supporting clinical trials, training customers, and integrating our technologies and know-how into customer manufacturing operations.
We work together with our customers to integrate the best possible drug delivery and surface modification technologies with their products, not only to meet their performance requirements, but also to perform services quickly so that the product may reach the market ahead of the competition. To quickly solve problems that might arise during the development and optimization process, we have developed extensive capabilities in analytical chemistry and surface characterization within our R&D organization. Ourstate-of-the-art instrumentation and extensive experience allow us to test the purity of coating reagents, to monitor the elution rate of drug from coatings, microparticles and implants, to measure coating thickness and smoothness, and to map the distribution of chemicals throughout coatings, microparticles and implants. We believe our capabilities far exceed those of our direct competitors, and sometimes even exceed those of our large-company customers. In order to better serve our customers, in November 2008 we announced the creation of a new centralized R&D function to serve the needs of the Company’s clinically and market focused business units, other than our SurModics Pharmaceuticals business unit, which continues to have its own R&D operations.
As medical products become more sophisticated and complex and as competition increases, we believe the need for drug delivery and surface modification will continue to grow. We intend to continue our development efforts to expand our drug delivery and surface modification technologies to provide additional optimized properties to meet these needs across multiple medical markets. In addition, we are expanding our drug delivery and surface modification technology expertise to capture more of the final product value. We are doing this by, in selected cases, developing or acquiring technologies or devices to develop from feasibility stage up to and including animal and human clinical testing stage. There can be no assurance that we will be successful in developing or acquiring additional technologies or devices.
After thorough consideration of each market opportunity, our technical strategy is to target selected formulation characteristics for further development, to facilitate and shorten the license cycle. We continue to perform research into applications for future products both on our own and in conjunction with some of our customers. Some of the R&D projects currently in progress include additional polymer systems for site specific and systemic drug delivery, including microparticles, nanoparticles and biodegradable technologies, as well as technologies to improve healing around implantable devices, technologies to deliver nucleic acids, proteins and cell therapies, advanced stabilization reagents, slide-based microarray technologies and drug delivery platforms for ophthalmic applications.
In fiscal 2010, 2009 2008 and 2007,2008, our R&D expenses were $36.1 million, $34.4 million $40.5 million and $28.5$40.5 million, respectively. Of the above amounts, $17.9 million, $21.2 million $21.3 million and $22.6$21.3 million were spent on internal R&D in fiscal 2010, 2009 2008 and 2007,2008, respectively, and $18.2 million, $13.2 million $19.2 million and $5.8$19.2 million in those years, respectively, were spent on customer-sponsored R&D, which includes technology optimization and other development work on customer product applications. We intend to continue investing in R&D to advance our drug delivery and surface modification technologies and to expand uses for our technology platforms. In addition, we continue to pursue access to products and technologies developed outside the Company as appropriate to complement our internal R&D efforts.
13
Patents and Proprietary Rights
Patents and other forms of proprietary rights are an essential part of the SurModics business model. We protect our extensive portfolio of technologies through a number of United States patentsfiling and maintaining patent rights covering a variety of coatings, drug delivery methods, reagents, and formulations, as well as particular clinical device applications. Generally, we seek patent protection in the United States for many of our proprietary technologies. We generallymay also file international patent applications in the locations matching the major markets of our customers (primarily in North America, Europe, and Japan) in parallel with. In fiscal 2010, we filed 46 United States applications. In fiscal 2009, we filed 38 United Statespatent applications, as well as 43 international patent applications, expanding the portfolio protection around our current technologies as well as enabling pursuit of new technology concepts, innovations, and directions.
12
Additionally, we have 3 pendinglicensed our Photolink® hydrophilic technology to a number of our customers for use in a variety of medical device applications, including those described in “SurModics’ Drug Delivery and Surface Modification Technologies — Applications” above. In particular, we have 8 issued U.S. patent applicationspatents, 13 issued international patents, and 64 pending international patent applications protecting various aspects of Bravo,these technologies, including compositions, methods of manufacturingmanufacture, and methods of coating products.devices. The expiration dates for these patents range from 2015 to 2020.
The Company aggressively pursues patent protection covering the proprietary technologies that we consider important to our business. In addition to seeking patent protection in the U.S., we also generally file patent applications in European countries and additional foreign countries, including Australia, Canada and Japan, on a selective basis. Generally, the expiration dates of our issued patents are determined based on the filing date of the earliest filed patent application from which the patent claims priority. We strategically manage our patent portfolio so as to ensure that we have valid and enforceable patent rights protecting our technological innovations.
As of September 30, 2009,2010, we had 164187 pending United States patent applications, 612 of which were exclusively licensed from others, and 283244 foreign patent applications, of which 3942 were exclusively licensed from others. Likewise, as of September 30, 2009,2010, we owned 124109 issued United States patents, 1316 of which were exclusively licensed from others, and 244192 international patents, of which 5065 were exclusively licensed from others.
We also rely upon trade secrets and other unpatented proprietary technologies. We seek to maintain the confidentiality of such information by requiring employees, consultants and other parties to sign confidentiality agreements and by limiting access by parties outside the Company to such information. There can be no assurance, however, that these measures will prevent the unauthorized disclosure or use of this information, or that others will not be able to independently develop such information. Additionally, there can be no assurance that any agreements regarding confidentiality and non-disclosure will not be breached, or, in the event of any breach, that adequate remedies would be available to us.
Marketing and Sales
We market our technologies and products throughout the world using a direct sales force consisting of dedicated sales professionals who focus on specific markets and companies. These sales professionals work in concert with business unit personnel to coordinate customer activities. We believe that our new organizational structure allows us to better understand and meet the needs of our customers by organizing our business around patient needs. The specialization of our sales professionals fosters an in-depth knowledge of the issues faced by our customers within these markets such as industry trends, technology changes, biomaterial changes and the regulatory environment. In addition, we enter into sales and marketing relationships with third-parties to distribute our diagnostic products around the world. See Note 11 to the consolidated financial statements for information regarding domestic and foreign revenue.
In general, we license our technologies on a non-exclusive basis to customers for use on specific products, or on an exclusive basis, but limited to a specific “field of use.” This strategy enables us to license our technologies to multiple customers in the same market. We also target new product applications with existing customers.
To support our marketing and sales activities, we publish technical literature on our various surface modification, drug delivery, andin vitrodiagnostics technologies and products. In addition, we exhibit at major trade shows and technical meetings, advertise in selected trade journals and through our website, and conduct direct mailings to appropriate target markets.
14
We also offer ongoing customer service and technical support throughout our licensees’ relationships with us. This service and support may begin with a feasibility study, and also may include additional services such as assistance in the transfer of the technology to the licensee, further optimization, process control and troubleshooting, preparation of product for clinical studies, and assistance with regulatory submissions for product approval. Most of these services are billable to customers.
Acquisitions and Investments
In order to further our strategic objectives and strengthen our existing businesses, we intend to continue to explore acquisitions, investments and strategic collaborations to diversify and grow our business. As a result, we expect to make future investments or acquisitions where we believe that we can broaden our technology offerings
13
and expand our sources of revenue and the number of markets in which we participate. See Note 2 to the consolidated financial statements for further information regarding our minority equity investments. Mergers and acquisitions of medical technology companies are inherently risky, and no assurance can be given that any of our previous or future acquisitions will be successful or will not materially adversely affect our consolidated results of operations, financial condition, or cash flows.
In July 2007, we acquired Brookwood Pharmaceuticals, Inc. (now known as SurModics Pharmaceuticals, Inc.) for an up-front payment, including fees, of $42.3 million and potential additional payments of up to $22 million based upon achievement of certain milestones. Since the acquisition, we have paid the sellers additional consideration of $5$5.8 million related to achievement of milestones.milestones, and the sellers are still eligible to receive up to $16.3 million in additional consideration. The additional cash consideration was recorded as an increase to goodwill.
In August 2007, we acquired BioFX Laboratories, Inc. (“BioFX”) for consideration consisting of an up-front payment, including fees, of $11.6 million and potential additional payments of up to $11.4 million based upon achievement of certain milestones. Since the acquisition, we have paid the sellers additional consideration of $1.1 million related to achievement of a milestone, and the sellers are still eligible to receive up to $7.6$3.5 million in additional consideration.
In November 2008, we extended our technology offerings by acquiring a portfolio of intellectual property and collaborative drug delivery projects from PR Pharmaceuticals, Inc., a drug delivery company specializing in injectable, biodegradable sustained release formulations for an up-front payment, including fees, of $3.2$3.1 million and potential payments of up to $6.0 million based upon achievement of certain milestones. Since the acquisition, we have paid the sellers additional consideration of $2.4 million related to achievement of certain milestones.
Significant Customers
We have two customers that each provided more than 10% of our revenue in fiscal 2009.2010. Revenue from Merck and Johnson & Johnson and Medtronic represented approximately 37%17% and 11%14%, respectively, of our total revenue for the year ended September 30, 2009.
2010. The loss of one or more of our largest customers could have a material adverse effect on our business, financial condition, results of operations, and cash flow as discussed in more detail below.
15
Competition
The ability for drug delivery and surface modification technologies to improve the performance of medical devices and drugs and to enable new product categories has resulted in increased competition in these markets. Some of our competitors offer drug delivery technologies, while others specialize in lubricious or hemocompatible coating technology. Some of these companies target ophthalmology applications, while others target cardiovascular or other medical device applications. In addition, because of the many product possibilities afforded by surface modification technologies, many of the large medical device manufacturers have developed, or are engaged in efforts to develop, internal competency in the area of drug delivery and surface modification. Many of our existing and potential competitors have greater financial, technical and marketing resources than we have.
We attempt to differentiate ourselves from our competitors by providing what we believe is a high value addedvalue-added approach to drug delivery and surface modification technology. We believe that the primary factors customers consider in choosing a particular technology include performance (e.g., flexibility, ability to fine tune drug elution profiles, biocompatibility, etc.), ease of manufacturing,time-to-market, intellectual property protection, ability to produce multiple properties from a single process, compliance with manufacturing regulations, ability to manufacture clinical and commercial products (especially for SurModics Pharmaceuticals customers), customer service and total cost of goods (including manufacturing process labor). We believe our technologies deliver exceptional performance in these areas, allowing us to compete favorably with respect to these factors. We believe that the cost and time required to obtain the necessary regulatory approvals significantly reduces the likelihood of a customer changing the manufacturing process it uses once a device or drug has been approved for sale.
Because a significant portion of our revenue depends on the receipt of royalties based on sales of medical devices incorporating our technologies, we are also affected by competition within the markets for such devices. We believe that
14
the intense competition within the medical device market creates opportunities for our technologies as medical device manufacturers seek to differentiate their products through new enhancements or to remain competitive with enhancements offered by other manufacturers. Because we seek to license our technologies on a non-exclusive basis, we may further benefit from competition within the medical device markets by offering our technologies to multiple competing manufacturers of a device. However, competition in the medical device market could also have an adverse effect on us. While we seek to license our products to established manufacturers, in certain cases our licensees may compete directly with larger, dominant manufacturers with extensive product lines and greater sales, marketing and distribution capabilities. We also are unable to control other factors that may impact commercialization of coated devices or drug products, such as regulatory approval, marketing and sales efforts of our licensees or competitive pricing pressures within the particular market. There can be no assurance that products employing our technologies will be successfully commercialized by our licensees or that such licensees will otherwise be able to compete effectively.
Competition in the diagnostics market is highly fragmented. In the product lines in which we compete (protein stabilization reagents, substrates, recombinant autoimmune antigens and surface chemistry technologies), we face an array of competitors ranging from large manufacturers with multiple business lines to small manufacturers that offer a limited selection of products. Many of our competitors have substantially more capital resources, marketing experience, research and development resources and production facilities than we do. We believe that our products compete on performance, stability (shelf life), sensitivity (lower levels detected, faster results), consistency and price. We believe that our continued competitive success will depend on our ability to develop or acquire new proprietary products, obtain patent or other protection for our products and successfully market our products directly or through partners.
Manufacturing
Historically, we have performed limited manufacturing activities for our customers.customers, other than the manufacture of ourin vitrodiagnostics products which we sell to our customers, all of which we manufacture in our Eden Prairie, Minnesota facility. In general, we do not coat medical devices that are intended for commercial sale by our customers, though we often support our customers by coating products intended for pre-clinical and clinical development, including human clinical trials.trials and on occasion, even commercial product. Some of our customers, particularly in the pharmaceutical and biotechnology industries, prefer to outsource the manufacturing of drug delivery formulations to partners. Accordingly, in April 2008, we acquired a facility in Birmingham, Alabama with approximately 286,000 square feet of warehouse and office space and constructed a cGMP manufacturing facility there in order to upgrade our manufacturing capabilities. This facility was opened and qualified in 2010. In December 2010, we announced that the Board of Directors of the Company had authorized the Company to explore strategic alternatives for the Company’s Pharmaceuticals business, including a potential sale of that business, divestiture of our manufacturing facility, or other transactions that could result in the cGMP facility not being available to us to meet our manufacturing needs.
16
We attempt to maintain multiple sources of supply for the key raw materials used to manufacture our products. We do, however, purchase some raw materials from single sources, but we believe that additional sources of supply are readily available. Further, to the extent additional sources of supply are not readily available, we believe that we could manufacture such raw materials.
We follow quality management procedures in accordance with applicable regulations and guidance for the development and manufacture of materials and pharmaceutical, device, biotechnology or combination products that support clinical trials and commercialization. In an effort to better meet our customers’ needs in this area, our Eden Prairie, Minnesota facility received ISO 1348513485:2003 and ISO 90019001:2000 certification in fiscal 2004 and has maintainedreceived updated certifications in each subsequent year. In fiscal 2010, our Birmingham, Alabama facility received ISO 9001:2008 and updated those certifications without interruption since.ISO 13485:2003 certification.
Government Regulation
Although our drug delivery and surface modification technologies themselves are not directly regulated by the Food and Drug Administration (FDA), the medical devices, pharmaceutical and biotechnology products incorporating our technologies are subject to FDA regulation. New medical devices utilizing our technologies can only be marketed in the United States after a 510(k) application has been cleared or a pre-market approval application (PMA) has been approved by the FDA. This process can take anywhere from three months for a 510(k) application, to two or three years or more for a PMA application. The burden of demonstrating to the FDA that a new device is
15
either substantially equivalent to a previously marketed device (510(k) marketing clearance process), or in the case of implantable devices, safe and effective (PMA process), rests with our customers as the medical device manufacturers. New pharmaceutical and biotechnology products utilizing our technologies can only be marketed in the United States after a New Drug Application (NDA) or Biologics License Application (BLA) has been approved by the FDA. The burden of obtaining FDA approval of the NDA or BLA rests with our customers.
In support of our customers’ regulatory filings, we maintain various confidential Drug Master Files, Device Master Files and Veterinary Master Files with the FDA and with other regulatory agencies outside the U.S. regarding the nature, chemical structure and biocompatibility of our reagents. Although our licensees generally do not have direct access to these files, they may, with our permission, reference these files in their various regulatory submissions to these agencies. This approach allows regulatory agencies to understand in confidence the details of our technologies without us having to share this highly confidential information with our customers.
U.S. legislation allows companies, prior to obtaining FDA clearance or approval to market a medical product in the U.S., to manufacture medical products in the U.S. and export them for sale in international markets. This generally allows us to realize earned royalties sooner. However, sales of medical products outside the U.S. are subject to international requirements that vary from country to country. The time required to obtain approval for sale internationally may be longer or shorter than that required by the FDA.
Employees
As of December 1, 2009,2010, we had 248215 employees, of whom 206166 were engaged in research, product development, quality, or manufacturing positions, with the remainder in sales, marketing, or administrative positions. Post-graduate degrees are held by 6469 of our employees, 3027 of whom hold Ph.D. degrees. We are not a party to any collective bargaining agreements, and we believe that our employee relations are good.
We believe that our future success will depend in part on our ability to attract and retain qualified technical, management and marketing personnel. Such experienced personnel are in high demand, and we must compete for their services with other firms that may be able to offer more favorable compensation packages or benefits.
Name | Age | Position | ||||
| Philip D. Ankeny | 47 | Interim Chief Executive Officer, Senior Vice President and Chief Financial Officer | ||||
| Timothy J. Arens | 43 | General Manager, In Vitro Diagnostics | ||||
| Charles W. Olson | 46 | Senior Vice President and General Manager, Medical Device | ||||
| Bryan K. Phillips | 39 | Senior Vice President, General Counsel and Secretary | ||||
| Eugene C. Rusch | 62 | Vice President, Manufacturing | ||||
| Joseph J. Stich | 45 | Vice President of Marketing, Corporate Development and Strategy | ||||
| Arthur J. Tipton, Ph.D. | 53 | Senior Vice President and General Manager, Pharmaceuticals | ||||
| Jan M. Webster | 51 | Vice President of Human Resources | ||||
Philip D. Ankenyjoined the Company do not relate strictlyas its Vice President and Chief Financial Officer in April 2003 with the additional responsibilities of Vice President, Business Development added in April 2004. He was promoted to historical orSenior Vice President and Chief Financial Officer in May 2006. In June 2010, Mr. Ankeny assumed the role of Interim Chief Executive Officer. Prior to joining SurModics, he served as Chief Financial Officer for Cognicity, Inc. from 1999 to 2002. Mr. Ankeny also serves on the Board of Directors of Innovex, Inc., which designs and manufactures flexible circuit interconnect solutions to original equipment manufacturers in the electronics industry.
16
Mr. Ankeny received an A.B. degree in economics and engineering from Dartmouth College in 1985 and an M.B.A. from Harvard Business School in 1989.
Timothy J. Arensjoined the Company in February 2007 as Director, Business Development. Mr. Arens became Director of Financial Planning and Analysis in October 2007. He was promoted to his current facts. As such, they are considered “forward-looking statements” that provide current expectations or forecastsrole as Senior Director of future events. These forward-looking statements are made pursuantFinancial Planning and Analysis and General Manager, In Vitro Diagnostics in October 2010. Prior to joining SurModics, Mr. Arens was employed at St. Jude Medical, a medical technology company, from 2003 to 2007 in positions of increasing responsibility related to business development and strategic planning functions. Mr. Arens received a B.S. in Finance from the University of Wisconsin Eau Claire in 1989 and an M.B.A. from the University of Minnesota’s Carlson School of Management in 1996.
Charles W. Olsonjoined the Company in July 2001 as Market Development Manager, was promoted in December 2002 to Director, Business Development, named General Manager of the Hydrophilic Technologies business unit in April 2004, and promoted to Vice President and General Manager, Hydrophilic Technologies in October 2004. In April 2005, the position of Vice President, Sales was added to his responsibilities. In November 2008, Mr. Olson was named Vice President of our Cardiovascular business unit, in March 2010 he was named Senior Vice President, Business Development and Marketing, and in October 2010, he was named Senior Vice President and General Manager, Medical Device. Prior to joining SurModics, Mr. Olson was employed as General Manager at Minnesota Extrusion from 1998 to 2001 and at Lake Region Manufacturing in project management and technical sales from 1993 to 1998. Mr. Olson received a B.S. degree in Marketing from Winona State University in 1987.
Bryan K. Phillipsjoined the Company in July 2005 as Patent Counsel and Assistant General Counsel. In January 2006, Mr. Phillips was appointed Corporate Secretary, and he was promoted to Deputy General Counsel in October 2007. He was promoted to Vice President, General Counsel and Corporate Secretary in September 2008 and was promoted to Senior Vice President in October 2010. Prior to joining SurModics, from 2001 to 2005, Mr. Phillips served as patent counsel at Guidant Corporation’s Cardiac Rhythm Management Group where he was responsible for developing and implementing intellectual property strategies and also for supporting the company’s business development function. He also practiced law at the Minneapolis-based law firm of Merchant & Gould P.C. Mr. Phillips received a B.S. degree in Mechanical Engineering from the University of Kansas in 1993 and a law degree from the University of Minnesota Law School in 1999. He is admitted to the safe harbor provisionsMinnesota bar and is registered to practice before the United States Patent and Trademark Office.
Eugene C. Ruschjoined the Company in March 2010 as Vice President of Manufacturing. Prior to joining SurModics, Mr. Rusch served Alkermes, Inc., a biotechnology company, as Vice President/General Manager-Manufacturing Operations beginning in 2004. Prior to that, he worked for over 20 years for Hoffmann-LaRoche Pharmaceutical in several managerial positions. Mr. Rusch received his B.S. degree in Microbiology from Rutgers University.
Joseph J. Stichjoined the Private Securities Litigation Reform ActCompany in March 2010 as Vice President of 1995. Such statements can be identifiedMarketing, Corporate Development and Strategy. Before joining SurModics, Mr. Stich was Vice President of Corporate Development for Abraxis BioScience, LLC, a biotechnology company focused on oncology therapeutics. Prior to joining Abraxis, he was a Vice President of MGI Pharma, Inc., a biopharmaceutical company, from 2005 to 2009. Mr. Stich’s prior experience also includes serving as President/COO of Pharmaceutical Corp. of America (a subsidiary of Publicis Healthcare Specialty Group), and positions of increasing responsibility in sales and marketing at Sanofi-Aventis Pharmaceuticals. He received his B.B.A. from the University of Wisconsin — Whitewater in 1988, and his M.B.A. from Rockhurst University in Kansas City in 1996.
Arthur J. Tipton, Ph.D.,became Vice President, SurModics and President, SurModics Pharmaceuticals, coincident with the acquisition of SurModics Pharmaceuticals by the useSurModics in July 2007. Dr. Tipton was named Senior Vice President and Chief Scientific Officer in February 2010 and in October 2010 he was named Senior Vice President, Pharmaceuticals. Dr. Tipton joined Southern Research Institute in 2004 as Vice President of terminology suchPharmaceutical Formulations and then became President and CEO of SurModics Pharmaceuticals in January 2005 when it was launched as “anticipate,” “believe,”a new company based on Southern Research Institute’s pharmaceutical formulations business. Prior to joining Southern Research Institute, Dr. Tipton served as Executive Vice President at Durect Corporation from 2001 to 2004. Dr. Tipton also held a variety of positions at Southern BioSystems (now part of Durect),
17
18
1918
| ITEM 1A. | RISK FACTORS. |
RISKS RELATING TO OUR BUSINESS, STRATEGY AND INDUSTRY
We are subject to changes in general economic conditions that are beyond our control including recession and declining consumer confidence.
During periods of economic slowdown or recession, such as the United States and world economies are currently experiencing, many of our customers are forced to delay or terminate some of their product development plans. Because we rely on licensing and commercialization of our technology by third parties, we may be severely impacted by the decreasing research and development budgets of our customers. In addition, in an environment of decreasing research and development spending, sales of our In Vitro TechnologiesDiagnostics products may similarly suffer as a result of the decreased utilization of research-focused products. Although we attempt to manage these risks, anyAny sustained period of decreased research and development spending by our customers and potential customers could adversely affect our financial position, liquidity, and results of operations.
The decrease in available financing for our customers and for new ventures that could potentially become our customers can reduce our potential opportunities.
One of the consequences of the economic slowdown has been a decrease in the availability of financing for bothstart-up and other developing ventures, which can impact our business in several ways. For example, some customers have been unable to obtain additional financing and were forced to cease their operations. Because our financial results depend substantially on the success of our customers in commercializing their products, a reduced ability by companies to take their products to market can substantially adversely affect our results of operations. In addition, the decrease in available financing has resulted in fewerstart-up medical device, specialty pharmaceutical, and biotechnology companies than in prior years. To the extent that fewer new companies are started, the number of potential customers for our technologies will be smaller, and we may be unable to meet our business goals, which could substantially affect our financial performance.
The loss of, or significant reduction in business from, one or more of our major customers could significantly reduce our revenue, earnings or other operating results.
We have two customers that each provided 10% or more of our revenue in fiscal 2009.2010. Revenue from Merck and Johnson & Johnson and Medtronic represented approximately 37%17% and 11%14%, respectively, of our total revenue for the fiscal year ended September 30, 2009. In addition, as discussed earlier, we recently entered into a License Agreement with Roche and Genentech which provided for an up front licensing fee of $3.5 million, potential payments of up to approximately $200 million in fees and milestone payments in the event of the successful development and commercialization of multiple products, and payment for development work done on these products.2010. The loss of one or more of our largest customers, or reductions in business from them, could have a material adverse effect on our business, financial condition, results of operations, and cash flow. For example, in December 2008, following a strategic review of its business and product portfolio, Merck terminated its collaboration with us relating to the development and potential commercialization of our I-vationtm intravitreal implant and we do not expect to have any revenue from Merck in fiscal 2010.implant. There can be no assurance that revenue from any customer will continue at their historical levels. If we cannot broaden our customer base, we will continue to depend on a small number of customers for a significant portion of our revenue.
The long-term success of our business may suffer if we are unable to expand our licensing base to reduce our reliance upon several major customers.
A significant portion of our revenue is derived from a relatively small number of customer products. We intend to continue pursuing a strategy of licensing our technologies to a diversified base of medical device and drug manufacturers and other customers, thereby expanding the commercialization opportunities for our technologies. Success will depend, in part, on our ability to attract new licensees, to enter into agreements for additional applications with existing licensees and to develop and market new applications. There can be no assurance that we will be able to identify, develop and adapt our technologies for new applications in a timely and cost effectivecost-effective manner; that new license agreements will be executed on terms favorable to us; that new applications will be
20
accepted by customers in our target markets; or that products incorporating newly licensed technology, including new applications, will gain regulatory approval, be commercialized or gain market acceptance. Delays or failures in these efforts could have an adverse effect on our business, financial condition and results of operations.
19
Drug delivery and surface modification are competitive markets and carry the risk of technological obsolescence.
We operate in a competitive and evolving field, and new developments are expected to continue at a rapid pace. Our success depends, in part, upon our ability to maintain a competitive position in the development of technologies and products in the field of drug delivery and surface modification. Our drug delivery and surface modification technologies compete with technologies developed by a number of other companies. In addition, many medical device manufacturers have developed, or are engaged in efforts to develop, drug delivery or surface modification technologies for use on their own devices.products. Some of our existing and potential competitors (especially medical device manufacturers pursuing coating solutions through their own research and development efforts) have greater financial and technical resources and production and marketing capabilities than us. Competitors may succeed in developing competing technologies or obtaining governmental approval for products before us. Products incorporating our competitors’ technologies may gain market acceptance more rapidly than products using ours. Developments by competitors may render our existing and potential products uncompetitive or obsolete. Furthermore, there can be no assurance that new products or technologies developed by others, or the emergence of new industry standards, will not render our products or technologies or licensees’ products incorporating our technologies uncompetitive or obsolete. Any new technologies that make our drug delivery or surface modification technologies less competitive or obsolete would have a material adverse effect on our business, financial condition and results of operations.
We could face adverse consequences as a result of the actions of a major stockholder.
According to a Schedule 13D, filed with the Securities Exchange Commission on November 17, 2010, by Ramius LLC and certain of its affiliates, Ramius beneficially owned approximately 12% of our common stock as of the date of the filing. Ramius has publicly and privately expressed opinions with respect to the operation of our business, our business strategy, and other matters. In addition, Ramius has nominated a slate of directors for election to our Board of Directors. To the extent that Ramius is successful in preventing the Company from executing on its long-term business strategy, our business and operating results could be negatively impacted. The uncertainty and negative publicity resulting from the activities of Ramius could also have a material adverse effect on our ability to attract new employees (or retain current employees), new customers and to do additional business with our existing customers. Finally, the expenses incurred in connection with, and management distraction caused by, the actions of Ramius and our responses to those actions could have a material adverse effect on our business, financial condition and results of operations.
Failure to identify strategic investment and acquisition opportunities may limit our growth.
An important part of our growth in the future may involve strategic investments and the acquisition of complementary businesses or technologies. Our identification of suitable investment opportunities and acquisition candidates involves risks inherent in assessing the technology, value, strengths, weaknesses, overall risks and profitability, if any, of investment and acquisition candidates. We may not be able to identify suitable investment and acquisition candidates. If we do not make suitable investments and acquisitions, we may find it more difficult to realize our growth objectives.
The acquisitions that we have made, or any future acquisitions that we undertake could be difficult to integrate, disrupt our business, dilute shareholder value, or harm our operating results.
In recent years we have made several significant acquisitions, including SurModics Pharmaceuticals, Inc. (formerly Brookwood Pharmaceuticals, Inc.), the largest acquisition in our history. The process of integrating acquired businesses into our operations poses numerous risks, including:
| • | an inability to assimilate acquired operations, personnel, technology, information systems, and internal control systems and products; | |
| • | diversion of management’s attention, including the need to manage several remote locations with a limited management team; | |
| • | difficulties and uncertainties in transitioning the customers or other business relationships from the acquired entity to us; and | |
| • | the loss of key employees of acquired companies. |
20
In addition, future acquisitions by us may be dilutive to our shareholders, and cause large one-time expenses or create goodwill or other intangible assets that could result in significant asset impairment charges in the future. For example, in the fourth quarter of fiscal 2010 we recognized a goodwill impairment charge of $13.8 million, which represented a full impairment of the remaining goodwill associated with our SurModics Pharmaceuticals acquisition. Strategic investments may result in impairment charges if the value of any such investment declines significantly. In addition, if we acquire entities that have not yet commercialized products but rather are developing technologies for future commercialization, our earnings per share may fluctuate as we expend significant funds for continued research and development efforts for acquired technology necessary to commercialize such acquired technology. We cannot
21
guarantee that we will be able to successfully complete any investments or acquisitions or that we will realize any anticipated benefits from investments or acquisitions that we complete.
Goodwill or other assets on our balance sheet may become impaired, which could have a material adverse effect on our operating results.
As a result of our acquisitions, we have recorded a significant amount of goodwill on our balance sheet. As required by the accounting guidance for goodwill, we evaluate at least annually the potential impairment of goodwill. Testing for impairment of goodwill involves the determination of the fair value of our reporting units. The estimation of fair values involves a high degree of judgment and subjectivity in the assumptions used. We also evaluate other assets on our balance sheet, including intangible assets, whenever events or changes in circumstances indicate that their carrying value may not be recoverable. Our estimate of the fair value of the assets may be based on fair value appraisals or discounted cash flow models using various inputs.
Future impairment of our goodwill or other assets could materially adversely affect our results of operations. For example, in the fourth quarter of fiscal 2010 we recognized an impairment charge of $13.8 million related to goodwill associated with our acquisition of SurModics Pharmaceuticals, Inc. In addition, in fiscal 2010, we recognized asset impairment charges totaling $4.9 million.
Research and development of new technologiescosts may adversely affect our operating results.
The success of our business depends on a number of factors, including our continued research and development of new technologies for future commercialization. In researching and developing such new technologies, we may incur significant expenses that may adversely affect our operating results, including our profitability. Additionally, these activities are subject to risks of failure that are inherent in the development of new medical technologies and as a result, may never result in commercially viable technologies.
Our failure to expand our management systems and controls to support anticipated growth orour business and integrate acquisitions could seriously harm our operating results and business.
We recognize revenue in accordance with various complex accounting standards, and changes in circumstances or interpretations may lead to accounting adjustments.
Our revenue recognition policies involve application of various complex accounting standards, including Securities and Exchange Commission Staff Accounting Bulletin No. 104 (SAB 104), and accounting guidance associated with revenue arrangements with multiple deliverables. Our compliance with such accounting standards often involves management’s judgment regarding whether the criteria set forth in the standards have been met such that we can recognize as revenue the amounts that we receive as payment for our products or services. We base our judgments on assumptions that we believe to be reasonable under the circumstances. However, these judgments, or the assumptions underlying them, may change over time. In addition, the SEC or the Financial Accounting Standards Board may issue new positions or revised guidance on the treatment of complex accounting matters. Changes in circumstances or third-party guidance could cause our judgments to
21
change with respect to our interpretations of these complex standards, and transactions recorded, including revenue recognized, for one or more prior reporting periods, which could be adversely affected.
RISKS RELATING TO OUR OPERATIONS AND RELIANCE ON THIRD PARTIES
We rely on third parties to market, distribute and sell themost products incorporating our technologies, and those third parties may not perform or agreements with those parties could be terminated.
