussions aboutfurther detail on acquisition accounting, see Note 1D. For further detail on the application oftechniques and methodologies that we use to estimate fair value, see the following: recent acquisitions (Note 2A1E); investments (Note 7A);. benefit planHistorically, intangible assets (Note 11D);have been the most significant fair values within our business combinations. We utilize an income approach to estimate the acquisition date fair value of each identifiable intangible asset. Some of the more significant estimates and assumptions inherent in this approach include the amount and timing of projected net cash flows, the discount rate, the tax rate, and, for IPR&D assets, the probability of technical and regulatory success (PTRS). All of these judgments and estimates can materially impact our results of operations. For further information on our process to estimate the fair value of intangible assets, see Asset Impairments below. We estimate the fair value of acquired inventory, including finished goods and work in process, by determining the estimated selling price when completed, less an estimate of costs to be incurred to complete and sell the inventory, and an estimate of a reasonable profit allowance for those manufacturing and selling efforts. The fair value of inventory is recognized in our results of operations as the inventory is sold. Some of the more significant estimates and assumptions inherent in the estimate of the fair value of inventory include stage of completion, costs to complete, costs to dispose and selling price.
We estimate the fair value of acquired PP&E using a combination of the cost and market approaches. Some of the more significant estimates and assumptions inherent in these approaches are the values of asset replacement costs, comparable assets and estimated remaining economic lives of the assets.
For the provisional amounts recognized for the Seagen assets acquired and liabilities assumed as of the acquisition date, see Note 2A. The estimated values are not yet finalized and are subject to change, which could be significant. We will finalize the amounts recognized as we obtain the information necessary to complete the analyses. We expect to finalize the amounts of assets acquired and liabilities assumed as soon as possible but no later than one year from the acquisition date. Revenues
Our gross product revenues are subject to a variety of deductions, which generally are estimated and recorded in the same period that the revenues are recognized. Such variable consideration represents chargebacks, rebates, sales allowances and sales returns. These deductions represent estimates of the related obligations and, as such, knowledge and judgment are required when estimating the impact of these revenue deductions on gross sales for a reporting period.
Historically, adjustments to these estimates to reflect actual results or updated expectations, have not been material to our overall business and generally have been less than 1% of revenues. Product-specific rebates, however, can have a significant impact on year-over-year individual product revenue growth trends. If any of our ratios, factors, assessments, experiences or judgments are not indicative or accurate estimates of our future experience, our results could be materially affected. The potential of our estimates to vary (sensitivity) differs by program, product, type of customer and geographic location. However, estimates associated with U.S. Medicare, Medicaid and performance-based contract rebates are most at risk for material adjustment because of the extensive time delay between the recording of the accrual and its ultimate settlement, an interval that can generally range up to one year. Because of this lag, our recording of adjustments to reflect actual amounts can incorporate revisions of several prior quarters.
Rebate accruals are product specific and, therefore for any period, are impacted by the mix of products sold as well as the forecasted channel mix for each individual product. For further information, see the
Asset Impairments
We review all of our long-lived assets for impairment indicators throughout the year. We perform impairment testing for indefinite-lived intangible assets and goodwill at least annually and for all other long-lived assets whenever impairment indicators are present. When necessary, we record charges for impairments of long-lived assets for the amount by which the fair value is less than the carrying value of these assets. Our impairment review processes are described in Note 1L.1M. Examples of events or circumstances that may be indicative of impairment include:
•A significant adverse change in legal factors or in the business climate that could affect the value of the asset. For example, a successful challenge of our patent rights would likely result in generic competition earlier than expected.
•A significant adverse change in the extent or manner in which an asset is used such as a restriction imposed by the FDA or other regulatory authorities that could affect our ability to manufacture or sell a product.
•An expectation of losses or reduced profits associated with an asset. This could result, for example, from a change in a government reimbursement program that results in an inability to sustain projected product revenues and profitability. This also could result from the introduction of a competitor’s product that impacts projected revenue growth, as well as the lack of acceptance of a product by patients, physicians and payers.payors. For IPR&D projects, this could result from, among other things, a change in outlook based on clinical trial data, a delay in the projected launch date or additional expenditures to commercialize the product.
Identifiable Intangible Assets
––We use an income approach, specifically the discounted cash flow method to determine the fair value of intangible assets, other than goodwill. We start with a forecast of all the expected net cash flows associated with the asset, which incorporates the consideration of a terminal value for indefinite-lived assets, and then we apply an asset-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions that impact our fair value estimates include: the amount and timing of the projected net cash flows, which includes the expected impact of competitive, legal and/or regulatory forces on the projections and the impact of technological advancements and risk associated with IPR&D assets, as well as the selection of a long-term growth rate; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic originjurisdictional mix of the projected cash flows.
While all intangible assets other than goodwill can face events and circumstances that can lead to impairment, those that are most at risk of impairment include IPR&D assets (approximately $3.2$23.2 billion as of December 31, 2020)2023) and newly acquired or recently impaired indefinite-lived brand assets. IPR&D assets are high-risk assets, given the uncertain nature of R&D. Newly acquired and recently impaired indefinite-lived assets are more vulnerable to impairment as the assets are recorded at fair value and are then subsequently measured at the lower of fair value or
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 28 |
carrying value at the end of each reporting period. As such, immediately after acquisition or impairment, even small declines in the outlook for these assets can negatively impact our ability to recover the carrying value and can result in an impairment charge.
Goodwill
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 34 |
Goodwill––Our goodwill impairment review work as of December 31, 20202023 concluded that none of our goodwill was impaired and we do not believe the risk of impairment is significant at this time.
In our review, we first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. Qualitative factors that we consider include, for example, macroeconomic and industry conditions, overall financial performance and other relevant entity-specific events. If we conclude that it is more likely than not that the fair value of a reporting unit is less than its carrying value, we then perform a quantitative fair value test.
When we are required to determine the fair value of a reporting unit, we mainlytypically use the income approach but may also use the market approach, or a weighted-average combination of both approaches.
•approach. The income approach is a forward-looking approach to estimating fair value and relies primarily on internal forecasts. Within the income approach, we use the discounted cash flow method. We start with a forecast of all the expected net cash flows for the reporting unit, which includes the application of a terminal value, and then we apply a reporting unit-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of technological risk and competitive, legal and/or regulatory forces on the projections, as well as the selection of a long-term growth rate; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
•The market approach is a historical approach to estimating fair value and relies primarily on external information. We may use two alternative methods within the market approach:
◦Guideline public company method—this method employs market multiples derived from market prices of stocks of companies that are engaged in the same or similar lines of business and that are actively traded on a free and open market and the application of the identified multiples to the corresponding measure of our reporting unit’s financial performance.
◦Guideline transaction method—this method relies on pricing multiples derived from transactions of significant interests in companies engaged in the same or similar lines of business and the application of the identified multiples to the corresponding measure of our reporting unit’s financial performance.
The market approach is only appropriate when the available external information is robust and deemed to be a reliable proxy for the specific reporting unit being valued; however, these assessments may prove to be incomplete or inaccurate. Some of the more significant estimates and assumptions inherent in this approach include: the selection of appropriate guideline companies and transactions and the determination of applicable premiums and discounts based on any differences in ownership percentages, ownership rights, business ownership forms or marketability between the reporting unit and the guideline companies and transactions.
Benefit Plans
For a description of our different benefit plans, see Note 11.
Effective January 1, 2018, accruals for future benefits under the PCPP (our largest U.S. defined benefit plan) and the defined benefit section of the Pfizer Group Pension Scheme (our largest pension plan in the U.K.) were frozen and resulted in elimination of future service costs for the plans. The Pfizer defined contribution savings plan provides additional annual contributions to those previously accruing benefits under the PCPP and active members of the Pfizer Group Pension Scheme started accruing benefits under the defined contribution section of that plan.
Our assumptions reflect our historical experiences and our judgment regarding future expectations that have been deemed reasonable by management. The judgments made in determining the costs of our benefit plans can materially impact our results of operations.
| The following provides (i) at the end of each year, the expected annual rate of return on plan assets for the following year, (ii) the actual annual rate of return on plan assets achieved in each year, and (iii) the weighted-average discount rate used to measure the benefit obligations at the end of each year for our U.S. qualified pension plans and our international pension plans(a): | | 2020 | | 2019 | | 2018 |
| U.S. Qualified Pension Plans | | | | | | |
The following provides (i) at the end of each year, the expected annual rate of return on plan assets for the following year, (ii) the actual annual rate of return on plan assets achieved in each year, and (iii) the weighted-average discount rate used to measure the benefit obligations at the end of each year for our U.S. pension plans and our international pension plans(a): | | The following provides (i) at the end of each year, the expected annual rate of return on plan assets for the following year, (ii) the actual annual rate of return on plan assets achieved in each year, and (iii) the weighted-average discount rate used to measure the benefit obligations at the end of each year for our U.S. pension plans and our international pension plans(a): |
| | 2023 | | | | 2023 | | 2022 | | 2021 |
| U.S. Pension Plans | |
| Expected annual rate of return on plan assets | |
| Expected annual rate of return on plan assets | |
| Expected annual rate of return on plan assets | Expected annual rate of return on plan assets | | 6.8 | % | | 7.0 | % | | 7.2 | % | | 8.0 | % | | 7.5 | % | | 6.3 | % |
| Actual annual rate of return on plan assets | Actual annual rate of return on plan assets | | 14.1 | | | 22.6 | | | (5.3) | |
| Discount rate used to measure the plan obligations | Discount rate used to measure the plan obligations | | 2.6 | | | 3.3 | | | 4.4 | |
| International Pension Plans | International Pension Plans | | | |
| Expected annual rate of return on plan assets | Expected annual rate of return on plan assets | | 3.4 | | | 3.6 | | | 3.9 | |
| Expected annual rate of return on plan assets | |
| Expected annual rate of return on plan assets | |
| Actual annual rate of return on plan assets | Actual annual rate of return on plan assets | | 9.7 | | | 10.7 | | | (0.9) | |
| Discount rate used to measure the plan obligations | Discount rate used to measure the plan obligations | | 1.5 | | | 1.7 | | | 2.5 | |
(a)For detailed assumptions associated with our benefit plans, see Note 11B.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 29 |
Expected Annual Rate of Return on Plan Assets
––The assumptions for the expected annual rate of return on all of our plan assets reflect our actual historical return experience and our long-term assessment of forward-looking return expectations by asset classes, which is used to develop a weighted-average expected return based on the implementation of our targeted asset allocation in our respective plans.
The expected annual rate of return on plan assets for our U.S. plans and the majority of our international plans is applied to the fair value of plan assets at each year-end and the resulting amount is reflected in our net periodic benefit costs in the following year. Differences between the actual rate of return on plan assets and the expected annual rate of return on plan assets are immediately recognized through earnings upon remeasurement.
| | | | | | | | | | | | | | |
| The following illustrates the sensitivity of net periodic benefit costs to a 50 basis point decline in our assumption for the expected annual rate of return on plan assets, holding all other assumptions constant (in millions, pre-tax): |
| Assumption | | Change | | Increase in 2021 2024 Net Periodic Benefit Costs |
Expected annual rate of return on plan assets(a) | | 50 basis point decline | | $11684 |
(a)The estimate excludes any potential mark-to-market adjustments.
The actual return on plan assets wasresulted in a net gain on our plan assets of approximately $2.9 billion$835 million during 20202023.
Discount Rate Used to Measure Plan Obligations
––The weighted-average discount rate used to measure the plan obligations for our U.S. defined benefit plans is determined at least annually and evaluated and modified, as required, to reflect the prevailing market rate of a portfolio of high-quality fixed income investments, rated AA/Aa or better, that reflect the rates at which the pension benefits could be effectively settled. The discount rate used to measure the plan obligations for our significant international plans is determined at least annually by reference to investment grade corporate bonds, rated AA/Aa or better, including, when there is sufficient data, a yield-curve approach. These discount rate determinations are made in consideration of local requirements.
The measurement of the plan obligations at the end of the year will affect (i) the actuarial (gains)/losses recognized in our net periodic benefit cost for that year and (ii) the amount of service cost and interest cost and amortization expense reflected in our net periodic benefit costs in the following year.
| | | | | | | | | | | | | | | | | | | | |
| The following illustrates the sensitivity of net periodic benefit costs and benefit obligations to a 10 basis point decline in our assumption for the discount rate, holding all other assumptions constant (in millions, pre-tax): |
| Assumption | | Change | | Increase in 2021 Net Periodic Benefit Costs | | 2020 Benefit Obligations |
| | | | Increase | | Increase |
| Discount rate | | 10 basis point decline | | $2 | | $483 |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 35 |
| | | | | | | | | | | | | | | | | | | | |
| The following illustrates the sensitivity of net periodic benefit costs and benefit obligations to a 10 basis point decline in our assumption for the discount rate, holding all other assumptions constant (in millions, pre-tax): |
| Assumption | | Change | | Decrease in 2024 Net Periodic Benefit Costs | | Increase to 2023 Benefit Obligations |
| Discount rate | | 10 basis point decline | | $5 | | $210 |
The change in the discount rates used in measuring our plan obligations as of December 31, 20202023 resulted in an increasea decrease in the measurement of our aggregate plan obligations by approximately $1.9 billion.$616 million.
Anticipated Change in Accounting Policy
We anticipate making a change in our pension accounting policy under which we would begin recognizing actuarial gains and losses immediately in the income statement compared to our current accounting policy that recognizes such gains and losses in stockholders’ equity and amortizes them as a component of net periodic benefit cost/(credit) over future periods. This anticipated change is expected to go into effect in the first quarter of 2021 and if adopted, will require recasting prior period amounts to conform to the new accounting policy.
Income Tax Assets and Liabilities
Contingencies
We and certain of our subsidiaries are subject to numerous contingencies arising in the ordinary course of business, including tax and legal contingencies, and guarantees and indemnifications. For additional information, seeSee Notes 1P1Q,1S, 1R,5D and 16.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 30 |
ANALYSIS OF THE CONSOLIDATED STATEMENTS OF INCOME
Total Revenues by Geography
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following presents worldwide revenues by geography: |
| | | Year Ended December 31, | | % Change |
| | | Worldwide | | U.S. | | International | | Worldwide | | U.S. | | International |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 | | 20/19 | | 19/18 | | 20/19 | | 19/18 | | 20/19 | | 19/18 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total revenues | | $ | 41,908 | | | $ | 41,172 | | | $ | 40,825 | | | $ | 21,712 | | | $ | 20,593 | | | $ | 20,119 | | | $ | 20,196 | | | $ | 20,579 | | | $ | 20,705 | | | 2 | | | 1 | | | 5 | | | 2 | | | (2) | | | (1) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The following presents worldwide Total revenues by geography: |
| | | Year Ended December 31, | | % Change |
| | | Worldwide | | U.S. | | International | | Worldwide | | U.S. | | International |
| (MILLIONS) | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 | | 23/22 | | 22/21 | | 23/22 | | 22/21 |
| Operating segments: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Biopharma | | $ | 57,186 | | | $ | 98,988 | | | $ | 79,557 | | | $ | 26,698 | | | $ | 42,083 | | | $ | 29,221 | | | $ | 30,488 | | | $ | 56,905 | | | $ | 50,336 | | | (42) | | | 24 | | | (37) | | | 44 | | | (46) | | | 13 | |
| Business Innovation | | 1,310 | | | 1,342 | | | 1,731 | | | 390 | | | 390 | | | 524 | | | 920 | | | 952 | | | 1,206 | | | (2) | | | (22) | | | — | | (26) | | | (3) | | | (21) | |
| Total revenues | | $ | 58,496 | | | $ | 100,330 | | | $ | 81,288 | | | $ | 27,088 | | | $ | 42,473 | | | $ | 29,746 | | | $ | 31,408 | | | $ | 57,857 | | | $ | 51,542 | | | (42) | | | 23 | | | (36) | | | 43 | | | (46) | | | 12 | |
2020 v. 2019
| | | | | | | | | | | | | | | | | | | | |
| The following provides an analysis of the change in worldwide revenues by geographic areas in 2020: |
| (MILLIONS OF DOLLARS) | | Worldwide | | U.S. | | International |
| Operational growth/(decline): | | | | | | |
Growth from Prevnar 13/Prevenar 13, Ibrance, Eliquis, Xeljanz, Vyndaqel/Vyndamax, Xtandi, Inlyta, Biosimilars and the Hospital therapeutic area, partially offset by Chantix/Champix. See the Analysis of the Consolidated Statements of Income––Revenues––Selected Product Discussion within MD&A for additional analysis | | $ | 3,479 | | | $ | 1,902 | | | $ | 1,577 | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| Impact of completion of the Consumer Healthcare JV transaction. Revenues in 2019 reflect seven months of Consumer Healthcare business domestic operations and eight months of international operations, and none in 2020 | | (2,082) | | | (988) | | | (1,094) | |
| Lower revenues for Enbrel internationally, primarily reflecting continued biosimilar competition in most developed Europe markets, as well as in Japan and Brazil, all of which is expected to continue | | (320) | | | — | | | (320) | |
| | | | | | |
| Other operational factors, net | | (10) | | | 205 | | | (214) | |
| Operational growth/(decline), net | | 1,068 | | | 1,119 | | | (50) | |
| | | | | | |
| Unfavorable impact of foreign exchange | | (331) | | | — | | | (331) | |
Revenues increase/(decrease) | | $ | 736 | | | $ | 1,119 | | | $ | (383) | |
Revenues for 2020 included an estimated unfavorable impact of approximately $700 million, or 2%, due to COVID-19, primarily reflecting lower demand for certain products in China and unfavorable disruptions to wellness visits for patients in the U.S., which negatively impacted prescribing patterns for certain products, partially offset by increased U.S. demand for certain sterile injectable products and increased adult uptake for Prevenar 13 in certain international markets, resulting from greater vaccine awareness for respiratory illnesses, and U.S. revenues for BNT162b2. | | | | | | | | | | | | | | | | | | | | |
The following provides an analysis of the worldwide change in Total revenues by geographic areas from 2022 to 2023: |
| (MILLIONS) | | Worldwide | | U.S. | | International |
| Operational growth/(decline): | | | | | | |
| Worldwide declines from Comirnaty | | $ | (26,423) | | | $ | (6,370) | | | $ | (20,053) | |
| Worldwide declines from Paxlovid | | (17,506) | | | (11,803) | | | (5,703) | |
| Worldwide growth from the Vyndaqel family, Eliquis, the Prevnar family and Inlyta, partially offset by worldwide declines from Ibrance, Xeljanz and Xtandi | | 1,016 | | | 1,018 | | | (2) | |
| Increase in revenues from Nurtec ODT/Vydura and Oxbryta, which were acquired in the fourth quarter of 2022 | | 972 | | | 949 | | | 23 | |
| Revenues from Abrysvo, primarily driven by launch of the older adult indication in the U.S. in July 2023 | | 890 | | | 888 | | | 2 | |
| Revenues from legacy Seagen products subsequent to the acquisition on December 14, 2023 | | 120 | | | 120 | | | — | |
| Other operational factors, net | | 120 | | | (185) | | | 305 | |
| Operational growth/(decline), net | | (40,812) | | | (15,385) | | | (25,428) | |
| | | | | | |
| Unfavorable impact of foreign exchange | | (1,022) | | | — | | | (1,022) | |
Total revenues increase/(decrease) | | $ | (41,834) | | | $ | (15,385) | | | $ | (26,449) | |
Emerging markets revenues decreased $456 million,$8.1 billion, or 5%40%, in 20202023 to $8.4$12.0 billion from $8.8$20.1 billion in 2019,2022, reflecting an operational decrease of $7.4 billion, or 37%, and were relatively flat operationally, reflecting an unfavorable impact offrom foreign exchange of 5% on3%. The operational decrease in emerging markets revenues. The relatively flat operational performancerevenues was primarily driven by declines from Comirnaty and Paxlovid, partially offset by growth from Eliquis, Prevenar 13, Ibrance andLorbrena, Zavicefta offset by lower revenues for Consumer Healthcare, reflecting the July 31, 2019 completion of the Consumer Healthcare JV transaction.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 31 |
2019 v. 2018
| | | | | | | | | | | | | | | | | | | | |
| The following provides an analysis of the change in worldwide revenues by geographic areas in 2019: |
| (MILLIONS OF DOLLARS) | | Worldwide | | U.S. | | International |
| Operational growth/(decline): | | | | | | |
| Growth from Ibrance, Eliquis, Xeljanz and Prevnar/Prevenar 13 | | $ | 2,495 | | | $ | 914 | | | $ | 1,581 | |
Higher revenues for certain Hospital products as a result of: •continued growth of anti-infective products in China, driven by increased demand for Sulperazon and new launches; •the 2018 U.S. launches of our immune globulin IV products (Panzyga and Octagam); and •the launches of certain anti-infectives products (Zavicefta, Zinforo and Cresemba) in international developed and emerging markets | | 472 | | | 174 | | | 298 | |
| Higher revenues for Inlyta, primarily in the U.S. driven by increased demand resulting from the second quarter of 2019 U.S. FDA approvals for the combinations of certain immune checkpoint inhibitors plus Inlyta for the first-line treatment of patients with advanced RCC | | 190 | | | 175 | | | 14 | |
| Higher revenues for Biosimilars, primarily in the U.S. | | 168 | | | 185 | | | (17) | |
Higher revenues for rare disease products driven by: •the U.S. launches in May 2019 of Vyndaqel and in September 2019 of Vyndamax for the treatment of ATTR-CM; •continued uptake for the transthyretin amyloid polyneuropathy indication, primarily in developed Europe; and •the March 2019 launch of the ATTR-CM indication in Japan, partially offset by: •lower revenues for certain rare disease products, including the hemophilia franchises (Refacto AF/Xyntha and BeneFIX), primarily due to competitive pressures, and Genotropin in developed markets, mainly due to unfavorable channel mix in the U.S. | | 159 | | | 108 | | | 51 | |
| Impact of completion of the Consumer Healthcare JV transaction. Revenues in 2019 only reflect seven months of Consumer Healthcare business domestic operations and eight months of international operations | | (1,436) | | | (889) | | | (547) | |
| Lower revenues from other Hospital products, primarily reflecting declines in developed markets, mostly due to the continued expected negative impact from generic competition for products that have previously lost marketing exclusivity | | (447) | | | (200) | | | (247) | |
| Lower revenues for Enbrel, primarily in most developed Europe markets due to continued biosimilar competition | | (292) | | | — | | | (292) | |
| Other operational factors, net | | 141 | | | 6 | | | 136 | |
| Operational growth, net | | 1,450 | | | 473 | | | 976 | |
| | | | | | |
| Unfavorable impact of foreign exchange | | (1,103) | | | — | | | (1,103) | |
| Revenues increase/(decrease) | | $ | 347 | | | $ | 473 | | | $ | (127) | |
Emerging markets revenues increased $210 million, or 2%, in 2019 to $8.8 billion, from $8.6 billion in 2018, reflecting an operational increase of $820 million, or 10%. Foreign exchange had an unfavorable impact of 7% on emerging markets revenues. The operational increase in emerging markets was primarily driven by Prevenar 13, Ibrance and Eliquis.
Product Revenue Deductions
Our gross product revenues are subject to a variety of deductions, which generally are estimated and recorded in the same period that the revenues are recognized. These deductions represent estimates of the related obligations and, as such, knowledge and judgment are required when estimating the impact of these product revenue deductions on gross sales for a reporting period.
Historically, adjustments to these estimates to reflect actual results or updated expectations, have not been material to our overall business and generally have been less than 1% of revenues. Product-specific rebates, however, can have a significant impact on year-over-year individual product revenue growth trends.
| | | | | | | | | | | | | | | | | | | | |
| The following presents information about revenue deductions: |
| | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 |
| Medicare rebates | | $ | 647 | | | $ | 628 | | | $ | 495 | |
| Medicaid and related state program rebates | | 1,136 | | | 1,259 | | | 984 | |
| Performance-based contract rebates | | 2,660 | | | 2,332 | | | 1,758 | |
| Chargebacks | | 4,531 | | | 3,411 | | | 2,954 | |
| Sales allowances | | 3,841 | | | 3,782 | | | 3,536 | |
| Sales returns and cash discounts | | 924 | | | 878 | | | 1,128 | |
| Total | | $ | 13,739 | | | $ | 12,290 | | | $ | 10,854 | |
Revenue | | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 36 |
| | | | | | | | | | | | | | | | | | | | |
| The following presents information about product revenue deductions: |
| | | Year Ended December 31, |
| (MILLIONS) | | 2023 | | 2022 | | 2021 |
| Medicare rebates | | $ | 997 | | | $ | 838 | | | $ | 726 | |
| Medicaid and related state program rebates | | 1,655 | | | 973 | | | 1,214 | |
| Performance-based contract rebates | | 5,159 | | | 3,575 | | | 3,253 | |
| Chargebacks | | 9,828 | | | 7,560 | | | 6,122 | |
| Sales allowances | | 6,790 | | | 5,460 | | | 4,809 | |
Sales returns and cash discounts(a) | | 5,619 | | | 1,290 | | | 1,054 | |
| Total | | $ | 30,048 | | | $ | 19,697 | | | $ | 17,178 | |
(a)The increase in sales returns and cash discounts in 2023 was primarily due to the revenue reversal of $3.5 billion in the fourth quarter of 2023, related to the expected return of an estimated 6.5 million treatment courses of EUA-labeled U.S. government Paxlovid inventory (see Note 17C). Product revenue deductions are primarily a function of product sales volume, mix of products sold, contractual or legislative discounts and rebates.
For information on our accruals for product revenue deductions, including the balance sheet classification of these accruals, see Note 1G.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 3237 |
Total Revenues—Selected Product Discussion
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Revenue | | | | | | |
| (MILLIONS OF DOLLARS) | | | | Year Ended Dec. 31, | | % Change | | |
| Product | | Global
Revenues | | Region | | 2020 | | 2019 | | Total | | Oper. | | Operational Results Commentary |
Prevnar 13/
Prevenar 13 | | $5,850
Up 1%
(operationally) | | U.S. | | $ | 2,930 | | $ | 3,209 | | | (9) | | | | | Operational growth internationally primarily reflects increased adult uptake in certain international markets resulting from greater vaccine awareness for respiratory illnesses, including specifically pneumococcal disease, due to the COVID-19 pandemic, as well as continued strong pediatric uptake in China, partially offset by a decline in the U.S., primarily driven by the expected unfavorable impact of disruptions to wellness visits for pediatric and adult patients due to COVID-19-related mobility restrictions or limitations as well as the continued impact of a lower remaining eligible adult population and the impact of the revised ACIP recommendation for the adult indication to shared clinical decision making, which means the decision to vaccinate should be made at the individual level between health care providers and their patients. |
| Int’l. | | 2,920 | | 2,638 | | | 11 | | | 13 | |
| Worldwide | | $ | 5,850 | | $ | 5,847 | | | — | | 1 | |
| Ibrance | | $5,392
Up 9% (operationally) | | U.S. | | $ | 3,634 | | $ | 3,250 | | | 12 | | | | | Primarily driven by continued strong volume growth in most markets, partially offset by pricing pressures in certain developed Europe markets. |
| Int’l. | | 1,758 | | 1,710 | | | 3 | | | 5 | | |
| Worldwide | | $ | 5,392 | | $ | 4,961 | | | 9 | | | 9 | | |
| Eliquis | | $4,949
Up 18%
(operationally) | | U.S. | | $ | 2,688 | | $ | 2,343 | | | 15 | | | | | Primarily driven by continued increased adoption in non-valvular atrial fibrillation as well as oral anti-coagulant market share gains, partially offset by a lower net price due to an increased impact from the Medicare “coverage gap” and unfavorable channel mix in the U.S. |
| Int’l. | | 2,260 | | 1,877 | | | 20 | | | 22 | | |
| Worldwide | | $ | 4,949 | | $ | 4,220 | | | 17 | | | 18 | | |
| Xeljanz | | $2,437
Up 9%
(operationally) | | U.S. | | $ | 1,706 | | $ | 1,636 | | | 4 | | | | | Higher volumes in the U.S. within the RA, PsA and UC indications driven by reaching additional patients through improvements in formulary access, partially offset by increased discounts from recently-signed contracts which were entered into in order to unlock access to additional patient lives. Also reflects operational growth internationally mainly driven by continued uptake in the RA indication and, to a lesser extent, from the recent launch of the UC indication in certain developed markets. |
| Int’l. | 731 | | 606 | | | 21 | | | 23 | | |
| Worldwide | $ | 2,437 | | $ | 2,242 | | | 9 | | | 9 | | |
Vyndaqel/
Vyndamax | | $1,288
*
| | U.S. | | $ | 613 | | $ | 191 | | | * | | | | Driven by the U.S. launches of Vyndaqel in May 2019 and Vyndamax in September 2019 for the treatment of ATTR-CM and by the March 2019 launch of the ATTR-CM indication in Japan and the February 2020 approval of the ATTR-CM indication in the EU. |
| Int’l. | 675 | | 282 | | * | * |
| Worldwide | $ | 1,288 | | $ | 473 | | * | * |
| Xtandi | | $1,024
Up 22%
(operationally) | | U.S. | | $ | 1,024 | | $ | 838 | | | 22 | | | | | Primarily driven by continued strong demand for Xtandi in the mCRPC and nmCRPC indications, as well as the mCSPC indication, which was approved in the U.S. in December 2019. |
| Int’l. | — | | — | | — | — |
| Worldwide | $ | 1,024 | | $ | 838 | | 22 | | 22 | |
Chantix/
Champix | | $919
Down 17%
(operationally) | | U.S. | | $ | 716 | | $ | 899 | | | (20) | | | | | Driven by the U.S. and primarily reflects expected lower demand resulting from reduced doctor visits, including wellness visits when Chantix is typically prescribed, due to COVID-19-related mobility restrictions or limitations as well as the loss of patent protection in the U.S. in November 2020, partially offset by increased demand in Spain as a result of government reimbursement starting in January 2020. |
| Int’l. | 203 | | 208 | | | (2) | | | (1) | | |
| Worldwide | $ | 919 | | $ | 1,107 | | | (17) | | | (17) | | |
| Inlyta | | $787
Up 66%
(operationally) | | U.S. | | $ | 523 | | $ | 295 | | | 78 | | | | | Primarily due to increased demand in the U.S. and certain developed international markets, following the approvals in 2019 for combinations of certain immune checkpoint inhibitors plus Inlyta for the first-line treatment of patients with advanced RCC. |
| Int’l. | 264 | | 182 | | | 45 | | | 47 | | |
| Worldwide | $ | 787 | | $ | 477 | | | 65 | | | 66 | | |
| Biosimilars | | $1,527
Up 68%
(operationally) | | U.S. | | $ | 899 | | $ | 451 | | | 99 | | | | | Primarily driven by recent oncology biosimilar launches in the U.S. and other global markets and continued growth from Retacrit, primarily in the U.S. |
| Int’l. | 628 | | 460 | | | 36 | | | 37 | | |
| Worldwide | $ | 1,527 | | $ | 911 | | | 68 | | | 68 | | |
| Hospital | | $7,961
Up 3%
(operationally) | | U.S. | | $ | 3,362 | | $ | 3,081 | | | 9 | | | | | Higher revenues in the U.S., primarily driven by increased demand for certain sterile injectable products utilized in the intubation and ongoing treatment of mechanically-ventilated COVID-19 patients, continued growth from Panzyga and recent anti-infective launches, as well as Pfizer CentreOne business in international markets, partially offset by lower demand for certain anti-infective products in China due to lower infection rates driven by fewer elective surgical procedures, shorter in-patient hospital stays and improved infection control. |
| Int’l. | 4,599 | | 4,691 | | | (2) | | | — | |
| Worldwide | $ | 7,961 | | $ | 7,772 | | | 2 | | | 3 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Revenue | | | | | | |
| (MILLIONS) | | | | Year Ended Dec. 31, | | % Change | | |
| Product | | Global
Revenues | | Region | | 2023 | | 2022 | | Total | | Oper. | | Operational Results Commentary |
Comirnaty(a) | | $11,220
Down 70%
(operationally) | | U.S. | | $ | 2,404 | | | $ | 8,775 | | | (73) | | | | | Declines largely driven by lower contracted deliveries and demand in international markets and lower U.S. government contracted deliveries, due to transition to new variant vaccines in most markets and the transition to traditional U.S. commercial market sales which began in September 2023. |
| Int’l. | | 8,816 | | | 29,032 | | | (70) | | | (69) | | |
| Worldwide | | $ | 11,220 | | | $ | 37,806 | | | (70) | | | (70) | | |
| Eliquis | | $6,747
Up 5%
(operationally) | | U.S. | | $ | 4,228 | | | $ | 3,822 | | | 11 | | | | | Growth driven primarily by continued oral anti-coagulant adoption and market share gains in the non-valvular atrial fibrillation indication in the U.S. and certain markets in Europe, partially offset by declines due to LOE and generic competition in certain international markets. |
| Int’l. | | 2,519 | | | 2,658 | | | (5) | | | (3) | | |
| Worldwide | | $ | 6,747 | | | $ | 6,480 | | | 4 | | | 5 | | |
| Prevnar family | | $6,440
Up 3%
(operationally) | | U.S. | | $ | 4,204 | | | $ | 4,032 | | | 4 | | | | | Growth primarily driven by the adult indications in the U.S. due to strong patient demand for Prevnar 20 for the eligible adult population, partially offset by the Prevnar pediatric indication in the U.S. driven by lower market share due to competitor entry. |
| Int’l. | | 2,236 | | | 2,305 | | | (3) | | | — | |
| Worldwide | | $ | 6,440 | | | $ | 6,337 | | | 2 | | | 3 | | |
| Ibrance | | $4,753
Down 6% (operationally) | | U.S. | | $ | 3,151 | | | $ | 3,370 | | | (6) | | | | | Declines primarily driven by lower demand globally due to competitive pressure, lower clinical trial purchases internationally, and planned price decreases in certain international developed markets. |
| Int’l. | | 1,602 | | | 1,751 | | | (8) | | | (6) | | |
| Worldwide | | $ | 4,753 | | | $ | 5,120 | | | (7) | | | (6) | | |
| Vyndaqel family | | $3,321
Up 36%
(operationally) | | U.S. | | $ | 1,863 | | | $ | 1,245 | | | 50 | | | | | Growth largely driven by continued strong uptake of the ATTR-CM indication, primarily in the U.S. and developed Europe, partially offset by a planned price decrease that went into effect in Japan in the second quarter of 2022. |
| Int’l. | 1,458 | | | 1,202 | | 21 | | 22 | |
| Worldwide | $ | 3,321 | | | $ | 2,447 | | 36 | | 36 | |
| Xeljanz | | $1,703
Down 4%
(operationally) | | U.S. | | $ | 1,154 | | | $ | 1,129 | | | 2 | | | | | Decline driven primarily by decreased prescription volumes globally resulting from ongoing shifts in prescribing patterns related to label changes, partially offset by higher net price in the U.S. due to favorable changes in channel mix. |
| Int’l. | | 549 | | | 668 | | | (18) | | | (15) | | |
| Worldwide | | $ | 1,703 | | | $ | 1,796 | | | (5) | | | (4) | | |
| Paxlovid | | $1,279
Down 92%
(operationally) | | U.S. | | $ | (1,289) | | | $ | 10,514 | | | * | | | | Declines primarily driven by: •a non-cash revenue reversal of $3.5 billion recorded in the fourth quarter of 2023, of which a portion was associated with sales recorded in 2022, related to the expected return of an estimated 6.5 million treatment courses of EUA-labeled U.S. government inventory (see Note 17C); and •lower contractual deliveries in most international markets, partially offset by: •strong demand in China under the temporary National Reimbursement Drug List (which ended on April 1, 2023) due to surge in COVID-19 infection during the first quarter of 2023; and •fourth quarter sales under traditional commercial markets following transition, primarily in the U.S. |
| Int’l. | 2,568 | | 8,419 | | (69) | | (68) | |
| Worldwide | $ | 1,279 | | $ | 18,933 | | (93) | | (92) | |
| Xtandi | | $1,191
Down 1%
(operationally) | | U.S. | | $ | 1,191 | | | $ | 1,198 | | | (1) | | | | | Decline driven by lower net price mainly due to unfavorable changes in channel mix, partially offset by higher demand. |
| Int’l. | — | | | — | | — | — |
| Worldwide | $ | 1,191 | | | $ | 1,198 | | (1) | | (1) | |
| Inlyta | | $1,036
Up 5%
(operationally) | | U.S. | | $ | 642 | | | $ | 618 | | | 4 | | | | | Growth primarily reflects continued growth in emerging markets and the U.S. driven by the adoption of combinations of certain immune checkpoint inhibitors and Inlyta for the first-line treatment of patients with advanced RCC, partially offset by lower volumes and lower net price in certain European markets. |
| Int’l. | 394 | | | 385 | | 3 | | 7 | | |
| Worldwide | $ | 1,036 | | | $ | 1,003 | | 3 | | 5 | | |
| Nurtec ODT/Vydura | | $928
* | | U.S. | | $ | 908 | | | $ | 211 | | | * | | | | Growth primarily driven by timing of the acquisition of Biohaven (fourth quarter of 2022) as well as strong patient demand in the U.S. See Note 2A. |
| Int’l. | 20 | | | 2 | | * | * | |
| Worldwide | $ | 928 | | | $ | 213 | | * | * | |
| | | | | | | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 38 |
Business Innovation
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Revenue | | | | | | |
| (MILLIONS) | | | | Year Ended Dec. 31, | | % Change | | |
| Operating Segment | | Global
Revenues | | Region | | 2023 | | 2022 | | Total | | Oper. | | Operational Results Commentary |
| Business Innovation | | $1,310
Down 2%
(operationally) | | U.S. | | $ | 390 | | | $ | 390 | | | — | | | | Decline primarily driven by a reduction in Comirnaty supply to BioNTech and lower revenues from our active pharmaceutical ingredient sales operation, partially offset by higher manufacturing activities performed on behalf of customers as well as an increase in R&D services to select innovative biotech companies under our Pfizer Ignite operations. |
| Int’l. | 920 | | | 952 | | | (3) | | | (3) | | |
| Worldwide | $ | 1,310 | | | $ | 1,342 | | | (2) | | | (2) | | |
(a)Comirnaty includes direct sales and Alliance revenues related to sales of the Pfizer-BioNTech COVID-19 vaccine, which are recorded within our Primary Care customer group. It does not include revenues for certain Comirnaty-related manufacturing activities performed on behalf of BioNTech, which are included in PC1, which is part of the Business Innovation operating segment. See Note 17C. * Calculation isIndicates calculation not meaningful or results are equal to or greater than 100%.
Seerights, Note 16 for a discussion of recent developments concerning patent and product litigation relating to certain of the products discussed above.Seeabove and Note 17B17C for additional information regarding the primary indications or class of the selected products discussed above.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 33 |
Product Developments
A comprehensive update of Pfizer’s development pipeline was published as of February 2, 2021Costs and is available at www.pfizer.com/science/drug-product-pipeline. It includes an overview of our research and a list of compounds in development with targeted indication and phase of development, as well as mechanism of action for some candidates in Phase 1 and all candidates from Phase 2 through registration.
The following provides information about significant marketing application-related regulatory actions by, and filings pending with, the FDA and regulatory authorities in the EU and Japan. The table below includes only approvals for products that have occurred in the last twelve months and does not include approvals that may have occurred prior to that time. The table includes filings with regulatory decisions pending (even if the filing occurred outside of the last twelve-month period).
| | | | | | | | | | | | | | |
PRODUCT | DISEASE AREA | APPROVED/FILED* |
U.S. | EU | JAPAN |
PF-07302048 (COVID-19 Vaccine)(a)
| Immunization to prevent COVID-19 (16 years of age and older) | EUA
Dec.
2020
| CMA
Dec.
2020
| Approved Feb.
2021
|
Bavencio
(avelumab)(b)
| First-line maintenance urothelial cancer | Approved
June
2020
| Approved
Jan.
2021
| Filed
May
2020
|
First-line RCC (combination with Inlyta (axitinib)) | | | Approved
Dec.
2019
|
Nyvepria (pegfilgrastim-apgf) | Neutropenia in patients undergoing cancer chemotherapy (biosimilar) | Approved
June
2020
| Approved
Nov.
2020
| |
Braftovi (encorafenib)(c)
| Second or third-line BRAFv600E-mutant mCRC (combination with Erbitux® (cetuximab))
| Approved
April
2020
| Approved
June
2020
| Approved
Nov.
2020
|
Braftovi (encorafenib) and Mektovi (binimetinib)(c)
| Second or third-line BRAFV600E-mutant mCRC (combination with Erbitux® (cetuximab))
| | | Approved
Nov.
2020
|
Xtandi
(enzalutamide)(d)
| mCSPC | Approved
Dec.
2019
| Filed
July
2019
| |
Abrilada (U.S.); Amsparity (EU)
(adalimumab-afzb)(e)
| RA (biosimilar) | Approved
Nov.
2019
| Approved Feb.
2020
| |
abrocitinib (PF-04965842) | Atopic dermatitis | Filed
Oct.
2020
| Filed
Oct.
2020
| Filed
Dec.
2020
|
Infliximab Pfizer (infliximab) | Ankylosing spondylitis (biosimilar) | | | Approved
Oct.
2020
|
Bevacizumab Pfizer (bevacizumab) | Non-small cell lung cancer (biosimilar) | | | Approved
Sept.
2020
|
Rituximab Pfizer (rituximab) | Chronic idiopathic thrombocytopenic purpura (biosimilar) | | | Approved
Aug.
2020
|
tanezumab(f)
| Chronic pain due to moderate-to-severe osteoarthritis | Filed
March
2020
| Filed
March
2020
| Filed
Aug.
2020
|
Bosulif
(bosutinib) | First-line chronic myelogenous leukemia | | | Approved
June
2020
|
Daurismo
(glasdegib) | Combination with low-dose cytarabine for AML | | Approved
June
2020
| |
Ruxience
(rituximab) | Follicular lymphoma (biosimilar) | | Approved
April
2020
| |
Staquis
(crisaborole) | Atopic dermatitis | | Approved
March
2020
| |
Vyndaqel
(tafamidis free acid)
| ATTR-CM | | Approved
Feb.
2020
| |
Xeljanz
(tofacitinib) | Modified release 11 mg tablet for RA (combination with methotrexate) | | Approved
Dec.
2019
| |
Ankylosing spondylitis | Filed
Aug.
2020
| | |
Relugolix(g)
| Uterine fibroids (combination with estradiol and norethindrone acetate) | Filed
Aug.
2020
| | |
Lorbrena
(lorlatinib)
| First- line ALK-positive non-small cell lung cancer | Filed
Dec.
2020
| | |
somatrogon (PF-06836922)(h)
| Pediatric growth hormone deficiency | Filed
Jan.
2021
| | |
PF-06482077 (Vaccine) | Invasive and non-invasive pneumococcal infections (adults) | Filed
Dec.
2020
| | |
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 34 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Costs and expenses follow: |
| | Year Ended December 31, | | % Change |
| (MILLIONS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Cost of sales | | $ | 24,954 | | | $ | 34,344 | | | $ | 30,821 | | | (27) | | | 11 | |
Percentage of Total revenues | | 42.7 | % | | 34.2 | % | | 37.9 | % | | | | |
| Selling, informational and administrative expenses | | 14,771 | | | 13,677 | | | 12,703 | | | 8 | | | 8 | |
| Research and development expenses | | 10,679 | | | 11,428 | | | 10,360 | | | (7) | | | 10 | |
| Acquired in-process research and development expenses | | 194 | | | 953 | | | 3,469 | | | (80) | | | (73) | |
| Amortization of intangible assets | | 4,733 | | | 3,609 | | | 3,700 | | | 31 | | | (2) | |
Restructuring charges and certain acquisition-related costs | | 2,943 | | | 1,375 | | | 802 | | | * | | 71 | |
Other (income)/deductions—net(a) | | (835) | | | 217 | | | (4,878) | | | * | | * |
*For the U.S., the filing date is the date on which the FDA accepted our submission. For the EU, the filing date is the date on which the EMA validated our submission.Indicates calculation not meaningful.
(a)PF-07302048 or BNT162b2 (Pfizer/BioNTech COVID-19 vaccine) received EUA from the FDABeginning in 2024, we will include royalty income in Total revenues and CMA from the EMA.
(b)Being developed in collaborationwill restate prior periods for consistency with Merck KGaA, Germany.
(c)Erbitux® is a registered trademark of ImClone LLC. In the EU, we are developing in collaboration with the Pierre Fabre Group. In Japan, we are developing in collaboration with Ono Pharmaceutical Co., Ltd.
(d)Being developed in collaboration with Astellas.
(e)We are working to make Abrilada available to U.S. patients as soon as feasible based on the terms of our agreement with AbbVie. Current plans are to launch Abrilada in 2023. We do not currently plan to commercialize Amsparity in the EU due to unfavorable market conditions.
(f)Being developed in collaboration with Lilly.
(g)Being developed in collaboration with Myovant.
(h)Being developed in collaboration with OPKO Health, Inc.2024 presentation.
In China, the following products received regulatory approvals in the last twelve months: Eucrisa for atopic dermatitis in July 2020 and Vyndaqel for cardiac amyloidosis in September 2020.
The following provides information about additional indications and new drug candidates in late-stage development:
| | | | | | | | |
| PRODUCT/CANDIDATE | PROPOSED DISEASE AREA |
LATE-STAGE CLINICAL PROGRAMS FOR ADDITIONAL USES AND DOSAGE FORMS
FOR IN-LINE AND IN-REGISTRATION PRODUCTS | Bavencio (avelumab)(a)
| First-line non-small cell lung cancer |
Ibrance (palbociclib)(b)
| ER+/HER2+ metastatic breast cancer |
Xtandi (enzalutamide)(c)
| Non-metastatic high-risk castration sensitive prostate cancer |
Talzenna (talazoparib) | Combination with Xtandi (enzalutamide) for first-line mCRPC |
PF-06482077 (Vaccine) | Invasive and non-invasive pneumococcal infections (pediatric) |
somatrogon (PF-06836922)(d)
| Adult growth hormone deficiency |
tanezumab(e)
| Cancer pain |
Braftovi (encorafenib) and Erbitux® (cetuximab)(f)
| First-line BRAFv600E-mutant mCRC
|
Relugolix(g)
| Combination with estradiol and norethindrone acetate for endometriosis |
| | |
NEW DRUG CANDIDATES IN LATE-STAGE DEVELOPMENT | aztreonam-avibactam
(PF-06947387) | Treatment of infections caused by Gram-negative bacteria for which there are limited or no treatment options |
fidanacogene elaparvovec (PF-06838435) | Hemophilia B |
Giroctocogene
fitelparvovec
(SB-525 or PF-07055480)
| Hemophilia A |
PF-06425090 (Vaccine) | Primary clostridioides difficile infection |
PF-06886992 (Vaccine) | Serogroups meningococcal (adolescent and young adults) |
PF-06928316 (Vaccine) | Respiratory syncytial virus infection (maternal) |
PF-07265803 | Dilated cardiomyopathy due to Lamin A/C gene mutation |
ritlecitinib (PF-06651600) | Alopecia areata |
sasanlimab (PF-06801591) | Non-muscle-invasive bladder cancer |
PF-06939926 | Duchenne muscular dystrophy |
marstacimab (PF-06741086) | Hemophilia |
(a)Being developed in collaboration with Merck KGaA, Germany.
(b)Being developed in collaboration with the Alliance Foundation Trial.
(c)Being developed in collaboration with Astellas.
(d)Being developed in collaboration with OPKO Health, Inc.
(e)Being developed in collaboration with Lilly.
(f)Erbitux® is a registered trademark of ImClone LLC. In the EU, we are developing in collaboration with the Pierre Fabre Group. In Japan, we are developing in collaboration with Ono Pharmaceutical Co., Ltd.
(g)Being developed in collaboration with Myovant.
For additional information about our R&D organization, see the Item 1. Business—Research and Development section of this Form 10-K.
COSTS AND EXPENSES
The changes in costs and expenses below reflect, among other things, a decline in expenses resulting from the July 31, 2019 completion of the Consumer Healthcare JV transaction (see Note 2C). In addition, the COVID-19 pandemic impacted certain operating expenses in 2020.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 35 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Costs and expenses follow: |
| | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 | | 20/19 | | 19/18 |
| Cost of sales | | $ | 8,692 | | | $ | 8,251 | | | $ | 8,987 | | | 5 | | | (8) | |
Percentage of Revenues | | 20.7 | % | | 20.0 | % | | 22.0 | % | | | | |
| Selling, informational and administrative expenses | | 11,615 | | | 12,750 | | | 12,612 | | | (9) | | | 1 | |
Percentage of Revenues | | 27.7 | % | | 31.0 | % | | 30.9 | % | | | | |
| Research and development expenses | | 9,405 | | | 8,394 | | | 7,760 | | | 12 | | | 8 | |
Percentage of Revenues | | 22.4 | % | | 20.4 | % | | 19.0 | % | | | | |
| Amortization of intangible assets | | 3,436 | | | 4,462 | | | 4,736 | | | (23) | | | (6) | |
Percentage of Revenues | | 8.2 | % | | 10.8 | % | | 11.6 | % | | | | |
Restructuring charges and certain acquisition-related costs | | 600 | | | 601 | | | 1,058 | | | — | | (43) | |
Percentage of Revenues | | 1.4 | % | | 1.5 | % | | 2.6 | % | | | | |
| Other (income)/deductions—net | | 669 | | | 3,314 | | | 2,077 | | | (80) | | | 60 | |
Cost of Sales
2020 v. 2019
Cost of sales increased $441 million,decreased $9.4 billion, primarily due to:
•increased sales volumes;
•the increase in royalty expenses,a reduction of $14.2 billion due to an increase inlower sales of related products;
•the unfavorable impact of incremental costs incurred in response to the COVID-19 pandemic;Comirnaty; and
•the unfavorable impacta reduction of foreign exchange and hedging activity on intercompany inventory,$1.5 billion due to lower sales of Paxlovid,
partially offset by:
•the favorable impactnon-cash charges of the July 31, 2019 completion of the Consumer Healthcare JV transaction.$6.2 billion for inventory write-offs and related charges ($5.0 billion for Paxlovid and $1.2 billion for Comirnaty).
The increase in Cost of salesas a percentage of Total revenues was mainly driven by the non-cash charge of $6.2 billion discussed above, and unfavorable changes in 2020, compared to 2019, wassales mix, primarily due to alllower sales of Paxlovid and Comirnaty, which includes the unfavorable impact of the factors discussed above, $3.5 billion non-cash Paxlovid revenue reversal.
Selling, Informational and Administrative Expenses
Selling, informational and administrative expenses increased $1.1 billion, mostly due to:
•an increase of $1.1 billion in marketing and promotional expenses for recently acquired and launched products;
•an increase of $280 million for the expected Paxlovid commercial launch;
•an increase of $210 million in our liability to be paid to participants of our supplemental savings plan; and
•an increase of $170 million in marketing and promotional expenses for rare disease products,
partially offset by an increase in alliance revenues, which have no associated costby:
•a decrease of sales.$690 million due to a lower provision for U.S. healthcare reform fees related to Comirnaty and Paxlovid.
2019 v. 2018Research and Development Expenses
Cost of salesResearch and development expenses decreased $736$749 million, primarily due to:
•the favorable impactlower spending of the July 31, 2019 completion of the Consumer Healthcare JV transaction;
•the favorable impact of foreign exchange;$870 million mainly for lower compensation-related expenses, and
•the favorable impact of hedging activity of intercompany inventory,
partially offset by:
•the unfavorable change in product mix; ongoing vaccine and
•the increase in royalty expenses, due to an increase in sales of related products.
The decrease in Cost of sales as a percentage of revenues in 2019, compared to 2018, was primarily due to all of the factors discussed above, hospital programs, as well an increase in alliance revenues, which have no associated cost of sales.
Selling, Informational and Administrative (SI&A) Expenses
2020 v. 2019
SI&A expenses decreased $1.1 billion, mostly due to:
•the favorable impact of the July 31, 2019 completion of the Consumer Healthcare JV transaction;
•lower spending for corporate enabling functions;
•lower spending on sales and marketing activities due to the impact of the COVID-19 pandemic; and
•lower investments across the Internal Medicine and Inflammation & Immunology portfolios,
partially offset by:
•the increase in external, incremental costs directly related to implementing our cost-reduction/productivity initiatives; and
•the increase in business and legal entity alignment costs.
2019 v. 2018
SI&A expenses increased $138 million, primarily due to:
•additional investment in emerging markets;
•additional investment in the Oncology portfolio in developed markets;
•increased employee deferred compensation as a result of savings plan gains;
•the increase due to the timing of expenses (i.e., insurance recoveries and product donations);
•marketing and promotional expenses for the U.S. launches of Vyndaqel in May 2019 and Vyndamax in September 2019;
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 36 |
•increased business and legal entity alignment costs;
•costs to separate Consumer Healthcare; and
•increased healthcare reform expenses,
partially offset by:
•the favorable impact of the July 31, 2019 completion of the Consumer Healthcare JV; and
•the favorable impact of foreign exchange.
Research and Development (R&D) Expenses
2020 v. 2019
R&D expenses increased $1.0 billion, mainly due to:
•costs related to our collaboration agreement with BioNTech to co-develop a COVID-19 vaccine, including an upfront payment to BioNTech;
•a net increase in upfront payments, mainly related to Myovant and Valneva; and
•increased investments towards building new capabilities and driving automation,
partially offset by:
•the net reductiondecrease of upfront and milestone payments associated with the acquisition of Therachon in July 2019 and Akcea in October 2019.
2019 v. 2018
R&D expenses increased $635$260 million mainly due to:
•upfront payments to Therachon and Akcea;
•increased investments towards building new capabilities and driving automation;
•increased spending on our Inflammation & Immunology and Rare Disease portfolios due to several Phase 3 programs and
investment in gene therapy;
•increased spending related to assets acquired from our acquisition of Array; and
•increased medical spend for new and growing products,
partially offset by:
•decreased spending across the Oncology, Vaccines and Internal Medicine portfolios, as select programs have reached completion;
•the decrease in the value of the portfolio performance share grants reflecting changesthe decrease in the price of Pfizer’s common stock,
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 39 |
partially offset by:
•increased investments of $345 million, mainly to develop certain acquired assets, as well as management’s assessment ofactivities to support upcoming product launches.
Acquired In-Process Research and Development Expenses
Acquired in-process research and development expenses decreased $758 million primarily reflecting the probability that the specified performance criteria will be achieved;non-recurrence of:
•an upfront payment of $426 million related to the discontinuationclosing of the Staphylococcus aureus vaccine trial;acquisition of ReViral Ltd. in 2022;
•the favorable impact of the July 31, 2019 completion of the Consumer Healthcare JV;an upfront payment to Biohaven and a premium paid on our equity investment in Biohaven totaling $263 million in 2022; and
•the favorable impact of foreign exchange.a $76 million premium paid on our equity investment in BioNTech to develop a potential mRNA vaccine against shingles, both recorded in 2022.
Amortization of Intangible Assets
2020 v. 2019
Amortization of intangible assets decreased $1.0 increased $1.1 billion, primarily due to:
•the non-recurrenceas a result of amortization2023 reflecting a full year of fully amortized assets and the impairment of Eucrisa in the fourth quarter of 2019,
partially offset by:
•the increase in amortization of intangible assets from our acquisitionacquisitions of Array.
2019 v. 2018
Amortization of intangible assets decreased $274 million, mainly due to:
•the non-recurrence of amortization as a result of the impairment of sterile injectable products in the fourth quarter of 2018;
•fully amortized assets;Biohaven and
•the contribution of our Consumer Healthcare business to the Consumer Healthcare JV,
partially offset by:
•the increase in amortization related to assets recorded as a result of the approval of Xtandi in the U.S. for the treatment of nmCRPC in July of 2018; and
• GBT, higher amortization of intangible assets from our acquisitionrelated to Prevnar, as well as reclassifications of Array.
For additional information, seeIPR&D to developed technology rights, partially offset by fully amortized assets. See Notes 2A, 2C, and 10A.
Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
Transforming to a More Focused Company Program
For a description of our program, as well as the anticipated and actual costs, see Note 3. ––The program savings discussed below may be rounded and represent approximations. In connection with the costs primarily related to therestructuring our corporate enabling functions, initiatives, we expectachieved gross cost savings of $1.0 billion, or net cost savings, excluding merit and inflation growth and certain real estate cost increases, of $700 million, in the two year period from 2021 through 2022. In connection with transforming our commercial go-to market strategy, we expect net cost savings of $1.4 billion, to be achieved primarily over the two-year period 2021-2022.from 2022 through 2024. In connection with manufacturing network optimization, including legacy cost reduction initiatives, we expect targetedachieved net cost savings of $300 million$550 million. In connection with optimizing our end-to-end R&D operations, we expect net cost savings of $2.3 billion to be achieved primarily from 20202023 through 2022.2025.
| | | | | | | | |
Pfizer Inc.This program is expected to deliver net cost savings of at least $4 billion, to be achieved primarily from 2023 through 2024. | 2020 Form 10-K | 37 |
Certain qualifying costs for this programthese programs were recorded in 2020,2023, 2022 and in the fourth quarter of 2019,2021, and are reflected as Certain Significant Items and excluded from our non-GAAP measure of Adjusted Income. See the Non-GAAP Financial Measure: Adjusted Income section of thiswithin MD&A.
For a description of our programs, as well as the anticipated and actual costs, see Note 3A, The program savings discussed above may be rounded and represent approximations. In addition to this program,these programs, we continuously monitor our operations for cost reduction and/or productivity opportunities, especially in light of the losses of exclusivity and the expiration of collaborative arrangements for various products.
Other (Income)/Deductions––Net
2020 v. 2019
Other deductions—The favorable period-over-period change of $1.1 billion was primarily driven by net decreased $2.6 billion, mainly due to:
•gains on equity securities in 2023 versus net losses recognized on equity securities in 2022 and lower net interest expense, partially offset by higher intangible asset impairment charges;
•charges. See
higher net periodic benefit credits other than service costs;
•lower business and legal entity alignment costs;
•higher Consumer Healthcare JV equity method income; and
•lower charges for certain legal matters,
partially offset by:
•higher net losses on asset disposals.
2019 v. 2018
Other deductions—net increased $1.2 billion, mainly due to:
•higher net periodic benefits costs other than service costs;
•lower income from collaborations, out-licensing arrangements and sales of compound/product rights;
•higher interest expense mainly as a result of an increased commercial paper balance due to the acquisition of Array, as well as the retirement of lower-coupon debt and the issuance of new debt with a higher coupon than the debt outstanding for the comparative prior year period; and
•higher business and legal entity alignment costs,
partially offset by:
•lower asset impairment charges.
See Note 4for additional information.Upjohn Separation Costs
PROVISION/(BENEFIT) FOR TAXES ON INCOMESince inception through December 31, 2023, we have incurred substantially all costs of approximately $700 million in connection with separating Upjohn, including costs and expenses related to separation of legal entities and transaction costs.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | % Change |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 | | 20/19 | | 19/18 |
| Provision/(benefit) for taxes on income | | $ | 477 | | | $ | 618 | | | $ | (266) | | | (23) | | | * |
| Effective tax rate on continuing operations | | 6.4 | % | | 5.4 | % | | (7.4) | % | | | | |
Provision/(Benefit) for Taxes on Income
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | % Change |
| (MILLIONS) | | 2023 | | 2022 | | 2021 | | 23/22 | | 22/21 |
| Provision/(benefit) for taxes on income | | $ | (1,115) | | | $ | 3,328 | | | $ | 1,852 | | | * | | 80 | |
| Effective tax rate on continuing operations | | (105.4) | % | | 9.6 | % | | 7.6 | % | | | | |
*Indicates calculation not meaningful or result is equal to or greater than 100%.meaningful.
For information about our effective tax rate and the events and circumstances contributing to the changes between periods, as well as details about discrete elements that impacted our tax provisions, see Note 5. DISCONTINUED OPERATIONS
For information about our discontinued operations, see Note 2B.
NON-GAAP FINANCIAL MEASURE: ADJUSTED INCOME
Adjusted income is an alternative measure of performance used by managementChanges in Tax Laws––Many countries outside the U.S. have enacted legislation for global minimum taxation resulting from the Organization for Economic Co-operation and Development’s (OECD) Base Erosion and Profit Shifting “Pillar 2” project. The EU has approved a directive requiring member states to evaluateincorporate the OECD provisions into their respective domestic laws, and other countries outside the EU are also enacting the provisions into their domestic law. The provisions are generally effective for Pfizer in 2024, though significant details and guidance around the provisions are still pending. Income tax expense could be adversely affected as the legislation becomes effective in countries in which we do business, and such impact could be material to our overall performance in conjunction with other performance measures. As such, we believe that investors’ understanding of our performance is enhanced by disclosing this measure. We use Adjusted income, certain components of Adjusted income and Adjusted diluted EPS to present the results of our major operations––the discovery, development, manufacture, marketing, salesoperations. We continue to monitor pending OECD guidance and distribution of biopharmaceutical products worldwide––prior to considering certain income statement elements as follows:
| | | | | | | | | | | | | | |
Measure | | Definition | | Illustrative Use |
Adjusted income | | Net income attributable to Pfizer Inc. common shareholders(a)
before the impact of purchase accounting for acquisitions, acquisition-related costs, discontinued operations and certain significant items
| | •Monthly managerial analysis of our operating results and our annual budgets are prepared using these non-GAAP measures
•Senior management’s compensation is determined, in part, using these non-GAAP measures(b)
|
Adjusted cost of sales, Adjusted selling, informational and administrative expenses, Adjusted research and development expenses, Adjusted other (income)/deductions––net
| | Cost of sales, Selling, informational and administrative expenses, Research and development expenses, Amortization of intangible assets and Other (income)/deductions––net (a), each before the impact of purchase accounting for acquisitions, acquisition-related costs, discontinued operations and certain significant items, which are components of the Adjusted income measure
| |
Adjusted diluted EPS | | EPS attributable to Pfizer Inc. common shareholders––diluted (a) before the impact of purchase accounting for acquisitions, acquisition-related costs, discontinued operations and certain significant items
| |
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 38 |
(a)Most directly comparable GAAP measure.
(b)The short-term incentive plans for substantially all non-sales-force employees worldwide are funded from a pool based on our performance, measured in significant part by three metrics, one of which is Adjusted diluted EPS, which is derived from Adjusted income and accounts for 40% of the bonus pool funding. Additionally, the payout for Performance Share Awards is determined in part by Adjusted net income, which is derived from Adjusted income. Effective for the 2020 performance year and consistent with shareholder feedback received in 2019, the Compensation Committee of the BOD approved adding an R&D pipeline achievement factor to the existing short-term incentive financial metrics.
Adjusted income, and its components and Adjusted diluted EPS, are non-GAAP financial measures that have no standardized meaning prescribed by GAAP and, therefore, are limited in their usefulness to investors. Because of their non-standardized definitions, they may not be comparable to the calculation of similar measures of other companies and are presented solely to permit investors to more fully understand how management assesses performance. A limitation of these measures is that they provide a view of our operations without including all events during a period, and do not provide a comparable view of our performance to peers. These measures are not, and should not be viewed as, substitutes for their directly comparable GAAP measures of Net income attributable to Pfizer Inc. common shareholders, components of Net income attributable to Pfizer Inc. common shareholders and EPS attributable to Pfizer Inc. common shareholders—diluted, respectively. See the accompanying reconciliations of certain GAAP reported to non-GAAP adjusted information for 2020, 2019 and 2018 below.
We also recognize that, as internal measures of performance, these measures have limitations, and we do not restrict our performance-management process solely to these measures. We also use other tools designed to achieve the highest levels of performance. For example, our R&D organization has productivity targets, upon which its effectiveness is measured. In addition, total shareholder return, both on an absolute basis and relative to a publicly traded pharmaceutical index, plays a significant role in determining payouts under certain of our incentive compensation plans.
Purchase Accounting Adjustments
Adjusted income excludes certain significant purchase accounting impacts resulting from business combinations and net asset acquisitions. These impacts can include the incremental charge to cost of sales from the sale of acquired inventory that was written up to fair value, amortization related to the increase in fair value of the acquired finite-lived intangible assets, and to a much lesser extent, depreciation related to the increase/decrease in fair value of the acquired fixed assets, amortization related to the increase in fair value of acquired debt, and the fair value changes for contingent consideration. Therefore, the Adjusted income measure includes the revenues earned upon the sale of the acquired products without considering the acquisition cost of those products.
The exclusion of amortization attributable to acquired intangible assets provides management and investors an alternative view of our results by providing a degree of parity to internally developed intangible assets for which R&D costs have been expensed. However, we have not factored in the impacts of any other differences that might have occurred if we had discovered and developed those intangible assets on our own, such as different R&D costs, timelines or resulting sales; accordingly, this approach does not intend to be representative of the results that would have occurred if we had discovered and developed the acquired intangible assets internally.
Acquisition-Related Costs
Adjusted income excludes acquisition-related costs, which are comprised of transaction, integration, restructuring charges and additional depreciation costs for business combinations because these costs are unique to each transaction and represent costs that were incurred to restructure and integrate businesses as a result of an acquisition. We have made no adjustments for resulting synergies.
The significant costs incurred in connection with a business combination result primarily from the need to eliminate duplicate assets, activities or employees––a natural result of acquiring a fully integrated set of activities. For this reason, we believe that such costs incurred can be viewed differently in the context of an acquisition from those costs incurred in other, more normal, business contexts. The integration and restructuring costs for a business combination may occur over several years, with the more significant impacts typically ending within three years of the relevant transaction. Because of the need for certain external approvals for some actions, the span of time needed to achieve certain restructuring and integration activities can be lengthy.
Discontinued Operations
For information about our discontinued operations, see Note 2B.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 40 |
PRODUCT DEVELOPMENTS
A comprehensive update of Pfizer’s development pipeline was published as of January 30, 2024 and is available at www.pfizer.com/science/drug-product-pipeline. It includes an overview of our research and a list of compounds in development with targeted indication and phase of development, as well as mechanism of action for some candidates in Phase 1 and all candidates from Phase 2 through registration.
This section provides information as of the date of this filing about significant marketing application-related regulatory actions by, and filings pending with, the FDA and regulatory authorities in the EU and Japan.
The tables below include filing and approval milestones for products that have occurred in the last twelve months and generally do not include approvals that may have occurred prior to that time. The tables include filings with regulatory decisions pending (even if the filing occurred outside of the last twelve-month period).
COVID-19 Vaccine Products
Beginning with the original monovalent Pfizer-BioNTech COVID-19 Vaccine, initially authorized for emergency use, to Comirnaty (COVID-19 Vaccine, mRNA, 2023-2024 Formula), approved by the FDA for individuals 12 years and older and the Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula) authorized by the FDA for emergency use for individuals 6 months through 11 years of age, efforts to stay current with circulating COVID-19 strains have resulted in the rapid development of targeted, adapted vaccines for licensure in the U.S., Europe, Japan and other markets. The adapted vaccines have included two bivalent formulations (Original and Omicron BA.1, not authorized in the U.S., and Original and Omicron BA.4/BA.5). As updated COVID-19 vaccines are formulated to more closely target currently circulating vaccines, prior vaccine formulations are generally no longer utilized in a majority of the markets.
The 2023-2024 Formula includes a monovalent (single) component that corresponds to the Omicron sub-variant XBB.1.5 of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The table below summarizes the approval of the 2023-2024 Formula in the markets indicated:
| | | | | | | | | | | | | | |
| PRODUCT | INDICATION | REGULATORY STATUS |
U.S.(a) | EU | JAPAN |
Comirnaty
(COVID-19 Vaccine,
mRNA, 2023-2024 Formula) | Active immunization to prevent COVID-19 caused by SARS-CoV-2 for individuals 6 months through 4 years of age | Authorized September 2023 | Approved August 2023 | Approved September 2023 |
| Active immunization to prevent COVID-19 caused by SARS-CoV-2 for individuals 5 through 11 years of age | Authorized September 2023 | Approved August 2023 | Approved September 2023 |
| Active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 12 years of age and older | Approved September 2023 | Approved August 2023 | Approved September 2023 |
(a)In September 2023, Pfizer and BioNTech announced the FDA approved a regulatory application for their Omicron XBB.1.5-adapted monovalent COVID-19 vaccine for individuals 12 years of age and older (Comirnaty (COVID-19 Vaccine, mRNA, 2023-2024 Formula)). The FDA also granted EUA for the Omicron XBB.1.5-adapted monovalent COVID-19 vaccine for individuals 6 months through 11 years of age (Pfizer-BioNTech COVID-19 Vaccine (2023-2024 Formula)).
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 41 |
Other Products
| | | | | | | | | | | | | | |
| PRODUCT | INDICATION OR PROPOSED INDICATION | APPROVED/FILED* |
| U.S. | EU | JAPAN |
Ngenla (somatrogon)(a) | Pediatric growth hormone deficiency | Approved June 2023 | Approved February 2022 | Approved January 2022 |
Prevnar 20/Apexxnar (Vaccine) | Active immunization to prevent pneumonia, invasive disease and otitis media caused by Streptococcus pneumoniae (adults) | Approved June 2021 | Approved February 2022 | Filed September 2023 |
| Active immunization to prevent pneumonia, invasive disease and otitis media caused by Streptococcus pneumoniae (pediatric) | Approved April 2023 | Filed November 2022 | Filed March 2023 |
TicoVac
(Vaccine) | Active immunization to prevent tick-borne encephalitis disease | Approved August 2021 | | Filed March 2023 |
Paxlovid(b)(nirmatrelvir and ritonavir) | COVID-19 in high-risk adults | Approved May 2023 | Approved February 2023 | Approved July 2023 |
Nurtec ODT/Vydura (rimegepant) | Acute treatment of migraine with or without aura (adults) | Approved February 2020 | Approved April 2022 | |
| Prevention of episodic migraine (adults) | Approved May 2021 | Approved April 2022 | |
Litfulo/Ritfulo
(ritlecitinib) | Alopecia areata | Approved June 2023 | Approved September 2023 | Approved June 2023 |
Zavzpret (zavegepant)
(intranasal) | Acute treatment of migraine with or without aura (adults) | Approved March 2023 | | |
Penbraya (PF-06886992)
(Vaccine) | Active immunization to prevent serogroups ABCWY meningococcal infections (adolescent and young adults) | Approved October 2023 | Filed June 2023 | |
Abrysvo
(Vaccine) | Active immunization to prevent RSV infection (maternal) | Approved August 2023 | Approved August 2023 | Approved January 2024 |
| Active immunization to prevent RSV infection (older adults) | Approved May 2023 | Approved August 2023 | Filed May 2023 |
| Velsipity (etrasimod) | Ulcerative colitis (moderately to severely active) | Approved October 2023 | Approved February 2024 | |
| Braftovi (encorafenib) and Mektovi (binimetinib) | BRAFV600E-mutant metastatic non-small cell lung cancer | Approved October 2023 | Filed October 2023(c) | |
| Elrexfio (elranatamab) | Multiple myeloma triple-class relapsed/refractory | Approved August 2023 | Approved December 2023 | Filed June 2023 |
| Talzenna (talazoparib) | Combination with Xtandi (enzalutamide) for adult patients with homologous recombination repair (HRR) gene-mutated mCRPC(d) | Approved June 2023 | Approved January 2024 | Approved January 2024 |
| Treatment of BRCA gene-mutated, HER2-negative, inoperable or recurrent breast cancer who have been treated with cancer chemotherapy | Approved October 2018 | Approved June 2019 | Approved January 2024 |
fidanacogene elaparvovec (PF-06838435)(e) | Hemophilia B (adults) | Filed June 2023 | Filed May 2023 | |
Xtandi (enzalutamide)(f) | nmCSPC with biochemical recurrence at high risk for metastasis (high-risk BCR) | Approved November 2023 | Filed September 2023 | |
| marstacimab (PF-06741086) | Hemophilia A and B | Filed December 2023 | Filed October 2023 | |
aztreonam-avibactam(g) (PF-06947387) | Treatment of infections caused by Gram-negative bacteria with limited or no treatment options | | Filed September 2023 | |
Padcev (enfortumab vedotin-ejfv)(h) | In combination with Keytruda(i) (pembrolizumab) for locally advanced or metastatic urothelial cancer (adults) | Approved December 2023 | Filed January 2024 | Filed January 2024 |
Tivdak (tisotumab vedotin-tftv)(j) | Recurrent or metastatic cervical cancer with disease progression on or after first-line therapy | Filed(k) January 2024 | Filed February 2024 | |
| Tukysa (tucatinib) | In combination with trastuzumab for HER2-positive metastatic colorectal cancer that has progressed following treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy | Approved January 2023 | | |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 42 |
*For the U.S., the filing date is the date on which the FDA accepted our submission. For the EU, the filing date is the date on which the EMA validated our submission.
(a)Being developed in collaboration with OPKO.
(b)Previously authorized under EUA in the U.S. (December 2021) and approved by the FDA in high-risk adults (May 2023). Remains under EUA for children (12-18 years of age; >88lbs) in the U.S.
(c)Pierre Fabre is the Marketing Authorization Holder for Braftovi (encorafenib) and Mektovi (binimetinib) in the EU.
(d)Listed indication applies to U.S. only. EU indication (all comers): mCRPC in whom chemotherapy is not clinically indicated; Japan indication: BRCA gene-mutated mCRPC.
(e)Being developed in collaboration with Spark Therapeutics, Inc.
(f)Being developed in collaboration with Astellas.
(g)Being developed in collaboration with AbbVie. AbbVie has the exclusive commercialization rights to this investigative therapy in the U.S. and Canada; Pfizer leads the joint development program and has commercialization rights in all other countries.
(h)Being developed in collaboration with Astellas.
(i)Keytruda is a registered trademark of Merck Sharp & Dohme Corp.
(j)Being developed in collaboration with Genmab.
(k)January 2024 filing date refers to application for conversion from accelerated to full approval.
The following provides information about additional indications and new drug candidates in late-stage development:
| | | | | | | | | | | |
| | PRODUCT/CANDIDATE | PROPOSED DISEASE AREA |
| LATE-STAGE CLINICAL PROGRAMS FOR ADDITIONAL USES AND DOSAGE FORMS FOR IN-LINE AND IN-REGISTRATION PRODUCTS | | Ibrance (palbociclib)(a) | ER+/HER2+ metastatic breast cancer |
| Talzenna (talazoparib) | Combination with Xtandi (enzalutamide) for DNA Damage Repair-deficient mCSPC |
Ngenla (somatrogon)(b) | Adult growth hormone deficiency |
Braftovi (encorafenib) and Erbitux® (cetuximab)(c) | First-line BRAFV600E-mutant mCRC |
| Paxlovid (nirmatrelvir; ritonavir) | COVID-19 in high-risk children (6-11 years of age; >88lbs) |
| Litfulo (ritlecitinib) | Vitiligo |
| Elrexfio (elranatamab) | Multiple myeloma double-class exposed |
| Newly diagnosed multiple myeloma post-transplant maintenance |
| Newly diagnosed multiple myeloma transplant-ineligible |
| Oxbryta (voxelotor) | Sickle cell disease (pediatric) |
Eliquis (apixaban)(d) | Venous thromboembolism (pediatric) |
| Abrysvo (vaccine) | Active immunization to prevent RSV infection in adults (18-59) |
Padcev (enfortumab vedotin)(e) | Cisplatin-ineligible/decline muscle-invasive bladder cancer |
| Cisplatin-eligible muscle-invasive bladder cancer |
| Tukysa (tucatinib) | HER2+ adjuvant breast cancer |
| 2nd line/3rd line HER2+ metastatic breast cancer |
| 1st line HER2+ metastatic colorectal cancer |
| NEW DRUG CANDIDATES IN LATE-STAGE DEVELOPMENT | | giroctocogene fitelparvovec (PF-07055480)(f) | Hemophilia A |
| PF-06425090 (Vaccine) | Immunization to prevent primary clostridioides difficile infection |
| sasanlimab (PF-06801591) | Combination with Bacillus Calmette-Guerin for non-muscle-invasive bladder cancer |
| fordadistrogene movaparvovec (PF-06939926) | Duchenne muscular dystrophy (ambulatory) |
VLA15 (PF-07307405) vaccine(g) | Immunization to prevent Lyme disease |
| PF-07252220 (quadrivalent mRNA-based vaccine) | Immunization to prevent influenza |
Vepdegestrant (PF-07850327)(h) | Breast cancer metastatic - 2nd line ER+/HER2- |
| inclacumab (PF-07940370) | Sickle cell disease |
Ibrance + vepdegestrant(h) | ER+/HER2- metastatic breast cancer |
| Dazukibart (PF-06823859) | Dermatomyositis, polymyositis |
Disitamab vedotin(i) | 1st line HER2 (≥IHC1+) metastatic urothelial cancer |
PF-07926307 (COVID/flu combo vaccine)(j) | Immunization to prevent COVID infection and influenza |
| sisunatovir (PF-07923568) | Respiratory syncytial virus infection (adults) |
Note: Braftovi/Mektovi/Keytruda previously listed as a late-stage clinical candidate is no longer considered registrational and has been removed.
