UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| |
☒
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
☒ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended: December 31, 20192022
OR
| |
☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to ��
Commission File Number: 0-24260
AMEDISYS, INC.
(Exact Name of Registrant as Specified in its Charter)
| | 11-3131700 | | | | | | |
Delaware | | 11-3131700 |
| (State or other jurisdiction of
incorporation or organization)
| | (I.R.S. Employer
Identification No.)
|
3854 American Way, Suite A,Baton Rouge,LA70816
(Address of principal executive offices, including zip code)
(225) (225) 292-2031 or (800) 467-2662
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
| | | | | | | | | | | | | |
| Title of Each Class | | Trading Symbol | | Name of Each Exchange on Which Registered |
| Common Stock, par value $0.001 per share | | AMED | | The NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☑ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
| | | | | | | | | | | | | |
| Large accelerated filer | ☑ | | Accelerated filer
| ☐ |
Non-accelerated filer
| ☐ | | Smaller reporting company
| ☐ |
| | | Emerging growth company
| ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.☑
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. □
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). □
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
The aggregate market value of the voting and non-voting common stock held by non-affiliates of the registrant, based on the last sale price as quoted by the NASDAQ Global Select Market on June 28, 201930, 2022 (the last business day of the registrant’s most recently completed second fiscal quarter) was $2.8$3.0 billion. For purposes of this determination, shares beneficially owned by executive officers, directors and ten percent stockholders have been excluded, which does not constitute a determination that such persons are affiliates.
As of February 14, 2020,10, 2023, the registrant had 32,313,98332,550,602 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement for its 20202023 Annual Meeting of Stockholders (the “2020“2023 Proxy Statement”) to be filed pursuant to the Securities Exchange Act of 1934 with the Securities and Exchange Commission within 120 days of December 31, 20192022 are incorporated herein by reference into Part III of this Annual Report on Form 10-K.
TABLE OF CONTENTS
SPECIAL CAUTION CONCERNING FORWARD-LOOKING STATEMENTS
When included in this Annual Report on Form 10-K, or in other documents that we file with the Securities and Exchange Commission (“SEC”) or in statements made by or on behalf of the Company, words like “believes,” “belief,” “expects,” “strategy,” “plans,” “anticipates,” “intends,” “projects,” “estimates,” “may,” “might,” “could,” “would,” “should” and similar expressions are intended to identify forward-looking statements as defined by the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve a variety of risks and uncertainties that could cause actual results to differ materially from those described therein. These risks and uncertainties include, but are not limited to the following: changes in Medicare and other medical payment levels; changes in payments and covered services by federal and state governments; future cost containment initiatives undertaken by third-party payors; changes in the episodic versus non-episodic mix of our payors or payment methodologies; changes in the case mix of our patients; staffing shortages driven by the competitive labor market; our ability to attract and retain qualified personnel; competition in the healthcare industry; our ability to maintain or establish new patient referral sources; changes in or our failure to comply with existing federal and state laws or regulations or the inability to comply with new government regulations on a timely basis,basis; the impact of the novel coronavirus pandemic ("COVID-19"), including the measures that have been and may be taken by governmental authorities to mitigate it, on our business, financial condition and results of operations; changes in Medicareestimates and other medical payment levels,judgments associated with critical accounting policies; our ability to consistently provide high-quality care; our ability to keep our patients and employees safe; our access to financing; our ability to meet debt service requirements and comply with covenants in debt agreements; business disruptions due to natural or man-made disasters, climate change or acts of terrorism, widespread protests or civil unrest; our ability to open care centers, acquire additional care centers and integrate and operate these care centers effectively, competition in the healthcare industry, changes in the case mix of patients and payment methodologies, changes in estimates and judgments associated with critical accounting policies,effectively; our ability to maintain or establish new patient referral sources, our ability to consistently provide high-quality care, our ability to attractrealize the anticipated benefits of acquisitions, investments and retain qualified personnel, changes in payments and covered services by federal and state governments, future cost containment initiatives undertaken by third-party payors, our access to financing, our ability to meet debt service requirements and comply with covenants in debt agreements, business disruptions due to natural disasters or acts of terrorism,joint ventures; our ability to integrate, manage and keep our information systems secure, our ability to realizesecure; the anticipated benefitsimpact of the acquisition of Compassionate Care Hospice ("CCH"), our ability to comply with requirements stipulated in the CCH corporate integrity agreement,inflation; and changes in lawlaws or developments with respect to any litigation relating to the Company, including various other matters, many of which are beyond our control, and such other factors as discussed throughout Part I, Item 1A. "Risk Factors" of this Annual Report on Form 10-K.
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely on any forward-looking statement as a prediction of future events. We expressly disclaim any obligation or undertaking and we do not intend to release publicly any updates or changes in our expectations concerning the forward-looking statements or any changes in events, conditions or circumstances upon which any forward-looking statement may be based, except as may be required by law. For a discussion of some of the factors discussed above as well as additional factors, see Part I, Item 1A, “Risk Factors” and Part II, Item 7, “Critical Accounting Estimates” within “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Unless otherwise provided, “Amedisys,” “we,” “us,” “our,” and the “Company”“the Company” refer to Amedisys, Inc. and our consolidated subsidiaries, and when we refer to 2019, 20182022, 2021 and 2017,2020, we mean the twelve month period then ended December 31, unless otherwise provided.
A copy of this Annual Report on Form 10-K for the year ended December 31, 20192022 as filed with the SEC, including all exhibits, is available on our internet website at http://www.amedisys.com on the “Investors” page under the “SEC Filings” link.
PART I
ITEM 1. BUSINESS
Overview
Amedisys, Inc. is a leading healthcare services company committed to helping our patients age in place by providing clinically excellent care and support in the home. Our operations involve serving patients across the United States through our threefour operating divisions: home health, hospice, personal care and personalhigh acuity care. We deliver clinically distinct care that best suits our patients' needs, whether that is home-based recovery and rehabilitation after an operation or injury or care that empowers patients to manage a chronic disease through our home health division, hospice care at the end of life, or providing assistance with daily activities through our personal care division or delivering the essential elements of inpatient hospital, palliative and skilled nursing facility ("SNF") care to patients in their homes through our high acuity care division.
We are among the largest pure play providers of home health and hospice care in the United States, with 479approximately 20,000 employees in 532 care centers in 3837 states within the United States and the District of Columbia. Our 21,000 employees deliver the highest quality care makingperforming more than 12.311.2 million visits tofor more than 415,000465,000 patients annually. Over 2,6003,000 hospitals and 67,000102,000 physicians nationwide have chosen us as a partner in post-acute care.
Due to the age demographics of our patient base, our services are primarily paid for by Medicare which has represented approximately 73%74% to 76%75% of our net service revenue over the last three years. We also remain focused on maintaining a profitable and strategically important managed care contract portfolio. We continuously work with our payors to structure innovative contracts which reward us for providing quality care to our patients.
Amedisys is headquartered in Baton Rouge, Louisiana, with an executive office in Nashville, Tennessee. Our common stock is currently traded on the NASDAQ Global Select Market under the trading symbol “AMED.” Founded and incorporated in Louisiana in 1982, Amedisys was reincorporated as a Delaware corporation prior to becoming a publicly traded company in August 1994.
Our strategy is to be the best choice for care wherever our patients call home. We accomplish this by providing clinically distinct care, being the employer of choice and delivering operational excellence and efficiency, which when combined, drive growth. Our mission is to provide best-in-class home health, hospice, personal care and personalhigh acuity care services allowing our patients to maintain a sense of independence, quality of life and dignity while delivering industry leading outcomes. We believe that our unwavering dedication to clinical quality and constant focus on both our patients and our employees differentiates us from our competitors.
Our Home Health Segment:
Amedisys Home HealthOur home health segment provides compassionate healthcare to help our patients recover from surgery or illness, live with chronic diseases and prevent avoidable hospital readmissions. Our home health footprint includes 321347 care centers located in 34 states within the United States and the District of Columbia. Within these care centers, we deploy our care teams which include skilled nurses who are trained, licensed and certified to administer medications, care for wounds, monitor vital signs and provide a wide range of other nursing services; rehabilitation therapists specialized in physical, speech and occupational therapy; and social workers and aides who assist our patients with completing important personal tasks.
We take an empowering approach to helping our patients and their families understand their medical conditions, how to manage them and how to maximize the quality of their lives while living with a chronic disease or other health condition. Our clinicians are trained to understand the whole patient – not just their medical diagnosis.
ThisOur commitment to clinical distinction is most evident in our clinical quality measures such as Star Ratings.the Quality of Patient Care and Patient Satisfaction star ratings. In the CenterCenters for Medicare and Medicaid Services (“CMS”) reports for the January 2020 release,April 2023 preview, the Quality of Patient Care star average across all Amedisys providers is 4.27was 4.49 with 86%99% of our providerscare centers at 4+ stars and 4346 care centers rated at 5+5 stars. Our Patient Satisfaction star average for the January 20202023 release was 3.71,3.57, outperforming the industry average by 6%1% (April 2023 preview data is not available for this metric). Our goal is to have all care centers achieve a 4.0 Quality Star Rating,of Patient Care star rating, and we are implementinghave implemented targeted action plans to continue to improve the quality of care we deliver for our patients and further our culture of quality.
Our Hospice Segment:
Hospice care is designed to provide comfort and support for those who are dealing with a terminal illness. It is a benevolent form of care that promotes dignity and affirms quality of life for the patient, family members and other loved ones. Individuals with a terminal illness such as cancer, heart disease, pulmonary disease Alzheimer’s or cancerAlzheimer’s may be eligible for hospice care if they have a life expectancy of six months or less.
We operate 138 Our hospice care centers in 33 states within the United States. Within these care centers, we deploy our care teams which include nurse practitioners and other skilled nurses, social workers, aides, bereavement counselors and chaplains.
At Amedisys Hospice, ourOur focus is on building and retaining an exceptional team, delivering the highest quality care and service to our patients and their families and establishing Amedisys as the preferred and preeminent hospice provider in each community we serve. In order to realize these goals, we invest in tailored training development and recognition programsdevelopment for our employees including medical record training, employee skills training and leadership development. Thiswhich has led to our team’s consistent achievement at or above the national average in family satisfaction results and quality scores, as well as the trust of the healthcare community.
Another element of our approach is our outreach strategy to more fully engage the entire community of eligible patients. These outreach efforts have built our hospice patient population to more accurately represent the causes of death in the communities we serve, with a specific focus on heart disease, lung disease and dementia in order to address the historical underrepresentation of non-cancer diagnoses.
By working to accept every eligible patient who seeks end-of-life care, we fulfill our hospice mission and strengthen our standing in the community.
During 2019, we acquired Compassionate Care Hospice ("CCH") and RoseRock Healthcare ("RoseRock"). On January 1, 2020, we acquired Asana Hospice. With these acquisitions, Amedisys now owns and operates 146 hospice care centers in 33 states, providing care to more than 12,000 patients daily as the third largest hospice provider in the United States.
Our Personal Care Segment:
Personal care provides assistance with the essential activities of daily living. We believe that personal care services are highly synergistic with our core skilled home health and hospice businesses, and that by expanding these capabilities, we will be able to provide our patients and payor partners with a true continuum of care.
Amedisys acquired its first personal care company in 2016 – an important step in executing our strategy of improving the continuity of care our patients receive as their clinical needs change. Weand continued our strategy to expand ourthe personal care segment with four additional acquisitions andacquisitions. We currently operate 1011 personal-care care centers in Massachusetts and one personal-care care center in botheach Florida and Tennessee.
In July 2019,On February 10, 2023, we signed ana definitive agreement with ClearCare, Inc. ("ClearCare"), the provider of theto sell our personal care industry’sbusiness (excluding the Florida operations). The divestment is expected to close during the second quarter of 2023. See Item 8, Note 6 - Assets Held For Sale for additional information.
Our High Acuity Care Segment:
The acquisition of Contessa Health ("Contessa") on August 1, 2021 established our high acuity care segment. Our high acuity care segment has the capability to deliver the essential elements of inpatient hospital, SNF care and palliative care to patients in their homes. In connection with the acquisition of Contessa, we obtained interests in a professional corporation that employs clinicians and several joint ventures with health system partners. Additionally, the acquisition provided the Company with an advanced claims analytic platform, network management and additional capabilities to enter into risk-based arrangements with managed care organizations.
Our joint venture partners in the high acuity care segment represent national and large regional healthcare systems, each of which view the ability to provide inpatient level care in patients’ homes as critical to relieving capacity constraints within their facilities, providing care in a more cost-effective setting and keeping patients engaged with their health system brand by providing a superior patient experience. The patients who utilize our home-based recovery services typically have one or more chronic conditions that have historically required frequent emergency department visits and inpatient hospital stays. Our patient satisfaction scores for these home-based programs have consistently exceeded 85%, and we have successfully reduced hospital and skilled nursing readmission rates compared to historical baselines for these episodes of care.
We provide management services to the joint ventures which include the development and implementation of clinical protocols to ensure the safe and efficient delivery of services in the home and high quality outcomes; an internally-developed technology platform that provides medical documentation, analytics and claims processing capabilities; provider network development services to ensure that all care resources are available to meet patient needs; and expertise in developing and negotiating contracts with third party health insurance payors to provide reimbursement for services in risk-based arrangements. Our expertise and capabilities in these areas deliver value to both the health system and the health insurance payor and give us the opportunity for future expansion within the healthcare continuum for chronically ill patients, including palliative care services, especially as the U.S. population ages and consumer preferences continue to shift to home-based care. Our joint venture partnership model with leading software platform, representing 4,000healthcare systems and our relationships with health plan insurers facilitate our ability to take and manage additional risk for this patient population in value-based arrangements.
Network Partnerships:
We have a Care Coordination Agreement with BrightStar Care to add its agencies to the Amedisys personal care agencies in every zip code innetwork, which helps facilitate the United States. Our agreement with ClearCare creates an opportunity to establishcoordination of care between our home health and hospice care centers and a network partnership between Amedisys and personal care agencies using ClearCare in order to better coordinate patient care. Long term, we believe this allows us to build a nation-wide network of personal care agencies and furtherspartners. We believe this agreement will further our efforts to provide patients with a true care continuum in the home. This relationship will also help us as we continue to have innovative payment conversations with Medicare Advantage plans who have begun to recognize the value that combined home health, hospice, personal care and personalhigh acuity care services bring to their members and care delivery infrastructure.
Responding to the Changing Regulatory and Reimbursement Environment:
As the government continues to seek opportunities to refine payment models, we believe that our strategy of becoming a leader in providing a range of serviceservices across the at-home continuum positions us well for the future. Our ability to provide quality home health, hospice, personal care and personalhigh acuity care allows us to partner with health systems and managed care organizations to improve care coordination, reduce hospitalizations and lower costs.
Acquisitions:Innovations:
On February 1, 2019,In the coming year, our core business innovations will consist of workforce optimization with a focus on new ways to engage, recruit and retain our clinical staff, clinical optimization and reorganization initiatives and continuing to differentiate our service offerings as we acquired Compassionate Care Hospice,build out our aging-in-place capabilities. The acquisition of Contessa in 2021 will also be a nationwide hospice provider headquartered in Parsippany, New Jersey,platform for a purchase pricecontinued innovations as we expand Contessa’s lines of $327.9 million, net of cash acquired of $6.7 million.business, including palliative care at home.
Acquisitions:
On April 1, 2019,2022, we acquired RoseRock15 home health care centers from Evolution Health, LLC, a division of Envision Healthcare, an Oklahoma based hospice provider, for a purchase pricedoing business as Guardian Healthcare, Gem City, and Care Connection of $17.5 million.
On January 1, 2020, we acquired Asana Hospice, a hospice provider withCincinnati ("Evolution") and two home health locations in Pennsylvania, Ohio, Texas, Missourifrom AssistedCare Home Health, Inc. and Kansas, for a purchase priceRH Homecare Services, LLC, doing business as AssistedCare Home Health and AssistedCare of $67.0 million.the Carolinas ("AssistedCare").
Financial Information:
Financial information for our home health, hospice, personal care and personalhigh acuity care segments can be found in our consolidated financial statements included in this Annual Report on Form 10-K.
Our Employeesemployees are critical to our vision to be the leading aging-in-place company. Taking care of our people is our top priority. Our success is directly correlated with our ability to continue to attract, develop and retain the most qualified and passionate employees. Our work is not just a job but a calling. Our workforce strategy emanates from our core values of SPIRIT - Service, Passion, Integrity, Respect, Innovation and Talent. We know that by taking great care of our people, they can continue to provide industry leading patient care.
As of February 14, 2020,10, 2023, we employed approximately 21,30020,000 people throughout the United States. We also utilize contract employees consistingin the normal course of approximately 11,600 home healthour business.
Diversity and Inclusion:
We endeavor to create a culture of caregiving where our employees 5,800 hospicefeel as cared for every day as our patients. Success means all team members feel a sense of belonging, support and empowerment to be their best selves personally and professionally. We have committed to giving our employees 3,000 personal carea voice and have instituted numerous formal listening programs including quarterly pulse surveys, focus groups and town halls to routinely gather feedback from our employees and 900 corporateaddress any concerns. Our commitment to diversity and divisionalinclusion is also broadly reflected across our policies and people practices. Under the leadership of our employee-led Diversity and Inclusion Council, over 1,100 leaders participated in diversity and inclusion training designed to support employees.a positive and inclusive work environment during 2022. Additionally, we have four Employee Resource Groups ("ERGs") which foster connection and community within our workforce: (1) Global Black Community, (2) LGBTQIA+, (3) disAbilities and (4) Military/Veterans. We are also committed to having a diverse Board of Directors. Women currently comprise over half of the directors on our Board.
Talent Acquisition, Retention and Development:
We strive to hire, develop and retain top talent. The core of our care delivery model is dependent upon attracting clinicians, predominately nurses. We compete for talent by offering a great culture, an opportunity to provide the highest quality clinical care and competitive market-based compensation. Our compensation plans are designed to deliver a competitive base pay as well as attractive incentive opportunities, primarily for leadership positions, but also to reward quality care. We provide significant opportunities for development and continuing education as we know that career development is a key component of attracting and retaining top talent. We continually monitor and assess employee metrics on hiring, retention and terminations to gain a deep understanding of our workforce and drive continuous improvement.
The impact of the novel coronavirus pandemic ("COVID-19") and demand for clinicians has generated continuing pressure on the labor markets. Clinicians have become harder to recruit and more costly to employ. Attracting the best people in healthcare and supporting our people with an unrivaled experience are key initiatives for the Company to ensure adequate clinical capacity for our patients.
Health and Safety:
The health and well-being of our employees is of utmost importance to us. We offer a comprehensive benefit package that provides employees and their families with access to a variety of innovative, flexible and convenient health and wellness programs that support their physical and mental health by providing tools and resources to help them improve or maintain their health status.
Payment for Our Services
Home Health Medicare
The Medicare home health benefit is available both for patients who need home care following discharge from a hospital and patients who suffer from chronic conditions that require ongoing, but intermittent, care.
As a condition of participation under Medicare, beneficiaries must be homebound (meaning that the beneficiary is unable to leave his/her home without a considerable and taxing effort), require intermittent skilled nursing, physical therapy or speech therapy services, and receive treatment under a plan of care established and periodically reviewed by a physician.
Until recently, Medicare payment rates were based on the severity of the patient’s condition, his or her service needs and other factors relating to the cost of providing services and supplies, bundled into 60-day episodes of care (see discussion of the Patient-Driven Groupings Model ("PDGM") below). An episode starts with the first day a billable visit is performed and ends 60 days later or upon discharge, if earlier. If a patient is still in treatment on the 60th day, a recertification assessment is undertaken to determine whether the patient needs additional care. If the patient’s physician determines that further care is necessary, another episode begins on the 61st day (regardless of whether a billable visit is rendered on that day) and ends 60 days later. The first day of a consecutive episode, therefore, is not necessarily the new episode’s first billable visit.
Annually, the Medicare program base rates are set through federal legislation, as follows:
|
| | | |
| Period | Base Episode Payment |
| January 1, 2017 through December 31, 2017 | $ | 2,990 |
|
| January 1, 2018 through December 31, 2018 | $ | 3,040 |
|
| January 1, 2019 through December 31, 2019 | $ | 3,154 |
|
| January 1, 2020 through December 31, 2020 (only applies to episodes beginning on December 31, 2019 or prior) | $ | 3,221 |
|
The CMS Calendar Year 2020 Home Health Final Rule, which became effective January 1, 2020, sets forth a change in the unit of payment from 60-day episodes of care to 30-day periods of care. The table below includes the 30-day payment rate.
|
| | | |
| Period | Base 30-Day Payment |
| January 1, 2020 through December 31, 2020 (only applies to episodes beginning on January 1, 2020 and thereafter) | $ | 1,864 |
|
Medicare payments may be adjusted up or down as a result of one or more of the following: (a) an outlier payment if a patient’s care was unusually costly (capped at 10% of total reimbursement per provider number); (b) a low utilization payment adjustment (“LUPA”) if the number of visits during the episode was four or fewer; (c) a partial payment if a patient transferred to another provider or we admitted a patient transferring from another provider before an episode was complete; (d) a payment adjustment based upon the level of therapy services required (with various incremental adjustments made for additional visits, with larger payment increases associated with the sixth, fourteenth and twentieth visit thresholds); (e) the number of episodes of care provided to a patient, regardless of whether the same home health provider provided care for the entire series of episodes; (f) changes in the base payments established by the Medicare program and (g) adjustments to the base payments for case mix and geographic wages.
In November 2018, CMS issued a final rule that updated the Medicare Home Health Prospective Payment System ("HHPPS") rates and wage index for calendar year ("CY") 2019. The final rule resulted in a 2.2 percent increase in payments to Home Health agencies ("HHA") in CY 2019. In October 2019, CMS issued a final rule that finalized the implementation of an alternative case-mix adjustment methodology, the PDGM, that became effective January 1, 2020. See "Home Health Payment Reform" below and Part II, Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations: Overview - Payment" for additional information on the most recent regulation from CMS.
As a Medicare provider, we are subject to periodic audits by the Medicare program, and that program has various rights and remedies against us if they assert that we have overcharged the program or failed to comply with program requirements. Home
Health providers are subject to pre- and post-payment reviews for compliance with Medicare coverage guidelines and medical necessity. Adjustments on this basis may include individual claims adjustments or overpayment determinations based on an extrapolated sample of claims. Medical necessity reviews evaluate whether services are clinically appropriate in terms of frequency, type, extent, site and duration. Technical billing and documentation reviews focus on documentation of services. Medicare and other payors may reject or deny claims for payment if the underlying paperwork does not support the medical necessity of services or fails to establish satisfaction of a coverage rule; such as if a provider is unable to perform periodic therapy assessments required by coverage criteria or cannot provide appropriate billing documentation, acceptable physician authorizations or face-to-face meeting documentation.
Medicare can reopen previously filed and reviewed claims and deny coverage of the services and require us to repay any overcharges, as well as make deductions from future amounts due to us. In the ordinary course of business, we appeal the Medicare and Medicaid program's denial of claims in an effort to recover the denied claims.
Home Health Non-Medicare
Payments from Medicaid and private insurance carriers are episodic-based rates or per-visit rates depending upon the terms and conditions established with such payors. Episodic-based rates paid by our non-Medicare payors are paid in a similar manner and subject to the same adjustments as discussed above for Medicare; however, these rates can vary based upon negotiated terms which generally range from 90% to 100% of Medicare rates. Approximately 15% to 20% of our negotiated managed care contract volume affords us the opportunity to receive additional payment if we achieve certain quality or process metrics as defined in each contract.
Hospice Medicare
The Medicare hospice benefit is available when a physician and specific clinical findings support a diagnosis of a terminal condition where the patient has a terminal timeline of six months or less. Hospice care is evaluated in benefit periods; two 90-day benefit periods followed by an unlimited number of 60-day benefit periods. Payments are based on daily rates for each day a beneficiary is enrolled in the hospice benefit. The daily payment rates are intended to cover costs that hospices incur in furnishing services identified in patients' care plans, based on specific levels of care. Payments are adjusted by a wage index to reflect health care labor costs across the country and are established annually through federal legislation. Payments are made according to a fee schedule that has four different levels of care: routine home care, continuous home care, inpatient respite care and general inpatient care.
Medicare payment is provided for two separate payment rates for routine care: payments for the first 60 days of care and care beyond 60 days. In addition to the two routine rates, on January 1, 2016, Medicare also began reimbursing for a service intensity add-on (“SIA”). The SIA is based on visits made in the last seven days of life by a registered nurse (“RN”) or medical social worker (“MSW”) for patients in a routine level of care.
Adjustments for medical necessity and technical billing requirements may be made to Medicare revenue based on the same claims processing or medical necessity reviews described above for Home Health services when we find we are unable to obtain appropriate billing documentation, authorizations or face-to-face documentation and other reasons unrelated to credit risk.
Two caps limit the amount and cost of care that any individual hospice provider number provides in a single year. Generally, each hospice care center has its own provider number; however, where we have created branch care centers to help our parent care centers serve a geographic location, the parent and branch have the same provider number.
| |
• | Inpatient Cap -One cap limits the number of days of inpatient care an agency may provide to not more than 20 percent of its total patient care days. The daily Medicare payment rate for any inpatient days of service that exceed the cap is set at the routine home care rate, and the provider is required to reimburse Medicare for any amounts it receives in excess of the cap.
|
| |
• | Overall Payment Cap -The other cap is an absolute dollar limit on the average annual payment per beneficiary a hospice agency can receive. This cap is calculated by the Medicare fiscal intermediary at the end of each hospice cap period to determine the maximum allowable payments per provider number. We estimate our potential cap exposure using information available for both inpatient day limits as well as per beneficiary cap amounts. The total cap amount for each provider is calculated by multiplying the number of beneficiaries electing hospice care during the period by a statutory amount that is indexed for inflation.
|
Payment rates for hospice care, the hospice cap amount and the hospice wage index are updated annually according to Section 1814(i)(1)(C)(ii)(VII) of the Social Security Act, which requires CMS to use the inpatient hospital market basket, adjusted for multifactor productivity (MFP) and other adjustments as specified in the Social Security Act, to determine the hospice payment update percentage. The caps are subject to annual and retroactive adjustments, which can cause providers to be required to reimburse
the Medicare program if such caps are exceeded. Our ability to stay within these caps depends on a number of factors, each determined on a provider number basis, including the average length of stay and mix in level of care.
Our revenues are derived in large part from governmental third-party payors. There are budget pressures from government and other payors to control health care costs and to reduce or limit increases in reimbursement rates for health care services. Governmental payment programs are subject to statutory and regulatory changes, retroactive rate adjustments, administrative or executive orders and government funding restrictions, all of which may materially increase or decrease the rate of program payments to us for our services. It is possible that future budget cuts in Medicare and Medicaid may be enacted by Congress and implemented by CMS. Therefore, we cannot assure you that payments from governmental or private payors will remain at levels comparable to present levels or will, in the future, be sufficient to cover the costs allocable to patients eligible for reimbursement pursuant to such programs. See Part II, Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations: Overview - Payment"– CMS Payment Updates" for additional information on the most recent regulationregulations from CMS.
Home Health Medicare
The Medicare home health benefit is available both for patients who need home care following discharge from a hospital and patients who suffer from chronic conditions that require ongoing, but intermittent, care.
As a condition of participation under Medicare, beneficiaries must be homebound (meaning that the beneficiary is unable to leave his/her home without a considerable and taxing effort), require intermittent skilled nursing, physical therapy or speech therapy services and receive treatment under a plan of care established and periodically reviewed by a physician. In order to provide greater flexibility during COVID-19, CMS has relaxed the definition of homebound status through the duration of the public health emergency. During the pandemic, a beneficiary is considered homebound if they have been instructed by a physician not to leave their house because of a confirmed or suspected COVID-19 diagnosis or if the patient has a condition that makes them more susceptible to contracting COVID-19.
Services under the Medicare home health benefit are bundled into 60-day episodes of care. An episode starts the first day a billable visit is performed and ends 60 days later or upon discharge, if earlier. If a patient is still in treatment on the 60th day, a recertification assessment is undertaken to determine whether the patient needs additional care. If the patient’s physician determines that further care is necessary, another episode begins on the 61st day (regardless of whether a billable visit is rendered on that day) and ends 60 days later.
Effective January 1, 2020, CMS implemented a revised case-mix adjustment methodology, the Patient-Driven Groupings Model ("PDGM"). PDGM uses a 30-day period of care rather than a 60-day episode of care as the unit of payment, eliminates the use of the number of therapy visits provided in determining payment and relies more heavily on clinical characteristics and other patient information. Under PDGM, each 60-day episode includes two 30-day payment periods. The table below includes the base 30-day payment rates.
| | | | | |
| Period | Base 30-Day Payment |
| January 1, 2020 through December 31, 2020 (only applies to episodes beginning on January 1, 2020 and thereafter) | $ | 1,864 | |
| January 1, 2021 through December 31, 2021 | $ | 1,901 | |
| January 1, 2022 through December 31, 2022 | $ | 2,032 | |
| January 1, 2023 through December 31, 2023 | $ | 2,011 | |
On October 31, 2022, CMS issued the Home Health Final Rule for Medicare home health providers for calendar year 2023. CMS estimates that the final rule will result in a 0.7% increase in payments to home health providers. This increase is the result of a 4.0% payment update (4.1% market basket adjustment less a 0.1% productivity adjustment) and an increase of 0.2% for the update to the fixed-dollar loss ratio used in determining outlier payments offset by a permanent adjustment of -3.5% based on the difference between assumed and actual behavioral changes resulting from the implementation of PDGM. The -3.5% permanent adjustment is derived from a -3.925% behavioral assumption adjustment. In the Calendar Year 2023 Preliminary Rule, CMS proposed a behavioral assumption adjustment of -7.69%. CMS revised the adjustment to -7.85% in the final rule and also reduced it by half (to -3.925%) in order to mitigate such a significant reduction to reimbursement in a single year. The remaining -3.925% behavioral assumption adjustment will be considered in future rulemaking. The final rule also finalizes a permanent 5% cap on negative wage index changes for home health agencies. Based on our analysis of the final rule, we expect our impact to be flat, which is less than the estimated 0.7% rate increase.
In addition to the permanent adjustments, CMS is also considering a temporary adjustment of approximately $2 billion to offset overpayments in calendar years 2020 and 2021. CMS has elected not to apply the temporary adjustment to calendar year 2023; however, CMS is still considering how to best apply the adjustment in future rulemaking.
PDGM uses timing, admission source, functional impairment levels and principal and other diagnoses to case-mix adjust payments. The case-mix adjusted payment for a 30-day period of care is subject to additional adjustments based on certain variables, including, but not limited to (a) an outlier payment if our patient's care was unusually costly (capped at 10% of total reimbursement per provider number); (b) a low utilization payment adjustment (“LUPA”) if the number of visits provided was less than the established threshold, which ranges from two to six visits and varies for every case-mix group under PDGM; (c) a partial payment if a patient transferred to another provider or from another provider before completing the 30-day period of care; and (d) the applicable geographic wage index. Payments for routine and non-routine supplies are included in the 30-day payment rate.
As a Medicare provider, we are subject to periodic audits by the Medicare program, and that program has various rights and remedies against us if they assert that we have overcharged the program or failed to comply with program requirements. Home health providers are subject to pre- and post-payment reviews for compliance with Medicare coverage guidelines and medical necessity. Adjustments on this basis may include individual claims adjustments or overpayment determinations based on an extrapolated sample of claims. Medical necessity reviews evaluate whether services are clinically appropriate in terms of frequency, type, extent, site and duration. Technical billing and documentation reviews focus on documentation of services. Medicare and other payors may reject or deny claims for payment if the underlying documentation does not support the medical necessity of services or fails to establish satisfaction of a coverage rule, such as if a provider is unable to perform periodic therapy assessments required by coverage criteria or cannot provide appropriate billing documentation, acceptable physician authorizations or face-to-face meeting documentation.
Medicare can reopen previously filed and reviewed claims and deny coverage of the services and require us to repay any overcharges, as well as make deductions from future amounts due to us. In the ordinary course of business, we appeal the Medicare and Medicaid program's denial of claims that we believe are inappropriate in an effort to recover the denied claims.
Home Health Non-Medicare
Payments from non-Medicare payors are either a percentage of Medicare rates, per-visit rates or case rates depending upon the terms and conditions established with such payors. Reimbursements from our non-Medicare payors that are based on Medicare rates are paid in a similar manner and subject to the same adjustments as discussed above for Medicare; however, these rates can vary based upon negotiated terms which generally range from 95% to 100% of Medicare rates. Approximately 30% of our managed care contract volume affords us the opportunity to receive additional payments if we achieve certain quality or process metrics as defined in each contract (e.g. star ratings and acute-care hospitalization rates).
Hospice Medicare
The Medicare hospice benefit is available when a physician and specific clinical findings support a diagnosis of a terminal condition where the patient has a terminal diagnosis of six months or less. Hospice care is evaluated in benefit periods: two 90-day benefit periods followed by an unlimited number of 60-day benefit periods. Payments are based on daily rates for each day a beneficiary is enrolled in the hospice benefit. Payments are made according to a fee schedule that has four different levels of care: routine home care, continuous home care, inpatient respite care and general inpatient care. The daily payment rates are intended to cover costs that hospices incur in furnishing services identified in patients' care plans, based on specific levels of care. Payments are adjusted by a wage index to reflect health care labor costs across the country and are established annually through federal legislation.
Medicare payments include two separate payment rates for routine care: payments for the first 60 days of care and care beyond 60 days. In addition to the two routine rates, Medicare also reimburses for a service intensity add-on (“SIA”). The SIA is based on visits made in the last seven days of life by a registered nurse or medical social worker for patients in a routine level of care.
Adjustments for eligibility and technical billing requirements may be made to Medicare revenue based on the same claims processing reviews described above for home health services when we find we are unable to obtain appropriate billing documentation, authorizations or face-to-face documentation and other reasons unrelated to credit risk.
Two caps limit the amount of payment that any individual hospice provider number can receive in a single year. Generally, each hospice care center has its own provider number; however, where we have created branch care centers to help our parent care centers serve a geographic location, the parent and branch have the same provider number.
•Inpatient Cap: The inpatient cap limits the number of days of inpatient care an agency may provide to not more than 20 percent of its total patient care days. The daily Medicare payment rate for any inpatient days of service that exceed the cap is set at the routine home care rate, and the provider is required to reimburse Medicare for any amounts it receives in excess of the cap.
•Overall Payment Cap: The overall payment cap is an absolute dollar limit on the average annual payment per beneficiary a hospice agency can receive. This cap is calculated by the Medicare Administrative Contractor at the end of each hospice cap period to determine the maximum allowable payments per provider number.
We estimate our potential cap exposure using information available for both inpatient day limits as well as per beneficiary cap amounts. The total cap amount for each provider is calculated by multiplying the number of beneficiaries electing hospice care during the period by a statutory amount that is indexed for inflation.
Payment rates for hospice care, the hospice cap amount and the hospice wage index are updated annually according to Section 1814(i)(1)(C)(ii)(VII) of the Social Security Act ("SSA"), which requires CMS to use the inpatient hospital market basket, adjusted for multifactor productivity and other adjustments as specified in the SSA, to determine the hospice payment update percentage. The caps are subject to annual and retroactive adjustments, which can cause providers to be required to reimburse the Medicare program if such caps are exceeded. Our ability to stay within these caps depends on a number of factors, each determined on a provider number basis, including the average length of stay and mix in level of care.
Hospice Non-Medicare
Non-Medicare payors pay at rates that differ from established Medicare rates for hospice services, and are based on separate, negotiated agreements. We bill and are paid by these non-Medicare payors based on such negotiated agreements.
Personal Care Non-Medicare
Personal care payments are received from payor clients, including state and local governmental agencies, managed care organizations, commercial insurers and private consumers, based on rates that are either contractual or fixed by legislation.
High Acuity Care
High acuity care payments are derived from health insurance plans, health system partners and Medicare and non-Medicare home health payors. Contracts with health insurance plans provide for fixed payment rates for a 30-day or 60-day episode of care indexed to assigned patient diagnoses in return for our obligation to assume risk for the coordination and payment of required medical services necessary to treat the medical condition for which the patient was diagnosed in a home-based setting. Contracts with health system partners provide for payments on a per diem basis at the contracted rate for each day during the remainder of an inpatient acuity stay serviced at the patient's home. Payments for home health services are similar to those described above.
The contracted payment rates with health insurance plans and health system partners are developed by our medical economics team using historical claims and inpatient admission data provided by the respective health insurance plan or health system partner. The data includes medical costs incurred outside of a patient’s historical inpatient stay that may be expected to continue under our program and an estimate of the cost of the medical services under our program which will replace the patient’s inpatient hospital stay. We mitigate the risk of excessive program medical costs by ensuring that we enroll eligible members into the plan, by effectuating clinically effective plans of care and by ensuring that all covered services are related to the condition for which the patient was admitted to the program. Additionally, we have purchased episodic stop-loss insurance for certain payor contracts.
Controls Over Our Business System Infrastructure
We establish and maintain processes and controls over coding, clinical operations, billing, patient recertifications and compliance to help monitor and promote adherence with Medicare requirements.
| |
• | •Coding – Specified international classification of disease ("ICD") diagnosis codes are assigned to each of our patients based on their particular health conditions (such as diabetes, coronary artery disease or congestive heart failure). Because coding regulations are complex and are subject to frequent change, we maintain controls surrounding our coding process. To reduce the associated risk of coding failures, we provide annual update training to clinical managers, as needed training to care center directors and clinical managers and training during orientation for new employees to ensure accurate information is gathered and provided to our coding team. In addition, our electronic medical records system (Homecare Homebase) includes automated edits for home health and hospice based on pre-defined compliance metrics. For home health, we also provide monthly specialized coding education, obtain outside expert coding instruction and have certified coders review all patient outcome and assessment information sets (“OASIS”) and assign the appropriate ICD code. Specified international classification of disease ("ICD") diagnosis codes are assigned to each of our patients based on their particular health conditions (such as diabetes, coronary artery disease or congestive heart failure). Because coding regulations are complex and are subject to frequent change, we maintain controls surrounding our coding process. To reduce the associated risk of coding failures, we provide annual update training to clinical managers, as needed training to care center directors and clinical managers and training during orientation for new employees to ensure accurate information is gathered and provided to our coding team. Our electronic medical records system (Homecare Homebase) includes automated home health coding edits based on pre-defined compliance metrics. For home health, we also provide monthly specialized coding education, obtain outside expert coding instruction and have certified clinician coders review all patient outcome and assessment information sets (“OASIS”) and assign the appropriate ICD code. Additional training for coders, clinicians, office staff and business development teams occurred throughout 2019 to ensure all coding practices and guidelines are being followed in preparation for the implementation of PDGM. |
| |
• | Clinical Operations – Regulatory requirements allow patients to be eligible for home health care benefits if through a face-to-face visit with a physician, they are considered homebound and it is determined that skilled nursing, physical therapy or speech therapy services are required. These clinical services may include: educating the patient about their disease, assessment and observation of disease status, delivery of clinical skills such as wound care, administration of injections or intravenous fluids, management and evaluation of a patient’s plan of care, physical therapy services to assist patients with functional limitations and speech therapy services for speech or swallowing disorders. Patients eligible for hospice care are terminally ill (with a life expectancy of six months or less if the illness runs its normal course). Our hospice program provides care and support to our terminally ill patients with a 6-month prognosis and their families with services including medical care, counseling, medication management and needed equipment and supplies for the terminal illness and all related conditions. We provide education on Medicare Guidelines for Coverage and Conditions of Participation and utilize outside expert regulatory services if necessary.
|
| |
• | Billing – We maintain controls over our billing processes to help promote accurate and complete billing; we have annual billing compliance testing; use formalized billing attestations; limit access to billing systems; use automated daily billing operational indicators; and take prompt corrective action with employees who knowingly fail to follow our billing policies and procedures in accordance with a "zero tolerance" policy.
|
| |
• | Patient Recertification – In order to be recertified for an additional episode of care, a patient must continue to meet qualifying criteria and have a continuing medical need. Changes in the patient’s condition may require changes to the patient’s medical regimen or modified care protocols within the episode of care. The patient’s progress towards established
|
•Billing – We maintain controls over our billing processes to help promote accurate and complete billing. Processes and controls have been implemented to ensure that prior to the submission of any bills, the visit/occurrence was completed, documented sufficiently by an appropriate clinician and/or provider, and that the billed claim complies with all regulatory and payor requirements. Examples of process monitoring controls include conducting annual billing compliance testing, user access reviews for billing systems and use of automated daily billing operational indicators. We take prompt corrective action with employees who knowingly fail to follow our billing policies and procedures.
•Patient Recertification – In order to be recertified for an additional home health episode of care, a patient must continue to meet qualifying criteria and have a continuing medical need that requires the skills of a nurse or therapist. Changes in the patient’s condition may require changes to the patient’s medical regimen or modified care protocols within the episode of care. The patient’s progress towards established goals is evaluated prior to recertification. As with the initial episode of care, a recertification requires orders from the patient’s physician. Before any employee recommends recertification to a physician, we conduct a care center level, multidisciplinary care team conference. Specific tools are used to ensure that the patient continues to meet coverage criteria prior to recertifying. Hospice recertification for additional benefit periods of care requires continued demonstration of a terminal prognosis as determined by the hospice physician in collaboration with the attending physician and the interdisciplinary care team.
| |
• | Compliance – We develop, implement and maintain ethics and compliance programs as a component of the centralized corporate services provided to our home health, hospice and personal-care care centers.
•Compliance – We develop, implement and maintain ethics and compliance programs as a component of the centralized corporate services provided to our home health, hospice, personal care and high acuity-care service lines. Our ethics and compliance program includes a Code of Conduct for our employees, officers, directors, contractors and affiliates and a disclosure program for reporting regulatory or ethical concerns to our compliance team through a confidential hotline, which is augmented by exit surveys of departing employees. We promote a culture of compliance within our company through educational presentations, regular newsletters and persistent messaging from our senior leadership to our employees stressing the importance of strict compliance with legal requirements and company policies and procedures. Additionally, we have mandatory compliance training and testing for all new employees upon hire and annually for all staff thereafter. We also maintain a robust compliance audit program focusing on key risk areas. |
Our Regulatory Environment
We are highly regulated by federal, state and local authorities. The healthcare industry is subject to numerous laws, regulations and rules including, among others, those related to government healthcare participation requirements, various licensure and accreditations, reimbursement for patient services, health information privacy and security rules and Medicare and Medicaid fraud and abuse prohibitions (including, but not limited to, federal statutes and regulations prohibiting kickbacks and other illegal inducements to potential referral sources, self-referrals by physicians and false claims submitted to federal health care programs). Regulations and policies frequently change, and we monitor changes through our internal government affairs department, as well as multiple trade and governmental publications and associations.
Our home health and hospice subsidiaries are certified by CMS and therefore are subject to the rules and regulations of the Medicare system. Additionally, all of our business lines are likewise subject to federal, state and local laws and regulations dealing with issues such as occupational safety, employment, medical leave, insurance, civil rights, discrimination, building codes, data privacy, data security and recordkeeping. We have set forth below a discussion of the regulations that we believe most significantly affect our home health and hospice businesses.
Licensure, Certificates of Need (CON)("CON") and Permits of Approval (POA)("POA")
Home health and hospice care centers operate under licenses granted by the health authorities of their respective states. Some states require health care providers (including hospice and home health agencies) to obtain prior state approval for the purchase, construction or expansion of health care locations, capital expenditures exceeding a prescribed amount, or changes in services. For those states that require a CON or POA, the provider must also complete a separate application process establishing a location and must receive required approvals.
CertainAdditionally, certain states, including a number in which we operate, carefully restrict new entrants into the market based on demographic and/or demonstrative usage of additional providers. These states limit the entry of new providers or services and the expansion of existing providers or services in their markets through a CON or POA process, which is periodically evaluated and updated as required by applicable state law. For those states that require a CON or POA, the provider must complete a separate application process establishing a location and must receive required approvals.
To the extent that we require a CON, POA or other similar approvals are required to expand our operations, our expansion could be adversely affected by the inability to obtain the necessary approvals, changes in the standards applicable to those approvals and possible delays and expenses associated with obtaining those approvals. In some instances, other providers in the market may file opposition to a CON or POA application and this could further delay an approval.
In every state where required, our care centers possess a license and/or a CON or POA issued by the state health authority that determines the local service area for the home health or hospice care centers. Currently, state health authorities in 17 states and the District of Columbia require a CON or, in the State of Arkansas, a POA, in order to establish and operate a home health care center, and state health authorities in 912 states and the District of Columbia require a CON to operate a hospice care center.
We operate 231 home health care centers and 47 hospice care centers in the following CON states: Alabama, Arkansas (POA), Georgia, Kentucky, Maine, Maryland, Mississippi, New Jersey, New York, North Carolina, Rhode Island, South Carolina, Tennessee, Washington and West Virginia,CON/POA states as well as the District of Columbia. We provide hospice related services in the following CON states: Alabama, Florida, Maryland, North Carolina, Tennessee and West Virginia.listed below.
| | | | | | | | | | | | | | |
| State | | Home Health | | Hospice |
| Alabama | | 29 | | 10 |
| Arkansas (POA) | | 7 | | — | |
| Florida | | — | | | 6 |
| Georgia | | 56 | | — | |
| Kentucky | | 17 | | — | |
| Maryland | | 9 | | 3 |
| Mississippi | | 8 | | — | |
| New Jersey | | 2 | | — | |
| New York | | 5 | | — | |
| North Carolina | | 13 | | 7 |
| South Carolina | | 26 | | — | |
| Tennessee | | 45 | | 15 |
| Washington | | 2 | | — | |
| West Virginia | | 11 | | 6 |
| Washington, DC | | 1 | | — | |
| Total Care Centers in CON/POA States | | 231 | | 47 |
Medicare Participation: Licensing, Certification and Accreditation
All providers are subject to compliance with various federal, state and local statues and regulations in the U.S. and receive periodic inspection by state licensing agencies to review standards of medical care, equipment and safety. We have dedicated internal resources and utilize external parties when necessary to monitor and ensure compliance with the various applicable federal, state and local laws, rules and regulations.
Our care centers must comply with regulations promulgated by the United States Department of Health and Human Services ("HHS") and CMS in order to participate in the Medicare program and receive Medicare payments. Sections 1861(o) and 1891 of the SSA, 42 CFR 484.1 et seq., establish the conditions that an HHAa home health agency ("HHA") must meet in order to participate in the Medicare program. Section 1861(d)(d)1861(dd) of the SSA, 42 CFR 418.1, et seq.seq., establishes the conditions that a hospice provider must meet in order to participate in the Medicare program. Among other things, these regulations, applicable to HHAs and hospices, respectively, known as “Conditionsconditions of Participation”participation and/or conditions of payment (“COPs”), relate to the type of facility, its personnel and its standards of medical care, as well as its compliance with federal, state and local laws and regulations.
New Additional COPs applicable to HHAs which went into effect on January 13, 2018, focus on the safe delivery of quality care provided to patients and the impact of that care on patient outcomes through the protection and promotion of patients' rights, care planning, delivery and coordination of services and streamlining of regulatory requirements.
CMS has adopted alternative sanction enforcement options which allow CMS (i) to impose temporary management, direct plans of correction or direct training and (ii) to impose payment suspensions and civil monetary penalties in each case on providers out of compliance with the COPs. CMS engages or has engaged a number of third party contractors, including Recovery Audit Contractors (“RACs”), Program Safeguard Contractors (“PSCs”), Zone Program Integrity Contractors (“ZPICs”), Uniform Program Integrity Contractors ("UPICs") and, Medicaid Integrity Contractors (“MICs”) and Supplemental Medical Review Contractors (“SMRCs”), to conduct extensive reviews of claims data and state and Federal Governmentfederal government health care program laws and regulations applicable to healthcare providers. These audits evaluate the appropriateness of billings submitted for payment. In addition to identifying overpayments, audit contractors can refer suspected violations of law to government enforcement authorities.
All providers are subject to compliance with various federal, state and local statutes and regulations in the United States and receive periodic inspection by state licensing agencies to review standards of medical care, equipment and safety. We have dedicated internal resources and utilize external parties when necessary to monitor and ensure compliance with the various applicable federal, state and local laws, rules and regulations, as well as requirements of applicable accrediting organizations.
If we fail to comply with applicable laws and regulations, we could be subjected to liabilities, including criminal penalties, civil penalties (including the loss of our licenses to operate one or more of our businesses) andand/or exclusion of a facility from participation in the Medicare, Medicaid and other federal and state health care programs. If any of our facilities were to lose its accreditation or otherwise lose its certification under the Medicare and Medicaid programs, the facility maywould be unable to receive reimbursement from the Medicare and Medicaid programs and other payors.payors until it gains recertification or accreditation. We believe our facilities are in substantial compliance with current applicable federal, state, local and independent review body regulations and standards. The requirements for licensure, certification and accreditation are subject to change and, in order to
remain qualified, it may become necessary for us to make changes in our facilities, equipment, personnel and services in the future, which could have a material adverse impact on operations.
Regulations and Other Factors
The healthcare industry is subject to numerous laws, regulations and rules including, among others, those related to government healthcare participation requirements, various licensure and accreditations, reimbursement for patient services, health information privacy and security rules, and Medicare and Medicaid fraud and abuse provisions (including, but not limited to, federal statutes and regulations prohibiting kickbacks and other illegal inducements to potential referral sources, false claims submitted to federal health care programs and self-referrals by physicians).
Providers that are found to have violated any of these laws and regulations may be excluded from participating in government healthcare programs, subjected to significant fines or penalties and/or required to repay amounts received from the government for previously billed patient services. Although we believe our policies, procedures and practices comply with governmental regulations, no assurance can be given that we will not be subjected to additional governmental inquiries or actions, or that we would not be faced with sanctions, fines or penalties if so subjected.operations.
Federal and State Anti-Fraud and Anti-KickbackAbuse Laws and Regulations
As a provider under the Medicare and Medicaid systems,programs, we are subject to various anti-fraud and abuse laws, including the Federal health care programs’ anti-kickback statuteAnti-Kickback Statute, the Stark or Physician Self-Referral Law, the False Claims Act, Civil Monetary Penalties Law and where applicable, itsvarious state law counterparts. Affected government health care programs includeanti-fraud and abuse laws. These laws govern any health care plans or programs that are funded by the United States government (other than certain federal employee health insurance benefits/programs), includingas well as certain state health care programs that receive federal funds, such as Medicaid. Our compliance and ethics program is designed to ensure Amedisys meets all applicable federal and state laws and regulations as well as industry standards.
Federal Anti-Kickback Statute ("AKS")
Subject to certain exceptions, these laws prohibitthe federal AKS prohibits any offer, payment, solicitation or receipt of any form of remuneration to induce or reward the referral of business payable under a government health care program or in return for the purchase, lease, order, arranging for, or recommendation of items or services covered under a government health care program. A relatedThe law also forbids the offer or transfer of anything of value, including certain waivers of co-payment obligations and deductible amounts, to a beneficiary of Medicare or Medicaid that is likely to influence the beneficiary’s selection of health care providers, again, subject to certain safe harbor exceptions. Violations of the federal anti-kickback statuteAKS can resulttrigger the False Claims Act and Civil Monetary Penalties Law, potentially resulting in imprisonment, the impositioncivil fines up to $25,076 for each violation, penalties of penalties topping $100,000,
up to $112,131 (last updated 2022) plus three times the amount of the improper remuneration, imprisonment and potentially, exclusion from furnishing services under any government health care program. In addition,There are also criminal penalties under the statesAKS, and providers found to be in which we operate generally have laws that prohibit certain direct or indirect payments or fee-splitting arrangements betweenviolation of the federal AKS can be excluded from participation in the federal health care providers where they are designed to obtain the referral of patients from a particular provider.programs.
Stark or Physician Self-Referral Law
The Social Security Act includes a provision commonlyStark Law, also known as the “Stark Law.” This lawPhysician Self-Referral Law, prohibits physicians from referring Medicare and Medicaid patients to entities for the provision of designated health services with which they or any of their immediate family members have a direct or indirect financial relationship, unless an exception to the law's prohibition is met. These types of referrals are known as “self-referrals.” Sanctions for violating the Stark Law include civil penalties of up to $25,372$27,750 for each violation and up to $169,153$185,009 (last updated 2022) for schemes to circumvent the Stark restrictions and up to $10,000 for each day an entity fails to report required information and exclusion from the federal health care programs.Law restrictions. There are a number of exceptions to the self-referral prohibition, including employment contracts and leases, and recruitment agreements that adheremay be used so long as the arrangement adheres to certain enumerated requirements.
Violations of the Stark Law may also result in payment denials, and may also triggerFalse Claims Act scrutiny, additional civil monetary penalties and federal program exclusion. Several
The False Claims Act
The federal False Claims Act ("FCA") prohibits false claims or requests for payment for health care services. Under the FCA, the government may penalize any person who knowingly submits, or participates in submitting, claims for payment to the Federal Government which are false or fraudulent, or which contain false or misleading information. Any person who knowingly makes or uses a false record or statement to avoid paying the Federal Government, or knowingly conceals or avoids an obligation to pay money to the Federal Government, may also be subject to fines under the FCA. Under the FCA, the term “person” means an individual, company or corporation. The term "knowingly" means the person (i) has actual knowledge of the information; (ii) acts in deliberate ignorance of the truth or falsity of the information; or (iii) acts in reckless disregard of the truth or falsity of the information.
The Federal Government has used the FCA to prosecute Medicare and other governmental program fraud in areas such as violations of the federal Anti-Kickback Statute or the Stark Laws, coding errors, billing for services not provided and submitting false cost reports. The FCA has also been used to prosecute people or entities that bill services at a higher reimbursement rate than is allowed and that bill for care that is not medically necessary. In addition to government enforcement, the FCA authorizes private citizens to bring qui tam or “whistleblower” lawsuits, greatly extending the practical reach of the FCA. The per-claim penalty range is between $23,607 and $25,076 (last updated 2022).
The Fraud Enforcement and Recovery Act of 2009 (“FERA”) amended the FCA with the intent of enhancing the powers of government enforcement authorities and whistleblowers to bring FCA cases. In particular, FERA attempts to clarify that liability may be established not only for false claims submitted directly to the government, but also for claims submitted to government contractors and grantees. FERA also seeks to clarify that liability exists for attempts to avoid repayment of overpayments, including improper retention of federal funds. FERA also included amendments to FCA procedures, expanding
the government’s ability to use the Civil Investigative Demand process to investigate defendants, and permitting government complaints and intervention to relate back to the filing of the whistleblower’s original complaint. FERA is likely to increase both the volume and liability exposure of FCA cases brought against health care providers.
In the Patient Protection and Affordable Care Act (enacted in 2010), Congress enacted requirements related to identifying and returning overpayments made under Medicare and Medicaid. CMS finalized regulations regarding this so-called “60-day rule,” which requires providers to report and return Medicare and Medicaid overpayments within 60 days of identifying the overpayment. A provider who retains identified overpayments beyond 60 days may be liable under the FCA. “Identification” occurs when a person “has, or should have through the exercise of reasonable diligence,” identified and quantified the amount of an overpayment. The final rule also established a six-year lookback period, meaning overpayments must be reported and returned if a person identifies the overpayment within six years of the date the overpayment was received. Providers must report and return overpayments even if they did not cause the overpayment.
In addition to the FCA, the Federal Government may use several criminal statutes to prosecute the submission of false or fraudulent claims for payment to the Federal Government. Many states have similar false claims statutes that impose liability for the types of acts prohibited by the False Claims Act. As part of the Deficit Reduction Act of 2005 (the “DRA”), Congress provides states an incentive to adopt state false claims acts consistent with the federal FCA. Additionally, the DRA requires providers who receive $5 million or more annually from Medicaid to include information on federal and state false claims acts, whistleblower protections and the providers’ own policies on detecting and preventing fraud in their written employee policies.
Civil Monetary Penalties Law
HHS may impose civil monetary penalties ("CMP") for a variety of civil offenses related to federal health care programs. They may be imposed upon any person or entity who presents, or causes to be presented, certain ineligible claims for medical items or services, for providing improper inducements to beneficiaries to obtain services, for payments to limit services to patients, and for offenses related to relationships with excluded individuals, among other things.
Maximum CMP amounts increased in 2022. For example, the penalty for knowing and willful solicitation, receipt, offer or payment of remuneration for referring an individual for a service or for purchasing, leasing or ordering an item to be paid for by a federal health care program increased from $105,563 to $112,131, and the CMP for beneficiary inducement increased from $21,113 to $22,427 per occurrence.
State Laws
In addition to federal laws, some states in which we conduct businessoperate generally have also enacted statutes similarlaws that prohibit kickbacks in scopeexchange for referrals, certain direct or indirect payments or fee-splitting arrangements between health care providers, improper physician referrals, beneficiary inducements and purpose to the federal fraud and abuse laws and the Stark Law. These state laws may mirror the federal Stark Lawfalse or may be different in scope.improperly billed claims. The available guidance and enforcement activity associated with such state laws varies considerably.
We monitor all aspects of our business and have developed a comprehensive ethics and compliance program that is designed to meet or exceed applicablevary considerably, but in some cases may be stricter than federal guidelines and industry standards. Nonetheless, because the law in this area is complex and constantly evolving, there can be no assurance that federal regulatory authorities will not determine that any of our arrangements with physicians violate the Stark Law.law.
Federal and State Privacy and Security Laws
The Health Insurance Portability and Accountability Act of 1996 or HIPAA,("HIPAA") requires us to comply with standards for the exchange of health information within our company and with third parties, such as payors, business associates and patients. These include standards for common health care transactions, such as:as claims information, plan eligibility, payment information and the use of electronic signatures; unique identifiers for providers, employers, health plans and individuals; and security, privacy, breach notification and enforcement.
The HIPAA transactionstransaction regulations establish form, format and data content requirements for most electronic health care transactions, such as health care claims that are submitted electronically. The HIPAA privacy regulations establish comprehensive requirements relating to the use and disclosure of protected health information. The HIPAA security regulations establish minimum standards for the protection of protected health information that is stored or transmitted electronically. The HIPAA breach notification regulations establish the applicable requirements for notifying individuals, the U.S. Department of Health and Human Services (HHS),HHS and the media in the event of a data breach affecting protected health information. Violations of the privacy, security and breach notification regulations are punishable by civil and criminal penalties.
The American Recovery and Economic Reinvestment Act of 2009 (“ARRA”) increased the amount ofCurrently, civil monetary penalties that can be imposed for violations of HIPAA, and the amounts are updated annually for inflation. For 2020, penalties for HIPAA violations can range from $119 to $1.785 million$127 per violation withto a maximum fine of $1.785$1.919 million for identicalmultiple violations of the same provision during a calendar year. In 2018, a nation-wide health benefit company paid $16 million toTo date, the largest penalty imposed by HHS following a data breach. Prior to this record payment, the largest HIPAA fine was $5.55breach is $16 million. ARRA also authorized stateState attorneys general tomay also bring civil enforcement actions under HIPAA, and attorney generalsattorneys general are actively engaged in enforcement. These penalties could be in addition to other penalties assessed by a state for a breach which would be considered reportable under thea particular state’s data breach notification laws.
The Health Information Technology for Economic and Clinical Health (“HITECH”) Act was enacted in conjunction with ARRA. Among other things, the HITECH Act makes business associates of covered entities directly liable for compliance with certain HIPAA requirements, strengthens the limitations on the use and disclosure of protected health information without individual authorizations, and adopts the additional HITECH Act enhancements, including enforcement of noncompliance with HIPAA due to willful neglect. The
Recent changes to HIPAA enacted as part of ARRA reflect a Congressional intent that HIPAA’s privacy and security provisions be more strictly enforced. These changes have stimulated increased enforcement activity and enhanced the potential that health care providers will be subject to financial penalties for violations of HIPAA. In addition, the Secretary of HHS is required to perform periodic audits to ensure covered entities (and their business associates, as that term is defined under HIPAA) comply with the applicable HIPAA requirements, increasing the likelihood that a HIPAA violation will result in an enforcement action.
In addition to the federal HIPAA regulations, most states also have laws that protect the confidentiality of health information and other personally identifiable information, and these laws may be broader in scope with respect to protected health information and other personal data. Certaininformation than HIPAA. Some of these laws grant individualindividuals rights with respect to their information, and wepersonal information. We may be required to expend significant resources to comply with these laws. Further, all 50 states and the District of Columbia have adopted data breach notification laws that impose, in varying degrees, an obligation to notify affected persons and/or state regulators in the event of a data breach or compromise, including when their personal information has or may have been accessed by an unauthorized person. Some state breach notification laws may also impose physical and electronic security requirements regarding the safeguarding of personal information, such as social security numbers and bank and credit card account numbers. Violation of state privacy, security and breach notification laws can trigger significant monetary penalties. In addition, certain states’ privacy, security and data breach laws, including, for example, the California Consumer Privacy Act, as amended by the California Privacy Rights Act, include a private rightrights of action that may expose us to private litigation regarding our privacy practices and significant damages awards or settlements in civil litigation.
The False Claims Act
The Federal False Claims Act ("FCA") prohibits false claims or requests for payment for health care services. Under the FCA, the government may penalize any person who knowingly submits, or participates in submitting, claims for payment to the Federal Government which are false or fraudulent, or which contain false or misleading information. Any person who knowingly makes or uses a false record or statement to avoid paying the Federal Government, or knowingly conceals or avoids an obligation to pay money to the Federal Government, may also be subject to fines under the FCA. Under the FCA, the term “person” means an individual, company or corporation.
The Federal Government has used the FCA to prosecute Medicare and other governmental program fraud in areas such as violations of the Federal anti-kickback statute or the Stark Laws, coding errors, billing for services not provided and submitting false cost reports. The FCA has also been used to prosecute people or entities that bill services at a higher reimbursement rate than is allowed and that bill for care that is not medically necessary. In addition to government enforcement, the FCA authorizes private citizens to bring qui tam or “whistleblower” lawsuits, greatly extending the practical reach of the FCA. In 2018, the Department of Justice ("DOJ") announced that the FCA penalties would once again be increasing. The per-claim penalty range is between $11,463 and $22,927 (last updated 2019).
The Fraud Enforcement and Recovery Act of 2009 (“FERA”) amended the False Claims Act with the intent of enhancing the powers of government enforcement authorities and whistleblowers to bring False Claims Act cases. In particular, FERA attempts to clarify that liability may be established not only for false claims submitted directly to the government, but also for claims submitted to government contractors and grantees. FERA also seeks to clarify that liability exists for attempts to avoid repayment of overpayments, including improper retention of federal funds. FERA also included amendments to False Claims Act procedures, expanding the government’s ability to use the Civil Investigative Demand process to investigate defendants, and permitting government complaints in intervention to relate back to the filing of the whistleblower’s original complaint. FERA is likely to increase both the volume and liability exposure of False Claims Act cases brought against health care providers.
In the Patient Protection and Affordable Care Act (discussed in more detail below), Congress enacted requirements related to identifying and returning overpayments made under Medicare and Medicaid. CMS finalized regulations regarding this so-called “60-day rule,” which requires providers to report and return Medicare and Medicaid overpayments within 60 days of identifying the same. A provider who retains identified overpayments beyond 60 days may be liable under the False Claims Act. “Identification” occurs when a person “has, or should have through the exercise of reasonable diligence,” identified and quantified the amount of an overpayment. The final rule also established a six-year lookback period, meaning overpayments must be reported and returned if a person identifies the overpayment within six years of the date the overpayment was received. Providers must report and return overpayments even if they did not cause the overpayment.
In addition to the False Claims Act, the Federal Government may use several criminal statutes to prosecute the submission of false or fraudulent claims for payment to the Federal Government. Many states have similar false claims statutes that impose liability for the types of acts prohibited by the False Claims Act. As part of the Deficit Reduction Act of 2005 (the “DRA”), Congress provided states an incentive to adopt state false claims acts consistent with the Federal False Claims Act. Additionally, the DRA required providers who receive $5 million or more annually from Medicaid to include information on federal and state false claims acts, whistleblower protections and the providers’ own policies on detecting and preventing fraud in their written employee policies.
Civil Monetary Penalties
The United States Department of Health and Human Services may impose civil monetary penalties ("CMP") for a variety of civil offenses related to federal health care programs. They may be imposed upon any person or entity who presents, or causes to be presented, certain ineligible claims for medical items or services, for providing improper inducements to beneficiaries to obtain
services, for payments to limit services to patients, and for offenses related to relationships with excluded individuals, among other things.
Maximum CMP amounts have been increased significantly as a result of the Bipartisan Budget Act of 2018, which was signed into law on February 9, 2018. The maximum CMP has increased to $102,522 for: (1) knowingly making or causing to be made a false statement, omission or misrepresentation of a material fact in any application, bid or contract to participate or enroll as a provider or supplier (42 CFR 1003.210(a)(6)), and (2) making or using a false record or statement that is material to a false or fraudulent claim (42 CFR 1003.210(a)(7)).
