QuickLinks-- Click here to rapidly navigate through this document
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark
(Mark One)
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE
ACT OF 1934
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2000
[_] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
2002
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _______________ to ____________
Commission file number:0-26642
-------
MYRIAD GENETICS, INC.
(Exact
(Exact name of registrant as specified in its charter)
Delaware 87-0494517
--------- ----------
(State or other jurisdiction (I.R.S. Employer Identification No.)
of incorporation or organization)
320 Wakara Way, Salt Lake City, UT 84108
- ---------------------------------- -----
(Address of principal executive offices) (Zip Code)
| Delaware (State or other jurisdiction of incorporation or organization) | 87-0494517 (I.R.S. Employer Identification No.) | |
320 Wakara Way, Salt Lake City, UT (Address of principal executive offices) | 84108 (Zip Code) |
Registrant's telephone number, including area code:(801) 584-3600
Securities registered pursuant to Section 12(b) of the Exchange Act: None
Securities registered pursuant to Section 12(g) of the Exchange Act:
Common Stock, $.01 Par Value Per Share
(Title
Preferred Share Purchase Rights
(Title of Class)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [ X ]ý No [_]o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [_]o
The aggregate market value of the registrant's voting stock held by non-
affiliatesnon-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) on September 1, 20002, 2002 was $1,621,749,204,$392,025,303, based on the last sale price as reported by The Nasdaq Stock Market.
As of September 1, 200018, 2002 the registrant had 22,269,64023,835,056 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated from the Registrant's Proxy Statement for the Annual Meeting of Stockholders to be held on November 17, 2000.
1
Overview
We are a leader inleading biopharmaceutical company focused on the usedevelopment and marketing of gene-based medicine to develop novel therapeutic and molecular diagnosticpredictive medicine products. We are focused on the emerging
field of proteomics, which involves establishing the relationship between
protein function and particular diseases by identifying disease-specific
proteins. We employhave developed a varietynumber of proprietary proteomic technologies which permit us to discover
important diseaseidentify genes, their related proteins and the biological pathways they form. We use this information to better understand the role these genes and their related proteins play in the onset and progression of human disease. We operate two wholly owned subsidiaries, Myriad Pharmaceuticals, Inc. and Myriad Genetic Laboratories, Inc., to commercialize our therapeutic and predictive medicine discoveries. Myriad Pharmaceuticals, Inc. develops and intends to market novel therapeutic products. Myriad Genetic Laboratories, Inc. focuses on the development and marketing of predictive medicine products that assess an individual's risk of developing a specific disease.
Myriad researchers have integrated these
technologies using powerful bioinformaticsmade important discoveries in the fields of cancer, viral diseases such as AIDS, and robotics systemsacute thrombosis. These discoveries point to conductnovel disease pathways and have paved the way for the development of new drugs. Additionally, our research efforts on a high-throughput basis. This integrated proteomics platform
has enabled us to identify numerous proteinspipeline of drug targets offers therapeutic opportunities for the treatment of diseases such as promising targets for new
proprietary drugsheart disease, rheumatoid arthritis, Alzheimer's disease and molecular diagnostic tests.
Using our proprietary technologies, weother central nervous system disorders. We have identified 22871 drug targets to date. We have delivered 13also established an extensive portfolio of drug candidates that are under development at Myriad. Fifteen of these drug targets tocandidates are in pre-clinical testing. Flurizan™, our strategic partners based
on our discoverylead therapeutic product for the treatment of genes involved in breast cancer, brain cancer, prostate cancer, heart disease, dementia and other disorders.is currently in a large, multi-center human clinical trial. We have received total
payments from our seven current strategic partners in excessalso recently submitted an Investigational New Drug (IND) application for the evaluation of $100 million.R-flurbiprofen (MPC-7869) for the treatment of Alzheimer's disease. We will receive additional milestone and royalty payments if our strategic partners
develop and commercialize drugs from the thirteen targets we have delivered to
them. Our current partners include Bayer Corporation, Eli Lilly and Company,
Hitachi Ltd., Hoffmann-LaRoche Inc., Pharmacia Corporation, Novartis
Corporation, Schering-Plough Corporation and Schering AG. We have also
established a portfolio of nine new drug targets that we have retained for our
own small molecule drug development program. We expectintend to independently develop test and, commercialize small molecule therapeutics from drug targets selected
fromsubject to regulatory approval, market our internal portfolio,therapeutic products, particularly in the area of cancer. Outside of the
oncology area, we will seek to enter into future strategic partnerships for the
clinical development of many of these targets.cancer and infectious diseases.
We also focus on developing, marketing and selling products used for
predictive medicine and personalized medicine. We have developed and commercialized twofive innovative molecular diagnostic tests, one ofpredictive medicine products: BRACAnalysis®, which is used for analyzingto assess a woman's risk of developing breast and ovarian cancer, susceptibilityCOLARIS™ and the otherCOLARIS AP™, which are used to determine a person's risk of developing colon cancer, MELARIS®, which is used to determine a person's risk of developing malignant melanoma, and CardiaRisk®, which is used for therapeutic management of hypertensive patients. In August 2000, we announced
the future launch of a predictive medicine test for hereditary colon cancer and
uterine cancer. We market these products using our own internal 106 person sales force in the United States and we have entered into marketing collaborations with other organizations in the United Kingdom, Ireland,Austria, Brazil, Canada, Germany, Japan, and Japan.Switzerland. Revenues from these proprietary tests, which we analyze in our CLIA approved laboratory,products grew approximately 70%57% from the prior year to $8.8$26.8 million in the fiscal year ended June 30, 2000.2002.
We believe that the future of medicine lies in the creation of new classes of drugs that prevent disease from occurring or progressing and that treat the cause, not just the symptoms, of the disease. In addition, we believe that advances in the emerging field of molecular diagnosticspredictive medicine will improve our ability to determine which patients are subject to a greater risk of developing these diseases and who therefore should receive these new preventativepreventive medicines.
Industry Background
We have devoted substantially all of our resources to maintaining our research and development programs, undertaking drug discovery and development, and operating our predictive medicine business. Our revenues have consisted primarily of sales of predictive medicine products and research payments received pursuant to collaborative agreements, upfront fees, and milestone payments. We have yet to attain profitability and, for the year ended June 30, 2002, we had a net loss of $14.0 million and as of June 30, 2002 had an accumulated deficit of $73.8 million.
We have formed strategic alliances with 12 major pharmaceutical or multinational companies including Abbott Laboratories, Bayer Corporation, E.I. du Pont de Nemours and Company (DuPont),
2
Eli Lilly and Company, Hitachi Ltd., Hoffmann-LaRoche Inc., Novartis Corporation, Oracle Corporation, Pharmacia Corporation, Schering AG, Schering-Plough Corporation, and Torrey Mesa Research Institute, a subsidiary of Syngenta. We intend to enter into additional collaborative relationships to discover genes, proteins, protein networks, and drug targets associated with common diseases as well as to continue to fund internal research projects. However, we may be unable to enter into additional collaborative relationships on terms acceptable to us.
In April 2001, we announced the formation of Myriad Proteomics, Inc., a new venture with Hitachi, Ltd. and Gene-Based Drug DevelopmentOracle Corporation to map the human proteome. Myriad Proteomics, which is 49 percent owned by the Company, intends to develop and market a proprietary map of the human proteome to pharmaceutical and biotechnology companies for therapeutic and diagnostic product development.
We expect to incur losses for at least the next several years, primarily due to expansion of our drug discovery and development efforts, expansion of our research and development programs, launch of new predictive medicine products, and expansion of our facilities. Additionally, we expect to incur substantial sales, marketing and other expenses in connection with building our pharmaceutical and predictive medicine businesses. We expect that losses will fluctuate from quarter to quarter and that such fluctuations may be substantial.
Business Strategy
Understanding the cause of a disease at the level of genes, proteins and biological pathways can be very helpful in determining how best to treat the disease. Historically, technologies used to discover treatments for the symptoms of diseases have been less effective against complex diseases that arise through a combination of genetic and environmental factors, such as cancer and heart disease. In order to treat complex diseases effectively, it is imperative to understand how the body uses its genetic information, how genetic mutationsthe disruption of important biological pathways can lead to disease, and how drugs can be developed to prevent, halt or reverse disease progression. As the scientific community learnswe learn more about the genetic basis of disease, we believe that the current methods of drug developmentwe will be revolutionized.
2
The majority of diseases are treated by modifying the activities of biological pathways through drugs that interact with the proteins produced by
the genes in affected cells and tissues. The quest for safer and more effective treatments for a wider range of diseases has led pharmaceutical companiesus to employ genomics and proteomics in theirour drug discovery and development programs.
Modern gene-based
Gene-based small molecule drug discovery and development programs at Myriad typically involve the following steps:
Target Discovery. Target discovery involves identifying genes and their proteins related to disease susceptibility, onset or progression. A better understanding of some diseases has resulted from the identification of disease-
relateddisease-related proteins and the subsequent understanding of their function.
Protein Function and Biological Pathway Determination. Proteins control virtually all cellular processes, including important disease processes. The determination of a protein's function clarifiesand clarifying the role of a protein in the biological pathway of a disease.disease, leads to the identification of key regulators in that pathway or drug target.
Target Validation. After identifying aan important disease-related protein, the decision must be made as to whether the protein can be a drug target. If a
protein is not qualified to serve as a target other proteins in the same
disease pathway can be examined as potential targets. A protein target that is
identified must be validated to confirm that the potential targetit is at a control point in a disease-related pathway and that a drug which interacts with the target is expected to have a beneficial effect. If through the validation process a protein is not qualified to serve as a drug target, other proteins in the same disease pathway can be examined as potential targets.
Assay Development and High-Throughput Screening. A specific assay must be developed for each validated drug target to identify compounds that inhibit or activate athe specific protein. To identify
3
potential drugs, a target is tested through high-throughput screening against a chemically diverse library, usually comprised of hundredsmillions of different small molecule compounds. The screening process frequently produces several compounds that interact with the identified drug target.
Drug Development. Compounds that may be suitable for development into potential drugs undergo selection and optimization. Once selected, the compound is optimized by synthesizing and testing a series of closely related compounds. Based on expected activity, safety and bioavailability, the most promising leads are selected. If the disease results from the loss of function of a specific protein, protein replacement therapy may represent an attractive alternative. Following optimization, lead compounds and protein therapeutics enter into preclinicalpre-clinical testing to establish their efficacy and safety in animals. If preclinicalpre-clinical tests are successful, candidate drugs enter clinical trials to determine their efficacy and safety in humans.
Predictive and Personalized Medicine
PredictiveMedicine. In predictive medicine identifies those individuals at risk for the
development of specific diseases, and guides the healthcare management of those
predisposed individuals to delay the onset or prevent the occurrence of specific
diseases. Once a predisposed individual is identified, that individual can make
more informed decisions in selecting the most appropriate surveillance measures
for prevention, and therapy. Personalized medicine establishes a genetic
response profile to drug therapy for specific individuals. Knowing how a patient
will likely respond to particular drugs may decrease the occurrence of adverse
side effects from medications while improving their effectiveness, possibly
leading to better outcomes and lower overall healthcare costs. Both predictive
and personalized medicine are of interest to healthcare payors who seek to lower
costs and improve the effectiveness of medical care.
Molecular Diagnostics. Molecular diagnostics is the analysis ofwe analyze genes and their proteins to predict individuals' risks for developing diseases and their responses to specific treatments. Armed with this risk assessment information, individuals can increase surveillance and take preventive action to prevent or delay the onset of disease. As drugs are developed and approved for use, knowledge about side effects and efficacy in specific individuals emerges. Using this pharmacogenomic knowledge, personal genetic profiles can be developed to predict responses of individuals to drugs.
Our Business Strategy
We believe that the future of medicine lies in the creation of new classes of drugs that are safer and more effective; drugs that not only treat disease but that also prevent disease from occurring. We also believe that the emerging field of predictive medicine will revolutionize the practice of medicine by identifying an individual's risk of developing diseases later in life.
Our business strategy is to understand the relationship between proteins and diseases in order to develop the next generation of therapeutic and molecular diagnosticpredictive medicine products. Through our proprietary technologies, we are 3
molecular diagnosticpredictive medicine products. Our business strategy includes the following key elements:
. Expand
- •
- Use our
proprietary proteomic databases.technologies to discover important disease genes and proteins, understand their functions and identify lead compounds. Wewill continueplan to expand ourexisting genetic and medical databases in Utah and Quebec. Theseproprietarydatabases not only enable us to accelerate our gene discovery efforts, they are also useful in target validation, pharmacogenomics and disease association studies. . Discover important disease genes, understand their function and identify lead compounds. We will expand ProNet(R), our proprietary proteomic technology,technologies to uncover additional disease pathways, discover functions for many of the proteins in these pathways and identify high quality drug targets. In addition, we will continue to employ ourProTrap(TM)high-throughput screening technologyfor high- throughput screeningin order to rapidlydevelop assays for our high throughput screening platform.identify novel small molecule drugs. We believe this will result in the identification of numerous lead compounds for potential drug development.. Selectively developBased on the specific characteristics of our drug targets, we will augment our small molecule drug development capability with protein replacement therapy and antibody therapy. - •
- Develop and commercialize therapeutic products. We intend to take selected compounds, particularly in the
areaareas of cancer and infectious diseases such as AIDS, through the clinical developmentprocess.process ourselves. We are focusing oncancerthese diseases due to the large unmet need for effective and less toxic drugs, and the oftentimes shorter and less expensive clinical trials resulting from the potential for fast track status that the U.S. Food and Drug Administration, or FDA, has typically afforded novelcancer drugs.drugs in these areas. Additionally, we will be able to leverage the expertise of our existing oncology sales force in the marketing ofthesenovel cancertherapies. .therapies and intend to expand our existing sales force to address the AIDS market as well. - •
- Grow and expand our predictive medicine business. We plan continue to increase the domestic and foreign market penetration of our existing predictive medicine products and create additional products to capitalize on the emerging areas of predictive medicine.
- •
- Capitalize on our strategic alliances with major pharmaceutical companies.We expect to maintain
and expandour strategic alliances focused on the discovery of novel drug targets.Moreover, as we identify and develop lead compounds, we plan to partner many of these compounds with major pharmaceutical companies prior to pursuing human clinical trials.This will shift much of the
4
financial risk associated with later
stage drug development to our partners, while permitting us to benefit from our partners' drug development expertise and marketing strength.
. Grow and expand our molecular diagnostic business. We will continue to
increase the domestic and foreign market penetration of our existing
molecular diagnostic tests and create additional tests to capitalize on
the emerging areas of predictive and personalized medicine.
Our Integrated Proteomic PlatformSet of Technologies
We have developed and integrated a powerful set of proteomic technologies
and databases that enable us to discover genes of commercial importance and understand their role in disease pathways. Our technology platform provides the basisknowledge to develop therapeutic and molecular diagnosticpredictive medicine products, based on a vastly improved understanding of the genetic basis of disease. Our proteomic
platform consists of the following key elements.
Genetic and Medical Databases
Our genetic databases, which are based on distinct populations, provide us
with a unique competitive advantage because they enable us to correlate the
inheritance of gene mutations through multiple generations with the occurrence
of disease. We have created an extensive computerized genealogical database
whose ancestries are centered on the pioneer families of Utah. This population
is valuable for genetic research because of its Northern European ancestry,
its large families, and its profound interest in recording its genealogy.
Information from this population, such as medical records, DNA samples,
genealogy and other health-related data, has been identified by our
researchers and collaborators and assembled into our computerized genealogy
database. This database has allowed us to discover genes involved in breast
cancer, ovarian cancer, melanoma, brain cancer, prostate cancer, heart
disease, and diabetes.
4
We have linked our database of Utah families to a disease registry from
Intermountain Health Care, which operates 40 hospitals and clinics in the
western United States. This information includes data such as laboratory tests,
prescription medications, drug allergies, surgical procedures and patient
criteria.
We have recently augmented this genetic medical information of the Utah
population by developing databases of individuals with specific diseases in
Quebec. This genetically isolated population complements the Utah population and
further strengthens our ability to more rapidly identify disease-causing genes.
A database of affected individuals from Quebec, a population that is twice as
old as Utah allows us to quickly identify important disease-causing genes. We
have worldwide exclusive rights and access to the Utah and Quebec databases.
Our high-throughput sequencing and mutation screening systems use a
robotics platform and bioinformatics software custom designed by our scientists
and software engineers. This integrated system has been expanded to incorporate
the introduction of a large number of genes and research populations, permitting
the rapid comparison of novel mutations in candidate genes between individuals
with diseases and healthy individuals drawn from the same population. This high-
throughput, automated system enables us to rapidly detect genes, which are
highly correlated with disease, and in many instances can be shown to be causal.
ProNet(R) Database
We believe that because virtually all cellular processes are controlled by proteins, including important disease processes, knowledge of protein interactions and functions can be extremely valuable in the identification of novel drug targets for therapeutic development. In order to determine the function of genes and their role in disease pathways, we use our proprietary ProNet(R) technologyProNet® and ProSpec™ technologies. These technologies enable us to develop our ProNet(R) database ofidentify human proteins, to discover the other proteins with which they interact and to improve our understanding of their involvement in important disease pathways. Each protein and its interacting partners form a network, which reads like a map, positioning the protein in the disease pathway and tracing the protein's role in that pathway.
Using our ProNet(R) technology,high-throughput proteomic technologies, we screen target proteins throughwith our proprietary libraries constructed from a variety of different tissues and organs, such as heart, brain, kidney, liver, breast and prostate. We have constructed over 1533 proprietary libraries each containing approximately 10 million protein fragments. We apply our proprietary automation and robotic capabilities to the protein search process to allow high-throughput processing of protein interactions. Our current capacity allows us to identify over 100hundreds of protein interactions each day. Every new interaction is entered into our
ProNet(R) database.
We believe that ProNet(R) provides aProNet® and ProSpec™ provide significant opportunityopportunities to identify and develop novel drug targets by:
.
- •
- discovering new proteins in the disease pathways;
. - •
- discovering functions for
manynovel proteins;. - •
- identifying new functions for known proteins;
. - •
- identifying proteins involved in critical interactions along the pathway; and
. - •
- selecting high quality drug
discoverytargets from disease pathways.Our clients access these data through secure Internet connections. We have created the following three types of ProNet(R) databases: . Proprietary ProNet(R) databases for specific pharmaceutical company clients. These databases address specific diseases and disease pathways of strategic importance to our pharmaceutical partners. Specific drug targets are selected by our partners for their proprietary drug discovery research. . Main ProNet(R) database of proteins and biological pathways, which contains proprietary interactions that we have discovered. These interactions are distinct from those identified for client companies. 5. ProNet(R) Online database of protein-protein interactions from the public domain. We use this database as a marketing tool and it is freely available to the public through the Internet at www.myriad- pronet.com. ProTrap(TM) Technology
We have developed a newproprietary drug screening technology platform called ProTrap(TM). The
ProTrap(TM) technologyProTrap™ which allows us quickly and cost effectively to build high-
throughputhigh-throughput drug screens using a yeast-based system. We believe that yeast-based screens offer a number of distinct cost and time advantages in comparison to the more commonly used mammalian or cell-free screens. Yeast are inexpensive and easy to grow and yeast screens can be run onthrough our liquid handling robots.robotics platform.
In the ProTrap(TM)ProTrap™ system, yeast are manipulated genetically so that they produce a human or viral protein. When the protein is produced in one of a variety of proprietary yeast strains, it causes the strain to change in a way that can be easily detected. Therefore, when a small molecular weight compound inhibits or activates the protein, a further change in the characteristics of the yeast strain is easily detectable.identified. The drug discovery screens are designed to be run in parallel, such that each screen controls for false positives in other screens. The result is greater efficiency and a higher screening throughput. Our ProTrap(TM)Additionally, our ProTrap™ technology has a wide variety of other potential
applications and can bebeen extended to complement our other target validation technologies by determining the functions of proteins. It complements ProNet(R)
by quickly finding new disease gene pathways. Finally, it can also determine the biological activity of a mutant proteinproteins that may have utility in pharmacogenomics.
Bioinformatics
Our high-throughput sequencing and Roboticsscreening systems use a robotics platform and bioinformatics software custom designed by our scientists and software engineers. This integrated system has been
5
expanded to incorporate the introduction of a large number of genes and research populations, permitting the rapid comparison of novel mutations in candidate genes between individuals with diseases and healthy individuals drawn from the same population. This high-throughput, automated system enables us to rapidly detect genes and proteins, which are highly correlated with disease, and in many instances can be shown to be causal.
The gene and drug discovery process generates vast amounts of information. Accordingly, we have designed proprietary bioinformatics systems, which provide significant analytical and data management capabilities. Our systems are based on integrated, protocol-driven database management software, which is used to track experiments and collect relevant data. In addition, we have developed a proprietary laboratory information management system. This system has the advantages of simplicity of design, ease of maintenance, and speed of development. To date, we have used our information management systemsoftware for our high-throughput systems for protein analysis, genotyping, genomic sequencing, mutation screening and compound screening. This has been of fundamental importance in sample tracking and quality assessment and quality control. We believe our strength in bioinformatics provides us with a substantial competitive advantage.
We employ state-of-the-art robotics platforms in all of our high-throughput systems. We use the same robotics software and hardware development and
maintenance teams to ensure efficiency throughout our operations. We operate
flexible robotics systems in our research and molecular diagnostics laboratories
and high-throughput robotics systems in our sequencing and drug screening
laboratories. Each of our robotics systems is connected continually in a real time interface with our proprietary laboratory information management system to maintain a high degree of precision in sample tracking. Our robotics systems have been designed to ensure that the sample volumes used for each of the applications are kept at minimum levels to maintain reagent cost savings in each of our operations. The high level of automation as well as the concerted effort in optimizing biochemistry and reducing reagent volumes allows us to produce data at a very competitive cost in the industry.
Therapeutic Product Development
The pharmaceutical industry has been successful in developing medicines to treat the symptoms of disease. However, as the current generation of compounds nears the end of its patent protection, the industry has begun to
6
some of those compounds in the oncology
areathese drug candidates through human clinical trials. If we developFor those therapeutic products in the area of cancer, then given the concentrated nature of the oncology market, we would be able to leverage the marketing efforts of our existing oncology sales force. OutsideGiven the concentrated nature of the oncology area,AIDS market, we intend to partner these lead compounds with
major pharmaceutical companies.expand our sales force to address this market ourselves.
We formed Myriad Pharmaceuticals, our wholly owned subsidiary, to use our proprietary proteomics technologies to discover and develop novel therapeutic products. We believe that our ProNet(R) database of important disease pathwaystechnology provides us with a significant advantage in drug discovery because it enables us to generate a large number of potential drug targets. Once these targets have been identified, our ProTrap(TM) technology enables us towe can rapidly screen a large number of these drug targets against our library of small molecule compounds. This integrated platform enables us to pursue a rapid and cost effective approach to identifying potentially valuable drug candidates
In contrast to the drug discovery model employed by much of the
biotechnology industry, which screens relatively few drug targets against large
libraries of compounds, we are able to screen large numbers of protein targets
against our diverse library of compounds and rigorously select those candidates
we believe to be the most promising. To date, we have 22 drug targets in
development. Of these 22, we have licensed 13 to our strategic partners for
further development and we have retained nine for internal development. Our
current in house drug discovery efforts target cancer, AIDS and rheumatoid
arthritis. In addition, we are exploring the biology around genes that we
believe are involved in a variety of disease areas, including arteriosclerosis,
chronic pain, chronic obstructive pulmonary disease and sleep disorders, and
have selected 110 proteins for further evaluation using our ProNet(R)
technology.
High-Throughput Screeningcandidates.
Our high-throughput screening is highly automated, using robot workstations and a proprietary computerized management system that monitors each step of the process, confirms that each step has been performed to eliminate operator errors and automatically correlates results with compound identity and drug target. Current capacity is approximately 3650 million screening data points per year.
We also build mammalian cell secondary assays to evaluate the initial compounds arising from the primary drug discovery screens. To date, we have completed the evaluation of these assays for colon
6
cancer, other solid tumors, AIDS and inflammatory diseases and have developed protocols to evaluate the mammalian toxicity of all compounds found in our drug discovery screens. We are exploring the biology around genes that we believe are involved in a variety of disease areas, including arteriosclerosis, chronic pain, chronic obstructive pulmonary disease and sleep disorders, and have selected 1,356 proteins for further evaluation using our ProNet® technology.
To date, we have discovered 871 drug targets. We have built drug discovery screens for eachmany of our nine proprietary drug targets and all nine have been runare screening them against our compound library.own library of 200,000 small molecule weight compounds. We have identified a number of proprietarynumerous candidate drug compounds from our drug discovery screens, including drug candidates for colon cancer, heart disease, rheumatoid arthritis, Alzheimer's disease, HCV, HBV, acute thrombosis, and HIV targets, which satisfy the initial criteria of showing selectivity for one molecular target without obvious toxicity. Furthermore, the compounds have been shown to display a good dose response curve, showing increased activity at higher concentrations and decreased activity at lower concentrations.
We have built mammalian cell15 drug candidates currently under development in pre-clinical studies. Flurizan™, our lead therapeutic product for the treatment of prostate cancer, is currently in a large, multi-center human clinical trial. We also recently submitted an Investigational New Drug (IND) application for the evaluation of R-flurbiprofen for the treatment of Alzheimer's disease. The following table outlines the status of our major drug development programs:
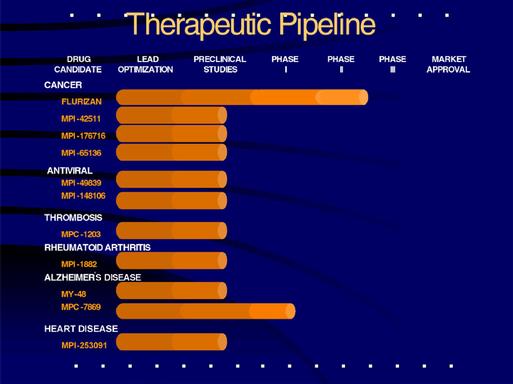
Flurizan: Candidate Drug for Prostate Cancer. Flurizan is a novel drug for the treatment of prostate cancer and is our most advanced therapeutic program. It has completed a phase II human clinical trial. In animal models of cancer, Flurizan demonstrated marked anti-tumor and anti-metastatic activity, significantly reducing the incidence of primary and secondary assaysprostate tumors. In humans, the drug was well tolerated in normal healthy subjects and in advanced prostate cancer patients who have relapsed. The drug has good bioavailability and would be given in pill form, once a day. Among relapsing prostate cancer patients, the level of prostate specific antigen (PSA) increases dramatically. After Flurizan was given to evaluate the initial
compounds arising from the primary drug discovery screens. To date, we have
completed the construction of severala group of these assayspatients, 52% experienced a reduction in the growth rate
7
of their PSA levels. Flurizan holds promise as an effective, safe drug for colon cancer, other
solid tumors, HIV and inflammatory diseases and have developed protocols to
evaluate the mammalian toxicity of all compounds found in our drug discovery
screens. We are currently working to build secondary screens for the remainder
of our drug targets.
MPI-42511 Candidate Therapeutic Compound for Colon Cancer
Our lead therapeutic development program addresses the treatment and prevention of colorectalprostate cancer. Based uponFour patents have issued on the drug.
MPI-176716: Candidate drug for Solid Tumors, Leukemia and Lymphoma. MPI-176716 is a novel small- molecule drug that inhibits an important step in the pathway controlling programmed cell death. As a result, most dividing cancer cell types tested to date are sensitive to this drug. We expect this drug candidate to address solid tumors as well as leukemia and lymphomas. These cancers account for an expected 1.3 million cases in 2001, according to the American Cancer Society. Drugs that have the potential to treat a common underlying mechanism of cancer have broad application to the treatment of disease and therefore, a very large market potential worldwide. Our cancer drug is in preclinical testing and if successful we plan to enter human clinical trials in cancer patients.
MPI-49839: Candidate Drug for AIDS. Our novel drug, MPI-49839, represents a new approach to the treatment of AIDS. The concept behind the drug may enable the creation of an entirely new class of therapeutics. The drug is distinct from the protease inhibitors and reverse-transcriptase inhibitors, which are the current generation of AIDS drugs, or fusion and integrase inhibitors, which are other classes of anti-HIV drugs being studied. Our anti-HIV drug is especially exciting in that it has the potential to improve on these current treatments for AIDS. With the evolution of multi-drug resistant strains of the virus comes an increased need for therapies that act through different mechanisms. Although current drugs have been quite successful in improving survival for AIDS patients, the drugs do not eliminate the virus, thus drug therapy becomes a life-long commitment. Researchers at the University of California recently estimated that an alarming 42% of HIV-infected individuals will be resistant to the current generation of drugs by 2005. The ability to establish long-term suppression of viral activity requires new drugs that are more impervious to viral resistance. Novel approaches such as Myriad's may well provide that extended therapeutic benefit to patients. MPI-49839 is in pre-clinical studies, and if successful, we plan to enter human clinical trials in AIDS patients.
