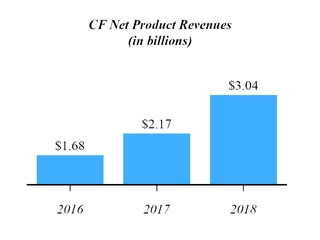
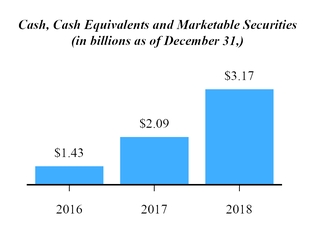
We are subject to data privacy and protection laws and regulations that apply to the collection, transmission, storage and use of proprietary information and personally-identifying information, which among other things, impose certain requirements relating to the privacy, security and transmission of individually identifiable health information. In addition, numerous other federal and state laws, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure and security of personal information. Various foreign countries also have, or are developing, laws governing the collection, use, disclosure, security, and cross-border transmission of personal information. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing focus on privacy and data protection issues with the potential to affect our business. For example, the E.U. General Data Protection Regulation went into effect in May 2018 and has imposed new obligations on us with respect to our processing of personal data and the cross-border transfer of such data. While we continue to address the implications of the recent changes to E.U. data privacy regulations, data privacy remains an evolving landscape at both the domestic and international level, with new regulations coming into effect and continued legal challenges and our efforts to comply with the evolving data protection rules may be unsuccessful. We must devote significant resources to understanding and complying with this changing landscape. Failure to comply with laws regarding data protection would expose us to risk of enforcement actions taken by data protection authorities in the E.U. and the potential for significant penalties if we are found to be non-compliant. Similarly, failure to comply with federal and state laws in the United States regarding privacy and security of personal information could expose us to penalties under such laws. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our business.40
The EMA has adopted a policy on publication of clinical data whereby it will publish clinical reports submitted as part of MAAs for drugs. The EMA aims to publish reports within 60 days after a decision on the application has been made by the European Commission. The ability of third-parties to review and/or analyze the raw data from our clinical trials may increase the risk of patient confidentiality breaches and could result in enhanced scrutiny of our clinical trials results. Such scrutiny could result in misconceptions being spread about our drugs and drug candidates, even if the underlying analysis of such review turns out to be flawed. These publications could also result in the disclosure of information to our competitors that we might otherwise deem confidential, which could harm our competitive position.
The use of social media platforms presents risks and challenges.
Social media is being used by third parties to communicate about our products and drug candidates and the diseases our therapies are designed to treat. We believe that members of the CF community may be more active on social media as compared to other patient populations due to the demographics of this patient population. Social media practices in the pharmaceutical and biotechnology industries are evolving, which creates uncertainty and risk of noncompliance with regulations applicable to our business. For example, patients may use social media platforms to comment on the effectiveness of, or adverse experiences with, a drug or a drug candidate, which could result in reporting obligations. In addition, our employees may engage on social media in ways that may not comply with our social media policy or with legal or regulatory requirements, which may give rise to liability, lead to the loss of trade secrets and other intellectual property, or result in public disclosure of protected personal information. There is a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us on any social networking website. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face restrictive regulatory actions or incur other harm to our business, including damage to our reputation.
Risks Related to Development, Clinical Testing and Regulation of Our Products and Drug Candidates
Our drug candidates remain subject to clinical testing and regulatory approval. Our future success is dependent on our ability to successfully develop additional drug candidates for both CF and non-CF indications.
Our business depends upon the successful development and commercialization of drug candidates. These drug candidates are in various stages of development and must satisfy rigorous standards of safety and efficacy before they can be approved for sale by the FDA or comparable foreign regulatory authorities. To satisfy these standards, we must allocate resources among our various development programs and must engage in expensive and lengthy testing of our drug candidates. Discovery and development efforts for new pharmaceutical products, including new combination therapies, are resource-intensive and may take 10 to 15 years or longer for each drug candidate. Despite our efforts, our drug candidates may not:
offer therapeutic or other improvement over existing competitive therapies;
show the level of safety and efficacy, including the level of statistical significance, required by the FDA or other regulatory authorities for approval of a drug candidate;
meet applicable regulatory standards;
be capable of being produced in commercial quantities at acceptable costs; or
if approved for commercial sale, be successfully marketed as pharmaceutical products.
We have recently completed and/or have ongoing or planned clinical trials for several of our drug candidates. The strength of our company’s product portfolio and pipeline will depend in large part upon the outcomes of these clinical trials and our ability to develop and commercialize combination treatments for CF, including our next-generation CFTR corrector compounds and develop treatments for other diseases. Results of our clinical trials and findings from our nonclinical studies, including toxicology findings in nonclinical studies conducted concurrently with clinical trials, could lead to abrupt changes in our development activities, including the possible cessation of development activities associated with a particular drug candidate or program. For example, in 2018 we discontinued development of VX-210 based on a recommendation by the data Safety Monitoring Board, or DSMB, to stop a clinical trial early due to futility.
Moreover, clinical data are often susceptible to varying interpretations, and many companies that have believed their drug candidates performed satisfactorily in clinical trials have nonetheless failed to obtain marketing approval of their drug candidate. Furthermore, results from our clinical trials may not meet the level of statistical significance or otherwise provide the level of evidence or safety and efficacy required by the FDA or other regulatory authorities for approval of a drug candidate.
Many companies in the pharmaceutical and biotechnology industries, including our company, have suffered significant setbacks in later-stage clinical trials even after achieving promising results in earlier-stage clinical trials. Accordingly, the results from completed preclinical studies and clinical trials may not be replicated in later clinical trials, and ongoing clinical trials for our drug candidates may not be predictive of the results we may obtain in later-stage clinical trials or of the likelihood of approval of a drug candidate for commercial sale. In addition, from time to time we report interim data from our clinical trials. Interim data from a clinical trial may not be predictive of final results from the clinical trial.
If we are unable to obtain regulatory approval, we will be unable to commercialize our drug candidates.
The time required to complete clinical trials and to satisfy the FDA and other countries’ regulatory review processes is uncertain and typically takes many years. Our analysis of data obtained from nonclinical and clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We also may encounter unanticipated delays or increased costs due to government regulation from future legislation or administrative action or changes in governmental policy during the period of drug development, clinical trials and governmental regulatory review.
We may seek a Fast Track and/or Breakthrough Therapy designation for some of our drug candidates. Drug candidates that receive one or both of these designations may be eligible for, among other things, a priority regulatory review. Each of these designations is within the discretion of the FDA. Accordingly, even if we believe one of our drug candidates meets the criteria for Fast Track and/or Breakthrough Therapy designation, the FDA may disagree and instead determine not to make such designation. The receipt of one or both of these designations for a drug candidate does not guarantee a faster development process, review or approval compared to drugs developed or considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if one or more of our drugs or drug candidates qualifies for Fast Track and/or Breakthrough Therapy designation, the FDA may later decide to withdraw such designation if it determines that the drug or drug candidate no longer meets the conditions for qualification.
Any failure to obtain regulatory approvals for a drug candidate would prevent us from commercializing that drug candidate. Any delay in obtaining required regulatory approvals could materially adversely affect our ability to successfully commercialize a drug candidate. Furthermore, any regulatory approval to market a drug may be subject to limitations that we do not expect on the indicated uses for which we may market the drug. Any such limitations could reduce the size of the market for the drug.
We also are subject to numerous foreign regulatory requirements governing the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. Non-U.S. jurisdictions have different approval procedures than those required by the FDA, and these jurisdictions may impose additional testing requirements for our drug candidates. The foreign regulatory approval process includes all of the risks associated with the FDA approval process described above, as well as risks attributable to the satisfaction of foreign requirements. Approval by the FDA does not ensure approval by regulatory authorities outside the United States and approval by a foreign regulatory authority does not ensure approval by the FDA. In addition, although the FDA may accept data from clinical trials conducted outside the United States, acceptance of this data is subject to conditions imposed by the FDA. For example, the clinical trial must be well designed and conducted and performed by qualified investigators in accordance with ethical principles. The trial population also must adequately represent the U.S. population, and the data must be applicable to the U.S. population and U.S. medical practice in ways that the FDA deems clinically meaningful. In addition, while these clinical trials are subject to the applicable local laws, FDA acceptance of the data will depend on its determination that the trials also complied with all applicable U.S. laws and regulations. If the FDA does not accept the data from any trial that we conduct outside the United States, it would likely result in the need for additional trials, which would be costly and time-consuming and delay or permanently halt our development of the applicable drug candidate.
If clinical trials are prolonged or delayed, our development timelines for the affected development program could be extended, our costs to develop the drug candidate could increase and the competitive position of the drug candidate could be adversely affected.
We cannot predict whether or not we will encounter problems with any of our completed, ongoing or planned clinical trials that will cause us or regulatory authorities to delay or suspend clinical trials, or delay the analysis of data from our completed or ongoing clinical trials. Among the factors that could delay our development programs are:
ongoing discussions with the FDA or comparable foreign authorities regarding the scope or design of our clinical trials and the number of clinical trials we must conduct;
delays in enrolling volunteers or patients into clinical trials, including as a result of low numbers of patients that meet the eligibility criteria for the trial;
a lower than anticipated retention rate of volunteers or patients in clinical trials;
the need to repeat clinical trials as a result of inconclusive results, unforeseen complications in testing or clinical investigator error;
inadequate supply or deficient quality of drug candidate materials or other materials necessary for the conduct of our clinical trials;
unfavorable FDA or foreign regulatory authority inspection and review of a manufacturing facility that supplied clinical trial materials or its relevant manufacturing records or a clinical trial site or records of any clinical or preclinical investigation;
unfavorable scientific results from clinical trials;
serious and unexpected drug-related side-effects experienced by participants in our clinical trials or by participants in clinical trials being conducted by our competitors to evaluate drug candidates with similar mechanisms of action or structures to drug candidates that we are developing;
favorable results in testing of our competitors’ drug candidates, or FDA or foreign regulatory authority approval of our competitors’ drug candidates; or
action by the FDA or a foreign regulatory authority to place a clinical hold or partial clinical hold on a trial or compound or deeming the clinical trial conduct as problematic.
Our ability to enroll patients in our clinical trials in sufficient numbers and on a timely basis is subject to a number of factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease, the number of other clinical trials ongoing and competing for patients in the same indication and the eligibility criteria for the clinical trial. In addition, patients may drop out of our clinical trials or may be lost to follow-up medical evaluation after treatment ends, and this could impair the validity or statistical significance of the trials. Delays in patient enrollment or unforeseen drop-out rates may result in increased costs and longer development times.
We, our collaborators, the FDA or other applicable regulatory authorities may suspend clinical trials of a drug candidate at any time if we or they believe the healthy volunteers or patients participating in such clinical trials are being exposed to unacceptable health risks or for other reasons. Any such suspension could materially adversely affect the development of a particular drug candidate and our business.
If our processes and systems are not compliant with regulatory requirements, we could be subject to restrictions on marketing our products or could be delayed in submitting regulatory filings seeking approvals for our drugproduct candidates.
We have a number of regulated processes and systems that are required both prior to and following approval of our drugs and drugproduct candidates. These processes and systems are subject to continual review and periodic inspection by the FDA and other regulatory bodies. In addition, the clinical research organizations and other third parties that we work with in our non-clinical studies and clinical trials and our oversight of such parties are subject to similar reviews and periodic inspection by the FDA and other regulatory bodies. If compliance issues are identified at any point in the development and approval process, we may experience delays in filing for regulatory approval for our drugproduct candidates, or delays in obtaining regulatory approval after filing, if at all. Any later discovery of previously unknown problems or safety issues with approved drugs or manufacturing processes, or failure to comply with regulatory requirements, may result in restrictions on such drugs or manufacturing processes, withdrawal of drugs from the market, the imposition of civil or criminal penalties or a refusal by the FDA and/or other regulatory bodies to approve pending applications for marketing approval of new drugs or supplements to approved applications, any of which could have a material adverse effect on our business. In addition, we are party to agreements that transfer responsibility for complying with specified regulatory requirements, such as filing and maintenance of marketing authorizations and safety reporting or compliance with manufacturing requirements, to our collaborators and third-party manufacturers. If our collaborators or third-party manufacturers do not fulfill these regulatory obligations, any drugs for which we or they obtain approval may be subject to later restrictions on manufacturing or sale, which could have a material adverse effect on our business.
We are subject to various and evolving laws and regulations governing the privacy and security of personal data, and our failure to comply could adversely affect our business, result in fines and/or criminal penalties, and damage our reputation.
Risks RelatedWe are subject to Collaborationsdata privacy and security laws and regulations in various jurisdictions that apply to the collection, storage, use, sharing, and security of personal data, including health information, and impose significant compliance obligations. In addition, numerous other Business Development Activities
Our abilityfederal and state laws, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, govern the collection, use, disclosure and security of personal information. The legislative and regulatory landscape for privacy and data protection continues to executeevolve, and there has been an increasing focus on our long-term strategy depends in part on our ability to acquire rights to additional drugs, drug candidatesprivacy and other technologies that havedata protection issues with the potential to add toaffect our pipeline or provide us with new commercial opportunities.business.
In order to achieve our long-term business objectives, our strategy is to supplement our internal pipeline by acquiring rights to additional drugs, drug candidates and other technologies that have the potential to provide us with new commercial opportunities, including in CF and in therapeutic areas outside of CF. We may not be able to acquire, in-license or otherwise obtain rights to additional drugs, drug candidates or other technologies on acceptable terms or at all. We have faced and will continue to face significant competition for the acquisition of rights to these types of drugs, drug candidates and other technologies from a variety of other companies with interests in the specialty pharmaceutical marketplace, many of which have significantly more financial resources and experience in business development activities than we have. In addition, non-profit organizations may be willing to provide capital to the companies that control additional drugs, drug candidates or technologies, which may provide incentives for companies to advance these drugs, drug candidates or technologies independently. Because of these competitive pressures, the cost of acquiring, in-licensing or otherwise obtaining rights to such drugs, drug candidates or other technologies has grown dramatically in recent years and may be at levels that we cannot afford or that we believe are not justified by market potential. This competition is most intense for approved drugs and late-stage drug candidates, which have the lowest risk and would have the most immediate effect on our financial performance.
We may not realize the anticipated benefits of potential acquisitions or licenses to businesses, drugs, drug candidates and other technologies, and the integration following any such acquisition or license may disrupt our business and management.
We may acquire a business or the rights to drugs, drug candidates or other technologies. In recent years we have entered into both acquisition and collaboration arrangements, including our acquisition of VX-561 from Concert, our agreement with CRISPR to collaborate on the discovery and development of potential new treatments aimed at the underlying genetic causes of human diseases using CRISPR-Cas9 gene editing technology and our collaboration agreement with Moderna, pursuant to which we are seeking to identify and develop mRNA therapeutics for the treatment of CF. With respect to each of these transactions and any additional acquisition of a business or rights to drugs, drug candidates or other technologies, we may not realize the anticipated benefits of such transaction, each of which involves numerous risks. These risks include:
failure to successfully develop and commercialize the drugs, drug candidates or technologies that we acquire or license or to achieve other strategic objectives;
inadequate or unfavorable data from clinical trials evaluating the acquired or licensed drug or drug candidates;
entry into markets in which we have no or limited direct prior experience or where competitors in such markets have stronger market positions;
disruption of our ongoing business and distraction of our management and employees from other opportunities and challenges;
potential failure of the due diligence processes to identify significant problems, liabilities or other shortcomings or challenges of an acquired company, or acquired or licensed drug, drug candidate or technology, including but not limited to, problems, liabilities or other shortcomings or challenges with respect to intellectual property, product quality, safety, accounting practices, employee, customer or third party relations and other known and unknown liabilities;
liability for activities of the acquired company or licensor before the acquisition or license, including intellectual property infringement claims, violations of laws, commercial disputes, tax liabilities, and other known and unknown liabilities;
exposure to litigation or other claims in connection with, or inheritance of claims or litigation risk as a result of an acquisition or license, including but not limited to, claims from terminated employees, customers, former equity holders or other third-parties;
difficulty in integrating the drugs, drug candidates, technologies, business operations and personnel of an acquired asset or company; and
difficulties in the integration of the acquired company’s departments, systems, including accounting, human resource and other administrative systems, technologies, books and records, and procedures, as well as in
maintaining uniform standards, controls, including internal control over financial reporting required by the Sarbanes-Oxley Act of 2002 and related procedures and policies.
Acquisitions and licensing arrangements are inherently risky, and ultimately, if we do not complete an announced acquisition or license transaction or integrate an acquired business, or an acquired or licensed drug, drug candidate or other technology successfully and in a timely manner, we may not realize the benefits of the acquisition or license to the extent anticipated and the perception of the effectiveness of our management team and our company may suffer in the marketplace. Additionally, we may later incur impairment charges related to assets acquired in any such transaction. For example, we enteredthe E.U. General Data Protection Regulation, or GDPR, went into a strategic collaborationeffect in 2018 and license agreement with Parion Sciences, Inc., or Parion, to develop ENaC inhibitors in 2015 and incurred an impairment charge related to this collaboration in the third quarter of 2017. In addition, even if we achieve the long-term benefits associated with strategic transactions, our expenses and short-term costs may increase materially and adversely affect our liquidity and short-term net income (loss). Future licenses or acquisitions could result in potentially dilutive issuances of equity securities, the incurrence of debt, the creation of contingent liabilities, impairment expenses related to goodwill, and impairment or amortization expenses related to other intangible assets, which could harm our financial condition.
We face risks in connection with existing and future collaborations with respect to the development, manufacture and commercialization of our products and drug candidates.
The risks that we face in connection with our current collaborations, including with Arbor, CRISPR, Janssen and Moderna, and any future collaborations, include the following:
Our collaborators may change the focus of their development and commercialization efforts or may have insufficient resources to effectively develop our drug candidates. The ability of some of our products and drug candidates to reach their potential could be limited if collaborators decrease or fail to increase development or commercialization efforts related to those products or drug candidates. Our collaboration agreements provide our collaborators with a level of discretion in determining the amount and timing of efforts and resources that they will apply to these collaborations.
Any future collaboration agreements may have the effect of limiting the areas of research and development that we may pursue, either alone or in collaboration with third parties.
Collaborators may develop and commercialize, either alone or with others, drugs that are similar to or competitive with the drugs or drug candidates that are the subject of their collaborations with us.
Disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the research, development or commercialization of drug candidates, might lead to additional responsibilities forhas imposed new obligations on us with respect to drug candidates,our processing of personal data and the cross-border transfer of such data, including higher standards of obtaining consent, more robust transparency requirements, data breach notification requirements, requirements for contractual language with our data processors, and stronger individual data rights. Different E.U. member states have interpreted the GDPR differently and many have imposed additional requirements, which add to the complexity of processing personal data in the E.U. The GDPR also imposes strict rules on the transfer of personal data to countries outside the E.U., including the U.S. and the U.K., and permits data protection authorities to impose large penalties for violations of the GDPR. The GDPR rules related to cross border data transfers continue to evolve based on E.U. court decisions and regulator guidance, which presents certain practical challenges to compliance. Compliance with the GDPR is a rigorous and time-intensive process that may increase our cost of doing business or might result in litigation or arbitration. Any such disagreements would divert management attention and resources and be time-consuming and expensive.
Collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or exposerequire us to potential litigation.change our business practices, and despite those efforts, there is a risk that we may be subject to fines and penalties, litigation, and reputational harm in connection with any activities falling within the scope of the GDPR.
Collaborators may infringeIn the intellectual propertyU.S., California has passed the California Consumer Privacy Act (the “CCPA”), which went into effect on January 1, 2020. In November 2020, California also passed the California Privacy Rights Act (the “CPRA”), which expands and builds upon the consumer privacy rights of third parties,the CCPA. Certain other states have also enacted legislation governing the protection of personal data and several other states and the federal government are actively considering similar proposed legislation. Additionally, Brazil passed the General Data Protection Law, which went into effect in August 2020. While we continue to address the implications of the new data privacy regulations, data privacy remains an evolving landscape at both the domestic and international level, with new regulations coming into effect and continued legal challenges. Each law is also subject to various interpretations by courts and regulatory agencies, creating even more uncertainty. While we have a global privacy program that addresses such laws and regulations, our efforts to comply with the evolving data protection rules may be unsuccessful.
We must devote significant resources to understanding and complying with the changing landscape in this area. Failure to comply with data protection laws may expose us to litigationrisk of enforcement actions taken by data protection authorities, private
rights of action in some jurisdictions, and potential liability.
Investigations and/or compliance or enforcement actions against a collaborator, which may expose us to indirect liability as a result of our partnership with such collaborator.
Our collaboration agreementssignificant penalties if we are subject to termination under various circumstances.
Additionally, if a collaborator werefound to be involved in a business combination, it might deemphasizenon-compliant. Failure to comply with the GDPR and applicable national data protection laws of European Economic Area member states could lead to fines of up to €20,000,000 or terminate the development or commercialization of any drug candidate licensedup to it by us. If one of our collaborators terminates its agreement with us, we may find it more difficult to attract new collaborators and our perception in the business and financial communities could be harmed.
We may not be able to attract collaborators or external funding for the development and commercialization of certain of our drug candidates.
As part of our ongoing strategy, we may seek additional collaborative arrangements or external funding for certain of our development programs and/or seek to expand existing collaborations to cover additional commercialization and/or development activities. We have a number of research programs and early-stage clinical development programs, some of which are being developed in collaboration with a third party. For example, we have an ongoing collaboration with Janssen, pursuant to which Janssen is developing drug candidates that resulted from our research activities. At any time, we may determine that in order to continue development of a drug candidate or program or successfully commercialize a drug we need to identify a collaborator or amend or expand an existing collaboration. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment4% of the collaborator’s resources and expertise, the terms and conditionstotal worldwide annual revenue of the proposed collaborationpreceding financial year, whichever is higher. Some of these laws and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject drug candidate, the costs and complexities of manufacturing and delivering such drug candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of the applicable intellectual property, which can exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. Potentially, and depending on the circumstances, we may desire that a collaborator either agree to fund portions of a drug development program led by us, or agree to provide all of the funding and directly lead the development and commercialization of a program. No assurance can be given that any efforts we make to seek additional collaborative arrangements will be successfully completed on a timely basis or at all. If we elect to fund and undertake development or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we are unable to enter into acceptable collaborative relationships, one or more of our development programs could be delayed or terminated andregulations also carry the possibility of our receiving a return on our investment incriminal sanctions. For example, while we are not directly subject to the program could be impaired.
Risks Related to Third-Party ManufacturingHealth Insurance Portability and Reliance on Third Parties
We depend on third-party manufacturers to manufacture our productsAccountability Act of 1996, as amended by the Health Information Technology for Economic and the materials we require for our clinical trials. We may not be able to maintain these relationships and could experience supply disruptions outside of our control.
We rely on a worldwide network of third-party manufacturers to manufacture our drugs for commercial use and our drug candidates for clinical trials. As a result of our reliance on these third-party manufacturers and suppliers,Clinical Health Act, or HIPAA, we could be subject to significant supply disruptions outside of our control. Our supply chain for sourcing raw materials and manufacturing drug product ready for distribution is a multi-step international endeavor. Third-party contract manufacturers,penalties, including some in China, perform different parts of our manufacturing process. Contract manufacturers may supply us with raw materials, convert these raw materials into drug substance and/criminal penalties if we knowingly obtain or convert the drug substance into final dosage form. Establishing and managing this global supply chain requires a significant financial commitment and the creation and maintenance of numerous third-party contractual relationships. Although we attempt to manage the business relationships with companies in our supply chain, we do not have control over their operations. Supply disruptions may resultdisclose individually identifiable health information from a HIPAA-covered health care provider or research institution that has not complied with HIPAA’s requirements for disclosing such information. In addition, the commercialization of cell and gene therapies requires the collection and processing of a greater amount of personal data than traditional therapies, potentially increasing risk. Furthermore, the number of factors, including shortages in product raw materials, labor or technical difficulties, regulatory inspections or restrictions, shipping or customs delays or any other performance failure by any third-party manufacturer ongovernment investigations related to data security incidents and privacy violations continue to increase and government investigations typically require significant resources and generate negative publicity, which we rely. Any supply disruptions could disrupt sales of our products and/or the timing of our clinical trials.
We require a supply for our medicines for commercial sale and a supply of our drug candidates for use in our clinical trials. While we have developed some internal capabilities, a majority of the manufacturing steps needed to produce our drug candidates and drug products are performed through a third-party manufacturing network. To ensure the stability of our supply chains we aim to develop additional sources of manufacture for all steps of our manufacturing processes at the time of, or shortly after, marketing approval. Therefore, at any point in time, we may have a limited number of single source manufacturers for certain steps in our manufacturing processes, particularly for recently launched products.
If we or our third-party manufacturers become unable or unwilling to continue manufacturing product on our behalf and we are not able to promptly identify another manufacturer, we could experience a disruption in the commercial supply of our then-marketed medicines, which would have a significant effect on patients,harm our business and our product revenues. Similarly, a disruption in the clinical supply of drug products could delay the completion of clinical trials and affect timelines for regulatory filings. There can be no assurance that we will be able to establish and maintain secondary manufacturers for all of our drug candidates and drug products on a timely basis or at all.reputation.
In the course of providing its services, a contract manufacturer may develop process technology relatedThe COVID-19 pandemic has added further complexity to the manufactureprocessing of our products or drug candidates that the manufacturer owns, either independently or jointly with us. This
would increase our reliance on that manufacturer or require us to obtain a license from that manufacturer in order to have our products or drug candidates manufactured by other suppliers utilizing the same process.
We rely on third parties to conduct certain pre-clinical work and clinical trials, and those third parties may not perform satisfactorily, including failing to meet established deadlines for the completion of such studies and/or trials or failing to satisfy regulatory requirements.
We rely on third parties such as contract research organizations to help manage certain pre-clinical work and our clinical trials and on medical institutions, clinical investigators and clinical research organizations such as the Therapeutic Development Network, which is primarily funded by the CFF, to assist in the design and review of, and to conduct our clinical trials, including enrolling qualified patients. Our reliance on these third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities.personal data. For example, safety measures and government health regulations intended to protect our employees, contractors, and other visitors to our sites may require the collection of certain personal data. Although we remain responsible forare focused on ensuring that each ofpersonal data is properly protected, our clinical trials is conducted in accordance with the general investigational plan and protocols for the clinical trial. Moreover, the FDA requires us to comply with standards, commonly referred to as good laboratory practices and good clinical practices, for conducting, recording and reporting the results of pre-clinical and clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, weefforts may be required to replace them. Although we believe that there are a number of other third-party contractorsunsuccessful and we could engage to continue these activities, it may result in a delay of the affected clinical trial or drug development program. If clinical trials are not conducted in accordance with our contractual expectations or regulatory requirements, action by regulatory authorities might significantly and adversely affect the conduct or progress of these clinical trials or in specific circumstances might result in a requirement that a clinical trial be redone. Accordingly, our efforts to obtain regulatory approvals for and commercialize our drug candidates could be delayed.
Risks Related to Intellectual Property
If our patents do not protect our drugs or our drugs infringe third-party patents, we couldunintentionally be subject to litigation which could result in injunctions preventing us from selling our productsunauthorized access or substantial liabilities.
We have numerous issued patents and pending patent applications in the United States, as well as counterparts in other countries. Our success will depend, in significant part, on our ability to obtain and defend U.S. and foreign patents covering our drugs, their uses and our processes, to preserve our trade secrets and to operate without infringing the proprietary rights of third parties. We cannot be certain that any patents will issue from our pending patent applications or, even if patents issue or have issued, that the issued claims will provide us with adequate protection against competitive products or otherwise be commercially valuable.
Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these patents, our ability to obtain, maintain and enforce patents is uncertain and involves complex legal and factual questions. Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents in the U.S. The Leahy-Smith America Invents Act, or the Leahy-Smith Act, includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The United States Patent Office developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, became effective in March 2013. The first to file provisions limit the rights of an inventor who is the first to invent an invention but is not the first to file an application claiming that invention. U.S. and foreign patent applications typically are maintained in confidence for a period of time after they initially are filed with the applicable patent office. Consequently, we cannot be certain that we were the first to invent, or the first to file patent applications on, our products or drug candidates or their use. If a third party also has filed a U.S. patent application relating to our drugs or drug candidates, their uses, or a similar invention, we may have to participate in legal or administrative proceedings to determine priority of invention. For applications governed by the Lahey-Smith Act, if a third-party has an earlier filed U.S. patent application relating to our drugs or drug candidates, their uses, or a similar invention, we may be unable to obtain an issued patent from our application.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability. Our patents may be challenged by third parties, resulting in the patent being deemed invalid, unenforceable or narrowed in scope, or the third party may circumvent any such issued patents. Also, our pending patent applications may not issue, and we may not receive any additional patents. Our patents might not contain claims that are sufficiently broad to prevent others from utilizing our technologies. For instance, the issued patents relating to our drugs or drug candidates may be limited to a particular molecule or molecules and may not cover similar molecules that have similar clinical properties. Consequently, our competitors may
independently develop competing products that do not infringe our patents or other intellectual property. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future products.
The laws of many foreign jurisdictions do not protect intellectual property rights to the same extent as in the United States and many companies in our segment of the pharmaceutical industry have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. If we encounter such difficulties in protecting or are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business could be substantially harmed.
Because of the extensive time required for the discovery, development, testing and regulatory review of drug candidates, it is possible that a patent may expire before a drug candidate can be commercialized, or a patent may expire or remain in effect for only a short period following commercializationdisclosure of such drug candidate. This would result in a minimal or non-existent period of patent exclusivity. If our drug candidates are not commercialized significantly ahead of the expiration date of any applicable patent, or if we have no patent protection on such drug candidates, then, to the extent available we would rely on other forms of exclusivity, such as regulatory exclusivity provided by the FDCA and its counterpart agencies in various jurisdictions, and/or orphan drug exclusivity.
Uncertainty over intellectual property in the pharmaceutical and biotechnology industry has been the source of litigation and other disputes, which is inherently costly and unpredictable.
There is considerable uncertainty within our industry about the validity, scope and enforceability of many issued patents in the United States and elsewhere in the world, and, to date, the law and practice remains in substantial flux both in the agencies that grant patents and in the courts. We cannot currently determine the ultimate scope and validity of patents which may be granted to third parties in the future or which patents might be asserted as being infringed by the manufacture, use and sale of our products.
There has been, and we expect that there may continue to be, significant litigation in the pharmaceutical industry regarding patents and other intellectual property rights. Litigation, arbitrations, administrative proceedings and other legal actions with private parties and governmental authorities concerning patents and other intellectual property rights may be protracted, expensive and distracting to management. Competitors may sue us as a way of delaying the introduction of our drugs or to remove our drugs from the market. Any litigation, including litigation related to Abbreviated New Drug Applications, or ANDA, litigation related to 505(b)(2) applications, interference proceedings to determine priority of inventions, derivations proceedings, inter partes review, oppositions to patents in foreign countries, litigation against our collaborators or similar actions, may be costly and time consuming and could harm our business. We expect that litigation may be necessary in some instances to determine the validity and scope of certain of our proprietary rights. Litigation may be necessary in other instances to determine the validity, scope or non-infringement of certain patent rights claimed by third parties to be pertinent to the manufacture, use or sale of our products. Ultimately, the outcome of such litigation could adversely affect the validity and scope of our patent or other proprietary rights, hinder our ability to manufacture and market our products, or result in the assessment of significant monetary damages against us that may exceed amounts, if any, accrued in our financial statements.
