UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
(Mark One) | | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 20212023
or | | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
COMMISSION FILE NO. 0-26224000-26224
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
(EXACT NAME OF REGISTRANT AS SPECIFIED IN ITS CHARTER)
| | | | | | | | | | | | | | |
| Delaware | | 51-0317849 |
(STATE OR OTHER JURISDICTION OF
INCORPORATION OR ORGANIZATION) | | (I.R.S. EMPLOYER
IDENTIFICATION NO.) |
| |
| 1100 Campus Road | | 08540 |
| Princeton | , | New Jersey | | (ZIP CODE) |
| (ADDRESS OF PRINCIPAL EXECUTIVE OFFICES) | |
REGISTRANT’S TELEPHONE NUMBER, INCLUDING AREA CODE: (609) 275-0500
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT: | | | | | | | | |
| Title of Each Class | Trading Symbol | Name of Exchange on Which Registered |
| Common Stock, Par Value $.01 Per Share | IART | Nasdaq Global Select Market |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT:
NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act. Yes ☐
No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one): | | | | | | | | | | | |
| Large accelerated filer | ☒ | Accelerated filer | ☐ |
| | | |
| Non-accelerated filer | ☐ | Smaller reporting company | ☐ |
| | | |
| Emerging growth company | ☐ | | |
If an emerging growth company, indicate by check if the registrant has elected not to use the extended transition period for complying with any new revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of June 30, 2021,2023, the aggregate market value of the registrant’s common stock held by non-affiliates was approximately $4,968.6$3,256.6 million based upon the closing sales price of the registrant’s common stock on The Nasdaq Global Select Market on such date. The number of shares of the registrant’s Common Stock, $0.01 par value, outstanding as of February 22, 202227, 2024 was 83,243,031.78,219,780.
DOCUMENTS INCORPORATED BY REFERENCE:
Certain portions of the registrant’s definitive proxy statement relating to its scheduled May 13, 20229, 2024 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission, are incorporated by reference in Part III of this report.Annual Report on Form 10-K.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
Unless otherwise stated or the context otherwise indicates, all references in this Annual Report on Form 10-K to “Integra LifeSciences,” “Integra,” “the Company,” “we,” “our,” and “us” refer to Integra LifeSciences Holdings Corporation, a Delaware corporation and its consolidated subsidiaries.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
We have made statements in this report, including statements under “Business” and “Management's Discussion and Analysis of Financial Condition and Results of Operations” that constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, ("the Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended ("the "Exchange Act"). These forward-looking statements are subject to a number of risks, uncertainties and assumptions about us including, among other things:
•the on-going and possible future effects of global challenges, including macroeconomic uncertainties, such as supply chain disruptions, inflation, bank failures, rising interest rates and availability of capital markets, the Israel-Hamas and Ukraine-Russia wars, other economic disruptions and U.S. and global recession concerns, on our customers and suppliers, and on our business, financial condition, results of operations and cash flows;
•general economic and business conditions, both domestically and in our international markets, including the effect of the continuing worldwide macroeconomic uncertainty and increasing trade regulations and tariffs;
•our expectations and estimates concerning future financial performance, financing plans and the impact of competition;
•anticipated trends in our business;
•anticipated demand for our products, particularly capital equipment;
•our ability to produce and deliver products in sufficient quantities to meet sales demands;
•the ongoing and possible future effects of supply chain constraints, including the availability of critical raw materials and components, as well as cost inflation in materials, packaging and transportation;
•our expectations concerning our ongoing restructuring, integration and manufacturing transfer and expansion activities;
•existing and future regulations affecting our business, and enforcement of those regulations;
•conducting business internationally;
•our failure to comply with the substantial regulation related to quality standards applicable to our manufacturing and quality processes could have an adverse effect on our business, financial condition, or results of operations;
•our ability to remediate all matters identified in United States Food & Drug (the "FDA") observations and warning letters that we received or may receive;
•our ability to obtain additional debt and equity financing to fund capital expenditures, working capital requirements and acquisitions;
•physicians' willingness to adopt our recently launched and planned products, third-party payors' willingness to provide or continue reimbursement for any of our products and our ability to secure regulatory approval for products in development;
•initiatives launched by our competitors;
•our ability to protect our intellectual property, including trade secrets;
•our ability to complete acquisitions, integrate operations post-acquisition and maintain relationships with customers of acquired entities;
•the ability to successfully manage ongoing organizational and strategic changes, including our ability to attract, motivate and retain key employees and maintain engagement and efficiency in remote work environments;
•anticipated trends relating to our financial condition or results of operations, including the impact of interest rate and foreign currency exchange fluctuations;
•the effect of any future public health crises, including the timing, scope and effect of U.S. and international governmental, regulatory, fiscal, monetary and public health responses to such crises; and
•other risk factors described in Item 1A. "Risk Factors" in this Annual Report on Form 10-K.
Forward-looking statements can be identified by forward-looking words such as “believe,” “may,” “could,” “might,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “expect,” “should,” “would,” “expect,” “target,” “pursue,” “forecast,” “hope” and similar expressions in this Annual Report on Form 10K. Forward-looking statements in this Annual Report on Form 10-K include, but are not limited to, statements regarding our five-pillar growth strategy; the closing of our pending acquisition of Acclarent, Inc. on anticipated terms and timing, or at all; the anticipated benefits of our pending acquisition of Acclarent, Inc.; expectations and plans with respect to strategic initiatives, product development and regulatory approvals, including the anticipated resumption of manufacturing at the Company’s Boston, Massachusetts facility. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by such forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this report. Except as required by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Factors that could cause or contribute to differences in our future financial results include the cautionary statements set forth herein and in our other filings with the Securities and Exchange Commission, including those set forth under “Risk Factors” set forth in Part I, Item 1A of this Annual Report on Form 10-K. We qualify all of our forward-looking statements by these cautionary statements.
PART I
ITEM 1. BUSINESS
OVERVIEW
The terms “we,” “our,” “us,” “Company” and “Integra” refer to Integra LifeSciences Holdings Corporation a Delaware corporation, and its subsidiaries, unless the context suggests otherwise.
The Company, headquartered in Princeton, New Jersey, is a world leader inleading global medical technology. The Company was foundedtechnology company innovating treatment pathways to advance patient outcomes and set new standards of surgical, neurologic and regenerative care.
Founded in 1989 with the acquisition of an engineered collagen technology platform used to repair and regenerate tissue. Since then, Integra hastissue, our common stock trades on the Nasdaq Global Select Market (“Nasdaq”) under the symbol “IART.” We have developed numerous product lines from this technology for applications ranging from burn and deep tissue wounds to the repair of dura mater in the brain, as well as nerves and tendons. The Company hasWe have expanded itsour base regenerative technology business to include surgical instruments, neurosurgical products and advanced wound care through global acquisitions and product development to meet the evolving needs of itsour customers and enhance patient care.
Integra products are sold in more than 130 countries through a direct sales force as well as distributors and wholesalers. We manufacture and sell medical technologies and products in two reportable business segments: Codman Specialty Surgical ("CSS") and Tissue Technologies ("TT"). The CSS segment, which represents approximately two-thirds of our total revenue, consists of market-leading technologies and instrumentation used for a wide range of specialties, such as neurosurgery, neurocritical care and otolaryngology. We are the world leader in neurosurgery and one of the top three providers in instruments used in precision, specialty, and general surgical procedures. Our TT segment generates about one-third of our overall revenue and focuses on three main areas: complex wound surgery, surgical reconstruction, and peripheral nerve repair.
We have key manufacturing and research facilities located in California, Indiana, Maryland, Massachusetts, New Jersey, Ohio, Puerto Rico, Tennessee, Utah, Canada, China, France, Germany, Ireland and Switzerland. We source most of our handheld surgical instruments and dural sealant products through specialized third-party vendors.
Vision
We aspire to continue to be a worldwide leader in neurosurgery and reconstructive surgery with a portfolio of leading businesses that delivers outstanding customer experiences through innovation, execution and teamwork to positively impact the lives of millions of patients and their families.
Strategy
Integra is committed to delivering high quality products that positively impactFollowing the lives of millions of patients and their families. We focus on four key pillarscompletion of our strategy: 1) enabling an execution-focused culture, 2) optimizing relevant scale, 3) advancing innovationstrategic refresh in 2023, we refocused our strategies around five pillars. Of these five pillars, we have identified three core growth drivers: (1) innovating for outcomes, (2) growing internationally, and agility,(3) broadening our impact on care pathways. Our execution of the core growth drivers is enabled by two key levers: (4) driving operational and 4) leadingcustomer excellence and (5) cultivating a high-performance culture. As outlined in customer experience. Wegreater detail below, we believe that by sharpeningthese five pillars will enable us to realize and advance our focus on these areas through improved planning and communication, optimization of our infrastructure, and strategically aligned acquisitions, we can build scale, increase competitiveness and achieve our long-term goals.integrated growth strategy.
To this end, theour executive leadership team has established the following key priorities aligned to the following areas of focus:five pillars:
Strategic AcquisitionsInnovating for Outcomes. An important part of the Company'sIntegra’s growth strategy is pursuing strategic transactionsintroducing new products to strengthen and licensing agreementsexpand our portfolio, including via acquisitions. For example, we entered into a stock purchase agreement to acquire Acclarent, Inc. (“Acclarent”) from Ethicon, Inc., a subsidiary of Johnson & Johnson in December 2023. Acclarent is an innovator and market leader in ear, nose and throat (“ENT”) procedures and we believe that increase relevant scalethe acquisition of Acclarent will provide Integra with the opportunity to become a leading provider of ENT products and technologies. Furthermore, we believe that, owing to the ENT segment being an anatomical adjacency to neurosurgery, the acquisition will allow Integra to deliver future innovation both within the ENT segment and across our other CSS technology platforms. Additionally, we seek clinical evidence to support regulatory approval and strong reimbursement of our product portfolio around the world, including new indications for existing technologies. For example, in 2021, we filed a pre-market approval (“PMA”) application for a specific indication for Surgimend® in the clinical areasuse of post-mastectomy breast reconstruction. In 2022, we acquired SIA, which is also pursuing a pre-market approval for DuraSorb for use in which Integra competes. During 2021, the Company acquired ACell Inc. ("ACell"implant-based breast reconstruction (“IBBR”), an innovative regenerative medicine company specializingand in June 2023 we completed enrollment in the manufacturing of porcine urinary bladder extracellular matrices. This acquisition not only expandedDuraSorb U.S. IDE clinical study for two-stage breast reconstruction; the Company’s product offering of regenerative technologies, but itprimary follow-up period is one year after device implantation. We hope to obtain FDA approvals for both products in 2025. We also supported the Company’s long-term growth and profitability strategy as this product line has a financial profile similar to Integra’s other regenerative tissue products. All critical components of ACell have been integrated into the Company’s TT segment. See Note 4, Acquisitions and Divestitures, to the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K) for additional details. In 2021, we continued to advance the development of pioneering neurosurgical technologies fromwith the expansion of our 2019 acquisitions, Arkis Biosciences, Inc.product offerings. In 2023 we launched the CUSA® Clarity Tips for use in surgical procedures requiring the controlled fragmentation, emulsification and Rebound Therapeutics Corporation.aspiration of bone as well as in laparoscopic liver surgery.
Portfolio Optimization Over the years, we have been significantly expanding our global footprint through investments in our commercial organization, the expansion and New Product Introductions. We are investing in innovative product development to drive a multi-generational pipeline for our key product franchises. Our product development efforts span across our key global franchises focused on potential for significant returns on investment. In addition toof international markets and new product development, we are funding studies to gather clinical evidence to support launches, ensure market access and improve reimbursement for existing products. In addition to acquisitions and organic reinvestment, we continually look to optimize our portfolio towards higher growth and higher margin businesses.introductions. As such, we may opportunistically divest businesses or discontinue products where we see limited runway for future value creation in line with our aspirations due in part to changes in the market, business fundamentals or the regulatory environment.
In January 2021, we completed the sale of our Extremity Orthopedics business to Smith & Nephew USD Limited ("Smith & Nephew"), a subsidiaryIn-China-For-China strategy, we continue the build out of Smith & Nephew plc, for approximately $240 millionour assembly capabilities in cash. This transaction enables us to increaseour new facility in Suzhou, China. Several new products were introduced in select international markets in 2023, including MicroMatrix® and Certas Plus® Programmable Valve which were launched in Europe, and CUSA Clarity Laparoscopic tip which was launched in Australia,
our investmentsNew Zealand, Japan, Canada, South Africa and Israel. In addition, DuraGen Secure, received approval in our core neurosurgeryJapan, while DuraGen Plus, an absorbable and tissuesutureless collagen onlay indicated as a dura substitute for the repair of dura mater, was approved in China.
Broadening Impact on Care Pathways. We seek ways to develop products and technologies businessesthat impact the lives of patients, starting with the journey that a patient takes from diagnosis and fund pipeline opportunitiestreatment planning to expand our addressable marketssurgery and postoperative care. We are well-established in acute care in the hospital setting and continue to strengthen our existing leadership positionsleverage that strong position to grow in these segmentsthis segment and drive future growth. See Note 4, Acquisitionsshape treatment pathways into preoperative care and Divestitures, to the Notes to Consolidated Financial Statements (Part II, Item 8additional sites of this Form 10-K) for details.care.
Commercial Channel InvestmentsDriving Operations and Customer Excellence.. Investing in our sales channels is a core part of our strategy to create specialization and greater focus on reaching new and existing customers and addressing their needs. To support our commercial efforts in Tissue Technologies, we expanded our two-tier specialist model to increase our presence in focused segments by creating a virtual selling organization to help serve the evolving needs of our customers. In addition, we continue to build upon our leadership brands across our product franchises in both CSS and TT to engage customers through enterprise-wide contracts with leading hospitals, integrated delivery networks and global purchasing organizations in the United States. Internationally, we We have increased our commercial resources significantly in key emerging markets and arebeen making investments to supportbuild more responsive and scalable processes, enhance the reliability of our sales organizationsupply chain, and maximize our commercial opportunities. These investments in our international sales channel position us well for expansiondrive productivity initiatives to further supply and long-term growth.
Customer Experience. We aspire to be ranked as a best-in-class provider and are committed to strengthen our relationships with all customers. Welower costs. Additionally, we continue to invest in technologies, systems and processes to enhance the customer experience. In 2023, we continued to invest in our capacity expansion. This includes ongoing projects of transferring our Boston manufacturing to a new location in Braintree, Massachusetts., validating manufacturing processes in our manufacturing facility in Plainsboro, New Jersey and increasing cleanroom capacity in our Memphis, Tennessee location.
Cultivating a High-Performance Culture. In seeking to sustain a culture of excellence and accountability, we have focused on employee empowerment and agility and building a diverse and inclusive workplace. These efforts resulted in our being named in several best workplace lists globally in 2023. Additionally, we launched digital toolshave been making further strides in advancing our environmental, social and programs, resources and virtual product traininggovernance ("ESG") agenda to drive continued customer familiarity withsustainability across the organization and recently published our growing portfoliosecond annual ESG report in the third quarter of medical technologies globally.2023. For more information on our ESG strategy, goals, performance, and achievements, please visit “Our Company—ESG Report” at https://www.integralife.com/esg-report. Information on our website is not incorporated by reference herein and is not part of this Annual Report on Form 10-K.
BUSINESS SEGMENTS
IntegraWe currently manufacturesmanufacture and sellssell our productsmedical technologies and technologiesproducts in the following two global reportable business segments: Codman Specialty Surgical and Tissue Technologies. We include financial information regarding our reportable business segments and certain geographic information under "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations", and Note 16, Segment and Geographic Information to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K).
Codman Specialty Surgical
Our CSS segment offers global, neurosurgery market-leading technologies, brands and instrumentation. The Codman Specialty Surgical business consistsproduct portfolio represents a continuum of a broad portfolio of market-leading brands, such as Codman®, DuraGen®, DuraSeal®, CUSA®, Mayfield® care from pre-operative, to the neurosurgery operating room, to the neuro-critical care unit and Bactiseal®, which are usedpost care for the management of multiple disease states, includingboth adult and pediatric patients suffering from brain tumors, traumatic brain injury, hydrocephaluscerebrospinal fluid pressure complications and other neurological conditions. The growthWe offer leading technologies in this business in the recent years has been fueled by geographic expansion and new product registrations in markets, such as China, Japan, and Europe, which we expect to continue in the near to long term.
We also expanded our product offerings in 2021 with the launch of our newdural repair, ultrasonic tissue ablation, intracranial pressure ("ICP") monitoring, system, CereLink™ in the U.S.hydrocephalus management, and Europe. It provides clinicians with advanced continuous ICP monitoring that until now, has not been available when treating patients with traumatic brain injuries
Moreover, we are expanding into minimally invasive surgery ("MIS")cranial stabilization systems, while providing a rich research and the surgical management of intracerebral hemorrhages ("ICH"), with the 2021 clinical launch of Aurora® Surgiscope®, a proprietary surgical solution with integrated visualization and capabilities designed specificallydevelopment pipeline for use in deep-seated brain lesions. We started gathering clinical evidence using this same technology for early surgical intervention for the treatment of ICH. We believe this technology offers the promise of transforming the standard of care in neurosurgery.growth.
Rounding out the portfolio is a catalog of surgical headlamps, and surgical instrumentation, as well as after-market service. With thousands of surgical instrument products, including specialty surgical instruments, we call on the central sterile processing unit of hospitals and acute care surgical centers. Additionally, through a strong U.S. distribution model, we can serve the needs of hundreds of medical offices.
Our global commercial network includes clinical specialists, a large direct global sales force and strategic partnerships and distributors that serve hospitals, integrated health networks, group purchasing organizations, clinicians, surgery centers and health care providers. Outside the U.S., we have a combination of direct and indirect sales channels in international markets to sell certain product lines.
Tissue Technologies
Following the sale of the Extremity Orthopedics business in the first quarter of 2021, we rebranded the OrthopedicsOur TT segment focuses on three main areas: complex wound surgery, surgical reconstruction, and Tissue Technologies segment as Tissue Technologies. See Note 4, Acquisitionsperipheral nerve repair and Divestitures, to the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K) for details.
The Tissue Technologies segment consists of fourfive unique regenerative technology areas - highly engineered bovine collagen, bovine dermis, porcine urinary bladder, and human amniotic tissue.tissue, and resorbable synthetic mesh. This broad regenerative platform, which includes multiple leading brands such as Integra® Dermal Matrices, AmnioExcel®, SurgiMend®, MicroMatrix® and NeuraGen®, primarily addresses the needs of plastic, reconstructive and general surgeons focused on the treatment of acute wounds, such as burns,
chronic wounds, including diabetic foot ulcers, and surgical tissue repair, such as hernia, tendon, peripheral nerve repair and protection.
We have a specialized sales organization composed of directly employed sales representatives, as well as specialty distributors, organized based upon their call point. Our wound reconstruction sales representatives call on surgeons doing procedures in limb salvage, trauma, wound reconstruction and burns, and chronic wounds primarily in the inpatient wound care clinic setting. We
also have a dedicated surgical reconstruction sales team focused on plastic and reconstructive surgery and hernia procedures with differentiated products. Finally, we have a distributor network focused on biologics. Outside the U.S., we have a combination of direct and indirect sales channels in international markets to sell certain product lines.
This business segment also includes private-label sales of a broad set of our regenerative and wound care technologies. Our customers are other medical technology companies that sell to end markets primarily in spine, surgical and wound care.
We anticipate new product introductionsCOMPETITION
The healthcare industry is highly competitive and new clinical indications will continue to contribute tocharacterized by continual change and improvements in technology. This is particularly the growth of the segment. In the third quarter of 2021, we filed the premarket approval application for a specific indication for SurgiMendcase in the use of post-mastectomy breast reconstruction, formarket segments in which we hopeoperate. A number of companies have developed or are expected to obtain FDA approvaldevelop products that compete or will compete with our products. Many of these competitors offer a broader product portfolio and have greater brand recognition than we do, which may make these competitors more attractive to hospitals, group purchasing organizations, laboratories, physicians and other potential customers. Competitors may develop superior products or products of similar quality for sale at the same or lower prices. Moreover, our products could be rendered obsolete by changes to industry standards or guidelines or advances in 2023.
COMPETITIONtechnology. We can give no assurance that we will be able to compete successfully with existing or new competitors
Our competitors for CSS areinclude divisions within Medtronic, Inc., Stryker Corporation, Becton DickinsonSteris PLC, and Company and Aesculap, a division of B. Braun Medical, Inc. In addition, we compete with many smaller specialized companies and larger companies that do not otherwise focus on the offerings of Codman Specialty Surgical technologies. We rely on the depth and breadth of our sales and marketing organization, our innovative technologies, and our procurement and manufacturing operations to maintain our competitive position.
Our competition incompetitors for TT includesinclude Smith & Nephew plc, Organogenesis Holdings Inc., MiMedx Group, Inc., LifeCell Corporation, a subsidiary of Allergan PLC, C.R. Bard, a subsidiary of Becton Dickinson and Company, and Axogen, Inc. We compete with many additional companies who partially participate in soft tissue reconstruction of complex wounds, peripheral nerve repair and surgical reconstruction.
In addition, our products also compete against medical practices that treat a condition without using a medical device or any particular product, such as medical practices that utilize autograft tissue instead of our dermal regeneration products, duraplasty products and nerve repair products. Depending on the product line, we compete based on our products' features, strength of our sales force or distributors, sophistication of our technology and cost effectiveness of our solution.
We believe that the success of our products depends on our ability to differentiate ourselves and to demonstrate that our products deliver the clinical and operational attributes that are most important and cost-effective to customers. These attributes include, but are not limited to, superiority in efficacy, ease of use, reliability, accuracy, quality and cost. We believe our continued success depends in large part upon our ability to invest in product enhancements and technologies that will help us distinguish our product portfolio from our competitors.
RESEARCH AND DEVELOPMENT STRATEGY
Our research and development activities focus on identifying unmet surgical needs and addressing those needs with innovative solutions and products. The markets in which we participate are characterized by rapid technological change, frequent product introductions and evolving customer requirements. Investment in research and development is critical to driving our future growth. Our research and development efforts are focused on the further development and improvement of our existing products, the design and development of new innovative medical technologies and regulatory compliance across all our business segments. We apply our core competency in regenerative technology to innovate products for neurosurgical, wound applications, plastic surgery, and reconstructive surgery and we have extensive R&D development programs for our core platforms of electromechanical technologies. Additionally, we conduct products and clinical studies to generate efficacy and health economic evidence.
Regenerative Technologies. Integra wasWe were the first Companycompany to receive a United States Food and Drug Administration ("FDA")an FDA claim for regeneration of dermal tissue and isare a world leader in regenerative technology. Because regenerative technology products represent a fast-growing, high-margin opportunity for us, we allocate a large portion of our research and development budget to these projects. Our regenerative technology development program applies our expertise in bioengineering to a range of biomaterials including natural materials such as purified collagen, andintact human or animal tissues, honey as well as synthetics such as polymers.resorbable synthetic polymers with our DuraSorband DuraSealproduct lines. These unique product designs are used for neurosurgical and orthopedicreconstructive surgical applications, as well as dermal regeneration, including the healing of chronic and acute wounds, tendon and nerve repair. Our regenerative technology platform includes our legacy Integra® Dermal Regeneration Template ("IDRT") products and complementary technologies that we have acquired. Our collagen manufacturing capability, combined with our history of innovation, including our launch of NeuraGen 3D, provides us with strong platform technologies for multiple indications.
In 2020, we announced positive clinical and economic data on Integra® Bilayer Wound Matrix ("IBWM") in complex lower extremity reconstruction based on two retrospective studies recently published in Plastic and Reconstructive Surgery, the official journal of the American Society of Plastic Surgeons. As surgeons look for ways to efficiently and effectively repair and close wounds, IBWM helps address the efficiency needed in operating rooms by reducing both the operating time and costs to hospitals and patients. In 2021, we completed one of the largest diabetic foot ulcers ("DFU"), randomized controlled trials of the PriMatrix® Dermal Repair Scaffold for the management of DFU. This multi-center study enrolled more than 225 patients with chronic DFU's over the course of 12-week treatments and 4-week follow-up phases. The results of this study, which was published in the Journal of Wound Care, demonstrated that PriMatrix plus standard of care ("SOC") consisting of sharp debridement, infection elimination, use of dressings and offloading was significantly more likely to achieve complete wound closure compared with SOC alone, with a median number of one application of the product.
In the second quarter of 2023, after consultation with the FDA, The Company initiated a voluntary global recall of all products manufactured at the Boston facility, including Primatrix®, Surgimend®, Revize™, and TissueMend™, distributed between March 1, 2018 and May 22, 2023. See Item 1A. Risk Factors, under the heading Risks Related to our Regulatory Environment and under Item 7. General Management's Discussion and Analysis of Financial Condition and Results of Operations - FDA Matters of this Annual Report on Form 10-K for further discussion.
In the third quarter of 2021, we filed a PMA application for a specific indication for Surgimend® in the use of post-mastectomy breast reconstruction. In 2022, we acquired SIA, which is also PMA for DuraSorb with IBBR, and in June 2023 we completed enrollment in the DuraSorb U.S. IDE clinical study for two-stage breast reconstruction; the primary follow-up period is one year after device implantation. By offering two distinct product solutions, we believe we have the opportunity to build a leading position in the IBBR market. We hope to obtain FDA approvals in 2025.
Additionally, in 2022 we launched NeuraGen 3D Nerve Guide Matrix, a resorbable implant for repair of peripheral nerve discontinuities and engineered to create an optimized environment for nerve regeneration. Following the completion of design control activities in 2022, we launched both Cytal and MicroMatrix in Europe in 2023. In 2023, the Company received 510(k) clearance from the FDA for MicroMatrix® Flex.
Electromechanical Technologies and Instrumentation. The CSS business consists of a broad portfolio of market-leading brands, such as Codman®, DuraGen®, DuraSeal®, CUSA®, Mayfield®,Bactiseal®, and Certas® Plus,which are used for the management of multiple disease states, including brain tumors, traumatic brain injury, hydrocephalus and other neurological conditions. The growth in this business in recent years has been fueled by geographic expansion and new product registrations in markets, such as China, Japan, and Europe, which we expect to continue in the near-to-long term. Because our electromechanical products and instruments address significant needs in surgical procedures and limit uncertainty for surgeons, we continue to invest in registrations, clearances, and approvals for new indications and next generation improvements to our market-leading products. We have several active programs focused on life cycle management and innovation for capital and disposable products in our portfolio. Our product development efforts are focused on core clinical applications in CSFcerebrospinal fluid ("CSF") management, neuro-critical care monitoring, minimally invasive instruments and electrosurgery and ultrasonic medical technologies, as well as our ambition to transform the standard of care in neurosurgery with product advancements in MISminimally invasive surgery ("MIS") and ICH.the surgical management of intracerebral hemorrhage ("ICH"). Our lighting franchise is among the most dynamic in the industry.
The Company benefitted from our product launches from prior years, including our new electrosurgery generator and irrigator system, an innovative customer-centric toolkit for our CertasTM Plus Programmable Valve along with additional shunt configurations. In Japan, we are experiencing strong growth as a result of the successful launch of DuraGen in mid-2019, which is the first and only collagen xenograft approved for use as a dural substitute in the country. We are focused on the development of core clinical applications in our electromechanical technologies portfolio. Also, weWe continue to update our CUSA Clarity platform by incorporating new ultrasonic handpiece surgical tips and integrated electrosurgical capabilities. In 2022, we made progress to several enhancements to our CUSA Clarity Tissue Ablation System. The extended laparoscopic tip was launched in the U.S. to enhance laparoscopic liver procedures. In addition, a single-sided bone tip received 510(k) clearance from the FDA. Commercial launch was completed successfully in early 2023. In August 2023, we launched a modified 23 kHz CUSA Electrosurgery Module (CEM) for Clarity handpieces that can be used with additional electrosurgery generators. We continue to work with several instrument partners to bring new surgical instrument platforms to the market.
In the third quarter of 2021,Throughout 2023 we launched our CereLink ICP Monitor System in the U.S. and Europe. CereLink provides enhanced accuracy, usability and advanced data presentation that provides clinicians with uncompromised, advanced continuous ICP monitoring that until now, has not been available when treating patients with traumatic brain injuries.
In 2021, wealso continued to advance the early-stage technology platforms we acquired in 2019. Through the acquisition of Arkis Biosciences, Inc. ("Arkis") we added a platform technology, CerebroFlo®CerebroFlo® external ventricular drainage ("EVD"), catheter with Endexo®Endexo® technology, a permanent additive designed to reduce the potential for catheter obstruction due to thrombus formation. The CerebroFlo EVD Cathetercatheter has demonstrated an average of 99% less thrombus accumulation onto its surface, in vitro, compared to a market leading EVD catheter. In 2019,Our work to combine our Bactiseal® antimicrobial technology with the Endexo anti-occlusive technology continues to progress for both a silicone-based hydrocephalus and EVD project.
Throughout 2023, we also acquiredcontinued to advance our innovation from the Rebound Therapeutics Corporation ("Rebound Therapeutics"), which was acquired in 2019. Rebound Therapeutics specializes in a Company that specialized in single-use medical device, known as the Aurora Surgiscope, which is the only tubular retractor system designed for cranial surgery with an integrated access channel, camera and lighting. The 9mm Surgiscope received 510(k) clearance from the FDA in the fourth quarter of 2023.
In the third quarter of 2021, we conductedlaunched our CereLink ICP Monitor System in the U.S. and Europe and continued the global rollout in the first half of 2022. On August 18, 2022, the Company, after consultation with the FDA and other regulatory authorities outside of the United States, initiated an immediate voluntary global product removal of all CereLink® intracranial pressure monitors. We believe that the out-of-range readings are principally caused by a combined interaction of electrical noise (originating from sources such as electrical components in the device, other devices set up near the CereLink Monitor, and the hospital power grid) and an electrical potential difference between the patient and monitor. We submitted a traditional 510(k) submission to the FDA on September 15, 2023 as a result of customer reports about monitors whose pressure readings were out of range. We have received 510(k) clearance from the FDA on February 4th, 2024. We plan to resume shipments of CereLink monitors in the U.S. in the first quarter of 2024. Shipments resumed in international markets with a limited clinical launchrelease in the third quarter of 2023. See Item 1A. Risk Factors, under the heading Risks Related to our Regulatory Environment and under Item 7. General Management's Discussion and Analysis of Financial Condition and Results of Operations - FDA Matters of this Annual Report on Form 10-K for further discussion.
MANUFACTURE AND AVAILABILITY OF RAW MATERIALS
We manufacture products at manufacturing facilities located in various countries throughout the world. We purchase many of the Aurora Surgiscope for usecomponents and raw materials used in minimally invasive neurosurgery as well as initiated a registry called MIRROR to collect data on early surgical intervention using this same technology platform for the treatment of ICH.manufacturing our products from numerous suppliers in various countries.
RESOURCES
In general, raw materials essential to our businesses are readily available from multiple sources. For reasons of quality assurance, availability, or cost effectiveness, certain components and raw materials are available only from one or a sole supplier. Ourlimited number of suppliers. We have established long-term supply contracts with many of our suppliers and our practice is to maintain sufficient inventory of components so that our production will not be significantly disrupted even if a particular component or material is not available for a period of time.Due to the high standards and FDA requirements applicable to manufacturing our products, such as the FDA's Quality System Regulation and Good Manufacturing Practices, we may not be able to quickly establish additional or replacement sources for certain components or materials. Some of our manufacturing operations are located outside of the U.S., including in Puerto Rico, Switzerland, Ireland and France. Those manufacturing operations are also subject to additional challenges and risks associated with international operations described under the caption “Risk Factors” set forth in Part I, Item 1A of this Annual Report on Form 10-K. In the event we are unable to obtain sufficient quantities of raw materials or components on commercially reasonable terms or in a timely manner, our ability to manufacture our products on a timely and cost-competitive basis may be compromised, which may have a material adverse effect on our business, financial condition and results of operations.
Certain of our products, including but not limited to our dermal regeneration products, duraplasty products, wound care products, and nerve and tendon repair products, contain material derived from bovine tissue. We take great care to provide products that are safe and free of agents that can cause disease. In particular, the collagen used in the products that we manufacture is derived from the deep flexor tendon of cattle less than 24 months24-months old from New Zealand, a country that has never had a reported case of bovine spongiform encephalopathy ("BSE") (otherwise known as mad cow disease), from the U.S. or from fetal bovine dermis. The World Health Organization classifies different types of cattle tissue for relative risk of BSE transmission. Deep flexor tendon and fetal bovine skin are in the lowest-risk category for BSE transmission, and therefore considered to have a negligible risk of containing the agent that causes BSE.
INTELLECTUAL PROPERTY
We seek patent and trademark protection for our key technology, products and product improvements, both in the U.S. and in selected foreign countries. When determined appropriate, we have enforced and plan to continue to enforce and defend our patent and trademark rights. In general, however, we do not rely solely on our patent and trademark estate to provide us with any significant competitive advantages as it relates to our existing product lines. We also rely upon trade secrets and continuing technological innovations to develop and maintain our competitive position. In an effort to protect our trade secrets, we have a policy requiring our employees, consultants and advisors to execute proprietary information and invention assignment agreements upon commencement of employment or consulting relationships with us. These agreements also provide that all confidential information developed or made known to the individual during the course of their relationship with us must be kept confidential, except in specified circumstances.
AccuDrain®, AmnioExcel®, Aquasonic®, Auragen®, Aurora® Surgiscope®, Bactiseal®, BioDFence®, BioDOptix®, Brainet®, Budde®, Buzz™, CereLink™CereLink®, CerebroFlo® EVD Catheter with Endexo® Technology, Codman®, Codman Accu-Flo®, Codman Bicol®, Codman® Certas® Plus, Codman® Hakim®Programmable valve, Codman Holter®, Codman ICP Express®, Codman Microsensor®, Codman VersaTru®, Codman VPV®, Contour-Flex®, Cranioplastic®, CRW®, CRW Precision™, Ctherm™, CUSA®, Cytal®, DirectLink®, DuraGen®, DuraSeal®, DuraSorb®,Gentrix®, HeliCote®, HeliPlug®, HeliTape®, HeliMend®, Helistat®, Helitene®, Hermetic™, Hy-Tape®, Integra®, IntegraLink®, Isocool®, Jarit®, Lead-Lok™, Licox®, LimiTorr��LimiTorr™, Luxtec®,
Luxtec®,Mayfield®, MatriStem UBM™, MediHoney®, MicroFrance®, MicroMatrix®, Miltex®, Mischler™, MoniTorr ICP™, Natus®, NeuraGen®, NeuraWrap™, Nicolet®, Omnigraft®, Omni-Tract®, OSV II®, Padgett®, PriMatrix®, Pureflow™, Q-Snor™, Redmond™, Revize™, Ruggles®, Signacreme®, SurgiMend®, TCC-EZ®, TenoGlide®, TissueMend®, Ultra VS™, VersaTru®, Xtrasorb®, zRIP™, and the Integra logo are some of the material trademarks of Integra LifeSciences Corporation and its subsidiaries. MAYFIELD® is a registered trademark of SM USA, Inc., and is used by Integra under license.
SEASONALITY
Revenues during our fourth quarter tend to be stronger than other quarters because many hospitals increase their purchases of our products during the fourth quarter to coincide with the end of their budget cycles in the U.S. In general, our first quarter usually has lower revenues than the preceding fourth quarter, the second and third quarters have higher revenues than the first quarter, and the fourth quarter revenues are the highest in the year. The main exceptions to this pattern occur because of material acquisitions as well as impacts of the COVID-19 pandemic.
Impact of COVID-19 Pandemic on our business
During the COVID-19 pandemic, the Company's focus remained on supporting patients, providing customers with life-saving products, and protecting the well-being of our employees. The rapid and evolving spread of the virus and subsequent variants have resulted in unprecedented challenges to the global healthcare industry. In response to the pandemic, we acted swiftly by implementing protocols to ensure continuity of our manufacturing and distribution sites around the world and to provide for the safety of our employees.
The COVID-19 pandemic continues to have widespread and unpredictable impacts and the Company has continued to manage risks in this uncertain environment. We remain confident that the underlying markets in which the Company competes remain attractive. We also remain focused on managing the business for the long-term. The Company's adaptability and resiliency in the face of this unprecedented crisis is made possible in part by prior investments in technology infrastructure and operations, as well as our talented and committed global workforce.
Capital markets and worldwide economies have also been significantly impacted by the COVID-19 pandemic, and it is possible that the pandemic could cause a local and/or global economic recession. Any such economic recession could have a material adverse effect on the Company's long-term business as hospitals curtail and reduce capital as well as overall spending. The COVID-19 pandemic and local actions, such as “shelter-in-place” orders and restrictions on travel and access to our customers or temporary closures of our facilities or the facilities of our suppliers and their contract manufacturers, disruption and/or higher costs to the Company’s supply chain, staffing shortages in hospitals and labor constraints in our facilities, could further impact our sales, margins and our ability to ship our products and supply our customers. Any of these events could negatively impact the number of surgical and medical intervention procedures performed and have a material adverse effect on our business, financial condition, results of operations, or cash flows.
Information pertaining to risk factors as it relates to the COVID-19 pandemic can be found in Item 1A. Risk Factors of this Annual Report on Form 10-K.acquisitions.
GOVERNMENT REGULATION AND COMPLIANCE
We are a manufacturer and marketer of medical devices and Human Tissue and Cell Based Products ("HCT/Ps") and therefore are subject to extensive regulation by the FDA, the Center for Medicare Services of the U.S. Department of Health and Human Services ("HHS"), other federal governmental agencies and, in some jurisdictions, by state and foreign governmental authorities. These regulations govern the introduction of new medical devices and HCT/Ps, the observance of certain standards with respect to the design, manufacture, testing, labeling, promotion and sales of the products, the maintenance of certain records, the ability to track devices, the reporting of potential product defects, the import and export of products, and other matters. FDA product approvals may be withdrawn or suspended if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
8Our business is also affected by patient and data privacy laws and government payer cost containment initiatives, as well as environmental health and safety laws and regulations.
United States Food and Drug Administration
Our products are subject to extensive regulation particularly as to safety, efficacy and adherence to FDA Quality System Regulation, and related manufacturing standards. Medical device products are subject to rigorous FDA and other governmental agency regulations in the United States and similar regulations of foreign agencies abroad. The FDA regulates the design, development, research, preclinical and clinical testing, introduction, manufacture, advertising, labeling, packaging, marketing, distribution, import and export, and record keeping for such products, in order to ensure that medical products distributed in the United States are safe and effective for their intended use. In addition, the FDA is authorized to establish special controls to provide reasonable assurance of the safety and effectiveness of most devices. Non-compliance with applicable requirements can result in import detentions, fines, civil and administrative penalties, injunctions, suspensions or losses of regulatory approvals, recall or seizure of products, operating restrictions, refusal of the government to approve product export applications or allow us to enter into supply contracts, and criminal prosecution. The regulatory process for obtaining product approvals and clearances can be onerous and costly. The FDA requires, as a condition to marketing a medical device in the U.S., that we secure a Premarket Notification clearance pursuant to Section 510(k) of
Under the Federal Food, Drug and Cosmetic Act (the "FD&C Act"), authorization to commercially distribute a new medical device in the U.S. is generally obtained in one of two primary ways. The first, known as pre-market notification or an approved premarketthe 510(k) process, requires us to demonstrate that our medical device is substantially equivalent to a legally marketed medical device. A 510(k) pre-market notification filing must contain information establishing that the device to be sold is substantially equivalent to a device commercially distributed prior to May 28, 1976 or to a device that has been determined by the FDA to be substantially equivalent. The second, more rigorous process, known as pre-market approval ("PMA"(“PMA”), application (or supplemental requires us to independently demonstrate that a medical device is safe and effective for its intended use. This process is generally much more time-consuming and expensive than the 510(k) process. The PMA application). Obtaining these approvalsprocess involves a complex and clearances can take uplengthy testing process that is subject to review by the FDA and may require several years to obtain. We may need to first obtain an investigational device exemption (for significant risk devices), known as an IDE, in order to conduct extensive clinical testing of the device to obtain the necessary clinical data for submission to the FDA. The FDA will approve a PMA only if after evaluating the supporting technical data it finds that the PMA contains sufficient, valid scientific evidence to assure that the device is safe and effective for its intended use(s). This approval may involve preclinical studies and clinical trials. be granted with post-approval requirements including inspection of manufacturing facilities and/or additional patient follow-up for an indefinite period of time.
The FDA also may require a post-approval clinical study as a condition of approval. To perform clinical trials for significant risk devices in the U.S. on an unapproved product, we are required to obtain an Investigational Device ExemptionIDE from the FDA. The FDA also may require a filing for approval prior to marketing products that are modifications of existing products or new indications for existing products. Moreover, after clearance/approval is given, if the product is shown to be hazardous or defective, the FDA and foreign regulatory agencies have the power to withdraw the clearance or approval, as the case may be, or require us to change the device, its manufacturing process or its labeling, to supply additional proof of its safety and effectiveness or to recall, repair,
replace or refund the cost of the medical device. Because we currently export medical devices manufactured in the U.S. that have not been approved by the FDA for distribution in the U.S., we are required to obtain approval/registration in the country to which we are exporting and maintain certain records relating to exports and make these available to the FDA for inspection, if required.
Human Cells, Tissues and Cellular and Tissue-Based Products
Integra, through the acquisition of Derma Sciences andits wholly-owned subsidiary BioD LLC ("BioD"(“BioD”), is involved with the recovery, processing, storage, transportation and distribution of donated amniotic tissue. The FDA has specific regulations governing HCT/Ps. An HCT/P is a product containing, or consisting of, human cells or tissue intended for transplantation into a human patient. Examples of HCT/PsHCTPs include bone, ligament, skin and cornea.
Some HCT/Ps fall within the definition of a biological product, medical device or drug regulated under the FD&C Act. These biologic, device or drug HCT/Ps must comply both with the requirements exclusively applicable to HCT/Ps and, in addition, with requirements applicable to biologics, devices or drugs, including premarket clearance or approval from the FDA.
Section 361 of the Public Health Service Act ("(“Section 361"361”) authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease. HCT/Ps regulated as “361” HCT/Ps are subject to requirements relating to registering facilities and listing products with the FDA, screening and testing for tissue donor eligibility, and Good Tissue Practices when processing, storing, labeling, and distributing HCT/Ps, including required labeling information, stringent record keeping, and adverse event reporting.
The American Association of Tissue Banks ("AATB"(“AATB”) has issued operating standards for tissue banking. Compliance with these standards is a requirement in order to become an AATB-accredited tissue establishment. In addition, some states have their own tissue banking regulations. We are licensed or have permits for tissue banking in California, Delaware, Illinois, Maryland, New York, Oregon, and Tennessee. In Tennessee, we are registered with the FDA Center for Biological Evaluations and Research.
Procurement of certain human organs and tissue for transplantation is subject to the restrictions of the National Organ Transplant Act, which prohibits the transfer of certain human organs, including skin and related tissue for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. BioD our wholly-owned subsidiary, is a registered Tissue Bank and is involved with the recovery, storage and transportation of donated human amniotic tissue.
Medical Device Regulations
The FDA requires that a manufacturer obtain 510(k) clearance or a PMA for a variety of reasons, such as introducing a new medical device or new indication for use of an existing medical device, before introducing it into the U.S. market. The type of marketing authorization is generally linked to the classification of the device. The FDA classifies medical devices into three classes based on risk. Regulatory control increases from Class I (lowest risk) to Class III (highest risk). The FDA generally must clear or approve the commercial sale of new medical devices in Classes II and III. Commercial sales of our Class II medical devices (except for Class II exempt devices) and Class III medical devices within the U.S. must be preceded by either a pre-market notification filing pursuant to Section 510(k) of the FD&C Act (Class II) or the granting of a pre-market approval, or PMA (Class III).
On June 22, 2015,The process of obtaining a Section 510(k) clearance generally requires the submission of performance data and may require clinical data, which in some cases can be extensive, to demonstrate that the device is “substantially equivalent” to another device that is currently marketed under a 510(k); a device that is referred to as “predicate device.” As a result, FDA clearance requirements may extend the development process for a considerable length of time. In the case of a PMA, the FDA issuedmay require additional review by an Untitled Letter (the "Untitled Letter") allegingadvisory panel, which can further lengthen the process. The PMA process, which is reserved for new devices that BioD's morselized amniotic membrane tissue-based products doare not meetsubstantially equivalent to any predicate device and for high-risk devices (i.e., Class III devices) that are used to support or sustain human life or which present a potential, unreasonable risk of illness or injury, may take several years and requires the criteriasubmission of extensive performance and clinical information.
Medical devices can be marketed only for regulation as HCT/Ps solely under Section 361 andthe indications for which they are cleared or approved. After a device has received 510(k) clearance for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a result, BioD would needsignificant change in the design, materials, method of manufacture or intended use, may require a biologics licensenew 510(k) clearance or PMA approval and payment of an FDA user fee. The determination as to lawfully market those morselized products. Sincewhether or not a modification could significantly affect the issuancedevice’s safety or effectiveness is initially left to the manufacturer using available FDA guidance; however, the FDA may review this determination to evaluate the regulatory status of the Untitled Letter, BioDmodified product at any time and may require the Company have made knownmanufacturer to cease marketing and recall the FDA their disagreement with the FDA’s assertion that certain products are more than minimally manipulated.modified device until 510(k) clearance or PMA approval is obtained. The FDA has not changed its position that certain of the BioD acquired products are not eligible for marketing solely under Section 361. In July 2020, the FDA issued the final guidance document related to human tissue titled, “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use” (the “2020 HCT/P Final Guidance”). The 2020 HCT/P Final Guidance document supersedes the November 2017 guidance by the same title.
The HCT/P Final Guidance maintains the FDA’s position that products such as the Company’s morselized amniotic membrane tissue-based products do not meet the criteria for regulation solely as HCT/Ps. In addition, in the November 2017 guidance, the FDA articulated a risk-based approach to enforcement and, while some uses for amniotic membrane tissue-based products would have as much as thirty-six months of enforcement discretion, other high risk uses couldmanufacturer may also be subject to immediate enforcement action. The 2020 HCT/P Final Guidance maintained this approach and extended the discretionary enforcement period to May 31, 2021.
Considering the risk of enforcement action, the Company discontinued the manufacturing of all morselized amniotic membrane tissue-based products prior to May 31, 2021. We no longer distribute these products. As of December 31, 2021, the Company has not received any further notice of enforcement action from the FDA regarding its morselized amniotic membrane tissue-based products.
Revenues from the now discontinued BioD morselized amniotic membrane-based products for the year ended December 31, 2021 were less than 1.0% of consolidated revenues.
Medical Device Regulations
We also are required to register with the FDA as a medical device manufacturer. As such,manufacturer and any devices we manufacture and distribute pursuant to clearance or approval by the FDA are subject to pervasive and continuing regulation by the FDA and certain state agencies. These include product listing and establishment registration requirements, which help facilitate FDA inspections and other regulatory actions, and our manufacturing sites are subject to periodic inspection by the FDA for compliance with the FDA's Quality System Regulations. These regulations require that we manufacture our products and maintain our documents in a prescribed manner with respect to design, manufacturing, testing and control activities. Further, we are required to comply with various FDA requirements and other legal requirements for labeling and promotion. FDA regulates unclassified devices via the 510(k) process and has the authority to classify these devices and/or require Special Controls, additional testing and submission of a new 510(k) as part of the classification process for unclassified devices that are currently on the market as 510(k) cleared products. If the FDA believes that a company is not in compliance with applicable regulations, it may issue a warning letter, institute proceedings to detain or seize products, issue a recall order, impose operating restrictions, enjoin future violations and assess civil penalties against that company, its officers or its employees and may recommend criminal prosecution to the U.S. Department of Justice. AllThe majority of Integra manufacturing facilities participate in the Medical Device Single Audit Program and are audited annually for compliance with the Quality System for US FDA, Canada, Australia, Brazil, and Japan.
Medical device regulations also are in effect in many of the countries in which we do business outside the U.S. In the European Economic Area ("EEA"), which is comprised of the 27 member states of the European Union (the "EU") plus Norway, Iceland and Liechtenstein, medical devices need to comply with specific requirements. These requirements were previously known as "Essential Requirements" under the former EU Medical Devices Directive (Council Directive 93/42/EEC, or MDD) and are now defined "General Safety and Performance Requirements (GSPR)" under the new EU Medical Devices Regulation (Regulation (EU) 2017/745, or "EU MDR"). Although the requirements set forth in the EU MDR are generally consistent with those laid out in the MDD (with a few exceptions), the EU MDR is intended, among other things, to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation. These laws range from comprehensive medical device approval and Quality System requirements for some or all of our medical device products to simpler requests for product data or certifications. Under the European Union Medical Device Directive,MDD, medical devices must meet the Medical Device DirectiveMDD standards and receive CE Mark Certification prior to marketing in the European Union ("EU"). In addition,EEA. Although we continue to transition our certification profile to meet the new EU MDR requirements, these stricter regulations set forth in the EU enactedMDR may pose additional challenges for Integra to continue marketing products in the EU Medical Device Regulation, which imposes stricter requirementsas these regulations come into force. See “Item 1A. Risk Factors - We are subject to stringent domestic and foreign medical device regulations and oversight and any adverse action may adversely affect our ability to compete in the marketplace and our financial condition and business operations” of this Annual Report on the marketing and sales of medical devices including but not limited to quality systems, labeling and clinical data. Form 10-K.
CE Mark Certification requires a comprehensive quality system program, technical documentation, clinical evaluation and data on the product which are then reviewed, by a Notified Body. A Notified Body is an organization designated by the national governments of the EU member states to make independent judgments about whether a product complies with the requirements established by each CE marking directive. The Medical Device Directive, Medical Device Regulation,MDD, MDR, ISO 9000 series and ISO 13485 are recognized international quality standards that are designed to ensure that we develop and manufacture quality medical devices. Other countries are also instituting regulations regarding medical devices or interpreting and enforcing existing regulations more strictly. Compliance with these regulations requires extensive documentation and clinical reports for our products, revisions to labeling, and other requirements such as facility inspections to comply with the registration requirements. A recognized Notified Body audits our facilities annually to verify our compliance with the ISO 13485 Quality System standard.
Certain countries, as well as the EU, have issued regulations that govern products that contain materials derived from animal sources. Regulatory authorities are particularly concerned with materials infected with the agent that causes BSE. These regulations affect our dermal regeneration products, duraplasty products, hernia repair products, biomaterial products for the spine, nerve and tendon repair products and certain other products, all of which contain material derived from bovine tissue. Although we take great care to provide that our products are safe and free of agents that can cause disease, products that contain materials derived from animals, including our products, may become subject to additional regulation, or even be banned in certain countries, because of concern over the potential for prion transmission. Significant new regulations, a ban of our products, or a movement away from bovine-derived products because of an outbreak of BSE could have a material, adverse effect on our current business or our ability to expand our business. See “Item 1A. Risk Factors -– Certain ofRisks Related to our products contain materials derived from animal sources and may become subject to additional regulationRegulatory Environment”" of this Annual Report on Form 10-K.
Postmarket Requirements.Requirements. After a device is cleared or approved for commercial distribution, numerous regulatory requirements apply. These include the FDA Quality System Regulations which cover the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of medical devices; the FDA's general prohibition against promoting products for unapproved or 'off-label' uses; the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and the Reports of Corrections and Removals regulation, which require manufacturers to report recalls and field corrective actions
to the FDA if initiated to reduce a risk to health posed by the device or to remedy a violation of the FD&C Act. Postmarket requirements are also followed globally where our products are registered and approved. These foreign jurisdictions have similar requirements to the FDA which include reporting requirements such as adverse events and recalls.
Regulations Governing Reimbursement
Market acceptance of our medical products in the U.S. and other countries is dependent upon the purchasing and procurement practices of our customers and patient need for our products and procedures and, the coverage and reimbursement of patients’ medical expenses by government healthcare programs, private insurers or other healthcare payors. The delivery of our devices is subject to regulation by the HHS and comparable state and non-U.S. agencies responsible for reimbursement and regulation of healthcare items and services. Healthcare providers that purchase medical devices generally rely on third-party payors, including, in the U.S., the Medicare and Medicaid programs and private payors, such as indemnity insurers, employer group health insurance programs and managed care plans, to reimburse all or part of the cost of the products. As a result, demand for our products is and will continue to be dependent in part on the coverage and reimbursement policies of these payors. The manner in which reimbursement is sought and obtained varies based upon the type of payor involved and the setting in which the product is furnished and utilized. Reimbursement from Medicare, Medicaid and other third-party payors may be subject to periodic adjustments as a result of legislative, regulatory and policy changes, as well as budgetary pressures. Possible reductions in, or eliminations of, coverage or reimbursement by third-party payors, or denial of, or provision of uneconomical reimbursement for new products may affect our customers' revenue and ability to purchase our products. Any changes in the healthcare regulatory, payment or enforcement landscape relative to our customers' healthcare services have the potential to significantly affect our operations and revenue.
Implementation of legislative or regulatory reforms to reimbursement systems, including price regulation, competitive bidding and tendering, coverage and payment policies, comparative effectiveness of therapies, technology assessments and managed-care arrangements, or adverse decisions relating to our products by administrators of these systems in coverage or reimbursement, could significantly reduce reimbursement or result in the denial of coverage, which could have an impact on the acceptance of and demand for our products and the prices that our customers are willing to pay for them.Other regulations
Anti-Bribery Laws. In the U.S., we are subject to laws and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws and physician self-referral laws that regulate the means by which companies in the health care industry may market their products to hospitals and health care professionals and may compete by discounting the prices of their products. Similar anti-bribery laws exist in many of the countries in which we sell our products outside the U.S., as well as the United States Foreign Corrupt Practices Act (which(the “FCPA”) which addresses the activities of U.S. companies in foreign markets).markets. Our products also are subject to regulation regarding reimbursement, and U.S. healthcare laws apply when a customer submits a claim for a product that is reimbursed under a federally funded healthcare program. These global laws require that we exercise care in designing our sales and marketing practices, including interactions with healthcare professionals, and customer discount arrangements. See “Item 1A. Risk Factors -– We are exposed to a variety of risks relating to our international sales and operations” of this Annual Report on Form 10-K for further details.
Import-export. Our international operations subject us to laws regarding sanctioned countries, entities and persons, customs, and import-export. Among other things, these laws restrict, and in some cases can prevent, U.S. companies from directly or indirectly selling goods, technology or services to people or entities in certain countries. In addition, these laws require that we exercise care in our business dealings with entities in and from foreign countries.In addition to our need to comply with such regulations in connection with our direct activities, we also sell and provide goods, technology and services to agents, representatives and distributors who may export such items to customers and end-users. If we, or the third parties through which we do business, are not in compliance with applicable import, export control or economic sanctions laws and regulations, we may be subject to civil or criminal enforcement action, and varying degrees of liability. Such actions may disrupt or delay sales of our products or services or result in restrictions on our distribution and sales of products or services that may materially impact our business
Hazardous materialsEnvironmental Health and Safety. Our research, development and manufacturing processes involve the controlled use of certain hazardous materials. We are subject to country-specific, federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials and certain waste products. We believe that our environmental, health and safety procedures for handling and disposing of these materials comply with the standards prescribed by the controlling laws and regulations. However, risk of accidental releases or injury from these materials is possible. These risks are managed to minimize or eliminate associated business impacts. In the event of this type of accident, we could be held liable for damages and face a liability that could exceed our resources. We could be subject to a regulatory shutdown of a facility that could prevent the distribution and sale of products manufactured there for a significant period of time, and we could suffer a casualty loss that could require a shutdown of the facility in order to repair it, any of which could have a material, adverse effect on our business. Although we continuously strive to maintain full compliance with respect to all applicable global
environmental, health and safety laws and regulations, we could incur substantial costs to fully comply with future laws and regulations, and our operations, business or assets may be negatively affected. Furthermore, global environmental, health and safety compliance is an ongoing process. Integra hasWe have compliance procedures in place for compliance with Employee Health & Safety laws, driven by a centrally led organizational structure that ensures proper implementation, which is essential to our overall business objectives.
In addition, to the above regulations, we are, and may be, subject to regulation under country-specific federal and state laws, including, but not limited to, requirements regarding record keeping, and the maintenance of personal information, including personal health information. As a public Company, we are subject to the securitiesnumerous federal, state, foreign and local laws relating to safe working conditions, environmental protection and fire hazard control, among others. We may be required to incur significant costs to comply with these laws and regulations including the Sarbanes-Oxley Act of 2002. We also are subject to other present and could be subject to possible future, local, state, federal and foreign regulations.
Third-Party Reimbursement. Healthcare providers that purchase medical devices generally rely on third-party payors, including, in the U.S., the Medicarefuture and Medicaid programscomplying with these laws may result in a material adverse effect upon our business, financial condition and private payors, such as indemnity insurers, employer group health insurance programs and managed care plans, to reimburse all or partresults of the cost of the products. As a result, demand for our products is and will continue to be dependent in part on the coverage and reimbursement policies of these payors. The manner in which reimbursement is sought and obtained varies based upon the type of payor involved and the setting in which the product is furnished and utilized. Reimbursement from Medicare, Medicaid and other third-party payors may be subject to periodic adjustments as a result of legislative, regulatory and policy changes, as well as budgetary pressures. Possible reductions in, or eliminations of, coverage or reimbursement by third-party payors, or denial of, or provision of uneconomical reimbursement for new products may affect our customers' revenue and ability to purchase our products. Any changes in the healthcare regulatory, payment or enforcement landscape relative to our customers' healthcare services have the potential to significantly affect our operations and revenue.operations.
Data Privacy and Cybersecurity Laws and Regulations. As a business with a significant global footprint, compliance with evolving regulations and standards in data privacy and cybersecurity (relating to the confidentiality and security of our information technology systems, products such as medical devices, and other services provided by us) may result in increased costs, lower revenue, new complexities in compliance, new challenges for competition, and the threat of increased regulatory enforcement activity. Our business relies on the secure electronic transmission, storage and hosting of sensitive information, including personal information, financial information, intellectual property, and other sensitive information related to our customers and workforce.
For example, in the U.S., the collection, maintenance, protection, use, transmission, disclosure and disposal of certain personal information and the security of medical devices are regulated at the U.S. federal and state, and industry levels. U.S. federal and
state laws protect the confidentiality of certain patient health information, including patient medical records, and restrict the use and disclosure of patient health information by health care providers. For example, in the U.S. we are obligated to comply with the requirements of the Health Insurance and Portability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (collectively, “HIPPA”). Under HIPAA, the HHS has issued regulations, including the HIPAA Privacy, Security and Breach Notification Rules, to protect the privacy and security of protected health information used or disclosed by covered entities including health care providers and their business associates, as well as covered subcontractors. HIPAA also regulates standardization of data content, codes and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include significant civil and criminal penalties for each violation. In addition, the FDA has issued guidance advising manufacturers to take cybersecurity risks into account in product design for connected medical devices and systems, to assure that appropriate safeguards are in place to reduce the risk of unauthorized access or modification to medical devices that contain software and reduce the risk of introducing threats into hospital systems that are connected to such devices. The FDA also issued guidance on post market management of cyber security in medical devices.
Outside the U.S., we are impacted by the privacy and data security requirements at the international, national and regional level, and on an industry specific basis. Legal requirements in these countries relating to the collection, storage, handling and transfer of personal data and, potentially, intellectual property continue to evolve with increasingly strict enforcement regimes. In Europe, for example, we are subject to EU General Data Protection Regulation ("GDPR") which requires member states to impose minimum restrictions on the collection, use and transfer of personal data and includes, among other things, a requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances and significant fines for non-compliance. The GDPR also requires companies processing personal data of individuals residing in the EU to comply with EU privacy and data protection rules.
Please refer to “Item 1A. Risk Factors -– We are subjectFailure to requirementscomply with laws relating to the confidentiality of sensitive personal information technology which could adversely affector standards related to the transmission of electronic health data, may require us to make significant changes to our business” products, or incur penalties or other liabilities” of this Annual Report on Form 10-K for additional discussion of the risks accompanying compliance with data privacy and cybersecurity laws and regulations.
These laws and regulations impact the ways in which we use and manage personal data, protected health information, and our information technology systems. They also impact our ability to move, store, and access data across geographic boundaries. Compliance with these requirements may require changes in business practices, complicate our operations, and add complexity and additional management and oversight needs. They also may complicate our clinical research activities, as well as product offerings that involve transmission or use of clinical data.
HUMAN CAPITALSPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Workforce DemographicsWe have made statements in this report, including statements under “Business” and “Management's Discussion and Analysis of Financial Condition and Results of Operations” that constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, ("the Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended ("the "Exchange Act"). These forward-looking statements are subject to a number of risks, uncertainties and assumptions about us including, among other things:
•the on-going and possible future effects of global challenges, including macroeconomic uncertainties, such as supply chain disruptions, inflation, bank failures, rising interest rates and availability of capital markets, the Israel-Hamas and Ukraine-Russia wars, other economic disruptions and U.S. and global recession concerns, on our customers and suppliers, and on our business, financial condition, results of operations and cash flows;
•general economic and business conditions, both domestically and in our international markets, including the effect of the continuing worldwide macroeconomic uncertainty and increasing trade regulations and tariffs;
•our expectations and estimates concerning future financial performance, financing plans and the impact of competition;
•anticipated trends in our business;
•anticipated demand for our products, particularly capital equipment;
•our ability to produce and deliver products in sufficient quantities to meet sales demands;
•the ongoing and possible future effects of supply chain constraints, including the availability of critical raw materials and components, as well as cost inflation in materials, packaging and transportation;
•our expectations concerning our ongoing restructuring, integration and manufacturing transfer and expansion activities;
•existing and future regulations affecting our business, and enforcement of those regulations;
•conducting business internationally;
•our failure to comply with the substantial regulation related to quality standards applicable to our manufacturing and quality processes could have an adverse effect on our business, financial condition, or results of operations;
•our ability to remediate all matters identified in United States Food & Drug (the "FDA") observations and warning letters that we received or may receive;
•our ability to obtain additional debt and equity financing to fund capital expenditures, working capital requirements and acquisitions;
•physicians' willingness to adopt our recently launched and planned products, third-party payors' willingness to provide or continue reimbursement for any of our products and our ability to secure regulatory approval for products in development;
•initiatives launched by our competitors;
•our ability to protect our intellectual property, including trade secrets;
•our ability to complete acquisitions, integrate operations post-acquisition and maintain relationships with customers of acquired entities;
•the ability to successfully manage ongoing organizational and strategic changes, including our ability to attract, motivate and retain key employees and maintain engagement and efficiency in remote work environments;
•anticipated trends relating to our financial condition or results of operations, including the impact of interest rate and foreign currency exchange fluctuations;
•the effect of any future public health crises, including the timing, scope and effect of U.S. and international governmental, regulatory, fiscal, monetary and public health responses to such crises; and
•other risk factors described in Item 1A. "Risk Factors" in this Annual Report on Form 10-K.
Forward-looking statements can be identified by forward-looking words such as “believe,” “may,” “could,” “might,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “expect,” “should,” “would,” “expect,” “target,” “pursue,” “forecast,” “hope” and similar expressions in this Annual Report on Form 10K. Forward-looking statements in this Annual Report on Form 10-K include, but are not limited to, statements regarding our five-pillar growth strategy; the closing of our pending acquisition of Acclarent, Inc. on anticipated terms and timing, or at all; the anticipated benefits of our pending acquisition of Acclarent, Inc.; expectations and plans with respect to strategic initiatives, product development and regulatory approvals, including the anticipated resumption of manufacturing at the Company’s Boston, Massachusetts facility. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by such forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this report. Except as required by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Factors that could cause or contribute to differences in our future financial results include the cautionary statements set forth herein and in our other filings with the Securities and Exchange Commission, including those set forth under “Risk Factors” set forth in Part I, Item 1A of this Annual Report on Form 10-K. We qualify all of our forward-looking statements by these cautionary statements.
PART I
ITEM 1. BUSINESS
OVERVIEW
Integra LifeSciences Holdings Corporation is a leading global medical technology company innovating treatment pathways to advance patient outcomes and set new standards of surgical, neurologic and regenerative care.
Founded in 1989 with the acquisition of an engineered collagen technology platform used to repair and regenerate tissue, our common stock trades on the Nasdaq Global Select Market (“Nasdaq”) under the symbol “IART.” We have developed numerous product lines from this technology for applications ranging from burn and deep tissue wounds to the repair of dura mater in the brain, as well as nerves and tendons. We have expanded our base regenerative technology business to include surgical instruments, neurosurgical products and advanced wound care through global acquisitions and product development to meet the evolving needs of our customers and enhance patient care.
Integra products are sold in more than 130 countries through a direct sales force as well as distributors and wholesalers. We manufacture and sell medical technologies and products in two reportable business segments: Codman Specialty Surgical ("CSS") and Tissue Technologies ("TT"). The CSS segment, which represents approximately two-thirds of our total revenue, consists of market-leading technologies and instrumentation used for a wide range of specialties, such as neurosurgery, neurocritical care and otolaryngology. We are the world leader in neurosurgery and one of the top three providers in instruments used in precision, specialty, and general surgical procedures. Our TT segment generates about one-third of our overall revenue and focuses on three main areas: complex wound surgery, surgical reconstruction, and peripheral nerve repair.
We have key manufacturing and research facilities located in California, Indiana, Maryland, Massachusetts, New Jersey, Ohio, Puerto Rico, Tennessee, Utah, France, Germany, Ireland and Switzerland. We source most of our handheld surgical instruments and dural sealant products through specialized third-party vendors.
Vision
We aspire to continue to be a worldwide leader in neurosurgery and reconstructive surgery with a portfolio of leading businesses that delivers outstanding customer experiences through innovation, execution and teamwork to positively impact the lives of millions of patients and their families.
Strategy
Following the completion of our strategic refresh in 2023, we refocused our strategies around five pillars. Of these five pillars, we have identified three core growth drivers: (1) innovating for outcomes, (2) growing internationally, and (3) broadening our impact on care pathways. Our execution of the core growth drivers is enabled by two key levers: (4) driving operational and customer excellence and (5) cultivating a high-performance culture. As outlined in greater detail below, we believe these five pillars will enable us to realize and advance our integrated growth strategy.
To this end, our executive leadership team has established the following key priorities aligned to the following five pillars:
Innovating for Outcomes. An important part of Integra’s growth strategy is introducing new products to strengthen and expand our portfolio, including via acquisitions. For example, we entered into a stock purchase agreement to acquire Acclarent, Inc. (“Acclarent”) from Ethicon, Inc., a subsidiary of Johnson & Johnson in December 31,2023. Acclarent is an innovator and market leader in ear, nose and throat (“ENT”) procedures and we believe that the acquisition of Acclarent will provide Integra with the opportunity to become a leading provider of ENT products and technologies. Furthermore, we believe that, owing to the ENT segment being an anatomical adjacency to neurosurgery, the acquisition will allow Integra to deliver future innovation both within the ENT segment and across our other CSS technology platforms. Additionally, we seek clinical evidence to support regulatory approval and strong reimbursement of our product portfolio around the world, including new indications for existing technologies. For example, in 2021, we had approximately 3,800 regular fullfiled a pre-market approval (“PMA”) application for a specific indication for Surgimend® in the use of post-mastectomy breast reconstruction. In 2022, we acquired SIA, which is also pursuing a pre-market approval for DuraSorb for use in implant-based breast reconstruction (“IBBR”), and part time employees and 900 contingent, subcontracted, and outsourced partners.
65%in June 2023 we completed enrollment in the DuraSorb U.S. IDE clinical study for two-stage breast reconstruction; the primary follow-up period is one year after device implantation. We hope to obtain FDA approvals for both products in 2025. We also continued to advance the development of pioneering neurosurgical technologies with the expansion of our employeesproduct offerings. In 2023 we launched the CUSA® Clarity Tips for use in surgical procedures requiring the controlled fragmentation, emulsification and aspiration of bone as well as in laparoscopic liver surgery.
Growing Internationally. Over the years, we have been significantly expanding our global footprint through investments in our commercial organization, the expansion and development of international markets and new product introductions. As part of our In-China-For-China strategy, we continue the build out of our assembly capabilities in our new facility in Suzhou, China. Several new products were introduced in select international markets in 2023, including MicroMatrix® and Certas Plus® Programmable Valve which were launched in Europe, and CUSA Clarity Laparoscopic tip which was launched in Australia,
New Zealand, Japan, Canada, South Africa and Israel. In addition, DuraGen Secure, received approval in Japan, while DuraGen Plus, an absorbable and sutureless collagen onlay indicated as a dura substitute for the repair of dura mater, was approved in China.
Broadening Impact on Care Pathways. We seek ways to develop products and technologies that impact the lives of patients, starting with the journey that a patient takes from diagnosis and treatment planning to surgery and postoperative care. We are well-established in acute care in the hospital setting and continue to leverage that strong position to grow in this segment and shape treatment pathways into preoperative care and additional sites of care.
Driving Operations and Customer Excellence. We have been making investments to build more responsive and scalable processes, enhance the reliability of our supply chain, and drive productivity initiatives to further supply and lower costs. Additionally, we continue to invest in technologies, systems and processes to enhance the customer experience. In 2023, we continued to invest in our capacity expansion. This includes ongoing projects of transferring our Boston manufacturing to a new location in Braintree, Massachusetts., validating manufacturing processes in our manufacturing facility in Plainsboro, New Jersey and increasing cleanroom capacity in our Memphis, Tennessee location.
Cultivating a High-Performance Culture. In seeking to sustain a culture of excellence and accountability, we have focused on employee empowerment and agility and building a diverse and inclusive workplace. These efforts resulted in our being named in several best workplace lists globally in 2023. Additionally, we have been making further strides in advancing our environmental, social and governance ("ESG") agenda to drive sustainability across the organization and recently published our second annual ESG report in the third quarter of 2023. For more information on our ESG strategy, goals, performance, and achievements, please visit “Our Company—ESG Report” at https://www.integralife.com/esg-report. Information on our website is not incorporated by reference herein and is not part of this Annual Report on Form 10-K.
BUSINESS SEGMENTS
We currently manufacture and sell our medical technologies and products in the following two reportable business segments: Codman Specialty Surgical and Tissue Technologies. We include financial information regarding our reportable business segments and certain geographic information under "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations" and Note 16, Segment and Geographic Information to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K).
Codman Specialty Surgical
Our CSS segment offers global, neurosurgery market-leading technologies, brands and instrumentation. The product portfolio represents a continuum of care from pre-operative, to the neurosurgery operating room, to the neuro-critical care unit and post care for both adult and pediatric patients suffering from brain tumors, brain injury, cerebrospinal fluid pressure complications and other neurological conditions. We offer leading technologies in dural repair, ultrasonic tissue ablation, intracranial pressure ("ICP") monitoring, hydrocephalus management, and cranial stabilization systems, while providing a rich research and development pipeline for growth.
Rounding out the portfolio is a catalog of surgical headlamps, surgical instrumentation, as well as after-market service. With thousands of surgical instrument products, including specialty surgical instruments, we call on the central sterile processing unit of hospitals and acute care surgical centers. Additionally, through a strong U.S. distribution model, we can serve the needs of hundreds of medical offices.
Our global commercial network includes clinical specialists, a large direct global sales force and strategic partnerships and distributors that serve hospitals, integrated health networks, group purchasing organizations, clinicians, surgery centers and health care providers. Outside the U.S., we have a combination of direct and indirect sales channels in international markets to sell certain product lines.
Tissue Technologies
Our TT segment focuses on three main areas: complex wound surgery, surgical reconstruction, and peripheral nerve repair and consists of five unique regenerative technology areas - highly engineered bovine collagen, bovine dermis, porcine urinary bladder, human amniotic tissue, and resorbable synthetic mesh. This broad regenerative platform, which includes multiple leading brands such as Integra® Dermal Matrices, AmnioExcel®, SurgiMend®, MicroMatrix® and NeuraGen®, primarily addresses the needs of plastic, reconstructive and general surgeons focused on the treatment of acute wounds, such as burns, chronic wounds, including diabetic foot ulcers, and surgical tissue repair, such as hernia, tendon, peripheral nerve repair and protection. Following our acquisition of SIA in 2022, we have also sought to expand our IBBR product offerings and in June 2023 we completed enrollment in the DuraSorb U.S. IDE clinical study for two-stage breast reconstruction.
We have a specialized sales organization composed of directly employed sales representatives, as well as specialty distributors, organized based upon their call point. Our wound reconstruction sales representatives call on surgeons doing procedures in limb salvage, trauma, wound reconstruction and burns, and chronic wounds primarily in the inpatient wound care clinic setting. We
also have a dedicated surgical reconstruction sales team focused on plastic and reconstructive surgery and hernia procedures with differentiated products. Finally, we have a distributor network focused on biologics. Outside the U.S., we have a combination of direct and indirect sales channels in international markets to sell certain product lines.
This business segment also includes private-label sales of a broad set of our regenerative and wound care technologies. Our customers are other medical technology companies that sell to end markets primarily in spine, surgical and wound care.
