PART I
ITEM 1. BUSINESS
OVERVIEW
The terms “we,” “our,” “us,” “Company”“Company,” "Integra LifeSciences," and “Integra” refer to Integra LifeSciences Holdings Corporation, a Delaware corporation, and its subsidiaries, unless the context suggests otherwise.
Integra headquartered in Princeton, New Jersey,LifeSciences is a worldglobal leader in medical technology. The Company was foundedregenerative tissue technologies and neurological solutions dedicated to limiting uncertainty for clinicians so they can focus on providing the best patient care. Founded in 1989 with the acquisition of an engineered collagen technology platform used to repair and regenerate tissue. Since then,tissue, Integra LifeSciences Holdings Corporation common stock trades on the Nasdaq Global Select Market (“Nasdaq”) under the symbol “IART.” Integra has developed numerous product lines from this technology for applications ranging from burn and deep tissue wounds to the repair of dura mater in the brain, as well as nerves and tendons. The Company has expanded its base regenerative technology business to include surgical instruments, neurosurgical products and advanced wound care and orthopedic hardware, through a combination of several global acquisitions and product development of products internally to further meet the evolving needs of its customers and impactenhance patient care.
Integra products are sold in more than 130 countries through a direct sales force as well as distributors and wholesalers. We manufacture and sell ourmedical technologies and products in two reportable business segments: Codman Specialty Surgical and Orthopedics and Tissue Technologies. Our Codman Specialty Surgical products comprise specialty surgical implants and instrumentation for a broad range of specialties. This segment includes products and solutions for dural access and repair, precision tools and instruments, advanced energy, cerebral spinal fluid ("CSF"CSS") management and neuro monitoring including market-leading product portfolios used in neurosurgery operation suites and critical care units. Our Orthopedics and Tissue Technologies product portfolios consist("TT"). The CSS segment, which represents approximately two-thirds of differentiated regenerative technology productsour total revenue, consists of market-leading technologies and instrumentation used for soft tissue repaira wide range of specialties, such as neurosurgery, neurocritical care and tissue regeneration products,otolaryngology. We are the world leader in neurosurgery and one of the top three providers in instruments used in precision, specialty, and general surgical procedures. Our TT segment generates about one-third of our overall revenue and focuses on three main areas: complex wound surgery, surgical reconstruction, and small bone fixation and joint replacement hardware products for both upper extremities and lower extremities. This business also includes private label sales of a broad set of our regenerative and wound care medicine technologies.peripheral nerve repair.
We have key manufacturing and research facilities located in California, Indiana, Maryland, Massachusetts, New Jersey, Ohio, Massachusetts,Puerto Rico, Tennessee, Texas, Canada,Utah, France, Germany, Ireland Switzerland, and Puerto Rico. Switzerland. We also source most of our handheld surgical instruments specialty metal and pyrocarbon implants, and dural sealant products through specialized third-party vendors.
We aspire to continue to be a worldwide leader in neurosurgery &and reconstructive surgery with a portfolio of leading businesses that delivers outstanding customer experienceexperiences through innovation, execution and teamwork to positively impact the lives of millions of patients and their families.
Integra is committed to delivering high quality products that positively impact the lives of millions of patients and their families. We focus on four key pillars of our strategy: 1) buildingenabling an execution-focused culture, 2) achievingoptimizing relevant scale, 3) improvingadvancing innovation and agility, and innovation, and 4) leading in customer experience. We believe that by sharpening our focus on these areas through improved planning and communication, optimization of our infrastructure, and strategically aligned tuck-in acquisitions, we can build scale, increase competitiveness and achieve our long-term goals.
To this end, the executive leadership team has established the following key priorities aligned to the following areas of focus:
Strategic Acquisitions. An important part of the our strategy is pursuing strategic transactions and licensing agreements that increase relevant scale in the clinical areas in which Integra competes. In 2019, we closed outOur growth strategy includes the acquisition of 45 transition service agreements, covering 90 countries, markingbusinesses, assets or products lines to increase the successful completionbreadth of our offerings, the integrationreach of our product portfolios and drive relevant scale to our customers. On December 6, 2022, the Codman NeurosurgeryCompany completed the acquisition of Surgical Innovation Associates, Inc. ("SIA"), which develops, markets and sells DuraSorb®, a resorbable synthetic matrix for plastic and reconstructive surgery. This acquisition will advance Integra’s global strategy in breast reconstruction, expanding plans to access the most significantU.S. market where SIA is pursuing pre-market approval for use in implant-based breast reconstruction ("IBBR"). We also continued to expand our product offering of regenerative technologies from our 2021 acquisition of ACell, Inc. ("ACell"), an innovative regenerative medicine company specializing in the Company’s history. This acquisition expanded our portfoliomanufacturing of neurosurgery productsporcine urinary bladder extracellular matrices. See Note 4, Acquisitions and established us asDivestitures, to the world leader in neurosurgery. It has also enabled usNotes to bring our entire product portfolio to a global market. In 2019, Integra acquired Arkis Biosciences, Inc. and Rebound Therapeutics Corporation, bothConsolidated Financial Statements (Part IV, Item 15 of which align with Company’s strategy to acquire and develop innovative technologies that address unmet needs in patient care.this Annual Report on Form 10-K) for additional details.
As such, we may opportunistically divest businesses or discontinue products where we see limited runway for future value creation in line with our aspirations due in part to changes in the market, business fundamentals or the regulatory environment.
4
In August 2022, we completed the sale of our non-core traditional wound care ("TWC") business to Gentell, LLC ("Gentall") for $28.8 million, which consists of $27.8 million in cash plus $1.0 million in contingent consideration which may be received upon the achievement of certain revenue-based performance milestones. In January 2021, we completed the sale of our Extremity Orthopedics business to Smith & Nephew USD Limited ("Smith & Nephew"), a subsidiary of Smith & Nephew plc, for approximately $240 million in cash. Our portfolio optimization actions over the past two years have allowed us to increase our focus on Integra’s core portfolio of market-leading products in neurosurgery, surgical instrumentation and product franchisesregenerative tissue and moves us closer to achieving our long-term organic growth and profitability targets. See Note 4, Acquisitions and Divestitures, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for discontinuation.additional details.
Commercial Channel Investments.Investments With acquisitions, new product introductions and a broader portfolio of products, investing. Investing in our sales channels is a core part of our strategy to create specialization and greater focus on reaching new and existing customers and addressing their needs. To support our commercial efforts in Tissue Technologies, we utilize a two-tier specialist model to increase our presence in focused segments to help serve the evolving needs of our customers. In addition, we continue to build upon our leadership brands across our product franchises in both CSS and TT to engage customers through enterprise-wide contracts with leading hospitals, integrated delivery networks and global purchasing organizations in the United States. Internationally, we have increased our commercial resources significantly in manykey emerging markets and are making investments to support our sales organization and maximize our commercial opportunities. We nowDomestically, we have a strongalso increased our TT sales force in the United States to support the expanded regenerative tissue product portfolio that includes ACell products. These investments in our international and domestic sales channel that will deliver our current portfolio as well as position us well for expansion. In addition, we continue to build upon our leadership brands across our product franchises to enable us to engage hospital systems through enterprise-wide contracts.expansion and long-term growth.
Customer Experience. We aspire to be ranked as a best-in-class provider and are committed to strengthening our relationships with all customers. We strive to consistently deliver outstanding customer service and continue to invest in technologies, systems and processes to improveenhance the way our customers do business with us. Additionally,customer experience. In 2022, we expectoutsourced certain transactional back-office finance and customer service activities to enhance customer quality, build on the success of our professional educationscale for future growth, and capture cost efficiencies. We also launched digital tools and programs, resources and virtual product training to drive continued customer familiarity with our growing portfolio of medical technologies globally. .
BUSINESS SEGMENTS
Codman Specialty Surgical
In 2022, we made progress to several enhancements to our CUSA Clarity Tissue Ablation System. The acquisitionextended laparoscopic tip was launched in the U.S. to enhance laparoscopic liver procedures. In addition, a single-sided bone tip received 510(k) clearance. Commercial launch is expected in the first quarter of Codman Neurosurgery from Johnson & Johnson increased2023. We continue to update our global direct sales representationCUSA Clarity platform by incorporating new ultrasonic handpiece and commercial presence. This acquisition expandedintegrated electrosurgical capabilities.
Moreover, we are expanding into minimally invasive surgery ("MIS") and the product portfoliosurgical management of intracerebral hemorrhages ("ICH"), with the 2021 clinical launch of Aurora® Surgiscope®, a proprietary surgical solution with integrated visualization and capabilities designed specifically for use in deep-seated brain lesions. We continue to gather clinical evidence using this same technology for early surgical intervention of ICH. We believe this technology offers the promise of transforming the standard of care in neurosurgery. In 2022, we launched the Aurora® Evacuator with Coagulation device in the U.S., designed to be used in conjunction with our well known, leading technologiesAurora Surgiscope to safely address and evacuate blood in dural repair, ultrasonic tissue ablation, intracranial pressure ("ICP") monitoring, hydrocephalus management, and cranial stabilization systems, while providing a rich research and development pipeline for growth.the brain caused by hemorrhagic stroke.
Rounding out the portfolio is a catalog of surgical headlamps and surgical instrumentation, as well as asset management software and support, and after-market service. With thousands of surgical instrument products, including specialty surgical instruments, we call on the central sterile processing unit of hospitals and acute care surgical centers. Additionally, through a strong U.S. distribution model, we can serve the needs of hundreds of physician, dentalmedical offices.
We also expanded our product offerings in 2021 with the launch of our new intracranial pressure ("ICP") monitoring system, CereLink® in the U.S. and veterinary offices.Europe and continued the global rollout in the first half of 2022. See additional discussion regarding certain matters with CereLinkunder "Item 1A. Risk Factors" under the heading Risks Related to our Regulatory Environment
5
and under "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations - General - FDA Matters" of this Annual Report on Form 10-K.
Our global commercial network includes clinical specialists, a large direct global sales force and strategic partnerships and distributors that serve hospitals, integrated health networks, group purchasing organizations, clinicians, surgery centers and health care providers.
Outside the U.S., we have a smallcombination of direct and indirect sales presence, primarilychannels in certain European countries, Australia, New Zealand, and Canada, and use distributors in other international markets to sell certain product lines.
This business segment also includes private-label sales of a broad set of our regenerative and wound care technologies. Our customers are other medical technology companies that sell to end markets primarily in orthopedics, spine, surgical and wound care.
COMPETITION
Our competitors for Codman Specialty SurgicalCSS are the Aesculap division of B. Braun Medical, Inc., Medtronic, Inc., Stryker Corporation, and Becton Dickinson and Company.Company, and B. Braun Medical, Inc. In addition, we compete with many smaller specialized companies and larger companies that do not otherwise focus on the offerings of Codman Specialty Surgical technologies. We rely on the depth and
breadth of our sales and marketing organization, our innovative technology,technologies, and our procurement and manufacturing operations to maintain our competitive position.
Our competition in Orthopedics and Tissue Technologiesfor TT includes the DePuy/Synthes business of Johnson & Johnson, ACell, Inc., Stryker Corporation, Wright Medical Group, N.V., Smith & Nephew plc, Organogenesis Holdings Inc., MiMedx Group, Inc., LifeCell Corporation, a subsidiary of Allergan PLC, Becton Dickinson and Zimmer Biomet Holdings,Company, and Axogen, Inc., as well as other major orthopedic We compete with many additional companies that carry a full line of small bone and joint fixation andwho partially participate in soft tissue products.
Our research and development activities focus on identifying unmet surgical needs and addressing those needs with innovative solutions and products. We apply our core competency in regenerative technology to innovate products for neurosurgical, wound applications, plastic surgery, and reconstructive surgery and we have extensive R&D development programs for our core platforms of electromechanical technologies. Additionally, we conduct products and clinical studies to generate efficacy and health economic evidence.
Regenerative Technologies.Integra was the first company to receive a FDA claim for regeneration of dermal tissue and is a world leader in regenerative technology. Because regenerative technology products represent a fast-growing, high-margin opportunity for us, we allocate a large portion of our research and development budget to these projects. Our regenerative technology development program applies our expertise in bioengineering to a range of biomaterials including natural materials such as purified collagen, intact human or animal tissues, honey as well as resorbable synthetic polymers with our DuraSorband DuraSealproduct lines. These unique product designs are used for neurosurgical and reconstructive surgical applications, as well as dermal regeneration, including the healing of chronic and acute wounds, tendon and nerve repair. Our regenerative
6
technology platform includes our legacy Integra® Dermal Regeneration Template ("IDRT") products and complementary technologies that we have acquired. Our collagen manufacturing capability, combined with our history of innovation, including our launch of NeuraGen 3D, provides us with strong platform technologies for multiple indications.
In 2020, we announced positive clinical and economic data on Integra® Bilayer Wound Matrix ("IBWM") in complex lower extremity reconstruction based on two retrospective studies recently published in Plastic and Reconstructive Surgery, the official journal of the American Society of Plastic Surgeons. As surgeons look for ways to efficiently and effectively repair and close wounds, IBWM helps address the efficiency needed in operating rooms by reducing both the operating time and costs to hospitals and patients. In 2021, we completed one of the largest diabetic foot ulcers ("DFU"), randomized controlled trials of the PriMatrix® Dermal Repair Scaffold for the management of DFU. This multi-center study enrolled more than 225 patients with chronic DFU's over the course of 12-week treatments and 4-week follow-up phases. The results of this study, which was published in the Journal of Wound Care, demonstrated that PriMatrix plus standard of care ("SOC") consisting of sharp debridement, infection elimination, use of dressings and offloading was significantly more likely to achieve complete wound closure compared with SOC alone, with a median number of one application of the product. Integra is currently pursuing pre-market approval for implant-based breast reconstruction with our Surgimend product. In 2022, we acquired SIA, which is also pursuing a pre-market approval for IBBR. By offering two distinct product solutions, we believe we have the opportunity to build a leading position in the market. We completed design control activities in 2022 for a Q1 2023 launch of Cytal and MicroMatrix in Europe and a pilot launch of the Cardion Pericardial Patch to gain clinical experience for finding a private label partner for that product.
Electromechanical Technologies and Instrumentation. Because our electromechanical products and instruments address significant needs in surgical procedures and limit uncertainty for surgeons, we continue to invest in approvals for new indications and next generation improvements to our market-leading products. We have several active programs focused on life cycle management and innovation for capital and disposable products in our portfolio. Our product development efforts are focused on core clinical applications in cerebrospinal fluid ("CSF") management, neuro-critical care monitoring, minimally invasive instruments and electrosurgery and ultrasonic medical technologies, as well as our ambition to transform the standard of care in neurosurgery with product advancements in MIS and ICH. Our lighting franchise is among the most dynamic in the industry.
We are focused on the development of core clinical applications in our electromechanical technologies portfolio. In 2022, we made progress to several enhancements to our CUSA Clarity Tissue Ablation System. The extended laparoscopic tip was launched in the U.S. to enhance laparoscopic liver procedures. In addition, a manufacturersingle-sided bone tip received 510(k) approval. Commercial launch is expected in the first quarter of 2023. We continue to update our CUSA Clarity platform by incorporating new ultrasonic handpiece and marketerintegrated electrosurgical capabilities. We continue to work with several instrument partners to bring new surgical instrument platforms to the market.
We are focused on the development of medical devices,core clinical applications in our electromechanical technologies portfolio. In June 2022, we launched the Neutus® EVD system, our first external ventricular drain (“EVD") in China. The Neutus EVD system is manufactured in China by Shanghai Haoju Medical Technology Co., Ltd. under an exclusive distribution arrangement. The device is used in the management of cerebrospinal fluid and therefore are subjectis highly complementary to extensive regulation byour Bactiseal® catheter and advanced intercranial pressure monitoring products.
In the third quarter of 2021, we launched our CereLink ICP Monitor System in the U.S. and Europe and continued the global rollout in the first half of 2022.On August 18, 2022, the Company, after consultation with the FDA the Center for Medicare Servicesand other regulatory authorities outside of the U.S. DepartmentUnited States, initiated an immediate voluntary global product removal of Health all CereLink® intracranial pressure monitors. See Item 1A. Risk Factors, under the heading Risks Related to our Regulatory Environment and Human Services, other federal governmental agenciesunder Item 7. General Management's Discussion and Analysis of Financial Condition and Results of Operations - FDA Matters of this Annual Report on Form 10-K for further discussion.
In 2022, we continued to advance the early-stage technology platforms we acquired in some jurisdictions, by state2019. Through the acquisition of Arkis Biosciences, Inc. ("Arkis") we added a platform technology, CerebroFlo® external ventricular drainage ("EVD"), catheter with Endexo® technology, a permanent additive designed to reduce the potential for catheter obstruction due to thrombus formation. The CerebroFlo EVD Catheter has demonstrated an average of 99% less thrombus accumulation onto its surface, in vitro, compared to a market leading EVD catheter. Our work to combine our bactiseal antimicrobial technology with the Endexo anti-occlusive technology obtained through our 2019 acquisition of Arkis continues to progress for both a silicone-based hydrocephalus and foreign governmental authorities. These regulations governEVD project.
In 2019, we also acquired Rebound Therapeutics Corporation ("Rebound Therapeutics"), a company that specialized in single-use medical device, known as Aurora Surgiscope, which is the introductiononly tubular retractor system designed for cranial surgery with an integrated access channel, camera and lighting. In the third quarter of new medical devices, the observance of certain standards with respect to the design, manufacture, testing, labeling, promotion and sales2021, we conducted a limited clinical launch of the devices,Aurora Surgiscope for use in minimally invasive neurosurgery as well as initiated a registry called MIRROR to collect data on early surgical intervention using this same technology platform for the maintenancetreatment of certain records,ICH. In 2022, we launched the ability to track devices, the reporting of potential product defects, the import and export of devices, and other matters.Aurora®
7
In November 2017, the FDA issued the final guidance document relatedgeneral, raw materials essential to human tissue titled, “Regulatory Considerationsour businesses are readily available from multiple sources. For reasons of quality assurance, availability, or cost effectiveness, certain components and raw materials are available only from a sole supplier. Our practice is to maintain sufficient inventory of components so that our production will not be significantly disrupted even if a particular component or material is not available for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use” (the “HCT/P Final Guidance”). The HCT/P Final Guidance maintains the FDA’s position that products such as the company’s morselized amniotic membrane tissue-based products do not meet the criteria for regulation solely as HCT/Ps. In addition, the FDA articulated a risk-based approach to enforcement and, while some uses for amniotic membrane tissue-based products would have as much as thirty-six monthsperiod of enforcement discretion, other high risk uses could be subject to immediate enforcement action. The company does not believe the uses for its amniotic membrane tissue-based products fall into the high-risk category. As of February 21, 2020, the company has not received any further notice of enforcement action from the FDA regarding its morselized amniotic tissue-based products. Nonetheless, we can make no assurances that the FDA will continue to exercise its enforcement discretion with respect to the company’s morselized amniotic membrane tissue-based products, and any potential action of the FDA could have a financial impact regarding the sales of such products. The company has been considering and continues to consider regulatory approval pathways for its morselized amniotic membrane tissue-based products.time.
INTELLECTUAL PROPERTY
We seek patent and trademark protection for our key technology, products and product improvements, both in the U.S. and in selected foreign countries. When determined appropriate, we have enforced and plan to continue to enforce and defend our patent and trademark rights. In general, however, we do not rely solely on our patent and trademark estate to provide us with any significant competitive advantages as it relates to our existing product lines. We also rely upon trade secrets and continuing technological innovations to develop and maintain our competitive position. In an effort to protect our trade secrets, we have a policy of requiring our employees, consultants and advisors to execute proprietary information and invention assignment agreements upon commencement of employment or consulting relationships with us. These agreements also provide that all confidential information developed or made known to the individual during the course of their relationship with us must be kept confidential, except in specified circumstances.
AccuDrain®, AmnioExcel®, AmnioMatrixAquasonic®, BioDFactorAuragen®, Aurora® Surgiscope®, Bactiseal®, BioDFence®, BioDOptix®, BioDRestore™, BioguardBrainet®, BioMotion®, Bold®, Budde®, Buzz™, CadenceCereLink®, Capture™,CerebroFlo® EVD Catheter with Endexo® Technology, Codman®, Codman Accu-Flo®, Codman Bicol®, Codman®Certas®Plus, Codman® Hakim®Programmable valve, Codman Holter®, Codman ICP Express®, Codman Microsensor®, Codman VersaTru®, Codman VPV®, Contour-Flex®, Cranioplastic®,CRW®, CRW Precision™, Ctherm™, CUSA®, DigiFuseCytal®, DirectLink®, DuraGen®, DuraSeal®, First ChoiceDuraSorb®,, Hallu Gentrix®, HeliCote®, HeliPlug®, HeliTape®,, HeliMend®, Helistat®, Helitene®, Hermetic™, Hy-Tape®, Integra®, IntegraLink®, , IPP-ON®, Isocool®, Jarit®, Lead-Lok™, Licox®, LimiTorr™, Luxtec®, Mayfield®, MediHoneyMatriStem UBM™, MediHone®, MemoFix®y®, MicroFrance®, MicroMatrix®,Miltex®, MovementMischler™, MoniTorr ICP™, Natus®, NeuraGen®, NeuraWrap™, NuGripNicolet®, Omnigraft®, Omni-Tract®, OSV II®, Qwix®, Padgett®, Panta®, PriMatrix®, PyroSphere®Pureflow™, Q-Snor™, Redmond™, Revize™, Ruggles®, SafeGuardSignacreme®, Salto Talaris®, Subtalar MBA®, SurgiMend®, TCC-EZ®, TenoGlide®, Ti6®, Tibiaxys®, TissueMend®, Titan™ Ultra VS™, TruArchVersaTru®, Uni-CP®, Uni-Clip®, Xtrasorb®, zRIP™, and the Integra logo are some of the material trademarks of Integra LifeSciences Corporation and its subsidiaries. MAYFIELD® is a registered trademark of SM USA, Inc., and is used by Integra under license.
SEASONALITY
Revenues during our fourth quarter tend to be stronger than other quarters because many hospitals increase their purchases of our products during the fourth quarter to coincide with the end of their budget cycles in the U.S. In general, our first quarter usually has lower revenues than the preceding fourth quarter, the second and third quarters have higher revenues than the first quarter, and the fourth quarter revenues are the highest in the year. The main exceptions to this pattern occur because of material intervening acquisitions.acquisitions as well as impacts of the COVID-19 pandemic.
GOVERNMENT REGULATION AND COMPLIANCE
We are a manufacturer and marketer of medical devices and Human Tissue and Cell Based Products ("HCT/Ps") and therefore are subject to extensive regulation by the FDA, the Center for Medicare Services of the U.S. Department of Health and Human Services, other federal governmental agencies and, in some jurisdictions, by state and foreign governmental authorities. These regulations govern the introduction of new medical devices and HCT/Ps, the observance of certain standards with respect to the design, manufacture, testing, labeling, promotion and sales of the products, the maintenance of certain records, the ability to track devices, the reporting of potential product defects, the import and export of products, and other matters.
8
United States Food and Drug Administration
Our products are subject to extensive regulation particularly as to safety, efficacy and adherence to FDA Quality System Regulation, and related manufacturing standards. Medical device products are subject to rigorous FDA and other governmental agency regulations in the United States and similar regulations of foreign agencies abroad. The FDA regulates the design, development, research, preclinical and clinical testing, introduction, manufacture, advertising, labeling, packaging, marketing, distribution, import and export, and record keeping for such products, in order to ensure that medical products distributed in the United States are safe and effective for their intended use. In addition, the FDA is authorized to establish special controls to provide reasonable assurance of the safety and effectiveness of most devices. Non-compliance with applicable requirements can result in import detentions, fines, civil and administrative penalties, injunctions, suspensions or losses of regulatory approvals, recall or seizure of products, operating restrictions, refusal of the government to approve product export applications or allow us to enter into supply contracts, and criminal prosecution. The regulatory process for obtaining product approvals and clearances can be onerous and costly. The FDA requires, as a condition to marketing a medical device in the U.S., that we secure a Premarket Notification clearance pursuant to Section 510(k) of the Federal Food, Drug and Cosmetic Act (the "FD&C Act"), or an approved PMA application (or supplemental PMA application). Obtaining these approvals and clearances can take up to several years and may involve preclinical studies and clinical trials. The FDA also may require a post-approval clinical study as a condition of approval. To perform clinical trials for significant risk devices in the U.S. on an unapproved product, we are required to obtain an Investigational Device Exemption from the FDA. The FDA also may require a filing for approval prior to marketing products that are modifications of existing products or new indications for existing products. Moreover, after clearance/approval is given, if the product is shown to be hazardous or defective, the FDA and foreign regulatory agencies have the power to withdraw the clearance or approval, as the case may be, or require us to change the device, its manufacturing process or its labeling, to supply additional proof of its safety and effectiveness or to recall, repair, replace or refund the cost of the medical device. Because we currently export medical devices manufactured in the U.S. that have not been approved by the FDA for distribution in the U.S., we are required to obtain approval/registration in the country to which we are exporting and maintain certain records relating to exports and make these available to the FDA for inspection, if required.
Human Cells, Tissues and Cellular and Tissue-Based Products
Integra, through its wholly-owned subsidiary BioD LLC ("BioD"), is involved with the recovery, processing, storage, transportation and distribution of donated amniotic tissue. The FDA has specific regulations governing HCT/Ps. An HCT/P is a product containing, or consisting of, human cells or tissue intended for transplantation into a human patient. Examples of HCT/Ps include bone, ligament, skin and cornea.
Some HCT/Ps fall within the definition of a biological product, medical device or drug regulated under the FD&C Act. These biologic, device or drug HCT/Ps must comply both with the requirements exclusively applicable to HCT/Ps and, in addition, with requirements applicable to biologics, devices or drugs, including premarket clearance or approval from the FDA.
Section 361 of the Public Health Service Act ("Section 361") authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease. HCT/Ps regulated as “361” HCT/Ps are subject to requirements relating to registering facilities and listing products with the FDA, screening and testing for tissue donor eligibility, and Good Tissue Practices when processing, storing, labeling, and distributing HCT/Ps, including required labeling information, stringent record keeping, and adverse event reporting.
The American Association of Tissue Banks ("AATB") has issued operating standards for tissue banking. Compliance with these standards is a requirement in order to become an AATB-accredited tissue establishment. In addition, some states have their own tissue banking regulations. We are licensed or have permits for tissue banking in California, Delaware, Illinois, Maryland, New York, Oregon, and Tennessee. In Tennessee, we are registered with the FDA Center for Biological Evaluations and Research.
Procurement of certain human organs and tissue for transplantation is subject to the restrictions of the National Organ Transplant Act, which prohibits the transfer of certain human organs, including skin and related tissue for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. BioD is a registered Tissue Bank and is involved with the recovery, storage and transportation of donated human amniotic tissue.
On June 22, 2015, the FDA issued an Untitled Letter (the "Untitled Letter") alleging that BioD's morselized amniotic membrane tissue-based products do not meet the criteria for regulation as HCT/Ps solely under Section 361 and that, as a result, BioD would need a biologics license to lawfully market those morselized products. Since the issuance of the Untitled Letter, BioD and the Company has made known to the FDA their disagreement with the FDA’s assertion that certain products are more than minimally manipulated. The FDA has not changed its position that certain of the BioD acquired products are not eligible for marketing solely under Section 361. In July 2020, the FDA issued the final guidance document related to human tissue titled, “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use” (the “2020 HCT/P Final Guidance”). The 2020 HCT/P Final Guidance document supersedes the November 2017 guidance by the same title.
9
The HCT/P Final Guidance maintains the FDA’s position that products such as the Company’s morselized amniotic membrane tissue-based products do not meet the criteria for regulation solely as HCT/Ps. In addition, in the November 2017 guidance, the FDA articulated a risk-based approach to enforcement and, while some uses for amniotic membrane tissue-based products would have as much as thirty-six months of enforcement discretion, other high risk uses could be subject to immediate enforcement action. The 2020 HCT/P Final Guidance maintained this approach and extended the discretionary enforcement period to May 31, 2021.
Considering the risk of enforcement action, the Company discontinued the manufacturing of all morselized amniotic membrane tissue-based products prior to May 31, 2021. We no longer distribute these products. As of December 31, 2022, the Company has not received any further notice of enforcement action from the FDA regarding its morselized amniotic membrane tissue-based products.
Medical Device Regulations
Unless an exemption applies, the FDA requires that a manufacturer introducing a new medical device or a new indication for use of an existing medical device obtain either a Section 510(k) premarket notification clearance or a PMA, before introducing it into the U.S. market. The type of marketing authorization is generally linked to the classification of the device. The FDA classifies medical devices into one of three classes (Class I, II or III) based on the degree of risk the FDA determines to be associated with a device and the level of regulatory control deemed necessary to ensure the device’s safety and effectiveness.
The process of obtaining a Section 510(k) clearance generally requires the submission of performance data and often clinical data, which in some cases can be extensive, to demonstrate that the device is “substantially equivalent” to a device that was on the market before 1976 or to a device that has been found by the FDA to be “substantially equivalent” to such a pre-1976 device, a predecessor device is referred to as “predicate device.” As a result, FDA clearance requirements may extend the development process for a considerable length of time. In addition, in some cases, the FDA may require additional review by an advisory panel, which can further lengthen the process. The PMA process, which is reserved for new devices that are not substantially equivalent to any predicate device and for high-risk devices or those that are used to support or sustain human life, may take several years and requires the submission of extensive performance and clinical information.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device has received 510(k) clearance for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, may require a new 510(k) clearance or PMA approval and payment of an FDA user fee. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance; however, the FDA may review this determination to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
We also are required to register with the FDA as a medical device manufacturer and any devices we manufacture and distribute pursuant to clearance or approval by the FDA are subject to pervasive and continuing regulation by the FDA and certain state agencies. These include product listing and establishment registration requirements, which help facilitate FDA inspections and other regulatory actions, and our manufacturing sites are subject to periodic inspection by the FDA for compliance with the FDA's Quality System Regulations. These regulations require that we manufacture our products and maintain our documents in a prescribed manner with respect to design, manufacturing, testing and control activities. Further, we are required to comply with various FDA requirements and other legal requirements for labeling and promotion. If the FDA believes that a company is not in compliance with applicable regulations, it may issue a warning letter, institute proceedings to detain or seize products, issue a recall order, impose operating restrictions, enjoin future violations and assess civil penalties against that company, its officers or its employees and may recommend criminal prosecution to the U.S. Department of Justice. All Integra manufacturing facilities participate in the Medical Device Single Audit Program and are audited annually for compliance with the Quality System for US FDA, Canada, Australia, Brazil, and Japan.
Medical device regulations also are in effect in many of the countries in which we do business outside the U.S. In the European Economic Area ("EEA"), which is comprised of the 27 member states of the European Union plus Norway, Iceland and Liechtenstein, medical devices need to comply with specific requirements. These requirements were previously known as "Essential Requirements" under the former EU Medical Devices Directive (Council Directive 93/42/EEC, or MDD) and are now defined "General Safety and Performance Requirements (GSPR)" under the new EU Medical Devices Regulation (Regulation (EU) 2017/745, or "EU MDR"). Although the requirements set forth in the EU MDR are generally consistent with those laid out in the MDD (with a few exceptions), the EU MDR is intended, among other things, to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation. These laws range from comprehensive medical device approval and Quality System requirements for some or all of our medical device products to simpler requests for product data or certifications. Under the European Union Medical Device Directive, medical devices must meet the Medical Device Directive standards and receive
10
CE Mark Certification prior to marketing in the EEA. Although we continue to transition our certification profile to meet the new EU MDR requirements, these stricter regulations set forth in the EU MDR may pose additional challenges for Integra to continue marketing products in the EU as these regulations come into force. See “Item 1A. Risk Factors - We are subject to stringent domestic and foreign medical device regulations and oversight and any adverse action may adversely affect our ability to compete in the marketplace and our financial condition and business operations” of this Annual Report on Form 10-K.
CE Mark Certification requires a comprehensive quality system program, technical documentation, clinical evaluation and data on the product which are then reviewed, by a Notified Body. A Notified Body is an organization designated by the national governments of the EU member states to make independent judgments about whether a product complies with the requirements established by each CE marking directive. The Medical Device Directive, Medical Device Regulation, ISO 9000 series and ISO 13485 are recognized international quality standards that are designed to ensure that we develop and manufacture quality medical devices. Other countries are also instituting regulations regarding medical devices or interpreting and enforcing existing regulations more strictly. Compliance with these regulations requires extensive documentation and clinical reports for our products, revisions to labeling, and other requirements such as facility inspections to comply with the registration requirements. A recognized Notified Body audits our facilities annually to verify our compliance with the ISO 13485 Quality System standard.
Certain countries, as well as the EU, have issued regulations that govern products that contain materials derived from animal sources. Regulatory authorities are particularly concerned with materials infected with the agent that causes BSE. These regulations affect our dermal regeneration products, duraplasty products, hernia repair products, biomaterial products for the spine, nerve and tendon repair products and certain other products, all of which contain material derived from bovine tissue. Although we take great care to provide that our products are safe and free of agents that can cause disease, products that contain materials derived from animals, including our products, may become subject to additional regulation, or even be banned in certain countries, because of concern over the potential for prion transmission. Significant new regulations, a ban of our products, or a movement away from bovine-derived products because of an outbreak of BSE could have a material, adverse effect on our current business or our ability to expand our business. See “Item 1A. Risk Factors – Risks Related to our Regulatory Environment" of this Annual Report on Form 10-K.
Postmarket Requirements. After a device is cleared or approved for commercial distribution, numerous regulatory requirements apply. These include the FDA Quality System Regulations which cover the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of medical devices; the FDA's general prohibition against promoting products for unapproved or 'off-label' uses; the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and the Reports of Corrections and Removals regulation, which require manufacturers to report recalls and field corrective actions to the FDA if initiated to reduce a risk to health posed by the device or to remedy a violation of the FD&C Act. Postmarket requirements are also followed globally where our products are registered and approved. These foreign jurisdictions have similar requirements to the FDA which include reporting requirements such as adverse events and recalls.
Other regulations
Anti-Bribery Laws. In the U.S., we are subject to laws and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws and physician self-referral laws that regulate the means by which companies in the health care industry may market their products to hospitals and health care professionals and may compete by discounting the prices of their products. Similar anti-bribery laws exist in many of the countries in which we sell our products outside the U.S., as well as the United States Foreign Corrupt Practices Act (which addresses the activities of U.S. companies in foreign markets). Our products also are subject to regulation regarding reimbursement, and U.S. healthcare laws apply when a customer submits a claim for a product that is reimbursed under a federally funded healthcare program. These global laws require that we exercise care in designing our sales and marketing practices, including interactions with healthcare professionals, and customer discount arrangements. See “Item 1A. Risk Factors – We are exposed to a variety of risks relating to our international sales and operations” of this Annual Report on Form 10-K for further details.
Import-export. Our international operations subject us to laws regarding sanctioned countries, entities and persons, customs, and import-export. Among other things, these laws restrict, and in some cases can prevent, U.S. companies from directly or indirectly selling goods, technology or services to people or entities in certain countries. In addition, these laws require that we exercise care in our business dealings with entities in and from foreign countries.
Hazardous materials. Our research, development and manufacturing processes involve the controlled use of certain hazardous materials. We are subject to country-specific, federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials and certain waste products. We believe that our environmental, health and safety procedures for handling and disposing of these materials comply with the standards prescribed by the controlling laws and regulations. However, risk of accidental releases or injury from these materials is possible. These risks are managed to minimize or eliminate associated business impacts. In the event of this type of accident, we could be held liable for damages
11
and face a liability that could exceed our resources. We could be subject to a regulatory shutdown of a facility that could prevent the distribution and sale of products manufactured there for a significant period of time, and we could suffer a casualty loss that could require a shutdown of the facility in order to repair it, any of which could have a material, adverse effect on our business. Although we continuously strive to maintain full compliance with respect to all applicable global environmental, health and safety laws and regulations, we could incur substantial costs to fully comply with future laws and regulations, and our operations, business or assets may be negatively affected. Furthermore, global environmental, health and safety compliance is an ongoing process. Integra has compliance procedures in place for compliance with Employee Health & Safety laws, driven by a centrally led organizational structure that ensures proper implementation, which is essential to our overall business objectives.
In addition to the above regulations, we are, and may be, subject to regulation under country-specific federal and state laws, including, but not limited to, requirements regarding record keeping, and the maintenance of personal information, including personal health information. As a public Company, we are subject to the securities laws and regulations, including the Sarbanes-Oxley Act of 2002. We also are subject to other present and could be subject to possible future, local, state, federal and foreign regulations.
Third-Party Reimbursement. Healthcare providers that purchase medical devices generally rely on third-party payors, including, in the U.S., the Medicare and Medicaid programs and private payors, such as indemnity insurers, employer group health insurance programs and managed care plans, to reimburse all or part of the cost of the products. As a result, demand for our products is and will continue to be dependent in part on the coverage and reimbursement policies of these payors. The manner in which reimbursement is sought and obtained varies based upon the type of payor involved and the setting in which the product is furnished and utilized. Reimbursement from Medicare, Medicaid and other third-party payors may be subject to periodic adjustments as a result of legislative, regulatory and policy changes, as well as budgetary pressures. Possible reductions in, or eliminations of, coverage or reimbursement by third-party payors, or denial of, or provision of uneconomical reimbursement for new products may affect our customers' revenue and ability to purchase our products. Any changes in the healthcare regulatory, payment or enforcement landscape relative to our customers' healthcare services have the potential to significantly affect our operations and revenue.
Data Privacy and Cybersecurity Laws and Regulations. As a business with a significant global footprint, compliance with evolving regulations and standards in data privacy and cybersecurity (relating to the confidentiality and security of our information technology systems, products such as medical devices, and other services provided by us) may result in increased costs, lower revenue, new complexities in compliance, new challenges for competition, and the threat of increased regulatory enforcement activity. Our business relies on the secure electronic transmission, storage and hosting of sensitive information, including personal information, financial information, intellectual property, and other sensitive information related to our customers and workforce.
For example, in the U.S., the collection, maintenance, protection, use, transmission, disclosure and disposal of certain personal information and the security of medical devices are regulated at the U.S. federal and state, and industry levels. U.S. federal and state laws protect the confidentiality of certain patient health information, including patient medical records, and restrict the use and disclosure of patient health information by health care providers. For example, in the U.S. we are obligated to comply with the requirements of the Health Insurance and Portability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (collectively, “HIPPA”). Under HIPAA, the Department of Health and Human Services has issued regulations, including the HIPAA Privacy, Security and Breach Notification Rules, to protect the privacy and security of protected health information used or disclosed by covered entities including health care providers and their business associates, as well as covered subcontractors. HIPAA also regulates standardization of data content, codes and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include significant civil and criminal penalties for each violation. In addition, the FDA has issued guidance advising manufacturers to take cybersecurity risks into account in product design for connected medical devices and systems, to assure that appropriate safeguards are in place to reduce the risk of unauthorized access or modification to medical devices that contain software and reduce the risk of introducing threats into hospital systems that are connected to such devices. The FDA also issued guidance on post market management of cyber security in medical devices.
Outside the U.S., we are impacted by the privacy and data security requirements at the international, national and regional level, and on an industry specific basis. Legal requirements in these countries relating to the collection, storage, handling and transfer of personal data and, potentially, intellectual property continue to evolve with increasingly strict enforcement regimes. In Europe, for example, we are subject to EU General Data Protection Regulation ("GDPR") which requires member states to impose minimum restrictions on the collection, use and transfer of personal data and includes, among other things, a requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances and significant fines for non-compliance. The GDPR also requires companies processing personal data of individuals residing in the EU to comply with EU privacy and data protection rules.
12
Please refer to “Item 1A. Risk Factors – We are subject to requirements relating to information technology which could adversely affect our business” of this Annual Report on Form 10-K for additional discussion of the risks accompanying compliance with data privacy and cybersecurity laws and regulations.
These laws and regulations impact the ways in which we use and manage personal data, protected health information, and our information technology systems. They also impact our ability to move, store, and access data across geographic boundaries. Compliance with these requirements may require changes in business practices, complicate our operations, and add complexity and additional management and oversight needs. They also may complicate our clinical research activities, as well as product offerings that involve transmission or use of clinical data.
HUMAN CAPITAL
Workforce Demographics
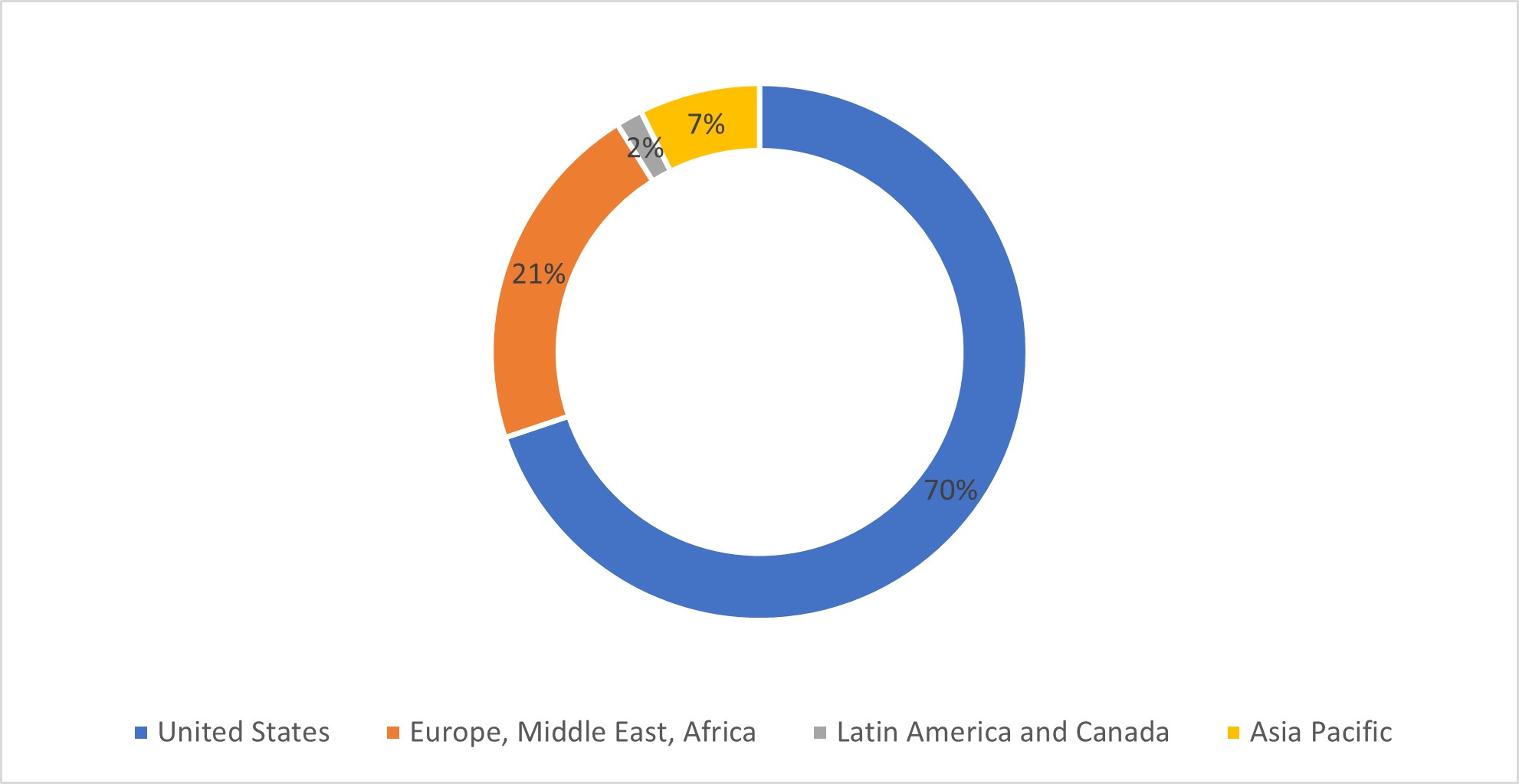
As of December 31, 2022, we had approximately 3,722 regular full and part time employees and 874 contingent, subcontracted, and outsourced partners.
70% of our employees are located in the United States, 21% in Europe, 2% in Latin America and Canada and 7% in Asia Pacific which includes Australia and New Zealand.
Diversity and Inclusion
A diverse workforce and an inclusive culture and work environment is a business priority and a key to our long-term success. Our commitment to diversity and inclusion starts at the top with our Board of Directors and CEO. At all levels of the Company, we focus on attracting, retaining, and developing our diverse talent.
Leadership Commitment and Accountability
Executive leadership set diversity and inclusion goals for the Company on an annual basis. Advancing diversity and inclusion initiatives to build stronger teams has been a company-wide goal and the direct engagement of executive leadership in advancing diversity and inclusion initiatives helps to promote awareness throughout the Company.
Leadership Councils, Employee Resource Groups and External Partnerships
We are accountable to our diversity commitment through our leadership councils, employee resource groups, and external partnerships.
•The Women’s Leadership Council, established in 2017 and chaired by our President & Chief Executive Officer, Jan De Witte, is a results-oriented advisory group comprised of ten of our senior women leaders across Integra. The
13
specific charter of the Council is to work together to identify ways to continue to attract and retain female talent, advance the development of our women into leadership roles, increase the cultural awareness of the value of inclusion and diversity in our Company, and create specific development forums for our high performing women at Integra.
•Employee Resources Groups encourage a culture of awareness and inclusion, assist in the attraction and retention of diverse talent, and help colleagues develop leadership skills. Members of the Executive Leadership Team serve as sponsors for each of Integra’s employee resource groups. Integra currently has six Employee Resources Groups:
◦Women of Integra Networks (WIN) with 20+ chapters globally
◦African American Affinity Group
◦Veteran Employee Resource Group
◦Indian American Network
◦Asian American and Pacific Islander Network
◦Integra PRIDE (LGBTQ+ Employee Resource Group)
•We reinforce our commitment to diversity by partnering with other organizations focused on driving inclusion in the workplace including the CEO Action for Diversity & Inclusion, the largest CEO-driven business commitment to advance diversity and inclusion in the work place and Healthcare Businesswomen’s Association, an association dedicated to further the advancement and impact of women in the business of healthcare.
Promoting an inclusive culture through learning opportunities
To help drive our culture of inclusion, our colleagues participate in programs focused on how to manage bias, value differences, and develop inclusive leadership skills.
•Members of our executive leadership, senior management team, and larger scope leaders participated in a ½ day Microinequities training. The content includes understanding unconscious bias and microinequities, how to identify microinequities in day-to-day decisions and actions as leaders, and ways to mitigate microinequities on an individual and organizational level.
•In 2020, we launched two foundational programs to promote diversity and inclusion: Introduction to Managing Unconscious Bias, a course that creates awareness of unconscious biases in the workplaces and tools to build-bias breaking skills and Practicing Inclusion which examines what practicing inclusion in the workplace looks like. These trainings are now mandatory for all new Integra hires.
•We regularly provide educational content and resources to aid our colleagues as they build cultural competency and inclusive leadership skills
Gender Diversity
We believe that our company is stronger and will deliver strong operating results, when we build diverse teams and leverage broad perspectives to meet the needs of our shareholders, customers, colleagues, and communities we serve.
The breakout of our colleagues by gender:
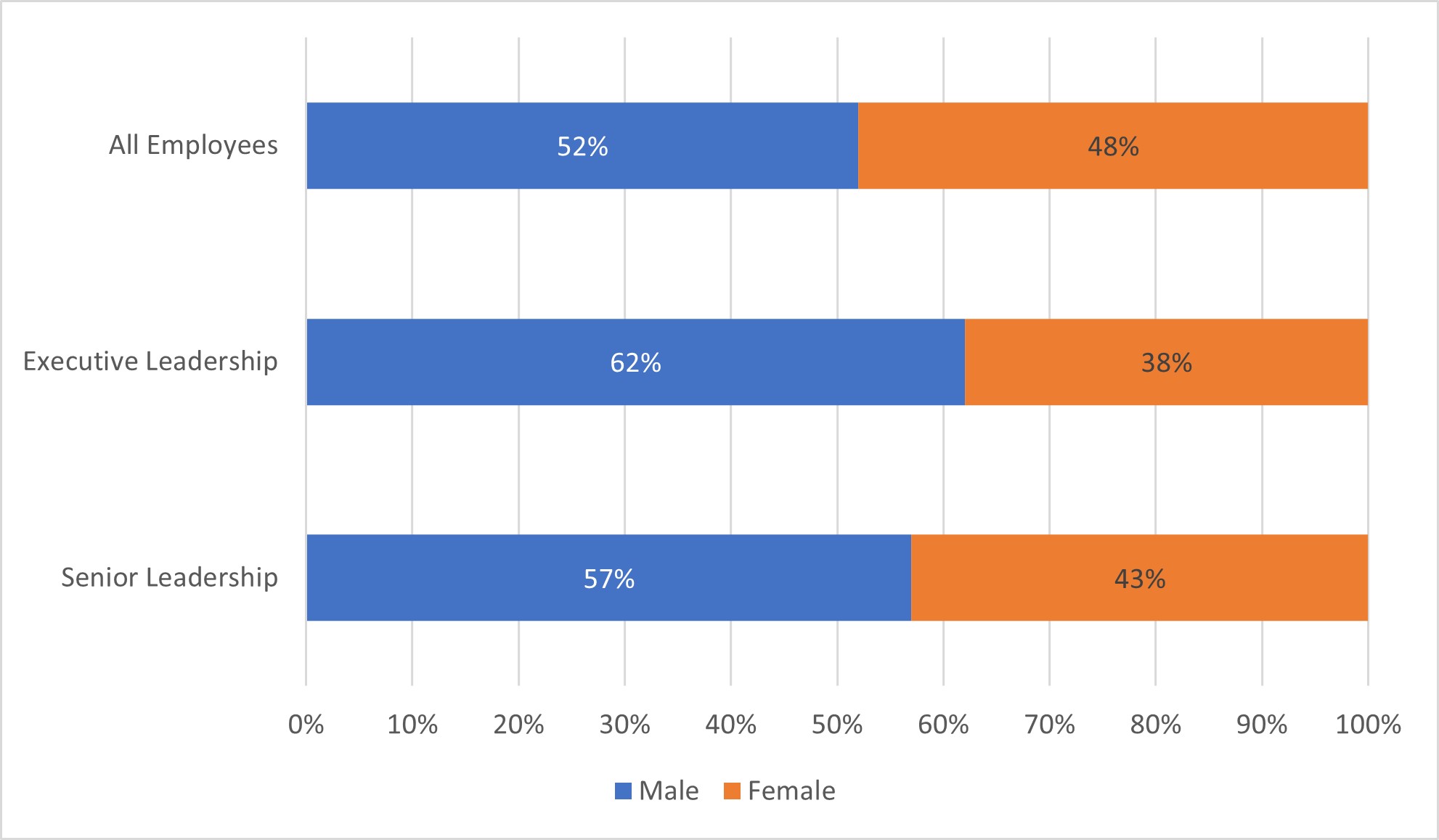
48% of Integra’s overall population is female, 52% male. We continue to strive to ensure our diversity in our leadership ranks is representative of our overall population. Through mentorship, sponsorship, recruitment efforts, and development programs we look to continue to grow our population of females in leadership roles at Integra. Currently, 38% of our executive leaders and 43% of senior leaders (non-executive vice presidents) are female.
14
In partnership with Leadership Edge, a company founded by women leaders and dedicated to growing and mentoring women. Integra sponsors the Excel Women’s Leadership Program. The program is designed to accelerate the development and advancement of high potential, mid-career female leaders into senior leadership roles. The program has assisted in further building our pipeline of women leaders with 60% of the program’s graduates being promoted into roles with increased responsibility.
Compensation and Benefits
Our compensation philosophy is designed to reinforce and align with our mission, business strategy, and financial needs. We invest in the physical, emotional and financial well-being of our employees through our robust compensation and benefit programs. We provide market-competitive compensation and benefits based on benchmarking surveys we conduct regularly for all position levels against relevant peer companies. Our annual and long-term incentive packages are linked directly to business and individual performance, with a balance of short- and long-term financial and strategic objectives. We have an employee stock purchase plan. Eligibility for non-salary benefits such as salary continuance, life insurance, health insurance, and similar benefits, follows local regulations and practices.
Integra is a pay-for-performance company committed to fair pay. All compensation decisions are made without regard to personal characteristics such as, but not limited to, gender, race, color, national or ethnic origin, age, disability, sexual orientation, gender identity or expression, genetic information, religion, or veteran status. As part of our commitment to compensation equity, Integra regularly conducts a pay equity analysis, reviewing how our organization compensates employees against external and internal data in conjunction with the role and scope of each position and making adjustments if necessary.
Talent Development and Retention
We have comprehensive and effective human capital development programs in place because we believe that the personal success of our employees is critical to the overall success of our business. To build a diverse and talented organization, we have invested in honing our recruiting and hiring processes to attract top talent and engage new hires from the very beginning of their experience at Integra.
We offer a variety of opportunities for our employees to learn and grow. Continued learning and development is a critical component of employee job satisfaction, retention, and career advancement—and ultimately, a driver of business success. We encourage and promote experiential, collaborative, and formal learning programs. Employees are also encouraged to discuss with their managers the skills, training, and experience needed to grow and develop. In addition to several skills-based trainings
15
available (technical, sales, leadership ability) to all employees, managers may recommend external job-specific development programs to employees. These programs are paid for directly by Integra.
Employee Health and Safety:
Integra is committed to providing a safe environment for all employees and visitors. We rely on our environmental, health and safety management systems as well as entrusting our managers to oversee and ensure health and safety at their respective sites and foster a workplace culture to achieve that end. We implement our approach globally by our systems and support at regional and country levels from colleagues that implement proper safety protocols, identify and correct hazards, and remain safety conscious at all times. Managers are expected to enforce health and safety regulations, including compliance with applicable federal, state and local laws. Our Environmental Health and Safety ("EH&S") organizational structure incorporates both workplace EH&S coordinators and compliance teams. We have developed an Incident Procedure Policy and General Safety Rules that guide our colleagues to improve our workplace environment, improve safety, and reduce risk and costs.