To the extent that valid present or future third-party patents or other intellectual property rights cover our drugs, drug candidates or technologies, we or our strategic collaborators may seek licenses or other agreements from the holders of such rights in order to avoid or settle legal claims. Such licenses may not be available on acceptable terms, which may hinder our ability to, or prevent us from being able to, manufacture and market our drugs. Payments under any licenses that we are able to obtain would reduce our profits derived from the covered products.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims.
In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third
parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to management.
Risks Related To Our Operations
Risks associated with operating in foreign countries could materially adversely affect our business.
We have expanded our international operations over the past several years in order to market our CF medicines and expand our research and development capabilities. New laws and industry codes in the E.U. and elsewhere have expanded transparency requirements regarding payments and transfers of value as well as patient-level clinical trialpersonal data. New laws in the E.U. also have expanded protections related to personal data and provided for increased sanctions for violations. Collectively, our expansion and these new requirements are adding to our compliance costs and expose us to potential sanctions for failing to meet the enhanced safeguards and reporting demands in these jurisdictions. In addition, a significant portion of our commercial supply chain, including sourcing of raw materials and manufacturing, is located in China and the E.U. Consequently, we are, and will continue to be, subject to risks related to operating in foreign countries, including risks relating to intellectual property protections and business interruptions. These risks are increased with respect to countries, such as China, that have substantially different local laws and business practices and weaker protections for intellectual property. Risks associated with conducting operations in foreign countries include:
differing regulatory requirements for drug approvals and regulation of approved drugs in foreign countries;
varying reimbursement regimes and difficulties or the inability to obtain reimbursement for our products in foreign countries in a timely manner;
differing patient treatment infrastructures, particularly since our business is focused on the treatment of serious diseases that affect relatively smaller numbers of patients and are typically prescribed by specialist physicians;
collectibility of accounts receivable;
changes in tariffs, trade barriers and regulatory requirements, the risks of which appear to have increased in the current political environment;
economic weakness, including recession and inflation, or political instability in particular foreign economies and markets;
differing levels of enforcement and/or recognition of contractual and intellectual property rights;
complying with local laws and regulations, which are interpreted and enforced differently across jurisdictions and which can change significantly over time;
foreign taxes, including withholding of payroll taxes;
foreign currency fluctuations, which could result in reduced revenues or increased operating expenses, and other obligations incident to doing business or operating in another country;
workforce uncertainty in countries where labor unrest is more common than in the United States;
import and export licensing requirements, tariffs, and other trade and travel restrictions;
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
business interruptions resulting from geo-political actions, including war and terrorism.
Our revenues are subject to foreign exchange rate fluctuations due to the global nature of our operations. Although we have foreign currency forward contracts to hedge forecasted product revenues denominated in foreign currencies, our efforts to reduce currency exchange losses may not be successful. As a result, currency fluctuations among our reporting currency, the U.S. dollar, and the currencies in which we do business will affect our operating results, often in unpredictable ways.
In addition, our international operations are subject to regulation under U.S. law. For example, the FCPA prohibits U.S. companies and their representatives from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad. In many countries, the health care professionals we regularly interact with may meet the definition of a foreign government official for purposes of the FCPA. We also are subject to import/export control laws. Failure to comply with domestic or foreign laws could result in various adverse consequences, including the possible delay in approval or refusal to approve a product, recalls, seizures, withdrawal of an approved product from the market, the imposition of civil or criminal sanctions, the prosecution of executives overseeing our international operations and corresponding bad publicity and negative perception of our company in foreign countries.
If we fail to manage our operations effectively, our business may suffer.
We have expanded and are continuing to expand our global operations and capabilities, which has placed, and will continue to place, significant demands on our management and our operational, research and development and financial infrastructure. To effectively manage our business, we need to:
implement and clearly communicate our corporate-wide strategies;
enhance our operational and financial infrastructure, including our controls over records and information;
enhance our operational, financial and management processes, including our cross-functional decision-making processes and our budget prioritization systems;
train and manage our global employee base; and
enhance our compliance and legal resources.
Risk relating to the Referendum of the United Kingdom’s Membership of the European Union.
Our European headquarters and European research facility are located in the United Kingdom, or the U.K., and a significant portion of our ex-U.S. net product revenues are derived from sales in the U.K. In June 2016, the U.K. held a referendum in which voters approved an exit from the E.U., commonly referred to as “Brexit.” The U.K. government provided official notice of withdrawal from the E.U. in March 2017 and has a period of two years from the date of its formal notification (such period ending March 29, 2019) to negotiate the terms of its withdrawal from, and future relationship with, the E.U., including the terms of trade between the U.K. and the E.U. and potentially other countries. If no formal withdrawal agreement is reached, then it is expected that the U.K.’s membership in the E.U. will automatically terminate two years after the submission of the notification of the U.K.’s intention to withdraw from the E.U., unless all remaining member states unanimously consent to an extension of this period. Discussions between the U.K. and the E.U. focused on finalizing withdrawal issues and transition agreements are ongoing. However, limited progress to date in these negotiations and ongoing uncertainty within the U.K. government increases the possibility of the U.K. leaving the E.U. on March 29, 2019 without a withdrawal agreement and associated transition period in place, which is likely to cause significant market and economic disruption, which may cause third-party payors, including governmental organizations, to closely monitor their costs and reduce their spending budgets. The effects of Brexit will depend on any agreements the U.K. makes to retain access to E.U. markets either during a transitional period or more permanently. Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the U.K. determines which E.U. laws to replace or replicate. Given the lack of comparable precedent, it is unclear what financial, trade, regulatory and legal implications the withdrawal of the U.K. from the E.U. would have and how such withdrawal would affect us. Any of these effects of Brexit, among others, could adversely affect our business, financial condition and operating results.
Our business has a substantial risk of product liability claims and other litigation liability. If we do not obtain appropriate levels of insurance, any potential claims could adversely affect our business.
We are or may be involved in various legal proceedings, including securities class action lawsuits and claims related to product liability, intellectual property and breach of contract. Such proceedings may involve claims for, or the possibility of, fines and penalties involving substantial amounts of money or other relief, including but not limited to civil or criminal fines and penalties. If any of these legal proceedings were to result in an adverse outcome, it could have a material adverse effect on our business.
With respect to product liability and clinical trial risks, in the ordinary course of business we are subject to liability claims and lawsuits, including potential class actions, alleging that our products or drug candidates have caused, or could cause, serious adverse events or other injury. We have product liability insurance and clinical trial insurance in amounts that we believe are adequate to cover this risk. However, our insurance may not provide adequate coverage against all potential
liabilities. If a claim is brought against us, we might be required to pay legal and other expenses to defend the claim, as well as pay uncovered damage awards resulting from a claim brought successfully against us and these damages could be significant and have a material adverse effect on our financial condition. Furthermore, whether or not we are ultimately successful in defending any such claims, we might be required to direct significant financial and managerial resources to such defense and adverse publicity is likely to result.
A breakdown or breach of our information technology systems could subject us to liability or interrupt the operation of our business.
We maintain and rely extensively on information technology systems and network infrastructures for the effective operation of our business. In the course of our business, we collect, store and transmit confidential information (including personal information and intellectual property), and it is critical that we do so in a secure manner to maintain the confidentiality and integrity of such confidential information. The size and complexity of our information technology and information security systems makes such systems potentially vulnerable to service interruptions or to security breaches. A disruption, infiltration or failure of our information technology systems or any of our data centers as a result of software or hardware malfunctions, computer viruses, cyber-attacks, employee theft or misuse, power disruptions, natural disasters, floods or accidents could cause breaches of data security and loss of critical data, which in turn could materially adversely affect our business and subject us to both private and governmental causes of action. While we have implemented security measures in an attempt to minimize these risks to our data and information technology systems and have adopted a business continuity plan to deal with a disruption to our information technology systems, cyber-attacks are increasing in their frequency, sophistication and intensity, and have become increasingly difficult to detect. There can be no assurance that our efforts to protect our data and information systems will prevent breakdowns or breaches in our systems that could adversely affect our business. In addition, our liability insurance may not be sufficient in type or amount to cover us against claims related to security breaches, cyber-attacks or other related liabilities.
If we fail to attract and retain skilled employees, our business could be materially harmed.
Because our drug discovery and development activities are highly technical in nature, we require the services of highly qualified and trained scientists who have the skills necessary to conduct these activities. In addition, we need to attract and retain employees with experience in marketing and commercialization of medicines. We have entered into employment agreements with some executives and provide compensation-related benefits to all of our key employees that vest over time and therefore induce them to remain with us. However, the employment agreements can be terminated by the executive on relatively short notice. The value to employees of stock-related benefits that vest over time — such as options and restricted stock units — is significantly affected by movements in our stock price, and may at any point in time be insufficient to counteract more lucrative offers from other companies. We face intense competition for our personnel from our competitors and other companies throughout our industry. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. Moreover, the growth of local biotechnology companies and the expansion of major pharmaceutical companies into the Boston area have increased competition for the available pool of skilled employees, especially in technical fields, and the high cost of living in Massachusetts makes it difficult to attract employees from other parts of the country to Massachusetts. In addition, the available pool of skilled employees would be further reduced if immigration laws change in a manner that increases restrictions on immigration. Our ability to commercialize our products, and achieve our research and development objectives, depends on our ability to respond effectively to these demands. If we are unable to hire and retain qualified personnel, there could be a material adverse effect on our business.
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research and development efforts involve the regulated use of hazardous materials, chemicals, and various controlled and radioactive compounds. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by state, federal and foreign regulations, the risk of loss of, or accidental contamination or injury from, these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could be substantial. We also are subject to numerous environmental, health, and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens, and the handling of biohazardous materials. Although we maintain workers’ compensation insurance to cover us for costs we may incur due to injuries to our employees resulting from the use of these materials, this insurance may not provide adequate coverage against potential liabilities. We maintain insurance to cover pollution conditions or other extraordinary or unanticipated events relating to our use and disposal of hazardous materials that we believe is appropriate based on the small amount of hazardous materials we generate. Additional federal, state and local laws and regulations affecting our operations may be adopted in the
future. We may incur substantial costs to comply with, and substantial fines or penalties if we violate, any of these laws or regulations.
Risks Related to Business Development Activities
Our ability to execute on our long-term strategy depends in part on our ability to engage in transactions and collaborations with other entities that add to our pipeline or provide us with new commercial opportunities.
In order to achieve our long-term business objectives, we seek to license or acquire products, product candidates and other technologies that have the potential to complement our ongoing research and development efforts, access emerging technologies and license or acquire pipeline assets. These transactions may be similar to prior transactions, may be structured differently than prior transactions, or may involve larger transactions or later-stage assets. We have faced and will continue to face significant competition for the acquisition of rights to these types of products, product candidates and other technologies from a variety of other companies, many of which have significantly more financial resources and experience in business development activities than we have. In addition, non-profit organizations may be willing to provide capital to the companies that control additional products, product candidates or technologies, which may provide incentives for companies to advance these products, product candidates or technologies independently. Also, the cost of acquiring, in-licensing or otherwise obtaining rights to such products, product candidates or other technologies has grown dramatically in recent years and may be at levels that we cannot afford or that we believe are not justified by market potential. As a result, we may not be able to acquire, in-license or otherwise obtain rights to additional products, product candidates or other technologies on acceptable terms or at all.
We may not realize the anticipated benefits of acquisitions of businesses or technologies, and the integration following any such acquisition may disrupt our business and management.
It is challenging to effectively integrate businesses and technologies that we acquire, including the acquisitions of Semma and Exonics and the exclusive licenses that we have acquired from CRISPR and Moderna, and we may not realize the benefits anticipated from such transactions. Achieving the anticipated benefits of any transaction and successfully integrating acquired businesses or technologies involves a number of risks, including:
•failure to successfully develop and commercialize the acquired products, product candidates or technologies or to achieve other strategic objectives;
•delays or inability to progress preclinical programs into clinical development or unfavorable data from clinical trials evaluating the acquired or licensed product or product candidates;
•difficulty in integrating the products, product candidates, technologies, business operations and personnel of an acquired asset or company;
•disruption of our ongoing business and distraction of our management and employees from daily operations or other opportunities and challenges;
•the potential loss of key employees of an acquired company;
•entry into markets in which we have no or limited direct prior experience or where competitors in such markets have stronger market positions;
•potential failure of the due diligence processes to identify significant problems, liabilities or challenges of an acquired company, or acquired or licensed products, product candidate or technology, including but not limited to, problems, liabilities or challenges with respect to intellectual property, clinical or non-clinical data, safety, accounting practices, employee, or third-party relations and other known and unknown liabilities;
•liability for activities of the acquired company or licensor before the acquisition or license, including intellectual property infringement claims, violations of laws, commercial disputes, tax liabilities, and other known and unknown liabilities;
•exposure to litigation or other claims in connection with, or inheritance of claims or litigation risk as a result of an acquisition or license, including but not limited to, claims from terminated employees, customers, former equity holders or other third parties; and
•difficulties in the integration of the acquired company’s departments, systems, including accounting, human resource and other administrative systems, technologies, books and records, and procedures, as well as in maintaining uniform standards, controls, including internal control over financial reporting required by the Sarbanes-Oxley Act of 2002 and related procedures and policies.
Acquisitions, licensing arrangements and other strategic transactions are inherently risky, and ultimately, if we do not complete an announced acquisition, collaboration or strategic transaction or integrate an acquired or licensed asset, business or technology successfully and in a timely manner, we may not realize the anticipated benefits of the strategic transaction.
We may later incur impairment charges related to assets acquired in any such transaction. Even if we achieve the long-term benefits associated with our strategic transactions, our expenses and short-term costs may increase materially and adversely affect our liquidity and short-term net income. Future strategic transactions could result in potentially dilutive issuances of equity securities, the incurrence of debt, the creation of contingent liabilities, impairment expenses related to goodwill, or impairment or amortization expenses related to other intangible assets, all of which could harm our financial condition.
We face risks in connection with existing and future collaborations with respect to the development, manufacture and commercialization of our products and product candidates.
The risks that we face in connection with our current collaborations, including CRISPR, and any future collaborations, include the following:
•Our collaborators may change the focus of their development and commercialization efforts or may have insufficient resources or expertise to effectively develop, manufacture or commercialize our product candidates.
•The ability of some of our therapies to reach their potential could be limited if collaborators are unable to effectively develop, manufacture or commercialize these therapies or product candidates or decrease or fail to increase development or commercialization efforts related to those therapies or product candidates. Our collaboration agreements allocate development, manufacturing and commercialization responsibilities between us and our collaborators and provide our collaborators with a level of discretion in determining the amount and timing of efforts and resources that they will apply to these collaborations.
•Our collaborators may have limited experience in developing, manufacturing and commercializing therapies, either generally, or in the specific therapeutic area.
•Collaboration agreements may have the effect of limiting the areas of research and development that we may pursue, either alone or in collaboration with third parties.
•Collaborators may develop and commercialize, either alone or with others, drugs that are similar to or competitive with the products or product candidates that are the subject of their collaborations with us.
•Disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development, might cause delays or termination of the research, development or commercialization of product candidates, might lead to additional responsibilities or costs for us with respect to product candidates, or might result in litigation or arbitration. Any such disagreements would divert management attention and resources and would be time-consuming and expensive.
•Collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation.
•Collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability.
•Investigations and/or compliance or enforcement actions against a collaborator, which may expose us to indirect liability as a result of our partnership with such collaborator.
•Our collaboration agreements are subject to termination under various circumstances.
•We may be unable to control the resources our collaborators devote to our programs, products or product candidates, and the priorities and strategic objectives of our collaborators may not align precisely with ours.
Additionally, if a collaborator were to be involved in a business combination with a third party, it might de-emphasize or terminate the development or commercialization of any product candidate licensed to it by us. If one of our collaborators terminates its agreement with us, we may find it more difficult to attract new collaborators and our perception in the business and financial communities could be harmed.
We may not be able to attract collaborators or external funding for the development and commercialization of certain of our product candidates.
As part of our ongoing strategy, we may seek additional collaborative arrangements or external funding for certain of our development programs and/or seek to expand existing collaborations to cover additional commercialization and/or development activities. We have a number of research programs and clinical development programs, some of which are being developed in collaboration with a third party. At any time, we may determine that in order to continue development of a product candidate or program or successfully commercialize a drug we need to identify a collaborator or amend or expand an
existing collaboration. For example, in April 2021, we amended and restated the original JDCA, positioning us to lead global development, manufacturing and commercialization of CTX001, with support from CRISPR.
Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA, EMA or other regulatory authorities, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of the applicable intellectual property, which can exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. Potentially, and depending on the circumstances, we may desire that a collaborator either agree to fund portions of a drug development program led by us, or agree to provide all of the funding and directly lead the development and commercialization of a program. No assurance can be given that any efforts we make to seek additional collaborative arrangements will be successfully completed on a timely basis or at all. If we elect to fund and undertake development or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we are unable to enter into acceptable collaborative relationships, one or more of our development programs could be delayed or terminated and the possibility of our receiving a return on our investment in the program could be impaired.
Risks Related to Supply, Manufacturing and Reliance on Third Parties
We depend on third-party manufacturers and our internal capabilities to manufacture our products and the materials we require for our clinical trials. We may not be able to maintain our third-party relationships and could experience supply disruptions outside of our control.
We rely on a worldwide network of third-party manufacturers and our internal capabilities, including our own manufacturing facility in Boston, to manufacture product candidates for clinical trials as well as our medicines for commercial use. We could be subject to significant supply interruptions as a result of disruptions to third party or our internal manufacturing capabilities. Our supply chain for sourcing raw materials and manufacturing drug product ready for distribution, including obtaining necessary supplies, is a multi-step international endeavor. Third-party contract manufacturers, including some in China, perform different parts of our manufacturing process. Contract manufacturers may supply us with raw materials, convert these raw materials into drug substance and/or convert the drug substance into final dosage form. Third parties are used for packaging, warehousing and distribution of products. In cell and genetic therapies, third parties also will be used to both manufacture and deliver our therapies, which requires significant expertise and capacity to meet our requirements. This capacity may be limited by the number of other clinical trials and commercial manufacturing ongoing for other companies seeking similar support.
If third parties are unwilling or unable to meet our requirements, including as a result of the COVID-19 pandemic or because of their own supply or capacity issues, we could experience supply disruptions outside of our control. Additionally, manufacturing facilities, both foreign and domestic, are subject to inspections by the FDA and other U.S. and foreign government authorities. Although we actively engage with regulatory authorities, the timing of regulatory approvals for each of these facilities may be delayed for a variety of reasons, including as a result of the COVID-19 pandemic. In addition, we and the third parties with whom we engage are required to maintain compliance with quality regulations globally. An inability to maintain compliance with such regulations, including cGMP requirements, could cause significant disruptions to our business and operations.
Additionally, establishing, managing and expanding our global supply chain requires a significant financial commitment and the creation and maintenance of our numerous third-party contractual relationships. Although we attempt to manage the business relationships with companies in our supply chain, we could be subject to supply disruptions outside of our control.
Supply disruptions may result from a number of factors, including shortages in product raw materials, labor or technical difficulties, regulatory inspections or restrictions, shipping or customs delays, general global supply chain disruptions, or any other performance failure by us or any third-party manufacturer on which we rely. We may also experience supply disruptions if regulatory agencies are unable to inspect the manufacturing facilities on which we rely. Any such disruptions could disrupt sales of our products and/or the timing or advancement of our clinical trials.
While we have developed internal capabilities to supply product candidates for use in our clinical trials as well as our
medicines for commercial sale, a majority of the manufacturing steps needed to produce our medicines, product candidates, and drug products are performed through a third-party manufacturing network. We expect that we will continue to rely on third parties to meet our commercial supply needs and a significant portion of our clinical supply needs for the foreseeable future.
If we or our third-party manufacturers become unable or unwilling to continue manufacturing product and we are not able to promptly identify another manufacturer, we could experience a disruption in the commercial supply of our then-marketed medicines, which would have a significant effect on patients, our business, and our product revenues. Similarly, a disruption in the clinical supply of product candidates could delay the completion of clinical trials and affect timelines for regulatory filings. We have a limited number of critical steps in our manufacturing process that are single sourced, including for recently launched products. To ensure the stability of our supply chains, we continue to develop alternatives for our manufacturing processes. However, there can be no assurance that we will be able to establish and maintain additional manufacturers or capacity for all of our product candidates and products on a timely basis or at all.
In the course of providing its services, a contract manufacturer may develop process technology related to the manufacture of our products or product candidates that the manufacturer owns, either independently or jointly with us. This would increase our reliance on that manufacturer or require us to obtain a license from that manufacturer in order to have our products or product candidates manufactured by other suppliers utilizing the same process.
We rely on third parties to conduct pre-clinical work, clinical trials and other activities, and those third parties may not perform satisfactorily, including failing to meet established deadlines for the completion of such studies and/or trials or failing to satisfy regulatory requirements.
We rely on third parties such as CROs to help manage certain pre-clinical work and our clinical trials and on medical institutions, clinical investigators, and clinical research organizations such as the Therapeutic Development Network, which is primarily funded by the CFF, to assist in the design and review of, and to conduct our clinical trials, including enrolling qualified patients. In addition, we engage third party contractors to support numerous other research, commercial and administrative activities. Our reliance on these third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities. For example, we remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the clinical trial. Moreover, the FDA requires us to comply with standards, commonly referred to as good laboratory practices and good clinical practices, for conducting, recording and reporting the results of pre-clinical and clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Such standards, particularly with respect to newer cell and genetic therapies, will continue to evolve and subject us and third parties to new or changing requirements.
If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be required to replace them. Although we believe that there are a number of other third-party contractors we could engage to continue the activities, it may result in a delay of the affected clinical trial, drug development program or applicable activity. If clinical trials are not conducted in accordance with our contractual expectations or regulatory requirements, action by regulatory authorities might significantly and adversely affect the conduct or progress of these clinical trials or in specific circumstances might result in a requirement that a clinical trial be redone. Accordingly, our efforts to obtain regulatory approvals for and commercialize our product candidates could be delayed. In addition, failure of any third-party contractor to conduct activities in accordance with our expectations, including as a result of the COVID-19 pandemic, could adversely affect the relevant research, development, commercial or administrative activity.
Risks Related to Intellectual Property
If our patents do not protect our products or our products infringe third-party patents, we could be subject to litigation which could result in injunctions preventing us from selling our products or substantial liabilities.
We own and/or control numerous issued patents and pending patent applications in the U.S., as well as counterparts in other countries. Our success will depend, in significant part, on our ability to obtain and defend U.S. and foreign patents covering our products, their uses and our processes, to preserve our trade secrets and to operate without infringing the proprietary rights of third parties. We cannot be certain that any patents will issue from our pending patent applications or, even if patents issue or have issued, that the issued claims will provide us with adequate protection against competitive products or otherwise be commercially valuable.
Due to evolving legal standards relating to the patentability, validity, and enforceability of patents covering pharmaceutical and biotechnological inventions and the scope of claims made under these patents, our ability to obtain, maintain and enforce patents is uncertain and involves complex legal and factual questions. Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents in the U.S. The Leahy-Smith America Invents Act, or the Leahy-Smith Act, made a number of significant changes to U.S. patent law in 2011. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. For example, the first to file provisions limit the rights of an inventor who is the first to invent an invention but is not the first to file an application claiming that invention. U.S. and foreign patent applications typically are maintained in confidence for a period of time after they initially are filed with the applicable patent office. Consequently, we cannot be certain that we were the first to invent, or the first to file patent applications on, our products or product candidates or their use. If a third party also has filed a U.S. patent application relating to our products or product candidates, their uses, or a similar invention, we may have to participate in legal or administrative proceedings to determine priority of invention. For applications governed by the Leahy-Smith Act, if a third-party has an earlier filed U.S. patent application relating to our products or product candidates, their uses, or a similar invention, we may be unable to obtain an issued patent from our application.
The issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability. Our patents may be challenged by third parties and certain of our patents have been challenged. This could result in the patent being deemed invalid, unenforceable or narrowed in scope, or the third party may circumvent any such issued patents. Also, our pending patent applications may not issue, and we may not receive any additional patents.
Our patents or patents we license might not contain claims that are sufficiently broad to prevent others from developing competing products. For instance, issued patents, or patents that may issue in the future, (i) relating to our small molecules may be limited to a particular molecule or molecules and may not cover similar molecules that have similar clinical properties, and (ii) relating to cell or genetic therapies may not cover similar technologies that would allow competitors to achieve similar results. Consequently, our competitors may independently develop competing products that do not infringe our patents or other intellectual property. In addition, CRISPR only has co-exclusive rights to the patent rights that protect the core CRISPR/Cas9 gene-editing technology.
The laws of many foreign jurisdictions do not protect intellectual property rights to the same extent as in the U.S. and many companies in our segment of the pharmaceutical industry have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. If we encounter such difficulties in protecting or are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business could be substantially harmed.
Because of the extensive time required for the discovery, development, testing and regulatory review of product candidates, it is possible that a patent may expire before a product candidate can be commercialized, or a patent may expire or remain in effect for only a short period following commercialization of such product candidate. This would result in a minimal or non-existent period of patent exclusivity. If our product candidates are not commercialized significantly ahead of the expiration date of any applicable patent, or if we have no patent protection on such product candidates, then, to the extent available we would rely on other forms of exclusivity, such as regulatory exclusivity provided by the FDCA and its counterpart agencies in various jurisdictions, and/or orphan drug exclusivity.
Uncertainty over intellectual property in the pharmaceutical and biotechnology industry has been the source of litigation and other disputes that are inherently costly and unpredictable.
There is considerable uncertainty within our industry about the validity, scope, and enforceability of many issued patents in the U.S. and elsewhere in the world, and, to date, the law and practice remains in substantial flux both in the agencies that grant patents and in the courts. We cannot currently determine the ultimate scope and validity of patents which may be granted to third parties in the future or which patents might be asserted as being infringed by the manufacture, use and sale of our products.
There has been, and we expect that there may continue to be, significant litigation in the pharmaceutical industry regarding patents and other intellectual property rights. Litigation, arbitrations, administrative proceedings, and other legal actions with private parties and governmental authorities concerning patents and other intellectual property rights may be protracted, expensive, and distracting to management. Competitors may sue us as a way of delaying the introduction of our products or to remove our products from the market. Any litigation, including litigation related to Abbreviated New Drug Applications, or ANDA, litigation related to 505(b)(2) applications, interference proceedings to determine priority of
inventions, derivations proceedings, inter partes review, oppositions to patents in foreign countries, litigation against our collaborators or similar actions, may be costly and time consuming and could harm our business. We expect that litigation may be necessary in some instances to determine the validity and scope of certain of our proprietary rights. Litigation may be necessary in other instances to determine the validity, scope or non-infringement of certain patent rights claimed by third parties to be pertinent to the manufacture, use or sale of our products. Ultimately, the outcome of such litigation could adversely affect the validity and scope of our patent or other proprietary rights, hinder our ability to manufacture and market our products, or result in the assessment of significant monetary damages against us that may exceed amounts, if any, accrued in our consolidated financial statements.
On July 24, 2020, we filed a lawsuit against Sun Pharmaceutical Industries Limited, or Sun, in the U.S. District Court for the District of Delaware, or the District Court, alleging infringement of U.S. Patent No. 10,646,481, or the ’481 patent. The lawsuit follows Vertex’s receipt of a Notice Letter on June 11, 2020, advising that Sun had submitted an ANDA to the FDA seeking approval to manufacture and market a generic version of the 150 mg tablet of KALYDECO in the U.S. The Notice Letter indicated that Sun submitted a “Paragraph IV” certification to the FDA in which Sun asserted that the ’481 patent is invalid or would not be infringed by Sun’s generic product. The ’481 patent, which expires in 2029, was issued on May 12, 2020, and listed in the Orange Book with respect to KALYDECO 150 mg tablets on June 1, 2020. Sun does not appear to challenge our other U.S. patents covering KALYDECO.
On July 13, 2021, we filed a lawsuit against Lupin Limited and Lupin Pharmaceuticals, Inc., or, collectively, Lupin, in the District Court alleging infringement of the ’481 patent. The lawsuit follows our receipt of a Notice Letter on June 2, 2021 advising that Lupin had submitted an ANDA to the FDA seeking approval to manufacture and market a generic version of the 150 mg tablet of KALYDECO in the U.S. The Notice Letter indicated that Lupin submitted a “Paragraph IV” certification to the FDA in which Lupin asserts that the ’481 patent is invalid or would not be infringed by Lupin’s generic product. Lupin does not appear to challenge our other U.S. patents covering KALYDECO.
On September 24, 2021, the District Court consolidated the cases against Sun and Lupin described above and scheduled trial for the consolidated cases beginning on October 23, 2023. We intend to vigorously enforce its intellectual property rights relating to KALYDECO, including the ’481 patent.
CRISPR has licensed certain rights to a worldwide patent portfolio that covers various aspects of the CRISPR/Cas9 editing platform technology including, for example, compositions of matter and methods of use, including their use in targeting or cutting DNA from Dr. Charpentier, one of the named inventors of this patent portfolio. The patent portfolio also has named inventors who assigned their rights to the CVC Group. For example, in connection with their collaboration, Novartis and Intellia Therapeutics, Inc. have reportedly obtained a license to this patent portfolio in certain fields. Patents and patent applications in this patent portfolio have been the subject of numerous contentious proceedings in the U.S., Europe, and other jurisdictions, including interference proceedings in the USPTO between the CVC Group and (separately) the Broad Institute, Sigma-Aldrich and ToolGen. Decisions rendered to date in these proceedings may be subject to appeal. To date, both the CVC Group and the Broad Institute have obtained granted patents that purport to cover aspects of CRISPR/Cas9 editing platform technology. The patents and patent applications within the patent portfolios of the CVC Group, the Broad Institute, Sigma-Aldrich, and/or ToolGen are, or may in the future be, involved in proceedings similar to interferences or priority disputes in Europe or other foreign jurisdictions. We can give no assurances to the ultimate outcome of these proceedings or the disputes between the CVC Group and the Broad Institute, Sigma-Aldrich and ToolGen.
In addition to the Broad Institute, other third parties have filed patent applications claiming CRISPR/Cas9-related inventions and may allege that they invented one or more of the inventions claimed by the CVC Group. Thus, the USPTO may, in the future, declare an interference between certain CVC Group patent applications and one or more patent applications. The Broad Institute, as well as other third parties, could seek to assert its issued patents against us based on our CRISPR/Cas9-based activities, including commercialization. Defense of these claims, regardless of their merit, could involve substantial litigation expense and could result in a substantial diversion of management and other employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. In that event, we could be unable to further develop and commercialize CTX001 or other products that we may develop using the CRISPR/Cas9 technology we license from CRISPR.
To the extent that valid present or future third-party patents or other intellectual property rights cover our products, product candidates or technologies, we or our strategic collaborators may seek licenses or other agreements from the holders of such rights in order to avoid or settle legal claims. Such licenses may not be available on acceptable terms, which may
hinder our ability to, or prevent us from being able to, manufacture and market our products. Payments under any licenses that we are able to obtain would reduce our profits derived from the covered products.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims.
