UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| | | |
þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | | For the fiscal year ended December 31, 20062007 |
or |
| | |
o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
COMMISSION FILE NUMBER 0-19871
STEMCELLS, INC.
(Exact name of Registrant as specified in its charter)
| | | |
DELAWAREA Delaware Corporation
| | 94-3078125 |
(State or other jurisdiction
of incorporation or organization) | | (I.R.S. Employer
Identification No.) |
3155 PORTER DRIVE, PALO ALTO, CA 94304
(Address of principal offices) (zip | | |
3155 PORTER DRIVE
PALO ALTO, CA
(Address of principal offices) | | 94304
(zip code) |
Registrant’s telephone number, including area code:
(650) 475-3100
Securities registered pursuant to Section 12(b) of the Act:
NONE
| | |
Title of Each Class | | Name of Each Exchange on Which Registered |
|
| Common Stock, $0.01 par value | | Nasdaq Global Market |
| Junior Preferred Stock Purchase Rights | | |
Securities registered pursuant to Section 12(g) of the Act:
COMMON STOCK, $.01 PAR VALUE
JUNIOR PREFERRED STOCK PURCHASE RIGHTS
NoneTitle of class
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 ofRegulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of thisForm 10-K or any amendment to thisForm 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a non-accelerated filer.smaller reporting company. See definitionthe definitions of “large accelerated filer,” “accelerated filerfiler” and large accelerated filer”“smaller reporting company” inRule 12b-2 of the Exchange Act. (Check one):
| | | | | | |
| oLarge accelerated filer | | þ Accelerated filer | | o Non-accelerated filer | | Accelerated Filer þo Smaller reporting company |
| Non-accelerated filer o | | | (Do not check if a smaller reporting company) | | |
Indicate by check mark whether the Registrant is a shell company (as defined inRule 12b-2 of the Exchange Act). Yes o No þ
Aggregate market value of Common Stockcommon stock held by non-affiliates at June 30, 2006: $157,013,768.2007: $182,141,740. Inclusion of shares held beneficially by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the direction of management policies of the registrant, or that such person is controlled by or under common control with the Registrant.
Common stock outstanding at February 23, 2007: 78,625,66729, 2008: 80,732,542 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement relating to the registrant’s 20072008 Annual Meeting of Stockholders to be filed with the Commission pursuant to Regulation 14A are incorporated by reference in Part III of this report.
FORWARD LOOKING STATEMENTS
THIS REPORT CONTAINS FORWARD-LOOKING STATEMENTS AS DEFINED UNDER THE FEDERAL SECURITIES LAWS. ACTUAL RESULTS COULD VARY MATERIALLY. FACTORS THAT COULD CAUSE ACTUAL RESULTS TO VARY MATERIALLY ARE DESCRIBED HEREIN AND IN OTHER DOCUMENTS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION. READERS SHOULD PAY PARTICULAR ATTENTION TO THE CONSIDERATIONS DESCRIBED IN THE SECTION OF THIS REPORT ENTITLED “MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS” AS WELL AS ITEM 1A UNDER THE HEADING “RISK FACTORS.”
Table of Contents
NOTE REGARDING REFERENCES TO OUR COMMON STOCK
Throughout thisForm 10-K, the words “we,” “us,” “our,” and “StemCells” refer to StemCells, Inc., including StemCells California, Inc., a wholly-owned subsidiary, and the owner or licensee of most of our intellectual property. “Common stock” refers to StemCells, Inc., common stock, $.01 par value.
2
PART I
Overview
StemCells, Inc. is focused onengaged in the discovery and development of cell-based therapeutics to treat damage to, or degeneration of, major organ systems such as the Central Nervous System, Liver and Pancreas. Many degenerative diseases are caused by the loss of normal cellular function in the particular organ.systems. Our aim is to restore or support organ function, improve patients’ lives and reduce the substantial health care costs associated with these diseases and disorders. We seek to identifydisorders by identifying and purify raredeveloping stem and progenitor cells develop methods and processes to expand and bank them as transplantable cells, and then demonstrate their safety and efficacy aspotential therapeutic agents. We currently have product development programs for two cell types: the human neural stem cell and human liver engrafting cells. In our CNS Program, we are currently conducting a Phase I clinical trial to evaluate the safety and preliminary efficacy of our leadHuCNS-SC® product thecandidate (purified human neural stem cell (HuCNS-SCtm),cells) as a treatment for infantile and late infantile neuronal ceroid lipofuscinosis (NCL), two forms of a group of disorders often referred to as Batten disease. As of February 2007, two patients out of a total of six plannedWe have completed enrollment and dosing in this six-patient trial have been enrolled and transplanted with HuCNS-SC.
Stem cells are cells that can produce all the functional mature cell types foundexpect it to be completed in normal organs of healthy individuals. Progenitor cells are cells that have already developed from the stem cells, but can still produce one or more types of mature cells within an organ. We use cells derived from donated fetal or adult tissue sources, which are supplied to us in compliance with all applicable state and federal regulations. At this time, we are not developing embryonic stem cells for therapeutic use nor are we involved in any activity directed toward human cloning. Our programs are all directed toward the use of tissue-derived cells for treating or curing diseases and injuries.
We have successfully identified, purified, and characterized the human neural stem cell. Our neural stem cell product, HuCNS-SC, is in a Phase I clinical trial for its first indication andearly 2009. In addition, we are conducting additional preclinical and IND-enabling work for additional indications. We have also identified a population of cells derived from liver tissue, thespinal cord injury, myelin disorders and retinal disorders. In our Liver Program, we are in preclinical development with, and are continuing to improve processes to isolate and purify, our human liver engrafting cells (hLEC),.
Many degenerative diseases are caused by the loss of normal cellular function in a particular organ. When cells are damaged or destroyed, they no longer produce, metabolize or accurately regulate substances, such as sugars, amino acids, neurotransmitters, and hormones, which when transplanted intoare essential to life. Although traditional pharmaceuticals and genetically engineered biologics may have some utility in addressing a mouse modeldegenerative condition, there is no technology existing today that can deliver these essential substances precisely to the sites of liver degeneration, show long-term engraftment, differentiate into human hepatocytes, secrete human hepatic proteinsaction, under the appropriate physiological regulation, in the appropriate quantity, or for the duration required to cure the degenerative condition. Cells, however, can do all this naturally. Thus, transplantation of stem or progenitor cells may prevent the loss of, or even generate new, functional cells and form structural elements ofpotentially maintain or restore organ function and the liver. Based on this data, we are developing the hLEC for potential therapeutic applications to liver diseases. Our goal is to initiate a clinical test of these cells in patients within the next twelve months. We have also identified a candidate stem cell of the pancreas and this program is at the research stage.patient’s health.
We believe that, if successfully developed, our cell technologies will create the basis for therapies that would address a number of conditions with significant unmet medical needs. Many neurodegenerative diseases involve the failure of an organ that cannot be transplanted, i.e., the brain or central nervous system. Otherspinal cord. Many liver diseases, such as hepatitis, and diabetes, involve organs, such as the liver or pancreas, that can be transplanted,addressed by a liver transplant, but of which there is atransplantable livers are in very limited supply available for transplant.supply. We estimate that these neural,degenerative conditions of the central nervous system (CNS) and the liver and pancreatic conditionstogether affect more than 5735 million people in the United States and account for more than $325nearly $200 billion annually in health care costs.(1)1
StemCells California, Inc., a California corporation, is our wholly-owned subsidiary, and the owner or licensee of most of our intellectual property. References in this annual report to “the Company,” “we,” “us,” and similar words include this subsidiary.
Stem Cell Therapy BackgroundThe Potential of Our Tissue-Derived Cell-Based Therapeutics
Cells maintain normal physiological functionWe are focused on identifying and purifying tissue-derived stem and progenitor cells for use in healthy individuals by secretinghomologous therapy. Tissue-derived stem cells are developmentally pre-programmed to become the mature functional cells of the organ from which they were derived. We believe that homologous use of purified, unmodified tissue-derived cells (i.e., use of brain-derived neural stem cells for treatment of CNS disorders and liver-derived cells for treatment of liver disorders) is the most direct way to provide for engraftment and differentiation into functional cells, and should minimize the risk of transplantation of unwanted cell types.
To our knowledge, no one has developed an effective therapy for replacing lost or metabolizing substances,damaged tissues from the human nervous system. Replacement of tissues in other areas of the human body is mainly limited to those few cases, such as sugars, amino acids, neurotransmitters and hormones, which are essential to life. Whenbone marrow or peripheral blood cell transplants, where transplantation of the patient’s own cells are damaged oris now feasible. In a few additional areas, including the liver, transplantation of donor organs is now used, but is limited by the scarcity of organs available through donation. More recently, investigators have isolated
(1)1 This estimate is based on information from the Alzheimer’s Association, the Alzheimer’s Disease Education & Referral Center (National Institute on Aging), the National Parkinson Foundation, the National Institutes of Health’s National Institute on Neurological Disorders and Stroke, the Foundation for Spinal Cord Injury Prevention, Care & Cure, the Travis Roy Foundation, the Centers for Disease Control and Prevention, the Wisconsin Chapter of the Huntington’s Disease Society of America, the American Liver Foundation, the Cincinnati Children’s Hospital Medical Center, and JAIDs.
3
destroyed, they no longer produce, metabolizesubpopulations of cells from a specific organ, such as hepatocytes from the liver or accurately regulate those substances. Cell loss and impaired cellular function are leading causes of degenerative diseases, and some ofislet cells from the specific substances or proteins that are deficient in some of these diseasespancreas, which have been identified. Although administeringtransplanted into patients with a measure of success. However, these substances or proteins has some advantages over traditional pharmaceuticals, such as specificity, there is no existing technology that can deliver them precisely totypes of cell transplants are also limited both by the sitesquality of action, under the appropriate physiological regulation, in the appropriate quantity, nor for the duration required to cure the degenerative condition. Cells, however, can do all this naturally. Thus, where failingharvested cells are no longer producing needed substances or proteins or where there has been irreversible tissue damage or organ failure, transplantation of stem or progenitor cells may slow or prevent the loss of functional cells or enable the generation of new functional cells, thus potentially maintaining or restoring organ function and the patient’s health.availability of suitable organs.
Stem cells are rare and only available in limited supply. They have two defining characteristics: (i) they produce all of the kinds of mature cells making up the particular organ;organ and (ii) they self renew — that is, some of the cells developed from stem cells are themselves new stem cells, thus permitting the process to occur again and again. Stem cells are knownBecause of this self-renewal property, we believe that cell-based therapeutics may facilitate the return to existproper function potentially for a number of systemsthe life of the patient. To date four human body, includingstem cells have been identified and characterizedin vivo: the bloodhemotopoietic stem cell, the mesenchymal stem cell, the neural stem cell, and immune system, the central and peripheral nervousembryonic stem cell. Many researchers believe stem cells exist in other organ systems, (including the brain), the skin, bone, and even hair. They are thought to exist for many others, including the liver, and pancreas endocrine systems, gut, muscle,system, and the heart. Stem cells can produce all the mature functional cell types found in normal organs. Progenitor cells are responsiblecells that have already developed from the stem cells, but can still produce one or more mature cell type within an organ. We use cells derived from donated fetal or adult tissue sources, which are supplied to us in compliance with all applicable state and federal regulations. We are not developing embryonic stem cells for organ regeneration during normal cell replacement and, to a greater or lesser extent, after injury.therapeutic use nor are we involved in any activity directed toward human cloning.
Stem cells are rare and only available in limited supply, whether from the patients themselves or from donors. Cells obtained from the same person who will receive them may be abnormal if the patient is ill or the tissue is contaminated with disease-causing cells. Also, such cells can often be obtained only through significant surgical procedures. Therefore, inIn order to develop cell-based therapeutics, three key challenges must be overcome: (i) identifying the stem or progenitor cells of a particular organ and testing them for therapeutic potential; (ii) creating processes to enable use of these rare cells in clinical applications, such as expanding and banking them in sufficient quantities to transplant into multiple patients, or purifying them for use in direct transplantation; and (iii) demonstrating the safety and efficacy of these potential therapeutics in human clinical trials.
The Potential of Our Tissue-Derived Cell-Based Therapeutics
We believe that, if successfully developed, stem and progenitor cell-based therapeutics have the potential to provide a broad therapeutic approach comparable in importance to traditional pharmaceuticals and genetically engineered biologics. Stem cells can self-renew and specialize into the different kinds of cells that are commonly lost in various diseases, and our preclinical research suggests that transplanted stem cells may also be able to migrate some distance to the proper location within the body, expand, specialize and protect or replace damaged or defective cells. Because of this, we believe that cell-based therapeutics may facilitate the return to proper function, potentially for the life of the patient.
To our knowledge, no one has developed an FDA-approved method for replacing lost or damaged tissues from the human nervous system. Replacement of tissues in other areas of the human body is mainly limited to those few cases, such as bone marrow or peripheral blood cell transplants, where transplantation of the patient’s own cells is now feasible. In a few additional areas, including the liver, transplantation of donor organs is now used, but is limited by the scarcity of organs available through donation. More recently, investigators have isolated subpopulations of cells from a specific organ, such as hepatocytes from the liver or islet cells from the pancreas, which have been transplanted into patients with a measure of success. However, these types of cell transplants are also limited by the availability of suitable organs.
StemCells has focused on identifying and purifying tissue-derived stem and progenitor cells for use in homologous therapy, that is, use of brain derived neural stem cells for treatment of brain disorders; pancreas derived cells for treatment of pancreas disorders; or liver derived cells for treatment of liver disorders. Tissue-derived stem cells are developmentally pre-programmed to become the specialized cells of the organ from which they are derived by responding directly to cues of the host environment. We believe the homologous use of purified, unmodified tissue-derived cells is the most direct way to provide for engraftment of the cells and differentiation into the mature specialized cells of the organ, and should minimize the risk of transplantation of unwanted cell types.
4
We have developed techniques for discovering novel monoclonal antibodies that can be used to label markers on cell surfaces to identify and isolate specific cell types, and particularly stem and progenitor cells. This methodology allows us to purify the stem cell population and eliminate other unwanted cell types. For example, we have discovered and patented the use of monoclonal antibodies to identify the human neural stem cell, as well as human liver engrafting cells and a candidate pancreatic stem/progenitor cell.
With respect to the human neural stem cell, our leadHuCNS-SC product candidate, we believe we have overcome the first two key challenges to developing a cell-based therapeutic.challenges. We have (i) identified and characterized the human neural stem cell and (ii) have developed proprietary and reproducible processes to identify, purify, expand and bank human neural stem cells from brain tissue. Because the product is a purified composition of normal human neural stem cells, it should be better suited for transplantation and should provide a safer and more effective alternative to therapies that are based on cells derived from cancer cells, animal-derived cells or are an unpurified mix of many different cell types. Furthermore, we have shown that these purified and expanded stem cells, when transplanted into immunodeficient mice, engraft, migrate, differentiate into neurons and glial cells and survive for as long as one year withno signof tumor formation or adverse effects on the animals; moreover, the cells were still dividing at the end of the test period. These findings show that our neural stem cells, when transplanted, adopt the characteristics of the host brain and act like normal stem cells, suggesting the possibility of a continual replenishment of normal human brain cells.
Business Strategy
We are seeking to develop and commercialize cell-based therapeutics to treat, and possibly cure, a range of human diseases. Our strategy is to be the first to identify, isolate and patent multiple types of human stem and progenitor cells with therapeutic and commercial importance; to develop techniques and processes either to reproducibly purify thethese cells for direct transplant or to enable the expansion and banking of thosethese cells; and then to take them into clinical development as transplantable therapeutics. To date, we have identified three rare cell types: the human neural stem cell, human liver engrafting cells, and a candidate pancreas stem cell. We have developed the methods and processes to expand and bank the neural stem cell and are now engaged in the first clinical trial of our neural stem cell product, HuCNS-SC.
We believe that patent protection will be available to the first to identify and isolate any of the finite number of different types of human stem cells, and the first to define methods to culture such cells, making the commercial development of cell-based treatments for currently intractable diseases financially feasible. Thus, a central element of our business strategy is to obtain patent protection for the compositions, processes and uses of these multiple types of cells. We have obtained rights to certain inventions relating to stem cells and progenitor cells from academic institutions. We expect to continue to expand our search for, and to seek to acquire rights from third parties relating to, new stem and progenitor cells, and to further develop our intellectual property positions with respect to these cells in-house and through research at scholarly institutions. Our portfolio of issued patents includes a method of culturing normal human central nervous system stem and progenitor cells in our proprietary chemically-defined media, and our published studies show that these cultured and expanded cells give rise to all three major cell types of the central nervous system. We also have patent applications pending in connection with our work on liver and pancreas stem and progenitor cells.
Research and Development Programs
Overview
We have devoted substantial resources to our research programs to isolate and develop stem and progenitor cells that we believe can serve as a basis for protecting or replacing diseased or injured cells. Our efforts to date have been directed at methods to identify, isolate and culture large quantities of stem and progenitor cells of the human nervous system, liver and pancreas and to develop therapeutics utilizing these cells.
The following table showssummarizes the current status of, and the potentialanticipated initial indications for, our primary research andtwo product development programs and projects. The table is qualified in its entirety by reference to theprograms. A more detailed descriptionsdiscussion of such programs and projects appearing elsewhere in this report. We continually evaluate our research and product development efforts and reallocate resources among existing programs or to new programs in lighteach of experimental results, commercial potential, availability of third party funding, likelihood of near-term
5
efficacy, collaboration success or significant technology enhancement, as well as other factors. Our research and product development programs are at relatively early stages of development and will require substantial resources to commercialize.these follows the table.
| | | |
Program Description and Objective | | Stage/Status(1)Status
|
| |
NeuralCNS Program | | Clinical |
| | | |
Cellular therapyCell-based therapeutics to restore or preserve function to central nervous system tissue by protecting, repairing or replacing dysfunctional or damaged cells. Initial indications are lysosomal storage diseases that affect the CNS, e.g., NCL, and disorders in which demyelination plays a central role, e.g. certain spinal cord injuries, cerebral palsy
| |
Neuronal Ceroid Lipofuscinosis (also known as Batten disease)
• DemonstratedEnrollment and dosing completed for six-patient Phase I clinical trial; we expect trial completion in vivoproof of principle showing in a mouse model for Infantile NCL that HuCNS-SC canearly 2009.