Throughout the COVID-19 pandemic, we have placed a high priority on employee health, providing resources to support our workforce. At the outset of the pandemic, we sought to protect the health and safety of our employees unable to work remotely, including those in research and development, quality, manufacturing, distribution and sales roles. Such measures included the institution of robust hygiene practices, distribution of personal protective equipment, and the adoption of increased sanitation and social distancing protocols. We continue to actively monitor the COVID-19 pandemic and its variants and respond based on guidance from U.S. and global health organizations, relevant governmental guidance, and evolving practices.
Employee Engagement & Wellbeing
We regularly seek employee feedback and sentiment about our workplace through global engagement surveys conducted on a bi-annual basis. After each survey is complete, we share detailed results with senior management and all employees within each department. Each function or division appoints survey administrators who work with their respective teams to understand the feedback and establish action items. We believe this process enables us to monitor employee engagement and create a continuously improving, satisfying work environment for our employees.
We are committed to improving the quality of life of our employees and their families. Our health and wellbeing programs differ by country and typical benefits include comprehensive health insurance, disability coverage, workplace accommodations, parental leave and other leaves of absence based on health or life events (e.g., bereavement), employee assistance programs, fitness reimbursement, and flu shots. We also provide on-demand health advocates to help employees navigate the health insurance system, access to digital health solutions, a weight management program, smoking cessation assistance, a substance use disorder helpline, a diabetes health program and other similar programs to drive healthy behaviors and awareness.
FINANCIAL INFORMATION ABOUT GEOGRAPHIC AREAS
Financial information about our geographical areas is set forth in our financial statements Note 16, Segment and Geographic Information, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K).
AVAILABLE INFORMATION
We are subject to the informational requirements of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”Act"). In accordance with the Exchange Act, we file annual, quarterly and special reports, proxy statements and other information with the Securities and Exchange Commission, (the "SEC"("the SEC"). Our financial information may be viewed, including the information contained in this report, and other reports we file with the SEC, on the Internet, without charge as soon as reasonably practicable after we file them with the SEC, in the “SEC Filings” page of the Investor Relations section of our website at www.integralife.com. A copy may also be obtained for any of these reports, without charge, from our Investor Relations department, 1100 Campus Road, Princeton, NJ 08540. Alternatively, reports filed may be viewed or obtained with the SEC atthrough the SEC's website at www.sec.gov.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
We have made statements in this report, including statements under “Business” and “Management's Discussion and Analysis of Financial Condition and Results of Operations” that constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, (the “Securities Act”("the Securities Act"), and Section 21E of the Exchange Act. These forward-looking statements are subject to a number of risks, uncertainties and assumptions about us including, among other things:
•the on-going and possible future effects of the COVID-19 pandemic and associated economic disruptions, including supply chain constraints and inflation, on our business, financial condition, results of operations and cash flows;
•general economic and business conditions, both nationally and in our international markets;markets, including the effect of the continuing worldwide macroeconomic uncertainty;
16
•our expectations and estimates concerning future financial performance, financing plans and the impact of competition;
•anticipated trends in our business;
•anticipated demand for our products, particularly capital equipment;
•our ability to produce regenerative-basedand deliver products in sufficient quantities to meet sales demands;
•our expectations concerning our ongoing restructuring, integration and manufacturing transfer and expansion activities;
•existing and future regulations affecting our business, and enforcement of those regulations;
•our failure to comply with the substantial regulation related to quality standards applicable to our manufacturing and quality processes could have an adverse effect on our business, financial condition, or results of operations;
•our ability to obtain additional debt and equity financing to fund capital expenditures, working capital requirements and acquisitions;
•physicians' willingness to adopt our recently launched and planned products, third-party payors' willingness to provide or continue reimbursement for any of our products and our ability to secure regulatory approval for products in development;
•initiatives launched by our competitors;
•our ability to protect our intellectual property, including trade secrets;
•our ability to complete acquisitions, integrate operations post-acquisition and maintain relationships with customers of acquired entities;
•our ability to remediate all matters identified in FDA observations and warning letters that we received or may receive; and
•other risk factors described in the section entitledItem 1A. "Risk Factors" in this report.Annual Report on Form 10-K.
Forward-looking statements can be identified by forward-looking words such as “believe,” “may,” “could,” “might,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “expect,” “should,” “would” and similar expressions in this report. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this report may not occur and actual results could differ materially from those anticipated or implied in the forward-looking statements.
ITEM 1A. RISK FACTORS
The continuing worldwide macroeconomic and geopolitical uncertainty may adversely affect our business and prospects.
Global economic disruptions, including the COVID-19 pandemic, have continued to impact the global supply chain, primarily through constraints on raw materials and electronic components. Additionally, we have observed a reduction in both inbound and outbound transportation capacity as a result of port closures and delays associated with the pandemic, which is causing longer lead times in receiving raw materials, as well as increased freight costs. These highly competitive and constrained supply chain conditions are increasing our cost of sales, which has and may continue to adversely impact our profitability. Given the ongoing uncertainty regarding the duration and extent of the COVID-19 pandemic, we are uncertain as to the duration and extent of constraint on our supply chain and are unable to predict the extent to which it will affect our global operations.
Continued concerns about the systemic impact of potential long-term and wide-spread recession and geopolitical issues, including the war in Ukraine, have contributed to increased market volatility and diminished expectations for economic growth in the world. Our Businessbusiness and results of operations have been and may continue to be adversely impacted by changes in macroeconomic conditions, including inflation, rising interest rates and the accessibility of capital markets. Uncertainty about global economic conditions may also cause decreased demand for our products and services and increased competition, which could result in lower sales volume and downward pressure on the prices for our products, longer sales cycles, and slower adoption of new technologies. A weakening of macroeconomic conditions may also adversely affect our suppliers, which could result in interruptions in supply.
Market acceptance of our medical products in the U.S. and other countries is dependent upon the medical equipment purchasing and procurement practices of our customers, patient need for our products and procedures and the reimbursement of patients' medical expenses by government healthcare programs and third-party payors. The continuing uncertainty surrounding global economic conditions and financial markets may cause the purchasers of medical equipment to decrease their procurement activities. Economic uncertainty, an increase in unemployment rates, as well as increasing health insurance premiums, co-payments and deductibles may adversely affect demand for our products and procedures. Furthermore, governments and other third-party payors around the world facing tightening budgets could move to further reduce the reimbursement rates or the scope of coverage offered, which could adversely affect sales of our products.
Public health crises, such as the COVID-19 pandemic, have had, and could in the future have, a negative effect on our business.
17
Our global operations and interactions with healthcare systems, providers and patients around the world expose us to risks associated with public health crises, including epidemics and pandemics such as COVID-19. In particular, the COVID-19 pandemic continues to cause significant volatility and uncertainty in the global and regional economies, leading to changes in consumer and business behavior, market fluctuations, materials and product shortages and restrictions on business and individual activities, all of which are materially impacting supply and demand in broad sectors of the world markets. Additionally, the COVID-19 pandemic, together with general macroeconomic conditions, have led to disruptions in the global supply chain, primarily through a lack of availability of raw materials and electronic components. We have experienced challenges associated with material and component availability for certain product lines, longer shipping and delivery times for raw materials and components, constrained logistics capacity related to the movement of our products, availability of skilled labor and increased costs of raw materials, components, labor, and freight and courier services. Regional COVID-19 case volumes (including those related to subsequent variants), actions taken by governmental authorities, private businesses and individuals, such as “shelter-in-place” orders and restrictions on travel and access to our customers or temporary closures of our facilities or the facilities of our suppliers, disruption and/or higher costs to the Company’s supply chain, staffing shortages in hospitals and labor constraints in our facilities, could further impact our gross margins and our ability to ship our products and supply our customers.
The emergence of new variants, vaccinations and public health measures are driving the pace of economic recovery unevenly in various regions. The direct and indirect disruptions caused by the pandemic and the responses of both governments and individuals could negatively impact the number of surgical and medical intervention procedures performed and have a material adverse effect on our business, financial condition, results of operations, or cash flows. The extent to which fear of exposure to or actual effects of COVID-19, new variants, disease outbreak, epidemic or a similar widespread health concern impacts our business will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the speed and extent of geographic spread of the disease, the duration of the outbreak, travel restrictions, the efficacy of vaccination and treatment; impact on the U.S. and international healthcare systems, the U.S. economy and worldwide economy; the timing, scope and effectiveness of U.S. and international governmental response; and the impact on the health, well-being and productivity of our employees.
RISKS RELATING TO OUR BUSINESS
Our operating results may fluctuate.
Our operating results, including components of operating results such as gross margin and cost of product sales,operating expenses, may fluctuate from time to time, and such fluctuations could affect our stock price. Our operating results have fluctuated in the past and can be expected to fluctuatedo so from time to time in the future. Some of the factors that may cause these fluctuations include:
•economic conditions worldwide, which could affect the ability of hospitals and other customers to purchase our products and could result in a reduction in elective and non-reimbursed operative procedures;
•the impact of acquisitions, and our ability to integrate acquisitions;
•risks related to COVID-19 and other epidemics or similar widespread health concerns;
•expenditures for major initiatives, including acquired businesses and integrations thereof and restructuring;
•the timing of significant customer orders, which tend to increase in the fourth quarter to coincidecoinciding with the end of budget cycles for many hospitals;cycles;
•increased competition for a wide range of customers across all our product lines in the markets our products are sold;
•market acceptance of our existing products, as well as products in development;
•retention of current employees and recruiting of new employees in light of market competition for talent and relevant skills;
•the timing of regulatory approvals as well as changes in country-specific regulatory requirements;
•changes in the exchange rates of exchange between the U.S. dollar and otherforeign currencies of foreign countries in which we do business;
•changes in the variable interest rates of our debt instruments which could impact debt service requirements;
•potential backorders, lost sales and expenses incurred in connection with product recalls or field corrective actions;
•disruption of our operations and sales resulting from extreme weather conditions or natural disasters that damage our manufacturing, distribution, or distributioninfrastructure of those facilities, or the suppliers and service providers for those facilities, or the infrastructure in the locations of those facilities;
•our ability to manufacture and ship our products efficiently or in sufficient quantities to meet sales demands;
•changes in the cost or decreases in the supply of raw materials and services, including sterilization, energy, steel pyrocarbon and honey;
•the timing of our research and development expenditures;
•reimbursement for our products by third-party payors such as Medicare, Medicaid, private and public health insurers and foreign governmental health systems;
•the ability to maintain existing distribution rights to and from certain third parties;
•the ability to maintain business if or when we opt to convert such business from distributors to a direct sales model;
•the ability of our commercial sales representatives to obtain sales targets in a reasonable time frame;
18
•the impact of changes to our sales organization, continued channel expansion, including increased specialization;
•peer-reviewed publications discussing the clinical effectiveness of the products we sell;
•inspections of our manufacturing facilities for compliance with Quality System Regulations (Good Manufacturing Practices), which could result in Form 483 observations, warning letters, injunctions or other adverse findings from the FDA or from equivalent regulatory bodies, and corrective actions, procedural changes and other actions that we determine are necessary or appropriate to address the results of those inspections, any of which may affect production and our ability to supply our customers with our products;
•changes in regulations or guidelines that impact the sales and marketing practices for products that we sell;
•the increased regulatory scrutiny of certain of our products, including products which we manufacture for others, could result in their being removedremoval from the market or involve field corrective actions that could affect the marketability of our products;
•enforcement or defense of intellectual property rights;
•changes in tax laws, or their interpretations; and
•the impact of goodwill and intangible asset impairment charges if future operating results of the acquired businesses are significantly less than the results anticipated at the time of the acquisitions.
The industry and market segments in which we operate are highly competitive, and we may be unable to compete effectively with other companies.
There is intense competition among medical device companies. We compete with established medical technology companies in many of our product areas. Competition also comes from early-stage companies, that have alternative technological solutions for our primary clinical targets, as well as universities, research institutions and other non-profit entities. In certain cases, our products compete primarily against medical practices that treat a condition without using a device or any particular product, such as the medical practices that use autograft tissue instead of our dermal regeneration products, duraplasty products and nerve repair products, or that use other technologies that cost less than our products. Many of our competitors have access to greater financial, technical, research and development, marketing, manufacturing, sales, distribution, administrative, consulting and other resources than we do. Our competitors may be more effective at developing commercial products. Our competitorsproducts or navigating the regulatory approval process in the markets in which we operate. They may be able to gain market share by offering lower-cost products or by offering products that enjoy better reimbursement from third-party payors such as Medicare, Medicaid, private and public health insurers and foreign governmental health systems.
Our competitive position depends on our ability to achieve market acceptance for our products, develop new products, implement production and marketing plans, secure regulatory approval for products under development, demonstrate clinical and economic effectiveness, obtain and maintain reimbursement coverage and funding under Medicare, Medicaid, private and public health insurersthird-party payors and foreign governmental health systems, obtain patent protection and produce products consistently in sufficient quantities to meet demand. We may need to develop new applications for our products to remain competitive. Technological advances by one or more of our current or future competitors or their achievement of superior reimbursement from Medicare, Medicaid, private and public health insurersthird-party payors and foreign governmental health systems could render our present or future products obsolete or uneconomical. Our future success will depend upon our ability to compete effectively against current technology as well as to respond effectively to technological advances, changes in customers' requirements or changes in payor or regulatory evidence requirements. Additionally, purchasing decisions of our customers may be based on clinical evidence or comparative effectiveness studies and, because of our vast array of products, we might not be able to fund the studies necessary to gain entry or maintain our position or provide the required information to compete effectively. Other companies may have more resources available to fund such studies. For example, competitors have launched and have beenare developing products to compete with our dural repair products, extremity reconstruction implants, regenerative skin, neuro critical care monitors and ultrasonic tissue ablation devices, among others. Further, inIn the current environment of managed care, consolidation among health care providers, increased competition, and declining reimbursement rates, we have been increasingly required to compete on the basis of price. Competitive pressures could adversely affect our profitability. Given these factors, we cannot guarantee that we will be able to compete effectively or continue our level of success in the areas in which we compete.
•several foreign countries have implemented reforms of their respective healthcare sectors in an effort to reduce healthcare spending, including restricting funding to only those medical technologies and procedures with proven effectiveness, increasing patient co-payments and providing for payback measures. Governmental health systems have revised and continue to consider revisions of healthcare budgets, which could result in stricter standards for implementing certain medical procedures, increased scrutiny of medical devices, and downward pricing pressure;
19
•Medicare, Medicaid, private and public health insurer and foreign governmental cutbacks could create downward pricing pressure on our products;
•in the U.S., Medicare and Medicaid coverage as well as commercial payor coverage determinations could reduce or eliminate reimbursement or coverage for certain of our wound matrix, amniotic, surgical reconstruction and advanced wound dressing products as well as other products in most regions, negatively affecting our market for these products, and future determinations could reduce or eliminate reimbursement or coverage for these products in other regions and could reduce or eliminate reimbursement or coverage for other products;
•there has been a consolidation among healthcare facilities and purchasers of medical devices in the U.S., some of whom prefer to limit the number of suppliers from whom they purchase medical products, and these entities may decide to stop purchasing our products or demand discounts on our prices;
•in the U.S., we are party to contracts with group purchasing organizations, which negotiate pricing for many member hospitals, require us to discount our prices for certain of our products and limit our ability to raise prices for certain of our products, particularly surgical instruments;
•there is economic pressure to contain healthcare costs in domestic and international markets, and, regardless of the consolidation discussed above, providers generally are exploring ways to cut costs by eliminating purchases or driving reductions in the prices that they pay for medical devices, or increasing clinical or economic evidence thresholds for product formularies;
•there are proposed and existing laws, regulations and industry policies in domestic and international markets regulating the sales and marketing practices and the pricing and profitability of companies in the healthcare industry;
•proposed laws or regulations may permit hospitals to provide financial incentives to doctors for reducing hospital costs, will award physician efficiency, and will encourage partnerships with healthcare service and goods providers to reduce prices; and
•there have been initiatives by third-party payors and foreign governmental health systems to challenge the prices charged for medical products that could affect our ability to sell products on a competitive basis.
Any and all of the above factors could materially and adversely affect our levels of revenue and our profitability.
Our current strategy involves growth through acquisitions, which requires us to incur substantial costs and potential liabilities for which we may never realize the anticipated benefits.benefits, and also requires us to successfully integrate acquired businesses into our business operations in order to avoid our business being materially and adversely affected.
In addition to internally generated growth, our current strategy involves growth through acquisitions. Between January 1, 20172020 and December 31, 2019,2022, we have acquired 5two businesses at a total cost of approximately $1.3 billion.$358.4 million which amount includes our acquisition of ACell, Inc. in January 2021 for $306.9 million and our acquisition of Surgical Innovation Associates, Inc. for $51.5 million in December 2022. Both of these acquisitions added products to our complex wound management and plastic and reconstructive surgery product portfolios, respectively, and provides additional growth opportunities for our TT segment.
We may be unable to continue to implement our growth strategy and our strategyit may ultimately may be unsuccessful. A significant portion of our growth in revenues has resulted from, and is expected to continue to result from, the acquisition of businesses or products complementary to our own. We engage in evaluations of potential acquisitions and are in various stages of discussion regarding possible acquisitions, certain of which, if consummated, could be significant to us. Any new acquisition could result in material transaction expenses, increased operating, amortization and interest and amortization expense, increased depreciation expense, increased operating expense,expenses, and possible in-process research and development charges for acquisitions that do not meet the definition of a “business,” any of which could have a material, adverse effect on our operating results. Certain businesses that we acquire may not have adequate financial, disclosure, regulatory, quality or other compliance controls at the time we acquire them and could require significant expenditures to address those controls or subject us to increased risk. As we grow by acquisition, we must manage and integrate the new businesses to bring them into our systems for financial, disclosure, compliance, regulatory and quality control, realize economies of scale, and control costs. If we cannotFailure to integrate acquired businesses and operations (including acquired employees and systems), retain key customers and suppliers of any acquired business or manage the cost of providing our products or price our products appropriately could preclude realization of the full benefits that we expect from there transactions. Our failure to meet the challenges involved in integrating the business in order to realize the anticipated benefits of the acquisitions could cause an interruption of, or loss of momentum in, our profitabilityactivities and could suffer.materially and adversely affect our results of operations. In addition, acquisitions involve other risks, including diversion of management resources otherwise available for the running of our business and the development of our business as well as risks associated with entering markets in which our marketing teams and sales force has limited experience or where experienced distribution alliances are not available. Some acquisitions may include the need for ongoing product development to occur consistent with time sensitive milestones in order for the Company to achieve its commercial projections for the acquisition. Our future profitability will depend in part upon our ability to develop further our resources to adapt to these new products or business areas and to identify and enter into or maintain satisfactory distribution networks. Further, asAs a result of our acquisitions of other healthcare businesses, we may be subject to the risk of unanticipated business uncertainties, regulatory and other compliance matters or legal liabilities relating to those acquired businesses for which the sellers of the acquired businesses may not indemnify us, for which we may not be able to obtain insurance (or adequate insurance), or for which the indemnification may not be sufficient to cover the ultimate liabilities. We
20
may not be able to identify suitable acquisition candidates in the future, obtain acceptable financing or consummate any future acquisitions. Certain potential acquisitions are subject to antitrust and competition laws, which laws could impact our ability to pursue strategic acquisitions and could result in mandated divestitures. If we are unsuccessful in our acquisition strategy, we may be unable to meet our financial targets and our financial performance could be materially and adversely affected.
These risks may be heightened in cases where the majority of the former businesses’ operations, employees and customers are located outside the U.S. Any one or all of these factors could increase operating costs or lower anticipated financial performance. Many of these factors are also outside of our control. In addition, dispositions of certain key products, technologies and other rights, including pursuant to conditions imposed on us to obtain regulatory approvals, may affect our business operations.
Even if the operations of the businesses are integrated successfully, we may not realize the full benefits of the acquisition, including the synergies, cost savings or sales or growth opportunities that we expect. These benefits may not be achieved within the anticipated time frame, or at all. Additional unanticipated costs could be incurred in the integration of the businesses. All of these factors could cause a reduction to our earnings per share, decrease or delay the expected accretive effect of the transaction, and negatively impact the price of our common stock.
Our future financial results could be adversely affected by impairments or other charges.
The guidance on long-lived assets requires that we assess the impairment of our long-lived assets, including finite-lived intangible assets, whenever events or changes in circumstances indicate that the carrying value may not be recoverable as measured by the sum of the expected future undiscounted cash flows. As of December 31, 2019, we had $337.4 million and $868.5 million of property, plant and equipment and finite-lived intangible assets, respectively.
The value of a medical device business is often volatile, and the assumptions underlying our estimates made in connection with our assessments under the guidance may change as a result of that volatility or other factors outside our control and may result in impairment charges. The amount of any such impairment charges could be significant and could have a material, adverse effect on our reported financial results for the period in which the charge is taken and could have an adverse effect on the market price of our securities, including the notes and the common stock into which they may be converted.
Lack of market acceptance for our products or market preference for technologies that compete with our products could reduce our revenues and profitability.
Market acceptance of our products depends on many factors, including our ability to convince prospective collaborators and customers that our technology is an attractive alternative to other technologies, to manufacture products in sufficient quantities and at acceptable costs, and to supply and service sufficient quantities of our products directly or through our distribution alliances. For example, the use of autograft tissue is a well-established means for repairing the dermis, and it competes for acceptance in the market with our collagen-based wound care products. In addition, unfavorable reimbursement methodologies,payment amounts or adverse coverage determinations of third-party payors, including Medicare, Medicaid, private and public health insurers, and foreign governmental health systems, regarding our products or third-party determinations that favor a competitor’s product over ours, could harm acceptance or continued use of our products.For example, greater market acceptance of our wound graft products may ultimately depend on our ability to demonstrate that coverage and reimbursement are available and favorable, or because they are an attractive, cost-effective alternative to other treatment options.
If there are negative events in the healthcare industry, whether real or perceived, there could be a negative impact on the industry as a whole. The industry is subject to rapid and continuous change arising from, among other things, consolidation, technological improvements, the pressure on governments, third-party payors and providers to reduce healthcare costs, and healthcare reform legislation and initiatives domestically and internationally. In addition, our future success depends, in part, on our ability to license and develop additional products. Even if we determine that a product candidate has medical benefits, the cost of commercializing, either through internal development or payments associated with licensing arrangements, could be too high to justify development and we could ultimately face competitors with more effective products and better reimbursement status that cost less and are ready for commercial introduction before our products. If we are unable to develop additional commercially viable products, our future prospects could be materially and adversely affected.
21
One or more of these factors could vary unpredictably, and such variations could have a material,
adverse effect on our competitive position. We may not be able to adjust our contemplated plan of development to meet changing market demands.
It could be difficult to replace some of our suppliers.
Outside vendors, some of whom are sole-source suppliers, provide key components and raw materials used in the manufacture of our products. Although we believe that alternative sources for many of these components and raw materials are available, any interruption in supply of a limited or sole-source component or raw material could harm our ability to manufacture our products until a new or alternative source of supply is identified and qualified. In addition, an uncorrected defect or supplier’s variation in a component or raw material, either unknown to us or incompatible with our manufacturing process, could harm our ability to manufacture products. We may not be able to find a sufficient alternative supplier in a reasonable time period, or on commercially reasonable terms, if at all, and our ability to produce and supply our products could be impaired. We believe that these factors are most likely to affect the following products that we manufacture:sell:
•our collagen-based products and bovine-based products, such as the Integra Dermal Regeneration Template and wound matrix products, the DuraGen®DuraGen® family of products, our Absorbable Collagen Sponges, PriMatrix® and SurgiMend products;
•our products made from silicone, such as our neurosurgical shunts and drainage systems and hemodynamic shunts;
•products which use many different specialty parts, electrical components, or chemicals from numerous suppliers, such as our intracranial monitors, shunts, catheters, tissue ablation, and headlights;
•our biosynthetic products, that use pyrolytic carbon (i.e., PyroCarbon) technology, such as certain of our reconstructive extremity orthopedic implants;including the DuraSeal sealant system and DuraSorb biosynthetic mesh scaffold;
•products which are amniotic tissue based;tissue-based
•products which are porcine tissue-based;
•products that use medical grade leptospermum honey, such as our Medihoney products; and
•our TCC-EZ® total contact cast system products. |
The availability of amniotic tissue-based products depends upon, among other factors, the availability of tissue from human donors. Access to donated amniotic tissue could also be adversely impacted by regulatory changes or evolving public perceptions of the donor process.
Additionally, many of our products require sterilization by third-party suppliers. To the extent these suppliers are unable to provide sterilization services, whether due to lack of capacity, regulatory requirements, environmental concerns such as those relating to ethylene oxide or otherwise, we may be unable to transition sterilization to other suppliers in a timely or cost effective manner, or at all, which could have an adverse impact on our operating results.
While it is our policy to maintain sufficient inventory of components so that our production will not be significantly disrupted even if a particular component or material is not available for a period of time, we remain at risk that we will not be able to qualify new components or materials quickly enough to prevent a disruption if one or more of our suppliers ceases production of important components or materials.
We may experience difficulties, delays, performance impact or unexpected costs from consolidation of facilities and transfer of manufacturing facilities.
In recent years, we consolidated several facilities or transferred manufacturing operations from third parties to our existing internal manufacturing facilities and may further undertake similar consolidations or transfers in the future in order to improve our cost structure, achieve increased operating efficiencies, and improve our competitive standing or results of operations and/or to address unfavorable economic conditions. As part of these initiatives, we may also lose favorable tax incentives or not be able to renew leases on acceptable terms. We may further reduce staff, make changes to certain capital projects, close certain production operations and abandon leases for certain facilities that will not be used in our operations. In conjunction with any actions, we will continue to make significant investments and build the framework for our future growth. We may not realize, in full or in part, the anticipated benefits and savings from these efforts because of unforeseen difficulties, delays, implementation issues or unexpected costs. If we are unable to achieve or maintain all of the resulting savings or benefits to our business or other unforeseen events occur, our business and results of operations may be adversely affected.
We may have significant product liability exposure and our insurance may not cover all potential claims.
We are exposed to product liability and other claims if our technologies or products are alleged to have caused harm. We may not be able to obtain insurance for the potential liability on acceptable terms with adequate coverage or at reasonable costs. Any potential product liability claims could exceed the amount of our insurance coverage or may be excluded from coverage under the terms of the policy. Our insurance may not be renewed at a cost and level of coverage comparable to that then in effect.
22
Economic and political instability around the world could adversely affect the ability of hospitals, other customers, suppliers and distributors to access funds or otherwise have available liquidity, which could reduce orders for our products or interrupt our production or distribution or result in a reduction in elective and non-reimbursed operative procedures.
Economic and political instability around the world could adversely affect the ability of hospitals and other customers to access funds to enable them to fund their operating and capital budgets. As a result, hospitals and other customers could reduce budgets or put all or part of their budgets on hold or close their operations, which could have a negative effect on our sales, particularly the sales of capital equipment such as our ultrasonic surgical aspirators, neuromonitors and cranial stabilization products, or result in a reduction in elective and non-reimbursed procedures. The occurrence of those economic conditions could make it more difficult for us to accurately forecast and plan our future business activities and depending on their severity, could have a material, adverse effect on our business, financial condition and results of operations.
Our private-label product lines depend significantly on key relationships with third parties, which we could be unable to establish and maintain.
Our private-label business depends in part on entering into and maintaining long-term supply agreements with third parties. The third parties with whom we have entered into agreements might terminate these agreements for a variety of reasons, including developing other sources for the products that we supply. Termination of our most important relationships could adversely affect our expectations for the growth of private-label products.
RISKS RELATED TO OUR REGULATORY ENVIRONMENT
The adoption of healthcare reform in the U.S. and initiatives sponsored by other governments may adversely affect our business, results of operations and/or financial condition.
Our operations may be substantially affected by potential fundamental changes in the global political, economic and regulatory landscape of the healthcare industry. Government and private sector initiatives to limit the growth of healthcare costs are continuing in the U.S., and in many other countries in which we do business, causing the marketplace to put increased emphasis on the delivery of more cost-effective treatments. These initiatives include price regulation, competitive pricing, coverage and payment policies, comparative effectiveness of therapies, technology assessments and managed-care arrangements. The adoption of some or all of these initiatives could have a material, adverse effect on our financial condition and results of operations.
In the United States, the Patient Protection and Affordable Care Act (the “ACA”), signed into law in March 2010, includes several provisions that impact our businesses in the U.S. The ACA includes provisions that, among other things, reduce and/or limit Medicare reimbursement, require all individuals to have health insurance (with limited exceptions), and require detailed disclosure of transfers of value made to healthcare professionals. Other legislative changes have been proposed and adopted since the Affordable Care Act was enacted, including The Budget Control Act of 2011, The American Taxpayer Relief Act of 2012 and Medicare Access and CHIP Reauthorization Act of 2015, which, among other things, have reduced payments under Medicare and Medicaid to certain healthcare providers or altered the formula by which Medicare makes annual payment adjustments. Congress also drafts and introduces, from time to time, legislation that could significantly change the statutory provisions governing the regulation of medical devices. In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. For example, over the last several years, the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k) clearance process for their products.
We cannot predict what impact ongoing uncertainty regarding federal and state health reform proposals, including the implementation or repeal of the ACA, judicial review and interpretation of the ACA and other healthcare laws, instability of the insurance markets, changes in the U.S. administration and policy, an expansion in government’s role in and/or additional proposals and/or changes to the U.S. health care system or its legislation will have on our customer’s purchasing decisions and/or reimbursement which could have a material adverse effect on our business. We expect that additional state and federal health care reform measures will be adopted in the future, including those initiatives affecting coverage and reimbursement for our products, any of which could limit the amounts that federal and state governments will pay for health care products and services, which could adversely affect the growth of the market for our products or demand for our products, or result in additional pricing pressures. We cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us. We continue to monitor the implementation of such legislation and, to the extent new market or industry trends or new governmental programs evolve, we will consider implementing or implement programs in response.
We are subject to stringent domestic and foreign medical device regulations and oversight and any adverse action may adversely affect our ability to compete in the marketplace and our financial condition and business operations.
Our products, development activities and manufacturing processes are subject to extensive and rigorous regulation by numerous government agencies, including the FDA and comparable foreign agencies, as discussed in “Part 1, Item 1. Business –
23
Government Regulation.” To varying degrees, each of these agencies monitors and enforces our compliance with laws and regulations governing the development, testing, manufacturing, labeling, marketing and distribution of our medical devices. We are also subject to regulations that may apply to certain of our products that are Drug/Device Combination products or are considered to be subject to pharmaceutical regulations outside the U.S. The process of obtaining marketing approval or clearance from the FDA and comparable foreign regulatory agencies for new products, or for enhancements or modifications to existing products could be costly, time consuming and burdensome, lead to failed clinical trials or weakened clinical evidence, involve modifications, repairs or replacements of our products and result in limitations on the indicated use of our products, which may negatively impact our ability to market our products and services, result in delays or prevent full commercial realization of future products or service. Furthermore, failure to obtain timely approvals or renewals may result in significant penalties and fines. Additional regulations govern the approval, initiation, conduct, monitoring, documentation and reporting of clinical studies to regulatory agencies in the countries or regions in which they are conducted. Failure to comply, could subject us to significant enforcement actions and sanctions, including halting the study, rejection of data generated in the study, seizure of investigational devices or data, sanctions against investigators, civil or criminal penalties, and other actions. In addition, without the data from one or more clinical studies, it may not be possible for us to secure the data necessary to support certain regulatory submissions, to secure reimbursement or demonstrate other requirements. We cannot assure that access to clinical investigators, sites and subjects, documentation and data will be available on the terms and timeframes necessary.
We are subject to extensive complex regulatory requirements by domestic and foreign government agencies and any failure to comply with our ongoing responsibilities under their applicable laws and regulations could result in a material adverse impact on our business. Failure to comply with applicable regulations could result in future product recalls, injunctions preventing the shipment of products or other enforcement actions that could have a material adverse effect on our business.
We also are subject to the European Medical Device Regulation, which was adopted by the European Union (“EU”) as a common legal framework for all EU member states. The implementation for Class I products occurred on May 26, 2021 and the EUDAMED Database was implemented on May 26, 2022. Under this regulation, companies that wish to manufacture and distribute medical devices in EU member states must meet certain quality system, and safety requirements as well as ongoing product monitoring responsibilities. Companies must also obtain a “CE” marking (i.e., a mandatory conformity marking for certain products sold within the European Economic Area) for their products. Complying with the requirements of these regulations may require us to incur significant expenditures. Expenditures for European Union Medical Device Regulation compliance activities amounted to $45.1 million for the year ended December 31, 2022 and we anticipate incurring additional expenditures in connection with our on-going efforts to obtain certification for our products under the European Medical Device Regulation. Various penalties exist for non-compliance with the laws implementing the European Medical Device Regulations which if incurred, could have a material adverse impact on our business, results of operations and cash flows.
Further, the regulatory environment in China continues to evolve, and officials in the Chinese government exercise broad discretion in deciding how to interpret and apply regulations. It is possible that the Chinese government's current or future interpretation and application of existing or new regulations will negatively impact our China operations, result in regulatory investigations or lead to fines or penalties.
In addition, we are subject to laws and regulations that govern the means by which companies in the healthcare industry may market their products to healthcare professionals and may compete by discounting the prices of their products, including for example, the federal Anti-Kickback Statute, the federal False Claims Act, the federal Health Insurance Portability and Accountability Act of 1996, state law equivalents to these federal laws that are meant to protect against fraud and abuse and analogous laws in foreign countries. Violations of these laws are punishable by criminal and civil sanctions, including, but not limited to, in some instances civil and criminal penalties, damages, fines, and exclusion from participation in federal and state healthcare programs, including Medicare and Medicaid. Although we exercise care in structuring our sales and marketing practices and customer discount arrangements to comply with those laws and regulations, we cannot assure that:
•government officials charged with responsibility for enforcing those laws will not assert that our sales and marketing practices or customer discount arrangements are in violation of those laws or regulations; or
•government regulators or courts will interpret those laws or regulations in a manner consistent with our interpretation.
We have in place policies and procedures for compliance that we believe are at least as stringent as those set forth in the AdvaMed Code of Ethics which was developed by AdvaMed, a trade association that represents the medical device industry, and which is intended to represent best practices with respect to medical device companies' interactions with healthcare providers. We regularly train our sales and marketing personnel on our policies regarding sales and marketing practices. Pursuant to the AdvaMed Code, we have certified our adoption of the AdvaMed Code. The sales and marketing practices of our industry have been the subject of increased scrutiny from federal and state government agencies, and we believe that this trend will continue. Various hospital organizations, medical societies and trade associations are establishing their own practices that may require detailed disclosures of relationships between healthcare professionals and medical device companies or ban or restrict certain marketing and sales practices such as gifts and business meals. Since these laws, regulations and ultimate enforcement continue to evolve, we cannot predict with certainty, what, if any, impact, changes to them may have on our business or our customers.
24
Outside of the U.S. we are subject to privacy and data security regulations at the international, national and regional level, as well as on an industry specific basis. For example, in Europe, we are subject to the EU General Data Protection Regulation ("GDPR") which is related to the collection, processing, storage, transfer and use of personal data. In the U.S., we are subject to the California Consumer Privacy Act of 2018 (“CCPA”) and other similar laws in the United States, at both the federal and state level. Noncompliance with GDPR could trigger fines of up to 4% of global annual revenues. In addition, we are subject to the new China Personal Information Protection Law that went into effect November 1, 2021 which focuses on protecting personal information and cross border transfers of the information. Compliance with these requirements, either individually or in the aggregate, may require changes in business practices added complexity and additional management oversight. They also may complicate our clinical research activities, as well as product offerings that involve transmission or use of clinical data. Non-compliance may result in proceedings against us by governmental or other entities and/or significant fines which could negatively impact our reputation and adversely affect our business.
Should we delay or fail to comply with one or more of the regulatory requirements we could have reduced sales, increased costs, delays to new product introductions, enhancements or our strategic plans, or harm to our reputation or competitiveness, which could have a material adverse effect on our business and financial results.
Our medical device products are subject to reporting requirements and recalls, even after receiving regulatory clearance, approval or certification, which could harm our reputation, business and financial results.
After a device is placed on the market, numerous regulatory requirements apply, which require manufacturers to follow, among other things, design, testing, production, control, documentation and other quality assurance procedures during the manufacturing process; labeling regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and medical device reporting regulations that require us to report to FDA or similar governmental bodies in other countries if our products may have caused or contributed to a death or serious injury or malfunction in a way that would be reasonably likely to contribute to death or serious injury if the malfunction were to recur. The FDA and similar governmental bodies in other countries have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture or in the event that a product poses an unacceptable risk to health. We may, under own initiative, recall a product if a reasonable possibility of serious injury or any material deficiency in a device is found, or withdraw a product to improve device performance or for other reasons.
Recalls of any of our products may divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. A recall could harm our reputation with customers and consumers which could reduce the sales of our products. In addition, the FDA or other foreign governmental agencies may implement enforcement actions in connection with a recall which could impair our product offerings and be harmful to our business and financial results.
Certain of our products contain materials derived from animal sources and may become subject to additional regulation.
Certain of our products are derived from bovine or porcine tissue sources.As a result, we may experience difficulties in processing and producing our bovine and porcine tissue products at scale, including problems related to yields, quality control and assurance, tissue availability, adequacy of control policies and procedures and availability of skilled personnel.
With respect to bovine, among other products, our dermal regeneration products, duraplasty products, wound care products, bone void fillers, nerve and tendon repair products and certain other products, contain material derived from bovine tissue. In 2022, 43.3% of our revenues derived from products containing material derived from bovine tissue. Products that contain materials derived from animal sources, including food, pharmaceuticals and medical devices, are subject to scrutiny in the media and by regulatory authorities. Regulatory authorities are concerned about the potential for the transmission of disease from animals to humans via those materials. This public scrutiny has been particularly acute in Japan and Western Europe with respect to products derived from animal sources, because of concern that materials infected with the agent that causes bovine spongiform encephalopathy, otherwise known as BSE or mad cow disease, may, if ingested or implanted, cause a variant of the human Creutzfeldt-Jakob Disease, an ultimately fatal disease with no known cure. The World Organization for Animal Health recognizes the U.S. as having a negligible risk for BSE, which is the highest status available.
We take care to provide that our products are safe and free of agents that can cause disease. In particular, we qualified a source of collagen from a country outside the U.S. that is considered BSE/TSE-free. The World Health Organization classifies different types of bovine tissue for relative risk of BSE transmission. Deep flexor tendon and bovine fetal skin, which are used in our products, are in the lowest-risk categories for BSE transmission and are therefore considered to have a negligible risk of containing the agent that causes BSE (an improperly folded protein known as a prion). Nevertheless, products that contain materials derived from animals, including our products, could become subject to additional regulation, or even be banned in certain countries, because of concern over the potential for the transmission of prions. Significant new regulations, or a ban of our products, could have a material, adverse effect on our current business or our ability to expand our business.
Certain countries, such as Japan, China, Taiwan and Argentina, have issued regulations that require our collagen products be sourced from countries where no cases of BSE have occurred, and the EU has requested that our dural replacement products and other products that are used in neurological tissue be sourced from a country where no cases of BSE have occurred. Currently, we source bovine fetal hides from the U.S. and purchase tendon from the U.S. and New Zealand. New Zealand has
25
never had a case of BSE. We received approval in the U.S., the EU, Japan, Taiwan, China, Argentina as well as other countries for the use of New Zealand-sourced tendon in the manufacturing of our products. If we cannot continue to use or qualify a source of tendon from New Zealand or another country that has never had a case of BSE, we could be prohibited from selling our collagen products in certain countries.
We are subject to current and potential future requirements relating to protection of the environment, such as hazardous materials regulations, which may impose significant compliance or other costs on us.
Certain of our processes in manufacturing and research and development involve the controlled use of certain hazardous materials. In addition, we own and/or lease a number of facilities at which hazardous materials have been used in the past. Finally, we have acquired various companies that historically have used certain hazardous materials and that have owned and/or leased facilities at which hazardous materials have been used. For all of these reasons, we are subject to federal, state, foreign, and local laws and regulations governing the use, manufacture, storage, transportation, handling, treatment, remediation, and disposal of hazardous materials and certain waste products (“Environmental, Health, Safety and Transportation Laws”). Although we believe that our procedures for handling, transporting, and disposing of hazardous materials comply with the Environmental, Health, Safety and Transportation Laws, such laws may be amended in ways that increase our cost of compliance, perhaps materially.
Furthermore, the potential risk of accidental contamination or injury from these materials cannot be eliminated, and there is also a risk that such contamination previously has occurred in connection with one of our facilities or in connection with one of the companies we have purchased. In the event of such an accident or contamination, we could be held liable for any damages that result and any related liability could exceed the limits or fall outside the coverage of our insurance and could exceed our resources. We may not be able to maintain insurance on acceptable terms or at all.
Our business and operations are subject to risks related to climate change.
The long-term effects of global climate change present both physical risks (from the increased frequency of extreme weather conditions or natural disasters) and transition risks (from regulatory requirements or technology changes). Such extreme weather conditions could pose physical risks to our facilities and disrupt operation of our supply chain and may impact operational costs. Concern over global climate change could result in new legal or regulatory requirements designed to mitigate the effects of climate change on the environment. If such laws or regulations are more stringent than current legal or regulatory requirements, we may experience increased compliance burdens and costs to meet the regulatory obligations and such measures may interrupt our operations or the operations of our suppliers, potentially leading to higher costs, and therefore negatively impact our results of operations.
We are subject to requirements relating to information technology which could adversely affect our business.
If we are unable to maintain reliable information technology systems and prevent disruptions, outages, or data breaches, we may suffer regulatory consequences in addition to business consequences. Our worldwide operations means that we are subject to laws and regulations, including data protection and cyber security laws and regulations, in many jurisdictions. The variety of U.S. and international privacy and cybersecurity laws and regulations impacting our operations are described in “Item 1. Business - Government Regulation - Other Factors - Data Privacy and Cybersecurity Laws and Regulations." We have programs to ensure compliance with such laws and regulations. However, there is no guarantee that we will avoid enforcement actions by governmental bodies. Enforcement actions may be costly and interrupt regular operations of our business. In addition, there has been a developing trend of civil lawsuits and class actions relating to breaches of consumer data held by large companies or incidents arising from other cyber-attacks. While Integra has not been named in any such suits, if a substantial breach or loss of data were to occur, we could become a target of such litigation.
If we do not retain our key personnel and attract and retain other highly skilled employees, our business could suffer.
If we fail to recruit, develop and retain the necessary personnel, our business and our ability to obtain new customers, develop new products and provide acceptable levels of customer service could suffer. The success of our business is heavily dependent on the leadership of our key management personnel. Our success also depends on our ability to recruit, develop and retain and motivate highly skilled sales, marketing, manufacturing and scientific personnel. Competition for these persons in our industry is intense, and we may not be able to successfully recruit, train or retain qualified personnel. We are experiencing increasing challenges in building and retaining our workforce in certain markets, where pressure from inflation and competition have exacerbated turnover and retention trends continuing from the COVID-19 pandemic. Labor shortages and competition for qualified personnel could cause disruptions in our business operations.
RISKS RELATED TO TAX AND DEBT
We may have additional tax liabilities.
We are subject to income taxes in the U.S. and many foreign jurisdictions and are commonly audited by various tax authorities. In the ordinary course of our business, there are many transactions and calculations where the ultimate tax determination is uncertain. Significant judgment is required in determining our worldwide provision for income taxes. Although we believe that our tax estimates are reasonable, the final determination of tax audits and any related litigation could be materially different
26
from our historical income tax provisions and accruals. The results of an audit or litigation could have a material, adverse effect on our financial statements in the period or periods for which that determination is made.
Changes in tax laws or exposures to additional tax liabilities could negatively impact the Company's operating results.
We are subject to income taxes, as well as taxes that are not income-based, in both the U.S. and many foreign jurisdictions. Taxes could significantly increase due to changes in tax laws or changes in our interpretation of those laws. For example, the Organization for Economic Co-operation and Development, a global policy forum, is developing a global tax framework that, if implemented, includes a global minimum tax rate of 15%. Taxes could also significantly increase due to changes in accounting guidance. Our future effective tax rate could be unfavorably affected by numerous factors including a change in, or the interpretation of, tax rules and regulations in the jurisdictions in which we operate (including changes in legislation currently being considered), a change in our geographic earnings mix, and/or to the jurisdictions in which we operate, or a change in the measurement of our deferred taxes.
Our leverage and debt service obligations could adversely affect our business.
Our leverage and debt service obligations could adversely affect our business. As of December 31, 2022, our total consolidated external debt was approximately $1.5 billion (See Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations and Note 5, Debt, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for a discussion of our consolidated external debt). We may also incur additional indebtedness in the future. Our substantial indebtedness could have material, adverse consequences, including:
•making it more difficult for us to satisfy our financial obligations;
•increasing our vulnerability to adverse economic, regulatory and industry conditions, and placing us at a disadvantage compared to our competitors that are less leveraged;
•limiting our ability to compete and our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; and
•limiting our ability to borrow additional funds for working capital, capital expenditures, acquisitions and general corporate or other purposes.
Our debt service obligations will require us to use a portion of our operating cash flow to pay interest and principal on indebtedness instead of for other corporate purposes, including funding future expansion of our business, acquisitions, and ongoing capital expenditures, which could impede our growth. In addition, our ability to comply with, renegotiate or extend the Company’s debt obligations will depend on our operating and financial performance, which in turn is subject to prevailing economic conditions and financial, business and other factors beyond our control. Any disruptions in our operations, the financial markets, or the overall economy, including as a result of COVID-19, may adversely affect the availability and cost of credit to us and/or our ability to comply with our existing obligations.
Changes in the calculation and or complete replacement of LIBOR could have an impact on our business.
The United Kingdom’s Financial Conduct Authority (“FCA”), which regulates LIBOR, announced in July 2017 that it will no longer persuade or require banks to submit rates for LIBOR. On March 5, 2021, the ICE Benchmark Administration, which administers LIBOR, and the FCA announced that all LIBOR settings will either cease to be provided by any administrator, or no longer be representative immediately after December 31, 2021, for all non-U.S. dollar LIBOR settings and one-week and two-month U.S. dollar LIBOR settings, and immediately after June 30, 2023 for the remaining U.S. dollar LIBOR settings. We have multiple debt facilities which utilizes a variable rate equal to Eurodollar LIBOR rate as a component of our interest rate.
Management expects all LIBOR-based contracts to be replaced by the Secured Overnight Financing Rate (“SOFR”), which is calculated based on overnight transactions under repurchase agreements backed by Treasury securities. The Alternative Reference Rates Committee, a group of private-market participants convened by the U.S. Federal Reserve Board and the New York Federal Reserve, has recommended the use of SOFR as a more robust reference rate alternative to LIBOR. The use of SOFR as a substitute for LIBOR is, however, voluntary and may not be suitable for all market participants. There can be no assurance that the replacement rate will be economically equivalent to LIBOR, which could result in higher interest rates for us under our debt facilities. There is no guarantee that a transition from LIBOR to SOFR will not result in financial market disruptions, significant increases in benchmark rates, or our borrowing costs, any of which could have an adverse effect on our business, results of operations and financial condition.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
Our intellectual property rights may not provide meaningful commercial protection for our products, potentially enabling third parties to use our technology or very similar technology and could reduce our ability to compete in the market.
To compete effectively, we depend, in part, on our ability to maintain the proprietary nature of our technologies and manufacturing processes, which includes the ability to obtain, protect and enforce patents on our technology and to protect our
27
trade secrets. We own or have licensed patents that cover aspects of some of our product lines. Our patents, however, may not provide us with any significant competitive advantage. Others may challenge our patents and, as a result, our patents could be narrowed, invalidated or rendered unenforceable. Competitors may develop products similar to ours that our patents do not cover. In addition, the approval or rejection of patent applications may take several years and our current and future patent applications may not result in the issuance of patents in the U.S. or foreign countries.
Our competitive position depends, in part, upon unpatented trade secrets, which we may be unable to protect.
Our competitive position also depends upon unpatented trade secrets, which are difficult to protect. We cannot assure that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets, that our trade secrets will not be disclosed or that we can effectively protect our rights to unpatented trade secrets.
In an effort to protect our trade secrets, we require our employees, consultants and advisors to execute confidentiality and invention assignment agreements upon commencement of employment or consulting relationships with us. These agreements provide that, except in specified circumstances, all confidential information developed or made known to the individual during the course of their relationships with us must be kept confidential. We cannot assure, however, that these agreements will provide meaningful protection for our trade secrets or other proprietary information in the event of the unauthorized use or disclosure of confidential information.
Our success will depend partly on our ability to operate without infringing or misappropriating the proprietary rights of others.
We may be sued for infringing the intellectual property rights of others. In addition, we may find it necessary, if threatened, to initiate a lawsuit seeking a declaration from a court that we do not infringe the proprietary rights of others or that their rights are invalid or unenforceable. If we do not prevail in any litigation, in addition to any damages we might have to pay, we would be required to stop the infringing activity (which could include a cessation of selling the products in question) or obtain a license for the proprietary rights involved. Any required license may be unavailable to us on acceptable terms, if at all. In addition, some licenses may be nonexclusive and allow our competitors to access the same technology we license.
If we fail to obtain a required license or are unable to design our products so as not to infringe on the proprietary rights of others, we may be unable to sell some of our products, and this potential inability could have a material, adverse effect on our revenues and profitability.
We may be involved in lawsuits relating to our intellectual property rights and promotional practices, which may be expensive.
To protect or enforce our intellectual property rights, we may have to initiate or defend legal proceedings, such as infringement suits or opposition proceedings, against or by third parties. In addition, we may have to institute proceedings regarding our competitors’ promotional practices or defend proceedings regarding our promotional practices. Legal proceedings are costly, and, even if we prevail, the cost of the legal proceedings could affect our profitability. In addition, litigation is time-consuming and
could divert management's attention and resources away from our business. Moreover, in response to our claims against other parties, those parties could assert counterclaims against us.
If any of our facilities or those of our suppliers were damaged and/or our manufacturing or business processes interrupted, we could experience lost revenues and our business could be seriously harmed.
Damage to our manufacturing, distribution, development and/or research facilities because of fire, extreme weather conditions, natural disaster, power loss, communications failure, geopolitical disruption, unauthorized entry or other events, such as a flu or other health epidemic, such as the novel coronavirus,COVID-19, could significantly disrupt our operations, the operations of suppliers and critical infrastructure and delay or prevent product manufacture and shipment during the time required to repair, rebuild or replace the damaged facilities. Certain of our manufacturing facilities are located in Puerto Rico, which in the past has experienced both severe earthquakeshurricanes and other natural disasters. We believeClimate change may increase both the risk associated with operating a manufacturing plant in Puerto Rico, post Hurricane Maria, has returnedfrequency and severity of extreme weather conditions and natural disasters and, consequently, risks to historical levels. While there are still some challenges with the energy systemour operations and service is occasionally disrupted for short periods, it has not impacted operations primarily due to the generator capacity at the plant.growth. Although we maintain property damage and business interruption insurance coverage on these facilities, our insurance might not cover all losses under such
circumstances, and we may not be able to renew or obtain such insurance in the future on acceptable terms with adequate coverage or at reasonable costs.
We are exposed to a variety of risks relating to our international sales and operations.
We generate significant revenues outside the U.S. in multiple foreign currencies, and in U.S. dollar-denominated transactions conducted with customers who generate revenue in currencies other than the U.S. dollar. For those foreign customers who purchase our products in U.S. dollars, currency fluctuations between the U.S. dollar and the currencies in which those
28
customers do business may have a negative impact on the demand for our products in foreign countries where the U.S. dollar has increased in value compared to the local currency.
Since we have operations based outside the U.S. and we generate revenues and incur operating expenses in multiple foreign currencies, we experience currency exchange risk with respect to those foreign currency-denominated revenues and expenses. Our most significant currency exchange risk relates to transactions conducted in Australian dollars, British pounds, Canadian dollars, Chinese yuan, euros,Euros, Japanese yen, and Swiss francs.
We cannot predict the consolidated effects of exchange rate fluctuations upon our future operating results because of the number of currencies involved, the variability of currency exposure and the potential volatility of currency exchange rates. Although we address currency risk management through regular operating and financing activities, and, on a limited basis, through the use of derivative financial instruments, those actions may not prove to be fully effective. For a description of our use of derivative financial instruments, see Note 6, Derivative Instruments in our consolidated financial statements. to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K).
Our international operations subject us to laws regarding sanctioned countries, entities and persons, customs, import-export, laws regarding transactions in foreign countries, the U.S. Foreign Corrupt Practices Act and local anti-bribery and other laws regarding interactions with healthcare professionals, and product registration requirements. Among other things, these laws restrict, and in some cases prevent, U.S. companies from directly or indirectly selling goods, technology or services to people or entities in certain countries. In addition, these laws require that we exercise care in structuring our sales and marketing practices and effecting product registrations in foreign countries.
The United Kingdom (UK) held a referendum in which voters approved anKingdom’s (“UK”) exit from the EU,European Union on January 31, 2020, commonly referred to as “Brexit.” As a result ofBrexit, has caused, and may continue to cause uncertainty in the referendum, the British government began negotiating the terms of the UK’s future relationship with the EU. The UK exited the EU on January 31, 2020 and entered a transition period which extends through
As we seek to continue to expand and strengthen our international operations, we may experience difficulty in growing our sales in certain new markets and other international markets in which we are attempting to increase our presence due to, among other things, customer acceptance, undeveloped and/or unfamiliar distribution channels, regulatory restrictions and changes, and business knowledge of these markets.