FDA Regulation
The U.S. Food and Drug Administration (“FDA”("FDA") Regulation
The FDA regulates medical device user facilities, which include home health care providers. FDA regulations require user facilities to report patient deaths and serious injuries to the FDA and/or the manufacturer of a device used by the facility if the device may have caused or contributed to the death or serious injury of any patient. FDA regulations also require user facilities to maintain files related to adverse events and to establish and implement appropriate procedures to ensure compliance with the above reporting and recordkeeping requirements. User facilities are subject to FDA inspection, and noncompliance with applicable requirements may result in warning letters or sanctions including civil monetary penalties, injunction, product seizure, criminal fines and/or imprisonment.
The Improving Medicare Post-Acute Care Transformation Act
In October 2014, the Improving Medicare Post-Acute Care Transformation Act (“IMPACT Act”) was signed into law requiring the reporting of standardized patient assessment data for quality improvement, payment and discharge planning purposes across the spectrum of post-acute care providers (“PACs”), including skilled nursing facilities and home health agencies. The IMPACT Act requires PACs to begin reporting:report: (1) standardized patient assessment data at admission and discharge by October 1, 2018 for post-acute care providers, including skilled nursing facilities and by January 1, 2019 for home health agencies;discharge; (2) new quality measures, including functional status, skin integrity, medication reconciliation, incidence of major falls and patient preference regarding treatment and discharge at various intervals between October 1, 2016 and January 1, 2019;discharge; and (3) resource use measures, including Medicare spending per beneficiary, discharge to community and hospitalization rates of potentially preventable readmissions by October 1, 2016 for post-acute care providers, including skilled nursing facilities and by October 1, 2017 for home health agencies.readmissions. Failure to report such data when required would subject a facility to a two percent reduction in market basket prices then in effect.
The IMPACT Act further requires HHS and the Medicare Payment Advisory Commission (“MedPAC”), a commission chartered by Congress to advise it on Medicare payment issues, to study alternative PAC payment models, including payment based upon individual patient characteristics and not care setting, with corresponding Congressional reports required based on such analysis. The IMPACT Act also includedincludes provisions impacting Medicare-certified hospices, including: (1) increasing survey frequency for Medicare-certified hospices to once every 36 months; (2) imposing a medical review process for facilities with a high percentage of stays in excess of 180 days; and (3) updating the annual aggregate Medicare payment cap.
See discussion of the effects of this law on our operations below.
Pre-Claim Review Choice Demonstration for Home Health Services
On June 8, 2016, CMS announced the implementation of a three-year Medicare pre-claim review ("PCR") demonstration for home health services provided to beneficiaries in the states of Illinois, Florida, Texas, Michigan and Massachusetts. The pre-claim review is a process through which a request for provisional affirmation of coverage is submitted for review before a final claim is submitted for payment. On April 1, 2017, CMS paused the PCR Demonstration for Home Health Services while CMS considered a number of changes. CMS revised the demonstration to incorporate more flexibility and choices for providers, as well as risk-based changes to reward providers who show compliance with Medicare home health policies.
On May 31, 2018, CMS issued a notice indicating its intention to re-launch an HHA pre-claim review demonstration project. The original program had drawn criticism that it created significant administrative burdens and reduced access to care. Now called theCMS's Review Choice Demonstration for Home Health Services ("RCD"), the revised demonstration will give gives HHAs in the demonstration states 3three options: pre-claim review of all claims, post-payment review of all claims, or minimal post-payment review with a 25% payment reduction for all home health services. Under the pre-claim review and post-payment review options, provider claims are reviewed for every episode of care until the appropriate claim approval rate (90% based on a minimum of 10ten pre-claim requests or claims submitted) is reached. Further, once the appropriate claim approval rate is reached, a provider can elect to opt-out of claim reviews except for a spot check of 5% of its claims to ensure continued compliance. Amedisys has elected the pre-claim review option. The demonstration initially applies to HHA providers in Florida, Illinois, North Carolina, Ohio and Texas, with the option to expand after 5five years to other states in the
Medicare Administrative Contractor Jurisdiction M (Palmetto). In an October 21, 2019 release, CMS announced that it will reschedule the next phaseAfter several delays, RCD has been fully implemented in all five states as of its RCD to allow agencies time to transition to PDGM. RCD implementation will resume on March 2, 2020 in Texas, followed by demonstrations in North Carolina and Florida on May 4, 2020. However, CMS officials have indicated that these dates are subject to change. The choice selection period began on January 15, 2020 and will end on February 13, 2020 for HHAs located in Texas. Following the close of the choice selection period, the demonstration is expected to begin in Texas on March 2, 2020, and all periods of care starting on or after this date will be subject to the requirements of the choice selected.April 1, 2022.
Following the start of the demonstration in Texas, the demonstration is expected to begin in North Carolina and Florida on May 4, 2020. CMS will monitor the transition to the PDGM and assess the need for any change to this date.
Home Health Value-Based Purchasing
On January 1, 2016, CMS implemented Home Health Value-Based Purchasing ("HHVBP"). The HHVBP model was designed to give Medicare-certified home health agencies incentives or penalties, through payment bonuses, to giveprovide higher quality and more efficient care. HHVBP was rolled out to nine pilot states: Arizona, Florida, Iowa, Maryland, Massachusetts, Nebraska, North Carolina, Tennessee and Washington, seveneight of which Amedisys currently has home health operations. Bonuses and penalties began in 2018 with the maximum of plus or minus 3% growing to plus or minus 8% by 2022. Payment adjustments arewere calculated based on performance in 20a variety of measures which include currentincluded Quality of Patient Care and Patient Satisfaction star measures, as well as measures based on submission of data to a CMS web portal. The measures used may be subject to modification or change by CMS.
Under the demonstration, agencies with higher performance receivereceived bonuses, while those with lower scores receivereceived lower payments relative to current levels. Agency performance iswas evaluated against separate improvement and attainment scores, with payment tied to the higher of these two scores. CMS used 2015 as the baseline year for performance, with 2016 as the first year for performance measurement. The first payment adjustment began January 1, 2018, based on 2016 performance data. Between 2018
In January 2021, CMS and the Center for Medicare and Medicaid Innovation announced its intention, through rulemaking, to expand HHVBP with an implementation date no earlier than January 2022. In November 2021, CMS issued the Calendar Year 2022 Home Health Final Rule for Medicare home health providers which provided for the expansion of the HHVBP model to all 50 states beginning January 1, 2023 with calendar year 2023 being the first performance year and calendar year 2025 being the first payment year with a proposed maximum payment adjustment, varies (upwardup or downward) from 3 percent to 8 percent.down, of 5%. In doing so, the final payment year of the HHVBP demonstration (2022) was cancelled.
Home Health Payment Reform
On February 9, 2018, Congress passed the Bipartisan Budget Act of 2018 ("BBA of 2018"), which funded government operations, set two-year government spending limits and enacted a variety of healthcare related policies. Specific to home health, the BBA of 2018 providesprovided for a targeted extension of the home health rural add-on payment, a reduction of the 2020 market basket update, modification of eligibility documentation requirements and reform to the HHPPS.Home Health Prospective Payment System ("HHPPS"). The HHPPS reform included the following parameters: for home health units of service beginning on January 1, 2020, a 30-day payment system will apply;was to be applied; the transition to the 30-day payment system mustwas to be budget neutral; and CMS mustwas to conduct at least one Technical Expert Panel during 2018, prior to any notice and comment rulemaking process, related to the design of any new case-mix adjustment model.
The final HHA regulations introduced by CMS (CMS-1689-FC)Calendar Year 2019 Home Health Final Rule updated the Medicare HHPPS and finalized the implementation of an alternative case-mix adjustment methodology, PDGM, thatwhich became effective on January 1, 2020. The PDGM adjusted payments to home health agencies providing home health services under Medicare Fee-For-Service based on patient characteristics for 30-day periods of care and also eliminated the use of therapy visits in the determination of payments. While the changes were to be implemented in a budget neutral manner to the industry, the ultimate impact will varyvaried by provider based on factors including patient mix and admission source. Additionally, CMS made assumptions about behavioral changes which were finalized in the Calendar Year 2020 Home Health Final Rule released on October 31, 2019. CMS assumed that home health agencies will change their documentation2019 and resulted in a 4.36% reduction to reimbursement. The behavioral changes were related to coding practices, low utilization payment adjustment ("LUPA") management and will put the highest paying diagnosis code as the principal diagnosis code in order to have a 30-day period be placed into a higher-paying clinical group. Initially, CMS proposed an 8.1% reduction in the base payment rate for a 30-day period of care to ensure overall budget neutrality in Medicare home health spending in CY 2020. In the 2020 Final Rule, CMS reduced that downward adjustment to 4.36%. Notably,co-morbidities. CMS is required by the law to analyze data for CYscalendar years 2020-2026, retrospectively, to determine the impact of the difference between assumed and actual behavior changes and to make any such payment changes as are necessary to offset or supplement the adjustments based on anticipated behavior. Additionally, in an effort to eliminate fraud risks, CMS is phasing outreduced the upfront payment associated with requests for anticipated payment ("RAPs") overto 20% in 2020 with the full elimination of RAPs in 2021.
On October 31, 2022, CMS issued the Home Health Final Rule for Medicare home health providers for calendar year 2023. CMS estimates that the final rule will result in a 0.7% increase in payments to home health providers. This increase is the result of a 4.0% payment update (4.1% market basket adjustment less a 0.1% productivity adjustment) and an increase of 0.2% for the update to the fixed-dollar loss ratio used in determining outlier payments offset by a permanent adjustment of -3.5% based on the difference between assumed and actual behavioral changes resulting from the implementation of PDGM. The -3.5% permanent adjustment is derived from a -3.925% behavioral assumption adjustment. In the Calendar Year 2023 Preliminary Rule, CMS proposed a behavioral assumption adjustment of -7.69%. CMS revised the adjustment to -7.85% in the final rule and also reduced it by half (to -3.925%) in order to mitigate such a significant reduction to reimbursement in a single year. The remaining -3.925% behavioral assumption adjustment will be considered in future rulemaking. The final rule also finalizes a permanent 5% cap on negative wage index changes for home health agencies. Based on our analysis of the final rule, we expect our impact to be flat, which is less than the estimated 0.7% rate increase.
In addition to the permanent adjustments, CMS is also considering a temporary adjustment of approximately $2 billion to offset overpayments in calendar years 2020 and 2021. CMS has elected not to apply the temporary adjustment to calendar year 2023; however, CMS is still considering how to best apply the adjustment in future rulemaking.
The BBA of 2018 also mandated the implementation of a new methodology for applying rural add-on payments for home health services (“rural add-on”). Unlike previous rural add-ons, which were applied to all rural areas uniformly, the extension provided varying add-on amounts depending on the rural county (or equivalent area) classification by classifying each rural county (or equivalent area) into one of three distinct categories: (1) rural counties and equivalent areas in the highest quartile of all counties and equivalent areas based on the number of Medicare home health episodes furnished per 100 individuals who are entitled to, or enrolled for, benefits under Part A of Medicare or enrolled for benefits under Part B of Medicare only, but not enrolled in a Medicare Advantage plan under Part C of Medicare (the "high utilization" category); (2) rural counties and equivalent areas with a population density of 6 individuals or fewer per square mile of land area that are not included in the "high utilization" category (the "low population density" category); and (3) rural counties and equivalent areas not in either the "high utilization" or "low population density" categories (the "all other" category).
In the Calendar Year ("CY") 2019 Home Health Final Rule, CMS finalized policies for the rural add-on payments for CY 2019 through CY 2022, in accordance with section 50208 of the BBA of 2018. The CY 2019 through CY 2022 rural add-on percentages outlined in the rule are shown in the table below.
| | | | | | | | | | | | | | |
| Rural Add-On Percentages, CYs 2019-2022 |
| Category | CY 2019 | CY 2020 | CY 2021 | CY 2022 |
| High utilization | 1.5% | 0.5% | None | None |
| Low population density | 4.0% | 3.0% | 2.0% | 1.0% |
| All other | 3.0% | 2.0% | 1.0% | None |
Environmental and Climate Change Matters
We are committed to transparency around our environmental footprint and climate-related risks and opportunities. We have adopted an integrated approach to address the impacts of climate change on our business, with cross-disciplinary teams responsible for managing climate-related activities, initiatives and policies. Strategies and progress toward our goals are reviewed with senior leadership and the Nominating and Corporate Governance Committee of our Board of Directors. During 2022, we engaged a third party expert to conduct our inaugural greenhouse gas (“GHG”) emissions inventory. We will establish interim GHG targets covering Scope 1 and 2 emissions in line with the Paris Agreement’s 1.5°C emissions reduction goal and report all relevant Scope 3 emissions and a timeline for establishing Scope 3 GHG reduction targets by December 31, 2023. Additional information about our environmental and climate activities can be found in our annual Environmental, Social and Governance Report, which is available on our website. For more information regarding climate change and its possible adverse impact on us, see “Item 1A. Risk Factors — Risks Related to Our Operations — Our operations could be impacted by war, terrorism, natural or man-made disasters and climate change” in this Annual Report on Form 10-K.
Our Competitors
There are few barriers to entry in the home health and hospice jurisdictions that do not require certificates of needa CON or permits of approval.POA. Our primary competition in these jurisdictions comes from local privately and publicly-owned and hospital-owned health care providers. We compete based on the quality of services, the availability of personnel, the quality of services, expertise of visiting staff, and, in certain instances, on the price of our services. In addition, we compete with a number of non-profit organizations that finance acquisitions and capital expenditures on a tax-exempt basis or receive charitable contributions that are unavailable to us.
Available Information
Our company website address is www.amedisys.com. We use our website as a channel of distribution for important company information. Important information, including press releases, analyst presentations and financial information regarding our company, is routinely posted on and accessible on the Investor Relations subpage of our website, which is accessible by clicking on the tab labeled “Investors” on our website home page. Visitors to our website can also register to receive automatic e-mail and other notifications alerting them when new information is made available on the Investor Relations subpage of our website. In addition, we make available on the Investor Relations subpage of our website (under the link “SEC Filings”), free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, ownership reports on Forms 3, 4 and 5 and any amendments to those reports as soon as reasonably practicable after we electronically file or furnish such reports with the SEC.Securities and Exchange Commission ("SEC"). Further, copies of our Certificate of Incorporation and Bylaws, our Code of Ethical Business Conduct, our Corporate Governance Guidelines and the charters for the Audit, Compensation, Quality of Care, Compliance and Ethics and Nominating and Corporate Governance Committees of our Board are also available on the Investor Relations subpage of our website (under the link “Governance”). Reference to our website does not constitute incorporation by reference of the information contained on the website and should not be considered part of this document.
Our electronically filed reports can also be obtained on the SEC’s internet site at http://www.sec.gov.
ITEM 1A. RISK FACTORS
The risks described below, and risks described elsewhere in this Form 10-K, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows and the actual outcome of matters as to which forward-looking statements are made in this Form 10-K. The risk factors described below and elsewhere in this Form 10-K are not the only risks faced by Amedisys. Our business and consolidated financial condition, results of operations and cash flows may also be materially adversely affected by factors that are not currently known to us, by factors that we currently consider immaterial or by factors that are not specific to us, such as general economic conditions.
If any of the following risks are actually realized, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected. In that case, the trading price of our common stock could decline.
You should refer to the explanation of the qualifications and limitations on forward-looking statements under “Special Caution Concerning Forward-Looking Statements.” All forward-looking statements made by us are qualified by the risk factors described below.
Risks Related to Reimbursement
Federal and state changes to reimbursement and other aspects of Medicare and Medicaid could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Our net service revenue is primarily derived from Medicare, which accounted for 74%, 73%75% and 76%75% of our consolidated net service revenue during 2019, 20182022, 2021 and 2017,2020, respectively. Payments received from Medicare are subject to changes made through federal legislation. When such changes are implemented, we must also modify our internal billing processes and procedures accordingly, which can require significant time and expense. These changes, as further detailed in Part I, Item 1, “Business: Payment for Our Services,” can include changes to base payments and adjustments for home health services, changes to cap limits and per diem rates for hospice services and changes to Medicare eligibility and documentation requirements or changes designed to restrict utilization. Any such changes, including retroactive adjustments, adopted in the future by the Center for Medicare and Medicaid Services (“CMS”)CMS could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
In April of 2015, Congress passed and President Obama signed the so-called “doc fix” in the form of the Medicare Access and CHIP Reauthorization Act of 2015 (“MACRA”). This law replaces a long-standing physician reimbursement formula with statutorily prescribed physician payment updates and provisions. MACRA provided for an increase of 3% of the payment amount
otherwise made for home health services furnished in rural areas and set Medicare reimbursements for post-acute care providers to increase by 1.0% in fiscal year 2018.
Section 6407 of the Affordable Care Act, as implemented by 42 CFR § 424.22, added new Medicare requirements for face-to-face encounters to support claims for home health services. Under 42 CFR § 424.22, the certifying physician must document a face-to-face encounter with the patient before making a certification that home health services are required under the Medicare home health benefit. A home health agency’s generated medical record documentation, by itself, is not sufficient in demonstrating the patient's eligibility for the home health benefit. Therefore, additional documentation, such as an admit summary, part of the OASIS, or a therapy evaluation/therapy notes, must be signed off by the certifying physician and incorporated into the medical record to support the face-to-face.
The requirements for face-to-face encounters continue to be one of the most complex issues in the industry and can be the source of claims denials if not fulfilled. Section 6407(d) of the Affordable Care Act also provided that the requirements for face-to-face encounters in the provisions described above shall apply in the case of physicians making certifications for home health services under title XIX of the Act (Medicaid) in the same manner and to the same extent as such requirements apply under title XVIII (Medicare). On February 2, 2016, CMS published a final rule, which is currently in effect, adding new requirements for Medicaid home health services. Among other things, the final rule requires that for the initial ordering of home health services, the physician must document that a face-to-face encounter that is related to the primary reason the beneficiary requires home health services occurred no more than 90 days before or 30 days after the start of services. The final rule also requires that for the initial ordering of certain medical equipment, the physician or authorized non-physician practitioner must document that a face-to-face encounter that is related to the primary reason the beneficiary requires medical equipment occurred no more than six months prior to the start of services.
On July 31, 2019, CMS issued a final rule to update hospice payment rates and the wage index for fiscal year 2020. The rule includes a rebasing of continuous home care, inpatient respite care and general inpatient care to better reflect the costs of care. This rebasing will be offset by a reduction in routine home care payments of 2.7% to achieve budget neutrality. In addition, CMS eliminated the one-year “lag” in the use of the hospital wage index in an effort to align with the Inpatient Prospective Payment System ("IPPS") and other payment systems. CMS estimates hospices serving Medicare beneficiaries would see an estimated 2.6% increase in payments. This increase is the result of a 3.0% market basket adjustment less a 0.4% productivity adjustment. We have estimated the impact of the final rule on us to be an increase in revenue of approximately 0.5%; however, we are expecting the impact on gross margin percentage to be a reduction of approximately 0.5% as the majority of the revenue increase will be passed through to the general inpatient and respite facilities. These estimates are subject to change as our mix of work changes.
In November 2018, CMS issued the Calendar Year 2019 Home Health Final Rule, which provided for the first payment rate increase for home health providers since 2010. In the 2019 rule, CMS also issued proposed payment changes for Medicare home health providers for 2020. These proposed changes included changes to the Home Health Prospective Payment System ("HHPPS") case-mix adjustment methodology through the use of a new Patient-Driven Groupings Model ("PDGM") for home health payments, a change in the unit of payment from a 60-day payment period to a 30-day payment period and the elimination of the use of therapy visits in the determination of payments. While the proposed changes are to be implemented in a budget neutral manner to the industry, the ultimate impact will vary by provider based on factors including patient mix and admission source.
The CMS Calendar Year 2020 Home Health Final Rule, released in October 2019, sets forth the implementation of PDGM and a change in the unit of payment from 60-day episodes of care to 30-day periods of care. Additionally, in an effort to eliminate fraud risks, CMS is phasing out requests for anticipated payment ("RAPs") over 2020 with the full elimination of RAPs in 2021. CMS estimates that the proposed rule will result in a 1.3% increase in payments to home health providers. The increase is the result of a statutorily mandated 1.5% market basket increase pursuant to the Bipartisan Budget Act of 2018, reduced by 0.2% for the rural add-on. CMS is also assuming that the industry will make certain behavioral changes resulting in a decrease in reimbursement of 4.36%. We have estimated the impact of the final rule on us to be a reduction in revenue of 2.8%. Our current view is that we can fully offset the impact via behavioral changes and a mix of cost levers which include clinical mix and utilization.
There are continuing efforts to reform governmental health care programs that could result in major changes in the health care delivery and reimbursement system on a national and state level, including changes directly impacting the reimbursement systems for our home health and hospice care centers. The U.S. federal budget is subject to change, and the Medicare program is frequently mentioned as a target for spending cuts. Within the Medicare program, the hospice benefit is often specifically targeted for cuts. The full impact on our business of any future cuts in Medicare or other programs is uncertain. Though we cannot predict what, if any, reform proposals will be adopted, health care reform and legislation may have a material adverse effect on our business and ourconsolidated financial condition, results of operations and cash flows through decreasing payments made for our services.
We could also be affected adversely by the continuing efforts of governmental payors to contain health care costs. We cannot assure you that reimbursement payments under governmental payor programs, including Medicare supplemental insurance policies, will remain at levels comparable to present levels or will be sufficient to cover the costs allocable to patients eligible for reimbursement
pursuant to these programs. Any such changes could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Future cost containment initiatives undertaken by private third party payors may limit our future revenue and profitability.
Our non-Medicare revenue and profitability are affected by continuing efforts of third party payors to maintain or reduce costs of health care by lowering payment rates, narrowing the scope of covered services, increasing case management review of services and negotiating pricing. There can be no assurance that third party payors will make timely payments for our services, and there is no assurance that we will continue to maintain our current payor or revenue mix. We are continuing our efforts to develop our non-Medicare sources of revenue. Any changes in payment levels from current or future third party payors could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Possible changes in the case mix of patients, as well as payor mix and payment methodologies, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Our revenue is determined by a number of factors, including our mix of patients and the rates of payment among payors. Changes in the case mix of our patients, payment methodologies or the payor mix among Medicare, Medicaid and private payors could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Our failure to negotiate favorable managed care contracts, or our loss of existing favorable managed care contracts, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
One of our strategies is to diversify our payor sources by increasing the business we do with managed care companies. We strive to put in place favorable contracts with managed care payors; however, we may not be successful in these efforts. Additionally, there is a risk that the favorable managed care contracts that we put in place may be terminated. Managed care contracts typically permit the payor to terminate the contract without cause, on very short notice, typically 60 days, which can provide payors leverage to reduce volume or obtain favorable pricing. Our failure to negotiate and put in place favorable managed care contracts, or our failure to maintain in place favorable managed care contracts, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Quality reporting requirements may negatively impact Medicare reimbursement.
Hospice quality reporting was mandated by PPACA,the Patient Protection and Affordable Health Care Act and the Health Care and Education Reconciliation Act ("PPACA"), which directs the Secretary to establish quality reporting requirements for hospice programs. Failure to submit required quality data will result in a 2 percentage point2% reduction to the market basket percentage increase for that fiscal year. This quality reporting program is currently “pay-for-reporting,” meaning it is the act of submitting data that determines compliance with program requirements.
Similarly,Section 1895(b)(3)(B)(v) of the Social Security Act requires the submission of quality data by home health agencies. Failure to submit quality data will result in a 2% reduction in the home health agency's annual home health payment update percentage. This pay-for-reporting requirement was implemented on January 1, 2007. In the Calendar Year 2015 Home Health Final Rule, CMS proposed to establishdefined a newmore explicit “Pay-for-Reporting Performance Requirement” withby which provider compliance with quality reporting program requirements can be measured. In the Calendar Year 2016 Home Health Final Rule, CMS required home health agencies that do not submit quality measure data to CMS are subject to a 2.0% reduction in their annual home health payment update percentage. Home health agencies are required to report prescribed quality assessment data for a minimum of 70.0%90% of all patients with episodes of care that occur on or after July 1, 2015. This compliance threshold increased by 10.0% in each of two subsequent periods - i.e., for episodes beginning on or after July 1, 2016 and before June 30, 2017, home health agencies must score at least 80%, and for episodes beginning on or after July 1, 2017 and thereafter, the required performance level is at least 90%.patients.
The Improving Medicare Post-Acute Care Transformation Act of 2014 (the “IMPACT Act”) requires the submission of standardized data by home health agencies and other providers. Specifically, the IMPACT Act requires, among other significant activities, the reporting of standardized patient assessment data with regard to quality measures, resource use and other measures. Failure to report data as required will subject providers to a 2% reduction in market basket prices then in effect.
There can be no assurance that all of our agencies will continue to meet quality reporting requirements in the future which may result in one or more of our agencies seeing a reduction in its Medicare reimbursements. Regardless, we, like other healthcare providers, are likely to incur additional expenses in an effort to comply with additional and changing quality reporting requirements.
Value-based purchasing may negatively impact Medicare reimbursement.
Both government and private payors are increasingly looking to value-based purchasing to contain costs. Value-based purchasing focuses on quality of outcomes and efficiency of care, rather than quantity of care. The first performance year of the expanded value-based purchasing model begins on January 1, 2023, and the model has been expanded to all 50 states. Under the expanded model, home health agencies receive adjustments to their Medicare fee-for-service payments based on their performance against a set of quality measures, relative to their peers' performance. Performance on these quality measures in a specified year (performance year) impacts payment adjustments in a later year (payment year). CMS may also create a similar plan for hospices in the future. Government and private payors’ implementation of value-based purchasing requirements could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Any economic downturn, deepening of an economic downturn, continued deficit spending by the Federal Government or state budget pressures may result in a reduction in payments and covered services.
Adverse developments in the United States could lead to a reduction in Federal Government expenditures, including governmentally funded programs in which we participate, such as Medicare and Medicaid. In addition, if at any time the Federal Government is not able to meet its debt payments unless the federal debt ceiling is raised, and legislation increasing the debt ceiling is not enacted, the Federal Government may stop or delay making payments on its obligations, including funding for government programs in which we participate, such as Medicare and Medicaid. Failure of the government to make payments under these programs could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Further, any failure by the United States Congress to complete the federal budget process and fund government operations may result in a Federal Government shutdown, potentially causing us to incur substantial costs without reimbursement under the Medicare program, which could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. As an example, the failure of the 2011 Joint Select Committee to meet its Deficit Reduction goal resulted in an automatic reduction in Medicare home health and hospice payments of 2% beginning April 1, 2013.2013 ("sequestration" - suspended from May 1, 2020 through March 31, 2022; reinstated at 1% for the period April 1, 2022 through June 30, 2022 and at 2% thereafter).
Historically, state budget pressures have resulted in reductions in state spending. Given that Medicaid outlays are a significant component of state budgets, we can expect continuing cost containment pressures on Medicaid outlays for our services.
In addition, sustained unfavorable economic conditions may affect the number of patients enrolled in managed care programs and the profitability of managed care companies, which could result in reduced payment rates and could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Future cost containment initiatives undertaken by private third party payorsRisks Related to our Operations
A shortage of qualified nursing staff and other clinicians, such as therapists and nurse practitioners, could materially impact our ability to attract, train and retain qualified personnel and could increase operating costs.
We compete for qualified personnel with other healthcare providers. Our ability to attract and retain clinicians depends on several factors, including our ability to provide these personnel with attractive assignments and competitive salaries and benefits. We cannot be assured we will succeed in any of these areas. In addition, there are shortages of qualified health care personnel in some of our markets. As a result, we may limit our future revenue and profitability.
Our non-Medicare revenue and profitability are affected by continuing efforts of third party payors to maintain or reduceface higher costs of health care by lowering payment rates, narrowing the scopeattracting clinicians and providing them with more attractive benefit packages than we originally anticipated or we may have to utilize contract clinicians, both of covered services, increasing case management review of services and negotiating pricing. There can be no assurance that third party payors will make timely payments for our services, and there is no assurance that we will continue to maintain our current payor or revenue mix. We are continuing our efforts to develop our non-Medicare sources of revenue and any changes in payment levels from current or future third party payorswhich could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Risks Related In addition, if we expand our operations into geographic areas where health care providers historically have been unionized, or if any of our care center employees become unionized, being subject to Laws and Government Regulations
We are operating under a Corporate Integrity Agreement. Violations of thiscollective bargaining agreement could result in substantial penalties or exclusion from participation in the Medicare program.
Corporate Integrity Agreement
On April 23, 2014, with no admissions of liabilitymay have a negative impact on our part,ability to timely and successfully recruit qualified personnel and may increase our operating costs. In some circumstances, we entered into a settlement agreement withmay have to hire contract clinicians to fulfill staffing needs, which could increase the U.S. Departmentrisk of Justice relatingan adverse patient event. Generally, if we are unable to certainattract and retain clinicians, the quality of our clinicalservices may decline and business operations. Concurrently with our entry into this agreement, we entered into a corporate integrity agreement (“CIA”) with the Office of Inspector General-HHS (“OIG”). The CIA formalized various aspects of our already existing ethicscould lose patients and compliance programs and contained other requirements designed to help ensure our ongoing compliance with federal health care program requirements. Among other things, the CIA required us to maintain our existing compliance program, executive compliance committee and compliance committee of the Board of Directors; provide certain compliance training; continue screening new and current employees to ensure they are eligible to participate in federal health care programs; engage an independent review organization to perform certain audits and reviews and prepare certain reports regarding our compliance with federal health care programs, our billing submissions to federal health care programs and our compliance and risk mitigation programs; and provide certain reports and management certifications to the OIG. Additionally, the CIA specifically required that we report substantial overpayments that we discovered we had received from federal health care programs, as well as probable violations of federal health care laws. The corporate integrity agreement had a term of five years that ended on April 21, 2019. We filed our final annual report on July 19, 2019.
Compassionate Care Hospice Corporate Integrity Agreement
On January 30, 2015, Compassionate Care Hospice (“CCH”) entered into a CIA with the OIG. The CIA required that CCH provide annual on-site compliance training; develop and implement policies to ensure compliance with federal health care program requirements; screen new and current employees to ensure that they are eligible to participate in federal health care programs; establish a compliance committee that contains both a Compliance Officer and a Chief Quality Officer; retain a Governing Authority expert who will periodically complete a compliance program review; and retain an independent review organization (IRO) to complete claims review for hospice services rendered in New York. The OIG waived the claims review for the final year of the CCH CIA based on the closure of the New York operations. Additionally, the CIA required that CCH report substantial overpayments that CCH discovered it received from federal health care programs, as well as probable violations of federal, criminal, civil or administrative health care laws. Upon breach of the CIA, CCH could have become liable for payment of certain stipulated penalties, or could have been excluded from participation in federal health care programs. The CIA has a term of five years that ended on January 30, 2020.
We are subject to extensive government regulation. Any changes to the laws and regulations governing our business, or to the interpretation and enforcement of those laws or regulations,referral sources, which could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Our industry is subjectbusiness may be materially adversely affected by the ongoing COVID-19 pandemic.
The outbreak of the COVID-19 pandemic has resulted in a general economic downturn and volatility in the stock market and has also caused and may continue to extensive federalcause a decrease in our patient volumes and state lawsrevenues, an increase in our costs, an inability to access our patients and regulations. See Part I, Item 1, “Our Regulatory Environment” for additional informationreferral sources, staffing shortages and medical supply shortages, any of which, or a combination of which, could have a material adverse effect on such laws and regulations. Federal and state laws and regulations impact how we conduct our business and financial results. The ultimate impact of COVID-19, including the servicesimpact on our liquidity, financial condition and results of operations, is uncertain and will depend on many factors and future developments, which are highly uncertain and cannot be predicted at this time, such as the severity, scope and length of time that the pandemic continues, including regional surges in COVID-19 cases at various times. In addition, the COVID-19 pandemic has resulted in widespread global supply chain disruptions to vendors including critical supply shortages, significant material cost inflation and extended lead times for items that are required for our operations. Continued disruptions could increase our costs and could limit the availability of products critical to our operations.
We may be more vulnerable to the effects of a public health emergency than other businesses due to the nature of our patient population and the physical proximity required by our operations, which could harm our business disproportionately to other businesses.
The majority of our patients are older individuals and/or individuals with complex medical challenges or multiple ongoing diseases, many of whom may be more vulnerable than the general public during a pandemic or in a public health emergency. Our employees are also at greater risk of contracting contagious diseases due to their increased exposure to vulnerable individuals. Our employees could also have difficulty attending to our patients if a program of social distancing or quarantine is instituted in response to a public health emergency. In addition, we offermay expand existing internal policies in a manner that may have a similar effect. If the virus that causes COVID-19 and its potentially more contagious variants cause an additional resurgence of infections of COVID-19, if new variants that are resistant to government approved COVID-19 vaccinations continue to emerge, or if an influenza or other pandemic were to occur, we could suffer significant losses to our interactions with patients,patient population or a reduction in the availability of our employees and caregivers, and we could be required to hire replacements for affected workers at an inflated cost. Accordingly, public health emergencies could have a disproportionate material adverse effect on our financial condition and results of operations.
Because we are limited in our ability to control rates received for our services, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected if we are not able to maintain or reduce our costs to provide such services.
As Medicare is our primary payor and rates are established through federal legislation, we have to manage our costs of providing care to achieve a desired level of profitability. Additionally, non-Medicare rates are difficult for us to negotiate as such payors are under pressure to reduce their own costs. As a result, we manage our costs in order to achieve a desired level of profitability including, but not limited to, centralization of various processes, the publicuse of technology and impose certain requirements on us such as:management of the number of employees utilized. If we are not able to continue to streamline our processes and reduce our costs, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected.
licensure and certification;
adequacy andIf we are unable to consistently provide high quality of health care, services;our business will be adversely impacted.
qualificationsProviding quality patient care is the cornerstone of health care and support personnel;
quality and safety of medical equipment;
confidentiality, maintenance and security issues associated with medical records and claims processing;
relationships withour business. We believe that hospitals, physicians and other referral sources;sources refer patients to us in large part because of our reputation for delivering quality care. Clinical quality is becoming increasingly important within our industry. Medicare imposes a financial penalty upon hospitals that have excessive rates of patient readmissions within 30 days from hospital discharge. We believe this regulation provides a competitive advantage to home health providers who can differentiate themselves based upon quality, particularly by achieving low patient acute care hospitalization readmission rates and by implementing disease management programs designed to be responsive to the needs of patients served by referring hospitals. We are focused intently upon improving our patient outcomes, particularly our patient acute care hospitalization readmission rates. If we should fail to attain our goals regarding acute care hospitalization readmission rates and other quality metrics, we expect our ability to generate referrals would be adversely impacted, which could have a material adverse effect upon our business and consolidated financial condition, results of operations and cash flows.
Additionally, Medicare has established consumer-facing websites, Home Health Compare and Hospice Compare, that present data regarding our performance on certain quality measures compared to state and national averages. Failure to achieve or exceed these averages may negatively affect our rates of reimbursement and our ability to generate referrals, which could have a material adverse effect upon our business and consolidated financial condition, results of operations and cash flows.
If we are unable to maintain relationships with existing patient referral sources, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected.
Our success depends on referrals from physicians, hospitals and other sources in the communities we serve and on our ability to maintain good relationships with existing referral sources. Our referral sources are not (and cannot be) contractually obligated to refer patients to us and may refer their patients to other providers. Our growth and profitability depend, in part, on our ability to establish and maintain close working relationships with these patient referral sources and to increase awareness and acceptance of the benefits of home health and hospice care by our referral sources and their patients. Our loss of, or failure to maintain, existing relationships or our failure to develop new referral relationships could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Our industry is highly competitive, with few barriers to entry in certain states.
There are few barriers to entry in home health and hospice markets that do not require a CON or POA. Our primary competition comes from local privately-owned, publicly-owned and hospital-owned health care providers. We compete based on the availability of personnel, the quality of services, expertise of visiting staff, and in certain instances, on the price of our services. In addition, we compete with a number of non-profit organizations and tax-supported governmental agencies that finance acquisitions and capital expenditures on a tax-exempt or tax-favorable basis or receive charitable contributions that are unavailable to us. Increased competition in the future may limit our ability to maintain or increase our market share.
Further, the introduction of new and enhanced service offerings by others, in combination with industry consolidation and the development of strategic relationships by our competitors (including mergers of competitors with each other and with insurers), could cause a decline in revenue or loss of market acceptance of our services or make our services less attractive.
Managed care organizations and other third party payors continue to consolidate, which enhances their ability to influence the delivery of health care services. Consequently, the health care needs of patients in the United States are increasingly served by a smaller number of managed care organizations. These organizations generally enter into service agreements with a limited number of providers. Our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected if these organizations terminate us as a provider and/or engage our competitors as a preferred or exclusive provider. In addition, should private payors, including managed care payors, seek to negotiate additional discounted fee structures or the assumption by health care providers of all or a portion of the financial risk through prepaid capitation arrangements, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected.
If we are unable to react competitively to new developments, our operating policiesresults may suffer. State CON or POA laws often limit the ability of competitors to enter into a given market, are not uniform throughout the United States and procedures;are frequently the subject of efforts to limit or repeal such laws. If states remove existing CONs or POAs, we could face increased competition in these states. There can be no assurances that other states will not seek to eliminate or limit their existing CON or POA programs, which could lead to increased competition in these states. Further, we cannot assure you that we will be able to compete successfully against current or future competitors, which could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
emergency preparednessThe success of our high acuity care segment depends on our ability to enter into capitation and other forms of risk-based contracts with managed care health plans. If we are unsuccessful in obtaining these contracts or if we are unsuccessful in managing costs associated with risk-based contracts, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected.
Our acquisition of Contessa not only established the foundation for our high acuity care segment, but it also added key infrastructure to enable us to more quickly and effectively enter into risk-based contracts with managed care health plans. Should our high acuity care joint venture partnerships not deliver sufficient perceived value to managed care health plans, those health plans may limit or forego opportunities to partner with us in expanded risk-based contracts. Additionally, assuming risk assessmentsfrom managed care health plans requires that the appropriate clinical and policiesoperating protocols be in place to actuarially assess eligible members and procedures;determine historical baseline healthcare expenditures, enroll eligible members into the program, effectuate a clinically effective plan of care to treat those patients primarily in a home-based setting and coordinate care throughout various phases of the member’s treatment including proactive primary care and palliative care services. Should we be ineffective in identifying and enrolling members into the program or should the clinical treatment plans we implement for enrolled members not result in reduced healthcare costs during the period in which those members are enrolled, we could incur significant additional costs under these contracts that exceed the revenues we receive. These negative outcomes could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
policiesOur business depends on our information systems. A cyber-attack, security breach or our inability to effectively integrate, manage and procedureskeep our information systems secure and operational could disrupt our operations.
Healthcare providers and health insurance plans must comply with the HIPAA regulations regarding employee relations;
additionthe privacy and security of facilitiesprotected health information. The HIPAA regulations impose significant requirements on providers with regard to how such protected health information may be used and services;
billing for services;
disclosed. Further, the regulations include extensive and complex requirements for utilizationproviders to establish reasonable and appropriate administrative, technical and physical safeguards to ensure the confidentiality, integrity and availability of services;protected health information. In the event the provider experiences a "breach" and the personal information is compromised, providers are obligated under HIPAA to notify individuals, the government, and in the event the breach involves 500 or more individuals, the media. HIPAA directs the Secretary of HHS to provide for periodic audits to ensure covered entities (and their business associates, as that term is defined under HIPAA) comply with the applicable HIPAA requirements.
In addition to federal regulators, state attorneys general are also enforcing information security breaches. All 50 states have breach notification laws; some of these laws also include proactive data security requirements. In addition to state laws regarding confidentiality of medical information, several states are now focused on expanding state privacy laws regarding personal information which is more broadly defined than medical information.
Our networks, systems and devices store sensitive information, including intellectual property, proprietary business information and personal information of our patients, partners and employees. We have installed privacy protection systems and devices on our network, systems and point of care tablets in an attempt to prevent unauthorized access to information created, received, transmitted and maintained by us. However, in the event of a sophisticated ransomware attack, malware, viruses, phishing, or social engineering, our technology may fail to adequately secure the protected health information and personal information we create, receive, transmit and maintain in our databases. In such circumstances, we may be held liable to our patients and regulators, which could result in fines, litigation or adverse publicity that could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Even if we are not held liable, any resulting negative publicity could harm our business and distract the attention of management.
Our business depends on effective, secure and operational information systems which include systems provided by or hosted by external contractors, partners and other service providers. For example, our care centers depend upon our information systems and software for patient care, accounting, billing, collections, risk management, quality assurance, human resources, payroll and patient care;other information considered to be sensitive and/or confidential. These third party vendors, or "business associates," comply with substantially the same HIPAA requirements as the healthcare provider. This is accomplished through the use of "Business Associate Agreements" with vendors. We believe that our subcontractors and vendors take precautionary measures to prevent problems that could affect our business operations as a result of failure or disruption to their information systems or networks. However, there is no guarantee such efforts will be successful in preventing a system disruption or security incident. The occurrence of any information system failure, breach or security incident, or a vendor's breach of the Business Associate Agreement could result in interruptions, delays, breaches of protected health information and personal information, loss or corruption of data and cessations or interruptions in the availability of these systems and the information they create, receive, transmit or maintain. All of these events or circumstances, among others, could have an adverse effect on our business and consolidated financial condition, results of operations and cash flows, and they could harm our business reputation.
reportingIn general, all information systems, including those we host or have hosted by third parties, are vulnerable to damage or interruption from fire, flood, power loss, telecommunications failure, human error or malicious acts, break-ins and maintaining records regarding adverseother intentional or unintentional events.
Our business is also at risk from and may be materially impacted and/or disrupted by information security incidents, such as ransomware, malware, viruses, phishing, social engineering and other security events. Such incidents can range from individual attempts to gain unauthorized access to information technology systems to more sophisticated security threats. These lawsevents can also result from internal compromises, such as human error or a rogue employee or contractor, and regulations,can occur on our systems or on the systems of our partners and their interpretations,subcontractors. Additionally, our current information systems are subject to change. Changesother non-environmental risks, including technological obsolescence, in existing laws and regulations, some instances, which may create increased security and/or their interpretations,operational risk.
Problems with, or the enactmentfailure of, newour technology and systems or any system upgrades or programming changes associated with such technology and systems could have a material adverse effect on our operations, patient care, data capture and integrity, medical documentation, billing, collections, assessment of internal controls and management and reporting capabilities. If we experience a reduction in the performance, reliability or availability of our information systems, our operations and ability to produce timely and accurate reports could be materially adversely affected.
Our information systems and applications also require continual maintenance, upgrading and enhancement to meet our operational and security needs. Our acquisition activity requires transitions and integration of various information systems. We regularly upgrade and expand our information systems’ capabilities. If we experience difficulties with the transition and integration of information systems or are unable to implement, maintain or expand our systems properly, we could suffer from, among other things, operational disruptions, regulatory investigations or audits and increases in administrative expenses.
As cyber threats continue to evolve, we may be required to expend significant capital and other resources to protect against the threat of security breaches or to mitigate and alleviate problems caused by security incidents, including unauthorized access to protected health information and personal information stored in our information systems and the introduction of computer viruses or other malicious software programs to our systems. If we don't expend capital and other resources to continually enhance our security systems, our security measures may be inadequate to prevent security breaches and our business operations and reputation could be materially adversely affected by federal and state fines and penalties, legal claims or proceedings, cancellation of contracts and loss of patients if security breaches are not prevented. The healthcare industry is currently a target for cyber criminals and is therefore experiencing increased scrutiny from federal and state regulators with
respect to compliance with regulations designed to safeguard protected health information and mitigate cyber-attacks. There are significant costs associated with a breach, including investigation costs, remediation and mitigation costs, notification costs, attorney fees, litigation and the potential for reputational harm and lost revenues due to a loss in confidence in the provider. We cannot predict the costs to comply with these laws or regulationsthe costs associated with a potential breach of protected health information, which could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows, by:and our business reputation.
increasing our administrative and other costs;
increasing or decreasing mandated services;
causing us to abandon business opportunities we might have otherwise pursued;
decreasing utilization of services;
forcing us to restructure our relationships with referral sources and providers; or
requiring us to implement additional or different programs and systems.
Additionally,If we are subject to various routinecyber-attacks or security breaches in the future, this could result in harm to patients; business interruptions and non-routine reviews, auditsdelays; the loss, misappropriation, corruption or unauthorized access of data; litigation and investigations by the Medicarepotential liability under privacy, security and Medicaid programsconsumer protection laws or other applicable laws; reputational damage and other federal and state governmental agencies, which have various rights and remedies against us if they establish that we have overcharged the programsinquiries. Any such problems or failed to comply with program requirements. Violation of the laws governing our operations, or changes in interpretations of those laws, could result in the imposition of fines, civil or criminal penalties,failures and the termination of our rights to participatecosts incurred in federal and state-sponsored programs and/correcting any such problems or the suspension or revocation of our licenses. If we become subject to material fines, or if other sanctions or other corrective actions are imposed on us, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected.
We face periodic and routine reviews, audits and investigations under our contracts with federal and state government agencies and private payors, and these audits could have adverse findings that may negatively impact our business.
As a result of our participation in the Medicare and Medicaid programs, we are subject to various governmental reviews, audits and investigations to verify our compliance with these programs and applicable laws and regulations. We also are subject to audits under various government programs, including the Recovery Audit Contractors (“RACs”), Zone Program Integrity Contractors (“ZPICs”), Uniform Program Integrity Contractors ("UPICs"), Program Safeguard Contractors (“PSCs”) and Medicaid Integrity Contractors (“MICs”) as well as in accordance with the requirements of our CIA, in which third party firms engaged by CMS or by the Company conduct extensive reviews of claims data and medical and other records to identify potential improper payments under the Medicare program. Private pay sources also reserve the right to conduct audits. If billing errors are identified in the sample of reviewed claims, the billing error can be extrapolated to all claims filed which could result in a larger overpayment than originally identified in the sample of reviewed claims. Our costs to respond to and defend reviews, audits and investigations may be significant andfailures, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Moreover, an adverse review, auditFurther, to the extent our external information technology contractors or investigation could result in:
required refundingother service providers have their own cyber-attack, security event or retroactive adjustment of amountsinformation technology failure, become insolvent or fail to support the software or systems we have been paid pursuantlicensed from them, our operations could be materially adversely affected. A failure to restore our information systems after the federal or state programs or from private payors;
state or federal agencies imposing fines, penalties and other sanctionsoccurrence of any of these events could have a material adverse effect on us;
loss of our right to participate in the Medicare program, state programs, or one or more private payor networks; or
damage to our business and consolidated financial condition, results of operations and cash flows. Because of the protected health information we store and transmit, loss of electronically stored information for any reason could expose us to risk of regulatory action and litigation and possible liability and loss.
We believe we have all the necessary licenses from third parties to use technology and software that we do not own. A third party could, however, allege that we are infringing its rights, which may deter our ability to obtain licenses on commercially reasonable terms from the third party, if at all, or cause the third party to commence litigation against us. In addition, we may find it necessary to initiate litigation to protect our trade secrets, to enforce our intellectual property rights and to determine the scope and validity of any proprietary rights of others. Any such litigation, or the failure to obtain any necessary licenses or other rights, could materially and adversely affect our business.
Our insurance liability coverage may not be sufficient for our business needs.
As a result of operating in the home health industry, our business entails an inherent risk of claims, losses and potential lawsuits alleging incidents involving our employees that may occur in a patient’s home. We maintain professional liability insurance to provide coverage to us and our subsidiaries against these risks. However, we cannot assure you claims will not be made in the future in excess of the limits of our insurance, nor can we assure you that any such claims, if successful and in excess of such limits, will not have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. In some states, state law may prohibit or limit insurance coverage for the risk of punitive damages arising from professional liability and general liability claims and/or litigation. As a result, we may be liable for punitive damage awards in these states that either are not covered or are in excess of our insurance policy limits. Our insurance coverage also includes fire, property damage, cyber security and general liability with varying limits. We cannot assure you that the insurance we maintain will satisfy claims made against us or that insurance coverage will continue to be available to us at commercially reasonable rates, in adequate amounts or on satisfactory terms. Any claims made against us, regardless of their merit or eventual outcome, could damage our reputation and business.
We may be subject to substantial malpractice or other similar claims.
The services we offer involve an inherent risk of professional liability and related substantial damage awards. As of February 10, 2023, we have approximately 20,000 employees (11,200 home health, 5,900 hospice, 1,900 personal care, 200 high acuity care and 1,000 corporate employees). In addition, we employ direct care workers on a contractual basis to support our existing workforce. Due to the nature of our business, we, through our employees and caregivers who provide services on our behalf, may be the subject of medical malpractice claims. A court could find these individuals should be considered our agents, and, as a result, we could be held liable for their acts or omissions. We cannot predict the effect that any claims of this nature, regardless of their ultimate outcome, could have on our business or reputation or on our ability to attract and retain patients and employees. While we maintain malpractice liability coverage that we believe is appropriate given the nature and breadth of our operations, any claims against us in various markets.
These resultsexcess of insurance limits, or multiple claims requiring us to pay deductibles, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
If awe are unable to maintain our corporate reputation, our business may suffer.
Our success depends on our ability to maintain our corporate reputation, including our reputation for providing quality patient care center failsand for compliance with Medicare requirements and the other laws to which we are subject. Adverse publicity surrounding any aspect of our business, including the death or disability of any of our patients due to our failure to provide proper care, or due to any failure on our part to comply with the conditions of participation in the Medicare program, that care centerrequirements, HIPAA requirements, or other laws to which we are
subject, could be subjected to sanctions or terminated from the Medicare program.
Each ofnegatively affect our care centers must comply with required conditions of participation in the Medicare program. If we fail to meet the conditions of participation at a care center, we may receive a notice of deficiency from the applicable state surveyor. If that care center then fails to institute an acceptable plan of correction to remediate the deficiency within the correction period provided by the state surveyor, that care center could be terminated from the Medicare program or subjected to alternative sanctions. CMS outlined its alternative sanction enforcement options for home health care centers through a regulation published in 2012; under
the regulation, CMS may impose temporary management, direct a plan of correction, direct training or impose payment suspensions and civil monetary penalties, in each case, upon providers who fail to comply with the conditions of participation. Termination of one or more of our care centers from the Medicare program for failure to satisfy the program’s conditions of participation, or the imposition of alternative sanctions, could disrupt operations, require significant attention by management, or have a material adverse effect on our business andCompany’s overall reputation and consolidated financial condition, resultsthe willingness of operations and cash flows.
We are subject to federal and state laws that govern our financial relationships with physicians and other health care providers, including potential or current referral sources.
We are required to comply with federal and state laws, generally referred to as “anti-kickback laws,” that prohibit certain direct and indirect payments or other financial arrangements between health care providers that are designed to encourage the referral of patients to a particular provider for medical services. In addition to these anti-kickback laws, the Federal Government has enacted specific legislation, commonly known as the “Stark Law,” that prohibits certain financial relationships, specifically including ownership interests and compensation arrangements, between physicians (and the immediate family members of physicians) and providers of designated health services, such as home health care centers, to whom the physicians refer patients. Some of these same financial relationships are also subject to additional regulation by states. Although we believe we have structured our relationships with physicians and other potential referral sources to comply with these laws where applicable, we cannot assure yourefer patients to us. Further, the poor performance, reputation or negative conduct of competitors may have spillover effects that courtsadversely affect the industry and our brand.
A write off of a significant amount of intangible assets or regulatory agencies will not interpret state and federal anti-kickback laws and/or the Stark Law and similar state laws regulating relationships between health care providers and physicians in ways that will adversely implicate our practices or that isolated instances of noncompliance will not occur. Violations of federal or state Stark or anti-kickback laws could lead to criminal or civil fines or other sanctions, including denials of government program reimbursement or even exclusion from participation in governmental health care programs, whichlong-lived assets could have a material adverse effect on our business and consolidated financial condition and results of operationsoperations.
A significant and sustained decline in our stock price and market capitalization, a significant decline in our expected future cash flows.
We may faceflows, a significant uncertaintyadverse change in the industrybusiness climate or slower growth rates could result in the need to perform an impairment analysis under Accounting Standards Codification (“ASC”) Topic 350 “Intangibles – Goodwill and Other” in future periods in addition to our annual impairment test. If we were to conclude that a write down of goodwill is necessary, then we would record the appropriate charge, which could result in material charges that are adverse to our consolidated financial condition and results of operations. See Part II, Item 8, Note 5 – Goodwill and Other Intangible Assets, Net to our consolidated financial statements for additional information.
Because we have grown in part through acquisitions, goodwill and other acquired intangible assets represent a substantial portion of our assets. Goodwill was $1.3 billion as of December 31, 2022 and if we make additional acquisitions, it is likely that we will record additional goodwill and intangible assets in our consolidated financial statements. We also have long-lived assets consisting of property and equipment and other identifiable intangible assets of $117.2 million as of December 31, 2022, which we review on a periodic basis as well as when events or circumstances indicate that the carrying amount of an asset may not be recoverable. If a determination that a significant impairment in value of our unamortized intangible assets or long-lived assets occurs, such determination could require us to write off a substantial portion of our assets. A write off of these assets could have a material adverse effect on our consolidated financial condition and results of operations.
Our operations could be impacted by war, terrorism, natural or man-made disasters and climate change.
The Company's business may be adversely affected by instability, disruption or destruction in a geographic region in which it operates, regardless of cause, including war, terrorism, riot, civil insurrection or social unrest, climate change, natural or man-made disasters and extreme weather conditions, such as hurricanes, tornadoes, wildfires, earthquakes and floods. Any such event in the markets in which we operate could not only impact the day-to-day operations of our care centers, but could also disrupt our relationships with patients, employees and referral sources located in the affected areas and, in the case of our corporate office, our ability to provide administrative support services, including billing and collection services. In addition, any episode of care that is not completed due to government health care reform.
The health care industrysuch an event will generally result in lower revenue for the United States is subject to fundamental changes due to ongoing health care reform effortsepisode. Our corporate office and related political, economic and regulatory influences. In March 2010, comprehensive health care reform legislation was signed into law in the United States through the passage of PPACA.
PPACA makes a number of changesour care centers are located in the southeastern United States and the Gulf Coast Region, increasing our exposure to Medicare payment rateshurricanes and also calls for a rebasingflooding. Moreover, global climate change could increase the intensity of individual hurricanes or the number of hurricanes that occur each year. Even if our facilities are not directly damaged, we may experience considerable disruptions in our operations due to property damage or electrical outages experienced in storm-affected areas by our care givers, payors, vendors and others. Additionally, long-term adverse weather conditions, whether caused by global climate change or otherwise, could cause an outmigration of people from the communities where our care centers are located. If any of the home health payment system. These reimbursement changes are described in detail in Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations: Overview – Payment.”
Regulations implementing the provisions of the PPACA and related initiatives may similarly increase our costs, decrease our revenues, expose us to expanded liability or require us to revise the ways in which we conduct our business.
PPACA also calls for a number of other changes to be made over time that will likely have a significant impact upon the health care delivery system. For example, PPACA mandates creation of a home health value-based purchasing program, the development of quality measures and decreases in home health reimbursement rates, including rebasing, as further described in Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations: Overview – Payment.”
In addition, various health care reform proposals similar to the federal reformscircumstances described above have also emerged at the state level, including in several states in which we operate. We cannot predict with certainty what health care initiatives, if any, willoccur, there could be implemented at the state level, or what the ultimatea harmful effect of federal health care reform or any future legislation or regulation may have on us or on our business and consolidated financial condition,our results of operations could be adversely affected.
Further, the current Russia-Ukraine conflict has created extreme volatility in the global financial markets and cash flows.
In addition to impacting our Medicare businesses, PPACA may also significantly affect our non-Medicare businesses. PPACA makes many changes to the underwriting and marketing practices of private payors. The resulting economic pressures could prompt these payors to seek to lower their rates of reimbursement for the services we provide. PPACA continuesis expected to have residual effects on our non-Medicare business.
Finally, efforts to repeal or substantially modify provisionsfurther global economic consequences, including disruptions of the PPACA continueglobal supply chain and energy markets. Any such volatility or disruptions may have adverse consequences on us or the third parties on whom we rely. If the equity and credit markets deteriorate, including as a result of political unrest or war, it may make any necessary debt or equity financing more difficult to obtain in Congressa timely manner or on favorable terms, more costly or more dilutive. Our business, financial condition and results of operations may be materially and adversely affected by any negative impact on the global economy resulting from the conflict in Ukraine or any other geopolitical tensions.
Inflation in the courts. The ultimate outcomeseconomy could negatively impact our business and results of legislative effortsoperations.
Recently, inflation has increased throughout the United States economy. Our operations have been materially impacted by the current inflationary environment as we have experienced higher labor costs and increases in supply costs, fuel costs and mileage reimbursements. Additionally, cost increases may outpace our expectations, causing us to repeal, substantially amend, eliminate or reduce funding for the PPACA is unknown. In addition to the prospect for legislative repeal or revision, the President and members of his administration hostile to the PPACA could seek to impose substantial changes upon the PPACA through administrative action, including revised regulationuse our cash and other Executive Branch action. The effectliquid assets faster than forecasted. If we are unable to successfully manage the effects of any major modification or repeal of the PPACA oninflation, our business, operations oroperating results, cash flows and financial condition cannotmay be predicted, but could be materially adverse.adversely affected.
Risks Related to our Growth Strategies
Our growth strategy depends on our ability to acquire additional care centers and integrate and operate these care centers effectively.effectively, make investments and enter into joint ventures and other strategic relationships. If our growth strategy is unsuccessful or we are not able to successfully integrate newly acquired care centers into our existing operations, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected.
We may not be able to fully integrate the operations of our acquired businesses with our current business structure in an efficient and cost-effective manner. Acquisitions, investments, joint ventures or strategic relationships involve significant risks and uncertainties, including difficultiesincluding:
•Difficulties in recouping partial episode payments and other types of misdirected payments for services from the previous owners; difficultiesowners in an acquisition;
•Difficulties integrating acquired personnel and business practices into our business; the
•The potential loss of key employees, referral sources or patients of acquired care centers; the
•The delay in payments associated with change in ownership, control and the internal processprocesses of the Medicare fiscal intermediary; and theAdministrative Contractors;
•The assumption of liabilities and exposure to unforeseen liabilities of acquired care centers. centers;
•The incurrence or assumption of significant debt, which could also cause a deterioration of our credit ratings, result in increased borrowing costs and interest expense and diminish our future access to the capital markets;
•Diverging interests from those of our joint venture partners or other strategic partners - we may not be able to direct the management and operations of the joint venture or other strategic relationship in the manner we believe is most appropriate, exposing us to additional risk;
•Variability in operating results which could cause our financial results to differ from our own expectations or the investment community’s expectations in any given period, or over the long-term; and
•Pre-closing and post-closing earnings charges which could adversely impact operating results in any given period.
As a result of our acquisitions and investments, we have recorded significant goodwill and other assets on our balance sheet. If we are not able to realize the value of these assets, or if the fair value of our investments declines, we may be required to record impairment charges which could have a material adverse effect on our consolidated financial condition and results of operations.
Further, the financial benefits we expect to realize from many of our acquisitions are largely dependent upon our ability to improve clinical performance, overcome regulatory deficiencies, improve the reputation of the acquired business in the community and control costs. As we expand our markets, our growth could strain our resources, including our management, information and accounting systems, regulatory compliance, logistics and other internal controls. The failure to accomplish any of these objectives, or to effectively integrate any of these businesses or to maintain a sufficient level of resources to match our growth could have a material adverse effecteffects on our business and consolidated financial condition, results of operations and cash flows.
The indemnification provisions of acquisition agreements by which we have acquired companies may not fully protect us, and as a result, we may face unexpected liabilities.
Certain of the acquisition agreements by which we have acquired companies require the former owners to indemnify us against certain liabilities related to the operation of the acquired company before we acquired it. In most of these agreements, however, the liability of the former owners is limited, and certain former owners may be unable to meet their indemnification responsibilities. We cannot assure you that these indemnification provisions will protect us fully or at all, and as a result, we may face unexpected liabilities that adversely affectcould have a material adverse effect on our business and consolidated financial statements.condition, results of operations and cash flows.
State efforts to regulate the establishment or expansion of health care providers could impair our ability to expand our operations.
Some states require health care providers (including skilled nursing facilities, hospice care centers, home health care centers and assisted living facilities) to obtain prior approval, known as a CON or POA, in order to commence operations. Seeoperations (see Part I, Item 1, “Our Regulatory Environment” for additional information on CONs and POAs.POAs). If we are not able to obtain such approvals, our ability to expand our operations could be impaired, which could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Federal regulation may impair our ability to consummate acquisitions or open new care centers.
Changes in federal laws or regulations may materially adversely impact our ability to acquire care centers or open new start-up care centers. For example, the Social Security Act provides the Secretary with the authority to impose temporary moratoria on the enrollment of new Medicare providers, if deemed necessary to combat fraud, waste or abuse under government programs. While there are no active Medicare moratoria, there can be no assurance that CMS will not adopt a moratorium on new providers in the future. Additionally, in 2010, CMS implemented and amended a regulation known as the “36 Month Rule” that is applicable to home health care center acquisitions. Subject to certain exceptions, the 36 Month Rule prohibits buyers of certain home health care centers - those that either enrolled in Medicare or underwent a change in majority ownership fewer than 36 months prior to the acquisition - from assuming the Medicare billing privileges of the acquired care center. The 36 Month Rule may restrict bona fide transactions and potentially block new investments in home health agencies. These changes in federal laws and regulations, and similar future changes, may further increase competition for acquisition targets and could have a material detrimental impact on our acquisition strategy.
Risks Related to our Operations
Because we are limited in our ability to control rates received for our services,Divestitures or other dispositions could negatively impact our business, and consolidated financial condition, results of operations and cash flows could be materially adversely affected if we are not able to maintain or reduce our costs to provide such services.
As Medicare is our primary payor and rates are established through federal legislation,contingent liabilities from businesses that we have to manage our costs of providing care to achieve a desired level of profitability. Additionally, non-Medicare rates are difficult for us to negotiate as such payors are under pressure to reduce their own costs. As a result, we manage our costs in order to achieve a desired level of profitability including, but not limited to, centralization of various processes, the use of technology and management of the number of employees
utilized. If we are not able to continue to streamline our processes and reduce our costs, our business and consolidated financial condition, results of operations and cash flowssold could be materially adversely affected.
Our industry is highly competitive, with few barriers to entry in certain states.
There are few barriers to entry in home health markets that do not require a CON or POA. Our primary competition comes from local privately-owned and hospital-owned health care providers. We compete based on the availability of personnel; the quality of services; expertise of visiting staff; and in certain instances, on the price of our services. Increased competition in the future may limit our ability to maintain or increase our market share.
Further, the introduction of new and enhanced service offerings by others, in combination with industry consolidation and the development of strategic relationships by our competitors (including mergers of competitors with each other and with insurers), could cause a decline in revenue or loss of market acceptance of our services or make our services less attractive. Additionally, we compete with a number of non-profit organizations that can finance acquisitions and capital expenditures on a tax-exempt basis or receive charitable contributions that are unavailable to us.
Managed care organizations and other third party payors continue to consolidate, which enhances their ability to influence the delivery of health care services. Consequently, the health care needs of patients in the United States are increasingly served by a smaller number of managed care organizations. These organizations generally enter into service agreements with a limited number of providers. Our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected if these organizations terminate us as a provider and/or engage our competitors as a preferred or exclusive provider. In addition, should private payors, including managed care payors, seek to negotiate additional discounted fee structures or the assumption by health care providers of all or a portion of the financial risk through prepaid capitation arrangements, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected.
If we are unable to react competitively to new developments, our operating results may suffer. State CON or POA laws often limit the ability of competitors to enter into a given market, are not uniform throughout the United States and are frequently the subject of efforts to limit or repeal such laws. If states remove existing CONs or POAs, we could face increased competition in these states. For example, New Hampshire repealed its CON laws in 2015, and legislation was recently introduced in South Carolina that would have limited the application of its CON program. There can be no assurances that other states will not seek to eliminate or limit their existing CON or POA programs, which could lead to increased competition in these states. Further, we cannot assure you that we will be able to compete successfully against current or future competitors, which could have a material adverse effect onaffect our business and consolidated financial condition, results of operations and cash flows.
If weWe continually assess the strategic fit of our existing businesses and may divest, spin-off or otherwise dispose of businesses that are unabledeemed not to maintain relationshipsfit with existing patient referral sources,our strategic plan or are not achieving the desired return on investment. These transactions pose risks and challenges that could negatively impact our business and consolidated financial condition, resultsstatements. For example, when we decide to sell or otherwise dispose of operationsa business or assets, we may be unable to do so on satisfactory terms within our anticipated timeframe or at all, and cash flows could be materially adversely affected.