• continuously produce the enzyme that is deficient in Infantile NCL
• protect host neurons from death
• extend the lifespan of the HuCNS-SC transplanted mice
• Phase 1 clinical trial ongoing at Oregon Health & Science University. As of February 2007, two patients out of a total of six planned have been enrolled and transplanted with HuCNS-SC
|
| | Preclinical |
| | |
| |
Spinal Cord Indications:
• Demonstratedin vivoproof of principle in a mouse model for spinal cord injury that transplanted HuCNS- SC
• restores motor function in injured animals
• directly contributes to the functional recovery; when human cells are ablated restored function is lost
• become specialized oligodendrocytes and neurons
• demonstrated in preclinical studies that transplanted HuCNS-SC
• does not contribute to scar formation
• does not induce abnormal pain
Myelin Disorders:
• Demonstratedin vivoproof of principle in the myelin deficient shiverer mouse and spinal cord injured mouse that transplanted HuCNS-SC
• shows integration of myelin producing oligodendrocytes into the mouse brain and spinal cord |
64
| | | |
Program Description and Objective | | Stage/Status(1)Status
|
| |
| | | |
| the CNS, such as NCL, and disorders in which demyelination plays a central role. | | • Demonstratedin vivoproof of principle by showing in a mouse model for infantile NCL that transplanted HuCNS-SC cells can:
• tight wrappingcontinuously produce the enzyme that is deficient in infantile NCL • protect host neurons from death
• extend the lifespan of the mouse nerve axons to form myelin sheathHuCNS-SC transplanted mice
|
| | | |
| | ResearchSpinal Cord Injury:
|
| | | |
| | |
• Demonstratedin vivoproof of principle by showing in a mouse model for spinal cord injury that transplanted HuCNS- SC cells can:
• restore motor function in injured animals
• directly contribute to the abilityfunctional recovery; when human cells are ablated restored function is lost
• become specialized oligodendrocytes and neurons
|
| | |
| | Myelin Disorders:
|
| | |
| | • Demonstratedin vivoproof of principle by showing in the myelin deficient shiverer mouse and spinal cord injured mouse that transplanted HuCNS-SC cells can:
• integrate myelin producing oligodendrocytes into the mouse brain and spinal cord
• tightly wrap the mouse nerve axons to reproducibly purifyform myelin sheath
Retinal Disorders:
|
| | |
| | • Established collaboration with Casey Eye Institute to obtainin vivoproof of principle that human neural stem cells protect retinal cells from degeneration and expand these cells to create cell banks
• Expanded and banked human neural stem cells engraftprevent or slow loss of vision in the brain and spinal cordsRoyal College of rodents to give rise to the 3 specialized cell types of the CNS with no evidence of tumor formationSurgeons rat model |
| | | |
| Liver Program | | Preclinical |
| | | |
Cellular therapy to restore function to liver tissue by replacing dysfunctional or damaged cells. Initial indications may include liver-based metabolic disorders
disorders. | |
• Demonstrated the engraftment and survival of the hLECshLEC in anin vivomouse liver degeneration model.model
• Detected human serum albumin and alpha-1-antitrypsin in serum of transplanted animals.animals
• Detected bile canaliculistructural elements of the liver (bile canaliculi)
• Identified cell surface markers and methods for selection of hLEC from livers of a broad age range, and including those deemed not suitable for liver protein exporttransplantation
• Identifiedin vitroculture assay for growth of hLEC containing cells that co-express markers for both bile duct cells and hepatocytes
|
| | | |
| | | |
| | |
| | |
| | |
| | Research |
| | |
| |
• Identified cell surface markers and methods for purification of human liver engrafting cells (hLECs) from livers, of a broad age range, deemed not suitable for liver transplantation.
• Identified in vitro culture assay for growth of human liver engrafting cells, containing cells that co-express markers for both bile duct cells and hepatocytes
|
| | |
Pancreas Program | | Research |
| | |
Cellular therapy to restore function to pancreas tissue by replacing dysfunctional or damaged cells
| |
• Identified cell surface markers and methods for purification of candidate human pancreatic stem/progenitor cells (hPSCs)
• Developedin vitroscreening assay for testing biological activity of hPSCs
|
5
| | |
(1) | | “Research” refers to early stage research and product development activitiesin vitro, including the selection and characterization of product candidates for preclinical testing. “Preclinical” refers to further testing of a defined product candidatein vitroand in animals prior to clinical studies. “Clinical” refers to the testing of a defined product candidate in humans. |
NeuralCNS Program
Many neurodegenerative diseases involve the failure of brain or other central nervous system tissue (i.e., the brain, spinal cord and eye) due to the loss of functional cells. We believe the transplantation of neural stem or progenitor cells may slow or prevent the
7
loss of functional cells or enable the generation of new functional cells, thus potentially maintaining or restoring organ function. Our NeuralCNS Program is initially focusing on developing clinical applications to prevent the loss of, or restore function to, neural cells affected by genetic disorders such as neuronal ceroid lipofuscinosis Type III Gaucher’s Disease, and certain other lysosomal storage diseases; diseases in which deficient myelination plays a central role, such as certain spinal cord injuries or brain disorders such as cerebral palsy; and traumatic or ischemic insults to the brain or spinal cord,cord; and disorders in which retinal degeneration play a central role, such as strokeage-related macular degeneration or traumatic brain injuries.retinitis pigmentosa. These disorders affect a significant number of people in the United States and there currently are no effective long-term therapies for them. We
Our lead product candidate, HuCNS-SC cells, is a purified composition of normal human neural stem cells. As such, we believe it is better suited for transplantation and should provide a safer and more effective alternative to therapies that therapeuticsare based on cells derived from cancer cells, animal-derived cells or are an unpurified mix of cell types. Furthermore, our processHuCNS-SC cells can be directly transplanted, unlike embryonic stem cells, which require a prerequisite differentiation step prior to administration in order to preclude teratoma formation (tumors of multiple differentiated cell types). Our preclinical research has shownin vivothat HuCNS-SC cells engraft, migrate, differentiate into neurons and glial cells, and survive for identifying, purifying and culturingas long as one year withno signof tumor formation or adverse effects; moreover, the HuCNS-SC cells were still producing progeny cells at the end of the test period. These findings show that our neural stem and progenitor cells, may be useful in treating such diseases.when transplanted, act like normal stem cells, suggesting the possibility of a continual replenishment of normal human neural cells.
StemCells Inc holdsWe hold a substantial portfolio of issued and allowed patents in the neural field. See “Patents,“Patents, Proprietary Rights and Licenses.Licenses,” below.
Neuronal Ceroid Lipofuscinosis (NCL; also known as Batten disease).
Neuronal ceroid lipofuscinosis (NCL), which is often referred to as Batten disease, is a neurodegenerative disease that affects infants and young children. All threeTwo forms of NCL — infantile and late infantile and juvenile —infantile— are caused by the lackdeficiency of a lysosomal enzyme, but all are genetically different.enzyme. Infantile and late infantile NCL are brought on by inherited genetic mutations in theCLN1gene, which codes for palmitoyl-protein thioesterase 1 (PPT1) and in theCLN2gene, which codes for tripeptidyl peptidase I (TPP-I), respectively. As a result of these mutations, the relevant enzyme is either defective or missing, leading to the accumulation of non-degraded lysosomal substratescellular waste product in various cell types. This accumulation eventually interferes with normal cellular and tissue function, and leads to seizures and progressive loss of motor skills, sight and mental capacity. Today, NCL is always fatal. To correct the major defect in NCL patients, the missing enzyme has to be provided to the brain where it can be taken up by the enzyme-deficient cells.
We are currently conductinghave completed enrollment and dosing of a six-patient Phase I clinical trial at Oregon Health & Science University Doernbecher Children’s Hospital to evaluate the safety and preliminary efficacy of our HuCNS-SC product candidate as a treatment for infantile and late infantile NCL. This trial is an open label study with two dose levels and is planned to enroll six patients. HuCNS-SC will be transplanted into theselevels. Under the trial protocol, patients who will then receive immunosuppression for one year following transplantation.transplantation of the HuCNS-SC cells. In addition to measuringevaluating the safety of HuCNS-SC cells, the trial will also evaluate the ability of HuCNS-SCthe cells to affect the progression of the disease. We expect the trial to be completed in early 2009. We believe this clinical trial is the first FDA-approved trial to use purified human neural stem cells as a potential therapeutic agent. As of February 2007, two patients have been enrolled and transplanted with HuCNS-SC.
Our preclinical data demonstrate that HuCNS-SC cells, when transplanted in a mouse model of infantile NCL, engraft, migrate throughout the brain, produce the missing PPT1 enzyme, measurably reduce the toxic storage material in the brain, and protect host neurons so that more of them survive; insurvive. In addition, we have shown that the lifetime of the mice transplanted with HuCNS-SC transplanted micecells is extended compared to the control group. We have also demonstratedin vitrothat HuCNS-SC cells produce TPP-I, the enzyme that is deficient in late infantile NCL.
Other Lysosomal Storage Diseases.
NCL, or Batten disease, is one of a group of approximately 46 lysosomal storage diseases (“LSDs”)(LSDs). Some of theseAll LSDs which are all caused by defective or missing enzymes,proteins involved in lysosomal function and some LSDs can be treated by enzyme replacement therapies. Examples of enzyme replacement products used in these therapiesalready approved are Cerezymetm for Gaucher disease, Fabryzymetm for Fabry disease, Myozyme® for Pompe disease, Aldurazymetm for MPS I, and
6
Naglazymetm for MPS VI. All of these approved products, however, address LSDs which primarily affect peripheral organs and not the central nervous system. About half of the lysosomal storage diseases, however, do primarily affect the central nervous system; consequently, enzyme replacement therapy is not currently a practical treatment option for this subset of LSDs because enzymes are typically too large to cross the blood-brain barrier. We believe that transplanting HuCNS-SC cells directly into the CNS may have the potential to treat some LSDs that affect the CNS by acting as cellular mini-pumps for the secretion and supply ofsupplying missing enzymes to the brain. To date,In addition to infantile and late infantile NCL, we have found that HuCNS-SC cells can produce the relevant enzyme in severala number of other LSDs that affect the CNS, including infantile and late infantile NCL.CNS. And we continue to test for others.
8
Spinal Cord Indications.Injury.
Stem cells may have the potential to treat various spinal cord indications. Using a mouse model of spinal cord injury, our collaborators, Drs. Aileen Anderson and Brian Cummings of the Reeve-Irvine Center at the University of California, have shown that HuCNS-SC cells have the potential to protect and regenerate damaged nerves and nerve fibers, and that injured mice transplanted with our human neural stem cells showed improved motor function compared to control animals. Inspection of the spinal cords from thesethe treated mice showed significant levels of human neural cells derived from the transplanted stem cells. Moreover, the humanSome of these cells that are found in the spinal cord of the transplanted mice matured intowere oligodendrocytes, the specialized neural cell that forms the myelin sheath around axons, insulating themwhile others had become neurons and enabling them to conduct signals to other axons.showed evidence of synapse formation, a requirement for proper neuronal function. Drs. Anderson and Cummings then selectively ablated the human cells, and found that the functional improvement was lost, thus demonstrating that the human cells had played a direct role in the functional recovery of the transplanted mice. We are currently conductingcontinuing preclinical development on our HuCNS-SC as a potential therapeuticproduct candidate for various spinal cord indications.indications and our goal is to initiate a clinical trial for a spinal cord indication in mid-2008.
Other Remyelination Indications.Myelin Disorders.
In addition to certain spinal cord indications, lossLoss of myelin characterizes conditions such as multiple sclerosis, cerebral palsy and certain genetic disorders (for example, Krabbe’s disease and metachormaticmetachromatic leukodystrophy)., and also plays a role in certain spinal cord indications. Myelin is the substance that wraps around and insulates nerve axons and allows proper signal conductivity along the nerves. We have shown that HuCNS-SC cells differentiate into oligodendrocytes, the myelin producing cells, and produce myelin. We have transplanted HuCNS-SC cells into the brain of the mutant shiverer mouse, which is deficient in myelin, and shown widespread engraftment of human cells that matured into oligodendrocytes. Furthermore, analysis of the brain tissue of these mice shows the myelin produced bythat the human cells ensheathesoligodendrocytes myelinated the mouse nerve, providing the proper layers of insulation. Further studies are in progress to demonstrate proper function of the newly produced myelin.axons. Pilot studies for understanding myelin production and repair wereare being conducted in collaboration with researchers at the Oregon Health & Science University and the Yale University School of Medicine.University. We are currently conducting preclinical development on our HuCNS-SC as a potential therapeuticproduct candidate for a brain indication characterized by myelin deficiency.deficiency and our goal is to initiate a clinical trial for such an indication by the end of 2008.
Retinal Disorders.
Published studies have shown that in a well-established animal model of retinal degeneration known as the Royal College of Surgeons (RCS) rat model, human neural stem cells protect retinal function and thereby preserve vision. These studies indicate that our HuCNS-SC cells could have potential clinical application as a treatment for retinal degeneration. The retina is a thin layer of neural cells that lines the back of the eye and is responsible for converting external light into neural signals; loss of function in retinal cells leads to impairment or loss of vision. The most common forms of retinal degeneration are age-related macular degeneration and retinitis pigmentosa. In January 2008, we entered into a research collaboration with the Oregon Health & Science University (OHSU) Casey Eye Institute to evaluate engraftment and function of our HuCNS-SC cells in the RCS rat model. If this research successfully demonstratesin vivoproof of principle with our proprietary cells, our goal would then be to prepare for and initiate a human clinical trial for a retinal disorder in 2009.
Other Neural Collaborations.Collaborations
We have established a number of research collaborations in the neural field to assess both thein vitropotential of the HuCNS-SC cells and the effects of transplanting HuCNS-SC cells into preclinical animal models, including a collaboration with
7
researchers at the Stanford University School of Medicine to evaluate our human neural stem cells in animal models of stroke. CollaborativeThe results of these studies regardingdemonstrate the formationtargeted migration of specific populations of neurons have also been donethe cells toward the stroke lesion and differentiation toward the neuronal lineage. Another study with researchers at The UniversityStanford’s School of Texas Medical BranchMedicine demonstrated that HuCNS-SC cells labeled with magnetic nanoparticles could non-invasively track the survival and migration of human cells within the University of California, San Diego.brain. In addition, we concluded an NIH-funded collaboration with Dr. George A. Carlson of the McLaughlin Research Institute to understandinvestigate the role of Alzheimer’s plaques in neuronal cell death in Alzheimer’s disease. Under the collaboration, Dr. Carson transplanted HuCNS-SC cells into mouse models of Alzheimer’s disease and the cells showed robust engraftment in an environment riddled with Alzheimer’s plaques. We plan to analyze the engrafted human cells in the brains of the transplanted mice.
Liver Program
Approximately 1 in 10According to the American Association for the study of Liver Diseases website, approximately 25 million Americans suffers from diseases and disorders of the liver for many of whichare afflicted with liver-related disease each year. To our knowledge there currently are no effective, long-term treatments.treatments for many of these. Liver stem or progenitor cells may be useful in the treatment of some of these diseases, such as hepatitis, liver failure, blood-clotting disorder, cirrhosis, of the liver and liver cancer. A source of defined human cells capable of engraftment and substantial liver regeneration could provide a cellular therapy or cell-based therapeutic product available to a wider patient base than whole liver (or organ) transplants.
We have identified and isolated a cell population that we call human Liver Engrafting Cells (hLEC) which shows promise as a potential therapeutic. The hLEC can be derived from all types of human livers, including those that would not otherwise be used for orthotopic liver transplantation. When tested in ourin vitroculture assay, these antibody-enriched cells, hLEC produce enzymes needed for normal liver function, such as human serum albumin. When transplanted into immunodeficient mice, the hLEC demonstrate robust engraftmentengraft and produce human proteins including albumin and alpha-1-antitrypsin and form structural elements of the liver. In September 2007, we entered into a research collaboration with Belgium’s Université Catholique de Louvain (UCL) and the UCL-affiliated Cliniques Universitaires Saint Luc to further the development of hLEC as a potential cell-based liver suggesting that once
9
transplanted,therapy. Under this collaboration, we are working with UCL to improve processes to isolate and purify hLEC, become functionalwhich we believe is an important step prior to initiating any clinical study of the cells. Based on these data, our Liver Program has a goal of initiating a clinical trial to test the hLEC in patients within the next twelve months, with theOur initial indication for clinical application will likely to be a liver-based metabolic disorder characterized by an enzyme deficiency.
Pancreas Program
We have used our monoclonal antibody-based search methodology to identifyhold a rare subsetportfolio of human pancreatic cells that may be candidate pancreatic stem/progenitor cells. If those cells can be differentiated into islet cells, the pancreas cells that produce insulin, they may be usefulissued and allowed patents in the treatment of Type 1 diabetesliver field. See “Patents, Proprietary Rights and those cases of Type 2 diabetes where insulin secretion is defective. We have filed a patent application on the monoclonal antibodies used and have developed what we believe to be an appropriate animal model to test the biological activity of the purified candidate pancreatic stem cells.
Note on State and Federal Grants
In November 2004, California State Proposition 71 (Prop. 71), the California Stem Cell Research and Cures Initiative, was adopted by the electorate. It is intended to encourage stem cell research in the State of California, and to finance such research with State funds of approximately $295 million annually for 10 years beginning with 2005. It is our understanding that the California Institute for Regenerative Medicine to be created under the Initiative will provide grants, primarily but not exclusively to academic institutions, to advance both embryonic stem cell research and adult stem cell research; the latter is the current and exclusive focus at StemCells. StemCells, Inc. is eligible to receive Prop. 71 generated funds and we do intend to apply for such funding. We also remain eligible for federal government support from the National Institute of Health (NIH) due to our focus on adult stem cells, although Prop. 71 funds will not go to any project that receives NIH funding.Licenses,” below.
Manufacturing
We have made considerable investments in our manufacturing operations. We believe we have the ability to process cells suitable for use in our ongoing and planned research and development activities and clinical trials.
Marketing
Because of the early stage of our stem and progenitor cell programs, we have not yet addressed questions of channels of distribution and marketing of potential future products.
Patents, Proprietary Rights and Licenses
We believe that proprietary protection of our inventions will be critical to our future business. We vigorously seek out intellectual property that we believe might be useful in connection with our products, and have an aggressiveactive program of protecting our intellectual property. We believe that our know-how will also provide a significant competitive advantage, and we intend to continue to develop and protect our proprietary know-how. We may also from time to time seek to acquire licenses to important externally developed technologies.
We have exclusive or non-exclusive rights to a portfolio of patents and patent applications related to various stem and progenitor cells and methods of deriving and using them. These patents and patent applications relate to compositions of matter, methods of obtaining such cells, and methods for preparing, transplanting and utilizing such cells. As of December 31, 2006,2007, our U.S. patent portfolio included forty-eightmore than 50 issued or allowed U.S. patents sevenfrom over 25 separate patent families. Four of whichour U.S. patents issued in 2006. Approximately 20 additional2007: (i) U.S. Patent No. 7,211,404,
8
(ii) U.S. Patent No. 7,166,277, (iii) U.S. Patent No. 7,217,565, and (iv) U.S. Patent No. 7,204,979. These new patents have further strengthened our already extensive patent applications were pending, fourportfolio, which, we believe, gives us a competitive advantage, especially in the emerging field of which had been allowed. In addition, weneural stem cells, because our patents broadly cover methods for identification, isolation, expansion, and transplantation of neural stem cells as well as their use in drug discovery and testing.