From time to time, proposals are made to significantly change existing trade agreements and relationships between the U.S. and other countries. For instance,In recent years, the U.S. government has implemented substantial changes to U.S. trade policies, including import restrictions, increased import tariffs and changes in U.S. participation in multilateral trade agreements, such as the United States-Mexico-Canada Agreement to replace the former North American Free Trade Agreement. The ongoing global economic competition and trade tensions between the U.S. and China have imposedhas resulted in the U.S. government assessing supplemental tariffs on certain goods imported from China and China’s assessment of retaliatory tariffs on certain imports of U.S. goods into China. In addition, the United States has assessed or proposed supplemental tariffs and quantitative restrictions on U.S. imports of certain products imported into their respective countries. While we currently do not anticipate that these tariffs will have a material impact on our business,from other countries as well. Owing to the list of items subject to these tariffs could changecomplex relationships between the U.S. and it is possible that they could adversely impact our supply chain costs or our ability to sell certain of our products in China. More generally, additional tariffssuch other countries, political, diplomatic, military, or other events could result in business disruptions, including increased regulatory enforcement against companies, tariffs, trade barriers imposed byembargoes, export restrictions and the U.S.termination or other countriesmodification of existing trade agreements. The imposition of such restrictions could increase the cost of the Company’s products and the components and raw materials that go into making them, require the Company to change its operations and the products it offers and negatively impact consumer confidence and spending, all of which, both individually and in the aggregate, could materially and adversely affect our business, results of operations and financial results.condition.
Cyber-attacks or other disruptions to our information technology systems could adversely affect our business.
We are increasingly dependent on sophisticated information technology for our infrastructure and to support business decisions. Our information systems require an ongoing commitment of significant resources to maintain, protect, and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards, the increasing need to protect patient and customer information, and changing customer
29
patterns. An experienced third party maintains the enterprise business system used to support our transaction processing, accounting and financial reporting, and supply chain and manufacturing processes. Any significant breakdown, intrusion, interruption, corruption, or destruction of these systems, as well as any data breaches, could have a material, adverse effect on our business.
We have programs, processes (including ongoing improvements) and technologies in place to prevent, detect, contain, respond to and mitigate security related threats and potential incidents. We undertake considerable ongoing improvements to our systems, connected devices and information-sharing products in order to minimize vulnerabilities, in accordance with industry and regulatory standards. Because the techniques used to obtain unauthorized access or interrupt services change frequently and can be difficult to detect, anticipating, identifying or preventing these intrusionsthreats or mitigating them if and when they occur, may be challenging.
We are also relydependent on third party vendors to supply and/or support certain aspects of our information technology systems. Third party systems which may contain defects in design or manufacture or other problems that could result in system disruption or unexpectedly compromise the information security of our own systems, and we are dependent on these third parties to provide reliable systems and software and to deploy appropriate security programs to protect their systems.
In addition, as we continue to grow in part through new business acquisitions. As a result of acquisitions we may face risks due to implementation, modification, or remediation of controls, procedures, and policies relating to data privacy and cybersecurity at the acquired business. We continue to consolidate and integrate the number of systems we operate, and to upgrade and expand our information system capabilities for stable and secure business operations.Despite our implementation of controls to protect our systems and sensitive, confidential or personal data or information, we may be vulnerable to material security breaches, theft, misplaced, lost or corrupted data, employee errors and/or malfeasance (including misappropriation by departing employees) that could potentially lead to the compromising of sensitive, confidential or personal data or information, improper use of our systems, software solutions or networks, unauthorized access, use, disclosure, modification or destruction of information, defective products, production downtimes and operational disruptions. In addition, a cyber-related attack could result in other negative consequences, including damage to our reputation or competitiveness, remediation or increased protection costs, litigation or regulatory action.
Environmental, social and corporate governance (ESG) issues, including those related to climate change and sustainability, may have an adverse effect on our business, financial condition and results of operations and damage our reputation.
There is an increasing focus from certain investors, customers, consumers, employees and other stakeholders concerning ESG matters. Additionally, public interest and legislative pressure related to public companies’ ESG practices continue to grow. Consistent with these developments, we published our inaugural ESG Report which includes performance highlights in key areas such as employee health and safety, diversity and inclusion, community impact, ethics and compliance, and environmental responsibility. In addition, we formally expanded Board oversight to include ESG strategy and reporting. If, however, our ESG practices fail to meet regulatory requirements or investor, customer, consumer, employee or other stakeholders' evolving expectations and standards for responsible corporate citizenship in areas including environmental stewardship, support for local communities, Board of Director and employee diversity, human capital management, employee health and safety practices, product quality, supply chain management, corporate governance and transparency, our reputation, brand and employee retention may be negatively impacted, and our customers and suppliers may be unwilling to continue to do business with us.
If we are unabledo not adapt to maintain reliable information technology systemsor comply with new regulations, or fail to meet evolving investor, industry or stakeholder expectations and prevent disruptions, outages, or data breaches, weconcerns regarding ESG issues, investors may suffer regulatory consequencesreconsider their capital investment in additionour Company, and customers may choose to business consequences. Our worldwide operations mean that we are subject to laws and regulations, including data protection and cyber security laws and regulations, in many jurisdictions. The variety of U.S. and international privacy and cybersecurity laws and regulations impactingstop purchasing our operations are described in “Item 1. Business - Government Regulation - Other Factors - Data Privacy and Cybersecurity Laws and Regulations." We have programs to ensure compliance with such laws and regulations. However, there is no guarantee that we will avoid enforcement actions by governmental bodies. Enforcement actions may be costly and interrupt regular operations of our business. In addition, there has been a developing trend of civil lawsuits and class actions relating to breaches of consumer data held by large companies or incidents arising from other cyber-attacks. While Integra has not been named in any such suits, if a substantial breach or loss of data were to occur, we could become a target of such litigation.
ITEM 1B. UNRESOLVED STAFF COMMENTS
As of the filing of this Annual Report on Form 10-K, we had no unresolved comments from the staff of the Securities and Exchange Commission that were received not less than 180 days before the end of our 20192022 fiscal year.
| ITEM 2. | PROPERTIES | ||||
As of December 31, 2019,2022, we lease approximately 166,991 square feet of space in Princeton, NJ, where we house our principal headquarters, sales operations, research and development, and support functions. This lease expires in 2036.2035.
30
We have key manufacturing and research facilities located in California, Indiana, Maryland, Massachusetts, New Jersey, Ohio, Massachusetts,Puerto Rico, Tennessee, Texas, Canada,Utah, France, Germany, Ireland Switzerland, California and Puerto Rico.Switzerland. Our instrument procurement operations are located in Germany. Our primary distribution centers are located in Kentucky, Nevada, Ohio, Kentucky, Australia, Belgium, Canada, Italy, Japan, and France.China. In addition, we lease several smaller facilities to support additional administrative, assembly, and distribution operations. Third parties own and operate the facilities in Nevada, Kentucky, Japan and Belgium. We own facilities in Biot, France, Saint Aubin Le Monial, France, Rietheim-Weilheim, Germany Ohio, and PennsylvaniaOhio and we lease all of our other facilities. We also have repair centers in California, Massachusetts, Ohio, Australia, France, Japan, China and Germany.Germany, and field service presence in Canada, Dubai, India, Italy, Netherlands, Singapore, Thailand and United Kingdom.
Our manufacturing facilities are registered with the FDA. Our facilities are subject to FDA inspection to ensure compliance with Quality System regulations. For further information regarding the status of FDA inspections, see the "Government Regulation"Item 1. Business –Government Regulation and "Management'sCompliance Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations - Update on Remediation Activities" sections– FDA Matters in this Annual Report on Form 10-K.
| ITEM 3. | LEGAL PROCEEDINGS | ||||
Information pertaining to legal proceedings can be found in Note 15. 15, Commitment and Contingencies in our 2019, to the Notes to Consolidated Financial Statements.Statements (Part IV, Item 15 of this Annual Report on Form 10-K).
| ITEM 4. | MINE SAFETY DISCLOSURES | ||||
Not applicable.
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | ||||
Market Information, Holders and Dividends
Our common stock trades on The NASDAQNasdaq Global Select Market under the symbol “IART.” The number of stockholders of record as of February 18, 202021, 2023 was approximately 892,approximately 779, which includes stockholders whose shares were held in nominee name.
Dividend Policy
We have not paid any cash dividends on our common stock since our formation. Our Senior Credit Facility (as defined below) limits the amount of dividends that we may pay. Any future determinations to pay cash dividends on our common stock will be at the discretion of the Board and will depend upon our financial condition, results of operations, cash flows and other factors deemed relevant by the Board.
Sales of Unregistered Securities
There were no sales of unregistered securities during the years ended December 31, 2019, 20182022, 2021 or 2017.2020.
Sale of Registered Securities
Issuer Purchases of Equity Securities
On December 11, 2018,January 12, 2022, the Company entered into a $125.0 million accelerated share repurchase ("2022 ASR") and received 1.48 million shares of the Company common stock at inception of the 2022 ASR, which represented approximately 80% of the expected total shares under the 2022 ASR. On March 24, 2022, the early exercise provision was exercised by 2022 ASR counterparty. Upon settlement on March 24, 2022, the Company received an additional 0.46 million shares determined using the volume-weighted average price of the Company's common stock during the term of the 2022 ASR.
On April 26, 2022, the Board of Directors authorized the Company to repurchase up to $225.0 million of the Company’s common stock. The program allows the Company to repurchase its shares opportunistically from time to time. The repurchase authorization expires in December 2020.2024. This stock repurchase authorization replaces the previous $225 million stock repurchase authorization, of which $100 million remained authorized at the time of its replacement, and which was otherwise set to expire on December 31, 2022. Purchases may be affected through one or more open market transactions, privately negotiated transactions, transactions structured through investment banking institutions, or a combination of the foregoing.
31
For the year ended December 31, 2019, 2018 or 2017.
2021, there were no repurchases of the Company’s common stock as part of the share repurchase authorization.
See Note 8, Treasury Stock to, in our consolidated financial statements the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for further details.
The information required by this item regarding our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K.
Stock Performance Graph
The graph below compares the Company offered and sold in a private placement $575.0 million of 0.5% convertible notes due in 2025. The Company intendsfive-year total return to use $100.0 millionstockholders on our common stock with the return of the net proceeds fromStandard & Poor’s (S&P) 500 Stock Index and the offeringS&P Healthcare Equipment Index. The Company’s cumulative shareholder return is based on an investment of $100 on December 31, 2017 and is compared to repurchase sharesthe cumulative total return of the Company's stock.S&P indices mentioned above over the period with a like amount invested.
Note: The stock price performance shown on the graph above is not indicative of future price performance. This includes up to approximately $7.6 million from certain purchasersgraph shall not be deemed filed for purposes of Section 18 of the convertible notesExchange Act or otherwise subject to the liabilities of that section nor shall it be deemed incorporated by reference in conjunction withany filing under the closingSecurities Act or the Exchange Act, regardless of the offering. Additionally, the Company intends to use $92.4 million of the proceeds to repurchase shares through an accelerated share repurchase transaction ("ASR").any general incorporation language in such filing.
| Years Ended December 31, | |||||||||||||||||||
| 2019 | 2018 | 2017 | 2016 | 2015 | |||||||||||||||
| (In thousands, except per share data) | |||||||||||||||||||
| Operating Results: | |||||||||||||||||||
| Total revenues, net | $ | 1,517,557 | $ | 1,472,441 | $ | 1,188,236 | $ | 992,075 | $ | 882,734 | |||||||||
| Costs and expenses | 1,423,797 | 1,361,443 | 1,143,432 | 876,735 | 803,147 | ||||||||||||||
| Operating income (4) | 93,760 | 110,998 | 44,804 | 115,340 | 79,587 | ||||||||||||||
| Interest expense, net (1) (2) | (43,178 | ) | (61,883 | ) | (34,764 | ) | (25,779 | ) | (23,504 | ) | |||||||||
| Other income, net | 9,522 | 8,288 | 1,345 | 845 | 4,588 | ||||||||||||||
| Income from continuing operations before income taxes | 60,104 | 57,403 | 11,385 | 90,406 | 60,671 | ||||||||||||||
| (Benefit from) provision for income taxes (4) (6) | 9,903 | (3,398 | ) | (53,358 | ) | 15,842 | 53,820 | ||||||||||||
| Net income from continuing operations | $ | 50,201 | $ | 60,801 | $ | 64,743 | $ | 74,564 | $ | 6,851 | |||||||||
| Loss from discontinued operations (net of tax benefit) | $ | — | $ | — | $ | — | $ | — | $ | (10,370 | ) | ||||||||
| Net income (loss) | $ | 50,201 | $ | 60,801 | $ | 64,743 | $ | 74,564 | $ | (3,519 | ) | ||||||||
| Diluted net income per common share from continuing operations | $ | 0.58 | $ | 0.72 | $ | 0.82 | $ | 0.94 | $ | 0.10 | |||||||||
| Diluted net loss per common share from discontinued operations | $ | — | $ | — | $ | — | $ | — | $ | (0.15 | ) | ||||||||
| Diluted net income (loss) per common share | $ | 0.58 | $ | 0.72 | $ | 0.82 | $ | 0.94 | $ | (0.05 | ) | ||||||||
| Weighted average common shares outstanding for diluted net income per share | 86,494 | 83,999 | 79,121 | 79,194 | 71,354 | ||||||||||||||
| As of December 31, | ||||||||||||||||||||
| 2019 | 2018 | 2017 | 2016 | 2015 | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Financial Position: | ||||||||||||||||||||
| Cash, cash equivalents | $ | 198,911 | $ | 138,838 | $ | 174,935 | $ | 102,055 | $ | 48,132 | ||||||||||
| Total assets (5) (7) | 3,303,240 | 3,107,887 | 3,211,257 | 1,807,954 | 1,774,224 | |||||||||||||||
| Short-term borrowings under the term loan of the Senior Credit Facility | 45,000 | 22,500 | 60,000 | — | 14,375 | |||||||||||||||
| Long-term borrowings including the revolving portion of the Senior Credit Facility (1) | 1,198,561 | 1,210,513 | 1,781,142 | 665,000 | 481,875 | |||||||||||||||
| Long-term debt (2) (8) | 104,500 | 121,200 | — | — | 218,240 | |||||||||||||||
| Retained earnings (4) | 398,574 | 348,373 | 285,186 | 220,443 | 145,879 | |||||||||||||||
| Stockholders’ equity (3) | 1,416,736 | 1,375,796 | 962,306 | 839,667 | 751,443 | |||||||||||||||
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | ||||
32
The following discussion and analysis ofprovides information management believes to be relevant to understanding our financial condition and results of operations. For a full understanding of financial condition and results of operations, it should be read together with the selected consolidated financial data and our financial statements andwith the related notes appearing elsewhere in this report. ThisThe discussion focuses on our financial results for the year ended December 31, 2022 and analysis contains2021. The comparison of fiscal 2021 to 2020 has been omitted from this Form 10-K, but can be referenced in our Form 10-K for the fiscal year ended December 31, 2021—“Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations” filed with the SEC on February 24, 2022.
We have made statements in this report which constitute forward-looking statements that involve risks, uncertaintieswithin the meaning of Section 27A of the Securities Act and assumptions. OurSection 21E of the Exchange Act. The Company’s actual results maycould differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those set forth under Item 1A. Risk Factors. We have made statements in this report which constitute forward-looking statements within the heading “Risk Factors.”meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act. These forward-looking statements are subject to a number of risks, uncertainties and assumptions about the Company and other matters. Please refer to “Special Note Regarding Forward-Looking Statements” and Item 1A. Risk Factors for a discussion of the factors that could cause actual results to differ materially from those projected in these statements. The following information concerning our business, results of operations and financial condition should also be read in conjunction with the information included under Item 1. Business, Item 1A. Risk Factors and Item 15. Financial Statements and Supplementary Data.
GENERAL
Integra headquartered in Princeton, New Jersey,LifeSciences is a worldglobal leader in medical technology. The Company was foundedregenerative tissue technologies and neurological solutions dedicated to limiting uncertainty for clinicians so they can focus on providing the best patient care. Founded in 1989 with the acquisition of an engineered collagen technology platform used to repair and regenerate tissue. Since then,tissue, Integra LifeSciences Holdings Corporation common stock trades on the Nasdaq Global Select Market (“Nasdaq”) under the symbol “IART.” Integra has developed numerous product lines from this technology for applications ranging from burn and deep tissue wounds to the repair of dura mater in the brain, and repair ofas well as nerves and tendons. The Company has expanded its base regenerative technology business to include surgical instruments, neurosurgical products and advanced wound care collagen matrix products for hernia and plastic & reconstructive surgery, and orthopedic hardware, through a combination of several global acquisitions and product development of products internally to further meet the evolving needs of its customers and impactenhance patient care.
We have key manufacturing and research facilities located in California, Indiana, Maryland, Massachusetts, New Jersey, Ohio, Massachusetts,Puerto Rico, Tennessee, Texas, Canada,Utah, France, Germany, Ireland Switzerland, and Puerto Rico. Switzerland. We also source most of our handheld surgical instruments specialty metal and pyrocarbon implants, and dural sealant products through specialized third-party vendors.
Integra is committed to delivering high quality products that positively impact the lives of millions of patients and their families. We focus on four key pillars of our strategy: 1) buildingenabling an execution-focused culture, 2) achievingoptimizing relevant scale, 3) improvingadvancing innovation and agility, and innovation, and 4) leading in customer experience. We believe that by sharpening our focus on these areas through improved planning and communication, optimization of our infrastructure, and strategically aligned tuck-in acquisitions, we can build scale, increase competitiveness and achieve our long-term goals.
To this end, the executive leadership team has established the following key priorities aligned to the following areas of focus:
Strategic Acquisitions. An important part of ourthe Company's strategy is pursuing strategic transactions and licensing agreements that increase relevant scale in the clinical areas in which Integra competes. In 2019, we closed outOur growth strategy includes the acquisition of 45 transition service agreements, covering 90 countries, markingbusinesses, assets or products lines to increase the successful completionbreadth of our offerings, the integrationreach of our product portfolios and drive relevant scale to our customers. On December 6, 2022, the Codman NeurosurgeryCompany completed the acquisition the most significant acquisition in the Company’s history.of SIA, which develops, markets and sells DuraSorb, a resorbable synthetic matrix for plastic and reconstructive surgery. This acquisition expandedwill advance Integra’s global strategy in breast reconstruction, expanding plans to access the U.S. market where SIA is pursuing pre-market approval for use in IBBR. We also continued to advance the development of pioneering neurosurgical technologies with the expansion our portfolioproduct offering of neurosurgery productsregenerative technologies from our 2021 ACell acquisition. See Note 4, Acquisitions and established us asDivestitures, to the world leader in neurosurgery. It has also enabled usNotes to bring our entire product portfolio to a global market. In 2019, we acquired Arkis Biosciences, Inc. and Rebound Therapeutics Corporation, bothConsolidated Financial Statements (Part IV, Item 15 of which align with Company’s strategy to acquire and develop innovative technologies that address unmet needs.this Annual Report on Form 10-K) for additional details.
33
Portfolio Optimization and New Product Introductions.Introductions. We are investing in innovative product development to drive a multi-generational pipeline for our key product franchises. Our product development efforts span across our key global franchises focused on potential technological innovationsfor significant returns on investment. In 2019, we launched ten new products across our key product franchises. In addition to new product development, we are funding studies to gather clinical evidence to support launches, ensure market access and improve reimbursement for existing products. In 2019,addition to acquisitions and organic reinvestment, we discontinued certain low-growth, low margin products. We continuecontinually look to identify ways of optimizingoptimize our portfolio including identifying low-growth, low-margintowards higher growth and higher margin businesses. As such, we may opportunistically divest businesses or discontinue products and product franchiseswhere we see limited runway for discontinuation.future value creation in line with our aspirations due in part to changes in the market, business fundamentals or the regulatory environment.
Commercial Channel Investments.Investments With acquisitions, new product introductions and a broader portfolio of products, investing. Investing in our sales channels is a core part of our strategy to create specialization and greater focus on reaching new and existing customers and addressing their needs. To support our commercial efforts in Tissue Technologies, we utilize a two-tier specialist model to increase our presence in focused segments to help serve the evolving needs of our customers. In addition, we continue to build upon our leadership brands across our product franchises in both CSS and TT to engage customers through enterprise-wide contracts with leading hospitals, integrated delivery networks and global purchasing organizations in the United States. Internationally, we have increased our commercial resources significantly in manykey emerging markets and are making investments to support our sales organization and maximize our commercial opportunities. We nowDomestically, we have a strongalso increased our TT sales force in the United States to support the expanded regenerative tissue product portfolio that includes ACell products. These investments in our international and domestic sales channel that will deliver our current portfolio as well as position us well for expansion. In addition, we continue to build upon our leadership brands across our product franchises to enable us to engage hospital systems through enterprise-wide contracts.expansion and long-term growth.
Customer Experience. We aspire to be ranked as a best-in-class provider and are committed to strengthenstrengthening our relationships with all customers. We strive to consistently deliver outstanding customer service and continue to invest in technologies, systems
and processes to improveenhance the way our customers do business with us. Additionally,customer experience. In 2022, we expectoutsourced certain transactional back-office finance and customer service activities to enhance customer quality, build on the success of our professional educationscale for future growth, and capture cost efficiencies. We also launched digital tools and programs, resources and virtual product training to drive continued customer familiarity with our growing portfolio of medical technologies globally. .
We continue to invest in collecting clinical evidence to support ourthe Company's existing products and new product launches, and to ensure that we obtain market access for broader and more cost-effective solutions.
In each area, we continue to benefit from products launched over the past several years, including our new electrosurgery generator and irrigator system, an innovative customer-centric toolkit for our Certas™Certas® Plus Programmable Valve along with additional shunt configurations. We launched DuraGen®In Japan, we are experiencing strong growth as a result of the successful launch of DuraGen in Japan. DuraGenmid-2019, which is the first and only non-autologous collagen xenograft approved for use as a dural substitute in Japan. the country.
In 2022, we continued to advance the two early-stage technology platforms we acquired in 2019. Through the acquisition of Arkiss, we added a platform technology, CerebroFlo EVD, which is a catheter with Endexo technology, a permanent additive designed to reduce the potential for catheter obstruction due to thrombus formation. The CerebroFlo EVD Catheter has demonstrated an average of 99% less thrombus accumulation onto its surface, in vitro, compared to a market leading EVD catheter. In 2019, we also acquired Rebound Therapeutics which specialized in a single-use medical device, known as Aurora Surgiscope, which is the only tubular retractor system designed for cranial surgery with an integrated access channel, camera and lighting. In 2021, we began and continued to conduct a limited clinical launch of the Aurora Surgiscope for use in minimally invasive neurosurgery as well as initiated a registry called MIRROR to collect data on early surgical intervention using this same technology platform for the treatment of ICH. In 2022, we have continued to execute on our growth initiatives. We launched the Aurora Evacuator with Coagulation device in the U.S., designed to be used in conjunction with our Aurora Surgiscope to safely address and evacuate blood in the brain caused by hemorrhagic stroke.
We are focused on the development of core clinical applications in our our electromechanical technologies portfolio. Also during 2019, we updated our CUSA Clarity platform to incorporate new ultrasonic tips and integrated electrosurgical capabilities.We continue to work with several instrument partners to bring new surgical instrument patterns to the market. This enables us to add new instruments with minimal expense and invest in ongoing development, such as our next generation of LED technology with our DUO LED Surgical Headlight System.
34
Within our TT segment, in 2022, we launched NeuraGen 3D Nerve Guide Matrix, a resorbable implant for repair of peripheral nerve discontinuities and engineered to create an optimized environment for nerve regeneration. During 2021, we completed one of the largest diabetic foot ulcers ("DFU"), randomized controlled trials of the PriMatrix Dermal Repair Scaffold for the management of DFU. This multi-center study enrolled more than 225 patients with chronic DFU's over the course of 12-week treatments and 4-week follow-up phases. The Panta II system is our new fusion nail usedresults of this study, which was published in ankle fixation. We also launchedthe Journal of Wound Care, demonstrated that PriMatrix plus standard of care SOC consisting of sharp debridement, infection elimination, use of dressings and offloading was significantly more likely to achieve complete wound closure compared with SOC alone, with a Small Post Baseplatemedian number of one application of the product. During 2020, we announced positive clinical and economic data on Integra® Bilayer Wound Matrix ("IBWM") in our Reverse Shoulder System that accommodates smallercomplex lower extremity reconstruction based on two retrospective studies recently published in Plastic and Reconstructive Surgery, the official journal of the American Society of Plastic Surgeons. As surgeons look for ways to efficiently and effectively repair and close wounds, IBWM helps address the efficiency needed in operating rooms by reducing both the operating time and costs to hospitals and patients. We initiated the limited market release of enhancements to our Salto Talaris® Total Ankle System. We continue to work on advanced shoulder products and are developing a pyrocarbon shoulder hemiarthroplasty product to add to our orthopedic reconstruction portfolio.
FDA Untitled LetterMatters
On June 22, 2015,August 18, 2022, the Company, after consultation with the FDA issued an Untitled Letter (the "Untitled Letter") alleging that BioD LLC's ("BioD") morselized amniotic membrane tissue based products do not meet the criteria for regulation as HCT/Ps solely under Section 361and other regulatory authorities outside of the Public Health Services Act ("Section 361") and that,United States, initiated an immediate voluntary global product removal of all CereLink intracranial pressure monitors as a result BioD would needof customer reports about monitors whose pressure readings were out of range. The Company believes that the out-of-range readings are principally caused by electrical interference from the external environment and/or interference from a biologics license to lawfully market those morselized products. Sincecomponent on the issuancecircuit board of the Untitled Letter, BioDmonitor. These out-of-range readings have occurred at a low incidence rate and more recentlyat a limited number of sites; however, out of an abundance of caution, the Company removed all CereLink monitors from the field.
The Company Is continuing its investigation into the matter in order to remedy the observed issue and plans to resume shipment of the CereLink monitors as soon as any such issues have been resolved. Based on outlook for returning the product to market and feedback from customers, the Company recorded a $1.9 million provision for product returns, as a reduction of net revenue, and a $0.8 million rework accrual in discussions with the FDA to communicate their disagreement with the FDA’s assertion that certaincost of goods sold in 2022.
We manufacture and distribute products are more than minimally manipulated. Thederived from human tissue for which FDA has not changed its position that certain of the BioD acquired products are not eligible for marketing solely under Section 361.
On March 7, 2019, TEI Biosciences, Inc. ("TEI"), a wholly-owned subsidiary of the Company received a Warning Letter (the “Warning Letter”), dated March 6, 2019, from the United States Food and Drug Administration (the “FDA”).FDA. The warning letter relatesrelated to quality systems issues at ourTEI's manufacturing facility located in Boston, Massachusetts. The letter resulted from an inspection held at that facility in October and November 2018 and did not identify any new observations that were not already provided in the Form 483 that followed the inspection. The Company has provided detailed responsessubmitted its initial response to the FDA Warning Letter on March 28, 2019 and provides regular progress reports to the FDA as to its corrective actions on a monthly basis and, since the conclusion of the inspection, has undertaken significant efforts to remediate the observations and continues to do so. On October 28, 2021 the FDA initiated an inspection of the facility and at the conclusion of the inspection issued a FDA Form 483 on November 12, 2021 (the "2021 Form 483"). The warning letter doesCompany provided an initial response to the inspection observations and will continue to provide responses to FDA. The Warning Letter and the 2021 FDA Form 483 do not restrict the Company’s ability to manufacture or ship products or require the recall of any products. Nor does itproducts, nor do they restrict our ability to seek FDA 510(k) clearance of products. The letter states that requests
Revenues of products manufactured in the TEI Boston facility for the year ended December 31, 20192022 were approximately 4.2%5.3% of consolidated revenues.
ACQUISITIONS & DIVESTITURES
Acquisitions
On July 29, 2019,December 6, 2022, the Company acquired Arkis BioSciences Inc. ("Arkis")completed its acquisition of SIA for an acquisition purchase price of $30.9$51.5 million. In addition to the purchase price, the acquisition includes two separate contingent considerations payments, which are dependent on 1) achieving certain revenue-based performance milestones in 2023, 2024, and 2025 (up to $50M in additional payments), as well as 2) the approval by the FDA of the PMA application for DuraSorb for certain uses by certain timing targets (up to $40M in additional payments). SIA was a privately-held company whose core technology, DuraSorb is a fully resorbable scaffold of a globally accepted polymer, cleared for use in hernia repair, abdominal wall, and other soft tissue reinforcement. DuraSorb sales will be reported within Integra’s TT segment as part of its Wound Reconstruction and Care franchise. See Note 4, Acquisitions and Divestitures, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details.
ACell Inc.
On January 20, 2021, the Company acquired ACell for an acquisition purchase price of $306.9 million (the "Arkis Acquisition") plus contingent consideration obligations of up to $25.5$100 million, that may be payable based on the successful completion ofupon achieving certain developmentrevenue-based performance milestones in 2022, 2023 and commercial milestones. The contingent consideration had an acquisition date fair value of $13.1 million. The estimated fair value as of December 31, 2019 was $14.2 million. This amount is included in other liabilities at December 31, 2019 in the consolidated balance sheets of the Company. Arkis2025. ACell was a privately-held company that marketedoffered a portfolio of regenerative products for complex wound management, including developing and commercializing products based on MatriStem Urinary Bladder Matrix, a technology platform derived from porcine urinary bladder extracellular matrix. See Note 4, Acquisitions and Divestitures, to the CerebroFlo® external ventricular drainage (EVD) catheter with Endexo® technology, a permanent additive designedNotes to reduce the potentialConsolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for catheter obstruction due to clotting.additional details.
On September 9, 2019,August 31, 2022, the Company acquired Rebound Therapeutics Corporation (“Rebound”), developerscompleted the sale of a single-use medical device known asits TWC business to Gentell for $28.8 million, which consists of $27.8 million in cash plus $1.0 million in contingent consideration which may be received upon achieving certain revenue-based performance milestones two years after the AURORA Surgiscope® System ("Aurora") which enables minimally invasive access, using optics closing date. The proceeds from the sale of the TWC business of $27.8 million is presented in the consolidated statement of cash flows net of cash transferred of $3.5 million and illumination, for visualization, diagnostic and therapeutic use in neurosurgery (the “Rebound transaction”)other transaction fees. The transaction was accounted forincluded the sale of the Company's TWC products, such as an asset acquisitionsponges, gauze and conforming bandages, and certain advanced wound care dressings, such as supportive, calcium alginate, hydrogel, and foam dressings. In connection with the sale, the Company concluded that it acquired primarily one asset. Underrecognized $0.6 million as a gain from the termssale of business in the Rebound transaction, the Company made an upfront paymentconsolidated statement of $67.1 million and committed to pay up to $35.0 million of contingent development milestones upon achievement of certain regulatory milestones. During the fourth quarter of 2019, the Company triggered a $5.0 million obligation to be paid to former shareholders of Rebound. The Company recorded the $5.0 million as an additional in-process research and development expense which was included in accrued liabilities atoperations for year ended December 31, 2019.2022. The transaction is subject to final working capital adjustments. See Note 4, Acquisitions and Divestitures, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Form 10-K) for details.
On January 4, 2019,2021, the Company entered into a licensing agreement with Integrated Shoulder Collaboration, Inc ("ISC"). Undercompleted its sale of its Extremity Orthopedics business to Smith & Nephew. The transaction included the termssale of the agreement, the Company paid ISC $1.7 million for the exclusive, worldwide license to commercialize its short stemCompany's upper and stemlesslower Extremity Orthopedics product portfolio, including ankle and shoulder system. A patent related to short stemarthroplasty and stemless shoulder systems was issued to ISC during the first quarter of 2019. ISC is eligible to receive royalties on sales of the short stemhand and stemless shoulder system upon commercialization.wrist product lines. The Company has the option to acquire ISC at a date four years subsequent to the first commercial sale, which becomes mandatory upon the achievement of a certain sales thresholds of the short stem and stemless shoulder system, for an amount not to exceed $80.0 million. The transaction was accounted for as an asset acquisition as the Company concluded that it acquired primarily one asset. The total upfront payment of $1.7 million was expensed as a component of research and development expense and the future milestone and option payments will be recorded if the corresponding events become probable.
36
OPTIMIZATION AND INTEGRATION ACTIVITIES
As a result of our ongoing acquisition strategy and significant growth in recent years, we have undertaken cost-saving initiatives to consolidate manufacturing operations, distribution facilities and transfer activities, implement a common ERP system, eliminate duplicative positions, realign various sales and marketing activities, and expand and upgrade production capacity for our regenerative technology products. These efforts are expected to continue and while we expect a positive impact from ongoing restructuring, integration, and manufacturing transfer and expansion activities, such results remain uncertain. In support of our continued focus on product margins during 2022, we closed a manufacturing facility located in France and began the transfer of production to the Company’s existing Switzerland facility. The transfer completed in the fourth quarter of 2022. In 2022, we outsourced certain transactional back-office finance and customer service activities to enhance customer quality, build scale for future growth, and capture cost efficiencies. This transition also completed in the fourth quarter of 2022. While the transition was complete in 2022, we are continuing to work with our outsource provider to transform our processes and gain efficiencies.
RESULTS OF OPERATIONS
Executive Summary
Income before taxes includes the following special charges:
| Years Ended December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
Acquisition, divestiture and integration-related charges (1) | $ | (18,849) | $ | (11,712) | |||||||
| Structural optimization charges | 23,072 | 20,762 | |||||||||
| EU medical device regulation | 45,147 | 24,375 | |||||||||
| Total | 49,370 | 33,425 | |||||||||
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| Acquisition and integration-related charges | $ | 124,665 | $ | 93,926 | $ | 117,947 | |||||
| Structural optimization charges | 17,582 | 19,598 | 7,461 | ||||||||
| Discontinued product lines charges | 9,168 | — | 1,156 | ||||||||
| EU medical device regulation | 6,221 | — | — | ||||||||
| Impairment charges | 5,764 | 4,941 | 3,290 | ||||||||
| Litigation matters | 96 | 4,598 | — | ||||||||
| Other | — | — | 5,538 | ||||||||
| Total | $ | 163,496 | $ | 123,063 | $ | 135,392 | |||||
The items reported above are reflected in the consolidated statements of operations as follows:
| Years Ended December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| Cost of goods sold | $ | 11,722 | $ | 32,334 | |||||||
| Research and development | 21,882 | 17,487 | |||||||||
| Selling, general and administrative | 20,584 | 31,013 | |||||||||
| Gain from the sale of business | (644) | (41,798) | |||||||||
| Other (income) expense | (4,174) | (5,611) | |||||||||
| Total | 49,370 | 33,425 | |||||||||
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
Cost of goods sold (1) | $ | 25,266 | $ | 34,563 | $ | 28,413 | |||||
| Research and development | 2,786 | — | — | ||||||||
| In-process research and development | 64,916 | — | — | ||||||||
| Selling, general and administrative | 67,265 | 87,709 | 107,361 | ||||||||
Intangible asset amortization (2) | 5,764 | — | — | ||||||||
| Other (income) expense | (2,501 | ) | 791 | (382 | ) | ||||||
| Total | $ | 163,496 | $ | 123,063 | $ | 135,392 | |||||
We typically define special charges as items for which the amounts and/or timing of such expenses may vary significantly from period to period, depending upon our acquisition, divestiture, integration and restructuring activities, and for which the amounts are non-cash in nature, or for which the amounts are not expected to recur at the same magnitude. We believe that given our ongoing strategy of seeking acquisitions, our continuing focus on rationalizing our existing manufacturing and distribution infrastructure and our continuing review of various product lines in relation to our current business strategy, some of the special charges discussed above could recur with similar materiality in the future.
We believe that the separate identification of these special charges provides important supplemental information to investors regarding financial and business trends relating to our financial condition and results of operations. Investors may find this information useful in assessing comparability of our operating performance from period to period, against the business model objectives that management has established, and against other companies in our industry. We provide this information to investors so that they can analyze our operating results in the same way that management does and to use this information in their assessment of our core business and valuation of Integra.
37
Revenues and Gross Margin
Our revenues and gross margin on product revenues were as follows:
| Years Ended December 31, | ||||||||||||||||||||||||||||
| Years Ended December 31, | ||||||||||||||||||||||||||||
| 2019 | 2018 | 2017 | ||||||||||||||||||||||||||
| Dollars in thousands | Dollars in thousands | 2022 | 2021 | |||||||||||||||||||||||||
| Segment Net Sales | (In thousands) | Segment Net Sales | ||||||||||||||||||||||||||
| Codman Specialty Surgical | $ | 996,206 | $ | 963,929 | $ | 720,301 | Codman Specialty Surgical | $ | 1,019,564 | $ | 1,025,232 | |||||||||||||||||
| Orthopedics and Tissue Technologies | 521,351 | 508,512 | 467,935 | |||||||||||||||||||||||||
| Tissue Technologies | Tissue Technologies | 538,102 | 517,216 | |||||||||||||||||||||||||
| Total revenues | 1,517,557 | 1,472,441 | 1,188,236 | Total revenues | 1,557,666 | 1,542,448 | ||||||||||||||||||||||
| Cost of goods sold | 564,681 | 571,496 | 435,511 | Cost of goods sold | 587,355 | 597,808 | ||||||||||||||||||||||
| Gross margin on total revenues | $ | 952,876 | $ | 900,945 | $ | 752,725 | Gross margin on total revenues | $ | 970,311 | $ | 944,640 | |||||||||||||||||
| Gross margin as a percentage of total revenues | 62.8 | % | 61.2 | % | 63.3 | % | Gross margin as a percentage of total revenues | 62.3 | % | 61.2 | % | |||||||||||||||||
Revenues
For the year ended December 31, 2019,2022, total revenues increased by $45.1$15.2 million, or 3.1%1.0%, to $1,517.6$1,557.7 million from $1,472.4$1,542.4 million during the prior year. DomesticThis increase is inclusive of an unfavorable foreign currency impact of $37.9 million, as well as a $10.2 million decrease that impacts both domestic and international revenues, related to the divestiture of the TWC business. Excluding the impacts of these items, domestic revenues increased $31.5by $41.6 million, or 3.0%,3.8%. International revenues increased by $21.7 million or 4.8%.
In the CSS segment, revenues were $1,019.6 million which was a decrease of $5.7 million, or 0.6% as compared to $1,077.4the prior-year period. This increase is inclusive of a $34.1 million unfavorable foreign currency impact on revenue. Excluding the impact of foreign currency, the CSS segment revenues increased $28.4 million as compared to the prior year period. This increase was driven primarily by low single digits growth in both our Neurosurgery and Instruments portfolios as compared to the same period in the prior year. The increase in our Neurosurgery portfolio was driven primarily by growth in advanced energy and CSF management, partially offset by the voluntary recall of the CereLink ICP monitoring system.
In the TT segment, revenues were $538.1 million, which was an increase of $20.9 million, or 4.0% as compared to the prior-year period.. Excluding the impact of foreign currency of $3.8 million and were 71.0%the divestiture impact of totalTWC of $10.2 million, the TT segment revenues in the third quarter increased by $34.9 million. This increase was driven by mid-single digit increases in our Wound Reconstruction business, led by Integra® Dermal Matrices, SurgiMendand ACell MicroMatrix and low double digit increased in our Private Label business driven by higher customer demand and favorable order timing.
Gross Margin
Gross margin was $970.3 million for the year ended December 31, 2019. International revenues increased to $440.2 million, compared to $426.5 million during 2018. The net increase of $45.1 million was a result of growth in both segments of $68.4 million offset by a $12.5 million unfavorable impact of foreign exchanges, which mainly impacts the Codman Specialty Surgical segment, and a $10.8 million unfavorable impact due to discontinued and divested products.
Gross margin as a percentage of revenues was 62.8%62.3% in 2019,2022 and 61.2% in 2018, and 63.3% in 2017.2021. The increase in gross margin percentage from 2018was due to 2019 resulted primarily fromhigher revenues and a reduction in Codman Neurosurgery acquisition and integration costs. The decrease in gross margin percentage of total revenue from 2017 to 2018 resulted primarily from dilution related to full-year product sales from the Codman Neurosurgery acquisition at lower margins than the Company's historical average. Additionally, there were higher net costs associated with the full yearinventory step up amortization of technology-based intangible assets capitalized in connection with the Codman Neurosurgery acquisition.acquisition of ACell in the prior year. These were partially offset by CereLink recall impacts, unfavorable regional mix, and higher material and labor costs.
Operating Expenses
The following is a summary of operating expenses as a percent of total revenues:
| Years Ended December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| Research and development | 6.5 | % | 6.0 | % | |||||||
| Selling, general and administrative | 39.6 | % | 41.3 | % | |||||||
| Intangible asset amortization | 0.9 | % | 1.1 | % | |||||||
| Total operating expenses | 47.0 | % | 48.4 | % | |||||||
| Years Ended December 31, | ||||||||
| 2019 | 2018 | 2017 | ||||||
| Research and development | 5.2 | % | 5.3 | % | 5.3 | % | ||
| In-process research and development | 4.3 | % | — | % | — | % | ||
| Selling, general and administrative | 45.3 | % | 46.9 | % | 52.5 | % | ||
| Intangible asset amortization | 1.8 | % | 1.4 | % | 1.7 | % | ||
38
The Company continues to manage and prioritize its operating costs to increase organic investments that will drive long-term growth including the support of new product development and introductions, clinical studies, geographic expansion and targeted U.S. sales channel expansion.
Research and Development
Research and development expenses totaled $79.6for the year ended December 31, 2022 increased by $8.1 million in 2019,as compared to $78.0 million in 2018 and $63.5 million in 2017. Thethe prior year. This increase in research and development expenses in 2018 compared to 2017 primarilyspending resulted from the full-year impact of the acquisitions of Derma Sciences and Codman Neurosurgery and additional spending on new product development, clinical studies and clinical studies.
Selling, general and administrative expenses for the year ended December 31, 2018 increased2022 decreased by $66.7$21.1 million or 10.7% to $690.7 million,as compared to $624.1 million in 2017. Selling and marketing expenses increased by $72.3 million, primarily resulting from the full-year impact of the Derma Sciences and Codman Neurosurgery acquisitions, higher headcount in our sales force compared
to the prior year higher commissiondriven primarily by the $18.1 million reduction in fair value of contingent consideration for ACell. The remainder of the decrease is a result of reduced employee related costs resulting from increasesand stock-based compensation, partially offset by increased selling costs on commissions and investment in revenue and channelsales force expansion. General and administrative costs decreased by $5.6 million, primarily resulting from one-time costs for the year ended December 31, 2017 related to acquiring and integrating the Derma Sciences and Codman Neurosurgery businesses in the year of acquisition.
Amortization expense (excluding amounts reported in cost of product revenues for technology-based intangible assets) in 20192022 was $27.0$13.9 million compared to $21.2$16.9 million in 2018. The increase is2021 primarily attributeddue to an impairment chargea reduction associated with the end of $5.8 million related tothe amortization period for a certain customer relationship intangible asset.
In 2018,addition, there was a decrease in amortization expense (excluding amounts reported in cost of product revenues for technology-based intangible assets) was $21.2 million, compared to $20.4 million in 2017. The increase primarily resulted from the full-year amortizationas a result of intangible assets capitalized as part of our Derma Sciences acquisition.sold with the TWC divestiture.
We may discontinue certain products in the future as we continue to assess the profitability of our product lines. As our profitability assessment evolves, we may make further decisions about our trade names and incur additional impairment charges or accelerated amortization. We expect total annual amortization expense (including amounts reported in cost of product revenues, but excluding any possible future amortization associated with acquired in-process research and development ("IPR&D")) to be approximately $74.6 million in 2020, $64.1 million in 2021, $60.6 million in 2022, $59.7$82.3 million in 2023, $58.9$81.7 million in 2024, $81.7 million in 2025, $81.5 million in 2026, $79.6 million in 2027 and $547.7$551.6 million thereafter.
Non-Operating Income and Expenses
The following is a summary of non-operating income and expenses:
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| Interest income | $ | 10,779 | $ | 2,800 | $ | 255 | |||||
| Interest expense | (53,957 | ) | (64,683 | ) | (35,019 | ) | |||||
| Other income, net | 9,522 | 8,288 | 1,345 | ||||||||
| Total non-operating income and expense | $ | (33,656 | ) | $ | (53,595 | ) | $ | (33,419 | ) | ||
| Years Ended December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| Interest income | $ | 11,917 | $ | 6,737 | |||||||
| Interest expense | (49,594) | (50,395) | |||||||||
| Gain from sale of business | 644 | 41,798 | |||||||||
| Other income, net | 12,007 | 19,307 | |||||||||
| Total non-operating income and expense | $ | (25,026) | $ | 17,447 | |||||||
Interest Income
Interest income for the year ended December 31, 2022 increased in 2019by $5.2 million as compared to 2018the same period last year primarily due to the full-year impact ofhigher interest rate differential on cross-currency swaps designated as net investment hedges.
Interest Expense
Interest expense was $54.0for the year ended December 31, 2022 decreased by $0.8 million $64.7 million and $35.0 million in 2019, 2018 and 2017, respectively. Interest expense decreased in 2019 as compared to 2018 primarily resulting from a decrease in our weighted average interest rate and a decrease in the outstanding balance of our Senior Credit Facility compared to the same period in 2018.
Gain from the sale of businesses
On August 31, 2022, the Company completed its previously announced sale of its TWC business to fundGentell and recognized $0.6 million as a gain from the acquisitionssale of Derma Sciences and Codman Neurosurgery in 2017.
Other Income, Net
Other income, net increased in 2019for the year ended December 31, 2022 decreased by $1.2 million as compared to 2018 primarily driven from a $3 million gain from a legal settlement.
39
Income Taxes
Our effective incomeincome tax rate was 16.5%, (5.9)%15.6% and (468.7)%21.2% of income before income taxes in 2019, 20182022 and 2017,2021, respectively. See Note 12, “Income13, Income Taxes”, in our consolidated financial statements for a reconciliation of the United States federal statutory rate to our effective tax rate.
Our effective tax rate could vary from year to year depending on, among other factors, tax law changes, the geographic and business mix and taxable earnings and losses. We consider these factors and others, including our history of generating taxable earnings, in assessing our ability to realize deferred tax assets. We estimate our worldwide effective income tax rate for 20202023 to be approximately 20.0%.20.7%, estimated based on existing tax laws.
At December 31, 2019,2022, the Company had $9.9$9.7 million of valuation allowance against the remaining $141.9$195.2 million of gross deferred tax assets recorded at December 31, 2019.2022. Our deferred tax asset valuation allowance increaseddecreased by $2.9$0.1 million in 2019 and decreased by $1.0 million2022, remaining substantially unchanged in 2018.2021. This valuation allowance relates to deferred tax assets for which the Company does not believe it has satisfied the more likely than not threshold for realization. The increase in valuation allowance in 2019 primarily resulted from certain assets from the Rebound and Arkis acquisitions. The decrease in valuation allowance in 2018 primarily resulted from the realization of certain deferred tax assets related to the acquisition of Derma Sciences and the impact of current year activity. If we determine that we would be able to realize more or less than the recorded amount of net deferred tax assets, we will record an adjustment to the deferred tax asset valuation allowance in the period such a determination is made.
At December 31, 2019,2022, we had net operating loss carryforwards of $130.1$79.5 million for federal income tax purposes, $37.5$75.5 million for foreign income tax purposes and $42.8$37.9 million for state income tax purposes to offset future taxable income.Theincome. The federal net operating loss carryforwards increased during 2019 from2022 due to the acquisition of Arkis and Rebound, offset by usage of federal net operating losses during 2019.SIA. Of the total federal net operating loss carryforwards, $111.2$60.9 million expire through 2037 and $18.9$18.6 million have an indefinite carryforward period. Regarding the foreign net operating loss carryforwards, $1.3 million expire through 2024, $0.9 million expire through 2025, and the remaining $35.3$16.4 million have an indefinite carryforwardcarryforward period. The state net operating loss carryforwards expire throughin 2036.
GEOGRAPHIC PRODUCT REVENUES AND OPERATIONS
| Years Ended December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| United States | $ | 1,126,810 | $ | 1,089,526 | |||||||
| Europe | 170,903 | 191,327 | |||||||||
| Asia Pacific | 176,477 | 182,034 | |||||||||
| Rest of World | 83,476 | 79,561 | |||||||||
| Total Revenues | $ | 1,557,666 | $ | 1,542,448 | |||||||
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| United States | $ | 1,077,379 | $ | 1,045,887 | $ | 894,260 | |||||
| Europe | 197,468 | 201,354 | 150,147 | ||||||||
| Asia Pacific | 157,391 | 144,253 | 80,636 | ||||||||
| Rest of World | 85,319 | 80,947 | 63,193 | ||||||||
| Total Revenues | $ | 1,517,557 | $ | 1,472,441 | $ | 1,188,236 | |||||
Domestic revenues increased to $1,077.4 million, or 71.0% of total revenues, for the year ended December 31, 2019, from $1,045.9 million, or 71.0% of total revenues. Growth in domestic revenues was driven by our dural repair, programmable valves, and our core tissue products. European sales decreased by $3.9$37.3 million for the year ended December 31, 20192022 compared to the same period last year. These increases are inclusive of $4.4 million related to the divestiture of the TWC business. European sales decreased by $20.4 million for the year resulting primarily from unfavorable impacts of foreign exchange partially offset by an increase in sales of our programmable valve and core tissue products.ended December 31, 2022 compared to the same period last year. Sales to customers in Asia Pacific anddecreased by $5.6 million for the year ended December 31, 2022 compared to the same period last year. The Rest of the World for the year ended December 31, 20192022 increased by $17.5$3.9 million primarily driven by increases in our dural repair products and CUSA® capital and related disposables partially offset by unfavorable impacts of foreign exchange.
LIQUIDITY AND CAPITAL RESOURCES
At December 31, 2019, our non-U.S. subsidiaries held approximately $143.7 million of cash and cash equivalents that are available for use by all of our operations around the world. The Company asserts that it has the ability and intent to indefinitely reinvest the undistributed earnings from its foreign operations unless there is a tax-free manner under which to remit the earnings.
| Year Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| Net cash provided by operating activities | $ | 231,433 | $ | 199,683 | $ | 114,544 | |||||
| Net cash used in investing activities | (162,668 | ) | (49,705 | ) | (1,221,335 | ) | |||||
| Net cash used (provided) by financing activities | (8,766 | ) | (180,872 | ) | 1,168,947 | ||||||
| Effect of exchange rate fluctuations on cash | 74 | (5,203 | ) | 10,724 | |||||||
| Net increase (decrease) in cash and cash equivalents | $ | 60,073 | $ | (36,097 | ) | $ | 72,880 | ||||
Cash and Marketable Securities
The Company had cash and cash equivalents totaling approximately $456.7 million and $513.4 million at December 31, 2022 and 2021, respectively, which are valued based on Level 1 measurements in the fair value hierarchy. At December 31, 2022, our non-U.S. subsidiaries held approximately $229.8 million of cash and cash equivalents that are available for use outside the U.S. The Company asserts that it has the ability and intends to indefinitely reinvest the undistributed earnings from its foreign operations unless there is no material tax cost to remit the earnings into the U.S.
| Year Ended December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| Net cash provided by operating activities | $ | 264,469 | $ | 312,427 | |||||||
| Net cash used in investing activities | (58,580) | (161,443) | |||||||||
| Net cash used (provided) by financing activities | (251,953) | (98,226) | |||||||||
| Effect of exchange rate fluctuations on cash | (10,723) | (9,476) | |||||||||
| Net increase (decrease) in cash and cash equivalents | $ | (56,787) | $ | 43,282 | |||||||
Cash Flows Provided by Operating Activities
Operating cash flows for the year ended December 31, 2022 decreased by $48.0 million compared to the same period in 2021. Net income after removing the impact of the gain on sale of businesses and Restatednon-cash adjustments increased for the year ended December 31, 2022, by approximately $9.6 million as compared to 2021 primarily due to earnings from higher revenues. The changes in assets and liabilities, net of business acquisitions, decreased cash flows by $39.4 million in 2022 as compared to the increase in cash flows of $18.2 million for the same period in 2021. The change in 2022 is mainly attributable to increases in inventory and accounts receivable. The increase in inventory is due to a build up of safety stock due to supply chain challenges. The increase in accounts receivable is due to increased sales as well as a decrease in days sales outstanding.
Operating cash flows for the year ended December 31, 2021 increased by $108.6 million compared to the same period in 2020. Net income after removing the impact of the gain on sale of business and non-cash adjustments increased for the year ended December 31, 2021, by approximately $49.1 million as compared to the same period in 2020 primarily due to the continuing revenue recovery in the current year as compared to the height of the COVID-19 pandemic in the prior year. The changes in assets and liabilities, net of business acquisitions, increased cash flows from operating activities in the current year by $18.2 million compared to the decrease of $41.3 million for the same period in 2020. The improvement in 2021 working capital is attributable to a decrease in inventory of $5.4 million due to investments in building safety stock made in the prior year where inventory increased by $48.3 million as well as improved sales in 2021.
Cash Flows Used in Investing Activities
During the year ended December 31, 2022, we paid $42.3 millionfor capital expenditures to support operations improvement initiatives at a number of our manufacturing facilities and other information technology investments, $51.5 million to acquire SIA, as well as the $4.7 million payment related to the final developmental milestone for Rebound Therapeutics Corporation. This was partially offset by the net proceeds from the sale of the TWC business of $24.0 million. The proceeds from the sale of the TWC business of $27.8 million is presented net of cash transferred of $3.5 million and other transaction fees. Additionally, the Company also received $4.9 million proceeds on cross-currency swaps designated as net investment hedge.
During the year ended December 31, 2021, we paid a net cash amount of $303.9 million in relation to the acquisition of ACell and received net proceeds of $190.5 million for the sale of the Extremity Orthopedics business. The Company also paid for $48.0 million capital expenditures to support operations improvement initiatives at a number of our manufacturing facilities and other information technology investments.
41
Cash Flows (Used in) Provided by Financing Activities
Uses of cash from financing activities for the year ended December 31, 2022 primarily related to the purchase of treasury stock of $125.0 million under the 2022 accelerated share repurchase agreement that was completed in the first quarter of 2022. In addition, the Company had $24.6 million in cash taxes paid in net equity settlements as a result of the departure of the former chief executive officer of the Company. The Company also had repayments of $148.6 million under our Senior Credit Agreement
Uses of cash from financing activities for the year ended December 31, 2021 were repayments of $125.5 million on the revolving portion of our Senior Credit Facility and Securitization Facility. In addition, the Company had $4.8 million in cash taxes paid in net equity settlements. These uses were offset by $6.8 million proceeds from the exercise of stock options and $25.5 million borrowings under our Senior Credit Facility and Securitization Facility.