Note: Zavzpret oral for the prevention of chronic migraine previously listed as a late-stage clinical candidate has been removed.
(a)Being developed in collaboration with The Alliance Foundation Trials, LLC.
(b)Being developed in collaboration with OPKO.
(c)Erbitux is a registered trademark of ImClone LLC. In the EU, we are developing in collaboration with the Pierre Fabre Group. In Japan, we are developing in collaboration with Ono.
(d)Being developed in collaboration with BMS.
(e)Being developed in collaboration with Astellas.
(f)Being developed in collaboration with Sangamo Therapeutics, Inc.
(g)Being developed in collaboration with Valneva.
(h)Vepdegestrant is being developed in collaboration with Arvinas.
(i)Being developed in collaboration with RemeGen Co., Ltd.
(j)Being developed in collaboration with BioNTech.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 43 |
NON-GAAP FINANCIAL MEASURE: ADJUSTED INCOME
Adjusted income is an alternative measure of performance used by management to evaluate our overall performance as a supplement to our GAAP Reported performance measures. As such, we believe that investors’ understanding of our performance is enhanced by disclosing this measure. We use Adjusted income, certain components of Adjusted income and Adjusted diluted EPS to present the results of our major operations––the discovery, development, manufacture, marketing, sale and distribution of biopharmaceutical products worldwide––prior to considering certain income statement elements as follows:
| | | | | | | | | | | | | | |
| Measure | | Definition | | Relevance of Metrics to Our Business Performance |
| Adjusted income | | Net income attributable to Pfizer Inc. common shareholders(a) before the impact of amortization of intangible assets, certain acquisition-related items, discontinued operations and certain significant items | | •Provides investors useful information to: ◦evaluate the normal recurring operational activities, and their components, on a comparable year-over-year basis ◦assist in modeling expected future performance on a normalized basis •Provides investors insight into the way we manage our budgeting and forecasting, how we evaluate and manage our recurring operations and how we reward and compensate our senior management(b) |
Adjusted cost of sales, Adjusted selling, informational and administrative expenses, Adjusted research and development expenses and Adjusted other (income)/deductions––net | | Cost of sales, Selling, informational and administrative expenses, Research and development expenses and Other (income)/deductions––net (a), each before the impact of amortization of intangible assets, certain acquisition-related items, discontinued operations and certain significant items, which are components of the Adjusted income measure | |
| Adjusted diluted EPS | | EPS attributable to Pfizer Inc. common shareholders––diluted (a) before the impact of amortization of intangible assets, certain acquisition-related items, discontinued operations and certain significant items | |
(a)Most directly comparable GAAP measure.
(b)The short-term incentive plans for substantially all non-sales-force employees worldwide are funded from a pool based on our performance, measured in significant part versus three budgeted metrics, one of which is Adjusted diluted EPS (as defined for annual incentive compensation purposes), which is derived from Adjusted income and accounts for 40% of the bonus pool funding tied to financial performance. Additionally, the payout for performance share awards is determined in part by Adjusted net income, which is derived from Adjusted income. Beginning in the first quarter of 2022, we no longer exclude any expenses for acquired IPR&D from our non-GAAP Adjusted results but we continue to exclude certain of these expenses for our financial results for annual incentive compensation purposes. The bonus pool funding, which is largely based on financial performance, is adjusted by our R&D pipeline performance, as measured by four metrics, and performance against certain of our ESG metrics, and may be further modified by our Compensation Committee’s assessment of other factors.
Adjusted income and its components and Adjusted diluted EPS are non-GAAP financial measures that have no standardized meaning prescribed by GAAP and, therefore, are limited in their usefulness to investors. Because of their non-standardized definitions, they may not be comparable to the calculation of similar measures of other companies and are presented to permit investors to more fully understand how management assesses performance. A limitation of these measures is that they provide a view of our operations without including all events during a period, and do not provide a comparable view of our performance to peers. These measures are not, and should not be viewed as, substitutes for their most directly comparable GAAP measures of Net income attributable to Pfizer Inc. common shareholders, components of Net income attributable to Pfizer Inc. common shareholders and EPS attributable to Pfizer Inc. common shareholders—diluted, respectively.
We also recognize that, as internal measures of performance, these measures have limitations, and we do not restrict our performance-management process solely to these measures. We also use other tools designed to achieve the highest levels of performance. For example, our R&D organization has productivity targets, upon which its effectiveness is measured. In addition, total shareholder return, both on an absolute basis and relative to a publicly traded pharmaceutical index, plays a significant role in determining payouts under certain of our incentive compensation plans.
Adjusted Income and Adjusted Diluted EPS
Amortization of Intangible Assets—Adjusted income excludes all amortization of intangible assets.
Acquisition-Related Items––Adjusted income excludes certain acquisition-related items, which are composed of transaction, integration, restructuring charges and additional depreciation costs for business combinations because these costs are unique to each transaction and represent costs that were incurred to restructure and integrate businesses as a result of an acquisition. We have made no adjustments for resulting synergies.
The significant costs incurred in connection with a business combination result primarily from the need to eliminate duplicate assets, activities or employees––a natural result of acquiring a fully integrated set of activities. For this reason, we believe that such costs incurred can be viewed differently in the context of an acquisition from those costs incurred in other, more normal, business contexts. The integration and restructuring costs for a business combination may occur over several years, with the more significant impacts typically ending within three years of the relevant transaction. Because of the need for certain external approvals for some actions, the span of time needed to achieve certain restructuring and integration activities can be lengthy.
Acquisition-related items may include purchase accounting impacts such as the incremental charge to cost of sales from the sale of acquired inventory that was written up to fair value, depreciation related to the increase/decrease in fair value of acquired fixed assets, amortization related to the increase in fair value of acquired debt, and the fair value changes for contingent consideration.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 44 |
Discontinued Operations––Adjusted income excludes the results of discontinued operations, as well as any related gains or losses on the disposal of such operations. We believe that this presentation is meaningful to investors because, while we review our therapeutic areas and product linesportfolio for strategic fit with our operations, we do not build or run our business with the intent to discontinue parts of our business. Restatements due to discontinued operations do not impact compensation or change the Adjusted income measure for the compensation in respect of the restated periods, but are presented for consistency across all periods.
Certain Significant Items
Adjusted income excludes certain significant items representing substantive and/or unusual items that are evaluated individually on a quantitative and qualitative basis. Certain significant items may be highly variable and difficult to predict. Furthermore, in some cases it is reasonably possible that they could reoccur in future periods. For example, although major non-acquisition-related cost-reduction programs are specific to an event or goal with a defined term, we may have subsequent programs based on reorganizations of the business, cost productivity or in response to LOE or economic conditions. Legal charges to resolve litigation are also related to specific cases, which are facts and circumstances specific and, in some cases, may also be the result of litigation matters at acquired companies that were inestimable, not probable or unresolved at the date of acquisition.acquisition, or legal matters related to divested products or businesses. Gains and losses on equity securities and pension and postretirement actuarial remeasurement gains and losses have a very high degree of inherent market volatility, which we do not control and cannot predict with any level of certainty, and we do not believe including these gains and losses assists investors in understanding our business or is reflective of our core operations and business. Unusual items represent items that are not part of our ongoing business; items that, either as a result of their nature or size, we would not expect to occur as part of our normal business on a regular basis; items that would be non-recurring; or items that relate to products we no longer sell. ForSee the Reconciliations of GAAP Reported to Non-GAAP Adjusted Information––Certain Line Items below for a non-inclusive list of certain significant items see Details of Income Statement Items Included in GAAP Reported but Excluded from Non-GAAP Adjusted Income below.items.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 39 |
ReconciliationReconciliations of GAAP Reported to Non-GAAP Adjusted Information––Certain Line Items
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2020 |
| IN MILLIONS, EXCEPT PER COMMON SHARE DATA | | GAAP Reported | | Purchase Accounting Adjustments(a) | | Acquisition-Related Costs(a) | | Discontinued Operations(a) | | Certain Significant Items(a) | | Non-GAAP Adjusted |
| Revenues | | $ | 41,908 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 41,908 | |
| Cost of sales | | 8,692 | | | 18 | | | — | | | — | | | (118) | | | 8,592 | |
| Selling, informational and administrative expenses | | 11,615 | | | (2) | | | — | | | — | | | (489) | | | 11,124 | |
| Research and development expenses | | 9,405 | | | 5 | | | — | | | — | | | (526) | | | 8,884 | |
| Amortization of intangible assets | | 3,436 | | | (3,152) | | | — | | | — | | | — | | | 284 | |
| Restructuring charges and certain acquisition-related costs | | 600 | | | — | | | (44) | | | — | | | (556) | | | — | |
| (Gain) on completion of Consumer Healthcare JV transaction | | (6) | | | — | | | — | | | — | | | 6 | | | — | |
| Other (income)/deductions––net | | 669 | | | (75) | | | — | | | — | | | (2,068) | | | (1,474) | |
| Income from continuing operations before provision/(benefit) for taxes on income | | 7,497 | | | 3,206 | | | 44 | | | — | | | 3,752 | | | 14,499 | |
Provision/(benefit) for taxes on income(b) | | 477 | | | 668 | | | 9 | | | — | | | 803 | | | 1,957 | |
| Income from continuing operations | | 7,021 | | | 2,537 | | | 35 | | | — | | | 2,948 | | | 12,541 | |
| Income from discontinued operations––net of tax | | 2,631 | | | — | | | — | | | (2,631) | | | — | | | — | |
| Net income attributable to noncontrolling interests | | 36 | | | — | | | — | | | — | | | — | | | 36 | |
| Net income attributable to Pfizer Inc. common shareholders | | 9,616 | | | 2,537 | | | 35 | | | (2,631) | | | 2,948 | | | 12,506 | |
| Earnings per common share attributable to Pfizer Inc. common shareholders––diluted | | 1.71 | | | 0.45 | | | 0.01 | | | (0.47) | | | 0.52 | | | 2.22 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, 2023 |
Data presented will not (in all cases) aggregate to totals. MILLIONS, EXCEPT PER SHARE DATA | | Cost of sales(a) | | Selling, informational and administrative expenses(a) | | Other (income)/deductions––net(a) | | Net income attributable to Pfizer Inc. common shareholders(a), (b), (c) | | Earnings per common share attributable to Pfizer Inc. common shareholders––diluted |
| GAAP Reported | | $ | 24,954 | | | | $ | 14,771 | | | | $ | (835) | | | | $ | 2,119 | | | $ | 0.37 | |
| Amortization of intangible assets | | — | | | | — | | | | — | | | | 4,733 | | | |
| Acquisition-related items | | (629) | | | | (11) | | | | (28) | | | | 1,874 | | | |
Discontinued operations(d) | | — | | | | — | | | | — | | | | (11) | | | |
| Certain significant items: | | | | | | | | | | | | | |
Restructuring charges/(credits) and implementation costs and additional depreciation—asset restructuring(e) | | (98) | | | | (290) | | | | — | | | | 2,227 | | | |
Certain asset impairments(f) | | — | | | | — | | | | (3,024) | | | | 3,024 | | | |
(Gains)/losses on equity securities(f) | | — | | | | — | | | | 1,588 | | | | (1,588) | | | |
| Actuarial valuation and other pension and postretirement plan (gains)/losses | | — | | | | — | | | | 265 | | | | (265) | | | |
| Other | | (238) | | (g) | | (24) | | | | (246) | | (h) | | 518 | | | |
| Income tax provision—Non-GAAP items | | | | | | | | (2,131) | | | |
| Non-GAAP Adjusted | | $ | 23,988 | | | | $ | 14,446 | | | | $ | (2,281) | | | | $ | 10,501 | | | $ | 1.84 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2019 |
| IN MILLIONS, EXCEPT PER COMMON SHARE DATA | | GAAP Reported | | Purchase Accounting Adjustments(a) | | Acquisition-Related Costs(a) | | Discontinued Operations(a) | | Certain Significant Items(a) | | Non-GAAP Adjusted |
| Revenues | | $ | 41,172 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 41,172 | |
| Cost of sales | | 8,251 | | | 19 | | | — | | | — | | | (208) | | | 8,062 | |
| Selling, informational and administrative expenses | | 12,750 | | | 2 | | | (2) | | | — | | | (263) | | | 12,488 | |
| Research and development expenses | | 8,394 | | | 4 | | | — | | | — | | | (663) | | | 7,736 | |
| Amortization of intangible assets | | 4,462 | | | (4,191) | | | — | | | — | | | — | | | 271 | |
| Restructuring charges and certain acquisition-related costs | | 601 | | | — | | | (183) | | | — | | | (418) | | | — | |
| (Gain) on completion of Consumer Healthcare JV transaction | | (8,086) | | | — | | | — | | | — | | | 8,086 | | | — | |
| Other (income)/deductions––net | | 3,314 | | | (21) | | | — | | | — | | | (3,563) | | | (270) | |
| Income from continuing operations before provision/(benefit) for taxes on income | | 11,485 | | | 4,186 | | | 185 | | | — | | | (2,971) | | | 12,885 | |
Provision/(benefit) for taxes on income(b) | | 618 | | | 823 | | | 59 | | | — | | | 539 | | | 2,039 | |
| Income from continuing operations | | 10,867 | | | 3,363 | | | 126 | | | — | | | (3,510) | | | 10,846 | |
| Income from discontinued operations––net of tax | | 5,435 | | | — | | | — | | | (5,435) | | | — | | | — | |
| Net income attributable to noncontrolling interests | | 29 | | | — | | | — | | | — | | | — | | | 29 | |
| Net income attributable to Pfizer Inc. common shareholders | | 16,273 | | | 3,363 | | | 126 | | | (5,435) | | | (3,510) | | | 10,817 | |
| Earnings per common share attributable to Pfizer Inc. common shareholders––diluted | | 2.87 | | | 0.59 | | | 0.02 | | | (0.96) | | | (0.62) | | | 1.91 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, 2022 |
Data presented will not (in all cases) aggregate to totals. MILLIONS, EXCEPT PER SHARE DATA | | Cost of sales(a) | | Selling, informational and administrative expenses(a) | | Other (income)/deductions––net(a) | | Net income attributable to Pfizer Inc. common shareholders(a), (b), (c) | | Earnings per common share attributable to Pfizer Inc. common shareholders––diluted |
| GAAP Reported | | $ | 34,344 | | | $ | 13,677 | | | | $ | 217 | | | | $ | 31,372 | | | $ | 5.47 | |
| Amortization of intangible assets | | — | | | — | | | | — | | | | 3,609 | | | |
| Acquisition-related items | | (119) | | | (7) | | | | (74) | | | | 832 | | | |
Discontinued operations(d) | | — | | | — | | | | — | | | | (21) | | | |
| Certain significant items: | | | | | | | | | | | | |
Restructuring charges/(credits) and implementation costs and additional depreciation—asset restructuring(e) | | (88) | | | (562) | | | | — | | | | 1,396 | | | |
Certain asset impairments(f) | | — | | | — | | | | (421) | | | | 421 | | | |
(Gains)/losses on equity securities(f) | | — | | | — | | | | (1,270) | | | | 1,270 | | | |
| Actuarial valuation and other pension and postretirement plan (gains)/losses | | — | | | — | | | | 230 | | | | (230) | | | |
| Other | | (40) | | | (59) | | | | (636) | | (h) | | 752 | | | |
| Income tax provision—Non-GAAP items | | | | | | | | (1,683) | | | |
| Non-GAAP Adjusted | | $ | 34,096 | | | $ | 13,049 | | | | $ | (1,954) | | | | $ | 37,717 | | | $ | 6.58 | |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 4045 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2018 |
| IN MILLIONS, EXCEPT PER COMMON SHARE DATA | | GAAP Reported | | Purchase Accounting Adjustments(a) | | Acquisition-Related Costs(a) | | Discontinued Operations(a) | | Certain Significant Items(a) | | Non-GAAP Adjusted |
| Revenues | | $ | 40,825 | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | 40,825 | |
| Cost of sales | | 8,987 | | | 3 | | | (10) | | | — | | | (105) | | | 8,874 | |
| Selling, informational and administrative expenses | | 12,612 | | | 2 | | | (2) | | | — | | | (191) | | | 12,420 | |
| Research and development expenses | | 7,760 | | | 3 | | | — | | | — | | | (47) | | | 7,716 | |
| Amortization of intangible assets | | 4,736 | | | (4,456) | | | — | | | — | | | — | | | 280 | |
| Restructuring charges and certain acquisition-related costs | | 1,058 | | | — | | | (299) | | | — | | | (759) | | | — | |
| (Gain) on completion of Consumer Healthcare JV transaction | | — | | | — | | | — | | | — | | | — | | | — | |
| Other (income)/deductions––net | | 2,077 | | | (182) | | | (7) | | | — | | | (2,520) | | | (631) | |
| Income from continuing operations before provision/(benefit) for taxes on income | | 3,594 | | | 4,630 | | | 318 | | | — | | | 3,622 | | | 12,164 | |
Provision/(benefit) for taxes on income(b) | | (266) | | | 888 | | | 54 | | | — | | | 1,509 | | | 2,185 | |
| Income from continuing operations | | 3,861 | | | 3,741 | | | 264 | | | — | | | 2,113 | | | 9,979 | |
| Income from discontinued operations––net of tax | | 7,328 | | | — | | | — | | | (7,328) | | | — | | | — | |
| Net income attributable to noncontrolling interests | | 36 | | | — | | | — | | | — | | | — | | | 36 | |
| Net income attributable to Pfizer Inc. common shareholders | | 11,153 | | | 3,741 | | | 264 | | | (7,328) | | | 2,113 | | | 9,944 | |
| Earnings per common share attributable to Pfizer Inc. common shareholders––diluted | | 1.87 | | | 0.63 | | | 0.04 | | | (1.23) | | | 0.35 | | | 1.66 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, 2021 |
Data presented will not (in all cases) aggregate to totals. MILLIONS, EXCEPT PER SHARE DATA | | Cost of sales(a) | | Selling, informational and administrative expenses(a) | | Other (income)/deductions––net(a) | | Net income attributable to Pfizer Inc. common shareholders(a), (b) | | Earnings per common share attributable to Pfizer Inc. common shareholders––diluted |
| GAAP Reported | | $ | 30,821 | | | $ | 12,703 | | | | $ | (4,878) | | | | $ | 21,979 | | | $ | 3.85 | |
| Amortization of intangible assets | | — | | | (38) | | | | (2) | | | | 3,746 | | | |
| Acquisition-related items | | 25 | | | (3) | | | | (114) | | | | 139 | | | |
Discontinued operations(d) | | — | | | — | | | | — | | | | 585 | | | |
| Certain significant items: | | | | | | | | | | | | |
Restructuring charges/(credits) and implementation costs and additional depreciation—asset restructuring(e) | | (108) | | | (450) | | | | — | | | | 1,309 | | | |
| Certain asset impairments | | — | | | — | | | | (86) | | | | 86 | | | |
(Gains)/losses on equity securities(f) | | — | | | — | | | | 1,338 | | | | (1,338) | | | |
| Actuarial valuation and other pension and postretirement plan (gains)/losses | | — | | | — | | | | 1,601 | | | | (1,601) | | | |
| Other | | (52) | | | (141) | | (i) | | (334) | | (h) | | 542 | | | |
| Income tax provision—Non-GAAP items | | | | | | | | (2,250) | | | |
| Non-GAAP Adjusted | | $ | 30,685 | | | $ | 12,071 | | | | $ | (2,475) | | | | $ | 23,196 | | | $ | 4.06 | |
(a)For details of adjustments, see Details of Income Statement Items Included inthat reconcile GAAP Reported but Excludedto non-GAAP Adjusted balances are shown pre-tax. Our effective tax rates for GAAP Reported income from Non-GAAPcontinuing operations were: (105.4)% in 2023, 9.6% in 2022 and 7.6% in 2021. See Note 5. Our effective tax rates for non-GAAP Adjusted Income.income were: 9.0% in 2023, 11.7% in 2022 and 14.5% in 2021. (b)The effective tax rate on Non-GAAP Adjusted income was 13.5% in 2020, 15.8% in 2019Includes reconciling amounts for Research and 18.0% in 2018. The decrease in 2020, compared with 2019, was primarily duedevelopment expenses that are not material to a favorable change in the jurisdictional mixour non-GAAP consolidated results of earnings as a result of operating fluctuations in the normal course of business. The decrease in 2019, compared with 2018, was primarily due to a favorable change in the jurisdictional mix of earnings as a result of operating fluctuations in the normal course of business, partially offset by a decrease in tax benefits for the resolution of certain tax positions, principally non-U.S., pertaining to prior years.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 41 |
Details of Income Statement Items Included in GAAP Reported but Excluded from Non-GAAP Adjusted Income
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 |
| Purchase accounting adjustments | | | | | | |
Amortization, depreciation and other(a) | | $ | 3,224 | | | $ | 4,205 | | | $ | 4,633 | |
| Cost of sales | | (18) | | | (19) | | | (3) | |
| Total purchase accounting adjustments—pre-tax | | 3,206 | | | 4,186 | | | 4,630 | |
Income taxes(b) | | (668) | | | (823) | | | (888) | |
| Total purchase accounting adjustments—net of tax | | 2,537 | | | 3,363 | | | 3,741 | |
| | | | | | |
| Acquisition-related items | | | | | | |
Restructuring charges/(credits)(c) | | — | | | (192) | | | 37 | |
Transaction costs(c) | | 10 | | | 63 | | | 1 | |
Integration costs and other(c) | | 34 | | | 311 | | | 260 | |
Net periodic benefit costs/(credits) other than service costs(d) | | — | | | — | | | 7 | |
Additional depreciation—asset restructuring(e) | | — | | | 3 | | | 12 | |
| Total acquisition-related items—pre-tax | | 44 | | | 185 | | | 318 | |
Income taxes(f) | | (9) | | | (59) | | | (54) | |
| Total acquisition-related items—net of tax | | 35 | | | 126 | | | 264 | |
| | | | | | |
| Discontinued operations | | | | | | |
| | | | | | |
| | | | | | |
Income from discontinued operations—net of tax(g) | | (2,631) | | | (5,435) | | | (7,328) | |
| | | | | | |
| Certain significant items | | | | | | |
Restructuring charges/(credits)––cost reduction initiatives(h) | | 556 | | | 418 | | | 759 | |
Implementation costs and additional depreciation—asset restructuring(i) | | 257 | | | 192 | | | 212 | |
Net (gains)/losses on asset disposals(d) | | 238 | | | — | | | — | |
Net (gains)/losses recognized during the period on equity securities(d) | | (557) | | | (415) | | | (586) | |
Certain legal matters, net(d) | | 24 | | | 291 | | | 84 | |
Certain asset impairments(d) | | 1,691 | | | 2,798 | | | 3,101 | |
Business and legal entity alignment costs(j) | | 270 | | | 412 | | | 63 | |
(Gain) on completion of Consumer Healthcare JV transaction(k) | | (6) | | | (8,086) | | | — | |
Other(l) | | 1,278 | | | 1,418 | | | (10) | |
| Total certain significant items—pre-tax | | 3,752 | | | (2,971) | | | 3,622 | |
Income taxes(m) | | (803) | | | (539) | | | (1,509) | |
| Total certain significant items—net of tax | | 2,948 | | | (3,510) | | | 2,113 | |
| Total purchase accounting adjustments, acquisition-related items, discontinued operations and certain significant items—net of tax, attributable to Pfizer Inc. | | $ | 2,890 | | | $ | (5,455) | | | $ | (1,209) | |
(a)Included primarily in Amortization of intangible assets.
(b)Included in Provision/(benefit) for taxes on income. Includes the tax effect of the associated pre-tax amounts, calculated by determining the jurisdictional location of the pre-tax amounts and applying that applicable tax rate.operations.
(c)Included inFor 2023, the total acquisition-related items of $1.9 billion include reconciling amounts for Restructuring charges and certain acquisition-related costs.of $1.2 billion, mainly composed of $785 million of integration costs and other charges, $190 million of transaction costs and $125 million of employee termination-related charges. For 2022, the total acquisition-related items of $832 million included reconciling amounts for Restructuring charges and certain acquisition-related costs of $631 million, composed of $348 million of integration costs and other charges, $144 million of transaction costs and $138 million of employee termination-related charges. See Note 3.3. (d)Included in Other (income)/deductions—net. See Note 42B. (e)In 2019, primarily included in Selling, informational and administrative expenses. In 2018, primarily included in Cost of sales. Represents the impact of changes in the estimated useful lives of assets involved in restructuring actions related to acquisitions.
(f)Included in Provision/(benefit) for taxes on income. Income taxes includes the tax effect of the associated pre-tax amounts, calculated by determining the jurisdictional location of the pre-tax amounts and applying the applicable tax rate. 2019 includes the impact of the non-taxable reversal of certain accruals related to our acquisition of Wyeth upon the effective favorable settlement of a U.S. IRS audit for multiple tax years.
(g)Included in Income from discontinued operations––net of tax and relates to the November 16, 2020 spin-off and combination of our Upjohn Business with Mylan. See Note 2B.
(h)Amounts relate toIncludes employee termination costs, asset impairments and other exit costs related to our cost-reduction and productivity initiatives not associated with acquisitions,acquisitions. See Note 3.(g)For 2023, the total of $238 million mainly includes $286 million in inventory losses, overhead costs related to the period in which the facility could not operate, and incremental costs resulting from tornado damage to our manufacturing facility in Rocky Mount, NC, partially offset by insurance recoveries.
(h)For 2023, the total of $246 million includes charges of (i) $474 million for certain legal matters, primarily representing certain product liability and other legal expenses related to products discontinued and/or divested by Pfizer, and to a lesser extent, legal obligations related to pre-acquisition matters, and (ii) $127 million mostly related to our equity-method accounting pro-rata share of intangible asset amortization and impairments, costs of separating from GSK and restructuring costs recorded by Haleon, partially offset by: (i) a $222 million gain on the divestiture of our early-stage rare disease gene therapy portfolio to Alexion, and (ii) dividend income of $211 million related to our investment in Nimbus resulting from Takeda’s acquisition of Nimbus’s oral, selective allosteric tyrosine kinase 2 (TYK2) inhibitor program subsidiary. For 2022, the total of $636 million included charges of (i) $307 million mostly representing our equity-method accounting pro rata share of restructuring charges and costs of separating from GSK recorded by Haleon/the Consumer Healthcare JV, and adjustments to our equity-method basis differences which are also related to the separation of Haleon/the Consumer Healthcare JV from GSK, and (ii) $230 million for certain legal matters, primarily representing certain product liability and other legal expenses related to products discontinued and/or divested by Pfizer. For 2021, the total of $334 million included in Restructuringcharges of (i) $185 million mostly representing our equity-method accounting pro rata share of restructuring charges and costs of separating from GSK recorded by the Consumer Healthcare JV, and (ii) $162 million for certain acquisition-related costslegal matters, primarily for c (see Note 3)ertain product liability expenses related to products discontinued and/or divested by Pfizer, and to a lesser extent, legal obligations related to pre-acquisition matters.
(i)Amounts relate to our cost-reduction/productivity initiatives not related to acquisitions (see Note 3). For 2020,2021, the total of $141 million primarily included in Cost of sales ($62 million) and Selling, informational and administrative expenses ($197 million). For 2019, included in Cost of sales ($89 million), Selling, informational and administrative expenses ($73 million) and Research and development expenses ($30 million). For 2018, included in Cost of sales ($101 million), Selling, informational and administrative expenses ($71 million) and Research and development expenses ($39 million).
(j)In 2020, included in Cost of sales ($51 million), Selling, informational and administrative expenses ($206 million) and Research and development expenses ($13 million) and primarily represents costs for consulting, legal, tax and advisory services associated with a non-recurring internal reorganization of legal entities. In 2019, primarily included in Cost of sales ($15 million), Selling, informational and administrative expenses ($96 million) and Other (income)/deductions––net ($300 million) and in 2018, included in Other (income)/deductions––net and represents costs for consulting, legal, tax and other advisory services associated with the design, planning and implementation of our then new business structure, effective in the beginning of 2019.
(k)Included in (Gain) on completion of Consumer Healthcare JV transaction (see Note 2C).
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 42 |
(l)For 2020, primarily included in Selling, informational and administrative expenses ($86 million), Research and development expenses ($515 million)and Other (income)/deductions––net ($672 million). For 2019, included in Cost of sales ($104 million), Selling, informational and administrative expenses ($94 million), Research and development expenses ($632 million) and Other (income)/deductions––net ($589 million). For 2018, primarily included in Selling, informational and administrative expenses ($120 million) and Other (income)/deductions––net ($142 million income). 2020 includes the following charges recorded in Research and development expenses: (i) $151 million, representing the expense portion of our upfront payment to Myovant, (ii) an upfront payment of $130 million to Valneva, (iii) a $75 million milestone payment to Akcea, (iv) a $72 million upfront payment to BioNTech and (v) a $50 million milestone payment to Therachon. 2020 also includes, among other things, the following charges recorded in Other (income)/deductions––net: (i) charges of $367 million,primarilyrepresenting our pro rata share of restructuring and business combination accounting charges recorded by the Consumer Healthcare JV, partially offset by gains from the divestiture of certain of the JV’s brands recorded by the Consumer Healthcare JV, and our write-off and amortization of equity method basis differences primarily related to those brand divestitures and to inventory, and (ii) $198 million of settlement losses within the U.S. PCPP. 2019 included, among other things, (i) a $337 million charge in Research and development expenses related to our acquisition of Therachon, (ii) an upfront license fee payment of $250 million to Akcea, recorded in Research and development expenses, (iii) charges of $240 million, primarily in Selling, informational and administrative expenses ($87 million) and Other (income)/deductions––net ($152 million), for external incremental costs, such as transaction costs and costs to separate our Consumer Healthcare business into a separate legal entity associated with the formation of the Consumer Healthcare JV, (iv) net losses on early retirement of debt of $138 million in Other (income)/deductions––net, (v)charges of $112 million recorded in Other (income)/deductions––net representing our pro rata share of primarily restructuring and business combination accounting charges recorded by the Consumer Healthcare JV and (vi) a $99 million charge in Cost of sales related to rivipansel, primarily for inventory manufactured for expected future sale. For 2018, included, among other things, (i) a non-cash $343 million pre-tax gain in Other (income)/deductions––net associated with our transaction with Bain Capital to create a new biopharmaceutical company, Cerevel, to continue development of a portfolio of clinical and preclinical stage neuroscience assets primarily targeting disorders of the central nervous system, (ii) an $88 million charge, in the aggregate, in Selling, informational and administrative expenses for a special, one-time bonus paid to virtually all Pfizer colleagues, excluding executives, which was one of several actions taken by us after evaluating the expected positive net impact of the December 2017 enactment of the TCJA and (iii) a non-cash $50 million pre-tax gain in Other (income)/deductions––net as a result of the contribution of our allogeneic CAR T therapy development program assets in connection with our contribution agreement entered into with Allogene (see Note 2B).
(m)Included in Provision/(benefit) for taxes on income. Income taxes includes the tax effect of the associated pre-tax amounts, calculated by determining the jurisdictional location of the pre-tax amounts and applying the applicable tax rate. The amount in 2020 was favorably impacted by tax benefits associated with intangible asset impairment charges (see Note 4). The amount in 2019 was favorably impacted by a benefit of $1.4 billion, representing tax and interest, resulting from the favorable settlement of a U.S. IRS audit for multiple tax years, the benefits related to certain tax initiatives for the implementation of our then new business structure, as well as the tax benefit recorded as a result of additional guidance issued by the U.S. Department of Treasury related to the TCJA and unfavorably impacted by the tax expense of approximately $2.7 billion associated with the gain related to the completion of the Consumer Healthcare JV transaction. The amount in 2018 was favorably impacted primarily by tax benefits related to the TCJA, including certain 2018 tax initiatives as well as adjustments to the provisional estimate of the legislation, reported and disclosed within the applicable measurement period, in accordance with guidance issued by the SEC.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 4346 |
ANALYSIS OF THE CONSOLIDATED STATEMENTS OF CASH FLOWS
For a discussion of the drivers of change for 2022 versus 2021 as well as cash flows from discontinued operations in 2021, see the Analysis of the Consolidated Statements of Cash Flows sectionwithin MD&A in our 2022 Form 10-K.
Cash Flows from Continuing Operations
| | | | | Year Ended December 31, | |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 | | Drivers of change |
| (MILLIONS) | |
| (MILLIONS) | |
| (MILLIONS) | | | 2023 | | 2022 | | 2021 | | Drivers of change 2023 v. 2022 |
| Cash provided by/(used in): | Cash provided by/(used in): | | | | | | | | |
| Operating activities from continuing operations | |
| Operating activities from continuing operations | |
| Operating activities from continuing operations | Operating activities from continuing operations | | $ | 10,586 | | | $ | 7,011 | | | $ | 8,875 | | | 2020 v. 2019 The change is driven mainly by higher net income adjusted for non-cash items, advanced payments in 2020 for BNT162b2 recorded in deferred revenue, the upfront cash payment associated with our acquisition of Therachon in 2019, and the upfront cash payment associated with our licensing agreement with Akcea in 2019, partially offset by an increase in benefit plan contributions. The change also reflects the impact of timing of receipts and payments in the ordinary course of business. The change in Other adjustments, net is driven primarily by an increase in equity method dividends received, partially offset by an increase in equity income and increases in net unrealized gains on equity securities. 2019 v. 2018 The change is driven mostly by the upfront cash payments in 2019 associated with our acquisition of Therachon and our licensing agreement with Akcea, partially offset by a decrease in benefit plan contributions. The change also reflects the impact of timing of receipts and payments in the ordinary course of business. The change in Other adjustments, net is driven primarily by a non-cash gain in 2018 associated with our transaction with Bain Capital to create a new biopharmaceutical company, Cerevel, and a non-cash gain in 2018 on the contribution of Pfizer’s allogeneic CAR T developmental program assets, partially offset by net gains on foreign exchange hedging of our intercompany inventory sales. | | $ | 8,700 | | | $ | | $ | 29,267 | | | $ | | $ | 32,922 | | | The change was driven primarily by a decrease in net income adjusted for non-cash items and the timing of receipts and payments in the ordinary course of business, partially offset by net changes in inventory greater than one year (see Note 8A). | | The change was driven primarily by a decrease in net income adjusted for non-cash items and the timing of receipts and payments in the ordinary course of business, partially offset by net changes in inventory greater than one year (see Note 8A). |
| Investing activities from continuing operations | Investing activities from continuing operations | | $ | (4,188) | | | $ | (3,852) | | | $ | 4,584 | | | 2020 v. 2019 The change is driven mostly by a $6.0 billion decrease in net proceeds from short-term investments with original maturities of three months or less and $2.7 billion in net purchases of short-term investments with original maturities of greater than three months in 2020 (compared to $2.3 billion net proceeds from short-term investments with original maturities of greater than three months in 2019), partially offset by the cash used to acquire Array, net of cash acquired, of $10.9 billion in 2019. 2019 v. 2018 The change is driven primarily by cash used for the acquisition of Array, net of cash acquired, of $10.9 billion in 2019, partially offset by an increase in net proceeds generated from the sale of investments of $2.9 billion for cash needs, including financing the acquisition of Array. | Investing activities from continuing operations | | $ | (32,278) | | | $ | | $ | (15,783) | | | $ | | $ | (22,534) | | | The change was driven mainly by $43.4 billion cash paid in 2023 for the acquisition of Seagen, net of cash acquired, compared with $23.0 billion cash paid in 2022 for acquisitions (Biohaven, $11.5 billion, Arena, $6.2 billion and GBT, $5.2 billion), net of cash acquired (see Note 2A), as well as a $4.0 billion dividend received from the Consumer Healthcare JV in 2022 that was allocated to investing activities (see Note 2C), partially offset by a $5.5 billion increase in net redemptions of short-term investments in 2023 and a $1.7 billion decrease in purchases of long-term investments. | | The change was driven mainly by $43.4 billion cash paid in 2023 for the acquisition of Seagen, net of cash acquired, compared with $23.0 billion cash paid in 2022 for acquisitions (Biohaven, $11.5 billion, Arena, $6.2 billion and GBT, $5.2 billion), net of cash acquired (see Note 2A), as well as a $4.0 billion dividend received from the Consumer Healthcare JV in 2022 that was allocated to investing activities (see Note 2C), partially offset by a $5.5 billion increase in net redemptions of short-term investments in 2023 and a $1.7 billion decrease in purchases of long-term investments. |
| Financing activities from continuing operations | Financing activities from continuing operations | | $ | (21,640) | | | $ | (8,485) | | | $ | (20,441) | | | 2020 v. 2019 The change is driven primarily by $14.0 billion net payments on short-term borrowings in 2020 (compared to $10.6 billion net proceeds raised from short-term borrowings in 2019) and an increase in cash dividends paid of $397 million, partially offset by a decrease in purchases of common stock of $8.9 billion, lower repayments on long-term debt of $2.8 billion, and an increase in issuances of long-term debt of $280 million. 2019 v. 2018 The change is driven mostly by $10.6 billion of net proceeds raised from short-term borrowings in 2019, primarily in connection with the acquisition of Array (compared to net payments on short-term borrowings of $2.3 billion in 2018) and lower purchases of common stock of $3.3 billion, partially offset by higher repayments on long-term debt of $3.2 billion and lower proceeds from the exercise of stock options of $864 million. | Financing activities from continuing operations | | $ | 26,066 | | | $ | | $ | (14,834) | | | $ | | $ | (9,816) | | | The change was driven mostly by $30.8 billion of proceeds from the issuance of long-term debt in May of 2023 and a $7.9 billion increase in net proceeds from the issuance of short-term borrowings. | | The change was driven mostly by $30.8 billion of proceeds from the issuance of long-term debt in May of 2023 and a $7.9 billion increase in net proceeds from the issuance of short-term borrowings. |
|
Cash Flows from Discontinued Operations
Cash flows from discontinued operations relate to the Upjohn Business (see Note 2B).In 2020, net cash provided by financing activities from discontinued operations primarily reflects issuances of long-term debt.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 44 |
ANALYSIS OF FINANCIAL CONDITION, LIQUIDITY, CAPITAL RESOURCES AND MARKET RISK
We rely largely onOur historically robust operating cash flows, short-term investments or commercial paper borrowings and long-term debtflow, which we expect to provide forcontinue over time, is a key strength of our liquidity requirements.and capital resources and our primary funding source. We continue our efforts to improve cash inflows through working capital efficiencies. We target specific areasbelieve as a result of focus including accounts receivable, inventories, accounts payable, and other working capital, which allows us to optimize our operating cash flows.
Due to our significant operating cash flows as well asthis, together with our financial assets, access to capital markets, revolving credit agreements, and available lines of credit, and revolving credit agreements, we believe that we have and will maintain the ability to meet our liquidity needs to support ongoing operations, our capital allocation objectives, and our contractual and other obligations for the foreseeable future, which can include, among others:future.
•theWe focus efforts to optimize operating cash flows through achieving working capital requirementsefficiencies that target accounts receivable, inventories, accounts payable, and other working capital. Excess cash from operating cash flows is invested in money market funds and available-for-sale debt securities which consist of our operations, including our R&D activities;
•investments in our business;
•dividend payments and potential increases in the dividend rate;
•share repurchases;
•the cash requirements for our cost-reduction/productivity initiatives;
•paying down outstanding debt;
•contributions to our pension and postretirement plans; and
•business development activities.
Our long-termprimarily high-quality, highly liquid, well-diversified debt is rated high-quality by both S&P and Moody’s. See the Credit Ratings section below.securities. We have taken, and will continue to take, a conservative approach to our financial investments and monitoring of our liquidity position in response to market changes. Our debt investments consist primarily of high-quality, highly liquid, well-diversified available-for-sale debt securities.
Debt Capacity—Lines of Credit
We have available lines of credit and revolving credit agreements with a group of banks and other financial intermediaries. We typically maintain cash and cash equivalent balances and short-term investments which, together with our available revolving credit facilities, are in excess of our commercial paper and other short-term borrowings. See Note 7C.
Selected Measures of Liquidity and Capital Resources
| | | | | | | | | | | | | | |
| The following presents certain relevant measures of our liquidity and capital resources: |
| | As of December 31, |
| (MILLIONS OF DOLLARS, EXCEPT RATIOS) | | 2020 | | 2019 |
Selected financial assets(a): | | | | |
| Cash and cash equivalents | | $ | 1,784 | | | $ | 1,121 | |
| Short-term investments | | 10,437 | | | 8,525 | |
| Long-term investments, excluding private equity securities at cost | | 2,973 | | | 2,258 | |
| | | 15,195 | | | 11,905 | |
| Debt: | | | | |
| Short-term borrowings, including current portion of long-term debt | | 2,703 | | | 16,195 | |
| Long-term debt | | 37,133 | | | 35,955 | |
| | | 39,835 | | | 52,150 | |
| Selected net financial liabilities | | $ | (24,641) | | | $ | (40,245) | |
| | | | |
Working capital(b) | | $ | 9,147 | | | $ | (4,501) | |
| Ratio of current assets to current liabilities | | 1.35:1 | | 0.88:1 |
| | | | |
(a)See Note 7 for a description of certain assets held and for a description of credit risk related toAdditionally, we may obtain funding through short-term or long-term sources from our financial instruments held.
(b)The increase in working capital was primarily driven by the use of Upjohn cash distribution proceeds to pay down short-term commercial paper borrowings. See Note 2B.
On November 16, 2020, we received $12.0 billion as partial consideration for the contribution of the Upjohn Business to Viatris (see Note 2B). In November 2020, we used the cash proceeds to pay down commercial paper and redeem, before the maturity date, the $1.15 billion aggregate principal amount outstanding of 1.95% senior unsecured notes that were due in June 2021 and $342 million aggregate principal amount of 5.80% senior unsecured notes that were due in August 2023.
In May 2020, we completed a public offering of $4.0 billion aggregate principal amount of senior unsecured notes.
In March 2020, we:
•completed a public offering of $1.25 billion aggregate principal amount of senior unsecured sustainability notes. The proceeds were initially used to repay outstanding commercial paper and subsequently will be used to help manage our environmental impact and support increased patient access to the capital markets, banking relationships and relationships with other financial intermediaries to meet our medicines and vaccines, especially among underserved populations, and strengthen healthcare systems; andliquidity needs.
•repurchased at par all $1.065 billion principal amount outstanding of senior unsecured notes that were due in 2047 before the maturity date.
For additional information about these issuances and retirements, see Note 7D. | | | | | | | | |
| Diverse sources of funds: | | Related disclosure presented in this Form 10-K |
| Internal sources: | | |
•Operating cash flows | | |
•Cash and cash equivalents | | |
•Money market funds | | |
•Available-for-sale debt securities | | |
•Equity investments | | |
| | |
| External sources: | | |
| Short-term funding: | | |
•Commercial paper | | |
•Revolving credit facilities | | |
•Lines of credit | | |
| | |
| Long-term funding: | | |
•Long-term debt | | |
•Equity | | |
Financing for Seagen Acquisition––As part of the financing for our acquisition of Seagen, we issued $31 billion of long-term debt in May 2023 and $8 billion of commercial paper in the fourth quarter of 2023. The net proceeds from long-term debt were invested in short-term investments in a combination of money market funds and available-for-sale debt securities until the completion of the acquisition.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 4547 |
Credit Ratings
Two major corporate debt-rating organizations,––The cost and availability of financing are influenced by credit ratings, and an increase or decrease in our credit rating could have a beneficial or adverse effect on financing. Our long-term debt is rated high-quality by both S&P and Moody’s. In March 2023, following the announcement of the proposed acquisition of Seagen, Moody’s andchanged its outlook on our long-term debt to Negative; S&P assign ratings todowngraded our short-term rating from A-1+ to A-1. In October 2023, following the announcement of the amended Paxlovid supply agreement with the U.S. government and updated 2023 guidance, S&P changed its outlook on our long-term debt.debt to Negative. In December 2023, following the release of 2024 guidance (i) Moody’s downgraded our long-term rating from A1 to A2 and changed its outlook on our long-term debt to Stable and (ii) S&P downgraded our long-term rating from A+ to A security rating isand changed its outlook on our long-term debt to Stable.
| | | | | | | | | | | | | | | | | | | | |
| As of the date of the filing of this Form 10-K, the following ratings have been assigned to our commercial paper and senior unsecured long-term debt: |
| NAME OF RATING AGENCY | | Pfizer Short-Term Rating | | Pfizer Long-Term Rating | | Outlook/Watch |
| Moody’s | | P-1 | | A2 | | Stable Outlook |
| S&P | | A-1 | | A | | Stable Outlook |
These ratings are not
a recommendation to buy, sell or hold securities and the rating isratings are subject to revision or withdrawal at any time by the rating organization. Each
rating should be evaluated independently of any other rating.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
The current ratings assigned to our commercial paper and senior unsecured long-term debt: |
NAME OF RATING AGENCY | | Pfizer
Short-Term | | Pfizer
Long-Term | | Outlook/Watch | | Date of Last Rating Change |
| Rating | | Rating | | |
Moody’s | | P-1 | | A2 | | Stable | | November 2020 |
S&P | | A-1+ | | A+ | | Stable | | November 2020 |
Both Moody’sCapital Allocation Framework––Our capital allocation framework is primarily devised to enhance shareholder value and S&P lowered Pfizer’s long-term debt rating one notch to ‘A2’is based on three core pillars: growing our dividend, reinvesting in the business and ‘A+’, respectively, upon completion of the Upjohn separation in November 2020. Pfizer’s short-term rating remained unchanged. Additionally, both rating agencies removed Pfizer’s long-term debt rating from “under review” and assigned a stable outlook.
LIBOR
Global Economic Conditions
Our Venezuela and Argentina operations function in hyperinflationary economies. The impact to Pfizer is not considered material. For additional information on the global economic environment, see the Item 1A. Risk Factors––Global Operations section in this Form 10-K.
Market Risk
The objective of our financial risk management program is to minimize the impact of foreign exchange rate and interest rate movements on our earnings. We address these exposures through a combination of operational means and financial instruments. We adapt our practices periodically as economic conditions change. For more information, see Notes 1F and 7E, as well as the Item 1A. Risk Factors—Global Operations section in this Form 10-K for key currencies in which we operate.
Foreign Exchange Risk—We are subject to foreign exchange risk in our commercial operations, assets and liabilities that are denominated in foreign currencies and our net investments in foreign subsidiaries.
On the commercial side, a significant portion of our revenues and earnings is exposed to changes in exchange rates. Where foreign exchange risk is not offset by other exposures, we may use foreign currency forward-exchange contracts and/or foreign currency swaps to manage that risk.
With respect to our financial assets and liabilities, our primary foreign exchange exposure arises from intercompany receivables and payables, and, to a lesser extent, from investments and debt denominated in currencies other than the functional currency of the business entity.
In addition, under certain market conditions, we may seek to protect against possible declines in the reported net investments of our foreign business entities. In these cases, we may use foreign exchange contracts and/or foreign currency debt.
The fair values of our financial instrument holdings are analyzed at year-end to determine their sensitivity to foreign exchange rate changes. In this analysis, holding all other assumptions constant and assuming that a change in one currency’s rate relative to the U.S. dollar would not have any effect on another currency’s rates relative to the U.S. dollar, if the dollar were to appreciate against all other currencies by 10%, as of December 31, 2020, the expected adverse impact on our net income would not be significant.
Interest Rate Risk—Our interest-bearing investments and borrowings are subject to interest rate risk which may have an impact on net income. Depending on market conditions, we may change the profile of our outstanding debt or investments by entering into derivative financial instruments like interest rate swaps, either to hedge or offset the exposure to changes in the fair value of hedged items with fixed interest rates, or to convert variable rate debt (or investments) to fixed rates.
The fair values of our financial instrument holdings are analyzed at year-end to determine their sensitivity to interest rate changes. In this analysis, holding all other assumptions constant and assuming a parallel shift in the interest rate curve for all maturities and for all instruments, if there were a one hundred basis point decrease in interest rates as of December 31, 2020, the expected adverse impact on our net income would not be significant.
Equity Price Risk––We hold equity securities with readily determinable fair values in life science companies as a result of certain business development transactions. While we are holding such securities, we are subject to equity price risk, and this may increase the volatility of our income in future periods due to changes in the fair value of equity investments. From time to time, we will sell such equity securities based on our business considerations, which may include limiting our price risk.
Our equity securities with readily determinable fair values are analyzed at year-end to determine their sensitivity to equity price rate changes. In this sensitivity analysis, the expected adverse impact on our net income would not be significant.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 46 |
Contractual Obligations
Payments due under contractual obligations as of December 31, 2020, mature as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | Years | | |
| (MILLIONS OF DOLLARS) | | Total | | 2021 | | 2022- 2023 | | 2024- 2025 | | There-after | | |
Long-term debt, including current portion(a) | | $ | 39,135 | | | $ | 2,002 | | | $ | 4,346 | | | $ | 3,068 | | | $ | 29,719 | | | Consists of senior unsecured notes (including fixed and floating rate, foreign currency denominated, and other notes). Commitments under financing leases are not significant. |
Interest payments on long-term debt obligations(a) | | 21,122 | | | 1,390 | | | 2,746 | | | 2,455 | | | 14,530 | | | Incorporates only current period assumptions for interest rates, foreign currency translation rates and hedging strategies, and assumes that interest is accrued through the maturity date or expiration of the related instrument. |
Other long-term liabilities(b) | | 2,070 | | | 383 | | | 451 | | | 381 | | | 855 | | | Includes expected payments relating to our unfunded U.S. supplemental (non-qualified) pension plans, postretirement plans and deferred compensation plans. Excludes amounts relating to our U.S. qualified pension plans and international pension plans, all of which have a substantial amount of plan assets, because the required funding obligations are not expected to be material and/or because such liabilities do not necessarily reflect future cash payments, as the impact of changes in economic conditions on the fair value of the pension plan assets and/or liabilities can be significant. Also, excludes $4.2 billion of liabilities related to the fair value of derivative financial instruments, legal matters and employee terminations, among other liabilities, most of which do not represent contractual obligations. |
Operating leases(c) | | 3,312 | | | 357 | | | 638 | | | 460 | | | 1,856 | | | Includes future minimum rental commitments under non-cancelable operating leases, including an agreement to lease space in an office building in New York City. |
Purchase obligations and other(d) | | 3,793 | | | 847 | | | 1,470 | | | 933 | | | 543 | | | Includes agreements to purchase goods and services that are enforceable and legally binding and includes amounts relating to advertising, information technology services, employee benefit administration services, and potential milestone payments deemed reasonably likely to occur. |
Other taxes payable—deemed repatriated accumulated post-1986 earnings of foreign subsidiaries(e) | | 9,000 | | | 700 | | | 1,700 | | | 3,700 | | | 2,900 | | | Represents estimated cash payments related to the TCJA repatriation tax liability. |
Uncertain tax positions(e) | | 42 | | | 42 | | | — | | | — | | | — | | | Includes only income tax amounts currently payable. We are unable to predict the timing of tax settlements related to our noncurrent obligations for uncertain tax positions as tax audits can involve complex issues and the resolution of those issues may span multiple years, particularly if subject to negotiation or litigation. |
(a)See Note 7.
(b)See Notes 3, 7A,11E and16.
(c)See Note 15.
(d)Also includes obligations to make guaranteed fixed annual payments over the next six years in connection with the U.S. and EU approvals for Besponsa ($401 million) and an obligation to make guaranteed fixed annual payments over the next seven years for Bosulif ($195 million), both associated with R&D arrangements.
(e)See Note 5.
The above table includes amounts for potential milestone payments under collaboration, licensing or other arrangements, if the payments are deemed reasonably likely to occur. Payments under these agreements generally become due and payable only upon the achievement of certain development, regulatory and/or commercialization milestones, which may span several years and which may never occur.
In 2021, we expect to spend approximately $3.0 billion on property, plant and equipment. We rely largely on operating cash flows to fund our capital investment needs.
Off-Balance Sheet Arrangements
In the ordinary course of business and in connection with the sale of assets and businesses and other transactions, we often indemnify our counterparties against certain liabilities that may arise in connection with the transaction or that are related to events and activities. For more information on guarantees and indemnifications, see Note 16B.
Additionally, certain of our co-promotion or license agreements give our licensors or partners the rights to negotiate for, or in some cases to obtain under certain financial conditions, co-promotion or other rights in specified countries with respect to certain of our products.
Share-Purchase Plans and Accelerated Share Repurchase Agreements
See Note 12 for information on the shares of our common stock purchased and the cost of purchases under our publicly announced share-purchase plans, including our accelerated share repurchase agreements. At December 31, 2020, our remaining share-purchase authorization was approximately $5.3 billion.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 47 |
Dividends on Common Stock
In December 2020, our BOD declared a first-quarter dividend of $0.39 per share, payable on March 5, 2021, to shareholders of record at the close of business on January 29, 2021. The first-quarter 2021 cash dividend will be our 329th consecutive quarterly dividend.
Our current and projected dividends provide a return to shareholders while maintaining sufficient capital to invest in growing our business. Our dividends are not restricted by debt covenants. While the dividend level remains a decision of Pfizer’s BOD and will continue to be evaluated in the context of future business performance, we currently believe that we can support future annual dividend increases, barring significant unforeseen events. ViatrisOn December 14, 2023, our BOD declared a first-quarter dividend of $0.42 per share, payable on March 1, 2024, to shareholders of record at the close of business on January 26, 2024. The first-quarter 2024 cash dividend will be our 341st consecutive quarterly dividend.
As of December 31, 2023, our remaining share-purchase authorization was approximately $3.3 billion.
Off-Balance Sheet Arrangements, Contractual, and Other Obligations––In the ordinary course of business, (i) we enter into off-balance sheet arrangements that may result in contractual and other obligations and (ii) in connection with the sale of assets and businesses and other transactions, we often indemnify our counterparties against certain liabilities that may arise in connection with the transaction or that are related to events and activities. For more information on guarantees and indemnifications, see Note 16B. Additionally, certain of our co-promotion or license agreements give our licensors or partners the rights to negotiate for, or in some cases to obtain under certain financial conditions, co-promotion or other rights in specified countries with respect to certain of our products. Furthermore, collaboration, licensing or other R&D arrangements may give rise to potential milestone payments. Payments under these agreements generally become due and payable only upon the achievement of certain development, regulatory and/or commercialization milestones, which may span several years and which may never occur.
Our significant contractual and other obligations as of December 31, 2023 consisted of:
•Long-term debt, including current portion (see Note 7D) and related interest payments; •Estimated cash payments related to the TCJA repatriation estimated tax liability (see Note 5). Estimated future payments related to the TCJA repatriation tax liability that will occur after December 31, 2023 total $6.0 billion, of which an estimated $1.5 billion is expected to begin paying a quarterly dividendbe paid in the second quarter of 2021, at which time Pfizer’s quarterly dividendnext twelve months and an estimated $4.5 billion is expected to be reducedpaid in periods thereafter. Our obligations may vary as a result of changes in our uncertain tax positions and/or availability of attributes such thatas foreign tax and other credit carryforwards; •Certain commitments totaling $5.2 billion, of which an estimated $1.3 billion is to be paid in the combined dividend dollar amount received bynext twelve months, and $3.9 billion in periods thereafter (seeNote 16C); •Purchases of PP&E (seeNote 9). In 2024, we expect to spend approximately $3.7 billion on PP&E; and •Future minimum rental commitments under non-cancelable operating leases (see Note 15). Global Economic Conditions––Venezuela, Argentina and Turkey operations function in a hyperinflationary economy. The impact to Pfizer shareholders, based uponis not considered material. See theItem 1A. Risk Factors––Global Operations section. Market Risk––We are subject to foreign exchange risk, interest rate risk, and equity price risk. The objective of our financial risk management program is to minimize the impact of foreign exchange rate and interest rate movements on our earnings. We address such exposures through a combination of continued Pfizer ownershipoperational means and approximately 0.124079 sharesfinancial instruments. For more information on how we manage our foreign exchange and interest rate risks, see Notes 1F and 7E, as well as the Item 1A. Risk Factors—Global Operationssection for key currencies in which we operate. Our sensitivity analyses of Viatris common stock whichsuch risks are discussed below. Foreign Exchange Risk—The fair values of our financial instrument holdings are analyzed at year-end to determine their sensitivity to foreign exchange rate changes. In this analysis, holding all other assumptions constant and assuming that a change in one currency’s rate relative to the U.S. dollar would not have any effect on another currency’s rates relative to the U.S. dollar, if the dollar were granted for each Pfizer shareto move against all other currencies by 10%, as of December 31, 2023, the expected impact on our net income would not be significant.
Interest Rate Risk—The fair values of our financial instrument holdings are analyzed at year-end to determine their sensitivity to interest rate changes. In this analysis, holding all other assumptions constant and assuming a parallel shift in the spin-off,interest rate curve for all maturities and for all instruments, if there were a one hundred basis point change in interest rates as of December 31, 2023, the expected impact on our net income would not be significant.
Equity Price Risk––We hold long-term investments in equity securities with readily determinable fair values in life science companies as a result of certain business development transactions (see Note 7B). While we are holding such securities, we are subject to equity price risk, and this may increase the volatility of our income in future periods due to changes in the fair value of equity investments. From time to time, we will equatesell
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 48 |
such equity securities based on our business considerations, which may include limiting our price risk. Our equity securities with readily determinable fair values are analyzed at year-end to Pfizer’s dividend amount in effect immediately priordetermine their sensitivity to equity price rate changes. In this sensitivity analysis, the initiation of the Viatris dividend.expected impact on our net income would not be significant.
NEW ACCOUNTING STANDARDS
Recently Adopted Accounting Standards
| | | | | | | | | | | | | | |
Recently Issued Accounting Standards, Not Adopted as of December 31, 20202023 |
| Standard/Description | | Effective Date | | Effect on the Financial Statements |
Accounting for income taxesIn June 2022, the FASB issued final guidance to clarify that a contractual restriction on the sale of an equity security eliminates certain exceptions tois not considered part of the guidance, related tounit of account of the approach for intraperiod tax allocation, the methodology for calculating income taxes in an interim periodequity security and, the recognitiontherefore, is not considered when measuring fair value. Recognizing a contractual sale restriction as a separate unit of deferred tax liabilities for outside basis differences. account is not permitted.
| | January 1, 2024, with early adoption permitted. | | The new guidance also simplifies aspects of the accounting for franchise taxesis consistent with our current policy, and enacted changes in tax laws or rates and clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. | | January 1, 2021. | | We doit will not expect this guidance to have a materialan impact on our consolidated financial statements. |
In November 2023, the FASB issued final guidance to improve transparency of segment disclosures. The final guidance requires the disclosure of significant segment expenses that are regularly provided to the chief operating decision maker and included within each reported measure of segment profit or loss, other segment items by reportable segment and a description of its composition, and requires all current annual disclosures be provided in interim periods. | | January 1, 2024 for annual reports and January 1, 2025 for interim reports. Early adoption is permitted. | | This new guidance will result in increased disclosures in the notes to our financial statements. |
ReferenceIn December 2023, the FASB issued final guidance to improve income tax disclosures. The final guidance requires enhanced disclosures primarily related to existing rate reform provides temporary optional expedientsreconciliation and exceptions to the guidance for contracts, hedging relationships, and other transactions that reference LIBOR or another reference rate expected to be discontinued after 2021 because of reference rate reform.income taxes paid information.
The
| | January 1, 2025, with early adoption permitted. | | This new guidance provides the following optional expedients:1.Simplify accounting analyses under current U.S. GAAP for contract modifications.
2.Simplify the assessment of hedge effectiveness and allow hedging relationships affected by reference rate reform to continue.
3.Allow a one-time election to sell or transfer debt securities classified as held to maturity that reference a rate affected by reference rate reform.
| | Elections can be adopted prospectively at any timewill result in increased disclosures in the first quarter of 2020 through December 31, 2022.
| | We are assessing the impact of the provisions of this new guidance onnotes to our consolidated financial statements. |
| | | | | |
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 4849 |
| | | | | |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Pfizer Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Pfizer Inc. and Subsidiary Companies (the Company) as of December 31, 20202023 and 2019,2022, the related consolidated statements of income, comprehensive income, equity, and cash flows for each of the years in the three-year period ended December 31, 2020,2023, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20202023 and 2019,2022, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2020,2023, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2020,2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 25, 202122, 2024 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Evaluation of the U.S. Medicare, Medicaid, and performance-based contract rebates accrual
As discussed in Note 1G to the consolidated financial statements, the Company records estimated deductions for Medicare, Medicaid, and performance-based contract rebates (collectively, U.S. rebates) as a reduction to gross product revenues. The accrual for U.S. rebates is recorded in the same period that the corresponding revenues are recognized. The length of time between when a sale is made and when the U.S. rebate is paid by the Company can be as long as one year, which increases the need for significant management judgment and knowledge of market conditions and practices in estimating the accrual. We identified the evaluation of the U.S. rebates accrual as a critical audit matter because the evaluation of the product-specific experience ratio assumption involved especially challenging auditor judgment. The product-specific experience ratio assumption relates to estimating which of the Company’s revenue transactions will ultimately be subject to a related rebate.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls over the Company’s U.S. rebates accrual process related to the development of the product-specific experience ratio assumptions. We estimated the U.S. rebates accrual using internal information and historical data and compared the result to the Company’s estimated U.S. rebates accrual. We evaluated the Company’s ability to accurately estimate the accrual for U.S. rebates by comparing historically recorded accruals to the actual amount that was ultimately paid by the Company.
Evaluation of gross unrecognized tax benefits
As discussed in Notes 5D and 1P,1Q, the Company’s tax positions are subject to audit by local taxing authorities in each respective tax jurisdiction, and the resolution of such audits may span multiple years. Since tax law is complex and often subject to varied interpretations and judgments, it is uncertain whether some of the Company’s tax positions will be sustained upon audit. As of December 31, 2020,2023, the Company has recorded gross unrecognized tax benefits, excluding associated interest, of $5.6$4.8 billion. We identified the evaluation of certain of the Company’s gross unrecognized tax benefits as a critical audit matter because a high degree of audit effort, including specialized skills and knowledge, and complex auditor judgment was required in evaluating the Company’s interpretation of tax law and its estimate of the ultimate resolution of its tax positions.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of an internal control over the Company’s liability for unrecognized tax position process related to (1) interpretation
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 49 |
Report of Independent Registered Public Accounting Firm
of tax law, (2) evaluation of which of the Company’s tax positions may not be sustained upon audit, and (3) estimation and recording of the gross
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 50 |
Report of Independent Registered Public Accounting Firm
unrecognized tax benefits. We involved tax and valuation professionals with specialized skills and knowledge who assisted in evaluating the Company’s interpretation of tax laws, including the assessment of transfer pricing practices in accordance with applicable tax laws and regulations. We inspected settlements with applicable taxing authorities, including assessing the expiration of statutes of limitations. We tested the calculation of the liability for uncertain tax positions, including an evaluation of the Company’s assessment of the technical merits of tax positions and estimates of the amount of tax benefits expected to be sustained.
Evaluation of product liability and other product-related litigation
As discussed in Notes 1R1S and 16 to the consolidated financial statements, the Company is involved in product liability and other product-related litigation, which can include personal injury, consumer, off-label promotion, securities, antitrust and breach of contract claims, among others. Certain of these pending product and other product-related legal proceedings could result in losses that could be substantial. The accrued liability and/or disclosure for the pending product liability and other product-related legal proceedings requires a complex series of judgments by the Company about future events, which involves a number of uncertainties. We identified the evaluation of product liability and other product-related litigation as a critical audit matter. Challenging auditor judgment was required to evaluate the Company’s judgments about future events and uncertainties.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls over the Company’s product liability and other product-related litigation processes, including controls related to (1) the evaluation of information from external and internal legal counsel, (2) forward-looking expectations, and (3) new legal proceedings, or other legal proceedings not currently reserved or disclosed. We read letters received directly from the Company’s external and internal legal counsel that described the Company’s probable or reasonably possible legal contingency to pending product liability and other product-related legal proceedings. We inspected the Company’s minutes from meetings of the Audit Committee, which included the status of key litigation matters. We evaluated the Company’s ability to estimate its monetary exposure to pending product and other product-related legal proceedings by comparing historically recorded liabilities to actual monetary amounts incurred upon resolution of prior legal matters. We analyzed relevant publicly available information about the Company, its competitors, and the industry.
Evaluation of the fair value measurement of the developed technology rights and in-process research and development intangible assets acquired in the Seagen business combination
As discussed in Note 2A to the consolidated financial statements, on December 14, 2023, the Company acquired Seagen Inc. and its subsidiaries (Seagen). The total fair value of consideration transferred was $44.2 billion. Of that, the Company provisionally recorded $7.5 billion of developed technology rights with an estimated weighted-average life of approximately 18 years and $20.8 billion of in-process research and development (IPR&D). We identified the evaluation of the fair value measurement of the acquired developed technology rights and IPR&D as a critical audit matter. A high degree of subjective auditor judgment was required to evaluate certain key assumptions used to estimate the acquisition-date fair value of the acquired developed technology rights and IPR&D. Specifically, the key assumptions for certain IPR&D assets, including revenue growth rates, probability of technical and regulatory success (PTRS) rates, and the discount rate, and the key assumptions for certain developed technology rights, including revenue growth rates and the discount rate, represented subjective determinations of future market and economic conditions. Changes to those assumptions could have had a significant effect on the determination of the fair value measurements.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls related to the Company’s acquisition-date valuation process, including controls related to the development of the key assumptions for certain IPR&D assets and developed technology rights. We performed sensitivity analyses over the key assumptions for certain IPR&D assets and developed technology rights to assess the impact of changes in those key assumptions on the Company’s determination of the fair value of the IPR&D and developed technology rights, respectively. We evaluated the reasonableness of the Company’s forecasted revenue growth rates by comparing them to historical results for comparable products and peer companies, analyst expectations, and industry related third-party data. Further, we evaluated the PTRS rates for certain IPR&D assets by considering the phase of development of the clinical projects and the Company's history of obtaining regulatory approval and comparing them to PTRS rates derived from analyst reports and other industry related third-party data. We evaluated the data sources used by management in determining the key assumptions for certain IPR&D assets and developed technology rights by comparing to industry standards and evidence obtained in other areas of the audit.In addition, we involved valuation professionals with specialized skills and knowledge, who assisted in:
(1)evaluating the discount rates used by the Company for certain IPR&D and developed technology rights by comparing them against discount rate ranges that were independently developed using publicly available market data for comparable entities
(2)testing the source information underlying the determination of the discount rates.
| | |
| KPMG LLP
|
We have not been able to determine the specific year that KPMG andwe or our predecessor firms began serving as the Company’s auditor, however, we are aware that KPMG andwe or our predecessor firms have served as the Company’s auditor since at least 1942. |
|
| New York, New York |
|
February 25, 202122, 2024 |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 5051 |
Consolidated Statements of Income
Pfizer Inc. and Subsidiary Companies
| | | | | | Year Ended December 31, |
| (MILLIONS, EXCEPT PER COMMON SHARE DATA) | | | 2020 | | 2019 | | 2018 |
| Revenues | | | $ | 41,908 | | | $ | 41,172 | | | $ | 40,825 | |
| | |
| | | | | Year Ended December 31, |
| (MILLIONS, EXCEPT PER SHARE DATA) | | (MILLIONS, EXCEPT PER SHARE DATA) | | | | | 2023 | | 2022 | | 2021 |
| Revenues: | |
Product revenues(a) | |
Product revenues(a) | |
Product revenues(a) | |
Alliance revenues(a) | |
| Total revenues | |
| Costs and expenses: | Costs and expenses: | | | | | | |
Cost of sales(a) | | | 8,692 | | | 8,251 | | | 8,987 | |
Selling, informational and administrative expenses(a) | | | 11,615 | | | 12,750 | | | 12,612 | |
Research and development expenses(a) | | | 9,405 | | | 8,394 | | | 7,760 | |
Cost of sales(b), (c) | |
Cost of sales(b), (c) | |
Cost of sales(b), (c) | |
Selling, informational and administrative expenses(b) | |
Research and development expenses(b) | |
| Acquired in-process research and development expenses | |
| Amortization of intangible assets | Amortization of intangible assets | | | 3,436 | | | 4,462 | | | 4,736 | |
| Restructuring charges and certain acquisition-related costs | Restructuring charges and certain acquisition-related costs | | | 600 | | | 601 | | | 1,058 | |
| (Gain) on completion of Consumer Healthcare JV transaction | | | (6) | | | (8,086) | | | 0 | |
| | Other (income)/deductions––net | |
| Other (income)/deductions––net | |
| Other (income)/deductions––net | Other (income)/deductions––net | | | 669 | | | 3,314 | | | 2,077 | |
| Income from continuing operations before provision/(benefit) for taxes on income | Income from continuing operations before provision/(benefit) for taxes on income | | | 7,497 | | | 11,485 | | | 3,594 | |
| Provision/(benefit) for taxes on income | Provision/(benefit) for taxes on income | | | 477 | | | 618 | | | (266) | |
| Income from continuing operations | Income from continuing operations | | | 7,021 | | | 10,867 | | | 3,861 | |
| | Income from discontinued operations––net of tax | | | 2,631 | | | 5,435 | | | 7,328 | |
| Discontinued operations––net of tax | |
| Discontinued operations––net of tax | |
| Discontinued operations––net of tax | |
| | | Net income before allocation to noncontrolling interests | |
| | Net income before allocation to noncontrolling interests | |
| | Net income before allocation to noncontrolling interests | Net income before allocation to noncontrolling interests | | | 9,652 | | | 16,302 | | | 11,188 | |
| Less: Net income attributable to noncontrolling interests | Less: Net income attributable to noncontrolling interests | | | 36 | | | 29 | | | 36 | |
| Net income attributable to Pfizer Inc. common shareholders | Net income attributable to Pfizer Inc. common shareholders | | | $ | 9,616 | | | $ | 16,273 | | | $ | 11,153 | |
| Earnings per common share––basic: | Earnings per common share––basic: | | | | | | | |
Earnings per common share––basic: | |
Earnings per common share––basic: | | | | | | | | |
| Income from continuing operations attributable to Pfizer Inc. common shareholders | Income from continuing operations attributable to Pfizer Inc. common shareholders | | | $ | 1.26 | | | $ | 1.95 | | | $ | 0.65 | |
| Income from discontinued operations––net of tax | | | 0.47 | | | 0.98 | | | 1.25 | |
| Discontinued operations––net of tax | |
| Net income attributable to Pfizer Inc. common shareholders | Net income attributable to Pfizer Inc. common shareholders | | | $ | 1.73 | | | $ | 2.92 | | | $ | 1.90 | |
Earnings per common share––diluted: | Earnings per common share––diluted: | | | | | | | |
| Income from continuing operations attributable to Pfizer Inc. common shareholders | Income from continuing operations attributable to Pfizer Inc. common shareholders | | | $ | 1.24 | | | $ | 1.91 | | | $ | 0.64 | |
| Income from discontinued operations––net of tax | | | 0.47 | | | 0.96 | | | 1.23 | |
| Income from continuing operations attributable to Pfizer Inc. common shareholders | |
| Income from continuing operations attributable to Pfizer Inc. common shareholders | |
| Discontinued operations––net of tax | |
| Net income attributable to Pfizer Inc. common shareholders | Net income attributable to Pfizer Inc. common shareholders | | | $ | 1.71 | | | $ | 2.87 | | | $ | 1.87 | |
| | Weighted-average shares––basic | Weighted-average shares––basic | | | 5,555 | | | 5,569 | | | 5,872 | |
| Weighted-average shares––basic | |
| Weighted-average shares––basic | |
| Weighted-average shares––diluted | Weighted-average shares––diluted | | | 5,632 | | | 5,675 | | | 5,977 | |
(b)Exclusive of amortization of intangible assets, except as disclosed in assets.
See Accompanying Notes.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 5152 |
Consolidated Statements of Comprehensive Income
Pfizer Inc. and Subsidiary Companies
| | Year Ended December 31, |
| | Year Ended December 31, | | | | Year Ended December 31, |
| (MILLIONS) | (MILLIONS) | | 2020 | | 2019 | | 2018 | (MILLIONS) | | 2023 | | 2022 | | 2021 |
| Net income before allocation to noncontrolling interests | Net income before allocation to noncontrolling interests | | $ | 9,652 | | | $ | 16,302 | | | $ | 11,188 | |
| | Foreign currency translation adjustments, net | Foreign currency translation adjustments, net | | $ | 957 | | | $ | 654 | | | $ | (799) | |
| Reclassification adjustments | | (17) | | | (288) | | | (22) | |
| Foreign currency translation adjustments, net | |
| Foreign currency translation adjustments, net | |
| | Unrealized holding gains/(losses) on derivative financial instruments, net | |
| | Unrealized holding gains/(losses) on derivative financial instruments, net | |
| | | | 940 | | | 366 | | | (821) | |
| Unrealized holding gains/(losses) on derivative financial instruments, net | Unrealized holding gains/(losses) on derivative financial instruments, net | | (582) | | | 476 | | | 220 | |
Reclassification adjustments for (gains)/losses included in net income(a) | Reclassification adjustments for (gains)/losses included in net income(a) | | 21 | | | (664) | | | 27 | |
| | | | (561) | | | (188) | | | 247 | |
| Unrealized holding gains/(losses) on available-for-sale securities, net | Unrealized holding gains/(losses) on available-for-sale securities, net | | 361 | | | (1) | | | (185) | |
Reclassification adjustments for (gains)/losses included in net income(b) | Reclassification adjustments for (gains)/losses included in net income(b) | | (188) | | | 39 | | | 124 | |
Reclassification adjustments for unrealized gains included in Retained earnings(c) | | 0 | | | 0 | | | (462) | |
| | 173 | | | 38 | | | (522) | |
| Benefit plans: actuarial gains/(losses), net | | (1,128) | | | (826) | | | (649) | |
| Reclassification adjustments related to amortization | | 276 | | | 241 | | | 242 | |
| Reclassification adjustments related to settlements, net | | 278 | | | 274 | | | 142 | |
| Other | | (189) | | | 22 | | | 112 | |
| | | (763) | | | (289) | | | (153) | |
| | (261) | |
| | (261) | |
| | (261) | |
| Benefit plans: prior service (costs)/credits and other, net | Benefit plans: prior service (costs)/credits and other, net | | 52 | | | (7) | | | (9) | |
| Reclassification adjustments related to amortization of prior service costs and other, net | Reclassification adjustments related to amortization of prior service costs and other, net | | (176) | | | (181) | | | (181) | |
| Reclassification adjustments related to curtailments of prior service costs and other, net | Reclassification adjustments related to curtailments of prior service costs and other, net | | 0 | | | (2) | | | (19) | |
| Other | | 0 | | | 1 | | | 2 | |
| | | | (124) | | | (189) | | | (207) | |
| Other comprehensive income/(loss), before tax | Other comprehensive income/(loss), before tax | | (335) | | | (262) | | | (1,457) | |
Tax provision/(benefit) on other comprehensive income/(loss)(d) | | (349) | | | 115 | | | 518 | |
| Tax provision/(benefit) on other comprehensive income/(loss) | |
| Other comprehensive income/(loss) before allocation to noncontrolling interests | Other comprehensive income/(loss) before allocation to noncontrolling interests | | $ | 14 | | | $ | (376) | | | $ | (1,975) | |
| | | | | | | | | | | |
| Comprehensive income before allocation to noncontrolling interests | | $ | 9,666 | | | $ | 15,926 | | | $ | 9,214 | |
| Comprehensive income/(loss) before allocation to noncontrolling interests | |
| Less: Comprehensive income/(loss) attributable to noncontrolling interests | Less: Comprehensive income/(loss) attributable to noncontrolling interests | | 27 | | | 18 | | | 16 | |
| Comprehensive income attributable to Pfizer Inc. | | $ | 9,639 | | | $ | 15,908 | | | $ | 9,198 | |
| Comprehensive income/(loss) attributable to Pfizer Inc. | |
(a)Reclassified into Other (income)/deductions—net and Cost of sales. See Note 7E.7E. (b)Reclassified into Other (income)/deductions—net.