Our success depends on referrals from physicians, hospitalseven after reaching a definitive agreement to sell or dispose a business, the sale is typically subject to satisfaction of pre-closing conditions which may not become satisfied. In addition, divestitures or other dispositions may dilute our earnings per share, have other adverse tax, financial and other sources in the communitiesaccounting impacts and distract management, and disputes may arise with buyers. In addition, we servemay retain responsibility for and/or agree to indemnify buyers against some known and onunknown contingent liabilities related to certain businesses or assets we sell or dispose. Any of these conditions or liabilities may negatively impact our ability to maintain good relationships with existing referral sources. Our referral sources are not contractually obligated to refer patients to us and may refer their patients to other providers. Our growth and profitability depend, in part, on our ability to establish and maintain close working relationships with these patient referral sources and to increase awareness and acceptance of the benefits of home health and hospice care by our referral sources and their patients. Our loss of, or failure to maintain, existing relationships or our failure to develop new referral relationships could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
If we are unableRisks Related to provide consistently high quality of care, our business will be adversely impacted.Laws and Government Regulations
Providing quality patient care is the cornerstone of our business. We believe that hospitals, physicians and other referral sources refer patients to us in large part because of our reputation for delivering quality care. Clinical quality is becoming increasingly important within our industry. Effective October 2012, Medicare began to impose a financial penalty upon hospitals that have excessive rates of patient readmissions within 30 days from hospital discharge. We believe this regulation provides a competitive advantage to home health providers who can differentiate themselves based upon quality, particularly by achieving low patient acute care hospitalization readmission rates and by implementing disease management programs designed to be responsive to the needs of patients served by referring hospitals. We are focused intently upon improving our patient outcomes, particularly our patient acute care hospitalization readmission rates. If we should fail to attain our goals regarding acute care hospitalization readmission rates and other quality metrics, we expect our ability to generate referrals would be adversely impacted, which could have a material adverse effect upon our business and consolidated financial condition, results of operations and cash flows.
Additionally, Medicare has established consumer-facing websites, Home Health Compare and Hospice Compare, that present data regarding our performance on certain quality measures compared to state and national averages. If we should fail to achieve or exceed these averages, it may affect our ability to generate referrals, which could have a material adverse effect upon our business and consolidated financial condition, results of operations and cash flows.
Our business depends on our information systems. A cyber-attack, security breach or our inability to effectively integrate, manage and keep our information systems secure and operational could disrupt our operations.
Our business depends on effective, secure and operational information systems which include systems provided by external contractors and other service providers. For example, our care centers depend upon our information systems for patient care, accounting, billing, collections, risk management, quality assurance, human resources, payroll and other information. Our networks and devices store sensitive information, including intellectual property, proprietary business information and personally identifiable information of our patients, partners, and employees.
In general, our information systems are vulnerable to damage or interruption from fire, flood, power loss, telecommunications failure, human acts, break-ins and similar events. Our business is at risk from and may be impacted by information security incidents, including ransomware, malware, phishing, social engineering, and other security events. Such incidents can range from individual attempts to gain unauthorized access to information technology systems to more sophisticated security threats. These events can also result from internal compromises, such as human error or malicious acts. These events can occur on our systems or on the systems of our partners and subcontractors.
Problems with, or the failure of, our technology and systems or any system upgrades or programming changes associated with such technology and systems could have a material adverse effect on our operations, patient care, data capture, medical documentation, billing, collections, assessment of internal controls and management and reporting capabilities. If we experience a reduction in the performance, reliability, or availability of our information systems, our operations and ability to produce timely and accurate reports could be materially adversely affected. If we are subject to cyber-attacksextensive government regulation. Any changes to the laws and regulations governing our business, or security breaches into the future, this could result in harm to patients; business interruptionsinterpretation and delays; the loss, misappropriation, corruption or unauthorized accessenforcement of data; litigation and potential liability under privacy, security and consumer protectionthose laws or other applicable laws; reputational damage and federal and state governmental inquiries. Any such problems or failures and the costs incurred in correcting any such problems or failures, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Further, to the extent our external information technology contractors or other service providers become insolvent or fail to support the software or systems we have licensed from them, our operations could be materially adversely affected. A failure to restore our information systems after the occurrence of any of these events could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Because of the confidential health information we store and transmit, loss of electronically stored information for any reason could expose us to a risk of regulatory action and litigation and possible liability and loss.
Our information systems and applications also require continual maintenance, upgrading and enhancement to meet our operational needs. Our acquisition activity requires transitions and integration of various information systems. We regularly upgrade and expand our information systems’ capabilities. If we experience difficulties with the transition and integration of information systems or are unable to implement, maintain, or expand our systems properly, we could suffer from, among other things, operational disruptions, regulatory problems and increases in administrative expenses.
As cyber threats continue to evolve, we may be required to expend significant capital and other resources to protect against the threat of security breaches or to mitigate and alleviate problems caused by breaches, including unauthorized access to patient data and personally identifiable information stored in our information systems, and the introduction of computer viruses or other malicious software programs to our systems. Our security measures may be inadequate to prevent security breaches and our business operations could be materially adversely affected by federal and state fines and penalties, legal claims or proceedings, cancellation of contracts and loss of patients if security breaches are not prevented. The healthcare industry is currently experiencing increased attention on compliance with regulations, designed to safeguard protected health information and mitigate cyber-attacks on entities. There are significant costs associated with a breach, including investigation costs, remediation and mitigation costs, notification costs, attorney fees, and the potential for reputational harm and lost revenues due to a loss in confidence in the provider. We cannot predict the costs to comply with these laws or the costs associated with a potential breach of protected health information, which could have a material adverse effect on our business, results of operations, financial position and cash flows, and our business reputation.
We have installed privacy protection systems and devices on our network and point of care tablets in an attempt to prevent unauthorized access to information in our database. However, our technology may fail to adequately secure the confidential health
information and personally identifiable information we maintain in our databases. In such circumstances, we may be held liable to our patients and regulators, which could result in fines, litigation or adverse publicity that could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Even if we are not held liable, any resulting negative publicity could harm our business and distract the attention of management.
We believe that our subcontractors and vendors take precautionary measures to prevent problems that could affect our business operations as a result of failure or disruption to their information systems. However, there is no guarantee such efforts will be successful in preventing a disruption, and it is possible that we may be impacted by information system failures. The occurrence of any information system failures could result in interruptions, delays, loss or corruption of data and cessations or interruptions in the availability of these systems. All of these events or circumstances, among others, could have an adverse effect on our business, results of operations, financial position and cash flows, and they could harm our business reputation.
We believe we have all the necessary licenses from third parties to use technology and software that we do not own. A third party could, however, allege that we are infringing its rights, which may deter our ability to obtain licenses on commercially reasonable terms from the third party, if at all, or cause the third party to commence litigation against us. In addition, we may find it necessary to initiate litigation to protect our trade secrets, to enforce our intellectual property rights and to determine the scope and validity of any proprietary rights of others. Any such litigation, or the failure to obtain any necessary licenses or other rights, could materially and adversely affect our business.
Possible changes in the case mix of patients, as well as payor mix and payment methodologies, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Our revenueindustry is determined by a number of factors, includingsubject to extensive federal and state laws and regulations. See Part I, Item 1, “Our Regulatory Environment” for additional information on such laws and regulations. Federal and state laws and regulations impact how we conduct our mix ofbusiness, the services we offer and our interactions with patients, our employees and the ratespublic and impose certain requirements on us such as:
•licensure and certification;
•adequacy and quality of payment among payors.health care services;
•qualifications of health care and support personnel;
•quality and safety of medical equipment;
•confidentiality, maintenance and security associated with medical records and claims processing;
•relationships with physicians and other referral sources;
•operating policies and procedures;
•emergency preparedness risk assessments and policies and procedures;
•policies and procedures regarding employee relations;
•addition of facilities and services;
•billing for services;
•requirements for utilization of services;
•documentation required for billing and patient care; and
•reporting and maintaining records regarding adverse events.
These laws and regulations, and their interpretations, are subject to change. Changes in existing laws and regulations, or their interpretations, or the case mixenactment of new laws or regulations could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows by:
•increasing our administrative and other costs;
•increasing or decreasing mandated services;
•causing us to abandon business opportunities we might have otherwise pursued;
•decreasing utilization of services;
•forcing us to restructure our relationships with referral sources and providers; or
•requiring us to implement additional or different programs and systems.
Additionally, we are subject to various routine and non-routine reviews, audits and investigations by the Medicare and Medicaid programs and other federal and state governmental agencies, which have various rights and remedies against us if they establish that we have overcharged the programs or failed to comply with program requirements. We are also subject to potential lawsuits under the federal False Claims Act and other federal and state whistleblower statutes designed to combat fraud and abuse in our industry. Violation of the laws governing our operations, or changes in interpretations of those laws, could result in the imposition of fines, civil or criminal penalties, the termination of our patients, payment methodologies rights to participate in federal and state-sponsored programs and/or the payor mix among Medicare, Medicaidsuspension or revocation of our licenses. If we become subject to material fines, or if other sanctions or other corrective actions are imposed on us, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected.
We face periodic and routine reviews, audits and investigations under our contracts with federal and state government agencies and private payors, and these audits could have adverse findings that may negatively impact our business.
As a result of our participation in the Medicare and Medicaid programs, we are subject to various governmental reviews, audits and investigations to verify our compliance with these programs and applicable laws and regulations. We also are subject to audits under various federal and state government programs in which third party firms engaged by CMS, including Recovery Audit Contractors (“RACs”), Zone Program Integrity Contractors (“ZPICs”), Uniform Program Integrity Contractors ("UPICs"), Program Safeguard Contractors (“PSCs”), Medicaid Integrity Contractors (“MICs”) and Supplemental Medical Review Contractors (“SMRCs”), conduct extensive reviews of claims data and medical and other records to identify potential improper payments under the Medicare program. Additionally, private pay sources reserve the right to conduct audits. If billing errors are identified in the sample of reviewed claims, the billing error can be extrapolated to all claims filed which could result in a larger overpayment than originally identified in the sample of reviewed claims. Our costs to respond to and defend reviews, audits and investigations may be significant and could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Moreover, an adverse review, audit or investigation could result in:
•required refunding or retroactive adjustment of amounts we have been paid pursuant to the federal or state programs or from private payors;
•state or federal agencies imposing fines, penalties and other sanctions on us;
•loss of our right to participate in the Medicare program, state programs or one or more private payor networks; or
•damage to our business and reputation in various markets.
These results could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
If a care center fails to comply with the conditions of participation in the Medicare program, that care center could be subjected to sanctions or terminated from the Medicare program.
Each of our care centers must comply with required conditions of participation in the Medicare program. If we fail to meet the conditions of participation at a care center, we may receive a notice of deficiency from the applicable state surveyor. If that care center then fails to institute an acceptable plan of correction to remediate the deficiency within the correction period provided by the state surveyor, that care center could be terminated from the Medicare program or subjected to alternative sanctions. CMS may impose temporary management, direct a plan of correction, direct training or impose payment suspensions and civil monetary penalties, in each case, upon providers who fail to comply with the conditions of participation. Termination of one or more of our care centers from the Medicare program for failure to negotiate favorable managed care contracts,satisfy the program’s conditions of participation, or our lossthe imposition of existing favorable managed care contracts,alternative sanctions, could disrupt operations, require significant attention by management or have a material adverse effect on our business and reputation and consolidated financial condition, results of operations and cash flows.
One ofWe are subject to federal and state laws that govern our strategies is to diversify our payor sources by increasing the business we dofinancial relationships with managed care companies, and we strive to put in place favorable contracts with managed care payors. However, we may not be successful in these efforts. Additionally, there is a risk that the favorable managed care contracts that we put in place may be terminated. Managed care contracts typically permit the payor to terminate the contract without cause, on very short notice, typically 60 days, which can provide payors leverage to reduce volume or obtain favorable pricing. Our failure to negotiate and put in place favorable managed care contracts, or our failure to maintain in place favorable managed care contracts, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
A write off of a significant amount of intangible assets or long-lived assets could have a material adverse effect on our consolidated financial condition and results of operations.
A significant and sustained decline in our stock price and market capitalization, a significant decline in our expected future cash flows, a significant adverse change in the business climate or slower growth rates could result in the need to perform an impairment analysis under Accounting Standard Codification (“ASC”) Topic 350 “Intangibles – Goodwill and Other” in future periods in addition to our annual impairment test. If we were to conclude that a write down of goodwill is necessary, then we would record the appropriate charge, which could result in material charges that are adverse to our consolidated financial condition and results of operations. See Part II, Item 8, Note 4 – Goodwill and Other Intangible Assets, Net to our consolidated financial statements for additional information.
Because we have grown in part through acquisitions, goodwillphysicians and other acquired intangible assets represent a substantial portionhealth care providers, including potential or current referral sources.
As stated in Part I, Item 1, "Our Regulatory Environment" of our assets. Goodwill was approximately $658.5 million as of December 31, 2019this document pertaining to Federal and ifState Anti-Fraud and Abuse Laws and Regulations, we make additional acquisitions, it is likely that we will record additional goodwillare required to comply with various federal anti-fraud and intangible assets in our consolidated financial statements. We also have long-lived assets consisting of propertyabuse laws, including the Anti-Kickback Statute, the Stark or Physician Self-Referral Law, the False Claims Act and equipment and other identifiable intangible assets of $92.9 million as of December 31, 2019, which we review on a periodic basisCivil Monetary Penalties Law, as well as when events or circumstances indicate that the carrying amount of an asset may not be recoverable. If a determination that a significant impairment in value ofstate laws and regulations.
Although we believe we have structured our unamortized intangible assets or long-lived assets occurs, such determination could require us to write off a substantial portion of our assets. A write off of these assets could have a material adverse effect on our consolidated financial condition and results of operations.
A shortage of qualified registered nursing staffrelationships with physicians and other clinicians, such as therapistsactual or potential referral sources to comply with these laws where applicable, the laws are complex, and nurse practitioners, could materially impact our abilitythe Stark Law contains a number of strict liability provisions under which no intent to attract, trainviolate the law is required for a violation to be found. It is possible that courts or regulatory agencies may interpret state and retain qualified personnelfederal anti-kickback laws and/or the Stark Law and could increase operating costs.
We compete for qualified personnel with other healthcare providers. Our ability to attract and retain clinicians depends on several factors, including our ability to provide these personnel with attractive assignments and competitive salaries and benefits. We cannot be assured we will succeed in any of these areas. In addition, there are shortages of qualifiedsimilar state laws regulating relationships between health care personnelproviders and physicians in someways that will adversely implicate our practices or that isolated instances of our markets. As a result, wenoncompliance may face higher costsoccur. Violations of attracting clinicians and providing them with attractive benefit packages than we originally anticipatedfederal or state Stark or anti-kickback laws could lead to criminal or civil fines or other sanctions, including repayment of federal health care program payments related to these arrangements, denials of government program reimbursement or even exclusion from participation in governmental health care programs, which could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. In addition, if we expand our operations into geographic areas where health care providers historically have been unionized,It is possible that a claim that results from a kickback or if any of our care center employees become unionized, being subject to a collective bargaining agreement may have a negative impact on our ability to timely and successfully recruit qualified personnel and may increase our operating costs. Generally, if we are unable to attract and retain clinicians, the quality of our services may decline and we could lose patients and referral sources, which could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Our insurance liability coverage may not be sufficient for our business needs.
As a result of operating in the home health industry, our business entails an inherent risk of claims, losses and potential lawsuits alleging incidents involving our employees that are likely to occur in a patient’s home. We maintain professional liability insurance to provide coverage to us and our subsidiaries against these risks. However, we cannot assure you claims will not beis made in the future in excessviolation of the limits of our insurance, nor can we assure you that any such claims, if successful and in excess of such limits, will not have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. Our insurance coverageStark Law also includes fire, property damage and generalmay render it false or fraudulent, creating further potential liability with varying limits. We cannot assure you thatunder the insurance we maintain will satisfy claims made against us or that insurance coverage will continue to be available to us at commercially reasonable rates, in adequate amounts or on satisfactory terms. Any claims made against us, regardless of their merit or eventual outcome, could damage our reputation and business.
We may be subject to substantial malpractice or other similar claims.federal False Claims Act, discussed above.
The services we offer involve an inherent risk of professional liabilityNo Surprises Act and related substantial damage awards. As of February 14, 2020, we have approximately 21,300 employees (11,600 home health, 5,800 hospice, 3,000 personal care and 900 corporate employees). In addition, we employ direct care workers on a contractual basis to support our existing workforce. Due to the nature of our business, we, through our employees and caregivers who provide services on our behalf, may be the subject of medical malpractice claims. A courtsimilar price transparency initiatives could find these individuals should be considered our agents, and, as a result, we could be held liable for their acts or omissions. We cannot predict the effect that any claims of this nature, regardless of their ultimate outcome, could have on our business or reputation or on our ability to attract and retain patients and employees. While we maintain malpractice liability coverage that we believe is appropriate given the nature and breadth of our operations, any claims against us in excess of insurance limits, or multiple claims requiring us to pay deductibles, could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
If we are unable to maintain our corporate reputation, our business may suffer.
Our success depends on our ability to maintain our corporate reputation, including our reputation for providing quality patient care and for compliance with Medicare requirements and the other laws to which we are subject. Adverse publicity surrounding any aspect of our business, including the death or disability of any of our patients due to our failure to provide proper care, or due to any failure on our part to comply with Medicare requirements or other laws to which we are subject, could negatively affect our Company’s overall reputation and the willingness of referral sources to refer patients to us.
We depend on the services of our executive officers and other key employees.
We depend greatly on the efforts of our executive officers and other key employees to manage our operations. The loss or departure of any one of these executives or other key employees could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
Our operations could be impacted by natural disasters.
The occurrence of natural disasters in the markets in which we operate could not only impact the day-to-day operations of our care centers, but could also disrupt our relationships with patients employees and referral sources located ininsurers.
Effective January 1, 2022, the affected areasNo Surprises Act, enacted as part of the Consolidated Appropriations Act, 2021, creates price transparency requirements, including (i) requiring providers to send to patients or their health plan a good faith estimate of the expected charges and indiagnostic codes prior to furnishing scheduled items or services and (ii) prohibiting providers from charging patients an amount beyond the case of our corporate office,in-network cost sharing amount for services rendered by out-of network providers, subject to limited exceptions. Price transparency initiatives such as the No Surprises Act may impact our ability to provide administrative support services, including billingobtain or maintain favorable contract terms, and collection services. In addition, any episode of care that is not completed due to themay impact of a natural disaster will generally result in lower revenue for the episode. For example, our corporate officecompetitive position and a number of our care centers are located in the southeastern United Statesrelationships with patients and the Gulf Coast Region, increasing our exposure to hurricanes and flooding. Future hurricanes or other natural disasters may have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.insurers.
Risks Related to Liquidity
Delays in payment may cause liquidity problems.
Our business is characterized by delays from the time we provide services to the time we receive payment for these services. If we have difficulty in obtaining documentation, such as physician orders, experience information system problems or experience other issues that arise with Medicare or other payors, we may encounter additional delays in our payment cycle.
In addition, timingTiming delays in billings and collections may cause working capital shortages. Working capital management, including prompt and diligent billing and collection, is an important factor in achieving our financial results and maintaining liquidity. It is possible that delays in obtaining documentation support such as physician orders, system problems, Medicare or other providerpayor issues or industry trends may extend our collection period, which may materially adversely affect our working capital, and our working capital management procedures may not successfully mitigate this risk. CMS's inability to have its systems ready to properly reimburse home health providers under the new PDGM could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
On May 31,29, 2018, CMS issued a notice indicating its intention to re-launch a home health agency ("HHA") pre-claim review demonstration project. Now called the Review Choice Demonstration for Home Health Services ("RCD") and fully implemented in five states as of April 1, 2022 (Florida, Illinois, North Carolina, Ohio and Texas), the revised demonstration will give HHAsgives home health agencies in the demonstration states 3three initial options: pre-claim review of all claims, post-payment review of all claims, or minimal post-payment review with a 25% payment reduction for all home health services. The demonstration initially will apply to HHA providersReduced review options are available for home health agencies that demonstrate compliance. Compliance with this process has resulted in Florida, Illinois, North Carolina, Ohio,increased administrative costs and Texas, with the option to expand after 5 years to other statesdelays in reimbursement for home health services in the Medicare Administrative Contractor Jurisdiction M (Palmetto). In an October 21, 2019 release, CMS announced that it will reschedule the next phase of its RCD to allow agencies time to transition to PDGM. RCD implementation will resume on March 2, 2020 in Texas, followed by demonstrations in North Carolina and Florida on May 4, 2020. However, CMS officials have indicated that these dates arestates subject to change. Following the start of the demonstration in Texas, the demonstration is expected to begin in North Carolina and Florida on May 4, 2020. CMS will monitor the transition to the PDGM and assess the need for any change to this date.demonstration. These delays could materially adversely affect our working capital.
Additionally, our hospice operations may experience payment delays. We have experienced payment delays when attempting to collect funds from state Medicaid programs in certain instances. Delays in receiving payments from these programs may also materially adversely affect our working capital.
Changes in units of payment for home health agencies could reduce our Medicare home health reimbursement levels.
As required byEffective January 1, 2020, CMS implemented a revised case-mix adjustment methodology, the Bipartisan Budget Act of 2018, the PDGM willPatient-Driven Groupings Model ("PDGM"). Although this change the unit of payment for home health agencies from a 60-day episode of care to 30-day periods of care. This change is proposedwas to be implemented in aan overall budget neutral manner. Thus,manner, the move to the PDGM is not supposed to result in lower net reimbursement. However,ultimate impact varied by provider based on factors including patient mix and admission source. Additionally, CMS has made assumptions about behavioral changes; for example, that home health agencies will change their documentationchanges which resulted in a 4.36% reduction to reimbursement. Accordingly, the adoption of PDGM had a negative impact on our Medicare revenue per episode in 2020. Additionally, in the Calendar Year 2023 Home Health Final Rule, CMS finalized a 3.5% permanent reduction in reimbursement based on the difference between assumed and coding practices and would put the highest paying diagnosis code as the principal diagnosis code in order to have a 30-day period be placed into a higher-paying clinical group. CMS has taken into account expectedactual behavioral effects of policy changes related to implementation of the PDGM, resulting in lower reimbursement levels in some cases. Accordingly,from the implementation of PDGM. The -3.5% permanent adjustment is derived from a -3.925% behavioral assumption adjustment which is half of the PDGMfull proposed adjustment of 7.85%. The remaining -3.925% behavioral assumption adjustment will be considered in future rulemaking. In addition to the permanent adjustments, CMS is also considering a temporary adjustment of $2 billion to offset overpayments in calendar years 2020 and 2021. Payment updates could continue to negatively impact our 2020 raterates of reimbursement in future years and have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows. See Part I, Item 1, “Our Regulatory Environment -– Home Health Payment Reform” for additional information on the PDGM.
The volatility and disruption of the capital and credit markets and adverse changes in the United States and global economies could impact our ability to access both available and affordable financing, and without such financing, we may be unable to achieve our objectives for strategic acquisitions and internal growth.
While we intend to finance strategic acquisitions and internal growth with cash flows from operations and borrowings under our revolving credit facility, we may require sources of capital in addition to those presently available to us. Uncertainty in the capital and credit markets may impact our ability to access capital on terms acceptable to us (i.e. at attractive/affordable rates) or at all, and this may result in our inability to achieve present objectives for strategic acquisitions and internal growth. Further, in the event we need additional funds, and we are unable to raise the necessary funds on acceptable terms, our business and consolidated financial condition, results of operations and cash flows could be materially adversely affected.
Our indebtedness could impact our financial condition and impair our ability to fulfill other obligations.
As of December 31, 2019,2022, we had total outstanding indebtedness, excluding finance leases, of approximately $242.3$436.1 million. Our level of indebtedness could have a material adverse effect on our business and consolidated financial position, results of operations and cash flows and could impair our ability to fulfill other obligations in several ways, including:
•it could require us to dedicate a portion of our cash flow from operations to payments on our indebtedness, which could reduce the availability of cash flow to fund acquisitions, start-ups, working capital, capital expenditures and other general corporate purposes;
•it could limit our ability to borrow money or sell stock for working capital, capital expenditures, debt service requirements and other purposes;
•it could limit our flexibility in planning for, and reacting to, changes in our industry or business;
•it could make us more vulnerable to unfavorable economic or business conditions; and
•it could limit our ability to make acquisitions or take advantage of other business opportunities.
In the event we incur additional indebtedness, the risks described above could increase.
The agreements governing our indebtedness contain various covenants that limit our discretion in the operation of our business and our failure to satisfy requirements in these agreements could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
The agreements governing our indebtedness (the “Debt Agreements”) contain certain obligations, including restrictive covenants that require us to comply with or maintain certain financial covenants and ratios and restrict our ability to:
•incur additional debt;
•redeem or repurchase stock, pay dividends or make other distributions;
•make certain investments;
•create liens;
•enter into transactions with affiliates;
•make acquisitions;
•enter into joint ventures;
•merge or consolidate;
•invest in foreign subsidiaries;
•amend acquisition documents;
•enter into certain swap agreements;
•make certain restricted payments;
•transfer, sell or leaseback assets; and
•make fundamental changes in our corporate existence and principal business.
Our Debt Agreements also limit our ability to reinvest the net cash proceeds from asset sales or subordinated debt issuances in certain circumstances. For example, in the event we or any of our subsidiaries receive more than $5 million in net cash proceeds from an asset sale, disposition or involuntary disposition, our Debt Agreements require us to prepay our term loan facility and revolving credit facility with all of such net cash proceeds, unless we elect to reinvest the net cash proceeds in fixed or capital assets related to our business.
In addition, events beyond our control could affect our ability to comply with the Debt Agreements. Any failure by us to comply with or maintain all applicable financial covenants and ratios and to comply with all other applicable covenants could result in an event of default with respect to the Debt Agreements. If we are unable to obtain a waiver from our lenders in the event of any non-compliance, our lenders could accelerate the maturity of any outstanding indebtedness and terminate the commitments to make further extensions of credit (including our ability to borrow under our revolving credit facility). Any failure to comply with these covenants could have a material adverse effect on our business and consolidated financial condition, results of operations and cash flows.
The potential cessation or modification of LIBOR may increase our interest expense or otherwise adversely affect us.
Our credit facility carries a floating interest rate which is tied to the Eurodollar rate (i.e., LIBOR) and the prime rate. On July 27, 2017, the United Kingdom’s Financial Conduct Authority, which regulates LIBOR, announced that it intends to stop persuading or compelling banks to submit LIBOR quotations after 2021 (the “FCA Announcement”). The FCA Announcement indicates that the continuation of LIBOR on the current basis cannot and will not be assured after 2021, and LIBOR may cease to exist or otherwise be unsuitable for use as a benchmark. Recent proposals for LIBOR reforms may result in the establishment of new methods of calculating LIBOR or the establishment of one or more alternative benchmark rates. Although our credit facility provides for alternative base rates, some of those alternative base rates are related to LIBOR, and the consequences of any potential cessation, modification or other reform of LIBOR cannot be predicted at this time. If LIBOR ceases to exist, we most likely will need to amend the credit facility, and we cannot predict what alternative interest rate(s) will be negotiated with our counterparties. As a result, our interest expense may increase, our ability to refinance some or all of our existing indebtedness may be impacted and our available cash flow may be adversely affected.
Risks Related to Ownership of Our Common Stock
The price of our common stock has been and may continue to be volatile.volatile, which could lead to securities litigation brought against us or cause investors to lose the value of their investment.
The price at which our common stock trades has experienced significant volatility and may continue to be volatile. The stock market from time to time experiences significant price and volume fluctuations that impactDuring 2022, the market prices of securities, particularly those of health care companies. The marketclosing price of our common stock ranged from a high of $178.09 per share to a low of $80.12 per share. Various factors have impacted, and may be influenced by many factors, including:continue to impact, the price of our common stock, including among others:
our operating and financial performance;
•variances in our quarterly financial results compared to research analyst expectations;
the depth•changes in financial estimates and liquidity of the market forrecommendations by securities analysts;
•changes in our common stock;estimates, guidance or business plans;
future purchases or sales of common stock by the Company or large stockholders or the perception that such purchases or sales could occur;•changes in management;
investor, analyst and media perception of our business and our prospects;
developments relating to litigation or governmental investigations;
•changes or proposed changes in health care laws or regulations or enforcement of these laws and regulations, or announcements relating to these matters;
departure of key personnel;
•changes in the Medicare, Medicaid and private insurance payment rates for home health and hospice;
•the operating and stock price performance of other comparable companies;
•announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments; or
•market and business conditions related to COVID-19;
•general economic and stock market conditions.conditions; or
•other factors described in this "Risk Factors" section and elsewhere in this Annual Report on Form 10-K.
In addition, the stock market in general, and the NASDAQ Global Select Market (“NASDAQ”) in particular, has experienced price and volume fluctuations that we believe have often been unrelated or disproportionate to the operating performance of
health care provider companies. These broad market and industry factors may materially reduce the market price of our common stock,
regardless of our operating performance. SecuritiesAs a result, investors may not be able to sell their common stock at or above the purchase price. In addition, securities class-action cases have often been brought against companies following periods of volatility in the market price of their securities. Such litigation, if instituted against us, could result in substantial costs and a diversion of management's attention and resources.
The activities of short sellers could reduce the price or prevent increases in the price of our common stock. “Short sale” is defined as the sale of stock by an investor that the investor does not own. Typically, investors who sell short believe the price of the stock will fall, and anticipate selling shares at a higher price than the purchase price at which they will buy the stock. As of December 31, 2019,2022, investors held a short position of approximately 1.31.6 million shares of our common stock which represented 4%5% of our outstanding common stock. The anticipated downward pressure on our stock price due to actual or anticipated sales of our stock by some institutions or individuals who engage in short sales of our common stock could cause our stock price to decline.
Our Board of Directors may use anti-takeover provisions or issue stock to discourage a change of control.
Our certificate of incorporation currently authorizes us to issue up to 60,000,000 shares of common stock and 5,000,000 shares of undesignated preferred stock. Our Board of Directors may cause us to issue additional stock to discourage an attempt to obtain control of our company. For example, shares of stock could be sold to purchasers who might support our Board of Directors in a control contest or to dilute the voting or other rights of a person seeking to obtain control. In addition, our Board of Directors could cause us to issue preferred stock entitling holders to vote separately on any proposed transaction, convert preferred stock into common stock, demand redemption at a specified price in connection with a change in control or exercise other rights designed to impede a takeover.
The issuance of additional shares may, among other things, dilute the earnings and equity per share of our common stock and the voting rights of common stockholders.
We have implemented other anti-takeover provisions or provisions that could have an anti-takeover effect, including advance notice requirements for director nominations and stockholder proposals, no cumulative voting for directors, a requirement that director vacancies are filled by remaining directors (including vacancies resulting from removal), and the number of directors is fixed by the Board of Directors, and the Board of Directors can increase or decrease the size of the Board of Directors without stockholder approval (within the range set forth in our Certificate of Incorporation and Bylaws). These provisions, and others that our Board of Directors may adopt hereafter, may discourage offers to acquire us and may permit our Board of Directors to choose not to entertain offers to purchase us, even if such offers include a substantial premium to the market price of our stock. Therefore, our stockholders may be deprived of opportunities to profit from a salechange of control.
Our Bylaws provide that unless we otherwise consent to the selection of an alternative forum, the Court of Chancery of the State of Delaware or, if the Court of Chancery does not have jurisdiction, the federal court for the District of Delaware, will be the sole and exclusive forum for any derivative action or proceeding brought on behalf of us, any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, employees or agents to us or our stockholders, any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law or our Certificate of Incorporation or Bylaws or any action asserting a claim governed by the internal affairs doctrine. This provision would not apply to claims brought to enforce a duty or liability created by the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or any other claim for which the federal courts have exclusive jurisdiction. In addition, our Bylaws provide that the federal district courts of the United States will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act of 1933, as amended (the “Securities Act”), unless we consent in writing to the selection of an alternative forum.
These exclusive forum provisions may limit the ability of our stockholders to bring a claim in a judicial forum that such stockholders find favorable for disputes with us or our directors or officers, which may discourage such lawsuits against us and our directors, officers, employees and agents.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our executive office is located in Nashville, Tennessee in a leased property consisting of 25,09720,759 square feet; our corporate headquarters is located in Baton Rouge, Louisiana in a leased property consisting of 85,955 square feet. We believe we have adequate space to accommodate our corporate staff located in these locations for the foreseeable future.
In addition to our executive office and corporate headquarters, we also lease facilities for our home health, hospice and personal-care care centers.centers and our high acuity care joint ventures. Generally, our leases have an initial term of five years, but range from one to ten years. Most of our leases also contain early termination options and renewal options. The following table shows the location of our 321347 Medicare-certified home health care centers, 138164 Medicare-certified hospice care centers, and 1213 personal-care care centers and 8 admitting high acuity care joint ventures at December 31, 2019:2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| State | | Home Health | | Hospice | | Personal Care | | High Acuity Care | | State | | Home Health | | Hospice | | Personal Care | | High Acuity Care |
| Alabama | | 29 | | | 10 | | | — | | | — | | | Nebraska | | 1 | | | 7 | | | — | | | — | |
| Arizona | | 3 | | | 1 | | | — | | | 1 | | | New Hampshire | | 3 | | | 3 | | | — | | | — | |
| Arkansas | | 7 | | | — | | | — | | | — | | | New Jersey | | 2 | | | 7 | | | — | | | — | |
| California | | 4 | | | 1 | | | — | | | — | | | New York | | 6 | | | — | | | — | | | 1 | |
| Connecticut | | 1 | | | 1 | | | — | | | — | | | North Carolina | | 13 | | | 7 | | | — | | | — | |
| Delaware | | 2 | | | 2 | | | — | | | — | | | Ohio | | 4 | | | 5 | | | — | | | — | |
| Florida | | 16 | | | 6 | | | 1 | | | — | | | Oklahoma | | 7 | | | 1 | | | — | | | — | |
| Georgia | | 56 | | | 9 | | | — | | | — | | | Oregon | | 3 | | | 1 | | | — | | | — | |
| Illinois | | 2 | | | — | | | — | | | — | | | Pennsylvania | | 8 | | | 20 | | | — | | | 2 | |
| Indiana | | 5 | | | 5 | | | — | | | — | | | Rhode Island | | 1 | | | 2 | | | — | | | — | |
| Iowa | | — | | | 1 | | | — | | | — | | | South Carolina | | 26 | | | 8 | | | — | | | 1 | |
| Kansas | | — | | | 1 | | | — | | | — | | | South Dakota | | — | | | 1 | | | | | |
| Kentucky | | 17 | | | — | | | — | | | — | | | Tennessee | | 45 | | | 15 | | | 1 | | | — | |
| Louisiana | | 8 | | | 5 | | | — | | | — | | | Texas | | 17 | | | 12 | | | — | | | — | |
| Maine | | 3 | | | 4 | | | — | | | — | | | Virginia | | 14 | | | 5 | | | — | | | — | |
| Maryland | | 9 | | | 3 | | | — | | | — | | | Washington | | 2 | | | — | | | — | | | — | |
| Massachusetts | | 6 | | | 10 | | | 11 | | | — | | | West Virginia | | 11 | | | 6 | | | — | | | — | |
| Michigan | | — | | | — | | | — | | | 1 | | | Wisconsin | | 1 | | | 3 | | | — | | | 2 | |
| Mississippi | | 8 | | | — | | | — | | | — | | | Washington, D.C. | | 1 | | | — | | | — | | | — | |
| Missouri | | 6 | | | 2 | | | — | | | — | | | Total | | 347 | | | 164 | | | 13 | | | 8 | |
|
| | | | | | | | | | | | | | | | | | | | |
| State | | Home Health | | Hospice | | Personal Care | | State | | Home Health | | Hospice | | Personal Care |
| Alabama | | 30 |
| | 7 |
| | — |
| | New Jersey | | 2 |
| | 7 |
| | — |
|
| Arkansas | | 5 |
| | — |
| | — |
| | Nebraska | | — |
| | 2 |
| | — |
|
| Arizona | | 3 |
| | 1 |
| | — |
| | New York | | 4 |
| | — |
| | — |
|
| California | | 4 |
| | 2 |
| | — |
| | New Hampshire | | 3 |
| | 4 |
| | — |
|
| Connecticut | | 4 |
| | 1 |
| | — |
| | North Carolina | | 8 |
| | 6 |
| | — |
|
| Delaware | | 2 |
| | 2 |
| | — |
| | Ohio | | 1 |
| | 3 |
| | — |
|
| Florida | | 18 |
| | 5 |
| | 1 |
| | Oklahoma | | 6 |
| | 1 |
| | — |
|
| Georgia | | 60 |
| | 10 |
| | — |
| | Oregon | | 3 |
| | 1 |
| | — |
|
| Illinois | | 3 |
| | 1 |
| | — |
| | Pennsylvania | | 7 |
| | 14 |
| | — |
|
| Indiana | | 5 |
| | 1 |
| | — |
| | Rhode Island | | 1 |
| | 2 |
| | — |
|
| Kansas | | 1 |
| | 2 |
| | — |
| | South Carolina | | 21 |
| | 8 |
| | — |
|
| Kentucky | | 17 |
| | — |
| | — |
| | South Dakota | | — |
| | 1 |
| | — |
|
| Louisiana | | 10 |
| | 5 |
| | — |
| | Tennessee | | 44 |
| | 11 |
| | 1 |
|
| Massachusetts | | 5 |
| | 10 |
| | 10 |
| | Texas | | 1 |
| | 10 |
| | — |
|
| Maine | | 2 |
| | 4 |
| | — |
| | Virginia | | 13 |
| | 4 |
| | — |
|
| Maryland | | 9 |
| | 3 |
| | — |
| | Washington | | 1 |
| | — |
| | — |
|
| Michigan | | — |
| | 1 |
| | — |
| | West Virginia | | 11 |
| | 6 |
| | — |
|
| Minnesota | | — |
| | 1 |
| | — |
| | Wisconsin | | 1 |
| | 1 |
| | — |
|
| Mississippi | | 9 |
| | — |
| | — |
| | Washington, D.C. | | 1 |
| | — |
| | — |
|
| Missouri | | 6 |
| | 1 |
| | — |
| | Total | | 321 |
| | 138 |
| | 12 |
|
ITEM 3. LEGAL PROCEEDINGS
See Part II, Item 8, Note 1012 – Commitments and Contingencies for information concerning our legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information and Holders
Our common stock trades on the NASDAQ Global Select Market under the trading symbol “AMED.” As of February 14, 2020,10, 2023, there were approximately 495478 holders of record of our common stock. This number of holders of record does not represent the actual number of beneficial owners of our common stock because shares are frequently held in “street name” by securities dealers and others for the benefit of individual owners who have the right to vote their shares.
Dividend Policy
We have not declared or paid any cash dividends on our common stock or any other of our securities and do not expect to pay cash dividends for the foreseeable future. We currently intend to retain our future earnings, if any, to fund the development and growth of our business. Future decisions concerning the payment of dividends will depend upon our results of operations, financial condition, capital expenditure plans and debt service requirements, as well as such other factors asthat our Board of Directors, in its sole discretion, may consider relevant. In addition, our outstanding indebtedness restricts, and we anticipate any additional future indebtedness may restrict, our ability to pay cash dividends; provided, however, that we may pay dividends (i) payable solely in our equity securities andor (ii) cash dividends if (1) no default or event of default under the Second Amended Credit Agreement shall have occurred and be continuing at the time of such dividend or would result therefrom, and (2) we demonstrate that, upon giving pro forma effect to such dividend, our consolidated leverage ratio (as defined in the Second Amended Credit Agreement) is less than 2.02.75 to 1.0 and (3) we demonstrate a minimum liquidity of $50 million upon giving effect to such dividend.1.0.
Purchases of Equity Securities
The following table provides the information with respect to purchases made by us of shares of our common stock during each of the months during the three-month period ended December 31, 2019:2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Period | | (a)
Total Number
of Shares (or Units)
Purchased | | | (b)
Average Price
Paid per Share (or Unit) | | (c)
Total Number of
Shares (or Units)
Purchased as Part of
Publicly Announced
Plans or Programs | | (d) Maximum Number (or
Approximate Dollar
Value) of Shares (or
Units) That May Yet Be
Purchased Under the
Plans or Programs | |
| October 1, 2022 to October 31, 2022 | 324 | | | | $ | 97.98 | | | — | | | $ | 82,648,900 | | |
| November 1, 2022 to November 30, 2022 | — | | | | — | | | — | | | 82,648,900 | | |
| December 1, 2022 to December 31, 2022 | — | | | | — | | | — | | | 82,648,900 | | |
| | 324 | | (1) | | $ | 97.98 | | | — | | | $ | 82,648,900 | | (2) | |
(1)Includes shares of common stock surrendered to us by certain employees to satisfy tax withholding and/or strike price obligations in connection with the vesting of non-vested stock and the exercise of stock options previously awarded to such employees under our 2008 and 2018 Omnibus Incentive Compensation Plans.
(2)Represents amounts remaining as of December 31, 2022 under the $100 million New Share Repurchase Program, which was authorized by our Board of Directors on August 2, 2021 and expired on December 31, 2022. Effective as of February 2, 2023, we are authorized to repurchase up to $100 million of our common stock through December 31, 2023 under the 2023 Share Repurchase Program. See Item 8, Note 17 – Subsequent Events for additional information on the 2023 Share Repurchase Program.
|
| | | | | | | | | | | | | | | |
| Period | | (a) Total Number of Shares (or Units) Purchased | | | (b) Average Price Paid per Share (or Unit) | | (c) Total Number of Shares (or Units) Purchased as Part of Publicly Announced Plans or Programs | | (d) Maximum Number (or Approximate Dollar Value) of Shares (or Units) That May Yet Be Purchased Under the Plans or Programs (2) |
| October 1, 2019 to October 31, 2019 | 1,272 |
| | | $ | 135.17 |
| | — |
| | $ | 100,000,000 |
|
| November 1, 2019 to November 30, 2019 | — |
| | | — |
| | — |
| | 100,000,000 |
|
| December 1, 2019 to December 31, 2019 | — |
| | | — |
| | — |
| | 100,000,000 |
|
| | | 1,272 |
| (1) | | $ | 135.17 |
| | — |
| | $ | 100,000,000 |
|
| |
(1) | Includes shares of common stock surrendered to us by certain employees to satisfy tax withholding obligations in connection with the vesting of non-vested stock previously awarded to such employees under our 2008 and 2018 Omnibus Incentive Compensation Plans. |
| |
(2) | On February 25, 2019, we announced that our board of directors authorized a stock repurchase program, under which we may repurchase up to $100 million of our outstanding common stock through March 1, 2020. As of December 31, 2019, we have not repurchased any shares pursuant to this stock repurchase program. |
Stock Performance Graph
The Performance Graph below compares the cumulative total stockholder return on our common stock, $0.001 par value per share, for the five-year period ended December 31, 2019,2022 with the cumulative total return on the NASDAQ composite index and an industry peer group over the same period (assuming the investment of $100 in our common stock, the NASDAQ composite index and the industry peer group on December 31, 20142017 and the reinvestment of dividends). The peer group we selected is comprised of: Addus Homecare Corporation ("ADUS"), Chemed Corporation ("CHE"), Encompass Health Corporation ("EHC"), LHC Group, Inc. (“LHCG”) and National Healthcare Corporation (“NHC”). The cumulative total stockholder return on the following graph is historical and is not necessarily indicative of future stock price performance. No cash dividends have been paid on our common stock.
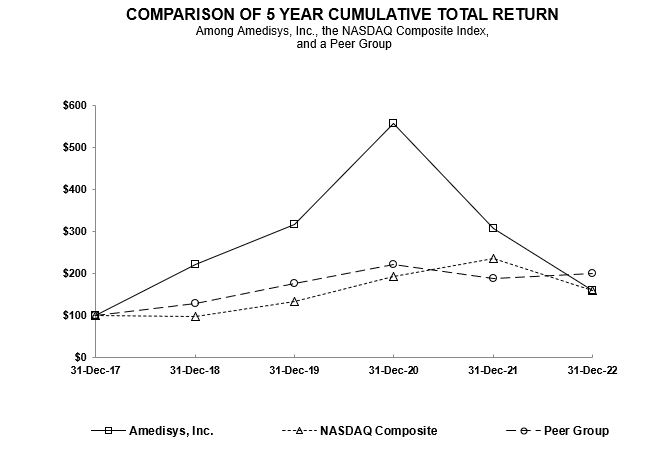
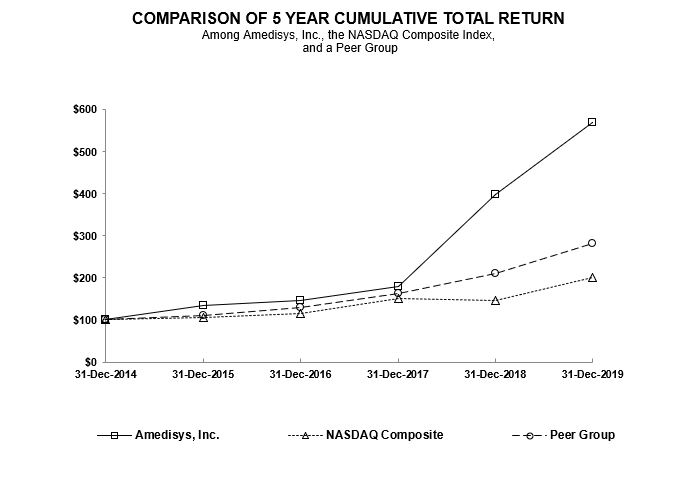
| | | | 12/31/2014 | | 12/31/2015 | | 12/31/2016 | | 12/31/2017 | | 12/31/2018 | | 12/31/2019 | | 12/31/2017 | | 12/31/2018 | | 12/31/2019 | | 12/31/2020 | | 12/31/2021 | | 12/31/2022 |
| Amedisys, Inc. | $ | 100.00 |
| | $ | 133.97 |
| | $ | 145.25 |
| | $ | 179.59 |
| | $ | 399.01 |
| | $ | 568.72 |
| Amedisys, Inc. | $ | 100.00 | | | $ | 222.18 | | | $ | 316.68 | | | $ | 556.50 | | | $ | 307.11 | | | $ | 158.49 | |
| NASDAQ Composite | $ | 100.00 |
| | $ | 106.96 |
| | $ | 116.45 |
| | $ | 150.96 |
| | $ | 146.67 |
| | $ | 200.49 |
| NASDAQ Composite | $ | 100.00 | | | $ | 97.16 | | | $ | 132.81 | | | $ | 192.47 | | | $ | 235.15 | | | $ | 158.65 | |
| Peer Group | $ | 100.00 |
| | $ | 111.06 |
| | $ | 129.16 |
| | $ | 162.93 |
| | $ | 210.21 |
| | $ | 281.40 |
| Peer Group | $ | 100.00 | | | $ | 129.43 | | | $ | 175.68 | | | $ | 221.32 | | | $ | 187.29 | | | $ | 200.24 | |
This stock performance information is “furnished” and shall not be deemed to be “soliciting material” or subject to Regulation 14A under the Securities Exchange Act, of 1934 (the “Exchange Act”), shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, and shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date of this report and irrespective of any general incorporation by reference language in any such filing, except to the extent we specifically incorporate the information by reference.
ITEM 6. SELECTED FINANCIAL DATA[RESERVED]
The selected consolidated financial data presented below is derived from our audited consolidated financial statements for the five-year period ended December 31, 2019. The financial data for the years ended December 31, 2019, 2018 and 2017 should be read together with our consolidated financial statements and related notes included in Item 8, “Financial Statements and Supplementary Data” and the information included in Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” herein.
|
| | | | | | | | | | | | | | | | | | | |
| | 2019 | | 2018 | | 2017 (1) | | 2016 (2) | | 2015 (3) |
| | (Amounts in thousands, except per share data) |
| Income Statement Data: | | | | | | | | | |
| Net service revenue | $ | 1,955,633 |
| | $ | 1,662,578 |
| | $ | 1,511,272 |
| | $ | 1,419,261 |
| | $ | 1,266,489 |
|
| Operating income (loss) | $ | 177,472 |
| | $ | 155,148 |
| | $ | 78,524 |
| | $ | 57,340 |
| | $ | (9,166 | ) |
| Net income (loss) attributable to Amedisys, Inc. | $ | 126,833 |
| | $ | 119,346 |
| | $ | 30,301 |
| | $ | 37,261 |
| | $ | (3,021 | ) |
| Net income (loss) attributable to Amedisys, Inc. per basic share | $ | 3.95 |
| | $ | 3.64 |
| | $ | 0.90 |
| | $ | 1.12 |
| | $ | (0.09 | ) |
| Net income (loss) attributable to Amedisys, Inc. per diluted share | $ | 3.84 |
| | $ | 3.55 |
| | $ | 0.88 |
| | $ | 1.10 |
| | $ | (0.09 | ) |
| |
(1) | During 2017, we recorded charges related to the Securities Class Action Lawsuit settlement and related legal fees in the amount of $29.8 million ($18.1 million, net of tax). Additionally, we recorded a charge in the amount of $21.4 million as the result of H.R. 1 (Tax Cuts and Jobs Act) enacted on December 22, 2017. |
| |
(2) | During 2016, we recorded Homecare Homebase (“HCHB”) implementation costs in the amount of $8.4 million ($5.1 million, net of tax) and recognized a non-cash charge to write off assets as a result of our conversion to the HCHB platform in the amount of $4.4 million ($2.7 million, net of tax). |
| |
(3) | During 2015, we recorded non-cash charges to write off the software costs incurred related to the development of AMS3 Home Health and Hospice in the amount of $75.2 million ($45.5 million, net of tax) and to reduce the carrying value of our corporate headquarters in the amount of $2.1 million ($1.2 million, net of tax). |
|
| | | | | | | | | | | | | | | | | | | |
| | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| | (Amounts in thousands) |
| Balance Sheet Data: | | | | | | | | | |
| Total assets | $ | 1,262,745 |
| | $ | 717,118 |
| | $ | 813,482 |
| | $ | 734,029 |
| | $ | 681,715 |
|
| Total debt, including current portion | $ | 242,183 |
| | $ | 7,387 |
| | $ | 88,841 |
| | $ | 93,029 |
| | $ | 96,630 |
|
| Total Amedisys, Inc. stockholders’ equity | $ | 640,450 |
| | $ | 481,582 |
| | $ | 515,321 |
| | $ | 460,203 |
| | $ | 409,568 |
|
| Cash dividends declared per common share | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
| | $ | — |
|
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis provides information we believe is relevant to an assessment and understanding of our results of operations and financial condition for 2019, 20182022, 2021 and 2017.2020. This discussion should be read in conjunction with our audited financial statements included in Item 8, “Financial"Financial Statements and Supplementary Data” and Part I, Item 1, “Business” of this Annual Report on Form 10-K. The following analysis contains forward-looking statements about our future revenues, operating results and expectations. See “Special Caution Concerning Forward-Looking Statements” for a discussion of the risks, assumptions and uncertainties affecting these statements as well as Part I, Item 1A, “Risk Factors.”
For a discussion of a comparison of the years ended December 31, 2021 and December 31, 2020, please refer to "Management's Discussion and Analysis of Financial Condition and Results of Operations" included in our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission on February 24, 2022.
Overview
We are a provider of high-quality in-home healthcare and related services to the chronic, co-morbid, aging American population, with approximately 74%, 73%75% and 76%75% of our consolidated net service revenue derived from Medicare for 2019, 20182022, 2021 and 2017,2020, respectively.
Our operations involve servicing patients through our threefour reportable business segments: home health, hospice, personal care and personalhigh acuity care. Our home health segment delivers a wide range of services in the homes of individuals who may be recovering from an illness, injury or surgery. Our hospice segment provides care that is designed to provide comfort and support for those who are facing a terminal illness. Our personal care segment provides patients with assistance with the essential activities of daily living. Our high acuity care segment, which was established with the acquisition of Contessa Health ("Contessa") on August 1, 2021, delivers the essential elements of inpatient hospital and skilled nursing facility ("SNF") care to patients in their homes. As of December 31, 2019,2022, we owned and operated 321347 Medicare-certified home health care centers, 138164 Medicare-certified hospice care centers, and 1213 personal-care care centers including unconsolidatedand 8 admitting high acuity care joint ventures in 3837 states within the United States and the District of Columbia.
Care Centers Summary (Includes Unconsolidated Joint Ventures)
| | | | | | | | | | | | | | | | | | | | | | | |
| Home Health | | Hospice | | Personal Care | | High Acuity Care (1) |
| At December 31, 2019 | 321 | | | 138 | | | 12 | | | — | |
| Acquisitions/Expansions/Denovos | 4 | | | 54 | | | 2 | | | — | |
| Closed/Consolidated | (5) | | | (12) | | | — | | | — | |
| At December 31, 2020 | 320 | | | 180 | | | 14 | | | — | |
| Acquisitions/Expansions/Denovos | 11 | | | 1 | | | — | | | 7 | |
| Closed/Consolidated | — | | | (6) | | | — | | | — | |
| At December 31, 2021 | 331 | | | 175 | | | 14 | | | 7 | |
| Acquisitions/Expansions/Denovos | 27 | | | — | | | — | | | 2 | |
| Closed/Consolidated | (11) | | | (11) | | | (1) | | | (1) | |
| At December 31, 2022 | 347 | | | 164 | | | 13 | | | 8 | |
|
| | | | | | | | |
| | Home Health | | Hospice | | Personal Care |
| At December 31, 2016 | 330 |
| | 81 |
| | 14 |
|
| Acquisitions/Start-Ups/De Novos | 3 |
| | 2 |
| | 7 |
|
| Closed/Consolidated | (10 | ) | | — |
| | (6 | ) |
| At December 31, 2017 | 323 |
| | 83 |
| | 15 |
|
| Acquisitions/Start-Ups/De Novos | 1 |
| | 1 |
| | 1 |
|
| Closed/Consolidated | (1 | ) | | — |
| | (4 | ) |
| At December 31, 2018 | 323 |
| | 84 |
| | 12 |
|
| Acquisitions/Start-Ups/De Novos | 3 |
| | 59 |
| | — |
|
| Closed/Consolidated | (5 | ) | | (5 | ) | | — |
|
| At December 31, 2019 | 321 |
| | 138 |
| | 12 |
|
When we refer to “same store business,” we mean2022 Developments
•Maintained the highest Quality of Patient Care star rating in the home health hospice and personal-care care centers that we have operated for at least the last twelve months and start-ups that are an expansionindustry of a same store care center; when we refer to “acquisitions,” we mean home health, hospice and personal-care care centers that we acquired within the last twelve months; and when we refer to “de novos,” we mean home health, hospice and personal-care care centers opened by us in the last twelve months which are not an expansion of a same store care center. Once a care center has been in operation for a twelve month period, the results for that particular care center are included as part of our same store business from that date forward.
2019 Developments
Continued to deliver on our goal of clinical distinction4.49 with 86%99% of our care centers at 4+ Stars in the January 2020 Home Health Compare ("HHC") release.
•Outperformed the industry on all Hospice Item Set ("HIS") measures.measures as well as the newly reported Hospice Care Index ("HCI") metric
•Released our inaugural Environmental, Social and Governance ("ESG") Report
•Performed over 12.311.2 million visits.visits
Lowered company voluntary turnover rate to 16.9%.
Acquired•Expanded our usage and successfully integrated Compassionate Care Hospice ("CCH") and RoseRock Healthcare ("RoseRock") and signed a definitive agreement to acquire Asana Hospice (subsequently closed on January 1, 2020) making Amedisys the third largest hospice company in the United States, exceeding 11,000 in hospice average daily census.
Successfully piloted several tools and data analytics platforms ofrelationship with Medalogix, a predictive data and analytics company, helping to further optimize our current business and enablingpositioning us to work more closely with Medicare Advantage payors.payors
Implemented pay practice changes and staffing model efficiencies•Executed an innovative case rate contract with a large national payor
•Continued to further drive operational excellence.grow our Contessa partnerships ending the year with 11 signed joint ventures
Invested in the business to prepare ourselves for the Patient-Driven Groupings Model ("PDGM").
Executed innovative personal care partnership with ClearCare, giving Amedisys access to personal care services nationwide.
Increased net service revenue 18% and operating income 14%.
Expanded•Grew our home health gross margin as a percentage of revenue by 150 basis points.footprint via the Evolution and AssistedCare acquisitions
Delivered over $200•Generated $133 million in cash flow from operations.operations
•Began to execute on a clinical optimization plan to gain efficiencies and clinical capacity
20202023 Strategy
Continue•Further advance our commitment to clinical distinction with a goalindustry leading Quality of all care centers achieving a minimum of 4.0 Quality Star Rating.Patient Care star scores in home health and drive best-in-class hospice quality as measured by the Hospice Care Index
•Continue to focus on consistent organic growth (de novos)better the communities and inorganic expansionpatients we serve by further incorporating ESG practices into our business operations
•Advance our culture and sense of belonging through diversity and inclusion initiatives
•Build a learning culture through world class leadership development
•Reduce turnover in all three segments.
| |
��� | Focus on recruitment and retention, applying more sophisticated analytics to understand what drives turnover. |
Successfully implement PDGM.
Invest in further expansion of Medalogix products.
Deliver on CCH expectations through realization of synergies.
Expand revenue in innovative payment relationships with Medicare Advantage payors.
Build infrastructure to provide care coordination to patients in need of home health, hospice or personal care.
Incubate new and innovative relationshipsroles, especially focused on expandingcritical clinician positions
•Further expand our breadthanalytics capabilities internally and depth inside the home.through our Medalogix investment
•Consistently grow all lines of business organically and inorganically
•Execute new hospital at home joint venture agreements and expand Contessa's service offering into new lines of business such as palliative care at home
•Continue to execute clinical optimization and reorganization initiatives
Financial Performance
Results forOn a consolidated basis, operating income decreased $71 million on a $9 million increase in net service revenue. Significant drivers of the year ended December 31, 2019 reflect$71 million decrease in operating income were the resultsreturn of sequestration ($23 million) and acquisitions ($34 million). Additionally, wage inflation and a shift in our continued focus on operational improvements and efficiencies and the successful acquisition and integration of our hospice acquisitions.home health volumes from episodic to non-episodic negatively impacted performance.
Our home health care centers experienced growthsegment's revenue and volume were impacted by COVID-19 early in the year, staffing shortages driven by the competitive labor market and a shift from episodic volumes and improvement in utilization and clinician mixwhich generate higher revenue to non-episodic volumes which, combined with the positive impactreturn of the 2019 changes in reimbursement,sequestration and labor pressures, led to the segment delivering a $27$38 million increasedecrease in operating income.income for the segment.
Our hospice segment completedexperienced declines in both our same store admissions and average daily census, which is the acquisitionsmain driver of CCHhospice revenue, primarily due to a decline in our length of stay resulting from a delay in the timing of patients coming onto service and RoseRock. These acquisitions contributed approximately $22 millionan increase in operating income to the hospice segment.discharge rate of our patients.
Our personal care segment contributed approximately $8 million in operating incomecontinued to be impacted by staffing shortages during 2019.2022.
Our high acuity care segment expanded its joint venture footprint and made significant investments to build the clinical, operational and technological infrastructure necessary to support the development and future growth of home recovery care programs on a national scale.
Economic and Industry Factors
Our home health, hospice and personal care segments operate in a highly fragmented and highly competitive industry. The degree of competitiveness for our home health and hospice care centers varies based upon whether our care centers operate in states that require a certificate of need (CON)("CON") or permit of approval (POA)("POA"). In such states, expansion by existing providers or entry into the market by new providers is permitted only where determination is made by state health authorities that a given amount of unmet healthcare need exists. Currently, 70%67% and 28%29% of our home health and hospice care centers, respectively, operate in CON/POA states.
As the Federal government continues to debate a reduction in expenditures and a reform of the Medicare system, our industry continues to face reimbursement pressures. These reform efforts could result in major changes in the health care delivery and reimbursement system on a national and state level, including changes directly impacting the reimbursement systems for our home health and hospice care centers.
PaymentWages and other expenses increase during periods of inflation and when labor shortages occur in the marketplace. The impact of inflation on the Company is primarily in the area of labor costs, supply costs, fuel costs and mileage reimbursements. The healthcare industry is labor intensive. We have experienced, and expect to continue to experience, increases in wage costs. In addition, increases in healthcare costs are typically higher than inflation and impact our costs under our employee benefit plans.
In July 2019, theThe Centers for Medicare and Medicaid Services ("CMS") Payment Updates
Hospice
On July 27, 2022, CMS issued athe final rule to update hospice payment rates and the wage index for fiscal year 2020. The rule includes a rebasing of continuous home care, inpatient respite care and general inpatient care to better reflect the costs of care. This rebasing resulted in a reduction in routine home care payments of 2.7% to achieve budget neutrality. In addition, CMS eliminated the one-year “lag” in the use of the hospital wage index in an effort to align with the Inpatient Prospective Payment System ("IPPS") and other payment systems.2023, effective for services provided beginning October 1, 2022. CMS estimates hospices serving Medicare beneficiaries wouldwill see an estimated 2.6%a 3.8% increase in payments. This increase is the result of a 3.0%4.1% market basket adjustment as required under the Patient Protection and Affordable Health Care Act and the Health Care and Education Reconciliation Act ("PPACA") less a 0.4%0.3% productivity adjustment. We have estimatedAdditionally, CMS increased the impactaggregate cap amount by 3.8% to $32,487. Based on our analysis of the final rule, on uswe expect our impact to be an increase in revenue of approximately 0.5%; however, we are expectingline with the impact on gross margin percentage to be a reduction of approximately 0.5% as3.8% increase.
Home Health
On October 31, 2022, CMS issued the majority of the revenue increase will be passed through to the general inpatient and respite facilities. These estimates are subject to change based on our mix of patients.
The CMS Calendar Year 2019 Home Health Final Rule released in November 2018, provided for the first payment rate increase for home health providers since 2010. In the 2019 rule, CMS also issued proposed payment changes for Medicare home health providers for 2020. These proposed changes included changes to the Home Health Prospective Payment System ("HHPPS") case-mix adjustment methodology through the use of a new PDGM for home health payments, a change in the unit of payment from a 60-day payment period to a 30-day payment period and the elimination of the use of therapy visits in the determination of payments.
The CMS Calendar Year 2020 Home Health Final Rule, released in October 2019, confirmed the implementation of PDGM effective January 1, 2020 as well as the change in the unit of payment. Additionally, in an effort to eliminate fraud risks, CMS is reducing requests for anticipated payment ("RAPs") for 2020 to 20% with the full elimination of RAPs in 2021.calendar year 2023. CMS estimates that the final rule will result in a 1.3%0.7% increase in payments to home health providers. TheThis increase is the result of a statutorily mandated 1.5%4.0% payment update (4.1% market basket adjustment less a 0.1% productivity adjustment) and an increase pursuant to the Bipartisan Budget Act of 2018, reduced by 0.2% for the rural add-on. In calculatingupdate to the impact, CMS alsofixed-dollar loss ratio used in determining outlier payments offset by a permanent adjustment of -3.5% based on the difference between assumed that the industry will make certainand actual behavioral changes relatedresulting from the implementation of PDGM. The -3.5% permanent adjustment is derived from a -3.925% behavioral assumption adjustment. In the Calendar Year 2023 Preliminary Rule, CMS proposed a behavioral assumption adjustment of -7.69%. CMS revised the adjustment to coding practices, low utilization payment-7.85% in the final rule and also reduced it by half (to -3.925%) in order to mitigate such a significant reduction to reimbursement in a single year. The remaining -3.925% behavioral adjustment ("LUPA") management and co-morbidities. Aswill be considered in future rulemaking. The final rule also finalizes a result, CMS reduced reimbursement by 4.36%. We have estimated the impactpermanent 5% cap on negative wage index changes for home health agencies. Based on our analysis of the final rule, on uswe expect our impact to be flat, which is less than the estimated 0.7% rate increase.
In addition to the permanent adjustments, CMS is also considering a reductiontemporary adjustment of approximately $2 billion to offset overpayments in revenue of 2.8%. Our current viewcalendar years 2020 and 2021. CMS has elected not to apply the temporary adjustment to calendar year 2023; however, CMS is still considering how to best apply the adjustment in future rulemaking.
Amedisys submitted formal comments to the Calendar Year 2023 Home Health Proposed Rule in mid-August and joined industry stakeholders in requesting that we can offset the impact via a mix of appropriate behavioral changes and cost levers which include clinician mix and utilization.CMS use an alternative methodology to determine budget neutrality.