A principal element of our business strategy is to enter into licensing arrangements with medical device, pharmaceutical, and biotechnology companies that manufacture products incorporating our technologies. For the fiscal years ended September 30, 2010, 2009 2008 and 2007,2008, we derived approximately 62%49%, 53%62% and 72%53% of our revenue, respectively, from royalties and license fees. Although we do market certain diagnostic products and reagents, we do not currently market, distribute or sell our own medical devices or pharmaceutical compounds, nor do we intend to do so in the foreseeable future. Thus, our prospects are greatly dependent on the receipt of royalties from licensees of our technologies. The amount and timing of such royalties are, in turn, dependent on the ability of our licensees to gain successful regulatory approval for, market and sell products incorporating our technologies. Failure of certain licensees to gain regulatory approval or market acceptance for such products could have a material adverse effect on our business, financial condition and results of operations.
Our customers market and sell (and most manufacture) the products incorporating our licensed technologies. If one or more of our licensees fail to pursue the development or marketing of these products as planned, our revenue and profits may not reach our expectations, or may decline. Additionally, our ability to generate positive operating
22
results in connection with the achievement of development or commercialization milestones may also suffer. For example, as discussed previously, Merck terminated their collaboration with us relating to the development and potential commercialization of our I-vationtm intravitreal implant following a strategic review of its business and product development portfolio in 2008. We do not control the timing and other aspects of the development or commercialization of products incorporating our licensed technologies because our customers may have priorities that differ from ours or their development or marketing efforts may be unsuccessful, resulting in delayed or discontinued products. Hence, the amount and timing of revenue we derive from our customers’ research and development as well as royalty payments received by us will fluctuate, and such fluctuations could have a material adverse effect on our business, financial condition and results of operations.
Under our standard license agreements, licensees can terminate the license for any reason upon 90 days’ prior written notice. Existing and potential licensees have no obligation to deal exclusively with us in obtaining drug delivery or surface modification technologies and may pursue parallel development or licensing of competing technological solutions on their own or with third parties. A decision by a licensee to terminate its relationship with us could materially adversely affect our business, financial condition and results of operations.
We have limited or no redundancy in our manufacturing facilities, and we may lose revenue and be unable to maintain our customer relationships if we lose our production capacity.capacity or are unable to successfully manage the transition of our BioFX manufacturing operations.
We manufacture all of the products we sell in our existing production labs in our Eden Prairie, Minnesota, Birmingham, Alabama, and Owings Mills, Maryland facilities. We have recently begun the process of migrating the production of our BioFX products from our leased facilities in Maryland to our headquarters in Eden Prairie, Minnesota. There are a number of risks associated with this move, including the loss of personnel associated with closing our Maryland facilities, damage to equipment, decreased efficiency associated with the relocation, product quality issues related to the transition, lack of continuity in key support functions and damaged customer relationships as a result of any of the above disruptions. If we experience any of the above issues in the relocation of our production operations, we could experience material adverse effects on our business, financial condition and results of operations.
In addition, if any of our existing production facilities becomes incapable of manufacturing products for any reason, we may be unable to meet production requirements, we may lose revenue and we may not be able to maintain our relationships with our customers, including certain of our licensees. In particular, because most of our customers use these reagents to create royalty-bearing products, failure by us to deliver products, including polymers and reagents, could result in decreased royalty revenue, as well as decreased revenue from the sale of products. Without our existing production facilities, we would have no other means of manufacturing products until we were able to restore the manufacturing capability at a particular facility or develop an alternative manufacturing
22
facility. Although we carry business interruption insurance to cover lost revenue and profits in an amount we consider adequate, this insurance does not cover all possible situations. In addition, our business interruption insurance would not compensate us for the loss of opportunity and potential adverse impact on relations with our existing customers resulting from our inability to produce products for them.
We have limited experience manufacturing pharmaceutical products, for commercial sale and use, and we may be subject to adverse consequences if we fail to comply with applicable regulations.regulations or contractual obligations.
Under the terms of certain of our licensing agreements, we may be obligated to manufacture pharmaceutical or biotechnology products for existing or future licensees under appropriate circumstances. In addition, certain potential customers may require that we be responsible for the manufacture of pharmaceutical or biotechnology products in order to enter into licensing agreements with us. In addition, as part of our strategy to utilize our cGMP manufacturing facility, we may manufacture products for customers other than our licensees, substantially increasing our exposure to risks related to manufacturing beyond those previously associated with our core business.
The manufacture of pharmaceutical or biotechnology products can be an expensive, time consuming, and complex process. process with which we have limited experience, and which we may not fully understand the operational or cost aspects of. For example, we have limited experience in the financial planning of contract manufacturing operations, and may not fully understand our cost structures, or may enter into contract manufacturing arrangements with customers based on financial assumptions that turn out not to be true. Any failure on our part to anticipate such issues could adversely affect our business, financial condition and results of operations.
Further, any manufacturer of pharmaceutical and biotechnology products is subject to applicable Current Good Manufacturing Practice (cGMP)cGMP regulations as prescribed by the Food and Drug AdministrationFDA or other rules and regulations prescribed by foreign regulatory authorities. Although we have purchased a facility in Alabama and have substantially completed upgrading the facility, weWe may be unable to maintain our facilities in compliance with cGMP or other applicable regulatory standards. Such a failure to comply with cGMP could result in significant time delays or inability to obtain (and maintain) marketing approval for any future products that we may be required to manufacture, which may result in financial penalties under the terms of license agreements, as well as damage our relationships with our customers in the future. Furthermore, we may be subject to sanctions, including fines and temporary or permanent suspension of operations, product recalls and marketing restrictions, if we fail to comply with the laws and regulations pertaining to our business.
We may face product liability claims related to participation in clinical trials, the use or misuse of our products or the manufacture and supply of pharmaceutical products.
The development and sale of medical devices and component products involves an inherent risk of product liability claims. Although we expect that devices incorporating our technologies will be manufactured by others and sold under their own labels, and in most cases our customer agreements provide indemnification against such claims,
23
there can be no guarantee that we will not become involved in the manufacture and supply of commercial quantities of products to licensees, that product liability claims will not be filed against us for such products, that parties indemnifying us will have the financial ability to honor their indemnification obligations or that such manufacturers will not seek indemnification or other relief from us for any such claims. Any product liability claims, with or without merit, could result in costly litigation, reduced sales, significant liabilities and diversion of our management’s time, attention and resources. We have obtained a level of liability insurance coverage that we believe is appropriate to our activities, however we cannot be sure that our product liability insurance coverage is adequate or that it will continue to be available to us on acceptable terms, if at all. Furthermore, we do not expect to be able to obtain insurance covering our costs and losses as a result of any recall of products or devices incorporating our technologies because of alleged defects, whether such recall is instituted by us, by a customer, or is required by a regulatory agency. A product liability claim, recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse effect on our business, financial condition and results of operations.
Our revenue will be harmed if we cannot purchase sufficient reagent components we use in our manufacture of reagents.
We currently purchase some of the components we use to manufacture reagents from sole suppliers. If any of our sole suppliers becomes unwilling to supply components to us, incursexperiences an interruption in its production or is otherwise unable to provide us with sufficient material to manufacture our reagents, we will experience production interruptions. If we lose our sole supplier of any particular reagent component or are otherwise unable to procure all
23
components required for our reagent manufacturing for an extended period of time, we may lose the ability to manufacture the reagents our customers require to commercialize products incorporating our technology. This could result in lost royalties and product sales, which would harm our financial results. Adding suppliers to our approved vendor list may require significant time and resources since we typically thoroughly review a supplier’s business and operations to become comfortable with the quality and integrity of the materials we purchase for use with our technology, including reviewing a supplier’s manufacturing processes and evaluating the suitability of materials and packaging procedures the supplier uses. We routinely attempt to maintain multiple suppliers of each of our significant materials, so we have alternative suppliers, if necessary. However, if the number of suppliers of a material is reduced, or if we are otherwise unable to obtain our material requirements on a timely basis and on favorable terms, our operations may be harmed.
We are dependent upon key personnel and may not be able to attract qualified personnel in the future.
Our success is dependent upon our ability to retain and attract highly qualified management and technical personnel. We face intense competition for such qualified personnel. We do not maintain key person insurance, norand we generally do we havenot enter into employment agreements, with the majority of our employees, except for with certain of our executive officers. Although we have non-compete agreements with most employees, there can be no assurance that such agreements will be enforceable or that they will serve to keep employees working for us.enforceable. The loss of the services of one or more key employees or the failure to attract and retain additional qualified personnel could have a material adverse effect on our business, financial condition and results of operations.
RISKS RELATING TO OUR INTELLECTUAL PROPERTY
If we cannot adequately protect our technologies and proprietary information, we may be unable to sustain a competitive advantage.
Our success depends, in large part, on our ability to obtain and maintain patents, operate without infringing on the proprietary rights of third parties and protect our proprietary rights against infringement by third parties. We have been granted U.S. and foreign patents and have U.S. and foreign patent applications pending related to our proprietary technologies. There can be no assurance that any pending patent application will be approved, that we will develop additional proprietary technologies that are patentable, that any patents issued will provide us with competitive advantages or will not be challenged or invalidated by third parties, or that the patents of others will not prevent the commercialization of products incorporating our technologies. Furthermore, there can be no assurance that others will not independently develop similar technologies, duplicate any of our technologies or design around our patents.
24
We may become involved in expensive and unpredictable patent litigation or other intellectual property proceedings which could result in liability for damages, or impair our development and commercialization efforts.
Our commercial success also will depend, in part, on our ability to avoid infringing patent or other intellectual property rights of third parties. There has been substantial litigation regarding patent and other intellectual property rights in the medical device and pharmaceutical industries, and intellectual property litigation may be used against us as a means of gaining a competitive advantage. Intellectual property litigation is complex, time consuming and expensive, and the outcome of such litigation is difficult to predict. If we were found to be infringing any third party patent or other intellectual property right, we could be required to pay significant damages, alter our products or processes, obtain licenses from others, which we may not be able to do on commercially reasonable terms, if at all, or cease commercialization of our products and processes. Any of these outcomes could have a material adverse effect on our business, financial condition and results of operations.
Patent litigation or U.S. Patent and Trademark Office interferencecertain other administrative proceedings may also be necessary to enforce any patents issued or licensed to us or to determine the scope and validity of third party proprietary rights. These activities could result in substantial cost to us, even if the eventual outcome is favorable to us. An adverse outcome of any such litigation or interference proceeding could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using our technology. Any action to defend or prosecute
24
intellectual property would be costly and result in significant diversion of the efforts of our management and technical personnel, regardless of outcome, and could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to keep our trade secrets confidential, our technology and proprietary information may be used by others to compete against us.
We rely significantly upon proprietary technology, information, processes and know-how that are not subject to patent protection. We seek to protect this information through trade secret or confidentiality agreements with our employees, consultants, potential licensees, or other parties as well as through other security measures. There can be no assurance that these agreements or any security measure will provide meaningful protection for our unpatented proprietary information. In addition, our trade secrets may otherwise become known or be independently developed by competitors.
If we or any of our licensees breach any of the agreements under which we have in-licensed intellectual property from others, we could be deprived of important intellectual property rights and future revenue.
We are a party to various agreements through which we have in-licensed or otherwise acquired from third parties rights to certain technologies that are important to our business. In exchange for the rights granted to us under these agreements, we agree to meet certain research, development, commercialization, sublicensing, royalty, indemnification, insurance, and other obligations. If we or one of our licensees fails to comply with these obligations set forth in the relevant agreement through which we have acquired rights, we may be unable to effectively use, license, or otherwise exploit the relevant intellectual property rights and may be deprived of current or future revenues that are associated with such intellectual property.
RISKS RELATING TO CLINICAL AND REGULATORY MATTERS
Healthcare policy changes, including pending proposalsnew legislation intended to reform the U.S. healthcare system, may have a material adverse effect on us.
Healthcare costs have risen significantly over the past decade. There have been and continue to be proposals by legislators, regulators, and third-party payors to keep these costs down. Certain proposals, if implemented, would impose limitations on the prices our customers will be able to charge for theirour products, or the amounts of reimbursement available for their products from governmental agencies or third-party payors. Because a portion of our revenue is typically derived from royalties on products which constitute a percentage of the selling price, these limitations could have an adverse effect on our revenue.
25
Products incorporating our technologies are subject to continuing regulations and extensive approval or clearance processes. If our licensees are unable to obtain or maintain the necessary regulatory approvals or clearances for such products, then our licensees will not be able to commercialize those products on a timely basis, if at all.
Medical devices, biotechnology products or pharmaceutical products incorporating theour technologies are subject to regulation by the Food and Drug Administration (FDA)FDA and other regulatory authorities. In order to obtain regulatory approval for products incorporating our technologies, extensive preclinical studies as well as clinical trials in humans may be required. Clinical development, including preclinical testing, is a long, expensive and uncertain process. The burden of securing regulatory approval for these products typically rests with our licensees, the medical device or
25
pharmaceutical customer.manufacturers. However, we have prepared Drug Master Files and Device Master Files which may be accessed by the FDA and other regulatory authorities to assist them in their review of the applications filed by our licensees.
The process of obtaining FDA and other required regulatory approvals is expensive and time-consuming. Historically, most medical devices incorporating our technologies have been subject to the FDA’s 510(k) marketing approval process, which typically lasts from six to nine months. Supplemental or full pre-market approval reviews require a significantly longer period, delaying commercialization. By contrast, pharmaceutical products incorporating our technologies are subject to the FDA’s New Drug Application process, which typically takes a number of years to complete. Additionally, biotechnology products incorporating our technologies are subject to the FDA’s Biologics License Application process, which also typically takes a number of years to complete. In addition, sales of medical devices and pharmaceutical or biotechnology products outside the U.S. are subject to international regulatory requirements that vary from country to country. The time required to obtain approval for sale internationally may be longer or shorter than that required for FDA approval.
There can be no assurance that our licensees will be able to obtain regulatory approval for their products on a timely basis, orif at all. Regulatory approvals, if granted, may include significant limitations on the indicated uses for which the product may be marketed. In addition, product approval could be withdrawn for failure to comply with regulatory standards or the occurrence of unforeseen problems following initial marketing. Changes in existing regulations or adoption of new governmental regulations or policies could prevent or delay regulatory approval of products incorporating our technologies or subject us to additional regulation. Failure or delay of our licensees in obtaining FDA and other necessary regulatory approval or clearance, or the loss of previously obtained approvals, could have a material adverse effect on our business, financial condition and results of operations.
We may face liability if we mishandle or improperly dispose of the hazardous materials used in some of our research, development and manufacturing processes.
Our research, development and manufacturing activities sometimes involve the controlled use of various hazardous materials. Although we believe that our safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. While we currently maintain insurance in amounts that we believe are appropriate, we could be held liable for any damages that might result from any such event. Any such liability could exceed our insurance and available resources and could have a material adverse effect on our business, financial condition and results of operations.
26
Additionally, certain of our activities are regulated by federal and state agencies in addition to the FDA. For example, activities in connection with disposal of certain chemical waste are subject to regulation by the U.S. Environmental Protection Agency. We could be held liable in the event of improper disposal of such materials, even if these acts were done by third parties. Some of our reagent chemicals must be registered with the agency, with basic information filed related to toxicity during the manufacturing process as well as the toxicity of the final product. Failure to comply with existing or future regulatory requirements could have a material adverse effect on our business, financial condition and results of operations.
RISKS RELATING TO OUR SECURITIES
Our stock price has been volatile and may continue to be volatile.
The trading price of our common stock has been, and is likely to continue to be, highly volatile, in large part attributable to developments and circumstances related to factors identified in “Forward-Looking Statements” and “Risk Factors.” The market value of shares of our common stock may rise or fall sharply at any time because of this volatility, as a result of large sales executed by significant holders of our stock, and also because of significant short positions taken by investors from time to time in our stock. In the fiscal year ended September 30, 2009,2010, the closing sale price for our common stock ranged from $15.96$10.67 to $31.69$30.69 per share. In addition, since the end of our fiscal year through November 30, 2010 the closing sale price for our common stock has ranged from $8.33 to $13.11 per share. The market prices for securities of medical technology, drug delivery and biotechnology companies historically have been highly volatile, and the market has experienced significant price and volume fluctuations that aremay be unrelated to the operating performance of particular companies.
26
| ITEM 1B. | UNRESOLVED STAFF COMMENTS. |
None.
| ITEM 2. | PROPERTIES. |
Our principal operations are located in Eden Prairie, a suburb of Minneapolis, Minnesota, where we own a building that has approximately 64,000 square feet of space. We also own an undeveloped parcel of land adjacent to our principal facility, which we intend to use to accommodate our growth needs.needs, and have leased additional warehouse space near our owned facility.
In addition to our Eden Prairie facility,facilities, we also own and lease facilities in Birmingham, Alabama in connection with our SurModics Pharmaceuticals operations. The facility which we acquired in the SurModics Pharmaceuticals acquisition consists of approximately 33,000 square feet. In April 2008, we acquired a second building in Birmingham, Alabama that has approximately 286,000 square feet in order to upgrade ourwhich we have constructed a cGMP (current good manufacturing capabilities. We also lease an approximately 14,000 square foot facility in Birmingham which contains three cleanroom suites primarily used for the manufacture of drug productspractice) development and a separate facility in Birmingham containing approximately 4,500 square feet of warehouse space.manufacturing facility. We also lease facilities in Owings Mills, Maryland in connection withthat are used for general office space and manufacturing for our BioFX operations andoperations. We also lease office space in Irvine, California, which we vacated and subleased in connection with our March 2010 reorganization. In December 2010, we announced that the Board of Directors of the Company had authorized the Company to explore strategic alternatives for use bythe Company’s Pharmaceuticals business, including a potential sale of that business, divestiture of our Ophthalmology business unit.manufacturing facility, or other transactions that could result in the cGMP facility not being available to us to meet our manufacturing needs.
| ITEM 3. | LEGAL PROCEEDINGS. |
See Note 9 to the Consolidated Financial Statements for information regarding commitments and contingencies.
27
| ITEM 4. |
28
29
3027
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
Our stock is traded on the Nasdaq Global Select Market under the symbol “SRDX.” The table below sets forth the range of high and low sale prices, by quarter, for our Common Stock, as reported by Nasdaq, in each of the last two fiscal years.
Fiscal Quarter Ended: | High | Low | High | Low | ||||||||||||
| September 30, 2010 | $ | 16.68 | $ | 10.62 | ||||||||||||
| June 30, 2010 | 22.25 | 15.00 | ||||||||||||||
| March 31, 2010 | 23.31 | 19.00 | ||||||||||||||
| December 31, 2009 | 31.00 | 22.05 | ||||||||||||||
| September 30, 2009 | $ | 25.14 | $ | 20.87 | 25.14 | 20.87 | ||||||||||
| June 30, 2009 | 23.40 | 17.95 | 23.40 | 17.95 | ||||||||||||
| March 31, 2009 | 27.42 | 15.96 | 27.42 | 15.96 | ||||||||||||
| December 31, 2008 | 31.69 | 18.95 | 31.69 | 18.95 | ||||||||||||
| September 30, 2008 | 45.06 | 28.05 | ||||||||||||||
| June 30, 2008 | 47.88 | 42.00 | ||||||||||||||
| March 31, 2008 | 55.40 | �� | 38.17 | |||||||||||||
| December 31, 2007 | 56.09 | 48.35 | ||||||||||||||
Our transfer agent is:
American Stock Transfer & Trust Company
59 Maiden Lane, Plaza Level
New York, New York 10038
(800) 937-5449
59 Maiden Lane, Plaza Level
New York, New York 10038
(800) 937-5449
According to the records of our transfer agent, as of December 7, 2009,9, 2010, there were 264217 holders of record of our Common Stockcommon stock and approximately 10,8256,493 beneficial owners of shares registered in nominee or street name.
We have never paid any cash dividends on our Common Stockcommon stock and do not anticipate doing so in the foreseeable future.
The following table presents information with respect to purchases of common stock of the Company made during the three months ended September 30, 2009,2010, by the Company or on behalf of the Company or any “affiliated purchaser” of the Company, as defined inRule 10b-18(a)(3) under the Exchange Act.
| (c) | (d) | |||||||||||||||
| Total Number | Approximate Dollar | |||||||||||||||
| of Shares | Value of | |||||||||||||||
| Purchased | Shares That | |||||||||||||||
| as Part of | May Yet Be | |||||||||||||||
| (a) | (b) | Publicly | Purchased | |||||||||||||
| Total Number | Average | Announced | Under the | |||||||||||||
| of Shares | Price Paid | Plans or | Plans or | |||||||||||||
Period | Purchased(1) | per Share(1) | Programs | Programs(2) | ||||||||||||
| 7/1/09 — 7/31/09 | 4,655 | $ | 22.93 | 0 | $ | 7,333,728 | ||||||||||
| 8/1/09 — 8/31/09 | 0 | NA | 0 | $ | 7,333,728 | |||||||||||
| 9/1/09 — 9/30/09 | 203 | $ | 24.02 | 0 | $ | 7,333,728 | ||||||||||
| Total | 4,858 | $ | 22.97 | 0 | $ | 7,333,728 | ||||||||||
| (c) | (d) | |||||||||||||||
| Total Number | Approximate Dollar | |||||||||||||||
| of Shares | Value of | |||||||||||||||
| Purchased | Shares That | |||||||||||||||
| as Part of | May Yet Be | |||||||||||||||
| (a) | (b) | Publicly | Purchased | |||||||||||||
| Total Number | Average | Announced | Under the | |||||||||||||
| of Shares | Price Paid | Plans or | Plans or | |||||||||||||
Period | Purchased(1) | per Share(1) | Programs | Programs(2) | ||||||||||||
| 7/1/10 — 7/31/10 | 10,769 | $ | 13.28 | 0 | $ | 5,302,113 | ||||||||||
| 8/1/10 — 8/31/10 | 0 | NA | 0 | $ | 5,302,113 | |||||||||||
| 9/1/10 — 9/30/10 | 841 | $ | 10.93 | 0 | $ | 5,302,113 | ||||||||||
| Total | 11,610 | $ | 13.11 | 0 | $ | 5,302,113 | ||||||||||
| (1) | The purchases in this column were repurchased by the Company to pay the exercise price and/or to satisfy tax withholding obligations in connection with so-called “stock swap exercises” related to the vesting of employee restricted stock | |
| (2) | On November 15, 2007, our Board of Directors announced the authorization of the repurchase of $35 million of our outstanding common stock. As of September 30, |
3128
Stock Performance Chart
The following chart compares the cumulative total shareholder return on the Company’s Common Stock with the cumulative total return on the Nasdaq Stock Market and the Nasdaq Medical Industry Index (Medical Devices, Instruments and Supplies). The comparison assumes $100 was invested on September 30, 20042005 and assumes reinvestment of dividends.

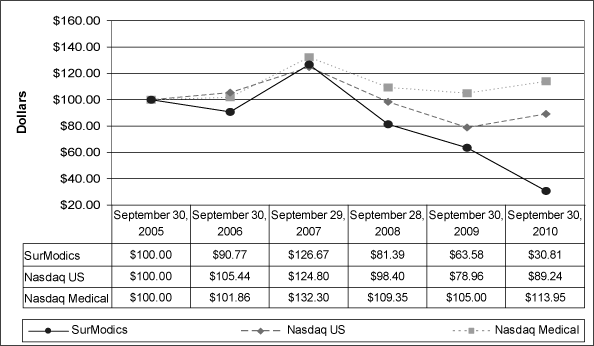
3229
| ITEM 6. | SELECTED FINANCIAL DATA. |
The data presented below as of and for the fiscal years ended September 30, 2010, 2009 2008 and 20072008 are derived from our audited consolidated financial statements included elsewhere in this report. The financial data as of and for the fiscal years ended September 30, 20062007 and 20052006 are derived from our audited financial statements which are not included in this report. The information set forth below should be read in conjunction with the Company’s consolidated financial statements and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in Item 7 of this report and our consolidated financial statements and related notes beginning onpage F-1 and other financial information included in this report.
| Fiscal Year | Fiscal Year | |||||||||||||||||||||||||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | 2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||||||||||||||||||||||
| (Dollars in thousands, except per share data) | (Dollars in thousands, except per share data) | |||||||||||||||||||||||||||||||||||||||
| Statements of Operations Data: | ||||||||||||||||||||||||||||||||||||||||
| Total revenue | $ | 121,534 | $ | 97,051 | $ | 73,164 | $ | 69,884 | $ | 62,381 | $ | 69,898 | $ | 121,534 | $ | 97,051 | $ | 73,164 | $ | 69,884 | ||||||||||||||||||||
| Operating income | 57,501 | 27,261 | 9,899 | 36,163 | 2,985 | |||||||||||||||||||||||||||||||||||
| Net income (loss) | 37,550 | 14,739 | 3,347 | 20,334 | (8,246 | ) | ||||||||||||||||||||||||||||||||||
| Diluted net income (loss) per share | 2.15 | 0.80 | 0.18 | 1.09 | (0.45 | ) | ||||||||||||||||||||||||||||||||||
| Operating (loss) income | (14,053 | ) | 57,501 | 27,261 | 9,899 | 36,163 | ||||||||||||||||||||||||||||||||||
| Net (loss) income | (21,089 | ) | 37,550 | 14,739 | 3,347 | 20,334 | ||||||||||||||||||||||||||||||||||
| Diluted net (loss) income per share | (1.21 | ) | 2.15 | 0.80 | 0.18 | 1.09 | ||||||||||||||||||||||||||||||||||
| Balance Sheet Data: | ||||||||||||||||||||||||||||||||||||||||
| Cash, short-term and long-term investments | $ | 47,868 | $ | 71,978 | $ | 70,225 | $ | 106,571 | $ | 73,319 | $ | 56,786 | $ | 47,868 | $ | 71,978 | $ | 70,225 | $ | 106,571 | ||||||||||||||||||||
| Total assets | 185,562 | 191,028 | 171,331 | 157,402 | 124,225 | 170,279 | 185,562 | 191,028 | 171,331 | 157,402 | ||||||||||||||||||||||||||||||
| Retained earnings | 103,989 | 66,439 | 51,620 | 48,273 | 27,914 | 82,900 | 103,989 | 66,439 | 51,620 | 48,273 | ||||||||||||||||||||||||||||||
| Total stockholders’ equity | 172,372 | 141,806 | 130,922 | 145,203 | 115,581 | 154,359 | 172,372 | 141,806 | 130,922 | 145,203 | ||||||||||||||||||||||||||||||
| Statements of Cash Flows Data: | ||||||||||||||||||||||||||||||||||||||||
| Net cash provided by operating activities | $ | 22,008 | $ | 31,321 | $ | 39,822 | $ | 50,715 | $ | 35,279 | ||||||||||||||||||||||||||||||
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
The following discussion and analysis of our financial condition, results of operations and trends for the future should be read together with “Selected Financial Data” and our audited consolidated financial statements and related notes appearing elsewhere in this report. Any discussion and analysis regarding trends in our future financial condition and results of operations are forward-looking statements that involve risks, uncertainties and assumptions, as more fully identified in “Forward-Looking Statements” and “Risk Factors.” Our actual future financial condition and results of operations may differ materially from those anticipated in the forward-looking statements.
Overview
SurModics is a leading provider of drug delivery and surface modification technologies to the healthcare industry. In November 2008,March 2010 we announced a change in our organizationaloperational structure into four clinically and market focused business units: Cardiovascular, Ophthalmology, SurModics Pharmaceuticals, and In Vitro Technologies. We believe that this structure improvesto better align functional expertise, which resulted in the visibility, marketing and adoptionelimination of the Company’s broad array of technologies within specific markets and helps our customers in the medical device, pharmaceutical and life science industries better solve unmet clinical needs. In addition, a new centralized research and development function has been formed to serve the needs of the Company’s clinically and market focused business units, other than the SurModics Pharmaceuticals business unit, which continues to maintain certain R&D operations.units.
The reorganizationorganizational change resulted indid not diminish the Company being comprised of newCompany’s continued market focused business units.focus. The “Therapeutic” contains:market includes revenue from: (1) the Cardiovascular, business unit, which provides drug delivery and surface modification technologies to customers in the cardiovascular market; (2) the Ophthalmology, business unit, which is dedicated tofocused on the advancement of treatments for eye diseases, such as age-related macular degeneration (AMD) and diabetic macular edema (DME), two of the leading causes of blindness; and (3) Other Markets, which is focused on a variety of clinical markets principally in the SurModics Pharmaceuticals business unit, which provides proprietary polymer-based drug delivery technologies to companies developing improved pharmaceutical products. Revenue results in Therapeutic are presented byand biotechnology industries. The “Diagnostic” market includes revenue from the clinical market areas in which our customers participate (Cardiovascular, Ophthalmology and Other Markets). “Diagnostic” contains the In Vitro Technologies business unit, which includes ourCompany’s microarray slide technologies, our stabilization products, antigens and substrates for immunoassay diagnostic tests, and ourin vitrodiagnostic format technology.
In October 2010, we announced initiatives intended to reduce our cost structure. As part of these initiatives, the Company implemented a change in its organizational structure to reflect our three complementary, but distinct business units: Medical Device, Pharmaceuticals, and In Vitro Diagnostics. Because this change occurred in fiscal 2011 and is therefore not useful in explaining our fiscal 2010 results, we will describe our business below as it was conducted in fiscal 2010. Beginning with the first quarter of fiscal 2011, we will describe our business under the new reporting structure.
3330
Our revenue is derived from three primary sources: (1) royalties and license fees from licensing our proprietary drug delivery and surface modification technologies andin vitrodiagnostic formats to customers; the vast majority (typically in excess of 90%) of revenue in the “royalties and license fees” category is in the form of royalties; (2) the sale of polymers and reagent chemicals, stabilization products, antigens, substrates and microarray slides to the diagnostics and biomedical research industry; and (3) research and development fees generated on customer projects. Revenue fluctuates from quarter to quarter depending on, among other factors: our customers’ success in selling products incorporating our technologies; the timing of introductions of licensed products by customers; the timing of introductions of products that compete with our customers’ products; the number and activity level associated with customer development projects; the number and terms of new license agreements that are finalized; the value of reagent chemicals and other products sold to customers; and the timing of future acquisitions we complete, if any.
For financial accounting and reporting purposes, we report our results in one reportable segment. We made this determination because each business unit has similar economic characteristics;we manage our sales and marketing efforts and our expenses on a company-wide basis. In addition, a significant percentage of our employees provide support services (including research and development) to each business unit; technologya variety of customers, and our technologies and products from each business unit are marketed to the same or similar customers; each business unit uses the same sales and marketing resources; and each business unit operates in the same regulatory environment.customers.
In June 2007, we entered into a License and Research Collaboration Agreement and separate Supply Agreement with Merck & Co., Inc. (“Merck”) related to our I-vationtm TA (triamcinolone acetonide) intravitreal implant. Under the terms of the Merck agreements, we received an up frontupfront license fee of $20 million and were eligible to receive up to an additional $288 million in fees and development milestones associated with the successful product development and attainment of appropriate U.S. and EU regulatory approvals, as well as payment for our research and development activities. In September 2008, following a strategic review of its business and product development portfolio, Merck gave notice that it was terminating the collaborative research and license agreement, as well as the supply agreement entered into in June 2007. This decision was not based on any concerns about the safety or efficacy of the I-vation system. The termination was effective in December 2008, and we have recognized revenue related to the termination of approximately $45 million in fiscal 2009, principally from amounts that previously had been deferred and amortized under the accounting treatment required by accounting guidance for revenue arrangements with multiple deliverables. The $45 million includes a $9 million milestone payment associated with the termination of the triamcinolone acetonide development program.
In November 2008, we acquired a portfolio of intellectual property and collaborative drug delivery projects from PR Pharmaceuticals, Inc., a drug delivery company specializing in injectable, biodegradable sustained release formulations. Total consideration paid through September 30, 20092010 was $5.6 million and PR Pharmaceuticals, Inc. is eligible to receive up to an additional $3.6 million in cash upon successful achievement of specified milestones. The proprietary technologies we acquired complement and enhance our existing portfolio of drug delivery capabilities by providing a broader toolkit for protein delivery and the ability to use smaller gauge needles for microparticle injections. In addition, the multiple customer development programs we assumed complement the diversified portfolio of customer projects at SurModics Pharmaceuticals, and we believe will further leverage the investment we are making in cGMP manufacturing.