MPI-42511: Candidate Drug for Colon Cancer. MPI-42511 is a novel small-molecule drug that inhibits a key regulator of a cancer pathway that is involved in 95% of all cases of colon cancer. Our scientists employed a rapid, high-throughput two-tier screening procedure to discover this potential colon cancer pathway
developed withdrug. Initially, we screened our ProNet technology, we identified a novel drug target, built
and implemented a high-throughput drug discovery screen that resulted inlibrary of small molecules for their ability to inhibit the
discovery of a small molecule compound. The compound, MPI-42511, showed
selectivity for the target both in the initial screen and in a human cell line
assay. Subsequently, we have demonstrated the anti-colon cancer activity of the compound against a
7
variety ofdrug target. We isolated several candidates, which were subsequently screened for the ability to specifically kill human colon cancer cells without harming normal cells. These compounds provide the potential to prevent unchecked cell lines. A rangegrowth during the progression of chemical analogues of MPI-
42511 have been generatedcolon cancer. The lead drug is now in pre-clinical evaluation, and evaluated for optimal drug characteristicsif successful, we plan to enter human clinical trials in colon cancer models. Pre-clinicalpatients.
MPC-1203: Candidate Drug for Acute Thrombosis. MPC-1203 is a proprietary recombinant form of the human protein, anti-thrombin III. Anti-thrombin III plays a critical role in helping to maintain the flow of blood by inhibiting clot formation. It is a circulating plasma protein that is produced in the liver. Following severe trauma or major surgery, this essential protein is degraded by enzymes, and can no longer prevent the blood from clotting. Our proprietary form effectively resists degradation by these enzymes, which are released during inflammatory events. By resisting inactivation, MPC-1203 remains in circulation, available to carry out its function in the body. Blood clotting is a major concern following orthopedic surgery such as hip replacement surgery, open heart surgery and other critical trauma to the body. Clotting of the blood is also a cause of organ failure and death following sepsis and cancer. MPC-1203 is the subject of two United States patent applications and eight foreign patent applications. Our protein drug is in pre-clinical testing, and if successful, we plan to enter human clinical trials.
MPC-7869: Candidate Drug for Alzheimer's Disease. We recently submitted an Investigational New Drug (IND) application to the FDA for the evaluation of MPC-7869 (R-flurbiprofen) in the treatment and prevention of Alzheimer's disease. In our Phase I human clinical trial, which has now been cleared
8
by the FDA for initiation, we intend to establish the safety profile and dosing regimens of MPC-7869 in healthy elderly volunteers. Alzheimer's disease is a degenerative neurological condition affecting up to 20% of all people aged 80 or older, with an estimated 4 million cases in the United States alone. Current approved treatments, such as acetylcholinesterase inhibitors, temporarily mitigate symptoms without meaningfully impacting progression of the underlying disease. Alzheimer's disease is marked by progressive cognitive decline and by the accumulation of amyloid plaques and neurofibrillary tangles in the brain. The major structural component of these plaques is amyloid beta protein, specifically Amyloid beta-42 (Ab42). Many researchers now believe that Ab42 plays an important role in the onset of Alzheimer's disease. Preclinical studies performed with MPI-42511NIH funding, at Mayo Clinic Jacksonville and UCSD have demonstrated that R-flurbiprofen substantially lowers the levels of Ab42 in colon cancer model
organisms have been initiated.
Candidate Therapeutic Compound for AIDSboth human cell lines and in animal models of Alzheimer's disease. We have established a substantial development programbelieve MPC-7869 holds promise as an effective, safe drug for the treatment and prevention of the Human Immunodeficiency Virus (HIV). The program was initiated from the
discovery, using ProNet(R), of an intriguing protein interaction between the HIV
virus and the human host cell. A high-throughput screen was constructed and has
identified compounds that showed selectivity against the target. The target is
neither of the protease inhibitor nor reverse-transcriptase inhibitor class and
thus represents the potential for novel drug therapy as the two common currently
prescribed drugs become less effective through increased viral resistance. These
compounds are now being further evaluated for their activity against the virus.
Molecular Diagnostics
We are committed to the development and marketing of novel molecular
diagnostic products for the emerging market opportunities of predictive medicine
and personalized medicine.Alzheimer's disease.
Predictive Medicine Products
Predictive medicine determines whichidentifies those individuals are at risk for the development of specific diseases, and guides the healthcare management of those predisposed individuals to delay the onset or prevent the occurrence of specific diseases. ThisOnce a predisposed individual is identified, that individual can make more informed decisions in selecting the most appropriate surveillance measures and therapeutic course of action. Because predictive medicine guides the healthcare management of those predisposed individuals, this allows healthcare resources to be focused on individuals who have the greatest need and may reduce waste in the healthcare system. Personalized medicine establishes an individual's genetic response
profile to a specific drug. Knowing how individual patients are likely to
respond to a particular drug may lead to more effective choice of medication,
reduced adverse side effects and lower overall healthcare costs.
Through our wholly owned subsidiary, Myriad Genetic Laboratories, we have
established a central molecular diagnostics facilityare committed to provide genetic
information services worldwide to healthcare providers.the development and marketing of novel products for the emerging market opportunities of predictive medicine. We have also developed a
clinical database of information on genetic mutations of each gene discovered,
including the frequency of occurrence in different ethnic population groups and
the clinical effect of these mutations. From these mutations we can identify an
individual's genetic predisposition to disease. Through our database of
mutations we can provide healthcare professionals with detailed genetic
information regarding the risk profile of these different ethnic groups. We also provide educational and support services to physicians and healthcare professionals as part of our genetic informationpredictive medicine business. The molecular
diagnostic testspredictive medicine products we have developed and currently market are not subject to FDA approval, but are subject to oversight and approval byunder the Clinical Laboratory Improvement Amendments, or CLIA. We have obtained all approvals required by CLIA.
Our strategy is to first introduce molecular diagnosticpredictive medicine products in the United States, and then to make them available worldwide through strategic marketing partnerships abroad. We have developed three molecular diagnosticfive predictive medicine products, BRACAnalysis(R)BRACAnalysis®, COLARIS™ and CardiaRisk(R)COLARIS AP®, that weMELARIS®, and CardiaRisk®. We are currently marketing these products in the United States directly through our own 106 person oncology sales force, as well as through a partnership with Laboratory Corporation of America Holdings (LabCorp). Through our partnership with LabCorp we intend to make our predictive medicine products broadly available to primary care physicians throughout the United States. LabCorp is our exclusive sales and COLARIS(TM), which
willdistribution partner, marketing the products through its 600-person U.S. sales force to more than 200,000 of LabCorp's physician customers. All of Myriad's predictive medicine products are included in this agreement.
9
The potential international market for our predictive medicine products is estimated to be launchedat least twice the size of the United States market. After introducing predictive medicine products in the fall of 2000.United States, we plan to introduce our products in foreign markets primarily through strategic marketing partners. We arehave completed marketing agreements with MDS Laboratory Services in the process of developing
additional molecular diagnostic tests for genetic susceptibility to prostate
cancerCanada, Falco Biosystems, Ltd. in Japan, Bioscientia, Ltd. in Germany, Austria and melanoma.
BRACAnalysis(R)Switzerland, and Laboratorio Fleury in Brazil.
BRACAnalysis®: Predictive Medicine Product for Breast and Ovarian Cancer
SusceptibilityCancer. It is estimated that each year, approximately 180,000203,500 women in the United States are diagnosed with breast cancer each year and approximately 25,00023,300 women are diagnosed with ovarian cancer annually.cancer. Each year in the United States, an estimated 43,00039,600 women will die from breast cancer, which has the second highest cancer mortality rate among women, and an estimated 14,50013,900 women will die of ovarian cancer. We reported the discovery of the BRCA1 breast and ovarian cancer
predisposing gene in the October 7, 1994, issue of the journal Science, and in
December 1995, announced the discovery of the complete sequence of BRCA2 breast
cancer gene, as reported in the journal Nature Genetics. BRCA1 and BRCA2 appear to be responsible for approximately 84% of the early onset hereditary breast cancer and approximately 90% of hereditary ovarian cancer. Hereditary breast
cancer is believed to account for approximately 10% of all cases of breast cancer. A study of women in the United States published in the American Journal of Human Genetics indicates that a woman with a BRCA1 mutation has an 86% risk of
8
BRACAnalysis(R),
BRACAnalysis® is a comprehensive analysis of the BRCA1 and BRCA2 genes for determining a woman's susceptibility to breast and ovarian cancer. BRACAnalysis(R)BRACAnalysis® provides important information that we believe will help the patient and her physician make better informed lifestyle, surveillance, chemoprevention and treatment decisions. The price perfor the test is currently $2,580$2,760 and is covered by most health maintenance organizations and health insurance providers in the United States. CardiaRisk(R)We have nine issued United States patents covering BRACAnalysis®.
COLARIS™: Predictive Medicine Product for Colon Cancer and Uterine Cancer. Colorectal cancer is the second leading cause of cancer deaths in the United States, with approximately 148,300 new cases expected to be diagnosed in the year 2002. Familial forms of colorectal cancer were estimated in 1997 to account for 10% to 30% of all cases according to the American Society of Clinical Oncologists. The health care management considerations in these hereditary syndromes are similar to those for breast and ovarian cancer at-risk individuals. Individuals who carry a mutation in one of the two colon cancer genes have a greater than 80% lifetime risk of developing colon cancer and women have a 60% life time chance of developing uterine cancer. Highly effective preventive measures include colonoscopy and the removal of precancerous polyps. To illustrate the predictive medicine value of molecular testing in colorectal cancer syndromes, it has been shown that individuals who carry gene mutations can lower their risk of developing cancer by more than 50% with appropriate preventive and surveillance measures.
COLARIS™ is a comprehensive analysis of the MLH1 and MSH2 genes for determining a person's risk of developing colon cancer or uterine cancer. COLARIS™ provides important information that we believe will help in the surveillance and possible prevention of colon cancer. The price for the test is $1,950 and is covered by most health maintenance organizations and health insurance providers in the United States.
COLARIS AP™: Predictive Medicine Product for Colon Cancer. In May 2002 we introduced our fifth predictive medicine product for genetic susceptibility to colon cancer. COLARIS AP™ detects mutations in the APC gene, which cause a colon polyp-forming syndrome known as familial adenomatous polyposis (FAP), and a more common variation of the syndrome known as attenuated FAP (aFAP). FAP may be responsible for as much as 20% of hereditary colorectal cancer, and aFAP
10
may underlie as much as 20% of all colon cancer cases. There are an estimated 32,000 possible cases of FAP and aFAP each year in the United States. Our other colon cancer product is COLARIS™, a predictive medicine test for hereditary colon cancer that is not associated with significant polyp formation. Together, COLARIS™ and COLARIS AP™ may account for approximately 90% of all hereditary colon cancer syndromes. The price for the test is $1,685 and is covered by most health maintenance organizations and health insurance providers in the United States.
MELARIS™: Predictive Medicine Product for Melanoma. In September 2001 we introduced our fourth predictive medicine product for genetic susceptibility to malignant melanoma, a deadly form of skin cancer. The incidence of melanoma, a malignant form of skin cancer, has increased approximately 4% per year since the early 1970's and is the second fastest growing cancer in the United States. This year 53,600 Americans are expected to be diagnosed with melanoma, according to the American Cancer Society. We discovered that mutations in the p16 gene are involved in cancer and can be inherited and predispose individuals to melanoma, as reported in the September 1994 issue of the journalNature Genetics. Melanoma is lethal within five years in 86% of cases where it has spread to another site in the body. However, when melanoma is diagnosed at an early stage, fewer than 10% of patients die within five years. We believe that approximately 10% of melanoma cases are hereditary.
MELARIS™, which assesses a person's risk of developing melanoma, provides important information that we believe will be useful in the surveillance and prevention of melanoma. Melanoma can be prevented through appropriate screening and a specific threshold of action for mutation carriers, in which precancerous lesions are removed before cancer can develop. We have six issued United States patents covering MELARIS™. The price for the test is $745 and is covered by most health maintenance organizations and health insurance providers in the United States.
CardiaRisk®: Personalized Medicine Product for Hypertension ManagementHypertension. Approximately 50 million people in the United States are hypertensive. Hypertension has a significant genetic component and is a major risk factor for cardiovascular disease, kidney failure and stroke. The angiotensinogen gene, or AGT gene, is believed to be involved in the salt-dependent form of hypertension, which accounts for approximately 35% of all hypertension. Therapy for these patients includes the use of a low-salt diet, other dietary regimens, and numerous drug therapies to control blood pressure. Results of a recent study of 1,509 patients by the National Institutes of Health showed that of all patients placed on a low-salt diet, only patients with the AGT mutation achieved a significant reduction in blood pressure over the three-year course of the study. Patients in this study with the variant form of the AGT gene were also found to be 42% more likely to experience hypertension earlier in life and more severely.
CardiaRisk(R)
CardiaRisk® identifies individuals likely to respond to specific high blood pressure therapies by screening for mutations of the AGT gene. Mutations
of this gene determine a patient's potential reaction to different courses of
therapy for hypertension. Using CardiaRisk(R)CardiaRisk® to help predict the specific therapies and drugs to which a patient will respond may improve patient compliance, reduce adverse side effects and decrease overall healthcare costs. CardiaRisk(R)CardiaRisk® is a fully automated test that we perform using DNA extracted from a patient's blood sample. The cost for the test is $295$315 and it is not currently reimbursed by health insurance. We believe CardiaRisk(R)CardiaRisk® is one of the first commercially available personalized medicine products. COLARIS(TM): Predicitve Medicine for Hereditary Colon Cancer and Uterine Cancer
We announced in August 2000 the launch of COLARIS(TM), a molecular
diagnostic test for genetic susceptibility to colorectal and uterine cancer.
Colorectal cancer is the second leading cause of cancer deaths in thehave six issued United States with 130,200 new cases expected to be diagnosed in the year 2000.
Familial forms of colorectal cancer were estimated in 1997 to account for 10% to
30% of all cases according to the American Society of Clinical Oncologists. The
molecular diagnostic considerations in these hereditary syndromes are similar to
those necessary for breast and ovarian cancer at-risk individuals, which we have
already commercialized. To illustrate the predictive medicine value of molecular
testing in colorectal cancer syndromes, it has been shown that individuals who
carry gene mutations can lower their risk of developing cancer by more than 50%
with appropriate surveillance measures.
Predictive Medicine Tests under Development
Prostate Cancer. We are preparing to introduce a molecular diagnostic test
for prostate cancer in calendar year 2001, based upon our discovery of the HPC2
prostate cancer gene. Prostate cancer is diagnosed in approximately 180,000 men
each year in the United States. According to the American Cancer Society, over
31,000 men will die of the disease in 2000, making it the second most common
cause of cancer death in men. Early diagnosis is effective in increasing the
survival for patients with prostate cancer. Diagnosis at the local and regional
stages is associated with a five-year survival rate approaching 100%, although
survival rates for more advanced tumors decline rapidly. Tests such as PSA have
had a positive effect on survival with prostate cancer, and serial PSA
determinations among patients identified as high risk from a molecular
diagnostic test offer potential for early diagnosis with longer
9
survival. Recent genetic studies suggest that approximately 10% of prostate
cancer is due to hereditary predisposition.
Melanoma. We are planning to introduce a molecular diagnostic test for
genetic susceptibility to melanoma in calendar year 2001. The incidence of
melanoma, a malignant form of skin cancer, has increased approximately 4% per
year since the early 1970's. In the year 2000, approximately 44,000 Americans
will be diagnosed with melanoma, according to the journal Science. We discovered
that mutations in the p16 gene are involved in cancer and can be inherited and
predispose individuals to melanoma, as reported in the September 1994 issue of
the journal Nature Genetics. Melanoma is lethal within five years in 86% of
cases where it has spread to another site in the body. However, when melanoma is
diagnosed at an early stage, fewer than 10% of patients die within five years.
We believe that approximately 10% of melanoma cases are hereditary. We have
substantial expertise in the genetic analysis of melanoma and have begun to
identify important disease-predisposing p16 mutations.
Sales and Marketing
We are currently marketing BRACAnalysis(R) and CardiaRisk(R), and we expect
to market other diagnostic products, including COLARIS(TM), in the United States
directly through our own direct sales force. The potential international market
for our molecular diagnostic products is estimated to be at least twice the size
of the United States market. After introducing molecular diagnostic products in
the United States, we plan to introduce our products in foreign markets
primarily through strategic marketing partners. We have recently completed
marketing agreements with the following foreign marketing partners:
Partner Territory
------- ---------
Falco Biosystems, Ltd. Japan
MDS Laboratory Services Canada
Rosgen Ltd. United Kingdom and Ireland
patents covering CardiaRisk®.
Strategic Alliances
In order to limit the financial risks associated with the development of therapeutic products, including costs associated with related clinical trials of such drugs, our strategy is to enter into alliances with corporate partners who assume such risks and other assorted financial costs. In addition to our current strategic alliances, we are actively pursuing other partners in areas that we believe may enhance our ability to develop and exploit our technology. The
financial structure of ourIn fiscal year 2002 we entered into three new strategic alliances varies with each agreementAbbott Laboratories, Pharmacia Corporation, and may include research payments, equity investments, milestone payments, upfront
payments, license fees, subscription fees, option payments,E.I. du Pont de Nemours and royalty payments
or profit sharing.
Events that trigger milestone payments to us may include:
. the discovery of a gene or protein;
. the determination of the function of a gene or protein;
. the identification of a lead compound;
. the filing of an investigational new drug application with the FDA;
. the commencement of Phase III clinical trials; and
. the submission of a new drug application with the FDA.Company.
11
We are dependent on each strategic partner to commercialize the therapeutic products identified during our collaboration. If our partner commercializes the product, we will receive a royalty on sales of the product or share in the profits derived from sales of the drug. If any of our strategic partners cease efforts to commercialize any 10
We have formed strategic alliances with 12 major pharmaceutical or multinational companies including Abbott Laboratories, Bayer Corporation, E.I. du Pont de Nemours and Company (DuPont), Eli Lilly and Company. In August 1992, we entered into a Research
Collaboration and License Agreement with Eli Lilly and its former subsidiary,
Hybritech Incorporated, under which Eli Lilly and Hybritech made an equity
investment in us, and provided funding over a three-year period to support our
research and development program to discover the BRCA1 gene. The total equity
investment, research funding and potential milestone payments under this
collaboration may provide us with up to $4,000,000. In addition, we may also
receive future milestone payments and future royalty payments on therapeutic and
diagnostic product sales. The research portion of this collaboration was
concluded successfully on schedule in August 1995.Company, Hitachi Ltd., Hoffmann-LaRoche Inc., Novartis Corporation. In April 1995, we commenced a five-year
collaborative research and development arrangement with Novartis Corporation.
The total equity investment, research funding and potential milestone payments
under this collaboration may provide us with up to $60,000,000. The research
effort focused on the discovery of genes and drug targets involved in the field
of cardiovascular disease. The research phase of the Novartis collaboration
concluded successfully on schedule in April 2000. In March 1998, we announced
that Novartis had licensed the therapeutic rights to the CHD1 heart disease
gene, triggering a milestone payment to us. In addition, we may receive future
royalty payments on therapeutic products sold by Novartis.
Bayer Corporation. In September 1995, we commenced a five-year
collaborative research and development arrangement with Bayer Corporation. The
total equity investment, research funding and potential milestone payments under
this collaboration may provide us with up to $71,000,000. In November 1997 and
again in December 1998, we announced expansions of our collaborative research
and development arrangement with Bayer. The expanded collaboration may provide
us with additional research funding and potential milestone payments of up to
$137,000,000. We are entitled to receive royalties from sales of therapeutic
products commercialized by Bayer.
Schering-Plough Corporation. In April 1997, we commenced a three-year
collaborative research and development arrangement with Schering-Plough
Corporation. The total equity investment, research funding, license fees and
potential milestone payments under this collaboration may provide us with up to
$60,000,000. The research phase of the Schering-Plough collaboration concluded
successfully on schedule in April 2000. In October 1997, we announced that
Schering had licensed the therapeutic rights to the MMAC1 cancer gene. In March
1998, we demonstrated the tumor-suppressor activity of the MMAC1 gene. Each
event triggered milestone payments from Schering to us. In May 2000, we
announced that Schering had licensed the therapeutic rights to the HPC2 prostate
cancer gene, triggering a milestone payment to us. In addition, we may receive
future royalty payments on therapeutic products sold by Schering-Plough.
Schering AG. In October 1998, we entered into a five-year collaboration
withCorporation, Oracle Corporation, Pharmacia Corporation, Schering AG, to utilize ProNet(R) for drug discoverySchering-Plough Corporation, and development. Under
the agreement, we will have an option to co-promote all new therapeutic products
in North America and receive 50 percentTorrey Mesa Research Institute, a subsidiary of the profits from North American sales
of all new drugs discovered with ProNet(R). The total research funding, license
fees, subscription fees, option payments and potential milestone payments under
this collaboration may provide us with up to $51,000,000. If we choose to co-
promote a drug developed by Schering AG as a 50 percent partner, we are required
to pay funds to Schering AG to establish equal ownership. In October 1999, we
announced the expansion of our collaboration with Schering AG to include
research in the field of cardiovascular disease.
Pharmacia Corporation. In November 1998, we entered into a 15 month
collaboration with Pharmacia Corporation (formerly Monsanto Company) to utilize
ProNet(R) for drug discovery and development. In December 1999, Pharmacia
exercised its option to extend the research term for an additional 12 months and
exercised its option to expand the research funding. The total research funding,
option payments, license fees and potential milestone payments under this
collaboration may provide us with up to $28,000,000. In addition, we are
entitled to receive royalties from sales of therapeutic products commercialized
by Pharmacia.
Novartis Agricultural Discovery Institute, Inc. In July 1999, we entered
into a two-year collaboration and license agreement with the Novartis
Agricultural Discovery Institute, Inc. ("NADII"). The genomic collaboration
will focus on the discovery of the genetic structure of cereal crops. The total
funding under this collaboration is
11
expected to provide us with up to $33,500,000. Upon completion, we and NADII
intend to jointly offer commercial access to the genomic databases and share
equally in any resulting proceeds.
Hoffmann-LaRoche Inc. In December 1999, we entered into a 12 month
collaboration with Hoffmann-LaRoche Inc. to utilize ProNet(R) for drug discovery
and development in the area of cardiovascular disease. The total research
funding, license fees and potential milestone payments under this collaboration
may provide us with up to $13,000,000. In addition, we are entitled to receive
royalties from sales of therapeutic products commercialized by Roche.
Hitachi Ltd. In May 2000, we entered into a three year strategic alliance
with Hitachi Ltd. Under the terms of the agreement, we will work with Hitachi
to exploit the ProNet(R) technology together in Japan and Hitachi will establish
a designated ProNet(R) facility to expedite the discovery of novel protein-
protein interactions for Japanese customers. Total payments under this
collaboration are expected to provide us with $26,000,000. In addition, we are
entitled to receive royalties from sales of therapeutic products commercialized
by Hitachi.Syngenta.
We intend to enter into additional collaborative relationships with other corporate partners to locate and sequence genes and proteins, to discover protein networks associated with other common diseases, and to identify lead compounds which may be developed into commercial therapeutic products by those partners.
Patents and Proprietary Rights
We intend to seek patent protection in the United States and major foreign jurisdictions for the genes, we discover, mutations and products of the genes and
related processes,proteins, protein interactions, antibodies, drug targets, drug compounds, transgenic animals, technology related methods and processes and other inventions which we believe are patentable and where we believe our interests would be best served by seeking patent protection. We also intend to seek patent protection or rely upon trade secret rights to protect certain other technologies which may be used in discovering and characterizing new genes and proteins and which may be used in the development of novel diagnosticpredictive medicine and therapeutic products. To protect our trade secrets and other proprietary information, we require that our employees and consultants enter into confidentiality and invention assignment agreements. TheseHowever, those confidentiality and invention assignment agreements may not provide us with adequate protection. In addition, any such patents may not issue, and the breadth or the degree of protection of any claims of such patents may not afford us with significant protection.
We own or have licensed rights to 2899 issued patents and numerous patent applications in the United States as well as numerousand foreign patent
applications relating to genes, proteins, and protein interactions associated
with cancer, heart disease, neurological disease and hypertension, processes for
identifying and sequencing genes, and other related gene discovery technologies.countries. However, any patent applications which we have filed or will file or to which we have licensed or will license rights may not issue, and patents that do issue may not contain commercially valuable claims. In addition, any patents issued to us or our licensors may not afford meaningful protection for our technology or products or may be subsequently circumvented, invalidated or narrowed.
Our processes and potential products may also conflict with patents which have been or may be granted to competitors, academic institutions or others. As the biotechnology industry expands and more patents are issued, the risk increases that our processes and potential products may give rise to interferences filed by others in the U.S. Patent and Trademark Office, or to claims of patent infringement by other companies, institutions or individuals. These entities or persons could bring legal actions against us claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the related product or process. If any of these actions are successful, in addition to any potential liability for damages, we could be required to cease the infringing activity or obtain a license in order to continue to manufacture or market the relevant product or process. We may not prevail in any such action and any license required under any such patent may not be made available on acceptable terms, if at all.
Our failure to obtain a license to any technology that we may require to commercialize our technologies or potential products could have a material adverse effect on our business. There is also considerable pressure on academic institutions to publish discoveries in the genetic field.genomic and proteomic fields. Such a publication by an academic collaborator of ours prior to the filing date of our
12
application, if it covers a genediscovery claimed in the application, may preclude the patent from issuing or the filing of foreign patent applications, or if a patent was issued, may invalidate the patent.
12
We also rely upon unpatented proprietary technology, and in the future may determine in some cases that our interests would be better served by reliance on trade secrets or confidentiality agreements rather than patents or licenses. These include some of our positional cloning, protein interaction, roboticsgenomic, proteomic, robotic and bioinformaticsbioinformatic technologies. We may not be able to protect our rights to such unpatented proprietary technology and others may independently develop substantially equivalent technologies. If we are unable to obtain strong proprietary rights to our processes or products after obtaining regulatory clearance, competitors may be able to market competing processes and products.
Others may obtain patents having claims which cover aspects of our products or processes which are necessary for or useful to the development, use or manufacture of our services or products. Should any other group obtain patent protection with respect to our discoveries, our commercialization of molecular
diagnostic servicespredictive medicine products and potential therapeutic products could be limited or prohibited.
In addition, we are a party to various license agreements which give us the rights to use certain technology in our research, development and testing processes. We may not be able to continue to license this technology on commercially reasonable terms, if at all. Our failure to maintain rights to this technology could have a material adverse effect on our business.
Competition
Competition is intense in our existing and potential markets. Our competitors in the United States and abroad are numerous and include, among others, major pharmaceutical and diagnostic companies, specialized biotechnology firms, universities and other research institutions. Many of our potential competitors have considerably greater financial, technical, marketing and other resources than we do. We expect competition to intensify in the fields in which we are involved as technical advances occur in these fields and become more widely known.
We expect to encounter significant competition with respect to any drugs that may be developed using our technologies. Companies that complete clinical trials, obtain required regulatory approvals and commence commercial sales of therapeutic products prior to us may achieve a significant competitive advantage. We may not be able to develop such products successfully and we may not obtain patents covering such products that provide protection against competitors. Moreover, competitors may succeed in developing therapeutic products that circumvent our products, our competitors may succeed in developing technologies or products that are more effective than those developed by us or that would render our technologies or products less competitive or obsolete.
The technologies for discovering genes that predispose persons to major diseases and approaches for commercializing those discoveries are new and rapidly evolving. Rapid technological developments could result in our potential services, products, or processes becoming obsolete before we recover a significant portion of our related research and development costs and associated capital expenditures. Our competitors in the United States and abroad are numerous and
include, among others, major pharmaceutical and diagnostic companies,
specialized biotechnology firms, universities and other research institutions.
Many of our potential competitors have considerably greater financial,
technical, marketing and other resources than we do, which may allow these
competitors to discover important genes before we can. If we do not discover additional disease-predisposing genes, characterize their functions, develop genetic testspredictive medicine products and related information services based on such discoveries, obtain regulatory and other approvals, and launch such services or products before our competitors, we could be adversely affected. Moreover, any molecular diagnostic
testspredictive medicine products that we may develop could be made obsolete by less expensive or more effective tests or methods that may be developed in the future. We expect
competition to intensify in the fields in which we are involved as technical
advances occur in these fields and become more widely known.
We also expect to encounter significant competition with respect to any
drugs that may be developed using our technologies. Companies that complete
clinical trials, obtain required regulatory approvals and commence commercial
sales of therapeutic products prior to us or our collaborative partners may
achieve a significant competitive advantage. We and our collaborative partners
may not be able to develop such products successfully and we may not obtain
patents covering such products that provide protection against competitors.