The following payment adjustments are effective for each of the years indicated based on CMS’s final rules:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Home Health | | Hospice |
| 2023 | | 2022 | | 2021 | | 2023 (1) | | 2022 | | 2021 |
| Market Basket Update | 4.1 | % | | 3.1 | % | | 2.0 | % | | 4.1 | % | | 2.7 | % | | 2.4 | % |
| Rural Add-On Adjustment | — | | | (0.1) | | | (0.1) | | | — | | | — | | | — | |
| Productivity Adjustment | (0.1) | | | (0.5) | | | — | | | (0.3) | | | (0.7) | | | — | |
| Behavioral Adjustment | (3.5) | | | — | | | — | | | — | | | — | | | — | |
| Fixed-Dollar Loss Ratio Adjustment | 0.2 | | | 0.7 | | | — | | | — | | | — | | | — | |
| Estimated Industry Impact | 0.7 | % | | 3.2 | % | | 1.9 | % | | 3.8 | % | | 2.0 | % | | 2.4 | % |
Estimated Company-Specific Impact (2) | — | % | | 3.2 | % | | 1.9 | % | | 3.8 | % | | 2.0 | % | | 2.4 | % |
|
| | | | | | | | | | | | | | | | | |
| | Home Health | | Hospice |
| | 2020 (1) | | 2019 | | 2018 (2) | | 2020 (3) | | 2019 | | 2018 |
| Market Basket Update | 1.5 | % | | 3.0 | % | | 1.0 | % | | 3.0 | % | | 2.9 | % | | 1.0 | % |
| Rural Add-On Adjustment | (0.2 | ) | | — |
| | — |
| | — |
| | — |
| | — |
|
| Nominal Case Mix Adjustment | — |
| | — |
| | (0.9 | ) | | — |
| | — |
| | — |
|
| PPACA Adjustment | — |
| | — |
| | — |
| | — |
| | (0.3 | ) | | — |
|
| Productivity Adjustment | — |
| | (0.8 | ) | | — |
| | (0.4 | ) | | (0.8 | ) | | — |
|
| Estimated Industry Impact | 1.3 | % | | 2.2 | % | | 0.1 | % | | 2.6 | % | | 1.8 | % | | 1.0 | % |
| Behavioral Assumptions | (4.4 | )% | | | | | | | | | | |
| Estimated Industry Impact Including Behavioral Assumptions | (3.1 | )% | |
| |
| |
| |
| |
|
Estimated Company-Specific Impact (4) | (2.8 | )% | | 1.2 | % | | (0.7 | )% | | 0.5 | % | | 1.6 | % | | 1.0 | % |
| |
(1) | The estimated industry impact of 1.3% only applies to episodes that started on or before December 31, 2019 and are scheduled to complete on or after January 1, 2020. The estimated industry impact including behavioral assumptions of (3.1)% only applies to episodes that started on or after January 1, 2020. |
| |
(2) | Includes the targeted extension of the home health rural add-on payment from the Bipartisan Budget Act of 2018. |
| |
(3) | Effective for services provided from October 1, 2019 to September 30, 2020. |
| |
(4) | (2)Our company-specific impact of the home health final rule differs depending on differences in the wage index and the impact of coding and outlier changes. Our company-specific impact of the hospice final rule differs based on our mix of patients. |
Payor Changes
Effective November 1, 2019, one of our episodic payors phased out their episodic plan offering, and the members were transferred to plans that pay per visit. We expect the overall financial impact of the change to be minimal.home health final rule could differ depending on differences in the wage index, our patient case mix and other factors, such as low utilization payment adjustments ("LUPAs") or outliers, which are described in more detail under Critical Accounting Estimates below. Our company-specific impact of the hospice final rule could differ based on our mix of patients and differences in the wage index.
PartnershipsSequestration
In July 2019, we signed an agreement with ClearCare, Inc.March 2020, Congress passed the bipartisan Coronavirus Aid, Relief and Economic Security Act ("ClearCare"CARES Act"), which provided for the providersuspension of the automatic 2% reduction of Medicare claim reimbursements ("sequestration") for the period May 1, 2020 through December 31, 2020. During 2020 and 2021, Congress passed additional COVID-19 relief legislation which extended the 2% suspension of sequestration through March 31, 2022; sequestration was reinstated as a 1% reduction to Medicare claim reimbursements for the period April 1, 2022 through June 30, 2022 and was fully reinstated as a 2% reduction to Medicare claim reimbursements effective July 1, 2022. The reinstatement of sequestration has resulted in a reduction of our net service revenue.
Novel Coronavirus Pandemic ("COVID-19")
Our operations and financial performance have been impacted by COVID-19. The financial impacts of COVID-19 are discussed in further detail under "Results of Operations" below. While we currently believe that we have a reasonable view of operations, the ultimate impact of COVID-19, including the impact on our liquidity, financial condition and results of operations is uncertain and will depend on many factors and future developments, which are highly uncertain and cannot be predicted at this time, such as the severity, scope and length of time that the pandemic continues, including regional surges in COVID-19 cases at various times. In addition, the COVID-19 pandemic has resulted in widespread global supply chain disruptions to vendors including critical supply shortages, significant material cost inflation and extended lead times for items that are required for our operations. Potential impacts of COVID-19 on our results include lower revenue; higher salary and wage expense related to quarantine pay, contract clinicians, wage inflation, increased costs to hire and retain employees and training; and increased supply costs related to supply chain constraints, personal protective equipment ("PPE") and COVID-19 testing. The impacts to net service revenue include the following:
•lower volumes due to interruption of the operations of our referral sources, patients' unwillingness to accept services and restrictions on access to facilities for hospice services;
•lower reimbursement due to missed visits resulting in an increase in LUPAs and lost billing periods; and
•lower hospice average daily census due to a decline in our average length of stay.
See Item 8, Note 3 – Novel Coronavirus Pandemic ("COVID-19") to our consolidated financial statements for additional information regarding COVID-19 and the CARES Act.
Network Developments
We have a Care Coordination Agreement with BrightStar Care to add its agencies to the Amedisys personal care industry’s leading software platform, representing 4,000network, which helps facilitate the coordination of care between our home health and hospice care centers and a network of personal care agencies in every zip code in the United States. Our agreement with ClearCare creates an opportunity to establish a network partnership between Amedisys and personal care agencies using ClearCare in order to better coordinate patient care.partners. Long term, we believe this allowsagreement will allow us to build a nation-wide network of personal care agencies and furthersfurther our efforts to provide patients with a true care continuum in the home. This relationship will also help us as we continue to have innovative payment conversations with Medicare Advantage plans who have begun to recognize the value that combined home health, hospice, personal care and personalhigh acuity care services bring to their members and care delivery infrastructure.
Governmental Inquiries and Investigations and Other Litigation
See Item 8, Note 1012 – Commitments and Contingencies to our consolidated financial statements for additional informationa discussion of and updates regarding our corporate integrity agreement ("CIA"), the CCH CIA, the subpoenalegal proceedings and civil investigative demands issued by the U.S. Department of Justice and the South Carolina and Florida Zone Program Integrity Contractor audits.investigations we are involved in. No assurances can be given as to the timing or outcome of these items.
Results of Operations
Consolidated
The following table summarizes our consolidated results of operations (amounts in millions):
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Net service revenue | $ | 2,223.2 | | | $ | 2,214.1 | | | $ | 2,071.5 | |
| Other operating income | — | | | 13.3 | | | 34.4 | |
| Cost of service, excluding depreciation and amortization | 1,260.4 | | | 1,233.4 | | | 1,185.4 | |
| Gross margin, excluding depreciation and amortization | 962.8 | | | 994.0 | | | 920.5 | |
| % of net service revenue | 43.3 | % | | 44.9 | % | | 44.4 | % |
| General and administrative expenses, excluding depreciation and amortization and impairment charge | 754.1 | | | 711.2 | | | 668.2 | |
| % of net service revenue | 33.9 | % | | 32.1 | % | | 32.3 | % |
| Depreciation and amortization | 24.9 | | | 30.9 | | | 28.8 | |
| Impairment charge | 3.0 | | | — | | | 4.2 | |
| Operating income | 180.8 | | | 251.9 | | | 219.3 | |
| Total other (expense) income, net | (20.5) | | | 28.3 | | | (8.4) | |
| Income tax expense | (42.5) | | | (70.1) | | | (25.6) | |
| Effective income tax rate | 26.5 | % | | 25.0 | % | | 12.2 | % |
| Net income | 117.7 | | | 210.2 | | | 185.2 | |
| | | | | |
| Net loss (income) attributable to noncontrolling interests | 0.9 | | | (1.1) | | | (1.6) | |
| Net income attributable to Amedisys, Inc. | $ | 118.6 | | | $ | 209.1 | | | $ | 183.6 | |
|
| | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Net service revenue | $ | 1,955.6 |
| | $ | 1,662.6 |
| | $ | 1,511.3 |
|
| Gross margin, excluding depreciation and amortization | 805.3 |
| | 669.7 |
| | 607.9 |
|
| % of revenue | 41.2 | % | | 40.3 | % | | 40.2 | % |
| Other operating expenses | 607.9 |
| | 501.3 |
| | 482.3 |
|
| % of revenue | 31.1 | % | | 30.1 | % | | 31.9 | % |
| Depreciation and amortization | 18.4 |
| | 13.3 |
| | 17.1 |
|
| Securities Class Action Lawsuit settlement, net | — |
| | — |
| | 28.7 |
|
| Asset impairment charge | 1.5 |
| | — |
| | 1.3 |
|
| Operating income | 177.5 |
| | 155.1 |
| | 78.5 |
|
| Total other (expense) income, net | (7.1 | ) | | 3.8 |
| | 2.3 |
|
| Income tax expense | (42.5 | ) | | (38.8 | ) | | (50.1 | ) |
| Effective income tax rate | 24.9 | % | | 24.4 | % | | 62.0 | % |
| Net income | 127.9 |
| | 120.1 |
| | 30.7 |
|
| Net income attributable to noncontrolling interests | (1.1 | ) | | (0.8 | ) | | (0.4 | ) |
| Net income attributable to Amedisys, Inc. | $ | 126.8 |
| | $ | 119.3 |
| | $ | 30.3 |
|
Year Ended December 31, 20192022 Compared to the Year Ended December 31, 20182021
Overall,On a consolidated basis, our operating income increased $22decreased approximately $71 million on a net service revenue increase of $293$9 million. Our 2019The year over year decrease in operating results includeincome is primarily due to the acquisitions of CCHContessa on August 1, 2021 and RoseRock whichEvolution and AssistedCare on April 1, 2022 (which combined contributed approximately $174$54 million in net service revenue and an operating loss of approximately $5 million and is inclusive of $14$44 million in acquisitionthe current year and integration costs and $6$4 million in intangibles amortization.
Additionally,net service revenue and an operating loss of $10 million in the prior year), a $9 million reduction to net service revenue related to our operating income was negatively impacted byInfinity Zone Program Integrity Contractors ("ZPIC") audits, a $7 million accrualfavorable adjustment recorded in the prior year related to settlement discussions with theour U.S. Department of Justice ("DOJ") matters (see Item 8, Note 10 -12 – Commitments and Contingencies to our consolidated financial statements for additional information)information regarding both the ZPIC and DOJ matters), a $3 million impairment charge recorded in connection with the wind down of operations of one of our high acuity care joint ventures and a $2greater benefit recognized in the prior year totaling $23 million assetassociated with the suspension of sequestration.
Excluding our acquisitions, the Infinity ZPIC audits, the DOJ matters, the impairment charge related toand the incremental sequestration benefit recognized in the prior year, our acquired names intangibles (see Item 8, Note 4 - Goodwilloperating income increased $5 million while net service revenue decreased $2 million. Our results were positively impacted by rate increases, improvements in clinician utilization, reductions in hospice staffing levels and Other Intangible Assets, Net tolower depreciation and amortization. These items were offset by a decrease in our consolidated financial statements for additional information).
Our year-to-date performance reflects growth and operating improvement in all three segments of our legacy operations. We expanded gross marginepisodic home health revenue as a percentage of total net service revenue, a decline in our hospice average daily census, which is the main driver of hospice revenue, a decrease in our other operating income due to the expiration of the CARES Act Provider Relief Fund ("PRF") funds, an increase in our cost of service resulting from planned wage increases and wage inflation and an increase in our general and administrative expenses. Additionally, our volumes have been and continue to be impacted by staffing shortages resulting from the competitive labor market.
As noted above, we received CARES Act PRF funds in 2020 which were used to cover COVID-19 expenses incurred by our home health and personal care segments. Bothhospice segments benefitted from rate increases with home health also delivering improvementsthrough June 30, 2021. We recorded income related to these funds totaling $13 million in clinician utilization and discipline mix. Our hospice segment's gross margin as a percentage of revenue decreased due to our acquisition activity. Additionally, our
other operating expenses asincome within our consolidated statements of operations during the year ended December 31, 2021. This income fully offset the COVID-19 costs incurred during the six-month period ended June 30, 2021, which totaled $13 million; however, we were not able to recognize any operating income during the six-month period ended December 31, 2021 to offset the $8 million of COVID-19 costs incurred during this period. Additionally, we were not able to recognize any operating income to offset the $9 million of COVID-19 costs incurred during the year ended December 31, 2022.
Our operating results reflect a percentage of revenue increased only 1%$43 million increase in our general and administrative expenses compared to 2018; this increase is inclusive of approximately $16prior year. Excluding our acquisitions, our general and administrative expenses increased $8 million in acquisition and integration costs. Excluding the acquisition and integration costs, our other operating expenses as a percentage of revenue remained relatively flat compared(1%) due to 2018 despite planned wage increases and the addition of resources to support growth.
Total other (expense) income, net includes the following items (amounts in millions):
| | | | | | | | | | | |
| For the Years Ended
December 31, |
| 2022 | | 2021 |
| Interest income | $ | 0.2 | | | $ | — | |
| Interest expense | (22.2) | | | (9.5) | |
| Equity in (loss) earnings from equity method investments | (0.1) | | | 4.9 | |
| Gain on equity method investments | — | | | 31.1 | |
| Miscellaneous, net | 1.6 | | | 1.8 | |
| Total other (expense) income, net | $ | (20.5) | | | $ | 28.3 | |
|
| | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 |
| Interest income | $ | 0.1 |
| | $ | 0.3 |
|
| Interest expense | (14.5 | ) | | (7.4 | ) |
| Equity in earnings from equity method investments | 5.3 |
| | 7.7 |
|
| Miscellaneous, net | 2.0 |
| | 3.2 |
|
| | $ | (7.1 | ) | | $ | 3.8 |
|
Interest expense increased $7$13 million in 2019 from 2018year over year as a result of an increaseinterest accrued in conjunction with the Inifnity ZPIC audits discussed above and increased borrowings and higher interest rates under our Second Amended Credit Agreement (see Item 8, Note 79 – Long-Term Obligations to our consolidated financial statements for additional information
regarding our Second Amended Credit Agreement). Equity in earnings fromGain on equity method investments includes gains of $2 million and $5 million for 2019 and 2018, respectively.
Year Ended December 31, 2018 Compared to the Year Ended December 31, 2017
Overall, our operating income increased $77 million on a revenue increase of $151 million. Our 2017 operating results were negatively impacted $40 million; these impacts include a $30 million charge for the Securities Class Action Lawsuit settlement and related legal fees,prior year includes a $7$31 million reduction in revenue as a result of the Florida ZPIC audit and charges of approximately $3 milliongain related to our home health closures and restructuring plan. Excluding these 2017 impacts, operating income increased $37 million, driven by continued growthinvestment in our home health and hospice segments, increases in clinical productivity in our home health segment and a continued focus on maintaining cost discipline, as our other operating expenses increased only 3% on a 10% increase in net service revenue. In addition, our gross margin as a percentage of revenue was relatively flat despite a net reduction of $3 million in net service revenue and gross margin resulting from the 2018 changes in reimbursement and planned wage increases.
Our 2018 operating results include the results of our acquisition of Christian Care at Home, which provided home health services to the state of Kentucky, East Tennessee Personal Care Services, which owned and operated one personal-care care center servicing the state of Tennessee, and certain personal care operations from Bring Care Home in Massachusetts. These three acquisitions accounted for approximately $5 million of our $151 million increase in revenue and $1 million of our $15 million increase in other operating expenses.
Total other income, net includes the impact of the following items (amounts in millions):
|
| | | | | | | |
| | For the Years Ended December 31, |
| | 2018 | | 2017 |
| Interest income | $ | 0.3 |
| | $ | 0.1 |
|
| Interest expense | (7.4 | ) | | (5.0 | ) |
| Equity in earnings from equity method investments | 7.7 |
| | 3.4 |
|
| Miscellaneous, net | 3.2 |
| | 3.8 |
|
| | $ | 3.8 |
| | $ | 2.3 |
|
Interest expense includes interest expense related to the Florida ZPIC audit of $2 million for 2018. Equity in earnings from equity method investments includes gains of $5 million and $1 million for 2018 and 2017, respectively. Miscellaneous, net includes proceeds from legal settlements of $1 million and $2 million for 2018 and 2017, respectively. Excluding these items, total other income, net increased $1 million in 2018 from 2017.
Our 2017 income tax expense includes a $21 million charge related to the remeasurement of our deferred tax assets and liabilities to the enacted corporate income tax rate of 21% as required by the enactment of H.R. 1 (Tax Cuts and Jobs Act), on December 22, 2017Medalogix (see Item 8, Note 8 - Income Taxes1 – Nature of Operations, Consolidation and Presentation of Financial Statements to our consolidated financial statements)statements for additional information).
Home Health DivisionSegment
The following table summarizes our home health segment results of operations:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
Financial Information (in millions): | | | | | |
| Medicare | $ | 891.3 | | | $ | 914.5 | | | $ | 847.3 | |
| Non-Medicare | 464.2 | | | 439.3 | | | 401.9 | |
| Net service revenue | 1,355.5 | | | 1,353.8 | | | 1,249.2 | |
| Other operating income | — | | | 7.3 | | | 20.2 | |
| Cost of service | 769.0 | | | 756.6 | | | 729.9 | |
| Gross margin | 586.5 | | | 604.5 | | | 539.5 | |
| Depreciation and amortization | 4.0 | | | 4.3 | | | 3.9 | |
| Impairment charge | — | | | — | | | 3.4 | |
| Other general and administrative expenses | 348.5 | | | 328.5 | | | 307.2 | |
| Operating income | $ | 234.0 | | | $ | 271.7 | | | $ | 225.0 | |
| Same Store Growth (1): | | | | | |
| Medicare revenue | (5 | %) | | 8 | % | | (1 | %) |
| Non-Medicare revenue | 2 | % | | 9 | % | | 1 | % |
| Total admissions | 3 | % | | 6 | % | | 1 | % |
| Total volume (2) | — | % | | 5 | % | | 2 | % |
| Key Statistical Data - Total (3): | | | | | |
| Admissions | 374,631 | | | 353,075 | | | 331,354 | |
| Recertifications | 178,101 | | | 183,134 | | | 177,631 | |
| Total volume | 552,732 | | | 536,209 | | | 508,985 | |
| | | | | |
| Medicare completed episodes | 304,012 | | | 311,531 | | | 301,856 | |
| Average Medicare revenue per completed episode (4) | $ | 3,010 | | | $ | 2,959 | | | $ | 2,836 | |
| Medicare visits per completed episode (5) | 12.9 | | | 13.9 | | | 14.9 | |
| | | | | |
| Visiting clinician cost per visit | $ | 99.90 | | | $ | 93.44 | | | $ | 89.62 | |
| Clinical manager cost per visit | 11.08 | | | 9.75 | | | 9.17 | |
| Total cost per visit | $ | 110.98 | | | $ | 103.19 | | | $ | 98.79 | |
| Visits | 6,929,137 | | | 7,331,935 | | | 7,388,549 | |
(1)Same store information represents the percent change in our Medicare, Non-Medicare and Total revenue, admissions or volume for the period as a percent of the Medicare, Non-Medicare and Total revenue, admissions or volume of the prior period. Same store is defined as care centers that we have operated for at least the last twelve months and startups that are an expansion of a same store care center.
(2)Total volume includes all admissions and recertifications.
(3)Total includes acquisitions, start-ups and denovos.
(4)Average Medicare revenue per completed episode is the average Medicare revenue earned for each Medicare completed episode of care. Average Medicare revenue per completed episode reflects the suspension of sequestration for the period May 1, 2020 through March 31, 2022 and the reinstatement of sequestration at 1% effective April 1, 2022 and at 2% effective July 1, 2022.
(5)Medicare visits per completed episode are the home health Medicare visits on completed episodes divided by the home health Medicare episodes completed during the period.
|
| | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
Financial Information (in millions): | | | | | |
| Medicare | $ | 859.2 |
| | $ | 830.8 |
| | $ | 793.3 |
|
| Non-Medicare | 397.2 |
| | 343.7 |
| | 290.6 |
|
| Net service revenue | 1,256.4 |
| | 1,174.5 |
| | 1,083.9 |
|
| Cost of service | 754.1 |
| | 722.1 |
| | 670.9 |
|
| Gross margin | 502.3 |
| | 452.4 |
| | 413.0 |
|
| Asset impairment charge | 1.5 |
| | — |
| | 1.3 |
|
| Other operating expenses | 301.4 |
| | 279.8 |
| | 281.9 |
|
| Operating income | $ | 199.4 |
| | $ | 172.6 |
| | $ | 129.8 |
|
| Same Store Growth (1): | | | | | |
| Medicare revenue | 4 | % | | 6 | % | | (4 | %) |
| Non-Medicare revenue | 16 | % | | 18 | % | | 17 | % |
| Total admissions | 7 | % | | 5 | % | | 2 | % |
| Total volume (2) | 5 | % | | 7 | % | | 4 | % |
| Key Statistical Data - Total (3): | | | | | |
| Medicare: | | | | | |
| Admissions | 195,513 |
| | 190,748 |
| | 190,132 |
|
| Recertifications | 110,460 |
| | 112,773 |
| | 106,774 |
|
| Total volume | 305,973 |
| | 303,521 |
| | 296,906 |
|
| | | | | | |
| Completed episodes | 300,551 |
| | 296,223 |
| | 290,227 |
|
| Visits | 5,207,563 |
| | 5,261,315 |
| | 5,067,436 |
|
| Average revenue per completed episode (4) | $ | 2,920 |
| | $ | 2,854 |
| | $ | 2,823 |
|
| Visits per completed episode (5) | 17.3 |
| | 17.6 |
| | 17.3 |
|
| Non-Medicare: | | | | | |
| Admissions | 133,180 |
| | 118,577 |
| | 107,665 |
|
| Recertifications | 62,108 |
| | 55,736 |
| | 46,364 |
|
| Total volume | 195,288 |
| | 174,313 |
| | 154,029 |
|
| Visits | 3,065,745 |
| | 2,772,339 |
| | 2,347,363 |
|
| Total (3): | | | | | |
| Visiting Clinician Cost per Visit | $ | 83.11 |
| | $ | 81.88 |
| | $ | 82.04 |
|
| Clinical Manager Cost per Visit | $ | 8.04 |
| | $ | 8.01 |
| | $ | 8.44 |
|
| Total Cost per Visit | $ | 91.15 |
| | $ | 89.89 |
| | $ | 90.48 |
|
| Visits | 8,273,308 |
| | 8,033,654 |
| | 7,414,799 |
|
| |
(1) | Same store information represents the percent change in our Medicare, Non-Medicare and Total revenue, admissions or volume for the period as a percent of the Medicare, Non-Medicare and Total revenue, admissions or volume of the prior period. Effective July 1, 2019, same store is defined as care centers that we have operated for at least the last twelve months and startups that are an expansion of a same store care center. |
| |
(2) | Total volume includes all admissions and recertifications. |
| |
(3) | Total includes acquisitions and de novos. |
| |
(4) | Average Medicare revenue per completed episode is the average Medicare revenue earned for each Medicare completed episode of care. |
| |
(5) | Medicare visits per completed episode are the home health Medicare visits on completed episodes divided by the home health Medicare episodes completed during the period. |
Year Ended December 31, 20192022 Compared to the Year Ended December 31, 20182021
Operating Results
Overall, our operating income increased $27decreased $38 million on an $82a $2 million increase in net service revenue. Our gross marginThe year over year results were impacted by the April 1, 2022 acquisitions of Evolution and AssistedCare (which contributed net service revenue of $35 million and an operating loss of $3 million to the year ended December 31, 2022), a $9 million reduction in net service revenue related to our Infinity ZPIC audits and a greater benefit recognized in the prior year totaling $14 million associated with the suspension of sequestration. Excluding these items, our operating income decreased $12 million on a $10 million decrease in net service revenue primarily due to a decrease in episodic revenue as a percentage of total net service revenue, was positively impacted byhigher revenue adjustments, the 2019 changes in reimbursement, growth in volumes, the acuity level of our patients, improved utilization and a focus on discipline mix. The impactexpiration of the 2019 change in reimbursement wasCARES Act PRF funds, planned wage increases, wage inflation and an increase in net service revenueour other general and gross margin of approximately $12 million.administrative expenses. These items were partially offset by the increase in reimbursement and improvement in our operating performance driven by improvements in clinician utilization.
Net Service Revenue
Our net service revenue increased $82 million (7%) on$2 million. Excluding our April 1, 2022 acquisitions of Evolution and AssistedCare, the Infinity ZPIC audits and the incremental sequestration benefit recognized in the prior year, our net service revenue decreased $10 million. We have experienced a 5%year over year decline in our episodic volumes, which generate higher revenue than our non-episodic volumes. Additionally, our volumes have been impacted by staffing shortages driven by the competitive labor market. These items, as well as an increase in total volume andrevenue adjustments, have resulted in a 2% increaseyear over year decline in Medicareour net service revenue per episode. The volume growthwhich was driven by a 7% increase in admissionspartially offset by lower recertification volume. The increase in Medicare revenue per episode is the result of a 1.2%3.2% increase in reimbursement effective January 1, 2022.
Other Operating Income
Other operating income consists of the recognition of funds received from the CARES Act PRF which were available for use through June 30, 2021. We recorded income related to these funds totaling $7 million during the year ended December 31, 2021. This income fully offset the COVID-19 costs incurred during the six-month period ended June 30, 2021, which totaled $7 million; however, we were not able to recognize any operating income during the six-month period ended December 31, 2021 to offset the $6 million of COVID-19 costs incurred during this period. Additionally, we were not able to recognize any operating income to offset the $7 million of COVID-19 costs incurred during the year ended December 31, 2022. The COVID-19 costs were associated with the remainder duepurchase of PPE, quarantine pay and COVID-19 testing and have been recorded to an increase in the acuity levelcost of service within our patients. Additionally, our non-Medicare (per visit and episodic) rates increased approximately 3% which is a combinationconsolidated statements of rate increases and increases in the acuity level of our patients. Revenue was also positively impacted by a reduction in our revenue adjustments.operations.
Cost of Service, Excluding Depreciation and Amortization
Our cost of service consists of costs associated with direct clinician care in the homes of our patients as well as the cost of clinical managers who monitor the overall delivery of care. OurOverall, our total cost of service increased 4% on a 3%2% primarily due to an 8% increase in total visits. Ourour total cost per visit increased approximately 1% aspartially offset by a 6% decrease in total visits resulting from improvements in clinician utilization as evidenced by a decline of 1.0 visit per Medicare completed episode year over year. The 2% increase in our total cost per visit is primarily due to planned wage increases, an increase in salaried employees (partially due to our recent acquisitions), wage inflation, increased costs to hire and optimization of disciplineretain employees, visit mix, higher fuel prices and mileage reimbursement partially offset planned wage increases. Additionally, changesby a decrease in COVID-19 costs. In addition, while we compensate our clinicians on a per visit basis, there is a fixed cost component of our cost structure which also resulted in an increase in our home health care center staffing resultedcost per visit due to the significant decline in a shift of some office staff from cost of service to other operating expenses totaling approximately $4 million.visits year over year.
Other OperatingGeneral and Administrative Expenses
Other operatinggeneral and administrative expenses increased approximately $22$20 million. Excluding our acquisitions, other general and administrative expenses increased $10 million primarily due to an increase in salaries and benefits expense as a result ofplanned wage increases, the addition of resources to support volume growth, planned wage increases and the home health staffing shifts referenced above.
Year Ended December 31, 2018 Compared to the Year Ended December 31, 2017
Operating Results
Overall, our operating income increased $43 million on a $91 million increase in net service revenue. The $43 million increase includes a $7 million reduction in revenue related to the Florida ZPIC audit in 2017. Our growth in volumes and increases in clinician productivity positively impacted our gross margin as a percentage of revenue, which increased despite the 2018 changes in reimbursement and planned wage increases. The impact of the 2018 changes in reimbursement was a reduction in net service revenue and gross margin of approximately $7 million.
Net Service Revenue
Our revenue increased $91 million on a 7% increase in total volume. The volume growth was driven by a 5% increase in admissions and a 130 basis point increase in our Medicare recertification rate. In addition to the increase in volume, our revenue per episode was up $31 per episode as a result of an increase in the acuity level of our patients which enabled us to overcome the 70 basis point reimbursement reduction effective January 1, 2018.
Cost of Service, Excluding Depreciation and Amortization
Our cost of service increased 8% on an 8% increase in total visits. Our increase in total visits was driven by growth in volumes as well as an increase in visits per completed episode which is the result of an increase in the acuity level of our patients. Our cost per visit decreased 1% as an increase in clinician productivity offset planned wage increases.
Other Operating Expenses
Other operating expenses decreased approximately $2 million on an 8% increase in net service revenue primarily due to a decrease in salaries and benefits expense as 2017 operating expenses included approximately $3 million in costs related to our home health restructuring plan. Additionally, we experienced decreases in rent expense, professional fees and telecommunications expense which were offset by increases in information technology expense andhigher travel and training expense.spend and higher information technology fees partially offset by lower incentive compensation costs.
Hospice DivisionSegment
The following table summarizes our hospice segment results of operations:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
Financial Information (in millions): | | | | | |
| Medicare | $ | 744.1 | | | $ | 750.1 | | | $ | 710.0 | |
| Non-Medicare | 43.7 | | | 41.7 | | | 40.1 | |
| Net service revenue | 787.8 | | | 791.8 | | | 750.1 | |
| Other operating income | — | | | 6.0 | | | 13.1 | |
| Cost of service | 426.5 | | | 425.2 | | | 400.6 | |
| Gross margin | 361.3 | | | 372.6 | | | 362.6 | |
| Depreciation and amortization | 2.3 | | | 2.7 | | | 2.2 | |
| Impairment charge | — | | | — | | | 0.8 | |
| Other general and administrative expenses | 203.3 | | | 198.4 | | | 175.4 | |
| Operating income | $ | 155.7 | | | $ | 171.5 | | | $ | 184.2 | |
| Same Store Growth (1): | | | | | |
| Medicare revenue | (1 | %) | | — | % | | 4 | % |
| | | | | |
| Hospice admissions | (1 | %) | | 2 | % | | 6 | % |
| Average daily census | (1 | %) | | (4 | %) | | 1 | % |
| Key Statistical Data - Total (2): | | | | | |
| Hospice admissions | 52,656 | | | 53,507 | | | 49,694 | |
| Average daily census | 13,091 | | | 13,271 | | | 13,081 | |
| Revenue per day, net | $ | 164.88 | | | $ | 163.47 | | | $ | 156.69 | |
| Cost of service per day | $ | 89.26 | | | $ | 87.77 | | | $ | 83.67 | |
| Average discharge length of stay | 91 | | | 94 | | | 99 | |
|
| | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
Financial Information (in millions): | | | | | |
| Medicare | $ | 586.6 |
| | $ | 390.2 |
| | $ | 350.7 |
|
| Non-Medicare | 30.6 |
| | 20.7 |
| | 17.1 |
|
| Net service revenue | 617.2 |
| | 410.9 |
| | 367.8 |
|
| Cost of service | 335.1 |
| | 212.0 |
| | 187.5 |
|
| Gross margin | 282.1 |
| | 198.9 |
| | 180.3 |
|
| Other operating expenses | 139.1 |
| | 85.7 |
| | 77.5 |
|
| Operating income | $ | 143.0 |
| | $ | 113.2 |
| | $ | 102.8 |
|
| Same Store Growth (1): | | | | | |
| Medicare revenue | 7 | % | | 11 | % | | 17 | % |
| Hospice admissions | 4 | % | | 8 | % | | 11 | % |
| Average daily census | 7 | % | | 11 | % | | 15 | % |
| Key Statistical Data - Total (2): | | | | | |
| Hospice admissions | 40,194 |
| | 27,596 |
| | 25,381 |
|
| Average daily census | 11,164 |
| | 7,588 |
| | 6,820 |
|
| Revenue per day, net | $ | 151.47 |
| | $ | 148.36 |
| | $ | 147.75 |
|
| Cost of service per day | $ | 82.24 |
| | $ | 76.53 |
| | $ | 75.31 |
|
| Average discharge length of stay | 98 |
| | 100 |
| | 93 |
|
| |
(1) | Same store information represents the percent change in our Medicare revenue, Hospice admissions or average daily census for the period as a percent of the Medicare revenue, Hospice admissions or average daily census of the prior period. Effective July 1, 2019, same store is defined as care centers that we have operated for at least the last twelve months and startups that are an expansion of a same store care center. |
| |
(2) | Total includes acquisitions and de novos. |
(2)Total includes acquisitions and denovos.
Year Ended December 31, 20192022 Compared to the Year Ended December 31, 20182021
Operating Results
On February 1, 2019, we acquired CCH, which owned and operated 53 hospice care centers. On April 1, 2019, we acquired RoseRock, which owned and operated one hospice care center. Acquisitions are included in our consolidated financial statements from their respective acquisition dates. As a result, our hospice segment operating results for 2019 and 2018 are not fully comparable.
Overall, our operating income increased $30decreased $16 million on a $206$4 million increasedecrease in net service revenue. Our operating income was negatively impacted byExcluding a $7 million reduction to revenue and gross marginfavorable adjustment recorded in the prior year related to settlement discussions with the U.S. Department of Justiceour DOJ matters (see Item 8, Note 10 -12 – Commitments and Contingencies to our consolidated financial statements for additional information). Our and a $9 million greater benefit recognized in the prior year associated with the suspension of sequestration, operating results were positively impacted by changesincome was flat as the increases in reimbursement effective October 1, 2021 and 2022, lower revenue adjustments, savings associated with clinical optimization and reorganization initiatives and reductions in staffing levels were offset by a decline in our hospice average daily census, which resulted inis the main driver of hospice revenue, planned wage increases, wage inflation and an increase in our other general and administrative expenses.
Net Service Revenue
Excluding the DOJ matters and incremental sequestration benefit recognized in the prior year, our net service revenue and gross margin of approximately $7increased $12 million and $6 million, respectively. The majority ofprimarily due to the revenue increase associated with the 2020 changeincreases in reimbursement which became effective October 1, 2019, will be passed through2021 and 2022 as well as lower revenue adjustments partially offset by a decline in our same store average daily census, which is the main driver of hospice revenue. Our same store average daily census was down 1% year over year primarily due to a decline in our general inpatientlength of stay resulting from a delay in the timing of patients coming onto service, an increase in the discharge rate of our patients and respite facilities. Our operating results were also positively impacted by continued growth and bya decline in our acquisitions which contributed approximately $174 million in net service revenue and $22 million inhospice admissions throughout the year.
Other Operating Income
Other operating income consists of the recognition of funds received from the CARES Act PRF which were available for use through June 30, 2021. We recorded income related to our hospice segment's results forthese funds totaling $6 million during the year ended December 31, 2019.
Net Service Revenue
Our hospice revenue increased $2062021. This income fully offset the COVID-19 costs incurred during the six-month period ended June 30, 2021, which totaled $6 million; approximately $174however, we were not able to recognize any operating income during the six-month period ended December 31, 2021 to offset the $2 million of which is attributableCOVID-19 costs incurred during this period. Additionally, we were not able to recognize any operating income to offset the $2 million of COVID-19 costs incurred during the year ended December 31, 2022. The COVID-19 costs were associated with the purchase of PPE, quarantine pay and COVID-19 testing and have been recorded to cost of service within our acquisition activities. The remaining $32 million increase is the resultconsolidated statements of a 7% increase in our average daily census and increases in reimbursement totaling 1.6% and 0.5% effective for services provided from October 1, 2018 and October 1, 2019, respectively, partially offset by an increase in our revenue adjustments, which include a $7 million reduction to revenue and gross margin related to the U.S. Department of Justice matter noted above.operations.
Cost of Service, Excluding Depreciation and Amortization
Our hospice cost of service increased $123 million, approximately $110 million of which is attributable to our acquisition activity. The remaining $13 million increase is primarily due toless than 1% as a 7%2% increase in average daily census, planned wage increases and an increase in our general inpatient and respite facility costs as the majority of the reimbursement increase, which became effective October 1, 2019, will be passed through to these facilities. Our cost of service per day increased 7%, largely drivenwas offset by a 1% decline in our acquisitions asaverage daily census. The increase in our same store cost of service per day remained relatively flat. We expect our acquisitions' cost of service per day to approximate our legacy metric as we fully integrate them during 2020.
Other Operating Expenses
Other operating expenses increased $53 million; approximately $42 million of the increase is related to our acquisition activity. The remaining $11 million increase is due to increases in other care center related expenses, primarily salaries and benefits expense due to the addition of resources to support census growth and planned wage increases, professional fees and travel and training expense.
Year Ended December 31, 2018 Compared to the Year Ended December 31, 2017
Operating Results
Overall, our operating income increased $10 million on a $43 million increase in net service revenue. The 12% increase in net service revenue was partially offset by a lower gross margin as a percentage of revenue primarily related to planned wage increases, an increase in revenue adjustmentswage inflation, increased costs to hire and amounts due back to Medicare for hospice capsretain employees and an increase in other operating expenses.
Net Service Revenue
Our hospice revenue increased $43 million on an 11% increase in our average daily censushigher fuel prices and a 1.0% and 1.6% increase in reimbursement effective for services provided from each October 1, 2017 and October 1, 2018, respectively. We experienced a $2 million increase in our revenue adjustments and cap whichmileage reimbursements partially offset the revenue increase for the year ended December 31, 2018.by lower COVID-19 costs, reductions in staffing levels and savings associated with clinical optimization and reorganization initiatives.
Cost of Service, Excluding DepreciationOther general and Amortization
Our hospice cost of serviceadministrative expenses increased $25$5 million, (13%) as the result of an 11% increase in average daily census. Our cost of service per day increased 2% primarily due to an increase in salary cost per day as a result of planned wage increases.
Other Operating Expenses
Other operating expenses increased $8 million on a 12% increase in net service revenue. The increase was related to other care center related expenses, primarily salaries and benefits expense, advertising expense, information technology expense, professional fees andincreases, higher travel and training expense as a result of the addition of resources to support census growth.spend, higher information technology fees and severance and lease termination costs associated with clinical optimization and reorganization initiatives.
Personal Care DivisionSegment
The following table summarizes our personal care segment results of operations:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
Financial Information (in millions): | | | | | |
| Medicare | $ | — | | | $ | — | | | $ | — | |
| Non-Medicare | 61.4 | | | 65.0 | | | 72.2 | |
| Net service revenue | 61.4 | | | 65.0 | | | 72.2 | |
| Other operating income | — | | | — | | | 1.1 | |
| Cost of service | 46.7 | | | 49.1 | | | 54.9 | |
| Gross margin | 14.7 | | | 15.9 | | | 18.4 | |
| Depreciation and amortization | 0.1 | | | 0.2 | | | 0.2 | |
| Other general and administrative expenses | 9.2 | | | 11.2 | | | 12.4 | |
| Operating income | $ | 5.4 | | | $ | 4.5 | | | $ | 5.8 | |
| Key Statistical Data - Total: | | | | | |
| Billable hours | 1,851,563 | | | 2,275,511 | | | 2,730,121 | |
| Clients served | 10,448 | | | 12,074 | | | 15,019 | |
| Shifts | 791,596 | | | 974,409 | | | 1,177,586 | |
| Revenue per hour | $ | 33.15 | | | $ | 28.54 | | | $ | 26.45 | |
| Revenue per shift | $ | 77.55 | | | $ | 66.66 | | | $ | 61.31 | |
| Hours per shift | 2.3 | | | 2.3 | | | 2.3 | |
|
| | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
Financial Information (in millions): | | | | | |
| Medicare | $ | — |
| | $ | — |
| | $ | — |
|
| Non-Medicare | 82.0 |
| | 77.2 |
| | 59.6 |
|
| Net service revenue | 82.0 |
| | 77.2 |
| | 59.6 |
|
| Cost of service | 61.1 |
| | 58.8 |
| | 45.0 |
|
| Gross margin | 20.9 |
| | 18.4 |
| | 14.6 |
|
| Other operating expenses | 12.5 |
| | 13.1 |
| | 9.7 |
|
| Operating income | $ | 8.4 |
| | $ | 5.3 |
| | $ | 4.9 |
|
| Key Statistical Data: | | | | | |
| Billable hours | 3,308,338 |
| | 3,248,304 |
| | 2,604,794 |
|
| Clients served | 17,364 |
| | 17,981 |
| | 16,774 |
|
| Shifts | 1,488,175 |
| | 1,468,541 |
| | 1,195,511 |
|
| Revenue per hour | 24.80 |
| | 23.75 |
| | 22.86 |
|
| Revenue per shift | 55.13 |
| | 52.54 |
| | 49.80 |
|
| Hours per shift | 2.2 |
| | 2.2 |
| | 2.2 |
|
Year Ended December 31, 20192022 Compared to the Year Ended December 31, 20182021
Operating income related to our personal care segment increased $3$1 million on a $5$4 million increasedecrease in net service revenue. The decrease in net service revenue is due to lower billable hours resulting from staffing shortages partially offset by rate increases. These results are inclusiveimpacts have been mitigated by a reduction in our cost of the acquisitionsservice as most of East Tennessee Personal Care Services (May 2018) and Bring Care Home (October 2018). As a result, our personal care operating results for 2019 and 2018employees are not fully comparable.
Gross marginpaid on an hourly basis as well as a percentagereduction in our other general and administrative expenses.
On February 10, 2023, we signed a definitive agreement to sell our personal care business (excluding the Florida operations). The divestment is expected to close during the second quarter of revenue increased 170 basis points as the2023. See Item 8, Note 6 - Assets Held For Sale for additional information.
High Acuity Care Segment
The following table summarizes our high acuity care segment benefited from rate increases combined with operating cost controls. Additionally, other operating expenses decreased approximately $1 million resulting in an increase in operating income.results of operations:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
Financial Information (in millions): | | | | | |
| Medicare | $ | 5.2 | | | $ | — | | | $ | — | |
| Non-Medicare | 13.3 | | | 3.5 | | | — | |
| Net service revenue | 18.5 | | | 3.5 | | | — | |
| Other operating income | — | | | — | | | — | |
| Cost of service | 18.2 | | | 2.5 | | | — | |
| Gross margin | 0.3 | | | 1.0 | | | — | |
| Depreciation and amortization | 3.3 | | | 1.3 | | | — | |
| Impairment charge | 3.0 | | | — | | | — | |
| Other general and administrative expenses | 33.1 | | | 10.0 | | | — | |
| Operating loss | $ | (39.1) | | | $ | (10.3) | | | $ | — | |
| Key Statistical Data - Total: | | | | | |
| Full risk admissions | 448 | | | 107 | | | — | |
| Limited risk admissions | 1,142 | | | 413 | | | — | |
| Total admissions | 1,590 | | | 520 | | | — | |
| | | | | |
| Full risk revenue per episode | $ | 11,273 | | | $ | 10,457 | | | $ | — | |
| Limited risk revenue per episode | $ | 5,553 | | | $ | 5,693 | | | $ | — | |
| | | | | |
| Number of admitting joint ventures (1) | 8 | | | 7 | | | — | |
(1) Prior year count has been recast to include admitting joint ventures only.
Year Ended December 31, 20182022 Compared to the Year Ended December 31, 20172021
Operating income related to our personalResults
Our high acuity care segment remained flat onresults include a full year of operations in the current year compared to five months of operations in the prior year. Our year over year results reflect revenue growth which was offset by an $18 million increase in netour cost of service revenue. 2018 revenues were positively impactedand other general and administrative expenses driven by the following acquisitions: Intercity Home Care (October 2017), East Tennessee Personal Care Services (May 2018) and Bring Care Home (October 2018). The segment experienced a decrease in gross margin as a percentage of revenue related to additional costs associated with these acquisitions and the Employer Medical Assistance Contribution program ("EMAC") that became effectiveinvestments in the statebusiness. We also recorded an impairment charge in connection with the wind down of Massachusetts on January 1, 2018. Otherthe operations of one of our joint ventures. Although we expect our high acuity care segment to continue to generate operating expenses increased $3 million on an $18 million increase in net service revenue. Acquisitions are includedlosses, we also expect improvement in our consolidatedoperating income as we leverage our operating structure through growth in current and future joint ventures and expansion into new lines of business such as palliative care at home.
Net Service Revenue
Our high acuity care segment provides home recovery care services for high acuity patients on either a full risk or limited risk basis, each with different reimbursement arrangements. Full risk admissions are admissions for which we assume the financial statements from their respective acquisition dates.risk for all related healthcare services during a 30-day or 60-day episodic period in exchange for a fixed contracted bundled rate. Limited risk admissions are admissions for which we assume the risk for certain healthcare services during a shorter acute phase period (equivalent to an inpatient hospital stay) in exchange for a contracted per diem payment.
Additionally, on March 23, 2022, our high acuity care segment entered into a transaction in which one of our health system partners contributed its home health operations to one of our existing joint ventures. As a result, our personalhigh acuity care operating results for 2018segment includes revenue totaling approximately $6 million related to this joint venture's home health operations.
Cost of Service, Excluding Depreciation and 2017 are not fully comparable.Amortization
Our cost of service consists primarily of medical costs associated with direct clinician care provided to our patients during the applicable episode period, costs associated with our virtual care unit ("VCU") which enables us to provide monitoring services and facilitates virtual patient rounding visits via telehealth and costs associated with resources to support future palliative care at home programs. We continue to invest in the infrastructure of our VCU in anticipation of future growth.
Other General and Administrative Expenses
Other general and administrative expenses primarily consist of salaries and benefits. We have made significant investments to build the clinical, operational and technological infrastructure necessary to support the development and future growth of home recovery care programs on a national scale. We have employees at both the local market level and at our corporate offices.
Corporate
The following table summarizes our corporate results of operations:
|
| | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
Financial Information (in millions): | | | | | |
| Other operating expenses | $ | 160.9 |
| | $ | 127.6 |
| | $ | 117.8 |
|
| Depreciation and amortization | 12.4 |
| | 8.4 |
| | 12.5 |
|
| Total operating expenses before Securities Class Action Lawsuit settlement, net | $ | 173.3 |
| | $ | 136.0 |
| | $ | 130.3 |
|
| Securities Class Action Lawsuit settlement, net | — |
| | — |
| | 28.7 |
|
| Total operating expenses | $ | 173.3 |
| | $ | 136.0 |
| | $ | 159.0 |
|
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
Financial Information (in millions): | | | | | |
| Other general and administrative expenses | $ | 160.0 | | | $ | 163.1 | | | $ | 173.2 | |
| Depreciation and amortization | 15.2 | | | 22.4 | | | 22.5 | |
| Total operating expenses | $ | 175.2 | | | $ | 185.5 | | | $ | 195.7 | |
Corporate expenses consist of costs relatingrelated to our executive management and corporate and administrative support functions, primarily information services, accounting, finance, billing and collections, legal, compliance, risk management, procurement, marketing, clinical administration, training, human resources and administration.
Year Ended December 31, 20192022 Compared to the Year Ended December 31, 20182021
During 2019, corporate operating expenses increased $37 million; approximately $27 million of which is attributable to the CCH acquisition: $7 million relates to CCH corporateCorporate other general and administrative support functions, $6expenses decreased approximately $3 million relates to CCH intangibles amortizationduring the year ended December 31, 2022. Excluding our acquisitions, corporate other general and approximately $14administrative expenses decreased $4 million relates to CCH acquisition and integration costs. Excluding the impact of the CCH acquisition, corporate operating expenses increased $10 million which represents 3% of our $293 million increase in revenue. This increase isyear over year primarily due to increases in salaries and benefits expense and information technology expense which were partially offset by decreases in professional fees and legal settlements as well ashigher gains on the sale of fleet vehicles.
Year Ended December 31, 2018 Compared to the Year Ended December 31, 2017
Excluding the Securities Class Action Lawsuit settlement during the year ended December 31, 2017, corporate operating expenses increased 4% onvehicles, lower incentive compensation costs and a 10% increase in net service revenue. Approximately $2 million of the increase was related to a reduction in our indemnity receivable related to the Florence, South Carolina third party audit (see Item 8, Note 10 - Commitments and Contingencies to our consolidated financial statements for additional information). The remaining increase was related to increases in salaries and benefits expense and travel and training expense whichfavorable legal settlement; these items were partially offset by a decrease in depreciationplanned wage increases, costs associated with our clinical optimization and amortization.reorganization initiatives and higher acquisition and integration costs.
Liquidity and Capital Resources
Cash Flows
The following table summarizes our cash flows for the periods indicated (amounts in millions):
| | | | For the Years Ended December 31, | | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 | | 2022 | | 2021 | | 2020 |
| Cash provided by operating activities | $ | 202.0 |
| | $ | 223.5 |
| | $ | 105.7 |
| Cash provided by operating activities | $ | 133.3 | | | $ | 188.9 | | | $ | 289.0 | |
| Cash used in investing activities | (352.9 | ) | | (22.2 | ) | | (44.0 | ) | Cash used in investing activities | (94.5) | | | (281.6) | | | (287.1) | |
| Cash provided by (used in) financing activities | 227.2 |
| | (267.4 | ) | | (5.5 | ) | |
| Cash (used in) provided by financing activities | | Cash (used in) provided by financing activities | (30.4) | | | 55.1 | | | (15.0) | |
| Net increase (decrease) in cash, cash equivalents and restricted cash | 76.3 |
| | (66.1 | ) | | 56.2 |
| Net increase (decrease) in cash, cash equivalents and restricted cash | 8.4 | | | (37.6) | | | (13.1) | |
| Cash, cash equivalents and restricted cash at beginning of period | 20.2 |
| | 86.4 |
| | 30.2 |
| Cash, cash equivalents and restricted cash at beginning of period | 45.8 | | | 83.4 | | | 96.5 | |
| Cash, cash equivalents and restricted cash at end of period | $ | 96.5 |
| | $ | 20.2 |
| | $ | 86.4 |
| Cash, cash equivalents and restricted cash at end of period | $ | 54.1 | | | $ | 45.8 | | | $ | 83.4 | |
Cash provided by operating activities totaled $202.0 million for 2019, $223.5 million for 20182022, 2021 and $105.7 million for 2017. During each year, we maintained2020 has provided sufficient liquidity to finance our capital expenditures, both routine and non-routine, and acquisitions. Changes in our cash provided by operating activities during the past three years were primarily the result of fluctuations in our net income, the collections of our accounts receivable and the timing of payments of accrued expenses. During 2017,Cash provided by operating cash flows were negatively impacted by approximately $30activities decreased $55.6 million relatedduring 2022 compared to 2021 primarily due to the Securities Class Action Lawsuit settlementpayment of a full year of operating expenses for our high acuity care segment compared to only
five months in the prior year, the repayment of $38.0 million in connection with our Infinity ZPIC audits (see Item 8, Note 10 –12 - Commitments and Contingencies to our consolidated financial statements).statements for additional information), lower collections due to the reinstatement of sequestration and an increase in days revenue outstanding. Cash provided by operating activities decreased $100.1 million during 2021 compared to 2020 primarily due to the deferral of payroll taxes and the receipts of CARES Act PRF funds in 2020 and an increase in days revenue outstanding in 2021 partially offset by an increase in operating income.
CashOur cash used in investing activities increased $330.7 million during 2019 compared to 2018 primarily due toconsists of the acquisitionspurchase of CCHproperty and RoseRock.equipment, investments and acquisitions. Cash used in investing activities decreased $21.8$187.1 million during 2018 compared to 20172022 primarily due to decreasesreductions in acquisition spend. Our 2020 cash paidflows from investing activities included proceeds from the sale of our investment in the Heritage Healthcare Innovation Fund, LP (see Item 8, Note 1 - Nature of Operations, Consolidation and Presentation of Financial Statements to our consolidated financial statements for acquisitions ($24.5 million)additional information). Excluding these proceeds, cash used in investing activities decreased $23.4 million during 2021 primarily due to reductions in acquisition spend.
Our financing activities primarily consist of borrowings under our term loan and/or revolving credit facility, repayments of borrowings, the remittance of taxes associated with shares withheld on non-cash compensation, proceeds related to the exercise of stock options, proceeds related to the purchase of stock under our employee stock purchase plan and capital expenditures ($4.1 million) offset by an increasethe purchase of company stock under our stock repurchase programs. Cash used in investments ($6.7 million).
Cashfinancing activities totaled $30.4 million during 2022; cash provided by financing activities totaled $227.2$55.1 million during 2019 and2021. The $85.5 million change is primarily relateddue to ourhigher borrowings under our Second Amended Credit Agreement to fund acquisitions. Cash usedacquisitions in financing activities increased $261.9 million during 2018 compared to 2017 primarily due to our repurchase of company stock and the repayments of borrowings under our Term Loan and Revolving Credit Facility offset by borrowings under our new Credit Agreement.2021.
Liquidity
Typically, our principal source of liquidity is the collection of our patient accounts receivable, primarily through the Medicare program. In addition to our collection of patient accounts receivable, from time to time, we can and do obtain additional sources of liquidity by the incurrence of additional indebtedness.
During 2019,2022, we spent $7.9$6.2 million in capital expenditures compared to $6.6$6.3 million and $10.7$5.3 million during 20182021 and 2017,2020, respectively. Our capital expenditures for 20202023 are expected to be approximately $6.0$13.0 million to $8.0$15.0 million, excluding the impact of any future acquisitions.
Additionally, during 2022, pursuant to our authorized stock repurchase program, we repurchased 150,000 shares of our common stock at a weighted average price of $115.64 per share and a total cost of approximately $17 million. The repurchased shares are classified as treasury shares.
As of December 31, 2019,2022, we had $30.3$40.5 million in cash and cash equivalents and $449.8$520.4 million in availability under our $550.0 million Revolving Credit Facility.
During 2017, we settled the Securities Class Action Lawsuit for approximately $43.7 million, of which approximately $15.0 million was paid by the Company's insurance carriers; we used cash on hand to make the required remaining $28.7 million payment.
Based on our operating forecasts and our new debt service requirements, we believe we will have sufficient liquidity to fund our operations, capital requirements and debt service requirements.requirements for the next twelve months and beyond.
Outstanding Patient Accounts Receivable
Our patient accounts receivable increased $48.6$21.8 million from December 31, 2018 to December 31, 20192021. Our Medicare patient accounts receivable increased $9.8 million primarily due to our acquisition activity.billing issues related to the Notice of Admissions ("NOAs") process and billing delays resulting from the pre-claim review process in the five Review Choice Demonstration ("RCD") states. Our non-Medicare patient accounts receivable increased $12.0 million as a result of the transition of episodic payor reimbursement models to per visit reimbursement methods. Our cash collection as a percentage of revenue was 105% and 104%100% for the twelve-month periods ended December 31, 20192022 and 2018, respectively.2021. Our days revenue outstanding, net at December 31, 20192022 was 40.946.1 days which is an increase of 2.9 days from December 31, 2018. Our acquisition activity has negatively impacted our days revenue outstanding by approximately 2.3 days.2021.
Our patient accounts receivable includes unbilled receivables and are aged based upon ourthe initial service date. We monitor unbilled receivables on a care center by care center basis to ensure that all efforts are made to bill claims within timely filing deadlines. Our unbilled patient accounts receivable canmay be impacted by acquisition activity, probepre-claim reviews required by the Medicare Administrative Contractors in the five RCD states, voluntary pre-bill edits orand review, efforts to secure needed documentation to bill (orders, consents, etc.), integrations of recent acquisitions, changes of ownership and any regulatory changes which result in additional information or procedures needed prior to billing.and procedural updates impacting claim submission. The timely filing deadline for Medicare is one year from the date of the episode was completed,last billable service in the 30-day billing period and varies by state for Medicaid-reimbursableMedicaid-reimburseable services and varies among insurance companies and other private payors.
The following schedules detail our patient accounts receivable, by payor class, aged based upon initial date of service (amounts in millions, except days revenue outstanding):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 0-90 | | 91-180 | | 181-365 | | Over 365 | | Total |
| At December 31, 2022: | | | | | | | | | |
| Medicare patient accounts receivable | $ | 179.9 | | | $ | 11.4 | | | $ | 5.1 | | | $ | 0.1 | | | $ | 196.5 | |
| Other patient accounts receivable: | | | | | | | | | |
| Medicaid | 16.3 | | | 1.4 | | | 0.7 | | | — | | | 18.4 | |
| Private | 67.5 | | | 8.7 | | | 5.7 | | | — | | | 81.9 | |
| Total | $ | 83.8 | | | $ | 10.1 | | | $ | 6.4 | | | $ | — | | | $ | 100.3 | |
| Total patient accounts receivable | | | | | | | | | $ | 296.8 | |
| Days revenue outstanding (1) | | | | | | | | | 46.1 | |
| | | | | | | | | | | | | | 0-90 | | 91-180 | | 181-365 | | Over 365 | | Total |
| | 0-90 | | 91-180 | | 181-365 | | Over 365 | | Total | |
| At December 31, 2019: | | | | | | | | | | |
| At December 31, 2021: | | At December 31, 2021: | | | | | | | | | |
| Medicare patient accounts receivable | $ | 115.2 |
| | $ | 13.8 |
| | $ | 6.8 |
| | $ | 1.0 |
| | $ | 136.8 |
| Medicare patient accounts receivable | $ | 176.7 | | | $ | 7.5 | | | $ | 1.1 | | | $ | 1.4 | | | $ | 186.7 | |
| Other patient accounts receivable: | | | | | | | | | | Other patient accounts receivable: | | | | | | | | | |
| Medicaid | 22.6 |
| | 5.7 |
| | 4.0 |
| | — |
| | 32.3 |
| Medicaid | 16.0 | | | 1.5 | | | 0.7 | | | — | | | 18.2 | |
| Private | 60.0 |
| | 6.3 |
| | 2.2 |
| | — |
| | 68.5 |
| Private | 59.7 | | | 8.7 | | | 1.7 | | | — | | | 70.1 | |
| Total | $ | 82.6 |
| | $ | 12.0 |
| | $ | 6.2 |
| | $ | — |
| | $ | 100.8 |
| Total | $ | 75.7 | | | $ | 10.2 | | | $ | 2.4 | | | $ | — | | | $ | 88.3 | |
| Total patient accounts receivable | | | | | | | | | $ | 237.6 |
| Total patient accounts receivable | | | | | | | | | $ | 275.0 | |
| Days revenue outstanding (1) | | | | | | | | | 40.9 |
| Days revenue outstanding (1) | | 43.2 | |
|
| | | | | | | | | | | | | | | | | | | |
| | 0-90 | | 91-180 | | 181-365 | | Over 365 | | Total |
| At December 31, 2018: | | | | | | | | | |
| Medicare patient accounts receivable | $ | 95.5 |
| | $ | 8.1 |
| | $ | 1.0 |
| | $ | 1.8 |
| | $ | 106.4 |
|
| Other patient accounts receivable: | | | | | | | | | |
| Medicaid | 13.1 |
| | 2.7 |
| | 1.1 |
| | — |
| | 16.9 |
|
| Private | 51.3 |
| | 6.7 |
| | 4.4 |
| | 3.3 |
| | 65.7 |
|
| Total | $ | 64.4 |
| | $ | 9.4 |
| | $ | 5.5 |
| | $ | 3.3 |
| | $ | 82.6 |
|
| Total patient accounts receivable | | | | | | | | | $ | 189.0 |
|
| Days revenue outstanding (1) | | | | | | | | | 38.0 |
|
| |
(1) | Our calculation of days revenue outstanding, net is derived by dividing our ending net patient accounts receivable at December 31, 2019 and 2018 by our average daily net patient revenue for the three-month periods ended December 31, 2019 and 2018, respectively. |
Indebtedness
FirstSecond Amendment to Amended and Restatedthe Credit Agreement
On February 4, 2019,July 30, 2021, we entered into the FirstSecond Amendment to theour Credit Agreement (as amended by the FirstSecond Amendment, the “Amended"Second Amended Credit Agreement”Agreement"). The Second Amended Credit Agreement provides for a senior secured credit facility in an initial aggregate principal amount of up to $725.0 million,$1.0 billion, which includes thea $550.0 million Revolving Credit Facility under the Credit Agreement, and a term loan facility in thewith a principal amount of up to $175.0$450.0 million (the “Term"Amended Term Loan Facility”Facility" and collectively with the Revolving Credit Facility, the “Credit Facility”"Amended Credit Facility"), which was added by.
Net proceeds from the First Amendment.
We borrowed the entire principal amount of the$450.0 million Amended Term Loan Facility on February 4, 2019 in orderwere used to fund a portion of the purchase price ofContessa acquisition.
Our weighted average interest rate for borrowings under our Amended Term Loan Facility was 3.2% for the CCH acquisition, withyear ended December 31, 2022 and 1.6% for the remainder of the purchase price and associated transactional fees and expenses funded by proceeds from the Revolving Credit Facility.
year ended December 31, 2021. Our weighted average interest rate for borrowings under our $550.0 million Revolving Credit Facility was 4.0%3.4% for the periodyear ended December 31, 2019. Our weighted average interest rate for borrowings under our $175.0 million Term Loan Facility was 3.8%2022 and 1.9% for the period February 4, 2019 toyear ended December 31, 2019.2021.
As of December 31, 2019,2022, our consolidated leverage ratio was 1.0 and1.7, our consolidated interest coverage ratio was 17.211.6 and we are in compliance with our covenants under the Second Amended Credit Agreement.
As of December 31, 2019,2022, our availability under our $550.0 million Revolving Credit Facility was $449.8$520.4 million as we have $70.0 millionno outstanding in borrowings and $30.2$29.6 million outstanding in letters of credit.
See Item 8, Note 7 -9 – Long Term Obligations to our consolidated financial statements for additional details on our outstanding long-term obligations.
2019 Stock Repurchase ProgramPrograms
On February 25, 2019,December 23, 2020, we announced that our Board of Directors authorized a stock repurchase program, under which we could repurchase up to $100 million of our outstanding common stock through December 31, 2021 (the "2021 Share Repurchase Program"). Pursuant to this program, we repurchased 446,832 shares of our common stock at a weighted average price of $223.49 per share and a total cost of approximately $100 million during the year ended December 31, 2021. We did not repurchase any shares pursuant to this stock repurchase program during the year ended December 31, 2020. The repurchased shares were classified as treasury shares. The 2021 Share Repurchase Program expired on December 31, 2021.
On August 2, 2021, our Board of Directors authorized a share repurchase program, under which we could repurchase up to $100 million of our outstanding common stock through December 31, 2022 to commence upon the completion of the Company's 2021 Share Repurchase Program (the "New Share Repurchase Program"). Pursuant to this program, we repurchased 150,000 shares of our common stock at a weighted average price of $115.64 per share and a total cost of approximately $17 million during the year ended December 31, 2022. The repurchased shares were classified as treasury shares. The New Share Repurchase Program expired on December 31, 2022.
Under the terms of the 2021 Share Repurchase Program and the New Share Repurchase Program, we were allowed to repurchase shares from time to time through open market purchases, unsolicited or solicited privately negotiated transactions, an accelerated stock repurchase program, and/or a trading plan in compliance with Exchange Act Rule 10b5-1. The timing and the amount of the repurchases were determined by management based on a number of factors, including but not limited to share price, trading volume and general market conditions, as well as on working capital requirements, general business conditions and other factors.
On February 2, 2023, our Board of Directors authorized a share repurchase program, under which we may repurchase up to $100 million of our outstanding common stock through March 1, 2020.December 31, 2023 (the "2023 Share Repurchase Program").
Under the terms of the program,2023 Share Repurchase Program, we are allowed to repurchase shares from time to time inthrough open market purchases, unsolicited or solicited privately negotiated transactions, block purchases an accelerated stock repurchase program, and/or a trading plan in private transactions in accordancecompliance with applicable federal securities laws and other legal requirements. We are allowed to enter intoExchange Act Rule 10b5-1 plans to effect some or all of the repurchases.10b5-1. The timing and the amount of the repurchases will be determined by management based on a number of factors, including but not limited to share price, trading volume and general market conditions, as well as on working capital requirements, general business conditions and other factors.
We did not repurchase any shares pursuant to this stock purchase program during 2019.