We also have foreign counterparts to many of the U.S. applications and patents; counterparts to thirteena majority of our U.S. patents or applicationsand applications; a substantial number of these have issued in various countries, making a total of over 130 individual150 granted or allowednon-U.S. patents from those thirteen cases. In 2003, one party filed an opposition to twoas of our issued European patent cases. Both oppositions were heard in 2005, and the patents were maintained in somewhat altered form by the Opposition Division of the European Patent Office. The time for appeal has run in each case. U.S. counterparts to these patents are part of our issued patent portfolio; they are not subject to opposition, since that procedure does not exist under U.S. patent law, although other types of proceedings may be available to third parties to contest our U.S. patents.December 31, 2007.
10
Among our significant patents are:U.S. patents:
| | |
| • | U.S. Patent No. 5,851,832, covering our methods for the human CNS cell cultures containing central nervous system stem cells, for compositions of human CNS cells expanded by these methods, and for use of these cultures in human transplantation. These human CNS stem and progenitor cells expanded in culture may be useful for repairing or replacing damaged central nervous system tissue, including the brain and the spinal cord; |
|
| | • | U.S. Patent No. 5,968,829, entitled “Human CNS Neural Stem Cells,” which covers our composition of matter for human CNS stem cells; |
| |
| | • | U.S. Patent No. 6,103,530, covering our media7,153,686, entitled “Enriched Central Nervous System Stem Cell and Progenitor Cell Populations, and Methods for culturing human CNSIdentifying, Isolating and Enriching such Populations,” which claims the composition of matter of various antibody-selected neural stem cells;cell populations; |
| |
| | • | U.S. Patent NumberNo. 6,777,233, entitled “Cultures of Human CNS Neural Stem Cells,” which discloses a neural stem cell culture with a doubling rate of 5 to 10 days; |
|
| • | U.S. Patent No. 6,497,872, entitled “Neural transplantation using proliferated multipotent neural stem cells and their progeny,” which covers transplanting any neural stem cells or their differentiated progeny, whether the cells have been cultured in suspension or as adherent cells, for the treatment of any disease; |
|
| • | U.S. Patent No. 6,468,794, entitled “Enriched central nervous system stem cell and progenitor cell populations, and methods for identifying, isolating and enriching for such populations,” which covers the identification and purification of the human CNS stem cell; |
| |
| | • | U.S. Patent Nos. 6,238,922 and 7,049,141, (“Useboth entitled “Use of collagenase in the preparation of neural stem cell cultures”),cultures,” which describe methods to advance thein vivo culture and passage of human CNS stem cells that result in a 100-fold increase in CNS stem and progenitor cell production after 6 passages. We believe the methodologies of these patents and the one mentioned immediately above together augment our leadership position in the stem cell field by providing a reproducible proprietary method for obtaining and expanding stem cells for therapeutic uses;passages; |
| |
| | • | U.S. Patent NumberNo. 5,851,832, entitled “In Vitrogrowth and proliferation of multipotent neural stem cells and their progeny,” which covers our methods for selecting the human CNS cell cultures containing central nervous system stem cells, for compositions of human CNS cells expanded by these methods, and for use of cells derived from these cultures in human transplantation; |
|
| • | U.S. Patent No. 6,294,346, (“Useentitled “Use of multipotent neural stem cells and their progeny for the screening of drugs and other biological agents”);agents,” which describes the use of human neural stem cells as a tool for screening the effects of drugs and other biological agents on such cells, such as small molecule toxicology studies; and |
| |
| | • | U.S. Patent Number 6,497,872,No. 7,211,404, entitled “Neural“Liver engrafting cells, assays, and uses thereof,” which covers the isolation of an enriched population of hepatic liver engrafting cells. These cells may be used for transplantation using proliferated multipotent neural stem cells and their progeny,” covers transplanting any neural stem cells or their differentiated progeny, whether the cells have been cultured in suspensionas well as targets for drug discovery, or as adherent cells, for the treatmenta source of any disease. We are the exclusive licensees of the patent, which gives us the right to exclude others from practicing the claimed invention.identifying liver specific genes. |
Among the recent patents issued or exclusively licensed to us are patents covering a method of drug screening using enriched populations of neural stem cells (U.S. Patent No. 7,105,150); methods for producing enriched populations of neural stem cells (U.S. Patent No. 7,037,719); in vitro cell culture compositions of enriched populations of neural stem cells (U.S. Patent No. 7,153,686); methods for the in vitro proliferation of neural stem cells (U.S. Patent No. 7,115,418); cDNA libraries obtained from neurospheres (U.S. Patent No. 7,105,342); and methods of screening for biological agents that affect proliferation, differentiation, survival, phenotype, or function of neural stem cells (U.S. Patent No. 7,101,709). In addition, we expect several other neural stem cell cases to issue shortly; we have also received indications that another application, covering methods of selecting for human liver engrafting cells, should be allowed soon.
These new patents have further strengthened our already extensive patent portfolio and, we believe, give StemCells the dominant intellectual property position in the field, covering methods for identification, isolation, expansion, and transplantation of neural stem cells as well as drug discovery and testing.
The following table lists our issued U.S. patents:
| | | | |
U.S. Patent Number
| | | | |
Owned by StemCells
| | Subject
| | Date of Expiration |
|
5,968,829 | | Human CNS neural stem cells | | 9/05/17 |
6,103,530 | | Human CNS neural stem cells — culture media | | 9/05/17 |
6,238,922 | | Use of collagenase in the preparation of neural stem cell cultures | | 2/26/19 |
6,468,794 | | Enriched neural stem cell populations, and methods for identifying, isolating and enriching for neural stem cells | | 10/21/19 |
6,498,018 | | Human CNS neural stem cells | | 9/05/17 |
6,777,233 | | Cultures of human CNS neural stem cells | | 9/05/17 |
7,037,719 | | Enriched neural stem cell populations, and methods for identifying, isolating and enriching for neural stem cells | | 10/21/19 |
11
| | | | |
U.S. Patent Number
| | | | |
Owned by StemCells
| | Subject
| | Date of Expiration |
|
7,049,141 | | Use of collagenase in the preparation of neural stem cell cultures | | 2/26/19 |
7,105,150 | | Drug screening & discovery using enriched neural stem cell populations | | 10/21/19 |
7,153,686 | | Enriched neural stem cell populations, and methods for identifying, isolating and enriching for neural stem cells | | 10/21/19 |
| | | | |
Licensed from
NeuroSpheres
| | | | |
5,750,376 | | In vitro genetic modification | | 5/12/15 |
5,851,832 | | In vitro proliferation | | 12/22/15 |
5,980,885 | | Methods for inducing in vivo proliferation of precursor cells | | 11/09/16 |
5,981,165 | | In vitro induction of dopaminergic cells from mammalian central nervous system multipotent stem cell compositions | | 11/09/16 |
6,071,889 | | Methods for in vivo transfer of a nucleic acid sequence to proliferating neural cells | | 6/06/17 |
6,093,531 | | Generation of hematopoietic cells from multipotent neural stem cells | | 6/19/18 |
6,165,783 | | Methods of inducing differentiation of multipotent neural stem cells | | 10/20/18 |
6,294,346 | | Methods for screening biological agents | | 9/25/18 |
6,368,854 | | Hypoxia-mediated neurogenesis | | 10/20/18 |
6,399,369 | | cDNA libraries derived from populations of non-primary neural cells | | 6/04/19 |
6,497,872 | | Neural transplantation using proliferated multipotent neural stem cells and their progeny | | 12/24/19 |
6,638,501 | | Use of multipotent neural stem cell progeny to augment non-neural tissues | | 6/19/18 |
6,897,060 B1 | | Generation of hematopoietic cells | | 6/19/18 |
6,924,142 B2 | | Hypoxia-mediated neurogenesis assay | | 10/20/18 |
7,101,709 | | Methods of making cDNA libraries, cell screening with cultures of neural stem cells | | 9/22/12 |
7,105,342 | | cDNA libraries derived from populations of non-primary neural cells | | 12/5/12 |
7,115,418 | | Methods of Proliferating Undifferentiated Neural Cells | | 11/30/12 |
| | | | |
U.S. Licensed from
NeuroSpheres
| | | | |
7,166,277 | | Remyelination | | 1/23/24 |
| | | | |
Licensed from the
California Institute
of Technology
| | | | |
5,589,376 | | Mammalian neural crest stem cells | | 12/31/13 |
5,629,159 | | Immortalization and disimmortalization of cells | | 5/13/14 |
5,654,183 | | Genetically engineered mammalian neural crest stem cells | | 8/05/14 |
5,672,499 | | Methods for immortalizing multipotent neural crest stem cells | | 9/30/14 |
5,693,482 | | In vitro neural crest stem cell assay | | 12/02/14 |
5,824,489 | | Methods for isolating mammalian multipotent neural crest stem cells | | 10/20/15 |
12
| | | | |
Licensed from the
| | | | |
California Institute
| | | | |
of Technology
| | Subject
| | |
|
5,849,553 | | Immortalizing and disimmortalizing multipotent neural crest stem cells | | 12/15/15 |
5,928,947 | | Mammalian multipotent neural crest stem cells | | 7/27/16 |
5,935,811 | | Neuron restrictive silencer factor proteins | | 8/10/16 |
6,001,654 | | Methods for differentiating neural stem cells to neurons or smooth muscle cells (TGFb) | | 7/27/12 |
6,033,906 | | Differentiating mammalian neural stem cells to glial cells using neuregulins | | 3/07/17 |
6,270,990 | | Neuron restrictive silencer factor proteins | | 3/03/15 |
6,555,337 | | Neurogenin | | 9/27/16 |
6,566,496 | | Neurogenin | | 9/27/16 |
6,824,774 | | Antibodies that bind neuron-restrictive silencer factor proteins | | 10/25/16 |
6,890,724 B2 | | Methods and Compositions for Neural Progenitor Cells (cRET) | | 9/06/16 |
| | | | |
Licensed from the
Scripps Research
Institute
| | | | |
6,242,666 | | An animal model for identifying a common stem/ progenitor to liver cells and pancreatic cells | | 12/16/18 |
6,541,251 | | Pancreatic progenitor 1 gene and its uses | | 4/26/21 |
6,753,153 | | Markers for identification and isolation of pancreatic islet alpha and beta progenitors | | 12/13/20 |
6,911,533 | | Pancreatic progenitor I gene and its uses | | 4/26/21 |
| | | | |
Licensed from
Oregon Health &
Science University
| | | | |
6,132,708 | | Liver regeneration using pancreas cells | | 10/10/17 |
We also rely upon trade-secret protection for our confidential and proprietary information and take active measures to control access to that information.
Our policy is to require our employees, consultants and significant scientific collaborators and sponsored researchers to execute confidentiality agreements upon the commencement of anany employment or consulting relationship with us. These agreements generally provide that all confidential information developed or made known to the individual by us during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees and consultants, the agreements generally provide that all inventions conceived by the individual in the course of rendering services to us shall be our exclusive property.
We have obtained rights from universities and research institutions to technologies, processes and compounds that we believe may be important to the development of our products. These agreements typically require us to pay license fees, meet certain diligence obligations and, upon commercial introduction of certain products, pay royalties. These include exclusive license agreements with The Scripps Institute, the California Institute of Technology and the Oregon Health & Science University, to certain patents and know-how regarding present and certain future developments in CNS, liver and pancreas stem cells. Our licenses may be canceled or converted to non-exclusive licenses if we fail to use the relevant technology or if we breach our agreements. Loss of such licenses could expose us to the risks of third party patentsand/or technology. There can be no assurance that any of these licenses will provide effective protection against our competitors. We have also obtained patent rights from cross-licensing certain of our patents to NeuroSpheres and to StemCell Therapeutics. See “Patents, Proprietary Rights and Licenses”
13
9
The patent positions of pharmaceutical and biotechnology companies, including ours, are uncertain and involve complex and evolving legal and factual questions. The coverage sought in a patent application can be denied or significantly reduced before or after the patent is issued. Consequently, we do not know whether any of our pending applications will result in the issuance of patents, or if any existing or future patents will provide significant protection or commercial advantage or will be circumvented by others. Because patent applications are secret until the applications are published (usually eighteen months after the earliest effective filing date), and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make the inventions covered by each of our pending patent applications or that we were the first to file patent applications for such inventions. There can be no assurance that patents will issue from our pending or future patent applications or, if issued, that such patents will be of commercial benefit to us, afford us adequate protection from competing products, or not be challenged or declared invalid.
In the event that a third party has also filed a patent application relating to inventions claimed in our patent applications, we may have to participate in interference proceedings declared by the United States Patent and Trademark Office to determine priority of invention, which could result in substantial uncertainties and cost for us, even if the eventual outcome is favorable to us. There can be no assurance that our patents, if issued, would be held valid by a court of competent jurisdiction.
A number of pharmaceutical, biotechnology and other companies, universities and research institutions have filed patent applications or have been issued patents relating to cell therapy, stem cells and other technologies potentially relevant to or required by our expected products. We cannot predict which, if any, of such applications will issue as patents or the claims that might be allowed.
If third party patents or patent applications contain claims infringed by our technology and such claims or claims in issued patents are ultimately determined to be valid, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost, if at all, or be able to develop or obtain alternative technology. If we are unable to obtain such licenses at a reasonable cost, we may not be able to develop certain products commercially. There can be no assurance that we will not be obliged to defend ourselves in court against allegations of infringement of third party patents. Patent litigation is very expensive and could consume substantial resources and create significant uncertainties. An adverse outcome in such a suit could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease using such technology.
License AgreementsLicenses with Research Institutions
We have entered into a number of research-plus-license agreements with academic organizations including The Scripps Research Institute (Scripps), the California Institute of Technology (Cal Tech), the Oregon Health & Science University (OHSU), and the University of Texas Medical Branch. The research components of these agreements have been concluded and have resulted in a number of licenses for resultant technology. Under thethese license agreements, we are typically subject to obligations of due diligence and the requirement to pay royalties on products that use patented technology licensed under suchthese agreements. The license agreements with these institutions relate largely to stem or progenitor cells and or to processes and methods for the isolation, identification, expansion, or culturing of stem or progenitor cells. Generally speaking, these license agreements will terminate upon expiration, revocation or invalidation of the patents licensed to us, unless governmental regulations require a shorter term. They also will terminate earlier if we breach our obligations under the agreement and do not cure the breach or if we declare bankruptcy, and webankruptcy. We can terminate thethese license agreements at any time upon notice.
In the case of Scripps, we must pay $50,000 upon the initiation of thea Phase II trial for our first product candidate using Scripps licensed technology, and upon completion of that Phase II trial we must pay Scripps an additional $125,000. Upon approval of the first product for sale in the market, we must pay Scripps $250,000.
Pursuant to the terms of our license agreement with Cal Tech, we must pay $10,000 upon the issuance of the first patent in each family licensed to us under the relevant agreement and $5,000 on the first anniversary of the issuance of each such patent, payable in cash or common stock at our option. We have paid $40,000$50,000 on account of these patents through December 31, 2006;2007; the $10,000 due in 20062007 was paid in common stock (3,848(3,865 shares). These amounts are creditable against royalties we must pay under the license agreements. The maximum royalties that we will have to pay to the California Institute of TechnologyCal Tech will be $2 million per year, with an overall maximum of $15 million.
14
Once we pay the $15 million maximum royalty, the licenses will become fully paid and irrevocable. In August 2002 we acquired an additional license from Cal Tech to different technology, pursuant to which we issued 27,535 shares of our common stock with a market value of approximately $35,000; we have also issued 9,535 shares of our common stock with a market value of approximately $15,000 to Cal Tech on the issuance of two patents covered under this additional license.
In 2002, we issued a license to BioWhittaker, Inc., for the exclusive right to make, sell and distribute one of our proprietary cells for the research market only. BioWhittaker was acquired by Cambrex, and the cognizant Cambrex division has now been acquired by Lonza. In 2003 and 2004 respectively, we issued non-exclusive licenses to StemCell Technologies, Inc. to make, use and sell certain proprietary mouse and rat neural stem cells and culture media for all mammalian neural stem cells, and to R&D Systems to make, use and sell certain stem cell expansion kits, also for the research market. These licenses are not expected to generate material revenues.
In July 2005, we entered into an agreementLicenses with ReNeuron Limited, a wholly owned subsidiary of ReNeuron Group plc, a listed UK corporation (collectively referred to as “ReNeuron”). As part of the agreement, we granted ReNeuron a license that allows ReNeuron to exploit their “c-mycER” conditionally immortalized adult human neural stem cell technology for therapy and other purposes. We received a 7.5% fully-diluted equity interest in ReNeuron, subject to certain anti-dilution provisions, as well as a cross-license to the exclusive use of ReNeuron’s technology for certain diseases and conditions, including lysosomal storage diseases, spinal cord injury, cerebral palsy and multiple sclerosis. The agreement also provides for full settlement of any potential claims that either we or ReNeuron might have had against the other in connection with any putative infringement of certain of each party’s patent rights prior to the effective date of the agreement. The agreement is Exhibit 10.71 to our Quarterly Report onForm 10-QCommercial Entities for the quarter ended June 30, 2005. See Note 2 and “Item 7a. Quantitative and Qualitative Disclosures about Market Risk” for more details on this transaction. In August 2006, we entered an agreement with Stem Cell Therapeutics (SCT), a Canadian corporation listed on the Toronto Stock Exchange, granting it a non-exclusive, royalty-bearing license to use several of our patents for treating specified diseases of the central nervous system; the grant does not include any rights to cell transplantation. SCT granted StemCells a royalty-free non-exclusive license to certain of its patents for research and development and a royalty-bearing non-exclusive for certain commercial purposes. SCT paid an up-front license fee; the license also provides for other payments including annual maintenance, milestones, and royalties.
NeuroSpheres, Ltd.