On October 5, 2009, we entered into a License and Development Agreement with F. Hoffmann-La Roche, Ltd. (“Roche”) and Genentech, Inc., a wholly-owned member of the Roche Group (“Genentech”). Under the terms of the License Agreement, Roche and Genentech will have an exclusive license to develop and commercialize a sustained drug delivery formulation of Lucentis® (ranibizumab injection) utilizing SurModics’ proprietary biodegradable microparticles drug delivery system. Under the terms of the agreement, we received an up frontupfront licensing fee of $3.5 million and are eligible to receive potential payments of up to approximately $200 million in fees and milestone payments in the event of the successful development and commercialization of multiple products, as well as payment for development work done on these products. Roche and Genentech will have the right to obtain manufacturing services from SurModics. In the event a commercial product is developed, we will also receive royalties on sales of such products.product.
34
Critical Accounting Policies
The discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the
31
United States of America. The preparation of these financial statements is based in part on the application of significant accounting policies, many of which require management to make estimates and assumptions (see Note 2 to the consolidated financial statements). Actual results may differ from these estimates under different assumptions or conditions and could materially impact our results of operations. We believe the following are critical areas in the application of our accounting policies that currently affect our financial condition and results of operations.
Revenue recognition. In accordance with accounting guidance, revenue is recognized when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) shipment has occurred or delivery has occurred if the terms specify destination; (3) the sales price is fixed or determinable; and (4) collectability is reasonably assured. However, when there are additional performance requirements, revenue is recognized when such requirements have been satisfied. The Company licenses technology to third parties and collects royalties. Royalty revenue is generated when a licensed customer sells products incorporating ourthe Company’s licensed technologies. Royalty revenue is recognized as our licensees report it to us, and payment is typically submitted concurrently with a quarterly report. This revenue recognition model is similar to usage fee accounting. Minimum royalty fees are recognized in the period earned, provided that collectability is reasonably assured. For stand-alone license agreements, up-front license fees are recognized over the economic life of the technology.
Revenue related to a performance milestone is recognized upon achievement of the milestone and meeting specific revenue recognition criteria. We recognize initial license fees over the term of the related agreement. Minimum royalty fees are recognized in the period earned. Product sales to third parties are recognized at the time of shipment, provided that an order has been received, the price is fixed or determinable, collectability of the resulting receivable is reasonably assured and returns can be reasonably estimated. Our sales terms provide no right of return outside of our standard warranty policy. Payment terms are generally set at30-45 days. Generally, revenue for research and development is recorded as performance progresses under the applicable contract. When we have revenue
Revenue arrangements with multiple deliverables we comply with currenthave been accounted for based on accounting guidance in existence at the time the arrangement commences. Prior to October 1, 2009, arrangements such as license and recognizedevelopment agreements were analyzed to determine whether the deliverables, which often include a license and performance obligations such as research and development, could be separated, or whether they must be accounted for as a single unit of accounting in accordance with accounting guidance. If the fair value of the undelivered performance obligations could be determined, such obligations would then be accounted for separately. If the license was considered to either (i) not have stand-alone value or (ii) have stand-alone value but the fair value of any of the undelivered performance obligations could not be determined, the arrangement would then be accounted for as a single unit of accounting, and the license payments and payments for performance obligations would be recognized as revenue over the estimated period of when the performance obligations are performed, or the economic life of the technology licensed to the customer. When we determined that an arrangement should be accounted for as a single unit of accounting, we recognized the related revenue on a time-based accounting model.
The Company had one significant multiple element arrangement prior to October 1, 2009 that was accounted for as a single unit of accounting resulting in deferral and recognition of all related payments received for license and research and development activities using a time-based model. This arrangement was terminated during the first quarter of fiscal 2009.
In October 2009, the accounting standards for multiple deliverable revenue arrangements were amended to:
(i) provide updated guidance on whether multiple deliverables exist, how the deliverables in an arrangement should be separated, and how the consideration should be allocated;
(ii) require an entity to allocate revenue in an arrangement using estimated selling prices (ESP) of deliverables if a vendor does not have vendor-specific objective evidence of selling price (VSOE) or third-party evidence of selling price (TPE); and
(iii) eliminate the use of the residual method and require an entity to allocate revenue using the relative selling price method.
We elected to early adopt this accounting guidance at the beginning of our first quarter of fiscal 2010, on a prospective basis, for applicable transactions originating or materially modified after October 1, 2009. In connection with the adoption of the amended accounting standard we also changed our policy prospectively
32
for multiple element arrangements, whereby we account for revenue using a multiple attribution model in which consideration allocated to research and development activities is recognized as performed, and milestone payments are recognized when the milestone events are achieved, when such activities and milestones are deemed substantive. Accordingly, in situations where a unit of accounting includes both a license and research and development activities, and when a license does not have stand-alone value, the Company applies a multiple attribution model in which consideration allocated to the license is recognized ratably, consideration allocated to research and development activities is recognized as performed and milestone payments are recognized when the milestone events are achieved, when such activities and milestones are deemed substantive.
The Company enters into license and development arrangements that may consist of multiple deliverables which could include a license(s) to SurModics’ technology, research and development activities, manufacturing services, and product sales based on the needs of its customers. For example, a customer may enter into an arrangement to obtain a license to SurModics’ intellectual property which may also include research and development activities, and supply of products manufactured by SurModics. For these services provided, SurModics could receive upfront license fees upon signing of an agreement and granting the license, fees for research and development activities as such activities are performed, milestone payments contingent upon advancement of the product through development and clinical stages to successful commercialization, fees for manufacturing services and supply of product, and royalty payments based on customer sales of product incorporating SurModics’ technology. Our license and development arrangements generally do not have refund provisions if the customer cancels or terminates the agreement. Typically all payments made are non-refundable.
We evaluate each deliverable in a multiple element arrangement for separability. We are then required to allocate revenue to each separate deliverable using a hierarchy of VSOE, TPE, or ESP. In certain instances, we are not able to establish VSOE for all deliverables in an arrangement with multiple elements which may be a result of SurModics infrequently selling each element as itseparately. When VSOE cannot be established, SurModics establishes a selling price of each element based on TPE. TPE is earned.determined based on competitor prices for similar deliverables when sold separately.
When we are unable to establish a selling price using VSOE or TPE, we use ESP in our allocation of arrangement consideration. The objective of ESP is to determine the price at which SurModics would transact a sale if the product or service were sold on a stand-alone basis. ESP is generally used for highly customized offerings.
SurModics determines ESP for undelivered elements by considering multiple factors including, but not limited to, market conditions, competitive landscape and past pricing arrangements with similar characteristics.
Costs related to products delivered are recognized in the period revenue is recognized except for services related to the Merck agreement, which have been recognized as incurred. Customer advances are accounted for as a liability until all criteria for revenue recognition have been met.
Valuation of long-lived assets. WeAccounting guidance requires us to periodically evaluate whether events and circumstances have occurred that may affect the estimated useful life or the recoverability of the remaining balance of long-lived assets, such as property and equipment.equipment and intangibles. If such events or circumstances were to indicate that the carrying amount of these assets would not be recoverable, we would estimate the future cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected future cash flows (undiscounted and without interest charges) or other measure of fair value were less than the carrying amount of the assets, we would recognize an impairment charge.
In fiscal 2010, we recognized asset impairment charges totaling $4.9 million. We wrote down facility-related assets in Alabama by $1.9 million to their fair value based on a decision to sell the assets, however based on further analysis of various factors associated with the consolidation of facilities we later decided not to sell the facility. The carrying value of the facility is $2.1 million at September 30, 2010, which is based on a real estate appraisal obtained during our negotiations. We also wrote down certain project- and technology-related assets totaling $1.7 million, as there were very limited business opportunities expected in light of current market conditions and general economic environment. SurModics also incurred a charge of $1.3 million associated with certain fixed assets in Minnesota given the current level of business activity and overall economic conditions. Each of these events included analysis of expected future cash flows or real estate market data which was compared to the carrying values of the assets to
33
determine the impairment charges that were recognized. The assets associated with these charges had limited remaining value and as such were written down to zero value at September 30, 2010.
Goodwill. Goodwill represents the excessWe record all assets and liabilities acquired in purchase acquisitions, including goodwill, at fair value as required by accounting guidance for business combinations. The initial recognition of the costgoodwill requires management to make subjective judgments concerning estimates of how the acquired entities overassets will perform in the fair value assigned to the assets purchased and liabilities assumed in connection with the Company’s acquisitions. future using valuation methods including discounted cash flow analysis.
Goodwill is not amortized but is subject, at a minimum, to annual tests for impairment in accordance with accounting guidance.guidance for goodwill. Under certain situations, interim impairment tests may be required if events occur or circumstances change indicating that would more likely than not reduce the fair value of a reporting unit below its carrying amount of goodwill may be impaired.amount.
Evaluating goodwill for impairment involves the determination of the fair value of our reporting units in which we have recorded goodwill. A reporting unit is a component of our resultsan operating segment for which discrete financial information is available and reviewed by management on a regular basis. SurModics has
We have determined that itsour reporting units are itsour SurModics Pharmaceuticals, business unit, a component within Therapeutics, andInc. (SurModics Pharma) subsidiary, the In Vitro TechnologiesDiagnostics operations and the SurModics drug delivery and hydrophilic coatings operations. The Company reorganized in March 2010 which resulted in the elimination of the Company’s business unit.units. The reporting units with goodwill resulted from the acquisitions of SurModics Pharma and BioFX Laboratories, Inc. in fiscal 2007. Inherent in the determination of fair value of our reporting units are certain estimates and judgments, including the interpretation of current economic indicators and market valuations as well as our strategic plans with regard to our operations.
We performed our annual impairment test of goodwill in the fourth quarter of fiscal 20092010 and recognized a non-cash goodwill impairment charge of $13.8 million, which represented a full impairment of the goodwill associated with our SurModics Pharma reporting unit. Prior to testing goodwill for impairment we tested our definite-lived assets, property, plant and equipment as well as intangible assets, under the provisions of the accounting guidance for impairment or disposal of long-lived assets, and determined that there were no impairments of these assets. We did not record any goodwill impairment charges during fiscal 2009 or 2008.
The goodwill impairment in fiscal 2010 reflected a significant decline in the estimated fair value of our reporting units, which resulted from a slowdown in business activity which was most pronounced in the fourth quarter of fiscal 2010, higher operating costs with our recently placed in-service cGMP manufacturing facility, and a significant decrease in our stock price during the year. Our stock price declined from $24.13 per share at October 1, 2009 to $12.03 per share at the date of our annual impairment test, which was August 31, 2010. While we continually evaluate whether any indications of impairment are present that would require an impairment charge. analysis on an interim basis, no such indicators were considered present prior to the fourth quarter of fiscal 2010. Prior to the fourth quarter, based on our outlook for future results and the fact that our market capitalization exceeded our book value by a margin of 64% at June 30, 2010, we did not believe that the events and circumstances in existence at our interim reporting dates indicated that it was more likely than not that the fair value of any of our reporting units would be less than its carrying amount.
In evaluating whether goodwill was impaired, we compared the fair value of the reporting units to which goodwill is assigned to their carrying value (step onevalues (Step 1 of the impairment test). In calculating fair value, we used the income approach as our primary indicator of fair value, with the market approach used as a test of reasonableness. The income approach is a valuation technique based on multiples of revenue and bookunder which we estimate future cash flows using the reporting units’ financial forecasts. Future estimated cash flows are discounted to their present value for comparable companies since the technique is consistent with the objective of measuringto calculate fair value. The comparisonmarket approach establishes fair value by comparing our company to other publicly traded guideline companies selected haveor by analysis of actual transactions of similar businesses or assets sold. The income approach is tailored to the circumstances of our business, and the market approach is completed as a secondary test to ensure that the results of the income approach are reasonable and in line with comparable companies in the industry. The summation of our reporting units’ fair values was compared and reconciled to our market capitalization as of the date of our impairment test.
In the situation where a reporting unit’s carrying amount exceeds its fair value, the amount of the impairment loss must be measured. The measurement of the impairment (Step 2 of the impairment test) is calculated by determining the implied fair value of a reporting unit’s goodwill. In calculating the implied fair value of goodwill,
3534
In determining the fair value of our SurModics Pharma reporting unit under the income approach, our expected cash flows are affected by various assumptions. Fair value on a discounted cash flow basis used forecasts over a ten year period with an estimation of residual growth rates thereafter. We use our business plans and projections as the basis for expected future cash flows. The most significant assumptions incorporated in these forecasts for the most recent goodwill impairment tests included annual revenue changes based on current customer programs and expected progression of programs into different phases of development. A discount rate of 15 percent was used in the 2010 analysis to reflect the relevant risks of the higher growth assumed for this reporting unit. Given the significant difference between the reporting unit’s fair value and carrying value, any change in the discount rate would not have changed the evaluation of impairment.
In estimating the fair value of our company under the market approach, we considered the relative merits of commonly applied market capitalization multiples based on the availability of data. Based on our analysis, we utilized the guideline public company method to support the valuation of the reporting units.
Based on the goodwill analysis performed as of August 31, 2010, the $13.8 million of goodwill in the SurModics Pharma reporting unit failed Step 1 of the impairment test, and Step 2 of the impairment test indicated that goodwill was fully impaired. We also anticipate that this reporting unit may achieve additional milestone obligations of $5.7 million in fiscal 2011 and we may record a goodwill impairment charge for this amount in fiscal 2011. The indicated excess in fair value over carrying value of the Company’s In Vitro Diagnostics reporting unit in Step 1 of the impairment test at August 31, 2010 was approximately 82% and as such the $8.0 million of goodwill related to this reporting unit is not impaired. To the extent that actual results or other assumptions about future economic conditions or potential for our growth and profitability in this business changes, it is possible that our conclusion regarding the goodwill could change, which could have a material effect on our financial position and results of operations. The SurModics drug delivery and hydrophilic coatings operations does not have any goodwill and was included in the analysis to assist in reconciling the fair value of all reporting units to the Company’s market capitalization at August 31, 2010. See Note 2 to the consolidated financial statements for which indefinite-lived assets were being evaluated.further information.
Investments. Investments consist principally of U.S. government and government agency obligations and mortgage-backed securities and are classified asavailable-for-sale orheld-to-maturity at September 30, 2009.2010. Our investment policy calls for no more than 5% of investments be held in any one credit issue, excluding U.S. government and government agency obligations, net of tax.obligations.Available-for-sale investments are reported at fair value with unrealized gains and losses excluded from operations and reported as a separate component of stockholders’ equity, except forother-than-temporary impairments, which are reported as a charge to current operations and result in a new cost basis for the investment in accordance with accounting guidance.investment. Our evaluation of theavailable-for-sale investments resulted in no loss recognition in fiscal 20092010 and loss recognition of $4.3 million related to our investment in OctoPlus N.V. (included in Other Assets in the consolidated balance sheets) in fiscal 2008, as we determined the loss to be another-than-temporary impairment based on a significant decline in the stock price as of September 30, 2008. The impairment of the OctoPlus N.V. investment resulted in a new cost basis.2009. Investments for which management has the intent and ability to hold to maturity are classified asheld-to-maturity and reported at amortized cost. If there iswas another-than-temporary impairment in the fair value of any individual security classified asheld-to-maturity, the Company willwould write down the security to fair value with a corresponding adjustment to other income (loss). Interest on debt securities, including amortization of premiums and accretion of discounts, is included in other income (loss). Realized gains and losses from the sales of debt securities, which are included in other income (loss), are determined using the specific identification method. See Notes 2 and 3 to the consolidated financial statements for further information.
Income tax accruals and valuation allowances. When preparing the consolidated financial statements, we are required to estimate the income taxestax obligations in each of the jurisdictions in which we operate. This process involves estimating the actual current tax obligations based on expected income, statutory tax rates and tax planning opportunities in the various jurisdictions. In the event there is a significant unusual or one-time item recognized in the results of operations, the tax attributable to that item would be separately calculated and recorded in the period the unusual or one-time item occurred. Tax law requires certain items to be included in our tax return at different times than the items are reflected in our results of operations. As a result, the annual effective tax rate reflected in
35
our results of operations is different than that reported on our tax return (i.e., our cash tax rate). Some of these differences are permanent, such as expenses that are not deductible in our tax return, and some are temporary differences that will reverse over time, such as depreciation expense on capital assets. These temporary differences result in deferred tax assets and liabilities, which are included in our consolidated balance sheets. Deferred tax assets generally represent items that can be used as a tax deduction or credit in our tax returnreturns in future years, for which we have already recorded the expense in our consolidated statements of income.operations. We must assess the likelihood that our deferred tax assets will be recovered from future taxable income, and to the extent we believe that recovery is not likely, we must establish a valuation allowance against those deferred tax assets. Deferred tax liabilities generally represent items for which we have already taken a deduction in our tax return, but we have not yet recognized the items as expense in our results of operations. Significant judgment is required in evaluating our tax positions, and in determining our provision for income taxes, our deferred tax assets and liabilities and any valuation allowance recorded against our deferred tax assets. We had total deferred tax assets in excess of total deferred tax liabilities of $2.9 million as of September 30, 2010 and 2009, and $12.2including valuation allowances of $6.5 million as of September 30, 2008, including valuation allowances of2010 and $3.3 million as of September 30, 2009 and $3.4 million as of September 30, 2008.2009. The valuation allowances related to impairment losses on investments and were recorded because the Company does not currently foresee future capital gains within the allowable carry-forward and carry-back periods to offset these capital losses when they are recognized. As such, no tax benefit has been recorded in the consolidated statements of income. In addition, we recorded a valuation allowance related to state net operating losses based on the uncertainty regarding the realization of the net operating losses in the carryforward periods.
The Company adopted accounting provisions on October 1, 2007 which defined new standards for recognizing the benefits of tax return positions in the financial statements as “more-likely-than-not” to be sustained by the taxing authorities based solely on the technical merits of the position. If the recognition threshold is met, the tax benefit is measured and recognized as the largest amount of tax benefit that, in our judgment, is greater than 50 percent likely to be realized. The total gross amount of unrecognized tax benefits as of September 30, 2010, 2009 and 2008 was $1.9 million, $2.0 million and $1.5 million, respectively, excluding accrued interest and penalties. Of these unrecognized tax benefits, $1.9 million, $2.0 million of these tax benefitsand $1.5 million would affect our effective tax rate if recognized.for fiscal years 2010, 2009 and 2008, respectively. Interest and penalties recorded for uncertain tax positions
36
are included in our income tax provision. As of September 30, 2010, 2009 and 2008, $0.7 million, $0.6 million and $0.4 million, respectively, of interest and penalties were accrued, excluding the tax benefits of deductible interest. The Internal Revenue Service has commenced an examination of our United States income tax return for fiscal 2009 in the first quarter of fiscal 2011. Fiscal years 2006, 2007 and 2008 remain subject to examination by federal tax authorities. Tax returns for state and local jurisdictions for fiscal years 2003 through 20082009 remain subject to examination by state and local tax authorities. In the event that we have determined not to file tax returns with a particular state or local jurisdiction, all years remain subject to examination by the tax authorities. The ultimate outcome of tax matters may differ from our estimates and assumptions. Unfavorable settlement of any particular issue would require the use of cash and could result in increased income tax expense. Favorable resolution could result in reduced income tax expense. Within the next 12 months, we do not expect that our unrecognized tax benefits will change significantly. See Note 8 to the consolidated financial statements for further information regarding the impact of adopting this new standard as well as changes in unrecognized tax benefits during fiscal 2010, 2009 and 2008.
36
Results of Operations
Years Ended September 30, 2010 and 2009
| Fiscal | Fiscal | Increase/ | ||||||||||||||
(Dollars in thousands) | 2010 | 2009 | (Decrease) | % Change | ||||||||||||
| Revenue: | ||||||||||||||||
| Therapeutic | ||||||||||||||||
| Cardiovascular | $ | 40,155 | $ | 39,841 | $ | 314 | 1 | % | ||||||||
| Ophthalmology | 7,617 | 52,102 | (44,485 | ) | (85 | )% | ||||||||||
| Other Markets | 10,932 | 13,114 | (2,182 | ) | (17 | )% | ||||||||||
| Total Therapeutic | 58,704 | 105,057 | (46,353 | ) | (44 | )% | ||||||||||
| Diagnostic | 11,194 | 16,477 | (5,283 | ) | (32 | )% | ||||||||||
| Total revenue | $ | 69,898 | $ | 121,534 | $ | (51,636 | ) | (42 | )% | |||||||
Revenue. Fiscal 2010 revenue was $69.9 million, a decrease of $51.6 million, or 42%, from fiscal 2009. The decreases in Therapeutic and Diagnostic revenue, as detailed in the table above, are further explained in the narrative below.
Therapeutic. Revenue in Therapeutic was $58.7 million in fiscal 2010, a 44% decrease compared with $105.1 million in the prior-year period. The decrease in total revenue principally reflects the recognition in fiscal 2009 of revenue of approximately $45 million associated with the Merck collaborative research and license agreement, which was terminated effective in the first quarter of fiscal 2009. Excluding this significant event-specific item in fiscal 2009, Therapeutic revenue decreased $1.4 million, or 2%.
Cardiovascular derives a substantial amount of revenue from royalties and license fees and product sales attributable to Cordis Corporation, a Johnson & Johnson company, on its CYPHER® Sirolimus-eluting Coronary Stent. The CYPHER® stent incorporates a proprietary SurModics polymer coating that delivers a therapeutic drug designed to reduce the occurrence of restenosis in coronary artery lesions. The CYPHER® stent faces continuing competition from Boston Scientific, Medtronic, and Abbott Laboratories. Stents from these companies compete directly with the CYPHER® stent both domestically and internationally. For the last several years, royalty and reagent product sales have decreased due to lower CYPHER stent sales. We anticipate that royalty revenue from the CYPHER® stent are likely to descrease in fiscal 2011 and beyond as the various marketers of drug-eluting stents compete, and as others enter the marketplace. We also receive a royalty on sales of delivery systems used to deliver the Medtronic Endeavor® and Endeavor® Resolute drug-eluting stents. These stent delivery systems incorporate our proprietary hydrophilic technology and are sold in the United States and internationally.
Cardiovascular revenue increased $0.3 million, or 1%, in fiscal 2010, compared with the prior-year principally as a result of higher license fees which included recognition of fees of $1.25 million associated with a terminated agreement, and higher reagent product sales, partially offset by lower research and development revenue. Cordis CYPHER® stent sales decreased approximately 26% during fiscal 2010 which resulted in lower royalty revenue from this agreement.
Ophthalmology revenue decreased $44.5 million, or 85%, in fiscal 2010, compared with the prior-year. The significant decrease relates to the recognition of previously deferred revenue associated with the terminated collaborative research and license agreement with Merck in fiscal 2009. In September 2008, following a strategic review of Merck’s business and product development portfolio, Merck gave notice that it was terminating the collaborative research and license agreement as well as the supply agreement entered into in June 2007. The termination became effective in December 2008. We recognized the revenue previously deferred totaling $34.8 million, and we received and recognized a $9 million milestone payment from Merck associated with the termination of the triamcinolone acetonide development program.
37
Ophthalmology revenue, when excluding the Merck event-specific items of fiscal 2009, increased by $0.5 million, or 7%, principally as a result of a license fee milestone event.
Other Markets revenue decreased $2.2 million, or 17%, in fiscal 2010, compared with the prior-year period. Lower research and development revenue was the primary reason for the decrease. Fiscal 2010, like fiscal 2009, continued to see selected customers delay, slow or cancel development projects as a result of various factors, including current economic conditions, financing challenges, and issues in the pharmaceutical industry. Other Markets revenue is derived from more than 50 customers.
Diagnostic. Diagnostic revenue was $11.2 million in fiscal 2010, a decrease of 32% compared with $16.5 million in the prior-year period. The decrease was attributable to lower royalties and license fees in fiscal 2010. In past years, Diagnostic derived a significant percentage of revenue from Abbott Laboratories. Royalty revenue from our diagnostic format patent license agreement with Abbott was $4.9 million in fiscal 2009. There was no royalty revenue from Abbott in fiscal 2010 because the patents had expired. In addition to the lower royalties and license fees, product sales decreased less than 1% compared with fiscal 2009 as customers continued to be cautious with their purchasing activity. We anticipate modest growth in product sales for fiscal 2011.
Product costs. Product costs were $9.4 million in fiscal 2010, a 26% increase from the prior year. Overall product margins averaged 53%, compared with 61% in the prior year. The decrease in product margins reflected the mix of products sold in fiscal 2010, as there were higher polymer product sales, which products carry lower margins than our reagent and diagnostic products. There was an inventory impairment charge totaling $0.4 million recognized in fiscal 2010. The gross margin, when adjusting for this impairment, was 55%.
Customer research and development expenses. Customer research and development (“Customer R&D”) expenses were $18.1 million, an increase of 38% compared with fiscal 2009. The increase principally reflects the impact of higher fixed costs attributable to our Alabama research and development operations. Customer R&D margins were negative 18%, compared with 51% in fiscal 2009. Fiscal 2009 margins were 32% after adjusting for Merck deferred revenue recognition and final billings. The increase in fiscal 2010 costs reflects the higher fixed overhead costs in Alabama as well as increased material costs. We anticipate fiscal 2011 costs associated with our cGMP facility to increase because of maintenance and validation activities.
Other research and development expenses. Other research and development (“Other R&D”) expenses were $17.9 million, a decrease of 15% compared with fiscal 2009. Overhead costs allocated to Other R&D decreased compared with fiscal 2009, and our research and development headcount decreased in fiscal 2010 compared with fiscal 2009 as a result of our March 2010 reorganization and attrition, resulting in lower labor costs. These reductions were partially offset by higher project material costs.
Selling, general and administrative expenses. Selling, general and administrative (“SG&A”) expenses were $18.5 million, an increase of 7% compared with fiscal 2009. The increase principally reflects higher professional services fees, higher bad debt expenses and additional operating costs with our Alabama facilities that are allocated to SG&A, partially offset by lower stock-based compensation expense and lower SG&A headcount.
Restructuring charges. In March 2010, we announced an organizational change designed to support future growth by better meeting customer needs, leveraging our multiple competencies across the organization, and building on our pharmaceutical industry experience. We eliminated approximately 4% of our workforce with the terminations occurring across various functions. SurModics recorded total restructuring charges of approximately $1.3 million in connection with the reorganization, consisting of $0.8 million associated with severance pay and benefits expenses and $0.5 million of facility-related costs. SurModics vacated and subleased its leased office facility in Irvine, California and a warehouse in Birmingham, Alabama.
In November 2008, we announced a functional reorganization which resulted in elimination of approximately 5% of our workforce. These employee terminations occurred across various functions, and the reorganization plan was completed by the end of the first quarter of fiscal 2009. The reorganization also resulted in SurModics vacating a leased office facility in Eden Prairie, Minnesota, and consolidating into our owned office and research facility also in Eden Prairie. We recorded total restructuring charges of $1.8 million in connection with this reorganization. These pre-tax charges consisted of $0.5 million of severance pay and benefits expenses and $1.3 million of facility-related costs.
38
Costs totaling $1.9 million have been paid associated with both restructurings, and we anticipate paying the remaining $1.2 million within the next three years, with the majority in the next twelve months.
We also announced an additional restructuring subsequent to fiscal 2010 which resulted in a reduction to our cost structure and renewed focus on business units. This change resulted in the elimination of approximately 13% of our workforce with anticipated restructuring charges in the first quarter of fiscal 2011 in the range of $1.3 million to $1.7 million.
Asset impairment charges. In fiscal 2010, we recorded a $1.9 million asset impairment charge associated with writing down one of our facilities in Alabama to fair value based on a decision to sell the facility, which we later determined not to sell. The $2.1 million carrying value of this facility is based on a real estate market appraisal obtained during our negotiations.
We also recorded a $1.3 million asset impairment charge associated with certain long-lived assets where very limited business is expected in the near term based on current market conditions. Furthermore, a $1.3 million asset impairment charge associated with certain fixed asset costs located in Minnesota and a $0.4 million asset impairment charge associated with prototypes and other equipment related to a development project for which very limited use is expected in the near term in light of current market conditions. The assets associated with these charges had limited remaining value and as such were written down to zero value.
Goodwill impairment charge. In fiscal 2010, we recorded a $13.8 million goodwill impairment charge associated with our SurModics Pharmaceuticals reporting unit. The goodwill impairment charge in fiscal 2010 reflected a significant decline in the estimated fair value of our reporting units, mainly our SurModics Pharmaceuticals reporting unit, which resulted from a slowdown in business activity most pronounced in the fourth quarter of fiscal 2010, higher operating costs with our recently placed in-service cGMP manufacturing facility, and a significant decrease in our stock price during the year. Our stock price declined from $24.13 per share at October 1, 2009 to $12.03 per share at the date of our annual impairment test, which was August 31, 2010. We continually evaluate whether any indications of impairment are present that would require an impairment analysis on an interim basis. Prior to the fourth quarter, based on our outlook for future results and the fact that our market capitalization exceeded our book value by a margin of 64% at June 30, 2010, we did not believe that the events and circumstances in existence at our interim reporting dates indicated that it was more likely than not that the fair value of any of our reporting units would be less than its carrying amount.
Other income (loss), net. Other loss was $6.6 million in fiscal 2010, compared with income of $2.0 million in fiscal 2009. Income from investments was $1.0 million in fiscal 2010, compared with $1.8 million in fiscal 2009. The decrease primarily reflects lower yields generated from our investment portfolio in fiscal 2010. The fiscal 2010 loss primarily reflects a total of $7.9 million of impairment losses in connection with our portfolio of strategic investments.
We recognized an impairment loss on our investment in Nexeon MedSystems totaling $5.3 million in the fourth quarter of fiscal 2010 based on the valuations associated with potential new rounds of financing. In addition, we recognized a $2.4 million loss on our investment in a medical technology company in the third quarter of fiscal 2010 based on market valuations and a pending financing round for this company. Another entity in which the Company had a strategic investment sold the majority of its assets in the third quarter of fiscal 2010 resulting in an impairment loss of $0.2 million.
Income tax expense. The income tax provision was $0.4 million in fiscal 2010, compared with $22.0 million in fiscal 2009. The effective tax rate in fiscal 2010 is not meaningful because a tax expense was recorded on a pretax loss. The effective tax rate, when excluding the impact of the goodwill impairment charge of $13.8 million and impairment losses on investments of $7.9 million was 39.3% since SurModics does not currently foresee offsetting capital gains that could offset these capital losses, and therefore no benefit has been recorded. The effective tax rate in fiscal 2009 was 36.9%. The increase in the effective tax rate, adjusted for the one-time items noted, is primarily a
39
result of non-deductible stock-based compensation expenses, offset partially by lower state taxes resulting from adjustments to state deferred taxes.
Years Ended September 30, 2009 and 2008
| Fiscal | Fiscal | Increase/ | Fiscal | Fiscal | Increase/ | |||||||||||||||||||||||||||
(Dollars in thousands) | 2009 | 2008 | (Decrease) | % Change | 2009 | 2008 | (Decrease) | % Change | ||||||||||||||||||||||||
| Revenue: | ||||||||||||||||||||||||||||||||
| Therapeutic | ||||||||||||||||||||||||||||||||
| Cardiovascular | $ | 39,841 | $ | 47,675 | $ | (7,834 | ) | (16 | )% | $ | 39,841 | $ | 47,675 | $ | (7,834 | ) | (16 | )% | ||||||||||||||
| Ophthalmology | 52,102 | 10,252 | 41,850 | 408 | % | 52,102 | 10,252 | 41,850 | 408 | % | ||||||||||||||||||||||
| Other Markets | 13,114 | 17,875 | (4,761 | ) | (27 | )% | 13,114 | 17,875 | (4,761 | ) | (27 | )% | ||||||||||||||||||||
| Total Therapeutic | 105,057 | 75,802 | 29,255 | 39 | % | 105,057 | 75,802 | 29,255 | 39 | % | ||||||||||||||||||||||
| Diagnostic | 16,477 | 21,249 | (4,772 | ) | (22 | )% | 16,477 | 21,249 | (4,772 | ) | (22 | )% | ||||||||||||||||||||
| Total revenue | $ | 121,534 | $ | 97,051 | $ | 24,483 | 25 | % | ||||||||||||||||||||||||
| Total Revenue | $ | 121,534 | $ | 97,051 | $ | 24,483 | 25 | % | ||||||||||||||||||||||||
Revenue. Fiscal 2009 revenue was $121.5 million, an increase of $24.5 million, or 25%, from fiscal 2008. The increase in Therapeutic and decrease in Diagnostic revenue, as detailed in the table above, are further explained in the narrative below.