Moreover, competitors may succeed in developing therapeutic products that
circumvent our products, our competitors may succeed in developing technologies
or products that are more effective than those developed by us and our
collaborative partners or that would render our and our competitors'
technologies or products less competitive or obsolete.
Governmental Regulation
Regulation by governmental authorities in the United States and foreign countries is a significant factor in the development, manufacture and marketing of our proposed products and services and in our ongoing research and development activities. The therapeutic products and molecular diagnostic testspredictive medicine
13
products developed by us will require regulatory approval by governmental agencies prior to commercialization. Various federal statutes and regulations also govern or influence the testing, manufacturing, safety, labeling, storage, record keeping, and marketing of therapeutic products. The process of obtaining these approvals and the subsequent compliance with applicable statutes and regulations require the expenditure of substantial time and financial resources. Any failure by us or our collaborators, licensors or licensees to obtain, or any delay in obtaining, regulatory approval could have a material adverse effect on our business.
13
Therapeutics. Under our current strategic alliances, our partners have the
right to develop certain therapeutic products based on our gene discoveries. We
also intend to develop independently therapeutic products based on gene
discoveries that we have not licensed to partners.our discoveries. Such products will be subject to regulation by the FDA and foreign regulatory authorities and require approval before they may be clinically tested and commercially marketed for human therapeutic use in the United States and other countries. The precise regulatory requirements with which we and our corporate partners will have to comply are undergoing frequent revisions and refinement. It is also uncertain whether the clinical data generated in such studies will be acceptable to the FDA such that the FDA will approve the marketing of such products. In addition, obtaining FDA approval for therapeutic products is a costly and time consuming process.
The steps required before a pharmaceutical agent may be marketed in the United States include:
. preclinical
- •
- pre-clinical laboratory,in vivo and formulation studies;
. - •
- the submission to the FDA of an Investigational New Drug, or IND, application, which must become effective before human clinical trials may commence;
. - •
- adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug;
. - •
- the submission of a New Drug Application, or NDA, to the FDA; and
. - •
- FDA approval of the NDA, including approval of all product labeling and advertising.
The testing and approval process requires substantial time, effort, and financial resources and we cannot be certain that any approvals for any of our products will be granted on a timely basis, if at all.
Human clinical trials are typically conducted in three sequential phases which may overlap:
.
- •
- PHASE I: The drug is initially introduced into healthy human subjects or patients and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion.
. - •
- PHASE II: Involves studies in a limited patient population to identify possible adverse effects and safety risks, to determine the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
. - •
- PHASE III: When Phase II evaluations demonstrate that a dosage range of the product is effective and has an acceptable safety profile, Phase III trials are undertaken to further evaluate dosage and clinical efficacy and to further test for safety in an expanded patient population at geographically dispersed clinical study sites.
In the case of products for severe or life-threatening diseases such as cancer, the initial human testing is often conducted in patients rather than in healthy volunteers. Since these patients already have the target disease, these studies may provide initial evidence of efficacy traditionally obtained in Phase II trials and thus these trials are frequently referred to as Phase I/II trials. We cannot be certain that we or any of our partners will successfully complete Phase I, Phase II or Phase III testing of any compound within any specific time period, if at all. Furthermore, the FDA or the sponsor may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
14
The results of product development, preclinicalpre-clinical studies and clinical studies are submitted to the FDA as part of a NDA. The FDA may deny a NDA if the applicable regulatory criteria are not satisfied or may require additional clinical data. Even if such data is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Once issued, the FDA may withdraw product approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products which have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs.
14
On November 21, 1997, President Clinton signed into law the Food and Drug Administration Modernization Act. That Act codified the FDA's policy of granting "fast track" approval for therapies intended to treat severe or life-
threatening diseases.life-threatening diseases such as cancer and AIDS. This new policy is intended to facilitate the study of life saving therapies and shorten the total time for marketing approvals; however, there can be no assurance that these fast track procedures will shorten the time of approval for any of our products.
Satisfaction of the above FDA requirements or similar requirements of state, local and foreign regulatory agencies typically takes several years and the actual time required may vary substantially, based upon the type, complexity and novelty of the product or indication. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures upon our or our partners' activities. The FDA or any other regulatory agency may not grant any approvals on a timely basis, if at all. Success in early stage clinical trials does not assure success in later stage clinical trials. Data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations which could delay, limit or prevent regulatory approval. Even if a product receives regulatory approval, the approval may be significantly limited to specific indications and dosages. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Delays in obtaining, or failures to obtain regulatory approvals may have a material adverse effect on our business. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
Any products manufactured or distributed by us or our partners pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with current good manufacturing practices, or cGMP, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP regulations and other FDA regulatory requirements.
Molecular Diagnostics.
Predictive Medicine. We are subject to governmental regulation at the federal, state, and local levels as a clinical laboratory. We have received CLIA certification from the Department of Health and Human Services. On the state level, New York has implemented regulations concerning molecular diagnostic testing and we have received approval from the State of New York for both breast and ovarian cancer susceptibility, colon and uterine cancer susceptibility and hypertension/heart disease risk. We are aware of several other states that require licensing or registration of general clinical laboratory activities. We believe that we have taken all steps required of us in such jurisdictions in order for us to conduct business in those jurisdictions. However, we may not be able to maintain state level regulatory compliance in all states where we may do business. Failure to maintain state regulatory compliance, or changes in state regulatory schemes, could result in a substantial curtailment or even prohibition of our clinical activities and could have a material adverse effect on our business.
15
CLIA authorizes the Department of Health and Human Services to regulate clinical laboratories. These regulations, which affect us, mandate that all clinical laboratories be certified to perform testing on human specimens and provide specific conditions for certification. These regulations also contain guidelines for the qualification, responsibilities, training, working conditions and oversight of clinical laboratory employees. In addition, specific standards are imposed for each type of test which is performed in a laboratory. CLIA and the regulations promulgated thereunder are enforced through quality inspections of test methods, equipment, instrumentation, materials and supplies on a periodic basis. Any change in CLIA or these regulations or in the interpretation thereof could have a material adverse effect on our business.
Our business is also subject to regulation under state and federal laws regarding environmental protection and hazardous substances control, including the Occupational Safety and Health Act, the Environmental Protection Act, and the Toxic Substance Control Act. We believe that we are in material compliance with these and other applicable laws and that our ongoing compliance will not have a material adverse effect on our business. However, statutes or regulations applicable to our business may be adopted which impose substantial additional costs to assure compliance or otherwise materially adversely affect our operations.
15
Human Resources
As of September 1, 2000,2002, we had 338491 full-time equivalent employees, including 4165 persons holding doctoral degrees and threeor medical doctors.doctor degrees. Most of our employees are engaged directly in research, development, production and marketing activities. We believe that the success of our business will depend, in part, on our ability to attract and retain qualified personnel.
Our employees are not covered by a collective bargaining agreement, and we consider our relations with our employees to be good.
Risk Factors
We are a company in the early stages of development and commercialization and may never achieve the goals of our business plan.
We may be unable to continue to successfully develop or commercialize our products. Certain of our products are still in the early stages of development. We began operations in 1991 and have been engaged primarily in research directed toward the discovery and sequencing of genes, proteins, and protein pathways that predispose people to common diseases and the development of therapeutic and predictive medicine products. In October 1996 we introduced for commercial use BRACAnalysis®, our first predictive medicine product. In January 1998 we introduced for commercial use CardiaRisk®, our second predictive medicine product. In August 2000, we introduced for commercial use COLARIS™, our third predictive medicine product. In September 2001, we introduced for commercial use MELARIS®, our fourth predictive medicine product. In May 2002, we introduced for commercial use COLARIS AP™, our most recent predictive medicine product.
16
Our lead therapeutic compound has recently entered a large, multi-center human clinical trial. We also recently submitted an Investigational New Drug (IND) application for the evaluation of R-flurbiprofen (MPC-7869) for the treatment of Alzheimer's disease. Other therapeutic products are in various stages of pre-clinical development. Any therapeutic products under development by us will take several more years to develop and undergo extensive pre-clinical and clinical testing. Additionally, therapeutic products are subject to substantial regulatory review. We may be unable to discover or develop any therapeutic or additional predictive medicine products through the utilization of our technologies. Even if we develop products for commercial use, we may not be able to develop products that:
- •
- meet applicable regulatory standards, in a timely manner or at all;
- •
- successfully compete with other technologies and products;
- •
- avoid infringing the proprietary rights of others;
- •
- are manufacturable in sufficient quantities or at reasonable cost; or
- •
- are successfully marketed.
We have a history of operating losses and expect to continue to incur losses in the future.
We have a limited operating history and have experienced operating losses since our inception. We expect these losses to continue for the next several years and we may never be profitable or achieve significant revenues. For example, we experienced net losses of $14.0 million during the fiscal year ended June 30, 2002, $7.2 million during the year ended June 30, 2001 and $8.7 million during the year ended June 30, 2000. We had an accumulated deficit of $73.8 million as of June 30, 2002. In order to develop and commercialize our products, we expect to incur significant increases in our expenses over the next several years. As a result, we expect to incur operating losses at least for the foreseeable future. Our ability to achieve significant revenues or profitability will depend upon numerous factors, including our ability to:
- •
- identify drug targets and lead compounds that may lead to future therapeutic products;
- •
- create and introduce additional marketable predictive medicine products; and
- •
- obtain and maintain strategic collaborations.
If we are unable to overcome financial and regulatory obstacles, including those that arise in connection with new technologies, then we may never be able to develop commercially viable therapeutic products.
We are currently initiating the development of potential therapeutic products, which will require significant research and development expenditures, extensive pre-clinical and clinical testing and regulatory approvals. Preclinical and clinical testing will require the expenditure of significant funds. Even after spending significant funds, we may not be able to develop or successfully commercialize any potential therapeutic products.
Therapeutic products that we may develop will be subject to the risks of failure inherent in the development of therapeutic products based on new technologies. These risks include the possibilities that:
- •
- potential therapeutic products will be found to be unsafe or ineffective or otherwise fail to receive necessary regulatory clearances;
- •
- the products, if safe and effective, will be difficult to manufacture on a large scale or uneconomical to market;
17
- •
- proprietary rights of third parties will preclude us or our partners from marketing our products; or
- •
- third parties will market superior or equivalent products.
In addition, before receiving all required FDA approvals to market any product, we will have to demonstrate that the product is safe and effective on the patient population and for the diseases that would be treated. The clinical testing, manufacturing and marketing of products are subject to the rigorous testing and approval process of the FDA and equivalent foreign regulatory authorities, which can take many years and requires the expenditure of substantial financial and other resources. We may never obtain regulatory approvals for any products that we develop. Moreover, if regulatory approval of a product is granted, this approval may impose limitations on the indicated uses for which it may be marketed. Further, even if such regulatory approval is obtained, a marketed product and its manufacturer are subject to continuing review, and the discovery of previously unknown problems with a product or manufacturer may result in restrictions on such product or manufacturer, including withdrawal of the product from the market.
Clinical trials or marketing of any potential therapeutic products may expose us to liability claims from the use of these therapeutic products. We may not be able to obtain product liability insurance or, if obtained, sufficient coverage may not be available at a reasonable cost. In addition, as we develop therapeutic products internally, we will have to make significant investments in therapeutic product development, marketing, sales and regulatory compliance resources. We will also have to establish or contract for the manufacture of products, including supplies of drugs used in clinical trials, under the current good manufacturing practices of the FDA, which can be time consuming and costly.
If we are unable to maintain relationships with current collaborative partners or enter into new collaborative arrangements, then our business will be harmed.
We currently depend heavily and will depend heavily in the future on third parties for support in product development, manufacturing, marketing and distribution. Part of our current business strategy is to form collaborative arrangements with strategic partners to develop and commercialize therapeutic products based on our gene discoveries. We may not be able to maintain our current collaborative arrangements or negotiate additional acceptable collaborative arrangements in the future.
The research phase of our collaborations expire after a fixed term. Any current or future collaborative arrangement may not be successful. Failure of any collaborative arrangement, or termination by any of our collaborative partners of their respective agreements, could have a material adverse effect on our business. Further, additional milestone payments and future potential royalty payments from our collaborators are dependent upon their continuing to develop products based on the potential therapeutic targets we delivered to them. These partners may decide not to develop any products based on these targets. Even if these partners commence such development, they could decide to terminate it at any time.
In addition, our collaborative partners may pursue alternative technologies or develop alternative products either on their own or in collaboration with others, including our competitors, as a means of developing diagnostic products or treatments for the diseases targeted by the collaborative programs. Our interests may not continue to coincide with those of our collaborative partners, and some of our collaborative partners may develop, independently or with third parties, therapeutic or diagnostic products that could compete with those developed in collaboration with our partners or independently. Additionally, disputes over rights or technology or other proprietary interests may arise. Such disputes or disagreements between us and our collaborative partners could lead to delays in collaborative research projects, or could result in litigation or arbitration, any of which could have a material adverse effect on our business. In addition, there have been a significant number of recent consolidations among pharmaceutical companies. These consolidations among the companies with which we are
18
collaborating could result in the diminution or termination of, or delays in, the development or commercialization of the products or research programs under one or more of our collaborative agreements.
Our current predictive medicine products and other predictive medicine or therapeutic products that we may develop may never achieve significant commercial market acceptance.
We may not succeed in achieving significant commercial market acceptance of any of our products. While we have marketed several of our predictive medicine products for several years and have gained some acceptance with oncologists and surgeons, we need to convince the larger group of obstetricians/gynecologists and primary care physicians of the benefits of our predictive medicine products in order to increase our sales of those products. Our ability to successfully commercialize our current predictive medicine products, as well as any other predictive medicine or therapeutic products that we may develop, will depend on several factors, including:
- •
- Our ability to convince the medical community of the safety and clinical efficacy of our products and their potential advantages over existing therapeutic products and predictive medicine techniques.
- •
- The agreement by third-party payors to provide full or even partial reimbursement coverage for our products, the scope and extent of which will affect patients willingness or ability to pay for our products and will likely heavily influence physicians' decisions to recommend our products. To date, no third-party payors have been willing to reimburse patients for CardiaRisk.
- •
- The willingness of physicians and patients to utilize predictive medicine products which are difficult to perform and interpret. This difficulty is caused by a combination of factors, including the large number, sometimes many hundreds, of different mutations in the genes which our tests analyze, the need to characterize each specific mutation, and the ability of our products to predict only as to a statistical probability, not certainty, that a tested individual will develop the disease for which the test has been completed.
These factors present obstacles to significant commercial acceptance of our products, which we will have to spend substantial time and money to overcome, if we can do so at all. Our inability to successfully do so will harm our business.
If we do not compete effectively with scientific and commercial competitors, we may not be able to successfully commercialize our products.
Research in the field of genomics and proteomics is intense and highly competitive. This research is characterized by rapid technological change. Our competitors in the United States and abroad are numerous and include, among others, major pharmaceutical and diagnostic companies, biotechnology firms, universities and other research institutions. Many of our potential competitors have considerably greater financial, technical, marketing and other resources than we do, which may allow these competitors to discover important genes and determine their function before we do. We could be adversely affected if we do not discover genes, proteins or protein pathways and characterize their function, develop therapeutic and predictive medicine products based on these discoveries, obtain regulatory and other approvals and launch these products and their related services before our competitors. We also expect to encounter significant competition with respect to any therapeutic or predictive medicine products that we may develop or commercialize. Those companies that complete clinical trials, obtain required regulatory approvals and commence commercial sales of therapeutic products before we do may achieve a significant competitive advantage in marketing and commercializing their products. We may not be able to develop therapeutic or predictive medicine products successfully and may not obtain patents covering these products that provide protection against our competitors. Moreover, our competitors may succeed in developing therapeutic or
19
predictive medicine products that circumvent our technologies or products. Or, our competitors may succeed in developing technologies or products that are more effective than those developed by us or that would render our technologies or products less competitive or obsolete. We expect competition to intensify in the fields in which we are involved as technical advances in these fields occur and become more widely known.
If our current collaborators or scientific advisors terminate their relationships with us or develop relationships with a competitor, our ability to discover genes and commercialize therapeutic and predictive medicine products could be adversely affected.
We have relationships with collaborators at academic and other institutions who conduct research at our request. These collaborators are not our employees. As a result, we have limited control over their activities and, except as otherwise required by our collaboration agreements, can expect only limited amounts of their time to be dedicated to our activities. We have established collaborations with the University of Utah, Intermountain Health Care, Galileo Genomics, Inc., Iceland Genomics Corporation, and PrecisionMed, Inc. to pursue the discovery of genes involved in cancer, cardiovascular disease, obesity, osteoporosis, asthma, and certain central nervous system disorders. Our ability to discover genes, proteins, and protein pathways involved in human disease and commercialize therapeutic and predictive medicine products will depend in part on the continuation of these collaborations. If any of these collaborations are terminated, we may not be able to enter into other acceptable collaborations. In addition, our existing collaborations may not be successful.
Our research collaborators and scientific advisors may have relationships with other commercial entities, some or all of which could compete with us. Our research collaborators and scientific advisors sign agreements which provide for the confidentiality of our proprietary information and the results of studies conducted at our request. We may not, however, be able to maintain the confidentiality of our technology and other confidential information in connection with every collaboration. The dissemination of our confidential information could have a material adverse effect on our business.
If our current operating plan changes and we find that our existing capital resources will not meet our needs, we may find it necessary to raise additional funding, which funding may not be available.
We anticipate that our existing capital resources will enable us to maintain currently planned operations for at least the next two years. However, we base this expectation on our current operating plan, which may change. We have incurred, and will continue to incur, significant costs in the discovery, development and marketing of current and prospective therapeutic and predictive medicine products. Our ongoing gene discovery programs and our efforts to develop therapeutic and predictive medicine products will require substantial cash resources. If, for example, a new disease gene is discovered through these efforts, we would require funds in addition to our current operating plan to develop and launch a new predictive medicine product. Additionally, if we discover a new drug target with promising therapeutic properties, we would require funding in addition to our current operating plan to move the candidate drug into pre-clinical studies and human clinical trials. If, due to changes in our current operating plan, adequate funds are not available, we may be required to raise additional funds. Sources of additional capital resources include, but are not limited to, public or private equity financings, establishing a credit facility, or selling convertible debt securities. This additional funding may not be available to us or, if available, it may not be on reasonable terms.
Because of our potential long-term capital requirements, we may access the public or private equity markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time. If additional funds are raised by issuing equity securities, existing shareholders may suffer significant dilution.
20
If we are unable to comply with applicable governmental regulations, we may not be able to continue our operations.
The establishment and operation of our predictive medicine laboratory and the production and marketing of services and products developed through our technologies, as well as our ongoing research and development activities, are subject to regulation by numerous federal, state and local governmental authorities in the United States and by comparable regulatory agencies in other countries where we might seek to market services and products that may be developed. On the state level, only New York has implemented regulations concerning predictive medicine products and we have been accredited under the Clinical Laboratory Evaluation Program by the Department of Health of the State of New York for BRACAnalysis®, CardiaRisk®, COLARIS™, MELARIS®, and COLARIS AP™. Failure to maintain state regulatory compliance, or changes in state regulatory schemes, could result in a substantial curtailment or even prohibition of Myriad Laboratories' clinical activities and could have a material adverse effect on our business. We have received federal accreditation from the Department of Health and Human Services under the Clinical Laboratory Improvement Amendments, or CLIA, to operate our predictive medicine laboratory. However, our accreditation may subsequently be revoked, suspended or limited, or our accreditation may not be renewed on an annual basis as required. Furthermore, while the FDA has elected not to substantially regulate the activities or diagnostic tests performed by laboratories like our clinical laboratory, the FDA has stated that it has the right to do so, and the FDA may seek to regulate or require clearance or approval of our products in the future. If the FDA should require that these products receive FDA approval prior to their use in our laboratory, this approval may not be received on a timely basis, if at all.
If groups such as insurance companies and employers discriminate against individuals with a genetic predisposition to a disease, then demand for our predictive medicine products may decrease.
Predictive medicine products have raised ethical issues regarding confidentiality and the appropriate uses of information provided by these products. For these reasons, governmental authorities place restrictions on, or regulate the use of, predictive medicine products. While largely prohibited through federal and state laws, it is possible that discrimination by insurance companies against patients shown to have a genetic predisposition to a particular disease could occur through the raising of premiums by insurers to prohibitive levels, outright cancellation of insurance or unwillingness to provide coverage. We could experience a delay in market penetration or a reduction in the size of our potential serviceable market, which would adversely affect future revenue, if insurance discrimination were to become a significant barrier to testing acceptance. Similarly, employers could discriminate against employees with a genetic predisposition to a disease due to the increased risk for disease resulting in possible cost increases for health insurance and the potential for lost employment time. Any of these scenarios could cause us to experience a delay or reduction in product acceptance, which could materially adversely affect our business.
If we are not able to protect our proprietary technology, our business will be harmed and we may not remain competitive.
Our success will depend, in part, on our ability to obtain patent protection, both in the United States and in other countries, for genes we discover, for the function of the proteins produced by the genes and related technologies, processes, methods and other inventions that we believe are patentable. Our ability to preserve our trade secrets and other intellectual property is also critical to our long-term success. The patent position of biotechnology firms generally is highly uncertain and involves complex legal and factual questions. To date there has not emerged from the United States Patent and Trademark Office ("PTO"), the United States courts, or from patent offices or courts in foreign countries, a consistent policy regarding the breadth of claims allowed in biotechnology patents. Our or our licensors' patent applications may never issue as patents, and the claims of any issued patents may
21
not afford meaningful protection for our technology or products. In addition, any patents issued to us or our licensors may be challenged, and subsequently narrowed, invalidated or circumvented.
Our products may also conflict with patents that have been or may be granted to others. As the biotechnology industry expands and more patent applications are filed and patents are issued, the risk increases that our products may give rise to a declaration of interference by the PTO, or to claims of patent infringement by other companies, institutions or individuals. These entities or persons could bring legal proceedings against us seeking damages or seeking to enjoin us from testing, manufacturing or marketing our products. Patent litigation is costly, and even if we prevail, the cost of such litigation could have a material adverse effect on us. If the other parties in any such actions are successful, in addition to any liability for damages, we could be required to cease the infringing activity or obtain a license. Any license required may not be available to us on acceptable terms, if at all. Our failure to obtain a license to any technology that we may require to commercialize our products could have a material adverse effect on our business. In addition, there is considerable pressure on academic institutions to publish discoveries in the genetic field. Such a publication by an academic collaborator of ours, prior to the filing of a patent application on this discovery, may compromise our ability to obtain U.S. and foreign patent protection for the discovery.
If a third party files a patent application with claims to a gene or protein we have discovered, the PTO may declare an interference between competing patent applications. If an interference is declared, we may not prevail in the interference. If the other party prevails in the interference, we may be precluded from commercializing services or products based on the gene or protein, or may be required to seek a license. A license may not be available to us on commercially acceptable terms, if at all.
We also rely upon unpatented proprietary technologies. We may not be able to adequately protect our rights in such unpatented proprietary technologies, which could have a material adverse effect on our business. For example, others may independently develop substantially equivalent proprietary information or techniques or otherwise gain access to our proprietary technologies or disclose our technologies to our competitors.
If we were sued for patent infringement by third parties, we might incur significant costs and delays in product introduction.
Our industry includes many organizations seeking to rapidly identify and characterize genes through the use of gene expression analysis and other technologies. To the extent any patents are issued to those organizations on partial or full-length genes or uses for such genes, the risk increases that the sale of our predictive medicine products currently being marketed or under development, and any sales of therapeutic drugs developed by us, may give rise to claims of patent infringement. Others may have filed and in the future are likely to file patent applications covering genes or gene products that are similar or identical to our products. Any of these patent applications may have priority over our patent applications. Any legal action against us or our collaborators claiming damages and seeking to enjoin commercial activities relating to the affected products and processes could, in addition to subjecting us to potential liability for damages, require us or our collaborators to obtain a license in order to continue to manufacture or market the affected products and processes or could enjoin us from continuing to manufacture or market the affected products and processes, thereby significantly increasing our costs associated with, and significantly delaying, product introduction and marketing. We may not prevail in any of these actions and any license required under any of these patents may not be available on commercially acceptable terms, if at all. We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights. If we become involved in this litigation, it could consume a substantial portion of our managerial and financial resources.
22
If we fail to retain our key personnel and hire, train and retain qualified employees and consultants, we may not be able to successfully continue our business.
Because of the specialized scientific nature of our business, we are highly dependent upon our ability to attract and retain qualified management, scientific and technical personnel. We are currently recruiting additional qualified management, scientific and technical personnel. Competition for such personnel is intense. Loss of the services of or failure to recruit additional key management, scientific and technical personnel would adversely affect our research and development programs and predictive medicine business and may have a material adverse effect on our business as a whole.
Our agreements with our employees generally provide for employment that can be terminated by either party without cause at any time, subject to specified notice requirements. Further, the non-competition provision to which each employee is subject expires on the applicable date of termination of employment.
We depend on a limited number of third parties for some of our supplies of equipment and reagents. If these supplies become unavailable, then we may not be able to successfully perform our research or operate our business at all or on a timely basis.
We currently rely on two suppliers to provide our gene sequencing machines and reagents required in connection with our research. We believe that currently there are limited alternative suppliers of gene sequencing machines and reagents. The gene sequencing machines or the reagents may not remain available in commercial quantities at acceptable costs. If we are unable to obtain when needed additional gene sequencing machines or an adequate supply of reagents or other ingredients at commercially reasonable rates, our ability to continue to identify genes and perform molecular diagnostic testing would be adversely affected.
If we were successfully sued for product liability, we could face substantial liabilities that exceed our resources.
Our business exposes us to potential liability risks inherent in the testing, marketing and processing of predictive medicine products, including possible misdiagnoses. Although we are insured against such risks up to a $13,000,000 annual aggregate limit in connection with the use of our products, our present product liability insurance may be inadequate. A successful product liability claim in excess of our insurance coverage could have a material adverse effect on our business. Any successful product liability claim may prevent us from obtaining adequate product liability insurance in the future on commercially desirable or reasonable terms. An inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of our products. Our business also may expose us to liability inherent in the testing, manufacturing and marketing of prospective therapeutic products. Liability claims may be asserted against us. We have obtained product liability and other related insurance, but we may not be able to maintain this insurance on acceptable terms.
Our business involves environmental risks that may result in liability for us.
In connection with our research and development activities, we are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens and wastes. Although we believe that we have complied with the applicable laws, regulations and policies in all material respects and have not been required to correct any material noncompliance, we may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Although we believe that our safety procedures for handling and disposing of controlled materials comply with the standards prescribed by state and federal regulations, accidental
23
contamination or injury from these materials may occur. In the event of such an occurrence, we could be held liable for any damages that result and any such liability could exceed our resources.
Our stock price is highly volatile and our stock may lose all or a significant part of its value.
The market prices for securities of biotechnology and genomic companies have been volatile. This volatility has significantly affected the market prices for these securities for reasons frequently unrelated to the operating performance of the specific companies. These broad market fluctuations may adversely affect the market price of our common stock. The market price for our common stock has fluctuated significantly since public trading commenced in October 1995, and it is likely that the market price will continue to fluctuate in the future. In addition, the stock market has experienced extreme price and volume fluctuations. Events or factors that may have a significant impact on our business and on the market price of our common stock include the following:
- •
- quarterly fluctuations in operating results;
- •
- announcements by us, our collaborative partners or our present or potential competitors;
- •
- technological innovations or new commercial products or services;
- •
- regulatory approval developments;
- •
- developments or disputes concerning patent or proprietary rights;
- •
- public concern regarding the safety, efficacy or other implications of our products or services; or
- •
- general market conditions out of our control.
Our headquarters and facilities are located in Salt Lake City, Utah. We currently lease a 92,000149,000 square foot building dedicated to research and development, administration and laboratory space whichthat has received federal certification under CLIA to serve as a genetic predisposition testing
laboratory.CLIA. Activity related to our research, drug development and molecular diagnosticspredictive medicine segments is performed at this location. Additionally, we lease 6,440 square feet for
various research support functions. The lease on our primary facility has a term of tenfifteen years, through March 2016, and provides for a renewal option for a term of up to ten additional years.
We believe that our existing facilities and equipment are well maintained and in good working condition. We believe our current facilities will provide adequate capacity for the foreseeable future. We continue to make investments in capital equipment as needed to meet the research requirements of our collaborative agreements, our lead compounddrug development requirements, and the anticipated demand for our molecular diagnostic tests.
predictive medicine products.
We are not a party to any material legal proceedings.
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
On August 15, 2000,
No matters were submitted during the Company held a Special Meeting of Shareholders (the
"Special Meeting"). A quorum of 14,464,640 shares of Common Stockfourth quarter of the Company
(of a total 21,870,706) outstanding shares, or approximately 66.14%) was
represented at the Special Meeting in person or by proxy, which was held to vote
on the following proposal.
1. To consider and act upon a proposal recommended by the Board of
Directors to amend the Company's Restated Certificate of Incorporation
to increase the Company's authorized common stock from 15 million
shares to 60 million shares.