In March 1994, we entered into a Contract Researchcontract research and License Agreementlicense agreement with NeuroSpheres, Ltd., which was clarified in a License Agreementlicense agreement dated as of April 1, 1997. Under the agreement as clarified, we obtained an exclusive patent license from NeuroSpheres in the field of transplantation, subject to a limited right of NeuroSpheres to purchase a nonexclusive license from us, which right was not exercised and has expired. We have developed additional intellectual property relating to the subject matter of the license. We entered into an additional license agreement with NeuroSpheres as of October 30, 2000, under which we obtained an exclusive license in the field of non-transplant uses, such as drug discovery and drug testing and clarified our rights under NeuroSpheres patents for generating cells of the blood and immune system from neural stem cells. Together, our rights under the licenses are exclusive for all uses of the technology. We made up-front payments to NeuroSpheres of 65,000 shares of our common stock in October 2000 and $50,000 in January 2001, and we will make additional cash payments when milestones are achieved under the terms of the October 2000 agreement. In addition, in October 2000 we reimbursed NeurospheresNeuroSpheres for patent costs amounting to $341,000. Milestone payments, payable at various stages in the development of potential products, would total $500,000 for each product that is approved for market. In addition, beginning in 2004, annual payments of $50,000 became due, payable by the last day of the year and fully creditable against royalties due to NeuroSpheres under the October 2000 Agreement. Our agreements with NeuroSpheres will terminate at the expiration of all patents licensed to us, but can terminate earlier if we breach our obligations under the agreement and do not cure the breach, or if we declare bankruptcy. We have a security interest in the licensed technology.
1510
Signal Pharmaceuticals, Inc.ReNeuron Limited
In December 1997,July 2005, we entered into two sublicense agreementsan agreement with Signal Pharmaceuticals (Signal), Inc. under which each party sublicensedReNeuron Limited, a wholly owned subsidiary of ReNeuron Group plc, a listed UK corporation (collectively referred to as “ReNeuron”). As part of the agreement, we granted ReNeuron a license that allows ReNeuron to exploit their “c-mycER” conditionally immortalized adult human neural stem cell technology for therapy and other purposes. We received a 7.5% fully-diluted equity interest in ReNeuron, subject to certain anti-dilution provisions, as well as a cross-license to the exclusive use of ReNeuron’s technology for certain diseases and conditions, including lysosomal storage diseases, spinal cord injury, cerebral palsy, and multiple sclerosis. The agreement also provides for full settlement of any potential claims that either we or ReNeuron might have had against the other in connection with any putative infringement of certain of each party’s patent rights prior to the effective date of the agreement. See Note 2 “Financial Instruments — ReNeuron” in Part I, Item 8 of thisForm 10-Kand biological materials“Quantitative and Qualitative Disclosures about Market Risk” in Part I, Item 7A of thisForm 10-K for further information.
Stem Cell Therapeutics Corp.
In August 2006, we entered into an agreement with Stem Cell Therapeutics Corp. (SCT), a Canadian corporation listed on the Toronto Stock Exchange, granting it a non-exclusive, royalty-bearing license to use in defined fields. Signal has now beenseveral of our patents for treating specified diseases of the central nervous system; the grant does not include any rights to cell transplantation. SCT granted us a royalty-free non-exclusive license to certain of its patents for research and development and a royalty-bearing non-exclusive license for certain commercial purposes. SCT paid an up-front license fee; the license also provides for other payments including annual maintenance, milestones and royalties.
Other Commercial Licenses
In 2002, we issued a license to BioWhittaker, Inc. for the exclusive right to make, sell and distribute one of our proprietary cells for the research market only. BioWhittaker was acquired by Celgene, whichCambrex Corporation, and the relevant Cambrex division was subsequently acquired by Lonza Group. This license is not expected to generate material revenue.
In 2003, we issued a non-exclusive license to StemCell Technologies, Inc. to make, use and sell certain proprietary mouse and rat neural stem cells and in 2004, relinquished itswe issued a non-exclusive license culture media for all mammalian neural stem cells, and to R&D Systems to make, use and sell certain stem cell expansion kits, also for the University of California, which then terminated the sublicenseresearch market. These licenses are not expected to StemCells for lack of diligence. Effective September 11, 2005, StemCells terminated the remaining sublicense.generate material revenue.
Competition
TheIn most instances, the targeted disease statesindications for our initial products in some instances currentlydevelopment have no effective long-term therapies.therapies at this time. However, we do expect that our initial products will have to compete with a variety of therapeutic products and procedures. MajorOther pharmaceutical and biotechnology companies currently offer a number of pharmaceutical products to treat lysosomal storage disorders,diseases, neurodegenerative and liver diseases, diabetes and other diseases for which our technologies may be applicable. Many pharmaceutical and biotechnology companies are investigating new drugs and therapeutic approaches for the same purposes, which may achieve new efficacy profiles, extend the therapeutic window for such products, alter the prognosis of these diseases, or prevent their onset. We believe that our products, when and if successfully developed, will compete with these products principally on the basis of improved and extended efficacy and safety and their overall economic benefit to the health care system. The market for therapeutic products that address degenerative diseases is large and competition is intense. We expect competition to increase. We believe that our most significant competitors will be fully integrated pharmaceuticalMany companies and more established biotechnology companies. Smaller companies may also be significant competitors, particularly through collaborative arrangements with large pharmaceutical or biotechnology companies. Many of these competitors have significant products approved or in development that could be competitive with our potential products. We expect competition to increase.
Competition for any stem and progenitor cell products that we may develop may be in the form of existing and new drugs, other forms of cell transplantation, ablative and simulative procedures, medical devices, and gene therapy. We believe that some of our competitors are also trying to develop stem and progenitor cell-based technologies. We expect that all of these products will compete with our potential stem and progenitor cell products based on efficacy, safety, cost and intellectual property positions.
We may also face competition from companies that have filed patent applications relating to the use of genetically modified cells to treat disease, disorder or injury. In the event our therapies should require the use of
11
such genetically modified cells, we may be required to seek licenses from these competitors in order to commercialize certain of our proposed products, and such licenses may not be granted.
If we develop products that receive regulatory approval, they would then have to compete for market acceptance and market share. For certain of our potential products, an important success factor will be the timing of market introduction of competitive products. This is a function of the relative speed with which we and our competitors can develop products, complete the clinical testing and approval processes, and supply commercial quantities of a product to market. These competitive products may also impact the timing of clinical testing and approval processes by limiting the number of clinical investigators and patients available to test our potential products.
We expect that all of these products will compete with our potential stem and progenitor cell-based products based on efficacy, safety, cost, and intellectual property positions. While we believe that these will be the primary competitive factors, will be product efficacy, safety, and the timing and scope of regulatory approvals, other factors include, in certain instances, obtaining marketing exclusivity under the Orphan Drug Act, availability of supply, manufacturing, marketing and sales expertise and capability, and reimbursement coverage, price, and patent and technology position.coverage.
Government Regulation
Our research and development activities and the future manufacturing and marketing of our potential products are, and will continue to be, subject to regulation for safety and efficacy by numerous governmental authorities in the United States and other countries.
16
U.S. Regulations
In the United States, pharmaceuticals, biologicals and medical devices are subject to rigorous regulation by the U.S. Food and Drug Administration or FDA, regulation.(FDA). The Federal Food, Drug and Cosmetic Act, as amended, and the Public Health Service Act, as amended, theapplicable FDA regulations, promulgated thereunder, and other Federalfederal and state statutes and regulations govern, among other things, the testing, manufacture, safety, efficacy, labeling, storage, export, record keeping, approval, marketing, advertising, and promotion of our potential products. Product development and approval within this regulatory framework takes a number of years and involves significant uncertainty combined with the expenditure of substantial resources. In addition, themany jurisdictions, both federal state, and other jurisdictionsstate, have restrictions on the use of fetal tissue.
FDA Marketing Approval
The steps required before our potential products may be marketed in the United States include:
| | | |
Steps | | Considerations |
| |
| 1. Preclinical laboratory and animal tests | | Preclinical tests include laboratory evaluation of the cells and the formulation intended for use in humans for quality and consistency.In vivostudies are performed in normal animals and specific disease models to assess the potential safety and efficacy of the cell therapy product. |
| | |
2. Submission to the FDA of an Investigational New Drug (IND) application (IND), which must become effective before U.S. human clinical trials may commence | | The IND is a regulatory document submitted to the FDA with the preclinical and manufacturing data, a proposed development plan and a proposed protocol for a study in humans. The IND becomes effective 30 days following receipt by the FDA, provided there are no questions, requests for delay or objections from the FDA. If the FDA has questions or concerns, it notifies the sponsor, and the IND will then be on clinical hold until the sponsor responds satisfactorily. In general an IND must become effective before U.S. human clinical trials may commence. |
12
| | | |
Steps | | Considerations |
|
3. Adequate and well-controlled humanHuman clinical trials to establish the safety and efficacy of the product | | Clinical trials involve the evaluation of a potential product under the supervision of a qualified physician, in accordance with a protocol that details the objectives of the study, the parameters to be used to monitor safety and the efficacy criteria to be evaluated. Each protocol is submitted to the FDA as part of the IND. The protocol for each clinical study must be approved by an independent Institutional Review Board (IRB) of the institution at which the study is conducted and the informed consent of all participants must be obtained. The IRB reviews the existing information on the product, considers ethical factors, the safety of human subjects, the potential benefits of the therapy, and the possible liability of the institution. The IRB is responsible for ongoing safety assessment of the subjects during the Clinical Investigation. |
| | clinical investigation. |
| | | Clinical development is traditionally conducted in three sequential phases, Phase 1, 2I, II and 3. |
| | III. |
| | | Phase 1I studies for a cell therapy product are designed to evaluate safety in a small number of subjects in a selected patient population by assessing adverse effects, and may |
17
| | |
Steps
| | Considerations
|
|
| | |
| | include multiple dose levels. This study may also gather preliminary evidence of a beneficial effect on the disease. |
| | | |
| | Phase 2 mayII studies typically involve studies in a larger, but still limited, patient population to determine biological and clinical effects of the investigational product and to identify possible adverse effects and safety risks of the product in the selected patient population. |
| | | |
| | Phase 3 trials would beIII studies are undertaken to conclusively demonstrate clinical benefit or effect in a statistically significant manner and to test further for safety within a broader patient population, generally at multiple study sites. |
| | |
| | | The FDA continually reviews the clinical trial plans and results and may suggest changes or may require discontinuance of the trials at any time if significant safety issues arise. |
| | |
4. Submission to the FDA of marketing authorization applicationsa Biologics Licensing Application (BLA) | | The results of the preclinical studies and clinical studies are submitted to the FDA in the form ofan application for marketing approval authorization applications. |
| | authorization. |
5. FDA approval of the application(s) prior to any commercial sale or shipment of the drug. Biologic product manufacturing establishments located in certain states also may be subject to separate regulatory and licensing requirementRegulatory Approval | | The testing and approval process will require substantial time, effort and expense. The time for approval is affected by a number of factors, including relative risks and benefits demonstrated in clinical trials, the availability of alternative treatments and the severity of the disease. Additional animal studies or clinical trials may be requested during the FDA review period, which might add to that time. FDA approval of the application(s) is required prior to any commercial sale or shipment of the therapeutic product. Biologic product manufacturing facilities located in certain states also may be subject to separate regulatory and licensing requirement. |
13
| | |
Steps | | Considerations |
|
| 6. Post-marketing studies | | After receiving FDA marketing approval for a product for an initial indication, further clinical trials may be required to gain approval for the use of the product for additional indications. The FDA may also require post-marketing testing and surveillance to monitor for adverse effects, which could involve significant expense, or the FDA may elect to grant only conditional approvals subject to collection of post-marketing data. In addition, the recently enacted FDA Amendments Act of 2007 provides the FDA with expanded authority over drug products after approval, including the authority to require post-approval studies and clinical trials, labeling changes based on new safety information, and compliance with risk evaluation and mitigation strategies approved by the FDA. |
After FDA approval for the product, the manufacturing and the initial indications, further clinical trials may be required to gain approval for the use of the product for additional indications. The FDA may also require unusual or restrictive post-marketing testing and surveillance to monitor for adverse effects, which could involve significant expense, or may elect to grant only conditional approvals.
FDA Manufacturing Requirements
Among the conditions for product licensure is the requirement that the prospective manufacturer’s quality control and manufacturing procedures conform to the FDA’s current good manufacturing practice (cGMP) requirements. Even after producta product’s licensure approval, theits manufacturer must comply with cGMP on a continuing basis, and what constitutes cGMP may change as the state of the art of manufacturing changes. Domestic manufacturing facilities are subject to regular FDA inspections for cGMP compliance, which are normally held at least every two years. Foreign manufacturing facilities are subject to periodic FDA inspections or inspections by the foreign regulatory authorities. Domestic manufacturing facilities may also be subject to inspection by foreign authorities.
Orphan Drug Act
The Orphan Drug Act provides incentives to drug manufacturers to develop and manufacture drugs for the treatment of diseases or conditions that affect fewer than 200,000 individuals in the United States. Orphan drug status can also be sought for treatments for diseases or conditions that affect more than 200,000 individuals in the United States if the sponsor does not realistically anticipate its product becoming profitable from sales in the United
18
States. We may apply for orphan drug status for certain of our therapies. Under the Orphan Drug Act, a manufacturer of a designated orphan product can seek tax benefits, and the holder of the first FDA approval of a designated orphan product will be granted a seven-year period of marketing exclusivity in the United States for that product for the orphan indication. While the marketing exclusivity of an orphan drug would prevent other sponsors from obtaining approval of the same compound for the same indication, it would not prevent other compounds or products from being approved for the same use including, in some cases, slight variations on the originally designated orphan product.
FDA Human Cell and Tissue Regulations
Our research and development is based on the use of human stem and progenitor cells. The FDA has initiated a risk-based approach to regulating Human Cell, Tissue and Cellular and Tissue-based (HCT/P) products and has published current Good Tissue Practice (cGTP) regulations. As part of this approach, the FDA has published final rules for registration of establishments that recover, process, store, label, package, or distribute HCT/P products or that screen or test the donor of HCT/P products, and for the listing of such products. In addition, the FDA has published rules for determining the suitability of donors of cells and tissue, the eligibility of the cells and tissues for clinical use and for current good tissue practice for manufacturers using them, which came into effect in May 2005. We cannot yet determine the full effects of this regulatory initiative, including precisely how it may affect the extent of regulatory obligations associated with multipotent stem cell research, and the manufacture and marketing of stem cell products.
14
Other Regulations
In addition to safety regulations enforced by the FDA, we are also subject to regulations under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, and other present and potential future foreign, Federal,federal, state, and local regulations.
International Law
Outside the United States, we will be subject to regulations that govern the import of drug products from the United States or other manufacturing sites and foreign regulatory requirements governing human clinical trials and marketing approval for our products. The requirements governing the conduct of clinical trials, product licensing, pricing, and reimbursements vary widely from country to country. In particular, the European Union, or EU, is revising its regulatory approach to biotechnology products, and representatives from the United States, Japan and the EU are in the process of harmonizing and making more uniform the regulations for the registration of pharmaceutical products in these three markets. This process increases uncertainty over regulatory requirements in our industry. Furthermore, human stem and progenitor cells may be regulated in the EU and other countries as transplant material or as a somatic cell therapy medicinal product, depending on the processing, indication and country.
Environment
We have made, and will continue to make, expenditures for environmental compliance and protection. Expenditures for compliance with environmental laws have not had, and are not expected to have, a material effect on our capital expenditures, results of operations or competitive position.
Reimbursement and Health Care Cost Control
Reimbursement for the costs of treatments and products such as ours from government health administration authorities, private health insurers and others, both in the United States and abroad, is a key element in the success of new health care products. Significant uncertainty often exists as to the reimbursement status of newly approved health care products.
The revenuesrevenue and profitability of some health care-related companies have been affected by the continuing efforts of governmental and third party payerspayors to contain or reduce the cost of health care through various means. PayersPayors are increasingly attempting to limit both coverage and the levels of reimbursement for new therapeutic products approved for marketing by the FDA, and are refusing, in some cases, to provide any coverage for uses of approved products for disease indications for which the FDA has not granted marketing approval. In certain foreign markets, pricing or profitability of prescription pharmaceuticals is subject to government control. In the United States, there have been a number of Federalfederal and state proposals to implement government control over health care costs.
19
Employees
As of December 31, 2006,2007, we had forty-six63 full-time employees, 17 of whom thirteen have Ph.D., M.D. or M.D.D.V.M. degrees. Thirty-five49 full-time employees work in research and development and laboratory support services. No employees are covered by collective bargaining agreements.