In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to management.
Risks Related To Our Operations
Risks associated with operating in foreign countries could materially adversely affect our business.
We have expanded our international operations over the past several years in order to market our CF medicines and expand our research and development capabilities. New laws and industry codes in the E.U. and elsewhere have expanded transparency requirements regarding payments and transfers of value to healthcare professionals, requirements surrounding patient-level clinical trial data, the protection of personal data and increased sanctions for violations. Collectively, our expansion and these new requirements are adding to our compliance costs and potentially exposes us to sanctions in the event of an infringement or failure to report in these jurisdictions. In addition, a significant portion of our commercial supply chain, including sourcing of raw materials and manufacturing, is located in China and the E.U. Consequently, we are, and will continue to be, subject to risks related to operating in foreign countries, including risks relating to intellectual property protections and business interruptions, including as a result of the COVID-19 pandemic. These risks are increased with respect to countries such as China that have substantially different local laws and business practices and weaker protections for intellectual property. Risks associated with operating a global biotechnology company include:
•differing regulatory requirements for drug approvals and regulation of approved drugs in foreign countries;
•varying reimbursement regimes and difficulties or the inability to obtain reimbursement for our products in foreign countries in a timely manner;
•differing patient treatment infrastructures, particularly since our business is focused on the treatment of serious diseases that affect relatively smaller numbers of patients and are typically prescribed by specialist physicians;
•collectability of accounts receivable;
•changes in tariffs, trade barriers, and regulatory requirements, the risks of which appear to have increased in the current political environment;
•economic weakness, including recession and inflation, or political instability in particular foreign economies and markets;
•differing levels of enforcement and/or recognition of contractual and intellectual property rights;
•complying with local laws and regulations, which can change significantly over time;
•foreign taxes, including withholding of payroll taxes;
•foreign currency fluctuations, which could result in reduced revenues or increased operating expenses, and other obligations incident to doing business or operating in another country;
•workforce uncertainty in countries where labor unrest is more common than in the U.S.;
•reliance on third-party vendors and suppliers;
•import and export licensing requirements, tariffs, and other trade and travel restrictions;
•global or regional public health emergencies that could affect our operations or business;
•production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
•business interruptions resulting from geo-political actions, including war and terrorism.
Our revenues are subject to foreign exchange rate fluctuations due to the global nature of our operations. Although we have foreign currency forward contracts to hedge forecasted product revenues denominated in foreign currencies, our efforts to reduce currency exchange losses may not be successful. As a result, currency fluctuations among our reporting currency, the U.S. dollar, and the currencies in which we do business will affect our operating results, often in unpredictable ways.
In addition, our international operations are subject to regulation under U.S. law. For example, the FCPA prohibits U.S. companies and their representatives from offering, promising, authorizing or making payments to foreign officials for the purpose of obtaining or retaining business abroad. In many countries, the health care professionals we regularly interact with may meet the definition of a foreign government official for purposes of the FCPA. We also are subject to import/export control laws. Failure to comply with domestic or foreign laws could result in various adverse consequences, including the possible delay in approval or refusal to approve a product, recalls, seizures, withdrawal of an approved product from the market, the imposition of civil or criminal sanctions, the prosecution of executives overseeing our international operations and corresponding bad publicity and negative perception of our company in foreign countries.
If we fail to attract and retain skilled employees, our business could be materially harmed.
Due to the highly technical nature of our drug discovery and development activities, we require the services of highly qualified and trained scientists who have the skills necessary to conduct these activities. In addition, we need to attract and retain employees with experience in marketing and commercialization of medicines. We have entered into employment agreements with some executives and provide stock-related compensation benefits to all of our key employees that vest over time and therefore induce them to remain with us. However, the employment agreements can be terminated by the executive on relatively short notice. The value to employees of stock-related benefits that vest over time can be significantly affected by movements in our stock price and business performance, and may, at any point in time, be insufficient to counteract more lucrative offers from other companies. We face intense competition for our personnel from our competitors and other companies throughout our industry, especially with respect to employees with expertise in cell or genetic therapies. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. Moreover, the growth of local biotechnology companies and the expansion of major pharmaceutical companies into the Boston area has increased competition for the available pool of skilled employees, especially in technical fields. The high cost of living can make it difficult to attract employees from other parts of the country to our Massachusetts headquarters. Current job market dynamics, caused in part by the effects of COVID-19 and other macro-level events, with many employers unable to fill existing openings at all levels of their organizations, could result in significant increases to our costs to recruit and retain employees. Challenges could adversely affect our operations and financial results if we do not have sufficient staff to perform necessary functions. In addition, the available pool of skilled employees would be further reduced if immigration laws change in a manner that increases restrictions on immigration. Our ability to continue to commercialize our products and achieve our research and development objectives depends on our ability to respond effectively to these demands. If we are unable to hire and retain qualified personnel, there could be a material adverse effect on our business.
We are subject to risks associated with the COVID-19 pandemic.
The COVID-19 pandemic has broadly affected the global economy, resulted in significant travel and work restrictions in
many regions and has put a significant strain on healthcare resources. COVID-19 has had, and we expect it will continue to have, an impact on our operations, an impact on the operations of our collaborators, third-party contractors and other entities, including governments, governmental agencies, and payors, with which we interact, and an impact on the people with CF who take our medicines. In addition, we have seen some delays in enrollment in certain clinical trials, supply chain delays, and regulatory delays due to the COVID-19 pandemic. To date, the most significant effect on our business operations has been the requirement that a majority of our employees work remotely.
We continue to monitor local COVID-19 trends and government guidance for each of our site locations and are utilizing a site-specific approach to assess and permit employee access to our sites. Currently, our sites are open where appropriate and permitted by local laws and guidelines. There can be no assurance that our sites will remain open, when additional employees will gain access to our sites, or whether we will be required to pause or delay enrollment and dosing at clinical trial sites. Any site closure, pause, or delay of a clinical trial could harm our operations and delay the development of our product candidates. In addition, even if sites or clinical trials are open for enrollment, COVID-19 may nevertheless impact clinical trial enrollment or participation, for example due to suspension of in-person procedures required for enrollment, government shut-down orders, or decreased patient willingness to participate compared to pre-COVID-19 pandemic levels. COVID-19 may also impact uptake of our medicines generally and patient retention in clinical trials, potentially resulting in higher drop-out rates or missed visits, which may negatively affect the strength of our clinical trial data.
Health regulatory agencies globally may experience disruptions in their operations as a result of the COVID-19 pandemic. The FDA and comparable foreign regulatory agencies may have slower response times or lack resources to continue to monitor our clinical trials or to engage in other activities related to review of regulatory submissions in drug development. In response to the COVID-19 pandemic and the public health emergency declaration in the U.S., on March 10, 2020, the FDA announced its intention to temporarily postpone most inspections of foreign manufacturing facilities and products, and it subsequently postponed routine surveillance inspections of domestic manufacturing facilities and provided guidance regarding the conduct of clinical trials. In July 2021, the FDA stated that it had begun transitioning back to standard operations for domestic inspections, while continuing to prioritize mission-critical work for foreign inspections. The FDA may not be able to maintain this pace and further delays or setbacks are possible in the future. As a result, review, inspection, and other timelines for our product candidates may be materially delayed for an unknown period of time.
In the future, the economic impacts of the COVID-19 pandemic could affect our business directly or indirectly, including potentially affecting the net prices for our products through changes in our payor mix as a result of increased unemployment in the U.S. or increased pressure on healthcare costs in the U.S. and around the world. The effects on our research, development, manufacturing, and commercialization activities, including the continued launch and uptake of our products, will be dependent on, among other things, the severity and duration of the COVID-19 pandemic and any worsening of the global economic environment as a result thereof, as well as the impact of the pandemic on our third-party manufacturers, suppliers, distributors, subcontractors and customers. While the ultimate impact of COVID-19 on our business is highly uncertain, any negative impacts that materialize could materially adversely affect our operations, financial performance and stock price. Any negative impacts of COVID-19, alone or in combination with others, could exacerbate other risk factors discussed herein. The full extent to which the COVID-19 pandemic will negatively affect our operations, financial performance, and stock price will depend on future developments that are highly uncertain and cannot be predicted, including the scope and duration of the pandemic and actions taken by governmental authorities and other third parties in response to the pandemic.
If we fail to manage our operations effectively, our business may suffer.
We have expanded and are continuing to expand our global operations and capabilities, which has placed, and will continue to place, significant demands on our management and our operational, research and development and financial infrastructure. To effectively manage our business, we need to:
•implement and clearly communicate our corporate-wide strategies;
•enhance our operational and financial infrastructure, including our controls over records and information;
•enhance our operational, financial and management processes, including our cross-functional decision-making processes and our budget prioritization systems;
•train and manage our global employee base; and
•enhance our compliance and legal resources.
Our business faces potential risks relating to the U.K.’s withdrawal from the E.U.
Our European headquarters and European research facility are located in the U.K. On January 31, 2020, the U.K. formally withdrew from the E.U., also known as Brexit. The U.K. and the E.U. negotiated a detailed post-Brexit Trade and Cooperating Agreement which went into effect on January 1, 2021. As of January 1, 2021, E.U. Treaties, E.U. free movement rights and the general principals of E.U. law no longer apply in relation to the U.K. By virtue of the E.U. (Withdrawal) Act 2018, E.U. relations will continue to apply in U.K. domestic law to the extent that they are not modified or revoked by regulations under that Act. Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the U.K. determines which E.U. laws to replace or replicate. Given the lack of comparable precedent, it is unclear what financial, trade, regulatory and legal implications the withdrawal of the U.K. from the E.U. would have and how such withdrawal would affect us. Any of these effects of Brexit, among others, could adversely affect our business, financial condition and operating results.
Our business has a substantial risk of product liability claims and other litigation liability.
We are or may be involved in various legal proceedings, including securities/shareholder matters and claims related to product liability, intellectual property, employment law, and breach of contract. Such proceedings may involve claims for, or the possibility of, damages or fines and penalties involving substantial amounts of money or other relief, including but not limited to civil or criminal fines and penalties. If any of these legal proceedings were to result in an adverse outcome, it could have a material adverse effect on our business.
With respect to product liability and clinical trial risks, in the ordinary course of business we are subject to liability claims and lawsuits, including potential class actions, alleging that our products or product candidates have caused, or could cause, serious adverse events or other injury. We have product liability insurance and clinical trial insurance in amounts that we believe are adequate to cover this risk. However, our insurance may not provide adequate coverage against all potential liabilities. If a claim is brought against us, we might be required to pay legal and other expenses to defend the claim, as well as pay uncovered damage awards resulting from a claim brought successfully against us and these damages could be significant and have a material adverse effect on our financial condition. Furthermore, whether or not we are ultimately successful in defending any such claims, we might be required to direct significant financial and managerial resources to such defense and adverse publicity is likely to result.
A breakdown or breach of our information technology systems could subject us to liability or interrupt the operation of our business.
We maintain and rely extensively on information technology systems and network infrastructures for the effective operation of our business. In the course of our business, we collect, store, and transmit confidential information (including personal information and intellectual property), and it is critical that we do so in a secure manner to maintain the confidentiality and integrity of such confidential information. A disruption, infiltration, or failure of our information technology systems or any of our data centers as a result of software or hardware malfunctions, computer viruses, cyber-attacks, employee theft or misuse, power disruptions, natural disasters, floods or accidents could cause breaches of data security and loss of critical data, which in turn could materially adversely affect our business and subject us to both private and governmental causes of action. While we have implemented security measures to minimize these risks to our data and information technology systems and have adopted a business continuity plan to deal with a disruption to our information technology systems, there can be no assurance that our efforts to protect our data and information systems will prevent breakdowns or breaches in our systems that could adversely affect our business. In addition, our liability insurance may not be sufficient in type or amount to cover us against claims related to security breaches, cyber-attacks or other related liabilities.
Cyber-attacks are increasing in their frequency, sophistication, and intensity, and are becoming increasingly difficult to detect. They are often carried out by well-resourced and skilled and parties, including nation states, organized crime groups, “hacktivists” and employees or contractors acting carelessly or with malicious intent. Cyber-attacks include deployment of harmful malware and key loggers, ransomware, denial-of-service attacks, malicious websites, the use of social engineering, and other means to affect the confidentiality, integrity and availability of our technology systems and data. Cyber-attacks also include manufacturing, hardware or software supply chain attacks, which could cause a delay in the manufacturing of products or products produced for contract manufacturing or lead to a data privacy or security breach. Our key business
partners face similar risks, and any security breach of their systems could adversely affect our security posture. In addition, our increased use of cloud technologies heightens these third party and other operational risks, and any failure by cloud or other technology service providers to adequately safeguard their systems and prevent cyber-attacks could disrupt our operations and result in misappropriation, corruption, or loss of confidential or propriety information. Risk of cyber-attack is increased with employees working remotely, including as a result of the ongoing COVID-19 pandemic. During this time, there is an increased risk that we may be vulnerable to cybersecurity-related events such as phishing attacks and other security threats as a result of our employees, third party vendors and collaborators working remotely from non-corporate managed networks.
If our facilities were to experience a catastrophic loss, our operations would be seriously harmed.
Most of our operations, including our research and development activities, are conducted in a limited number of facilities. If any of our major facilities were to experience a catastrophic loss, due to an earthquake, severe storms, fire or similar event, our operations could be seriously harmed. For example, our corporate headquarters, as well as additional leased space that we use for certain logistical and laboratory operations and manufacturing, are located in a flood zone along the Massachusetts coast. We have adopted a business continuity plan to address most crises. However, if we are unable to fully implement our business continuity plans, we may experience delays in recovery of data and/or an inability to perform vital corporate functions, which could result in a significant disruption in our research, development, manufacturing and/or commercial activities, large expenses to repair or replace the facility and/or the loss of critical data, which wouldcould have a material adverse effect on our business.
The use of social media platforms presents risks and challenges.
Social media is being used by third parties to communicate about our products and product candidates and the diseases our therapies are designed to treat. We believe that members of the CF community may be more active on social media as compared to other patient populations due to the demographics of this patient population. Social media practices in the pharmaceutical and biotechnology industries are evolving, which creates uncertainty and risk of noncompliance with regulations applicable to our business. For example, patients may use social media platforms to comment on the effectiveness of, or adverse experiences with, a product or a product candidate, which could result in reporting obligations. In addition, our employees may engage on social media in ways that may not comply with legal or regulatory requirements, which may give rise to liability, lead to the loss of trade secrets and other intellectual property, or result in public disclosure of protected personal information. There is a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us on any social networking website. Certain data protection regulations, such as the GDPR, apply to personal data contained on social media. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face regulatory actions or incur harm to our business, including damage to our reputation.
Risks Related to Financial Results and Holding Our Common Stock
Our stock price may fluctuate.
Market prices for securities of companies such as ours are highly volatile. From January 1, 20182021 to December 31, 2018,2021, our common stock traded between $144.07$176.36 and $194.92$242.99 per share. The market for our stock, like that of other companies in the biotechnology industry, has experienced significant price and volume fluctuations. The future market price of our securities could be significantly and adversely affected by factors such as:
•the information contained in our quarterly earnings releases, including updates regarding our commercialized products or our product candidates, our net product revenues and operating expenses for completed periods and guidance regarding future periods;
•announcements of FDA actions with respect to our drugstherapies or those of our competitors’ drugs,competitors, or regulatory filings for our drug candidatestherapies or those of our competitors, or announcements of interim or final results of clinical trials or nonclinical studies relating to our drugs, drug candidatestherapies or those of our competitors;
•developments in domestic and international governmental policy or regulation, for example, relating to drug pricing or intellectual property rights;pricing;
•technological innovations or the introduction of new drugs by our competitors;
•government regulatory action;
•public concern as to the safety of drugs developed by us or our competitors;
•developments in patent or other intellectual property rights or announcements relating to these matters;
•information disclosed by third parties regarding our business or products;
•developments relating specifically to other companies and market conditions for pharmaceutical and biotechnology stocks or stocks in general;
•business development, capital structuring or financing activities; and
•general worldwide or national economic, political and capital market conditions.conditions, including as a result of the ongoing COVID-19 pandemic.
Following periods of volatility in the market price of a company'scompany’s securities, stockholder derivative lawsuits and securities class action litigation are common. Such litigation, if instituted against us or our officers and directors, could result in substantial costs and a diversion of management'smanagement’s attention and resources.
Our effective tax rate fluctuates, and changes in tax laws, regulations and treaties, unfavorable resolution of tax contingencies or exposure to additional income tax liabilities could have a material impact on our future taxable income.
Our effective tax rate is derived from a combination of applicable tax rates in the various places that we operate globally. Our effective tax rate may be different than experienced in the past due to numerous factors, including changes in the mix of our profitability from country to country, the results of tax authority examinations/audits of our tax filings, adjustments to the value of our uncertain tax positions, changes in accounting for income taxes, and changes in tax laws or modifications of treaties in various jurisdictions. For example, changes to the U.S. tax code are anticipated under the current administration. Any of these factors could cause us to experience an effective tax rate that is significantly different from previous periods or our current expectations.
We assess the impact of various tax reform proposals and modifications to existing tax treaties in all jurisdictions where we have operations to determine the potential effect on our business and any assumptions we have made about our future taxable income. We cannot predict whether any specific proposals will be enacted, the terms of any such proposals or what effect, if any, such proposals would have on our business if they were to be enacted. Beginning in 2022, the Tax Cuts and Jobs Act of 2017 eliminates the currently available option to deduct research and development expenditures and requires taxpayers to amortize them over five years. The U.S. Congress is considering legislation that would defer the amortization requirement to future periods, however, we have no assurance that the provision will be repealed or otherwise modified. If the requirement is not repealed or modified, it will have a material impact on our cash flows beginning in 2022.
Recommendations from the Organization for Economic Co-operation and Development that are part of the base erosion and profit shifting framework could result in changes in tax laws in jurisdictions in which we do business and adversely affect our provision for income taxes and our current rate. If these recommendations, or other changes in law, were adopted by the jurisdictions in which we do business, it could adversely affect our provision for income tax and our current rate.
We are subject to ongoing tax audits in various jurisdictions, and local tax authorities may disagree with certain positions we have taken and assess additional taxes. We regularly assess the probable outcomes of these audits to determine the appropriateness of our tax provision, and we have established contingency reserves for material tax exposures. However, the calculation of our tax exposures involves the application of complex tax laws and regulations in many jurisdictions, as well as interpretations as to the legality under E.U. state aid rules of tax advantages granted in certain jurisdictions. Therefore, there can be no assurance that we will accurately predict the outcomes of these disputes or other tax audits or that issues raised by tax authorities will be resolved at a financial cost that does not exceed our related reserves and the actual outcomes of these disputes and other tax audits could have a material impact on our results of operations or financial condition.
Our quarterly operating results are subject to significant fluctuation.
Our operating results have fluctuated from quarter to quarter in the past, and we expect that they will continue to do so in the future. Our revenues are primarily dependent on the levelamount of net product revenues from sales of our CF medicines. Our total net product revenues could vary on a quarterly basis based on, among other factors, the timing of orders from our
significant customers. Additional factors that have caused quarterly fluctuations to our operating results in recent years include variable amounts of revenues, expenses related to business development activities, changes in the fair value of our strategic investments, impairment charges, charges for excess and obsolete inventories, changes in the fair value of derivative
instruments and the consolidation or deconsolidation of variable interest entities. Our revenues also are subject to foreign exchange rate fluctuations due to the global nature of our operations. Although we have foreign currency forward contracts to hedge forecasted product revenues denominated in foreign currencies, our efforts to reduce currency exchange losses may not be successful. As a result, currency fluctuations among our reporting currency, the U.S. dollar, and the currencies in which we do business may affect our operating results, often in unpredictable ways. Our quarterly results also could be materially affected by significant charges, which may or may not be similar to charges we have experienced in the past. Most of our operating expenses relate to our research and development activities, do not vary directly with the amount of revenues and are difficult to adjust in the short term. As a result, if revenues in a particular quarter are below expectations, we are unlikely to reduce operating expenses proportionately for that quarter. These examples are only illustrative and other risks, including those discussed in these “Risk Factors,” could also cause fluctuations in our reported financial results. Our operating results during any one period do not necessarily suggest the results of future periods.
We expect that results from our clinical development activities and the clinical development activities of our competitors will continue to be released periodically, and may result in significant volatility in the price of our common stock.
Any new information regarding our products and drugproduct candidates or competitive products or potentially competitive drugproduct candidates can substantially affect investors’ perceptions regarding our future prospects. We, our collaborators, and our competitors periodically provide updates regarding drug development programs, typically through press releases, conference calls and presentations at medical conferences. These periodic updates often include interim or final results from clinical trials conducted by us or our competitors and/or information about our or our competitors’ expectations regarding regulatory filings and submissions as well as future clinical development of our products or drugproduct candidates, competitive products or potentially competitive drugproduct candidates. The timing of the release of information by us regarding our drug development programs is often beyond our control and is influenced by the timing of receipt of data from our clinical trials and by the general preference among pharmaceutical companies to disclose clinical data during medical conferences. In addition, the information disclosed about our clinical trials, or our competitors’ clinical trials, may be based on interim rather than final data that may involve interpretation difficulties and may in any event not accurately predict final results.
Changes The release of such information may result in tax laws, regulations and treaties could affect our future taxable income.
A change in tax laws, treaties or regulations, or their interpretation, of any country in which we operate could materially affect us if we generate taxable income in a future period. On December 22, 2017, the United States enacted H.R.1., known as the Tax Cuts and Jobs Act, which represented a substantial change to tax lawsvolatility in the United States, but which did not have a material impact on our financial statements because we maintained a valuation allowance on the majorityprice of our net operating losses and other deferred tax assets as of December 31, 2017, which was only released at the end of 2018. However, over the next several years we expect to utilize our net operating losses and other deferred assets, and any future changes in tax laws could have a material effect on our business.common stock.
We continue to assess the impact of various tax reform proposals and modifications to existing tax treaties in all jurisdictions where we have operations to determine the potential effect on our business and any assumptions we have made about our future taxable income. We cannot predict whether any specific proposals will be enacted, the terms of any such proposals or what effect, if any, such proposals would have on our business if they were to be enacted.General Risk Factors
We may need to raise additional capital that may not be available.
We may need to raise additional capital in the future. Any potential public offering, private placement or debt financing may or may not be similar to the transactions that we entered into in the past. Any debt financing may be on terms that, among other things, include conversion features that could result in dilution to our then-existing security holders and restrict our ability to pay interest and dividends—although we do not intend to pay dividends for the foreseeable future. Additionally, our pledge of specified assets as collateral to secure our obligations under our credit agreement may limit our ability to obtain additional debt financing. Any equity financings would result in dilution to our then-existing security holders. If adequate funds are not available on acceptable terms, or at all, we may be required to curtail significantly or discontinue one or more of our research, drug discovery or development programs, including clinical trials, incur significant cash exit costs, or attempt to obtain funds through arrangements with collaborators or others that may require us to relinquish rights to certain of our technologies, drugsproducts or drugproduct candidates. Based on many factors, including general economic conditions, additional financing may not be available on acceptable terms, if at all.
Future indebtedness could materially and adversely affect our financial condition, and the terms of our credit agreementagreements impose restrictions on our business, reducing our operational flexibility and creating default risks.
In October 2016,2019, we entered into a credit agreement providing for a $500$500.0 million revolving facility, $300 millionfacility. In September 2020, we entered into a second credit agreement providing for a $2.0 billion revolving facility. Each of which was drawn at closing and subsequently paid off in February 2017. Thethe credit agreementagreements provides that, subject to the
satisfaction of certain conditions, we may request that the borrowing capacity under the credit agreement be increased by an additional $300.0$500.0 million. All outstanding borrowings under the credit agreement mature on October 13, 2021. If we borrow under our current credit agreements or any future credit agreement, such indebtedness could have important consequences to our business, including increasing our vulnerability to general adverse financial, business, economic and industry conditions, as well as other factors that are beyond our control. The credit agreement requiresagreements require that we comply with certain financial covenants, including (i) a consolidated leverage ratio covenant and (ii) a consolidated EBITDAinterest coverage ratio covenant, in each case to be measured on a quarterly basis. Further, the credit agreement includesagreements include negative
covenants, subject to exceptions, restricting or limiting our ability and the ability of our subsidiaries to, among other things, incur additional indebtedness, grant liens, engage in certain investment, acquisition and disposition transactions, pay dividends, repurchase capital stock and enter into transactions with affiliates. As a result, we may be restricted from engaging in business activities that may otherwise improve our business. Failure to comply with the covenants could result in an event of default that could trigger acceleration of our indebtedness, which would require us to repay all amounts owing under the credit agreementagreements and/or our capitalfinance leases and could have a material adverse effect on our business. Additionally, our obligations under the credit agreementagreements are unconditionally guaranteed by certain of our domestic subsidiaries. All obligations under the credit agreement, and the guarantees of those obligations, are secured by substantially all of our assets and the assets of all guarantors (excluding intellectual property, owned and leased real property and certain other excluded property), including the pledge of all or a portion of the equity interests of certain of our subsidiaries. If we fail to satisfy our obligations under the credit agreement or are unable to obtain sufficient funds to make payments, the lenders could foreclose on our pledged collateral.
Issuances of additional shares of our common stock could cause the price of our common stock to decline.
As of December 31, 2018,2021, we had 255.2254.5 million shares of common stock issued and outstanding. As of December 31, 2018,2021, we also had outstanding options to purchase 8.63.6 million shares of common stock with a weighted-average exercise price of $111.46$141.76 per share. Outstanding vested options are likely to be exercised if the market price of our common stock exceeds the applicable exercise price, and, in the future, we expect to issue additional options and restricted stock unitsequity awards to directors and employees. In addition, we may issue additional common stock or restricted securities in the future as part of financing activities or business development activities and any such issuances may have a dilutive effect on our then-existing shareholders. Sales of substantial amounts of our common stock in the open market, or the availability of such shares for sale, could adversely affect the price of our common stock. The issuance of restricted common stock or common stock upon exercise of any outstanding options would be dilutive, and may cause the market price for a share of our common stock to decline.
There can be no assurance that we will repurchase shares of common stock or that we will repurchase shares at favorable prices.
OurIn June 2021, our Board of Directors has authorized a share repurchase program ofpursuant to which we are authorized to repurchase up to $500 million to repurchase shares$1.5 billion of our common stock. stock by December 31, 2022. As of December 31, 2021, we had repurchased $1.0 billion of common stock and had $0.5 billion of remaining authorization for additional share repurchases pursuant to this program.
Our stock repurchases will depend upon, among other factors, our cash balances and potential future capital requirements, results of operations, financial condition, and other factors that we may deem relevant. We can provide no assurance that we will repurchase stock at favorable prices, if at all.
We have adopted anti-takeover provisions and are subject to Massachusetts corporate laws that may frustrate any attempt to remove or replace our current management or effectuate a business combination involving Vertex.
Our corporate charter and by-law provisions and Massachusetts state laws may discourage certain types of transactions involving an actual or potential change of control of Vertex that might be beneficial to us or our security holders. Although we amended our charter to eliminate staggered terms for our Board of Directors, our shareholders will not have the ability to vote for all members of the Board of Directors on an annual basis until 2020. Our by-laws grant the directors a right to adjourn annual meetings of shareholders, and certain provisions of our by-laws may be amended only with an 80% shareholder vote. We may issue shares of any class or series of preferred stock in the future without shareholder approval and upon such terms as our Board of Directors may determine. The rights of the holders of common stock will be subject to, and may be adversely affected by, the rights of the holders of any class or series of preferred stock that may be issued in the future. Massachusetts state law prohibits us from engaging in specified business combinations, unless the combination is approved or consummated in a prescribed manner, and prohibits voting by any shareholder who acquires 20% or more of our voting stock without shareholder approval. As a result, shareholders or other parties may find it more difficult to remove or replace our current management.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, and, in particular,including the descriptiondescriptions of our Business set forth in Part I, Item 1, theour Risk Factors set forth in thisPart I, Item 1A, and our Management’s Discussion and Analysis of Financial Condition and Results of Operations set forth in Part II, Item 7, contain or incorporate a numbercontains forward-looking statements. Forward-looking statements are not purely historical and may be accompanied by words such as “anticipates,” “may,” “forecasts,” “expects,” “intends,” “plans,” “potentially,” “believes,” “seeks,” “estimates,” and other words and terms of forward-lookingsimilar meaning. Such statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding:may relate to:
•our expectations regarding the amount of, timing of, and trends with respect to our financial performance, including revenues, costs and expenses, and other gains and losses, including those related to our CF net product revenues;losses;
•our expectations regarding clinical trials, including expectations for patient enrollment, development timelines, and regulatory authority filings and submissions for ivacaftor, lumacaftor, tezacaftor, VX-659, VX-445, VX-150 and the timelines for regulatory filings for a triple combination regimen;
our ability to obtain reimbursement for our medicines in ex-U.S. markets and our ability to otherwise successfully market our medicines or any drug candidates for which we obtain regulatory approval;
our expectations regarding the timing and structure of clinical trials of our drugs and drug candidates and the expected timing of our receipt of data from our ongoing and planned clinical trials;trials, and regulatory authority filings and other submissions for our therapies;
•our ability to maintain and obtain adequate reimbursement for our products, our ability to launch, commercialize and market our products or any of our other therapies for which we obtain regulatory approval and our ability to obtain label expansions for existing therapies;
•our expectations regarding our ability to continue to grow our CF business by increasing the number of people with CF eligible and able to receive our medicines and providing improved treatment options for people who are already eligible for one of our medicines;
•the data that will be generated by ongoing and planned clinical trials and the ability to use that data to advance compounds, continue development or support regulatory filings;
•our beliefs regarding the support provided by clinical trials and preclinical and nonclinical studies of our drug candidatestherapies for further investigation, clinical trials or potential use as a treatment;
•our planplans to continue investing in our research and development programs, including anticipated timelines for our programs, and our strategy to develop our drug candidates,pipeline programs, alone or with third party-collaborators;
•our beliefs regarding the approximate patient populations for the disease areas on which we focus;
•the potential benefits and therapeutic scope of our acquisitions and collaborations;
•the establishment, development and maintenance of collaborative relationships;relationships, including potential milestone payments or other obligations;
•potential business development activities;activities, including the identification of potential collaborative partners or acquisition targets;
•our ability to expand and protect our intellectual property portfolio and otherwise maintain exclusive rights to products;
•potential fluctuations in foreign currency exchange rates;rates and the effectiveness of our foreign currency management program;
•our expectations regarding our provision for or benefit from income taxes and the utilization of our deferred tax assets;
•our ability to use our research programs to identify and develop new drugproduct candidates to address serious diseases and significant unmet medical needs;
•our plans to expand, strengthen, and invest in our global supply chains and manufacturing infrastructure and capabilities, including for cell and gene therapies;
•our ability to attract and retain skilled personnel;
•our expectations involving governmental cost containment and other regulatory efforts;
•our expectations surrounding the competitive landscape facing our products and product candidates;
•our expectations regarding the effect of the COVID-19 pandemic on, among other things, our financial performance, liquidity, business and operations, including manufacturing, supply chain, research and development activities and pipeline programs; and
•our liquidity and our expectations regarding the possibility of raising additional capital.