Therapeutic. Revenue in Therapeutic was $105.1 million in fiscal 2009, a 39% increase compared with $75.8 million in the prior-year period.prior-year. The increase in total revenue reflects the recognition of revenue of approximately $45 million associated with the terminated Merck collaborative research and license agreement. Excluding these significant event-specific items, Therapeutic revenue decreased $15.7 million, or 21%.
Cardiovascular derives a substantial amount of revenue from royalties and license fees and product sales attributable to Cordis Corporation, a Johnson & Johnson company, on its CYPHER® Sirolimus-eluting Coronary Stent. The CYPHER® stent incorporates a proprietary SurModics polymer coating that delivers a therapeutic drug designed to reduce the occurrence of restenosis in coronary artery lesions. The CYPHER® stent faces continuing competition from Boston Scientific, Medtronic, and Abbott Laboratories. Stents from these companies compete directly with the CYPHER® stent both domestically and internationally. Future royalty and reagent sales revenue could decrease as a result of lower CYPHER® stent sales as a result of the ongoing and expected future competition. We anticipate that royalty revenue from the CYPHER® stent may be volatile throughout fiscal 2010 and beyond as the various marketers of drug-eluting stents compete in the marketplace and as others enter the marketplace. We also receive a royalty on sales of the Medtronic Endeavor® drug-eluting stent delivery system incorporating our hydrophilic technology, which is sold in the United States and internationally and commenced sales in Japan in May 2009.
Cardiovascular revenue decreased $7.8 million, or 16%, in fiscal 2009, compared with the prior-year period principally as a result of lower royalties and license fees and research and development revenue. Our royalty revenue from Cordis decreased approximately 35% as a result of the decrease in CYPHER® stent sales.
37
Ophthalmology revenue increased $41.9 million, or 408%, in fiscal 2009, compared with the prior-year period.prior-year. The significant increase principally reflects the recognition of approximately $45 million of previously deferred revenue associated with the terminated collaborative research and license agreement with Merck and a milestone payment associated with the termination of the triamcinolone acetonide development program.
Ophthalmology revenue, excluding the Merck event-specific items of fiscal 2009 and amortization of revenue in fiscal 2008, was unchanged at $7.1 million in both fiscal years.
Other Markets revenue decreased $4.8 million, or 27%, in fiscal 2009, compared with the prior-year period.prior-year. Lower research and development revenue was the primary reason for the decrease. Selected customers have delayed, slowed or cancelled development projects in fiscal 2009 as a result of various factors including current economic conditions. Other Markets revenue is derived from more than 50 customers.
Diagnostic. Revenue in Diagnostic was $16.5 million in fiscal 2009, a decrease of 22% compared with $21.2 million in the prior-year period.prior-year. This decrease was attributable to lower royalties and license fees in fiscal 2009. In past years, Diagnostic derived a significant percentage of revenue from Abbott Laboratories. Fiscal 2009 was the last year in which we received royalty revenue from our diagnostic format patent license agreement with Abbott Laboratories. Royalty revenue from Abbott was $4.9 million in fiscal 2009, compared with $8.7 million in fiscal 2008. Product sales in Diagnostic decreased 4% compared with fiscal 2008, as customers slowed purchasing activity in early fiscal 2009.
40
Product costs. Product costs were $7.5 million in fiscal 2009, an 11% decrease from the prior year. Overall product margins averaged 61%, compared with 58% in the prior year. The increase in product margins reflected the mix of products sold in fiscal 2009 as we had a decrease in sales of our SurModics Pharmaceuticals polymer products, which carry lower margins than our reagent and diagnostic products.
Customer research and development expenses. Customer research and development (“Customer R&D”)&D expenses were $13.2 million, a decrease of 31% compared with fiscal 2008. The decrease principally reflects the impact of lower research and development revenue, adjusted for Merck. Customer R&D margins were 51%, compared with 24% in fiscal 2008. The margins were 32% and 21% for fiscal 2009 and 2008, respectively, after adjusting for Merck deferred revenue recognition in both periods. The increase in fiscal 2009 margins reflects lower labor and material costs incurred on projects, as well as lower overhead costs allocated to Customer R&D.
Other research and development expenses. Other research and development (“Other R&D”)&D expenses were $21.2 million, essentially unchanged compared with $21.3 million in fiscal 2008. Our research and development headcount decreased in fiscal 2009 as a result of our November 2008 reorganization, resulting in lower labor costs, which were offset by higher overhead costs being allocated to Other R&D.
Selling, general and administrative expenses. Selling, general and administrative expenses were $17.2 million, a decrease of 17% compared with fiscal 2008. The decrease principally reflects lower employee compensation costs related to our annual incentive compensation program and lower stock-based compensation expense, as fiscal 2008 included costs related to transitions on our Board of Directors.
Purchased in-process research and development. In November 2008, we acquired certain assets comprised of intellectual property and collaborative programs from PR Pharmaceuticals, Inc. The fair value of $3.2 million associated with the in-process research and development intangible asset was determined by management and recognized as an expense.
Restructuring charges. In November 2008, we announced a functional reorganization to better serve our customers and improve our operating performance. As a result of the reorganization, we eliminated 15 positions, or approximately 5% of our workforce. These employee terminations occurred across various functions, and the reorganization plan was completed by the end of the first quarter of fiscal 2009. The reorganization also resulted in SurModics vacating a leased office facility in Eden Prairie, Minnesota, and consolidating into our owned office and research facility also in Eden Prairie.
We recorded total restructuring charges of $1.8 million in connection with the reorganization. These pre-tax charges consisted of $0.5 million of severance pay and benefits expenses and $1.3 million of facility-related costs.
38
Other income (loss), net. Other income was $2.0 million in fiscal 2009, compared with a loss of $0.4 million in fiscal 2008. Income from investments was $1.8 million in fiscal 2009, compared with $3.3 million in fiscal 2008. The decrease primarily reflects lower investment balances in fiscal 2009. The fiscal 2008 loss primarily reflects a $4.3 million impairment loss on our investment in OctoPlus N.V., based on a significant decline in the stock price as of September 30, 2008.
Income tax expense. The income tax provision was $22.0 million in fiscal 2009, compared with $12.2 million in fiscal 2008. The effective tax rate in fiscal 2009 was 36.9% compared with 45.2% in fiscal 2008. ExcludingThe effective tax rate, when excluding the impact of the $4.3 million impairment loss in fiscal 2008 (sincewas 38.9% since the Company doesdid not currently foresee offsetting capital gains that could offset this capital loss, no tax benefit has been recorded), the effective tax rate was 38.9%.recorded. The decrease in the effective tax rate, adjusted for the one-time item noted, is primarily a result of lower state taxes and the tax reserve associated with uncertain tax positions.
| Fiscal | Fiscal | |||||||||||||||
(Dollars in thousands) | 2008 | 2007 | Increase | % Change | ||||||||||||
| Revenue: | ||||||||||||||||
| Therapeutic | ||||||||||||||||
| Cardiovascular | $ | 47,675 | $ | 46,487 | $ | 1,188 | 3 | % | ||||||||
| Ophthalmology | 10,252 | 2,453 | 7,799 | 318 | % | |||||||||||
| Other Markets | 17,875 | 4,041 | 13,834 | 342 | % | |||||||||||
| Total Therapeutic | 75,802 | 52,981 | 22,821 | 43 | % | |||||||||||
| Diagnostic | 21,249 | 20,183 | 1,066 | 5 | % | |||||||||||
| Total revenue | $ | 97,051 | $ | 73,164 | $ | 23,887 | 33 | % | ||||||||
39
40
Liquidity and Capital Resources
Operating Activities.As of September 30, 2009,2010, the Company had working capital of $29.0$29.8 million, of which $20.6$20.5 million consisted of cash, cash equivalents and short-term investments. Working capital decreased $5.0increased $0.8 million from the September 30, 20082009 level, driven principally by lower cash, accounts receivable andhigher income taxes receivable balances, offset by lower accrued compensation and deferred revenueaccounts receivable balances. Deferred revenue balances have decreasedincreased as a result of the termination of$3.5 million upfront payment associated with the Merck arrangement license and development agreement with Roche and Genentech
41
in fiscal 2009. In addition, accrued annual incentive compensation decreased because fiscal 2009 objectives were not achieved.2010. Our cash, cash equivalents and short-term and long-term investments totaled $56.8 million at September 30, 2010, an increase of $8.9 million from $47.9 million at September 30, 2009, a decrease of $24.1 million from $72.0 million at September 30, 2008.2009. The decreaseincrease was principally driven by redemptions which were used to finance investment in our new manufacturing facility in Alabama, which totaled $24.4 million and for our stock repurchase program, which totaled $15.0 million.cash from operations. The Company’s investments principally consist of U.S. government and government agency obligations and investment grade, interest-bearing corporate debt securities with varying maturity dates, the majority of which are five years or less. The Company’s policy requires that no more than 5% of investments be held in any one credit issue, excluding U.S. government and government agency obligations. The primary investment objective of the portfolio is to provide for the safety of principal and appropriate liquidity while meeting or exceeding a benchmark (Merrill Lynch 1-3 Year Government-Corporate Index) total rate of return. Management plans to continue to direct its investment advisors to manage the Company’s investments primarily for the safety of principal for the foreseeable future as it assesses other investment opportunities and uses of its investments.
The Company had positive cash flows from operating activities of approximately $22.0 million in fiscal 2010, compared with $31.3 million in fiscal 2009, compared with $39.8 million in fiscal 2008.2009. The following table depicts our cash flows from operations for each of fiscal 20092010 and 2008:2009:
| For the Years Ended | For the Years Ended September 30, | |||||||||||||||
| September 30, | 2010 | 2009 | ||||||||||||||
| 2009 | 2008 | (In thousands) | ||||||||||||||
| (Dollars in thousands) | ||||||||||||||||
| Net income | $ | 37,550 | $ | 14,739 | ||||||||||||
| Net (loss) income | $ | (21,089 | ) | $ | 37,550 | |||||||||||
| Depreciation and amortization | 5,912 | 6,071 | 7,818 | 5,912 | ||||||||||||
| Stock-based compensation | 6,853 | 9,652 | 5,875 | 6,853 | ||||||||||||
| Purchased in-process research and development | 3,200 | — | — | 3,200 | ||||||||||||
| Impairment loss on investment | — | 4,314 | ||||||||||||||
| Asset impairment charges | 4,896 | — | ||||||||||||||
| Goodwill impairment charge | 13,810 | — | ||||||||||||||
| Impairment loss on investments | 7,943 | — | ||||||||||||||
| Deferred taxes and other net operating activities | 10,433 | (3,938 | ) | 774 | 8,672 | |||||||||||
| Net change in deferred revenue | (36,050 | ) | 11,452 | 2,632 | (36,050 | ) | ||||||||||
| Net change in other operating assets and liabilities | 3,423 | (2,468 | ) | (651 | ) | 5,184 | ||||||||||
| Net cash provided by operating activities | $ | 31,321 | $ | 39,822 | $ | 22,008 | $ | 31,321 | ||||||||
Net (loss) income in fiscal 2009 increased2010 decreased compared with fiscal 2008, however2009, which also resulted in lower cash provided by operating activities was lower.activities. The decrease in cash from operations reflects lower CYPHER® stent royalties and no Abbott royalties in fiscal 2010 as well as increased operating expenses associated with our facilities in Alabama. Net income was higher in fiscal 2009 principally as a result of the recognition of previously deferred revenue associated with the Merck agreement, which is a non-cash item. The Merck termination also resulted in a reduction in deferred tax asset balances, which are non-cash. The decrease in cash from operations also reflects lower CYPHER® stent royalties and Abbott royalties in fiscal 2009.
Investing Activities.We conduct a significant majority of our operations at our Eden Prairie, Minnesota headquarters and at our SurModics Pharmaceuticals subsidiary located in Birmingham, Alabama. In addition to our Eden Prairie and Birmingham locations, we lease approximately 4,800 square feet of commercial office space in Irvine, California, where our Ophthalmology business unit conducts a portion of its operations, and approximately 73,000 square feet of office and warehouse space in Eden Prairie. In December 2008, we consolidated all of our Eden Prairie personnel into our owned facility as part of our reorganization efforts, and subsequently subleased our leased space in Eden Prairie to another company. In April 2008, we purchased a building for $12.2 million with approximately 286,000 square feet of space near our present location inoriginal Birmingham, Alabama.Alabama location. We have invested an additional
41
In July 2007, we made equity investments in Paragon Intellectual Properties, LLC (“Paragon”) and Apollo Therapeutics, LLC (“Apollo”), a Paragon subsidiary. The Paragon and Apollo investments totaled $3.5 million. SurModics made an additional equity investment of $2.5 million, based upon successful completion of specified development milestones, in fiscal 2008. In October 2008, Paragon announced that it had restructured, moving from a limited liability company with seven subsidiaries to a single C-corporation named Nexeon MedSystems, Inc. (“Nexeon”). We continued to account for our investment in Paragon and Apollo under the equity method in the first quarter of fiscal 2009, as both entities report results to us on a one-quarter lag. Commencing with the second quarter of fiscal 2009, we account for our investment in Nexeon under the cost method, as our ownership is less than 20%. and we do not exert significant influence over the medical technology company’s operating or financial activities.
42
SurModics made an additional cash investment in Nexeon of $500,000 in fiscal 2009. In the fourth quarter of fiscal 2010 we determined that this investment wasother-than-temporarily impaired and recognized a $5.3 million impairment loss. We held discussions with Nexeon management to understand the business status and outlook, valuations associated with potential new rounds of financing, operating metrics and other industry factors which impacted our assessment of the carrying value of this investment.
In July 2007, we entered into a stock purchase agreement with Southern Research Institute whereby we acquired 100% of the capital stock of SurModics Pharmaceuticals, Inc. (formerly known as Brookwood Pharmaceuticals, Inc.) (“SurModics Pharmaceuticals”) for $40 million in cash on the closing date, and up to an additional $22 million in cash upon the successful achievement of specified milestones. In fiscal 2009 and 2008,Additional milestones were achieved and $5through fiscal 2010, such that $5.8 million of additional purchase price was recorded as an increase to goodwill. The sellers are still eligible to receive up to $16.3 million in additional consideration through calendar 2011. Based in Birmingham, Alabama, SurModics Pharmaceuticals specializes in proprietary injectable microparticles and implants to provide sustained delivery of drugs being developed by leading pharmaceutical, biotechnology and medical device clients as well as emerging companies. This acquisition has helped us broaden our technology offerings to our customers, diversify the range of markets in which we participate, expand our customer base, and enhance our pipeline of potential revenue generating opportunities. See Note 4The goodwill recognized through fiscal 2010, $13.8 million, was impaired as a result of our annual goodwill impairment testing performed at August 31, 2010. In addition, we are likely to the consolidated financial statements for further information.incur certain milestone payment obligations totaling a minimum of $5.7 million in fiscal 2011. Assuming we do, we expect to record an additional goodwill impairment charge in fiscal 2011.
In August 2007, we entered into a stock purchase agreement to acquire 100% of the capital stock of BioFX Laboratories, Inc. (“BioFX”) for $11.3 million in cash on the closing date, and up to an additional $11.4 million in cash upon the successful achievement of specified milestones. In fiscal 2008, a milestone was achieved and $1.1 million of additional purchase price was recorded as an increase to goodwill. The sellers are still eligible to receive up to $7.6$3.5 million in additional consideration.consideration through calendar 2011. Based in Owings Mills, Maryland, BioFX is a leading manufacturer of substrates, a critical component of diagnostic test kits used to detect and signal that a certain reaction has taken place. The acquisition of BioFX has broadened our product portfolio in the in vitro diagnostics market. See Note 4 to the consolidated financial statements for further information.
In November 2008, our SurModics Pharmaceuticals subsidiary entered into an asset purchase agreement with PR Pharmaceuticals, Inc. (“PR Pharma”) whereby it acquired certain contracts and assets of PR Pharma for $2.9 million in cash on the closing date, $0.3 million in transaction costs, and up to an additional $6.0 million upon the successful achievement of specified milestones. In fiscal 2009, $2.4 million of additional purchase price was paid based on achievement of certain milestones. PR Pharma is eligible to receive up to $3.6 million in cash upon the successful achievement of additional milestones. We believe this acquisition strengthens our portfolio of drug delivery technologiesSee Note 4 to the consolidated financial statements for the pharmaceutical and biotechnology industries.further information.
42
In August 2009, the Company invested $2.0 million in a medical technology company.company with an additional $0.5 million invested in March 2010. In the third quarter of fiscal 2010, a new round of financing was completed for this entity which resulted in significantly lower valuation when compared with previous financings. We assessed the latest information and recognized a $2.4 million impairment loss on this investment. We account for this investment following the cost method, as our ownership level is below 20%. and we do not exert significant influence over the medical technology company’s operating or financial activities.
Financing Activities.In November 2007, our Board of Directors authorized the repurchase of up to $35 million of the Company’s common stock in open-market transactions, private transactions, tender offers, or other transactions. The repurchase authorization does not have a fixed expiration date. During fiscal 2009,2010, we purchased 623,800102,533 shares of common stock for $15.0$2.0 million at an average price of $24.05$19.81 per share. Under the current authorization, the Company has $7.3$5.3 million remaining available for authorized share repurchases as of September 30, 2009.2010.
43
We do not have any other credit agreements and believe that our existing cash, cash equivalents and investments, together with cash flow from operations, will provide liquidity sufficient to meet the below stated needs and fund our operations for the next twelve months. There can be no assurance, however, that SurModics’ business will continue to generate cash flows at current levels, and disruptions in financial markets may negatively impact the Company’s ability to access capital in a timely manner and on attractive terms. Our anticipated liquidity needs for fiscal 20102011 may include, but are not limited to, the following: capital expenditures related to equipment purchases for the Alabama cGMP facility in the range of $5 million to $6 million; general capital expenditures in the range of $4 million to $6 million; contingent consideration payments of $5.7 million to $6.4 million related to our acquisition of SurModics Pharmaceuticals; contingent consideration payments, if any, related to our acquisitionsacquisition of SurModics Pharmaceuticals and BioFX as well as the purchase of certain assets from PR Pharmaceuticals; and any amounts associated with the repurchase of common stock under the authorization discussed above.
Off-Balance Sheet Arrangements
and Contractual Obligations.As of September 30, 2009,2010, the Company did not have any off-balance sheet arrangements with any unconsolidated entities.
Presented below is a summary of contractual obligations and payments due by period (in thousands). See Note 9 to the consolidated financial statements for additional information regarding the below obligations.
| Less than | More than | |||||||||||||||||||
| Total | 1 Year | 1-3 Years | 3-5 Years | 5 Years | ||||||||||||||||
| Operating leases | $ | 889 | $ | 422 | $ | 303 | $ | 164 | $ | — | ||||||||||
| Less than | More than | |||||||||||||||||||
| Total | 1 Year | 1-3 Years | 3-5 Years | 5 Years | ||||||||||||||||
| Operating Leases | $ | 515 | $ | 258 | $ | 117 | $ | 124 | $ | 16 | ||||||||||
| Other long-term Liabilities(1) | 336 | — | 187 | 21 | 128 | |||||||||||||||
| Total | $ | 851 | $ | 258 | $ | 304 | $ | 145 | $ | 144 | ||||||||||
| (1) | Other long-term liability contractual obligations primarily relate to payments associated with terminated operating leases as part of our restructuring activities in fiscal 2009 and 2010. |
As of September 30, 2010, our gross liability for uncertain tax positions was $2.7 million. We are not able to reasonably estimate the amount by which the liability will increase or decrease over an extended period of time or whether a cash settlement of the liability will be required. Therefore, these amounts have been excluded from the schedule of contractual obligations.
43
New Accounting Pronouncements.No other new accounting pronouncement issued or effective has had, or is expected to have, a material impact on the Company’s consolidated financial statements.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK. |
The Company’s investment policy requires investments with high credit quality issuers and limits the amount of credit exposure to any one issuer. The Company’s investments principally consist of U.S. government and government agency obligations and investment-grade, interest-bearing corporate debt securities with varying maturity dates, the majority of which are five years or less. Because of the credit criteria of the Company’s investment policies, the primary market risk associated with these investments is interest rate risk. SurModics does not use derivative financial instruments to manage interest rate risk or to speculate on future changes in interest
44
rates. A one percentage point increase in interest rates would result in an approximate $591,000$0.7 million decrease in the fair value of the Company’savailable-for-sale andheld-to-maturity securities as of September 30, 2009,2010, but no material impact on the results of operations or cash flows.
Management believes that a reasonable change in raw material prices would not have a material impact on future earnings or cash flows because the Company’s inventory exposure is not material.
44
Although we conduct business in foreign countries, our international operations consist primarily of sales of reagent and stabilization chemicals. Additionally, all sales transactions are denominated in U.S. dollars. Accordingly, we do not expect to be subject to material foreign currency risk with respect to future costs or cash flows from our foreign sales. To date, we have not entered into any foreign currency forward exchange contracts or other derivative financial instruments to hedge the effects of adverse fluctuations in foreign currency exchange.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
The consolidated balance sheets as of September 30, 20092010 and 20082009 and the consolidated statements of income,operations, stockholders’ equity and cash flows for each of the three years in the period ended September 30, 2009,2010, together with Report of Independent Registered Public Accounting Firm and related footnotes (including selected unaudited quarterly financial data) begin onpage F-1 of thisForm 10-K.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES. |
| 1. | Disclosure Controls and Procedures. |
As of the end of the period covered by this report, the Company conducted an evaluation under the supervision and with the participation of the Company’s management, including the Company’s interim Chief Executive Officer and Chief Financial Officer regarding the effectiveness of the design and operation of the Company’s disclosure controls and procedures pursuant toRule 13a-15(b) of the Securities Exchange Act of 1934 (the “Exchange Act”). Based upon that evaluation, the interim Chief Executive Officer and Chief Financial Officer concluded that the Company’s disclosure controls and procedures are effective to ensure that information required to be disclosed by the Company in reports that it files under the Exchange Act is recorded, processed, summarized and reported within the time period specified in the Securities Exchange Commission rules and forms, and to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosures.
| 2. | Internal Control over Financial Reporting. |
45
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
SurModics, Inc.
Eden Prairie, Minnesota
We have audited the internal control over financial reporting of SurModics, Inc. and subsidiaries (the “Company”) as of September 30, 2009,2010, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of September 30, 2009,2010, based on the criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements and financial statement schedule as of and for the year ended September 30, 20092010 of the Company and our report dated December 11, 2009,14, 2010, expressed an unqualified opinion on those financial statements.
/s/ DELOITTE & TOUCHE LLP
Minneapolis, Minnesota
December 11, 200914, 2010
46
| 3. | Changes in Internal Controls. |
There was no change in our internal control over financial reporting that occurred during the fourth quarter of the year covered by thisForm 10-K that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION. |
None.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE. |
The information required by Item 10 relating to directors, our audit committee, the nature of changes, if any, to procedures by which our shareholders may recommend nominees for directors, codesour code of ethics and compliance with Section 16(a) of the Securities Exchange Act of 1934 is incorporated herein by reference to the sections entitled “Election of Directors,” “Section 16(a) Beneficial Ownership Reporting Compliance,” “Corporate Governance — Code of Ethics and Business Conduct”Conduct,” “Corporate Governance — Corporate Governance and Nominating Committee; Procedures & Policies” and “Audit Committee Report,” which appear in the Company’s definitive Proxy Statement for its 20102011 Annual Meeting of Shareholders. The information required by Item 10 relating to executive officers appears in Part I of thisForm 10-K.
| ITEM 11. | EXECUTIVE COMPENSATION. |
The information required by Item 11 is incorporated herein by reference to the sections entitled “Executive Compensation and Other Information,” “Compensation Discussion and Analysis,” “Director Compensation forDuring Fiscal 2009”2010” and “Compensation Committee Report,” which appear in the Company’s definitive Proxy Statement for its 20102011 Annual Meeting of Shareholders.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
The information required by Item 12 is incorporated herein by reference to the sections entitled “Principal Shareholders,” and “Management Shareholdings” and “Equity Compensation Plan Information” which appear in the Company’s definitive Proxy Statement for its 20102011 Annual Meeting of Shareholders.
Equity Compensation Plan Information
The following table provides information related to the Company’s equity compensation plans in effect as of September 30, 2010:
| (c) | ||||||||||||
| Number of Securities | ||||||||||||
| (a) | (b) | Remaining Available for | ||||||||||
| Number of Securities to | Weighted-Average | Future Issuance Under | ||||||||||
| be Issued Upon Exercise | Exercise Price of | Equity Compensation | ||||||||||
| of Outstanding Options, | Outstanding Options, | Plans (Excluding Securities | ||||||||||
Plan Category | Warrants and Rights | Warrants and Rights | Reflected in Column (a)) | |||||||||
| Equity compensation plans approved by shareholders | 1,611,333 | (1) | $ | 31.02 | (1) | 2,001,609 | (2) | |||||
| Equity compensation plans not approved by shareholders | 0 | N/A | 0 | |||||||||
| Total | 1,611,333 | $ | 31.02 | 2,001,609 | ||||||||
| (1) | Excludes shares that may be issued under the Company’s amended and restated 1999 Employee Stock Purchase Plan, but includes amounts reserved for previously-granted restricted stock and performance share awards under the 2009 Equity Incentive Plan. | |
| (2) | Includes 1,818,801 shares available for future issuance under the 2009 Equity Incentive Plan. There are 182,808 shares available under the amended and restated 1999 Employee Stock Purchase Plan. |
47
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
The information required by Item 13 is incorporated herein by reference to the sections entitled “Corporate Governance — Related Person Transaction Approval Policy,” “Corporate Governance — Transactions With Related Parties” and “Corporate Governance — Majority of Independent Directors; Committees of Independent Directors,” which appear in the Company’s definitive Proxy Statement for its 20102011 Annual Meeting of Shareholders.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES. |
The information required by Item 14 is incorporated herein by reference to the section entitled “Independent Registered Public Accounting Firm,” which appears in the Company’s definitive Proxy Statement for its 20102011 Annual Meeting of Shareholders.
4748
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES. |
(a) 1. Financial Statements
The following statements are included in this report on the pages indicated:
| Page (s) | ||
| F-1 | ||
| F-2 | ||
| F-3 | ||
| F-4 | ||
| F-5 | ||
| F-6 to |
2. Financial Statement Schedules.See Schedule II — “Valuation and Qualifying Accounts” in this section of thisForm 10-K. All other schedules are omitted because they are inapplicable, not required, or the information is in the consolidated financial statements or related notes.
3. Listing of Exhibits.The exhibits which are filed with this report or which are incorporated herein by reference are set forth in the Exhibit Index following the signature page.
SurModics, Inc.
Schedule II
Valuation and Qualifying Accounts
Valuation and Qualifying Accounts
| Column B | Column C | Column D | Column E | Column B | Column C | Column D | Column E | |||||||||||||||||||||||||
| Balance at | Additions | Deductions | Balance at | Balance at | Additions | Deductions | Balance at | |||||||||||||||||||||||||
Column A | Beginning of | Charged to | From | End of | Beginning of | Charged to | From | End of | ||||||||||||||||||||||||
Description | Period | Expenses | Reserves | Period | Period | Expenses | Reserves | Period | ||||||||||||||||||||||||
| Year Ended September 30, 2007 | ||||||||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 40 | $ | 7 | $ | 7 | (a) | $ | 40 | |||||||||||||||||||||||
| Year Ended September 30, 2008 | ||||||||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 40 | $ | 228 | $ | 133 | (a) | $ | 135 | $ | 40 | $ | 228 | $ | 133 | (a) | $ | 135 | ||||||||||||||
| Year Ended September 30, 2009 | ||||||||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 135 | $ | (34 | ) | $ | 19 | (a) | $ | 82 | $ | 135 | $ | (34 | ) | $ | 19 | (a) | $ | 82 | ||||||||||||
| Restructuring accrual | $ | — | $ | 1,763 | $ | 808 | (b) | $ | 955 | $ | — | $ | 1,763 | $ | 808 | (b) | $ | 955 | ||||||||||||||
| Year Ended September 30, 2010 | ||||||||||||||||||||||||||||||||
| Allowance for doubtful accounts | $ | 82 | $ | 367 | $ | (12 | )(a) | $ | 461 | |||||||||||||||||||||||
| Restructuring accrual | $ | 955 | $ | 1,306 | $ | 1,078 | (b) | $ | 1,183 | |||||||||||||||||||||||
| (a) | Uncollectible accounts written off and adjustments to the allowance. | |
| (b) | Adjustments to the accrual account reflect payments or non-cash charges associated with the accrual. |
4849
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
SURMODICS, INC.
| By: | /s/ |
Interim Chief Executive Officer, Senior Vice
President and Chief Financial Officer
Dated: December 11, 200914, 2010
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant, in the capacities, and on the dates indicated.
(Power of Attorney)
Each person whose signature appears below authorizes BRUCE J BARCLAY and PHILIP D. ANKENY, and constitutes and appoints said personsperson as his or her true and lawful attorneys-in-factattorney-in-fact and agents, each acting alone,agent, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any or all amendments to this Annual Report onForm 10-K and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, authorizing said personsperson and granting unto said attorneys-in-factattorney-in-fact and agents, each acting alone,agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all said attorneys-in-factattorney-in-fact and agents, each acting alone,agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue thereof.
Signature | Title | Date | ||||
/s/ | ||||||
Philip D. Ankeny | Interim Chief Executive Officer, Senior Vice President and Chief Financial Officer (principal executive and financial officer) | December | ||||
/s/ Mark A. Lehman Mark A. Lehman | Corporate Controller (principal accounting officer) | December | ||||
/s/ José H. Bedoya José H. Bedoya | Director | December | ||||
/s/ John W. Benson John W. Benson | Director | December | ||||
/s/ Mary K. Brainerd Mary K. Brainerd | Director | December | ||||
/s/ Robert C. Buhrmaster Robert C. Buhrmaster | Director | December 14, 2010 | ||||
4950
Signature | Title | Date | ||||
/s/ | Director | December | ||||
| Director | ||||||
/s/ | Director | December | ||||
/s/ | Director | December | ||||
/s/ | Director | December | ||||
5051
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
EXHIBIT INDEX TOFORM 10-K
For the Fiscal Year Ended September 30, 20092010
SURMODICS, INC.