Scientific Advisory Board
Members of our Scientific Advisory Board (SAB) provide us with strategic guidance in regard to our research and product development programs, as well as assistance in recruiting employees and collaborators. Each SABScientific Advisory Board member has entered into a consulting agreement with us. These consulting agreements specify the compensation to be paid to the consultant and require that all information about our products and technology be kept confidential. All of the SABScientific Advisory Board members are employed by employers other than us and may have commitments to, or consulting or advising agreements with, other entities that limit their availability to us. The SABScientific Advisory Board members have generally agreed, however, for so long as they serve as consultants to us,
15
not to provide any services to any other entities that would conflict with the services the member provides to us. We are entitled to terminate the arrangements if we determine that there is such a conflict. Members of the SABour Scientific Advisory Board offer consultation on specific issues encountered by us as well as general advice on the directions of appropriate scientific inquiry for us. In addition, SABthe Scientific Advisory Board members assist us in assessing the appropriateness of moving our projects to more advanced stages. The following persons are members of our SAB:Scientific Advisory Board:
| | |
| | • | Irving L. Weissman, M.D., Chairman of our Scientific Advisory Board, is the Virginia and Daniel K. Ludwig Professor of Cancer Research, Professor of Pathology and Professor of Developmental Biology at Stanford University, Stanford California, and Director of the Stanford University Institute for Stem Cell Biology and Regenerative Medicine, as well asand Director of the Stanford Comprehensive Cancer Center.Center, all in Stanford, California. Dr. Weissman’s lab was responsible for the discovery and isolation of the first ever mammalian tissue stem cell, the hematopoietic (blood-forming) stem cell. Dr. Weissman was responsible for the formation of three stem cell companies, SyStemix, Inc., StemCells, Inc., and Cellerant, Inc. He is a member of the Board of Directors and Chairman of the Scientific Advisory Boards of StemCells and Cellerant. Dr. Weissman co-discovered the mammalian and human hematopoietic stem cells and the human neural stem cell. He has extended these stem cell discoveries to cancer and leukemia, discovering the leukemic stem cells in human and mouse acute or blast crisis myeloid leukemias, and has enriched the cancer stem cells in several human brain cancers as well as human head and neck squamous cell carcinoma. Past achievements of Dr. Weissman’s laboratory include identification of the states of development between stem cells and mature blood cells, the discovery and molecular isolation and characterization of lymphocyte and stem cell homing receptors, and identification of the states of thymic lymphocyte development. His laboratory at Stanford has developed accurate mouse models of human leukemias, and has shown the central role of inhibition of programmed cell death in that process. He has also established the evolutionary origins of pre-vertebrate stem cells, and identified and cloned the transplantation genes that prevent their passage from one organism to another. Dr. Weissman has been elected to the National Academy of Science, the Institute of Medicine of the National Academies, the American AssociationAcademy of the Arts and Sciences, the American Society of Microbiology, and several other societies..societies. He has received the Kaiser Award for Excellence in Preclinical Teaching, the Pasarow Foundation Award for Cancer Research, the California Scientist of the Year (2002), the Kovalenko Medal of the National Academy of Sciences, the Elliott Joslin Medal for Diabetes Research, the de Villiers Award for Leukemia Research, the Irvington Award for Immunologist of the Year, the Bass Award of the Society of Neurosurgeons, the New York Academy of Medicine Award for Medical Research, the Alan Cranston Award for Aging Research, the Linus Pauling Award for Biomedical Research, the E. Donnall Thomas Award for Hematology Research, the van Bekkum Award for Stem Cell Research, the Outstanding Investigator Award from the National Institutes of Health, and many other awards. |
| |
| | • | David J. Anderson, Ph.D., is Roger W. Sperry Professor of Biology, California Institute of Technology, Pasadena, California and Investigator, Howard Hughes Medical Institute. His laboratory was the first to isolate a multipotent, self-renewing, stem cell for the peripheral nervous system, the first to identify instructive signals that promote the differentiation of these stem cells along various lineages, and the first to accomplish a direct purification of peripheral neural stem cells from uncultured tissue. Dr. Anderson’s |
20
| | |
| | laboratory also was the first to isolate transcription factors that act as master regulators of neuronal fate. More recently, he has identified signals that tell a neural stem cell to differentiate to oligodendrocytes, the myelinating glia of the central nervous system, as well as factors for astrocyte differentiation. Dr. Anderson is a co-founder of StemCellsthe Company and a member of its SAB,our Scientific Advisory Board, and was a founding SAB member of the scientific advisory board of the International Society for Stem Cell Research. Dr. Anderson also serves on the SABscientific advisory board of Allen Institute for Brain Science. He has held a presidential Young Investigator Award from the National Science Foundation, a Sloan foundation Fellowship in Neuroscience, and has been Donald D. Matson lecturer at Harvard Medical School. He has received the Charles Judson Herrick Award from the American Association of Anatomy, the 1999 W. Alden Spencer Award in Neurobiology from Columbia University, and the Alexander von Humboldt Foundation Award. Dr. Anderson has been elected to the National Academy of Science and is a member of the American Academy of Arts and Sciences. |
16
| | |
| | • | Fred H. Gage, Ph.D., is Professor, Laboratory of Genetics, The Salk Institute for Biological Studies, La Jolla, California and Adjunct Professor, Department of Neurosciences, University of California, San Diego, California. Dr. Gage’s lab was the first to discover Neurogenesis in the adult human brain. His research focus is on the development of strategies to induce recovery of function following central nervous system (CNS) damage. Dr. Gage is a co-founder of StemCells and of BrainCells, Inc., and a member of the SABscientific advisory board of each. Dr. Gage also serves on the Scientific Advisory Board of Ceregene, Inc.Inc, and he is a founding member of the scientific advisory board of the International Society for Stem Cell Research. Dr. Gage has been the recipient of numerous awards, including the 1993 Charles A. Dana Award for Pioneering Achievements in Health and Education, the Christopher Reeves Medal, the Decade of the Brain Medal, the Max-Planck research Prize, and the Pasarow Foundation Award. Professor Gage is a member of the Institute of Medicine, a member of the National Academy of Science, and a Fellow of the American Academy of Arts and Science. |
21
AVAILABLE INFORMATIONAvailable Information
Our principal executive offices are located at 3155 Porter Drive, Palo Alto, CA 94304, and our main telephone number is(650) 475-3100. InvestorsThe following information can obtain access to this annual report onForm 10-K, our quarterly reports onForm 10-Q, our current reports onForm 8-K and all amendments to these reports,be obtained free of charge onthrough our website athttp://www.stemcellsinc.com as soon as reasonably practicable after such filings are electronically filed with the SEC. www. stemcellsinc.com or by sending ane-mail message to irpr@stemcellsinc.com:
| | |
| • | our annual report onForm 10-K, quarterly reports onForm 10-Q, current reports onForm 8-K, and all amendments to these reports as soon as reasonably practicable after such material is electronically filed with the Securities and Exchange Commission; |
|
| • | our policies related to corporate governance, including StemCells’ Code of Conduct and Ethics and Procedure for Submission of Complaints; and |
|
| • | the charters of the Audit Committee, the Compensation & Stock Option Committee and the Corporate Governance & Nominating Committee of our Board of Directors. |
The public may read and copy any material we file with the SEC at the SEC’s Public Reference Room at 450 Fifth100 F Street, N.W.N.E., Washington D.C., 20549. The public may obtain information on the operations of the Public Reference Room by calling the SEC at1-800-SEC-0330. The SEC maintains an Internet site,http://www.sec.gov, which contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
| |
ItemITEM 1A. | Risk FactorsRISK FACTORS |
This annual report ofForm 10-K contains forward looking statements that involve risks and uncertainties. Our business, operating results, financial performance, and share price may be materially adversely affected by a number of factors, including but not limited to the following risk factors, any one of which could cause actual results to vary materially from anticipated results or from those expressed in any forward-looking statements made by us in this annual report ofForm 10-K or in other reports, press releases or other statements issued from time to time. Additional factors that may cause such a difference are set forth elsewhere in this annual report ofForm 10-K.
Risks Related to our Business
Any adverse development in the initialrelating to our HuCNS-SC product candidate, such as a significant clinical trial for our stem cell technologyfailure, could substantially depress our stock price and prevent us from raising the capital we will need to further develop our stem cell technology.additional capital.
To an unusual extent,At present our ability to progress as a company is significantly dependent on a single product candidate, our HuCNS-SC cells (purified human neural stem cells), and on a single early stage clinical trial.trial, our Phase I clinical trial for neuronal ceroid lipofuscinosis (NCL, also often referred to as Batten disease). Any clinical, regulatory or other development that preventssignificantly delays or delaysprevents us from conducting our initial clinicalcompleting this trial, for NCL (Batten disease), or any material safety issue or adverse side effect to any patient that occurs during thestudy participant in this trial, or the failure of this initial trial to enroll patients and proceed to completion as anticipated or to show the results expected by investors, would likely significantly depress our stock price significantly and could prevent us from raising the substantial additional capital we will requireneed to further develop our stem cellcellular technologies. Moreover, any material adverse occurrence in our first clinical trial for Batten disease could substantially impair our ability to initiate clinical trials to test our HuCNS-SC cells in patients with spinal cord injuries, myelin disorders or other potential indications. This, in turn, could
17
adversely impact our ability to raise additional capital and pursue our planned research and development efforts in both our CNS and liver programs.
Our financial situation is precarious and, based on currently estimated operating expenses, our existingWe have limited capital resources and we may not be sufficientobtain the significant additional capital needed to fundsustain our operations beyond the next eighteen months.research and development efforts.
We have incurred significant operating losses and negative cash flows since inception. We have not achieved profitability and may not be able to realize sufficient revenues to achieve or sustain profitability in the future. We do not expect to be profitable in the next several years, but rather expect to incur additional and increasing operating losses. We have limited liquidity and capital resources and must obtain significant additional capital resources in order to sustain our product development efforts, and for acquisition ofacquire businesses, technologies and intellectual property rights which may be important to our business, continue preclinical and clinical testing of our anticipatedinvestigative products, pursuit ofpursue regulatory approvals, acquisition ofacquire capital equipment, laboratory and office facilities, establishment ofestablish production capabilities, maintainingmaintain and enforcingenforce our intellectual property portfolio, and support our general and administrative expenses and other working capital requirements. We rely on cash reserves and proceeds from equity and debt offerings, proceeds from the transfer, license, lease, or sale of our intellectual property rights, equipment, facilities, or investments, and government grants and funding from collaborative arrangements, if obtainable, to fund our operations. If we exhaust our cash reserves and are unable to realize adequate financing, we may be unable to meet operating obligations and be required to initiate bankruptcy proceedings. Our existing capital resources may not be sufficient to fund our operations beyond the next eighteen months.
We intend to pursue opportunities to obtainfor additional financingfundraising in the future through equity andor debt financings, corporate alliances or combinations, grants andor collaborative research arrangements. Thearrangements, or any combination of these. However, the source, timing and availability of any future financingfundraising will depend principally upon market conditions, interest rates and, more specifically, on our progress in our exploratory,research, preclinical and future clinical development programs. Funding may not be available when needed — at all or on terms acceptable to us. Lack of necessary fundsWhile we actively manage our programs and resources in order to conserve cash between fundraising opportunities, our existing capital resources may require usnot be sufficient to fund our operations beyond the next twelve to eighteen months. If we exhaust our cash reserves and are unable to realize adequate additional fundraising, we may be unable to meet operating obligations and be required to initiate bankruptcy proceedings or delay, scale back or eliminate some or all of our research and product development programsand/or our capital expenditures or to license our potential products or technologies to third parties.programs.
Our product development programs are based on novel technologies and are inherently risky.
We are subject to the risks of failure inherent in the development of products based on new technologies. The novel nature of thethese therapies creates significant challenges in regardsregard to product development and optimization, manufacturing, government regulation, third party reimbursement, and market acceptance. For example, the FDA
22
has relatively little experience with stem cell-based therapeutics, and the pathway to regulatory approval for cell-based therapies, including our product candidates, may accordingly be more complex and lengthy than the pathway for new conventional drugs. These challenges may prevent us from developing and commercializing products on a timely or profitable basis or at all.
Our technology is at an early stage of discovery and development, and we may fail to develop any commercially acceptable or profitable products.
We have incurred significant operating losses and negative cash flows since inception. We have not achieved profitability and may not be able to realize sufficient revenue to achieve or sustain profitability in the future. We have yet to develop any products that have been approved for marketing.marketing and we do not expect to become profitable within the next several years, but rather expect to incur additional and increasing operating losses. Before we may marketcommercializing any medical product, we mustwill need to obtain regulatory approval from the FDA andor from equivalent foreign agencies after conducting extensive preclinical studies and clinical trials that demonstrate that ourthe product candidates arecandidate is safe and effectiveeffective. Except for each disease for which we seek approval. We have no experience in conducting clinical trials prior to the current NCL trial currently being conducted at the Oregon Health & Science University (OHSU)., we have had no experience conducting human clinical trials. We expect that none of our cell-based therapeutic product candidates will be commercially available for several years, if at all.
Our programs are still at the preclinical phase for our human liver engrafting cell, and at the discovery phase for our candidate human pancreas stem cell. While the U.S. Food and Drug Administration (FDA)FDA has permitted us to go forward withinitiate our Phase I clinical trial of our proprietary neural stem cell therapyHuCNS-SC product — HuCNS SC —candidate in NCL, and the Institutional Review Board of OHSU has approved the protocol and we have completed dosing the six patients planned for the trial, there can be no assurance that the clinical investigatorstrial will be able to identify suitable candidates for completion of the trial (which is intended to enroll six subject, only of whom two have received transplants so far),completed or ofresult in a successful outcome of the trial if candidates are enrolled. We may fail to discover the stem cells we are seeking, to develop any products, to obtain regulatory approvals, to enter clinical trials, or to commercialize any products.outcome. We may elect to delay or discontinue preclinicalother studies or clinical trials based on unfavorable results. Any product using stem cell technologydeveloped from or based on cellular technologies may fail to:
| | |
| | • | survive and persist in the desired location; |
18
| | |
| | • | provide the intended therapeutic benefits;benefit; |
| |
| | • | properly integrateengraft into existing tissue in the desired manner; or |
| |
| | • | achieve therapeutic benefits equal to, or better than, the standard of treatment at the time of testing. |
In addition, our products may cause undesirable side effects. Results of preclinical research in animals may not be indicative of thefuture clinical results that will be obtained in later stages of preclinical or clinical research. Ifhumans.
Ultimately if regulatory authorities do not approve our products or if we fail to maintain regulatory compliance, we would be unable to commercialize our products, and our business and results of operations would be harmed. Even if we do succeed in developing products, we will face many potential obstacles such as the need to develop or obtain manufacturing, marketing and distribution capabilities. Furthermore, because transplantation of stem cells is a new form of therapy, the marketplace may not accept any products we may develop. If we do succeed in developing products, we will face many potential obstacles such as the need to obtain regulatory approvals and to develop or obtain manufacturing, marketing and distribution capabilities. In addition, we will face substantial additional risks such as product liability claims.
Moreover, because our cell-based therapeutic products will be derived from tissue of individuals other than the patient (that is, they will be “non-self” or “allogeneic” transplant products), patients will likely require the use of immunosuppressive drugs such as cyclosporine, FK506, or others to prevent rejection of the cells.drugs. While immunosuppression is now standard in connection with allogeneic transplants of various kinds, such as heart or liver transplants, long-term maintenance on immunosuppressive drugs can produceresult in complications that includesuch as infection, cancer, cardiovascular disease, and renal dysfunction and other side effects depending upon which immunosuppressive regimen is employed.dysfunction. Immunosuppression is currently being tested with our therapeutic product candidate in our Phase I clinical trial for NCL (Batten disease).NCL.
Our success will depend in large part on our ability to develop and commercialize products that treat diseases other than Batten disease.neuronal ceroid lipofuscinosis (Batten disease).
Although we have initially focused on evaluating our neural stem cell product for the treatment of infantile and late infantile NCL (Batten disease), this disease is rare and the market for treating this disease is small.
23
Accordingly, even if we obtain marketing approval for our HuCNS-SC product candidate for infantile and late infantile NCL, in order to achieve profitability, if at all, we will likely need to obtain approval for HuCNS-SC and other potential products to treat additional diseases that present more significant market opportunities.
Acquisitions of companies, businesses or technologies may substantially dilute our stockholders and increase our operating losses.
We may make acquisitions of businesses, technologies or intellectual property rights or otherwise expand our business activities in ways we believe to be necessary, useful or complementary to our current product development efforts and cell-based therapeutics business. Any such acquisition or change in business activities may require assimilation of the operations, products or product candidates and personnel of the acquired business and the training and integration of its employees, and could substantially increase our operating costs, without any offsetting increase in revenue. Acquisitions may not provide the intended technological, scientific or business benefits and could disrupt our operations and divert our limited resources and management’s attention from our current operations, which could harm our existing product development efforts. We would likely issue equity securities to pay for any future acquisitions. The issuance of equity securities for an acquisition could be substantially dilutive to our stockholders. In addition, our results of operations may suffer because of acquisition-related costs or the post-acquisition costs of funding the development of an acquired technology or product candidates or operation of the acquired business, or due to amortization or impairment costs for acquired goodwill and other intangible assets. Any investment made in, or funds advanced to, a potential acquisition target could also significantly adversely affect our results of operation and could further reduce our limited capital resources. Any acquisition or action taken in anticipation of a potential acquisition or other change in business activities could substantially depress the price of our stock.
We have payment obligations resulting from real property owned or leased by us in Rhode Island, which diverts funding from our stem cellcell-based therapeutics research and development.
Prior to our reorganization in 1999 and the consolidation of our business in California, we carried out our former encapsulated cell therapy programs in Lincoln, Rhode Island, where we also had our administrative offices. Although we have vacated the Rhode Island facilities, we remain obligated to make lease payments and payments
19
for operating costs for our former science and administrative facility, which we have leased through June 30, 2013. These costs, beforesub-tenant rental income, amounted to approximately $1,550,000$1,523,000 in 2006;2007; our rent payments will increase over the term of the lease, and our operating costs may increase as well. In addition to these costs of our former science and administrative facility, we are obligated to make debt service payments and payments for operating costs of approximately $450,000$400,000 per year for our former encapsulated cell therapy pilot manufacturing facility, which we own. We have currently subleased a portion of the science and administrative facility, and we are seeking to sublease the remaining portion, but we cannot be sure that we will be able to keep any part of the facility subleased for the duration of our obligation. We haveare currently subleasedseeking to sublease the entire pilot manufacturing facility, to a privately-held biotechnology company, but may not be able to sublease or sell the facility in the future once the current sublease agreements expire.future. These continuing costs significantly reduce our cash resources and adversely affect our ability to fund further development of our stem cell technology.cellular technologies. In addition, changes in real estate market conditions and assumptions regarding the length of time it may take us to either fully sublease, assign or sell our remaining interest in the our former research facility in Rhode Island may have a significant impact on and cause large variations in our quarter to quarter results of operations. In 1999, in connection with exiting our former research facility in Rhode Island, we created a reserve for the estimated lease payments and operating expenses related to it. The reserve is periodically re-evaluated and adjusted based on assumptions relevant to real estate market conditions and the estimated time until we can either, fully sublease, assign or sell our remaining interests in the property. At December 31, 2006,2007, the reserve was $6,750,000. In 2006 and 2005,$6,143,000. For the year 2007, we incurred $1,295,000 and $1,079,000$1,420,000 in operating expenses net ofsub-tenant income for this facility. Expenses for this facility will fluctuate based on changes in tenant occupancy rates and other operating expenses related to the lease. Even though it is our intent to sublease, assign, sell, or otherwise divest ourselves of our interests in the facility at the earliest possible time, we cannot determine with certainty a fixed date by which such events will occur. In light of this uncertainty, based on estimates, we will periodically re-evaluate and adjust the reserve, as necessary, and we may make significant adverse adjustments to the reserve in the future.
We may need but failbe unable to obtain partners to support our stem cellcell-based therapeutic product development efforts andwhen needed to commercialize our technology.technologies.
Equity and debt financings alone may not be sufficient to fund the cost of developing our stem cellcellular technologies, and we may need to rely on partnering or other arrangements to provide financial support for our stem cellcellular discovery and development efforts. In addition, in order to successfully develop and commercialize our technology,technologies, we may need to enter into a wide variety ofvarious arrangements with corporate sponsors, pharmaceutical companies, universities, research groups, and others. While we have engaged, and expect to continue to engage, in discussions regarding such arrangements, we have not reached any agreement, and we may fail to obtain any such agreement on terms acceptable to us. Even if we enter into such arrangements, we may not be able to satisfy our obligations under them or renew or replace them after their original terms expire. Furthermore, these arrangements may require us to grant rights to third parties, such as exclusive marketing rights to one or more products, may require us to issue securities to our collaborators and may contain other terms that are burdensome to us. If we enter collaboration agreements and any of our collaborators terminates its relationship with us or fails to perform its obligationsresult in a timely manner, the development or commercialization of our technology and potential products may be adversely affected.