AnyForward-looking statements are subject to certain risks, uncertainties, or all of our forward-looking statements in this Annual Report on Form 10-K may turn outother factors that are difficult to be wrong. They can be affected by inaccurate assumptions or by known or unknown riskspredict and uncertainties. Many factors mentioned in this Annual Report on Form 10-K will be important in determining future results. Consequently, no forward-looking statement can be guaranteed. Actual future results may vary materially from expected results. We also provide a cautionary discussion of risks and uncertainties under “Risk Factors” above in this Item 1A. These are factors and uncertainties that we think could cause our actual results to differ materially from expected results. Other factors and uncertainties besides those listed there could also adversely affect us.
Without limiting the foregoing, the words “believes,” “anticipates,” “plans,” “intends,” “expects” and similar expressions are intended to identify forward-looking statements. There are a number of factors and uncertainties that could cause actual events or results to differ materially from those indicated byin any such statements.These risks, uncertainties, and other factors include, but are not limited to, those described in our Risk Factors, set forth in Part I, Item 1A, and elsewhere in this report and those described from time to time in our future reports filed with the Securities and Exchange Commission.
Any such forward-looking statements manyare made on the basis of which are beyond our control, including the factorsviews and uncertainties set forth under “Risk Factors” above in this Item 1A. In addition, the forward-looking statements contained herein represent our estimate onlyassumptions as of the date of thisthe filing and shouldare not be relied uponestimates of future performance. Except as representing our estimate as of any subsequent date. Whilerequired by law, we may elect to update these forward-looking statements at some point in the future, we specifically disclaim anyundertake no obligation to do sopublicly update any forward-looking statements. The reader is cautioned not to reflect actual results, changes in assumptions or changes in other factors affectingplace undue reliance on any such forward-looking statements.
| |
ITEM 1B. | UNRESOLVED STAFF COMMENTS |
ITEM 1B.UNRESOLVED STAFF COMMENTS
We did not receive any written comments from the Securities and Exchange Commission prior to the date 180 days before the end of the fiscal year ended December 31, 20182021 regarding our filings under the Securities Exchange Act of 1934, as amended, that have not been resolved.
ITEM 2.PROPERTIES
Corporate Headquarters
We lease approximately 1.1 million square feet of office and laboratory space at our corporate headquarters in Boston, Massachusetts in two buildings pursuant to two leases that we entered into in May 2011. TheThese leases commenced in December 2013 and will extend until December 2028. We have an option to extend the term of the leases for an additional ten years. In addition, we have a lease for approximately 100,000 square feet of space in the Boston Marine Industrial Park, in close proximity to our corporate headquarters. We are using this additional space for certain logistical and laboratory operations and manufacturing equipment that complement the office and laboratory facilities at our corporate headquarters.
Additional United States and Worldwide Locations
In addition to our facilities in Massachusetts,corporate headquarters, we lease an aggregate of approximately 300,000728,000 square feet of space. We lease approximately 170,000 square feet of officespace globally. This space includes logistical, laboratory, commercial and laboratory space in San Diego, California to a lease that expires in 2035. Our other facilities includemanufacturing operations, as well as laboratory and office space to support our research and development organizations Milton Park, Abingdon, England, and office spaceorganizations. We also own approximately 213,000 square feet at our continuous manufacturing facility in many of the countries in which we sell our products.Massachusetts.
ITEM 3.LEGAL PROCEEDINGS
We are not currently subject to any material legal proceedings.
| |
ITEM 4. | MINE SAFETY DISCLOSURES |
ITEM 4.MINE SAFETY DISCLOSURES
Not applicable.
PART II
| |
ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
ITEM 5.MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is traded on The Nasdaq Global Select Market under the symbol “VRTX.”
Shareholders
As of January 31, 2019,2022, there were 1,277107 holders of record of our common stock.
Performance Graph
We became part of the Standard & Poor’s 500 (“S&P 500®”) Stock Index in 2013.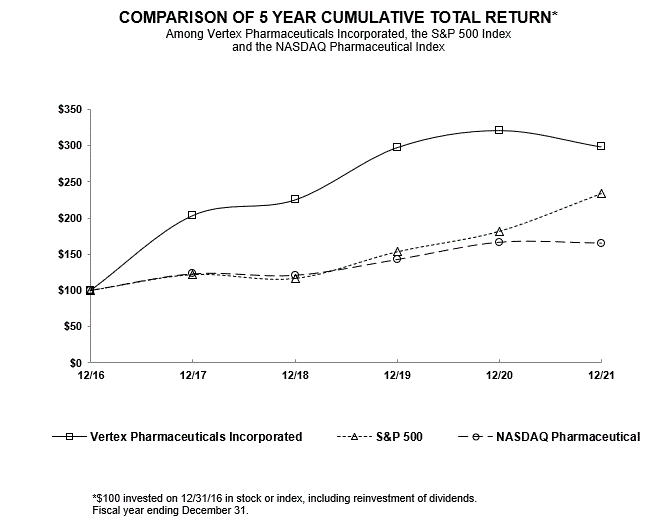
Dividends
We currently expect that any future earnings will be retained for use in our business. Any future determination to declare cash dividends will be subject to the discretion of our board of directors and applicable law and will depend on various factors, including our results of operations, financial condition, prospects and any other factors deemed relevant by our board of directors. In addition, our credit agreement limits our ability to pay cash dividends on our common stock.
Issuer Repurchases of Equity Securities
We haveIn June 2021, our board of directors approved a share repurchase program announced in January 2018, under(the “2021 Share Repurchase Program”), pursuant to which we arewere authorized to repurchase up to $500.0 million$1.5 billion of our common stock by December 31, 2019. As of September 30, 2018, we had repurchased $211.0 million of common stock under this program and had remaining available $289.0 million to repurchase additional shares under this program. 2022.
The table set forth below shows repurchases of securities by us during the three months ended December 31, 2018, including shares repurchased2021 under our share repurchase program and a small number of restricted2021 Share Repurchase Program.
| | | | | | | | | | | | | | | | | | | | | | | |
| Period | Total Number
of Shares Purchased | | Average Price
Paid per Share | | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs (1) | | Approximate dollar value of Shares that May Yet be Purchased Under the Plans or Programs (1) |
| Oct. 1, 2021 to Oct. 31, 2021 | 1,984,142 | | | $ | 180.33 | | | 1,984,142 | | | $ | 500,000,086 | |
| Nov. 1, 2021 to Nov. 30, 2021 | 1,900 | | | $ | 180.00 | | | 1,900 | | | $ | 499,658,094 | |
| Dec. 1, 2021 to Dec. 31, 2021 | — | | | $ | — | | | — | | | $ | 499,658,094 | |
| Total | 1,986,042 | | | $ | 180.33 | | | 1,986,042 | | | $ | 499,658,094 | |
(1)Under our 2021 Share Repurchase Program, we are authorized to purchase shares repurchased by us from employeestime to time through open market or privately negotiated transactions. Such purchases may be made pursuant to Rule 10b5-1 plans or other means as determined by our equity programs. Asmanagement and in accordance with the requirements of December 31, 2018, we hadthe Securities and Exchange Commission. The approximate dollar value of shares that may yet be repurchased $350.0 million of common stockis based solely on shares that may be repurchased under the share repurchase program and had remaining available $150.0 million to repurchase additionalexcludes any shares pursuant to this program.that may be repurchased under our employee equity programs.
ITEM 6.[RESERVED]
|
| | | | | | | | | | | |
| Period | | Total Number
of Shares Purchased (1) | Average Price
Paid per Share | Total Number of Shares
Purchased as Part of
Publicly Announced
Plans or Programs (2) | Maximum Number of
Shares (or approximate dollar amount) that May Yet be Purchased Under
the Plans or Programs (2) |
| Oct. 1, 2018 to Oct. 31, 2018 | 278,920 |
| $ | 174.24 |
| 275,351 |
| $ | 240,390,554 |
|
| Nov. 1, 2018 to Nov. 30, 2018 | 346,159 |
| $ | 165.80 |
| 338,979 |
| $ | 182,995,963 |
|
| Dec. 1, 2018 to Dec. 31, 2018 | 197,930 |
| $ | 166.70 |
| 196,878 |
| $ | 150,000,059 |
|
| Total | 823,009 |
| $ | 168.88 |
| 811,208 |
| $ | 150,000,059 |
|
| |
(1) | Consists of 811,208 shares repurchased pursuant to our share repurchase program (described in footnote 2 below) at an average price of $171.33 per share and 11,801 restricted shares repurchased for $0.01 per share from our employees pursuant to our equity plans. While we have restricted shares that are continuing to vest under our equity plans that are subject to repurchase rights upon termination of service, we have transitioned our equity program to granting restricted stock units. Unvested restricted stock units are forfeited upon termination of service and do not result in an issuer repurchase that would be reflected in this table. |
| |
(2) | Our board of directors has approved a share repurchase program pursuant to which we are authorized to repurchase up to $500.0 million of our common stock by December 31, 2019; the program was announced on January 31, 2018. Under the share repurchase program, we are authorized to purchase shares from time to time through open market or privately negotiated transactions and such purchases may be made pursuant to Rule 10b5-1 plans or other means as determined by our management and in accordance with the requirements of the Securities and Exchange Commission. The approximate dollar value of shares that may yet be repurchased is based solely on shares that may be repurchased under the share repurchase program and excludes any shares that may be repurchased under our employee equity programs. |
| |
ITEM 6. | SELECTED FINANCIAL DATA |
The following unaudited selected consolidated financial data are derived from our audited consolidated financial statements. These data should be read in conjunction with our audited consolidated financial statements and related notes that are included elsewhere in this Annual Report on Form 10-K and with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in Item 7.
|
| | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| | 2018 | | 2017 | | 2016 | | 2015 | | 2014 |
| Consolidated Statements of Operations Data: | (in thousands, except per share amounts) |
| Product revenues, net | $ | 3,038,325 |
| | $ | 2,165,480 |
| | $ | 1,683,632 |
| | $ | 1,000,324 |
| | $ | 487,821 |
|
| Collaborative and royalty revenues (1) | 9,272 |
| | 323,172 |
| | 18,545 |
| | 32,012 |
| | 92,594 |
|
| Total revenues | 3,047,597 |
| | 2,488,652 |
| | 1,702,177 |
| | 1,032,336 |
| | 580,415 |
|
| Total costs and expenses (2) | 2,412,447 |
| | 2,365,409 |
| | 1,692,241 |
| | 1,499,215 |
| | 1,272,827 |
|
| (Benefit from) provision for income taxes (3) | (1,486,862 | ) | | (107,324 | ) | | 16,665 |
| | 30,381 |
| | 6,958 |
|
| Net income (loss) attributable to Vertex | $ | 2,096,896 |
| | $ | 263,484 |
| | $ | (112,052 | ) | | $ | (556,334 | ) | | $ | (738,555 | ) |
| Diluted income (loss) from continuing operations per share attributable to Vertex common shareholders | $ | 8.09 |
| | $ | 1.04 |
| | $ | (0.46 | ) | | $ | (2.31 | ) | | $ | (3.14 | ) |
| Shares used in per diluted share calculations | 259,185 |
| | 253,225 |
| | 244,685 |
| | 241,312 |
| | 235,307 |
|
| | | | | | | | | | |
| | As of December 31, |
| | 2018 | | 2017 | | 2016 | | 2015 | | 2014 |
| Consolidated Balance Sheet Data: | (in thousands) |
| Cash, cash equivalents and marketable securities | $ | 3,168,242 |
| | $ | 2,088,666 |
| | $ | 1,434,557 |
| | $ | 1,042,462 |
| | $ | 1,387,106 |
|
| Deferred tax assets (3) | 1,499,672 |
| | — |
| | — |
| | — |
| | — |
|
| Total assets | 6,245,898 |
| | 3,546,014 |
| | 2,896,787 |
| | 2,498,587 |
| | 2,334,679 |
|
| Total current liabilities (4) | 1,120,292 |
| | 807,260 |
| | 792,537 |
| | 506,167 |
| | 368,254 |
|
| Long-term debt obligations, excluding current portion | — |
| | — |
| | — |
| | 223,863 |
| | 280,569 |
|
| Construction financing lease obligation, excluding current portion (5) | 561,892 |
| | 563,406 |
| | 486,359 |
| | 472,611 |
| | 473,073 |
|
| Other long-term obligations | 128,511 |
| | 133,042 |
| | 279,700 |
| | 202,318 |
| | 116,600 |
|
| |
(1) | In 2017, we recorded $230.0 million of collaborative and royalty revenues related to an upfront payment made to us pursuant to our collaboration agreement with Merck KGaA, Darmstadt, Germany. See Note B, “Collaborative Arrangements and Acquisitions.”
|
| |
(2) | Total costs and expenses included (i) in 2018 and 2017, intangible asset impairment charges of $29.0 million and $255.3 million, respectively, (ii) $111.9 million collaborative expenses in 2018 primarily related to strategic license agreements; $168.7 million collaborative expenses in 2017 primarily related to an asset acquisition and (iii) in 2014, $50.9 million of restructuring charges primarily related to the relocation of our corporate headquarters. See Note J, “Intangible Assets and Goodwill,” and Note B, “Collaborative Arrangements and Acquisitions.” |
| |
(3) | In 2018, we released the valuation allowance on the majority of our net operating losses and other deferred tax assets resulting in a benefit from income taxes of $1.56 billion in the fourth quarter of 2018 and we recorded a $1.50 billion deferred tax asset on our consolidated balance sheet as of December 31, 2018. In 2018 and 2017, we recorded benefits from income taxes related to the impairment of intangible assets. See Note J, “Intangible Assets and Goodwill.”
|
| |
(4) | As of December 31, 2018 and 2017, we had $354.4 million and $232.4 million, respectively, recorded as current liabilities related to cash received by us for sales of ORKAMBI in France for which the price has not been established. See Note A, “Nature of Business and Accounting Policies.”
|
| |
(5) | We have entered into several leases in which we are deemed to be the owner for accounting purposes. See Note L, “Long-term Obligations.”
|
| |
ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
ITEM 7.MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Our discussion and analysis of our financial condition and results of operations for 2021 as compared to 2020 are discussed below. For a discussion of our financial condition and results of operations for 2020 as compared to 2019, please refer to Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our 2020 Annual Report on Form 10-K, except as set forth below.
OVERVIEW
We invest in scientific innovation to create transformative medicines for people with serious diseases. Our business is focuseddiseases with a focus on developing and commercializing therapies for the treatment ofspecialty markets. We have four approved medicines to treat cystic fibrosis, or CF, a life-threatening genetic disease, and advancingare focused on increasing the number of people with CF eligible and able to receive our medicines through label expansions, approval of new medicines and expanded reimbursement. We are broadening our pipeline into additional disease areas through internal research efforts and accessing external innovation through business development programstransactions.
Our triple combination regimen, TRIKAFTA/KAFTRIO was approved in other serious diseases. Our marketed products2019 in the United States, or U.S., and in 2020 in the European Union, or E.U. Collectively, our four medicines are SYMDEKO/SYMKEVI (tezacaftor in combination with ivacaftor), ORKAMBI (lumacaftor in combination with ivacaftor) and KALYDECO (ivacaftor), which are collectively approved to treat approximately halfbeing used by the majority of the 75,000approximately 83,000 people with CF patients in North America, Europe and Australia. Our triple combination regimens, if approved, would significantlyWe are evaluating our medicines in additional patient populations, including younger children, with the goal of having small molecule treatments for approximately 90% of people with CF.
We continue to research and develop product candidates for the treatment of serious diseases, including genetic therapies to address the remaining approximately 10% of people with CF, sickle cell disease, beta thalassemia, APOL1-mediated kidney disease, type 1 diabetes, pain, alpha-1 antitrypsin deficiency, Duchenne muscular dystrophy, and myotonic dystrophy type 1.
Financial Highlights
| | | | | |
| Revenues | In 2021, our net product revenues continued to increase due to the uptake of KAFTRIO in Europe and continued strong performance of TRIKAFTA in the U.S., including the expanded indication of TRIKAFTA for children with CF 6 through 11 years of age. |
| Expenses | Our total R&D and SG&A expenses increased to $3.9 billion as compared to $2.6 billion in 2020 primarily due to a $900.0 million upfront payment we made to CRISPR in connection with an amendment to our CTX001 collaboration. In 2021, cost of sales was 12% of our net product revenues. |
| Cash | Our cash, cash equivalents and marketable securities increased to $7.5 billion as of December 31, 2021 as compared to $6.7 billion as of December 31, 2020 primarily due to our net product revenues and profitability, offset by repurchases of our common stock and the $900.0 million payment to CRISPR. |
Business Updates
Marketed Products
We expect to continue to grow our CF business by increasing the number of people with CF patientseligible and able to receive our medicines and providing improved treatment options for people who are already eligible for one of our medicines. Since the beginning of 2021, we have made significant progress in activities supporting these efforts.
•The U.S. Food and Drug Administration, or the FDA, approved the use of TRIKAFTA for children with CF 6 through 11 years of age who have at least one F508del mutation or at least one mutation that is responsive to TRIKAFTA.
•In January 2022, the European Commission and the U.K.’s Medicines and Healthcare products Regulatory Agency granted marketing authorization for KAFTRIO in the treatment of children with CF 6 through 11 years of age who have at least one F508del mutation in the CFTR gene.
•TRIKAFTA/KAFTRIO is now approved and could provide an improvedreimbursed or accessible in more than 20 countries outside the U.S.
•Our Phase 3 clinical trial evaluating ORKAMBI for the treatment option forof children with CF 12 through 24 months of age met its primary endpoint. Based on these data, we plan to submit regulatory filings in the U.S. and Europe in the first and second quarters of 2022, respectively.
Pipeline
We continue to advance a majoritypipeline of potentially transformative small molecule, and cell and genetic therapies aimed at treating serious diseases. Since the patients currently eligible for our products. beginning of 2021, we have made important progress in activities supporting these programs.
Cystic Fibrosis
•In November 2018,the third quarter of 2021, we reported positive data, including interim data, fromannounced the initiation of Phase 3 clinical trials evaluating thea once-daily investigational triple combination of VX-659, tezacaftorVX-121/tezacaftor/VX-561 (deutivacaftor). Enrollment is underway in these two Phase 3 clinical trials, and ivacaftorwe expect to complete enrollment in both trials by late 2022 or early 2023.
•We are conducting enabling studies for CF messenger ribonucleic acid, or mRNA, therapeutics designed to treat the underlying cause of CF by enabling cells in the lungs to produce functional CFTR protein for the treatment of the approximately 10% of people with CF who do not produce any CFTR protein. We expect to submit an Investigational New Drug Application, or IND, for this program in 2022.
Sickle Cell Disease and Beta Thalassemia
•We are evaluating the use of a non-viral ex vivo CRISPR gene-editing therapy, CTX001, for the treatment of severe sickle cell disease, or SCD, and transfusion-dependent beta thalassemia, or TDT. Enrollment is complete in the ongoing clinical trials evaluating CTX001 in severe SCD and TDT.
•Data presented to date support the profile of CTX001 as a potential one-time functional cure for people with severe SCD and TDT. CTX001 safety data to date is generally consistent with an autologous stem cell transplant and myeloablative conditioning. We anticipate regulatory submissions of CTX001 in late 2022.
APOL1-Mediated Kidney Disease
•In December 2021, we announced that patients with APOL1-mediated focal segmental glomerulosclerosis, or FSGS, treated with VX-147, a copysmall molecule inhibitor of the F508del mutationAPOL1 function, on top of standard of care achieved a statistically significant, substantial and clinically meaningful reduction of proteinuria in their CFTR genea Phase 2 proof-of-concept clinical trial. We anticipate completing our end of Phase 2 meetings with regulators and a second mutation that resultsadvancing VX-147 into pivotal development in minimal CFTR function, whom we refer to as F508del/Min patients; and who have two copies of the F508del mutation, whom we refer to as F508del homozygous patients. Inpeople with APOL1-mediated kidney disease, or AMKD, including APOL1-mediated FSGS, in the first quarter of 2019,2022.
Type 1 Diabetes
•VX-880 is a stem cell-derived, allogeneic, fully differentiated, insulin-secreting islet cell replacement therapy, using standard immunosuppression to protect the implanted cells. Our Phase 1/2 clinical trial evaluating VX-880 as a potential treatment for type 1 diabetes, or T1D, is ongoing at multiple clinical sites in the U.S. In January 2022, we announced positive Day 150 data for the first T1D patient in this clinical trial, including restoration of islet cell function and rapid improvements in multiple measures. In this first patient, the safety of VX-880 was generally consistent with the immunosuppressive regimen used in this study. We will continue to dose patients in 2022.
•We also are pursuing additional programs in T1D, in which these stem cell-derived, fully differentiated, insulin-secreting islet cells are encapsulated and implanted in an immunoprotective device or modified to produce hypoimmune cells. We are conducting IND-enabling studies for the cells and device program, and we expect to report data from the Phase 3 clinical trials evaluating the triple combination of VX-445, tezacaftor and ivacaftor. We expect that this data in conjunction with the VX-659 data that was reported in November 2018 will enable us to choose the better of the two regimens to submit for regulatory approval. We expect to submit aan Investigational New Drug Application, or NDA,IND, for this program in 2022.
Pain
•Two Phase 2 dose ranging acute pain clinical trials evaluating VX-548, a selective small molecule inhibitor of NaV1.8, are underway; one following bunionectomy surgery and the other following abdominoplasty surgery. We expect to obtain data from the United States Foodclinical trials evaluating VX-548 in the first quarter of 2022.
Alpha-1 Antitrypsin, or AAT, Deficiency
•We plan to advance one or more novel small molecule Z-AAT correctors into the clinic in 2022.
Investments in External Innovation
•Pursuant to a collaboration with CRISPR that we amended in 2021, we now lead global development, manufacturing and Drug Administration, or FDA,commercialization of CTX001, with support from CRISPR.
•We entered into research collaborations with Obsidian Therapeutics, Inc., Arbor Biotechnologies, Inc., and Mammoth Biosciences, Inc.
Our Business Environment
Our net product revenues come from the sale of our medicines for the treatment of CF. Our CF strategy involves continuing to develop and obtain approval and reimbursement for treatment regimens that will provide benefits to all people with CF and increasing the number of people with CF eligible and able to receive our medicines, including through label expansions, expanded reimbursement, and the development of new medicines. We are actively pursuing a triple combination regimen no later than mid-2019. We also are developing drugpipeline of product candidates for the treatment of pain, beta-thalassemia, sickle cell disease and alpha-1 antitrypsin deficiency.
2018 Financial Highlights
Revenues:
In 2018, our CF net product revenues continuedserious diseases outside of CF. Our strategy is to increase due to the approval of our third CF medicine, SYMDEKO/SYMKEVI, and increasing KAYLDECO net product revenues. In 2019, we expect our CF net product revenues to continue to increase due to full-year revenues from SYMDEKO/SYMKEVI and further CF net product revenue growth will be dependent on if, and when, we are able to able to obtain approval to market a triple combination regimen for patients with CF.
Expenses
In 2018, combined R&D and SG&A expenses increased by 8% from $1.82 billion in 2017 to $1.97 billion in 2018. In 2018, cost of sales was approximately 13.5% of our CF net product revenues.
Balance Sheet
Increased balance sheet strength driven by earnings.
2018 Business Highlights
Cystic Fibrosis
Announced positive data from two Phase 3 clinical trials evaluating the triple combination of VX-659, tezacaftor and ivacaftor in F508del/Min patients and F508del homozygous patients 12 years of age or older.
Completed enrollment in two Phase 3 clinical trials evaluating the triple combination of VX-445, tezacaftor and ivacaftor in F508del/Min patients and F508del homozygous patients 12 years of age or older. Data from these clinical trials is expectedcombine transformative advances in the first quarterunderstanding of 2019.
Obtained approval for SYMDEKO inhuman disease biology and the United States in the first quarterscience of 2018 for F508del homozygous patients 12 years of age or older.
Successfully launched SYMDEKO in the United States.
Obtained approval for SYMKEVI in the European Union in the fourth quarter of 2018 for F508del homozygous patients 12 years of age or older.
Obtained approvals from the FDA and EMA for label expansions for KALYDECO and ORKAMBI for younger patient groups.
Entered into innovative long-term access agreements in ex-U.S markets, including Australia and Denmark.
Initiated a Phase 1 clinical trial to evaluate VX-121, an additional next-generation CFTR corrector.
Expanding Pipeline
Demonstrated proof-of-concept for VX-150, a NaV1.8 inhibitor, in acute and neuropathic pain and initiated a Phase 2b dosing ranging clinical trial.
Initiated first clinical trials of CTX001, an investigational gene-editing treatment that we are evaluating as a potential treatment for beta-thalassemia and sickle cell disease.
Initiated a Phase 1 clinical trial for a novel drug candidate for alpha-1 antitrypsin deficiency.
Established a collaboration with Arbor Biotechnologies to enhance our ongoing efforts to develop innovative gene-editing therapies for a range of serious diseases.
Research
We plan to continue investing in our research programs and fostering scientific innovationtherapeutics in order to identifydiscover and develop transformativenew medicines. This approach includes advancing multiple compounds from each program, spanning multiple modalities, into early clinical trials and evaluating patient data to inform discovery and development of additional compounds, with the goal of bringing first-in-class and best-in-class therapies to patients, and to provide durable clinical and commercial success.
In addition to continuing ourpursuit of new product candidates and therapies in specialty markets, we invest in research in CF, our current research programs include programs targeting pain, alpha-1 antitrypsin and focal segmental glomerulosclerosis.development. We believe that pursuing research in diverse areas allows us to balance the risks inherent in drugproduct development and may provide drugproduct candidates that will form our pipeline in future years. To supplement our internal research programs, we acquire technologies and programs and collaborate with biopharmaceutical and technology companies, leading academic research institutions, government laboratories, foundations and other organizations, as needed, to advance research in our areas of therapeutic interest and to access technologies needed to execute on our strategy.
Drug Discovery and Development
Discovery and development of a new pharmaceutical or biological product is a difficult and lengthy process that requires significant financial resources along with extensive technical and regulatory expertise. Potential drug candidates are subjected to rigorous evaluations, driven in part by stringent regulatory considerations, designed to generate information concerning efficacy, side-effects, proper dosage levels and a variety of other physical and chemical characteristics that are important in determining whether a drug candidate should be approved for marketing as a pharmaceutical product. Most chemical compounds that are investigated as potential drug or biological product candidates never progress into development, and most drugproduct candidates that do advance into development never receive marketing approval. Because ourOur investments in drugproduct candidates are subject to considerable risks, werisks. We closely monitor the results of our discovery, research, clinical trials and nonclinical studies and frequently evaluate our drugproduct development programs in light of new data and scientific, business and commercial insights, with the objective of balancing risk and potential. This process can result in abruptrapid changes in focus and priorities as new
information becomes available and as we gain additional understanding of our ongoing programs and potential new programs, as well as those of our competitors.
IfOur business also requires ensuring appropriate manufacturing and reimbursement of our products. As we believe that data fromadvance our product candidates through clinical development toward commercialization and market and sell our approved products, we build and maintain our supply chain and quality assurance resources. We rely on a completed registration program support approvalglobal network of a drug candidate, we submit an NDAthird parties and our internal capabilities to the FDA requesting approval to market the drug candidate in the United Statesmanufacture and seek analogous approvals from comparable regulatory authorities in jurisdictions outside the United States. To obtain approval, we must, among other things, demonstrate with evidence gathered in nonclinical studiesdistribute our products for commercial sale and well-controlledpost-approval clinical trials thatand to manufacture and distribute our product candidates for clinical trials. In addition to establishing supply chains for each new approved product, we adapt our supply chain for existing products to include additional formulations or to increase scale of production for existing products as needed. The processes for cell and genetic therapies can be more complex than those required for small molecule drugs and require different systems, equipment, facilities and expertise. We are focused on ensuring the drug candidate is safe and effective for the disease it is intended to treat and that the manufacturing facilities, processes and controls for the manufacturestability of the drug candidate are adequate. The FDA and ex-U.S. regulatory authorities have substantial discretion in deciding whether or not a drug candidate should be granted approval based on the benefits and risks of the drug candidate in the treatment of a particular disease, and could delay, limit or deny regulatory approval. If regulatory delays are significant or regulatory approval is limited or denied altogether,supply chains for our financial results and the commercial prospects for the drug candidate involved will be harmed.
Regulatory Compliance
Our marketing of pharmaceuticalcurrent products, is subject to extensive and complex laws and regulations. We have a corporate compliance program designed to actively identify, prevent and mitigate risk through the implementation of compliance policies and systems and through the promotion of a culture of compliance. Among other laws, regulations and standards, we are subject to various U.S. federal and state laws, and comparable laws in other jurisdictions, pertaining to health care fraud and abuse, including anti-kickback and false claims laws, and laws prohibiting the promotion of drugs for unapproved or off-label uses. Anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, receive or pay any remuneration to induce the referral of business, including the purchase or prescription of a particular drug that is reimbursed by a state or federal program. False claims laws prohibit anyone from knowingly or willfully presenting for payment to third-party payors, including Medicare and Medicaid, claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. We are subject to laws and regulations that regulate the sales and marketing practices of pharmaceutical manufacturers, as well as laws such as the U.S. Foreign Corrupt Practices Act, which governfor our international business practices with respect to payments to government officials. We expect to continue to devote substantial resources to maintain, administer and expand these compliance programs globally.
Sales of our products depend, to a large degree, on the extent to which our products are reimbursed by third-party payors, such as government health programs, commercial insurance and managed health care organizations. Reimbursement for our products, including our potential pipeline therapies, cannot be assured and may take significant periods of time to obtain. We dedicate substantial management and other resources in order to obtain and maintain appropriate levels of reimbursement for our products from third-party payors, including governmental organizations, in the United StatesU.S. and ex-U.S. markets.
In the United States,U.S., we have worked successfully with third party-payorsthird-party payors in order to promptly obtain appropriate levels of reimbursement for our CF medicines, and as such, more than 95% of patients across the U.S. have accessmedicines. We plan to our medicines through their insurance plans. We continue to engage in discussions with numerous commercial insurers and managed health care organizations, along with government health programs that are typically managed by authorities in the individual states, to ensure that payors recognize the significant benefits that our medicines provide by treating the underlying causeand provide patients with appropriate levels of cystic fibrosis and continue to provide access to our current medicines.
medicines now and in the future. In Europe and other ex-U.S. markets, we seek government reimbursement for our medicines on a country-by-country basis.or region-by-region basis, as required. This is necessary for each new medicine, as well as for label expansions for our current medicines in most countries.medicines. We successfully obtainedexpect to continue to focus significant resources to obtain expanded reimbursement for KALYDECO in each significant ex-U.S. market within two years of approval. We are experiencing significant challenges in obtaining reimbursement for ORKAMBI in certain ex-U.S. markets. Specifically, we have been discussing potential reimbursement for ORKAMBI in the United Kingdom and France, which represent significant potential markets for our CF medicines since its approvaland, ultimately, pipeline therapies in 2015.U.S. and ex-U.S. markets.