COMPETITION
The healthcare industry is highly competitive and characterized by continual change and improvements in technology. This is particularly the case in the market segments in which we operate. A number of companies have developed or are expected to develop products that compete or will compete with our products. Many of these competitors offer a broader product portfolio and have greater brand recognition than we do, which may make these competitors more attractive to hospitals, group purchasing organizations, laboratories, physicians and other potential customers. Competitors may develop superior products or products of similar quality for sale at the same or lower prices. Moreover, our products could be rendered obsolete by changes to industry standards or guidelines or advances in technology. We can give no assurance that we will be able to compete successfully with existing or new competitors
Our competitors for CSS include divisions within Medtronic, Inc., Stryker Corporation, Steris PLC, and B. Braun Medical, Inc. In addition, we compete with many smaller specialized companies and larger companies that do not otherwise focus on the offerings of Codman Specialty Surgical technologies. We rely on the depth and breadth of our sales and marketing organization, our innovative technologies, and our procurement and manufacturing operations to maintain our competitive position.
Our competitors for TT include Smith & Nephew plc, Organogenesis Holdings Inc., MiMedx Group, Inc., Allergan PLC, Becton Dickinson and Company, and Axogen, Inc. We compete with additional companies who partially participate in soft tissue reconstruction of complex wounds, peripheral nerve repair and surgical reconstruction. In addition, our products also compete against medical practices that treat a condition without using a medical device or any particular product, such as medical practices that utilize autograft tissue instead of our dermal regeneration products, duraplasty products and nerve repair products. Depending on the product line, we compete based on our products' features, strength of our sales force or distributors, sophistication of our technology and cost effectiveness of our solution.
We believe that the success of our products depends on our ability to differentiate ourselves and to demonstrate that our products deliver the clinical and operational attributes that are most important and cost-effective to customers. These attributes include, but are not limited to, superiority in efficacy, ease of use, reliability, accuracy, quality and cost. We believe our continued success depends in large part upon our ability to invest in product enhancements and technologies that will help us distinguish our product portfolio from our competitors.
RESEARCH AND DEVELOPMENT STRATEGY
Our research and development activities focus on identifying unmet surgical needs and addressing those needs with innovative solutions and products. The markets in which we participate are characterized by rapid technological change, frequent product introductions and evolving customer requirements. Investment in research and development is critical to driving our future growth. Our research and development efforts are focused on the further development and improvement of our existing products, the design and development of new innovative medical technologies and regulatory compliance across all our business segments. We apply our core competency in regenerative technology to innovate products for neurosurgical, wound applications, plastic surgery, and reconstructive surgery and we have extensive R&D development programs for our core platforms of electromechanical technologies. Additionally, we conduct products and clinical studies to generate efficacy and health economic evidence.
Regenerative Technologies.We were the first company to receive an FDA claim for regeneration of dermal tissue and are a world leader in regenerative technology. Our regenerative technology development program applies our expertise in bioengineering to a range of biomaterials including natural materials such as purified collagen, intact human or animal tissues, honey as well as resorbable synthetic polymers with our DuraSorband DuraSealproduct lines. These unique product designs are used for neurosurgical and reconstructive surgical applications, as well as dermal regeneration, including the healing of chronic and acute wounds, tendon and nerve repair. Our regenerative technology platform includes our legacy Integra® Dermal Regeneration Template ("IDRT") products and complementary technologies that we have acquired. Our collagen manufacturing capability, combined with our history of innovation, including our launch of NeuraGen 3D, provides us with strong platform technologies for multiple indications.
In the second quarter of 2023, after consultation with the FDA, The Company initiated a voluntary global recall of all products manufactured at the Boston facility, including Primatrix®, Surgimend®, Revize™, and TissueMend™, distributed between March 1, 2018 and May 22, 2023. See Item 1A. Risk Factors, under the heading Risks Related to our Regulatory Environment and under Item 7. General Management's Discussion and Analysis of Financial Condition and Results of Operations - FDA Matters of this Annual Report on Form 10-K for further discussion.
In the third quarter of 2021, we filed a PMA application for a specific indication for Surgimend® in the use of post-mastectomy breast reconstruction. In 2022, we acquired SIA, which is also PMA for DuraSorb with IBBR, and in June 2023 we completed enrollment in the DuraSorb U.S. IDE clinical study for two-stage breast reconstruction; the primary follow-up period is one year after device implantation. By offering two distinct product solutions, we believe we have the opportunity to build a leading position in the IBBR market. We hope to obtain FDA approvals in 2025.
Additionally, in 2022 we launched NeuraGen 3D Nerve Guide Matrix, a resorbable implant for repair of peripheral nerve discontinuities and engineered to create an optimized environment for nerve regeneration. Following the completion of design control activities in 2022, we launched both Cytal and MicroMatrix in Europe in 2023. In 2023, the Company received 510(k) clearance from the FDA for MicroMatrix® Flex.
Electromechanical Technologies and Instrumentation. The CSS business consists of a broad portfolio of market-leading brands, such as Codman®, DuraGen®, DuraSeal®, CUSA®, Mayfield®,Bactiseal®, and Certas® Plus,which are used for the management of multiple disease states, including brain tumors, traumatic brain injury, hydrocephalus and other neurological conditions. The growth in this business in recent years has been fueled by geographic expansion and new product registrations in markets, such as China, Japan, and Europe, which we expect to continue in the near-to-long term. Because our electromechanical products and instruments address significant needs in surgical procedures and limit uncertainty for surgeons, we continue to invest in registrations, clearances, and approvals for new indications and next generation improvements to our market-leading products. We have several active programs focused on life cycle management and innovation for capital and disposable products in our portfolio. Our product development efforts are focused on core clinical applications in cerebrospinal fluid ("CSF") management, neuro-critical care monitoring, minimally invasive instruments and electrosurgery and ultrasonic medical technologies, as well as our ambition to transform the standard of care in neurosurgery with product advancements in minimally invasive surgery ("MIS") and the surgical management of intracerebral hemorrhage ("ICH"). Our lighting franchise is among the most dynamic in the industry.
We are focused on the development of core clinical applications in our electromechanical technologies portfolio. We continue to update our CUSA Clarity platform by incorporating new ultrasonic handpiece and integrated electrosurgical capabilities. In 2022, we made progress to several enhancements to our CUSA Clarity Tissue Ablation System. The extended laparoscopic tip was launched in the U.S. to enhance laparoscopic liver procedures. In addition, a single-sided bone tip received 510(k) clearance from the FDA. Commercial launch was completed successfully in early 2023. In August 2023, we launched a modified 23 kHz CUSA Electrosurgery Module (CEM) for Clarity handpieces that can be used with additional electrosurgery generators. We continue to work with several instrument partners to bring new surgical instrument platforms to the market.
Throughout 2023 we also continued to advance the early-stage technology platforms we acquired in 2019. Through the acquisition of Arkis Biosciences, Inc. ("Arkis") we added a platform technology, CerebroFlo® external ventricular drainage ("EVD"), catheter with Endexo® technology, a permanent additive designed to reduce the potential for catheter obstruction due to thrombus formation. The CerebroFlo EVD catheter has demonstrated an average of 99% less thrombus accumulation onto its surface, in vitro, compared to a market leading EVD catheter. Our work to combine our Bactiseal® antimicrobial technology with the Endexo anti-occlusive technology continues to progress for both a silicone-based hydrocephalus and EVD project.
Throughout 2023, we continued to advance our innovation from the Rebound Therapeutics Corporation ("Rebound Therapeutics"), which was acquired in 2019. Rebound Therapeutics specializes in a single-use medical device, known as the Aurora Surgiscope, which is the only tubular retractor system designed for cranial surgery with an integrated access channel, camera and lighting. The 9mm Surgiscope received 510(k) clearance from the FDA in the fourth quarter of 2023.
In the third quarter of 2021, we launched our CereLink ICP Monitor System in the U.S. and Europe and continued the global rollout in the first half of 2022. On August 18, 2022, the Company, after consultation with the FDA and other regulatory authorities outside of the United States, initiated an immediate voluntary global product removal of all CereLink® intracranial pressure monitors. We believe that the out-of-range readings are principally caused by a combined interaction of electrical noise (originating from sources such as electrical components in the device, other devices set up near the CereLink Monitor, and the hospital power grid) and an electrical potential difference between the patient and monitor. We submitted a traditional 510(k) submission to the FDA on September 15, 2023 as a result of customer reports about monitors whose pressure readings were out of range. We have received 510(k) clearance from the FDA on February 4th, 2024. We plan to resume shipments of CereLink monitors in the U.S. in the first quarter of 2024. Shipments resumed in international markets with a limited release in the third quarter of 2023. See Item 1A. Risk Factors, under the heading Risks Related to our Regulatory Environment and under Item 7. General Management's Discussion and Analysis of Financial Condition and Results of Operations - FDA Matters of this Annual Report on Form 10-K for further discussion.
MANUFACTURE AND AVAILABILITY OF RAW MATERIALS
We manufacture products at manufacturing facilities located in various countries throughout the world. We purchase many of the components and raw materials used in manufacturing our products from numerous suppliers in various countries.
In general, raw materials essential to our businesses are readily available from multiple sources. For reasons of quality assurance, availability, or cost effectiveness, certain components and raw materials are available only from one or a limited number of suppliers. We have established long-term supply contracts with many of our suppliers and our practice is to maintain sufficient inventory of components so that our production will not be significantly disrupted even if a particular component or material is not available for a period of time. Due to the high standards and FDA requirements applicable to manufacturing our products, such as the FDA's Quality System Regulation and Good Manufacturing Practices, we may not be able to quickly establish additional or replacement sources for certain components or materials. Some of our manufacturing operations are located outside of the U.S., including in Puerto Rico, Switzerland, Ireland and France. Those manufacturing operations are also subject to additional challenges and risks associated with international operations described under the caption “Risk Factors” set forth in Part I, Item 1A of this Annual Report on Form 10-K. In the event we are unable to obtain sufficient quantities of raw materials or components on commercially reasonable terms or in a timely manner, our ability to manufacture our products on a timely and cost-competitive basis may be compromised, which may have a material adverse effect on our business, financial condition and results of operations.
Certain of our products, including but not limited to our dermal regeneration products, duraplasty products, wound care products, and nerve and tendon repair products, contain material derived from bovine tissue. We take great care to provide products that are safe and free of agents that can cause disease. In particular, the collagen used in the products that we manufacture is derived from the deep flexor tendon of cattle less than 24-months old from New Zealand, a country that has never had a reported case of bovine spongiform encephalopathy ("BSE") (otherwise known as mad cow disease), from the U.S. or from fetal bovine dermis. The World Health Organization classifies different types of cattle tissue for relative risk of BSE transmission. Deep flexor tendon and fetal bovine skin are in the lowest-risk category for BSE transmission, and therefore considered to have a negligible risk of containing the agent that causes BSE.
INTELLECTUAL PROPERTY
We seek patent and trademark protection for our key technology, products and product improvements, both in the U.S. and in selected foreign countries. When determined appropriate, we have enforced and plan to continue to enforce and defend our patent and trademark rights. In general, however, we do not rely solely on our patent and trademark estate to provide us with any significant competitive advantages as it relates to our existing product lines. We also rely upon trade secrets and continuing technological innovations to develop and maintain our competitive position. In an effort to protect our trade secrets, we have a policy requiring our employees, consultants and advisors to execute proprietary information and invention assignment agreements upon commencement of employment or consulting relationships with us. These agreements also provide that all confidential information developed or made known to the individual during the course of their relationship with us must be kept confidential, except in specified circumstances.
AccuDrain®, AmnioExcel®, Aquasonic®, Auragen®, Aurora® Surgiscope®, Bactiseal®, BioDFence®, BioDOptix®, Brainet®, Budde®, Buzz™, CereLink®, CerebroFlo® EVD Catheter with Endexo® Technology, Codman®, Codman Accu-Flo®, Codman Bicol®, Codman® Certas® Plus, Codman® Hakim®Programmable valve, Codman Holter®, Codman ICP Express®, Codman Microsensor®, Codman VersaTru®, Codman VPV®, Contour-Flex®, Cranioplastic®, CRW®, CRW Precision™, Ctherm™, CUSA®, Cytal®, DirectLink®, DuraGen®, DuraSeal®, DuraSorb®, Gentrix®, HeliCote®, HeliPlug®, HeliTape®, HeliMend®, Helistat®, Helitene®, Hermetic™, Hy-Tape®, Integra®, IntegraLink®, Isocool®, Jarit®, Lead-Lok™, Licox®, LimiTorr™,
Luxtec®, Mayfield®, MatriStem UBM™, MediHoney®, MicroFrance®, MicroMatrix®, Miltex®, Mischler™, MoniTorr ICP™, Natus®, NeuraGen®, NeuraWrap™, Nicolet®, Omnigraft®, Omni-Tract®, OSV II®, Padgett®, PriMatrix®, Pureflow™, Q-Snor™, Redmond™, Revize™, Ruggles®, Signacreme®, SurgiMend®, TCC-EZ®, TenoGlide®, TissueMend®, Ultra VS™, VersaTru®, Xtrasorb®, zRIP™, and the Integra logo are some of the material trademarks of Integra LifeSciences Corporation and its subsidiaries. MAYFIELD® is a registered trademark of SM USA, Inc., and is used by Integra under license.
SEASONALITY
Revenues during our fourth quarter tend to be stronger than other quarters because many hospitals increase their purchases of our products during the fourth quarter to coincide with the end of their budget cycles in the U.S. In general, our first quarter usually has lower revenues than the preceding fourth quarter, the second and third quarters have higher revenues than the first quarter, and the fourth quarter revenues are the highest in the year. The main exceptions to this pattern occur because of material acquisitions.
GOVERNMENT REGULATION AND COMPLIANCE
We are a manufacturer and marketer of medical devices and Human Tissue and Cell Based Products ("HCT/Ps") and therefore are subject to extensive regulation by the FDA, the Center for Medicare Services of the U.S. Department of Health and Human Services ("HHS"), other federal governmental agencies and, in some jurisdictions, by state and foreign governmental authorities. These regulations govern the introduction of new medical devices and HCT/Ps, the observance of certain standards with respect to the design, manufacture, testing, labeling, promotion and sales of the products, the maintenance of certain records, the ability to track devices, the reporting of potential product defects, the import and export of products, and other matters. FDA product approvals may be withdrawn or suspended if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
Our business is also affected by patient and data privacy laws and government payer cost containment initiatives, as well as environmental health and safety laws and regulations.
United States Food and Drug Administration
Our products are subject to extensive regulation particularly as to safety, efficacy and adherence to FDA Quality System Regulation, and related manufacturing standards. Medical device products are subject to rigorous FDA and other governmental agency regulations in the United States 23%and similar regulations of foreign agencies abroad. The FDA regulates the design, development, research, preclinical and clinical testing, introduction, manufacture, advertising, labeling, packaging, marketing, distribution, import and export, and record keeping for such products, in Europe, Middle Eastorder to ensure that medical products distributed in the United States are safe and Africa, 4%effective for their intended use. In addition, the FDA is authorized to establish special controls to provide reasonable assurance of the safety and effectiveness of most devices. Non-compliance with applicable requirements can result in Latin Americaimport detentions, fines, civil and Canadaadministrative penalties, injunctions, suspensions or losses of regulatory approvals, recall or seizure of products, operating restrictions, refusal of the government to approve product export applications or allow us to enter into supply contracts, and 8%criminal prosecution. The regulatory process for obtaining product approvals and clearances can be onerous and costly.
Under the Federal Food, Drug and Cosmetic Act (the "FD&C Act"), authorization to commercially distribute a new medical device in Asia Pacific which includes Australiathe U.S. is generally obtained in one of two primary ways. The first, known as pre-market notification or the 510(k) process, requires us to demonstrate that our medical device is substantially equivalent to a legally marketed medical device. A 510(k) pre-market notification filing must contain information establishing that the device to be sold is substantially equivalent to a device commercially distributed prior to May 28, 1976 or to a device that has been determined by the FDA to be substantially equivalent. The second, more rigorous process, known as pre-market approval (“PMA”) requires us to independently demonstrate that a medical device is safe and New Zealand.effective for its intended use. This process is generally much more time-consuming and expensive than the 510(k) process. The PMA process involves a complex and lengthy testing process that is subject to review by the FDA and may require several years to obtain. We may need to first obtain an investigational device exemption (for significant risk devices), known as an IDE, in order to conduct extensive clinical testing of the device to obtain the necessary clinical data for submission to the FDA. The FDA will approve a PMA only if after evaluating the supporting technical data it finds that the PMA contains sufficient, valid scientific evidence to assure that the device is safe and effective for its intended use(s). This approval may be granted with post-approval requirements including inspection of manufacturing facilities and/or additional patient follow-up for an indefinite period of time.
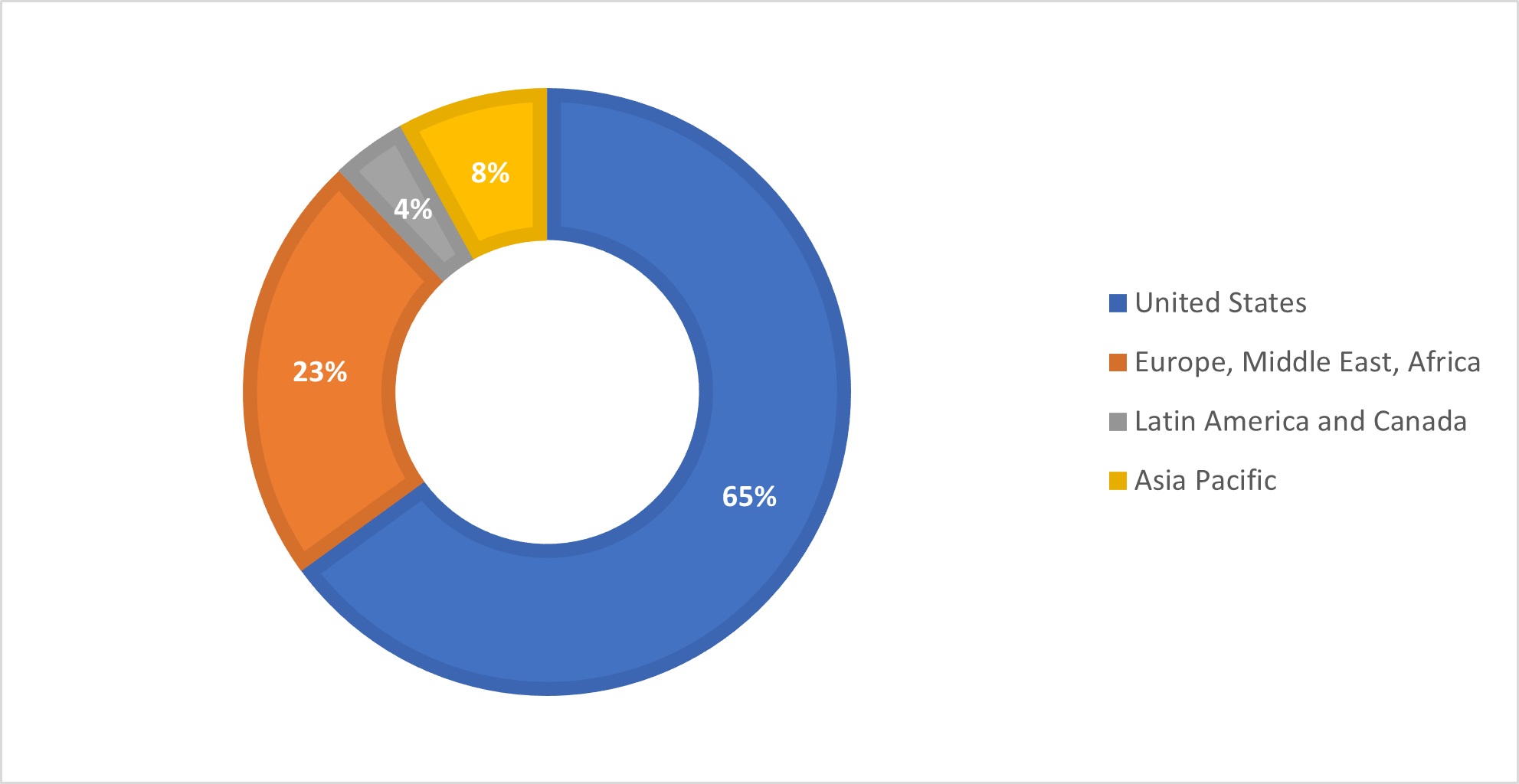
replace or refund the cost of the medical device. Because we currently export medical devices manufactured in the U.S. that have not been approved by the FDA for distribution in the U.S., we are required to obtain approval/registration in the country to which we are exporting and maintain certain records relating to exports and make these available to the FDA for inspection, if required.
Human Cells, Tissues and Cellular and Tissue-Based Products
Integra, through its wholly-owned subsidiary BioD LLC (“BioD”), is involved with the recovery, processing, storage, transportation and distribution of donated amniotic tissue. The FDA has specific regulations governing HCT/Ps. An HCT/P is a product containing, or consisting of, human cells or tissue intended for transplantation into a human patient. Examples of HCTPs include bone, ligament, skin and cornea.
Some HCT/Ps fall within the definition of a biological product, medical device or drug regulated under the FD&C Act. These biologic, device or drug HCT/Ps must comply both with the requirements exclusively applicable to HCT/Ps and, in addition, with requirements applicable to biologics, devices or drugs, including premarket clearance or approval from the FDA.
Section 361 of the Public Health Service Act (“Section 361”) authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease. HCT/Ps regulated as “361” HCT/Ps are subject to requirements relating to registering facilities and listing products with the FDA, screening and testing for tissue donor eligibility, and Good Tissue Practices when processing, storing, labeling, and distributing HCT/Ps, including required labeling information, stringent record keeping, and adverse event reporting.
The American Association of Tissue Banks (“AATB”) has issued operating standards for tissue banking. Compliance with these standards is a requirement in order to become an AATB-accredited tissue establishment. In addition, some states have their own tissue banking regulations. We are licensed or have permits for tissue banking in California, Delaware, Illinois, Maryland, New York, Oregon, and Tennessee. In Tennessee, we are registered with the FDA Center for Biological Evaluations and Research.
Procurement of certain human organs and tissue for transplantation is subject to the restrictions of the National Organ Transplant Act, which prohibits the transfer of certain human organs, including skin and related tissue for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. BioD is a registered Tissue Bank and is involved with the recovery, storage and transportation of donated human amniotic tissue.
Medical Device Regulations
The FDA requires that a manufacturer obtain 510(k) clearance or a PMA for a variety of reasons, such as introducing a new medical device or new indication for use of an existing medical device, before introducing it into the U.S. market. The type of marketing authorization is generally linked to the classification of the device. The FDA classifies medical devices into three classes based on risk. Regulatory control increases from Class I (lowest risk) to Class III (highest risk). The FDA generally must clear or approve the commercial sale of new medical devices in Classes II and III. Commercial sales of our Class II medical devices (except for Class II exempt devices) and Class III medical devices within the U.S. must be preceded by either a pre-market notification filing pursuant to Section 510(k) of the FD&C Act (Class II) or the granting of a pre-market approval, or PMA (Class III).
The process of obtaining a Section 510(k) clearance generally requires the submission of performance data and may require clinical data, which in some cases can be extensive, to demonstrate that the device is “substantially equivalent” to another device that is currently marketed under a 510(k); a device that is referred to as “predicate device.” As a result, FDA clearance requirements may extend the development process for a considerable length of time. In the case of a PMA, the FDA may require additional review by an advisory panel, which can further lengthen the process. The PMA process, which is reserved for new devices that are not substantially equivalent to any predicate device and for high-risk devices (i.e., Class III devices) that are used to support or sustain human life or which present a potential, unreasonable risk of illness or injury, may take several years and requires the submission of extensive performance and clinical information.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device has received 510(k) clearance for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, may require a new 510(k) clearance or PMA approval and payment of an FDA user fee. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance; however, the FDA may review this determination to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
DiversityWe also are required to register with the FDA as a medical device manufacturer and Inclusion
A diverse workforceany devices we manufacture and an inclusive culturedistribute pursuant to clearance or approval by the FDA are subject to pervasive and work environment iscontinuing regulation by the FDA and certain state agencies. These include product listing and establishment registration requirements, which help facilitate FDA inspections and other regulatory actions, and our manufacturing sites are subject to periodic inspection by the FDA for compliance with the FDA's Quality System Regulations. These regulations require that we manufacture our products and maintain our documents in a business priorityprescribed manner with respect to design, manufacturing, testing and control activities. Further, we are required to comply with various FDA requirements and other legal requirements for labeling and promotion. FDA regulates unclassified devices via the 510(k) process and has the authority to classify these devices and/or require Special Controls, additional testing and submission of a key to our long-term success. Our commitment to diversity and inclusion ("D&I") starts at the top with our Board of Directors and CEO. At all levelsnew 510(k) as part of the classification process for unclassified devices that are currently on the market as 510(k) cleared products. If the FDA believes that a company we focus on attracting, retaining,is not in compliance with applicable regulations, it may issue a warning letter, institute proceedings to detain or seize products, issue a recall order, impose operating restrictions, enjoin future violations and developing our diverse talent.
Leadership Commitmentassess civil penalties against that company, its officers or its employees and Accountability
Executive leadership team members setmay recommend criminal prosecution to the D&I goalsU.S. Department of Justice. The majority of Integra manufacturing facilities participate in the Medical Device Single Audit Program and are audited annually for compliance with the companyQuality System for US FDA, Canada, Australia, Brazil, and advancing diversity and inclusion initiatives to build stronger teams remains a company-wide goal.
Leadership Councils, Employee Resource Groups and External Partnerships:
We are accountable to our D&I commitment through our leadership councils, employee resource groups, and external partnerships.Japan.
•Our Women’s Leadership Council, since its establishmentMedical device regulations also are in 2017,effect in many of the countries in which we do business outside the U.S. In the European Economic Area ("EEA"), which is an action and results-oriented advisory group comprised of fifteenthe 27 member states of the European Union (the "EU") plus Norway, Iceland and Liechtenstein, medical devices need to comply with specific requirements. These requirements were previously known as "Essential Requirements" under the former EU Medical Devices Directive (Council Directive 93/42/EEC, or MDD) and are now defined "General Safety and Performance Requirements (GSPR)" under the new EU Medical Devices Regulation (Regulation (EU) 2017/745, or "EU MDR"). Although the requirements set forth in the EU MDR are generally consistent with those laid out in the MDD (with a few exceptions), the EU MDR is intended, among other things, to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation. These laws range from comprehensive medical device approval and Quality System requirements for some or all of our senior women leaders across Integra. The specific charter ofmedical device products to simpler requests for product data or certifications. Under the Council isMDD, medical devices must meet the MDD standards and receive CE Mark Certification prior to work together to identify ways tomarketing in the EEA. Although we continue to attract and retain female talent, advance the development oftransition our women into leadership roles, increase the cultural awareness of the value of inclusion and diversity in our company, and create specific development forums for our high performing women at Integra.
•Employee resources groups encourage a culture of awareness and inclusion, assist in the attraction and retention of diverse talent, and help colleagues develop leadership skills. Members of the executive leadership team serve as sponsors for each of Integra’s employee resource groups. Integra has five Employee Resources Groups:
◦Women of Integra Networks ("WIN") with 20+ chapters globally
◦African American Affinity Group
◦Veteran Employee Resource Group
◦Indian American Professional Network
◦Asian American and Pacific Islander Employee Resource Group
•We reinforce our commitment to diversity by partnering with other organizations focused on driving inclusion in the workplace including the CEO Action for Diversity & Inclusion, the largest CEO-driven business commitment to advance D&I in the workplace and Healthcare Businesswomen’s Association, an association dedicated to further the advancement and impact of women in the business of healthcare.
Promoting an inclusive culture through learning opportunities:
To help drive our culture of inclusion, our colleagues participate in programs focused on how to manage bias and value differences.
•Members of our executive leadership, senior management team, and larger scope leaders participate in a half-day microinequities training. The content includes understanding unconscious bias and microinequities, how to identify microinequities in day-to-day decisions and actions as leaders, and ways to mitigate microinequities on an individual and organizational level.
•Upon joining Integra, colleagues globally participate in two programs to promote inclusion: a course that creates awareness of unconscious biases in the workplaces and tools to build-bias breaking skills and a course that examines what practicing inclusion in the workplace looks like.
Gender Diversity:
We believe that our company is stronger and will deliver strong operating results when we build diverse teams and leverage broad perspectivescertification profile to meet the needsnew EU MDR requirements, these stricter regulations set forth in the EU MDR may pose additional challenges for Integra to continue marketing products in the EU as these regulations come into force. See “Item 1A. Risk Factors - We are subject to stringent domestic and foreign medical device regulations and oversight and any adverse action may adversely affect our ability to compete in the marketplace and our financial condition and business operations” of this Annual Report on Form 10-K.
CE Mark Certification requires a comprehensive quality system program, technical documentation, clinical evaluation and data on the product which are then reviewed, by a Notified Body. A Notified Body is an organization designated by the national governments of the EU member states to make independent judgments about whether a product complies with the requirements established by each CE marking directive. The MDD, MDR, ISO 9000 series and ISO 13485 are recognized international quality standards that are designed to ensure that we develop and manufacture quality medical devices. Other countries are also instituting regulations regarding medical devices or interpreting and enforcing existing regulations more strictly. Compliance with these regulations requires extensive documentation and clinical reports for our products, revisions to labeling, and other requirements such as facility inspections to comply with the registration requirements. A recognized Notified Body audits our facilities annually to verify our compliance with the ISO 13485 Quality System standard.
Certain countries, as well as the EU, have issued regulations that govern products that contain materials derived from animal sources. Regulatory authorities are particularly concerned with materials infected with the agent that causes BSE. These regulations affect our dermal regeneration products, duraplasty products, hernia repair products, biomaterial products for the spine, nerve and tendon repair products and certain other products, all of which contain material derived from bovine tissue. Although we take great care to provide that our products are safe and free of agents that can cause disease, products that contain materials derived from animals, including our products, may become subject to additional regulation, or even be banned in certain countries, because of concern over the potential for prion transmission. Significant new regulations, a ban of our shareholders, customers, colleagues, and communities we serve.
products, or a movement away from bovine-derived products because of an outbreak of BSE could have a material, adverse effect on our current business or our ability to expand our business. See “Item 1A. Risk Factors –
TPostmarket Requirementshe breakout.After a device is cleared or approved for commercial distribution, numerous regulatory requirements apply. These include the FDA Quality System Regulations which cover the procedures and documentation of our colleagues by gender asthe design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of December 31, 2021:
48%medical devices; the FDA's general prohibition against promoting products for unapproved or 'off-label' uses; the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and the Reports of Integra’s overall population is female, 52% male. We continueCorrections and Removals regulation, which require manufacturers to strive to ensure our diversity in our leadership roles is representative of our overall population. Through mentorship, sponsorship, recruitment efforts,report recalls and development programs we look to continue to grow our population of females in leadership roles at Integra. Currently, 42% of our executive leaders and 45% of senior leaders (non-executive vice presidents) are female.
to the FDA if initiated to reduce a risk to health posed by the device or to remedy a violation of the FD&C Act. Postmarket requirements are also followed globally where our products are registered and approved. These foreign jurisdictions have similar requirements to the FDA which include reporting requirements such as adverse events and recalls.
Regulations Governing Reimbursement
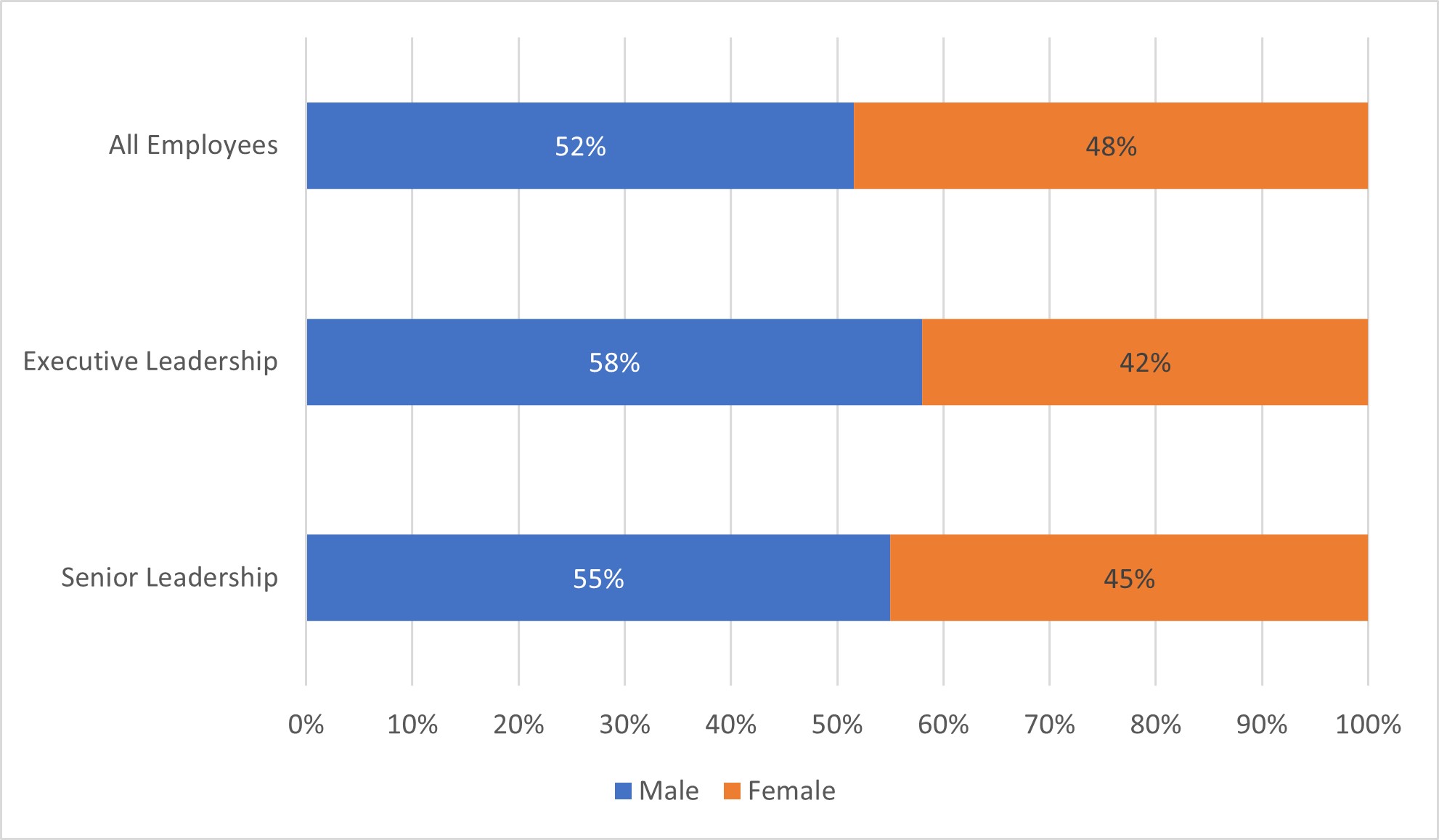
Other regulations
In partnership with Leadership Edge, a company founded by women leadersEnvironmental Health and dedicated to growing and mentoring women, Integra sponsors the Excel Women’s Leadership Program. The program is designed to accelerate theSafety. Our research, development and advancementmanufacturing processes involve the controlled use of high potential, mid-career female leaders into senior leadership roles. The program has assisted in further building our pipelinecertain hazardous materials. We are subject to country-specific, federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of women leaders with 50% of the program’s graduates being promoted into roles with increased responsibility.
Employee Healththese materials and Safety:
Integra LifeSciences is committed to providing a safe environment for all employees and visitors.certain waste products. We rely onbelieve that our environmental, health and safety management systems as well as entrustingprocedures for handling and disposing of these materials comply with the standards prescribed by the controlling laws and regulations. However, risk of accidental releases or injury from these materials is possible. These risks are managed to minimize or eliminate associated business impacts. In the event of this type of accident, we could be held liable for damages and face a liability that could exceed our managersresources. We could be subject to overseea regulatory shutdown of a facility that could prevent the distribution and ensure healthsale of products manufactured there for a significant period of time, and safety at their respective sites and fosterwe could suffer a workplace culturecasualty loss that could require a shutdown of the facility in order to achieve that end. We implementrepair it, any of which could have a material, adverse effect on our approach globally by our systems and support at regional and country levels from colleagues that implement proper safety protocols, identify and correct hazards, and remain safety conscious at all times. Managers are expectedbusiness. Although we continuously strive to enforce health and safety regulations, includingmaintain full compliance with respect to all applicable federal, state and local laws. Our Environmental Health and Safety ("EH&S") organizational structure incorporates both workplace EH&S coordinators and compliance teams. We have developed an Incident Procedure Policy and General Safety Rules that guide our colleagues to improve our workplace environment, improve safety, and reduce risk and costs.
As we navigate the COVID-19 pandemic and its variants, we have placed a high priority on employee health, providing resources to support our workforce through this challenging time. To help limit exposure to the coronavirus, we acted to ensure employees in business-critical functions who cannot work from home are protected, including those in research and development, quality, manufacturing, distribution, and sales. Personal protective equipment, increased sanitation and social distancing guidance are provided to protect our employees. We continue to actively monitor the COVID-19 pandemic and its variants and respond based on guidance from U.S. and global health organizations, relevant governmental guidance, and evolving practices.
FINANCIAL INFORMATION ABOUT GEOGRAPHIC AREAS
Financial information about our geographical areas is set forth in our financial statements Note 16, Segment and Geographic Information, to the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K).
AVAILABLE INFORMATIONenvironmental, health and safety laws and regulations, we could incur substantial costs to fully comply with future laws and regulations, and our operations, business or assets may be negatively affected. Furthermore, global environmental, health and safety compliance is an ongoing process. We have compliance procedures in place for compliance with Employee Health & Safety laws, driven by a centrally led organizational structure that ensures proper implementation, which is essential to our overall business objectives.
WeIn addition, we are subject to numerous federal, state, foreign and local laws relating to safe working conditions, environmental protection and fire hazard control, among others. We may be required to incur significant costs to comply with these laws and regulations in the informationalfuture and complying with these laws may result in a material adverse effect upon our business, financial condition and results of operations.
Data Privacy and Cybersecurity Laws and Regulations. As a business with a significant global footprint, compliance with evolving regulations and standards in data privacy and cybersecurity (relating to the confidentiality and security of our information technology systems, products such as medical devices, and other services provided by us) may result in increased costs, lower revenue, new complexities in compliance, new challenges for competition, and the threat of increased regulatory enforcement activity. Our business relies on the secure electronic transmission, storage and hosting of sensitive information, including personal information, financial information, intellectual property, and other sensitive information related to our customers and workforce.
For example, in the U.S., the collection, maintenance, protection, use, transmission, disclosure and disposal of certain personal information and the security of medical devices are regulated at the U.S. federal and state, and industry levels. U.S. federal and state laws protect the confidentiality of certain patient health information, including patient medical records, and restrict the use and disclosure of patient health information by health care providers. For example, in the U.S. we are obligated to comply with the requirements of the Securities ExchangeHealth Insurance and Portability Act of 1934,1996, as amended (the “Exchange Act"by the Health Information Technology for Economic and Clinical Health Act of 2009 (collectively, “HIPPA”). In accordance withUnder HIPAA, the Exchange Act, we file annual, quarterly and special reports, proxy statements and other information with the Securities and Exchange Commission, ("the SEC"). Our financial information may be viewed,HHS has issued regulations, including the HIPAA Privacy, Security and Breach Notification Rules, to protect the privacy and security of protected health information containedused or disclosed by covered entities including health care providers and their business associates, as well as covered subcontractors. HIPAA also regulates standardization of data content, codes and formats used in this report,health care transactions and other reportsstandardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include significant civil and criminal penalties for each violation. In addition, the FDA has issued guidance advising manufacturers to take cybersecurity risks into account in product design for connected medical devices and systems, to assure that appropriate safeguards are in place to reduce the risk of unauthorized access or modification to medical devices that contain software and reduce the risk of introducing threats into hospital systems that are connected to such devices. The FDA also issued guidance on post market management of cyber security in medical devices.
Outside the U.S., we fileare impacted by the privacy and data security requirements at the international, national and regional level, and on an industry specific basis. Legal requirements in these countries relating to the collection, storage, handling and transfer of personal data and, potentially, intellectual property continue to evolve with the SEC,increasingly strict enforcement regimes. In Europe, for example, we are subject to EU General Data Protection Regulation ("GDPR") which requires member states to impose minimum restrictions on the Internet, without charge as soon as reasonably practicable after we file them with the SEC,collection, use and transfer of personal data and includes, among other things, a requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances and significant fines for non-compliance. The GDPR also requires companies processing personal data of individuals residing in the “SEC Filings” pageEU to comply with EU privacy and data protection rules.
Please refer to “Item 1A. Risk Factors – Failure to comply with laws relating to the confidentiality of sensitive personal information or standards related to the transmission of electronic health data, may require us to make significant changes to our products, or incur penalties or other liabilities” of this Annual Report on Form 10-K for additional discussion of the Investor Relations section of our website at www.integralife.com. A copy may also be obtained for any of these reports, without charge, from our Investor Relations department, 1100 Campus Road, Princeton, NJ 08540. Alternatively, reports filed may be viewed or obtained through the SEC's website at www.sec.govrisks accompanying compliance with data privacy and cybersecurity laws and regulations.
These laws and regulations impact the ways in which we use and manage personal data, protected health information, and our information technology systems. They also impact our ability to move, store, and access data across geographic boundaries. Compliance with these requirements may require changes in business practices, complicate our operations, and add complexity and additional management and oversight needs. They also may complicate our clinical research activities, as well as product offerings that involve transmission or use of clinical data.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
We have made statements in this report, including statements under “Business” and “Management's Discussion and Analysis of Financial Condition and Results of Operations” that constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, ("the Securities Act"), and Section 21E of the Securities Exchange Act.Act of 1934, as amended ("the "Exchange Act"). These forward-looking statements are subject to a number of risks, uncertainties and assumptions about us including, among other things:
•the COVID-19 pandemic;on-going and possible future effects of global challenges, including macroeconomic uncertainties, such as supply chain disruptions, inflation, bank failures, rising interest rates and availability of capital markets, the Israel-Hamas and Ukraine-Russia wars, other economic disruptions and U.S. and global recession concerns, on our customers and suppliers, and on our business, financial condition, results of operations and cash flows;
•general economic and business conditions, both nationallydomestically and in our international markets;markets, including the effect of the continuing worldwide macroeconomic uncertainty and increasing trade regulations and tariffs;
•our expectations and estimates concerning future financial performance, financing plans and the impact of competition;
•anticipated trends in our business;
•anticipated demand for our products, particularly capital equipment;
•our ability to produce and deliver products in sufficient quantities to meet sales demands;
•the ongoing and possible future effects of supply chain constraints, including the availability of critical raw materials and components, as well as cost inflation in materials, packaging and transportation;
•our expectations concerning our ongoing restructuring, integration and manufacturing transfer and expansion activities;
•existing and future regulations affecting our business, and enforcement of those regulations;
•conducting business internationally;
•our failure to comply with the substantial regulation related to quality standards applicable to our manufacturing and quality processes could have an adverse effect on our business, financial condition, or results of operations;
•our ability to remediate all matters identified in United States Food & Drug (the "FDA") observations and warning letters that we received or may receive;
•our ability to obtain additional debt and equity financing to fund capital expenditures, working capital requirements and acquisitions;
•physicians' willingness to adopt our recently launched and planned products, third-party payors' willingness to provide or continue reimbursement for any of our products and our ability to secure regulatory approval for products in development;
•initiatives launched by our competitors;
•our ability to protect our intellectual property, including trade secrets;
•our ability to complete acquisitions, integrate operations post-acquisition and maintain relationships with customers of acquired entities;
•the ability to successfully manage ongoing organizational and strategic changes, including our ability to remediate all matters identifiedattract, motivate and retain key employees and maintain engagement and efficiency in FDA observationsremote work environments;
•anticipated trends relating to our financial condition or results of operations, including the impact of interest rate and warning letters that we received or may receive;foreign currency exchange fluctuations;
•the effect of any future public health crises, including the timing, scope and effect of U.S. and international governmental, regulatory, fiscal, monetary and public health responses to such crises; and
•other risk factors described in the section entitledItem 1A. "Risk Factors" in this report.Annual Report on Form 10-K.
Forward-looking statements can be identified by forward-looking words such as “believe,” “may,” “could,” “might,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “seek,” “plan,” “expect,” “should,” “would”“would,” “expect,” “target,” “pursue,” “forecast,” “hope” and similar expressions in this Annual Report on Form 10K. Forward-looking statements in this Annual Report on Form 10-K include, but are not limited to, statements regarding our five-pillar growth strategy; the closing of our pending acquisition of Acclarent, Inc. on anticipated terms and timing, or at all; the anticipated benefits of our pending acquisition of Acclarent, Inc.; expectations and plans with respect to strategic initiatives, product development and regulatory approvals, including the anticipated resumption of manufacturing at the Company’s Boston, Massachusetts facility. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by such forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this report. WeExcept as required by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. In lightFactors that could cause or contribute to differences in our future financial results include the cautionary statements set forth herein and in our other filings with the Securities and Exchange Commission, including those set forth under “Risk Factors” set forth in Part I, Item 1A of this Annual Report on Form 10-K. We qualify all of our forward-looking statements by these risks and uncertainties, the forward-looking events and circumstances discussed in this report may not occur and actual results could differ materially from those anticipated or implied in the forward-lookingcautionary statements.
PART I
ITEM 1B.1. UNRESOLVED STAFF COMMENTSBUSINESS
AsOVERVIEW
Integra LifeSciences Holdings Corporation is a leading global medical technology company innovating treatment pathways to advance patient outcomes and set new standards of surgical, neurologic and regenerative care.
Founded in 1989 with the acquisition of an engineered collagen technology platform used to repair and regenerate tissue, our common stock trades on the Nasdaq Global Select Market (“Nasdaq”) under the symbol “IART.” We have developed numerous product lines from this technology for applications ranging from burn and deep tissue wounds to the repair of dura mater in the brain, as well as nerves and tendons. We have expanded our base regenerative technology business to include surgical instruments, neurosurgical products and advanced wound care through global acquisitions and product development to meet the evolving needs of our customers and enhance patient care.
Integra products are sold in more than 130 countries through a direct sales force as well as distributors and wholesalers. We manufacture and sell medical technologies and products in two reportable business segments: Codman Specialty Surgical ("CSS") and Tissue Technologies ("TT"). The CSS segment, which represents approximately two-thirds of our total revenue, consists of market-leading technologies and instrumentation used for a wide range of specialties, such as neurosurgery, neurocritical care and otolaryngology. We are the world leader in neurosurgery and one of the filingtop three providers in instruments used in precision, specialty, and general surgical procedures. Our TT segment generates about one-third of our overall revenue and focuses on three main areas: complex wound surgery, surgical reconstruction, and peripheral nerve repair.
We have key manufacturing and research facilities located in California, Indiana, Maryland, Massachusetts, New Jersey, Ohio, Puerto Rico, Tennessee, Utah, France, Germany, Ireland and Switzerland. We source most of our handheld surgical instruments and dural sealant products through specialized third-party vendors.
Vision
We aspire to continue to be a worldwide leader in neurosurgery and reconstructive surgery with a portfolio of leading businesses that delivers outstanding customer experiences through innovation, execution and teamwork to positively impact the lives of millions of patients and their families.
Strategy
Following the completion of our strategic refresh in 2023, we refocused our strategies around five pillars. Of these five pillars, we have identified three core growth drivers: (1) innovating for outcomes, (2) growing internationally, and (3) broadening our impact on care pathways. Our execution of the core growth drivers is enabled by two key levers: (4) driving operational and customer excellence and (5) cultivating a high-performance culture. As outlined in greater detail below, we believe these five pillars will enable us to realize and advance our integrated growth strategy.
To this end, our executive leadership team has established the following key priorities aligned to the following five pillars:
Innovating for Outcomes. An important part of Integra’s growth strategy is introducing new products to strengthen and expand our portfolio, including via acquisitions. For example, we entered into a stock purchase agreement to acquire Acclarent, Inc. (“Acclarent”) from Ethicon, Inc., a subsidiary of Johnson & Johnson in December 2023. Acclarent is an innovator and market leader in ear, nose and throat (“ENT”) procedures and we believe that the acquisition of Acclarent will provide Integra with the opportunity to become a leading provider of ENT products and technologies. Furthermore, we believe that, owing to the ENT segment being an anatomical adjacency to neurosurgery, the acquisition will allow Integra to deliver future innovation both within the ENT segment and across our other CSS technology platforms. Additionally, we seek clinical evidence to support regulatory approval and strong reimbursement of our product portfolio around the world, including new indications for existing technologies. For example, in 2021, we filed a pre-market approval (“PMA”) application for a specific indication for Surgimend® in the use of post-mastectomy breast reconstruction. In 2022, we acquired SIA, which is also pursuing a pre-market approval for DuraSorb for use in implant-based breast reconstruction (“IBBR”), and in June 2023 we completed enrollment in the DuraSorb U.S. IDE clinical study for two-stage breast reconstruction; the primary follow-up period is one year after device implantation. We hope to obtain FDA approvals for both products in 2025. We also continued to advance the development of pioneering neurosurgical technologies with the expansion of our product offerings. In 2023 we launched the CUSA® Clarity Tips for use in surgical procedures requiring the controlled fragmentation, emulsification and aspiration of bone as well as in laparoscopic liver surgery.
Growing Internationally. Over the years, we have been significantly expanding our global footprint through investments in our commercial organization, the expansion and development of international markets and new product introductions. As part of our In-China-For-China strategy, we continue the build out of our assembly capabilities in our new facility in Suzhou, China. Several new products were introduced in select international markets in 2023, including MicroMatrix® and Certas Plus® Programmable Valve which were launched in Europe, and CUSA Clarity Laparoscopic tip which was launched in Australia,
New Zealand, Japan, Canada, South Africa and Israel. In addition, DuraGen Secure, received approval in Japan, while DuraGen Plus, an absorbable and sutureless collagen onlay indicated as a dura substitute for the repair of dura mater, was approved in China.
Broadening Impact on Care Pathways. We seek ways to develop products and technologies that impact the lives of patients, starting with the journey that a patient takes from diagnosis and treatment planning to surgery and postoperative care. We are well-established in acute care in the hospital setting and continue to leverage that strong position to grow in this segment and shape treatment pathways into preoperative care and additional sites of care.
Driving Operations and Customer Excellence. We have been making investments to build more responsive and scalable processes, enhance the reliability of our supply chain, and drive productivity initiatives to further supply and lower costs. Additionally, we continue to invest in technologies, systems and processes to enhance the customer experience. In 2023, we continued to invest in our capacity expansion. This includes ongoing projects of transferring our Boston manufacturing to a new location in Braintree, Massachusetts., validating manufacturing processes in our manufacturing facility in Plainsboro, New Jersey and increasing cleanroom capacity in our Memphis, Tennessee location.
Cultivating a High-Performance Culture. In seeking to sustain a culture of excellence and accountability, we have focused on employee empowerment and agility and building a diverse and inclusive workplace. These efforts resulted in our being named in several best workplace lists globally in 2023. Additionally, we have been making further strides in advancing our environmental, social and governance ("ESG") agenda to drive sustainability across the organization and recently published our second annual ESG report in the third quarter of 2023. For more information on our ESG strategy, goals, performance, and achievements, please visit “Our Company—ESG Report” at https://www.integralife.com/esg-report. Information on our website is not incorporated by reference herein and is not part of this Annual Report on Form 10-K.
BUSINESS SEGMENTS
We currently manufacture and sell our medical technologies and products in the following two reportable business segments: Codman Specialty Surgical and Tissue Technologies. We include financial information regarding our reportable business segments and certain geographic information under "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations" and Note 16, Segment and Geographic Information to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K).
Codman Specialty Surgical
Our CSS segment offers global, neurosurgery market-leading technologies, brands and instrumentation. The product portfolio represents a continuum of care from pre-operative, to the neurosurgery operating room, to the neuro-critical care unit and post care for both adult and pediatric patients suffering from brain tumors, brain injury, cerebrospinal fluid pressure complications and other neurological conditions. We offer leading technologies in dural repair, ultrasonic tissue ablation, intracranial pressure ("ICP") monitoring, hydrocephalus management, and cranial stabilization systems, while providing a rich research and development pipeline for growth.
Rounding out the portfolio is a catalog of surgical headlamps, surgical instrumentation, as well as after-market service. With thousands of surgical instrument products, including specialty surgical instruments, we call on the central sterile processing unit of hospitals and acute care surgical centers. Additionally, through a strong U.S. distribution model, we can serve the needs of hundreds of medical offices.
Our global commercial network includes clinical specialists, a large direct global sales force and strategic partnerships and distributors that serve hospitals, integrated health networks, group purchasing organizations, clinicians, surgery centers and health care providers. Outside the U.S., we have a combination of direct and indirect sales channels in international markets to sell certain product lines.
Tissue Technologies
Our TT segment focuses on three main areas: complex wound surgery, surgical reconstruction, and peripheral nerve repair and consists of five unique regenerative technology areas - highly engineered bovine collagen, bovine dermis, porcine urinary bladder, human amniotic tissue, and resorbable synthetic mesh. This broad regenerative platform, which includes multiple leading brands such as Integra® Dermal Matrices, AmnioExcel®, SurgiMend®, MicroMatrix® and NeuraGen®, primarily addresses the needs of plastic, reconstructive and general surgeons focused on the treatment of acute wounds, such as burns, chronic wounds, including diabetic foot ulcers, and surgical tissue repair, such as hernia, tendon, peripheral nerve repair and protection. Following our acquisition of SIA in 2022, we have also sought to expand our IBBR product offerings and in June 2023 we completed enrollment in the DuraSorb U.S. IDE clinical study for two-stage breast reconstruction.
We have a specialized sales organization composed of directly employed sales representatives, as well as specialty distributors, organized based upon their call point. Our wound reconstruction sales representatives call on surgeons doing procedures in limb salvage, trauma, wound reconstruction and burns, and chronic wounds primarily in the inpatient wound care clinic setting. We
also have a dedicated surgical reconstruction sales team focused on plastic and reconstructive surgery and hernia procedures with differentiated products. Finally, we have a distributor network focused on biologics. Outside the U.S., we have a combination of direct and indirect sales channels in international markets to sell certain product lines.
This business segment also includes private-label sales of a broad set of our regenerative and wound care technologies. Our customers are other medical technology companies that sell to end markets primarily in spine, surgical and wound care.
COMPETITION
The healthcare industry is highly competitive and characterized by continual change and improvements in technology. This is particularly the case in the market segments in which we operate. A number of companies have developed or are expected to develop products that compete or will compete with our products. Many of these competitors offer a broader product portfolio and have greater brand recognition than we do, which may make these competitors more attractive to hospitals, group purchasing organizations, laboratories, physicians and other potential customers. Competitors may develop superior products or products of similar quality for sale at the same or lower prices. Moreover, our products could be rendered obsolete by changes to industry standards or guidelines or advances in technology. We can give no assurance that we will be able to compete successfully with existing or new competitors
Our competitors for CSS include divisions within Medtronic, Inc., Stryker Corporation, Steris PLC, and B. Braun Medical, Inc. In addition, we compete with many smaller specialized companies and larger companies that do not otherwise focus on the offerings of Codman Specialty Surgical technologies. We rely on the depth and breadth of our sales and marketing organization, our innovative technologies, and our procurement and manufacturing operations to maintain our competitive position.
Our competitors for TT include Smith & Nephew plc, Organogenesis Holdings Inc., MiMedx Group, Inc., Allergan PLC, Becton Dickinson and Company, and Axogen, Inc. We compete with additional companies who partially participate in soft tissue reconstruction of complex wounds, peripheral nerve repair and surgical reconstruction. In addition, our products also compete against medical practices that treat a condition without using a medical device or any particular product, such as medical practices that utilize autograft tissue instead of our dermal regeneration products, duraplasty products and nerve repair products. Depending on the product line, we compete based on our products' features, strength of our sales force or distributors, sophistication of our technology and cost effectiveness of our solution.
We believe that the success of our products depends on our ability to differentiate ourselves and to demonstrate that our products deliver the clinical and operational attributes that are most important and cost-effective to customers. These attributes include, but are not limited to, superiority in efficacy, ease of use, reliability, accuracy, quality and cost. We believe our continued success depends in large part upon our ability to invest in product enhancements and technologies that will help us distinguish our product portfolio from our competitors.
RESEARCH AND DEVELOPMENT STRATEGY
Our research and development activities focus on identifying unmet surgical needs and addressing those needs with innovative solutions and products. The markets in which we participate are characterized by rapid technological change, frequent product introductions and evolving customer requirements. Investment in research and development is critical to driving our future growth. Our research and development efforts are focused on the further development and improvement of our existing products, the design and development of new innovative medical technologies and regulatory compliance across all our business segments. We apply our core competency in regenerative technology to innovate products for neurosurgical, wound applications, plastic surgery, and reconstructive surgery and we have extensive R&D development programs for our core platforms of electromechanical technologies. Additionally, we conduct products and clinical studies to generate efficacy and health economic evidence.
Regenerative Technologies.We were the first company to receive an FDA claim for regeneration of dermal tissue and are a world leader in regenerative technology. Our regenerative technology development program applies our expertise in bioengineering to a range of biomaterials including natural materials such as purified collagen, intact human or animal tissues, honey as well as resorbable synthetic polymers with our DuraSorband DuraSealproduct lines. These unique product designs are used for neurosurgical and reconstructive surgical applications, as well as dermal regeneration, including the healing of chronic and acute wounds, tendon and nerve repair. Our regenerative technology platform includes our legacy Integra® Dermal Regeneration Template ("IDRT") products and complementary technologies that we have acquired. Our collagen manufacturing capability, combined with our history of innovation, including our launch of NeuraGen 3D, provides us with strong platform technologies for multiple indications.
In the second quarter of 2023, after consultation with the FDA, The Company initiated a voluntary global recall of all products manufactured at the Boston facility, including Primatrix®, Surgimend®, Revize™, and TissueMend™, distributed between March 1, 2018 and May 22, 2023. See Item 1A. Risk Factors, under the heading Risks Related to our Regulatory Environment and under Item 7. General Management's Discussion and Analysis of Financial Condition and Results of Operations - FDA Matters of this Annual Report on Form 10-K for further discussion.
In the third quarter of 2021, we had no unresolved commentsfiled a PMA application for a specific indication for Surgimend® in the use of post-mastectomy breast reconstruction. In 2022, we acquired SIA, which is also PMA for DuraSorb with IBBR, and in June 2023 we completed enrollment in the DuraSorb U.S. IDE clinical study for two-stage breast reconstruction; the primary follow-up period is one year after device implantation. By offering two distinct product solutions, we believe we have the opportunity to build a leading position in the IBBR market. We hope to obtain FDA approvals in 2025.
Additionally, in 2022 we launched NeuraGen 3D Nerve Guide Matrix, a resorbable implant for repair of peripheral nerve discontinuities and engineered to create an optimized environment for nerve regeneration. Following the completion of design control activities in 2022, we launched both Cytal and MicroMatrix in Europe in 2023. In 2023, the Company received 510(k) clearance from the staffFDA for MicroMatrix® Flex.
Electromechanical Technologies and Instrumentation. The CSS business consists of a broad portfolio of market-leading brands, such as Codman®, DuraGen®, DuraSeal®, CUSA®, Mayfield®,Bactiseal®, and Certas® Plus,which are used for the management of multiple disease states, including brain tumors, traumatic brain injury, hydrocephalus and other neurological conditions. The growth in this business in recent years has been fueled by geographic expansion and new product registrations in markets, such as China, Japan, and Europe, which we expect to continue in the near-to-long term. Because our electromechanical products and instruments address significant needs in surgical procedures and limit uncertainty for surgeons, we continue to invest in registrations, clearances, and approvals for new indications and next generation improvements to our market-leading products. We have several active programs focused on life cycle management and innovation for capital and disposable products in our portfolio. Our product development efforts are focused on core clinical applications in cerebrospinal fluid ("CSF") management, neuro-critical care monitoring, minimally invasive instruments and electrosurgery and ultrasonic medical technologies, as well as our ambition to transform the standard of care in neurosurgery with product advancements in minimally invasive surgery ("MIS") and the surgical management of intracerebral hemorrhage ("ICH"). Our lighting franchise is among the most dynamic in the industry.
We are focused on the development of core clinical applications in our electromechanical technologies portfolio. We continue to update our CUSA Clarity platform by incorporating new ultrasonic handpiece and integrated electrosurgical capabilities. In 2022, we made progress to several enhancements to our CUSA Clarity Tissue Ablation System. The extended laparoscopic tip was launched in the U.S. to enhance laparoscopic liver procedures. In addition, a single-sided bone tip received 510(k) clearance from the FDA. Commercial launch was completed successfully in early 2023. In August 2023, we launched a modified 23 kHz CUSA Electrosurgery Module (CEM) for Clarity handpieces that can be used with additional electrosurgery generators. We continue to work with several instrument partners to bring new surgical instrument platforms to the market.
Throughout 2023 we also continued to advance the early-stage technology platforms we acquired in 2019. Through the acquisition of Arkis Biosciences, Inc. ("Arkis") we added a platform technology, CerebroFlo® external ventricular drainage ("EVD"), catheter with Endexo® technology, a permanent additive designed to reduce the potential for catheter obstruction due to thrombus formation. The CerebroFlo EVD catheter has demonstrated an average of 99% less thrombus accumulation onto its surface, in vitro, compared to a market leading EVD catheter. Our work to combine our Bactiseal® antimicrobial technology with the Endexo anti-occlusive technology continues to progress for both a silicone-based hydrocephalus and EVD project.
Throughout 2023, we continued to advance our innovation from the Rebound Therapeutics Corporation ("Rebound Therapeutics"), which was acquired in 2019. Rebound Therapeutics specializes in a single-use medical device, known as the Aurora Surgiscope, which is the only tubular retractor system designed for cranial surgery with an integrated access channel, camera and lighting. The 9mm Surgiscope received 510(k) clearance from the FDA in the fourth quarter of 2023.
In the third quarter of 2021, we launched our CereLink ICP Monitor System in the U.S. and Europe and continued the global rollout in the first half of 2022. On August 18, 2022, the Company, after consultation with the FDA and other regulatory authorities outside of the SecuritiesUnited States, initiated an immediate voluntary global product removal of all CereLink® intracranial pressure monitors. We believe that the out-of-range readings are principally caused by a combined interaction of electrical noise (originating from sources such as electrical components in the device, other devices set up near the CereLink Monitor, and Exchange Commissionthe hospital power grid) and an electrical potential difference between the patient and monitor. We submitted a traditional 510(k) submission to the FDA on September 15, 2023 as a result of customer reports about monitors whose pressure readings were out of range. We have received 510(k) clearance from the FDA on February 4th, 2024. We plan to resume shipments of CereLink monitors in the U.S. in the first quarter of 2024. Shipments resumed in international markets with a limited release in the third quarter of 2023. See Item 1A. Risk Factors, under the heading Risks Related to our Regulatory Environment and under Item 7. General Management's Discussion and Analysis of Financial Condition and Results of Operations - FDA Matters of this Annual Report on Form 10-K for further discussion.
MANUFACTURE AND AVAILABILITY OF RAW MATERIALS
We manufacture products at manufacturing facilities located in various countries throughout the world. We purchase many of the components and raw materials used in manufacturing our products from numerous suppliers in various countries.
In general, raw materials essential to our businesses are readily available from multiple sources. For reasons of quality assurance, availability, or cost effectiveness, certain components and raw materials are available only from one or a limited number of suppliers. We have established long-term supply contracts with many of our suppliers and our practice is to maintain sufficient inventory of components so that were receivedour production will not be significantly disrupted even if a particular component or material is not available for a period of time. Due to the high standards and FDA requirements applicable to manufacturing our products, such as the FDA's Quality System Regulation and Good Manufacturing Practices, we may not be able to quickly establish additional or replacement sources for certain components or materials. Some of our manufacturing operations are located outside of the U.S., including in Puerto Rico, Switzerland, Ireland and France. Those manufacturing operations are also subject to additional challenges and risks associated with international operations described under the caption “Risk Factors” set forth in Part I, Item 1A of this Annual Report on Form 10-K. In the event we are unable to obtain sufficient quantities of raw materials or components on commercially reasonable terms or in a timely manner, our ability to manufacture our products on a timely and cost-competitive basis may be compromised, which may have a material adverse effect on our business, financial condition and results of operations.
Certain of our products, including but not limited to our dermal regeneration products, duraplasty products, wound care products, and nerve and tendon repair products, contain material derived from bovine tissue. We take great care to provide products that are safe and free of agents that can cause disease. In particular, the collagen used in the products that we manufacture is derived from the deep flexor tendon of cattle less than 180 days before24-months old from New Zealand, a country that has never had a reported case of bovine spongiform encephalopathy ("BSE") (otherwise known as mad cow disease), from the endU.S. or from fetal bovine dermis. The World Health Organization classifies different types of cattle tissue for relative risk of BSE transmission. Deep flexor tendon and fetal bovine skin are in the lowest-risk category for BSE transmission, and therefore considered to have a negligible risk of containing the agent that causes BSE.
INTELLECTUAL PROPERTY
We seek patent and trademark protection for our 2021 fiscal year.key technology, products and product improvements, both in the U.S. and in selected foreign countries. When determined appropriate, we have enforced and plan to continue to enforce and defend our patent and trademark rights. In general, however, we do not rely solely on our patent and trademark estate to provide us with any significant competitive advantages as it relates to our existing product lines. We also rely upon trade secrets and continuing technological innovations to develop and maintain our competitive position. In an effort to protect our trade secrets, we have a policy requiring our employees, consultants and advisors to execute proprietary information and invention assignment agreements upon commencement of employment or consulting relationships with us. These agreements also provide that all confidential information developed or made known to the individual during the course of their relationship with us must be kept confidential, except in specified circumstances.
AccuDrain®, AmnioExcel®, Aquasonic®, Auragen®, Aurora® Surgiscope®, Bactiseal®, BioDFence®, BioDOptix®, Brainet®, Budde®, Buzz™, CereLink®, CerebroFlo® EVD Catheter with Endexo® Technology, Codman®, Codman Accu-Flo®, Codman Bicol®, Codman® Certas® Plus, Codman® Hakim®Programmable valve, Codman Holter®, Codman ICP Express®, Codman Microsensor®, Codman VersaTru®, Codman VPV®, Contour-Flex®, Cranioplastic®, CRW®, CRW Precision™, Ctherm™, CUSA®, Cytal®, DirectLink®, DuraGen®, DuraSeal®, DuraSorb®, Gentrix®, HeliCote®, HeliPlug®, HeliTape®, HeliMend®, Helistat®, Helitene®, Hermetic™, Hy-Tape®, Integra®, IntegraLink®, Isocool®, Jarit®, Lead-Lok™, Licox®, LimiTorr™,
ITEM 1A. LuxtecRISK FACTORS®, Mayfield®, MatriStem UBM™, MediHoney®, MicroFrance®, MicroMatrix®, Miltex®, Mischler™, MoniTorr ICP™, Natus®, NeuraGen®, NeuraWrap™, Nicolet®, Omnigraft®, Omni-Tract®, OSV II®, Padgett®, PriMatrix®, Pureflow™, Q-Snor™, Redmond™, Revize™, Ruggles®, Signacreme®, SurgiMend®, TCC-EZ®, TenoGlide®, TissueMend®, Ultra VS™, VersaTru®, Xtrasorb®, zRIP™, and the Integra logo are some of the material trademarks of Integra LifeSciences Corporation and its subsidiaries. MAYFIELD® is a registered trademark of SM USA, Inc., and is used by Integra under license.
RISKS RELATED TO COVID-19SEASONALITY
Revenues during our fourth quarter tend to be stronger than other quarters because many hospitals increase their purchases of our products during the fourth quarter to coincide with the end of their budget cycles in the U.S. In general, our first quarter usually has lower revenues than the preceding fourth quarter, the second and third quarters have higher revenues than the first quarter, and the fourth quarter revenues are the highest in the year. The main exceptions to this pattern occur because of material acquisitions.
GOVERNMENT REGULATION AND COMPLIANCE
We are a manufacturer and marketer of medical devices and Human Tissue and Cell Based Products ("HCT/Ps") and therefore are subject to extensive regulation by the FDA, the Center for Medicare Services of the U.S. Department of Health and Human Services ("HHS"), other federal governmental agencies and, in some jurisdictions, by state and foreign governmental authorities. These regulations govern the introduction of new medical devices and HCT/Ps, the observance of certain standards with respect to the design, manufacture, testing, labeling, promotion and sales of the products, the maintenance of certain records, the ability to track devices, the reporting of potential product defects, the import and export of products, and other matters. FDA product approvals may be withdrawn or suspended if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
Our business is also affected by patient and data privacy laws and government payer cost containment initiatives, as well as environmental health and safety laws and regulations.
United States Food and Drug Administration
Our products are subject to extensive regulation particularly as to safety, efficacy and adherence to FDA Quality System Regulation, and related manufacturing standards. Medical device products are subject to rigorous FDA and other governmental agency regulations in the United States and similar regulations of foreign agencies abroad. The FDA regulates the design, development, research, preclinical and clinical testing, introduction, manufacture, advertising, labeling, packaging, marketing, distribution, import and export, and record keeping for such products, in order to ensure that medical products distributed in the United States are safe and effective for their intended use. In addition, the FDA is authorized to establish special controls to provide reasonable assurance of the safety and effectiveness of most devices. Non-compliance with applicable requirements can result in import detentions, fines, civil and administrative penalties, injunctions, suspensions or losses of regulatory approvals, recall or seizure of products, operating restrictions, refusal of the government to approve product export applications or allow us to enter into supply contracts, and criminal prosecution. The regulatory process for obtaining product approvals and clearances can be onerous and costly.
Under the Federal Food, Drug and Cosmetic Act (the "FD&C Act"), authorization to commercially distribute a new medical device in the U.S. is generally obtained in one of two primary ways. The first, known as pre-market notification or the 510(k) process, requires us to demonstrate that our medical device is substantially equivalent to a legally marketed medical device. A 510(k) pre-market notification filing must contain information establishing that the device to be sold is substantially equivalent to a device commercially distributed prior to May 28, 1976 or to a device that has been determined by the FDA to be substantially equivalent. The second, more rigorous process, known as pre-market approval (“PMA”) requires us to independently demonstrate that a medical device is safe and effective for its intended use. This process is generally much more time-consuming and expensive than the 510(k) process. The PMA process involves a complex and lengthy testing process that is subject to review by the FDA and may require several years to obtain. We may need to first obtain an investigational device exemption (for significant risk devices), known as an IDE, in order to conduct extensive clinical testing of the device to obtain the necessary clinical data for submission to the FDA. The FDA will approve a PMA only if after evaluating the supporting technical data it finds that the PMA contains sufficient, valid scientific evidence to assure that the device is safe and effective for its intended use(s). This approval may be granted with post-approval requirements including inspection of manufacturing facilities and/or additional patient follow-up for an indefinite period of time.
The effectsFDA also may require a post-approval clinical study as a condition of approval. To perform clinical trials for significant risk devices in the U.S. on an unapproved product, we are required to obtain an IDE from the FDA. The FDA also may require a filing for approval prior to marketing products that are modifications of existing products or new indications for existing products. Moreover, after clearance/approval is given, if the product is shown to be hazardous or defective, the FDA and foreign regulatory agencies have the power to withdraw the clearance or approval, as the case may be, or require us to change the device, its manufacturing process or its labeling, to supply additional proof of its safety and effectiveness or to recall, repair,
replace or refund the cost of the COVID-19 pandemicmedical device. Because we currently export medical devices manufactured in the U.S. that have not been approved by the FDA for distribution in the U.S., we are required to obtain approval/registration in the country to which we are exporting and maintain certain records relating to exports and make these available to the FDA for inspection, if required.
Human Cells, Tissues and Cellular and Tissue-Based Products
Integra, through its wholly-owned subsidiary BioD LLC (“BioD”), is involved with the recovery, processing, storage, transportation and distribution of donated amniotic tissue. The FDA has specific regulations governing HCT/Ps. An HCT/P is a product containing, or consisting of, human cells or tissue intended for transplantation into a human patient. Examples of HCTPs include bone, ligament, skin and cornea.
Some HCT/Ps fall within the definition of a biological product, medical device or drug regulated under the FD&C Act. These biologic, device or drug HCT/Ps must comply both with the requirements exclusively applicable to HCT/Ps and, in addition, with requirements applicable to biologics, devices or drugs, including premarket clearance or approval from the FDA.
Section 361 of the Public Health Service Act (“Section 361”) authorizes the FDA to issue regulations to prevent the introduction, transmission or spread of communicable disease. HCT/Ps regulated as “361” HCT/Ps are subject to requirements relating to registering facilities and listing products with the FDA, screening and testing for tissue donor eligibility, and Good Tissue Practices when processing, storing, labeling, and distributing HCT/Ps, including required labeling information, stringent record keeping, and adverse event reporting.
The American Association of Tissue Banks (“AATB”) has issued operating standards for tissue banking. Compliance with these standards is a requirement in order to become an AATB-accredited tissue establishment. In addition, some states have their own tissue banking regulations. We are licensed or have permits for tissue banking in California, Delaware, Illinois, Maryland, New York, Oregon, and Tennessee. In Tennessee, we are registered with the FDA Center for Biological Evaluations and Research.