2018 Share Repurchase
On June 4, 2018, we purchased 2,418,304 of our common shares from affiliates of KKR Credit Advisors (US) LLC ("KKR"), representing one-half of KKR's then current holdings in the Company and 7.1% of the aggregate outstanding shares of the Company's common stock for a total purchase price of $181.4 million including related direct costs. The Company repurchased the shares at $73.96 which represents 96% of the closing stock price of the Company's common stock on June 4, 2018. The repurchased shares are classified as treasury shares.
Contractual Obligations
Our future contractual obligations at December 31, 20192022 were as follows (amounts in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Payments Due by Period |
| Total | | Less than
1 Year | | 2-3
Years | | 4-5
Years | | After
5 Years |
| Long-term obligations | $ | 436.1 | | | $ | 14.3 | | | $ | 44.9 | | | $ | 376.9 | | | $ | — | |
| Interest on long-term obligations (1) | 85.3 | | | 25.3 | | | 47.3 | | | 12.7 | | | — | |
| Finance leases | 2.3 | | | 1.2 | | | 1.1 | | | — | | | — | |
| Operating leases | 109.6 | | | 36.1 | | | 51.7 | | | 19.4 | | | 2.4 | |
| Purchase obligations (2) | 5.2 | | | 3.4 | | | 1.8 | | | — | | | — | |
| $ | 638.5 | | | $ | 80.3 | | | $ | 146.8 | | | $ | 409.0 | | | $ | 2.4 | |
(2)Purchase obligations are primarily related to information technology contracts and software licenses. We have a significant information technology contract that will be renewed in 2023. The table above does not reflect any amounts related to this contract.
Inflation
Our operations have been materially impacted by the current inflationary environment as we have experienced higher labor costs and increases in supply costs, fuel costs and mileage reimbursements. We expect inflation to continue to impact our operations in 2023. As of December 31, 2022, the impacts of inflation on our results of operations have been partially mitigated by rate increases, improvements in clinician utilization and reductions in hospice staffing levels. No assurance can be given as to our ability to offset the impacts of inflation in the future.
|
| | | | | | | | | | | | | | | | | | | |
| | Payments Due by Period |
| | Total | | Less than 1 Year | | 1-3 Years | | 4-5 Years | | After 5 Years |
| Long-term obligations | $ | 242.3 |
| | $ | 8.2 |
| | $ | 17.5 |
| | $ | 216.6 |
| | $ | — |
|
| Interest on long-term obligations (1) | 22.3 |
| | 6.9 |
| | 10.3 |
| | 5.1 |
| | — |
|
| Finance leases | 3.6 |
| | 1.9 |
| | 1.7 |
| | — |
| | — |
|
| Operating leases | 90.7 |
| | 30.2 |
| | 39.3 |
| | 14.2 |
| | 7.0 |
|
| Capital commitments | 0.3 |
| | 0.3 |
| | — |
| | — |
| | — |
|
| Purchase obligations | 11.1 |
| | 4.2 |
| | 6.6 |
| | 0.3 |
| | — |
|
| Uncertain tax positions | 2.7 |
| | — |
| | 2.7 |
| | — |
| | — |
|
| | $ | 373.0 |
| | $ | 51.7 |
| | $ | 78.1 |
| | $ | 236.2 |
| | $ | 7.0 |
|
| |
(1) | Interest on debt with variable rates was calculated using the current rate for that particular debt instrument at December 31, 2019. |
Critical Accounting Estimates
The discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principlesGenerally Accepted Accounting Principles (“U.S. GAAP”). The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses and related disclosures of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates, including those related to revenue recognition, collectability of accounts receivable, reserves related to insurance and litigation, business combinations, goodwill, intangible assets, income taxes and contingencies. We base these estimates on our historical experience and various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results experienced may vary materially and adversely from our estimates. To the extent there are material differences between our estimates and the actual results, our future results of operations may be affected.
We believe the following critical accounting policies represent our most significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
We account for revenue from contracts with customers in accordance with Accounting Standards UpdateCodification ("ASU"ASC") 2014-09,606, Revenue from Contracts with Customers (Topic 606) and ASU 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date (collectively, "ASC 606"), and as such, we recognize revenue in the period in which we satisfy our performance obligations under our contracts by transferring our promised services to our customers in amounts that reflect the consideration to which we expect to be entitled in exchange for providing patient care, which are the transaction prices allocated to the distinct services. The Company'sOur cost of obtaining contracts is not material.
Revenues are recognized as performance obligations are satisfied, which varies based on the nature of the services provided. Our performance obligation is the delivery of patient care services in accordance with the nature and frequency of services outlined in physicians' orders, which are determined by a physician based on a patient's specific goals.
The Company'sOur performance obligations relate to contracts with a duration of less than one year; therefore, the Company haswe have elected to apply the optional exemption provided by ASC 606 and isare not required to disclose the aggregate amount of the transaction price allocated to performance obligations that are unsatisfied or partially unsatisfied as of the end of the reporting period. The unsatisfied or partially unsatisfied performance obligations are generally completed when the patients are discharged, which generally occurs within days or weeks of the end of the reporting period.
We determine the transaction price based on gross charges for services provided, reduced by estimates for contractual and non-contractual revenue adjustments. Contractual revenue adjustments include adjustments providedare recorded for the difference between our standard rates and the contracted rates to be realized from patients, and third-party payors based on contracted rates.and others for services provided. Non-contractual revenue adjustments include discounts provided to self-pay, uninsured patients or other payors, adjustments resulting from payment reviews and adjustments arising from our inability to obtain appropriate billing documentation, authorizations or face-to-face documentation. Subsequent changes to the estimate of the transaction price are recorded as adjustments to net service revenue in the period of change.
Contractual revenue adjustments are recorded for the difference between our standard rates and the contracted rates to be realized from patients, third party payors and others for services provided. Non-contractual revenue adjustments are recorded for self-pay, uninsured patients and other payors by major payor class based on our historical collection experience, aged accounts receivable
by payor and current economicindustry conditions. Non-contractualThe non-contractual revenue adjustments represent the difference between amounts billed and amounts we expect to collect based on our collection history with similar payors. The Company assesses itsWe assess our ability to collect for the healthcare services provided at the time of patient admission based on the Company'sour verification of the patient's insurance coverage under Medicare, Medicaid, and other commercial or managed care insurance programs. Medicare represents approximately 74% of the Company'sour consolidated net service revenue.
Amounts due from third-party payors, primarily commercial health insurers and government programs (Medicare and Medicaid), include variable consideration for retroactive revenue adjustments due to settlements of audits and payment reviews. We determine our estimates for non-contractual revenue adjustments related to audits and payment reviews based on our historical experience and success rates in the claim appeals and adjudication process.
We determine our estimates for non-contractual revenue adjustments related to our inability to obtain appropriate billing documentation, authorizations or face-to-face documentation based on our historical experience which primarily includes a historical collection rate of over 99% on Medicare claims. Revenue is recorded at amounts we estimate to be realizable for services provided.
Home Health Revenue Recognition
Medicare Revenue
Net service revenue is recorded underEffective January 1, 2020, the Centers for Medicare prospectiveand Medicaid Services ("CMS") implemented a revised case-mix adjustment methodology, the Patient-Driven Groupings Model ("PDGM"). PDGM uses 30-day periods of care rather than 60-day episodes of care as the unit of payment, system (“PPS”) based on an established Federal Medicare home health episode payment rate, that is subject to adjustment based on certain variables including, but not limited to: (a) an outlier payment if a patient’s care was unusually costly (capped at 10%eliminates the use of total reimbursement per provider number); (b) a LUPA if the number of therapy visits was four or fewer; (c) a partialprovided in determining payment if a patient transferred to another provider or we admitted a patient transferring from another provider before completing the episode; (d) a payment adjustment based upon the level of therapy services required (with various incremental adjustments made for additional visits, with larger payment increases associated with the sixth, fourteenth and twentieth visit thresholds); (e) the number of episodes of care provided to a patient, regardless of whether the same home health provider provided care for the entire series of episodes; (f) changes in the base episode payments established by the Medicare Program; and (g) adjustments to the base episode payments for case mix and geographic wages. Medicare rates are basedrelies more heavily on the severity of the patient's condition, service needs and goals,clinical characteristics and other factors relating to the cost of providing services and supplies, bundled into an episode of care, not to exceed 60 days. An episode starts the first day a billable visit is performed and ends 60 days later or upon discharge, if earlier, with multiple continuous episodes allowed.patient information.
The Medicare home health benefit requires that beneficiaries be homebound (meaning that the beneficiary is unable to leave their home without a considerable and taxing effort), require intermittent skilled nursing, physical therapy or speech therapy services, and receive treatment under a plan of care established and periodically reviewed by a physician. All Medicare contracts are required to have a signed plan of care which represents a single performance obligation, comprisingcomprised of the delivery of a series of distinct services that are substantially similar and have a similar pattern of transfer to the customer. Accordingly, the Company accountswe account for the series of services ("episode") as a single performance obligation satisfied over time, as the customer simultaneously receives and consumes the benefits of the goods and services provided. Expected MedicareAn episode starts the first day a billable visit is performed and ends 60 days later or upon discharge, if earlier, with multiple continuous episodes allowed. Each 60-day episode includes two 30-day payment periods.
Net service revenue per episode is recognizedrecorded based on the established Federal Medicare home health payment rate for a pro-rated service output method, utilizing30-day period of care. ASC 606 notes that if an entity has a right to consideration from a customer in an amount that corresponds directly with the value of the entity’s performance completed to date, the entity may recognize revenue in the amount to which the entity has a right to invoice. We have elected to apply the "right to invoice" practical expedient, and therefore, our revenue recognition is based on the reimbursement we are entitled to for each 30-day period of care. We utilize our historical average length of episode priorstay for each 30-day period of care as the measure of progress towards the satisfaction of our performance obligation.
PDGM uses timing, admission source, functional impairment levels and principal and other diagnoses to discharge.
case-mix adjust payments. The base episodecase-mix adjusted payment can be adjustedfor a 30-day period of care is subject to additional adjustments based on eachcertain variables, including, but not limited to (a) an outlier payment if our patient's health including clinical condition, functional abilitiescare was unusually costly (capped at 10% of total reimbursement per provider number); (b) a low utilization payment adjustment (“LUPA”) if the number of visits provided was less than the established threshold, which ranges from two to six visits and service needs, as well asvaries for every case-mix group; (c) a partial payment if a patient is transferred to another provider or from another provider before completing the 30-day period of care; and (d) the applicable geographic wage index, low utilization, patient transfersindex. Payments for routine and other factors. The services covered bynon-routine supplies are included in the episode30-day payment include all disciplines of care in addition to medical supplies. rate.
Medicare can also make various adjustments to payments received if we are unable to produce appropriate billing documentation or acceptable authorizations. We estimate the impact of such adjustments based on our historical experience, which primarily includes a historical collection rate of over 99% on Medicare claims, and record this estimate during the period in which services are rendered as a reduction to revenue andwith a corresponding reduction to patient accounts receivable. A 0.1% change in our Medicare collection rate would impact our annual Medicare revenue by approximately $0.9 million.
A portionAmounts due from Medicare include variable consideration for retroactive revenue adjustments due to settlements of audits and payment reviews. We determine our estimates for non-contractual revenue adjustments related to audits and payment reviews based on our historical experience and success rates in the claim appeals and adjudication process.
The Medicare home health benefit requires that beneficiaries be homebound (meaning that the beneficiary is unable to leave his/her home without a considerable and taxing effort), require intermittent skilled nursing, physical therapy or speech therapy services and receive treatment under a plan of care established and periodically reviewed by a physician. In order to provide greater flexibility during the novel coronavirus pandemic ("COVID-19"), CMS relaxed the definition of homebound status through the duration of the public health emergency. During the pandemic, a beneficiary is considered homebound if they have been instructed by a physician not to leave their home because of a confirmed or suspected COVID-19 diagnosis or if the patient has a condition that makes them more susceptible to contracting COVID-19.
During 2020, 20% of the reimbursement from each Medicare episode is30-day payment rate was billed near the start of each episode,30-day period of care, referred to as a request for anticipated payment ("RAP"), and cash iswas typically received before all services arewere rendered. The amount of revenue recognized for episodes of care which are incomplete at period end is based on the company's average percentage of days complete on episodes as of the end of the year. As of December 31, 2019, the difference between theAny cash received from Medicare for a requestRAP for anticipated payment (“RAP”) on episodes in progress anda 30-day period of care that exceeded the associated estimated revenue earned was recorded to accrued expenses within our consolidated balance sheet.sheets. CMS fully eliminated all upfront payments associated with RAPs effective January 1, 2021. Effective January 1, 2022, CMS implemented a new one-time Notice of Admission ("NOA") process. The NOA process requires a one-time submission that establishes the home health period of care and covers all contiguous 30-day periods of care until the patient is discharged from Medicare home health services. If the NOA is not submitted timely, a payment reduction will be applied equal to 1/30 of the payment amount for each day from the home health start of care date until the date the NOA is submitted.
Non-Medicare Revenue
Payments from non-Medicare payors are either a percentage of Medicare rates, per-visit rates or case rates depending upon the terms and conditions established with such payors. Approximately 30% of our managed care contract volume affords us the opportunity to receive additional payments if we achieve certain quality or process metrics as defined in each contract (e.g. star ratings and acute-care hospitalization rates).
Episodic-based Revenue. We recognize revenue in a similar manner as we recognize Medicare revenue for episodic-based ratesamounts that are paid by other insurance carriers, including Medicare Advantage programs; however, these ratesamounts can vary based upon the negotiated terms which generally range from 90%95% to 100% of Medicare rates.
Non-episodic based Revenue. GrossFor our per visit contracts, gross revenue is recorded on an accrual basis based upon the date of service at amounts equal to our established or estimated per-visit rates. For our case rate contracts, gross revenue is recorded over our historical average length of stay using the established case rate for each admission. Contractual revenue adjustments are recorded for the difference between our standard rates and the contracted rates to be realized from patients, third parties and others for services provided and are deducted from gross revenue to determine net service revenue. We also make non-contractual revenue adjustments to non-episodic revenue based on our historical experience to reflect the estimated transaction price.Weprice. We receive a minimal amount of our net service revenue from patients who are either self-insured or are obligated for an insurance co-payment.
Under our case rate contracts, we may receive reimbursement before all services are rendered. Any cash received that exceeds the associated revenue earned is recorded to deferred revenue in accrued expenses within our consolidated balance sheets.
Hospice Revenue Recognition
Hospice Medicare Revenue
Gross revenue is recorded on an accrual basis based upon the date of service at amounts equal to the estimated payment rates. The estimated payment rates are predetermined daily or hourly rates for each of the four levels of care we deliver. The four levels of care are routine care, general inpatient care, continuous home care and respite care. Routine care accounted for 99%, 97% and 97% of our total net Medicare hospice service revenue for each of 2019, 20182022, 2021 and 2017,2020, respectively. There are two separate payment rates for routine care: payments for the first 60 days of care and care beyond 60 days. In addition to the two routine rates, we may also receive a service intensity add-on (“SIA”). The SIA is based on visits made in the last seven days of life by a registered nurse (“RN”) or medical social worker (“MSW”) for patients in a routine level of care.
The performance obligation is the delivery of hospice services to the patient, as determined by a physician, each day the patient is on hospice care.
We make adjustments to Medicare revenue for non-contractual revenue adjustments, which include our inability to obtain appropriate billing documentation or acceptable authorizations and other reasons unrelated to credit risk. We estimate the impact of these non-contractual revenue adjustments based on our historical experience, which primarily includes a historical collection rate of over 99% on Medicare claims, and record it during the period services are rendered. A 0.1% change in our Medicare collection rate would impact our annual Medicare revenue by approximately $0.7 million.
Additionally, our hospice service revenue is subject to certain limitations on payments from Medicare which are considered variable consideration. We are subject to an inpatient cap limit and an overall Medicare payment cap for each provider number. We monitor these caps on a provider-by-provider basis and estimate amounts due back to Medicare if we estimate a cap has been exceeded. We record these adjustments as a reduction to revenue and an increase in accrued expenses within our consolidated balance sheet. Beginning for the cap year ending October 31, 2017, providerssheets. Providers are required to self-report and pay their estimated cap liability by February 28th28th of the following year. As of December 31, 2019, we have settled our Medicare hospice reimbursements for all fiscal years through October 31, 2012. As of December 31, 2019,2022, we have recorded $5.7 million in accrued expenses for estimated amounts due back to Medicare for the Federal cap years ended October 31, 2013 through September 30, 2020; approximately $1.9 million of this amount is related to the cap liability acquired as part of the CCH acquisition. As of December 31, 2018, we had recorded $1.7$4.3 million for estimated amounts due back to Medicare in accrued expenses for the Federal cap years ended October 31, 20132016 through September 30, 2019.2023. As of December 31, 2021, we had recorded $4.5 million for estimated amounts due back to Medicare in accrued expenses for the Federal cap years ended October 31, 2016 through September 30, 2022.
Hospice Non-Medicare Revenue
Gross revenue is recorded on an accrual basis based upon the date of service at amounts equal to our established rates or estimated per day rates, as applicable. Contractual revenue adjustments are recorded for the difference between our standard rates and the contractual rates to be realized from patients, third partythird-party payors and others for services provided and are deducted from gross revenue to determine our net service revenue. We also make non-contractual revenue adjustments to non-Medicare revenue based on our historical experience to reflect the estimated transaction price.
Personal Care Revenue Recognition
Personal Care Revenue
We generate net service revenuesrevenue by providing our services directly to patients based on authorized hours, visits or units determined by the relevant agency, at a rate that is either contractual or fixed by legislation. Net service revenue is recognized at the time services are rendered based on gross charges for the services provided, reduced by estimates for contractual and non-contractual revenue adjustments. We receive payment for providing such services from payors, including state and local governmental agencies, managed care organizations, commercial insurers and private consumers. Payors include the following elder service agencies: Aging Services Access Points (ASAPs)("ASAPs"), Senior Care Options (SCOs)("SCOs"), Program of All-Inclusive Care for the Elderly (PACE)("PACE") and the Veterans Administration (VA)("VA").
High Acuity Care Revenue Recognition
High Acuity Care Revenue
Our revenues are derived from contracts with (1) health insurance plans for the coordination and provision of home recovery care services to clinically-eligible patients who are enrolled members in those insurance plans, (2) health system partners for the coordination and provision of home recovery care services to clinically-eligible patients who are discharged early from a health system facility to complete their inpatient stay at home and (3) Medicare and other payors for the provision of home health services.
Under our health insurance plan contracts, we provide home recovery care services, which include hospital-equivalent ("H@H") and skilled nursing facility ("SNF") equivalent services ("SNF@H"), for high acuity care patients on a full risk basis whereby we assume the financial risk for the coordination and payment of all hospital or SNF replacement medical services necessary to treat the medical condition for which the patient was diagnosed in a home-based setting for a 30-day (H@H) or 60-
day (SNF@H) episode of care in exchange for a fixed contracted bundled rate. For H@H programs, the fixed rate is based on the assigned diagnosis related group ("DRG") and the 30-day post-discharge related spend. For SNF@H programs, the fixed rate is based on the 60-day post-discharge related spend. Our performance obligation is the coordination and provision of patient care in accordance with physicians’ orders over either a 30-day or 60-day episode of care. The majority of our care coordination services and direct patient care is provided in the first five to seven days of the episode period (the "acute phase"). Monitoring services and follow-up direct patient care, as deemed necessary by the treating physician, are provided throughout the remainder of the episode. Since the majority of our services are provided during the acute phase, we recognize net service revenue over the acute phase based on gross charges for the services provided per the applicable managed care contract rates, reduced by estimates for revenue adjustments.
Under our contracts with health system partners, we provide home recovery care services for high acuity patients on a limited risk basis whereby we assume the risk for certain healthcare services during the remainder of an inpatient acute stay serviced at the patient’s home in exchange for a contracted per diem rate. The performance obligation is the coordination and provision of required medical services, as determined by the treating physician, for each day the patient receives inpatient-equivalent care at home. As such, revenues are recognized as services are administered and as our performance obligations are satisfied on a per diem basis, reduced by estimates for revenue adjustments.
We recognize adjustments to revenue during the period in which changes to estimates of assigned patient diagnoses or episode terminations become known, in accordance with the applicable managed care contracts. For certain health insurance plans, revenue is reduced by amounts owed by enrollees to healthcare providers under deductible, coinsurance or copay provisions of health insurance plan policies, since those amounts are repaid to the health insurance plans by us as part of a retrospective reconciliation process.
In March 2022, our high acuity care segment entered into a transaction in which one of our health system partners contributed its home health operations to one of our existing high acuity care joint ventures. We recognize Medicare and non-Medicare revenue in a manner that is consistent with our home health segment revenue recognition policy described above.
Business Combinations
We account for acquisitions using the acquisition method of accounting in accordance with ASC 805, Business Combinations. Acquisitions are accounted for as purchases and are included in our consolidated financial statements from their respective acquisition dates. Assets acquired, liabilities assumed and noncontrolling interests, if any, are measured at fair value on the acquisition date using the appropriate valuation method. Goodwill generated from acquisitions is recognized for the excess of the purchase price over tangible and identifiable intangible assets. In determining the fair value of identifiable intangible assets and any noncontrolling interests, we use various valuation techniques including the income approach, the cost approach and the
market approach. These valuation methods require us to make estimates and assumptions surrounding projected revenues and costs, growth rates and discount rates.
Goodwill and Other Intangible Assets
As of December 31, 2022, we had a goodwill balance of $1,287.4 million. Goodwill represents the amount of the purchase price in excess of the fair values assigned to the underlying identifiable net assets of acquired businesses. Goodwill is not amortized, but is subject to an annual impairment test. Tests are performed more frequently if events occur or circumstances change that would more likely than not reduce the fair value of the reporting unit below its carrying amount. These events or circumstances include, but are not limited to, a significant adverse change in the business environment, regulatory environment or legal factors, or a substantial decline in the market capitalization of our stock.
Generally Accepted Accounting Principles ("GAAP")U.S. GAAP allows for impairment testing to be done on either a quantitative or qualitative basis. During 2019,2022, we utilizedperformed a qualitative analysis for our annual impairment test and determined that there were no triggering events that would indicate thatassessment to determine if it is "moremore likely than not"not that the carrying valuesfair value of our reporting units are higherless than their respective fair values. As a result,carrying values by evaluating relevant events and circumstances including financial performance, market conditions and share price. Based on this assessment, we did not record any goodwill impairment charges and none ofconcluded that the goodwill associated with our varioushome health, hospice and high acuity care reporting units was not considered at risk of impairment as of October 31, 2019.2022. In addition to the qualitative assessment, we also performed a quantitative analysis for our personal care reporting unit due to the decline in revenues resulting from staffing shortages using an income and market approach. Based on this analysis, we concluded that the goodwill associated with our personal care reporting unit was not considered at risk of impairment as of October 31, 2022. Since the date of our last annual goodwill impairment test, there have been no material developments, events, changes in operating performance or other circumstances that would cause management to believe it is more likely than not that the fair value of any of our reporting units would be less than their carrying amounts.
As of December 31, 2022, we had an other intangible assets balance of $101.2 million. Intangible assets consist of certificates of need, licenses, acquired names, non-compete agreements and non-compete agreements.technology. We amortize non-compete agreements and acquired names that we do not intend to use in the futureindefinitely on a straight-line basis over their estimated useful lives, which isare generally two to three years for non-compete agreements and up to fivethree years for acquired names. We amortize technology over its estimated useful service life, which is generally up to seven years. Our indefinite-lived intangible assets are reviewed for impairment annually or more frequently if events occur or circumstances change that would more likely than not reduce the fair value of the intangible asset below its carrying amount. During 2019, weWe performed a qualitative assessment to determine if any of our indefinite-lived intangible assets were impaired; as a result of this analysis, we wrote off approximately $1.5 million of acquired names during the three-month period ended December 31, 2019. There2022 and determined that there have been no material developments, events, changes in operating performance or other circumstances that would cause management to believe it is more likely than not that the fair value of any of our remainingindefinite-lived intangible assets would be less than their carrying amounts.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risk from fluctuations in interest rates. Our Term Loan and Revolving Credit Facility carry a floating interest rate which is tied to the Eurodollar rate (i.e. LIBOR) and the Prime Rate, and therefore, our consolidated statements of operations and our consolidated statements of cash flows are exposed to changes in interest rates. Our Second Amended Credit Agreement provides for the replacement of LIBOR with the daily or term secured overnight financing rate ("SOFR") whenever LIBOR is discontinued. As of December 31, 2019,2022, the total amount of outstanding debt subject to interest rate fluctuations was $241.7$435.9 million. A 1.0% interest rate change would cause interest expense to change by approximately $2.4$4.4 million annually, assuming the Company makes no principal repayments.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Amedisys, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Amedisys, Inc. and subsidiaries (the Company) as of December 31, 20192022 and 2018,2021, the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for each of the years in the three‑yearthree-year period ended December 31, 2019,2022, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 20192022 and 2018,2021, and the results of its operations and its cash flows for each of the years in the three‑yearthree-year period ended December 31, 2019,2022, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2019,2022, based on criteria established in Internal Control -– Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 19, 202016, 2023 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Change in Accounting Principle
As discussed in Note 1 to the consolidated financial statements, the Company changed the manner in which it accounts for leases in 2019 due to the adoption of Accounting Standards Update (ASU) 2016-02, Leases (Topic 842); ASU 2018-01, Land Easement Practical Expedient for Transition to Topic 842; ASU 2018-10, Codification Improvements to Topic 842, Leases; and ASU 2018-11, Targeted Improvements (collectively, Topic 842).
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit MattersMatter
The critical audit mattersmatter communicated below are mattersis a matter arising from the current period audit of the consolidated financial statements that werewas communicated or required to be communicated to the audit committee and that: (1) relaterelates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical mattersaudit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit mattersmatter below, providing a separate opinionsopinion on the critical audit mattersmatter or on the accounts or disclosures to which they relate.it relates.
Acquisition of Compassionate Care Hospice - Valuation of certain intangible assets
As discussed in Notes 2 and 3 to the consolidated financial statements, the Company accounts for acquisitions using the acquisition method of accounting. Assets acquired are measured at fair value on the acquisition date using various valuation methods. The Company acquired Compassionate Care Hospice (CCH) for the purchase price of $327.9 million, net of cash acquired of $6.7 million, on February 1, 2019. Intangible assets acquired in connection with this transaction include Medicare licenses, certificates of need, trade name and non-compete agreements.
We identified the valuation of certain intangible assets, which consist of Medicare licenses, trade name, and non-compete agreements, acquired in the CCH transaction as a critical audit matter. Subjective auditor judgment was required to evaluate the identification of intangible assets acquired, valuation methodologies, and significant assumptions used in the valuation of these certain intangible assets. Specifically, the significant assumptions include projected revenue growth rates, projected earnings before interest, taxes, depreciation and amortization (EBITDA), and the discount rate. These assumptions relate to the future performance of the acquired business and changes to these assumptions could have a significant effect on the Company’s estimate of fair value of the intangible assets.
The primary procedures we performed to address this critical audit matter included the following. We tested certain internal controls over the Company’s acquisition accounting process, including controls over the identification of intangible assets acquired, assessment of the valuation methodologies, and the development of the significant assumptions used in the valuation of the intangible assets. We read the purchase agreement to identify the significant terms, conditions, and intangible assets acquired. We evaluated the Company’s projected revenue growth rates and projected EBITDA by comparing such assumptions to those of CCH’s peers and to industry reports. Additionally, we compared the Company’s projected revenue growth rates and projected EBITDA to CCH’s and the Company’s historical actual results. We involved valuation professionals with specialized skills and knowledge, who assisted in:
Evaluating the Company’s identification of intangible assets acquired;
Assessing the valuation methodologies used by the Company in the valuation analysis; and
Evaluating the weighted average cost of capital (WACC), which was used by the Company to determine the discount rate, by comparing the Company's inputs to the WACC to publicly available data for comparable entities and assessing the resulting WACC.
Revenue recognition - Evaluation of the non-contractual revenue adjustment estimates for Home Health and Hospice
As discussed in Note 2 to the consolidated financial statements, the Company determines the transaction price for revenue contracts based on gross charges for services provided, reduced by estimates for contractual revenue adjustments and an estimate for non-contractual revenue adjustments. Non-contractual revenue adjustments are recorded forinclude discounts provided to self-pay, uninsured patients andor other payors, by major payor class based on historical collection experience, evaluated for current economic conditions. Adjustmentsadjustments resulting from payment reviews and adjustments arising from the Company’s inability to obtain applicableappropriate billing documentation, authorizations or face-to-face documentationdocumentation. Non-contractual revenue adjustments are factors that are relevant torecorded based on the estimate of ultimate collection.Company’s historical collection experience, aged accounts receivable by payor and current industry conditions. The non-contractual revenue adjustments represent the difference between amounts billed and amounts the Company expects to collect based on its collection history with similar payors.
We identified the evaluation of the non-contractual revenue adjustment estimates noted above for the Home Health and Hospice segments as a critical audit matter. Subjective and complex auditor judgment was required to evaluate the method and historical collection experience used by the Company when developing the non-contractual revenue adjustment estimate. Specifically, the significant judgments relaterelated to evaluating the relevance and reliability of historical collection experience to the determination of the estimate, includewhich included evaluation of current conditions, trends, historical adjustment experience, and other factors.
The following are the primary procedures we performed to address this critical audit matter includedmatter. We evaluated the following. Wedesign and tested the operating effectiveness of certain internal controls related to the Company’s revenue process, including controls over the method and significant judgments for estimating non-contractual revenue adjustments noted above. We assessed the outcome of the estimation of non-contractual revenue adjustments in the prior period consolidated financial statements to identify circumstances or conditions that are relevant to the determination of the current year estimate. WeTo assess the current year method and the relevance of the historical collection experience, we also evaluated current conditions, trends, historical adjustment experience, and other factors relevant to the estimation of ultimate collection to assess the current year methodology and relevance of historical collection experience in determining the current year estimate.non-contractual revenue adjustments.
/s/ KPMG LLP
We have served as the Company's auditor since 2002.
Baton Rouge, Louisiana
February 19, 2020
AMEDISYS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share data)
| | | | As of December 31, | | As of December 31, |
| | 2019 | | 2018 | | 2022 | | 2021 |
| ASSETS | | | | ASSETS | | | |
| Current assets: | | | | Current assets: | |
| Cash and cash equivalents | $ | 30,294 |
| | $ | 20,229 |
| Cash and cash equivalents | $ | 40,540 | | | $ | 42,694 | |
| Restricted cash | 66,196 |
| | — |
| Restricted cash | 13,593 | | | 3,075 | |
| Patient accounts receivable | 237,596 |
| | 188,972 |
| Patient accounts receivable | 296,785 | | | 274,961 | |
| Prepaid expenses | 8,243 |
| | 7,568 |
| Prepaid expenses | 11,628 | | | 10,356 | |
| Other current assets | 8,225 |
| | 7,349 |
| Other current assets | 26,415 | | | 25,598 | |
| Total current assets | 350,554 |
| | 224,118 |
| Total current assets | 388,961 | | | 356,684 | |
| Property and equipment, net of accumulated depreciation of $96,137 and $95,472 | 28,113 |
| | 29,449 |
| |
| Property and equipment, net of accumulated depreciation of $101,364 and $96,937 | | Property and equipment, net of accumulated depreciation of $101,364 and $96,937 | 16,026 | | | 18,435 | |
| Operating lease right of use assets | 84,791 |
| | — |
| Operating lease right of use assets | 102,856 | | | 101,257 | |
| Goodwill | 658,500 |
| | 329,480 |
| Goodwill | 1,287,399 | | | 1,196,090 | |
| Intangible assets, net of accumulated amortization of $7,044 and $693 | 64,748 |
| | 44,132 |
| |
| Deferred income taxes | 21,427 |
| | 35,794 |
| |
| Intangible assets, net of accumulated amortization of $14,604 and $19,900 | | Intangible assets, net of accumulated amortization of $14,604 and $19,900 | 101,167 | | | 111,190 | |
| Deferred income tax assets | | Deferred income tax assets | — | | | 289 | |
| Other assets | 54,612 |
| | 54,145 |
| Other assets | 79,836 | | | 73,023 | |
| Total assets | $ | 1,262,745 |
| | $ | 717,118 |
| Total assets | $ | 1,976,245 | | | $ | 1,856,968 | |
| LIABILITIES AND EQUITY | | | | LIABILITIES AND EQUITY | | | |
| Current liabilities: | | | | Current liabilities: | |
| Accounts payable | $ | 31,259 |
| | $ | 28,531 |
| Accounts payable | $ | 43,735 | | | $ | 38,217 | |
| Payroll and employee benefits | 120,877 |
| | 92,858 |
| Payroll and employee benefits | 125,387 | | | 141,001 | |
| Accrued expenses | 137,111 |
| | 99,475 |
| Accrued expenses | 137,390 | | | 150,836 | |
| Current portion of long-term obligations | 9,927 |
| | 1,612 |
| Current portion of long-term obligations | 15,496 | | | 12,995 | |
| Current portion of operating lease liabilities | 27,769 |
| | — |
| Current portion of operating lease liabilities | 33,521 | | | 31,233 | |
| Total current liabilities | 326,943 |
| | 222,476 |
| Total current liabilities | 355,529 | | | 374,282 | |
| Long-term obligations, less current portion | 232,256 |
| | 5,775 |
| Long-term obligations, less current portion | 419,420 | | | 432,075 | |
| Operating lease liabilities, less current portion | 56,128 |
| | — |
| Operating lease liabilities, less current portion | 69,504 | | | 69,309 | |
| Deferred income tax liabilities | | Deferred income tax liabilities | 20,411 | | | — | |
| Other long-term obligations | 5,905 |
| | 6,234 |
| Other long-term obligations | 4,808 | | | 4,979 | |
| Total liabilities | 621,232 |
| | 234,485 |
| Total liabilities | 869,672 | | | 880,645 | |
| Commitments and Contingencies – Note 10 | | | | |
| Commitments and Contingencies – Note 12 | | Commitments and Contingencies – Note 12 | | | |
| Equity: | | | | Equity: | |
| Preferred stock, $0.001 par value, 5,000,000 shares authorized; none issued or outstanding | — |
| | — |
| Preferred stock, $0.001 par value, 5,000,000 shares authorized; none issued or outstanding | — | | | — | |
| Common stock, $0.001 par value, 60,000,000 shares authorized; 36,638,021 and 36,252,280 shares issued; and 32,284,051 and 31,973,505 shares outstanding | 37 |
| | 36 |
| |
| Common stock, $0.001 par value, 60,000,000 shares authorized; 37,891,186 and 37,674,868 shares issued; and 32,518,278 and 32,509,969 shares outstanding | | Common stock, $0.001 par value, 60,000,000 shares authorized; 37,891,186 and 37,674,868 shares issued; and 32,518,278 and 32,509,969 shares outstanding | 38 | | | 38 | |
| Additional paid-in capital | 645,256 |
| | 603,666 |
| Additional paid-in capital | 755,063 | | | 728,118 | |
| Treasury stock at cost 4,353,970 and 4,278,775 shares of common stock | (251,241 | ) | | (241,685 | ) | |
| Accumulated other comprehensive income | 15 |
| | 15 |
| |
| Treasury stock at cost, 5,372,908 and 5,164,899 shares of common stock | | Treasury stock at cost, 5,372,908 and 5,164,899 shares of common stock | (461,200) | | | (435,868) | |
| Retained earnings | 246,383 |
| | 119,550 |
| Retained earnings | 757,672 | | | 639,063 | |
| Total Amedisys, Inc. stockholders’ equity | 640,450 |
| | 481,582 |
| Total Amedisys, Inc. stockholders’ equity | 1,051,573 | | | 931,351 | |
| Noncontrolling interests | 1,063 |
| | 1,051 |
| Noncontrolling interests | 55,000 | | | 44,972 | |
| Total equity | 641,513 |
| | 482,633 |
| Total equity | 1,106,573 | | | 976,323 | |
| Total liabilities and equity | $ | 1,262,745 |
| | $ | 717,118 |
| Total liabilities and equity | $ | 1,976,245 | | | $ | 1,856,968 | |
The accompanying notes are an integral part of these consolidated financial statements.
AMEDISYS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except per share data)
| | | | For the Years Ended December 31, | | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 | | 2022 | | 2021 | | 2020 |
| Net service revenue | $ | 1,955,633 |
| | $ | 1,662,578 |
| | $ | 1,511,272 |
| Net service revenue | $ | 2,223,199 | | | $ | 2,214,112 | | | $ | 2,071,519 | |
| Other operating income | | Other operating income | — | | | 13,300 | | | 34,372 | |
| Cost of service, excluding depreciation and amortization | 1,150,337 |
| | 992,863 |
| | 903,377 |
| Cost of service, excluding depreciation and amortization | 1,260,425 | | | 1,233,356 | | | 1,185,369 | |
| General and administrative expenses: | | | | | | General and administrative expenses: | |
| Salaries and benefits | 394,452 |
| | 316,522 |
| | 305,938 |
| Salaries and benefits | 508,791 | | | 474,718 | | | 449,448 | |
| Non-cash compensation | 25,040 |
| | 17,887 |
| | 16,295 |
| Non-cash compensation | 16,560 | | | 23,809 | | | 26,730 | |
| Other | 188,434 |
| | 166,897 |
| | 159,980 |
| Other | 228,707 | | | 212,713 | | | 192,122 | |
| Depreciation and amortization | 18,428 |
| | 13,261 |
| | 17,123 |
| Depreciation and amortization | 24,935 | | | 30,901 | | | 28,802 | |
| Asset impairment charge | 1,470 |
| | — |
| | 1,323 |
| |
| Securities Class Action Lawsuit settlement, net | — |
| | — |
| | 28,712 |
| |
| Impairment charge | | Impairment charge | 3,009 | | | — | | | 4,152 | |
| Operating expenses | 1,778,161 |
| | 1,507,430 |
| | 1,432,748 |
| Operating expenses | 2,042,427 | | | 1,975,497 | | | 1,886,623 | |
| Operating income | 177,472 |
| | 155,148 |
| | 78,524 |
| Operating income | 180,772 | | | 251,915 | | | 219,268 | |
| Other income (expense): | | | | | | Other income (expense): | |
| Interest income | 78 |
| | 278 |
| | 158 |
| Interest income | 178 | | | 49 | | | 292 | |
| Interest expense | (14,515 | ) | | (7,370 | ) | | (5,031 | ) | Interest expense | (22,228) | | | (9,525) | | | (11,038) | |
| Equity in earnings from equity method investments | 5,338 |
| | 7,692 |
| | 3,381 |
| |
| Equity in (loss) earnings from equity method investments | | Equity in (loss) earnings from equity method investments | (45) | | | 4,949 | | | 3,966 | |
| Gain (loss) on equity method investments | | Gain (loss) on equity method investments | — | | | 31,098 | | | (2,980) | |
| Miscellaneous, net | 2,037 |
| | 3,240 |
| | 3,769 |
| Miscellaneous, net | 1,567 | | | 1,745 | | | 1,311 | |
| Total other (expense) income, net | (7,062 | ) | | 3,840 |
| | 2,277 |
| Total other (expense) income, net | (20,528) | | | 28,316 | | | (8,449) | |
| Income before income taxes | 170,410 |
| | 158,988 |
| | 80,801 |
| Income before income taxes | 160,244 | | | 280,231 | | | 210,819 | |
| Income tax expense | (42,503 | ) | | (38,859 | ) | | (50,118 | ) | Income tax expense | (42,545) | | | (70,065) | | | (25,635) | |
| Net income | 127,907 |
| | 120,129 |
| | 30,683 |
| Net income | 117,699 | | | 210,166 | | | 185,184 | |
| Net income attributable to noncontrolling interests | (1,074 | ) | | (783 | ) | | (382 | ) | |
| Net loss (income) attributable to noncontrolling interests | | Net loss (income) attributable to noncontrolling interests | 910 | | | (1,094) | | | (1,576) | |
| Net income attributable to Amedisys, Inc. | $ | 126,833 |
| | $ | 119,346 |
| | $ | 30,301 |
| Net income attributable to Amedisys, Inc. | $ | 118,609 | | | $ | 209,072 | | | $ | 183,608 | |
| Basic earnings per common share: | | | | | | Basic earnings per common share: | | | | | |
| Net income attributable to Amedisys, Inc. common stockholders | $ | 3.95 |
| | $ | 3.64 |
| | $ | 0.90 |
| Net income attributable to Amedisys, Inc. common stockholders | $ | 3.65 | | | $ | 6.41 | | | $ | 5.64 | |
| Weighted average shares outstanding | 32,142 |
| | 32,791 |
| | 33,704 |
| Weighted average shares outstanding | 32,517 | | | 32,642 | | | 32,559 | |
| Diluted earnings per common share: | | | | | | Diluted earnings per common share: | |
| Net income attributable to Amedisys, Inc. common stockholders | $ | 3.84 |
| | $ | 3.55 |
| | $ | 0.88 |
| Net income attributable to Amedisys, Inc. common stockholders | $ | 3.63 | | | $ | 6.34 | | | $ | 5.52 | |
| Weighted average shares outstanding | 32,990 |
| | 33,609 |
| | 34,304 |
| Weighted average shares outstanding | 32,653 | | | 32,972 | | | 33,268 | |
|
The accompanying notes are an integral part of these consolidated financial statements.
AMEDISYS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(Amounts in thousands)
| | | | For the Years Ended December 31, | | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 | | 2022 | | 2021 | | 2020 |
| Net income | $ | 127,907 |
| | $ | 120,129 |
| | $ | 30,683 |
| Net income | $ | 117,699 | | | $ | 210,166 | | | $ | 185,184 | |
| Other comprehensive income | — |
| | — |
| | — |
| Other comprehensive income | — | | | — | | | — | |
| Comprehensive income | 127,907 |
| | 120,129 |
| | 30,683 |
| Comprehensive income | 117,699 | | | 210,166 | | | 185,184 | |
| Comprehensive income attributable to non-controlling interests | (1,074 | ) | | (783 | ) | | (382 | ) | |
| Comprehensive loss (income) attributable to non-controlling interests | | Comprehensive loss (income) attributable to non-controlling interests | 910 | | | (1,094) | | | (1,576) | |
| Comprehensive income attributable to Amedisys, Inc. | $ | 126,833 |
| | $ | 119,346 |
| | $ | 30,301 |
| Comprehensive income attributable to Amedisys, Inc. | $ | 118,609 | | | $ | 209,072 | | | $ | 183,608 | |
The accompanying notes are an integral part of these consolidated financial statements.
AMEDISYS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(Amounts in thousands, except common stock shares)
| | | | | | | | | | | | | | | | | | | | Total | | Common Stock | | Additional
Paid-in
Capital | | Treasury
Stock | | Accumulated
Other
Comprehensive
Income | | Retained
Earnings | | Noncontrolling
Interests |
| | Total | | Common Stock | | Additional Paid-in Capital | | Treasury Stock | | Accumulated Other Comprehensive Income | | Retained Earnings (Deficit) | | Noncontrolling Interests | Shares | | Amount | |
| Shares | | Amount | | |
| Balance, December 31, 2016 | $ | 461,142 |
| | 35,253,577 |
| | $ | 35 |
| | $ | 537,472 |
| | $ | (46,774 | ) | | $ | 15 |
| | $ | (30,545 | ) | | $ | 939 |
| |
| Issuance of stock – employee stock purchase plan | 2,382 |
| | 53,848 |
| | — |
| | 2,382 |
| | — |
| | — |
| | — |
| | — |
| |
| Issuance of stock – 401(k) plan | 8,223 |
| | 156,487 |
| | — |
| | 8,223 |
| | — |
| | — |
| | — |
| | — |
| |
| Issuance/(cancellation) of non-vested stock | — |
| | 139,016 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| |
| Exercise of stock options | 4,554 |
| | 144,206 |
| | — |
| | 4,554 |
| | — |
| | — |
| | — |
| | — |
| |
| Non-cash compensation | 16,295 |
| | — |
| | — |
| | 16,295 |
| | — |
| | — |
| | — |
| | — |
| |
| Tax benefit from stock options exercised and restricted stock vesting | 448 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 448 |
| | — |
| |
| Surrendered shares | (6,939 | ) | | — |
| | — |
| | — |
| | (6,939 | ) | | — |
| | — |
| | — |
| |
| Noncontrolling interest distribution | (216 | ) | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (216 | ) | |
| Assets contributed to equity investment | (146 | ) | | — |
| | — |
| | (146 | ) | | — |
| | — |
| | — |
| | — |
| |
| Net income | 30,683 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 30,301 |
| | 382 |
| |
| Balance, December 31, 2017 | 516,426 |
| | 35,747,134 |
| | 35 |
| | 568,780 |
| | (53,713 | ) | | 15 |
| | 204 |
| | 1,105 |
| |
| Balance, December 31, 2019 | | Balance, December 31, 2019 | $ | 641,513 | | | 36,638,021 | | | $ | 37 | | | $ | 645,256 | | | $ | (251,241) | | | $ | 15 | | | $ | 246,383 | | | $ | 1,063 | |
| Issuance of stock – employee stock purchase plan | 2,429 |
| | 38,961 |
| | — |
| | 2,429 |
| | — |
| | — |
| | — |
| | — |
| Issuance of stock – employee stock purchase plan | 3,562 | | | 21,561 | | | — | | | 3,562 | | | — | | | — | | | — | | | — | |
| Issuance of stock – 401(k) plan | 9,232 |
| | 129,451 |
| | — |
| | 9,232 |
| | — |
| | — |
| | — |
| | — |
| Issuance of stock – 401(k) plan | 3,057 | | | 18,312 | | | — | | | 3,057 | | | — | | | — | | | — | | | — | |
| Issuance/(cancellation) of non-vested stock | — |
| | 174,044 |
| | 1 |
| | (1 | ) | | — |
| | — |
| | — |
| | — |
| Issuance/(cancellation) of non-vested stock | — | | | 169,489 | | | — | | | — | | | — | | | — | | | — | | | — | |
| Exercise of stock options | 5,953 |
| | 162,690 |
| | — |
| | 5,953 |
| | — |
| | — |
| | — |
| | — |
| Exercise of stock options | 6,325 | | | 622,829 | | | 1 | | | 6,324 | | | — | | | — | | | — | | | — | |
| Non-cash compensation | 17,887 |
| | — |
| | — |
| | 17,887 |
| | — |
| | — |
| | — |
| | — |
| Non-cash compensation | 26,730 | | | — | | | — | | | 26,730 | | | — | | | — | | | — | | | — | |
| Surrendered shares | (6,570 | ) | | — |
| | — |
| | — |
| | (6,570 | ) | | — |
| | — |
| | — |
| Surrendered shares | (54,493) | | | — | | | — | | | 13,358 | | | (67,851) | | | — | | | — | | | — | |
| Shares repurchased | (181,402 | ) | | — |
| | — |
| | — |
| | (181,402 | ) | | — |
| | — |
| | — |
| |
| Noncontrolling interest distribution | (1,090 | ) | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (1,090 | ) | |
| Repurchase of noncontrolling interest | (361 | ) | | — |
| | — |
| | (614 | ) | | — |
| | — |
| | — |
| | 253 |
| |
| Noncontrolling interest distributions | | Noncontrolling interest distributions | (1,122) | | | — | | | — | | | — | | | — | | | — | | | — | | | (1,122) | |
| Write-off of other comprehensive income | | Write-off of other comprehensive income | (15) | | | — | | | — | | | — | | | — | | | (15) | | | — | | | — | |
| Net income | 120,129 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 119,346 |
| | 783 |
| Net income | 185,184 | | | — | | | — | | | — | | | — | | | — | | | 183,608 | | | 1,576 | |
| Balance, December 31, 2018 | 482,633 |
| | 36,252,280 |
| | 36 |
| | 603,666 |
| | (241,685 | ) | | 15 |
| | 119,550 |
| | 1,051 |
| |
| Balance, December 31, 2020 | | Balance, December 31, 2020 | 810,741 | | | 37,470,212 | | | 38 | | | 698,287 | | | (319,092) | | | — | | | 429,991 | | | 1,517 | |
| Issuance of stock – employee stock purchase plan | 3,187 |
| | 30,483 |
| | — |
| | 3,187 |
| | — |
| | — |
| | — |
| | — |
| Issuance of stock – employee stock purchase plan | 3,968 | | | 20,823 | | | — | | | 3,968 | | | — | | | — | | | — | | | — | |
| Issuance of stock – 401(k) plan | 9,753 |
| | 79,056 |
| | — |
| | 9,753 |
| | — |
| | — |
| | — |
| | — |
| |
| Issuance/(cancellation) of non-vested stock | — |
| | 189,134 |
| | 1 |
| | (1 | ) | | — |
| | — |
| | — |
| | — |
| Issuance/(cancellation) of non-vested stock | — | | | 151,365 | | | — | | | — | | | — | | | — | | | — | | | — | |
| Exercise of stock options | 3,611 |
| | 87,068 |
| | — |
| | 3,611 |
| | — |
| | — |
| | — |
| | — |
| Exercise of stock options | 2,054 | | | 32,468 | | | — | | | 2,054 | | | — | | | — | | | — | | | — | |
| Non-cash compensation | 25,040 |
| | — |
| | — |
| | 25,040 |
| | — |
| | — |
| | — |
| | — |
| Non-cash compensation | 23,809 | | | — | | | — | | | 23,809 | | | — | | | — | | | — | | | — | |
| Surrendered shares | (9,556 | ) | | — |
| | — |
| | — |
| | (9,556 | ) | | — |
| | — |
| | — |
| Surrendered shares | (16,898) | | | — | | | — | | | — | | | (16,898) | | | — | | | — | | | — | |
| Noncontrolling interest distribution | (1,062 | ) | | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (1,062 | ) | |
| Shares repurchased | | Shares repurchased | (99,878) | | | — | | | — | | | — | | | (99,878) | | | — | | | — | | | — | |
| Noncontrolling interest contributions | | Noncontrolling interest contributions | 250 | | | — | | | — | | | — | | | — | | | — | | | — | | | 250 | |
| Noncontrolling interest distributions | | Noncontrolling interest distributions | (1,747) | | | — | | | — | | | — | | | — | | | — | | | — | | | (1,747) | |
| Acquired noncontrolling interest | | Acquired noncontrolling interest | 43,858 | | | — | | | — | | | — | | | — | | | — | | | — | | | 43,858 | |
| Net income | 127,907 |
| | — |
| | — |
| | — |
| | — |
| | — |
| | 126,833 |
| | 1,074 |
| Net income | 210,166 | | | — | | | — | | | — | | | — | | | — | | | 209,072 | | | 1,094 | |
| Balance, December 31, 2019 | $ | 641,513 |
| | 36,638,021 |
| | $ | 37 |
| | $ | 645,256 |
| | $ | (251,241 | ) | | $ | 15 |
| | $ | 246,383 |
| | $ | 1,063 |
| |
| Balance, December 31, 2021 | | Balance, December 31, 2021 | 976,323 | | | 37,674,868 | | | 38 | | | 728,118 | | | (435,868) | | | — | | | 639,063 | | | 44,972 | |
| Issuance of stock – employee stock purchase plan | | Issuance of stock – employee stock purchase plan | 3,848 | | | 36,206 | | | — | | | 3,848 | | | — | | | — | | | — | | | — | |
| Issuance/(cancellation) of non-vested stock | | Issuance/(cancellation) of non-vested stock | — | | | 142,477 | | | — | | | — | | | — | | | — | | | — | | | — | |
| Exercise of stock options | | Exercise of stock options | 2,304 | | | 37,635 | | | — | | | 2,304 | | | — | | | — | | | — | | | — | |
| Non-cash compensation | | Non-cash compensation | 16,560 | | | — | | | — | | | 16,560 | | | — | | | — | | | — | | | — | |
| Surrendered shares | | Surrendered shares | (7,981) | | | — | | | — | | | — | | | (7,981) | | | — | | | — | | | — | |
| Shares repurchased | | Shares repurchased | (17,351) | | | — | | | — | | | — | | | (17,351) | | | — | | | — | | | — | |
| Noncontrolling interest contributions | | Noncontrolling interest contributions | 12,401 | | | — | | | — | | | — | | | — | | | — | | | — | | | 12,401 | |
| Noncontrolling interest distributions | | Noncontrolling interest distributions | (1,561) | | | — | | | — | | | — | | | — | | | — | | | — | | | (1,561) | |
| Sale of noncontrolling interest | | Sale of noncontrolling interest | 4,331 | | | — | | | — | | | 4,233 | | | — | | | — | | | — | | | 98 | |
| Net income | | Net income | 117,699 | | | — | | | — | | | — | | | — | | | — | | | 118,609 | | | (910) | |
| Balance, December 31, 2022 | | Balance, December 31, 2022 | $ | 1,106,573 | | | 37,891,186 | | | $ | 38 | | | $ | 755,063 | | | $ | (461,200) | | | $ | — | | | $ | 757,672 | | | $ | 55,000 | |
The accompanying notes are an integral part of these consolidated financial statements.
AMEDISYS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands) | | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Cash Flows from Operating Activities: | | | | | |
| Net income | $ | 117,699 | | | $ | 210,166 | | | $ | 185,184 | |
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | |
| Depreciation and amortization | 24,935 | | | 30,901 | | | 28,802 | |
| Non-cash compensation | 16,560 | | | 23,809 | | | 26,730 | |
| Amortization and impairment of operating lease right of use assets | 46,029 | | | 40,364 | | | 39,140 | |
| Loss (gain) on disposal of property and equipment | 519 | | | (124) | | | (30) | |
| (Gain) loss on equity method investments | — | | | (31,098) | | | 2,980 | |
| Write-off of other comprehensive income | — | | | — | | | (15) | |
| Deferred income taxes | 23,377 | | | 44,582 | | | (26,560) | |
| Equity in loss (earnings) from equity method investments | 45 | | | (4,949) | | | (3,966) | |
| Amortization of deferred debt issuance costs/debt discount | 991 | | | 917 | | | 869 | |
| Return on equity method investments | 5,163 | | | 5,343 | | | 5,444 | |
| Impairment charge | 3,009 | | | — | | | 4,152 | |
| Changes in operating assets and liabilities, net of impact of acquisitions: | | | | | |
| Patient accounts receivable | (14,230) | | | (18,030) | | | 2,114 | |
| Other current assets | (3,525) | | | (12,202) | | | (7,181) | |
| Other assets | 438 | | | (1,017) | | | 31 | |
| Accounts payable | 4,894 | | | (4,353) | | | 1,941 | |
| Accrued expenses | (39,382) | | | (26,915) | | | 39,839 | |
| Other long-term obligations | (8,822) | | | (28,796) | | | 27,717 | |
| Operating lease liabilities | (41,175) | | | (36,645) | | | (34,695) | |
| Operating lease right of use assets | (3,242) | | | (3,060) | | | (3,544) | |
| Net cash provided by operating activities | 133,283 | | | 188,893 | | | 288,952 | |
| Cash Flows from Investing Activities: | | | | | |
| Proceeds from the sale of deferred compensation plan assets | 252 | | | 135 | | | 101 | |
| Proceeds from the sale of property and equipment | 66 | | | 144 | | | 80 | |
| Purchases of property and equipment | (6,165) | | | (6,302) | | | (5,332) | |
| Investments in technology assets | (1,050) | | | (419) | | | — | |
| Investment in equity method investee | (637) | | | (200) | | | (875) | |
| Proceeds from sale of equity method investment | — | | | — | | | 17,876 | |
| Purchase of cost method investment | (15,000) | | | (5,000) | | | — | |
| Acquisitions of businesses, net of cash acquired | (71,952) | | | (269,965) | | | (298,958) | |
| Net cash used in investing activities | (94,486) | | | (281,607) | | | (287,108) | |
| Cash Flows from Financing Activities: | | | | | |
| Proceeds from issuance of stock upon exercise of stock options | 2,304 | | | 2,054 | | | 6,325 | |
| Proceeds from issuance of stock to employee stock purchase plan | 3,848 | | | 3,968 | | | 3,562 | |
| Shares withheld to pay taxes on non-cash compensation | (7,981) | | | (16,898) | | | (54,493) | |
| Noncontrolling interest contributions | 3,501 | | | 250 | | | — | |
| Noncontrolling interest distributions | (1,561) | | | (1,747) | | | (1,122) | |
| Proceeds from sale of noncontrolling interest | 5,817 | | | — | | | — | |
| Proceeds from borrowings under term loan | — | | | 290,312 | | | — | |
| Proceeds from borrowings under revolving line of credit | 534,500 | | | 500,700 | | | 684,200 | |
| Repayments of borrowings under revolving line of credit | (534,500) | | | (551,700) | | | (703,200) | |
| Principal payments of long-term obligations | (13,296) | | | (9,143) | | | (10,249) | |
| Debt issuance costs | — | | | (2,792) | | | — | |
| Provider relief fund advance | — | | | (60,000) | | | 60,000 | |
| Purchase of company stock | (17,351) | | | (99,878) | | | — | |
| Payment of accrued contingent consideration | (5,714) | | | — | | | — | |
| Net cash (used in) provided by financing activities | (30,433) | | | 55,126 | | | (14,977) | |
| Net increase (decrease) in cash, cash equivalents and restricted cash | 8,364 | | | (37,588) | | | (13,133) | |
| Cash, cash equivalents and restricted cash at beginning of period | 45,769 | | | 83,357 | | | 96,490 | |
| Cash, cash equivalents and restricted cash at end of period | $ | 54,133 | | | $ | 45,769 | | | $ | 83,357 | |
| | | | | |
|
| | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Cash Flows from Operating Activities: | | | | | |
| Net income | $ | 127,907 |
| | $ | 120,129 |
| | $ | 30,683 |
|
| Adjustments to reconcile net income to net cash provided by operating activities: | | | | | |
| Depreciation and amortization | 18,428 |
| | 13,261 |
| | 17,123 |
|
| Non-cash compensation | 25,040 |
| | 17,887 |
| | 16,295 |
|
| 401(k) employer match | 10,509 |
| | 8,976 |
| | 8,754 |
|
| Amortization and impairment of operating lease right of use assets | 35,905 |
| | — |
| | — |
|
| Loss on disposal of property and equipment | 141 |
| | 714 |
| | — |
|
| Deferred income taxes | 13,466 |
| | 20,271 |
| | 52,178 |
|
| Equity in earnings from equity method investments | (5,338 | ) | | (7,692 | ) | | (3,381 | ) |
| Amortization of deferred debt issuance costs/debt discount | 873 |
| | 797 |
| | 735 |
|
| Return on equity investment | 4,955 |
| | 6,158 |
| | 5,321 |
|
| Asset impairment charge | 1,470 |
| | — |
| | 1,323 |
|
| Changes in operating assets and liabilities, net of impact of acquisitions: | | | | | |
| Patient accounts receivable | (24,146 | ) | | 12,224 |
| | (34,672 | ) |
| Other current assets | (2,682 | ) | | 8,679 |
| | (4,940 | ) |
| Other assets | 832 |
| | 2,947 |
| | (12,749 | ) |
| Accounts payable | (11,329 | ) | | 3,165 |
| | (2,843 | ) |
| Accrued expenses | 42,096 |
| | 13,524 |
| | 31,843 |
|
| Other long-term obligations | (329 | ) | | 2,443 |
| | 61 |
|
| Operating lease liabilities | (32,295 | ) | | — |
| | — |
|
| Operating lease right of use assets | (3,503 | ) | | — |
| | — |
|
| Net cash provided by operating activities | 202,000 |
| | 223,483 |
| | 105,731 |
|
| Cash Flows from Investing Activities: | | | | | |
| Proceeds from sale of deferred compensation plan assets | 448 |
| | 715 |
| | 622 |
|
| Proceeds from the sale of property and equipment | 162 |
| | 54 |
| | 249 |
|
| Purchases of property and equipment | (7,888 | ) | | (6,558 | ) | | (10,707 | ) |
| Investments in equity method investees | (210 | ) | | (7,144 | ) | | (476 | ) |
| Acquisitions of businesses, net of cash acquired | (345,460 | ) | | (9,260 | ) | | (33,715 | ) |
| Net cash used in investing activities | (352,948 | ) | | (22,193 | ) | | (44,027 | ) |
| Cash Flows from Financing Activities: | | | | | |
| Proceeds from issuance of stock upon exercise of stock options | 3,611 |
| | 5,953 |
| | 4,554 |
|
| Proceeds from issuance of stock to employee stock purchase plan | 3,187 |
| | 2,429 |
| | 2,382 |
|
| Shares withheld upon stock vesting | (9,556 | ) | | (6,570 | ) | | (6,939 | ) |
| Non-controlling interest distribution | (1,062 | ) | | (1,090 | ) | | (216 | ) |
| Proceeds from borrowings under term loan | 175,000 |
| | — |
| | — |
|
| Proceeds from borrowings under revolving line of credit | 262,500 |
| | 138,000 |
| | — |
|
| Repayments of borrowings under revolving line of credit | (200,000 | ) | | (130,500 | ) | | — |
|
| Principal payments of long-term obligations | (5,624 | ) | | (91,450 | ) | | (5,319 | ) |
| Debt issuance costs | (847 | ) | | (2,433 | ) | | — |
|
| Purchase of company stock | — |
| | (181,402 | ) | | — |
|
| Repurchase of noncontrolling interest | — |
| | (361 | ) | | — |
|
| Net cash provided by (used in) financing activities | 227,209 |
| | (267,424 | ) | | (5,538 | ) |
| Net increase (decrease) in cash, cash equivalents and restricted cash | 76,261 |
| | (66,134 | ) | | 56,166 |
|
| Cash, cash equivalents and restricted cash at beginning of period | 20,229 |
| | 86,363 |
| | 30,197 |
|
| Cash, cash equivalents and restricted cash at end of period | $ | 96,490 |
| | $ | 20,229 |
| | $ | 86,363 |
|
| Supplemental Disclosures of Cash Flow Information: | | | | | |
| Cash paid for interest | $ | 9,628 |
| | $ | 3,522 |
| | $ | 2,697 |
|
| Cash paid for income taxes, net of refunds received | $ | 29,522 |
| | $ | 14,278 |
| | $ | 315 |
|
| Supplemental Disclosures of Non-Cash Financing Activities: | | | | | |
| Note payable issued for software licenses | $ | — |
| | $ | 418 |
| | $ | — |
|
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Supplemental Disclosures of Cash Flow Information: | | | | | |
| Cash paid for interest | $ | 14,939 | | | $ | 5,291 | | | $ | 6,207 | |
| Cash paid for Infinity ZPIC interest | $ | 12,755 | | | $ | — | | | $ | — | |
| Cash paid for income taxes, net of refunds received | $ | 24,013 | | | $ | 34,097 | | | $ | 50,721 | |
| Supplemental Disclosures of Non-Cash Activity: | | | | | |
| Accrued contingent consideration | $ | 19,195 | | | $ | — | | | $ | — | |
| Noncontrolling interest contribution | $ | 8,900 | | | $ | — | | | $ | — | |
The accompanying notes are an integral part of these consolidated financial statements.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 20192022
1. NATURE OF OPERATIONS, CONSOLIDATION AND PRESENTATION OF FINANCIAL STATEMENTS
Amedisys, Inc., a Delaware corporation (together with its consolidated subsidiaries, referred to herein as “Amedisys,” “we,” “us,” or “our”), is a multi-state provider of home health, hospice, personal care and personalhigh acuity care services with approximately 74%, 73%75% and 76%75% of our consolidated net service revenue derived from Medicare for 2019, 20182022, 2021 and 2017,2020, respectively. As of December 31, 2019,2022, we owned and operated 321347 Medicare-certified home health care centers, 138164 Medicare-certified hospice care centers, and 1213 personal-care care centers and 8 admitting high acuity care joint ventures in 3837 states within the United States and the District of Columbia.
Recently Adopted Accounting Pronouncements
On January 1, 2019,During 2021, the Company adopted Accounting Standards Update ("ASU") 2016-02, Leases (Topic 842); ASU 2018-01, Land Easement Practical Expedient for Transition to Topic 842; ASU 2018-10,2020-10, Codification Improvements, which included minor technical corrections and clarifications to Topic 842, Leases;improve consistency and ASU 2018-11, Targeted Improvements (collectively, "Topic 842") using a modified retrospective transition approach, which requiresclarify the new standards to be applied to all leases existing atapplication of various provisions of the date of initial application. Under Topic 842, lessees are required to recognize a lease liability and right-of-use asset ("ROU asset") for all leases with a term greater than twelve months and to disclose key information about leasing arrangements. Additionally, leases will be classified as either financing or operating;codification by amending the classification will determine the pattern of expense recognition and classification within the statement of operations. We are using the standards' effective date as our date of initial application. Consequently, our financial information was not updated and the disclosures required under the new standard are not provided for dates and periods prior to January 1, 2019. The new standard provides several optional practical expedients that can be adopted at transition. We have elected the "package of practical expedients," which allows us to not reassess our prior conclusions regarding lease identification, lease classification and initial direct costs. We did not elect the use-of-hindsight or the practical expedient pertaining to land easements; the latter not being applicable to us. The most significant effects related to this adoption relate to (1) the recognition of new ROU assets and lease liabilities on our balance sheet for our real estate and fleet operating leases; and (2) significant new disclosures about our leasing activities. Upon adoption, we recognized approximately $80 million in operating leases liabilities with corresponding ROU assets of approximately the same amount. The new standard also provides practical expedients for an entity’s ongoing accounting. We have elected the practical expedient that allows us to not separate lease and non-lease components for all of our leases. We are applying the short-term lease recognition exemption to certain information technology leases; therefore, we have not recognized ROU assets and lease liabilities for these leases.