The proposal was adopted, with 11,706,506 voting FOR, 2,746,418 voting
AGAINST and 11,716 abstentions.
16
24
Item 5. MARKET FOR REGISTRANT'S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
The Company's
Our Common Stock began trading on the Nasdaq National Market on October 6, 1995 under the symbol "MYGN". The following table sets forth, for the last two fiscal years, the high and low sales prices for the Common Stock, as reported by the Nasdaq National Market:
High Low
---- ---
Fiscal 2000:
Fourth Quarter......................... $ 76.063 $19.00
Third Quarter.......................... $116.063 $21.313
Second Quarter......................... $ 25.375 $ 8.25
First Quarter.......................... $ 9.75 $ 4.323
Fiscal 1999:
Fourth Quarter......................... $ 6.188 $ 4.375
Third Quarter.......................... $ 5.75 $ 4.25
Second Quarter......................... $ 6.25 $ 3.938
First Quarter ........................ $ 8.00 $ 2.875
| | High | Low | |||||
|---|---|---|---|---|---|---|---|
| Fiscal 2002: | |||||||
| Fourth Quarter | $ | 35.00 | $ | 16.30 | |||
| Third Quarter | $ | 53.20 | $ | 30.11 | |||
| Second Quarter | $ | 63.64 | $ | 28.70 | |||
| First Quarter | $ | 62.50 | $ | 24.75 | |||
| Fiscal 2001: | |||||||
| Fourth Quarter | $ | 79.85 | $ | 29.50 | |||
| Third Quarter | $ | 81.75 | $ | 31.25 | |||
| Second Quarter | $ | 138.00 | $ | 67.188 | |||
| First Quarter | $ | 92.813 | $ | 53.00 | |||
As of September 1, 2000,August 28, 2002, there were approximately 140165 stockholders of record of the Common Stock and, according to the Company'sour estimates, approximately 2,50019,358 beneficial owners of the Common Stock. The Company hasWe have not paid dividends to itsour stockholders since itsour inception and doeswe do not plan to pay cash dividends in the foreseeable future. The CompanyWe currently intendsintend to retain earnings, if any, to finance the growth of the Company.
our growth.
Sale of Unregistered Securities
During the three months ended June 30, 2000, the Company issued a total of
2,742 shares of Common Stock to a director and a consultant of the Company
pursuant to the exercise of stock options at a weighted average price of $0.49
per share.
On June 15, 2000 the Company sold 600,000 shares of Common Stock for an
aggregate purchase price of $24,000,000. The sale was made to an accredited
investor in a private placement that was exempt from registration under
Regulation S of the Securities Act of 1933 (the "Securities Act").
No person acted as an underwriter with respect to the transactions set
forth above. In each of the foregoing instances, the Company relied on Section
4(2) of the Securities Act or Regulation S or Rule 701 promulgated under the
Securities Act for the exemption from the registration requirements of the
Securities Act, since no public offerings or offerings inside the United
States were involved.
17
None.
25
Item 6. SELECTED CONSOLIDATED FINANCIAL DATA
The following table sets forth our consolidated financial data as of and for each of the five years ended June 30, 2000.2002. The selected consolidated financial data as of and for each of the five years ended June 30, 20002002 have been derived from our consolidated financial statements. The consolidated financial statements and the report thereon for the year ended June 30, 20002002 are included elsewhere in this Annual Report on Form 10-K. The information below should be read in conjunction with the consolidated financial statements (and notes thereon) and "Management's Discussion and Analysis of Financial Condition and Results of Operations," included in Item 7.
| | Years Ended June 30, | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2002 | 2001 | 2000 | 1999 | 1998 | |||||||||||||
| Consolidated Statement of Operations Data: | ||||||||||||||||||
| Research revenue | $ | 27,015,167 | $ | 28,071,252 | $ | 25,219,766 | $ | 20,093,057 | $ | 20,999,598 | ||||||||
| Predictive medicine revenue | 26,821,332 | 17,091,139 | 8,793,272 | 5,220,349 | 2,210,983 | |||||||||||||
| Total revenues | 53,836,499 | 45,162,391 | 34,013,038 | 25,313,406 | 23,210,581 | |||||||||||||
| Costs and expenses: | ||||||||||||||||||
| Predictive medicine cost of revenue | 10,716,761 | 7,402,906 | 3,986,473 | 3,066,354 | 1,391,368 | |||||||||||||
| Research and development expense | 36,294,669 | 33,818,144 | 28,098,769 | 23,452,220 | 23,002,340 | |||||||||||||
| Selling, general and administrative expense | 25,484,836 | 17,077,846 | 13,474,923 | 11,105,520 | 11,807,023 | |||||||||||||
| Total costs and expenses | 72,496,266 | 58,298,896 | 45,560,165 | 37,624,094 | 36,200,731 | |||||||||||||
| Operating loss | (18,659,767 | ) | (13,136,505 | ) | (11,547,127 | ) | (12,310,688 | ) | (12,990,150 | ) | ||||||||
| Other income (expense): | ||||||||||||||||||
| Interest income | 5,384,802 | 6,850,479 | 3,208,506 | 2,348,827 | 3,223,683 | |||||||||||||
| Interest expense | — | — | — | (6,278 | ) | (32,681 | ) | |||||||||||
| Other | (214,405 | ) | (305,134 | ) | (383,481 | ) | (27,314 | ) | 2,113 | |||||||||
| Loss before income taxes | (13,489,370 | ) | (6,591,160 | ) | (8,722,102 | ) | (9,995,453 | ) | (9,797,035 | ) | ||||||||
| Income taxes | 500,000 | 583,333 | — | — | — | |||||||||||||
| Net loss | $ | (13,989,370 | ) | $ | (7,174,493 | ) | $ | (8,722,102 | ) | $ | (9,995,453 | ) | $ | (9,797,035 | ) | |||
| Basic and diluted net loss per share | $ | (0.59 | ) | $ | (0.31 | ) | $ | (0.43 | ) | $ | (0.53 | ) | $ | (0.53 | ) | |||
| Basic and diluted weighted average shares outstanding | 23,660,127 | 22,815,035 | 20,220,446 | 18,782,244 | 18,578,962 | |||||||||||||
| | As of June 30, | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2002 | 2001 | 2000 | 1999 | 1998 | ||||||||||
| Consolidated Balance Sheet Data: | |||||||||||||||
| Cash, cash equivalents and marketable investment securities | $ | 124,242,908 | $ | 145,954,968 | $ | 88,655,844 | $ | 38,926,459 | $ | 53,109,493 | |||||
| Working capital | 56,833,907 | 104,615,236 | 57,263,118 | 8,348,224 | 21,806,290 | ||||||||||
| Total assets | 157,390,080 | 172,145,355 | 106,375,305 | 53,550,940 | 67,391,972 | ||||||||||
| Stockholders' equity | 128,869,500 | 139,561,798 | 77,706,647 | 48,215,736 | 57,481,013 | ||||||||||
26
Quarterly Financial Data (Unaudited)
| | Quarters Ended, | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | June 30, 2002 | March 31, 2002 | December 31, 2001 | September 30, 2001 | |||||||||||
| Consolidated Statement of Operations Data: | |||||||||||||||
| Research revenue | $ | 6,431,912 | $ | 5,803,255 | $ | 7,107,309 | $ | 7,672,691 | |||||||
| Predictive medicine revenue | 7,680,539 | 7,255,065 | 6,368,132 | 5,517,596 | |||||||||||
| Total revenues | 14,112,451 | 13,058,320 | 13,475,441 | 13,190,287 | |||||||||||
| Costs and expenses: | |||||||||||||||
| Predictive medicine cost of revenue | 3,031,148 | 2,848,591 | 2,565,334 | 2,271,689 | |||||||||||
| Research and development expense | 10,680,968 | 8,739,952 | 8,612,388 | 8,261,360 | |||||||||||
| Selling, general and administrative expense | 7,867,977 | 5,911,925 | 6,080,473 | 5,624,462 | |||||||||||
| Total costs and expenses | 21,580,093 | 17,500,468 | 17,258,195 | 16,157,511 | |||||||||||
| Operating loss | (7,467,642 | ) | (4,442,148 | ) | (3,782,754 | ) | (2,967,224 | ) | |||||||
| Other income (expense): | |||||||||||||||
| Interest income | 958,656 | 1,076,435 | 1,418,554 | 1,931,156 | |||||||||||
| Other | (214,190 | ) | (5,655 | ) | 29,491 | (24,050 | ) | ||||||||
| Loss before income taxes | (6,723,176 | ) | (3,371,368 | ) | (2,334,709 | ) | (1,060,118 | ) | |||||||
| Income taxes | 125,000 | 125,000 | 125,000 | 125,000 | |||||||||||
| Net loss | $ | (6,848,176 | ) | $ | (3,496,368 | ) | $ | (2,459,709 | ) | $ | (1,185,118 | ) | |||
| Basic and diluted net loss per share | $ | (0.29 | ) | $ | (0.15 | ) | $ | (0.10 | ) | $ | (0.05 | ) | |||
| Basic and diluted weighted average shares outstanding | 23,790,574 | 23,763,165 | 23,607,694 | 23,482,735 | |||||||||||
| | Quarters Ended, | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | June 30, 2001 | March 31, 2001 | December 31, 2000 | September 30, 2000 | |||||||||||
| Consolidated Statement of Operations Data: | |||||||||||||||
| Research revenue | $ | 5,661,695 | $ | 6,652,289 | $ | 7,988,017 | $ | 7,769,251 | |||||||
| Predictive medicine revenue | 5,160,282 | 4,914,950 | 3,965,898 | 3,050,009 | |||||||||||
| Total revenues | 10,821,977 | 11,567,239 | 11,953,915 | 10,819,260 | |||||||||||
| Costs and expenses: | |||||||||||||||
| Predictive medicine cost of revenue | 2,221,517 | 2,149,029 | 1,726,998 | 1,305,362 | |||||||||||
| Research and development expense | 7,247,908 | 8,428,402 | 9,351,036 | 8,790,797 | |||||||||||
| Selling, general and administrative expense | 4,806,659 | 4,247,554 | 4,080,244 | 3,943,390 | |||||||||||
| Total costs and expenses | 14,276,084 | 14,824,985 | 15,158,278 | 14,039,549 | |||||||||||
| Operating loss | (3,454,107 | ) | (3,257,746 | ) | (3,204,363 | ) | (3,220,289 | ) | |||||||
| Other income (expense): | |||||||||||||||
| Interest income | 1,372,919 | 2,057,167 | 2,022,100 | 1,398,293 | |||||||||||
| Other | (22,021 | ) | (27,465 | ) | (7,183 | ) | (248,465 | ) | |||||||
| Loss before income taxes | (2,103,209 | ) | (1,228,044 | ) | (1,189,446 | ) | (2,070,461 | ) | |||||||
| Income taxes | 83,333 | 500,000 | — | — | |||||||||||
| Net loss | $ | (2,186,542 | ) | $ | (1,728,044 | ) | $ | (1,189,446 | ) | $ | (2,070,461 | ) | |||
| Basic and diluted net loss per share | $ | (0.09 | ) | $ | (0.07 | ) | $ | (0.05 | ) | $ | (0.09 | ) | |||
| Basic and diluted weighted average shares outstanding | 23,323,937 | 23,219,841 | 22,698,098 | 22,032,596 | |||||||||||
27
Item 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
OPERATIONS
Overview
We are a leader in the emerging field of proteomics and gene-based medicine
focusingleading biopharmaceutical company focused on the development and marketing of novel therapeutic and molecular diagnosticpredictive medicine products. We have developed and will continue to expand upon, a number of proprietary proteomic databasestechnologies which permit us through the use of our bioinformatics and
robotics technologies, to identify human genes, andtheir related proteins that mayand the biological pathways they form. We use this information to better understand the role proteins play a role in the onset orand progression of major human diseases.disease. We formedoperate two wholly owned subsidiaries, Myriad Pharmaceuticals, Inc. and Myriad Genetic Laboratories, Inc., to commercialize our therapeutic and molecular diagnosticpredictive medicine discoveries. Myriad Pharmaceuticals, Inc. independentlydevelops and in conjunction with
collaborative partners, focuses on the discovery and development ofintends to market novel therapeutic products. Myriad Genetic Laboratories, Inc. focuses on the development and marketing of molecular diagnosticpredictive medicine products that accessassess an individual's risk of developing a specific disease.
Myriad researchers have made important discoveries in the fields of cancer, viral diseases such as AIDS, and acute thrombosis. These discoveries point to novel disease pathways and have paved the way for the development of new drugs. Additionally, our pipeline of drug targets offers therapeutic opportunities for the treatment of diseases such as heart disease, rheumatoid arthritis, Alzheimer's disease and other central nervous system disorders. We have identified 871 drug targets to date. We have also established an extensive portfolio of drug candidates that are under development at Myriad. Fifteen of these drug candidates are in pre-clinical testing. Flurizan™, our lead therapeutic product for the treatment of prostate cancer, is currently in a large, multi-center human clinical trial. We also recently submitted an Investigational New Drug (IND) application for the evaluation of R-flurbiprofen (MPC-7869) for the treatment of Alzheimer's disease. We intend to independently develop and, subject to regulatory approval, market our therapeutic products, particularly in the area of cancer and infectious diseases.
We also have developed and commercialized five innovative predictive medicine products: BRACAnalysis®, which is used to assess a woman's risk of developing breast and ovarian cancer, COLARIS™ and COLARIS AP™, which are used to determine a person's risk of developing colon cancer, MELARIS®, which is used to determine a specificperson's risk of developing malignant melanoma, and CardiaRisk®, which is used for therapeutic management of hypertensive patients. We market these products using our own internal 106 person sales force in the United States and we have entered into marketing collaborations with other organizations in Austria, Brazil, Canada, Germany, Japan, and Switzerland. Revenues from these proprietary products grew approximately 57% from the prior year to $26.8 million in the fiscal year ended June 30, 2002.
We believe that the future of medicine lies in the creation of new classes of drugs that prevent disease from occurring or progressing and permits physiciansthat treat the cause, not just the symptoms, of disease. In addition, we believe that advances in the emerging field of predictive medicine will improve our ability to determine which patients are subject to a greater risk of developing these diseases and their patients to take appropriate
health care measures to reduce the risk.who therefore should receive these new preventive medicines.
We have devoted substantially all of our resources to maintaining our research and development programs, supporting collaborative research agreements,
operating a molecular diagnostic laboratory, establishing genomic sequencing,
establishing high-throughput screening, and undertaking drug discovery and development.development, and operating our predictive medicine business. Our revenues have consisted primarily of sales of predictive medicine products and research payments received pursuant to collaborative agreements, upfront fees, and milestone payments, and
sales of molecular diagnostic products.payments. We have yet to attain profitability and, for the year ended June 30, 2000,2002, we had a net loss of $8,722,102$14.0 million and as of June 30, 20002002 had an accumulated deficit of $52,661,982.
In April 1995, we commenced a five-year collaborative research$73.8 million.
We have formed strategic alliances with 12 major pharmaceutical or multinational companies including Abbott Laboratories, Bayer Corporation, E.I. du Pont de Nemours and development arrangement withCompany (DuPont), Eli Lilly and Company, Hitachi Ltd., Hoffmann-LaRoche Inc., Novartis Corporation. The total equity investment,
research funding and potential milestone payments under this collaboration may
provide us with up to $60,000,000. The research phase of the Novartis
collaboration concluded successfully on schedule in April 2000. We are entitled
to receive royalties from sales of therapeutic products commercialized by
Novartis.
In September 1995, we commenced a five-year collaborative research and
development arrangement with Bayer Corporation. The total equity investment,
research funding and potential milestone payments under this collaboration may
provide us with up to $71,000,000. In November 1997 and again in December 1998,
we announced expansions of our collaborative research and development
arrangement with Bayer. The expanded collaboration may provide us with
additional research funding and potential milestone payments of up to
$137,000,000. We are entitled to receive royalties from sales of therapeutic
products commercialized by Bayer.
In October 1996, we announced the introduction of BRACAnalysis(R), a
comprehensive BRCA1 and BRCA2 gene sequence analysis for susceptibility to
breast and ovarian cancer. In January 1998, we announced the introduction of
CardiaRisk(R), which may assist physicians both in identifying which
hypertensive patients are at a significantly increased risk of developing
cardiovascular disease and identifying which patients are likely to respond to
low salt diet therapy and antihypertensive drug therapy. In August 2000, we
announced the future launch of COLARIS(TM), a predicitive medicine test for
hereditary colon cancer and uterine cancer. We plan to begin accepting
COLARIS(TM) samples in the fall of 2000. We, through our wholly owned subsidiary
Myriad Genetic Laboratories, Inc., recognized molecular diagnostic revenues,
primarily from BRACAnalysis(R), of $8,793,272 for the year ended June 30, 2000.
In April 1997, we commenced a three-year collaborative research and
development arrangement with Schering-Plough Corporation. The total equity
investment, research funding, license fees and potential milestone payments
under this collaboration may provide us with up to $60,000,000. The research
phase of the Schering-Plough collaboration concluded successfully on schedule in
April 2000. We are entitled to receive royalties from sales of therapeutic
products commercialized by Schering-Plough.
In October 1998, we entered into a five-year collaboration withCorporation, Oracle
28
Corporation, Pharmacia Corporation, Schering AG, to utilize our protein interaction technology, ProNet(R), for drug discoverySchering-Plough Corporation, and development. Under the agreement, we will have an option to co-promote all new
therapeutic products in North America and receive 50%Torrey Mesa Research Institute, a subsidiary of the profits from North
American sales of
19
all new drugs discovered with ProNet(R). The total research funding, license
fees, subscription fees, option payments and potential milestone payments under
this collaboration may provide us with up to $51,000,000. If we choose to co-
promote a drug developed by Schering AG as a 50% partner, we may be required to
pay funds to Schering AG to establish equal ownership.
In November 1998, we entered into a 15 month collaboration with Pharmacia
Corporation (formerly Monsanto Company) to utilize ProNet(R) for drug discovery
and development. In December 1999, Pharmacia exercised its option to extend the
research term for an additional 12 months and exercised its option to expand the
research funding. The total research funding, option payments, license fees and
potential milestone payments under this collaboration may provide us with up to
$28,000,000. We are entitled to receive royalties from sales of therapeutic
products commercialized by Pharmacia.
In July 1999, we entered into a two-year collaboration and license
agreement with the Novartis Agricultural Discovery Institute, Inc. The genomic
collaboration will focus on the discovery of the genetic structure of cereal
crops. The total funding under this collaboration is expected to provide us with
$33,500,000. Upon completion, we intend to jointly offer with NADII commercial
access to the genomic databases and share equally in any resulting proceeds.
In October 1999, we announced the expansion of our collaboration with
Schering AG to include research in the field of cardiovascular disease. We also
entered into a Securities Purchase Agreement and a Standstill Agreement with
Schering Berlin Venture Corporation to sell to Schering Berlin 606,060 shares of
our common stock for an aggregate purchase price of $5,000,000.
In December 1999, we entered into a 12 month collaboration with Hoffmann-
LaRoche Inc. to utilize ProNet(R) for drug discovery and development in the area
of cardiovascular disease. The total research funding, license fees and
potential milestone payments under this collaboration may provide us with up to
$13,000,000. In addition, we are entitled to receive royalties from sales of
therapeutic products commercialized by Roche.
In May 2000, we entered into a three year strategic alliance with Hitachi
Ltd. Under the terms of the agreement, we will work with Hitachi to exploit the
ProNet(R) technology together in Japan and Hitachi will establish a designated
ProNet(R) facility to expedite the discovery of novel protein-protein
interactions for Japanese customers. Total research and license payments under
this collaboration are expected to provide us with $26,000,000. In addition, we
are entitled to receive royalties from sales of therapeutic products
commercialized by Hitachi.Syngenta. We intend to enter into additional collaborative relationships to locate
and sequencediscover genes, and discoverproteins, protein networks, and drug targets associated with other common diseases as well as to continue to fund internal research projects. WeHowever, we may be unable to enter into additional collaborative relationships on terms acceptable to us.
In April 2001, we announced the formation of Myriad Proteomics, Inc., a new venture with Hitachi, Ltd. and Oracle Corporation to map the human proteome. Myriad Proteomics, which is 49 percent owned by the Company, intends to develop and market a proprietary map of the human proteome to pharmaceutical and biotechnology companies for therapeutic and diagnostic product development.
We expect to incur losses for at least the next several years, primarily due to expansion of our research and development programs, expansion of our drug discovery and development efforts, increased staffing costsexpansion of our research and development programs, launch of new predictive medicine products, and expansion of our facilities. Additionally, we expect to incur substantial sales, marketing and other expenses in connection with building our molecular diagnostic business.pharmaceutical and predictive medicine businesses. We expect that losses will fluctuate from quarter to quarter and that such fluctuations may be substantial.
Critical Accounting Policies
Critical accounting policies are those policies which are both important to the portrayal of a company's financial condition and results and require management's most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Our critical accounting policies are as follows:
- •
- revenue recognition;
- •
- investments in privately-held companies;
- •
- investment in Myriad Proteomics, Inc.;
Revenue Recognition. We apply the provisions of Securities and Exchange Commission (SEC) Staff Accounting Bulletin No. 101,Revenue Recognition (SAB 101) to all our revenue transactions. Research revenues include revenues from research and technology licensing agreements. We recognize revenue from research contracts in accordance with the percentage-of-completion method of accounting and following the guidance in Statement of Position 81-1,Accounting for Performance of Construction-Type and Certain Production-Type Contracts. Percent complete is estimated based on costs incurred relative to total estimated contract costs. We make adjustments, if necessary, to the estimates used in the percentage-of-completion method of accounting as work progresses and we gain experience. Our estimates of total contract costs include assumptions, such as estimated research hours to complete, materials costs, and other direct and indirect costs. Actual results may vary significantly from our estimates. Revenues related to up-front payments and technology license fees when continuing involvement or research services are required of us are recognized over the period of performance.
Predictive medicine revenues include revenues from the sale of predictive medicine products and related marketing agreements. Predictive medicine revenue is recognized upon completion of the test and communication of results. Up-front payments related to marketing agreements are recognized ratably over the life of the agreement.
Investments in Privately-Held Companies. We review the valuation of our investments in privately-held biotechnology and pharmaceutical companies for possible impairment as changes in facts and circumstances indicate that impairment should be assessed. The amount of impairment, if any, and valuation of these investments are based on our estimates and, in certain circumstances, the completion of independent, third-party appraisals of the investments. Inherent in these estimates and appraisals are
29
assumptions such as the comparability of the investee to similar publicly traded companies, the value of the investee's underlying research and development efforts, the likelihood that the investee's current research projects will result in a marketable product, and the investee's expected future cash flows. Accordingly, the amount recognized by us upon ultimate liquidation of these investments may vary significantly from the estimated fair values at June 30, 2002.
Investment In Myriad Proteomics. In April 2001, we announced the formation of a new alliance with Hitachi, Ltd. (Hitachi), Friedli Corporate Finance A.G. (Friedli), and Oracle Corporation (Oracle) to map the human proteome. The newly formed entity, Myriad Proteomics, Inc. (Myriad Proteomics) intends to develop and market its proprietary proteomic information to pharmaceutical and biotechnology companies for therapeutic and diagnostic product development.
As part of the formation of Myriad Proteomics we entered into administrative and scientific outsourcing agreements with Myriad Proteomics. These agreements expired on June 30, 2002 and new agreements covering subsequent limited outsourcing services have been established. Charges to Myriad Proteomics for services incurred related to the administrative and scientific outsourcing agreements were based on actual time and expenses that we incurred on behalf of Myriad Proteomics and were not recorded as revenues but as a contra research expense.
RESULTS OF OPERATIONS
Years ended June 30, 20002002 and 1999.2001
Research revenues for our fiscal year ended June 30, 20002002 were $25,219,766
as$27.0 million compared to $20,093,057$28.1 million for the fiscal year ended June 30, 1999. The
increase2001. Research revenue is comprised of 26%research payments received pursuant to collaborative agreements, amortization of license fees and milestone payments. This decrease of 4% in our research revenue is primarily attributable to revenue
recognized fromgreater emphasis on our internal research and drug development programs, performing research for Myriad Proteomics, and the NADII collaboration that began in July 1999,successful completion of the Roche
collaboration which beganBayer and TMRI collaborations in December 1999,2001. Partially offsetting the overall decrease in research revenue were revenues from our new collaborations with Abbott Laboratories and the Hitachi collaboration which
beganDuPont, both entered into in May 2000.March 2002. Research revenue from theour research collaboration agreements is generally recognized as related costs are incurred. Consequently, as these programs progress and costs increase or decrease, revenues increase or decrease proportionately.
20
Molecular diagnostic
Predictive medicine revenues of $8,793,272 were recognized in thefor our fiscal year ended June 30, 2000,2002 were $26.8 million, an increase of 68%57% or $3,572,923$9.7 million over the prior fiscal year. Molecular diagnosticPredictive medicine revenue is comprised of sales of molecular diagnostic tests
resultingpredictive medicine products and fees and royalties from our discovery of disease genes. Salespredictive medicine product marketing partners. Increased sales and marketing efforts since
that time, together with increased demand as a result ofand wider acceptance of the
testour products by the medical community have given rise to theresulted in increased revenues for the fiscal year ended June 30, 2000. There2002. However, there can be no assurance however that molecular diagnosticpredictive medicine revenues will continue to increase at the historical rate.rates.
Research and development expenses for the fiscal year ended June 30, 2000
increased2002 were $36.3 million compared to $28,098,769 from $23,452,220$33.8 million for the prior year, anfiscal year. The increase of 20%. This increase7% was primarily due to an increaseincreased costs associated with our ongoing clinical trial for Flurizan™ and increased research spending for our ongoing drug discovery efforts in Myriad Pharmaceuticals. Research and development expenses were partially offset by reimbursement for research activitieswe performed for Myriad Proteomics as part of a scientific outsourcing agreement. For the fiscal year ended June 30, 2002, research and development expenses were reduced by $5.5 million as a result of our recent collaborations with NADII, Roche, and Hitachi as well as
those programs we fund internally. The increased level of research spending also
includes the ongoing drug discovery efforts of Myriad Pharmaceuticals, our
wholly-owned subsidiary, continued development and utilization of ProNet, and
third-party sponsored research programs.these scientific outsourcing services.
Selling, general and administrative expenses for the fiscal year ended June 30, 20002002 were $13,474,923$25.5 million compared to $11,105,520$17.1 million for the prior fiscal year ended June
year. Selling, general and administrative expenses consist primarily of salaries, commissions and related personnel costs for sales, marketing, executive, legal, finance, accounting, human resources, information technology, and business
30 1999. This
development personnel, allocated facilities expenses and other corporate expenses. The increase of 21%49% was primarily attributable to costs associated
with the ongoing promotion ofincreases in our molecular diagnostic business including
preparations forsales force from 75 to 106 sales representatives, the launch of COLARIS, atwo new predictive medicine test for
hereditary colonproducts, and uterine cancer scheduledmarketing costs related to be available in the fall of
2000. Increased costs also resulted from the establishment of international
license agreements and the related costs of increasing our infrastructuredirect-to-consumer campaign to support increased molecular diagnostic testing volumes.our predictive medicine business. We expect this larger sales force and related marketing efforts to enable us to increase awareness of our predictive medicine business. We expect our selling, general and administrative expenses will continue to fluctuate as needed in
supportdependent on the number and scope of our molecular diagnostic businessnew product launches and our researchdrug discovery and development efforts.
Cash, cash equivalents, and marketable investment securities were
$88,655,844decreased $21.7 million or 15% from $146.0 million at June 30, 2000 as compared2001 to $38,926,459$124.2 million at June 30, 1999.2002. This increasedecrease in our cash, cash equivalents, and marketable investment securities wasis primarily attributable to the private sale of approximately $34,000,0000 worth
ofincreased expenditures for our Common Stock during the year, as well as receipt of license payments,
milestone paymentsinternal drug development programs and advance research payments from our collaborators. These
cash receipts were offset byother expenditures we incurred in the ordinary course of business. As a result of the our increaseddecreased cash position and declining interest rates, interest income for the fiscal year ended June 30, 20002002 was $3,208,506 as$5.4 million compared to $2,348,827$6.9 million for the fiscal year ended June 30, 1999. The loss on disposition2001, a decrease of assets of $383,481
in the22%.
Years ended June 30, 2001 and 2000
Research revenues for our fiscal year ended June 30, 2000 was primarily the result of our retiring
unproductive assets.
Years ended June 30, 1999 and 1998.
Research revenues for the Company's fiscal year ended June 30, 19992001 were $20,093,057 as$28.1 million compared to $20,999,598$25.2 million for the fiscal year ended June 30, 1998.