Procurement of certain human organs and tissue for transplantation is subject to the restrictions of the National Organ Transplant Act, which prohibits the transfer of certain human organs, including skin and related tissue for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. BioD is a registered Tissue Bank and is involved with the recovery, storage and transportation of donated human amniotic tissue.
Medical Device Regulations
The FDA requires that a manufacturer obtain 510(k) clearance or a PMA for a variety of reasons, such as introducing a new medical device or new indication for use of an existing medical device, before introducing it into the U.S. market. The type of marketing authorization is generally linked to the classification of the device. The FDA classifies medical devices into three classes based on risk. Regulatory control increases from Class I (lowest risk) to Class III (highest risk). The FDA generally must clear or approve the commercial sale of new medical devices in Classes II and III. Commercial sales of our Class II medical devices (except for Class II exempt devices) and Class III medical devices within the U.S. must be preceded by either a pre-market notification filing pursuant to Section 510(k) of the FD&C Act (Class II) or the granting of a pre-market approval, or PMA (Class III).
The process of obtaining a Section 510(k) clearance generally requires the submission of performance data and may require clinical data, which in some cases can be extensive, to demonstrate that the device is “substantially equivalent” to another device that is currently marketed under a 510(k); a device that is referred to as “predicate device.” As a result, FDA clearance requirements may extend the development process for a considerable length of time. In the case of a PMA, the FDA may require additional review by an advisory panel, which can further lengthen the process. The PMA process, which is reserved for new devices that are not substantially equivalent to any predicate device and for high-risk devices (i.e., Class III devices) that are used to support or sustain human life or which present a potential, unreasonable risk of illness or injury, may take several years and requires the submission of extensive performance and clinical information.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device has received 510(k) clearance for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, may require a new 510(k) clearance or PMA approval and payment of an FDA user fee. The determination as to whether or not a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance; however, the FDA may review this determination to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties.
We also are required to register with the FDA as a medical device manufacturer and any devices we manufacture and distribute pursuant to clearance or approval by the FDA are subject to pervasive and continuing regulation by the FDA and certain state agencies. These include product listing and establishment registration requirements, which help facilitate FDA inspections and other regulatory actions, and our manufacturing sites are subject to periodic inspection by the FDA for compliance with the FDA's Quality System Regulations. These regulations require that we manufacture our products and maintain our documents in a prescribed manner with respect to design, manufacturing, testing and control activities. Further, we are required to comply with various FDA requirements and other legal requirements for labeling and promotion. FDA regulates unclassified devices via the 510(k) process and has the authority to classify these devices and/or require Special Controls, additional testing and submission of a new 510(k) as part of the classification process for unclassified devices that are currently on the market as 510(k) cleared products. If the FDA believes that a company is not in compliance with applicable regulations, it may issue a warning letter, institute proceedings to detain or seize products, issue a recall order, impose operating restrictions, enjoin future violations and assess civil penalties against that company, its officers or its employees and may recommend criminal prosecution to the U.S. Department of Justice. The majority of Integra manufacturing facilities participate in the Medical Device Single Audit Program and are audited annually for compliance with the Quality System for US FDA, Canada, Australia, Brazil, and Japan.
Medical device regulations also are in effect in many of the countries in which we do business outside the U.S. In the European Economic Area ("EEA"), which is comprised of the 27 member states of the European Union (the "EU") plus Norway, Iceland and Liechtenstein, medical devices need to comply with specific requirements. These requirements were previously known as "Essential Requirements" under the former EU Medical Devices Directive (Council Directive 93/42/EEC, or MDD) and are now defined "General Safety and Performance Requirements (GSPR)" under the new EU Medical Devices Regulation (Regulation (EU) 2017/745, or "EU MDR"). Although the requirements set forth in the EU MDR are generally consistent with those laid out in the MDD (with a few exceptions), the EU MDR is intended, among other things, to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation. These laws range from comprehensive medical device approval and Quality System requirements for some or all of our medical device products to simpler requests for product data or certifications. Under the MDD, medical devices must meet the MDD standards and receive CE Mark Certification prior to marketing in the EEA. Although we continue to significantly impact global economic conditionstransition our certification profile to meet the new EU MDR requirements, these stricter regulations set forth in the EU MDR may pose additional challenges for Integra to continue marketing products in the EU as these regulations come into force. See “Item 1A. Risk Factors - We are subject to stringent domestic and have affected,foreign medical device regulations and oversight and any adverse action may continue toadversely affect our operations, supply chain, distribution, sales force,ability to compete in the marketplace and our financial condition and business operations” of this Annual Report on Form 10-K.
CE Mark Certification requires a comprehensive quality system program, technical documentation, clinical evaluation and data on the product which are then reviewed, by a Notified Body. A Notified Body is an organization designated by the national governments of the EU member states to make independent judgments about whether a product complies with the requirements established by each CE marking directive. The MDD, MDR, ISO 9000 series and ISO 13485 are recognized international quality standards that are designed to ensure that we develop and manufacture quality medical devices. Other countries are also instituting regulations regarding medical devices or interpreting and enforcing existing regulations more strictly. Compliance with these regulations requires extensive documentation and clinical reports for our products, revisions to labeling, and other requirements such as facility inspections to comply with the registration requirements. A recognized Notified Body audits our facilities annually to verify our compliance with the ISO 13485 Quality System standard.
Certain countries, as well as the financial stabilityEU, have issued regulations that govern products that contain materials derived from animal sources. Regulatory authorities are particularly concerned with materials infected with the agent that causes BSE. These regulations affect our dermal regeneration products, duraplasty products, hernia repair products, biomaterial products for the spine, nerve and tendon repair products and certain other products, all of hospitalswhich contain material derived from bovine tissue. Although we take great care to provide that our products are safe and free of agents that can cause disease, products that contain materials derived from animals, including our products, may become subject to additional regulation, or even be banned in certain countries, because of concern over the potential for prion transmission. Significant new regulations, a ban of our products, or a movement away from bovine-derived products because of an outbreak of BSE could have a material, adverse effect on our current business or our ability to expand our business. See “Item 1A. Risk Factors – Risks Related to our Regulatory Environment" of this Annual Report on Form 10-K.
Postmarket Requirements.After a device is cleared or approved for commercial distribution, numerous regulatory requirements apply. These include the FDA Quality System Regulations which cover the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of medical devices; the FDA's general prohibition against promoting products for unapproved or 'off-label' uses; the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur; and the Reports of Corrections and Removals regulation, which require manufacturers to report recalls and field corrective actions
to the FDA if initiated to reduce a risk to health posed by the device or to remedy a violation of the FD&C Act. Postmarket requirements are also followed globally where our products are registered and approved. These foreign jurisdictions have similar requirements to the FDA which include reporting requirements such as adverse events and recalls.
Regulations Governing Reimbursement
Market acceptance of our medical products in the U.S. and other countries is dependent upon the purchasing and procurement practices of our customers and have causedpatient need for our products and could again cause a reduction in procedures which could materially adversely affectand, the coverage and reimbursement of patients’ medical expenses by government healthcare programs, private insurers or other healthcare payors. The delivery of our business, resultsdevices is subject to regulation by the HHS and comparable state and non-U.S. agencies responsible for reimbursement and regulation of operations, financial condition,healthcare items and stock price.
On March 11, 2020, the World Health Organization characterized the Novel Coronavirus Disease 2019 (“COVID-19”) as a pandemic.services. The COVID-19 pandemic continues to have widespread and unpredictable impactsHealthcare providers that purchase medical devices generally rely on global society, economies, financial markets, and business practices and negatively impact business and healthcare activity globally. To date, and in continuing efforts to control the spread of COVID-19 (including subsequent surges and variants), governments around the world,third-party payors, including, in the U.S., havethe Medicare and continueMedicaid programs and private payors, such as indemnity insurers, employer group health insurance programs and managed care plans, to implement various preventative measures including quarantines, “shelter in place” orders, “stay at home” orders, travel restrictions, business operation restrictions, school closures, and other similar types of measures. Even as efforts to contain the pandemic have made progress, new variantsreimburse all or part of the virus are causing additional surges or outbreaks. The COVID-19 pandemic has impacted and may continue to impact our business operations, including our employees, customers, suppliers, distributors, other service providers and communities in which we operate, and there is substantial uncertainty in the nature and degree of its continued effects over time.
In response to the COVID-19 pandemic and related mitigation efforts, similar to many other employers in the U.S., the Company has and continues to encourage many employees to work remotely. The Company has continued to operate certain manufacturing facilities to date in compliance with federal, state and local orders regarding COVID-19. The healthcost of the Company’s workforce is our top concern and the Company has procured equipment and implemented safety protocols in an effort to maintain the health and safety of our employees.A number of our network of business partners have been adversely affected by the COVID-19 pandemic. These impacts could impair our ability to move our products through distribution channels to end customers, and any such delay or shortage in the supply of materials or products mayproducts. As a result, in our inability to satisfy consumer demand for certain of our products in a timely manner or at all, which could harm our reputation, future sales and profitability.
It is not possible to predict with precision how future demand for our products is and will continue to be impacted bydependent in part on the COVID-19 pandemic ascoverage and reimbursement policies of these payors. The manner in which reimbursement is sought and obtained varies based upon the scope and durationtype of the pandemic and its impact on our businesspayor involved and the marketssetting in which we operate remain unpredictable. The Company has implemented extensive business contingency plans across its global organizationthe product is furnished and networkutilized. Reimbursement from Medicare, Medicaid and other third-party payors may be subject to periodic adjustments as a result of third parties throughlegislative, regulatory and policy changes, as well as budgetary pressures. Possible reductions in, or eliminations of, coverage or reimbursement by third-party payors, or denial of, or provision of uneconomical reimbursement for new products may affect our customers' revenue and ability to purchase our products. Any changes in the healthcare regulatory, payment or enforcement landscape relative to our customers' healthcare services have the potential to significantly affect our operations and revenue.
Implementation of legislative or regulatory reforms to reimbursement systems, including price regulation, competitive bidding and tendering, coverage and payment policies, comparative effectiveness of therapies, technology assessments and managed-care arrangements, or adverse decisions relating to our products by administrators of these systems in coverage or reimbursement, could significantly reduce reimbursement or result in the denial of coverage, which the Company conducts its business which helps limit some of the impact of the COVID-19 pandemic but does not completely prevent or avoid a negativecould have an impact on the business. In addition, COVID-19 has impactedacceptance of and may further impact the global economy and capital markets, including by negatively impacting access to capital markets, foreign currency exchange rates, and interest rates, each of which may adversely impact our business and liquidity.
The extent to which the COVID-19 pandemic will negatively affect the Company's operations and financial position will depend on future developments that remain uncertain and cannot be predicted with precision. For example, including, without limitation, the pandemic could cause:
•Continued fluctuations in our operational results, revenues, and cash flows which may negatively impact our stock price;
•Impact on our operations and sales including but not limited to delays in orders, ability to market, sell, deliver and service our products;
•Reductions in demand for our products and services duethe prices that our customers are willing to pay for them.
Other regulations
Anti-Bribery Laws. In the impact of COVID-19 onU.S., we are subject to laws and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws and physician self-referral laws that regulate the means by which companies in the health care industry may market their products to hospitals and customers suchhealth care professionals and may compete by discounting the prices of their products. Similar anti-bribery laws exist in many of the countries in which we sell our products outside the U.S., as continued or future postponement or cancellationswell as the United States Foreign Corrupt Practices Act (the “FCPA”) which addresses the activities of procedures, hospital postponement or cancellationU.S. companies in foreign markets. Our products also are subject to regulation regarding reimbursement, and U.S. healthcare laws apply when a customer submits a claim for a product that is reimbursed under a federally funded healthcare program. These global laws require that we exercise care in designing our sales and marketing practices, including interactions with healthcare professionals, and customer discount arrangements. See “Item 1A. Risk Factors – We are exposed to a variety of capital purchases, or eliminationrisks relating to our international sales and operations” of services;this Annual Report on Form 10-K for further details.
•Import-exportDisruption. Our international operations subject us to manufacturing operationslaws regarding sanctioned countries, entities and persons, customs, and import-export. Among other things, these laws restrict, and in some cases can prevent, U.S. companies from directly or indirectly selling goods, technology or services to people or entities in certain countries. In addition, these laws require that we exercise care in our business dealings with entities in and from foreign countries. In addition to our need to comply with such regulations in connection with our direct activities, we also sell and provide goods, technology and services to agents, representatives and distributors who may export such items to customers and end-users. If we, or the third parties through which we do business, are not in compliance with applicable import, export control or economic sanctions laws and regulations, we may be subject to civil or criminal enforcement action, and varying degrees of liability. Such actions may disrupt or delay sales of our products or services or result in restrictions on our distribution supply chains;and sales of products or services that may materially impact our business
•Environmental Health and SafetyIncreased challenges or restraints in obtaining necessary products or components from our suppliers. Our research, development and vendors;
•Reduction or interruption to our manufacturing processes involve the controlled use of certain hazardous materials. We are subject to country-specific, federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials and certain waste products. We believe that our environmental, health and safety procedures for handling and disposing of these materials comply with the standards prescribed by the controlling laws and regulations. However, risk of accidental releases or injury from these materials is possible. These risks are managed to minimize or eliminate associated business impacts. In the event of this type of accident, we could be held liable for damages and face a liability that could exceed our resources. We could be subject to a regulatory shutdown of a facility that could prevent the distribution and sale of products manufactured there for a significant period of time, and we could suffer a casualty loss that could require a shutdown of the facility in order to repair it, any of which could have a material, adverse effect on our business;business. Although we continuously strive to maintain full compliance with respect to all applicable global
environmental, health and safety laws and regulations, we could incur substantial costs to fully comply with future laws and regulations, and our operations, business or assets may be negatively affected. Furthermore, global economic instabilityenvironmental, health and inflationsafety compliance is an ongoing process. We have compliance procedures in place for compliance with Employee Health & Safety laws, driven by a centrally led organizational structure that ensures proper implementation, which is essential to our overall business objectives.
In addition, we are subject to numerous federal, state, foreign and recessions, whichlocal laws relating to safe working conditions, environmental protection and fire hazard control, among others. We may be required to incur significant costs to comply with these laws and regulations in the future and complying with these laws may result in hospitals and customers reducing capital spending and could materially affecta material adverse effect upon our business, financial condition and results of operations.
Data Privacy and Cybersecurity Laws and Regulations. As a business with a significant global footprint, compliance with evolving regulations and standards in data privacy and cybersecurity (relating to the confidentiality and security of our information technology systems, products such as medical devices, and other services provided by us) may result in increased costs, lower revenue, new complexities in compliance, new challenges for competition, and the threat of increased regulatory enforcement activity. Our business relies on the secure electronic transmission, storage and hosting of sensitive information, including personal information, financial information, intellectual property, and other sensitive information related to our customers and workforce.
For example, in the U.S., the collection, maintenance, protection, use, transmission, disclosure and disposal of certain personal information and the security of medical devices are regulated at the U.S. federal and state, and industry levels. U.S. federal and state laws protect the confidentiality of certain patient health information, including patient medical records, and restrict the use and disclosure of patient health information by health care providers. For example, in the U.S. we are obligated to comply with the requirements of the Health Insurance and Portability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (collectively, “HIPPA”). Under HIPAA, the HHS has issued regulations, including the HIPAA Privacy, Security and Breach Notification Rules, to protect the privacy and security of protected health information used or disclosed by covered entities including health care providers and their business associates, as well as covered subcontractors. HIPAA also regulates standardization of data content, codes and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include significant civil and criminal penalties for each violation. In addition, the FDA has issued guidance advising manufacturers to take cybersecurity risks into account in product design for connected medical devices and systems, to assure that appropriate safeguards are in place to reduce the risk of unauthorized access or modification to medical devices that contain software and reduce the risk of introducing threats into hospital systems that are connected to such devices. The FDA also issued guidance on post market management of cyber security in medical devices.
Outside the U.S., we are impacted by the privacy and data security requirements at the international, national and regional level, and on an industry specific basis. Legal requirements in these countries relating to the collection, storage, handling and transfer of personal data and, potentially, intellectual property continue to evolve with increasingly strict enforcement regimes. In Europe, for example, we are subject to EU General Data Protection Regulation ("GDPR") which requires member states to impose minimum restrictions on the collection, use and transfer of personal data and includes, among other things, a requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances and significant fines for non-compliance. The GDPR also requires companies processing personal data of individuals residing in the EU to comply with EU privacy and data protection rules.
Please refer to “Item 1A. Risk Factors – Failure to comply with laws relating to the confidentiality of sensitive personal information or standards related to the transmission of electronic health data, may require us to make significant changes to our products, or incur penalties or other liabilities” of this Annual Report on Form 10-K for additional discussion of the risks accompanying compliance with data privacy and cybersecurity laws and regulations.
These laws and regulations impact the ways in which we use and manage personal data, protected health information, and our information technology systems. They also impact our ability to move, store, and access data across geographic boundaries. Compliance with these requirements may require changes in business practices, complicate our operations, and add complexity and additional management and oversight needs. They also may complicate our clinical research activities, as well as product offerings that involve transmission or use of clinical data.
HUMAN CAPITAL
Our people are our greatest asset and we view human capital management and the strength of our employees as integral to the long-term success of our business. We understand that we rely on our employees worldwide to propel our organization forward with great ideas, innovations and leadership.
Workforce Demographics
As of December 31, 2023, we had approximately 3,946 regular full and part time employees and 1383 contingent, subcontracted, and outsourced partners.
Approximately 70% of our employees are located in the United States, 21% in Europe, 2% in Latin America and Canada and 7% in Asia Pacific which includes Australia and New Zealand.
Diversity and Inclusion
A diverse workforce and an inclusive culture and work environment is a business priority and a key to our long-term success. We believe our company is stronger when we build diverse teams and leverage broad perspectives. Diverse teams meet the needs of our shareholders, customers, colleagues and communities we serve. Our commitment to diversity and inclusion starts at the top with our Board of Directors and Chief Executive Officer. At all levels of the Company, we focus on attracting, retaining, and developing our diverse talent. We have implemented initiatives to promote awareness of our corporate commitment to diversity and inclusion and employ trainings and other educational programs to inform and educate our workforce – forming communities of advocates and allies to help advance a culture of inclusion – develop inclusive leadership skills and identify and minimize the impact of unconscious bias. Through our Employee Resource Groups (ERGs), leadership councils and external partnerships, we provide opportunities for colleagues to create a welcoming culture, advance diversity and inclusion in the workplace and to provide feedback to our executive team. In fiscal year 2023, we expanded the number of Integra-sponsored ERGs to seven (7) as we believe our ERGs, which are employee-led groups, provide career and leadership development and networking opportunities for members and strengthen ties between employees of many different backgrounds, cultures, and interests.
Compensation and Benefits
Our compensation philosophy is designed to reinforce and align with our mission, business strategy, and financial needs. We invest in the physical, emotional and financial well-being of our employees through our robust compensation and benefit programs. We provide market-competitive compensation and benefits based on benchmarking surveys we conduct regularly for all position levels against relevant peer companies. Our annual and long-term incentive packages are linked directly to business and individual performance, with a balance of short- and long-term financial and strategic objectives. We have an employee stock purchase plan. Eligibility for non-salary benefits such as salary continuance, life insurance, health insurance, and similar benefits, follows local regulations and practices.
We are a pay-for-performance company committed to fair pay. All compensation decisions are made without regard to personal characteristics such as, but not limited to, our future access to capital, and negatively impact the valuegender, race, color, national or ethnic origin, age, disability, sexual orientation, gender identity or expression, genetic information, religion, or veteran status. As part of our stock;commitment to compensation equity, Integra regularly conducts a pay equity analysis, reviewing how our organization compensates employees against external and internal data in conjunction with the role and scope of each position and making adjustments if necessary.
•Talent Development and Retention
We have comprehensive and effective human capital development programs in place because we believe that the personal success of our employees is critical to the overall success of our business. To build a diverse and talented organization, we have invested in honing our recruiting and hiring processes to attract top talent and engage new hires from the very beginning of their experience at Integra.
We offer a variety of opportunities for our employees to learn and grow. Continued limitationslearning and development is a critical component of employee job satisfaction, retention, and career advancement—and ultimately, a driver of business success. We encourage and promote experiential, collaborative, and formal learning programs. Employees are also encouraged to discuss with their managers the skills, training, and experience needed to grow and develop. In addition to several skills-based trainings available (technical, sales, leadership ability) to all employees, managers may recommend external job-specific development programs to employees. These programs are paid for directly by Integra.
Employee Health and Safety:
We are committed to providing a safe environment for all employees and visitors. We rely on our operations due to restrictions associated with “shelter in place” orders and travel restrictions;
•Distraction of management time and focus;
•Increased risk that insurance coverage will not provide protection for all of the COVID-19-related disruption;
•Continued and/or increased risks related to theenvironmental, health and safety ofmanagement systems as well as entrusting our employees (and retention issues), volatility of foreign currency exchange rates,managers to oversee and risk of cybersecurity attacksensure health and breaches;
•Possible liquidity constraintssafety at their respective sites and credit impact;foster a workplace culture to achieve that end. We implement our approach globally by our systems and support at regional and country levels from colleagues that implement proper safety protocols, identify and correct hazards, and remain safety conscious at all times. Managers are expected to enforce health and safety regulations, including compliance with applicable federal, state and local laws. Our Environmental Health and Safety ("EH&S") organizational structure incorporates both
workplace EH&S coordinators and compliance teams. We have developed an Incident Procedure Policy and General Safety Rules that guide our colleagues to improve our workplace environment, improve safety, and reduce risk and costs.
Employee Engagement and Wellbeing
We regularly seek employee feedback and sentiment about our workplace through global engagement surveys conducted on at least a bi-annual basis. After each survey is complete, we share detailed results with senior management and all employees within each department. We are incorporating employee survey results into our corporate strategies – across company, division and function levels – and have further used this employee feedback to modify corporate programs and initiatives. We believe this process enables us to monitor employee engagement and create a continuously improving, satisfying work environment for our employees.
We are committed to improving the quality of life of our employees and their families. Our health and wellbeing programs differ by country and typical benefits include comprehensive health insurance, disability coverage, workplace accommodations, parental leave and other leaves of absence based on health or life events (e.g., bereavement), employee assistance programs, fitness reimbursement, and flu shots. We also provide on-demand health advocates to help employees navigate the health insurance system, access to digital health solutions, a weight management program, smoking cessation assistance, a substance use disorder helpline, a diabetes health program and other similar programs to drive healthy behaviors and awareness.
FINANCIAL INFORMATION ABOUT GEOGRAPHIC AREAS
•Financial information about our geographical areas is set forth in our financial statements Note 16, Delays in obtaining regulatory clearances, approvalSegment and Geographic Information, to market products, quality inspections, or delaysthe Notes to clinical trial activity;Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K).
AVAILABLE INFORMATION
•We are subject to the informational requirements of the Exchange Act of 1934. In accordance with the Exchange Act, we file annual reports on Form 10K, quarterly reports on Form 10Q, current reports on Form 8-K, and any amendments to those reports, proxy statements and other information with the Securities and Exchange Commission, ("the SEC"). Our financial information may be viewed, including the information contained in this report, and other reports we file with the SEC, on the Internet, without charge as soon as reasonably practicable after we file them with the SEC, in the “SEC Filings” page of the Investor Relations section of our website at Delaysinvestor.integralife.com. A copy may also be obtained for any of these reports, without charge, from our Investor Relations department, 1100 Campus Road, Princeton, NJ 08540. Alternatively, reports filed may be viewed or obtained through the SEC's website at www.sec.gov.
Investors and others should note that we announce material financial information to our investors using our investor relations website (investor.integralife.com), SEC filings, press releases, public conference calls and webcasts. We use these channels as well as social media to communicate with the public about our Company, our services and other issues. It is possible that the information we post on social media could be deemed to be material information. Therefore, we encourage investors, the media, and others interested in coverage decisionsour Company to review the information we post on the social media channels listed on our investor relations website. We have used, and intend to continue to use, our investor relations website as means of disclosing material non-public information and for complying with our disclosure obligations under Regulation FD. Additional corporate governance information, including our certificate of incorporation, bylaws, corporate governance guidelines, board committee charters, and global code of conduct, is also available on our investor relations website under the heading “Corporate Governance.” The contents of our websites are not intended to be incorporated by privatereference into this Annual Report on Form 10-K or in any other report or document we file with the SEC, and public health insurersany references to our website are intended to be inactive textual references only.
ITEM 1A. RISK FACTORS
GLOBAL CHALLENGES AND MACROECONOMIC CONDITIONS
The continuing worldwide macroeconomic and geopolitical uncertainty may adversely affect our business and prospects.
Geopolitical instability and other macroeconomic factors, including inflation, supply chain disruptions, interest rate and foreign governmental health systems;
•Delayscurrency rate fluctuations, and volatility in the completion of supportive clinical studies for payer coverage decisions or clinical and economic decision makers due to slowed study enrollments;
•Delays to acquisition plans, increased risks to the operations andcapital markets could negatively impact our business, financial condition, and results of newly acquired businesses,operations. Global economic disruptions have continued to impact the global supply chain, primarily through constraints on raw materials and electronic components. Additionally, we have observed a reduction in both inbound and outbound transportation capacity as a result of port closures, shipping lane disruptions and delays since the Coronavirus Disease ("COVID-19") pandemic, all of which is causing longer lead times in receiving raw materials, as well as increased costs or delaysfreight costs. These highly competitive and constrained supply chain conditions are increasing our cost of sales, which has and may continue to integration of newly acquired businesses;adversely impact our profitability.
•TheContinued concerns about the systemic impact of any reprioritizationpotential long-term and wide-spread recession and geopolitical issues, including wars and acts of capital allocations on our abilityterrorism, have contributed to achieve our strategic objectives overincreased market volatility and diminished expectations for economic growth in the mediumworld. Our business and long-term; and
•Write downs or impairments of investments in third parties, goodwill or intangible assets from recently acquired businesses, accounts receivable, or other assets.
As the situation surrounding the COVID-19 pandemic remains fluid, it is difficult to predict, with any certainty, the duration and extent of its impact which depends on future developments that cannot be accurately predicted at this time, such as the severity and transmission rate of the virus (including any variant strains), the extent and effectiveness of containment actions including the effectiveness of the vaccine against future variants and the availability of new antiviral medicines for the treatment of COVID-19, and the impact of these and other factors on our employees, customers, suppliers, distributors and other service providers.If COVID-19, or a variant strain, continues to spread and escalate domestically or internationally, or if governments impose additional measures intended to mitigate the spread and related effects of the pandemic, the risks described above could be elevated significantly. Should that occur, and the COVID-19 pandemic persist for a prolonged time, the above factors and others that are currently unknown could have a material adverse impact on our business, results of operations financialhave been and may continue to be adversely impacted by changes in macroeconomic conditions, including inflation, rising interest rates, bank failures and the accessibility of capital markets. Uncertainty about global economic conditions may also cause decreased demand for our products and services and increased competition, which could result in lower sales volume and downward pressure on the prices for our products, longer sales cycles, and slower adoption of new technologies. A weakening of macroeconomic conditions may also adversely affect our suppliers, which could result in interruptions in supply.
Market acceptance of our medical products in the U.S. and other countries is dependent upon the medical equipment purchasing and procurement practices of our customers, patient need for our products and procedures and the reimbursement of patients' medical expenses by government healthcare programs and third-party payors. The continuing uncertainty surrounding global economic conditions and prospectsfinancial markets may cause the purchasers of medical equipment to decrease their procurement activities. Economic uncertainty, an increase in unemployment rates, as well as increasing health insurance premiums, co-payments and deductibles may adversely affect demand for our products and procedures. Furthermore, governments and other third-party payors around the world facing tightening budgets could elevate known risks described in this Item 1A. Risk Factors. Information pertainingmove to further reduce the potential impactreimbursement rates or the scope of the COVID-19 pandemic and associated economic disruptions, and the actual operational and financial impacts that we have experienced to date can be found in Management's Discussion and Analysiscoverage offered, which could adversely affect sales of Financial Position and Results of Operations.our products.
RISKS RELATING TO OUR BUSINESS
Our operating results may fluctuate.
Our operating results, including components of operating results such as gross margin and cost of product sales,operating expenses, may fluctuate from time to time, and such fluctuations could affect our stock price.time. Our operating results have fluctuated in the past and can be expected to do so from time to time in the future. Some of the factors that may cause these fluctuations include:
•risks related to COVID-19;
•economic conditions worldwide, which could affect the ability of hospitals and other customers to purchase our products and could result in a reduction in elective and non-reimbursed operative procedures;
•the impact of acquisitions, our ability to integrate acquisitions, and our restructuring activities including portfolio rationalization, divestitures and product lifecycle management;divestitures;
•expenditures for major initiatives, including acquired businesses and integrations thereof and restructuring;
•the timing of significant customer orders, which tend to increase in the fourth quarter coinciding with the end of budget cycles;
•increased competition for a wide range of customers across all our product lines in the markets our products are sold;
•market acceptance of our existing products, as well as products in development;
•retention of current employees and recruiting of new employees in light of market competition for talent and relevant skills;
•the timing of regulatory approvals as well as changes in country-specific regulatory requirements;
•changes in the exchange rates between the U.S. dollar and foreign currencies of countries in which we do business;
•changes in the variable interest rates of our debt instruments which could impact debt service requirements;
•potential backorders, lost sales and expenses incurred in connection with product recalls or field corrective actions;
•disruption of our operations and sales resulting from political instability, war, insurrections, extreme weather conditions, orthe outbreak of disease, natural disasters, or other events outside our control that damage our manufacturing, distribution, or infrastructure of those facilities, or the suppliers and service providers for those facilities;
•our ability to manufacture and ship our products efficiently or in sufficient quantities to meet sales demands;
•changes in the cost or decreases in the supply of raw materials and services, including sterilization, energy, steel and honey;
•the timing of our research and development expenditures;
•reimbursement for our products by third-party payors such as Medicare, Medicaid, private and public health insurers and foreign governmental health systems;
•risks related to epidemics or similar widespread health concerns;
•the ability to maintain existing distribution rights to and from certain third parties;
•the ability to maintain business if or when we opt to convert such business from distributors to a direct sales model;
•the ability of our commercial sales representatives to obtain sales targets in a reasonable time frame;
•the impact of changes to our sales organization, continued channel expansion, including increased specialization;
•peer-reviewed publications discussing the clinical effectiveness of the products we sell;
•inspections of our manufacturing facilities for compliance with Quality System Regulations (Good Manufacturing Practices), which could result in Form 483 observations, warning letters, injunctions or other adverse findings from the FDA or from equivalent regulatory bodies, and corrective actions, procedural changes and other actions that we determine are necessary or appropriate to address the results of those inspections, any of which may affect production and our ability to supply our customers with our products;
•changes in regulations or guidelines that impact the sales and marketing practices for products that we sell;
•the increased regulatory scrutiny of certain of our products, including products which we manufacture for others, could result in removal from the market or involve field corrective actions that could affect the marketability of our products;
•enforcement or defense of intellectual property rights;
•changes in tax laws, or their interpretations; and
•the impact of goodwill and intangible asset impairment charges if future operating results of the acquired businesses are significantly less than the results anticipated at the time of the acquisitions.
Fluctuations in our operating results, including any of the above factors, may cause the market price of our common stock to fluctuate.
The industry and market segments in which we operate are highly competitive, and we may be unable to compete effectively with other companies.
There is intense competition among medical device companies. We compete with established medical technology companies in many of our product areas. Competition also comes from early-stage companies, universities, research institutions and other non-profit entities. In certain cases, our products compete primarily against medical practices that treat a condition without using a device or any particular product, such as the medical practices that use autograft tissue instead of our dermal regeneration products, duraplasty products and nerve repair products, or that use other technologies that cost less than our products. Many of our competitors have access to greater financial, technical, research and development, marketing, manufacturing, sales, distribution, administrative, consulting and other resources than we do. Our competitors may be more effective at developing commercial products.products or navigating the regulatory approval process in the markets in which we operate. They may be able to gain market share by offering lower-cost products or products that enjoy better reimbursement from third-party payors and foreign governmental health systems.
Our competitive position depends on our ability to achieve market acceptance for our products, develop new products, enhance existing products, implement production and marketing plans, secure regulatory approval for products under development and maintain previously-obtained approvals, demonstrate clinical and economic effectiveness, obtain and maintain reimbursement coverage and funding under third-party payors and foreign governmental health systems, obtain patent protection and produce products consistently in sufficient quantities to meet demand. We may need to develop new applications for our products to remain competitive. Technological advances by one or more of our current or future competitors or their achievement of superior reimbursement from third-party payors and foreign governmental health systems could render our present or future products obsolete or uneconomical. Our future success will depend upon our ability to compete effectively against current technology as well as to respond effectively to technological advances, changes in customers' requirements or in payor or regulatory evidence requirements. Additionally, purchasing decisions of our customers may be based on clinical evidence or comparative effectiveness studies and, because of our vast array of products, we might not be able to fund the studies necessary to gain entry or maintain our position or provide the required information to compete effectively. Other companies may have more resources available to fund such studies. For example, competitors have launched and are developing products to compete with our dural repair products, regenerative skin, neuro critical care monitors and ultrasonic tissue ablation devices, among others. In the current environment of managed care, consolidation among health care providers, increased competition, and declining reimbursement rates, we have been increasingly required to compete on the basis of price. Competitive pressures could adversely affect our profitability. Given these factors, we cannot guarantee that we will be able to compete effectively or continue our level of success in the areas in which we compete.
Changes in the healthcare industry may require us to decrease the selling price for our products, may reduce the size of the market for our products, or may eliminate a market, any of which could have a negative impact on our financial performance.
Trends toward managed care, healthcare cost containment and other changes in government and private sector initiatives in the U.S. and other countries in which we do business are placing increased emphasis on the delivery of more cost-effective medical therapies that could adversely affect the sale and/or the prices of our products. For example:
•third-party payors of hospital services and hospital outpatient services, including Medicare, Medicaid, private and public health insurers and foreign governmental health systems, annually revise their payment methodologies, which can result in stricter standards for reimbursement of hospital charges for certain medical procedures or the elimination of reimbursement;
•several foreign countries have implemented reforms of their respective healthcare sectors in an effort to reduce healthcare spending, including restricting funding to only those medical technologies and procedures with proven effectiveness, and increasing patient co-payments.co-payments and providing for payback measures. Governmental health systems have revised and continue to consider revisions of healthcare budgets, which could result in stricter standards for implementing certain medical procedures, increased scrutiny of medical devices, and downward pricing pressure;
•Medicare, Medicaid, private and public health insurer and foreign governmental cutbacks could create downward pricing pressure on our products;
•in the U.S., Medicare and Medicaid coverage as well as commercial payor coverage determinations could reduce or eliminate reimbursement or coverage for certain of our wound matrix, amniotic, surgical reconstruction and advanced wound dressing products as well as other products in most regions, negatively affecting our market for these products, and future determinations could reduce or eliminate reimbursement or coverage for these products in other regions and could reduce or eliminate reimbursement or coverage for other products;
•there has been a consolidation among healthcare facilities and purchasers of medical devices in the U.S., some of whom prefer to limit the number of suppliers from whom they purchase medical products, and these entities may decide to stop purchasing our products or demand discounts on our prices;
•in the U.S., we are party to contracts with group purchasing organizations, which negotiate pricing for many member hospitals, require us to discount our prices for certain of our products and limit our ability to raise prices for certain of our products, particularly surgical instruments;
•there is economic pressure to contain healthcare costs in domestic and international markets, and, regardless of the consolidation discussed above, providers generally are exploring ways to cut costs by eliminating purchases or driving reductions in the prices that they pay for medical devices, implementing national and provincial tender pricing, as recently implemented in China, or increasing clinical or economic evidence thresholds for product formularies;
•there are proposed and existing laws, regulations and industry policies in domestic and international markets regulating the sales and marketing practices and the pricing and profitability of companies in the healthcare industry;
•proposed laws or regulations may permit hospitals to provide financial incentives to doctors for reducing hospital costs, will award physician efficiency, and will encourage partnerships with healthcare service and goods providers to reduce prices; and
•there have been initiatives by third-party payors and foreign governmental health systems to challenge the prices charged for medical products that could affect our ability to sell products on a competitive basis.
Any and all of the above factors could materially and adversely affect our levels of revenue and our profitability.
Our current strategy involves growth through acquisitions, which requires us to incur substantial costs and potential liabilities for which we may never realize the anticipated benefits, and also requires us to successfully integrate acquired businesses into our business operations in order to avoid our business being materially and adversely affected.
In addition to internally generated growth, our current strategy involves growth through acquisitions. Between January 1, 20192021 and December 31, 2021,2023, we have acquired 3two businesses at a total cost of approximately $404.3$358.4 million which amount includes our acquisition of ACell, Inc. in January 2021 for $306.9 million. Thismillion and our acquisition of Surgical Innovation Associates, Inc. for $51.5 million in December 2022. Both of these acquisitions added products to our complex wound management and plastic and reconstructive surgery product portfolioportfolios, respectively, and provides additional growth opportunities for our TT segment.
In December 2023, we entered into a definitive agreement to acquire Acclarent from Johnson & Johnson, for $275.0 million in cash, subject to customary purchase price adjustments, and a cash payment of $5.0 million upon the achievement of a regulatory milestone. Completion of our pending acquisition of Acclarent is conditioned upon the receipt of certain regulatory approvals, and we cannot provide assurance that these approvals will be obtained. If any conditions, including with respect to divestitures, or changes to the proposed structure of the acquisition are required to obtain these regulatory approvals, they may have the effect of jeopardizing or delaying completion of the pending acquisition or reducing the anticipated benefits of the pending acquisition. If we are required to agree to any material conditions in order to obtain any approvals required to complete the pending acquisition, the business and results of operations of our company following the closing may be adversely affected.
We may be unable to continue to implement our growth strategy and it may ultimately be unsuccessful. A significant portion of our growth in revenues has resulted from, and is expected to continue to result from, the acquisition of businesses or products complementary to our own. We engage in evaluations of potential acquisitions and are in various stages of discussion regarding possible acquisitions, certain of which, if consummated, could be significant to us. Failure to complete the Acclarent acquisition, on a timely basis or at all, could negatively impact our future business and financial results and those of the acquired business. Any new acquisition could result in material transaction expenses, increased operating, amortization and interest expenses, and possible in-process research and development charges for acquisitions that do not meet the definition of a “business,” any of which could have a material, adverse effect on our operating results. Certain businesses that we acquire may not have adequate financial, disclosure, regulatory, quality or other compliance controls at the time we acquire them and could require significant expenditures to address those controls or subject us to increased risk. As we grow by acquisition, we must manage and integrate the new businesses to bring them into our systems for financial, disclosure, compliance, regulatory and quality control, realize economies of scale, and control costs. Failure to integrate acquired businesses and operations (including acquired employees and systems), retain key customers and suppliers of any acquired business or manage the cost of providing our products or price our products appropriately could preclude realization of the full benefits that we expect from there transactions. Our failure to meet the challenges involved in integrating the business in order to realize the anticipated benefits of the acquisitions could cause an interruption of, or loss of momentum in, our activities and could materially and adversely affect our results of operations. In addition, acquisitions involve other risks, including diversion of management resources otherwise available for the running of our business and the development of our business as well as risks associated with entering markets in which our marketing teams and sales force has limited experience or where experienced distribution alliances are not available. Some acquisitions may include the need for ongoing product development to occur consistent with time sensitive milestones in order for the Company to achieve its commercial projections for the acquisition. Our future profitability will depend in part upon our
ability to develop our resources to adapt to these new products or business areas and to identify and enter into or maintain satisfactory distribution networks. As a result of our acquisitions of other healthcare businesses, we may be subject to the risk of unanticipated business uncertainties, regulatory and other compliance matters or legal liabilities relating to those acquired businesses for which the sellers of the acquired businesses may not indemnify us, for which we may not be able to obtain insurance (or adequate insurance), or for which the indemnification may not be sufficient to cover the ultimate liabilities. We may not be able to identify suitable acquisition candidates in the future, obtain acceptable financing or consummate any future acquisitions. Certain potential acquisitions are subject to antitrust and competition laws, which laws could impact our ability to pursue strategic acquisitions and could result in mandated divestitures. If we are unsuccessful in our acquisition strategy, we may be unable to meet our financial targets and our financial performance could be materially and adversely affected.
These risks may be heightened in cases where the majority of the former businesses’ operations, employees and customers are located outside the U.S. Any one or all of these factors could increase operating costs or lower anticipated financial performance. Many of these factors are also outside of our control. In addition, dispositions of certain key products, technologies and other rights, including pursuant to conditions imposed on us to obtain regulatory approvals, may affect our business operations.
These risks may be heightened in cases where a substantial portion of an acquired businesses’ operations, employees or customers are located outside the U.S. Any one or all of these factors could complicate the integration of acquired employees and operations, increase operating costs or lower anticipated financial performance. Many of these factors are also outside of our control. For example, following the anticipated consummation of the Acclarent acquisition, the ongoing conflict in Israel, including any escalation or expansion thereof, and the measures enacted by the Israeli and other governments in response may make it more difficult for us to both integrate Acclarent and realize the anticipated benefits of the acquisition.
Even if the operations of the businesses are integrated successfully, we may not realize the full benefits of the acquisition, including the synergies, cost savings or sales or growth opportunities that we expect. These benefits may not be achieved within the anticipated time frame, or at all. Additional unanticipated costs could be incurred in the integration of the businesses. All of these factors could cause a reduction to our earnings per share, decrease or delay the expected accretive effect of the transaction, and negatively impact the price of our common stock.
Our global business exposes us to operational and economic risks.
A significant portion of our current operations are conducted and located outside the United States, and our growth strategy involves expanding our existing foreign operations and entering into new foreign jurisdictions. We have significant manufacturing and distribution sites in North America and Europe.
Our international operations subject us to laws regarding sanctioned countries, entities and persons, customs, import-export, laws regarding transactions in foreign countries, the U.S. Foreign Corrupt Practices Act and local anti-bribery and other laws regarding interactions with healthcare professionals, and product registration requirements. Among other things, these laws restrict, and in some cases prevent, U.S. companies from directly or indirectly selling goods, technology or services to people or entities in certain countries. In addition, these laws require that we exercise care in structuring our sales and marketing practices and effecting product registrations in foreign countries.
As we seek to continue to expand and strengthen our international operations, we may experience difficulty in growing our sales in certain new markets and other international markets in which we are attempting to increase our presence due to, among other things, customer acceptance, undeveloped and/or unfamiliar distribution channels, regulatory restrictions and changes, and business knowledge of these markets.
From time to time, proposals are made to significantly change existing trade agreements and relationships between the U.S. and other countries. In recent years, the U.S. government has implemented substantial changes to U.S. trade policies, including import restrictions, increased import tariffs and changes in U.S. participation in multilateral trade agreements, such as the United States-Mexico-Canada Agreement to replace the former North American Free Trade Agreement. The ongoing global economic competition and trade tensions between the U.S. and China has resulted in the U.S. government assessing supplemental tariffs on certain goods imported from China and China’s assessment of retaliatory tariffs on certain imports of U.S. goods into China. In addition, the United States has assessed or proposed supplemental tariffs and quantitative restrictions on U.S. imports of certain products from other countries as well. Owing to the complex relationships between the U.S. and such other countries, political, diplomatic, military, or other events could result in business disruptions, including increased regulatory enforcement against companies, tariffs, trade embargoes, export restrictions and the termination or modification of existing trade agreements. The imposition of such restrictions could increase the cost of the Company’s products and the components and raw materials that go into making them, require the Company to change its operations and the products it offers and negatively impact consumer confidence and spending, all of which, both individually and in the aggregate, could materially and adversely affect our business, results of operations and financial condition.
The Russia-Ukraine conflict and resulting sanctions and export restrictions are creating barriers to doing business in Russia and adversely impacting global supply chains. While we have no manufacturing, distribution or direct material suppliers in the region, we are closely monitoring the potential raw material or supplier impact in both Russia and Ukraine. Materials like palladium and neon, which are both dependent on Russian supply, are part of broader semiconductor shortages in industry. Additional sanctions, export restrictions, and potential countermeasures within Russia may lead to greater uncertainty and geopolitical shifts in Asia that could cause additional adverse impacts on global supply chains and our business, results of operations, financial condition and cash flows.
Exchange rate fluctuations and foreign currency hedges could adversely affect our financial results.
We generate significant revenues outside the U.S. in multiple foreign currencies, and in U.S. dollar-denominated transactions conducted with customers who generate revenue in currencies other than the U.S. dollar. For those foreign customers who purchase our products in U.S. dollars, currency fluctuations between the U.S. dollar and the currencies in which those customers do business may have a negative impact on the demand for our products in foreign countries where the U.S. dollar has increased in value compared to the local currency.
Since we have operations based outside the U.S. and we generate revenues and incur operating expenses in multiple foreign currencies, we experience currency exchange risk with respect to those foreign currency-denominated revenues and expenses. Our most significant currency exchange risk relates to transactions conducted in Australian dollars, British pounds, Canadian dollars, Chinese yuan, Euros, Japanese yen, and Swiss francs.
We cannot predict the consolidated effects of exchange rate fluctuations upon our future operating results because of the number of currencies involved, the variability of currency exposure and the potential volatility of currency exchange rates. Although we address currency risk management through regular operating and financing activities, and, on a limited basis, through the use of derivative financial instruments, those actions may not prove to be fully effective. For a description of our use of derivative financial instruments, see Note 6, Derivative Instruments to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K).
Our future financial results could be adversely affected by impairments or other charges.
We are required to test both goodwill and indefinite-lived intangible assets for impairment on an annual basis based upon a fair value approach, rather than amortizing them over time. We are also required to test goodwill and indefinite-lived intangible assets for impairment between annual tests if an event occurs such as a significant decline in revenues or cash flows for certain products, or the discount rates used in the calculations of discounted cash flows change significantly, or circumstances change that would more likely than not reduce our enterprise fair value below its book value. If such a decline, rate change or circumstance were to materialize, we may record an impairment of these intangible assets that could be material to the financial
statements. See Item 7 “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Critical Accounting Estimates” of this report.Annual Report on Form 10K, and Note 7, Goodwill and Other Intangible Assets to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Annual Report on Form 10-K).
The guidance on long-lived assets requires that we assess the impairment of our long-lived assets, including finite-lived intangible assets, whenever events or changes in circumstances indicate that the carrying value may not be recoverable as measured by the sum of the expected future undiscounted cash flows.
Also, Company decisions and other economic factors relating to our trade names may occur over time. For instance, we may discontinue certain products in the future as we continue to assess the profitability of our product lines. As a result, we may need to record impairment chargescharges or accelerate amortization on certain trade names or technology-related intangible assets in the future.
The value of a medical device business is often volatile, and the assumptions underlying our estimates made in connection with our assessments under the guidance may change as a result of that volatility or other factors outside our control and may result in impairment charges. The amount of any such impairment charges could be significant and have a material, adverse effect on our reported financial results for the period in which the charge is taken and could have an adverse effect on the market price of our securities, including the notes and the common stock into which they may be converted.
Lack of market acceptance for our products or market preference for technologies that compete with our products could reduce our revenues and profitability.
Market acceptance of our products depends on many factors, including our ability to convince prospective customers that our technology is an attractive alternative to other technologies, to manufacture products in sufficient quantities and at acceptable costs, and to supply and service sufficient quantities of our products directly or through our distribution alliances. For example, the use of autograft tissue is a well-established means for repairing the dermis, and it competes for acceptance in the market with our collagen-based wound care products. In addition, unfavorable payment amounts or adverse coverage determinations of third-party payors, including Medicare, Medicaid, private and public health insurers, and foreign governmental health systems, regarding our products or third-party determinations that favor a competitor’s product over ours, could harm acceptance or continued use of our products.For example, greater market acceptance of our wound graft products may ultimately depend on our ability to demonstrate that coverage and reimbursement are available and favorable, or because they are an attractive, cost-effective alternative to other treatment options.
If there are negative events in the healthcare industry, whether real or perceived, there could be a negative impact on the industry as a whole. The industry is subject to rapid and continuous change arising from, among other things, consolidation, technological improvements, the pressure on governments, third-party payors and providers to reduce healthcare costs, and healthcare reform legislation and initiatives domestically and internationally. In addition, our future success depends, in part, on our ability to license and develop additional products. Even if we determine that a product candidate has medical benefits, the cost of commercializing, either through internal development or payments associated with licensing arrangements, could be too high to justify development and we could ultimately face competitors with more effective products and better reimbursement status that cost less and are ready for commercial introduction before our products. If we are unable to develop additional commercially viable products, our future prospects could be materially and adversely affected.
One or more of these factors could vary unpredictably, and such variations could have a material, adverse effect on our competitive position. We may not be able to adjust our contemplated plan of development to meet changing market demands.
It could be difficult to replace some of our suppliers.
Outside vendors, some of whom are sole-source suppliers, provide key components and raw materials used in the manufacture of our products. Although we believe that alternative sources for many of these components and raw materials are available, any interruption in supply of a limited or sole-source component or raw material could harm our ability to manufacture our products until a new or alternative source of supply is identified and qualified. In addition, an uncorrected defect or supplier’s variation in a component or raw material, either unknown to us or incompatible with our manufacturing process, could harm our ability to manufacture products. We may not be able to find a sufficient alternative supplier in a reasonable time period, or on commercially reasonable terms, if at all, and our ability to produce and supply our products could be impaired. We believe that these factors are most likely to affect the following products that we sell:
•our collagen-based products and bovine-based products, such as the Integra Dermal Regeneration Template and wound matrix products, the DuraGen® family of products, our Absorbable Collagen Sponges, PriMatrix® and SurgiMend® products;
•our products made from silicone, such as our neurosurgical shunts and drainage systems and hemodynamic shunts;
•products which use many different specialty parts, electrical components, or chemicals from numerous suppliers, such as our intracranial monitors, shunts, catheters, tissue ablation, and headlights;
•our biosynthetic products, including the DuraSeal sealant system and DuraSorb biosynthetic mesh scaffold;
•products which are amniotic tissue-based
•products which are porcine tissue-based;
•products that use medical grade leptospermum honey, such as our Medihoney products; and
•our TCC-EZ® total contact cast system products.
The availability of amniotic tissue-based products depends upon, among other factors, the availability of tissue from human donors. Access to donated amniotic tissue could also be adversely impacted by regulatory changes or evolving public perceptions of the donor process.
Additionally, many of our products require sterilization by third-party suppliers. To the extent these suppliers are unable to provide sterilization services, whether due to lack of capacity, regulatory requirements, environmental concerns such as those relating to ethylene oxide or otherwise, we may be unable to transition sterilization to other suppliers in a timely or cost effective manner, or at all, which could have an adverse impact on our operating results.
Our supply chain and our cost of goods also may be negatively impacted by unanticipated price increases due to factors such as global economic disruptions, electronic component shortages, fear of future or ongoing pandemics, inflation, including wage inflation, or to supply restrictionsrecessionary conditions and geopolitical events, including the wars in Ukraine and Israel, all of which are beyond our control or the control of our suppliers.
While it is our policy to maintain sufficient inventory of components so that our production will not be significantly disrupted even if a particular component or material is not available for a period of time, we remain at risk that we will not be able to qualify new components or materials quickly enough to prevent a disruption if one or more of our suppliers ceases production of important components or materials.
We may experience difficulties, delays, performance impact or unexpected costs from consolidation of facilities and transfer of manufacturing facilities.
In recent years, we consolidated several facilities or transferred manufacturing operations from third parties to our existing internal manufacturing facilities and may further undertake similar consolidations or transfers in the future in order to improve our cost structure, achieve increased operating efficiencies, and improve our competitive standing or results of operations and/or to address unfavorable economic conditions. As part of these initiatives, we may also lose favorable tax incentives or not be able to renew leases on acceptable terms. We may further reduce staff, make changes to certain capital projects, close certain production operations and abandon leases for certain facilities that will not be used in our operations. In conjunction with any actions, we will continue to make significant investments and build the framework for our future growth. We may not realize, in full or in part, the anticipated benefits and savings from these efforts because of unforeseen difficulties, delays, implementation issues or unexpected costs. If we are unable to achieve or maintain all of the resulting savings or benefits to our business or other unforeseen events occur, our business and results of operations may be adversely affected.
If any of our facilities or those of our suppliers were damaged and/or our manufacturing or business processes interrupted, we could experience lost revenues and our business could be seriously harmed.
Damage to our manufacturing, distribution, development and/or research facilities because of fire, extreme weather conditions, natural disaster, power loss, communications failure, geopolitical disruption, unauthorized entry or other events, such as a flu or other health epidemic, could significantly disrupt our operations, the operations of suppliers and critical infrastructure and delay or prevent product manufacture and shipment during the time required to repair, rebuild or replace the damaged facilities. Certain of our manufacturing facilities are located in Puerto Rico, which in the past has experienced both severe hurricanes and other natural disasters. Climate change may increase both the frequency and severity of extreme weather conditions and natural disasters and, consequently, risks to our operations and growth. Although we maintain property damage and business interruption insurance coverage on these facilities, our insurance might not cover all losses under such circumstances, and we may not be able to renew or obtain such insurance in the future on acceptable terms with adequate coverage or at reasonable costs.
Global supply constraints have and may continue to adversely affect our ability to meet customer demand, and increase our costs to manufacture, transport and warehouse a certain subset of our products. In addition, global supply constraints have resulted in increases to the costs of production of certain of our products that we may not be able to pass on to our customers. We expect these factors will continue to impact us in the future and obtaining alternative sources of raw materials and components could involve significant costs and regulatory challenges and may not be available to us on commercially reasonable terms, if at all.
We may have significant product liability exposure and our insurance may not cover all potential claims.
We are exposed to product liability and other claims if our technologies or products are alleged to have caused harm. We may not be able to obtain insurance for the potential liability on acceptable terms with adequate coverage or at reasonable costs. Any potential product liability claims could exceed the amount of our insurance coverage or may be excluded from coverage under the terms of the policy. Our insurance may not be renewed at a cost and level of coverage comparable to that then in effect.
Economic and political instability around the world could adversely affect the ability of hospitals, other customers, suppliers and distributors to access funds or otherwise have available liquidity, which could reduce orders for our products or interrupt our production or distribution or result in a reduction in elective and non-reimbursed operative procedures.
Economic and political instability around the world could adversely affect the ability of hospitals and other customers to access funds to enable them to fund their operating and capital budgets. As a result, hospitals and other customers could reduce budgets or put all or part of their budgets on hold or close their operations, which could have a negative effect on our sales, particularly the sales of capital equipment such as our ultrasonic surgical aspirators, neuromonitorsneuro monitors and stereotacticcranial stabilization products, or result in a reduction in elective and non-reimbursed procedures. The occurrence of those economic conditions could make it more difficult for us to accurately forecast and plan our future business activities and depending on their severity, could have a material, adverse effect on our business, financial condition and results of operations.
Our private-labelprivate label product lines depend significantly on key relationships with third parties, which we could be unable to establish and maintain.
Our private-labelprivate label business depends in part on entering into and maintaining long-term supply agreements with third parties. The third parties with whom we have entered into agreements might terminate these agreements for a variety of reasons, including developing other sources for the products that we supply. TerminationIn addition, the voluntary global recall of all products manufactured in our Boston, Massachusetts facility ("the Boston recall") and manufacturing stoppage impacted certain of our private label products and, following the anticipated resumption of the commercialization of products manufactured at the Boston facility, we are unable to predict the effect that the Boston recall will have on our relationships for such private label products. The diminution or termination of our most important relationships could adversely affect our expectations for the growth of private-labelprivate label products.
RISKS RELATED TO OUR REGULATORY ENVIRONMENT
The adoption of healthcare reform in the U.S. and initiatives sponsored by other governments may adversely affect our business, results of operations and/or financial condition.
Our operations may be substantially affected by potential fundamental changes in the global political, economic and regulatory landscape of the healthcare industry. Government and private sector initiatives to limit the growth of healthcare costs are continuing in the U.S., and in many other countries in which we do business, causing the marketplace to put increased emphasis on the delivery of more cost-effective treatments. These initiatives include price regulation, competitive pricing, coverage and payment policies, comparative effectiveness of therapies, technology assessments and managed-care arrangements. The adoption of some or all of these initiatives could have a material, adverse effect on our financial condition and results of operations.
In the United States, the Patient Protection and Affordable Care Act (the “ACA”), signed into law in March 2010, includes several provisions that impact our businesses in the U.S. The ACA includes provisions that, among other things, reduce and/or limit Medicare reimbursement, require all individuals to have health insurance (with limited exceptions), and require detailed disclosure of transfers of value made to healthcare professionals. Other legislative changes have been proposed and adopted since the Affordable Care Act was enacted, including The Budget Control Act of 2011, The American Taxpayer Relief Act of 2012 and Medicare Access and CHIP Reauthorization Act of 2015, which, among other things, have reduced payments under Medicare and Medicaid to certain healthcare providers or altered the formula by which Medicare makes annual payment adjustments.
We cannot predict what impact ongoing uncertainty regarding federal and state health reform proposals, including the implementation or repeal of the ACA, judicial review and interpretation of the ACA and other healthcare laws, instability of the insurance markets, changes in the U.S. administration and policy, an expansion in government’s role in and/or additional proposals and/or changes to the U.S. health care system or its legislation will have on our customer’s purchasing decisions and/or reimbursement which could have a material adverse effect on our business. We expect that additional state and federal health care reform measures will be adopted in the future, including those initiatives affecting coverage and reimbursement for our products, any of which could limit the amounts that federal and state governments will pay for health care products and services, which could adversely affect the growth of the market for our products or demand for our products, or result in additional pricing pressures. We cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us. We continue to monitor the implementation of such legislation and, to the extent new market or industry trends or new governmental programs evolve, we will consider implementing or implement programs in response.
We are subject to stringent domestic and foreign medical device regulations and oversight and any adverse action may adversely affect our ability to compete in the marketplace and our financial condition and business operations.
Our products,medical devices and technologies, development activities and manufacturing processes are subject to extensive and rigorous regulation by numerous government agencies, including the FDA and comparable foreign agencies, as discussed in “Part 1, Item 1. Business – Government Regulation.”Regulation” of this Annual Report on Form 10-K. To varying degrees, each of these agencies monitors and enforces our compliance with laws and regulations governing the development, testing, manufacturing, labeling, marketing and distribution of our medical devices. We are also subject to regulations that may apply to certain of our products that are Drug/Device Combination products or are considered to be subject to pharmaceutical regulations outside the U.S. Before a new medical device, or a new use of an existing product can be marketed in the United States, it must first receive either premarket clearance under Section 510(k) of the FD&C Act, a grant of a request for de novo classification, or a PMA from the FDA, unless an exemption applies. The process of obtaining marketing approval or clearance from the FDA and comparable foreign regulatory agencies for new products, or for enhancements or modifications to existing products could be costly, time consuming and burdensome, lead to failed clinical trials or weakened clinical evidence, involve modifications, repairs or replacements of our products and result in limitations on the indicated use of our products, which may negatively impact our ability to market our products and services, result in delays or prevent full commercial realization of future products or service. Furthermore, failure to obtain timely approvals or renewals may result in significant penalties and fines. Additional regulations govern the approval, initiation, conduct, monitoring, documentation and reporting of clinical studies to regulatory agencies in the countries or regions in which they are conducted. Failure to comply, could subject us to significant enforcement actions and sanctions, including halting the study, rejection of data generated in the study, seizure of investigational devices or data, sanctions against investigators, civil or criminal penalties, and other actions. In addition, without the data from one or more clinical studies, it may not be possible for us to secure the data necessary to support certain regulatory submissions, to secure reimbursement or demonstrate other requirements. We cannot assure you that access to clinical investigators, sites and subjects, documentation and data will be available on the terms and timeframes necessary.
We are subject to extensive complex regulatory requirements by domestic and foreign government agencies and any failure to comply with our ongoing responsibilities under their applicable laws and regulations could result in a material adverse impact on our business. Failure to comply with applicable regulations could result in future product recalls, injunctions preventing the shipment of products or other enforcement actions that could have a material adverse effect on our business. In addition, the FDA has taken the position that device manufacturers are prohibited from promoting their products other than for the uses and indications set forth in the approved product labeling, and any failure to comply could subject us to significant civil or criminal
exposure, administrative obligations and costs, and/or other potential penalties from, and/or agreements with, the federal government.
We also are also subject to the European Medical Device Regulation (“MDR”), which was adopted by the European Union (“EU”) as a common legal framework for all EU member states. The implementation for Class I products occurred on May 26, 2021 and the EUDAMED Database is scheduledEuropean Commission recently extended the implementation period to the end of 2027 for May 26, 2022.high-risk devices and to the end of 2028 for medium and low risk devices. Under this regulation,the EU MDR, companies that wish to manufacture and distribute medical devices in EU member states must meet certain quality system, and safety requirements as well as ongoing product monitoring responsibilities. Companies must also obtain a “CE” marking (i.e., a mandatory conformity marking for certain products sold within the European Economic Area) for their products. Complying with the requirements of these regulations may require us to incur significant expenditures. Expenditures for EU MDR compliance activities amounted to $46.6 million for the year ended December 31, 2023 and we anticipate incurring additional expenditures in connection with our on-going efforts to obtain certification for our products under the European Medical Device Regulation. Various penalties exist for non-compliance with the laws implementing the European Medical Device Regulations which if incurred, could have a material adverse impact on our business, results of operations and cash flows.
Further, the regulatory environment in China continues to evolve, and officials in the Chinese government exercise broad discretion in deciding how to interpret and apply regulations. It is possible that the Chinese government's current or future interpretation and application of existing or new regulations will negatively impact our China operations, result in regulatory investigations or lead to fines or penalties.
Should we delay or fail to comply with one or more of the regulatory requirements we could have reduced sales, increased costs, delays to new product introductions, enhancements or our strategic plans, or harm to our reputation or competitiveness, which could have a material adverse effect on our business and financial results.
In addition, maintaining compliance with multiple regulators, and multiple centers within the FDA, adds complexity and cost to our manufacturing processes. Our manufacturing facilities and those of our contract manufacturers are subject to periodic regulatory inspections by the FDA and other regulatory agencies, and these facilities are subject to the FDA's Quality System Regulation and Good Manufacturing Practices. Please refer to “Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations – FDA Matters" (Part II, Item 7 of this Annual Report on Form 10-K) for more information relating to the warning letter we received from the FDA related to inspection observations of the quality systems at our Boston, Massachusetts manufacturing facility and our remediation efforts and expectations regarding the resumption of commercial distribution of products manufactured at the Boston facility. We or our contractors may fail to satisfy these regulatory requirements in the future, and any failure to do so may prevent us from selling our products.
Some of our activities may subject us to risks under federal and state laws prohibiting “kickbacks” and false or fraudulent claims.
We are subject to laws and regulations that govern the means by which companies in the healthcare industry may market their products to healthcare professionals and may compete by discounting the prices of their products, including for example, the federal Anti-Kickback Statute, the federal False Claims Act, the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), state law equivalents to these federal laws that are meant to protect against fraud and abuse and analogous laws in foreign countries. Violations of these laws are punishable by criminal and civil sanctions, including, but not limited to, in some instances civil and criminal penalties, damages, fines, and exclusion from participation in federal and state healthcare programs, including Medicare and Medicaid. Although
Our international operations are subject to the provisions of the U.S. Foreign Corrupt Practices Act of 1977, as amended (“FCPA”), which prohibits U.S. companies and their representatives from offering or making improper payments to foreign officials for the purpose of obtaining or retaining business. In many countries, the healthcare professionals we exercise careregularly interact with may meet the definition of a foreign official for purposes of the FCPA. Our international operations are also subject to various other international anti-bribery laws such as the UK Anti-Bribery Act. In addition, the Chinese government recently launched a campaign to combat corruption in structuringhealthcare with a focus on pharmaceutical and medical device companies covering production, supply, sales, usage, and reimbursement.The target of the campaign is kickbacks to hospitals and healthcare professionals with a focus on transfers of value to healthcare professionals in the form of grants, donations, event sponsorships, honoraria, and consulting fees. Despite meaningful measures that we undertake to facilitate lawful conduct, which include training and compliance programs and internal policies and procedures, we may not always prevent unauthorized, reckless or criminal acts by our salesemployees or agents, or employees or agents of businesses or operations we may acquire. Violations of these laws, or allegations of such violations, could disrupt our operations, involve significant management distraction and marketing practiceshave a material adverse effect on our business, financial condition and customer discount arrangementsresults of operations. We also could be subject to adverse publicity, severe penalties, including criminal and civil penalties, disgorgement, further changes or enhancements to our procedures, policies and controls, personnel changes and other remedial actions. Moreover, our failure to comply with thosedomestic or foreign laws could result in various adverse consequences, including possible delay in approval or refusal to approve a product, recalls, seizures, and regulations, we cannot assure that:
•government officials charged with responsibility for enforcing those laws will not assert that our sales and marketing practices or customer discount arrangements are in violationwithdrawal of those laws or regulations; oran approved product from the market.
•
government regulators or courts will interpret those laws or regulations in a manner consistent with our interpretation.
We have in place policies and procedures for compliance that we believe are at least as stringent as those set forth in the AdvaMed Code of Ethics which was developed by AdvaMed, a trade association that represents the medical device industry, and which is intended to represent best practices with respect to medical device companies' interactions with healthcare providers. We regularly train our sales and marketing personnel on our policies regarding sales and marketing practices. Pursuant to the AdvaMed Code, we have certified our adoption of the AdvaMed Code. The sales and marketing practices of our industry have been the subject of increased scrutiny from federal and state government agencies, and we believe that this trend will continue. Various hospital organizations, medical societies and trade associations are establishing their own practices that may require detailed disclosures of relationships between healthcare professionals and medical device companies or ban or restrict certain marketing and sales practices such as gifts and business meals. Since these laws, regulations and ultimate enforcement continue to evolve, we cannot predict with certainty, what, if any, impact, changes to them may have on our business or our customers.
Outside of the U.S. weOur medical device products are subject to privacyreporting requirements and data securityrecalls, even after receiving regulatory clearance, approval or certification, which could harm our reputation, business and financial results.
Both before and after a device is placed on the market, numerous regulatory requirements apply, which require manufacturers to follow, among other things, design, testing, production, control, documentation and other quality assurance procedures during the manufacturing process; labeling regulations, which prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and medical device reporting regulations that require us to report to FDA or similar governmental bodies in other countries if our products are ineffective or may have caused or contributed to a death or serious injury or malfunction in a way that would be reasonably likely to contribute to death or serious injury if the malfunction were to recur. The FDA and similar governmental bodies in other countries have the authority to require the recall, repair, replacement, or refund of such products, refuse to grant pending pre-market approval applications or require certificates of non-U.S. governments for exports, and/or require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health, and in certain rare circumstances, ban medical devices. We may, under own initiative, recall a product if a reasonable possibility of serious injury or any material deficiency in a device is found, or withdraw a product to improve device performance or for other reasons. For example, in May 2023, after consultation with the FDA, we initiated a voluntary global recall of all products manufactured in our Boston, Massachusetts facility distributed between March 1, 2018 and May 22, 2023. For more information concerning the Boston recall, including our remediation efforts and expectations regarding the resumption of commercial distribution of products manufactured at the international, nationalBoston facility, please see "Item 7. Management's Discussion and regional level, as well asAnalysis of Financial Condition and Results of Operations - FDA Matters" in this Annual Report on Form 10-K.
Recalls of any of our products may divert managerial and financial resources and have an industry specificadverse effect on our financial condition and results of operations and cash flows. A recall could harm our reputation with customers and consumers which could reduce the sales of our products. In addition, the FDA or other foreign governmental agencies may implement enforcement actions Any adverse regulatory action, depending on its magnitude, may restrict us from effectively marketing and selling our products and limit our ability to obtain future pre-market clearances or approvals, and could result in a substantial modification to our business practices and operations.
The adoption of healthcare reform in the U.S. and initiatives sponsored by other governments may adversely affect our business, results of operations and/or financial condition.
Our operations may be substantially affected by potential fundamental changes in the global political, economic and regulatory landscape of the healthcare industry. Government and private sector initiatives to limit the growth of healthcare costs are continuing in the U.S., and in many other countries in which we do business, causing the marketplace to put increased emphasis on the delivery of more cost-effective treatments. These initiatives include price regulation, competitive pricing, coverage and payment policies, comparative effectiveness of therapies, technology assessments and managed-care arrangements. For example, Congress also drafts and introduces, from time to time, legislation that could significantly change the statutory provisions governing the regulation of medical devices. In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. For example, in Europe, we are subjectover the last several years, the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the EU General Data Protection Regulation ("GDPR") which is related to the collection, processing, storage, transfer510(k) clearance process for their products. The adoption of some or all of these initiatives could have a material, adverse effect on our financial condition and useresults of personal data. In the U.S., we are subject to the California Consumer Privacy Act of 2018 (“CCPA”) and other similar laws in the United States, at both theoperations.
We cannot predict what impact ongoing uncertainty regarding federal and state level. Noncompliance with GDPR could trigger fineshealth reform proposals, instability of up to 4% of global annual revenues. In addition, we are subjectthe insurance markets, changes in the U.S. administration and policy, an expansion in government’s role in and/or additional proposals and/or changes to the new China Personal Information Protection Law that went into effect November 1, 2021 which focusesU.S. health care system or its legislation will have on protecting personal information and cross border transfers of the information. Compliance with these requirements, either individually or in the aggregate, may require changes in business practices added complexity and additional management oversight. They also may complicate our clinical research activities, as well as product offerings that involve transmission or use of clinical data. Non-compliance may result in proceedings against us by governmental or other entitiescustomer’s purchasing decisions and/or significant fines which could negatively impact our reputation and adversely affect our business.
Should we delay or fail to comply with one or more of the regulatory requirements we could have reduced sales, increased costs, delays to new product introductions, enhancements or our strategic plans, or harm to our reputation or competitiveness,reimbursement which could have a material adverse effect on our businessbusiness. We expect that additional state and financial results.federal and
foreign health care reform measures will be adopted in the future, including those initiatives affecting coverage and reimbursement for our products, any of which could limit the amounts that federal and state governments will pay for health care products and services, which could adversely affect the growth of the market for our products or demand for our products, or result in additional pricing pressures. We cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us. We continue to monitor the implementation of such legislation and, to the extent new market or industry trends or new governmental programs evolve, we will consider implementing or implement programs in response.
Certain of our products contain materials derived from animal sources and may become subject to additional regulation.
Certain of our products are derived from bovine or porcine tissue sources. As a result, we may experience difficulties in processing and producing our bovine and porcine tissue products at scale, including problems related to yields, quality control and assurance, tissue availability, adequacy of control policies and procedures and availability of skilled personnel.
With respect to bovine, among other products, our dermal regeneration products, duraplasty products, wound care products, bone void fillers, nerve and tendon repair products and certain other products, contain material derived from bovine tissue. In 2021, approximately 43.3%2023, 43.4% of our revenues derived from products containing material derived from bovine tissue. Products that contain materials derived from animal sources, including food, pharmaceuticals and medical devices, are subject to scrutiny in the media and by regulatory authorities. Regulatory authorities are concerned about the potential for the transmission of disease from animals to humans via those materials. This public scrutiny has been particularly acute in Japan and Western Europe with respect to products derived from animal sources, because of concern that materials infected with the agent that causes bovine spongiform encephalopathy, otherwise known as BSE or mad cow disease, may, if ingested or implanted, cause a variant of the human Creutzfeldt-Jakob Disease, an ultimately fatal disease with no known cure. The World Organization for Animal Health recognizes the U.S. as having a negligible risk for BSE, which is the highest status available.
We take care to provide that our products are safe and free of agents that can cause disease. In particular, we qualified a source of collagen from a country outside the U.S. that is considered BSE/TSE-free. The World Health Organization classifies different types of bovine tissue for relative risk of BSE transmission. Deep flexor tendon and bovine fetal skin, which are used in our products, are in the lowest-risk categories for BSE transmission and are therefore considered to have a negligible risk of containing the agent that causes BSE (an improperly folded protein known as a prion). Nevertheless, products that contain materials derived from animals, including our products, could become subject to additional regulation, or even be banned in certain countries, because of concern over the potential for the transmission of prions. Significant new regulations, or a ban of our products, could have a material, adverse effect on our current business or our ability to expand our business.
Certain countries, such as Japan, China, Taiwan and Argentina, have issued regulations that require our collagen products be sourced from countries where no cases of BSE have occurred, and the EU has requested that our dural replacement products and other products that are used in neurological tissue be sourced from a country where no cases of BSE have occurred. Currently, we source bovine fetal hides from the U.S. and purchase tendon from the U.S. and New Zealand. New Zealand has never had a case of BSE. We received approval in the U.S., the EU, Japan, Taiwan, China, Argentina as well as other countries for the use of New Zealand-sourced tendon in the manufacturing of our products. If we cannot continue to use or qualify a source of tendon from New Zealand or another country that has never had a case of BSE, we could be prohibited from selling our collagen products in certain countries.
We are subject to current and potential future requirements relating to protection of the environment, such as hazardous materials regulations, which may impose significant compliance or other costs on us.