24
Because the patient population for NCL, or Batten disease, is very small, we may encounter difficulties in enrolling subjectsdecrease in our first clinical trial.
The first clinical application we are pursuing — NCL (also known as Batten disease) — has a very small patient population. From this small population, we must locate and enroll patients that satisfy the specific enrollment criteria for our Phase I clinical trial for this indication. This clinical trial may be delayed significantly or terminated if we are unable to enroll a sufficient number of qualified patients.
We have a history of operating losses, and we may fail to obtain revenues or become profitable.
We expect to continue to incur substantial operating losses in the future in order to conduct our research and development activities, and, if those activities are successful, to fund clinical trials and other expenses. These expenses include the cost of acquiring technology, product testing, acquiring regulatory approvals, establishing production, marketing, sales and distribution programs and administrative expenses. We have not earned any revenues from sales of any product. All of our past revenues have been derived from, and any revenues we may obtain for the foreseeable future are expected to be derived from, license agreements, cooperative agreements, research grants, investments and interest on invested capital. We currently have no cooperative agreements or research grants and we may not obtain any such agreements or additional grants in the future or receive any revenues from them.stock price.
If we are unable to protect our patents and proprietary rights, our business, financial condition and results of operations willmay be materially harmed.
We either own or license a number of patents and pending patent applications related to various stem and progenitor cells, andincluding human neural stem cell cultures, as well as methods of deriving and using them, including human neural stem cell cultures. Patentthem. The process of obtaining patent protection for products such as those we propose to develop is highly uncertain and involves complex and continually evolving factual and legal questions. The governmental authorities that consider patent applications can deny or significantly reduce the patent coverage requested in an application either before or after issuing the patent. Consequently, we do not know whether any of our pending applications will result in the issuance of patents, if any existing or future patents will provide sufficient protection or significant commercial advantage or if others will circumvent these patents. We cannot be certain that we were the first to discover the inventions covered by each of our pending patent applications or that we were the first to file patent applications for such inventions because patent applications are secret until they are published, and because publication of discoveries in the scientific or patent literature often lags behind actual discoveries. Patents may not issue from our pending or future patent applications or, if issued, may not be of commercial benefit to us. In addition, our patents may not afford us adequate protection from competing products. Third parties may challenge our patents or governmental authorities may declare them invalid or reduce their scope. In the event that a third party has also filed a patent application relating to inventions claimed in our patent applications, we may have to participate in proceedings to determine priority of invention. Even if a patent issues, a court could decide that the patent was issued invalidly. Because patents issue for a limited term, our patents may expire before we utilize them profitably. Our most important patents begin to expire in 2015. UnderFor example, under the procedures of the European Patent Office, third parties may oppose our issued European patents during the relevant opposition period. These proceedings and oppositions could result in substantial uncertainties and cost for us, even if the eventual outcome is favorable to us, and the outcome might not be favorable to us. One party has opposed twoIn the United States, third parties may seek to invalidate issued patents through a U.S. PTO reexamination process or through the courts. In addition, changes to the laws protecting intellectual property rights could adversely impact the perceived or actual value of our granted European patents. Both oppositions were heardCompany. Consequently, we do not know whether any of our pending applications will result in 2005, andthe issuance of patents, whether any of our issued patents will be invalidated or restricted, whether any existing or future patents will provide sufficient protection or significant
20
commercial advantage, or whether others will circumvent these patents, whether or not lawfully. In addition, our patents may not afford us adequate protection from competing products. Moreover, because patents issue for a limited term, our patents may expire before we can commercialize a product covered by the issued patent claims or before we can utilize the patents were maintainedprofitably. Some of our most important patents begin to expire in somewhat altered form. Although the time for appeal has now run, and the .U.S. counterparts to these patents are not subject to opposition, since that procedure does not exist under U.S. patent law, other types of proceedings may be available to third parties to contest our U.S. patents. See “Item 1. Business — Patents, Proprietary Rights and Licenses” and “Item 3. Legal proceedings.”2015.
If we learn of third parties who infringe our patent rights, we may needdecide to initiate legal proceedings to enforce these rights. Patent litigation is inherently unpredictable and highly risky and may result in unanticipated challenges to the validity or enforceability of our patentintellectual property, which could result in the loss of these rights. TheseIn general litigation proceedings may entail significant costs,are also very costly and these thirdthe parties we bring actions against may have significantly greater financial resources than us.our own. We may not prevail in these proceedings. See “Item 1. Business — Patents, Proprietary Rights and Licenses” and “Item 3. Legal proceedings.”
25
Proprietary trade secrets and unpatented know-how are also important to our research and development activities. We cannot be certain that others will not independently develop the same or similar technologies on their own or gain access to our trade secrets or disclose such technology or that we will be able to meaningfully protect our trade secrets and unpatented know-how. We require our employees, consultants and significant scientific collaborators and sponsored researchers to execute confidentiality agreements upon the commencement of an employment or consulting relationship with us. These agreements may, however, fail to provide meaningful protection or adequate remedies for us in the event of unauthorized use, transfer or disclosure of such information or technology.
If others are first to discover and patent the stem cells we are seeking to discover, we could be blocked from further work on those stem cells.
Because the first person or entity to discover and obtain a valid patent to a particular stem or progenitor cell may effectively block all others, it will be important for us or our collaborators to be the first to discover any stem cell that we are seeking to discover. Failure to be the first could prevent us from commercializing all of our research and development affected by that patent.
If we are unable to obtain necessary licenses to third-party patents and other rights, we may not be able to commercially develop our expected products.
A number of pharmaceutical, biotechnology and other companies, universities and research institutions have filed patent applications or have received patents relating to cell therapy, stem and progenitor cells and other technologies potentially relevant to, or necessary for, our expected products. We cannot predict which, if any, of thethese applications will issue as patents or how many of these issued patents will be found valid and thereenforceable. There may also be existing issued patents of which we are currently unaware of which would be infringed by the commercialization of one or more of our product candidates would infringe.candidates. If third party patents or patent applications contain valid claims that our technology infringes upon their technology,so, we may be prevented from commercializing that technologythese products unless the third party is willing to grant a license to us. We may be unable to obtain licenses to the relevant patents at a reasonable cost, if at all, and may also be unable to develop or obtain alternative non-infringing technology. If we are unable to obtain such licenses or develop non-infringing technology at a reasonable cost, our business could be significantly harmed. Also, any infringement lawsuits commenced against us may result in significant costs, divert our management’s attention and result in an award against us for substantial damages.damages, or potentially prevent us from continuing certain operations.
We are aware of intellectual property rights held by third parties that relate to products or technologies we are developing. For example, some aspects of our stem cellcell-based therapeutic product candidates involve the use of growth factors, antibodies and other reagents that may, in certain cases, be the subject of third party rights. Before we commercialize any product using these growth factors, antibodies or reagents, we may need to obtain license rights from third parties or use alternative growth factors, antibodies and reagents that are not then the subject of third party patent rights. We currently believe that the commercialization of our products as currently planned will not infringe these third party rights, or, alternatively, that we will be able to obtain necessary licenses or otherwise use alternatealternative non-infringing technology. However, third parties may nonetheless bring suit against us claiming infringement. If we are unable to prove that our technology does not infringe their patents, or if we are unable to obtain necessary licenses or otherwise use alternative non-infringing technology, we may not be able to commercialize any products. Also, if we use alternative non-infringing technology, we may need to demonstrate comparability in subsequent clinical trials.
We have obtained rights from companies, universities and research institutions to technologies, processes and compounds that we believe may be important to the development of our products. These licensors, however, may cancel our licenses or convert them to non-exclusive licenses if we fail to use the relevant technology or otherwise breach these agreements. Loss of these licenses could expose us to the risk that our technology infringes the rights of third parties. We can give no assurance that any of these licenses will provide effective protection against our competitors.
2621
We compete with companies that have significant advantages over us.
The market for therapeutic products to treat diseases of, or injuries to, the central nervous system (CNS) is large and competition is intense. The majority of the products currently on the market or in development are small molecule pharmaceutical compounds. Many of the world’s largecompounds, and many pharmaceutical companies including Merck, Pfizer, Abbott, Bristol-Myers Squibb, Novartis and GlaxoSmithKline, have made significant commitments to the CNS field. AnyWe believe cellular therapies, if proven safe and effective, will have unique properties that will make them desirable over small molecule drugs, none of which currently replace damaged tissue. However, any cell-based therapytherapeutic to treat diseases of, or injuries to, the CNS is likely to face intense competition from the small molecule sector. In addition, a number of biotechnology companies with resources far greater than ours may also emergesector, biologics, as competitors. These include Genzyme, Amgen, Cephalon, Shire Pharmaceuticals, BioMarin, Celgene, Biogen Idec, and Titan Pharmaceuticals/Schering AG. Finally, we alsowell as medical devices. We expect to compete with smaller biotechnologya host of companies, such as NeuralStem, Geron, NeuroNova, ReNeuron, and ES Cell International, some of which are privately owned.
We believe that our human neural stem cells may have application to many or most of the Lysosomal Storage Diseases (“LSDs”) with CNS involvement. We are currently conducting a Phase I clinical trial at Doernbecher Children’s Hospital at Oregon Health & Safety University to treat infantileowned and late infantile NCL (also known as Batten disease), which are among the LSDs that affect the CNS. There can be no assurance that the trial will demonstrate either safety or efficacy of our HuCNS-SC. There are, so far as we know, no approved therapies for NCL or any of the other CNS-specific LSDs, but other companies, including Genzyme, BioMarin, and Shire, have products approved to treat peripheral aspects of some of the other LSDs, and other products are in clinical trials.which have resources far greater than ours.
In the liver field, there are no broad-based therapies for the treatment of liver disease at present. The primary therapy is liver transplantation, which is limited by the availability of matched donor organs. Liver-assist devices, when and if they become available, could also be used to help patients while they await suitably matched organs for transplantation. Liver transplantation may remain the standard of care even if we successfully develop a cellular therapy. In addition, new therapies may become available before we successfully develop a cell-based therapy for liver disease.
In the field of diabetes, a number of major companies currently market products for the treatment of diabetes and are also engaged in the research and development of new therapies. Such companies include Eli Lilly, Novo Nordisk, J&J, Amylin, ViaCell, and Serono. Consequently, should we successfully develop a cell-based therapy for diabetes, we would expect to face severe competition from these and similar companies.
Development of our technologytechnologies is subject to, and restricted by, extensive government regulation, which could impede our business.
Our research and development efforts, as well as any future clinical trials, and the manufacturing and marketing of any products we may develop, will be subject to, and restricted by, extensive regulation by governmental authorities in the United States and other countries. The process of obtaining U.S. Food and Drug AdministrationFDA and other necessary regulatory approvals is lengthy, expensive and uncertain. We or our collaborators may fail to obtain the necessary approvals to commence or continue clinical testing or to manufacture or market our potential products in reasonable time frames, if at all. In addition, the U.S. Congress and other legislative bodies may enact regulatory reforms or restrictions on the development of new therapies that could adversely affect the regulatory environment in which we operate or the development of any products we may develop.
We base our research and development on the use of human stem and progenitor cells obtained from human tissue, including fetal tissue. The federal and state governments and other jurisdictions impose restrictions on the use of fetal tissue, including those incorporated in the recent federal current Good Tissue Practice, or cGTP, regulations. These regulatory and other constraints could prevent us from obtaining cells and other components of our products in the quantity or quality needed for their development or commercialization. These restrictions change from time to time and may become more onerous. Additionally, we may not be able to identify or develop reliable sources for the cells necessary for our potential products — that is, sources that follow all state and federal guidelines for cell procurement. Certain components used to manufacture our stem and progenitor cell product candidates will need to be manufactured in compliance with the FDA’s Good Manufacturing Practices, or cGMP. Accordingly, we will need to enter into supply agreements with companies that manufacture these components to cGMP standards.
27
Although we do not use embryonic stem cells, government regulation and threatened regulation of embryonic tissue may lead top researchers to leave the field of stem cell research, or the country, in order to assure that their careers will not be impeded by restrictions on their work. Similarly, these factors may induce the best graduate students to choose other fields less vulnerable to changes in regulatory oversight, thus exacerbating the risk, discussed below, that we may not be able to attract and retain the scientific personnel we need in face of the competition among pharmaceutical, biotechnology and health care companies, universities and research institutions for what may become a shrinking class of qualified individuals. In addition, we cannot be certain that constraints on the use of embryonic stem cells will not be extended to use of fetal stem cells. Moreover, it is possible that concerns regarding research using embryonic stem cells will negatively impact our stock price and our ability to attract collaborators and investors.
We may apply for status under the Orphan Drug Act for some of our therapies to gain a seven-year period of marketing exclusivity for those therapies. The U.S. Congress in the past has considered, and in the future again may consider, legislation that would restrict the extent and duration of the market exclusivity of an orphan drug. If enacted, such legislation could prevent us from obtaining some or all of the benefits of the existing statute even if we were to apply for and obtain orphan drug status with respect to a potential product.
We are dependent on the services of key personnel.
We are highly dependent on the principal members of our management and scientific staff and some of our outside consultants, including the members of our scientific advisory board, our chief executive officer, our chief operating officer, our vice presidents, and the heads of key departments or functions within the company. Although we have entered into employment agreements with some of these individuals, they may terminate their agreements at any time. In addition, our operations are dependent upon our ability to attract and retain additional qualified scientific and management personnel. We may not be able to attract and retain the personnel we need on acceptable terms given the competition for experienced personnel among pharmaceutical, biotechnology and health care companies, universities and research institutions.
22
Our activities involve hazardous materials and experimental animal testing; improper handling of these animals and materials by our employees or agents could expose us to significant legal and financial penalties.
Our research and development activities involve the controlled use of test animals as well as hazardous chemicals and potentially hazardous biological materials such as human tissue and animals.tissue. Their use subjects us to environmental and safety laws and regulations such as those governing laboratory procedures, exposure to blood-borne pathogens, use of laboratory animals, and the handling of biohazardous materials. Compliance with current or future laws and regulations may be expensive and the cost of compliance could adversely affect us.
Although we believe that our safety procedures for using, handling, storing, and disposing of hazardous and potentially hazardous materials comply with the standards prescribed by California and federal regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. In the event of such an accident or of any violation of these or future laws and regulations, state or federal authorities could curtail our use of these materials; we could be liable for any civil damages that result, the cost of which could be substantial; and we could be subjected to substantial fines or penalties. In addition, any failure by us to control the use, disposal, removal, or storage, or to adequately restrict the discharge, or to assist in the cleanup, of hazardous chemicals or hazardous, infectious or toxic substances could subject us to significant liability. Any such liability could exceed our resources and could have a material adverse effect on our business, financial condition and results of operations. Moreover, an accident could damage our research and manufacturing facilities and operations and result in serious adverse effects on our business.
The manufacture, development, manufacturing and commercialization of stem cellcell-based therapeutic products expose us to product liability claims, which could lead to substantial liability.
By developing and, ultimately, commercializing medical products, we are exposed to the risk of product liability claims. Product liability claims against us could entailresult in substantial litigation costs and damage awards
28
against us. We have obtained liability insurance that covers our clinical trials, and we will need to increase our insurance coverage if and when we begin commercializing products. We may not be able to obtain insurance on acceptable terms, if at all, and the policy limits on our insurance policies may be insufficient to cover our liability.
SinceThe manufacture of cell-based therapeutic products is novel, highly regulated, critical to our business, and dependent upon specialized key materials.
The proliferation and manufacture of cell-based therapeutic products are complicated and difficult processes, dependent upon substantial know-how and subject to the need for continual process improvements to be competitive. Our manufacturing experience is limited and the technologies are comparatively new. In addition, our ability toscale-up manufacturing to satisfy the requirements of our planned clinical trials is uncertain. Manufacturing disruptions may occur and despite efforts to regulate and control all aspects of manufacturing, the potential for human or system failure remains. Manufacturing irregularities or lapses in quality control could have a serious adverse effect on our reputation and business, which could cause a significant loss of stockholder value. Many of the materials that we use to prepare our cell-based products are highly specialized and available from a limited number of suppliers. At present, some of our material requirements are single sourced, and the loss of one or more of these sources may adversely affect our business if we are unable to obtain alternatives or alternative sources at all or upon terms that are acceptable to us.
Because health care insurers and other organizations may not pay for our products or may impose limits on reimbursements, our ability to become profitable could be reduced.adversely affected.
In both domestic and foreign markets, sales of potential products are likely to depend in part upon the availability and amounts of reimbursement from third-party health care payor organizations, including government agencies, private health care insurers and other health care payors, such as health maintenance organizations and self-insured employee plans. There is considerable pressure to reduce the cost of therapeutic products, and governmentproducts. Government and other third party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for new therapeutic products and by refusing, in some cases, to provide any coverage for
23
uses of approved products for disease indications for which the U.S. Food and Drug AdministrationFDA or other relevant authority has not granted marketing approval. Moreover, in some cases, government and other third party payors have refused to provide reimbursement for uses of approved products for disease indications for which the FDA or other relevant authorityhasgranted marketing approval. Significant uncertainty exists as to the reimbursement status of newly approved health care products or novel therapies such as ours. Even if we obtain regulatory approval to market our products, we can give no assurance that reimbursement will be provided by such payors at all or without substantial delay or, if such reimbursement is provided, that the approved reimbursement amounts will be sufficient to enable us to sell products we develop on a profitable basis. Changes in reimbursement policies could also adversely affect the willingness of pharmaceutical companies to collaborate with us on the development of our stem cell technology.cellular technologies. In certain foreign markets, pricing or profitability of prescription pharmaceuticals is subject to government control. We also expect that there will continue to be a number of federal and state proposals to implement government control over health care costs. Efforts at health care reformto change regulatory and reimbursement standards are likely to continue in future legislative sessions. We do not know what legislative proposals federal or state governments will adopt or what actions federal, state or private payerspayors for health care goods and services may take in response to health care reformsuch proposals or legislation. We cannot predict the effect of government control and other health care reforms may havereimbursement practices on our business.
We have limited liquidity and capital resources and may not obtain the significant capital resources we will need to sustain our research and development efforts.
We have limited liquidity and capital resources and must obtain substantial additional capital to support our research and development programs, for acquisition of technology and intellectual property rights and, to the extent we decide to undertake these activities ourselves, for preclinical and clinical testing of our anticipated products, pursuit of regulatory approvals, establishment of production capabilities, maintaining and enforcing our intellectually property portfolio, establishment of marketing and sales capabilities and distribution channels, and general administrative expenses. If we do not obtain the necessary capital resources, we may have to delay, reduce or eliminate some or all of our research and development programs or license our technology or any potential products to third parties rather than commercialize them ourselves. We intend to pursue our needed capital resources through equity and debt financings, corporate alliances, grants and collaborative research arrangements. We may fail to obtain the necessary capital resources from any such sources when needed or on terms acceptable to us. Our ability to complete successfully any such arrangements will depend upon market conditions and, more specifically, on continued progress in our research and development efforts.