Exhibit | ||||
| 2 | .1 | Agreement of Merger, dated January 18, 2005, with InnoRx, Inc. — incorporated by reference to Exhibit 2.1 to the Company’s Current Report onForm 8-K dated January 18, 2005, SEC FileNo. 0-23837. | ||
| 2 | .2 | Stock Purchase Agreement, dated July 31, 2007, between SurModics, Inc. and Southern Research Institute. — incorporated by reference to Exhibit 2.1 to the Company’s Current Report onForm 8-K dated July 31, 2007, SEC FileNo. 0-23837. | ||
| 3 | .1 | Restated Articles of Incorporation, as amended — incorporated by reference to Exhibit 3.1 to the Company’s Quarterly Report onForm 10-QSB for the quarter ended December 31, 1999,SEC File No. 0-23837. | ||
| 3 | .2 | Restated Bylaws of the Company, as amended.** | ||
| 10 | .1* | Company’s Incentive 1997 Stock Option Plan, including specimen of Incentive Stock Option Agreement — incorporated by reference to Exhibit 10.3 to the Company’s Registration Statement onform SB-2, Reg.No. 333-43217. | ||
| 10 | .2* | Form of Restricted Stock Agreement under 1997 Plan — incorporated by reference to Exhibit 10.4 to the Company’s Registration Statement onform SB-2, Reg.No. 333-43217. | ||
| 10 | .3* | Form of Non-qualified Stock Option Agreement under 1997 Plan — incorporated by reference to Exhibit 10.5 to the Company’s Registration Statement onform SB-2, Reg.No. 333-43217. | ||
| 10 | .4 | Adjusted License Agreement by and between the Company and Cordis Corporation effective as of January 1, 2003 — incorporated by reference to Exhibit 10.11 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2002, SEC FileNo. 0-23837. | ||
| 10 | .5 | Reagent Supply Agreement by and between the Company and Cordis Corporation effective as of January 1, 2003 — incorporated by reference to Exhibit 10.12 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2002, SEC FileNo. 0-23837. | ||
| 10 | .6* | Form of officer acceptance regarding employment/compensation — incorporated by reference to Exhibit 10.9 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2005, SEC FileNo. 0-23837. | ||
| 10 | .7* | 2003 Equity Incentive Plan (as amended and restated December 13, 2005) (adopted December 13, 2005 by the board of directors and approved by the shareholders on January 30, 2006) — incorporated by reference to Exhibit 10.1 to the Company’sForm 8-K filed February 3, 2006, SEC FileNo. 0-23837. | ||
| 10 | .8* | Form of SurModics, Inc. 2003 Equity Incentive Plan Nonqualified Stock Option Agreement — incorporated by reference to Exhibit 99.1 to the Company’s8-K filed March 20, 2006,SEC File No. 0-23837. | ||
| 10 | .9* | Form of SurModics, Inc. 2003 Equity Incentive Plan Incentive Stock Option Agreement — incorporated by reference to Exhibit 99.2 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .10* | Form of SurModics, Inc. 2003 Equity Incentive Plan Restricted Stock Agreement — incorporated by reference to Exhibit 99.3 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .11* | Form of SurModics, Inc. 2003 Equity Incentive Plan Performance Share Award Agreement — incorporated by reference to Exhibit 99.4 to the Company’s8-K filed March 20, 2006,SEC File No. 0-23837. | ||
| 10 | .12* | Form of SurModics, Inc. 2003 Equity Incentive Plan Performance Unit Award (cash settled) Agreement — incorporated by reference to Exhibit 99.5 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .13* | Form of SurModics, Inc. 2003 Equity Incentive Plan Restricted Stock Unit Agreement — incorporated by reference to Exhibit 99.6 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .14* | Form of SurModics, Inc. 2003 Equity Incentive Plan Stock Appreciation Rights (cash settled) Agreement — incorporated by reference to Exhibit 99.7 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
Exhibit | ||||
| 2 | .1 | Agreement of Merger, dated January 18, 2005, with InnoRx, Inc. — incorporated by reference to Exhibit 2.1 to the Company’s Current Report onForm 8-K dated January 18, 2005, SEC FileNo. 0-23837. | ||
| 2 | .2 | Stock Purchase Agreement, dated July 31, 2007, between SurModics, Inc. and Southern Research Institute. — incorporated by reference to Exhibit 2.1 to the Company’s Current Report onForm 8-K dated July 31, 2007, SEC FileNo. 0-23837. | ||
| 3 | .1 | Restated Articles of Incorporation, as amended — incorporated by reference to Exhibit 3.1 to the Company’s Quarterly Report onForm 10-QSB for the quarter ended December 31, 1999,SEC File No. 0-23837. | ||
| 3 | .2 | Restated Bylaws of the Company, as amended — incorporated by reference to Exhibit 3.2 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2009,SEC File No. 0-23837. | ||
| 10 | .1* | Company’s Incentive 1997 Stock Option Plan, including specimen of Incentive Stock Option Agreement — incorporated by reference to Exhibit 10.3 to the Company’s Registration Statement onform SB-2, Reg.No. 333-43217. | ||
| 10 | .2* | Form of Restricted Stock Agreement under 1997 Plan — incorporated by reference to Exhibit 10.4 to the Company’s Registration Statement onform SB-2, Reg.No. 333-43217. | ||
| 10 | .3* | Form of Non-qualified Stock Option Agreement under 1997 Plan — incorporated by reference to Exhibit 10.5 to the Company’s Registration Statement onform SB-2, Reg.No. 333-43217. | ||
| 10 | .4+ | Adjusted License Agreement by and between the Company and Cordis Corporation effective as of January 1, 2003 — incorporated by reference to Exhibit 10.11 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2002, SEC FileNo. 0-23837. | ||
| 10 | .5+ | Reagent Supply Agreement by and between the Company and Cordis Corporation effective as of January 1, 2003 — incorporated by reference to Exhibit 10.12 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2002, SEC FileNo. 0-23837. | ||
| 10 | .6* | Form of officer acceptance regarding employment/compensation — incorporated by reference to Exhibit 10.9 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2005, SEC FileNo. 0-23837. | ||
| 10 | .7* | 2003 Equity Incentive Plan (as amended and restated December 13, 2005) (adopted December 13, 2005 by the board of directors and approved by the shareholders on January 30, 2006) — incorporated by reference to Exhibit 10.1 to the Company’sForm 8-K filed February 3, 2006, SEC FileNo. 0-23837. | ||
| 10 | .8* | Form of SurModics, Inc. 2003 Equity Incentive Plan Nonqualified Stock Option Agreement — incorporated by reference to Exhibit 99.1 to the Company’s8-K filed March 20, 2006,SEC File No. 0-23837. | ||
| 10 | .9* | Form of SurModics, Inc. 2003 Equity Incentive Plan Incentive Stock Option Agreement — incorporated by reference to Exhibit 99.2 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .10* | Form of SurModics, Inc. 2003 Equity Incentive Plan Restricted Stock Agreement — incorporated by reference to Exhibit 99.3 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .11* | Form of SurModics, Inc. 2003 Equity Incentive Plan Performance Share Award Agreement — incorporated by reference to Exhibit 99.4 to the Company’s8-K filed March 20, 2006,SEC File No. 0-23837. | ||
| 10 | .12* | Form of SurModics, Inc. 2003 Equity Incentive Plan Performance Unit Award (cash settled) Agreement — incorporated by reference to Exhibit 99.5 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .13* | Form of SurModics, Inc. 2003 Equity Incentive Plan Restricted Stock Unit Agreement — incorporated by reference to Exhibit 99.6 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .14* | Form of SurModics, Inc. 2003 Equity Incentive Plan Stock Appreciation Rights (cash settled) Agreement — incorporated by reference to Exhibit 99.7 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
5152
Exhibit | ||||
| 10 | .15* | Form of SurModics, Inc. 2003 Equity Incentive Plan Stock Appreciation Rights (stock settled) Agreement — incorporated by reference to Exhibit 99.8 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .16* | Change in Control Agreement with Bruce J Barclay, dated April 19, 2006 — incorporated by reference to Exhibit 99.1 to the Company’sForm 8-K filed April 25, 2006, SEC FileNo. 0-23837. | ||
| 10 | .17* | Change in Control Agreement with Philip D. Ankeny, dated April 19, 2006 — incorporated by reference to Exhibit 99.2 to the Company’sForm 8-K filed April 25, 2006, SEC FileNo. 0-23837. | ||
| 10 | .18 | The Company’s Board Compensation Policy, Amended as of November 17, 2008.** | ||
| 10 | .19* | Change in Control Agreement with Paul A. Lopez, dated November 15, 2006 — incorporated by reference to Exhibit 10.27 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2006, SEC FileNo. 0-23837. | ||
| 10 | .20* | Description of certain retirement benefits for Dale R. Olseth — incorporated by reference to Exhibit 10.28 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2006, SEC FileNo. 0-23837. | ||
| 10 | .21+ | Exclusive License and Research Collaboration Agreement with Merck & Co., Inc. dated June 26, 2007 — incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report onForm 10-Q for the quarter ended June 30, 2007, SEC FileNo. 0-23837. | ||
| 10 | .22+ | Supply Agreement with Merck & Co., Inc. dated June 26, 2007 — incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report onForm 10-Q for the quarter ended June 30, 2007, SEC FileNo. 0-23837. | ||
| 10 | .23* | Employment Agreement of Arthur J. Tipton, Ph.D., dated July 31, 2007 — incorporated by reference to Exhibit 10.27 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2007. | ||
| 10 | .24 | Purchase Agreement with Vest Mykyng LLC, dated August 24, 2007 — incorporated by reference to Exhibit 10.28 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2007. | ||
| 10 | .25 | Real Estate Purchase and Sale Agreement dated March 11, 2008 by and between Belk, Inc. and SurModics Pharmaceuticals, Inc. — incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report onForm 10-Q for the quarter ended March 31, 2008, SEC FileNo. 0-23837. | ||
| 10 | .26 | Credit Agreement dated as of February 27, 2009, by and between SurModics, Inc. and Wells Fargo Bank, National Association as Sole Lead Arranger and Administrative Agent — incorporated by reference to Exhibit 10.1 to the Company’sForm 8-K filed March 4, 2009, SEC FileNo. 0-23837. | ||
| 10 | .27* | Amendment to Change of Control Agreement, dated as of December 23, 2008, between SurModics, Inc. and Bruce J Barclay.** | ||
| 10 | .28* | Amendment to Change of Control Agreement, dated as of December 23, 2008, between SurModics, Inc. and Philip D. Ankeny.** | ||
| 10 | .29* | Second Amendment to Change of Control Agreement, dated as of April 19, 2009, between SurModics, Inc. and Bruce J Barclay.** | ||
| 10 | .30* | Second Amendment to Change of Control Agreement, dated as of April 19, 2009, between SurModics, Inc. and Philip D. Ankeny.** | ||
| 21 | Subsidiaries of the Registrant.** | |||
| 23 | Consent of Deloitte & Touche LLP.** | |||
| 24 | Power of Attorney (included on signature page of thisForm 10-K).** | |||
| 31 | .1 | Certification of Chief Executive Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002.** | ||
| 31 | .2 | Certification of Chief Financial Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002.** | ||
| 32 | .1 | Certification of Chief Executive Officer Pursuant to Section 906 of Sarbanes-Oxley Act of 2002.** | ||
| 32 | .2 | Certification of Chief Financial Officer Pursuant to Section 906 of Sarbanes-Oxley Act of 2002.** | ||
Exhibit | ||||
| 10 | .15* | Form of SurModics, Inc. 2003 Equity Incentive Plan Stock Appreciation Rights (stock settled) Agreement — incorporated by reference to Exhibit 99.8 to the Company’s8-K filed March 20, 2006, SEC FileNo. 0-23837. | ||
| 10 | .16* | Change in Control Agreement with Philip D. Ankeny, dated April 19, 2006 — incorporated by reference to Exhibit 99.2 to the Company’sForm 8-K filed April 25, 2006, SEC FileNo. 0-23837. | ||
| 10 | .17 | The Company’s Board Compensation Policy, Amended and Restated as of February 8, 2010.** | ||
| 10 | .18 | Credit Agreement dated as of February 27, 2009, by and between SurModics, Inc. and Wells Fargo Bank, National Association as Sole Lead Arranger and Administrative Agent — incorporated by reference to Exhibit 10.1 to the Company’sForm 8-K filed March 4, 2009, SEC FileNo. 0-23837. | ||
| 10 | .19* | Amendment to Change of Control Agreement, dated as of December 23, 2008, between SurModics, Inc. and Philip D. Ankeny. — incorporated by reference to Exhibit 10.28 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2009, SEC FileNo. 0-23837. | ||
| 10 | .20* | Second Amendment to Change of Control Agreement, dated as of April 19, 2009, between SurModics, Inc. and Philip D. Ankeny. — incorporated by reference to Exhibit 10.30 to the Company’s Annual Report onForm 10-K for the fiscal year ended September 30, 2009, SEC FileNo. 0-23837. | ||
| 10 | .21+ | License and Development Agreement between Genentech, Inc., F. Hoffmann-La Roche, Ltd., and SurModics, Inc., dated October 5, 2009 — incorporated by reference to Exhibit 10.1 to the Company’sForm 10-Q filed February 5, 2010, SEC FileNo. 0-23837. | ||
| 10 | .22+ | Master Services Agreement by and between Genentech, Inc., F. Hoffmann-La Roche, Ltd. and SurModics, Inc., dated October 5, 2009 — incorporated by reference to Exhibit 10.2 to the Company’sForm 10-Q filed February 5, 2010, SEC FileNo. 0-23837. | ||
| 10 | .23* | SurModics, Inc. 2009 Equity Incentive Plan. — incorporated by reference to Exhibit 10.3 to the Company’sForm 10-Q filed May 7, 2010, SEC FileNo. 0-23837. | ||
| 10 | .24* | SurModics, Inc. 1999 Employee Stock Purchase Plan (as amended and restated November 30, 2009). — incorporated by reference to Exhibit 10.2 to the Company’sForm 10-Q filed May 7, 2010,SEC File No. 0-23837. | ||
| 10 | .25* | Form of Incentive Stock Option Agreement for the SurModics, Inc. 2009 Equity Incentive Plan — incorporated by reference to Exhibit 10.4 to the Company’sForm 10-Q filed May 7, 2010, SEC FileNo. 0- 23837. | ||
| 10 | .26* | Form of Non-Statutory Stock Option Agreement for the SurModics, Inc. 2009 Equity Incentive Plan — incorporated by reference to Exhibit 10.5 to the Company’sForm 10-Q filed May 7, 2010,SEC File No. 0-23837. | ||
| 10 | .27* | Form of Performance Share Agreement for the SurModics, Inc. 2009 Equity Incentive Plan — incorporated by reference to Exhibit 10.6 to the Company’sForm 10-Q filed May 7, 2010,SEC File No. 0-23837. | ||
| 10 | .28* | Form of Restricted Stock Agreement for the SurModics, Inc. 2009 Equity Incentive Plan — incorporated by reference to Exhibit 10.7 to the Company’sForm 10-Q filed May 7, 2010, SEC FileNo. 0-23837. | ||
| 21 | Subsidiaries of the Registrant.** | |||
| 23 | Consent of Deloitte & Touche LLP.** | |||
| 24 | Power of Attorney (included on signature page of thisForm 10-K).** | |||
| 31 | .1 | Certification of Chief Executive Officer and Chief Financial Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002.** | ||
| 32 | .1 | Certification of Chief Executive Officer and Chief Financial Officer Pursuant to Section 906 of Sarbanes-Oxley Act of 2002.** | ||
| * | Management contract or compensatory plan or arrangement |
| ** | Filed herewith | |
| + | Confidential treatment requested as to portions of the exhibit. Confidential portions omitted and provided separately to the Securities and Exchange Commission. |
5253
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
SurModics, Inc.
Eden Prairie, Minnesota
We have audited the accompanying consolidated balance sheets of SurModics, Inc. and subsidiaries (the “Company”) as of September 30, 20092010 and 2008,2009, and the related consolidated statements of income,operations, stockholders’ equity, and cash flows for each of the three years in the period ended September 30, 2009.2010. Our audits also includeincluded the financial statement schedule listed in the Index at Item 15. These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on the financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of SurModics, Inc. and subsidiaries as of September 30, 20092010 and 2008,2009, and the results of their operations and their cash flows for each of the three years in the period ended September 30, 2009,2010, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of September 30, 2009,2010, based on the criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated December 11, 200914, 2010, expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ DELOITTE & TOUCHE LLP
Minneapolis, Minnesota
December 11, 200914, 2010
F-1
SurModics, Inc. and Subsidiaries
As of September 30
| 2009 | 2008 | 2010 | 2009 | |||||||||||||
| (In thousands, except share data) | (In thousands, except share data) | |||||||||||||||
ASSETS | ASSETS | ASSETS | ||||||||||||||
| Current Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 11,636 | $ | 15,376 | $ | 11,391 | $ | 11,636 | ||||||||
| Short-term investments | 8,932 | 9,251 | 9,105 | 8,932 | ||||||||||||
| Accounts receivable, net of allowance for doubtful accounts of $82 and $135 as of | ||||||||||||||||
| September 30, 2009 and 2008, respectively | 11,320 | 14,589 | ||||||||||||||
| Accounts receivable, net of allowance for doubtful accounts of $461 and $82 as of September 30, 2010 and 2009, respectively | 8,987 | 11,320 | ||||||||||||||
| Inventories | 3,330 | 2,651 | 3,047 | 3,330 | ||||||||||||
| Deferred tax asset | 353 | 1,058 | 247 | 353 | ||||||||||||
| Prepaids and other | 1,443 | 3,584 | 4,701 | 1,443 | ||||||||||||
| Total Current Assets | 37,014 | 46,509 | 37,478 | 37,014 | ||||||||||||
| Property and equipment, net | 66,915 | 41,897 | 65,395 | 66,915 | ||||||||||||
| Long-term investments | 27,300 | 47,351 | 36,290 | 27,300 | ||||||||||||
| Deferred tax asset | 2,548 | 11,099 | 2,606 | 2,548 | ||||||||||||
| Intangible assets, net | 17,458 | 16,870 | 15,257 | 17,458 | ||||||||||||
| Goodwill | 21,070 | 18,001 | 8,010 | 21,070 | ||||||||||||
| Other assets, net | 13,257 | 9,301 | 5,243 | 13,257 | ||||||||||||
| Total Assets | $ | 185,562 | $ | 191,028 | $ | 170,279 | $ | 185,562 | ||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||||||
| Current Liabilities | ||||||||||||||||
| Accounts payable | $ | 3,468 | $ | 3,466 | $ | 3,341 | $ | 3,468 | ||||||||
| Accrued liabilities: | ||||||||||||||||
| Compensation | 926 | 3,015 | 930 | 926 | ||||||||||||
| Accrued income taxes payable | 186 | — | — | 186 | ||||||||||||
| Accrued other | 1,637 | 1,407 | 1,753 | 1,637 | ||||||||||||
| Deferred revenue | 905 | 4,335 | 562 | 905 | ||||||||||||
| Other current liabilities | 862 | 303 | 1,061 | 862 | ||||||||||||
| Total Current Liabilities | 7,984 | 12,526 | 7,647 | 7,984 | ||||||||||||
| Deferred revenue, less current portion | 623 | 33,243 | 3,598 | 623 | ||||||||||||
| Other long-term liabilities | 4,583 | 3,453 | 4,675 | 4,583 | ||||||||||||
| Total Liabilities | 13,190 | 49,222 | 15,920 | 13,190 | ||||||||||||
| Commitments and Contingencies (Note 9) | ||||||||||||||||
| Stockholders’ Equity | ||||||||||||||||
| Series A preferred stock — $.05 par value, 450,000 shares authorized; | ||||||||||||||||
| no shares issued and outstanding | — | — | — | — | ||||||||||||
| Common stock — $.05 par value, 45,000,000 shares authorized; 17,471,472 | ||||||||||||||||
| and 18,030,270 shares issued and outstanding | 874 | 901 | ||||||||||||||
| Common stock — $.05 par value, 45,000,000 shares authorized; 17,423,601 and 17,471,472 shares issued and outstanding | 871 | 874 | ||||||||||||||
| Additional paid-in capital | 66,005 | 74,573 | 69,702 | 66,005 | ||||||||||||
| Accumulated other comprehensive income (loss) | 1,504 | (107 | ) | |||||||||||||
| Accumulated other comprehensive income | 886 | 1,504 | ||||||||||||||
| Retained earnings | 103,989 | 66,439 | 82,900 | 103,989 | ||||||||||||
| Total Stockholders’ Equity | 172,372 | 141,806 | 154,359 | 172,372 | ||||||||||||
| Total Liabilities and Stockholders’ Equity | $ | 185,562 | $ | 191,028 | $ | 170,279 | $ | 185,562 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-2
SurModics, Inc. and Subsidiaries
For the Years Ended September 30
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| (In thousands, except net | (In thousands, except net | |||||||||||||||||||||||
| income per share) | income (loss) per share) | |||||||||||||||||||||||
| Revenue | ||||||||||||||||||||||||
| Royalties and license fees | $ | 75,464 | $ | 51,788 | $ | 52,679 | $ | 34,277 | $ | 75,464 | $ | 51,788 | ||||||||||||
| Product sales | 19,333 | 20,052 | 13,543 | 20,184 | 19,333 | 20,052 | ||||||||||||||||||
| Research and development | 26,737 | 25,211 | 6,942 | 15,437 | 26,737 | 25,211 | ||||||||||||||||||
| Total revenue | 121,534 | 97,051 | 73,164 | 69,898 | 121,534 | 97,051 | ||||||||||||||||||
| Operating Costs and Expenses | ||||||||||||||||||||||||
| Operating costs and expenses | ||||||||||||||||||||||||
| Product | 7,508 | 8,476 | 5,584 | 9,425 | 7,508 | 8,476 | ||||||||||||||||||
| Customer research and development | 13,183 | 19,187 | 5,840 | 18,147 | 13,183 | 19,187 | ||||||||||||||||||
| Other research and development | 21,179 | 21,311 | 22,625 | 17,916 | 21,179 | 21,311 | ||||||||||||||||||
| Selling, general and administrative | 17,200 | 20,816 | 13,643 | 18,451 | 17,200 | 20,816 | ||||||||||||||||||
| Purchased in-process research and development | 3,200 | — | 15,573 | — | 3,200 | — | ||||||||||||||||||
| Restructuring charges | 1,763 | — | — | 1,306 | 1,763 | — | ||||||||||||||||||
| Asset impairment charges | 4,896 | — | — | |||||||||||||||||||||
| Goodwill impairment charge | 13,810 | — | — | |||||||||||||||||||||
| Total operating costs and expenses | 64,033 | 69,790 | 63,265 | 83,951 | 64,033 | 69,790 | ||||||||||||||||||
| Income from Operations | 57,501 | 27,261 | 9,899 | |||||||||||||||||||||
| (Loss) income from operations | (14,053 | ) | 57,501 | 27,261 | ||||||||||||||||||||
| Other Income (Loss) | ||||||||||||||||||||||||
| Other (loss) income | ||||||||||||||||||||||||
| Investment income, net | 1,839 | 3,329 | 4,844 | 1,023 | 1,839 | 3,329 | ||||||||||||||||||
| Impairment loss on investment | — | (4,314 | ) | — | ||||||||||||||||||||
| Other income (loss), net | 184 | 616 | (75 | ) | ||||||||||||||||||||
| Impairment loss on investments | (7,943 | ) | — | (4,314 | ) | |||||||||||||||||||
| Other income, net | 314 | 184 | 616 | |||||||||||||||||||||
| Other income (loss), net | 2,023 | (369 | ) | 4,769 | ||||||||||||||||||||
| Other (loss) income, net | (6,606 | ) | 2,023 | (369 | ) | |||||||||||||||||||
| Income Before Income Taxes | 59,524 | 26,892 | 14,668 | |||||||||||||||||||||
| Income Tax Provision | (21,974 | ) | (12,153 | ) | (11,321 | ) | ||||||||||||||||||
| (Loss) income before income taxes | (20,659 | ) | 59,524 | 26,892 | ||||||||||||||||||||
| Income tax provision | (430 | ) | (21,974 | ) | (12,153 | ) | ||||||||||||||||||
| Net Income | $ | 37,550 | $ | 14,739 | $ | 3,347 | ||||||||||||||||||
| Net (loss) income | $ | (21,089 | ) | $ | 37,550 | $ | 14,739 | |||||||||||||||||
| Basic net income per share | $ | 2.15 | $ | 0.82 | $ | 0.19 | ||||||||||||||||||
| Diluted net income per share | $ | 2.15 | $ | 0.80 | $ | 0.18 | ||||||||||||||||||
| Weighted Average Shares Outstanding | ||||||||||||||||||||||||
| Basic net (loss) income per share | $ | (1.21 | ) | $ | 2.15 | $ | 0.82 | |||||||||||||||||
| Diluted net (loss) income per share | $ | (1.21 | ) | $ | 2.15 | $ | 0.80 | |||||||||||||||||
| Weighted average shares outstanding | ||||||||||||||||||||||||
| Basic | 17,435 | 18,026 | 18,033 | 17,372 | 17,435 | 18,026 | ||||||||||||||||||
| Dilutive effect of outstanding stock options | 34 | 304 | 184 | |||||||||||||||||||||
| Dilutive effect of outstanding stock options and non-vested stock | — | 34 | 304 | |||||||||||||||||||||
| Diluted | 17,469 | 18,330 | 18,217 | 17,372 | 17,469 | 18,330 | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
SurModics, Inc. and Subsidiaries
For the Years Ended September 30, 2010, 2009 2008 and 20072008
| Accumulated | Accumulated | |||||||||||||||||||||||||||||||||||||||||||||||
| Additional | Other | Total | Additional | Other | Total | |||||||||||||||||||||||||||||||||||||||||||
| Common Stock | Paid-in | Comprehensive | Retained | Stockholders’ | Common Stock | Paid-In | Comprehensive | Retained | Stockholders’ | |||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Capital | Income (Loss) | Earnings | Equity | Shares | Amount | Capital | Income (Loss) | Earnings | Equity | |||||||||||||||||||||||||||||||||||||
| (In thousands) | (In thousands) | |||||||||||||||||||||||||||||||||||||||||||||||
| Balance September 30, 2006 | 18,830 | $ | 942 | $ | 96,281 | $ | (293 | ) | $ | 48,273 | $ | 145,203 | ||||||||||||||||||||||||||||||||||||
| Components of comprehensive income, net of tax: | ||||||||||||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | 3,347 | 3,347 | ||||||||||||||||||||||||||||||||||||||||||
| Unrealized holding gains on available-for-sale securities arising during the period | — | — | — | 1,999 | — | 1,999 | ||||||||||||||||||||||||||||||||||||||||||
| Add reclassification for losses included in net income, net of tax benefit of $10 | — | — | — | 17 | — | 17 | ||||||||||||||||||||||||||||||||||||||||||
| Comprehensive income | — | — | — | — | — | 5,363 | ||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock | 14 | 1 | 457 | — | — | 458 | ||||||||||||||||||||||||||||||||||||||||||
| Common stock repurchased | (1,008 | ) | (50 | ) | (34,980 | ) | — | — | (35,030 | ) | ||||||||||||||||||||||||||||||||||||||
| Common stock options exercised, net | 217 | 11 | 4,778 | — | — | 4,789 | ||||||||||||||||||||||||||||||||||||||||||
| Purchase of common stock to pay employee taxes | 112 | 5 | (379 | ) | — | — | (374 | ) | ||||||||||||||||||||||||||||||||||||||||
| Excess tax benefit from exercise of stock options | — | — | 466 | — | — | 466 | ||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | 10,312 | — | — | 10,312 | ||||||||||||||||||||||||||||||||||||||||||
| Other | — | — | (265 | ) | — | — | (265 | ) | ||||||||||||||||||||||||||||||||||||||||
| Balance September 30, 2007 | 18,165 | 909 | 76,670 | 1,723 | 51,620 | 130,922 | 18,165 | $ | 909 | $ | 76,670 | $ | 1,723 | $ | 51,620 | $ | 130,922 | |||||||||||||||||||||||||||||||
| Components of comprehensive income, net of tax: | ||||||||||||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | 14,739 | 14,739 | — | — | — | — | 14,739 | 14,739 | ||||||||||||||||||||||||||||||||||||
| Unrealized holding losses on available-for-sale securities arising during the period | — | — | — | (5,882 | ) | — | (5,882 | ) | — | — | — | (5,882 | ) | — | (5,882 | ) | ||||||||||||||||||||||||||||||||
| Add reclassification for losses included in net income, net of tax provision of $167 | — | — | — | 4,052 | — | 4,052 | — | — | — | 4,052 | — | 4,052 | ||||||||||||||||||||||||||||||||||||
| Comprehensive income | — | — | — | — | — | 12,909 | — | — | — | — | — | 12,909 | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock | 16 | 1 | 516 | — | — | 517 | 16 | 1 | 516 | — | — | 517 | ||||||||||||||||||||||||||||||||||||
| Common stock repurchased | (342 | ) | (17 | ) | (13,954 | ) | — | — | (13,971 | ) | (342 | ) | (17 | ) | (13,954 | ) | — | — | (13,971 | ) | ||||||||||||||||||||||||||||
| Common stock options exercised, net | 114 | 4 | 2,514 | — | — | 2,518 | 114 | 4 | 2,514 | — | — | 2,518 | ||||||||||||||||||||||||||||||||||||
| Purchase of common stock to pay employee taxes | 77 | 4 | (1,678 | ) | — | — | (1,674 | ) | 77 | 4 | (1,678 | ) | — | — | (1,674 | ) | ||||||||||||||||||||||||||||||||
| Excess tax benefit from exercise of stock options | — | — | 1,081 | — | — | 1,081 | ||||||||||||||||||||||||||||||||||||||||||
| Excess tax benefit from stock-based compensation plans | — | — | 1,081 | — | — | 1,081 | ||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | 9,652 | — | — | 9,652 | — | — | 9,652 | — | — | 9,652 | ||||||||||||||||||||||||||||||||||||
| Other | — | — | (228 | ) | — | — | (228 | ) | — | — | (228 | ) | — | — | (228 | ) | ||||||||||||||||||||||||||||||||
| Accounting change for income taxes | — | — | — | — | 80 | 80 | — | — | — | — | 80 | 80 | ||||||||||||||||||||||||||||||||||||
| Balance September 30, 2008 | 18,030 | 901 | 74,573 | (107 | ) | 66,439 | 141,806 | 18,030 | 901 | 74,573 | (107 | ) | 66,439 | 141,806 | ||||||||||||||||||||||||||||||||||
| Components of comprehensive income, net of tax: | ||||||||||||||||||||||||||||||||||||||||||||||||
| Components of comprehensive income, | ||||||||||||||||||||||||||||||||||||||||||||||||
| net of tax: | ||||||||||||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | 37,550 | 37,550 | — | — | — | — | 37,550 | 37,550 | ||||||||||||||||||||||||||||||||||||
| Unrealized holding gains on available-for-sale securities arising during the period | — | — | — | 2,123 | — | 2,123 | — | — | — | 2,123 | — | 2,123 | ||||||||||||||||||||||||||||||||||||
| Add reclassification for gains included in net income, net of tax provision of $299 | — | — | — | (512 | ) | — | (512 | ) | — | — | — | (512 | ) | — | (512 | ) | ||||||||||||||||||||||||||||||||
| Comprehensive income | — | — | — | — | — | 39,161 | — | — | — | — | — | 39,161 | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock | 40 | 2 | 611 | — | — | 613 | 40 | 2 | 611 | — | — | 613 | ||||||||||||||||||||||||||||||||||||
| Common stock repurchased | (624 | ) | (31 | ) | (14,967 | ) | — | — | (14,998 | ) | (624 | ) | (31 | ) | (14,967 | ) | — | — | (14,998 | ) | ||||||||||||||||||||||||||||
| Common stock options exercised, net | 15 | 1 | 65 | — | — | 66 | 15 | 1 | 65 | — | — | 66 | ||||||||||||||||||||||||||||||||||||
| Purchase of common stock to pay employee taxes | 10 | 1 | (569 | ) | — | — | (568 | ) | 10 | 1 | (569 | ) | — | — | (568 | ) | ||||||||||||||||||||||||||||||||
| Excess tax benefit from exercise of stock options | — | — | (366 | ) | — | — | (366 | ) | ||||||||||||||||||||||||||||||||||||||||
| Excess tax benefit from stock-based compensation plans | — | — | (366 | ) | — | — | (366 | ) | ||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | 6,853 | — | — | 6,853 | — | — | 6,853 | — | — | 6,853 | ||||||||||||||||||||||||||||||||||||
| Other | — | — | (195 | ) | — | — | (195 | ) | — | — | (195 | ) | — | — | (195 | ) | ||||||||||||||||||||||||||||||||
| Balance September 30, 2009 | 17,471 | $ | 874 | $ | 66,005 | $ | 1,504 | $ | 103,989 | $ | 172,372 | 17,471 | 874 | 66,005 | 1,504 | 103,989 | 172,372 | |||||||||||||||||||||||||||||||
| Components of comprehensive loss, | ||||||||||||||||||||||||||||||||||||||||||||||||
| net of tax: | ||||||||||||||||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | (21,089 | ) | (21,089 | ) | ||||||||||||||||||||||||||||||||||||||||
| Unrealized holding losses on available-for-sale securities arising during the period | — | — | — | (437 | ) | — | (437 | ) | ||||||||||||||||||||||||||||||||||||||||
| Add reclassification for gains included in net loss, net of tax benefit of $118 | — | — | — | (181 | ) | — | (181 | ) | ||||||||||||||||||||||||||||||||||||||||
| Comprehensive loss | (21,707 | ) | ||||||||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock | 40 | 2 | 608 | — | — | 610 | ||||||||||||||||||||||||||||||||||||||||||
| Common stock repurchased | (102 | ) | (6 | ) | (2,026 | ) | — | — | (2,032 | ) | ||||||||||||||||||||||||||||||||||||||
| Common stock options exercised, net | 14 | 1 | 281 | — | — | 282 | ||||||||||||||||||||||||||||||||||||||||||
| Purchase of common stock to pay employee taxes | 1 | — | (545 | ) | (545 | ) | ||||||||||||||||||||||||||||||||||||||||||
| Excess tax benefit from stock-based compensation plans | — | — | (496 | ) | — | — | (496 | ) | ||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | 5,875 | — | — | 5,875 | ||||||||||||||||||||||||||||||||||||||||||
| Balance September 30, 2010 | 17,424 | $ | 871 | $ | 69,702 | $ | 886 | $ | 82,900 | $ | 154,359 | |||||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
SurModics, Inc. and Subsidiaries
For the Years Ended September 30
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| (In thousands) | (In thousands) | |||||||||||||||||||||||
| Operating Activities | ||||||||||||||||||||||||
| Net income | $ | 37,550 | $ | 14,739 | $ | 3,347 | ||||||||||||||||||
| Adjustments to reconcile net income to net cash provided by | ||||||||||||||||||||||||
| operating activities | ||||||||||||||||||||||||
| Net (loss) income | $ | (21,089 | ) | $ | 37,550 | $ | 14,739 | |||||||||||||||||
| Adjustments to reconcile net (loss) income to net cash provided by | ||||||||||||||||||||||||
| operating activities: | ||||||||||||||||||||||||
| Depreciation and amortization | 5,912 | 6,071 | 4,214 | 7,818 | 5,912 | 6,071 | ||||||||||||||||||
| (Gain) loss on equity method investments and sales of investments | (103 | ) | 415 | 75 | (299 | ) | (103 | ) | 415 | |||||||||||||||
| Amortization of premium (discount) on investments | 139 | 70 | (1,388 | ) | ||||||||||||||||||||
| Impairment loss on investment | — | 4,314 | — | |||||||||||||||||||||
| Amortization of premium on investments | 128 | 139 | 70 | |||||||||||||||||||||
| Impairment loss on investments | 7,943 | — | 4,314 | |||||||||||||||||||||
| Stock-based compensation | 6,853 | 9,652 | 10,312 | 5,875 | 6,853 | 9,652 | ||||||||||||||||||
| Purchased in-process research & development | 3,200 | — | 15,573 | — | 3,200 | — | ||||||||||||||||||
| Restructuring charges | 1,763 | — | — | |||||||||||||||||||||
| Asset impairment charges | 4,896 | — | — | |||||||||||||||||||||
| Goodwill impairment charge | 13,810 | — | — | |||||||||||||||||||||
| Deferred tax | 8,229 | (3,428 | ) | (9,434 | ) | 446 | 8,229 | (3,428 | ) | |||||||||||||||
| Excess tax benefit from exercise of stock options | 366 | (1,081 | ) | (466 | ) | |||||||||||||||||||
| Excess tax benefit from stock-based compensation plans | 496 | 366 | (1,081 | ) | ||||||||||||||||||||
| Loss on disposals of property and equipment | 291 | 78 | 379 | 3 | 291 | 78 | ||||||||||||||||||
| Other | (250 | ) | — | — | — | (250 | ) | — | ||||||||||||||||
| Change in operating assets and liabilities: | ||||||||||||||||||||||||
| Accounts receivable | 3,269 | 1,548 | 1,940 | 2,333 | 3,269 | 1,548 | ||||||||||||||||||
| Inventories | (679 | ) | (154 | ) | (850 | ) | 284 | (679 | ) | (154 | ) | |||||||||||||
| Accounts payable and accrued liabilities | (2,387 | ) | (264 | ) | 2,594 | 1,135 | (624 | ) | (264 | ) | ||||||||||||||
| Income taxes | 2,656 | (5,003 | ) | 5,501 | (4,121 | ) | 2,656 | (5,003 | ) | |||||||||||||||
| Deferred revenue | (36,050 | ) | 11,452 | 19,166 | 2,632 | (36,050 | ) | 11,452 | ||||||||||||||||
| Prepaids and other | 562 | 1,413 | (248 | ) | (282 | ) | 562 | 1,413 | ||||||||||||||||
| Net cash provided by operating activities | 31,321 | 39,822 | 50,715 | 22,008 | 31,321 | 39,822 | ||||||||||||||||||
| Investing Activities | ||||||||||||||||||||||||
| Purchases of property and equipment | (29,364 | ) | (23,866 | ) | (3,626 | ) | (9,679 | ) | (29,364 | ) | (23,866 | ) | ||||||||||||
| Sales of property and equipment | — | 32 | 37 | — | — | 32 | ||||||||||||||||||
Purchases ofavailable-for-sale investments | (33,568 | ) | (22,857 | ) | (136,498 | ) | (34,919 | ) | (33,568 | ) | (22,857 | ) | ||||||||||||
Sales/maturities ofavailable-for-sale investments | 55,263 | 29,258 | 185,075 | |||||||||||||||||||||
| Sales/maturities of investments | 25,986 | 55,263 | 29,258 | |||||||||||||||||||||
Purchases ofheld-to-maturity investments | — | (6,485 | ) | — | — | — | (6,485 | ) | ||||||||||||||||
| Investment in other strategic assets | (2,500 | ) | (2,562 | ) | (5,749 | ) | (500 | ) | (2,500 | ) | (2,562 | ) | ||||||||||||
| Purchase of licenses and patents | (631 | ) | (2,452 | ) | (1,355 | ) | (210 | ) | (631 | ) | (2,452 | ) | ||||||||||||
| Acquisitions, net of cash acquired | (8,585 | ) | (3,219 | ) | (49,112 | ) | (750 | ) | (8,585 | ) | (3,219 | ) | ||||||||||||
| Repayment of notes receivable | — | 5,870 | 530 | — | — | 5,870 | ||||||||||||||||||
| Other investing activities | (187 | ) | (228 | ) | (265 | ) | — | (187 | ) | (228 | ) | |||||||||||||
| Net cash used in investing activities | (19,572 | ) | (26,509 | ) | (10,963 | ) | (20,072 | ) | (19,572 | ) | (26,509 | ) | ||||||||||||
| Financing Activities | ||||||||||||||||||||||||
| Excess tax benefit from exercise of stock options | (366 | ) | 1,081 | 466 | ||||||||||||||||||||
| Excess tax benefit from stock-based compensation plans | (496 | ) | (366 | ) | 1,081 | |||||||||||||||||||
| Issuance of common stock | 679 | 3,037 | 5,247 | 892 | 679 | 3,037 | ||||||||||||||||||
| Repurchase of common stock | (14,998 | ) | (13,971 | ) | (35,030 | ) | (2,032 | ) | (14,998 | ) | (13,971 | ) | ||||||||||||
| Purchase of common stock to pay employee taxes | (568 | ) | (1,674 | ) | (374 | ) | (545 | ) | (568 | ) | (1,674 | ) | ||||||||||||
| Repayment of notes payable | (236 | ) | (222 | ) | — | — | (236 | ) | (222 | ) | ||||||||||||||
| Net cash used in financing activities | (15,489 | ) | (11,749 | ) | (29,691 | ) | (2,181 | ) | (15,489 | ) | (11,749 | ) | ||||||||||||
| Net change in cash and cash equivalents | (3,740 | ) | 1,564 | 10,061 | (245 | ) | (3,740 | ) | 1,564 | |||||||||||||||
| Cash and Cash Equivalents | ||||||||||||||||||||||||
| Beginning of year | 15,376 | 13,812 | 3,751 | 11,636 | 15,376 | 13,812 | ||||||||||||||||||
| End of year | $ | 11,636 | $ | 15,376 | $ | 13,812 | $ | 11,391 | $ | 11,636 | $ | 15,376 | ||||||||||||
| Supplemental Information | ||||||||||||||||||||||||
| Cash paid for income taxes | $ | 11,285 | $ | 21,058 | $ | 14,930 | $ | 4,105 | $ | 11,285 | $ | 21,058 | ||||||||||||
| Noncash transaction — acquisition of property, | ||||||||||||||||||||||||
| plant, and equipment on account | $ | 1,247 | $ | 1,745 | $ | 252 | $ | 565 | $ | 1,247 | $ | 1,745 | ||||||||||||
| Noncash transaction — acquisition of intangibles on account | $ | 210 | $ | — | $ | — | $ | — | $ | 210 | $ | — | ||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
SurModics, Inc. and Subsidiaries
September 30, 20092010 and 20082009
| 1. | Description |
SurModics, Inc. and subsidiaries (the “Company”) develops, manufactures and markets innovative drug delivery and surface modification technologies for the healthcare industry. The Company’s revenue is derived from three primary sources: (1) royalties and license fees from licensing its patented drug delivery and surface modification technologies andin vitrodiagnostic formats to customers; (2) the sale of polymers and reagent chemicals to licensees; substrates, antigens and stabilization products to the diagnostics industry; microarray slides to the diagnostic and biomedical research markets; and (3) research and development fees generated on projects for customers.