Certain of our processes in manufacturing and research and development involve the controlled use of certain hazardous materials. In addition, we own and/or lease a number of facilities at which hazardous materials have been used in the past. Finally, we have acquired various companies that historically have used certain hazardous materials and that have owned and/or leased facilities at which hazardous materials have been used. For all of these reasons, we are subject to federal, state, foreign, and local laws and regulations governing the use, manufacture, storage, transportation, handling, treatment, remediation, and disposal of hazardous materials and certain waste products (“Environmental, Health, Safety and Transportation Laws”). Although we believe that our procedures for handling, transporting, and disposing of hazardous materials comply with the Environmental, Health, Safety and Transportation Laws, such laws may be amended in ways that increase our cost of compliance, perhaps materially.
Furthermore, the potential risk of accidental contamination or injury from these materials cannot be eliminated, and there is also a risk that such contamination previously has occurred in connection with one of our facilities or in connection with one of the companies we have purchased. In the event of such an accident or contamination, we could be held liable for any damages that result and any related liability could exceed the limits or fall outside the coverage of our insurance and could exceed our resources.resources and could have a material impact on our operations and cash flows. We may not be able to maintain insurance on acceptable terms or at all.
Our business and operations are subject to risks related to climate change.
The long-term effects of global climate change present both physical risks (from the increased frequency of extreme weather conditions or natural disasters) and transition risks (from regulatory requirements or technology changes). Such extreme weather conditions could pose physical risks to our facilities and disrupt operation of our supply chain and may impact operational costs. Concern over global climate change could result in new legal or regulatory requirements designed to mitigate the effects of climate change on the environment. If such laws or regulations are more stringent than current legal or regulatory requirements, we may experience increased compliance burdens and costs to meet the regulatory obligations and such measures may interrupt our operations or the operations of our suppliers, potentially leading to higher costs, and therefore negatively impact our results of operations.
WeEnvironmental, social and corporate governance (ESG) issues, including those related to climate change and sustainability, may have an adverse effect on our business, financial condition and results of operations and damage our reputation.
There is an increasing focus from certain investors, customers, consumers, employees and other stakeholders concerning ESG matters. Additionally, public interest and legislative pressure related to public companies’ ESG practices continue to grow. While we may create and publish voluntary disclosures regarding ESG matters from time to time, many of the statements in those voluntary disclosures are based on hypothetical expectations and assumptions that may or may not be representative of current or actual risks or events or forecasts of expected risks or events, including the costs associated therewith. Such expectations and assumptions are necessarily uncertain and may be prone to error or subject to requirements relatingmisinterpretation given the long timelines involved and the lack of an established single approach to information technologyidentifying, measuring and reporting on many ESG matters. If we do not adapt to or comply with new regulations, or fail to meet evolving investor, industry or stakeholder expectations and concerns regarding ESG issues, investors may reconsider their capital investment in our Company, and customers may choose to stop purchasing our products, which could adversely affecthave a material adverse effect on our business.reputation, business or financial condition.
If we are unabledo not retain our key personnel and attract and retain other highly skilled employees, our business could suffer.
If we fail to maintain reliable information technology systemsrecruit, develop and prevent disruptions, outages, or data breaches,retain the necessary personnel, our business and our ability to obtain new customers, develop new products and provide acceptable levels of customer service could suffer. The success of our business is heavily dependent on the leadership of our key management personnel. Our success also depends on our ability to recruit, develop and retain and motivate highly skilled sales, marketing, manufacturing and scientific personnel. Competition for these persons in our industry is intense, and we may suffer regulatory consequences innot be able to successfully recruit, train or retain qualified personnel. In addition, to business consequences. Our worldwide operations meanswe recognize that we are subject to lawsattracting, retaining and regulations, including data protection and cyber security laws and regulations, in many jurisdictions. The variety of U.S. and international privacy and cybersecurity laws and regulations impacting our operations are described in “Item 1. Business - Government Regulation - Other Factors - Data Privacy and Cybersecurity Laws and Regulations." We have programs to ensure compliance with such laws and regulations. However, theredeveloping a diverse workforce is no guarantee that we will avoid enforcement actions by governmental bodies. Enforcement actions may be costly and interrupt regular operations ofa critical success factor for our business. In addition,that regard, we are continuously facing significant competition in our markets and at all levels in the workforce. We also continue to face the challenges of maintaining employee well-being, recognizing that the continued additional financial, family and health burdens that many employees may be experiencing due to macroeconomic uncertainties, including inflation, and other factors, may adversely impact job performance, employee engagement and employee retention. Additionally, in our industry, there has been a developing trendis substantial competition for key personnel in the regions in which we operate. Labor shortages and competition for qualified personnel, particularly as employees are increasingly able to work remotely, could cause disruptions in our business operations. If we fail to effectively manage any organizational and/or strategic changes, our financial condition, results of civil lawsuitsoperations, and class actions relatingreputation, as well as our ability to breaches of consumer data held by large companies or incidents arising from other cyber-attacks. While Integra has not been named in any such suits, if a substantial breach or loss of data were to occur, wesuccessfully attract, motivate and retain key employees, could become a target of such litigation.be harmed.
RISKS RELATED TO TAX AND DEBT
We may have additional tax liabilities.
We are subject to income taxes in the U.S. and many foreign jurisdictions and are commonly audited by various tax authorities. In the ordinary course of our business, there are many transactions and calculations where the ultimate tax determination is uncertain. Significant judgment is required in determining our worldwide provision for income taxes. Although we believe that our tax estimates are reasonable, tax authorities may disagree with certain positions we have taken and the final determination of tax audits and any related litigation could be materially different from our historical income tax provisions and accruals.In addition, economic and political pressures to increase tax revenue in various jurisdictions may make resolving tax disputes favorably more difficult. The results of an audit or litigation could have a material, adverse effect on our financial statements in the period or periods for which that determination is made.made and could result in the imposition of fines and penalties.
Changes in tax laws or exposures to additional tax liabilities could negatively impact the Company's operating results.
We are subject to income taxes, as well as taxes that are not income-based, in both the U.S. and many foreign jurisdictions.Taxes could significantly increase due to changes in tax laws or changes in our interpretation of those laws. For example, the Organization for Economic Co-operation and Development, a global policy forum, released model rules related to a new 15% global minimum tax regime. Several of the jurisdictions that we operate in have already adopted these rules, which could impact the amount of taxes that we pay. Taxes could also significantly increase due to changes in accounting guidance. Our future effective tax rate could be unfavorably affected by numerous factors including a change in, or the interpretation of, tax rules and regulations in the jurisdictions in which we operate (including changes in legislation currently being considered), the expiration of or disputes
about certain tax agreements in a particular jurisdiction, a change in our geographic earnings mix, and/or to the jurisdictions in which we operate, or a change in the measurement of our deferred taxes.
Our leverage and debt service obligations could adversely affect our business.
Our leverage and debt service obligations could adversely affect our business. As of December 31, 2021,2023, our total consolidated external debt was approximately $1.6$1.4 billion (See Item 77. Management's Discussion and Analysis of Financial Condition and Results of Operations and Note 5,55, Debt of, to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for a discussion of our consolidated external debt). We may also incur additional indebtedness in the future. Our substantial indebtedness could have material, adverse consequences, including:
•making it more difficult for us to satisfy our financial obligations;
•increasing our vulnerability to adverse economic, regulatory and industry conditions, and placing us at a disadvantage compared to our competitors that are less leveraged;
•limiting our ability to compete and our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; and
•limiting our ability to borrow additional funds for working capital, capital expenditures, acquisitions and general corporate or other purposes.
Our debt service obligations will require us to use a portion of our operating cash flow to pay interest and principal on indebtedness instead of for other corporate purposes, including funding future expansion of our business, acquisitions, and ongoing capital expenditures, which could impede our growth. In addition, our ability to comply with, renegotiate or extend the Company’s debt obligations will depend on our operating and financial performance, which in turn is subject to prevailing economic conditions and financial, business and other factors beyond our control.Anycontrol. Any disruptions in our operations, the financial markets, or the overall economy, including as a result of COVID-19, may adversely affect the availability and cost of credit to us and/or our ability to comply with our existing obligations.
ChangesGLOBAL PUBLIC HEALTH CONCERNS
Public health crises, such as the COVID-19 pandemic, have had, and could in the calculation and or complete replacement of LIBOR couldfuture have, an impacta negative effect on our business.
The United Kingdom’s Financial Conduct Authority (“FCA”),Our global operations and interactions with healthcare systems, providers and patients around the world expose us to risks associated with public health crises, including epidemics and pandemics. Such pandemics or disease outbreaks, such as the COVID-19 pandemic, have created and may continue to create significant volatility, uncertainty and economic disruption in the markets in which regulates LIBOR, announcedwe sell our products and in July 2017 that it will no longer persuade or require bankswhich we operate. In response to submit rates for LIBOR. On March 5, 2021, the ICE Benchmark Administration, which administers LIBOR,COVID-19 pandemic, governments around the world imposed measures designed to reduce the transmission of COVID-19 and individuals responded to the FCA announced that all LIBOR settings will either ceasefear of contracting COVID-19.
Additionally, the impact of the COVID-19 pandemic and its aftermath on general macroeconomic conditions has led to be provided by any administrator, or no longer be representative immediately after December 31, 2021, for all non-U.S. dollar LIBOR settings and one-week and two-month U.S. dollar LIBOR settings, and immediately after June 30, 2023 for the remaining U.S. dollar LIBOR settings. We have multiple debt facilities which utilizes a variable rate equal to Eurodollar LIBOR rate as a component of our interest rate. This transition away from LIBOR as a common reference ratedisruptions in the global financial marketsupply chain, primarily through a lack of availability of raw materials and electronic components. We have experienced challenges associated with material and component availability for certain product lines, longer shipping and delivery times for raw materials and components, constrained logistics capacity related to the movement of our products, availability of skilled labor and increased costs of raw materials, components, labor, and freight and courier services.
The direct and indirect disruptions caused by the pandemic and the responses of both governments and individuals could negatively impact the number of surgical and medical intervention procedures performed and have a material adverse effect on our business. Management continuesbusiness, financial condition, results of operations, or cash flows. The extent to monitorwhich fear of exposure to or actual effects of COVID-19, new variants, disease outbreak, epidemic or a similar widespread health concern impacts our business will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the statusspeed and discussions regarding LIBOR.extent of geographic spread of the disease, the duration of the outbreak, travel restrictions, the efficacy of vaccination and treatment; impact on the U.S. and international healthcare systems, the U.S. economy and worldwide economy; the timing, scope and effectiveness of U.S. and international governmental response; and the impact on the health, well-being and productivity of our employees.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
Our intellectual property rights may not provide meaningful commercial protection for our products, potentially enabling third parties to use our technology or very similar technology and could reduce our ability to compete in the market.
To compete effectively, we depend, in part, on our ability to maintain the proprietary nature of our technologies and manufacturing processes, which includes the ability to obtain, protect and enforce patents on our technology and to protect our trade secrets. We own or have licensed patents that cover aspects of some of our product lines. Our patents, however, may not
provide us with any significant competitive advantage. Others may challenge our patents and, as a result, our patents could be narrowed, invalidated or rendered unenforceable. Competitors may develop products similar to ours that our patents do not cover. In addition, the approval or rejection of patent applications may take several years and our current and future patent applications may not result in the issuance of patents in the U.S. or foreign countries.
Our competitive position depends, in part, upon unpatented trade secrets, which we may be unable to protect.
Our competitive position also depends upon unpatented trade secrets, which are difficult to protect. We cannot assure that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets, that our trade secrets will not be disclosed or that we can effectively protect our rights to unpatented trade secrets.
In an effort to protect our trade secrets, we require our employees, consultants and advisors to execute confidentiality and invention assignment agreements upon commencement of employment or consulting relationships with us. These agreements provide that, except in specified circumstances, all confidential information developed or made known to the individual during the course of their relationships with us must be kept confidential. We cannot assure, however, that these agreements will provide meaningful protection for our trade secrets or other proprietary information in the event of the unauthorized use or disclosure of confidential information.
Our success will depend partly on our ability to operate without infringing or misappropriating the proprietary rights of others.
We may be sued for infringing the intellectual property rights of others. In addition, we may find it necessary, if threatened, to initiate a lawsuit seeking a declaration from a court that we do not infringe the proprietary rights of others or that their rights are invalid or unenforceable. If we do not prevail in any litigation, in addition to any damages we might have to pay, we would be required to stop the infringing activity (which could include a cessation of selling the products in question) or obtain a license for the proprietary rights involved. Any required license may be unavailable to us on acceptable terms, if at all. In addition, some licenses may be nonexclusive and allow our competitors to access the same technology we license.
If we fail to obtain a required license or are unable to design our products so as not to infringe on the proprietary rights of others, we may be unable to sell some of our products, and this potential inability could have a material, adverse effect on our revenues and profitability.profitability and cash flows.
We may be involved in lawsuits relating to our intellectual property rights and promotional practices, which may be expensive.
ToThe medical device industry is characterized by extensive intellectual property litigation and to protect or enforce our intellectual property rights, we may have to initiate or defend legal proceedings, such as infringement suits or opposition proceedings, against or by third parties. In addition, we may have to institute proceedings regarding our competitors’ promotional practices or defend proceedings regarding our promotional practices. Legal proceedings are costly, and, even if we prevail, the cost of the legal proceedings could affect our profitability.profitability and cash flows. In addition, litigation is time-consuming and could divert management's attention and resources away from our business. Moreover, in response to our claims against other parties, those parties could assert counterclaims against us.
RISKS RELATED TO GLOBAL OPERATIONS
If any of our facilities or those of our suppliers were damaged and/or our manufacturing or business processes interrupted, we could experience lost revenues and our business could be seriously harmed.
Damage to our manufacturing, distribution, development and/or research facilities because of fire, extreme weather conditions, natural disaster, power loss, communications failure, geopolitical disruption, unauthorized entry or other events, such as a flu or other health epidemic, such as COVID-19, could significantly disrupt our operations, the operations of suppliers and critical infrastructure and delay or prevent product manufacture and shipment during the time required to repair, rebuild or replace the damaged facilities. Certain of our manufacturing facilities are located in Puerto Rico, which in the past has experienced both severe earthquakes and other natural disasters.Climate change may increase both the frequency and severity of extreme weather conditions and natural disasters and, consequently, risks to our operations and growth. Although we maintain property damage and business interruption insurance coverage on these facilities, our insurance might not cover all losses under such circumstances, and we may not be able to renew or obtain such insurance in the future on acceptable terms with adequate coverage or at reasonable costs.
We are exposed to a variety of risks relating to our international sales and operations.
We generate significant revenues outside the U.S. in multiple foreign currencies, and in U.S. dollar-denominated transactions conducted with customers who generate revenue in currencies other than the U.S. dollar. For those foreign customers who purchase our products in U.S. dollars, currency fluctuations between the U.S. dollar and the currencies in which those customers do business may have a negative impact on the demand for our products in foreign countries where the U.S. dollar has increased in value compared to the local currency.
Since we have operations based outside the U.S. and we generate revenues and incur operating expenses in multiple foreign currencies, we experience currency exchange risk with respect to those foreign currency-denominated revenues and expenses. Our most significant currency exchange risk relates to transactions conducted in Australian dollars, British pounds, Canadian dollars, Chinese yuan, euros, Japanese yen, and Swiss francs.
We cannot predict the consolidated effects of exchange rate fluctuations upon our future operating results because of the number of currencies involved, the variability of currency exposure and the potential volatility of currency exchange rates. Although we address currency risk management through regular operating and financing activities, and, on a limited basis, through the use of derivative financial instruments, those actions may not prove to be fully effective. For a description of our use of derivative financial instruments, see Note 6, Derivative Instruments of the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K).
Our international operations subject us to laws regarding sanctioned countries, entities and persons, customs, import-export, laws regarding transactions in foreign countries, the U.S. Foreign Corrupt Practices Act and local anti-bribery and other laws regarding interactions with healthcare professionals, and product registration requirements. Among other things, these laws restrict, and in some cases prevent, U.S. companies from directly or indirectly selling goods, technology or services to people or entities in certain countries. In addition, these laws require that we exercise care in structuring our sales and marketing practices and effecting product registrations in foreign countries.
The United Kingdom’s (“UK”) exit from the European Union on January 31, 2020, commonly referred to as Brexit, has caused, and may continue to cause uncertainty in the global political markets. It is possible that Brexit could, among other things, affect the legal and regulatory environments to which our business is subject, impose greater restrictions on imports and exports between the UK and the EU and other parties, and create economic and political uncertainty in the region.
From time to time, proposals are made to significantly change existing trade agreements and relationships between the U.S. and other countries. Owing to the complex relationships between the U.S. and such other countries, political, diplomatic, military, or other events could result in business disruptions, including increased regulatory enforcement against companies, tariffs, trade embargoes, and export restrictions. The imposition of such restrictions could increase the cost of the Company’s products and the components and raw materials that go into making them, require the Company to change its operations and the products it offers and negatively impact consumer confidence and spending, all of which, both individually and in the aggregate, could materially and adversely affect our operations and financial results.
GENERAL RISK FACTORSCYBERSECURITY AND DATA PRIVACY
Cyber-attacks or other disruptions to our information technology systems could adversely affect our business.
We are increasingly dependent on sophisticated information technology for our infrastructure and to support business decisions. Our information systems require an ongoing commitment of significant resources to maintain, protect, and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards, the increasing need to protect patient and customer information, and changing customer patterns. An experienced third party maintains the enterprise business system used to support our transaction processing, accounting and financial reporting, and supply chain and manufacturing processes. Any significant breakdown, intrusion, interruption, corruption, or destruction of these systems, as well as any data breaches, could have a material, adverse effect on our business.
Third parties may attempt to breach our systems and may obtain data relating to patients, proprietary or sensitive information. As a result of the COVID-19 pandemic, weWe may face increased cybersecurity risks due to our reliance on internet technology and the number of our employees who are working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. If we, or third parties on whom we rely, fail to maintain or protect our information systems and data integrity effectively, we could lose existing customers, have difficulty attracting new customers, suffer backlash from negative public relations, have regulatory sanctions or penalties imposed, have increases in operating expenses, incur expenses or lose revenues as a result of a data privacy breach, or suffer other adverse consequences.
We have programs, processes (including ongoing improvements) and technologies in place to prevent, detect, contain, respond to and mitigate security related threats and potential incidents. Because the techniques used to obtain unauthorized access or interrupt services change frequently and can be difficult to detect, anticipating, identifying or preventing these threats or mitigating them if and when they occur, may be challenging. We are also dependent on third party vendors to supply and/or support certain aspects of our information technology systems which may contain defects in design or manufacture or other problems that could result in system disruption or unexpectedly compromise the information security of our own systems. In addition, as we grow in part through new acquisitions we may face risks due to implementation, modification, or remediation of controls, procedures, and policies relating to data privacy and cybersecurity at the acquired business. We continue to consolidate and integrate the number of systems we operate, and to upgrade and expand our information system capabilities for stable and secure business operations. Despite our implementation of controls to protect our systems and sensitive, confidential or personal data or information, we may be vulnerable to material security breaches, theft, misplaced, lost or corrupted data, employee errors and/or malfeasance (including misappropriation by departing employees) that could potentially lead to the compromising of sensitive, confidential or personal data or information, improper use of our systems, software solutions or networks, unauthorized access, use, disclosure, modification or destruction of information, defective products, production downtimes and operational disruptions. In addition, a cyber-related attack could result in other negative consequences, including damage to our reputation or competitiveness, remediation or increased protection costs, litigation or regulatory action.
Failure to comply with laws relating to the confidentiality of sensitive personal information or standards related to the transmission of electronic health data, may require us to make significant changes to our products, or incur penalties or other liabilities.
State, federal and foreign laws, such as HIPAA or the California Consumer Privacy Act of 2018, regulate the confidentiality of sensitive personal information and the circumstances under which such information may be released. These measures may govern the disclosure and use of personal and patient medical record information and may require users of such information to implement specified security measures, and to notify individuals in the event of privacy and security breaches. Evolving laws and regulations in this area could restrict the ability of our customers to obtain, use or disseminate patient information, or could require us to incur significant additional costs to re-design our products in a timely manner, either of which could have an adverse impact on our results of operations. Other health information standards, such as regulations under HIPAA, establish standards regarding electronic health data transmissions and transaction code set rules for specified electronic transactions, for example transactions involving submission of claims to third-party payors. These standards also continue to evolve and are often unclear and difficult to apply. We have incurred and expect that we will continue to incur significant costs implementing additional security measures to protect against new or enhanced data security or privacy threats, or to comply with current and new federal, state and international laws governing the unauthorized disclosure or exfiltration of confidential and personal information which are continuously being enacted and proposed. Outside the U.S., we are impacted by privacy and data security requirements at the international, national and regional level, and on an industry specific basis. More privacy and security laws and regulations are being adopted, and more are being enforced, with potential for significant financial penalties. In the EU, increasingly stringent data protection and privacy rules have been enacted. The EU General Data Protection Regulation (GDPR) applies uniformly across the EU and includes, among other things, a requirement for prompt notice of data breaches to data subjects and supervisory authorities in certain circumstances. The GDPR also requires companies processing personal data of individuals residing in the EU to comply with EU privacy and data protection rules. Failure to maintain the confidentiality of sensitive personal information in accordance with the applicable regulatory requirements, or to abide by electronic health data transmission standards, could expose us to breach of contract claims, fines and penalties, costs for remediation and harm to our reputation.
ITEM 1B. UNRESOLVED STAFF COMMENTS
As of the filing of this Annual Report on Form 10-K, we had no unresolved comments from the staff of the SEC that were received not less than 180 days before the end of our 2023 fiscal year.
ITEM 1C. CYBERSECURITY
Information Technology and Cybersecurity
Our business relies on the secure electronic transmission, storage and hosting of sensitive information, including personal information, financial information, intellectual property, and other sensitive information related to our customers and workforce. Given the importance of cybersecurity to our business, we maintain a comprehensive information technology and cybersecurity program to increase both the effectiveness of our systems and our preparedness for cybersecurity risks, including security monitoring for internal and external threats to bolster the confidentiality, integrity and availability of our information assets. We regularly perform evaluations of our cybersecurity program, including periodic internal and external audits, penetration tests and incident response simulations, and our information technology infrastructure and cybersecurity management system are subject to external program assessments on a regular basis. In 2017, we adopted the National Institute
of Standards and Technology Cybersecurity Framework (NIST CSF) to bolster our cybersecurity management system and reduce cybersecurity risks.
We engage multiple independent third-party cybersecurity services and consulting firms to review our cybersecurity program and we have entered into partnerships with entities such as the Health Information Sharing and Analysis Center, the Cybersecurity & Infrastructure Security Agency, InfraGard, the Department of Homeland Security, the Cyber Fraud Task Forces and the Center for Internet Security to complement our program and bolster our data protection and privacy efforts. To monitor and minimize the risks from cybersecurity threats associated with our use of third-party service providers, we require the completion of standardized information gathering questionnaires from service providers prior to entering any engagement for services. Further, we utilize security ratings from industry-recognized sources to provide an external analysis of such third-party service providers. We work closely with these industry-recognized sources to interpret the security ratings results in the context of the specific characteristics of our information technology and cybersecurity systems, which helps inform our assessment of the efficacy and reliability of the third-party vendors we use. We also conduct periodic internal reviews of the performance and reliability of the third-parties we have engaged for cybersecurity services.
Management and Board Oversight
The Board has overall responsibility for the oversight of risk management at the Company, which includes overseeing our process for identifying, assessing and mitigating significant financial, operational, strategic, cybersecurity and other risks that may affect the Company. Our Chief Information Officer, or CIO, leads our cybersecurity program and our Director, Cybersecurity leads our cybersecurity team. Our CIO provides periodic reports relating to cybersecurity matters to the Board, as well as our Chief Executive Officer and other members of our senior management, as appropriate. Our executive leadership team and Board provide principal oversight and guidance of our cybersecurity risk management programs and processes. We have established a cybersecurity executive steering committee to review and discuss cybersecurity issues and review our security metrics. The committee is comprised of a cross-functional group of senior executives, including our Chief Executive Officer, Chief Financial Officer, Chief Legal Officer, Chief Information Officer and Director, Cybersecurity, and is responsible for the implementation and oversight of the processes and systems we use to assess and manage risk from cybersecurity threats as well as cybersecurity incidents. Our CIO and committee members have significant work experience related to cybersecurity issues or oversight and members of our cybersecurity team hold vendor-neutral and vendor-specific certifications from organizations such as the Information Systems Audit and Control Association (ISACA), the Computing Technology Industry Association (CTIA) and the International Information System Security Certification Consortium (ISC2). In addition, we require all new employees to complete cybersecurity training so they are better able to understand how to identify, protect, and preserve sensitive data and minimize risks related to cybersecurity matters. We supplement this new hire training with annual training and certification programs, which includes social engineering simulations. We continue to expand and improve our global training programs to raise employee awareness of security obligations and members of senior management regularly provide employees with communications regarding the cybersecurity environment to increase employee awareness of cybersecurity trends and emerging risks.
Processes for Assessing, Identifying and Managing Material Risks from Cybersecurity Threats
Our monitoring capabilities, including our internal auditing procedures, internal control over financial reporting and corporate compliance programs, are designed in part to inform management about our material risks, including those related to cybersecurity risks. In the event of an incident which jeopardizes the confidentiality, integrity, or availability of our information assets, and our risk management systems, we maintain a regularly tested incident response program. Pursuant to the program and its escalation protocols, designated personnel are responsible for assessing the severity of the incident and associated threat, containing the threat, remediating the threat, including recovery or data and access to systems, analyzing the reporting and disclosure obligations associated with the incident, and performing post-incident analysis and program improvements. Although the particular personnel assigned to an incident response team will depend on the particular facts and circumstances, the team is generally led by the CIO or another member of the cybersecurity executive steering committee and will include other information technology and legal personnel. In the event of a potentially material incident, the incident response team regularly reports to both the Company’s Board and members of senior management, including the Chief Executive Officer, Chief Financial Officer and Chief Legal Officer to assist in making determinations regarding applicable SEC reporting requirements.
In addition, our Board receives regular reports from management on matters relating to strategic and operational initiatives, financial performance, cybersecurity and legal developments. The Company’s Enterprise Risk Management program, which has been adopted by the Company to further enhance oversight of risks inherent to our business and allow members of the Board and management to gain a greater understanding of the efforts being undertaken to manage the risks confronting the Company, covers cybersecurity risks.
Our management believes that our current systems and practice of implementing regular updates positions us well to support current needs and future growth. We use a strategic information systems multi-year planning process that involves senior management and is integrated into our overall business planning. Information systems projects are prioritized based upon strategic, financial, regulatory, risk and other business advantage criteria.
Cybersecurity Risks
As of December 31, 2023, we have not had any material cybersecurity incidents. However, we face risks associated with cybersecurity incidents, whether through cyber-attacks or cyber intrusions over the Internet, ransomware and other forms of malware, computer viruses, attachment to emails, phishing attempts or other scams. Although we make efforts to maintain the security and integrity of our networks and systems, and the proprietary, confidential and personal information that resides on or is transmitted through them, and we have implemented various cybersecurity policies and procedures to manage the risk of a security incident or disruption, there can be no assurance that our security efforts and measures will be effective or that attempted security incidents or disruptions would not be successful or damaging. We also carry insurance that provides protection against the potential losses arising from a cybersecurity incident. See “Risk Factors–Risks Related to Cybersecurity and Data Privacy—Cyber-attacks or other disruptions to our information technology systems could adversely affect our business” and “—Failure to comply with laws relating to the confidentiality of sensitive personal information or standards related to the transmission of electronic health data, may require us to make significant changes to our products, or incur penalties or other liabilities."
As of December 31, 2021,2023, we lease approximately 166,991 square feet of space in Princeton, NJ, where we house our principal headquarters, sales operations, and support functions. This lease expires in 2035.
We haveOur segments utilize key manufacturing and research facilities located in California, Indiana, Maryland, Massachusetts, New Jersey, Ohio, Illinois, Puerto Rico, Tennessee, Utah, Canada, China, France, Germany, Ireland, and Switzerland. Our instrument procurement operations are located in Germany. Our primary distribution centers are located in Kentucky, Nevada, Ohio, Kentucky, Australia, Belgium, Canada, Italy, Japan, and France.China. In addition, we lease several smaller facilities to support additional administrative, assembly, and distribution operations. Third parties own and operate the facilities in Nevada, Kentucky, Japan and Belgium. We own facilities in Biot, France, Saint Aubin Le Monial, France, Rietheim-Weilheim, Germany and Ohio and we lease all of our other facilities. We also have repair centers in Ohio,United States, Canada, Australia, France, Japan, China and Germany, and field service presence covering regions within Europe, Asia Pacific and Latin America with direct teams based in the United States, Canada, Dubai,France, Germany, Austria, Switzerland, Korea, Taiwan, India, Italy, Belgium, Luxembourg, Netherlands, Singapore, Thailand, Australia, New Zealand, and United Kingdom.
Our manufacturing facilities are registered with the FDA. Our facilities are subject to FDA inspection to ensure compliance with Quality System regulations. For further information regarding the status of FDA inspections, see the "Government Regulation""Item 1. Business –Government Regulation and "Management'sCompliance" and "Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations - Update on Remediation Activities" sections– FDA Matters" in this Annual Report on Form 10-K.
Information pertaining to legal proceedings can be found in Note 15. Commitment15, Commitments and Contingencies of, to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K).
| | | | | |
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
PART II | | | | | |
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information, Holders and Dividends
Our common stock trades on The NASDAQNasdaq Global Select Market under the symbol “IART.” The number of stockholders of record as of February 22, 202227, 2024 was approximately 777,749, which includes stockholders whose shares were held in nominee name.
Dividend Policy
We have not paid any cash dividends on our common stock since our formation. Our Senior Credit Facility (as defined below) limits the amount of dividends that we may pay. Any future determinations to pay cash dividends on our common stock will be at the discretion of the Board and will depend upon our financial condition, results of operations, cash flows and other factors deemed relevant by the Board.
Sales of Unregistered Securities
There were no sales of unregistered securities during the years ended December 31, 2021, 20202023, 2022 or 2019.2021.
Sale of Registered Securities
There were no sales of registered securities during the years ended December 31, 2021, 20202023, 2022 or 2019.2021.
Issuer Purchases of Equity Securities
The following table provides information about purchases by the Company during the quarter ended December 31, 2023 of equity securities that are registered by us pursuant to Section 12 of the Exchange Act. Subject to applicable law, share repurchases may be made from time to time in open market transactions, privately negotiated transactions including accelerated share repurchase agreements, or pursuant to instruments and plans complying with Rule 10b5-1 under the Exchange Act, among other types of transactions and arrangements.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuer purchases of equity securities |
| Period | | Total number of shares purchased by month | | Average price paid per share | | Total number of shares purchased by month as part of publicly announced repurchase programs (1) | | Approximate dollar value of shares that may yet be purchased under the plans or program |
| | | |
| 10/01/23 - 10/31/23 | | 928,485 | | | $ | 37.17 | | | 928,485 | | | $ | 100,000,000 | |
| 11/01/23 - 11/30/23 | | — | | | $ | — | | | — | | | 100,000,000 | |
| 12/01/23 - 12/31/23 | | — | | | $ | — | | | — | | | 100,000,000 | |
| | 928,485 | | | $ | 37.17 | | | 928,485 | | | |
(1)On December 7, 2020,July 18, 2023, the Board of Directors authorized the Companya stock repurchase program, which expires on December 31, 2023, to repurchase up to $225 million of the Company’s outstanding common stock.stock, effective as of the close of trading on September 23, 2022. As of December 31, 2023, $100.0 million remained unused under this program. The program allowsdoes not obligate the Company to repurchase itsacquire a minimum amount of shares. Under the program, shares opportunistically from time to time. The repurchase authorization expires in December 2022. This stock repurchase authorization replaces the previous $225 million stock repurchase authorization, of which $125 million remained authorized at the time of its replacement, and which was otherwise set to expire on December 31, 2020. Purchases may be affected through one repurchased in privately negotiated and/or more open market transactions, privately negotiated transactions, transactions structured through investment banking institutions, or a combination ofincluding under plans complying with Rule 10b5-1 under the foregoing.Exchange Act.
For the year ended December 31, 2021, there were no repurchases of the Company’s common stock as part of the share repurchase authorization.
On January 12, 2022,August 15, 2023, the Company entered into a $125.0$125 million accelerated share repurchase ("2022August 2023 ASR") and received 1.52.3 million shares of Companythe common stock at inception of the 2022August 2023 ASR, which represented approximately 80% of the expected total shares under the 2022August 2023 ASR. The remaining 20% ofOn October 18, 2023 the expected total shares is expected to settle inearly exercise provision was exercised by the first half of 2022, upon which additional shares of common stock may be delivered to the Company or, under certain circumstances, the Company may be required to make a cash payment or may elect to deliver shares of our common stock to the 2022August 2023 ASR counterparty in each case pursuant to the terms of the 2022 ASR agreement between the Company and the 2022 ASR counter party.counterparty. The total number of shares to be delivered or the amount of such payment, as well as the final average price per share, will be based on the volume-weighted average price, less a discount, of the Company's common stock during the term of the transaction. As a result of this transaction, $100.0 million remains available under the $225.0 million stock repurchase authorization.
During the twelve months ended December 31, 2020, the Company repurchased 2.1 million shares of Integra’s common stock as part of the previous share repurchase authorization. The Company utilized $100.0 million of net proceeds from the offering of convertible notes to execute the share repurchase transactions. This included $7.6 million from certain purchasers of the convertible notes in conjunction with the closing of the offering. On February 5, 2020, the Company entered into a $92.4 million accelerated share repurchase ("2020 ASR") to complete the remaining $100.0 million of share repurchase. The Company received 1.3 million shares at inception of the 2020 ASR, which represented approximately 80% of the expected total shares. Upon settlement of the 2020 ASR in June 2020, the Company received an additional 0.60.9 million shares determined using the volume-weighted average price of the Company's common stock during the term of the transaction.August 2023 ASR.
On January 26, 2023, the Company entered into a $150 million accelerated share repurchase ("January 2023 ASR") and received 2.1 million shares of common stock at inception of the January 2023 ASR, which represented approximately 80% of the expected total shares under the January 2023 ASR. The settlement of the January ASR agreement was completed in two separate transactions on April 26, 2023 and May 4, 2023, where the Company received an additional 0.30 million and 0.31 million shares respectively, determined using the volume-weighted average price of the Company's common stock during the term of the January 2023 ASR.
On August 16, 2022, the Inflation Reduction Act of 2022 (the “Act”) was signed into law. The Act implemented a new excise tax of 1% on the net share repurchases made by the Company effective for share repurchases performed January 1, 2023, or after. The Company accrued $2.5 million of excise tax related to the two ASR agreements during 2023.
On July 18, 2023, the Board of Directors authorized a new $225 million share repurchase program, replacing the existing $225 million program authorized in April 2022, of which $100 million remained authorized as of the year ended December 31, 2023. The program authorized in July 2023, which expires on December 31, 2025, allows the Company to repurchase its shares opportunistically from time to time. The Company may utilize various methods to effect any repurchases, including open market transactions, privately negotiated transactions, transactions structured through investment banking institutions, including accelerated share repurchases, or a combination of the foregoing, some of which may be effected through Rule 10b5-1 plans. The price and timing of any future purchases under the share repurchase program will depend on factors such as levels of cash generation from operations, the volume of stock option exercises by employees, cash requirements for acquisitions, dividends, economic and market conditions and stock price, and such repurchases may be discontinued at any time.
See Note 8, Treasury Stock toof the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for further details.
Securities Authorized for Issuance under Equity Compensation Plan
The information required by this item regarding our equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report on Form 10-K.
Stock Performance Graph
The graph below compares the five-year total return to stockholders on our common stock with the return of the Standard & Poor’s (S&P) 500 Stock Index and the S&P Healthcare Equipment Index for the five years ended December 31, 2023. The Company’s cumulative shareholder return is based on an investment of $100 on December 31, 2018 and is compared to the cumulative total return of the S&P indices mentioned above over the period with a like amount invested. Measurement points are the last trading day of each respective fiscal year.
Note: The stock price performance shown on the graph above is not indicative of future price performance. This graph shall not be deemed filed for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, regardless of any general incorporation language in such filing.
| | | | | |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis provides information management believes to be relevant to understanding our financial condition and results of operations. For a full understanding of financial condition and results of operations, it should be read together with the selected unaudited consolidated financial data and our financial statements with the related notes appearing elsewhere in this report. The discussion focuses on our financial results for the year ended December 31, 20212023 and 2020.2022. The comparison of fiscal 20202022 to 20192021 has been omitted from this Form 10-K, but can be referenced in our Form 10-K for the fiscal year ended December 31, 2020—2022—“Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations” filed with the SEC on February 23, 2021.22, 2023.
We have made statements in this report which constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934 (the “Exchange Act”).Act. These forward-looking statements are subject to a number of risks, uncertainties and assumptions about the Company and other matters. These forward-looking statements include, but are not limited to, statements related to the Company's expectations regarding the potential impacts of the COVID-19 pandemic on our business, financial condition, and results of operations. These statements should, therefore, be considered in light of various important factors, including, but not limited to, the following: the risk that the COVID-19 pandemic could lead to further material delays and cancellations of, or reduced demand for, procedures; delayed capital spending by the Company's customers; disruption and/or higher costs to theThe Company’s supply chain; staffing shortages in hospitals; labor impacts in our facilities; delays in gathering clinical evidence; diversion of management and other resources to respond to the COVID-19 outbreak; the impact of global and regional economic and credit market conditions on healthcare spending; the risk that the COVID-19 virus disrupts local economies and causes economies in our key markets to enter prolonged recessions. The Company's actual results could differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those set forth under Item 1A. Risk Factors. Please refer to “Special Note Regarding Forward-Looking Statements” and Item 1A. Risk Factors for a discussion of the heading “Risk Factors.”factors that could cause actual results to differ materially from those projected in these statements. The following information concerning our business, results of operations and financial condition should also be read in conjunction with the information included under Item 1. Business, Item 1A. Risk Factors and Item 15. Exhibits and Financial Statement Schedules.
GENERAL
Integra, headquartered We are a leading global medical technology company innovating treatment pathways in Princeton, New Jersey, is a world leader in medical technology. The Company was foundedsurgical, neurologic and regenerative care to advance patient outcomes and set new standards of surgical, neurologic and regenerative care. Founded in 1989 with the acquisition of an engineered collagen technology platform used to repair and regenerate tissue. Since then, Integra hastissue, our common stock trades on the Nasdaq Global Select Market (“Nasdaq”) under the symbol “IART.” We have developed numerous product lines from this technology for applications ranging from burn and deep tissue wounds to the repair of dura mater in the brain, as well as nerves and tendons. The Company hasWe have expanded itsour base regenerative technology business to include surgical instruments, neurosurgical products and advanced wound care through a combination of several global acquisitions and product development to meet the evolving needs of itsour customers and impactenhance patient care.
Integra manufacturesOur products are sold in more than 130 countries through a direct sales force as well as distributors and sellswholesalers. We manufacture and sell medical technologies and products in two reportable business segments: Codman Specialty Surgical ("CSS"(“CSS”) and Tissue Technologies ("TT"(“TT”). The CSS segment, which represents approximately two-thirds of our total revenue, consists of market-leading technologies and instrumentation used for a wide range of specialties, such as neurosurgery, neurocritical care and otolaryngology. We are the world leader in neurosurgery and one of the top three providers in instruments used in precision, specialty, and general surgical procedures. Our TT segment generates about one-third of our overall revenue and focuses on three main areas: complex wound surgery, surgical reconstruction, and peripheral nerve repair.
We have key manufacturing and research facilities located in California, Indiana, Maryland, Massachusetts, New Jersey, Ohio, Puerto Rico, Tennessee, Utah, Canada, China, France, Germany, Ireland and Switzerland. We also source most of our handheld surgical instruments and dural sealant products through specialized third-party vendors.
Integra is committed to delivering high quality products that positively impactFollowing the lives of millions of patients and their families. We focus on four key pillarscompletion of our strategy: 1) enabling an execution-focused culture, 2) optimizing relevant scale, 3) advancing innovationstrategic refresh in 2023, we refocused our strategies around five pillars. Of these five pillars, we have identified three core growth drivers: (1) innovating for outcomes, (2) growing internationally, and agility,(3) broadening our impact on care pathways. Our execution of the core growth drivers is enabled by two key levers: (4) driving operational and 4) leadingcustomer excellence and (5) cultivating a high-performance culture. As outlined in customer experience. Wegreater detail below, we believe that by sharpeningthese five pillars will enable us to realize and advance our focus on these areas through improved planning and communication, optimization of our infrastructure, and strategically aligned tuck-in acquisitions, we can build scale, increase competitiveness and achieve our long-term goals.integrated growth strategy.
To this end, the executive leadership team has established the following key priorities aligned to the following areas of focus:five pillars:
Strategic AcquisitionsInnovating for Outcomes. An important part of the Company'sIntegra’s growth strategy is pursuing strategic transactionsintroducing new products to strengthen and licensing agreementsexpand our portfolio, including via acquisitions. For example, we entered into a stock purchase agreement to acquire Acclarent, Inc. (“Acclarent”) from Ethicon, Inc., a subsidiary of Johnson & Johnson in December 2023. Acclarent is an innovator and market leader in ear, nose and throat (“ENT”) procedures and we believe that increase relevant scalethe acquisition of Acclarent will provide Integra with the opportunity to become a leading provider of ENT products and technologies. Furthermore, we believe that, owing to the ENT segment being an anatomical adjacency to neurosurgery, the acquisition will allow Integra to deliver future innovation both within the ENT segment and across our other CSS technology platforms. Additionally, we seek clinical evidence to support regulatory approval and strong reimbursement of our product portfolio around the world, including new indications for existing technologies. For example, in 2021, we filed a pre-market approval (“PMA”) application for a specific indication for Surgimend® in the clinical areasuse of post-mastectomy breast reconstruction. In 2022, we acquired SIA, which is also pursuing a pre-market approval for DuraSorb for use in which Integra competes. During 2021, the Company acquired ACell Inc. ("ACell"implant-based breast reconstruction (“IBBR”), an innovative regenerative medicine company specializingand in June 2023 we completed enrollment in the manufacturing of porcine urinary bladder extracellular matrices. This acquisition not only expandedDuraSorb U.S. IDE clinical study for two-stage breast reconstruction; the Company’s product offering of regenerative technologies, but itprimary follow-up period is one year after device implantation. We hope to obtain FDA approvals for both products in 2025. We also supported the Company’s long-term growth and profitability strategy as this product line has a financial profile similar to Integra’s other regenerative tissue products. All critical components of ACell have been integrated into the Company’s TT segment. See Note 4, Acquisitions and Divestitures, to the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K) for additional details. In 2021, we continued to advance the development of pioneering neurosurgical technologies from our 2019 acquisitions, Arkis Biosciences, Inc. and Rebound Therapeutics Corporation.
Portfolio Optimization and New Product Introductions. We are investing in innovative product development to drive a multi-generational pipeline for our key product franchises. Our product development efforts span across our key global franchises focused on potential for significant returns on investment. In addition to new product development, we are funding studies to gather clinical evidence to support launches, ensure market access and improve reimbursement for existing products. In addition to acquisitions and organic reinvestment, we continually look to optimize our portfolio towards higher growth and higher margin businesses. As such, we may opportunistically divest businesses or discontinue products where we see limited runway for future value creation in line with our aspirations due in part to changes in the market, business fundamentals or the regulatory environment.
In January 2021, we completed the saleexpansion of our Extremity Orthopedics business to Smith & Nephew USD Limited ("Smith & Nephew"), a subsidiaryproduct offerings. In 2023 we launched the CUSA® Clarity Tips for use in surgical procedures requiring the controlled fragmentation, emulsification and aspiration of Smith & Nephew plc, for approximately $240 millionbone as well as in cash. This transaction enables us to increase our investments in our core neurosurgery and tissue technologies businesses and fund pipeline opportunities to expand our addressable markets to strengthen our existing leadership positions in these segments and drive future growth. See Note 4, Acquisitions and Divestitures, to the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K) for details.
Commercial Channel Investments. Investing in our sales channels is a core part of our strategy to create specialization and greater focus on reaching new and existing customers and addressing their needs. To support our commercial efforts in Tissue Technologies, we expanded our two-tier specialist model to increase our presence in focused segments by creating a virtual selling organization to help serve the evolving needs of our customers. In addition, we continue to build upon our leadership brands across our product franchises in both CSS and TT to engage customers through enterprise-wide contracts with leading hospitals, integrated delivery networks and global purchasing organizations in the United States. Internationally, we have increased our commercial resources significantly in key emerging markets and are making investments to support our sales organization and maximize our commercial opportunities. These investments in our international sales channel position us well for expansion and long-term growth.laparoscopic liver surgery.
Customer ExperienceGrowing Internationally: Over the years, we have been significantly expanding our global footprint through investments in our commercial organization, the expansion and development of international markets and new product introductions. As part of our In-China-For-China strategy, we continue the build out of our assembly capabilities in our new facility in Suzhou, China. Several new products were introduced in select international markets in 2023, including MicroMatrix® and Certas Plus® Programmable Valve which were launched in Europe, and CUSA Clarity Laparoscopic tip which was launched in Australia, New Zealand, Japan, Canada, South Africa and Israel. In addition, DuraGen Secure, received approval in Japan, while DuraGen Plus, an absorbable and sutureless collagen onlay indicated as a dura substitute for the repair of dura mater, was approved in China.
Broadening Impact on Care Pathways. We aspireseek ways to be ranked asdevelop products and technologies that impact the lives of patients, starting with the journey that a best-in-class providerpatient takes from diagnosis and treatment planning to surgery and postoperative care. We are committedwell-established in acute care in the hospital setting and continue to strengthenleverage that strong position to grow in this segment and shape treatment pathways into preoperative care and additional sites of care.
Driving Operations and Customer Excellence. We have been making investments to build more responsive and scalable processes, enhance the reliability of our relationships with all customers. Wesupply chain, and drive productivity initiatives to further supply and lower costs. Additionally, we continue to invest in technologies, systems and processes to enhance the customer experience. In 2023, we continued to invest in our capacity expansion. This includes ongoing projects of transferring our Boston manufacturing to a new location in Braintree, Massachusetts., validating manufacturing processes in our manufacturing facility in Plainsboro, New Jersey and increasing cleanroom capacity in our Memphis, Tennessee location.
Cultivating a High-Performance Culture. In seeking to sustain a culture of excellence and accountability, we have focused on employee empowerment and agility and building a diverse and inclusive workplace. These efforts resulted in our being named in several best workplace lists globally in 2023. Additionally, we launched digital toolshave been making further strides in advancing our environmental, social and programs, resources and virtual product traininggovernance ("ESG") agenda to drive continued customer familiarity withsustainability across the organization and recently published our growing portfoliosecond annual ESG report in the third quarter of medical technologies globally.2023. For more information on our ESG strategy, goals, performance, and achievements, please visit “Our Company—ESG Report” at https://www.integralife.com/esg-report. Information on our website is not incorporated by reference herein and is not part of this Annual Report on Form 10-K.
ClinicalNew Product Introductions and ProductResearch and Development ActivitiesUpdates
We continue to invest in collecting clinical evidence to support the Company'sour existing products and new product launches, and to ensure that we obtain market access for broader and more cost-effective solutions. In each area,
Electromechanical Technologies and Instrumentation. The CSS business consists of a broad portfolio of market-leading brands, such as Codman®, DuraGen®, DuraSeal®, CUSA®, Mayfield®,Bactiseal®, and Certas® Plus,which are used for the management of multiple disease states, including brain tumors, traumatic brain injury, hydrocephalus and other neurological conditions. The growth in this business in recent years has been fueled by geographic expansion and new product registrations in markets, such as China, Japan, and Europe, which we expect to continue in the near-to-long term. Because our electromechanical products and instruments address significant needs in surgical procedures and limit uncertainty for surgeons, we continue to benefit frominvest in registrations, clearances, and approvals for new indications and next generation improvements to our market-leading products. We have several active programs focused on life cycle management and innovation for capital and disposable products launched overin our portfolio. Our product development efforts are focused on core clinical applications in cerebrospinal fluid ("CSF") management, neuro-critical care monitoring, minimally invasive instruments and electrosurgery and ultrasonic medical technologies, as well as our ambition to transform the past several years, including our new electrosurgery generatorstandard of care in neurosurgery with product advancements in minimally invasive surgery ("MIS") and irrigator system, an innovative customer-centric toolkit for our Certas Plus Programmable Valve along with additional shunt configurations. In Japan, we are experiencing strong growth as a resultthe surgical management of intracerebral hemorrhage ("ICH"). Our lighting franchise is among the successful launch of DuraGen in mid-2019, which is the first and only collagen xenograft approved for use as a dural substitutemost dynamic in the country. industry.
We are focused on the development of core clinical applications in our electromechanical technologies portfolio. Also, weWe continue to update our CUSA Clarity platform by incorporating new ultrasonic handpiece surgical tips and integrated electrosurgical capabilities. In 2022, we made progress to several enhancements to our CUSA Clarity Tissue Ablation System. The extended laparoscopic tip was launched in the U.S. to enhance laparoscopic liver procedures. In addition, a single-sided bone tip received 510(k) clearance from the FDA. Commercial launch was completed successfully in early 2023. In August 2023, we launched a modified 23 kHz CUSA Electrosurgery Module (CEM) for Clarity handpieces that can be used with additional electrosurgery generators. We continue to work with several instrument partners to bring new surgical instrument platforms to the market.
In the third quarter of 2021, our CereLink ICP Monitor System was launched in the U.S. and Europe. CereLink provides enhanced accuracy, usability and advanced data presentation that provides clinicians with uncompromised, advanced continuous ICP monitoring that until now, has not been available when treating patients with traumatic brain injuries.
WeThroughout 2023 we also continued to advance the early-stage technology platforms we acquired in 2019. Through the acquisition of Arkis Biosciences, Inc. ("Arkis") we added a platform technology, CerebroFlo®CerebroFlo® external ventricular drainage ("EVD"), catheter with Endexo®Endexo® technology, a permanent additive designed to reduce the potential for catheter obstruction due to thrombus formation. The CerebroFlo EVD Cathetercatheter has demonstrated an average of 99% less thrombus accumulation onto its surface, in vitro, compared to a market leading EVD catheter. In 2019,Our work to combine our Bactiseal® antimicrobial technology with the Endexo anti-occlusive technology continues to progress for both a silicone-based hydrocephalus and EVD project.
Throughout 2023, we also acquiredcontinued to advance our innovation from the Rebound Therapeutics a Company that specializedCorporation ("Rebound Therapeutics"), which was acquired in 2019. Rebound Therapeutics specializes in a single-use medical device, known as the Aurora Surgiscope, which is the only tubular retractor system designed for cranial surgery with an integrated access channel, camera and lighting. The 9mm Surgiscope received 510(k) clearance from the FDA in the fourth quarter of 2023.
In the third quarter of 2021, we conductedlaunched our CereLink ICP Monitor System in the U.S. and Europe and continued the global rollout in the first half of 2022. On August 18, 2022, the Company, after consultation with the FDA and other regulatory authorities outside of the United States, initiated an immediate voluntary global product removal of all CereLink® intracranial pressure monitors. We believe that the out-of-range readings are principally caused by a combined interaction of electrical noise (originating from sources such as electrical components in the device, other devices set up near the CereLink Monitor, and the hospital power grid) and an electrical potential difference between the patient and monitor. We submitted a traditional 510(k) submission to the FDA on September 15, 2023 as a result of customer reports about monitors whose pressure readings were out of range. We have received 510(k) clearance from the FDA on February 4th, 2024. We plan to resume shipments of CereLink monitors in the U.S. in the first quarter of 2024. Shipments resumed in international markets with a limited clinical launchrelease in the third quarter of 2023.
Regenerative Technologies.We were the Aurora Surgiscopefirst company to receive an FDA claim for useregeneration of dermal tissue and are a world leader in minimally invasive neurosurgeryregenerative technology. Our regenerative technology development program applies our expertise in bioengineering to a range of biomaterials including natural materials such as purified collagen, intact human or animal tissues, honey as well as initiated a registry called MIRROR to collect data on earlyresorbable synthetic polymers with our DuraSorband DuraSealproduct lines. These unique product designs are used for neurosurgical and reconstructive surgical intervention using this sameapplications, as well as dermal regeneration, including the healing of chronic and acute wounds, tendon and nerve repair. Our regenerative technology platform for the treatment of ICH.
Withinincludes our TT segment, during 2020, we announced positive clinical and economic data onlegacy Integra® Bilayer Wound Matrix ("IBWM") in complex lower extremity reconstruction based on two retrospective studies recently published in Plastic and Reconstructive Surgery, the official journal of the American Society of Plastic Surgeons. As surgeons look for ways to efficiently and effectively repair and close wounds, IBWM helps address the efficiency needed in operating rooms by reducing both the operating time and costs to hospitals and patients. In 2021, we completed one of the largest diabetic foot ulcers ("DFU"), randomized controlled trials of the PriMatrix® Dermal Repair ScaffoldRegeneration Template ("IDRT") products and complementary technologies that we have acquired. Our collagen manufacturing capability, combined with our history of innovation, including our launch of NeuraGen 3D, provides us with strong platform technologies for multiple indications.
In the managementsecond quarter of DFU. This multi-center study enrolled more than 225 patients2023, after consultation with chronic DFU's over the courseFDA, The Company initiated a voluntary global recall of 12-week treatmentsall products manufactured at the Boston facility, including Primatrix®, Surgimend®, Revize™, and 4-week follow-up phases. The resultsTissueMend™, distributed between March 1, 2018 and May 22, 2023.
In the third quarter of this study, which was published2021, we filed a PMA application for a specific indication for Surgimend® in the Journal of Wound Care, demonstrated that PriMatrix plus standard of care ("SOC") consisting of sharp debridement, infection elimination, use of dressingspost-mastectomy breast reconstruction. In 2022, we acquired SIA, which is also PMA for DuraSorb with IBBR, and offloading was significantly more likelyin June 2023 we completed enrollment in the DuraSorb U.S. IDE clinical study for two-stage breast reconstruction; the primary follow-up period is one year after device implantation. By offering two distinct product solutions, we believe we have the opportunity to achieve complete wound closure compared with SOC alone, withbuild a median numberleading position in the IBBR market. We hope to obtain FDA approvals in 2025.
Additionally, in 2022 we launched NeuraGen 3D Nerve Guide Matrix, a resorbable implant for repair of one applicationperipheral nerve discontinuities and engineered to create an optimized environment for nerve regeneration. Following the completion of design control activities in 2022, we launched both Cytal and MicroMatrix in Europe in 2023. In 2023, the product.Company received 510(k) clearance from the FDA for MicroMatrix® Flex.
COVID-19 Pandemic
During this global crisis, the Company's focus remained on supporting patients, providing customers with life-saving products, and protecting the well-beingAs part of our employees. The global COVID-19 pandemic, together withongoing efforts to remain compliant, the preventative and precautionary measures taken by businesses, communities and governments, has resulted in an unprecedented challenge to the global healthcare industry. In response to the pandemic, we acted swiftly by implementing protocols to ensure continuity of our manufacturing and distribution sites around the world and to provide for the safety of our employees.
The COVID-19 pandemicCompany continues to have widespread and unpredictable impacts andwork towards European Union Medical Device Regulation ("EU MDR") certifications. In 2023 the Company has continued to managereceived EU MDR certification in the risks in this uncertain environment. We remain confident that the underlying markets in whichCSS segment for Hakim Programmable Valves, Certas Plus without Bactiseal catheters, and DuraSeal Dural. Additionally, the Company competes remain attractive. We also remain focused on managing the business for the long-term. The Company's adaptability and resiliencyhas received EU MDR certification in the face of this unprecedented crisis is made possible in part by prior investments in technology infrastructureTT segment for IDRT and operations, as well as our talented and committed global workforce.BioPatch.
Capital markets and worldwide economies have also been significantly impacted by the COVID-19 pandemic, and it is possible that it could cause a local and/or global economic recession. Any such economic recession could have a material adverse effect on the Company's long-term business as hospitals curtail and reduce capital as well as overall spending. The COVID-19 pandemic and local actions, such as “shelter-in-place” orders and restrictions on travel and access to our customers or temporary closures of our facilities or the facilities of our suppliers and their contract manufacturers, disruption and/or higher costs to the Company’s supply chain, staffing shortages in hospitals and labor constraints in our facilities, could further impact our sales and our ability to ship our products and supply our customers. Any of these events could negatively impact the number of surgical and medical intervention procedures performed and have a material adverse effect on our business, financial condition, results of operations, or cash flows.
FDA Matters
On August 18, 2022, we, after consultation with the FDA and other regulatory authorities outside of the United States, initiated an immediate voluntary global product removal of all CereLink intracranial pressure monitors as a result of customer reports about monitors whose pressure readings were out of range. We manufacturebelieve that the out-of-range readings are principally caused by a combined interaction of electrical noise (originating from sources such as electrical components in the device, other devices set up near the CereLink Monitor, and distribute products derivedthe hospital power grid) and an electrical potential difference between the patient and monitor. These out-of-range readings have occurred at a low incidence rate and at a limited number of sites; however, out of an abundance of caution, we removed all CereLink monitors from human tissue for whichthe field.
We submitted a traditional 510(k) premarket notification to the FDA has specific regulations governing human cells, tissueson September 15, 2023 and cellular and tissue-based products ("HCT/Ps"). An HCT/P isreceived 510(k) clearance on February 4th, 2024 from the FDA. The submission included design changes to remedy the previously-observed issues identified above. We plan to resume shipments of CereLink monitors in the U.S. in the first quarter of 2024. We resumed shipments in international markets with a product containing or consistinglimited release in the third quarter of human cells or tissue intended for transplantation into a human patient. Refer to Item 1. 2023.
Business and Item 1A. Risk Factors for further details around these FDA regulations and their potential effect on the Company's portfolio of morselized amniotic material-based products as well as the impact on consolidated revenues.
On March 7, 2019, TEI Biosciences, Inc. ("TEI") a, one of our wholly-owned subsidiary of the Companysubsidiaries, received a Warning Letter (the “Warning“2019 Warning Letter”), dated March 6, 2019, from the FDA. The warning letter2019 Warning Letter related to quality systems issues at TEI's manufacturing facility located in Boston, Massachusetts. The Boston facility manufactures extracellular bovine matrix products in our Tissue Technologies segment that are sold both in wound reconstruction and care and in private label channels. The letter resulted from an inspection held at that facility in October and November 2018 and did not identify any new observations that were not already provided in the Form 483 that followed the inspection. The CompanyWe submitted itsour initial response to the FDA2019 Warning Letter on March 28, 2019 and providesprovide regular progress reports to the FDA as to its corrective actions and, since the conclusion of the inspection, has undertaken significant efforts to remediate the observations and continues to do so.actions. On October 28, 2021, the FDA initiated an inspection of the facility and at the conclusion of the inspection, issued aan FDA Form 483 on November 12, 2021 (the "2021 Form 483"). The CompanyWe provided an initial response to the inspectional observationsinspection observations. On March 1, 2023, the FDA commenced an inspection of the Boston facility, and will continueissued an FDA Form 483 at the conclusion of this inspection (the “2023 Form 483”). In May 2023, after consultation with the FDA, the Company initiated a voluntary recall of products manufactured in the Boston facility distributed between March 1, 2018 and May 22, 2023, and extended the temporary halt of manufacturing at the facility to provide responses to FDA. Theimplement additional detection and quality controls. On July 19, 2023, TEI received a Warning Letter, dated July 17, 2023, from the FDA related to quality system issues at the Boston facility (the “2023 Warning Letter”). The 2023 Warning Letter did not identify any new observations that had not already been provided in the 2023 Form 483. The Company has submitted an initial response to the FDA for both the 2023 Form 483 and the 20212023 Warning Letter. We are committed to resolving the matters identified in the Warning Letters and Form 483483s and are continuing our significant efforts to remediate the observations.
Following implementation of upgraded Good Laboratory Practices and expertise and a standardization of corrective and preventative action processes and governance, the Company resumed manufacturing at its Boston facility in the fourth quarter of 2023. We anticipate submitting a final external audit to the FDA by the end of the first quarter of 2024 with commercialization targeted for the second half of 2024.
Although the Warning Letters do not restrict the Company’s ability to manufacture or ship products or require the recall of any products, nor do they restrict our ability to seek FDA 510(k) clearance of products. The Warning Letter states that requests for Certificates to Foreign Governments would not be granted. However, due to our monthly progress reports, the FDA agreed to issue Certificates to Foreign Governments for the products, manufactured at TEI due to substantial progress and the length of time it takes to resolve the Warning Letter. Additionally, premarket approval applicationsPMAs for Class III devices to which the Quality Systemquality system regulation violations are reasonably related will not be approved until the violations have been corrected. The TEI Boston facility manufactures extracellular bovine matrix products.addressed. We cannot give any assurances that the FDA will be satisfied with our response to the Warning Letterissues identified by the FDA or as to the expected date of the resolution of the matters included in the letter.such issues. Until the issues cited inby the letterFDA are resolved to the FDA’s satisfaction, the FDA may initiate additional regulatory action without further notice. Any adverse regulatory action, depending on its magnitude, may restrict us from effectively manufacturing, marketing and selling our products and could have a material adverse effect on our business, financial condition and results of operations.
We continue to work with our customers in wound reconstruction and care and in private label as we move toward commercialization in the second half of 2024. Revenues of products manufactured in the TEI Boston facility for the year ended December 31, 20212022 were approximately 4.7%5.3% of consolidated revenues.
ACQUISITIONS & DIVESTITURES
Divestiture
On January 4, 2021, the Company completed its sale of its Extremity Orthopedics business to Smith & Nephew. The transaction included the sale of the Company's upper and lower Extremity Orthopedics product portfolio, including ankle and shoulder arthroplasty and hand and wrist product lines. The Company received an aggregate purchase price of $240.0 million from Smith & Nephew and concurrently paid $41.5 million to the Consortium of Focused Orthopedists, LLC ("CFO"), effectively terminating the licensing agreement between Integra and CFO relating to the development of shoulder arthroplasty products. The Company recognized a gain of $41.8 million in connection with the sale that is presented in "Gain from the sale of business" in the consolidated statement of operations for For the year ended December 31, 2021. See Note 4, Acquisitions and Divestitures2023, due to the Boston recall, the Company recorded a $18.7 million provision for product returns, as a reduction of net revenue. Of this amount, $9.9 million was credited to customers in the Notesyear ended December 31, 2023. The Company also recorded a $24.6 million write off of inventory that was no longer able to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K) for details.be sold.
ACQUISITIONS & DIVESTITURES
Acquisitions
Our growth strategy includes the acquisition of businesses, assets or products lines to increase the breadth of our offerings and the reach of our product portfolios and drive relevant scale to our customers. As a result of several acquisitions from 2019 through 2021, in 2022, our financial results for the year ended December 31, 20212023 may not be directly comparable to those of the corresponding prior-year periods. See Note 4, Acquisitions and Divestitures, of the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for a further discussion.
ACellSurgical Innovation Associates, Inc.
On January 20, 2021, the Company acquired ACell, Inc.December 6, 2022, we completed its acquisition of SIA for an acquisition purchase price of $306.9 million plus$51.5 million. In addition to the purchase price, the acquisition includes two separate contingent consideration obligations of up to $100 million, that may be payable uponconsiderations payments, which are dependent on 1) achieving certain revenue-based performance milestones in 2022, 2023, 2024, and 2025. ACell2025 (up to $50M in additional payments), as well as 2) the approval by the FDA of the PMA application for DuraSorb for certain uses by certain timing targets (up to $40M in additional payments). On June 28, 2023, we announced the completion of patient enrollment in the DuraSorb U.S. IDE clinical study for two-stage breast reconstruction. Prior to our acquisition, SIA was a privately-held company that offeredwhose core technology, DuraSorb, is a portfoliofully resorbable scaffold of regenerative productsa globally accepted polymer, cleared for complex wound management, including developinguse in hernia repair, abdominal wall, and commercializing products based on MatriStem Urinary Bladder Matrix ("UBM"), a technology platform derived from porcine urinary bladder extracellular matrix.other soft tissue reinforcement. DuraSorb sales are reported within Integra’s TT segment as part of its Wound Reconstruction and Care franchise. See Note 4, Acquisitions and Divestitures of, to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for details.
Acclarent Inc.
In December 2023, we entered into a definitive agreement to acquire Acclarent, Inc. from Ethicon, Inc., a Johnson & Johnson MedTech company for $275 million in cash at closing, subject to customary purchase price adjustments, and an additional $5 million upon the achievement of certain regulatory milestones. Acclarent is an innovator and market leader in ENT procedures and we believe that the acquisition of Acclarent will provide Integra with the opportunity to become a leading provider of ENT products and technologies. Furthermore, we believe that, owing to the ENT segment being an anatomical adjacency to neurosurgery, the acquisition will allow Integra to deliver future innovation both within the ENT segment and across our other CSS technology platforms.
Divestitures
On August 31, 2022, the Company completed the sale of its TWC business to Gentell, Inc. (“Gentell”) for $28.8 million, which consists of $27.8 million in cash plus $1.0 million in contingent consideration which may be received upon achieving certain revenue-based performance milestones two years after the closing date. The proceeds from the sale of the TWC business of $27.8 million is presented in the consolidated statement of cash flows net of cash transferred of $3.5 million and other transaction fees. The transaction included the sale of the Company's TWC products, such as sponges, gauze and conforming bandages, and certain advanced wound care dressings, such as supportive, calcium alginate, hydrogel, and foam dressings. In connection with the sale, the Company recognized $0.6 million as a gain from the sale of business in the consolidated statement of operations for the fiscal year ended December 31, 2022. The transaction is subject to final working capital adjustments. See Note 4, Acquisitions and Divestitures, to the Notes to Consolidated Financial Statements (Part IV, Item 15 of this Form 10-K) for details.
Arkis BioSciences Inc.
On July 29, 2019, the Company acquired Arkis BioSciences Inc. ("Arkis") for an acquisition purchase price of $30.6 million (the "Arkis Acquisition") plus contingent consideration of up to $25.5 million, that may be payable based on the successful completion of certain development and commercial milestones. The Company estimated the fair value of the contingent consideration to be $13.1 million at the acquisition date. The Company estimated fair value of the contingent consideration as of December 31, 2021 to be $15.1 million. The Company recorded $3.7 million in accrued expenses and other current liabilities and $11.4 million in other liabilities at December 31, 2021 in the consolidated balance sheets of the Company. Arkis was a privately-held company that marketed the CerebroFlo external ventricular drainage ("EVD") catheter with Endexo technology, a permanent additive designed to reduce the potential for catheter obstruction due to clotting. See Note 4, Acquisitions and Divestitures of the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K) for details.
Rebound Therapeutics Corporation
On September 9, 2019, the Company acquired Rebound Therapeutics Corporation (“Rebound”), developers of a single-use medical device known as the Aurora which enables minimally invasive access, using optics and illumination, for visualization, diagnostic and therapeutic use in neurosurgery (the “Rebound transaction”). Under the terms of the Rebound transaction, the Company made an upfront payment of $67.1 million and committed to pay up to $35.0 million of contingent development milestones upon achievement of certain regulatory milestones. The acquisition of Rebound was primarily concentrated in one single identifiable asset and thus, for accounting purposes, the Company concluded that the acquired assets did not meet the accounting definition of a business. The initial payment was allocated primarily to Aurora, resulting in a $59.9 million in-process research and development ("IPR&D") expense. The balance of approximately $7.2 million, which included $2.1 million of cash and cash equivalents and a net deferred tax asset of $4.2 million, was allocated to the remaining net assets acquired. The deferred tax asset primarily resulted from a federal net operating loss carryforward. See Note 4, Acquisitions and Divestitures of the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K) for details.
OPTIMIZATION AND INTEGRATION ACTIVITIES
As a result of our ongoing acquisition strategy and significant growth in recent years, in 2023 we have undertaken cost-saving initiatives to consolidate manufacturing operations, distribution facilities and transfer activities, implement a common ERP system, eliminate duplicative positions, realign various sales and marketing activities, and expand and upgrade production capacity for our regenerative technology products. These efforts are expected to continue and while we expect a positive impact from ongoing restructuring, integration, and manufacturing transfer and expansion activities, such results remain uncertain. In support of our continued focus on product margins we closed a manufacturing facility located in France in 2022, and transferred production to our existing Switzerland facility. In addition to closing the manufacturing facility, we outsourced certain transactional back-office finance and customer service activities to enhance customer quality, build scale for future growth, and capture cost efficiencies.
RESULTS OF OPERATIONS
Executive Summary
Net income for the year ended December 31, 20212023 was $169.1$67.7 million, or $1.98$0.84 per diluted share, compared to $133.9$180.6 million, or $1.57$2.16 per diluted share for the year ended December 31, 2020.2022. The increasedecrease in net income for the year ended December 31, 2021,2023, was primarily driven by higher revenues across most franchises driven by continued recovery in surgical procedure volumesimpacts from prior year COVID-19 pandemic levels.the Boston recall. This was partially offset by higher operating expenses as costs continued to normalize after 2020 cost reduction actions. Within non-operating income and expense, the Company benefited from lower interest expense, higher other incomeincludes inventory write-offs of $24.6 million, and a gainprovision for product returns of $41.8$18.7 million as a result offor the sale of its Extremity Orthopedics business to Smith & Nephew. The Company also had a net tax benefit in 2020 due to the impact of the intra-entity transfer of certain intellectual property which resulted in the recognition of a deferred tax benefit in the amount of $59.2 million.year ended December 31, 2023.
Special Charges
Income before taxes includes the following special charges:
| | | | Years Ended December 31, | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | Dollars in thousands | 2023 | | 2022 |
Acquisition, divestiture and integration-related charges (1) | Acquisition, divestiture and integration-related charges (1) | $ | (11,712) | | | $ | 32,906 | |
| Structural optimization charges | Structural optimization charges | 20,385 | | | 15,363 | |
Boston recall expenses(2) | |
| EU medical device regulation | EU medical device regulation | 24,375 | | | 9,372 | |
| Discontinued product lines charges | 377 | | | 6,342 | |
| Expenses related to debt refinancing | — | | | 6,168 | |
COVID-19 pandemic related charges (2) | — | | | 3,482 | |
| Convertible debt non-cash interest expense | — | | | 15,415 | |
| EU medical device regulation | |
| EU medical device regulation | |
| | Total | Total | 33,425 | | | 89,048 | |
| | Total | |
| | Total | |
(1) The Company completed its sale of its Extremity Orthopedics business and recognized a gain of $41.8 million for the year ended December 31, 2021 which was partially offset by other acquisition, divestiture and integration-related charges. See Note 4, Acquisitions and Divestitures of the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for details.
(2) Charges relate to business interruptionsThis includes inventory write-offs and costs associated with the COVID-19 pandemic which impacted the Company's operations globally, partially offset by Coronavirus government relief programs.idle capacity charges.
The items reported above are reflected in the consolidated statements of operations as follows:
| | | | Years Ended December 31, | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | Dollars in thousands | 2023 | | 2022 |
| Cost of goods sold | Cost of goods sold | $ | 32,334 | | | $ | 34,557 | |
| Research and development | Research and development | 17,487 | | | 3,163 | |
| Selling, general and administrative | Selling, general and administrative | 31,013 | | | 29,745 | |
Interest expense (1) | — | | | 21,583 | |
| Gain from the sale of business | Gain from the sale of business | (41,798) | | | — | |
| Other (income) expense | Other (income) expense | (5,611) | | | — | |
| Total | Total | 33,425 | | | 89,048 | |
(1) Upon adoption of ASU No. 2020-06, the Company will no longer incur non-cash interest expense for the amortization of debt discount. See Note 1, Basis of Presentation of the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K), for details.
We typically define special charges as items for which the amounts and/or timing of such expenses may vary significantly from period to period, depending upon our acquisition, divestiture, integration and restructuring activities, and for which the amounts are non-cash in nature, or for which the amounts are not expected to recur at the same magnitude. We believe that given our ongoing strategy of seeking acquisitions, our continuing focus on rationalizing our existing manufacturing and distribution infrastructure and our continuing review of various product lines in relation to our current business strategy, some of the special charges discussed above could recur with similar materiality in the future.
We believe that the separate identification of these special charges provides important supplemental information to investors regarding financial and business trends relating to our financial condition and results of operations. Investors may find this information useful in assessing comparability of our operating performance from period to period, against the business model objectives that management has established, and against other companies in our industry. We provide this information to investors so that they can analyze our operating results in the same way that management does and to use this information in their assessment of our core business and valuation of Integra.