Greater2000. The increase in our research revenue of 11% was primarily attributable to increased revenue recognized during the fiscal year ended June 30, 1998
versus the fiscal year ended June 30, 1999 is the result of $3,950,000 in
research milestonesfrom both our Hitachi and contract expansion payments we received 1998. Excluding
the milestone and contract expansion payments, our ongoing research revenue
increased $3,043,459 for the fiscal year ended June 30, 1999 versus fiscal 1998.TMRI collaborations. Research revenue from theour research collaboration agreements is generally recognized as related costs are incurred. Consequently, as these programs progress and costs increase or decrease, revenues increase or decrease proportionately.
Molecular diagnostic
Predictive medicine revenues of $5,220,349$17.1 million were recognized in the fiscal year ended June 30, 1999,2001, an increase of 136%94% or $3,009,366$8.3 million over the fiscal year
ended June 30, 1998. Molecular diagnosticprior year. Predictive medicine revenue is comprised of sales of diagnostic testspredictive medicine products resulting from the our discovery of important disease genes. We launched
the test for genetic predisposition to breast and ovarian cancer in October 1996
and we launched the test for heart disease and hypertension risk in January
1998. SalesThe successful launch of COLARIS™, as well as increased sales and marketing efforts, since that time have giventogether with wider acceptance of our products by the medical community, gave rise to the increased revenues for the fiscal year ended June 30, 1999.2001. However, there can be no assurance that predictive medicine revenues will continue to increase at historical rates.
Research and development expenses for the year ended June 30, 19992001 increased to $23,452,220$33.8 million from $23,002,340$28.1 million for the prior year.year, an increase of 20%. This increase was primarily due to an increase in research activities as a resultthe drug discovery and drug development efforts of Myriad Pharmaceuticals, Inc., our collaborations with Novartis, Bayer, Schering, Schering AG, and Pharmacia,wholly-owned subsidiary, as well as those programs we funded internally. The increased level of research spending includes ongoing development of the Company's ProNet(TM) and mutation
screening technologies, third-party sponsored research programs, and the
formation of Myriad Pharmaceuticals, Inc. ("Myriad Pharmaceuticals"). Myriad
Pharmaceuticals,activities relating to our wholly-owned subsidiary, was
21
created to develop therapeutic lead compounds for selected common diseases with
large potential markets that are under-served by current therapeutic options.strategic collaborations.
Selling, general and administrative expenses for the fiscal year ended June 30, 1999 decreased $701,503 from2001 were $17.1 million compared to $13.5 million for the fiscal year ended June 30, 1998. During2000. This increase of 27% was primarily attributable to costs associated with the fiscal year endedongoing promotion of our predictive medicine business, including the launch of COLARIS™, that was introduced in September 2000. We also bolstered our sales force to 75 full time employees, to allow us to increase awareness of our predictive medicine business through direct contact with health care professionals. We expect that our selling, general and administrative expenses will continue to fluctuate as needed in support of our predictive medicine business and our drug discovery and development efforts.
Cash, cash equivalents, and marketable investment securities increased $57.3 million, or 65%, from $88.7 million at June 30, 1998, we pursued a plan2000 to dramatically increase our
sales force. Start-up expenses for the sales staff included training,
relocation, and sales supplies. For the fiscal year ended$146.0 million at June 30, 1999, we
maintained a steady, well-trained sales force which resulted2001. This increase in fewer selling
expenses. In addition,our cash, cash equivalents and marketable investment securities was primarily attributable to the sale of approximately $68.6 million of our Common Stock in private placements during the fiscal year, ended June 30, 1998, we incurred
significant expenses in defenseas well as receipt of approximately $10 million from license fees and milestone payments. As a result of our intellectual property, including the
successful settlement of legal actions with OncorMed. Such expenses were
drastically reduced during the fiscal year ended June 30, 1999.
Interestincreased cash position, interest income for the fiscal year ended June 30, 1999 decreased2001 was $6.9 million compared to $2,348,827 from $3,223,683$3.2 million for the fiscal year ended June 30, 1998. Cash, cash
equivalents, and marketable investment securities were $38,926,459 at June 30,
1999 as compared to $53,109,493 at June 30, 1998. This decrease in cash, cash
equivalents and marketable investment securities was attributable to
expenditures incurred2000, an increase of 114%. The loss on disposition of
31
assets of $0.3 million in the ordinary course of business and has resulted in
reduced interest income. Interest expense for thefiscal year ended June 30, 1999,
amounting to $6,278,2001 was due entirely to borrowings underprimarily the Company's
equipment financing facility.
result of our retiring unproductive assets.
LIQUIDITY AND CAPITAL RESOURCES
Net cash provided byused in operating activities was $17,163,535$16.3 million during the fiscal year ended June 30, 2000 as2002 compared to $14,137,559$3.8 million used in operating activities during the prior fiscal year. Trade receivables increased $1,100,765$3.8 million between June 30, 19982001 and June 30, 1999.
This increase is2002, primarily attributabledue to the 68%57% increase in molecular
diagnostic revenuepredictive medicine sales during fiscal 2000. Trade receivables as a percentage of
molecular diagnostic revenue continues to be in the 25-27% range for bothsame period. Prepaid expenses increased $0.6 million between June 30, 20002001 and June 30, 1999. Other receivables decreased $1,456,749 during the
fiscal year ended June 30, 2000 primarily as a result of our receipt of
collaborative partner payments for research work performed in the prior year.
Prepaid expenses increased $2,056,284 during the fiscal year ended June 30,
2000. The increase is primarily2002 due to advance payments to purchase lab supplies at a discount, advanced royalties, and insurance premiums. Accounts payable and
accrued expenses increased by $4,495,772 during the fiscal year endeddiscount. Related party receivables decreased $1.8 million between June 30, 20002001 and June 30, 2002, due to reimbursement for services provided to Myriad Proteomics. Other assets increased $0.6 million between June 30, 2001 and June 30, 2002, primarily as a resultdue to the acquisition of our effortspatents. Related party payables increased $1.0 million between June 30, 2001 and June 30, 20002 due to manage cash flows and extend
payment terms as well as increased accrued year end payroll related expenses,
and accrued broker fees.equipment purchased from Myriad Proteomics. Deferred revenue, representing the difference in collaborative payments we have received and research revenue which we have
recognized, increaseddecreased by $18,837,682$5.4 million between June 30, 2001 and June 30, 2002.
Our investing activities provided cash of $38.1 million during the fiscal year ended June 30, 2000
in large part due to upfront payments from NADII2002 and Hitachi as well as
marketing license fees we received from recent molecular diagnostic license
agreements.
The Company's investing activities used $4,335,576 of cash in the fiscal
year ended June 30, 2000 and provided cash of $4,506,423 in$85.1 million during the prior fiscal year
ended June 30, 1999.year. Investing activities were comprised primarily of changes to marketable investment securities and capital expenditures for research equipment, office furniture, and facility improvements
and changes to marketable investment securities.equipment. During the fiscal year ended June 30, 2000,2002, we investedshifted a portion of our investments from marketable investment securities to cash received from private equity placements,
collaborative research payments, upfront payments, milestone payments, marketing
license payments and molecular diagnostic salescash equivalents due to short-term and long-term
investmentschanges in order to take advantage of higher interest rates. These funds
were investedDuring the fiscal year ended June 30, 2002 other assets increased $2.5 million due to an investment in accordance witha privately held pharmaceutical company, and was partially offset $0.6 million due to the sale of part of our investment guidelines as established by our
Boardin a separate privately held biotechnology company. Additional investing activities included capital expenditures of Directors.$6.9 million for research equipment and facility improvements.
Financing activities provided $37,981,833$3.4 million during the fiscal year ended June 30, 2000. We recognized proceeds from three separate financings during2002, due to the year.
In September 1999,exercise of stock options.
On November 9, 2001, we entered intofiled a Subscription Agreement pursuant to which we
sold 710,000 shares our unregistered Common Stock for a purchase price of
$4,987,750. In conjunction with the Subscription Agreement, we issued a 3-year
warrant to purchase an additional 35,500 shares at a premium of 10%. In October
1999, we entered into a Securities Purchase Agreement and a Standstill Agreement
with Schering Berlin to sell to Schering Berlin 606,060 shares of unregistered
Common Stock. Schering Berlin agreed to acquire the shares for an aggregate
purchase price of $5,000,0000. In June 2000, we sold 600,000 shares of
unregistered Common Stock to a European pharmaceutical company that resulted in
proceeds of $24,000,000. We have no obligation to register the shares associated
with the September 1999 Financing and the June 2000 FinancingForm S-3 shelf registration statement with the Securities and Exchange Commission. Additional cash was provided fromCommission for the exercisesale of stock options duringup to $250 million of various types of securities, which the fiscal year ended June 30, 2000.
22
We believe that with our existing capital resources, we will have adequate funds to maintain our current and planned operations for at least the next two years, although no assurance can be given that changes will not occur that would consume available capital resources before such time. Our future capital requirements will be substantial and will depend on many factors, including:
.
- •
- the progress of our preclinical and clinical activities;
- •
- the progress of our research and development programs;
. - •
- the progress of our drug discovery and drug development programs;
. - •
- the cost of developing and launching additional
molecular diagnostic tests; .predictive medicine products; - •
- the costs of filing, prosecuting and enforcing patent claims;
. - •
- the costs associated with competing technological and market developments;
. - •
- the payments received under collaborative agreements and changes in collaborative research relationships;
. - •
- the costs associated with potential commercialization of our
genediscoveries, if any, including the development of manufacturing, marketing and sales capabilities; and.
32
- •
- the cost and availability of third-party financing for capital expenditures and administrative and legal expenses.
Because of our significant long-term capital requirements, we intend to raise funds when conditions are favorable, even if we do not have an immediate need for additional capital at such time.
Subsequent Events
In August 2000, we announced
Effects of Inflation
We do not believe that inflation has had a stock split to be effected inmaterial impact on our business, sales, or operating results during the form of a
stock dividend of one new share for each share of Common Stock outstanding. The
record date for the split was set as August 28, 2000 and the distribution date
was set as September 11, 2000. All references to the number of common shares and
per share amounts in this Annual Report on Form 10-K have been restated to
reflect the effect of the split for all periods presented.
In August 2000, we also closed on an equity financing with Acqua Wellington
North American Equities Fund, Ltd. We sold 350,000 shares of our Common Stock to
Acqua Wellington for gross proceeds in excess of $22 million. We have agreed to
register these shares with the Securities and Exchange Commission.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The Company maintains
We maintain an investment portfolio in accordance with itsour Investment Policy. The primary objectives of the Company'sour Investment Policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. The Company'sOur Investment Policy specifies credit quality standards for the Company'sour investments and limits the amount of credit exposure to any single issue, issuer or type of investment.
The Company's
Our investments consist of securities of various types and maturities of three years or less, with a maximum average maturity of 12 months. These securities are classified either as available-for-sale or held-to-
maturity.held-to-maturity. Available-for-sale securities are recorded on the balance sheet at fair market value with unrealized gains or losses reported as part of accumulated other comprehensive loss. Held-to-maturity securities are recorded at amortized cost, adjusted for the amortization or accretion of premiums or discounts. Gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned. A decline in the market value of any available-for-sale or held-to-
maturityheld-to-maturity security below
23
The securities held in the Company'sour investment portfolio are subject to interest rate risk. Changes in interest rates affect the fair market value of the available-for-salemarketable investment securities. After a review of the Company'sour marketable securities as of June 30, 2000, the Company has2002, we have determined that in the event of a hypothetical ten percent increase in interest rates, the resulting decrease in fair market value of the Company'sour marketable investment securities would be insignificant to the consolidated financial statements as a whole.
Certain Factors That May Affect Future Results of Operations
Some of the matters discussed in this
The Securities and Exchange Commission encourages companies to disclose forward-looking information so that investors can better understand a company's future prospects and make informed investment decisions. This Annual Report on Form 10-K include
forward-looking statements as that term is defined incontains such "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases you canThese statements may be made directly in this Annual Report, and they may also be made a part of this Annual Report by reference to other documents filed with the Securities and Exchange Commission, which is known as "incorporation by reference."
Words such as "may," "anticipate," "estimate," "expects," "projects," "intends," "plans," "believes" and words and terms of similar substance used in connection with any discussion of future operating or financial performance, identify forward-looking statements by terminology such as "may", "will", "should", "potential",
"continue", "expects", "anticipates", "intends", "plans", "believes",
"estimates", and similar expressions. We have based thesestatements. All forward-looking statements on our currentare management's present expectations and projections aboutof future events. We
caution investors that actual results may vary significantlyevents and are subject to a number of factorsrisks and uncertainties including, but not limitedthat could cause actual results to differ materially from those described in the following: intense competition relatedforward-looking statements. These risks and uncertainties include, among other things: our inability to the discovery of disease-related genesfurther identify, develop and achieve commercial success for new products and technologies; the possibility that others may discover,of delays in the research and we may not be abledevelopment necessary to gain
rights with respect to, genes important to the establishment of a successful
genetic testing business; difficulties inherentselect drug development candidates and delays in developing genetic tests once
genes have been discovered; our limited experience in operating a genetic
testing laboratory; our limited marketing and sales experience and
33
clinical trials; the risk that tests which we have or may developclinical trials may not be marketed at acceptable prices or
receive commercial acceptanceresult in marketable products; the marketsrisk that we are targeting or expectmay be unable to target; uncertainty as to whether there will exist adequate reimbursement for
our services from government, private healthcare insurerssuccessfully finance and third-party
payors; uncertainties as to the extent of future government regulation of our
business; uncertainties as to whether we and our collaborators will be
successful in developing and obtainingsecure regulatory approval for,of and commercial
acceptancemarket our drug candidates; our dependence upon pharmaceutical and biotechnology collaborations; the levels and timing of therapeutics based on the discovery of disease-related genes and
proteins;payments under our collaborative agreements; uncertainties as toabout our ability to develop therapeutic lead compounds,
which is aobtain new business area for us;corporate collaborations and acquire new technologies on satisfactory terms, if at all; the risk that markets will not exist
for therapeutic lead compounds that we develop or if such markets exist, that we
will not be abledevelopment of competing systems; our ability to sell compounds which we develop at acceptable prices.
These forward-looking statements are made asprotect our proprietary technologies; patent-infringement claims; and risks of new, changing and competitive technologies and regulations in the dateUnited States and internationally. Please also see the discussion of risks and uncertainties under "Risk Factors" in Item 1 of this report, and we
assume no obligation to update them or to explain the reasons why actual results
may differ.Report.
In light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained in this Annual Report on Form 10-Kor in any document incorporated by reference might not occur. Stockholders are cautioned not to place undue reliance on the forward-looking statements, which speak only of the date of this Annual Report or the date of the document incorporated by reference in this Annual Report. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to the Company or to any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
34
MYRIAD GENETICS, INC.
| Index to Financial Statements | Number | |
|---|---|---|
| Independent Auditors' | F-1 | |
| Consolidated Balance Sheets as of June 30, | F-2 | |
| Consolidated Statements of Operations for the Years Ended June 30, | F-3 | |
| Consolidated Statements of Stockholders' Equity and Comprehensive Loss for the Years Ended June 30, | F-4 | |
| Consolidated Statements of Cash Flows for the Years Ended June 30, | F-5 | |
| Notes to Consolidated Financial | F-6 |
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
25
PART III
Item 10. DIRECTORS AND OFFICERS OF THE REGISTRANT
The response to this item is incorporated by reference from the discussion
responsive thereto under the captions "Management" and "Section 16(a) Beneficial
Ownership Reporting Compliance" in the Company's Proxy Statement for the 2000
Annual Meeting of Stockholders.
Item 11. EXECUTIVE COMPENSATION
The response to this item is incorporated by reference from the discussion
responsive thereto under the caption "Executive Compensation" in the Company's
Proxy Statement for the 2000 Annual Meeting of Stockholders.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The response to this item is incorporated by reference from the discussion
responsive thereto under the caption "Share Ownership" in the Company's Proxy
Statement for the 2000 Annual Meeting of Stockholders.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
The response to this item is incorporated by reference from the discussion
responsive thereto under the caption "Executive Compensation--Employment
Agreements, Termination of Employment and Change of Control Arrangements" in the
Company's Proxy Statement for the 2000 Annual Meeting of Stockholders.
26
PART IV
Item 14. EXHIBITS, FINANCIAL STATEMENT SCHEDULES, AND REPORTS ON FORM 8-K
Item 14(a). The following documents are filed as part of this annual report
on Form 10-K.
Item 14(a)(1). See "Index to Consolidated Financial Statements and Financial
and (2) Statement Schedules" at Item 8 to this Annual Report on Form 10-
K. Other financial statement schedules have not been included
because they are not applicable or the information is included
in the financial statements or notes thereto.
Item 14(a)(3) Exhibits
--------
The following is a list of exhibits filed as part of this Annual
Report on Form 10-K.
Exhibit
Number Description
- ------ -----------
(3.1)p - Restated Certificate of Incorporation of the Registrant (Filed
as Exhibit 3.1)
(3.1 (a)) - Restated Certificate of Incorporation of the Registrant
(3.1 (b)) - Certificate of Amendment of Restated Certificate of
Incorporation
(3.2)p - Restated By-Laws of the Registrant (Filed as Exhibit 3.2)
(4.1)p - See Exhibits 3.1, 3.1(a), 3.1(b) and 3.2 (Filed as Exhibit 4.1)
(4.2)* - Form of Common Stock Certificate (Filed as Exhibit 4.2)
(10.1)z$ - 1992 Employee, Director and Consultant Stock Option Plan as
amended and restated September 24, 1999 (Filed as Exhibit 10.1)
(10.2)*$ - Employee Stock Purchase Plan (Filed as Exhibit 10.2)
(10.3)*$ - Employment Agreement between Myriad Genetics, Inc., Myriad
Genetic Laboratories, Inc. and Peter D. Meldrum, dated May 15,
1993 (Filed as Exhibit 10.3)
(10.4)*$ - Employment Agreement between Myriad Genetics, Inc., Myriad
Genetic Laboratories, Inc. and Mark H. Skolnick, Ph.D., dated
January 1, 1994 (Filed as Exhibit 10.4)
(10.5)*$ - Employment Agreement between Myriad Genetics, Inc., Myriad
Genetic Laboratories, Inc. and Jay M. Moyes, dated July 12,
1993 (Filed as Exhibit 10.5)
(10.6)* - Form of Registration Agreement executed in connection with the
private placement of Series A Preferred Stock (Filed as Exhibit
10.6)
(10.7)* - Stock Purchase Agreement for Series C Convertible Preferred
Stock between the Registrant and Novartis Corporation, dated
April 27, 1995 (Filed as Exhibit 10.7)
(10.8)* - Standstill Agreement between the Registrant and Novartis
Corporation, dated April 27, 1995 (Filed as Exhibit 10.8)
(10.9)* - Voting Agreement between the Registrant and Novartis
Corporation, dated April 27, 1995 (Filed as Exhibit 10.9)
(10.10)# - Collaborative Research and License Agreement between the
Registrant and Novartis Corporation, dated April 27, 1995
(Cardiovascular Diseases) (Filed as Exhibit 10.10)
(10.11)# - Research Collaboration and License Agreement between the
Registrant, Eli Lilly & Company and Hybritech Incorporated,
dated August 1, 1992 (Breast Cancer--BRCA1) (Filed as Exhibit
10.11)
(10.12)# - Collaborative Agreement between the Registrant and Hybritech
Incorporated, dated March 5, 1993 (BRCA1 Test Kits) (Filed as
Exhibit 10.12)
(10.13)# - Exclusive License Agreement between the Registrant and the
University of Utah Research Foundation, dated August 4 1993, as
amended (Genes Predisposing to Cancer) (Filed as Exhibit 10.14)
(10.14)# - Standard Research Agreement and Form of License Agreement
between the Registrant and the University of Utah, effective
January 1, 1993, as amended (Genes Predisposing to Cancer)
(Filed as Exhibit 10.14)
(10.15)# - Exclusive License Agreement between the Registrant and the
University of Utah Research Foundation, dated August 4, 1993
(Angiotensinogen Variants and Predisposition to Hypertension)
(Filed as Exhibit 10.15)
(10.16)# - Exclusive License Agreement between the Registrant and the
University of Utah Research Foundation, dated June 21, 1994
(MTS1 or p16) (Filed as Exhibit 10.16)
27
(10.17)# - Exclusive License Agreement between the Registrant and the
University of Utah Research Foundation, dated November 23, 1994
(Breast Cancer--BRCA2) (Filed as Exhibit 10.17)
(10.18)# - Standard Research Agreement dated May 1, 1995 between the
Registrant and the University of Utah (Cardiovascular Disorders
and Coronary Heart Disease Database) (Filed as Exhibit 10.18)
(10.19)# - Exclusive License Agreement dated May 1, 1995 between the
Registrant and the University of Utah Research Foundation
(Cardiovascular Disorders and Coronary Heart Disease Database)
(Filed as Exhibit 10.19)
(10.20)# - Standard Research Agreement dated July 31, 1995 between the
Registrant and the University of Utah (Obesity Database) (Filed
as Exhibit 10.20)
(10.21)# - Exclusive License Agreement dated July 31, 1995 between the
Registrant and the University of Utah Research Foundation
(Obesity Database) (Filed as Exhibit 10.21)
(10.22)# - Co-Exclusive License Agreement among the Registrant, the
University of Utah Research Foundation and Institut National de
la Sante et de la Recherche Medicale, dated October 6, 1993
(Angiotensinogen and Predisposition to Essential Hypertension)
(Filed as Exhibit 10.22)
(10.23)# - License Agreement between the Registrant and California
Institute of Technology, dated April 21, 1994 (MTS1 or p16)
(Filed as Exhibit 10.23)
(10.24)* - Research Agreement between the Registrant and California
Institute of Technology, dated June 3, 1994 (MTS1 or p16)
(Filed as Exhibit 10.24)
(10.25)* - Stock Purchase Agreement for Series D Convertible Preferred
Stock between the Registrant and Bayer Corporation, dated
September 11, 1995 (Filed as Exhibit 10.25)
(10.26)* - Standstill Agreement between the Registrant and Bayer
Corporation, dated September 11, 1995 (Filed as Exhibit 10.26)
(10.27)* - Voting Agreement between the Registrant and Bayer Corporation,
dated September 11, 1995 (Filed as Exhibit 10.27)
(10.28)# - Collaborative Research and License Agreement between the
Registrant and Bayer Corporation, dated September 11, 1995
(Filed as Exhibit 10.28)
(10.29)# - Standard Research Agreement between the Registrant and IHC
Health Services, Inc., dated as of September 1, 1995 (Filed as
Exhibit 10.29)
(10.30)@ - Research Agreement between the Registrant and IHC Health
Services, Inc., dated as of June 24, 1996
(10.31)!@ - Patent and Technology License Agreement dated September 26,
1996 among the Board of Regents of the University of Texas
System, the University of Texas M.D. Anderson Cancer Center and
the Registrant (Filed as Exhibit 10.1)
(10.32)! - Lease Agreement, dated October 12, 1995, between the Boyer
Research Park Associates V, by its general partner, the Boyer
Company and the Registrant (Filed as Exhibit 10.2)
(10.33)! - Amendment to Lease Agreement, dated March 29, 1996 between the
Boyer Research Park Associates V, by its general partner, the
Boyer Company and the Registrant (Filed as Exhibit 10.3)
(10.34)!@ - Letter Agreement, dated March 4, 1996, among the University of
Utah, Genetic Epidemiology and the Registrant regarding
Extension of Standard Research agreement and Form of License
Agreement between the Registrant and the University of Utah,
effective January 1, 1993, as amended (Genes Predisposing to
Cancer) (Filed as Exhibit 10.4)
(10.35)q@ - Patent and Technology License Agreement dated December 2, 1996
among the Board of Regents of the University of Texas System,
the University of Texas M.D. Anderson Cancer Center and the
Registrant (Filed as Exhibit 10.1)
(10.36)=@ - Collaborative Research and License Agreement among the
Registrant, Schering Corporation and Schering-Plough, Ltd.,
dated April 22, 1997 (Prostate and Other Cancers) (Filed as
Exhibit 10.36)
(10.37)= - Standstill Agreement between the Registrant and Schering
Corporation, dated April 22, 1997 (Filed as Exhibit 10.37)
(10.38)= - Stock Purchase Agreement for Common Stock between the
Registrant and Schering Corporation, dated April 22, 1997
(Filed as Exhibit 10.38)
(10.39)++@ - Standard Research Agreement between the Company and Valley
Mental Health dated September 1, 1997 (central nervous system
disorders) (Filed as Exhibit 10.1)
(10.40)++ - International Swap Dealers Association, Inc. Master Agreement
("ISDA Master Agreement") between the Registrant and Swiss Bank
Corporation, London Branch dated October 8, 1997 (Filed as
Exhibit 10.2)
(10.41)++ - Schedule to ISDA Master Agreement between the Registrant and
Swiss Bank Corporation, London Branch dated October 8, 1997
(Filed as Exhibit 10.3)
(10.42)++ - Confirmation for Contract A entered into pursuant to ISDA
Master Agreement between the Registrant
28
and Swiss Bank Corporation, London Branch dated October 8, 1997
(Filed as Exhibit 10.4)
(10.42)++ - Confirmation for Contract B entered into pursuant to ISDA
Master Agreement between the Registrant and Swiss Bank
Corporation, London Branch dated October 8, 1997 (Filed as
Exhibit 10.5)
(10.43)%@ - Amendment and Supplement to Collaborative Research and License
Agreement dated November 19, 1997 between Bayer Corporation and
the Registrant (Filed as Exhibit 10.1)
(10.44)k - Lease Agreement-Research Park Building Phase II, dated March 6,
1998, between the Research Park Associated VI, by its general
partner, the Boyer Company, L.C. and the Registrant
(10.45)& - Memorandum of Lease between the Company and Boyer Foothill
Associates, Ltd. dated August 24, 1998 (Filed as Exhibit 10.1)
(10.46)& - Memorandum of Lease between the Company and Boyer Research Park
Associates VI, L.C. dated August 24, 1998 (Filed as Exhibit
10.2)
(10.47)& - Subordination Agreement and Estoppel, Attornment and Non-
Disturbance Agreement (Lease to Deed of Trust) between the
Company and Wells Fargo Bank, National Association dated June
24, 1998 (Filed as Exhibit 10.3 )
(10.48)w - Master Lease Agreement dated December 31, 1998 between General
Electric Capital Corporation and the Company (Filed as Exhibit
10.1)
(10.49)w - Addendum No. 1 to Master Lease Agreement dated December 31,
1998 between General Electric Capital Corporation and the
Company (Filed as Exhibit 10.2)
(10.50)w - Addendum No. 2 to Master Lease Agreement dated December 31,
1998 between General Electric Capital Corporation and the
Company (Filed as Exhibit 10.3)
(10.51)w - Biotech Equipment Schedule Schedule No. 001 dated December 31,
1998 to Master Lease Agreement dated December 31, 1998 between
General Electric Corporation and the Company (Filed as Exhibit
10.4)
(10.52)w - Annex A to Equipment Schedule No. 001 to Master Lease Agreement
dated December 31, 1998 between General Electric Corporation
and the Company (Filed as Exhibit 10.5)
(10.53)w - Annex B to Equipment Schedule No. 001 to Master Lease Agreement
dated December 31, 1998 between General Electric Corporation
and the Company (Filed as Exhibit 10.6)
(10.54)w - Addendum to Schedule No. 001 to Master Lease Agreement dated as
of December 31, 1998 between General Electric Corporation and
the Company (Filed as Exhibit 10.7)
(10.55)w@ - Collaborative Research, License and Co-Promotion agreement
dated as of October 5, 1998 between Schering Aktiengesellschaft
and the Company (Filed as Exhibit 10.8)
(10.56)w@ - Collaborative ProNet Research and License Agreement dated as of
November 11, 1998 between Monsanto Company and the Company
(Filed as Exhibit 10.9)
(10.57)w@ - Letter Amendment to the Collaborative Research and License
Agreement dated as of November 30, 1998 between Bayer
Corporation and the Company (Filed as Exhibit 10.10)
(10.58)m@ - Collaboration and License Agreement between the Company and
Novartis Agricultural Discovery Institute, Inc. dated July 27,
1999 (Filed as Exhibit 10.1)
(10.59)m - Subscription Agreement between the Company and Peter Friedli
dated September 30, 1999 (Filed as Exhibit 10.2)
(10.60)m - Securities Purchase Agreement and Standstill Agreement between
the Company and Schering Berlin Venture Corporation dated
October 15, 1999 (Filed as Exhibit 10.3)
(10.61)f - Mater Lease Agreement dated October 25 between Comdisco
Laboratory and Scientific Group, a Division of Comdisco
Healthcare Group, Inc. and the Company (Filed as Exhibit 10.1)
(10.62)f - Addendum to the Master Lease Agreement dated October 25, 1999
between Comdisco Laboratory and Scientific Group, a Division of
Comdisco Healthcare Group, Inc. and the Company (Filed as
Exhibit 10.2)
(10.63)f - Amendment No. 1 to the Master Lease Agreement dated October 25,
1999 between Comdisco Laboratory and Scientific Group, a
Division of Comdisco Healthcare Group, Inc. and the Company
(Filed as Exhibit 10.3)
(10.64)f - Equpment Schedule No. SG01 dated November 10, 1999 to the
Master Lease Agreement dated October 25, 1999 between Comdisco
Laboratory and Scientific Group, a Division of Comdisco
Healthcare Group, Inc. and the Company (Filed as Exhibit 10.4)
(10.65) - Purchase Agreement dated as of August 28, 2000 between the
Registrant and Acqua Wellington North American Equities Fund,
Ltd.