(c)See Note 1B in our 2018 Financial Report.
(d)See Note 5E.
See Accompanying Notes.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 5253 |
Consolidated Balance Sheets
Pfizer Inc. and Subsidiary Companies
| | As of December 31, |
| (MILLIONS, EXCEPT PREFERRED STOCK ISSUED AND PER COMMON SHARE DATA) | | 2020 | | 2019 |
| | As of December 31, | | | | As of December 31, |
| (MILLIONS, EXCEPT PER SHARE DATA) | | (MILLIONS, EXCEPT PER SHARE DATA) | | 2023 | | 2022 |
| | Assets | Assets | | | |
| Assets | |
| Assets | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Cash and cash equivalents | Cash and cash equivalents | | $ | 1,784 | | | $ | 1,121 | |
| Short-term investments | Short-term investments | | 10,437 | | | 8,525 | |
| Trade accounts receivable, less allowance for doubtful accounts: 2020—$508; 2019—$493 | | 7,930 | | | 6,772 | |
| Trade accounts receivable, less allowance for doubtful accounts: 2023—$470; 2022—$449 | |
| Inventories | Inventories | | 8,046 | | | 7,068 | |
| Current tax assets | Current tax assets | | 3,264 | | | 2,736 | |
| Other current assets | Other current assets | | 3,438 | | | 2,357 | |
| Current assets of discontinued operations and other assets held for sale | | 167 | | | 4,224 | |
| | Total current assets | |
| Total current assets | |
| Total current assets | Total current assets | | 35,067 | | | 32,803 | |
| Equity-method investments | Equity-method investments | | 16,856 | | | 17,133 | |
| Long-term investments | Long-term investments | | 3,406 | | | 3,014 | |
| Property, plant and equipment | Property, plant and equipment | | 13,900 | | | 12,969 | |
| Identifiable intangible assets | Identifiable intangible assets | | 28,471 | | | 33,936 | |
| Goodwill | Goodwill | | 49,577 | | | 48,202 | |
| Noncurrent deferred tax assets and other noncurrent tax assets | Noncurrent deferred tax assets and other noncurrent tax assets | | 2,383 | | | 1,911 | |
| Other noncurrent assets | Other noncurrent assets | | 4,569 | | | 4,199 | |
| Noncurrent assets of discontinued operations | | 0 | | | 13,427 | |
| Total assets | Total assets | | $ | 154,229 | | | $ | 167,594 | |
| | Liabilities and Equity | Liabilities and Equity | | | | |
| Short-term borrowings, including current portion of long-term debt: 2020—$2,002; 2019—$1,462 | | $ | 2,703 | | | $ | 16,195 | |
| Liabilities and Equity | |
| Liabilities and Equity | | | | | |
| Short-term borrowings, including current portion of long-term debt: 2023—$2,254; 2022—$2,560 | |
| Trade accounts payable | Trade accounts payable | | 4,309 | | | 3,887 | |
| Dividends payable | Dividends payable | | 2,162 | | | 2,104 | |
| Income taxes payable | Income taxes payable | | 1,049 | | | 980 | |
| Accrued compensation and related items | Accrued compensation and related items | | 3,058 | | | 2,390 | |
| Deferred revenues | |
| Other current liabilities | |
| | Other current liabilities | | 12,640 | | | 9,334 | |
| Current liabilities of discontinued operations | | 0 | | | 2,413 | |
| Total current liabilities | |
| Total current liabilities | |
| Total current liabilities | Total current liabilities | | 25,920 | | | 37,304 | |
| | Long-term debt | Long-term debt | | 37,133 | | | 35,955 | |
| Pension benefit obligations | | 4,766 | | | 5,291 | |
| Postretirement benefit obligations | | 645 | | | 926 | |
| Long-term debt | |
| Long-term debt | |
| Pension and postretirement benefit obligations | |
| Noncurrent deferred tax liabilities | Noncurrent deferred tax liabilities | | 4,063 | | | 5,652 | |
| Other taxes payable | Other taxes payable | | 11,560 | | | 12,126 | |
| Other noncurrent liabilities | Other noncurrent liabilities | | 6,669 | | | 6,894 | |
| Total liabilities | Total liabilities | | 90,756 | | | 104,148 | |
| | Commitments and Contingencies | Commitments and Contingencies | | 0 | | 0 |
| Commitments and Contingencies | |
| Commitments and Contingencies | | | | | |
| | Preferred stock, no par value, at stated value; 27 shares authorized; issued: 2020—0; 2019—431 | | 0 | | | 17 | |
| Common stock, $0.05 par value; 12,000 shares authorized; issued: 2020—9,407; 2019—9,369 | | 470 | | | 468 | |
| Preferred stock, no par value, at stated value; 27 shares authorized; no shares issued or outstanding as of December 31, 2023 and December 31, 2022 | |
| Preferred stock, no par value, at stated value; 27 shares authorized; no shares issued or outstanding as of December 31, 2023 and December 31, 2022 | |
| Preferred stock, no par value, at stated value; 27 shares authorized; no shares issued or outstanding as of December 31, 2023 and December 31, 2022 | |
| Common stock, $0.05 par value; 12,000 shares authorized; issued: 2023—9,562; 2022—9,519 | |
| Additional paid-in capital | Additional paid-in capital | | 88,674 | | | 87,428 | |
| | Treasury stock, shares at cost: 2020—3,840; 2019—3,835 | | (110,988) | | | (110,801) | |
| Treasury stock, shares at cost: 2023—3,916; 2022—3,903 | |
| Treasury stock, shares at cost: 2023—3,916; 2022—3,903 | |
| Treasury stock, shares at cost: 2023—3,916; 2022—3,903 | |
| Retained earnings | Retained earnings | | 96,770 | | | 97,670 | |
| Accumulated other comprehensive loss | Accumulated other comprehensive loss | | (11,688) | | | (11,640) | |
| Total Pfizer Inc. shareholders’ equity | Total Pfizer Inc. shareholders’ equity | | 63,238 | | | 63,143 | |
| Equity attributable to noncontrolling interests | Equity attributable to noncontrolling interests | | 235 | | | 303 | |
| Total equity | Total equity | | 63,473 | | | 63,447 | |
| Total liabilities and equity | Total liabilities and equity | | $ | 154,229 | | | $ | 167,594 | |
See Accompanying Notes.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 5354 |
Consolidated Statements of Equity
Pfizer Inc. and Subsidiary Companies
| | | | | PFIZER INC. SHAREHOLDERS | | |
| Preferred Stock | | Common Stock | | | | Treasury Stock | | | | | | | |
| (MILLIONS, EXCEPT PREFERRED SHARES AND PER SHARE AMOUNTS) | | Shares | | Stated Value | | Shares | | Par Value | | Add’l
Paid-In
Capital | | Shares | | Cost | | Retained Earnings | | Accum.
Other
Comp. Loss | | Share -
holders’
Equity | | Non-controlling Interests | | Total
Equity |
| Balance, January 1, 2018 | | 524 | | | $ | 21 | | | 9,275 | | | $ | 464 | | | $ | 84,278 | | | (3,296) | | | $ | (89,425) | | | $ | 85,291 | | | $ | (9,321) | | | $ | 71,308 | | | $ | 348 | | | $ | 71,656 | |
| | | | | | | | | PFIZER INC. SHAREHOLDERS | | |
| | (MILLIONS, EXCEPT PER SHARE DATA) | |
| (MILLIONS, EXCEPT PER SHARE DATA) | |
| (MILLIONS, EXCEPT PER SHARE DATA) | | | | | | | Shares | | Par Value | | Add’l
Paid-In
Capital | | Shares | | Cost | | Retained Earnings | | Accum.
Other
Comp. Loss | | Share -
holders’
Equity | | Non-controlling Interests | | Total
Equity |
| Balance, January 1, 2021 | |
| Net income | Net income | | 11,153 | | | 11,153 | | | 36 | | | 11,188 | |
| Other comprehensive income/(loss), net of tax | Other comprehensive income/(loss), net of tax | | (1,955) | | | (1,955) | | | (20) | | | (1,975) | |
| Cash dividends declared, per share: $1.38 | |
| Cash dividends declared, per share: $1.57 | |
| Common stock | Common stock | | (8,060) | | | (8,060) | | | (8,060) | |
| Preferred stock | | (1) | | | (1) | | | (1) | |
| Common stock | |
| Common stock | |
| | Noncontrolling interests | |
| Noncontrolling interests | |
| Noncontrolling interests | |
| Share-based payment transactions | |
| | Other | |
| | Other | |
| | Other | |
| Balance, December 31, 2021 | |
| Net income | |
| Other comprehensive income/(loss), net of tax | |
| Cash dividends declared, per share: $1.61 | |
| Common stock | |
| Common stock | |
| Common stock | |
| | Noncontrolling interests | |
| Noncontrolling interests | |
| Noncontrolling interests | Noncontrolling interests | | — | | | (12) | | | (12) | |
| Share-based payment transactions | Share-based payment transactions | | 57 | | | 3 | | | 1,977 | | | (12) | | | 13 | | | 1,993 | | | 1,993 | |
| Purchases of common stock | Purchases of common stock | | (307) | | | (12,198) | | | (12,198) | | | (12,198) | |
| Preferred stock conversions and redemptions | | (46) | | | (2) | | | (3) | | | — | | | — | | | (4) | | | (4) | |
Other(a) | | — | | | — | | | 1,172 | | | 1,172 | | | — | | | 1,172 | |
| Balance, December 31, 2018 | | 478 | | | 19 | | | 9,332 | | | 467 | | | 86,253 | | | (3,615) | | | (101,610) | | | 89,554 | | | (11,275) | | | 63,407 | | | 351 | | | 63,758 | |
| | Other | |
| Other | |
| Other | |
| Balance, December 31, 2022 | |
| Net income | Net income | | 16,273 | | | 16,273 | | | 29 | | | 16,302 | |
| Other comprehensive income/(loss), net of tax | Other comprehensive income/(loss), net of tax | | (365) | | | (365) | | | (11) | | | (376) | |
| Cash dividends declared, per share: $1.46 | |
| Cash dividends declared, per share: $1.65 | |
| Common stock | Common stock | | (8,174) | | | (8,174) | | | (8,174) | |
| Preferred stock | | (1) | | | (1) | | | (1) | |
| Common stock | |
| Common stock | |
| | Noncontrolling interests | Noncontrolling interests | | — | | | (6) | | | (6) | |
| Share-based payment transactions | | 37 | | | 2 | | | 1,219 | | | (8) | | | (326) | | | 894 | | | 894 | |
| Purchases of common stock | | (213) | | | (8,865) | | | (8,865) | | | (8,865) | |
| Preferred stock conversions and redemptions | | (47) | | | (2) | | | (3) | | | — | | | 1 | | | (4) | | | (4) | |
| Other | | (40) | | | — | | | — | | | 19 | | | (21) | | | (60) | | | (81) | |
| Balance, December 31, 2019 | | 431 | | | 17 | | | 9,369 | | | 468 | | | 87,428 | | | (3,835) | | | (110,801) | | | 97,670 | | | (11,640) | | | 63,143 | | | 303 | | | 63,447 | |
| Net income | | | 9,616 | | | 9,616 | | | 36 | | | 9,652 | |
| Other comprehensive income/(loss), net of tax | | | 23 | | | 23 | | | (9) | | | 14 | |
| Cash dividends declared, per share: $1.53 | | |
| Common stock | | | (8,571) | | | (8,571) | | | (8,571) | |
| Preferred stock | | | — | | | — | | | — | |
| Noncontrolling interests | |
| Noncontrolling interests | Noncontrolling interests | | | — | | | (91) | | | (91) | |
| Share-based payment transactions | Share-based payment transactions | | | 37 | | | 2 | | | 1,261 | | | (6) | | | (218) | | | 1,044 | | | 1,044 | |
| Preferred stock conversions and redemptions(b) | | (431) | | | (17) | | | (15) | | | 1 | | | 31 | | | (1) | | | (1) | |
Distribution of Upjohn Business(c) | | | (1,944) | | | (71) | | | (2,015) | | | (3) | | | (2,018) | |
| | Other | Other | | | — | | | — | | | — | | | — | | | (1) | | | (1) | |
| Balance, December 31, 2020 | | 0 | | | $ | 0 | | | 9,407 | | | $ | 470 | | | $ | 88,674 | | | (3,840) | | | $ | (110,988) | | | $ | 96,770 | | | $ | (11,688) | | | $ | 63,238 | | | $ | 235 | | | $ | 63,473 | |
| | Other | |
| | Other | |
| Balance, December 31, 2023 | |
(a)Primarily represents the cumulative effect of the adoption of new accounting standards in 2018 for revenues, financial assets and liabilities, income tax accounting, and the reclassification of certain tax effects. See Note 1B in our 2018 Financial Report.
(b)See Note 12.
(c)See Note 2B.
See Accompanying Notes.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 5455 |
Consolidated Statements of Cash Flows
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, |
| (MILLIONS) | | 2020 | | 2019 | | 2018 |
| | | | | | |
| Operating Activities | | | | | | |
| Net income before allocation to noncontrolling interests | | $ | 9,652 | | | $ | 16,302 | | | $ | 11,188 | |
| Income from discontinued operations—net of tax | | 2,631 | | | 5,435 | | | 7,328 | |
| Net income from continuing operations before allocation to noncontrolling interests | | 7,021 | | | 10,867 | | | 3,861 | |
| Adjustments to reconcile net income before allocation to noncontrolling interests to net cash provided by operating activities: | | | | | | |
| Depreciation and amortization | | 4,777 | | | 5,795 | | | 6,150 | |
| Asset write-offs and impairments | | 2,049 | | | 2,941 | | | 3,398 | |
| TCJA impact | | 0 | | | (323) | | | (596) | |
Gain on completion of Consumer Healthcare JV transaction, net of cash conveyed(a) | | (6) | | | (8,233) | | | 0 | |
| Deferred taxes from continuing operations | | (1,468) | | | 596 | | | (2,204) | |
| | | | | | |
| Share-based compensation expense | | 756 | | | 688 | | | 923 | |
| Benefit plan contributions in excess of expense/income | | (1,790) | | | (288) | | | (1,057) | |
| | | | | | |
| Other adjustments, net | | (478) | | | (1,080) | | | (1,266) | |
| Other changes in assets and liabilities, net of acquisitions and divestitures: | | | | | | |
| Trade accounts receivable | | (1,249) | | | (1,140) | | | (458) | |
| Inventories | | (736) | | | (1,080) | | | (432) | |
| Other assets | | (146) | | | 840 | | | (52) | |
| Trade accounts payable | | 353 | | | (340) | | | 404 | |
| Other liabilities | | 2,741 | | | 851 | | | 367 | |
| Other tax accounts, net | | (1,238) | | | (3,084) | | | (163) | |
| Net cash provided by operating activities from continuing operations | | 10,586 | | | 7,011 | | | 8,875 | |
| Net cash provided by operating activities from discontinued operations | | 3,817 | | | 5,576 | | | 6,952 | |
| Net cash provided by operating activities | | 14,403 | | | 12,588 | | | 15,827 | |
| | | | | | |
| Investing Activities | | | | | | |
| Purchases of property, plant and equipment | | (2,252) | | | (2,072) | | | (1,984) | |
| Purchases of short-term investments | | (13,805) | | | (6,835) | | | (11,677) | |
| Proceeds from redemptions/sales of short-term investments | | 11,087 | | | 9,183 | | | 17,581 | |
| Net (purchases of)/proceeds from redemptions/sales of short-term investments with original maturities of three months or less | | 920 | | | 6,925 | | | (3,917) | |
| Purchases of long-term investments | | (597) | | | (201) | | | (1,797) | |
| Proceeds from redemptions/sales of long-term investments | | 723 | | | 232 | | | 6,244 | |
| Acquisitions of businesses, net of cash acquired | | 0 | | | (10,861) | | | 0 | |
| Acquisitions of intangible assets | | (539) | | | (418) | | | (152) | |
Other investing activities, net(a) | | 274 | | | 195 | | | 287 | |
| Net cash provided by/(used in) investing activities from continuing operations | | (4,188) | | | (3,852) | | | 4,584 | |
| Net cash provided by/(used in) investing activities from discontinued operations | | (82) | | | (94) | | | (60) | |
| Net cash provided by/(used in) investing activities | | (4,271) | | | (3,945) | | | 4,525 | |
| | | | | | |
| Financing Activities | | | | | | |
| Proceeds from short-term borrowings | | 12,352 | | | 16,455 | | | 3,711 | |
| Principal payments on short-term borrowings | | (22,197) | | | (8,378) | | | (4,437) | |
| Net (payments on)/proceeds from short-term borrowings with original maturities of three months or less | | (4,129) | | | 2,551 | | | (1,617) | |
| Proceeds from issuance of long-term debt | | 5,222 | | | 4,942 | | | 4,974 | |
| Principal payments on long-term debt | | (4,003) | | | (6,806) | | | (3,566) | |
| Purchases of common stock | | 0 | | | (8,865) | | | (12,198) | |
| Cash dividends paid | | (8,440) | | | (8,043) | | | (7,978) | |
| Proceeds from exercise of stock options | | 425 | | | 394 | | | 1,259 | |
| Other financing activities, net | | (869) | | | (736) | | | (588) | |
| Net cash provided by/(used in) financing activities from continuing operations | | (21,640) | | | (8,485) | | | (20,441) | |
| Net cash provided by/(used in) financing activities from discontinued operations | | 11,991 | | | 0 | | | 0 | |
| Net cash provided by/(used in) financing activities | | (9,649) | | | (8,485) | | | (20,441) | |
| Effect of exchange-rate changes on cash and cash equivalents and restricted cash and cash equivalents | | (8) | | | (32) | | | (116) | |
| Net increase/(decrease) in cash and cash equivalents and restricted cash and cash equivalents | | 475 | | | 125 | | | (205) | |
| Cash and cash equivalents and restricted cash and cash equivalents, at beginning of period | | 1,350 | | | 1,225 | | | 1,431 | |
| Cash and cash equivalents and restricted cash and cash equivalents, at end of period | | $ | 1,825 | | | $ | 1,350 | | | $ | 1,225 | |
|
| - Continued - |
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, |
| (MILLIONS) | | 2023 | | 2022 | | 2021 |
| | | | | | |
| Operating Activities | | | | | | |
| Net income before allocation to noncontrolling interests | | $ | 2,158 | | | $ | 31,407 | | | $ | 22,025 | |
| Discontinued operations—net of tax | | (15) | | | 6 | | | (434) | |
| Net income from continuing operations before allocation to noncontrolling interests | | 2,172 | | | 31,401 | | | 22,459 | |
Adjustments to reconcile net income before allocation to noncontrolling interests to net cash provided by/(used in) operating activities: | | | | | | |
| Depreciation and amortization | | 6,290 | | | 5,064 | | | 5,191 | |
| Asset write-offs and impairments | | 3,408 | | | 550 | | | 276 | |
| Deferred taxes | | (3,442) | | | (3,764) | | | (4,293) | |
| | | | | | |
| Share-based compensation expense | | 525 | | | 872 | | | 1,182 | |
| Benefit plan contributions in excess of expense/income | | (787) | | | (1,158) | | | (3,123) | |
Inventory write-offs and related charges associated with COVID-19 products(a) | | 6,199 | | | 1,183 | | | — | |
| | | | | | |
| Other adjustments, net | | (3,492) | | | 758 | | | (1,573) | |
| Other changes in assets and liabilities, net of acquisitions and divestitures: | | | | | | |
| Trade accounts receivable | | 347 | | | 261 | | | (3,811) | |
Inventories(a) | | (1,169) | | | (591) | | | (1,125) | |
Other assets(b) | | (663) | | | (4,506) | | | (1,057) | |
| Trade accounts payable | | (300) | | | 1,191 | | | 1,242 | |
Other liabilities(c) | | 595 | | | (1,449) | | | 18,721 | |
| Other tax accounts, net | | (982) | | | (545) | | | (1,166) | |
| Net cash provided by/(used in) operating activities from continuing operations | | 8,700 | | | 29,267 | | | 32,922 | |
| Net cash provided by/(used in) operating activities from discontinued operations | | — | | | — | | | (343) | |
| Net cash provided by/(used in) operating activities | | 8,700 | | | 29,267 | | | 32,580 | |
| | | | | | |
| Investing Activities | | | | | | |
| Purchases of property, plant and equipment | | (3,907) | | | (3,236) | | | (2,711) | |
| Purchases of short-term investments | | (30,974) | | | (36,384) | | | (38,457) | |
| Proceeds from redemptions/sales of short-term investments | | 39,264 | | | 44,821 | | | 27,447 | |
| Net (purchases of)/proceeds from redemptions/sales of short-term investments with original maturities of three months or less | | 5,174 | | | (483) | | | (8,088) | |
| Purchases of long-term investments | | (204) | | | (1,913) | | | (1,068) | |
| Proceeds from redemptions/sales of long-term investments | | 1,979 | | | 641 | | | 649 | |
| Acquisitions of businesses, net of cash acquired | | (43,430) | | | (22,997) | | | — | |
Dividend received from the Consumer Healthcare JV(d) | | — | | | 3,960 | | | — | |
| Other investing activities, net | | (179) | | | (192) | | | (305) | |
| Net cash provided by/(used in) investing activities from continuing operations | | (32,278) | | | (15,783) | | | (22,534) | |
| Net cash provided by/(used in) investing activities from discontinued operations | | — | | | — | | | (12) | |
| Net cash provided by/(used in) investing activities | | (32,278) | | | (15,783) | | | (22,546) | |
| | | | | | |
| Financing Activities | | | | | | |
| Proceeds from short-term borrowings | | 4,525 | | | 3,891 | | | — | |
| Payments on short-term borrowings | | (3) | | | (3,887) | | | — | |
| Net (payments on)/proceeds from short-term borrowings with original maturities of three months or less | | 3,161 | | | (222) | | | (96) | |
| Proceeds from issuances of long-term debt | | 30,831 | | | — | | | 997 | |
| Payments on long-term debt | | (2,569) | | | (3,298) | | | (2,004) | |
| Purchases of common stock | | — | | | (2,000) | | | — | |
| Cash dividends paid | | (9,247) | | | (8,983) | | | (8,729) | |
| Other financing activities, net | | (631) | | | (335) | | | 16 | |
| | | | | | |
| | | | | | |
| Net cash provided by/(used in) financing activities | | 26,066 | | | (14,834) | | | (9,816) | |
| Effect of exchange-rate changes on cash and cash equivalents and restricted cash and cash equivalents | | (40) | | | (165) | | | (59) | |
| Net increase/(decrease) in cash and cash equivalents and restricted cash and cash equivalents | | 2,448 | | | (1,515) | | | 159 | |
| Cash and cash equivalents and restricted cash and cash equivalents, at beginning of period | | 468 | | | 1,983 | | | 1,825 | |
| Cash and cash equivalents and restricted cash and cash equivalents, at end of period | | $ | 2,917 | | | $ | 468 | | | $ | 1,983 | |
|
| - Continued - |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 5556 |
Consolidated Statements of Cash Flows
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | |
|
| | Year Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| Supplemental Cash Flow Information | | | | | | |
| Cash paid (received) during the period for: | | | | | | |
| Income taxes | | $ | 3,153 | | | $ | 3,664 | | | $ | 3,655 | |
| Interest paid | | 1,641 | | | 1,587 | | | 1,311 | |
| Interest rate hedges | | (20) | | | (42) | | | (38) | |
| | | | | | |
| Non-cash transactions: | | | | | | |
32% equity-method investment in the Consumer Healthcare JV received in exchange for contributing Pfizer’s Consumer Healthcare business(a) | | $ | 0 | | | $ | 15,711 | | | $ | 0 | |
| Equity investment in Allogene received in exchange for Pfizer's allogeneic CAR T developmental program assets | | 0 | | | 0 | | | 92 | |
| Equity investment in Cerevel in exchange for Pfizer’s portfolio of clinical and preclinical neuroscience assets | | 0 | | | 0 | | | 343 | |
| | | | | | | | | | | | | | | | | | | | |
|
| | Year Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| Supplemental Cash Flow Information | | | | | | |
| Cash paid/(received) during the period for: | | | | | | |
| Income taxes | | $ | 3,147 | | | $ | 7,867 | | | $ | 7,427 | |
| Interest paid | | 2,215 | | | 1,442 | | | 1,467 | |
| Interest rate hedges | | 134 | | | 54 | | | (2) | |
| | | | | | |
| Non-cash transaction: | | | | | | |
| Right-of-use assets obtained in exchange for lease liabilities | | $ | 614 | | | $ | 752 | | | $ | 1,943 | |
(a)The $8.2 billionSee Gain on completion of Consumer Healthcare JV transaction, net of cash conveyedNotes 8A reflects the receipt of a 32% equity-method investment in the new company initially valued at $15.7 billion in exchange for net assets contributed of $7.6 billion and is presented in operating activities net of $146 million cash conveyed that is reflected in Other investing activities, net17A. See See Accompanying Notes.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 5657 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 1. Basis of Presentation and Significant Accounting Policies
A. Basis of Presentation
The consolidated financial statements include the accounts of our parent company and all subsidiaries and are prepared in accordance with U.S. GAAP. The decision of whether or not to consolidate an entity for financial reporting purposes requires consideration of majority voting interests, as well as effective economic or other control over the entity. Typically, we do not seek control by means other than voting interests. For subsidiaries operating outside the U.S., the financial information is included as of and for the year ended November 30 for each year presented. Pfizer's fiscal year-end for U.S. subsidiaries is as of and for the year ended December 31 for each year presented. Substantially all unremitted earnings of international subsidiaries are free of legal and contractual restrictions. All significant transactions among our subsidiaries have been eliminated.
On November 16, 2020,December 14, 2023, we completed the spin-off andacquisition of Seagen. On December 31, 2021, we completed the combinationsale of our Upjohn Business with Mylan. Prior to the separation of the Upjohn Business, beginning in 2020, the Upjohn Business, Meridian which issubsidiary, the manufacturer of EpiPen and other auto-injector products, and a pre-existing strategic collaboration between Pfizer and Mylan for generic drugs in Japan (the Mylan-Japan collaboration) were managed as part of our former Upjohn operating segment. Revenues and expenses associated with Meridian and the Mylan-Japan collaboration were included in the Upjohn operating segment results along with the results of operations of the Upjohn Business in Pfizer’s historical consolidated financial statements. Meridian, which remains with Pfizer, supplies EpiPen Auto-Injectors to Viatris under a supply agreement expiring December 31, 2024, with an option for Viatris to extend for an additional one-year term. On December 21, 2020, which falls in Pfizer’s international 2021 fiscal year, Pfizer and Viatris completed the termination, under the previously disclosed agreement dated November 13, 2020, of the Mylan-Japan collaboration and we transferred related inventories and operations that were part of the Mylan-Japan collaboration to Viatris. Beginning in the fourth quarter of 2020, the financial results of the Upjohn Business and the Mylan-Japan collaboration are reflected as discontinued operations for all periods presented. The financial results of Meridian are now included in our Hospital therapeutic area for all periods presented. Upon completion of the spin-off of the Upjohn Business on November 16, 2020, the Upjohn assets and liabilities were derecognized from our consolidated balance sheet and are reflected in Retained Earnings–Distribution of Upjohn Business in the consolidated statement of equity. The assets and liabilities associated with the Upjohn Business and the Mylan-Japan collaboration are classified as assets and liabilities of discontinued operations. Certain prior year amounts have been reclassified to conform with the current year presentation.products. In addition, other acquisitions and business development activities completed in 2020, 20192023, 2022 and 2018, including the acquisitions of Array and Therachon, and the contribution of our Consumer Healthcare business to the Consumer Healthcare JV,2021 impacted financial results in the periods presented. See Note 2.
Prior to the separation of the Upjohn Business, we managed our commercial operations through 3 distinct business segments: (i) our innovative science-based biopharmaceutical products business (Biopharma); (ii) our global, primarily off-patent branded and generics business (Upjohn); and (iii) through July 31, 2019, Pfizer’s consumer healthcare business. With the formation of the Consumer Healthcare JV in 2019 and the completion of the spin-off of our Upjohn Business in the fourth quarter of 2020, Pfizer has transformed into a more focused, global leader in science-based innovative medicines and vaccines. We now operate as a single operating segment engaged in the discovery, development, manufacturing, marketing, sales and distribution of biopharmaceutical products worldwide. Regional commercial organizations market, distribute and sell our products. Our commercial organization is supported by global platform functions that are responsible for the research, development, manufacturing and supply of our products. The business is also supported by global corporate enabling functions. Our determination that we operate as a single segment is consistent with the financial information regularly reviewed by the chief operating decision maker for purposes of evaluating performance, allocating resources, setting incentive compensation targets, and planning and forecasting for future periods. Our chief operating decision maker allocates resources and assesses financial performance on a consolidated basis. Prior-period information has been restated to reflect our current organizational structure following the separation of the Upjohn Business. For information about product and geographic revenues, see Note 172.
We have made certain reclassification adjustments to conform prior-period amounts to the current presentation. Certain amounts in the consolidated financial statements and associated notes may not add due to rounding. All percentages have been calculated using unrounded amounts.
B. New Accounting Standards Adopted in 20202023
On January 1, 2020,2023, we adopted the following accounting standards:
Credit Losses on Financial Instruments––We adopted a new accounting standard for credit lossessupplier finance programs which requires increased disclosures in the notes to our financial statements. See Note 8C.In the second quarter of 2023, we adopted new accounting standards on financial instruments, which replacesreference rate reform that provide temporary optional expedients and exceptions to the probable initial recognition threshold for incurred loss estimates under prior guidance with a methodology that reflects expected credit loss estimates. The standard generally impacts financial assets that have a contractual right to receive cash and are not accounted for at fair value through net income, such as accounts receivable and held-to-maturity debt securities. The new guidance requires us to identify, analyze, document and support new methodologies for quantifying expected credit loss estimates for certain financial instruments, using information such as historical experience, current economic conditions and information, and the use of reasonable and supportable forecasted information. The standard also amends existing impairment guidance for available-for-sale debt securitiescontracts, hedging relationships, and other transactions that reference LIBOR or another reference rate that were discontinued after June 30, 2023. We applied certain of the optional expedients related to incorporate a credit loss allowancehedge accounting relationships. The main purpose of the expedients is to allow hedge accounting to continue uninterrupted and allows for reversals of credit impairments inmake it easier to apply the eventrequirements to maintain hedge accounting during the issuer’s credit improves.
We adopted the new accounting standard utilizing the modified retrospective method and, therefore, no adjustments were made to priortransition period financial statements. The cumulative effect of adopting the standard as an adjustment to the opening balance of Retained earnings was not material. The adoption of this standard did not have a material impact on our consolidated statement of income or consolidated statement of cash flows for the year endedthrough December 31, 2020, nor on our consolidated balance sheet as of December 31, 2020. For additional information, see Note 1G.
Goodwill Impairment Testing––We prospectively adopted the new standard, which eliminates the requirement to perform a hypothetical purchase price allocation to measure goodwill impairment. Under the new guidance, the goodwill impairment test is performed by comparing the fair value of a reporting unit with its carrying amount, and recognizing an impairment charge for the amount by which the carrying amount
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 57 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
of the reporting unit exceeds its fair value. There was no impact to our consolidated financial statements from the adoption of this new standard.
Implementation Costs in a Cloud Computing Arrangement––We prospectively adopted the new standard related to customers’ accounting for implementation costs incurred in a cloud computing arrangement that is considered a service contract. The new guidance aligns the requirements for capitalizing implementation costs in such arrangements with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. The adoption of this guidance did not have a material impact on our consolidated financial statements.
Collaboration Agreements––We prospectively adopted the new standard, which provides guidance clarifying the interaction between the accounting for collaborative arrangements and revenue from contracts with customers. There was no impact to our consolidated financial statements from the adoption of this new standard.2024.
C. Estimates and Assumptions
In preparing these financial statements, we use certain estimates and assumptions that affect reported amounts and disclosures. These estimates and assumptions can impact all elements of our financial statements. For example, in the consolidated statements of income, estimates are used when accounting for deductions from revenues, determining the cost of inventory that is sold, allocating cost in the form of depreciation and amortization, and estimating restructuring charges and the impact of contingencies, as well as determining provisions for taxes on income. On the consolidated balance sheets, estimates are used in determining the valuation and recoverability of assets, and in determining the reported amounts of liabilities, all of which also impact the consolidated statements of income. Certain estimates of fair value and amounts recorded in connection with acquisitions, revenue deductions, impairment reviews, restructuring-associated charges, investments and financial instruments, valuation allowances, pension and postretirement benefit plans, contingencies, share-based compensation, and other calculations can result from a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions.
Our estimates are often based on complex judgments and assumptions that we believe to be reasonable, but that can be inherently uncertain and unpredictable. If our estimates and assumptions are not representative of actual outcomes, our results could be materially impacted. As future events and their effects cannot be determined with precision, our estimates and assumptions may prove to be incomplete or inaccurate, or unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions. We are subject to risks and uncertainties that may cause actual results to differ from estimated amounts, such as changes in the healthcare environment, competition, litigation, legislation and regulations. We regularly evaluate our estimates and assumptions using historical experience and expectations about the future. We adjust our estimates and assumptions when facts and circumstances indicate the need for change.
D. Acquisitions
Our consolidated financial statements include the operations of acquired businesses after the completion of the acquisitions. We account for acquired businesses using the acquisition method of accounting, which requires, among other things, that most assets acquired and liabilities assumed be recognized at their estimated fair values as of the acquisition date and that the fair value of acquired IPR&D be recorded on the balance sheet. Transaction costs are expensed as incurred. Any excess of the consideration transferred over the assigned values of the net assets acquired is recorded as goodwill. When we acquire net assets that do not constitute a business, as defined in U.S. GAAP, no goodwill is recognized and acquired IPR&D is expensed.
expensed in
Contingent consideration in a business combination is included as part of the acquisition cost and is recognized at fair value as of the acquisition date. Fair value is generally estimated by using a probability-weighted discounted cash flow approach. See Note 16D. Any liability resulting from contingent consideration is remeasured to fair value at each reporting date until the contingency is resolved. These changes in fair value are recognized in earnings in Other (income)/deductions––net.
E. Fair Value
We measure certain assets and liabilities at fair value, either upon initial recognition or for subsequent accounting or reporting. We estimate fair value using an exit price approach, which requires, among other things, that we determine the price that would be received to sell an asset or paid to transfer a liability in an orderly market. The determination of an exit price is considered from the perspective of market participants,
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 58 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
considering the highest and best use of non-financial assets and, for liabilities, assuming that the risk of non-performance will be the same before and after the transfer.
When estimating fair value, depending on the nature and complexity of the asset or liability, we may use one or all of the following techniques:
•Income approach, which is based on the present value of a future stream of net cash flows.
•Market approach, which is based on market prices and other information from market transactions involving identical or comparable assets or liabilities.
•Cost approach, which is based on the cost to acquire or construct comparable assets, less an allowance for functional and/or economic obsolescence.
Our fair value methodologies depend on the following types of inputs:
•Quoted prices for identical assets or liabilities in active markets (Level 1 inputs).
•Quoted prices for similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active, or inputs other than quoted prices that are directly or indirectly observable, or inputs that are derived principally from, or corroborated by, observable market data by correlation or other means (Level 2 inputs).
•Unobservable inputs that reflect estimates and assumptions (Level 3 inputs).
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 58 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The following inputs and valuation techniques are used to estimate the fair value of our financial assets and liabilities:
•Available-for-sale debt securities—third-party matrix-pricing model that uses significant inputs derived from or corroborated by observable market data and credit-adjusted yield curves.
•Equity securities with readily determinable fair values—quoted market prices and observable NAV prices.
•Derivative assets and liabilities—third-party matrix-pricing model that uses inputs derived from or corroborated by observable market data. Where applicable, these models use market-based observable inputs, including interest rate yield curves to discount future cash flow amounts, and forward and spot prices for currencies. The credit risk impact to our derivative financial instruments was not significant.
•Money market funds—observable NAV prices.
We periodically review the methodologies, inputs and outputs of third-party pricing services for reasonableness. Our procedures can include, for example, referencing other third-party pricing models, monitoring key observable inputs (like benchmark interest rates) and selectively performing test-comparisons of values with actual sales of financial instruments.
F. Foreign Currency Translation
For most of our international operations, local currencies have been determined to be the functional currencies. We translate functional currency assets and liabilities to their U.S. dollar equivalents at exchange rates in effect as of the balance sheet date and income and expense amounts at average exchange rates for the period. The U.S. dollar effects that arise from changing translation rates are recorded in Other comprehensive income/(loss). The effects of converting non-functional currency monetary assets and liabilities into the functional currency are recorded in Other (income)/deductions––net. For operations in highly inflationary economies, we translate monetary items at rates in effect as of the balance sheet date, with translation adjustments recorded in Other (income)/deductions––net, and we translate non-monetary items at historical rates.
G. G. Revenues and Trade Accounts Receivable
Revenue Recognition––We record revenues from product sales when there is a transfer of control of the product from us to the customer. We typically determine transfer of control based on when the product is shipped or delivered and title passes to the customer. For certain contracts, the finished product may temporarily be stored at our or our third-party subcontractors’ locations under a bill-and-hold arrangement. Revenue is recognized on bill-and-hold arrangements at the point in time when the customer obtains control of the product and all of the following criteria have been met: the arrangement is substantive; the product is identified separately as belonging to the customer; the product is ready for physical transfer to the customer; and we do not have the ability to use the product or direct it to another customer. In bill-and-hold arrangements which are part of the U.S. Government Strategic National Stockpile, we recognize revenue for the product sale when the product is initially placed into the Stockpile and we provide a rotation service to maintain an agreed upon level of shelf life for product in the stockpile. In determining when the customer obtains control of the product, we consider certain indicators, including whether we have a present right to payment from the customer, whether title and/or significant risks and rewards of ownership have transferred to the customer and whether customer acceptance has been received.
In the fourth quarter of 2023, we began reporting Product revenues and Alliance revenues as separate line items in our consolidated statements of income. Prior-period amounts have been reclassified to conform to the current presentation.
Our Sales Contracts––Sales on credit are typically under short-term contracts. Collections are based on market payment cycles common in various markets, with shorter cycles in the U.S. Sales are adjusted for sales allowances, chargebacks, rebates and sales returns and cash discounts. Sales returns may occur due to LOE, product recalls or a changing competitive environment.
Deductions from Revenues––Our gross product revenues are subject to a variety of deductions, which generally are estimated and recorded in the same period that the revenues are recognized. Such variable consideration represents chargebacks, rebates, sales allowances and sales returns. These deductions represent estimates of the related obligations and, as such, knowledge and judgment is required when estimating the impact of these product revenue deductions on gross sales for a reporting period.
Provisions for pharmaceutical sales returns––Provisions are based on a calculation for each market that incorporates the following, as appropriate: local returns policies and practices; historical returns as a percentage of sales; an understanding of the reasons for past returns; estimated shelf life by product; an estimate of the amount of time between shipment and return or lag time; and any other factors that could
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 59 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
impact the estimate of future returns, such as LOE, product recalls or a changing competitive environment. Generally, returned products are destroyed, and customers are refunded the sales price in the form of a credit.
We record sales incentives as a reduction of revenues at the time the related revenues are recorded or when the incentive is offered, whichever is later. We estimate the cost of our sales incentives based on our historical experience with similar incentives programs to predict customer behavior.
The following outlines our common sales arrangements:
•Customers––Our prescription biopharmaceutical products, with the exception of Paxlovid in 2022 and 2023, are sold principally to wholesalers, but we also sell directly to retailers, hospitals, clinics, government agencies and pharmacies. In 2022 and 2023, we principally sold Paxlovid globally to government agencies. Our vaccines in the U.S., we are primarily sell our vaccines productssold directly to the federal government CDC,(including the CDC), wholesalers, individual provider offices, retail pharmacies and integrated delivery networks. Outsidesystems. Our vaccines outside the U.S., we are primarily sell our vaccinessold to government and non-government institutions. Customers for our consumer healthcare business, which were part of the business that was combined with GSK’s Consumer Healthcare business included retailers and, to a lesser extent, wholesalers and distributors.
BiopharmaceuticalPrescription pharmaceutical products that ultimately are used by patients are generally covered under governmental programs, managed care programs and insurance programs, including those managed through PBMs, and are subject to sales allowances and/or rebates payable directly to those programs. Those sales allowances and rebates are generally negotiated, but government programs may have legislated amounts by type of product (e.g., patented or unpatented).
Specifically:
•In the U.S., we sell our products principally to distributors and hospitals. We also have contracts with managed care programs or PBMs and legislatively mandated contracts with the federal and state governments under which we provide rebates based on medicines utilized by the lives they cover. We record provisions for Medicare, Medicaid, and performance-based contract pharmaceutical rebates based upon our experience ratio of rebates paid and actual prescriptions written during prior periods. We apply the experience ratio to the respective period’s sales to determine the rebate accrual and related expense. This experience ratio is evaluated regularly to ensure that the historical trends are as current as practicable. We estimate discounts on branded prescription drug sales to Medicare Part D participants in the Medicare “coverage gap,” also known as the “doughnut hole,” based on the historical experience of beneficiary prescriptions and consideration of the utilization that is expected to result from the discount in the coverage gap. We evaluate this estimate regularly to ensure that the historical trends and future expectations are as current as practicable. For performance-based contract rebates, we also consider current contract terms, such as changes in formulary status and rebate rates.
•Outside the U.S., the majority of our pharmaceutical sales allowances are contractual or legislatively mandated and our estimates are based on actual invoiced sales within each period, which reduces the risk of variations in the estimation process. In certain European countries,
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 59 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
rebates are calculated on the government’s total unbudgeted pharmaceutical spending or on specific product sales thresholds and we apply an estimated allocation factor against our actual invoiced sales to project the expected level of reimbursement. We obtain third-party information that helps us to monitor the adequacy of these accruals.
•Provisions for pharmaceutical chargebacks (primarily reimbursements to U.S. wholesalers for honoring contracted prices and legislated discounts to third parties) closely approximate actual amounts incurred, as we settle these deductions generally within two to five weeks of incurring the liability.
We recorded direct product sales and/or allianceAlliance revenues of more than $1 billion for each of sevennine products in 2020,2023, for each of sixten products in 20192022 and for each of sevennine products in 2018.2021. In the aggregate, these direct productsproduct sales and/or alliance productAlliance revenues represent 53%represented 64%, 82% and 75% of our Total revenues in 2020, 49% of our revenues in 20192023, 2022 and 47%of our revenues in 2018.2021, respectively. See Note 17B 17Cfor additional information.. The loss or expiration of intellectual property rights can have a significant adverse effect on our revenues as our contracts with customers will generally be at lower selling prices and lower volumes due to added competition and wegeneric competition. We generally provide for higher sales returns during the period in which individual markets begin to near the loss or expiration of intellectual property rights. | Our accruals for Medicare, Medicaid and related state program and performance-based contract rebates, chargebacks, sales allowances and sales returns and cash discounts are as follows: | | | | | As of December 31, | | | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 |
| (MILLIONS) | | (MILLIONS) | | 2023 | | 2022 |
Reserve against Trade accounts receivable, less allowance for doubtful accounts | Reserve against Trade accounts receivable, less allowance for doubtful accounts | | $ | 861 | | | $ | 823 | |
| Other current liabilities: | Other current liabilities: | | | |
Other current liabilities: | |
Other current liabilities: | |
| Accrued rebates | |
| Accrued rebates | |
| Accrued rebates | Accrued rebates | | 3,017 | | | 2,512 | |
| Other accruals | Other accruals | | 436 | | | 379 | |
| | Other noncurrent liabilities | Other noncurrent liabilities | | 399 | | | 384 | |
| Other noncurrent liabilities | |
| Other noncurrent liabilities | |
| Total accrued rebates and other sales-related accruals | Total accrued rebates and other sales-related accruals | | $ | 4,712 | | | $ | 4,098 | |
Taxes collected from customers relating to product sales and remitted to governmental authorities are excluded from RevenuesProduct revenues.
Trade Accounts Receivable—Trade accounts receivable are stated at their net realizable value. The allowance for credit losses reflects our best estimate of expected credit losses of the receivables portfolio determined on the basis of historical experience, current information, and forecasts of future economic conditions. In developing the estimate for expected credit losses, trade accounts receivables are segmented into pools of assets depending on market (U.S. versus international), delinquency status, and customer type (high risk versus low risk and government versus non-government), and fixed reserve percentages are established for each pool of trade accounts receivables.
In determining the reserve percentages for each pool of trade accounts receivables, we considered our historical experience with certain customers and customer types, regulatory and legal environments, country and political risk, and other relevant current and future forecasted macroeconomic factors. These credit risk indicators are monitored on a quarterly basis to determine whether there have been any changes in the economic environment that would indicate the established reserve percentages should be adjusted, and are considered on a regional basis to reflect more geographic-specific metrics. Additionally, write-offs and recoveries of customer receivables are tracked against collections
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 60 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
on a quarterly basis to determine whether the reserve percentages remain appropriate. When management becomes aware of certain customer-specific factors that impact credit risk, specific allowances for these known troubled accounts are recorded. Trade accounts receivable are written off after all reasonable means to collect the full amount (including litigation, where appropriate) have been exhausted.
During 2020,2023 and 2022, additions to the allowance for credit losses, write-offs and recoveries of customer receivables were not material to our consolidated financial statements.
H. Collaborative Arrangements
Payments to and from our collaboration partners are presented in our consolidated statements of income based on the nature of the arrangement (including its contractual terms), the nature of the payments and applicable accounting guidance. Under co-promotionco-commercialization agreements, we record the amounts received for our share of gross profits from our collaboration partners as allianceAlliance revenues, a component of Revenues, when our collaboration partners are the principal in the transaction and we receive a share of their net sales or profits. Alliance revenues are recorded as we perform co-promotion activities for the collaboration and the collaboration partners sell the products to their customers. The related expenses for selling and marketing these products including reimbursements to or from our collaboration partners for these costs are included in Selling, informational and administrative expenses. In collaborative arrangements where we manufacture a product for our collaboration partners, we record revenues when we transfer control of the product to our collaboration partners. In collaboration arrangements where we are the principal in the transaction, we record amounts paid to collaboration partners for their share of net sales or profits earned, and all royalty payments to collaboration partners as Cost of sales. Royalty payments received from collaboration partners are included in Other (income)/deductions—net.
Reimbursements to or from our collaboration partners for development costs are typically recorded in Research and development expenses. Upfront payments and pre-approval milestone payments due from us to our collaboration partners in development stage collaborations are recorded as ResearchAcquiredin-process research and development expenses. Milestone payments due from us to our collaboration partners after regulatory approval has been attained for a medicine are recorded in Identifiable intangible assets—Developedassets—developed technology rights.rights. Upfront and pre-approval milestone payments earned from our collaboration partners by us are recognized in Other (income)/deductions—net over the development period for the products, when our performance obligations include providing R&D services to our collaboration partners. Upfront, pre-approval and post-approval milestone payments earned by us may be recognized in Other (income)/deductions—net immediately when earned or over other periods depending upon the nature of our performance obligations in the applicable collaboration. Where the milestone event is regulatory approval for a medicine, we generally recognize milestone payments due to us in the transaction price when regulatory approval in the applicable jurisdiction has been attained. We may recognize milestone payments due to us in the transaction price earlier than the milestone event in certain circumstances when recognition of the income would not be probable of a significant reversal.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 60 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
I. Cost of Sales and Inventories
Inventories are recorded at the lower of cost or net realizable value. The cost of finished goods, work in process and raw materials is determined using average actual cost. We regularly review our inventories for impairment and reserves are established when necessary.
Inventories that are not expected to be sold within 12 months are classified as
J. Selling, Informational and Administrative Expenses
Selling, informational and administrative costs are expensed as incurred. Among other things, these expenses include the internal and external costs of marketing, advertising, shipping and handling, information technologydigital and legal defense. Advertising expenses totaled approximately $1.8$3.7 billion in 2020, $2.42023, $2.8 billion in 20192022 and $2.7$2.0 billion in 2018.2021. Production costs are expensed as incurred and the costs of TV, radio, and other electronic media and publications are expensed when the related advertising occurs.
K. Research and Development Expenses
R&D costs are expensed as incurred. These expenses include the costs of our proprietary R&D efforts, as well as costs incurredR&D activities performed in connection with certain licensing arrangements.
L. Acquired In-Process Research and Development Expenses
Before a compound receives regulatory approval, we record upfront and milestone payments we make to third parties under licensing and collaboration arrangements as expense. Upfront payments are recorded when incurred, and milestone payments are recorded when the specific milestone has been achieved. Once a compound receives regulatory approval, we record any milestone payments in Identifiable intangible assets, less accumulated amortization and, unless the asset is determined to have an indefinite life, we typically amortize the payments on a straight-line basis over the remaining agreement term or the expected product life cycle, whichever is shorter.Acquired in-process research and development expenses includes costs incurred in connection with (a) all upfront and milestone payments on collaboration and in-license agreements, including premiums on equity securities and (b) asset acquisitions of acquired IPR&D.
L.M. Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets
Long-lived assets include:
•Property, plant and equipment, less accumulated depreciation—These assets are recorded at cost, including any significant improvements after purchase, less accumulated depreciation. Property, plant and equipment assets, other than land and construction in progress, are depreciated on a straight-line basis over the estimated useful life of the individual assets. Depreciation begins when the asset is ready for its intended use. For tax purposes, accelerated depreciation methods are used as allowed by tax laws.
•Identifiable intangible assets, less accumulated amortization—These assets are recorded at fair value at acquisition. Intangible assets with finite lives are amortized on a straight-line basis over their estimated useful lives. Intangible assets with indefinite lives are not amortized until a useful life can be determined.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 61 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
•Goodwill—Goodwill represents the excess of the consideration transferred for an acquired business over the assigned values of its net assets. Goodwill is not amortized.
Amortization of finite-lived acquired intangible assets that contribute to our ability to sell, manufacture, research, market and distribute products, compounds and intellectual property is included in Amortization of intangible assets as these intangible assets benefit multiple business functions. Amortization of intangible assets that are for a single function and depreciation of property, plant and equipment are included in Cost of sales, Selling, informational and administrative expenses and/or Research and development expenses, as appropriate.assets.
We review our long-lived assets for impairment indicators throughout the year. We perform impairment testing for indefinite-lived intangible assets and goodwill at least annually and for all other long-lived assets whenever impairment indicators are present. When necessary, we record impairments of long-lived assets for the amount by which the fair value is less than the carrying value of these assets.
Specifically:
•For finite-lived intangible assets, such as developed technology rights, and for other long-lived assets, such as property, plant and equipment, whenever impairment indicators are present, we calculate the undiscounted value of the projected cash flows for the asset, or asset group, and compare this estimated amount to the carrying amount. If the carrying amount is greater, we record an impairment loss for the excess of book value over fair value. In addition, in all cases of an impairment review, we reevaluate the remaining useful lives of the assets and modify them, as appropriate.
•For indefinite-lived intangible assets, such as Brandsbrands and IPR&D assets, when necessary, we determine the fair value of the asset and record an impairment loss, if any, for the excess of book value over fair value. In addition, in all cases of an impairment review other than for IPR&D assets, we re-evaluate whether continuing to characterize the asset as indefinite-lived is appropriate.
•For goodwill, when necessary, we determine the fair value of each reporting unit and record an impairment loss, if any, for the excess of the book value of the reporting unit over the implied fair value.
M.N. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
We may incur restructuring charges in connection with acquisitions when we implement plans to restructure and integrate the acquired operations or in connection with our cost-reduction and productivity initiatives.
•In connection with acquisition activity, we typically incur costs associated with executing the transactions, integrating the acquired operations (which may include expenditures for consulting and the integration of systems and processes), and restructuring the combined company (which may include charges related to employees, assets and activities that will not continue in the combined company); and
•In connection with our cost-reduction/productivity initiatives, we typically incur costs and charges for site closings and other facility rationalization actions, workforce reductions and the expansion of shared services, including the development of global systems.
Included in Restructuring charges and certain acquisition-related costs are all restructuring charges, as well as certain other costs associated with acquiring and integrating an acquired business.company. If the restructuring action results in a change in the estimated useful life of an asset, that incremental impact is classified in Cost of sales, Selling, informational and administrative expenses and/or Research and development
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 61 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
expenses, as appropriate. Employee termination costs are generally recorded when the actions are probable and estimable and include accrued severance benefits, pension and postretirement benefits, many of which may be paid out during periods after termination. Transaction costs, such as banking, legal, accounting and other similar costs incurred in connection with a business acquisition are expensed as incurred.
Our business and platform functions may be impacted by these actions, including sales and marketing, manufacturing and R&D, as well as our corporate enabling functions (such as digital, global real estate operations, legal, finance, human resources, worldwide public affairs, compliance and worldwide procurement).functions.
N.O. Cash Equivalents and Statement of Cash Flows
Cash equivalents include items almost as liquid as cash, such as certificates of deposit and time deposits with maturity periods of three months or less when purchased. If items meeting this definition are part of a larger investment pool, we classify them as Short-term investments.
Cash flows for financial instruments designated as fair value or cash flow hedges may be included in operating, investing or financing activities, depending on the classification of the items being hedged. Cash flows for financial instruments designated as net investment hedges are classified according to the nature of the hedgehedging instrument. Cash flows for financial instruments that do not qualify for hedge accounting treatment are classified according to their purpose and accounting nature.
O.P. Investments and Derivative Financial Instruments
The classification of an investment depends on the nature of the investment, our intent and ability to hold the investment, and the degree to which we may exercise influence. Our investments are primarily comprised of the following:
•Public equity securities with readily determinable fair values, which are carried at fair value, with changes in fair value reported in Other (income)/deductions—net.
•Available-for-sale debt securities, which are carried at fair value, with changes in fair value reported in Other comprehensive income/(loss) until realized.
•Held-to-maturity debt securities, which are carried at amortized cost.
•Private equity securities without readily determinable fair values and where we have no significant influence are measured at cost minus any impairment and plus or minus changesadjustments resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer.
•For equity investments in common stock or in-substance common stock where we have significant influence over the financial and operating policies of the investee, we use the equity-method of accounting. Under the equity-method, we record our share of the investee’s income and expenses in Other (income)/deductions—net. The excess of the cost of the investment over our share of the underlying equity in the net assets of the investee as of the acquisition date is allocated to the identifiable assets and liabilities of the investee, with any remaining
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 62 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
excess amount allocated to goodwill. Such investments are initially recorded at cost, which is the fair value of consideration paid and typically does not include contingent consideration.
Realized gains or losses on sales of investments are determined by using the specific identification cost method.
We regularly evaluate all of our financial assets for impairment. For investments in debt and equity, if and when a decline in fair value if any, is determined, an impairment charge is recorded and a new cost basis in the investment is established.
Derivative financial instruments are carried at fair value in variouscertain balance sheet categories (see Note 7A), with changes in fair value reported in Netnet income or, for derivative financial instruments in certain qualifying hedging relationships, in Other comprehensive income/(loss) (see Note 7E).
P. Q. Tax Assets and Liabilities and Income Tax Contingencies
Tax Assets and Liabilities
Current tax assets primarily includesinclude (i) tax effects for intercompany transfers of inventory within our combined group, which are recognized in the consolidated statements of income when the inventory is sold to a third party and (ii) income tax receivables that are expected to be recovered either via refunds from taxing authorities or reductions to future tax obligations.
Deferred tax assets and liabilities are recognized for the expected future tax consequences of differences between the financial reporting and tax bases of assets and liabilities using enacted tax rates and laws. We provide a valuation allowance when we believe that our deferred tax assets are not recoverable based on an assessment of estimated future taxable income that incorporates ongoing, prudent and feasible tax-planning strategies, that would be implemented, if necessary, to realize the deferred tax assets. Amounts recorded for valuation allowances requires judgments about future income which can depend heavily on estimates and assumptions. All deferred tax assets and liabilities within the same tax jurisdiction are presented as a net amount in the noncurrent section of our consolidated balance sheet.
Other non-current tax assets primarily represent our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction. These potential benefits generally result from cooperative efforts among taxing authorities, as required by tax treaties to minimize double taxation, commonly referred to as the competent authority process. The recoverability of these assets, which we believe to be more likely than not, is dependent upon the actual payment of taxes in one tax jurisdiction and, in some cases, the successful petition for recovery in another tax jurisdiction.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 62 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Other taxes payable as of December 31, 20202023 and 20192022 include liabilities for uncertain tax positions and the noncurrent portion of the repatriation tax liability for which we elected payment over eight years through 2026. For additional information, seeSee Note 5D for uncertain tax positions and Note 5A for the repatriation tax liability and other estimates and assumptions in connection with the TCJA.
Income Tax Contingencies
––We account for income tax contingencies using a benefit recognition model. If we consider that a tax position is more likely than not to be sustained upon audit, based solely on the technical merits of the position, we recognize all or a portion of the benefit. We measure the benefit by determining the amount that is greater than 50% likely of being realized upon settlement, presuming that the tax position is examined by the taxing authority with full knowledge of all relevant information.
We regularly monitor our position and subsequently recognize the unrecognized tax benefit: (i) if there are changes in tax law, analogous case law or there is new information that sufficiently raise the likelihood of prevailing on the technical merits of the position to “more likely than not”; (ii) if the statute of limitations expires; or (iii) if there is a completion of an audit resulting in a favorable settlement of that tax year with the appropriate agency. Liabilities for uncertain tax positions are classified as current only when we expect to pay cash within the next 12 months. Interest and penalties, if any, are recorded in Provision/(benefit) for taxes on income and are classified on our consolidated balance sheet with the related tax liability.
Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes, and variation from such estimates could materially affect our financial statements in the period of settlement or when the statutes of limitations expire, as we treat these events as discrete items in the period of resolution.
Q.R. Pension and Postretirement Benefit Plans
The majority of our employees worldwide are covered by defined benefit pension plans, defined contribution plans or both. In the U.S., we have both IRC-qualified and supplemental (non-qualified) defined benefit plans and defined contribution plans, as well as other postretirement benefit plans consisting primarily of medical insurance for retirees and their eligible dependents. Net periodic pension and postretirement benefit costs other than the service costs are recognized in Other (income)/deductions—net. We immediately recognize actuarial gains and losses arising from the remeasurement of our pension and postretirement plans (mark-to-market accounting). Each time a pension or postretirement plan is remeasured, the actuarial gain or loss is recognized immediately and classified as Other (income)/deductions––net. We recognize the overfunded or underfunded status of each of our defined benefit plans as an asset or liability. The obligations are generally measured at the actuarial present value of all benefits attributable to employee service rendered, as provided by the applicable benefit formula. Our pension and other postretirement obligations may be determined using assumptions such as discount rate, expected annual rate of return on plan assets, expected employee turnover and participant mortality. For our pension plans, the obligation may also include assumptions as to future compensation levels. For our other postretirement benefit plans, the obligation may include assumptions as to the expected cost of providing medical insurance benefits, as well as the extent to which those costs are shared with the employee or others (such as governmental programs). Plan assets are measured at fair value. Net periodic pension and postretirement benefit costs other than the service costs are recognized in Other (income)/deductions—net.
R. | | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 63 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
S. Legal and Environmental Contingencies
We and certain of our subsidiaries are subject to numerous contingencies arising in the ordinary course of business, such as patent litigation, product liability and other product-related litigation, commercial litigation,and other asserted or unasserted matters, environmental claims and proceedings, government investigations and guarantees and indemnifications. WeIn assessing contingencies related to legal and environmental proceedings that are pending against the Company, or unasserted claims that are probable of being asserted, we record accruals for these contingencies to the extent that we conclude that a loss is both probable and reasonably estimable. If some amount within a range of loss appears to be a better estimate than any other amount within the range, we accrue that amount. Alternatively, when no amount within a range of loss appears to be a better estimate than any other amount, we accrue the lowest amount in the range. We record anticipated recoveries under existing insurance contracts when recovery is assured.
S.T. Share-Based Payments
Our compensation programs can include share-based payments. Generally, grants under share-based payment programs are accounted for at fair value and these fair values are generally amortized on a straight-line basis or on an accelerated attribution approach over the vesting terms with the related costs recorded in Cost of sales, Selling, informational and administrative expenses and/or Research and development expenses, as appropriate.
Note 2. Acquisitions, Divestitures, Equity-Method Investments, Licensing Arrangement, Collaborative Arrangements and Collaborative ArrangementsResearch and Development Arrangement
A. Acquisitions
Array
Seagen––On July 30, 2019,December 14, 2023 (the acquisition date), we acquired Array,Seagen, a commercial stage biopharmaceuticalglobal biotechnology company focused on the discovery, developmentthat discovers, develops and commercialization of targeted small moleculecommercializes transformative cancer medicines, to treat cancer and other diseases of high unmet need, for $48$229 per share in cash. The total fair value of the consideration transferred was $11.2$44.2 billion ($10.943.4 billion, net of cash acquired). In addition, $157in connection with the acquisition $476 million in post-closing compensation expense for Seagen employee incentive awards was recorded in Restructuring charges and certain acquisition-related costs (see Note 3). The combination of local Pfizer and Seagen entities may be pending in various jurisdictions and integration is subject to completion of various local legal and regulatory steps. Seagen’s principal business was the development, manufacture, marketing and distribution of targeted cancer therapeutics, primarily using antibody-drug conjugate technology. Seagen’s portfolio includes four approved medicines as well as a pipeline of product candidates. Clinical development programs are ongoing for each of these approved medicines for potential new or expanded indications and for several product candidates. We believe our acquisition of Seagen will strengthen our oncology capabilities by allowing us to combine Seagen’s antibody-drug conjugate technology with the resources and scale of the Pfizer enterprise and to advance more potential breakthroughs to patients with cancer.
The following table summarizes the provisional amounts recognized for assets acquired and liabilities assumed as of the acquisition date. The estimated values are not yet finalized (see below) and are subject to change, which could be significant. We will finalize the amounts recognized as we obtain the information necessary to complete the analyses. We expect to finalize these amounts as soon as possible but no later than one year from the acquisition date.
| | | | | | | | |
| (MILLIONS) | | Amounts Recognized as of Acquisition Date (Provisional) |
Working capital, excluding inventories(a) | | $ | 736 | |
Inventories(b) | | 4,195 | |
| Property, plant and equipment | | 524 | |
Identifiable intangible assets, excluding in-process research and development(c) | | 7,970 | |
| In-process research and development | | 20,800 | |
| Other noncurrent assets | | 174 | |
Net income tax accounts(d) | | (6,123) | |
| Other noncurrent liabilities | | (167) | |
| Total identifiable net assets | | 28,108 | |
| Goodwill | | 16,126 | |
| Net assets acquired/total consideration transferred | | $ | 44,234 | |
(a)Includes cash and cash equivalents, accounts receivable, other current assets, accounts payable, accrued compensation and other current liabilities.
(b)Comprised of $1.0 billion current inventories and $3.1 billion noncurrent inventories.
(c)Comprised mainly of $7.5 billion of finite-lived developed technology rights with an estimated weighted-average life of approximately 18 years.
(d)As of the acquisition date, included primarily in Noncurrent deferred tax liabilities.
The following items are subject to change:
•Amounts for certain balances included in working capital (excluding inventories), and certain legal contingencies, pending receipt of certain information that could affect provisional amounts recorded. We do not believe any adjustments for legal contingencies will have a material impact on our consolidated financial statements.
•Amounts for identifiable intangible assets, inventories, contractual commitments, PP&E, and operating lease ROU assets and liabilities, pending finalization of valuation efforts, the completion of certain physical inventory counts and the confirmation of the physical existence and condition of certain PP&E assets.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 64 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
•Amounts for income tax assets, receivables and liabilities, pending the filing of Seagen’s pre-acquisition tax returns and the receipt of information, including but not limited to that from taxing authorities, which may change certain estimates and assumptions used.
As of the acquisition date, the fair value of accounts receivable approximated the book value acquired. The gross contractual amount receivable was $597 million.
In the ordinary course of business, Seagen may incur liabilities for environmental, legal and tax matters, as well as guarantees and indemnifications. These matters may include contingencies. Except as specifically excluded by the relevant accounting standard, contingencies are required to be measured at fair value as of the acquisition date if the acquisition-date fair value of the asset or liability arising from a contingency can be determined. If the acquisition-date fair value of the asset or liability cannot be determined, the asset or liability would be recognized at the acquisition date if both of the following criteria are met: (i) it is probable that an asset existed or that a liability had been incurred at the acquisition date, and (ii) the amount of the asset or liability can be reasonably estimated.
•Environmental Matters—In the ordinary course of business, Seagen may incur liabilities for environmental matters such as remediation work, asset retirement obligations and environmental guarantees and indemnifications.
•Legal Matters—Seagen is involved in various legal proceedings, including patent, intellectual property, and product liability matters of a nature considered normal to its business. The contingencies arising from legal matters are not significant to our consolidated financial statements.
•Tax Matters—In the ordinary course of business, Seagen incurs liabilities for income taxes. Income taxes are exceptions to both the recognition and fair value measurement principles associated with the accounting for business combinations. Reserves for income tax contingencies continue to be measured under the benefit recognition model previously used by Seagen (see Note 1Q). Net liabilities for income taxes as of the acquisition date were $6.1 billion, including $56 million for uncertain tax positions. The net tax liability includes $7.5 billion for the tax impact of fair value adjustments, partially offset by $1.4 billion for deferred tax assets on which Seagen had recognized a valuation allowance. Goodwill is calculated as the excess of the consideration transferred over the net assets recognized and represents the future economic benefits arising from other assets acquired that could not be individually identified and separately recognized. Specifically, the goodwill recorded as part of the acquisition of Seagen includes the following:
•the expected specific synergies and other benefits that we believe will result from combining the operations of Seagen with the operations of Pfizer;
•any intangible assets that do not qualify for separate recognition, as well as future, as yet unidentified projects and products; and
•the value of the going-concern element of Seagen’s existing businesses (the higher rate of return on the assembled collection of net assets versus if Pfizer had acquired all of the net assets separately).
Goodwill is not amortized and is not deductible for tax purposes. All of the goodwill related to the acquisition of Seagen is related to our Biopharma segment (see Note 10). Actual and Pro Forma Impact of Acquisition—The following table presents information for Seagen’s operations that are included in Pfizer’s consolidated statements of income beginning from the acquisition date, December 14, 2023, through Pfizer’s year-end in 2023:
| | | | | | | | | |
| (MILLIONS) | | | December 31, 2023 |
| Revenues | | | $ | 120 | |
Net loss attributable to Pfizer Inc. common shareholders(a) | | | (746) | |
(a)Includes restructuring, integration and acquisition-related costs ($614 million pre-tax) and purchase accounting charges related to (i) the preliminary fair value adjustment for acquisition-date inventory estimated to have been sold ($109 million pre-tax); (ii) amortization expense related to the preliminary fair value of identifiable intangible assets acquired from Seagen ($25 million pre-tax); as well as (iii) depreciation expense related to the preliminary fair value adjustment of fixed assets acquired from Seagen ($2 million pre-tax).
The following table provides unaudited U.S. GAAP supplemental pro forma information as if the acquisition of Seagen had occurred on January 1, 2022:
| | | | | | | | | | | | | | | | | | |
| | | | | | Unaudited Supplemental Pro Forma Consolidated Results |
| | | | Year Ended December 31, |
| (MILLIONS, EXCEPT PER SHARE DATA) | | | | | | 2023 | | 2022 |
| Revenues | | | | | | $ | 60,632 | | | $ | 102,127 | |
| Net income/(loss) attributable to Pfizer Inc. common shareholders | | | | | | (1,474) | | | 27,938 | |
| Diluted earnings/(loss) per share attributable to Pfizer Inc. common shareholders | | | | | | (0.26) | | | 4.87 | |
The unaudited supplemental pro forma consolidated results do not purport to reflect what the combined company’s results of operations would have been had the acquisition occurred on January 1, 2022, nor do they project the future results of operations of the combined company or reflect the expected realization of any cost savings associated with the acquisition. The actual results of operations of the combined company may differ significantly from the pro forma adjustments reflected here due to many factors.
The unaudited supplemental pro forma financial information includes various assumptions, including those related to the preliminary purchase price allocation of the assets acquired and the liabilities assumed from Seagen. The historical U.S. GAAP financial information of Pfizer and Seagen was adjusted, primarily for the following pre-tax adjustments:
•Additional amortization expense (approximately $503 million in 2023 and $526 million in 2022) related to the preliminary estimate of the fair value of identifiable intangible assets acquired.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 65 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
•Additional expense related to the preliminary estimate of the fair value adjustment to acquisition-date inventory estimated to have been sold (approximately $796 million in 2023 and $887 million in 2022).
•Additional interest expense (approximately $984 million in 2023 and $2.0 billion in 2022) related to the estimated debt issued by Pfizer and the commercial paper borrowings to partially finance the acquisition.
•Elimination of interest income (approximately $1.2 billion in 2023 and $267 million in 2022) related to the debt issuance proceeds that were invested prior to the acquisition date and associated with money market funds under the assumption that a portion of these funds would have been liquidated to partially fund the acquisition.
•Adjustment to move Seagen royalty income received from collaboration partners (approximately $203 million in 2023 and $165 million in 2022) from total revenues to other (income)/deductions, which is consistent with Pfizer’s presentation in 2023.
The above adjustments were then adjusted for the applicable tax impact using an estimated weighted-average statutory tax rate applied to the applicable pro forma adjustments.
The acquisition of Seagen had no impact on Pfizer’s weighted-average shares as no shares were issued.
GBT––On October 5, 2022, we acquired GBT, a biopharmaceutical company dedicated to the discovery, development and delivery of life-changing treatments for underserved patient communities, starting with sickle cell disease, for $68.50 per share in cash. The total fair value of the consideration transferred was $5.7 billion ($5.2 billion, net of cash acquired). In addition, $136 million in payments to ArrayGBT employees for the fair value of previously unvested stock optionslong-term incentive awards was recognized as post-closing compensation expense and recorded in Restructuring charges and certain acquisition-related costs(see (see Note 3). We financed the majority of the transaction with debt and the balance with existing cash. Array’s portfolio includes Braftovi (encorafenib) and Mektovi (binimetinib), a broad pipeline of targeted cancer medicines in different stages of R&D, as well as a portfolio of out-licensed medicines, which may generate milestones and royalties over time.
The final allocation of the consideration transferred to the assets acquired and the liabilities assumed was completed in 2020.2023. In connection with this acquisition,business combination, we recorded: (i) $6.3$4.4 billion in Identifiable intangible assets, consisting of $2.0$3.0 billion of IPR&D and $1.4 billion of developed technology rights with a useful life of six years, (ii) $1.1 billion of DevelopedGoodwill, (iii) $644 million of inventories to be sold over approximately three years, (iv) $516 million of net deferred tax liabilities and (v) $331 million of assumed long-term debt that was paid in full in the fourth quarter of 2022.
Biohaven––On October 3, 2022, we acquired Biohaven, the maker of Nurtec ODT/Vydura (rimegepant), an innovative therapy approved for both acute treatment of migraine and prevention of episodic migraine in adults. The transaction included the acquisition of Biohaven’s CGRP programs, including rimegepant, zavegepant and a portfolio of five pre-clinical CGRP assets. Under the terms of the agreement, we acquired all outstanding common shares of Biohaven not already owned by us for $148.50 per share, in cash, for payments of approximately $11.5 billion, plus repayment of third-party debt of $863 million and redemption of Biohaven’s redeemable preferred stock for $495 million. Effective immediately prior to the closing of the acquisition, Biohaven completed the spin-off of Biohaven Ltd. (NYSE: BHVN), distributing Biohaven Ltd.’s shares to Biohaven shareholders. Biohaven Ltd. became a new publicly traded company that retained Biohaven’s non-CGRP development stage pipeline compounds. Pfizer, a Biohaven shareholder, received a pro rata portion of Biohaven Ltd.’s shares in the distribution and owns approximately 1.3% of Biohaven Ltd. as of December 31, 2023.
This acquisition follows on the November 2021 collaboration for the commercialization of rimegepant and zavegepant outside the U.S., in connection with which Pfizer acquired 2.6% of Biohaven’s common stock (see Note 2E). Biohaven Ltd. also has the right to receive tiered royalties from Pfizer on any annual net sales of rimegepant and zavegepant in the U.S. in excess of $5.25 billion. This contingent consideration was determined to have no fair value as of the acquisition date. Pfizer also acquired Biohaven’s commitments for payment of high single digit to mid-teen percentage tiered royalties on world-wide net sales excluding China and low to high single digit royalties on net sales in China of rimegepant and zavegepant as well as certain regulatory approval and commercial milestone payments associated with rimegepant and zavegepant of up to $1.1 billion under pre-existing third-party license and other agreements. These milestone amounts have been reduced by $608 million since the acquisition due to payments made and renegotiation of certain of the applicable agreements. The total fair value of the consideration transferred was $11.8 billion, which includes the fair value of Pfizer’s previous investment in Biohaven on the acquisition date of approximately $300 million. The final allocation of the consideration transferred to the assets acquired and the liabilities assumed was completed in 2023. In connection with this business combination, we recorded: (i) $12.1 billion in Identifiable intangible assets, consisting of $11.6 billion of developed technology rights with a useful life of 11 years and $450 million of IPR&D, (ii) $823 million of Goodwill, (iii) $813 million of inventories to be sold over approximately two years, (iv) $398 million of trade accounts receivable, (v) $1.4 billion of assumed long-term debt that was paid in full in the fourth quarter of 2022, (vi) $544 million of net deferred tax liabilities and (vii) $526 million of Other current liabilities.