Amended and Restated Senior Credit Agreement, Convertible Senior Notes, Securitization and Related Hedging Activities
See Note 5, Debt,to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for further informationa discussion of our Amended and Restated Senior Credit Agreement, the 2025 Notes and Securitization Facility and Note 6, Derivative Instruments to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for discussion of our hedging activities. We are forecasting that sales and earnings for the next twelve months will be sufficient to remain in compliance with our financial covenants under the terms of the February 2020 Amendment and July 2020 Amendment to the Senior Credit Facility.
Share Repurchase Plan
On December 11, 2018,January 12, 2022, the Company entered into a $125.0 million accelerated share repurchase ("2022 ASR") and received 1.48 million shares of the Company common stock at inception of the 2022 ASR, which represented approximately 80% of the expected total shares under the 2022 ASR. On March 24, 2022, the early exercise provision was exercised by 2022 ASR counterparty. Upon settlement on March 24, 2022, the Company received an additional 0.46 million shares determined using the volume-weighted average price of the Company's common stock during the term of the 2022 ASR.
On April 26, 2022, the Board of Directors authorized the Company to repurchase up to $225$225.0 million of the Company’s common stock. The program allows the Company to repurchase its shares opportunistically from time to time. The repurchase authorization expires in December 2020.2024. This stock repurchase authorization replaces the previous $225 million stock repurchase authorization, of which $100 million remained authorized at the time of its replacement, and which was otherwise set to expire on December 31, 2022. Purchases may be affected through one or more open market transactions, privately negotiated transactions, transactions structured through investment banking institutions, or a combination of the foregoing. This stock repurchase authorization replaces
On January 26, 2023, the previousCompany entered into a $150 million stockaccelerated share repurchase authorization which was approved by the Board in 2016.
For the year ended December 31, 2019.
See Note 8, Treasury Stock, to approximately $7.6 million from certain purchasersthe Notes to Consolidated Financial Statements (Part IV, Item 15 of the convertible notes in conjunction with the closing of the offering. Additionally, the Company intends to use $92.4 million of the proceeds will be used to repurchase shares through an accelerated share repurchase transaction ("ASR"). Total shares repurchased through February 21, 2020 were 1,438,615.this Annual Report on Form 10-K) for further details.
Dividend Policy
We have not paid any cash dividends on our common stock since our formation. Our Senior Credit Facility limits the amount of dividends that we may pay. Any future determinations to pay cash dividends on our common stock will be at the discretion of ourthe Board of Directors and will depend upon our financial condition, results of operations, cash flows and other factors deemed relevant by the Board of Directors.Board.
We believe that our cash and Commitments
| Payments Due by Calendar Year | |||||||||||||||||||
| Total | 2020 | 2021-2022 | 2023-2024 | Thereafter | |||||||||||||||
| (In millions) | |||||||||||||||||||
| Senior Credit Facility - Revolver (1) | $ | 375.0 | $ | — | $ | — | $ | 375.0 | $ | — | |||||||||
| Senior Credit Facility - Term Loan | 877.5 | 45.0 | 123.8 | 708.8 | — | ||||||||||||||
| Interest on Term loan (2) | $ | 83.2 | 27.0 | 48.7 | 7.5 | ||||||||||||||
| Securitization Facility (1) | 104.5 | — | 104.5 | — | — | ||||||||||||||
| Operating Leases (3) | 155.4 | 12.4 | 27.3 | 23.0 | 92.7 | ||||||||||||||
| Purchase Obligations | 10.7 | 10.7 | — | — | — | ||||||||||||||
| Others | 5.7 | 2.1 | 0.7 | 1.7 | 1.1 | ||||||||||||||
| Total | $ | 1,612.0 | $ | 97.2 | $ | 305.0 | $ | 1,116.0 | $ | 93.8 | |||||||||
42
Off-Balance Sheet Arrangements
We will continue to have cash requirements to support seasonal working capital needs and capital expenditures, to pay interest, to service debt, and to fund acquisitions. As part of our ongoing operations, we enter into contractual arrangements that obligate us to make future cash payments.
Our primary obligations include principal and interest payments on revolving portion and Term Loan component of the Senior Credit Facility, Securitization Facility and Convertible Securities. See Note 5, Debt,to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details. The Company also leases some of our manufacturing facilities and office buildings which have future minimum lease payments associated. See Note 11, Leases and Related Party Leases, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for a schedule of our future minimum lease payments. Amounts related to the Company's other obligations, including employment agreements and purchase obligations were not material.
The Company has contingent consideration obligations related to prior and current year acquisitions and future pension contribution obligations. See Note 10, Retirement Benefit Plans,and Note 15, Commitments and Contingenciesto the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details. The associated obligations are not fixed. The Company also has a liability for uncertain tax benefits including interest and penalties. See Note 12, Income Taxesto the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details. The Company cannot make a reliable estimate of the period in which the uncertain tax benefits may be realized.
Employee Termination Benefits
The Company incurred employee termination costs on restructuring activities associated with a closure of a manufacturing facility located in France, outsourcing plans for select transactional back office activities, and executive reorganization in the consolidated statement of operations for the year ended December 31, 2022. In 2021, the Company incurred employee termination costs on restructuring activities associated with the closure of the manufacturing facility in France. Restructuring costs were included in accrued expenses and other current liabilities in the consolidated balance sheet for the year ended December 31, 2022 and 2021. See Note 2, Summary of Significant Accounting Policies,of the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for further details.
Our discussioneffective income tax rate was 15.6% and analysis21.2% of financial conditionincome before income taxes in 2022 and results of operations is based upon2021, respectively. See Note 13, Income Taxes, in our consolidated financial statements which have been prepared in accordance with accounting principles generally accepted infor a reconciliation of the United States federal statutory rate to our effective tax rate. Our effective tax rate could vary from year to year depending on, among other factors, tax law changes, the geographic and business mix and taxable earnings and losses. We consider these factors and others, including our history of America.generating taxable earnings, in assessing our ability to realize deferred tax assets.
Our effective tax rate could vary from year to year depending on, among other factors, tax law changes, the geographic and business mix and taxable earnings and losses. We consider these factors and others, including our history of generating taxable earnings, in assessing our ability to realize deferred tax assets. We estimate our worldwide effective income tax rate for 2023 to be approximately 20.7%, estimated based on existing tax laws.
At December 31, 2022, the Company had $9.7 million of valuation allowance against the remaining $195.2 million of gross deferred tax assets recorded at December 31, 2022. Our deferred tax asset valuation allowance decreased by $0.1 million in 2022, remaining substantially unchanged in 2021. This valuation allowance relates to deferred tax assets for which the Company does not believe it has satisfied the more likely than not threshold for realization.
At December 31, 2022, we had net operating loss carryforwards of $79.5 million for federal income tax purposes, $75.5 million for foreign income tax purposes and $37.9 million for state income tax purposes to offset future taxable income. The preparationfederal net operating loss carryforwards increased during 2022 due to the acquisition of these financial statements requires usSIA. Of the total federal net operating loss carryforwards, $60.9 million expire through 2037 and $18.6 million have an indefinite carryforward period. Regarding the foreign net operating loss carryforwards, $16.4 million have an indefinite carryforward period. The state net operating loss carryforwards expire in 2036.
As of December 31, 2022, the Company has not provided deferred income taxes on unrepatriated earnings from foreign subsidiaries as they are deemed to make estimatesbe indefinitely reinvested unless there is a manner under which to remit the earnings with no material tax cost. Such taxes would primarily be attributable to foreign withholding taxes and assumptionslocal income taxes when such earnings are distributed.
GEOGRAPHIC PRODUCT REVENUES AND OPERATIONS
The Company attributes revenues to geographic areas based on the location of the customer. Total revenue by major geographic area consisted of the following:
| Years Ended December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| United States | $ | 1,126,810 | $ | 1,089,526 | |||||||
| Europe | 170,903 | 191,327 | |||||||||
| Asia Pacific | 176,477 | 182,034 | |||||||||
| Rest of World | 83,476 | 79,561 | |||||||||
| Total Revenues | $ | 1,557,666 | $ | 1,542,448 | |||||||
The Company generates significant revenues outside the U.S., a portion of which are U.S. dollar-denominated transactions conducted with customers that generate revenue in currencies other than the U.S. dollar. As a result, currency fluctuations between the U.S. dollar and the currencies in which those customers do business could have an impact on the demand for the Company's products in foreign countries. Local economic conditions, regulatory compliance or political considerations, the effectiveness of our sales representatives and distributors, local competition and changes in local medical practice all may combine to affect our sales into markets outside the reported amountsU.S.
Domestic revenues increased by $37.3 million for the year ended December 31, 2022 compared to the same period last year. These increases are inclusive of $4.4 million related to the divestiture of the TWC business. European sales decreased by $20.4 million for the year ended December 31, 2022 compared to the same period last year. Sales to customers in Asia Pacific decreased by $5.6 million for the year ended December 31, 2022 compared to the same period last year. The Rest of the World for the year ended December 31, 2022 increased by $3.9 million compared to the same period last year. The international revenues were impacted by a $37.9 million unfavorable foreign exchange impact as well as $5.8 million related to the divestiture of the TWC business.
40
LIQUIDITY AND CAPITAL RESOURCES
Working Capital
At December 31, 2022 and December 31, 2021, working capital was $840.6 million and $813.7 million, respectively. Working capital consists of total current assets less total current liabilities as presented in the consolidated balance sheets.
Cash and Marketable Securities
The Company had cash and cash equivalents totaling approximately $456.7 million and $513.4 million at December 31, 2022 and 2021, respectively, which are valued based on Level 1 measurements in the fair value hierarchy. At December 31, 2022, our non-U.S. subsidiaries held approximately $229.8 million of cash and cash equivalents that are available for use outside the U.S. The Company asserts that it has the ability and intends to indefinitely reinvest the undistributed earnings from its foreign operations unless there is no material tax cost to remit the earnings into the U.S.
Cash Flows
| Year Ended December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| Net cash provided by operating activities | $ | 264,469 | $ | 312,427 | |||||||
| Net cash used in investing activities | (58,580) | (161,443) | |||||||||
| Net cash used (provided) by financing activities | (251,953) | (98,226) | |||||||||
| Effect of exchange rate fluctuations on cash | (10,723) | (9,476) | |||||||||
| Net increase (decrease) in cash and cash equivalents | $ | (56,787) | $ | 43,282 | |||||||
Cash Flows Provided by Operating Activities
Operating cash flows for the year ended December 31, 2022 decreased by $48.0 million compared to the same period in 2021. Net income after removing the impact of the gain on sale of businesses and non-cash adjustments increased for the year ended December 31, 2022, by approximately $9.6 million as compared to 2021 primarily due to earnings from higher revenues. The changes in assets and liabilities, net of business acquisitions, decreased cash flows by $39.4 million in 2022 as compared to the disclosureincrease in cash flows of contingent liabilities,$18.2 million for the same period in 2021. The change in 2022 is mainly attributable to increases in inventory and accounts receivable. The increase in inventory is due to a build up of safety stock due to supply chain challenges. The increase in accounts receivable is due to increased sales as well as a decrease in days sales outstanding.
Operating cash flows for the reported amountsyear ended December 31, 2021 increased by $108.6 million compared to the same period in 2020. Net income after removing the impact of revenuesthe gain on sale of business and expenses. Significant estimates affecting amounts reported or disclosednon-cash adjustments increased for the year ended December 31, 2021, by approximately $49.1 million as compared to the same period in 2020 primarily due to the continuing revenue recovery in the consolidatedcurrent year as compared to the height of the COVID-19 pandemic in the prior year. The changes in assets and liabilities, net of business acquisitions, increased cash flows from operating activities in the current year by $18.2 million compared to the decrease of $41.3 million for the same period in 2020. The improvement in 2021 working capital is attributable to a decrease in inventory of $5.4 million due to investments in building safety stock made in the prior year where inventory increased by $48.3 million as well as improved sales in 2021.
Cash Flows Used in Investing Activities
During the year ended December 31, 2022, we paid $42.3 millionfor capital expenditures to support operations improvement initiatives at a number of our manufacturing facilities and other information technology investments, $51.5 million to acquire SIA, as well as the $4.7 million payment related to the final developmental milestone for Rebound Therapeutics Corporation. This was partially offset by the net proceeds from the sale of the TWC business of $24.0 million. The proceeds from the sale of the TWC business of $27.8 million is presented net of cash transferred of $3.5 million and other transaction fees. Additionally, the Company also received $4.9 million proceeds on cross-currency swaps designated as net investment hedge.
During the year ended December 31, 2021, we paid a net cash amount of $303.9 million in relation to the acquisition of ACell and received net proceeds of $190.5 million for the sale of the Extremity Orthopedics business. The Company also paid for $48.0 million capital expenditures to support operations improvement initiatives at a number of our manufacturing facilities and other information technology investments.
41
Cash Flows (Used in) Provided by Financing Activities
Uses of cash from financing activities for the year ended December 31, 2022 primarily related to the purchase of treasury stock of $125.0 million under the 2022 accelerated share repurchase agreement that was completed in the first quarter of 2022. In addition, the Company had $24.6 million in cash taxes paid in net equity settlements as a result of the departure of the former chief executive officer of the Company. The Company also had repayments of $148.6 million under our Senior Credit Facility and Securitization Facility offset by $40.8 million borrowings under our Senior Credit Facility and Securitization Facility. The Company also had $5.5 million proceeds from the exercise of stock options. In this Annual Report on Form 10-K, we refer to the sixth amendment and restatement of our Senior Credit Facility with a syndicate of lending banks with Bank of America, N.A., as Administrative Agent as the "Senior Credit Facility."
Uses of cash from financing activities for the year ended December 31, 2021 were repayments of $125.5 million on the revolving portion of our Senior Credit Facility and Securitization Facility. In addition, the Company had $4.8 million in cash taxes paid in net equity settlements. These uses were offset by $6.8 million proceeds from the exercise of stock options and $25.5 million borrowings under our Senior Credit Facility and Securitization Facility.
Amended and Restated Senior Credit Agreement, Convertible Senior Notes, Securitization and Related Hedging Activities
See Note 5, Debt,to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for a discussion of our Amended and Restated Senior Credit Agreement, the 2025 Notes and Securitization Facility and Note 6, Derivative Instruments to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for discussion of our hedging activities. We are forecasting that sales and earnings for the next twelve months will be sufficient to remain in compliance with our financial statements include allowancescovenants under the terms of the February 2020 Amendment and July 2020 Amendment to the Senior Credit Facility.
Share Repurchase Plan
On January 12, 2022, the Company entered into a $125.0 million accelerated share repurchase ("2022 ASR") and received 1.48 million shares of the Company common stock at inception of the 2022 ASR, which represented approximately 80% of the expected total shares under the 2022 ASR. On March 24, 2022, the early exercise provision was exercised by 2022 ASR counterparty. Upon settlement on March 24, 2022, the Company received an additional 0.46 million shares determined using the volume-weighted average price of the Company's common stock during the term of the 2022 ASR.
On April 26, 2022, the Board of Directors authorized the Company to repurchase up to $225.0 million of the Company’s common stock. The program allows the Company to repurchase its shares opportunistically from time to time. The repurchase authorization expires in December 2024. This stock repurchase authorization replaces the previous $225 million stock repurchase authorization, of which $100 million remained authorized at the time of its replacement, and which was otherwise set to expire on December 31, 2022. Purchases may be affected through one or more open market transactions, privately negotiated transactions, transactions structured through investment banking institutions, or a combination of the foregoing.
On January 26, 2023, the Company entered into a $150 million accelerated share repurchase agreement ("2023 ASR") and received 2.1 million shares of the Company common stock at inception of the 2023 ASR, which represented approximately 80% of the expected total shares of under the 2023 SAR. The remaining repurchase transactions are expected to be completed in the first half of 2023.
For the year ended December 31, 2021, there were no repurchases of the Company’s common stock as part of the shar e repurchase authorization.
See Note 8, Treasury Stock, tothe Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for doubtful accounts receivablefurther details.
Dividend Policy
We have not paid any cash dividends on our common stock since our formation. Our Senior Credit Facility limits the amount of dividends that we may pay. Any future determinations to pay cash dividends on our common stock will be at the discretion of the Board and sales returns and allowances, net realizable valuewill depend upon our financial condition, results of inventories, valuation of intangible assets including in-process research and development, amortization periods for acquired intangible assets, estimates of projectedoperations, cash flows and discount rates used to value intangible assets and test goodwill and intangible assets for impairment, estimates of projected cash flows and depreciation and amortization periods for long-lived assets, computation of taxes, computation of valuation allowances recorded against deferred tax assets, valuation of stock-based compensation, valuation of pension assets and liabilities, valuation of derivative instruments, valuation ofother factors deemed relevant by the equity component of convertible debt instruments, valuation of debt instruments and loss contingencies. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the current circumstances. Actual results could differ from these estimates.Board.
Capital Resources
We believe that our cash and available borrowings under the following accounting policies, which formSenior Credit Facility are sufficient to finance our operations and capital expenditures over the basis for developing these estimates, are those that are most critical tonext twelve months. Our future capital requirements will depend on many factors, including the presentationgrowth of our consolidated financial statementsbusiness, the timing and require the more difficult subjectiveintroduction of new products and complex judgments:
42
We do not have excess quantitiesany off–balance sheet financing arrangements during the year-ended December 31, 2022 that have or are reasonably likely to have, a current or future effect on our financial condition, changes in inventory. To the extent that we determine there are excessfinancial condition, revenues or obsolete quantities or quantities with a shelf life that is too near its expiration for us to reasonably expect that we can sell those products prior to their expiration, we adjust their carrying value to estimated net realizable value. If future demand or market conditions are lower than our projections, or if we are unable to rework excess or obsolete quantities into other products, we may record further adjustments to the carrying value of inventory through a charge to cost of product revenues in the period the revision is made.
Contractual Obligations and Commitments
We will continue to have cash requirements to support seasonal working capital needs and capital expenditures, to pay interest, to service debt, and to fund acquisitions. As part of our ongoing operations, we enter into contractual arrangements that obligate us to make future cash payments.
Our primary obligations include principal and interest payments on revolving portion and Term Loan component of the respective acquisition dates. Net assets acquiredSenior Credit Facility, Securitization Facility and Convertible Securities. See Note 5, Debt,to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details. The Company also leases some of our manufacturing facilities and office buildings which have future minimum lease payments associated. See Note 11, Leases and Related Party Leases, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for a schedule of our future minimum lease payments. Amounts related to the Company's other obligations, including employment agreements and purchase obligations were not material.
The Company has contingent consideration obligations related to prior and current year acquisitions and future pension contribution obligations. See Note 10, Retirement Benefit Plans,and Note 15, Commitments and Contingenciesto the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details. The associated obligations are recorded at fair value atnot fixed. The Company also has a liability for uncertain tax benefits including interest and penalties. See Note 12, Income Taxesto the dateNotes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details. The Company cannot make a reliable estimate of the acquisition. Any purchase priceperiod in excess of these net assets is recorded as goodwill. The fair values of net assets acquiredwhich the uncertain tax benefits may be subject to revision basedrealized.
Employee Termination Benefits
The Company incurred employee termination costs on restructuring activities associated with a closure of a manufacturing facility located in France, outsourcing plans for select transactional back office activities, and executive reorganization in the final determinationconsolidated statement of fair values duringoperations for the measurement period, which may be up to one year fromended December 31, 2022. In 2021, the acquisition date.
Significant Accounting PoliciesThe excess ,of the cost over the fair valueNotes to Consolidated Financial Statements (Part IV, Item 15 of net assets of acquired businesses is recorded as goodwill. Goodwill is not subject to amortization, but is reviewedthis Annual Report on Form 10-K) for impairment at the reporting unit level annually, or more frequently if impairment indicators arise. Our assessment of the recoverability of goodwill is based upon a comparison of the carrying value of goodwill with its estimated fair value. We review goodwill for impairment annually as of July 31 and whenever events or changes in circumstances indicate the carrying value of goodwill may not be recoverable. Refer to Note 7 - Goodwill and Other Intangible Assets for more information on reportable segments.further details.
Income Taxes
Our effective income tax rate was 15.6% and 21.2% of income before income taxes in 2022 and 2021, respectively. See Note 13, Income Taxes, in our consolidated financial statements for a reconciliation of the United States federal statutory rate to our effective tax rate. Our effective tax rate could vary from year to year depending on, among other factors, tax law changes, the geographic and business mix and taxable earnings and losses. We consider these factors and others, including our history of generating taxable earnings, in assessing our ability to realize deferred tax assets.
Our effective tax rate could vary from year to year depending on, among other factors, tax law changes, the geographic and business mix and taxable earnings and losses. We consider these factors and others, including our history of generating taxable earnings, in assessing our ability to realize deferred tax assets. We estimate our worldwide effective income tax rate for 2023 to be approximately 20.7%, estimated based on existing tax laws.
At December 31, 2022, the Company had $9.7 million of valuation allowance against the remaining $195.2 million of gross deferred tax assets recorded at December 31, 2022. Our deferred tax asset valuation allowance decreased by $0.1 million in 2022, remaining substantially unchanged in 2021. This valuation allowance relates to deferred tax assets for which the Company does not believe it has satisfied the more likely than not threshold for realization.
At December 31, 2022, we had net operating loss carryforwards of $79.5 million for federal income tax purposes, $75.5 million for foreign income tax purposes and $37.9 million for state income tax purposes to offset future taxable income. The federal net operating loss carryforwards increased during 2022 due to the acquisition of SIA. Of the total federal net operating loss carryforwards, $60.9 million expire through 2037 and $18.6 million have an indefinite carryforward period. Regarding the foreign net operating loss carryforwards, $16.4 million have an indefinite carryforward period. The state net operating loss carryforwards expire in 2036.
As of December 31, 2022, the Company has not provided deferred income taxes on unrepatriated earnings from foreign subsidiaries as they are deemed to be indefinitely reinvested unless there is a manner under which to remit the earnings with no material tax cost. Such taxes would primarily be attributable to foreign withholding taxes and local income taxes when such earnings are distributed.
GEOGRAPHIC PRODUCT REVENUES AND OPERATIONS
The Company attributes revenues to geographic areas based on the location of the customer. Total revenue by major geographic area consisted of the following:
| Years Ended December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| United States | $ | 1,126,810 | $ | 1,089,526 | |||||||
| Europe | 170,903 | 191,327 | |||||||||
| Asia Pacific | 176,477 | 182,034 | |||||||||
| Rest of World | 83,476 | 79,561 | |||||||||
| Total Revenues | $ | 1,557,666 | $ | 1,542,448 | |||||||
The Company generates significant revenues outside the U.S., a portion of which are U.S. dollar-denominated transactions conducted with customers that generate revenue in currencies other than the U.S. dollar. As a result, currency fluctuations between the U.S. dollar and the currencies in which those customers do business could have an impact on the demand for the Company's products in foreign countries. Local economic conditions, regulatory compliance or political considerations, the effectiveness of our sales representatives and distributors, local competition and changes in local medical practice all may combine to affect our sales into markets outside the U.S.
Domestic revenues increased by $37.3 million for the year ended December 31, 2022 compared to the same period last year. These increases are inclusive of $4.4 million related to the divestiture of the TWC business. European sales decreased by $20.4 million for the year ended December 31, 2022 compared to the same period last year. Sales to customers in Asia Pacific decreased by $5.6 million for the year ended December 31, 2022 compared to the same period last year. The Rest of the World for the year ended December 31, 2022 increased by $3.9 million compared to the same period last year. The international revenues were impacted by a $37.9 million unfavorable foreign exchange impact as well as $5.8 million related to the divestiture of the TWC business.
40
LIQUIDITY AND CAPITAL RESOURCES
Working Capital
At December 31, 2022 and December 31, 2021, working capital was $840.6 million and $813.7 million, respectively. Working capital consists of total current assets less total current liabilities as presented in the consolidated balance sheets.
Cash and Marketable Securities
The Company had cash and cash equivalents totaling approximately $456.7 million and $513.4 million at December 31, 2022 and 2021, respectively, which are valued based on Level 1 measurements in the fair value hierarchy. At December 31, 2022, our non-U.S. subsidiaries held approximately $229.8 million of cash and cash equivalents that are available for use outside the U.S. The Company asserts that it has the ability and intends to indefinitely reinvest the undistributed earnings from its foreign operations unless there is no material tax cost to remit the earnings into the U.S.
Cash Flows
| Year Ended December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| Net cash provided by operating activities | $ | 264,469 | $ | 312,427 | |||||||
| Net cash used in investing activities | (58,580) | (161,443) | |||||||||
| Net cash used (provided) by financing activities | (251,953) | (98,226) | |||||||||
| Effect of exchange rate fluctuations on cash | (10,723) | (9,476) | |||||||||
| Net increase (decrease) in cash and cash equivalents | $ | (56,787) | $ | 43,282 | |||||||
Cash Flows Provided by Operating Activities
Operating cash flows for the year ended December 31, 2022 decreased by $48.0 million compared to the same period in 2021. Net income after removing the impact of the gain on sale of businesses and non-cash adjustments increased for the year ended December 31, 2022, by approximately $9.6 million as compared to 2021 primarily due to earnings from higher revenues. The changes in assets and liabilities, net of business acquisitions, decreased cash flows by $39.4 million in 2022 as compared to the increase in cash flows of $18.2 million for the same period in 2021. The change in 2022 is mainly attributable to increases in inventory and accounts receivable. The increase in inventory is due to a build up of safety stock due to supply chain challenges. The increase in accounts receivable is due to increased sales as well as a decrease in days sales outstanding.
Operating cash flows for the year ended December 31, 2021 increased by $108.6 million compared to the same period in 2020. Net income after removing the impact of the gain on sale of business and non-cash adjustments increased for the year ended December 31, 2021, by approximately $49.1 million as compared to the same period in 2020 primarily due to the continuing revenue recovery in the current year as compared to the height of the COVID-19 pandemic in the prior year. The changes in assets and liabilities, net of business acquisitions, increased cash flows from operating activities in the current year by $18.2 million compared to the decrease of $41.3 million for the same period in 2020. The improvement in 2021 working capital is attributable to a decrease in inventory of $5.4 million due to investments in building safety stock made in the prior year where inventory increased by $48.3 million as well as improved sales in 2021.
Cash Flows Used in Investing Activities
During the year ended December 31, 2022, we paid $42.3 millionfor capital expenditures to support operations improvement initiatives at a number of our manufacturing facilities and other information technology investments, $51.5 million to acquire SIA, as well as the $4.7 million payment related to the final developmental milestone for Rebound Therapeutics Corporation. This was partially offset by the net proceeds from the sale of the TWC business of $24.0 million. The proceeds from the sale of the TWC business of $27.8 million is presented net of cash transferred of $3.5 million and other transaction fees. Additionally, the Company also received $4.9 million proceeds on cross-currency swaps designated as net investment hedge.
During the year ended December 31, 2021, we paid a net cash amount of $303.9 million in relation to the acquisition of ACell and received net proceeds of $190.5 million for the sale of the Extremity Orthopedics business. The Company also paid for $48.0 million capital expenditures to support operations improvement initiatives at a number of our manufacturing facilities and other information technology investments.
41
Cash Flows (Used in) Provided by Financing Activities
Uses of cash from financing activities for the year ended December 31, 2022 primarily related to the purchase of treasury stock of $125.0 million under the 2022 accelerated share repurchase agreement that was completed in the first quarter of 2022. In addition, the Company had $24.6 million in cash taxes paid in net equity settlements as a result of the departure of the former chief executive officer of the Company. The Company also had repayments of $148.6 million under our Senior Credit Facility and Securitization Facility offset by $40.8 million borrowings under our Senior Credit Facility and Securitization Facility. The Company also had $5.5 million proceeds from the exercise of stock options. In this Annual Report on Form 10-K, we refer to the sixth amendment and restatement of our Senior Credit Facility with a syndicate of lending banks with Bank of America, N.A., as Administrative Agent as the "Senior Credit Facility."
Uses of cash from financing activities for the year ended December 31, 2021 were repayments of $125.5 million on the revolving portion of our Senior Credit Facility and Securitization Facility. In addition, the Company had $4.8 million in cash taxes paid in net equity settlements. These uses were offset by $6.8 million proceeds from the exercise of stock options and $25.5 million borrowings under our Senior Credit Facility and Securitization Facility.
Amended and Restated Senior Credit Agreement, Convertible Senior Notes, Securitization and Related Hedging Activities
See Note 5, Debt,to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for a discussion of our Amended and Restated Senior Credit Agreement, the 2025 Notes and Securitization Facility and Note 6, Derivative Instruments to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for discussion of our hedging activities. We are forecasting that sales and earnings for the next twelve months will be sufficient to remain in compliance with our financial covenants under the terms of the February 2020 Amendment and July 2020 Amendment to the Senior Credit Facility.
Share Repurchase Plan
On January 12, 2022, the Company entered into a $125.0 million accelerated share repurchase ("2022 ASR") and received 1.48 million shares of the Company common stock at inception of the 2022 ASR, which represented approximately 80% of the expected total shares under the 2022 ASR. On March 24, 2022, the early exercise provision was exercised by 2022 ASR counterparty. Upon settlement on March 24, 2022, the Company received an additional 0.46 million shares determined using the volume-weighted average price of the Company's common stock during the term of the 2022 ASR.
On April 26, 2022, the Board of Directors authorized the Company to repurchase up to $225.0 million of the Company’s common stock. The program allows the Company to repurchase its shares opportunistically from time to time. The repurchase authorization expires in December 2024. This stock repurchase authorization replaces the previous $225 million stock repurchase authorization, of which $100 million remained authorized at the time of its replacement, and which was otherwise set to expire on December 31, 2022. Purchases may be affected through one or more open market transactions, privately negotiated transactions, transactions structured through investment banking institutions, or a combination of the foregoing.
On January 26, 2023, the Company entered into a $150 million accelerated share repurchase agreement ("2023 ASR") and received 2.1 million shares of the Company common stock at inception of the 2023 ASR, which represented approximately 80% of the expected total shares of under the 2023 SAR. The remaining repurchase transactions are expected to be completed in the first half of 2023.
For the year ended December 31, 2021, there were no repurchases of the Company’s common stock as part of the shar e repurchase authorization.
See Note 8, Treasury Stock, tothe Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for further details.
Dividend Policy
We have not paid any cash dividends on our common stock since our formation. Our Senior Credit Facility limits the amount of dividends that we may pay. Any future determinations to pay cash dividends on our common stock will be at the discretion of the Board and will depend upon our financial condition, results of operations, cash flows and other factors deemed relevant by the Board.
Capital Resources
We believe that our cash and available borrowings under the Senior Credit Facility are sufficient to finance our operations and capital expenditures over the next twelve months. Our future capital requirements will depend on many factors, including the growth of our business, the timing and introduction of new products and investments, strategic plans and acquisitions, among others. Additional sources of liquidity available to us include short term borrowings and the issuance of long term debt and equity securities.
42
Off-Balance Sheet Arrangements
We do not have any off–balance sheet financing arrangements during the year-ended December 31, 2022 that have or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our interests.
Contractual Obligations and Commitments
We will continue to have cash requirements to support seasonal working capital needs and capital expenditures, to pay interest, to service debt, and to fund acquisitions. As part of our ongoing operations, we enter into contractual arrangements that obligate us to make future cash payments.
Our primary obligations include principal and interest payments on revolving portion and Term Loan component of the Senior Credit Facility, Securitization Facility and Convertible Securities. See Note 5, Debt,to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details. The Company also leases some of our manufacturing facilities and office buildings which have future minimum lease payments associated. See Note 11, Leases and Related Party Leases, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for a schedule of our future minimum lease payments. Amounts related to the Company's other obligations, including employment agreements and purchase obligations were not material.
The Company has contingent consideration obligations related to prior and current year acquisitions and future pension contribution obligations. See Note 10, Retirement Benefit Plans,and Note 15, Commitments and Contingenciesto the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details. The associated obligations are not fixed. The Company also has a liability for uncertain tax benefits including interest and penalties. See Note 12, Income Taxesto the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details. The Company cannot make a reliable estimate of the period in which the uncertain tax benefits may be realized.
Employee Termination Benefits
The Company incurred employee termination costs on restructuring activities associated with a closure of a manufacturing facility located in France, outsourcing plans for select transactional back office activities, and executive reorganization in the consolidated statement of operations for the year ended December 31, 2022. In 2021, the Company incurred employee termination costs on restructuring activities associated with the closure of the manufacturing facility in France. Restructuring costs were included in accrued expenses and other current liabilities in the consolidated balance sheet for the year ended December 31, 2022 and 2021. See Note 2, Summary of Significant Accounting Policies,of the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for further details.
CRITICAL ACCOUNTING POLICIES AND THE USE OF ESTIMATES
Our discussion and analysis of financial conditions and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America ("GAAP"). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities, and the reported amounts of revenues and expenses. Significant estimates affecting amounts reported or disclosed in the consolidated financial statements include allowances for doubtful accounts receivable and sales returns and allowances, net realizable value of inventories, valuation of intangible assets including amortization periods for acquired intangible assets, discount rates and estimated projected cash flows used to value and test impairments of long-lived assets and goodwill, estimates of projected cash flows and depreciation and amortization periods for long-lived assets, computation of taxes, valuation allowances recorded against deferred tax assets, the valuation of stock-based compensation, valuation of derivative instruments, valuation of contingent liabilities, the fair value of debt instruments and loss contingencies. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the current circumstances.
As we continue to navigate the COVID-19 pandemic and recent variants of the virus, as well as the adverse impacts to global economic conditions, supply chain and our operations, there may be impact to future estimates including, but not limited to, inventory valuations, fair value measurements, goodwill and long-lived asset impairments, the effectiveness of the Company’s hedging instruments, deferred tax valuation allowances, and allowances for doubtful accounts receivable.
We believe that the following accounting policies, which form the basis for developing these estimates, are those that are most critical to the presentation of our consolidated financial statements and require the more difficult subjective and complex judgments, often because of the need to make estimates about the effect of matters that are inherently uncertain. Because of this uncertainty, actual results could differ from these estimates.
43
Inventories
Inventories, consisting of purchased materials, direct labor and manufacturing overhead, are stated at the lower of cost (determined by the first-in, first-out method) or net realizable value. At each balance sheet date, we evaluate ending inventories for excess quantities, obsolescence or shelf-life expiration. Our evaluation includes an analysis of historical sales levels by product, projections of future demand by product, the risk of technological or competitive obsolescence for our products, general market conditions, a review of the shelf-life expiration dates for our products, and the feasibility of reworking or using excess or obsolete products or components in the production or assembly of other products that are not obsolete or for which we do not have excess quantities in inventory. To the extent that we determine there are excess or obsolete quantities or quantities with a shelf life that is too near its expiration for us to reasonably expect that we can sell those products prior to their expiration, we adjust their carrying value to estimated net realizable value. If future demand or market conditions are lower than our projections, or if we are unable to rework excess or obsolete quantities into other products, we may record further adjustments to the carrying value of inventory through a charge to cost of product revenues in the period the revision is made. As of December 31, 2022, our reserve for inventory obsolesce is 6% of total inventory on our consolidated balance sheets.
The Company capitalizes inventory costs associated with certain products prior to regulatory approval, based on management's judgment of probable economic benefit. The Company could be required to expense previously capitalized costs related to pre-approval inventory upon a change in such judgment, due to, among other potential factors, a denial or delay of approval by necessary regulatory bodies or a decision by management to discontinue the related development program.
Acquisitions
Results of operations of acquired companies are included in the Company’s results of operations as of the respective acquisition dates. The Company accounts for the acquisition of a business in accordance with ASC Topic 805, Business Combinations ("ASC Topic 805"). Amounts paid to acquire a business are allocated to the assets acquired and liabilities assumed based on the fair values at the date of acquisition. Any excess of the purchase price over the fair value of the net assets acquired in recorded as goodwill. Transaction costs and costs to restructure the acquired company are expensed as incurred.
Contingent consideration is recorded at fair value as measured on the date of acquisition. The value recorded is based on estimates of future financial projections under various potential scenarios using either a Monte Carlo simulation or the probability-weighted income approach derived from revenue estimates and probability assessment with respect to the likelihood of achieving contingent obligations. Contingent payments related to acquisitions consist of development, regulatory, and commercial milestone payments, in addition to sales-based payments, and are valued using discounted cash flow techniques. Each quarter until such contingent amounts are earned, the fair value of the liability is remeasured at each reporting period and adjusted as a component of operating expenses based on changes to the underlying assumptions. The change in the fair value of sales-based payments is based upon future revenue estimates and increases or decreases as revenue estimates or expectation of timing of payment charges. The estimates used to determine the fair value of the contingent consideration liability are subject to significant judgment and actual results are likely to differ from the amounts originally recorded.
The Company determines the fair value of acquired intangible assets based on detailed valuations that use certain information and assumptions provided by management. The Company allocates any excess purchase price over the fair value of the net tangible and intangible assets acquired to goodwill. Determining the fair value of these intangible assets, acquired as part of a business combination requires the Company to make significant estimates. These estimates include the amount and timing of projected future cash flows, the discount rate used to discount those cash flows to present value, the assessment of the asset’s life cycle, and the consideration of legal, technical, regulatory, economic, and competitive risks. The fair value assigned to other intangible assets is determined by estimating the future cash flows of each project or technology and discounting the net cash flows back to their present values. The discount rate used is determined at the time of measurement in accordance with accepted valuation methodologies.
In our most recent acquisition of SIA, the key areas of judgement relating to the valuation of the acquired definite-lived developed technology intangible assets were the net revenue growth rates, cost of sales, selling and marketing costs, discounts rates, and asset useful life. The key areas of judgement relating to the valuation of the contingent consideration are the inputs to the Monte-Carlo model including revenue-adjusted discount rate, counterpart discount rate, revenue volatility and forecasted revenue, earnings before income taxes and fixed costs. These assumptions were developed with the assistance of a third-party valuation expert.
44
Acquired IPR&D is recognized at fair value and initially characterized as an indefinite-lived intangible asset, irrespective of whether the acquired IPR&D has an alternative future use. The Company uses the income approach to determine the fair value of developed technology and IPR&D acquired in a business combination. This approach determines fair value by estimating the after-tax cash flows attributable to the respective asset over its useful life and then discounting these after-tax cash flows back to a present value. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each product including net revenues, cost of sales, R&D costs, selling and marketing costs, the appropriate discount rate to select in order to measure the risk inherent in each future cash flow stream, the assessment of each asset’s life cycle, and competitive trends impacting the asset and each cash flow stream. The Company also uses the income approach, as described above, to determine the estimated fair value of certain other identifiable intangible assets including customer relationships, trade names and business licenses. Customer relationships represent established relationships with customers, which provide a ready channel for the sale of additional products and services. Trade names represent acquired company and product names.
IPR&D acquired in a business combination is capitalized as an indefinite-lived intangible asset. Development costs incurred after the acquisition are expensed as incurred. Upon receipt of regulatory approval, the indefinite-lived intangible asset is then accounted for as a finite-lived intangible asset and amortized on a straight-line basis or accelerated basis, as appropriate, over its estimated useful life. If the research and development project is subsequently abandoned, the indefinite-lived intangible asset is charged to expense. IPR&D acquired outside of a business combination is expensed immediately.
Due to the uncertainty associated with research and development projects, there is risk that actual results will differ materially from the original cash flow projections and that the research and development project will result in a successful commercial product. The risks associated with achieving commercialization include, but are not limited to, delay or failure to obtain regulatory approvals to conduct clinical trials, delay or failure to obtain required market clearances, delays or issues with patent issuance, or validity and litigation.
If the acquired net assets do not constitute a business under the acquisition method of accounting, the transaction is accounted for as an asset acquisition and no goodwill is recognized. In an asset acquisition, the amount allocated to acquired IPR&D with no alternative future use is charged to expense at the acquisition date. Payments that would be recognized as contingent consideration in a business combination are expensed when probable in an asset acquisition. Refer to Note 4, Acquisitions and Divestituresto the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for details.
Valuation of Goodwill
The excess of the cost over the fair value of net assets of acquired businesses is recorded as goodwill. Goodwill is not subject to amortization but is reviewed for impairment at the reporting unit level annually, or more frequently if impairment indicators arise. The Company's assessment of the recoverability of goodwill is based upon a comparison of the carrying value of goodwill with its estimated fair value. Key assumptions used to estimate the fair value of goodwill include the Company's discounts rate and forecasted operating results. The Company had goodwill on the balance sheet of $1 billion as of December 31, 2022. The Company reviews goodwill for impairment in the third quarter every year in accordance with ASC Topic 350, Intangibles - Goodwill and Other ("ASC Topic 350"), and whenever events or changes in circumstances indicate the carrying value of goodwill may not be recoverable. Refer to Note 7, Goodwill and Other Intangibles, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for more information.
Valuation of Identifiable Intangible Assets
The Company tests intangible assets with indefinite lives for impairment annually in the third quarter in accordance with ASC Topic 350. Additionally, the Company may perform interim tests if an event occurs or circumstances change that could potentially reduce the fair value of a indefinite lived intangible asset below its carrying amount. The Company tests for impairment by either performing a qualitative evaluation or a quantitative test. The qualitative evaluation is an assessment of factors, including specific operating results as well as industry, market and general economic conditions, to determine whether it is more likely than not that the fair values of the intangible asset is less than its carrying amount. The Company may elect to bypass this qualitative evaluation and perform a quantitative test. There were no changes to identifiable intangible assets as a result of the Company's assessments.
Product rights and other definite-lived intangible assets are tested periodically for impairment in accordance with ASC Topic 360, Property, Plant and Equipment, ("ASC Topic 360") when events or changes in circumstances indicate that an asset's carrying value may not be recoverable. The impairment test involves comparing the carrying amount of the asset or asset group to the forecasted undiscounted future cash flows. In the event the carrying value of the asset exceeds the undiscounted future cash flows, the carrying value is considered not recoverable and impairment exists. An impairment loss is measured as the excess of the asset's carrying value over its fair value, calculated using discounted future cash flows. The computed impairment loss is recognized in the period that the impairment occurs.
45
As of December 31, 2022, the Company has $1.1 billion of identifiable intangible assets, net on the balance sheets.
Income Taxes
Since we conduct operations on a global basis, our effective tax rate has and will depend upon the geographic distribution of our pre-tax earnings among locations with varying tax rates. Changes in the tax rates of the various jurisdictions in which we operate affect our profits. In addition, we maintain a reserve for uncertain tax benefits, changes to which could impact our effective tax rate in the period such changes are made. The effective tax rate can also be impacted by changes in valuation allowances of deferred tax assets, and tax law changes.
Our provision for income taxes may change period-to-period based on specific events, such as the settlement of income tax audits and changes in tax laws, as well as general factors, including the geographic mix of income before taxes, state and local taxes and the effects of the Company's global income tax strategies. We maintain strategic management and operational activities in overseas subsidiaries. See Note 12, Income Taxes, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K), in our consolidated financial statements for disclosures related to foreign and domestic pretax income, foreign and domestic income tax expense (benefit) and the effect foreign taxes have on our overall effective tax rate.
We recognize a tax benefit from an uncertain tax position only if it is more likely than not to be sustained upon examination based on the technical merits of the position. The amount of the accrual for which an exposure exists is measured by determining the
amount that has a greater than 50 percent likelihood of being realized upon ultimate settlement of the position. Components of the reserve are classified as a long-term liability in the consolidated balance sheets. We record interest and penalties accrued in relation to uncertain tax benefits as a component of income tax expense.
We believe that we have identified all reasonably identifiable exposures and that the reserve we have established for identifiable exposures is appropriate under the circumstances; however, it is possible that additional exposures exist and that exposures will be settled at amounts different from the amounts reserved. It is also possible that changes in facts and circumstances could cause us to either materially increase or reduce the carrying amount of our tax reserves.
Our deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and their basis for income tax purposes, and the temporary differences created by the tax effects of capital loss, net operating loss and tax credit carryforwards. We record valuation allowances to reduce deferred tax assets to the amounts that arewhen it is more likely than not tothat some portion or all of the deferred tax assets will not be realized. We could recognize no benefit from our deferred tax assets or we could recognize some or all of the future benefit depending on the amount and timing of taxable income we generate in the future.
We intend to indefinitely reinvest substantially all of our foreign earnings in our foreign subsidiaries unless there is a tax–free manner under which to remit the earnings. The current analysis indicates that we have sufficient U.S. liquidity, including borrowing capacity, to fund foreseeable U.S. cash needs without requiring the repatriation of foreign cash. The 2017 Tax Act imposed a Toll Tax on a deemed repatriation of undistributed earnings of foreign subsidiaries. One time or unusual items that may impact our ability or intent to keep the foreign earnings and cash indefinitely reinvested include significant U.S. acquisitions, loans from a foreign subsidiary, and changes in tax laws.
As of December 31, 2019,2022, the Company has not provided deferred income taxes on unrepatriated earnings from foreign subsidiaries as they are deemed to be indefinitely reinvested.reinvested unless there is a manner under which to remit the earnings with no material tax cost. Such taxes would primarily be attributable to foreign withholding taxes and local income taxes when such earnings are distributed. As such, the Company has determined the tax impact of repatriating these earnings would not be material as of December 31, 2019.
| As of December 31, | |||||
| 2019 | 2018 | ||||
| Discount rate | 0.40 | % | 1.00 | % | |
| Expected return on plan assets | 3.33 | % | 3.40 | % | |
| Rate of compensation increase | 2.25 | % | 1.70 | % | |
Recently Issued and Adopted Accounting Standards
Refer to Note 2, Summary of Significant Accounting Policies,to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K), to the consolidated financial statements for recently adopted accounting pronouncements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to various market risks, including changes in foreign currency exchange rates and interest rates that could adversely affect our results of operations and financial condition. To manage the volatility relating to these typical business exposures, we may enter into various derivative transactions when appropriate. We do not hold or issue derivative instruments for trading or other speculative purposes.
46
Foreign Currency Exchange and Other Rate Risks
We operate on a global basis and are exposed to the risk that changes in foreign currency exchange rates could adversely affect our financial condition, results of operations and cash flows. We are primarily exposed to foreign currency exchange rate risk with respect to transactions and net assets denominated in Euros, ("EUR"), British pounds, ("GBP"), Swiss francs, ("CHF"), Canadian dollars, Japanese yen, Mexican pesos, Brazilian reais, Australian dollars and Chinese yuan. We manage the foreign currency exposure centrally, on a combined basis, which allows us to net exposures and to take advantage of any natural offsets. To mitigate the impact of currency fluctuations on transactions denominated in nonfunctional currencies, we periodically enter into derivative financial instruments in the form of foreign currency exchange forward contracts with major financial institutions. We temporarily record realized and unrealized gains and losses on these contracts that qualify as cash flow hedges in other comprehensive income, and then recognize them in other income or expense when the hedged item affects net earnings.
From time to time, we enter into foreign currency forward exchange contracts to manage currency exposures for transactions denominated in a currency other than an entity’s functional currency. As a result, the impact of foreign currency gains/losses recognized in earnings are partially offset by gains/losses on the related foreign currency forward exchange contracts in the same reporting period. Refer to Note 6, Derivative Instruments, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K) for furtheradditional information.
We maintain written policies and procedures governing our risk management activities. With respect to derivatives, changes in hedged items are generally expected to be completely offset by changes in the fair value of hedge instruments. Consequently, foreign currency exchange contracts would not subject us to material risk due to exchange rate movements, because gains and losses on these contracts offset gains and losses on the assets, liabilities or transactions being hedged.
The results of operations discussed herein have not been materially affected by inflation.
Interest Rate Risk
Cash and Cash Equivalents - We are exposed to the risk of interest rate fluctuations on the interest income earned on our cash and cash equivalents. A hypothetical 100 basis points movement in interest rates applicable to our cash and cash equivalents outstanding at December 31, 20192022 would increase interest income by approximately $2.0$5.1 million on an annual basis. No significant decrease in interest income would be expected as our cash balances are earning interest at rates of approximately 2one basis points. We are subject to foreign currency exchange risk with respect to cash balances maintained in foreign currencies.
Debt - Our interest rate risk relates primarily to U.S. dollar LIBOR-indexed borrowings. We use interest rate swap derivative instruments to manage our earnings and cash flow exposure to changes in interest rates. These interest rate swaps fix the interest rate on a portion of our expected LIBOR-indexed floating-rate borrowings. The Company held the following interest rate swaps as of December 31, 20192022 (dollar amounts in thousands):
| Hedged Item | Notional Amount | Designation Date | Effective Date | Termination Date | Fixed Interest Rate | Estimated Fair Value | ||||||||||||||||||||||||||||||||
| Assets (Liabilities) | ||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 150,000 | December 13, 2017 | July 1, 2019 | June 30, 2024 | 2.423 | % | 5,012 | |||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 200,000 | December 13, 2017 | January 1, 2018 | December 31, 2024 | 2.313 | % | 8,380 | |||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.220 | % | 1,831 | |||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.199 | % | 1,905 | |||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.209 | % | 1,970 | |||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.885 | % | 4,252 | |||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.867 | % | 4,153 | |||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 575,000 | December 15, 2020 | July 31, 2025 | December 31, 2027 | 1.415 | % | 23,742 | |||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 125,000 | December 15, 2020 | July 1, 2025 | December 31, 2027 | 1.404 | % | 5,467 | |||||||||||||||||||||||||||||||
| $ | 1,475,000 | $ | 56,712 | |||||||||||||||||||||||||||||||||||
47
| Hedged Item | Current Notional Amount | Designation Date | Effective Date | Termination Date | Fixed Interest Rate | Estimated Fair Value | ||||||||||
| Assets (Liabilities) | ||||||||||||||||
| 3-month USD LIBOR | 50,000 | February 6, 2017 | June 30, 2017 | June 30, 2020 | 1.834 | % | (2 | ) | ||||||||
| 1-month USD LIBOR | 100,000 | February 6, 2017 | June 30, 2017 | June 30, 2020 | 1.652 | % | 12 | |||||||||
| 1-month USD LIBOR | 100,000 | March 27, 2017 | December 31, 2017 | June 30, 2021 | 1.971 | % | (581 | ) | ||||||||
| 1-month USD LIBOR | 150,000 | December 13, 2017 | January 1, 2018 | December 31, 2022 | 2.201 | % | (2,880 | ) | ||||||||
| 1-month USD LIBOR | 150,000 | December 13, 2017 | January 1, 2018 | December 31, 2022 | 2.201 | % | (2,880 | ) | ||||||||
| 1-month USD LIBOR | 100,000 | December 13, 2017 | July 1, 2019 | June 30, 2024 | 2.423 | % | (3,517 | ) | ||||||||
| 1-month USD LIBOR | 50,000 | December 13, 2017 | July 1, 2019 | June 30, 2024 | 2.423 | % | (1,778 | ) | ||||||||
| 1-month USD LIBOR | 200,000 | December 13, 2017 | January 1, 2018 | December 31, 2024 | 2.313 | % | (6,595 | ) | ||||||||
| 1-month USD LIBOR | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.220 | % | (5,750 | ) | ||||||||
| 1-month USD LIBOR | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.199 | % | (5,747 | ) | ||||||||
| 1-month USD LIBOR | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.209 | % | (5,807 | ) | ||||||||
| 1-month USD LIBOR | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.885 | % | (4,930 | ) | ||||||||
| 1-month USD LIBOR | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.867 | % | (4,691 | ) | ||||||||
| Total interest rate derivatives designated as cash flow hedge | $ | 1,325,000 | (45,145 | ) | ||||||||||||
These interest rate swaps were designated as cash flow hedges as of December 31, 2019.2022. The total notional amount ofamounts related to the Company’s interest rate swaps in effectwere $1.5 billion and with $775.0 million effective as of December 31, 2019 was $900 million.2022. Based on our outstanding borrowings at December 31, 2019,2022, a 100 basis points change in interest rates would have impacted interest expense on the unhedged portionportion of the debt by $4.6by $1.1 million onon an annualized basis.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | ||||
Financial statements and the financial statement schedule specified by this Item, together with the report thereon of PricewaterhouseCoopers LLP, are presented following Item 1515. Exhibits and Financial Statement Schedule of this report.Annual Report on Form 10-K.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES | ||||
Not applicable.
48
| ITEM 9A. | CONTROLS AND PROCEDURES | ||||
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow for timely decisions regarding required disclosure. Disclosure controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Management has designed our disclosure controls and procedures to provide reasonable assurance of achieving the desired control objectives.
As required by Exchange Act Rule 13a-15(b), we have carried out an evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2019.2022. Based upon this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of December 31, 20192022 to provide such reasonable assurance.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Securities Exchange Act of 1934, as amended. Internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America (“GAAP”). We recognize that because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies and procedures may deteriorate.
To evaluate the effectiveness of our internal control over financial reporting, management used the criteria described in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based upon this evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2019.2022.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 20192022 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) that occurred during the quarter ended December 31, 20192022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION | ||||
The following disclosure is intended to satisfy any obligation to provide disclosures pursuant to Item 5.03 of Form 8-K.
Effective as of February 21, 2023, based on the recommendation of the Nominating and Corporate Governance Committee of the Board of Directors (the “Board”) of Integra LifeSciences Holdings Corporation (the “Company”), in connection with the new Securities and Exchange Commission rules and changes to the Securities Exchange Act of 1934, as amended (the "Exchange Act"), regarding universal proxy cards, certain recent changes to the Delaware General Corporation Law (the "DGCL"), and a periodic review of corporate governance matters, the Board approved amendments to the Company’s Second Amended and Restated Bylaws, as amended (the “Third A&R Bylaws”).