Ethical and other concerns surrounding the use of stem or progenitor-based cell therapy may negatively affect regulatory approval or public perception of our product candidates, which could reduce demand for our products.products or depress our stock price.
The use of stem cells for research and therapy has been the subject of debate regarding related ethical, legal and social issues. Although these concerns have mainly been directed to the use of embryonic stem cells, which we do not use, the distinction between embryonic and non-embryonic stem cells is frequently overlooked; moreover, our use of human stem or progenitor cells from fetal sources might raise these or similar concerns. Negative public attitudes toward stem cell therapy could result in greater governmental regulation of stem cell therapies, which could harm our business. For example, concerns regarding such possible regulation could impact our ability to attract collaborators and investors. Also, existing regulatory constraints on the use of embryonic stem cells may in the future be extended to use of fetal stem cells, and these constraints might prohibit or restrict us from conducting research or
29
from commercializing products. Government regulationExisting and threatenedpotential U.S. government regulation of embryonic tissue could also harm our ability to attract and retain qualified scientific personnel by causing topmay lead researchers to leave the country or the field of stem cell research altogether; andor the country altogether, in order to assure that their careers will not be impeded by encouraging the bestrestrictions on their work. Similarly, these factors may induce graduate students to choose other fields that are less vulnerable to changes in regulatory oversight.oversight, thus exacerbating the risk that we may not be able to attract and retain the scientific personnel we need in face of the competition among pharmaceutical, biotechnology and health care companies, universities and research institutions for what may become a shrinking class of qualified individuals.
Our corporate documents and Delaware law contain provisions that maycould make it difficult for us to be acquired in a transaction that wouldmight be beneficial to our shareholders.stockholders.
Our board of directors has the authority to issue shares of preferred stock and to fix the rights, preferences, privileges, and restrictions of these shares without shareholderstockholder approval. In addition, we have adopted a rights plan that generally permits our existing shareholdersstockholders to acquire additional shares at a substantial discount to the market price in the event of certain attempts by third parties to acquire us. These rights, along with certain provisions in our corporate documents and Delaware law, may make it more difficult for a third party to acquire us or discourage a third party from attempting to acquire us, even if the acquisition might be beneficial to our shareholders.stockholders.
24
Risks Related to the Securities Market
Our stock price has been, and will likely continue to be, highly volatile, which may negatively affect our ability to obtain additional financing in the future.
The market price per share of our common stock has been and is likely to continue to be highly volatile due to the risks and uncertainties described in this section of thethis Annual Report onForm 10-K, as well as other factors, including:
| | |
| | • | our ability to develop and test our technology;technologies; |
| |
| | • | our ability to patent or obtain licenses to necessary technology;technologies; |
| |
| | • | conditions and publicity regarding the industry in which we operate, as well as the specific areas our product candidates seek to address; |
candidates seek to address;
| | |
| | • | competition in our industry; |
| |
| | • | price and volume fluctuations in the stock market at large that are unrelated to our operating performance; and |
| |
| | • | comments by securities analysts, or our failure to meet market expectations. |
Over the two-year period ended December 31, 2006,2007, the trading price of our common stock as reported on the Nasdaq MarketsGlobal Market ranged from a high of $6.77$4.06 to a low of $1.77.$1.40. As a result of this volatility, your investment in our stock is subject to substantial risk. Furthermore, the volatility of our stock price could negatively impact our ability to raise capital in the future.or acquire businesses or technologies.
We are contractually obligated to issue shares in the future, diluting the interest of current shareholders.stockholders.
As of December 31, 2006,2007, there were outstanding warrants to purchase 1,930,6581,355,000 shares of our common stock, at a weighted average exercise price of $1.86$1.85 per share. AsAlso as of December 31, 2006,2007, there were also outstanding options to purchase 8,501,5039,028,810 shares of our common stock, at a weighted average exercise price of $2.88$2.36 per share. Moreover, we expect to issue additional options to purchase shares of our common stock to compensate employees, consultants and directors, and may issue additional shares to raise capital, to acquire other companies or technologies, to pay for services, or for other corporate purposes. Any such issuances will have the effect of diluting the interest of current shareholders.stockholders.
| |
| Item 1B. | Unresolved Staff CommentsUNRESOLVED STAFF COMMENTS |
None.As part of a review by the staff of the Securities and Exchange Commission (the “Staff”) of our Annual Report onForm 10-K for the year ended December 31, 2006, we have received and responded to comments from the Staff. The Staff’s comments pertain to (i) our determination as to which of our license agreements are considered material contracts required to be filed as exhibits, (ii) our determination as to which of our consulting agreements are considered material contracts required to be filed as exhibits, (iii) our description of certain patent related disputes, including the patents at issue in those proceedings, and (iv) our disclosures with respect to our research and development activities. We filed our response to the Staff on February 29, 2008, but as of the date of the filing of this Annual Report onForm 10-K, the Staff’s comments remain unresolved.
30
| |
| Item 2. | PropertiesPROPERTIES |
We entered into a5-year lease, as of February 1, 2001, for a 40,000 square foot facility, located in the Stanford Research Park in Palo Alto, California. This facility includes space for animals as well as laboratories, offices and a suite designed to be used to manufacture materials for clinical trials. Effective July 1, 2006, under an agreement that extends the lease through March 31, 2010, we leased the remainder of the building, adding approximately 27,500 square feet to our leased premises. The facility will better enable us to achieve our goal of utilizing human stem and progenitor cells for the treatment of disorders of the nervous system, liver, and pancreas. We have a space-sharing agreement with Stanford University for part of the animal facility not needed for our own use.
25
We continue to lease the following facilities in Lincoln, Rhode Island obtained in connection with our former encapsulated cell technology: our former research laboratory and corporate headquarters building which contains 62,500 square feet of wet labs, specialty research areas and administrative offices held on a lease agreement that goes through June 2013, as well as a 21,000 square-foot pilot manufacturing facility and a 3,000 square-foot cell processing facility financed by bonds issued by the Rhode Island Industrial Facilities Corporation. We have subleased the 21,000 square-foot and the 3,000 square foot facilities. We have also subleased small portions of the 62,500 square foot facility, amounting to approximately ten21 percent for most of 2006.the total space. We are actively seeking to sublease, assign or sell our remaining interests in these properties.
| |
| Item 3. | Legal ProceedingsLEGAL PROCEEDINGS |
In December 2003, Geron Corporation filed oppositions to two of our European patents that relate to neural stem cells and their uses, alleging that each patent should be revoked on multiple grounds. Both oppositions were heard in 2005, and the patents were maintained in somewhat altered form by the Opposition Division of the European Patent Office, and the time for appeal has run. U.S. counterparts to both of these patents are part of our issued patent portfolio; they are not subject to opposition, since that procedure does not exist under U.S. patent law, but other types of proceedings may be available to third parties to contest our U.S. patents.
In July 2006, we filed suit against Neuralstem, Inc., in the Federal District Court for the District of Maryland, alleging that itsNeuralstem’s activities violate claims in four of our patents.the patents we exclusively licensed from NeuroSpheres. Neuralstem has filed a motion for dismissal or summary judgment in the alternative, citing Title 35, Section 271(e)(1) of the United States Code, which says that it is not an act of patent infringement to make, use or sell a patented invention “solely for uses reasonably related to the development and submission of information” to the FDA. Neuralstem argues that sincebecause it does not have any therapeutic products on the market yet, the activities complained of fall within the protection of Section 271(e)(1) — that is, basically, that the suit is premature. This issue will be decided after discovery is complete. Subsequent to filing its motion to dismiss, in December 2006, Neuralstem petitioned the U.S. Patent and Trademark Office (PTO) to reexamine two of the patents in our infringement action against Neuralstem, namely U.S. Patent No. 6,294,346 (claiming the use of human neural stem cells for drug screening) and U.S. Patent No. 7,101,709 (claiming the use of human neural stem cells for screening biological agents). In April 2007, Neuralstem petitioned the PTO to reexamine the remaining two patents in the suit, namely U.S. Patent No. 5,851,832 (claiming methods for proliferating human neural stem cells) and U.S. Patent No. 6,497,872 (claiming methods for transplanting human neural stem cells). These requests were granted by the PTO and, in June 2007, the parties voluntarily agreed to stay the pending litigation while the PTO considers these reexamination requests. In October 2007, Neuralstem petitioned the PTO to reexamine a fifth patent, namely U.S. Patent No. 6,103,530, which claims a culture medium for proliferating mammalian neural stem cells. In September 2007, the PTO issued first office actions in each of the first four reexaminations. The Company has since filed its first responses to each of these, and expects all four patents to re-issue in 2008.
In 2003, Geron Corporation filed an opposition to two of our issued European patent cases, namely EP0594669 (claiming, among other things, methods for proliferating and using human neural stem cells as therapeutic and drug screening agents) and EP0669973 (claiming, among other things, methods for proliferating and differentiating human neural stem cells). Both oppositions were heard in 2005, and the patents were maintained in somewhat altered form by the Opposition Division of the European Patent Office. In essence the scope of each patent was limited to proliferation using specific growth factors and each had to disclaim derivation of human neural stem cells from human embryonic tissue in order to comply with the European law which precludes the patenting of embryonic stem cells. The time for appeal has run in each case. U.S. counterparts to these patents are part of our issued patent portfolio; they are not subject to opposition, because that procedure does not exist under U.S. patent law, although other types of proceedings may be available to third parties to contest our U.S. patents.
| |
| Item 4. | Submission Of Matters ToSUBMISSION OF MATTERS TO A Vote Of Security HoldersVOTE OF SECURITY HOLDERS |
None.
26
PART II
| |
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity SecuritiesMARKET FOR REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
(a) Market Priceprice and dividend information
In September 2005 the Nasdaq Stock Market approved our application to move the listing of our commonOur stock from the Nasdaq Capital Market (previously known as the Nasdaq SmallCap Market) to the Nasdaq National Market (now known asis traded on the Nasdaq Global Market). The stock began trading on the Nasdaq National Market on
31
September 30, 2005 under the same symbol STEM. The quarterly ranges of high and low bid prices for the last two fiscal years as reported by NASDAQNasdaq are shown below:
| | | | | | | | | | | | | |
| | | | 2006 | | High | | | Low | |
| |
| | | | | First Quarter | | $ | 4.06 | | | $ | 3.45 | |
| | | | | Second Quarter | | $ | 3.58 | | | $ | 1.77 | |
| | | | | Third Quarter | | $ | 2.55 | | | $ | 1.90 | |
| | | | | Fourth Quarter | | $ | 3.49 | | | $ | 2.05 | |
| | | | | 2005 | | | | | | | | |
| | | | | First Quarter | | $ | 6.76 | | | $ | 3.00 | |
| | | | | Second Quarter | | $ | 4.60 | | | $ | 2.58 | |
| | | | | Third Quarter | | $ | 6.57 | | | $ | 4.19 | |
| | | | | Fourth Quarter | | $ | 5.53 | | | $ | 3.40 | |
| | | | | | | | | |
2007 | | High | | | Low | |
| |
| First Quarter | | $ | 3.63 | | | $ | 2.36 | |
| Second Quarter | | $ | 3.09 | | | $ | 2.27 | |
| Third Quarter | | $ | 2.45 | | | $ | 1.90 | |
| Fourth Quarter | | $ | 2.53 | | | $ | 1.40 | |
| | | | | | | | | |
2006 | | | | | | |
| |
| First Quarter | | $ | 4.06 | | | $ | 3.45 | |
| Second Quarter | | $ | 3.58 | | | $ | 1.77 | |
| Third Quarter | | $ | 2.55 | | | $ | 1.90 | |
| Fourth Quarter | | $ | 3.49 | | | $ | 2.05 | |
No cash dividends have been declared on the Company’sour common stock since the Company’sour inception.
3227
PERFORMANCE GRAPH
The following graph comparesWe show below the cumulative5-year total return to shareholders of StemCells, Inc.’s common stock relativeour stockholders during the period from December 31, 2002 through December 31, 20071 in comparison to the cumulative total returns ofreturn on the SStandard & PPoor’s 500 Index and the Amex Biotechnology Stock Index. The graph assumesIndex during that the value of the investment in the company’s common stock and in each of the indexes (including reinvestment of dividends) was $100 on December 31, 2001 and tracks it through December 31, 2006.same period.
The stock price performance shown on the graph below is not necessarily indicative of future stock price performance.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN
Among StemCells, Inc., S & P 500 Index and the Amex Biotechnology Stock Index
for the period from December 31, 2001 until December 31, 2006(1)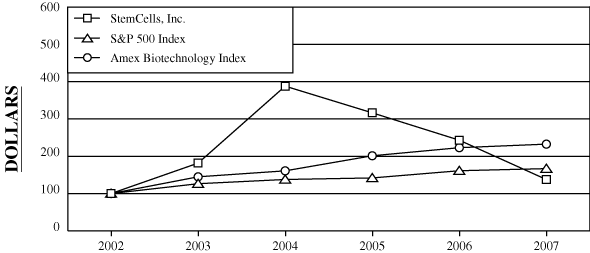
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | December 31,
| | | December 31,
| | | December 31,
| | | December 31,
| | | December 31,
| | | December 31,
| | | December 31,
| | | December 31,
| | | December 31,
| | | December 31,
| | | December 31,
| | | December 31,
|
| | | | 2001 | | | 2002 | | | 2003 | | | 2004 | | | 2005 | | | 2006 | | | 2002 | | | 2003 | | | 2004 | | | 2005 | | | 2006 | | | 2007 |
| StemCells, Inc. | | | $ | 100.00 | | | | $ | 31.23 | | | | $ | 56.73 | | | | $ | 121.20 | | | | $ | 98.85 | | | | $ | 75.93 | | | | $ | 100.00 | | | | $ | 181.65 | | | | $ | 388.07 | | | | $ | 316.51 | | | | $ | 243.12 | | | | $ | 137.61 | |
| S&P 500 Index | | | $ | 100.00 | | | | $ | 76.63 | | | | $ | 96.85 | | | | $ | 105.56 | | | | $ | 108.73 | | | | $ | 123.54 | | | | $ | 100.00 | | | | $ | 126.38 | | | | $ | 137.75 | | | | $ | 141.88 | | | | $ | 161.20 | | | | $ | 166.89 | |
| AMEX BIOTECH STOCK INDEX | | | $ | 100.00 | | | | $ | 58.26 | | | | $ | 84.42 | | | | $ | 93.74 | | | | $ | 117.28 | | | | $ | 129.91 | | |
| Amex Biotechnology Index | | | | $ | 100.00 | | | | $ | 144.91 | | | | $ | 160.92 | | | | $ | 201.32 | | | | $ | 223.01 | | | | $ | 232.54 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Based on the closing price of the company’s common stock on the first day of trading on the NASDAQ Global Market. Cumulative total returns assume reinvestment of all dividends andassumes a hypothetical investment of $100 on December 31, 2001.2002. |
The information under “Performance Graph” is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference in any filing of StemCells, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this10-K and irrespective of any general incorporation language in those filings.
(b) Approximate Number of Holders of Common Stock
As of February 28, 2007,29, 2008, there were approximately 592582 holders of record of theour common stock and as of the same date the closing price per share of theour common stock on the NASDAQNasdaq Global Market was $2.83.$1.51.
(c) Recent SaleSales of Unregistered Securities (last three years ending December 31, 2006)2007)
The CompanyWe issued the following unregistered securities in 2004:2007:
| | |
| | • | In August 2004, StemCellsJune 2007, we issued 9,5353,865 shares of common stock to the California Institute of Technology (Cal Tech) asfor payment forof annual fees of $10,000 and $5,000 that were due on the issuancefor each of two patents to which StemCells holdswe hold a license from Cal Tech, that were payable in cash or stock at our choice. We elected to pay the Company’s option.fees in stock. The shares were issued in a transaction not involving any public offering pursuant to Section 4(2) of the Securities Act of 1933, as amended. |
3328
We issued the following unregistered securities in 2006:
| | |
| | • | In December 2004, StemCellsAugust 2006, we issued 1,8163,848 shares of common stock to inventorsthe California Institute of Technology (Cal Tech) as payment of annual fees of $5,000 for each of two patents to which we hold a technology as part payment for approximately $2,800 oflicense from Cal Tech, payable in cash or stock at our choice. We elected to pay the total option fee of $25,000 to acquire an exclusive license to the technology from the Board of Trustees of The Leland Stanford Junior University.fees in stock. The shares were issued in a transaction not involving any public offering pursuant to Section 4(2) of the Securities Act of 1933, as amended. |
No unregistered securities were issued in 2005.