Revenues and Gross Margin
Our revenues and gross margin on product revenues were as follows:
| | | |
| | | | Years Ended December 31, | |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | |
| Dollars in thousands | |
| Dollars in thousands | |
| Segment Net Sales | |
| Segment Net Sales | |
| Segment Net Sales | Segment Net Sales | | |
| Codman Specialty Surgical | Codman Specialty Surgical | $ | 1,025,232 | | | $ | 894,831 | | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | |
| Tissue Technologies | |
| Tissue Technologies | |
| Tissue Technologies | Tissue Technologies | 517,216 | | | 477,037 | | |
| Total revenues | Total revenues | 1,542,448 | | | 1,371,868 | | |
| Total revenues | |
| Total revenues | |
| Cost of goods sold | |
| Cost of goods sold | |
| Cost of goods sold | Cost of goods sold | 597,808 | | | 520,834 | | |
| Gross margin on total revenues | Gross margin on total revenues | $ | 944,640 | | | $ | 851,034 | | |
| Gross margin on total revenues | |
| Gross margin on total revenues | |
| Gross margin as a percentage of total revenues | Gross margin as a percentage of total revenues | 61.2 | % | | 62.0 | % | |
| Gross margin as a percentage of total revenues | |
| Gross margin as a percentage of total revenues | |
Revenues
For the year ended December 31, 2021,2023, total revenues increaseddecreased by $170.6$16.1 million, or 12.4%1.0%, to $1,542.4$1,541.6 million from $1,371.9$1,557.7 million during the prior year. DomesticThis decrease was primarily driven by the impact of the Boston recall, which was comprised of $18.7 million return reserve recorded, as well as a decline in revenue of $61.9 million. This decrease is inclusive of an unfavorable foreign currency impact of $6.8 million, as well as a $16.3 million decrease that impacts both domestic and international revenues, related to the divestiture of the TWC business. This also includes an increase of $9.8 million related to the SIA acquisition. Excluding the impacts of the Boston recall, foreign currency impact, and TWC divestiture, domestic revenues increased by $117.6$38.4 million or 12.1%, to $1,089.5 million and were 70.6% of total revenues for the year ended December 31, 2021.3.7%. International revenues increased by $53.0$39.4 million or 13.3% to $452.9 million, compared to $399.9 million during 2020.9.6%. The increase in domestic revenues was primarily driven by recovery experienced from the COVID-19 pandemic across most franchises compared to the prior year. Foreign exchange fluctuations had a favorable impact of $9.8 million onincreases in our Instruments and Neurosurgery portfolio. The increase in international revenues for the year.was primarily driven by our Asia Pacific region including China, Japan, and Australia.
In the CSS segment, revenues were $1,025.2$1,059.0 million which was an increase of $130.4$39.4 million, or 14.6%3.9% as compared to the prior-year period. This increase is inclusive of a $6.4 million unfavorable foreign currency impact on revenue. Excluding the impact of foreign currency, the CSS segment revenues increased $45.8 million as compared to the prior year period. This increase was driven primarily by mid single digit growth in both our Neurosurgery and Instruments portfolios as compared to the same period in the prior year. The increase was driven primarily by growth in dural access & repair, CSF management, as well as instruments.
In the TT segment, revenues were $482.6 million, which was a decrease of $55.5 million, or 10.3% as compared to the prior-year period, asinclusive of a result$0.4 million unfavorable foreign currency impact on revenue, a $16.3 million decrease that impacts both domestic and international revenues related to the divestiture of the continued recovery experienced fromTWC business, and a $9.8 million increase related to the COVID-19 pandemic,SIA acquisition. This also includes the impact of the Boston recall of $18.7 million return reserve and $61.9 million decline in revenue. Excluding the launchimpact of our new CereLink™ ICP monitoring system inthese items, the third quarter in U.S. and European markets. The Company saw growth within our Neurosurgery portfolio increasing low double digits primarily due to sales in neuromonitoring, advanced energy and dural access and repair. Sales in our instruments portfolioTT segment increased low double digitsby $32.0 million as compared to the same period in the prior year, driven by order recovery.
In the TT segment, revenues were $517.2 million, which was an increase of $40.2 million, or 8.4% as comparedattributable to the prior-year period. Within TT, our Extremity Orthopedics business was divested on January 4, 2021strong sales in IDRT and the acquisition of ACell was completed on January 20, 2021. Sales in our Wound Reconstruction business, excluding the impact of the divestitureMicroMatrix® and acquisition, increased low double digits. Sales in our Private Label business increased low double digits as a result of continued recovery experienced from the COVID-19 pandemic.
As we look forward to 2022 and beyond, we continue to closely monitor local, regional, and global COVID-19 surges as well as recent variants of the virus for an impact on procedures. The reallocation of hospital resources to treat COVID-19 and staffing shortages may continue to cause a financial strain on healthcare systems and reduce procedural volumes.Cytal®
Gross Margin
Gross margin was $944.6$884.7 million for the year ended December 31, 2021, an increase2023, a decrease of $93.6$85.6 million from $851.0$970.3 million for the same period last year. Gross margin as a percentage of revenues was 61.2%57.4% in 20212023 and 62.0%62.3% in 2020.2022. The decrease in gross margin percentage was due to increased amortizationprimarily associated with technology-based intangible assetsthe Boston recall, which includes inventory write-offs of $24.6 million, a provision for product returns of $18.7 million, and inventory step-up amortization in connection withidle capacity at the acquisitionBoston facility of ACell.$15.0 million for the year ended December 31, 2023.
Operating Expenses
The following is a summary of operating expenses as a percent of total revenues:
| | | | | | | | | | | |
| | Years Ended December 31, |
| | 2021 | | 2020 |
| Research and development | 6.0 | % | | 5.6 | % |
| Selling, general and administrative | 41.3 | % | | 43.3 | % |
| Intangible asset amortization | 1.1 | % | | 2.0 | % |
| Total operating expenses | 48.4 | % | | 50.9 | % |
| | | | | | | | | | | |
| | Years Ended December 31, |
| | 2023 | | 2022 |
| Research and development | 6.8 | % | | 6.5 | % |
| Selling, general and administrative | 42.6 | % | | 39.6 | % |
| Intangible asset amortization | 0.8 | % | | 0.9 | % |
| Total operating expenses | 50.2 | % | | 47.0 | % |
Total operating expenses, which consist of research and development, selling, general and administrative, and intangible asset amortization expenses, increased by $47.7$41.8 million or 6.8%5.7% to $747.4$773.2 million in 2021,2023, compared to $699.7$731.4 million in the prior year. The increase in operating expenses compared to the prior year reflects costs associated with higher employee related costs, increased research and development as well as increased outside spending as revenue recovered. We also benefited from reduced operating expenses from the sale of the Extremity Orthopedics business and cost synergies as a result of the acquisition of ACell.
The Company continues to manage and prioritize its operating costs to increase organic investments that will drive long-term growth including the support of new product development and introductions, clinical studies, geographic expansion and targeted U.S. sales channel expansion.
Research and Development
Research and development expenses for the year ended December 31, 20212023 increased by $15.7$3.0 million as compared to the prior year. This increase in spending resulted from additional spending onexpenses related to the SIA acquisition, new product development and clinical studies and spending related to European Union Medical Device Regulation compliance activities.studies.
Selling, General and Administrative
Selling, general and administrative expenses for the year ended December 31, 20212023 increased by $42.9$40.3 million as compared to the prior year driven primarily due to increased costs associated with the SIA acquisition, and higher employeespend in commercial selling activities. Current year expenses included an increase in the fair value of contingent considerations of $12.9 million, primarily related costs, higher incentive and stock-based compensation, as well as increased outside spending as revenue recovered.to SIA. Prior year expenses included a reduction in the fair value of contingent consideration for ACell, Inc. of $18.1 million.
Intangible Asset Amortization
Amortization expense (excluding amounts reported in cost of product revenues for technology-based intangible assets) in 20212023 was $16.9$12.4 million compared to $27.8$13.9 million in 2020 primarily due to2022, a reduction in amortization expense associated withdecrease resulting from intangible assets sold in conjunction with the sale of the Extremity Orthopedics business during the current year as well as accelerated amortization expense associated with an intangible asset which was recorded in the prior year.TWC divestiture.
We may discontinue certain products in the future as we continue to assess the profitability of our product lines. As our profitability assessment evolves, we may make further decisions about our trade names and incur additional impairment charges or accelerated amortization. We expect total annual amortization expense to be approximately $79.1 million in 2022, $78.4 million in 2023, $77.7$82.7 million in 2024, $77.7$82.7 million in 2025, $77.6$82.5 million in 2026, $80.6 million in 2027, $79.0 million in 2028 and $585.8$481.2 million thereafter.
Non-Operating Income and Expenses
The following is a summary of non-operating income and expenses:
| | | | Years Ended December 31, | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | Dollars in thousands | 2023 | | 2022 |
| Interest income | Interest income | $ | 6,737 | | | $ | 9,297 | |
| Interest expense | Interest expense | (50,395) | | | (71,581) | |
| Gain from sale of business | Gain from sale of business | 41,798 | | | — | |
| Other income, net | Other income, net | 19,307 | | | 4,434 | |
| Total non-operating income and expense | Total non-operating income and expense | $ | 17,447 | | | $ | (57,850) | |
Interest Income
Interest income for the year ended December 31, 2021 decreased2023 increased by $2.6$5.3 million as compared to the same period last year primarily due to the settlement of cross-currency swaps designated as net investment hedges during Q4 2020.higher interest rates.
Interest Expense
Interest expense for the year ended December 31, 2021 decreased2023 increased by $21.2$1.8 million as compared to the same period last year primarily due to incremental borrowing on the elimination of the non-cash interest expense as the result of the adoption ASU 2020-06 and the expenses associated with Amended and Restated Senior Credit Agreement which occurredrevolver in the prior period. See Note 2, Summarysecond half of Significant Accounting Policies2023.
of the Notes to Consolidated Financial Statements (Part II, Item 8 of this Form 10-K) for details in relation to the adoption of ASU 2020-06.
Gain from the saleSale of businessBusinesses
On January 4, 2021,August 31, 2022, the Company completed itsthe sale of its Extremity OrthopedicsTWC business to Gentell and recognized a gain of $41.8$0.6 million in the first quarter of the current year.
Other Income, Net
Other income, net for the year ended December 31, 2021 increased2023 decreased by $14.9$8.3 million, primarily due to income associated with the transition services agreement with Smith & Nephew, favorable impact of foreign exchange in the current year and higherlower Transition Service Agreement ("TSA") income from additional cross currency swaps that were entered into during Q4 2020.our recent divestitures.
Income Taxes
Our effective incomeincome tax rate was 21.2%16.4% and (43.2)%15.6% of income before income taxes in 20212023 and 2020,2022, respectively. See Note 13,12, Income Taxes, in our consolidated financial statements for a reconciliation of the United States federal statutory rate to our effective tax rate. Our effective tax rate could vary from year to year depending on, among other factors, tax law changes, the geographic and business mix and taxable earnings and losses. We consider these factors and others, including our history of generating taxable earnings, in assessing our ability to realize deferred tax assets.
In December 2020, the Company completed an intra-entity transfer of certain intellectual property rights to one of its subsidiaries in Switzerland. While the transfer did not result in a taxable gain; the Company’s Swiss subsidiary received a step-up in tax basis based on the fair value of the transferred intellectual property rights. The Company determined the fair value using a discounted cash flow model based on expectations of revenue growth rates, royalty rates, discount rates, and useful lives of the intellectual property. The Company recorded a $59.2 million deferred tax benefit in Switzerland related to the amortizable tax basis in the transferred intellectual property.
Our effective tax rate could vary from year to year depending on, among other factors, tax law changes, the geographic and business mix and taxable earnings and losses. We consider these factors and others, including our history of generating taxable earnings, in assessing our ability to realize deferred tax assets. We estimate our worldwide effective income tax rate for 20222024 to be approximately 18.4%.19.8%, estimated based on existing tax laws.
At December 31, 2021,2023, the Company had $9.8$12.5 million of valuation allowance against the remaining $190.7$239.6 million of gross deferred tax assets recorded at December 31, 2021.2023. Our deferred tax asset valuation allowance decreasedincreased by $0.1$2.8 million in 2021 and2023, primarily driven by a $3.3 million increase related to the new Swiss tax credit. The valuation allowance for 2022 had remained substantially unchanged in 2020.as compared to 2021. This valuation allowance relates to deferred tax assets for which the Company does not believe it has satisfied the more likely than not threshold for realization.
At December 31, 2021,2023, we had net operating loss carryforwards of $71.7$64.7 million for federal income tax purposes, $26.6$98.4 million for foreign income tax purposes and $39.0$19.2 million for state income tax purposes to offset future taxable income. The federal net operating loss carryforwards decreased during 20212023 due to usage during the use of net operating losses.year. Of the total federal net operating loss carryforwards, $67.5$55.8 million expire through 2037 and $4.1$8.9 million have an indefinite carryforward period. Regarding the foreign net operating loss carryforwards, $26.6$81.0 million expire through 2028 and $17.4 million have an indefinite carryforwardcarryforward period. The state net operating loss carryforwards expire in 2036.
As of December 31, 2021,2023, the Company has not provided deferred income taxes on unrepatriated earnings from foreign subsidiaries as they are deemed to be indefinitely reinvested.reinvested unless there is a manner under which to remit the earnings with no material tax cost. Such taxes would primarily be attributable to foreign withholding taxes and local income taxes when such earnings are distributed. As such, the Company has determined the tax impact of repatriating these earnings would not be material as of December 31, 2021.
GEOGRAPHIC PRODUCT REVENUES AND OPERATIONS
The Company attributes revenues to geographic areas based on the location of the customer. Total revenue by major geographic area consisted of the following:
| | | | Years Ended December 31, | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | Dollars in thousands | 2023 | | 2022 |
| United States | United States | $ | 1,089,526 | | | $ | 971,975 | |
| Europe | Europe | 191,327 | | | 172,689 | |
| Asia Pacific | Asia Pacific | 182,034 | | | 157,174 | |
| Rest of World | Rest of World | 79,561 | | | 70,030 | |
| Total Revenues | Total Revenues | $ | 1,542,448 | | | $ | 1,371,868 | |
The Company generatesWe generate significant revenues outside the U.S., a portion of which are U.S. dollar-denominated transactions conducted with customers that generate revenue in currencies other than the U.S. dollar. As a result, currency fluctuations between the U.S. dollar and the currencies in which those customers do business could have an impact on the demand for the Company'sour products in foreign countries. Local economic conditions, regulatory compliance or political considerations, the effectiveness of our sales representatives and distributors, local competition and changes in local medical practice all may combine to affect our sales into markets outside the U.S.
Domestic revenues increaseddecreased by $117.6$26.1 million for the year ended December 31, 20212023 compared to the same period last year. European sales increaseddecreased by $18.6$5.7 million for the year ended December 31, 20212023 compared to the same period last year. Sales to customers in Asia Pacific increased by $24.9$16.6 million for the year ended December 31, 20212023 compared to the same period last year. The Rest of the World for the year ended December 31, 2021 increased2023 decreased by $9.5$1.0 million compared to the same period last year. The increaseinternational revenues were impacted by a $6.8 million unfavorable foreign exchange impact, with the larger impact in Europe. The decrease in global revenues globally wasis primarily driventhe result of the Boston recall which affected both domestic and international markets. We continue to see growth in our Asia Pacific Market by the continued recovery experienced from the COVID-19 pandemic across all franchises compared to the prior year due to rebound in surgical procedure volumes. Salesleveraging our existing portfolios, specifically CUSA® and DuraGen®, in China Japan Canada, Europe and our indirect markets continue to drive international growth. The Company also benefited from the launch of the CereLink ICP Monitor which occurred during the second half of the 2021.Japan.
LIQUIDITY AND CAPITAL RESOURCES
Working Capital
At December 31, 20212023 and December 31, 2020,2022, working capital was $813.7$751.1 million and $836.2$840.6 million, respectively. Working capital consists of total current assets less total current liabilities as presented in the consolidated balance sheets.
Cash and Marketable Securities
The Company had cash and cash equivalents totaling approximately $513.4$276.4 million and $470.2$456.7 million at December 31, 20212023 and 2020,2022, respectively, which are valued based on Level 1 measurements in the fair value hierarchy. At December 31, 2021,2023, our non-U.S. subsidiaries held approximately $245.2$246.9 million of cash and cash equivalents that are available for use outside the U.S. The Company asserts that it has the ability and intends to indefinitely reinvest the undistributed earnings from its foreign operations unless there is no material tax cost to remit the earnings into the U.S.
Short Term Investments
The Company had short term investments totaling approximately $32.7 million at December 31, 2023 and $0.0 million at December 31, 2022.
Cash Flows
| | | | Year Ended December 31, | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | Dollars in thousands | 2023 | | 2022 |
| Net cash provided by operating activities | Net cash provided by operating activities | $ | 312,427 | | | $ | 203,832 | |
| Net cash used in investing activities | Net cash used in investing activities | (161,443) | | | (68,073) | |
| Net cash used (provided) by financing activities | Net cash used (provided) by financing activities | (98,226) | | | 121,625 | |
| Effect of exchange rate fluctuations on cash | Effect of exchange rate fluctuations on cash | (9,476) | | | 13,871 | |
| Net increase (decrease) in cash and cash equivalents | Net increase (decrease) in cash and cash equivalents | $ | 43,282 | | | $ | 271,255 | |
Cash Flows Provided by Operating Activities
Operating cash flows for the year ended December 31, 2021 increased2023 decreased by $108.6$124.5 million compared to the same period in 2020.2022. Net income after removing the impact of the gain on sale of business and non-cash adjustments increaseddecreased for the year ended December 31, 2021,2023, by approximately $49.1$82.4 million as compared to the same period in 20202022 primarily duedue to lower revenues and inventory write-off attributable to the continuing revenue recovery in the current year as compared to the height of the COVID-19 pandemic in the prior year. The changes in assets and liabilities, net of business acquisitions, increased cash flows from operating activities in the current year by $18.2 million compared to the decrease of $41.3 million for the same period in 2020. The improvement in 2021 working capital is attributable to a decrease in inventory of $5.4 million due to investments in building safety stock made in the prior year where inventory increased by $48.3 million as well as improved sales in 2021.
Operating cash flows for the year ended December 31, 2020 decreased compared to the same period in 2019. Net income after non-cash adjustments increased by approximately $0.8 million to $245.1 million from $245.9 million. Boston recall along with higher selling expenses. The changes in assets and liabilities, net of business acquisitions, decreased cash flows from operating activities by $41.3$81.6 million in the year ended December 31, 20202023 as compared to athe decrease in cash flows of $14.5$39.4 million for the same period in 2019.2022. The decreasechange in 20202023 is mainly attributable to anincreases in inventory.
Operating cash flows for the year ended December 31, 2022 decreased by $48.0 million compared to the same period in 2021. Net income after removing the impact of the gain on sale of businesses and non-cash adjustments increased for the year ended December 31, 2022, by approximately $9.6 million as compared to 2021 primarily due to earnings from higher revenues. The changes in assets and liabilities, net of business acquisitions, decreased cash flows by $39.4 million in 2022 as compared to the increase in cash flows of $18.2 million for the same period in 2021. The change in 2022 is mainly attributable to increases in inventory and accounts receivable. The increase in inventory is due to improvea build up of safety stock of select products. In addition, decreases were also driven by reduced payables offset by decreasesdue to supply chain challenges. The increase in accounts receivable is due to lower revenues and continued collection efforts.increased sales as well as a increase in days sales outstanding.
Cash Flows Used in Investing Activities
During the year ended December 31, 2023, we paid $66.9 millionfor capital expenditures to support operations improvement initiatives at a number of our manufacturing facilities, including our Braintree facility in Boston, and other information technology investments. In addition, we paid $32.7 million related to short-term investments. This was partially offset by $5.4 million of proceeds on cross-currency swaps designated as net investment hedge.
During the year ended December 31, 2021,2022, we paid a net cash amount of $303.9$42.3 million in relation to the acquisition of ACell and received net proceeds of $190.5 million for the sale of the Extremity Orthopedics business. The Company also paid for $48.0 million capital expenditures to support operations improvement initiatives at a number of our manufacturing facilities and other information technology investments.
Duringinvestments, $51.5 million to acquire SIA, as well as the year ended December 31, 2020, we paid $38.9$4.7 million for capital expenditures, most of which were directed to our facilities located in Mansfield, MA; Boston, MA; Memphis, TN; and Princeton, NJ and $25.0 million associated with achieving developmental milestones paidpayment related to the former shareholdersfinal developmental milestone for Rebound Therapeutics Corporation. This was partially offset by the net proceeds from the sale of Rebound.the TWC business of $24.0 million. The proceeds from the sale of the TWC business of $27.8 million is presented net of cash transferred of $3.5 million and other transaction fees. Additionally, the Company also received $4.9 million proceeds on cross-currency swaps designated as net investment hedge.
Cash Flows (Used in) Provided by Financing Activities
Uses of cash from financing activities for the year endedActivitiesDecember 31, 2023primarily related to the purchase of treasury stock of $275.0 million under the accelerated share repurchase agreements that were completed during the year. In addition, the Company had $5.9 million in cash taxes paid in net equity settlements related to the vesting of annual grants. The Company also had repayments of $110.6 million under our Senior Credit Facility (as defined below) and Securitization Facility offset by $165.1 million borrowings under our Senior Credit Facility and Securitization Facility. The Company also had $4.3 million proceeds from the exercise of stock options.
Uses of cash from financing activities for the year ended December 31, 2021 were repayments2022 primarily related to the purchase of $125.5treasury stock of $125.0 million onunder the revolving portion2022 accelerated share repurchase agreement that was completed in the first quarter of our Senior Credit Facility and Securitization Facility. 2022. In addition, the Company had $4.8$24.6 million in cash taxes paid in net equity settlements. These uses weresettlements, $16.8 million of which resulted from the departure of the former chief executive officer of the Company. The Company also had repayments of $148.6 million under our Senior Credit Facility and Securitization Facility offset by $6.8 million proceeds from the exercise of stock options and $25.5$40.8 million borrowings under our Senior Credit Facility and Securitization Facility.
Our principal sources of cash from financing activities for the year ended December 31, 2020 were $515.3 The Company also had $5.5 million in proceeds from the issuanceexercise of Convertible Senior Notes including the call and warrant transactions and $171.5 million borrowing under our Senior Credit Facility and Securitization Facility. These were offset by repayments of $441.0 million on the revolving portion of our Senior Credit Facility and Securitization Facility, $24.3 million in debt issuance costs related to the Amended and Restated Senior Credit Agreement and the issuance of Convertible Senior Notes and $100.0 million in purchases of treasury stock.stock options.
Amended and Restated Senior Credit Agreement, Convertible Senior Notes, Securitization and Related Hedging Activities
See Note 5, Debt, to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for a discussionfurther details of our Amended and Restated Senior Credit Agreement, the 2025 Notes and Securitization Facility and Note 6, Derivative Instruments to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for discussionfurther details of our hedging activities. We are forecasting that sales and earnings for the next twelve months will be sufficient to remain in compliance with our financial covenants under the terms of the February 2020 Amendment and July 2020 Amendment to the Senior Credit Facility.
Share Repurchase Plan
On December 7, 2020, the Board of Directors authorized the Company to repurchase up to $225 million of the Company’s common stock. The program allows the Company to repurchase its shares opportunistically from time to time. The repurchase authorization expires in December 2022. This stock repurchase authorization replaces the previous $225 million stock repurchase authorization, of which $125 million remained authorized at the time of its replacement, and which was otherwise set to expire on December 31, 2021.
For the year ended December 31, 2021, there were no repurchases of the Company’s common stock as part of the share repurchase authorization.
On January 12, 2022, the Company entered into a $125.0 million accelerated share repurchase ("2022 ASR") and received 1.5 million shares of the Company common stock at inception of the 2022 ASR, which represented approximately 80% of the expected total shares under the 2022 ASR. The remaining 20% of the expected total shares is expected to settle in the first half of 2022, upon which additional shares of common stock may be delivered to the Company or, under certain circumstances, the Company may be required to make a cash payment or may elect to deliver shares of our common stock to the 2022 ASR counterparty in each case pursuant to the terms of the 2022 ASR agreement between the Company and the 2022 ASR counterparty. The total number of shares to be delivered or the amount of such payment, as well as the final average price per share, will be based on the volume-weighted average price, less a discount, of the Company's common stock during the term of the transaction. As a result of this transaction, $100.0 million remains available under the $225.0 million stock repurchase authorization.
During the twelve months ended December 31, 2020, the Company repurchased 2.1 million shares of Integra’s common stock as part of the previous share repurchase authorization. The Company utilized $100.0 million of net proceeds from the offering of convertible notes to execute the share repurchase transactions. This included $7.6 million from certain purchasers of the convertible notes in conjunction with the closing of the offering. On February 5, 2020, the Company entered into a $92.4 million accelerated share repurchase ("2020 ASR") to complete the remaining $100.0 million of share repurchase. The Company received 1.3 million shares at inception of the 2020 ASR, which represented approximately 80% of the expected total shares. Upon settlement of the 2020 ASR in June 2020, the Company received an additional 0.6 million shares determined using the volume-weighted average price of the Company's common stock during the term of the transaction.
See Note 8, Treasury Stockof,to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for further details.details of our share repurchase programs.
Dividend Policy
We have not paid any cash dividends on our common stock since our formation. Our Senior Credit Facility limits the amount of dividends that we may pay. Any future determinations to pay cash dividends on our common stock will be at the discretion of the Board and will depend upon our financial condition, results of operations, cash flows and other factors deemed relevant by the Board.
Capital Resources
We believe that our cash and available borrowings under the Senior Credit Facility are sufficient to finance our operations and capital expenditures forover the foreseeable future.next twelve months. Our future capital requirements will depend on many factors, including the growth of our business, the timing and introduction of new products and investments, strategic plans and acquisitions, among others. Additional sources of liquidity available to us include short term borrowings and the issuance of long term debt and equity securities.
Off-Balance Sheet Arrangements
We do not have any off–balance sheet financing arrangements during the year-ended December 31, 20212023 that have or are reasonably likely to have, a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our interests.
Contractual Obligations and Commitments
We will continue to have cash requirements to support seasonal working capital needs and capital expenditures, to pay interest, to service debt, and to fund acquisitions. As part of our ongoing operations, we enter into contractual arrangements that obligate us to make future cash payments.
Our primary obligations include principal and interest payments on the revolving portion and Term Loan component of the Senior Credit Facility, Securitization Facility and Convertible Securities. See Note 5, Debt, to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for details. The Company also leases some of our manufacturing facilities and office buildings which have required future minimum lease payments associated.payments. See Note 11, Leases and Related Party Leases, to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for a schedule of our future minimum lease payments. Amounts related to the Company's other obligations, including employment agreements and purchase obligations were not material.
The Company has contingent consideration obligationobligations related to prior and current year acquisitions and future pension contribution obligations. See Note 10, Retirement Benefit Plans, and Note 15, Commitments and Contingencies to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for details. The associated obligations are not fixed. The Company also has a liability for uncertain tax benefits including interest and penalties. See Note 12, Income Taxes to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for details. The Company cannot make a reliable estimate of the period in which the uncertain tax benefits may be realized.
Employee Termination Benefits
The Company incurred employee termination costs on sales force restructuring costs of $3.4 million and $4.9 million in cost of goods sold, $0.5 million and $1.2 million in selling, general and administrative and $0.3 million and $0.3 million in research and development related to employee terminations associated with a future plant closureactivities in the consolidated statement of operations for the yearsyear ended December 31, 2021 and 2020, respectively.2023. In 2022, the Company incurred employee termination costs on restructuring activities associated with the closure of the manufacturing facility in France. Restructuring costs of $10.2 million were included in accruedaccrued expenses and other current liabilities and $6.4 million were included in other liabilities in the consolidated balance sheet for the year ended December 31, 20212023 and 2020, respectively. 2022. See Note 2, Summary of Significant Accounting Policies, of the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for further details.
CRITICAL ACCOUNTING POLICIES AND THE USE OF ESTIMATES
Our discussion and analysis of financial conditions and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America ("GAAP"). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities, and the reported amounts of revenues and expenses. Significant estimates affecting amounts reported or disclosed in the consolidated financial statements include allowances for doubtful accounts receivable and sales returns and allowances, net realizable value of inventories, valuation of intangible assets including amortization periods for acquired intangible assets, discount rates and estimated projected cash flows used to value and test impairments of long-lived assets and goodwill, estimates of projected cash flows and depreciation and amortization periods for long-lived assets, computation of taxes, valuation allowances recorded against deferred tax assets, the valuation of stock-based compensation, valuation of derivative instruments, valuation of contingent liabilities, the fair value of debt instruments and loss contingencies. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the current circumstances.
As we continue to navigate the COVID-19 pandemic and recent variants of the virus, as well as the adverse impacts to global economic conditions, supply chain and our operations, there may be impact to future estimates including, but not limited to, inventory valuations, fair value measurements, goodwill and long-lived asset impairments, the effectiveness of the Company’s hedging instruments, deferred tax valuation allowances, and allowances for doubtful accounts receivable.
We believe that the following accounting policies, which form the basis for developing these estimates, are those that are most critical to the presentation of our consolidated financial statements and require the more difficult subjective and complex judgments, often because of the need to make estimates about the effect of matters that are inherently uncertain. Because of this uncertainty, actual results could differ from these estimates.
Allowances for Doubtful Accounts Receivable and Sales Returns and Allowances
We evaluate the collectability of accounts receivable based on a combination of factors. The Company recognizes a provision for doubtful accounts that reflects the Company’s estimate of expected credit losses for trade accounts receivable. In circumstances where a specific customer is unable to meet its financial obligations to us, we record an allowance against amounts due to reduce the net recognized receivable to the amount that we reasonably expect to collect. For all other customers, the Company evaluates measurement of all expected credit losses for trade receivables held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. If the financial condition of customers or the length of time that receivables are past due were to change, we may change the recorded amount of allowances for doubtful accounts in the future through charges or reductions to selling, general and administrative expense.
We record a provision for estimated sales returns and allowances on revenues in the same period as the related revenues are recorded. We base these estimates on historical sales returns and allowances and other known factors. If actual returns or allowances differ from our estimates and the related provisions for sales returns and allowances, we may change the provision in the future through an increase or decrease in revenues.
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. We adopted this guidance on January 1, 2020 using a modified retrospective transition method which requires a cumulative-effect adjustment to the opening balance of retained earnings to be recognized on the date of adoption with no change to financial results reported in prior periods. The cumulative-effect adjustment recorded on January 1, 2020 was not material. The adoption of this ASU did not have a significant impact on our consolidated financial statements and related disclosures. Our exposure to credit losses may increase if its customers are adversely affected by changes in healthcare laws, coverage, and reimbursement, economic pressures or uncertainty associated with local or global economic recessions, disruption associated with the COVID-19 pandemic and recent variants of the virus, and other customer-specific factors. Although we have historically not experienced significant credit losses, it is possible that there could be an adverse impact due to customer and governmental responses to the COVID-19 pandemic.
Inventories
Inventories, consisting of purchased materials, direct labor and manufacturing overhead, are stated at the lower of cost (determined by the first-in, first-out method) or net realizable value. At each balance sheet date, we evaluate ending inventories for excess quantities, obsolescence or shelf-life expiration. Our evaluation includes an analysis of historical sales levels by product, projections of future demand by product, the risk of technological or competitive obsolescence for our products, general market conditions, a review of the shelf-life expiration dates for our products, and the feasibility of reworking or using excess or obsolete products or components in the production or assembly of other products that are not obsolete or for which we do not have excess quantities in inventory. To the extent that we determine there are excess or obsolete quantities or quantities with a shelf life that is too near its expiration for us to reasonably expect that we can sell those products prior to their expiration, we adjust their carrying value to estimated net realizable value. If future demand or market conditions are lower than our projections, or if we are unable to rework excess or obsolete quantities into other products, we may record further adjustments to the carrying value of inventory through a charge to cost of product revenues in the period the revision is made. As of December 31, 2023, our reserve for inventory excess and obsolescence is 9% of total inventory on our consolidated balance sheet.
The Company capitalizes inventory costs associated with certain products prior to regulatory approval, based on management's judgment of probable economic benefit. The Company could be required to expense previously capitalized costs related to pre-approval inventory upon a change in such judgment, due to, among other potential factors, a denial or delay of approval by necessary regulatory bodies or a decision by management to discontinue the related development program.
Acquisitions
Results of operations of acquired companies are included in the Company’s results of operations as of the respective acquisition dates. The Company accounts for the acquisition of a business in accordance with ASC Topic 805, Business Combinations (ASC 805) ("ASC Topic 805"). Amounts paid to acquire a business are allocated to the assets acquired and liabilities assumed based on the fair values at the date of acquisition. Any excess of the purchase price over the fair value of the net assets acquired in recorded as goodwill. Transaction costs and costs to restructure the acquired company are expensed as incurred.
Contingent consideration is recorded at fair value as measured on the date of acquisition. The value recorded is based on estimates of future financial projections under various potential scenarios using either a Monte Carlo simulation or the probability-weighted income approach derived from revenue estimates and probability assessment with respect to the likelihood of achieving contingent obligations. Contingent payments related to acquisitions consist of development, regulatory, and commercial milestone payments, in addition to sales-based payments, and are valued using discounted cash flow techniques. Each quarter until such contingent amounts are earned, the fair value of the liability is remeasured at each reporting period and adjusted as a component of operating expenses based on changes to the underlying assumptions. The change in the fair value of sales-based payments is based upon future revenue estimates and increases or decreases as revenue estimates or expectation of timing of payment charges. The estimates used to determine the fair value of the contingent consideration liability are subject to significant judgment and actual results are likely to differ from the amounts originally recorded.
The Company determines the fair value of acquired intangible assets based on detailed valuations that use certain information and assumptions provided by management. The Company allocates any excess purchase price over the fair value of the net tangible and intangible assets acquired to goodwill. Determining the fair value of these intangible assets, acquired as part of a business combination requires the Company to make significant estimates. These estimates include the amount and timing of projected future cash flows, the discount rate used to discount those cash flows to present value, the assessment of the asset’s life cycle, and the consideration of legal, technical, regulatory, economic, and competitive risks. The fair value assigned to other intangible assets is determined by estimating the future cash flows of each project or technology and discounting the net cash flows back to their present values. The discount rate used is determined at the time of measurement in accordance with accepted valuation methodologies.
In our most recent acquisition of SIA, the key areas of judgement relating to the valuation of the acquired definite-lived developed technology intangible assets were the net revenue growth rates, cost of sales, selling and marketing costs, discounts rates, and asset useful life. The key areas of judgement relating to the valuation of the contingent consideration are the inputs to the Monte-Carlo model including revenue-adjusted discount rate, counterpart discount rate, revenue volatility and forecasted revenue, earnings before income taxes and fixed costs. These assumptions were developed with the assistance of a third-party valuation expert.
Acquired IPR&D is recognized at fair value and initially characterized as an indefinite-lived intangible asset, irrespective of whether the acquired IPR&D has an alternative future use. The Company uses the income approach to determine the fair value of developed technology and IPR&D acquired in a business combination. This approach determines fair value by estimating the after-tax cash flows attributable to the respective asset over its useful life and then discounting these after-tax cash flows back to a present value. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each product including net revenues, cost of sales, R&D costs, selling and marketing costs, the appropriate discount rate to select in order to measure the risk inherent in each future cash flow stream, the assessment of each asset’s life cycle, and competitive trends impacting the asset and each cash flow stream. The Company also uses the income approach, as described above, to determine the estimated fair value of certain other identifiable intangible assets including customer relationships, trade names and business licenses. Customer relationships represent established relationships with customers, which provide a ready channel for the sale of additional products and services. Trade names represent acquired company and product names.
IPR&D acquired in a business combination is capitalized as an indefinite-lived intangible asset. Development costs incurred after the acquisition are expensed as incurred. Upon receipt of regulatory approval, the indefinite-lived intangible asset is then accounted for as a finite-lived intangible asset and amortized on a straight-line basis or accelerated basis, as appropriate, over its estimated useful life. If the research and development project is subsequently abandoned, the indefinite-lived intangible asset is charged to expense. IPR&D acquired outside of a business combination is expensed immediately.
Due to the uncertainty associated with research and development projects, there is risk that actual results will differ materially from the original cash flow projections and that the research and development project will result in a successful commercial product. The risks associated with achieving commercialization include, but are not limited to, delay or failure to obtain regulatory approvals to conduct clinical trials, delay or failure to obtain required market clearances, delays or issues with patent
issuance, or validity and litigation.
If the acquired net assets do not constitute a business under the acquisition method of accounting, the transaction is accounted for as an asset acquisition and no goodwill is recognized. In an asset acquisition, the amount allocated to acquired IPR&D with no alternative future use is charged to expense at the acquisition date. Payments that would be recognized as contingent consideration in a business combination are expensedrecognized when probable in an asset acquisition. Refer to Note 4, Acquisitions and Divestitures to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for details.
Valuation of Goodwill
The excess of the cost over the fair value of net assets of acquired businesses is recorded as goodwill. Goodwill is not subject to amortization but is reviewed for impairment at the reporting unit level annually, or more frequently if impairment indicators arise. The Company's assessment of the recoverability of goodwill is based upon a comparison of the carrying value of goodwill with its estimated fair value. Key assumptions used to estimate the fair value of goodwill include the Company's discounts rate and forecasted operating results. The Company had goodwill on the balance sheet of $1.1 billion as of December 31, 2023. The Company reviews goodwill for impairment in the third quarter every year in accordance with ASC Topic 350, Intangibles - Goodwill and Other ("ASC Topic 350"), and whenever events or changes in circumstances indicate the carrying value of goodwill may not be recoverable.
In the second quarter of 2023, due to the Boston recall, as well as the associated drop in the Company's stock price in that quarter, the Company elected to perform a quantitative analysis, using a combination of a discounted cash flow method and guideline public company method for its TT reporting unit. The quantitative test utilized key assumptions of revenue growth rate, a terminal growth rate of 2%, a discount rate of 10%, and the range and application of the company guideline multiples. The Company determined, after performing the quantitative analysis, that the fair value of the goodwill of the reporting unit was not less than the carrying amount, with more than 20% headroom.
During the third quarter of 2023, the Company elected to perform a qualitative analysis for its three reporting units. The Company determined, after performing the qualitative analysis, that there was no evidence that it is more likely than not that the fair value was less that the carrying amounts, therefore, it was not necessary to perform a quantitative impairment test. Refer to Note 7, Goodwill and Other Intangiblesof, to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K) for more information.
Valuation of Identifiable Intangible Assets
The Company tests intangible assets with indefinite lives for impairment annually in the third quarter in accordance with ASC Topic 350. Additionally, the Company may perform interim tests if an event occurs or circumstances change that could potentially reduce the fair value of a indefinite lived intangible asset below its carrying amount. The Company tests for impairment by either performing a qualitative evaluation or a quantitative test. The qualitative evaluation is an assessment of factors, including specific operating results as well as industry, market and general economic conditions, to determine whether it is more likely than not that the fair values of the intangible asset is less than its carrying amount. The Company may elect to bypass this qualitative evaluation and perform a quantitative test. There were no changes to identifiable intangible assets as a result of the Company's assessments.
Product rights and other definite-lived intangible assets are tested periodically for impairment in accordance with ASC Topic 360,Property, Plant and Equipment, ("ASC Topic 360") when events or changes in circumstances indicate that an asset's carrying value may not be recoverable. The impairment test involves comparing the carrying amount of the asset or asset group to the forecasted undiscounted future cash flows. In the event the carrying value of the asset exceeds the undiscounted future cash flows, the carrying value is considered not recoverable and impairment exists. An impairment loss is measured as the excess of the asset's carrying value over its fair value, calculated using discounted future cash flows. The computed impairment loss is recognized in the period that the impairment occurs.
Derivatives
We develop, manufacture, and sell medical devices globally. Our earnings and cash flows are exposed to market risk from changes in interest rates and currency exchange rates. We address these risks through a risk management program that includes the useAs of derivative financial instruments and operate the program pursuant to documented corporate risk management policies. All derivative financial instruments are recognized in the financial statements at fair value in accordance with the authoritative guidance. Under the guidance, for those instruments that are designated and qualify as hedging instruments, the hedging instrument must be designated as a fair value hedge, cash flow hedge, or a hedge of a net investment in a foreign operation, based on the exposure being hedged. The accounting for changes in the fair value of a derivative instrument depends on whether it has been designated and qualifies as part of a hedging relationship and, further, on the type of hedging relationship. Our derivative instruments do not subject our earnings or cash flows to material risk, and gains and losses on these derivatives generally offset losses and gains on the item being hedged. We have not entered into derivative transactions for speculative purposes. From time to time, we may enter into derivatives that are not designated as hedging instruments in order to protectDecember 31, 2023, the Company from currency volatility due to intercompany balances.
All derivative instruments are recognized at their fair values as eitherhas $1.1 billion of identifiable intangible assets, or liabilitiesnet on the balance sheet. We determine the fair value of our derivative instruments, by considering the estimated amount we would receive to sell or transfer these instruments at the reporting date and by taking into account expected forward interest rates, currency exchange rates, the creditworthiness of the counterparty for assets, and our creditworthiness for liabilities. In certain instances, we may utilize a discounted cash flow model to measure fair value. Generally, we use inputs that include quoted prices for similar assets or liabilities in active markets, other observable inputs for the asset or liability, and inputs that are derived principally from, or corroborated by, observable market data by correlation or other means.sheets.
Income Taxes
Since we conduct operations on a global basis, our effective tax rate has and will depend upon the geographic distribution of our pre-tax earnings among locations with varying tax rates. Changes in the tax rates of the various jurisdictions in which we operate affect our profits. In addition, we maintain a reserve for uncertain tax benefits, changes to which could impact our effective tax rate in the period such changes are made. The effective tax rate can also be impacted by changes in valuation allowances of deferred tax assets, and tax law changes.
Our provision for income taxes may change period-to-period based on specific events, such as the settlement of income tax audits and changes in tax laws, as well as general factors, including the geographic mix of income before taxes, state and local taxes and the effects of the Company's global income tax strategies. We maintain strategic management and operational activities in overseas subsidiaries. See Note 12, Income Taxesof, to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K), in our consolidated financial statements for disclosures related to foreign and domestic pretax income, foreign and domestic income tax expense (benefit) and the effect foreign taxes have on our overall effective tax rate.
We recognize a tax benefit from an uncertain tax position only if it is more likely than not to be sustained upon examination based on the technical merits of the position. The amount of the accrual for which an exposure exists is measured by determining the amount that has a greater than 50 percent likelihood of being realized upon ultimate settlement of the position. Components of the reserve are classified as a long-term liability in the consolidated balance sheets. We record interest and penalties accrued in relation to uncertain tax benefits as a component of income tax expense.
We believe that we have identified all reasonably identifiable exposures and that the reserve we have established for identifiable exposures is appropriate under the circumstances; however, it is possible that additional exposures exist and that exposures will be settled at amounts different from the amounts reserved. It is also possible that changes in facts and circumstances could cause us to either materially increase or reduce the carrying amount of our tax reserves.
Our deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and their basis for income tax purposes, and the temporary differences created by the tax effects of capital loss, net operating loss and tax credit carryforwards. We record valuation allowances when it is more likely than not that some portion or all of the deferred tax assets will not be realized. We could recognize no benefit from our deferred tax assets or we could recognize some or all of the future benefit depending on the amount and timing of taxable income we generate in the future.
We intend to indefinitely reinvest substantially all of our foreign earnings in our foreign subsidiaries unless there is a tax–free manner under which to remit the earnings. The current analysis indicates that we have sufficient U.S. liquidity, including borrowing capacity, to fund foreseeable U.S. cash needs without requiring the repatriation of foreign cash. One time or unusual items that may impact our ability or intent to keep the foreign earnings and cash indefinitely reinvested include significant U.S. acquisitions, loans from a foreign subsidiary, and changes in tax laws.
As of December 31, 2021,2023, the Company has not provided deferred income taxes on unrepatriated earnings from foreign subsidiaries as they are deemed to be indefinitely reinvested.reinvested unless there is a manner under which to remit the earnings with no material tax cost. Such taxes would primarily be attributable to foreign withholding taxes and local income taxes when such earnings are distributed. As such, the Company has determined the tax impact of repatriating these earnings would not be material as of December 31, 2021.
Loss Contingencies
We are subject to claims and lawsuits in the ordinary course of our business, including claims by employees or former employees, and claims with respect to our products and involving commercial disputes. We accrue for loss contingencies when it is deemed probable that a loss has been incurred and that loss is estimable. The amounts accrued are based on the full amount of the estimated loss before considering insurance proceeds, if applicable, and do not include an estimate for legal fees expected to be incurred in connection with the loss contingency. We consistently accrue legal fees expected to be incurred in connection with loss contingencies as those fees are incurred by outside counsel as a period cost. Our financial statements do not reflect any material amounts related to possible unfavorable outcomes of claims and lawsuits to which we are currently a party because we currently believe that such claims and lawsuits are not expected, individually or in the aggregate, to result in a material, adverse effect on our financial condition. However, it is possible that these contingencies could materially affect our results of operations, financial position and cash flows in a particular period if we change our assessment of the likely outcome of these matters.
Pension Benefits
The Company maintains defined benefit pension plans that cover certain employees in France, Japan, Germany and Switzerland. Various factors are considered in determining the pension liability, including the number of employees expected to be paid their salary levels and years of service, the expected return on plan assets, the discount rate used to determine the benefit obligations, the timing of benefit payments and other actuarial assumptions. If the actual results and events for the pension plans differ from current assumptions, the benefit obligation may be over or under valued. We recognize the underfunded status of the defined benefit pension plans as an asset or a liability in the balance sheet, with changes in the funded status recorded through other comprehensive income in the year in which those changes occur.
The Company’s discount rates are determined by considering current yield curves representing high quality, long-term fixed income instruments. The resulting discount rates are consistent with the duration of plan liabilities. In 2021, the discount rate was prescribed as the current yield on corporate bonds with an average rating of AA or AAA of equivalent currency and term to the liabilities.
The expected return on plan assets represents the average rate of return expected to be earned on plan assets over the period the benefits included in the benefit obligation are to be paid. In developing the expected rate of return, the Company considers returns of historical market data as well as actual returns on the plan assets. Using this reference information, the long-term return expectations for each asset category are developed according to the allocation among those investment categories.
The net plan assets of the pension plans are invested in common trusts as of December 31, 2021. Common trusts are classified as Level 2 in fair value hierarchy. The fair value of common trusts are valued at net asset value based on the fair values of the underlying investments of the trusts as determined by the sponsor of the trusts.
The following weighted average assumptions were used to develop net periodic pension benefit cost and the actuarial present value of projected pension benefit obligations for the year ended December 31, 2021 and 2020, respectively:
| | | | | | | | | | | | | |
| As of December 31, | | |
| 2021 | | 2020 | | |
| Discount rate | 0.37 | % | | 0.34 | % | | |
| Expected return on plan assets | 3.59 | % | | 2.04 | % | | |
| Rate of compensation increase | 2.10 | % | | 2.14 | % | | |
| Interest crediting rate for cash balance plans | 1.0 | % | | 1.0 | % | | |
A change of plus (minus) 25 basis points on expected rate of return on plan assets, with other assumptions held constant, would have an estimated $0.1 million favorable (unfavorable) impact on pension plan costs. As of December 31, 2021, contributions expected to be paid to the plan in 2022 are $2.3 million.
We use the corridor approach in the valuation of defined benefit pension benefit plans. The corridor approach defers all actuarial gains and losses resulting from variances between actual results and actuarial assumptions. Those unrecognized gains and losses are amortized when the net gains and losses exceed 10% of the greater of the market-related value of plan assets or the projected benefit obligation at the beginning of the year. The amount in excess of the corridor is amortized over the average remaining service period to retirement date of active plan participants.
Stock-based Compensation
We apply the authoritative guidance for stock-based compensation. This guidance requires companies to recognize the expense related to the fair value of their stock-based compensation awards. Stock-based compensation expense for stock option awards is based on the grant date fair value on using the binomial distribution model. The Company recognizes compensation expense for stock option awards, restricted stock awards, performance stock awards and contract stock awards on a ratable basis over the requisite service period of the award. All excess tax benefits and taxes and tax deficiencies from stock-based compensation are included in the provision for income taxes in the consolidated statement of operations.
Recently Issued and Adopted Accounting Standards
Refer to Note 2, Summary of Significant Accounting Policiesof,to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K), to the consolidated financial statements for recently adopted accounting pronouncements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to various market risks, including changes in foreign currency exchange rates and interest rates that could adversely affect our results of operations and financial condition. To manage the volatility relating to these typical business exposures, we may enter into various derivative transactions when appropriate. We do not hold or issue derivative instruments for trading or other speculative purposes.
Foreign Currency Exchange and Other Rate Risks
We operate on a global basis and are exposed to the risk that changes in foreign currency exchange rates could adversely affect our financial condition, results of operations and cash flows. We are primarily exposed to foreign currency exchange rate risk with respect to transactions and net assets denominated in Euros, British pounds, Swiss francs, Canadian dollars, Japanese yen, Mexican pesos, Brazilian reais, Australian dollars and Chinese yuan. We manage the foreign currency exposure centrally, on a combined basis, which allows us to net exposures and to take advantage of any natural offsets. To mitigate the impact of currency fluctuations on transactions denominated in nonfunctional currencies, we periodically enter into derivative financial instruments in the form of foreign currency exchange forward contracts with major financial institutions. We temporarily record realized and unrealized gains and losses on these contracts that qualify as cash flow hedges in other comprehensive income, and then recognize them in other income or expense when the hedged item affects net earnings.
From time to time, we enter into foreign currency forward exchange contracts to manage currency exposures for transactions denominated in a currency other than an entity’s functional currency. As a result, the impact of foreign currency gains/losses recognized in earnings are partially offset by gains/losses on the related foreign currency forward exchange contracts in the same reporting period. Refer to Note 6, Derivative Instrumentsfor further information of, to the Notes to Consolidated Financial Statements (Part II,IV, Item 815 of this Annual Report on Form 10-K). for additional information.
We maintain written policies and procedures governing our risk management activities. With respect to derivatives, changes in hedged items are generally expected to be completely offset by changes in the fair value of hedge instruments. Consequently, foreign currency exchange contracts would not subject us to material risk due to exchange rate movements, because gains and losses on these contracts offset gains and losses on the assets, liabilities or transactions being hedged.
The results of operations discussed herein have not been materially affected by inflation.
Interest Rate Risk
Cash and Cash Equivalents - We are exposed to the risk of interest rate fluctuations on the interest income earned on our cash and cash equivalents. A hypothetical 100 basis points movement in interest rates applicable to our cash and cash equivalents outstanding at December 31, 20212023 would increase interest income by approximately $5.1$2.8 million on an annual basis. No significant decrease in interest income would be expected as our cash balances are earning interest at rates of approximately one basis points. We are subject to foreign currency exchange risk with respect to cash balances maintained in foreign currencies.
Short-Term Investments- We are exposed to the risk of interest rate fluctuations on the interest income earned on our short-term investments. A hypothetical 100 basis points movement in interest rates applicable to our short-term investments outstanding at December 31, 2023 would increase or decrease interest income by approximately $0.3 million on an annual basis.
Debt - OurThe Company’s interest rate risk relates primarily to U.S. dollar LIBOR-indexeddenominated variable interest rate borrowings. We useThe Company uses interest rate swap derivative instruments to manage our earnings and cash flow exposure toresulting from changes in interest rates. These interest rate swaps fix theapply a fixed interest rate on a portion of ourthe Company's expected LIBOR-indexed floating-rateSOFR-indexed borrowings. TheIn connection with the March 2023 Amendment to the Senior Credit Facility, the Company held the followingamended its interest rate from LIBOR to SOFR-indexed interest. In March 2023, the Company entered into a basis swap where the Company receives Term SOFR and pays daily compounded SOFR to convert the portfolio of swaps asfrom daily compounded SOFR to term SOFR. See Note 6, Derivative Instrumentsto the Notes to Consolidated Financial Statements (Part IV, Item 15 of December 31, 2021 (dollar amounts in thousands):this Annual Report on Form 10-K) for a detail of current interest rate swap derivative instruments.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Hedged Item | | Notional Amount | | Designation Date | | Effective Date | | Termination Date | | Fixed Interest Rate | | Estimated Fair Value |
| | | | | | | | | | | | Assets (Liabilities) |
| 1-month USD LIBOR Loan | | 300,000 | | | December 13, 2017 | | January 1, 2018 | | December 31, 2022 | | 2.201 | % | | (5,268) | |
| 1-month USD LIBOR Loan | | 150,000 | | | December 13, 2017 | | July 1, 2019 | | June 30, 2024 | | 2.423 | % | | (5,520) | |
| 1-month USD LIBOR Loan | | 200,000 | | | December 13, 2017 | | January 1, 2018 | | December 31, 2024 | | 2.313 | % | | (7,421) | |
| 1-month USD LIBOR Loan | | 75,000 | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.220 | % | | (5,512) | |
| 1-month USD LIBOR Loan | | 75,000 | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.199 | % | | (5,464) | |
| 1-month USD LIBOR Loan | | 75,000 | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.209 | % | | (5,494) | |
| 1-month USD LIBOR Loan | | 100,000 | | | December 18, 2018 | | December 30, 2022 | | December 31, 2027 | | 2.885 | % | | (6,886) | |
| 1-month USD LIBOR Loan | | 100,000 | | | December 18, 2018 | | December 30, 2022 | | December 31, 2027 | | 2.867 | % | | (6,764) | |
| 1-month USD LIBOR Loan | | 575,000 | | | December 15, 2020 | | July 31, 2025 | | December 31, 2027 | | 1.415 | % | | 3,552 | |
| 1-month USD LIBOR Loan | | 125,000 | | | December 15, 2020 | | July 1, 2025 | | December 31, 2027 | | 1.404 | % | | 821 | |
| | $ | 1,775,000 | | | | | | | | | | | $ | (43,957) | |
These interest rate swaps were designated as cash flow hedges as of December 31, 2021.2023. The total notional amounts related to the Company’s interest rate swaps were $1.8$1.5 billion and with $875.0 million$0.8 billion effective as of December 31, 2021.2023. Based on our outstanding borrowings at December 31, 2021,2023, a 100 basis points change in interest rates would have impacted interest expense on the unhedged portionportion of the debt by $1.1$1.6 million on an annualized basis.
| | | | | |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Financial statements and the financial statement schedule specified by this Item, together with the report thereon of PricewaterhouseCoopers LLP, are presented following Item 1515. Exhibits and Financial Statement Schedule of this report.Annual Report on Form 10-K.
| | | | | |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES |
Not applicable.
| | | | | |
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow for timely decisions regarding required disclosure. Disclosure controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Management has designed our disclosure controls and procedures to provide reasonable assurance of achieving the desired control objectives.
As required by Exchange Act Rule 13a-15(b), we have carried out an evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2021.2023. Based upon this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of December 31, 20212023 to provide such reasonable assurance.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Securities Exchange Act of 1934, as amended. Internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America (“GAAP”). We recognize that because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies and procedures may deteriorate.
To evaluate the effectiveness of our internal control over financial reporting, management used the criteria described in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based upon this evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2021.2023.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 20212023 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) that occurred during the quarter ended December 31, 20212023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| | | | | |
| ITEM 9B. | OTHER INFORMATION |
Not applicable.
| | | | | |
| ITEM 9C. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
Not applicable.
PART III
INCORPORATION BY REFERENCE
The information called for by Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities relating to equity compensation plans, Item 10. Directors, Executive Officers and Corporate Governance, Item 11. Executive Compensation, Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters, Item 13. Certain Relationships and Related Transactions, and Director Independence and Item 14. Principal Accountant Fees and Services is incorporated herein by reference to the Company’s definitive proxy statement for its Annual Meeting of Stockholders scheduledscheduled to be held on May 13, 2022, which9, 2024, which definitive proxy statement is expected to be filed with the Commission not later than 120 days after the end of the fiscal year to which this report relates.
PART IV
| | | | | |
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULE |
(a) Documents filed as a part of this report:
1. Financial Statements.
The following financial statements and financial statement schedules are filed as a part of this report: | | | | | |
| |
| |
| |
| |
| |
| |
| |
| |
2. Financial Statement Schedule. | |
| |
| |
| |
All other schedules not listed above have been omitted, because they are not applicable or are not required, or because the required information is included in the consolidated financial statements or notes thereto.
3. Exhibits required to be filed by Item 601 of Regulation S-K. | | | | | | | | |
| 2.1 | |
| | |
| 2.1(a) | | | |
| | | |
| 2.1(b) | | | |
| | | |
2.22.1(c)+ | | Stock and Asset Purchase Agreement, by anddated December 12, 2023, among Medtronic,Ethicon, Inc., Medtronic Xomed Instrumentation, SAS, and Integra LifeSciences Corporation, dated as of September 12, 2014 (Incorporated by reference to Exhibit 2.1 to the Company's Current Report on Form 8-K filed on October 27, 2014) |
| | |
2.3 | | | |
| | |
| 2.4 | |
| | |
2.5 | | |
| | |
| | | | | | | | |
2.6 | | |
| | |
2.7 | | |
| | |
2.8(a) | | |
| | |
2.8(b) | | |
| | |
| 3.1(a) | | | |
| | |
| 3.1(b) | | | |
| | | |
| 3.1(c) | | | |
| | | |
| 3.1(d) | | | |
| | | |
3.2(a)3.2 | | |
| |
3.2(b) | | | |
| | | |
| 4.1 | | | |
| | | |
| | | | | | | | | | | |
| 4.2 | | | |
| | |
| 4.3 | | | |
| | | |
4.44.4+ | | | |
| | | |
| 10.1(a) | | |
| |
10.1(b) | | | |
| | | |
10.1(c)10.1(b) | | | |
| | | |
| 10.2(a)* | | |
| |
| | | | | | | | |
10.2(b) | | |
| | |
10.3 | | |
| | |
10.4 | | |
| |
10.5 | | |
| |
10.6(a) | | | |
| | |
10.6(b)10.2(b)* | | | |
| | |
| | |
10.7(a)10.3(a)* | | | |
| | |
10.7(b)10.3(b)* | | | |
| | | |
10.7(c)10.3(c)* | | | |
| | | |
10.7(d)10.3(d)* | | | |
| | | |
10.7(e)10.3(e)* | | | |
| | | |
10.7(f)10.3(f)* | | | |
| | | |
10.7(g)10.3(g)* | | | |
| | |
10.810.3(h)* | | | |
| | | |
| 10.3(i)* | | | |
| | | |
| 10.3(j)* | | | |
| | | |
| 10.3(k)* | | | |
| | | |
| 10.3(l)* | | | |
| | | | | | | | | | | |
| | | |
| 10.4* | | |
| | |
10.9(a) | | | |
| | | |
10.9(b)10.5* | | |
| |
10.10 | | |
| |
10.11(a) | | | |
| | | |
10.11(b)10.6* | | |
| | |
10.12 | | |
| | |
| | | | | | | | |
10.13(a) | | |
| |
10.13(b) | | |
| |
10.13(c) | | |
| |
10.14 | | |
| |
10.15(a) | | |
| |
10.15(b) | | |
| |
10.16(a) | | |
| |
10.16(b) | | |
| |
10.17 | | |
| |
10.18(a) | | |
| |
10.18(b) | | |
| |
10.18(c) | | |
| |
10.18(d) | | |
| |
10.19 | | |
| |
10.20 | | |
| |
10.21 | | |
| | |
10.22(a) | | |
| | |
| | | | | | | | |
10.22(b) | | |
| |
10.22(c) | | |
| | |
10.23 | | |
| | |
10.24 | | |
| | |
10.25(a) | | |
| | |
10.25(b) | | |
| | |
10.25(c) | | |
| | |
10.25(d) | | |
| | |
10.25(e) | | |
| | |
10.26 | | |
| | |
10.27(a) | | |
| | |
10.27(b) | | | |
| | | |
10.2810.7* | | |
| | |
10.29 | | |
| | |
10.30 | | |
| | |
10.31 | | |
| | |
10.32 | | |
| | |
10.33 | | |
| | |
10.34 | | |
| | |
10.35 | | | |
| | | |
10.36(a)10.8* | | |
| | |
| | | | | | | | |
10.36(b) | | |
| | |
10.37(a) | | |
| | |
10.37(b) | | |
| | |
10.37(c) | | |
| | |
10.38(a) | | |
| | |
10.38(b) | | |
| | |
10.38(c) | | |
| | |
10.39 | | |
| | |
10.40 | | |
| | |
10.41 | | |
| | |
10.42 | | |
| | |
10.43 | | | |
| | | |
10.44(a)10.9* | | | |
| | | |
10.44(b)10.10* | | | |
| | | |
10.44(c)10.11(a)* | | | |
| | | |
| 10.11(b)* | | | |
| | | |
10.45(a)10.12(a) | | Receivables Financing Agreement, dated as of December 21, 2018, by and among Integra Receivables LLC, Integra LifeSciences Sales LLC, as Servicer, PNC Bank, National Association, as Administrative Agent, PNC Capital Markets LLC, as Structuring Agent, and certain lenders and group agents that are parties thereto from time to time (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on December 28, 2018) | |
| | | |
10.45(b)10.12(b) | | Amendment No. 1 to Receivables Financing Agreement and Reaffirmation of Performance Guaranty, dated as of March 29, 2019, by and among Integra Receivables LLC, Integra LifeSciences Sales LLC, as Servicer, PNC Bank, National Associations, as Administrative Agent, Committed Lender and Group Agent, Mizuho Bank, Ltd., as Committed Lender and Group Agent and PNC Capital Markets LLC, as Structuring Agent, and certain lenders and group agents that are parties thereto from time to time (Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2021) | |
| | |
| | | | | | | | |
10.45(c)10.12(c) | | Amendment No. 2 to Receivables Financing Agreement and Reaffirmation of Performance Guaranty, dated as of July 17, 2020, by and among, Integra Receivables LLC, Integra LifeSciences Sales LLC, as Servicer, PNC Bank, National Associations, as Administrative Agent, Committed Lender and Group Agent, Mizuho Bank, Ltd., as Committed Lender and Group Agent and PNC Capital Markets LLC, as Structuring Agent, and certain lenders and group agents that are parties thereto from time to time (Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2021) | |
| | | |
10.45(d)10.12(d) | | Amendment No. 3 to Receivables Financing Agreement and Reaffirmation of Performance Guaranty, dated as of May 28, 2021, by and among, Integra Receivables LLC, Integra LifeSciences Sales LLC, as Servicer, PNC Bank, National Associations, as Administrative Agent, PNC Capital Markets LLC, as Structuring Agent, Committed Lender and Group Agent, and certain lenders and group agents that are parties thereto from time to time (Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2021) | |
| | | |
10.4610.12(e) | | Amendment No. 4 to Receivables Financing Agreement and Reaffirmation of Performance Guaranty, dated as of April 17, 2023, by and among, Integra Receivables LLC, Integra LifeSciences Sales LLC, as Servicer, PNC Bank, National Associations, as Administrative Agent, PNC Capital Markets LLC, as Structuring Agent, Committed Lender and Group Agent, and certain lenders and group agents that are parties thereto from time to time (Incorporated by reference to Exhibit 10.4 to the Company Quarterly Report on Form 10-Q for the quarter ended March 31, 2023) | |
| | | |
| | | | | | | | | | | |
| 10.12(f)+ | | Amendment No. 5 to Receivables Financing Agreement and Reaffirmation of Performance Guaranty, dated as of December 15, 2023, by and among, Integra Receivables LLC, Integra LifeSciences Sales LLC, as Servicer, PNC Bank, National Associations, as Administrative Agent, PNC Capital Markets LLC, as Structuring Agent, Committed Lender and Group Agent, The Bank of Nova Scotia, as Committed Lender and Group Agent, and certain lenders and group agents that are parties thereto from time to time | |
| | | |
| 10.13 | | | |
| | | |
10.47(a)10.14 | | SixthSeventh Amended and Restated Credit Agreement, dated as of February 3, 2020,March 24, 2023, among Integra LifeSciences Holdings Corporation, the lenders party thereto, Bank of America, N.A., as Administrative Agent, Swing Line Lender and an L/C Issuer, Citibank N.A., JPMorgan Chase Bank, N.A., Morgan Stanley MUFG Loan Partners, LLC, PNC Bank, N.A., Truist Securities, Inc. and Wells Fargo Bank, N.A., as Co-Syndication Agents, and PNC Bank, N.A.,The Bank of Nova Scotia, BMO Harris Bank of the West, BBVA USA,N.A., BNP Paribas, Capital One, National Association, Citizens Bank, N.A., DNB Capital LLC,Bank ASA, New York Branch, Santander Bank, N.A., and TD Bank, N.A. and Truist Bank,, as Co-Documentation Agents. (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 3, 2020).March 24, 2023) | |
| | | |
10.47(b)10.15 | | Amendment, dated July 14, 2020, to that Sixth Amended and Restated Credit Agreement, among Integra LifeSciences Holdings Corporation, a syndicate of lending banks, Bank of America, N.A., as Administrative Agent, Swing Line Lender and L/C Issuer, Citibank N.A., Morgan Stanley MUFG Loan Partners, LLC and Wells Fargo Bank, N.A. as Co-Syndication Agents, and PNC Bank, N.A., Bank of Nova Scotia, Bank of the West, BBVA USA, Capital One, National Association, Citizens Bank, N.A., DNB Capital LLC, Santander Bank, N.A., T.D. Bank, N.A. and Truist Bank, as Co-Documentation Agents (as amended, restated, modified and supplemented from time to time prior to the date hereof, the “Credit Agreement”) (Incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on July 20, 2020). |
| | |
10.48 | | | |
| | | |
10.4910.16 | | Ratification Agreement, dated as of February 3, 2020,March 24, 2023, between Integra LifeSciences Holdings Corporation, the Subsidiary Guarantors of Integra LifeSciences Holdings Corporation and Bank of America, N.A., as Administrative Agent (Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on February 3, 2020)March 24, 2023) | |
| | | |
10.5010.17 | | | |
| | | |
10.5110.18 | | | |
| | | |
10.5210.19 | | | |
| | | |
10.5310.20 | | | |
| | | |
10.5410.21 | | | |
| | | |
10.5510.22 | | | |
| | |
| | | | | | | | |
10.5610.23 | | | |
| | | |
10.5710.24 | | | |
| | | |
10.5810.25 | | | |
| | | |
10.5910.26 | | | |
| | | |
| | | | | | | | | | | |
10.6010.27 | | | |
| | | |
10.6110.28 | | | |
| | | |
10.6210.29 | | | |
| | | |
10.6310.30 | | | |
| | | |
10.6410.31 | | | |
| | | |
10.6521.1+ | | |
| | |
21.1 | | | |
| | | |
23.123.1+ | | | |
| | | |
31.131.1+ | | | |
| | | |
31.231.2+ | | | |
| | | |
32.132.1+ | | | |
| | | |
32.232.2+ | | | |
| | | |
99.197.1+ | | | |
| | | |
99.2101.INS+# | | Inline XBRL Instance Document | |
| | | |
99.3101.SCH+# | | |
99.4 | | |
| | | | | | | | |
| | |
99.5 | | |
| | |
99.6 | | |
| | |
99.7 | | |
| | |
99.8 | | |
| | |
99.9 | | |
| | |
101.INS | | XBRL Instance Document+# |
| | |
101.SCH | | Inline XBRL Taxonomy Extension Schema Document+#Document | |
| | | |
101.CAL101.CAL+# | | Inline XBRL Taxonomy Extension Calculation Linkbase Document+#Document | |
| | | |
| 101.DEF | | Inline XBRL Definition Linkbase Document | |
| | | |
101.LAB101.LAB+# | | Inline XBRL Taxonomy Extension Labels Linkbase Document+#Document | |
| | | |
101.PRE101.PRE+# | | Inline XBRL Taxonomy Extension Presentation Linkbase Document+#Document | |
| | | |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | |
| | | | | |
| * | Indicates a management contract or compensatory plan or arrangement. |
| | | | | |
| + | Indicates this document is filed as an exhibit herewith. |
| | | | | |
| # | The financial information of Integra LifeSciences Holdings Corporation Annual Report on Form 10-K for the year ended December 31, 20212023 filed on February 24, 202228, 2024 formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statements of Operations, (ii) the Consolidated Statement of Comprehensive Income (Loss), (iii) the Consolidated Balance Sheets, (iv) Parenthetical Data to the Consolidated Balance Sheets, (v) the Consolidated Statements of Cash Flows, (vi) the Consolidated Statements of Changes in Stockholders’ Equity, and (vii) Notes to Consolidated Financial Statements, is furnished electronically herewith. |
The Company’s Commission File Number for Reports on Form 10-K, Form 10-Q and Form 8-K is 0-26224.000-26224.
| | | | | |
| ITEM 16. | FORM 10-K SUMMARY |
None.
SIGNATURES
Pursuant to the requirements of Section 13 of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | |
| |
| INTEGRA LIFESCIENCES HOLDINGS CORPORATION |
| |
| By: | /s/ Jan De Witte |
| Jan De Witte |
| President and Chief Executive Officer, and Director |
| (Principal Executive Officer) |
| |
| By: | /s/ Carrie L. AndersonLea Knight |
| Carrie L. AndersonLea Knight |
| Executive Vice President and Chief Financial Officer |
| (Principal Financial Officer) |
| |
| By: | /s/ Jeffrey A. Mosebrook |
| Jeffrey A. Mosebrook |
| Senior Vice President, Finance |
| (Principal Accounting Officer) |
| |
Date: February 24, 202228, 2024
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons, on behalf of the registrant in the capacities indicated. | | | | | | | | | | | | | | |
| | | | |
| Signature | | Title | | Date |
| | | | |
| /s/ Jan De Witte | | President and Chief Executive Officer, | | February 24, 202228, 2024 |
| Jan De Witte | | and Director (Principal Executive Officer) | | |
| | | | |
/s/ Carrie L. AndersonLea Knight | | Executive Vice President and Chief Financial Officer | | February 24, 202228, 2024 |
Carrie L. AndersonLea Knight | | (Principal(Principal Financial Officer) | | |
| | | | |
| /s/ Jeffrey A. Mosebrook | | Senior Vice President, Finance | | February 24, 202228, 2024 |
| Jeffrey A. Mosebrook | | (Principal Accounting Officer) | | |
| | | | |
| /s/ Stuart M. Essig, Ph.D. | | Chairman of the Board | | February 24, 202228, 2024 |
| Stuart M. Essig, Ph.D. | | | | |
| | | | |
/s/ Rhonda Germany Ballintyn | | Director | | February 24, 2022 |
Rhonda Germany Ballintyn | | | | |
| | | | |
| /s/ Keith Bradley, Ph.D. | | Director | | February 24, 202228, 2024 |
| Keith Bradley, Ph.D. | | | | |
| | | | |
| /s/ Shaundra Clay | | Director | | February 24, 202228, 2024 |
| Shaundra Clay | | | | |
| | | | |
| /s/ Jeffrey A. Graves | | Director | | February 28, 2024 |
| Jeffrey A. Graves | | | | |
| | | | |
| /s/ Barbara B. Hill | | Director | | February 24, 202228, 2024 |
| Barbara B. Hill | | | | |
| | | | |
/s/ Donald E. Morel, Jr., Ph.D.Renee W. Lo | | Director | | February 24, 202228, 2024 |
Donald E. Morel, Jr., Ph.D.Renee W. Lo | | | | |
| | | | |
| /s/ Raymond G. Murphy | | Director | | February 24, 202228, 2024 |
| Raymond G. Murphy | | | | |
| | | | |
| /s/ Christian S. Schade | | Director | | February 24, 202228, 2024 |
| Christian S. Schade | | | | |
| | | | |
| | | | |
| | | | |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Integra LifeSciences Holdings Corporation
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Integra LifeSciences Holdings Corporation and its subsidiaries (the “Company”) as of December 31, 20212023 and 2020,2022, and the related consolidated statements of operations, of comprehensive income, of changes in stockholders’ equity, and of cash flows for each of the three years in the period ended December 31, 2021,2023, including the related notes and financial statement schedule listed in the index appearing under Item 15(a)(2) (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2021,2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 20212023 and 2020,2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 20212023 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2021,2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Change in Accounting Principle
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for convertible instruments in 2021 and leases in 2019.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that
(i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
ACell, Inc. AcquisitionInterim Goodwill Impairment Assessment - Valuation of Developed Technology Intangible AssetsTissue Technologies Reporting unit
As described in Notes 2 4 and 7 to the consolidated financial statements, in January 2021, the Company acquired ACell Inc.has three reporting units and the total goodwill balance was $1,055 million as of December 31, 2023, of which $388.5 million relates to the Tissue Technologies reporting unit. Goodwill is tested by management for an acquisition purchaseimpairment at the reporting unit level annually during the third quarter every year, or more frequently if impairment indicators arise. Management's assessment of the recoverability of goodwill is based upon a comparison of the carrying value of goodwill with its estimated fair value. As disclosed by management, in the second quarter of 2023, due to the voluntary global recall of all products manufactured at the Boston facility, as well as the associated drop in the Company's stock price in that quarter, management elected to perform a quantitative analysis, using a combination of $306.9 million plus contingent considerationa discounted cash flow method and guideline public company method for its Tissue Technologies reporting unit. The quantitative test utilized assumptions of up to $100 million, which resulted in $245Mrevenue growth rate, terminal growth rate, discount rate, and the range and application of developed technology intangible assets being recorded. The estimatedcompany guideline multiples. Management determined, after performing the quantitative analysis, that the fair value of the developed technology acquired was determined by management using the multi-period excess earnings methodgoodwill of the income approach. Management’s significant assumptions used inreporting unit was not less than the estimate of fair value included the estimated net cash flows, net revenues, cost of sales, selling and marketing costs, discount rates and each asset’s life cycle.carrying amount.