The Third A&R Bylaws, among other things:
•Address matters relating to Rule 14a-19 under the Exchange Act (the "Universal Proxy Rules"), including requiring: (a) the stockholder’s nomination notice to include a representation that it intends to solicit proxies from stockholders representing at least 67% of the voting power of shares entitled to vote on the election of directors; (b) the stockholder to comply with the Universal Proxy Rules and provide reasonable evidence thereof prior to the stockholder meeting; and (c) the stockholder to use a proxy card color other than white, which is reserved for the exclusive use of the Board. (Article 2, Sections 2.03, 2.08, and 2.10)
•Enhance the informational and procedural requirements in connection with stockholder proposals and stockholder director nominations, including: (a) requiring additional information about the stockholder making the director
49
nomination or proposal; (b) requiring additional information about the stockholder proposed business and/or director nominee; and (c) providing that the number of nominees a stockholder may nominate for election at the annual meeting of the stockholders may not exceed the number of directors to be elected at such annual meeting. (Article 2, Sections 2.03 and 2.10)
•Modify the provisions relating to adjournment procedures, availability of lists of stockholders entitled to vote at stockholder meetings, and electronic notices, in each case, to reflect amendments to the DGCL. (Article 2, Sections 2.04 and 2.05; Article 4, Section 4.01)
•Provide that any proxies received for disqualified or withdrawn Board nominees will be treated as abstentions. (Article 2, Section 2.08)
•Add a provision for stockholder actions by written consent that requires the Company to engage an independent inspector of election to perform a review of the validity of the applicable consents and revocations. (Article 2, Section 2.09)
•Clarify the powers of the chair of a stockholder meeting, including with respect to the chair’s ability to prescribe rules and regulations for the conduct of the meeting. (Article 2, Section 2.11)
•Add a federal forum provision to designate the federal district courts as the exclusive forum for matters arising under the Securities Act of 1933, as amended, and the Exchange Act. (Article 8, Section 8.02)
•Make various other updates, including ministerial and conforming changes and changes to clarify the Company’s ability to conduct business by means of remote communication.
The foregoing description of the Third A&R Bylaws is qualified in its entirety by the full text of the Third A&R Bylaws filed as Exhibit 3.3 hereto and incorporated herein by reference.
| ITEM 9C. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS | ||||
Not applicable.
PART III
INCORPORATION BY REFERENCE
The information called for by Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities relating to equity compensation plans, Item 10. Directors, Executive Officers and Corporate Governance, Item 11. Executive Compensation, Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters, Item 13. Certain Relationships and Related Transactions, and Director Independence and Item 14. Principal Accountant Fees and Services is incorporated herein by reference to the Company’s definitive proxy statement for its Annual Meeting of Stockholders scheduledscheduled to be held on May 13, 2020, which12, 2023, which definitive proxy statement is expected to be filed with the Commission not later than 120 days after the end of the fiscal year to which this report relates.
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULE | ||||
(a) Documents filed as a part of this report.report:
1. Financial Statements.
The following financial statements and financial statement schedules are filed as a part of this report:
50
All other schedules not listed above have been omitted, because they are not applicable or are not required, or because the required information is included in the consolidated financial statements or notes thereto.
3. Exhibits required to be filed by Item 601 of Regulation S-K.
| 10.3(a) | ||||||||
51
Third Amended and Restated 2003 Equity Incentive Plan effective May 22, 2015 (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 29, | ||||||||
| 10.3(i) | |||||||||
| 10.3(j) | |||||||||
| 10.3(k) | |||||||||
| 10.3(l) | |||||||||
| 10.4 | |||||||||
| 10.5 | |||||||||
| 10.6 | |||||||||
52
| 10.13(a) | ||||||||
| 10.13(c) | ||||||||
| 10.13(d) | ||||||||
| 10.14 | ||||||||
| 10.16 | ||||||||
53
| 31.1 | ||||||||
| 31.2 | ||||||||
| 32.1 | ||||||||
| 32.2 | ||||||||
| XBRL Instance Document+# | ||||||||
54
| 101.SCH | XBRL Taxonomy Extension Schema Document+# | |||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document+# | |||||||
| 101.DEF | XBRL Definition Linkbase Document | |||||||
| 101.LAB | XBRL Taxonomy Extension Labels Linkbase Document+# | |||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document+# | |||||||
| Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) | ||||||||
| * | Indicates a management contract or compensatory plan or arrangement. | ||||
| + | Indicates this document is filed as an exhibit herewith. | ||||
| # | The financial information of Integra LifeSciences Holdings Corporation Annual Report on Form 10-K for the year ended December 31, | ||||
The Company’s Commission File Number for Reports on Form 10-K, Form 10-Q and Form 8-K is 0-26224.000-26224.
| ITEM 16. | FORM 10-K SUMMARY | ||||
None.
55
SIGNATURES
Pursuant to the requirements of Section 13 of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| INTEGRA LIFESCIENCES HOLDINGS CORPORATION | |||||
| By: | /s/ | ||||
| President and Chief Executive Officer | |||||
| (Principal Executive Officer) | |||||
| By: | |||||
| /s/ Jeffrey A. Mosebrook | |||||
| Jeffrey A. Mosebrook | |||||
| Senior Vice President, Finance | |||||
| (Principal Financial Officer and Principal Accounting Officer) | |||||
Date: February 21, 202022, 2023
56
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons, on behalf of the registrant in the capacities indicated.
| Signature | Title | Date | ||||||||||||
| /s/ | President and Chief Executive Officer, | February | ||||||||||||
| and Director (Principal Executive Officer) | ||||||||||||||
| /s/ Jeffrey A. Mosebrook | Senior Vice President, Finance | February | ||||||||||||
| Jeffrey A. Mosebrook | (Principal Financial Officer and Principal Accounting Officer) | |||||||||||||
| /s/ Stuart M. Essig, Ph.D. | Chairman of the Board | February | ||||||||||||
| Stuart M. Essig, Ph.D. | ||||||||||||||
| /s/ Keith Bradley, Ph.D. | Director | February | ||||||||||||
| Keith Bradley, Ph.D. | ||||||||||||||
| /s/ Shaundra Clay | Director | February 22, 2023 | ||||||||||||
| Shaundra Clay | ||||||||||||||
| /s/ Barbara B. Hill | Director | February | ||||||||||||
| Barbara B. Hill | ||||||||||||||
| /s/ | Director | February | ||||||||||||
| /s/ Donald E. Morel, Jr., Ph.D. | Director | February | ||||||||||||
| Donald E. Morel, Jr., Ph.D. | ||||||||||||||
| /s/ Raymond G. Murphy | Director | February | ||||||||||||
| Raymond G. Murphy | ||||||||||||||
| /s/ Christian S. Schade | Director | February | ||||||||||||
| Christian S. Schade | ||||||||||||||
57
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Integra LifeSciences Holdings Corporation
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Integra LifeSciences Holdings Corporation and its subsidiaries(the (the “Company”) as of December 31, 20192022 and 2018,2021, and the related consolidated statements of operations, of comprehensive income, of changes in stockholders’ equity and of cash flows for each of the three years in the period ended December 31, 2019,2022, including the related notes and financial statement schedule listed in the index appearing under Item 15(a)(2) (collectively referred to as the “consolidated financial statements”).We also have audited the Company's internal control over financial reporting as of December 31, 2019,2022, based on criteria established in Internal Control - Integrated Framework (2013)issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 20192022 and 2018,2021, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 20192022 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019,2022, based on criteria established in Internal Control - Integrated Framework(2013) issued by the COSO.
Change in Accounting Principle
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for leasesconvertible instruments in 2019 and revenues from contracts with customers in 2018.2021.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management'sManagement’s Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidatedfinancial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and
F-1
that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit mattersmatter communicated below are mattersis a matter arising from the current period audit of the consolidated financial statements that werewas communicated or required to be communicated to the audit committee and that (i) relaterelates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit mattersmatter below, providing a separate opinionsopinion on the critical audit mattersmatter or on the accounts or disclosures to which they relate.it relates.
As described in Note 74 to the consolidated financial statements, on December 6, 2022, the Codman tradenameCompany completed its acquisition of Surgical Innovation Associates, Inc. (SIA) for an acquisition purchase price of $51.5 million, and will pay up to an additional $50 million upon the achievement of certain revenue-based performance milestones in 2023, 2024, and 2025 and up to an additional $40.0M upon the approval by the FDA of the Premarketing Approval Application for DuraSorb. The acquisition also resulted in a $75 million developed technology intangible asset balancebeing recorded. The estimated fair value of the developed technology acquired was $163.1 million asdetermined by management using the multi-period excess earnings method of December 31, 2019. During the third quarterincome approach. Management’s significant assumptions used in the estimate of 2019, management elected to bypassfair value included the qualitative impairment assessment for its Codman tradename intangibleestimated net cash flows, including net revenues, cost of sales, research and development costs, selling and marketing costs, working capital, contributory asset charges, discount rate, the asset’s life cycle, and competitive trends impacting the asset and perform a quantitative impairment test. In performing this test, management utilized projected sales growth rates, a royalty rate of 5%, a range of tax rates between 17.4-20.7%, and discount rate of 12.5%.the cash flow stream.
The principal considerations for our determination that performing procedures relating to the indefinite livedvaluation of developed technology intangible asset impairment assessmentfrom the acquisition of the Codman tradenameSurgical Innovation Associates, Inc. is a critical audit matter are (i) there wasthe significant judgment by management when developing the fair value measurementestimate of the Codman tradename, which in turn led toacquired developed technology intangible asset, (ii) a high degree of auditor judgment and, subjectivity, in performing procedures relating to the fair value measurement; (ii) there was significant auditand effort in performing procedures to evaluate the fair value measurement and evaluating management’s significant assumptions includingrelated to net revenues, cost of sales, discount rate and the projected sales growth rates, royalty rate, tax rates, and discount rateasset’s life cycle; and (iii) the audit effort involved the use of professionals with specialized skill and knowledge to assist in performing these procedures and evaluating the audit evidence obtained.knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the indefinite lived intangible asset impairment assessment,acquisition accounting, including controls over management’s valuation of the acquired developed technology intangible asset and controls over the development of significant assumptions usedrelated to estimatenet revenues, cost of sales, discount rate and the fair value of the intangible asset.asset’s life cycle. These procedures also included, among others (i) reading the purchase agreement and (ii) testing management’s process for developing the estimated fair value measurement;of the developed technology intangible asset. Testing management’s process included evaluating the appropriateness of the method;multi-period excess earnings method, testing the completeness accuracy, and relevanceaccuracy of underlying data used in the impairment assessment;method, and evaluating the reasonableness of the significant assumptions including projectedused by management related to net revenues, cost of sales, growth rates, royaltydiscount rate tax rates, and discount rate.the asset’s life cycle. Evaluating the reasonableness of management’s assumptions related to projectednet revenues, cost of sales, growth rates, royalty rate, and tax ratesthe asset’s life cycle involved evaluating whether the assumptions used by management were reasonable considering the(i) current and past performance of the businessproduct associated with the developed technology acquired; (ii) the consistency with external market and industry data; and (iii) whether thesethe assumptions wereare consistent with evidence obtained in other areas of the audit and industry data.audit. Professionals with specialized skill and knowledge were used to assist in evaluating (i) the appropriateness of the Company’s impairment assessmentmulti-period excess earnings method and (ii) the reasonableness of certain significant assumptions, including the discount rate.
/s/ PricewaterhouseCoopers LLP
Florham Park, New Jersey
February 21, 202022, 2023
We have served as the Company’s auditor since 1989.
F-2
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(Dollars in thousands, except per share amounts)
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands, except per share amounts) | |||||||||||
| Total revenue, net | $ | 1,517,557 | $ | 1,472,441 | $ | 1,188,236 | |||||
| Costs and expenses: | |||||||||||
| Cost of goods sold | 564,681 | 571,496 | 435,511 | ||||||||
| Research and development | 79,573 | 78,041 | 63,455 | ||||||||
| In-process research and development | 64,916 | — | — | ||||||||
| Selling, general and administrative | 687,599 | 690,746 | 624,096 | ||||||||
| Intangible asset amortization | 27,028 | 21,160 | 20,370 | ||||||||
| Total costs and expenses | 1,423,797 | 1,361,443 | 1,143,432 | ||||||||
| Operating income | 93,760 | 110,998 | 44,804 | ||||||||
| Interest income | 10,779 | 2,800 | 255 | ||||||||
| Interest expense | (53,957 | ) | (64,683 | ) | (35,019 | ) | |||||
| Other income, net | 9,522 | 8,288 | 1,345 | ||||||||
| Income before income taxes | 60,104 | 57,403 | 11,385 | ||||||||
| Provision (benefit) for income taxes | 9,903 | (3,398 | ) | (53,358 | ) | ||||||
| Net income per share | $ | 50,201 | $ | 60,801 | $ | 64,743 | |||||
| Basic | $ | 0.59 | $ | 0.73 | $ | 0.84 | |||||
| Diluted | $ | 0.58 | $ | 0.72 | $ | 0.82 | |||||
| Weighted average common shares outstanding (See Note 13): | |||||||||||
| Basic | 85,637 | 82,857 | 76,897 | ||||||||
| Diluted | 86,494 | 83,999 | 79,121 | ||||||||
| Years Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Total revenue, net | $ | 1,557,666 | $ | 1,542,448 | $ | 1,371,868 | |||||||||||
| Costs and expenses: | |||||||||||||||||
| Cost of goods sold | 587,355 | 597,808 | 520,834 | ||||||||||||||
| Research and development | 101,193 | 93,051 | 77,381 | ||||||||||||||
| Selling, general and administrative | 616,316 | 637,445 | 594,526 | ||||||||||||||
| Intangible asset amortization | 13,882 | 16,914 | 27,757 | ||||||||||||||
| Total costs and expenses | 1,318,746 | 1,345,218 | 1,220,498 | ||||||||||||||
| Operating income | 238,920 | 197,230 | 151,370 | ||||||||||||||
| Interest income | 11,917 | 6,737 | 9,297 | ||||||||||||||
| Interest expense | (49,594) | (50,395) | (71,581) | ||||||||||||||
| Gain from sale of businesses | 644 | 41,798 | — | ||||||||||||||
| Other income, net | 12,007 | 19,307 | 4,434 | ||||||||||||||
| Income before income taxes | 213,894 | 214,677 | 93,520 | ||||||||||||||
| Provision (benefit) for income taxes | 33,344 | 45,602 | (40,372) | ||||||||||||||
| Net income | $ | 180,550 | $ | 169,075 | $ | 133,892 | |||||||||||
| Net income per share | |||||||||||||||||
| Basic | $ | 2.18 | $ | 2.00 | $ | 1.58 | |||||||||||
| Diluted | $ | 2.16 | $ | 1.98 | $ | 1.57 | |||||||||||
| Weighted average common shares outstanding (See Note 13): | |||||||||||||||||
| Basic | 82,997 | 84,698 | 84,650 | ||||||||||||||
| Diluted | 83,516 | 85,485 | 85,228 | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| Net income | $ | 50,201 | $ | 60,801 | $ | 64,743 | |||||
| Other comprehensive income (loss), before tax: | |||||||||||
| Change in foreign currency translation adjustments | (174 | ) | (19,159 | ) | 37,454 | ||||||
| Unrealized gain (loss) on derivatives | |||||||||||
| Unrealized derivative (loss) gain arising during period | (13,671 | ) | 11,709 | (3,425 | ) | ||||||
| Less: Reclassification adjustments for gains included in net income | 14,865 | 13,400 | 2,958 | ||||||||
| Unrealized loss on derivatives | (28,536 | ) | (1,691 | ) | (6,383 | ) | |||||
| Defined benefit pension plan - net (loss) arising during period | (8,973 | ) | (643 | ) | (57 | ) | |||||
| Total other comprehensive income (loss), before tax | (37,683 | ) | (21,493 | ) | 31,014 | ||||||
| Income tax benefit (expense) related to items in other comprehensive loss | 6,724 | (143 | ) | 2,333 | |||||||
| Total other comprehensive income (loss), net of tax | (30,959 | ) | (21,636 | ) | 33,347 | ||||||
| Comprehensive income, net of tax | $ | 19,242 | $ | 39,165 | $ | 98,090 | |||||
| Years Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Net income | $ | 180,550 | $ | 169,075 | $ | 133,892 | |||||||||||
| Other comprehensive (loss) income, before tax: | |||||||||||||||||
| Change in foreign currency translation adjustments | (17,807) | (17,362) | 53,363 | ||||||||||||||
| Unrealized gain (loss) on derivatives | |||||||||||||||||
| Unrealized derivative gain (loss) arising during period | 104,351 | 68,192 | (96,837) | ||||||||||||||
| Less: Reclassification adjustments for gain (loss) included in net income | 18,859 | 17,024 | (24,442) | ||||||||||||||
| Unrealized gain (loss) on derivatives | 85,492 | 51,168 | (72,395) | ||||||||||||||
| Defined benefit pension plan - net gain (loss) arising during period | 7,429 | 6,998 | 4,604 | ||||||||||||||
| Total other comprehensive gain (loss), before tax | 75,114 | 40,804 | (14,428) | ||||||||||||||
| Income tax (expense) benefit related to items in other comprehensive gain (loss) | (19,694) | (11,900) | 16,771 | ||||||||||||||
| Total other comprehensive gain (loss), net of tax | 55,420 | 28,904 | 2,343 | ||||||||||||||
| Comprehensive income, net of tax | $ | 235,970 | $ | 197,979 | $ | 136,235 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED BALANCE SHEETS
(Dollars in thousands, except per share amounts)
| December 31, | |||||||
| 2019 | 2018 | ||||||
| (In thousands) | |||||||
| ASSETS | |||||||
| Current Assets: | |||||||
| Cash and cash equivalents | $ | 198,911 | $ | 138,838 | |||
| Trade accounts receivable, net of allowances of $4,303 and $3,719 | 275,296 | 265,737 | |||||
| Inventories, net | 316,054 | 280,347 | |||||
| Prepaid expenses and other current assets | 67,907 | 90,160 | |||||
| Total current assets | 858,168 | 775,082 | |||||
| Property, plant and equipment, net | 337,404 | 300,112 | |||||
| Right of use asset - operating leases | 94,530 | — | |||||
| Intangible assets, net | 1,031,591 | 1,079,496 | |||||
| Goodwill | 954,280 | 926,475 | |||||
| Deferred tax assets | 12,623 | 6,805 | |||||
| Other assets | 14,644 | 19,917 | |||||
| Total assets | $ | 3,303,240 | $ | 3,107,887 | |||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
| Current Liabilities: | |||||||
Current portion of borrowings under senior credit facility | $ | 45,000 | $ | 22,500 | |||
Current portion of lease liability - operating leases | 12,253 | — | |||||
| Accounts payable, trade | 113,090 | 76,050 | |||||
| Contract liabilities | 4,772 | 3,764 | |||||
| Accrued compensation | 79,385 | 75,693 | |||||
| Accrued expenses and other current liabilities | 76,809 | 84,545 | |||||
| Total current liabilities | 331,309 | 262,552 | |||||
| Long-term borrowings under senior credit facility | 1,198,561 | 1,210,513 | |||||
| Long-term borrowings under securitization facility | 104,500 | 121,200 | |||||
| Lease liability - operating leases | 97,504 | — | |||||
| Deferred tax liabilities | 36,553 | 57,778 | |||||
| Other liabilities | 118,077 | 80,048 | |||||
| Total liabilities | 1,886,504 | 1,732,091 | |||||
| Commitments and contingencies (Refer to Note 15) | |||||||
| Stockholders’ Equity: | |||||||
| Preferred Stock; no par value; 15,000 authorized shares; none outstanding | — | — | |||||
| Common stock; $0.01 par value; 240,000 authorized shares; 88,735 and 88,044 issued at December 31, 2019 and 2018, respectively | 887 | 880 | |||||
| Additional paid-in capital | 1,213,620 | 1,192,601 | |||||
| Treasury stock, at cost; 2,865 and 2,881 shares at December 31, 2019 and 2018, respectively | (119,943 | ) | (120,615 | ) | |||
| Accumulated other comprehensive loss | (76,402 | ) | (45,443 | ) | |||
| Retained earnings | 398,574 | 348,373 | |||||
| Total stockholders’ equity | 1,416,736 | 1,375,796 | |||||
| Total liabilities and stockholders’ equity | $ | 3,303,240 | $ | 3,107,887 | |||
| December 31, | |||||||||||
| 2022 | 2021 | ||||||||||
| ASSETS | |||||||||||
| Current Assets: | |||||||||||
| Cash and cash equivalents | $ | 456,661 | $ | 513,448 | |||||||
| Trade accounts receivable, net of allowances of $4,304 and $4,735 | 263,465 | 231,831 | |||||||||
| Inventories, net | 324,583 | 317,386 | |||||||||
| Prepaid expenses and other current assets | 116,789 | 91,051 | |||||||||
| Total current assets | 1,161,498 | 1,153,716 | |||||||||
| Property, plant and equipment, net | 311,302 | 311,703 | |||||||||
| Right of use asset - operating leases | 148,284 | 84,543 | |||||||||
| Intangible assets, net | 1,126,609 | 1,145,573 | |||||||||
| Goodwill | 1,038,881 | 1,013,458 | |||||||||
| Deferred tax assets, net | 45,994 | 56,950 | |||||||||
| Other assets | 57,190 | 16,440 | |||||||||
| Total assets | $ | 3,889,758 | $ | 3,782,383 | |||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||
| Current Liabilities: | |||||||||||
| Current portion of borrowings under senior credit facility | $ | 38,125 | $ | 45,000 | |||||||
| Current portion of lease liability - operating leases | 14,624 | 14,775 | |||||||||
| Accounts payable, trade | 102,100 | 61,837 | |||||||||
| Contract liabilities | 7,253 | 5,295 | |||||||||
| Accrued compensation | 78,771 | 92,656 | |||||||||
| Accrued expenses and other current liabilities | 80,033 | 120,458 | |||||||||
| Total current liabilities | 320,906 | 340,021 | |||||||||
| Long-term borrowings under senior credit facility | 733,149 | 824,257 | |||||||||
| Long-term borrowings under securitization facility | 104,700 | 112,500 | |||||||||
| Long-term convertible securities | 567,341 | 564,426 | |||||||||
| Lease liability - operating leases | 157,420 | 90,329 | |||||||||
| Deferred tax liabilities | 63,338 | 45,788 | |||||||||
| Other liabilities | 138,501 | 120,258 | |||||||||
| Total liabilities | 2,085,355 | 2,097,579 | |||||||||
| Stockholders’ Equity: | |||||||||||
| Preferred Stock; no par value; 15,000 authorized shares; none outstanding | — | — | |||||||||
| Common stock; $0.01 par value; 240,000 authorized shares; 90,477 and 89,600 issued at December 31, 2022 and 2021, respectively | 905 | 896 | |||||||||
| Additional paid-in capital | 1,276,977 | 1,264,943 | |||||||||
| Treasury stock, at cost; 6,823 and 4,899 shares at December 31, 2022 and 2021, respectively | (362,862) | (234,448) | |||||||||
| Accumulated other comprehensive income (loss) | 10,265 | (45,155) | |||||||||
| Retained earnings | 879,118 | 698,568 | |||||||||
| Total stockholders’ equity | 1,804,403 | 1,684,804 | |||||||||
| Total liabilities and stockholders’ equity | $ | 3,889,758 | $ | 3,782,383 | |||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| OPERATING ACTIVITIES: | |||||||||||
| Net income | $ | 50,201 | $ | 60,801 | $ | 64,743 | |||||
| Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||
| Depreciation and amortization | 109,462 | 110,730 | 88,945 | ||||||||
| Non-cash in-process research and development expense | 64,916 | — | — | ||||||||
| Non-cash impairment charges | 5,764 | 4,941 | 3,290 | ||||||||
| Deferred income tax benefit | (19,046 | ) | (8,184 | ) | (67,304 | ) | |||||
| Share-based compensation | 21,255 | 20,779 | 21,550 | ||||||||
| Amortization of debt issuance costs | 5,390 | 6,270 | 2,722 | ||||||||
| Non-cash lease expense | 5,060 | — | — | ||||||||
| Realized loss on sale of short-term investments | — | — | 2,287 | ||||||||
| Loss on disposal of property and equipment | 1,821 | 1,385 | 6,989 | ||||||||
| Gain on divestiture of business | — | — | (2,645 | ) | |||||||
| Change in fair value of contingent consideration and others | 1,119 | 1,214 | (4,710 | ) | |||||||
| Accounts receivable | (9,428 | ) | (17,021 | ) | (89,698 | ) | |||||
| Inventories | (43,308 | ) | 8,300 | 99 | |||||||
| Prepaid expenses and other current assets | 13,071 | 3,933 | (33,808 | ) | |||||||
| Other non-current assets | 13,156 | 1,052 | (914 | ) | |||||||
| Accounts payable, accrued expenses and other current liabilities | 14,666 | 3,588 | 95,321 | ||||||||
| Contract liabilities | (607 | ) | 1,504 | 3,874 | |||||||
| Other non-current liabilities | (2,059 | ) | 391 | 23,803 | |||||||
| Net cash provided by operating activities | 231,433 | 199,683 | 114,544 | ||||||||
| INVESTING ACTIVITIES: | |||||||||||
| Proceeds from sale of short-term investments | — | — | 16,951 | ||||||||
| Proceeds from note receivable | 752 | 910 | 483 | ||||||||
| Cash used in business acquisitions, net of cash acquired | (30,509 | ) | 26,704 | (1,241,946 | ) | ||||||
| Acquired in-process research and development | (64,995 | ) | — | — | |||||||
| Purchases of property and equipment | (69,537 | ) | (77,741 | ) | (43,503 | ) | |||||
| Proceeds from sales of property and equipment | 37 | 422 | 293 | ||||||||
| Proceeds from divestiture of business | — | — | 46,387 | ||||||||
| Net proceeds on swaps designated as net investment hedges | 1,584 | — | — | ||||||||
| Net cash used in investing activities | (162,668 | ) | (49,705 | ) | (1,221,335 | ) | |||||
| FINANCING ACTIVITIES: | |||||||||||
| Proceeds from borrowings of long-term indebtedness | 236,900 | 171,200 | 1,307,000 | ||||||||
| Payments on debt | (246,100 | ) | (660,000 | ) | (117,000 | ) | |||||
| Net cash paid for contingent consideration | — | (38,196 | ) | (4,661 | ) | ||||||
| Proceeds from the issuance of common stock, net of issuance costs | — | 349,590 | — | ||||||||
| Debt issuance costs | — | (5,037 | ) | (19,043 | ) | ||||||
| Proceeds from exercised stock options | 6,948 | 9,392 | 9,774 | ||||||||
| Cash taxes paid in net equity settlement | (6,514 | ) | (7,821 | ) | (7,123 | ) | |||||
| Net cash provided by (used in) financing activities | (8,766 | ) | (180,872 | ) | 1,168,947 | ||||||
| Effect of exchange rate changes on cash and cash equivalents | 74 | (5,203 | ) | 10,724 | |||||||
| Net increase (decrease) in cash and cash equivalents | 60,073 | (36,097 | ) | 72,880 | |||||||
| Cash and cash equivalents at beginning of period | 138,838 | 174,935 | 102,055 | ||||||||
| Cash and cash equivalents at end of period | $ | 198,911 | $ | 138,838 | $ | 174,935 | |||||
| Years Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| OPERATING ACTIVITIES: | |||||||||||||||||
| Net income | $ | 180,550 | $ | 169,075 | $ | 133,892 | |||||||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||||||||
| Depreciation and amortization | 118,299 | 119,836 | 116,031 | ||||||||||||||
| Non-cash in-process research and development expense | — | — | 519 | ||||||||||||||
| Non-cash impairment charges | — | 2,754 | — | ||||||||||||||
| Deferred income tax (benefit) provision | (4,585) | (2,755) | (64,138) | ||||||||||||||
| Share-based compensation | 27,725 | 36,210 | 19,590 | ||||||||||||||
| Amortization of debt issuance costs and expenses associated with debt refinancing | 6,845 | 7,030 | 12,076 | ||||||||||||||
| Non-cash lease expense | 2,816 | 3,834 | 2,955 | ||||||||||||||
| Accretion of bond issuance discount | — | — | 15,415 | ||||||||||||||
| Loss (Gain) on disposal of property and equipment and construction in-progress | (6,813) | 2,240 | 7,855 | ||||||||||||||
| Gain from the sale of businesses | (644) | (41,798) | — | ||||||||||||||
| Change in fair value of contingent consideration and others | (20,304) | (2,162) | 951 | ||||||||||||||
| Changes in assets and liabilities: | |||||||||||||||||
| Accounts receivable | (33,905) | 7,265 | 52,105 | ||||||||||||||
| Inventories | (29,124) | 5,374 | (48,348) | ||||||||||||||
| Prepaid expenses and other current assets | 8,612 | (21,143) | 1,632 | ||||||||||||||
| Other non-current assets | (2,182) | 7,875 | 13,735 | ||||||||||||||
| Accounts payable, accrued expenses and other current liabilities | 17,343 | 32,874 | (57,512) | ||||||||||||||
| Contract liabilities | 4,274 | 28 | (37) | ||||||||||||||
| Other non-current liabilities | (4,438) | (14,110) | (2,889) | ||||||||||||||
| Net cash provided by operating activities | 264,469 | 312,427 | 203,832 | ||||||||||||||
| INVESTING ACTIVITIES: | |||||||||||||||||
| Purchases of property and equipment | (42,343) | (48,022) | (38,890) | ||||||||||||||
| Proceeds from sale of business | 23,960 | 190,468 | — | ||||||||||||||
| Acquired in-process research and development and intangibles | (4,742) | (58) | (25,000) | ||||||||||||||
| Cash paid for business acquisitions, net of cash acquired | (51,509) | (303,910) | — | ||||||||||||||
| Proceeds from sales of property and equipment | 11,145 | 3 | 3,657 | ||||||||||||||
| Net proceeds (payments) on swaps designated as net investment hedges | 4,909 | 76 | (7,840) | ||||||||||||||
| Net cash used in investing activities | (58,580) | (161,443) | (68,073) | ||||||||||||||
| FINANCING ACTIVITIES: | |||||||||||||||||
| Proceeds from borrowings of long-term indebtedness | 40,750 | 25,500 | 171,500 | ||||||||||||||
| Payments on debt | (148,550) | (125,500) | (441,000) | ||||||||||||||
| Purchase of option hedge on convertible notes | — | — | (104,248) | ||||||||||||||
| Proceeds from convertible notes issuance | — | — | 575,000 | ||||||||||||||
| Proceeds from sale of stock purchase warrants | — | — | 44,563 | ||||||||||||||
| Payment of debt issuance costs | — | (249) | (24,347) | ||||||||||||||
| Purchase of treasury stock | (125,000) | — | (100,000) | ||||||||||||||
| Proceeds from exercised stock options | 5,465 | 6,824 | 5,232 | ||||||||||||||
| Cash taxes paid in net equity settlement | (24,618) | (4,801) | (5,075) | ||||||||||||||
| Net cash (used in) provided by financing activities | (251,953) | (98,226) | 121,625 | ||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | (10,723) | (9,476) | 13,871 | ||||||||||||||
| Net increase (decrease) in cash and cash equivalents | (56,787) | 43,282 | 271,255 | ||||||||||||||
| Cash and cash equivalents at beginning of period | 513,448 | 470,166 | 198,911 | ||||||||||||||
| Cash and cash equivalents at end of period | $ | 456,661 | $ | 513,448 | $ | 470,166 | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY
| Common Stock | Treasury Stock | Additional Paid-In Capital | Accumulated Other Comprehensive Income (Loss) | Retained Earnings | Total Equity | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||||||||
| Balance, January 1, 2017 | 77,666 | $ | 777 | (2,946 | ) | $ | (123,051 | ) | $ | 798,652 | $ | (57,154 | ) | $ | 220,443 | $ | 839,667 | |||||||||||||
| Net income | — | — | — | — | — | — | 64,743 | 64,743 | ||||||||||||||||||||||
| Other comprehensive income (loss), net of tax | — | — | — | — | — | 33,347 | — | 33,347 | ||||||||||||||||||||||
| Issuance of common stock through employee stock purchase plan | 12 | — | — | — | 509 | — | — | 509 | ||||||||||||||||||||||
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | 788 | 8 | 19 | 1,407 | 723 | — | — | 2,138 | ||||||||||||||||||||||
| Exercise of warrants | 2,840 | 28 | — | — | (28 | ) | — | — | — | |||||||||||||||||||||
| Share-based compensation | — | — | — | — | 21,902 | — | — | 21,902 | ||||||||||||||||||||||
| Balance, December 31, 2017 | 81,306 | $ | 813 | (2,927 | ) | $ | (121,644 | ) | $ | 821,758 | $ | (23,807 | ) | $ | 285,186 | $ | 962,306 | |||||||||||||
| Adoption of Update No. 2014-09 | — | — | — | — | — | — | 1,854 | 1,854 | ||||||||||||||||||||||
| Adoption of Update No. 2018-02 | — | — | — | — | — | — | 532 | 532 | ||||||||||||||||||||||
| Net income | — | — | — | — | — | — | 60,801 | 60,801 | ||||||||||||||||||||||
| Other comprehensive income (loss), net of tax | — | — | — | — | — | (21,636 | ) | (21,636 | ) | |||||||||||||||||||||
| Issuance of common stock through employee stock purchase plan | — | — | — | — | 553 | — | — | 553 | ||||||||||||||||||||||
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | 700 | 4 | 46 | 1,030 | 52 | — | — | 1,086 | ||||||||||||||||||||||
| Equity offering | 6,038 | 60 | — | — | 349,529 | — | — | 349,589 | ||||||||||||||||||||||
| Share-based compensation | — | 3 | — | — | 20,709 | — | — | 20,712 | ||||||||||||||||||||||
| Balance, December 31, 2018 | 88,044 | 880 | (2,881 | ) | (120,615 | ) | 1,192,601 | (45,443 | ) | 348,373 | 1,375,796 | |||||||||||||||||||
| Net income | — | — | — | — | — | — | 50,201 | 50,201 | ||||||||||||||||||||||
| Other comprehensive income (loss), net of tax | — | — | — | — | — | (30,959 | ) | — | (30,959 | ) | ||||||||||||||||||||
| Issuance of common stock through employee stock purchase plan | 17 | — | — | — | — | 716 | — | 716 | ||||||||||||||||||||||
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | 674 | 7 | 16 | 672 | (961 | ) | (282 | ) | ||||||||||||||||||||||
| Share-based compensation | — | — | — | — | 21,264 | — | — | 21,264 | ||||||||||||||||||||||
| Balance, December 31, 2019 | 88,735 | 887 | (2,865 | ) | (119,943 | ) | 1,213,620 | (76,402 | ) | 398,574 | 1,416,736 | |||||||||||||||||||
| Common Stock | Treasury Stock | Additional Paid-In Capital | Accumulated Other Comprehensive Income | Retained Earnings | Total Equity | ||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||||||||||||||||||||
| Balance, January 1, 2020 | 88,735 | $ | 887 | (2,865) | $ | (119,943) | $ | 1,213,620 | $ | (76,402) | $ | 398,574 | $ | 1,416,736 | |||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | — | — | 133,892 | 133,892 | |||||||||||||||||||||||||||||||||||||||
| Other comprehensive loss, net of tax | — | — | — | — | — | 2,343 | — | 2,343 | |||||||||||||||||||||||||||||||||||||||
| Issuance of common stock through employee stock purchase plan | 13 | — | — | — | 694 | — | — | 694 | |||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | 503 | 2 | 11 | 526 | (1,066) | — | — | (538) | |||||||||||||||||||||||||||||||||||||||
| Share-based compensation | — | 4 | — | — | 19,397 | — | — | 19,401 | |||||||||||||||||||||||||||||||||||||||
| Share repurchase and equity component of the convertible note issuance, net | — | — | — | — | 42,539 | — | — | 42,539 | |||||||||||||||||||||||||||||||||||||||
| Accelerated shares repurchased | — | — | (2,060) | (115,724) | 15,724 | — | — | (100,000) | |||||||||||||||||||||||||||||||||||||||
| Adoption of Update No. 2016-13 | — | — | — | — | — | — | (200) | (200) | |||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2020 | 89,251 | 893 | (4,914) | (235,141) | 1,290,908 | (74,059) | 532,266 | 1,514,867 | |||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | — | — | 169,075 | 169,075 | |||||||||||||||||||||||||||||||||||||||
| Other comprehensive loss, net of tax | — | — | — | — | — | 28,904 | — | 28,904 | |||||||||||||||||||||||||||||||||||||||
| Issuance of common stock through employee stock purchase plan | 18 | — | — | 1,127 | — | — | 1,127 | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | 331 | 1 | 15 | 693 | 201 | — | — | 895 | |||||||||||||||||||||||||||||||||||||||
| Share-based compensation | — | 2 | — | — | 35,981 | — | — | 35,983 | |||||||||||||||||||||||||||||||||||||||
| Adoption of Update No. 2020-06 | — | — | — | — | (63,274) | — | (2,773) | (66,047) | |||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2021 | 89,600 | $ | 896 | (4,899) | $ | (234,448) | $ | 1,264,943 | $ | (45,155) | $ | 698,568 | $ | 1,684,804 | |||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | — | — | 180,550 | 180,550 | |||||||||||||||||||||||||||||||||||||||
| Other comprehensive income (loss), net of tax | — | — | — | — | — | 55,420 | — | 55,420 | |||||||||||||||||||||||||||||||||||||||
| Issuance of common stock through employee stock purchase plan | 17 | — | — | — | 1,078 | — | — | 1,078 | |||||||||||||||||||||||||||||||||||||||
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | 859 | 7 | 14 | 738 | (20,974) | — | — | (20,229) | |||||||||||||||||||||||||||||||||||||||
| Share-based compensation | — | 2 | — | — | 27,778 | — | — | 27,780 | |||||||||||||||||||||||||||||||||||||||
| Accelerated shares repurchased | — | — | (1,938) | (129,152) | 4,152 | — | — | (125,000) | |||||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2022 | 90,476 | 905 | (6,823) | (362,862) | 1,276,977 | 10,265 | 879,118 | 1,804,403 | |||||||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. BUSINESS
Integra LifeSciences Holdings Corporation (the “Company”) was incorporated in Delaware in 1989. The Company is a worldworldwide leader in medical devices, is dedicatedtechnology. The Company was founded with the acquisition of an engineered collagen technology platform used to limiting uncertaintyrepair and regenerate tissue. Since then, Integra has developed numerous product lines from this technology for surgeonsapplications ranging from burn and deep tissue wounds to the repair of dura mater in the brain, as well as nerves and tendons. The Company has expanded its base regenerative technology business to include surgical instruments, neurosurgical products and advanced wound care through global acquisitions and product development to meet the development, manufacturing,evolving needs of its customers and marketing of cost-effective surgical implants and medical instruments. Its products are used primarily in neurosurgery, extremity reconstruction, orthopedics and general surgery.
enhance patient care. The Company sells its products directly through various sales forces and through a variety of other distribution channels.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF PRESENTATION
These financial statements and the accompanying notes are prepared in accordance with accounting principles generally accepted in the United States of America and conform to Regulation S-X under the Securities Exchange Act of 1934, as amended.
PRINCIPLES OF CONSOLIDATION
The consolidated financial statements include the accounts of the Company and its subsidiaries, all of which are wholly owned. All intercompanywholly-owned subsidiaries. Intercompany accounts and transactions arehave been eliminated in consolidation. See Note 4, Acquisitions and Pro Forma ResultsDivestitures, for details of new subsidiaries included in the consolidation.
USE OF ESTIMATES
The preparation of consolidated financial statements is in conformity with generally accepted accounting principles in the United States ("GAAP") which requires management to make estimates and assumptions that affect the reported amountsamount of assets and liabilities, the disclosure of contingent liabilities, and the reported amounts of revenues and expenses. Significant estimates affecting amounts reported or disclosed in the consolidated financial statements include allowances for doubtful accounts receivable and sales returns and allowances, net realizable value of inventories, valuation of intangible assets and in-process research and development ("IPR&D"),including amortization periods for acquired intangible assets, discount rates and estimated projected cash flows used to value and test impairments of long-lived assets and goodwill, estimates of projected cash flows and depreciation and amortization periods for long-lived assets, computation of taxes, valuation allowances recorded against deferred tax assets, the valuation of stock-based compensation, valuation of pension assets and liabilities,derivative instruments, valuation of derivative instruments, and valuationcontingent liabilities, the fair value of debt instruments and loss contingencies. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the current circumstances. Actual results could differ from these estimates.
CASH AND CASH EQUIVALENTS
The Company considers all short-term, highly liquid investments purchased with original maturities of three months or less to be cash equivalents. These investments are carried at cost, which approximates fair value.
TRADE ACCOUNTS RECEIVABLE AND ALLOWANCES FOR DOUBTFUL ACCOUNTS RECEIVABLE
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The Company grants credit to customers in the normal course of business, but generally does not require collateral or any other security to support its receivables.
The Company evaluates the collectability of accounts receivable based on a combination of factors. The Company recognizes a provision for doubtful accounts that reflects the Company’s estimate of expected credit losses for trade accounts receivable. In circumstances where a specific customer is unable to meet its financial obligations to the Company, a provision to the allowances for doubtful accounts is recorded against amounts due to reduce the net recognized receivable to the amount that is reasonably expected to be collected. For all other customers, the Company evaluates measurement of all expected credit losses for trade receivables held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts.
F - 8
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The Company adopted this guidance on January 1, 2020 using a provisionmodified retrospective transition method which requires a cumulative-effect adjustment to the allowances for doubtful accounts isopening balance of retained earnings to be recognized on the date of adoption with no change to financial results reported in prior periods. The cumulative-effect adjustment recorded based on factors including the lengthJanuary 1, 2020 was not material. The adoption of time the receivables are past due, the current business environment andthis ASU did not have a significant impact on the Company's historical experience. consolidated financial statements and related disclosures. The Company's exposure to credit losses may increase if its customers are adversely affected by changes in healthcare laws, coverage, and reimbursement, economic pressures or uncertainty associated with local or global economic recessions, disruption associated with the COVID-19 pandemic and recent variants of the virus, and other customer-specific factors. Although the Company has historically not experienced significant credit losses, it is possible that there could be an adverse impact due to customer and governmental responses to the COVID-19 pandemic.
Provisions to the allowances for doubtful accounts are recorded to selling, general and administrative expenses. Account balances are charged off against the allowance when it is probable that the receivable will not be recovered. Provision for doubtful accounts, net of recoveries, associated with accounts receivable, included in selling, general and administrative expense, were $2.1was charges of $0.2 million $0.6for the year ended December 31, 2022, recoveries of $1.1 million, and $2.0charges of $3.6 million for the years ended December 31, 2019, 20182021 and 2017,2020, respectively.
| Balance at Beginning of Period | Charged to Costs and Expenses | Other | Deductions | Balance at End of Period | |||||||||||||||||||||||||
| Dollars in thousands | |||||||||||||||||||||||||||||
| Year Ended: | |||||||||||||||||||||||||||||
| December 31, 2022 | $ | 4,735 | 238 | — | (669) | $ | 4,304 | ||||||||||||||||||||||
| December 31, 2021 | $ | 6,439 | (1,059) | 341 | (986) | $ | 4,735 | ||||||||||||||||||||||
| December 31, 2020 | $ | 4,303 | 3,635 | — | (1,499) | $ | 6,439 | ||||||||||||||||||||||
INVENTORIES
Inventories, consisting of purchased materials, direct labor and manufacturing overhead, are stated at the lower of cost, the value determined by the first-in, first-out method, or net realizable value. Inventories consisted of the following:
| December 31, | ||||||
| 2019 | 2018 | |||||
| (In thousands) | ||||||
| Finished goods | 201,870 | $ | 179,885 | |||
| Work in process | 48,333 | 47,715 | ||||
| Raw materials | 65,851 | 52,747 | ||||
| Total inventories, net | 316,054 | $ | 280,347 | |||
| December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| Finished goods | 172,088 | $ | 162,528 | ||||||||
| Work in process | 70,598 | 65,323 | |||||||||
| Raw materials | 81,897 | 89,535 | |||||||||
| Total inventories, net | $ | 324,583 | $ | 317,386 | |||||||
At each balance sheet date, the Company evaluates inventories for excess quantities, obsolescence or shelf life expiration. This evaluation includes analysis of historical sales levels by product, projections of future demand, the risk of technological or competitive obsolescence for products, general market conditions, a review of the shelf life expiration dates for products, as well as the feasibility of reworking or using excess or obsolete products or components in the production or assembly of other products that are not obsolete or for which there are not excess quantities in inventory. To the extent that management determines there are excess or obsolete inventory or quantities with a shelf life that is too near its expiration for the Company to reasonably expect that it can sell those products prior to their expiration, the Company adjusts the carrying value to estimated net realizable value.
The Company capitalizes inventory costs associated with certain products prior to regulatory approval, based on management's judgment of probable economic benefit. The Company could be required to expense previously capitalized costs related to pre-approval inventory upon a change in such judgment, due to, among other potential factors, a denial or delay of approval by necessary regulatory bodies or a decision by management to discontinue the related development program. NaNNo such amounts were capitalized at December 31, 20192022 or 2018.2021.
F-9
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment are stated at historical cost less accumulated depreciation and any impairment charges. The Company provides for depreciation using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are amortized over the lesser of the lease term or the useful life. The cost of major additions and improvements is capitalized, while maintenance and repair costs that do not improve or extend the lives of the respective assets are charged to operations as incurred. The cost of computer software developed or obtained for internal use is accounted for in accordance with the Accounting Standards Codification 350-40, Internal-Use Software.
Property, plant and equipment balances and corresponding lives were as follows:
| December 31, | |||||||||
| 2019 | 2018 | Useful Lives | |||||||
| (In thousands) | |||||||||
| Land | $ | 1,476 | $ | 1,837 | |||||
| Buildings and building improvements | 16,262 | 20,472 | 5-40 years | ||||||
| Leasehold improvements | 114,941 | 105,063 | 1-20 years | ||||||
| Machinery and production equipment | 155,313 | 143,921 | 3-20 years | ||||||
| Surgical instrument kits | 33,104 | 31,231 | 4-5 years | ||||||
| Information systems and hardware | 138,398 | 129,962 | 1-7 years | ||||||
| Furniture, fixtures, and office equipment | 22,145 | 17,731 | 1-15 years | ||||||
| Construction-in-progress | 140,366 | 105,075 | |||||||
| Total | 622,005 | 555,292 | |||||||
| Less: Accumulated depreciation | (284,601 | ) | (255,180 | ) | |||||
| Property, plant and equipment, net | $ | 337,404 | $ | 300,112 | |||||
| December 31, | |||||||||||||||||
| Dollars in thousands | 2022 | 2021 | Useful Lives | ||||||||||||||
| Land | $ | 966 | $ | 1,512 | |||||||||||||
| Buildings and building improvements | 14,710 | 19,032 | 5-40 years | ||||||||||||||
| Leasehold improvements | 164,292 | 155,495 | 1-20 years | ||||||||||||||
| Machinery and production equipment | 181,780 | 183,270 | 3-20 years | ||||||||||||||
| Demonstration equipment | 3,792 | 2,791 | 4-5 years | ||||||||||||||
| Information systems and hardware | 151,330 | 148,706 | 1-7 years | ||||||||||||||
| Furniture, fixtures, and office equipment | 20,286 | 20,921 | 1-15 years | ||||||||||||||
| Construction-in-progress | 103,875 | 94,850 | |||||||||||||||
| Total | 641,031 | 626,577 | |||||||||||||||
| Less: Accumulated depreciation | (329,729) | (314,874) | |||||||||||||||
| Property, plant and equipment, net | $ | 311,302 | $ | 311,703 | |||||||||||||
Depreciation expense associated with property, plant and equipmentequipment was $42.6$40.1 million $44.1, $39.4 million, and $36.1$42.1 million for the years ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively.
CAPITALIZED INTEREST
The interest cost on capital projects, including facilities build-out and internal use software, is capitalized and included in the cost of the project. Capitalization commences with the first expenditure for the project and continues until the project is substantially complete and ready for its intended use. When no debt is incurred specifically for a project, interest is capitalized on project
expenditures using the weighted average cost of the Company's outstanding borrowings. For the years ended December 31, 20192022 and 2018,2021, respectively, the Company capitalized $3.1$1.4 million and $2.3$1.2 million of interest expense into property, plant and equipment.
ACQUISITIONS
Results of operations of acquired companies are included in the Company’s results of operations as of the respective acquisition dates. Acquired businesses are accountedThe Company accounts for using the acquisition method of accounting, which requires thata business in accordance with ASC 805, Business Combinations ("ASC Topic 805"). Amounts paid to acquire a business are allocated to the assets acquired and liabilities assumed be recordedbased on their fair values at fair value, with limited exceptions.the date of acquisition. Any excess of the purchase price over the fair value of the net assets acquired is recorded as goodwill. Transaction costs and costs to restructure the acquired Companycompany are expensed as incurred. The operating results of the acquired business are reflected in the consolidated financial statements after
Contingent consideration is recorded at fair value as measured on the date of acquisition. Acquired in-process researchThe value recorded is based on estimates of future financial projections under various potential scenarios using either a Monte Carlo simulation or the probability-weighted income approach derived from revenue estimates and development (“IPR&D”) is recognized at fair value and initially characterized as an indefinite-lived intangible asset, irrespective of whether the acquired IPR&D has an alternative future use. Contingent consideration is recognized at the estimated fair value on the acquisition date. Subsequent changesprobability assessment with respect to the fair valuelikelihood of achieving contingent payments are recognized in selling, general and administrative expense in consolidated statements of operations.obligations. Contingent payments related to acquisitions consist of development, regulatory, and commercial milestone payments, in addition to sales-based payments, and are valued using discounted cash flow techniques. TheEach quarter until such contingent amounts are earned, the fair value of development, regulatory,the liability is remeasured at each reporting period and commercial milestone payments reflects management’s expectationsadjusted as a component of operating expenses based on changes to the probability of payment and increases or decreases asunderlying assumptions. The change in the probability of payment or expectation of timing of payments changes. The fair value of sales-based payments is based upon probability-weighted future revenue estimates and increases or decreases as revenue estimates or expectation of timing of payments changes.
F-10
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company determines the fair value of acquired intangible assets based on detailed valuations that use certain information and assumptions provided by management. The Company allocates any excess of the costpurchase price over the fair value of the net assets of acquired businesses is recorded as goodwill. Goodwill is not subject to amortization but is reviewed for impairment at the reporting unit level annually, or more frequently if impairment indicators arise. The Company's assessment of the recoverability of goodwill is based upon a comparison of the carrying value of goodwill with its estimated fair value. The Company reviews goodwill for impairment in the third quarter every year in accordance with ASC Topic 350tangible and whenever events or changes in circumstances indicate the carrying value of goodwill may not be recoverable. Refer to Note 7, Goodwill and Other Intangibles for more information.
Acquired IPR&D is recognized at fair value and initially characterized as an indefinite-lived intangible asset, irrespective of whether the acquired IPR&D has an alternative future use. The Company uses the income approach to determine the fair value of developed technology and IPR&D acquired in a business combination. This approach determines fair value by estimating the after-tax cash flows attributable to the respective asset over its useful life and then discounting these after-tax cash flows back to a present value. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each product including net revenues, cost of sales, R&D costs, selling and marketing costs, the appropriate discount rate to select in order to measure the risk inherent in each future cash flow stream, the assessment of each asset’s life cycle, and competitive trends impacting the asset and each cash flow stream. The Company also uses the income approach, as described above, to determine the estimated fair value of certain other identifiable intangible assets including customer relationships, trade names and business licenses. Customer relationships represent established relationships with customers, which provide a ready channel for the sale of additional products and services. Trade names represent acquired company and product names.
IPR&D acquired in a business combination is capitalized as an indefinite-lived intangible asset. Development costs incurred after the acquisition are expensed as incurred. Upon receipt of regulatory approval, the indefinite-lived intangible asset is then accounted for as a finite-lived intangible asset and amortized on a straight-line basis or accelerated basis, as appropriate, over its estimated useful life. If the research and development project is subsequently abandoned, the indefinite-lived intangible asset is charged to expense. IPR&D acquired outside of a business combination is expensed immediately.
Due to the uncertainty associated with research and development projects, there is risk that actual results will differ materially from the original cash flow projections and that the research and development project will result in a successful commercial product. The risks associated with achieving commercialization include, but are not limited to, delay or failure to obtain regulatory approvals to conduct clinical trials, delay or failure to obtain required market clearances, delays or issues with patent
issuance, or validity and litigation.
If the acquired net assets do not constitute a business under the acquisition method of accounting, the transaction is accounted for as an asset acquisition and no goodwill is recognized. In an asset acquisition, the amount allocated to acquired IPR&D with no alternative future use is charged to expense at the acquisition date. Payments that would be recognized as contingent consideration in a business combination are expensed when probable in an asset acquisition. Refer to Note 4, Acquisitions and Divestituresfor more information.
GOODWILL AND OTHER INTANGIBLE ASSETS
The excess of the cost over the fair value of net assets of acquired businesses is recorded as goodwill. Goodwill is not subject to amortization but is reviewed for impairment at the reporting unit level annually, or more frequently if impairment indicators arise. The Company's assessment of the recoverability of goodwill is based upon a comparison of the carrying value of goodwill with its estimated fair value. The Company reviews goodwill for impairment in the third quarter every year in accordance with ASC Topic 350, Intangibles - Goodwill and Other ("ASC Topic 350") and whenever events or changes in circumstances indicate the carrying value of goodwill may not be recoverable. Refer to Note 7, Goodwill and Other Intangibles for more information.
The Company has two reportable segments with three underlying reporting units. Refer to Note 16, Segment and Geographic Information for more information on reportable segments.
Other intangible assets include patents, trademarks, purchased technology, and supplier and customer relationships. Identifiable intangible assets are initially recorded at fair market value at the time of acquisition generally using an income or cost approach. The Company capitalizes costs incurred to renew or extend the term of recognized intangible assets and amortizes those costs
over their expected useful lives.
F-11
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Product rights and other definite-lived intangible assets are tested periodically for impairment in accordance with ASC 350 Topic when events or changes in circumstances indicate that an asset's carrying value may not be recoverable. The impairment testing involves comparing the carrying amount of the asset or asset group to the forecasted undiscounted future cash flows. In the event the carrying value of the asset exceeds the undiscounted future cash flows, the carrying value is considered not recoverable and impairment exists. An impairment loss is measured as the excess of the asset's carrying value over its fair value, calculated using discounted future cash flows. The computed impairment loss is recognized in the period that the impairment occurs.