Basis of Presentation
The consolidated financial statements include all accounts and wholly owned subsidiaries, and have been prepared in accordance with accounting principles generally accepted in the United States of America (GAAP). All significant inter-company transactions have been eliminated.
Subsequent Events
Subsequent events have been evaluated through December 11, 2009, the date the financial statements were issued.
On October 5, 2009,14, 2010 the Company enteredannounced an organizational change to reduce its cost structure and renew its focus on business units. The Company reorganized into three market-focused business units: Medical Device, Pharmaceuticals and In Vitro Diagnostics (IVD). Previously the Company operated under a licensefunctional expertise alignment. As a result of these organizational changes, which included a 13% reduction in total workforce, the Company will incur a one-time restructuring charge of approximately $1.3 million to $1.7 million in the first quarter of fiscal 2011. Beginning with the first quarter of fiscal 2011, the Company will describe its business under the new reporting structure.
The Company has incurred a $0.8 million milestone payment obligation related to the SurModics Pharmaceuticals, Inc. (SurModics Pharmaceuticals) acquisition in the first quarter of fiscal 2011.
In November 2010, the Company received notice from the Internal Revenue Service that it was awarded approximately $1.3 million of federal grants for qualified investments made in qualified therapeutic discovery projects. These grants will be recognized in fiscal 2011 as a reduction to the Company’s Other Research and development agreementDevelopment expenses.
In addition, in December 2010, we announced that the Board of Directors of the Company had authorized the Company to explore strategic alternatives for the Company’s Pharmaceuticals business, including a potential sale of that business. This decision by the Board reflects our focus on returning the Company to profitable growth, and our renewed commitment to pursuing growth opportunities and investments in our Medical Device and In Vitro Diagnostics businesses. We have retained Piper Jaffray & Co. as our financial adviser in connection with F. Hoffmann-La Roche, Ltd. (“Roche”)this process. The Company has made no decision to enter into any transaction regarding the Pharmaceuticals business, and Genentech, Inc.,there can be no assurance that we will enter into such a wholly ownedtransaction in the future. Additional details concerning this announcement can be found in the Company’s Current Report onForm 8-K expected to be filed with the Securities and Exchange Commission on or before December 20, 2010.
In December 2010, we also announced that Gary R. Maharaj has been named President and Chief Executive Officer of the Company, with such appointment to be effective December 27, 2010. Mr. Maharaj will also serve as a member of the Roche Group (“Genentech”)Company’s Board of Directors. Mr. Maharaj previously served as President and Chief Executive Officer of Arizant Inc., associateda provider of patient temperature management in hospital operating rooms. Additional details concerning this announcement can be found in the Company’s Current Report onForm 8-K expected to be filed with the Company’s proprietary biodegradable microparticles drug delivery system. Securities and Exchange Commission on or before December 20, 2010.
F-6
SurModics, received an up front licensing fee of $3.5 million, could be eligibleInc. and Subsidiaries
Notes to receive up to approximately $200 million in fees and milestone payments in the event of the successful development and commercialization of multiple products, and will be paid for development work done on these products. Roche and Genentech will have the right to obtain manufacturing services from SurModics. In the event a commercial product is developed, the Company will also receive royalties on sales of such products.Consolidated Financial Statements — (Continued)
| 2. | Summary of Significant Accounting Policies and Select Balance Sheet Information |
Cash and Cash Equivalents
Cash and cash equivalents consist of financial instruments with original maturities of three months or less and are stated at cost which approximates fair value.
Investments
Investments consist principally of U.S. government and government agency obligations and mortgage-backed securities and are classified asavailable-for-sale orheld-to-maturity at September 30, 20092010 and 2008.2009.Available-for-sale investments are reported at fair value with unrealized gains and losses net of tax excluded from operations and reported as a separate component of stockholders’ equity, except forother-than-temporary impairments, which are reported as a charge to current operations. A loss would be recognized when there is another-than-temporary impairment in the fair value of any individual security classified asavailable-for-sale, with the associated net unrealized loss reclassified out of accumulated other comprehensive income with a corresponding adjustment to other income (loss). This adjustment results in a new cost basis for the investment. Investments that management has the intent and ability to hold to maturity are classified asheld-to-maturity and reported at amortized cost. If there is another-than-temporary impairment in the fair value of any individual security classified asheld-to-maturity, the Company will write down the security to fair value with a corresponding adjustment to other income (loss). Interest on debt securities, including amortization of premiums and accretion of discounts, is
F-6
included in other income (loss). Realized gains and losses from the sales of debt securities, which are included in other income (loss), are determined using the specific identification method.
The original cost, unrealized holding gains and losses, and fair value ofavailable-for-sale investments as of September 30 were as follows(in thousands):
| 2009 | 2010 | |||||||||||||||||||||||||||||||
| Original Cost | Unrealized Gains | Unrealized Losses | Fair Value | Original Cost | Unrealized Gains | Unrealized Losses | Fair Value | |||||||||||||||||||||||||
| U.S. government obligations | $ | 10,837 | $ | 253 | $ | — | $ | 11,090 | $ | 25,968 | $ | 395 | $ | (34 | ) | $ | 26,329 | |||||||||||||||
| Mortgage-backed securities | 7,938 | 177 | (106 | ) | 8,009 | 4,711 | 164 | (48 | ) | 4,827 | ||||||||||||||||||||||
| Municipal bonds | 7,210 | 232 | — | 7,442 | 3,079 | 72 | — | 3,151 | ||||||||||||||||||||||||
| Asset-backed securities | 2,334 | 65 | (143 | ) | 2,256 | 1,146 | 8 | (42 | ) | 1,112 | ||||||||||||||||||||||
| Corporate bonds | 1,181 | 3 | — | 1,184 | 5,828 | 24 | — | 5,852 | ||||||||||||||||||||||||
| Total | $ | 29,500 | $ | 730 | $ | (249 | ) | $ | 29,981 | $ | 40,732 | $ | 663 | $ | (124 | ) | $ | 41,271 | ||||||||||||||
| 2008 | 2009 | |||||||||||||||||||||||||||||||
| Original Cost | Unrealized Gains | Unrealized Losses | Fair Value | Original Cost | Unrealized Gains | Unrealized Losses | Fair Value | |||||||||||||||||||||||||
| U.S. government obligations | $ | 18,440 | $ | 91 | $ | (87 | ) | $ | 18,444 | $ | 10,837 | $ | 253 | $ | — | $ | 11,090 | |||||||||||||||
| Mortgage-backed securities | 10,147 | 46 | (179 | ) | 10,014 | 7,938 | 177 | (106 | ) | 8,009 | ||||||||||||||||||||||
| Municipal bonds | 11,022 | 153 | (3 | ) | 11,172 | 7,210 | 232 | — | 7,442 | |||||||||||||||||||||||
| Asset-backed securities | 6,193 | 2 | (171 | ) | 6,024 | 2,334 | 65 | (143 | ) | 2,256 | ||||||||||||||||||||||
| Corporate bonds | 4,582 | 8 | (33 | ) | 4,557 | 1,181 | 3 | — | 1,184 | |||||||||||||||||||||||
| Total | $ | 50,384 | $ | 300 | $ | (473 | ) | $ | 50,211 | $ | 29,500 | $ | 730 | $ | (249 | ) | $ | 29,981 | ||||||||||||||
F-7
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
The original cost and fair value of investments by contractual maturity at September 30, 20092010 were as follows(in thousands):
| Original Cost | Fair Value | Amortized Cost | Fair Value | |||||||||||||
| Debt securities due within: | ||||||||||||||||
| One year | $ | 6,830 | $ | 6,911 | $ | 8,075 | $ | 8,092 | ||||||||
| One to five years | 14,297 | 14,749 | 27,046 | 27,541 | ||||||||||||
| Five years or more | 8,373 | 8,321 | 5,611 | 5,638 | ||||||||||||
| Total | $ | 29,500 | $ | 29,981 | $ | 40,732 | $ | 41,271 | ||||||||
The following table summarizes sales ofavailable-for-sale securities for the years ended September 30, 2010, 2009 2008 and 20072008(in thousands):
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Proceeds from sales | $ | 55,263 | $ | 29,258 | $ | 185,075 | $ | 23,986 | $ | 55,263 | $ | 29,258 | ||||||||||||
| Gross realized gains | $ | 823 | $ | 454 | $ | 7 | $ | 302 | $ | 823 | $ | 454 | ||||||||||||
| Gross realized losses | $ | (12 | ) | $ | (26 | ) | $ | (34 | ) | $ | (3 | ) | $ | (12 | ) | $ | (26 | ) | ||||||
At September 30, 2009,2010, the amortized cost and fair market value ofheld-to-maturity debt securities were $6.3$4.1 million and $6.4$4.3 million, respectively. Investments in securities designated asheld-to-maturity consist of tax-exempt municipal bonds and have maturity dates ranging between three months and three years from September 30, 2009.2010. At September 30, 2008,2009, the amortized cost and fair market value ofheld-to-maturity debt securities were $6.3 million and $6.4 million, and $6.3 million, respectively.
F-7
Inventories
Inventories are principally stated at the lower of cost or market using the specific identification method and include direct labor, materials and overhead. Inventories consisted of the following as of September 30(in thousands):
| 2009 | 2008 | 2010 | 2009 | |||||||||||||
| Raw materials | $ | 1,287 | $ | 1,308 | $ | 1,140 | $ | 1,287 | ||||||||
| Finished products | 2,043 | 1,343 | 1,907 | 2,043 | ||||||||||||
| Total | $ | 3,330 | $ | 2,651 | $ | 3,047 | $ | 3,330 | ||||||||
Property and Equipment
Property and equipment are stated at cost and are depreciated using the straight-line method over 1 to 32 years, the estimated useful lives of the assets. The Company recorded depreciation expense of $6.2 million, $3.8 million $3.1 million and $2.2$3.1 million for the years ended September 30, 2010, 2009 2008 and 2007,2008, respectively.
The September 30, 20092010 and 20082009 balances inconstruction-in-progress include the cost of enhancing the capabilities of the Company’s Eden Prairie, Minnesota and Birmingham, Alabama facilities. As assets are placed in service,construction-in-progress is transferred to the specific property and equipment categories and depreciated over the estimated useful lives of the assets.
In April 2008,fiscal 2010, the Company acquiredrecorded a 286,000 square foot$1.9 million asset impairment charge associated with writing down one of our facilities in Alabama to fair value based on a decision to sell the facility, situated on 42 acreswhich decision was reversed later in Birmingham, Alabama for $12.2 million.fiscal 2010 ($0.5 million related to Land, $1.2 million related to Building and improvements and $0.2 million related to Laboratory fixtures and equipment). The Company has been renovating the existing facility to accommodate research and development, clinical manufacturing and commercial manufacturing of drug delivery products for pharmaceutical and biotechnology customers. The building is currently classified asconstruction-in-progress until renovation and remodeling is completed. The value of the landalso recognized a $0.8 million asset impairment charge associated with the purchase is classified as part of the total land carrying value.
| Useful Life | 2009 | 2008 | ||||||||||
| (In years) | ||||||||||||
| Land | $ | 7,409 | $ | 7,409 | ||||||||
| Laboratory fixtures and equipment | 3 to 12 | 19,549 | 15,767 | |||||||||
| Building and improvements | 1 to 32 | 15,911 | 15,025 | |||||||||
| Office furniture and equipment | 3 to 10 | 4,550 | 4,156 | |||||||||
| Construction-in-progress | 40,210 | 16,931 | ||||||||||
| Less accumulated depreciation | (20,714 | ) | (17,391 | ) | ||||||||
| Property and equipment, net | $ | 66,915 | $ | 41,897 | ||||||||
F-8
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
business is expected in the near term based on current market conditions. In addition, the Company recorded a $1.3 million asset impairment charge associated with certain fixed asset costs located in Minnesota that were included inConstruction-in-progress at September 30, 2009.
Property and equipment consisted of the following components as of September 30(in thousands):
| Useful Life | 2010 | 2009 | ||||||||||
| (In years) | ||||||||||||
| Land | $ | 6,886 | $ | 7,409 | ||||||||
| Laboratory fixtures and equipment | 3 to 12 | 25,958 | 19,549 | |||||||||
| Building and improvements | 1 to 39 | 47,084 | 15,911 | |||||||||
| Office furniture and equipment | 3 to 10 | 5,879 | 4,550 | |||||||||
| Construction-in-progress | 4,386 | 40,210 | ||||||||||
| Less accumulated depreciation | (24,798 | ) | (20,714 | ) | ||||||||
| Property and equipment, net | $ | 65,395 | $ | 66,915 | ||||||||
Other Assets
Other assets consist principally of strategic investments. In fiscal 2010, the balance in other assets decreased primarily as a result of impairments of three investments.
Other assets consisted of the following components as of September 30(in thousands):
| 2010 | 2009 | |||||||
| Investment in OctoPlus | $ | 2,624 | $ | 3,700 | ||||
| Investment in Nexeon MedSystems | 285 | 5,651 | ||||||
| Investment in ThermopeutiX | 1,185 | 1,185 | ||||||
| Investment in ViaCyte (formerly Novocell) | 559 | 559 | ||||||
| Other | 590 | 2,162 | ||||||
| Other assets, net | $ | 5,243 | $ | 13,257 | ||||
In January 2005, the Company made an initial equity investment of approximately $3.9 million in OctoPlus N.V. (OctoPlus), a company based in the Netherlands active in the development of pharmaceutical formulations incorporating novel biodegradable polymers. Subsequent investments brought the Company’s total investment to $6.0 million. In October 2006, OctoPlus common stock began trading on an international exchange following an initial public offering of its common stock. With a readily determinable fair market value, the Company now treats the investment in OctoPlus as anavailable-for-sale investment rather than a cost method investment.Available-for-sale investments are reported at fair value with unrealized gains and losses reported as a separate component of stockholders’ equity, except forother-than-temporary impairments, which are reported as a charge to current operations, recorded in the other income (loss) section of the consolidated statements of income, and result in a new cost basis for the investment. As of September 30, 2009,2010, the investment in OctoPlus represented an ownership interest of less than 10%. The Company recorded no realized gain or loss related to this investment in fiscal 2010 and 2009. The Company recognized an impairment loss on the investment totaling $4.3 million in fiscal 2008 based on a significant decline in the stock price of OctoPlus as a result of market conditions. The cost basis in the Company’s investment in OctoPlus is $1.7 million.
Beginning in May 2005, the Company has invested $1.2 million in ThermopeutiX, Inc. (“ThermopeutiX”)(ThermopeutiX), a California-based early stage company developing novel medical devices for the treatment of vascular and neurovascular diseases. In addition to the investment, SurModics has licensed its hydrophilic and hemocompatible
F-9
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
coating technologies to ThermopeutiX for use with its devices. The Company’s investment in ThermopeutiX, which is accounted for under the cost method, represents an ownership interest of less than 20%.
The Company has invested a total of $5.2 million in ViaCyte, Inc., (ViaCyte), formerly Novocell, Inc. (“Novocell”), a privately-held California-based biotechnology firm that is developing a unique treatment for diabetes using coated islet cells, the cells that produce insulin in the human body. In fiscal 2006, the Company determined its investment in NovocellViaCyte was impaired and that the impairment wasother-than-temporary. Accordingly, the Company recorded an impairment loss of $4.7 million. The balance of the investment, $559,000, which is accounted for under the cost method, represents less than a 5% ownership interest.
In July 2007, the Company made equity investments in Paragon Intellectual Properties, LLC (“Paragon”)(Paragon) and Apollo Therapeutics, LLC (“Apollo”)(Apollo), a Paragon subsidiary, totaling $3.5 million. SurModics made an additional equity investment in fiscal 2008 totaling $2.5 million, based upon successful completion of specified development milestones. In addition to the investments, the Company has licensed its Finaletm prohealing coating technology and provides development services on a time and materials basis to Apollo. In October 2008, Paragon announced that it had restructured, moving from a limited liability company with seven subsidiaries to a single C-corporation named Nexeon MedSystems, Inc. (“Nexeon”)(Nexeon). SurModics continued to account for the investments in Paragon and Apollo under the equity method in the first quarter of fiscal 2009, as both entities reportreported results to us on a one-quarter lag. Commencing with the second quarter of fiscal 2009, SurModics accounted for the investment in Nexeon under the cost method as the Company’s ownership level is less than 20%., and the Company did not exert significant influence over Nexeon’s operating or financial activities. The Company made an additional investment of $500,000 in Nexeon in fiscal 2009. In the fourth quarter of fiscal 2010 the Company held discussions with Nexeon management to understand the business status and outlook, valuations associated with potential new rounds of financing, operating metrics and other industry factors which impacted the Company’s assessment of the carrying value of this investment. As a result of its assessment, the Company recognized a $5.3 million impairment loss on this investment as it was determined the investment wasother-than-temporarily impaired.
In August 2009, the Company invested $2.0 million in a medical technology company and made a follow-on investment of $0.5 million in March 2010. The Company recognized an impairment loss on this investment totaling $2.4 million in fiscal 2010, based on market valuations and a pending financing round for this company. The Company’s investment in the medical technology company is accounted for under the cost method, as the Company’s ownership interest is less than 20%. This and the Company did not exert significant influence over the medical technology company’s operating or financial activities. Another entity in which the Company had a strategic investment issold the majority of its assets in fiscal 2010, resulting in an impairment loss of $0.2 million to the Company. These investments are included in the category titled “Other” in the table below.
F-9
| 2009 | 2008 | |||||||
| Investment in OctoPlus | $ | 3,700 | $ | 1,714 | ||||
| Investment in Nexeon MedSystems | 5,651 | 5,388 | ||||||
| Investment in ThermopeutiX | 1,185 | 1,185 | ||||||
| Investment in Novocell | 559 | 559 | ||||||
| Other | 2,162 | 455 | ||||||
| Other assets, net | $ | 13,257 | $ | 9,301 | ||||
above.
In the years ended September 30, 2010, 2009 2008 and 2007,2008, the Company recognized revenue of $1.5 million, $1.4 million and $4.1 million, and $909,000, respectively, from activity with companies in which it had a strategic investment.
Intangible Assets
Intangible assets consist principally of acquired patents and technology, customer relationships, licenses, and trademarks. The Company recorded amortization expense of $1.6 million, $2.1 million, $3.0 million, and $2.0$3.0 million for the years ended September 30, 2010, 2009 2008 and 2007,2008, respectively.
In fiscal 2009,2010, the Company acquiredrecognized an asset impairment charge of $0.7 million associated with certain patent rights. Management applied the accounting guidance associated with long-lived assets of PR Pharmaceuticals,and determined an impairment occurred for these assets as very limited business is expected in the near term based on current market conditions.
F-10
SurModics, Inc., which resulted in an increase and Subsidiaries
Notes to intangible assets. See Note 4 for further information regarding the acquisition.Consolidated Financial Statements — (Continued)
Intangible assets consisted of the following as of September 30(in thousands):
| Useful Life | 2009 | 2008 | Useful Life | 2010 | 2009 | |||||||||||||||||||
| (In years) | (In years) | |||||||||||||||||||||||
| Customer lists | 9-11 | $ | 8,657 | $ | 7,340 | 9-11 | $ | 8,657 | $ | 8,657 | ||||||||||||||
| Abbott license | 4 | — | 7,037 | |||||||||||||||||||||
| Core technology | 8-18 | 8,330 | 6,930 | 8-18 | 8,330 | 8,330 | ||||||||||||||||||
| Patents and other | 2-20 | 3,076 | 3,398 | 2-20 | 2,376 | 3,076 | ||||||||||||||||||
| Trademarks | �� | 600 | 580 | 600 | 600 | |||||||||||||||||||
| Less accumulated amortization | (3,205 | ) | (8,415 | ) | (4,706 | ) | (3,205 | ) | ||||||||||||||||
| Intangible assets, net | $ | 17,458 | $ | 16,870 | $ | 15,257 | $ | 17,458 | ||||||||||||||||
| 2010 | $ | 1,627 | ||||||
| 2011 | 1,604 | $ | 1,546 | |||||
| 2012 | 1,602 | 1,544 | ||||||
| 2013 | 1,602 | 1,544 | ||||||
| 2014 | 1,602 | 1,544 | ||||||
| 2015 | 1,533 | |||||||
Goodwill
The following table summarizes the changes in the carrying amount of goodwill(in thousands):
| Balance at October 1, 2008 | $ | 18,001 | ||
| Acquisitions | 3,016 | |||
| Adjustment | 53 | |||
| Balance at September 30, 2009 | 21,070 | |||
| Acquisitions | 750 | |||
| Goodwill Impairment | (13,810 | ) | ||
| Balance at September 30, 2010 | $ | 8,010 | ||
Goodwill represents the excess of the cost of the acquired entities over the fair value assigned to the assets purchased and liabilities assumed in connection with the Company’s acquisitions (see Note 4 for further information).acquisitions. The carrying amount of goodwill is evaluated annually, and between annual evaluations if events occur or circumstances change indicating that the carrying amount of goodwill may be impaired.
The Company has determined that the reporting units are the SurModics Pharmaceuticals, Inc. subsidiary, the In Vitro Diagnostics operations and the SurModics drug delivery and hydrophilic coatings operations. The reporting units with goodwill resulted from the acquisitions of SurModics Pharma and BioFX Laboratories, Inc. in fiscal 2007. Inherent in the determination of fair value of the reporting units are certain estimates and judgments, including the interpretation of current economic indicators and market valuations as well as the Company’s strategic plans with regard to its operations.
The Company performed its annual impairment test of goodwill in the fourth quarter of fiscal 2010 and recognized a goodwill impairment charge of $13.8 million, which represented a full impairment of the goodwill associated with the SurModics Pharma reporting unit. Prior to testing goodwill for impairment the Company tested its definite-lived assets, property, plant and equipment as well as intangible assets, under the provisions of the accounting guidance for impairment or disposal of long-lived assets, and determined that there were no impairments of these assets. The Company did not record any goodwill impairment charges during fiscal 2009 or 2008.
F-10F-11
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
In evaluating whether goodwill was impaired, the Company compared the fair value of the reporting units to which goodwill is assigned to their respective carrying values (Step 1 of the impairment test). In calculating fair value, the Company used the income approach as the primary indicator of fair value with the market approach used as a test of reasonableness. The income approach is a valuation technique under which the Company estimates future cash flows using the reporting units’ financial forecasts. Future estimated cash flows are discounted to their present value to calculate fair value. The market approach establishes fair value by comparing SurModics Pharmaceuticals,to other publicly traded guideline companies or by analysis of actual transactions of similar businesses or assets sold. The income approach is tailored to the circumstances of the Company’s business, and $3the market approach is completed as a secondary test to ensure that the results of the income approach are reasonable and in line with comparable companies in the industry. The summation of the reporting units’ fair values were compared and reconciled to the Company’s market capitalization as of the date of the impairment test.
In the situation where a reporting unit’s carrying amount exceeds its fair value, the amount of the impairment loss must be measured. The measurement of the impairment (Step 2 of the impairment test) is calculated by determining the implied fair value of a reporting unit’s goodwill. In calculating the implied fair value of goodwill, the fair value of the reporting unit is allocated to all other assets and liabilities of that unit based on their fair values. The excess of the fair value of a reporting unit over the amount assigned to its other assets and liabilities is the implied fair value of goodwill. The goodwill impairment is measured as the excess of the carrying amount of goodwill over its implied fair value.
In determining the fair value of the SurModics Pharma reporting unit under the income approach, the SurModics Pharma expected cash flows are affected by various assumptions. Fair value on a discounted cash flow basis used forecasts over a ten year period with an estimation of residual growth rates thereafter. The Company uses its business plans and projections as the basis for expected future cash flows. The most significant assumptions incorporated in these forecasts for the most recent goodwill impairment tests included annual revenue changes based on current customer programs and expected progression of programs into different phases of development. A discount rate of 15 percent was used in the 2010 analysis to reflect the relevant risks of the higher growth assumed for this reporting unit. Given the significant difference between the reporting unit’s fair value and carrying value any change in the discount rate would not have changed the evaluation of impairment.