Arena––On March 11, 2022, we acquired Arena, a clinical stage company with development-stage therapeutic candidates in gastroenterology, dermatology and cardiology, for $100 per share in cash. The total fair value of the consideration transferred was $6.6 billion ($6.2 billion, net of cash acquired). In addition, $138 million in payments to Arena employees for the fair value of previously unvested long-term incentive awards was recognized as post-closing compensation expense and recorded in Restructuring charges and certain acquisition-related costs (see Note 3). The final allocation of the consideration transferred to the assets acquired and the liabilities assumed was completed in 2023. In connection with this business combination, we recorded: (i) $5.5 billion in Identifiable intangible assets, consisting of $5.0 billion of IPR&Dand $460 million of indefinite-lived licensing agreements and other, (ii) $1.0 billionof Goodwill and (iii) $490 million of net deferred tax liabilities.
ReViral––On June 9, 2022, we acquired ReViral, a privately held, clinical-stage biopharmaceutical company focused on discovering, developing and commercializing novel antiviral therapeutics that target respiratory syncytial virus, for a total consideration of up to $536 million, including upfront payments of $436 million upon closing (including a base payment of $425 million plus working capital adjustments) and an additional $100 million contingent upon a future development milestone for a secondary pipeline asset. It was subsequently determined the applicable milestone was not achieved.
We accounted for the transaction as an asset acquisition since the lead asset, sisunatovir, represented substantially all of the fair value of the gross assets acquired. At the acquisition date, we recorded a $426 million charge representing an acquired IPR&D asset with no alternative
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 6366 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
a useful life of 16 yearsuse in , $2.8 billion of IPR&D Acquired in-process research and $1.5 billion of Licensing agreements ($1.2 billion for technology in development––indefinite-lived licensing agreements and $360 million for developed technology––finite-lived licensing agreements with a useful life of 10 years), (ii) $6.1 billion of Goodwill expenses, (iii) $1.1 billion of net deferred taxwhich is presented as a cash outflow from operating activities. Other assets acquired and liabilities and (iv) $451 million of assumed long-term debt, which was paid in full in 2019.were not significant.
In 2020, we recorded measurement period adjustments to the estimated fair values initially recorded in 2019, which resulted in a reduction in Trillium––Identifiable intangible assets of approximately $900 million with a corresponding change to Goodwill and net deferred tax liabilities. The measurement period adjustments were recorded to better reflect market participant assumptions about facts and circumstances existing as of the acquisition date and did not have a material impact on our consolidated statement of income for the year ended December 31, 2020.
Therachon
On July 1, 2019,November 17, 2021, we acquired all the remaining shares of Therachon, a privately-held clinical-stage biotechnology company focused on rare diseases, with assets in development for the treatment of achondroplasia, a genetic condition and the most common form of short-limb dwarfism, for $340 million upfront, plus potential milestone payments of up to $470 million contingent on the achievement of key milestones in the development and commercialization of the issued and outstanding common stock not already owned by Pfizer of Trillium, a clinical stage immuno-oncology company developing therapies targeting cancer immune evasion pathways and specific cell targeting approaches, for $18.50 per share in cash, for total consideration of $2.0 billion, net of cash acquired. As a result, Trillium became our wholly owned subsidiary. We previously held a 2% ownership investment in Trillium. Trillium’s lead asset. In 2018, we acquired approximately 3%program, TTI-622, is an investigational fusion protein that is designed to block the inhibitory activity of Therachon’s outstanding shares for
$5 million. CD47, a molecule that is overexpressed by a wide variety of tumors.
We accounted for the transaction as an asset acquisition since the lead asset, TTI-622, represented substantially all of the fair value of the gross assets acquired, which exclude cash acquired. The total fair valueAt the acquisition date, we recorded a $2.1 billion charge representing an acquired IPR&D asset with no alternative future use in Acquired in-process research and development expenses, of which the $2.0 billion net cash consideration transferred for Therachon was $322 million, which consisted of $317 million ofis presented as a cash and our previous $5 million investment in Therachon.outflow from operating activities. In connection with this asset acquisition, we recorded $256 million of assets acquired primarily consisting of cash and investments. Liabilities assumed were approximately $81 million.
Pro forma information for the aforementioned acquisitions (except for Seagen) has not been presented because these acquisitions were not material to our consolidated financial statements.
B. Divestitures
Divestiture of Early-Stage Rare Disease Gene Therapy Portfolio––On September 19, 2023, we completed an agreement with Alexion, under which Alexion purchased and licensed the assets of our early-stage rare disease gene therapy portfolio. This agreement is consistent with our previously announced strategy to pivot from viral capsid-based gene therapy approaches to harnessing new platform technologies that we believe can have a chargetransformative impact on patients, such as mRNA or in vivo gene editing. Under the terms of $337the agreement, Alexion will pay us total consideration of up to $1 billion, consisting of an upfront payment of $300 million which was paid at closing and future contingent milestone payments, plus tiered royalties based on annual net sales of the assets. In connection with the closing of the transaction, Pfizer recognized a $222 million pre-tax gain in Other (income)/deductions––net (see Note 4). Discontinued Operations
Meridian––On December 31, 2021, we completed the sale of our Meridian subsidiary for approximately $51 million in cash and recognized a loss of approximately $167 million, net of tax, in ResearchDiscontinued operations––net of tax. In connection with the sale, Pfizer and development expenses.the purchaser of Meridian entered into various agreements to provide a framework for our relationship after the sale, including interim TSAs and an MSA. Services under the TSAs are completed as of December 31, 2023. The MSA is for a term of three years post sale with a two year extension period. Amounts recorded under the interim TSAs and MSA in 2023 and 2022 were not material to our operations. No amounts were recorded under these arrangements in 2021.
B. Divestitures
Upjohn Separation and Combination with Mylan
Mylan––
On November 16,In connection with the 2020 we completed the spin-off and the combination of the Upjohn Business with Mylan (the Transactions) to form Viatris. The Transactions were structured as an all-stock, Reverse Morris Trust transaction. Specifically, (i) we contributed the Upjohn Business to a wholly owned subsidiary, which was renamed Viatris, so that the Upjohn Business was separated from the remainder of our business (the Separation), (ii) following the Separation, we distributed, on a pro rata basis, all of the shares of Viatris common stock held by Pfizer to Pfizer stockholders as of the November 13, 2020 record date, such that each Pfizer stockholder as of the record date received approximately 0.124079 shares of Viatris common stock per share of Pfizer common stock (the Distribution); and (iii) immediately after the Distribution, the Upjohn Business combined with Mylan in a series of transactions in which Mylan shareholders received 1 share of Viatris common stock for each Mylan ordinary share held by such shareholder, subject to any applicable withholding taxes (the Combination). Prior to the Distribution, Viatris made a cash payment to Pfizer equal to $12.0 billion as partial consideration for the contribution of the Upjohn Business to Viatris. As of the closing of the Combination, Pfizer stockholders owned approximately 57% of the outstanding shares of Viatris common stock, and Mylan shareholders owned approximately 43% of the outstanding shares of Viatris common stock, in each case on a fully diluted, as-converted and as-exercised basis. The Transactions are generally expected to be tax free to Pfizer and Pfizer stockholders for U.S. tax purposes. Beginning November 16, 2020, Viatris operates both the Upjohn Business and Mylan as an independent publicly traded company, which is traded under the symbol “VTRS” on the NASDAQ.
In connection with the Transactions, in June 2020, Upjohn Inc. and Upjohn Finance B.V. completed privately placed debt offerings of $7.45 billion and €3.60 billion aggregate principal amounts, respectively, (approximately $11.4 billion) of senior unsecured notes and entered into other financing arrangements, including a $600 million delayed draw term loan agreement and a revolving credit facility agreement for up to $4.0 billion. Proceeds from the debt offerings and other financing arrangements were used to fund the $12.0 billion cash distribution Viatris made to Pfizer prior to the Distribution. We used the cash distribution proceeds to pay down commercial paper borrowings and redeem the $1.15 billion aggregate principal amount outstanding of our 1.95% senior unsecured notes that were due in June 2021 and $342 million aggregate principal amount outstanding of our 5.80% senior unsecured notes that were due in August 2023, before the maturity date. Interest expense for the $11.4 billion in debt securities incurred during 2020 is included in Income from discontinued operations––net of tax. Following the Separation and Combination of the Upjohn Business with Mylan, we are no longer the obligor or guarantor of any Upjohn debt or Upjohn financing arrangements.
As a result of the separation of Upjohn, we incurred separation-related costs of $434 million in 2020 and $83 million in 2019, which are included in Income from discontinued operations––net of tax. These costs primarily relate to professional fees for regulatory filings and separation activities within finance, tax, legal and information system functions as well as investment banking fees.
In connection with the Transactions, Pfizer and Viatris entered into various agreements, to effect the Separation and Combination to provide a framework for our relationship after the Combination, including a separation and distribution agreement, manufacturing and supply agreements (MSAs), transition service agreements (TSAs),interim operating models, including agency arrangements, MSAs, TSAs, a tax matters agreement, and an employee matters agreement, among others. The interim agency operating model arrangements primarily include billings, collections and remittance of rebates that we are performing on a transitional basis on behalf of Viatris. Under the MSAs, Pfizer or Viatris, as the case may be, manufactures, labels and packages products for the other party. The terms of the MSAs range in initial duration from 4four to 7seven years post-Separation. Thepost-separation. Services under the TSAs primarily involve Pfizer providing services to Viatris related to finance, information technology and human resource infrastructure and are generally expected to be for termswere largely completed as of no more than 3 years post-Separation. In addition, we are also party to various commercial agreements with Viatris. The amounts billed for net manufacturing supply and transition services providedDecember 31, 2023. Amounts recorded under the above agreements as well as sales toin 2023, 2022 and purchases from Viatris are2021 were not material to our results of continuing operations in 2020.
Included in our consolidated balance sheetoperations. Net amounts due to Viatris under the above agreements were $33 million as of December 31, 20202023 and $94 million as of December 31, 2022. The cash flows associated with the above agreements are net amounts dueincluded in Net cash provided by operating activities from Viatris primarily related to various interim agency operating models and transitional services, partially offset by net amounts duecontinuing operations, except fora $277 million payment to Viatris for unsettled intercompany balances as of the closing date of the spin-off, transaction-related indemnifications and a contractual cash paymentmade in 2021 pursuant to terms of the separation and distribution agreement, totaling approximately $401 million. The interim agency operating model primarily includes billings, collections and remittance of rebates that we are performing on a transitional basis on behalf of Viatris.
The operating results of the Upjohn Business arewhich is reported asin Income from discontinued operations––Other financing activities, net of tax through November 16, 2020, the date of the spin-off and combination with Mylan. In addition, as of December 31, 2019, the assets and liabilities associated with this business are classified as assets and liabilities of discontinued operations. Prior-period financial information has been restated, as appropriate..
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 6467 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| Components of Income from discontinued operations––net of tax: | | | | Year Ended December 31,(a) |
| (MILLIONS OF DOLLARS) | | | 2020 | | 2019 | | 2018 |
| Revenues | | | $ | 7,314 | | | $ | 10,578 | | | $ | 12,822 | |
Components of Discontinued operations––net of tax: | |
Components of Discontinued operations––net of tax: | |
Components of Discontinued operations––net of tax: | |
| | | | Year Ended December 31,(a) | | | | | | Year Ended December 31,(a) |
| (MILLIONS) | | (MILLIONS) | | | | 2023 | | 2022 | | 2021 |
| Total revenues | |
| Costs and expenses: | Costs and expenses: | | | | |
| Cost of sales | |
| Cost of sales | |
| Cost of sales | Cost of sales | | | 1,899 | | | 1,976 | | | 2,261 | |
| Selling, informational and administrative expenses | Selling, informational and administrative expenses | | | 1,665 | | | 1,599 | | | 1,842 | |
| Research and development expenses | Research and development expenses | | | 212 | | | 255 | | | 246 | |
| Acquired in-process research and development expenses | |
| Amortization of intangible assets | Amortization of intangible assets | | | 136 | | | 148 | | | 157 | |
| Restructuring charges and certain acquisition-related costs | Restructuring charges and certain acquisition-related costs | | | 7 | | | 146 | | | (14) | |
| Other (income)/deductions––net | Other (income)/deductions––net | | | 400 | | | 253 | | | 30 | |
| Pre-tax income from discontinued operations | | | 2,995 | | | 6,201 | | | 8,300 | |
| Provision for taxes on income | | | 364 | | | 766 | | | 973 | |
| | Income from discontinued operations––net of tax | | | $ | 2,631 | | | $ | 5,435 | | | $ | 7,328 | |
| Pre-tax income/(loss) from discontinued operations | |
| Provision/(benefit) for taxes on income | |
| Income/(loss) from discontinued operations––net of tax | |
| Pre-tax gain/(loss) on sale of discontinued operations | |
| Provision/(benefit) for taxes on income | |
| Gain/(loss) on sale of discontinued operations––net of tax | |
| Discontinued operations––net of tax | |
(a)Virtually allIn 2023 and 2022, Income from discontinued operations––Discontinued operations—net of tax relates to post-close adjustments. In 2021, Discontinued operations—net of tax primarily includes (i) the Upjohn Businessoperations of Meridian prior to its sale on December 31, 2021 recognized in Income/(loss) from discontinued operations—net of tax, which includes a pre-tax expense to resolve an MDL relating to EpiPen against the Company in the U.S. District Court for the District of Kansas for $345 million; and (ii) the after tax loss of $167 million related to the sale of Meridian recognized in Gain/(loss) on sale of discontinued operations––net of tax. To a much lesser extent, Discontinued operations—net of tax in 2021 also includes the operations of the Mylan-Japan collaboration prior to its termination on December 21, 2020 and post-close adjustments directly related to our former Upjohn and Nutrition discontinued businesses, including adjustments for tax, benefits and legal-related matters recognized in all periods presented.Income/(loss) from discontinued operations—net of tax.
| | | | | | | | | | | | | | | | |
| Components of assets and liabilities of discontinued operations and other assets held for sale: |
| | | | As of December 31,(a) |
| (MILLIONS OF DOLLARS) | | | | 2020 | | 2019 |
| Cash and cash equivalents | | | | $ | 0 | | | $ | 184 | |
| Trade accounts receivable, less allowance for doubtful accounts | | | | 0 | | | 1,952 | |
| Inventories | | | | 86 | | | 1,215 | |
| Other current assets | | | | 0 | | | 852 | |
| Other assets held for sale | | | | 82 | | | 21 | |
| Current assets of discontinued operations and other assets held for sale | | | | $ | 167 | | | $ | 4,224 | |
| | | | | | |
| Property, plant and equipment | | | | $ | 0 | | | $ | 998 | |
| Identifiable intangible assets | | | | 0 | | | 1,434 | |
| Goodwill | | | | 0 | | | 10,451 | |
| Other noncurrent assets | | | | 0 | | | 544 | |
| Noncurrent assets of discontinued operations | | | | $ | 0 | | | $ | 13,427 | |
| | | | | | |
| Trade accounts payable | | | | $ | 0 | | | $ | 334 | |
| Accrued compensation and related items | | | | 0 | | | 330 | |
| Other current liabilities | | | | 0 | | | 1,749 | |
| Current liabilities of discontinued operations | | | | $ | 0 | | | $ | 2,413 | |
| | | | | | |
| Pension and postretirement benefit obligations | | | | $ | 0 | | | $ | 545 | |
| Other noncurrent liabilities | | | | 0 | | | 403 | |
Noncurrent liabilities of discontinued operations(b) | | | | $ | 0 | | | $ | 948 | |
C. Equity-Method Investments
(a) Haleon/Consumer Healthcare JV––Amounts relate to discontinued operationsOn July 18, 2022, GSK completed a demerger of the Upjohn Business and the Mylan-Japan collaboration, except for amounts in Other assets held for sale,Consumer Healthcare JV which represent unrelated property, plant and equipment held for sale.
(b) Included in Other noncurrent liabilities.
As a result of the spin-off of the Upjohn Business, we distributed net assets of $1.9 billion as of November 16, 2020, which has been reflected as a reduction to Retained earnings. Of this amount, $412 million represents cash transferred to the Upjohn Business, with the remainder considered a non-cash activity in the consolidated statement of cash flows for the year ended December 31, 2020. The spin-off also resulted in a net increase to Accumulated other comprehensive loss of $71 million for the derecognition of net gains on foreign currency translation adjustments of $397 million and actuarial losses net of prior service credits associated with benefit plans of $326 million, which were reclassified to Retained earnings.
Contribution Agreement Between Pfizer and Allogene
In April 2018, Pfizer and Allogene announced that the two companies entered into a contribution agreement for Pfizer’s portfolio of assets related to allogeneic CAR T therapy,became Haleon, an investigational immune cell therapy approach to treating cancer. Under this agreement, we received an equity investment in Allogene and Allogene received our rights to pre-clinical and clinical CAR T assets, all of which were previously licensed to us from French cell therapyindependent, publicly traded company Cellectis, beginning in 2014 and French pharmaceutical company, Servier, beginning in 2015. Allogene assumed responsibility for all potential financial obligations to both Cellectis and Servier. In connection with the Allogene transaction, we recognized a non-cash $50 million pre-tax gain in Other (income)/deductions––net in the second quarter of 2018, representing the difference between the $127 million fair value of the equity investment received and the book value of assets transferred (including an allocation of goodwill) (see Note 4).
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 65 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
As of December 31, 2020, we held a 15.7% equity stake in Allogene, and our investment in Allogene is being measured at fair value with changes in fair value recognized in net income.
Sale of Phase 2b Ready AMPA Receptor Potentiator for CIAS to Biogen
In April 2018, we sold our Phase 2b ready AMPA receptor potentiator for CIAS to Biogen. We received $75 million upfront which was recognized in Other (income)/deductions––net (see Note 4) and may receive up to $515 million in total development and commercialization milestones, as well as tiered royalties in the low-to-mid-teen percentages.
Divestiture of Neuroscience Assets
In September 2018, we and Bain Capital entered into a transaction to create a new biopharmaceutical company, Cerevel (formerly known as Cerevel Therapeutics, LLC), to continue development of a portfolio of clinical and preclinical stage neuroscience assets primarily targeting disorders of the central nervous system including Parkinson’s disease, epilepsy, Alzheimer’s disease, schizophrenia and addiction. In connection with this transaction, we out-licensed the portfolio to Cerevel in exchange for a 25% ownership stake in Cerevel’s parent company, Cerevel Therapeutics, Inc., and potential future regulatory and commercial milestone payments and royalties. In connection with the transaction, we recognized a non-cash $343 million pre-tax gain in Other (income)/deductions––net in the third quarter of 2018, representing the fair value of the equity investment received as the assets transferredhad a book value of $0 (see Note 4). On October 27, 2020, Cerevel Therapeutics, Inc. completed a merger with ARYA Sciences Acquisition Corp II, a publicly-traded special purpose acquisition corporation, and a concurrent private investment in public equity “PIPE” transaction to form Cerevel Therapeutics Holdings, Inc. Our existing shares in Cerevel Therapeutics, Inc. converted into common shares of Cerevel Therapeutics Holdings, Inc. as part of the merger transaction, and we purchased an additional $12 million in common shares as part of the PIPE transaction. The common shares of Cerevel Therapeutics Holdings, Inc. trade publiclylisted on the NASDAQ stock market (ticker symbol CERE). AsLondon Stock Exchange that holds the joint historical consumer healthcare business of December 31, 2020, weGSK and Pfizer following the demerger. We continue to hold a 21.5% equity stake in Cerevel Therapeutics Holdings, Inc. for which we have elected the fair value option and which we measure at fair value with changes in fair value recognized in net income. In the fourth quarterown 32% of 2020, we remeasured our investment based on the market price of Cerevel Therapeutics Holdings, Inc. common sharesHaleon as of December 31, 2020 less a discount for lack of marketability, and we recognized a gain of $20 million in Other income/(deductions)––net.2023.
C. Equity-Method Investments
Formation of Consumer Healthcare JV
On July 31, 2019, we completed a transaction in which we and GSK combined our respective consumer healthcare businesses into a new JV that operates globally under the GSK Consumer Healthcare name. In exchange, we received a 32% equity stake in the new company and GSK owns the remaining 68%. Upon closing, we deconsolidated our Consumer Healthcare business and recognized a pre-tax gain of $8.1 billion ($5.4 billion, net of tax) in the third quarter of 2019 in (Gain) on completion of Consumer Healthcare JV transaction for the difference in the fair value of our 32% equity stake and the carrying value of our Consumer Healthcare business. Our financial results and our Consumer Healthcare segment’s operating results for 2019 reflect seven months of Consumer Healthcare segment domestic operations and eight months of Consumer Healthcare segment international operations. The financial results for 2020 do not reflect any contribution from the Consumer Healthcare business.
In valuing our investment in the Consumer Healthcare JV, we used discounted cash flow techniques. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which include the expected impact of competitive, legal or regulatory forces on the products; the long-term growth rate, which seeks to project the sustainable growth rate over the long term; the discount rate, which seeks to reflect our best estimate of the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
We are accounting for our interest in the Consumer Healthcare JV as an equity-method investment. The carrying value of our investment in the Consumer Healthcare JV is $16.7 billionHaleon as of December 31, 20202023 and $17.0December 31, 2022 was $11.5 billion and $10.8 billion, respectively, and is reported in Equity-method investments. The fair value of our investment in Haleon as of December 31, 2019 and is reported as a private equity investment in Equity-method investments as2023, based on quoted market prices of December 31, 2020 and 2019. TheHaleon stock, was $12.1 billion. Haleon/the Consumer Healthcare JV is a foreign investee whose reporting currency is the U.K. pound, and therefore we translate its financial statements into U.S. dollars and recognize the impact of foreign currency translation adjustments in the carrying value of our investment and in other comprehensive income. The decreaseincrease in the value of our investment from December 31, 20192022 to December 31, 20202023 is primarily due to dividends of $932 million, which were received from the Consumer Healthcare JV in June, September and November 2020, largely offset by our share of the JV’sHaleon’s earnings of $417$489 million and $345as well as $280 million in pre-tax foreign currency translation adjustments (see Note 6)., partially offset by $153 million in dividends. We record our share of earnings from Haleon/the Consumer Healthcare JV on a quarterly basis on a one-quarter lag in Other (income)/deductions––net commencing from August 1, 2019.. Our total share of Haleon’s earnings generated in the fourth quarter of 2022 and the first nine months of 2023, which we recorded in our operating results in 2023, was $489 million. Our total share of Haleon/the Consumer Healthcare JV’s earnings generated in the fourth quarter of 2021 and the first nine months of 2022, which we recorded in our operating results in 2022, was $536 million. Our total share of the JV’s earnings generated in the fourth quarter of 20192020 and the first nine months of 2020,2021, which we recorded in our operating results in 2020,2021, was $417$495 million. Our total share of two monthsAs part of the JV’s earnings generated in the third quarter of 2019, which we recorded in our operating results in the fourth quarter of 2019, was $47 million. As of the July 31, 2019 closing date, we estimated that the fair value ofinitial accounting for our investment in the Consumer Healthcare JV was $15.7 billion andin 2019, we determined that 32%the difference between the initial fair value of theour investment less our underlying equity in the carrying value of the net assets of the Consumer Healthcare JV was $11.2 billion, resultingresulted in an initial excess basis difference of approximately $4.5$4.8 billion. InWe allocated the fourth quarter of 2019, we preliminarily completed the allocation of the basis difference which resulted from the excess of the initial fair value of our investment over the underlying equity in the carrying value of the net assets of the JV, primarily to inventory, definite-lived intangible assets, indefinite-lived intangible assets, related deferred tax liabilities, and equity method goodwill within the investment account. During the fourth quarter of 2019, the Consumer Healthcare JV revised the initial carrying value of the net assets of the JV and our 32% share of the underlying equity in the carrying value of the net assets of the Consumer Healthcare JV was reduced to $11.0 billion and our initial basis difference was increased to $4.8 billion. The adjustment was allocated to equity method goodwill within the investment account.equity-method goodwill. We began recording therecognize amortization of these basis differences allocated to inventory, definite-lived intangible assets and related deferred tax liabilities in Other (income)/deductions––net commencing August 1, 2019. During the third and fourth quarters of 2020, we recognized write-offs of a portion of our basis differences allocated to indefinite-lived and definite-lived intangible assets and related deferred tax liabilities for the divestiture of certain brands by the Consumer Healthcare JV during its second quarter of 2020. The total amortization and write-off of these basis differences for the fourth quarter of 2019 and the first nine months of 2020, which was included in Other (income)/deductions—
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 66 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
net in 2020, was $119 million of expense. The amortization of basis differences for two months of the third quarter of 2019 totaling approximately $31 million is included in our operating results in the fourth quarter of 2019. See Note 4. . Amortization of basis differences on inventory and related deferred tax liabilities was completely recognized by the second quarter of 2020. Basis differences on definite-lived intangible assets and related deferred tax liabilities are being amortized over the lives of the underlying assets, which range from 8 to 20 years. In 2022, our equity-method income included in Other (income)/ deductions––net also included charges of $100 million, primarily for adjustments to our equity-method basis differences related to the separation of Haleon/the Consumer Healthcare JV from GSK. The total amortization and adjustment of basis differences resulting from the excess of the initial fair value of our investment over the underlying equity in the carrying value of the net assets of Haleon/the Consumer Healthcare JV was not material to our results of operations in 2023 and 2021. See Note 4.While we have received our full 32%
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 68 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | |
| Summarized financial information for our equity-method investee, Haleon/the Consumer Healthcare JV, as of September 30, 2023, the most recent period available, and as of September 30, 2022 and for the periods ending September 30, 2023, 2022, and 2021 is as follows: |
| (MILLIONS) | | | | September 30, 2023 | | September 30, 2022 |
| Current assets | | | | $ | 5,876 | | | $ | 5,932 | |
| Noncurrent assets | | | | 36,954 | | | 35,204 | |
| Total assets | | | | $ | 42,830 | | | $ | 41,137 | |
| | | | | | |
| Current liabilities | | | | $ | 6,117 | | | $ | 5,235 | |
| Noncurrent liabilities | | | | 15,744 | | | 17,220 | |
| Total liabilities | | | | $ | 21,862 | | | $ | 22,455 | |
| | | | | | |
| Equity attributable to shareholders | | | | $ | 20,719 | | | $ | 18,455 | |
| Equity attributable to noncontrolling interests | | | | 249 | | | 227 | |
| Total net equity | | | | $ | 20,968 | | | $ | 18,682 | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | For the Twelve Months Ending |
| (MILLIONS) | | | | September 30, 2023 | | September 30, 2022 | | September 30, 2021 |
| Net sales | | | | $ | 13,921 | | | $ | 13,566 | | | $ | 12,836 | |
| Cost of sales | | | | (5,580) | | | (5,081) | | | (4,755) | |
| Gross profit | | | | $ | 8,341 | | | $ | 8,486 | | | $ | 8,081 | |
| Income from continuing operations | | | | 1,606 | | | 1,745 | | | 1,614 | |
| Net income | | | | 1,606 | | | 1,745 | | | 1,614 | |
| Income attributable to shareholders | | | | 1,528 | | | 1,675 | | | 1,547 | |
In connection with GSK’s previously announced planned demerger of at least 80% of GSK’s 68% equity interest in the Consumer Healthcare JV, as of the July 31, 2019 closing and transferred control of our Consumer Healthcare business toin March 2022 the Consumer Healthcare JV completed its offering of a total aggregate principal amount of $8.75 billion in U.S. dollar-denominated senior notes of various maturities, €2.35 billion in euro-denominated senior notes of various maturities and £700 million in U.K. pound-denominated senior notes of various maturities (collectively, the contribution“notes”). The notes were guaranteed by GSK generally up to and excluding the date of the businessdemerger (the “Guarantee Assumption Date”). We agreed to indemnify GSK for 32% (representing our pro rata equity interest in the Consumer Healthcare JV) of any amount payable by GSK pursuant to its guarantee of the notes. Our indemnity was not completed in certain non-U.S. jurisdictions due to temporary regulatory or operational constraints. In these jurisdictions, we have continued to operate the businessprovided solely for the net economic benefit of GSK. Neither we nor any of our subsidiaries were an issuer or guarantor of any of the notes.
Following its issuance of the notes in March 2022, which fell in our international second quarter of 2022, the Consumer Healthcare JV loaned to us and GSK the net proceeds received from the notes on a pro rata equity ownership basis, for which we are indemnified against risks associated with such operationsreceived a loan of £2.9 billion ($3.7 billion as of the end of our second quarter of 2022), at an interest rate of 1.365% per annum payable semi-annually in the interim period, subject to our obligations under the definitive transaction agreements. We expect the contribution in these jurisdictions to be completed by the second half of 2021. As such, we have treated these jurisdictions as sold for accounting purposes.
arrears. In connectionconjunction with the contribution,demerger, we entered into certain transitional agreements designedreceived £3.5 billion ($4.2 billion) in dividends from the JV in July 2022, of which $4.0 billion related to facilitatea one-time pre-separation dividend, which decreased the orderly transitioncarrying value of our investment and are included in Net cash provided by/(used in) investing activities. Simultaneous with the receipt of the business todividends, we repaid the Consumer Healthcare£2.9 billion loan from the JV. These agreements primarily relate to administrative services, which are generally to be provided for a periodGSK similarly received pro rata dividends and simultaneously repaid its pro rata loan from the JV. In conjunction with these transactions, our indemnification of up to 24 months after closing. We will also manufacture and supply certain consumer products for the Consumer Healthcare JV and the Consumer Healthcare JV will manufacture and supply certain retained Pfizer products for us after closing, generally for a term of up to six years. These agreements are not material to Pfizer.
As a part of Pfizer, pre-tax income on a management basis for the Consumer Healthcare businessGSK’s guarantee discussed above was $654 million through July 31, 2019 and $977 million in 2018.
| | | | | | | | | | | | | | | | |
| Summarized financial information for our equity method investee, the Consumer Healthcare JV, as of and for the twelve months ending September 30, 2020, the most recent period available, and as of and for the two months ending September 30, 2019 is as follows: |
| (MILLIONS OF DOLLARS) | | | | September 30, 2020 | | September 30, 2019 |
| Current assets | | | | $ | 6,614 | | | $ | 7,505 | |
| Noncurrent assets | | | | 38,361 | | | 38,575 | |
| Total assets | | | | $ | 44,975 | | | $ | 46,081 | |
| | | | | | |
| Current liabilities | | | | $ | 5,246 | | | $ | 5,241 | |
| Noncurrent liabilities | | | | 5,330 | | | 5,536 | |
| Total liabilities | | | | $ | 10,576 | | | $ | 10,776 | |
| | | | | | |
| Equity attributable to shareholders | | | | $ | 34,154 | | | $ | 35,199 | |
| Equity attributable to noncontrolling interests | | | | 245 | | | 105 | |
| Total net equity | | | | $ | 34,400 | | | $ | 35,304 | |
| | | | | | | | | | | | | | | | |
| | | | For the Twelve Months Ending | | For the Two Months
Ending |
| (MILLIONS OF DOLLARS) | | | | September 30, 2020 | | September 30, 2019 |
| Net sales | | | | $ | 12,720 | | | $ | 2,161 | |
| Cost of sales | | | | (5,439) | | | (803) | |
| Gross profit | | | | $ | 7,281 | | | $ | 1,358 | |
| Income from continuing operations | | | | 1,350 | | | 152 | |
| Net income | | | | 1,350 | | | 152 | |
| Income attributable to shareholders | | | | 1,307 | | | 148 | |
Investment in ViiV
In 2009, we and GSK created ViiV, which is focused on research, development and commercialization of human immunodeficiency virus (HIV) medicines. We own approximately 11.7% of ViiV, and prior to 2016 we accounted for our investment under the equity method due to the significant influence that we have over the operations of ViiV through our board representation and minority veto rights. We suspended application of the equity method to our investment in ViiV in 2016 when the carrying value of our investment was reduced to 0zero due to the recognition of cumulative equity methodequity-method losses and dividends.dividends, and therefore we no longer record our proportionate share of ViiV’s net income (loss) in our results of operations. Since 2016, we have recognized dividends from ViiV as income in Other (income)/deductions––net when earned, including dividends of $278$265 million in 2020, $2202023, $314 million in 20192022 and $253$166 million in 20182021 (see Note 4). | | | | | | | | | | | | | | | | |
| Summarized financial information for our equity-method investee, ViiV, as of December 31, 2023 and 2022 and for the years ending December 31, 2023, 2022, and 2021 is as follows: |
| | | | As of December 31, |
| (MILLIONS) | | | | 2023 | | 2022 |
| Current assets | | | | $ | 4,237 | | | $ | 4,043 | |
| Noncurrent assets | | | | 3,009 | | | 3,014 | |
| Total assets | | | | $ | 7,245 | | | $ | 7,057 | |
| | | | | | |
| Current liabilities | | | | $ | 4,085 | | | $ | 3,780 | |
| Noncurrent liabilities | | | | 5,998 | | | 5,996 | |
| Total liabilities | | | | $ | 10,083 | | | $ | 9,777 | |
| | | | | | |
| Total net equity/(deficit) attributable to shareholders | | | | $ | (2,838) | | | $ | (2,720) | |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 6769 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | |
| Summarized financial information for our equity method investee, ViiV, as of December 31, 2020 and 2019 and for the years ending December 31, 2020, 2019, and 2018 is as follows: |
| | | | As of December 31, |
| (MILLIONS OF DOLLARS) | | | | 2020 | | 2019 |
| Current assets | | | | $ | 3,283 | | | $ | 3,839 | |
| Noncurrent assets | | | | 3,381 | | | 3,437 | |
| Total assets | | | | $ | 6,664 | | | $ | 7,276 | |
| | | | | | |
| Current liabilities | | | | $ | 3,028 | | | $ | 2,904 | |
| Noncurrent liabilities | | | | 6,370 | | | 5,860 | |
| Total liabilities | | | | $ | 9,398 | | | $ | 8,765 | |
| | | | | | |
| Total net equity/(deficit) attributable to shareholders | | | | $ | (2,734) | | | $ | (1,489) | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | Year Ended December 31, |
| (MILLIONS) | | | | 2023 | | 2022 | | 2021 |
| Net sales | | | | $ | 7,845 | | | $ | 6,955 | | | $ | 6,380 | |
| Cost of sales | | | | (1,060) | | | (819) | | | (682) | |
| Gross profit | | | | $ | 6,785 | | | $ | 6,135 | | | $ | 5,698 | |
| Income from continuing operations | | | | 3,090 | | | 3,108 | | | 2,040 | |
| Net income | | | | 3,090 | | | 3,108 | | | 2,040 | |
| Income attributable to shareholders | | | | 3,090 | | | 3,108 | | | 2,040 | |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | | | 2020 | | 2019 | | 2018 |
| Net sales | | | | $ | 6,224 | | | $ | 6,139 | | | $ | 6,219 | |
| Cost of sales | | | | (574) | | | (516) | | | (462) | |
| Gross profit | | | | $ | 5,650 | | | $ | 5,623 | | | $ | 5,757 | |
| Income from continuing operations | | | | 2,012 | | | 3,398 | | | 2,154 | |
| Net income | | | | 2,012 | | | 3,398 | | | 2,154 | |
| Income attributable to shareholders | | | | 2,012 | | | 3,398 | | | 2,154 | |
D. Licensing ArrangementsArrangement
Agreement with Valneva
2Valneva––On April 30, 2020,In June 2022, we signedentered into an agreementEquity Subscription Agreement, under which we invested €90.5 million ($95 million) in Valneva to further support our arrangement to co-develop and commercialize Valneva’s Lyme disease vaccine candidate, VLA15, which covers six serotypes that are prevalentwe originally entered into with Valneva in North America and Europe. Valneva and Pfizer will work closely together throughout the development of VLA15. Valneva is eligible to receive a total of up to $308 million in cash payments from us consisting of a $130 million upfront payment, which was paid and recorded in Research and development expenses in our second quarter of 2020, as well as $35 million in development milestones and $143 million in early commercialization milestones. Under2020. In addition, we updated the terms of theour existing co-development and commercialization agreement for VLA15. Valneva will now fund 30%40% of allthe remaining shared development costs, through completion of the development program, and in return we will pay Valneva tiered royalties. Weroyalties ranging from 14% to 22%, compared to royalties starting at 19% in the initial agreement. In addition, the royalties will lead late-stage development and have sole control over commercialization.
Agreement with BioNTech
In August 2018, a multi-year R&D arrangement went into effect between BioNTech and Pfizer to develop mRNA-based vaccines for prevention of influenza (flu). In relation to this R&D arrangement, in September 2018, we made an upfront payment of $50 million to BioNTech, which was recorded in Research and development expenses, and BioNTech became eligible to receivebe complemented by up to $325$100 million in development and sales-based milestones and royalty payments associated with worldwidepayable to Valneva based on cumulative sales. Other early commercialization milestones are unchanged. As part of the transaction,December 31, 2023, we also purchased 169,670 newly-issued ordinary sharesheld a 6.9% equity stake of BioNTech for $50 million in the third quarter of 2018.Valneva.
Akcea
On October 4, 2019, we entered into a worldwide exclusive licensing agreement for AKCEA-ANGPTL3-LRx, an investigational antisense therapy being developed to treat patients with certain cardiovascular and metabolic diseases, with Akcea, a wholly-owned subsidiary of Ionis. The transaction closed in November 2019 and we made an upfront payment of $250 million to Akcea, which was recorded in Research and development expenses in our fourth quarter of 2019. We may be required to make development, regulatory and sales milestone payments of up to $1.3 billion and pay tiered, double-digit royalties on annual worldwide net sales upon marketing approval of AKCEA-ANGPTL3-LRx.
E. Collaborative Arrangements
In the normal course of business, weWe enter into collaborative arrangements with respect to in-line medicines, as well as medicines in development that require completion of research and regulatory approval. Collaborative arrangements are contractual agreements with third parties that involve a joint operating activity, typically a research and/or commercialization effort, where both we and our partner are active participants in the activity and are exposed to the significant risks and rewards of the activity. Our rights and obligations under our collaborative arrangements vary. For example, we have agreements to co-promote pharmaceutical products discovered by us or other companies, and we have agreements where we partner to co-develop and/or participate together in commercializing, marketing, promoting, manufacturing and/or distributing a drug product.product or vaccine.
AgreementCollaboration with Myovant
On December 26, 2020,Biohaven––In November 2021, we entered into a collaboration and license agreement and related sublicense agreement with Biohaven and certain of its subsidiaries to jointly developcommercialize rimegepant and commercialize Orgovyx™ (relugolix) in advanced prostate cancerzavegepant for the treatment and if approved, relugolix combination tablet (relugolix 40 mg, estradiol 1.0 mg, and norethindrone acetate 0.5 mg) in women’s health inprevention of migraines outside of the U.S. and Canada. We will also receive an exclusive option, subject to commercialize relugolix in oncology outside the U.S. and Canada, excluding certain Asian countries.regulatory approval. Under the terms of the agreement, Biohaven would lead R&D globally and we would have the companies will equally share profits and allowable expenses for Orgovyx and the relugolix combination tablet inexclusive right to commercialization globally, outside of the U.S. and Canada, with Myovant bearing our shareUpon the closing of allowable expenses up to a maximum
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 68 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
of $100the transaction on January 4, 2022, we paid Biohaven $500 million, in 2021 and up to a maximum of $50 million in 2022. We will record our share of gross profits as Alliance revenue. Myovant will remain responsible for regulatory interactions and drug supply and continue to lead clinical development for the relugolix combination tablet. Myovant will be entitled to receive up to $4.35 billion, including an upfront payment of $650$150 million whichand an equity investment of $350 million. We recognized $263 million for the upfront payment and premium paid on our equity investment in Acquired in-process research and development expenses. In October 2022, we acquired all outstanding common shares of Biohaven not already owned by us for $148.50 per share, in cash, for payments of approximately $11.5 billion. See Note 2A. This acquisition represented a settlement of the pre-existing relationship, and we determined that no gain or loss was made inrequired to be recognized.Collaborations with BioNTech––On December 2020, $20030, 2021, we entered into a research, development and commercialization agreement to develop a potential first mRNA-based vaccine for the prevention of shingles (herpes zoster virus) based on BioNTech’s proprietary mRNA technology and our antigen technology. Under the terms of the agreement, we agreed to pay BioNTech $225 million, in potentialincluding an upfront cash payment of $75 million and an equity investment of $150 million. BioNTech is eligible to receive future regulatory milestones for FDA approvals for relugolix combination tablet in women’s health, and tiered sales milestonesmilestone payments of up to $3.5 billion$200 million. In return, BioNTech agreed to pay us $25 million for prostate cancer and also for the combined women’s health indications. If we exercise the optionour proprietary antigen technology. The net upfront payment to commercialize relugolix in oncology outside of the U.S. and Canada, excluding certain Asian countries, Myovant will receive $50 million and be entitledBioNTech was recorded to receive double-digit royalties on sales. In connection with this transaction, we recognized $499 million in Identifiable intangible assets––Developed technologyrights and $151 million in ResearchAcquired in-process research and development expenses representing the relative fair valuein our fourth quarter of the portion of the upfront payment allocated2021. We and BioNTech share development costs. We will have commercialization rights to the approved indicationpotential vaccine worldwide, excluding Germany, Turkey and unapproved indications of the product, respectively.
Agreement with CStone
On September 29, 2020, we entered into a strategic collaboration with CStone to address oncological needs in China. The collaboration encompasses our $200 million upfront equity investment in CStone, a collaboration between the companies for the developmentcertain developing countries where BioNTech will have commercialization rights. We and BioNTech will share gross profits from commercialization of CStone’s sugemalimab (CS1001, PD-L1 antibody) in mainland China, and a framework between the companies to bring additional oncology assets to the Greater China market. The transaction closed on October 9, 2020.any product. As of December 31, 2020,2023, we held a 9.9%an equity stake in CStone.
Agreement with BioNTechof 2.7% of BioNTech.
On April 9, 2020, we signed a global agreement with BioNTech to co-develop a mRNA-based coronavirus vaccine program BNT162b2, aimed at preventing COVID-19 disease. The collaboration rapidly advanced a COVID-19 vaccine candidate into human clinical testing based on BioNTech’s proprietary mRNA vaccine platforms, and the vaccine has been granted EUAinfection, which resulted in the U.S., the EU and the U.K., among other countries. We are working with BioNTech to manufacture and help ensure rapid worldwide access to the vaccine. The collaboration leverages our broad expertise in vaccine R&D, regulatory capabilities, and global manufacturing and distribution network. In connection with the April 2020 agreement, we paid BioNTech an upfront cash paymentdevelopment of $72 million, which was recorded in Research and development expenses in our second quarter of 2020, and we made an additional equity investment of $113 million in common stock of BioNTech. BioNTech became eligible to receive potential milestone payments of up to $563 million for a total consideration of $748 million. Under the terms of this agreement, we and BioNTech will share gross profits and development costs equally after the vaccine is approved and successfully commercialized, and we were responsible for all of the development costs until commercialization of the vaccine. Thereafter, BioNTech was to repay us its 50 percent share of these development costs through reductions in gross profit sharing and milestone payments to BioNTech over time.Comirnaty. On January 29, 2021, we and BioNTech signed an amended version of the April 2020 agreement. Under the January 2021 agreement, BioNTech will paypaid us their 50 percent share of prior development costs in a lump sum payment during the first quarter of 2021. Further R&D costs will beare being shared equally. We have commercialization rights to the vaccine worldwide, (excludingexcluding Germany and Turkey where BioNTech will marketmarkets and distributedistributes the vaccine under the agreement with us, and excluding China, Hong Kong, Macau and Taiwan, which are subject to a separate collaboration between BioNTech and Shanghai Fosun Pharmaceutical (Group) Co., Ltd).Ltd. We recognize Revenuesrevenues and Costcost of sales on a gross basis in markets where we are commercializing the vaccine and we will record our share of gross profits related to sales of the vaccine by BioNTech in Germany and Turkey in Alliance revenues.
WeCollaboration with Beam––On December 24, 2021, we entered into a multi-year research collaboration with Beam to utilize Beam’s in vivo base editing programs, which use mRNA and lipid nanoparticles, for three targets for rare genetic diseases of the liver, muscle and central nervous system. Under the terms of the agreement, Beam conducts all research activities through development candidate selection for three undisclosed targets, which are not included in Beam’s existing programs, and we may opt in to obtain exclusive licenses to each development candidate. Beam has a right to opt in, at the end of phase 1/2 studies, upon the payment by Beam of an option exercise fee, to a global co-development and co-commercialization agreement with respect to one program licensed under the collaboration pursuant to which we and Beam would share net profits as well as development and commercialization costs in a 65%/35% ratio (Pfizer/Beam). Upon entering into the agreement, we recorded $300 million in Acquired in-process research and development expenses in the fourth quarter of 2021 for an upfront payment due to Beam, and if we exercise our opt in to licenses for all three targets, Beam will be eligible for up to an additional $1.05 billion in development, regulatory and commercial milestone payments for a potential total deal consideration of up to $1.35 billion. Beam is also eligible to receive royalties on global net sales for each licensed program.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 70 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Collaboration with Arvinas––On July 21, 2021, we entered into a global collaboration with Arvinas to develop and commercialize ARV-471, an investigational oral PROTAC® (PROteolysis TArgeting Chimera) estrogen receptor protein degrader. The estrogen receptor is a well-known disease driver in most breast cancers. In connection with the agreement, we made an additionalupfront cash payment of $650 million to Arvinas and we made a $350 million equity investment of $50 million in the common stock of BioNTech as partArvinas. We recognized $706 million for the upfront payment and a premium paid on our equity investment in Acquired in-process research and development expenses in our third quarter of an underwritten equity offering by BioNTech, which closed2021. Arvinas is also eligible to receive up to $400 million in July 2020.approval milestones and up to $1 billion in commercial milestones. The companies equally share worldwide development costs, commercialization expenses and profits. As of December 31, 2020,2023, we held ana 5.1% equity stake of 2.5% in BioNTech.Arvinas.
Summarized Financial Information for Collaborative Arrangements
| | The following provides the amounts and classification of payments (income/(expense)) between us and our collaboration partners: | The following provides the amounts and classification of payments (income/(expense)) between us and our collaboration partners: | The following provides the amounts and classification of payments (income/(expense)) between us and our collaboration partners: |
| Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 |
Revenues—Revenues(a) | | $ | 284 | | | $ | 305 | | | $ | 268 | |
Revenues—Alliance revenues(b) | | 5,418 | | | 4,648 | | | 3,838 | |
| | Year Ended December 31, | | | | Year Ended December 31, |
| (MILLIONS) | | (MILLIONS) | | 2023 | | 2022 | | 2021 |
Product revenues(a) | |
Alliance revenues(b) | |
| Total revenues from collaborative arrangements | Total revenues from collaborative arrangements | | $ | 5,703 | | | $ | 4,953 | | | $ | 4,107 | |
Cost of sales(c) | Cost of sales(c) | | $ | (61) | | | $ | (52) | | | $ | (34) | |
Selling, informational and administrative expenses(d) | Selling, informational and administrative expenses(d) | | (194) | | | (176) | | | (92) | |
Research and development expenses(e) | Research and development expenses(e) | | (192) | | | 104 | | | 162 | |
Acquired in-process research and development expenses(f) | |
Other income/(deductions)—net(f)(g) | Other income/(deductions)—net(f)(g) | | 567 | | | 362 | | | 281 | |
(a)Represents sales to our partners of products manufactured by us.
(b)Substantially all relates to amounts earned from our partners under co-promotion agreements. The decrease in 2023 was primarily driven by a decline in Alliance revenues from Comirnaty, partially offset by an increase in Alliance revenues from Eliquis. The increase in 2022 was primarily driven by increases in each of the periods presented reflect increases in allianceAlliance revenues from Eliquis, Comirnaty and Xtandi.Bavencio.
(c)Primarily relates to amounts paid to collaboration partners for their share of net sales or profits earned in collaboration arrangements where we are the principal in the transaction, and cost of sales for inventory purchased from our partners. The decreases in 2023 and in 2022 primarily relate to Comirnaty.
(d)Represents net reimbursements to our partners for selling, informational and administrative expenses incurred.
(e)Represents net reimbursements from our partners for research and development expenses incurred.
(f)Primarily relates to upfront payments and pre-approval milestone payments earned byto our partners as well as net reimbursements.premiums paid on our equity investments in the common stock of our partners.
(f)(g)Primarily relates to royalties from our collaboration partners.
The amounts outlined in the above table do not include transactions with third parties other than our collaboration partners, or other costs for the products under the collaborative arrangements.
F. Research and Development Arrangement
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 69 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 33.. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives
A. Restructuring Programs
Transforming to a More Focused Company Program––In 2019, we substantially completed several multi-year initiatives focused on positioning us for future growth and creating a simpler, more efficient operating structure within each business.
announced that we would be incurring costs associated with our Transforming to a More Focused Company Program,
With the formation of the Consumer Healthcare JV in 2019 and the spin-off of our Upjohn Business in the fourth quarter of 2020, Pfizer has transformed into a more focused, global leader in science-based innovative medicines and vaccines. We have undertaken effortsmulti-year effort to ensure our cost base alignsaligned appropriately with our revenue base. While certain direct costs transferredoperating structure following Pfizer’s transformation into a more focused, innovative science-based global biopharmaceutical business. This program included activities to the Consumer Healthcare JV and to the Upjohn Business in connection with the spin-off, there are indirect costs which did not transfer. In addition, we are taking steps to(i) restructure our corporate enabling functions to appropriately support our operating structure; (ii) transform our commercial go-to-market model; and drive the purpose of(iii) optimize our focused innovative biopharmaceutical products businessmanufacturing network and R&D and PGS platform functions.operations. The program costs discussed below may be rounded and represent approximations.
We expect costs for this program, primarily related to restructure our corporate enabling functions, to be incurred from 2020 through 2022 and to total $1.6 billion on a pre-tax basis, with substantially all costs to be cash expenditures. Actions will include, among others, changes in location of certain activities, expanded useoptimize our R&D operations and co-location of centers of excellence and shared services, and increased use of digital technologies. The associated actions and the specific costs will primarily include severance and benefit plan impacts, exit costsreduce cycle times, as well as associatedto further prioritize our internal R&D portfolio, primarily included severance and implementation costs.
Also as part of this program, we expect The costs to incur costs related tooptimize our manufacturing network optimization, including certain legacy cost-reduction initiatives, of $500 million, with approximately 20% of the costs to be non-cash. The costs for this effort are expected to be incurred primarily from 2020 through 2022, and will include, among other things,largely included severance, implementation costs, product transfer costs, site exit costs, as well asand accelerated depreciation.
From the start of this program in the fourth quarter of 2019 through December 31, 2020,2023, we incurred costs of $900 million.
Key Activities
In 2020, we incurred costs$4.0 billion, of $896 million, composed primarilywhich $1.5 billion ($1.0 billion of the Transforming to a More Focused Company program. In 2019, we incurred costs of $820 million composed of $548 million for the 2017-2019 and Organizing for Growth initiatives, $288 million for the integration of Array, $94 million for the integration of Hospira, and $87 million for the Transforming to a More Focused Company program, partially offset by income of $197 million, primarily due to the reversal of certain accruals upon the effective favorable settlement of an IRS audit for multiple tax years and other acquisition-related initiatives.
| | | | | | | | | | | | | | | | | | | | | | | | |
| The following summarizes acquisitions and cost-reduction/productivity initiatives costs and credits: |
| | | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | | | | | 2020 | | 2019 | | 2018 |
| Restructuring charges/(credits): | | | | | | | | | | |
| Employee terminations | | | | | | $ | 474 | | | $ | 108 | | | $ | 473 | |
Asset impairments(a) | | | | | | 88 | | | 69 | | | 290 | |
| Exit costs/(credits) | | | | | | (6) | | | 50 | | | 33 | |
Restructuring charges(b) | | | | | | 556 | | | 227 | | | 796 | |
Transaction costs(c) | | | | | | 10 | | | 63 | | | 1 | |
Integration costs and other(d) | | | | | | 34 | | | 311 | | | 260 | |
| Restructuring charges and certain acquisition-related costs | | | | | | 600 | | | 601 | | | 1,058 | |
Net periodic benefit costs recorded in Other (income)/deductions––net | | | | | | 39 | | | 23 | | | 144 | |
Additional depreciation––asset restructuring recorded in our consolidated statements of income as follows(e): | | | | | | | | | | |
| Cost of sales | | | | | | 23 | | | 29 | | | 36 | |
| Selling, informational and administrative expenses | | | | | | 0 | | | 3 | | | 2 | |
| Research and development expenses | | | | | | (3) | | | 8 | | | 0 | |
| Total additional depreciation––asset restructuring | | | | | | 19 | | | 40 | | | 38 | |
| | | | | | | | | | |
Implementation costs recorded in our consolidated statements of income as follows(f): | | | | | | | | | | |
| Cost of sales | | | | | | 40 | | | 61 | | | 75 | |
| Selling, informational and administrative expenses | | | | | | 197 | | | 73 | | | 71 | |
| Research and development expenses | | | | | | 1 | | | 22 | | | 39 | |
| | | | | | | | | | |
| Total implementation costs | | | | | | 238 | | | 156 | | | 186 | |
| Total costs associated with acquisitions and cost-reduction/productivity initiatives | | | | | | $ | 896 | | | $ | 820 | | | $ | 1,426 | |
(a)2018 charges are largely for cost-reduction initiatives notrestructuring charges) was associated with acquisitions.
(b)Represents acquisition-related costs ($192 million credit in 2019,our Biopharma segment and $37 million charge in 2018) and cost reduction initiatives ($556 million charge in 2020, $418 million charge in 2019, and $759 million charge in 2018). 2020 charges mainly represent employee termination costs for our Transforming to a More Focused Company cost-reductionhave substantially completed this program. 2019 restructuring charges mainly represent employee termination costs for cost-reduction and productivity initiatives, partially offset by the reversal of certain accruals related to our acquisition of Wyeth upon the effective favorable settlement of an IRS audit for multiple tax years (see Note 5B). 2018 charges were primarily related to employee termination costs and asset write downs. The employee termination costs for
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 7071 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2019Realigning our Cost Base Program––In the fourth quarter of 2023, we announced that we launched a multi-year, enterprise-wide cost realignment program that aims to realign our costs with our longer-term revenue expectations. We expect costs associated with this multi-year effort to continue through 2024 and 2018 wereto total approximately $3.0 billion, primarily representing cash expenditures for severance and implementation costs, of which $1.1 billion is associated with our improvements to operational effectiveness as partBiopharma segment.
In 2023, we incurred costs under this program of the realignment$1.7 billion, of which $674 million (including $665 million of restructuring charges) is associated with our business structure,Biopharma segment.
B. Key Activities
| | | | | | | | | | | | | | | | | | | | | | | | |
| The following summarizes costs and credits for acquisitions and cost-reduction/productivity initiatives: |
| | | | Year Ended December 31, |
| (MILLIONS) | | | | | | 2023 | | 2022 | | 2021 |
| Restructuring charges/(credits): | | | | | | | | | | |
| Employee terminations | | | | | | $ | 1,622 | | | $ | 776 | | | $ | 680 | |
| Asset impairments | | | | | | 227 | | | 52 | | | 53 | |
| Exit costs/(credits) | | | | | | 119 | | | 54 | | | 8 | |
Restructuring charges/(credits)(a) | | | | | | 1,968 | | | 882 | | | 741 | |
Transaction costs(b) | | | | | | 190 | | | 144 | | | 20 | |
Integration costs and other(c) | | | | | | 785 | | | 348 | | | 41 | |
| Restructuring charges and certain acquisition-related costs | | | | | | 2,943 | | | 1,375 | | | 802 | |
Net periodic benefit costs/(credits) recorded in Other (income)/deductions––net | | | | | | (7) | | | (9) | | | (63) | |
Additional depreciation––asset restructuring recorded in our consolidated statements of income as follows(d): | | | | | | | | | | |
| Cost of sales | | | | | | 31 | | | 34 | | | 63 | |
| Selling, informational and administrative expenses | | | | | | 1 | | | 2 | | | 23 | |
| | | | | | | | | | |
| Total additional depreciation––asset restructuring | | | | | | 32 | | | 36 | | | 87 | |
| | | | | | | | | | |
Implementation costs recorded in our consolidated statements of income as follows(e): | | | | | | | | | | |
| Cost of sales | | | | | | 67 | | | 54 | | | 45 | |
| Selling, informational and administrative expenses | | | | | | 289 | | | 560 | | | 426 | |
| Research and development expenses | | | | | | 101 | | | 2 | | | 1 | |
| | | | | | | | | | |
| Total implementation costs | | | | | | 457 | | | 616 | | | 472 | |
| Total costs associated with acquisitions and cost-reduction/productivity initiatives | | | | | | $ | 3,426 | | | $ | 2,018 | | | $ | 1,298 | |
(a)Primarily represents cost-reduction initiatives. Amounts associated with our Biopharma segment: $672 million for 2023 (including charges of $665 million for Realigning our Cost Base Program and credits of $20 million for 2019, also includes employee termination costs for the Transforming to a More Focused Company cost-reduction program.program), $354 million for 2022 (including charges of $291 million for Transforming to a More Focused Company program) and $610 million for 2021 (including charges of $612 million for Transforming to a More Focused Company program).
(c)(b)Represents external costs for banking, legal, accounting and other similar services.
(d)(c)Represents external, incremental costs directly related to integrating acquired businesses, such as expenditures for consulting and the integration of systems and processes, and certain other qualifying costs. 20202023 costs mostly relate to our acquisition of Seagen, including $476 million that was recognized as a post-closing compensation expense for payments to Seagen employees in the fourth quarter of 2023 for the fair value of long-term incentive awards that vested upon closing and the expense for employee incentive awards issued in contemplation of the merger. 2022 costs mostly related to our acquisitions of Arena and GBT, including $138 million in payments to Arena employees in the first quarter of 2022 and $136 million in payments to GBT employees in the fourth quarter of 2022 for the fair value of previously unvested long-term incentive awards that was recognized as post-closing compensation expense. See Note 2A. 2021 costs primarily related to our acquisition of Array. 2019 costs mainly related to our acquisitions of Array, including $157 million in payments to Array employees for the fair value of previously unvested stock options that was recognized as post-closing compensation expense (see Note 2A), and Hospira. 2018 costs mostly related to our acquisition of Hospira.Trillium.(e)(d)Represents the impact of changes in the estimated useful lives of assets involved in restructuring actions.
(f)(e)Represents external, incremental costs directly related to implementing our non-acquisition-related cost-reduction/productivity initiatives.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following summarizes the components and changes in restructuring accruals: |
| (MILLIONS OF DOLLARS) | | Employee
Termination
Costs | | Asset
Impairment
Charges | | Exit Costs | | Accrual |
| Balance, January 1, 2019 | | $ | 1,113 | | | $ | 0 | | | $ | 49 | | | $ | 1,161 | |
Provision(a) | | 108 | | | 69 | | | 50 | | | 227 | |
Utilization and other(b) | | (450) | | | (69) | | | (53) | | | (572) | |
Balance, December 31, 2019(c) | | 770 | | | 0 | | | 46 | | | 816 | |
| Provision | | 474 | | | 88 | | | (6) | | | 556 | |
Utilization and other(b) | | (462) | | | (88) | | | (25) | | | (575) | |
Balance, December 31, 2020(d) | | $ | 782 | | | $ | 0 | | | $ | 15 | | | $ | 798 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following summarizes the components and changes in restructuring accruals: |
| (MILLIONS) | | Employee
Termination
Costs | | Asset
Impairment
Charges | | Exit Costs | | Accrual |
| Balance, January 1, 2022 | | $ | 1,014 | | | $ | — | | | $ | 57 | | | $ | 1,071 | |
| Provision | | 776 | | | 52 | | | 54 | | | 882 | |
Utilization and other(a) | | (594) | | | (52) | | | (103) | | | (750) | |
Balance, December 31, 2022(b) | | 1,196 | | | — | | | 8 | | | 1,204 | |
| Provision | | 1,622 | | | 227 | | | 119 | | | 1,968 | |
Utilization and other(a) | | (840) | | | (227) | | | (116) | | | (1,184) | |
Balance, December 31, 2023(c) | | $ | 1,978 | | | $ | — | | | $ | 11 | | | $ | 1,988 | |
(a)Includes the reversal of certain accruals related to our acquisition of Wyeth upon the favorable settlement of an IRS audit for multiple tax years. See Note 5D.
(b)IncludesOther activity includes adjustments for foreign currency translation.translation that are not material to our consolidated financial statements.
(b)Included in Other current liabilities ($991 million) and Other noncurrent liabilities ($213 million).
(c)Included in Other current liabilities ($641 million) and Other noncurrent liabilities ($175 million).
(d)Included in Other current liabilities ($628 million)1.3 billion) and Other noncurrent liabilities ($169663 million).
Note 4. Other (Income)/Deductions—Net
| | | | | | | | | | | | | | | | | | | | |
Components of Other (income)/deductions––net include: |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 |
| Interest income | | $ | (73) | | | $ | (225) | | | $ | (333) | |
Interest expense(a) | | 1,449 | | | 1,573 | | | 1,316 | |
| Net interest expense | | 1,376 | | | 1,348 | | | 983 | |
| Royalty-related income | | (770) | | | (646) | | | (485) | |
| Net (gains)/losses on asset disposals | | 237 | | | (32) | | | (71) | |
Net (gains)/losses recognized during the period on equity securities(b) | | (540) | | | (454) | | | (586) | |
Net realized (gains)/losses on sales of investments in debt securities(c) | | 0 | | | 0 | | | 141 | |
Income from collaborations, out-licensing arrangements and sales of compound/product rights(d) | | (326) | | | (168) | | | (476) | |
Net periodic benefit costs/(credits) other than service costs(e) | | (236) | | | 72 | | | (270) | |
Certain legal matters, net(f) | | 28 | | | 292 | | | 84 | |
Certain asset impairments(g) | | 1,691 | | | 2,843 | | | 3,115 | |
Business and legal entity alignment costs(h) | | 0 | | | 300 | | | 63 | |
Consumer Healthcare JV equity method (income)/loss(i) | | (298) | | | (17) | | | 0 | |
Other, net(j) | | (493) | | | (226) | | | (421) | |
| Other (income)/deductions––net | | $ | 669 | | | $ | 3,314 | | | $ | 2,077 | |
(a)Capitalized interest totaled $96 million in 2020, $88 million in 2019 and $73 million in 2018.
(b)2020 gains include, among other things, unrealized gains of $405 million related to investments in BioNTech and SpringWorks Therapeutics, Inc. (SpringWorks). 2019 gains included, among other things, unrealized gains of $295 million related to investments in Cortexyme, Inc. and SpringWorks. 2018 gains included unrealized gains on equity securities of $477 million, reflecting the adoption of a new accounting standard in 2018 and were primarily driven by unrealized gains of $466 million related to our investment in Allogene. See Notes 2B and 7B.
(c)2018 primarily included gross realized losses on sales of available-for-sale debt securities of $402 million and a net loss of $18 million from derivative financial instruments used to hedge the foreign exchange component of the matured available-for-sale debt securities, partially offset by gross realized gains on sales of available-for-sale debt securities of $280 million. Proceeds from the sale of available-for-sale debt securities were $5.7 billion in 2018.
(d)2020 includes, among other things, (i) an upfront payment to us of $75 million from our sale of our CK1 assets to Biogen, (ii) $40 million of milestone income from Puma Biotechnology, Inc. related to Neratinib regulatory approvals in the EU, (iii) $30 million of milestone income from Lilly related to the first commercial sale in the U.S. of LOXO-292 for the treatment of RET fusion-positive NSCLC and (iv) $108 million in milestone income from multiple licensees. 2019 includes, among other things, $78 million in milestone income from Mylan Pharmaceuticals Inc. related to the FDA’s approval and launch of Wixela Inhub®, a generic of Advair Diskus®(fluticasone propionate and salmeterol inhalation powder) and $52 million in milestone income from multiple licensees. 2018 includes, among other things, (i) $118 million in milestone income from multiple licensees, (ii) $110 million in milestone payments received from Shire, of which $75 million related to their first dosing of a patient in a Phase 3 clinical trial for the treatment of UC and $35 million related to their first dosing of a patient in a Phase 3 clinical trial
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 7172 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
for the treatment of Crohn’s disease, (iii) an upfront payment to us and a recognized milestone totaling $85 million for the sale of an AMPA receptor potentiator for CIAS to Biogen, (iv) $50Note 4. Other (Income)/Deductions—Net
| | | | | | | | | | | | | | | | | | | | |
Components of Other (income)/deductions––net include: |
| | Year Ended December 31, |
| (MILLIONS) | | 2023 | | 2022 | | 2021 |
| Interest income | | $ | (1,624) | | | $ | (251) | | | $ | (36) | |
Interest expense(a) | | 2,209 | | | 1,238 | | | 1,291 | |
Net interest expense(b) | | 585 | | | 987 | | | 1,255 | |
| Royalty-related income | | (1,058) | | | (845) | | | (857) | |
| | | | | | |
Net (gains)/losses recognized during the period on equity securities(c) | | (1,590) | | | 1,273 | | | (1,344) | |
Income from collaborations, out-licensing arrangements and sales of compound/product rights(d) | | (154) | | | (188) | | | (396) | |
| Net periodic benefit costs/(credits) other than service costs | | (610) | | | (849) | | | (2,547) | |
Certain legal matters, net(e) | | 474 | | | 230 | | | 182 | |
Certain asset impairments(f) | | 3,024 | | | 421 | | | 86 | |
| | | | | | |
Haleon/Consumer Healthcare JV equity method (income)/loss(g) | | (505) | | | (436) | | | (471) | |
Other, net(h) | | (1,002) | | | (378) | | | (786) | |
| Other (income)/deductions––net | | $ | (835) | | | $ | 217 | | | $ | (4,878) | |
(a)Capitalized interest totaled $160 million in 2023, $124 million in 2022 and $108 million in 2021.
(b)The decrease in net interest expense in 2023 reflects higher interest expense driven by our $31 billion aggregate principal amount of senior unsecured notes issued in May 2023 as part of the financing for our acquisition of Seagen, which was more than offset by higher interest income on the investment of the net proceeds from the debt issuance.
(c)2023 net gains primarily include, among other things, a realized gain of $1.7 billion related to salesour investment in Telavant Holdings, Inc. and unrealized gains of compound/product rights$297 million related to our investment in Cerevel Therapeutics Holdings, Inc (Cerevel), partially offset by unrealized losses of $292 million related to our investment in BioNTech. 2022 net losses included, among other things, unrealized losses of $986 million related to investments in BioNTech, Allogene Therapeutics, Inc. and (v) a $40Arvinas. 2021 net gains included, among other things, unrealized gains of $1.6 billion related to investments in BioNTech and Cerevel.
(d)2021 included, among other things, $188 million milestone paymentof net collaboration income from Merck & Co., Inc. in conjunction with the approval of ertugliflozin in the EU.BioNTech related to Comirnaty.
(e) See Note 11. In 2019,2023 primarily includes certain product liability and other non-service cost components’ activitylegal expenses related to the Consumer Healthcare JV transaction, such as gain on settlements, were recorded in (Gain) on completion of Consumer Healthcare JV transaction.products discontinued and/or divested by Pfizer and legal obligations related to pre-acquisition matters. 2022 primarily included certain product liability and other legal expenses related to products discontinued and/or divested by Pfizer. 2021 primarily included certain product liability expenses related to products discontinued and/or divested by Pfizer, and to a lesser extent, legal obligations related to pre-acquisition matters.
(f)2019 mostly included legal reserves for certain pending legal matters. 20182023 primarily included legal reserves for certain pending legal matters, partially offset by the reversal of a legal accrual where a loss was no longer deemed probable.
(g)2020 primarily includesrepresents intangible asset impairment charges of $1.7$3.0 billion, of which $2.9 billion is associated with our Biopharma segment ($2.8 billion recorded in the fourth quarter), including: $1.4 billion for etrasimod (Velsipity) IPR&D, based on a change in development plans for additional indications and overall revenue expectations, $964 million for Prevnar 13 developed technology rights ($834 million for pediatric and $130 million for adult), due to updated commercial forecasts mainly composed of: (i) $900reflecting a transition to higher serotype coverage, and $486 million related tofor various other IPR&D assets for unapproved indications of certain cancer medicines, acquired in our Array acquisition, and reflect, among other things, updated commercial forecasts; (ii) $528 million related to Eucrisa, a finite-lived developed technology right acquired in our Anacor acquisition, and reflectsrights, due to updated commercial forecasts mainly reflecting competitive pressures; and (iii) $263pressures and/or prioritization decisions. 2023 also includes $128 million associated with Other business activities, related to finite-livedIPR&D and developed technology rights for certain generic sterile injectables acquired in our Hospira acquisition,software assets and reflects unfavorable pivotal trial results and updated commercial forecasts mainly reflecting competitive pressures.
2019 primarily includedforecasts. 2022 represented intangible asset impairment charges of $2.8 billion, mainly composed of $2.6 billion, related to Eucrisa, and reflects updated commercial forecasts mainly reflecting competitive pressures.
2018 primarily included intangible asset impairment charges of $3.1 billion, mainly composed of (i) $2.6 billion related to developed technology rights, $242associated with our Biopharma segment of: $200 million related to licensing agreements and $80 million related tofor an IPR&D allasset for the unapproved indication of which were acquired in our Hospira acquisition, for generic sterile injectable products associated with various indications; and (ii) $117 million relatedsymptomatic dilated cardiomyopathy due to a multi-antigen vaccine IPR&D program for adults undergoing elective spinal fusion surgery. The intangible asset impairment charges for the generic sterile injectable products reflect, among other things, updated commercial forecasts, reflecting an increased competitive environment as well as higher manufacturing costs, largely stemming from manufacturing and supply issues. The intangible asset impairment charge for the multi-antigen vaccine IPR&D program was the resultmutation of the gene encoding the lamin A/C protein that resulted from the Phase 2b3 trial reaching futility at a pre-planned interim analysis.
(h)Mainly represents incremental costsanalysis and $171 million for the design, planningdeveloped technology rights due to updated commercial forecasts mainly reflecting competitive pressures. 2022 also included intangible asset impairment charges of $50 million associated with PC1, related to finite-lived licensing agreements and implementation of our then new business structure, effective in the beginning of 2019, and primarily includes consulting, legal, tax and other advisory services.reflected updated contract manufacturing forecasts reflecting changes to market dynamics.
(j)(h)20202023 includes, among other things, (i) dividend income of $278$265 million from our investment in ViiV and $211 million from our investment in Nimbus resulting from Takeda’s acquisition of Nimbus’s oral, selective allosteric tyrosine kinase 2 (TYK2) inhibitor program subsidiary and (ii) a $222 million gain on the divestiture of our early-stage rare disease gene therapy portfolio to Alexion. 2022 included, among other things, (i) dividend income of $314 million from our investment in ViiV, (ii) income net of costs associated with TSAs of $142 million and (iii) charges of $105$77 million, reflecting the change in the fair value of contingent consideration. 20192021 included, among other things, (i) income net of costs associated with TSAs of $288 million, (ii) dividend income of $220$166 million from our investment in ViiV; (ii)ViiV and (iii) charges of $152 million for external incremental costs, such as transaction costs and costs to separate our Consumer Healthcare business into a separate legal entity, associated with the formation of the Consumer Healthcare JV; and (iii) net losses on early retirement of debt of $138 million. 2018 included, among other things, (i) a non-cash $343 million pre-tax gain associated with our transaction with Bain Capital to create a new biopharmaceutical company, Cerevel, to continue development of a portfolio of clinical and preclinical stage neuroscience assets primarily targeting disorders of the central nervous system; (ii) dividend income of $253 million from our investment in ViiV; (iii) a non-cash $50 million pre-tax gain related to our contribution agreement entered into with Allogene (see Note 2B); (iv) charges of $207$142 million, reflecting the change in the fair value of contingent consideration, and (vi) charges of $112 million for external incremental costs, such as transaction costs and costs to separate our Consumer Healthcare business into a separate legal entity, associated with the formation of the Consumer Healthcare JV.consideration.
The asset impairment charges included in Other (income)/deductions––net are based on estimates of fair value.
| Additional information about the intangible assets that were impaired during 2020 (impairment recorded in Other (income)/deductions–net) follows: | | Additional information about the intangible assets that were impaired during 2023 follows: | | Additional information about the intangible assets that were impaired during 2023 follows: |
| | | | Year Ended | | | | | | Year Ended |
| | Fair Value(a) | | | | Fair Value(a) | | December 31, 2023 |
| (MILLIONS) | | (MILLIONS) | | Amount | | Level 1 | | Level 2 | | Level 3 | | Impairment |
| | | | Year Ended December 31, |
| Fair Value(a) | | 2020 |
| (MILLIONS OF DOLLARS) | | Amount | | Level 1 | | Level 2 | | Level 3 | | Impairment |
Intangible assets––IPR&D(b) | | $ | 1,100 | | | $ | 0 | | | $ | 0 | | | $ | 1,100 | | | $ | 900 | |
Intangible assets––IPR&D(b) | |
Intangible assets––IPR&D(b) | |
Intangible assets––IPR&D(b) | |
Intangible assets––Developed technology rights(b) | Intangible assets––Developed technology rights(b) | | 740 | | | 0 | | | 0 | | | 740 | | | 791 | |
Intangible assets––Licensing agreements and other(b) | |
| Total | Total | | $ | 1,840 | | | $ | 0 | | | $ | 0 | | | $ | 1,840 | | | $ | 1,691 | |
(a)The fair value amount isamounts are presented as of the date of impairment, as these assets are not measured at fair value on a recurring basis. See also Note 1E.1E. (b)Reflects intangible assets written down to fair value in 2020.2023. Fair value was determined using the income approach, specifically the multi-period excess earnings method, also known as the discounted cash flow method. We started with a forecast of all the expected net cash flows for the asset and then applied an asset-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of competitive, legal and/or regulatory forces on the product; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
Note 5. Tax Matters
A. Taxes on Income from Continuing Operations
| | | | | | | | | | | | | | | | | | | | |
Components of Income from continuing operations before provision/(benefit) for taxes on income include: |
| | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 |
| United States | | $ | (2,488) | | | $ | 7,064 | | | $ | (6,111) | |
| International | | 9,986 | | | 4,420 | | | 9,706 | |
Income from continuing operations before provision/(benefit) for taxes on income(a), (b) | | $ | 7,497 | | | $ | 11,485 | | | $ | 3,594 | |
(a)2020 v. 2019––The domestic loss in 2020 versus domestic income in 2019 was mainly related to the non-recurrence of the gain on the completion of the Consumer Healthcare JV transaction as well as higher certain asset impairments and higher R&D expenses. The increase in the international income was primarily related to the non-recurrence of the write off of assets contributed to the Consumer Healthcare JV as well as lower certain asset impairments and lower amortization of intangible assets.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 7273 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 5. Tax Matters
A. Taxes on Income from Continuing Operations
| | | | | | | | | | | | | | | | | | | | |
Components of Income from continuing operations before provision/(benefit) for taxes on income include: |
| | | Year Ended December 31, |
| (MILLIONS) | | 2023 | | 2022 | | 2021 |
| United States | | $ | (4,411) | | | $ | 5,032 | | | $ | 6,064 | |
| International | | 5,469 | | | 29,697 | | | 18,247 | |
Income from continuing operations before provision/(benefit) for taxes on income(a), (b) | | $ | 1,058 | | | $ | 34,729 | | | $ | 24,311 | |
(b)(a)20192023 v. 20182022––The domestic loss in 2023 versus domestic income in 2019 versus domestic loss2022 and the decrease in 2018international income in 2023 was mainly relatedprimarily attributable to the completionlower revenues, higher intangible asset impairment charges, and increases in Restructuring charges and certain acquisition-related costs, Amortization of the Consumer Healthcare JV transaction as well as lower certain asset impairments,intangible assets, and Selling, informational and administrative expenses, partially offset by higher businessa decrease in Cost of sales and legal entity alignment costs as well as increased costs related to certain legal matters. net gains on equity securities in 2023 versus net losses on equity securities in 2022.