LONG-LIVED ASSETS
Long-lived assets held and used by the Company, including property, plant and equipment, intangible assets, and leases are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. For purposes of evaluating the recoverability of long-lived assets to be held and used, a recoverability test is performed using projected undiscounted net cash flows applicable to the long-lived assets. If an impairment exists, the amount of such impairment is calculated based on the estimated fair value of the asset. Impairments to long-lived assets to be disposed of are recorded based upon the difference between the carrying value and the fair value of the applicable assets.
INTEGRA FOUNDATION
The Company may periodically make contributions to the Integra Foundation, Inc. The Integra Foundation was incorporated in 2002 exclusively for charitable, educational, and scientific purposes and qualifies under IRC 501(c)(3) as an exempt private foundation. Under its charter, the Integra Foundation engages in activities that promote health, the diagnosis and treatment of disease, and the development of medical science through grants, contributions and other appropriate means. The Integra Foundation is a separate legal entity and is not a subsidiary of the Company; therefore, its results are not included in these consolidated financial statements. The Company contributed $0.3$0.0 million, $0.8$1.2 million and $0.5$0.8 million to the Integra Foundation during the years ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively. These contributions were recorded in selling, general, and administrative expense.
DERIVATIVES
The Company develops, manufactures, and sells medical devices globally and its earnings and cash flows are exposed to market risk from changes in interest rates and currency exchange rates. The Company addresses these risks through a risk management program that includes the use of derivative financial instruments and operates the program pursuant to documented corporate risk management policies. All derivative financial instruments are recognized in the financial statements at fair value in accordance with the authoritative guidance. Under the guidance, for those instruments that are designated and qualify as hedging instruments, the hedging instrument must be designated as a fair value hedge, cash flow hedge, or a hedge of a net investment in a foreign operation, based on the exposure being hedged. The accounting for changes in the fair value of a derivative instrument depends on whether it has been designated and qualifies as part of a hedging relationship and, further, on the type of hedging relationship. The Company's derivative instruments do not subject its earnings or cash flows to material risk, and gains and losses on these derivatives generally offset losses and gains on the item being hedged. The Company has not entered into derivative transactions for speculative purposes and frompurposes. From time to time, the Company may enter into derivatives that are not designated as hedging instruments in order to protect itself from currency volatility due to intercompany balances.
All derivative instruments are recognized at theirthe fair values as either assets or liabilities on the balance sheet. The Company determines the fair value of its derivative instruments using the framework prescribed by the authoritative guidance, by considering the estimated amount the Company would receive to sell or transfer these instruments at the reporting date and by taking into account:account expected forward interest rates, currency exchange rates, the creditworthiness of the counterparty for assets, and its creditworthiness for liabilities. In certain instances, the Company utilizes a discounted cash flow model to measure fair value. Generally, the Company uses inputs that include quoted prices for similar assets or liabilities in active markets, other observable inputs for the asset or liability and inputs derived principally from, or corroborated by, observable market data by correlation or other means. The Company has classified all of its derivative assets and liabilities within Level 2 of the fair value hierarchy because observable inputs are available for substantially the full term of its derivative instruments. The Company classifies derivatives designated as hedges in the same category as the item being hedged for cash flow presentation purposes.
F-12
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company entered into a foreign currency forward contractand foreign currency swap contracts that isare not designated as a hedging instrumentinstruments for accounting purposes. This contract isThese contracts are recorded at fair value, with the changes in fair value recognized into other income, net, on the consolidated financial statements. Refer to Note 6, Derivative Instruments for more information.
FOREIGN CURRENCY
All assets and liabilities of foreign subsidiaries which have a functional currency other than the U.S. dollar are translated at the rate of exchange at year-end, while elements of the income statement are translated at the average exchange rates in effect during the year. The net effect of these translation adjustments is shown as a component of accumulated other comprehensive income (loss). These currency translation adjustments are not currently adjusted for income taxes as they relate to permanent investments in non-U.S. subsidiaries. Foreign currency transaction lossnet losses of $0.3$3.3 million, $1.7net gains of less than $0.1 million, and $2.9net losses $1.6 million are reported in other income, net in the statements of operations, for the year ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively.
INCOME TAXES
Income taxes are accounted for by using the asset and liability method. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis. A valuation allowance is provided when it is more likely than not that some portion
or all of the deferred tax assets will not be realized. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period when the change is enacted.
The Company recognizes a tax benefit from an uncertain tax position only if it is more likely than not to be sustained upon examination based on the technical merits of the position. Reserves are established for positions that don't meet this recognition threshold. The reserve is measured as the largest amount of benefit determined on a cumulative probability basis that the Company believes is more likely than not to be realized upon ultimate settlement of the position. These reserves are classified as long-term liabilities in the consolidated balance sheets of the Company, unless the reserves are expected to be paid in cash during the next twelve months, in which case they are classified as current liabilities. The Company also records interest and penalties accrued in relation to uncertain tax benefits as a component of income tax expense.
While the Company believes it has identified all reasonably identifiablereasonable exposures and the reserve it has established for identifiable exposures is appropriate under the circumstances, it is possible that additional exposures exist and that exposures may be settled at amounts different than the amounts reserved. It is also possible that changes in facts and circumstances could cause the Company to either materially increase or reduce the carrying amount of its tax reserve.
The Company continues to indefinitely reinvest substantially all of its foreign earnings.earnings unless there is a manner under which to remit the earnings without a material tax cost. The current provisional analysis indicates that the Company has sufficient U.S. liquidity, including borrowing capacity, to fund foreseeable U.S. cash needs without requiring the repatriation of foreign cash. The Tax Cuts and Jobs Act (the “2017 Tax Act”), enacted in December 2017, imposed a toll tax on a deemed repatriation of undistributed earnings of foreign subsidiaries. One time or unusual items that may impact the ability or intent to keep the foreign earnings and cash indefinitely reinvested include significant U.S. acquisitions, loans from a foreign subsidiary and changes in tax laws.
REVENUE RECOGNITION
Revenue is recognized upon the transfer of control of promised products or services to the customers in an amount that reflects the consideration the Company expects to receive in exchange for those products and services.
Total revenue, net, includes product sales, product royalties and other revenues, such as fees received from services.
For products shipped with FOB shipping point terms, the control of the product passes to the customer at the time of shipment. For shipments in which the control of the product is transferred when the customer receives the product, the Company recognizes revenue upon receipt by the customer. Certain products that the Company produces for private label customers have no alternative use and the Company has a right of payment for performance to date. Revenues from those products are recognized over the period that the Company manufactures these products, which is typically one month to three months. The Company uses the input method to measure the manufacturing activities completed to date, which depicts the progress of the Company's performance obligation of transferring control of goods being manufactured for private label customers.
A portion of the Company's product revenue is generated from consigned inventory maintained at hospitals and distributors, and also from inventory physically held by field sales representatives. For these types of products sales, the Company retains control until the product has been used or implanted, at which time revenue is recognized.
F-13
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Revenues from sale of products and services are evidenced by either a contract with the customer or a valid purchase order and an invoice which includes all relevant terms of sale. For product sales, invoices are generally issued upon the transfer of control (or upon the completion of the manufacturing in the case of the private label transactions recognized over time) and are typically payable 30 days after the invoice date. The Company performs a review of each specific customer's creditworthiness and ability to pay prior to acceptance as a customer. Further, the Company performs periodic reviews of its customers' creditworthiness prospectively. Refer to Note 3, Revenue From Contracts With Customers for more information. The Company also maintains a provision for estimated returns and allowances in the same period that the related revenue is recorded. This reserve is based upon an analysis of actual credit memos issued for pricing issues or returned goods over an extended period, as well as assumptions about outstanding accounts receivable and judgment in interpreting the data.
RESEARCH AND DEVELOPMENT
Research and development costs, including salaries, depreciation, consultant and other external fees, and facility costs directly attributable to research and development activities, are expensed in the period in which they are incurred.
EMPLOYEE TERMINATION BENEFITS
The Company does not have a written severance plan, and it does not offer similar termination benefits to affected employees in all restructuring initiatives. Accordingly, in situations where minimum statutory termination benefits must be paid to the affected employees, the Company records employee severance costs associated with these restructuring activities in accordance with the authoritative guidance for non-retirement post-employment benefits. Charges associated with these activities are recorded when the payment of benefits is probable and can be reasonably estimated. In all other situations where the Company pays out termination benefits, including supplemental benefits paid in excess of statutory minimum amounts and benefits offered to affected employees based on management's discretion, the Company records these termination costs in accordance with the authoritative guidance for ASC Topic 712 Compensation-NonretirementCompensation - Nonretirement Benefits and ASC Topic 420 One-time Employee Termination Benefits.
The timing of the recognition of charges for employee severance costs other than minimum statutory benefits depends on whether the affected employees are required to render service beyond their legal notification period in order to receive the benefits. If affected employees are required to render service beyond their legal notification period, charges are recognized over the future service period. Otherwise, charges are recognized when management has approved a specific plan and employee communication requirements have been met.
The Company incurred employee termination costs on restructuring activities associated with a closure of a manufacturing facility in France and other reorganization projects in the consolidated statement of operations for the year ended December 31, 2022. In 2021, the Company incurred employee termination costs on restructuring activities associated with the closure of a manufacturing facility in France. The following table summarizes our restructuring related accrual balances included within accrued expenses and other current liabilities in the consolidated balance sheet for the year ended December 31, 2022 and 2021.
| Years Ended December 31, | ||||||||||||||
| (Dollars in thousands) | 2022 | 2021 | ||||||||||||
| Balance, beginning of the year | $ | 10,226 | $ | 6,372 | ||||||||||
| Charges: | ||||||||||||||
| Cost of Goods Sold | $ | 1,494 | $ | 3,436 | ||||||||||
| Research and development | 72 | 288 | ||||||||||||
| Selling, general and administrative | $ | 5,582 | $ | 466 | ||||||||||
| Payments and other adjustments | $ | (12,267) | $ | (336) | ||||||||||
| Balance, end of the year | $ | 5,107 | $ | 10,226 | ||||||||||
Included in the accrual balance as of December 31, 2022 is $2.0 million related to the closure of a manufacturing facility located in France, and other reorganization projects of $3.1M.Included in the accrual balance as of December 31, 2021 is $10.2 million related to the closure of the manufacturing facility located in France.
STOCK-BASED COMPENSATION
F-14
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
PENSION BENEFITS
The Company maintains defined benefit pension plans that cover certain employees in Austria, France, Japan, Germany and Switzerland. Various factors are considered in determining the pension liability, including the number of employees expected to be paid their salary levels and years of service, the expected return on plan assets, the discount rate used to determine the benefit obligations, the timing of benefit payments and other actuarial assumptions.
Retirement benefit plan assumptions are reassessed on an annual basis or more frequently if changes in circumstances indicate a re-evaluation of assumptions are required. The key benefit plan assumptions are the discount rate and expected rate of return on plan assets. The discount rate is based on average rates on bonds that matched the expected cash outflows of the benefit plans. The expected rate of return is based on historical and expected returns on the various categories of plan assets.
The Company uses the corridor approach in measuring the amount of net periodic benefit pension cost to recognize each period. The corridor approach defers all actuarial gains and losses resulting from variances between actual results and actuarial assumptions. Those unrecognized gains and losses are amortized when the net gains and losses exceed 10% of the greater of the market-related value of plan assets or the projected benefit obligation at the beginning of the year. The amount in excess of the corridor is amortized over the average remaining service period to retirement date of active plan participants.
Deferred Compensation Plan
The Company maintains a deferred compensation plan in which certain employees of the Company may defer the payment and taxation of up to 75% of their base salary and up to 100% of bonus amounts and other eligible cash compensation.
This deferred compensation is invested in funds offered under the Plan and is valued based on Level 1 measurements in the fair value hierarchy. The purpose of the plan is to retain key employees by providing them with an opportunity to defer a portion of their compensation as elected by the participant in accordance with the plan. Any amounts set aside to defray the liabilities assumed by the Company will remain the general assets of the Company until such amounts are distributed to the participants. Assets of the Company's deferred compensation plan are included in Other current assets and recorded at fair value based on their quoted market prices.
CONCENTRATION OF CREDIT RISK
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of cash and cash equivalents, which are held at major financial institutions, investment-grade marketable debt securities and trade receivables.
The Company's products are sold on an uncollateralized basis and on credit terms based upon a credit risk assessment of each customer. A portion of the Company's trade receivables to customers outside the United States includes sales to foreign distributors, who then sell to government owned or supported healthcare systems.
None of the Company's customers accounted for 10% or more of the consolidated net sales during the years ended December 31, 2019, 20182022, 2021 and 2017.2020.
RECENT ACCOUNTING PRONOUNCEMENTS
In December 2019, the FASB issued ASU No. 2019-12,Income Taxes:Taxes: Simplifying the Accounting for Income Taxes, intended to simplify the accounting for income taxes by eliminating certain exceptions related to the approach for intraperiodintra-period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. The newThis guidance also simplifies aspects of the accounting for franchise taxes and enacted changes in tax laws or rates and clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. The standard is effective for annual periods beginning after December 15, 2020 and interim periods within, with early adoption permitted. The Company adopted ASU 2019-12 as of January 1, 2021. Adoption of the standard requires certain changes to be made prospectively, with some changes to be made retrospectively. The adoption of this guidance did not have a significant impact on the Company's consolidated financial statements and related disclosures.
F-15
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
In March 2020, the FASB issued ASU 2020-04, Reference Rate Reform (Topic 848), and subsequent amendment to the initial guidance: ASU 2021-01, Reference Rate Reform (Topic 848): Scope (collectively, “Topic 848”). Topic 848 provides optional expedients and exceptions for applying GAAP to contracts, hedging relationships, and other transactions affected by reference rate reform if certain criteria are met. The amendments apply only to contracts, hedging relationships, and other transactions that reference London Inter-Bank Offered Rate ("LIBOR") or another reference rate expected to be discontinued because of reference rate reform. The guidance generally can be applied from March 12, 2020 through December 31, 2022. On October 5, 2022, the FASB approved an extension of the sunset date of the reference rate reform from December 31, 2022 to December 31, 2024, past LIBOR’s end date. The Company currently has contracts that are indexed to LIBOR and are continuing to evaluate the scope of impacted contracts and potential risk. The Company expects all LIBOR-based contracts to be replaced by the Secured Overnight Financing Rate (“SOFR”), which is calculated based on overnight transactions under repurchase agreements backed by Treasury securities. The Alternative Reference Rates Committee, a group of private-market participants convened by the U.S. Federal Reserve Board and the New York Federal Reserve, has recommended the use of SOFR as a more robust reference rate alternative to LIBOR. The use of SOFR as a substitute for LIBOR is, however, voluntary and may not be suitable for all market participants. There can be no assurance that the replacement rate will be economically equivalent to LIBOR, which could result in higher interest rates for us under our debt facilities. There is no guarantee that a transition from LIBOR to SOFR will not result in financial market disruptions, significant increases in benchmark rates, or our borrowing costs, any of which could have an adverse effect on our business, results of operations and financial condition.
In August 2020, the FASB issued ASU 2020-06, Debt- Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity's Own Equity (Subtopic 815-40):Accounting for Convertible Instruments and Contracts in an Entity's Own Equity. The guidance simplifies accounting for convertible instruments by removing major separation models required under current GAAP. Consequently, more convertible debt instruments will be reported as a single liability instrument with no separate accounting for embedded conversion features. The ASU removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception, which will permit more equity contracts to qualify. The guidance also simplifies the diluted net income per share calculation in certain areas. The ASU will be effective for annual and interim periods beginning after December 15, 2021, and early adoption is permitted for fiscal years beginning after December 15, 2020, and interim periods within those fiscal years using either the modified retrospective or full retrospective method.
As detailed in Note 5, Debt, on February 4, 2020, the Company issued $575.0 million aggregate principal amount of its 0.5% Convertible Senior Notes due 2025 (the "2025 Notes"). The 2025 Notes are subject to the guidance included in ASU 2020-06. The Company adopted this guidance on January 1, 2021 using the modified retrospective approach which resulted in a cumulative-effect adjustment that increased (decreased) the following consolidated balance sheet accounts:
| ADJUSTMENT | CONSOLIDATED BALANCE SHEET CLASSIFICATION | AMOUNT (in millions) | ||||||||||||
| Deferred tax impact of cumulative-effect adjustment | Deferred tax liabilities | $ | (20.6) | |||||||||||
| Debt discount reclassification | Long-term convertible securities | 89.1 | ||||||||||||
| Equity issuance costs reclassification | Long-term convertible securities | (2.5) | ||||||||||||
| Debt discount amortization and equity costs reclassification, net of tax | Retained Earnings | (2.8) | ||||||||||||
| Net impact of cumulative-effect adjustment | Additional paid-in capital | (63.3) | ||||||||||||
On December 9, 2020, the Company made an irrevocable election under the indenture to require the principal portion of its 2025 Notes to be settled in cash and any excess in shares. Following the irrevocable notice, only the amounts settled in excess of the principal will be considered in diluted earnings per share under the “if-converted” method. Upon adoption of ASU 2020-06, the Company’s 2025 Notes were reflected entirely as a liability since the embedded conversion feature will no longer be separately presented within stockholders’ equity. Additionally, from January 1, 2021, the Company is currently assessingno longer incurring non-cash interest expense for the impactamortization of debt discount.
In October 2020, the FASB issued ASU 2020-10, Codification Improvements, which updates various codification topics by clarifying or improving disclosure requirements to align with the regulations of the U.S. Securities and Exchange Commission (the "SEC") . The ASU has been effective for the Company for annual and interim periods beginning after January 1, 2021. The Company adopted this standard on the January 1, 2021. The adoption of this guidance did not have a significant impact on the Company's consolidated financial conditionstatements and resultsrelated disclosures.
F-16
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
In May 2021, the FASB issued ASU 2021-04, Earnings Per Share (Topic 260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718), and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Issuer’s Accounting for Certain Modifications or Exchanges of operations.Freestanding Equity-Classified Written Call Options which provides guidance to clarify and reduce diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options (for example, warrants) that remain equity classified after modification or exchange. The amendments in this ASU No. 2021-04 are effective for all entities for fiscal years beginning after December 15, 2021, and interim periods within those fiscal years, with early adoption permitted, including interim periods within those fiscal years. The amendment currently has no impact to the Company as the effect will largely depend on the terms of written call options or financings issued or modified in the future.
There are no other recently issued accounting pronouncements that are expected to have any significant effect on the Company's financial position, results of operations or cash flows.
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION
Cash paid for interest during the years ended December 31, 2019, 20182022, 2021 and 20172020 was $48.9$42.2 million (net of $3.1$1.4 million that was capitalized into construction in progress), $58.3$43.2 million (net of $1.2 million that was capitalized into construction in progress) and $47.3 million (net of $2.3 million that was capitalized into construction in progress) and $32.3 million (net of $1.1 million that was capitalized into construction in progress), respectively.
Cash paid for income taxes, net of refunds, for the years ended December 31, 2019, 20182022, 2021 and 20172020 was $16.2$35.9 million, $10.4$49.5 million and $14.6$29.8 million, respectively.
NON-CASH INVESTING AND FINANCING ACTIVITIES
Property and equipment purchases included in liabilities at December 31, 2022, 2021 and 2020 were $10.5 million, $4.7 million and $1.6 million, respectively.
During the fourth quarter of 2019,2021, the Company achieved the firstits final developmental milestone which triggered a $5.0 million obligation to be paid to former shareholders of Rebound. In addition, theRebound Therapeutics Corporation ("Rebound"). The Company recorded $5.0 million as in-process research and development expensean intangible asset in the consolidated statements of operations.balance sheet upon achieving the milestone. The remaining obligation was included in accrued liabilities at December 31, 20192021 in the consolidated balance sheets. The milestone was fully paid in 2022.
During the fourth quarter of 2020, the Company achieved another developmental milestone which triggered a $20.0 million obligation to be paid to the former shareholders of Rebound. The Company recorded $20.0 million as an intangible asset in the consolidated balance sheet upon achieving the milestone. The milestone was paid during the firstfourth quarter of 2020.
3. REVENUES FROM CONTRACTS WITH CUSTOMERS
Summary of Accounting Policies on Revenue Recognition
Revenue is recognized upon the transfer of control of promised products or services to the customers in an amount that reflects the consideration the Company expects to receive in exchange for those products and services.
Performance Obligations
The Company's performance obligations consist mainly of transferring control of goods and services identified in the contracts, purchase orders, or invoices. The Company has no significant multi-element contracts with customers.
Significant Judgments
Usage-based royalties and licenses are estimated based on the provisions of contracts with customers and recognized in the same period that the royalty-based products are sold by the Company's strategic partners. The Company estimates and recognizes royalty revenue based upon communication with licensees, historical information, and expected sales trends. Differences between actual reported licensee sales and those that were estimated are adjusted in the period in which they become known, which is typically the following quarter. Historically, such adjustments have not been significant.
The Company estimates returns, price concessions, and discount allowances using the expected value method based on historical trends and other known factors. Rebate allowances are estimated using the most likely method based on each customer contract.
The Company's return policy, as set forth in its product catalogs and sales invoices, requires review and authorization in advance prior to the return of product. Upon the authorization, a credit will be issued for the goods returned within a set amount of days from the shipment, which is generally ninety90 days.
F-17
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company disregards the effects of a financing component if the Company expects, at contract inception, that the period between the transfer and customer payment for the goods or services will be one year or less. The Company has no significant revenues recognized on payments expected to be received more than one year after the transfer of control of products or services to customers.
Contract Asset and Liability
Revenues recognized from the Company's private label business that are not invoiced to the customers as a result of recognizing revenue over time are recorded as a contract asset included in the prepaid expenses and other current assets account in the consolidated balance sheet. Upon invoicing to the customer, the balance is recorded in trade receivable, net in the consolidated balance sheet.
Other operating revenues may include fees received under service agreements. Non-refundable fees received under multiple-period service agreements are recognized as revenue as the Company satisfies the performance obligations to the other party. A portion of the transaction price allocated to the performance obligations to be satisfied in the future periods is recognized as contract liability.
The following table summarized the changes in the contract asset and liability balances for the year ended December 31, 2019:2022:
| Total | ||||
| (amounts in thousands) | ||||
| Contract Asset | ||||
| Contract asset, January 1, 2019 | $ | 4,193 | ||
Transferred to trade receivable of contract asset included in beginning of the year contract asset | (4,193 | ) | ||
| Contract asset, net of transferred to trade receivables on contracts during the period | 8,680 | |||
| Contract asset, December 31, 2019 | $ | 8,680 | ||
| Contract Liability | ||||
| Contract liability, January 1, 2019 | $ | 12,716 | ||
| Recognition of revenue included in beginning of year contract liability | (5,613 | ) | ||
| Contract liability, net of revenue recognized on contracts during the period | 4,872 | |||
| Foreign currency translation | (29 | ) | ||
| Contract liability, December 31, 2019 | $ | 11,946 | ||
| Dollars in thousands | Total | ||||
| Contract Asset | |||||
| Contract asset, January 1, 2022 | $ | 11,412 | |||
| Transferred to trade receivable from contract asset included in beginning of the year contract asset | (11,412) | ||||
| Contract asset, net of transferred to trade receivables on contracts during the period | 10,122 | ||||
| Contract asset, December 31, 2022 | $ | 10,122 | |||
| Contract Liability | |||||
| Contract liability, January 1, 2022 | $ | 11,946 | |||
| Recognition of revenue included in beginning of year contract liability | (5,349) | ||||
| Contract liability, net of revenue recognized on contracts during the period | 9,596 | ||||
| Foreign currency translation | (66) | ||||
| Contract liability, December 31, 2022 | $ | 16,127 | |||
At December 31, 2019,2022, the short-term portion of the contract liability of 4.8$7.3 million and the long-term portion of 7.1$8.8 million wereis included in accrued expenses and other current liabilities and other liabilities, respectively, in the consolidated balance sheet.
As of December 31, 2019,2022, the Company is expected to recognize revenue of approximately $4.8 million in 2020, $2.8 million in 2021, $1.9 million in 2022, $0.8$7.3 million in 2023, $0.5$4.2 million in 2024, $2.7 million in 2025, $1.1 million in 2026, $0.7 million in 2027, and $1.2$0.1 million thereafter.
Shipping and Handling Fees
The Company elected to account for shipping and handling activities as a fulfillment cost rather than a separate performance obligation. Amounts billed to customers for shipping and handling are included as part of the transaction price and recognized as revenue when control of underlying products is transferred to the customer. The related shipping and freight charges incurred by the Company are included in the cost of goods sold.
Product Warranties
Certain of the Company's medical devices, including monitoring systems and neurosurgical systems, are designed to operate over long periods of time. These products are sold with warranties which may extend for up to two years from the date of purchase. The warranties are not considered a separate performance obligation. The Company estimates its product warranties using the expected value method based on historical trends and other known factors. The Company includes them in accrued expenses and other current liabilities in the consolidated balance sheet.
Taxes Collected from Customers
The Company elected to exclude from the measurement of the transaction price all taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction and collected by the entity from a customer.
F-18
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Disaggregated Revenue
The following table presents revenues disaggregated by the major sources of revenues for years-ended December 31, 2019, 20182022, 2021 and 2017 (amounts2020 (dollar amounts in thousands):
| Year Ended December 31, 2022 | Year Ended December 31, 2021 | Year Ended December 31, 2020 | ||||||||||||||||||
| Neurosurgery | $ | 794,017 | $ | 802,959 | $ | 716,339 | ||||||||||||||
| Instruments | 225,547 | 222,273 | 178,492 | |||||||||||||||||
| Total Codman Specialty Surgical | 1,019,564 | 1,025,232 | 894,831 | |||||||||||||||||
Wound Reconstruction and Care(2)(3) | 406,689 | 392,463 | 293,038 | |||||||||||||||||
Extremity Orthopedics(1) | — | — | 78,316 | |||||||||||||||||
| Private Label | 131,413 | 124,753 | 105,683 | |||||||||||||||||
| Total Tissue Technologies | 538,102 | 517,216 | 477,037 | |||||||||||||||||
| Total revenue | $ | 1,557,666 | $ | 1,542,448 | $ | 1,371,868 | ||||||||||||||
| Year Ended December 31, 2019 | Year Ended December 31, 2018 | Year Ended December 31, 2017 | |||||||
| (amounts in thousands) | |||||||||
| Neurosurgery | 707,011 | 684,148 | 446,994 | ||||||
| Precision Tools and Instruments | 289,195 | $ | 279,781 | $ | 273,307 | ||||
| Total Codman Specialty Surgical | 996,206 | 963,929 | 720,301 | ||||||
| Wound Reconstruction | 322,739 | 311,565 | 274,398 | ||||||
| Extremity Orthopedics | 90,082 | 90,588 | 93,546 | ||||||
| Private Label | 108,530 | 106,359 | 99,991 | ||||||
| Total Orthopedics and Tissue Technologies | 521,351 | 508,512 | 467,935 | ||||||
| Total revenue | $ | 1,517,557 | $ | 1,472,441 | $ | 1,188,236 | |||
(3) On August 31, 2022, the Company completed the sale of its non-core traditional wound care ("TWC") business. See Note4, Acquisitions and Divestitures
See Note 16, Segment and Geographical Information, for details of revenues based on the location of the customer.
| Year Ended December 31, 2018 | ||||||
| As Reported | Excluding Impact of Topic 606 | |||||
| (Amounts in thousands) | ||||||
| Statement of Operations | ||||||
| Total revenue, net | $ | 1,472,441 | $ | 1,468,075 | ||
| Cost of goods sold | 571,496 | 570,028 | ||||
| Income tax benefit | (3,398 | ) | (4,119 | ) | ||
| Net income | 60,801 | 58,624 | ||||
4. ACQUISITIONS AND PRO FORMA RESULTSDIVESTITURES
On July 29, 2019,December 6, 2022, the Company acquired Arkis BioSciencescompleted its acquisition of Surgical Innovation Associates, Inc. ("Arkis"SIA") for an acquisition purchase price of $30.9$51.5 million. In addition to the purchase price, the acquisition includes two separate contingent considerations payments, which are dependent on 1) achieving certain revenue-based performance milestones in 2023, 2024, and 2025 (up to $50.0 million (the "Arkis Acquisition"in additional payments), as well as 2) the approval by the FDA of the Premarket Approval (“PMA”) plus contingent considerationApplication for DuraSorb for certain uses by certain timing targets (up to $40.0 million in additional payments). SIA's core technology, DuraSorb, is a fully resorbable scaffold of up to $25.5 million, that maya globally accepted polymer, which is cleared for use in hernia repair, abdominal wall, and other soft tissue reinforcement. DuraSorb sales will be payable based on the successful completionreported within Integra’s Tissue Technologies segment as part of certain developmentits Wound Reconstruction and commercial milestones. The contingent consideration had an acquisition date fair value of $13.1 million. Arkis was a privately-held company that marketed the CerebroFlo® external ventricular drainage (EVD) catheter with Endexo® technology, a permanent additive designed to reduce the potential for catheter obstruction due to thrombus formation.Care franchise.
Assets Acquired and Liabilities Assumed at Fair Value
The ArkisSIA Acquisition has been accounted for using the acquisition method of accounting. This method requires that assets acquired, and liabilities assumed in a business combination to be recognized at their fair values as of the acquisition date. As of December 31, 2019, certain amounts relating to tax related matters have not been finalized. The finalization of these matters could result in changes to goodwill.
F-19
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following table summarizes the fair values of the assets acquired and liabilities assumed at the acquisition date:
| Preliminary Valuation as of December 31, 2019 | Weighted Average Life | |||
| (Dollars in thousands) | ||||
| Cash | $ | 90 | ||
| Other current assets | 751 | |||
| Property, plant and equipment | 159 | |||
| Deferred Tax Assets | 1,535 | |||
| Intangible assets: | ||||
| CerebroFlo developed Technology | 20,100 | 15 years | ||
| Enabling technology license | 1,980 | 14 years | ||
| Goodwill | 27,600 | |||
| Total assets acquired | 52,215 | |||
| Accounts Payable, accrued expenses and other liabilities | 2,926 | |||
| Contingent consideration | 13,100 | |||
| Deferred tax liabilities | 5,305 | |||
| Net assets acquired | $ | 30,884 | ||
| Dollars in thousands | Valuation as of December 6, 2022 | Weighted Average Life | ||||||||||||||||||
| Current assets: | ||||||||||||||||||||
| Cash | $ | 4,438 | ||||||||||||||||||
| Trade accounts receivable, net | 1,551 | |||||||||||||||||||
| Inventories, net | 2,900 | |||||||||||||||||||
| Prepaid expenses and other current assets | 1,654 | |||||||||||||||||||
| Total current assets | $ | 10,543 | ||||||||||||||||||
| Intangible assets | 75,000 | 14 years | ||||||||||||||||||
| Goodwill | 41,854 | |||||||||||||||||||
| Total assets acquired | $ | 127,397 | ||||||||||||||||||
| Current liabilities: | ||||||||||||||||||||
| Accounts payable and accrued expenses | $ | 2,044 | ||||||||||||||||||
| Total current liabilities | $ | 2,044 | ||||||||||||||||||
| Deferred Tax Liability | 11,799 | |||||||||||||||||||
| Contingent consideration | 57,607 | |||||||||||||||||||
| Total liabilities assumed | 71,450 | |||||||||||||||||||
| Net assets acquired | $ | 55,947 | ||||||||||||||||||
Developed Technology
The estimated fair value of the intangible assetsdeveloped technology was determined using the multi-period excess earnings method of the income approach, which is a valuation technique that provides an estimate ofestimates value based on the fairpresent value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life.future economic benefits. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each asset (includingproduct including net revenues, cost of sales, R&D costs, selling and marketing costs, working capital, and working capital/contributory asset charges),charges, the appropriate discount rate to select in order to
measure the risk inherent in each future cash flow stream, the assessment of eachthe asset’s life cycle, and competitive trends impacting the asset and eachthe cash flow stream.
The Company used a discount rate of 14.5%18% to arrive at the present value for the acquired intangible assets to reflect the rate of return a market participant would expect to earn and incremental commercial uncertainty in the cash flow projections. No assurances can be given that the underlying assumptions used to prepare the discounted cash flow analysis will not change. For these and other reasons, actual results may vary significantly from estimated results.
Goodwill
The Company allocated goodwill related to the ArkisSIA Acquisition to the Codman Specialty Surgical segment. Goodwill is the excess of the consideration transferred over the net assets recognized and represents the expected revenue and cost synergies of the combined company and assembled workforce. One of the key factors that contributes to the recognition of goodwill, and a driver for the Company's acquisition of Arkis, is the planned expansion of the Endexo technology with the existing products within the Codman Specialty Surgical segment. Goodwill recognized as a result of this acquisition is non-deductible for income tax purposes.
| Final Valuation | Weighted Average Life | ||||
| (Dollars in thousands) | |||||
| Inventory | 74,962 | ||||
| Assets held for sale | 30,813 | ||||
| Other current assets | 8,202 | ||||
| Property, plant and equipment | 41,339 | ||||
| Intangible assets: | |||||
| Codman corporate trade name | 162,900 | Indefinite | |||
| Completed technology | 375,200 | 22 years | |||
| Goodwill | 342,322 | ||||
| Total assets acquired | 1,035,738 | ||||
| Accrued expenses | 1,730 | ||||
| Pension liabilities | 19,917 | ||||
| Net assets acquired | $ | 1,014,091 | |||
| Final Valuation | Weighted Average Life | ||||
| (Dollars in thousands) | |||||
| Cash and cash equivalents | $ | 16,512 | |||
| Short-term investments | 19,238 | ||||
| Accounts receivable | 8,949 | ||||
| Inventory | 17,977 | ||||
| Prepaid expenses and other current assets | 4,369 | ||||
| Property, plant and equipment | 4,311 | ||||
| Intangible assets: | |||||
| Customer relationship | 78,300 | 14 years | |||
| Trademarks/brand names | 13,500 | 15 years | |||
| Completed technology | 11,600 | 14 years | |||
| Non-compete agreement | 280 | 1 year | |||
| Goodwill | 73,765 | ||||
| Deferred tax assets | 14,524 | ||||
| Other assets | 101 | ||||
| Total assets acquired | 263,426 | ||||
| Accounts payable | 4,560 | ||||
| Accrued expenses and other current liabilities | 7,409 | ||||
| Contingent liability | 37,174 | ||||
| Other liabilities | 3,805 | ||||
| Net assets acquired | $ | 210,478 | |||
Contingent Consideration
The Company assumeddetermines the acquisition date fair value of contingent consideration incurred by Derma Sciences, Inc. ("Derma Sciences") relatedobligations based on a probability-weighted income approach derived from revenue estimates and a probability assessment with respect to its acquisitionsthe likelihood of BioDachieving contingent obligations. The fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement as defined using the intellectual property related to Medihoney products.fair value concepts in ASC 820. The Company accounted forresulting most likely payouts are discounted using an appropriate effective annual interest rate. At each reporting date, the contingent liabilities by recording theirconsideration obligation will be revalued to estimated fair value onand changes in fair value will be reflected as income or expense in the dateconsolidated statement of operations. Changes in the fair value of the contingent considerations may result from changes in discount periods and rates and changes in the timing and amount of revenue estimates. Changes in assumptions utilized in the contingent consideration fair value estimates could result in an increase in the contingent consideration obligation and a corresponding charge to operating results.
As part of the acquisition, based on a probability weighted income approach. Thethe Company has paid $33.3 million relatedis required to pay to the aforementioned contingent liabilities. NaN contingent liability remainsshareholder of SIA up to $90.0 million for two separate payments, which relatesare dependent on 1) achieving certain revenue-based performance milestones in 2023, 2024, and 2025 (up to net sales of Medihoney products exceeding certain amounts defined$50.0 million in additional payments), as well as 2) the agreement between the Company and Derma Sciences. The potential maximum undiscounted payment amounts to $3.0 million. The estimated fair value as of December 31, 2019 and December 31, 2018 was $0.2 million included in other liabilities, in the consolidated balance sheets.
| Year Ended December 31, 2017 | |||
| (Pro forma) | |||
| (In thousands except per share amounts) | |||
| Total revenue from continuing operations | $ | 1,428,491 | |
| Net income from continuing operations | $ | 81,730 | |
| Basic earnings per share from continuing operations | $ | 1.06 | |
F-20
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Deferred Tax Liabilities
Deferred tax liabilities result from identifiable intangible assets’ fair value adjustments. These adjustments create excess book basis over tax basis which is tax-effected by the Federal Trade Commission and the antitrust authoritystatutory tax rates of Spain.applicable jurisdictions.
Sale of non-core traditional wound care business
On October 6, 2017,August 31, 2022, the Company completed its sale of its non-core traditional wound care ("TWC") business to Gentell, LLC ("Gentell") for $28.8 million, which consists of $27.8 million in cash plus $1.0 million in contingent consideration which may be received upon achieving certain revenue-based performance milestones two years after the terms and subject toclosing date. The proceeds from the conditionssale of the Divestiture Agreement,TWC business of $27.8 million is presented in the Divestiture was completedconsolidated statement of cash flows net of cash transferred of $3.5 million and Natus paid an aggregate purchase price of $46.4 million.
| Inventories | $ | 8,348 | |
| Prepaid expenses and other current assets | 36 | ||
| Assets held for sale | 30,813 | ||
| Property, plant and equipment, net | 1,122 | ||
| Goodwill | 2,861 | ||
| Total assets divested | $ | 43,180 | |
| Deferred revenue | $ | 1,082 | |
| Accrued compensation | 209 | ||
| Total liabilities divested | $ | 1,291 | |
The transitional supply agreement with Natus requires the Company to provide to Natus certain assets defined in the transitional supply agreement upon termination. The Company recognized a liability of $1.3 million, included in other liabilities in consolidated balance sheet, related to estimated cost of assets to be provided to Natus upon termination of transitional supply agreement.
On January 4, 2021, the Company completed the sale of its Extremity Orthopedics business to Smith & Nephew USD Limited ("Smith & Nephew"). The transaction included the sale of the Company's upper and lower Extremity Orthopedics product portfolio, including ankle and shoulder arthroplasty and hand and wrist product lines. The Company received an aggregate purchase price of $240.0 million from Smith & Nephew and concurrently paid $41.5 million to the Consortium of Focused Orthopedists, LLC ("CFO") effectively terminating the licensing agreement between Integra and CFO relating to the development of shoulder arthroplasty products.
F-21
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Assets and liabilities divested consisted of the following as of December 31, 2020 (dollar amounts in thousands):
| Prepaid expenses and other current assets | $ | 713 | |||
| Right of use asset - operating leases and Other assets | 3,186 | ||||
| Deferred tax assets | 6,589 | ||||
| Intangible assets, net | 13,332 | ||||
| Property, plant and equipment, net | 37,893 | ||||
| Goodwill | 47,546 | ||||
| Inventories | 52,845 | ||||
| Total assets held for sale | $ | 162,104 | |||
| Other liabilities | $ | 336 | |||
| Current portion of lease liability - operating leases | 539 | ||||
| Accrued compensation | 1,767 | ||||
| Deferred tax liabilities | 3,440 | ||||
| Lease liability - operating leases | 5,669 | ||||
| Total liabilities held for sale | $ | 11,751 | |||
The divestiture did not represent a strategic shift that had a major effect on the Company's operations and financial statements. Goodwill was allocated to the assets and liabilities divested using the relative fair value method of the Extremity Orthopedics business to the Company's Tissue Technologies reporting unit. In connection with the sale, the Company recognized a gain of $41.8 million that is presented in Gain from the sale of business in the consolidated statement of operations for the year ended December 31, 2021. The Company finalized the net working capital to Smith & Nephew as of December 31, 2021.
The Company also entered into a transition services agreement ("TSA") with Smith & Nephew which requires the Company to provide certain services on behalf of Smith & Nephew for the duration of the period subsequent to the sale of the business as defined in the TSA. The Company recognized a payable due to Smith & Nephew of $2.3 million as of December 31, 2022, which is included in the consolidated balance sheet within accrued expenses and other current liabilities. The TSA includes services such as invoicing and cash collections from customers on behalf of Smith & Nephew. As of December 31, 2022, the Company has concluded the majority of the transition services agreement, pending final payment.
ACell, Inc. Acquisition
On January 20, 2021, the Company acquired ACell, Inc. (the "ACell Acquisition") for an acquisition purchase price of $306.9 million plus contingent considerations of up to $100 million, that may be payable upon achieving certain revenue-based performance milestones in 2022, 2023 and 2025. The final working capital adjustments of $1.3 million was finalized and paid as of June 30, 2021. ACell was a privately-held company that offered a portfolio of regenerative products for complex wound management, including developing and commercializing products based on MatriStem Urinary Bladder Matrix, a technology platform derived from porcine urinary bladder extracellular matrix.
Assets Acquired and Liabilities Assumed at Fair Value
The ACell Acquisition has been accounted for using the acquisition method of accounting. This method requires that assets acquired and liabilities assumed in a business combination are recognized at their fair values as of the acquisition date.
F-22
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following table summarizes the final fair values of the assets acquired and liabilities assumed at the acquisition date:
| Dollars in thousands | Final Valuation | Weighted Average Life | ||||||||||||||||||
| Current assets: | ||||||||||||||||||||
| Cash | $ | 2,726 | ||||||||||||||||||
| Trade accounts receivable, net | 16,469 | |||||||||||||||||||
| Inventories, net | 18,299 | |||||||||||||||||||
| Prepaid expenses and other current assets | 1,498 | |||||||||||||||||||
| Total current assets | $ | 38,992 | ||||||||||||||||||
| Property, plant and equipment, net | 13,769 | |||||||||||||||||||
| Intangible assets | 245,000 | 13-14 years | ||||||||||||||||||
| Goodwill | 94,147 | |||||||||||||||||||
| Right of use asset - operating leases | 9,259 | |||||||||||||||||||
| Deferred tax assets | 7,465 | |||||||||||||||||||
| Other assets | 148 | |||||||||||||||||||
| Total assets acquired | $ | 408,780 | ||||||||||||||||||
| Current liabilities: | ||||||||||||||||||||
| Accounts payable | $ | 718 | ||||||||||||||||||
| Accrued expenses | 5,966 | |||||||||||||||||||
| Current portion of lease liability - operating leases | 1,673 | |||||||||||||||||||
| Total current liabilities | $ | 8,357 | ||||||||||||||||||
| Other long-term liability | 276 | |||||||||||||||||||
| Lease liability - operating leases | 7,585 | |||||||||||||||||||
| Deferred tax liability | 61,724 | |||||||||||||||||||
| Contingent consideration | 23,900 | |||||||||||||||||||
| Total liabilities assumed | 101,842 | |||||||||||||||||||
| Net assets acquired | $ | 306,938 | ||||||||||||||||||
Intangible Assets
The estimated fair value of the developed technology acquired was determined using the multi-period excess earnings method of the income approach, which estimates value based on the present value of future economic benefits. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each product including net revenues, cost of sales, R&D costs, selling and marketing costs, the appropriate discount rate to select in order to measure the risk inherent in each future cash flow stream, the assessment of each asset’s life cycle, and competitive trends impacting the asset and each cash flow stream.
The Company used a discount rate of 8.5% to arrive at the present value for the acquired intangible assets to reflect the rate of return a market participant would expect to earn and incremental commercial uncertainty in the cash flow projections. No assurances can be given that the underlying assumptions used to prepare the discounted cash flow analysis will not change. For these and other reasons, actual results may vary significantly from estimated results.
Goodwill
The Company allocated goodwill related to the ACell acquisition to the Tissue Technologies segment. Goodwill is the excess of the consideration transferred over the net assets recognized and represents the expected synergies of the combined company and assembled workforce. Goodwill recognized as a result of this acquisition is non-deductible for income tax purposes.
F-23
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Contingent Consideration
As part of the acquisition, the Company is required to make payments to the former shareholders of ACell up to $100 million based on the achievement of certain revenue-based performance milestones in 2022, 2023, and 2025. Based on revenue performance in 2022, no payment will be made for the first performance milestone. The Company used iterations of the Monte Carlo simulation to calculate the fair value of the contingent consideration that considered the possible outcomes of scenarios related to each specific milestone. The Company estimated the fair value of the contingent consideration to be $23.9 million at the acquisition date. The Company recorded $3.7 million and $21.8 million in other liabilities as of December 31, 2022 and 2021, respectively, in the consolidated balance sheets of the Company. The change in the fair value of the contingent obligation was primarily as a result of changes in the timing and amount of revenue estimates.
The Company determined the acquisition date fair value of contingent consideration obligations using a Monte Carlo simulation, as well as significant unobservable inputs, reflecting the Company’s assessment of the assumptions market participants would use to value these liabilities. The fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement as defined using the fair value concepts in ASC 820. The resultant most likely payouts are discounted using an appropriate effective annual interest rate. At each reporting date, the contingent consideration obligations is revalued to estimated fair value and changes in fair value will be reflected as income or expense in our consolidated statement of operations. Changes in the fair value of the contingent considerations may result from changes in discount periods and rates and changes in the timing and amount of revenue estimates.
Deferred Tax Liabilities
Deferred tax liabilities result from identifiable intangible assets’ fair value adjustments. These adjustments create excess book basis over tax basis which is tax-effected by the statutory tax rates of applicable jurisdictions.
5. DEBT
On MayFebruary 3, 2018,2020, the Company entered into the fifthsixth amendment and restatement (the "May 2018"February 2020 Amendment") of its Senior Credit Facility (the "Senior Credit Facility") with a syndicate of lending banks with Bank of America, N.A., as Administrative Agent. The May 2018February 2020 Amendment extended the maturity date to MayFebruary 3, 2023 and decreased the applicable rate, as described below.2025. The Company continues to have the aggregate principal amount of up to approximately $2.2 billion available to it through the following facilities:
The Company’s maximum consolidated total leverage ratio in the financial covenants (as defined in the Senior Credit Facility) was modified tois the following:
| Fiscal Quarter | Maximum Consolidated Total Leverage Ratio | |||||||
| 4.00 | ||||||||
Borrowings under the Senior Credit Facility bear interest, at the Company'sCompany’s option, at a rate equal to the following:
The applicable rates are based on the Company’s consolidated total leverage ratio (defined as the ratio of (a) consolidated funded indebtedness as of such date less cash that is not subject to any restriction on the use or investment thereof to (b) consolidated EBITDA at(as defined by the timeJuly 2020 amendment), for the period of the applicable borrowing)four consecutive fiscal quarters ending on such date).
F-24
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company will also pay an annual commitment fee (ranging from 0.15% to 0.35%0.30%), based on the Company’sCompany's consolidated total leverage ratio, on the amount available for borrowing under the revolving credit facility.
The Senior Credit Facility is collateralized by substantially all of the assets of the Company’s U.S. subsidiaries, excluding intangible assets. The Senior Credit Facility is subject to various financial and negative covenants and at December 31, 20192022, the Company was in compliance with all such covenants. TheIn connection with the February 2020 Amendment, the Company capitalized $4.2$4.6 million of financing costs in connection with modification of the Senior Credit Facility and wrote off $1.2 million of previously capitalized financing costs during the first quarter of 2020. In connection with the July 2020 amendment, the Company expensed $3.3 million of incremental financing costs in 2018 in connection with the modificationsmodification of the Senior Credit Facility.Facility during the third quarter of 2020.
There was no balance outstanding at December 31, 2022 under the revolving portion of the Senior Credit Facility and as of December 31, 2021, there was $31.3 million, outstanding under the revolving portion of the Senior Credit Facility at weighted average interest rate of 1.4%. At December 31, 2022 and 2021, there was $771.3 million and $843.8 million, respectively, outstanding, under the Term Loan component of the Senior Credit Facility at weighted average interest rate of 5.6% and 1.4%, respectively. At December 31, 2022 and 2021, there was $38.1 million and $45.0 million, respectively, of the Term Loan component of the Senior Credit Facility was classified as current on the consolidated balance sheets.
The fair value of outstanding borrowings of the Senior Credit Facility's Term Loan components at December 31, 2022 was $800.8 million. This fair values were determined by using a discounted cash flow model based on current market interest rates available to the Company. These inputs are corroborated by observable market data for similar liabilities and therefore classified within Level 2 of the fair value hierarchy. Level 2 inputs represent inputs that are observable for the asset or liability, either directly or indirectly, and are other than active market observable inputs that reflect unadjusted quoted prices for identical assets or liabilities
Letters of credit outstanding as of December 31, 2022 and 2021 totaled $1.6 million. There were no amounts drawn as of December 31, 2022.
Contractual repayments of the Term Loan component of the Senior Credit Facility are due as follows:
| Year-ended December 31, 2022 | Principal Repayment | |||||||
| Dollars in thousands | ||||||||
| 2023 | $ | 38,125 | ||||||
| 2024 | $ | 67,500 | ||||||
| 2025 | $ | 669,375 | ||||||
| $ | 775,000 | |||||||
| Year-ended December 31, 2019 | Principal Repayment | ||
| (In thousands) | |||
| 2020 | $ | 45,000 | |
| 2021 | 56,250 | ||
| 2022 | 67,500 | ||
| 2023 | 708,750 | ||
| $ | 877,500 | ||
The outstanding balance of the revolving credit component of the Senior Credit Facility is due on MayFebruary 3, 2023.2025.
Convertible Senior Notes
On February 4, 2020, the Company issued $575.0 million aggregate principal amount of its 0.5% Convertible Senior Notes due 2025 (the "2025 Notes"). The 2025 Notes will mature on August 15, 2025 and bear interest at a rate of 0.5% per annum payable semi-annually in arrears, unless earlier converted, repurchased or redeemed in accordance with the terms of the 2025 Notes. The portion of debt proceeds that was classified as equity at the time of the offering was $104.5 million. The effective interest rate implicit in the liability component was 4.2%. In connection with this offering, the Company capitalized $13.2 million of financing fees.
The 2025 Notes are senior, unsecured obligations of the Company, and are convertible into cash and shares of its common stock based on initial conversion rate, subject to adjustment of 13.5739 shares per $1,000 principal amounts of the 2025 Notes (which represents an initial conversion price of $73.67 per share). The 2025 Notes convert only in the following circumstances: (1) if the closing price of the Company's common stock has been at least 130% of the conversion price during the period; (2) if the average trading price per $1,000 principal amount of the 2025 Notes is less than or equal to 98% of the average conversion value of the 2025 Notes during a period as defined in the indenture; (3) at any time on or after February 20, 2023; or (4) if specified corporate transactions occur. As of December 31, 2022, none of these conditions existed with respect to the 2025 Notes and as a result the 2025 Notes are classified as long term.
F-25
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
On December 9, 2020, the Company entered into the First Supplemental Indenture to the original agreement dated as of February 4, 2020 between the Company and Citibank, N.A., as trustee, governing the Company’s outstanding 2025 Notes. The Company irrevocably elected (1) to eliminate the Company’s option to choose physical settlement on any conversion of the 2025 Notes that occurs on or after the date of the First Supplemental Indenture and (2) with respect to any Combination Settlement for a conversion of the 2025 Notes, the Specified Dollar Amount that will be settled in cash per $1,000 principal amount of the 2025 Notes shall be no lower than $1,000.
Holders of the Notes will have the right to require the Company to repurchase for cash all or a portion of their Notes at 100% of their principal amount, plus any accrued and unpaid interest, upon the occurrence of a fundamental change (as defined in the indenture relating to the Notes). The Company will also be required to increase the conversion rate for holders who convert their Notes in connection with certain fundamental changes occurring prior to the maturity date or following delivery by the Company of a notice of redemption.
In connection with the issuance of the 2025 Notes, the Company entered into call transactions and warrant transactions, primarily with affiliates of the initial purchasers of the 2025 Notes (the “hedge participants”). The cost of the call transactions was $104.2 million for the 2025 Notes. The Company received $44.5 million of proceeds from the warrant transactions for the 2025 Notes. The call transactions involved purchasing call options from the hedge participants, and the warrant transactions involved selling call options to the hedge participants with a higher strike price than the purchased call options. The initial strike price of the call transactions was $73.67, subject to anti-dilution adjustments substantially similar to those in the 2025 Notes. The initial strike price of the warrant transactions was $113.34 for the 2025 Notes, subject to customary anti-dilution adjustments.
At December 31, 2022, the carrying amount of the liability was $575.0 million. The fair value of the 2025 Notes at December 31, 2022 was $560.5 million. Factors that the Company considered when estimating the fair value of the 2025 Notes included recent quoted market prices or dealer quote. The level of the 2025 Notes is considered as Level 1.
As a result of the adoption of ASU 2020-06, for both the years ended December 31, 2022 and 2021, the Company recognized only cash interest related to the contractual interest coupon on the 2025 Notes of $2.9 million.
Securitization Facility
During the fourth quarter of 2018, the Company entered into an accounts receivable securitization facility (the "Securitization Facility") under which accounts receivable of certain domestic subsidiaries are sold on a non-recourse basis to a special purpose entity (“SPE”), which is a bankruptcy-remote, consolidated subsidiary of the Company. Accordingly, the assets of the SPE are not available to satisfy the obligations of the Company or any of its subsidiaries. From time to time, the SPE may finance such accounts receivable with a revolving loan facility secured by a pledge of such accounts receivable. The amount of outstanding borrowings on the Securitization Facility at any one time is limited to $150.0 million. The Securitization Facility agreement is for an initial three-year term and may be extended. The agreementAgreement ("Securitization Agreement") governing the Securitization Facility contains certain covenants and termination events. An occurrence of an event of default or a termination event under this Securitization FacilityAgreement may give rise to the right of its counterparty to terminate this facility. AtAs of December 31, 2019,2022, the Company was in compliance with the covenants and none of the termination events had occurred.
On May 28, 2021, the Company entered into an amendment (the "May 2021 Amendment") of the Securitization Facility which extended the maturity date from December 21, 2021 to May 28, 2024. The May 2021 Amendment does not increase the Company’s total indebtedness.