(10.66) - Registration Rights Agreement dated as of August 28, 2000
between the Registrant and Acqua Wellington North American
Equities Fund, Ltd.
(21.1) - List of Subsidiaries of the Registrant
(23.1) - Consent of KPMG LLP
29
(27.1) - Financial Data Schedule
* Previously filed with the Commission as Exhibits to, and incorporated
herein by reference from, the Company's Registration Statement filed on
Form S-1, File No. 33-95970
# Previously filed with the Commission as Exhibits to, and incorporated
herein by reference from, the Company's Registration Statement filed on
Form S-1, File No. 33-95970, and for which Confidential Treatment has
been granted by the Securities and Exchange Commission as to certain
portions.
@ Confidential Treatment requested as to certain portions, which portions
are omitted and filed separately with the Commission.
p Previously filed and incorporated herein by reference from the Form 10-
Q for the period ending September 30, 1995.
$ Management contract or compensatory plan or arrangement required to be
filed as an exhibit to this Form 10-K pursuant to Item 14(c) of this
report.
! Previously filed and incorporated herein by reference from the Form 10-
Q for the period ending September 30, 1996.
q Previously filed and incorporated herein by reference from the Form 10-
Q for the period ending December 31, 1996.
= Previously filed and incorporated herein by reference from the Form 10-
K for the period ending June 30, 1997.
++ Previously filed and incorporated herein by reference from the Form 10-
Q for the period ending September 30, 1997.
% Previously filed and incorporated herein by reference from the Form 10-
Q for the period ending December 31, 1997.
z Previously filed and incorporated herein by reference from the
Company's Registration Statement filed on Form S-8, effective December
22, 1999, File No. 333-93363.
k Previously filed and incorporated herein by reference from the Form 10-
K for the period ending June 30, 1998.
& Previously filed and incorporated herein by reference from the Form 10-
Q for the period ending September 30, 1998.
w Previously filed and incorporated herein by reference from the Form 10-
Q for the period ending December 31, 1998.
m Previously filed and incorporated herein by reference from the Form 10-
Q for the period ending September 30, 1999.
f Previously filed and incorporated herein by reference from the Form 10-
Q for the period ending December 31, 1999.
Where a document is incorporated by reference from a previous filing, the
Exhibit number of the document in that previous filing is indicated in
parentheses after the description of such document.
Item 14(b) Reports on Form 8-K
-------------------
30
No reports on Form 8-K were filed during the last quarter of the year ended
June 30, 2000.
31
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities
Exchange Act of 1934, the Registrant has duly caused this report to be signed on
its behalf by the undersigned, thereunto duly authorized, in Salt Lake City,
Utah on September 13, 2000.
MYRIAD GENETICS, INC.
By: /s/ Peter D. Meldrum
--------------------
Peter D. Meldrum
President and Chief Executive Officer
Pursuant to the requirements of the Securities Exchange Act of 1934, this
report has been signed below by the following persons on behalf of the
registrant and in the capacities indicated below and on the dates indicated.
Signatures Title Date
---------- ----- ----
By: /s/ Peter D. Meldrum President and Chief Executive September 13, 2000
--------------------------- Officer and Director
Peter D. Meldrum (principal executive officer)
By: /s/ Jay M. Moyes Vice President of Finance September 13, 2000
--------------------------- (principal financial and
Jay M. Moyes accounting officer)
By: /s/ John J. Horan Chairman of the Board September 13, 2000
---------------------------
John J. Horan
By: /s/ Walter Gilbert Vice Chairman of the Board September 13, 2000
---------------------------
Walter Gilbert, Ph.D.
By: /s/ Mark H. Skolnick Chief Scientific Officer and September 13, 2000
--------------------------- Director
Mark H. Skolnick, Ph.D.
By: /s/ Arthur H. Hayes, Jr. Director September 13, 2000
---------------------------
Arthur H. Hayes, Jr., M.D.
By: /s/ Dale A. Stringfellow Director September 13, 2000
---------------------------
Dale A. Stringfellow, Ph.D.
By: /s/ Alan J. Main Director September 13, 2000
---------------------------
Alan J. Main, Ph.D.
By: /s/ Michael J. Berendt Director September 13, 2000
---------------------------
Michael J. Berendt, Ph.D.
By: /s/ Linda S. Wilson Director September 13, 2000
---------------------------
Linda S. Wilson, Ph.D.
35
The Board of Directors and Stockholders
Myriad Genetics, Inc.:
We have audited the accompanying consolidated balance sheets of Myriad Genetics, Inc. and subsidiaries as of June 30, 20002002 and 1999,2001, and the related consolidated statements of operations, stockholders' equity and comprehensive loss, and cash flows for each of the years in the three-year period ended June 30, 2000.2002. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Myriad Genetics, Inc. and subsidiaries as of June 30, 20002002 and 1999,2001, and the results of their operations and their cash flows for each of the years in the three-year period ended June 30, 2000,2002, in conformity with accounting principles generally accepted in the United States of America.
KPMG LLP
Salt Lake City, Utah KPMG LLP
August 22, 2000
23, 2002
F-1
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Balance Sheets
June 30,
--------------------------------------
Assets 2000 1999
---------------- -----------------
Current assets:
Cash and cash equivalents $ 56,214,736 5,404,944
Marketable investment securities 24,286,955 4,477,138
Prepaid expenses 2,678,984 622,700
Trade accounts receivables, less allowance for doubtful
accounts of $145,000 in 2000 and $73,439 in 1999 2,352,154 1,322,950
Other receivables 398,947 1,855,696
---------------- -----------------
Total current assets 85,931,776 13,683,428
---------------- -----------------
Equipment and leasehold improvements:
Equipment 16,965,545 13,351,229
Leasehold improvements 4,005,729 3,520,253
---------------- -----------------
20,971,274 16,871,482
Less accumulated depreciation and amortization 9,719,556 6,871,981
---------------- -----------------
Net equipment and leasehold improvements 11,251,718 9,999,501
Long-term marketable investment securities 8,154,153 29,044,377
Other assets 1,037,658 823,634
---------------- -----------------
$ 106,375,305 53,550,940
================ =================
Liabilities and Stockholders' Equity
Current liabilities:
Accounts payable $ 4,262,359 2,917,810
Accrued liabilities 4,905,857 1,754,634
Deferred revenue 19,500,442 662,760
---------------- -----------------
28,668,658 5,335,204
---------------- -----------------
Commitments and contingencies
Stockholders' equity:
Preferred stock, $0.01 par value. Authorized 5,000,000 shares;
no shares issued and outstanding -- --
Common stock, $0.01 par value. Authorized 60,000,000 shares;
issued and outstanding 21,866,482 shares in 2000 and 18,857,464
shares in 1999 218,666 188,575
Additional paid-in capital 130,235,403 92,283,661
Accumulated other comprehensive loss (85,440) (68,846)
Deferred compensation -- (247,774)
Accumulated deficit (52,661,982) (43,939,880)
---------------- -----------------
Total stockholders' equity 77,706,647 48,215,736
---------------- -----------------
$ 106,375,305 53,550,940
================ =================
June 30, 2002 and 2001
| | 2002 | 2001 | ||||||
|---|---|---|---|---|---|---|---|---|
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 61,066,953 | 35,936,817 | |||||
| Marketable investment securities | 12,007,946 | 91,282,481 | ||||||
| Prepaid expenses | 4,826,825 | 4,219,037 | ||||||
| Trade accounts receivable, less allowance for doubtful accounts of $505,000 in 2002 and $255,000 in 2001 | 7,233,162 | 3,634,370 | ||||||
| Other receivables | 219,601 | 314,571 | ||||||
| Related party receivables | — | 1,811,517 | ||||||
| Total current assets | 85,354,487 | 137,198,793 | ||||||
| Equipment and leasehold improvements: | ||||||||
| Equipment | 26,409,275 | 21,425,910 | ||||||
| Leasehold improvements | 5,383,989 | 3,721,345 | ||||||
| 31,793,264 | 25,147,255 | |||||||
| Less accumulated depreciation and amortization | 16,360,166 | 12,416,209 | ||||||
| Net equipment and leasehold improvements | 15,433,098 | 12,731,046 | ||||||
| Long-term marketable investment securities | 51,168,009 | 18,735,670 | ||||||
| Other assets | 5,434,486 | 3,479,846 | ||||||
| $ | 157,390,080 | 172,145,355 | ||||||
| Liabilities and Stockholders' Equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 9,461,575 | 9,657,385 | |||||
| Related party payable | 1,037,446 | — | ||||||
| Accrued liabilities | 3,591,189 | 3,082,799 | ||||||
| Deferred revenue | 14,430,370 | 19,843,373 | ||||||
| Total current liabilities | 28,520,580 | 32,583,557 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders' equity: | ||||||||
| Preferred stock, $0.01 par value. Authorized 5,000,000 shares; no shares issued and outstanding | — | — | ||||||
| Common stock, $0.01 par value. Authorized 60,000,000 shares; issued and outstanding 23,817,098 shares in 2002 and 23,441,659 shares in 2001 | 238,171 | 234,417 | ||||||
| Additional paid-in capital | 202,149,210 | 198,800,273 | ||||||
| Accumulated other comprehensive income | 307,964 | 363,583 | ||||||
| Accumulated deficit | (73,825,845 | ) | (59,836,475 | ) | ||||
| Total stockholders' equity | 128,869,500 | 139,561,798 | ||||||
| $ | 157,390,080 | 172,145,355 | ||||||
See accompanying notes to consolidated financial statements.
F-2
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Operations
Years ended June 30,
-----------------------------------------------------
2000 1999 1998
--------------- --------------- ---------------
Research revenue $ 25,219,766 20,093,057 20,999,598
Molecular diagnostic revenue 8,793,272 5,220,349 2,210,983
--------------- -------------- --------------
Total revenues 34,013,038 25,313,406 23,210,581
Costs and expenses:
Molecular diagnostic cost of revenue 3,986,473 3,066,354 1,391,368
Research and development expense 28,098,769 23,452,220 23,002,340
Selling, general, and administrative expenses 13,474,923 11,105,520 11,807,023
--------------- -------------- --------------
Total cost and expenses 45,560,165 37,624,094 36,200,731
--------------- -------------- --------------
Operating loss (11,547,127) (12,310,688) (12,990,150)
Other income (expense):
Interest income 3,208,506 2,348,827 3,223,683
Interest expense -- (6,278) (32,681)
Other (383,481) (27,314) 2,113
--------------- -------------- --------------
2,825,025 2,315,235 3,193,115
--------------- -------------- --------------
Net loss $ (8,722,102) (9,995,453) (9,797,035)
=============== ============== ==============
Basic and diluted loss per common share $ (0.43) (0.53) (0.53)
=============== ============== ==============
Basic and diluted weighted average shares outstanding 20,220,446 18,782,244 18,578,962
=============== ============== ==============
Years ended June 30, 2002, 2001, and 2000
| | 2002 | 2001 | 2000 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Research revenue | $ | 27,015,167 | 28,071,252 | 25,219,766 | ||||||
| Predictive medicine revenue | 26,821,332 | 17,091,139 | 8,793,272 | |||||||
| Total revenues | 53,836,499 | 45,162,391 | 34,013,038 | |||||||
| Costs and expenses: | ||||||||||
| Predictive medicine cost of revenue | 10,716,761 | 7,402,906 | 3,986,473 | |||||||
| Research and development expense | 36,294,669 | 33,818,144 | 28,098,769 | |||||||
| Selling, general, and administrative expense | 25,484,836 | 17,077,846 | 13,474,923 | |||||||
| Total costs and expenses | 72,496,266 | 58,298,896 | 45,560,165 | |||||||
| Operating loss | (18,659,767 | ) | (13,136,505 | ) | (11,547,127 | ) | ||||
| Other income (expense): | ||||||||||
| Interest income | 5,384,802 | 6,850,479 | 3,208,506 | |||||||
| Other | (214,405 | ) | (305,134 | ) | (383,481 | ) | ||||
| Loss before income taxes | (13,489,370 | ) | (6,591,160 | ) | (8,722,102 | ) | ||||
| Income taxes | 500,000 | 583,333 | — | |||||||
| Net loss | $ | (13,989,370 | ) | (7,174,493 | ) | (8,722,102 | ) | |||
| Basic and diluted loss per common share | $ | (0.59 | ) | (0.31 | ) | (0.43 | ) | |||
| Basic and diluted weighted average shares outstanding | 23,660,127 | 22,815,035 | 20,220,446 | |||||||
See accompanying notes to consolidated financial statements.
F-3
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Stockholders' Equity and Comprehensive Loss
Years ended June 30, 2000, 1999,2002, 2001, and 1998
Accumu-
lated
other
Additi- compre- Compre-
tional hensive Deferred Accum- hensive Stock-
Common stock paid-in income compen- ulated income holders'
----------------------
Shares Amount capital (loss) sation deficit (loss) equity
----------- ---------- ----------- --------- ----------- ------------ ------------- -----------
Balances at
June 30, 1997 18,445,104 184,451 91,513,514 5,382 (1,376,980) (24,147,392) 66,178,975
Issuance of
common stock for
cash upon exercise
of options and
warrants 211,408 2,114 392,071 -- -- -- 394,185
Issuance of common
stock for cash 18,490 185 178,074 -- -- -- 178,259
Amortization of
deferred
compensation -- -- -- -- 530,534 -- 530,534
Forfeiture of deferred
compensation -- -- (270,000) -- 270,000 -- --
Net loss -- -- -- -- -- (9,797,035) (9,797,035) (9,797,035)
Unrealized gains (losses)
on marketable
investment securities:
Unrealized holding
gains arising
during period -- -- -- -- -- -- 13,064 --
Less: classification
adjustment for
gains included
in net loss -- -- -- -- -- -- (16,969) --
------------
Other comprehensive loss -- -- -- (3,905) -- -- (3,905) (3,905)
------------
Comprehensive loss -- -- -- -- -- -- (9,800,940) --
============
----------- ---------- ----------- --------- ----------- ------------ -----------
Balances at
June 30, 1998 18,675,002 186,750 91,813,659 1,477 (576,446) (33,944,427) 57,481,013
Issuance of
common stock for
cash upon exercise
of options and warrants 137,654 1,377 364,918 -- -- -- 366,295
Issuance of common
stock for cash 44,808 448 203,146 -- -- -- 203,594
Amortization of
deferred compensation -- -- -- -- 230,610 -- 230,610
Forfeiture of deferred
compensation -- -- (98,062) -- 98,062 -- --
Net loss -- -- -- -- -- (9,995,453) (9,995,453) (9,995,453)
Unrealized losses
on marketable
investment securities:
Unrealized holding
losses arising
during period -- -- -- -- -- -- (115,287) --
Less: classification
adjustment for
losses included
in net loss -- -- -- -- -- -- 44,964 --
------------
Other comprehensive loss -- -- -- (70,323) -- -- (70,323) (70,323)
------------
Comprehensive loss -- -- -- -- -- -- (10,065,776) --
============
----------- ---------- ----------- --------- ----------- ------------ -----------
Balances at
June 30, 1999 18,857,464 188,575 92,283,661 (68,846) (247,774) (43,939,880) 48,215,736
F-4 (Continued)
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Stockholders' Equity and Comprehensive Loss
Years ended June 30, 2000 1999, and 1998
Accumu-
lated
other
Additi- compre- Compre-
tional hensive Deferred Accum- hensive Stock-
Common stock paid-in income compen- ulated income holders'
---------------------
Shares Amount capital (loss) sation deficit (loss) equity
---------- --------- ----------- --------- ---------- ---------- ---------- ----------
Issuance of
common stock for
cash upon exercise
of options and warrants 1,092,958 $ 10,930 6,525,622 -- -- -- -- 6,536,552
Issuance of common
stock for cash, net of
offering costs 1,916,060 19,161 31,426,120 -- -- -- -- 31,445,281
Amortization of
deferred compensation -- -- -- -- 247,774 -- -- 247,774
-- --
Net loss -- -- -- -- -- (8,722,102) (8,722,102) (8,722,102)
Unrealized losses
on marketable
investment securities:
Unrealized holding
losses arising
during period -- -- -- -- -- -- (63,638) --
Less: classification
adjustment for
losses included
in net loss -- -- -- -- -- -- 47,044
----------
Other comprehensive loss -- -- -- (16,594) -- -- (16,594) (16,594)
----------
Comprehensive loss -- -- -- -- -- -- (8,738,696) --
==========
---------- --------- ----------- --------- ---------- ---------- ----------
Balances at
June 30, 2000 21,866,482 $ 218,666 130,235,403 (85,440) -- (52,661,982) 77,706,647
========== ========= =========== ========= ========== ========== ==========
| | Common stock | | Accumulated other comprehensive income (loss) | | | | | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Additional paid-in capital | Deferred compensation | Accumulated deficit | Comprehensive income (loss) | Stockholders' equity | |||||||||||||||
| | Shares | Amount | ||||||||||||||||||
| Balances at June 30, 1999 | 18,857,464 | $ | 188,575 | 92,283,661 | (68,846 | ) | (247,774 | ) | (43,939,880 | ) | 48,215,736 | |||||||||
| Issuance of common stock for cash upon exercise of options and warrants | 1,092,958 | 10,930 | 6,525,622 | — | — | — | — | 6,536,552 | ||||||||||||
| Issuance of common stock for cash, net of offering costs | 1,916,060 | 19,161 | 31,426,120 | — | — | — | — | 31,445,281 | ||||||||||||
| Amortization of deferred compensation | — | — | — | — | 247,774 | — | — | 247,774 | ||||||||||||
| Net loss | — | — | — | — | — | (8,722,102 | ) | (8,722,102 | ) | (8,722,102 | ) | |||||||||
| Unrealized gains (losses) on marketable investment securities: | ||||||||||||||||||||
| Unrealized holding losses arising during year | — | — | — | — | — | — | (63,638 | ) | — | |||||||||||
| Less classification adjustment for losses included in net loss | — | — | — | — | — | — | 47,044 | — | ||||||||||||
| Other comprehensive loss | — | — | — | (16,594 | ) | — | — | (16,594 | ) | (16,594 | ) | |||||||||
| Comprehensive loss | — | — | — | — | — | — | $ | (8,738,696 | ) | |||||||||||
| Balances at June 30, 2000 | 21,866,482 | 218,666 | 130,235,403 | (85,440 | ) | — | (52,661,982 | ) | 77,706,647 | |||||||||||
| Issuance of common stock for cash upon exercise of options and warrants | 811,219 | 8,112 | 4,960,754 | — | — | — | — | 4,968,866 | ||||||||||||
| Issuance of common stock for cash, net of offering costs | 763,958 | 7,639 | 63,604,116 | — | — | — | — | 63,611,755 | ||||||||||||
| Net loss | — | — | — | — | — | (7,174,493 | ) | (7,174,493 | ) | (7,174,493 | ) | |||||||||
| Unrealized gains (losses) on marketable investment securities: | ||||||||||||||||||||
| Unrealized holding gains arising during year | — | — | — | — | — | 449,023 | — | |||||||||||||
| Less classification adjustment for losses included in net loss | — | — | — | — | — | — | — | — | ||||||||||||
| Other comprehensive gain | — | — | — | 449,023 | — | — | 449,023 | 449,023 | ||||||||||||
| Comprehensive loss | — | — | — | — | — | — | $ | (6,725,470 | ) | — | ||||||||||
| Balances at June 30, 2001 | 23,441,659 | 234,417 | 198,800,273 | 363,583 | — | (59,836,475 | ) | 139,561,798 | ||||||||||||
| Issuance of common stock for cash | 375,439 | $ | 3,754 | 3,348,937 | — | — | — | — | 3,352,691 | |||||||||||
| Net loss | — | — | — | — | — | (13,989,370 | ) | (13,989,370 | ) | (13,989,370 | ) | |||||||||
| Unrealized gains (losses) on marketable investment securities: | ||||||||||||||||||||
| Unrealized holding losses arising during period | — | — | — | — | — | — | (63,515 | ) | — | |||||||||||
| Less classification adjustment for gains included in net loss | — | — | — | — | — | — | 7,896 | — | ||||||||||||
| Other comprehensive loss | — | — | — | (55,619 | ) | — | — | (55,619 | ) | (55,619 | ) | |||||||||
| Comprehensive loss | — | — | — | — | — | — | $ | (14,044,989 | ) | — | ||||||||||
| Balances at June 30, 2002 | 23,817,098 | $ | 238,171 | 202,149,210 | 307,964 | — | (73,825,845 | ) | 128,869,500 | |||||||||||
See accompanying notes to consolidated financial statements.
F-5
F-4
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Cash Flows
Years ended June 30,
------------------------------------------------
2000 1999 1998
-------------- -------------- --------------
Cash flows from operating activities:
Net loss $ (8,722,102) (9,995,453) (9,797,035)
Adjustments to reconcile net loss to net cash provided by
(used in) operating activities:
Depreciation and amortization 3,284,734 3,223,779 3,272,936
Loss (gain) on sale of equipment 383,481 (17,650) 14,856
Loss (gain) on sale of investment securities 47,044 44,964 (16,969)
Bad debt expense 71,561 7,439 66,000
Changes in operating assets:
Trade receivables (1,100,765) (859,062) (354,161)
Prepaid expenses (2,056,284) (356,021) 179,581
Other receivables 1,456,749 (1,738,643) 177,914
Other assets 465,663 -- (941,405)
Accounts payable and accrued expenses 4,495,772 (2,387,557) 3,346,712
Deferred revenue 18,837,682 (2,059,355) (2,977,312)
-------------- -------------- --------------
Net cash provided by (used in) operating activities 17,163,535 (14,137,559) (7,028,883)
-------------- -------------- --------------
Cash flows from investing activities:
Proceeds from sale of equipment 14,851 3,604,579 4,133
Capital expenditures (4,617,196) (3,975,813) (3,185,906)
Investment in biotechnology company (750,000) -- --
Purchase of investment securities held-to-maturity (4,126,628) (17,462,407) (117,237,699)
Maturities of investment securities held-to-maturity 5,957,410 20,001,804 117,100,138
Purchase of investment securities available-for-sale (19,857,144) (274,244,194 (723,380,886)
Sale of investment securities available-for-sale 19,043,131 276,582,454 724,018,727
-------------- -------------- --------------
Net cash provided by (used in) investing activities (4,335,576) 4,506,423 (2,681,493)
-------------- -------------- --------------
Cash flows from financing activities:
Payments on notes payable -- (128,843) (342,797)
Net proceeds from issuance of common stock 37,981,833 569,889 572,444
-------------- -------------- --------------
Net cash provided by financing activities 37,981,833 441,046 229,647
-------------- -------------- --------------
Net increase (decrease) in cash and cash equivalents 50,809,792 (9,190,090) (9,480,729)
Cash and cash equivalents at beginning of year 5,404,944 14,595,034 24,075,763
-------------- -------------- --------------
Cash and cash equivalents at end of year $ 56,214,736 5,404,944 14,595,034
============== ============== ==============
Supplemental disclosure of cash flow information:
Interest paid $ -- 6,278 32,681
Supplemental disclosures of noncash investing and financing activities:
Decrease in additional paid-in capital as a result of
forfeitures of stock options $ -- (98,062) (270,000)
Fair value adjustment on marketable investment securities
charged to stockholders' equity (16,594) (70,323) (3,905)
Years ended June 30, 2002, 2001, and 2000
| | 2002 | 2001 | 2000 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cash flows from operating activities: | |||||||||||||
| Net loss | $ | (13,989,370 | ) | (7,174,493 | ) | (8,722,102 | ) | ||||||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | |||||||||||||
| Depreciation and amortization | 4,496,146 | 3,728,563 | 3,284,734 | ||||||||||
| Loss on disposition/impairment of assets | 222,301 | 305,134 | 383,481 | ||||||||||
| Loss (gain) on sale of investment securities | (7,896 | ) | — | 47,044 | |||||||||
| Bad debt expense | 250,000 | 110,000 | 71,561 | ||||||||||
| Changes in operating assets: | |||||||||||||
| Trade receivables | (3,848,792 | ) | (1,392,216 | ) | (1,100,765 | ) | |||||||
| Prepaid expenses | (607,788 | ) | (1,540,053 | ) | (2,056,284 | ) | |||||||
| Other receivables | 94,970 | 84,376 | 1,456,749 | ||||||||||
| Related party receivables | 1,811,517 | (1,811,517 | ) | — | |||||||||
| Other assets | (670,154 | ) | — | 465,663 | |||||||||
| Accounts payable and accrued expenses | 312,580 | 3,571,968 | 4,495,772 | ||||||||||
| Related party payable | 1,037,446 | — | — | ||||||||||
| Deferred revenue | (5,413,003 | ) | 342,931 | 18,837,682 | |||||||||
| Net cash provided by (used in) operating activities | (16,312,043 | ) | (3,775,307 | ) | 17,163,535 | ||||||||
| Cash flows from investing activities: | |||||||||||||
| Proceeds from sale of equipment | — | — | 14,851 | ||||||||||
| Capital expenditures | (6,852,742 | ) | (5,255,213 | ) | (4,617,196 | ) | |||||||
| Investments in other companies | (2,482,243 | ) | (2,700,000 | ) | (750,000 | ) | |||||||
| Proceeds from sale of investments in other companies | 630,000 | — | — | ||||||||||
| Purchases of investment securities held-to-maturity | (8,513,715 | ) | (119,683,435 | ) | (4,126,628 | ) | |||||||
| Maturities of investment securities held-to-maturity | 14,123,075 | 126,610,618 | 5,957,410 | ||||||||||
| Purchases of investment securities available-for-sale | (81,243,183 | ) | (129,650,517 | ) | (19,857,144 | ) | |||||||
| Sales of investment securities available-for-sale | 122,428,296 | 45,595,314 | 19,043,131 | ||||||||||
| Net cash provided by (used in) investing activities | 38,089,488 | (85,083,233 | ) | (4,335,576 | ) | ||||||||
| Cash flows from financing activities: | |||||||||||||
| Net proceeds from issuance of common stock | 3,352,691 | 68,580,621 | 37,981,833 | ||||||||||
| Net cash provided by financing activities | 3,352,691 | 68,580,621 | 37,981,833 | ||||||||||
| Net increase (decrease) in cash and cash equivalents | 25,130,136 | (20,277,919 | ) | 50,809,792 | |||||||||
| Cash and cash equivalents at beginning of year | 35,936,817 | 56,214,736 | 5,404,944 | ||||||||||
| Cash and cash equivalents at end of year | $ | 61,066,953 | 35,936,817 | 56,214,736 | |||||||||
| Supplemental disclosures of noncash investing and financing activities: | |||||||||||||
| Fair value adjustment on marketable investment securities charged to stockholders' equity | $ | (55,619 | ) | 449,023 | (16,594 | ) | |||||||
See accompanying notes to consolidated financial statements.
F-6
F-5
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2000, 1999,2002, 2001, and 1998
2000
(1) Summary of Significant Accounting Policies
- (a)
- Organization and Business Description
- (b)
- Principles of Consolidation
Myriad Genetics, Inc. and subsidiaries (collectively, the Company) is a genomicsleading biopharmaceutical company focusedfocusing on the development of therapeutic and
diagnostic products based on the discovery of major common human
disease genes and their biological pathways. The Company utilizes
analyses of extensive family histories and genetic material, as well
as a number of proprietary technologies, to identify inherited gene
mutations which increase the risk to individuals of developing these
diseases. The discovery of disease-predisposing genes and their
biochemical pathways provides the Company with three significant
commercial opportunities: (i) the development and marketing of molecular diagnosticnovel therapeutic and predictive medicine products. The Company has developed a number of proprietary proteomic technologies that permit it to identify genes, their related proteins, and the biological pathways they form. The Company uses this information services, (ii)to understand the marketing of
subscriptions torole they play in the ProNet database of protein interactions,onset and (iii) the development of therapeutic products for the treatment and
preventionprogression of major diseases associated with these geneshuman disease. The Company operates two wholly owned subsidiaries, Myriad Pharmaceuticals, Inc. and their
biochemical pathways.Myriad Genetic Laboratories, Inc., to commercialize its therapeutic and predictive medicine discoveries. The Company's operations are located in Salt Lake City, Utah.
- (c)
- Cash Equivalents
- (d)
- Equipment and Leasehold Improvements
The consolidated financial statements presented herein include the accounts of Myriad Genetics, Inc., and its wholly owned subsidiaries, Myriad Genetic Laboratories, Inc., Myriad Pharmaceuticals, Inc., and Myriad Financial, Inc. All intercompany amounts have been eliminated in consolidation.