(b)2022 v. 2021––The decrease in the internationaldomestic income wasis primarily related to net losses on equity securities in 2022 versus net gains on equity securities in 2021, lower net periodic benefit credits and higher restructuring charges and certain asset impairments as well as the write off of assets contributedacquisition-related costs, partially offset by Paxlovid income and lower acquired IPR&D expenses. The increase in international income is primarily related to the Consumer Healthcare JV.Paxlovid and Comirnaty income partially offset by lower net periodic benefit credits.
| Components of Provision/(benefit) for taxes on income based on the location of the taxing authorities include: | Components of Provision/(benefit) for taxes on income based on the location of the taxing authorities include: | Components of Provision/(benefit) for taxes on income based on the location of the taxing authorities include: |
| | | | Year Ended December 31, | | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 |
| (MILLIONS) | | (MILLIONS) | | 2023 | | 2022 | | 2021 |
| United States | United States | | | | | | |
| Current income taxes: | Current income taxes: | | | |
| Current income taxes: | |
| Current income taxes: | |
| Federal | |
| Federal | |
| Federal | Federal | | $ | 371 | | | $ | (1,886) | | | $ | 388 | |
| State and local | State and local | | 58 | | | (187) | | | (49) | |
| Deferred income taxes: | Deferred income taxes: | | | |
| Federal | Federal | | (1,061) | | | 1,193 | | | (1,641) | |
| Federal | |
| Federal | |
| State and local | State and local | | (115) | | | 266 | | | 15 | |
| Total U.S. tax benefit | | (747) | | | (613) | | | (1,287) | |
TCJA(a) | | | | | | |
| Current income taxes | | 0 | | | (135) | | | (3,035) | |
| Deferred Income taxes | | 0 | | | (187) | | | 2,439 | |
| Total TCJA tax benefit | | 0 | | | (323) | | | (596) | |
| Total U.S. tax provision/(benefit) | |
| | International | |
| | International | |
| | International | International | | | | | | |
| Current income taxes | Current income taxes | | 1,517 | | | 2,418 | | | 2,195 | |
| Current income taxes | |
| Current income taxes | |
| Deferred income taxes | Deferred income taxes | | (292) | | | (863) | | | (579) | |
| Total international tax provision | | 1,224 | | | 1,555 | | | 1,617 | |
| Total international tax provision/(benefit) | |
| Provision/(benefit) for taxes on income | Provision/(benefit) for taxes on income | | $ | 477 | | | $ | 618 | | | $ | (266) | |
(a)The 2018 current tax benefit and deferred tax expense primarily relate to the utilization of tax credit carryforwards against the repatriation tax liability associated with the enactment of the TCJA. See discussion below.
Amounts discussed below are rounded to the nearest hundred million and represent approximations.
In 2018, we finalized our provisional accounting for the tax effects of the TCJA, based on our best estimates of available information and data. We reported and disclosed the impacts within the applicable measurement period,changes in accordance with SEC guidance, and recorded a favorable adjustment of $100 million to Provision/(benefit) for taxes on income impacting the effective tax rate year-over-year are summarized below:
2023 v. 2022
The tax benefit of $1.1 billion for 2023 compared to the tax provision of $3.3 billion for 2022 was primarily a result of changes in the jurisdictional mix of earnings and the resolution of uncertain tax positions in various markets. The 2023 pre-tax income included a greater percentage of expenses taxed at higher rates as compared to the 2022 pre-tax income, resulting in a 2023 tax benefit compared to the 2022 tax provision. These expenses included amortization expense, acquisition-related costs, restructuring charges and intangible asset impairment charges. The tax benefit for 2023 and the tax provision for 2022 included tax benefits related to global income tax resolutions in multiple tax jurisdictions spanning multiple tax years. The tax provision for 2022 also included the closing of U.S. IRS audits covering five tax years.
2022 v. 2021
The higher effective tax rate in 2022 was mainly the result of:
•the non-recurrence of certain initiatives executed in 2021 associated with our investment in the Consumer Healthcare JV with GSK based on estimates and assumptions that we believe to be reasonable,
partially offset by:
•tax benefits in 2022 related to global income tax resolutions in multiple tax jurisdictions spanning multiple tax years that included the closing of U.S. IRS audits covering five tax years.
In all years, federal, state and international net tax liabilities assumed or established as part of a business acquisition are not included in Provision/(benefit) for taxes on income (see Note 2A). We elected, with the filing of our 2018 U.S. Federal Consolidated Income Tax Return, to pay our initial estimated $15 billion repatriation tax liability on accumulated post-1986 foreign earnings over eight years through 2026. The thirdfifth annual installment of this liability whichwas paid by its April 18, 2023 due date. The sixth annual installment is due to be paid in April 2021,15, 2024 and is reported in current Income taxes payable, and theas of December 31, 2023. The remaining liability is reported in noncurrent Other taxes payablepayable. as of December 31, 2020. Our obligations may vary as a result of changes in our uncertain tax positions and/or availability of attributes such as foreign tax and other credit carryforwards.
The TCJA subjects a U.S. shareholder to current tax on global intangible low-taxed income earned by certain foreign subsidiaries. The FASB Staff Q&A, Topic 740, No. 5, Accounting for Global Intangible Low-Taxed Income, states that we are permitted to make an accounting policy election to either recognize deferred taxes for temporary basis differences expected to reverse as global intangible low-taxed income in future years or provide for the tax expense related to such income in the year the tax is incurred. We elected to recognize deferred taxes for temporary differences expected to reverse as global intangible low-taxed income in future years.
On March 27, 2020, the Coronavirus Aid, Relief, and Economic Security Act (CARES Act) was signed into law in the U.S. to provide certain relief as a result of the COVID-19 pandemic. In addition, governments around the world have enacted or implemented various forms of tax relief measures in response to the economic conditions in the wake of COVID-19. As of December 31, 2020, neither the CARES Act nor changes to income tax laws or regulations in other jurisdictions had a significant impact on our effective tax rate.
The changes in Provision/(benefit) for taxes on income impacting the effective tax rate year-over-year are summarized below:
2020 v. 2019
The higher effective tax rate in 2020 was mainly the result of:
•the non-recurrence of the $1.4 billion tax benefits, representing taxes and interest, recorded in 2019 due to the favorable settlement of an IRS audit for multiple tax years;
•the non-recurrence of the tax benefits related to certain tax initiatives associated with the implementation of our then new business structure; and
•the non-recurrence of the tax benefits recorded in 2019 as a result of additional guidance issued by the U.S. Department of Treasury related to the TCJA, as well as:
•lower tax benefits related to the impairment of intangible assets,
partially offset by:
•the non-recurrence of the tax expense of $2.7 billion recorded in the third quarter of 2019 associated with the gain related to the completion of the Consumer Healthcare JV transaction; and
•the favorable change in the jurisdictional mix of earnings as a result of operating fluctuations in the normal course of business.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 7374 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2019 v. 2018B. Tax Rate Reconciliation
| | | | | | | | | | | | | | | | | | | | |
The reconciliation of the U.S. statutory income tax rate to our effective tax rate for Income from continuing operations follows: |
| | | Year Ended December 31, |
| | 2023* | | 2022 | | 2021 |
| U.S. statutory income tax rate | | 21.0 | % | | 21.0 | % | | 21.0 | % |
| | | | | | |
Taxation of non-U.S. operations(a), (b) | | (21.1) | | | (5.0) | | | (4.3) | |
Tax settlements and resolution of certain tax positions(c) | | (40.3) | | | (3.0) | | | (0.4) | |
Foreign-Derived Intangible Income deduction(d) | | (33.1) | | | (1.9) | | | (0.6) | |
State & local taxes(e) | | (22.4) | | | — | | | (0.5) | |
| Charitable contributions | | (7.3) | | | (0.5) | | | (0.6) | |
| | | | | | |
Certain Consumer Healthcare JV initiatives(c) | | — | | | — | | | (6.0) | |
| U.S. R&D tax credit | | (15.8) | | | (0.6) | | | (0.5) | |
Interest(f) | | 13.5 | | | 0.2 | | | 0.4 | |
All other, net(g) | | 0.2 | | | (0.6) | | | (0.7) | |
| Effective tax rate for income from continuing operations | | (105.4) | % | | 9.6 | % | | 7.6 | % |
The higher effective tax rate was primarilypercentages for the result of:
•the tax expense of $2.7 billion associated with the gain related to the completion of the Consumer Healthcare JV transaction; and
•the non-recurrence of certain tax initiatives and favorable adjustments to the provisional estimate of the TCJA,
partially offset by:
•an increase in tax benefits associated with the resolution of certain tax positions pertaining to prior years, primarily due to a benefit of $1.4 billion, representing tax and interest, resulting from the favorable settlement of an IRS audit;
•benefits related to certain tax initiatives associated with the implementation of our then new business structure;
•the tax benefits recorded as a result of additional guidance issued2023 reconciling items are significantly impacted by the U.S. Department of Treasury related to the enactment of the TCJA; and
•the favorable change in the jurisdictional mix of earnings as a result of operating fluctuations in the normal course of business.
In all years, federal, statelower domestic and international net tax liabilities assumed or established as part of a business acquisition are not included in Provision/Income from continuing operations before provision/(benefit) for taxes on income (see Note 2A5A).
B. Tax Rate Reconciliation
| | | | | | | | | | | | | | | | | | | | |
The reconciliation of the U.S. statutory income tax rate to our effective tax rate for Income from continuing operations follows: |
| | | Year Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| U.S. statutory income tax rate | | 21.0 | % | | 21.0 | % | | 21.0 | % |
TCJA impact(a) | | 0 | | | (2.8) | | | (16.6) | |
Taxation of non-U.S. operations (b), (c) | | (9.6) | | | (4.5) | | | 1.2 | |
Tax settlements and resolution of certain tax positions(d) | | (2.5) | | | (13.8) | | | (19.3) | |
Completion of Consumer Healthcare JV transaction(d) | | 0 | | | 8.2 | | | 0 | |
U.S. Healthcare Legislation(e) | | 0.1 | | | 0 | | | (1.1) | |
| U.S. R&D tax credit | | (1.3) | | | (0.8) | | | (2.2) | |
Interest(f) | | 1.1 | | | 0.6 | | | 5.7 | |
| | | | | | |
All other, net(g) | | (2.4) | | | (2.5) | | | 3.9 | |
| Effective tax rate for income from continuing operations | | 6.4 | % | | 5.4 | % | | (7.4) | % |
(a)See Note 5A.
(b)For taxation of non-U.S. operations, this rate impact reflects the income tax rates and relative earnings in the locations where we do business outside the U.S., together with the U.S. tax cost on our international operations, changes in uncertain tax positions not included in the reconciling item called “Tax settlements and resolution of certain tax positions,” as well as changes in valuation allowances. Specifically: (i) the jurisdictional location of earnings is a significant component of our effective tax rate each year, and the rate impact of this component is influenced by the specific location of non-U.S. earnings and the level of such earnings as compared to our total earnings; (ii) the U.S. tax implications of our foreign operations is a significant component of our effective tax rate each year and generally offsets some of the reduction to our effective tax rate each year resulting from the jurisdictional location of earnings; (iii) the impact of certain tax initiatives; and (iv) the impact of changes in uncertain tax positions not included in the reconciling item called “Tax settlements and resolution of certain tax positions” is a component of our effective tax rate each year that can result in either an increase or decrease to our effective tax rate. The jurisdictional mix of earnings, which includes the impact of the location of earnings as well as the U.S. tax cost on our international operations, can vary as a result of operating fluctuations in the normal course of business and as a result of the extent and location of other income and expense items, such as restructuring charges, asset impairments and gains and losses on strategic business decisions. See also Note 5A for the components of pre-tax income and Provision/(benefit) for taxes on income, which is based on the location of the taxing authorities, and for information about settlements and other items impacting Provision/(benefit) for taxes on income.(c)(b)In all years, the impact on our effective tax rate is the result of the jurisdictional location of earnings. In 2020 and 2019, the reduction in our effective tax rate resulting fromis a result of the jurisdictional location of earnings and is largely due to lower tax rates in certain jurisdictions, as well as manufacturing and other incentives for our subsidiaries in Singapore and, to a lesser extent, in Puerto Rico. We benefit from Puerto Rican tax incentives pursuant to a grant that expires during 2029.2053. Under such grant, we are partially exempt from income, property and municipal taxes. In Singapore, we benefit from incentive tax rates effective through 20452048 on income from manufacturing and other operations.
(d)For a discussion aboutThe higher rate benefit from the Foreign-Derived Intangible Income deduction in 2022 is mainly the result of the TCJA requirement to capitalize R&D costs for tax settlements and resolution of certain tax positions andyears beginning after December 31, 2021.
(e)Includes the impact of U.S. state and local taxes and changes in the gain onstate valuation allowances including those related to the completionacquisition of the Consumer Healthcare JV transaction, see Note 5A.
(e)The favorable rate impact in 2018 is a result of the updated 2017 invoice received from the federal government, which reflected a lower expense than what was previously estimated for invoiced periods, as well as certain tax initiatives.Seagen.
(f)Includes changes in interest related to our uncertain tax positions not included in the reconciling item called “Tax settlements and resolution of certain tax positions”.
(g)All other, net is primarily due to routine business operations.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 7475 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
C. Deferred Taxes
| Components of our deferred tax assets and liabilities, shown before jurisdictional netting, follow: | | 2020 Deferred Tax* | | 2019 Deferred Tax* |
| (MILLIONS OF DOLLARS) | | Assets | | (Liabilities) | | Assets | | (Liabilities) |
| | 2023 Deferred Tax* | | | | 2023 Deferred Tax* | | 2022 Deferred Tax* |
| (MILLIONS) | | (MILLIONS) | | Assets | | (Liabilities) | | Assets | | (Liabilities) |
Prepaid/deferred items(a) | Prepaid/deferred items(a) | | $ | 3,094 | | | $ | (352) | | | $ | 1,918 | | | $ | (204) | |
| Inventories | | 276 | | | (25) | | | 267 | | | (10) | |
Intangible assets(b) | | 793 | | | (5,355) | | | 718 | | | (6,784) | |
| Accrued/deferred royalties | |
Deferred revenues(b) | |
Inventories(c) | |
Intangible assets(d) | |
| Property, plant and equipment | Property, plant and equipment | | 211 | | | (1,219) | | | 177 | | | (1,204) | |
| Employee benefits | | 1,981 | | | (127) | | | 2,115 | | | (37) | |
Employee benefits(e) | |
| Restructurings and other charges | Restructurings and other charges | | 291 | | | — | | | 212 | | | — | |
| Legal and product liability reserves | Legal and product liability reserves | | 382 | | | — | | | 469 | | | — | |
Net operating loss/tax credit carryforwards(c) | | 1,761 | | | — | | | 2,003 | | | — | |
Research and development(f) | |
Net operating loss/tax credit carryforwards(g), (h) | |
| Unremitted earnings | Unremitted earnings | | — | | | (46) | | | — | | | (77) | |
| State and local tax adjustments | State and local tax adjustments | | 171 | | | — | | | 152 | | | — | |
Investments(d) | | 128 | | | (3,545) | | | 11 | | | (3,318) | |
Investments(i) | |
| All other | All other | | 102 | | | (57) | | | 167 | | | (9) | |
| 9,189 | | | (10,726) | | | 8,208 | | | (11,643) | |
| | 19,037 | |
| Valuation allowances | Valuation allowances | | (1,586) | | | — | | | (1,526) | | | — | |
| Total deferred taxes | Total deferred taxes | | $ | 7,603 | | | $ | (10,726) | | | $ | 6,682 | | | $ | (11,643) | |
Net deferred tax liability(e) | | | | $ | (3,123) | | | | | $ | (4,961) | |
Net deferred tax asset/(liability)(j), (k) | |
*The deferred tax assets and liabilities associated with global intangible low-taxed income are included in the relevant categories. See Note 5A1Q. (a)The increase in 2020net deferred tax assets in 2023 is primarily related to temporary differences associated with the capitalizationtiming of certain R&D-related expenses.cash tax payments made and accruals recorded in the ordinary course of business.
(b)The increase in deferred tax assets in 2023 is primarily related to temporary differences associated with the non-cash revenue reversal for Paxlovid recorded in the fourth quarter of 2023. See Note 17C. (c)The decrease in 2020net deferred tax assets in 2023 is primarily due to the resultacquisition of inventories related to Seagen, partially offset by the temporary differences associated with the non-cash charges for inventory write-offs for Paxlovid and Comirnaty.
(d)The increase in net deferred tax liabilities in 2023 is primarily due to the acquisition of intangible assets related to Seagen, partially offset by the amortization of intangible assets and certain impairment charges.
(c)(e)The decrease in net deferred tax assets in 2023 is primarily due to changes in pension and postretirement benefit obligations, as well as the performance of plan assets reported in the period. See Note 11.(f)The increase in deferred tax assets in 2023 is primarily related to the acquisition of capitalized R&D costs related to Seagen and the TCJA requirement to capitalize R&D costs for tax years beginning after December 31, 2021.
(g)The increase in deferred tax assets in 2023 is primarily due to the acquisition of net operating loss carryforwards and credit carryforwards related to Seagen. See Note 2A. (h)The amounts in 20202023 and 20192022 are reduced for unrecognized tax benefits of $3.0$1.3 billion and $2.9$1.2 billion, respectively, where we have net operating loss carryforwards, similar tax losses, and/or tax credit carryforwards that are available, under the tax law of the applicable jurisdiction, to settle any additional income taxes that would result from the disallowance of a tax position.
(d)(i)The amountsincrease in 2020 and 2019 arenet deferred tax liabilities in 2023 is primarily due to the impact of foreign currency translation adjustments related to our equity-method investment in Haleon/the Consumer Healthcare JV. See Note 2C.2C.(e)(j)In 2020,2023, Noncurrent deferred tax assets and other noncurrent tax assets ($0.91.8 billion), and Noncurrent deferred tax liabilities ($4.10.6 billion). In 2019,2022, Noncurrent deferred tax assets and other noncurrent tax assets ($0.74.8 billion), and Noncurrent deferred tax liabilities ($5.71.0 billion).
We have carryforwards, primarily related to net operating and capital losses, general business credits, foreign tax credits and charitable contributions, which are available to reduce future U.S. federal and/or state, as well as international, income taxes payable with either an indefinite life or expiring at various times from 20212024 to 2040.2043. Certain of our U.S. net operating losses and general business credits are subject to limitations under IRC Section 382.
As of December 31, 2020,2023, we have not made a U.S. tax provision on $55.0$49.0 billion of unremitted earnings of our international subsidiaries. As these earnings are intended to be indefinitely reinvested overseas, the determination of a hypothetical unrecognized deferred tax liability as of December 31, 20202023 is not practicable. The amount of indefinitely reinvested earnings is based on estimates and assumptions and subject to management evaluation, and is subject to change in the normal course of business based on operational cash flow, completion of local statutory financial statements and the finalization of tax returns and audits, among other things. Accordingly, we regularly update our earnings and profits analysis for such events.
D. Tax Contingencies
For a description of our accounting policies associated with accounting for income tax contingencies, see Note 1P.1Q.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 76 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Uncertain Tax Positions
As tax law is complex and often subject to varied interpretations, it is uncertain whether some of our tax positions will be sustained upon audit. As of December 31, 2020,2023, we had $4.3 $3.1 billion and as of December 31, 2019,2022, we had $4.2$2.9 billion in net unrecognized tax benefits, excluding associated interest.
•Tax assets for uncertain tax positions primarily represent our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction. These potential benefits generally result from cooperative efforts among taxing authorities, as required by tax treaties to minimize double taxation, commonly referred to as the competent authority process. The recoverability of these assets, which we believe to be more likely than not, is dependent upon the actual payment of taxes in one tax jurisdiction and, in some cases, the successful petition for recovery in another tax jurisdiction. As of December 31, 2020,2023, we had $1.3$1.7 billion in assets associated with uncertain tax positions. These amounts were included in Noncurrent deferred tax assets and other noncurrent tax assets ($1.11.6 billion), Noncurrent deferred tax liabilities ($122 million) and Other taxes payable ($4645 million). As of December 31, 2019,2022, we had $1.2$1.5 billion in assets associated with uncertain tax positions. These amounts were included in Noncurrent deferred tax assets and other noncurrent tax assets ($1.01.5 billion) and Noncurrent deferred tax liabilitiesOther taxes payable ($10945 million).
•Substantially all of these unrecognized tax benefits, if recognized, would impact our effective income tax rate.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 75 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | |
| The reconciliation of the beginning and ending amounts of gross unrecognized tax benefits follows: |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 |
| Balance, beginning | | $ | (5,381) | | | $ | (6,259) | | | $ | (6,558) | |
Acquisitions(a) | | 37 | | | (44) | | | 0 | |
Divestitures(b) | | 265 | | | 0 | | | 0 | |
Increases based on tax positions taken during a prior period(c) | | (232) | | | (36) | | | (192) | |
Decreases based on tax positions taken during a prior period(c), (d) | | 64 | | | 1,109 | | | 561 | |
Decreases based on settlements for a prior period(e) | | 15 | | | 100 | | | 123 | |
Increases based on tax positions taken during the current period(c) | | (411) | | | (383) | | | (370) | |
| Impact of foreign exchange | | (72) | | | 25 | | | 56 | |
Other, net(c), (f) | | 120 | | | 107 | | | 121 | |
Balance, ending(g) | | $ | (5,595) | | | $ | (5,381) | | | $ | (6,259) | |
| | | | | | | | | | | | | | | | | | | | |
| The reconciliation of the beginning and ending amounts of gross unrecognized tax benefits follows: |
| (MILLIONS) | | 2023 | | 2022 | | 2021 |
| Balance, beginning | | $ | (4,494) | | | $ | (6,068) | | | $ | (5,595) | |
| Acquisitions | | (46) | | | (52) | | | — | |
| | | | | | |
Increases based on tax positions taken during a prior period(a) | | (158) | | | (67) | | | (111) | |
Decreases based on tax positions taken during a prior period(a), (b) | | 310 | | | 1,339 | | | 103 | |
Decreases based on settlements for a prior period(b), (c) | | 85 | | | 842 | | | 24 | |
Increases based on tax positions taken during the current period(a) | | (515) | | | (701) | | | (550) | |
| Impact of foreign exchange | | (44) | | | 90 | | | 22 | |
Other, net(a), (d) | | 58 | | | 122 | | | 40 | |
Balance, ending(e) | | $ | (4,802) | | | $ | (4,494) | | | $ | (6,068) | |
(a)For 2020 and 2019, primarily related to the acquisition of Array (goodwill adjustment made within the measurement period). See Note 2A.
(b)For 2020, related to the separation of Upjohn. See Note 2B.
(c)Primarily included in Provision/(benefit) for taxes on income.
(d)(b)Primarily related to effectively settling certain issues with the U.S. and foreign tax authorities. See Note 5A.Note 5A.(e)(c)Primarily related to cash payments and reductions of tax attributes.
(f)(d)Primarily related to decreases as a result of a lapse of applicable statutes of limitations.
(g)(e)In 2020,2023, included in Income taxes payable ($3494 million), Other current assets ($1 million), Noncurrent deferred tax assets and other noncurrent tax assets ($18 million)1.3 billion), Noncurrent deferred tax liabilities ($3.0 billion)4 million) and Other taxes payable ($2.53.4 billion). In 2019,2022, included in Income taxes payable ($10840 million),Current tax Other current assets ($23 million), Noncurrent deferred tax assets and other noncurrent tax assets ($51 million)1.2 billion), Noncurrent deferred tax liabilities ($2.8 billion)5 million) and Other taxes payable ($2.43.2 billion).
•Interest related to our unrecognized tax benefits is recorded in accordance with the laws of each jurisdiction and is recorded primarily in Provision/(benefit) for taxes on income. In 2020,2023, we recorded a net increase in interest of $89$64 million. In 2019,2022, we recorded a net decrease in interest of $564 million, resulting primarily from a settlement with the IRS; and in 2018,$17 million. In 2021, we recorded a net increase in interest of $103$108 million. Gross accrued interest totaled $493$605 million as of December 31, 20202023 (reflecting a decrease of $5$11 million as a result of cash payments and a decrease of $75 million relating to the separation of Upjohn)payments) and gross accrued interest totaled $485$552 million as of December 31, 20192022 (reflecting a decrease of $13$31 million as a result of cash payments). In 2020, this amount was2023 and 2022, these amounts were substantially all included in Income taxes payable ($7 million) and Other taxes payablepayable. ($486 million). In 2019, this amount was included in Income taxes payable ($20 million)and Other taxes payable ($465 million). Accrued penalties are not significant. See also Note 5A.5A
Status of Tax AuditsMatters and Potential Impact on Accruals for Uncertain Tax Positions
The U.S. is one of our major tax jurisdictions, and we are regularly audited by the IRS. With respect to Pfizer, the IRS has issued a Revenue Agent’s Report (RAR) for tax years 2011-2013. We2016-2018 are not in agreement with the RAR and are currently appealing certain disputed issues. Tax years 2014-2015 are currently under audit. Tax years 2016-20202019-2023 are open but not under audit. All other tax years are closed.
In addition to the open audit years in the U.S., we have open audit years and certain related audits, appeals and investigations in othercertain major international tax jurisdictions such as Canada (2013-2020), Japan (2017-2020)(2017-2023), Europe (2011-2020,(2012-2023, primarily reflectingin Ireland, the U.K., France, Italy, Spain and Germany), Asia Pacific (2013-2023, primarily in Australia, China, Japan and Singapore) and Latin America (1998-2020,(1998-2023, primarily reflectingin Brazil) and Puerto Rico (2016-2020).
Any settlements or statutes of limitations expirations could result in a significant decrease in our uncertain tax positions. We estimate that it is reasonably possible that within the next 12 months, our gross unrecognized tax benefits, exclusive of interest, could decrease by as much as $50$100 million, as a result of settlements with taxing authorities or the expiration of the statutes of limitations. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes, and variation from such estimates could materially affect our financial statements in the period of settlement or when the statutes of limitations expire, as we treat these events as discrete items in the period of resolution. Finalizing audits with the relevant taxing authorities can include formal administrative and legal proceedings, and, as a result, it is difficult to estimate the timing and range of possible changes related to our uncertain tax positions, and such changes could be significant.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 7677 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
E. Tax Provision/(Benefit) on Other Comprehensive Income/(Loss)
| Components of the Tax provision/(benefit) on other comprehensive income/(loss) include: | Components of the Tax provision/(benefit) on other comprehensive income/(loss) include: | |
Components of the Tax provision/(benefit) on other comprehensive income/(loss) include: | |
| | | | | Year Ended December 31, | | | | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | | 2020 | | 2019 | | 2018 |
| (MILLIONS) | | (MILLIONS) | | | | | | 2023 | | 2022 | | 2021 |
Foreign currency translation adjustments, net(a) | Foreign currency translation adjustments, net(a) | | | $ | (79) | | | $ | 254 | | | $ | 94 | |
| | Unrealized holding gains/(losses) on derivative financial instruments, net | Unrealized holding gains/(losses) on derivative financial instruments, net | | | (88) | | | 83 | | | 21 | |
| Unrealized holding gains/(losses) on derivative financial instruments, net | |
| Unrealized holding gains/(losses) on derivative financial instruments, net | |
| Reclassification adjustments for (gains)/losses included in net income | Reclassification adjustments for (gains)/losses included in net income | | | (25) | | | (125) | | | 27 | |
Reclassification adjustments of certain tax effects from AOCI to Retained earnings(b) | | | 0 | | | 0 | | | 1 | |
| | | |
| | |
| | | | | (113) | | | (42) | | | 50 | |
| Unrealized holding gains/(losses) on available-for-sale securities, net | Unrealized holding gains/(losses) on available-for-sale securities, net | | | 45 | | | 0 | | | (23) | |
| Reclassification adjustments for (gains)/losses included in net income | Reclassification adjustments for (gains)/losses included in net income | | | (24) | | | 5 | | | 16 | |
Reclassification adjustments for tax on unrealized gains from AOCI to Retained earnings(c) | | | 0 | | | 0 | | | (45) | |
| | | | | 22 | | | 5 | | | (53) | |
| Benefit plans: actuarial gains/(losses), net | | | (281) | | | (169) | | | (141) | |
| Reclassification adjustments related to amortization | | | 62 | | | 55 | | | 55 | |
| Reclassification adjustments related to settlements, net | | | 65 | | | 65 | | | 33 | |
Reclassification adjustments of certain tax effects from AOCI to Retained earnings(b) | | | 0 | | | 0 | | | 637 | |
| Other | | | (8) | | | (10) | | | 29 | |
| | |
| | |
| | | | | (161) | | | (58) | | | 612 | |
| Benefit plans: prior service (costs)/credits and other, net | Benefit plans: prior service (costs)/credits and other, net | | | 12 | | | (1) | | | 2 | |
| Reclassification adjustments related to amortization of prior service costs and other, net | Reclassification adjustments related to amortization of prior service costs and other, net | | | (31) | | | (43) | | | (39) | |
| Reclassification adjustments related to curtailments of prior service costs and other, net | Reclassification adjustments related to curtailments of prior service costs and other, net | | | 0 | | | (1) | | | (4) | |
Reclassification adjustments of certain tax effects from AOCI to Retained earnings(b) | | | 0 | | | 0 | | | (144) | |
| Other | | | 1 | | | 0 | | | 0 | |
| | | |
| | | |
| | | | | | (17) | | | (45) | | | (185) | |
| Tax provision/(benefit) on other comprehensive income/(loss) | Tax provision/(benefit) on other comprehensive income/(loss) | | | $ | (349) | | | $ | 115 | | | $ | 518 | |
(a)Taxes are not provided for foreign currency translation adjustments relating to investments in international subsidiaries that are expected to be held indefinitely.
(b)For additional information on the adoption of a new accounting standard related to reclassification of certain tax effects from AOCI, see Note 1B in our 2018 Financial Report.
(c)For additional information on the adoption of a new accounting standard related to financial assets and liabilities, see Note 1B in our 2018 Financial Report.
Note 6. Accumulated Other Comprehensive Loss, Excluding Noncontrolling Interests
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The following summarizes the changes, net of tax, in Accumulated other comprehensive loss: |
| | Net Unrealized Gains/(Losses) | | Benefit Plans | | |
| (MILLIONS OF DOLLARS) | | Foreign Currency Translation Adjustments | | Derivative Financial Instruments | | Available-For-Sale Securities | | Actuarial Gains/(Losses) | | Prior Service (Costs)/ Credits and Other | | Accumulated Other Comprehensive Income/(Loss) |
| Balance, January 1, 2018 | | $ | (5,180) | | | $ | (30) | | | $ | 401 | | | $ | (5,262) | | | $ | 750 | | | $ | (9,321) | |
Other comprehensive income/(loss) due to the adoption of new accounting standards(a) | | (2) | | | (1) | | | (416) | | | (637) | | | 144 | | | (913) | |
Other comprehensive income/(loss)(b) | | (893) | | | 198 | | | (53) | | | (128) | | | (166) | | | (1,041) | |
| Balance, December 31, 2018 | | (6,075) | | | 167 | | | (68) | | | (6,027) | | | 728 | | | (11,275) | |
Other comprehensive income/(loss)(b) | | 123 | | | (146) | | | 33 | | | (231) | | | (144) | | | (365) | |
| Balance, December 31, 2019 | | (5,952) | | | 20 | | | (35) | | | (6,257) | | | 584 | | | (11,640) | |
Other comprehensive income/(loss)(b) | | 1,028 | | | (448) | | | 151 | | | (602) | | | (106) | | | 23 | |
Distribution of Upjohn Business(c) | | (397) | | | 0 | | | 0 | | | 352 | | | (26) | | | (71) | |
| Balance, December 31, 2020 | | $ | (5,321) | | | $ | (428) | | | $ | 116 | | | $ | (6,507) | | | $ | 452 | | | $ | (11,688) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The following summarizes the changes, net of tax, in Accumulated other comprehensive loss: |
| | Net Unrealized Gains/(Losses) | | Benefit Plans | | |
| (MILLIONS) | | Foreign Currency Translation Adjustments(a) | | Derivative Financial Instruments | | Available-For-Sale Securities | | Prior Service (Costs)/Credits and Other | | Accumulated Other Comprehensive Income/(Loss) |
| Balance, January 1, 2021 | | $ | (5,450) | | | $ | (428) | | | $ | 116 | | | $ | 452 | | | $ | (5,310) | |
Other comprehensive income/(loss)(b) | | (722) | | | 547 | | | (336) | | | (75) | | | (587) | |
| | | | | | | | | | |
| Balance, December 31, 2021 | | (6,172) | | | 119 | | | (220) | | | 377 | | | (5,897) | |
Other comprehensive income/(loss)(b) | | (2,188) | | | (531) | | | 440 | | | (129) | | | (2,407) | |
| Balance, December 31, 2022 | | (8,360) | | | (412) | | | 220 | | | 248 | | | (8,304) | |
Other comprehensive income/(loss)(b) | | 497 | | | 195 | | | (229) | | | (120) | | | 343 | |
| Balance, December 31, 2023 | | $ | (7,863) | | | $ | (217) | | | $ | (9) | | | $ | 128 | | | $ | (7,961) | |
(a)Represent the cumulative effect adjustments as of January 1, 2018 from the adoption of accounting standards related to (i) financial assets and liabilities and (ii) the reclassification of certain tax effects from AOCI. See Note 1B in our 2018 Financial Report.
(b)Amounts do not include foreign currency translation adjustments attributable to noncontrolling interests of $9 million loss in 2020, $11 million loss in 2019 and $20 million loss in 2018. interests.
(b)Foreign currency translation adjustments include net losses in 2020 primarily include gains from the strengthening of the euro, Japanese yen, Australian dollar2023, 2022 and U.K. pound against the U.S. dollar, and net gains2021 related to foreign currency translation adjustments related to our equity method investment in the Consumer Healthcare JV (see Note 2C), partially offset by the impact of our net investment hedging program. Foreign currency translation adjustments in 2019 primarily include a gain of approximately $1.3 billion pre-tax ($978 million after-tax) related to foreign currency translation adjustments attributable toprogram and our equity methodequity-method investment in Haleon/the Consumer Healthcare JV (see Note 2C), partially offset by the strengthening of the U.S. dollar against the euro and the Australian dollar, and the results of our net investment hedging program. Amounts in 2018 primarily reflect the strengthening of the U.S. dollar against the euro, U.K. pound and Chinese renminbi.. (c)For more information, see Note 2B.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 7778 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 7. Financial Instruments
A. Fair Value Measurements
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis and Fair Value Hierarchy, using a Market Approach:
| | As of December 31, 2020 | | | As of December 31, 2019 |
| (MILLIONS OF DOLLARS) | | Total | | Level 1 | | Level 2 | | | Total | | Level 1 | | Level 2 |
| | As of December 31, 2023 | |
| | As of December 31, 2023 | |
| | As of December 31, 2023 | | | | | As of December 31, 2022 |
| (MILLIONS) | | (MILLIONS) | | Total | | Level 1 | | Level 2 | | | | Total | | Level 1 | | Level 2 |
| Financial assets: | Financial assets: | | | | | | | | | | | | | |
| Short-term investments | Short-term investments | | | | | |
| Classified as equity securities with readily determinable fair values: | | | | | |
| Short-term investments | |
| Short-term investments | |
| Equity securities with readily determinable fair values: | |
| Equity securities with readily determinable fair values: | |
| Equity securities with readily determinable fair values: | |
| Money market funds | |
| Money market funds | |
| Money market funds | Money market funds | | $ | 567 | | | $ | 0 | | | $ | 567 | | | | $ | 705 | | | $ | 0 | | | $ | 705 | |
| | Classified as available-for-sale debt securities: | | | | | |
| Available-for-sale debt securities: | |
| Available-for-sale debt securities: | |
| Available-for-sale debt securities: | |
| Government and agency—non-U.S. | |
| Government and agency—non-U.S. | |
| Government and agency—non-U.S. | Government and agency—non-U.S. | | 7,719 | | | 0 | | | 7,719 | | | | 4,863 | | | 0 | | | 4,863 | |
| Government and agency—U.S. | Government and agency—U.S. | | 982 | | | 0 | | | 982 | | | | 811 | | | 0 | | | 811 | |
| Corporate and other | Corporate and other | | 1,008 | | | 0 | | | 1,008 | | | | 1,013 | | | 0 | | | 1,013 | |
| 9,709 | | | 0 | | | 9,709 | | | | 6,687 | | | 0 | | | 6,687 | |
| | 4,400 | |
| Total short-term investments | Total short-term investments | | 10,276 | | | 0 | | | 10,276 | | | | 7,392 | | | 0 | | | 7,392 | |
| Other current assets | Other current assets | | | | | | | | | | | | | |
| Derivative assets: | Derivative assets: | | | | | |
| Interest rate contracts | | 18 | | | 0 | | | 18 | | | | 53 | | | 0 | | | 53 | |
| Derivative assets: | |
| Derivative assets: | |
| | Foreign exchange contracts | |
| | Foreign exchange contracts | |
| | Foreign exchange contracts | Foreign exchange contracts | | 234 | | | 0 | | | 234 | | | | 413 | | | 0 | | | 413 | |
| Total other current assets | Total other current assets | | 251 | | | 0 | | | 251 | | | | 465 | | | 0 | | | 465 | |
| Long-term investments | Long-term investments | | | | | | | | | | | | | |
Classified as equity securities with readily determinable fair values(a) | | 2,809 | | | 2,776 | | | 32 | | | | 1,902 | | | 1,863 | | | 39 | |
Equity securities with readily determinable fair values(a) | |
Equity securities with readily determinable fair values(a) | |
Equity securities with readily determinable fair values(a) | |
| | Classified as available-for-sale debt securities: | | | | | |
| Available-for-sale debt securities: | |
| Available-for-sale debt securities: | |
| Available-for-sale debt securities: | |
| Government and agency—non-U.S. | Government and agency—non-U.S. | | 6 | | | 0 | | | 6 | | | | 0 | | | 0 | | | 0 | |
| Government and agency—U.S. | | 121 | | | 0 | | | 121 | | | | 303 | | | 0 | | | 303 | |
| Government and agency—non-U.S. | |
| Government and agency—non-U.S. | |
| | Corporate and other | Corporate and other | | 0 | | | 0 | | | 0 | | | | 11 | | | 0 | | | 11 | |
| 128 | | | 0 | | | 128 | | | | 315 | | | 0 | | | 315 | |
| Corporate and other | |
| Corporate and other | |
| | 150 | |
| Total long-term investments | Total long-term investments | | 2,936 | | | 2,776 | | | 160 | | | | 2,216 | | | 1,863 | | | 354 | |
| Other noncurrent assets | Other noncurrent assets | | | | | | | | | | | | | |
| Derivative assets: | Derivative assets: | | | | | |
| Derivative assets: | |
| Derivative assets: | |
| Interest rate contracts | |
| Interest rate contracts | |
| Interest rate contracts | Interest rate contracts | | 117 | | | 0 | | | 117 | | | | 266 | | | 0 | | | 266 | |
| Foreign exchange contracts | Foreign exchange contracts | | 5 | | | 0 | | | 5 | | | | 261 | | | 0 | | | 261 | |
| Total derivative assets | Total derivative assets | | 122 | | | 0 | | | 122 | | | | 526 | | | 0 | | | 526 | |
Insurance contracts(b) | Insurance contracts(b) | | 693 | | | 0 | | | 693 | | | | 575 | | | 0 | | | 575 | |
| Total other noncurrent assets | Total other noncurrent assets | | 814 | | | 0 | | | 814 | | | | 1,102 | | | 0 | | | 1,102 | |
| Total assets | Total assets | | $ | 14,278 | | | $ | 2,776 | | | $ | 11,501 | | | | $ | 11,176 | | | $ | 1,863 | | | $ | 9,313 | |
| | Financial liabilities: | Financial liabilities: | | | | | |
| Financial liabilities: | |
| Financial liabilities: | |
| Other current liabilities | |
| Other current liabilities | |
| Other current liabilities | Other current liabilities | | | | | |
| Derivative liabilities: | Derivative liabilities: | | | | | |
| | Derivative liabilities: | |
| Derivative liabilities: | |
| Interest rate contracts | |
| Interest rate contracts | |
| Interest rate contracts | |
| Foreign exchange contracts | Foreign exchange contracts | | $ | 501 | | | $ | 0 | | | $ | 501 | | | | $ | 114 | | | $ | 0 | | | $ | 114 | |
| Total other current liabilities | Total other current liabilities | | 501 | | | 0 | | | 501 | | | | 114 | | | 0 | | | 114 | |
| Other noncurrent liabilities | Other noncurrent liabilities | | | | | | | | | | | | | |
| Derivative liabilities: | Derivative liabilities: | | | | | |
| | Derivative liabilities: | |
| Derivative liabilities: | |
| Interest rate contracts | |
| Interest rate contracts | |
| Interest rate contracts | |
| Foreign exchange contracts | Foreign exchange contracts | | 599 | | | 0 | | | 599 | | | | 604 | | | 0 | | | 604 | |
| Total other noncurrent liabilities | Total other noncurrent liabilities | | 599 | | | 0 | | | 599 | | | | 604 | | | 0 | | | 604 | |
| Total liabilities | Total liabilities | | $ | 1,100 | | | $ | 0 | | | $ | 1,100 | | | | $ | 718 | | | $ | 0 | | | $ | 718 | |
(a)Long-term equity securities of $190$130 million as of December 31, 20202023 and $176$143 million as of December 31, 20192022 were held in restricted trusts for U.S. non-qualified employee benefit plans.
(b)Includes life insurance policies held in restricted trusts for U.S. non-qualified employee benefit plans. The underlying invested assets in these contracts are marketable securities, which are carried at fair value, with changes in fair value recognized in Other (income)/deductions—net (see Note 4).
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 78 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Financial Assets and Liabilities Not Measured at Fair Value on a Recurring Basis
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Carrying values and estimated fair values using a market approach: | | |
| | As of December 31, 2020 | | | | As of December 31, 2019 |
| | Carrying Value | | Estimated Fair Value | | | | Carrying Value | | Estimated Fair Value |
| (MILLIONS OF DOLLARS) | | | | Total | | Level 2 | | | | | | Total | | Level 2 | | |
| Financial Liabilities | | | | | | | | | | | | | | | | |
| Long-term debt, excluding the current portion | | $ | 37,133 | | | $ | 45,533 | | | $ | 45,533 | | | | | $ | 35,955 | | | $ | 40,842 | | | $ | 40,842 | | | |
The differences between the estimated fair values and carrying values forof held-to-maturity debt securities, private equity securities, long-term receivables and short-term borrowings not measured at fair value on a recurring basis were not significant as of December 31, 20202023 and 2019.2022. The fair value measurements of our held-to-maturity debt securities and short-term borrowings are based on Level 2 inputs. The fair value measurements of our long-term receivables and private equity securities are based on Level 3 inputs using a market approach.inputs.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 79 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
B. Investments
Total Short-Term, and Long-Term Investments and Equity-Method Investments
| | The following summarizes our investments by classification type: | The following summarizes our investments by classification type: | The following summarizes our investments by classification type: |
| As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 |
| | As of December 31, | | | | As of December 31, |
| (MILLIONS) | | (MILLIONS) | | 2023 | | 2022 |
| Short-term investments | Short-term investments | | | | |
Equity securities with readily determinable fair values(a) | |
Equity securities with readily determinable fair values(a) | |
Equity securities with readily determinable fair values(a) | Equity securities with readily determinable fair values(a) | | $ | 567 | | | $ | 705 | |
| Available-for-sale debt securities | Available-for-sale debt securities | | 9,709 | | | 6,687 | |
| Held-to-maturity debt securities | Held-to-maturity debt securities | | 161 | | | 1,133 | |
| Total Short-term investments | Total Short-term investments | | $ | 10,437 | | | $ | 8,525 | |
| | Long-term investments | Long-term investments | | | |
| Equity securities with readily determinable fair values | | $ | 2,809 | | | $ | 1,902 | |
| Long-term investments | |
| Long-term investments | |
Equity securities with readily determinable fair values(b) | |
Equity securities with readily determinable fair values(b) | |
Equity securities with readily determinable fair values(b) | |
| Available-for-sale debt securities | Available-for-sale debt securities | | 128 | | | 315 | |
| Held-to-maturity debt securities | Held-to-maturity debt securities | | 37 | | | 42 | |
Private equity securities at cost(b) | Private equity securities at cost(b) | | 432 | | | 756 | |
| Total Long-term investments | Total Long-term investments | | $ | 3,406 | | | $ | 3,014 | |
| Equity-method investments | Equity-method investments | | 16,856 | | | 17,133 | |
| Total long-term investments and equity-method investments | Total long-term investments and equity-method investments | | $ | 20,262 | | | $ | 20,147 | |
| Held-to-maturity cash equivalents | Held-to-maturity cash equivalents | | $ | 89 | | | $ | 163 | |
(a)As of December 31, 2020 and 2019, includesRepresent money market funds primarily invested in U.S. Treasury and government debt.
(b)Represent investments in the life sciences sector.
Debt Securities
| At December 31, 2020, our investment securities portfolio consisted of diverse, primarily investment-grade, debt securities. The contractual maturities, or estimated maturities, of the debt securities are as follows: | | As of December 31, 2020 | As of December 31, 2019 |
| | Gross Unrealized | | Maturities (in Years) | | Gross Unrealized | |
| (MILLIONS OF DOLLARS) | | Amortized Cost | | Gains | | Losses | | Fair Value | | Within 1 | | Over 1
to 5 | | Over 5 | | Amortized Cost | | Gains | | Losses | | Fair Value |
| Our investment portfolio consists of investment-grade debt securities issued across diverse governments, corporate and financial institutions: | | Our investment portfolio consists of investment-grade debt securities issued across diverse governments, corporate and financial institutions: |
| | As of December 31, 2023 | | | | As of December 31, 2023 | As of December 31, 2022 |
| | | | Gross Unrealized | |
| (MILLIONS) | |
| (MILLIONS) | |
| (MILLIONS) | | | Amortized Cost | | Gains | | Losses | | Fair Value | | Within 1 | | Over 1
to 5 | | Over 5 | | Amortized Cost | | Gains | | Losses | | Fair Value |
| Available-for-sale debt securities | Available-for-sale debt securities | | | | | | | | | | | | | | | | |
Government and agency––non-U.S. | Government and agency––non-U.S. | | $ | 7,593 | | | $ | 136 | | | $ | (4) | | | $ | 7,725 | | | $ | 7,719 | | | $ | 6 | | | $ | 0 | | | $ | 4,895 | | | $ | 6 | | | $ | (38) | | | $ | 4,863 | |
Government and agency––non-U.S. | |
Government and agency––non-U.S. | |
Government and agency––U.S. | Government and agency––U.S. | | 1,104 | | | 0 | | | (1) | | | 1,103 | | | 982 | | | 121 | | | 0 | | | 1,120 | | | 0 | | | (6) | | | 1,114 | |
Corporate and other(a) | | 1,006 | | | 2 | | | 0 | | | 1,008 | | | 1,008 | | | 0 | | | 0 | | | 1,027 | | | 0 | | | (2) | | | 1,025 | |
| Corporate and other | |
| Held-to-maturity debt securities | Held-to-maturity debt securities | | | |
| Time deposits and other | |
| Time deposits and other | |
| Time deposits and other | Time deposits and other | | 283 | | | 0 | | | 0 | | | 283 | | | 251 | | | 9 | | | 24 | | | 535 | | | 0 | | | 0 | | | 535 | |
Government and agency––non-U.S. | Government and agency––non-U.S. | | 5 | | | 0 | | | 0 | | | 5 | | | 0 | | | 0 | | | 5 | | | 803 | | | 0 | | | 0 | | | 803 | |
| Total debt securities | Total debt securities | | $ | 9,991 | | | $ | 138 | | | $ | (5) | | | $ | 10,124 | | | $ | 9,959 | | | $ | 136 | | | $ | 29 | | | $ | 8,380 | | | $ | 6 | | | $ | (47) | | | $ | 8,340 | |
(a)Primarily issued by a diverse group of corporations.
For our portfolio of available-for-sale and held-to-maturity debt securities, anyAny expected credit losses to these portfolios would be immaterial to theour financial statements.
Equity Securities
| | | | | | | | | | | | | | | | | | | | |
| The following presents the calculation of the portion of unrealized (gains)/losses that relates to equity securities, excluding equity-method investments, held at the reporting date: |
| | Year Ended December 31, |
| (MILLIONS) | | 2023 | | 2022 | | 2021 |
Net (gains)/losses recognized during the period on equity securities(a) | | $ | (1,590) | | | $ | 1,273 | | | $ | (1,344) | |
| Less: Net (gains)/losses recognized during the period on equity securities sold during the period | | (1,754) | | | (126) | | | (80) | |
Net unrealized (gains)/losses during the reporting period on equity securities still held at the reporting date(b) | | $ | 165 | | | $ | 1,400 | | | $ | (1,264) | |
(a)Reported in Other (income)/deductions––net. See Note 4. (b)Included in net unrealized (gains)/losses are observable price changes on equity securities without readily determinable fair values. As of December 31, 2023, there were cumulative impairments and downward adjustments of $259 million and upward adjustments of $213 million. Impairments, downward and upward adjustments were not material to our operations in 2023, 2022 and 2021.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 80 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
C. Short-Term Borrowings
| | | | | | | | | | | | | | |
| Short-term borrowings include: |
| | As of December 31, |
| (MILLIONS) | | 2023 | | 2022 |
Commercial paper, principal amount(a) | | $ | 7,965 | | | $ | — | |
| Current portion of long-term debt, principal amount | | 2,250 | | | 2,550 | |
Other short-term borrowings, principal amount(b) | | 252 | | | 385 | |
| Total short-term borrowings, principal amount | | 10,467 | | | 2,935 | |
| Net fair value adjustments related to hedging and purchase accounting | | 5 | | | 11 | |
| Net unamortized discounts, premiums and debt issuance costs | | (121) | | | (1) | |
Total Short-term borrowings, including current portion of long-term debt, carried at historical proceeds, as adjusted | | $ | 10,350 | | | $ | 2,945 | |
(a)Issued in the fourth quarter of 2023 as part of the financing for our acquisition of Seagen (see Note 2A). The weighted-average effective interest rate on commercial paper outstanding was approximately 5.37% as of December 31, 2023. (b)Primarily includes cash collateral. See Note 7F. As of December 31, 2023, we had access to a total of $15 billion in committed U.S. revolving credit facilities, consisting of an $8 billion facility maturing in October 2024 and a $7 billion facility maturing in October 2028, which may be used for general corporate purposes including to support our global commercial paper borrowings. In addition to the U.S. revolving credit facilities, our lenders have provided us an additional $305 million in lines of credit, of which $274 million expire within one year. Essentially all lines of credit were unused as of December 31, 2023.
D. Long-Term Debt
| | | | | | | | | | | | | | |
| The following outlines our senior unsecured long-term debt* and the weighted-average stated interest rate by maturity: |
| | As of December 31, |
| (MILLIONS) | | 2023 | | 2022 |
Notes due 2024 (3.9% for 2022)(a) | | $ | — | | | $ | 2,250 | |
| Notes due 2025 (3.9% for 2023 and 0.8% for 2022) | | 3,750 | | | 750 | |
| Notes due 2026 (3.7% for 2023 and 2.9% for 2022) | | 6,000 | | | 3,000 | |
| Notes due 2027 (2.1% for 2023 and 2022) | | 1,029 | | | 1,000 | |
| Notes due 2028 (4.6% for 2023 and 4.8% for 2022) | | 5,660 | | | 1,660 | |
| Notes due 2029 (3.5% for 2023 and 2022) | | 1,750 | | | 1,750 | |
| Notes due 2030-2034 (4.1% for 2023 and 2.9% for 2022) | | 12,000 | | | 4,000 | |
| Notes due 2035-2039 (5.8% for 2023 and 2022) | | 8,048 | | | 8,017 | |
| Notes due 2040-2044 (4.1% for 2023 and 3.6% for 2022) | | 7,995 | | | 4,903 | |
| Notes due 2045-2049 (4.1% for 2023 and 2022) | | 3,500 | | | 3,500 | |
| Notes due 2050-2063 (5.0% for 2023 and 2.7% for 2022) | | 11,250 | | | 1,250 | |
| Total long-term debt, principal amount | | 60,982 | | | 32,080 | |
| Net fair value adjustments related to hedging and purchase accounting | | 1,039 | | | 959 | |
| Net unamortized discounts, premiums and debt issuance costs | | (483) | | | (175) | |
| Other long-term debt | | — | | | 20 | |
| Total long-term debt, carried at historical proceeds, as adjusted | | $ | 61,538 | | | $ | 32,884 | |
| Current portion of long-term debt, carried at historical proceeds, as adjusted (not included above (3.9% for 2023 and 3.7% for 2022)) | | $ | 2,254 | | | $ | 2,560 | |
*Our long-term debt is generally redeemable by us at any time at varying redemption prices plus accrued and unpaid interest.
(a)Reclassified to the current portion of long-term debt.
Issuances
| | | | | | | | | | | | | | |
In May 2023, we issued, through our wholly-owned finance subsidiary, PIE, the following senior unsecured notes as part of the financing for our acquisition of Seagen(a), (b): |
| (MILLIONS) | | | | Principal |
| Interest Rate | | Maturity Date | | December 31, 2023 |
| 4.65% | | May 19, 2025 | | $ | 3,000 | |
| 4.45% | | May 19, 2026 | | 3,000 | |
| 4.45% | | May 19, 2028 | | 4,000 | |
| 4.65% | | May 19, 2030 | | 3,000 | |
| 4.75% | | May 19, 2033 | | 5,000 | |
| 5.11% | | May 19, 2043 | | 3,000 | |
| 5.30% | | May 19, 2053 | | 6,000 | |
| 5.34% | | May 19, 2063 | | 4,000 | |
Total long-term debt issued in 2023(c) | | | | $ | 31,000 | |
| | | | | | | | |
| Pfizer Inc. | 792023 Form 10-K | 81 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Equity Securities
| | | | | | | | | | | | | | | | | | | | |
| The following presents the calculation of the portion of unrealized (gains)/losses that relate to equity securities, excluding equity method investments, held at the reporting date: |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 |
Net (gains)/losses recognized during the period on equity securities(a) | | $ | (540) | | | $ | (454) | | | $ | (586) | |
| Less: Net (gains)/losses recognized during the period on equity securities sold during the period | | (24) | | | (25) | | | (109) | |
Net unrealized (gains)/losses during the reporting period on equity securities still held at the reporting date(b) | | $ | (515) | | | $ | (429) | | | $ | (477) | |
(a)Reported in Other (income)/deductions––net. See Note 4.
(b)Included in net unrealized gains are observable price changes on equity securities without readily determinable fair values. Since January 1, 2018, there were cumulative impairments and downward adjustments of $81 million and upward adjustments of $61 million. Impairments, downward and upward adjustments were not significant in 2020, 2019 and 2018.
C. Short-Term Borrowings
| | | | | | | | | | | | | | |
| Short-term borrowings include: |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 |
Commercial paper(a) | | $ | 556 | | | $ | 13,915 | |
Current portion of long-term debt, principal amount(b) | | 2,004 | | | 1,458 | |
Other short-term borrowings, principal amount(c) | | 145 | | | 860 | |
| Total short-term borrowings, principal amount | | 2,705 | | | 16,233 | |
| Net fair value adjustments related to hedging and purchase accounting | | 0 | | | 5 | |
| Net unamortized discounts, premiums and debt issuance costs | | (2) | | | (43) | |
Total Short-term borrowings, including current portion of long-term debt, carried at historical proceeds, as adjusted | | $ | 2,703 | | | $ | 16,195 | |
(a)See Note 2B.
(b)See Note 7D.
(c)Primarily includes cash collateral. See Note 7F.
The weighted-average effective interest rate on commercial paper outstanding was approximately 0.13% as of December 31, 2020 and 1.92% as of December 31, 2019.
As of December 31, 2020, we had access to a total of $11 billion in U.S. revolving credit facilities consisting of a $7 billion facility expiring in 2025 and a $4 billion facility expiring in September 2021, which may be used to support our commercial paper borrowings. In January 2021, the $4 billion facility was terminated at our request. In addition to the U.S. revolving credit facilities, our lenders have provided us an additional $332 million in lines of credit, of which $300 million expire within one year. Of these total lines of credit, $11.3 billion were unused as of December 31, 2020.
D. Long-Term Debt
| | | | | | | | | | | | | | |
| The following outlines our senior unsecured long-term debt and the weighted-average stated interest rate by maturity: |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 |
Notes due 2021 (2.4% for 2019)(a) | | $ | 0 | | | $ | 3,153 | |
| Notes due 2022 (1.0% for 2020 and 2019) | | 1,728 | | | 1,624 | |
| Notes due 2023 (3.2% for 2020 and 3.7% for 2019) | | 2,550 | | | 2,892 | |
| Notes due 2024 (3.9% for 2020 and 2019) | | 2,250 | | | 2,250 | |
| Notes due 2025 (0.8% for 2020) | | 750 | | | 0 | |
| Notes due 2026 (2.9% for 2020 and 2019) | | 3,000 | | | 3,000 | |
| Notes due 2027-2030 (3.1% for 2020 and 3.6% for 2019) | | 6,781 | | | 4,453 | |
| Notes due 2034-2036 (5.3% for 2020 and 2019) | | 2,250 | | | 2,250 | |
| Notes due 2037-2040 (5.6% for 2020 and 6.0% for 2019) | | 8,086 | | | 7,066 | |
| Notes due 2043-2046 (3.7% for 2020 and 2019) | | 4,878 | | | 4,818 | |
| Notes due 2047-2050 (3.6% for 2020 and 4.1% for 2019) | | 3,500 | | | 3,315 | |
| Total long-term debt, principal amount | | 35,774 | | | 34,820 | |
| Net fair value adjustments related to hedging and purchase accounting | | 1,562 | | | 1,305 | |
| Net unamortized discounts, premiums and debt issuance costs | | (207) | | | (176) | |
| Other long-term debt | | 4 | | | 5 | |
| Total long-term debt, carried at historical proceeds, as adjusted | | $ | 37,133 | | | $ | 35,955 | |
| Current portion of long-term debt, carried at historical proceeds, as adjusted (not included above (2.6% and 1.2%)) | | $ | 2,002 | | | $ | 1,462 | |
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 80 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
(a) ReclassifiedThe notes are fully and unconditionally guaranteed on a senior unsecured basis by Pfizer Inc. PIE was formed to finance a portion of the consideration for the acquisition of Seagen and has no assets or operations, and will have no assets or operations, other than as related to the current portionissuance, administration and repayment of long-term debt.
Our long-termthe notes and any other debt outlinedsecurities that it may issue in the above table is generally redeemable by us at any time at varying redemption prices plus accrued and unpaid interest.
Issuances
| | | | | | | | | | | | | | |
In 2020, we issued the following: |
(MILLIONS OF DOLLARS) | | | | Principal |
Interest Rate | | Maturity Date | | As of
December 31, 2020 |
0.800%(a)
| | May 28, 2025 | | $ | 750 | |
1.700%(a)
| | May 28, 2030 | | 1,000 | |
2.550%(a)
| | May 28, 2040 | | 1,000 | |
2.700%(a)
| | May 28, 2050 | | 1,250 | |
| | | | $ | 4,000 | |
| | | | |
2.625%(b)
| | April 1, 2030 | | $ | 1,250 | |
(a)May be redeemed by us at any time, in whole, or in part, at varying redemption prices plus accrued and unpaid interest. The weighted-average effective interest rate for the notes at issuance was 2.11%.future.
(b)MayThe notes may be redeemed by us at any time, in whole, or in part, at a make-whole redemption price plus accrued and unpaid interest.
(c)The weighted average effective interest rate for the notes at issuance was 2.67%4.93%.
In March 2019,August 2021, we completed a public offering of $5.0$1.0 billion aggregate principal amount of senior unsecured notes with a weighted-averagedue 2031 at an effective interest rate of 3.57%1.79%.
In September 2018, we completed a public offering of $5.0 billion aggregate principal amount of senior unsecured notes with a weighted-average effective interest rate of 3.56%.
Retirements
In November 2020, we repurchased all $1.15 billion and $342 million principal amount outstanding of the 1.95% senior unsecured notes due June 2021 and 5.80% senior unsecured notes due August 2023 and recorded a total net loss of $36 million, in Other (income)/deductions––net. See Note 2B.
In March 2020, we repurchased at par all $1.065 billion principal amount outstanding of our senior unsecured notes due in 2047.
In January 2019, we repurchased all €1.1 billion ($1.3 billion) principal amount outstanding of the 5.75% euro-denominated debt due June 2021 at a redemption value of €1.3 billion ($1.5 billion). We recorded a net loss of $138 million in Other (income)/deductions––net, which included the related termination of cross currency swaps.
E. Derivative Financial Instruments and Hedging Activities
Foreign Exchange Risk
Risk––
A significant portion of our revenues, earnings and net investments in foreign affiliates is exposed to changes in foreign exchange rates. WeWhere foreign exchange risk is not offset by other exposures, we manage our foreign exchange risk predominatelyprincipally through the use of derivative financial instruments and foreign currency debt. These financial instruments serve to mitigate the impact on net income as a result of remeasurement into another currency, or against the impact of translation into U.S. dollars of certain foreign exchange-denominated transactions.
The derivative financial instruments primarily hedge or offset exposures in the euro, U.K. pound, Japanese yen, Swedish kronaCanadian dollar, and Canadian dollar. Additionally, we hedgeChinese renminbi, and include a portion of our forecasted foreign exchange-denominated intercompany inventory sales denominated in euro, Japanese yen, Chinese renminbi, Canadian dollar, U.K. pound and Australian dollar forhedged up to two years.
Changes in fair value are reported in earnings or in Other comprehensive income/(loss), depending on the nature and purpose of the financial instrument (hedge or offset relationship). For certain foreign exchange contracts, we exclude an amount from the assessment of hedge effectiveness and recognize the excluded amount through an amortization approach in earnings. The hedge relationships are as follows:
•Generally, we recognize the gains and losses on foreign exchange contracts that are designated as fair value hedges in earnings upon the recognition of the change in fair value of the hedged item. We also recognize the offsetting foreign exchange impact attributable to the hedged item in earnings.
•Generally, we record in Other comprehensive income/(loss) gains or losses on foreign exchange contracts that are designated as cash flow hedges and reclassify those amounts into earnings in the same period or periods during which the hedged transaction affects earnings.
•We record in Other comprehensive income/(loss)––Foreign currency translation adjustments, net the foreign exchange gains and losses related to foreign exchange-denominated debt and foreign exchange contracts designated as a hedge of our net investments in foreign subsidiaries and reclassify those amounts into earnings upon the sale or substantial liquidation of our net investments.
•For certain foreign exchange contracts not designated as hedging instruments, we recognize the gains and losses on contracts that are used to offset foreign currency assets or liabilities immediately into earnings along with the earnings impact of the items they generally offset. These contracts essentially take the opposite currency position of that reflected inon the month-end balance sheet to counterbalance the effect of any currency movement.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 81 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Interest Rate Risk
Risk––
Our interest-bearing investments and borrowings are subject to interest rate risk. Depending on market conditions, we may change the profile of our outstanding debt or investments by entering into derivative financial instruments like interest rate swaps, either to hedge or offset the exposure to changes in the fair value of hedged items with fixed interest rates, or to convert variable rate debt or investments to fixed rates. The derivative financial instruments primarily hedge U.S. dollar fixed-rate debt.
We recognize the gains and losseschange in fair value on interest rate contracts that are designated as fair value hedges in earnings, uponas well as the recognition of the change in fair valueoffsetting earnings impact of the hedged risk. We also recognize the offsetting earnings impactrisk attributable to the hedged item.
| The following summarizes the fair value of the derivative financial instruments and the related notional amounts (including those reported as part of discontinued operations): | | (MILLIONS OF DOLLARS) | | As of December 31, 2020 | | As of December 31, 2019 |
| | | Fair Value | | | Fair Value |
| Notional | | Asset | | Liability | | Notional | | Asset | | Liability |
| The following summarizes the fair value of the derivative financial instruments and notional amounts: | | The following summarizes the fair value of the derivative financial instruments and notional amounts: |
| (MILLIONS) | | (MILLIONS) | | As of December 31, 2023 | | As of December 31, 2022 |
| | | | Fair Value | | | | | | Fair Value | | | | Fair Value |
| | Notional | | | | Notional | | Asset | | Liability | | Notional | | Asset | | Liability |
| Derivatives designated as hedging instruments: | Derivatives designated as hedging instruments: | | | | | | | | | | | | |
Foreign exchange contracts(a) | Foreign exchange contracts(a) | | $ | 24,369 | | | $ | 145 | | | $ | 1,005 | | | $ | 25,193 | | | $ | 591 | | | $ | 662 | |
Foreign exchange contracts(a) | |
Foreign exchange contracts(a) | |
| Interest rate contracts | Interest rate contracts | | 1,950 | | | 135 | | | 0 | | | 6,645 | | | 318 | | | 0 | |
| | 280 | | | 1,005 | | | 909 | | | 662 | |
| | | | 546 | |
| | Derivatives not designated as hedging instruments: | Derivatives not designated as hedging instruments: | | | |
| Derivatives not designated as hedging instruments: | |
| Derivatives not designated as hedging instruments: | |
| Foreign exchange contracts | |
| Foreign exchange contracts | |
| Foreign exchange contracts | Foreign exchange contracts | | $ | 15,063 | | | 94 | | | 95 | | | $ | 19,623 | | | 82 | | | 55 | |
| | Total | Total | | | $ | 373 | | | $ | 1,100 | | | $ | 992 | | | $ | 718 | |
| Total | |
| Total | |
(a)The notional amount of outstanding foreign exchange contracts hedging our intercompany forecasted inventory sales was $5.0$4.9 billion as of December 31, 20202023 and $5.9$4.4 billion as of December 31, 2019.2022.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following summarizes information about the gains/(losses) incurred to hedge or offset operational foreign exchange or interest rate risk (including gains/(losses) reported as part of discontinued operations). |
| | | Amount of
Gains/(Losses)
Recognized in OID(a) | | Amount of Gains/(Losses)
Recognized in OCI(a) | | Amount of Gains/(Losses)
Reclassified from
OCI into OID and COS(a) |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2020 | | 2019 | | 2020 | | 2019 |
| Derivative Financial Instruments in Cash Flow Hedge Relationships: | | | | | | | | | | | | |
Foreign exchange contracts(b) | | $ | — | | | $ | — | | | $ | (649) | | | $ | 339 | | | $ | (77) | | | $ | 525 | |
Amount excluded from effectiveness testing recognized in earnings based on an amortization approach(c) | | — | | | — | | | 55 | | | 136 | | | 57 | | | 140 | |
| | | | | | | | | | | | |
| Derivative Financial Instruments in Fair Value Hedge Relationships: | | | | | | | | | | | | |
| Interest rate contracts | | 369 | | | 900 | | | — | | | — | | | — | | | — | |
| Hedged item | | (369) | | | (900) | | | — | | | — | | | — | | | — | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| Derivative Financial Instruments in Net Investment Hedge Relationships: | | | | | | | | | | | | |
| Foreign exchange contracts | | — | | | — | | | (501) | | | (313) | | | 0 | | | 0 | |
The portion on foreign exchange contracts excluded from the assessment of hedge effectiveness(c) | | — | | | — | | | 181 | | | 188 | | | 154 | | | 144 | |
| | | | | | | | | | | | |
| Non-Derivative Financial Instruments in Net Investment Hedge Relationships: | | | | | | | | | | | | |
| Foreign currency short-term borrowings | | — | | | — | | | 8 | | | 34 | | | 0 | | | 0 | |
Foreign currency long-term debt(d) | | — | | | — | | | (183) | | | 36 | | | 0 | | | 0 | |
| | | | | | | | | | | | |
| Derivative Financial Instruments Not Designated as Hedges: | | | | | | | | | | | | |
| Foreign exchange contracts | | 178 | | | (172) | | | — | | | — | | | — | | | — | |
All other net(c) | | — | | | — | | | 12 | | | 0 | | | (1) | | | (1) | |
| | $ | 178 | | | $ | (172) | | | $ | (1,077) | | | $ | 421 | | | $ | 133 | | | $ | 808 | |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 82 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following summarizes information about the gains/(losses) incurred to hedge or offset operational foreign exchange or interest rate risk exposures: |
| | |
Gains/(Losses)
Recognized in OID(a)
| | Gains/(Losses)
Recognized in OCI(a) | | Gains/(Losses)
Reclassified from
OCI into OID and COS(a) |
| | Year Ended December 31, |
| (MILLIONS) | | 2023 | | 2022 | | 2023 | | 2022 | | 2023 | | 2022 |
| Derivative Financial Instruments in Cash Flow Hedge Relationships: | | | | | | | | | | | | |
| Interest rate contracts | | $ | — | | | $ | — | | | $ | 68 | | | $ | — | | | $ | 1 | | | $ | — | |
Foreign exchange contracts(b) | | — | | | — | | | 380 | | | 1,296 | | | 236 | | | 1,916 | |
Amount excluded from effectiveness testing and amortized into earnings(c) | | — | | | — | | | 178 | | | 148 | | | 177 | | | 145 | |
| | | | | | | | | | | | |
| Derivative Financial Instruments in Fair Value Hedge Relationships: | | | | | | | | | | | | |
| Interest rate contracts | | 196 | | | (337) | | | — | | | — | | | — | | | — | |
| Hedged item | | (196) | | | 337 | | | — | | | — | | | — | | | — | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| Derivative Financial Instruments in Net Investment Hedge Relationships: | | | | | | | | | | | | |
| Foreign exchange contracts | | — | | | — | | | (393) | | | 816 | | | — | | | — | |
Amount excluded from effectiveness testing and amortized into earnings(c) | | — | | | — | | | 137 | | | 73 | | | 136 | | | 129 | |
| | | | | | | | | | | | |
Non-Derivative Financial Instruments in Net Investment Hedge Relationships(d): | | | | | | | | | | | | |
| Foreign currency short-term borrowings | | — | | | — | | | — | | | 26 | | | — | | | — | |
| Foreign currency long-term debt | | — | | | — | | | (29) | | | 51 | | | — | | | — | |
| | | | | | | | | | | | |
| Derivative Financial Instruments Not Designated as Hedges: | | | | | | | | | | | | |
| Foreign exchange contracts | | 164 | | | (1,153) | | | — | | | — | | | — | | | — | |
| | | | | | | | | | | | |
| | $ | 164 | | | $ | (1,153) | | | $ | 341 | | | $ | 2,409 | | | $ | 549 | | | $ | 2,190 | |
(a)OID = Other (income)/deductions—net, included in Other (income)/deductions—net in the consolidated statements of income. COS = Cost of Sales, included in Cost of sales in the consolidated statements of income. OCI = Other comprehensive income/(loss), included in the consolidated statements of comprehensive income.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 82 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
(b)The amounts reclassified from OCI into COS were:
•were a net gain of $172$253 million in 2020 (including a gain of $22 million reported in Income from discontinued operations––net of tax);2023 and
•a net gain of $247$375 million in 2019 (including a gain of $46 million reported in Income from discontinued operations––net of tax).
2022. The remaining amounts were reclassified from OCI into OID. Based on year-end foreign exchange rates that are subject to change, we expect to reclassify a pre-tax lossgain of $341$11 million within the next 12 months into income. The maximum length of time over which we are hedging our exposure to the variability in future foreign exchange cash flowflows is approximately 19 years and relates to our $1.8 billion U.K. pound debt maturing in 2043.foreign currency debt.
(c)The amounts reclassified from OCI were reclassified into OID.
(d)Long-term debt includes foreign currency borrowings withwhich are used as net investment hedges; the related carrying values of $2.1 billion as of December 31, 2020, which are used as hedging instruments in net investment hedge relationships.2023 and December 31, 2022 were $824 million and $795 million, respectively.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following summarizes the amounts recorded in our consolidated balance sheet related to cumulative basis adjustments for fair value hedges: |
| | As of December 31, 2020 | | As of December 31, 2019 |
| | | | Cumulative Amount of Fair
Value Hedging Adjustment
Increase/(Decrease) to
Carrying Amount | | | | Cumulative Amount of Fair Value Hedging Adjustment Increase/(Decrease) to
Carrying Amount |
| (MILLIONS OF DOLLARS) | | Carrying Amount of Hedged Assets/Liabilities(a) | | Active
Hedging
Relationships | | Discontinued Hedging Relationships | | Carrying Amount of Hedged Assets/Liabilities(a) | | Active Hedging Relationships | | Discontinued Hedging Relationships |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| Long-term debt | | $ | 2,016 | | | $ | 117 | | | $ | 1,149 | | | $ | 7,092 | | | $ | 266 | | | $ | 690 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following summarizes cumulative basis adjustments to our long-term debt in fair value hedges: |
| | As of December 31, 2023 | | As of December 31, 2022 |
| | | | Cumulative Amount of Fair
Value Hedging Adjustment
Increase/(Decrease) to
Carrying Amount | | | | Cumulative Amount of Fair
Value Hedging Adjustment Increase/(Decrease) to
Carrying Amount |
| (MILLIONS) | | Carrying Amount of Hedged Assets/Liabilities(a) | | Active
Hedging
Relationships | | Discontinued Hedging Relationships | | Carrying Amount of Hedged Assets/Liabilities(a) | | Active Hedging Relationships | | Discontinued Hedging Relationships |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| Short-term borrowings, including current portion of long-term debt | | $ | — | | | $ | — | | | $ | 4 | | | $ | — | | | $ | — | | | $ | 10 | |
| Long-term debt | | $ | 7,196 | | | $ | (131) | | | $ | 957 | | | $ | 2,235 | | | $ | (321) | | | $ | 1,042 | |
(a)Carrying amounts exclude the cumulative amount of fair value hedging adjustments.
F.F. Credit Risk
On an ongoing basis, we monitor and review the credit risk of our customers, financial institutions and exposures in our investment portfolio.
With respect to our trade accounts receivable, we monitor the creditworthiness of our customers to which we grant credit in the normal course of business. In general, there is no requirement for collateral from customers. For additional information on our trade accounts receivable and allowance for credit losses, see Note 1G1G. A significant portion of our trade accounts receivable balances are due from drug wholesalers.wholesalers and governments. For additional information on our trade accounts receivables with significant customers, see Note 17B17C.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 83 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
With respect to our investments, we monitor concentrations of credit risk associated with government, government agency, and corporate issuers of securities. Investments are placed in instruments that are investment grade and are primarily short in duration. Exposure limits are established to limit a concentration with any single credit counterparty. As of December 31, 2020,2023, the largest investment exposures in our portfolio representconsisted primarily of U.S. government money market funds, as well as sovereign debt instruments issued by the U.S., France, Canada, Japan, Sweden and Germany.
With respect to our derivative financial instrument agreements with financial institutions, we do not expect to incur a significant loss from failure of any counterparty. Derivative financial instruments are executed under International Swaps and Derivatives Association (ISDA) master agreements with credit-support annexes that contain zero threshold provisions requiring collateral to be exchanged daily depending on levels of exposure. As a result, there are no significant concentrations of credit risk with any individual financial institution. As of December 31, 2020,2023, the aggregate fair value of these derivative financial instruments that are in a net payable position was $946$768 million, for which we have posted collateral of $821$771 million with a corresponding amount reported in Short-term investments. As of December 31, 2020,2023, the aggregate fair value of our derivative financial instruments that are in a net receivable position was $137$225 million, for which we have received collateral of $142$221 million with a corresponding amount reported in Short-term borrowings, including current portion of long-term debt.