At December 31, 2022 and 2021, the Company had $104.5$104.7 million and $121.2$112.5 million, of outstanding borrowings under its Securitization Facility at a weighted average interest rate of 2.8%5.0% and 3.4% as of December 31, 2019 and 2018, respectively.
F-26
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
6. DERIVATIVE INSTRUMENTS
Interest Rate Hedging
The Company’s interest rate risk relates to U.S. dollar denominated variable interest rate borrowings. The Company uses interest rate swap derivative instruments to manage earnings and cash flow exposure resulting from changes in interest rates. These interest rate swaps apply a fixed interest rate on a portion of ourthe Company's expected LIBOR-indexed floating-rate borrowings.
The Company held the following interest rate swaps as of December 31, 20192022 and 2021 (dollar amounts in thousands):
| Hedged Item | Current Notional Amount | Designation Date | Effective Date | Termination Date | Fixed Interest Rate | Estimated Fair Value | |||||||||||
| Assets (Liabilities) | |||||||||||||||||
| 3-month USD LIBOR | 50,000 | February 6, 2017 | June 30, 2017 | June 30, 2020 | 1.834 | % | (2 | ) | |||||||||
| 1-month USD LIBOR | 100,000 | February 6, 2017 | June 30, 2017 | June 30, 2020 | 1.652 | % | 12 | ||||||||||
| 1-month USD LIBOR | 100,000 | March 27, 2017 | December 31, 2017 | June 30, 2021 | 1.971 | % | (581 | ) | |||||||||
| 1-month USD LIBOR | 150,000 | December 13, 2017 | January 1, 2018 | December 31, 2022 | 2.201 | % | (2,880 | ) | |||||||||
| 1-month USD LIBOR | 150,000 | December 13, 2017 | January 1, 2018 | December 31, 2022 | 2.201 | % | (2,880 | ) | |||||||||
| 1-month USD LIBOR | 100,000 | December 13, 2017 | July 1, 2019 | June 30, 2024 | 2.423 | % | (3,517 | ) | |||||||||
| 1-month USD LIBOR | 50,000 | December 13, 2017 | July 1, 2019 | June 30, 2024 | 2.423 | % | (1,778 | ) | |||||||||
| 1-month USD LIBOR | 200,000 | December 13, 2017 | January 1, 2018 | December 31, 2024 | 2.313 | % | (6,595 | ) | |||||||||
| 1-month USD LIBOR | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.220 | % | (5,750 | ) | |||||||||
| 1-month USD LIBOR | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.199 | % | (5,747 | ) | |||||||||
| 1-month USD LIBOR | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.209 | % | (5,807 | ) | |||||||||
| 1-month USD LIBOR | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.885 | % | (4,930 | ) | |||||||||
| 1-month USD LIBOR | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.867 | % | (4,691 | ) | |||||||||
| Total interest rate derivatives designated as cash flow hedge | $ | 1,325,000 | (45,145 | ) | |||||||||||||
| Hedged Item | Current Notional Amount | Designation Date | Effective Date | Termination Date | Fixed Interest Rate | Estimated Fair Value | |||||||||||
| Assets (Liabilities) | |||||||||||||||||
| 3-month USD LIBOR | 50,000 | June 22, 2016 | December 31, 2016 | June 30, 2019 | 1.062 | % | $ | 410 | |||||||||
| 3-month USD LIBOR | 50,000 | June 22, 2016 | December 31, 2016 | June 30, 2019 | 1.062 | % | 415 | ||||||||||
| 1-month USD LIBOR | 50,000 | July 12, 2016 | December 31, 2016 | June 30, 2019 | 0.825 | % | 418 | ||||||||||
| 3-month USD LIBOR | 50,000 | February 6, 2017 | June 30, 2017 | June 30, 2020 | 1.834 | % | 619 | ||||||||||
| 1-month USD LIBOR | 100,000 | February 6, 2017 | June 30, 2017 | June 30, 2020 | 1.652 | % | 1,287 | ||||||||||
| 1-month USD LIBOR | 100,000 | March 27, 2017 | December 31, 2017 | June 30, 2021 | 1.971 | % | 1,246 | ||||||||||
| 1-month USD LIBOR | 150,000 | December 13, 2017 | January 1, 2018 | December 31, 2022 | 2.201 | % | 1,491 | ||||||||||
| 1-month USD LIBOR | 150,000 | December 13, 2017 | January 1, 2018 | December 31, 2022 | 2.201 | % | 1,460 | ||||||||||
| 1-month USD LIBOR | 100,000 | December 13, 2017 | July 1, 2019 | June 30, 2024 | 2.423 | % | 418 | ||||||||||
| 1-month USD LIBOR | 50,000 | December 13, 2017 | July 1, 2019 | June 30, 2024 | 2.423 | % | 162 | ||||||||||
| 1-month USD LIBOR | 200,000 | December 13, 2017 | January 1, 2018 | December 31, 2024 | 2.313 | % | 2,076 | ||||||||||
| 1-month USD LIBOR | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.220 | % | (2,594 | ) | |||||||||
| 1-month USD LIBOR | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.199 | % | (2,551 | ) | |||||||||
| 1-month USD LIBOR | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.209 | % | (2,568 | ) | |||||||||
| 1-month USD LIBOR | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.885 | % | (797 | ) | |||||||||
| 1-month USD LIBOR | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.867 | % | (873 | ) | |||||||||
| Total interest rate derivatives designated as cash flow hedges | $ | 1,475,000 | $ | 619 | |||||||||||||
| December 31, 2022 | December 31, 2021 | December 31, 2022 | December 31, 2021 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hedged Item | Notional Amount | Designation Date | Effective Date | Termination Date | Fixed Interest Rate | Estimated Fair Value | ||||||||||||||||||||||||||||||||||||||||||||
| Asset (Liability) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | — | 300,000 | December 13, 2017 | January 1, 2018 | December 31, 2022 | 2.201 | % | $ | — | $ | (5,268) | |||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 150,000 | 150,000 | December 13, 2017 | July 1, 2019 | June 30, 2024 | 2.423 | % | 5,012 | (5,520) | |||||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 200,000 | 200,000 | December 13, 2017 | January 1, 2018 | December 31, 2024 | 2.313 | % | 8,380 | (7,421) | |||||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 75,000 | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.220 | % | 1,831 | (5,512) | |||||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 75,000 | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.199 | % | 1,905 | (5,464) | |||||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 75,000 | 75,000 | October 10, 2018 | July 1, 2020 | June 30, 2025 | 3.209 | % | 1,970 | (5,494) | |||||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 100,000 | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.885 | % | 4,252 | (6,886) | |||||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 100,000 | 100,000 | December 18, 2018 | December 30, 2022 | December 31, 2027 | 2.867 | % | 4,153 | (6,764) | |||||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 575,000 | 575,000 | December 15, 2020 | July 31, 2025 | December 31, 2027 | 1.415 | % | 23,742 | 3,552 | |||||||||||||||||||||||||||||||||||||||||
| 1-month USD LIBOR Loan | 125,000 | 125,000 | December 15, 2020 | July 1, 2025 | December 31, 2027 | 1.404 | % | 5,467 | 821 | |||||||||||||||||||||||||||||||||||||||||
| $ | 1,475,000 | $ | 1,775,000 | $ | 56,712 | $ | (43,956) | |||||||||||||||||||||||||||||||||||||||||||
The Company has designated these derivative instruments as cash flow hedges. The Company assesses the effectiveness of these derivative instruments and has recorded the changes in the fair value of the derivative instrument designated as a cash flow hedge as unrealized gains or losses in accumulated other comprehensive lossincome (“AOCL”AOCI”), net of tax, until the hedged item affected earnings, at which point any gain or loss was reclassified to earnings. If the hedged cash flow does not occur, or if it becomes probable that it will not occur, the Company will reclassify the remaining amount of any gain or loss on the related cash flow hedge recorded in AOCLAOCI to interest expense at that time.
Foreign Currency Hedging
From time to time, the Company enters into foreign currency hedge contracts intended to protect the U.S. dollar value of certain forecasted foreign currency denominated transactions. The Company assesses the effectiveness of the contracts that are designated as hedging instruments. The changes in fair value of foreign currency cash flow hedges are recorded in AOCL,AOCI, net of tax, untiltax. Those amounts are subsequently reclassified to earnings from AOCI as impacted by the hedged item affects earnings. Oncewhen the related hedged item affects earnings, the Company reclassifies amounts recorded in AOCL to earnings. If the hedged forecasted transaction does not occur, or if it becomes probable that it will not occur, the Company will reclassify the amount of any gain or loss on the related cash flow hedge to earnings at that time. For contracts not designated as hedging instruments, the changes in fair value of the contracts are recognized in other income, (expense), net in the consolidated statements of operation, along with the offsetting foreign currency gain or loss on the underlying assets or liabilities.
The success of the Company’s hedging program depends, in part, on forecasts of certain activity denominated in foreign currency. The Company may experience unanticipated currency exchange gains or losses to the extent that there are differences between forecasted and actual activities during periods of currency volatility. In addition, changes in currency exchange rates related to any unhedged transactions may affect earnings and cash flows.
Cross-Currency Rate Swaps
On October 2, 2017, the Company entered into cross currency swap agreements to convert a notional amount of $300.0 million equivalent to 291.2 million of CHFSwiss Francs ("CHF") denominated intercompany loans into U.S. dollars. The CHFCHF- denominated intercompany loans were the result of the purchase of intellectual property by a subsidiary in Switzerland as part of an acquisition. On September 26, 2022, the Codman Acquisition. Company amended the CHF-denominated intercompany loan to extend the termination date to September 2023 and as a result, the Company early terminated the cross-currency swap designated as cash flow hedge of an intercompany loan with aggregate notional amount of $50.0 million. Simultaneously, the Company entered into a cross-currency swap agreement to convert a notional amount of CHF 48.5 million equivalent to $49.1 million of this
F-27
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
amended intercompany loan into U.S. dollars. The loss recorded by the Company upon the settlement of the swap was not material for the period. As of December 31, 2022, $49.1 million of the $300.0 million notional amount remain outstanding.
On December 21, 2020, the Company entered into cross-currency swap agreements to convert a notional amount of $471.6 million equivalent to 420.1 million of a CHF-denominated intercompany loan into U.S. dollars. The CHF-denominated intercompany loan was the result of an intra-entity transfer of certain intellectual property rights to a subsidiary in Switzerland completed during the fourth quarter of 2020. The intercompany loan requires quarterly payments of CHF 5.8 million plus accrued interest. As a result, the aggregate notional amount of the related cross-currency swaps will decrease by a corresponding amount.
The objective of these cross-currency swaps is to reduce volatility of earnings and cash flows associated with changes in the foreign currency exchange rate. Under the terms of these contracts, which have been designated as cash flow hedges, the Company will make interest payments in Swiss Francs and receive interest in U.S. dollars. Upon the maturity of these contracts, the Company will pay the principal amount of the loans in Swiss Francs and receive U.S. dollars from the counterparties.
The Company held the following cross-currency rate swaps designated as cash flow hedges as of December 31, 20192022 and 2021 (dollar amounts in thousands):
| 2019 | ||||||||||||||
| Effective Date | Termination Date | Fixed Rate | Aggregate Notional Amount | Fair Value Asset (Liability) | ||||||||||
| Pay CHF | October 2, 2017 | October 2, 2020 | 1.75% | CHF | 32,355 | $ | (101 | ) | ||||||
| Receive U.S.$ | 4.38% | $ | 33,333 | |||||||||||
| Pay CHF | October 2, 2017 | October 2, 2021 | 1.85% | CHF | 48,533 | (119 | ) | |||||||
| Receive U.S.$ | 4.46% | $ | 50,000 | |||||||||||
| Pay CHF | October 2, 2017 | October 2, 2022 | 1.95% | CHF | 145,598 | (289 | ) | |||||||
| Receive U.S.$ | 4.52% | $ | 150,000 | |||||||||||
| Total | $ | (509 | ) | |||||||||||
| December 31, 2022 | December 31, 2021 | December 31, 2022 | December 31, 2021 | ||||||||||||||||||||||||||||||||||||||
| Effective Date | Termination Date | Fixed Rate | Aggregate Notional Amount | Fair Value Asset (Liability) | |||||||||||||||||||||||||||||||||||||
| Pay CHF | October 2, 2017 | October 2, 2022 | 1.95% | CHF | — | 145,598 | — | (8,283) | |||||||||||||||||||||||||||||||||
| Receive U.S.$ | 4.52% | $ | — | 150,000 | |||||||||||||||||||||||||||||||||||||
| Pay CHF | December 21, 2020 | December 22, 2025 | 3.00% | CHF | 374,137 | 397,137 | (4,241) | 41 | |||||||||||||||||||||||||||||||||
| Receive U.S.$ | 3.98% | $ | 420,001 | 445,821 | |||||||||||||||||||||||||||||||||||||
| Pay CHF | September 28, 2022 | September 29, 2023 | 1.95% | CHF | 48,532 | — | (3,528) | — | |||||||||||||||||||||||||||||||||
| Receive U.S.$ | 5.32% | $ | 49,142 | — | |||||||||||||||||||||||||||||||||||||
| Total | $ | (7,769) | $ | (8,242) | |||||||||||||||||||||||||||||||||||||
On October 4, 2021 in accordance with the termination date, the Company settled a cross-currency swap designated as a cash flow hedge of an intercompany loan with an aggregate notional amount of $66.7$50.0 million. The original maturity dates were October 2, 2020,
| 2018 | ||||||||||||||
| Effective Date | Termination Date | Fixed Rate | Aggregate Notional Amount | Fair Value Asset (Liability) | ||||||||||
| Pay CHF | October 2, 2017 | October 2, 2020 | 1.75% | CHF | 97,065 | $ | (215 | ) | ||||||
| Receive U.S.$ | 4.38% | $ | 100,000 | |||||||||||
| Pay CHF | October 2, 2017 | October 2, 2021 | 1.85% | CHF | 48,533 | (422 | ) | |||||||
| Receive U.S.$ | 4.46% | $ | 50,000 | |||||||||||
| Pay CHF | October 2, 2017 | October 2, 2022 | 1.95% | CHF | 145,598 | (2,193 | ) | |||||||
| Receive U.S.$ | 4.52% | $ | 150,000 | |||||||||||
| Total | $ | (2,830 | ) | |||||||||||
The cross- currencycross-currency swaps are carried on the consolidated balance sheet at fair value, and changes in the fair values are recorded as unrealized gains or losses in AOCL. For the years ended December 31, 2019 and 2018, the Company recorded a loss of $4.0 million and gain $2.2 million, respectively, in other income, net related to change in fair value related to the foreign currency rate translation to offset the gains or losses recognized on the intercompany loan.AOCI.
Net Investment Hedges
The Company manages certain foreign exchange risks through a variety of strategies, including hedging. The Company is exposed to foreign exchange risk from its international operations through foreign currency purchases, net investments in foreign subsidiaries, and foreign currency assets and liabilities created in the normal course of business. On October 1, 2018 ,December 16, 2020 and May 26, 2022, the Company entered into cross-currency swap agreements designated as net investment hedges to partially offset the effects of foreign currency on foreign subsidiaries.
F-28
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company held the following cross-currency rate swaps designated as net investment hedges as of December 31, 20192022 and December 31, 2018, respectively2021 (dollar amounts in thousands):
| December 31, 2022 | December 31, 2021 | December 31, 2022 | December 31, 2021 | ||||||||||||||||||||||||||||||||||||||||||||
| Effective Date | Termination Date | Fixed Rate | Aggregate Notional Amount | Fair Value Asset (Liability) | |||||||||||||||||||||||||||||||||||||||||||
| Pay EUR | October 3, 2018 | September 30, 2023 | —% | EUR | 51,760 | 51,760 | 4,713 | 2,503 | |||||||||||||||||||||||||||||||||||||||
| Receive U.S.$ | 2.57% | $ | 60,000 | 60,000 | |||||||||||||||||||||||||||||||||||||||||||
| Pay EUR | October 3, 2018 | September 30, 2025 | —% | EUR | 38,820 | 38,820 | 4,307 | 2,147 | |||||||||||||||||||||||||||||||||||||||
| Receive U.S.$ | 2.19% | $ | 45,000 | 45,000 | |||||||||||||||||||||||||||||||||||||||||||
| Pay CHF | December 16, 2020 | December 16, 2027 | —% | CHF | — | 222,300 | — | (792) | |||||||||||||||||||||||||||||||||||||||
| Receive USD | 1.10% | $ | — | 250,000 | |||||||||||||||||||||||||||||||||||||||||||
| Pay CHF | May 26, 2022 | December 16, 2028 | —% | CHF | 288,210 | — | (14,663) | — | |||||||||||||||||||||||||||||||||||||||
| Receive U.S.$ | 1.94% | $ | 300,000 | — | |||||||||||||||||||||||||||||||||||||||||||
| Total | $ | (5,643) | $ | 3,858 | |||||||||||||||||||||||||||||||||||||||||||
| December 31, 2019 | ||||||||||||||
| Effective Date | Termination Date | Fixed Rate | Aggregate Notional Amount | Fair Value Asset (Liability) | ||||||||||
| Pay EUR | October 3, 2018 | September 30, 2021 | — | EUR | 44,859 | 2,459 | ||||||||
| Receive U.S.$ | 3.01% | $ | 52,000 | |||||||||||
| Pay EUR | October 3, 2018 | September 30, 2023 | — | EUR | 51,760 | 3,087 | ||||||||
| Receive U.S.$ | 2.57% | $ | 60,000 | |||||||||||
| Pay EUR | October 3, 2018 | September 30, 2025 | — | EUR | 38,820 | 2,032 | ||||||||
| Receive U.S.$ | 2.19% | $ | 45,000 | |||||||||||
| Pay GBP | October 3, 2018 | September 30, 2025 | 1.67% | GBP | 128,284 | (154 | ) | |||||||
| Receive U.S.$ | 2.71% | $ | 167,500 | |||||||||||
| Pay CHF | October 3, 2018 | September 30, 2025 | — | CHF | 165,172 | 1,221 | ||||||||
| Receive GBP | 1.67% | GBP | 128,284 | |||||||||||
| Total | $ | 8,645 | ||||||||||||
| December 31, 2018 | ||||||||||||||
| Effective Date | Termination Date | Fixed Rate | Aggregate Notional Amount | Fair Value Asset (Liability) | ||||||||||
| Pay EUR | October 3, 2018 | September 30, 2021 | — | EUR | 70,738 | 1,359 | ||||||||
| Receive U.S.$ | 3.01% | $ | 82,000 | |||||||||||
| Pay EUR | October 3, 2018 | September 30, 2023 | — | EUR | 51,760 | (421 | ) | |||||||
| Receive U.S.$ | 2.57% | $ | 60,000 | |||||||||||
| Pay EUR | October 3, 2018 | September 30, 2025 | — | EUR | 38,820 | (150 | ) | |||||||
| Receive U.S.$ | 2.19% | $ | 45,000 | |||||||||||
| Pay GBP | October 3, 2018 | September 30, 2025 | 1.67% | GBP | 128,284 | 2,360 | ||||||||
| Receive U.S.$ | 2.71% | $ | 167,500 | |||||||||||
| Pay CHF | October 3, 2018 | September 30, 2025 | — | CHF | 165,172 | (3,780 | ) | |||||||
| Receive GBP | 1.67% | GBP | 128,284 | |||||||||||
| Total | $ | (632 | ) | |||||||||||
On September 30, 2021, in accordance with the termination date, the Company settled cross-currency swaps designated as net investment hedge with an aggregate notional amount of $52 million equivalent to 44.9 million Euros. As a result of the settlement, the Company recorded a gain of $0.1 million in AOCL.AOCI.
The cross-currency swaps were carried on the consolidated balance sheet at fair value and changes in the fair values were recorded as unrealized gains or losses in AOCL. For the year ended December 31, 2019 and 2018, the Company recorded a gain of $20.5 million and $1.7 million, respectively, in AOCL related to the change in fair value of the cross-currency swaps.AOCI.
Counterparty Credit Risk
The Company manages its concentration of counterparty credit risk on its derivative instruments by limiting acceptable counterparties to a group of major financial institutions with investment grade credit ratings, and by actively monitoring their credit ratings and outstanding positions on an ongoing basis. Therefore, the Company considers the credit risk of the counterparties to be low. Furthermore, none of the Company’s derivative transactions are subject to collateral or other security arrangements, and none contain provisions that depend upon the Company’s credit ratings from any credit rating agency.
Fair Value of Derivative Instruments
The Company has classified all of its derivative instruments within Level 2 of the fair value hierarchy because observable inputs are available for substantially the full term of the derivative instruments. The fair values of the interest rate swaps and cross-currency swaps were developed using a market approach based on publicly available market yield curves and the terms of the swap. The Company performs ongoing assessments of counterparty credit risk.
F-29
| Fair Value as of December 31, | |||||||
| 2019 | 2018 | ||||||
Location on Balance Sheet (1): | (In thousands) | ||||||
| Derivatives designated as hedges — Assets: | |||||||
| Prepaid expenses and other current assets | |||||||
| Cash Flow Hedges | |||||||
Interest rate swap(2) | $ | 12 | $ | 4,654 | |||
| Cross-currency swap | 5,032 | 7,615 | |||||
| Net Investment Hedges | |||||||
| Cross-currency swap | $ | 7,952 | $ | 8,888 | |||
| Other assets | |||||||
| Cash Flow Hedges | |||||||
Interest rate swap(2) | — | 5,350 | |||||
| Net Investment Hedges | |||||||
| Cross-currency swap | $ | 3,465 | $ | 1,774 | |||
| Total Derivatives designated as hedges — Assets | $ | 16,461 | $ | 28,281 | |||
| Derivatives designated as hedges — Liabilities | |||||||
| Accrued expenses and other current liabilities | |||||||
| Cash Flow Hedges | |||||||
Interest rate swap(2) | $ | 6,635 | $ | — | |||
| Cross-currency swap | 101 | — | |||||
| Other liabilities | |||||||
| Cash Flow Hedges | |||||||
Interest rate swap(2) | 38,522 | 9,385 | |||||
| Cross-currency swap | 5,440 | 10,445 | |||||
| Net Investment Hedges | |||||||
| Cross-currency swap | $ | 2,772 | $ | 11,294 | |||
| Total Derivative designated as hedges — Liabilities | $ | 53,470 | $ | 31,124 | |||
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following table summarizes the fair value for derivatives designated as hedging instruments in the consolidated balance sheets as of December 31, 2022 and 2021:
| Fair Value as of December 31, | |||||||||||||||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||||||||||||||
Location on Balance Sheet (1): | |||||||||||||||||||||||
| Derivatives designated as hedges — Assets: | |||||||||||||||||||||||
| Prepaid expenses and other current assets | |||||||||||||||||||||||
| Cash Flow Hedges | |||||||||||||||||||||||
| Cross-currency swap | 4,497 | 4,900 | |||||||||||||||||||||
Interest rate swap(2) | 16,682 | — | |||||||||||||||||||||
| Net Investment Hedges | |||||||||||||||||||||||
| Cross-currency swap | 11,653 | 5,120 | |||||||||||||||||||||
| Other assets | |||||||||||||||||||||||
| Cash Flow Hedges | |||||||||||||||||||||||
Interest rate swap(2) | 40,030 | 4,373 | |||||||||||||||||||||
| Net Investment Hedges | |||||||||||||||||||||||
| Cross-currency swap | 3,311 | 2,104 | |||||||||||||||||||||
| Total derivatives designated as hedges — Assets | $ | 76,173 | $ | 16,497 | |||||||||||||||||||
| Derivatives designated as hedges — Liabilities | |||||||||||||||||||||||
| Accrued expenses and other current liabilities | |||||||||||||||||||||||
| Cash Flow Hedges | |||||||||||||||||||||||
Interest rate swap(2) | $ | — | $ | 18,187 | |||||||||||||||||||
| Cross-currency swap | 3,528 | 8,283 | |||||||||||||||||||||
| Net Investment Hedges | |||||||||||||||||||||||
| Cross-currency swap | — | — | |||||||||||||||||||||
| Other liabilities | |||||||||||||||||||||||
| Cash Flow Hedges | |||||||||||||||||||||||
Interest rate swap(2) | — | 30,143 | |||||||||||||||||||||
| Cross-currency swap | 8,738 | 4,859 | |||||||||||||||||||||
| Net Investment Hedges | |||||||||||||||||||||||
| Cross-currency swap | 20,608 | 3,366 | |||||||||||||||||||||
| Total derivatives designated as hedges — Liabilities | 32,874 | 64,838 | |||||||||||||||||||||
(1)The Company classifies derivative assets and liabilities as current based on the cash flows expected to be incurred within the following 12 months.
(2)At December 31, 2022 and 2021, the total notional amounts related to the Company’s interest rate swaps were $1.5 billion and $1.8 billion respectively.
F-30
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following presents the effect of derivative instruments designated as cash flow hedges and net investment hedges on the accompanying consolidated statementsstatement of operations during the years ended December 31, 20192022 and 2021:
| Dollars in thousands | Balance in AOCI Beginning of Year | Amount of Gain (Loss) Recognized in AOCI | Amount of Gain (Loss) Reclassified from AOCI into Earnings | Balance in AOCI End of Year | Location in Statements of Operations | ||||||||||||||||||||||||
| Year Ended December 31, 2022 | |||||||||||||||||||||||||||||
| Cash Flow Hedges | |||||||||||||||||||||||||||||
| Interest rate swap | $ | (43,956) | $ | 93,308 | $ | (7,360) | $ | 56,712 | Interest expense | ||||||||||||||||||||
| Cross-currency swap | (9,688) | 8,847 | 19,430 | (20,271) | Other income, net | ||||||||||||||||||||||||
| Net Investment Hedges | |||||||||||||||||||||||||||||
| Cross-currency swap | (2,321) | 2,196 | 6,789 | (6,914) | Interest income | ||||||||||||||||||||||||
| $ | (55,965) | $ | 104,351 | $ | 18,859 | $ | 29,527 | ||||||||||||||||||||||
| Year Ended December 31, 2021 | |||||||||||||||||||||||||||||
| Cash Flow Hedges | |||||||||||||||||||||||||||||
| Interest rate swap | $ | (93,769) | $ | 27,402 | $ | (22,411) | $ | (43,956) | Interest expense | ||||||||||||||||||||
| Cross-currency swap | (1,073) | 24,275 | 32,890 | (9,688) | Other income, net | ||||||||||||||||||||||||
| Net Investment Hedges | |||||||||||||||||||||||||||||
| Cross-currency swap | (12,291) | 16,515 | 6,545 | (2,321) | Interest income | ||||||||||||||||||||||||
| $ | (107,133) | $ | 68,192 | $ | 17,024 | $ | (55,965) | ||||||||||||||||||||||
For the years ended December 31, 2022 and 2021, the Company recorded a gain of $11.1 million and gain of 2018$23.8 million:, respectively, in other income, net related to change in fair value related to the foreign currency rate translation of the cross-currency swaps, designated as cash flow hedges, to offset the gains or losses recognized on the intercompany loans.
For the years ended December 31, 2022 and 2021, the Company recorded gains of $8.4 million and $9.1 million, respectively, in other income, net included in the consolidated statements of operations related to the interest rate differential of the cross-currency swaps designated as cash flow hedges.
The estimated gain that is expected to be reclassified to other income, net from AOCI as of December 31, 2022, for the cross-currency swaps designated as cash flow hedges within the next twelve months is $4.5 million. As of December 31, 2022, the Company does not expect any gains or losses will be reclassified into earnings as a result of the discontinuance of these cash flow hedges because the original forecasted transaction will not occur.
The estimated gain that is expected to be reclassified to interest income from AOCI as of December 31, 2022 for the cross-currency swaps designated as net investment hedges, within the next twelve months is $11.7 million.
Derivative Instruments not designated hedges:
During the fourth quarter of 2020, the Company entered into foreign currency forward contracts, with a notional amount of $4.2 million, to mitigate the foreign exchange risk related to certain intercompany loans denominated in Canadian Dollar ("CAD"). These contracts were settled in third quarter of 2022.
During the second quarter of 2021, the Company entered into a foreign currency swap, with a notional amount of $7.3 million, to mitigate the risk from fluctuations in foreign currency exchange rates associated with an intercompany loan denominated in JPY. In a foreign currency swap transaction, the Company agrees with another party to exchange, at specified intervals, the difference between one currency and another currency at a fixed exchange rate, generally set at inception, calculated by reference to an agreed upon notional amount. The notional amount of each currency is exchanged at the inception and termination of the currency swap by each party. The Company subsequently paid down a portion of this swap, bringing the notional amount down to $6.4 million.
The following table summarizes the gains (losses) of derivative instruments not designated as hedges on the condensed consolidated statements of income, which was included in other income:
Balance in AOCL Beginning of Year | Amount of Gain (Loss) Recognized in AOCL | Amount of Gain (Loss) Reclassified from AOCL into Earnings | Balance in AOCL End of Year | Location in Statements of Operations | |||||||||||||
| (In thousands) | |||||||||||||||||
| Year Ended December 31, 2019 | |||||||||||||||||
| Cash Flow Hedges | |||||||||||||||||
| Interest rate swap | $ | 619 | $ | (43,493 | ) | $ | 2,271 | $ | (45,145 | ) | Interest expense | ||||||
| Cross-currency swap | (6,190 | ) | 9,334 | 2,967 | 177 | Other income, net | |||||||||||
| Net Investment Hedges | |||||||||||||||||
| Cross-currency swap | (632 | ) | 20,488 | 9,627 | 10,229 | Interest income | |||||||||||
| $ | (6,203 | ) | $ | (13,671 | ) | $ | 14,865 | $ | (34,739 | ) | |||||||
| Year Ended December 31, 2018 | |||||||||||||||||
| Cash Flow Hedges | |||||||||||||||||
| Interest rate swap | $ | 592 | $ | 924 | $ | 897 | $ | 619 | Interest expense | ||||||||
| Cross-currency swap | (5,104 | ) | 9,062 | 10,148 | (6,190 | ) | Other income, net | ||||||||||
| Net Investment Hedges | |||||||||||||||||
| Cross-currency swap | — | 1,723 | 2,355 | (632 | ) | Interest income | |||||||||||
| $ | (4,512 | ) | $ | 11,709 | $ | 13,400 | $ | (6,203 | ) | ||||||||
F-31
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| Dollars in thousands | December 31, | |||||||||||||
| 2022 | 2021 | |||||||||||||
| Foreign currency forward contracts | $ | — | $ | (174) | ||||||||||
| Foreign currency swaps | 1,258 | 629 | ||||||||||||
| Total | $ | 1,258 | $ | 455 | ||||||||||
7. GOODWILL AND OTHER INTANGIBLE ASSETS
Goodwill
The Company tests goodwill for impairment by either performing a qualitative evaluation or a quantitative test.
The qualitative evaluation is an assessment of factors including reporting unit specific operating results as well as industry, market and general economic conditions, to determine whether it is more likely than not that the fair values of a reporting unit is less than its carrying amount, including goodwill. The Company may elect to bypass the qualitative assessment for its 3three reporting units and perform a quantitative test. The assumptions used in evaluating goodwill for impairment are subject to change and are tracked against historical results by management.
The quantitative test estimates the fair value of its 3the three reporting units using a discounted cash flow model, which incorporates significant estimates and assumptions made by management which, by their nature, are characterized by uncertainty. Inputs used to fair value the Company's reporting units are considered inputs of the fair value hierarchy. For Level 3 measurements, significant increases or decreases in long-term growth rates or discount rates in isolation or in combination could result in a significantly lower or higher fair value measurement. The key assumptions impacting the valuation included the following:
•The reporting unit's financial projections, which are based on management's assessment of regional and macroeconomic variables, industry trends and market opportunities, and the Company's strategic objectives and future growth plans.
•The projected terminal value for the reporting unit, which represents the present value of projected cash flows beyond the last period in the discounted cash flow analysis. The terminal value reflects the Company's assumptions related to long-term growth rates and profitability, which are based on several factors, including local and macroeconomic variables, market opportunities, and future growth plans.
•The discount rate used to measure the present value of the projected future cash flows is set using a weighted-average cost of capital method that considers market and industry data as well as the Company's specific risk factors that are likely to be considered by a market participant. The weighted-average cost of capital is the Company's estimate of the overall after-tax rate of return required by equity and debt holders of a business enterprise.
Changes in the carrying amount of goodwill in 20192022 and 20182021 were as follows:
| Codman Specialty Surgical | Orthopedics and Tissue Technologies | Total | |||||||||
| (In thousands) | |||||||||||
| Goodwill at January 1, 2018 | $ | 634,767 | $ | 303,138 | $ | 937,905 | |||||
Codman acquisition measurement period adjustments | (3,964 | ) | — | (3,964 | ) | ||||||
Foreign currency translation | (5,043 | ) | (2,423 | ) | (7,466 | ) | |||||
| Goodwill at December 31, 2018 | $ | 625,760 | $ | 300,715 | $ | 926,475 | |||||
| Arkis Acquisition | 27,600 | — | 27,600 | ||||||||
| Foreign currency translation | 140 | 65 | 205 | ||||||||
| Goodwill at December 31, 2019 | $ | 653,500 | $ | 300,780 | $ | 954,280 | |||||
| Dollars in thousands | Codman Specialty Surgical | Tissue Technologies | Total | ||||||||||||||
| Goodwill at January 1, 2021 | $ | 671,975 | $ | 260,392 | $ | 932,367 | |||||||||||
| ACell Acquisition | — | 94,147 | 94,147 | ||||||||||||||
| Foreign currency translation | (8,547) | (4,509) | (13,056) | ||||||||||||||
| Balance at December 31, 2021 | $ | 663,428 | $ | 350,030 | $ | 1,013,458 | |||||||||||
| Sale of non-core traditional wound care business | — | (5,019) | (5,019) | ||||||||||||||
| SIA Acquisition | — | 41,855 | 41,855 | ||||||||||||||
| Foreign currency translation | (7,209) | (4,204) | (11,413) | ||||||||||||||
| Balance at December 31, 2022 | $ | 656,219 | $ | 382,662 | $ | 1,038,881 | |||||||||||
F-32
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Other Intangible Assets
The components of the Company's identifiable intangible assets were as follows:
| December 31, 2022 | |||||||||||||||||||||||
| Dollars in thousands | Weighted Average Life | Cost | Accumulated Amortization | Net | |||||||||||||||||||
| Completed technology | 18 years | $ | 1,204,325 | $ | (370,968) | $ | 833,357 | ||||||||||||||||
| Customer relationships | 12 years | 193,081 | (144,040) | 49,041 | |||||||||||||||||||
| Trademarks/brand names | 28 years | 97,265 | (34,674) | 62,591 | |||||||||||||||||||
| Codman trade name | Indefinite | 166,693 | — | 166,693 | |||||||||||||||||||
| Supplier relationships | 30 years | 30,211 | (17,170) | 13,041 | |||||||||||||||||||
| All other | 11 years | 5,957 | (4,071) | 1,886 | |||||||||||||||||||
| $ | 1,697,532 | $ | (570,923) | $ | 1,126,609 | ||||||||||||||||||
| December 31, 2021 | ||||||||||||||||||||||||||||||||||||
Weighted Average Life | December 31, 2019 | |||||||||||||||||||||||||||||||||||
| Cost | Accumulated Amortization | Net | ||||||||||||||||||||||||||||||||||
| (Dollars in Thousands) | ||||||||||||||||||||||||||||||||||||
| Dollars in thousands | Dollars in thousands | Weighted Average Life | Cost | Accumulated Amortization | Net | |||||||||||||||||||||||||||||||
| Completed technology | 19 years | $ | 880,623 | $ | (213,702 | ) | $ | 666,921 | Completed technology | 18 years | $ | 1,132,954 | $ | (307,013) | $ | 825,941 | ||||||||||||||||||||
| Customer relationships | 12 years | 222,575 | (119,393 | ) | 103,182 | Customer relationships | 12 years | 211,344 | (142,755) | 68,589 | ||||||||||||||||||||||||||
| Trademarks/brand names | 28 years | 103,873 | (28,514 | ) | 75,359 | Trademarks/brand names | 28 years | 98,367 | (31,468) | 66,899 | ||||||||||||||||||||||||||
| Codman trade name | Indefinite | 163,126 | — | 163,126 | Codman trade name | Indefinite | 167,758 | — | 167,758 | |||||||||||||||||||||||||||
| Supplier relationships | 27 years | 34,721 | (17,947 | ) | 16,774 | Supplier relationships | 30 years | 30,211 | (16,192) | 14,019 | ||||||||||||||||||||||||||
All other (1) | 4 years | 10,869 | (4,640 | ) | 6,229 | |||||||||||||||||||||||||||||||
| All other | All other | 11 years | 6,258 | (3,891) | 2,367 | |||||||||||||||||||||||||||||||
| $ | 1,415,787 | $ | (384,196 | ) | $ | 1,031,591 | $ | 1,646,892 | $ | (501,319) | $ | 1,145,573 | ||||||||||||||||||||||||
Weighted Average Life | December 31, 2018 | ||||||||||||
| Cost | Accumulated Amortization | Net | |||||||||||
| (Dollars in Thousands) | |||||||||||||
| Completed technology | 19 years | $ | 855,679 | $ | (167,384 | ) | $ | 688,295 | |||||
| Customer relationships | 13 years | 231,448 | (106,859 | ) | 124,589 | ||||||||
| Trademarks/brand names | 28 years | 104,061 | (24,764 | ) | 79,297 | ||||||||
| Codman trade name | Indefinite | 162,054 | — | 162,054 | |||||||||
| Supplier relationships | 27 years | 34,721 | (16,519 | ) | 18,202 | ||||||||
All other (1) | 4 years | 10,958 | (3,899 | ) | 7,059 | ||||||||
| $ | 1,398,921 | $ | (319,425 | ) | $ | 1,079,496 | |||||||
The companyCompany tests intangible assets with indefinite lives for impairment annually in the third quarter in accordance with ASC Topic 350. Additionally, the Company may perform interim tests if an event occurs or circumstances change that could potentially reduce the fair value of a indefinite lived intangible asset below its carrying amount. The Company electedtests for impairment by either performing a qualitative evaluation or a quantitative test. The qualitative evaluation is an assessment of factors, including specific operating results as well as industry, market and general economic conditions, to determine whether it is more likely than not that the fair values of the intangible asset is less than its carrying amount. The Company may elect to bypass thethis qualitative evaluation for its Codman tradename intangible asset and perform a quantitative test duringtest.
During the third quarter of 2019. In performing this test,2022, the Company utilizedelected to perform a range of projected sales growth rates,qualitative analysis for its intangible asset with indefinite lives. The Company determined, after performing the qualitative analysis, that there was no evidence that it is more likely than not that the fair value was less that the carrying amounts, therefore, it was not necessary to perform a royalty rate of 5.0%, a range of tax rates between 17.4-20.7%, and a discount rate of 12.5%. The assumptions used in evaluating the Codman tradename forquantitative impairment are subject to change and are tracked against historical results by management. Based on the results of the quantitative test, the Company recorded 0 impairment to the Codman tradename intangible asset.test.
Product rights and other definite-lived intangible assets are tested periodically for impairment in accordance with ASC Topic 360 when events or changes in circumstances indicate that an asset’sasset's carrying value may not be recoverable. The impairment testing involves comparing the carrying amount of the asset or asset group to the forecasted undiscounted future cash flows. In the event the carrying value of the asset exceeds the undiscounted future cash flows, the carrying value is considered not recoverable and an impairment exists. An impairment loss is measured as the excess of the asset’sasset's carrying value over its fair value, calculated using discounted future cash flows. The computed impairment loss is recognized in the period that the impairment occurs.
F-33
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
8. TREASURY STOCK
On December 11, 2018,January 26, 2023, the Company entered into a $150 million accelerated share repurchase ("2023 ASR") and received 2.1 million shares of the Company common stock at inception of the 2023 ASR, which represented approximately 80% of the expected total shares of under the 2023 SAR. The remaining repurchase transactions are expected to be completed in the first half of 2023.
On April 26 2022, the Board of Directors authorized the Company to repurchase up to $225 million of the Company’s common stock. The program allows the Company to repurchase its shares opportunistically from time to time. The repurchase authorization expires in December 2020.2024. This stock repurchase authorization replaces the previous $225 million stock repurchase authorization, of which $100 million remained authorized at the time of its replacement, and which was otherwise set to expire on December 31, 2022. Purchases may be affected through one or more open market transactions, privately negotiated transactions, transactions structured through investment banking institutions, or a combination of the foregoing. This
On January 12, 2022, the Company entered into a $125 million accelerated share repurchase ("2022 ASR") and received 1.48 million shares of Company common stock repurchase authorization replacesat inception of the previous $150.0 million stock repurchase authorization, approved2022 ASR, which represented approximately 80% of the expected total shares under the 2022 ASR. In March 24, 2022, the early exercise provision was exercised by the Board in 2016.
For the year ended December 31, 2019 and 2018.2021, there were no repurchases of the Company’s common stock as part of the share repurchase authorization.
On August 16, 2022, the Inflation Reduction Act of 2022 (the “Act”) was signed into law. The Act implements a new excise tax of 1% on the net share repurchases made by the company effective for share repurchases performed January 1, 2023, or after.
9. STOCK-BASED COMPENSATION
Stock-based compensation expense - all related to employees and members of the Board of Directors - recognized under the authoritative guidance was as follows:
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| Selling, general and administrative | $ | 19,153 | $ | 18,721 | $ | 19,785 | |||||
| Research and development | 1,785 | 1,609 | 1,273 | ||||||||
| Cost of goods sold | 317 | 449 | 492 | ||||||||
| Total stock-based compensation expense | 21,255 | 20,779 | 21,550 | ||||||||
| Total estimated tax benefit related to stock-based compensation expense | 9,420 | 10,430 | 15,448 | ||||||||
| Net effect on net income | $ | 11,835 | $ | 10,349 | $ | 6,102 | |||||
| Years Ended December 31, | |||||||||||||||||
| Dollars in thousands | 2022 | 2021 | 2020 | ||||||||||||||
| Cost of goods sold | 549 | 470 | 344 | ||||||||||||||
| Research and development | 1,739 | 1,644 | 1,471 | ||||||||||||||
| Selling, general and administrative | $ | 25,437 | $ | 34,096 | $ | 17,776 | |||||||||||
| Total stock-based compensation expense | 27,725 | 36,210 | 19,591 | ||||||||||||||
| Total estimated tax benefit related to stock-based compensation expense | 10,574 | 13,804 | 6,221 | ||||||||||||||
| Net effect on net income | $ | 17,151 | $ | 22,406 | $ | 13,370 | |||||||||||
EMPLOYEE STOCK PURCHASE PLAN
The purpose of the Employee Stock Purchase Plan (the “ESPP”) is to provide eligible employees of the Company with the opportunity to acquire shares of common stock at periodic intervals by means of accumulated payroll deductions. The ESPP is a non-compensatory plan. Under the ESPP, a total of 3.0 million shares of common stock are reserved for issuance. These shares will be made available either from the Company’s authorized but unissued shares of common stock or from shares of common stock reacquired by the Company as treasury stock. At December 31, 2019,2022, 2.0 million shares remain available for purchase under the ESPP. During the years ended December 31, 2019, 20182022, 2021 and 2017,2020, the Company issued 12,53120,780 shares, 16,72116,948 shares and 12,16818,284 shares under the ESPP for $0.7$1.1 million, $0.7$1.1 million and $0.6$1.1 million, respectively.
F-34
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
EQUITY AWARD PLANS
As of December 31, 2019,2022, the Company had stock options, restricted stock awards, performance stock awards, contract stock awards and restricted stock unit awards outstanding under 3 plans, the 2000 Equity Incentive Plan (the “2000 Plan”), the 2001 Equity Incentive Plan (the “2001 Plan”),Integra LifeSciences Holdings Corporation Fifth Amended and theRestated 2003 Equity Incentive Plan (the “2003 Plan,”Plan”). The 2000 and collectively, (the “Plans”)).2001 Equity Incentive Plans were terminated as of February 19, 2021, and no further awards may be issued under the plans.
In May 2010 and May 2017, the stockholders of the Company approved amendments to the 2003 Plan to increase by 3.5 million and 1.7 million, respectively, the number of shares of common stock that may be issued under the 2003 Plan. The Company has reserved 4.0 million shares under each of the 2000 Plan and the 2001 Plan, and 14.7 million shares under the 2003 Plan. The Plans permit the Company to grant incentive and non-qualified stock options, stock appreciation rights, restricted stock, contract stock, performance stock, or dividend equivalent rights to designated directors, officers, employees and associates of the Company.
Stock options issued under the Plans become2003 Plan became exercisable over specified periods, generally within four years from the date of grant for officers and employees, and within one year from the date of the grant for members of the Board of Directors. The awards generally expire eight years from the grant date for employees and from six to ten years for directors and certain executive officers.officers, except in certain instances that result in accelerated vesting due to death, disability, retirement age or change in control provisions within their grant agreements. Restricted stock issued under the Plans2003 Plan vests ratably over specified periods, generally three years after the date of grant. The vesting of performance stock issued under the 2003 Plan is subject to service and performance conditions.
Stock Options
The Company values stock option grants using the binomial distribution model. Management believes that the binomial distribution model is preferable to the Black-Scholes model because it is a more flexible model that gives consideration to the impact of non-transferability and vesting provisions in valuing employee stock options.
In determining the value of stock options granted, the Company considered that it has never paid cash dividends and does not currently intend to pay cash dividends, and thus has assumed a 0% dividend yield. Expected volatilities are based on the historical volatility of the Company’s stock price. The expected life of stock options is estimated based on historical data on exercise of stock options, post-vesting forfeitures and other factors to estimate the expected term of the stock options granted. The risk-free interest rates are derived from the U.S. Treasury yield curve in effect on the date of grant for instruments with a remaining term similar to the expected life of the options. The Company accounts for forfeitures as they occur.
The following weighted-average assumptions were used in the calculation of fair value:
| Years Ended December 31, | |||||
| 2019 | 2018 | 2017 | |||
| Dividend yield | 0% | 0% | 0% | ||
| Expected volatility | 28% | 28% | 30% | ||
| Risk free interest rate | 2.51% | 2.79% | 2.18% | ||
| Expected life of option from grant date | 7 years | 8 years | 8 years | ||
| Years Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Dividend yield | 0% | 0% | 0% | ||||||||||||||
| Expected volatility | 30% | 29% | 27% | ||||||||||||||
| Risk free interest rate | 2.01% | 1.30% | 0.89% | ||||||||||||||
| Expected life of option from grant date | 7 years | 7 years | 7 years | ||||||||||||||
| Weighted average grant date fair value of options granted | $23.15 | $22.59 | $13.03 | ||||||||||||||
The following table summarizes the Company’s stock option activity.
| Shares | Weighted Average Exercise Price | Weighted Average Contractual Term in Years | Aggregate Intrinsic Value | ||||||||||||||||||||
| Stock Options | (In thousands) | (In thousands) | |||||||||||||||||||||
| Outstanding at January 1, 2022 | 1,225 | $ | 45.11 | 4.30 | $26,970 | ||||||||||||||||||
| Granted | 146 | 65.11 | — | — | |||||||||||||||||||
| Exercised | (155) | 28.32 | — | — | |||||||||||||||||||
| Forfeited or Expired | (14) | 50.68 | — | — | |||||||||||||||||||
| Outstanding at December 31, 2022 | 1,202 | $ | 49.63 | 4.14 | $ | 10,772 | |||||||||||||||||
| Exercisable at December 31, 2022 | 871 | $ | 45.82 | 3.30 | $ | 9,635 | |||||||||||||||||
| Weighted Average Exercise Price | Weighted Average Contractual Term in Years | Aggregate Intrinsic Value | |||||||||||
| Shares | |||||||||||||
| Stock Options | (In thousands) | (In thousands) | |||||||||||
| Outstanding at January 1, 2019 | 1,448 | $ | 27.91 | — | — | ||||||||
| Granted | 203 | 55.91 | |||||||||||
| Exercised | (350 | ) | 17.83 | ||||||||||
| Forfeited or Expired | (17 | ) | 46.52 | ||||||||||
| Outstanding at December 31, 2019 | 1,284 | $ | 34.83 | 4.03 | $ | 30,128 | |||||||
| Vested or expected to vest at December 31, 2019 | 1,284 | $ | 34.83 | 4.03 | $ | 30,128 | |||||||
| Exercisable at December 31, 2019 | 961 | $ | 28.49 | 3.15 | $ | 28,639 | |||||||
F-35
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company recognized $3.0$3.5 million, $2.6$5.0 million and $3.0$3.2 million in expense related to stock options during the years ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively. The intrinsic value of options exercised for the years ended December 31, 2019, 20182022, 2021 and 20172020 were $14.6$4.0 million, $16.9$11.1 million and $16.2$8.7 million, respectively. The weighted average grant date fair value of options granted during the years ended December 31, 2019, 2018 and 2017 was $18.74, $21.78 and $16.95, respectively. Cash received from option exercises and employee stock purchase plan was $6.9$5.5 million, $9.4$6.8 million and $9.8$5.2 million, for the years
ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively. The realized tax benefit from options exercised were $3.0 million, $3.1were $0.6 million, $2.2 million and $6.2$1.7 million for the years ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively.
As of December 31, 2019,2022, there was approximately $4.5$3.5 million of total unrecognized compensation costs related to unvested stock options. These costs are expected to be recognized over a weighted-average period of approximately two years.
Awards of Restricted Stock, Performance Stock and Contract Stock
The following table summarizes the Company’s awards of restricted stock, performance stock and contract stock for the year ended December 31, 2019.2022.
| Restricted Stock Awards | Performance Stock and Contract Stock Awards | |||||||||||
| Shares | Weighted Average Grant Date Fair Value Per Share | Shares | Weighted Average Grant Date Fair Value Per Share | |||||||||
| (In thousands) | (In thousands) | |||||||||||
| Unvested, January 1, 2019 | 417 | $ | 48.97 | 85 | 45.56 | |||||||
| Granted | 303 | 55.41 | 157 | 55.86 | ||||||||
| Adjustments for performance achievement related to award target | — | — | 19 | 50.36 | ||||||||
| Cancellations | (59 | ) | 50.31 | (27 | ) | — | ||||||
| Performance stock awards vested in 2018 and released in 2019 | — | 273 | 42.94 | |||||||||
| Released | (201 | ) | 46.08 | (175 | ) | 56.03 | ||||||
| Vested but not released | — | — | (140 | ) | 50.36 | |||||||
| Unvested, December 31, 2019 | 460 | $ | 54.31 | 192 | 55.38 | |||||||
| Restricted Stock Awards | Performance Stock and Contract Stock Awards | ||||||||||||||||||||||
| Shares | Weighted Average Grant Date Fair Value Per Share | Shares | Weighted Average Grant Date Fair Value Per Share | ||||||||||||||||||||
| (In thousands) | (In thousands) | ||||||||||||||||||||||
| Unvested, January 1, 2022 | 422 | $ | 58.78 | 442 | 60.62 | ||||||||||||||||||
| Granted | 334 | 62.88 | 245 | 62.89 | |||||||||||||||||||
| Adjustments for performance achievement related to award target | — | — | (18) | 60.76 | |||||||||||||||||||
| Cancellations | (34) | 62.29 | (10) | 59.88 | |||||||||||||||||||
| Released | (239) | 58.27 | (252) | 59.38 | |||||||||||||||||||
| Unvested, December 31, 2022 | 483 | $ | 61.63 | 407 | 62.88 | ||||||||||||||||||
The Company recognized $18.1$24.3 million, $18.1$31.2 million and $18.5$16.4 million in expense related to such awards during the years ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively. The total fair market value of shares vested and released in 2019, 20182022, 2021 and 20172020 was $21.1$65.0 million, $24.8$15.7 million and $22.2$17.3 million, respectively. Vested awards include shares that have been fully earned but had not been delivered as of December 31, 2019.2022.
Performance stock awards have performance features associated with them. Performance stock, restricted stock and contract stock awards generally have requisite service periods of three years. The fair value of these awards is being expensed on a straight-line basis over the vesting period. As of December 31, 2022, there were 129,399 performance stock units ("PSU's") subject to vest and be released based on 2022 performance achievement.
As of December 31, 2019,2022, there was approximately $23.4$29.7 million of total unrecognized compensation costs related to unvested restricted stock, performance stock and contract stock awards. These costs are expected to be recognized over a weighted-average period of approximately two years.
At December 31, 2019, there are approximately 0.5 million vested Restricted Units and 0.1 million vested performance share units held by various employees for which the related shares have not yet been issued. The final determination of the number of shares to be issued is made by the Company's Compensation Committee of the Board of Directors which is is contingent upon achieving certain revenue and organic revenue growth performance metric.
The Company capitalized into inventory, share based compensation costs of $0.3$0.6 million, $0.4$0.5 million and $0.5$0.4 million for the years ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively. Such share-based compensation was recognized as cost of goods sold when related inventory was sold.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
10. RETIREMENT BENEFIT PLANS
DEFINED BENEFIT PLANS
The Company has various defined benefit plans which covers certain employees in Austria, France, Japan, Germany and Switzerland.