The principal considerations for our determination that performing procedures relating to the valuationinterim goodwill impairment assessment of developed technology intangible assets from the acquisition of ACell Inc.Tissue Technologies reporting unit is a critical audit matter are the (i) the significant judgment by management inwhen developing the fair value estimate of the acquired developed technology intangible assets,reporting unit; (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’smanagement's significant assumptions related to net revenues, costthe revenue growth rate, terminal growth rate, discount rate, and the range and application of sales, selling and marketing costs, discount rates and each asset’s life cycle;company guideline multiples; and (iii) the audit effort involved in the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the acquisition accounting,management's goodwill impairment assessment, including controls over management’sthe valuation of the intangible assets and controls over the development of significant assumptions related to net revenues, cost of sales, selling and marketing costs, discount rates and each asset’s life cycle.Tissue Technologies reporting unit. These procedures also included, among others (i) reading the purchase agreement and (ii) testing management’smanagement's process for developing the fair value estimate of the developed technology intangible assets. Testing management’s process includedTissue Technologies reporting unit; (ii) evaluating the appropriateness of the multi-period excess earnings method,discounted cash flow and guideline public company methods used by management; (iii) testing the completeness accuracy, and relevanceaccuracy of underlying data used by management in the method,discounted cash flow and guideline public company methods; and (iv) evaluating the reasonableness of the significant assumptions used by management in the discounted cash flow method related to net revenues, costthe revenue growth rate, terminal growth rate, and discount rate, and the significant assumption used by management in the guideline public company method related to the range and application of sales, selling and marketing costs, discount rates and each asset’s life cycle.company guideline multiples. Evaluating the reasonableness of management’smanagement's assumptions related to net revenues, cost of sales, sellingthe revenue growth rates and marketing costs and each asset’s life cycleterminal growth rate involved evaluating whether the assumptions used by management were reasonable considering (i) the current and past performance of the products associated with the developed technology acquired, as well as the performance of the reporting unit that assumed the products associated with the developed technology acquired;unit; (ii) the consistency with external market and industry data; and (iii) and whether the assumptions arewere consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in evaluating (i) the evaluationappropriateness of the Company’s multi-period excess earnings methoddiscounted cash flow and guideline public company methods and (ii) the reasonableness of the discount rate and the discount ratesrange and each asset’s life cycleapplication of company guideline multiples assumptions.
/s/ PricewaterhouseCoopers LLP
Florham Park, New Jersey
February 24, 202228, 2024
We have served as the Company’s auditor since 1989.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(Dollars in thousands, except per share amounts)
| | | | Years Ended December 31, | | Years Ended December 31, |
| | | 2021 | | 2020 | | 2019 | | 2023 | | 2022 | | 2021 |
| Total revenue, net | Total revenue, net | $ | 1,542,448 | | | $ | 1,371,868 | | | $ | 1,517,557 | |
| | Costs and expenses: | Costs and expenses: | |
| Costs and expenses: | |
| Costs and expenses: | |
| Cost of goods sold | |
| Cost of goods sold | |
| Cost of goods sold | Cost of goods sold | 597,808 | | | 520,834 | | | 564,681 | |
| Research and development | Research and development | 93,051 | | | 77,381 | | | 79,573 | |
| In-process research and development | — | | | — | | | 64,916 | |
| Selling, general and administrative | Selling, general and administrative | 637,445 | | | 594,526 | | | 687,599 | |
| Intangible asset amortization | Intangible asset amortization | 16,914 | | | 27,757 | | | 27,028 | |
| Total costs and expenses | Total costs and expenses | 1,345,218 | | | 1,220,498 | | | 1,423,797 | |
| Operating income | Operating income | 197,230 | | | 151,370 | | | 93,760 | |
| Interest income | Interest income | 6,737 | | | 9,297 | | | 10,779 | |
| Interest expense | Interest expense | (50,395) | | | (71,581) | | | (53,957) | |
| Gain from sale of business | 41,798 | | | — | | | — | |
| Gain from sale of businesses | |
| Other income, net | Other income, net | 19,307 | | | 4,434 | | | 9,522 | |
| Income before income taxes | Income before income taxes | 214,677 | | | 93,520 | | | 60,104 | |
| Provision (benefit) for income taxes | 45,602 | | | (40,372) | | | 9,903 | |
| Provision for income taxes | |
| Net income | Net income | $ | 169,075 | | | $ | 133,892 | | | $ | 50,201 | |
| | Net income per share | Net income per share | |
| Net income per share | |
| Net income per share | |
| Basic | |
| Basic | |
| Basic | Basic | $ | 2.00 | | | $ | 1.58 | | | $ | 0.59 | |
| Diluted | Diluted | $ | 1.98 | | | $ | 1.57 | | | $ | 0.58 | |
| | Weighted average common shares outstanding (See Note 13): | Weighted average common shares outstanding (See Note 13): | |
| Weighted average common shares outstanding (See Note 13): | |
| Weighted average common shares outstanding (See Note 13): | |
| Basic | |
| Basic | |
| Basic | Basic | 84,698 | | | 84,650 | | | 85,637 | |
| Diluted | Diluted | 85,485 | | | 85,228 | | | 86,494 | |
The accompanying notes are an integral part of these consolidated financial statements.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(Dollars in thousands)
| | | | Years Ended December 31, | | Years Ended December 31, |
| | | 2021 | | 2020 | | 2019 | | 2023 | | 2022 | | 2021 |
| Net income | Net income | $ | 169,075 | | | $ | 133,892 | | | $ | 50,201 | |
| | Other comprehensive (loss) income, before tax: | Other comprehensive (loss) income, before tax: | |
| Other comprehensive (loss) income, before tax: | |
| Other comprehensive (loss) income, before tax: | |
| Change in foreign currency translation adjustments | |
| Change in foreign currency translation adjustments | |
| Change in foreign currency translation adjustments | Change in foreign currency translation adjustments | (17,362) | | | 53,363 | | | (174) | |
| | Unrealized gain (loss) on derivatives | Unrealized gain (loss) on derivatives | |
| Unrealized gain (loss) on derivatives | |
| Unrealized gain (loss) on derivatives | |
| Unrealized derivative gain (loss) arising during period | |
| Unrealized derivative gain (loss) arising during period | |
| Unrealized derivative gain (loss) arising during period | Unrealized derivative gain (loss) arising during period | 68,192 | | | (96,837) | | | (13,671) | |
| Less: Reclassification adjustments for gain (loss) included in net income | Less: Reclassification adjustments for gain (loss) included in net income | 17,024 | | | (24,442) | | | 14,865 | |
| Unrealized gain (loss) on derivatives | Unrealized gain (loss) on derivatives | 51,168 | | | (72,395) | | | (28,536) | |
| | Defined benefit pension plan - net gain (loss) arising during period | Defined benefit pension plan - net gain (loss) arising during period | 6,998 | | | 4,604 | | | (8,973) | |
| Defined benefit pension plan - net gain (loss) arising during period | |
| Defined benefit pension plan - net gain (loss) arising during period | |
| | Total other comprehensive gain (loss), before tax | Total other comprehensive gain (loss), before tax | 40,804 | | | (14,428) | | | (37,683) | |
| Income tax (expense) benefit related to items in other comprehensive loss | (11,900) | | | 16,771 | | | 6,724 | |
| Total other comprehensive gain (loss), before tax | |
| Total other comprehensive gain (loss), before tax | |
| Income tax (expense) benefit related to items in other comprehensive gain (loss) | |
| Total other comprehensive gain (loss), net of tax | Total other comprehensive gain (loss), net of tax | 28,904 | | | 2,343 | | | (30,959) | |
| | Comprehensive income, net of tax | Comprehensive income, net of tax | $ | 197,979 | | | $ | 136,235 | | | $ | 19,242 | |
| Comprehensive income, net of tax | |
| Comprehensive income, net of tax | |
The accompanying notes are an integral part of these consolidated financial statements.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED BALANCE SHEETS
(Dollars in thousands, except per share amounts)
| | December 31, |
| 2021 | | 2020 |
| December 31, | | | December 31, |
| 2023 | | | 2023 | | 2022 |
| ASSETS | ASSETS | | | |
| Current Assets: | Current Assets: | |
| Current Assets: | |
| Current Assets: | |
| Cash and cash equivalents | Cash and cash equivalents | $ | 513,448 | | | $ | 470,166 | |
| Trade accounts receivable, net of allowances of $4,735 and $6,439 | 231,831 | | | 225,532 | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Short-term investments | |
| Trade accounts receivable, net of allowances of $4,879 and $4,304 | |
| Inventories, net | Inventories, net | 317,386 | | | 310,117 | |
| Prepaid expenses and other current assets | 91,051 | | | 69,282 | |
| Assets held for sale | — | | | 162,105 | |
| Prepaid Expenses | |
| Other Current Assets | |
| Total current assets | Total current assets | 1,153,716 | | | 1,237,202 | |
| Property, plant and equipment, net | Property, plant and equipment, net | 311,703 | | | 287,529 | |
| Right of use asset - operating leases | Right of use asset - operating leases | 84,543 | | | 83,635 | |
| Intangible assets, net | Intangible assets, net | 1,145,573 | | | 989,436 | |
| Goodwill | Goodwill | 1,013,458 | | | 932,367 | |
| Deferred tax assets, net | Deferred tax assets, net | 56,950 | | | 73,690 | |
| Other assets | Other assets | 16,440 | | | 11,277 | |
| Total assets | Total assets | $ | 3,782,383 | | | $ | 3,615,136 | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | LIABILITIES AND STOCKHOLDERS’ EQUITY | | | |
| Current Liabilities: | Current Liabilities: | |
| Current Liabilities: | |
| Current Liabilities: | |
| Current portion of borrowings under senior credit facility | Current portion of borrowings under senior credit facility | $ | 45,000 | | | $ | 33,750 | |
| Current portion of borrowings under securitization facility | — | | | 112,500 | |
| Current portion of borrowings under senior credit facility | |
| Current portion of borrowings under senior credit facility | |
| Current portion of lease liability - operating leases | Current portion of lease liability - operating leases | 14,775 | | | 12,818 | |
| Accounts payable, trade | Accounts payable, trade | 61,837 | | | 54,608 | |
| Contract liabilities | Contract liabilities | 5,295 | | | 5,275 | |
| Accrued compensation | Accrued compensation | 92,656 | | | 76,117 | |
| Accrued expenses and other current liabilities | Accrued expenses and other current liabilities | 120,458 | | | 94,194 | |
| Liabilities held for sale | — | | | 11,751 | |
| Total current liabilities | Total current liabilities | 340,021 | | | 401,013 | |
| Long-term borrowings under senior credit facility | Long-term borrowings under senior credit facility | 824,257 | | | 933,387 | |
| Long-term borrowings under securitization facility | Long-term borrowings under securitization facility | 112,500 | | | — | |
| Long-term convertible securities | Long-term convertible securities | 564,426 | | | 474,834 | |
| Lease liability - operating leases | Lease liability - operating leases | 90,329 | | | 88,118 | |
| Deferred tax liabilities | Deferred tax liabilities | 45,788 | | | 16,190 | |
| Other liabilities | Other liabilities | 120,258 | | | 186,727 | |
| Total liabilities | Total liabilities | 2,097,579 | | | 2,100,269 | |
| Stockholders’ Equity: | Stockholders’ Equity: | |
| Preferred Stock; no par value; 15,000 authorized shares; none outstanding | Preferred Stock; no par value; 15,000 authorized shares; none outstanding | — | | | — | |
| Common stock; $0.01 par value; 240,000 authorized shares; 89,600 and 89,251 issued at December 31, 2021 and 2020, respectively | 896 | | | 893 | |
| Preferred Stock; no par value; 15,000 authorized shares; none outstanding | |
| Preferred Stock; no par value; 15,000 authorized shares; none outstanding | |
| Common stock; $0.01 par value; 240,000 authorized shares; 90,920 and 90,477 issued at December 31, 2023 and 2022, respectively | |
| Additional paid-in capital | Additional paid-in capital | 1,264,943 | | | 1,290,909 | |
| Treasury stock, at cost; 4,899 and 4,914 shares at December 31, 2021 and 2020, respectively | (234,448) | | | (235,141) | |
| Accumulated other comprehensive loss | (45,155) | | | (74,059) | |
| Treasury stock, at cost; 12,751 and 6,823 shares at December 31, 2023 and 2022, respectively | |
| Accumulated other comprehensive income (loss) | |
| Retained earnings | Retained earnings | 698,568 | | | 532,265 | |
| Total stockholders’ equity | Total stockholders’ equity | 1,684,804 | | | 1,514,867 | |
| Total liabilities and stockholders’ equity | Total liabilities and stockholders’ equity | $ | 3,782,383 | | | $ | 3,615,136 | |
The accompanying notes are an integral part of these consolidated financial statements.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Dollars in thousands) | | | | Years Ended December 31, | | Years Ended December 31, |
| | | 2021 | | 2020 | | 2019 | | 2023 | | 2022 | | 2021 |
| OPERATING ACTIVITIES: | OPERATING ACTIVITIES: | | | | | |
| Net income | Net income | $ | 169,075 | | | $ | 133,892 | | | $ | 50,201 | |
| Net income | |
| Net income | |
| Adjustments to reconcile net income to net cash provided by operating activities: | Adjustments to reconcile net income to net cash provided by operating activities: | |
| Depreciation and amortization | Depreciation and amortization | 119,836 | | | 116,031 | | | 109,462 | |
| Non-cash in-process research and development expense | — | | | 519 | | | 64,916 | |
| Depreciation and amortization | |
| Depreciation and amortization | |
| | Non-cash impairment charges | |
| Non-cash impairment charges | |
| Non-cash impairment charges | Non-cash impairment charges | 2,754 | | | — | | | 5,764 | |
| Deferred income tax (benefit) provision | Deferred income tax (benefit) provision | (2,755) | | | (64,138) | | | (19,046) | |
| Share-based compensation | Share-based compensation | 36,210 | | | 19,590 | | | 21,255 | |
| Amortization of debt issuance costs and expenses associated with debt refinancing | Amortization of debt issuance costs and expenses associated with debt refinancing | 7,030 | | | 12,076 | | | 5,390 | |
| Non-cash lease expense | Non-cash lease expense | 3,834 | | | 2,955 | | | 5,060 | |
| Accretion of bond issuance discount | — | | | 15,415 | | | — | |
| Loss on disposal of property and equipment and construction in-progress | 2,240 | | | 7,855 | | | 1,821 | |
| Gain from the sale of business | (41,798) | | | — | | | — | |
| | Loss (Gain) on disposal of property and equipment and construction in-progress | |
| Loss (Gain) on disposal of property and equipment and construction in-progress | |
| Loss (Gain) on disposal of property and equipment and construction in-progress | |
| Gain from the sale of businesses | |
| Change in fair value of contingent consideration and others | Change in fair value of contingent consideration and others | (2,162) | | | 951 | | | 1,119 | |
| Changes in assets and liabilities: | Changes in assets and liabilities: | |
| Accounts receivable | |
| Accounts receivable | |
| Accounts receivable | Accounts receivable | 7,265 | | | 52,105 | | | (9,428) | |
| Inventories | Inventories | 5,374 | | | (48,348) | | | (43,308) | |
| Prepaid expenses and other current assets | Prepaid expenses and other current assets | (21,143) | | | 1,632 | | | 13,071 | |
| Other non-current assets | Other non-current assets | 7,875 | | | 13,735 | | | 13,156 | |
| Accounts payable, accrued expenses and other current liabilities | Accounts payable, accrued expenses and other current liabilities | 32,874 | | | (57,512) | | | 14,666 | |
| Contract liabilities | Contract liabilities | 28 | | | (37) | | | (607) | |
| Other non-current liabilities | Other non-current liabilities | (14,110) | | | (2,889) | | | (2,059) | |
| Net cash provided by operating activities | Net cash provided by operating activities | 312,427 | | | 203,832 | | | 231,433 | |
| INVESTING ACTIVITIES: | INVESTING ACTIVITIES: | | | | | |
| Purchases of property and equipment | Purchases of property and equipment | (48,022) | | | (38,890) | | | (69,537) | |
| Proceeds from sale of Extremity Orthopedics Business | 190,468 | | | — | | | — | |
| Purchases of property and equipment | |
| Purchases of property and equipment | |
| Proceeds from sale of business | |
| Acquired in-process research and development and intangibles | Acquired in-process research and development and intangibles | (58) | | | (25,000) | | | (64,995) | |
| Proceeds from note receivable | — | | | — | | | 752 | |
| Purchases of Investments | |
| Cash paid for business acquisitions, net of cash acquired | Cash paid for business acquisitions, net of cash acquired | (303,910) | | | — | | | (30,509) | |
| Proceeds from sales of property and equipment | Proceeds from sales of property and equipment | 3 | | | 3,657 | | | 37 | |
| Net proceeds (payments) on swaps designated as net investment hedges | 76 | | | (7,840) | | | 1,584 | |
| | Net proceeds on swaps designated as net investment hedges | |
| Net proceeds on swaps designated as net investment hedges | |
| Net proceeds on swaps designated as net investment hedges | |
| Net cash used in investing activities | Net cash used in investing activities | (161,443) | | | (68,073) | | | (162,668) | |
| FINANCING ACTIVITIES: | FINANCING ACTIVITIES: | | | | | |
| Proceeds from borrowings of long-term indebtedness | Proceeds from borrowings of long-term indebtedness | 25,500 | | | 171,500 | | | 236,900 | |
| Proceeds from borrowings of long-term indebtedness | |
| Proceeds from borrowings of long-term indebtedness | |
| Payments on debt | Payments on debt | (125,500) | | | (441,000) | | | (246,100) | |
| Purchase of option hedge on convertible notes | — | | | (104,248) | | | — | |
| Proceeds from convertible notes issuance | — | | | 575,000 | | | — | |
| Proceeds from sale of stock purchase warrants | — | | | 44,563 | | | — | |
| | Payment of debt issuance costs | |
| | Payment of debt issuance costs | |
| | Payment of debt issuance costs | Payment of debt issuance costs | (249) | | | (24,347) | | | — | |
| Purchase of treasury stock | Purchase of treasury stock | — | | | (100,000) | | | — | |
| Proceeds from exercised stock options | Proceeds from exercised stock options | 6,824 | | | 5,232 | | | 6,948 | |
| Cash taxes paid in net equity settlement | Cash taxes paid in net equity settlement | (4,801) | | | (5,075) | | | (6,514) | |
| Net cash (used in) provided by financing activities | (98,226) | | | 121,625 | | | (8,766) | |
| Net cash used in financing activities | |
| Effect of exchange rate changes on cash and cash equivalents | Effect of exchange rate changes on cash and cash equivalents | (9,476) | | | 13,871 | | | 74 | |
| Net increase in cash and cash equivalents | 43,282 | | | 271,255 | | | 60,073 | |
| Net increase (decrease) in cash and cash equivalents | |
| Cash and cash equivalents at beginning of period | Cash and cash equivalents at beginning of period | 470,166 | | | 198,911 | | | 138,838 | |
| Cash and cash equivalents at end of period | Cash and cash equivalents at end of period | $ | 513,448 | | | $ | 470,166 | | | $ | 198,911 | |
The accompanying notes are an integral part of these consolidated financial statements.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY
(Dollars in thousands)
| | Common Stock | | Treasury Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Loss | | Retained Earnings | | Total Equity |
| Shares | | Amount | Shares | | Amount |
| Balance, January 1, 2019 | 88,044 | | | $ | 880 | | | (2,881) | | | $ | (120,615) | | | $ | 1,192,601 | | | $ | (45,443) | | | $ | 348,373 | | | $ | 1,375,796 | |
| Common Stock | | | Common Stock | | Treasury Stock | | Additional Paid-In Capital | | Accumulated Other Comprehensive Income | | Retained Earnings | | Total Equity |
| Shares | | Amount | Shares | | Amount |
| Balance, January 1, 2021 | |
| Net income | Net income | — | | | — | | | — | | | — | | | — | | | — | | | 50,201 | | | 50,201 | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | — | | | — | | | (30,959) | | | — | | | (30,959) | |
| Issuance of common stock through employee stock purchase plan | 17 | | | — | | | — | | | — | | | 716 | | | — | | | — | | | 716 | |
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | 674 | | | 7 | | | 16 | | | 672 | | | (961) | | | — | | | — | | | (282) | |
| Share-based compensation | — | | | — | | | — | | | — | | | 21,264 | | | — | | | — | | | 21,264 | |
| Balance, December 31, 2019 | 88,735 | | | 887 | | | (2,865) | | | (119,943) | | | 1,213,620 | | | (76,402) | | | 398,574 | | | 1,416,736 | |
| Net income | — | | | — | | | — | | | — | | | — | | | — | | | 133,892 | | | 133,892 | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | — | | | — | | | 2,343 | | | — | | | 2,343 | |
| Issuance of common stock through employee stock purchase plan | 13 | | | — | | | — | | | — | | | 694 | | | — | | | — | | | 694 | |
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | 503 | | | 2 | | | 11 | | | 526 | | | (1,066) | | | — | | | — | | | (538) | |
| Share-based compensation | — | | | 4 | | | — | | | — | | | 19,397 | | | — | | | — | | | 19,401 | |
| Share repurchase and equity component of the convertible note issuance, net | — | | | — | | | — | | | — | | | 42,539 | | | — | | | — | | | 42,539 | |
| Accelerated shares repurchased | — | | | — | | | (2,060) | | | (115,724) | | | 15,724 | | | — | | | — | | | (100,000) | |
| Adoption of Update No. 2016-13 | — | | | — | | | — | | | — | | | — | | | — | | | (200) | | | (200) | |
| Balance, December 31, 2020 | 89,251 | | | 893 | | | (4,914) | | | (235,141) | | | 1,290,908 | | | (74,059) | | | 532,266 | | | 1,514,867 | |
| Net income | — | | | — | | | — | | | — | | | — | | | — | | | 169,075 | | | 169,075 | |
| Other comprehensive loss, net of tax | — | | | — | | | — | | | — | | | — | | | 28,904 | | | — | | | 28,904 | |
| | Other comprehensive income (loss), net of tax | |
| Treasury shares retirement | |
| Issuance of common stock through employee stock purchase plan | Issuance of common stock through employee stock purchase plan | 18 | | | — | | | — | | | 1,127 | | | — | | | — | | | 1,127 | |
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | 331 | | | 1 | | | 15 | | | 693 | | | 201 | | | — | | | — | | | 895 | |
| Share-based compensation | Share-based compensation | — | | | 2 | | | — | | | — | | | 35,981 | | | — | | | — | | | 35,983 | |
| Adoption of Update No. 2020-06 | Adoption of Update No. 2020-06 | — | | | — | | | — | | | — | | | (63,274) | | | — | | | (2,773) | | | (66,047) | |
| Balance, December 31, 2021 | Balance, December 31, 2021 | 89,600 | | | $ | 896 | | | (4,899) | | | $ | (234,448) | | | $ | 1,264,943 | | | $ | (45,155) | | | $ | 698,568 | | | $ | 1,684,804 | |
| Net income | |
| Other comprehensive income (loss), net of tax | |
| | Issuance of common stock through employee stock purchase plan | |
| Issuance of common stock through employee stock purchase plan | |
| Issuance of common stock through employee stock purchase plan | |
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | |
| Share-based compensation | |
| Accelerated shares repurchased | |
| Balance, December 31, 2022 | |
| Net income | |
| Other comprehensive income (loss), net of tax | |
| | Issuance of common stock through employee stock purchase plan | |
| Issuance of common stock through employee stock purchase plan | |
| Issuance of common stock through employee stock purchase plan | |
| Issuance of common stock for vesting of share-based awards, net of shares withheld for taxes | |
| Share-based compensation | |
| Accelerated shares repurchased | |
| Balance, December 31, 2023 | |
The accompanying notes are an integral part of these consolidated financial statements.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. BUSINESS
Integra LifeSciences Holdings Corporation (the “Company”) was incorporated in Delaware in 1989. The Company is a worldwide leader in medical technology. The Company was founded with the acquisition of an engineered collagen technology platform used to repair and regenerate tissue. Since then, Integra has developed numerous product lines from this technology for applications ranging from burn and deep tissue wounds to the repair of dura mater in the brain, as well as nerves and tendons. The Company has expanded its base regenerative technology business to include surgical instruments, neurosurgical products and advanced wound care through global acquisitions and product development to meet the evolving needs of its customers and enhance patient care. The Company sells its products directly through various sales forces and through a variety of other distribution channels.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF PRESENTATION
These financial statements and the accompanying notes are prepared in accordance with accounting principles generally accepted in the United States of America and conform to Regulation S-X under the Securities Exchange Act of 1934, as amended.
PRINCIPLES OF CONSOLIDATION
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. Intercompany accounts and transactions have been eliminated in consolidation. See Note 4, Acquisitions and Divestitures, for details of new subsidiaries included in the consolidation.
USE OF ESTIMATES
The preparation of consolidated financial statements is in conformity with generally accepted accounting principles in the United States ("GAAP") which requires management to make estimates and assumptions that affect the reported amount of assets and liabilities, the disclosure of contingent liabilities, and the reported amounts of revenues and expenses. Significant estimates affecting amounts reported or disclosed in the consolidated financial statements include allowances for doubtful accounts receivable and sales returns and allowances, net realizable value of inventories, valuation of intangible assets including amortization periods for acquired intangible assets, discount rates and estimated projected cash flows used to value and test impairments of long-lived assets and goodwill, estimates of projected cash flows and depreciation and amortization periods for long-lived assets, computation of taxes, valuation allowances recorded against deferred tax assets, the valuation of stock-based compensation, valuation of derivative instruments, valuation of contingent liabilities, the fair value of debt instruments and loss contingencies. These estimates are based on historical experience and on various other assumptions that are believed to be reasonable under the current circumstances. Actual results could differ from these estimates.As the Company continues to navigate the novel coronavirus ("COVID-19") pandemic and recent variants of the virus as well as the adverse impacts to global economic conditions, supply chain and the operations, there may be impact to future estimates including, but not limited to, inventory valuations, fair value measurements, goodwill and long-lived asset impairments, the effectiveness of the Company’s hedging instruments, deferred tax valuation allowances, and allowances for doubtful accounts receivable.
RECLASSIFICATIONS
Certain amounts from the prior year's financial statements have been reclassified in order to conform to the current year's presentation.
CASH AND CASH EQUIVALENTS
The Company considers all short-term, highly liquid investments purchased with original maturities of three months or less to be cash equivalents. These investments are carried at cost, which approximates fair value.
SHORT TERM INVESTMENTS
Short-term investments are securities with original maturities greater than 90 days that are available for use in our operations in the next twelve months. The short-term investments, primarily consisting of time deposits, are recorded at cost, which approximates fair value, which is estimated based on the net asset value of these investments. Interest and dividends are recorded in income when earned.
TRADE ACCOUNTS RECEIVABLE AND ALLOWANCES FOR DOUBTFUL ACCOUNTS RECEIVABLE
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The Company grants credit to customers in the normal course of business, but generally does not require collateral or any other security to support its receivables.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company evaluates the collectability of accounts receivable based on a combination of factors. The Company recognizes a provision for doubtful accounts that reflects the Company’s estimate of expected credit losses for trade accounts receivable. In circumstances where a specific customer is unable to meet its financial obligations to the Company, a provision to the allowances for doubtful accounts is recorded against amounts due to reduce the net recognized receivable to the amount that is reasonably expected to be collected. For all other customers, the Company evaluates measurement of all expected credit losses for trade receivables held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts.
In June 2016, the FASB issued ASU 2016-13, Financial InstrumentsF - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments8
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
. The Company adopted this guidance on January 1, 2020 using a modified retrospective transition method which requires a cumulative-effect adjustment to the opening balance of retained earnings to be recognized on the date of adoption with no change to financial results reported in prior periods. The cumulative-effect adjustment recorded on January 1, 2020 was not material. The adoption of this ASU did not have a significant impact on the Company's consolidated financial statements and related disclosures.
The Company's exposure to credit losses may increase if its customers are adversely affected by changes in healthcare laws, coverage, and reimbursement, economic pressures or uncertainty associated with local or global economic recessions, disruption associated with the COVID-19 pandemic and recent variants of the virus, and other customer-specific factors. Although the Company has historically not experienced significant credit losses, it is possible that there could be an adverse impact due to customer and governmental responses to the COVID-19 pandemic.recessions.
Provisions to the allowances for doubtful accounts are recorded to selling, general and administrative expenses. Account balances are charged off against the allowance when it is probable that the receivable will not be recovered. Provision for doubtful accounts, net of recoveries, associated with accounts receivable, included in selling, general and administrative expense, was recoveriescharges of $1.1$3.0 million for the year ended December 31, 2021, and2023, charges of $3.6$0.2 million, and $2.1recoveries of $1.1 million for the years ended December 31, 20202022 and 2019,2021, respectively.
The below table shows the roll forward of the allowance for doubtful accounts for the years ended December 31, 2023, 2022 and 2021:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at Beginning of Period | | Charged to Costs and Expenses | | Other | | Deductions | | Balance at End of Period |
| Dollars in thousands | | | | |
| Year Ended: | | | | | | | | | |
| December 31, 2023 | $ | 4,304 | | | 2,963 | | | — | | | (2,388) | | | $ | 4,879 | |
| December 31, 2022 | $ | 4,735 | | | 238 | | | — | | | (669) | | | $ | 4,304 | |
| December 31, 2021 | $ | 6,439 | | | (1,059) | | | 341 | | | (986) | | | $ | 4,735 | |
(1)Deductions primarily relates to allowance for doubtful accounts written off during the year, net of recoveries and other adjustments.
INVENTORIES
Inventories, consisting of purchased materials, direct labor and manufacturing overhead, are stated at the lower of cost, the value determined by the first-in, first-out method, or net realizable value. Inventories consisted of the following:
| | | | | | | | | | | |
| | December 31, |
| Dollars in thousands | 2021 | | 2020 |
| Finished goods | 162,528 | | | $ | 180,301 | |
| Work in process | 65,323 | | | 53,336 | |
| Raw materials | 89,535 | | | 76,480 | |
| Total inventories, net | $ | 317,386 | | | $ | 310,117 | |
At December 31, 2020, $52.8 million of inventories, net was presented separately as "Assets held for sale" in conjunction with the sale of the Extremity Orthopedics business. See Note 4, Acquisitions and Divestitures. | | | | | | | | | | | |
| | December 31, |
| Dollars in thousands | 2023 | | 2022 |
| Finished goods | 196,402 | | | $ | 172,088 | |
| Work in process | 74,035 | | | 70,598 | |
| Raw materials | 119,171 | | | 81,897 | |
| Total inventories, net | $ | 389,608 | | | $ | 324,583 | |
At each balance sheet date, the Company evaluates inventories for excess quantities, obsolescence or shelf life expiration. This evaluation includes analysis of historical sales levels by product, projections of future demand, the risk of technological or competitive obsolescence for products, general market conditions, a review of the shelf life expiration dates for products, as well as the feasibility of reworking or using excess or obsolete products or components in the production or assembly of other products that are not obsolete or for which there are not excess quantities in inventory. To the extent that management determines there are excess or obsolete inventory or quantities with a shelf life that is too near its expiration for the Company to reasonably expect that it can sell those products prior to their expiration, the Company adjusts the carrying value to estimated net realizable value.
The Company capitalizes inventory costs associated with certain products prior to regulatory approval, based on management's judgment of probable economic benefit. The Company could be required to expense previously capitalized costs related to pre-approval inventory upon a change in such judgment, due to, among other potential factors, a denial or delay of approval by necessary regulatory bodies or a decision by management to discontinue the related development program. No such amounts were capitalized at December 31, 20212023 or 2020.2022.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
In the twelve months ended December 31, 2023, due to the Boston recall, the Company recorded a $24.6 million write off of inventory to cost of goods sold that was no longer able to be sold.
PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment are stated at historical cost less accumulated depreciation and any impairment charges. The Company provides for depreciation using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are amortized over the lesser of the lease term or the useful life. The cost of major additions and improvements is capitalized, while maintenance and repair costs that do not improve or extend the lives of the respective assets are charged to operations as incurred. The cost of computer software developed or obtained for internal use is accounted for in accordance with the Accounting Standards Codification 350-40, Internal-Use Software.
Property, plant and equipment balances and corresponding lives were as follows:
| | | | | | | | | | | | | | | | | |
| | December 31, | | |
| Dollars in thousands | 2021 | | 2020 | | Useful Lives |
| Land | $ | 1,512 | | | $ | 1,541 | | | |
| Buildings and building improvements | 19,032 | | | 17,345 | | | 5-40 years |
| Leasehold improvements | 155,495 | | | 144,852 | | | 1-20 years |
| Machinery and production equipment | 183,270 | | | 166,973 | | | 3-20 years |
| Surgical instrument kits | 2,791 | | | 1,164 | | | 4-5 years |
| Information systems and hardware | 148,706 | | | 143,770 | | | 1-7 years |
| Furniture, fixtures, and office equipment | 20,921 | | | 20,843 | | | 1-15 years |
| Construction-in-progress | 94,850 | | | 73,890 | | | |
| Total | 626,577 | | | 570,378 | | | |
| Less: Accumulated depreciation | (314,874) | | | (282,849) | | | |
| Property, plant and equipment, net | $ | 311,703 | | | $ | 287,529 | | | |
At December 31, 2020, $37.9 million of property, plant and equipment, net was presented separately as "Assets held for sale" in conjunction with the sale of the Extremity Orthopedics business. See Note 4, Acquisitions and Divestitures. | | | | | | | | | | | | | | | | | |
| | December 31, | | |
| Dollars in thousands | 2023 | | 2022 | | Useful Lives |
| Land | $ | 978 | | | $ | 966 | | | |
| Buildings and building improvements | 14,859 | | | 14,710 | | | 5-40 years |
| Leasehold improvements | 171,062 | | | 163,342 | | | 1-20 years |
| Machinery and production equipment | 198,127 | | | 182,730 | | | 3-20 years |
| Demonstration equipment | 3,896 | | | 3,792 | | | 4-5 years |
| Information systems and hardware | 160,899 | | | 151,330 | | | 1-7 years |
| Furniture, fixtures, and office equipment | 20,549 | | | 20,286 | | | 1-15 years |
| Construction-in-progress | 137,276 | | | 103,875 | | | |
| Total | 707,646 | | | 641,031 | | | |
| Less: Accumulated depreciation | (367,447) | | | (329,729) | | | |
| Property, plant and equipment, net | $ | 340,199 | | | $ | 311,302 | | | |
Depreciation expense associated with property, plant and equipment was $39.4$40.9 million, $42.1$40.1 million, and $42.6$39.4 million for the years ended December 31, 2021, 20202023, 2022 and 2019,2021, respectively.
During the fourth quarter of 2020, the Company wrote-off certain construction in progress of $6.7 million related to a manufacturing project that the Company decided to discontinue. The Company determined that the carrying amounts of these assets were not recoverable.
CAPITALIZED INTEREST
The interest cost on capital projects, including facilities build-out and internal use software, is capitalized and included in the cost of the project. Capitalization commences with the first expenditure for the project and continues until the project is substantially complete and ready for its intended use. When no debt is incurred specifically for a project, interest is capitalized on project expenditures using the weighted average cost of the Company's outstanding borrowings. For the years ended December 31, 20212023 and 2020,2022, respectively, the Company capitalized $1.2$2.4 million and $2.3$1.4 million of interest expense into property, plant and equipment.
ACQUISITIONS
Results of operations of acquired companies are included in the Company’s results of operations as of the respective acquisition dates. The Company accounts for the acquisition of a business in accordance with ASC 805, Business Combinations (ASC 805) ("ASC Topic 805"). Amounts paid to acquire a business are allocated to the assets acquired and liabilities assumed based on their fair values at the date of acquisition. Any excess of the purchase price over the fair value of the net assets acquired inis recorded as goodwill. Transaction costs and costs to restructure the acquired company are expensed as incurred.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Contingent consideration is recorded at fair value as measured on the date of acquisition. The value recorded is based on estimates of future financial projections under various potential scenarios using either a Monte Carlo simulation or the probability-weighted income approach derived from revenue estimates and probability assessment with respect to the likelihood of achieving contingent obligations. Contingent payments related to acquisitions consist of development, regulatory, and commercial milestone payments, in addition to sales-based payments, and are valued using discounted cash flow techniques. Each quarter until such contingent amounts are earned, the fair value of the liability is remeasured at each reporting period and adjusted as a component of operating expenses based on changes to the underlying assumptions. The change in the fair value of sales-based payments is based upon future revenue estimates and increases or decreases as revenue estimates or expectation of timing of payment charges. The estimates used to determine the fair value of the contingent consideration liability are subject to significant judgment and actual results are likely to differ from the amounts originally recorded.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company determines the fair value of acquired intangible assets based on detailed valuations that use certain information and assumptions provided by management. The Company allocates any excess purchase price over the fair value of the net tangible and intangible assets acquired to goodwill. Determining the fair value of these intangible assets acquired as part of a business combination requires the Company to make significant estimates. These estimates include the amount and timing of projected future cash flows, the discount rate used to discount those cash flows to present value, the assessment of the asset’s life cycle, and the consideration of legal, technical, regulatory, economic, and competitive risks. The fair value assigned to other intangible assets is determined by estimating the future cash flows of each project or technology and discounting the net cash flows back to their present values. The discount rate used is determined at the time of measurement in accordance with accepted valuation methodologies.
Acquired IPR&D is recognized at fair value and initially characterized as an indefinite-lived intangible asset, irrespective of whether the acquired IPR&D has an alternative future use. The Company uses the income approach to determine the fair value of developed technology and IPR&D acquired in a business combination. This approach determines fair value by estimating the after-tax cash flows attributable to the respective asset over its useful life and then discounting these after-tax cash flows back to a present value. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each product including net revenues, cost of sales, R&D costs, selling and marketing costs, the appropriate discount rate to select in order to measure the risk inherent in each future cash flow stream, the assessment of each asset’s life cycle, and competitive trends impacting the asset and each cash flow stream. The Company also uses the income approach, as described above, to determine the estimated fair value of certain other identifiable intangible assets including customer relationships, trade names and business licenses. Customer relationships represent established relationships with customers, which provide a ready channel for the sale of additional products and services. Trade names represent acquired company and product names.
IPR&D acquired in a business combination is capitalized as an indefinite-lived intangible asset. Development costs incurred after the acquisition are expensed as incurred. Upon receipt of regulatory approval, the indefinite-lived intangible asset is then accounted for as a finite-lived intangible asset and amortized on a straight-line basis or accelerated basis, as appropriate, over its estimated useful life. If the research and development project is subsequently abandoned, the indefinite-lived intangible asset is charged to expense. IPR&D acquired outside of a business combination is expensed immediately.
Due to the uncertainty associated with research and development projects, there is risk that actual results will differ materially from the original cash flow projections and that the research and development project will result in a successful commercial product. The risks associated with achieving commercialization include, but are not limited to, delay or failure to obtain regulatory approvals to conduct clinical trials, delay or failure to obtain required market clearances, delays or issues with patent
issuance, or validity and litigation.
If the acquired net assets do not constitute a business under the acquisition method of accounting, the transaction is accounted for as an asset acquisition and no goodwill is recognized. In an asset acquisition, the amount allocated to acquired IPR&D with no alternative future use is charged to expense at the acquisition date. Payments that would be recognized as contingent consideration in a business combination are expensedrecognized when probable in an asset acquisition. Refer to Note 4, Acquisitions and Divestitures for more information.
GOODWILL AND OTHER INTANGIBLE ASSETS
The excess of the cost over the fair value of net assets of acquired businesses is recorded as goodwill. Goodwill is not subject to amortization but is reviewed for impairment at the reporting unit level annually, or more frequently if impairment indicators arise. The Company's assessment of the recoverability of goodwill is based upon a comparison of the carrying value of goodwill with its estimated fair value. The Company reviews goodwill for impairment in the third quarter every year in accordance with ASC Topic 350, Intangibles - Goodwill and Other ("ASC Topic 350") and whenever events or changes in circumstances indicate the carrying value of goodwill may not be recoverable. Refer to Note 7, Goodwill and Other Intangibles for more information.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company has 2two reportable segments with 3three underlying reporting units. Refer to Note 16, Segment and Geographic Information for more information on reportable segments.
Other intangible assets include patents, trademarks, purchased technology, and supplier and customer relationships. Identifiable intangible assets are initially recorded at fair market value at the time of acquisition generally using an income or cost approach. The Company capitalizes costs incurred to renew or extend the term of recognized intangible assets and amortizes those costs
over their expected useful lives.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company tests intangible assets with indefinite lives for impairment annually in the third quarter in accordance with ASC Topic 350. Additionally, the Company may perform interim tests if an event occurs or circumstances change that could potentially reduce the fair value of aan indefinite lived intangible asset below its carrying amount. The Company tests for impairment by either performing a qualitative evaluation or a quantitative test. The qualitative evaluation is an assessment of factors, including specific operating results as well as industry, market and general economic conditions, to determine whether it is more likely than not that the fair values of the intangible asset is less than its carrying amount. The Company may elect to bypass this qualitative evaluation and perform a quantitative test.
Product rights and other definite-lived intangible assets are tested periodically for impairment in accordance with ASC Topic 360 when events or changes in circumstances indicate that an asset's carrying value may not be recoverable. The impairment testing involves comparing the carrying amount of the asset or asset group to the forecasted undiscounted future cash flows. In the event the carrying value of the asset exceeds the undiscounted future cash flows, the carrying value is considered not recoverable and impairment exists. An impairment loss is measured as the excess of the asset's carrying value over its fair value, calculated using discounted future cash flows. The computed impairment loss is recognized in the period that the impairment occurs.
LONG-LIVED ASSETS
Long-lived assets held and used by the Company, including property, plant and equipment, intangible assets, and leases are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. For purposes of evaluating the recoverability of long-lived assets to be held and used, a recoverability test is performed using projected undiscounted net cash flows applicable to the long-lived assets. If an impairment exists, the amount of such impairment is calculated based on the estimated fair value of the asset. Impairments to long-lived assets to be disposed of are recorded based upon the difference between the carrying value and the fair value of the applicable assets.
INTEGRA FOUNDATION
The Company may periodically make contributions to the Integra Foundation, Inc. The Integra Foundation was incorporated in 2002 exclusively for charitable, educational, and scientific purposes and qualifies under IRC 501(c)(3) as an exempt private foundation. Under its charter, the Integra Foundation engages in activities that promote health, the diagnosis and treatment of disease, and the development of medical science through grants, contributions and other appropriate means. The Integra Foundation is a separate legal entity and is not a subsidiary of the Company; therefore, its results are not included in these consolidated financial statements. The Company contributed $1.2 million, $0.8 million and $0.3 millionmade no contributions to the Integra Foundation during the years ended December 31, 2021, 20202023 and 2019, respectively.2022 and $1.2 million during the year ended December 31, 2021. These contributions were recorded in selling, general, and administrative expense.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
DERIVATIVES
The Company develops, manufactures, and sells medical devices globally and its earnings and cash flows are exposed to market risk from changes in interest rates and currency exchange rates. The Company addresses these risks through a risk management program that includes the use of derivative financial instruments and operates the program pursuant to documented corporate risk management policies. All derivative financial instruments are recognized in the financial statements at fair value in accordance with the authoritative guidance. Under the guidance, for those instruments that are designated and qualify as hedging instruments, the hedging instrument must be designated as a fair value hedge, cash flow hedge, or a hedge of a net investment in a foreign operation, based on the exposure being hedged. The accounting for changes in the fair value of a derivative instrument depends on whether it has been designated and qualifies as part of a hedging relationship and, further, on the type of hedging relationship. The Company's derivative instruments do not subject its earnings or cash flows to material risk, and gains and losses on these derivatives generally offset losses and gains on the item being hedged. The Company has not entered into derivative transactions for speculative purposes. From time to time, the Company may enter into derivatives that are not designated as hedging instruments in order to protect itself from currency volatility due to intercompany balances.
All derivative instruments are recognized at the fair values as either assets or liabilities on the balance sheet. The Company determines the fair value of its derivative instruments using the framework prescribed by the authoritative guidance, by considering the estimated amount the Company would receive to sell or transfer these instruments at the reporting date and by taking into account:account expected forward interest rates, currency exchange rates, the creditworthiness of the counterparty for assets, and its creditworthiness for liabilities. In certain instances, the Company utilizes a discounted cash flow model to measure fair value. Generally, the Company uses inputs that include quoted prices for similar assets or liabilities in active markets, other observable inputs for the asset or liability and inputs derived principally from, or corroborated by, observable market data by correlation or other means. The Company has classified all of its derivative assets and liabilities within Level 2 of the fair value hierarchy because observable inputs are available for substantially the full term of its derivative instruments. The Company classifies derivatives designated as hedges in the same category as the item being hedged for cash flow presentation purposes.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company entered into foreign currency forward and foreign currency swap contracts that are not designated as a hedging instruments for accounting purposes. These contracts are recorded at fair value, with the changes in fair value recognized into other income, net, on the consolidated financial statements. Refer to Note 6, Derivative Instruments for more information.
FOREIGN CURRENCY
All assets and liabilities of foreign subsidiaries which have a functional currency other than the U.S. dollar are translated at the rate of exchange at year-end, while elements of the income statement are translated at the average exchange rates in effect during the year. The net effect of these translation adjustments is shown as a component of accumulated other comprehensive income (loss). These currency translation adjustments are not currently adjusted for income taxes as they relate to permanent investments in non-U.S. subsidiaries. Foreign currency transaction net losses of $4.4 million, net losses of $3.3 million, and net gains of less than $0.1 million, and net losses of $1.6 million and $0.3 million are reported in other income, net in the statements of operations, for the year ended December 31, 2021, 20202023, 2022 and 2019,2021, respectively.
INCOME TAXES
Income taxes are accounted for by using the asset and liability method. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis. A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period when the change is enacted.
The Company recognizes a tax benefit from an uncertain tax position only if it is more likely than not to be sustained upon examination based on the technical merits of the position. Reserves are established for positions that don't meet this recognition threshold. The reserve is measured as the largest amount of benefit determined on a cumulative probability basis that the Company believes is more likely than not to be realized upon ultimate settlement of the position. These reserves are classified as long-term liabilities in the consolidated balance sheets of the Company, unless the reserves are expected to be paid in cash during the next twelve months, in which case they are classified as current liabilities. The Company also records interest and penalties accrued in relation to uncertain tax benefits as a component of income tax expense.
While the Company believes it has identified all reasonable exposures and the reserve it has established is appropriate under the circumstances, it is possible that additional exposures exist and that exposures may be settled at amounts different than the amounts reserved. It is also possible that changes in facts and circumstances could cause the Company to either materially increase or reduce the carrying amount of its tax reserve.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company continues to indefinitely reinvest substantially all of its foreign earnings.earnings unless there is a manner under which to remit the earnings without a material tax cost. The current provisional analysis indicates that the Company has sufficient U.S. liquidity, including borrowing capacity, to fund foreseeable U.S. cash needs without requiring the repatriation of foreign cash. One time or unusual items that may impact the ability or intent to keep the foreign earnings and cash indefinitely reinvested include significant U.S. acquisitions, loans from a foreign subsidiary and changes in tax laws.
REVENUE RECOGNITION
Revenue is recognized upon the transfer of control of promised products or services to the customers in an amount that reflects the consideration the Company expects to receive in exchange for those products and services.
Total revenue, net, includes product sales, product royalties and other revenues, such as fees received from services.
For products shipped with FOB shipping point terms, the control of the product passes to the customer at the time of shipment. For shipments in which the control of the product is transferred when the customer receives the product, the Company recognizes revenue upon receipt by the customer. Certain products that the Company produces for private label customers have no alternative use and the Company has a right of payment for performance to date. Revenues from those products are recognized over the period that the Company manufactures these products, which is typically one month to three months. The Company uses the input method to measure the manufacturing activities completed to date, which depicts the progress of the Company's performance obligation of transferring control of goods being manufactured for private label customers.
A portion of the Company's product revenue is generated from consigned inventory maintained at hospitals and distributors, and also from inventory physically held by field sales representatives. For these types of products sales, the Company retains control until the product has been used or implanted, at which time revenue is recognized.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Revenues from sale of products and services are evidenced by either a contract with the customer or a valid purchase order and an invoice which includes all relevant terms of sale. For product sales, invoices are generally issued upon the transfer of control (or upon the completion of the manufacturing in the case of the private label transactions recognized over time) and are typically payable 30 days after the invoice date. The Company performs a review of each specific customer's creditworthiness and ability to pay prior to acceptance as a customer. Further, the Company performs periodic reviews of its customers' creditworthiness prospectively. Refer to Note 3, Revenue From Contracts With Customers for more information. The Company also maintains a provision for estimated returns and allowances in the same period that the related revenue is recorded. This reserve is based upon an analysis of actual credit memos issued for pricing issues or returned goods over an extended period, as well as assumptions about outstanding accounts receivable and judgment in interpreting the data.
RESEARCH AND DEVELOPMENT
Research and development costs, including salaries, depreciation, consultant and other external fees, and facility costs directly attributable to research and development activities, are expensed in the period in which they are incurred.
EMPLOYEE TERMINATION BENEFITS
The Company does not have a written severance plan, and it does not offer similar terminationbut has a history of providing benefits to affectedfor employees in all restructuring initiatives. Accordingly, inthe case of involuntary termination. In situations whereoutside the US, there are minimum statutory termination benefits requirements by country that must be paid to the affected employees, theemployees. The Company records employee severance costs associated with these restructuring activities in accordance with the authoritative guidance for non-retirement post-employment benefits. Charges associated with these activities are recorded when the payment of benefits is probable and can be reasonably estimated. In all other situations where the Company pays out termination benefits including supplemental benefits paid in excess of statutory minimum amounts and benefits offered to affected employees based on management's discretion, the Company records these termination costs in accordance withonce communication is made to the authoritative guidance for ASC Topic 712 Compensation-Nonretirement Benefits and ASC Topic 420 One-time Employee Termination Benefits.affected employees.
The timing of the recognition of charges for employee severance costs other than minimum statutory benefits depends on whether the affected employees are required to render service beyond their legal notification period in order to receive the benefits. If affected employees are required to render service beyond their legal notification period, charges are recognized over the future service period. Otherwise, charges are recognized when management has approved a specific plan and employee communication requirements have been met.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company incurred employee termination costs on sales force restructuring costs of $3.4 million and $4.9 million in cost of goods sold, $0.5 million and $1.2 million in selling, general and administrative and $0.3 million and $0.3 million in research and development related to employee terminations associated with a future plant closureactivities in the consolidated statement of operations for the yearsyear ended December 31, 20212023. In addition, the Company incurred employee termination costs on restructuring activities associated with a closure of a manufacturing facility in France and 2020, respectively. Restructuring costsother reorganization projects in the consolidated statement of $10.2 million wereoperations for the year ended December 31, 2022. The following table summarizes the restructuring related accrual balances included in accruedwithin accrued expenses and other current liabilities and $6.4 million were included in other liabilities in the consolidated balance sheet for the year ended December 31, 20212023 and 2020, respectively.2022.
| | | | | | | | | | | | | | |
| | Years Ended December 31, |
| (Dollars in thousands) | | 2023 | | 2022 |
| Balance, beginning of the year | | $ | 5,107 | | | $ | 10,226 | |
| Charges: | | | | |
| Cost of Goods Sold | | — | | | 1,494 | |
| Research and development | | — | | | 72 |
| Selling, general and administrative | | 1,048 | | | 5,582 | |
| Payments and other adjustments | | (4,042) | | | (12,267) | |
| Balance, end of the year | | $ | 2,113 | | | $ | 5,107 | |
STOCK-BASED COMPENSATION
Relevant authoritative guidance requires companies to recognize the expense related to the fair value of their stock-based compensation awards. Stock-based compensation expense for stock option awards are based on the grant date fair value using the binomial distribution model. The Company recognizes compensation expense for stock option awards, restricted stock awards, performance stock awards and contract stock awards over the requisite service period of the award. All excess tax benefits and taxes and tax deficiencies from stock-based compensation are included in provision for income taxes in the consolidated statement of operations. Refer to Note 9, Stock-based Compensation for more information.
PENSION BENEFITS
The Company maintains defined benefit pension plans that cover certain employees in France, Japan, Germany and Switzerland. Various factors are considered in determining the pension liability, including the number of employees expected to be paid their salary levels and years of service, the expected return on plan assets, the discount rate used to determine the benefit obligations, the timing of benefit payments and other actuarial assumptions.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Retirement benefit plan assumptions are reassessed on an annual basis or more frequently if changes in circumstances indicate a re-evaluation of assumptions are required. The key benefit plan assumptions are the discount rate and expected rate of return on plan assets. The discount rate is based on average rates on bonds that matched the expected cash outflows of the benefit plans. The expected rate of return is based on historical and expected returns on the various categories of plan assets.
The Company uses the corridor approach in measuring the amount of net periodic benefit pension cost to recognize each period. The corridor approach defers all actuarial gains and losses resulting from variances between actual results and actuarial assumptions. Those unrecognized gains and losses are amortized when the net gains and losses exceed 10% of the greater of the market-related value of plan assets or the projected benefit obligation at the beginning of the year. The amount in excess of the corridor is amortized over the average remaining service period to retirement date of active plan participants.
Deferred Compensation Plan
The Company maintains a deferred compensation plan in which certain employees of the Company may defer the payment and taxation of up to 75% of their base salary and up to 100% of bonus amounts and other eligible cash compensation.
This deferred compensation is invested in funds offered under the Plan and is valued based on Level 1 measurements in the fair value hierarchy. The purpose of the plan is to retain key employees by providing them with an opportunity to defer a portion of their compensation as elected by the participant in accordance with the plan. Any amounts set aside to defray the liabilities assumed by the Company will remain the general assets of the Company until such amounts are distributed to the participants. Assets of the Company's deferred compensation plan are included in Other current assets and recorded at fair value based on their quoted market prices.
CONCENTRATION OF CREDIT RISK
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of cash and cash equivalents, which are held at major financial institutions, investment-grade marketable debt securities and trade receivables.
The Company's products are sold on an uncollateralized basis and on credit terms based upon a credit risk assessment of each customer. A portion of the Company's trade receivables to customers outside the United States includes sales to foreign distributors, who then sell to government owned or supported healthcare systems.
None of the Company's customers accounted for 10% or more of the consolidated net sales during the years ended December 31, 2023, 2022 and 2021 2020.
RECENT ACCOUNTING PRONOUNCEMENTS
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures which enhances the transparency of income tax disclosures by expanding annual disclosure requirements related to the rate reconciliation and 2019.income taxes paid. The amendments are effective for fiscal years beginning after December 15, 2023, and for interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The amendments should be applied retrospectively to all prior periods presented in the financial statements. The Company is currently evaluating this ASU to determine its impact on the Company's disclosures.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which updates reportable segment disclosure requirements primarily through enhanced disclosures about significant segment expenses. The amendments are effective for fiscal years beginning after December 15, 2023, and for interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. The amendments should be applied retrospectively to all prior periods presented in the financial statements. The Company does not plan to early adopt, but is currently evaluating this ASU to determine its impact on the Company's disclosures.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
RECENT ACCOUNTING PRONOUNCEMENTS
In August 2018, the FASB issued ASU 2018-14, Compensation-Retirement Benefits-Defined Benefit Plans-General (Subtopic 715-20): Disclosure Framework-Changes to the Disclosure Requirements for Defined Benefit Plans. This guidance modifies the disclosure requirements for employers that sponsor defined benefit pension or other postretirement plans. The ASU is effective for fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. Early adoption was permitted. The Company adopted this guidance during the year ended December 31, 2020. The adoption of this guidance did not have a significant impact on the Company's consolidated financial statements and related disclosures.
In August 2018, the FASB issued ASU 2018-15, Intangibles - Goodwill and Other - Internal-Use Software (Subtopic 350-40), relating to a customer's accounting for implementation, set-up, and other upfront costs incurred in a cloud computing arrangement that is hosted by a vendor (e.g., a service contract). Under this guidance, a customer will apply the same criteria for capitalizing implementation costs as it would for an arrangement that has a software license. The new guidance also prescribes the balance sheet, income statement, and cash flow classification of the capitalized implementation costs and related amortization expense, and requires additional quantitative and qualitative disclosures. The ASU is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. The Company adopted this guidance on January 1, 2020 using a prospective transition method. The adoption of this guidance did not have a significant impact on the Company's consolidated financial statements and related disclosures.
In December 2019, the FASB issued ASU 2019-12, Income Taxes: Simplifying the Accounting for Income Taxes, intended to simplify the accounting for income taxes by eliminating certain exceptions related to the approach for intra-period tax allocation, the methodology for calculating income taxes in an interim period and the recognition of deferred tax liabilities for outside basis differences. This guidance also simplifies aspects of the accounting for franchise taxes and enacted changes in tax laws or rates and clarifies the accounting for transactions that result in a step-up in the tax basis of goodwill. The standard is effective for annual periods beginning after December 15, 2020 and interim periods within, with early adoption permitted. The Company adopted ASU 2019-12 as of January 1, 2021. Adoption of the standard requires certain changes to be made prospectively, with some changes to be made retrospectively. The adoption of this guidance did not have a significant impact on the Company's consolidated financial statements and related disclosures.
In March 2020, the FASBFinancial Accounting Standards Board ("FASB") issued ASU 2020-04, Reference Rate Reform (Topic 848), whichand subsequent amendment to the initial guidance: ASU 2021-01, Reference Rate Reform (Topic 848): Scope (collectively, “Topic 848”). Topic 848 provides optional guidanceexpedients and exceptions for a limited period of timeapplying GAAP to ease the potential burden in accounting for (or recognizing the effects of)contracts, hedging relationships, and other transactions affected by reference rate reform on financial reporting. This amendment applies to all entities, subject to meetingif certain criteria that haveare met. The amendments apply only to contracts, hedging relationships, and other transactions that reference London Inter-Bank Offered Rate ("LIBOR") or another reference rate expected to be discontinued because of reference rate reform. This ASU is effective immediately and mayThe guidance generally can be applied prospectivelythrough December 31, 2024. The Alternative Reference Rates Committee, a group of private-market participants convened by the U.S. Federal Reserve Board and the New York Federal Reserve, has recommended the use of the Secured Overnight Financing Rate ("SOFR") as a more robust reference rate alternative to contract modifications made and hedging relationshipsLIBOR. On March 24, 2023, the Company entered into or evaluatedthe seventh amendment and restatement (the "March 2023 Amendment") of its Senior Credit Facility (the “Senior Credit Facility”) with a syndicate of lending banks with Bank of America, N.A., as Administrative Agent. In connection with the March 2023 Amendment, the Company replaced all LIBOR-based contracts with SOFR, which is calculated based on or before December 31, 2022.overnight transactions under repurchase agreements backed by Treasury securities. In January 2021,addition, on April 17, 2023 the FASB also issued ASU 2021-01, Reference Rate Reform- Scope which clarified certain optional expedients and exceptions to entities that are affected becauseCompany entered into an amendment (the "April 2023 Amendment") of the referenceSecuritization Facility (as defined below) and amended the interest rate reform. The amendments in this ASU affectfrom LIBOR to SOFR indexed rate. (See Note 6). In March 2023, the guidance in ASU 2020-04Company entered into a basis swap where the Company receives Term SOFR and are effectivepays LIBOR to convert the portfolio of interest rate swaps from LIBOR to SOFR. Integra has elected to adopt the optional expedient under ASC 848, which will allow the interest rate swap hedging relationship to continue, without de-designation, due to the change in the same timeframe as ASU 2020-04. The Company currently has contracts that are indexed rate from LIBOR to LIBOR and are continuing to monitor this activity and evaluate the associated risk. The Company is continuing to evaluate the scope of impacted contracts and the potential impact. The Company is also monitoring the developments regarding alternative rates and may amend certain contracts to accommodate those rates if the contract does not already specify a replacement rate. While the notional value of agreements potentially indexed to LIBOR is material, the Company does not expect a material impact consolidated financial statements and related disclosures associated with this transition.SOFR.
In August 2020, the FASB issued ASU 2020-06, Debt- Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging-Contracts in Entity's Own Equity (Subtopic 815-40):Accounting for Convertible Instruments and Contracts in an Entity's Own Equity. The guidance simplifies accounting for convertible instruments by removing major separation models required under current GAAP. Consequently, more convertible debt instruments will be reported as a single liability instrument with no separate accounting for embedded conversion features. The ASU removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception, which will permit more equity contracts to qualify. The guidance also simplifies the diluted net income per share calculation in certain areas. The ASU will be effective for annual and interim periods beginning after December 15, 2021, and early adoption is permitted for fiscal years beginning after December 15, 2020, and interim periods within those fiscal years using either the modified retrospective or full retrospective method.
As detailed in Note 5, Debt, on February 4, 2020, the Company issued $575.0 million aggregate principal amount of its 0.5% Convertible Senior Notes due 2025 (the "2025 Notes"). The 2025 Notes are subject to the guidance included in ASU 2020-06. The Company adopted this guidance on January 1, 2021 using the modified retrospective approach which resulted in a cumulative-effect adjustment that increased (decreased) the following consolidated balance sheet accounts:
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | | |
ADJUSTMENT | | CONSOLIDATED BALANCE SHEET CLASSIFICATION | | AMOUNT
(in millions) |
Deferred tax impact of cumulative-effect adjustment | | Deferred tax liabilities | | $ | (20.6) | |
Debt discount reclassification | | Long-term convertible securities | | 89.1 |
Equity issuance costs reclassification | | Long-term convertible securities | | (2.5) | |
Debt discount amortization and equity costs reclassification, net of tax | | Retained Earnings | | (2.8) | |
Net impact of cumulative-effect adjustment | | Additional paid-in capital | | (63.3) | |
On December 9, 2020, the Company made an irrevocable election under the indenture to require the principal portion of its 2025 Notes to be settled in cash and any excess in shares. Following the irrevocable notice, only the amounts settled in excess of the principal will be considered in diluted earnings per share under the “if-converted” method. Upon adoption of ASU 2020-06, the Company’s 2025 Notes were reflected entirely as a liability since the embedded conversion feature will no longer be separately presented within stockholders’ equity. Additionally, from January 1, 2021, the Company is no longer incurring non-cash interest expense for the amortization of debt discount, therefore the interest expense for the 2025 Notes, which is included in the interest expense on the consolidated statements of operations and comprehensive loss, is lower as compared to the fiscal year of 2020.
In October 2020, the FASB issued ASU 2020-10, Codification Improvements, which updates various codification topics by clarifying or improving disclosure requirements to align with the regulations of the U.S. Securities and Exchange Commission (the "SEC") . The ASU has been effective for the Company for annual and interim periods beginning after January 1, 2021. The Company adopted this standard on the January 1, 2021. The adoption of this guidance did not have a significant impact on the Company's consolidated financial statements and related disclosures.
In May 2021, the FASB issued ASU 2021-04, Earnings Per Share (Topic 260), Debt—Modifications and Extinguishments (Subtopic 470-50), Compensation—Stock Compensation (Topic 718), and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options which provides guidance to clarify and reduce diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options (for example, warrants) that remain equity classified after modification or exchange. The amendments in this ASU No. 2021-04 are effective for all entities for fiscal years beginning after December 15, 2021, and interim periods within those fiscal years, with early adoption permitted, including interim periods within those fiscal years. The amendment currently has no impact to the Company as the effect will largely depend on the terms of written call options or financings issued or modified in the future.
There are no other recently issued accounting pronouncements that are expected to have any significant effect on the Company's financial position, results of operations or cash flows.
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION
Cash paid for interest during the years ended December 31, 2023, 2022 and 2021 2020 and 2019 was $43.2$44.3 million (net of $1.2$2.4 million that was capitalized into construction in progress), $47.3$42.2 million (net of $2.3$1.4 million that was capitalized into construction in progress) and $48.9$43.2 million (net of $3.1$1.2 million that was capitalized into construction in progress), respectively.
Cash paid for income taxes, net of refunds, for the years ended December 31, 2021, 20202023, 2022 and 20192021 was $49.5$23.6 million, $29.835.9 million and $16.2$49.5 million, respectively.
NON-CASH INVESTING AND FINANCING ACTIVITIES
Property and equipment purchases included in liabilities at December 31, 2023, 2022 and 2021 2020 and 2019 were $4.7$10.0 million, $1.6$10.5 million and $11.0$4.7 million, respectively.
During the fourth quarter of 2021, the Company achieved its final developmental milestone which triggered a $5.0 million obligation to be paid to former shareholders of Rebound Therapeutics Corporation ("Rebound"). The Company recorded $5.0 million as an intangible asset in the consolidated balance sheet upon achieving the milestone. The remaining obligation was included in accrued liabilities at December 31, 2021 in the consolidated balance sheets. The milestone is expected to bewas fully paid during the first quarter ofin 2022.
During the fourth quarter of 2020, the Company achieved another developmental milestone which triggered a $20.0 million obligation to be paid to the former shareholders of Rebound. The Company recorded $20.0 million as an intangible asset in the consolidated balance sheet upon achieving the milestone. The milestone was paid during the fourth quarter of 2020.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
During the fourth quarter of 2019, the Company achieved the first developmental milestone which triggered a $5.0 million obligation to be paid to former shareholders of Rebound. In addition, the Company recorded $5.0 million as in-process research and development expense in the consolidated statements of operations. The obligation was included in accrued liabilities at December 31, 2019 in the consolidated balance sheets. The milestone was paid during the first quarter of 2020.
3. REVENUES FROM CONTRACTS WITH CUSTOMERS
Summary of Accounting Policies on Revenue Recognition
Revenue is recognized upon the transfer of control of promised products or services to the customers in an amount that reflects the consideration the Company expects to receive in exchange for those products and services.
Performance Obligations
The Company's performance obligations consist mainly of transferring control of goods and services identified in the contracts, purchase orders, or invoices. The Company has no significant multi-element contracts with customers.
Significant JudgmentsEstimates
Usage-based royalties and licenses are estimated based on the provisions of contracts with customers and recognized in the same period that the royalty-based products are sold by the Company's strategic partners. The Company estimates and recognizes royalty revenue based upon communication with licensees, historical information, and expected sales trends. Differences between actual reported licensee sales and those that were estimated are adjusted in the period in which they become known, which is typically the following quarter. Historically, such adjustments have not been significant.
The Company estimates returns, price concessions, and discount allowances using the expected value method based on historical trends and other known factors. Rebate allowances are estimated using the most likely method based on each customer contract.
The Company's return policy, as set forth in its product catalogs and sales invoices, requires review and authorization in advance prior to the return of product. Upon the authorization, a credit will be issued for the goods returned within a set amount of days from the shipment, which is generally ninety90 days.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Due to the voluntary recall in 2023 of all products manufactured at the Boston facility, including Primatrix®, Surgimend®, Revize™, and TissueMend™, the Boston recall, the Company recorded a total of $18.7 million provision for product returns, as a reduction of net revenue, and has credited $9.9 million to customers during the year ended December 31, 2023. As of December 31, 2023, the return reserve was $8.8 million.
The Company disregards the effects of a financing component if the Company expects, at contract inception, that the period between the transfer and customer payment for the goods or services will be one year or less. The Company has no significant revenues recognized on payments expected to be received more than one year after the transfer of control of products or services to customers.
Contract Asset and Liability
Revenues recognized from the Company's private label business that are not invoiced to the customers as a result of recognizing revenue over time are recorded as a contract asset included in the prepaid expenses and other current assets account in the consolidated balance sheet. Upon invoicing to the customer, the balance is recorded in trade receivable, net in the consolidated balance sheet.
Other operating revenues may include fees received under service agreements. Non-refundable fees received under multiple-period service agreements are recognized as revenue as the Company satisfies the performance obligations to the other party. A portion of the transaction price allocated to the performance obligations to be satisfied in the future periods is recognized as a contract liability.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following table summarized the changes in the contract asset and liability balances for the year ended December 31, 2021:2023:
| | | | | |
| Dollars in thousands | Total |
| Contract Asset | |
Contract asset, January 1, 20212023 | $ | 7,43010,122 | |
Transferred to trade receivable offrom contract asset included
| (7,430)(7,743) | |
| Written off from beginning of the year contract asset due to Boston recall | (2,379) | |
| Contract asset, net of transferred to trade receivables on contracts during the period | 11,4129,233 | |
Contract asset, December 31, 20212023 | $ | 11,4129,233 | |
| |
| Contract Liability | |
Contract liability, January 1, 20212023 | $ | 11,96116,127 | |
| Recognition of revenue included in beginning of year contract liability | (5,164)(6,834) | |
| Contract liability, net of revenue recognized on contracts during the period | 5,1756,951 | |
| Foreign currency translation | (26)8 | |
Contract liability, December 31, 20212023 | $ | 11,94616,252 | |
At December 31, 2021,2023, the short-term portion of the contract liability of $5.3$8.5 million and the long-term portion of $6.7$7.7 million is included in current liabilities and other liabilities, respectively, in the consolidated balance sheet.
As of December 31, 2021,2023, the Company is expected to recognize revenue from unsatisfied or partially satisfied performance obligations of approximately $5.3 million in 2022, $2.9 million in 2023, $1.8$8.5 million in 2024, $0.9$4.2 million in 2025, $0.6$2.1 million in 2026, $1.1 million in 2027, $0.2 million in 2028, and $0.4$0.1 million thereafter.
Shipping and Handling Fees
The Company elected to account for shipping and handling activities as a fulfillment cost rather than a separate performance obligation. Amounts billed to customers for shipping and handling are included as part of the transaction price and recognized as revenue when control of underlying products is transferred to the customer. The related shipping and freight charges incurred by the Company are included in the cost of goods sold.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Product Warranties
Certain of the Company's medical devices, including monitoring systems and neurosurgical systems, are designed to operate over long periods of time. These products are sold with warranties which may extend for up to two years from the date of purchase. The warranties are not considered a separate performance obligation. The Company estimates its product warranties using the expected value method based on historical trends and other known factors. The Company includes them in accrued expenses and other current liabilities in the consolidated balance sheet.
Taxes Collected from Customers
The Company elected to exclude from the measurement of the transaction price all taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction and collected by the entity from a customer.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Disaggregated Revenue
The following table presents revenues disaggregated by the major sources of revenues for years-ended December 31, 2021, 20202023, 2022 and 20192021 (dollar amounts in thousands):
| | | Year Ended December 31, 2021 | Year Ended December 31, 2020 | Year Ended December 31, 2019 |
| | | | Year Ended December 31, 2023 | |
| | | | Year Ended December 31, 2023 | |
| | | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | Year Ended December 31, 2021 |
| Neurosurgery | Neurosurgery | | 802,959 | | 716,339 | | 767,793 | |
| Instruments | Instruments | | 222,273 | | $ | 178,492 | | $ | 228,413 | |
| Total Codman Specialty Surgical | Total Codman Specialty Surgical | | 1,025,232 | | 894,831 | | 996,206 | |
| Wound Reconstruction and Care(2) | | 392,463 | | 293,038 | | 322,739 | |
Extremity Orthopedics(1) | | — | | 78,316 | | 90,082 | |
Wound Reconstruction and Care(1)(2) | |
Wound Reconstruction and Care(1)(2) | |
Wound Reconstruction and Care(1)(2) | |
| Private Label | Private Label | | 124,753 | | 105,683 | | 108,530 | |
| Total Tissue Technologies | Total Tissue Technologies | | 517,216 | | 477,037 | | 521,351 | |
| Total revenue | Total revenue | | $ | 1,542,448 | | $ | 1,371,868 | | $ | 1,517,557 | |
(1) On January 4, 2021, the Company completed its sale of its Extremity Orthopedics business. In conjunction with the sale of this business, the Company rebranded the Orthopedics and Tissue Technologies segment as Tissue Technologies in the first quarter of 2021. See Note 4. Acquisitions and Divestitures, for details.
(2) See Note 4. Acquisitions and Divestitures, for details around the ACell acquisition.and SIA acquisitions.
Prior period amounts were reclassified between categories within(2) On August 31, 2022, the Codman Specialty Surgical segment to conform toCompany completed the current period presentation.sale of its non-core traditional wound care ("TWC") business. See Note4, Acquisitions and Divestitures
See Note 16, Segment and Geographical Information, for details of revenues based on the location of the customer.
4. ACQUISITIONS AND DIVESTITURES
Sale of Extremity Orthopedics BusinessSurgical Innovation Associates, Inc. Acquisition
On January 4, 2021,December 6, 2022, the Company completed the saleits acquisition of its Extremity Orthopedics business to Smith & Nephew USD LimitedSurgical Innovation Associates, Inc. ("Smith & Nephew"). The transaction included the sale of the Company's upper and lower Extremity Orthopedics product portfolio, including ankle and shoulder arthroplasty and hand and wrist product lines. The Company received an aggregate purchase price of $240.0 million from Smith & Nephew and concurrently paid $41.5 million to the Consortium of Focused Orthopedists, LLC ("CFO") effectively terminating the licensing agreement between Integra and CFO relating to the development of shoulder arthroplasty products.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Assets and liabilities divested consisted of the following as of December 31, 2020 (dollar amounts in thousands):
| | | | | |
Prepaid expenses and other current assets | $ | 713 | |
Right of use asset - operating leases and Other assets | 3,186 | |
Deferred tax assets | 6,589 | |
Intangible assets, net | 13,332 | |
Property, plant and equipment, net | 37,893 | |
Goodwill | 47,546 | |
Inventories | 52,845 | |
Total assets held for sale | $ | 162,104 | |
| |
Other liabilities | $ | 336 | |
Current portion of lease liability - operating leases | 539 | |
Accrued compensation | 1,767 | |
Deferred tax liabilities | 3,440 | |
Lease liability - operating leases | 5,669 | |
Total liabilities held for sale | $ | 11,751 | |
The divestiture does not represent a strategic shift that will have a major effect on the Company's operations and financial statements. Goodwill was allocated to the assets and liabilities divested using the relative fair value method of the Extremity Orthopedics business to the Company's Tissue Technologies reporting unit. In connection with the sale, the Company recognized a gain of $41.8 million that is presented in Gain from the sale of business in the consolidated statement of operations for the year ended December 31, 2021. The Company finalized the net working capital and paid an additional $1.3 million to Smith & Nephew as of December 31, 2021.