Note 8. Other Financial Information
A. Inventories
| The following summarizes the components of Inventories: | The following summarizes the components of Inventories: | The following summarizes the components of Inventories: |
| As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 |
| | As of December 31, | | | | As of December 31, |
| (MILLIONS) | | (MILLIONS) | | 2023 | | 2022 |
| Finished goods | Finished goods | | $ | 2,878 | | | $ | 2,265 | |
| Work in process | | 4,430 | | | 4,131 | |
| Work-in-process | |
| Raw materials and supplies | Raw materials and supplies | | 738 | | | 672 | |
Inventories(a) | Inventories(a) | | $ | 8,046 | | | $ | 7,068 | |
Noncurrent inventories not included above(b) | Noncurrent inventories not included above(b) | | $ | 890 | | | $ | 638 | |
(a)The changeincrease from December 31, 20192022 of $1.2 billion reflects an increase of approximately $1.0 billion representing acquired Seagen inventory, inclusive of the fair value step-up (see Note 2A), and increases for certain products including inventory build fordue to new product launches, supply recovery and changes in net market demanddemand. These increases were offset to a large extent by $1.0 billion in inventory write-offs for Paxlovid and network strategy, and an increase due to foreign exchange.Comirnaty. (b)Included in Other noncurrent assets. ThereThe decrease from December 31, 2022 of $1.3 billion is primarily driven by inventory write-offs for Paxlovid of $4.2 billion and, to a lesser extent, inventory write-offs for Comirnaty of $0.7 billion, offset to a large extent by an increase of approximately $3.1 billion representing acquired Seagen inventory, inclusive of the fair value step-up (see Note 2A). The charges and corresponding inventory write-offs were based on our analysis of Paxlovid and Comirnaty inventory levels as of December 31, 2023 in relation to our commercial outlook for both products. Based on current estimates and assumptions, there are no recoverability issues for these amounts. B. Other Current Liabilities
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 83 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Note 9. Property, Plant and Equipment
| | | | | | | | | | | | | | | | | | | | |
The following summarizes the components of Property, plant and equipment: |
| | | Useful Lives | | As of December 31, |
| (MILLIONS OF DOLLARS) | | (Years) | | 2020 | | 2019 |
| Land | | - | | $ | 444 | | | $ | 495 | |
| Buildings | | 33-50 | | 9,022 | | | 9,181 | |
| Machinery and equipment | | 8-20 | | 11,153 | | | 10,648 | |
| Furniture, fixtures and other | | 3-12.5 | | 4,541 | | | 4,840 | |
| Construction in progress | | - | | 3,552 | | | 2,794 | |
| | | | 28,711 | | | 27,959 | |
| Less: Accumulated depreciation | | | | 14,812 | | | 14,990 | |
| Property, plant and equipment | | | | $ | 13,900 | | | $ | 12,969 | |
| | | | | | | | | | | | | | |
| The following provides long-lived assets by geographic area: |
| | | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 |
| Property, plant and equipment | | | | |
| United States | | $ | 7,821 | | | $ | 7,194 | |
| Developed Europe | | 4,775 | | | 4,238 | |
| Developed Rest of World | | 413 | | | 453 | |
| Emerging Markets | | 890 | | | 1,083 | |
| Property, plant and equipment | | $ | 13,900 | | | $ | 12,969 | |
Note 10. Identifiable Intangible Assets and Goodwill
A. Identifiable Intangible Assets
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The following summarizes the components of Identifiable intangible assets: |
| | | As of December 31, 2020 | | As of December 31, 2019 |
| (MILLIONS OF DOLLARS) | | Gross
Carrying
Amount | | Accumulated
Amortization | | Identifiable
Intangible
Assets, less
Accumulated
Amortization | | Gross
Carrying
Amount | | Accumulated
Amortization | | Identifiable
Intangible
Assets, less
Accumulated
Amortization |
| Finite-lived intangible assets | | | | | | | | | | | | |
Developed technology rights(a) | | $ | 73,545 | | | $ | (50,902) | | | $ | 22,643 | | | $ | 72,449 | | | $ | (47,092) | | | $ | 25,357 | |
| Brands | | 922 | | | (774) | | | 148 | | | 922 | | | (741) | | | 181 | |
Licensing agreements and other(b) | | 2,292 | | | (1,186) | | | 1,106 | | | 1,687 | | | (1,108) | | | 579 | |
| | 76,759 | | | (52,862) | | | 23,896 | | | 75,058 | | | (48,941) | | | 26,117 | |
| Indefinite-lived intangible assets | | | | | | | | | | | | |
| Brands | | 827 | | | | | 827 | | | 827 | | | | | 827 | |
IPR&D(c) | | 3,175 | | | | | 3,175 | | | 5,919 | | | | | 5,919 | |
| | | | | | | | | | | | |
Licensing agreements and other(b) | | 573 | | | | | 573 | | | 1,073 | | | | | 1,073 | |
| | 4,575 | | | | | 4,575 | | | 7,819 | | | | | 7,819 | |
Identifiable intangible assets(d) | | $ | 81,334 | | | $ | (52,862) | | | $ | 28,471 | | | $ | 82,877 | | | $ | (48,941) | | | $ | 33,936 | |
(a)The increase in the gross carrying amount primarily reflects the transfer of $600 million from IPR&D to Developed technology rights to reflect the approval of Braftovi in combination with Erbitux® (cetuximab), for the treatment of BRAFV600E-mutant mCRC after prior therapy, as well as a $499 million capitalized portion of an upfront payment to Myovant (see Note 2E) and an increase from a $200 million measurement period adjustment related to the acquisition of Array (see Note2A),partially offset by a $528 million impairment of Eucrisa (see Note 4) and a $263 million impairment of certain generic sterile injectables acquired in connection with our acquisition of Hospira (see Note 4).
(b)The changes in the gross carrying amounts primarily reflect the transfer of $600 million from indefinite-lived Licensing agreements and other to finite-lived Licensing agreements and other to reflect the approval in the U.S. of several products subject to out-licensing arrangements acquired from Array, as well as measurement period adjustments related to the acquisition of Array.
(c)The decrease in the gross carrying amount primarily reflects a decrease from a $1.2 billion measurement period adjustment related to the acquisition of Array, a $900 million impairment of IPR&D (see Note 4), and the transfer of $600 million from IPR&D to Developed technology rights to reflect the approval of Braftovi in combination with Erbitux® (cetuximab), for the treatment of BRAFV600E-mutant mCRC after prior therapy.
(d)The decrease is primarily due to amortization, impairments, and measurement period adjustments related to the acquisition of Array, partially offset by the capitalization of an upfront payment to Myovant (see Note 2E).
Nearly all of our identifiable intangible assets are managed by our commercial organization, with only 9% of total cost of IPR&D managed by our R&D organization. | | | | | | | | | | | | | | | | | | | | |
The following summarizes the components of Property, plant and equipment: |
| | | Useful Lives | | As of December 31, |
| (MILLIONS) | | (Years) | | 2023 | | 2022 |
| Land | | - | | $ | 353 | | | $ | 368 | |
| Buildings | | 33-50 | | 9,046 | | | 8,832 | |
| Machinery and equipment | | 8-20 | | 14,263 | | | 12,881 | |
| Furniture, fixtures and other | | 3-12.5 | | 5,399 | | | 4,491 | |
| Construction in progress | | - | | 5,925 | | | 4,875 | |
| | | | 34,985 | | | 31,448 | |
| Less: Accumulated depreciation | | | | 16,045 | | | 15,174 | |
| Property, plant and equipment | | | | $ | 18,940 | | | $ | 16,274 | |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 84 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | |
| The following provides long-lived assets by geographic area: |
| | | As of December 31, |
| (MILLIONS) | | 2023 | | 2022 |
| United States | | $ | 10,674 | | | $ | 9,179 | |
| Developed Europe | | 6,221 | | | 5,389 | |
| Developed Rest of World | | 290 | | | 293 | |
| Emerging Markets | | 1,756 | | | 1,413 | |
| Property, plant and equipment | | $ | 18,940 | | | $ | 16,274 | |
Note 10. Identifiable Intangible Assets and Goodwill
A. Identifiable Intangible Assets
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following summarizes the components of Identifiable intangible assets: |
| | | As of December 31, 2023 | | As of December 31, 2022 |
| (MILLIONS) | | Gross
Carrying
Amount | | Accumulated
Amortization | | Identifiable
Intangible
Assets, less
Accumulated
Amortization | | Gross
Carrying
Amount | | Accumulated
Amortization | | Identifiable
Intangible
Assets, less
Accumulated
Amortization |
| Finite-lived intangible assets | | | | | | | | | | | | |
Developed technology rights(a) | | $ | 99,267 | | | $ | (60,493) | | | $ | 38,773 | | | $ | 85,604 | | | $ | (56,307) | | | $ | 29,297 | |
| Brands | | 922 | | | (877) | | | 45 | | | 922 | | | (844) | | | 78 | |
Licensing agreements and other(b) | | 2,756 | | | (1,458) | | | 1,297 | | | 2,237 | | | (1,397) | | | 841 | |
| | 102,944 | | | (62,828) | | | 40,116 | | | 88,763 | | | (58,548) | | | 30,215 | |
| Indefinite-lived intangible assets | | | | | | | | | | | | |
| Brands | | 827 | | | | | 827 | | | 827 | | | | | 827 | |
IPR&D(c) | | 23,193 | | | | | 23,193 | | | 11,357 | | | | | 11,357 | |
| | | | | | | | | | | | |
| Licensing agreements and other | | 763 | | | | | 763 | | | 971 | | | | | 971 | |
| | 24,784 | | | | | 24,784 | | | 13,155 | | | | | 13,155 | |
Identifiable intangible assets(d) | | $ | 127,728 | | | $ | (62,828) | | | $ | 64,900 | | | $ | 101,919 | | | $ | (58,548) | | | $ | 43,370 | |
(a)The increase in the gross carrying amount primarily includes, among other things: (i) $7.5 billion for the acquisition of Seagen (see Note 2A); (ii) the transfer of IPR&D to developed technology rights of $3.6 billion for etrasimod (Velsipity), $2.1 billion for Padcev, $1.1 billion for Braftovi/Mektovi, and $450 million as a result of the approval in the U.S. for Zavzpret nasal spray; and (iii) $495 million of capitalized milestones as a result of the approval in the U.S. for Zavzpret nasal spray, partially offset by (iv) impairments of $964 million for Prevnar 13 (see Note 4). (b)The increase in the gross carrying amount primarily reflects $450 million for the acquisition of Seagen (see Note 2A). (c)The increase in the gross carrying amount mainly reflects $20.8 billion for the acquisition of Seagen (see Note 2A), partially offset by the transfer from IPR&D to developed technology rights as mentioned in note (a) above, and impairments of $1.4 billion for etrasimod (Velsipity). (d)The increase is primarily due to $28.8 billion for the acquisition of Seagen (see Note 2A) and the $495 million of capitalized milestones described in note (a) above, partially offset by amortization expense of $4.7 billion and impairments of $3.0 billion (see Note 4). Developed Technology Rights
Rights––
Developed technology rights represent the cost for developed technology acquired from third parties and can include the right to develop, use, market, sell and/or offer for sale the product, compounds and intellectual property that we have acquired with respect to products, compounds and/or processes that have been completed. We possess a well-diversified portfolio of hundreds of developed technology rights across therapeutic categories, representing our commercialized products. The significant components of developed technology rights are the following: Nurtec ODT/Vydura, Adcetris, Xtandi, Prevnar 13/Prevenar 13 Infant,etrasimod (Velsipity), Padcev, Braftovi/Mektovi, Premarin, Prevnar 13/Prevenar 13 Adult, Eucrisa, Orgovyx,family and to a lesser extent Zavicefta, Tygacil, Merrem/Meronem, Refacto AF/Xyntha, Pristiq and Bosulif.Oxbryta. Also included in this category are the post-approval milestone payments made under our alliance agreements for certain biopharmaceuticalprescription pharmaceutical products.
Brands
Brands represent the cost for tradenames and know-how, as the products themselves do not receive patent protection. Indefinite-lived brands include Medrol and Depo-Medrol, while finite-lived brands include Depo-ProveraZavedos and Zavedos.
IPR&DDepo-Provera.
IPR&D assets represent the acquisition date fair value (less impairments) of R&D assets acquired through business combinations that have not yet received regulatory approval in a major market.market which could include both new investigational products and additional indications for in-line products. The significant components of IPR&D are the following: the program for the oral poly ADP ribose polymerase inhibitor for the treatment of patients with germline BRCA-mutated advanced breast cancer acquired as part of the Medivation acquisitionSGN-B6A, Disitamab vedotin, GBT601, Tukysa, Padcev and assets acquired in connection with the Array acquisition.talazoparib. IPR&D assets are required to be classified as indefinite-lived assets until the successful completion or the abandonment of the associated R&D effort. Accordingly, during the development period after the date of acquisition, these assets are not amortized until approval is obtained in a major market, typically either the U.S. or the EU, or in a series of other countries, subject to certain specified conditions and management judgment. At that time, we will determine the useful life of the asset, reclassify it out of IPR&D and begin amortization. If the associated R&D effort is abandoned, the related IPR&D assets will likely be written-off, and we will record an impairment charge.
IPR&D assets are high-risk assets, given the uncertain nature of R&D. Accordingly, we expect that many of these IPR&D assets willmay become impaired andand/or be written-off at some time in the future.
Licensing Agreements
Licensing agreements for developed technology and for technology in development primarily relate to out-licensing arrangements acquired from third parties, including the Array, acquisition.Arena and Seagen acquisitions. These assets represent the cost for the license, where we acquired the right to future royalties and/or milestones upon development or commercialization by the licensing partner.partners. A
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 85 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
significant component of the licensing arrangements are for out-licensing arrangements with a number of partners for oncology technology in varying stages of development that have not yet received regulatory approval in a major market.partners. Accordingly, during the development period after the date of acquisition, each of these assets is classified as indefinite-lived intangible assets and will not be amortized until approval is obtained in a major market. At that time we will determine the useful life of the asset, reclassify the respective licensing arrangement asset to finite-lived intangible asset and begin amortization. If the development effort is abandoned, the related licensing asset will likely be written-off, and we will record an impairment charge.
Amortization
The weighted-average life for each of our total finite-lived intangible assets is approximately 11 years, and for the largest component, developed technology rights, is approximately 911 years. Total amortization expense for finite-lived intangible assets was $3.5 billion in 2020, $4.5 billion in 2019 and $4.8 billion in 2018.
| | The following provides the expected annual amortization expense: | The following provides the expected annual amortization expense: | The following provides the expected annual amortization expense: |
| (MILLIONS OF DOLLARS) | | 2021 | | 2022 | | 2023 | | 2024 | | 2025 |
| (MILLIONS) | | (MILLIONS) | | 2024 | | 2025 | | 2026 | | 2027 | | 2028 |
| Amortization expense | Amortization expense | | $ | 3,372 | | | $ | 3,249 | | | $ | 2,921 | | | $ | 2,642 | | | $ | 2,492 | |
B. Goodwill
At the beginning of 2019, we reorganized our commercial operations and began to manage our businesses through 3 different operating segments––Biopharma, Upjohn and Consumer Healthcare. As a result of the reorganization of our commercial operations, our remaining goodwill was required to be reallocated amongst the then new Biopharma and Upjohn operating segments by determining the fair value of each reporting unit under our old and new management structure and the portions being transferred. We completed this re-allocation based on relative fair value in the second quarter of 2019 and retrospectively presented goodwill according to the operating structure.
Our Consumer Healthcare business was classified as held for sale as of December 31, 2018 and, upon closing of the transaction with GSK during the third quarter of 2019, we deconsolidated our Consumer Healthcare business and derecognized Consumer Healthcare goodwill. For additional information, see Note 2C. On November 16, 2020, we completed the spin-off and the combination of our Upjohn Business with Mylan. Upon closing, we deconsolidated the Upjohn business and derecognized $10.6 billion in Upjohn goodwill. In addition, at December 31, 2019, the goodwill associated with the Upjohn Business was classified as Noncurrent assets of discontinued operations. For additional information, see Note 2B.
| | | | | | | | |
| Pfizer Inc. | 2020 Form 10-K | 85
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | |
The following summarizes the components and changes in the carrying amount of Goodwill: |
(MILLIONS OF DOLLARS)(MILLIONS) | | | | | | | | | | Total(a) |
Balance, January 1, 20192022 | | | | | | | | | | $ | 42,92749,208 | |
Additions(a) | | | | | | | | | | 5,411 | |
Other(b)
| | | | | | | | | | (136)2,917 | |
| Impact of foreign exchange | | | | | | | | | | (750) | |
Balance, December 31, 20192022 | | | | | | | | | | 48,20251,375 | |
Additions(c)(b) | | | | | | | | | | 72716,117 | |
Other(b)
Impact of foreign exchange and other | | | | | | | | | | 648292 | |
Balance, December 31, 20202023 | | | | | | | | | | $ | 49,57767,783 | |
(a)Our goodwill balance continues to be assigned within the Biopharma reportable segment.
(b)Additions in 2022 relate to our acquisitions of GBT, Arena and Biohaven, and in 2023 primarily related to our acquisition of Array (seeSeagen. See Note 2A). (b)Other represents the impact of foreign exchange.
(c)Additions primarily represent the impact of measurement period adjustments related to our Array acquisition (see Note 2A).
Note 11.11. Pension and Postretirement Benefit Plans and Defined Contribution Plans
The majority of our employees worldwide are eligible for retirement benefits provided through defined benefit pension plans, defined contribution plans or both. In the U.S., we sponsor both IRC-qualified and supplemental (non-qualified) defined benefit plans and defined contribution plans. A qualified plan meets the requirements of certain sections of the IRC, and, generally, contributions to qualified plans are tax deductible. A qualified plan typically provides benefits to a broad group of employees with restrictions on discriminating in favor of highly compensated employees with regard to coverage, benefits and contributions. A supplemental (non-qualified) plan provides additional benefits to certain employees. In addition, we provide medical insurance benefits to certain retirees and their eligible dependents through our postretirement plans.
A. Components of Net Periodic Benefit Costs and Changes in Other Comprehensive Income/(Loss)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The following summarizes the components of net periodic benefit cost/(credit) and the changes in Other comprehensive income/(loss) for our benefit plans: |
| | |
| | Pension Plans | | Postretirement Plans |
| | U.S. | | International | | |
| | Year Ended December 31, |
| (MILLIONS) | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
| Service cost | | $ | — | | | $ | — | | | $ | — | | | $ | 85 | | | $ | 116 | | | $ | 130 | | | $ | 12 | | | $ | 29 | | | $ | 36 | |
| Interest cost | | 589 | | | 534 | | | 455 | | | 287 | | | 157 | | | 146 | | | 21 | | | 27 | | | 29 | |
| Expected return on plan assets | | (778) | | | (862) | | | (1,052) | | | (304) | | | (296) | | | (327) | | | (44) | | | (47) | | | (39) | |
| Amortization of prior service cost/(credit) | | 2 | | | 2 | | | (2) | | | — | | | (1) | | | (1) | | | (119) | | | (130) | | | (151) | |
Actuarial (gains)/losses(a) | | (410) | | | 225 | | | (684) | | | 102 | | | (11) | | | (690) | | | 51 | | | (440) | | | (167) | |
| Curtailments | | — | | | — | | | — | | | (2) | | | (11) | | | (4) | | | (12) | | | (18) | | | (82) | |
| Special termination benefits | | 6 | | | 18 | | | 17 | | | — | | | 1 | | | — | | | — | | | 1 | | | 2 | |
| Net periodic benefit cost/(credit) reported in income | | (592) | | | (84) | | | (1,265) | | | 169 | | | (45) | | | (746) | | | (90) | | | (578) | | | (372) | |
Cost/(credit) reported in Other comprehensive income/(loss) | | (2) | | | (2) | | | 2 | | | 31 | | | (1) | | | 4 | | | 128 | | | 169 | | | 107 | |
Cost/(credit) recognized in Comprehensive income | | $ | (594) | | | $ | (86) | | | $ | (1,264) | | | $ | 199 | | | $ | (46) | | | $ | (742) | | | $ | 38 | | | $ | (410) | | | $ | (265) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The following provides the annual (credit)/cost (including costs reported as part of discontinued operations) and changes in Other comprehensive income/(loss) for our benefit plans: |
| | |
| | Pension Plans | | |
| | U.S.
Qualified | | U.S.
Supplemental
(Non-Qualified) | | International | | Postretirement
Plans |
| | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 |
| Service cost | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 146 | | | $ | 125 | | | $ | 136 | | | $ | 38 | | | $ | 37 | | | $ | 39 | |
| Interest cost | | 499 | | | 629 | | | 598 | | | 34 | | | 47 | | | 55 | | | 164 | | | 215 | | | 212 | | | 49 | | | 75 | | | 72 | |
| Expected return on plan assets | | (1,015) | | | (890) | | | (1,040) | | | 0 | | | 0 | | | 0 | | | (306) | | | (317) | | | (360) | | | (36) | | | (33) | | | (37) | |
| Amortization of: | | | | | | | | | | | | | | | | | | | | | | | | |
| Actuarial losses | | 136 | | | 147 | | | 120 | | | 15 | | | 11 | | | 13 | | | 125 | | | 80 | | | 101 | | | 0 | | | 3 | | | 7 | |
| Prior service cost/(credit) | | (3) | | | (3) | | | 2 | | | (1) | | | (1) | | | (1) | | | (3) | | | (4) | | | (4) | | | (170) | | | (173) | | | (178) | |
| Curtailments | | 0 | | | 0 | | | 12 | | | 0 | | | 0 | | | 1 | | | 0 | | | (1) | | | (4) | | | 0 | | | (47) | | | (17) | |
| Settlements | | 223 | | | 230 | | | 113 | | | 49 | | | 27 | | | 26 | | | 6 | | | 16 | | | 4 | | | 0 | | | (10) | | | 0 | |
| Special termination benefits | | (1) | | | 4 | | | 6 | | | 2 | | | 17 | | | 10 | | | 0 | | | 0 | | | 0 | | | 0 | | | 2 | | | 2 | |
| Net periodic benefit cost/(credit) reported in income | | (161) | | | 116 | | | (189) | | | 99 | | | 100 | | | 103 | | | 132 | | | 115 | | | 84 | | | (118) | | | (146) | | | (111) | |
(Credit)/cost reported in Other comprehensive income/(loss) | | 640 | | | (246) | | | 361 | | | 95 | | | 115 | | | (189) | | | 202 | | | 570 | | | 84 | | | (50) | | | 38 | | | 105 | |
(Credit)/cost recognized in Comprehensive income | | $ | 479 | | | $ | (129) | | | $ | 171 | | | $ | 194 | | | $ | 215 | | | $ | (86) | | | $ | 333 | | | $ | 685 | | | $ | 168 | | | $ | (168) | | | $ | (107) | | | $ | (6) | |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 86 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
The components of net periodic benefit cost/(credit) other than the service cost component are primarily included in Other (income)/deductions––net (see Note 4). B. Actuarial Assumptions
| | | | | | | | | | | | | | | | | | | | |
| The following provides the weighted-average actuarial assumptions of our benefit plans: |
| | Year Ended December 31, |
| (PERCENTAGES) | | 2020 | | 2019 | | 2018 |
| Weighted-average assumptions used to determine benefit obligations | | | | | | |
| Discount rate: | | | | | | |
| U.S. qualified pension plans | | 2.6 % | | 3.3 | % | | 4.4 | % |
| U.S. non-qualified pension plans | | 2.4 % | | 3.2 | % | | 4.3 | % |
| International pension plans | | 1.5 % | | 1.7 | % | | 2.5 | % |
| Postretirement plans | | 2.5 % | | 3.2 | % | | 4.3 | % |
Rate of compensation increase(a): | | | | | | |
| | | | | | |
| | | | | | |
| International pension plans | | 2.9 % | | 1.4 | % | | 1.4 | % |
| Weighted-average assumptions used to determine net periodic benefit cost | | | | | | |
| Discount rate: | | | | | | |
| U.S. qualified pension plans | | 3.3 % | | 4.4 | % | | 3.8 | % |
| U.S. non-qualified pension plans | | 3.2 % | | 4.3 | % | | 3.7 | % |
| International pension plans interest cost | | 1.5 % | | 2.2 | % | | 2.0 | % |
| International pension plans service cost | | 1.6 % | | 2.4 | % | | 2.3 | % |
| Postretirement plans | | 3.2 % | | 4.3 | % | | 3.7 | % |
| Expected return on plan assets: | | | | | | |
| U.S. qualified pension plans | | 7.0 % | | 7.2 | % | | 7.5 | % |
| International pension plans | | 3.6 % | | 3.9 | % | | 4.4 | % |
| Postretirement plans | | 7.0 % | | 7.3 | % | | 7.5 | % |
| Rate of compensation increase: | | | | | | |
U.S. qualified pension plans(a) | | 0 | | | 0 | | | 2.8 | % |
U.S. non-qualified pension plans(a) | | 0 | | | 0 | | | 2.8 | % |
| International pension plans | | 2.9 % | | 1.4 | % | | 2.5 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Pension Plans | | Postretirement Plans |
| | U.S. | | International | | |
| | Year Ended December 31, |
| (PERCENTAGES) | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
| Weighted-average assumptions used to determine net periodic benefit cost: | | | | | | | | | | | | | | | | | | |
| Discount rate: | | | | | | | | | | | | | | | | | | |
| Pension plans/postretirement plans | | 5.4 | % | | 2.9 | % | | 2.6 | % | | | | | | | | 5.5 | % | | 2.9 | % | | 2.5 | % |
| Interest cost | | | | | | | | 3.8 | % | | 1.5 | % | | 1.2 | % | | | | | | |
| Service cost | | | | | | | | 3.6 | % | | 1.7 | % | | 1.4 | % | | | | | | |
| Expected return on plan assets | | 7.5 | % | | 6.3 | % | | 6.8 | % | | 4.5 | % | | 3.1 | % | | 3.4 | % | | 7.5 | % | | 6.3 | % | | 6.8 | % |
Rate of compensation increase(a) | | | | | | | | 3.0 | % | | 2.8 | % | | 2.9 | % | | | | | | |
| | | | | | | | | | | | | | | | | | |
| Weighted-average assumptions used to determine benefit obligations at fiscal year-end: | | | | | | | | | | | | | | | | | | |
| Discount rate | | 5.4 | % | | 5.4 | % | | 2.9 | % | | 4.4 | % | | 3.8 | % | | 1.6 | % | | 5.4 | % | | 5.5 | % | | 2.9 | % |
Rate of compensation increase(a) | | | | | | | | 3.2 | % | | 3.0 | % | | 2.8 | % | | | | | | |
(a)Effective January 1, 2018, we froze the defined benefit plans to future benefit accruals in the U.S. and members’ accrued benefits to that date no longer increase in line with future compensation increases. The rate of compensation increase is therefore no longer an assumptionnot used to determine the benefit obligation and net periodic benefit cost and benefit obligation for the U.S. qualified and non-qualified pension plans.plans as these plans are frozen.
The assumptions above are used to develop the benefit obligations at each fiscal year-end. All of the assumptions are reviewed on at least an annual basis.annually. We revise these assumptions based on an annual evaluation of long-term trends as well as market conditions that may have an impact on the cost of providing retirement benefits.
The weighted-average discount rate for our U.S. defined benefit plans is determined annually and evaluated and modifiedset with reference to reflect at year-end the prevailing market rate of a portfolio of high-quality fixed income investments, rated AA/Aa or better that reflect the rates at which the pension benefits could be effectively settled. For our international plans, the discount rates are set by benchmarking against investment grade corporate bonds rated AA/Aa or better, including, when there is sufficient data, a yield curve approach. These rate determinations are made consistent with local requirements. Overall, the yield curves used to measure the benefit obligations at year-end 20202023 resulted in lowerbroadly unchanged discount rates for the U.S. pension and postretirement plans and higher discount rates for the international pension plans as compared to the prior year.
| | | | | | | | | | | | | | |
| The following provides the healthcare cost trend rate assumptions for our U.S. postretirement benefit plans: |
| | As of December 31, |
| | 2020 | | 2019 |
| | | | |
| Healthcare cost trend rate assumed for next year (up to age 65) | | 5.4 % | | 5.6 | % |
| Healthcare cost trend rate assumed for next year (age 65 and older) | | 5.6 % | | 6.0 | % |
| Rate to which the cost trend rate is assumed to decline | | 4.5 % | | 4.5 | % |
| Year that the rate reaches the ultimate trend rate | | 2037 | | | 2037 | |
| | | | | | | | | | | | | | |
| The following provides the healthcare cost trend rate assumptions for our U.S. postretirement benefit plans: |
| | As of December 31, |
| | 2023 | | 2022 |
| | | | |
| Healthcare cost trend rate assumed for next year | | 7.9 | % | | 6.4 | % |
| | | | |
| Rate to which the cost trend rate is assumed to decline | | 4.0 | % | | 4.0 | % |
| Year that the rate reaches the ultimate trend rate | | 2047 | | | 2045 | |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 87 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
C. Obligations and Funded Status
| The following provides an analysis of the changes in our benefit obligations, plan assets and funded status of our benefit plans (including those reported as part of discontinued operations): | The following provides: (i) an analysis of the changes in our benefit obligations, plan assets and funded status of our benefit plans, (ii) the funded status recognized in our consolidated balance sheets and (iii) the pre-tax components of cumulative amounts recognized in Accumulated other comprehensive loss: | | The following provides: (i) an analysis of the changes in our benefit obligations, plan assets and funded status of our benefit plans, (ii) the funded status recognized in our consolidated balance sheets and (iii) the pre-tax components of cumulative amounts recognized in Accumulated other comprehensive loss: |
| | | | Pension Plans | | Postretirement Plans |
| | | | U.S. Qualified | | U.S. Supplemental
(Non-Qualified) | | International | | Postretirement
Plans |
| Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2020 | | 2019 | | 2020 | | 2019 | | 2020 | | 2019 |
| | Year Ended December 31, | |
| | Year Ended December 31, | |
| | Year Ended December 31, | |
| (MILLIONS) | | (MILLIONS) | | 2023 | | 2022 | | 2023 | | 2022 | | 2023 | | 2022 |
Change in benefit obligation(a) | Change in benefit obligation(a) | | | | | | | | | | | | | | | | |
| | Benefit obligation, beginning | |
| Benefit obligation, beginning | |
| Benefit obligation, beginning | Benefit obligation, beginning | | $ | 16,535 | | | $ | 15,141 | | | $ | 1,351 | | | $ | 1,280 | | | $ | 11,059 | | | $ | 9,952 | | | $ | 1,667 | | | $ | 1,870 | |
| Service cost | Service cost | | 0 | | | 0 | | | 0 | | | 0 | | | 146 | | | 125 | | | 38 | | | 37 | |
| Interest cost | Interest cost | | 499 | | | 629 | | | 34 | | | 47 | | | 164 | | | 215 | | | 49 | | | 75 | |
| Employee contributions | Employee contributions | | 0 | | | 0 | | | 0 | | | 0 | | | 8 | | | 7 | | | 88 | | | 84 | |
| Plan amendments | Plan amendments | | 2 | | | 0 | | | 0 | | | 0 | | | 2 | | | 18 | | | (56) | | | (56) | |
Changes in actuarial assumptions and other(b) | Changes in actuarial assumptions and other(b) | | 1,953 | | | 2,001 | | | 159 | | | 152 | | | 702 | | | 1,224 | | | (132) | | | (87) | |
| Foreign exchange impact | Foreign exchange impact | | 0 | | | 0 | | | 0 | | | 0 | | | 646 | | | (33) | | | 2 | | | (1) | |
Upjohn spin-off(c) | | (1,016) | | | 0 | | | 0 | | | 0 | | | (320) | | | 0 | | | (218) | | | 0 | |
| Acquisitions/divestitures/other, net | | 0 | | | (4) | | | 0 | | | (1) | | | 0 | | | (55) | | | 0 | | | (36) | |
| Curtailments | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | | | (2) | | | 0 | | | 0 | |
| Settlements | | (650) | | | (692) | | | (117) | | | (70) | | | (34) | | | (34) | | | 0 | | | 0 | |
| Special termination benefits | | (1) | | | 4 | | | 2 | | | 17 | | | 0 | | | 0 | | | 0 | | | 2 | |
| Upjohn spin-off | |
| Acquisitions/divestitures, net | |
| Curtailments and special termination benefits | |
Settlements(c) | |
| Benefits paid | Benefits paid | | (383) | | | (544) | | | (62) | | | (74) | | | (372) | | | (360) | | | (201) | | | (221) | |
Benefit obligation, ending(a) | Benefit obligation, ending(a) | | 16,940 | | | 16,535 | | | 1,366 | | | 1,351 | | | 12,001 | | | 11,059 | | | 1,238 | | | 1,667 | |
| | Change in plan assets | Change in plan assets | | | | | | | | | |
| Fair value of plan assets, beginning | Fair value of plan assets, beginning | | 14,586 | | | 13,051 | | | 0 | | | 0 | | | 8,956 | | | 8,215 | | | 519 | | | 469 | |
| Actual gain/(loss) on plan assets | | 1,974 | | | 2,760 | | | — | | | — | | | 868 | | | 873 | | | 69 | | | 50 | |
| Fair value of plan assets, beginning | |
| Fair value of plan assets, beginning | |
| Actual return on plan assets | |
| Company contributions | Company contributions | | 1,253 | | | 11 | | | 179 | | | 144 | | | 197 | | | 230 | | | 113 | | | 137 | |
| Employee contributions | Employee contributions | | 0 | | | 0 | | | 0 | | | 0 | | | 8 | | | 7 | | | 88 | | | 84 | |
| Foreign exchange impact | Foreign exchange impact | | 0 | | | 0 | | | 0 | | | 0 | | | 462 | | | 42 | | | 0 | | | 0 | |
Upjohn spin-off(c) | | (687) | | | 0 | | | 0 | | | 0 | | | (270) | | | 0 | | | 0 | | | 0 | |
| Upjohn spin-off | |
| Acquisitions/divestitures, net | Acquisitions/divestitures, net | | 0 | | | — | | | — | | | — | | | (6) | | | (16) | | | 0 | | | 0 | |
| Settlements | | (650) | | | (692) | | | (117) | | | (70) | | | (34) | | | (34) | | | 0 | | | 0 | |
Settlements(c) | |
| Benefits paid | Benefits paid | | (383) | | | (544) | | | (62) | | | (74) | | | (372) | | | (360) | | | (201) | | | (221) | |
| Fair value of plan assets, ending | Fair value of plan assets, ending | | 16,094 | | | 14,586 | | | 0 | | | 0 | | | 9,811 | | | 8,956 | | | 588 | | | 519 | |
| Funded status—Plan assets less than benefit obligation | | $ | (845) | | | $ | (1,949) | | | $ | (1,366) | | | $ | (1,351) | | | $ | (2,191) | | | $ | (2,103) | | | $ | (651) | | | $ | (1,148) | |
| Funded status | |
| Amounts recorded in our consolidated balance sheet: | |
| Noncurrent assets | |
| Noncurrent assets | |
| Noncurrent assets | |
| Current liabilities | |
| Noncurrent liabilities | |
| Funded status | |
Pre-tax components of cumulative amounts recognized in Accumulated other comprehensive loss: | |
| | Prior service (costs)/credits | |
| | Prior service (costs)/credits | |
| | Prior service (costs)/credits | |
Information related to the funded status of pension plans with an ABO in excess of plan assets(d): | |
| | Fair value of plan assets | |
| | Fair value of plan assets | |
| | Fair value of plan assets | |
| ABO | |
| ABO | |
| ABO | |
Information related to the funded status of pension plans with a PBO in excess of plan assets(d): | |
Information related to the funded status of pension plans with a PBO in excess of plan assets(d): | |
Information related to the funded status of pension plans with a PBO in excess of plan assets(d): | |
| | Fair value of plan assets | |
| | Fair value of plan assets | |
| | Fair value of plan assets | |
| PBO | |
| PBO | |
| PBO | |
|
(a)The PBO represents the present value of the benefit obligation earned through the end of the year and factors in future compensation increases. The ABO is similar to the PBO but does not factor in future compensation increases. For the U.S. qualified and supplemental (non-qualified) pension plans, the benefit obligation is both the PBO which is also equal to the ABO.and ABO as these plans are frozen and future benefit accruals no longer increase with future compensation increases. For the international pension plans, the benefit obligation is the PBO. The ABO for our international pension plans was $11.5$7.0 billion in 20202023 and $10.6$7.2 billion in 2019.2022. For the postretirement plans, the benefit obligation is the ABO.
(b)PrimarilyFor 2023, primarily includes actuarial lossesgains resulting from decreasesincreases in discount rates for the international pension plans. For 2022, primarily includes actuarial gains resulting from increases in 2020 and 2019.discount rates, offset by increases in inflation assumptions for the international plan.
(c)For more information, see Note 2B.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following provides information as to how the funded status is recognized in our consolidated balance sheets: |
| | |
| | | Pension Plans | | | | |
| | | U.S. Qualified | | U.S. Supplemental
(Non-Qualified) | | International | | Postretirement
Plans |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2020 | | 2019 | | 2020 | | 2019 | | 2020 | | 2019 |
Noncurrent assets(a) | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 0 | | | $ | 522 | | | $ | 453 | | | $ | 0 | | | $ | 0 | |
Current liabilities(b) | | 0 | | | 0 | | | (127) | | | (189) | | | (31) | | | (30) | | | (6) | | | (24) | |
Noncurrent liabilities(c) | | (845) | | | (1,949) | | | (1,239) | | | (1,162) | | | (2,681) | | | (2,526) | | | (645) | | | (1,124) | |
| Funded status | | $ | (845) | | | $ | (1,949) | | | $ | (1,366) | | | $ | (1,351) | | | $ | (2,191) | | | $ | (2,103) | | | $ | (651) | | | $ | (1,148) | |
(a)IncludedAs a result of a group annuity contract entered into between Pfizer and a third-party insurance company in Other noncurrentJuly 2022, the third party insurance company assumed future benefit obligations and responsibility for the annuity payments of certain retirees in the Pfizer Consolidated Pension Plan. Benefit obligations of $586 million and plan assets. of $588 million were associated with this contract. In February 2024, regulatory approval was received for this contract.
(b)(d)Included in Accrued compensationOur main U.S. qualified plan, U.S. postretirement plan and related items.
(c)Included in Pension benefit obligations, Postretirement benefit obligations, and Other noncurrent liabilities, many of our larger funded international plans were overfunded as appropriate.of December 31, 2023.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 88 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The following provides the pre-tax components of cumulative amounts recognized in Accumulated other comprehensive loss: |
| | |
| | Pension Plans | | | | |
| | U.S. Qualified | | U.S. Supplemental
(Non-Qualified) | | International | | Postretirement
Plans |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2020 | | 2019 | | 2020 | | 2019 | | 2020 | | 2019 |
Actuarial losses(a) | | $ | (5,062) | | | $ | (4,812) | | | $ | (579) | | | $ | (484) | | | $ | (3,056) | | | $ | (2,921) | | | $ | 58 | | | $ | (76) | |
| Prior service (costs)/credits | | (3) | | | (2) | | | (1) | | | 0 | | | (31) | | | (21) | | | 688 | | | 830 | |
Total(b) | | $ | (5,065) | | | $ | (4,814) | | | $ | (580) | | | $ | (485) | | | $ | (3,087) | | | $ | (2,942) | | | $ | 746 | | | $ | 754 | |
(a)Primarily represent the impact of changes in discount rates and other assumptions that result in cumulative changes in our PBO, as well as the cumulative difference between the expected return and actual return on plan assets. These accumulated actuarial losses are recognized in Accumulated other comprehensive loss and are amortized into net periodic benefit costs primarily over the average remaining service period for active participants for plans that are not frozen or the average life expectancy of plan participants for frozen plans, primarily using the corridor approach.
(b)The change from December 31, 2019 includes the derecognition of $388 million of pre-tax actuarial losses, net of prior service credits associated with benefit plans distributed as a result of the spin-off and the combination of the Upjohn Business with Mylan on November 16, 2020.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following provides information related to the funded status of selected benefit plans (including those reported as part of liabilities of discontinued operations): |
| | |
| | |
| | U.S. Qualified | | U.S. Supplemental (Non-Qualified) | | International |
| | As of December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 | | 2020 | | 2019 | | 2020 | | 2019 |
| Pension plans with an ABO in excess of plan assets: | | | | | | | | | | | | |
| Fair value of plan assets | | $ | 16,094 | | | $ | 14,586 | | | $ | — | | | $ | — | | | $ | 6,674 | | | $ | 5,843 | |
| ABO | | 16,940 | | | 16,535 | | | 1,366 | | | 1,351 | | | 8,961 | | | 7,960 | |
| Pension plans with a PBO in excess of plan assets: | | | | | | | | | | | | |
| Fair value of plan assets | | 16,094 | | | 14,586 | | | — | | | — | | | 6,735 | | | 5,947 | |
| PBO | | 16,940 | | | 16,535 | | | 1,366 | | | 1,351 | | | 9,447 | | | 8,503 | |
All of our U.S. plans and many of our international plans were underfunded as of December 31, 2020.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 89 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
D. Plan Assets
| The following provides the components of plan assets (including those reported as part of discontinued operations): | | The following provides the components of plan assets: | | The following provides the components of plan assets: |
| | | | As of December 31, 2023 | | | | | | As of December 31, 2023 | | As of December 31, 2022 |
| | | | | | Fair Value | | | | Fair Value | |
| (MILLIONS OF DOLLARS) | | As of December 31, 2020 | | Level 1 | | Level 2 | | Level 3 | | Assets Measured at NAV(a) | | As of December 31, 2019 | | Level 1 | | Level 2 | | Level 3 | | Assets Measured at NAV(a) |
| U.S. qualified pension plans | | | | | | | | | | | | | | | | | | | | |
| (MILLIONS EXCEPT TARGET ALLOCATION PERCENTAGE) | |
| (MILLIONS EXCEPT TARGET ALLOCATION PERCENTAGE) | |
| (MILLIONS EXCEPT TARGET ALLOCATION PERCENTAGE) | | | Target Allocation Percentage | | Total | | Level 1 | | Level
2 | | Level 3 | | Assets Measured at NAV(a) | | Total | | Level 1 | | Level
2 | | Level 3 | | Assets Measured at NAV(a) |
| U.S. pension plans | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Cash and cash equivalents | Cash and cash equivalents | | $ | 781 | | | $ | 70 | | | $ | 711 | | | $ | 0 | | | $ | 0 | | | $ | 363 | | | $ | 80 | | | $ | 284 | | | $ | 0 | | | $ | 0 | |
| Equity securities: | Equity securities: | | | |
| Global equity securities | |
| Global equity securities | |
| Global equity securities | Global equity securities | | 3,241 | | | 3,213 | | | 27 | | | 1 | | | 0 | | | 3,464 | | | 3,406 | | | 57 | | | 0 | | | 0 | |
| Equity commingled funds | Equity commingled funds | | 1,325 | | | 0 | | | 1,110 | | | 0 | | | 215 | | | 1,179 | | | 0 | | | 819 | | | 0 | | | 360 | |
| Fixed income securities: | Fixed income securities: | | | |
| Corporate debt securities | Corporate debt securities | | 6,499 | | | 23 | | | 6,476 | | | 0 | | | 0 | | | 5,292 | | | 10 | | | 5,281 | | | 1 | | | — | |
| Corporate debt securities | |
| Corporate debt securities | |
Government and agency obligations(b) | Government and agency obligations(b) | | 1,555 | | | 0 | | | 1,555 | | | 0 | | | 0 | | | 1,799 | | | 0 | | | 1,799 | | | 0 | | | 0 | |
| Fixed income commingled funds | Fixed income commingled funds | | 23 | | | — | | | 23 | | | 0 | | | 0 | | | 6 | | | 0 | | | 6 | | | 0 | | | 0 | |
| Other investments: | Other investments: | | | |
Partnership investments(c) | |
Partnership investments(c) | |
Partnership investments(c) | Partnership investments(c) | | 1,431 | | | 0 | | | — | | | 0 | | | 1,431 | | | 1,212 | | | 0 | | | 0 | | | 0 | | | 1,212 | |
| Insurance contracts | Insurance contracts | | 190 | | | 0 | | | 190 | | | 0 | | | 0 | | | 196 | | | 0 | | | 196 | | | 0 | | | 0 | |
Other commingled funds(d) | Other commingled funds(d) | | 1,049 | | | 0 | | | 11 | | | 0 | | | 1,038 | | | 1,075 | | | 0 | | | 9 | | | 0 | | | 1,066 | |
| Total | Total | | $ | 16,094 | | | $ | 3,306 | | | $ | 10,103 | | | $ | 1 | | | $ | 2,684 | | | $ | 14,586 | | | $ | 3,496 | | | $ | 8,451 | | | $ | 1 | | | $ | 2,638 | |
| International pension plans | International pension plans | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | Cash and cash equivalents | | $ | 407 | | | $ | 61 | | | $ | 346 | | | $ | 0 | | | $ | 0 | | | $ | 221 | | | $ | 33 | | | $ | 187 | | | $ | 0 | | | $ | 0 | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Equity securities: | Equity securities: | | | |
| | Equity commingled funds | |
| Equity commingled funds | |
| Equity commingled funds | Equity commingled funds | | 2,051 | | | 0 | | | 1,681 | | | 0 | | | 370 | | | 1,922 | | | 0 | | | 1,548 | | | 0 | | | 374 | |
| Fixed income securities: | Fixed income securities: | | | |
| Corporate debt securities | |
| Corporate debt securities | |
| Corporate debt securities | Corporate debt securities | | 925 | | | 0 | | | 925 | | | 0 | | | 0 | | | 796 | | | 0 | | | 796 | | | 0 | | | 0 | |
Government and agency obligations(b) | Government and agency obligations(b) | | 1,334 | | | 0 | | | 1,334 | | | 0 | | | 0 | | | 1,200 | | | 0 | | | 1,200 | | | 0 | | | 0 | |
| Fixed income commingled funds | Fixed income commingled funds | | 2,484 | | | 0 | | | 1,217 | | | 0 | | | 1,267 | | | 2,201 | | | 0 | | | 1,031 | | | 0 | | | 1,171 | |
| Other investments: | Other investments: | | | |
Partnership investments(c) | Partnership investments(c) | | 69 | | | 0 | | | 3 | | | 0 | | | 66 | | | 66 | | | 0 | | | 3 | | | 0 | | | 63 | |
Partnership investments(c) | |
Partnership investments(c) | |
| Insurance contracts | Insurance contracts | | 1,027 | | | 0 | | | 57 | | | 969 | | | 1 | | | 1,027 | | | 0 | | | 82 | | | 944 | | | 1 | |
Other(d) | Other(d) | | 1,514 | | | 0 | | | 117 | | | 393 | | | 1,003 | | | 1,524 | | | 0 | | | 82 | | | 398 | | | 1,043 | |
| Total | Total | | $ | 9,811 | | | $ | 61 | | | $ | 5,681 | | | $ | 1,362 | | | $ | 2,707 | | | $ | 8,956 | | | $ | 33 | | | $ | 4,929 | | | $ | 1,342 | | | $ | 2,652 | |
U.S. postretirement plans(e) | U.S. postretirement plans(e) | | | | | | | | | | | | | | | | | | | | |
| | Cash and cash equivalents | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Insurance contracts | Insurance contracts | | $ | 588 | | | $ | 0 | | | $ | 588 | | | $ | — | | | $ | — | | | $ | 519 | | | $ | — | | | $ | 519 | | | $ | 0 | | | $ | 0 | |
| | Total | |
(a)Certain investments that are measured at NAV per share (or its equivalent) have not been classified in the fair value hierarchy. The NAV amounts presented in this table are intended to permit reconciliation of the fair value hierarchy to the amounts presented for the total pension benefits plan assets.
(b)Government and agency obligations are inclusive of repurchase agreements.
(c)Mainly includes investments in private equity, private debt public equity limited partnerships, and to a lesser extent, real estate and venture capital.estate.
(d)Mostly includes investments in hedge funds and real estate.
(e)Reflects postretirement plan assets, which support a portion of our U.S. retiree medical plans.
| The following provides an analysis of the changes in our more significant investments valued using significant unobservable inputs (including those reported as part of discontinued operations): | | The following provides an analysis of the changes in our more significant investments valued using significant unobservable inputs: | |
| The following provides an analysis of the changes in our more significant investments valued using significant unobservable inputs: | |
| The following provides an analysis of the changes in our more significant investments valued using significant unobservable inputs: | |
| | | | International Pension Plans |
| | | Insurance contracts | | Other |
| | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | | 2020 | | 2019 | | 2020 | | 2019 |
| | | | International Pension Plans | |
| | | | International Pension Plans | |
| | | | International Pension Plans | |
| | | | | | | | | | Year Ended December 31, | | | | | | | | | | | | Year Ended December 31, |
| (MILLIONS) | | (MILLIONS) | | | | | | | | | | 2023 | | 2022 |
| Fair value, beginning | Fair value, beginning | | | $ | 944 | | | $ | 684 | | | $ | 398 | | | $ | 382 | |
| Actual return on plan assets: | Actual return on plan assets: | | | | | | |
| Assets held, ending | Assets held, ending | | | 32 | | | 50 | | | (10) | | | 6 | |
| | Assets held, ending | |
| Assets held, ending | |
| Assets sold during the period | |
| Purchases, sales, and settlements, net | Purchases, sales, and settlements, net | | | (38) | | | (40) | | | (10) | | | 6 | |
| Transfer into/(out of) Level 3 | Transfer into/(out of) Level 3 | | | (11) | | | 247 | | | (2) | | | 0 | |
| Exchange rate changes | Exchange rate changes | | | 42 | | | 2 | | | 16 | | | 4 | |
| Fair value, ending | Fair value, ending | | | $ | 969 | | | $ | 944 | | | $ | 393 | | | $ | 398 | |
| | | |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 9089 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Equity securities, Fixed income securities and Other investments may each be combined into commingled funds. Most commingled funds are valued to reflect the interest in the fund based on the reported year-end NAV. Partnership and Other investments are valued based on year-end reported NAV (or its equivalent), with adjustments as appropriate for lagged reporting of up to three months.
The following methods and assumptions were used to estimate the fair value of our pension and postretirement plans’ assets:
•Cash and cash equivalents: Level 1 investments may include cash, cash equivalents and foreign currency valued using exchange rates. Level 2 investments may include short-term investment funds which are commingled funds priced at a stable NAV by the administrator of the funds.
•Equity securities: Level 1 investments may include individual securities that are valued at the closing price or last trade reported on the major market on which they are traded. Level 1 and Level 2 investments may include commingled funds that have a readily determinable fair value based on quoted prices on an exchange or a published NAV derived from the quoted prices in active markets of the underlying securities. Level 3 investments may include individual securities that are unlisted, delisted, suspended, or illiquid and are typically valued using their last available price.
•Fixed income securities: Level 1 investments may include individual securities that are valued at the closing price or last trade reported on the major market on which they are traded. Level 2 investments may include commingled funds that have a readily determinable fair value based on observable prices of the underlying securities. Level 2 investments may include corporate bonds, government and government agency obligations and other fixed income securities valued using bid evaluation pricing models or quoted prices of securities with similar characteristics. Level 3 investments may include securities that are valued using alternative pricing sources, such as investment managers or brokers, which use proprietary pricing models that incorporate unobservable inputs.
•Other investments: Level 1 investments may include individual securities that are valued at the closing price or last trade reported on the major market on which they are traded. Level 2 investments may include Insuranceinsurance contracts which invest in interest bearing cash, U.S. government securities and corporate debt instruments. Level 3 investments may include securities or insurance contracts that are valued using alternative pricing sources, such as investment managers or brokers, which use proprietary pricing models that incorporate unobservable inputs.
Equity securities, Fixed income securities and Other investments may each be combined into commingled funds. Most commingled funds are valued to reflect the interest in the fund based on the reported year-end NAV. Partnership and Other investments are valued based on year-end reported NAV (or its equivalent), with adjustments as appropriate for lagged reporting of up to three months.
Certain investments are authorized to include derivatives, such as equity or bond futures, swaps, options and currency futures or forwards for managing risks and exposures.
| | | | | | | | | | | | | | | | | | | | |
| The following provides the long-term target asset allocations ranges and the percentage of the fair value of plan assets for benefit plans: |
| | |
| | | Target
Allocation Percentage | | Percentage of Plan Assets |
| | As of December 31, |
| (PERCENTAGES) | | 2020 | | 2020 | | 2019 |
| U.S. qualified pension plans | | | | | | |
| Cash and cash equivalents | | 0-10% | | 4.9 % | | 2.5 | % |
| Equity securities | | 35-55% | | 28.4 % | | 31.8 | % |
| Fixed income securities | | 28-53% | | 50.2 % | | 48.7 | % |
| Other investments | | 5-20% | | 16.6 % | | 17.0 | % |
| Total | | 100 | % | | 100 % | | 100 | % |
| International pension plans | | | | | | |
| Cash and cash equivalents | | 0-10% | | 4.2 | % | | 2.5 | % |
| Equity securities | | 20-40% | | 20.9 | % | | 21.5 | % |
| Fixed income securities | | 35-60% | | 48.4 | % | | 46.9 | % |
| Other investments | | 10-35% | | 26.6 | % | | 29.2 | % |
| Total | | 100 | % | | 100 % | | 100 | % |
| U.S. postretirement plans | | | | | | |
| Cash and cash equivalents | | 0-5% | | 0 | | | 0 | |
| | | | | | |
| | | | | | |
| Other investments | | 95-100% | | 100 | % | | 100 | % |
| Total | | 100 | % | | 100 % | | 100 | % |
Global plan assets are managed with the objective of generating returns that will enable the plans to meet their future obligations, while seeking to manage net periodic benefit costs and cash contributions over the long-term. We utilize long-term asset allocation ranges in the management of our plans’ invested assets. Our long-term return expectations are developed based on a diversified, global investment strategy that takes into account historical experience, as well as the impact of portfolio diversification, active portfolio management, and our view of current and future economic and financial market conditions. As market conditions and other factors change, we may adjust our targets accordingly and our asset allocations may vary from the target allocations.
Our long-term asset allocation ranges reflect our asset class return expectations and tolerance for investment risk within the context of the respective plans’ long-term benefit obligations. These ranges are supported by analysis that incorporates historical and expected returns by asset class, as well as volatilities and correlations across asset classes and our liability profile.
Each pension plan is overseen by a local committee or board that is responsible for the overall investment of the pension plan assets. In determining investment policies and associated target allocations, each committee or board considers a wide variety of factors. As such, the target asset allocation for each of our international pension plans is set on a standalone basis by the relevant board or committee. The target
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 91 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
asset allocation ranges shown for the international pension plans seek to reflect the combined target allocations across all such plans, while also showing the range within which the target allocations for each plan typically falls.
The investment managers of certain separately managed accounts, commingled funds and private equity funds may be permitted to use repurchase agreements and derivative securities, including U.S. Treasury and equity futures contracts as described in each respective investment management, subscription, partnership or other governing agreement.
E. Cash Flows
It is our practice to fund amounts for our qualified pension plans that are at least sufficient to meet the minimum requirements set forth in applicable employee benefit laws and local tax laws.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following provides the expected future cash flow information related to our benefit plans: |
| | | Pension Plans | | |
| (MILLIONS OF DOLLARS) | | U.S. Qualified | | U.S. Supplemental
(Non-Qualified) | | International | | Postretirement Plans |
| Expected employer contributions: | | | | | | | | |
| 2021 | | $ | 0 | | | $ | 127 | | | $ | 282 | | | $ | 90 | |
| Expected benefit payments: | | | | | | | | |
| 2021 | | $ | 1,139 | | | $ | 127 | | | $ | 371 | | | $ | 97 | |
| 2022 | | 1,036 | | | 121 | | | 375 | | | 94 | |
| 2023 | | 1,032 | | | 116 | | | 375 | | | 92 | |
| 2024 | | 1,030 | | | 106 | | | 385 | | | 89 | |
| 2025 | | 986 | | | 100 | | | 393 | | | 86 | |
| 2026–2030 | | 4,625 | | | 424 | | | 2,086 | | | 430 | |
| | | | | | | | | | | | | | | | | | | | |
| The following provides the expected future cash flow information related to our benefit plans: |
| | | Pension Plans | | Postretirement Plans |
| (MILLIONS) | | U.S. | | International | | |
| Expected employer contributions: | | | | | | |
| 2024 | | $ | 94 | | | $ | 162 | | | $ | 39 | |
| Expected benefit payments: | | | | | | |
| 2024 | | $ | 1,009 | | | $ | 372 | | | $ | 43 | |
| 2025 | | 907 | | | 361 | | | 45 | |
| 2026 | | 894 | | | 371 | | | 46 | |
| 2027 | | 875 | | | 384 | | | 47 | |
| 2028 | | 858 | | | 386 | | | 47 | |
| 2029–2033 | | 4,004 | | | 2,073 | | | 218 | |
The above table reflects the total U.S. and international plan benefits projected to be paid from the plans or from our general assets under the current actuarial assumptions used for the calculation of the benefit obligation and, therefore, actual benefit payments may differ from projected benefit payments.obligation.
F. Defined Contribution Plans
We have defined contribution plans in the U.S. and several other countries. For the majority of the U.S. defined contribution plans, employees may contribute a portion of their salaries and bonuses to the plans, and we match, in cash, a portion of the employee contributions. Beginning on January 1, 2011, for newly hired non-union employees, rehires and transfers to the U.S. or Puerto Rico, we no longer offer a defined benefit pension plan and, instead,We also offer a Retirement Savings Contribution (RSC) in the defined contribution plan. The RSCwhich is an annual non-contributory employer contribution (that is not dependent upon the participant making a contribution) determined based on each employee’s eligible compensation, age and years of service. Beginning on January 1, 2018, all non-union employees in the U.S. and Puerto Rico defined benefit plans transitioned to the RSC in the defined contribution plans.Rico. We recorded charges related to the employer contributions to global defined contribution plans of $685$843 million in 2020, $6592023, $770 million in 20192022 and $622$732 million in 2018.2021.
Note 12. Equity
A. Common Stock Purchases
We purchase our common stock through privately negotiated transactions or in the open market as circumstances and prices warrant. Purchased shares under each of thea share-purchase plans,plan, which areis authorized by our BOD, are available for general corporate purposes. In December 2015, the BOD authorized an $11 billion share repurchase program, which was exhausted in the third quarter of 2018. In December 2017, the BOD authorized an additional $10 billion share repurchase program, which was exhausted in the first quarter of 2019. In December
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 90 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2018, the BOD authorized anothera $10 billion share repurchase program to be utilized over time and share repurchases commenced thereunder in the first quarter of 2019.
In March 2018,the first quarter of 2022, we entered into an accelerated share repurchase agreement (ASR) with Citibank, N.A. to repurchase $4 billion of our common stock pursuant to our previously announced share repurchase authorization. We paid $4 billion and received an initial delivery of 87 million shares of stock at a price of $36.61 per share, which represented approximately 80% of the notional amount of the ASR. In September 2018, the ASR was completed resulting in Citibank owing us an additional 21purchased 39 million shares of our common stock. The average price paid for allstock at a cost of the shares delivered$2 billion under the ASR was $36.86 per share. The common stock received is included in Treasury stock.
In February 2019, we entered into an ASR with Goldman Sachs & Co. LLC to repurchase $6.8 billion of our common stock pursuant to our previouslypublicly announced share repurchase authorization. We paid $6.8 billion and received an initial delivery of 130 million shares of common stock, which represented approximately 80% of the notional amount of the ASR. In August 2019, the ASR with Goldman Sachs & Co. LLC was completed resulting in Goldman Sachs & Co. LLC owing us an additional 33.5 million shares of our common stock. The average price paid for all of the shares delivered under the ASR was $41.42 per share. The common stock received is included in Treasury stock.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 92 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | |
| The following provides the number of shares of our common stock purchased and the cost of purchases under our publicly announced share purchase plans, including our ASRs: |
| | Year Ended December 31, |
| (SHARES IN MILLIONS, DOLLARS IN BILLIONS) | | 2020 | | 2019(a) | | 2018(b) |
| Shares of common stock purchased | | 0 | | | 213 | | | 307 | |
| Cost of purchase | | $ | 0 | | | $ | 8.9 | | | $ | 12.2 | |
(a)Represents shares purchased pursuant to the ASR withGoldman Sachs & Co. LLC entered into in February 2019, as well as open market share repurchases of $2.1 billion.
(b)Represents shares purchased pursuant to the ASR with Citibank entered into in March 2018, as well as open market share repurchases of $8.2 billion.
share-purchase plan. Our remaining share-purchase authorization was approximately $5.3$3.3 billion atas of December 31, 2020.2023.
B. Preferred Stock and Employee Stock Ownership Plans
Prior to May 4, 2020, our Series A convertible perpetual preferred stock (the Series A Preferred Stock) was held by an ESOP trust (the Trust). All outstanding shares of Series A Preferred Stock were converted, at the direction of the independent fiduciary under the Trust and in accordance with the certificate of designations for the Series A Preferred Stock, into shares of our common stock on May 4, 2020. The Trust received an aggregate of 1,070,369 shares of our common stock upon conversion, with 0 shares of Series A Preferred Stock remaining outstanding as a result of the conversion. In December 2020, we filed a certificate of elimination and a restated certificate of incorporation with the Delaware Secretary of State, which eliminated the Series A Preferred Stock.
Since May 4, 2020, weWe have 1one ESOP that holds common stock of the Company (Common ESOP). Prior to that there was also an ESOP that held the Series A Preferred Stock. As of December 31, 2020,2023, all shares of common stock held by the Common ESOP have been allocated to the Pfizer U.S. defined contribution plan participants. The compensation cost related to the Common ESOP was $19 million in 2020, $20 million in 2019for 2023 and $19 million in 2018.for each of 2022 and 2021.
Note 13. Share-Based Payments
Our compensation programs can include share-based payment awards with value that is determined by reference to the fair value of our shares and that provide for the grant of shares or options to acquire shares or similar arrangements. Our share-based awards are designed based on competitive survey data or industry peer groups used for compensation purposes;purposes, and are allocated between different long-term incentive awards, generally in the form of Total Shareholder Return Units (TSRUs), Restricted Stock Units (RSUs), Portfolio Performance Shares (PPSs), Performance Share Awards (PSAs), Breakthrough Performance Awards (BPAs) and Stock Options,stock options, as determined by the Compensation Committee.
The 2019 Stock Plan (2019 Plan) replaced and superseded the 2014 Plan. It provides for 400 million shares in addition to shares remaining under the 2014 Plan, to be authorized for grants. The 2019 Plan provides that the number of stock options, TSRUs, RSUs, or performance-based awards that may be granted to any one individual during any 36-month period is limited to 20 million shares. RSUs count as three shares, and that RSUs, PPSs, PSAs and PSAsBPAs count as 3three shares times the maximum potential payout, while TSRUs and stock options count as 1one share, toward the maximum shares available under the 2019 Plan. As of December 31, 2020, 4112023, 248 million shares were available for award.award, including 68 million shares that we assumed from the remaining shares available from the stock plan of Seagen which can be issued to legacy employees of Seagen and newly hired employees after the date of acquisition once such shares are registered on Form S-8. Although not required to do so, we have used authorized and unissued shares and, to a lesser extent, treasury stock to satisfy our obligations under these programs.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 91 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
A summary of the awards and valuation details:
| | | | | | | | | | | |
| Awarded to | Terms | Valuation | Recognition and Presentation |
Total Shareholder Return Units (TSRUs)(a), (b) |
| Senior and other key management and select employees | •Entitle the holder to receive shares of our common stock with a value equal to the difference between the defined settlement price and the grant price, plus the dividendsdividend equivalents accumulated during the five or seven-year term, if and to the extent the total value is positive. •Settlement price is the average closing price of our common stock during the 20 trading days ending on the fifth or seventh anniversary of the grant, as applicable; the grant price is the closing price of our common stock on the date of the grant. •Automatically settledsettle on the fifth or seventh anniversary of the grant but vest on the third anniversary of the grant. •Retirement-eligible holders can convert their TSRUs, when vested, into Profit Units (PTUs) with a conversion ratio based on a calculation used to determine the shares at TSRU settlement. The PTUs are entitled to earn Dividend Equivalent Units (DEUs), and the PTUs and DEUs will be settled in our common stock on the TSRUs’ original settlement date and will be subject to the terms and conditions of the original grant after which time there is no longer a substantial likelihood of forfeiture.including forfeiture provisions. | As of the grant date using a Monte Carlo simulation model | Amortized on a straight-line basis over the vesting term into Cost of sales, Selling, informational and administrative expenses, and/or Research and development expenses, as appropriate. |
| Restricted Stock Units (RSUs) |
| Select employees | •Entitle the holder to receive a specified number of shares of our common stock, including shares resulting from dividend equivalents paid on suchthat are reinvested into additional RSUs. •For RSUs granted during the periods presented,before 2022, generally in virtually all instances, the units vest afteron the third anniversary of the grant date assuming continuous service from the grant date. Beginning in 2022, generally in all instances, the units vest and distribute one-third per year for three years on each of the three yearsannual anniversaries from the date of grant assuming continuous service from the grant date. | As of the grant date using the closing price of our common stock | Amortized on a straight-line basis for RSUs granted before 2022, and on an accelerated attribution approach for RSUs granted beginning in 2022, over the vesting term into Cost of sales, Selling, informational and administrative expenses, and/or Research and development expenses, as appropriate. |
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 93 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | |
Awarded to | Terms | Valuation | Recognition and Presentation |
| Portfolio Performance Shares (PPSs) |
| Select employees | •Entitle the holder to receive, at the end of the performance period, shares of our common stock, if any, including shares resulting from dividend equivalents paidearned on such shares. •For PPSs granted, during the period presented, the awards vest after three yearson the third anniversary of the grant assuming continuous service from the grant date and the number of shares paid, if any, depends on the achievement of predetermined goals related to Pfizer’s long-term product portfolio during a three or five-year performance period from the year of the grant date.date, as applicable. •The number of shares that may be earned ranges from 0% to 200% of the initial award depending on goal achievement over the performance period. | As of the grant date using the intrinsic value method using the closing price of our common stock | Amortized on a straight-line basis over the probable vesting term into Cost of sales, Selling, informational and administrative expenses and/or Research and development expenses, as appropriate, and adjusted each reporting period, as necessary, to reflect changes in the price of our common stock, changes in the number of shares that are probable of being earned, and changes in management’s assessment of the probability that the specified performance criteria will be achieved and/or changes in management’sachieved. assessment of the probable vesting term.
|
| Performance Share Awards (PSAs) |
| Senior and other key management | •Entitle the holder to receive, at the end of the performance period, shares of our common stock (retirees) earned, if any, or an equal value in cash (active colleagues), including shares resulting from dividend equivalents on shares earned, dependent upon the achievement of predetermined goals related to 2two measures: a.Adjusted operating income (for performance years through 2018) or adjusted net income (for 2019 and later years, except for the 2017 PSAs) over 3 one-yearthree one-year periods; and b.TSR as compared to the NYSE ARCA Pharmaceutical Index (DRG Index) over the three-year performance period. •PSAs vest after three yearson the third anniversary of the grant assuming continuous service from the grant date. •The number of sharesaward that may be earned ranges from 0% to 200% of the initialtarget award depending on goal achievement over the performance period. | As of the grant date using the intrinsic value method using the closing price of our common stock | Amortized on a straight-line basis over the vesting term into Cost of sales, Selling, informational and administrative expenses, and/or Research and development expenses, as appropriate, and adjusted each reporting period, as necessary, to reflect changes in the price of our common stock, the number of shares that are probable of being earned and management’s assessment of the probability that the specified performance criteria will be achieved. |
| Breakthrough Performance Awards (BPAs) |
| Select employees identified as instrumental in delivering medicines to patients (excluding executive officers) | •Entitle the holder to receive, at the end of the performance period, shares of our common stock, if any, including shares resulting from dividend equivalents earned on such shares. •For BPAs granted, the awards, if earned/vested, are settled at the end of the performance period, but no earlier than the one-year anniversary of the date of grant and dependent upon the achievement of the respective predetermined performance goals related to advancing Pfizer’s product pipeline during the performance period. •The number of shares that may be earned ranges from 0% to 600% of the target award depending on the level and timing of goal achievement over the performance period. | As of the grant date using the intrinsic value method using the closing price of our common stock | Amortized on a straight-line basis over the probable vesting term into Cost of sales, Selling, informational and administrative expenses, and/or Research and development expenses, as appropriate, and adjusted each reporting period, as necessary, to reflect changes in the price of our common stock, changes in the number of shares that are probable of being earned and changes in management’s assessment of the probability that the specified performance criteria will be achieved.achieved and/or management’s assessment of the probable vesting term. |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 92 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | |
| Awarded to | Terms | Valuation | Recognition and Presentation |
| Stock Options |
| Select employees | •Entitle the holder to purchase a specified number of shares of our common stock at a price per share equal to the closing market price of our common stock on the date of grant, for a period of time when vested. •Beginning inSince 2016, only a limited set of non-U.S. employees received stock option grants. NaNNo stock options were awarded to senior and other key management in any period presented. •Stock options vest after three yearson the third anniversary of the grant assuming continuous service from the grant date and have a contractual term of 10 years. | As of the grant date using the Black-Scholes-Merton option-pricing model | Amortized on a straight-line basis over the vesting term into Cost of sales, Selling, informational and administrative expenses, and/or Research and development expenses, as appropriate. |
(a)Retirement-eligible holders, as defined in the grant terms, can convert their TSRUs, when vested, into Profit Units (PTUs) with a conversion ratio based on a calculation used to determine the shares at TSRU settlement. The PTUs are entitled to earn Dividend Equivalent Units (DEUs), and the PTUs and DEUs will be settled in our common stock on the TSRUs’ original settlement date and will be subject to the terms and conditions of the original grant including forfeiture provisions.
(b)In 2017, Performance Total Shareholder Return Units (PTSRUs) were awarded to the Former Chairman and Chief Executive Officer (1,444,395 PTSRUs) and 361,099 PTSRUs were awarded to the Group President, Chief Business Officer (former role Group President Pfizer Innovative Health) at a grant price of $30.31 and at a GDFV of $5.54 per PTSRU. All these amounts have been adjusted for the Upjohn spin-off discussed in Note 2B. In addition to having the same characteristics and valuation methodology of TSRUs, PTSRU grants require special service and performance conditions.
| | The following provides data related to all TSRU, RSU, PPS, PSA and stock option activity: | The following provides data related to all TSRU, RSU, PPS, PSA and stock option activity: | The following provides data related to all TSRU, RSU, PPS, PSA and stock option activity: |
| (MILLIONS OF DOLLARS, EXCEPT FAIR VALUE OF SHARES VESTED PER TSRU AND STOCK OPTION) | | TSRUs | | RSUs | | PPSs | | PSAs | | Stock Options |
| (MILLIONS, EXCEPT FAIR VALUE OF SHARES VESTED PER TSRU AND STOCK OPTION) | | (MILLIONS, EXCEPT FAIR VALUE OF SHARES VESTED PER TSRU AND STOCK OPTION) | | TSRUs | | RSUs | | PPSs | | PSAs | | Stock Options |
| Year Ended December 31, | Year Ended December 31, | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 | Year Ended December 31, | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
Total fair value of shares vested(a) | Total fair value of shares vested(a) | | $6.22 | | $8.52 | | $7.42 | | $334 | | $454 | | $146 | | $119 | | $136 | | $169 | | $25 | | $64 | | $4 | | $3.56 | | $5.98 | | $5.06 | Total fair value of shares vested(a) | | $10.71 | | $11.72 | | $7.26 | | $505 | | $345 | | $304 | | $116 | | $145 | | $181 | | $58 | | $57 | | $33 | | $7.88 | | $9.44 | | $4.86 |
| Total intrinsic value of options exercised or share units converted | Total intrinsic value of options exercised or share units converted | | $84 | | $175 | | $151 | | | | $224 | | $245 | | $194 | | | | $293 | | $261 | | $625 | Total intrinsic value of options exercised or share units converted | | $755 | | $1,131 | | $594 | | | | | | | | $250 | | $280 | | $228 | | | | | | | | $102 | | $247 | | $584 |
| Cash received upon exercise | Cash received upon exercise | | | | | | | | | | $425 | | $394 | | $1,259 | Cash received upon exercise | | | | | | | | | | | | | | | | | | | | | | | | | | $181 | | $260 | | $795 |
| Tax benefits realized from exercise | Tax benefits realized from exercise | | | | | | | | | | $55 | | $47 | | $115 | Tax benefits realized from exercise | | | | | | | | | | | | | | | | | | | | | | | | | | $20 | | $46 | | $106 |
Compensation cost recognized, pre-tax(b) | | $287 | | $294 | | $302 | | $272 | | $275 | | $286 | | $180 | | $114 | | $276 | | $31 | | $28 | | $62 | | $6 | | $7 | | $12 |
| Compensation cost recognized/(reduced), pre-tax | | Compensation cost recognized/(reduced), pre-tax | | $244 | | $255 | | $259 | | $437 | | $402 | | $281 | | $(138) | | $144 | | $535 | | $(5) | | $73 | | $76 | | $4 | | $4 | | $5 |
| Total compensation cost related to nonvested awards not yet recognized, pre-tax | Total compensation cost related to nonvested awards not yet recognized, pre-tax | | $224 | | $229 | | $246 | | $228 | | $241 | | $256 | | $104 | | $87 | | $102 | | $32 | | $34 | | $41 | | $4 | | $5 | | $5 | Total compensation cost related to nonvested awards not yet recognized, pre-tax | | $192 | | $179 | | $187 | | $212 | | $266 | | $271 | | $81 | | $135 | | $175 | | $22 | | $38 | | $54 | | $4 | | $3 |
| Weighted-average period over which cost is expected to be recognized (years) | Weighted-average period over which cost is expected to be recognized (years) | | 1.6 | | 1.6 | | 1.6 | | 1.7 | | 1.7 | | 1.7 | | 1.8 | | 1.8 | | 1.8 | | 1.9 | | 1.8 | | 1.8 | | 1.7 | | 1.6 | | 1.7 | Weighted-average period over which cost is expected to be recognized (years) | | 1.7 | | 1.7 | | 1.6 | | 1.8 | | 1.7 | | 1.8 | | 1.8 | | 1.7 | | 1.8 | | 1.8 | | 1.8 | | 1.7 | | 1.7 | | 1.6 |
(a)Weighted-average GDFV per TSRUs and stock options.
(b)TSRU includes expense for PTSRUs, which is not significant for all years presented.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 94 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Total share-based payment expense was $780$525 million, $718$872 million and $949 million$1.2 billion in 2020, 20192023, 2022 and 2018, respectively, which includes pre-tax share-based payment expense included in Income from discontinued operations––net of tax of $23 million, $30 million and $27 million in 2020, 2019 and 2018,2021, respectively. Tax benefit for share-based compensation expense was $141$93 million, $137$160 million and $180$227 million in 2020, 20192023, 2022 and 2018,2021, respectively.