Net periodic benefit costs for the Company’s defined benefit pension plans for the years ended December 31, 20192022 and 20182021 included the following (amounts in thousands):
| Year ended December 31, | |||||||
| 2019 | 2018 | ||||||
| Service cost | $ | 3,815 | $ | 2,704 | |||
| Interest cost | 517 | 351 | |||||
| Expected return on plan assets | (1,047 | ) | (944 | ) | |||
| Amortization of prior service cost (credit) | (259 | ) | — | ||||
| Recognized actuarial losses | 65 | 8 | |||||
| Settlements | 602 | — | |||||
| Net period benefit cost | $ | 3,693 | $ | 2,119 | |||
| Year ended December 31, | |||||||||||||||||||||||
| 2022 | 2021 | ||||||||||||||||||||||
| Service cost | $ | 2,419 | $ | 2,741 | |||||||||||||||||||
| Interest cost | 194 | 100 | |||||||||||||||||||||
| Expected return on plan assets | (1,381) | (893) | |||||||||||||||||||||
| Amortization of prior service cost (credit) | (326) | (281) | |||||||||||||||||||||
| Recognized actuarial losses | 9 | 186 | |||||||||||||||||||||
| Settlements | — | 51 | |||||||||||||||||||||
| Net period benefit cost | $ | 915 | $ | 1,904 | |||||||||||||||||||
The following weighted average assumptions were used to develop net periodic pension benefit costs and the actuarial present values of projected pension benefit obligations for the years ended December 31, 20192022 and 2018,2021, respectively:
| As of December 31, | |||||
| 2019 | 2018 | ||||
| Discount rate | 0.40 | % | 1.00 | % | |
| Expected return on plan assets | 3.33 | % | 3.40 | % | |
| Rate of compensation increase | 2.25 | % | 1.70 | % | |
| As of December 31, | |||||||||||||||||||||||
| 2022 | 2021 | ||||||||||||||||||||||
| Discount rate | 2.44 | % | 0.37 | % | |||||||||||||||||||
| Expected return on plan assets | 3.61 | % | 3.59 | % | |||||||||||||||||||
| Rate of compensation increase | 1.97 | % | 2.10 | % | |||||||||||||||||||
| Interest crediting rate for cash balance plans | 1.00 | % | 1.00 | % | |||||||||||||||||||
The Company’s discount rates are determined by considering current yield curves representing high quality, long-term fixed income instruments. The resulting discount rates are consistent with the duration of plan liabilities. In 20192022 and 2018,2021, the discount rates were prescribed as the current yield on corporate bonds with an average rating of AA or AAA of equivalent currency and term to the liabilities. The expected returns on plan assets represent the average rate of return expected to be earned on plan assets over the period the benefits included in the benefit obligation are to be paid. In developing the expected rates of return, the Company considers returns of historical market data as well as actual returns on the plan assets. Using this reference information, the long-term return expectations for each asset category are developed according to the allocation among those investment categories.
The assessment is determined using projections from external financial sources, long-term historical averages, actual returns by asset class and the various asset class allocations by market.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following sets forth the change in projected benefit obligations and the change in plan assets for the years ended December 31, 20192022 and 20182021 and a reconciliation of the funded status at December 31, 20192022 and 2018,2021, respectively (amounts in thousands):
| Year ended December 31, | |||||||
| 2019 | 2018 | ||||||
| Change In Projected Benefit Obligations | |||||||
| Projected benefit obligations, beginning of year | $ | 52,542 | $ | 47,661 | |||
| Interest cost | 517 | 351 | |||||
| Service cost | 3,815 | 2,704 | |||||
| Actuarial loss | 12,188 | 762 | |||||
| Plan amendments | (3,133 | ) | — | ||||
| Plan settlements | (2,664 | ) | — | ||||
| Employee contribution | 899 | 641 | |||||
| Premiums paid | (395 | ) | — | ||||
| Benefit payment | (635 | ) | (1,483 | ) | |||
| Plans transferred in | 3,199 | 2,280 | |||||
| Effect of foreign currency exchange rates | 639 | (374 | ) | ||||
| Projected benefit obligations, end of year | $ | 66,972 | $ | 52,542 | |||
| Year ended December 31, | |||||||
| 2019 | 2018 | ||||||
| Change In Plan Assets | |||||||
| Plan assets at fair value, beginning of year | $ | 31,103 | $ | 26,943 | |||
| Actual return on plan assets | (152 | ) | 1,802 | ||||
| Employer contributions | 2,189 | 1,720 | |||||
| Employee contributions | 899 | 641 | |||||
| Plan settlements | (2,645 | ) | — | ||||
| Benefits paid | (635 | ) | (1,463 | ) | |||
| Premiums paid | (395 | ) | — | ||||
| Plans transferred in | — | 1,589 | |||||
| Effect of foreign currency exchange rates | 406 | (129 | ) | ||||
| Plan assets at fair value, end of year | $ | 30,770 | $ | 31,103 | |||
| Year ended December 31, | |||||||||||||||||
| 2022 | 2021 | ||||||||||||||||
| Change In Projected Benefit Obligations | |||||||||||||||||
| Projected benefit obligations, beginning of year | $ | 65,184 | $ | 72,869 | |||||||||||||
| Interest cost | 194 | 100 | |||||||||||||||
| Service cost | 2,419 | 2,741 | |||||||||||||||
| Actuarial (gain) loss | (14,822) | (5,044) | |||||||||||||||
| Plan amendments | (390) | (586) | |||||||||||||||
| Plan settlements | (20) | (655) | |||||||||||||||
| Employee contribution | 999 | 917 | |||||||||||||||
| Premiums paid | (391) | (373) | |||||||||||||||
| Benefit payment | (999) | (2,128) | |||||||||||||||
| Effect of foreign currency exchange rates | (1,810) | (2,657) | |||||||||||||||
| Projected benefit obligations, end of year | $ | 50,364 | $ | 65,184 | |||||||||||||
| Year ended December 31, | |||||||||||||||||
| 2022 | 2021 | ||||||||||||||||
| Change In Plan Assets | |||||||||||||||||
| Plan assets at fair value, beginning of year | $ | 39,914 | $ | 37,825 | |||||||||||||
| Actual return on plan assets | (2,863) | 3,371 | |||||||||||||||
| Employer contributions | 2,356 | 2,254 | |||||||||||||||
| Employee contributions | 999 | 917 | |||||||||||||||
| Plan settlements | — | (633) | |||||||||||||||
| Benefits paid | (998) | (2,128) | |||||||||||||||
| Premiums paid | (391) | (373) | |||||||||||||||
| Effect of foreign currency exchange rates | (964) | (1,319) | |||||||||||||||
| Plan assets at fair value, end of year | $ | 38,053 | $ | 39,914 | |||||||||||||
| Year ended December 31, | |||||||
| 2019 | 2018 | ||||||
| Reconciliation Of Funded Status | |||||||
| Fair value of plan assets | $ | 30,770 | $ | 31,103 | |||
| Benefit obligations | 66,972 | 52,542 | |||||
| Unfunded benefit obligations | $ | 36,202 | $ | 21,439 | |||
| Year ended December 31, | |||||||||||||||||
| 2022 | 2021 | ||||||||||||||||
| Reconciliation Of Funded Status | |||||||||||||||||
| Fair value of plan assets | $ | 38,053 | $ | 39,914 | |||||||||||||
| Benefit obligations | 50,364 | 65,184 | |||||||||||||||
| Unfunded benefit obligations | $ | 12,311 | $ | 25,270 | |||||||||||||
The unfunded benefit obligations are included in other liabilities in the consolidated balance sheets at December 31, 20192022 and 2018,2021, respectively.
During the periods ended December 31, 20192022 and 2018,2021, the Company had a net lossesgain of $9.0$7.4 million and $0.6 $7.0 million, respectively, recognized within accumulated other comprehensive loss that has not been recognized as a component of net periodic benefit cost. The lossgain recognized during the period ended December 31, 2019,2021, is primarily attributed to a change in the discount rate used to estimate the projected benefit obligation for defined benefit plans which cover certain employees in Switzerland. The combined accumulated benefit obligations for the defined benefit plans was $61.1$46.4 million and $49.6$60.3 million as of December 31, 20192022 and 2018,2021, respectively.
Unrecognized gains and losses are amortized over the average remaining future service for each plan. For plans with no active employees, they are amortized over the average life expectancy. The amortization of gains and losses is determined by using a 10% corridor of the greater of the market value of assets or the accumulated benefit obligation. Total unamortized gains and losses in excess of the corridor are amortized over the average remaining future service.
Prior service costs/benefits for the pension plans are amortized over the average remaining future service of plan participants at the time of the plan amendment.
F-38
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The net plan assets of the pension plans are invested in common trusts. Common trusts are classified as Level 2 in fair value hierarchy. The fair value of common trusts is valued at net asset value based on the fair values of the underlying investments of the trusts as determined by the sponsor of the trusts. The investment strategy of the Company's defined benefit plans is both to meet the liabilities of the plans as they fall due and to maximize the return on invested assets within appropriate risk profile.
The investment strategy for the Company’s defined benefit plans is both to meet the liabilities of the plans as they fall due and to maximize the return on invested assets within appropriate risk tolerances. The benefit plans in Austria, France and Germany had 0no assets at December 31, 2019.2022.
As of December 31, 2019, 02022, no plan assets are expected to be returned to the Company in the next twelve months.
The following table is the summary of expected future benefit payments (in thousands):
| 2020 | $ | 1,702 | |
| 2021 | 1,743 | ||
| 2022 | 1,499 | ||
| 2023 | 1,650 | ||
| 2024 | 2,115 | ||
| Next five years | 10,157 | ||
| 2023 | $ | 2,211 | |||||||||
| 2024 | $ | 2,045 | |||||||||
| 2025 | $ | 2,050 | |||||||||
| 2026 | $ | 1,929 | |||||||||
| 2027 | $ | 1,868 | |||||||||
| Next five years | $ | 11,068 | |||||||||
As of December 31, 2019,2022, contributions expected to be paid to the plan in 20202022 is $2.1$2.6 million.
DEFINED CONTRIBUTION PLANS
The Company also has various defined contribution savings plans that cover substantially all employees in the United States, Belgium, Canada, France, Japan, Netherlands, the U.K. and Puerto Rico. The Company matches a certain percentage of each employee’s contributions as per the provisions of the plans. Total contributions by the Company to the plans were $8.6$9.8 million, $8.1$8.8 million and $7.2$6.7 million for the years ended December 31, 2019, 20182022, 2021 and 2017,2020, respectively.
The Company maintains a Deferred Compensation Plan in which certain employees of the Company may defer the payment and taxation of up to 75% of their base salary and up to 100% of bonus amounts and other eligible cash compensation.
This deferred compensation is invested in funds offered under this plan and is valued based on Level 1 measurements in the fair value hierarchy. Assets of the Company's deferred compensation plan are included in Other current assets and recorded at fair value based on their quoted market prices. The fair value of these assets at December 31, 2022 and 2021 was $4.7 million and $3.8 million. Offsetting liabilities relating to the deferred compensation plan are included in Other liabilities.
11. LEASES AND RELATED PARTY LEASES
The Company leases administrative, manufacturing, research and distribution facilities and vehicles through operating lease agreements. The Company has no finance leases as of December 31, 2019.2022. Many of the Company's leases include both lease (e.g., fixed payments including rent) and non-lease components (e.g., common-area or other maintenance costs). For vehicles, the Company has elected the practical expedient to group lease and non-lease components.
Most facility leases include 1one or more options to renew. The exercise of lease renewal options is typically at the Company's sole discretion, therefore, the majority of renewals to extend the lease terms are not included in the ROU assets and lease liabilities as they are not reasonably certain of exercise. The Company regularly evaluates renewal options and when they are reasonably certain of exercise, the renewal period is included in the lease term.
As most of the Company's leases do not provide an implicit rate, the Company uses a collateralized incremental borrowing rate based on the information available at the lease commencement date in determining the present value of the lease payments.
Total operating lease expense for the year ended December 31, 20192022 and 2021, was $19.6$22.6 million and $20.3 million, respectively, which includes $0.3 million, in related party operating lease expense.
F-39
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| December 31, 2019 | |||
| (In thousands, except lease term and discount rate) | |||
| ROU assets | $ | 94,530 | |
| Current lease liabilities | 12,253 | ||
| Non-current lease liabilities | 97,504 | ||
| Total lease liabilities | $ | 109,757 | |
| Weighted average remaining lease term (in years): | |||
| Leased facilities | 12.8 years | ||
| Leased vehicles | 2.6 years | ||
| Weighted average discount rate: | |||
| Leased facilities | 5.4 | % | |
| Leased vehicles | 3.2 | % | |
| December 31, 2022 | December 31, 2021 | ||||||||||||||||||||||
| (In thousands, except lease term and discount rate) | |||||||||||||||||||||||
| ROU assets | $ | 148,284 | $ | 84,543 | |||||||||||||||||||
| Current lease liabilities | 14,624 | 14,775 | |||||||||||||||||||||
| Non-current lease liabilities | 157,420 | 90,329 | |||||||||||||||||||||
| Total lease liabilities | $ | 172,044 | $ | 105,104 | |||||||||||||||||||
| Weighted average remaining lease term (in years): | |||||||||||||||||||||||
| Leased facilities | 16.9 years | 10.4 years | |||||||||||||||||||||
| Leased vehicles | 2.0 years | 2.1 years | |||||||||||||||||||||
| Weighted average discount rate: | |||||||||||||||||||||||
| Leased facilities | 5.4 | % | 5.1 | % | |||||||||||||||||||
| Leased vehicles | 2.7 | % | 2.6 | % | |||||||||||||||||||
Supplemental cash flow information related to leases was as follows for the year ended December 31, 2019 (in thousands):follows:
| December 31, 2019 | |||
| (In thousands) | |||
| Cash paid for amounts included in the measurement of lease liabilities: | |||
| Operating cash flows from operating leases | $ | 11,469 | |
| ROU assets obtained in exchange for lease liabilities: | |||
| Operating leases | 41,423 | ||
| December 31, 2022 | December 31, 2021 | ||||||||||
| (In thousands) | |||||||||||
| Cash paid for amounts included in the measurement of lease liabilities: | |||||||||||
| Operating cash flows from operating leases | $ | 17,442 | $ | 15,077 | |||||||
| ROU assets obtained in exchange for lease liabilities: | |||||||||||
| Operating leases | 72,169 | 12,610 | |||||||||
Future minimum lease payments under operating leases at December 31, 20192022 were as follows:
| Related Parties | Third Parties | Total | |||||||||
| (In thousands) | |||||||||||
| 2020 | 296 | 12,100 | 12,396 | ||||||||
| 2021 | 296 | 12,951 | 13,247 | ||||||||
| 2022 | 296 | 13,753 | 14,049 | ||||||||
| 2023 | 296 | 11,386 | 11,682 | ||||||||
| 2024 | 296 | 11,060 | 11,356 | ||||||||
| Thereafter | 1,428 | 91,235 | 92,663 | ||||||||
| Total minimum lease payments | $ | 2,908 | $ | 152,485 | $ | 155,393 | |||||
| Less: Imputed interest | $ | 45,636 | |||||||||
| Total lease liabilities | 109,757 | ||||||||||
| Less: Current lease liabilities | 12,253 | ||||||||||
| Long-term lease liabilities | 97,504 | ||||||||||
| Related Parties | Third Parties | Total | |||||||||||||||
| (In thousands) | |||||||||||||||||
| 2023 | 296 | 20,024 | 20,320 | ||||||||||||||
| 2024 | 296 | 20,869 | 21,165 | ||||||||||||||
| 2025 | 296 | 19,198 | 19,494 | ||||||||||||||
| 2026 | 296 | 16,830 | 17,126 | ||||||||||||||
| 2027 | 296 | 15,886 | 16,182 | ||||||||||||||
| Thereafter | 542 | 164,622 | 165,164 | ||||||||||||||
| Total minimum lease payments | $ | 2,022 | $ | 257,429 | $ | 259,451 | |||||||||||
| Less: Imputed interest | $ | 87,407 | |||||||||||||||
| Total lease liabilities | 172,044 | ||||||||||||||||
| Less: Current lease liabilities | 14,624 | ||||||||||||||||
| Long-term lease liabilities | 157,420 | ||||||||||||||||
There were 0no future minimum lease payments under finance leases at December 31, 2019.
F-40
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| Related Parties | Third Parties | Total | |||||||||
| (In thousands) | |||||||||||
| 2019 | $ | 296 | $ | 16,472 | $ | 16,768 | |||||
| 2020 | 296 | 13,510 | 13,806 | ||||||||
| 2021 | 296 | 12,197 | 12,493 | ||||||||
| 2022 | 296 | 12,937 | 13,233 | ||||||||
| 2023 | 296 | 10,707 | 11,003 | ||||||||
| Thereafter | 1,724 | 100,675 | 102,399 | ||||||||
| Total minimum lease payments | $ | 3,204 | $ | 166,498 | $ | 169,702 | |||||
Related Party Leases
The Company also leases its manufacturing facility in Plainsboro, New Jersey, from a general partnership that is 50% owned by a corporation whose shareholdersstockholders are trusts, whose beneficiaries include family members of the Company’s principal ownerstockholder and former Chairman and director. The term of the current lease agreement is through October 31, 20322029 at an annual rate of approximately $0.3$0.3 million per year. The current lease agreement also provides (i) a 5-year5-year renewal option for the Company to extend the lease from November 1, 20322029 through October 31, 20372034 at the fair market rental rate of the premises, and (ii) another 5-year5-year renewal option to extend the lease from November 1, 20372034 through October 31, 20422039 at the fair market rental rate of the premises.
12. INCOME TAXES
Income (Loss) before income taxes consisted of the following:
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| United States operations | $ | (38,359 | ) | $ | (21,218 | ) | $ | (32,640 | ) | ||
| Foreign operations | 98,463 | 78,621 | 44,025 | ||||||||
| Total | $ | 60,104 | $ | 57,403 | $ | 11,385 | |||||
| Years Ended December 31, | |||||||||||||||||
| Dollars in thousands | 2022 | 2021 | 2020 | ||||||||||||||
| United States operations | $ | 92,642 | $ | 91,150 | $ | 15,082 | |||||||||||
| Foreign operations | 121,252 | 123,527 | 78,438 | ||||||||||||||
| Total | $ | 213,894 | $ | 214,677 | $ | 93,520 | |||||||||||
A reconciliation of the U.S. Federal statutory rate to the Company’s effective tax rate is as follows:
| Years Ended December 31, | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Federal statutory rate | 21.0 | % | 21.0 | % | 21.0 | % | |||||||||||
| Increase (decrease) in income taxes resulting from: | |||||||||||||||||
| State income taxes, net of federal tax benefit | 0.1 | % | 1.9 | % | 1.2 | % | |||||||||||
| Foreign operations | (3.9) | % | (4.0) | % | (7.9) | % | |||||||||||
| Excess tax benefits from stock compensation | (2.4) | % | (1.2) | % | (1.0) | % | |||||||||||
| Intercompany profit in inventory | 0.3 | % | (0.2) | % | 1.2 | % | |||||||||||
| Nondeductible facilitative costs | 0.2 | % | 0.3 | % | 1.1 | % | |||||||||||
| Contingent Consideration | (2.0) | % | (0.2) | % | 0.2 | % | |||||||||||
| Research and development credit | (1.4) | % | (1.2) | % | (1.6) | % | |||||||||||
| Return to provision | (0.5) | % | (0.7) | % | (2.3) | % | |||||||||||
| Global intangible low-taxed income ("GILTI") | 2.8 | % | 0.7 | % | 2.5 | % | |||||||||||
| Nondeductible executive compensation | 1.8 | % | 0.9 | % | 2.4 | % | |||||||||||
| Fair market value step up on intra-entity transfer of intellectual property | — | % | — | % | (63.3) | % | |||||||||||
| Gain from sale of business - book to tax differences | — | % | 3.9 | % | 2.8 | % | |||||||||||
| Other | (0.4) | % | — | % | 0.5 | % | |||||||||||
| Effective tax rate | 15.6 | % | 21.2 | % | (43.2) | % | |||||||||||
Our effective tax rate was 15.6% and 21.2% of income before income taxes for the years ended December 31, 2022 and December 31, 2021, respectively. In 2022, the Company’s lower effective tax rate was driven by a $5.1 million income tax benefit related to stock compensation and a $2.4 million income tax benefit related to the filing of amended federal and state returns for prior years. In 2021, the Company's higher effective tax rate was driven in part by an $8.5 million income tax expense for nondeductible goodwill related to the sale of the Extremity Orthopedics business, offset by a $3.1 million income tax benefit related to excess tax benefits from stock compensation. In 2020, the Company’s lower worldwide effective tax rate was primarily driven by an $59.2 million income tax benefit on an intra-entity transfer of certain intellectual property, substantially completed during the fourth quarter in 2020. Excluding this transaction, the effective worldwide tax rate for 2020 was 20.2%.
F-41
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| Years Ended December 31, | ||||||||
| 2019 | 2018 | 2017 | ||||||
| Federal statutory rate | 21.0 | % | 21.0 | % | 35.0 | % | ||
| Increase (decrease) in income taxes resulting from: | ||||||||
| State income taxes, net of federal tax benefit | 1.0 | % | (0.4 | )% | (17.0 | )% | ||
| Foreign operations | (20.0 | )% | (21.8 | )% | (112.7 | )% | ||
| Excess tax benefits from stock compensation | (5.6 | )% | (7.8 | )% | (57.9 | )% | ||
| Charitable contributions | (0.6 | )% | (1.2 | )% | (10.6 | )% | ||
| Nondeductible meals and entertainment | 1.5 | % | 1.6 | % | 8.8 | % | ||
| Intercompany profit in inventory | 1.2 | % | 6.2 | % | 11.6 | % | ||
| Nondeductible facilitative costs | 0.8 | % | — | % | 22.5 | % | ||
| Changes in valuation allowances | 0.2 | % | 0.2 | % | 8.0 | % | ||
| Uncertain tax positions | 0.2 | % | 0.4 | % | (4.6 | )% | ||
| Research and development credit | (2.9 | )% | (2.6 | )% | (13.2 | )% | ||
| Return to provision | 1.7 | % | (2.9 | )% | (4.3 | )% | ||
| Global intangible low-taxed income ("GILTI") | 7.6 | % | 3.5 | % | — | % | ||
| Nondeductible executive compensation | 3.0 | % | 1.6 | % | — | % | ||
| Carryback of Federal net operating loss ("NOL") | 0.1 | % | (3.7 | )% | — | % | ||
| Other | 0.4 | % | — | % | 0.8 | % | ||
| Swiss tax holiday | (15.7 | )% | — | % | — | % | ||
| In-process research and development | 22.7 | % | — | % | — | % | ||
| Reduction of book gain on sale of assets | — | % | — | % | (4.6 | )% | ||
| Tax reform — Toll Tax | — | % | — | % | 48.1 | % | ||
| Tax reform — remeasurement of deferred tax assets and liabilities | — | % | — | % | (378.6 | )% | ||
| Effective tax rate | 16.5 | % | (5.9 | )% | (468.7 | )% | ||
During 2019,2022, the Company'sCompany’s foreign operations generated a $5.7$0.4 million decreaseincrease in income tax expense when compared with 2018,to the same period in 2021, because of geographic and business mix of taxable earnings and losses, among other factors. The 20192022 foreign effective tax rate is 3.5%15.9%, compared to 11.6%15.2% in 2018.2021. The Company'sCompany’s foreign tax rate is primarily based upon statutory rates.
During 2021, the Company’s foreign operations generated a $63.6 million increase in income tax expense when compared to the same period in 2020, because of the intra-entity transfer of certain intellectual property in 2020, geographic and business mix of taxable earnings and losses, among other factors. The 2021 foreign effective tax rate is 15.2%, compared to (57.1)% in 2020. The Company’s foreign tax rate is primarily based upon statutory rates and is also impacted by the intra-entity transfer of certain intellectual property as described above for 2020.
Changes to income tax holidaylaws and regulations, in Switzerland, described below.
The provision for income taxes consisted of approximately 2.0% over the rate in 2017. The Company's foreign tax rate is primarily based upon statutory rates.following:
| Years Ended December 31, | |||||||||||||||||
| Dollars in thousands | 2022 | 2021 | 2020 | ||||||||||||||
| Current: | |||||||||||||||||
| Federal | $ | 24,201 | $ | 31,938 | $ | 6,184 | |||||||||||
| State | 3,835 | 11,377 | 5,029 | ||||||||||||||
| Foreign | 9,893 | 5,042 | 12,553 | ||||||||||||||
| Total current | $ | 37,929 | $ | 48,357 | $ | 23,766 | |||||||||||
| Deferred: | |||||||||||||||||
| Federal | (11,591) | (12,830) | (5,079) | ||||||||||||||
| State | (2,316) | (3,688) | (1,760) | ||||||||||||||
| Foreign | 9,322 | 13,763 | (57,299) | ||||||||||||||
| Total deferred | $ | (4,585) | $ | (2,755) | $ | (64,138) | |||||||||||
| Provision for income taxes | $ | 33,344 | $ | 45,602 | $ | (40,372) | |||||||||||
F-42
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| Current: | |||||||||||
| Federal | $ | 14,597 | $ | (3,880 | ) | $ | 6,644 | ||||
| State | 3,447 | 1,609 | 1,233 | ||||||||
| Foreign | 10,905 | 7,057 | 6,069 | ||||||||
| Total current | $ | 28,949 | $ | 4,786 | $ | 13,946 | |||||
| Deferred: | |||||||||||
| Federal | (10,889 | ) | (7,202 | ) | (66,466 | ) | |||||
| State | (666 | ) | (3,048 | ) | (758 | ) | |||||
| Foreign | (7,491 | ) | 2,066 | (80 | ) | ||||||
| Total deferred | $ | (19,046 | ) | $ | (8,184 | ) | $ | (67,304 | ) | ||
| Provision for income taxes | $ | 9,903 | $ | (3,398 | ) | $ | (53,358 | ) | |||
The income tax effects of significant temporary differences that give rise to deferred tax assets and liabilities, shown before jurisdictional netting, are presented below:
| December 31, | |||||||
| 2019 | 2018 | ||||||
| (In thousands) | |||||||
| Assets: | |||||||
| Doubtful accounts | $ | 2,426 | $ | 1,507 | |||
| Inventory related items | 39,548 | 28,245 | |||||
| Tax credits | 19,134 | 9,072 | |||||
| Accrued vacation | 3,206 | 2,761 | |||||
| Accrued bonus | 6,017 | 5,515 | |||||
| Stock compensation | 8,347 | 10,093 | |||||
| Deferred revenue | 1,805 | 2,173 | |||||
| Net operating loss carryforwards | 37,418 | 33,350 | |||||
| Capitalization of research and development expenses | 9,781 | — | |||||
| Unrealized foreign exchange loss | 8,105 | 1,405 | |||||
| Charitable contributions carryforward | 235 | 1,994 | |||||
| Others | 5,900 | 8,835 | |||||
| Total deferred tax assets | 141,922 | 104,950 | |||||
| Less valuation allowance | (9,865 | ) | (6,973 | ) | |||
| Deferred tax assets after valuation allowance | $ | 132,057 | $ | 97,977 | |||
| Liabilities: | |||||||
| Intangible and fixed assets | (150,879 | ) | (144,861 | ) | |||
| Others | (5,108 | ) | (4,089 | ) | |||
| Total deferred tax liabilities | $ | (155,987 | ) | $ | (148,950 | ) | |
| Total net deferred tax liabilities | $ | (23,930 | ) | $ | (50,973 | ) | |
| December 31, | |||||||||||
| Dollars in thousands | 2022 | 2021 | |||||||||
| Assets: | |||||||||||
| Doubtful accounts | $ | 2,261 | $ | 2,029 | |||||||
| Inventory related items | 31,950 | 31,841 | |||||||||
| Tax credits | 13,084 | 13,319 | |||||||||
| Accrued vacation | 2,175 | 3,042 | |||||||||
| Accrued bonus | 4,944 | 7,415 | |||||||||
| Stock compensation | 10,175 | 13,955 | |||||||||
| Deferred revenue | 2,130 | 1,742 | |||||||||
| Net operating loss carryforwards | 30,707 | 26,198 | |||||||||
| Capitalization of research and development expenses | 51,542 | 36,770 | |||||||||
| Unrealized foreign exchange gain | 6,228 | 12,849 | |||||||||
| Charitable contributions carryforward | 180 | 206 | |||||||||
| Leases and Other | 39,788 | 41,371 | |||||||||
| Total deferred tax assets | 195,164 | 190,737 | |||||||||
| Less valuation allowance | (9,651) | (9,767) | |||||||||
| Deferred tax assets after valuation allowance | $ | 185,513 | $ | 180,970 | |||||||
| Liabilities: | |||||||||||
| Intangible and fixed assets | (166,891) | (152,150) | |||||||||
| Unrealized foreign exchange loss | (12,991) | — | |||||||||
| Leases and Other | (22,975) | (17,658) | |||||||||
| Total deferred tax liabilities | $ | (202,857) | $ | (169,808) | |||||||
| Total net deferred tax assets (liabilities) | $ | (17,344) | $ | 11,162 | |||||||
The 2017 U.S. Tax Cuts and Jobs Act contained a provision which requires, for tax purposes, the capitalization and amortization of research and development expenses; effective for years beginning after December 31, 2021. The Company’s deferred tax assets and liabilities are measured based onincreased by $20.2 million within the enacted tax rates that apply in years in which the temporary differences are expectedtable above, related to be realized or incurred. The Company remeasured its deferred tax assets and liabilities as a result of the 2017 Tax Act, using a provisional estimate under SAB No. 118 during 2017. The primary impact of the re-measurement was a decrease in the net deferred tax liability for the reduction of the U.S. statutory income tax rate from 35.0% to 21.0%. There were no material changes to the provisional amounts when the amounts were finalized in December of 2018.Act.
At December 31, 2019,2022, the Company had net operating loss carryforwards of $130.1$79.5 million for federal income tax purposes, $37.5$75.5 million for foreign income tax purposes and $42.8$37.9 million for state income tax purposes to offset future taxable income. The majority of the federal net operating loss carryforwards expire through 2037, while $18.9$18.6 million have an indefinite carry forward period. For foreign net operating loss carryforwards, $1.3$59.1 million will expire through 2024, $0.9 million expire through 2025, and2028, while the remaining $35.3$16.4 million have an indefinite carry forward period. The state net operating loss carryforwards expire through 2036.
The valuation allowance relates to deferred tax assets for certain items that will be deductible for income tax purposes under very limited circumstances and for which the Company believes it will not satisfy the more likely than not threshold for realization of the associated tax benefit. In the event that the Company determines that it would be able to realize more or less than the recorded amount of net deferred tax assets, an adjustment to the deferred tax asset valuation allowance would be recorded in the period such a determination is made.
The Company’s valuation allowance increased by $2.9 million, decreased by $1.0 million and increased by $4.4 million at December 31, 2019, 20182022 and 2017, respectively. The 2019 overall increase2021 primarily remained unchanged from respective prior periods; decreasing by an immaterial amount in the valuation allowance primarily resulted from certain assets from the Rebound and Arkis acquisitions.both periods.
F-43
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| Balance at Beginning of Period | Charged to Costs and Expenses | Other | Deductions | Balance at End of Period | |||||||||||||||||||||||||
| Description | |||||||||||||||||||||||||||||
| Dollars in thousands | |||||||||||||||||||||||||||||
| Year ended December 31, 2022 | |||||||||||||||||||||||||||||
| Deferred tax assets valuation allowance | 15,258 | (515) | (71) | 14,672 | |||||||||||||||||||||||||
| Year ended December 31, 2021 | |||||||||||||||||||||||||||||
| Deferred tax assets valuation allowance | 13,825 | 1,444 | 89 | (100) | 15,258 | ||||||||||||||||||||||||
| Year ended December 31, 2020 | |||||||||||||||||||||||||||||
| Deferred tax assets valuation allowance | 12,069 | 1,617 | — | 139 | 13,825 | ||||||||||||||||||||||||
As of December 31, 2019,2022, the Company has not provided deferred income taxes on unrepatriated earnings from foreign subsidiaries as they are deemed to be indefinitely reinvested. Suchreinvested unless there is a manner under which to remit the earnings with no material tax cost. Material taxes would primarily be attributable to foreign withholding taxes and local income taxes when such earnings are distributed. As such,The Company will repatriate foreign earnings when there is no need for reinvestment overseas and no material tax cost to bring the Company has determinedearnings back to the tax impact of repatriating these earningsUnited States. Reinvestment considerations would 0t be material as of December 31, 2019.include future acquisitions, transactions, and capital expenditure plans.
A reconciliation of the beginning and ending amount of uncertain tax benefits is as follows:
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| Balance, beginning of year | $ | 676 | $ | 424 | $ | 754 | |||||
| Gross increases: | |||||||||||
| Current year tax positions | 53 | 273 | 402 | ||||||||
| Prior years' tax positions | — | — | — | ||||||||
| Gross decreases: | |||||||||||
| Prior years' tax positions | — | — | (777 | ) | |||||||
| Statute of limitations lapses | — | (21 | ) | (17 | ) | ||||||
| Other | (53 | ) | — | 62 | |||||||
| Balance, end of year | $ | 676 | $ | 676 | $ | 424 | |||||
| Years Ended December 31, | |||||||||||||||||
| Dollars in thousands | 2022 | 2021 | 2020 | ||||||||||||||
| (In thousands) | |||||||||||||||||
| Balance, beginning of year | $ | 676 | $ | 702 | $ | 676 | |||||||||||
| Gross increases: | |||||||||||||||||
| Current year tax positions | 37 | — | — | ||||||||||||||
| Prior years' tax positions | — | — | 26 | ||||||||||||||
| Other | — | (26) | — | ||||||||||||||
| Balance, end of year | $ | 713 | $ | 676 | $ | 702 | |||||||||||
Approximately $0.7 million of the balance at December 31, 20192022 relates to uncertain tax positions that, if recognized, would affect the annual effective tax rate. There are 0 amounts within the balanceThe Company has $0.3 million of uncertain tax positions at December 31, 20192022 related to tax positions for which it is reasonably possible that the amounts could be reduced during the twelve months following December 31, 2019.2022.
The Company recognizes interest and penalties relating to uncertain tax positions in income tax expense. The Company recognized a minimal benefit for the years ended December 31, 2019, 20182022, 2021 and 2017.2020. The Company had minimal interest and penalties accrued for the years ended December 31, 20192022 and 20182021 and 2017.2020.
The Company files Federal income tax returns, as well as multiple state, local and foreign jurisdiction tax returns. The Company is no longer subject to examinations of its U.S. consolidated Federal income tax returns by the IRS through fiscal year 2015. Additionally, the Company is no longer subject to examinations by the IRS for fiscal year 2017. All significant state and local matters have been concluded through fiscal year 2014. All significant foreign matters have been settled through fiscal 2012.
F-44
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
13. NET INCOME PER SHARE
Basic and diluted net income per share was as follows:
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands, except per share amounts) | |||||||||||
| Basic net income per share: | |||||||||||
| Net income | $ | 50,201 | $ | 60,801 | $ | 64,743 | |||||
| Weighted average common shares outstanding | 85,637 | 82,857 | 76,897 | ||||||||
| Basic net income per common share | $ | 0.59 | $ | 0.73 | $ | 0.84 | |||||
| Diluted net income per share: | |||||||||||
| Net income | $ | 50,201 | $ | 60,801 | $ | 64,743 | |||||
| Weighted average common shares outstanding — Basic | 85,637 | 82,857 | 76,897 | ||||||||
| Effect of dilutive securities: | |||||||||||
| Warrants | — | — | 971 | ||||||||
| Stock options and restricted stock | 857 | 1,142 | 1,253 | ||||||||
| Weighted average common shares for diluted earnings per share | 86,494 | 83,999 | 79,121 | ||||||||
| Diluted net income per common share | $ | 0.58 | $ | 0.72 | $ | 0.82 | |||||
| Years Ended December 31, | |||||||||||||||||
| Dollars in thousands, except per share amounts | 2022 | 2021 | 2020 | ||||||||||||||
| Basic net income per share: | |||||||||||||||||
| Net income | $ | 180,550 | $ | 169,075 | $ | 133,892 | |||||||||||
| Weighted average common shares outstanding | 82,997 | 84,698 | 84,650 | ||||||||||||||
| Basic net income per common share | $ | 2.18 | $ | 2.00 | $ | 1.58 | |||||||||||
| Diluted net income per share: | |||||||||||||||||
| Net income | $ | 180,550 | $ | 169,075 | $ | 133,892 | |||||||||||
| Weighted average common shares outstanding — Basic | 82,997 | 84,698 | 84,650 | ||||||||||||||
| Effect of dilutive securities: | |||||||||||||||||
| Stock options and restricted stock | 519 | 787 | 577 | ||||||||||||||
| Weighted average common shares for diluted earnings per share | 83,516 | 85,485 | 85,228 | ||||||||||||||
| Diluted net income per common share | $ | 2.16 | $ | 1.98 | $ | 1.57 | |||||||||||
Common stock of approximately 0.40.3 million and 0.1 million shares at December 31, 2019,2022, and 20182021 that are issuable through exercise of dilutive securities, respectively, and were not included in the computation of diluted net income per share because their effect would have been anti-dilutive.
14. ACCUMULATED OTHER COMPREHENSIVE LOSSINCOME (LOSS)
Changes in accumulated other comprehensive loss by component between December 31, 20192022 and 20182021 are presented in the table below, net of tax:
| Gains and Losses on Derivatives | Defined Benefit Pension Items | Foreign Currency Items | Total | |||||||||||||
| (In thousands) | ||||||||||||||||
| Balance at December 31, 2018 | $ | (4,813 | ) | $ | (736 | ) | $ | (39,894 | ) | $ | (45,443 | ) | ||||
| Other comprehensive loss, net | (10,420 | ) | (8,973 | ) | (174 | ) | (19,567 | ) | ||||||||
| Less: Amounts reclassified from accumulated other comprehensive income, net | 11,392 | — | — | 11,392 | ||||||||||||
| Net current-period other comprehensive loss | (21,812 | ) | (8,973 | ) | (174 | ) | (30,959 | ) | ||||||||
| Balance at December 31, 2019 | $ | (26,625 | ) | $ | (9,709 | ) | $ | (40,068 | ) | $ | (76,402 | ) | ||||
| Dollars in thousands | Gains and Losses on Derivatives | Defined Benefit Pension Items | Foreign Currency Items | Total | ||||||||||||||||||||||
| Balance at December 31, 2021 | $ | (42,981) | $ | 1,893 | $ | (4,067) | $ | (45,155) | ||||||||||||||||||
| Other comprehensive gain (loss) | 80,335 | 7,429 | (17,807) | 69,957 | ||||||||||||||||||||||
| Less: Amounts reclassified from accumulated other comprehensive income, net | 14,537 | — | — | 14,537 | ||||||||||||||||||||||
| Net current-period other comprehensive gain (loss) | 65,798 | 7,429 | (17,807) | 55,420 | ||||||||||||||||||||||
| Balance at December 31, 2022 | $ | 22,817 | $ | 9,322 | $ | (21,874) | $ | 10,265 | ||||||||||||||||||
For the year ended December 31, 2019,2022, the Company reclassified gainsa gain of $2.3 million, $7.4$15.0 million and $1.7a loss of $0.4 million from AOCLaccumulated other comprehensive loss to other income, net and interest income, and interest expense, respectively.
15. COMMITMENTS AND CONTINGENCIES
In consideration for certain technology, manufacturing, distribution, and selling rights and licenses granted to the Company, the Company has agreed to pay royalties on sales of certain products that it sells. The royalty payments that the Company made under these agreements were not significant for any of the periods presented.
The Company is subject to various claims, lawsuits and proceedings in the ordinary course of the Company's business, including claims by current or former employees, distributors and competitors and with respect to its products and product liability claims, lawsuits and proceedings, some of which have been settled by the Company. In the opinion of management, such claims are either adequately covered by insurance or otherwise indemnified, or are not expected, individually or in the aggregate, to result in a material, adverse effect on the Company's financial condition. However, it is possible that the
F-45
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Company's results of operations, financial position and cash flows in a particular period could be materially affected by these contingencies.
The Company accrues for loss contingencies when it is deemed probable that a loss has been incurred and that loss is estimable. The amounts accrued are based on the full amount of the estimated loss before considering insurance proceeds and do not include an estimate for legal fees expected to be incurred in connection with the loss contingency. The Company consistently accrues legal fees expected to be incurred in connection with loss contingencies as those fees are incurred by outside counsel as a period cost.
Contingent Consideration
The Company determined the fair value of contingent consideration during the twelve-month period ended December 31, 20192022 and 20182021 to reflect the change in estimate, additions, payments, transfers and the time value of money during the period.
A reconciliation of the opening balances to the closing balances of these Level 3 measurements for the yearyears ended December 31, 20192022 and 20182021 is as follows (in thousands):
| Year ended December 31, 2022 | Contingent Consideration Liability Related to Acquisition of: | ||||||||||||||||||||||||||||||||||||||||||||||
| Arkis (See Note 4) | Location in Financial Statements | Derma Sciences | ACell Inc. (See Note 4) | SIA | Location in Financial Statements | ||||||||||||||||||||||||||||||||||||||||||
| Short-term | Long-term | Long-term | Short-term | Long-term | Long-term | ||||||||||||||||||||||||||||||||||||||||||
| Balance as of January 1, 2022 | $ | 3,691 | $ | 11,408 | $ | 230 | $ | — | $ | 21,800 | $ | — | |||||||||||||||||||||||||||||||||||
| Additions | — | — | — | — | 57,607 | ||||||||||||||||||||||||||||||||||||||||||
| Transfers from long-term to current portion | — | — | — | 4,885 | (4,885) | — | |||||||||||||||||||||||||||||||||||||||||
| Change in fair value of contingent consideration liabilities | (846) | (1,358) | Research and development | — | (4,885) | (13,215) | — | Selling, general and administrative | |||||||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2022 | $ | 2,845 | $ | 10,050 | $ | 230 | $ | — | $ | 3,700 | $ | 57,607 | $ | — | |||||||||||||||||||||||||||||||||
| Year ended December 31, 2021 | Contingent Consideration Liability Related to Acquisition of: | |||||||||||||||||||||||||||||||||||||||||||
| Arkis (See Note 4) | Location in Financial Statements | Derma Sciences | ACell Inc. (See Note 4) | Location in Financial Statements | ||||||||||||||||||||||||||||||||||||||||
| Short-term | Long-term | Long-term | Long-term | |||||||||||||||||||||||||||||||||||||||||
| Balance as of January 1, 2021 | $ | 3,415 | $ | 11,746 | $ | 230 | $ | — | ||||||||||||||||||||||||||||||||||||
| Additions | — | — | — | 23,900 | ||||||||||||||||||||||||||||||||||||||||
| Transfers from long-term to current portion | 276 | (276) | — | — | ||||||||||||||||||||||||||||||||||||||||
| Change in fair value of contingent consideration liabilities | — | $ | (62) | Research and development | — | (2,100) | Selling, general and administrative | |||||||||||||||||||||||||||||||||||||
| Balance as of December 31, 2021 | $ | 3,691 | $ | 11,408 | $ | 230 | $ | 21,800 | ||||||||||||||||||||||||||||||||||||
Derma Sciences
The Company assumed contingent consideration incurred by Derma Sciences, Inc. ("Derma Sciences") related to its acquisitions of BioD and the intellectual property related to Medihoney products. The Company accounted for the contingent liabilities by recording their fair value on the date of the acquisition based on a probability weighted income approach. The Company has already paid $33.3 million related to the aforementioned contingent liabilities. One contingent milestone remains which relates to net sales of Medihoney™ products exceeding certain amounts defined in the agreement between the Company and Derma Sciences. The potential maximum undiscounted payment amounts to $3.0 million. The estimated fair value as of December 31, 2022 and 2021 was $0.2 million.
F-46
| Year Ended December 31, 2019 | Contingent Consideration Liability Related to Acquisition of Arkis (See Note 4) | Contingent Consideration Liability Related to Acquisition of Derma Sciences (See Note 4) | Location in Financial Statements | ||||||
| Long-term | Long-term | ||||||||
| Balance as of January 1, 2019 | $ | — | $ | 230 | |||||
| Additions from acquisition of Arkis | 13,100 | — | |||||||
| Payments | — | — | |||||||
| Loss from change in fair value of contingent consideration liabilities | 1,110 | — | Research and development | ||||||
| Balance as of December 31, 2019 | $ | 14,210 | $ | 230 | |||||
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| Year Ended December, 2018 | Contingent Consideration Liabilities Related to Acquisition of Derma Sciences (See Note 4) | Contingent Consideration Liability Related to Acquisition of Confluent Surgical, Inc. | Location in Financial Statements | ||||||||||
| Short-term | Long-term | Short-term | |||||||||||
| Balance as of January 1, 2018 | $ | 315 | $ | 1,387 | $ | 22,478 | |||||||
| Transfers from long-term to current portion | 1,387 | (1,387 | ) | — | |||||||||
| Payments | (2,000 | ) | — | (24,000 | ) | ||||||||
| Loss from change in fair value of contingent consideration liabilities | 298 | 230 | 1,522 | Selling, general and administrative | |||||||||
| Balance as of December 31, 2018 | $ | — | $ | 230 | $ | — | |||||||
As part of Confluent Surgical, Inc., ("Confluent Surgical"). The purchase price included contingent consideration. The potential maximum undiscounted contingent consideration of $30.0 million consisted of $25.0 million upon obtaining certain U.S. governmental approvals (the "U.S. Contingent Consideration") and $5.0 million upon obtaining certain European governmental approvals, both related to the completion of the transition of the Confluent Surgical business. The fair values of contingent consideration related to the acquisition of Confluent Surgical were estimated using a discounted cash flow model using a discount rate of 2.2%. During the first quarter of 2018,Arkis BioSciences, Inc. ("Arkis"), the Company receivedis required to pay the U.S. governmental approvalsformer shareholders of Arkis up to $25.5 million based on the timing of certain development milestones of $10 million and adjustedcommercial sales milestones of $15.5 million, respectively. The Company used a probability weighted income approach to calculate the relatedfair value of the contingent consideration liabilitythat considered the possible outcomes of scenarios related to $19.0 million, which the Company paid in April 2018. During the third quarter of 2018, the Company received certain European governmental approvals.each specified milestone. The Company paidestimated the remaining $5.0 millionfair value of the contingent consideration related to Confluent Surgicalbe $13.1 million at the acquisition date. The estimated the fair value as of December 31, 2022 was $12.9 million. The Company recorded $2.8 million in Octoberaccrued expenses and other current liabilities and $10.1 million in other liabilities at December 31, 2022 in the consolidated balance sheets of 2018.the Company.
16. SEGMENT AND GEOGRAPHIC INFORMATION
The Company internally manages 2two global reportable segments and reports the results of its businesses to its chief operating decision maker. The 2two reportable segments and their activities are described below.
•The Codman Specialty Surgical segment includes (i) the Neurosurgery business, which sells a full line of products for neurosurgery and neuro critical care such as tissue ablation equipment, dural repair products, cerebral spinal fluid management devices, intracranial monitoring equipment, and cranial stabilization equipment and (ii) the precision tools and instrumentsInstruments business, which sells more than 40,000 instrument patterns and surgical and lighting products to hospitals, surgery centers, dental, podiatry, and veterinary offices.
•The Orthopedics and Tissue Technologies segment includes such offerings as skin and wound repair, bone and joint fixation implants in the upper and lower extremities,plastics & surgical reconstruction products, bone grafts, and nerve and tendon repair products.
The Corporate and other category includes (i) various executive, finance, human resource, information systems and legal functions, (ii) brand management, and (iii) share-based compensation costs.
The operating results of the various reportable segments as presented are not comparable to one another because (i) certain operating segments are more dependent than others on corporate functions for unallocated general and administrative and/or operational manufacturing functions, and (ii) the Company does not allocate certain manufacturing costs and general and administrative costs to the operating segment results. Net sales and profit by reportable segment for the years ended December 31, 2019, 20182022, 2021 and 20172020 are as follows:
| Years Ended December 31, | |||||||||||||||||
| Dollars in thousands | 2022 | 2021 | 2020 | ||||||||||||||
| Segment Net Sales | |||||||||||||||||
| Codman Specialty Surgical | $ | 1,019,564 | $ | 1,025,232 | $ | 894,831 | |||||||||||
| Tissue Technologies | 538,102 | 517,216 | 477,037 | ||||||||||||||
| Total revenues | $ | 1,557,666 | $ | 1,542,448 | $ | 1,371,868 | |||||||||||
| Segment Profit | |||||||||||||||||
| Codman Specialty Surgical | $ | 417,873 | $ | 439,471 | $ | 356,657 | |||||||||||
| Tissue Technologies | 233,802 | 228,199 | 159,630 | ||||||||||||||
| Segment profit | 651,675 | 667,670 | 516,287 | ||||||||||||||
| Amortization | (13,882) | (16,914) | (27,757) | ||||||||||||||
| Corporate and other | (398,873) | (453,526) | (337,160) | ||||||||||||||
| Operating income | $ | 238,920 | $ | 197,230 | $ | 151,370 | |||||||||||
F-47
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| Years Ended December 31, | |||||||||||
| 2019 | 2018 | 2017 | |||||||||
| (In thousands) | |||||||||||
| Segment Net Sales | |||||||||||
| Codman Specialty Surgical | $ | 996,206 | $ | 963,929 | $ | 720,301 | |||||
| Orthopedics and Tissue Technologies | 521,351 | 508,512 | 467,935 | ||||||||
| Total revenues | $ | 1,517,557 | $ | 1,472,441 | $ | 1,188,236 | |||||
| Segment Profit | |||||||||||
| Codman Specialty Surgical | $ | 395,019 | $ | 363,336 | $ | 292,971 | |||||
| Orthopedics and Tissue Technologies | 144,638 | 149,510 | 129,697 | ||||||||
| Segment profit | 539,657 | 512,846 | 422,668 | ||||||||
| Amortization | (27,028) | (21,160) | (20,370) | ||||||||
| Corporate and other | (418,869 | ) | (380,688 | ) | (357,494 | ) | |||||
| Operating income | $ | 93,760 | $ | 110,998 | $ | 44,804 | |||||
The Company does not allocate any assets to the reportable segments. No asset information is reported to the chief operating decision maker and disclosed in the financial information for each segment.
The Company attributes revenue to geographic areas based on the location of the customer. Total revenue, net and long-lived assets (tangible) by major geographic area are summarized below:
| United States* | Europe | Asia Pacific | Rest of the World | Consolidated | |||||||||||||||
| (In thousands) | |||||||||||||||||||
| Total revenue, net: | |||||||||||||||||||
| 2019 | $ | 1,077,379 | $ | 197,468 | $ | 157,391 | $ | 85,319 | $ | 1,517,557 | |||||||||
| 2018 | 1,045,887 | 201,354 | 144,253 | 80,947 | 1,472,441 | ||||||||||||||
| 2017 | 894,260 | 150,147 | 80,636 | 63,193 | 1,188,236 | ||||||||||||||
| Total long-lived assets: | |||||||||||||||||||
| 2019 | $ | 383,652 | $ | 47,325 | $ | 8,598 | $ | 7,143 | $ | 446,718 | |||||||||
| 2018 | 280,382 | 32,679 | 3,765 | 3,203 | 320,029 | ||||||||||||||
| Dollars in thousands | United States(1) | Europe | Asia Pacific | Rest of the World | Consolidated | ||||||||||||||||||||||||
| Total revenue, net: | |||||||||||||||||||||||||||||
| 2022 | $ | 1,126,810 | $ | 170,903 | $ | 176,477 | $ | 83,476 | $ | 1,557,666 | |||||||||||||||||||
| 2021 | 1,089,526 | 191,327 | 182,034 | 79,561 | 1,542,448 | ||||||||||||||||||||||||
| 2020 | 971,975 | 172,689 | 157,174 | 70,030 | 1,371,868 | ||||||||||||||||||||||||
| Total long-lived assets: | |||||||||||||||||||||||||||||
| 2022 | $ | 440,223 | $ | 60,857 | $ | 12,975 | $ | 2,721 | $ | 516,776 | |||||||||||||||||||
| 2021 | 339,535 | 55,026 | 11,289 | 6,836 | 412,686 | ||||||||||||||||||||||||
| (In thousands, except per share data) | |||||||||||||||||||
| Quarter | Total revenue, net | Gross margin | Net income (loss) | Per Share - Basic (1) | Per Share - Diluted (1) | ||||||||||||||
| 2019 | |||||||||||||||||||
| First | 359,690 | 230,778 | 32,756 | $ | 0.38 | $ | 0.38 | ||||||||||||
| Second | 383,645 | 239,974 | 29,736 | 0.35 | 0.34 | ||||||||||||||
| Third | 379,095 | 236,459 | (27,610 | ) | (0.32 | ) | (0.32 | ) | |||||||||||
| Fourth | 395,127 | 245,665 | 15,319 | 0.18 | 0.18 | ||||||||||||||
| 1,517,557 | 952,876 | 50,201 | |||||||||||||||||
| 2018 | |||||||||||||||||||
| First | $ | 357,082 | $ | 212,860 | $ | 10,992 | $ | 0.14 | $ | 0.14 | |||||||||
| Second | 366,190 | 228,625 | 11,376 | 0.14 | 0.14 | ||||||||||||||
| Third | 365,854 | 222,609 | 13,295 | 0.16 | 0.15 | ||||||||||||||
| Fourth | 383,315 | 236,851 | 25,138 | 0.29 | 0.29 | ||||||||||||||
| $ | 1,472,441 | $ | 900,945 | $ | 60,801 | ||||||||||||||
| Balance at Beginning of Period | Charged to Costs and Expenses | Other | Deductions | Balance at End of Period | |||||||||||||||
| Description | |||||||||||||||||||
| (In thousands) | |||||||||||||||||||
| Year ended December 31, 2019 | |||||||||||||||||||
| Allowance for doubtful accounts | 3,719 | 2,126 | — | (1,542 | ) | (2) | 4,303 | ||||||||||||
| Deferred tax assets valuation allowance | 6,973 | 3,848 | 1,291 | (4) | (43 | ) | 12,069 | ||||||||||||
| Year ended December 31, 2018 | |||||||||||||||||||
| Allowance for doubtful accounts | $ | 8,882 | $ | 557 | $ | (4,649 | ) | (3) | $ | (1,071 | ) | (2) | $ | 3,719 | |||||
| Deferred tax assets valuation allowance | 7,961 | (894 | ) | — | (94 | ) | 6,973 | ||||||||||||
| Year ended December 31, 2017 | |||||||||||||||||||
| Allowance for doubtful accounts and sales returns and allowances | $ | 6,319 | $ | 4,920 | $ | 1,518 | (1) | (3,875 | ) | (2) | $ | 8,882 | |||||||
| Deferred tax assets valuation allowance | 3,604 | 740 | 3,617 | (1) | — | 7,961 | |||||||||||||