Cash equivalents of $27,205,844$48,101,712 and $1,595,446$15,376,672 at June 30, 20002002 and 1999,2001, respectively, consist of short-term securities. The Company considers all highly liquid debt instruments with maturities at date of purchase of 90 days or less to be cash equivalents.
- (e)
- Impairment of Long-Lived Assets
Equipment and leasehold improvements are stated at cost. Depreciation and amortization are computed using the straight-line method based on the lesser of estimated useful lives of the related assets or lease terms. Equipment and leasehold improvements have depreciable lives which range from five to seven years.
The Company accounts for long-lived assets in accordance with the provisions of Statement of Financial Accounting Standards (SFAS) No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets. This Statement requires that long-lived assets be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
F-6
- (f)
- Income Taxes
- (g)
- Revenue Recognition
Income taxes are recorded using the asset and liability method. Under the asset and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
(Continued)
F-7
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2000, 1999,
- (h)
- Net Loss
Perper Common and Common Equivalent ShareLoss
Research revenues include revenues from research and 1998
(f) Revenue Recognitiontechnology licensing agreements. The Company recognizes revenue from research contracts in accordance with the termspercentage-of-completion method of accounting and following the contractguidance in Statement of Position 81-1,Accounting for Performance of Construction-Type and the related research activities
undertaken. This includes recognizing research revenue from research
contracts over time as researchCertain Production-Type Contracts. Percent complete is performed using the percentage-of-
completion methodestimated based on costs incurred relative to total estimated contract costs. The Company makes adjustments, if necessary, to the estimates used in the percentage-of-completion method of accounting as work progresses and the Company gains experience. Payments to the Company under these agreements cover the Company's direct costs and an allocation for overhead and general and administrative expenses. Payments received on uncompleted long-
termlong-term research contracts may be greater than or less than incurred costs and estimated earnings and have been recorded as other receivables or deferred revenues in the accompanying consolidated balance sheets. Molecular diagnosticRevenues related to technology license fees when continuing involvement or research services by the Company are required are recognized over the period of performance.
Predictive medicine revenues include revenues from the sale of predictive medicine products and related marketing agreements. Predictive medicine revenue is recognized upon completion of the test and communication of results. Payments received in advance of molecular diagnosticpredictive medicine work performed are recorded as deferred revenue. Revenues related to technology license fees when continuing
involvement or services by the Company are required, are generally
recognized over the period of performance. Up-front payments related to marketing agreements are generally recognized ratably over the life of the agreement.
(g)
Revenues are recognized in accordance with the provisions of Securities and Exchange Commission (SEC) Staff Accounting Bulletin No. 101,Revenue Recognition, (SAB 101). In December of 1999, the SEC staff released SAB 101 to provide guidance on the recognition, presentation and disclosure of revenue in financial statements. The Company adopted SAB 101 during the second quarter of fiscal 2001. The adoption of SAB 101 did not have an impact on the Company's results of operations or financial position.
Net loss per common share is computed based on the weighted-averageweighted average number of common shares and, as appropriate, dilutive potential common shares outstanding during the period. Stock options are considered to be potential common shares.
F-7
- (i)
- Use of Estimates
Basic loss per common share is the amount of loss for the period available to each share of common stock outstanding during the reporting period. Diluted loss per share is the amount of loss for the period available to each share of common stock outstanding during the reporting period and to each share that would have been outstanding assuming the issuance of common shares for all dilutive potential common shares outstanding during the period.
In calculating loss per common and common-equivalent share the net loss and the weighted average common and common-equivalent shares outstanding were the same for both the basic and diluted calculation.
For the years ended June 30, 2000, 1999,2002, 2001, and 1998,2000, there were antidilutive potential common shares of 3,892,248, 4,144,330,4,176,135, 4,121,061, and 4,137,440,3,892,248, respectively. Accordingly, these potential common shares were not included in the computation of diluted earningsloss per share for the years presented, but may be dilutive to future basic and diluted earnings per share.
(h)
- (j)
- Marketable Investment Securities
Management of the Company has made a number of estimates and assumptions relating to the reporting of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
(Continued)
F-8
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2000, 1999, and 1998
(i)
- (k)
- Fair Value Disclosure
The Company accounts for marketable investment securities by grouping them into one of two categories: held-to-maturity or available-for-
sale.available-for-sale. Held-to-maturity securities are those securities that the Company has the ability and intent to hold until maturity. All other securities are classified as available-for-sale.
Held-to-maturity securities are recorded at amortized cost, adjusted for the amortization or accretion of premiums or discounts. Available-
for-saleAvailable-for-sale securities are recorded at fair value. Unrealized holdingsholding gains and losses, net of the related tax effect, on available-for-sale securities are excluded from earnings and are reported as a separate component of stockholders' equity until realized.
Gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned. A decline in the market value of any available-for-sale or held-to-maturity security below cost that is deemed other than temporary results in a charge to earnings and establishes a new-costnew cost basis for the security. Premiums and discounts are amortized or accreted over the life of the related held-to-
maturityheld-to-maturity security as an adjustment to yield using the effective-
interesteffective-interest method.
(j)
At June 30, 2000,2002, the book valueconsolidated financial statements' carrying amount of the Company's financial instruments approximates fair value except as disclosed in note 2.
(k)
F-8
- (l)
- Stock-Based Compensation
- (m)
- Other Assets
The Company has adopted the disclosure provisions of Statement of
Financial Accounting StandardsSFAS No. 123,Accounting for Stock-Based Compensation (SFAS 123)123). SFAS 123 permits entities to adopt a fair
valuefair-value based method of accounting for stock options or similar equity instruments. However, it also allows an entity to continue measuring compensation cost for stock basedstock-based compensation using the intrinsic-
valueintrinsic-value method of accounting prescribed by Accounting Principles Board (APB) Opinion No. 25,Accounting for Stock Issued to Employees (APB 25). The Company has elected to continue to apply the provisions of APB 25 and provide pro forma disclosures required by SFAS 123.
(l)
- (n)
- Recent Accounting Pronouncements
Other assets are comprised of a purchased patent, security depositsintellectual property and an investmentinvestments in a privately held biotechnology company.
Amortization of the patent is computed using the straight-line method
over the estimated useful life of nine years. Accumulated amortization
related to the patent totaled $79,688 and $42,188 at June 30, 2000 and
1999, respectively.pharmaceutical companies. The private biotechnology and pharmaceutical company investment represents a 15
percent ownership interest and isinvestments are both accounted for under the cost method. Management reviews the valuation of both the patent and investmentinvestments for possible impairment as changes in facts and circumstances indicate that impairment should be assessed. For the year ended June 30, 2002, the Company recognized an impairment loss of $217,757 related to its investment in a privately held pharmaceutical company, which is included in other expenses in the accompanying consolidated statements of operations. The amount of impairment and valuation of this investment were based on management's estimates and the completion of an ongoing basisindependent, third-party appraisal of the investment. Accordingly, the amount recognized by comparing the carrying
value to undiscounted future cash flowsCompany upon the ultimate liquidation of this investment may vary significantly from the related assets.
(Continued)
F-9
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statementsestimated fair value at June 30, 2000, 1999, and 1998
(m) Accrued Liabilities
At June 30, 2000, accrued liabilities are comprised of accrued payroll
of $1,390,563, accrued vacation of $673,631, and other accrued
liabilities of $2,841,663. At June 30, 1999, the balance was comprised
of accrued payroll of $690,221, accrued vacation of $498,670, and
other accrued liabilities of $565,743.
2002.
In June 1998,May 2002, the Financial Accounting Standards Board (FASB) issued SFAS No. 145,Rescission of FASB Statements Nos. 4, 44, and 64, Amendment of FASB Statement No. 13, and Technical Corrections (SFAS 145). SFAS 145 eliminates Statement 4 (and Statement 64, as it amends Statement 4), which requires gains and losses from extinguishments of Financial Accounting Standardsdebt to be aggregated and, if material, classified as an extraordinary item, net of the related income tax effect. As a result, the criteria in APB Opinion No. 133, Accounting30 will now be used to classify those gains and losses. SFAS 145 amends FASB Statement No. 13 to require that certain lease modifications that have economic effects similar to sale-leaseback transactions are accounted for
Derivative Instruments and Hedging Activities (SFAS 133), that
establishes new accounting and reporting standards for companies to
report information about derivative instruments, including certain
derivative instruments embedded in other contracts (collectively
referred to as derivatives), and for hedging activities. It requires
that an entity recognize all derivatives as either assets or
liabilities in the balance sheet and measure those instruments at fair
value. For a derivative not designatedsame manner as a hedging instrument,
changes in the fair value of the derivative are recognized in earnings
in the period of change.sale-leaseback transactions. In addition, SFAS 145 makes technical corrections to some existing pronouncements. The Company willis required to adopt SFAS 133the provisions related to the rescission of Statements 4 and 64 on July 1, 2000. Management2002, and for all transactions entered into beginning May 15, 2002, adopt the provision related to the amendment of Statement 13. The Company is currently evaluating this statement but does not believe the adoption of SFAS 133expect that it will have a material effect on the Company'sits business, results of operations, financial position, or liquidity.
In July 2002, the FASB issued SFAS No. 146,Accounting for Costs Associated with Exit or Disposal Activities (SFAS 146). SFAS 146 addresses financial accounting and reporting for costs associated with exit or disposal activities and nullifies Emerging Issues Task Force (EITF) No. 94-3,Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (including Certain Costs Incurred in a Restructuring). SFAS 146 requires recognition of a liability for a cost associated with an exit or disposal activity when the liability is incurred, as opposed to when
F-9
the entity commits to an exit plan under EITF No. 94-3. The Company is required to adopt the provisions of SFAS 146 for exit or disposal activities that are initiated after December 1999, the Securities and Exchange Commission staff
released Staff Accounting Bulletin No. 101, Revenue Recognition, (SAB
101) to provide guidance on the recognition, presentation and
disclosure of revenue in financial statements; however, SAB 10131, 2002. The Company is currently evaluating this statement but does not change existing literature on revenue recognition. SAB 101
explains the staff's general framework for revenue recognition,
statingexpect that four criteria need to be met in order to recognize
revenue. The four criteria, all of which must be met, are the
following:
. There must be persuasive evidence of an arrangement;
. Delivery must have occurred or services must have been rendered;
. The selling price must be fixed or determinable; and
. Collectibility must be reasonably assured.
The Company will adopt SAB 101 during the quarter ended December 31,
2000. The Company believes that its current revenue recognition policy
is in compliance with this guidance; however, the Company continues to
evaluate the impact, if any, of SAB 101 and any possible, subsequent
interpretations of SAB 101 on the Company's policies and procedures.
(Continued)
F-10
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2000, 1999, and 1998
The FASB issued Interpretation No. 44, Accounting for Certain
Transactions Involving Stock Compensation - an Interpretation of APB
Opinion No. 25 (FIN 44) in March 2000. The interpretation clarifies
the application of APB 25 for only certain issues such as the
following: (a) the definition of employee for purposes of applying APB
25, (b) the criteria for determining whether a plan qualifies as a
noncompensatory plan, (c) the accounting consequence of various
modifications to the terms of a previously fixed stock option or
award, and (d) the accounting for an exchange of stock compensation
awards in a business combination. The Company will adopt FIN 44 on
July 1, 2000. Management does not believe that the interpretationit will have a material effect on the Company'sits business, results of operations, financial position, or liquidity.
(2) Marketable Investment Securities
The amortized cost, gross unrealized holding gains, gross unrealized holding losses, and fair value for available-for-sale and held-to-maturity securities by major security type and class of security at June 30, 20002002 and 1999,2001 were as follows:
| | Amortized cost | Gross unrealized holding gains | Gross unrealized holding losses | Fair value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| At June 30, 2002: | |||||||||||
| Held-to-maturity: | |||||||||||
| U.S. government obligations | $ | 2,543,229 | 3,675 | — | 2,546,904 | ||||||
| Corporate bonds and notes | 2,208,278 | 22,208 | — | 2,230,486 | |||||||
| $ | 4,751,507 | 25,883 | — | 4,777,390 | |||||||
| Available-for-sale: | |||||||||||
| Corporate bonds and notes | $ | 51,852,191 | 371,980 | (78,546 | ) | 52,145,625 | |||||
| Euro dollar bonds | 6,264,293 | 22,720 | (8,190 | ) | 6,278,823 | ||||||
| $ | 58,116,484 | 394,700 | (86,736 | ) | 58,424,448 | ||||||
At June 30, 2001: | |||||||||||
| Held-to-maturity: | |||||||||||
| Auction rate securities | $ | 2,005,912 | — | — | 2,005,912 | ||||||
| U.S. government obligations | 7,633,745 | 12,529 | — | 7,646,274 | |||||||
| Corporate bonds and notes | 721,210 | — | (385 | ) | 720,825 | ||||||
| $ | 10,360,867 | 12,529 | (385 | ) | 10,373,011 | ||||||
| Available-for-sale: | |||||||||||
| Commercial paper | $ | 39,103,968 | 25,900 | (2,165 | ) | 39,127,703 | |||||
| Corporate bonds and notes | 38,882,060 | 203,281 | (20,748 | ) | 39,064,593 | ||||||
| Certificates of deposit | 6,013,253 | 433 | (6,185 | ) | 6,007,501 | ||||||
| Asset-backed securities | 134,257 | 1,157 | (4,108 | ) | 131,306 | ||||||
| Euro dollar bonds | 15,160,163 | 170,770 | (4,752 | ) | 15,326,181 | ||||||
| $ | 99,293,701 | 401,541 | (37,958 | ) | 99,657,284 | ||||||
F-10
Maturities of debt securities classified as available-for-sale and held-to-
maturityheld-to-maturity are as follows at June 30, 2000. (Maturities of mortgage backed
securities have been presented based upon estimated cash flows assuming no
change in the current interest rate environment):
| | Amortized cost | Fair value | ||||
|---|---|---|---|---|---|---|
| Held-to-maturity: | ||||||
| Due within one year | $ | 1,475,711 | 1,490,081 | |||
| Due after one year through three years | 3,275,796 | 3,287,309 | ||||
| $ | 4,751,507 | 4,777,390 | ||||
| Available-for-sale: | ||||||
| Due within one year | $ | 10,475,005 | 10,532,235 | |||
| Due after one year through three years | 47,641,479 | 47,892,213 | ||||
| $ | 58,116,484 | 58,424,448 | ||||
(3) Leases
The Company leases office and laboratory space and equipment under three noncancelable operating leases. Future minimum lease payments under these leases as of June 30, 20002002 are as follows:
| Fiscal year ending: | ||||
| 2003 | $ | 4,964,082 | ||
| 2004 | 3,933,628 | |||
| 2005 | 3,018,926 | |||
| 2006 | 3,018,916 | |||
| 2007 | 2,313,059 | |||
| Thereafter | 14,104,933 | |||
| $ | 31,353,544 | |||
Rental expense was $4,604,885 in 2002, $4,447,203 in 2001, and $3,777,738 in 2000, $1,855,679 in 1999, and $1,282,308
in 1998.
The Company sold certain fixed assets for $3,551,784 in December of 1998.
The assets were leased back from the purchaser over a period of four years.
There was no gain or loss on this transaction and the resulting lease is
being accounted for as an operating lease.
F-12 (Continued)
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2000, 1999, and 1998
2000.
(4) Stock-Based Compensation
Prior to 1992, the Company granted nonqualified stock options to directors, employees, and other key individuals providing services to the Company. In 1992, the Company adopted the "1992 Employee, Director, and Consultant Fixed Stock Option Plan" (subsequently renamed the 2002 Amended and Restated Employee, Director and Consultant Stock Option Plan) and has reserved 6,000,0008,000,000 shares of common stock for issuance upon the exercise of options that the Company plans to grant from time to time under this plan. The exercise price of options granted in 2002, 2001, and 2000 is equivalent to the estimated fair market value of the stock at the date of grant. The number of shares, terms, and exercise period are determined by the Boardboard of Directorsdirectors on an option-by-option basis. Options generally vest ratably over four or five years and expire ten years from date of grant. As of June 30, 2000, 1,048,7482002, 1,676,260 shares are reserved for future grant under the 19922002 plan. For financial statement presentation purposes, the Company has
recorded as deferred compensation the excess of the deemed value of the
common stock at the date of grant over the exercise price. All deferred
compensation was amortized ratably over the vesting period. Amortization
expense was $247,774, $230,610, and $530,534 for the years ended June 30,
2000, 1999, and 1998, respectively.
F-11
A summary of activity is as follows:
| | 2002 | 2001 | 2000 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Number of shares | Weighted average exercise price | Number of shares | Weighted average exercise price | Number of shares | Weighted average exercise price | ||||||||||
| Options outstanding at beginning of year | 4,055,561 | $ | 34.03 | 3,826,748 | $ | 16.48 | 3,909,582 | $ | 6.32 | |||||||
| Plus options granted | 825,764 | 39.00 | 1,299,784 | 71.03 | 1,286,850 | 36.51 | ||||||||||
| Less: | ||||||||||||||||
| Options exercised | (344,073 | ) | 7.40 | (805,528 | ) | 6.36 | (1,007,232 | ) | 5.92 | |||||||
| Options canceled or expired | (426,617 | ) | 56.34 | (265,443 | ) | 46.17 | (362,452 | ) | 7.24 | |||||||
| Options outstanding at end of year | 4,110,635 | $ | 34.94 | 4,055,561 | $ | 34.03 | 3,826,748 | $ | 16.48 | |||||||
| Options exercisable at end of year | 1,526,064 | 25.45 | 1,039,248 | 14.14 | 1,093,510 | 6.17 | ||||||||||
| Weighted average fair value of options granted during the year | $ | 28.23 | $ | 56.35 | $ | 27.51 | ||||||||||
The following table summarizes information about fixed stock options outstanding at June 30, 2000:
| | Options outstanding | | | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Options exercisable | |||||||||||
| | | Weighted average remaining contractual life | | |||||||||
| Range of exercise prices | Number outstanding at June 30, 2002 | Weighted average exercise price | Number exercisable at June 30, 2002 | Weighted average exercise price | ||||||||
| $1.75 - 9.31 | 1,051,710 | 5.73 | $ | 5.47 | 586,680 | $ | 5.25 | |||||
| 9.69 - 25.06 | 1,038,927 | 6.91 | 20.38 | 560,527 | 17.42 | |||||||
| 25.36 - 59.37 | 1,028,401 | 9.27 | 42.79 | 82,925 | 49.70 | |||||||
| 59.74 - 93.81 | 991,597 | 8.49 | 73.33 | 295,932 | 73.93 | |||||||
| 4,110,635 | 1,526,064 | |||||||||||
The Company accounts for these plans under APB Opinion No. 25, under which no compensation cost has been recognized for those options granted whose exercise price was equivalent to the estimated fair market value at the date of grant. Had compensation cost for these plans been determined consistent with SFAS 123, the Company's net loss and loss per share would have been the following pro forma amounts:
| | 2002 | 2001 | 2000 | |||||
|---|---|---|---|---|---|---|---|---|
| Net loss: | ||||||||
| As reported | $ | 13,989,370 | 7,174,493 | 8,722,102 | ||||
| Pro forma | 35,067,233 | 19,400,559 | 13,565,122 | |||||
| Basic and diluted loss per share: | ||||||||
| As reported | 0.59 | 0.31 | 0.43 | |||||
| Pro forma | 1.48 | 0.85 | 0.67 | |||||
The fair value of each option grant is estimated on the date of the grant using the Black-Scholes option option—pricing model with the following weighted-weighted average assumptions used for grants in 2000, 1999, 2002, 2001,
F-12
and 1998,2000, respectively: risk-free interest rates of 6.3 percent, 4.8 percent,4.3%, 5.2%, and 5.5 percent;6.3%, expected dividend yields of zero percent0% for all years; expected lives of 5.46.0 years, 4.36.3 years, and 5.6 years;5.4 years, and expected volatility of 89 percent,
69 percent, and 63 percent.
During the year ended June 30, 1999, the Company issued options to purchase
223 shares of its wholly owned subsidiary Myriad Pharmaceuticals, Inc. to
the president of that subsidiary. The exercise price was equal to the fair
market value at the date of grant. The underlying shares are convertible to
150,048 shares of the Company's common stock.
F-14 (Continued)
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2000, 1999, and 1998
On October 22, 1998, the Board of Directors authorized a stock option
repricing amendment. Option holders electing to participate in the
repricing of eligible options were required to surrender one option for
every four options held. Under the repricing amendment, 1,178,388 options
were surrendered in exchange for 883,924 repriced options. The exercise
price of the repriced options is equal to the fair market value of the
Company's common stock on October 22, 1998. Directors'82%, executive
officers'93%, and outside consultants' options were excluded from the
repricing.
89%, respectively.
(5) Income Taxes
There was
The Company recorded $500,000 and $583,333 of foreign income tax expense in 2002 and 2001, respectively, and no income tax expense in 2000, 1999, or 1998 due to net operating
losses.2000. The difference between the expected tax benefit for all periods presented and the actual tax benefitexpense is primarily attributable to the effect of net operating losses being offset by an increase in the Company's valuation allowance.allowance, plus the effect of foreign income taxes in 2002 and 2001.
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities at June 30, 20002002 and 1999,2001 are presented below:
2000 1999
------------ ------------
Deferred tax assets:
Net operating loss carryforwards $ 27,109,000 21,288,000
Unearned revenue 7,274,000 247,000
Research and development credits 1,463,000 604,000
Accrued expenses 851,000 408,000
Capital loss carryforwards 28,000 -
------------ ------------
Total gross deferred tax assets 36,725,000 22,547,000
Less valuation allowance (36,123,000) (21,009,000)
------------ ------------
Net deferred tax assets 602,000 1,538,000
Deferred tax liability - equipment,
principally due to differences in
depreciation 602,000 1,538,000
------------ ------------
Total gross deferred tax liability 602,000 1,538,000
------------ ------------
Net deferred tax liability $ - -
============ ============
| | 2002 | 2001 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Deferred tax assets: | |||||||||
| Net operating loss carryforwards | $ | 54,265,000 | 44,469,000 | ||||||
| Unearned revenue | 5,383,000 | 7,402,000 | |||||||
| Research and development credits | 3,853,000 | 1,749,000 | |||||||
| Accrued expenses and other | 1,104,000 | 936,000 | |||||||
| Capital loss carryforwards | 34,000 | 34,000 | |||||||
| Total gross deferred tax assets | 64,639,000 | 54,590,000 | |||||||
| Less valuation allowance | (63,718,000 | ) | (54,138,000 | ) | |||||
| Net deferred tax assets | 921,000 | 452,000 | |||||||
| Deferred tax liability: | |||||||||
| Equipment, principally due to differences in depreciation | 921,000 | 452,000 | |||||||
| Total gross deferred tax liability | 921,000 | 452,000 | |||||||
| Net deferred tax liability | $ | — | — | ||||||
The net change in the total valuation allowance for the years ended June 30, 20002002 and 1999,2001 was an increase of $15,114,000$9,580,000 and $3,464,000,$18,015,000, respectively. Of the subsequently recognized tax benefits relating to the
valuation allowance forApproximately $35,947,000 of deferred tax assets as ofat June 30, 2000,
approximately $16,870,0002002, if recognizable in future years, will be recognized as additional paid-in capital, and the remainder will be allocated as an income tax benefit to be reported in the consolidated statement of operations.
At June 30, 2000,2002, the Company had total tax net operating losses of approximately $72,679,000$145,482,000 and total research and development credit carryforwards of approximately $1,463,000,$3,853,000, which can be carried forward to reduce federal income taxes. If not utilized, the tax loss and research and development credit carryforwards expire beginning in 2007 through 2020.
F-15 (Continued)
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2000, 1999, and 19982022.
Under the rules of the Tax Reform Act of 1986, the Company has undergone changes of ownership, and consequently, the availability of the Company's net operating loss and research and experimentation credit carryforwards in any one year isare limited. The maximum amount of
F-13
carryforwards available in a given year is limited to the product of the Company's value on the date of ownership change and the federal long-term tax-exempt rate, plus any limited carryforward not utilized in prior years. Management believes that
these limitations will not prevent these net operating losses from being
utilized.
(6) Common Stock Warrants
During the year ended June 30, 2000, the Company completed private placements of common stock wherein the placement agents received warrants to purchase 65,500 shares of the Company's common stock through the year 2003 at a weighted average price of $22.51, of which 65,500 are all still outstanding at June 30, 2000.
2002.
(7) Employee Deferred Savings Plan and Stock Purchase Plan
The Company has a deferred savings plan which qualifies under Section 401(k) of the Internal Revenue Code. Substantially all of the Company's employees are covered by the plan. The Company makes matching contributions of 50 percent50% of each employee's contribution with the employer's contribution not to exceed four percent4% of the employee's compensation. The Company's contributioncontributions to the plan waswere $703,530, $531,174, and $379,930 $358,325,for the years ended June 30, 2002, 2001, and $273,851 in
2000, 1999, and 1998, respectively.
The Company has an Employee Stock Purchase Plan (the Plan) which was adopted and approved by the Boardboard of Directorsdirectors and stockholders in December 1994, under which a maximum of 400,000 shares of common stock may be purchased by eligible employees. At June 30, 2000, 113,4362002, 157,409 shares of common stock had been purchased under the Plan. Because the discount allowed to employees under the Plan approximates the Company's cost to issue equity instruments, the Plan is not deemed to be compensatory and, therefore, is excluded from the pro forma loss shown in note 4.
(8) Collaborative Research Agreements
In March 2002, the Company formed a $34 million drug discovery collaboration to identify novel drug targets for the diagnosis and treatment of depression. The collaboration will merge the Company's integrated drug target identification and validation technologies with the collaborator's drug discovery and development expertise. The agreement provides the collaborator with license rights and specifies an upfront payment to the Company, plus guaranteed research funding, potential milestones and royalties. Revenue related to the license agreement is being recognized ratably over the service period and revenue related to this research collaboration is being recognized as the research is performed on a percent-complete basis.
Also in March 2002, the Company formed a $24 million research collaboration whereby the Company will apply its high-speed genomic sequencing capability and bioinformatics expertise to deliver molecular genetic information to the collaborator. Revenue related to this research collaboration is being recognized as the research is performed on a percent-complete basis.
In May 2000, the Company entered into a $26.0 million license agreement and research collaboration to utilize the Company's protein interaction technology (ProNet(TM)(ProNet®). Under the agreement, the licensee will receive a nonexclusive, fully paid, world-wideworldwide license to utilize ProNet(TM)ProNet® and receive support and related upgrades from the Company on a when-and-if-
availablewhen-and-if-available basis over the support period. Revenue related to the license agreement is being recognized ratably over the service period
F-14
and revenue related to the research collaboration areis being recognized as the costs of the contract are incurred on a percent completepercent-complete basis.
In December 1999, the Company entered into a 12 month collaboration to
utilize ProNet(TM) for drug discovery and development in the area of
cardiovascular disease. The Company may receive up to $13.0 million in
total research funding, license fees and potential milestone payments.
Revenue related to this research collaboration is being recognized as the
research is performed on a percent complete basis.
In August 1999, and as expanded in December 2000, the Company entered into a two-year collaboration to perform research related to cereal crop genomics. The Company expects to
receivereceived $33.5 million over the term of the agreement.from this collaboration, which was completed in December 2001. Revenue related to this research collaboration is being recognized as the research is
performed on a percent complete basis.
F-16 (Continued)
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2000, 1999, and 1998
In April 1995, the Company entered into a five-year collaborative research
and license agreement with a pharmaceutical company. Under the agreement,
the Company received $5.0 million per year which was recognized as revenue as the research was performed on a percent completepercent-complete basis. This
collaboration was completed in April of 2000.
In September 1995, the Company entered into a collaborative research and license agreement to perform various research for a pharmaceutical company. This agreement was expanded in 1997 and 1998. Under the agreement, as expanded, the Company expects to receive $42.7received $38.7 million through December 2002, which is being2001 when the project was completed. Revenue related to this project was recognized as revenue as the research iswas performed on a percent completepercent-complete basis.
Under some agreements the Company may license to the collaborator certain rights to therapeutic applications. The Company is entitled to receive royalties from sales of therapeutic products made by its collaborators. Revenue from research collaborations is recognized as research is performed using the percentage-of-completion method based on costs incurred relative to total estimated contract costs.
Because the Company has granted therapeutic rights to some of its collaborative licensees, the success of the programs is partially dependent upon the efforts of the licensees. Each of the above agreements may be terminated early. If any of the licensees terminatesterminate the above agreements, such termination may have a material adverse effect on the Company's operations.
(9) License Agreements
The Company has entered into license agreements with certain organizations
and academic institutions. The agreements grant the Company exclusive
worldwide licenses to certain technologies and patent applications that the
Company believes will be useful in the development of therapeutic and
molecular diagnostic products. Under the agreements, the Company may be
required to make future milestone payments upon achievement of certain
events. The Company is also required to make royalty payments based on net
sales of products subject to a minimum royalty upon commencement of sales.