COVID-19
We continue to monitor the impacts of the COVID-19 global pandemic on our business, including in our clinical trials, manufacturing facilities and capabilities, and ability to access necessary resources. COVID-19 has not materially affected our supply chain or the demand for our medicines, and we believe that we will be able to continue to supply all of our approved medicines to patients globally. We adjusted our business operations in response to COVID-19 and have continued to monitor local COVID-19 trends and government guidance for each of our site locations. We are utilizing a site-specific approach to assess and permit employee access to our sites. Currently, our sites are open to certain employees where appropriate and permitted by local laws and guidelines.
Strategic Transactions
Acquisitions
As part of our business strategy, we seek to acquire products, product candidates and other technologies and businesses that are aligned with our corporate and research and development strategies and complement and advance our ongoing research and development efforts. In 2019, we invested significantly in business development transactions designed to augment our pipeline, including the acquisition of Semma Therapeutics, Inc., or Semma, a privately-held company focused on the use of stem cell-derived human islets as a treatment for T1D, and Exonics Therapeutics, Inc., or Exonics, a privately-held company focused on creating transformative gene-editing therapies to repair mutations that cause Duchenne muscular dystrophy, or DMD, and other ex-U.S. markets,severe neuromuscular diseases, including Australia, Denmark, Germany, Ireland, Swedenmyotonic dystrophy type 1, or DM1. In the Semma acquisition, we paid approximately $950.0 million in cash to Semma equity holders. In the Exonics acquisition, we paid approximately $245.0 million upfront to Exonics equity holders and Italy,agreed to additional payments based upon successful achievement of specified development and regulatory milestones. We expect to continue to identify and evaluate potential acquisitions and may include larger transactions or later-stage assets.
Both of our 2019 acquisitions were accounted for as business combinations. As of the acquisition date for each transaction, the cash payments, as well as the fair value of contingent consideration for Exonics, were allocated primarily to
goodwill and the fair value of several in-process research and development assets that we acquired. The fair value of contingent consideration related to Exonics was recorded as a liability and continues to be adjusted on a quarterly basis. As a result, these acquisitions are primarily reflected in additional assets and liabilities on our consolidated balance sheet. Operating expenses incurred by Exonics and Semma after the acquisition dates and specific expenses associated with the acquisitions are reflected in our consolidated statement of operations.
Please refer to our critical accounting policies, “Acquisitions,” for further information regarding the significant judgments and estimates related to our acquisitions.
Collaboration and Licensing Arrangements
We enter into arrangements with third parties, including collaboration and licensing arrangements, for the development, manufacture and commercialization of products, product candidates, and other technologies that have the potential to complement our ongoing research and development efforts. We expect to continue to identify and evaluate collaboration and licensing opportunities that may be similar to or different from the collaborations and licenses that we have reached pricing and reimbursement agreements for ORKAMBI. In some of these countries, we have innovative reimbursement arrangements that provide a pathway to access and rapid reimbursement for certain future CF medicines, including arrangementsengaged in Ireland, Denmark and Australia.
Collaboration Arrangements and Strategic Investmentspreviously.
In-License Agreements
We have entered into collaborations with biotechnology and pharmaceutical companies in order to acquire rights or to license drugproduct candidates or technologies that enhance our pipeline and/or our research capabilities. Over the last several years, we entered into collaboration agreements with:
CRISPR Therapeutics AG, or CRISPR, pursuant to which we are collaborating on the discovery and developmentwith a number of potential new treatments aimed at the underlying genetic causes of human diseases using CRISPR-Cas9 gene editing technology;
companies, including Arbor Biotechnologies, Inc., or Arbor, pursuant to which we are collaborating on the discovery of novel proteins, including DNA endonucleases, to advance the development of new gene-editing therapies; and
ModernaCRISPR, Kymera Therapeutics, Inc., orMammoth Biosciences, Inc., Moderna, pursuant to which we are seeking to identifyInc., and develop messenger ribonucleic acid, or mRNA, therapeutics for the treatment of CF.
Obsidian Therapeutics, Inc. Generally, when we in-license a technology or drugproduct candidate, we make upfront payments to the collaborator, assume the costs of the program, andand/or agree to make contingent payments, which could consist of milestone, royalty, and option payments. DependingMost of these collaboration payments are expensed as research and development expenses; however, depending on many factors, including the structure of the collaboration, the significance of the drugin-licensed product candidate that we license
to the collaborator’s operations and the other activities in which our collaborators are engaged, the accounting for these transactions can vary significantly. Our research and development expenses included $1.1 billion in 2021, $184.6 million in 2020 and $318.3 million in 2019 related to upfront, milestone and other payments pursuant to our collaboration agreements and other business development agreements. The increase in these payments in 2021 was primarily related to the $900.0 million upfront payment we made to CRISPR that is described below.
For example,Joint Development and Commercialization Agreement with CRISPR
In 2017, we entered into a joint development and commercialization agreement, or the Original JDCA, with CRISPR pursuant to which we are developing and preparing to commercialize CTX001 for TDT and SCD. The Original JDCA was entered into following our exercise of an option to co-develop and co-commercialize the hemoglobinopathies program that was contained in a collaboration agreement that we entered into with CRISPR in 2015.
In April 2021, we and CRISPR entered into an amendment and restatement of the Original JDCA, or the A&R JDCA. In June 2021, we made a $900.0 million upfront payments and expenses incurredpayment to CRISPR in connection with ourthe closing of the transactions contemplated by the A&R JDCA. We concluded that we did not have any alternative future use for the acquired in-process research and development and recorded this upfront payment to “Research and development expenses.” Under the terms of the A&R JDCA, we are leading worldwide development, manufacturing, and commercialization of CTX001. As of July 1, 2021, 60% of the net profits and net losses for CTX001 are allocated to us and 40% of the net profits and net losses for CTX001 are allocated to CRISPR. CRISPR may earn an additional one-time $200.0 million milestone payment upon regulatory approval of CTX001. We concluded that the Original JDCA and Moderna collaborationsthe A&R JDCA are cost-sharing arrangements, which result in the net impact of the arrangements being expensed as researchrecorded in “Research and development expenses because the collaboration represents a small portion of each of these collaborator’s overall business. CRISPR and Moderna’s activities unrelated to our collaborations have no effect on our consolidated financial statements. In contrast, Parion Sciences, Inc., or Parion, and BioAxone Biosciences, Inc., or BioAxone, have historically been accounted for as variable interest entities, or VIEs, and historically have been included” in our consolidated financial statements due to (i) the significance of the respective licensed programs to Parion and BioAxone as a whole, (ii) our power to control the significant activities of the entities under each collaboration and (iii) our obligation to absorb losses and right to receive benefits that potentially could be significant. In 2017 and 2018, we determined that the above conditions were no longer satisfied with respect to Parion and BioAxone, respectively. As a result, we deconsolidated Parion and BioAxone from our consolidated financial statements as of September 30, 2017 and December 31, 2018, respectively.
A collaborator that we account for as a VIE may engage in activities unrelated to our collaboration. The revenues and expenses unrelated to the programs we in-license from our VIEs have historically been immaterial to our consolidated financial statements. With respect to each of Parion and BioAxone, the activities unrelated to our collaborations with these entities represented approximately 2% or less of our total revenues and total expenses on an annual basis during the periods that we consolidated these collaborators. As a result of these deconsolidations, these amounts decreased in 2018 compared to 2017 and we do not expect to have similar items in 2019 based on our current collaborations. For consolidated VIEs, we evaluated the fair value of the contingent payments payable by us on a quarterly basis. Changes in the fair value of these contingent future payments affected net income attributable to Vertex on a dollar-for-dollar basis, with increases in the fair value of contingent payments payable by us to a VIE resulting in a decrease in net income attributable to Vertex (or an increase in net loss attributable to Vertex) and decreases in the fair value of contingent payments payable by us to a VIE resulting in an increase in net income attributable to Vertex (or decrease in net loss attributable to Vertex). For additional information regarding our VIEs see Note B, “Collaborative Arrangements and Acquisitions,” and our critical accounting policies “Collaborations; Intangible Assets and Variable Interest Entities.”operations.
Out-License Agreements
We also have out-licensed internally-developed programs to collaborators who are leading the development of these programs. These out-license arrangements include our collaboration agreements with:
Janssen Pharmaceuticals, Inc., or Janssen, which is evaluating pimodivir in Phase 3 clinical trials for the treatment of influenza; and
agreement with Merck KGaA, Darmstadt, Germany, which licensed oncology research and development programs from us in early 2017.
Pursuant to these out-licensing arrangements, our collaborators are responsible for the research, development, and commercialization costs associated with these programs, and we are entitled to receive contingent milestone and/or royalty payments. As a result, we do not expect to incur significant
expenses in connection with these programs and have the potential for future collaborative and royalty revenues resulting from these programs.
Please refer to Note B, “Collaborative and Other Arrangements,” for further information regarding our in-license agreements and out-license agreements.
Strategic Investments
In connection with our business development activities, we have periodically made equity investments in our collaborators. As of December 31, 2018,2021, we held strategic equity investments in CRISPR, acertain public company, and Moderna, which became a publicly traded company in December 2018, and certain private companies, and we mayexpect to make additional strategic equity investments in the future. While we invest the majority of our cash, cash equivalents, and marketable securities in instruments with low risk that meet specific credit quality standards and limit our exposure to any one issue or type of instrument, our strategic investments are maintained and managed separately from our other cash, cash equivalents, and marketable securities.
Until December 31, 2017, changes As discussed below in the fair value“Other Income (Expense), Net” in our Results of these strategic investments were reflected on our consolidated balance sheet, but did not affect our net income until the related gains or losses were realized. As a result of new accounting guidance, effective January 1, 2018, Operations,any changes in the fair value of equity investments with readily determinable fair values (including publicly traded securities such as CRISPR and Moderna)securities) are recorded to other income (expense), net in our consolidated statement of operations. For equity investments without readily determinable fair values (including private equity investments), each reporting period we are required to re-evaluate the carrying value of the investment, which may result in other income (expense).
In 2018, we recorded within other income (expense), net an unrealized gain of $2.6 million related to changes in the fair value of our investments in CRISPR and Moderna, which are included in our net income. In 2018, income and expenses related to these investments did not have a material effect on our annual other income (expense), but had material effects on our quarterly other income (expense). To the extent that we continue to hold strategic investments and in particular strategic investments in publicly traded companies, we will record on a quarterly basis other income (expense) related to these strategic investments. Due to the high volatility of stocks in the biotechnology industry, we expect the value of these strategic investments to fluctuate and that the increases or decreases in the fair value of these strategic investments will continue to have material impacts on our net income (expense) and our profitability under GAAP on a quarterly and/or annual basis.
RESULTS OF OPERATIONS
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | 2018/2017
Comparison | | 2017/2016
Comparison |
| | | | | | | | Increase/(Decrease) | | Increase/(Decrease) |
| | 2018 | | 2017 | | 2016 | | $ | | % | | $ | | % |
| | (in thousands) | | (in thousands, except percentages) |
| Revenues | $ | 3,047,597 |
| | $ | 2,488,652 |
| | $ | 1,702,177 |
| | $ | 558,945 |
| | 22 | % | | $ | 786,475 |
| | 46 | % |
| Operating costs and expenses | 2,412,447 |
| | 2,365,409 |
| | 1,692,241 |
| | 47,038 |
| | 2 | % | | 673,168 |
| | 40 | % |
| Other items, net | 1,461,746 |
| | 140,241 |
| | (121,988 | ) | | 1,321,505 |
| | ** |
| | 262,229 |
| | ** |
|
| Net income (loss) attributable to Vertex | $ | 2,096,896 |
| | $ | 263,484 |
| | $ | (112,052 | ) | | $ | 1,833,412 |
| | ** |
| | $ | 375,536 |
| | ** |
|
| | | | | | | | | | | | | | |
| Net income (loss) per diluted share attributable to Vertex common shareholders | $ | 8.09 |
| | $ | 1.04 |
| | $ | (0.46 | ) | | | | ** Not meaningful |
| Diluted shares used in per share calculations | 259,185 |
| | 253,225 |
| | 244,685 |
| | | | | | | | |
Net Income (Loss) Attributable to Vertex
Comparison of Net Income Attributable to Vertex 2018 vs. 2017
Net income attributable to Vertex was $2.10 billion in 2018 as compared to $263.5 million in 2017. In the fourth quarter of 2018, we recorded a one-time non-cash benefit from income taxes of $1.56 billion when we released the valuation allowance on the majority of our net operating losses and other deferred tax assets. This one-time benefit is included in other items, net in the preceding table and substantially increased the net income attributable to Vertex in 2018.
Our total revenues increased in 2018 as compared to 2017 primarily due to an $872.8 million increase in CF net product revenues, partially offset by $230.0 million in one-time collaborative revenues recorded in 2017 related to an upfront payment from Merck KGaA, Darmstadt, Germany.
Our operating costs and expenses increased in 2018 as compared to 2017 due to increased costs of sales related to our increased product revenues and increased expenses related to our ongoing research and development efforts. Our operating expenses in each of 2018 and 2017 were affected by expenses related to business development activities. In 2018, we incurred $111.6 million in research expenses primarily related to upfront payments for collaborations and license agreements that we entered into during 2018. In 2017, we incurred a $255.3 million impairment charge related to Parion’s pulmonary ENaC platform and $160.0 million in development expenses incurred in connection with our acquisition of VX-561 from Concert Pharmaceuticals, Inc., or Concert, each of which is included in operating costs and expenses in the preceding table.
Comparison of Net Income (Loss) Attributable to Vertex 2017 vs. 2016
Net income attributable to Vertex was $263.5 million in 2017 as compared to a net loss attributable to Vertex of $(112.1) million in 2016. Our revenues increased in 2017 as compared to 2016 primarily due to increased ORKAMBI and KALYDECO net product revenues and $230.0 million in one-time collaborative revenues related to the strategic collaboration and license agreement we established with Merck KGaA, Darmstadt, Germany, in 2017. Our operating costs and expenses increased in 2017 as compared to 2016 primarily due to increases in our cost of sales related to our increased net product revenues, increases in our research and development expenses, which included $160.0 million in development expenses incurred in connection with the acquisition of VX-561 from Concert, increases in our sales and administrative expenses and a $255.3 million intangible asset impairment charge related to Parion’s pulmonary ENaC platform. Other items, net in 2017 primarily reflect a benefit from income taxes and certain other benefits associated with the impairment of Parion’s pulmonary ENaC platform, for which there were no comparable benefits in 2016, and a decrease in interest expense, net to $57.6 million. Other items, net in 2016 primary reflects interest expense, net of $81.4 million, a provision for income taxes of $16.7 million and net income attributable to noncontrolling interest of $28.0 million.
Earnings Per Share
In 2018, 2017 and 2016, net income (loss) attributable to Vertex was $8.09, $1.04, $(0.46), respectively, per diluted share. The increase in our diluted earnings per share in 2018 compared to 2017 was due to, among other things, the increased net product revenues and the benefit from income taxes as a result of the release of our valuation allowance on the majority
of our net operating losses and other deferred tax assets. The release of the valuation allowance increased net income attributable to Vertex by $6.03 per diluted share.
In 2018, 2017 and 2016, the number of diluted shares used to calculate net income (loss) per common share was 259.2 million, 253.2 million and 244.7 million, respectively. The increase in diluted shares in each year was primarily due to our issuance of shares of common stock pursuant to our employee equity programs. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2021 | | % Change | | 2020 | | % Change | | 2019 | | | | |
| (in millions, except percentages and per share amounts) | | | | |
| Revenues | $ | 7,574.4 | | | 22% | | $ | 6,205.7 | | | 49% | | $ | 4,162.8 | | | | | |
| Operating costs and expenses | 4,792.3 | | | 43% | | 3,349.4 | | | 13% | | 2,965.3 | | | | | |
| Income from operations | 2,782.1 | | | (3)% | | 2,856.3 | | | 139% | | 1,197.5 | | | | | |
| Other non-operating (expense) income, net | (51.7) | | | ** | | 260.6 | | | 32% | | 197.4 | | | | | |
| Provision for income taxes | 388.3 | | | (4)% | | 405.2 | | | 86% | | 218.1 | | | | | |
| Net income | $ | 2,342.1 | | | (14)% | | $ | 2,711.7 | | | 130% | | $ | 1,176.8 | | | | | |
| | | | | | | | | | | | | |
| Net income per diluted common share | $ | 9.01 | | | | | $ | 10.29 | | | | | $ | 4.51 | | | |
| Diluted shares used in per share calculations | 259.9 | | | | | 263.4 | | | | | 260.7 | | | | | |
| | | | | | | ** Not meaningful | | |
Revenues
| | | | | | | | | | | | | | | | | | 2021 | | % Change | | 2020 | | % Change | | 2019 |
| | | | | | | | 2018/2017
Comparison | | 2017/2016
Comparison | | (in millions, except percentages) |
| | | | | | | | Increase/(Decrease) | | Increase/(Decrease) | |
| | 2018 | | 2017 | | 2016 | | $ | | % | | $ | | % | |
| | (in thousands) | | (in thousands, except percentages) | |
| TRIKAFTA/KAFTRIO | | TRIKAFTA/KAFTRIO | $ | 5,697.2 | | | 47% | | $ | 3,863.8 | | | 820% | | $ | 420.1 | |
| SYMDEKO/SYMKEVI | | SYMDEKO/SYMKEVI | 420.4 | | | (33)% | | 628.6 | | | (56)% | | 1,417.7 | |
| ORKAMBI | | ORKAMBI | 771.6 | | | (15)% | | 907.5 | | | (32)% | | 1,331.9 | |
| KALYDECO | | KALYDECO | 684.2 | | | (15)% | | 802.9 | | | (19)% | | 991.0 | |
| Product revenues, net | $ | 3,038,325 |
| | $ | 2,165,480 |
| | $ | 1,683,632 |
| | $ | 872,845 |
| | 40 | % | | $ | 481,848 |
| | 29 | % | Product revenues, net | 7,573.4 | | | 22% | | 6,202.8 | | | 49% | | 4,160.7 | |
| Collaborative and royalty revenues | 9,272 |
| | 323,172 |
| | 18,545 |
| | (313,900 | ) | | ** |
| | 304,627 |
| | ** |
| |
| Other revenues | | Other revenues | 1.0 | | | ** | | 2.9 | | | ** | | 2.1 | |
| Total revenues | $ | 3,047,597 |
| | $ | 2,488,652 |
| | $ | 1,702,177 |
| | $ | 558,945 |
| | 22 | % | | $ | 786,475 |
| | 46 | % | Total revenues | $ | 7,574.4 | | | 22% | | $ | 6,205.7 | | | 49% | | $ | 4,162.8 | |
| | | | | | | | | | ** Not meaningful | | | | | | ** Not meaningful |
Product Revenues, Net
|
| | | | | | | | | | | |
| | 2018 | | 2017 | | 2016 |
| | (in thousands) |
| SYMDEKO/SYMKEVI | $ | 768,657 |
| | $ | — |
| | $ | — |
|
| ORKAMBI | 1,262,166 |
| | 1,320,850 |
| | 979,590 |
|
| KALYDECO | 1,007,502 |
| | 844,630 |
| | 703,432 |
|
| Total CF product revenues, net | $ | 3,038,325 |
| | $ | 2,165,480 |
| | $ | 1,683,022 |
|
In 2018,2021, our total CF net product revenues increased by $872.8 million$1.4 billion, or 22%, as compared to 2017. The increase2020 primarily due to the launch of KAFTRIO in totalmultiple countries internationally, which was approved in the E.U. in the third quarter of 2020, and the performance of TRIKAFTA in the U.S., including the launch of TRIKAFTA in June 2021 for children with CF 6 through 11 years of age. Decreases in revenues for our products other than TRIKAFTA/KAFTRIO were primarily the result of patients switching from these medicines to TRIKAFTA/KAFTRIO.
Our net product revenues wasfrom the U.S. and from ex-U.S. markets were as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2021 | | % Change | | 2020 | | % Change | | 2019 |
| (in millions, except percentages) |
| United States | $ | 5,287.3 | | | 10% | | $ | 4,826.4 | | | 58% | | $ | 3,060.3 | |
| ex-U.S. | 2,286.1 | | | 66% | | 1,376.4 | | | 25% | | 1,100.4 | |
| Product revenues, net | $ | 7,573.4 | | | 22% | | $ | 6,202.8 | | | 49% | | $ | 4,160.7 | |
We expect that our net product revenues will increase in 2022 due to the increasing numbernumbers of patientspeople being treated as awith our medicines. The increase is expected to result from continued performance of KAFTRIO outside the approval of SYMDEKOU.S. and TRIKAFTA in the U.S., label expansions for KALYDECO and ORKAMBIour previously approved products, and expanded access in ex-U.S. markets. We believe thatto our total CF net product revenues will increase in 2019 due primarily to increases in SYMDEKO/SYMKEVI net product revenues and further CF net product revenue growth will be dependent on when, and if, we obtain approval for our triple combination regimens. medicines.
Other Revenues
Our net product revenues are also dependent on, if, and when, we obtain additional reimbursement agreements for our CF medicines in ex-U.S. markets, particularly in the United Kingdom and France.
SYMDEKO/SYMKEVI
SYMDEKO/SYMKEVI net productother revenues were $768.7$1.0 million and $2.9 million in 2018. SYMDEKO was approved by the FDA in February 20182021 and SYMKEVI was approved in the European Union in November 2018. In 2018, SYMDEKO net product revenues increased each quarter as new patients initiated treatment. We did not recognize significant net product revenues from sales of SYMKEVI during 2018. We expect SYMDEKO/SYMKEVI net product revenues to continue to increase in 2019 as compared to 2018 due to the full year impact of SYMDEKO sales in the United States and as patients begin to obtain access to SYMKEVI in ex-U.S. markets.
ORKAMBI
The approval of SYMDEKO/SYMKEVI has had a negative effect on the net product revenues from ORKAMBI as a portion of the patients who were being treated with ORKAMBI switched to SYMDEKO/SYMKEVI. Due primarily to patients switching from ORKAMBI to SYMDEKO in the United States, ORKAMBI net product revenues decreased by 4.4% in 2018 as compared to 2017. In 2018, ORKAMBI net product revenues were $1.26 billion, including $310.5 million of net product revenues from ex-U.S. markets, compared to ORKAMBI net product revenues of $1.32 billion in 2017, including $167.6 million of net product revenues from ex-U.S. markets. In 2016, ORKAMBI net product revenues were $979.6 million, including $76.4 million of net product revenues from ex-U.S. markets.
Our consolidated balance sheet includes $354.4 million collected as of December 31, 2018 in France2020, respectively, related to ORKAMBI supplied under early access programs at the invoiced price. Pursuant to the revenue recognition guidancecollaborative milestones that
became effective under GAAP on January 1, 2018, we have recognized limited net product revenues to date on sales of ORKAMBI in France due to ongoing pricing discussions regarding the reimbursement rate for ORKAMBI. Please refer to “Critical Accounting Policies - Revenue Recognition” below for a discussion of our accounting treatment for our early access program for ORKAMBI in France.
KALYDECO
In 2018, KALYDECO net product revenues were $1.01 billion, including $363.5 million of net product revenues from ex-U.S. markets, compared to KALYDECO net product revenues of $844.6 million in 2017, including $334.2 million of net product revenues from ex-U.S. markets. In 2016, KALYDECO net product revenues were $703.4 million, including $303.9 million of net product revenues from ex-U.S. markets. The increases year-over-year were primarily due to additional patients being treated with KALYDECO as we completed reimbursement discussions in various ex-U.S. jurisdictions and as we increased the number of patients eligible to receive KALYDECO through label expansions.
Collaborative and Royalty Revenues
earned. Our collaborative and royalty revenues were $9.3 million, $323.2 million and $18.5 million in 2018, 2017 and 2016, respectively. In 2017, our collaborative and royalty revenues primarily included (i) $230.0 million in revenues related to the one-time upfront payment earned in 2017 from Merck KGaA, Darmstadt, Germany, and (ii) a $25.0 million milestone related to our license agreement with Janssen, Inc. for the treatment of influenza. Our 2017 collaborative and royalty revenues also included $40.0 million in revenues related to upfront and milestone payments earned by Parion in 2017 pursuant to a license agreement Parion entered into with a third party. We are not a party to the Parion license agreement and have no economic interest in either the license or these milestone payments. These revenues were included in our consolidated financial statements because we were consolidating Parion as a VIE during the first three quarters of 2017. Parion was deconsolidated as a VIE as of September 30, 2017 and any future payments received by Parion pursuant to this license agreement will no longer be recognized by us as collaborative revenue. In 2016 through 2018, our collaborative and royalty revenues also include a small amount of revenues related to a cash payment we received in 2008 when we sold our rights to certain HIV royalties and reimbursements for research and development activities and milestones related to our collaborative arrangements.
Our collaborativeother revenues have historically fluctuated significantly from one period to another based on our collaborative out-license activities, and may continue to fluctuate in the future. Our future royalty revenues will be dependent on if, and when, our collaborators, including Janssen, Inc. and Merck KGaA, Darmstadt, Germany are able to successfully develop drug candidates that we have out-licensed to them.
Operating Costs and Expenses
| | | | | | | | | | 2018/2017
Comparison | | 2017/2016
Comparison | |
| | | | | | | | Increase/(Decrease) | | Increase/(Decrease) | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2018 | | 2017 | | 2016 | | $ | | % | | $ | | % | | 2021 | | % Change | | 2020 | | % Change | | 2019 |
| | (in thousands) | | (in thousands, except percentages) | | (in millions, except percentages) |
| Cost of sales | $ | 409,539 |
| | $ | 275,119 |
| | $ | 210,460 |
| | $ | 134,420 |
| | 49 | % | | $ | 64,659 |
| | 31 | % | Cost of sales | $ | 904.2 | | | 23% | | $ | 736.3 | | | 34% | | $ | 547.8 | |
| Research and development expenses | 1,416,476 |
| | 1,324,625 |
| | 1,047,690 |
| | 91,851 |
| | 7 | % | | 276,935 |
| | 26 | % | Research and development expenses | 3,051.1 | | | 67% | | 1,829.5 | | | 4% | | 1,754.5 | |
| Sales, general and administrative expenses | 557,616 |
| | 496,079 |
| | 432,829 |
| | 61,537 |
| | 12 | % | | 63,250 |
| | 15 | % | |
| Restructuring (income) expenses | (184 | ) | | 14,246 |
| | 1,262 |
| | (14,430 | ) | | ** |
| | 12,984 |
| | ** |
| |
| Intangible asset impairment charges | 29,000 |
| | 255,340 |
| | — |
| | (226,340 | ) | | ** |
| | 255,340 |
| | ** |
| |
| Selling, general and administrative expenses | | Selling, general and administrative expenses | 840.1 | | | 9% | | 770.5 | | | 17% | | 658.5 | |
| Change in fair value of contingent consideration | | Change in fair value of contingent consideration | (3.1) | | | ** | | 13.1 | | | ** | | 4.5 | |
| Total costs and expenses | $ | 2,412,447 |
| | $ | 2,365,409 |
| | $ | 1,692,241 |
| | $ | 47,038 |
| | 2 | % | | $ | 673,168 |
| | 40 | % | Total costs and expenses | $ | 4,792.3 | | | 43% | | $ | 3,349.4 | | | 13% | | $ | 2,965.3 | |
| | | | | | | | | | ** Not meaningful | | | | | | ** Not meaningful |
Cost of Sales
Our cost of sales primarily consists of third-party royalties payable on net sales of our products as well as the cost of producing inventories that corresponded to product revenues for the reporting period, plus the third-party royalties payable on our net sales of our products.inventories. Pursuant to our agreement with the CFF,Cystic Fibrosis Foundation, our tiered third-party royalties on sales of TRIKAFTA/KAFTRIO, SYMDEKO/SYMKEVI, KALYDECO, and ORKAMBI, calculated as a percentage of net sales, range from the single digits to the sub-teens. As a resultsub-teens, with royalties on sales of the tiered royalty rate, which resets annually,TRIKAFTA/KAFTRIO slightly lower than for our cost of sales as a percentage of CF net product revenues are lower at the beginning of each calendar year.
other products. Over the last several years, our cost of sales has been increasing primarily due to increased net product revenues. Our costscost of sales as a percentage of CFour net product revenues increased from 12.7%was 12% in 2017 to 13.5% in 2018 due to the tiered royalties that we pay to the CFF.each of 2021 and 2020. In 2019,2022, we expect our total cost of sales will increase due to expected increases in our CF net product revenues and that our cost of sales as a percentage of total CFour net product revenues will be similar to our cost of sales as a percentage of total CF net product revenues in 2018.2021 and 2020.
Research and Development Expenses
| | | | | | | | | | 2018/2017
Comparison | | 2017/2016
Comparison | |
| | | | | | | | Increase/(Decrease) | | Increase/(Decrease) | | | | | | | | | | | | | | | | | | | | | | | | | |
| | 2018 | | 2017 | | 2016 | | $ | | % | | $ | | % | | 2021 | | % Change | | 2020 | | % Change | | 2019 |
| | (in thousands) | | (in thousands, except percentages) | | (in millions, except percentages) |
| Research expenses | $ | 438,360 |
| | $ | 311,206 |
| | $ | 314,602 |
| | $ | 127,154 |
| | 41 | % | | $ | (3,396 | ) | | (1 | )% | Research expenses | $ | 617.7 | | | (3)% | | $ | 636.7 | | | (13)% | | $ | 732.7 | |
| Development expenses | 978,116 |
| | 1,013,419 |
| | 733,088 |
| | (35,303 | ) | | (3 | )% | | 280,331 |
| | 38 | % | Development expenses | 2,433.4 | | | 104% | | 1,192.8 | | | 17% | | 1,021.8 | |
| Total research and development expenses | $ | 1,416,476 |
| | $ | 1,324,625 |
| | $ | 1,047,690 |
| | $ | 91,851 |
| | 7 | % | | $ | 276,935 |
| | 26 | % | Total research and development expenses | $ | 3,051.1 | | | 67% | | $ | 1,829.5 | | | 4% | | $ | 1,754.5 | |
Our research and development expenses include internal and external costs incurred for research and development of our drugsproducts and drugproduct candidates and expenses related to certain technologytechnologies that we acquire or license through business development transactions. We do not assign our internal costs, such as salary and benefits, stock-based compensation expense, laboratory supplies and other direct expenses and infrastructure costs, to individual drugsproducts or drugproduct candidates, because the employees within our research and development groups typically are deployed across multiple research and development programs. These internal costs are greater than ourWe assign external costs, such as the costs of services provided to us by clinical research organizations and other outsourced research which we allocate by individual program. Apart from upfront, milestone, and other payments related to our business development activities, our internal costs are significantly greater than our external costs. All research and development costs for our drugsproducts and drugproduct candidates are expensed as incurred.
Over the past three years, we have incurred $3.8$6.6 billion in research and development expenses associated with drugproduct discovery and development. The successful development of our drugproduct candidates is highly uncertain and subject to a number of risks. In addition, the duration of clinical trials may vary substantially according to the type, complexity and novelty of the drugproduct candidate and the disease indication being targeted. The FDA and comparable agencies in foreign countries impose substantial requirements on the introduction of therapeutic pharmaceutical products, typically requiring lengthy and detailed laboratory and clinical testing procedures, sampling activities and other costly and time-consuming procedures. Data obtained from nonclinical and clinical activities at any step in the testing process may be adverse and lead to discontinuation or redirection of development activities. Data obtained from these activities also are susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The duration and cost of discovery, nonclinical
studies and clinical trials may vary significantly over the life of a project and are difficult to predict. Therefore, accurate and meaningful estimates of the ultimate costs to bring our drugproduct candidates to market are not available.