The Company also entered into a transition services agreement with Smith & Nephew which requires the Company to provide certain services on behalf of Smith & Nephew for the duration of the period subsequent to the sale of the business as defined in the agreement. The Company recognized a payable due to Smith & Nephew of $9.1 million as of December 31, 2021, included in the consolidated balance sheet within accrued expenses and other current liabilities.
ACell, Inc. Acquisition
On January 20, 2021, the Company acquired ACell, Inc. (the "ACell Acquisition"SIA") for an acquisition purchase price of $306.9$51.5 million (the "SIA Acquisition") plus contingent considerationsconsideration of up to $100 million, that may be payable upon$90.0 million. In addition to the purchase price, the acquisition includes two separate contingent considerations payments, which are dependent on 1) achieving certain revenue-based performance milestones in 2022, 2023, 2024, and 2025. The final working capital adjustments2025 (up to $50.0 million in additional payments), as well as 2) the approval by the FDA of $1.3the Premarket Approval (“PMA”) Application for DuraSorb for certain uses by certain timing targets (up to $40.0 million was finalizedin additional payments). SIA's core technology, DuraSorb, is a fully resorbable scaffold of a globally accepted polymer, which is cleared for use in hernia repair, abdominal wall, and paidother soft tissue reinforcement. DuraSorb sales will be reported within Integra’s Tissue Technologies segment as part of June 30, 2021. ACell was a privately-held company that offered a portfolio of regenerative products for complex wound management, including developingits Wound Reconstruction and commercializing products based on MatriStem Urinary Bladder Matrix, a technology platform derived from porcine urinary bladder extracellular matrix.Care franchise.
Assets Acquired and Liabilities Assumed at Fair Value
The ACellSIA Acquisition has been accounted for using the acquisition method of accounting. This method requires that assets acquired, and liabilities assumed in a business combination areto be recognized at their fair values as of the acquisition date.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following table summarizes the final fair values of the assets acquired and liabilities assumed at the acquisition date:
| | | | | | | | | | | | | | |
| Dollars in thousands | Final Valuation | | Weighted Average Life | | | |
| Current assets: | | | | | | |
| Cash | $ | 2,7264,438 | | | | | | |
| Trade accounts receivable, net | 16,4691,551 | | | | | | |
| Inventories, net | 18,2992,900 | | | | | | |
| Prepaid expenses and other current assets | 1,4981,654 | | | | | | |
| Total current assets | $ | 38,99210,543 | | | | | | |
Property, plant and equipment, net | 13,769 | | | | | | |
| Intangible assets | 245,00075,000 | | | 14 years | | | 13-14 years | | |
| Goodwill | 94,14741,380 | | | | | | |
Right of use asset - operating leases | 9,259 | | | | | | |
Deferred tax assets | 7,465 | | | | | | |
Other assets | 148 | | | | | | |
| Total assets acquired | $ | 408,780126,923 | | | | | | |
| | | | | | |
| Current liabilities: | | | | | | |
| Accounts payable and accrued expenses | $ | 7182,044 | | | | | | |
Accrued expenses | 5,966 | | | | | | |
Current portion of lease liability - operating leases | 1,673 | | | | | | |
| Total current liabilities | $ | 8,3572,044 | | | | | | |
Other long-term liability | 276 | | | | | | |
Lease liability - operating leases | 7,585 | | | | | | |
Deferred tax liabilityTax Liability | 61,72411,325 | | | | | | |
| Contingent consideration | 23,90057,607 | | | | | | |
| Total liabilities assumed | 101,84270,976 | | | | | | |
| | | | | | |
| Net assets acquired | $ | 306,93855,947 | | | | | | |
Intangible Assets
Developed Technology
The estimated fair value of the developed technology acquired was determined using the multi-period excess earnings method of the income approach, which estimates value based on the present value of future economic benefits. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each product including net revenues, cost of sales, R&D costs, selling and marketing costs, working capital, and contributory asset charges, the appropriate discount rate to select in order to measure the risk inherent in each future cash flow stream, the assessment of eachthe asset’s life cycle, and competitive trends impacting the asset and eachthe cash flow stream.
The Company used a discount rate of 8.5%18% to arrive at the present value for the acquired intangible assets to reflect the rate of return a market participant would expect to earn and incremental commercial uncertainty in the cash flow projections. No assurances can be given that the underlying assumptions used to prepare the discounted cash flow analysis will not change. For these and other reasons, actual results may vary significantly from estimated results.
Goodwill
The Company allocated goodwill related to the ACell acquisitionSIA Acquisition to the Tissue Technologies segment. Goodwill is the excess of the consideration transferred over the net assets recognized and represents the expected revenue and cost synergies of the combined company and assembled workforce. A key factor that contributes to the recognition of goodwill, and a driver for the Company’s acquisition of SIA, is the attractive growth opportunities presented by the surgical matrix business in the breast reconstruction market. Goodwill recognized as a result of this acquisition is non-deductible for income tax purposes.
Contingent Consideration
As part of the acquisition, the Company is required to make payments to the former shareholders of ACell up to $100 million based on the achievement of certain revenue-based performance milestones in 2022, 2023, and 2025. The Company used iterations of the Monte Carlo simulation to calculate the fair value of the contingent consideration that considered the possible outcomes of scenarios related to each specific milestone. The Company estimated the fair value of the contingent consideration to be $23.9 million at the acquisition date. The estimated fair value as of December 31, 2021 was $21.8 million. This amount is included in other liabilities at December 31, 2021 in the consolidated balance sheets of the Company.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company determineddetermines the acquisition date fair value of contingent consideration obligations usingbased on a Monte Carlo simulation, as well as significant unobservable inputs, reflectingprobability-weighted income approach derived from revenue estimates and a probability assessment with respect to the Company’s assessmentlikelihood of the assumptions market participants would use to value these liabilities.achieving contingent obligations. The fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement as defined using the fair value concepts in ASC 820. The resultantresulting most likely payouts are discounted using an appropriate effective annual interest rate. At each reporting date, the contingent consideration obligations isobligation will be revalued to estimated fair value and changes in fair value will be reflected as income or expense in ourthe consolidated statement of operations. Changes in the fair value of the contingent considerations may result from changes in discount periods and rates and changes in the timing and amount of revenue estimates. Changes in assumptions utilized in the contingent consideration fair value estimates could result in an increase in the contingent consideration obligation and a corresponding charge to operating results.
As part of the acquisition, the Company is required to pay to the shareholder of SIA up to $90.0 million for two separate payments, which are dependent on 1) achieving certain revenue-based performance milestones in 2023, 2024, and 2025 (up to $50.0 million in additional payments), as well as 2) the approval by the FDA of the PMA for DuraSorb for certain uses by certain timing targets (up to $40.0 million in additional payments). The Company used iterations of the Monte Carlo simulation
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
to calculate the fair value of the contingent consideration for the revenue-based milestone that considered the possible outcomes of scenarios related to each specific milestone for the revenue based performance milestone. The Company used probabilities of achieving the conditions to calculate the fair value of the contingent consideration for the PMA approval milestone. For the twelve-month period ended December 31, 2023, the company estimates the fair value of contingent consideration for the revenue based milestone to be $41.8 million and PMA approval to be $26.9 million. This is compared to $32.6 million and $25.0 million, respectively at the acquisition date.
Deferred Tax Liabilities
Deferred tax liabilities result from identifiable intangible assets’ fair value adjustments. These adjustments create excess book basis over tax basis which is tax-effected by the statutory tax rates of applicable jurisdictions.
Pro Forma Results (unaudited)
Pro forma revenuesSale of non-core traditional wound care business
On August 31, 2022, the Company completed its sale of its non-core traditional wound care ("TWC") business to Gentell, LLC ("Gentell") for $28.8 million, which consists of $27.8 million in cash plus $1.0 million in contingent consideration which may be received upon achieving certain revenue-based performance milestones two years after the closing date. The proceeds from the sale of the TWC business of $27.8 million is presented in the consolidated statement of cash flows net of cash transferred of $3.5 million and other transaction fees. The transaction included the sale of the Company's TWC products, such as sponges, gauze and conforming bandages, and certain advanced wound care dressings, such as supportive, calcium alginate, hydrogel, and foam dressings.
The divestiture did not represent a strategic shift that had a major effect on the Company's operations and financial statements. Goodwill was allocated to the assets and liabilities divested using the relative fair value method of the TWC business to the Company's Tissue Technologies reportable business segment. In connection with the sale, the Company recognized $0.6 million as a gain from the sale of the business in the consolidated statement of operations for the year ended December 31, 20212022. The transaction is subject to final working capital adjustments.
In addition to the purchase and 2020 were $1,547.0sale agreement, the Company also entered into a contract manufacturing agreement with Gentell. Under the terms of the agreement, Gentell received inventory, equipment, and tooling to manufacture certain MediHoney® and TCC-EZ® products on behalf of the Company. On the close date of this transaction, the Company transferred all inventory associated with these products to Gentell and recognized an asset of $11.1 million, as a form of a deposit for the inventory transferred, which based on the expected timing of inventory purchases, was primarily included within prepaid expenses and $1,466.8other current assets in the consolidated balance sheet. This deposit will be utilized by the Company on future orders placed to Gentell for such products. As of December 31, 2023, the Company had a deposit remaining of $0.4 million respectively. Pro forma net incomewhich is included in prepaid assets. In addition, there are outstanding balances related to the Company's ongoing purchase of MediHoney® and earnings per share are not presented for this acquisition as they are not material.TCC-EZ® products subsequent to the close of the TWC divestiture..
Arkis BioSciencesDefinitive Agreement to Acquire Acclarent Inc.
In December 2023, the Company entered into a definitive agreement to acquire Acclarent, Inc. from Ethicon, Inc., a Johnson & Johnson MedTech company for $275 million in cash at closing, subject to customary purchase price adjustments, and an additional $5 million upon the achievement of certain regulatory milestones. Acclarent is an innovator and market leader in Ear, Nose, Throat ("ENT") procedures and upon closing, Integra will be one of the leading providers of ENT products and technologies. The transaction is expected to close by the second quarter of 2024.
Sale of Extremity Orthopedics Business
On July 29, 2019,January 4, 2021, the Company completed the sale of its Extremity Orthopedics business to Smith & Nephew USD Limited ("Smith & Nephew"). The transaction included the sale of the Company's upper and lower Extremity Orthopedics product portfolio, including ankle and shoulder arthroplasty and hand and wrist product lines. The Company received an aggregate purchase price of $240.0 million from Smith & Nephew and concurrently paid $41.5 million to the Consortium of Focused Orthopedists, LLC ("CFO") effectively terminating the licensing agreement between Integra and CFO relating to the development of shoulder arthroplasty products.
The divestiture did not represent a strategic shift that had a major effect on the Company's operations and financial statements. Goodwill was allocated to the assets and liabilities divested using the relative fair value method of the Extremity Orthopedics business to the Company's Tissue Technologies reporting unit. In connection with the sale, the Company recognized a gain of $41.8 million that is presented in Gain from the sale of business in the consolidated statement of operations for the year ended December 31, 2021. The Company finalized the net working capital to Smith & Nephew as of December 31, 2021.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
ACell, Inc. Acquisition
On January 20, 2021, the Company acquired Arkis BioSciencesACell, Inc. ("Arkis"(the "ACell Acquisition") for an acquisition purchase price of $30.6$306.9 million (the "Arkis Acquisition") plus contingent considerationconsiderations of up to $25.5$100 million, that may be payable based on the successful completionupon achieving certain revenue-based performance milestones in 2022, 2023 and 2025. The final working capital adjustments of certain development$1.3 million was finalized and commercial milestones. The contingent consideration had an acquisition date fair valuepaid as of $13.1 million. ArkisJune 30, 2021. ACell was a privately-held company that marketed the CerebroFlo® external ventricular drainage (EVD) catheter with Endexo®offered a portfolio of regenerative products for complex wound management, including developing and commercializing products based on MatriStem Urinary Bladder Matrix, a technology a permanent additive designed to reduce the potential for catheter obstruction due to thrombus formation.platform derived from porcine urinary bladder extracellular matrix.
Assets Acquired and Liabilities Assumed at Fair Value
The ArkisACell Acquisition has been accounted for using the acquisition method of accounting. This method requires that assets acquired and liabilities assumed in a business combination to beare recognized at their fair values as of the acquisition date.
The following table summarizes the final fair values of the assets acquired and liabilities assumed at the acquisition date:
| | | | | | | | | | | | | | |
| Dollars in thousands | Final Valuation | | Weighted Average Life | | | |
| Current assets: | | | | | | |
| Cash | $ | 902,726 | | | | | | |
OtherTrade accounts receivable, net | 16,469 | | | | | | |
| Inventories, net | 18,299 | | | | | | |
| Prepaid expenses and other current assets | 7511,498 | | | | | | |
| Total current assets | $ | 38,992 | | | | | | |
| Property, plant and equipment, net | 45713,769 | | | | | | |
| Intangible assets | 245,000 | | | 13-14 years | | | |
| Goodwill | 94,147 | | | | | | |
| Right of use asset - operating leases | 9,259 | | | | | | |
| Deferred tax assets | 1,6977,465 | | | | | | |
Intangible assets: | | |
CerebroFlo developed technologyOther assets | 20,100148 | | | 15 years
| Enabling technology license | 1,980 | | 14 years
Goodwill | 27,153 | | |
| Total assets acquired | 52,228 $ | 408,780 | | | | | | |
| | | | | | |
| Current liabilities: | | | | | | |
Accounts payable accrued | $ | 718 | | | | | | |
Accrued expenses and other | 5,966 | | | | | | |
| Current portion of lease liability - operating leases | 1,673 | | | | | | |
| Total current liabilities | 2,926 $ | 8,357 | | | | | | |
| Other long-term liability | 276 | | | | | | |
| Lease liability - operating leases | 7,585 | | | | | | |
| Deferred tax liability | 61,724 | | | | | | |
| Contingent consideration | 13,10023,900 | | | | | | |
Deferred taxTotal liabilities assumed | 5,603101,842 | | | | | | |
| | | | | | |
| Net assets acquired | $ | 30,599306,938 | | | | | | |
Intangible Assets
The estimated fair value of the intangible assetsdeveloped technology acquired was determined using the multi-period excess earnings method of the income approach, which is a valuation technique that provides an estimate ofestimates value based on the fairpresent value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life.future economic benefits. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each asset (includingproduct including net revenues, cost of sales, R&D costs, selling and marketing costs, and working capital/contributory asset charges), the appropriate discount rate to select in order to measure the risk inherent in each future cash flow stream, the assessment of each asset’s life cycle, and competitive trends impacting the asset and each cash flow stream.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company used a discount rate of 14.5%8.5% to arrive at the present value for the acquired intangible assets to reflect the rate of return a market participant would expect to earn and incremental commercial uncertainty in the cash flow projections. No assurances can be given that the underlying assumptions used to prepare the discounted cash flow analysis will not change. For these and other reasons, actual results may vary significantly from estimated results.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Goodwill
The Company allocated goodwill related to the Arkis AcquisitionACell acquisition to the Codman Specialty SurgicalTissue Technologies segment. Goodwill is the excess of the consideration transferred over the net assets recognized and represents the expected revenue and cost synergies of the combined company and assembled workforce. One of the key factors that contributes to the recognition of goodwill, and a driver for the Company's acquisition of Arkis, is the planned expansion of the Endexo technology with the existing products within the Codman Specialty Surgical segment. Goodwill recognized as a result of this acquisition is non-deductible for income tax purposes.
Contingent Consideration
The Company determined the acquisition date fair value of contingent consideration obligations based on a probability-weighted income approach derived from revenue estimates and a probability assessment with respect to the likelihood of achieving contingent obligations. The fair value measurement is based on significant inputs not observable in the market and thus represents a Level 3 measurement as defined using the fair value concepts in ASC 820. The resultant probability-weighted cash flows are discounted using an appropriate effective annual interest rate. At each reporting date, the contingent consideration obligation is revalued to estimated fair value and changes in fair value will be reflected as income or expense in our consolidated statement of operations. Changes in the fair value of the contingent consideration obligations may result from changes in discount periods and rates, changes in the timing and amount of revenue estimates and changes in probability assumptions with respect to the likelihood of achieving the various contingent payment obligations. Adverse changes in assumptions utilized in the contingent consideration fair value estimates could result in an increase in the contingent consideration obligation and a corresponding charge to operating results.
As part of the acquisition of ACell (the "ACell Acquisition"), the Company is required to paymake payments to the former shareholders of ArkisACell up to $25.5$50 million based on the timingachievement by the Company of certain developmentrevenue-based performance milestones of $10in 2023 and $50 million and commercial sales milestones of $15.5 million, respectively.in 2025. The 2023 milestone was not achieved, leaving only one contingent milestone remaining. The Company used a probability weighted income approachiterations of the Monte Carlo simulation to calculate the fair value of the contingent consideration that considered the possible outcomes of scenarios related to each specifiedspecific milestone. The Company estimatedFor the twelve-month period ended December 31, 2023, the company estimates the fair value of the contingent consideration obligation to be $13.1$0.3 million. This is compared to $23.9 million at the acquisition date. The estimated the fair value as of December 31, 2021 was $15.1 million. The Company recorded $3.7date, and $3.7 million in accrued expenses and other current liabilities and $11.4 million in other liabilities at December 31, 2021 in the consolidated balance sheets of the Company.2022.
Deferred Tax Liabilities
Deferred tax liabilities result from identifiable intangible assets’ fair value adjustments. These adjustments create excess book basis over tax basis which is tax-effected by the statutory tax rates of applicable jurisdictions.
The pro forma results are not presented for this acquisition as they are not material.
Rebound Therapeutics Corporation
On September 9, 2019, the Company acquired Rebound Therapeutics Corporation (“Rebound”), developers of a single-use medical device known as the Aurora® Surgiscope® System ("Aurora") which enables minimally invasive access, using optics and illumination, for visualization, diagnostic and therapeutic use in neurosurgery (the “Rebound transaction”). Under the terms of the Rebound transaction, the Company made an upfront payment of $67.1 million and are committed to pay up to $35.0 million of contingent development milestones upon achievement of certain regulatory milestones. The acquisition of Rebound was primarily concentrated in 1 single identifiable asset and thus, for accounting purposes, the Company has concluded that the acquired assets do not meet the accounting definition of a business. The initial payment was allocated primarily to Aurora, resulting in a $59.9 million IPR&D expense. The balance of approximately $7.2 million, which included $2.1 million of cash and cash equivalents and a net deferred tax asset of $4.2 million, was allocated to the remaining net assets acquired. The deferred tax asset primarily resulted from a federal net operating loss carry forward.
During the fourth quarter of 2019, the Company achieved the first developmental milestone which triggered a $5.0 million obligation to be paid to former shareholders of Rebound. The Company recorded $5.0 million as IPR&D expense in the consolidated statements of operations. The obligation was included in accrued expenses and other current liabilities at December 31, 2019 in the consolidated balance sheets. The milestone was paid during the first quarter of 2020.
During the fourth quarter of 2020, the Company achieved another developmental milestone which triggered a $20.0 million obligation to be paid to the former shareholders of Rebound. The Company recorded $20.0 million as an intangible asset in the consolidated balance sheet upon achieving the milestone. The milestone was paid during the fourth quarter of 2020.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
During the fourth quarter of 2021, the Company achieved its final developmental milestone which triggered a $5.0 million obligation to be paid to the former shareholders of Rebound. The Company recorded $5.0 million as an intangible asset in the consolidated balance sheet upon achieving the milestone. The remaining obligation was included in accrued expenses and other current liabilities at December 31, 2021 in the consolidated balance sheets. The milestone is expected to be fully paid during the first quarter of 2022.
Integrated Shoulder Collaboration, Inc.
On January 4, 2019, the Company entered into a licensing agreement with Integrated Shoulder Collaboration, Inc ("ISC"). Under the terms of the agreement, the Company paid ISC $1.7 million for the exclusive, worldwide license to commercialize its short stem and stemless shoulder system. A patent related to short stem and stemless shoulder systems was issued to ISC during the first quarter of 2019. ISC is eligible to receive royalties on sales of the short stem and stemless shoulder system. The Company has the option to acquire ISC at a date four years subsequent to the first commercial sale, which becomes mandatory upon the achievement of a certain sales thresholds of the short stem and stemless shoulder system, for an amount not to exceed $80.0 million. The transaction was accounted for as an asset acquisition as the Company concluded that it acquired primarily 1 asset. The total upfront payment of $1.7 million was expensed as a component of research and development expense and the future milestone and option payments will be recorded if the corresponding events become probable.
In connection with the sale of the Company's Extremity Orthopedic business, on January 4, 2021 the Company paid $41.5 million to CFO pursuant to the terms of certain agreements between the Company and CFO relating to the sale of shares of ISC effectively terminating our licensing agreement with ISC.
5. DEBT
Amendment to the SixthSeventh Amended and Restated Senior Credit Agreement
On February 3, 2020,March 24, 2023, the Company entered into the sixthseventh amendment and restatement (the "February 2020"March 2023 Amendment") of itsthe Senior Credit Facility (the "Senior Credit Facility") with a syndicate of lending banks with Bank of America, N.A., as Administrative Agent. The February 2020March 2023 Amendment extended the maturity date to February 3, 2025.March 24, 2028, amended the contractual repayments of the term loan component, and amended the interest rate from LIBOR to SOFR-indexed interest. The Company continues to have the aggregate principal amount of up to approximately $2.2$2.1 billion available to it through the following facilities: (i) a $877.5$775.0 million Term Loanterm loan facility, and (ii) a $1.3 billion revolving credit facility, which includes a $60 million sublimit for the issuance of standby letters of credit and a $60 million sublimit for swingline loans.
On July 14, 2020, the Company entered into an amendment (the "July 2020 Amendment") to the February 2020 Amendment of the Senior Credit Facility to increase financial flexibility through June 30, 2021, in light of the unprecedented impact and uncertainty of the COVID-19 pandemic on the global economy. The July 2020 amendment did not increase the Company’s total indebtedness.
In connection with the July 2020 amendment, the Company’s maximum consolidated total leverage ratio in the financial covenants (as defined in the Senior Credit Facility) was modified tois the following:
| | | | | | | | |
| Fiscal Quarter Ending | | Maximum Consolidated Total Leverage Ratio |
| | |
| March 31, 2023 through December 31, 2024 | | 4.50 to 1.00 |
Execution of July 2020 AmendmentMarch 31, 2025 through June 30, 20212026 | | 5.504.25 to 1.00 |
September 30, 2021 through June 30, 2022 | | 5.00 to 1.00 |
September 30, 2022 through June 30, 2023 | | 4.50 to 1.00 |
September 30, 20232026 and the last day of each fiscal quarter thereafter | | 4.00 to 1.00 |
Borrowings under the Senior Credit Facility bear interest, at the Company’s option, at a rate equal to the following:
i.the Eurodollar Rate (as defined in the amendment and restatement)Term SOFR in effect from time to time plus 0.10% plus the applicable rate (ranging from 1.00% to 2.25%1.75%), or
ii.the highest of:
1.the weighted average overnight Federal funds rate, as published by the Federal Reserve Bank of New York, plus 0.50%
2.the prime lending rate of Bank of America, N.A. or
3.the one-month Eurodollar RateTerm SOFR plus 1.00%
The applicable rates are based on the Company’s consolidated total leverage ratio (defined as the ratio of (a) consolidated funded indebtedness as of such date less cash that is not subject to any restriction on the use or investment thereof to
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
(b) consolidated EBITDA (as defined by the July 2020 amendment)amended Seventh Amended and Restated Credit Agreement (the "Credit Agreement")), for the period of four consecutive fiscal quarters ending on such date).
The Company will pay an annual commitment fee (ranging from 0.15% to 0.30%), based on the Company's consolidated total leverage ratio, on the amount available for borrowing under the revolving credit facility.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Senior Credit Facility is collateralized by substantially all of the assets of the Company’s U.S. subsidiaries, excluding intangible assets. The Senior Credit Facility is subject to various financial and negative covenants and at December 31, 2021,2023, the Company was in compliance with all such covenants. In connection with the February 2020 Amendment, theThe Company capitalized $4.6$7.6 million of financing costs in connection with modification of the Senior Credit Facility and wrote off $1.2 million of previously capitalized financing costs during the first quarter of 2020. In connection with the July 2020 amendment, the Company expensed $3.3 million of incrementaldeferred financing costs in connection with the modification of the Senior Credit Facility and wrote off $0.2 million of previously capitalized financing costs during the third quarter of 2020.year ended 2023.
At There was $70.0 million outstanding at December 31, 2021 and 2020, there was2023 $31.3 million and $97.5 million, respectively, outstanding under the revolving portion of the Senior Credit Facility at a weighted average interest ratesrate of 1.4% and 1.5%, respectively. At6.8%. As of December 31, 2021 and 2020,2022, there was $843.8no balance outstanding under the revolving portion of the Senior Credit Facility. At December 31, 2023 and 2022, there was $775.0 million and $877.5 million, respectively, outstanding under the Term Loan component of the Senior Credit Facility at weighted average interest rate of 1.4%6.8% and 1.5%5.6%, respectively. At December 31, 20212023 and 2020,2022, there was $45.0$14.5 million and $33.8$38.1 million, respectively, of the Term Loan component of the Senior Credit Facility was classified as current on the consolidated balance sheets.
The fair value of outstanding borrowings of the Senior Credit Facility's revolving credit and Term Loan components at December 31, 20212023 were $31.0was $762.9 million and $838.4 million, respectively. These. This fair values were determined by using a discounted cash flow model based on current market interest rates available to the Company. These inputs are corroborated by observable market data for similar liabilities and therefore classified within Level 2 of the fair value hierarchy. Level 2 inputs represent inputs that are observable for the asset or liability, either directly or indirectly, and are other than active market observable inputs that reflect unadjusted quoted prices for identical assets or liabilities
Letters of credit outstanding as of December 31, 20212023 and 20202022 totaled $1.7 million, and $1.6 million.million respectively. There were no amounts drawn as of December 31, 2021.2023.
Contractual repayments of the Term Loan component of the Senior Credit Facility are due as follows:
| | Year-ended December 31, 2021 | | Principal Repayment |
| Year Ended December 31, 2023 | | Year Ended December 31, 2023 | | Principal Repayment |
| Dollars in thousands | Dollars in thousands | | |
| 2022 | | $ | 45,000 | |
| 2023 | | $ | 61,875 | |
| 2024 | |
| 2024 | |
| 2024 | 2024 | | $ | 67,500 | |
| 2025 | 2025 | | $ | 669,375 | |
| $ | 843,750 | |
| 2026 | |
| Thereafter | |
| | $ | |
Future interest payments on the term loan component of the Senior Credit Facility based on current interest rates are expected to approximate $11.0 million in 2022, $10.3 million in 2023, $9.4$52.4 million in 2024, and $0.9$50.5 million in 2025.2025, $47.9 million in 2026, and $54.7 million thereafter. Interest is calculated on the term loan portion of the Senior Credit Facility based on LIBORSOFR plus the spread paid bycertain amounts set forth in the Company.Credit Agreement. As the revolving credit facility and Securitization Facility (defined below) can be repaid at any time, no interest has been included in the calculation.
TheAny outstanding balance ofborrowings on the revolving credit component of the Senior Credit Facility is due on February 3, 2025.March 24, 2028.
Convertible Senior Notes
On February 4, 2020, the Company issued $575.0 million aggregate principal amount of its 0.5% Convertible Senior Notes due 2025 (the "2025 Notes"). The 2025 Notes will mature on August 15, 2025 and bear interest at a rate of 0.5% per annum payable semi-annually in arrears, unless earlier converted, repurchased or redeemed in accordance with the terms of the 2025 Notes. The portion of debt proceeds that was classified as equity at the time of the offering was $104.5 million. The effective interest rate implicit in the liability component was 4.2%. In connection with this offering, the Company capitalized $13.2 million of financing fees.
The 2025 Notes are senior, unsecured obligations of the Company, and are convertible into cash and shares of its common stock based on initial conversion rate, subject to adjustment of 13.5739 shares per $1,000 principal amounts of the 2025 Notes (which represents an initial conversion price of $73.67 per share). The 2025 Notes convert only in the following circumstances: (1) if the closing price of the Company's common stock has been at least 130% of the conversion price during the period; (2) if the average trading price per $1,000 principal amount of the 2025 Notes is less than or equal to 98% of the average conversion
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
value of the 2025 Notes during a period as defined in the indenture; (3) at any time on or after February 20, 2023;if the Company calls the notes for optional redemption as defined in the indenture; or (4) if specified corporate transactions occur. As of December 31, 2021,2023, none of these conditions existed with respect to the 2025 Notes and as a result the 2025 Notes are classified as long term.
On December 9, 2020, the Company entered into the First Supplemental Indenture to the original agreement dated as of February 4, 2020 between the Company and Citibank, N.A., as trustee, governing the Company’s outstanding 2025 Notes. The Company irrevocably elected (1) to eliminate the Company’s option to choose physical settlement on any conversion of the 2025 Notes that occurs on or after the date of the First Supplemental Indenture and (2) with respect to any Combination
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Settlement for a conversion of the 2025 Notes, the Specified Dollar Amount that will be settled in cash per $1,000 principal amount of the 2025 Notes shall be no lower than $1,000.
Holders of the Notes will have the right to require the Company to repurchase for cash all or a portion of their Notes at 100% of their principal amount, plus any accrued and unpaid interest, upon the occurrence of a fundamental change (as defined in the indenture relating to the Notes). The Company will also be required to increase the conversion rate for holders who convert their Notes in connection with certain fundamental changes occurring prior to the maturity date or following delivery by the Company of a notice of redemption.
In connection with the issuance of the 2025 Notes, the Company entered into call transactions and warrant transactions, primarily with affiliates of the initial purchasers of the 2025 Notes (the “hedge participants”). The cost of the call transactions was $104.2 million for the 2025 Notes. The Company received $44.5 million of proceeds from the warrant transactions for the 2025 Notes. The call transactions involved purchasing call options from the hedge participants, and the warrant transactions involved selling call options to the hedge participants with a higher strike price than the purchased call options. The initial strike price of the call transactions was $73.67, subject to anti-dilution adjustments substantially similar to those in the 2025 Notes. The initial strike price of the warrant transactions was $113.34 for the 2025 Notes, subject to customary anti-dilution adjustments.
At December 31, 2020, the carrying amount of the liability component was $485.9 million, the remaining unamortized discount was $89.1 million, and the principal amount outstanding was $575.0 million. On January 1, 2021, the Company adopted ASU 2020-06 using the modified retrospective method. See Note 2, Summary of Significant Accounting Policies2023, for further details. At December 31, 2021, the carrying amount of the liability was $575.0 million. The fair value of the 2025 Notes at December 31, 2021 was $640.52023 was $541.2 million. Factors that the Company considered when estimating the fair value of the 2025 Notes included recent quoted market prices or dealer quote. The level of the 2025 Notes is considered as Level 1.
As a resultSecuritization Facility
On December 15, 2023, the Company entered into an amendment (the "December 2023 Amendment") of the adoptionSecuritization Facility which extended the maturity date from May 28, 2024 to December 15, 2026. The company incurred approximately $0.3 million of ASU 2020-06,new issuance costs associated with the Company recognized only cash interest relatedamendment which will be amortized over 3 years, the length of the agreement. Due to the contractual interest couponincrease in borrowing capacity, the remaining $0.1 million of $2.9 million onunamortized costs from the 2025 Notes forprevious agreement will be amortized over the year ended December 31, 2021. Prior to the adoption, during the year ended December 31, 2020, the Company recognized cash interest of $2.6 million and amortizationlength of the discountamended agreement, 3 years. In addition, on April 17, 2023 the liability componentcompany entered into an amendment (the "April 2023 Amendment") of $15.4 million for a total interest charge of $18.0 million on the 2025 Notes.
Securitization Facility and amended the interest rate from LIBOR to SOFR indexed rate. The December 2023 and April 2023 Amendments do not increase the Company’s total indebtedness.
During the fourth quarter of 2018, the Company entered into an accounts receivable securitization facility (the "Securitization Facility") under which accounts receivable of certain domestic subsidiaries are sold on a non-recourse basis to a special purpose entity (“SPE”), which is a bankruptcy-remote, consolidated subsidiary of the Company. Accordingly, the assets of the SPE are not available to satisfy the obligations of the Company or any of its subsidiaries. From time to time, the SPE may finance such accounts receivable with a revolving loan facility secured by a pledge of such accounts receivable. The amount of outstanding borrowings on the Securitization Facility at any one time is limited to $150.0 million. The Securitization Facility Agreement ("Securitization Agreement") governing the Securitization Facility contains certain covenants and termination events. An occurrence of an event of default or a termination event under this Securitization Agreement may give rise to the right of its counterparty to terminate this facility. As of December 31, 2021,2023, the Company was in compliance with the covenants and none of the termination events had occurred.
On May 28, 2021, the Company entered into an amendment (the "May 2021 Amendment") of the Securitization Facility which extended the maturity date from December 21, 2021 to May 28, 2024. The May 2021 Amendment does not increase the Company’s total indebtedness.
At both December 31, 20212023 and 2020,2022, the Company had $112.5$89.2 million and $104.7 million, of outstanding borrowings under its Securitization Facility at a weighted average interest rate of 1.1%5.9% and 1.3%5.0%, respectively. The fair value of the outstanding borrowing of the Securitization Facility at December 31, 20212023 was $111.8$87.1 million. These fair values were determined by using a discounted cash flow model based on current market interest rates available to the Company. These inputs are corroborated by observable market data for similar liabilities and therefore classified within Level 2 of the fair value hierarchy. Level 2 inputs represent inputs that are observable for the asset or liability, either directly or indirectly, and are other than active market observable inputs that reflect unadjusted quoted prices for identical assets or liabilities.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
6. DERIVATIVE INSTRUMENTS
Interest Rate Hedging
The Company’s interest rate risk relates to U.S. dollar denominated variable interest rate borrowings. The Company uses interest rate swap derivative instruments to manage earnings and cash flow exposure resulting from changes in interest rates. These interest rate swaps apply a fixed interest rate on a portion of the Company's expected LIBOR-indexed floating-rateSOFR-indexed borrowings. In connection with the March 2023 Amendment to the Senior Credit Facility, the Company amended its interest rate from LIBOR to SOFR-indexed interest. In March 2023, the Company entered into a basis swap where the Company receives Term SOFR and pays daily compounded SOFR to convert the portfolio of swaps from daily compounded SOFR to term SOFR.
The Company held the following interest rate swaps as of December 31, 20212023 and 20202022 (dollar amounts in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2021 | | December 31, 2020 | | | | | | | | | | December 31, 2021 | | December 31, 2020 |
| Hedged Item | | Notional Amount | | Designation Date | | Effective Date | | Termination Date | | Fixed Interest Rate | | Estimated Fair Value |
| | | | | | | | | | | | | | Asset (Liability) |
| 1-month USD LIBOR Loan | | — | | | 100,000 | | | March 27, 2017 | | December 31, 2017 | | June 30, 2021 | | 1.971 | % | | $ | — | | | $ | (929) | |
| 1-month USD LIBOR Loan | | 300,000 | | | 300,000 | | | December 13, 2017 | | January 1, 2018 | | December 31, 2022 | | 2.201 | % | | (5,268) | | | (12,557) | |
| 1-month USD LIBOR Loan | | 150,000 | | | 150,000 | | | December 13, 2017 | | July 1, 2019 | | June 30, 2024 | | 2.423 | % | | (5,520) | | | (11,502) | |
| 1-month USD LIBOR Loan | | 200,000 | | | 200,000 | | | December 13, 2017 | | January 1, 2018 | | December 31, 2024 | | 2.313 | % | | (7,421) | | | (16,243) | |
| 1-month USD LIBOR Loan | | 75,000 | | | 75,000 | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.220 | % | | (5,512) | | | (9,836) | |
| 1-month USD LIBOR Loan | | 75,000 | | | 75,000 | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.199 | % | | (5,464) | | | (9,826) | |
| 1-month USD LIBOR Loan | | 75,000 | | | 75,000 | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.209 | % | | (5,494) | | | (9,783) | |
| 1-month USD LIBOR Loan | | 100,000 | | | 100,000 | | | December 18, 2018 | | December 30, 2022 | | December 31, 2027 | | 2.885 | % | | (6,886) | | | (10,407) | |
| 1-month USD LIBOR Loan | | 100,000 | | | 100,000 | | | December 18, 2018 | | December 30, 2022 | | December 31, 2027 | | 2.867 | % | | (6,764) | | | (10,431) | |
| 1-month USD LIBOR Loan | | 575,000 | | | 575,000 | | | December 15, 2020 | | July 31, 2025 | | December 31, 2027 | | 1.415 | % | | 3,552 | | | (1,907) | |
| 1-month USD LIBOR Loan | | 125,000 | | | 125,000 | | | December 15, 2020 | | July 1, 2025 | | December 31, 2027 | | 1.404 | % | | 821 | | | (348) | |
| | $ | 1,775,000 | | | $ | 1,875,000 | | | | | | | | | | | $ | (43,957) | | | $ | (93,769) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | December 31, 2023 | | | | | | | | | | | | December 31, 2023 | | |
| Hedged Item | | | | Notional Amount | | Designation Date | | Effective Date | | Termination Date | | Fixed Interest Rate | | Estimated Fair Value |
| | | | | | | | | | | | | | | | Asset (Liability) |
| 1-month Term SOFR Loan | | | | 150,000 | | | | | December 13, 2017 | | July 1, 2019 | | June 30, 2024 | | 2.423 | % | | 2,105 | | | |
| 1-month Term SOFR Loan | | | | 200,000 | | | | | December 13, 2017 | | January 1, 2018 | | December 31, 2024 | | 2.313 | % | | 4,978 | | | |
| 1-month Term SOFR Loan | | | | 75,000 | | | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.220 | % | | 1,349 | | | |
| 1-month Term SOFR Loan | | | | 75,000 | | | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.199 | % | | 1,312 | | | |
| 1-month Term SOFR Loan | | | | 75,000 | | | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.209 | % | | 1,346 | | | |
| 1-month Term SOFR Loan | | | | 100,000 | | | | | December 18, 2018 | | December 30, 2022 | | December 31, 2027 | | 2.885 | % | | 3,015 | | | |
| 1-month Term SOFR Loan | | | | 100,000 | | | | | December 18, 2018 | | December 30, 2022 | | December 31, 2027 | | 2.867 | % | | 3,052 | | | |
| 1-month Term SOFR Loan | | | | 575,000 | | | | | December 15, 2020 | | July 31, 2025 | | December 31, 2027 | | 1.415 | % | | 22,965 | | | |
| 1-month Term SOFR Loan | | | | 125,000 | | | | | December 15, 2020 | | July 1, 2025 | | December 31, 2027 | | 1.404 | % | | 5,263 | | | |
Basis Swap (1) | | | | — | | | | | March 31, 2023 | | March 24, 2023 | | December 31, 2027 | | N/A | | (1,829) | | | |
| | | | $ | 1,475,000 | | | | | | | | | | | | | $ | 43,556 | | | |
(1) The notional of the basis swap amortizes to match the total notional of the interest rate swap portfolio over time | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | December 31, 2022 | | | | | | | | | | | | December 31, 2022 |
| Hedged Item | | | | | Notional Amount | | Designation Date | | Effective Date | | Termination Date | | Fixed Interest Rate | | | | Estimated Fair Value |
| | | | | | | | | | | | | | | | | | Asset (Liability) |
| 1-month USD LIBOR Loan | | | | | | 150,000 | | | December 13, 2017 | | July 1, 2019 | | June 30, 2024 | | 2.423 | % | | | | 5,012 | |
| 1-month USD LIBOR Loan | | | | | | 200,000 | | | December 13, 2017 | | January 1, 2018 | | December 31, 2024 | | 2.313 | % | | | | 8,380 | |
| 1-month USD LIBOR Loan | | | | | | 75,000 | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.220 | % | | | | 1,831 | |
| 1-month USD LIBOR Loan | | | | | | 75,000 | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.199 | % | | | | 1,905 | |
| 1-month USD LIBOR Loan | | | | | | 75,000 | | | October 10, 2018 | | July 1, 2020 | | June 30, 2025 | | 3.209 | % | | | | 1,970 | |
| 1-month USD LIBOR Loan | | | | | | 100,000 | | | December 18, 2018 | | December 30, 2022 | | December 31, 2027 | | 2.885 | % | | | | 4,252 | |
| 1-month USD LIBOR Loan | | | | | | 100,000 | | | December 18, 2018 | | December 30, 2022 | | December 31, 2027 | | 2.867 | % | | | | 4,153 | |
| 1-month USD LIBOR Loan | | | | | | 575,000 | | | December 15, 2020 | | July 31, 2025 | | December 31, 2027 | | 1.415 | % | | | | 23,742 | |
| 1-month USD LIBOR Loan | | | | | | 125,000 | | | December 15, 2020 | | July 1, 2025 | | December 31, 2027 | | 1.404 | % | | | | 5,467 | |
| | | | | | | | | | | | | | | | | | |
| | | | | | $ | 1,475,000 | | | | | | | | | | | | | $ | 56,712 | |
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The interest rate swaps were carried on the consolidated balance sheet at fair value and changes in the fair values were recorded as unrealized gains or losses in accumulated other comprehensive income (“AOCI”). For the years ended December 31, 2023 and 2022, the Company recorded gains of $4.9 million and $93.3 million, respectively, in AOCI related to the change in fair value of the interest rate swaps.
For the years ended December 31, 2023 and 2022, the Company recorded a gain of $18.1 million and a loss of $7.4 million, respectively, in interest income included in the consolidated statements of operations related to the interest rate differential of the interest rate swaps. The estimated gain that is expected to be reclassified to interest income from AOCI as of December 31, 2023 within the next twelve months is $14.1 million.
The Company has designated these derivative instruments as cash flow hedges. The Company assesses the effectiveness of these derivative instruments and has recorded the changes in the fair value of the derivative instrument designated as a cash flow hedge as unrealized gains or losses in accumulated other comprehensive loss (“AOCL”),AOCI, net of tax, until the hedged item affected earnings, at which point any gain or loss was reclassified to earnings. If the hedged cash flow does not occur, or if it becomes probable that it will not occur, the Company will reclassify the remaining amount of any gain or loss on the related cash flow hedge recorded in AOCLAOCI to interest expense at that time.
Foreign Currency Hedging
From time to time, the Company enters into foreign currency hedge contracts intended to protect the U.S. dollar value of certain forecasted foreign currency denominated transactions. The Company assesses the effectiveness of the contracts that are designated as hedging instruments. The changes in fair value of foreign currency cash flow hedges are recorded in AOCL,AOCI, net of tax. Those amounts are subsequently reclassified to earnings from AOCLAOCI as impacted by the hedged item when the hedged item affects earnings. If the hedged forecasted transaction does not occur, or if it becomes probable that it will not occur, the Company will reclassify the amount of any gain or loss on the related cash flow hedge to earnings at that time. For contracts not designated as hedging instruments, the changes in fair value of the contracts are recognized in other income, net in the consolidated statements of operation, along with the offsetting foreign currency gain or loss on the underlying assets or liabilities.
During the fourth quarter of 2020, the Company entered into foreign currency forward contracts, with a notional amount of $9.7 million, to mitigate the foreign exchange risk related to certain intercompany loans denominated in Canadian Dollar ("CAD") and intercompany receivables denominated in Japanese Yen ("JPY"). The contracts are not designated as hedging instruments. The Company subsequently settled its foreign currency forward contracts associated with the intercompany receivables denominated in JPY during the first quarter of 2021. The Company recognized a $0.2 million loss from the change in fair value of the contracts, which was included in other income, net in the consolidated statement of operations as of December 31, 2021 and 2020, respectively. The fair value of the foreign currency forward contracts denominated in CAD was $0.2 million as of December 31, 2021. The fair value of the foreign currency forward contracts was $0.2 million as of December 31, 2020.
During the second quarter of 2021, the Company entered into a foreign currency swap, with a notional of $7.3 million to mitigate the risk from fluctuations in foreign currency exchange rates associated with certain intercompany loan denominated in
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Japanese Yen ("JPY"). In a foreign currency swap transaction, the Company agrees with another party to exchange, at specified intervals, the difference between one currency and another currency at a fixed exchange rate, generally set at inception, calculated by reference to an agreed upon notional amount. The notional amount of each currency is exchanged at the inception and termination of the currency swap by each party. The change in fair value of the foreign currency swap was $0.6 million as of December 31, 2021.
The success of the Company’s hedging program depends, in part, on forecasts of certain activity denominated in foreign currency. The Company may experience unanticipated currency exchange gains or losses to the extent that there are differences between forecasted and actual activities during periods of currency volatility. In addition, changes in currency exchange rates related to any unhedged transactions may affect earnings and cash flows.
Cross-Currency Rate Swaps
The objective of these cross-currency swaps is to reduce volatility of earnings and cash flows associated with changes in the foreign currency exchange rate. Under the terms of these contracts, which have been designated as cash flow hedges, the Company will make interest payments in Swiss Francs and receive interest in U.S. dollars. Upon the maturity of these contracts, the Company will pay the principal amount of the loans in Swiss Francs and receive U.S. dollars from the counterparties.
On October 2, 2017,September 22, 2023, the Company amended the Swiss Franc ("CHF")-denominated intercompany loan to partially settle CHF 20.0 million and extend the termination date to September 2024 and as a result, the Company terminated the cross-currency swap designated as cash flow hedge of an intercompany loan with aggregate notional amount of $48.5 million. Simultaneously, the Company entered into cross currencya cross-currency swap agreementsagreement to converthedge a notional amount of $300.0CHF 28.5 million equivalent to 291.2$31.5 million of Swiss Francs ("CHF") denominatedthis amended intercompany loansloan into U.S. dollars. The CHF- denominated intercompany loans wereloss recorded by the resultCompany upon the settlement of the purchase of intellectual property by a subsidiary in Switzerland as part of an acquisition.swap was not material for the period.
On December 21, 2020, the Company entered into cross-currency swap agreements to convert a notional amount of $471.6 million equivalent to 420.1 million of a CHF-denominated intercompany loan into U.S. dollars. The CHF-denominated intercompany loan was the result of an intra-entity transfer of certain intellectual property rights to a subsidiary in Switzerland completed during the fourth quarter of 2020. The intercompany loan requires quarterly payments of CHF 5.8 million plus accrued interest. As a result, the aggregate notional amount of the related cross-currency swaps will decrease by a corresponding amount.
The objective of these cross-currency swaps is to reduce volatility of earnings and cash flows associated with changes in the foreign currency exchange rate. Under the terms of these contracts, which have been designated as cash flow hedges, the Company will make interest payments in Swiss Francs and receive interest in U.S. dollars. Upon the maturity of these contracts, the Company will pay the principal amount of the loans in Swiss Francs and receive U.S. dollars from the counterparties.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The Company held the following cross-currency rate swaps as of December 31, 20212023 and 20202022 (dollar amounts in thousands):
| | | | | | | | | | December 31, 2023 | | | | | | | | | | | December 31, 2023 | December 31, 2022 | | December 31, 2023 | | December 31, 2022 |
| | Effective Date | | | | Effective Date | | Termination Date | | Fixed Rate | | Aggregate Notional Amount | | Fair Value
Asset (Liability) |
| | December 31, 2021 | December 31, 2020 | | December 31, 2021 | December 31, 2020 |
| Pay CHF | |
| Pay CHF | |
| Pay CHF | |
| Receive U.S.$ | |
| | Effective Date | | Termination Date | | Fixed Rate | | Aggregate Notional Amount | | Fair Value Asset (Liability) |
| Pay CHF | |
| | | | | | | | Pay CHF | |
| | Pay CHF | Pay CHF | | October 2, 2017 | | October 4, 2021 | | 1.85% | | CHF | — | | 48,533 | | | — | | (4,335) | |
| Receive U.S.$ | Receive U.S.$ | | 4.46% | | $ | — | | 50,000 | | |
| | Pay CHF | Pay CHF | | October 2, 2017 | | October 2, 2022 | | 1.95% | | CHF | 145,598 | | 145,598 | | | (8,283) | | (11,262) | |
| Receive U.S.$ | | 4.52% | | $ | 150,000 | | 150,000 | | |
| | Pay CHF | |
| | Pay CHF | Pay CHF | | December 21, 2020 | | December 22, 2025 | | 3.00% | | CHF | 397,137 | | 420,137 | | | 41 | | (7,843) | |
| Receive U.S.$ | Receive U.S.$ | | 3.98% | | $ | 445,821 | | 471,640 | | |
| | Total | Total | | $ | (8,242) | | $ | (23,441) | |
| | Total | |
| | Total | |
On October 4, 2021 in accordance with the termination date, the Company settled a cross-currency swap designated as a cash flow hedge of an intercompany loan with an aggregate notional amount of $50.0 million. The gain recorded by the Company upon the settlement of the swap was not material for the period.
On October 2, 2020 in accordance with the termination date, the Company settled a cross-currency swap designated as a cash flow hedge of an intercompany loan with an aggregate notional amount of $33.3 million. As a result of the settlement, the Company recorded a loss of $0.3 million in other income, net in the consolidated statement of operations.
The cross-currency swaps are carried on the consolidated balance sheet at fair value, and changes in the fair values are recorded as unrealized gains or losses in AOCL.AOCI. For the years ended December 31, 20212023 and 2020,2022 the Company recorded a loss of $37.4 million and a gain of $23.8 million and loss of $21.7$11.1 million, respectively, in other income, net related to change in fair value related to the foreign currency rate translationtranslation to offset the gains or losses recognized on the intercompany loans.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
For the years ended December 31, 20212023 and 2020,2022, the Company recordedrecorded a loss of $27.4 million and a gain of $24.3$8.8 million and a loss of $17.1 million,in AOCI, respectively, in AOCL related to change in fair value of the cross-currency swaps.
For the years ended December 31, 20212023 and 2020,2022, the Company recordedrecorded gains of $9.1$5.5 million and $5.8and $8.4 million, respectively, in other income, net included in the consolidated statements of operations related to the interest rate differential of the cross-currency swaps.
The estimated loss that is expected to be reclassified to other income (expense), net from AOCLAOCI as of December 31, 20212023 within the next twelve months is $3.4$4.3 million. As of December 31, 2021,2023, the Company does not expect any gains or losses will be reclassified into earnings as a result of the discontinuance of these cash flow hedges because the original forecasted transactiontransactions will not occur.
Net Investment Hedges
The Company manages certain foreign exchange risks through a variety of strategies, including hedging. The Company is exposed to foreign exchange risk from its international operations through foreign currency purchases, net investments in foreign subsidiaries, and foreign currency assets and liabilities created in the normal course of business. On October 1, 2018 and December 16, 2020, the Company entered into cross-currency swap agreements designated as net investment hedges to partially offset the effects of
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
foreign currency on foreign subsidiaries.
The Company held the following cross-currency rate swaps designated as net investment hedges as of December 31, 20212023 and 20202022 (dollar amounts in thousands):
| | | | | | | | | | December 31, 2023 | | | | | | | | | | | December 31, 2023 | | December 31, 2022 | | December 31, 2023 | | December 31, 2022 |
| | Effective Date | | | | Effective Date | | Termination Date | | Fixed Rate | | Aggregate Notional Amount | | Fair Value
Asset (Liability) |
| | | | December 31, 2021 | December 31, 2020 |
| Effective Date | | Termination Date | | Fixed Rate | | Aggregate Notional Amount | | Fair Value
Asset (Liability) |
| | Pay EUR | |
| Pay EUR | |
| Pay EUR | Pay EUR | | October 3, 2018 | | September 30, 2021 | | —% | | EUR | 44,859 | | | $ | — | | $ | (1,884) | |
| Receive U.S.$ | Receive U.S.$ | | 3.01% | | $ | 52,000 | | |
| | Pay EUR | Pay EUR | | October 3, 2018 | | September 30, 2023 | | —% | | EUR | 51,760 | | | 2,503 | | (450) | |
| Receive U.S.$ | | 2.57% | | $ | 60,000 | | |
| | Pay EUR | |
| | Pay EUR | Pay EUR | | October 3, 2018 | | September 30, 2025 | | —% | | EUR | 38,820 | | | 2,147 | | 92 | |
| Receive U.S.$ | Receive U.S.$ | | 2.19% | | $ | 45,000 | | |
| | Pay CHF | Pay CHF | | December 16, 2020 | | December 16, 2027 | | —% | | CHF | 222,300 | | | (792) | | (3,794) | |
| Receive USD | | 1.10% | | $ | 250,000 | | |
| | Pay CHF | |
| | Pay CHF | |
| Receive U.S.$ | |
| | Pay CHF | |
| | Pay CHF | |
| | Pay CHF | |
| Receive U.S.$ | |
| | Total | Total | | $ | 3,858 | | $ | (6,036) | |
| | Total | |
| | Total | |
On September 30, 2021, in accordance with the termination date, the Company settled cross-currency swaps designated as net investment hedge with an aggregate notional amount of $52 million equivalent to 44.9 million Euros. As a result of the settlement, the Company recorded a gain of $0.1 million in AOCL.
During the year ended December 31, 2020, the Company settled cross-currency swaps designated as net investment hedge with an aggregate notional amount of $167.5 million and 128.3 million Pound Sterling respectively as a result of an intra-entity transfer of certain intellectual property rights to a subsidiary. The original settlement date was September 30, 2025. As a result of the settlement, the Company recorded a loss of $7.8 million in AOCL.
The cross-currency swaps were carried on the consolidated balance sheet at fair value and changes in the fair values were recorded as unrealized gains or losses in AOCL.AOCI. For the yearyears ended December 31, 20212023 and 2020,2022, the Company recorded a gainloss of $16.5$30.7 million and a loss gain of $14.9$2.2 million, respectively, in AOCLAOCI related to the change in fair value of the cross-currency swaps.
For the years ended December 31, 20212023 and 2020,2022, the Company recorded a gaingains of $6.5$7.8 million and $7.6and $6.8 million, respectively, in interest income included in the consolidated statements of operations related to the interest rate differential of the cross-currency swaps.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The estimated gain that is expected to be reclassified to interest income from AOCLAOCI as of December 31, 20212023 within the next twelve months is $5.1 million.immaterial.
Foreign Currency Forward Contract
The Company has entered into a hedge for forecasted intercompany purchases denominated in foreign currencies through the use of forward contracts designated as cash flow hedges. To the extent these forward contracts meet hedge accounting criteria, changes in their fair value are included in AOCI. These changes in fair value will be recognized into earnings as a component of cost of sales when the forecasted-transaction occurs.
During 2023, the Company entered into foreign currency forward contracts to mitigate the risk of foreign currency on intercompany purchases in CHF. These contracts typically settle at various dates within twelve months of execution. As of December 31, 2023 there were no outstanding foreign currency forward contracts. During the year ended December 31, 2023 the Company recorded a gain of $0.4 million in AOCI related to the change in fair value of the foreign currency forward contracts. During the year ended December 31, 2023 the company recorded a gain of $0.4 million in cost of goods sold included in the consolidated statements of operations related to the foreign currency forward contracts.
Counterparty Credit Risk
The Company manages its concentration of counterparty credit risk on its derivative instruments by limiting acceptable counterparties to a group of major financial institutions with investment grade credit ratings, and by actively monitoring their credit ratings and outstanding positions on an ongoing basis. Therefore, the Company considers the credit risk of the counterparties to be low. Furthermore, none of the Company’s derivative transactions are subject to collateral or other security arrangements, and none contain provisions that depend upon the Company’s credit ratings from any credit rating agency.
Fair Value of Derivative Instruments
The Company has classified all of its derivative instruments within Level 2 of the fair value hierarchy because observable inputs are available for substantially the full term of the derivative instruments. The fair values of the interest rate swaps and cross-currency swaps were developed using a market approach based on publicly available market yield curves and the terms of the swap. The Company performs ongoing assessments of counterparty credit risk.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Effects of Derivative Instruments on Financial Position and Results of Operations
The following table summarizes the fair value for derivatives designated as hedging instruments in the consolidated balance sheets as of December 31, 20212023 and 2020:2022:
| | | | Fair Value as of December 31, | | | Fair Value as of December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | | Dollars in thousands | 2023 | | 2022 |
Location on Balance Sheet (1): | Location on Balance Sheet (1): | | |
| Derivatives designated as hedges — Assets: | Derivatives designated as hedges — Assets: | | |
| Derivatives designated as hedges — Assets: | |
| Derivatives designated as hedges — Assets: | |
| Derivatives designated as hedges — Assets: | |
| Derivatives designated as hedges — Assets: | |
| Derivatives designated as hedges — Assets: | |
| Derivatives designated as hedges — Assets: | |
| Derivatives designated as hedges — Assets: | |
| Derivatives designated as hedges — Assets: | |
| Prepaid expenses and other current assets | |
| Prepaid expenses and other current assets | |
| Prepaid expenses and other current assets | Prepaid expenses and other current assets | | |
| Cash Flow Hedges | Cash Flow Hedges | | |
| Cash Flow Hedges | |
| Cash Flow Hedges | |
Interest rate swap(2) | |
Interest rate swap(2) | |
Interest rate swap(2) | |
| Cross-currency swap | Cross-currency swap | 4,900 | | | 7,623 | | |
| | Net Investment Hedges | Net Investment Hedges | | |
| Net Investment Hedges | |
| Net Investment Hedges | |
| Cross-currency swap | |
| Cross-currency swap | |
| Cross-currency swap | Cross-currency swap | 5,120 | | | 5,297 | | |
| Other assets | Other assets | | |
| Cash Flow Hedges | Cash Flow Hedges | | |
| Cash Flow Hedges | |
| Cash Flow Hedges | |
Interest rate swap(2) | Interest rate swap(2) | 4,373 | | | — | | |
Interest rate swap(2) | |
Interest rate swap(2) | |
| | Net Investment Hedges | Net Investment Hedges | | |
| Net Investment Hedges | |
| Net Investment Hedges | |
| Cross-currency swap | |
| Cross-currency swap | |
| Cross-currency swap | Cross-currency swap | 2,104 | | | — | | |
| Total derivatives designated as hedges — Assets | Total derivatives designated as hedges — Assets | $ | 16,497 | | | $ | 12,920 | | |
| | Derivatives designated as hedges — Liabilities | Derivatives designated as hedges — Liabilities | | |
| Derivatives designated as hedges — Liabilities | |
| Derivatives designated as hedges — Liabilities | |
| Accrued expenses and other current liabilities | |
| Accrued expenses and other current liabilities | |
| Accrued expenses and other current liabilities | Accrued expenses and other current liabilities | | |
| Cash Flow Hedges | Cash Flow Hedges | | |
| Cash Flow Hedges | |
| Cash Flow Hedges | |
Interest rate swap(2) | |
Interest rate swap(2) | |
Interest rate swap(2) | |
| Cross-currency swap | |
| | Net Investment Hedges | |
| Net Investment Hedges | |
| Net Investment Hedges | |
| Cross-currency swap | |
| Cross-currency swap | |
| Cross-currency swap | |
| Other liabilities | |
| Cash Flow Hedges | |
| Cash Flow Hedges | |
| Cash Flow Hedges | |
Interest rate swap(2) | |
Interest rate swap(2) | |
Interest rate swap(2) | Interest rate swap(2) | $ | 18,187 | | | $ | 22,033 | | |
| Cross-currency swap | Cross-currency swap | 8,283 | | | 4,335 | | |
| Net Investment Hedges | Net Investment Hedges | | |
| Cross-currency swap | Cross-currency swap | — | | | 1,884 | | |
| Other liabilities | | |
| Cash Flow Hedges | | |
Interest rate swap(2) | 30,143 | | | 71,736 | | |
| Cross-currency swap | Cross-currency swap | 4,859 | | | 26,728 | | |
| Net Investment Hedges | | |
| Cross-currency swap | Cross-currency swap | 3,366 | | | 9,449 | | |
| Total derivatives designated as hedges — Liabilities | Total derivatives designated as hedges — Liabilities | 64,838 | | | 136,165 | | |
(1)The Company classifies derivative assets and liabilities as current based on the cash flows expected to be incurred within the following 12 months.
(2)At December 31, 20212023 and 2020,2022, the total notional amounts related to the Company’s interest rate swaps were $1.8 billion and $1.9 billion, respectively.$1.5 billion.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following presents the effect of derivative instruments designated as cash flow hedges and net investment hedges on the accompanying consolidated statement of operations during the years ended December 31, 20212023 and 2020:2022:
| | Dollars in thousands | Dollars in thousands | Balance in AOCL
Beginning of
Year | | Amount of
Gain (Loss)
Recognized in
AOCL | | Amount of Gain (Loss)
Reclassified from
AOCL into
Earnings | | Balance in AOCL
End of Year | | Location in
Statements of
Operations | Dollars in thousands | Balance in AOCI
Beginning of
Year | | Amount of
Gain (Loss)
Recognized in
AOCI | | Amount of Gain (Loss)
Reclassified from
AOCI into
Earnings | | Balance in AOCI
End of Year | | Location in
Statements of
Operations |
| Year Ended December 31, 2021 | | | | | | | | | |
| Year Ended December 31, 2023 | |
| Cash Flow Hedges | Cash Flow Hedges | |
| Cash Flow Hedges | |
| Cash Flow Hedges | |
| Interest rate swap | |
| Interest rate swap | |
| Interest rate swap | | $ | 56,712 | | | $ | 4,899 | | | $ | 18,055 | | | $ | 43,556 | | | Interest expense |
| Cross-currency swap | | Cross-currency swap | (20,271) | | | (27,406) | | | (31,914) | | | (15,763) | | | Other income, net |
| Forward Currency Forward Contracts | | Forward Currency Forward Contracts | — | | | 436 | | | 436 | | | — | | | Cost of sales |
| Net Investment Hedges | |
| Cross-currency swap | |
| Cross-currency swap | |
| Cross-currency swap | | (6,914) | | | (30,738) | | | 7,846 | | | (45,498) | | | Interest income |
| $ | |
| Year Ended December 31, 2022 | |
| Year Ended December 31, 2022 | |
| Year Ended December 31, 2022 | |
| Cash Flow Hedges | |
| Cash Flow Hedges | |
| Cash Flow Hedges | |
| Interest rate swap | |
| Interest rate swap | |
| Interest rate swap | Interest rate swap | $ | (93,769) | | | $ | 27,402 | | | $ | (22,411) | | | $ | (43,956) | | | Interest expense | $ | (43,956) | | | $ | | $ | 93,308 | | | $ | | $ | (7,360) | | | $ | | $ | 56,712 | | | Interest expense | | Interest expense |
| Cross-currency swap | Cross-currency swap | (1,073) | | | 24,275 | | | 32,890 | | | (9,688) | | | Other income, net | Cross-currency swap | (9,688) | | | 8,847 | | 8,847 | | | 19,430 | | 19,430 | | | (20,271) | | (20,271) | | | Other income, net | | Other income, net |
| Net Investment Hedges | Net Investment Hedges | |
| Cross-currency swap | Cross-currency swap | (12,291) | | | 16,515 | | | 6,545 | | | (2,321) | | | Interest income |
| $ | (107,133) | | | $ | 68,192 | | | $ | 17,024 | | | $ | (55,965) | | |
| Year Ended December 31, 2020 | | | | | | | | |
| Cash Flow Hedges | |
| Interest rate swap | $ | (45,145) | | | $ | (64,778) | | | $ | (16,154) | | | $ | (93,769) | | | Interest expense |
| Cross-currency swap | Cross-currency swap | 177 | | | (17,147) | | | (15,897) | | | (1,073) | | | Other income, net |
| Net Investment Hedges | |
| Cross-currency swap | Cross-currency swap | 10,229 | | | (14,911) | | | 7,609 | | | (12,291) | | | Interest income | (2,321) | | | 2,196 | | 2,196 | | | 6,789 | | 6,789 | | | (6,914) | | (6,914) | | | Interest income | | Interest income |
| $ | (34,739) | | | $ | (96,836) | | | $ | (24,442) | | | $ | (107,133) | | |
| $ | |
Derivative Instruments not designated hedges:
During the second quarter of 2021, the Company entered into a foreign currency swap, with a notional amount of $7.3 million, to mitigate the risk from fluctuations in foreign currency exchange rates associated with an intercompany loan denominated in JPY. In a foreign currency swap transaction, the Company agrees with another party to exchange, at specified intervals, the difference between one currency and another currency at a fixed exchange rate, generally set at inception, calculated by reference to an agreed upon notional amount. The notional amount of each currency is exchanged at the inception and termination of the currency swap by each party. The Company subsequently paid down a portion of this swap in the second quarter of 2023, bringing the notional amount down to $5.5 million as of December 31, 2023.
The following table summarizes the gains (losses) of derivative instruments not designated as hedges on the consolidated statements of income, which was included in other income:
| | | | | | | | | | | | | | |
| Dollars in thousands | | December 31, |
| | 2023 | | 2022 |
| | | | |
| Foreign currency swaps | | 566 | | | 1,258 | |
| Total | | $ | 566 | | | $ | 1,258 | |
7. GOODWILL AND OTHER INTANGIBLE ASSETS
Goodwill
The Company tests goodwill for impairment by either performing a qualitative evaluation or a quantitative test.
The qualitative evaluation is an assessment of factors including reporting unit specific operating results as well as industry, market and general economic conditions, to determine whether it is more likely than not that the fair values of a reporting unit is less than its carrying amount, including goodwill. The Company may elect to bypass the qualitative assessment for its 3three reporting units and perform a quantitative test. The assumptions used in evaluating goodwill for impairment are subject to change and are tracked against historical results by management.
The quantitative test estimates the fair value of the 3three reporting units using a discounted cash flow model, which incorporates significant estimates and assumptions made by management which, by their nature, are characterized by uncertainty. Inputs
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
used to fair value the Company's reporting units are considered inputs of the fair value hierarchy. For Level 3 measurements, significant increases or decreases in long-term growth rates or discount rates in isolation or in combination could result in a significantly lower or higher fair value measurement. The key assumptions impacting the valuation included the following:
•The reporting unit's financial projections, which are based on management's assessment of regional and macroeconomic variables, industry trends and market opportunities, and the Company's strategic objectives and future growth plans.
•The projected terminal value for the reporting unit, which represents the present value of projected cash flows beyond the last period in the discounted cash flow analysis. The terminal value reflects the Company's assumptions related to long-term growth rates and profitability, which are based on several factors, including local and macroeconomic variables, market opportunities, and future growth plans.
•The discount rate used to measure the present value of the projected future cash flows is set using a weighted-average cost of capital method that considers market and industry data as well as the Company's specific risk factors that are likely to be considered by a market participant. The weighted-average cost of capital is the Company's estimate of the overall after-tax rate of return required by equity and debt holders of a business enterprise.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
In the second quarter of 2023, due to the Boston recall, as well as the associated drop in the Company's stock price in that quarter, the Company elected to perform a quantitative analysis, using a combination of a discounted cash flow method and guideline public company method for its TT reporting unit. The quantitative test utilized key assumptions of revenue growth rate, a terminal growth rate of 2%, a discount rate of 10%, and the range and application of the company guideline multiples. The Company determined, after performing the quantitative analysis, that the fair value of the goodwill of the reporting unit was not less than the carrying amount, with more than 20% headroom.During the third quarter of 2021,2023, the Company elected to perform a qualitative analysis for its 3three reporting units. The Company determined, after performing the qualitative analysis, that there was no evidence that it is more likely than not that the fair value was less that the carrying amounts, therefore, it was not necessary to perform a quantitative impairment test.
Changes in the carrying amount of goodwill in 20212023 and 20202022 were as follows:
| | | | | | | | | | | | | | | | | |
| Dollars in thousands | Codman Specialty Surgical | | Tissue Technologies | | Total |
| Goodwill at January 1, 2020 | $ | 653,500 | | | $ | 300,780 | | | $ | 954,280 | |
| Transfer to assets held for sale | — | | | (47,546) | | | (47,546) | |
| Foreign currency translation | 18,475 | | | 7,158 | | | 25,633 | |
| Goodwill at December 31, 2020 | $ | 671,975 | | | $ | 260,392 | | | $ | 932,367 | |
| ACell Acquisition | — | | | 94,147 | | | 94,147 | |
| Foreign currency translation | (8,547) | | | (4,509) | | | (13,056) | |
| Goodwill at December 31, 2021 | $ | 663,428 | | | $ | 350,030 | | | $ | 1,013,458 | |
On January 4, 2021, the Company completed its sale of its Extremity Orthopedics business. In conjunction with the sale of this business, the Company rebranded the Orthopedics and Tissue Technologies segment as Tissue Technologies in the first quarter of 2021. See Note 4. Acquisitions and Divestitures, for details.
Other Intangible Assets
The components of the Company's identifiable intangible assets were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2021 |
| Dollars in thousands | Weighted
Average
Life | | Cost | | Accumulated Amortization | | Net |
| Completed technology | 18 years | | $ | 1,132,954 | | | $ | (307,013) | | | $ | 825,941 | |
| Customer relationships | 12 years | | 211,344 | | | (142,755) | | | 68,589 | |
| Trademarks/brand names | 28 years | | 98,367 | | | (31,468) | | | 66,899 | |
| Codman trade name | Indefinite | | 167,758 | | | — | | | 167,758 | |
| Supplier relationships | 30 years | | 30,211 | | | (16,192) | | | 14,019 | |
| All other | 11 years | | 6,258 | | | (3,891) | | | 2,367 | |
| | | $ | 1,646,892 | | | $ | (501,319) | | | $ | 1,145,573 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2020 |
| Dollars in thousands | Weighted
Average
Life | | Cost | | Accumulated Amortization | | Net |
| Completed technology | 19 years | | $ | 896,478 | | | $ | (248,088) | | | $ | 648,390 | |
| Customer relationships | 12 years | | 213,270 | | | (132,838) | | | 80,432 | |
| Trademarks/brand names | 28 years | | 104,209 | | | (31,767) | | | 72,442 | |
| Codman trade name | Indefinite | | 170,226 | | | — | | | 170,226 | |
| Supplier relationships | 27 years | | 30,211 | | | (15,203) | | | 15,008 | |
All other (1) | 4 years | | 6,693 | | | (3,755) | | | 2,938 | |
| | | $ | 1,421,087 | | | $ | (431,651) | | | $ | 989,436 | |
(1)Prior period amounts were reclassified as it relates to All Other within this table to conform to the current period presentation
At December 31, 2020, $13.3 million of Intangible assets, net were presented separately as "Assets held for sale" in conjunction with the sale of the Extremity Orthopedics business.
The increase in the Company's identifiable intangible assets at December 31, 2021 as compared to the year ended December 31, 2020, was primarily driven from intangible assets acquired in conjunction with the ACell Acquisition. See Note 4, Acquisitions and Divestitures, for details. | | | | | | | | | | | | | | | | | |
| Dollars in thousands | Codman Specialty Surgical | | Tissue Technologies | | Total |
| Goodwill at January 1, 2022 | $ | 663,428 | | | $ | 350,030 | | | $ | 1,013,458 | |
| Sale of non-core traditional wound care business | — | | | (5,019) | | | (5,019) | |
| SIA Acquisition | — | | | 41,855 | | | 41,855 | |
| Foreign currency translation | (7,209) | | | (4,204) | | | (11,413) | |
| Balance at December 31, 2022 | $ | 656,219 | | | $ | 382,662 | | | $ | 1,038,881 | |
| Sale of non-core traditional wound care business | — | | | — | | | — | |
| SIA Acquisition | — | | | (382) | | | (382) | |
| Foreign currency translation | 10,718 | | | 6,245 | | | 16,963 | |
| Balance at December 31, 2023 | $ | 666,937 | | | $ | 388,525 | | | $ | 1,055,462 | |
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Other Intangible Assets
The components of the Company's identifiable intangible assets were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2023 |
| Dollars in thousands | Weighted
Average
Life | | Cost | | Accumulated Amortization | | Net |
| Completed technology | 18 years | | $ | 1,226,128 | | | $ | (448,519) | | | $ | 777,609 | |
| Customer relationships | 12 years | | 193,895 | | | (152,160) | | | 41,735 | |
| Trademarks/brand names | 28 years | | 98,892 | | | (38,754) | | | 60,138 | |
| Codman trade name | Indefinite | | 174,531 | | | — | | | 174,531 | |
| Supplier relationships | 30 years | | 30,211 | | | (18,148) | | | 12,063 | |
| All other | 11 years | | 6,180 | | | (4,423) | | | 1,757 | |
| | | $ | 1,729,837 | | | $ | (662,004) | | | $ | 1,067,833 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2022 |
| Dollars in thousands | Weighted
Average
Life | | Cost | | Accumulated Amortization | | Net |
| Completed technology | 18 years | | $ | 1,204,325 | | | $ | (370,968) | | | $ | 833,357 | |
| Customer relationships | 12 years | | 193,081 | | | (144,040) | | | 49,041 | |
| Trademarks/brand names | 28 years | | 97,265 | | | (34,674) | | | 62,591 | |
| Codman trade name | Indefinite | | 166,693 | | | — | | | 166,693 | |
| Supplier relationships | 30 years | | 30,211 | | | (17,170) | | | 13,041 | |
| All other | 11 years | | 5,957 | | | (4,071) | | | 1,886 | |
| | | $ | 1,697,532 | | | $ | (570,923) | | | $ | 1,126,609 | |
Intangible Assets with Indefinite Lives
The Company tests intangible assets with indefinite lives for impairment annually in the third quarter in accordance with ASC Topic 350. Additionally, the Company may perform interim tests if an event occurs or circumstances change that could potentially reduce the fair value of a indefinite lived intangible asset below its carrying amount. The Company tests for impairment by either performing a qualitative evaluation or a quantitative test. The qualitative evaluation is an assessment of factors, including specific operating results as well as industry, market and general economic conditions, to determine whether it is more likely than not that the fair values of the intangible asset is less than its carrying amount. The Company may elect to bypass this qualitative evaluation and perform a quantitative test.