In June 2018, the FASB issued ASU 2018-07, Compensation - Stock Compensation (Topic 718): Improvements to Nonemployees Share-Based Payment Accounting which expands the scope of Topic 718codification to include share-based payments issuedall disclosure guidance in the appropriate disclosure sections and by amending and adding new headings, cross referencing to nonemployees for goodsother guidance and refining or services.correcting terminology. Our adoption of this standard on January 1, 2019 did not have ana material effect on our consolidated financial statements.
On January 1, 2018,During 2021, the Company adopted ASU 2014-09,2021-10, Revenue from Contracts with CustomersGovernment Assistance (Topic 606) and ASU 2015-14, Revenue from Contracts with Customers (Topic 606)832): Deferral of the Effective Date (collectively, "ASC 606"), the new accounting standards issuedDisclosures by the Financial Accounting Standards Board ("FASB") on revenue recognition, using the full retrospective method. ASC 606 outlines a single comprehensive model to use in accounting for revenue arising from contracts with customers. The standards supersede existing revenue recognition requirements and eliminate most industry-specific guidance from U.S. Generally Accepted Accounting Principles ("U.S. GAAP"). The core principle of the revenue recognition standard is to require an entity to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which it expects to be entitled in exchange for those goods or services. As a result of the Company's adoption of ASC 606, the revenue and related estimated uncollectible amounts owed to us by non-Medicare payors that were historically classified as provision for doubtful accounts are now considered a revenue adjustment in determining net service revenue. Accordingly, the Company reports estimated uncollectible balances due from third-party payors and uncollectible balances associated with patient responsibility as a reduction of the transaction price and therefore, as a reduction in net service revenue (or as it relates to Hospice room and board, an increase in cost of service, excluding depreciation and amortization) when historically these amounts were classified as provision for doubtful accounts within operating expenses within our consolidated statements of operations. In addition, the adoption of ASC 606 resulted in increased disclosure, including qualitative and quantitative disclosuresBusiness Entities about the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers.
In January 2017, the FASB issued ASU 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a BusinessGovernment Assistance, which provides guidancewas intended to assist entities with evaluating whether transactions should be accountedincrease transparency around financial reporting regarding government assistance by requiring disclosure of information about (1) the types of government assistance received, (2) an entity's accounting for as an acquisition (or disposal) of assets or a business. We adopted this ASU effective January 1, 2018, on a prospective basis. The impact on our consolidated financial statementsthe government assistance received and related disclosures will depend on(3) the facts and circumstances of any specific future transactions as evaluated under the new framework.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
In January 2017, the FASB issued ASU 2017-04, Intangibles - Goodwill and Other (Topic 350) - Simplifying the Test for Goodwill Impairment, which eliminates the requirement to calculate the implied fair value of goodwill to measure a goodwill impairment charge (Step 2effect of the goodwill impairment test). Instead, impairment will be measured using the difference between the carrying amount and the fair value of the reporting unit.assistance on an entity's financial statements. The ASU iswas effective for annual and interim periods beginning after December 15, 2019. Early2021, with early adoption is permitted. We adoptedSee Note 3 – Novel Coronavirus Pandemic ("COVID-19") for the disclosures associated with this ASU effective January 1, 2018, on a prospective basis and will apply this guidance to our future tests of goodwill impairment.standard.
In August 2016,During 2020, the FASB issuedCompany adopted ASU 2016-15,2016-13, Statement of Cash FlowsFinancial Instruments - Credit Losses (Topic 230): Classification of Certain Cash Receipts and Cash Payments326), which provides specificprovided guidance for measuring credit losses on eight cash flow classification issues not specifically addressed by U.S. GAAP. The ASU is effective for annual and interim periods beginning after December 15, 2017. The standard should be applied using a retrospective transition method unless it is impractical to do so for some of the issues. In such case, the amendments for those issues would be applied prospectively as of the earliest date practicable.financial instruments. Our adoption of this standard on January 1, 2018, using a retrospective transition method for each period presented, did not have ana material effect on our consolidated financial statements.
In March 2016,During 2020, the FASB issued ASU 2016-09, Compensation – Stock Compensation (Topic 718): Improvement to Employee Share-Based Payment Accounting, which simplified the accounting for share-based payment award transactions, including income tax consequences, classification of awards as either equity or liability and classification within the statement of cash flows. The ASU was effective for annual and interim periods beginning after December 15, 2016. WeCompany adopted this ASU effective January 1, 2017, and as a result, we recorded a $0.4 million increase to our non-current deferred tax asset and retained earnings for tax benefits that were not previously recognized under the prior rules. Additionally, on a prospective basis, we recorded excess tax benefits totaling $3.2 million as a discrete item in our income tax provision within our consolidated statements of operations for the year ended December 31, 2017. Historically, these amounts were recorded as additional paid-in capital in our consolidated balance sheet. We also elected to prospectively apply the change to the presentation of cash payments made to taxing authorities on the employees' behalf for shares withheld upon stock vesting within our consolidated statement of cash flows and to continue our current policy of estimating forfeitures of stock-based compensation awards at grant date and revising in subsequent periods to reflect actual forfeitures.
Recently Issued Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740) - Simplifying the Accounting for Income Taxes, which simplifieseliminated certain exceptions related to the approach for intraperiod tax allocation, the methodology for calculating taxes during the interim periods and the recognition of deferred tax liabilities for outside basis differences. This guidance also simplified aspects of the accounting for incomefranchise taxes, by removing certain exceptions toenacted changes in tax laws or rates and clarified the general principlesaccounting for transactions that result in Topic 740 and improves consistent application by clarifying and amending existing guidance.a step-up in the tax basis of goodwill. The ASU isguidance was effective for annualinterim and interimannual periods beginning after December 15, 2020. Early2020, with early adoption is permitted. While the Company does not expect a material impact uponOur adoption of this standard on a prospective basis was not material to the Company’s consolidated financial statements.
Recently Issued Accounting Pronouncements
In March 2020, the Financial Accounting Standards Board ("FASB") issued ASU 2019-12, we are still evaluating2020-04, Reference Rate Reform (Topic 848): Facilitation of the effectEffects of Reference Rate Reform on Financial Reporting, which provides optional expedients and exceptions for applying U.S. Generally Accepted Accounting Principles ("U.S. GAAP") to contract modifications and hedging relationships that reference the standard willLondon Inter-Bank Offered Rate ("LIBOR") or another reference rate expected to be discontinued, subject to meeting certain criteria. In January 2021, the FASB issued ASU 2021-01, Reference Rate Reform (Topic 848): Scope, which adds implementation guidance to ASU 2020-04 to clarify certain optional expedients in Topic 848. The guidance in ASU 2020-04 and ASU 2021-01 was effective upon issuance and may generally be applied prospectively through December 31, 2022. In December 2022, the FASB issued ASU 2022-06, Reference Rate Reform (Topic 848): Deferral of the Sunset Date of Topic 848, which deferred the sunset date of Topic 848 from December 31, 2022 to December 31, 2024. These standards did not have an effect on our consolidated financial statements and related disclosures and ongoing financial reporting.statements.
Use of Estimates
Our accounting and reporting policies conform with U.S. GAAP. In preparing the consolidated financial statements, we are required to make estimates and assumptions that impact the amounts reported in the consolidated financial statements and accompanying notes. Actual results could materially differ from those estimates.
Principles of Consolidation
These consolidated financial statements include the accounts of Amedisys, Inc. and our wholly owned subsidiaries. All significant intercompany accounts and transactions have been eliminated in our accompanying consolidated financial
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
statements, and business combinations accounted for as purchases have been included in our consolidated financial statements from their respective dates of acquisition. In addition to our wholly owned subsidiaries, we also have certain equity investments that are accounted for as set forth below.
Investments
We consolidate investments when the entity is a variable interest entity ("VIE") and we are the primary beneficiary or if we have controlling interests in the entity, which is generally ownership in excess of 50%. Third party equity interests in our consolidated joint ventures are reflected as noncontrolling interests in our consolidated financial statements. During 2016, we sold a 30% interest in one of our care centers while maintaining a controlling interest in the newly formed joint venture; we repurchased the 30% interest during 2018.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
We account for investments in entities in which we have the ability to exercise significant influence under the equity method if we hold 50% or less of the voting stock and the entity is not a variable interest entityVIE in which we are the primary beneficiary. During 2018, we made a $7.0 million investment in a healthcare analytics company; this investment is accounted for under the equity method. The book value of investments that we account for under the equity method of accounting totaled $35.7$40.5 million and $35.1$48.1 million as of December 31, 20192022 and 2018,2021, respectively, and is reflected in other assets within our consolidated balance sheets.
During the three-month period ended December 31, 2022, we sold a 49% interest in two of our home health care centers while maintaining a controlling interest in the newly formed joint venture. We are consolidating this joint venture. The total cash consideration received for the 49% noncontrolling interest was $1.9 million. In connection with the transaction, we recorded an after-tax gain of $1.4 million; this gain was recorded to additional paid-in capital within our consolidated balance sheet. During the three-month period ended September 30, 2022, we sold a 30% interest in two of our home health care centers while maintaining a controlling interest in the newly formed joint venture. We are consolidating this joint venture. The total cash consideration received for the 30% noncontrolling interest was $3.9 million. In connection with the transaction, we recorded an after-tax gain of $2.9 million; this gain was recorded to additional paid-in capital within our consolidated balance sheet. During 2021, a third-party acquired a majority of the issued and outstanding membership interests of one of our equity method investments, Medalogix, for cash, with the remaining membership interests rolling over into a newly formed entity that includes Medalogix as well as another healthcare predictive data and analytics company. We rolled over 100% of our ownership interest in Medalogix to the newly formed entity, and in connection with this transaction, we recognized a $31.1 million gain based on the purchase price of Medalogix, which is reflected in gain on equity method investments within our consolidated statements of operations.
In connection with the acquisition of Contessa Health ("Contessa") on August 1, 2021, we obtained interests in several joint ventures with health system partners and a professional corporation that employs clinicians. Each of these entities meets the criteria to be classified as a VIE. As of December 31, 2022, we are consolidating all of our admitting joint ventures with health system partners as well as the professional corporation as we have concluded that we are the primary beneficiary of these VIEs. We have management agreements in place with each of these entities whereby we manage the entities and run the day-to-day operations. As such, we possess the power to direct the activities that most significantly impact the economic performance of the VIEs. The significant activities include, but are not limited to, negotiating provider and payor contracts, establishing patient care policies and protocols, making employment and compensation decisions, developing the operating and capital budgets, performing marketing activities and providing accounting support. We also have the obligation to absorb any expected losses and the right to receive benefits. Additionally, from time to time we may be required to provide joint venture funding. Our high acuity care segment also includes two non-admitting joint ventures with health system partners that are accounted for under the equity method of accounting. Operations of one of these joint ventures have ceased, and we are currently awaiting claims runout to complete financial reconciliations with our health plan partner; we recorded a $3.0 million impairment charge related to our investment in this joint venture during the three-month period ended September 30, 2022.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
The terms of the agreements with each VIE prohibit us from using the assets of the VIE to satisfy the obligations of other entities. The carrying amount of the VIEs’ assets and liabilities included in our consolidated balance sheets are as follows (amounts in millions):
| | | | | | | | | | | |
| As of December 31, 2022 | | As of December 31, 2021 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 15.6 | | | $ | 3.1 | |
| Patient accounts receivable | 6.1 | | | 2.4 | |
| Other current assets | 0.6 | | | 0.1 | |
| Total current assets | 22.3 | | | 5.6 | |
| Property and equipment | 0.1 | | | 0.1 | |
| Operating lease right of use assets | 0.1 | | | — | |
| Goodwill | 8.5 | | | — | |
| Intangible assets | 0.4 | | | — | |
| Other assets | 0.2 | | | — | |
| Total assets | $ | 31.6 | | | $ | 5.7 | |
| LIABILITIES | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 0.1 | | | $ | — | |
| Payroll and employee benefits | 0.5 | | | 0.3 | |
| Accrued expenses | 5.8 | | | 3.4 | |
| Operating lease liabilities | 0.1 | | | — | |
| Current portion of long-term obligations | 0.2 | | | 0.8 | |
| Total liabilities | $ | 6.7 | | | $ | 4.5 | |
During 2020, we sold our investment in the Heritage Healthcare Innovation Fund, LP via a secondary transaction for $17.9 million which resulted in a $3.0 million loss which is reflected in gain (loss) on equity method investments within our consolidated statement of operations for the year ended December 31, 2020. The Company's original investment was made in 2010 and no longer fit within our strategic areas of focus. Proceeds from the sale were used to pay down debt and fund capital needs.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Revenue Recognition
We account for revenue from contracts with customers in accordance with ASC 606,Revenue from Contracts with Customers, and as such, we recognize revenue in the period in which we satisfy our performance obligations under our contracts by transferring our promised services to our customers in amounts that reflect the consideration to which we expect to be entitled in exchange for providing patient care, which are the transaction prices allocated to the distinct services. The Company'sOur cost of obtaining contracts is not material.
Revenues are recognized as performance obligations are satisfied, which varies based on the nature of the services provided. Our performance obligation is the delivery of patient care services in accordance with the nature and frequency of services outlined in physicians' orders, which are determined by a physician based on a patient's specific goals.
The Company'sOur performance obligations relate to contracts with a duration of less than one year; therefore, the Company haswe have elected to apply the optional exemption provided by ASC 606 and isare not required to disclose the aggregate amount of the transaction price allocated to performance obligations that are unsatisfied or partially unsatisfied as of the end of the reporting period. The unsatisfied or partially unsatisfied performance obligations are generally completed when the patients are discharged, which generally occurs within days or weeks of the end of the reporting period.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
We determine the transaction price based on gross charges for services provided, reduced by estimates for contractual and non-contractual revenue adjustments. Contractual revenue adjustments include adjustments providedare recorded for the difference between our standard rates and the contracted rates to be realized from patients, and third-party payors based on contracted rates.and others for services provided. Non-contractual revenue adjustments include discounts provided to self-pay, uninsured patients or other payors, adjustments resulting from payment reviews and adjustments arising from our inability to obtain appropriate billing documentation, authorizations or face-to-face documentation. Subsequent changes to the estimate of the transaction price are recorded as adjustments to net service revenue in the period of change.
Contractual revenue adjustments are recorded for the difference between our standard rates and the contracted rates to be realized from patients, third party payors and others for services provided. Non-contractual revenue adjustments are recorded for self-pay, uninsured patients and other payors by major payor class based on our historical collection experience, aged accounts receivable by payor and current economicindustry conditions. The non-contractual revenue adjustments represent the difference between amounts billed and amounts we expect to collect based on our collection history with similar payors. The Company assesses itsWe assess our ability to collect for the healthcare services provided at the time of patient admission based on the Company'sour verification of the patient's insurance coverage under Medicare, Medicaid, and other commercial or managed care insurance programs. Medicare represents approximately 74% of the Company'sour consolidated net service revenue.
Amounts due from third-party payors, primarily commercial health insurers and government programs (Medicare and Medicaid), include variable consideration for retroactive revenue adjustments due to settlements of audits and payment reviews. We determine our estimates for non-contractual revenue adjustments related to audits and payment reviews based on our historical experience and success rates in the claim appeals and adjudication process.
We determine our estimates for non-contractual revenue adjustments related to our inability to obtain appropriate billing documentation, authorizations or face-to-face documentation based on our historical experience which primarily includes a historical collection rate of over 99% on Medicare claims. Revenue is recorded at amounts we estimate to be realizable for services provided.
Revenue by payor class as a percentage of total net service revenue is as follows:
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
|
| | | | | | | | |
| | As of December 31, |
| | 2019 | | 2018 | | 2017 |
| Home Health: | | | | | |
| Medicare | 44 | % | | 50 | % | | 53 | % |
| Non-Medicare - Episodic-based | 9 | % | | 9 | % | | 8 | % |
| Non-Medicare - Non-episodic based | 12 | % | | 12 | % | | 11 | % |
| Hospice (1): | | | | | |
| Medicare | 30 | % | | 23 | % | | 23 | % |
| Non-Medicare | 1 | % | | 1 | % | | 1 | % |
| Personal Care | 4 | % | | 5 | % | | 4 | % |
| | 100 | % | | 100 | % | | 100 | % |
| | | | | | | | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 | | 2020 |
| Home Health: | | | | | |
| Medicare | 40 | % | | 41 | % | | 41 | % |
| Non-Medicare - Episodic-based | 8 | % | | 8 | % | | 7 | % |
| Non-Medicare - Non-episodic based | 13 | % | | 12 | % | | 13 | % |
| Hospice: | | | | | |
| Medicare | 33 | % | | 34 | % | | 34 | % |
| Non-Medicare | 2 | % | | 2 | % | | 2 | % |
| Personal Care | 3 | % | | 3 | % | | 3 | % |
| High Acuity Care (1) | 1 | % | | — | % | | — | % |
| 100 | % | | 100 | % | | 100 | % |
(1) Acquired Compassionate Care HospiceContessa Health on FebruaryAugust 1, 2019 and RoseRock Healthcare on April 1, 2019.2021.
Home Health Revenue Recognition
Medicare Revenue
Net service revenue is recorded underEffective January 1, 2020, the Centers for Medicare prospectiveand Medicaid Services ("CMS") implemented a revised case-mix adjustment methodology, the Patient-Driven Groupings Model ("PDGM"). PDGM uses 30-day periods of care rather than 60-day episodes of care as the unit of payment, system (“PPS”) based on an established Federal Medicare home health episode payment rate, that is subject to adjustment based on certain variables including, but not limited to: (a) an outlier payment if a patient’s care was unusually costly (capped at 10%eliminates the use of total reimbursement per provider number); (b) a low utilization payment adjustment (“LUPA”) if the number of therapy visits was four or fewer; (c) a partialprovided in determining payment if a patient transferred to another provider or we admitted a patient transferring from another provider before completing the episode; (d) a payment adjustment based upon the level of therapy services required (with various incremental adjustments made for additional visits, with larger payment increases associated with the sixth, fourteenth and twentieth visit thresholds); (e) the number of episodes of care provided to a patient, regardless of whether the same home health provider provided care for the entire series of episodes; (f) changes in the base episode payments established by the Medicare Program; and (g) adjustments to the base episode payments for case mix and geographic wages. Medicare rates are basedrelies more heavily on the severity of the patient's condition, service needs and goals,clinical characteristics and other factors relating to the cost of providing services and supplies, bundled into an episode of care, not to exceed 60 days. An episode starts the first day a billable visit is performed and ends 60 days later or upon discharge, if earlier, with multiple continuous episodes allowed.patient information.
The Medicare home health benefit requires that beneficiaries be homebound (meaning that the beneficiary is unable to leave their home without a considerable and taxing effort), require intermittent skilled nursing, physical therapy or speech therapy services, and receive treatment under a plan of care established and periodically reviewed by a physician. All Medicare contracts are required to have a signed plan of care which represents a single performance obligation, comprisingcomprised of the delivery of a series of distinct services that are substantially similar and have a similar pattern of transfer to the customer. Accordingly, the Company accountswe account for the series of services ("episode") as a single performance obligation satisfied over time, as the customer simultaneously receives and consumes the benefits of the goods and services provided. Expected MedicareAn episode starts the first day a billable visit is performed and ends 60 days later or upon discharge, if earlier, with multiple continuous episodes allowed. Each 60-day episode includes two 30-day payment periods.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
Net service revenue per episode is recognizedrecorded based on the established Federal Medicare home health payment rate for a pro-rated service output method, utilizing30-day period of care. ASC 606 notes that if an entity has a right to consideration from a customer in an amount that corresponds directly with the value of the entity’s performance completed to date, the entity may recognize revenue in the amount to which the entity has a right to invoice. We have elected to apply the "right to invoice" practical expedient, and therefore, our revenue recognition is based on the reimbursement we are entitled to for each 30-day period of care. We utilize our historical average length of episode priorstay for each 30-day period of care as the measure of progress towards the satisfaction of our performance obligation.
PDGM uses timing, admission source, functional impairment levels and principal and other diagnoses to discharge.
case-mix adjust payments. The base episodecase-mix adjusted payment can be adjustedfor a 30-day period of care is subject to additional adjustments based on eachcertain variables, including, but not limited to (a) an outlier payment if our patient's health including clinical condition, functional abilitiescare was unusually costly (capped at 10% of total reimbursement per provider number); (b) a low utilization payment adjustment (“LUPA”) if the number of visits provided was less than the established threshold, which ranges from two to six visits and service needs, as well asvaries for every case-mix group; (c) a partial payment if a patient transferred to another provider or from another provider before completing the 30-day period of care; and (d) the applicable geographic wage index, low utilization, patient transfersindex. Payments for routine and other factors. The services covered bynon-routine supplies are included in the episode30-day payment include all disciplines of care in addition to medical supplies. rate.
Medicare can also make various adjustments to payments received if we are unable to produce appropriate billing documentation or acceptable authorizations. We estimate the impact of such adjustments based on our historical experience, which primarily includes a historical collection rate of over 99% on Medicare claims, and record this estimate during the period in which services are rendered as a reduction to revenue andwith a corresponding reduction to patient accounts receivable.
A portionAmounts due from Medicare include variable consideration for retroactive revenue adjustments due to settlements of audits and payment reviews. We determine our estimates for non-contractual revenue adjustments related to audits and payment reviews based on our historical experience and success rates in the claim appeals and adjudication process.
The Medicare home health benefit requires that beneficiaries be homebound (meaning that the beneficiary is unable to leave his/her home without a considerable and taxing effort), require intermittent skilled nursing, physical therapy or speech therapy services and receive treatment under a plan of care established and periodically reviewed by a physician. In order to provide greater flexibility during the novel coronavirus pandemic ("COVID-19"), CMS relaxed the definition of homebound status through the duration of the public health emergency. During the pandemic, a beneficiary is considered homebound if they have been instructed by a physician not to leave their home because of a confirmed or suspected COVID-19 diagnosis or if the patient has a condition that makes them more susceptible to contracting COVID-19.
During 2020, 20% of the reimbursement from each Medicare episode is30-day payment period was billed near the start of each episode,30-day period of care, referred to as a request for anticipated payment ("RAP"), and cash iswas typically received before all services arewere rendered. The amount of revenue recognized for episodes of care which are incomplete at period end is based on the company's average percentage of days complete on episodes as of the end of the year. As of December 31, 2019, the difference between theAny cash received from Medicare for a requestRAP for anticipated payment (“RAP”) on episodes in progress anda 30-day period of care that exceeded the associated estimated revenue earned was recorded to accrued expenses within our consolidated balance sheets.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
Non-Medicare Revenue
Payments from non-Medicare payors are either a percentage of Medicare rates, per-visit rates or case rates depending upon the terms and conditions established with such payors. Approximately 30% of our managed care contract volume affords us the opportunity to receive additional payments if we achieve certain quality or process metrics as defined in each contract (e.g. star ratings and acute-care hospitalization rates).
Episodic-based Revenue. We recognize revenue in a similar manner as we recognize Medicare revenue for episodic-based ratesamounts that are paid by other insurance carriers, including Medicare Advantage programs; however, these ratesamounts can vary based upon the negotiated terms, the majority of which generally range from 90%95% to 100% of Medicare rates.
Non-episodic based Revenue. GrossFor our per visit contracts, gross revenue is recorded on an accrual basis based upon the date of service at amounts equal to our established or estimated per-visit rates. For our case rate contracts, gross revenue is recorded over our historical average length of stay using the established case rate for each admission. Contractual revenue adjustments are recorded for the difference between our standard rates and the contracted rates to be realized from patients, third parties and others for services provided and are deducted from gross revenue to determine net service revenue. We also make non-contractual revenue adjustments to non-episodic revenue based on our historical experience to reflect the estimated transaction
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
price. We receive a minimal amount of our net service revenue from patients who are either self-insured or are obligated for an insurance co-payment.
Under our case rate contracts, we may receive reimbursement before all services are rendered. Any cash received that exceeds the associated revenue earned is recorded to deferred revenue in accrued expenses within our consolidated balance sheets.
Hospice Revenue Recognition
Hospice Medicare Revenue
Gross revenue is recorded on an accrual basis based upon the date of service at amounts equal to the estimated payment rates. The estimated payment rates are predetermined daily or hourly rates for each of the four levels of care we deliver. The four levels of care are routine care, general inpatient care, continuous home care and respite care. Routine care accounted for 99%, 97% and 97% of our total net Medicare hospice service revenue for each of 2019, 20182022, 2021 and 2017,2020, respectively. There are two separate payment rates for routine care: payments for the first 60 days of care and care beyond 60 days. In addition to the two routine rates, we may also receive a service intensity add-on (“SIA”). The SIA is based on visits made in the last seven days of life by a registered nurse (“RN”) or medical social worker (“MSW”) for patients in a routine level of care.
The performance obligation is the delivery of hospice services to the patient, as determined by a physician, each day the patient is on hospice care.
We make adjustments to Medicare revenue for non-contractual revenue adjustments, which include our inability to obtain appropriate billing documentation or acceptable authorizations and other reasons unrelated to credit risk. We estimate the impact of these non-contractual revenue adjustments based on our historical experience, which primarily includes a historical collection rate of over 99% on Medicare claims, and record it during the period services are rendered.
Amounts due from Medicare include variable consideration for retroactive revenue adjustments due to settlements of audits and payment reviews. We determine our estimates for non-contractual revenue adjustments related to audits and payment reviews based on our historical experience and success rates in the claim appeals and adjudication process.
Additionally, our hospice service revenue is subject to certain limitations on payments from Medicare which are considered variable consideration. We are subject to an inpatient cap limit and an overall Medicare payment cap for each provider number. We monitor these caps on a provider-by-provider basis and estimate amounts due back to Medicare if we estimate a cap has been exceeded. We record these adjustments as a reduction to revenue and an increase in accrued expenses within our consolidated balance sheet. Beginning for the cap year ending October 31, 2017, providerssheets. Providers are required to self-report and pay their estimated cap liability by February 28th28th of the following year. As of December 31, 2019, we have settled our Medicare hospice reimbursements for all fiscal years through October 31, 2012. As of December 31, 2019,2022, we have recorded $5.7$4.3 million for estimated amounts due back to Medicare in accrued expenses for the Federal cap years ended October 31, 20132016 through September 30, 2020; approximately $1.9 million of this amount is related to the cap liability acquired as part of the Compassionate Care Hospice ("CCH") acquisition.2023. As of December 31, 2018,2021, we had recorded $1.7$4.5 million for estimated amounts due back to Medicare in accrued expenses for the Federal cap years ended October 31, 20132016 through September 30, 2019.2022.
Hospice Non-Medicare Revenue
Gross revenue is recorded on an accrual basis based upon the date of service at amounts equal to our established rates or estimated per day rates, as applicable. Contractual revenue adjustments are recorded for the difference between our standard rates and the contractual rates to be realized from patients, third partythird-party payors and others for services provided and are deducted from gross revenue to determine our net service revenue. We also make non-contractual adjustments to non-Medicare revenue based on our historical experience to reflect the estimated transaction price.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
Personal Care Revenue Recognition
Personal Care Revenue
We generate net service revenues by providing our services directly to patients based on authorized hours, visits or units determined by the relevant agency, at a rate that is either contractual or fixed by legislation. Net service revenue is recognized at the time services are rendered based on gross charges for the services provided, reduced by estimates for contractual and non-contractual revenue adjustments. We receive payment for providing such services from payors, including state and local governmental agencies, managed care organizations, commercial insurers and private consumers. Payors include the following elder service agencies: Aging Services Access Points (ASAPs)("ASAPs"), Senior Care Options (SCOs)("SCOs"), Program of All-Inclusive Care for the Elderly (PACE)("PACE") and the Veterans Administration (VA)("VA").
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
High Acuity Care Revenue Recognition
High Acuity Care Revenue
Our revenues are derived from contracts with (1) health insurance plans for the coordination and provision of home recovery care services to clinically-eligible patients who are enrolled members in those insurance plans, (2) health system partners for the coordination and provision of home recovery care services to clinically-eligible patients who are discharged early from a health system facility to complete their inpatient stay at home and (3) Medicare and other payors for the provision of home health services.
Under our health insurance plan contracts, we provide home recovery care services, which include hospital-equivalent ("H@H") and skilled nursing facility ("SNF") equivalent services ("SNF@H"), for high acuity care patients on a full risk basis whereby we assume the financial risk for the coordination and payment of all hospital or SNF replacement medical services necessary to treat the medical condition for which the patient was diagnosed in a home-based setting for a 30-day (H@H) or 60-day (SNF@H) episode of care in exchange for a fixed contracted bundled rate. For H@H programs, the fixed rate is based on the assigned diagnosis related group ("DRG") and the 30-day post-discharge related spend. For SNF@H programs, the fixed rate is based on the 60-day post-discharge related spend. Our performance obligation is the coordination and provision of patient care in accordance with physicians’ orders over either a 30-day or 60-day episode of care. The majority of our care coordination services and direct patient care is provided in the first five to seven days of the episode period (the "acute phase"). Monitoring services and follow-up direct patient care, as deemed necessary by the treating physician, are provided throughout the remainder of the episode. Since the majority of our services are provided during the acute phase, we recognize net service revenues over the acute phase based on gross charges for the services provided per the applicable managed care contract rates, reduced by estimates for revenue adjustments.
Under our contracts with health system partners, we provide home recovery care services for high acuity patients on a limited risk basis whereby we assume the risk for certain healthcare services during the remainder of an inpatient acute stay serviced at the patient’s home in exchange for a contracted per diem rate. The performance obligation is the coordination and provision of required medical services, as determined by the treating physician, for each day the patient receives inpatient-equivalent care at home. As such, revenues are recognized as services are administered and as our performance obligations are satisfied on a per diem basis, reduced by estimates for revenue adjustments.
We recognize adjustments to revenue during the period in which changes to estimates of assigned patient diagnoses or episode terminations become known, in accordance with the applicable managed care contracts. For certain health insurance plans, revenue is reduced by amounts owed by enrollees to healthcare providers under deductible, coinsurance or copay provisions of health insurance plan policies, since those amounts are repaid to the health insurance plans by us as part of a retrospective reconciliation process.
In March 2022, our high acuity care segment entered into a transaction in which one of our health system partners contributed its home health operations to one of our existing high acuity care joint ventures. We recognize Medicare and non-Medicare revenue in a manner that is consistent with our home health segment revenue recognition policy described above.
Government Grants
We account for government grants in accordance with ASU 2021-10, Government Assistance (Topic 832), by applying the grant model in accordance with International Accounting Standard ("IAS") 20, Accounting for Government Grants and Disclosure of Government Assistance, and as such, we recognize grant income on a systematic basis in line with the recognition of expenses or the loss of revenues for which the grants are intended to compensate. We recognize grants once both of the following conditions are met: (1) we are able to comply with the relevant conditions of the grant and (2) the grant will be received. See Note 3 – Novel Coronavirus Pandemic ("COVID-19") for additional information on our accounting for government funds received under the Coronavirus Aid, Relief and Economic Security Act ("CARES Act") and the Mass Home Care ASAP COVID-19 Provider Sustainability Program.
Cash, Cash Equivalents and Restricted Cash
Cash and cash equivalents include certificates of deposit and all highly liquid debt instruments with maturities of three months or less when purchased. Restricted cash includes cash that is not available for ordinary business use. As of December 31, 2019,2022 and 2021, we had $66.2$13.6 million ofand $3.1 million, respectively, classified as restricted cash that wasrelated to funds placed into an escrow accountaccounts in connection with the indemnity, closing payment and other provisions within the purchase agreements of our acquisitions. The increase in restricted cash from December 31, 2021 to fund the acquisitionDecember 31, 2022 is related to our acquisitions of Asana HospiceEvolution Health, LLC ("Evolution") and Assisted Care Home Health, Inc. and RH Homecare Services, LLC ("Assisted Care") on JanuaryApril 1, 2020.2022. See Note 4 – Acquisitions for additional information.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
The following table summarizes the balances related to our cash, cash equivalents and restricted cash for 2019 and 2018 (amounts in millions):
|
| | | | | | | |
| | As of December 31, |
| | 2019 | | 2018 |
| Cash and cash equivalents | $ | 30.3 |
| | $ | 20.2 |
|
| Restricted cash | 66.2 |
| | — |
|
| Cash, cash equivalents and restricted cash | $ | 96.5 |
| | $ | 20.2 |
|
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| Cash and cash equivalents | $ | 40.5 | | | $ | 42.7 | |
| Restricted cash | 13.6 | | | 3.1 | |
| Cash, cash equivalents and restricted cash | $ | 54.1 | | | $ | 45.8 | |
Patient Accounts Receivable
We report accounts receivable from services rendered at their estimated transaction price, which includes contractual and non-contractual revenue adjustments based on the amounts expected to be due from payors. Our patient accounts receivable are uncollateralized and consist of amounts due from Medicare, Medicaid, other third-party payors and patients. Our non-Medicare third-party payor base is comprised of a diverse group of payors that are geographically dispersed across the country. As of December 31, 2019,2022, there is only oneno single payor, other than Medicare, that accounts for more than 10% of our total outstanding patient receivables (approximately 10.4%).receivables. Thus, we believe there are no other significant concentrations of receivables that would subject us to any significant credit risk in the collection of our patient accounts receivable. We write off accounts on a monthly basis once we have exhausted our collection efforts and deem an account to be uncollectible. We believe the collectibilitycollectability risk associated with our Medicare accounts, which represent 58%represented 67% and 56%68% of our net patient accounts receivable at December 31, 20192022 and December 31, 2018,2021, respectively, is limited due to our historical collection rate of over 99% from Medicare and the fact that Medicare is a U.S. government payor.
We do not believe there are any significant concentrations of revenues from any payor that would subject us to any significant credit risk in the collection of our accounts receivable.
Medicare Home Health
For our home health patients (within both our home health and high acuity care segments), our pre-billing process includes verifying that we are eligible for payment from Medicare for the services that we provide to our patients. Our Medicare billing begins with a process to ensure that our billings are accurate through the utilization of an electronic Medicare claim review. We submit a RAP for 60%bill Medicare following the end of our estimated payment for the initial episode at the starteach 30-day period of care or 50% ofupon discharge, if earlier, for the estimated payment for any subsequent episodes of care contiguous withservices provided to the first episode for a particular patient. The full amount of the episode is billed after the episode has been completed (“final billed”). The RAP received for that particular episode is then deducted from our final payment. If a final bill is not submitted within the greater of 120 days from the start of the episode, or 60 days from the date the RAP was paid, any RAPs received for that episode will be recouped by Medicare from any other claims in process for that particular provider number. The RAP and final claim must then be resubmitted.
Medicare Hospice
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
For our hospice patients, our pre-billing process includes verifying that we are eligible for payment from Medicare for the services that we provide to our patients. Our Medicare billing begins with a process to ensure that our billings are accurate through the utilization of an electronic Medicare claim review. We bill Medicare on a monthly basis for the services provided to the patient.
Non-Medicare Home Health, Hospice, Personal Care and PersonalHigh Acuity Care
For our non-Medicare patients, our pre-billing process primarily begins with verifying a patient’s eligibility for services with the applicable payor. Once the patient has been confirmed for eligibility, we will provide services to the patient and bill the applicable payor. Our review and evaluation of non-Medicare accounts receivable includes a detailed review of outstanding balances and special consideration to concentrations of receivables from particular payors or groups of payors with similar characteristics that would subject us to any significant credit risk.
Property and Equipment
Property and equipment is stated at cost and depreciated on a straight-line basis over the estimated useful lives of the assets or life of the lease, if shorter. Additionally, we have internally developed computer software for our own use. Additions and improvements (including interest costs for construction of qualifying long-lived assets) are capitalized. Maintenance and repair expenses are charged to expense as incurred. The cost of property and equipment sold or disposed of and the related accumulated depreciation are eliminated from the property and equipment and related accumulated depreciation accounts, and any gain or loss is credited or charged to other general and administrative expenses.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
We assess the impairment of a long-lived asset group whenever events or changes in circumstances indicate that the asset’s carrying value may not be recoverable. Factors we consider important that could trigger an impairment review include but are not limited to the following:
| |
• | •A significant change in the extent or manner in which the long-lived asset group is being used. • |
A significant change in the business climate that could affect the value of the long-lived asset group.
•A significant change in the market value of the assets included in the asset group.
If we determine that the carrying value of long-lived assets may not be recoverable, we compare the carrying value of the asset group to the undiscounted cash flows expected to be generated by the asset group. If the carrying value exceeds the undiscounted cash flows, an impairment charge is indicated. An impairment charge is recognized to the extent that the carrying value of the asset group exceeds its fair value.
We generally provide for depreciation over the following estimated useful service lives.
| | | | | |
| Years |
| Buildings | Years39 |
Building | 39 |
| Leasehold improvements | Lesser of lease term or expected useful life |
| Equipment and furniture | 3 to 7 |
| Vehicles | 3 to 5 |
| Computer software | 2 to 7 |
| Finance leases | 3 |
During 2018, we reviewed the balances of our property and equipment and as a result, eliminated those asset balances and related accumulated depreciation for which the asset was no longer in service.
The following table summarizes the balances related to our property and equipment for 20192022 and 20182021 (amounts in millions):
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
|
| | | | | | | |
| | As of December 31, |
| | 2019 | | 2018 |
| Building and leasehold improvements | $ | 8.7 |
| | $ | 8.7 |
|
| Equipment and furniture | 55.6 |
| | 53.4 |
|
| Finance leases | 5.2 |
| | 2.9 |
|
| Computer software | 54.7 |
| | 59.9 |
|
| | 124.2 |
| | 124.9 |
|
| Less: accumulated depreciation | (96.1 | ) | | (95.5 | ) |
| | $ | 28.1 |
| | $ | 29.4 |
|
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| Buildings and leasehold improvements | $ | 9.7 | | | $ | 9.1 | |
| Equipment and furniture | 56.9 | | | 54.7 | |
| Finance leases | 4.1 | | | 4.5 | |
| Computer software | 46.7 | | | 47.0 | |
| 117.4 | | | 115.3 | |
| Less: Accumulated depreciation | (101.4) | | | (96.9) | |
| $ | 16.0 | | | $ | 18.4 | |
Depreciation expense for 2019, 20182022, 2021 and 20172020 was $11.6$11.5 million, $10.8$12.1 million and $14.4$12.1 million, respectively.
Business Combinations
We account for acquisitions using the acquisition method of accounting in accordance with ASC 805, Business Combinations. Acquisitions are accounted for as purchases and are included in our consolidated financial statements from their respective acquisition dates. Assets acquired, liabilities assumed and noncontrolling interests, if any, are measured at fair value on the acquisition date using the appropriate valuation method. Goodwill generated from acquisitions is recognized for the excess of the purchase price over tangible and identifiable intangible assets. In determining the fair value of identifiable intangible assets and any noncontrolling interests, we use various valuation techniques including the income approach, the cost approach and the market approach. These valuation methods require us to make estimates and assumptions surrounding projected revenues and costs, growth rates and discount rates.
Goodwill and Other Intangible Assets
As of December 31, 2022, we had a goodwill balance of $1,287.4 million. Goodwill represents the amount of the purchase price in excess of the fair values assigned to the underlying identifiable net assets of acquired businesses. Goodwill is not amortized, but is subject to an annual impairment test. Tests are performed more frequently if events occur or circumstances change that would more likely than not reduce the fair value of the reporting unit below its carrying amount. These events or circumstances include, but are not limited to, a significant adverse change in the business environment, regulatory environment or legal factors, or a substantial decline in the market capitalization of our stock.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
Each of our operating segments described in Note 1415 – Segment Information is considered to represent an individual reporting unit for goodwill impairment testing purposes. We consider each of our home health care centers to constitute an individual business for which discrete financial information is available. However, since these care centers have substantially similar operating and economic characteristics and resource allocationallocations and since significant investment decisions concerning these businesses are centralized and the benefits broadly distributed, we have aggregated these care centers and deemed them to constitute a single reporting unit. We have applied this same aggregation principle to our hospice and personal-care care centers and high acuity care joint ventures and have also deemed each of them to be a single reporting unit.
During 2019,2022, we performed a qualitative assessment to determine if it is more likely than not that the fair value of theour reporting units are less than their carrying values by evaluating relevant events and circumstances including financial performance, market conditions and share price. Based on this assessment, we did not record any goodwill impairment charges and none ofconcluded that the goodwill associated with our varioushome health, hospice and high acuity care reporting units was not considered at risk of impairment as of October 31, 2019.2022. In addition to the qualitative assessment, we also performed a quantitative analysis for our personal care reporting unit due to the decline in revenues resulting from staffing shortages using an income and market approach. Based on this analysis, we concluded that the goodwill associated with our personal care reporting unit was not considered at risk of impairment as of October 31, 2022. Since the date of our last annual goodwill impairment test,analysis, there have been no material developments, events, changes in operating performance or other circumstances that would cause management to believe it is more likely than not that the fair value of any of our reporting units would be less than their carrying amounts.
As of December 31, 2022, we had an other intangibles assets balance of $101.2 million. Intangible assets consist of certificates of need, licenses, acquired names, non-compete agreements and non-compete agreements.technology. We amortize non-compete agreements and acquired names that we do not intend to use in the futureindefinitely on a straight-line basis over their estimated useful lives, which isare generally two to three years for non-compete agreements and up to fivethree years for acquired names. We amortize technology over its estimated useful service life, which is generally up to seven years. Our indefinite-lived intangible assets are reviewed for impairment annually or more frequently if events occur or circumstances change that would more likely than not reduce the fair value of the intangible asset below its carrying amount. During 2019, weWe performed a qualitative assessment to determine if any of our indefinite-lived intangible assets were impaired; as a result of this analysis, we wrote off approximately $1.5 million of acquired names during the three-month period ended December 31, 2019. There2022 and determined that there have been no material developments, events, changes in operating performance or other circumstances that would cause management to believe it is more likely than not that the fair value of any of our remainingindefinite-lived intangible assets would be less than their carrying amounts.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
Debt Issuance Costs
During 2019,2021, we recorded $0.8$2.8 million in deferred debt issuance costs as a reduction to long-term obligations, less current portion in our consolidated balance sheet in connection with our entry into the Second Amended Credit Agreement (See Note 79 - Long-Term Obligations). As of December 31, 20192022 and 2018,2021, we had unamortized debt issuance costs of $3.5 million and $4.5 million, respectively, recorded as a reduction to long-term obligations, less current portion in our accompanying consolidated balance sheets. We amortize deferred debt issuance costs related to our long-term obligations over the term of the obligation through interest expense, unless the debt is extinguished, in which case unamortized balances are immediately expensed. The unamortized debt issuance costs of $3.5 million at December 31, 20192022 will be amortized over a weighted-average amortization period of 4.13.6 years.
Fair Value of Financial Instruments
The following details our financial instruments where the carrying value and the fair value differ (amounts in millions):
|
| | | | | | | | | | | | | | | |
| | Fair Value at Reporting Date Using |
| Financial Instrument | Carrying Value as of
December 31, 2019 | | Quoted Prices in Active Markets for Identical Items (Level 1) | | Significant Other Observable Inputs (Level 2) | | Significant Unobservable Inputs (Level 3) |
| Long-term obligations | $ | 242.3 |
| | $ | — |
| | $ | 240.8 |
| | $ | — |
|
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | Fair Value at Reporting Date Using |
| Financial Instrument | Carrying Value as of
December 31, 2022 | | Quoted Prices in Active
Markets for Identical
Items
(Level 1) | | Significant Other
Observable Inputs
(Level 2) | | Significant
Unobservable Inputs
(Level 3) |
| Long-term obligations | $ | 436.1 | | | $ | — | | | $ | 428.6 | | | $ | — | |
The fair value hierarchy is based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value. The three levels of inputs are as follows:
| |
• | •Level 1 – Quoted prices in active markets for identical assets and liabilities.
AMEDISYS, INC. AND SUBSIDIARIES NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS December 31, 2022 • |
Level 2 – Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities;liabilities, quoted prices in markets that are not active;active or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
•Level 3 – Unobservable inputs that are supported by little or no market activity and are significant to the fair value of the assets or liabilities.
Our deferred compensation plan assets are recorded at fair value and are considered a level 2 measurement. For our other financial instruments, including our cash and cash equivalents, patient accounts receivable, accounts payable, payroll and employee benefits and accrued expenses, we estimate the carrying amounts approximate fair value.
Income Taxes
We use the asset and liability approach for measuring deferred tax assets and liabilities based on temporary differences existing at each balance sheet date using currently enacted tax rates. Our deferred tax calculation requires us to make certain estimates about future operations. Deferred tax assets are reduced by a valuation allowance when we believe it is more likely than not that some portion or all of the deferred tax assets will not be realized. The effect of a change in tax rate is recognized as income or expense in the period that includes the enactment date. As of December 31, 2019 and 2018, our2022, we had net deferred tax liabilities of $20.4 million. As of December 31, 2021, we had net deferred tax assets were $21.4 million and $35.8 million, respectively.of $0.3 million.
Management regularly assesses the ability to realize deferred tax assets recorded in the Company’s entities based upon the weight of available evidence, including such factors as the recent earnings history and expected future taxable income. In the event future taxable income is below management’s estimates or is generated in tax jurisdictions different than projected, we could be required to increase the valuation allowance for deferred tax assets. This would result in an increase in our effective tax rate.
Share-Based Compensation
We record all share-based compensation as expense in the financial statements measured at the fair value of the award. We recognize compensation cost on a straight-line basis over the requisite service period for each separately vesting portion of the award. Upon adoption of ASU 2016-09 in 2017, we started recording the excess tax benefits related to stock option exercises as operating cash flows; these amounts were previously classified as financing cash flows. Share-based compensation expense for 2019, 20182022, 2021 and 20172020 was $25.0$16.6 million, $17.9$23.8 million and $16.3$26.7 million, respectively, and the total income tax benefit recognized for these expenses was $4.6 million, $4.3 million, $6.0 million and $6.4$4.7 million, respectively.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
respectively, prior to the application of the income tax compensation rules under Internal Revenue Code section 162(m) ("162(m)"). As of December 31, 2019
Weighted-Average Shares OutstandingOutstanding.
Net income per share attributable to Amedisys, Inc. common stockholders, calculated on the treasury stock method, is based on the weighted average number of shares outstanding during the period. The following table sets forth, for the periods indicated, shares used in our computation of weighted-average shares outstanding, which are used to calculate our basic and diluted net income attributable to Amedisys, Inc. common stockholders (amounts in thousands):
|
| | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Weighted average number of shares outstanding – basic | 32,142 |
| | 32,791 |
| | 33,704 |
|
| Effect of dilutive securities: | | | | | |
| Stock options | 545 |
| | 502 |
| | 281 |
|
| Non-vested stock and stock units | 303 |
| | 316 |
| | 319 |
|
| Weighted average number of shares outstanding – diluted | 32,990 |
| | 33,609 |
| | 34,304 |
|
| Anti-dilutive securities | 117 |
| | 50 |
| | 271 |
|
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Weighted average number of shares outstanding – basic | 32,517 | | | 32,642 | | | 32,559 | |
| Effect of dilutive securities: | | | | | |
| Stock options | 39 | | | 122 | | | 420 | |
| Non-vested stock and stock units | 97 | | | 208 | | | 289 | |
| Weighted average number of shares outstanding – diluted | 32,653 | | | 32,972 | | | 33,268 | |
| Anti-dilutive securities | 303 | | | 114 | | | 25 | |
Advertising Costs
We expense advertising costs as incurred. Advertising expense for 2019, 20182022, 2021 and 20172020 was $8.5$7.3 million, $7.0$7.4 million and $6.5 million, respectively.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
3. NOVEL CORONAVIRUS PANDEMIC ("COVID-19")
In March 2020, the World Health Organization declared COVID-19 a pandemic. As a healthcare at home company, we have been and will continue to be impacted by the effects of COVID-19; however, we remain committed to carrying out our mission of caring for our patients. We will continue to closely monitor the impact of COVID-19 on all aspects of our business, including the impacts to our employees, patients and suppliers; however, at this time, we are unable to estimate the ultimate impact the pandemic will have on our consolidated financial condition, results of operations or cash flows.
On March 27, 2020, the CARES Act was signed into legislation. The CARES Act provided for $175 billion to healthcare providers, including hospitals on the front lines of the COVID-19 pandemic. Of this total allocated amount, $30 billion was distributed immediately to providers based on their proportionate share of Medicare fee-for-service reimbursements in 2019. Healthcare providers were required to sign an attestation confirming receipt of the Provider Relief Fund ("PRF") funds and agree to the terms and conditions of payment. Our home health and hospice segments received approximately $100 million from the first $30 billion of funds distributed to healthcare providers in April 2020, which is inclusive of $2 million related to our joint venture care centers (equity method investments). We also acquired approximately $6 million of PRF funds in connection with the acquisition of AseraCare Hospice ("AseraCare"). Under the terms and conditions for receipt of the payment, we were allowed to use the funds to cover lost revenues and health care costs related to COVID-19 through June 30, 2021, and we were required to properly and fully document the use of these funds in reports to the U.S. Department of Health and Human Services ("HHS"). All required reporting was completed during the three-month period ended September 30, 2021, and our audit report was submitted to HHS on September 26, 2022.
For our wholly-owned subsidiaries, we utilized PRF funds to the extent we had qualifying COVID-19 expenses; we did not use PRF funds to cover lost revenues resulting from COVID-19. The grant income associated with the COVID-19 expenses incurred through June 30, 2021 is reflected in other operating income within our consolidated statements of operations.
We did not fully utilize the funds received; all unutilized funds were repaid in October 2021. In summary, the total funds that we received from the CARES Act PRF were accounted for as follows (amounts in millions):
| | | | | |
| Amount |
| Funds utilized through June 30, 2021 by consolidated entities | $ | 46.6 | |
| Funds repaid to the government by consolidated entities (excludes $0.2 million of interest repaid) | 58.3 | |
| Funds utilized through June 30, 2021 by unconsolidated joint ventures | 1.3 | |
| Funds repaid to the government by unconsolidated joint ventures | 0.6 | |
| $ | 106.8 | |
The CARES Act also provided for the temporary suspension of the automatic 2% reduction of Medicare claim reimbursements ("sequestration") for the period May 1, 2020 through December 31, 2020. During 2020 and 2021, Congress passed additional COVID-19 relief legislation which extended the 2% suspension of sequestration through March 31, 2022; sequestration was reinstated as a 1% reduction to Medicare claim reimbursements effective April 1, 2022 and a 2% reduction to Medicare claim reimbursements effective July 1, 2022. We recognized benefits to net service revenue totaling $13 million, $36 million and $23 million during 2022, 2021 and 2020, respectively.
Additionally, the CARES Act provided for the deferral of the employer share of social security tax (6.2%), effective for payments due after the enactment date through December 31, 2020. During 2020, we deferred approximately $55 million of social security taxes. Approximately $27 million was paid during December 2021; the remaining balance was paid during December 2022.
Our personal care segment did not receive funds under the CARES Act; however, it did receive funds totaling $1 million from the Mass Home Care ASAP COVID-19 Provider Sustainability Program, which were used during 2020 to cover costs related to COVID-19. The grant income associated with the funds received is reflected in other operating income within our consolidated statements of operations.
4. ACQUISITIONS
We complete acquisitions from time to time in order to pursue our strategy of increasing our market presence by expanding our service base and enhancing our position in certain geographic areas as a leading provider of home health, hospice, personal care and personalhigh acuity care services. The purchase price paid for acquisitions is negotiated through arm’s length transactions, with consideration based on our analysis of, among other things, comparable acquisitions and expected cash flows. Acquisitions are
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
accounted for as purchases and are included in our consolidated financial statements from their respective acquisition dates. Goodwill generated from acquisitions is recognized for the excess of the purchase price over tangible and identifiable intangible assets because of the expected contributions of the acquisitions to our overall corporate strategy. We typically engage outside appraisal firms to assist in the fair value determination of identifiable intangible assets and noncontrolling interests, if any, for significant acquisitions. The preliminary purchase price allocation is adjusted, as necessary, up to one year after the acquisition closing date if management obtains more information regarding asset valuation and liabilities assumed.
20192022 Acquisitions
Hospice DivisionOn March 23, 2022, we entered into a transaction with one of our high acuity care health system partners in which we contributed cash and our health system partner contributed its home health operations to one of our existing high acuity care joint ventures. As a result of this transaction, we recorded goodwill of $8.5 million, other intangibles of $0.4 million (certificate of need and licenses) and noncontrolling interest of $8.9 million within our consolidated balance sheet. The fair value of noncontrolling interest was determined using an income approach and a market approach.
On FebruaryApril 1, 2019,2022, we acquired CCH,15 home health care centers from Evolution Health, LLC, a national hospice care provider headquartered in New Jersey,division of Envision Healthcare, doing business as Guardian Healthcare, Gem City, and Care Connection of Cincinnati ("Evolution"), for aan estimated purchase price of $327.9$67.8 million. A portion of the purchase price ($51.1 million) was paid to the seller with cash on hand and proceeds from borrowings under our Revolving Credit Facility. The remainder ($16.7 million) was placed into an escrow account in accordance with the closing payment, indemnity and other provisions within the purchase agreement and recorded as restricted cash within our consolidated balance sheet. Corresponding liabilities were also recorded to accrued expenses and other long-term obligations within our consolidated balance sheet related to these contingent consideration arrangements.
Of the total $16.7 million placed into escrow, $1.0 million was set aside for the closing payment adjustment. The closing payment calculated on the acquisition date included estimates for cash, working capital and various other items. Under the purchase agreement, the purchase price was subject to an adjustment for any differences between estimated amounts included in the closing payment and actual amounts at close. The closing payment adjustment, which was finalized during the three-month period ended September 30, 2022, decreased the purchase price by $1.3 million from $67.8 million to $66.5 million. The remaining $15.7 million placed into escrow relates to certain outstanding matters existing as of the acquisition date as well as potential losses the Company may incur for which the seller has an obligation to indemnify the Company. This amount will either be paid to third parties as outstanding matters are resolved or to the seller at certain intervals in the future. As of December 31, 2022, $5.7 million of the $16.7 million has been released from escrow.
We expect $15 million of goodwill recorded for this acquisition to be deductible for income tax purposes over approximately 15 years.
Evolution contributed $29.4 million in net service revenue and an operating loss of cash acquired of $6.7 million.$5.3 million during the year ended December 31, 2022.
The Company has finalized its valuationis in the process of reviewing the fair value of the assets acquired and liabilities assumed. TheDuring the post-acquisition period ended December 31, 2022, total assets acquired decreased by $2.1 million (primarily patient accounts receivable and property and equipment) and total liabilities assumed (specifically, the deferred income tax liability) decreased by $0.3 million as a result of our review. These adjustments, combined with the closing payment adjustment of $1.3 million described above, resulted in a $0.5 million increase in goodwill. Based on the Company's preliminary valuation, which may be revised as additional information becomes available during the measurement period, the total consideration of $327.9$66.5 million has been allocated to assets acquired and liabilities assumed as of the acquisition date as follows (amounts in millions):
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 201976
|
| | | |
| | Amount |
| Patient accounts receivable | $ | 24.5 |
|
| Prepaid expenses | 0.8 |
|
| Other current assets | 0.1 |
|
| Property and equipment | 0.2 |
|
| Intangible assets | 27.2 |
|
| Operating lease right of use assets | 3.4 |
|
| Other assets | 1.1 |
|
| Total assets acquired | 57.3 |
|
| Accounts payable | (14.9 | ) |
| Payroll and employee benefits | (11.7 | ) |
| Accrued expenses | (11.7 | ) |
| Deferred tax liability | (0.9 | ) |
| Operating lease liabilities | (3.4 | ) |
| Total liabilities acquired | (42.6 | ) |
| Net identifiable assets acquired | 14.7 |
|
| Goodwill | 313.2 |
|
| Total estimated consideration | $ | 327.9 |
|
Intangible assets acquired include licenses, certificates of need, acquired names and non-compete agreements. The acquired names and non-compete agreements will be amortized over a weighted-average period of 2.0 and 2.3 years, respectively.
CCH contributed approximately $167.4 million in net service revenue and an operating loss of $5.6 million (inclusive of acquisition and integration costs totaling $14.5 million) during the year ended December 31, 2019.
We expect $278.8 million of goodwill recorded for this acquisition to be deductible for income tax purposes over approximately 15 years.
The following table contains unaudited pro forma condensed consolidated statement of operations information for the years ended December 31, 2019 and 2018 assuming that the CCH acquisition closed on January 1, 2018 (amounts in millions, except per share data):
|
| | | | | | | |
| | For the Year Ended December 31, |
| | 2019 | | 2018 |
| Net service revenue | $ | 1,971.7 |
| | $ | 1,852.8 |
|
| Operating income | 183.8 |
| | 175.7 |
|
| Net income attributable to Amedisys, Inc. | 130.5 |
| | 124.6 |
|
| Basic earnings per share | 4.06 |
| | 3.80 |
|
| Diluted earnings per share | $ | 3.96 |
| | $ | 3.71 |
|
The pro forma information presented above includes adjustments for (i) amortization of identifiable intangible assets, (ii) interest on additional debt required to fund the CCH acquisition, (iii) non-recurring transaction costs and (iv) income taxes based on the Company’s statutory tax rate. This pro forma information is presented for illustrative purposes only and may not be indicative of the results of operations that would have actually occurred. In addition, future results may vary significantly from the results reflected in the pro forma information.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 20192022
| | | | | |
| Amount |
| ASSETS | |
| Patient accounts receivable | $ | 7.6 | |
| Prepaid expenses | 0.2 | |
| Other current assets | 0.1 | |
| Property and equipment | 1.9 | |
| Operating lease right of use assets | 3.2 | |
| Intangible assets (licenses) | 1.3 | |
| Other assets | 0.1 | |
| Total assets acquired | $ | 14.4 | |
| LIABILITIES | |
| Accounts payable | $ | (0.8) | |
| Payroll and employee benefits | (2.7) | |
| Accrued expenses | (2.4) | |
| Operating lease liabilities | (2.8) | |
| Deferred income tax liability | (0.1) | |
| Current portion of long-term obligations | (0.6) | |
| Total liabilities assumed | (9.4) | |
| Net identifiable assets acquired | $ | 5.0 | |
| Goodwill | 61.5 | |
| Total consideration | $ | 66.5 | |
On April 1, 2019,2022, we acquired RoseRock Healthcaretwo home health locations from AssistedCare Home Health, Inc. and RH Homecare Services, LLC, doing business as AssistedCare Home Health and AssistedCare of the Carolinas ("RoseRock"AssistedCare"), an Oklahoma based hospice provider,respectively, for a purchase price of $17.5$24.7 million. TheA portion of the purchase price ($22.2 million) was paid to the seller with cash on hand onand proceeds from borrowings under our Revolving Credit Facility. The remainder ($2.5 million) was placed into an escrow account in accordance with the date ofindemnity provisions within the transaction. purchase agreement and is reflected in restricted cash within our consolidated balance sheet. A corresponding liability was also recorded to other long-term obligations within our consolidated balance sheet related to this contingent consideration arrangement. The $2.5 million will either be paid to third parties or to the seller at certain intervals in the future.
Based on the Company's preliminary valuation, we have recorded goodwill ($15.8 million)of $24.0 million and other intangibles includingof $0.7 million in connection with the acquisition. Intangible assets acquired include licenses ($0.5 million), certificates of need ($0.2 million) and acquired names ($1.0 million) and non-compete agreements ($0.7(less than $0.1 million). The acquired names and non-compete agreement will each be amortized over a weighted-averageweighted average period of 3.0 years. RoseRock contributed approximately $6.8 million in net service revenue and $0.8 million in operating income for the year ended December 31, 2019. one year.
We expect the entire amount of goodwill recorded for this acquisition to be deductible for income tax purposes over approximately 15 years.
2018AssistedCare contributed $6.1 million in net service revenue and operating income of $0.8 million during the year ended December 31, 2022.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
2021 Acquisitions
Home Health Division
On MarchMay 1, 2018,2021, we acquired the regulatory assets of Christian Care at Home which provideda home health services to the state of Kentuckyprovider in Randolph County, North Carolina for a total purchase price of $2.3$2.5 million. The purchase price was paid with cash on hand on the date of the transaction. We recorded goodwill ($2.1 million)of $2.4 million and other intangibles - certificate(certificate of need ($0.2 million)need) of $0.1 million in connection with the acquisition. We expect the entire amount of goodwill recorded for this acquisition to be deductible for income tax purposes over approximately 15 years.
Personal Care Division
On MayJuly 1, 2018,2021, we acquired the assets of East Tennessee Personal Care Services which ownedVisiting Nurse Association ("VNA"), a home health and operated 1 personal-care care center servicing the state of Tennesseehospice provider with locations in Nebraska and Iowa for a total purchase price of $2.0 million (subject to certain adjustments, of which $0.2 million was placed in a promissory note to be paid over 24 months, subject to any offsets or withholds for indemnification purposes).$20.1 million. The purchase price was paid with cash on hand on the date of the transaction. We recorded goodwill ($1.9 million)of $19.7 million and other intangibles - non-compete agreements ($0.1 million)(licenses) of $0.4 million in connection with the acquisition. We expect the entire amount of goodwill recorded for this acquisition to be deductible for income tax purposes over approximately 15 years.
On October 1, 2018,July 12, 2021, we acquired the regulatory assets of Bring Care Home which serviced the state of Massachusettsa home health provider in New York for a total purchase price of $5.7 million (subject to certain adjustments, of which $0.6 million was placed in a promissory note to be paid over 24 months, subject to any offsets or withholds for indemnification purposes).$1.5 million. The purchase price was paid with cash on hand on the date of the transaction. We recorded goodwill ($5.5 million)of $1.4 million and other intangibles - non-compete agreements ($0.2 million)(certificate of need) of $0.1 million in connection with the acquisition.
On August 1, 2021, we acquired Contessa, a leader in hospital-at-home and skilled nursing facility at-home services for an estimated purchase price of $240.7 million, net of cash acquired. The Contessa purchase price included estimates for cash, working capital and other items. Under the purchase agreement, the purchase price was subject to a closing payment adjustment for any differences between estimated amounts included in the closing payment and actual amounts at close. The closing payment adjustment, which was finalized during the three-month period ended December 31, 2021, increased the purchase price by $0.6 million from $240.7 million to $241.3 million.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
The Company has finalized its valuation of the assets acquired, liabilities assumed and noncontrolling interests. During the year ended December 31, 2022, the deferred income tax liability was adjusted downward by $2.8 million resulting in a $2.8 million decrease in goodwill. The total consideration of $241.3 million has been allocated to assets acquired, liabilities assumed and noncontrolling interests as of the acquisition date as follows (amounts in millions):
| | | | | |
| Amount |
| ASSETS | |
| Patient accounts receivable | $ | 1.5 | |
| Prepaid expenses | 0.3 | |
| Other current assets | 0.1 | |
| Property and equipment | 0.3 | |
| Operating lease right of use assets | 0.8 | |
| Intangible assets | 54.3 | |
| Other assets | 3.1 | |
| Total assets acquired | $ | 60.4 | |
| LIABILITIES AND EQUITY | |
| Accounts payable | $ | (0.1) | |
| Payroll and employee benefits | (0.6) | |
| Accrued expenses | (3.4) | |
| Operating lease liabilities | (0.8) | |
| Deferred income tax liability | (0.3) | |
| Current portion of long-term obligations | (0.9) | |
| Other long-term obligations | (0.2) | |
| Total liabilities assumed | (6.3) | |
| Noncontrolling interests | (43.9) | |
| Total equity assumed | (43.9) | |
| Total liabilities and equity assumed | $ | (50.2) | |
| Net identifiable assets acquired | $ | 10.2 | |
| Goodwill | 231.1 | |
| Total consideration | $ | 241.3 | |
Intangible assets acquired include acquired names ($28.3 million), technology ($19.8 million) and non-compete agreements ($6.2 million). The non-compete agreements will be amortized over a weighted-average period of 2.0 years, and the technology will be amortized over a weighted-average period of 7.0 years. The fair value of noncontrolling interest ($43.9 million) was determined using an income approach.
We do not expect any of the entire amount of goodwill recorded for this acquisition to be deductible for income tax purposes over approximately 15 years.purposes.