In estimating the fair value of the Company under the market approach, management considered the relative merits of commonly applied market capitalization multiples based on the availability of data. Based on the analysis, the Company utilized the guideline public company method to support the valuation of the reporting units.
Based on the goodwill analysis performed as of August 31, 2010, goodwill in the SurModics Pharma reporting unit failed Step 1 of the impairment test and Step 2 of the impairment test indicated that goodwill was fully impaired. The indicated excess in fair value over carrying value of the Company’s In Vitro Diagnostics reporting unit in Step 1 of the impairment test at August 31 2010 was approximately 82% and as such the $8.0 million of additional purchase price was recorded as an increasegoodwill related to goodwill.this reporting unit is not impaired. The SurModics drug delivery and hydrophilic coatings
F-12
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
operations does not have any goodwill and was included in the analysis to assist in reconciling the fair value of all reporting units to the Company’s market capitalization at August 31, 2010.
Impairment of Long-Lived Assets
The Company periodically evaluates whether events and circumstances have occurred that may affect the estimated useful life or the recoverability of the remaining balance of long-lived assets, such as property and equipment, intangible assets and investments. If such events or circumstances were to indicate that the carrying amount of these assets would not be recoverable, the Company would estimate the future cash flows expected to result from the use of the assets and their eventual disposition. If the sum of the expected future cash flows (undiscounted and without interest charges) or other measure of fair value were less than the carrying amount of the assets, the Company would recognize an impairment loss reducing the carrying value to fair market value. See the Property and Equipment, Other Assets and Intangible Assets sections in Note 2 for further information on impairments that were recognized in fiscal 2010.
Revenue Recognition
The Company’s revenue is derived from three primary sources: (1) royalties and license fees from licensing patentedits proprietary drug delivery and surface modification technologies andin vitrodiagnostic formats to customers; (2) the sale of polymers and reagent chemicals, stabilization products, antigens, substrates and microarray slides to the diagnostics and biomedical research industries; and (3) research and development fees generated on customer projects.
Taxes collected from customers and remitted to governmental authorities are excluded from revenue and amounted to $187,000, $309,000$0.1 million, $0.2 million and $170,000$0.3 million for the years ended September 30, 2010, 2009 2008 and 2007,2008, respectively.
Royalties & License Fees.and licenses fees. The Company licenses technology to third parties and collects royalties. Royalty revenue is generated when a customer sells products incorporating the Company’s licensed technologies. Royalty revenue is recognized as licensees report it to the Company, and payment is typically submitted concurrently with the report. Generally, licenseThis revenue recognition model is similar to usage fee accounting. Minimum royalty fees are recognized as revenue whenin the Company receives payment and the contract priceperiod earned, provided that collectability is fixed or determinable.reasonably assured. For stand-alone license agreements, up-front license fees are recognized over the termeconomic life of the related licensing agreement. Minimum royalty fees are recognized in the period earned.technology.
Milestone payments.Revenue related to a performance milestone is recognized based upon the achievement of the milestone, as defined in the respective agreements and provided the following conditions have been met:
| • | The milestone payment is | |
| • | The milestone | |
| • | Accomplishment of the milestone | |
| • | The amount of the milestone payment is commensurate with the related effort and | |
| • | The milestone payment is reasonable in comparison to all of the deliverables and payment terms in the arrangement; and | |
| • | A reasonable amount of time |
F-11F-13
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
If these conditions have not been met, the milestone payment is deferred and recognized over the termeconomic life of the agreement.technology.
Product Sales.sales. Product sales to third parties are recognized at the time of shipment, provided that an order has been received, the price is fixed or determinable, collectability of the resulting receivable is reasonably assured and returns can be reasonably estimated. The Company’s sales terms provide no right of return outside of the standard warranty policy. Payment terms are generally set at30-45 days.
Research and Development.development. The Company performs third party research and development activities, which are typically provided on a time and materials basis. Generally, revenue for research and development is recorded as performance progresses under the applicable contract.agreement.
Arrangements with multiple deliverables. ArrangementsPrior to October 1, 2009, arrangements such as license and development agreements arewere analyzed to determine whether the deliverables, which often include a license and performance obligations such as research and development, cancould be separated, or whether they must be accounted for as a single unit of accounting in accordance with accounting guidance. The Company recognizes up-front license payments under these agreements over the economic life of the technology licensed. If the fair value of the undelivered performance obligations cancould be determined, such obligations would then be accounted for separately. If the license iswas considered to either (i) not have stand-alone value or (ii) have stand-alone value but the fair value of any of the undelivered performance obligations cannotcould not be determined, the arrangement would then be accounted for as a single unit of accounting, and the license payments and payments for performance obligations would be recognized as revenue over the estimated period of when the performance obligations are performed, or the economic life of the technology licensed to the customer. When the Company determinesdetermined that an arrangement should be accounted for as a single unit of accounting, it recognizesrecognized the related revenue based on a time-based accounting model. Revenue
The Company had one significant multiple element arrangement prior to October 1, 2009 that was accounted for as a single unit of accounting resulting in deferral and recognition of all related payments received for license and research and development activities using a time-based model. This arrangement was terminated during the first quarter of fiscal 2009 as described in Note 1 above.
In October 2009, the accounting standards for multiple deliverable revenue arrangements were amended to:
(i) provide updated guidance on whether multiple deliverables exist, how the deliverables in an arrangement should be separated, and how the consideration should be allocated;
(ii) require an entity to allocate revenue in an arrangement using estimated selling prices (ESP) of deliverables if a vendor does not have vendor-specific objective evidence of selling price (VSOE) or third-party evidence of selling price (TPE); and
(iii) eliminate the use of the residual method and require an entity to allocate revenue using the relative selling price method.
The Company elected to early adopt this accounting guidance at the beginning of its first quarter of fiscal 2010, on a prospective basis, for applicable transactions originating or materially modified after October 1, 2009. In connection with the adoption of the amended accounting standard the Company also changed its policy prospectively for multiple element arrangements, whereby the Company accounts for revenue using a multiple attribution model in which consideration allocated to research and development activities is recognized as performed, and milestone payments are recognized when the milestone events are achieved, when such activities and milestones are deemed substantive. Accordingly, in situations where a unit of accounting includes both a license and research and development activities, and when a license does not have stand-alone value, the Company applies a multiple attribution model in which consideration allocated to the license is recognized ratably, consideration allocated to research and development activities is recognized as performed and milestone payments are recognized when the milestone events are achieved, when such activities and milestones are deemed substantive.
F-14
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
The Company enters into license and development arrangements that may consist of multiple deliverables which could include a license(s) to SurModics technology, research and development activities, manufacturing services, and product sales based on the needs of its customers. For example, a customer may enter into an arrangement to obtain a license to SurModics’ intellectual property which may also include research and development activities, and supply of products manufactured by SurModics. For these services provided, SurModics could receive upfront license fees upon signing of an agreement and granting the license, fees for research and development activities as such activities are performed, milestone payments contingent upon advancement of the product through development and clinical stages to successful commercialization, fees for manufacturing services and supply of product, and royalty payments based on customer sales of product incorporating SurModics’ technology. The Company’s license and development arrangements generally do not have refund provisions if the customer cancels or terminates the agreement. Typically all payments made are non-refundable.
The Company evaluates each deliverable in a multiple element arrangement for separability. The Company is then required to allocate revenue to each separate deliverable using a hierarchy of VSOE, TPE, or ESP. In certain instances, the Company is not able to establish VSOE for all deliverables in an arrangement with multiple elements which may be a result of the Company infrequently selling each element separately. When VSOE cannot be established, the Company establishes a selling price of each element based on TPE. TPE is determined based on competitor prices for similar deliverables when sold separately.
When the Company is unable to establish a selling price using VSOE or TPE, the Company uses ESP in its allocation of arrangement consideration. The objective of ESP is to determine the price at which the Company would transact a sale if the product or service were sold on a stand-alone basis. ESP is generally used for highly customized offerings.
The Company determines ESP for undelivered elements by considering multiple factors including, but not limited to, market conditions, competitive landscape and past pricing arrangements with similar characteristics.
Net sales as reported and pro forma net sales that would have been reported for the year ended September 30, 2010, if the transactions entered into or materially modified after September 30, 2009 were subject to the Company’s accounting policies under the previous accounting guidance, are shown in the following table (in thousands):
| Pro Forma Basis as if the | ||||||||
| Previous Accounting | ||||||||
| As Reported | Guidance Were in Effect | |||||||
| Total multiple element arrangement revenue | $ | 4,232 | $ | 378 | ||||
The impact to revenue for the fiscal year ended September 30, 2010 associated with arrangements with multiple deliverables totaled $45.3 million, $4.2 millionadoption of the new accounting guidance was primarily related to research and $0.3 milliondevelopment activities. The Company’s accounting policies under the previous accounting guidance would have resulted in fiscal 2009, 2008 and 2007, respectively. The fiscal 2009 revenue associated with multiple deliverable arrangements is reflected in royalties and license fees revenue ($37.6 million) and inpartial recognition of the research and development revenue ($7.7 million) in the current periods with the remainder deferred and recognized over the economic life of the technology. Under the new accounting guidance, the Company is recognizing research and development revenue as the activities are performed. The Company notes that this new accounting guidance will result in current revenue recognition of research and development activities in the period the activities are performed with the revenue generated changing from period to period based on the stage of project development. The amount of revenue that is recognized could be material in any reporting period.
In April 2010, the FASB issued updated authoritative accounting guidance which provides a consistent framework for applying the milestone method of revenue recognition in arrangements that include research or development deliverables. The amendments are effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010 with early adoption permitted. The Company is evaluating the guidance and does not expect the adoption to have a material impact on the Company’s consolidated statements of income.financial statements.
F-15
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Merck Agreement. On June 27, 2007 the Company announced a license and research collaboration agreement with Merck & Co., Inc. (“Merck”)(Merck). The agreement called for SurModics and Merck to pursue the joint development and commercialization of SurModics’ I-vation sustained drug delivery system with TA (triamcinolone acetonide), and other products combining certain of Merck’s proprietary drug compounds and the I-vation system for the treatment of serious retinal diseases. Under the terms of the agreement, Merck led and funded development and commercialization activities. SurModics received an up-front license fee of $20 million in fiscal 2007 and additional license fees totaling $11 million in fiscal 2008. In addition, the Company was paid for its activities in researching and developing the combination products. Research and development fees totaling $5.8 million were billed in fiscal 2008. The Company recognizedout-of-pocket reimbursements, totaling $1.6 million in fiscal 2008, as revenue in the period since the related costs were incurred when commensurate value was transferred to Merck in exchange for the reimbursement received.
In September 2008, following a strategic review of Merck’s business and product development portfolio, Merck gave notice to SurModics of its intent to terminate the collaborative research and license agreement as well as the supply agreement entered into in June 2007. The termination was effective December 2008. The Company recognized all remaining deferred revenue related to the Merck agreement, totaling $34.8 million, as revenue in fiscal 2009. The Company also recognized a $9 million milestone payment from Merck associated with the termination of the triamcinolone acetonide development program in fiscal 2009. As of September 30, 2009, there were no deferred
The Company recognized revenue amounts from Merck, compared with $34.8 million ofthe up-front license fee, additional license fees and research and development fees in deferred revenue asover the economic life of September 30, 2008.
F-12
the technology licensed to Merck, which was 16 years, prior to termination of the contract.
Deferred Revenue
Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue in the accompanying consolidated balance sheets, with deferred revenue to be recognized beyond one year being classified as non-current deferred revenue. As of September 30, 20092010 and 2008,2009, the Company had deferred revenue of $1.5$4.2 million and $37.6$1.5 million, respectively.
Costs related to products and services delivered are recognized in the period revenue is recognized except for services related to the Merck agreement, which were recognized as incurred. Customer advances are accounted for as a liability until all criteria for revenue recognition have been met.
Research and Development Costs
Research and development costs are expensed as incurred. Some research and development costs are related to third party contracts, and the related revenue is recognized as described in “Revenue Recognition” above. The research and development costs are presented in the consolidated statements of incomeoperations in two categories; those associated with customer relatedcustomer-related projects and those associated with other research and development costs.
Costs associated with customer relatedcustomer-related research and development include specific project direct labor costs and material expenses as well as an allocation of overhead costs based on direct labor dollars.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Ultimate results could differ from those estimates.
F-13F-16
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
No other new accounting pronouncement issued or effective has had, or is expected to have, a material impact on the Company’s consolidated financial statements.
| 3. | Fair Value Measurements |
Fair Value Hierarchy
Level 1 — Quoted (unadjusted) prices in active markets for identical assets or liabilities.
F-14
The Company’s Level 1 asset consists of its investment in OctoPlus (see Note 2 for further information). The fair market value of this investment is based on the quoted price of OctoPlus shares as traded on the Amsterdam Stock Exchange.
Level 2 — Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the asset or liability.
The Company’s Level 2 assets consist of money market funds, U.S. Treasury securities, corporate bonds, municipal bonds, U.S. agency securities, agency and municipal securities and certain asset-backed securities and mortgage-backed securities. Fair market values for these assets are based on quoted vendor prices and broker pricing where all significant inputs are observable.
Level 3 — Unobservable inputs to the valuation methodology that are supported by little or no market activity and that are significant to the measurement of the fair value of the assets or liabilities. Level 3 assets and liabilities include those whose fair value measurements are determined using pricing models, discounted cash flow methodologies or similar valuation techniques, as well as significant management judgment or estimation.
The Company’s Level 3 assets include a U.S. government agency security and certain asset-backed and mortgage-backed securities. The fair market values of these investments were determined by broker pricing where not all significant inputs were observable.
In valuing assets and liabilities, the Company is required to maximize the use of quoted market prices and minimize the use of unobservable inputs.
We did not significantly change our valuation techniques from prior periods.
F-17
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Assets and Liabilities Measured at Fair Value on a Recurring Basis
In instances where the inputs used to measure fair value fall into different levels of the fair value hierarchy, the fair value measurement has been determined based on the lowest level input that is significant to the fair value measurement in its entirety. The Company’s assessment of the significance of a particular item to the fair value measurement in its entirety requires judgment, including the consideration of inputs specific to the asset or liability. The following table presents information about the Company’s financial assets and liabilities measured at fair value on a recurring basis as of September 30, 20092010(in thousands):
| Quoted Prices | Quoted Prices | |||||||||||||||||||||||||||||||
| in Active | Significant | in Active | Significant | |||||||||||||||||||||||||||||
| Markets for | Other | Significant | Total Fair | Markets for | Other | Significant | Total Fair | |||||||||||||||||||||||||
| Identical | Observable | Unobservable | Value as of | Identical | Observable | Unobservable | Value as of | |||||||||||||||||||||||||
| Instruments | Inputs | Inputs | September 30, | Instruments | Inputs | Inputs | September 30, | |||||||||||||||||||||||||
| (Level 1) | (Level 2) | (Level 3) | 2009 | (Level 1) | (Level 2) | (Level 3) | 2010 | |||||||||||||||||||||||||
| Assets: | ||||||||||||||||||||||||||||||||
| Cash equivalents | $ | — | $ | 9,108 | $ | — | $ | 9,108 | $ | — | $ | 10,128 | $ | — | $ | 10,128 | ||||||||||||||||
| Short-term investments | — | 6,911 | — | 6,911 | ||||||||||||||||||||||||||||
| Long-term investments | — | 21,867 | 1,203 | 23,070 | ||||||||||||||||||||||||||||
| Available for sale debt securities | ||||||||||||||||||||||||||||||||
| US government obligations | — | 25,626 | 704 | 26,330 | ||||||||||||||||||||||||||||
| Mortgage backed securities | — | 4,757 | 69 | 4,826 | ||||||||||||||||||||||||||||
| Municipal bonds | — | 3,150 | — | 3,150 | ||||||||||||||||||||||||||||
| Asset backed securities | — | 1,113 | — | 1,113 | ||||||||||||||||||||||||||||
| Corporate bonds | — | 5,852 | — | 5,852 | ||||||||||||||||||||||||||||
| Other assets | 3,700 | — | — | 3,700 | 2,624 | — | — | 2,624 | ||||||||||||||||||||||||
| Total assets measured at fair value | $ | 3,700 | $ | 37,886 | $ | 1,203 | $ | 42,789 | $ | 2,624 | $ | 50,626 | $ | 773 | $ | 54,023 | ||||||||||||||||
Short-term and long-term investments disclosed in the consolidated balance sheets includeheld-to-maturity investments totaling $6.3$4.1 million as of September 30, 2009 and 2008.2010.Held-to-maturity investments are carried at an amortized cost.
The following table presents information about the Company’s financial assets and liabilities measured at fair value on a recurring basis as of September 30, 2009(in thousands):
| Quoted Prices | ||||||||||||||||
| in Active | Significant | |||||||||||||||
| Markets for | Other | Significant | Total Fair | |||||||||||||
| Identical | Observable | Unobservable | Value as of | |||||||||||||
| Instruments | Inputs | Inputs | September 30, | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | 2009 | |||||||||||||
| Assets: | ||||||||||||||||
| Cash equivalents | $ | — | $ | 9,108 | $ | — | $ | 9,108 | ||||||||
| Available for sale debt securities | ||||||||||||||||
| US government obligations | — | 9,960 | 1,130 | 11,090 | ||||||||||||
| Mortgage backed securities | — | 7,935 | 73 | 8,008 | ||||||||||||
| Municipal bonds | — | 7,443 | — | 7,443 | ||||||||||||
| Asset backed securities | — | 2,256 | — | 2,256 | ||||||||||||
| Corporate bonds | — | 1,184 | — | 1,184 | ||||||||||||
| Other assets | 3,700 | — | — | 3,700 | ||||||||||||
| Total assets measured at fair value | $ | 3,700 | $ | 37,886 | $ | 1,203 | $ | 42,789 | ||||||||
F-15F-18
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Changes in Level 3 Instruments Measured at Fair Value on a Recurring Basis
The following table istables (in thousands) provide a reconciliation of fiscal 2010 and 2009 financial assets and liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3)(in thousands):. Transfers of instruments into and out of Level 3 are based on beginning of year values.
| 2009 | ||||
| Balance, beginning of year | $ | 264 | ||
| Total realized and unrealized gains included in other comprehensive income | 25 | |||
| Purchases, sales and maturities, net | 339 | |||
| Transfer in (out) of Level 3 | 575 | |||
| Balance, end of year | $ | 1,203 | ||
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) | ||||||||||||
| For the Year Ended September 30, 2010 | ||||||||||||
| Available-for-Sale Debt Securities | ||||||||||||
| U.S. Government | Mortgage | |||||||||||
| Obligations | Backed | Total | ||||||||||
| Balance, September 30, 2009 | $ | 1,130 | $ | 73 | $ | 1,203 | ||||||
| Transfers into Level 3 | — | 148 | 148 | |||||||||
| Transfers out of Level 3 | (36 | ) | (145 | ) | (181 | ) | ||||||
| Total realized and unrealized gains (losses): | ||||||||||||
| Included in other comprehensive (loss) income | (33 | ) | 3 | (30 | ) | |||||||
| Purchases, issuances, sales and settlements, net | (357 | ) | (10 | ) | (367 | ) | ||||||
| Balance, September 30, 2010 | $ | 704 | $ | 69 | $ | 773 | ||||||
| Fair Value Measurements Using Significant | ||||||||||||||||
| Unobservable Inputs (Level 3) | ||||||||||||||||
| For the Year Ended September 30, 2009 | ||||||||||||||||
| Available-for-Sale Debt Securities | ||||||||||||||||
| U.S. Government | Mortgage | |||||||||||||||
| Obligations | Corporate | Backed | Total | |||||||||||||
| Balance, September 30, 2008 | $ | 74 | $ | 190 | $ | — | $ | 264 | ||||||||
| Transfers into Level 3 | 1,273 | — | 79 | 1,352 | ||||||||||||
| Transfers out of Level 3 | (581 | ) | (199 | ) | — | (780 | ) | |||||||||
| Total realized and unrealized gains (losses): | ||||||||||||||||
| Included in other comprehensive income (loss) | 15 | 9 | 1 | 25 | ||||||||||||
| Purchases, issuances, sales and settlements, net | 349 | — | (7 | ) | 342 | |||||||||||
| Balance, September 30, 2009 | $ | 1,130 | $ | — | $ | 73 | $ | 1,203 | ||||||||
As of September 30, 2009,2010, marketable securities measured at fair value using Level 3 inputs waswere comprised of $36,000$0.7 million of aan Other U.S. government agency security $73,000and $0.1 million of a mortgage-backed security and $1,094,000 of asset-backed securities within the Company’savailable-for-sale investment portfolio. These securities were measured using observable market data and Level 3 inputs as a result of the lack of market activity and liquidity. The fair value of these securities was based on the Company’s assessment of the underlying collateral and the creditworthiness of the issuer of the securities.
Assets and Liabilities Measured at Fair Value on a Non-Recurring Basis
The Company’s investments in non-marketable securities of private companies are accounted for using the cost method as the Company does not exert significant influence over the investees’ operating or equity method.financial activities. These investments, as well asheld-to-maturity securities, are measured at fair value on a non-recurring basis when they are deemed to beother-than-temporarily impaired. In determining whether a decline in value of non-marketable equity investments in private companies has occurred and isother-than-temporary, an assessment is made by considering available evidence, including the general market conditions in the investee’s industry, the investee’s product development status and subsequent rounds of financing and the related valuationand/or the Company’s participation in such financings. The Company also assesses the investee’s ability to meet business milestones and the financial condition and near-term prospects of the individual investee, including the rate at
F-19
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
which the investee is using its cash and the investee’s potential need for possible additional funding at a possiblypotentially lower valuation. The valuation methodology for determining the decline in value of non-marketable equity securities is based on inputs that require management judgment and are Level 3 inputs. The Company wrote down three investments totaling $7.9 million in the year ended September 30, 2010, as the investments were deemed to beother-than-temporarily impaired. A pending round of financing at a substantially lower valuation at one of the private companies resulted in impairment loss of $2.4 million. Another company sold off assets in light of current market conditions and this action resulted in impairment loss of $0.2 million. In addition, an impairment loss of $5.3 million was recognized related to a third company, which continues to face operational and financing difficulties and potential rounds of financing at lower valuations. Management utilized Level 3 inputs which included information about pending financings as well as market input to determine the fair value of these investments.
The Company also incurred long-lived asset impairment charges totaling $4.9 million in fiscal 2010. Fair value measurements used in the impairment reviews of property and equipment and intangible assets are Level 3 measurements that require management judgment. The Company recorded a $1.9 million asset impairment charge associated with writing down one of its facilities in Alabama to fair value based on a decision to sell the facility, which decision was reversed later in fiscal 2010. The $2.1 million carrying value of this facility is based on a real estate market appraisal obtained during the Company’s negotiations.
The Company also recorded a $1.3 million asset impairment charge associated with certain long-lived assets where very limited business is expected in the near term based on current market conditions. Furthermore, a $1.3 million asset impairment charge associated with certain fixed asset costs located in Minnesota and a $0.4 million asset impairment charge associated with prototypes and other equipment related to a development project for which very limited business is expected in the near term in light of current market conditions were also recognized. The assets associated with these charges had limited remaining value and as such were written down to zero value.
See Note 2 for additional information related to these impairments
| 4. | Acquisitions |
PR Pharmaceuticals, Inc. On November 4, 2008, the Company’s SurModics Pharmaceuticals, Inc. (formerly known as Brookwood Pharmaceuticals, Inc.) subsidiary entered into an asset purchase agreement with PR Pharmaceuticals, Inc. (“PR Pharma”)(PR Pharma), whereby it acquired certain contracts and assets of PR Pharma for $5.6 million consisting of $2.9 million in cash on the closing date, additional consideration of $2.4 million upon successful achievement of specified milestones and $0.3 million in transaction costs. PR Pharma is eligible to receive up to an additional $3.6 million in cash upon the successful achievement of milestones for contract signing and invoicing, successful patent issuances and product development. Management believes this acquisition strengthens the Company’s portfolio of drug delivery technologies for the pharmaceutical and biotechnology industries. The purchase price was allocated as follows as of November 4, 2008(in thousands):
| Core technology | $ | 1,400 | ||
| Customer relationships | 900 | |||
| In-process research and development | 3,200 | |||
| Trade names | 20 | |||
| Non-compete agreements | 50 | |||
| Total purchase price | $ | 5,570 | ||
| Core technology | $ | 1,400 | ||
| Customer relationships | 900 | |||
| In-process research and development | 3,200 | |||
| Trade names | 20 | |||
| Non-compete agreements | 50 | |||
| Total purchase price | $ | 5,570 | ||
F-16
The acquired developed technology is being amortized on a straight-line basis over 18 years, customer relationships are being amortized over 9 years, and non-compete agreements are being amortized over 2 years. The trade names havehad a life of less than one year and were fully amortized in fiscal 2009. As part of the acquisition, the
F-20
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Company recognized fair value associated with in-process research and development (IPR&D) of $3.2 million. The IPR&D was expensed on the date of acquisition and relates to polymer-based drug delivery systems. The value assigned to IPR&D is related to projects for which the related products have not achieved commercial feasibility and have no future alternative use. The amount of purchase price allocated to IPR&D was based on estimating the future cash flows of each project and discounting the net cash flows back to their present values. The discount rate used was determined at the time of acquisition in accordance with accepted valuation methods. These methodologies include consideration of the risk of the project not achieving commercial feasibility. The research efforts ranged from 5% to 50% complete at the date of acquisition. The Company used the Relief from Royalty valuation method to assess the fair value of the projects with a risk-adjusted discount rate of 25%. The Company determined the method was appropriate based on the nature of the projects and future cash flow streams. The research and development work performed is billed to customers, in most cases, using standard commercial billing rates which include a reasonable markup. Accordingly, the Company has no fixed cost obligations to carry projects forward. There have been no significant changes to the development plans for the acquired incomplete projects. Significant net cash inflows would commence with the commercial launch of customer products that are covered by the intellectual property rights and related agreements acquired from PR Pharma.
F-17
| Pro forma revenue | $ | 89,708 | ||
| Pro forma income from operations | $ | 28,034 | ||
| Pro forma net income | $ | 17,735 | ||
| Pro forma basic earnings per share | $ | 0.98 | ||
| Pro forma diluted earnings per shares | $ | 0.98 |
| 5. | Revolving Credit Facility |
In February 2009, the Company entered into a two-year $25.0 million unsecured revolving credit facility. Borrowings under the credit facility, if any, will bear interest at a benchmark rate plus an applicable margin based upon the Company’s funded debt to EBITDA ratio. In connection with the credit facility, the Company is required to maintain certain financial and nonfinancial covenants. As of September 30, 2009,2010, the Company had no debt outstanding under this credit facility and was not in compliance with allcertain covenants. The Company is working with the bank to obtain waivers and expects to complete these activities by the end of the second quarter of fiscal 2011. The Company believes that noncompliance will not cause liquidity issues given the Company’s investment holdings and cash flow generated by operations.
| 6. | Stockholders’ Equity |
The Company has stock-based compensation plans under which it grants stock options, and restricted stock awards and performance share awards. Accounting guidance requires all share-based payments to be recognized as an operating expense, based on their fair values, over the requisite service period. The Company’s stock-based compensation expenses for the years ended September 30 were allocated as followsto the following expense categories(in thousands):
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Product | $ | 87 | $ | 161 | $ | 96 | $ | 139 | $ | 87 | $ | 161 | ||||||||||||
| Research and development | 3,621 | 3,793 | 5,188 | |||||||||||||||||||||
| Customer research and development | 772 | 815 | 1,794 | |||||||||||||||||||||
| Other research and development | 2,399 | 2,806 | 1,999 | |||||||||||||||||||||
| Selling, general and administrative | 3,145 | 5,698 | 5,028 | 2,565 | 3,145 | 5,698 | ||||||||||||||||||
| Total | $ | 6,853 | $ | 9,652 | $ | 10,312 | $ | 5,875 | $ | 6,853 | $ | 9,652 | ||||||||||||
As of September 30, 2009,2010, approximately $8.7$4.8 million of total unrecognized compensation costs related to non-vested awards is expected to be recognized over a weighted average period of approximately 2.62.4 years. The unrecognized compensation costs include $2.8above exclude $1.2 million associated with performance share awards that are currently not anticipated to be fully expensed because the performance conditions are not expected to be met.
F-21
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
Stock Option Plans
The Company uses the Black-Scholes option pricing model to determine the weighted average grant date fair value of stock options granted. The weighted average per share fair value of stock options granted during fiscal 2010, 2009, and 2008 was $6.78, $8.95, and 2007 was $8.95, $14.85, and $17.42, respectively. The assumptions used as inputs in the model for the years ended September 30 were as follows:
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Risk-free interest rates | 2.30 | % | 2.80 | % | 4.50 | % | 1.95 | % | 2.30 | % | 2.80 | % | ||||||||||||
| Expected life | 4.8 years | 4.6 years | 5.4 years | 4.8 years | 4.8 years | 4.6 years | ||||||||||||||||||
| Expected volatility | 40 | % | 37 | % | 45 | % | 41 | % | 40 | % | 37 | % | ||||||||||||
| Dividend yield | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | 0 | % | ||||||||||||
The risk-free interest rate assumption was based on the U.S. Treasury’s rates for U.S. Treasury zero-coupon bonds with maturities similar to those of the expected term of the award. The expected life of options granted is
F-18
determined based on the Company’s experience. Expected volatility is based on the Company’s stock price movement.movement over a period approximating the expected term. Based on management’s judgment, dividend rates are expected to be zero for the expected life of the options. The Company also estimates forfeitures of options granted, which are based on historical experience.