The table above excludes total expense due to the modification for share-based awards in connection with our cost reduction/productivity initiatives, which was not significant for all years presented and is recorded in Restructuring charges and certain acquisition-related costs (see Note 3). Amounts capitalized as part of inventory cost were not significant for any period presented. | | Summary of the weighted-average assumptions used in the valuation of TSRUs and stock options: | Summary of the weighted-average assumptions used in the valuation of TSRUs and stock options: | Summary of the weighted-average assumptions used in the valuation of TSRUs and stock options: |
| TSRUs | | Stock Options |
| | TSRUs | | | | TSRUs | | Stock Options |
| Year Ended December 31, | Year Ended December 31, | | 2020 | | 2019 | | 2018 | | 2020 | | 2019 | | 2018 | Year Ended December 31, | | 2023 | | 2022 | | 2021 | | 2023 | | 2022 | | 2021 |
Expected dividend yield (based on a constant dividend yield during the expected term) | Expected dividend yield (based on a constant dividend yield during the expected term) | | 4.36 | % | | 3.27 | % | | 3.73 | % | | 4.36 | % | | 3.27 | % | | 3.73 | % | Expected dividend yield (based on a constant dividend yield during the expected term) | | 3.80 | % | | 3.42 | % | | 4.51 | % | | 3.80 | % | | 3.42 | % | | 4.51 | % |
Risk-free interest rate (based on interpolated yield on U.S. Treasury zero-coupon issues) | Risk-free interest rate (based on interpolated yield on U.S. Treasury zero-coupon issues) | | 1.15 | % | | 2.55 | % | | 2.60 | % | | 1.25 | % | | 2.66 | % | | 2.85 | % | Risk-free interest rate (based on interpolated yield on U.S. Treasury zero-coupon issues) | | 4.08 | % | | 1.87 | % | | 0.93 | % | | 4.03 | % | | 1.93 | % | | 1.27 | % |
Expected stock price volatility (based on implied volatility, after consideration of historical volatility) | Expected stock price volatility (based on implied volatility, after consideration of historical volatility) | | 20.99 | % | | 18.34 | % | | 20.00 | % | | 20.97 | % | | 18.34 | % | | 20.02 | % | Expected stock price volatility (based on implied volatility, after consideration of historical volatility) | | 23.23 | % | | 29.20 | % | | 26.53 | % | | 23.23 | % | | 29.21 | % | | 26.54 | % |
TSRUs contractual/stock options expected term, years (based on historical exercise and post-vesting termination patterns for stock options) | TSRUs contractual/stock options expected term, years (based on historical exercise and post-vesting termination patterns for stock options) | | 5.12 | | 5.13 | | 5.12 | | 6.75 | | 6.75 | | 6.75 | TSRUs contractual/stock options expected term, years (based on historical exercise and post-vesting termination patterns for stock options) | | 5.15 | | 5.17 | | 5.15 | | 6.50 | | 6.50 | | 6.75 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Summary of all TSRU, RSU, PPS and PSA activity during 2020 (with the shares granted representing the maximum award that could be achieved for PPSs and PSAs): |
| | TSRUs | | RSUs | | PPSs(a) | | PSAs |
| TSRUs | | Per TSRU, Weighted Average | | Shares | | Weighted Avg. GDFV per share | | Shares | | Weighted Avg. Intrinsic Value per share | | Shares | | Weighted Avg. Intrinsic Value per share |
| | | | | |
| (Thousands) | | GDFV | | Grant Price | | (Thousands) | | | (Thousands) | | (Thousands) | |
Nonvested, December 31, 2019(b) | 122,654 | | $ | 7.53 | | | $ | 38.01 | | | 23,407 | | $ | 37.54 | | | 17,694 | | $ | 39.18 | | | 5,061 | | $ | 39.18 | |
Granted(b) | 51,158 | | 6.22 | | | 34.12 | | | 8,423 | | 34.22 | | | 8,150 | | 34.10 | | | 1,713 | | 34.10 | |
Vested(b) | (45,757) | | | 6.40 | | | 34.11 | | | (9,321) | | | 34.70 | | | (6,393) | | | 34.73 | | | (728) | | | 34.65 | |
Reinvested dividend equivalents(b) | | | | | | | 955 | | | 37.32 | | | | | | | | | |
Forfeited(b) | (4,782) | | | 7.27 | | | 37.20 | | | (999) | | | 37.91 | | | (713) | | | 36.78 | | | (1,052) | | | 35.00 | |
Upjohn spin-off adjustment(c) | 6,571 | | 6.88 | | | 32.94 | | | 1,228 | | | 35.55 | | | 1,338 | | 36.69 | | | 270 | | | 36.69 | |
| Nonvested, December 31, 2020 | 129,844 | | $ | 6.90 | | | $ | 32.94 | | | 23,692 | | $ | 35.50 | | | 20,077 | | $ | 36.81 | | | 5,264 | | $ | 36.81 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Summary of all TSRU, RSU, PPS and PSA activity during 2023 (with the shares granted representing the maximum award that could be achieved for PPSs and PSAs): |
| | TSRUs | | RSUs | | PPSs(a) | | PSAs | | |
| TSRUs | | Per TSRU, Weighted Average | | Shares | | Weighted Avg. GDFV per share | | Shares | | Weighted Avg. Intrinsic Value per share | | Shares | | Weighted Avg. Intrinsic Value per share | | | | |
| | | | | | | |
| (Thousands) | | GDFV | | Grant Price | | (Thousands) | | | (Thousands) | | (Thousands) | | | | |
| Nonvested, December 31, 2022 | 101,693 | | $ | 7.58 | | | $ | 35.26 | | | 27,826 | | $ | 38.26 | | | 22,322 | | $ | 51.24 | | | 5,018 | | $ | 51.24 | | | | | |
| Granted | 26,631 | | 10.71 | | | 42.29 | | | 10,007 | | 42.11 | | | 8,751 | | 42.30 | | | 1,623 | | 42.30 | | | | | |
| Vested | (48,277) | | | 6.08 | | | 31.38 | | | (12,330) | | | 37.15 | | | (7,736) | | | 40.78 | | | (1,428) | | | 40.74 | | | | | |
| Reinvested dividend equivalents | | | | | | | 1,195 | | | 36.07 | | | | | | | | | | | | | |
| Forfeited | (2,374) | | | 9.99 | | | 40.86 | | | (855) | | | 41.25 | | | (1,112) | | | 36.09 | | | (479) | | | 38.47 | | | | | |
| Nonvested, December 31, 2023 | 77,673 | | $ | 9.67 | | | $ | 39.92 | | | 25,844 | | $ | 40.08 | | | 22,225 | | $ | 28.79 | | | 4,734 | | $ | 28.79 | | | | | |
(a)Vested and non-vested shares outstanding, but not paid as of December 31, 20202023 were 33.935.8 million.
(b)Activity prior to the Upjohn Business spin-off has not been adjusted.
(c)In connection with the Upjohn Business spin-off, the Company made adjustments to preserve the intrinsic value of the awards immediately before and after the spin-off. The terms of the outstanding awards remain the same and continue to vest over the original vesting periods. Certain outstanding awards at the time of the spin-off held by employees of Upjohn were prorated for services performed and the remaining portion forfeited at the time of the separation. The share-based awards held as of November 16, 2020 were adjusted as follows:
•The number of outstanding TSRUs was increased and the grant price was decreased.
•The number of shares of common stock subject to each outstanding RSUs, PPSs, and PSAs was increased.
The adjustments to the stock-based compensation awards did not result in additional compensation cost.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Summary of TSRU and PTU information as of December 31, 2020(a), (b): |
| | TSRUs (Thousands) | | PTUs (Thousands) | | Weighted-Average
Grant Price
Per TSRU | | Weighted-Average Remaining Contractual Term (Years) | | Aggregate Intrinsic Value (Millions) |
| TSRUs Outstanding | | 230,539 | | | — | | | $ | 29.57 | | | 2.3 | | $ | 1,737 | |
| TSRUs Vested | | 100,696 | | | — | | | 25.22 | | | 0.8 | | 1,168 | |
TSRUs Expected to vest(c) | | 124,594 | | | — | | | 32.94 | | | 3.3 | | 547 | |
| TSRUs exercised and converted to PTUs | | — | | | 1,467 | | | $ | — | | | 0.3 | | $ | 54 | |
(a)In 2020, we settled 5,478,547 TSRUs with a weighted-average grant price of $30.93 per unit.
(b)In 2020, 2,217,044 TSRUs with a weighted-average grant price of $29.26 per unit were converted into 757,285 PTUs.
(c)The number of TSRUs expected to vest takes into account an estimate of expected forfeitures.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 9593 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Summary of all stock option activity during 2020: |
| | Shares (Thousands) | | Weighted-Average
Exercise Price
Per Share | | Weighted-Average Remaining Contractual Term (Years) | | Aggregate Intrinsic Value(a) (Millions) |
Outstanding, December 31, 2019(b) | | 88,600 | | | $ | 28.39 | | | | | |
Granted(b) | | 1,755 | | | 34.10 | | | | | |
Exercised(b) | | (18,492) | | | 23.05 | | | | | |
Forfeited(b) | | (160) | | | 35.49 | | | | | |
Expired(b) | | (326) | | | 24.91 | | | | | |
Upjohn spin-off adjustment(c) | | 4,024 | | | 28.08 | | | | | |
| Outstanding, December 31, 2020 | | 75,402 | | | 28.31 | | | 3.1 | | $ | 645 | |
Vested and expected to vest, December 31, 2020(d) | | 75,226 | | | 28.30 | | | 3.0 | | 645 | |
| Exercisable, December 31, 2020 | | 71,732 | | | $ | 27.97 | | | 2.8 | | $ | 635 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Summary of TSRU and PTU information as of December 31, 2023(a), (b): |
| | TSRUs (Thousands) | | PTUs (Thousands) | | Weighted-Average
Grant Price
Per TSRU | | Weighted-Average Remaining Contractual Term (Years) | | Aggregate Intrinsic Value(c) (Millions) |
| TSRUs Outstanding | | 163,572 | | | | | $ | 36.83 | | | 2.0 | | $ | 131 | |
| TSRUs Vested | | 85,899 | | | | | 34.05 | | | 0.8 | | 131 | |
TSRUs Expected to vest(d) | | 75,276 | | | | | $ | 39.82 | | | 3.2 | | — | |
| Outstanding PTUs converted from TSRUs exercised | | | | 1,060 | | | | | 0.6 | | $ | 31 | |
(a)In 2023, we settled 38,957,175 TSRUs with a weighted-average grant price of $29.80 per unit.
(b)In 2023, 1,827,019 TSRUs with a weighted-average grant price of $31.73 per unit were converted into 679,742 PTUs.
(c)Market price of our underlying common stock less exercise price.
(d)The number of TSRUs expected to vest takes into account an estimate of expected forfeitures.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Summary of all stock option activity during 2023: |
| | Shares (Thousands) | | Weighted-Average
Exercise Price
Per Share | | Weighted-Average Remaining Contractual Term (Years) | | Aggregate Intrinsic Value(a) (Millions) |
| Outstanding, December 31, 2022 | | 35,280 | | | $ | 31.47 | | | | | |
| Granted | | 635 | | | 42.30 | | | | | |
| Exercised | | (6,709) | | | 27.47 | | | | | |
| Forfeited | | (36) | | | 39.37 | | | | | |
| Expired | | (718) | | | 31.25 | | | | | |
| Outstanding, December 31, 2023 | | 28,452 | | | 32.66 | | | 1.7 | | $ | — | |
Vested and expected to vest, December 31, 2023(b) | | 28,385 | | | 32.63 | | | 1.7 | | — | |
| Exercisable, December 31, 2023 | | 26,667 | | | $ | 32.19 | | | 1.3 | | $ | — | |
(a)Market price of our underlying common stock less exercise price.
(b)Activity prior to the Upjohn Business spin-off has not been adjusted.
(c)In connection with the Upjohn business spin-off discussed above, the number of shares of common stock subject to each outstanding stock option was increased and the exercise price was decreased. These adjustments did not result in additional compensation cost.
(d)The number of options expected to vest takes into account an estimate of expected forfeitures.
Note 14. Earnings Per Common Share Attributable to Pfizer Inc. Common Shareholders
| | | | | | | | | | | | | | | | | | | | |
The following presents the detailed calculation of EPS: |
| | | Year Ended December 31, |
| (IN MILLIONS) | | 2020 | | 2019 | | 2018 |
| EPS Numerator––Basic | | | | | | |
| Income from continuing operations attributable to Pfizer Inc. | | $ | 6,985 | | | $ | 10,838 | | | $ | 3,825 | |
| Less: Preferred stock dividends––net of tax | | 0 | | | 1 | | | 1 | |
| Income from continuing operations attributable to Pfizer Inc. common shareholders | | 6,984 | | | 10,837 | | | 3,824 | |
| | | | | | |
| | | | | | |
| Income from discontinued operations––net of tax | | 2,631 | | | 5,435 | | | 7,328 | |
| Net income attributable to Pfizer Inc. common shareholders | | $ | 9,616 | | | $ | 16,272 | | | $ | 11,152 | |
| EPS Numerator––Diluted | | | | | | |
| Income from continuing operations attributable to Pfizer Inc. common shareholders and assumed conversions | | $ | 6,985 | | | $ | 10,838 | | | $ | 3,825 | |
| Income from discontinued operations––net of tax, attributable to Pfizer Inc. common shareholders and assumed conversions | | 2,631 | | | 5,435 | | | 7,328 | |
| Net income attributable to Pfizer Inc. common shareholders and assumed conversions | | $ | 9,616 | | | $ | 16,273 | | | $ | 11,153 | |
| EPS Denominator | | | | | | |
| Weighted-average number of common shares outstanding––Basic | | 5,555 | | | 5,569 | | | 5,872 | |
| Common-share equivalents: stock options, stock issuable under employee compensation plans convertible preferred stock and accelerated share repurchase agreements | | 77 | | | 106 | | | 105 | |
| Weighted-average number of common shares outstanding––Diluted | | 5,632 | | | 5,675 | | | 5,977 | |
Anti-dilutive common stock equivalents(a) | | 4 | | | 2 | | | 2 | |
| | | | | | | | | | | | | | | | | | | | |
The following presents the detailed calculation of EPS: |
| | | Year Ended December 31, |
| (IN MILLIONS) | | 2023 | | 2022 | | 2021 |
| EPS Numerator | | | | | | |
| | | | | | |
| | | | | | |
| Income from continuing operations attributable to Pfizer Inc. common shareholders | | $ | 2,134 | | | $ | 31,366 | | | $ | 22,414 | |
| | | | | | |
| | | | | | |
| Discontinued operations––net of tax | | (15) | | | 6 | | | (434) | |
| Net income attributable to Pfizer Inc. common shareholders | | $ | 2,119 | | | $ | 31,372 | | | $ | 21,979 | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| EPS Denominator | | | | | | |
| Weighted-average number of common shares outstanding––Basic | | 5,643 | | | 5,608 | | | 5,601 | |
| Common-share equivalents | | 66 | | | 125 | | | 107 | |
| Weighted-average number of common shares outstanding––Diluted | | 5,709 | | | 5,733 | | | 5,708 | |
Anti-dilutive common stock equivalents(a) | | 9 | | | 1 | | | 2 | |
(a)These common stock equivalents were outstanding for the periods presented, but were not included in the computation of diluted EPS for those periods because their inclusion would have had an anti-dilutive effect.
Allocated shares held by the Common ESOP, including reinvested dividends, are considered outstanding for EPS calculations and the eventual conversion of allocated preferred shares held by the Preferred ESOP was assumed in the diluted EPS calculation until the conversion date, which occurred in May 2020. See Note 12.
Note 15. Leases
We lease real estate, fleet, and equipment for use in our operations. Our leases generally have lease terms of 1 to 30 years, some of which include options to terminate or extend leases for up to 5 to 10 years or on a month-to-month basis. We include options that are reasonably certain to be exercised as part of the determination of lease terms. We may negotiate termination clauses in anticipation of any changes in market conditions, but generally these termination options have not been exercised. Residual value guarantees are generally not included within our operating leases with the exception of some fleet leases. In addition to base rent payments, the leases may require us to pay directly for taxes and other non-lease components, such as insurance, maintenance and other operating expenses, which may be dependent on usage or vary month-to-month. Variable lease payments amounted to $380$444 million in 2020 and $3272023, $536 million in 2019.2022 and $381 million in 2021. We elected the practical expedient in the new standard to not separate non-lease components from lease components in calculating the amounts of ROU assets and lease liabilities for all underlying asset classes.
We determine if an arrangement is a lease at inception of the contract and we perform the lease classification test as of the lease commencement date. ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 9694 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
based on the present value of lease payments over the lease term. As most of our leases do not provide an implicit rate, we use our estimated incremental borrowing rate based on the information available at commencement date in determining the present value of future payments.
| | For operating leases, the ROU assets and liabilities in our consolidated balance sheets follows: | For operating leases, the ROU assets and liabilities in our consolidated balance sheets follows: | For operating leases, the ROU assets and liabilities in our consolidated balance sheets follows: |
| As of December 31, |
| (MILLIONS OF DOLLARS) | | Balance Sheet Classification | | 2020 | | 2019 |
| | | | As of December 31, | | | | | | As of December 31, |
| (MILLIONS) | | (MILLIONS) | | Balance Sheet Classification | | 2023 | | 2022 |
| ROU assets | ROU assets | | Other noncurrent assets | | $ | 1,393 | | | $ | 1,289 | |
| Lease liabilities (short-term) | Lease liabilities (short-term) | | Other current liabilities | | 321 | | | 269 | |
| Lease liabilities (long-term) | Lease liabilities (long-term) | | Other noncurrent liabilities | | 1,114 | | | 1,030 | |
| | Components of total lease cost includes: | Components of total lease cost includes: | Components of total lease cost includes: |
| Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | 2020 | | 2019 |
| | Year Ended December 31, | | | | Year Ended December 31, |
| (MILLIONS) | | (MILLIONS) | | 2023 | | 2022 | | 2021 |
| Operating lease cost | Operating lease cost | | $ | 433 | | | $ | 422 | |
| | Variable lease cost | Variable lease cost | | 380 | | | 327 | |
| | Variable lease cost | |
| | Variable lease cost | |
| Sublease income | Sublease income | | (40) | | | (45) | |
| Total lease cost | Total lease cost | | $ | 773 | | | $ | 704 | |
| | | | | | | | | | | | | | | | | | | | |
| Other supplemental information for 2020 follows: |
| | Weighted-Average Remaining Contractual Lease Term (Years) | | Weighted-Average Discount Rate | | |
| (MILLIONS OF DOLLARS) | | As of December 31, 2020 | | Year Ended December 31, 2020 |
| Operating leases | | 6.9 | | 2.9 | % | | |
| | | | | | |
| | | | | | |
| | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | |
| | | | | | |
| Operating cash flows from operating leases | | | | | | $ | 334 | |
| | | | | | |
| (Gains)/losses on sale and leaseback transactions, net | | | | | | (3) | |
| ROU assets obtained in exchange for new operating lease liabilities | | | | | | 413 | |
| | | | | | | | | | | | | | |
| Other supplemental information follows: |
| | As of December 31, |
| (MILLIONS) | | 2023 | | 2022 |
| Operating leases | | | | |
| Weighted-Average Remaining Contractual Lease Term (Years) | | 10.8 | | 11 |
| Weighted-Average Discount Rate | | 3.8 | % | | 3.0 | % |
| | | | | | | | | | | | | | | | | | | | |
| Other supplemental information for 2019 follows: |
| | Weighted-Average Remaining Contractual Lease Term (Years) | | Weighted-Average Discount Rate | | |
| (MILLIONS OF DOLLARS) | | As of December 31, 2019 | | Year Ended December 31, 2019 |
| Operating leases | | 6.9 | | 3.5 | % | | |
| | | | | | |
| | | | | | |
| | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | |
| | | | | | |
| Operating cash flows from operating leases | | | | | | $ | 339 | |
| | | | | | |
| (Gains)/losses on sale and leaseback transactions, net | | | | | | (29) | |
| ROU assets obtained in exchange for new operating lease liabilities | | | | | | 318 | |
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (MILLIONS) | | 2023 | | 2022 | | 2021 |
| | | | | | |
| | | | | | |
| | | | | | |
| Cash paid for amounts included in the measurement of lease liabilities: | | | | | | |
| | | | | | |
| Operating cash flows from operating leases | | $ | 744 | | | $ | 617 | | | $ | 387 | |
| | | | | | |
| (Gains)/losses on sale and leaseback transactions, net | | (49) | | | 11 | | | 1 | |
| | | | | | | | |
The following reconciles the undiscounted cash flows for the first five years and total of the remaining years to the operating lease liabilities recorded in the consolidated balance sheet as of December 31, 2020:2023: |
(MILLIONS OF DOLLARS)(MILLIONS) | | |
| Period | | Operating Lease Liabilities |
Next one year(a) | | $ | 357639 | |
| 1-2 years | | 299474 | |
| 2-3 years | | 250387 | |
| 3-4 years | | 167319 | |
| 4-5 years | | 137262 | |
| Thereafter | | 4081,743 | |
| Total undiscounted lease payments | | 1,6183,824 | |
| Less: Imputed interest | | 183671 | |
| Present value of minimum lease payments | | 1,4353,153 | |
| Less: Current portion | | 321527 | |
| Noncurrent portion | | $ | 1,1142,626 | |
(a)Reflects lease payments due within 12 months subsequent to the balance sheet date.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 97 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
In April 2018, we entered an agreement to lease space in an office building in New York City. We expect to take control of the property in 2021 and relocate our global headquarters to this new office building in 2022. Our future minimum rental commitment under this 20-year lease is approximately $1.6 billion.
Prior to our adoption of the new lease standard, rental expense, net of sublease income, was $301 million in 2018.
Note 16. Contingencies and Certain Commitments
We and certain of our subsidiaries are subject to numerous contingencies arising in the ordinary course of business, including tax and legal contingencies.contingencies, guarantees and indemnifications. The following outlines our legal contingencies.contingencies, guarantees and indemnifications. For a discussion of our tax contingencies, see Note 5B.5D
A. Legal Proceedings
Our legal contingencies include, but are not limited to, the following:
•Patent litigation, which typically involves challenges to the coverage and/or validity of patents on various products, processes or dosage forms. An adverse outcome could result in loss of patent protection for a product, a significant loss of revenues from a product or impairment of the value of associated assets. We are the plaintiff in the majority of these actions. An adverse outcome in actions in which we are the plaintiff could result in loss of patent protection for a drug, a significant loss of revenues from that drug or impairment of the value of associated assets.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 95 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
•Product liability and other product-related litigation related to current or former products, which can include personal injury, consumer, off-label promotion, securities, antitrust and breach of contract claims, among others, and often involves highly complex issues relating to medical causation, label warnings and reliance on those warnings, scientific evidence and findings, actual, provable injury and other matters.
•Commercial and other asserted or unasserted matters, which can include acquisition-, licensing-, intellectual property-, collaboration- or co-promotion-related and product-pricing claims and environmental claims and proceedings, and can involve complexities that will vary from matter to matter.
•Government investigations, which often are related to the extensive regulation of pharmaceutical companies by national, state and local government agencies in the U.S. and in other jurisdictions.
Certain of these contingencies could result in increased expenses and/or losses, including damages, royalty payments, fines and/or civil penalties, which could be substantial, and/or criminal charges.
We believe that our claims and defenses in matters in which we are a defendant are substantial, but litigation is inherently unpredictable and excessive verdicts do occur. We do not believe that any of these matters will have a material adverse effect on our financial position. However, we could incur judgments, enter into settlements or revise our expectations regarding the outcome of matters, which could have a material adverse effect on our results of operations and/or our cash flows in the period in which the amounts are accrued or paid.
We have accrued for losses that are both probable and reasonably estimable. Substantially all of our contingencies are subject to significant uncertainties and, therefore, determining the likelihood of a loss and/or the measurement of any loss can be complex. Consequently, we are unable to estimate the range of reasonably possible loss in excess of amounts accrued. Our assessments, which result from a complex series of judgments about future events and uncertainties, are based on estimates and assumptions that have been deemed reasonable by management, but that may prove to be incomplete or inaccurate, and unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions.
Amounts recorded for legal and environmental contingencies can result from a complex series of judgments about future events and uncertainties and can rely heavily on estimates and assumptions. In August 2020, the SEC amended its disclosure rules regarding the threshold for disclosure ofFor proceedings under environmental laws to which a governmental authority is a party. In accordance with the amended rule,party, we have adopted a disclosure threshold for such proceedings of $1 million in potential or actual governmental monetary sanctions.
The principal pending matters to which we are a party are discussed below. In determining whether a pending matter is a principal matter, we consider both quantitative and qualitative factors to assess materiality, such as, among others, the amount of damages and the nature of other relief sought, if specified; our view of the merits of the claims and of the strength of our defenses; whether the action purports to be, or is, a class action and, if not certified, our view of the likelihood that a class will be certified by the court; the jurisdiction in which the proceeding is pending; whether related actions have been transferred to multidistrict litigation; any experience that we or, to our knowledge, other companies have had in similar proceedings; whether disclosure of the action would be important to a reader of our financial statements, including whether disclosure might change a reader’s judgment about our financial statements in light of all of the information that is available to the reader; the potential impact of the proceeding on our reputation; and the extent of public interest in the matter. In addition, with respect to patent matters in which we are the plaintiff, we consider, among other things, the financial significance of the product protected by the patent(s) at issue. Some of the matters discussed below include those which management believes that the likelihood of possible loss in excess of amounts accrued is remote.
A1. Legal Proceedings––Patent Litigation
We are involved in suits relating to our patents (or those of our collaboration/licensing partners to which we have licenses or co-promotion rights), including but not limited to, those discussed below. Most involveWe face claims by generic drug manufacturers that patents covering our products (or those of our collaboration/licensing partners to which we have licenses or co-promotion rights and to which we may or may not be a party), processes or dosage forms are invalid and/or do not cover the product of the generic drug manufacturer. Also, counterclaims, as well as various independent actions, have been filed alleging that our assertions of, or attempts to enforce, patent rights with respect to certain products constitute unfair competition and/or violations of antitrust laws. In addition to the challenges to the U.S. patents that are discussed below, patent rights to certain of our products or those of our collaboration/licensing partners are being challenged in various other jurisdictions. Some of our collaboration or licensing partners face challenges to the validity of their patent rights in non-U.S. jurisdictions. For example, in April 2022, the U.K. High Court issued a judgment finding invalid a BMS patent related to Eliquis due to expire in 2026. In May 2023, the Court of Appeal dismissed BMS’s appeal and in October 2023, the Supreme Court refused BMS’s permission to appeal. Additional challenges are pending in other jurisdictions. Also, in July 2022, CureVac AG (CureVac) brought a patent infringement action against BioNTech and certain of its subsidiaries in the German Regional Court alleging that Comirnaty infringes certain German utility model patents and certain expired and unexpired European patents. Additional challenges involving Comirnaty patents may be filed against us and/or BioNTech in other jurisdictions in the future. Adverse decisions in these matters could have a material adverse effect on our results of operations.We are also party to patent damages suits in various jurisdictions pursuant to which generic drug manufacturers, payers,payors, governments or other parties are seeking damages from us for allegedly causing delay of generic entry. Additionally, our licensing and collaboration partners face challenges by generic drug manufacturers to patents covering products for which we have licenses or co-promotion rights.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 98 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
We also are often involved in other proceedings, such as inter partes review, post-grant review, re-examination or opposition proceedings, before the U.S. Patent and Trademark Office, the European Patent Office, or other foreign counterparts, as well as court proceedings relating to our intellectual property or the intellectual property rights of others.others, including challenges to such rights initiated by us. Also, if one of our patents (or one of our collaboration/licensing partner’s patents) is found to be invalid by such proceedings, generic or competitive products could be introduced into the market resulting in the erosion of sales of our existing products. For example, several of the patents in our pneumococcal vaccine portfolio werehave been challenged in inter partes review and post-grant review proceedings in the U.S. In 2017, the Patent Trial and Appeal Board (PTAB) initiated proceedings, which remain pending, with respect to 2 of our pneumococcal vaccine patents. However, the PTAB declined to initiate proceedingsTrademark Office, as to two other pneumococcal vaccine patents. Various legal challenges to other pneumococcal vaccine patents remain pending in jurisdictionswell as outside the U.S.The invalidation of allany of the patents in our pneumococcal portfolio could potentially allow a competitor’s pneumococcal vaccine intoadditional competitor vaccines, if approved, to enter the marketplace.marketplace earlier than anticipated. In the event that any of the patents are found valid and infringed, a competitor’s pneumococcal vaccine, if approved, might be prohibited from entering the market or a competitor might be required to pay us a royalty.
We are also subject to patent litigation pursuant to which one or more third parties seek damages and/or injunctive relief to compensate for alleged infringement of its patents by our commercial or other activities. For example, our Hospira subsidiaries are involved in patent disputes over their attempts to bring generic pharmaceutical and biosimilar products to market. If one of our marketed products (or a product of our collaboration/
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 96 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
licensing partners to which we have licenses or co-promotion rights) is found to infringe valid patent rights of a third party, such third party may be awarded significant damages or royalty payments, or we may be prevented from further sales of that product. Such damages may be enhanced as much as three-fold if we or one of our subsidiaries is found to have willfully infringed valid patent rights of a third party.
Actions In Which We Are The Plaintiff
EpiPen
In 2010, King, which we acquired in 2011 and is a wholly-owned subsidiary, brought a patent-infringement action against Sandoz in the U.S. District Court for the District of New Jersey in connection with Sandoz’s abbreviated new drug application (ANDA) filed with the FDA seeking approval to market an epinephrine injectable product. Sandoz is challenging patents, which expire in 2025, covering the next-generation autoinjector for use with epinephrine that is sold under the EpiPen brand name.
Xeljanz (tofacitinib)
Beginning in 2017, we brought patent-infringement actions against several generic manufacturers that filed separate ANDAsabbreviated new drug applications (ANDAs) with the FDA seeking approval to market their generic versions of tofacitinib tablets in one or both of 5 mg and 10 mg dosage strengths, and in both immediate and extended release forms. To date, we have settled actions with several generic manufacturers on terms not material to Pfizer.us. The remaining actions continue in the U.S. District Court for the District of Delaware as described below.
In 2017,October 2021, we brought a separate patent-infringement action against Zydus Pharmaceuticals (USA)Sinotherapeutics Inc. and Cadila Healthcare Ltd. (collectively, Zydus)(Sinotherapeutics) asserting the infringement and validity of 3 patents: theour patent covering the active ingredient expiring in December 2025 (the 2025 Patent), the patent covering an enantiomerextended release formulations of tofacitinib expiring in 2022, and the patent covering a polymorphic form of tofacitinib expiring in 2023 (the 2023 Patent), which Zydusthat was challenged by Sinotherapeutics in its ANDA seeking approval to market a generic version of tofacitinib 511 mg extended release tablets. In November 2020,2022, we settled the case against Zydus on terms not material to Pfizer. In February 2021, we brought a separatefiled an additional patent-infringement action against Zydus asserting the infringement and validitySinotherapeutics relating to its challenge of our compositionextended release formulation and method of matter and crystalline formtreatment patents challenged by Zydus in its ANDA seeking approval to market a generic version of tofacitinib 22 mg extended release tablets.
In 2018,June 2023, we brought a separate patent infringementpatent-infringement action against Teva PharmaceuticalsAurobindo Pharma Limited and Aurobindo Pharma USA, Inc. (Teva)(collectively Aurobindo) asserting the infringement and validity of our basic compound patent, covering extended release formulations of tofacitinib that was challenged by Teva in itsconnection with Aurobindo’s ANDA seeking approval to market a generic version of tofacitinib 11 mg extended release tablets.
In January 2021,December 2023, we broughtreached a separate patent-infringement action againstsettlement agreement with Aurobindo Pharma Limited (Aurobindo) assertingon terms not material to the infringement and validity of the 2025 Patent and the 2023 Patent, which Aurobindo challenged in its ANDA seeking approval to market a generic version of tofacitinib 5 mg and 10 mg tablets.
Inlyta (axitinib)
In 2019, Glenmark Pharmaceuticals Limited (Glenmark) notified us that it had filed an ANDA with the FDA seeking approval to market a generic version of Inlyta. Glenmark asserts the invalidity and non-infringement of the crystalline form patent for Inlyta that expires in 2030. In June 2019, we filed suit against Glenmark in the U.S. District Court for the District of Delaware, asserting the validity and infringement of the crystalline form patent for Inlyta.Company.
Ibrance (palbociclib)
In March 2019,Beginning in January 2021, several generic companies notified us that they had filed ANDAs with the FDA seeking approval to market generic versions of Ibrance.Ibrance tablets. We have settled with one of these generic companies on terms not material to us, and have dismissed the patent infringement actions against all other generic companies except for the action against Synthon Pharmaceuticals Inc. and its affiliated entities (collectively, Synthon), in which we have asserted the infringement and validity of the composition of matter patent, expiring in 2027. In December 2023, we reached a settlement agreement with Synthon on terms not material to the Company.
Mektovi (binimetinib)
Beginning in August 2022, several generic companies notified us that they had filed ANDAs with the FDA seeking approval to market generic versions of Mektovi. The generic companies assert the invalidity and non-infringement of 2 compositiontwo method of matteruse patents one of which expiresexpiring in 2023 and one of which expires in 2027, as a result of a U.S. Patent Term Extension certificate issued in January 2021, and2030, a method of use patent covering palbociclib, which expiresexpiring in 2023. In April 2019,2031, two method of use patents expiring in 2033, and a product by process patent expiring in 2033. Beginning in September 2022, we brought patent infringement actions against each of the generic filers in various federal courts,the U.S. District Court for the District of Delaware, asserting the validity and infringement of all six patents.
In August 2022 we received notice from Teva Pharmaceuticals, Inc. (Teva) that it had filed an ANDA seeking approval to market a generic version of Mektovi. Teva asserts the invalidity and non-infringement of two method of use patents expiring in 2033 and a product by process patent expiring in 2033. In June 2023, we brought a patent infringement action against Teva in the U.S. District Court for the District of Delaware, asserting the validity and infringement of the three patents.
Vyndaqel-Vyndamax(tafamidis/tafamidis meglumine)
Beginning in June 2023, several generic companies notified us that they had filed ANDAs with the FDA seeking approval to market generic versions of tafamidis capsules (61 mg) or tafamidis meglumine capsules (20 mg), challenging some or all of the patents listed in the FDA’s Orange Book for Vyndamax (tafamidis) and Vyndaqel (tafamidis meglumine). Scripps Research Institute (Scripps) owns the composition of matter patent and the method of treatment patents covering the products, and Pfizer is the exclusive licensee. Pfizer separately owns the crystalline form patent. Beginning in August 2023, we and Scripps brought patent infringement actions against the generic filers in the U.S. District Court for the District of Delaware, asserting the validity and infringement of the patents challenged byin suit. Pfizer is the generic companies. Beginningsole plaintiff in September 2020, we received correspondence from several generic companies notifying usactions that they would seek approval to market generic versions of Ibrance. The generic companies assert only the invalidityinfringement and non-infringement of our crystalline form patent which expires in 2034. Beginning in October 2020, we brought patent infringement actions against each of these generic companies in various federal courts, asserting the validity and infringement of the crystalline form patent.
Lyrica (pregabalin)Actions in Which We are the Defendant
•U.K.Comirnaty
In June 2014, Generics (U.K.) Ltd (tradingMarch 2022, Alnylam Pharmaceuticals, Inc. (Alnylam) filed a complaint in the U.S. District Court for the District of Delaware against Pfizer and Pharmacia & Upjohn Company LLC, our wholly owned subsidiary, alleging that Comirnaty infringes a U.S. patent issued in February 2022, and seeking unspecified monetary damages. In July 2022, Alnylam filed a second complaint in the U.S. District Court for the District of Delaware against Pfizer, Pharmacia & Upjohn Company LLC, BioNTech and BioNTech Manufacturing GmbH, alleging that Comirnaty infringes a U.S. patent issued in July 2022, and seeking unspecified monetary damages. In May 2023, Alnylam filed a separate complaint in the U.S. District Court for the District of Delaware against Pfizer and Pharmacia & Upjohn Company LLC alleging that Comirnaty infringes four additional U.S. patents issued on various dates in 2023 and seeking unspecified monetary damages.
In August 2022, ModernaTX, Inc. (ModernaTX) and Moderna US, Inc. (Moderna) sued Pfizer, BioNTech, BioNTech Manufacturing GmbH and BioNTech US Inc. in the U.S. District Court for the District of Massachusetts, alleging that Comirnaty infringes three U.S. patents. In its complaint, Moderna stated that it is seeking damages for alleged infringement occurring after March 7, 2022.
In August 2022, ModernaTX filed a patent infringement action in Germany against Pfizer and certain subsidiary companies, as Mylan)well as BioNTech and certain subsidiary companies, alleging that Comirnaty infringes two European patents. The German infringement action was stayed in December 2023 pending further action from the European Patent Office on the patents at issue. In September 2022, ModernaTX filed patent infringement actions in the U.K. and in the Netherlands against Pfizer and certain subsidiary companies, as well as BioNTech and certain subsidiary companies, on the same two European patents. In its complaints, ModernaTX stated that it is seeking damages for alleged infringement occurring after March 7, 2022. In the U.K., Pfizer and BioNTech brought an invalidity action against ModernaTX seeking to revoke these two European patents, which was consolidated with the Lyrica pain useSeptember 2022 action filed by ModernaTX. In November 2023, one of the European patents was revoked by the European Patent Office. In December 2023, the other European patent was declared invalid by a court in the High Court of Justice in London. Subsequently, Actavis Group PTC ehf filed an invalidity action in the same court, and Pfizer sued Actavis Group PTC ehf, Actavis U.K. Ltd and Caduceus Pharma Ltd (together, Actavis) for infringement and requested preliminary relief. Our request for preliminary relief was denied in a January 2015 hearing, and the denial subsequently was confirmed on appeal.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 9997 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
In February 2015,Netherlands (the invalidity decision is limited to the National Health Service (NHS) England was ordered by the High Court, as an intermediary, to issue guidance for prescribers and pharmacists directing the prescription and dispensing of Lyrica by brand when pregabalin was prescribed for the treatment of neuropathic pain. NHS Wales and NHS Northern IrelandNetherlands). ModernaTX has also issued prescribing guidance. The guidance to prescribe and dispense Lyrica for neuropathic pain was withdrawn uponfiled additional patent expiration in July 2017.
We also filed infringement actions against (i) Teva UK Ltd,Pfizer and (ii) Dr. Reddy’s Laboratories (UK) LtdBioNTech in certain other ex-U.S. jurisdictions.
In April 2023, Arbutus Biopharma Corporation (Arbutus) and Caduceus Pharma Ltd (together, Dr. Reddy’s) in February 2015, seeking the same relief asGenevant Sciences GmbH (Genevant) filed a complaint in the actionU.S. District Court for the District of New Jersey against Actavis. Dr. Reddy’sPfizer and BioNTech alleging that Comirnaty and its manufacture infringe five U.S. patents, and seeking unspecified monetary damages.
Paxlovid
In June 2022, Enanta Pharmaceuticals, Inc. filed an invalidity counterclaim. These actions were stayed pending the outcome of the Mylan and Actavis cases.
The Mylan and Actavis invalidity actions were hearda complaint in the HighU.S. District Court atfor the same time as the Actavis infringement action. The High Court ruledDistrict of Massachusetts against us, holdingPfizer alleging that the asserted claims were either not infringed or invalid,active ingredient in Paxlovid, nirmatrelvir, infringes a U.S. patent issued in June 2022, and appeals followed. In November 2018, the U.K. Supreme Court ruled that all the relevant claims directed to neuropathic pain were invalid.seeking unspecified monetary damages.
Abrysvo
In October 2015, after Sandoz GmbHAugust 2023, GlaxoSmithKline Biologics SA and Sandoz Ltd (together, Sandoz) launched a full label generic pregabalin product, we obtained from the High Court a preliminary injunction enjoining Sandoz from further sales of the product and ordering Sandoz to identify the parties holding its product. Sandoz identified wholesaler AAH Pharmaceuticals Ltd and pharmacy chain Lloyds Pharmacy Ltd (supplied by AAH), and we requested that these parties cease further sales and withdraw the Sandoz full label product. In October 2015, Lloyds was added to the Sandoz action, and we obtained a preliminary order from the High Court requiring Lloyds to advise its pharmacists that the Sandoz full label product should not be dispensed. In November 2015, the High Court confirmed the preliminary injunction against Sandoz and Lloyds. Sandoz filed an invalidity counterclaim. Upon agreement of the parties, in December 2015, the proceedings against Lloyds were discontinued, and the proceedings against Sandoz were stayed pending outcome of the Mylan and Actavis cases. The preliminary injunction against Sandoz remained in place until patent expiration in July 2017.
In May 2020, Dr. Reddy’sGlaxoSmithKline LLC (collectively, GSK Group) filed a claim for damages in connection with the above-referenced legal actions. In July 2020, the Scottish Ministers and 14 Scottish Health Boards (together, NHS Scotland) filed a claim for damages in connection with the above-referenced legal action concerning Sandoz. In September 2020, Teva, Sandoz, Ranbaxy, Inc. (Ranbaxy), Actavis, and the Secretary of State for Health and Social Care, together with 32 other National Health Service entities (together, NHS England, Wales, and Northern Ireland) filed claims for damages in the above-referenced legal actions. In November 2020, we and Mylan completed the transaction to spin-off our Upjohn Business and combine it with Mylan to form Viatris. As part of the transaction, Viatris has agreed to assume, and to indemnify Pfizer for, liabilities arising out of this matter.
◦Japan
In January 2017, Sawai Pharmaceutical Company Limited (a Japanese generic company) (Sawai) filed an invalidation action against the Lyrica pain use patent in the Japanese Patent Office (JPO). Hexal AG has filed a separate invalidation action that was stayed pending the result of the Sawai action. Multiple parties were allowed to intervene in the Sawai case. In July 2020, the JPO recognized the validity of certain amended claims of the patent covering Lyrica. We are appealing the decision. In August 2020, the Japanese regulatory authority granted regulatory approval to multiple generic companies and we filed legal actions against the generic companies seeking preliminary and permanent injunctions to prevent infringement of our patent. In November 2020, we and Mylan completed the transaction to spin-off our Upjohn Business and combine it with Mylan to form Viatris. As part of the transaction, Viatris has agreed to assume, and to indemnify Pfizer for, liabilities arising out of this matter.
Matter Involving Our Collaboration/Licensing Partners
Eliquis
In February, March, and April 2017, NaN generic companies sent BMS Paragraph-IV certification letters informing BMS that they had filed ANDAs seeking approval of generic versions of Eliquis, challenging the validity and infringement of one or more of the 3 patents listed in the Orange Book for Eliquis. NaN of the patents expired in December 2019 and the remaining patents currently are set to expire in 2026 and 2031. Eliquis has been jointly developed and is being commercialized by BMS and Pfizer. In April 2017, BMS and Pfizer filed patent-infringement actions against all generic filerscomplaint in the U.S. District Court for the District of Delaware against Pfizer alleging that the active ingredient in Abrysvo infringes four U.S. patents. The complaint seeks unspecified monetary damages and a permanent injunction against sales of Abrysvo for use in adults over 60 years of age. In November 2023, GSK Group amended its complaint to assert infringement of two additional patents. In addition, we have challenged certain of GSK’s RSV vaccine patents in certain ex-U.S. jurisdictions, including the U.K., the Netherlands and Belgium, and GSK has asserted that Abrysvo infringes these patents.
Matters Involving Pfizer and its Collaboration/Licensing Partners
Comirnaty
In July 2022, Pfizer, BioNTech and BioNTech Manufacturing GmbH filed a declaratory judgment complaint against CureVac in the U.S. District Court for the District of West Virginia, asserting that eachMassachusetts seeking a judgment of non-infringement for three U.S. patents relating to Comirnaty. In May 2023, the generic companies’ proposed products would infringe each of the patent(s) that each generic filer challenged. Some generic filers challenged only the 2031 patent, some challenged both the 2031 and 2026 patent, and one generic company challenged all 3 patents. In August 2020,case was transferred to the U.S. District Court for the Eastern District of Delaware ruledVirginia. Also in May 2023, CureVac asserted that bothComirnaty infringes the 2026 patentthree patents that were the subject of our declaratory judgment complaint, and in May and July 2023, CureVac asserted that Comirnaty infringes a number of additional U.S. patents.
In the 2031 patent are valid and infringed by the proposed generic products. In August and September 2020, the generic filers appealed the District Court’s decision to the U.S. Court of Appeals for the Federal Circuit. Prior to the August 2020 ruling, we and BMS settled with certain of the generic companies on terms not material toU.K., Pfizer and weBioNTech have sued CureVac seeking a judgment of invalidity of several patents and BMS may settle with other generic companies in the future.CureVac has made certain infringement counterclaims.
A2. Legal Proceedings––Product Litigation
We are defendants in numerous cases, including but not limited to those discussed below, related to our pharmaceutical and other products. Plaintiffs in these cases seek damages and other relief on various grounds for alleged personal injury and economic loss.
Asbestos
Between 1967 and 1982, Warner-Lambert owned American Optical Corporation (American Optical), which manufactured and sold respiratory protective devices and asbestos safety clothing. In connection with the sale of American Optical in 1982, Warner-Lambert agreed to indemnify the purchaser for certain liabilities, including certain asbestos-related and other claims. Warner-Lambert was acquired by Pfizer in 2000 and is a wholly owned subsidiary of Pfizer. Warner-Lambert is actively engaged in the defense of, and will continue to explore various means of resolving, these claims.
Numerous lawsuits against American Optical, Pfizer and certain of its previously owned subsidiaries are pending in various federal and state courts seeking damages for alleged personal injury from exposure to products allegedly containing asbestos and other allegedly hazardous materials sold by Pfizer and certain of its previously owned subsidiaries.
There also are a small number of lawsuits pending in various federal and state courts seeking damages for alleged exposure to asbestos in facilities owned or formerly owned by Pfizer or its subsidiaries.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 100 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Effexor
Beginning in May 2011, actions, including purported class actions, were filed in various federal courts against Wyeth and, in certain of the actions, affiliates of Wyeth and certain other defendants relating to Effexor XR, which is the extended-release formulation of Effexor. The plaintiffs in each of the class actions seek to represent a class consisting of all persons in the U.S. and its territories who directly purchased, indirectly purchased or reimbursed patients for the purchase of Effexor XR or generic Effexor XR from any of the defendants from June 14, 2008 until the time the defendants’ allegedly unlawful conduct ceased. The plaintiffs in all of the actions allege delay in the launch of generic Effexor XR in the U.S. and its territories, in violation of federal antitrust laws and, in certain of the actions, the antitrust, consumer protection and various other laws of certain states, as the result of Wyeth fraudulently obtaining and improperly listing certain patents for Effexor XR in the Orange Book, enforcing certain patents for Effexor XR and entering into a litigation settlement agreement with a generic drug manufacturer with respect to Effexor XR. Each of the plaintiffs seeks treble damages (for itself in the individual actions or on behalf of the putative class in the purported class actions) for alleged price overcharges for Effexor XR or generic Effexor XR in the U.S. and its territories since June 14, 2008. All of these actions have been consolidated in the U.S. District Court for the District of New Jersey.
In October 2014, the District Court dismissed the direct purchaser plaintiffs’ claims based on the litigation settlement agreement, but declined to dismiss the other direct purchaser plaintiff claims. In January 2015, the District Court entered partial final judgments as to all settlement agreement claims, including those asserted by direct purchasers and end-payerend-payor plaintiffs, which plaintiffs appealed to the U.S. Court of Appeals for the Third Circuit. In August 2017, the U.S. Court of Appeals for the Third Circuit reversed the District Court’s decisions and remanded the claims to the District Court.
Lipitor
•Antitrust Actions
Beginning in November 2011, purported class actions relating to Lipitor were filed in various federal courts against, among others, Pfizer, certain Pfizer affiliates, and, in most of the actions, Ranbaxy Laboratories Limited (Ranbaxy) and certain Ranbaxy affiliates. The plaintiffs in these various actions seek to represent nationwide, multi-state or statewide classes consisting of persons or entities who directly purchased, indirectly purchased or reimbursed patients for the purchase of Lipitor (or, in certain of the actions, generic Lipitor) from any of the defendants from March 2010 until the cessation of the defendants’ allegedly unlawful conduct (the Class Period). The plaintiffs allege delay in the launch of generic Lipitor, in violation of federal antitrust laws and/or state antitrust, consumer protection and various other laws, resulting from (i) the
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 98 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2008 agreement pursuant to which Pfizer and Ranbaxy settled certain patent litigation involving Lipitor and Pfizer granted Ranbaxy a license to sell a generic version of Lipitor in various markets beginning on varying dates, and (ii) in certain of the actions, the procurement and/or enforcement of certain patents for Lipitor. Each of the actions seeks, among other things, treble damages on behalf of the putative class for alleged price overcharges for Lipitor (or, in certain of the actions, generic Lipitor) during the Class Period. In addition, individual actions have been filed against Pfizer, Ranbaxy and certain of their affiliates, among others, that assert claims and seek relief for the plaintiffs that are substantially similar to the claims asserted and the relief sought in the purported class actions described above. These various actions have been consolidated for pre-trial proceedings in a Multi-District Litigation (In re Lipitor Antitrust Litigation MDL-2332)MDL in the U.S. District Court for the District of New Jersey.
In September 2013 and 2014, the District Court dismissed with prejudice the claims of the direct purchasers. In October and November 2014, the District Court dismissed with prejudice the claims of all other Multi-District LitigationMDL plaintiffs. All plaintiffs have appealed the District Court’s orders dismissing their claims with prejudice to the U.S. Court of Appeals for the Third Circuit. In addition, the direct purchaser class plaintiffs appealed the order denying their motion to amend the judgment and for leave to amend their complaint to the Court of Appeals. In August 2017, the Court of Appeals reversed the District Court’s decisions and remanded the claims to the District Court.
Also, in January 2013, the State of West Virginia filed an action in West Virginia state court against Pfizer and Ranbaxy, among others, that asserts claims and seeks relief on behalf of the State of West Virginia and residents of that state that are substantially similar to the claims asserted and the relief sought in the purported class actions described above.
•Personal Injury ActionsEpiPen (Direct Purchaser)
A number of individual and multi-plaintiff lawsuits have been filed against Pfizer in various federal and state courts alleging that the plaintiffs developed type 2 diabetes purportedly as a result of the ingestion of Lipitor. Plaintiffs seek compensatory and punitive damages.
In February 2014, the federal actions were transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re Lipitor (Atorvastatin Calcium) Marketing, Sales Practices and Products Liability Litigation (No. II) MDL-2502) in the U.S. District Court for the District of South Carolina. Since 2016, certain cases in the Multi-District Litigation were remanded to certain state courts. In January 2017, the District Court granted our motion for summary judgment, dismissing substantially all of the remaining cases pending in the Multi-District Litigation. In January 2017, the plaintiffs appealed the District Court’s decision to the U.S. Court of Appeals for the Fourth Circuit. In June 2018, the Court of Appeals affirmed the District Court’s decision. In November 2020, we and Mylan completed the transaction to spin-off our Upjohn Business and combine it with Mylan to form Viatris. As part of the transaction, Viatris has agreed to assume, and to indemnify Pfizer for, liabilities arising out of this matter.
Viagra
Since April 2016, a Multi-District Litigation has been pending in the U.S. District Court for the Northern District of California (In Re: Viagra (Sildenafil Citrate) Products Liability Litigation, MDL-2691), in which plaintiffs allege that they developed melanoma and/or the exacerbation of melanoma purportedly as a result of the ingestion of Viagra. Additional cases filed against Lilly with respect to Cialis have also been consolidated in the Multi-District Litigation (In re: Viagra (Sildenafil Citrate) and Cialis (Tadalafil) Products Liability Litigation, MDL-2691). In January 2020, the District Court granted our and Lilly’s motion to exclude all of plaintiffs’ general causation opinions. As a result, in April 2020, the District Court entered summary judgment in favor of defendants and dismissed all of plaintiffs’ claims. In April 2020, plaintiffs filed a notice of appeal in the U.S. Court of Appeals for the Ninth Circuit. In November 2020, we and Mylan completed the transaction to spin-off our Upjohn Business and combine it with Mylan to form Viatris. As part of the transaction, Viatris has agreed to assume, and to indemnify Pfizer for, liabilities arising out of this matter.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 101 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
EpiPen
Beginning in February 2017, purported class actions were filed in various federal courts by indirect purchasers of EpiPen against Pfizer, and/or its affiliates King and Meridian, and/or various entities affiliated with Mylan, and Mylan Chief Executive Officer, Heather Bresch. The plaintiffs in these actions seek to represent U.S. nationwide classes comprising persons or entities who paid for any portion of the end-user purchase price of an EpiPen between 2009 until the cessation of the defendants’ allegedly unlawful conduct. In February 2020, a similar lawsuit was filed in the U.S. District Court for the District of Kansas against Pfizer, its current and former affiliates King and Meridian, and thevarious Mylan entities, on behalf of a purported U.S. nationwide class of direct purchaser plaintiffs who purchased EpiPen devices directly from the defendants (the 2020 Lawsuit). Against Pfizer and/or its affiliates, plaintiffsdefendants. Plaintiffs in these actionsthis action generally allege that Pfizer’s and/or its affiliates’Pfizer and Mylan conspired to delay market entry of generic EpiPen through the settlement of patent litigation regarding EpiPen, and thereby delayed market entry of generic EpiPen in violation of federal antitrust laws and various state antitrust laws. At least 1 lawsuit also alleges that Pfizer and/or Mylan violated the federal Racketeer Influenced and Corrupt Organizations Act (RICO). Plaintiffs also filed various federal antitrust, state consumer protection and unjust enrichment claims against, and relating to conduct attributable solely to, Mylan and/or its affiliates regarding EpiPen.law. Plaintiffs seek treble damages for alleged overcharges for EpiPen since 2011. In July 2021, the District Court granted defendants’ motion to dismiss the direct purchaser complaint, without prejudice. In September 2021, plaintiffs filed an amended complaint. In August 2017, all2022, the District Court granted Pfizer’s motion to dismiss the complaint, and plaintiffs appealed to the U.S. Court of these actions, exceptAppeals for the 2020 Lawsuit, were consolidated for coordinated pre-trial proceedings in a Multi-District Litigation (Tenth Circuit. In re: EpiPen (Epinephrine Injection, USP) Marketing, Sales Practices and Antitrust Litigation, MDL-2785) inOctober 2023, the U.S. District Court forparties reached an agreement to settle the District of Kansas with other EpiPen-related actions against Mylan and/or its affiliateslitigation on terms not material to which Pfizer, King and Meridian are not parties.
In July 2020, a new lawsuit was filed in the U.S. District Court for the District of Colorado on behalf of indirect purchasers. Plaintiff represents a putative U.S. nationwide class of persons or entities who paid for any portion of the end-user purchase price of certain refill or replacement EpiPens since 2010. Plaintiff alleges that Pfizer and Meridian misrepresented the shelf-life and expiration date of EpiPen, in violation of the federal RICO statute. Plaintiff seeks treble damages for alleged unnecessary replacement or refill purchases of EpiPens by members of the putative class.
Nexium 24HR and Protonix
A number of individual and multi-plaintiff lawsuits have been filed against Pfizer, certain of its subsidiaries and/or other pharmaceutical manufacturers in various federal and state courts alleging that the plaintiffs developed kidney-related injuries purportedly as a result of the ingestion of certain proton pump inhibitors.Pfizer. The cases against Pfizer involve Protonix and/or Nexium 24HR and seek compensatory and punitive damages and, in some cases, treble damages, restitution or disgorgement. In August 2017, the federal actions were ordered transferred for coordinated pre-trial proceedingssettlement is subject to a Multi-District Litigation (In re: Proton-Pump Inhibitor Products Liability Litigation (No. II)) in the U.S. District Court for the District of New Jersey. In 2019, we and GSK combined our respective consumer healthcare businesses into a new Consumer Healthcare JV that operates globally under the GSK Consumer Healthcare name. As part of the JV transaction, the JV has agreed to assume, and to indemnify Pfizer for, liabilities arising out of such litigation to the extent related to Nexium 24HR.court approval.
Docetaxel
•Personal Injury Actions
A number of lawsuits have been filed against Hospira and Pfizer in various federal and state courts alleging that plaintiffs who were treated with Docetaxel developed permanent hair loss. The significant majority of the cases also name other defendants, including the manufacturer of the branded product, Taxotere. Plaintiffs seek compensatory and punitive damages. Additional lawsuits have been filed in which plaintiffs allege they developed blocked tear ducts following their treatment with Docetaxel.
In October 2016, the federal cases were transferred for coordinated pre-trial proceedings to a Multi-District Litigation (In re Taxotere (Docetaxel) Products Liability Litigation, MDL-2740)MDL in the U.S. District Court for the Eastern District of Louisiana. In 2022, the eye injury cases were transferred for coordinated pre-trial proceedings to a MDL in the U.S. District Court for the Eastern District of Louisiana.
•Mississippi Attorney General Government Action
In October 2018, the Attorney General of Mississippi filed a complaint in Mississippi state court against the manufacturer of the branded product and 8eight other manufacturers including Pfizer and Hospira, alleging, with respect to Pfizer and Hospira, a failure to warn about a risk of permanent hair loss in violation of the Mississippi Consumer Protection Act. The action seeks civil penalties and injunctive relief.
Array Securities Litigation
In November 2017, 2 purported class actions were filed in the U.S. District Court for the District of Colorado alleging that Array, which we acquired in July 2019 and is our wholly owned subsidiary, and certain of its former officers violated federal securities laws in connection with certain disclosures made, or omitted, by Array regarding the NRAS-mutant melanoma program. In March 2018, the actions were consolidated into a single proceeding.
Zantac
A number of lawsuits have been filed against Pfizer in various federal and state courts alleging that plaintiffs developed various types of cancer, or face an increased risk of developing cancer, purportedly as a result of the ingestion of Zantac. The significant majority of these cases also name other defendants that have historically manufactured and/or sold Zantac. Pfizer has not sold Zantac since 2006, and only sold an OTC version of the product. In 2006, Pfizer sold the consumer business that included its Zantac OTC rights to Johnson & Johnson and transferred the assets and liabilities related to Zantac OTC to Johnson & Johnson in connection with the sale. Plaintiffs in these cases seek compensatory and punitive damages and, in some cases, treble damages, restitution or disgorgement.damages.
In February 2020, the federal actions were transferred for coordinated pre-trial proceedings to a Multi-District Litigation (In re Zantac/Ranitidine NDMA Litigation, MDL-2924)MDL in the U.S. District Court for the Southern District of Florida. From June to December 2020: (i) plaintiffsFlorida (the Federal MDL Court). Plaintiffs in the Multi-District LitigationMDL filed against Pfizer and many other defendants a master personal injury complaint, a consolidated consumer class action complaint alleging, among other things, violations of the RICO statute andclaims under consumer protection statutes of all 50 states, and a consolidated third-party payor class actionmedical monitoring complaint alleging violationseeking to certify medical monitoring classes under the laws of 13 states. In December 2022, the RICO statuteFederal MDL Court granted defendants’ Daubert motions to exclude plaintiffs’ expert testimony and seeking reimbursementmotion for payments made forsummary judgment on general causation, which has resulted in the prescription versiondismissal of Zantac; (ii)all complaints in the litigation. Plaintiffs have appealed the Federal MDL Court’s rulings.
In addition, (i) Pfizer has received service of 2 Canadian class action complaints naming Pfizer and other defendants, and seeking compensatory and punitive damages for personal injury and economic loss, allegedly arising from the defendants’ sale of Zantac in Canada; (iii)and (ii) the State of New Mexico and the Mayor and City Council of Baltimore separately filed a civil actionactions against Pfizer and many other defendants in state courts, alleging various state statutory and common law claims in connection with the defendants’ alleged sale of Zantac in New Mexico; and (iv) Pfizer received servicethose jurisdictions. In April 2021, a Judicial Council Coordinated Proceeding was created in the Superior Court of a suit filed by the Mayor and City Council of Baltimore namingCalifornia in Alameda County to coordinate personal injury actions against Pfizer and other defendants alleging various claims under Maryland law.filed in California state court. Coordinated proceedings have also been created in other state courts. The large majority of the state court cases have been filed in the Superior Court of Delaware in New Castle County.
Many of these Zantac-related cases have been outstanding for a number of years and could take many more years to resolve. From time to time, Pfizer has explored and will continue to explore opportunistic settlements of these matters.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 10299 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Chantix
Beginning in August 2021, a number of putative class actions have been filed against Pfizer in various U.S. federal courts following Pfizer’s voluntary recall of Chantix due to the presence of a nitrosamine, N-nitroso-varenicline. Plaintiffs assert that they suffered economic harm purportedly as a result of purchasing Chantix or generic varenicline medicines sold by Pfizer. Plaintiffs seek to represent nationwide and state-specific classes and seek various remedies, including damages and medical monitoring. In December 2022, the federal actions were transferred for coordinated pre-trial proceedings to a MDL in the U.S. District Court for the Southern District of New York. Similar putative class actions have been filed in Canada and Israel, where the product brand is Champix.
A3. Legal Proceedings––Commercial and Other Matters
Monsanto-Related Matters
In 1997, Monsanto Company (Former Monsanto) contributed certain chemical manufacturing operations and facilities to a newly formed corporation, Solutia Inc. (Solutia), and spun off the shares of Solutia. In 2000, Former Monsanto merged with Pharmacia & Upjohn Company to form Pharmacia. Pharmacia then transferred its agricultural operations to a newly created subsidiary, named Monsanto Company (New Monsanto), which it spun off in a two-stage process that was completed in 2002. Pharmacia was acquired by Pfizer in 2003 and is a wholly owned subsidiary of Pfizer.
In connection with its spin-off that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities related to Pharmacia’s former agricultural business. New Monsanto has defended and/or is defending Pharmacia in connection with various claims and litigation arising out of, or related to, the agricultural business, and has been indemnifying Pharmacia when liability has been imposed or settlement has been reached regarding such claims and litigation.
In connection with its spin-off in 1997, Solutia assumed, and agreed to indemnify Pharmacia for, liabilities related to Former Monsanto’s chemical businesses. As the result of its reorganization under Chapter 11 of the U.S. Bankruptcy Code, Solutia’s indemnification obligations relating to Former Monsanto’s chemical businesses are primarily limited to sites that Solutia has owned or operated. In addition, in connection with its spin-off that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities primarily related to Former Monsanto’s chemical businesses, including, but not limited to, any such liabilities that Solutia assumed. Solutia’s and New Monsanto’s assumption of, and agreement to indemnify Pharmacia for, these liabilities apply to pending actions and any future actions related to Former Monsanto’s chemical businesses in which Pharmacia is named as a defendant, including, without limitation, actions asserting environmental claims, including alleged exposure to polychlorinated biphenyls. Solutia and/or New Monsanto are defending Pharmacia in connection with various claims and litigation arising out of, or related to, Former Monsanto’s chemical businesses, and have been indemnifying Pharmacia when liability has been imposed or settlement has been reached regarding such claims and litigation.
Environmental Matters
In 2009, as part of our acquisition of Wyeth, we submitted toassumed responsibility for environmental remediation at the U.S. Environmental Protection Agency (EPA) a corrective measures study report with regard to Pharmacia’s discontinued industrial chemical facility in North Haven, Connecticut. In September 2010, our corrective measures study report was approved by the EPA, and we commenced construction of the site remedy in late 2011 under an Updated Administrative Order on Consent with the EPA. In September 2019, the EPA acknowledged that construction of the site remedy has been completed.
Also in 2009, we submitted a revised site-wide feasibility study with regard to Wyeth Holdings Corporation’sLLC (formerly known as Wyeth Holdings Corporation and American Cyanamid Company) discontinued industrial chemical facility in Bound Brook, New Jersey. In July 2011, Wyeth Holdings CorporationSince that time, we have executed an Administrative Settlement Agreement and Orderor have become a party to a number of administrative settlement agreements, orders on Consent for Removal Action (the 2011 Administrative Settlement Agreement)consent, and/or judicial consent decrees, with the EPA with regardU.S. Environmental Protection Agency, the New Jersey Department of Environmental Protection and/or federal and state natural resource trustees to perform remedial design, removal and remedial actions, and related environmental remediation activities, and to resolve alleged damages to natural resources, at the Bound Brook facility. In accordance with the 2011 Administrative Settlement Agreement, we completed construction of an interim remedy to address the discharge of impacted groundwater from the facility to the Raritan River. In September 2012, the EPA issued a final remediation plan for the Bound Brook facility’s main plant area, which is generally in accordance with one of the remedies evaluated in our revised site-wide feasibility study. In March 2013, Wyeth Holdings Corporation (now Wyeth Holdings LLC) entered into an Administrative Settlement Agreement and Order on Consent with the EPA to allow us to undertake detailed engineering design of the remedy for the main plant area and to perform a focused feasibility study for 2 adjacent lagoons. In September 2015, the U.S., on behalf of the EPA, filed a complaint and consent decree with the federal District Court for the District of New Jersey that allows Wyeth Holdings LLC to complete the design and to implement the remedy for the main plant area. The consent decree (which supersedes the 2011 Administrative Settlement Agreement) was entered by the District Court in December 2015. In September 2018, the EPA issued a final remediation plan for the 2 adjacent lagoons, which is generally in accordance with one of the remedies evaluated in our focused feasibility study, and, in September 2019, Wyeth Holdings LLC entered into an Administrative Settlement Agreement and Order on Consent with the EPA to allow us to undertake detailed engineering design of the remedy for the lagoons.
We have accrued for the currently estimated costs of the site remedies for the North Haven and Bound Brook facilities.these activities.
We are aalso party to a number of other proceedings brought under the Comprehensive Environmental Response, Compensation, and Liability Act of 1980, as amended, and other state, local or foreign laws in which the primary relief sought is the cost of past and/or future remediation.
Contracts with Iraqi Ministry of Health
In October 2017, a number of U.S. service members, civilians, and their families brought a complaint in the U.S. District Court for the District of Columbia against a number of pharmaceutical and medical devices companies, including Pfizer and certain of its subsidiaries, alleging that the defendants violated the U.S. Anti-Terrorism Act. The complaint alleges that the defendants provided funding for terrorist organizations through their sales practices pursuant to pharmaceutical and medical device contracts with the Iraqi Ministry of Health, and seeks monetary relief. In July 2020, the District Court granted defendants’ motions to dismiss and dismissed all of plaintiffs’ claims. The plaintiffs are appealingIn January 2022, the Court of Appeals reversed the District Court’s decision. In February 2022, the defendants filed for en banc review of the Court of Appeals’ decision. In February 2023, the Court of Appeals denied defendants’ en banc petitions.
Allergan Complaint for Indemnity
In August 2018,2019, Pfizer was named as a defendant in a third-party complaint, for indemnity, along with King, filed by Allergan Finance LLC (Allergan) in a Multi-District Litigation (In re National Prescription Opiate Litigation MDL 2804) in the U.S. DistrictSupreme Court forof the Northern DistrictState of Ohio. The lawsuit assertedNew York, asserting claims for indemnity related to Kadian, which was owned for a short period by King in 2008, prior to Pfizer's acquisition of King in 2010. In December 2018, the District Court dismissed the lawsuit. In February 2019, Allergan filed a similar complaint in the Supreme Court of the State of New York, asserting claims for indemnity related to Kadian. ThatThis suit was voluntarily discontinued without prejudice in January 2021.
Viatris Securities Litigation
In October 2021, a putative class action was filed in the Court of Common Pleas of Allegheny County, Pennsylvania on behalf of former Mylan N.V. shareholders who received Viatris common stock in exchange for Mylan shares in connection with the spin-off of the Upjohn Business and its combination with Mylan (the Transactions). Viatris, Pfizer, and certain of each company’s current and former officers, directors and employees are named as defendants. An amended complaint was filed in January 2023, and alleges that the defendants violated certain provisions of the Securities Act of 1933 in connection with certain disclosures made in or omitted from the registration statement and related prospectus issued in connection with the Transactions, as well as related communications. Plaintiff seeks damages, costs and expenses and other equitable and injunctive relief. In November 2023, the parties reached an agreement to settle the litigation on terms not material to Pfizer. The settlement is subject to court approval.
Breach of Contract–Contract –Xalkori/Lorbrena Comirnaty
We are a defendantIn 2023, Pfizer and BioNTech Manufacturing GmbH initiated separate formal proceedings against the Republic of Poland, the Republic of Romania and Hungary in a breach of contract action brought by New York University (NYU) in the SupremeBelgium’s Court of First Instance of Brussels. Pfizer and BioNTech are seeking an order from the StateCourt holding those countries to their commitments for COVID-19 vaccine orders, which were placed as part of New York (Supreme Court). NYU alleges that it is entitled to royalties on Pfizer’s sales ofXalkori under the terms of a Research and License Agreement between NYU and Sugen, Inc. Sugen, Inc. was acquired by Pharmaciatheir contracts signed in August 1999, and 2021.
Pharmacia was acquired by Pfizer in 2003 and is a wholly owned subsidiary of Pfizer. The action was originally filed in 2013. In December 2015, the Supreme Court dismissed the action and, in
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 103100 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
May 2017, the New York State Appellate Division reversed the decision and remanded the proceedings to the Supreme Court. In January 2020, the Supreme Court denied both parties’ summary judgment motions.
In October 2020, NYU filed a separate breach of contract action against Pfizer alleging that it is entitled to royalties on sales of Lorbrena under the terms of the same NYU-Sugen, Inc. Research and Licensing Agreement.
A4. Legal Proceedings––Government Investigations
We are subject to extensive regulation by government agencies in the U.S., other developed markets and multiple emerging markets in which we operate. Criminal charges, substantial fines and/or civil penalties, limitations on our ability to conduct business in applicable jurisdictions, corporate integrity or deferred prosecution agreements, as well as reputational harm and increased public interest in the matter could result from government investigations in the U.S. and other jurisdictions in which we do business. These matters often involve government requests for information on a voluntary basis or through subpoenas after which the government may seek additional information through follow-up requests or additional subpoenas. In addition, in a qui tam lawsuit in which the government declines to intervene, the relator may still pursue a suit for the recovery of civil damages and penalties on behalf of the government. Among the investigations by government agencies are the matters discussed below.
Greenstone Investigations
•U.S. Department of Justice Antitrust Division Investigation
Since July 2017, the U.S. Department of Justice'sJustice’s Antitrust Division has been investigating our former Greenstone generics business. We believe this is related to an ongoing broader antitrust investigation of the generic pharmaceutical industry. The government has been obtaining information from GreenstoneWe have produced records relating to this investigation.
•State Attorneys General and Multi-District Generics Antitrust Litigation
In April 2018, Greenstone received requests for information from the Antitrust Department of the Connecticut Office of the Attorney General. In May 2019, Attorneys General of more than 40 states plus the District of Columbia and Puerto Rico filed a complaint against a number of pharmaceutical companies, including Greenstone and Pfizer. The matter has been consolidated with a Multi-District Litigation (In re: Generic Pharmaceuticals Pricing Antitrust Litigation MDL No. 2724) in the Eastern District of Pennsylvania. As to Greenstone and Pfizer, the complaint alleges anticompetitive conduct in violation of federal and state antitrust laws and state consumer protection laws. In June 2020, the State Attorneys General filed a new complaint against a large number of companies, including Greenstone and Pfizer, making similar allegations, but concerning a new set of drugs. This complaint was transferred to the Multi-District LitigationMDL in July 2020. The MDL also includes civil complaints filed by private plaintiffs and state counties against Pfizer, Greenstone and a significant number of other defendants asserting allegations that generally overlap with those asserted by the State Attorneys General.
Subpoena & Civil Investigative Demand relating to Manufacturing of Tris Pharma/Quillivant XR
In October 2018, we received a subpoena from the U.S. Attorney’s Office for the Southern District of New York (SDNY) seeking records relating to our relationship with another drug manufacturer and its production and manufacturing of drugs including, but not limited to, Quillivant XR. We responded to that subpoena in full and have had no communication with the SDNY in connection with the subpoena since June 2019. Additionally, in September 2020, we received a Civil Investigative Demand (CID) from the Texas Attorney General’s office seeking records of a similar nature to those requested by the SDNY. We produced records pursuantin response to this request. In November 2023, the subpoena.investigation culminated in a qui tam litigation brought by the State of Texas. The investigation is now closed.
Government Inquiries relating to Meridian Medical Technologies
In February 2019, we received a civil investigative demandCID from the U.S. Attorney’s Office for the SDNY. The civil investigative demandCID seeks records and information related to alleged quality issues involving the manufacture of auto-injectors at ourthe Meridian site. In August 2019, we received a HIPAA subpoena fromissued by the U.S. Attorney’s Office for the Eastern District of Missouri, in coordination with the Department of Justice’s Consumer Protection Branch, seeking similar records and information. We are producinghave produced records in response to these and subsequent requests.
U.S. Department of Justice/SEC Inquiry relating to Russian Operations
In June 2019, we received an informal request from the U.S. Department of Justice’s Foreign Corrupt Practices Act (FCPA) Unit seeking documents relating to our operations in Russia. In September 2019, we received a similar request from the SEC’s FCPA Unit. We have produced records pursuant to these requests.
Docetaxel––Mississippi Attorney General Government Investigation
See Note 16A2. Contingencies and Certain Commitments: Legal Proceedings––Product Litigation––Docetaxel––Mississippi Attorney General Government InvestigationAction above for information regarding a government investigation related to Docetaxel marketing practices.
U.S. Department of Justice Inquiries relating to India Operations
In March 2020, we received an informal request from the U.S. Department of Justice'sJustice’s Consumer Protection Branch seeking documents relating to our manufacturing operations in India, including at our former facility located at Irrungattukottai in India. In April 2020, we received a similar request from the U.S. Attorney’s Office for the SDNY regarding a civil investigation concerning operations at our facilities in India. We are producing records pursuant to these requests.
U.S. Department of Justice/SEC Inquiry relating to China Operations
In June 2020, we received an informal request from the U.S. Department of Justice'sJustice’s FCPA Unit seeking documents relating to our operations in China. In August 2020, we received a similar request from the SEC’s FCPA Unit. We are producinghave produced records pursuant to these requests.
Zantac––State of New Mexico and Mayor and City Council of Baltimore Civil ActionActions
See Note 16A2. Contingencies and Certain Commitments: Legal Proceedings––Product Litigation––Zantac above for information regarding a civil actionactions separately filed by the State of New Mexico and the Mayor and City Council of Baltimore alleging various state statutory and common law claims in connection with the defendants’ alleged sale of Zantac in New Mexico.those jurisdictions.
A5. Legal Proceedings––Matters Resolved During 2020Government Inquiries relating to Biohaven
DuringIn June 2022, the full-year 2020, certain matters, includingU.S. Department of Justice's Commercial Litigation Branch and the matter discussed below, were resolved or becameU.S. Attorney’s Office for the subjectWestern District of definitive settlement agreements or settlement agreements-in-principle.New York issued a CID relating to Biohaven. The CID seeks records and information related to, among other things, engagements with healthcare professionals and co-pay coupons cards. In March 2023, the California Department of Insurance issued a subpoena seeking records similar to those requested by the CID. Biohaven is a wholly-owned subsidiary that we acquired in October 2022. We are producing records in response to these requests.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 104101 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Hormone Therapy Consumer Class ActionU.S. Department of Justice Inquiry relating to Mexico Operations
A certified consumer class action was pending against Wyeth inIn March 2023, we received an informal request from the U.S. District CourtDepartment of Justice’s FCPA Unit seeking documents relating to our operations in Mexico. We are producing records pursuant to this request.