In 2016, 2017 and 2018, costs related to our CF programs represented the largest portion of our development costs. Any estimates regarding development and regulatory timelines for our drugproduct candidates are highly subjective and subject to change. In the fourth quarter of 2018,Until we obtained positivehave data from two Phase 3 clinical trials, evaluating the triple combination of VX-659, tezacaftor and ivacaftor and we plan to submit an NDA to the FDA for a triple combination regimen with VX-659 or VX-445 no later than mid-2019. We cannot make a meaningful estimate regarding when, or if, ever, our othera clinical development programsprogram will generate revenues and cash flows.
Research Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2021 | | Change % | | 2020 | | Change % | | 2019 |
| (in millions, except percentages) |
| Research Expenses: | | | | | | | | | |
| Salary and benefits | $ | 136.7 | | | 5% | | $ | 129.8 | | | (4)% | | $ | 134.6 | |
| Stock-based compensation expense | 77.3 | | | (10)% | | 85.6 | | | 23% | | 69.4 | |
| Outsourced services and other direct expenses | 160.0 | | | 38% | | 116.2 | | | —% | | 116.6 | |
| Collaborative payments | 105.4 | | | (43)% | | 184.6 | | | (40)% | | 307.8 | |
| Infrastructure costs | 138.3 | | | 15% | | 120.5 | | | 16% | | 104.3 | |
| Total research expenses | $ | 617.7 | | | (3)% | | $ | 636.7 | | | (13)% | | $ | 732.7 | |
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | 2018/2017
Comparison | | 2017/2016
Comparison |
| | | | | | | | Increase/(Decrease) | | Increase/(Decrease) |
| | 2018 | | 2017 | | 2016 | | $ | | % | | $ | | % |
| | (in thousands) | | (in thousands, except percentages) |
| Research Expenses: | | | | | | | | | | | | | |
| Salary and benefits | $ | 87,773 |
| | $ | 81,229 |
| | $ | 80,845 |
| | $ | 6,544 |
| | 8 | % | | $ | 384 |
| | <1% |
|
| Stock-based compensation expense | 62,925 |
| | 60,122 |
| | 51,034 |
| | 2,803 |
| | 5 | % | | 9,088 |
| | 18 | % |
| Laboratory supplies and other direct expenses | 50,578 |
| | 45,822 |
| | 43,151 |
| | 4,756 |
| | 10 | % | | 2,671 |
| | 6 | % |
| Outsourced services | 38,777 |
| | 39,497 |
| | 33,682 |
| | (720 | ) | | (2 | )% | | 5,815 |
| | 17 | % |
| Collaboration and asset acquisition expenses | 111,600 |
| | 8,425 |
| | 33,000 |
| | 103,175 |
| | ** |
| | (24,575 | ) | | ** |
|
| Infrastructure costs | 86,707 |
| | 76,111 |
| | 72,890 |
| | 10,596 |
| | 14 | % | | 3,221 |
| | 4 | % |
| Total research expenses | $ | 438,360 |
| | $ | 311,206 |
| | $ | 314,602 |
| | $ | 127,154 |
| | 41 | % | | $ | (3,396 | ) | | (1 | )% |
| | | | | | | | | | ** Not meaningful |
We maintain a substantial investment in research activities. Our research expenses have been affected, and are expected to continue to be affected, by research expenses associated with our business development activities. In particular, in 2018 our research expenses increased primarily due to $111.6 million in research expenses associated with our business development activities, including upfront payments for certain collaboration agreements, which are reflected in collaboration and asset acquisition expenses in the table above. Collaboration and asset acquisition expenses in 2018 primarily related to agreements we entered into with Arbor Biotechnologies and Merck KGaA, Darmstadt, Germany. Collaboration and asset acquisition expenses in 2016 included expenses related to a collaboration we entered into with Moderna. We expect to continue to invest in our research programs with a focus on identifying drug candidates with the goal of creating transformative medicines for serious diseases. Our research expenses have historically fluctuated, and are expected to continue to fluctuate, from one period to another due to upfront, milestone and certain other payments related to our business development activities that are reflected in the preceding table as collaborative payments. Our research expenses, apart from these collaborative payments, have been increasing over the last several years as we have invested in our pipeline and expanded our cell and genetic therapy capabilities.
Development Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2021 | | Change % | | 2020 | | Change % | | 2019 |
| (in millions, except percentages) |
| Development Expenses: | | | | | | | | | |
| Salary and benefits | $ | 347.6 | | | 18% | | $ | 295.7 | | | 18% | | $ | 249.9 | |
| Stock-based compensation expense | 191.0 | | | 8% | | 177.1 | | | 14% | | 155.2 | |
| Outsourced services and other direct expenses | 629.4 | | | 23% | | 512.2 | | | 21% | | 425.0 | |
| Collaborative payments | 1,007.9 | | | ** | | — | | | ** | | 10.5 | |
| Infrastructure costs | 257.5 | | | 24% | | 207.8 | | | 15% | | 181.2 | |
| Total development expenses | $ | 2,433.4 | | | 104% | | $ | 1,192.8 | | | 17% | | $ | 1,021.8 | |
| | | | | ** Not meaningful |
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | 2018/2017
Comparison | | 2017/2016
Comparison |
| | | | | | | | Increase/(Decrease) | | Increase/(Decrease) |
| | 2018 | | 2017 | | 2016 | | $ | | % | | $ | | % |
| | (in thousands) | | (in thousands, except percentages) |
| Development Expenses: | | | | | | | | | | | | | |
| Salary and benefits | $ | 220,128 |
| | $ | 208,769 |
| | $ | 177,399 |
| | $ | 11,359 |
| | 5 | % | | $ | 31,370 |
| | 18 | % |
| Stock-based compensation expense | 140,187 |
| | 121,778 |
| | 102,417 |
| | 18,409 |
| | 15 | % | | 19,361 |
| | 19 | % |
| Laboratory supplies and other direct expenses | 84,900 |
| | 45,594 |
| | 42,861 |
| | 39,306 |
| | 86 | % | | 2,733 |
| | 6 | % |
| Outsourced services | 344,339 |
| | 337,901 |
| | 282,137 |
| | 6,438 |
| | 2 | % | | 55,764 |
| | 20 | % |
| Collaboration and asset acquisition expenses | 250 |
| | 160,250 |
| | — |
| | (160,000 | ) | | ** |
| | 160,250 |
| | ** |
|
| Drug supply costs | 42,099 |
| | 13,660 |
| | 12,510 |
| | 28,439 |
| | 208 | % | | 1,150 |
| | 9 | % |
| Infrastructure costs | 146,213 |
| | 125,467 |
| | 115,764 |
| | 20,746 |
| | 17 | % | | 9,703 |
| | 8 | % |
| Total development expenses | $ | 978,116 |
| | $ | 1,013,419 |
| | $ | 733,088 |
| | $ | (35,303 | ) | | (3 | )% | | $ | 280,331 |
| | 38 | % |
| | | | | | | | | | ** Not meaningful |
In 2021 and 2020, costs related to our CF programs represented the largest portion of our development costs, apart from the $900.0 million upfront payment to CRISPR in 2021, which is included in the preceding table under collaborative payments. Our development expenses decreasedincreased by $35.3 million,$1.2 billion, or 3%104%, in 20182021 as compared to 2017 and increased by $280.3 million, or 38%, in 2017 as compared to 2016. The decrease in 2018 as compared to 2017 was2020, primarily due to the $160.0 million payment to Concert in connection with the acquisition of VX-561 in 2017 for which there were no comparableCRISPR and increased expenses in 2018, partially offset by increased costs associated with ongoingrelated to our diversifying pipeline, including clinical trials, including Phase 3 clinical trials evaluatingheadcount, and infrastructure costs. We expect our next-generation CFTR corrector compoundsdevelopment expenses, apart from payments related to our business development activities, to continue to increase in 2022 as parta result of triple combination treatment regimens.
our diversifying pipeline.
The increase in 2017 as compared to 2016 was primary due to the $160.0 million payment in 2017 to Concert and to increased outsourced services related to clinical trials.
Sales,Selling, General and Administrative Expenses
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
| | | | | | | | | |
| 2021 | | % Change | | 2020 | | % Change | | 2019 |
| (in millions, except percentages) |
| Selling, general and administrative expenses | $ | 840.1 | | | 9% | | $ | 770.5 | | | 17% | | $ | 658.5 | |
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | 2018/2017
Comparison | | 2017/2016
Comparison |
| | | | | | | | Increase/(Decrease) | | Increase/(Decrease) |
| | 2018 | | 2017 | | 2016 | | $ | | % | | $ | | % |
| | (in thousands) | | (in thousands, except percentages) |
| Sales, general and administrative expenses | $ | 557,616 |
| | $ | 496,079 |
| | $ | 432,829 |
| | $ | 61,537 |
| | 12 | % | | $ | 63,250 |
| | 15 | % |
Sales,Selling, general and administrative expenses increased by 12%9% in 20182021 as compared to 2017, and by 15% in 2017 as compared to 2016. These increases were2020, primarily due to the continued investment to support the commercialization of our medicines and increased global support for our pipeline products. We expect our sales,selling, general and administrative expenses to continue to increase in 2019.2022.
Restructuring ExpensesContingent Consideration
In 2018, 2017 and 2016, we recorded restructuring (income) expensesThe change in the fair value of $(0.2)our contingent consideration potentially payable to Exonics’ former equity holders was a $3.1 million $14.2 million and $1.3 million, respectively. Our restructuring expensesdecrease in 2017 were primarily related to our decision to consolidate our research activities into our Boston, Milton Park and San Diego locations and to close our research site in Canada.
Intangible Asset Impairment Charge
In 2018, we recorded a $29.0 million impairment charge attributable to non-controlling interest related to VX-210 that was licensed from BioAxone in 2014. In 2017, we recorded a $255.3 million impairment charge related to Parion’s pulmonary ENaC platform that we licensed from Parion in 20152021 and a benefit from income taxes$13.1 million increase in 2020. In future periods, we expect the fair value of $97.7 million relatedcontingent consideration to this impairment charge attributable to non-controlling interest. There were no corresponding intangible asset impairment chargesincrease or decrease based on, among other things, our estimates of the probability of achieving and the timing of these contingent development and regulatory milestone payments, as well as the time value of money and changes in 2016.market interest rates.
Other Items,Non-Operating Income (Expense), Net
Interest Expense, NetIncome
Our interest expense, net relates primarily to interest expenses associated with certain of our real estate leases and outstanding debt, if any, partially offset by interestInterest income from the investment of our cash equivalents and marketable securities. In 2018, 2017 and 2016, interest expense, net was $34.1$4.9 million $57.6 million and $81.4 million, respectively. The decrease in interest expense, net in 2018 as compared to 20172021, which was primarily due to an increase inlower than our interest income resulting fromof $22.2 million in 2020, due to a decrease in prevailing market interest rates, despite an increase in our cash equivalents and marketableavailable-for-sale debt securities. The decrease inOur future interest expense, net in 2017 as compared to 2016 was primarily due to the repayment of the $300.0 million outstanding under our revolving credit facility in February 2017. In 2019, we expect that weincome will incur approximately $52 million in interest expenses related to our real estate leases, including a decrease in 2019 as compared to 2018 of approximately $13 million based on updated accounting guidance related to aspects of lease accounting that became effective January 1, 2019. In addition to the updated accounting guidance, our future net interest expense will also be dependent on whether, and to what extent, we reborrow amounts under our credit facility and the amount of, and prevailing market interest rates on, our outstanding cash equivalents and marketableavailable-for-sale debt securities.
Interest Expense
Interest expense was $61.5 million in 2021 as compared to $58.2 million in 2020. The majority of our interest expense in these periods was related to imputed interest expense associated with our leased corporate headquarters in Boston.
Other Income (Expense), Net
In 2018, we recorded net other expense of $0.8 million. In 2017, we recorded net other expense of $81.4 million primarily related to the deconsolidation of Parion. In 2016,2021 and 2020, we recorded net other income of $4.1$4.9 million and $296.6 million, respectively, primarily related to foreign exchange gains.
In 2018,net gains of $17.1 million and $311.9 million in 2021 and 2020, respectively, resulting from changes in the fair value and sales of certain of our other income (expense), net fluctuated significantly on a quarterly basis based onstrategic investments. As of December 31, 2021, the fair value of our investments in publicly traded companies was $230.9 million. To the extent that we continue to hold strategic investments. While the annual effect on ourinvestments, particularly strategic investments in publicly traded companies, we will record other income (expense), net from our related to these strategic investments in CRISPR and Moderna wason a gain of $2.6 million, the value of these investments increased by $149.4 million in the first half of 2018 and decreased by $146.8 million in the second half of 2018.quarterly basis. We expect that due to the volatility of the stock price of
biotechnology companies, our other income (expense), net will fluctuate in future periods based on increases or decreases in the fair value of our strategic investments including CRISPR and Moderna.investments.
Income Taxes
In 2018, we recorded a benefit from income taxes of $1.49 billion. In 2017 and 2018, we were profitable from a U.S. federal income tax perspective and have used a portion of our net operating losses to offset this income since becoming profitable. Until the fourth quarter of 2018, we maintained a valuation allowance on the majority of our net operating losses and other deferred tax assets. Due to this valuation allowance, we did not record a significantOur provision for income taxes in 2016, 2017was $388.3 million for 2021 and $405.2 million for 2020. Our effective tax rate of 14% for 2021 was lower than the nine months ended September 30, 2018. In the fourth quarter of 2018, we released the valuation allowance resulting in a non-cash credit to net income of $1.56 billion. Further information on the release of the valuation allowance and significant judgments related to its release can be found below in “Critical Accounting Policies - Income Taxes.”
In 2019, we expect to continue to utilize our net operating losses to offset income, but would begin recording a significant provision for income taxes reflecting the utilization of the deferred tax assets. The majority of this provision for income taxes will be a non-cash expense until our net operating losses are fully utilized.
In 2017, we recorded a benefit from income taxes of $107.3 million,U.S. statutory rate primarily due to discrete tax benefits of (i) $94.8 million associated with an increase in the U.K.’s corporate tax rate from 19% to 25%, which was enacted in June 2021 and will become effective in April 2023, and (ii) $44.1 million resulting from an R&D tax credit study that we completed in 2021.
Our effective tax rate of 13% for 2020 was lower than the U.S. statutory rate primarily due to (i) a totaldiscrete tax benefit of $209.0 million associated with the transfer of intellectual property rights to the U.K., (ii) a discrete tax benefit associated with the write-off of a long-term intercompany receivable, (iii) a discrete tax benefit associated with an increase in the U.K.’s corporate tax rate from income taxes of $114.1 million attributable17% to noncontrolling interest19%, which was enacted and became effective in July 2020, and (iv) excess tax benefits related to the impairmentstock-based compensation. The impact of Parion’s pulmonary ENaC platformthese items was partially offset by U.S. income tax on foreign earnings.
Net Income
In summary, our net income decreased to $2.3 billion in 2021 as compared to $2.7 billion in 2020 primarily due to (i) our $900.0 million upfront payment to CRISPR in 2021 and decrease(ii) less other income derived from changes in the fair value of the contingent payments payable by usour strategic investments in 2021 as compared to Parion. In 2016, we recorded a provision for income taxes of $16.7 million, principally due to income taxes payable by our VIEs.
Noncontrolling Interest (VIEs)
The net loss (income) attributable to noncontrolling interest (VIEs) recorded on our consolidated statements of operations reflects Parion (through September 30, 2017) and BioAxone’s net (income) loss for the reporting period, adjusted for any changes in the noncontrolling interest holders’ claim to net assets, including contingent milestone, royalty and option payments. A summary of net (income) loss attributable to noncontrolling interest related to our VIEs for the three years ended December 31, 2018 is as follows:
|
| | | | | | | | | | | |
| | 2018 | | 2017 | | 2016 |
| | (in thousands) |
| Loss attributable to noncontrolling interest before (benefit from) provision for income taxes and changes in fair value of contingent payments | $ | 31,191 |
| | $ | 223,379 |
| | $ | 10,086 |
|
| (Benefit from) provision for income taxes | (3,668 | ) | | (114,090 | ) | | 16,743 |
|
| (Increase) decrease in fair value of contingent payments | (17,730 | ) | | 62,560 |
| | (54,850 | ) |
| Net loss (income) attributable to noncontrolling interest | $ | 9,793 |
| | $ | 171,849 |
| | $ | (28,021 | ) |
In 2018, the net loss attributable to noncontrolling interest was primarily related to the $29.0 million impairment charge related to VX-2102020, (iii) partially offset by an increaseincreased operating income in 2021, apart from the fair value of the contingent payments payable by uspayment to BioAxone of $17.7 million primarily due to the expiration ofCRISPR, resulting from our option to purchase BioAxone in 2018. In 2017, the net loss attributable to noncontrolling interest was primarily related to the $255.3 million impairment charge related to Parion’s pulmonary ENaC platform, a decrease in fair value of the contingent payments payable by us to Parion of $69.6 million upon deconsolidation and benefit from income taxes of $126.2 million related to these charges. In 2016, the net income attributable to noncontrolling interest was primarily related to an increase in the fair value of contingent payments based on a Phase 2 clinical trial of VX-371 achieving its primary safety endpoint.product revenues.
LIQUIDITY AND CAPITAL RESOURCES
The following table summarizes the components of our financial condition as of December 31, 20182021 and 2017:2020:
| | | | | | | | | | | | | | | | | |
| 2021 | | 2020 | | % Change |
| (in millions) | | |
| Cash, cash equivalents and marketable securities | $ | 7,524.9 | | | $ | 6,658.9 | | | 13% |
| Working Capital: | | | | | |
| Total current assets | $ | 9,560.6 | | | $ | 8,133.4 | | | 18% |
| Total current liabilities | (2,142.0) | | | (1,877.5) | | | 14% |
| Total working capital | $ | 7,418.6 | | | $ | 6,255.9 | | | 19% |
|
| | | | | | | | | | | | | | |
| | | | | | Increase/(Decrease) |
| | 2018 | | 2017 | | $ | | % |
| | (in thousands, except percentages) |
| Cash, cash equivalents and marketable securities | $ | 3,168,242 |
| | $ | 2,088,666 |
| | $ | 1,079,576 |
| | 52 | % |
| Working Capital | | | | | | | |
| Total current assets | $ | 3,843,109 |
| | $ | 2,648,963 |
| | $ | 1,194,146 |
| | 45 | % |
| Total current liabilities | (1,120,292 | ) | | (807,260 | ) | | (313,032 | ) | | 39 | % |
| Total working capital | $ | 2,722,817 |
| | $ | 1,841,703 |
| | $ | 881,114 |
| | 48 | % |
As of December 31, 2018,2021, total working capital was $7.4 billion, which represented an increase of $1.2 billion from $6.3 billion as of December 31, 2020. The increase in total working capital in 2021 was primarily related to $2.6 billion of cash provided by operations, which was net of our $900.0 million payment to CRISPR, partially offset by $1.4 billion of cash used to repurchase our common stock pursuant to our share repurchase programs and purchases of property and equipment of $235.0 million.
Sources and Uses of Liquidity
As of December 31, 2021, we had cash, cash equivalents, and marketable securities of $3.2$7.5 billion, which represented an increase of $1.1 billion$866.0 million from $2.1$6.7 billion as of December 31, 2017. In 2018, our cash, cash equivalents and marketable securities balance increased primarily due to cash receipts from product sales and $289.3 million of cash received from issuances of common stock under our employee benefit plans partially offset by cash expenditures to fund our operations and $350.0 million of cash used to repurchase shares of our common stock. We expect that our future cash flows will be substantially dependent on our CF product sales.
As of December 31, 2018, total working capital was $2.7 billion, which represented an increase of $881.1 million from $1.8 billion as of December 31, 2017. The most significant items that increased total working capital in 2018 were $1.3 billion of cash provided by operations and $289.3 million of cash received from issuances of common stock under our employee benefit plans partially offset by $350.0 million of cash used to repurchase shares of our common stock and expenditures for property and equipment of $95.5 million as well as other expenditures.
Sources of Liquidity
2020. We intend to rely on our existing cash, cash equivalents and marketable securities together with cash flows from product sales as our primary source of liquidity. We are receiving cash flows from sales of KALYDECO and ORKAMBI in the United States and ex-U.S. markets and from SYMDEKO in the United States. We will begin receiving cash flows from sales of SYMKEVI in the European Union in 2019. Future net product revenues from ex-U.S. markets will be dependent on, among other things, the timing of and our ability to complete reimbursement discussions in European countries.
We may borrow up to $500.0 million pursuant to a revolving credit facility that we entered into in 2016. We may repay and reborrow amounts under the revolving credit agreement without penalty. Subject to certain conditions, we may request that the borrowing capacity under this credit agreement be increased by an additional $300.0 million.
In 2018 and 2017, we received significant proceeds from the issuance of common stock under our employee benefit plans and more limited proceeds from employee benefit plans in 2016. The amount and timing of future proceeds from employee benefits plans is uncertain. In 2018, the value of our strategic investment in CRISPR fluctuated on a quarterly basis. The future value of our strategic investments, including our investments in CRISPR and Moderna, is uncertain. Other possible sources of future liquidity include strategic collaborative agreements that include research and/or development funding, commercial debt, public and private offerings of our equity and debt securities, development milestones and royalties on sales of products, software and equipment leases, strategic sales of assets or businesses and financial transactions. Negative covenants in our credit agreement may prohibit or limit our ability to access these sources of liquidity.
Future Capital Requirements
We have significant future capital requirements including:
incurring substantial operating expenses to conduct research and development activities and to operate our organization; and
having substantial facility and capital lease obligations, including leases for two buildings in Boston, Massachusetts that continue through 2028 and a lease in San Diego, California that continues through 2034.
In addition,
As of December 31, 2018, we have accrued approximately $354.4 million from ORKAMBI early access programs in France. We expect we will be required to repay a portion of the collected amounts to the French government
based on the difference between the invoiced price of ORKAMBI and the final price for ORKAMBI in France once we conclude our ongoing pricing discussions with the French government.
We have entered into certain collaboration agreements with third parties that include the funding of certain research, development and commercialization efforts with the potential for future milestone and royalty payments by us upon the achievement of pre-established developmental and regulatory targets and/or commercial targets, and we may enter into additional business development transactions, including acquisitions, collaborations and equity investments, that require additional capital. For example, in 2018 and 2017, we made $100.4 million and $168.7 million of upfront and milestone payments related to collaborations and asset acquisitions.
To the extent we borrow amounts under the credit agreement we entered into in October 2016, we would be required to repay any outstanding principal amounts in 2021.
In January 2018, we announced a share repurchase program to repurchase up to $500.0 million of shares of our common stock through December 31, 2019. As of December 31, 2018, $150.0 million remained available to fund repurchases under the share repurchase program.
We expect that cash flows from our CF products together with our current cash, cash equivalents and marketable securities will be sufficient to fund our operations for at least the next twelve months. The adequacy of our available funds to meet our future operating and capital requirements will depend on many factors, including the amounts of future revenues generated by CFour products, and the potential introduction of one or more of our other drugproduct candidates to the market, including a triple combination regimen for patients with CF, the level of our business development activities and the number, breadth, cost and prospects of our research and development programs.
Credit Facilities & Financing Strategy
We may borrow up to a total of $2.5 billion pursuant to two revolving credit facilities. We may repay and reborrow amounts under these revolving credit agreements without penalty. Subject to certain conditions, we may request that the borrowing capacity for each of the credit agreements be increased by an additional $500.0 million, for a total of $3.5 billion collectively. Negative covenants in our credit agreement may prohibit or limit our ability to access these sources of liquidity. As of December 31, 2021, we were in compliance with these covenants.
We may also raise additional capital by borrowing under credit agreements, through public offerings or private placements of our securities or securing new collaborative agreements or other methods of financing. We will continue to manage our capital structure and will consider all financing opportunities, whenever they may occur, that could strengthen our long-term liquidity profile. There can be no assurance that any such financing opportunities will be available on acceptable terms, if at all.
CONTRACTUAL COMMITMENTS AND OBLIGATIONS71
The following table sets forth our commitments and obligations as of December 31, 2018:
|
| | | | | | | | | | | | | | | | | | | |
| | Payments Due by Period |
| | 2019 | | 2020-2021 | | 2022-2023 | | 2024 and later | | Total |
| | (in thousands) |
| Fan Pier Leases | $ | 66,540 |
| | $ | 145,178 |
| | $ | 145,178 |
| | $ | 389,855 |
| | $ | 746,751 |
|
| Facility leases, excluding Fan Pier Leases | 18,531 |
| | 45,053 |
| | 42,654 |
| | 185,336 |
| | 291,574 |
|
| Capital lease obligations | 10,770 |
| | 12,931 |
| | 5,274 |
| | 3,085 |
| | 32,060 |
|
| Research, development and drug supply costs | 20,579 |
| | 3,316 |
| | 314 |
| | — |
| | 24,209 |
|
| Other | 5,563 |
| | 2,995 |
| | 106 |
| | 5,619 |
| | 14,283 |
|
| Total contractual commitments and obligations | $ | 121,983 |
| | $ | 209,473 |
| | $ | 193,526 |
| | $ | 583,895 |
| | $ | 1,108,877 |
|
Leases | | | | | | | | | | | | | | | | | | | | | |
| 2021 | | | | 2020 | | | | 2019 |
| (in millions) |
| Net cash provided by (used in): | | | | | | | | | |
| Operating activities | $ | 2,643.5 | | | | | $ | 3,253.5 | | | | | $ | 1,569.3 | |
| Investing activities | $ | (340.9) | | | | | $ | 99.4 | | | | | $ | (1,235.3) | |
| Financing activities | $ | (1,478.0) | | | | | $ | (505.3) | | | | | $ | 126.8 | |
We lease two buildings that are located at Fan PierOperating Activities
Cash provided by operating activities was $2.6 billion in Boston, Massachusetts. We commenced lease payments on these two buildings2021 as compared to $3.3 billion in December 20132020 primarily due to a $369.6 million decrease in our net income resulting from the $900.0 million upfront payment we made to CRISPR in 2021. Cash provided by operating activities was $3.3 billion in 2020 as compared to $1.6 billion in 2019 primarily due to a $1.5 billion increase in our net income resulting from increased net product revenues.
Investing Activities
Cash used in investing activities was $340.9 million in 2021, primarily related to purchases of property and the initial lease periods endequipment, and, to a lesser extent, purchases of notes receivable and strategic investments. In 2020, our investing activities primarily related to $437.6 million of proceeds from sales of our strategic investments, partially offset by purchases of property and equipment. In 2019, we spent $1.2 billion to acquire Semma and Exonics.
Financing Activities
Cash used in December 2028. We also lease officefinancing activities was $1.5 billion in 2021 and laboratory space$505.3 million in San Diego, California2020 as compared to cash provided by financing activities of $126.8 million in 2019. In 2021 and will commence base rent payments for this building in the second quarter of 20192020, aggregate share repurchases pursuant to a 16 year lease. The future minimum rental payments that we are obligated to payour share repurchase programs were $1.4 billion and $539.1 million, respectively, which represented the largest portion of our financing activities. In 2019, our financing activities provided $126.8 million of cash related to the San Diego building are included in “Facility leases, excluding Fan Pier Leases.” The table also reflects leasesissuance of equipment that are accountedcommon stock pursuant to our employee benefit plans, partially offset by repurchases of our common stock pursuant to our share repurchase programs.
Future Capital Requirements
We have significant future capital requirements including:
•significant expected operating expenses to conduct research and development activities and to operate our organization;
•substantial facility and finance lease obligations as described below;
•royalties we pay to the Cystic Fibrosis Foundation on sales of our CF products; and
•cash paid for as capital leases.income taxes.
Research, Development and Drug Supply Costs
The amounts reflected in “Research, development and drug supply costs,” do not include certain payments we anticipate making to clinical research organizations, or CROs, because these contracts are cancelable, at our option, with notice. However, we historically have not cancelled such contracts. As of December 31, 2018, we had accrued $53.1 million related
to these contracts for costs incurred for services provided through December 31, 2018, andIn addition, we have approximately $177.0 million in cancelablesignificant potential future commitments based on existing contracts as of December 31, 2018. These amounts reflect planned expenditures based on existing contracts and do not reflect any future modifications to, or terminations of, existing contracts or anticipated or potential new contracts.capital requirements including:
Collaborative Arrangements and Asset Acquisitions
•We have entered into certain research and development collaboration agreements with third parties and acquired certain assets that include the funding of certain research, development, manufacturing and commercialization efforts withefforts. Certain of our business development transactions, including collaborations and acquisitions, include the potential for future milestone and royalty payments by us upon the achievement of pre-established developmental and regulatory targets and/or commercial targets. Our obligation to fund these research and development and commercialization efforts and to pay these potential milestone and royalties is contingent upon continued involvement in the programs and/or the lack of any adverse events that could cause the discontinuance of the programs. These payments include:
CFF: CFF has the right to tiered royalties ranging from single digits to sub-teens on any approved drugs first synthesized and/or tested during a research term on or before February 28, 2014, including KALYDECO, ORKAMBI, SYMDEKO/SYMKEVI, lumacaftor, ivacaftor and tezacaftor and royalties ranging from low single digits to mid-single digits on potential sales of certain compounds first synthesized and/or tested between March 1, 2014 and August 31, 2016, including VX-659 and VX-445. For combination products, such as ORKAMBI and SYMDEKO/SYMKEVI, sales are allocated equally to each of the active pharmaceutical ingredients in the combination product.
Research and Development Milestones: Ourprograms associated with our collaborations and certain otheracquisitions. We may enter into additional business development arrangements,transactions, including our asset acquisition from Concert, have milestoneacquisitions, collaborations, and royalty payments payable by us upon the successful achievement of pre-established developmental, regulatory and/or commercial targets or net sales.equity investments, that require additional capital.
Contingent payments•To the extent we borrow amounts under theseour existing credit agreements, become due and payable only upon achievement of certain milestones and are not includedwe would be required to repay any outstanding principal amounts in the contractual obligations table above.2022 or 2024.
Tax-related Obligations
We exclude liabilities pertaining to uncertain tax positions from our summary of contractual obligations as we cannot make a reliable estimate of the period of cash settlement with the respective taxing authorities. •As of December 31, 2018,2021, we had $0.5 billion available under our liabilities associated with uncertain tax positions were $19.5 million.2021 Share Repurchase Program.
Other Funding CommitmentsAdditional information on several of our future capital requirements is provided below.
Research and Development Costs
At any point in time, we have several ongoing clinical trials at various stages of clinical development. Our clinical trial costs are dependent on, among other things, our research activities advancing to later-stage clinical development as well as the size, number, and length of our clinical trials.
Leases
Finance Leases
Our table detailing contractual commitmentscorporate headquarters is in two buildings that we lease at Fan Pier in Boston, Massachusetts. We commenced lease payments on these buildings in 2013 and obligations does not include severance payment obligationsthe initial lease periods end in December 2028. We also lease office and laboratory space in San Diego, California. We commenced lease payments for this building in 2019 pursuant to certainan initial 16 year lease term. We account for each of these buildings as finance leases.