(10) Segment and Related Information
The Company's business units have been aggregated into two reportable segments: (i) research and (ii) molecular diagnostics.predictive medicine. The research segment is focused on the discovery and sequencing of genes related to major common diseases, the discovery of proteins and their related biological pathways, and the development of therapeutic products for the treatment and prevention of major diseases. The molecular diagnosticspredictive medicine segment provides testing to determine predisposition to common diseases.
F-17 (Continued)
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Notes to Consolidated Financial Statements
June 30, 2000, 1999, and 1998
The accounting policies of the segments are the same as those described in the summary of significant accounting policies (note 1). The Company evaluates segment performance based on loss
F-15
from operations before interest income and expense and other income and expense. The Company's assets are not identifiable by segment.
| | Research | Predictive medicine | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Year ended June 30, 2002: | ||||||||
| Revenues | $ | 27,015,167 | 26,821,332 | 53,836,499 | ||||
| Depreciation and amortization | 2,894,434 | 1,601,712 | 4,496,146 | |||||
| Segment operating loss | 14,244,330 | 4,415,437 | 18,659,767 | |||||
| Year ended June 30, 2001: | ||||||||
| Revenues | 28,071,252 | 17,091,139 | 45,162,391 | |||||
| Depreciation and amortization | 2,597,297 | 1,131,266 | 3,728,563 | |||||
| Segment operating loss | 7,460,775 | 5,675,730 | 13,136,505 | |||||
| Year ended June 30, 2000: | ||||||||
| Revenues | 25,219,766 | 8,793,272 | 34,013,038 | |||||
| Depreciation and amortization | 2,494,333 | 790,401 | 3,284,734 | |||||
| Segment operating loss | 5,373,891 | 6,173,236 | 11,547,127 | |||||
| | 2002 | 2001 | 2000 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total operating loss for reportable segments | $ | (18,659,767 | ) | (13,136,505 | ) | (11,547,127 | ) | |||
| Unallocated amounts: | ||||||||||
| Interest income | 5,384,802 | 6,850,479 | 3,208,506 | |||||||
| Other | (214,405 | ) | (305,134 | ) | (383,481 | ) | ||||
| Income taxes | (500,000 | ) | (583,333 | ) | — | |||||
| Net loss | $ | (13,989,370 | ) | (7,174,493 | ) | (8,722,102 | ) | |||
All of the Company's revenues were derived from research and testing performed in the United States. Additionally, all of the Company's long-
livedlong-lived assets are located in the United States. All of the Company's research segment revenue was generated from seven, four,six, and threeseven collaborators in fiscal 2002, 2001, and 2000, 1999, and 1998, respectively. Additionally,Further, revenue from two of the seven collaborators was in excess of ten percent10% of the Company's consolidated revenues for each year presented.
F-18 (Continued)
(11) Common Stock Split
On August 16, 2000,
(10) Investment in Myriad Proteomics, Inc.
In April 2001, the BoardCompany announced the formation of Directors declared a two-for-one stock
splitnew alliance with Hitachi, Ltd. (Hitachi), Friedli Corporate Finance A.G. (Friedli), and Oracle Corporation (Oracle) to map the human proteome. The newly formed entity, Myriad Proteomics, Inc. (Myriad Proteomics), will market its proprietary proteomic information to pharmaceutical and biotechnology companies for therapeutic and diagnostic product development. The Company contributed technology to Myriad Proteomics in exchange for a 49% ownership interest and Hitachi, Friedli, and Oracle contributed a combined $82 million in cash in exchange for the remaining 51% ownership in Myriad Proteomics.
The Company is accounting for its investment in Myriad Proteomics using the equity method. Because the Company's initial investment in Myriad Proteomics consisted of technology with a carrying value of $0 on the Company's common stock. All references to the number of common
shares and per share amounts in the consolidated financial statements, and related footnotes have been restated to reflectgiven the effectuncertainty of the split for
all periods presented.
(12) Subsequent Events
In August 2000,realizability of the difference between the $82 million carrying amount and the Company's
F-16
proportionate share of the net assets of Myriad Proteomics, the Company's initial investment in Myriad Proteomics was recorded as $0. The Company allocated $41 million of this difference to technology and this amount is being reduced as the related technology charges, including in-process research and development, are incurred at Myriad Proteomics. At June 30, 2002, the remaining technology basis difference is estimated to be $14 million. The remaining $41 million of unallocated basis difference is being accreted to income over the period of expected benefit of 15 years.
As part of the formation of Myriad Proteomics, the Company entered into administrative and scientific outsourcing agreements with Myriad Proteomics. The original terms of these agreements expired on December 31, 2001, but were extended until June 30, 2002 at the option of Myriad Proteomics.
Charges to Myriad Proteomics for services incurred related to the administrative and scientific outsourcing agreements are based on actual time and expenses incurred by the Company on behalf of Myriad Proteomics. During the years ended June 30, 2002 and 2001, the Company provided $6,253,394 and $1,644,498, respectively, of administrative and scientific services to Myriad Proteomics. As of June 30, 2002, the Company has received $22 millionall but $292,585 of payments from Myriad Proteomics for these outsourcing services. This amount has been recorded as a reduction of a $1,330,031 payable to Myriad Proteomics for equipment purchased by the Company from Myriad Proteomics, resulting in $1,037,446 included as a related party payable on the accompanying consolidated balance sheets.
Summarized balance sheet information as of June 30, 2002 and 2001 for Myriad Proteomics is as follows:
| | 2002 | 2001 | |||
|---|---|---|---|---|---|
| | (unaudited) | | |||
| Current assets | $ | 50,703,000 | 72,437,000 | ||
| Noncurrent assets | 62,301,000 | 65,758,000 | |||
| Current liabilities | 2,783,000 | 1,812,000 | |||
| Noncurrent liabilities | 18,575,000 | 20,697,000 | |||
| Stockholders' equity | 91,646,000 | 115,686,000 | |||
Summarized statement of operations information for Myriad Proteomics for the years ended June 30, 2002 and 2001 (the years in which the Company had an investment in Myriad Proteomics) is as follows:
| | 2002 | 2001 | |||
|---|---|---|---|---|---|
| | (unaudited) | | |||
| Total revenues | $ | — | — | ||
| In-process research and development | — | 46,316,000 | |||
| Other operating costs and expenses | 28,478,000 | 3,068,000 | |||
| Net loss | 24,288,000 | 48,205,000 | |||
F-17
Item 10. DIRECTORS AND OFFICERS OF THE REGISTRANT
The response to this item is incorporated by reference from the private placementdiscussion responsive thereto under the captions "Management" and "Section 16(a) Beneficial Ownership Reporting Compliance" in our Proxy Statement for the 2002 Annual Meeting of 350,000Stockholders to be held on November 13, 2002.
Item 11. EXECUTIVE COMPENSATION
The response to this item is incorporated by reference from the discussion responsive thereto under the caption "Executive Compensation" in our Proxy Statement for the 2002 Annual Meeting of Stockholders to be held on November 13, 2002.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Except as set forth below, the response to this item is incorporated by reference from the discussion responsive thereto under the caption "Security Ownership of Certain Beneficial Owners and Management" in our Proxy Statement for the 2002 Annual Meeting of Stockholders to be held on November 13, 2002.
Equity Compensation Plan Information
As of June 30, 2002, we have two equity compensation plans in effect. Prior to 1992, we granted nonqualified stock options to directors, employees, and other key individuals providing services to us (Pre-1992 Plan). The Pre-1992 Plan was not approved by shareholders, and no shares were granted or issued upon exercise of outstanding options during the fiscal year ended June 30, 2002. In 1992, we adopted and received shareholder approval for the "1992 Employee, Director, and Consultant Fixed Stock Option Plan", which was subsequently renamed the "2002 Amended and Restated Employee, Director, and Consultant Stock Option Plan" (2002 Plan) upon approval of our Board of Directors to extend the plan until November 2003. We have reserved 8,000,000 shares of common stock.
F-19
EXHIBIT INDEX
Exhibit
Number Descriptionstock for issuance upon the exercise of options that we plan to grant from time to time under the 2002 Plan. Additional equity compensation plan information is as follows:
| | a | b | c | ||||
|---|---|---|---|---|---|---|---|
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants, and rights | Weighted-average exercise price of outstanding options, warrants, and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column a) | ||||
| Equity compensation plans approved by security holders | 4,040,035 | $ | 35.52 | 1,676,260 | |||
| Equity compensation plans not approved by security holders | 70,600 | 1.75 | 805,392 | ||||
| Total | 4,110,635 | $ | 34.94 | 2,481,652 | |||
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
The response to this item is incorporated by reference from the discussion responsive thereto under the caption "Executive Compensation—Employment Agreements, Termination of Employment and Change of Control Arrangements" in our Proxy Statement for the 2002 Annual Meeting of Stockholders to be held on November 13, 2002.
36
Item 14. EXHIBITS, FINANCIAL STATEMENT SCHEDULES, AND REPORTS ON FORM 8-K
Item 14(a).The following documents are filed as part of this annual report on Form 10-K.
Item 14(a)(1). and (2)See "Index to Consolidated Financial Statements and Financial Statement Schedules" at Item 8 to this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto.
Item 14(a)(3)Exhibits
- ------ -----------------------
(3.1 (a)) --
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
| Exhibit Number | Description | |||
|---|---|---|---|---|
| (3.1 (a)) | — | Restated Certificate of Incorporation of the Registrant | ||
(3.1 (b)) | — | Certificate of Amendment of Restated Certificate of Incorporation | ||
(3.1 (c)) | — | Certificate of Designations | ||
(3.2)p | — | Restated By-Laws of the Registrant (Filed as Exhibit 3.2) | ||
(4.1) | — | See Exhibits 3.1(a), 3.1(b), 3.1(c) and 3.2 | ||
(4.2) | — | Form of Common Stock Certificate | ||
(4.3)g | — | Rights Agreement, dated as of July 17, 2001, between the Registrant and Mellon Investor Services LLC (Filed as Exhibit 4.1) | ||
(4.4) | — | Agreement of Substitution and Amendment of Common Shares Rights Agreement by and between the Registrant and American Stock Transfer and Trust Company dated August 16, 2002 | ||
(10.1)$ | — | 2002 Amended and Restated Employee, Director and Consultant Stock Option | ||
(10.2)*$ | — | Employee Stock Purchase Plan (Filed as Exhibit 10.2) | ||
(10.3)*$ | — | Employment Agreement between Myriad Genetics, Inc., Myriad Genetic Laboratories, Inc. and Peter D. Meldrum, dated May 15, 1993 (Filed as Exhibit 10.3) | ||
(10.4)*$ | — | Employment Agreement between Myriad Genetics, Inc., Myriad Genetic Laboratories, Inc. and Mark H. Skolnick, Ph.D., dated January 1, 1994 (Filed as Exhibit 10.4) | ||
(10.5)*$ | — | Employment Agreement between Myriad Genetics, Inc., Myriad Genetic Laboratories, Inc. and Jay M. Moyes, dated July 12, 1993 (Filed as Exhibit 10.5) | ||
(10.6)# | — | Collaborative Research and License Agreement between the Registrant and Novartis Corporation, dated April 27, 1995 (Cardiovascular Diseases) (Filed as Exhibit 10.10) | ||
(10.7)# | — | Research Collaboration and License Agreement between the Registrant, Eli Lilly & Company and Hybritech Incorporated, dated August 1, 1992 (Breast Cancer—BRCA1) (Filed as Exhibit 10.11) | ||
37
(10.8)# | — | Collaborative Agreement between the Registrant and Hybritech Incorporated, dated March 5, 1993 (BRCA1 Test Kits) (Filed as Exhibit 10.12) | ||
(10.9)# | — | Exclusive License Agreement between the Registrant and the University of Utah Research Foundation, dated October 8, 1991, as amended (Breast Cancer—BRCA1) (Filed as Exhibit 10.13) | ||
(10.10)# | — | Standard Research Agreement and Form of License Agreement between the Registrant and the University of Utah, effective January 1, 1993, as amended (Genes Predisposing to Cancer) (Filed as Exhibit 10.14) | ||
(10.11)# | — | Exclusive License Agreement between the Registrant and the University of Utah Research Foundation, dated August 4, 1993 (Angiotensinogen Variants and Predisposition to Hypertension) (Filed as Exhibit 10.15) | ||
(10.12)# | — | Exclusive License Agreement between the Registrant and the University of Utah Research Foundation, dated June 21, 1994 (MTS1 or p16) (Filed as Exhibit 10.16) | ||
(10.13)# | — | Exclusive License Agreement between the Registrant and the University of Utah Research Foundation, dated November 23, 1994 (Breast Cancer—BRCA2) (Filed as Exhibit 10.17) | ||
(10.14)# | — | Standard Research Agreement dated May 1, 1995 between the Registrant and the University of Utah (Cardiovascular Disorders and Coronary Heart Disease Database) (Filed as Exhibit 10.18) | ||
(10.15)# | — | Exclusive License Agreement dated May 1, 1995 between the Registrant and the University of Utah Research Foundation (Cardiovascular Disorders and Coronary Heart Disease Database) (Filed as Exhibit 10.19) | ||
(10.16)# | — | Standard Research Agreement dated July 31, 1995 between the Registrant and the University of Utah (Obesity Database) (Filed as Exhibit 10.20) | ||
(10.17)# | — | Exclusive License Agreement dated July 31, 1995 between the Registrant and the University of Utah Research Foundation (Obesity Database) (Filed as Exhibit 10.21) | ||
(10.18)# | — | Co-Exclusive License Agreement among the Registrant, the University of Utah Research Foundation and Institut National de la Sante et de la Recherche Medicale, dated October 6, 1993 (Angiotensinogen and Predisposition to Essential Hypertension) (Filed as Exhibit 10.22) | ||
(10.19)# | — | License Agreement between the Registrant and California Institute of Technology, dated April 21, 1994 (MTS1 or p16) (Filed as Exhibit 10.23) | ||
(10.20)* | — | Research Agreement between the Registrant and California Institute of Technology, dated June 3, 1994 (MTS1 or p16) (Filed as Exhibit 10.24) | ||
(10.21)# | — | Collaborative Research and License Agreement between the Registrant and Bayer Corporation, dated September 11, 1995 (Filed as Exhibit 10.28) | ||
(10.22)z@ | — | Research Agreement between the Registrant and IHC Health Services, Inc., dated as of June 24, 1996 | ||
(10.23)! | — | Lease Agreement, dated October 12, 1995, between the Boyer Research Park Associates V, by its general partner, the Boyer Company and the Registrant (Filed as Exhibit 10.2) | ||
38
(10.24)! | — | Amendment to Lease Agreement, dated March 29, 1996 between the Boyer Research Park Associates V, by its general partner, the Boyer Company and the Registrant (Filed as Exhibit 10.3) | ||
(10.25)!@ | — | Letter Agreement, dated March 4, 1996, among the University of Utah, Genetic Epidemiology and the Registrant regarding Extension of Standard Research agreement and Form of License Agreement between the Registrant and the University of Utah, effective January 1, 1993, as amended (Genes Predisposing to Cancer) (Filed as Exhibit 10.4) | ||
(10.26)q@ | — | Patent and Technology License Agreement dated December 2, 1996 among the Board of Regents of the University of Texas System, the University of Texas M.D. Anderson Cancer Center and the Registrant (Filed as Exhibit 10.1) | ||
(10.27)=@ | — | Collaborative Research and License Agreement among the Registrant, Schering Corporation and Schering-Plough, Ltd., dated April 22, 1997 (Prostate and Other Cancers) (Filed as Exhibit 10.36) | ||
(10.28)++@ | — | Standard Research Agreement between the Company and Valley Mental Health dated September 1, 1997 (central nervous system disorders) (Filed as Exhibit 10.1) | ||
(10.29)%@ | — | Amendment and Supplement to Collaborative Research and License Agreement dated November 19, 1997 between Bayer Corporation and the Registrant (Filed as Exhibit 10.1) | ||
(10.30)k | — | Lease Agreement-Research Park Building Phase II, dated March 6, 1998, between the Research Park Associated VI, by its general partner, the Boyer Company, L.C. and the Registrant | ||
(10.31)& | — | Memorandum of Lease between the Company and Boyer Foothill Associates, Ltd. dated August 24, 1998 (Filed as Exhibit 10.1) | ||
(10.32)& | — | Memorandum of Lease between the Company and Boyer Research Park Associates VI, L.C. dated August 24, 1998 (Filed as Exhibit 10.2) | ||
(10.33)& | — | Subordination Agreement and Estoppel, Attornment and Non-Disturbance Agreement (Lease to Deed of Trust) between the Company and Wells Fargo Bank, National Association dated June 24, 1998 (Filed as Exhibit 10.3) | ||
(10.34)w | — | Master Lease Agreement dated December 31, 1998 between General Electric Capital Corporation and the Company (Filed as Exhibit 10.1) | ||
(10.35)w | — | Addendum No. 1 to Master Lease Agreement dated December 31, 1998 between General Electric Capital Corporation and the Company (Filed as Exhibit 10.2) | ||
(10.36)w | — | Addendum No. 2 to Master Lease Agreement dated December 31, 1998 between General Electric Capital Corporation and the Company (Filed as Exhibit 10.3) | ||
(10.37)w | — | Biotech Equipment Schedule Schedule No. 001 dated December 31, 1998 to Master Lease Agreement dated December 31, 1998 between General Electric Corporation and the Company (Filed as Exhibit 10.4) | ||
(10.38)w | — | Annex A to Equipment Schedule No. 001 to Master Lease Agreement dated December 31, 1998 between General Electric Corporation and the Company (Filed as Exhibit 10.5) | ||
39
(10.39)w | — | Annex B to Equipment Schedule No. 001 to Master Lease Agreement dated December 31, 1998 between General Electric Corporation and the Company (Filed as Exhibit 10.6) | ||
(10.40)w | — | Addendum to Schedule No. 001 to Master Lease Agreement dated as of December 31, 1998 between General Electric Corporation and the Company (Filed as Exhibit 10.7) | ||
(10.41)w@ | — | Collaborative Research, License and Co-Promotion agreement dated as of October 5, 1998 between Schering Aktiengesellschaft and the Company (Filed as Exhibit 10.8) | ||
(10.42)w@ | — | Collaborative ProNet Research and License Agreement dated as of November 11, 1998 between Monsanto Company and the Company (Filed as Exhibit 10.9) | ||
(10.43)w@ | — | Letter Amendment to the Collaborative Research and License Agreement dated as of November 30, 1998 between Bayer Corporation and the Company (Filed as Exhibit 10.10) | ||
(10.44)m@ | — | Collaboration and License Agreement between the Company and Novartis Agricultural Discovery Institute, Inc. dated July 27, 1999 (Filed as Exhibit 10.1) | ||
(10.45)m | — | Subscription Agreement between the Company and Peter Friedli dated September 30, 1999 (Filed as Exhibit 10.2) | ||
(10.46)m | — | Securities Purchase Agreement and Standstill Agreement between the Company and Schering Berlin Venture Corporation dated October 15, 1999 (Filed as Exhibit 10.3) | ||
(10.47)f | — | Master Lease Agreement dated October 25 between Comdisco Laboratory and Scientific Group, a Division of Comdisco Healthcare Group, Inc. and the Company (Filed as Exhibit 10.1) | ||
(10.48)f | — | Addendum to the Master Lease Agreement dated October 25, 1999 between Comdisco Laboratory and Scientific Group, a Division of Comdisco Healthcare Group, Inc. and the Company (Filed as Exhibit 10.2) | ||
(10.49)f | — | Amendment No. 1 to the Master Lease Agreement dated October 25, 1999 between Comdisco Laboratory and Scientific Group, a Division of Comdisco Healthcare Group, Inc. and the Company (Filed as Exhibit 10.3) | ||
(10.50)f | — | Equipment Schedule No. SG01 dated November 10, 1999 to the Master Lease Agreement dated October 25, 1999 between Comdisco Laboratory and Scientific Group, a Division of Comdisco Healthcare Group, Inc. and the Company (Filed as Exhibit 10.4) | ||
(10.51)v | — | Purchase Agreement dated as of August 28, 2000 between the Registrant and Acqua Wellington North American Equities Fund, Ltd. | ||
(10.52)v | — | Registration Rights Agreement dated as of August 28, 2000 between the Registrant and Acqua Wellington North American Equities Fund, Ltd. | ||
(10.53)s | — | Purchase Agreement dated as of October 27, 2000 between the Registrant and Acqua Wellington North America Equities, Ltd. (Filed as Exhibit 10.1) | ||
40
(10.54)s | — | Registration Rights Agreement dated as of October 27, 2000 between the Registrant and Acqua Wellington North America Equities, Ltd. (Filed as Exhibit 10.2) | ||
(10.55)e | — | Lease Agreement, dated March 31, 2001 between the Registrant and Boyer Research Park Associates VI, by it general partner, The Boyer Company, L.C. (Filed as Exhibit 10.1) | ||
(10.56)e | — | Agreement, dated March 31, 2001, between the Registrant and Boyer Research Park Associates VI, by its general partner, The Boyer Company, L.C. (Filed as Exhibit 10.2) | ||
(10.57)e@ | — | License Agreement, dated December 7, 2000, between the Registrant and Encore Pharmaceuticals, Inc. (Filed as Exhibit 10.3) | ||
(21.1) | — | List of Subsidiaries of the Registrant | ||
(23.1) | — | Consent of KPMG LLP |
- *
- Previously filed with the Commission as Exhibits to, and incorporated herein by reference from, the Company's Registration Statement filed on Form S-1, File No. 33-95970.
- #
- Previously filed with the Commission as Exhibits to, and incorporated herein by reference from, the Company's Registration Statement filed on Form S-1, File No. 33-95970, and for which Confidential Treatment has been granted by the Securities and Exchange Commission as to certain portions.
- @
- Confidential Treatment has been granted by the Securities and Exchange Commission as to certain portions.
- p
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending September 30, 1995.
- $
- Management contract or compensatory plan or arrangement required to be filed as an exhibit to this Form 10-K pursuant to Item 14(c) of this report.
- z
- Previously filed and incorporated herein by reference from the Form 10-K for the period ending June 30, 1996.
- !
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending September 30, 1996.
- q
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending December 31, 1996.
- =
- Previously filed and incorporated herein by reference from the Form 10-K for the period ending June 30, 1997.
- ++
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending September 30, 1997.
- %
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending December 31, 1997.
- k
- Previously filed and incorporated herein by reference from the Form 10-K for the period ending June 30, 1998.
41
- &
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending September 30, 1998.
- w
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending December 31, 1998.
- m
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending September 30, 1999.
- f
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending December 31, 1999.
- v
- Previously filed and incorporated herein by reference from the Form 10-K for the period ending June 30, 2000.
- s
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending December 31, 2000.
- e
- Previously filed and incorporated herein by reference from the Form 10-Q for the period ending March 31, 2001.
- g
- Previously filed and incorporated herein by reference from the Form 8-K filed on July 18, 2001.
Where a document is incorporated by reference from a previous filing, the Exhibit number of the document in that previous filing is indicated in parentheses after the description of such document.
We filed a Current Report on Form 8-K, on April 30, 2002, to disclose that we had publicly disseminated a press release announcing that we had entered into a new collaboration with DuPont to better understand the genetics that drive the proprietary products of Pioneer, a subsidiary of DuPont.
We filed a Current Report on Form 8-K on May 22, 2002 to disclose that we had publicly disseminated a press release announcing that we had introduced a new predictive medicine product for risk of hereditary colon cancer, called COLARIS AP™.
42
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant (3.1 (b)) -- Certificatehas duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in Salt Lake City, Utah on September 26, 2002.
MYRIAD GENETICS, INC. | ||||
By: | /s/ PETER D. MELDRUM Peter D. Meldrum President and Chief Executive Officer | |||
Pursuant to the requirements of Amendmentthe Securities Exchange Act of Restated Certificate1934, this report has been signed below by the following persons on behalf of Incorporation
(10.65) -- Purchase Agreement datedthe registrant and in the capacities indicated below and on the dates indicated.
| Signatures | Title | Date | ||||
|---|---|---|---|---|---|---|
| By: | /s/ PETER D. MELDRUM Peter D. Meldrum | President and Chief Executive Officer and Director (principal executive officer) | September 26, 2002 | |||
By: | /s/ JAY M. MOYES Jay M. Moyes | Vice President of Finance (principal financial and accounting officer) | September 26, 2002 | |||
By: | /s/ HUGH A. D'ANDRADE Hugh A. D'Andrade | Chairman of the Board | September 26, 2002 | |||
By: | /s/ WALTER GILBERT Walter Gilbert, Ph.D. | Vice Chairman of the Board | September 26, 2002 | |||
By: | /s/ MARK H. SKOLNICK Mark H. Skolnick, Ph.D. | Chief Scientific Officer and Director | September 26, 2002 | |||
By: | /s/ ARTHUR H. HAYES, JR. Arthur H. Hayes, Jr., M.D. | Director | September 26, 2002 | |||
By: | /s/ DALE A. STRINGFELLOW Dale A. Stringfellow, Ph.D. | Director | September 26, 2002 | |||
By: | /s/ LINDA S. WILSON Linda S. Wilson, Ph.D. | Director | September 26, 2002 |
43
I, Peter D. Meldrum, certify that:
- 1.
- I have reviewed this annual report on Form 10-K of Myriad Genetics, Inc.;
- 2.
- Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report; and
- 3.
- Based on my knowledge, the financial statements, and other financial information included in this annual report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of,
August 28, 2000 betweenand for, theRegistrantperiods presented in this annual report.
Date: September 26, 2002 | ||
/s/ PETER D. MELDRUM Peter D. Meldrum President and Chief Executive Officer |
I, Jay M. Moyes, certify that:
- 1.
- I have reviewed this annual report on Form 10-K of Myriad Genetics, Inc.;
- 2.
- Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report; and
Acqua Wellington North American Equities, Ltd. (10.66) -- Registration Rights Agreement dated - 3.
- Based on my knowledge, the financial statements, and other financial information included in this annual report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of,
August 28, 2000 betweenand for, theRegistrant and Acqua Wellington North American Equities, Ltd. (21.1) -- List of Subsidiariesperiods presented in this annual report.
Date: September 26, 2002 | ||
/s/ JAY M. MOYES Jay M. Moyes Vice President of Finance and Chief Financial Officer |
44
Certification
Pursuant to Section 906 of the Registrant
(23.1) -- ConsentSarbanes-Oxley Act of KPMG LLP
(27.1) --2002
(Subsections (a) and (b) of Section 1350, Chapter 63 of Title 18, United States Code)
Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code), each of the undersigned officers of Myriad Genetics, Inc., a Delaware corporation (the "Company"), does hereby certify, to such officer's knowledge, that:
The Annual Report on Form 10-K for the year ended June 30, 2002 (the "Form 10-K") of the Company fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, and information contained in the Form 10-K fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: September 26, 2002 | ||
/s/ PETER D. MELDRUM Peter D. Meldrum President and Chief Executive Officer | ||
Date: September 26, 2002 | ||
/s/ JAY M. MOYES Jay M. Moyes Vice President of Finance and Chief Financial Officer |
The foregoing certification is being furnished solely pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code) and is not being filed as a separate disclosure document.
| Exhibit Number | Description of Exhibits | |||
|---|---|---|---|---|
| (4.2) | — | Form of Common Stock Certificate | ||
(4.4) | — | Agreement of Substitution and Amendment of Common Shares Rights Agreement by and between the Registrant and American Stock Transfer and Trust Company | ||
(10.1) | — | 2002 Amended and Restated Employee, Director and Consultant Stock Option Plan | ||
(21.1) | — | List of Subsidiaries of the Registrant | ||
(23.1) | — | Consent of KPMG LLP |
PART I
- Item 1. BUSINESS
- Item 2. FACILITIES
Item 3. LEGAL PROCEEDINGS
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
- Item 5. MARKET FOR REGISTRANT'S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Item 6. SELECTED CONSOLIDATED FINANCIAL DATA
Item 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
- Item 8. FINANCIAL STATEMENTS
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
MYRIAD GENETICS, INC. AND SUBSIDIARIES Consolidated Balance Sheets June 30, 2002 and 2001
MYRIAD GENETICS, INC. AND SUBSIDIARIES Consolidated Statements of Operations Years ended June 30, 2002, 2001, and 2000
MYRIAD GENETICS, INC. AND SUBSIDIARIES Consolidated Statements of Stockholders' Equity and Comprehensive Loss Years ended June 30, 2002, 2001, and 2000
MYRIAD GENETICS, INC. AND SUBSIDIARIES Consolidated Statements of Cash Flows Years ended June 30, 2002, 2001, and 2000
MYRIAD GENETICS, INC. AND SUBSIDIARIES Notes to Consolidated Financial