During the third quarter of 2021,2023, the Company elected to perform a qualitative analysis for its intangible asset with indefinite lives. The Company determined, after performing the qualitative analysis, that there was no evidence that it is more likely than not that the fair value was less that the carrying amounts, therefore, it was not necessary to perform a quantitative impairment test.
Intangible Assets with Definite Lives
Product rights and other definite-lived intangible assets are tested periodically for impairment in accordance with ASC Topic 360 when events or changes in circumstances indicate that an asset's carrying value may not be recoverable. The impairment testing involves comparing the carrying amount of the asset or asset group to the forecasted undiscounted future cash flows. In the event the carrying value of the asset exceeds the undiscounted future cash flows, the carrying value is considered not recoverable and impairment exists. An impairment loss is measured as the excess of the asset's carrying value over its fair value, calculated using discounted future cash flows. The computed impairment loss is recognized in the period that the impairment occurs.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
In the second quarter of 2023, due to the Boston recall, the Company elected to perform impairment testing on certain definite lived intangibles. The intangible components associated with the recalled products include completed technology and customer relationships with a net book value of $28.8 million and $7.6 million, respectively, as of December 31, 2023. The company used an undiscounted cash flow methodology and obtained revenue projections through the useful life of the intangibles. After performing the analysis, no impairment was noted. The Company will continue to monitor these intangibles as we return to the market and evaluate any changes that would impact our sales of these products.
Amortization expense (including amounts reported in cost of product revenues) for the years ended December 31, 2023, 2022 and 2021 2020 and 2019 was $83.3$82.8 million, $74.5$78.3 million and $72.8$83.3 million, respectively.
Annual amortization expense is expected to approximate $79.1 million in 2022, $78.4 million in 2023, $77.7$82.7 million in 2024, $77.7$82.7 million in 2025, $77.6$82.5 million in 2026, $80.6 million in 2027, $79.0 million in 2028 and $585.8$481.2 million thereafter. Amortization of product technology based intangible assets totaled $66.5$70.4 million, $46.7$64.4 million and $45.8$66.5 million for the years ended December 31, 2021, 20202023, 2022 and 2019,2021, respectively, and is presented by the Company within cost of goods sold.
8. TREASURY STOCK
As of December 31, 20212023 and 2020,2022, there were 4.912.8 million and 6.8 million shares of treasury stock outstanding with a cost of $234.4$647.3 million and $235.1$362.9 million, respectively, at a weighted average cost per share of $47.86.$50.76 and $53.18, respectively.
On December 7, 2020,August 15, 2023, the Company entered into a $125 million accelerated share repurchase ("August 2023 ASR") and received 2.3 million shares of the common stock at inception of the August 2023 ASR, which represented approximately 80% of the expected total shares under the August 2023 ASR. On October 18, 2023 the early exercise provision was exercised by the August 2023 ASR counterparty. The Company received an additional 0.9 million shares determined using the volume-weighted average price of the Company's common stock during the term of the August 2023 ASR.
On January 26, 2023, the Company entered into a $150 million accelerated share repurchase ("January 2023 ASR") and received 2.1 million shares of common stock at inception of the January 2023 ASR, which represented approximately 80% of the expected total shares under the January 2023 ASR. The settlement of the January ASR agreement was completed in two separate transactions on April 26, 2023 and May 4, 2023, where the Company received an additional 0.30 million and 0.31 million shares respectively, determined using the volume-weighted average price of the Company's common stock during the term of the January 2023 ASR.
On August 16, 2022, the Inflation Reduction Act of 2022 (the “Act”) was signed into law. The Act implemented a new excise tax of 1% on the net share repurchases made by the Company effective for share repurchases performed January 1, 2023, or after. The Company accrued $2.5 million of excise tax related to the two ASR agreements during 2023.
On July 18, 2023, the Board of Directors authorized the Company to repurchase up toa new $225 million share repurchase program, replacing the existing $225 million program authorized in April 2022. As of the Company’s common stock.December 31, 2023,$100 million remained authorized. The program authorized in July 2023, and which expires on December 31, 2025, allows the Company to repurchase its shares opportunistically from time to time. The repurchase authorization expires in December 2022.Company may utilize various methods to effect any repurchases, including open market transactions, privately negotiated transactions, transactions structured through investment banking institutions, including accelerated share repurchases, or a combination of the foregoing, some of which may be effected through Rule 10b5-1 plans. The price and timing of any future purchases under the share repurchase program will depend on factors such as levels of cash generation from operations, the volume of stock option exercises by employees, cash requirements for acquisitions, dividends, economic and market conditions and stock price, and such repurchases may be discontinued at any time.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
For the year ended December 31, 2021, there were no repurchases of the Company’s common stock as part of the share repurchase authorization.
On January 12, 2022, the Company entered into a $125.0$125 million accelerated share repurchase ("2022 ASR") and received 1.51.48 million shares of Company common stock at inception of the 2022 ASR, which represented approximately 80% of the expected total shares under the 2022 ASR. The remaining 20% ofIn March 24, 2022, the expected total shares is expected to settle in the first half of 2022, upon which additional shares of common stock may be delivered to the Company or, under certain circumstances, the Company may be required to make a cash payment or may elect to deliver shares of our common stock to the 2022 ASR counterparty in each case pursuant to the terms of the 2022 ASR agreement between the Company andearly exercise provision was exercised by the 2022 ASR counterparty. The total number of shares to be delivered or the amount of such payment, as well as the final average price per share, will be based on the volume-weighted average price, less a discount, of the Company's common stock during the term of the transaction. As a result of this transaction, $100 million remains available under the $225 million stock repurchase authorization.
During the twelve months ended December 31, 2020, the Company repurchased 2.1 million shares of Integra’s common stock as part of the previous share repurchase authorization. The Company utilized $100.0 million of net proceeds from the offering of the Convertible Senior Notes to execute the share repurchase transactions. This included $7.6 million from certain purchasers of the convertible notes in conjunction with the closing of the offering. On February 5, 2020, the Company entered into a $92.4 million accelerated share repurchase ("2020 ASR") to complete the remaining $100.0 million of share repurchase. The Company received 1.3 million shares at inception of the 2020 ASR, which represented approximately 80% of the expected total shares. Upon settlement of the 2020 ASR in June 2020,on March 24, 2022, the Company received an additional 0.60.46 million shares determined using the volume-weighted average price of the Company's common stock during the term of the transaction.2022 ASR.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
9. STOCK-BASED COMPENSATION
Stock-based compensation expense - all related to employees and members of the Board of Directors - recognized under the authoritative guidance was as follows:
| | Years Ended December 31, |
| Years Ended December 31, | | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | | 2019 | Dollars in thousands | 2023 | | 2022 | | 2021 |
| Cost of goods sold | Cost of goods sold | 470 | | | 344 | | | 317 | |
| Research and development | Research and development | 1,644 | | | 1,471 | | | 1,785 | |
| Selling, general and administrative | Selling, general and administrative | $ | 34,096 | | | $ | 17,776 | | | $ | 19,153 | |
| Total stock-based compensation expense | Total stock-based compensation expense | 36,210 | | | 19,591 | | | 21,255 | |
| Total estimated tax benefit related to stock-based compensation expense | Total estimated tax benefit related to stock-based compensation expense | 13,804 | | | 6,221 | | | 9,420 | |
| Net effect on net income | Net effect on net income | $ | 22,406 | | | $ | 13,370 | | | $ | 11,835 | |
EMPLOYEE STOCK PURCHASE PLAN
The purpose of the Employee Stock Purchase Plan (the “ESPP”) is to provide eligible employees of the Company with the opportunity to acquire shares of common stock at periodic intervals by means of accumulated payroll deductions. The ESPP is a non-compensatory plan. Under the ESPP, a total of 3.0 million shares of common stock are reserved for issuance. These shares will be made available either from the Company’s authorized but unissued shares of common stock or from shares of common stock reacquired by the Company as treasury stock. At December 31, 2021, 2.0 million shares remain available for purchase under the ESPP. During the years ended December 31, 2021, 2020 and 2019, the Company issued 16,948 shares, 18,284 shares and 12,531 shares under the ESPP for $1.1 million, $1.1 million and $0.7 million, respectively.
EQUITY AWARD PLANS
As of December 31, 2021,2023, the Company had stock options, restricted stock awards, performance stock awards, contract stock awards and restricted stock unit awards outstanding under the Integra LifeSciences Holdings Corporation Fifth Amended and Restated 2003 Equity Incentive Plan (the “2003 Plan”). The 2000 and 2001 Equity Incentive Plans were terminated as of February 19, 2021, and no further awards may be issued under the plans.
In May 2010 and May 2017, the stockholders of the Company approved amendments to the 2003 Plan to increase by 3.5 million and 1.7 million, respectively, the number of shares of common stock that may be issued under the 2003 Plan. The Company has reserved 4.0 million shares under each of the 2000 Plan and the 2001 Plan, and 14.7 million shares under the 2003 Plan. The Plans permit the Company to grant incentive and non-qualified stock options, stock appreciation rights,
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
restricted stock, contract stock, performance stock, or dividend equivalent rights to designated directors, officers, employees and associates of the Company.
Stock options issued under the 2003 Plan became exercisable over specified periods, generally within four years from the date of grant for officers and employees, and within one year from the date of the grant for members of the Board of Directors. The awards generally expire eight years from the grant date for employees and from six to ten years for directors and certain executive officers, except in certain instances that result in accelerated vesting due to death, disability, retirement age or change in control provisions within their grant agreements. Restricted stock issued under the 2003 Plan vests ratably over specified periods, generally three years after the date of grant. The vesting of performance stock issued under the 2003 Plan is subject to service and performance conditions.
Stock Options
The Company values stock option grants using the binomial distribution model. Management believes that the binomial distribution model is preferable to the Black-Scholes model because it is a more flexible model that gives consideration to the impact of non-transferability and vesting provisions in valuing employee stock options.
In determining the value of stock options granted, the Company considered that it has never paid cash dividends and does not currently intend to pay cash dividends, and thus has assumed a 0% dividend yield. Expected volatilities are based on the historical volatility of the Company’s stock price. The expected life of stock options is estimated based on historical data on exercise of stock options, post-vesting forfeitures and other factors to estimate the expected term of the stock options granted. The risk-free interest rates are derived from the U.S. Treasury yield curve in effect on the date of grant for instruments with a remaining term similar to the expected life of the options. The Company accounts for forfeitures as they occur.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following weighted-average assumptions were used in the calculation of fair value:
| | Years Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Years Ended December 31, | | | Years Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Dividend yield | Dividend yield | 0% | | 0% | | 0% | Dividend yield | 0% | | 0% |
| Expected volatility | Expected volatility | 29% | | 27% | | 28% | Expected volatility | 30% | | 30% | | 29% |
| Risk free interest rate | Risk free interest rate | 1.30% | | 0.89% | | 2.51% | Risk free interest rate | 3.86% | | 2.01% | | 1.30% |
| Expected life of option from grant date | Expected life of option from grant date | 7 years | | 7 years | | 7 years | Expected life of option from grant date | 7 years | | 7 years |
| Weighted average grant date fair value of options granted | Weighted average grant date fair value of options granted | $22.59 | | $13.03 | | $18.74 | Weighted average grant date fair value of options granted | $21.58 | | $23.15 | | $22.59 |
The following table summarizes the Company’s stock option activity.
| | Shares | | Weighted Average Exercise Price | | Weighted Average Contractual Term in Years | | Aggregate Intrinsic Value |
| Shares | | | Shares | | Weighted Average Exercise Price | | Weighted Average Contractual Term in Years | | Aggregate Intrinsic Value |
| Stock Options | Stock Options | (In thousands) | | | | | | (In thousands) | Stock Options | (In thousands) | | | | | | (In thousands) |
| Outstanding at January 1, 2021 | 1,346 | | | $ | 39.25 | | | 4.41 | | $34,560 |
| Outstanding at January 1, 2023 | | Outstanding at January 1, 2023 | 1,202 | | | $ | 49.63 | | | 4.14 | | $10,772 |
| Granted | Granted | 150 | | | 68.10 | | | — | | | — | |
| Exercised | Exercised | (230) | | | 24.74 | | | — | | | — | |
| Forfeited or Expired | Forfeited or Expired | (41) | | | 51.20 | | | — | | | — | |
| Outstanding at December 31, 2021 | 1,225 | | | $ | 45.11 | | | 4.30 | | $ | 26,970 | |
| Exercisable at December 31, 2021 | 858 | | | $ | 41.07 | | | 3.38 | | $ | 22,242 | |
| Outstanding at December 31, 2023 | |
| Exercisable at December 31, 2023 | |
The Company recognized $5.0$1.4 million, $3.2$3.5 million and $3.0$5.0 million in expense related to stock options during the years ended December 31, 2021, 20202023, 2022 and 2019,2021, respectively. The intrinsic value of options exercised for the years ended December 31, 2023, 2022 and 2021 2020 and 2019 were $11.1$1.8 million, $8.7$4.0 million and $14.6$11.1 million, respectively. Cash received from option exercises and employee stock purchase plan was $6.8$4.3 million, $5.2$5.5 million and $6.9$6.8 million, for the years ended December 31, 2021, 20202023, 2022 and 2019,2021, respectively. The realized tax benefit from options exercised were $2.2 million, $1.7were $0.1 million, $0.6 million and $3.0$2.2 million for the years ended December 31, 2023, 2022 and 2021, 2020 and 2019, respectively.
As of December 31, 2021,2023, there was approximately $2.9$3.8 million of total unrecognized compensation costs related to unvested stock options. These costs are expected to be recognized over a weighted-average period of approximately twothree years.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Awards of Restricted Stock, Performance Stock and Contract Stock
The following table summarizes the Company’s awards of restricted stock, performance stock and contract stock for the year ended December 31, 2021.2023.
| | Restricted Stock Awards | | Performance Stock and Contract Stock Awards |
| Shares | | Weighted Average Grant Date Fair Value Per Share | | Shares | | Weighted Average Grant Date Fair Value Per Share |
| (In thousands) | | | | (In thousands) | | |
| Unvested, January 1, 2021 | 472 | | | $ | 50.02 | | | 197 | | | 47.66 | |
| Restricted Stock Awards | | | Restricted Stock Awards | | Performance Stock and Contract Stock Awards |
| Shares | | | Shares | | Weighted Average Grant Date Fair Value Per Share | | Shares | | Weighted Average Grant Date Fair Value Per Share |
| (In thousands) | |
| Unvested, January 1, 2023 | |
| Unvested, January 1, 2023 | |
| Unvested, January 1, 2023 | |
| Granted | Granted | 242 | | | 68.31 | | | 223 | | | 67.91 | |
| Adjustments for performance achievement related to award target | Adjustments for performance achievement related to award target | — | | | — | | | 96 | | | 58.18 | |
| Cancellations | Cancellations | (78) | | | 54.94 | | | (152) | | | — | |
| Released | Released | (214) | | | 51.63 | | | (20) | | | 67.84 | |
| Vested but not released | — | | | — | | | (289) | | | 58.06 | |
| Unvested, December 31, 2021 | 422 | | | $ | 58.78 | | | 55 | | | 70.74 | |
| Unvested, December 31, 2023 | |
The Company recognized $31.2$18.7 million, $16.4$24.3 million and $18.1$31.2 million in expense related to such awards during the years ended December 31, 2021, 20202023, 2022 and 2019,2021, respectively. The total fair market value of shares vested and released in 2023, 2022
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
and 2021 2020 and 2019 was $15.7$18.2 million, $17.3$65.0 million and $21.1$15.7 million, respectively. Vested awards include shares that have been fully earned but had not been delivered as of December 31, 2021.2023.
Performance stock awards have performance features associated with them. Performance stock, restricted stock and contract stock awards generally have requisite service periods of three years. The fair value of these awards is being expensed on a straight-line basis over the vesting period. As of December 31, 2023, there were 51,241 performance stock units ("PSU's") granted in 2021 subject to vest and be released in 2024 based on PSU catch-up opportunity. No additional PSU's were subject to vest based on 2023 performance achievement.
As of December 31, 2021,2023, there was approximately $21.1$30.5 million of total unrecognized compensation costs related to unvested restricted stock, performance stock and contract stock awards. These costs are expected to be recognized over a weighted-average period of approximately two years.
As ofAt December 31, 2021,2023, there were approximately 0.5 million vested Restricted Units and 0.1 million vested performance share units held by various employees for which the related shares have not yet been issued. The final determination of the number of shares to be issued is made by the Company's Compensation Committee of the Board of Directors which is contingent upon achieving certain revenue and organic revenue growth performance metric.
At December 31, 2021, there were approximately 3.52.7 million shares available for grant under the 2003 Plan.
The Company capitalized into inventory, share based compensation costs of $0.5$0.6 million, $0.4$0.6 million and $0.3$0.5 million for the years ended December 31, 2021, 20202023, 2022 and 2019,2021, respectively. Such share-based compensation was recognized as cost of goods sold when related inventory was sold.
EMPLOYEE STOCK PURCHASE PLAN
INTEGRA LIFESCIENCES HOLDINGS CORPORATIONThe purpose of the Employee Stock Purchase Plan (the “ESPP”) is to provide eligible employees of the Company with the opportunity to acquire shares of common stock at periodic intervals by means of accumulated payroll deductions. The ESPP is a non-compensatory plan. Under the ESPP, a total of 3.0 million shares of common stock are reserved for issuance. These shares will be made available either from the Company’s authorized but unissued shares of common stock or from shares of common stock reacquired by the Company as treasury stock. At December 31, 2023, 1.9 million shares remain available for purchase under the ESPP. During the years ended December 31, 2023, 2022 and 2021, the Company issued 23,337 shares, 20,780 shares and 16,948 shares under the ESPP for $1.0 million, $1.1 million and $1.1 million, respectively.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
10. RETIREMENT BENEFIT PLANS
DEFINED BENEFIT PLANS
The Company has various defined benefit plans which covers certain employees in France, Japan, Germany and Switzerland.
Net periodic benefit costs for the Company’s defined benefit pension plans for the years ended December 31, 20212023 and 20202022 included the following (amounts in thousands):
| | Year ended December 31, | |
| 2021 | | 2020 | |
| Year ended December 31, | |
| Year ended December 31, | |
| Year ended December 31, | |
| 2023 | |
| 2023 | |
| 2023 | |
| Service cost | |
| Service cost | |
| Service cost | Service cost | $ | 2,741 | | | $ | 4,029 | | |
| Interest cost | Interest cost | 100 | | | 219 | | |
| Interest cost | |
| Interest cost | |
| Expected return on plan assets | |
| Expected return on plan assets | |
| Expected return on plan assets | Expected return on plan assets | (893) | | | (652) | | |
| Amortization of prior service cost (credit) | Amortization of prior service cost (credit) | (281) | | | (274) | | |
| Amortization of prior service cost (credit) | |
| Amortization of prior service cost (credit) | |
| Recognized actuarial losses | |
| Recognized actuarial losses | |
| Recognized actuarial losses | Recognized actuarial losses | 186 | | | 787 | | |
| Settlements | Settlements | 51 | | | (102) | | |
| Settlements | |
| Settlements | |
| Net period benefit cost | Net period benefit cost | $ | 1,904 | | | $ | 4,007 | | |
| Net period benefit cost | |
| Net period benefit cost | |
The following weighted average assumptions were used to develop net periodic pension benefit costs and the actuarial present values of projected pension benefit obligations for the years ended December 31, 20212023 and 2020,2022, respectively:
| | As of December 31, | |
| 2021 | | 2020 | |
| As of December 31, | |
| As of December 31, | |
| As of December 31, | |
| 2023 | |
| 2023 | |
| 2023 | |
| Discount rate | |
| Discount rate | |
| Discount rate | Discount rate | 0.37 | % | | 0.34 | % | |
| Expected return on plan assets | Expected return on plan assets | 3.59 | % | | 2.04 | % | |
| Expected return on plan assets | |
| Expected return on plan assets | |
| Rate of compensation increase | |
| Rate of compensation increase | |
| Rate of compensation increase | Rate of compensation increase | 2.10 | % | | 2.14 | % | |
| Interest crediting rate for cash balance plans | Interest crediting rate for cash balance plans | 1.00 | % | | 1.00 | % | |
| Interest crediting rate for cash balance plans | |
| Interest crediting rate for cash balance plans | |
The Company’s discount rates are determined by considering current yield curves representing high quality, long-term fixed income instruments. The resulting discount rates are consistent with the duration of plan liabilities. In 20212023 and 2020,2022, the
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
discount rates were prescribed as the current yield on corporate bonds with an average rating of AA or AAA of equivalent currency and term to the liabilities. The expected returns on plan assets represent the average rate of return expected to be earned on plan assets over the period the benefits included in the benefit obligation are to be paid. In developing the expected rates of return, the Company considers returns of historical market data as well as actual returns on the plan assets. Using this reference information, the long-term return expectations for each asset category are developed according to the allocation among those investment categories.
The assessment is determined using projections from external financial sources, long-term historical averages, actual returns by asset class and the various asset class allocations by market.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The following sets forth the change in projected benefit obligations and the change in plan assets for the years ended December 31, 20212023 and 20202022 and a reconciliation of the funded status at December 31, 20212023 and 2020,2022, respectively (amounts in thousands):
| | Year ended December 31, | |
| 2021 | | 2020 | |
| Year Ended December 31, | |
| Year Ended December 31, | |
| Year Ended December 31, | |
| 2023 | |
| 2023 | |
| 2023 | |
| Change In Projected Benefit Obligations | |
| Change In Projected Benefit Obligations | |
| Change In Projected Benefit Obligations | Change In Projected Benefit Obligations | | | | |
| Projected benefit obligations, beginning of year | Projected benefit obligations, beginning of year | $ | 72,869 | | | $ | 66,972 | | |
| Projected benefit obligations, beginning of year | |
| Projected benefit obligations, beginning of year | |
| Interest cost | |
| Interest cost | |
| Interest cost | Interest cost | 100 | | | 219 | | |
| Service cost | Service cost | 2,741 | | | 4,029 | | |
| Service cost | |
| Service cost | |
| Actuarial (gain) loss | |
| Actuarial (gain) loss | |
| Actuarial (gain) loss | Actuarial (gain) loss | (5,044) | | | (3,347) | | |
| Plan amendments | Plan amendments | (586) | | | — | | |
| Plan amendments | |
| Plan amendments | |
| Plan settlements | |
| Plan settlements | |
| Plan settlements | Plan settlements | (655) | | | (77) | | |
| Employee contribution | Employee contribution | 917 | | | 883 | | |
| Employee contribution | |
| Employee contribution | |
| Premiums paid | |
| Premiums paid | |
| Premiums paid | Premiums paid | (373) | | | (388) | | |
| Benefit payment | Benefit payment | (2,128) | | | (1,537) | | |
| Plans transferred in | — | | | — | | |
| Benefit payment | |
| Benefit payment | |
| Effect of foreign currency exchange rates | |
| Effect of foreign currency exchange rates | |
| Effect of foreign currency exchange rates | Effect of foreign currency exchange rates | (2,657) | | | 6,115 | | |
| Projected benefit obligations, end of year | Projected benefit obligations, end of year | $ | 65,184 | | | $ | 72,869 | | |
| Projected benefit obligations, end of year | |
| Projected benefit obligations, end of year | |
| | Year Ended December 31, | |
| | Year ended December 31, | |
| Year Ended December 31, | |
| | 2021 | | 2020 | |
| Year Ended December 31, | |
| 2023 | |
| 2023 | |
| 2023 | |
| Change In Plan Assets | |
| Change In Plan Assets | |
| Change In Plan Assets | Change In Plan Assets | | | | |
| Plan assets at fair value, beginning of year | Plan assets at fair value, beginning of year | $ | 37,825 | | | $ | 30,770 | | |
| Plan assets at fair value, beginning of year | |
| Plan assets at fair value, beginning of year | |
| Actual return on plan assets | |
| Actual return on plan assets | |
| Actual return on plan assets | Actual return on plan assets | 3,371 | | | 2,882 | | |
| Employer contributions | Employer contributions | 2,254 | | | 2,274 | | |
| Employer contributions | |
| Employer contributions | |
| Employee contributions | |
| Employee contributions | |
| Employee contributions | Employee contributions | 917 | | | 883 | | |
| Plan settlements | Plan settlements | (633) | | | (56) | | |
| Plan settlements | |
| Plan settlements | |
| Benefits paid | |
| Benefits paid | |
| Benefits paid | Benefits paid | (2,128) | | | (1,537) | | |
| Premiums paid | Premiums paid | (373) | | | (388) | | |
| Premiums paid | |
| Premiums paid | |
| Effect of foreign currency exchange rates | |
| Effect of foreign currency exchange rates | |
| Effect of foreign currency exchange rates | Effect of foreign currency exchange rates | (1,319) | | | 2,997 | | |
| Plan assets at fair value, end of year | Plan assets at fair value, end of year | $ | 39,914 | | | $ | 37,825 | | |
| Plan assets at fair value, end of year | |
| Plan assets at fair value, end of year | |
| | Year ended December 31, | |
| 2021 | | 2020 | |
| Year Ended December 31, | |
| Year Ended December 31, | |
| Year Ended December 31, | |
| 2023 | |
| 2023 | |
| 2023 | |
| Reconciliation Of Funded Status | |
| Reconciliation Of Funded Status | |
| Reconciliation Of Funded Status | Reconciliation Of Funded Status | | | | |
| Fair value of plan assets | Fair value of plan assets | $ | 39,914 | | | $ | 37,825 | | |
| Fair value of plan assets | |
| Fair value of plan assets | |
| Benefit obligations | |
| Benefit obligations | |
| Benefit obligations | Benefit obligations | 65,184 | | | 72,869 | | |
| Unfunded benefit obligations | Unfunded benefit obligations | $ | 25,270 | | | $ | 35,044 | | |
| Unfunded benefit obligations | |
| Unfunded benefit obligations | |
The unfunded benefit obligations are included in other liabilities in the consolidated balance sheets at December 31, 20212023 and 2020,2022, respectively.
During the periods ended December 31, 20212023 and 20202022, the Company had a net loss of $6.6 million and a net gain of $7.0 million and $4.6$7.4 million, respectively, recognized within accumulated other comprehensive loss that has not been recognized as a component of net periodic benefit cost. The gain recognized during the period ended December 31, 2021, is primarily attributed to a change in the discount rate used to estimate the projected benefit obligation for defined benefit plans which cover certain employees in Switzerland. The combined accumulated benefit obligations for the defined benefit plans was $60.3$62.8 million and $61.5$46.4 million as of December 31, 20212023 and 2020,2022, respectively.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Unrecognized gains and losses are amortized over the average remaining future service for each plan. For plans with no active employees, they are amortized over the average life expectancy. The amortization of gains and losses is determined by using a 10% corridor of the greater of the market value of assets or the accumulated benefit obligation. Total unamortized gains and losses in excess of the corridor are amortized over the average remaining future service.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Prior service costs/benefits for the pension plans are amortized over the average remaining future service of plan participants at the time of the plan amendment.
The net plan assets of the pension plans are invested in common trusts. Common trusts are classified as Level 2 in fair value hierarchy. The fair value of common trusts is valued at net asset value based on the fair values of the underlying investments of the trusts as determined by the sponsor of the trusts. The investment strategy of the Company's defined benefit plans is both to meet the liabilities of the plans as they fall due and to maximize the return on invested assets within appropriate risk profile.
The benefit plans in France and Germany had no assets at December 31, 2021.2023.
As of December 31, 2021,2023, no plan assets are expected to be returned to the Company in the next twelve months.
The following table is the summary of expected future benefit payments (in thousands):
| | | | 2022 | $ | 1,934 | | |
| 2023 | $ | 1,796 | | |
| 2024 | |
| | 2024 | |
| | 2024 | 2024 | $ | 1,831 | | |
| 2025 | 2025 | $ | 2,073 | | |
| 2025 | |
| 2025 | |
| 2026 | 2026 | $ | 2,003 | | |
| 2026 | |
| 2026 | |
| 2027 | |
| 2027 | |
| 2027 | |
| 2028 | |
| 2028 | |
| 2028 | |
| Next five years | Next five years | $ | 10,395 | | |
| Next five years | |
| Next five years | |
As of December 31, 2021,2023, contributions expected to be paid to the plan in 20222024 is $2.3$3.0 million.
DEFINED CONTRIBUTION PLANS
The Company also has various defined contribution savings plans that cover substantially all employees in the United States, Belgium, Canada, France, Japan, Netherlands, the U.K. and Puerto Rico. The Company matches a certain percentage of each employee’s contributions as per the provisions of the plans. Total contributions by the Company to the plans were $8.8$10.4 million, $6.7$9.8 million and $8.6$8.8 million for the years ended December 31, 2023, 2022 and 2021, 2020 and 2019, respectively.
DEFERRED COMPENSATION PLAN
The Company maintains a Deferred Compensation Plan in which certain employees of the Company may defer the payment and taxation of up to 75% of their base salary and up to 100% of bonus amounts and other eligible cash compensation.
During the first quarter of 2020, employees participating in the Company's deferred compensation plan began to defer their compensation. This deferred compensation is invested in funds offered under this plan and is valued based on Level 1 measurements in the fair value hierarchy. Assets of the Company's deferred compensation plan are included in Other current assets and recorded at fair value based on their quoted market prices. The fair value of these assets at December 31, 20212023 and 20202022 was $3.8$6.1 million and $2.0$4.7 million. Offsetting liabilities relating to the deferred compensation plan are included in Other liabilities.
11. LEASES AND RELATED PARTY LEASES
The Company leases administrative, manufacturing, research and distribution facilities and vehicles through operating lease agreements. The Company has no finance leases as of December 31, 2021.2023. Many of the Company's leases include both lease (e.g., fixed payments including rent) and non-lease components (e.g., common-area or other maintenance costs). For vehicles, the Company has elected the practical expedient to group lease and non-lease components.
Most facility leases include 1one or more options to renew. The exercise of lease renewal options is typically at the Company's sole discretion, therefore, the majority of renewals to extend the lease terms are not included in the ROU assets and lease liabilities as they are not reasonably certain of exercise. The Company regularly evaluates renewal options and when they are reasonably certain of exercise, the renewal period is included in the lease term.
As most of the Company's leases do not provide an implicit rate, the Company uses a collateralized incremental borrowing rate based on the information available at the lease commencement date in determining the present value of the lease payments.
Total operating lease expense for the year ended December 31, 20212023 and December 31, 2020,2022, was $20.3$24.0 million and $19.7$22.6 million, respectively, which includes $0.3 million, in related party operating lease expense.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Supplemental balance sheet information related to operating leases at December 31, 20212023 were as follows:
| | | December 31, 2021 | | December 31, 2020 |
| | (In thousands, except lease term and discount rate) |
| | | | | December 31, 2023 | |
| | | | | December 31, 2023 | |
| | | | | December 31, 2023 | | | December 31, 2022 |
| | | | | (In thousands, except lease term and discount rate) | | | | | | | (In thousands, except lease term and discount rate) |
| | ROU assets | ROU assets | | $ | 84,543 | | | $ | 83,635 | |
| ROU assets | |
| ROU assets | |
| | Current lease liabilities | |
| Current lease liabilities | |
| Current lease liabilities | Current lease liabilities | | 14,775 | | | 12,818 | |
| Non-current lease liabilities | Non-current lease liabilities | | 90,329 | | | 88,118 | |
| Total lease liabilities | Total lease liabilities | | $ | 105,104 | | | $ | 100,936 | |
| | Weighted average remaining lease term (in years): | Weighted average remaining lease term (in years): | | |
| Weighted average remaining lease term (in years): | |
| Weighted average remaining lease term (in years): | |
| | Leased facilities | |
| | Leased facilities | |
| | Leased facilities | Leased facilities | | 10.4 years | | 11.6 years | | | | | 16.3 years | | 16.9 years |
| Leased vehicles | Leased vehicles | | 2.1 years | | 2.3 years | Leased vehicles | | | | | 1.9 years | | 2.0 years |
| | Weighted average discount rate: | Weighted average discount rate: | | |
| Weighted average discount rate: | |
| Weighted average discount rate: | |
| Leased facilities | |
| Leased facilities | |
| Leased facilities | Leased facilities | | 5.1 | % | | 4.6 | % | | | | | 5.9 | % | | 5.4 | % |
| Leased vehicles | Leased vehicles | | 2.6 | % | | 2.3 | % | Leased vehicles | | | | | 2.7 | % | | 2.7 | % |
Supplemental cash flow information related to leases was as follows:
| | December 31, 2021 | | December 31, 2020 |
| (In thousands) |
| December 31, 2023 | | | December 31, 2023 | | December 31, 2022 |
| (In thousands) | | | (In thousands) |
| Cash paid for amounts included in the measurement of lease liabilities: | Cash paid for amounts included in the measurement of lease liabilities: | |
| Operating cash flows from operating leases | |
| Operating cash flows from operating leases | |
| Operating cash flows from operating leases | Operating cash flows from operating leases | $ | 15,077 | | | $ | 15,226 | |
| | ROU assets obtained in exchange for lease liabilities: | ROU assets obtained in exchange for lease liabilities: | |
| ROU assets obtained in exchange for lease liabilities: | |
| ROU assets obtained in exchange for lease liabilities: | |
| Operating leases | |
| Operating leases | |
| Operating leases | Operating leases | 12,610 | | | 6,027 | |
|
Future minimum lease payments under operating leases at December 31, 20212023 were as follows:
| | Related Parties | | Third Parties | | Total |
| (In thousands) |
| 2022 | 296 | | | 17,678 | | | 17,974 | |
| 2023 | 296 | | | 14,611 | | | 14,907 | |
| Related Parties | | | Related Parties | | Third Parties | | Total |
| (In thousands) | | | (In thousands) |
| 2024 | 2024 | 296 | | | 12,784 | | | 13,080 | |
| 2025 | 2025 | 296 | | | 11,289 | | | 11,585 | |
| 2026 | 2026 | 296 | | | 9,889 | | | 10,185 | |
| 2027 | |
| 2028 | |
| Thereafter | Thereafter | 838 | | | 67,992 | | | 68,830 | |
| Total minimum lease payments | Total minimum lease payments | $ | 2,318 | | | $ | 134,243 | | | $ | 136,561 | |
| Less: Imputed interest | Less: Imputed interest | | | | | $ | 31,457 | |
| Total lease liabilities | Total lease liabilities | | 105,104 | |
| Less: Current lease liabilities | Less: Current lease liabilities | | 14,775 | |
| Long-term lease liabilities | Long-term lease liabilities | | 90,329 | |
There were no future minimum lease payments under finance leases at December 31, 2021.2023.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
Related Party Leases
The Company leases its manufacturing facility in Plainsboro, New Jersey, from a general partnership that is 50% owned by a corporation whose stockholders are trusts, whose beneficiaries include family membersprincipal stockholder of the Company’s principal stockholder and former director.Company. The term of the current lease agreement is through October 31, 2029 at an annual rate of approximately $0.3 million per year.million. The current lease agreement also provides (i) a 5-year renewal option for the Company to extend the lease from November 1, 2029 through October 31, 2034 at the fair market rental rate of the premises, and (ii) another 5-year renewal option to extend the lease from November 1, 2034 through October 31, 2039 at the fair market rental rate of the premises.
12. INCOME TAXES
Income (Loss) before income taxes consisted of the following: | | Years Ended December 31, |
| Years Ended December 31, | | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | | 2019 | Dollars in thousands | 2023 | | 2022 | | 2021 |
| United States operations | United States operations | $ | 91,150 | | | $ | 15,082 | | | $ | (38,359) | |
| Foreign operations | Foreign operations | 123,527 | | | 78,438 | | | 98,463 | |
| Total | Total | $ | 214,677 | | | $ | 93,520 | | | $ | 60,104 | |
A reconciliation of the U.S. Federal statutory rate to the Company’s effective tax rate is as follows:
| | Years Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Years Ended December 31, | | | Years Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Federal statutory rate | Federal statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % | Federal statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| Increase (decrease) in income taxes resulting from: | Increase (decrease) in income taxes resulting from: | |
| State income taxes, net of federal tax benefit | State income taxes, net of federal tax benefit | 1.9 | % | | 1.2 | % | | 1.0 | % |
| Foreign operations | (4.0) | % | | (7.9) | % | | (20.0) | % |
| Excess tax benefits from stock compensation | (1.2) | % | | (1.0) | % | | (5.6) | % |
| State income taxes, net of federal tax benefit | |
| State income taxes, net of federal tax benefit | | 2.9 | % | | 0.1 | % | | 1.9 | % |
| Benefit derived from foreign operations | | Benefit derived from foreign operations | (17.2) | % | | (1.6) | % | | (3.3) | % |
| Nondeductible meals and entertainment | Nondeductible meals and entertainment | 0.1 | % | | 0.4 | % | | 1.5 | % | Nondeductible meals and entertainment | 1.1 | % | | 0.1 | % | | 0.1 | % |
| | Intercompany profit in inventory | Intercompany profit in inventory | (0.2) | % | | 1.2 | % | | 1.2 | % |
| Nondeductible facilitative costs | 0.2 | % | | 1.4 | % | | 0.8 | % |
| Intercompany profit in inventory | |
| Intercompany profit in inventory | | 3.3 | % | | 0.3 | % | | (0.2) | % |
| | Research and development credit | Research and development credit | (1.2) | % | | (1.6) | % | | (2.9) | % |
| Return to provision | (0.7) | % | | (2.3) | % | | 1.7 | % |
| Global intangible low-taxed income ("GILTI") | 0.7 | % | | 2.5 | % | | 7.6 | % |
| Nondeductible executive compensation | 0.9 | % | | 2.4 | % | | 3.0 | % |
| Fair market value step up on intra-entity transfer of intellectual property | — | % | | (63.3) | % | | — | % |
| | Research and development credit | |
| | Research and development credit | | (5.7) | % | | (1.4) | % | | (1.2) | % |
| | Nondeductible executive compensation & stock compensation shortfall | |
| | Nondeductible executive compensation & stock compensation shortfall | |
| | Nondeductible executive compensation & stock compensation shortfall | | 2.3 | % | | (0.6) | % | | (0.3) | % |
| Transaction and deal related costs | | Transaction and deal related costs | 3.3 | % | | (1.8) | % | | 0.1 | % |
| Gain from sale of business - book to tax differences | Gain from sale of business - book to tax differences | 3.9 | % | | 2.8 | % | | — | % | Gain from sale of business - book to tax differences | — | % | | — | % | | 3.9 | % |
| Swiss tax holiday | — | % | | — | % | | (15.7) | % |
| Nondeductible R&D expense | — | % | | — | % | | 22.7 | % |
| Changes in valuation allowances | | Changes in valuation allowances | 4.9 | % | | — | % | | 0.1 | % |
| Other | Other | (0.2) | % | | — | % | | 0.2 | % | Other | 0.5 | % | | (0.5) | % | | (0.9) | % |
| Effective tax rate | Effective tax rate | 21.2 | % | | (43.2) | % | | 16.5 | % | Effective tax rate | 16.4 | % | | 15.6 | % | | 21.2 | % |
Our effective tax rate was 21.2%16.4% and (43.2)%15.6% of income before income taxes for the years ended December 31, 20212023 and December 31, 2020,2022, respectively. In 2023, the Company’s higher effective tax rate was driven by the the inclusion of Global Intangible Low-Taxed Income ("GILTI"), offset by a $5.8 million income tax benefit related to a four-year tax credit received by a Swiss subsidiary. The Company received an extension of the 2018 Swiss tax grant for three years, until the 2027 tax year. The net benefit of the tax credit, recorded as of December 31, 2023, was based on projections of use of the incremental tax grant. The Company’s Swiss subsidiary may offset the tax credit against cantonal and communal income and capital taxes during tax years 2024 through 2027. Any unused balance at the end of the 2027 tax period will be forfeited.
In 2022, the Company’s lower effective tax rate was driven by a $5.1 million income tax benefit related to stock compensation and a $2.4 million income tax benefit related to the filing of amended federal and state returns for prior years. In 2021, the Company's higher effective tax rate was driven in part by an $8.5 million income tax expense for nondeductible goodwill related to the sale of the Extremity Orthopedics business, offset by a $3.1 million income tax benefit related to excess tax benefits from stock compensation. In 2020,
During 2023, the Company’s lower worldwideforeign operations generated a $0.7 million decrease in income tax expense when compared to the same period in 2022, because of geographic and business mix of taxable earnings and losses, among other factors. The 2023 foreign effective tax rate was primarily driven by an $59.2 million income tax benefit on an intra-entity transfer of certain intellectual property, substantially completed during the fourth quarteris 16.4%, compared to 15.9% in 2020. Excluding this transaction, the effective worldwide tax rate for 2020 was 20.2%.2022.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
In December 2020, the Company completed an intra-entity transfer of certain intellectual property rights to one of its subsidiaries in Switzerland. While the transfer did not result in a taxable gain, the Company’s Swiss subsidiary received a step-up in tax basis based on the fair value of the transferred intellectual property rights. The Company determined the fair value using a discounted cash flow model based on expectations of revenue growth rates, royalty rates, discount rates, and useful lives of the intellectual property. The Company recorded a $59.2 million deferred tax benefit in Switzerland related to the amortizable tax basis in the transferred intellectual property.
During 2021,2022, the Company’s foreign operations generated a $63.6$0.4 million increase in income tax expense when compared to the same period in 2020,2021, because of the intra-entity transfer of certain intellectual property in 2020, geographic and business mix of taxable earnings and losses, among other factors. The 20212022 foreign effective tax rate is 15.2%15.9%, compared to (57.1)%15.2% in 2020.2021. The Company’s foreign tax rate is primarily based upon statutory rates and is also impacted by the intra-entity transfer of certain intellectual property as described above for 2020.
During 2020, the Company’s foreign operations generated a $48.2 million decrease in income tax expense when compared to the same period in 2019 due to the intra-entity transfer of certain intellectual property, geographic and business mix of taxable earnings and losses, among other factors. The 2020 foreign effective tax rate is (57.1)%, compared to 3.5% in 2019. The Company’s foreign tax rate is primarily based upon statutory rates and is also impacted by the intra-entity transfer of certain intellectual property as described above for 2020. During 2019, the Company finalized negotiations related to tax holidays in Switzerland, on a federal, cantonal, and communal level. The Company received a federal tax credit in Switzerland of $12.1 million ($0.14 per share), which may be used over a seven-year period, ending in 2024. The Company also received a reduction in its rate for the cantonal and communal level taxes during the third quarter of 2019, pursuant to tax reform in Switzerland.rates.
Changes to income tax laws and regulations, in any of the tax jurisdictions in which the Company operates, could impact the effective tax rate. Various governments, both U.S. and non-U.S., are increasingly focused on tax reform and revenue-raising legislation. The current U.S. administration has proposedOn August 16, 2022, the Inflation Reduction Act of 2022 (the “Act”) was signed into law, for which the company did not experience a material impact on the company’s effective tax reform which, if enacted, may increase the Company’s U.S. federal income tax liability.rate. Further, legislation in foreign jurisdictions may be enacted, in continued response to the base erosion and profit-sharing (BEPS) project begun by the Organization for Economic Cooperation and Development (OECD).
The OECD recently finalized major reform of the international tax system with respectreleased model rules related to implementing a new 15% global minimum tax rate.regime (“Pillar 2”). Several of the jurisdictions that we operate in have already adopted some form of the model rules, which could impact the amount of taxes that the Company pays after 2023. However, the rules are complex and provide for delays during the early transition years, if certain conditions are met. The Company will continue to analyze the law to determine potential impacts. At this time, the Company does not expect the Pillar 2 legislation to have a material impact on its consolidated financial statements. Such changes in U.S. and Non-U.S. jurisdictions could have an adverse effect on the Company’s effective tax rate.
The provision for income taxes consisted of the following:
| | Years Ended December 31, |
| Years Ended December 31, | | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | | 2019 | Dollars in thousands | 2023 | | 2022 | | 2021 |
| Current: | Current: | | | | | |
| Federal | |
| Federal | |
| Federal | Federal | $ | 31,938 | | | $ | 6,184 | | | $ | 14,597 | |
| State | State | 11,377 | | | 5,029 | | | 3,447 | |
| Foreign | Foreign | 5,042 | | | 12,553 | | | 10,905 | |
| Total current | Total current | $ | 48,357 | | | $ | 23,766 | | | $ | 28,949 | |
| Deferred: | Deferred: | |
| Federal | Federal | (12,830) | | | (5,079) | | | (10,889) | |
| Federal | |
| Federal | |
| State | State | (3,688) | | | (1,760) | | | (666) | |
| Foreign | Foreign | 13,763 | | | (57,299) | | | (7,491) | |
| Total deferred | Total deferred | $ | (2,755) | | | $ | (64,138) | | | $ | (19,046) | |
| Provision for income taxes | Provision for income taxes | $ | 45,602 | | | $ | (40,372) | | | $ | 9,903 | |
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The income tax effects of significant temporary differences that give rise to deferred tax assets and liabilities, shown before jurisdictional netting, are presented below:
| | December 31, |
| December 31, | | | December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | Dollars in thousands | 2023 | | 2022 |
| Assets: | Assets: | | | |
| Doubtful accounts | |
| Doubtful accounts | |
| Doubtful accounts | Doubtful accounts | $ | 2,029 | | | $ | 2,207 | |
| Inventory related items | Inventory related items | 31,841 | | | 47,034 | |
| Tax credits | Tax credits | 13,319 | | | 18,319 | |
| Accrued vacation | Accrued vacation | 3,042 | | | 3,403 | |
| Accrued bonus | Accrued bonus | 7,415 | | | 4,883 | |
| Stock compensation | Stock compensation | 13,955 | | | 6,160 | |
| Deferred revenue | Deferred revenue | 1,742 | | | 1,665 | |
| Net operating loss carryforwards | Net operating loss carryforwards | 26,198 | | | 29,335 | |
| Capitalization of research and development expenses | Capitalization of research and development expenses | 36,770 | | | 13,044 | |
| Unrealized foreign exchange loss | 12,849 | | | 23,798 | |
| Unrealized foreign exchange gain | |
| Charitable contributions carryforward | Charitable contributions carryforward | 206 | | | 203 | |
| | Leases and Other | |
| Leases and Other | |
| Leases and Other | Leases and Other | 41,371 | | | 23,205 | |
| Total deferred tax assets | Total deferred tax assets | 190,737 | | | 173,256 | |
| Less valuation allowance | Less valuation allowance | (9,767) | | | (9,897) | |
| Deferred tax assets after valuation allowance | Deferred tax assets after valuation allowance | $ | 180,970 | | | $ | 163,359 | |
| Liabilities: | Liabilities: | | | |
| Intangible and fixed assets | Intangible and fixed assets | (152,150) | | | (90,274) | |
| Intangible and fixed assets | |
| Intangible and fixed assets | |
| Unrealized foreign exchange loss | |
| Leases and Other | Leases and Other | (17,658) | | | (15,585) | |
| Total deferred tax liabilities | Total deferred tax liabilities | $ | (169,808) | | | $ | (105,859) | |
| | Total net deferred tax assets (liabilities) | Total net deferred tax assets (liabilities) | $ | 11,162 | | | $ | 57,500 | |
| Total net deferred tax assets (liabilities) | |
| Total net deferred tax assets (liabilities) | |
Prior period amounts were re-classed, as it relates to Leases and Other, between tax assets and liabilities within this table, to conform to the current period presentation.
The 2017 U.S. Tax Cuts and Jobs Act contained a provision which requires, for tax purposes, the capitalization and amortization of research and development expenses; effective for years beginning after December 31, 2021. The Company’s deferred tax assets increased by $14.4 million and $20.3 million at December 31, 2023 and December 31, 2022 respectively within the table above, related to the 2017 Tax Act.
At December 31, 2021,2023, the Company had net operating loss carryforwards of $71.7$64.7 million for federal income tax purposes, $26.6$98.4 million for foreign income tax purposes and $39.0$19.2 million for state income tax purposes to offset future taxable income. The majority ofFor the federal net operating loss carryforwards, $55.8 million will expire through 2037,2037; while $4.1$8.9 million have an indefinite carry forward period. For foreign net operating loss carryforwards, $81.0 million will expire through 2028, while the remaining $26.6$17.4 million have an indefinite carry forward period. The state net operating loss carryforwards expire through 2036.
The valuation allowance relates to deferred tax assets for certain items that will be deductible for income tax purposes under very limited circumstances and for which the Company believes it will not satisfy the more likely than not threshold for realization of the associated tax benefit. In the event that the Company determines that it would be able to realize more or less than the recorded amount of net deferred tax assets, an adjustment to the deferred tax asset valuation allowance would be recorded in the period such a determination is made.
The Company’s valuation allowance decreased by less than $0.1 million, increased by less than $0.1 million and increased by $2.9 million at December 31, 2021, 2020 and 2019, respectively.2023 increased by $2.8 million, as compared to 2022, primarily driven by a $3.3 million increase related to the new Swiss tax credit. The 2021 and 2020 valuation allowance primarilyfor 2022 had remained substantially unchanged, from the prior period.as compared to 2021.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at Beginning of Period | | Charged to Costs and Expenses | | Other | | Deductions | | Balance at End of Period |
| Description | | | | |
| Dollars in thousands | | | |
| Year ended December 31, 2023 | | | | | | | | | |
| Deferred tax assets valuation allowance | 14,672 | | | 3,069 | | | 26 | | | 56 | | | 17,823 | |
| Year ended December 31, 2022 | | | | | | | | | |
| Deferred tax assets valuation allowance | 15,258 | | | (515) | | | — | | | (71) | | | 14,672 | |
| Year ended December 31, 2021 | | | | | | | | | |
| Deferred tax assets valuation allowance | 13,825 | | | 1,444 | | | 89 | | | (100) | | | 15,258 | |
As of December 31, 2021,2023, the Company has not provided deferred income taxes on unrepatriated earnings from foreign subsidiaries as they are deemed to be indefinitely reinvested unless there is a manner under which to remit the earnings with no material tax cost. Material taxes would primarily be attributable to foreign withholding taxes and local income taxes when such earnings are distributed. The Company will repatriate foreign earnings when there is no need for reinvestment overseas and no material tax cost to bring the earnings back to the United States. Reinvestment considerations would include future acquisitions, transactions, and capital expenditure plans. As such, the Company has determined the tax impact of repatriating these earnings would not be material as of December 31, 2021. The Company does not anticipate the need to repatriate earnings from foreign subsidiaries as a result of the impact of the COVID-19 pandemic.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
A reconciliation of the beginning and ending amount of uncertain tax benefits is as follows:
| | Years Ended December 31, |
| Years Ended December 31, | | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | | 2019 | Dollars in thousands | 2023 | | 2022 | | 2021 |
| (In thousands) |
| (In thousands) | | | (In thousands) |
| Balance, beginning of year | Balance, beginning of year | $ | 702 | | | $ | 676 | | | $ | 676 | |
| Gross increases: | Gross increases: | |
| Current year tax positions | Current year tax positions | — | | | — | | | 53 | |
| Current year tax positions | |
| Current year tax positions | |
| Prior years' tax positions | Prior years' tax positions | — | | | 26 | | | — | |
| Lapse of statute | |
| Other | Other | (26) | | | — | | | (53) | |
| Balance, end of year | Balance, end of year | $ | 676 | | | $ | 702 | | | $ | 676 | |
Approximately $0.7$0.8 million of the balance at December 31, 20212023 relates to uncertain tax positions that, if recognized, would affect the annual effective tax rate. There areThe Company has no amounts within the balance of uncertain tax positions at December 31, 20212023 related to tax positions for which it is reasonably possible that the amounts could be reduced during the twelve months following December 31, 2021.2023.
The Company recognizes interest and penalties relating to uncertain tax positions in income tax expense. The Company recognized a minimal benefitexpense for the years ended December 31, 2021, 20202023, 2022 and 2019.2021. The Company had minimal interest and penalties accrued for the years ended December 31, 20212023 and 20202022 and 2019.2021.
The Company files Federal income tax returns, as well as multiple state, local and foreign jurisdiction tax returns. The Company is no longer subject to examinations of its U.S. consolidated Federal income tax returns by the IRS through fiscal year 2017. All significant state and local matters have been concluded through fiscal 2015.year 2018. All significant foreign matters have been settled through fiscal 2012.2017.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
13. NET INCOME PER SHARE
Basic and diluted net income per share was as follows:
| | | | Years Ended December 31, | | Years Ended December 31, |
| Dollars in thousands, except per share amounts | Dollars in thousands, except per share amounts | 2021 | | 2020 | | 2019 | Dollars in thousands, except per share amounts | 2023 | | 2022 | | 2021 |
| Basic net income per share: | Basic net income per share: | | | | | |
| Net income | Net income | $ | 169,075 | | | $ | 133,892 | | | $ | 50,201 | |
| Net income | |
| Net income | |
| Weighted average common shares outstanding | Weighted average common shares outstanding | 84,698 | | | 84,650 | | | 85,637 | |
| Basic net income per common share | Basic net income per common share | $ | 2.00 | | | $ | 1.58 | | | $ | 0.59 | |
| | Diluted net income per share: | Diluted net income per share: | |
| Diluted net income per share: | |
| Diluted net income per share: | |
| Net income | |
| Net income | |
| Net income | Net income | $ | 169,075 | | | $ | 133,892 | | | $ | 50,201 | |
| | Weighted average common shares outstanding — Basic | Weighted average common shares outstanding — Basic | 84,698 | | | 84,650 | | | 85,637 | |
| Weighted average common shares outstanding — Basic | |
| Weighted average common shares outstanding — Basic | |
| Effect of dilutive securities: | Effect of dilutive securities: | |
| Stock options and restricted stock | |
| Stock options and restricted stock | |
| Stock options and restricted stock | Stock options and restricted stock | 787 | | | 577 | | | 857 | |
| Weighted average common shares for diluted earnings per share | Weighted average common shares for diluted earnings per share | 85,485 | | | 85,228 | | | 86,494 | |
| Diluted net income per common share | Diluted net income per common share | $ | 1.98 | | | $ | 1.57 | | | $ | 0.58 | |
Common stock of approximately 0.10.6 million and 0.3 million shares at December 31, 2021,2023, and 20202022 that are issuable through exercise of dilutive securities, respectively, and were not included in the computation of diluted net income per share because their effect would have been anti-dilutive.
Performance Shares
014. ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS)
Comprehensive income for the years ended December 31, 2023 and Restricted Units that entitle the holders to approximately 0.5 million shares of common stock2022:
| | | | | | | | | | | | | | | | | | | | |
| Dollars in thousands | | 2023 | | 2022 | | 2021 |
| Net income | | $ | 67,741 | | | $ | 180,550 | | | $ | 169,075 | |
| Foreign currency translation adjustment, net of tax | | (12,103) | | | (17,807) | | | (17,362) | |
| Change in unrealized loss/(gain) on derivatives, net of tax | | (6,658) | | | 65,798 | | | 39,268 | |
| Pension liability adjustment, net of tax | | (6,610) | | | 7,429 | | | 6,998 | |
| Comprehensive income, net | | 42,370 | | | 235,970 | | | 197,979 | |
Changes in accumulated other comprehensive loss by component between December 31, 2023 and 2022 are includedpresented in the basictable below, net of tax:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Dollars in thousands | | Gains and Losses on Derivatives | | Defined Benefit Pension Items | | Foreign Currency Items | | Total |
| Balance at December 31, 2022 | | $ | 28,147 | | | $ | 9,322 | | | $ | (27,204) | | | $ | 10,265 | |
| Other comprehensive gain (loss) | | (16,991) | | | (6,610) | | | (5,901) | | | (29,502) | |
| Less: Amounts reclassified from accumulated other comprehensive income, net | | (10,333) | | | — | | | 6,202 | | | (4,131) | |
| Net current-period other comprehensive gain (loss) | | (6,658) | | | (6,610) | | | (12,103) | | | (25,371) | |
| Balance at December 31, 2023 | | $ | 21,489 | | | $ | 2,712 | | | $ | (39,307) | | | $ | (15,106) | |
For the year ended December 31, 2023, the Company reclassified a loss of $24.2 million and diluted weighted average shares outstanding calculationa gain of $20.1 million from their date of issuance because no further consideration is due relatedaccumulated other comprehensive loss to the issuance of the underlying common shares.other income, net and interest income, respectively.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
14. ACCUMULATED OTHER COMPREHENSIVE LOSS
Changes in accumulated other comprehensive loss by component between December 31, 2021 and 2020 are presented in the table below, net of tax:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Dollars in thousands | | Gains and Losses on Derivatives | | Defined Benefit Pension Items | | Foreign Currency Items | | Total |
| Balance at December 31, 2020 | | $ | (82,249) | | | $ | (5,105) | | | $ | 13,295 | | | $ | (74,059) | |
| Other comprehensive gain (loss) | | 52,359 | | | 6,998 | | | (17,362) | | | 41,995 | |
| Less: Amounts reclassified from accumulated other comprehensive income, net | | 13,091 | | | — | | | — | | | 13,091 | |
| Net current-period other comprehensive gain (loss) | | 39,268 | | | 6,998 | | | (17,362) | | | 28,904 | |
| Balance at December 31, 2021 | | $ | (42,981) | | | $ | 1,893 | | | $ | (4,067) | | | $ | (45,155) | |
For the year ended December 31, 2021, the Company reclassified a gain of $25.3 million and a loss of $12.2 million from accumulated other comprehensive loss to other income, net and interest income, respectively.
15. COMMITMENTS AND CONTINGENCIES
In consideration for certain technology, manufacturing, distribution, and selling rights and licenses granted to the Company, the Company has agreed to pay royalties on sales of certain products that it sells. The royalty payments that the Company made under these agreements were not significant for any of the periods presented.
The Company is subject to various claims, lawsuits and proceedings in the ordinary course of the Company's business, including claims by current or former employees, distributors and competitors and with respect to its products and product liability claims, lawsuits and proceedings, some of which have been settled by the Company. In the opinion of management, such claims are either adequately covered by insurance or otherwise indemnified, or are not expected, individually or in the aggregate, to result in a material, adverse effect on the Company's financial condition. However, it is possible that the Company's results of operations, financial position and cash flows in a particular period could be materially affected by these contingencies.
The Company accrues for loss contingencies when it is deemed probable that a loss has been incurred and that loss is estimable. The amounts accrued are based on the full amount of the estimated loss before considering insurance proceeds and do not include an estimate for legal fees expected to be incurred in connection with the loss contingency. The Company consistently accrues legal fees expected to be incurred in connection with loss contingencies as those fees are incurred by outside counsel as a period cost.
On December 21, 2023, Fortis Advisors, LLC (representative of the securityholders of ACell, Inc.) filed for arbitration against Integra Life Sciences claiming breach of contract related to the earnout consideration from the 2021 acquisition of Acell. Refer to Note 4, Acquisitions and Divestitures, for additional information on the ACell Contingent Considerations. The Company believes that it has strong defenses to the allegations in the arbitration and intends to defend the matter vigorously.
On September 12, 2023, a securities class action complaint, captioned Pembroke Pines Firefighters & Police Officers Pension Fund v. Integra LifeSciences Holdings Corporation, No. 23-cv-20321 (D.N.J.), was filed by a purported stockholder of the Company in the United States District Court for the District of New Jersey (the “Pembroke Litigation”) against the Company and certain of the Company’s current and former executive officers. The Pembroke Litigation, filed on behalf of a putative class of stockholders who purchased or acquired the Company’s common stock between March 11, 2019 and May 22, 2023, inclusive, alleges violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, and Rule 10b-5 promulgated thereunder, on the basis of purportedly materially false and misleading statements and omissions relating to certain quality systems issues identified by the U.S. Food and Drug Administration at the Company’s Boston, Massachusetts manufacturing facility, the Company’s efforts to remediate those issues, and the Company’s forecasts for certain products in its Tissue Technologies segment. The complaint seeks, among other things, compensatory damages, attorneys’ fees, expert fees, and other costs. The Company believes that it has strong defenses to the allegations in the Pembroke Litigation, as we intend to defend the matter vigorously.
Contingent Consideration
The Company determined the fair value of contingent consideration during the twelve-month period ended December 31, 20212023 and 20202022 to reflect the change in estimate, additions, payments, transfers and the timefair value of money during the period.
A reconciliation of the opening balances to the closing balances of these Level 3 measurements for the years ended December 31, 20212023 and 20202022 is as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended December 31, 2021 | | Contingent Consideration Liability Related to Acquisition of: | | |
| Arkis
(See Note 4) | | Location in Financial Statements | | Derma Sciences | | | ACell Inc.
(See Note 4) | | Location in Financial Statements |
| | Short-term | | Long-term | | | | Long-term | | | Long-term | | |
| Balance as of January 1, 2021 | | $ | 3,415 | | | $ | 11,746 | | | | | $ | 230 | | | | $ | — | | | |
| Additiions | | — | | | — | | | | | — | | | | 23,900 | | | |
| Transfers from long-term to current portion | | 276 | | | (276) | | | | | — | | | | — | | | |
| Change in fair value of contingent consideration liabilities | | — | | | $ | (62) | | | Research and development | | — | | | | (2,100) | | | Selling, general and administrative |
| Balance as of December 30, 2021 | | $ | 3,691 | | | $ | 11,408 | | | | | $ | 230 | | | | $ | 21,800 | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Contingent Consideration Liability Related to Acquisition of: | | |
| Arkis | | Location in Financial Statements | | Derma Sciences | | ACell | | Surgical Innovations Associates, Inc. (FN 4) | | Location in Financial Statements |
| Balance as of January 1, 2023 | | $ | 12,895 | | | | | $ | 230 | | | $ | 3,700 | | | | | 57,607 | | | |
| Change in fair value of contingent consideration liabilities | | 2,860 | | | Research and development | | 2,327 | | | (3,400) | | | | | 11,093 | | | Selling, general and administrative |
| Balance as of December 31, 2023 | | 15,755 | | | | | 2,557 | | | 300 | | | | | 68,700 | | | |
| | | | | | | | | | | | | | |
| Short-Term | | $ | 7,778 | | | | | $ | — | | | $ | — | | | | | $ | 13,400 | | | Accrued expenses and other current liabilities |
| Long-Term | | 7,977 | | | | | 2,557 | | | 300 | | | | | 55,300 | | | Other liabilities |
| Total | | $ | 15,755 | | | | | $ | 2,557 | | | $ | 300 | | | | | $ | 68,700 | | | |
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | | | | | | | | | | | | | | | | | | | | |
| | Contingent Consideration Liability Related to Acquisition of: | |
| Year Ended December 31, 2020 | | Arkis
(See Note 4) | | Derma Sciences | Location in Financial Statements |
| | Short-term | | Long-term | | Long-term | |
| Balance as of January 1, 2020 | | $ | — | | | $ | 14,210 | | | $ | 230 | | |
| Transfers from long-term to current portion | | 3,415 | | | (3,415) | | | — | | |
| Loss from change in fair value of contingent consideration liabilities | | — | | | 951 | | | — | | Research and development |
| Balance as of December 31, 2020 | | $ | 3,415 | | | $ | 11,746 | | | $ | 230 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Contingent Consideration Liability Related to Acquisition of: | | |
| Arkis | | Location in Financial Statements | | Derma Sciences | | | | ACell Inc. (FN 4) | | Surgical Innovations Associates, Inc. (FN 4) | | Location in Financial Statements |
| Balance as of January 1, 2022 | | $ | 15,099 | | | | | $ | 230 | | | | | $ | 21,800 | | | $ | — | | | |
| Additions | | — | | | | | — | | | | | — | | | 57,607 | | | |
| Change in fair value of contingent consideration liabilities | | (2,204) | | | Research and development | | — | | | | | (18,100) | | | | | Selling, general and administrative |
| Balance as of December 31, 2022 | | $ | 12,895 | | | | | $ | 230 | | | | | $ | 3,700 | | | $ | 57,607 | | | |
| | | | | | | | | | | | | | |
| Short-Term | | $ | 2,845 | | | | | $ | — | | | | | $ | — | | | $ | — | | | |
| Long-Term | | 10,050 | | | | | 230 | | | | | 3,700 | | | 57,607 | | | Accrued expenses and other current liabilities |
| Total | | $ | 12,895 | | | | | $ | 230 | | | | | $ | 3,700 | | | $ | 57,607 | | | Other liabilities |
Arkis BioSciences Inc.
As part of the acquisition of Arkis BioSciences Inc. ("Arkis"), the Company is required to pay the former shareholders of Arkis up to $25.5 million based on the timing of certain development milestones of $10.0 million and commercial sales milestones of $15.5 million, respectively. The Company used a probability weighted income approach to calculate the fair value of the contingent consideration that considered the possible outcomes of scenarios related to each specified milestone. The Company estimated the fair value of the contingent consideration to be $13.1 million at the acquisition date.
Derma Sciences
The Company assumed contingent consideration incurred by Derma Sciences, Inc. ("Derma Sciences") related to its acquisitions of BioD and the intellectual property related to Medihoney products. The Company accounted for the contingent liabilities by recording their fair value on the date of the acquisition based on a probability weighted income approach. The Company has already paid $33.3 million related to the aforementioned contingent liabilities. One contingent milestone remains which relates to net sales of Medihoney™ products exceeding certain amounts defined in the agreement between the Company and Derma Sciences. The potential maximum undiscounted payment amounts to $3.0 million. The estimated fair value as of December 31, 2021 and 2020 was $0.2 million.
16. SEGMENT AND GEOGRAPHIC INFORMATION
The Company internally manages 2two global reportable segments and reports the results of its businesses to its chief operating decision maker. The 2two reportable segments and their activities are described below.
•The Codman Specialty Surgical segment includes (i) the Neurosurgery business, which sells a full line of products for neurosurgery and neuro critical care such as tissue ablation equipment, dural repair products, cerebral spinal fluid management devices, intracranial monitoring equipment, and cranial stabilization equipment and (ii) the Instruments business, which sells more than 40,000 instrument patterns and surgical and lighting products to hospitals, surgery centers, dental, podiatry, and veterinary offices.
•The Tissue Technologies segment includes such offerings as skin and wound repair, plastics & surgical reconstruction products, bone grafts, and nerve and tendon repair products. In conjunction with the sale of the Extremity Orthopedics business, the Company rebranded the Orthopedics and Tissue Technologies segment as Tissue Technologies in the first quarter of 2021.
The Corporate and other category includes (i) various executive, finance, human resource, information systems and legal functions, (ii) brand management, and (iii) share-based compensation costs.
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
The operating results of the various reportable segments as presented are not comparable to one another because (i) certain operating segments are more dependent than others on corporate functions for unallocated general and administrative and/or operational manufacturing functions, and (ii) the Company does not allocate certain manufacturing costs and general and administrative costs to the operating segment results. Net sales and profit by reportable segment for the years ended December 31, 2021, 20202023, 2022 and 20192021 are as follows:
INTEGRA LIFESCIENCES HOLDINGS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
| | | | Years Ended December 31, | | Years Ended December 31, |
| Dollars in thousands | Dollars in thousands | 2021 | | 2020 | | 2019 | Dollars in thousands | 2023 | | 2022 | | 2021 |
| Segment Net Sales | Segment Net Sales | | | | | |
| Codman Specialty Surgical | Codman Specialty Surgical | $ | 1,025,232 | | | $ | 894,831 | | | $ | 996,206 | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | |
| Tissue Technologies | Tissue Technologies | 517,216 | | | 477,037 | | | 521,351 | |
| Total revenues | Total revenues | $ | 1,542,448 | | | $ | 1,371,868 | | | $ | 1,517,557 | |
| Segment Profit | Segment Profit | | | | | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | |
| Codman Specialty Surgical | Codman Specialty Surgical | $ | 439,471 | | | $ | 356,657 | | | $ | 395,019 | |
| Tissue Technologies | Tissue Technologies | 228,199 | | | 159,630 | | | 144,638 | |
| Segment profit | Segment profit | 667,670 | | | 516,287 | | | 539,657 | |
| Amortization | Amortization | (16,914) | | (27,757) | | (27,028) | Amortization | (12,376) | | (13,882) | | (16,914) |
| Corporate and other | Corporate and other | (453,526) | | | (337,160) | | | (418,869) | |
| Operating income | Operating income | $ | 197,230 | | | $ | 151,370 | | | $ | 93,760 | |
The Company does not allocate any assets to the reportable segments. No asset information is reported to the chief operating decision maker and disclosed in the financial information for each segment. The Company attributes revenue to geographic areas based on the location of the customer. Total revenue, net and long-lived assets (tangible) by major geographic area are summarized below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Dollars in thousands | United States(1) | | Europe | | Asia Pacific | | Rest of the World | | Consolidated |
| Total revenue, net: | | | | | | | | | |
| 2021 | $ | 1,089,526 | | | $ | 191,327 | | | $ | 182,034 | | | $ | 79,561 | | | $ | 1,542,448 | |
| 2020 | 971,975 | | | 172,689 | | | 157,174 | | | 70,030 | | | 1,371,868 | |
| 2019 | 1,077,379 | | | 197,468 | | | 157,391 | | | 85,319 | | | 1,517,557 | |
| Total long-lived assets: | | | | | | | | | |
| 2021 | $ | 339,535 | | | $ | 55,026 | | | $ | 11,289 | | | $ | 6,836 | | | $ | 412,686 | |
| 2020 | 324,893 | | | 38,812 | | | 13,121 | | | 5,577 | | | 382,403 | |
(1) Includes long-lived assets in Puerto Rico.
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance at Beginning of Period | | Charged to Costs and Expenses | | Other | | Deductions | | Balance at End of Period |
| Description | | | | |
| Dollars in thousands | | | |
| Year ended December 31, 2021 | | | | | | | | | |
| Allowance for doubtful accounts | $ | 6,439 | | | $ | (1,059) | | (4) | $ | 341 | | (3) | $ | (986) | | (1) | $ | 4,735 | |
| Deferred tax assets valuation allowance | 13,825 | | | 1,444 | | | 89 | | | (100) | | | 15,258 | |
| Year ended December 31, 2020 | | | | | | | | | |
| Allowance for doubtful accounts | $ | 4,303 | | | $ | 3,635 | | | $ | — | | | $ | (1,499) | | (1) | $ | 6,439 | |
| Deferred tax assets valuation allowance | 12,069 | | | 1,617 | | | $ | — | | | 139 | | 13,825 | |
| Year ended December 31, 2019 | | | | | | | | | |
| Allowance for doubtful accounts | $ | 3,719 | | | $ | 2,126 | | | $ | — | | | $ | (1,542) | | (1) | $ | 4,303 | |
| Deferred tax assets valuation allowance | 6,973 | | | 3,848 | | | 1,291 | | (2) | (43) | | | 12,069 | |
(1)Deductions primarily relates to allowance for doubtful accounts written off during the year, net of recoveries and other adjustments.
(2)The above amount primarily relates to amounts acquired through the acquisition of Arkis and a charge recorded in 2019 to valuation allowance related to the non-deductibility of executive compensation.
(3)The above amount primarily relates to amounts acquired through the acquisition of ACell.
(4)Deduction primarily relates to a decrease to the allowance for doubtful accounts as a result of collections in the period and accounts written off during the year, net of recoveries. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Dollars in thousands | United States(1) | | Europe | | Asia Pacific | | Rest of the World | | Consolidated |
| Total revenue, net: | | | | | | | | | |
| 2023 | $ | 1,100,730 | | | $ | 165,221 | | | $ | 193,096 | | | $ | 82,526 | | | $ | 1,541,573 | |
| 2022 | 1,126,810 | | | 170,903 | | | 176,477 | | | 83,476 | | | 1,557,666 | |
| 2021 | 1,089,526 | | | 191,327 | | | 182,034 | | | 79,561 | | | 1,542,448 | |
| Total long-lived assets: | | | | | | | | | |
| 2023 | $ | 481,508 | | | $ | 51,730 | | | $ | 19,842 | | | $ | 1,497 | | | $ | 554,577 | |
| 2022 | 440,223 | | | 60,857 | | | 12,975 | | | 2,721 | | | 516,776 | |