Operating Leases
The remainder of our executive officersreal estate leases are accounted for as operating leases, including office and laboratory space at our Innovation Square facility near our corporate headquarters. Base rent payments commenced in the event of a not-for-cause employment termination under existing employment contracts. We will provide information regarding these obligations annually in our proxy statement2021 pursuant to an initial 15 year lease term for this building.
Our total future minimum lease payments for our annual meetingfinance and operating leases for each of shareholders.the next five years and in total are included in Note L, “Leases.” The total future undiscounted minimum lease payments were $796.2 million and $482.2 million related to our finance and operating leases, respectively, as of December 31, 2021.
In addition, as discussed below, we began distributing ORKAMBI through early access programs in France in 2015 at the invoiced price and are engaged in ongoing pricing discussions regarding the final price for ORKAMBI in France. We expect the difference between the amounts collected at the invoiced price and the final price for ORKAMBI in France will be returned to the French government and that this amount could be material.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements prepared in accordance with generally accepted accounting principles in the United States.U.S. The preparation of these financial statements requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reported periods. These items are monitored and analyzed by management for changes in facts and circumstances, and material changes in these estimates could occur in the future. Changes in estimates are reflected in reported results for the period in which the change occurs. We base our estimates on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from our estimates if past experience or other assumptions do not turn out to be substantially accurate.
We believe that our application of the following accounting policies, each of which requires significant judgments and estimates on the part of management, are the most critical to aid in fully understanding and evaluating our reported financial results:
•revenue recognition;
income taxes;
research and development accruals;
leases; and
collaborations;•acquisitions, including intangible assets, goodwill and variable interest entities.contingent consideration; and
•income taxes.
Our accounting policies, including the ones discussed below, are more fully described in the Notes to our consolidated financial statements, including Note A, “Nature of Business and Accounting Policies,” included in this Annual Report on Form 10-K.
Revenue Recognition
Product Revenues, Net
We generate product revenues from sales in the United StatesU.S. and in international markets. We sell our products principally to a limited number of specialty pharmacy and specialty distributors in the United States,U.S., which account for the largest portion of our total revenues, andrevenues. We make international sales primarily to specialty distributors and retail chains, as well as hospitals and clinics, many of which are government-owned or supported customers, collectively, our customers. Our customers in the United StatesU.S. subsequently resell our products to patients and health care providers. We contract with government agencies so that our products will be eligible for purchase by, or partial or full reimbursement from, such third-party payors. We recognize net product revenues from sales of our products when our customers obtain control of our products, which typically occurs upon delivery to our customers. Revenues from our product sales are recorded at the net sales price, or “transaction price,” which requires us to make several significant estimates regarding the net sales price.
The most significant estimate we are required to make is related to government and private payor rebates, chargebacks, discounts and fees, collectively rebates. The value of the rebates provided to third-party payors per course of treatment vary significantly and are based on government-mandated discounts and our arrangements with other third-party payors. In order to estimate our total rebates, we estimate the percentage of prescriptions that will be covered by each third-party payor, which is referred to as the payor mix. We track available information regarding changes, if any, to the payor mix for our products, to our contractual terms with third-party payors and to applicable governmental programs and regulations and levels of our products in the distribution channel. We adjust our estimated rebates based on new information, including information regarding actual rebates for our products, as it becomes available. Claims by third-party payors for rebates are submitted to us significantly after the related sales, potentially resulting in adjustments in the period in which the new information becomes known. Our credits to revenue related to prior period sales, apart from an adjustment to the transaction price for ORKAMBI distributed through early access programs in France in 2019, have not been significant (typically less than 1% of gross product revenues) and primarily related to U.S. rebates.
The following table summarizes activity related to our accruals for rebates (including a refund liability to the French government related to ORKAMBI distributed through early access programs in France, which was paid in 2020) for the three years ended December 31, 2018:2021, 2020 and 2019:
|
| | | |
| | (in thousands) |
| Balance as of December 31, 2015 | $ | 44,669 |
|
| Provision related to current period sales | 134,198 |
|
| Adjustments related to prior period sales | 154 |
|
| Credits/payments made | (97,094 | ) |
| Balance as of December 31, 2016 | $ | 81,927 |
|
| | |
| Provision related to current period sales | 176,996 |
|
| Adjustments related to prior period sales | (8,943 | ) |
| Credits/payments made | (137,765 | ) |
| Balance as of December 31, 2017 | $ | 112,215 |
|
| | |
| Provision related to current period sales | 330,883 |
|
| Adjustments related to prior period sales | (22,099 | ) |
| Credits/payments made | (229,361 | ) |
| Balance as of December 31, 2018 | $ | 191,638 |
|
| | | | | |
| (in millions) |
| Balance as of December 31, 2018 | $ | 545.1 | |
| Provision related to 2019 sales | 656.0 | |
| Adjustments related to prior year(s) sales | (95.5) | |
| Credits/payments made | (469.9) | |
| Balance as of December 31, 2019 | $ | 635.7 | |
| Provision related to 2020 sales | 1,284.1 | |
| Adjustments related to prior year(s) sales | 0.6 | |
| Credits/payments made | (1,144.8) | |
| Balance as of December 31, 2020 | $ | 775.6 | |
| Provision related to 2021 sales | 2,126.1 | |
| Adjustments related to prior year(s) sales | (27.6) | |
| Credits/payments made | (2,035.5) | |
| Balance as of December 31, 2021 | $ | 838.6 | |
We have also entered into annual contracts with government-owned and supported customers in international markets that limit the amount of annual reimbursement we can receive. Upon exceeding the annual reimbursement amount, products are provided free of charge.charge, which is a material right. We defer a portion of the consideration received, which includeincludes upfront paymentpayments and fees, for shipments made up to the annual reimbursement limit and theas “Other current liabilities.” The deferred amount is recognized as revenue when the free products are shipped. In order to estimate the portion of the consideration received to recognize as revenue and the portion of the amount to defer, we rely on our forecast of the number of units we will distribute during the applicable annual period in each international market in which our contracts with
government-owned and supported customers limit the amount of annual reimbursement we can receive. Our forecasts are based on, among other things, our historical experience.
The preceding estimates and judgments materially affect our recognition of net product revenues. Changes in our estimates of net product revenues could have a material affecteffect on net product revenues recorded in the period in which we determine that change occurs.
French Early Access Programs
WeIn 2015, we began distributing ORKAMBI through early access programs in France in 2015 and areremained engaged in ongoing pricingreimbursement discussions regardingwith the final priceFrench government for ORKAMBI, in France. Our consolidated balance sheets included $354.4 million and $232.4 million collected as of December 31, 2018 and 2017, respectively, in France related toincluding ORKAMBI that are classified as “Early access sales accrual” related to amounts collected in France as payment for shipments of ORKAMBI under thedistributed through early access programs, atuntil November 2019, when we reached an agreement with the invoiced price. We expectFrench government. From the time we began distributing ORKAMBI through early access programs in France, we expected that the difference between the amounts collected atbased on the invoiced priceamount and the final priceamount for ORKAMBI in France willdistributed through these programs would be returned to the French government. Our refund liability related to the early access programs in France was classified in “Accrued expenses” on our consolidated balance sheets.
We did not recognize ORKAMBIFrom the first quarter of 2018 through the third quarter of 2019, we recognized net product revenues fromfor ORKAMBI sales in France under the accounting guidanceearly access programs based on a transaction price that was applicable until December 31, 2017. Pursuant to ASC 606, which we adopted on January 1, 2018, we recorded an $8.3 million cumulative effect adjustment to “Accumulated deficit” primarily related to ORKAMBI net product revenues from sales in France and we began recognizing ORKAMBI net product revenues based onreflected our estimate of consideration we expectexpected to retain that will not be subject to a significant reversal, which results in recognized revenue that represents a small percentage of the invoiced price. If our estimates regarding the amounts we will receive pursuant to these programs change, we will reflect the effect of the change in estimate in net product revenues in the period in which the change in estimate occurs and will include adjustments to all prior sales of ORKAMBI under the early access programs. Depending on the final price of ORKAMBI and because the current estimate is based on the amount that willwould not be subject to a significant reversal in amounts recognized, this adjustment could be material.
Collaborative and Royalty Revenues
We recognize collaborative and royalty revenues generated through collaborative research, development and/or commercialization agreements. The terms of these agreements typically include payment to us of one or more of the following: nonrefundable, upfront license fees; development and commercial milestone payments; funding of research and/or development activities; and royalties on net sales of licensed products.
In connection, with upfront license fees and milestone payments we receive from these agreements we are required to determine the amount and timing ofwhich resulted in revenue recognition. These payments can either be recognized immediately or as we complete the performance obligations that we identify as required by the applicable accounting standard. In order to make this determination, we identify all material performance obligations, which may include a license to intellectual property and know-how, research and development activities and/or transition activities. In order to determine the transaction price, in addition to any upfront payment, we estimate the amount of variable consideration at the outset of the contract utilizing either the expected value method or most likely amount method, depending on the facts and circumstances relative to the contract. We constrain (reduce) the estimate of variable consideration such that it is probable that a significant reversal of previously recognized revenue will not occur throughout the life of the contract. When determining if variable consideration should be constrained, we consider whether there are factors outside our control that could result in a significant reversal of revenue. In making these assessments, we consider the likelihood and magnitude of a potential reversal of revenue.
Once the estimated transaction price is established, amounts are allocated to the performance obligations that have been identified. The transaction price is generally allocated to each separate performance obligation on a relative standalone selling price basis. In order to account for these agreements, we must develop assumptions that require judgment to determine the standalone selling price, which may include (i) the probability of obtaining marketing approval for the drug candidate, (ii) estimates regarding the timing of and the expected costs to develop and commercialize the drug candidate, (iii) estimates of future cash flows from potential product sales with respect to the drug candidate and (iv) appropriate discount and tax rates.
Income Taxes
We were engaged in research and development activities and incurred significant net operating losses for a number of years before recently becoming profitable. Since we started generating profits, we have usedrepresenting a portion of our net operating losses and maintained a valuation allowance on the majority of our net operating losses and other deferred tax assets until December 31, 2018. Accordingly, we have not reported any tax benefits relating to our net operating loss carryforwards and income tax credit carryforwards that are availableinvoiced amount.
Upon reaching an agreement with the French government for utilizationORKAMBI, including the final amount for ORKAMBI distributed through early access programs in future periods. As of December 31, 2018, we released the valuation allowance on the majority of our net operating losses and other deferred tax assets resulting in a non-cash benefit from income taxes of $1.56 billionFrance in the fourth quarter of 2018.2019, we updated the transaction price related to ORKAMBI distributed through early access programs and recognized net product revenues of $155.8 million related to these shipments, which occurred from 2015 through the date of our agreement with the French government, because the final amount for these shipments exceeded our previous estimate.
Acquisitions
We provide a valuation allowance whenare required to make several significant judgments and estimates in order to calculate the purchase price for our business combinations and then allocate it is more likely than notto the assets that deferred tax assets will not be realized. On a periodic basis, we reassess our valuation allowanceshave acquired and the liabilities that we have assumed on our deferred tax assets, weighing positiveconsolidated balance sheet. The most significant judgments and negative evidenceestimates relate to assess the recoverabilityfair value of the deferred tax assets. in-process research and development assets and contingent consideration liabilities related to these business combinations. Based on these judgments and estimates, the fair value of the goodwill that we record as a result of these business combinations may be material. Once recorded, these assets are subject to quarterly impairment analysis and our contingent consideration liability is adjusted quarterly, which requires similar judgments and estimates.
Intangible Assets
In the fourth quarter2019, we recorded in-process research and development assets related to our acquisitions of 2018, we reassessed our valuation allowancesExonics and considered positive evidence including significant cumulative consolidated and U.S. income over the three years ended December 31, 2018, revenue growth, clinical program progression, including the advancement and clinical trial data from our triple combination regimens, and expectations regarding future profitability, and negative evidence, including potential impact of competitionSemma totaling $400.0 million on our projections and cumulative losses inconsolidated balance sheet, which remained on the jurisdictions. After assessing both the positive evidence and the negative evidence, we released the valuation allowance on the majority of our net operating losses and other deferred tax assetsconsolidated balance sheet as of December 31, 2018.2021. Each of these assets is accounted for as an indefinite-lived intangible asset and is maintained on our consolidated balance sheet until either the project underlying it is completed or the asset becomes impaired. When we determine that an asset has become impaired or we abandon a project, we write down the carrying value of the related intangible asset to its fair value and record an impairment charge in the period in which the impairment occurs.
To determine the fair value of our in-process research and development assets, we utilize the multi-period excess earnings method of the income approach, which requires us to make estimates of the probability of technical and regulatory success, development cost assumptions, revenue projections and growth rates, commercial cost estimates and appropriate discount rates. These assumptions require significant management judgment and reasonable changes in the assumptions can cause material changes to the fair value of the intangible assets. Due to the early stage of Exonics and Semma’s programs, these significant assumptions could be affected by future economic and market conditions.
Contingent Consideration
As of December 31, 2021 and 2020, we had $186.5 million and $189.6 million, respectively, of liabilities on our consolidated balance sheet attributable to the fair value of the contingent development and regulatory payments that we may
owe to Exonics’ former equity holders upon the achievement of certain events.
We record an increase or a decrease in the fair value of the contingent consideration liability on our consolidated balance sheet and in our consolidated statement of operations on a quarterly basis. We determine the fair value of our contingent consideration liability using a probability weighted discounted cash flow method of the income approach, which requires us to make estimates of the timing of regulatory and commercial milestone achievement and the corresponding estimated probability of technical and regulatory success rates. Significant judgment is requiredused in makingdetermining the appropriateness of these assessments to maintain or reverse our valuation allowances and,assumptions during each reporting period. Reasonable changes in these assumptions can cause material changes to the extent our future expectations change we would have to assess the recoverability of these deferred tax assets at that time. The determination to release the majorityfair value of our valuation allowances increasedcontingent consideration liability. Due to the early stage of Exonics’ DMD and DM1 programs, these significant assumptions could be affected by future economic and market conditions.
Goodwill
In 2021 and 2020, we did not have any business combinations; therefore, we did not record any additional goodwill on our net income by $1.56 billion, or $6.03 per share in 2018.consolidated balance sheet. In 2019, duewe recorded goodwill of $554.6 million and $397.1 million related to our releaseacquisitions of Semma and Exonics, respectively. Goodwill reflects the difference between the fair value of the majorityconsideration transferred and the fair value of the net assets acquired. Thus, the goodwill that we record is dependent on the significant judgments and estimates inherent in the fair value of our valuation allowance in 2018, we expectin-process research and development assets and contingent consideration liabilities. We have one reporting unit for goodwill reporting purposes. We have not identified any goodwill impairment to continue to utilize our net operating losses to offset income, but expect to begin recording a significant provision for income taxes reflecting the utilization of our deferred tax assets.date.
Income Taxes
We utilize the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement carrying amounts and tax basis of assets and liabilities using enacted tax rates in effect for years in which the temporary differences are expected to reverse. If our estimate of the tax effect of reversing temporary differences is (i) not reflective of actual outcomes, (ii) modified to reflect new developments or interpretations of the tax law, or (iii) revised to incorporate new accounting principles, or changes in the expected timing or manner of the reversal, our results of operations could be materially impacted.
ResearchWe provide a valuation allowance when it is more likely than not that deferred tax assets will not be realized. On a periodic basis, we reassess our valuation allowances on our deferred tax assets, weighing positive and Development Accruals
Research and development expenses, including amounts funded through research and development collaborations, are expensed as incurred. When third-party service providers’ billing terms do not coincide with our period-end, we are requirednegative evidence to make estimates of our obligations to those third parties, including clinical trial and pharmaceutical development costs, contractual services costs, costs for drug supply, marketing expenses and infrastructure expenses incurred in a given
accounting period and record accruals atassess the endrecoverability of the period. We basedeferred tax assets. Significant judgment is required in making these assessments to maintain or reverse our estimates on our knowledge of the researchvaluation allowances and, development programs, services performed for the period, experience with related activities and the expected duration of the third-party service contract, where applicable. Due to the scope of the research and development activities being conducted and the complexity ofextent our clinical expense models, the estimates and judgments that we make affect the timing of our research and development expense for a reporting period and the related accruals on our consolidated balance sheet. These estimates are not likely to significantly affect the total expense we record over the course of a clinical trial or other research and development activity.
Leases
Our leases, and in particular the leases for our primary facilities, require significant judgment in order to determine the levels of operating expenses and interest expenses we record to our consolidated statement of operations and the amounts recorded on our consolidated balance sheet associated with the leases:
Until December 31, 2018, we were required to determine whetherfuture expectations change we would be deemed for accounting purposeshave to beassess the ownerrecoverability of these buildings as they were constructed. Upon completion of these buildings, we were required to determine whether or not the underlying leases met the criteria for “sale-leaseback” treatment.
Beginning on January 1, 2019, in accordance with ASC 842, Leases (“ASC 842”), we will be required to determine whether the leases associated with these buildings will be reflected as financing leases or operating leases.
deferred tax assets at that time. As of December 31, 2018, because our corporate headquarters2021, we maintained a valuation allowance of $220.4 million related primarily to U.S. state and San Diego building did not qualify for sale-leaseback treatment when construction was completed, we recorded the cost of construction of these buildings as “Property and equipment, net” and the related lease obligations as “Construction financing lease obligation,” on our consolidated balance sheets. Accordingly, we depreciated the assets and incurred interest expense associated with the financing obligation for these buildings. foreign tax attributes.
We bifurcated our lease payments pursuant to the leases into (i) a portion that is allocated to the buildings and (ii) a portion that is allocated to the land on which the buildings were constructed. In 2018, we incurred approximately (1) $15 million in depreciation expense and $7 million in rent expense, each of which is included in our operating expenses and (2) $65 million in interest expenses,record liabilities related to these leases. The amounts reflected on our consolidated balance sheetuncertain tax positions by prescribing a minimum recognition threshold and expenses incurred on our consolidated statement of operations required estimates regarding the useful life of the building and appropriate discount rates.
Beginning on January 1, 2019, we are accounting for our primary facilities under ASC 842. ASC 842 continues to require us to use significant judgment to determine whether these buildings should be accounted for as financing or operating leases including the fair value of the buildings at the inception of the lease and appropriate discount rates. Whether the buildings are recorded as financing or operating leases will result in significant shifts in the classification of expenses between operating expenses and interest expense on our consolidated statement of operations. This determination will also impact the amount and classification of assets and liabilities on our consolidated balance sheet.
Under ASC 842, we will account for our corporate headquarters and San Diego buildings as financing leases requiring us to account for these buildings over their respective lease terms, which are significantly shorter than these buildings’ useful lives. Under previous guidance, we utilized an estimated useful life of 40 years for these buildings consistent with a useful life an owner would apply to its buildings. As a result, we depreciated the buildings over 40 years, which also impacted the amount of interest expense that we recorded on an annual basis. As a result, in 2019, we expect we will record the following related to our corporate headquarters and San Diego leases: (i) operating expenses of approximately $48 million (an increase of approximately $26 million compared to 2018) and (ii) interest expense of approximately $52 million (a decrease of approximately $13 million compared to 2018).
Collaborations; Intangible Assets and Variable Interest Entities
Our collaborations require us to apply accounting policies that involve significant judgments and that have a material effect on our consolidated financial statements. For example, in 2017, we deconsolidated Parion, a VIE that we had consolidated since 2015, and recorded an intangible asset impairment charge of $255.3 million related to Parion’s pulmonary ENaC platform.
We review each collaboration agreement pursuant to which we license assets owned by a collaborator in order to determine whether we have a variable interest via the license agreement with the collaborator and if the variable interest is a variable interest in the collaborator as a whole. In connection with this assessment, we consider and make judgments regarding the following, among other factors: (1) whether the collaborator is a business; (2) the purpose and design of the collaborator; (3) the value of the licensed asset(s) as compared to the value of the collaborator as a whole; and (4) which party has the power to direct the activities that most significantly affect the collaborator’s economic performance.
We evaluate on a quarterly basis if we continue to have a variable interest in each VIE and are the primary beneficiary of the VIE, and if we later determine that we no longer have a variable interest or are no longer the primary beneficiary, we deconsolidate the applicable VIE. This evaluation involves an assessment of the activities being conducted pursuant to our collaboration agreement with the collaborator, the collaborator’s financial statements, discussions with the collaborator’s management regarding its other activities, including any new collaborations, financing activities, clinical data and the collaborator’s other programs.
We believe that the following effects of the consolidation and deconsolidation of VIEs on our consolidated financial statements are the most significant:
In periods in which we consolidate a VIE, we record net income (loss) attributable to our VIEs’ noncontrolling interest. This net income (loss) reflects our VIEs’ net income (loss)measurement attribute for the period as adjusted for gainsfinancial statement recognition and lossesmeasurement of a tax position taken or expected to be taken in the fair value of the contingent payments, which consist of milestone, royalty and option payments, payable by usa tax return. We adjust our liability to our VIEs. Thereflect any subsequent changes in the fair value of contingent payments decrease or increase our net loss attributable to Vertex on a dollar-for-dollar basis.
We recorded $255.3 millionrelevant facts and $29.0 million, respectively, of intangible assets on our consolidated balance sheet based on our estimate ofcircumstances surrounding the fair value of Parion’s and BioAxone’s indefinite-lived in-process research and development assets as of the applicable transaction date. We maintain these assets on our consolidated balance sheet until either the research and development project underlying it is completed or the asset becomes impaired. When we determine that an asset has become impaired or we abandon a project, we write down the carrying value of the related intangible asset to its fair value and record an impairment charge in the period in which the impairment occurs. We assess the fair value of these assets using a variety of methods, including present-value models that are based on multiple probability-weighted scenarios involving the development and potential commercialization of the underlying drug candidates. In 2017 and 2018, we recorded full impairment changes for the indefinite-lived in-process research and development assets that were related to our collaborations with Parion and BioAxone, respectively. As a result, we did not have any indefinite-lived intangible assets recorded on our consolidated balance sheet as of December 31, 2018.
In order to account for the fair value of the intangible assets and contingent payments related to collaborations with our VIEs, we use present-value models based on assumptions regarding the probability of achieving the relevant milestones, estimates regarding the timing of achieving the milestones, estimates of future product sales and the appropriate discount rates.uncertain positions. Significant judgment is usedrequired in determiningmaking this assessment, and, therefore, we re-evaluate uncertain tax positions and consider various factors, including, but not limited to, changes in tax law, the appropriatenessmeasurement of these assumptions during each reporting period. Changestax positions taken or expected to be taken in these assumptions could havetax returns, and changes in facts or circumstances related to a material effect on the fair value of the contingent payments and affect the analysis of whether or not an intangible asset is impaired.tax position.
The revenues, research and development expenses and sales, general and administrative expenses of our VIEs that are unrelated to the programs that we in-license from our VIEs and that are consolidated into our financial statements are set forth in the table below and represent approximately 2% or less of our revenues, research and development expenses and sales, general and administrative expenses in each period:
|
| | | | | | | | | | | |
| | 2018 | | 2017 | | 2016 |
| | (in thousands) |
| Revenues | $ | 1,840 |
| | $ | 43,376 |
| | $ | 944 |
|
| Research and development expenses | (2,114 | ) | | (7,729 | ) | | (6,762 | ) |
| Sales, general and administrative expenses | (2,029 | ) | | (3,826 | ) | | (4,160 | ) |
| Other items, net | (28,888 | ) | | (255,200 | ) | | (108 | ) |
| Loss attributable to noncontrolling interest before (benefit from) provision for income taxes and changes in fair value of contingent payments | $ | (31,191 | ) | | $ | (223,379 | ) | | $ | (10,086 | ) |
RECENT ACCOUNTING PRONOUNCEMENTS
Refer to Note A, “Nature of Business and Accounting Policies,” in the accompanying notes to the consolidated financial statements for a discussion of recent accounting pronouncements and new accounting pronouncements adopted during 2018.2021.
| |
ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
ITEM 7A.QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As part of our investment portfolio, we own financial instruments that are sensitive to market risks. The investment portfolio is used to preserve our capital until it is required to fund operations, including our research and development
activities.capital. None of these market risk-sensitive instruments are held for trading purposes. We do not have derivative financial instruments in our investment portfolio.
Interest Rate Risk
We invest our cash in a variety of financial instruments, principally securities issued by the U.S. government and its agencies, investment-grade corporate bonds and commercial paper, and money market funds. These investments are denominated in U.S. Dollars. All of our interest-bearing securities are subject to interest rate risk and could decline in value if interest rates fluctuate.fluctuate, including potential fluctuations as a result of COVID-19. Substantially all of our investment portfolio consists of marketable securities with active secondary or resale markets to help ensure portfolio liquidity, and we have implemented guidelines limiting the term-to-maturity of our investment instruments. Due to the conservative nature of these instruments, we do not believe that we have a material exposure to interest rate risk. If interest rates were to increase or decrease by 1%, the fair value of our investment portfolio would increase or decrease by an immaterial amount.
In 2016, weWe entered into a credit agreement.agreement in each of 2020 and 2019. Loans under thethese credit agreementagreements bear interest, at our option, at either a base rate or a EurodollarEurocurrency rate, in each case plus an applicable margin. Themargin based on our consolidated leverage ratio (the ratio of our total consolidated funded indebtedness to our consolidated EBITDA for the most recently completed four fiscal quarter period). Pursuant to the credit agreement that we entered into in 2019, the applicable margin on base rate loans ranges from 0.75%0.125% to 1.50%0.500% and the applicable margin on EurodollarEurocurrency loans ranges from 1.75%1.125% to 2.50%, in each case, based on our consolidated leverage ratio (as defined in1.500%. Pursuant to the credit agreement)agreement that we entered into in 2020, the applicable margin on base rate loans ranges from 0.500% to 0.875% and the applicable margin on Eurocurrency loans ranges from 1.500% to 1.875%. We do not believe that changes in interest rates related to theeither credit agreement would have a material effect on our consolidated financial statements. As of December 31, 2018,2021, we had no principal or interest outstanding.outstanding under either of our existing credit facilities. A portion of our “Interest expense, net”expense” in 20192022 will be dependent on whether, and to what extent, we reborrowborrow amounts under thethese existing facility.facilities.
Foreign Exchange Market Risk
As a result of our foreign operations, we face exposure to movements in foreign currency exchange rates, primarily the Euro and British Pound against the U.S. Dollar. Fluctuations in the global markets, including as a result of COVID-19, may have a positive or negative effect on our foreign exchange rate exposure. The current exposures arise primarily from cash, accounts receivable, intercompany receivables and payables, payables and accruals and inventories. Both positive and negative effects to our net revenues from international product sales from movements in exchange rates are partially mitigated by the natural, opposite effect that exchange rates have on our international operating costs and expenses.
We have a foreign currency management program with the objective of reducing the effect of exchange rate fluctuations on our operating results and forecasted revenues and expenses denominated in foreign currencies. We currently have cash flow hedges for the Euro, British Pound, Canadian Dollar, Swiss Franc and Australian Dollar related to a portion of our forecasted product revenues that qualify for hedge accounting treatment under U.S. GAAP. We do not seek hedge accounting treatment for our foreign currency forward contracts related to monetary assets and liabilities that impact our operating results. As of December 31, 2018,2021, we held foreign exchange forward contracts that were designated as cash flow hedges with notional amounts totaling $505.2 million and had$1.9 billion representing a net fair value of $20.1$38.2 million recorded on our consolidated balance sheet.
Although not predictive in nature, we believe a hypothetical 10% threshold reflects a reasonably possible near-term change in exchange rates. Assuming thatIf the December 31, 20182021 exchange rates were to change by a hypothetical 10%, the fair value recorded on our consolidated balance sheet related to our foreign exchange forward contracts that were designated as cash flow hedges as of December 31, 20182021 would change by approximately $50.5$189.3 million. However, since these contracts hedge a specific portion of our forecasted product revenues denominated in certain foreign currencies, any change in the fair value of these contracts is recorded in “Accumulated other comprehensive income (loss)” on our consolidated balance sheet and is reclassified to earnings in the same periods during which the underlying product revenues affect earnings. Therefore, any change in the fair value of these contracts that would result from a hypothetical 10% change in exchange rates would be entirely offset by the change in value associated with the underlying hedged product revenues resulting in no impact on our future anticipated earnings and cash flows with respect to the hedged portion of our forecasted product revenues.
Equity Price Risk
Information required by this section is incorporated by reference from the discussion in the “Strategic Investments” section of this Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| |
ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
ITEM 8.FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this Item 8 is contained on pages F-1 through F-53F-45 of this Annual Report on Form 10-K.
| |
ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
ITEM 9.CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
| |
ITEM 9A. | CONTROLS AND PROCEDURES |
ITEM 9A.CONTROLS AND PROCEDURES
(1) Evaluation of Disclosure Controls and Procedures. The Company’sOur chief executive officer and chief financial officer, after evaluating the effectiveness of the Company’sour disclosure controls and procedures (as defined in Rule 13a-15(e) and Rule 15d-15(e) promulgated under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this Annual Report on Form 10-K, have concluded that, based on such evaluation, the Company’sour disclosure controls and procedures were effective. In designing and evaluating the disclosure controls and procedures, the Company’s management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and the Company’s management necessarily was required to apply itsour judgment in evaluating the cost-benefit relationship of possible controls and procedures.
(2) Management’s Annual Report on Internal Control Over Financial Reporting. The management of the CompanyManagement is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and Rule 15d-15(f) promulgated under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of, the Company’sour principal executive and principal financial officers and effected by the Company’sour board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company’sOur internal control over financial reporting includesinclude those policies and procedures that:
• pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company;our assets;
• provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company;our directors; and
• provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’sour assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s managementManagement assessed the effectiveness of the Company’sour internal control over financial reporting as of December 31, 2018.2021. In making this assessment, itwe used the criteria set forth in the Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework)(COSO). Based on itsour assessment, the Company’s management has concluded that, as of December 31, 2018, the Company’s2021, our internal control over financial reporting is effective based on those criteria.
The Company’sOur independent registered public accounting firm, Ernst & Young LLP, issued an attestation report on the Company’sour internal control over financial reporting. See Section 4 below.
(3) Changes in Internal Controls.During the quarter ended December 31, 2018,2021, there were no changes in the Company’sour internal control over financial reporting that materially affected, or are reasonably likely to materially affect, the Company’sour internal control over financial reporting.
(4) Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of
Vertex Pharmaceuticals Incorporated
Opinion on Internal Control over Financial Reporting
We have audited Vertex Pharmaceuticals Incorporated’s internal control over financial reporting as of December 31, 2018,2021, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Vertex Pharmaceuticals Incorporated (the “Company”)Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2018,2021, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the 2021 consolidated balance sheets of Vertex Pharmaceuticals Incorporated as of December 31, 2018 and 2017, the related consolidatedfinancial statements of operations, comprehensive income (loss), shareholders’ equity and noncontrolling interest, and cash flows for each of the three years in the period ended December 31, 2018, and the related notesCompany and our report dated February 13, 20199, 2022, expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission ofand the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Overover Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Boston, Massachusetts
February 13, 2019
9, 2022
| |
ITEM 9B. | ITEM 9B. OTHER INFORMATION |
Not applicable.