The Company’s Incentive Stock Options (ISO) are granted at a price of at least 100% of the fair market value of the Common Stockcommon stock of the Company on the date of the grant or 110% with respect to optionees who own more than 10% of the total combined voting power of all classes of stock. ISOs generally expire in seven years or upon termination of employment and generally are exercisable at a rate of 20% per year commencing one year after the date of grant. NonqualifiedNon-qualified stock options are granted at fair market value on the date of grant. NonqualifiedNon-qualified stock options expire in 7 to 10 years or upon termination of employment or service as a Board member. NonqualifiedNon-qualified stock options granted prior to May 2008 generally become exercisable with respect to 20% of the shares on each of the first five anniversaries following the grant date, such that the entire option is fully vested five years after date of grant, and nonqualified stock options granted subsequent to May 2008 generally become exercisable with respect to 25% on each of the first four anniversaries following the grant date suchdate. Shareholders approved the 2009 Equity Incentive Plan (2009 Plan) at the February 8, 2010 Annual Meeting of Shareholders. The 2009 Plan has 1,500,000 shares authorized, plus the number of shares that the entire option is fully vested four years after the grant date. The Company has authorized 2,400,000 shares for granthave not yet been awarded under the 2003 Equity Incentive Plan, or were awarded and subsequently returned to the pool of which 51,000 remain available for future awards. In September 2009, the Company granted 29,066 performance share awards to officersshares under the 2003 Equity Incentive Plan and 229,552 stock optionspursuant to officers under the 2009 Equity Incentive Plan. The 2009 Equity Incentive Plan is subject to shareholder approval at the Februaryits terms. At September 30, 2010, Annual Meeting of Shareholders.there were 1,819,000 shares available for future awards. As of September 30, 2009,2010, the aggregate intrinsic value of the option shares outstanding and option shares exercisable was $0.7 millionnot meaningful, as the Company’s stock price of $11.92 per share on September 30, 2010 was below the value of option shares outstanding and $0.6 million, respectively.exercisable. At September 30, 2009,2010, the average remaining contractual life of options outstanding and options exercisable was 4.3 and 3.23.3 years, respectively. There was no intrinsic value associated with options exercised during fiscal 2010 as the Company’s stock price of $11.92 per share on September 30, 2010 was below the value of options exercised. The intrinsic value of options exercised during fiscal 2009 and 2008 and 2007 was $235,000, $2.9$0.2 million and $4.4$2.9 million, respectively.
| Weighted | ||||||||
| Number of | Average | |||||||
| Shares | Exercise Price | |||||||
| Outstanding at September 30, 2006 | 1,510,780 | $ | 29.69 | |||||
| Granted | 166,400 | 37.85 | ||||||
| Exercised | (253,060 | ) | 25.82 | |||||
| Forfeited | (22,700 | ) | 33.71 | |||||
| Outstanding at September 30, 2007 | 1,401,420 | 31.29 | ||||||
| Granted | 392,917 | 41.86 | ||||||
| Exercised | (163,297 | ) | 27.45 | |||||
| Forfeited | (108,250 | ) | 33.59 | |||||
| Outstanding at September 30, 2008 | 1,522,790 | 34.26 | ||||||
| Granted | 268,700 | 24.06 | ||||||
| Exercised | (17,600 | ) | 8.82 | |||||
| Forfeited | (104,320 | ) | 35.33 | |||||
| Outstanding at September 30, 2009 | 1,669,570 | $ | 32.82 | |||||
| Exercisable at September 30, 2009 | 902,589 | $ | 32.07 | |||||
F-22
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
| Weighted | ||||||||
| Number of | Average | |||||||
| Shares | Exercise Price | |||||||
| Outstanding at September 30, 2007 | 1,401,420 | $ | 31.29 | |||||
| Granted | 392,917 | 41.86 | ||||||
| Exercised | (163,297 | ) | 27.45 | |||||
| Forfeited | (108,250 | ) | 33.59 | |||||
| Outstanding at September 30, 2008 | 1,522,790 | $ | 34.26 | |||||
| Granted | 268,700 | 24.06 | ||||||
| Exercised | (17,600 | ) | 8.82 | |||||
| Forfeited | (104,320 | ) | 35.33 | |||||
| Outstanding at September 30, 2009 | 1,669,570 | $ | 32.82 | |||||
| Granted | 388,635 | 22.88 | ||||||
| Exercised | (20,350 | ) | 20.74 | |||||
| Forfeited | (545,534 | ) | 30.58 | |||||
| Outstanding at September 30, 2010 | 1,492,321 | $ | 31.22 | |||||
| Exercisable at September 30, 2010 | 843,112 | $ | 33.10 | |||||
Restricted Stock Awards
The Company has entered into restricted stock agreements with certain key employees, covering the issuance of Common Stockcommon stock (Restricted Stock). Under accounting guidance these shares are considered to be non-vested shares. The Restricted Stock will be released to the key employees if they are employed by the Company at the end of the vesting period. Compensation has been recognized for the estimated fair value of the 100,89541,072 common shares awarded and is being charged to income over the vesting term. The stock-based compensation table includes the Restricted Stock expenses recognized related to these awards, which totaled $1.0 million, $1.8 million and $2.2 million during fiscal 2010, 2009 and 2008, respectively.
| Weighted | ||||||||
| Number of | Average | |||||||
| Shares | Grant Price | |||||||
| Balance at September 30, 2007 | 206,191 | $ | 35.89 | |||||
| Granted | 12,383 | 42.18 | ||||||
| Vested | (40,336 | ) | 38.76 | |||||
| Forfeited | (21,109 | ) | 32.83 | |||||
| Balance at September 30, 2008 | 157,129 | $ | 36.06 | |||||
| Granted | 7,700 | 23.93 | ||||||
| Vested | (59,047 | ) | 34.44 | |||||
| Forfeited | (4,887 | ) | 41.91 | |||||
| Balance at September 30, 2009 | 100,895 | $ | 35.80 | |||||
| Granted | 30,440 | 18.49 | ||||||
| Vested | (83,195 | ) | 36.32 | |||||
| Forfeited | (7,068 | ) | 33.39 | |||||
| Balance at September 30, 2010 | 41,072 | $ | 22.33 | |||||
F-19F-23
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
| Weighted | ||||||||
| Number of | Average | |||||||
| Shares | Grant Price | |||||||
| Balance at September 30, 2006 | 153,000 | $ | 32.14 | |||||
| Granted | 83,027 | 42.07 | ||||||
| Vested | (24,836 | ) | 37.87 | |||||
| Forfeited | (5,000 | ) | 34.56 | |||||
| Balance at September 30, 2007 | 206,191 | 35.89 | ||||||
| Granted | 12,383 | 42.18 | ||||||
| Vested | (40,336 | ) | 38.76 | |||||
| Forfeited | (21,109 | ) | 32.83 | |||||
| Balance at September 30, 2008 | 157,129 | 36.06 | ||||||
| Granted | 7,700 | 23.93 | ||||||
| Vested | (59,047 | ) | 34.44 | |||||
| Forfeited | (4,887 | ) | 41.91 | |||||
| Balance at September 30, 2009 | 100,895 | $ | 35.80 | |||||
Performance Share Awards
The Company has entered into Performance Share agreements with certain key employees, covering the issuance of Common Stockcommon stock (Performance Shares). The Performance Shares vest upon the achievement of all or a portion of certain performance objectives, which must be achieved during the performance period. Compensation is recognized in each period based on management’s best estimate of the achievement level of the grants’ specified performance objectives and the resulting vesting amounts. In fiscal 2010, the Company recognized expense of $32,000 related to specific performance objectives achieved by certain individuals. In fiscal 2009, the Company reversed expenses previously recognized of $207,000 relating to three-year Performance Shares awarded in May 2008 and one-year Performance Shares awarded in September 2008, which was partially offset by an expense of $164,000 related to the estimated value of Performance Shares awarded to individuals based on likely achievement of specific performance objectives. The Company recorded compensation expense of $1.9 million in fiscal 2008 related to 30,552 one-year Performance Shares and 30,552 three-year Performance Shares awarded in May 2008 and 7,600 Performance Shares that vested for certain individuals that met various specific performance objectives. The Company recorded compensation expense of $4.8 million in fiscal 2007 related to 132,375 Performance Shares. The stock-based compensation table includes the Performance Shares expenses.
1999 Employee Stock Purchase Plan
Under the 1999 Employee Stock Purchase Plan (Stock Purchase Plan), the Company is authorized to issue up to 200,000400,000 shares of Common Stock.common stock. The number of authorized shares was increased by 200,000 effective with shareholder approval at the February 8, 2010 Annual Meeting. All full-time and part-time employees can choose to have up to 10% of their annual compensation withheld, with a limit of $25,000, to purchase the Company’s Common Stockcommon stock at purchase prices defined within the provisionsprovision of the Stock Purchase Plan. As of September 30, 20092010 and 2008,2009, there were $276,000$321,000 and $355,000$376,000 of employee contributions, respectively, included in accrued liabilities in the accompanying consolidated balance sheets. Stock compensation expense recognized related to the Stock Purchase Plan totaled $250,000, $265,000, $199,000 and $156,000$199,000 during fiscal 2010, 2009, 2008 and 2007,2008, respectively. The stock-based compensation table includes the Stock Purchase Plan expenses.
F-20
| 7. | Restructuring Charges |
In March 2010, the Company announced an organizational change designed to support future growth by better meeting customer needs, leveraging its multiple competencies across the organization, and building on its pharmaceutical industry experience. As a result of the reorganization, the Company eliminated 11 positions, or approximately 4% of the Company’s workforce. These employee terminations occurred across various functions and the reorganization plan was completed by the end of the third quarter of fiscal 2010. The Company also announced that it was vacating its leased sales office in Irvine, California and a leased warehouse in Birmingham, Alabama, as part of the reorganization plan. Both leased spaces were vacated by March 31, 2010.
The Company recorded total restructuring charges of approximately $1.3 million in connection with the fiscal 2010 reorganization. These pre-tax charges consisted of $0.8 million of severance pay and benefits expenses and $0.5 million of facility-related costs.
In November 2008, the Company announced a functional reorganization to allow the Company to better serve its customers and improve its operating performance. As a result of the reorganization, the Company eliminated 15 positions, or approximately five percent of the Company’s workforce. These employee terminations occurred across various functions and the reorganization plan was completed by the end of the first quarter of fiscal 2009. The Company also vacated a leased facility in Eden Prairie, Minnesota, consolidating into its owned office and research facility also in Eden Prairie, as part of the reorganization plan.
The Company recorded total restructuring charges of approximately $1.8 million in connection with the reorganization. These pre-tax charges consisted of $0.5 million of severance pay and benefits expenses and $1.3
F-24
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
$1.3 million of facility-related costs which were recorded in fiscal 2009. The restructuring iswas expected to result in approximately $2.0 million in annualized cost savings.
Cash payments related to both restructuring events totaled $1.1 million in fiscal 2010, resulting in a balance of $1.2 million at September 30, 2010.
The following table summarizes the restructuring accrual activity for fiscal 20092010(in thousands):
| Employee | Facility- | Employee | Facility- | |||||||||||||||||||||
| Severance | Related | Severance | Related | |||||||||||||||||||||
| and Benefits | Costs | Total | and Benefits | Costs | Total | |||||||||||||||||||
| Balance at September 30, 2008 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Accruals during the year | 513 | 1,250 | 1,763 | 513 | 1,250 | 1,763 | ||||||||||||||||||
| Cash Payments | (513 | ) | (295 | ) | (808 | ) | ||||||||||||||||||
| Cash payments | (513 | ) | (295 | ) | (808 | ) | ||||||||||||||||||
| Balance at September 30, 2009 | $ | — | $ | 955 | $ | 955 | $ | — | $ | 955 | $ | 955 | ||||||||||||
| Accruals during the year | 818 | 488 | 1,306 | |||||||||||||||||||||
| Cash payments | (814 | ) | (264 | ) | (1,078 | ) | ||||||||||||||||||
| Balance at September 30, 2010 | $ | 4 | $ | 1,179 | $ | 1,183 | ||||||||||||||||||
The charges above have been shown separately as restructuring charges on the consolidated statements of income.operations. The remaining accrual as of September 30,for both the fiscal 2010 and 2009 relates to facility-related costs that arerestructurings is expected to be paid within the next 1539 months. As such, the current portion totaling $0.9$1.0 million is recorded as a current liability within other accrued liabilities and the long-term portion totaling $0.1$0.2 million is recorded as a long-term liability within other long-term liabilities on the consolidated balance sheets.
F-25
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
| 8. | Income Taxes |
The Company accounts for income taxes under the asset and liability method prescribed in accounting guidance. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. The ultimate realization of deferred tax assets depends on the generation of future taxable income during the period in which related temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in this assessment. Deferred tax assets and liabilities are measured using the enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date of such change.
F-21
Income taxes in the accompanying consolidated statements of incomeoperations for the fiscal years ended September 30 are as follows(in thousands):
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Current provision: | ||||||||||||||||||||||||
| Federal | $ | 12,257 | $ | 13,534 | $ | 19,069 | $ | (331 | ) | $ | 12,257 | $ | 13,534 | |||||||||||
| State and foreign | 1,362 | 1,516 | 1,732 | 277 | 1,362 | 1,516 | ||||||||||||||||||
| Total current provision | 13,619 | 15,050 | 20,801 | (54 | ) | 13,619 | 15,050 | |||||||||||||||||
| Deferred provision (benefit): | ||||||||||||||||||||||||
| Federal | 7,483 | (2,832 | ) | (8,573 | ) | 1,019 | 7,483 | (2,832 | ) | |||||||||||||||
| State | 872 | (65 | ) | (907 | ) | (535 | ) | 872 | (65 | ) | ||||||||||||||
| Total deferred provision (benefit) | 8,355 | (2,897 | ) | (9,480 | ) | 484 | 8,355 | (2,897 | ) | |||||||||||||||
| Total provision | $ | 21,974 | $ | 12,153 | $ | 11,321 | $ | 430 | $ | 21,974 | $ | 12,153 | ||||||||||||
The reconciliation of the difference between amounts calculated at the statutory federal tax rate for the fiscal years ended September 30 and the Company’s effective tax rate is as follows(in thousands):
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Amount at statutory federal income tax rate | $ | 20,833 | $ | 9,387 | $ | 5,067 | $ | (7,231 | ) | $ | 20,833 | $ | 9,387 | |||||||||||
| Change because of the following items: | ||||||||||||||||||||||||
| State taxes | 1,206 | 715 | 736 | (209 | ) | 1,206 | 715 | |||||||||||||||||
| Other | (481 | ) | 223 | (241 | ) | (20 | ) | (481 | ) | 223 | ||||||||||||||
| Stock-based compensation | 416 | 239 | 262 | 276 | 416 | 239 | ||||||||||||||||||
| Valuation allowance | — | 1,589 | — | 2,780 | — | 1,589 | ||||||||||||||||||
| Write-off of in-process research and development | — | — | 5,497 | |||||||||||||||||||||
| Goodwill impairment | 4,834 | — | — | |||||||||||||||||||||
| Income tax provision | $ | 21,974 | $ | 12,153 | $ | 11,321 | $ | 430 | $ | 21,974 | $ | 12,153 | ||||||||||||
F-26
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
The components of deferred income taxes consisted of the following as of September 30 and result from differences in the recognition of transactions for income tax and financial reporting purposes(in thousands):
| 2009 | 2008 | 2010 | 2009 | |||||||||||||
| Depreciable assets | $ | (2,951 | ) | $ | (4,325 | ) | $ | (5,795 | ) | $ | (2,951 | ) | ||||
| Deferred revenue | 261 | 11,005 | 1,666 | 261 | ||||||||||||
| Accruals and reserves | 526 | 523 | 780 | 526 | ||||||||||||
| Stock options | 5,258 | 4,397 | 5,947 | 5,258 | ||||||||||||
| Impaired asset | 3,264 | 3,318 | ||||||||||||||
| Unrealized (losses) gains on investments | (962 | ) | 66 | |||||||||||||
| Impaired investments | 6,130 | 3,264 | ||||||||||||||
| Unrealized losses on investments | (563 | ) | (962 | ) | ||||||||||||
| Other | 844 | 571 | 1,211 | 844 | ||||||||||||
| Valuation allowance | (3,339 | ) | (3,398 | ) | (6,523 | ) | (3,339 | ) | ||||||||
| Total deferred tax asset | 2,901 | 12,157 | 2,853 | 2,901 | ||||||||||||
| Less current deferred tax asset | (353 | ) | (1,058 | ) | (247 | ) | (353 | ) | ||||||||
| Noncurrent deferred tax asset | $ | 2,548 | $ | 11,099 | $ | 2,606 | $ | 2,548 | ||||||||
In fiscal 2008,2010, the Company recorded a $1.6$3.1 million valuation allowance against thewhich primarily relates to potential capital losslosses created by the impairment of the Company’s investmentinvestments in OctoPlusNexeon and two additional medical technology companies (see Note 23 for further information). The valuation allowance was recorded because the Company does not currently foresee future capital gains within the
F-22
On October 1, 2007, the Company adopted new accounting guidance on the accounting for uncertainty in income taxes. The adoption of the new guidance resulted in an increase to retained earnings as of October 1, 2007, of $80,000, which was reflected as a cumulative effect of a change in accounting principle, with a corresponding decrease to the net liability for unrecognized tax expenses. Unrecognized tax benefits are the differences between a tax position taken, or expected to be taken in a tax return, and the benefit recognized for accounting purposes pursuant to accounting guidance. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows(in thousands):
| 2009 | 2008 | 2010 | 2009 | 2008 | ||||||||||||||||
| Beginning of fiscal year | $ | 1,540 | $ | 1,120 | $ | 2,042 | $ | 1,540 | $ | 1,120 | ||||||||||
| Increases in tax positions for prior years | 273 | 194 | ||||||||||||||||||
| Increase in tax positions for prior years | — | 280 | 194 | |||||||||||||||||
| Decrease in tax positions for prior years | (104 | ) | (7 | ) | — | |||||||||||||||
| Increases in tax positions for current year | 260 | 237 | 92 | 260 | 237 | |||||||||||||||
| Settlements with taxing authorities | — | — | — | — | — | |||||||||||||||
| Lapse of the statute of limitations | (31 | ) | (11 | ) | (82 | ) | (31 | ) | (11 | ) | ||||||||||
| End of fiscal year | $ | 2,042 | $ | 1,540 | $ | 1,948 | $ | 2,042 | $ | 1,540 | ||||||||||
The total amount of unrecognized tax benefits including interest and penalties that, if recognized, would affect the effective tax rate as of September 30, 2010, 2009 and 2008, respectively, are $1.9 million, $2.0 million and $1.3$1.5 million. Currently, the Company does not expect the liability for unrecognized tax benefits to change significantly in the next twelve months with the above balances classified on the consolidated balance sheets as a part of long-term liabilities. Interest and penalties related to unrecognized tax benefits are recorded in income tax expense. As of September 30, 2010, 2009 and 2008, a gross balance of $605,000$0.7 million, $0.6 million and $397,000,$0.4 million, respectively, has been accrued related to the unrecognized tax benefits balance for interest and penalties.
The Company files tax returns, including returns for its subsidiaries, in the United States (U.S.) federal jurisdiction and in various state jurisdictions. Uncertain tax positions are related to tax years that remain subject to examination. The Internal Revenue Service has commenced an examination of the Company’s U.S. income tax returnsreturn for fiscal 2009 in the first quarter of fiscal 2011. Fiscal years ended September 30, 2006, 2007 and 2008 remain subject to examination by
F-27
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
federal tax authorities. Tax returns for state and local jurisdictions for fiscal years ended September 30, 2003 through 20082009 remain subject to examination by state and local tax authorities.
| 9. | Commitments and Contingencies |
Litigation. From time to time, the Company has been, and may become, involved in various legal actions involving its operations, products and technologies, including intellectual property and employment disputes. The outcomes of these legal actions are not within the Company’s complete control and may not be known for prolonged periods of time. In some actions, the claimants seek damages, as well as other relief, including injunctions barring the sale of products that are the subject of the lawsuit, which, if granted, could require significant expenditures or result in lost revenues. The Company records a liability in the consolidated financial statements for these actions when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate, the minimum amount of the range is accrued. If a loss is possible but not known or probable, and can be reasonably estimated, the estimated loss or range of loss is disclosed. In most cases, significant judgment is required to estimate the amount and timing of a loss to be recorded.
F-23
SRI Litigation. On July 31, 2009, the Company’s SurModics Pharmaceuticals business unitsubsidiary was named as a defendant in litigation pending in the circuit court of Jefferson County, Alabama, between SRI and two of SRI’s former employees (the “Plaintiffs”)Plaintiffs). In the litigation, the Plaintiffs allege that they contributed to or invented certain intellectual property while they were employed at SRI, and pursuant to SRI’s policies then in effect, they are entitled to, among other things, a portion of the purchase price consideration paid by the Company to SRI as part the Company’s acquisition of Brookwood Pharmaceuticals, Inc., pursuant to a stock purchase agreement made effective on July 31, 2007 (the “StockStock Purchase Agreement”)Agreement). A trial has not yet been scheduled. Pursuant to the Stock Purchase Agreement, the Company has certain rights of indemnification against losses (including without limitation, damages, expenses and costs) incurred as a result of the litigation. The Company’s consolidated financial statements do not include any expenses or liabilities related to the above litigation as the probability of the outcome is currently not determinable and any potential loss is not estimable. The Company believes that it has meritorious defenses to the Plaintiffs’Plaintiff’s claims and will vigorously defend and prosecute this matter.
InnoRx, Inc. In January 2005, the Company entered into a merger agreement whereby SurModics acquired all of the assets of InnoRx, Inc. (InnoRx), an early stage company developing drug delivery devices and therapies for the ophthalmology market. SurModics will be required to issue up to approximately 480,059 additional shares of its common stock to the stockholders of InnoRx upon the successful completion of the remaining development and commercial milestones involving InnoRx technology acquired in the transaction.
BioFX Laboratories, Inc. In August 2007, the Company acquired 100% of the capital stock of BioFX Laboratories, Inc. (BioFX), a provider of substrates to thein vitrodiagnostics industry. The sellers of BioFX are still eligible to receive up to $3.5 million in additional consideration based on specific revenue targets through calendar 2011.
SurModics Pharmaceuticals, Inc. In July 2007, the Company acquired 100% of the capital stock of Brookwood Pharmaceuticals Inc. (now known as SurModics Pharmaceuticals, Inc.) (SurModics Pharmaceuticals), a drug delivery company that provides proprietary polymer-based technologies to companies developing pharmaceutical products. The sellers of SurModics Pharmaceuticals are still eligible to receive up to $16.3 million in additional consideration based on successful achievement of specific milestones through calendar 2011. A project milestone event was achieved in the first quarter of fiscal 2011 and as such an obligation of $0.8 million was recognized.
Alabama Jobs Commitment. In April 2008, the Company purchased a 286,000 square foot office and warehouse facility to support cGMP manufacturing needs of customers. At the same time, SurModics Pharmaceuticals entered into an agreement with various governmental authorities to obtain financial incentives associated with creation of jobs in Alabama. Some of the governmental agencies have recapture rights in connection with the financial incentives if the number of full-time employees are not hired by June 2012, with an extension to June 2013
F-28
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
if circumstances or events occur that are beyond the control of SurModics Pharmaceuticals or could not have been reasonably anticipated by SurModics Pharmaceuticals. As of September 30, 2010, SurModics Pharmaceuticals has received $1.7 million in connection with the agreement, and the Company has recorded the payment in other long-term liabilities.
Operating Leases. The Company leases certain facilities under noncancelable operating lease agreements. Rent expense for the years ended September 30, 2010, 2009, and 2008 was $0.3 million, $1.0 million, and 2007 was $994,000, $773,000 and $140,000,$0.8 million, respectively. Annual commitments pursuant to operating lease agreements are as follows:
Year Ended September 30, | ||||||||
| 2010 | $ | 422,000 | ||||||
| 2011 | 177,000 | $ | 258,000 | |||||
| 2012 | 126,000 | 57,000 | ||||||
| 2013 | 131,000 | 60,000 | ||||||
| 2014 | 33,000 | 62,000 | ||||||
| 2015 | 62,000 | |||||||
| Thereafter | — | 16,000 | ||||||
| Total minimum lease payments | $ | 889,000 | $ | 515,000 | ||||
| 10. | Defined Contribution Plans |
The Company has a 401(k) retirement and savings plan for the benefit of qualifying employees. The Company has matchedmatches 50% of each dollar ofemployee contributions on the first 6% of the tax deferral elected by each employee.eligible compensation. Effective April 1, 2009, the Company changed its matching contribution to a discretionary approach and the Company ceased matching contributions. Effective April 1, 2010, the Company re-instated its matching contribution at the previous level. Company contributions totaling $243,000, $539,000$0.2 million, $0.2 million, and $356,000$0.5 million have been expensed for the years ended September 30, 2010, 2009, and 2008, and 2007, respectively. The expense increase in fiscal 2008 principally reflects the addition of employees eligible for this benefit as a result of the SurModics Pharmaceuticals acquisition.
| 11. | Operating Segments |
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker, or decision making group, in deciding
F-24
how to allocate resources and in assessing performance. In November 2008,October 2010, the Company announced it was changing its operational structure to renew focus on business units and the Company will now be organized into three business units: Medical Device, Pharmaceuticals and In Vitro Diagnostics (IVD). Beginning in the first quarter of fiscal 2011, the Company will describe its business under the new reporting structure.
In March 2010, prior to the fiscal 2011 change noted above, the Company announced it changed its operationalorganizational structure so thatto better align functional expertise, which also resulted in the Company is now organized into four clinically and market focusedelimination of the company’s business units: Cardiovascular, Ophthalmology, SurModics Pharmaceuticals, and In Vitro Technologies.units. The Company believes that this structure will improve the visibility, marketingevaluates revenue results and adoption of the Company’s broad array of technologies within specific markets and help its customers in the medical device, pharmaceutical and life science industries solve unmet clinical needs. In addition, a new centralized research and development function has been formed to serve the needs of the Company’s clinically and market focused business units, other than the SurModics Pharmaceuticals business unit, which continues to maintain certain R&D operations.
For fiscal years ended September 30, 2010, 2009, 2008 and 2007,2008, the Company’s results are aggregated into one reportable segment, as each business unit has similar economic characteristics, technology, manufacturing processes, customers, regulatory environments, and shared infrastructures. Thethe Company manages its expenses on a company-wide basis, as many costswell as its sales and activities are shared among the business units. The focus of the business units is providing solutionsmarketing efforts.
F-29
SurModics, Inc. and Subsidiaries
Notes to customers and maximizing financial performance over the long term.Consolidated Financial Statements — (Continued)
The table below presents revenue from the markets identified above, with Therapeutic broken out further by focus area, for the years ended September 30, as follows (in thousands):
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Therapeutic | ||||||||||||||||||||||||
| Cardiovascular | $ | 39,841 | $ | 47,675 | $ | 46,487 | $ | 40,155 | $ | 39,841 | $ | 47,675 | ||||||||||||
| Ophthalmology | 52,102 | 10,252 | 2,453 | 7,617 | 52,102 | 10,252 | ||||||||||||||||||
| Other Markets | 13,114 | 17,875 | 4,041 | 10,932 | 13,114 | 17,875 | ||||||||||||||||||
| Total Therapeutic | 105,057 | 75,802 | 52,981 | 58,704 | 105,057 | 75,802 | ||||||||||||||||||
| Diagnostic | 16,477 | 21,249 | 20,183 | 11,194 | 16,477 | 21,249 | ||||||||||||||||||
| Total revenue | $ | 121,534 | $ | 97,051 | $ | 73,164 | $ | 69,898 | $ | 121,534 | $ | 97,051 | ||||||||||||
Major Customers
Revenue from customers that equaled or exceeded 10% of total revenue was as follows for the years ended September 30:
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Johnson & Johnson | 17 | % | 11 | % | 20 | % | ||||||||||||||||||
| Medtronic, Inc. | 14 | % | ** | ** | ||||||||||||||||||||
| Merck & Company | 37 | % | <10 | % | ** | ** | 37 | % | ** | |||||||||||||||
| Johnson & Johnson | 11 | % | 20 | % | 33 | % | ||||||||||||||||||
| Abbott Laboratories | <10 | % | 10 | % | 16 | % | ** | ** | 10 | % | ||||||||||||||
| ** | - less than |
F-25
The revenue from the customers listed is derived from all three primary sources: royalties and license fees,licensing, product sales, and research and development fees.development.
Geographic Revenue
Geographic revenue was as follows for the years ended September 30:
| 2009 | 2008 | 2007 | 2010 | 2009 | 2008 | |||||||||||||||||||
| Domestic | 84 | % | 79 | % | 81 | % | 78 | % | 84 | % | 79 | % | ||||||||||||
| Foreign | 16 | % | 21 | % | 19 | % | 22 | % | 16 | % | 21 | % | ||||||||||||
F-30
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
| 12. | Quarterly Financial Data (Unaudited) |
The following is a summary of the unaudited quarterly results for the years ended September 30, 2010, 2009, 2008 and 20072008(in thousands, except per share data).
| First | Second | Third | Fourth | First | Second | Third | Fourth | |||||||||||||||||||||||||
| Quarter | Quarter | Quarter | Quarter | Quarter | Quarter | Quarter | Quarter | |||||||||||||||||||||||||
Fiscal 2010 | ||||||||||||||||||||||||||||||||
| Revenue | $ | 17,381 | $ | 18,360 | $ | 18,608 | $ | 15,549 | ||||||||||||||||||||||||
| Income (loss) from operations | 2,768 | (952 | ) | 2,220 | (18,089 | ) | ||||||||||||||||||||||||||
| Net income (loss) | 1,917 | (427 | ) | (916 | ) | (21,663 | ) | |||||||||||||||||||||||||
| Net income (loss) per share(1): | ||||||||||||||||||||||||||||||||
| Basic | 0.11 | (0.02 | ) | (0.05 | ) | (1.25 | ) | |||||||||||||||||||||||||
| Diluted | 0.11 | (0.02 | ) | (0.05 | ) | (1.25 | ) | |||||||||||||||||||||||||
Fiscal 2009 | ||||||||||||||||||||||||||||||||
| Revenue | $ | 63,216 | $ | 20,925 | $ | 18,186 | $ | 19,207 | $ | 63,216 | $ | 20,925 | $ | 18,186 | $ | 19,207 | ||||||||||||||||
| Income from operations | 42,667 | 6,200 | 4,661 | 3,973 | 42,667 | 6,200 | 4,661 | 3,973 | ||||||||||||||||||||||||
| Net income | 27,085 | 4,216 | 3,539 | 2,710 | 27,085 | 4,216 | 3,539 | 2,710 | ||||||||||||||||||||||||
| Net income per share(1): | ||||||||||||||||||||||||||||||||
| Basic | 1.53 | 0.24 | 0.20 | 0.16 | 1.53 | 0.24 | 0.20 | 0.16 | ||||||||||||||||||||||||
| Diluted | 1.53 | 0.24 | 0.20 | 0.16 | 1.53 | 0.24 | 0.20 | 0.16 | ||||||||||||||||||||||||
Fiscal 2008 | ||||||||||||||||||||||||||||||||
| Revenue | $ | 23,829 | $ | 25,707 | $ | 24,276 | $ | 23,239 | $ | 23,829 | $ | 25,707 | $ | 24,276 | $ | 23,239 | ||||||||||||||||
| Income from operations | 7,571 | 7,181 | 7,184 | 5,325 | 7,571 | 7,181 | 7,184 | 5,325 | ||||||||||||||||||||||||
| Net income (loss) | 5,646 | 5,107 | 4,800 | (814 | ) | 5,646 | 5,107 | 4,800 | (814 | ) | ||||||||||||||||||||||
| Net income (loss) per share(1): | ||||||||||||||||||||||||||||||||
| Basic | 0.31 | 0.28 | 0.27 | (0.05 | ) | 0.31 | 0.28 | 0.27 | (0.05 | ) | ||||||||||||||||||||||
| Diluted | 0.31 | 0.28 | 0.26 | (0.05 | ) | 0.31 | 0.28 | 0.26 | (0.05 | ) | ||||||||||||||||||||||
Fiscal 2007 | ||||||||||||||||||||||||||||||||
| Revenue | $ | 16,740 | $ | 17,362 | $ | 17,762 | $ | 21,300 | ||||||||||||||||||||||||
| Income (loss) from operations | 8,109 | 8,085 | 7,518 | (13,813 | ) | |||||||||||||||||||||||||||
| Net income (loss) | 5,992 | 5,675 | 5,587 | (13,907 | ) | |||||||||||||||||||||||||||
| Net income (loss) per share(1): | ||||||||||||||||||||||||||||||||
| Basic | 0.32 | 0.31 | 0.31 | (0.78 | ) | |||||||||||||||||||||||||||
| Diluted | 0.32 | 0.31 | 0.31 | (0.78 | ) | |||||||||||||||||||||||||||
| (1) | The sum of the quarterly earnings per share may not equal the annual earnings per share because of changes in the average shares outstanding. |
In the second quarter of fiscal 2010, the Company recorded a restructuring charge of $1.3 million, associated with a functional reorganization and an asset impairment charge of $2.1 million, associated with consolidation of the Company’s multiple facilities in Birmingham, Alabama.
In the third quarter of fiscal 2010, the Company recorded a $2.6 million non-cash impairment loss on its investment in two private medical technology companies and adjusted the asset impairment charge associated with the Birmingham, Alabama facilities by $0.2 million
In the fourth quarter of fiscal 2010, the Company recorded a $0.4 million non-cash inventory impairment charge, a $1.3 million in non-cash asset impairment charge associated with long-lived assets, a $1.3 million non-cash asset impairment loss associated with certain fixed assets costs in Minnesota, a $13.8 million non-cash goodwill impairment charge associated with the Company’s SurModics Pharmaceuticals reporting unit, and a $5.3 million non-cash impairment loss on its investment in Nexeon MedSystems.
F-26F-31
SurModics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements — (Continued)
In the first quarter of fiscal 2009, the Company recorded income that had previously been deferred of $34.8 million associated with the Merck contract termination, a $9 million milestone payment from Merck associated with the termination of the triamcinolone acetonide development program, a $3.2 million charge for in-process research and development acquired in connection with the purchase of certain contracts and assets of PR Pharma, as well as a $1.8 million restructuring charge associated with a functional reorganization.
In the fourth quarter of fiscal 2009, the Company recorded $1.3 million in royalty income in connection with the settlement of previously disclosed litigation involving Abbott Laboratories and Church & Dwight Co, Inc.
In the fourth quarter of fiscal 2008, the Company recorded a $4.3 million non-cash impairment loss on its investment in OctoPlus.
F-27F-32