Contessa contributed $18.5 million in net service revenue and an operating loss of $39.1 million (inclusive of technology intangibles amortization totaling $3.0 million) during the year ended December 31, 2022 and $3.5 million in net service revenue and an operating loss of $10.3 million (inclusive of technology intangibles amortization totaling $1.2 million) during the year ended December 31, 2021.
4.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
5. GOODWILL AND OTHER INTANGIBLE ASSETS, NET
Goodwill
During 2019, 20182022, 2021 and 2017,2020, we did not record any goodwill impairment charges as a result of our annual impairment test and none of the goodwill associated with our various reporting units was considered at risk of impairmentimpaired as of October 31st of each respective year (the date of our annual goodwill impairment test). Since the date of our last annual goodwill impairment test, there have been no material developments, events, changes in operating performance or other circumstances that would cause management to believe it is more likely than not that the fair value of any of our reporting units would be less than their carrying amounts.
The following table summarizes the activity related to our goodwill for 20192022 and 20182021 (amounts in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Goodwill |
| Home Health | | Hospice | | Personal Care | | High Acuity Care | | Total |
| Balances at December 31, 2020 (1) | $ | 90.4 | | | $ | 799.2 | | | $ | 43.1 | | | $ | — | | | $ | 932.7 | |
| Additions | 27.8 | | | 1.7 | | | — | | | 233.9 | | | 263.4 | |
| Balances at December 31, 2021 | 118.2 | | | 800.9 | | | 43.1 | | | 233.9 | | | 1,196.1 | |
| Additions | 85.6 | | | — | | | — | | | 8.5 | | | 94.1 | |
| Adjustments (2) | — | | | — | | | — | | | (2.8) | | | (2.8) | |
| Balances at December 31, 2022 | $ | 203.8 | | | $ | 800.9 | | | $ | 43.1 | | | $ | 239.6 | | | $ | 1,287.4 | |
|
| | | | | | | | | | | | | | | |
| | Goodwill |
| | Home Health | | Hospice | | Personal Care | | Total |
| Balances at December 31, 2017 (1) | $ | 85.0 |
| | $ | 199.3 |
| | $ | 35.6 |
| | $ | 319.9 |
|
| Additions | 2.1 |
| | — |
| | 7.5 |
| | 9.6 |
|
| Balances at December 31, 2018 | 87.1 |
| | 199.3 |
| | 43.1 |
| | 329.5 |
|
| Additions | — |
| | 329.0 |
| | — |
| | 329.0 |
|
| Balances at December 31, 2019 | $ | 87.1 |
| | $ | 528.3 |
| | $ | 43.1 |
| | $ | 658.5 |
|
| |
(1) | Net of prior years' accumulated impairment losses of $733.7 million, which is inclusive of write-offs related to the sale and closure of care centers. |
(2)The Company finalized its valuation of the assets acquired, liabilities assumed and noncontrolling interests in connection with the acquisition of Contessa on August 1, 2021. See Note 4 – Acquisitions for additional information.
Other Intangible Assets, Net
During 2017,2022 and 2021, we recorded a non-cashdid not record any impairment charges related to our other intangible assets impairment charge of $1.3 million related to care centers that were closed or consolidated during 2017 as discussed in Note 13 - Exit and Restructuring Activities. During 2019, we recorded a non-cash other intangible assets impairment charge of $1.5 million related to acquired names which are no longer in use or are associated with care centers that were closed.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
The following table summarizes the activity related to our other intangible assets, net for 20192022 and 20182021 (amounts in millions):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Other Intangible Assets, Net |
| Certificates of Need and Licenses | | Acquired
Names -Unamortizable | | Acquired
Names -Amortizable | | Non-Compete
Agreements (3) | | Technology (3) | | Total |
| Balances at December 31, 2020 (1) | $ | 47.0 | | | $ | 13.9 | | | $ | 5.5 | | | $ | 7.8 | | | $ | — | | | $ | 74.2 | |
| Additions | 0.8 | | | 28.3 | | | — | | | 6.2 | | | 20.2 | | | 55.5 | |
| Reclass to amortizable intangible | — | | | (6.6) | | | 6.6 | | | — | | | — | | | — | |
| Amortization (2) | (0.7) | | | — | | | (9.0) | | | (7.6) | | | (1.2) | | | (18.5) | |
| Balances at December 31, 2021 | 47.1 | | | 35.6 | | | 3.1 | | | 6.4 | | | 19.0 | | | 111.2 | |
| Additions | 2.4 | | | — | | | — | | | — | | | 1.1 | | | 3.5 | |
| Amortization (2) | (2.8) | | | — | | | (3.1) | | | (4.6) | | | (3.0) | | | (13.5) | |
| Balances at December 31, 2022 | $ | 46.7 | | | $ | 35.6 | | | $ | — | | | $ | 1.8 | | | $ | 17.1 | | | $ | 101.2 | |
|
| | | | | | | | | | | | | | | | | | | |
| | Other Intangible Assets, Net |
| | Certificates of Need and Licenses | | Acquired Names -Unamortizable | | Acquired Names -Amortizable (3) | | Non-Compete Agreements (3) | | Total |
| Balances at December 31, 2017 (2) | $ | 23.7 |
| | $ | 19.6 |
| | $ | — |
| | $ | 2.8 |
| | $ | 46.1 |
|
| Additions | 0.2 |
| | — |
| | — |
| | 0.3 |
| | 0.5 |
|
| Amortization | — |
| | — |
| | — |
| | (2.5 | ) | | (2.5 | ) |
| Balances at December 31, 2018 | 23.9 |
| | 19.6 |
| | — |
| | 0.6 |
| | 44.1 |
|
| Additions | 13.7 |
| | — |
| | 10.0 |
| | 5.2 |
| | 28.9 |
|
| Write-off (1) | — |
| | (1.5 | ) | | — |
| | — |
| | (1.5 | ) |
| Amortization | — |
| | — |
| | (4.4 | ) | | (2.4 | ) | | (6.8 | ) |
| Balances at December 31, 2019 | $ | 37.6 |
| | $ | 18.1 |
| | $ | 5.6 |
| | $ | 3.4 |
| | $ | 64.7 |
|
(2)Amortization of certificates of need and licenses is related to care centers that were closed during 2021 and 2022.
(3)The weighted average remaining amortization period of our amortizable non-compete agreements and technology is 0.6 years and 5.6 years, respectively.
| |
(1) | Write-off of intangible assets related to our annual impairment analysis as discussed above. |
| |
(2) | Net of prior years' accumulated amortization of $5.0 million for non-compete agreements. |
| |
(3) | The weighted average remaining amortization period of our amortizable acquired names and non-compete agreements is 1.2 years and 1.5 years, respectively. |
AMEDISYS, INC. AND SUBSIDIARIES
See Note 3 – Acquisitions for further details on additions to goodwill and other intangible assets, net.NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
The estimated aggregate amortization expense related to intangible assets for each of the five succeeding years is as follows (amounts in millions):
| | | | | |
| Intangible Asset Amortization |
| 2023 | $ | 4.8 | |
| 2024 | 3.0 | |
| 2025 | 3.0 | |
| 2026 | 3.0 | |
| 2027 | 3.0 | |
| $ | 16.8 | |
|
| | | |
| | Intangible Asset Amortization |
| 2020 | $ | 7.3 |
|
| 2021 | 1.5 |
|
| 2022 | 0.2 |
|
| 2023 | — |
|
| 2024 | — |
|
| | $ | 9.0 |
|
On February 10, 2023, we signed a definitive agreement to sell our personal care business (excluding the Florida operations) for a purchase price of $50 million. The divestment is expected to close during the second quarter of 2023.
The carrying amount of the assets and liabilities associated with our personal care reporting unit (which approximate fair value) included in our consolidated balance sheets are as follows (amounts in millions):
| | | | | | | | | | | |
| As of December 31, 2022 | | As of December 31, 2021 |
| ASSETS | | | |
| Current assets: | | | |
| Patient accounts receivable | $ | 9.6 | | | $ | 8.7 | |
| Prepaid expenses | 0.1 | | | 0.1 | |
| Other current assets | 9.7 | | | 8.8 | |
| Property and equipment | 0.1 | | | 0.2 | |
| Operating lease right of use assets | 2.5 | | | 2.8 | |
| Goodwill | 43.1 | | | 43.1 | |
| Intangible assets | — | | | 1.8 | |
| Total assets | $ | 55.4 | | | $ | 56.7 | |
| LIABILITIES | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 0.4 | | | $ | 0.3 | |
| Payroll and employee benefits | 0.6 | | | 2.5 | |
| Accrued expenses | 1.8 | | | 0.1 | |
| Current portion of operating lease liabilities | 0.6 | | | 0.7 | |
| Total current liabilities | 3.4 | | | 3.6 | |
| Operating lease liabilities, less current portion | 1.9 | | | 2.2 | |
| Total liabilities | $ | 5.3 | | | $ | 5.8 | |
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 20192022
5.7. DETAILS OF CERTAIN BALANCE SHEET ACCOUNTS
Additional information regarding certain balance sheet accounts is presented below (amounts in millions):
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| Other current assets: | | | |
| Payroll tax escrow | $ | 7.6 | | | $ | 7.9 | |
| Income tax receivable | 8.8 | | | 8.2 | |
| Due from joint ventures | 3.6 | | | 3.9 | |
| Other | 6.4 | | | 5.6 | |
| $ | 26.4 | | | $ | 25.6 | |
| Other assets: | | | |
| Workers’ compensation deposits | $ | 0.3 | | | $ | 0.3 | |
| Health insurance deposits | 0.9 | | | 0.9 | |
| Other miscellaneous deposits | 1.0 | | | 1.1 | |
| Indemnity receivable | 13.6 | | | 13.6 | |
| Equity method investments | 40.5 | | | 48.1 | |
| Cost method investments | 20.0 | | | 5.0 | |
| Other | 3.5 | | | 4.0 | |
| $ | 79.8 | | | $ | 73.0 | |
| Accrued expenses: | | | |
| Health insurance | $ | 16.2 | | | $ | 16.2 | |
| Workers’ compensation | 40.6 | | | 40.3 | |
| Florida ZPIC audit, gross liability | — | | | 17.4 | |
| Legal settlements and other audits | 32.1 | | | 27.5 | |
| Charity care | 1.9 | | | 1.4 | |
| Estimated Medicare cap liability | 4.3 | | | 4.5 | |
| Hospice accruals (room and board, general in-patient and other) | 19.1 | | | 23.6 | |
| | | |
| Patient and payor liabilities | 6.7 | | | 6.0 | |
| Accrued contingent consideration | 10.5 | | | — | |
| Accrued interest | 0.2 | | | 8.1 | |
| Other | 5.8 | | | 5.8 | |
| $ | 137.4 | | | $ | 150.8 | |
| Other long-term obligations: | | | |
| Reserve for uncertain tax positions | $ | — | | | $ | 3.4 | |
| Deferred compensation plan liability | 0.6 | | | 1.0 | |
| Accrued contingent consideration | 3.2 | | | — | |
| Other | 1.0 | | | 0.6 | |
| $ | 4.8 | | | $ | 5.0 | |
|
| | | | | | | |
| | As of December 31, |
| | 2019 | | 2018 |
| Other current assets: | | | |
| Payroll tax escrow | $ | 1.5 |
| | $ | 1.5 |
|
| Income tax receivable | 2.0 |
| | 1.6 |
|
| Due from joint ventures | 2.0 |
| | 1.9 |
|
| Other | 2.7 |
| | 2.3 |
|
| | $ | 8.2 |
| | $ | 7.3 |
|
| Other assets: | | | |
| Workers’ compensation deposits | $ | 0.2 |
| | $ | 0.4 |
|
| Health insurance deposits | 0.5 |
| | 0.5 |
|
| Other miscellaneous deposits | 1.0 |
| | 0.8 |
|
| Indemnity receivable | 13.6 |
| | 14.2 |
|
| Equity method investments | 35.7 |
| | 35.1 |
|
| Other | 3.6 |
| | 3.1 |
|
| | $ | 54.6 |
| | $ | 54.1 |
|
| Accrued expenses: | | | |
| Health insurance | $ | 15.8 |
| | $ | 12.4 |
|
| Workers’ compensation | 33.4 |
| | 30.9 |
|
| Florida ZPIC audit, gross liability | 17.4 |
| | 17.4 |
|
| Legal settlements and other audits | 19.0 |
| | 13.0 |
|
| Income tax payable | 0.5 |
| | — |
|
| Charity care | 2.7 |
| | 1.7 |
|
| Estimated Medicare cap liability | 5.7 |
| | 1.7 |
|
| Hospice cost of revenue | 24.4 |
| | 9.9 |
|
| Patient liability | 9.4 |
| | 6.3 |
|
| Other | 8.8 |
| | 6.2 |
|
| | $ | 137.1 |
| | $ | 99.5 |
|
| Other long-term obligations: | | | |
| Reserve for uncertain tax positions | $ | 3.1 |
| | $ | 2.9 |
|
| Deferred compensation plan liability | 1.0 |
| | 1.3 |
|
| Other | 1.8 |
| | 2.0 |
|
| | $ | 5.9 |
| | $ | 6.2 |
|
6.8. LEASES
We determine whether an arrangement is a lease at inception. We have operating leases, primarily for offices and fleet, that expire at various dates over the next nineseven years. We also have finance leases covering certain office equipment that expire at various dates over the next three years. Our leases do not contain any restrictive covenants.
Our office leases generally contain renewal options for periods ranging from one to five years. Because we are not reasonably certain to exercise these renewal options, the options are not considered in determining the lease term, and payments associated with the option years are excluded from lease payments. Our office leases also generally include termination options, which allow for early termination of the lease after the first one to three years. Because we are not reasonably certain to exercise these termination options, the options are not considered in determining the lease term; payments for the full lease term are included in lease payments. Our office leases do not contain any material residual value guarantees.
Our fleet leases include a term of 367 days with monthly renewal options thereafter. Our fleet leases also include terminal rental adjustment clauses (“TRAC”), which provide for a final rental payment adjustment at the end of the lease, typically based on
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
the amount realized from the sale of the vehicle. The TRAC is structured such that it will almost always result in a significant payment by us to the lessor if the renewal option is not exercised. Based on the significance of the TRAC adjustment at the initial lease
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
expiration, we believe that it is reasonably certain that we will exercise the monthly renewal options; therefore, the renewal options are considered in determining the lease term, and payments associated with the renewal options are included in lease payments.
For our fleet and office equipment leases, we use the implicit rate in the lease as the discount rate. For our office leases, the implicit rate is typically not available, so we use our incremental borrowing rate as the discount rate. Our lease agreements include both lease and non-lease components. We have elected the practical expedient that allows us to not separate lease and non-lease components for all of our leases.
Payments due under our operating and finance leases include fixed payments as well as variable payments. For our office leases, variable payments include amounts for our proportionate share of operating expenses, utilities, property taxes, insurance, common area maintenance and other facility-related expenses. For our vehicle and equipment leases, variable payments consist of sales tax.
The components of lease cost for the yearyears ended December 31, 20192022 and 2021 are as follows (amounts in millions):
| | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 |
| Operating lease cost: | | | |
| Operating lease cost | $ | 43.9 | | | $ | 40.3 | |
| Impairment of operating lease ROU assets | 2.1 | | | 0.1 | |
| Total operating lease cost | 46.0 | | | 40.4 | |
| | | |
| Finance lease cost: | | | |
| Loss on termination | 0.5 | | | — | |
| Amortization of ROU assets | 1.8 | | | 2.0 | |
| Interest on lease liabilities | 0.1 | | | 0.1 | |
| Total finance lease cost | 2.4 | | | 2.1 | |
| | | |
| Variable lease cost | 3.4 | | | 3.3 | |
| Short-term lease cost | — | | | — | |
| Total lease cost | $ | 51.8 | | | $ | 45.8 | |
|
| | | |
| | For the Year Ended December 31, 2019 |
| Operating lease cost: | |
| Operating lease cost | $ | 35.0 |
|
| Impairment of operating lease ROU assets | 0.9 |
|
| Total operating lease cost | 35.9 |
|
| | |
| Finance lease cost: | |
| Amortization of ROU assets | 1.7 |
|
| Interest on lease liabilities | 0.2 |
|
| Total finance lease cost | 1.9 |
|
| | |
| Variable lease cost | 2.6 |
|
| Short-term lease cost | 0.2 |
|
| | |
| Total lease cost | $ | 40.6 |
|
Amounts reported in the consolidated balance sheetsheets as of December 31, 20192022 and 2021 for our operating leases are as follows (amounts in millions):
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| Operating lease ROU assets | $ | 102.9 | | | $ | 101.3 | |
| | | |
| Current portion of operating lease liabilities | 33.5 | | | 31.2 | |
| Operating lease liabilities, less current portion | 69.5 | | | 69.3 | |
| Total operating lease liabilities | $ | 103.0 | | | $ | 100.5 | |
|
| | | |
| | December 31, 2019 |
| Operating lease ROU assets | $ | 84.8 |
|
| | |
| Current portion of operating lease liabilities | 27.8 |
|
| Operating lease liabilities, less current portion | 56.1 |
|
| Total operating lease liabilities | $ | 83.9 |
|
Amounts reported in the consolidated balance sheetsheets as of December 31, 20192022 and 2021 for finance leases are included in the table below. The finance lease ROU assets are recorded within property and equipment, net of accumulated depreciation within our consolidated balance sheet.sheets. The finance lease liabilities are recorded within current portion of long-term obligations and long-term obligations, less current portion within our consolidated balance sheet.sheets.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 20192022
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| Finance lease ROU assets | $ | 4.1 | | | $ | 4.5 | |
| Accumulated amortization | (1.8) | | | (2.8) | |
| Finance lease ROU assets, net | $ | 2.3 | | | $ | 1.7 | |
| | | |
| Current installments of obligations under finance leases | $ | 1.2 | | | $ | 0.9 | |
| Long-term portion of obligations under finance leases | 1.1 | | | 0.7 | |
| Total finance lease liabilities | $ | 2.3 | | | $ | 1.6 | |
|
| | | |
| | December 31, 2019 |
| Finance lease ROU assets | $ | 5.2 |
|
| Accumulated amortization | (1.8 | ) |
| Finance lease ROU assets, net | 3.4 |
|
| | |
| Current installments of obligations under finance leases | 1.7 |
|
| Long-term portion of obligations under finance leases | 1.7 |
|
| Total finance lease liabilities | $ | 3.4 |
|
Supplemental cash flow information and non-cash activity related to our leases are as follows (amounts in millions):
| | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 |
| Cash paid for amounts included in the measurement of lease liabilities and ROU assets: | | | |
| Operating cash flow from operating leases | $ | (44.4) | | | $ | (39.7) | |
| Financing cash flow from finance leases | (1.5) | | | (2.0) | |
| | | |
| ROU assets obtained in exchange for lease obligations: | | | |
| Operating leases | $ | 45.1 | | | $ | 46.1 | |
| Finance leases | 2.1 | | | 0.9 | |
| | | |
| Reductions to ROU assets resulting from reductions to lease obligations: | | | |
| Operating leases | $ | (4.2) | | | $ | (1.7) | |
| Finance leases | (0.6) | | | — | |
|
| | | |
| | For the Year Ended December 31, 2019 |
| Cash paid for amounts included in the measurement of lease liabilities and ROU assets: | |
| Operating cash flow from operating leases | $ | (35.8 | ) |
| Financing cash flow from finance leases | (1.7 | ) |
| | |
| ROU assets obtained in exchange for lease obligations: | |
| Operating leases | 116.0 |
|
| Finance leases | 2.9 |
|
| | |
| Reductions to ROU assets resulting from reductions to lease obligations: | |
| Operating leases | (1.7 | ) |
| Finance leases | — |
|
Amounts disclosed for ROU assets obtained in exchange for lease obligations include amounts added to the carrying amount of ROU assets resulting from lease modifications and reassessments.
Weighted average remaining lease terms and discount rates for our leases as of December 31, 20192022 and 2021 are as follows:
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| Weighted average remaining lease term (years): | | | |
| Operating leases | 3.5 | | 3.7 |
| Finance leases | 2.1 | | 1.7 |
| | | |
| Weighted average discount rate: | | | |
| Operating leases | 3.4 | % | | 2.7 | % |
| Finance leases | 5.3 | % | | 5.2 | % |
|
| | |
| Years |
Weighted average remaining lease term: | |
Operating leases | 3.9 |
|
Finance leases | 2.1 |
|
| Rate |
Weighted average discount rate: | |
Operating leases | 3.9 | % |
Finance leases | 5.3 | % |
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 20192022
Maturities of lease liabilities as of December 31, 20192022 are as follows (amounts in millions):
| | | | | | | | | | | |
| Operating
Leases | | Finance
Leases |
| 2023 | $ | 36.1 | | | $ | 1.2 | |
| 2024 | 30.9 | | | 0.9 | |
| 2025 | 20.8 | | | 0.3 | |
| 2026 | 12.7 | | | — | |
| 2027 | 6.7 | | | — | |
| Thereafter | 2.4 | | | — | |
| Total undiscounted lease payments | 109.6 | | | 2.4 | |
| Less: Imputed interest | (6.6) | | | (0.1) | |
| Total lease liabilities | $ | 103.0 | | | $ | 2.3 | |
|
| | | | | | | |
| | Operating Leases | | Finance Leases |
| 2020 | $ | 30.2 |
| | $ | 1.9 |
|
| 2021 | 24.3 |
| | 1.4 |
|
| 2022 | 15.0 |
| | 0.3 |
|
| 2023 | 9.2 |
| | — |
|
| 2024 | 5.0 |
| | — |
|
| Thereafter | 7.0 |
| | — |
|
| Total undiscounted lease payments | 90.7 |
| | 3.6 |
|
| Less: Imputed interest | (6.8 | ) | | (0.2 | ) |
| Total lease liabilities | $ | 83.9 |
| | $ | 3.4 |
|
7.9. LONG-TERM OBLIGATIONS
Long-term debt consists of the following for the periods indicated (amounts in millions):
|
| | | | | | | |
| | As of December 31, |
| | 2019 | | 2018 |
| $175.0 million Term Loan; interest rate at Base Rate plus Applicable Rate or Eurodollar Rate plus the Applicable Rate (3.3% at December 31, 2019); due February 4, 2024 | $ | 171.7 |
| | $ | — |
|
| $550.0 million Revolving Credit Facility; interest only payments; interest rate at Base Rate plus Applicable Rate or Eurodollar Rate plus the Applicable Rate (5.3% at December 31, 2019); due February 4, 2024 | 70.0 |
| | 7.5 |
|
| Promissory notes | 0.6 |
| | 1.1 |
|
| Finance leases | 3.4 |
| | 2.3 |
|
| Principal amount of long-term obligations | 245.7 |
| | 10.9 |
|
| Deferred debt issuance costs | (3.5 | ) | | (3.5 | ) |
| | 242.2 |
| | 7.4 |
|
| Current portion of long-term obligations | (9.9 | ) | | (1.6 | ) |
| Total | $ | 232.3 |
| | $ | 5.8 |
|
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| $450.0 million Term Loan; interest rate at Base Rate plus Applicable Rate or Eurodollar Rate plus Applicable Rate (5.9% at December 31, 2022); due July 30, 2026 | $ | 435.9 | | | $ | 447.2 | |
| $550.0 million Revolving Credit Facility; interest only payments; interest rate at Base Rate plus Applicable Rate or Eurodollar Rate plus Applicable Rate; due July 30, 2026 | — | | | — | |
| Promissory notes | 0.2 | | | 0.8 | |
| Finance leases | 2.3 | | | 1.6 | |
| Principal amount of long-term obligations | 438.4 | | | 449.6 | |
| Deferred debt issuance costs | (3.5) | | | (4.5) | |
| 434.9 | | | 445.1 | |
| Current portion of long-term obligations | (15.5) | | | (13.0) | |
| Long-term obligations, less current portion | $ | 419.4 | | | $ | 432.1 | |
Maturities of debt as of December 31, 20192022 are as follows (amounts in millions):
|
| | | |
| | |
| | Long-term obligations |
| 2020 | $ | 10.0 |
|
| 2021 | 10.1 |
|
| 2022 | 9.0 |
|
| 2023 | 82.0 |
|
| 2024 | 134.6 |
|
| | $ | 245.7 |
|
| | | | | |
| |
| Long-term
obligations |
| 2023 | $ | 15.5 | |
| 2024 | 23.3 | |
| 2025 | 22.7 | |
| 2026 | 376.9 | |
| 2027 | — | |
| $ | 438.4 | |
Credit Agreement
On June 29, 2018, we entered into our Amended and Restated Credit Agreement ("Credit(the "Credit Agreement") that provideswhich provided for a senior secured revolving credit facility in an initial aggregate principal amount of up to $550.0 million (the "Revolving Credit Facility"). The Revolving Credit Facility providesprovided for and includesincluded within its $550.0 million limit a $25.0 million swingline facility and commitments for up to $60.0 million in letters of credit. Upon lender approval, we maycould increase the aggregate loan amount under the Revolving Credit Facility by either i) $125.0 million or ii)plus an unlimited amount subject to a leverage limit of 0.5x under the maximum allowable consolidated leverage ratio which is currentlywas 3.0x per the Credit Agreement.
The funds available underfinal maturity of the Revolving Credit Facility was June 29, 2023, and there was no mandatory amortization on the outstanding principal balances which were payable in full upon maturity. The Revolving Credit Facility was used to pay off our existing indebtedness under our prior credit agreement, dated as of August 28, 2015 (the "Prior Credit Agreement"), with a principal balance of $127.5 million. The finalprovide
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 20192022
maturity of the Revolving Credit Facility is June 29, 2023 and there is no mandatory amortization on the outstanding principal balances which are payable in full upon maturity. The Revolving Credit Facility may be used to provide ongoing working capital needs and for general corporate purposes of the Company and our subsidiaries, including permitted acquisitions, as defined in the Credit Agreement.
First Amendment to Amended and Restatedthe Credit Agreement
On February 4, 2019, we entered into the First Amendment to the Credit Agreement (as amended by the First Amendment, the “Amended Credit Agreement”). The Amended Credit Agreement providesprovided for a senior secured credit facility in an initial aggregate principal amount of up to $725.0 million, which includesincluded the $550.0 million Revolving Credit Facility under the Credit Agreement, and a term loan facility with a principal amount of up to $175.0 million (the “Term Loan Facility” and collectively with the Revolving Credit Facility, the “Credit Facility”), which was added by the First Amendment.
We borrowed the entire principal amount of the Term Loan Facility on February 4, 2019 in order to fund a portion of the purchase price of the CCHCompassionate Care Hospice ("CCH") acquisition, with the remainder of the purchase price and associated transactional fees and expenses funded by proceeds from the Revolving Credit Facility.
Second Amendment to the Credit Agreement
On July 30, 2021, we entered into the Second Amendment to our Credit Agreement (as amended by the Second Amendment, the "Second Amended Credit Agreement"). The Second Amended Credit Agreement provides for a senior secured credit facility in an initial aggregate principal amount of up to $1.0 billion, which includes the $550.0 million Revolving Credit Facility and a term loan facility with a principal amount of up to $450.0 million (the "Amended Term Loan Facility" and collectively with the Revolving Credit Facility, the "Amended Credit Facility").
Net proceeds from the $450.0 million Amended Term Loan Facility were used to fund the Contessa acquisition.
The loans issued under the Amended Credit Facility bear interest on a per annum basis, at our election, at either: (i) the Base Rate plus the Applicable Rate or (ii) the Eurodollar Rate plus the Applicable Rate. The “Base Rate” means a fluctuating rate per annum equal to the highest of (a) the federal funds rate plus 0.50% per annum, (b) the prime rate of interest established by the Administrative Agent, and (c) the Eurodollar Rate plus 1% per annum. The “Eurodollar Rate” means the quoted rate per annum equal to the London Interbank Offered Rate ("LIBOR") or a comparable successor rate approved by the Administrative Agent for an interest period of one, two, three or six months (as selected by us). The “Applicable Rate” is based on the consolidated leverage ratio and is presented in the table below. As of December 31, 2019,2022, the Applicable Rate is 0.50% per annum for Base Rate Loans and 1.50% per annum for Eurodollar Rate Loans. Our Second Amended Credit Agreement provides for the replacement of LIBOR with the daily or term secured overnight financing rate ("SOFR") whenever LIBOR is discontinued. We are also subject to a commitment fee and letter of credit fee under the terms of the Second Amended Credit Agreement, as presented in the table below.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Pricing Tier | Consolidated Leverage Ratio | | Base Rate Loans | | Eurodollar Rate Loans and Daily Floating LIBOR Rate Loans | | Commitment
Fee | | Letter of
Credit Fee |
| I | > 3.00 to 1.0 | | 1.00 | % | | 2.00 | % | | 0.30 | % | | 1.75 | % |
| II | < 3.00 to 1.0 but > 2.00 to 1.0 | | 0.75 | % | | 1.75 | % | | 0.25 | % | | 1.50 | % |
| III | < 2.00 to 1.0 but > 0.75 to 1.0 | | 0.50 | % | | 1.50 | % | | 0.20 | % | | 1.25 | % |
| IV | < 0.75 to 1.0 | | 0.25 | % | | 1.25 | % | | 0.15 | % | | 1.00 | % |
|
| | | | | | | | | | | | | |
| Pricing Tier | Consolidated Leverage Ratio | | Base Rate Loans | | Eurodollar Rate Loans | | Commitment Fee | | Letter of Credit Fee |
| I | ≥ 3.00 to 1.0 | | 1.00 | % | | 2.00 | % | | 0.35 | % | | 1.75 | % |
| II | < 3.00 to 1.0 but ≥ 2.00 to 1.0 | | 0.75 | % | | 1.75 | % | | 0.30 | % | | 1.50 | % |
| III | < 2.00 to 1.0 but ≥ 0.75 to 1.0 | | 0.50 | % | | 1.50 | % | | 0.25 | % | | 1.25 | % |
| IV | < 0.75 to 1.0 | | 0.25 | % | | 1.25 | % | | 0.20 | % | | 1.00 | % |
The final maturity date of the Amended Credit Facility is February 4, 2024.July 30, 2026. The Revolving Credit Facility will terminate and be due and payable as of the final maturity date. The Amended Term Loan Facility, however, is subject to quarterly amortization of principal in the amount of (i) 0.625% for the period commencing on February 4, 2019July 30, 2021 and ending on March 31, 2020,September 30, 2023, and (ii) 1.250% for the period commencing on April 1, 2020 and ending on March 31, 2023, and (iii) 1.875% for the period commencing on AprilOctober 1, 2023 and ending on February 4, 2024.July 30, 2026. The remaining balance of the Amended Term Loan Facility must be paid upon the final maturity date. In addition to the scheduled amortization of the Amended Term Loan Facility, and subject to customary exceptions and reinvestment rights, we are required to prepay the Amended Term Loan Facility first and the Revolving Credit Facility second with 100% of all net cash proceeds received by any loan party or any subsidiary thereof in connection with (a) any asset sale or disposition where such loan party receives net cash proceeds in excess of $5 million or (b) any debt issuance that is not permitted under the Second Amended Credit Agreement.
The Second Amended Credit Agreement requires maintenance of two financial covenants: (i) a consolidated leverage ratio of funded indebtedness to Earnings Before Interest, Taxes, Depreciation and Amortization ("EBITDA"), as defined in the Second Amended Credit Agreement, and (ii) a consolidated interest coverage ratio of EBITDA to cash interest charges, as defined in the Second Amended Credit Agreement. Each of these covenants is calculated over rolling four-quarter periods and also is subject to certain exceptions and baskets. The Second Amended Credit Agreement also contains customary covenants,
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
including, but not limited to, restrictions on: incurrence of liens, incurrence of additional debt, sales of assets and other fundamental corporate changes, investments and declarations of dividends. These covenants contain customary exclusions and baskets as detailed in the Second Amended Credit Agreement. In connection with our entry into the Second Amended Credit Agreement during the year ended December 31, 2021, we recorded $0.8$2.8 million in deferred debt issuance costs as long-term obligations, less current portion within our consolidated balance sheet during the year ended December 31, 2019.sheet.
The Revolving Credit Facility is guaranteed by substantially all of our wholly-owned direct and indirect subsidiaries. The Second Amended Credit Agreement requires at all times that we (i) provide guarantees from wholly-owned subsidiaries that in the aggregate represent not less than 95% of our consolidated net revenues and adjusted EBITDA from all wholly-owned subsidiaries and (ii) provide guarantees from subsidiaries that in the aggregate represent not less than 70% of consolidated adjusted EBITDA, subject to certain exceptions.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
Our weighted average interest rate for borrowings under our $175.0 millionAmended Term Loan Facility was 3.8%3.2% for the period February 4, 2019 toyear ended December 31, 2019.2022 and 1.6% for the year ended December 31, 2021. Our weighted average interest rate for borrowings under our $550.0 million Revolving Credit Facility was 4.0%3.4% for the periodyear ended December 31, 20192022 and 3.8%1.9% for the period June 29, 2018 toyear ended December 31, 2018.2021.
As of December 31, 2019,2022, our consolidated leverage ratio was 1.0,1.7, our consolidated interest coverage ratio was 17.211.6 and we are in compliance with our covenants under the Second Amended Credit Agreement. In the event we are not in compliance with our debt covenants in the future, we would pursue various alternatives in an attempt to successfully resolve the non-compliance, which might include, among other things, seeking debt covenant waivers or amendments.
As of December 31, 2019,2022, our availability under our $550.0 million Revolving Credit Facility was $449.8$520.4 million as we have $70.0 millionno outstanding in borrowings and $30.2$29.6 million outstanding in letters of credit.
Joinder AgreementAgreements
In connection with the CCH acquisition, we entered into a Joinder Agreement, dated as of February 4, 2019 (the “CCH Joinder”), pursuant to which CCH and its subsidiaries were made parties to, and became subject to the terms and conditions of, the Amended Credit Agreement (now the Second Amended Credit Agreement), the Amended and Restated Security Agreement, dated as of June 29, 2018 (the “Amended and Restated Security Agreement”), and the Amended and Restated Pledge Agreement, dated as of June 29, 2018. Pursuant2018 (the “Amended and Restated Pledge Agreement”). In connection with the AseraCare acquisition, we entered into a Joinder Agreement, dated as of June 12, 2020, pursuant to which the AseraCare entities were made parties to, and became subject to the Joinder,terms and conditions of, the Amended Credit Agreement (now the Second Amended Credit Agreement), the Amended and Restated Security Agreement and the Amended and Restated Pledge Agreement (the “AseraCare Joinder"). In connection with the Contessa acquisition and the Second Amendment, we entered into a Joinder Agreement, dated as of September 3, 2021, pursuant to which Contessa and its subsidiaries and Asana, which we acquired on January 1, 2020, and its subsidiaries were made parties to, and became subject to the terms and conditions of, the Second Amended Credit Agreement, the Amended and Restated Security Agreement and the Amended and Restated Pledge Agreement (the “Contessa and Asana Joinder,” and together with the CCH Joinder and the AseraCare Joinder, the “Joinders”).
Pursuant to the Joinders, the Amended and Restated Security Agreement and the Amended and Restated Pledge Agreement, CCH and its subsidiaries, the AseraCare entities, Contessa and its subsidiaries and Asana and its subsidiaries granted in favor of the Administrative Agent a first lien security interest in substantially all of their personal property assets and pledged to the Administrative Agent each of their respective subsidiaries’subsidiaries' issued and outstanding equity interests. CCH and its subsidiaries, the AseraCare entities, Contessa and its subsidiaries and Asana and its subsidiaries also guaranteed our obligations, whether now existing or arising after the respective effective datedates of the Joinder,Joinders, under the Second Amended Credit Agreement pursuant to the terms of the JoinderJoinders and the Second Amended Credit Agreement.
Promissory Notes
Our outstanding promissory notes outstandingnote totaling $0.2 million, obtained through the acquisition of $0.6 million, issued in conjunction with acquisitions and software licenses, bearContessa on August 1, 2021, bears an interest rates ranging from 4.3% to 7.0%rate of 6.5%.
Finance Leases
Our outstanding finance leases outstanding of $3.4totaling $2.3 million relate to leased equipment and bear interest rates ranging from 5.2%2.1% to 14.3%5.3%.
8.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
10. INCOME TAXES
Income taxes attributable to continuing operations consist of the following (amounts in millions):
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Current income tax expense/(benefit): | | | | | |
| Federal | $ | 12.2 | | | $ | 20.3 | | | $ | 41.6 | |
| State and local | 7.0 | | | 5.2 | | | 10.6 | |
| 19.2 | | | 25.5 | | | 52.2 | |
| Deferred income tax expense/(benefit): | | | | | |
| Federal | 20.4 | | | 35.9 | | | (22.5) | |
| State and local | 2.9 | | | 8.7 | | | (4.1) | |
| 23.3 | | | 44.6 | | | (26.6) | |
| Income tax expense | $ | 42.5 | | | $ | 70.1 | | | $ | 25.6 | |
|
| | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Current income tax expense/(benefit): | | | | | |
| Federal | $ | 24.2 |
| | $ | 16.4 |
| | $ | (2.0 | ) |
| State and local | 4.8 |
| | 2.1 |
| | (0.1 | ) |
| | 29.0 |
| | 18.5 |
| | (2.1 | ) |
| Deferred income tax expense/(benefit): | | | | | |
| Federal | 9.5 |
| | 14.5 |
| | 51.2 |
|
| State and local | 4.0 |
| | 5.8 |
| | 1.0 |
|
| | 13.5 |
| | 20.3 |
| | 52.2 |
|
| Income tax expense | $ | 42.5 |
| | $ | 38.8 |
| | $ | 50.1 |
|
Total income tax expense for the years ended December 31, 2019, 20182022, 2021 and 20172020 was allocated as follows (amounts in millions):
|
| | | | | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Income from continuing operations | $ | 42.5 |
| | $ | 38.8 |
| | $ | 50.1 |
|
| Interest expense | 0.3 |
| | 0.1 |
| | — |
|
| Stockholders’ equity | — |
| | — |
| | (0.3 | ) |
| Goodwill | 0.9 |
| | — |
| | — |
|
| | $ | 43.7 |
| | $ | 38.9 |
| | $ | 49.8 |
|
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Income from continuing operations | $ | 42.5 | | | $ | 70.1 | | | $ | 25.6 | |
| Interest expense | (0.7) | | | 0.1 | | | 0.2 | |
| Goodwill | (2.7) | | | 3.1 | | | — | |
| Tax expense recorded to additional paid-in-capital | 1.5 | | | — | | | — | |
| Total | $ | 40.6 | | | $ | 73.3 | | | $ | 25.8 | |
A reconciliation of significant differences between the reported amount of income tax expense and the expected amount of income tax expense that would result from applying the U.S. federal statutory income tax rate of 21% in 2019 and 2018 and 35% in 2017 to income before income taxes is as follows:
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Income tax expense at U.S. federal statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| State and local income taxes, net of federal income tax benefit (1) | 5.6 | | | 5.0 | | | 2.4 | |
| Excess tax benefits from share-based compensation (1) | 0.3 | | | (2.1) | | | (12.7) | |
| Non-deductible executive compensation | 0.8 | | | 1.2 | | | 2.1 | |
| Unrecognized tax benefits (2) | (1.7) | | | — | | | — | |
| Other items, net (3) | 0.5 | | | (0.1) | | | (0.6) | |
| Income tax expense | 26.5 | % | | 25.0 | % | | 12.2 | % |
|
| | | | | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Income tax expense at U.S. federal statutory rate (1) | 21.0 | % | | 21.0 | % | | 35.0 | % |
| State and local income taxes, net of federal income tax benefit | 4.8 |
| | 4.8 |
| | 3.8 |
|
| Excess tax benefits from share-based compensation | (1.9 | ) | | (1.6 | ) | | (3.5 | ) |
| Tax rate change (2) | — |
| | — |
| | 26.5 |
|
| Other items, net (3) | 1.0 |
| | 0.2 |
| | 0.2 |
|
| Income tax expense | 24.9 | % | | 24.4 | % | | 62.0 | % |
| |
(1) | On December 22, 2017, H.R. 1, originally known as the Tax Cuts and Jobs Act was enacted, which eliminated the progressive U.S. federal corporate tax rate structure with a maximum corporate tax rate of 35% and replaced it with a flat tax rate of 21%, effective January 1, 2018. |
| |
(2) | According to Accounting Standard Codification ("ASC") 740, Income Taxes, deferred tax assets and liabilities are remeasured to reflect the effects of enacted changes in tax rates at the date of enactment, even though the tax rate changes are not effective until a future period. The Company's remeasurement of its deferred tax assets and liabilities to reflect the enacted reduced tax rate, pursuant to the Tax Cuts and Jobs Act, resulted in a $21.4 million deferred income tax expense during 2017.(2)For the year ended December 31, 2022, the Company recognized $2.7 million of federal uncertain tax positions due to a lapse of the statute of limitations.
AMEDISYS, INC. AND SUBSIDIARIES NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS December 31, 2022 (3)Includes various items such as non-deductible expenses, non-taxable income, tax credits, valuation allowance, uncertain tax positions and return-to-accrual adjustments. |
| |
(3) | Includes various items such as non-deductible expenses, non-taxable income, tax credits, valuation allowance, uncertain tax positions and return-to-accrual adjustments. |
As of December 31, 20192022 and 2018,2021, the Company had income taxes receivable of $2.0$8.8 million and $1.6$8.2 million, respectively, included in other current assets within our consolidated balance sheets.
Deferred tax assets (liabilities) consist of the following components (amounts in millions):
|
| | | | | | | |
| | As of December 31, |
| | 2019 | | 2018 |
| Deferred tax assets: | | | |
| Allowance for doubtful accounts | $ | — |
| | $ | 5.6 |
|
| Accrued payroll & employee benefits | 15.1 |
| | 11.2 |
|
| Workers’ compensation | 9.0 |
| | 8.3 |
|
| Amortization of intangible assets | — |
| | 14.7 |
|
| Share-based compensation | 7.9 |
| | 6.9 |
|
| Compliance matters | 4.8 |
| | 2.2 |
|
| Net operating loss carryforwards | 3.7 |
| | 5.9 |
|
| Tax credit carryforwards | 3.1 |
| | 2.8 |
|
| Other | 0.8 |
| | 0.7 |
|
| Gross deferred tax assets | 44.4 |
| | 58.3 |
|
| Less: valuation allowance | (0.4 | ) | | (0.7 | ) |
| Net deferred tax assets | 44.0 |
| | 57.6 |
|
| Deferred tax liabilities: | | | |
| Property and equipment | (4.3 | ) | | (4.4 | ) |
| Amortization of intangible assets | (0.3 | ) | | — |
|
| Deferred revenue | (13.5 | ) | | (13.5 | ) |
| Investment in partnerships | (3.3 | ) | | (3.1 | ) |
| Other liabilities | (1.2 | ) | | (0.8 | ) |
| Gross deferred tax liabilities | (22.6 | ) | | (21.8 | ) |
| Net deferred tax assets (liabilities) | $ | 21.4 |
| | $ | 35.8 |
|
| | | | | | | | | | | |
| As of December 31, |
| 2022 | | 2021 |
| Deferred tax assets: | | | |
| Accrued payroll & employee benefits | $ | 14.1 | | | $ | 13.2 | |
| Workers’ compensation | 10.6 | | | 10.5 | |
| Share-based compensation | 5.7 | | | 6.2 | |
| Legal & compliance matters | 4.7 | | | 6.2 | |
| Lease liability | 27.8 | | | 27.3 | |
| Deferred social security taxes (1) | — | | | 6.9 | |
| Net operating loss carryforwards | 11.6 | | | 13.6 | |
| Tax credit carryforwards | 2.9 | | | 2.5 | |
| Other assets | 0.2 | | | 0.5 | |
| Gross deferred tax assets | 77.6 | | | 86.9 | |
| Less: valuation allowance | (5.2) | | | (3.3) | |
| Net deferred tax assets | 72.4 | | | 83.6 | |
| Deferred tax liabilities: | | | |
| Property and equipment | (6.6) | | | (8.1) | |
| Amortization of intangible assets | (48.5) | | | (32.3) | |
| Deferred revenue | — | | | (4.5) | |
| Investment in partnerships | (10.0) | | | (10.8) | |
| Right-of-use asset | (27.0) | | | (26.7) | |
| Other liabilities | (0.7) | | | (0.9) | |
| Gross deferred tax liabilities | (92.8) | | | (83.3) | |
| Deferred income taxes | $ | (20.4) | | | $ | 0.3 | |
(1)The CARES Act provided for the deferral of the employer share of social security tax (6.2%), effective for payments due after the enactment date through December 31, 2020. Fifty percent of the deferred payroll taxes were due on December 31, 2021 with the remaining amounts due on December 31, 2022. As of December 31, 2021, the Company utilized itshad a remaining U.S. federal net operating loss ("NOL") carryforwards, research and developmentbalance of deferred social security taxes of $27 million, reflected within our consolidated balance sheets, which was paid in December 2022. For income tax credits and employmentpurposes, the deferred social security taxes are deductible when paid, leaving no remaining deferred tax credits in 2018; however,asset as of December 31, 2019, the Company has $0.1 million of federal NOL carryforwards acquired from the acquisition of CCH.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
As of December 31, 2019,2022, we have state NOLU.S. net operating loss (“NOL”) carryforwards of $73.9$20.9 million that are available to reduce future taxable income and $3.9may be carried forward indefinitely. While the NOL carryforwards are not subject to expiration, the annual NOL amount that is available to offset future taxable income is subject to limitation. The NOL carryforwards were acquired as part of the stock purchase of Contessa on August 1, 2021. Under Section 382 of the Internal Revenue Code of 1986, as amended ("Section 382"), substantial changes in a Company’s ownership may limit the amount of NOL carryforwards that can be utilized annually to offset future taxable income. As a result of the ownership change, the Company determined that there is an annual limitation, pursuant to Section 382, on the amount of NOL carryforwards that may be utilized to offset future taxable income.
As of December 31, 2022, we have state NOL carryforwards of $144.7 million ofthat are available to reduce future taxable income and various state tax credits totaling $3.7 million available to reduce future state income taxes. The state NOL and tax credit carryforwards expire at various times.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
As of December 31, 20192022 and 2018,2021, the valuation allowance for deferred tax assets, which is primarily related to certain state NOLs, and state tax credit carryforwards, was $0.4$5.2 million and $0.7$3.3 million, respectively. The net change in the total valuation allowance for the yearyears ended December 31, 20192022 and 2021 was a decreasean increase of $0.3 million; there was no change$1.9 million and an increase of $3.2 million, respectively. The $1.9 million increase in the total valuation allowance for the year ended December 31, 2018.2022 is due to Contessa's creation of state NOL carryforwards in jurisdictions that require separate company reporting and where the Company does not expect to have sufficient separate company future taxable income available to offset the state NOL carryforwards.
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income in those jurisdictions during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities (including the impact of available carryback and carryforward periods), projected future taxable income and tax-planning strategies in making this assessment. In order to fully realize the deferred tax assets, the Company will need to generate future taxable income before the expiration of the carryforwards governed by the tax code. Based on the current level of pretaxpre-tax earnings, the Company will generate the minimum amount of future taxable income needed to support the realization of the deferred tax assets. As a result, as of December 31, 2019,2022, management believes that it is more likely than not that we will realize the benefits of these deferred tax assets, net of the existing valuation allowances. The amount of the deferred tax asset considered realizable, however, could be reduced in the near term if estimates of future taxable income during the carryforward period are reduced.
Uncertain Tax Positions
We account for uncertain tax positions in accordance with the authoritative guidance for uncertain tax positions. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (amounts in millions):
| | | | For the Years Ended December 31, | | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 | | 2022 | | 2021 | | 2020 |
| Balance at beginning of period | $ | 2.7 |
| | $ | 2.7 |
| | $ | 4.1 |
| Balance at beginning of period | $ | 2.7 | | | $ | 2.7 | | | $ | 2.7 | |
| Additions for tax positions related to current year | — |
| | — |
| | — |
| Additions for tax positions related to current year | — | | | — | | | — | |
| Additions for tax positions related to prior year | — |
| | — |
| | — |
| Additions for tax positions related to prior year | — | | | — | | | — | |
| Reductions for tax positions related to prior years | — |
| | — |
| | — |
| Reductions for tax positions related to prior years | — | | | — | | | — | |
| Lapse of statute of limitations | — |
| | — |
| | (0.3 | ) | Lapse of statute of limitations | (2.7) | | | — | | | — | |
| Change in statutory tax rate (1) | — |
| | — |
| | (1.1 | ) | |
| Settlements | — |
| | — |
| | — |
| Settlements | — | | | — | | | — | |
| Balance at end of period | $ | 2.7 |
| | $ | 2.7 |
| | $ | 2.7 |
| Balance at end of period | $ | — | | | $ | 2.7 | | | $ | 2.7 | |
| |
(1) | The Company's remeasurement of its deferred tax assets and liabilities to reflect the enacted reduced tax rate as a result of the Tax Cuts and Jobs Act resulted in a $1.1 million reduction in its uncertain tax positions recorded in net deferred tax assets at December 31, 2017. |
As of December 31, 2019 and 2018,2021, there iswas $2.7 million of unrecognized tax benefits recorded in other long-term obligations within the consolidated balance sheet that,sheets. During 2022, the statute of limitations lapsed, ultimately removing the uncertainty surrounding the Company's ability to recognize the tax positions, if challenged under audit. As a result, the Company recognized a $2.7 million income tax benefit and corresponding reduction in future periods, would impact our effective tax rate.rate for the period ended December 31, 2022.
We recognized $0.3$0.1 million and $0.1$0.2 million of interest as components of interest expense in connection with our reserve for uncertain tax positions during the years ended December 31, 20192021 and 2018, respectively; we recognized2020, respectively. For the period ended December 31, 2022, the Company recorded a benefit of less than $0.1$0.7 million of interestbenefit as a component of interest expense, in connection with ouras a result of the lapse of the statute of limitations and corresponding release of the reserve for uncertain tax positions during the year ended December 31, 2017. Interestpositions. Accrued interest related to uncertain tax positions included in the consolidated balance sheet at December 31, 2019 and 20182021 was $0.4 million and $0.1 million, respectively.$0.7 million. There was no accrued interest related to uncertain tax positions included in the consolidated balance sheet at December 31, 2022.
We are subject to income taxes in the U.S. and in many individual states, with significant operations in Louisiana, South Carolina, Alabama, Georgia, Massachusetts and Tennessee. We are open to examination in the U.S. and in various individual states for the tax years ended December 31, 2014 through December 31, 2019.2022. We are also open to examination in various states for the years ended 2004 – 20192007 through 2022 resulting from net operating lossesNOLs generated and available for carryforward from those years.
9. CAPITAL STOCK AND SHARE-BASED COMPENSATION
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 20192022
We are authorized by our Certificate of Incorporation to issue 60,000,000 shares of common stock, $0.001 par value and 5,000,000 shares of preferred stock, $0.001 par value. As of December 31, 2019,2022, there were 36,638,02137,891,186 and 32,284,05132,518,278 shares of common stock issued and outstanding, respectively, and 0no shares of preferred stock issued or outstanding. Our Board of Directors is authorized to fix the dividend rights and terms, conversion and voting rights, redemption rights and other privileges and restrictions applicable to our preferred stock.
Share-Based Awards
On March 29, 2018, our Board of Directors and the Compensation Committee approved, subject to stockholder approval, the Amedisys, Inc. 2018 Omnibus Incentive Compensation Plan (the “2018 Plan”). On June 6, 2018, our stockholders approved the 2018 Plan at the Company's annual meeting of stockholders. The 2018 Plan replaces our 2008 Omnibus Incentive Compensation Plan (the “2008 Plan”), which terminated on June 6, 2018 when the stockholders approved the 2018 Plan. The 2018 Plan, as amended to date, authorizes the grant of various types of equity-based awards, such as stock awards, restricted stock units, stock appreciation rights and stock options to eligible participants, which include all of our employees and all employees of our 50% or more owned subsidiaries, our non-employee directors and certain consultants. The vesting terms of the awards may be tied to continued employment (or, for our non-employee directors, continued service on the Board of Directors) and/or achievement of certain pre-determined performance goals. We refer to stock awards subject to service-based vesting conditions as “non-vested stock” and restricted stock units subject to service-based or a combination of service-based and performance-based vesting conditions as “non-vested stock units.” The 2018 Plan is administered by the Compensation Committee of our Board of Directors, which determines, within the provisions of the 2018 Plan, those eligible participants to whom, and the times at which, awards shall be granted. The Compensation Committee, in its discretion, may delegate its authority and duties under the 2018 Plan to specified officers; however, only the Compensation Committee may approve the terms of awards to our executive officers.
Equity-based awards may be granted for a number of shares not to exceed, in the aggregate, approximately 2.5 million shares of common stock. We had approximately 2.01.7 million shares available at December 31, 2019.2022. The price per share for stock options shall be no less than the greater of (a) 100% of the fair value of a share of common stock on the date the option is granted or (b) the aggregate par value of the shares of our common stock on the date the option is granted. If a stock option is granted to any owner of 10% or more of the total combined voting power of us and our subsidiaries, the price is to be at least 110% of the fair value of a share of our common stock on the date the award is granted. Each equity-based award vests ratably over a 12 monthone year to four year period, with the exception of those issued under contractual arrangements that specify otherwise, and may be exercised during a period as determined by our Compensation Committee or as otherwise approved by our Compensation Committee. The contractual terms of stock options exercised shall not exceed ten years from the date such option is granted. The Company analyzes historical data of forfeited awards to develop an estimated forfeiture rate that is applied to the Company's non-cash compensation expense; however, all non-cash compensation expense is adjusted to reflect actual vestings and forfeitures.
Employee Stock Purchase Plan (“ESPP”)
We have a plan whereby our eligible employees may purchase our common stock at 85% of the market price at the time of purchase. On June 7, 2012, our stockholders ratified an amendment adopted by our Board of Directors to increase theThe total number of shares of our common stock authorized for issuance under our ESPP from 2,500,000 shares tois 4,500,000, shares, and as of December 31, 2019,2022, there were 1,348,3271,264,302 shares available for future issuance. The following is a detail of the purchases that werehave been made under the plan:
| | | | | | | | | | | |
| Employee Stock Purchase Plan Period | Shares Issued | | Price |
| 2020 and Prior | 3,171,373 | | | $ | 17.89 | |
| January 1, 2021 to March 31, 2021 | 4,060 | | | 225.07 | |
| April 1, 2021 to June 30, 2021 | 5,095 | | | 208.19 | |
| July 1, 2021 to September 30, 2021 | 7,466 | | | 126.74 | |
| October 1, 2021 to December 31, 2021 | 7,161 | | | 137.60 | |
| January 1, 2022 to March 31, 2022 | 6,184 | | | 146.45 | |
| April 1, 2022 to June 30, 2022 | 10,814 | | | 89.35 | |
| July 1, 2022 to September 30, 2022 | 12,047 | | | 82.27 | |
| October 1, 2022 to December 31, 2022 | 11,498 | | | 71.01 | |
| 3,235,698 | | | |
|
| | | | | | |
| Employee Stock Purchase Plan Period | Shares Issued | | Price |
| 2017 and Prior | 3,089,489 |
| | $ | 15.25 |
|
| January 1, 2018 to March 31, 2018 | 10,913 |
| | 51.29 |
|
| April 1, 2018 to June 30, 2018 | 8,673 |
| | 72.64 |
|
| July 1, 2018 to September 30, 2018 | 6,052 |
| | 106.22 |
|
| October 1, 2018 to December 31, 2018 | 7,856 |
| | 99.54 |
|
| January 1, 2019 to March 31, 2019 | 7,181 |
| | 104.77 |
|
| April 1, 2019 to June 30, 2019 | 8,230 |
| | 103.20 |
|
| July 1, 2019 to September 30, 2019 | 7,216 |
| | 111.36 |
|
| October 1, 2019 to December 31, 2019 | 6,063 |
| | 141.88 |
|
| | 3,151,673 |
| | |
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
ESPP expense included in general and administrative expense in our accompanying consolidated statements of operations was $0.7 million, $0.7 million and $0.6 million $0.5 millionfor 2022, 2021 and $0.4 million for each of 2019, 2018 and 2017,2020, respectively.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
Stock Options
On August 10, 2020, Paul B. Kusserow, Chief Executive Officer and Chairman of the Board of Amedisys, exercised 500,000 stock options previously awarded to him under the 2008 Plan. In connection with the exercise, Mr. Kusserow surrendered 231,683 shares of common stock to us to satisfy tax withholding and strike price obligations and elected to hold the net 268,317 shares issued to him. The surrendered shares are classified as treasury shares. This transaction resulted in a cash outflow of $40.4 million, reflected within financing activities in our consolidated statement of cash flows, related to the remittance of tax withholding obligations. In addition, Mr. Kusserow's stock option exercise resulted in a $24.0 million income tax benefit that was recorded in our consolidated statement of operations during the year ended December 31, 2020. See Note 10 – Income Taxes for additional details.
We use the Black-Scholes option pricing model to estimate the fair value of our stock options. There were 142,122, 163,66633,656, 40,788 and 308,29243,249 options granted during 2019, 20182022, 2021 and 2017,2020, respectively. Stock option compensation expense included in general and administrative expense in our accompanying consolidated statements of operations was $6.2$1.7 million, $5.7$3.6 million and $5.6$4.3 million for 2019, 20182022, 2021 and 2017,2020, respectively.
The fair values of the stock option awards were estimated using the following assumptions for each 2019, 20182022, 2021 and 2017:2020:
|
| | | | | |
| | For the Years Ended December 31, |
| | 2019 | | 2018 | | 2017 |
| Risk Free Rate | 1.44% - 2.53% | | 2.56% - 3.04% | | 1.99% - 2.16% |
| Expected Volatility | 42.46% - 43.83% | | 42.00% - 45.32% | | 50.18% - 51.81% |
| Expected Term | 6.00 - 6.25 years | | 4.12 - 6.25 years | | 5.78 - 6.25 years |
| Weighted Average Fair Value | $54.42 | | $42.48 | | $28.02 |
| Dividend Yield | —% | | —% | | —% |
| | | | | | | | | | | | | | | | | |
| For the Years Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Risk Free Rate | 1.91% | | 0.80% - 1.35% | | 0.38% - 1.51% |
| Expected Volatility | 40.97% | | 39.84% - 41.40% | | 40.15% - 42.80% |
| Expected Term | 6.25 years | | 6.25 years | | 6.25 years |
| Weighted Average Fair Value | $61.31 | | $107.45 | | $86.72 |
| Dividend Yield | —% | | —% | | —% |
We used the simplified method to estimate the expected term for the stock options granted during 2019, 20182022, 2021 and 20172020 as adequate historical experience is not available to provide a reasonable estimate.
The following table presents our stock option activity for 2019:2022:
|
| | | | | | | | |
| | Number of Shares | | Weighted Average Exercise Price | | Weighted Average Contractual Life (Years) |
| Outstanding options at January 1, 2019 | 833,315 |
| | $ | 36.79 |
| | 6.76 |
| Granted | 142,122 |
| | 121.32 |
| | |
| Exercised | (87,068 | ) | | 41.48 |
| | |
| Canceled, forfeited or expired | (12,395 | ) | | 66.18 |
| | |
| Outstanding options at December 31, 2019 | 875,974 |
| | $ | 49.62 |
| | 6.26 |
| Exercisable options at December 31, 2019 | 570,224 |
| | $ | 29.73 |
| | 5.31 |
| | | | | | | | | | | | | | | | | |
| Number of
Shares | | Weighted
Average Exercise
Price | | Weighted
Average Contractual
Life (Years) |
| Outstanding options at January 1, 2022 | 273,973 | | | $ | 137.54 | | | 7.21 |
| Granted | 33,656 | | | 143.25 | | | |
| Exercised | (37,635) | | | 61.23 | | | |
| Canceled, forfeited or expired | (51,382) | | | 174.57 | | | |
| Outstanding options at December 31, 2022 | 218,612 | | | $ | 142.86 | | | 6.56 |
| Exercisable options at December 31, 2022 | 163,286 | | | $ | 122.54 | | | 6.04 |
The aggregate intrinsic value of our outstanding options and exercisable options at December 31, 20192022 was $102.7$0.7 million and $78.2$0.7 million, respectively. Total intrinsic value of options exercised was $7.3$1.5 million, $9.7$5.1 million and $3.9$121.1 million for 2019, 20182022, 2021 and 2017,2020, respectively. The tax benefit from stock options exercised during the period amounted to $1.3$0.4 million, $1.6$1.0 million and $0.3$27.9 million for 2019, 20182022, 2021 and 2017,2020, respectively.
The following table presents our non-vested stock option activity for 2019:2022:
| | | | | | | | | | | |
| Number of
Shares | | Weighted Average
Grant Date Fair Value |
| Non-vested stock options at January 1, 2022 | 129,439 | | | $ | 182.45 | |
| Granted | 33,656 | | | 143.25 | |
| Vested | (64,496) | | | 150.79 | |
| Forfeited | (43,273) | | | 173.11 | |
| Non-vested stock options at December 31, 2022 | 55,326 | | | $ | 202.81 | |
|
| | | | | | |
| | Number of Shares | | Weighted Average Grant Date Fair Value |
| Non-vested stock options at January 1, 2019 | 370,470 |
| | $ | 30.97 |
|
| Granted | 142,122 |
| | 54.42 |
|
| Vested | (194,638 | ) | | 31.19 |
|
| Forfeited | (12,204 | ) | | 32.84 |
|
| Non-vested stock options at December 31, 2019 | 305,750 |
| | $ | 41.66 |
|
92
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
At December 31, 2019,2022, there was $6.1$2.0 million of unrecognized compensation cost related to stock options that we expect to be recognized over a weighted-average period of 1.8 years.
Non-Vested Stock
We issue shares of non-vested stock with a vesting term of one year. The compensation expense is determined based on the market price of our common stock at the date of grant applied to the total number of shares that are anticipated to fully vest. Non-vested stock compensation expense included in general and administrative expenses in our accompanying consolidated statements of operations was $1.2 million, $1.4 million and $1.7 million for 2019, 2018 and 2017, respectively.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
The following table presents our non-vested stock activity for 2019:
|
| | | | | | |
| | Number of Shares | | Weighted Average Grant Date Fair Value |
| Non-vested stock at January 1, 2019 | 14,904 |
| | $ | 80.54 |
|
| Granted | 9,859 |
| | 119.12 |
|
| Vested | (14,904 | ) | | 80.54 |
|
| Canceled, forfeited or expired | — |
| | — |
|
| Non-vested stock at December 31, 2019 | 9,859 |
| | $ | 119.12 |
|
The weighted average grant date fair value of non-vested stock granted was $119.12, $80.54 and $62.67 in 2019, 2018 and 2017, respectively.
At December 31, 2019, there was $0.8 million of unrecognized compensation cost related to non-vested stock awards that we expect to be recognized over a weighted average period of 0.4 years.
Non-Vested Stock Units
We issue non-vested stock unit awards that are service-based, performance-based or a combination of both with vesting terms ranging from one to four years. Based on the terms and conditions of these awards, we determine if the awards should be recorded as either equity or liability instruments. The compensation expense is determined based on the market price of our common stock at the date of grant, applied to the total number of units that are anticipated to vest, unless the award specifies differently. We account for such awards similar to our non-vested stock awards; however, no sharesShares of stock are not issued to the recipient until the stock unit awards have vested and after the pre-determined delivery date has occurred.
Non-Vested Stock Units – Service-Based ("Service-Based Non-Vested Stock Units")
Service-based non-vested stock unit compensation expense included in general and administrative expenses in our accompanying consolidated statements of operations was $8.7$12.1 million, $4.5$9.4 million and $3.6$7.5 million for 2019, 20182022, 2021 and 2017,2020, respectively.
The following table presents our service-based non-vested stock units activity for 2019:2022:
|
| | | | | | |
| | Number of Shares | | Weighted Average Grant Date Fair Value |
| Non-vested stock units at January 1, 2019 | 240,400 |
| | $ | 69.49 |
|
| Granted | 82,605 |
| | 123.70 |
|
| Vested | (78,119 | ) | | 58.75 |
|
| Canceled, forfeited or expired | (13,468 | ) | | 79.68 |
|
| Non-vested stock units at December 31, 2019 | 231,418 |
| | $ | 91.87 |
|
| | | | | | | | | | | |
| Number of
Shares | | Weighted Average
Grant Date Fair
Value |
| Non-vested stock units at January 1, 2022 | 180,823 | | | $ | 195.25 | |
| Granted | 211,361 | | | 115.07 | |
| Vested | (59,006) | | | 146.76 | |
| Canceled, forfeited or expired | (70,025) | | | 194.68 | |
| Non-vested stock units at December 31, 2022 | 263,153 | | | $ | 141.62 | |
The weighted average grant date fair value of service-based non-vested stock units granted was $123.70, $95.14$115.07, $234.42 and $53.79$206.10 in 2019, 20182022, 2021 and 2017,2020, respectively.