On February 7, 2022, the Company entered into an amendment to Dr. Jeffrey Leiden’s employment agreement, which was scheduled to expire on March 31, 2023. Among other things, the amendment extends the term for one year through March 31, 2024 and provides that Dr. Leiden’s equity compensation during the final year of the amended employment agreement will be equivalent to his equity compensation in the preceding year. The foregoing description of the amendment to Dr. Leiden’s employment agreement does not purport to be complete and is qualified in its entirety by reference to the full text of such agreement, which is filed as Exhibit 10.24 to this Annual Report on Form 10-K and incorporated by reference herein.
PART III
Portions of our definitive Proxy Statement for the 20192022 Annual Meeting of Shareholders, or 20192022 Proxy Statement, are incorporated by reference into this Part III of our Annual Report on Form 10-K.
| |
ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
ITEM 10.DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information regarding directors required by this Item 10 will be included in our 20192022 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under “Election of Directors,” “Corporate Governance and Risk Management,” “Shareholder Proposals for the 20192022 Annual Meeting and Nominations for Director,” “Section“Delinquent Section 16(a) Beneficial Ownership Reporting Compliance”Reports” and “Code of Conduct.” The information regarding executive officers required by this Item 10 as well as certain information regarding our directors is included in Part I of this Annual Report on Form 10-K.
| |
ITEM 11. | EXECUTIVE COMPENSATION |
ITEM 11.EXECUTIVE COMPENSATION
The information required by this Item 11 will be included in the 20192022 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under “Compensation Committee Interlocks and Insider Participation,” “Compensation Discussion and Analysis,” “Compensation and Equity Tables,” “Director Compensation,” “Management Development and Compensation Committee Report” and/or “Corporate Governance and Risk Management.”
| |
ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
ITEM 12.SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item 12 will be included in the 20192022 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information.”
| |
ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
ITEM 13.CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item 13 will be included in the 20192022 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under “Election of Directors,” “Corporate Governance and Risk Management,” and “Audit and Finance Committee.”
| |
ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
ITEM 14.PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item 14 will be included in the 20192022 Proxy Statement and is incorporated herein by reference. We expect this information to be provided under “Ratification of the Appointment of Independent Registered Public Accounting Firm.”
PART IV
| |
ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
ITEM 15.EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)(1) The Financial Statements required to be filed by Items 8 and 15(c) of Form 10-K, and filed herewith, are as follows:
| | | | | |
| |
| Page Number in
this Form 10-K |
| Report of Independent Registered Public Accounting Firm (PCAOB ID: 42) | |
Consolidated Statements of Operations for the years ended December 31, 2018, 20172021, 2020 and 20162019 | |
Consolidated Statements of Comprehensive Income (Loss) for the years ended December 31, 2018, 20172021, 2020 and 20162019 | |
Consolidated Balance Sheets as of December 31, 20182021 and 20172020 | |
Consolidated Statements of Shareholders’ Equity and Noncontrolling Interest for the years ended December 31, 2018, 20172021, 2020 and 20162019 | |
Consolidated Statements of Cash Flows for the years ended December 31, 2018, 20172021, 2020 and 20162019 | |
| Notes to Consolidated Financial Statements | |
(a)(2) Financial Statement Schedules have been omitted because they are either not applicable or the required information is included in the consolidated financial statements or notes thereto listed in (a)(1) above.
(a)(3) Exhibits.
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
|
| | | | | | | | | | | | | | | | |
| Exhibit Number | Exhibit Description | Filed with
this report | Incorporated by
Reference herein
from—Form
or Schedule | Filing Date/
Period Covered | SEC File/
Reg. Number |
3.1Plan of Acquisition | | | |
| 2.1 | Agreement and Plan of Merger, dated as of June 6, 2019, among Vertex Pharmaceuticals Incorporated, VXP Merger Sub, Inc., Exonics Therapeutics, Inc. and Shareholder Representative Services LLC, solely in its Capacity as Shareholders’ Representative, as amended by the Amendment to Agreement and Plan of Merger, dated as of June 12, 2019, among Vertex Pharmaceuticals Incorporated, VXP Merger Sub, Inc., Exonics Therapeutics, Inc. and Shareholder Representative Services LLC, solely in its Capacity as Shareholders’ Representative.† | | 10-Q
(Exhibit 10.1) | August 1, 2019 | 000-19319 |
| Governance Documents | | | |
| 3.1 | | | 10-Q
(Exhibit 3.1)
| July 26, 2018 | 000-19319 |
| 3.2 | | | 10-Q
(Exhibit 3.2)
| July 26, 2018May 1, 2020 | 000-19319 |
| Stock Certificate | | | |
| 4.1 | | | 10-K (Exhibit
(Exhibit 4.1) | February 15, 2018 | 000-19319 |
Collaboration Agreement4.2 | | | 10-K
(Exhibit 4.2) | February 13, 2020 | 000-19319 |
| Collaboration Agreement | | | |
| 10.1 | | | 10-Q/A
10-Q
(Exhibit 10.2) 10.1) | August 19, 2011November 3, 2021 | 000-19319 |
| 10.2 | | | 10-K
10-Q
(Exhibit 10.9) 10.2) | March 16, 2006November 3, 2021 | 000-19319 |
| 10.3 | | | 10-Q/A
(Exhibit 10.6)
| August 19, 2011 | 000-19319 |
| 10.4 | | | 10-Q
(Exhibit 10.3)
| August 9, 2011November 3, 2021 | 000-19319 |
| | | | | | | | | | | | | | | | | |
| Exhibit Number | Exhibit Description | Filed with this report | Incorporated by
Reference herein from—Form or Schedule | Filing Date/
Period Covered | SEC File/Reg. Number |
| 10.5 | | | 10-K
10-Q
(Exhibit 10.05) 10.4) | February 23, 2017November 3, 2021 | 000-19319 |
Leases10.6 | | | 10-Q
(Exhibit 10.1) | July 30, 2021 | 000-19319 |
10.6Leases | | | | | |
| 10.7 | | | 10-Q
(Exhibit 10.4)
10.2) | August 9, 2011July 30, 2021 | 000-19319 |
10.710.8 | | | 10-Q
(Exhibit 10.5)
10.3) | August 9, 2011July 30, 2021 | 000-19319 |
10.8 | | | 10-K
(Exhibit 10.10)
| February 16, 2016 | 000-19319Financing Agreements |
| 10.9 | | | 10-Q
(Exhibit 10.3)
| April 28, 2017 | 000-19319 |
|
| | | | | |
Exhibit Number | Exhibit Description | Filed with
this report | Incorporated by
Reference herein
from—Form
or Schedule | Filing Date/
Period Covered | SEC File/
Reg. Number |
Financing Agreements |
10.10 | | | 10-K
(Exhibit 10.12)
| February 23, 2017 | 000-19319 |
10.11 | | | 10-K
10-Q
(Exhibit 10.13) 10.1) | February 23, 2017October 31, 2019 | 000-19319 |
Equity Plans10.10 | | | 10-K
(Exhibit 10.10) | February 11, 2021 | 000-19319 |
10.1210.11 | | | 10-Q
(Exhibit 10.1) | October 30, 2020 | 000-19319 |
| Equity Plans | | | |
| 10.12 | | | 10-Q
(Exhibit 10.1)
| October 25, 2018 | 000-19319 |
| 10.13 | | | 8-K
(Exhibit 10.2)
| May 15, 2006 | 000-19319 |
| 10.14 | | | 10-K
(Exhibit 10.20)
| February 13, 2015 | 000-19319 |
| 10.15 | | | 10-Q
DEF 14A
(Exhibit 10.2) Appendix A) | October 25, 2018April 26, 2019 | 000-19319 |
| 10.16 | | | 10-K
(Exhibit 10.17)
| February 13, 2015 | 000-19319 |
| 10.17 | | | 10-K
(Exhibit 10.18)
| February 13, 2015 | 000-19319 |
| 10.18 | | | 10-K
(Exhibit 10.25)
| February 16, 2016 | 000-19319 |
| 10.19 | | | 10-K
(Exhibit 10.19)
| February 13, 2015 | 000-19319 |
| 10.20 | | | 10-K
(Exhibit 10.17) | February 13, 2020 | 000-19319 |
| 10.21 | | | 10-K
(Exhibit 10.27)
| February 16, 2016 | 000-19319 |
10.2110.22 | | | 10-Q
DEF 14A
(Exhibit 10.1) Appendix B) | August 1, 2016April 26, 2019 | 000-19319 |
| Agreements with Executive Officers and Directors | | | |
10.2210.23 | | | 8-K
(Exhibit 10.1)
| December 2, 2016April 1, 2020 | 000-19319 |
10.2310.24 | | X | | | |
| 10.25 | | | 10-K
(Exhibit 10.35)
| February 22, 2012 | 000-19319 |
10.2410.26 | | | 8-K
(Exhibit 10.1) | July 25, 2019 | 000-19319 |
| 10.27 | | | 8-K
(Exhibit 10.2) | July 25, 2019 | 000-19319 |
| 10.28 | | | 10-Q
(Exhibit 10.1)
| November 6, 2012 | 000-19319 |
| | | | | | | | | | | | | | | | | |
10.25Exhibit Number | Exhibit Description | Filed with this report | Incorporated by
Reference herein from—Form or Schedule | Filing Date/
Period Covered | SEC File/Reg. Number |
| 10.29 | | | 10-Q
(Exhibit 10.2)
| November 6, 2012 | 000-19319 |
10.2610.30 | | | 10-K
(Exhibit 10.34)
| February 16, 2016 | 000-19319 |
10.2710.31 | | | 10-K
(Exhibit 10.35)
| February 16, 2016 | 000-19319 |
10.2810.32 | | | 10-Q
(Exhibit 10.13)
| November 9, 2004 | 000-19319 |
10.29 | | | 10-K
(Exhibit 10.66)
| February 17, 2009 | 000-19319 |
10.32 | | | 10-K
(Exhibit 10.40)
| February 23, 2017 | 000-19319 |
10.33 | | | 10-K
(Exhibit 10.41)
| February 23, 2017 | 000-19319 |
10.34 | | | 10-K
(Exhibit 10.42)
| February 23, 2017 | 000-19319 |
10.3510.33 | | | 10-K
(Exhibit 10.43)
| February 23, 2017 | 000-19319 |
10.3610.34 | | | 10-Q
(Exhibit 10.1)
| October 30, 2017May 1, 2019 | 000-19319 |
10.3710.35 | | | 10-Q
(Exhibit 10.2)
| May 1, 2019 | 000-19319 |
| 10.36 | | October 30, 2017X | 000-19319 |
| |
| 10.37 | | X | | | |
| 10.38 | | | 10-K
(Exhibit 10.46) | February 15, 2018 | 000-19319 |
| 10.39 | | X | | | |
| Subsidiaries | | | | |
Exhibit Number | Exhibit Description | Filed with
this report | Incorporated by
Reference herein
from—Form
or Schedule | Filing Date/
Period Covered | SEC File/
Reg. Number |
10.3821.1 | | | 10-K (Exhibit 10.46) | February 15, 2018 | 000-19319 |
10.39 | | X | | | |
Subsidiaries | | | | |
21.1 | | X | | | |
| Consent | | | | |
| 23.1 | | X | | | |
| Certifications | | | | |
| 31.1 | | X | | | |
| 31.2 | | X | | | |
| 32.1 | | X | | | |
| 101.INS | XBRL Instance | X | | | |
| 101.SCH | XBRL Taxonomy Extension Schema | X | | | |
| 101.CAL | XBRL Taxonomy Extension Calculation | X | | | |
| 101.LAB | XBRL Taxonomy Extension Labels | X | | | |
| 101.PRE | XBRL Taxonomy Extension Presentation | X | | | |
| 101.DEF | XBRL Taxonomy Extension Definition | X | | | |
| 104 | Cover Page Interactive Data File––the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | X | | | |
* Management contract, compensatory plan or agreement.
| |
† | Confidential portions of this document have been filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment. |
SIGNATURES
Pursuant† Confidential portions of this document have been redacted according to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.applicable rules.
|
| | |
| Vertex Pharmaceuticals Incorporated |
| | |
February 13, 2019 | By: | /s/ Jeffrey M. Leiden |
| | Jeffrey M. Leiden
Chief Executive Officer
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
| | | | | | | | | | | | | |
| | Name | | | | Title | | | | | Date | | |
| | |
/s/ Jeffrey M. Leiden | | |
Jeffrey M. Leiden | Chair of the Board, President and Chief Executive Officer (Principal Executive Officer) | February 13, 2019 |
| | |
/s/ Paul M. Silva | | |
Paul M. Silva | Senior Vice President, Corporate Controller and Interim Chief Financial Officer (Principal Accounting Officer and Principal Financial Officer) | February 13, 2019 |
| | |
/s/ Sangeeta N. Bhatia | | |
Sangeeta N. Bhatia | Director | February 13, 2019 |
| | |
/s/ Alan Garber | | |
Alan Garber | Director | February 13, 2019 |
| | |
/s/ Terrence C. Kearney | | |
Terrence C. Kearney | Director | February 13, 2019 |
| | |
/s/ Yuchun Lee | | |
Yuchun Lee | Director | February 13, 2019 |
| | |
/s/ Margaret G. McGlynn | | |
Margaret G. McGlynn | Director | February 13, 2019 |
| | |
/s/ Bruce I. Sachs | | |
Bruce I. Sachs | Director | February 13, 2019 |
| | |
/s/ Elaine S. Ullian | | |
Elaine S. Ullian | Director | February 13, 2019 |
|
/s/ William D. Young | | |
William D. Young | Director | February 13, 2019 |
ItemITEM 16.FORM 10-K SUMMARY
Not applicable.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | |
| Vertex Pharmaceuticals Incorporated |
| | |
| February 9, 2022 | By: | /s/ Reshma Kewalramani |
| | Reshma Kewalramani Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Name | | | | Title | | | | Date | |
| | |
| /s/ Reshma Kewalramani | | |
| Reshma Kewalramani | President, Chief Executive Officer and Director (Principal Executive Officer) | February 9, 2022 |
| | |
| /s/ Charles F. Wagner, Jr. | | |
| Charles F. Wagner, Jr. | Executive Vice President and Chief Financial Officer (Principal Financial Officer) | February 9, 2022 |
| | |
| /s/ Kristen C. Ambrose | | |
| Kristen C. Ambrose | Senior Vice President and Chief Accounting Officer (Principal Accounting Officer) | February 9, 2022 |
| | |
| /s/Jeffrey M. Leiden | | |
| Jeffrey M. Leiden | Executive Chairman | February 9, 2022 |
| | |
| /s/ Sangeeta N. Bhatia | | |
| Sangeeta N. Bhatia | Director | February 9, 2022 |
| | |
| /s/ Lloyd Carney | | |
| Lloyd Carney | Director | February 9, 2022 |
| | |
| /s/ Alan Garber | | |
| Alan Garber | Director | February 9, 2022 |
| | |
| /s/ Terrence C. Kearney | | |
| Terrence C. Kearney | Director | February 9, 2022 |
| | |
| /s/ Yuchun Lee | | |
| Yuchun Lee | Director | February 9, 2022 |
| | |
| /s/ Margaret G. McGlynn | | |
| Margaret G. McGlynn | Director | February 9, 2022 |
| | | | | | | | | | | | | |
| /s/ Diana McKenzie | | |
| Diana McKenzie | Director | February 9, 2022 |
| | |
| /s/ Bruce I. Sachs | | |
| Bruce I. Sachs | Director | February 9, 2022 |
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of
Vertex Pharmaceuticals Incorporated
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Vertex Pharmaceuticals Incorporated (the “Company”)Company) as of December 31, 20182021 and 2017,2020, the related consolidated statements of operations, comprehensive income, (loss), shareholders’ equity, and noncontrolling interest, and cash flows for each of the three years in the period ended December 31, 2018,2021, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 20182021 and 2017,2020, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2018,2021, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”)(PCAOB), the Company’s internal control over financial reporting as of December 31, 2018,2021, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 13, 20199, 2022, expressed an unqualified opinion thereon.
Adoption of New Accounting Standards
ASU No. 2014-09
As discussed in Note A to the consolidated financial statements, the Company changed its method for recognizing revenue as a result of the adoption of Accounting Standard Update (ASU) No. 2014-09, Revenue from Contracts with Customers (Topic 606), and the amendments in ASUs 2015-14, 2016-08, 2016-10 and 2016-12 effective January 1, 2018.
ASU No. 2016-18
As discussed in Note A to the consolidated financial statements, on January 1, 2018, the Company retrospectively changed its method of presenting changes in restricted cash in the accompanying consolidated statements of cash flows as a result of the adoption of ASU No. 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash.
ASU No. 2016-01
As discussed in Note A to the consolidated financial statements, on January 1, 2018, the Company changed its method of presenting changes in the fair value of its investments in corporate equity securities as a result of the adoption of ASU No. 2016-01, Financial Instruments (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities.
ASU No. 2016-16
As discussed in Note A to the consolidated financial statements, on January 1, 2018, the Company changed its method for recognizing current and deferred income tax expenses or benefits related to the transfer of assets, other than inventory, within the consolidated entity as a result of the adoption of ASU No. 2016-16, Income Taxes (Topic 740): Intra-Entity Transfers of Assets Other Than Inventory.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included
evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
| | | | | | | | |
| | Revenue recognition - Payor Mix Impact on Measuring Variable Consideration |
| Description of the Matter | | As discussed in Note A to the Company’s consolidated financial statements, the Company records product sales at the net sales price, or “transaction price,” which requires the Company to make several significant estimates regarding the net sales price. The most significant estimates relate to government rebates, chargebacks, discounts and fees, collectively rebates. Due to the delay in receipt of claims by third-party payors, the Company estimates the percentage of prescriptions that will be covered by each third-party payor, which is referred to as the payor mix. Rebate accruals inclusive of estimated amounts due for claims not yet received or processed are recorded within accrued expenses on the Company’s consolidated balance sheet. Auditing the measurement of the Company’s net product revenues was complex and judgmental due to the significant estimation required in determining the amount of consideration that will be collected net of estimates for payor rebates. In particular, the net sales price is affected by assumptions in payor behavior such as changes in payor mix, payor collections, current customer contractual requirements, and experience with ultimate collection from third-party payors. |
| How We Addressed the Matter in Our Audit | | We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over the Company’s revenue recognition process, including controls over the underlying assumptions and inputs used by management to estimate amounts due to third-party payors and the completeness and accuracy of the data used in the estimates. We also tested the Company’s controls to assess the completeness and accuracy of the current and historical data that supports the estimate. Our audit procedures to test the Company’s recognition of net product revenues included, among others, assessing the methodology used to determine the estimate and testing the significant assumptions and the underlying data used by the Company in its analysis, which included historical claims data. To assess the payor mix assumptions we tested contracted rates, historical claims and payment data and related trends, and other relevant factors. We also assessed the historical accuracy of the Company’s estimates of third-party payor rebates. |
/s/ Ernst & Young LLP
| We have served as the Company’s auditor since 2005.
Boston, Massachusetts
February 13, 20199, 2022
VERTEX PHARMACEUTICALS INCORPORATED VERTEX PHARMACEUTICALS INCORPORATED Consolidated Statements of Operations (in thousands, except per share amounts) |
| | | | | | | | | | | |
| | Year Ended December 31, |
| | 2018 | | 2017 | | 2016 |
| Revenues: | | | | | |
| Product revenues, net | $ | 3,038,325 |
| | $ | 2,165,480 |
| | $ | 1,683,632 |
|
| Collaborative and royalty revenues | 9,272 |
| | 323,172 |
| | 18,545 |
|
| Total revenues | 3,047,597 |
| | 2,488,652 |
| | 1,702,177 |
|
| Costs and expenses: | | | | | |
| Cost of sales | 409,539 |
| | 275,119 |
| | 210,460 |
|
| Research and development expenses | 1,416,476 |
| | 1,324,625 |
| | 1,047,690 |
|
| Sales, general and administrative expenses | 557,616 |
| | 496,079 |
| | 432,829 |
|
| Restructuring (income) expenses | (184 | ) | | 14,246 |
| | 1,262 |
|
| Intangible asset impairment charges | 29,000 |
| | 255,340 |
| | — |
|
| Total costs and expenses | 2,412,447 |
| | 2,365,409 |
| | 1,692,241 |
|
| Income from operations | 635,150 |
| | 123,243 |
| | 9,936 |
|
| Interest expense, net | (34,119 | ) | | (57,550 | ) | | (81,432 | ) |
| Other (expense) income, net | (790 | ) | | (81,382 | ) | | 4,130 |
|
| Income (loss) before (benefit from) provision for income taxes | 600,241 |
| | (15,689 | ) | | (67,366 | ) |
| (Benefit from) provision for income taxes | (1,486,862 | ) | | (107,324 | ) | | 16,665 |
|
| Net income (loss) | 2,087,103 |
| | 91,635 |
| | (84,031 | ) |
| Loss (income) attributable to noncontrolling interest | 9,793 |
| | 171,849 |
| | (28,021 | ) |
| Net income (loss) attributable to Vertex | $ | 2,096,896 |
| | $ | 263,484 |
| | $ | (112,052 | ) |
| | | | | | |
| Amounts per share attributable to Vertex common shareholders: | | | | | |
| Net income (loss): | | | | | |
| Basic | $ | 8.24 |
| | $ | 1.06 |
| | $ | (0.46 | ) |
| Diluted | $ | 8.09 |
| | $ | 1.04 |
| | $ | (0.46 | ) |
| Shares used in per share calculations: | | | | | |
| Basic | 254,292 |
| | 248,858 |
| | 244,685 |
|
| Diluted | 259,185 |
| | 253,225 |
| | 244,685 |
|
(in millions, except per share amounts)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Revenues: | | | | | |
| Product revenues, net | $ | 7,573.4 | | | $ | 6,202.8 | | | $ | 4,160.7 | |
| Other revenues | 1.0 | | | 2.9 | | | 2.1 | |
| Total revenues | 7,574.4 | | | 6,205.7 | | | 4,162.8 | |
| Costs and expenses: | | | | | |
| Cost of sales | 904.2 | | | 736.3 | | | 547.8 | |
| Research and development expenses | 3,051.1 | | | 1,829.5 | | | 1,754.5 | |
| Selling, general and administrative expenses | 840.1 | | | 770.5 | | | 658.5 | |
| Change in fair value of contingent consideration | (3.1) | | | 13.1 | | | 4.5 | |
| Total costs and expenses | 4,792.3 | | | 3,349.4 | | | 2,965.3 | |
| Income from operations | 2,782.1 | | | 2,856.3 | | | 1,197.5 | |
| Interest income | 4.9 | | | 22.2 | | | 63.7 | |
| Interest expense | (61.5) | | | (58.2) | | | (58.5) | |
| Other income, net | 4.9 | | | 296.6 | | | 192.2 | |
| Income before provision for income taxes | 2,730.4 | | | 3,116.9 | | | 1,394.9 | |
| Provision for income taxes | 388.3 | | | 405.2 | | | 218.1 | |
| Net income | $ | 2,342.1 | | | $ | 2,711.7 | | | $ | 1,176.8 | |
| | | | | |
| Net income per common share: | | | | | |
| Basic | $ | 9.09 | | | $ | 10.44 | | | $ | 4.58 | |
| Diluted | $ | 9.01 | | | $ | 10.29 | | | $ | 4.51 | |
| Shares used in per share calculations: | | | | | |
| Basic | 257.7 | | | 259.8 | | | 256.7 | |
| Diluted | 259.9 | | | 263.4 | | | 260.7 | |
The accompanying notes are an integral part of the consolidated financial statements.
VERTEX PHARMACEUTICALS INCORPORATED VERTEX PHARMACEUTICALS INCORPORATED Consolidated Statements of Comprehensive Income (Loss) (in thousands) |
| | | | | | | | | | | |
| | Year ended December 31, |
| | 2018 | | 2017 | | 2016 |
| Net income (loss) | $ | 2,087,103 |
| | $ | 91,635 |
| | $ | (84,031 | ) |
| Changes in other comprehensive income (loss): | | | | | |
| Unrealized holding gains on marketable securities, net of tax of zero, $(2.7) million and $(3.8) million, respectively | 58 |
| | 6,954 |
| | 17,395 |
|
| Unrealized gains (losses) on foreign currency forward contracts, net of tax of $(7.1) million, $3.4 million and $(3.9) million, respectively | 27,438 |
| | (26,530 | ) | | 7,736 |
|
| Foreign currency translation adjustment | 8,855 |
| | (13,169 | ) | | (5,782 | ) |
| Total changes in other comprehensive income (loss) | 36,351 |
| | (32,745 | ) | | 19,349 |
|
| Comprehensive income (loss) | 2,123,454 |
| | 58,890 |
| | (64,682 | ) |
| Comprehensive loss (income) attributable to noncontrolling interest | 9,793 |
| | 171,849 |
| | (28,021 | ) |
| Comprehensive income (loss) attributable to Vertex | $ | 2,133,247 |
| | $ | 230,739 |
| | $ | (92,703 | ) |
(in millions)
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2021 | | 2020 | | 2019 |
| Net income | $ | 2,342.1 | | | $ | 2,711.7 | | | $ | 1,176.8 | |
| Other comprehensive income: | | | | | |
| Unrealized holding (losses) gains on marketable securities, net | (0.8) | | | (0.2) | | | 1.0 | |
| Unrealized gains (losses) on foreign currency forward contracts, net of tax of $(21.8), $14.3 and $7.0, respectively | 83.2 | | | (51.6) | | | (14.0) | |
| Foreign currency translation adjustment | 2.0 | | | (14.7) | | | 10.3 | |
| Total other comprehensive income (loss) | 84.4 | | | (66.5) | | | (2.7) | |
| Comprehensive income | $ | 2,426.5 | | | $ | 2,645.2 | | | $ | 1,174.1 | |
The accompanying notes are an integral part of the consolidated financial statements.
VERTEX PHARMACEUTICALS INCORPORATED VERTEX PHARMACEUTICALS INCORPORATED Consolidated Balance Sheets (in thousands, except share and per share amounts) |
| | | | | | | |
| | December 31, |
| | 2018 | | 2017 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 2,650,134 |
| | $ | 1,665,412 |
|
| Marketable securities | 518,108 |
| | 423,254 |
|
| Accounts receivable, net | 409,688 |
| | 281,343 |
|
| Inventories | 124,360 |
| | 111,830 |
|
| Prepaid expenses and other current assets | 140,819 |
| | 167,124 |
|
| Total current assets | 3,843,109 |
| | 2,648,963 |
|
| Property and equipment, net | 812,005 |
| | 789,437 |
|
| Intangible assets | — |
| | 29,000 |
|
| Goodwill | 50,384 |
| | 50,384 |
|
| Deferred tax assets | 1,499,672 |
| | 834 |
|
| Other assets | 40,728 |
| | 27,396 |
|
| Total assets | $ | 6,245,898 |
| | $ | 3,546,014 |
|
| Liabilities and Shareholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 110,987 |
| | $ | 73,994 |
|
| Accrued expenses | 604,495 |
| | 443,961 |
|
| Capital lease obligations, current portion | 9,817 |
| | 22,531 |
|
| Early access sales accrual | 354,404 |
| | 232,401 |
|
| Other liabilities, current portion | 40,589 |
| | 34,373 |
|
| Total current liabilities | 1,120,292 |
| | 807,260 |
|
| Capital lease obligations, excluding current portion | 19,658 |
| | 20,496 |
|
| Deferred tax liabilities | — |
| | 6,341 |
|
| Construction financing lease obligation, excluding current portion | 561,892 |
| | 563,406 |
|
| Advance from collaborator, excluding current portion | 82,573 |
| | 78,431 |
|
| Other liabilities, excluding current portion | 26,280 |
| | 27,774 |
|
| Total liabilities | 1,810,695 |
| | 1,503,708 |
|
| Commitments and contingencies | — |
| | — |
|
| Shareholders’ equity: | | | |
| Preferred stock, $0.01 par value; 1,000,000 shares authorized; none issued and outstanding | — |
| | — |
|
| Common stock, $0.01 par value; 500,000,000 shares authorized, 255,172,328 and 253,253,362 shares issued and outstanding, respectively | 2,546 |
| | 2,512 |
|
| Additional paid-in capital | 7,421,476 |
| | 7,157,362 |
|
| Accumulated other comprehensive income (loss) | 659 |
| | (11,572 | ) |
| Accumulated deficit | (2,989,478 | ) | | (5,119,723 | ) |
| Total Vertex shareholders’ equity | 4,435,203 |
| | 2,028,579 |
|
| Noncontrolling interest | — |
| | 13,727 |
|
| Total shareholders’ equity | 4,435,203 |
| | 2,042,306 |
|
| Total liabilities and shareholders’ equity | $ | 6,245,898 |
| | $ | 3,546,014 |
|
(in millions, except share data)
| | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 6,795.0 | | | $ | 5,988.2 | |
| Marketable securities | 729.9 | | | 670.7 | |
| Accounts receivable, net | 1,136.8 | | | 885.4 | |
| Inventories | 353.1 | | | 280.8 | |
| Prepaid expenses and other current assets | 545.8 | | | 308.3 | |
| Total current assets | 9,560.6 | | | 8,133.4 | |
| Property and equipment, net | 1,094.1 | | | 958.5 | |
| Goodwill | 1,002.2 | | | 1,002.2 | |
| Intangible assets | 400.0 | | | 400.0 | |
| Deferred tax assets | 934.5 | | | 882.8 | |
| Operating lease assets | 330.3 | | | 325.6 | |
| Other assets | 110.8 | | | 49.3 | |
| Total assets | $ | 13,432.5 | | | $ | 11,751.8 | |
| Liabilities and Shareholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 195.0 | | | $ | 155.1 | |
| Accrued expenses | 1,678.6 | | | 1,405.0 | |
| Other current liabilities | 268.4 | | | 317.4 | |
| Total current liabilities | 2,142.0 | | | 1,877.5 | |
| Long-term finance lease liabilities | 509.8 | | | 539.0 | |
| Long-term operating lease liabilities | 377.4 | | | 350.5 | |
| Long-term contingent consideration | 186.5 | | | 189.6 | |
| Other long-term liabilities | 116.8 | | | 108.4 | |
| Total liabilities | 3,332.5 | | | 3,065.0 | |
| Commitments and contingencies | — | | | — | |
| Shareholders’ equity: | | | |
| Preferred stock, $0.01 par value; 1,000,000 shares authorized; none issued and outstanding | — | | | — | |
| Common stock, $0.01 par value; 500,000,000 shares authorized, 254,479,046 and 259,889,549 shares issued and outstanding, respectively | 2.5 | | | 2.6 | |
| Additional paid-in capital | 6,880.8 | | | 7,894.0 | |
| Accumulated other comprehensive income (loss) | 15.9 | | | (68.5) | |
| Retained earnings | 3,200.8 | | | 858.7 | |
| Total shareholders’ equity | 10,100.0 | | | 8,686.8 | |
| Total liabilities and shareholders’ equity | $ | 13,432.5 | | | $ | 11,751.8 | |
The accompanying notes are an integral part of the consolidated financial statements.
The accompanying notes are an integral part of the consolidated financial statements.