Government Inquiries relating to Xeljanz
In April 2023, we received a HIPAA subpoena issued by the U.S. Attorney’s Office for the SouthernWestern District of California based onVirginia, in coordination with the alleged off-label marketingDepartment of its hormone therapy products. The case was originally filedJustice’s Commercial Litigation Branch, seeking records and information related to programs Pfizer sponsored in December 2003. The class consisted of California consumers who purchased Wyeth’s hormone-replacement products between January 1995 and January 2003 and who did not seek personal injury damages therefrom. The class sought compensatory and punitive damages, including a full refund of the purchase price. In March 2020, the parties reached an agreement, and obtained preliminary court approval,retail pharmacies relating to resolveXeljanz. We are producing records pursuant to this matter for $200 million, which was paid in full in the second quarter of 2020.request.
B. Guarantees and Indemnifications
In the ordinary course of business and in connection with the sale of assets and businesses and other transactions, we often indemnify our counterparties against certain liabilities that may arise in connection with the transaction or that are related to events and activities prior to or following a transaction. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we may be required to reimburse the loss. These indemnifications are generally subject to various restrictions and limitations. Historically, we have not paid significant amounts under these provisions and, as of December 31, 2020,2023, the estimated fair value of these indemnification obligations wasis not significant.material to Pfizer. See Note 2C for a description of the March 2022 indemnity provided by Pfizer to GSK in connection with the issuance of notes by the Consumer Healthcare JV. In conjunction with the completion of GSK’s demerger transactions in July 2022, GSK’s guarantee and our related indemnification of GSK’s guarantee were terminated. In addition, in connection with our entry into certain agreements and other transactions, our counterparties may agreebe obligated to indemnify us. For example, our collaborationglobal agreement with EMD Serono, Inc.BioNTech to co-promote Rebif in the U.S. expiredco-develop a mRNA-based coronavirus vaccine program aimed at the end of 2015 and includedpreventing COVID-19 infection, includes certain indemnity provisions. Patent litigation brought by Biogen Idec MA Inc. against EMD Serono Inc.provisions pursuant to which each of BioNTech and Pfizer is pendinghas agreed to indemnify the other for certain liabilities that may arise in connection with certain third-party claims relating to Comirnaty.
SeeNote 7Dfor information on Pfizer Inc.’s guarantee of the U.S. District Court for the District of New Jersey and the United States Court of Appeals for the Federal Circuit. EMD Serono Inc. has acknowledged that it is obligated to satisfy any award of damages.debt issued by PIE in May 2023. We have also guaranteed the long-term debt of certain companies that we acquired and that now are subsidiaries of Pfizer. See Note 7D. C. Certain Commitments
•As of December 31, 2020,2023, we had agreementscommitments totaling $3.8$5.2 billion to purchase goods and services that are enforceable and legally binding and enforceable. These commitments include amountspayments relating to advertising, information technology services, employee benefit administration services, and potential milestone payments deemed reasonably likely to occur.occur, and purchase obligations for goods and services.
•See Note 5A for information on the TCJA repatriation tax liability.D. Contingent Consideration for Acquisitions
We may be required to make payments to sellers for certain prior business combinations that are contingent upon future events or outcomes. See NoteNote 1D. The estimated fair value of contingent consideration as of December 31, 20202023 is $689$692 million, of which $123$179 million is recorded in Other current liabilities and $566$512 million in Other noncurrent liabilities, and $711as of December 31, 2022 was $645 million, of which $160$42 million iswas recorded in Other current liabilities and $551$603 million in Other noncurrent liabilitiesas of December 31, 2019.. The decreaseincrease in the contingent consideration balance from December 31, 20192022 is primarily due to fair value adjustments, partially offset by payments made upon the achievement of certain sales-based milestones, partially offset by fair value adjustments.milestones. E. Insurance
Our insurance coverage reflects market conditions (including cost and availability) existing at the time it is written, and our decision to obtain insurance coverage or to self-insure varies accordingly. Depending upon the cost and availability of insurance and the nature of the risk involved, the amount of self-insurance may be significant. The cost and availability of coverage have resulted in self-insuring certain exposures, including product liability. If we incur substantial liabilities that are not covered by insurance or substantially exceed insurance coverage and that are in excess of existing accruals, there could be a material adverse effect on our cash flows or results of operations in the period in which the amounts are paid and/or accrued.
Note 1717.. Product,Segment, Geographic and Other Revenue Information
A. Segment Information
We regularly review our operating segments and the approach used by management to evaluate performance and allocate resources. In 2023, we managed our commercial operations through two operating segments, each led by a single manager: Biopharma, our innovative science-based biopharmaceutical business, and Business Innovation, an operating segment established in the first quarter of 2023 that includes PC1, our contract development and manufacturing organization and a leading supplier of specialty active pharmaceutical ingredients, and Pfizer Ignite, an offering that provides strategic guidance and end-to-end R&D services to select innovative biotech companies that align with Pfizer’s R&D focus areas. Biopharma is the only reportable segment. Each operating segment has responsibility for its commercial activities. Regional commercial organizations market, distribute and sell our products and are supported by global platform functions that are responsible for the research, development, manufacturing and supply of our products and global corporate enabling functions. Each operating segment has a geographic footprint across developed and emerging markets. Our chief operating decision maker uses the revenues and earnings of the operating segments, among other factors, for performance evaluation and resource allocation. Beginning in July 2023, in consideration of planned future investments in oncology, including the December 2023 acquisition of Seagen, we reorganized our R&D platform operations. Discovery to late-phase clinical development for oncology is performed by a new end-to-end ORD organization and discovery to late-phase clinical development for all remaining therapeutic areas is consolidated into the end-to-end PRD organization. ORD and PRD replace our former WRDM and GPD organizations, where, prior to July 2023, research units within WRDM were generally responsible for research and early-stage development assets and, prior to July 2023, GPD was generally responsible for the clinical development strategy and operational execution of clinical trials for both early- and late-stage clinical assets in Pfizer’s pipeline. In 2023, Biopharma received R&D services from
A. | | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 102 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
ORD, PRD and the predecessor WRDM and GPD organizations. These services included IPR&D projects for new investigational products and additional indications for in-line products.
Other Business Activities––Other business activities include the operating results of Business Innovation as well as certain pre-tax costs not allocated to our operating segment results, such as costs associated with:
•ORD––the R&D expenses managed by our ORD organization, which is responsible for discovery to late-phase clinical development for oncology research projects for our global Biopharma portfolio along with facilitating regulatory submissions and interactions with regulatory agencies for these projects. R&D spending may include upfront and milestone payments for intellectual property rights for oncology projects.
•PRD––the R&D expenses managed by our PRD organization, which is responsible for discovery to late-phase clinical development research projects for all therapeutic areas other than oncology for our global Biopharma portfolio, along with facilitating regulatory submissions and interactions with regulatory agencies for these projects. R&D spending may include upfront and milestone payments for intellectual property rights related to non-oncology projects. The PRD organization also has responsibility for certain science-based and other platform-services organizations, which provide end-to-end technical expertise and other services to both ORD and PRD R&D projects, as well as the Worldwide Medical and Safety group, which helps ensure that Pfizer provides all stakeholders––including patients, healthcare providers, pharmacists, payors and health authorities––with complete and up-to-date information on the risks and benefits associated with Pfizer products so that they can make appropriate decisions on how and when to use Pfizer’s medicines.
•Corporate and other unallocated––the costs associated with (i) corporate enabling functions (such as digital, global real estate operations, legal, finance, human resources, worldwide public affairs, compliance and worldwide procurement, among others) and other corporate costs, including, but not limited to, all strategy, business development and portfolio management capabilities and certain compensation, as well as interest income and expense, and gains and losses on investments; (ii) overhead costs primarily associated with our manufacturing operations (which include manufacturing variances associated with production) that are not directly assessed to an operating segment, as business unit (segment) management does not manage these costs; and (iii) our share of earnings from Haleon/the Consumer Healthcare JV.
Reconciling Items––The following items, transactions and events are not allocated to our operating segment results: (i) all amortization of intangible assets; (ii) acquisition-related items, where we incur costs for executing the transaction, integrating the acquired operations and restructuring the combined company, and which may also include purchase accounting impacts, such as the incremental charge to cost of sales from the sale of acquired inventory that was written up to fair value, depreciation related to the increase/decrease in fair value of acquired fixed assets, amortization related to the increase in fair value of acquired debt, and the fair value changes for contingent consideration; and (iii) certain significant items, representing substantive and/or unusual, and in some cases recurring, items that are evaluated on an individual basis by management and that, either as a result of their nature or size, would not be expected to occur as part of our normal business on a regular basis. Such certain significant items can include, but are not limited to, pension and postretirement actuarial remeasurement gains and losses, non-acquisition-related restructuring costs, net gains and losses on investments in equity securities, as well as costs incurred for legal settlements, asset impairments and disposals of assets or businesses, including, as applicable, any associated transition activities.
Segment Assets––We manage our assets on a total company basis, not by operating segment, as our operating assets are shared or commingled. Therefore, our chief operating decision maker does not regularly review any asset information by operating segment and, accordingly, we do not report asset information by operating segment. Total assets were $227 billion as of December 31, 2023 and $197 billion as of December 31, 2022.
Selected Income Statement Information
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| The following table provides selected income statement information by reportable segment: |
| | | Total Revenues(a) | | | | Earnings(a) | | Depreciation and Amortization(b) |
| | Year Ended December 31, | | | | Year Ended December 31, | | Year Ended December 31, |
| (MILLIONS) | | 2023 | | 2022 | | | 2021 | | | | | | | | | 2023 | | 2022 | | | 2021 | | 2023 | | 2022 | | | 2021 |
| Reportable Segment: | | | | | | | | | | | | | | | | | | | | | | | | |
| Biopharma | | $ | 57,186 | | | $ | 98,988 | | | $ | 79,557 | | | | | | | | | $ | 30,632 | | | $ | 57,148 | | | $ | 40,647 | | | $ | 882 | | | $ | 813 | | | $ | 789 | |
Other business activities(c) | | 1,310 | | | 1,342 | | | 1,731 | | | | | | | | | (19,050) | | | (14,370) | | | (13,455) | | | 654 | | | 626 | | | 590 | |
| Reconciling Items: | | | | | | | | | | | | | | | | | | | | | | | | |
| Amortization of intangible assets | | | | | | | | | | | | | | (4,733) | | | (3,609) | | | (3,746) | | | 4,733 | | | 3,609 | | | 3,746 | |
| Acquisition-related items | | | | | | | | | | | | | | (1,874) | | | (832) | | | (139) | | | (11) | | | (20) | | | (21) | |
Certain significant items(d) | | | | | | | | | | | | | | (3,917) | | | (3,608) | | | 1,003 | | | 32 | | | 36 | | | 87 | |
| | $ | 58,496 | | | $ | 100,330 | | | $ | 81,288 | | | | | | | | | $ | 1,058 | | | $ | 34,729 | | | $ | 24,311 | | | $ | 6,290 | | | $ | 5,064 | | | $ | 5,191 | |
(a)Earnings = Income from continuing operations before provision/(benefit) for taxes on income. Biopharma’s revenues and earnings in 2023 reflect a non-cash revenue reversal of $3.5 billion (see Note 17C). Biopharma’s earnings also include dividend income from our investment in ViiV of $265 million in 2023, $314 million in 2022 and $166 million in 2021. (b)Certain production facilities are shared. Depreciation is allocated based on estimates of physical production.
(c)Other business activities include revenues and costs associated with Business Innovation and costs that we do not allocate to our operating segments, per above, including acquired IPR&D expenses in the periods presented (see Notes 2A and 2E). In 2023, earnings include approximately $6.2 billion of inventory write-offs and related charges to Cost of sales mainly due to lower-than-expected demand for our COVID-19 products. In 2022, earnings included COVID-19-related charges of approximately $1.7 billion to Cost of sales, composed of (i) inventory write-offs of approximately $1.2 billion related to COVID-19 products that exceeded or were expected to exceed their approved shelf-lives prior to being used and (ii) charges of approximately $0.5 billion, primarily related to excess raw materials for Paxlovid. (d)Certain significant items are substantive and/or unusual, and in some cases recurring, items (as noted above). Earnings in 2023 include, among other items: (i) intangible asset impairment charges of $3.0 billion recorded in Other (income)/deductions––net and (ii) restructuring charges/(credits) and implementation costs
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 103 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
and additional depreciation—asset restructuring of $2.2 billion ($290 million recorded in Selling, informational and administrative expenses and the remaining amount primarilyrecorded in Restructuring charges and certain acquisition-related costs), partially offset by (iii) net gains on equity securities of $1.6 billion recorded in Other (income)/deductions––net.Earnings in 2022 included, among other items: (i) restructuring charges/(credits) and implementation costs and additional depreciation—asset restructuring of $1.4 billion ($562 million recorded in Selling, informational and administrative expenses and the remaining amount primarilyrecorded in Restructuring charges and certain acquisition-related costs) and (ii) net losses on equity securities of $1.3 billion recorded in Other (income)/deductions––net. Earnings in 2021 included, among other items: (i) actuarial valuation and other pension and postretirement plan gains of $1.6 billion recorded in Other (income)/deductions––net and (ii) net gains on equity securities of $1.3 billion recorded in Other (income)/deductions––net, partially offset by (iii) restructuring charges/(credits) and implementation costs and additional depreciation—asset restructuring of $1.3 billion ($450 million recorded in Selling, informational and administrative expenses and the remaining amount primarily recorded in Restructuring charges and certain acquisition-related costs). See Notes 3and 4. B. Geographic Information
| The following summarizes revenues by geographic area: | | The following summarizes revenues by geographic area: | |
| The following summarizes revenues by geographic area: | |
| | | | | Year Ended December 31, | | | | | Year Ended December 31, |
| (MILLIONS OF DOLLARS) | | | 2020 | | 2019 | | 2018 |
| (MILLIONS) | | (MILLIONS) | | | | | | | | 2023 | | 2022 | | 2021 |
| United States | United States | | | $ | 21,712 | | | $ | 20,593 | | | $ | 20,119 | |
| Developed Europe | Developed Europe | | | 7,788 | | | 7,729 | | | 7,997 | |
| Developed Rest of World | Developed Rest of World | | | 4,036 | | | 4,022 | | | 4,090 | |
| Emerging Markets | Emerging Markets | | | 8,372 | | | 8,828 | | | 8,618 | |
| Revenues | | | $ | 41,908 | | | $ | 41,172 | | | $ | 40,825 | |
| Total revenues | |
Revenues exceeded $500 million in each of 8, 1014, 24 and 1021 countries outside the U.S. in 2020, 20192023, 2022 and 2018,2021, respectively. The U.S. is the only country to contribute more than 10% of total revenue in 2020, 20192023, 2022 and 2018.2021. As a percentage of revenues, our two largest national marketscountry outside the U.S. were China, which contributed 6% of total revenue in each of 2020, 2019 and 2018, andwas Japan, which contributed 6% of total revenue in 2023, 8% of total revenue in 2022 and 9% of total revenue in 2021.
C. Other Revenue Information
Significant Customers
We and our collaboration partner, BioNTech, have entered into agreements to supply pre-specified doses of Comirnaty with multiple developed and emerging nations around the world and are continuing to deliver doses of Comirnaty under such agreements. This includes supply agreements entered into in November 2020 and 5%February and May 2021 with the EC for Comirnaty on behalf of the different EU member states and certain other countries. Each EU member state submits its own Comirnaty vaccine order to us and is responsible for payment pursuant to terms of the supply agreements negotiated by the EC. In May 2023, we and BioNTech amended our contract with the EC to deliver COVID-19 vaccines to the EU. The amended agreement includes rephasing of delivery of doses annually through 2026 and an aggregate volume reduction, providing additional flexibility for those EU member states who agreed to the amended agreement. The EC will maintain access to future adapted COVID-19 vaccines and the ability to donate doses, in eachalignment with the original agreement.
In 2022 and 2023, we had entered into agreements to supply pre-specified treatment courses of 2019Paxlovid with government and 2018.government sponsored customers in multiple developed and emerging nations around the world, which represented most Paxlovid revenues in 2022 and 2023, while commercialization began in some markets in 2023. In October 2023, we announced an amended agreement with the U.S. government, which facilitated the transition of Paxlovid to traditional commercial markets starting in November 2023, with prices negotiated with commercial payors and a copay assistance program for eligible privately insured patients, as the U.S. government began to discontinue the distribution of EUA-labeled Paxlovid. We ensured commercial readiness by providing NDA-labeled commercial supply by the end of 2023. However, EUA-labeled Paxlovid remained available free-of-charge to all eligible patients until the end of 2023, and therefore, there was only minimal uptake of NDA-labeled commercial product before January 1, 2024. In connection with this agreement, we recorded a non-cash revenue reversal of $3.5 billion in the fourth quarter of 2023, of which a portion was associated with sales recorded in 2022, related to the expected return of an estimated 6.5 million treatment courses of EUA-labeled U.S. government inventory. We will convert these treatment courses previously purchased by the U.S. government to a volume-based credit, based on the actual number of treatment courses that are returned by the U.S. government, which will support continued access to Paxlovid through a U.S. government patient assistance program operated by Pfizer. Therefore, we expect the patient assistance program will provide an estimated 6.5 million treatment courses of FDA-approved, NDA-labeled Paxlovid free of charge to all eligible uninsured, Medicare and Medicaid patients through 2024, and to eligible uninsured and underinsured patients through 2028. We also agreed to create, in 2024, a U.S. Strategic National Stockpile of 1.0 million treatment courses to enable future pandemic preparedness through 2028, which will be managed and supplied by Pfizer at no cost to the U.S. government or taxpayers. While we will recognize revenue as the estimated 7.5 million treatment courses are delivered, there is no remaining cash consideration for these treatment courses.
| | | | | | | | | | | | | | | | | | | | |
The following summarizes revenue, as a percentage of Total revenues, for our three largest U.S. wholesaler customers and the U.S. government, which was concentrated in our Biopharma operating segment: |
| | | Year Ended December 31, |
| | 2023 | | 2022 | | 2021 |
| McKesson, Inc. | | 17 | % | | 8 | % | | 9 | % |
| Cencora, Inc. (formerly AmerisourceBergen Corporation) | | 12 | % | | 5 | % | | 7 | % |
| Cardinal Health, Inc. | | 10 | % | | 4 | % | | 5 | % |
| | | | | | |
U.S. government(a) | | — | | | 23 | % | | 13 | % |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 104 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Collectively, our three largest U.S. wholesaler customers represented 44% and 32% of total trade accounts receivable as of December 31, 2023 and December 31, 2022, respectively. Accounts receivable from the U.S. government as of December 31, 2023 and December 31, 2022 were not material to our consolidated financial statements.
Significant Revenues by Product
The following provides detailed revenue information for several of our major products:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (MILLIONS) | | | | Year Ended December 31, |
| PRODUCT | | PRIMARY INDICATION OR CLASS | | 2023 | | 2022 | | 2021 |
| | | | | | |
| TOTAL REVENUES | | | | $ | 58,496 | | | $ | 100,330 | | | $ | 81,288 | |
| GLOBAL BIOPHARMACEUTICALS BUSINESS (BIOPHARMA) | | $ | 57,186 | | | $ | 98,988 | | | $ | 79,557 | |
| Primary Care | | $ | 30,589 | | | $ | 73,023 | | | $ | 52,029 | |
Comirnaty direct sales and alliance revenues(a) | | Active immunization to prevent COVID-19 | | 11,220 | | | 37,806 | | | 36,781 | |
| Eliquis alliance revenues and direct sales | | Nonvalvular atrial fibrillation, deep vein thrombosis, pulmonary embolism | | 6,747 | | | 6,480 | | | 5,970 | |
| Prevnar family | | Active immunization to prevent pneumonia, invasive disease and otitis media caused by Streptococcus pneumoniae | | 6,440 | | | 6,337 | | | 5,272 | |
Paxlovid(b) | | COVID-19 in certain high-risk patients | | 1,279 | | | 18,933 | | | 76 | |
| Nurtec ODT/Vydura | | Acute treatment of migraine and prevention of episodic migraine | | 928 | | | 213 | | | — | |
| Abrysvo | | Active immunization to prevent RSV infection | | 890 | | | — | | | — | |
| Premarin family | | Symptoms of menopause | | 397 | | | 455 | | | 563 | |
| BMP2 | | Bone graft for spinal fusion | | 338 | | | 277 | | | 266 | |
| FSME-IMMUN/TicoVac | | Active immunization to prevent tick-borne encephalitis disease | | 268 | | | 200 | | | 185 | |
| Nimenrix | | Active immunization against invasive meningococcal ACWY disease | | 179 | | | 268 | | | 193 | |
| Trumenba | | Active immunization to prevent invasive disease caused by Neisseria meningitidis group B | | 126 | | | 123 | | | 118 | |
| All other Primary Care | | Various | | 1,777 | | | 1,932 | | | 2,604 | |
| Specialty Care | | $ | 14,970 | | | $ | 13,833 | | | $ | 15,194 | |
| Vyndaqel family | | ATTR-CM and polyneuropathy | | 3,321 | | | 2,447 | | | 2,015 | |
| Xeljanz | | RA, PsA, UC, active polyarticular course juvenile idiopathic arthritis, ankylosing spondylitis | | 1,703 | | | 1,796 | | | 2,455 | |
| Enbrel (Outside the U.S. and Canada) | | RA, juvenile idiopathic arthritis, PsA, plaque psoriasis, pediatric plaque psoriasis, ankylosing spondylitis and nonradiographic axial spondyloarthritis | | 830 | | | 1,003 | | | 1,185 | |
| Sulperazon | | Bacterial infections | | 757 | | | 786 | | | 683 | |
Ig Portfolio(c) | | Various | | 584 | | | 491 | | | 430 | |
| Genotropin | | Replacement of human growth hormone | | 539 | | | 360 | | | 389 | |
| Zavicefta | | Bacterial infections | | 511 | | | 412 | | | 413 | |
| Inflectra | | Crohn’s disease, pediatric Crohn’s disease, UC, pediatric UC, RA in combination with methotrexate, ankylosing spondylitis, PsA and plaque psoriasis | | 490 | | | 532 | | | 657 | |
| BeneFIX | | Hemophilia B | | 424 | | | 425 | | | 438 | |
| Zithromax | | Bacterial infections | | 406 | | | 331 | | | 278 | |
| Medrol | | Anti-inflammatory glucocorticoid | | 339 | | | 328 | | | 432 | |
| Oxbryta | | Sickle cell disease | | 328 | | | 73 | | | — | |
| Somavert | | Acromegaly | | 267 | | | 268 | | | 277 | |
| Fragmin | | Treatment/prevention of venous thromboembolism | | 238 | | | 269 | | | 305 | |
| ReFacto AF/Xyntha | | Hemophilia A | | 230 | | | 239 | | | 304 | |
| Cresemba | | Fungal infections | | 195 | | | 155 | | | 142 | |
| Vfend | | Fungal infections | | 187 | | | 225 | | | 267 | |
| Bicillin | | Bacterial infections | | 158 | | | 146 | | | 120 | |
| Cibinqo | | Atopic dermatitis | | 128 | | | 27 | | | — | |
| All other Anti-infectives | | Various | | 1,092 | | | 1,171 | | | 1,572 | |
| All other Specialty Care | | Various | | 2,244 | | | 2,350 | | | 2,830 | |
| Oncology | | $ | 11,627 | | | $ | 12,132 | | | $ | 12,333 | |
| Ibrance | | HR-positive/HER2-negative metastatic breast cancer | | 4,753 | | | 5,120 | | | 5,437 | |
| Xtandi alliance revenues | | mCRPC, nmCRPC, mCSPC, nmCSPC | | 1,191 | | | 1,198 | | | 1,185 | |
| Inlyta | | Advanced RCC | | 1,036 | | | 1,003 | | | 1,002 | |
| Bosulif | | Philadelphia chromosome–positive chronic myelogenous leukemia | | 645 | | | 575 | | | 540 | |
| Lorbrena | | ALK-positive metastatic NSCLC | | 539 | | | 343 | | | 266 | |
| Zirabev | | Treatment of mCRC; unresectable, locally advanced, recurrent or metastatic NSCLC; recurrent glioblastoma; metastatic RCC; and persistent, recurrent or metastatic cervical cancer | | 424 | | | 562 | | | 444 | |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 105 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
B. Other Revenue Information | | | | | | | | | | | | | | | | | | | | | | | | | | |
| (MILLIONS) | | | | Year Ended December 31, |
| PRODUCT | | PRIMARY INDICATION OR CLASS | | 2023 | | 2022 | | 2021 |
| | | | | | |
| Ruxience | | Non-hodgkin’s lymphoma, chronic lymphocytic leukemia, granulomatosis with polyangiitis (Wegener’s Granulomatosis) and microscopic polyangiitis | | 390 | | | 458 | | | 491 | |
| Xalkori | | ALK-positive and Proto-Oncogene 1, Receptor Tyrosine Kinase-positive advanced NSCLC | | 374 | | | 465 | | | 493 | |
| Retacrit | | Anemia | | 340 | | | 394 | | | 444 | |
| Aromasin | | Post-menopausal early and advanced breast cancer | | 301 | | | 248 | | | 211 | |
| Besponsa | | Relapsed or refractory B-cell acute lymphoblastic leukemia | | 236 | | | 219 | | | 192 | |
| Braftovi | | In combination with Mektovi for metastatic melanoma in patients with a BRAFV600E/K mutation and for metastatic NSCLC in patients with a BRAFV600E mutation; and In combination with Erbitux (cetuximab)(d) for the treatment of BRAFV600E-mutant mCRC after prior therapy | | 213 | | | 194 | | | 187 | |
Bavencio alliance revenues(e) | | Locally advanced or metastatic urothelial carcinoma; metastatic Merkel cell carcinoma; immunotherapy and tyrosine kinase inhibitor combination for patients with advanced RCC | | 190 | | | 271 | | | 178 | |
| Sutent | | Advanced and/or metastatic RCC, adjuvant RCC, refractory gastrointestinal stromal tumors (after disease progression on, or intolerance to, imatinib mesylate) and advanced pancreatic neuroendocrine tumor | | 180 | | | 347 | | | 673 | |
| Mektovi | | In combination with Braftovi for metastatic melanoma in patients with a BRAFV600E/K mutation and for metastatic NSCLC in patients with a BRAFV600E mutation | | 174 | | | 176 | | | 155 | |
| Trazimera | | HER2-positive breast cancer and metastatic stomach cancers | | 91 | | | 203 | | | 197 | |
Padcev(f) | | Locally advanced or metastatic urothelial cancer | | 52 | | | — | | | — | |
Adcetris(f) | | Hodgkin lymphoma and certain T-cell lymphomas | | 46 | | | — | | | — | |
Tukysa(f) | | Unresectable or metastatic HER2-positive breast cancer; RAS wild-type, HER2-positive unresectable or metastatic colorectal cancer | | 17 | | | — | | | — | |
Tivdak(f) | | Recurrent or metastatic cervical cancer | | 4 | | | — | | | — | |
| All other Oncology | | Various | | 433 | | | 357 | | | 238 | |
BUSINESS INNOVATION(g) | | $ | 1,310 | | | $ | 1,342 | | | $ | 1,731 | |
Pfizer CentreOne(h) | | Various | | 1,265 | | | 1,335 | | | 1,731 | |
| Pfizer Ignite | | Various | | 44 | | | 7 | | | — | |
| | | | | | |
| Total Alliance revenues included above | | $ | 7,582 | | | $ | 8,537 | | | $ | 7,652 | |
Significant Customers
We sell our biopharmaceutical products primarily to customersExcludes revenues for certain Comirnaty-related manufacturing activities performed on behalf of BioNTech, which are included in the wholesale sector.PC1 contract development and manufacturing organization. See footnote (h) below.
| | | | | | | | | | | | | | | | | | | | |
| The following summarizes revenue, as a percentage of total revenues, for our three largest U.S. wholesaler customers: |
| | | Year Ended December 31, |
| | 2020 | | 2019 | | 2018 |
| McKesson, Inc. | | 16 | % | | 15 | % | | 13 | % |
| AmerisourceBergen Corporation | | 13 | % | | 11 | % | | 8 | % |
| Cardinal Health, Inc. | | 10 | % | | 9 | % | | 8 | % |
Collectively,(d)Erbitux is a registered trademark of ImClone LLC.
(e)In March 2023, it was announced that our three largest U.S. wholesaleralliance with Merck KGaA to co-develop and co-commercialize Bavencio (avelumab) would terminate. Effective June 30, 2023, Merck KGaA took full control of the global commercialization of Bavencio. Beginning in the third quarter of 2023, the related profit share was replaced by a 15% royalty to Pfizer on net sales of Bavencio, which was recorded in Other (income)/deductions––net. We and Merck KGaA continue to operationalize our respective ongoing clinical trials for Bavencio; and Merck KGaA controls all future R&D activities. Bavencio is a registered trademark of Merck KGaA.
(f)Represents revenues from legacy Seagen products subsequent to the acquisition on December 14, 2023. See Note 2A. (g)See Note 17A above for information about Business Innovation. Prior-period financial information has been revised to reflect the current period presentation. (h)PC1 includes revenues from our contract manufacturing, including certain Comirnaty-related manufacturing activities performed on behalf of BioNTech ($33 million for 2023, $188 million for 2022, and $320 million for 2021), and revenues from our active pharmaceutical ingredient sales operation, as well as revenues related to our manufacturing and supply agreements with former legacy Pfizer businesses/partnerships.
Remaining Performance Obligations––Contracted revenue expected to be recognized from remaining performance obligations for firm orders in long-term contracts to supply Comirnaty and Paxlovid to our customers represented 30%, 25%totaled approximately $6 billion and 29% of total trade accounts receivable$3.4 billion, respectively, as of December 31, 2020, 20192023, which includes amounts received in advance and 2018.deferred, as well as amounts that will be invoiced as we deliver these products to our customers in future periods. Of these amounts, current contract terms provide for expected delivery of product with contracted revenue from 2024 through 2028, the timing of which may be renegotiated. Remaining performance obligations are based on foreign exchange rates as of the end of our fiscal fourth quarter of 2023 and exclude arrangements with an original expected contract duration of less than one year. Remaining performance obligations associated with contracts for other products and services were not significant as of December 31, 2023 or 2022.
Significant Product RevenuesOur deferred revenues primarily relate to advance payments received or receivable from various government or government sponsored customers for supply of Paxlovid and Comirnaty.
The following provides detailed revenue information for severaldeferred revenues related to Paxlovid totaled $3.4 billion as of December 31, 2023, with $1.5 billion and $1.9 billion recorded in current liabilities and noncurrent liabilities, respectively, while deferred revenues related to Paxlovid were not material as of December 31, 2022. The increase in Paxlovid deferred revenues during 2023 was primarily driven by the reversal of Paxlovid revenues and conversion of previously purchased EUA-labeled Paxlovid treatment courses into a volume-based credit under our major products:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (MILLIONS OF DOLLARS) | | | | Year Ended December 31, |
| PRODUCT | | PRIMARY INDICATION OR CLASS | | 2020 | | 2019 | | 2018 |
| | | | | | |
TOTAL REVENUES(a) | | | | $ | 41,908 | | | $ | 41,172 | | | $ | 40,825 | |
Internal Medicine(a) | | $ | 9,003 | | | $ | 8,790 | | | $ | 8,548 | |
| Eliquis alliance revenues and direct sales | | Nonvalvular atrial fibrillation, deep vein thrombosis, pulmonary embolism | | 4,949 | | | 4,220 | | | 3,434 | |
| Chantix/Champix | | An aid to smoking cessation treatment in adults 18 years of age or older | | 919 | | | 1,107 | | | 1,085 | |
| Premarin family | | Symptoms of menopause | | 680 | | | 734 | | | 832 | |
| BMP2 | | Development of bone and cartilage | | 274 | | | 287 | | | 279 | |
| Toviaz | | Overactive bladder | | 252 | | | 250 | | | 271 | |
| All other Internal Medicine | | Various | | 1,930 | | | 2,192 | | | 2,648 | |
| Oncology | | $ | 10,867 | | | $ | 9,014 | | | $ | 7,471 | |
| Ibrance | | Metastatic breast cancer | | 5,392 | | | 4,961 | | | 4,118 | |
| Xtandi alliance revenues | | mCRPC, nmCRPC, mCSPC | | 1,024 | | | 838 | | | 699 | |
| Sutent | | Advanced and/or metastatic RCC, adjuvant RCC, refractory GIST (after disease progression on, or intolerance to, imatinib mesylate) and advanced pancreatic neuroendocrine tumor | | 819 | | | 936 | | | 1,049 | |
| Inlyta | | Advanced RCC | | 787 | | | 477 | | | 298 | |
| Xalkori | | ALK-positive and ROS1-positive advanced NSCLC | | 544 | | | 530 | | | 524 | |
| Bosulif | | Philadelphia chromosome–positive chronic myelogenous leukemia | | 450 | | | 365 | | | 296 | |
Retacrit(b) | | Anemia | | 386 | | | 225 | | | 82 | |
| Lorbrena | | ALK-positive metastatic NSCLC | | 204 | | | 115 | | | 11 | |
Ruxience(b) | | Non-hodgkin’s lymphoma, chronic lymphocytic leukemia, granulomatosis with polyangiitis (Wegener’s Granulomatosis) and microscopic polyangiitis | | 170 | | | (1) | | | 0 | |
| Braftovi | | In combination with Mektovi for metastatic melanoma for patients who test positive for a BRAF genetic mutation and, in combination with Erbitux® (cetuximab), for the treatment of BRAFV600E-mutant mCRC after prior therapy | | 160 | | | 48 | | | 0 | |
Zirabev(b) | | Treatment of mCRC; unresectable, locally advanced, recurrent or metastatic NSCLC; recurrent glioblastoma; metastatic RCC; and persistent, recurrent or metastatic cervical cancer | | 143 | | | 1 | | | 0 | |
| Mektovi | | In combination with Braftovi for metastatic melanoma for patients who test positive for a BRAF genetic mutation | | 142 | | | 49 | | | 0 | |
| All other Oncology | | Various | | 645 | | | 470 | | | 395 | |
Hospital(a), (c) | | $ | 7,961 | | | $ | 7,772 | | | $ | 7,955 | |
| Sulperazon | | Bacterial infections | | 618 | | | 684 | | | 613 | |
| Medrol | | Anti-inflammatory glucocorticoid | | 402 | | | 469 | | | 493 | |
EpiPen(a) | | Epinephrine injection used in treatment of life-threatening allergic reactions | | 297 | | | 303 | | | 303 | |
| Zithromax | | Bacterial infections | | 276 | | | 336 | | | 326 | |
| Vfend | | Fungal infections | | 270 | | | 346 | | | 392 | |
| Panzyga | | Primary humoral immunodeficiency | | 269 | | | 183 | | | 39 | |
| Precedex | | Sedation agent in surgery or intensive care | | 260 | | | 155 | | | 213 | |
| Fragmin | | Treatment/prevention of venous thromboembolism | | 252 | | | 253 | | | 293 | |
| Zyvox | | Bacterial infections | | 222 | | | 251 | | | 236 | |
| Zavicefta | | Bacterial infections | | 212 | | | 108 | | | 46 | |
Pfizer CentreOne(d) | | Various | | 926 | | | 810 | | | 755 | |
| All other Anti-infectives | | Various | | 1,455 | | | 1,592 | | | 1,661 | |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 106 |
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| (MILLIONS OF DOLLARS) | | | | Year Ended December 31, |
| PRODUCT | | PRIMARY INDICATION OR CLASS | | 2020 | | 2019 | | 2018 |
| | | | | | |
All other Hospital(c) | | Various | | 2,502 | | | 2,281 | | | 2,584 | |
| Vaccines | | $ | 6,575 | | | $ | 6,504 | | | $ | 6,332 | |
| Prevnar 13/Prevenar 13 | | Pneumococcal disease | | 5,850 | | | 5,847 | | | 5,802 | |
| Nimenrix | | Meningococcal disease | | 221 | | | 230 | | | 140 | |
| FSME/IMMUN-TicoVac | | Tick-borne encephalitis disease | | 196 | | | 220 | | | 184 | |
| BNT162b2 | | Active immunization to prevent COVID-19 in individuals 16 years of age and older | | 154 | | | 0 | | | 0 | |
| All other Vaccines | | Various | | 154 | | | 207 | | | 206 | |
| Inflammation & Immunology (I&I) | | $ | 4,567 | | | $ | 4,733 | | | $ | 4,720 | |
| Xeljanz | | RA, PsA, UC, active polyarticular course juvenile idiopathic arthritis | | 2,437 | | | 2,242 | | | 1,774 | |
| Enbrel (Outside the U.S. and Canada) | | RA, juvenile idiopathic arthritis, PsA, plaque psoriasis, pediatric plaque psoriasis, ankylosing spondylitis and nonradiographic axial spondyloarthritis | | 1,350 | | | 1,699 | | | 2,112 | |
Inflectra/Remsima(b) | | Crohn’s disease, pediatric Crohn’s disease, UC, pediatric UC, RA in combination with methotrexate, ankylosing spondylitis, PsA and plaque psoriasis | | 659 | | | 625 | | | 642 | |
| All other I&I | | Various | | 121 | | | 167 | | | 192 | |
| Rare Disease | | $ | 2,936 | | | $ | 2,278 | | | $ | 2,211 | |
| Vyndaqel/Vyndamax | | ATTR-cardiomyopathy and polyneuropathy | | 1,288 | | | 473 | | | 148 | |
| BeneFIX | | Hemophilia B | | 454 | | | 488 | | | 554 | |
| Genotropin | | Replacement of human growth hormone | | 427 | | | 498 | | | 558 | |
| Refacto AF/Xyntha | | Hemophilia A | | 370 | | | 426 | | | 514 | |
| Somavert | | Acromegaly | | 277 | | | 264 | | | 267 | |
| All other Rare Disease | | Various | | 120 | | | 129 | | | 170 | |
Consumer Healthcare Business(e) | | $ | 0 | | | $ | 2,082 | | | $ | 3,587 | |
| | | | | | | | |
| Total Alliance revenues | | $ | 5,418 | | | $ | 4,648 | | | $ | 3,838 | |
Total Biosimilars(b) | | $ | 1,527 | | | $ | 911 | | | $ | 769 | |
Total Sterile Injectable Pharmaceuticals(a). (f) | | $ | 5,315 | | | $ | 5,013 | | | $ | 5,173 | |
(a)On November 16, 2020, we completed the spin-off and the combination of our Upjohn Business with Mylan to form Viatris. On December 21, 2020, Pfizer and Viatris completed the termination of a pre-existing strategic collaboration between Pfizer and Mylan for generic drugs in Japan (Mylan-Japan) and we transferred the operations that were part of the Mylan-Japan collaboration to Viatris. Beginning in the fourth quarter of 2020, the financial results of the Upjohn Business and the Mylan-Japan collaboration are reported as Income from discontinued operations––net of tax for all periods presented. Prior-period financial information has been restated, as appropriate. Prior to the separation of the Upjohn Business, and beginning in 2020, Upjohn began managing our Meridian subsidiary, the manufacturer of EpiPen and other auto-injector products, and the Mylan-Japan collaboration. As a result, revenues associated with our Meridian subsidiary, except for product revenues for EpiPen sold in Canada, and Mylan-Japan were reported in Upjohn beginning in the first quarter of 2020. Beginning in the fourth quarter of 2020, the results of our Meridian subsidiary are reported in the Hospital therapeutic area for all periods presented in our consolidated financial statements.
(b)Biosimilars are highly similar versions of approved and authorized biological medicines and primarily include revenues from Inflectra/Remsima, Retacrit, Ruxience and Zirabev.
(c)Hospital is a therapeutic area that commercializes our global portfolio of sterile injectable and anti-infective medicines. Hospital also includes Pfizer CentreOne(d). All other Hospital primarily includes revenues from legacy Sterile Injectable Pharmaceuticals (SIP) products (that are not anti-infective products) and, to a much lesser extent, solid oral dose products (that are not anti-infective products). SIP anti-infective products that are not individually listed above are recorded in “All other Anti-infectives”.
(d)Pfizer CentreOne includes revenues from our contract manufacturing and active pharmaceutical ingredient sales operation, including sterile injectables contract manufacturing, andThe deferred revenues related to our manufacturing and supply agreements.
(e)On July 31, 2019, our Consumer Healthcare business, an OTC medicines business, was combined with GSK’s consumer healthcare business to form a new consumer healthcare JV. See Note 2C.
(f)Total Sterile Injectable Pharmaceuticals represents the total of all branded and generic injectable products in the Hospital therapeutic area, including anti-infective sterile injectable pharmaceuticals.
Contract Liabilities
Our contract liabilities primarily relate to advance payments received or receivable in connection with contracts that we entered into during 2020 with various government or government sponsored customers in international markets for supply of BNT162b2. The deferred revenue associated with these advance payments totals approximately $957 millionComirnaty totaled $1.7 billion as of December 31, 20202023, with $1.1 billion and are$552 million recorded in Other current liabilities. and noncurrent liabilities, respectively. The deferred revenues related to Comirnaty totaled $2.5 billion as of December 31, 2022, with $2.4 billion and $77 million recorded in current liabilities and noncurrent liabilities, respectively. The decrease in Comirnaty deferred revenues during 2023 was primarily the result of amounts recognized in Product revenues as we delivered the products to our customers, partially offset by additional advance payments received as we entered into amended contracts, as well as the impact of foreign exchange. During 2023, we recognized revenue of approximately $2.2 billion that was included in the balance of Comirnaty deferred revenues as of December 31, 2022.
The Paxlovid and Comirnaty deferred revenues as of December 31, 2023 will be recognized in RevenuesProduct revenues proportionately as we deliver dosestransfer control of the vaccineproducts to our customers and satisfy our performance obligationobligations under the contracts, which we expectwith the amounts included in current liabilities expected to fully occur during 2021. Contractbe recognized in Product revenues within the next 12 months, and the amounts included in noncurrent liabilities expected to be recognized in Product revenues from December 2024 (which falls in our international first quarter of 2025) through 2028. Deferred revenues associated with contracts for other customer contractsproducts were not significant as of December 31, 20202023 or 2019.2022.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 107 |
Selected Quarterly Financial Data (Unaudited)
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Quarter |
| (MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA) | | First | | Second | | Third | | Fourth |
2020(a) | | | | | | | | |
| Revenues | | $ | 10,083 | | | $ | 9,864 | | | $ | 10,277 | | | $ | 11,684 | |
Costs and expenses(b) | | 7,219 | | | 6,559 | | | 8,716 | | | 11,323 | |
| Restructuring charges and certain acquisition-related costs | | 54 | | | 360 | | | 2 | | | 184 | |
| (Gain) on completion of Consumer Healthcare JV transaction | | (6) | | | — | | | — | | | — | |
| Income/(loss) from continuing operations before provision/(benefit) for taxes on income/(loss) | | 2,817 | | | 2,944 | | | 1,559 | | | 178 | |
| Provision/(benefit) for taxes on income/(loss) | | 355 | | | 396 | | | (104) | | | (170) | |
| Income/(loss) from continuing operations | | 2,462 | | | 2,548 | | | 1,663 | | | 348 | |
Income from discontinued operations––net of tax(c) | | 948 | | | 887 | | | 539 | | | 257 | |
| Net income/(loss) before allocation to noncontrolling interests | | 3,410 | | | 3,434 | | | 2,202 | | | 605 | |
| Less: Net income attributable to noncontrolling interests | | 9 | | | 8 | | | 8 | | | 11 | |
| Net income/(loss) attributable to Pfizer Inc. common shareholders | | $ | 3,401 | | | $ | 3,426 | | | $ | 2,194 | | | $ | 594 | |
| Earnings/(loss) per common share—basic: | | | | | | | | |
| Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | | $ | 0.44 | | | $ | 0.46 | | | $ | 0.30 | | | $ | 0.06 | |
Income from discontinued operations––net of tax(c) | | 0.17 | | | 0.16 | | | 0.10 | | | 0.05 | |
| Net income/(loss) attributable to Pfizer Inc. common shareholders | | $ | 0.61 | | | $ | 0.62 | | | $ | 0.39 | | | $ | 0.11 | |
| Earnings/(loss) per common share—diluted: | | | | | | | | |
| Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | | $ | 0.44 | | | $ | 0.45 | | | $ | 0.29 | | | $ | 0.06 | |
Income from discontinued operations––net of tax(c) | | 0.17 | | | 0.16 | | | 0.10 | | | 0.05 | |
| Net income/(loss) attributable to Pfizer Inc. common shareholders | | $ | 0.61 | | | $ | 0.61 | | | $ | 0.39 | | | $ | 0.10 | |
(a)Business development activities impacted our results of operations in 2020. See Note 1A.
(b)The fourth quarter historically reflects higher costs in Cost of sales, Selling, informational and administrative expenses and Research and development expenses. Certain asset impairments totaled $900 million in the third quarter of 2020 and $791 million in the fourth quarter of 2020 recorded in Other (income)/deductions—net. See Note 4.
(c)Operating results of the Upjohn Business and the Mylan-Japan collaboration are presented as discontinued operations in all periods presented following the November 16, 2020 spin-off and combination of our Upjohn Business with Mylan and the December 21, 2020 termination of the Mylan-Japan collaboration. See Note 2B.
Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 108 |
Selected Quarterly Financial Data (Unaudited)
Pfizer Inc. and Subsidiary Companies
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Quarter |
| (MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA) | | First | | Second | | Third | | Fourth |
2019(a) | | | | | | | | |
| Revenues | | $ | 9,957 | | | $ | 10,363 | | | $ | 10,402 | | | $ | 10,449 | |
Costs and expenses(b) | | 7,839 | | | 8,257 | | | 8,695 | | | 12,380 | |
Restructuring charges and certain acquisition-related costs(c), (d) | | 39 | | | (122) | | | 351 | | | 333 | |
(Gain) on completion of Consumer Healthcare JV transaction(d) | | — | | | — | | | (8,087) | | | 1 | |
| Income/(loss) from continuing operations before provision/(benefit) for taxes on income/(loss) | | 2,079 | | | 2,228 | | | 9,442 | | | (2,264) | |
Provision/(benefit) for taxes on income/(loss)(e) | | 142 | | | (1,169) | | | 2,866 | | | (1,221) | |
| Income/(loss) from continuing operations | | 1,937 | | | 3,397 | | | 6,576 | | | (1,043) | |
Income from discontinued operations––net of tax(f) | | 1,952 | | | 1,659 | | | 1,107 | | | 716 | |
| Net income/(loss) before allocation to noncontrolling interests | | 3,889 | | | 5,056 | | | 7,684 | | | (327) | |
| Less: Net income attributable to noncontrolling interests | | 6 | | | 10 | | | 4 | | | 10 | |
| Net income/(loss) attributable to Pfizer Inc. common shareholders | | $ | 3,884 | | | $ | 5,046 | | | $ | 7,680 | | | $ | (337) | |
| Earnings/(loss) per common share—basic: | | | | | | | | |
| Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | | $ | 0.34 | | | $ | 0.61 | | | $ | 1.19 | | | $ | (0.19) | |
Income from discontinued operations––net of tax(f) | | 0.35 | | | 0.30 | | | 0.20 | | | 0.13 | |
| Net income/(loss) attributable to Pfizer Inc. common shareholders | | $ | 0.69 | | | $ | 0.91 | | | $ | 1.38 | | | $ | (0.06) | |
| Earnings/(loss) per common share—diluted: | | | | | | | | |
| Income/(loss) from continuing operations attributable to Pfizer Inc. common shareholders | | $ | 0.34 | | | $ | 0.60 | | | $ | 1.16 | | | $ | (0.19) | |
Income from discontinued operations––net of tax(f) | | 0.34 | | | 0.29 | | | 0.20 | | | 0.13 | |
| Net income/(loss) attributable to Pfizer Inc. common shareholders | | $ | 0.68 | | | $ | 0.89 | | | $ | 1.36 | | | $ | (0.06) | |
(a)Business development activities impacted our results of operations in 2019. See Note 1A.
(b)The fourth quarter historically reflects higher costs in Cost of sales, Selling, informational and administrative expenses and Research and development expenses. The fourth quarter of 2019 includes $2.6 billion in certain asset impairments recorded in Other (income)/deductions—net. See Note 4.
(c)The second quarter of 2019 includes the reversal of certain accruals related to our acquisition of Wyeth upon the effective favorable settlement of an IRS audit from multiple tax years. See Note 5B. The third quarter of 2019 includes $217 million of integration costs and other, primarily including $157 million in payments to Array employees for the fair value of previously unvested stock options that was recognized as post-closing compensation expense. See Note 2A. The fourth quarter of 2019 primarily includes employee termination costs, asset impairments and other exit costs associated with cost reduction initiatives. The employee termination costs are mostly associated with our improvements to operational effectiveness as part of the realignment of our organizational structure and for the Transforming to a More Focused Company program. See Note 3.
(d)See Note 2C.
(e)During the second quarter of 2019, Pfizer reached settlement of disputed issues at the IRS Office of Appeals, thereby settling all issues related to U.S. tax returns of Pfizer for the years 2009-2010. As a result of settling these years, in the second quarter of 2019 we recorded a benefit of approximately $1.4 billion, representing tax and interest. The third quarter of 2019 reflects tax expense of approximately $2.7 billion associated with the gain related to the completion of the Consumer Healthcare JV.
(f)Operating results of the Upjohn Business and the Mylan-Japan collaboration are presented as discontinued operations in all periods presented following the November 16, 2020 spin-off and combination of our Upjohn Business with Mylan and the December 21, 2020 termination of the Mylan-Japan collaboration. See Note 2B.
Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year.
| | | | | |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| | | | | |
| ITEM 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
As of the end of the period covered by this Form 10-K, we carried out an evaluation, under the supervision and with the participation of our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures are effective in alerting them in a timely manner to material information required to be disclosed in our periodic reports filed with the SEC.
Changes in Internal Controls
DuringOn December 14, 2023, we acquired Seagen. Other than the addition of Seagen’s operations to our most recent fiscal quarter,internal control over financial reporting and any related changes in control to integrate Seagen into Pfizer, there has not been any change in the Company’s internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 109107 |
Report of Independent Registered Public Accounting Firm on
Internal Control Over Financial Reporting
TheTo the Board of Directors and Shareholders of
Pfizer Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited Pfizer Inc. and Subsidiary Companies’ (the Company) internal control over financial reporting as of December 31, 2020,2023, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2020,2023, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of Pfizer Inc. and Subsidiary Companiesthe Company as of December 31, 20202023 and 2019, and2022, the related consolidated statements of income, comprehensive income, equity, and cash flows for each of the years in the three-year period ended December 31, 2020,2023, and the related notes (collectively, the consolidated financial statements), and our report dated February 25, 202122, 2024 expressed an unqualified opinion on those consolidated financial statements.
The scope of management’s assessment of the effectiveness of internal control over financial reporting includes all of the Company’s consolidated operations except for the operations of Seagen Inc. and its subsidiaries (Seagen), which the Company acquired on December 14, 2023. Seagen’s operations represent 0.2% of the Company’s consolidated revenues for the year ended December 31, 2023, and assets associated with Seagen’s operations represent 22% of the Company’s consolidated total assets, as of December 31, 2023. Our audit of internal control over financial reporting of the Company also excluded an evaluation of the internal control over financial reporting of Seagen.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting.Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| | |
KPMG LLP |
| New York, New York |
|
February 25, 202122, 2024 |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 110108 |
Management’s Report on Internal Control Over Financial Reporting
Management’s Report
We prepared and are responsible for the financial statements that appear in this Form 10-K. These financial statements are in conformity with accounting principles generally accepted in the United States of America and, therefore, include amounts based on informed judgments and estimates. We also accept responsibility for the preparation of other financial information that is included in this document.
Report on Internal Control Over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. The Company’s internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate. Management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2020.2023. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control—Integrated Framework (2013). Based on our assessment and those criteria, management believes that the Company maintained effective internal control over financial reporting as of December 31, 2020.2023.
The scope of management’s assessment of the effectiveness of internal control over financial reporting includes all of the Company’s consolidated operations except for the operations of Seagen Inc. and its subsidiaries (Seagen), which the Company acquired on December 14, 2023. Seagen’s operations represent 0.2% of the Company’s consolidated revenues for the year ended December 31, 2023, and assets associated with Seagen’s operations represent 22% of the Company’s consolidated total assets, as of December 31, 2023.
The Company’s independent auditors have issued their auditors’ report on the Company’s internal control over financial reporting. That report appears above in this Form 10-K.
| | | | | | | | |
| | |
| Albert Bourla | | |
| Chairman and Chief Executive Officer | | |
| | | | | | | | |
| | |
Frank D’AmelioDavid M. Denton | | Jennifer B. Damico |
| Principal Financial Officer | | Principal Accounting Officer |
| | |
February 25, 202122, 2024 | | |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 111109 |
| | | | | |
| ITEM 9B. | OTHER INFORMATION |
During the three months ended December 31, 2023, none of our directors or officers adopted or terminated a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408 of Regulation S-K.
| | | | | |
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Information about our Directors is incorporated by reference from the discussion under the heading Item 1—Election of Directors in our Proxy Statement. Information about the Pfizer Policies on Business Conduct governing our employees, including our Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer, and the Code of Business Conduct and Ethics for Members of the Board of Directors, is incorporated by reference from the discussions under the headings Governance Overview—Pfizer Policies on Business Conduct and —Code of Conduct for Directors in our Proxy Statement. Information regarding the procedures by which our shareholders may recommend nominees to our Board of Directors is incorporated by reference from the discussion under the headings Item 1—Election of Directors—Criteria for Board Membership and Annual Meeting Information—Submitting Proxy Proposals and Director Nominations for the 20222025 Annual Meeting in our Proxy Statement. Information about our Audit Committee, including the members of the Committee, and our Audit Committee financial experts, is incorporated by reference from the discussion under the heading Governance Overview—Board Information—Board and Committee Information—Board Committees—The Audit Committee in our Proxy Statement. The balance of the information required by this item is contained in the discussion entitled Information about Our Executive Officers in this Form 10-K. | | | | | |
| ITEM 11. | EXECUTIVE COMPENSATION |
Information about Director and executive compensation is incorporated by reference from the discussion under the headings Non-Employee Director Compensation; Executive Compensation; and Governance—Board Information—Governance Overview—Board and Committee Information—Board Committees—The Compensation Committee—Compensation Committee Interlocks and Insider Participation in our Proxy Statement.
| | | | | |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Information required by this item is incorporated by reference from the discussion under the headings Executive Compensation—Compensation Tables—Equity Compensation Plan Information and Securities Ownership in our Proxy Statement.
| | | | | |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Information about certain relationships and transactions with related parties is incorporated by reference from the discussion under the headings Governance Overview—Other Governance Practices and Policies—Related Person Transactions and Indemnificationand—Transactions with Related Persons in our Proxy Statement. Information about director independence is incorporated by reference from the discussion under the heading GovernanceItem 1—Other Governance Practices and PoliciesElection of Directors—Director Independence in our Proxy Statement.
| | | | | |
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
Our independent registered public accounting firm is KPMG LLP, New York, NY, Auditor Firm ID: 185. Information about the fees for professional services rendered by our independent registered public accounting firm in 20202023 and 20192022 is incorporated by reference from the discussion under the heading Item 2—Ratification of Selection of Independent Registered Public Accounting Firm—Audit and Non-Audit Fees in our Proxy Statement. Our Audit Committee’s policy on pre-approval of audit and permissible non-audit services of our independent registered public accounting firm is incorporated by reference from the discussion under the heading Item 2—Ratification of Selection of Independent Registered Public Accounting Firm—Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Registered Public Accounting Firmin our Proxy Statement.
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 112 |
| | | | | |
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
15(a)(1) Financial Statements. The following consolidated financial statements, related notes and report of independent registered public accounting firm and supplementary data are set forth in Item 8. Financial Statements and Supplementary Data in this Form 10-K: •Selected Quarterly Financial Data (Unaudited)
15(a)(2) Financial Statement Schedules. Schedules are omitted because they are not required or because the information is provided elsewhere in the financial statements. The financial statements of unconsolidated subsidiaries are omitted because, considered in the aggregate, they would not constitute a significant subsidiary.
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 110 |
15(a)(3) Exhibits. These exhibits are available upon request. Requests should be directed to our Corporate Secretary, Pfizer Inc., 23566 Hudson Boulevard East, 42nd Street, New York, New York 10017.10001-2192. The exhibit numbers preceded by an asterisk (*) indicate exhibits filed with this Form 10-K. All other exhibit numbers indicate exhibits filed by incorporation by reference. Exhibit numbers 10.1 through 10.4410.50 are management contracts or compensatory plans or arrangements.
| | | | | | | | |
|
| | Stock and Asset Purchase Agreement, dated December 19, 2018, by and among us, GlaxoSmithKline plc and GlaxoSmithKline Consumer Healthcare Holdings Limited is incorporated by reference from our 2018 Annual Report on Form 10-K. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the SEC upon request any omitted schedule or exhibit to the Stock and Asset Purchase Agreement.) |
| | |
| | Business Combination Agreement and Plan of Merger, by and among Pfizer Inc., Aris Merger Sub, Inc. and Seagen Inc., dated as of July 29, 2019, by and among us, Upjohn Inc., Utah Acquisition Sub Inc., Mylan N.V., Mylan I B.V. and Mylan II B.V.March 12, 2023 is incorporated by reference from our Current Report on Form 8-K filed on July 29, 2019. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the SEC upon request any omitted schedule or exhibit to the Business Combination Agreement.)March 13, 2023. |
| | |
| | Amendment No. 1 to the Business Combination Agreement, dated as of May 29, 2020, by and among us, Upjohn Inc., Utah Acquisition Sub Inc., Mylan N.V., Mylan I B.V. and Mylan II B.V. is incorporated by reference from our Current Report on Form 8-K filed on June 1, 2020. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the SEC upon request any omitted schedule or exhibit to the Amendment No. 1 to the Business Combination Agreement.) |
| | |
| | Separation and Distribution Agreement, dated as of July 29, 2019, by and between us and Upjohn Inc. is incorporated by reference from our Current Report on Form 8-K filed on July 29, 2019. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the SEC upon request any omitted schedule or exhibit to the Separation and Distribution Agreement.) |
| | |
| | Amendment No. 1 to the Separation and Distribution Agreement, dated as of February 18, 2020, by and between us and Upjohn Inc. is incorporated by reference from our 2019 Annual Report on Form 10-K. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the SEC upon request any omitted schedule or exhibit to the Amendment No. 1 to the Separation and Distribution Agreement.) |
| | |
| | Amendment No. 2 to the Separation and Distribution Agreement, dated as of May 29, 2020, by and between us and Upjohn Inc. is incorporated by reference from our Current Report on Form 8-K filed on June 1, 2020. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the SEC upon request any omitted schedule or exhibit to the Amendment No. 2 to the Separation and Distribution Agreement.) |
| | |
| | Amendment No. 3 to the Separation and Distribution Agreement, dated as of September 18, 2020, by and between us and Upjohn Inc. is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended September 27, 2020. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the SEC upon request any omitted schedule or exhibit to the Amendment No. 3 to the Separation and Distribution Agreement.) |
| | |
| | Amendment No. 4 to the Separation and Distribution Agreement, dated as of November 15, 2020, by and between us and Upjohn Inc. (Pursuant to Item 601(b)(2) of Regulation S-K, the registrant hereby agrees to supplementally furnish to the SEC upon request any omitted schedule or exhibit to the Amendment No. 4 to the Separation and Distribution Agreement.) |
| | |
| | Our Restated Certificate of Incorporation dated December 14, 2020, is incorporated by reference from our Current Report on Form 8-K filed on December 14, 2020. |
| | |
| | Our By-laws, as amended on December 18, 2017,9, 2022, are incorporated by reference from our Current Report on Form 8-K filed on December 21, 2017.13, 2022. |
| | |
| | Indenture, dated as of January 30, 2001, between us and The Chase Manhattan Bank, is incorporated by reference from our Current Report on Form 8-K filed on January 30, 2001. |
| | |
| | First Supplemental Indenture, dated as of March 24, 2009, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended June 28, 2009. |
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 113 |
| | | | | | | | |
| | |
| | Second Supplemental Indenture, dated as of June 2, 2009, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K filed on June 3, 2009. |
| | |
| | Third Supplemental Indenture, dated as of June 3, 2013, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K filed on June 3, 2013. |
| | |
| | Fourth Supplemental Indenture, dated as of May 15, 2014, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on May 15, 2014. |
| | |
| | Fifth Supplemental Indenture, dated as of October 5, 2015, between us and The Bank of New York Mellon (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank)), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on October 6, 2015. |
| | |
| | Sixth Supplemental Indenture, dated as of June 3, 2016, between us and The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on June 3, 2016. |
| | |
| | Seventh Supplemental Indenture, dated as of November 21, 2016, between us and The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on November 21, 2016. |
| | |
| | Eighth Supplemental Indenture, dated as of March 17, 2017, among us, The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (successor to the Chase Manhattan Bank (National Association)))), as trustee, and The Bank of New York Mellon, London Branch, as paying agent, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on March 17, 2017. |
| | |
| | Ninth Supplemental Indenture, dated as of March 6, 2017, among us, The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, and The Bank of New York Mellon, London Branch, as paying agent and calculation agent, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on March 6, 2017. |
| | |
| | Tenth Supplemental Indenture, dated as of December 19, 2017, among us, The Bank of New York Mellon (formerly The Bank of New York (successor to JPMorgan Chase Bank, N.A. (formerly JPMorgan Chase Bank, formerly The Chase Manhattan Bank (National Association)))), as trustee, and The Bank of New York Mellon, London Branch, as paying agent, to Indenture dated as of January 30, 2001, is incorporated by reference from our Current Report on Form 8-K report filed on December 19, 2017. |
| | |
| | Indenture, dated as of April 10, 1992, between Wyeth (formerly American Home Products Corporation) and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Registration Statement on Form S-3, filed on January 18, 1995. |
| | |
| | Supplemental Indenture, dated as of October 13, 1992, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Registration Statement on Form S-3, filed on January 18, 1995. |
| | |
| | Fifth Supplemental Indenture, dated as of December 16, 2003, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s 2003 Annual Report on Form 10-K. |
| | |
| | Sixth Supplemental Indenture, dated as of November 14, 2005, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Current Report on Form 8-K filed on November 15, 2005. |
| | |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 111 |
| | | | | | | | |
| | Seventh Supplemental Indenture, dated as of March 27, 2007, between Wyeth and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, N.A.), as trustee, is incorporated by reference from Wyeth’s Current Report on Form 8-K filed on March 28, 2007. |
| | |
| | Eighth Supplemental Indenture, dated as of October 30, 2009, between Wyeth, us and The Bank of New York Mellon (as successor to JPMorgan Chase Bank, formerly The Chase Manhattan Bank), as trustee, to Indenture dated as of April 10, 1992 (as amended on October 13, 1992), is incorporated by reference from our Current Report on Form 8-K filed on November 3, 2009. |
| | |
| | Indenture, dated as of September 7, 2018, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on September 7, 2018. |
| | |
| | First Supplemental Indenture, dated as of September 7, 2018, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on September 7, 2018. |
| | |
| | Second Supplemental Indenture, dated as of March 11, 2019, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on March 11, 2019. |
| | |
| | Third Supplemental Indenture, dated as of March 27, 2020, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on March 27, 2020. |
| | |
| | Fourth Supplemental Indenture, dated as of May 28, 2020, between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on May 28, 2020. |
| | |
| | Fifth Supplemental Indenture, dated as of August 18, 2021 between us and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on August 18, 2021. |
| | Indenture, dated as of May 19, 2023, among Pfizer Investment Enterprises Pte. Ltd., Pfizer Inc. and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on May 19, 2023. |
| | |
| | First Supplemental Indenture, dated as of May 19, 2023, among Pfizer Investment Enterprises Pte. Ltd., Pfizer Inc. and The Bank of New York Mellon, as trustee, is incorporated by reference from our Current Report on Form 8-K filed on May 19, 2023. |
| | |
| | Description of Pfizer’s Securities. |
| | |
| | | | | | | | |
Pfizer Inc.4.27 | | 2020 Form 10-K114 |
| | | | | | | | |
4.24 | | Except as set forth in Exhibits 4.1-224.1-4.26 above, the instruments defining the rights of holders of long-term debt securities of the Company and its subsidiaries have been omitted. We agree to furnish to the SEC, upon request, a copy of each instrument with respect to issuances of long-term debt of the Company and its subsidiaries. |
| | |
| | 2001 Stock and Incentive Plan is incorporated by reference from our Proxy Statement for the 2001 Annual Meeting of Shareholders. |
| | |
| | Pfizer Inc. 2004 Stock Plan, as Amended and Restated is incorporated by reference from our 2011 Annual Report on Form 10-K. |
| | |
| | Amendment No. 1 to Pfizer 2004 Stock Plan.Plan is incorporated by reference from our 2020 Annual Report on Form 10-K. |
| | |
| | Pfizer Inc. 2014 Stock Plan is incorporated by reference from our Proxy Statement for the 2014 Annual Meeting of Shareholders. |
| | |
| | Amendment No. 1 to Pfizer Inc. 2014 Stock Plan.Plan is incorporated by reference from our 2020 Annual Report on Form 10-K. |
| | |
| | Form of Acknowledgment and Consent and Summary of Key Terms for Grants of RSUs, TSRUs, PPSs and PSAs is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended March 29, 2020.April 2, 2023. |
| | |
| | Form of Executive Grant Letter is incorporated by reference from our 2015 Annual Report on Form 10-K. |
| | |
| | Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees is incorporated by reference from our 2017 Annual Report on Form 10-K. |
| | |
| | Amendment No. 1 to the Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees is incorporated by reference from our 2018 Annual Report on Form 10-K. |
| | |
| | Amendment No. 2 to the Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees.Employees is incorporated by reference from our 2020 Annual Report on Form 10-K. |
| | |
| | Amendment No. 3 to the Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees is incorporated by reference from our 2022 Annual Report on Form 10-K. |
| | |
| | Amendment No. 4 to the Pfizer Consolidated Supplemental Pension Plan for United States and Puerto Rico Employees. |
| | Pfizer Supplemental Savings Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended April 3, 2016. |
| | |
| | Amendment No. 1 to the Pfizer Supplemental Savings Plan (Amended and Restated as of January 1, 2016), is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended October 1, 2017. |
| | |
| | Amendment No. 2 to the Pfizer Supplemental Savings Plan is incorporated by reference from our 2017 Annual Report on Form 10-K. |
| | |
| | Amendment No. 3 to the Pfizer Supplemental Savings Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended September 30, 2018. |
| | |
| | Amendment No. 4 to the Pfizer Supplemental Savings Plan is incorporated by reference from our 2018 Annual Report on Form 10-K. |
| | |
| | Amendment No. 5 to the Pfizer Supplemental Savings Plan is incorporated by reference from our 2018 Annual Report on Form 10-K. |
| | |
| | Amendment No. 6 to the Pfizer Supplemental Savings Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended June 30, 2019. |
| | |
| | Amendment No. 7 to the Pfizer Supplemental Savings Plan is incorporated by reference from our 2019 Annual Report on Form 10-K. |
| | |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 112 |
| | | | | | | | |
| | Amendment No. 8 to the Pfizer Supplemental Savings Plan.Plan is incorporated by reference from our 2020 Annual Report on Form 10-K. |
| | |
| | Amendment No. 9 to the Pfizer Supplemental Savings Plan.Plan is incorporated by reference from our 2020 Annual Report on Form 10-K. |
| | |
| | Amendment No. 10 to the Pfizer Supplemental Savings Plan is incorporated by reference from our 2022 Annual Report on Form 10-K. |
| | |
| | Amended and Restated Pfizer Inc. Global Performance Plan.Plan is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended October 1, 2023. |
| | |
| | Amended and Restated Deferred Compensation Plan is incorporated by reference from our 2012 Annual Report on Form 10-K. |
| | |
| | Amendment to Amended and Restated Deferred Compensation Plan, dated June 20, 2013, is incorporated by reference from our 2013 Annual Report on Form 10-K. |
|
| | Amendment No. 2 to Amended and Restated Deferred Compensation Plan, dated April 27, 2016, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended July 3, 2016. |
| | |
| | Amendment No. 3 to Amended and Restated Deferred Compensation Plan.Plan is incorporated by reference from our 2020 Annual Report on Form 10-K. |
| | |
| | Amendment No. 4 to Amended and Restated Deferred Compensation Plan. |
| | Wyeth 2005 (409A) Deferred Compensation Plan (frozen as of January 2012), together with certain Amendments, is incorporated by reference from our 2013 Annual Report on Form 10-K. |
| | |
| | Amendment No. 2 to Wyeth 2005 (409A) Deferred Compensation Plan.Plan is incorporated by reference from our 2020 Annual Report on Form 10-K. |
| | |
| | Amended and Restated Wyeth Supplemental Employee Savings Plan (effective as of January 1, 2005 and frozen as of January 2012), together with all material Amendments is incorporated by reference from our 2011 Annual Report on Form 10-K. |
| | |
| | Amendment to Amended and Restated Wyeth Supplemental Employee Savings Plan, dated June 20, 2013, is incorporated by reference from our 2013 Annual Report on Form 10-K. |
| | | | | | | | |
Pfizer Inc. | 2020 Form 10-K | 115 |
| | | | | | | | |
| | |
| | The form of Indemnification Agreement with each of our non-employee Directors is incorporated by reference from our 1996 Annual Report on Form 10-K. |
| | |
| | The form of Indemnification Agreement with each of the Named Executive Officers identified in our Proxy Statement for the 20202023 Annual Meeting of Shareholders is incorporated by reference from our 1997 Annual Report on Form 10-K. |
| | |
| | Letter to Frank A. D’Amelio regarding replacement pension benefit dated August 22, 2007 is incorporated by reference from our Current
Report on Form 8-K filed on August 22, 2007. |
| | |
| | Pfizer Inc. Executive Severance Plan is incorporated by referenced from our Current Report on Form 8-K filed on February 20, 2009. |
| | |
| | Amendment No. 1 to the Pfizer Inc. Executive Severance Plan is incorporated by reference from our 2018 Annual Report on Form 10-K. |
| | |
| | Amendment No. 2 to the Pfizer Inc. Executive Severance Plan is incorporated by reference from our 2019 Annual Report on Form 10-K. |
| | |
| | Amendment No. 3 to the Pfizer Inc. Executive Severance Plan.Plan is incorporated by reference from our 2020 Annual Report on Form 10-K. |
| | |
| | Amendment No. 4 to the Pfizer Inc. Executive Severance Plan is incorporate by reference from our 2022 Annual Report on Form 10-K. |
| | |
| | Annual Retainer Unit Award Plan (for Non-Employee Directors) (frozen as of March 1, 2006) as amended, is incorporated by reference from our 2008 Annual Report on Form 10-K. |
| | |
| | Nonfunded Deferred Compensation and Unit Award Plan for Non-Employee Directors, as amended, is incorporated by reference from our Quarterly2022 Annual Report on Form 10-Q for the period ended September 28, 2014.10-K. |
| | |
| | Form of Special Award Letter Agreement is incorporated by reference from our Current Report on Form 8-K filed on October 28, 2009. |
| | |
| | Offer Letter to G. Mikael Dolsten, dated April 6, 2009, is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended April 3, 2011. |
| | |
| | Form of Special Performance-Based Incentive Award Letter is incorporated by reference from our 2017 Annual Report on
Form 10-K. |
| | |
| | Form of Special Performance-Based Incentive Grant Letter is incorporated by reference from our 2017 Annual Report on
Form 10-K. |
| | |
| | Pfizer Inc. 2019 Stock Plan is incorporated by reference from our Proxy Statement for the 2019 Annual Meeting of Shareholders. |
| | |
| | Time Sharing Agreement, dated July 9, 2020, between Pfizer Inc. and Albert Bourla is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended June 28, 2020. |
| | |
| | Pfizer Inc. Executive Officer Cash Severance Policy is incorporated by reference from our Quarterly Report on Form 10-Q for the period ended July 2, 2023 |
| | |
| | Subsidiaries of the Company. |
| | |
| | Subsidiary Issuers of Guaranteed Securities. |
| | |
| | Consent of Independent Registered Public Accounting Firm. |
| | |
| | Power of Attorney (included as part of signature page). |
| | |
| | Certification by the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | |
| | | | | | | | |
| Pfizer Inc. | 2023 Form 10-K | 113 |
| | | | | | | | |
| | Certification by the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| | |
| | Certification by the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | |
| | Certification by the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| | |
| | Pfizer Inc. Recoupment Policy. |
| | |
| Exhibit 101: | | |
| *101.INS | | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| | |
| *101.SCH | | Inline XBRL Taxonomy Extension Schema |
| | |
| *101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase |
| | |
| *101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase |
| | |
| *101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase |
| | |
| *101.DEF | | Inline XBRL Taxonomy Extension Definition Document |
| | |
| 104 | | Cover Page Interactive Data File - the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| | | | | |
| ITEM 16. | FORM 10-K SUMMARY |
None.
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 116114 |
SIGNATURES
Under the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, this report was signed on behalf of the Registrant by the authorized person named below.
| | | | | | | | | | | |
| Pfizer Inc. |
| | |
Dated: February 25, 202122, 2024 | By: | | /S/ MARGARET M. MADDEN |
| | | Margaret M. Madden
Senior Vice President and Corporate Secretary
Chief Governance Counsel |
We, the undersigned directors and officers of Pfizer Inc., hereby severally constitute Douglas M. Lankler and Margaret M. Madden, and each of them singly, our true and lawful attorneys with full power to them and each of them to sign for us, in our names in the capacities indicated below, any and all amendments to this Annual Report on Form 10-K filed with the Securities and Exchange Commission.
Under the requirements of the Securities Exchange Act of 1934, this report was signed by the following persons on behalf of the Registrant and in the capacities and on the date indicated.
| | | | | | | | | | | | | | |
| Signature | | Title | | Date |
| | |
/S/ ALBERT BOURLA Albert Bourla | Chairman, and Chief Executive Officer and Director
(Principal Executive Officer) | February 23, 202120, 2024 |
| | |
/S/ FRANK A. D’AMELIODAVID M. DENTON Frank A. D’AmelioDavid M. Denton
| Chief Financial Officer, and Executive Vice President Global Supply
(Principal Financial Officer) | February 23, 202120, 2024 |
| | |
/S/ JENNIFER B. DAMICO Jennifer B. Damico | Senior Vice President and Controller
(Principal Accounting Officer) | February 24, 202120, 2024 |
| | |
/S/ RONALD E. BLAYLOCK
Ronald E. Blaylock | Director | February 24, 202121, 2024 |
| | |
/S/ SUSAN DESMOND-HELLMANN Susan Desmond-Hellmann | Director | February 23, 202121, 2024 |
| | |
/S/ JOSEPH J. ECHEVARRIA Joseph J. Echevarria | Director | February 23, 202120, 2024 |
| | |
/S/ SCOTT GOTTLIEB Scott Gottlieb | Director | February 23, 202121, 2024 |
| | |
/S/ HELEN H. HOBBS Helen H. Hobbs | Director | February 23, 202120, 2024 |
| | |
/S/ SUSAN HOCKFIELD Susan Hockfield | Director | February 23, 202120, 2024 |
| | |
/S/ DAN R. LITTMAN Dan R. Littman | Director | February 23, 202120, 2024 |
| | |
/S/ SHANTANU NARAYEN Shantanu Narayen | Director | February 23, 202120, 2024 |
| | |
/S/ SUZANNE NORA JOHNSON Suzanne Nora Johnson | Director | February 23, 202120, 2024 |
| | |
/S/ JAMES QUINCEY James Quincey | Director | February 23, 202121, 2024 |
| | |
/S/ JAMES C. SMITH James C. Smith | Director | February 23, 202120, 2024 |
| | | | | | | | |
| Pfizer Inc. | 20202023 Form 10-K | 117115 |