At December 31, 2019,2022, there was $11.4$22.6 million of unrecognized compensation cost related to our service-based non-vested stock units that we expect to be recognized over a weighted average period of 1.92.2 years.
Non-Vested Stock Units – Service-Based and Performance-Based Awards ("Performance-Based Non-Vested Stock Units")
During 2019,2022, we awarded performance-based awards to certain employees. The target level established by the award, which is based on the Company’s 20192022 adjusted earnings before interest, taxes, depreciation and amortization (“Adjusted EBITDA”), provided for the recipients to receive 102,585an aggregate of 71,349 non-vested stock units if the target was achieved. For a select group of employees, if the target objective iswas surpassed to the point of achieving the projected maximum payout, the recipients willwould receive an additional 18,327aggregate of 32,048 non-vested stock units during the three-month period ending March 31, 2020.2023. The target number of shares to be potentially awarded has beenwas reduced by forfeitures as indicated in the table below. On February 1, 2023, the Compensation Committee determined that the 2022 performance-based objective established by the award was not satisfied, and as a result, the target number of non-vested stock units will be forfeited. Performance-based non-vested stock units compensation expense included in general and administrative expenses in our consolidated statements of operations was $8.4$2.2 million, $5.8$10.2 million and $5.0$13.5 million for 2019, 20182022, 2021 and 2017,2020, respectively.
The following table presents our performance-based non-vested stock units activity for 2022:
| | | | | | | | | | | |
| Number of
Shares | | Weighted Average
Grant Date Fair
Value |
| Non-vested stock units at January 1, 2022 | 186,951 | | | $ | 206.36 | |
| Granted | 71,349 | | | 133.70 | |
| Vested | (85,767) | | | 156.18 | |
| Canceled, forfeited or expired | (104,486) | | | 237.30 | |
| Non-vested stock units at December 31, 2022 | 68,047 | | | $ | 144.55 | |
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 20192022
The following table presents our performance-based non-vested stock units activity for 2019:
|
| | | | | | |
| | Number of Shares | | Weighted Average Grant Date Fair Value |
| Non-vested stock units at January 1, 2019 | 226,677 |
| | $ | 65.76 |
|
| Granted | 102,585 |
| | 128.89 |
|
| Vested | (101,156 | ) | | 61.12 |
|
| Canceled, forfeited or expired | (20,682 | ) | | 82.72 |
|
| Non-vested stock units at December 31, 2019 | 207,424 |
| | $ | 97.55 |
|
The weighted average grant date fair value of performance-based non-vested stock units granted was $128.89, $79.59$133.70, $262.67 and $52.99$201.90 in 2019, 20182022, 2021 and 2017,2020, respectively.
At December 31, 2019,2022, there was $13.2$1.1 million in unrecognized compensation costs related to our performance-based non-vested stock units that we expect to be recognized over a weighted average period of 2.01.1 years.
10.12. COMMITMENTS AND CONTINGENCIES
Legal Proceedings – Ongoing
We are involved in legal actions in the followingnormal course of business, some of which seek monetary damages, including claims for punitive damages. Based on information available to us as of the date of this filing, we do not believe that these normal course actions, when finally concluded and determined, will have a material impact on our consolidated financial condition, results of operations or cash flows.
Legal fees related to all legal actions:matters are expensed as incurred.
Legal Proceedings - Completed
Subpoena Duces Tecum and Civil Investigative Demands Issued by the U.S. Department of Justice
On May 7, 2021, the U.S. Department of Justice notified the Company that they were closing their investigation into the below-referenced Subpoena Duces Tecum ("Subpoena") and civil investigative demands ("CIDs"). At the time, we had $6.5 million recorded to accrued expenses in our consolidated balance sheets related to these matters. We reversed this accrual during the three-month period ended June 30, 2021.
On May 21, 2015, we received a Subpoena Duces Tecum (“Subpoena”) issued by the U.S. Department of Justice. The Subpoena requestsrequested the delivery of information regarding 53 identified hospice patients to the United States Attorney’s Office for the District of Massachusetts. It also requestsrequested the delivery of documents relating to our hospice clinical and business operations and related compliance activities. The Subpoena generally coverscovered the period from January 1, 2011 through May 21, 2015. We are fully cooperating with the U.S. Department of Justice with respect to this investigation.
On November 3, 2015, we received a civil investigative demand (“CID”)CID issued by the U.S. Department of Justice pursuant to the federal False Claims Act relating to claims submitted to Medicare and/or Medicaid for hospice services provided through designated facilities in the Morgantown, West Virginia area. The CID requestsrequested the delivery of information to the United States Attorney’s Office for the Northern District of West Virginia regarding 66 identified hospice patients, as well as documents relating to our hospice clinical and business operations in the Morgantown area. The CID generally coverscovered the period from January 1, 2009 through August 31, 2015. We are fully cooperating with the U.S. Department of Justice with respect to this investigation.
On June 27, 2016, we received a CID issued by the U.S. Department of Justice pursuant to the federal False Claims Act relating to claims submitted to Medicare and/or Medicaid for hospice services provided through designated facilities in the Parkersburg, West Virginia area. The CID requestsrequested the delivery of information to the United States Attorney’s Office for the Southern District of West Virginia regarding 68 identified hospice patients, as well as documents relating to our hospice clinical and business operations in the Parkersburg area. The CID generally coverscovered the period from January 1, 2011 through June 20, 2016. We are fully cooperating with the U.S. Department of Justice with respect to this investigation.
Based on our analysis of sample claims data in connection with preliminary settlement discussions with the U.S. Department of Justice regarding the above matters, we have recorded a total of $6.5 million to accrued expenses in our consolidated balance sheet related to this matter. Due to the ongoing nature of the investigations and current stage of the settlement discussions, we are unable to estimate a range of potential loss at this time, and we cannot predict the timing or outcome of these investigations.
In addition to the matters referenced in this note, we are involved in legal actions in the normal course of business, some of which seek monetary damages, including claims for punitive damages. We do not believe that these normal course actions, when finally concluded and determined, will have a material impact on our consolidated financial condition, results of operations or cash flows.
Legal fees related to all legal matters are expensed as incurred.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
Legal Proceedings – Settled
Securities Class Action Lawsuits
As previously disclosed, between June 10 and July 28, 2010, several putative securities class action complaints were filed in the United States District Court for the Middle District of Louisiana (the “District Court”) against the Company and certain of our former senior executives. The cases were consolidated into the first-filed action Bach, et al. v. Amedisys, Inc., et al. Case No. 3:10-cv-00395, and the District Court appointed as co-lead plaintiffs the Public Employees’ Retirement System of Mississippi and the Puerto Rico Teachers’ Retirement System (the “Co-Lead Plaintiffs”).
The Plaintiffs were granted leave to file a First Amended Consolidated Complaint (the “First Amended Securities Complaint”) on behalf of all purchasers or acquirers of Amedisys’ securities between August 2, 2005 and September 30, 2011. The First Amended Securities Complaint alleges that the Company and seven individual defendants violated Section 10(b), Section 20(a), and Rule 10b-5 of the Securities Exchange Act of 1934 by materially misrepresenting the Company’s financial results and concealing a scheme to obtain higher Medicare reimbursements and additional patient referrals by (1) providing medically unnecessary care to patients, including certifying and re-certifying patients for medically unnecessary 60-day treatment episodes; (2) implementing clinical tracks such as “Balanced for Life” and wound care programs that provided a pre-set number of therapy visits irrespective of medical need; (3) “upcoding” patients’ Medicare forms to attribute a “primary diagnosis” to a medical condition associated with higher billing rates; and (4) providing improper and illegal remuneration to physicians to obtain patient certifications or re-certifications. The First Amended Securities Complaint sought certification of the case as a class action and an unspecified amount of damages, as well as interest and an award of attorneys’ fees.
On June 12, 2017, the Company reached an agreement-in-principle to settle this matter. All parties to the action executed a binding term sheet that, subject to final documentation and court approval, provided in part for a settlement payment of approximately $43.7 million, and the dismissal with prejudice of the litigation. Approximately $15.0 million of the settlement amount was paid by the Company’s insurance carriers. The net of these two amounts, $28.7 million, was recorded as a charge in our consolidated statements of operations and paid with cash on hand during 2017. On December 19, 2017, the Court entered the final order and judgment on the case.
Other Investigative Matters – Ongoing
Corporate Integrity Agreement
On April 23, 2014, with no admissions of liability on our part, we entered into a settlement agreement with the U.S. Department of Justice relating to certain of our clinical and business operations. Concurrently with our entry into this agreement, we entered into a corporate integrity agreement (“CIA”) with the Office of Inspector General-HHS (“OIG”). The CIA formalized various aspects of our already existing ethics and compliance programs and contained other requirements designed to help ensure our ongoing compliance with federal health care program requirements. Among other things, the CIA required us to maintain our existing compliance program, executive compliance committee and compliance committee of the Board of Directors; provide certain compliance training; continue screening new and current employees to ensure they are eligible to participate in federal health care programs; engage an independent review organization to perform certain audits and reviews and prepare certain reports regarding our compliance with federal health care programs, our billing submissions to federal health care programs and our compliance and risk mitigation programs; and provide certain reports and management certifications to the OIG. Additionally, the CIA specifically required that we report substantial overpayments that we discovered we had received from federal health care programs, as well as probable violations of federal health care laws. The corporate integrity agreement had a term of five years that ended on April 21, 2019. We filed our final annual report on July 19, 2019.
Compassionate Care Hospice Corporate Integrity Agreement
On January 30, 2015, CCH entered into a CIA with the OIG. The CIA required that CCH provide annual on-site compliance training; develop and implement policies to ensure compliance with federal health care program requirements; screen new and current employees to ensure that they are eligible to participate in federal health care programs; establish a compliance committee that contains both a Compliance Officer and a Chief Quality Officer; retain a Governing Authority expert who will periodically complete a compliance program review; and retain an independent review organization (IRO) to complete claims review for hospice services rendered in New York. The OIG waived the claims review for the final year of the CCH CIA based on the closure of the New York operations. Additionally, the CIA required that CCH report substantial overpayments that CCH discovered it received from federal health care programs, as well as probable violations of federal criminal, civil or administrative health care laws. Upon breach of the CIA, CCH could have become liable for payment of certain stipulated penalties, or could have been excluded from participation in federal health care programs. The CIA had a term of five years that ended on January 30, 2020.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
Third Party Audits – Ongoing
From time to time, in the ordinary course of business, we are subject to audits under various governmental programs in which third party firms engaged by CMS, including Recovery Audit Contractors (“RACs”), Zone Program Integrity Contractors (“ZPICs”), Uniform Program Integrity Contractors ("UPICs"(“UPICs”), Program Safeguard Contractors (“PSCs”) and, Medicaid Integrity Contractors (“MICs”), in which third party firms engaged bySupplemental Medical Review Contractors (“SMRCs”) and the Centers for Medicare and Medicaid Services (“CMS”Office of the Inspector General ("OIG"), conduct extensive reviews of claims data to identify potential improper payments. We cannot predict the ultimate outcome of any regulatory reviews or other governmental audits and investigations.
In July 2010, our subsidiary that provides hospice services in Florence, South Carolina received from a ZPIC a request for records regarding a sample of 30 beneficiaries who received services from the subsidiary during the period of January 1, 2008 through March 31, 2010 (the “Review Period”) to determine whether the underlying services met pertinent Medicare payment requirements. We acquired the hospice operations subject to this review on August 1, 2009; the Review Period covers time periods both before and after our ownership of these hospice operations. Based on the ZPIC’s findings for 16 beneficiaries, which were extrapolated to all claims for hospice services provided by the Florence subsidiary billed during the Review Period, on June 6, 2011, the Medicare Administrative Contractor ("MAC") for the subsidiary issued a notice of overpayment seeking recovery from our subsidiary of an alleged overpayment. We dispute these findings, and our Florence subsidiary has filed appeals through the Original Medicare Standard Appeals Process, in which we are seeking to have those findings overturned.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
An administrative law judge ("ALJ") hearing was held in early January 2015. On January 18, 2016, we received a letter dated January 6, 2016 referencing the ALJ hearing decision for the overpayment issued on June 6, 2011. The decision was partially favorable with a new overpayment amount of $3.7 million with a balance owed of $5.6 million including interest based on 9 disputed claims (originally 16). We filed an appeal to the Medicare Appeals Council on the remaining 9 disputed claims and also argued that the statistical method used to select the sample was not valid. No assurances can be given as to the timing or outcome of the Medicare Appeals Council decision. As of December 31, 2019,2022, Medicare has withheld payments of $5.7 million (including additional interest) as part of their standard procedures once this level of the appeal process has been reached. In the event we are not able to recoup this alleged overpayment, we are entitled to be indemnified by the prior owners of the hospice operations for amounts relating to the period prior to August 1, 2009. On January 10, 2019, an arbitration panel from the American Health Lawyers Association determined that the prior owners' liability for their indemnification obligation was $2.8 million. Accordingly, the Company reduced its indemnity receivable from $4.9 million to $2.8 million. The $2.1 million impact wasThis amount is recorded to general and administrative expenses, other within our consolidated statements of operations during the year ended December 31, 2018. As of December 31, 2019, we haveas an indemnity receivable of approximately $2.8 million for the amount withheld related to the period prior to August 1, 2009.within other assets in our consolidated balance sheets.
In July 2016, the Company received a request for medical records from SafeGuard Services, L.L.C (“SafeGuard”), a ZPIC, related to services provided by some of the care centers that the Company acquired from Infinity Home Care, L.L.C. The review period coverscovered time periods both before and after our ownership of the care centers, which were acquired on December 31, 2015. In August 2017, the Company received Requests for Repayment from Palmetto GBA, LLC (“Palmetto”) regarding Infinity Home Care of Lakeland, LLC, (“Lakeland Care Centers”) and Infinity Home Care of Pinellas, LLC, (“Clearwater Care Center”). The Palmetto letters arewere based on a statistical extrapolation performed by SafeGuard which alleged an overpayment of $34.0 million for the Lakeland Care Centers on a universe of 72 Medicare claims totaling $0.2 million in actual claims payments using a 100% error rate and an overpayment of $4.8 million for the Clearwater Care Center on a universe of 70 Medicare claims totaling $0.2 million in actual claims payments using a 100% error rate.
The Lakeland Request for Repayment coverscovered claims between January 2, 2014 and September 13, 2016. The Clearwater Request for Repayment coverscovered claims between January 2, 2015 and December 9, 2016. As a result of partially successful Level I and Level II Administrative Appeals, the alleged overpayment for the Lakeland Care Centers has beenwas reduced to $26.0 million and the alleged overpayment for the Clearwater Care Center has beenwas reduced to $3.3 million. The Company has now filed Level III Administrative Appeals, and will continue to vigorously pursue its appeal rights, which include contesting the methodology used byALJ hearings regarding the ZPIC contractor to perform statistical extrapolation. Lakeland Request for Repayment and the Clearwater Request for Repayment were held in April 2022.
The Company is contractually entitled to indemnification byreceived the prior owners for all claims prior to December 31, 2015, for up to $12.6 million.
At this stageresults of the review, based onALJ hearings for the information currently available to the Company, the Company cannot predict the timing or outcome of this review. The Company estimates a low-end potential range of loss related to this review of $6.5 million (assuming the Company is successful in seeking indemnity from the prior ownersClearwater Care Center and unsuccessful in demonstrating that the extrapolation method used by SafeGuard was erroneous). The Company has reduced its high-end potential range of loss from $38.8 million (the maximum amount Palmetto claims has been overpaid for both the Lakeland Care Centers on June 23, 2022 and June 30, 2022, respectively. The ALJ decisions for both the Clearwater Care Center and the Lakeland Care Centers were partially favorable for the claims that were reviewed, but the extrapolations were upheld. As a result, we increased our total accrual related to these matters from $17.4 million to $25.8 million during the three-month period ended June 30, 2022. The net of which amount $12.6these two amounts, $8.4 million, is subjectwas recorded as a reduction to indemnification bynet service revenue in our consolidated statement of operations during the prior owners)three-month period ended June 30, 2022. We received demands for repayment from Palmetto for both the Clearwater Care Center and the Lakeland Care Centers during the three-month period ended September 30, 2022. The demands were slightly less than our estimated accrual of $25.8 million. During the three-month period ended September 30, 2022, we adjusted our accrual to $29.3$25.2 million based onto reflect the partial success achieved byfinal amounts owed, excluding interest. The repayment for the Company in prosecuting its Level I and II Administrative Appeals.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Lakeland Care Centers totaling $34.3 million ($22.8 million extrapolated repayment plus $11.5 million accrued interest) was made during the three-month period ended September 30, 2022. The repayment for the Clearwater Care Center totaling $3.7 million ($2.4 million extrapolated repayment plus $1.2 million accrued interest) was made during the three-month period ended December 31, 2019
As2022. Additionally, we wrote off $1.5 million of December 31, 2019, we have an accrued liability of approximately $17.4 million related to this matter.receivables that were impacted by these matters. We expect to be indemnified by the prior owners, upon exhaustion of the parties' appeal rights, for approximately $10.9 million of the total $12.6 million available indemnification related to this matter and have recorded this amount within other assets in our consolidated balance sheet as of December 31, 2019. The net of these two amounts, $6.5 million, was recorded as a reduction in revenue in our consolidated statements of operations during 2017. As of December 31, 2019, $1.5 million of net receivables have been impacted by this payment suspension.sheets.
Insurance
We are obligated for certain costs associated with our insurance programs, including employee health, workers’ compensation and professional liability. While we maintain various insurance programs to cover these risks, we are self-insured for a substantial portion of our potential claims. We recognize our obligations associated with these costs, up to specified deductible limits in the period in which a claim is incurred, including with respect to both reported claims and claims incurred but not reported. These costs have generally been estimated based on historical data of our claims experience. Such estimates, and the resulting reserves, are reviewed and updated by us on a quarterly basis.
The following table presents details of our insurance programs, including amounts accruedrecorded, for the periods indicated (amounts in millions) inwithin accrued expenses in our accompanyingconsolidated balance sheets. The amounts accrued below represent our total estimated liability for individual
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
claims that are less than our noted insurance coverage amounts, which can include outstanding claims and claims incurred but not reported.reported (amounts in millions).
|
| | | | | | | |
| | As of December 31, |
| Type of Insurance | 2019 | | 2018 |
| Health insurance | $ | 15.8 |
| | $ | 12.4 |
|
| Workers’ compensation | 33.4 |
| | 30.9 |
|
| Professional liability | 5.1 |
| | 4.3 |
|
| | 54.3 |
| | 47.6 |
|
| Less: long-term portion | (1.3 | ) | | (1.1 | ) |
| | $ | 53.0 |
| | $ | 46.5 |
|
| | | | | | | | | | | |
| As of December 31, |
| Type of Insurance | 2022 | | 2021 |
| Health insurance | $ | 16.2 | | | $ | 16.2 | |
| Workers’ compensation | 40.8 | | | 40.5 | |
| Professional liability | 5.0 | | | 5.2 | |
| 62.0 | | | 61.9 | |
| Less: long-term portion | (0.2) | | | (0.2) | |
| $ | 61.8 | | | $ | 61.7 | |
Our health insurance has an exposure limit of $1.3 million for any individual covered life. Our workers compensation insurance has a retention limit of $1.0$2.0 million per incident, and our professional liability insurance has a retention limit of $0.3 million per incident.
Severance
We have commitments related to our Key Executive Severance Planseverance plans applicable to a number of our senior executives as well as the employment agreement entered into with our Chief Executive Officer, each ofand senior management, which generally commit us to pay severance benefits under certain circumstances.
Other
We are subject to various other types of claims and disputes arising in the ordinary course of our business. While the resolution of such issues is not presently determinable, we believe that the ultimate resolution of such matters will not have a significant effect on our consolidated financial condition, results of operations andor cash flows.
11.13. EMPLOYEE BENEFIT PLANS
401(k) Benefit Plan
We maintain a plan qualified under Section 401(k) of the Internal Revenue Code for all employees who have reached 21 years of age, effective the first month after their hire date. Under the plan, eligible employees may elect to defer a portion of their compensation, subject to Internal Revenue Service limits.
Effective January 1, 2017, ourOur match of contributions to be made to each eligible employee contribution is $0.44 for every $1.00 contributed up to the first 6% of theirthe employee's salary. The match is discretionary and thus is subject to change at the discretion of management. TheseOur match of contributions areis made in the form of our common stock, valued based upon the fair value of the stock as of the end of each calendar quarter end.cash. We expensed approximately $10.5$18.6 million, $9.0$17.0 million and $8.8$12.9 million related to our 401(k) benefit plan for 2019, 20182022, 2021 and 2017,2020, respectively.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
Deferred Compensation Plan
We had a Deferred Compensation Plan for additional tax-deferred savings for a select group of management or highly compensated employees. Amounts credited under the Deferred Compensation Plan were funded into a rabbi trust, which is managed by a trustee. The trustee has the discretion to manage the assets of the Deferred Compensation Plan as deemed fit, thus, the assets are not necessarily reflective of the same investment choices that would have been made by the participants.
Effective January 1, 2015, all prospective salary deferrals ceased. Participants will beare allowed to make transactions with any remaining account balances as they wish per plan guidelines.
12.14. SHARE REPURCHASEREPURCHASES
2019 Stock Repurchase Program
On February 25, 2019,December 23, 2020, we announced that our Board of Directors authorized a stock repurchase program, under which we could repurchase up to $100 million of our outstanding common stock through December 31, 2021 (the "2021 Share Repurchase Program"). Pursuant to this program, we repurchased 446,832 shares of our common stock at a weighted average price of $223.49 per share and a total cost of approximately $100 million during the year ended December 31, 2021. We did not repurchase any shares pursuant to this stock repurchase program during the year ended December 31, 2020. The repurchased shares were classified as treasury shares. The 2021 Share Repurchase Program expired on December 31, 2021.
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2022
On August 2, 2021, our Board of Directors authorized a share repurchase program, under which we could repurchase up to $100 million of our outstanding common stock through December 31, 2022 to commence upon the completion of the Company's 2021 Share Repurchase Program (the "New Share Repurchase Program"). Pursuant to this program, we repurchased 150,000 shares of our common stock at a weighted average price of $115.64 per share and a total cost of approximately $17 million during the year ended December 31, 2022. The repurchased shares were classified as treasury shares. The New Share Repurchase Program expired on December 31, 2022.
Under the terms of the 2021 Share Repurchase Program and the New Share Repurchase Program, we were allowed to repurchase shares from time to time through open market purchases, unsolicited or solicited privately negotiated transactions, an accelerated stock repurchase program, and/or a trading plan in compliance with Exchange Act Rule 10b5-1. The timing and the amount of the repurchases were determined by management based on a number of factors, including but not limited to share price, trading volume and general market conditions, as well as on working capital requirements, general business conditions and other factors.
On February 2, 2023, our Board of Directors authorized a share repurchase program, under which we may repurchase up to $100 million of our outstanding common stock through March 1, 2020December 31, 2023 ("the 2023 Share Repurchase Program"). See Note 17 - Subsequent Events for additional information on the newly authorized share repurchase program.
Under the terms of the program,2023 Share Repurchase Program, we are allowed to repurchase shares from time to time inthrough open market purchases, unsolicited or solicited privately negotiated transactions, block purchases an accelerated stock repurchase program, and/or a trading plan in private transactions in accordancecompliance with applicable federal securities laws and other legal requirements. We are allowed to enter intoExchange Act Rule 10b5-1 plans to effect some or all of the repurchases.10b5-1. The timing and the amount of the repurchases will be determined by management based on a number of factors, including but not limited to share price, trading volume and general market conditions, as well as on working capital requirements, general business conditions and other factors.
We did not repurchase any shares pursuant to this stock repurchase program during the year ended December 31, 2019.
2018 Share Repurchase
On June 4, 2018, we purchased 2,418,304 of our common shares from affiliates of KKR Credit Advisors (US) LLC ("KKR"), representing one-half of KKR's then current holdings in the Company and 7.1% of the aggregate outstanding shares of the Company's common stock for a total purchase price of $181.4 million including related direct costs. The Company repurchased the shares at $73.96 which represents 96% of the closing stock price of the Company's common stock on June 4, 2018. The repurchased shares are classified as treasury shares.
13. EXIT AND RESTRUCTURING ACTIVITIES
During 2017, we closed 4 Florida home health care centers, consolidated another 3 Florida home health care centers with care centers servicing the same markets and implemented a plan to restructure our home health division. As a result of these actions, we recorded non-cash charges of $1.3 million in asset impairment expense related to the write-off of intangible assets, $0.6 million in other general and administrative expenses related to lease termination costs and $3.0 million in salaries and benefits related to severance costs which was offset by a reduction in non-cash compensation of approximately $1.0 million within our consolidated statements of operations for 2017.
l4.15. SEGMENT INFORMATION
Our operations involve servicing patients through our 3four reportable business segments: home health, hospice, personal care and personalhigh acuity care. Our home health segment delivers a wide range of services in the homes of individuals who may be recovering from surgery, have a chronic disabilityan illness, injury or terminal illness or need assistance with completing important tasks.surgery. Our hospice segment provides palliative care that is designed to provide comfort and comfort to terminally ill patients and their families.support for those who are facing a terminal illness. Our personal care segment provides patients with assistance with the essential activities of daily living. Our high acuity care segment, which was established with the acquisition of Contessa on August 1, 2021, delivers the essential elements of inpatient hospital and SNF care to patients in their homes. The “other” column in the following tables consists of costs relating to executive management and administrative support functions, primarily information services, accounting, finance, billing and collections, legal, compliance, risk management, procurement, marketing, clinical administration, training, human resources and administration.
Management evaluates performance and allocates resources based on the operating income of the reportable segments, which includes an allocation of corporate expenses attributable to the specific segment as well asand includes revenues and all other costs directly attributable to the specific segment. Segment assets are not reviewed by the company’s chief operating decision maker and therefore are not disclosed below (amounts in millions).
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| For the Year Ended December 31, 2022 |
| Home Health | | Hospice | | Personal Care | | High Acuity Care | | Other | | Total |
| Net service revenue | $ | 1,355.5 | | | $ | 787.8 | | | $ | 61.4 | | | $ | 18.5 | | | $ | — | | | $ | 2,223.2 | |
| Cost of service, excluding depreciation and amortization | 769.0 | | | 426.5 | | | 46.7 | | | 18.2 | | | — | | | 1,260.4 | |
| General and administrative expenses, excluding depreciation and amortization and impairment charge | 348.5 | | | 203.3 | | | 9.2 | | | 33.1 | | | 160.0 | | | 754.1 | |
| Depreciation and amortization | 4.0 | | | 2.3 | | | 0.1 | | | 3.3 | | | 15.2 | | | 24.9 | |
| Impairment charge | — | | | — | | | — | | | 3.0 | | | — | | | 3.0 | |
| Operating expenses | 1,121.5 | | | 632.1 | | | 56.0 | | | 57.6 | | | 175.2 | | | 2,042.4 | |
| Operating income (loss) | $ | 234.0 | | | $ | 155.7 | | | $ | 5.4 | | | $ | (39.1) | | | $ | (175.2) | | | $ | 180.8 | |
|
| | | | | | | | | | | | | | | | | | | |
| | For the Year Ended December 31, 2019 |
| | Home Health | | Hospice | | Personal Care | | Other | | Total |
| Net service revenue | $ | 1,256.4 |
| | $ | 617.2 |
| | $ | 82.0 |
| | $ | — |
| | $ | 1,955.6 |
|
| Cost of service, excluding depreciation and amortization | 754.1 |
| | 335.1 |
| | 61.1 |
| | — |
| | 1,150.3 |
|
| General and administrative expenses | 297.2 |
| | 137.5 |
| | 12.3 |
| | 160.9 |
| | 607.9 |
|
| Depreciation and amortization | 4.2 |
| | 1.6 |
| | 0.2 |
| | 12.4 |
| | 18.4 |
|
| Asset impairment charge | 1.5 |
| | — |
| | — |
| | — |
| | 1.5 |
|
| Operating expenses | 1,057.0 |
| | 474.2 |
| | 73.6 |
| | 173.3 |
| | 1,778.1 |
|
| Operating income (loss) | $ | 199.4 |
| | $ | 143.0 |
| | $ | 8.4 |
| | $ | (173.3 | ) | | $ | 177.5 |
|
|
| | | | | | | | | | | | | | | | | | | |
| | For the Year Ended December 31, 2018 |
| | Home Health | | Hospice | | Personal Care | | Other | | Total |
| Net service revenue | $ | 1,174.5 |
| | $ | 410.9 |
| | $ | 77.2 |
| | $ | — |
| | $ | 1,662.6 |
|
| Cost of service, excluding depreciation and amortization | 722.1 |
| | 212.0 |
| | 58.8 |
| | — |
| | 992.9 |
|
| General and administrative expenses | 276.3 |
| | 84.6 |
| | 12.8 |
| | 127.6 |
| | 501.3 |
|
| Depreciation and amortization | 3.5 |
| | 1.1 |
| | 0.3 |
| | 8.4 |
| | 13.3 |
|
| Operating expenses | 1,001.9 |
| | 297.7 |
| | 71.9 |
| | 136.0 |
| | 1,507.5 |
|
| Operating income (loss) | $ | 172.6 |
| | $ | 113.2 |
| | $ | 5.3 |
| | $ | (136.0 | ) | | $ | 155.1 |
|
|
| | | | | | | | | | | | | | | | | | | |
| | For the Year Ended December 31, 2017 |
| | Home Health | | Hospice | | Personal Care | | Other | | Total |
| Net service revenue | $ | 1,083.9 |
| | $ | 367.8 |
| | $ | 59.6 |
| | $ | — |
| | $ | 1,511.3 |
|
| Cost of service, excluding depreciation and amortization | 670.9 |
| | 187.5 |
| | 45.0 |
| | — |
| | 903.4 |
|
| General and administrative expenses | 278.4 |
| | 76.6 |
| | 9.5 |
| | 117.8 |
| | 482.3 |
|
| Depreciation and amortization | 3.5 |
| | 0.9 |
| | 0.2 |
| | 12.5 |
| | 17.1 |
|
| Securities Class Action Lawsuit settlement, net | — |
| | — |
| | — |
| | 28.7 |
| | 28.7 |
|
| Asset impairment charge | 1.3 |
| | — |
| | — |
| | — |
| | 1.3 |
|
| Operating expenses | 954.1 |
| | 265.0 |
| | 54.7 |
| | 159.0 |
| | 1,432.8 |
|
| Operating income (loss) | $ | 129.8 |
| | $ | 102.8 |
| | $ | 4.9 |
| | $ | (159.0 | ) | | $ | 78.5 |
|
15. UNAUDITED SUMMARIZED QUARTERLY FINANCIAL INFORMATION
|
| | | | | | | | | | | | | | | |
| | | | | | Net Income Attributable to Amedisys, Inc. Common Stockholders (1) |
| | Revenue | | Net Income Attributable to Amedisys, Inc. | | Basic | | Diluted |
| 2019 | | | | | | | |
| 1st Quarter | $ | 467.3 |
| | $ | 31.3 |
| | $ | 0.98 |
| | $ | 0.95 |
|
| 2nd Quarter | 493.0 |
| | 33.7 |
| | 1.05 |
| | 1.02 |
|
| 3rd Quarter | 494.6 |
| | 34.1 |
| | 1.06 |
| | 1.03 |
|
| 4th Quarter | 500.7 |
| | 27.7 |
| | 0.86 |
| | 0.83 |
|
| | $ | 1,955.6 |
| | $ | 126.8 |
| | $ | 3.95 |
| | $ | 3.84 |
|
| 2018 | | | | | | | |
| 1st Quarter | $ | 399.3 |
| | $ | 27.2 |
| | $ | 0.80 |
| | $ | 0.79 |
|
| 2nd Quarter | 411.6 |
| | 33.3 |
| | 1.00 |
| | 0.98 |
|
| 3rd Quarter | 417.3 |
| | 31.4 |
| | 0.99 |
| | 0.96 |
|
| 4th Quarter | 434.4 |
| | 27.5 |
| | 0.86 |
| | 0.84 |
|
| | $ | 1,662.6 |
| | $ | 119.3 |
| | $ | 3.64 |
| | $ | 3.55 |
|
| |
(1) | Because of the method used in calculating per share data, the quarterly per share data may not necessarily total to the per share data as computed for the entire year. |
AMEDISYS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
December 31, 20192022
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| For the Year Ended December 31, 2021 |
| Home Health | | Hospice | | Personal Care | | High Acuity Care | | Other | | Total |
| Net service revenue | $ | 1,353.8 | | | $ | 791.8 | | | $ | 65.0 | | | $ | 3.5 | | | $ | — | | | $ | 2,214.1 | |
| Other operating income | 7.3 | | | 6.0 | | | — | | | — | | | — | | | 13.3 | |
| Cost of service, excluding depreciation and amortization | 756.6 | | | 425.2 | | | 49.1 | | | 2.5 | | | — | | | 1,233.4 | |
| General and administrative expenses, excluding depreciation and amortization and impairment charge | 328.5 | | | 198.4 | | | 11.2 | | | 10.0 | | | 163.1 | | | 711.2 | |
| Depreciation and amortization | 4.3 | | | 2.7 | | | 0.2 | | | 1.3 | | | 22.4 | | | 30.9 | |
| Operating expenses | 1,089.4 | | | 626.3 | | | 60.5 | | | 13.8 | | | 185.5 | | | 1,975.5 | |
| Operating income (loss) | $ | 271.7 | | | $ | 171.5 | | | $ | 4.5 | | | $ | (10.3) | | | $ | (185.5) | | | $ | 251.9 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| For the Year Ended December 31, 2020 |
| Home Health | | Hospice | | Personal Care | | High Acuity Care | | Other | | Total |
| Net service revenue | $ | 1,249.2 | | | $ | 750.1 | | | $ | 72.2 | | | $ | — | | | $ | — | | | $ | 2,071.5 | |
| Other operating income | 20.2 | | | 13.1 | | | 1.1 | | | — | | | — | | | 34.4 | |
| Cost of service, excluding depreciation and amortization | 729.9 | | | 400.6 | | | 54.9 | | | — | | | — | | | 1,185.4 | |
| General and administrative expenses, excluding depreciation and amortization and impairment charge | 307.2 | | | 175.4 | | | 12.4 | | | — | | | 173.2 | | | 668.2 | |
| Depreciation and amortization | 3.9 | | | 2.2 | | | 0.2 | | | — | | | 22.5 | | | 28.8 | |
| Impairment charge | 3.4 | | | 0.8 | | | — | | | — | | | — | | | 4.2 | |
| Operating expenses | 1,044.4 | | | 579.0 | | | 67.5 | | | — | | | 195.7 | | | 1,886.6 | |
| Operating income (loss) | $ | 225.0 | | | $ | 184.2 | | | $ | 5.8 | | | $ | — | | | $ | (195.7) | | | $ | 219.3 | |
16. RELATED PARTY TRANSACTIONS
During 2018, we made a $7.0 millionWe have an investment in Medalogix, a healthcare predictive data and analytics company; this investmentcompany, which is accounted for under the equity method. During the yearyears ended December 31, 2019,2022, 2021 and 2020, we incurred costs of approximately $0.5$9.4 million,$5.7 million and $3.9 million, respectively, in connection with theour usage of Medalogix's analytics platforms. We believe that the terms of these transactions are consistent with those negotiated at arm’s length.
On June 4, 2018, we purchased 2,418,304 of our common shares from affiliates of KKR, representing one-half of KKR's holdings in the Company and 7.1% of the aggregate outstanding shares of the Company's common stock for a total purchase price of $181.4 million including related direct costs. The Company repurchased the shares at $73.96 which represents 96% of the closing stock price of the Company's common stock on June 4, 2018. At the time of the transaction, KKR held approximately 14.2% of the Company's outstanding shares of common stock. As of December 31, 2019, KKR is no longer a shareholder of the Company's stock.
17. SUBSEQUENT EVENTS
2023 Share Repurchase Program
On February 2, 2023, our Board of Directors authorized a share repurchase program, under which we may repurchase up to $100 million of our outstanding common stock through December 31, 2023 (the "2023 Share Repurchase Program"). Under the terms of the 2023 Share Repurchase Program, we are allowed to repurchase shares from time to time through open market purchases, unsolicited or solicited privately negotiated transactions, an accelerated stock repurchase program, and/or a trading plan in compliance with Exchange Act Rule 10b5-1. The timing and the amount of the repurchases will be determined by management based on a number of factors, including but not limited to share price, trading volume and general market conditions, as well as on working capital requirements, general business conditions and other factors. Effective January 1, 2020,2023, repurchases are subject to a 1% excise tax under the Inflation Reduction Act.
Assets Held For Sale
On February 10, 2023, we acquired 100%signed a definitive agreement to sell our personal care business (excluding the Florida operations). The divestment is expected to close during the second quarter of the membership interests in Asana Hospice, a hospice provider with locations in Pennsylvania, Ohio, Texas, Missouri and Kansas2023. See Note 6 - Assets Held For Sale for a purchase price of $67.0 million.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We have established disclosure controls and procedures which are designed to provide reasonable assurance of achieving their objectives and to ensure that information required to be disclosed in our reports filed under the Exchange Act is recorded, processed, summarized, disclosed and reported within the time periods specified in the SEC’s rules and forms. This information is also accumulated and communicated to our management, including our principal executive officer and principal financial officer, and Board of Directors to allow timely decisions regarding required disclosure.
In connection with the preparation of this Annual Report on Form 10-K, as of December 31, 2019,2022, under the supervision and with the participation of our principal executive officer and principal financial officer, our management conducted an evaluation of the effectiveness of our disclosure controls and procedures, as such term is defined under Rules 13a-15(e) and 15d-15(e) promulgated under the Exchange Act.
Based on this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective at a reasonable assurance level as of December 31, 2019,2022, the end of the period covered by this Annual Report on Form 10-K.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act. Under the supervision and with the participation of our principal executive officer and our principal financial officer, our management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation under the framework in Internal Control – Integrated Framework (2013), our management concluded our internal control over financial reporting was effective as of December 31, 2019.2022.
Under guidelines established by the SEC, companies are allowed to exclude acquisitions from their assessment of internal control over financial reporting during the first year of an acquisition while integrating the acquired company. Accordingly, our assessment of internal controls excluded our acquisitionacquisitions of CompassionateEvolution Health, LLC, a division of Envision Healthcare, doing business as Guardian Healthcare, Gem City, and Care HospiceConnection of Cincinnati ("CCH"Evolution") and Assisted Care Home Health, Inc. and RH Homecare Services, LLC doing business as AssistedCare Home Health and AssistedCare of the Carolinas ("AssistedCare"), completed on FebruaryApril 1, 2019.2022. See Item 8, Note 34 - Acquisitions to our consolidated financial statements for additional information on our acquisitionacquisitions of CCH.Evolution and AssistedCare. Operations from this acquisitionthese acquisitions represented approximately 3%1% of total assets and 9%approximately 2% of total revenue as of and for the year ended December 31, 2019.2022.
KPMG LLP, the independent registered public accounting firm that audited our consolidated financial statements included in this Form 10-K, has issued a report on our internal control over financial reporting, which is included herein.
Changes in Internal Controls
There were no changes in our internal control over financial reporting (as defined in Exchange Act Rule 13a-15(f)) that have occurred during the quarter ended December 31, 20192022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including our principal executive officer and principal financial officer, does not expect that our disclosure controls or our internal controls over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls’ effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies and procedures. Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives and, based on an evaluation of our controls and procedures, our principal executive officer and our principal financial officer concluded our disclosure controls and procedures were effective at a reasonable assurance level as of December 31, 2019,2022, the end of the period covered by this Annual Report.
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Amedisys, Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited Amedisys, Inc. and subsidiaries’subsidiaries' (the Company) internal control over financial reporting as of December 31, 2019,2022, based on criteria established in Internal Control -– Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019,2022, based on criteria established in Internal Control -– Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 20192022 and 2018,2021, the related consolidated statements of operations, comprehensive income, stockholders’ equity, and cash flows for each of the years in the three‑yearthree-year period ended December 31, 2019,2022, and the related notes (collectively, the consolidated financial statements), and our report dated February 19, 202016, 2023 expressed an unqualified opinion on those consolidated financial statements.
The Company acquired CompassionateEvolution Health, LLC, a division of Envision Healthcare, doing business as Guardian Healthcare, Gem City, and Care HospiceConnection of Cincinnati (Evolution) and Assisted Care Home Health, Inc. and RH Homecare Services, LLC doing business as AssistedCare Home Health and AssistedCare of the Carolinas (AssistedCare) during 2019,2022, and management excluded from its assessment of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2019, Compassionate2022, Evolution Health, LLC, a division of Envision Healthcare, doing business as Guardian Healthcare, Gem City, and Care Hospice’sConnection of Cincinnati (Evolution) and Assisted Care Home Health, Inc. and RH Homecare Services, LLC doing business as AssistedCare Home Health and AssistedCare of the Carolinas (AssistedCare)’s internal control over financial reporting associated with 3%approximately 1% of total assets and 9%approximately 2% of total revenue included in the consolidated financial statements of the Company as of and for the year ended December 31, 2019.2022. Our audit of internal control over financial reporting of the Company also excluded an evaluation of the internal control over financial reporting of CompassionateEvolution Health, LLC, a division of Envision Healthcare, doing business as Guardian Healthcare, Gem City, and Care Hospice.Connection of Cincinnati (Evolution) and Assisted Care Home Health, Inc. and RH Homecare Services, LLC doing business as AssistedCare Home Health and AssistedCare of the Carolinas (AssistedCare).
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’sManagement's Annual Report on Internal Control over Financial Reporting.Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the
company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
Baton Rouge, Louisiana
February 19, 2020
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item is incorporated by reference to the 20202023 Proxy Statement to be filed with the SEC within 120 days after the end of the year ended December 31, 2019.2022.
Code of Conduct and Ethics
We have adopted a code of ethics that applies to all of our directors, officers and employees, including our principal executive officer, principal financial officer and principal accounting officer. This code of ethics which is entitled Code of Ethical Business Conduct, is posted at our internet website, http://www.amedisys.com. Any amendments to, or waivers of, the code of ethics will be disclosed on our website promptly following the date of such amendment or waiver.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item is incorporated by reference to the 20202023 Proxy Statement to be filed with the SEC within 120 days after the end of the year ended December 31, 2019.2022.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is incorporated by reference to the 20202023 Proxy Statement to be filed with the SEC within 120 days after the end of the year ended December 31, 2019.2022.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item is incorporated by reference to the 20202023 Proxy Statement to be filed with the SEC within 120 days after the end of the year ended December 31, 2019.2022.
ITEM 14. PRINCIPAL ACCOUNTINGACCOUNTANT FEES AND SERVICES
Our independent registered public accounting firm is KPMG LLP, Baton Rouge, Louisiana, Auditor Firm ID: 185
The information required by this item is incorporated by reference to the 20202023 Proxy Statement to be filed with the SEC within 120 days after the end of the year ended December 31, 2019.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
| | | | | | | | | | | | | |
| (a) | | 1. | | Financial Statements |
| | | | All financial statements are set forth under Part II, Item 8 of this report. |
| | | | |
| | 2. | | Financial Statement Schedules |
| | | | There are no financial statement schedules included in this report as they are either not applicable or included in the financial statements. |
| | | | |
| | 3. | | Exhibits |
| | | | The Exhibits are listed in the Exhibit Index required by Item 601 of Regulation S-K preceding the signature page of this report. |
ITEM 16. FORM 10-K SUMMARY
None.
EXHIBIT INDEX
The exhibits marked with the cross symbol (†) are filed and the exhibits marked with a double cross (††) are furnished with this Form 10-K. Any exhibits marked with the asterisk symbol (*) are management contracts or compensatory plans or arrangements filed pursuant to Item 601(b)(10)(iii) of Regulation S-K. The registrant agrees to furnish to the Commission supplementally upon request a copy of any schedules or exhibits omitted pursuant to Item 601(a)(5) of Regulation S-K of any exhibit set forth below.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Document Description | | Report or Registration Statement | | SEC File or
Registration
Number | | Exhibit
or Other
Reference |
| 2.1 | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 | | 0-24260 | | 2.1 |
| | | | | | | | |
| 2.2 | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 2.3 | | | | The Company's current Report on Form 8-K filed on June 4, 2018 | | 0-24260 | | 2.1 |
| | | | | | | | |
| 2.4 | | Stock Purchase Agreement, dated as of October 9, 2018, by and among Milton Heching, the Heching 2012 Exempt Irrevocable Trust, Amedisys Hospice, L.L.C., Compassionate Care Hospice Group, Inc., and solely for purposes of Sections 3.4, 4.3(a), 4.15 and Article VIII thereof, Amedisys, Inc. | | The Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2018 | | 0-24260 | | 2.1 |
| | | | | | | | |
| 2.5 | | | | The Company's Current Report on Form 8-K filed on April 27, 2020 | | 0-24260 | | 2.1 |
| | | | | | | | |
| 2.6 | | Agreement and Plan of Merger, dated as of June 27, 2021, by and among Amedisys Holding, L.L.C., Amedisys Commodore, L.L.C., Contessa Health, Inc., Shareholder Representative Services LLC, and, solely for purposes of Section 10.17, Amedisys, Inc. (Immaterial schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The registrant agrees to furnish supplementally a copy of any omitted schedule or exhibit to the U.S. Securities and Exchange Commission upon request)
| | The Company’s Current Report on Form 8-K filed on August 4, 2021 | | 0-24260 | | 2.1 |
| | | | | | | | |
| 3.1 | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2007 | | 0-24260 | | 3.1 |
| | | | | | | | |
| 3.2 | | | | The Company’s Current Report on Form 8-K filed on December 16, 2022 | | 0-24260 | | 3.1 |
| | | | | | | | |
| 4.1 | | | | The Company’s Registration Statement on Form S-3 filed August 20, 2007 | | 333-145582 | | 4.8 |
| | | | | | | | |
| 4.2 | | | | The Company's Annual Report on Form 10-K for the year ended December 31, 2021 | | 0-24260 | | 4.2 |
|
| | | | | | | | |
Exhibit Number | | Document Description | | Report or Registration Statement | | SEC File or Registration Number | | Exhibit or Other Reference |
| 2.1 | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 | | 0-24260 | | 2.1 |
| | | | | | | | | |
| 2.2 | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 | | 0-24260 | | 10.1 |
| | | | | | | | | |
| 2.3 | | | | The Company's current Report on Form 8-K filed on June 4, 2018 | | 0-24260 | | 2.1 |
| | | | | | | | | |
| 2.4 | | Stock Purchase Agreement, dated as of October 9, 2018, by and among Milton Heching, the Heching 2012 Exempt Irrevocable Trust, Amedisys Hospice, L.L.C., Compassionate Care Hospice Group, Inc., and solely for purposes of Sections 3.4, 4.3(a), 4.15 and Article VIII thereof, Amedisys, Inc. | | The Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2018 | | 0-24260 | | 2.1 |
| | | | | | | | | |
| 3.1 | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2007 | | 0-24260 | | 3.1 |
| | | | | | | | | |
| 3.2 | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2019 | | 0-24260 | | 3.2 |
| | | | | | | | | |
| 4.1 | | | | The Company’s Registration Statement on Form S-3 filed August 20, 2007 | | 333-145582 | | 4.8 |
| | | | | | | | | |
| †4.2 | | | | | | | | |
| | | | | | | | | |
| 10.1 | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2008 | | 0-24260 | | 10.1 |
| | | | | | | | | |
| 10.2* | | | | The Company’s Current Report on Form 8-K filed June 8, 2012 | | 0-24260 | | 10.1 |
| | | | | | | | | |
| †10.3* | | Composite Amedisys, Inc. 2008 Omnibus Incentive Compensation Plan (inclusive of Plan amendments dated June 7, 2012, October 25, 2012, April 23, 2015 and June 4, 2015, January 20, 2017, February 22, 2017 and September 25, 2018 and the full text of the Amedisys, Inc. 2008 Omnibus Incentive Compensation Plan) | | | | | | |
| | | | | | | | | |
| 10.4* | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008 | | 0-24260 | | 10.3 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Document Description | | Report or Registration Statement | | SEC File or
Registration
Number | | Exhibit
or Other
Reference |
| 10.1 | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2008 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.2* | | | | The Company’s Current Report on Form 8-K filed June 8, 2012 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.3* | | Composite Amedisys, Inc. 2008 Omnibus Incentive Compensation Plan (inclusive of Plan amendments dated June 7, 2012, October 25, 2012, April 23, 2015 and June 4, 2015, January 20, 2017, February 22, 2017 and September 25, 2018 and the full text of the Amedisys, Inc. 2008 Omnibus Incentive Compensation Plan) | | The Company's Annual Report on Form 10-K for the year ended December 31, 2019 | | 0-24260 | | 10.3 |
| | | | | | | | |
| 10.4* | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2014 | | 0-24260 | | 10.6 |
| | | | | | | | |
| 10.5* | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2014 | | 0-24260 | | 10.7 |
| | | | | | | | |
| 10.6* | | | | The Company's Annual Report on Form 10-K for the year ended December 31, 2018 | | 0-24260 | | 10.10 |
| | | | | | | | |
| 10.7* | | | | The Company's Annual Report on Form 10-K for the year ended December 31, 2018 | | 0-24260 | | 10.11 |
| | | | | | | | |
| 10.8* | | | | The Company's Annual Report on Form 10-K for the year ended December 31, 2018 | | 0-24260 | | 10.12 |
| | | | | | | | |
| 10.9* | | | | The Company’s Current Report on Form 8-K filed on October 3, 2018 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.10* | | | | The Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2019 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.11* | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2018 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.12* | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2020 | | 0-24260 | | 10.16 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Document Description | | Report or Registration Statement | | SEC File or
Registration
Number | | Exhibit
or Other
Reference |
| | | | | | | | |
| 10.13 | | Amended and Restated Credit Agreement dated as of June 29, 2018, among the Company and Amedisys Holding, L.L.C., as borrowers, certain subsidiaries of the Company party thereto as guarantors, Bank of America, N.A., as Administrative Agent, Swingline Lender and L/C Issuer, JPMorgan Chase Bank, N.A., as Syndication Agent, Capital One Bank National Association, Citizens Bank, N.A., Compass Bank, Fifth Third Bank, Hancock Whitney Bank, Regions Bank, and Wells Fargo Bank, National Association, as Co-Documentation Agents, the lenders party thereto, Merrill Lynch, Pierce Fenner & Smith Incorporated, Citizens Bank N.A., Fifth Third Bank and JPMorgan Chase Bank, N.A., as Joint Lead Arrangers, and Merrill Lynch, Pierce, Fenner & Smith Incorporated and JPMorgan Chase Bank, N.A., as Joint Bookrunners | | The Company’s current Report on Form 8-K filed on July 2, 2018 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.14 | | | | The Company’s current Report on Form 8-K filed on July 2, 2018 | | 0-24260 | | 10.2 |
| | | | | | | | |
| 10.15 | | | | The Company’s current Report on Form 8-K filed on July 2, 2018 | | 0-24260 | | 10.3 |
| | | | | | | | |
| 10.16 | | Agreement and Plan of Merger dated October 31, 2015 by and among Amedisys Health Care West, L.L.C., IHC Acquisitions, L.L.C., Infinity Home Care, L.L.C., Axiom HealthEquity Holdings Management, LLC, Infinity Healthcare Holdings, LLC, and Amedisys, Inc.
| | The Company’s Annual Report on Form 10-K for the year ended December 31, 2015 | | 0-24260 | | 10.27 |
| | | | | | | | |
| 10.17 | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2015 | | 0-24260 | | 10.28 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Document Description | | Report or Registration Statement | | SEC File or
Registration
Number | | Exhibit
or Other
Reference |
| | | | | | | | |
| 10.18 | | First Amendment to Amended and Restated Credit Agreement, dated as of February 4, 2019, by and among the Amedisys, Inc. and Amedisys Holding, L.L.C., as the borrowers, certain subsidiaries of the Company party thereto as guarantors, Bank of America, N.A., as the administrative agent, swingline lender and letter of credit issuer, JPMorganChase Bank, N.A., as syndication agent, Capital One Bank, National Association, Citizens Bank, N.A., Compass Bank, Fifth Third Bank, Hancock Whitney Bank, Regions Bank, and Wells Fargo Bank, National Association, as co-documentation agents, the lenders party thereto, Merrill Lynch, Pierce, Fenner & Smith Incorporated, Citizens Bank, N.A., Fifth Third Bank and JPMorgan Chase Bank, N.A., as joint lead arrangers, and Merrill Lynch, Pierce, Fenner & Smith Incorporated and JPMorgan Chase Bank, N.A., as joint bookrunners
| | The Company’s Current Report on Form 8-K filed on February 4, 2019 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.19 | | Joinder Agreement, dated as of February 4, 2019, by and among Amedisys, Inc. and Amedisys Holding, L.L.C., as the borrowers, each of the new subsidiary guarantors party thereto, and Bank of America, N.A., as the administrative agent | | The Company’s Current Report on Form 8-K filed on February 4, 2019 | | 0-24260 | | 10.2 |
| | | | | | | | |
| 10.20 | | | | The Company’s Current Report on Form 8-K filed on February 19, 2019 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.21 | | Joinder Agreement, dated as of June 12, 2020, by and among Amedisys, Inc. and Amedisys Holding, L.L.C., as the borrowers, each of the new subsidiary guarantors party thereto, and Bank of America, N.A., as the administrative agent (The schedules to the Joinder have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Company will furnish copies of the omitted schedules to the Securities and Exchange Commission upon request.) | | The Company's Current Report on Form 8-K filed on June 15, 2020 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.22* | | | | The Company's Annual Report on Form 10-K for the year ended December 31, 2020 | | 0-24260 | | 10.26 |
| | | | | | | | |
| 10.23* | | | | The Company’s Current Report on Form 8-K filed on February 24, 2021 | | 0-24260 | | 10.1 |
| | | | | | | | |
| 10.24 | | Second Amendment to Amended and Restated Credit Agreement, dated as of July 30, 2021, by and among Amedisys, Inc. and Amedisys Holding, L.L.C., as the borrowers, the Guarantors party thereto, the Lenders party thereto, Bank of America, N.A., as Administrative Agent, Swingline Lender and L/C Issuer, and the other L/C Issuers party thereto (Immaterial schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Company agrees to furnish supplementally a copy of any omitted schedule or exhibit to the U.S. Securities and Exchange Commission upon request.) | | The Company’s Current Report on Form 8-K filed on August 4, 2021 | | 0-24260 | | 10.1 |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Document Description | | Report or Registration Statement | | SEC File or
Registration
Number | | Exhibit
or Other
Reference |
| | | | | | | | |
| 10.25* | | Exhibit
| | Document Description | | Report or Registration Statement | | SEC File or
Registration
Number
| | Exhibit
or Other
Reference
|
10.5* | | | | The Company’s QuarterlyCurrent Report on Form 10-Q for the quarter ended June 30, 20088-K filed on January 10, 2022 | | 0-24260 | | 10.4 |
| | | | | | | | |
10.6* | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2014 | | 0-24260 | | 10.6 |
| | | | | | | | |
10.7* | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2014 | | 0-24260 | | 10.7 |
| | | | | | | | |
10.8* | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2014 | | 0-24260 | | 10.8 |
| | | | | | | | |
10.9* | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2014 | | 0-24260 | | 10.9 |
| | | | | | | | |
10.10* | | | | The Company's Annual Report on Form 10-K for the year ended December 31, 2018 | | 0-24260 | | 10.10 |
| | | | | | | | |
10.11* | | | | The Company's Annual Report on Form 10-K for the year ended December 31, 2018 | | 0-24260 | | 10.11 |
| | | | | | | | |
10.12* | | | | The Company's Annual Report on Form 10-K for the year ended December 31, 2018 | | 0-24260 | | 10.12 |
| | | | | | | | |
10.13* | | | | The Company’s Registration Statement on Form S-8 filed June 22, 2007 | | 333-143967 | | 4.2 |
| | | | | | | | |
10.14* | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2005 | | 0-24260 | | 10.4 |
|
| | | | | | | | |
Exhibit
Number
10.26* | | Document Description | | Report or Registration Statement | | SEC File or
Registration
Number
| | Exhibit
or Other
Reference
|
| | | | | | | | |
10.15* | | | | The Company’s CurrentCompany's Quarterly Report on Form 8-K filed on October 3, 201810-Q for the quarter ended September 30, 2022 | | 0-24260 | | 10.1 |
| | | | | | | | |
10.16*†10.27* | | | | The Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2019 | | 0-24260 | | 10.1 |
| | | | | | | | |
10.17* | | | | The Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2017 | | 0-24260 | | 10.1 |
| | | | | | | | |
10.18* | | | | The Company's Quarterly Report on Form 10-Q for the quarter ended September 30, 2017 | | 0-24260 | | 10.2 |
| | | | | | | | |
10.19* | | | | The Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2018 | | 0-24260 | | 10.1 |
| | | | | | | | |
†10.20* | | | | | | | | |
|
| | | | | | | | |
Exhibit Number | | Document Description | | Report or Registration Statement | | SEC File or Registration Number | | Exhibit or Other Reference |
| 10.21 | | Amended and Restated Credit Agreement dated as of June 29, 2018, among the Company and Amedisys Holding, L.L.C., as borrowers, certain subsidiaries of the Company party thereto as guarantors, Bank of America, N.A., as Administrative Agent, Swingline Lender and L/C Issuer, JPMorgan Chase Bank, N.A., as Syndication Agent, Capital One Bank National Association, Citizens Bank, N.A., Compass Bank, Fifth Third Bank, Hancock Whitney Bank, Regions Bank, and Wells Fargo Bank, National Association, as Co-Documentation Agents, the lenders party thereto, Merrill Lynch, Pierce Fenner & Smith Incorporated, Citizens Bank N.A., Fifth Third Bank and JPMorgan Chase Bank, N.A., as Joint Lead Arrangers, and Merrill Lynch, Pierce, Fenner & Smith Incorporated and JPMorgan Chase Bank, N.A., as Joint Bookrunners | | The Company’s current Report on Form 8-K filed on July 2, 2018 | | 0-24260 | | 10.1 |
| | | | | | | | | |
| 10.22 | | | | The Company’s current Report on Form 8-K filed on July 2, 2018 | | 0-24260 | | 10.2 |
| | | | | | | | | |
| 10.23 | | | | The Company’s current Report on Form 8-K filed on July 2, 2018 | | 0-24260 | | 10.3 |
| | | | | | | | | |
| 10.24 | | | | The Company’s Current Report on Form 8-K filed on April 24, 2014 | | 0-24260 | | 10.1 |
| | | | | | | | | |
| 10.25 | | | | The Company’s Current Report on Form 8-K filed on April 24, 2014 | | 0-24260 | | 10.2 |
| | | | | | | | | |
| †10.26 | | | | | | | | |
| | | | | | | | | |
| 10.27 | | Agreement and Plan of Merger dated October 31, 2015 by and among Amedisys Health Care West, L.L.C., IHC Acquisitions, L.L.C., Infinity Home Care, L.L.C., Axiom HealthEquity Holdings Management, LLC, Infinity Healthcare Holdings, LLC, and Amedisys, Inc.
| | The Company’s Annual Report on Form 10-K for the year ended December 31, 2015 | | 0-24260 | | 10.27 |
|
| | | | | | | | |
Exhibit Number | | Document Description | | Report or Registration Statement | | SEC File or Registration Number | | Exhibit or Other Reference |
| | | | | | | | | |
| 10.28 | | | | The Company’s Annual Report on Form 10-K for the year ended December 31, 2015 | | 0-24260 | | 10.28 |
| | | | | | | | | |
| 10.29 | | First Amendment to Amended and Restated Credit Agreement, dated as of February 4, 2019, by and among the Amedisys, Inc. and Amedisys Holding, L.L.C., as the borrowers, certain subsidiaries of the Company party thereto as guarantors, Bank of America, N.A., as the administrative agent, swingline lender and letter of credit issuer, JPMorganChase Bank, N.A., as syndication agent, Capital One Bank, National Association, Citizens Bank, N.A., Compass Bank, Fifth Third Bank, Hancock Whitney Bank, Regions Bank, and Wells Fargo Bank, National Association, as co-documentation agents, the lenders party thereto, Merrill Lynch, Pierce, Fenner & Smith Incorporated, Citizens Bank, N.A., Fifth Third Bank and JPMorgan Chase Bank, N.A., as joint lead arrangers, and Merrill Lynch, Pierce, Fenner & Smith Incorporated and JPMorgan Chase Bank, N.A., as joint bookrunners
| | The Company’s Current Report on Form 8-K filed on February 4, 2019 | | 0-24260 | | 10.1 |
| | | | | | | | | |
| 10.30 | | Joinder Agreement, dated as of February 4, 2019, by and among Amedisys, Inc. and Amedisys Holding, L.L.C., as the borrowers, each of the new subsidiary guarantors party thereto, and Bank of America, N.A., as the administrative agent | | The Company’s Current Report on Form 8-K filed on February 4, 2019 | | 0-24260 | | 10.2 |
| | | | | | | | | |
| 10.31 | | | | The Company’s Current Report on Form 8-K filed on February 19, 2019
| | 0-24260 | | 10.1 |
| | | | | | | | | |
| †21.1 | | | | | | | | |
| | | | | | | | | |
| †23.1 | | | | | | | | |
| | | | | | | | | |
| †31.1 | | | | | | | | |
| | | | | | | | | |
| †31.2 | | | | | | | | |
| | | | | | | | | |
| ††32.1 | | | | | | | | |
| | | | | | | | | |
| ††32.2 | | | | | | | | |
|
| | | | | | | | |
| †21.1 | | Exhibit
| | | | | | |
| | | | | | | | |
| †23.1 | | | | | | | | |
| | | | | | | | |
| †31.1 | | | | | | | | |
| | | | | | | | |
| †31.2 | | | | | | | | |
| | | | | | | | |
| ††32.1 | | | | | | | | |
| | | | | | | | |
| ††32.2 | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Document Description | | Report or Registration Statement | | SEC File or
Registration
Number
| | Exhibit
or Other
Reference
|
| †101.INS | | Inline XBRL Instance - The instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | | | | | | |
| | | | | | | | |
| †101.SCH | | Inline XBRL Taxonomy Extension Schema Document | | | | | | |
| | | | | | | | |
| †101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document | | | | | | |
| | | | | | | | |
| †101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase | | | | | | |
| | | | | | | | |
| †101.LAB | | Inline XBRL Taxonomy Extension LabelsLabel Linkbase Document | | | | | | |
| | | | | | | | |
| †101.PRE | | Inline XBRL Taxonomy Extension Presentation Linkbase Document | | | | | | |
| | | | | | | | |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | | | | | | |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | | | | | | |
AMEDISYS, INC. |
|
| By: | | /S/ PAUL B. KUSSEROW |
| | Paul B. Kusserow, |
| | President, Chief Executive Officer and |
| | Chairman of the Board |
Date: February 19, 202016, 2023
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the date indicated:
|
| | | | | | | | | | | | | |
| Signature | | Title | | Date |
| | | | |
/S/ PAUL B. KUSSEROW | | President, Chief Executive Officer
and Chairman of the Board (Principal
Executive Officer)
| | February 19, 202016, 2023 |
| Paul B. Kusserow | | | |
| | | | |
/S/ SCOTT G. GINN | | Acting Chief Operating Officer, Executive Vice President and Chief Financial Officer (Principal
Financial Officer and Principal Accounting Officer)
| | February 19, 202016, 2023 |
| Scott G. Ginn | | | |
| | | | |
/S/ VICKIE L. CAPPS | | Director | | February 19, 202016, 2023 |
| Vickie L. Capps | | | |
| | | | |
/S/ MOLLY COYE, MD | | Director | | February 19, 202016, 2023 |
| Molly Coye, MD | | | |
| | | | |
/S/ JULIE D. KLAPSTEIN | | Lead Independent Director | | February 19, 202016, 2023 |
| Julie D. Klapstein | | | |
| | | | |
/S/ TERESA L. KLINE | | Director | | February 19, 202016, 2023 |
| Teresa L. Kline | | | |
| | | | |
/S/ RICHARD A. LECHLEITER
| | Lead Independent Director | | February 19, 2020 |
Richard A. Lechleiter | | | |
| | | | |
/S/ JAKE L. NETTERVILLE
| | Director | | February 19, 2020 |
Jake L. Netterville | | | |
| | | | |
/S/ BRUCE D. PERKINS | | Director | | February 19, 202016, 2023 |
| Bruce D. Perkins | | | |
| | | | |
/S/ JEFFREY A. RIDEOUT, MD | | Director | | February 19, 202016, 2023 |
| Jeffrey A. Rideout, MD | | | |
| | | | |
/S/ DONALD A. WASHBURNIVANETTA D. SAMUELS | | Director | | February 19, 202016, 2023 |
Donald A. WashburnIvanetta D. Samuels | | | |