The Company issued the following unregistered securities in 2006:
| | |
| • | In August 2006, StemCells issued 3,848 shares of common stock to the California Institute of Technology (Cal Tech) as payment of annual fees of $5,000 on each for two patents to which StemCells holds a license from Cal Tech, payable in cash or stock at the Company’s choice. The Company elected to pay the fees in stock. The shares were issued in a transaction not involving any public offering pursuant to Section 4(2) of the Securities Act of 1933, as amended. |
Equity Compensation Plan Information
The following table provides certain information with respect to all of the Company’sour equity compensation plans in effect as of December 31, 2006.2007.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Equity Compensation Plan Information | | | Equity Compensation Plan Information | |
| | | | | | | Number of Securities
| | | | | | | Number of Securities
| |
| | | Number of Securities to
| | | | Remaining Available for
| | | Number of Securities to
| | | | Remaining Available for
| |
| | | be Issued Upon
| | Weighted-average
| | Future Issuance Under Equity
| | | be Issued Upon
| | Weighted-average
| | Future Issuance Under Equity
| |
| | | Exercise of
| | Exercise Price of
| | Compensation Plans
| | | Exercise of
| | Exercise Price of
| | Compensation Plans
| |
| | | Outstanding Options,
| | Outstanding Options,
| | (Excluding Securities
| | | Outstanding Options,
| | Outstanding Options,
| | (Excluding Securities
| |
| | | Warrants and Rights
| | Warrants and rights
| | Reflected in Column(a))
| | | Warrants and Rights
| | Warrants and rights
| | Reflected in Column(a))
| |
Plan category | | (a) | | (b) | | (c) | | | (a) | | (b) | | (c) | |
| |
Equity compensation plans approved by security holders | | | 8,501,503 | (1) | | $ | 2.88 | | | | 5,320,935 | | | | 9,028,810 | | | $ | 2.36 | | | | 3,024,408 | |
Equity compensation arrangements not approved by security holders | | | 100,000 | (2) | | $ | 1.20 | | | | N/a | | | | 100,000 | | | $ | 1.20 | | | | N/A | |
| | | | | | | | | | | | | | | |
| Totals | | | 8,601,503 | | | $ | 2.86 | | | | 5,320,935 | | | | 9,128,810 | | | $ | 2.33 | | | | 3,024,408 | |
| | | | | | | | | | | | | | | |
| | |
| (1) | | Consists of options issued to employees and directors and options issued as compensation to consultants for consultation services. These options were issued under the Company’sour 1992 Equity Incentive Plan, its Directors’ Stock Option Plan, its StemCells, Inc. Stock Option Plan, or itsour 2001, 2004 and 2006 Equity Incentive Plans. |
|
| (2) | | Represents the portion outstanding of a fully vested warrant issued in January 2003 to purchase 200,000 shares with an exercise price of $1.20 per share and exercisable, in whole or in part, for five years from the date of issuance. The warrant which constitutes an equity compensation arrangement not approved by security holders was issued in exchange for advisory services by non-employees. |
34
| |
| Item 6. | Selected Financial DataSELECTED FINANCIAL DATA |
The following selected financial and operating data are derived from our audited consolidated financial statements. The selected financial and operating data should be read in conjunction with “Item 7. Management’s
29
Discussion and Analysis of Financial Condition and Results of Operation” and the consolidated financial statements and notes thereto contained elsewhere in thisForm 10-K.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, | | | Year Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | | 2003 | | 2002 | | | 2007 | | 2006 | | 2005 | | 2004 | | 2003 | |
| | | (In thousands, except per share amounts) | | | (In thousands, except per share amounts) | |
| |
| Consolidated Statement of Operations | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Revenue from collaborative and licensing agreements | | $ | 55 | | | $ | 20 | | | $ | 22 | | | $ | 18 | | | $ | 40 | | |
| Revenue from grants | | | 38 | | | | 186 | | | | 119 | | | | 255 | | | | 375 | | |
| Total revenue | | | 93 | | | | 206 | | | | 141 | | | | 273 | | | | 415 | | |
| Revenue from licensing agreements and grants | | | $ | 57 | | | $ | 93 | | | $ | 206 | | | $ | 141 | | | $ | 273 | |
| Research and development expenses | | | 13,600 | | | | 8,226 | | | | 7,844 | | | | 5,479 | | | | 6,732 | | | | 19,937 | | | | 13,600 | | | | 8,226 | | | | 7,844 | | | | 5,479 | |
| General and administrative expenses | | | 7,154 | | | | 5,540 | | | | 4,870 | | | | 4,056 | | | | 4,009 | | | | 7,927 | | | | 7,154 | | | | 5,540 | | | | 4,870 | | | | 4,056 | |
| Wind-down expense(1) | | | 709 | | | | 2,827 | | | | 2,827 | | | | 2,885 | | | | 1,164 | | |
| Wind-down expenses(1) | | | | 783 | | | | 709 | | | | 2,827 | | | | 2,827 | | | | 2,885 | |
| License & settlement agreement income, net(2) | | | 103 | | | | 3,736 | | | | — | | | | — | | | | — | | | | 551 | | | | 103 | | | | 3,736 | | | | — | | | | — | |
| Gain on sale of marketable securities | | | | 716 | | | | — | | | | — | | | | — | | | | — | |
| Loss before deemed dividends and cumulative effect of change in accounting principle | | | (18,948 | ) | | | (11,738 | ) | | | (15,330 | ) | | | (12,291 | ) | | | (11,644 | ) | | | (25,023 | ) | | | (18,948 | ) | | | (11,738 | ) | | | (15,330 | ) | | | (12,291 | ) |
| Net loss | | | (18,948 | ) | | | (11,738 | ) | | | (15,330 | ) | | | (14,425 | ) | | | (13,276 | ) | | | (25,023 | ) | | | (18,948 | ) | | | (11,738 | ) | | | (15,330 | ) | | | (14,425 | ) |
| Basic and diluted loss per share | | $ | (0.25 | ) | | $ | (0.18 | ) | | $ | (0.31 | ) | | $ | (0.45 | ) | | $ | (0.53 | ) | | $ | (0.31 | ) | | $ | (0.25 | ) | | $ | (0.18 | ) | | $ | (0.31 | ) | | $ | (0.45 | ) |
| Shares used in computing basic and Diluted loss per share amounts | | | 74,611 | | | | 63,643 | | | | 49,606 | | | | 32,080 | | | | 25,096 | | |
| Shares used in computing basic and diluted loss per share amounts | | | | 79,772 | | | | 74,611 | | | | 63,643 | | | | 49,606 | | | | 32,080 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, | | | December 31, | |
| | | 2006 | | 2005 | | 2004 | | 2003 | | 2002 | | | 2007 | | 2006 | | 2005 | | 2004 | | 2003 | |
| | | (In thousands) | | | (In thousands) | |
| |
| Consolidated Balance Sheet | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 51,795 | | | $ | 34,541 | | | $ | 41,060 | | | $ | 13,082 | | | $ | 4,236 | | | $ | 9,759 | | | $ | 51,795 | | | $ | 34,541 | | | $ | 41,060 | | | $ | 13,082 | |
| Marketable securities | | | 7,266 | | | | 3,721 | | | | — | | | | — | | | | — | | | | 29,847 | | | | 7,266 | | | | 3,721 | | | | — | | | | — | |
| Total assets | | | 66,857 | | | | 44,839 | | | | 47,627 | | | | 19,786 | | | | 11,329 | | | | 48,283 | | | | 66,857 | | | | 44,839 | | | | 47,627 | | | | 19,786 | |
| Accrued wind-down expenses and deferred rent(1) | | | 6,750 | | | | 7,306 | | | | 5,528 | | | | 3,823 | | | | 1,931 | | |
| Accrued wind-down expenses | | | | 6,143 | | | | 6,750 | | | | 7,306 | | | | 5,528 | | | | 3,823 | |
| Long-term debt, including capital leases | | | 1,145 | | | | 1,351 | | | | 1,646 | | | | 1,850 | | | | 2,087 | | | | 1,034 | | | | 1,145 | | | | 1,351 | | | | 1,646 | | | | 1,850 | |
| Redeemable preferred stock(3) | | | — | | | | — | | | | — | | | | — | | | | 2,660 | | |
| Stockholders’ equity | | | 54,376 | | | | 32,376 | | | | 36,950 | | | | 10,964 | | | | 1,933 | | | | 35,212 | | | | 54,376 | | | | 32,376 | | | | 36,950 | | | | 10,964 | |
| | |
| (1) | | Relates to wind-down expenses in respect of the Company’sour Rhode Island facility. See Note 87 “Wind-down and exit costs” in the consolidated financial statements.Notes to the Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information. |
| |
| (2) | | Relates to an agreement with ReNeuron Limited. See Note 2 “Financial Instruments” in the consolidated financial statements. |
|
(3) | | See Note 10 in the consolidated financial statements.Notes to Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information. |
| |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of OperationsMANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion of our financial condition and results of operations should be read in conjunction with the accompanying financial statements and the related footnotes thereto.
This report contains forward looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act that involve substantial risks and uncertainties. Such statements include, without limitation, all statements as to expectation or belief and statements as to our future results of
35
operations, operations; the progress of our research, product development and clinical programs,programs; the need for, and timing of, additional capital and capital expenditures,expenditures; partnering prospects,prospects; costs of manufacture of products,products; the protection of, and the need for, additional intellectual property rights,rights; effects of regulations,regulations; the need for additional facilitiesfacilities; and potential market opportunities. Our actual results may vary materially from those contained in such forward-looking statements because of risks to which we are subject, including uncertainty as to whether the U.S. Food and Drug Administration (FDA) or other applicable regulators or review boardsregulatory authorities will permit us to proceed with clinical testing of proposed products despite the novel and unproven nature of our technology;technologies; the risk that although it has been allowed to go forward by the FDA and is now in progress, our initial clinical trial and any other clinical trials or studies could be substantially delayed beyond itstheir expected dates or cause us to incur substantial unanticipated costs; uncertainties regardingin our ability to obtain the capital resources needed to continue our current research and development operations and to conduct the research, preclinical development and clinical trials necessary for regulatory approvals; failurethe uncertainty regarding our ability to obtain a corporate partner or partners, if
30
needed, to support the development and commercialization of our stem cell programs;potential cell-based therapeutics products; the uncertainty regarding the outcome of theour Phase I clinical trial in NCL and any other clinical trials or studies we may conduct in the future; including uncertainty as to whether results obtained in the animal models of infantile neuronal ceroid lipofuscinosis (NCL), spinal cord injury, or other diseases and conditions will be able to be translated into treatment for humans; the uncertainty regarding the validity and enforceability of our issued patents; the uncertainty whether HuCNS-SC and any other products that may be generated in our stem cellcell-based therapeutics programs will prove clinically effectivesafe and not cause tumors or other side effects;effective; the uncertainty whether we will achieve revenuesrevenue from product sales or become profitable; our likely increase in the use of cash as compared to our historical use of cash; uncertainties regarding our obligations in regardwith respect to our former encapsulated cell therapy facilities in Rhode Island; obsolescence of our technology;technologies; competition from third parties; intellectual property rights of third parties; litigation risks; and other risks to which we are subject. SeeAll forward-looking statements attributable to us or to persons acting on our behalf are expressly qualified in their entirety by the cautionary statements and risk factors set forth in “Risk Factors” underin Part I, Item 1A above.of thisForm 10-K.
Overview
Since our inception in 1988, we have been primarily engaged inThe Company
Our research and development of human therapeutic products.(R&D) programs are focused on identifying and developing potential cell-based therapeutics which can either restore or support organ function. Since the second half ofwe relocated our corporate headquarters and research laboratories to California in 1999 our sole focus hasR&D efforts have primarily been ondirected at refining our methods for identifying, isolating, culturing, and purifying the human neural stem cell technology.and human liver engrafting cells (hLEC) and developing these as potential cell-based therapeutics for the central nervous system (CNS) and the liver, respectively. We are currently conducting a Phase I clinical trial of our HuCNS-SC® product candidate (purified human neural stem cellscells) as a treatment for infantile and late infantile neuronal ceroid lipofuscinosis (NCL), a fatal neurodegenerative disease often referred to as Batten disease. TheWe have completed enrollment and dosing for this six-patient trial and expect it to be completed in early 2009. Our CNS Program is beingcontinuing basic research and preclinical development for additional potential indications in the CNS field. We are targeting to initiate clinical trials to test our HuCNS-SC product candidate for a spinal cord indication by mid-2008 and for a myelin disorder in the brain by the end of 2008. In our Liver Program, we are in preclinical development with our human liver engrafting cells and are exploring their applicability as a cellular therapy to restore function to liver tissue by replacing dysfunctional or damaged cells. See Overview “Research and Development Programs” in the Business Section of Part I, Item 1 of thisForm 10-K for a brief description of our significant research and development programs. We have also conducted at Oregon Health & Science University’s Doernbecher Children’s Hospitalresearch on several other cell types and in Portland, Oregon.other areas, which could lead to other possible product candidates, process improvements or further research activities.
We have not derived any revenuesrevenue or cash flows from the sale or commercialization of any products apart fromexcept for license revenue for the research use of certain of our patented cells and media andfor use in research. As a result, we do not expect to receive revenues from product sales for at least several years. We have not commercialized any product and in order to do so we must, among other things, substantially increase our research and development expenditures as research and product development efforts accelerate and clinical trials are initiated. We had expenditures for screening and enrolling patients and for preparing HuCNS-SC doses for our Phase I clinical trial and will incur more such expenditures for any future clinical trials. We previously had expenditures for toxicology and other studies in preparation for submitting the Investigational New Drug application (IND) for our Phase I trial for NCL to the FDA and getting it cleared by the FDA, and will incur more such expenditures for any future INDs. We have incurred annual operating losses since inception and expect to incur substantial operating losses in the future. As a result,Therefore, we are dependent upon external financing from equity and debt offerings and revenuesrevenue from collaborative research arrangements with corporate sponsors to finance our operations. There areWe have no such collaborative research arrangements at this time and there can be no assurance that such financing or partnering revenuesrevenue will be available when needed or on terms acceptable to us.
Significant eventsBefore we can derive revenue or cash inflows from the commercialization of any of our product candidates, we will need to: (i) conduct substantialin vitrotesting and characterization of our proprietary cell types, (ii) undertake preclinical and clinical testing for specific disease indications; (iii) develop, validate andscale-up manufacturing processes to produce these cell-based therapeutics, and (iv) pursue required regulatory approvals. These steps are risky, expensive and time consuming.
Overall, we expect our R&D expenses to be substantial and to increase for the foreseeable future as we continue the development and clinical investigation of our current and future product candidates. However, expenditures on R&D programs are subject to many uncertainties, including whether we develop our product candidates with a partner or independently. We cannot forecast with any degree of certainty which of our current product candidates will be subject to future collaboration, when such collaboration agreements will be secured, if at all, and to what degree such arrangements would affect our development plans and capital requirements. In addition, there are numerous factors associated with the successful commercialization of any of our cell-based therapeutics, including future trial design and regulatory requirements, many of which cannot be determined with accuracy at this
31
time given the stage of our development and the novel nature of stem cell technologies. The regulatory pathways, both in the United States and internationally, are complex and fluid given the novel and, in general, clinically unproven nature of stem cell technologies. At this time, due to such uncertainties and inherent risks, we cannot estimate in a meaningful way the duration of, or the costs to complete, our R&D programs or whether, when or to what extent we will generate revenues or cash inflows from the commercialization and sale of any of our product candidates. While we are currently focused on advancing each of our product development programs, our future R&D expenses will depend on the determinations we make as to the scientific and clinical prospects of each product candidate, as well as our ongoing assessment of the past year include these:regulatory requirements and each product candidate’s commercial potential.
Given the early stage of development of our product candidates, any estimates of when we may be able to commercialize one or more of these products would not be meaningful. Moreover, any estimate of the time and investment required to develop potential products based upon our proprietary HuCNS-SC and hLEC technologies will change depending on the ultimate approach or approaches we take to pursue them, the results of preclinical and clinical studies, and the content and timing of decisions made by the FDA and other regulatory authorities. There can be no assurance that we will be able to develop any product successfully, or that we will be able to recover our development costs, whether upon commercialization of a developed product or otherwise. We cannot provide assurance that any of these programs will result in products that can be marketed or marketed profitably. If certain of our development-stage programs do not result in commercially viable products, our results of operations could be materially adversely affected.
Significant Events
In November, 2006, the first patient in ourJanuary 2008, we completed enrollment and dosing of a six-patient Phase I clinical trial was transplanted withof our proprietary human neural stem cellHuCNS-SC product — HuCNS-SCtm — at Oregon Health & Science University’s (OHSU) Doernbecher Children’s Hospital. Since then, a second patient has also been transplanted with HuCNS-SC at OHSU. This study is designed to evaluate the safety and preliminary efficacy of HuCNS-SCcandidate as a treatment for infantile and late infantile NCL (also knownneuronal ceroid lipofuscinosis (NCL) at Oregon Health & Science University (OHSU) Doernbecher Children’s Hospital.
In January 2008, we entered into a research collaboration with the OHSU Casey Eye Institute to evaluate our HuCNS-SC product candidate as Batten Disease)a potential treatment for retinal degeneration, a leading cause of blindness.
In December 2007, we announced that we were exploring the possible acquisition of privately held Progenitor Cell Therapy, LLC (PCT), a provider of cGMP-quality cell processing services headquartered in Hackensack, NJ. PCT agreed to a period of exclusivity to allow for due diligence and negotiations, and in consideration thereof, we agreed to make a totalsecured loan of six patientsup to $3.8 million to PCT, of which $1.0 million was lent. In March 2008, we terminated discussions to acquire PCT because the parties were unable to reach mutually agreeable terms and conditions. We anticipate the $1.0 million loan will be repaid in accordance with its terms.
In September 2007, we entered into a research collaboration with Belgium’s Université Catholique de Louvain (UCL) and the UCL-affiliated Cliniques Universitaires Saint Luc to further the development of hLEC as a potential cell-based liver therapy. Under the collaboration, we are plannedworking with UCL to be enrolled.improve processes to isolate and purify hLEC, which we believe is an important step prior to initiating any clinical study of the cells.
In June 2007, we announced that we had successfully completed enrollment and dosing of the low-dose cohort in our Phase I trial. A review of the trial data to that point, conducted by an independent Data Safety Monitoring Committee comprised of experts in pediatric neurosurgery, pediatric neurology, solid organ transplantation, and genetics, identified no safety issues to preclude advancing to the next dose level.
In June 2007, we announced the publication of a paper describing a new technique for non-invasive tracking of human neural stem cells transplanted into the brains of mice. The technique, which involves tagging the human neural stem cells with Feridex®, a commonly used magnetic resonance imaging agent approved by the FDA for use in humans, does not appear to alter the stem cells’ function or viability.
In February 2007, we raised net proceeds of approximately $3 million through the sale of 5,275,000 ordinary shares of ReNeuron Group plc. We received the shares as part of a license and settlement agreement we entered into with ReNeuron in 2005 in which we granted ReNeuron a license under some of our patents to exploit their conditionally immortalized adult human neural stem cell technology for therapy and other purposes.
3632
In April, 2006, we sold 11,750,820 shares of common stock to a limited number of institutional investors at a price of $3.05 per share, for gross proceeds of $35.8 million. We received total proceeds, net of offering expenses and placement agency fees, of approximately $33.4 million.
In June 2006 and February 2007, we received a combined total of approximately 1.3 million additional shares of ReNeuron Group plc, pursuant to the anti-dilution provisions of the license agreement entered into with ReNeuron in July 2005 as they applied to two ReNeuron financings. In February 2007, we sold approximately 5,275,000 shares of ReNeuron for net proceeds of approximately $3.1 million. As of February 26, 2007, we owned approximately 4.8 million shares of ReNeuron. See Note 2 and “Item 7a. Quantitative and Qualitative Disclosures about Market Risk.”
In 2006, the U.S. Patent and Trademark Office issued seven new patents that are owned by or exclusively licensed to StemCells, Inc., further strengthening what the Company believes is the dominant intellectual property position in the neural stem cell field, with claims covering methods for identification, isolation, expansion, and transplantation of neural stem cells as well as methods for drug discovery and testing. See “Patents, Proprietary Rights and Licenses.”
In August, 2006, we licensed certain rights to our intellectual property to Stem Cell Therapeutics Corp., a Canadian biotechnology company engaged in treating certain central nervous system disorders by stimulating endogenous neural stem cells. Under the agreement, we received an up-front fee and will receive license maintenance fees, as well as milestone and royalty payments. SeeLicense Agreementsunder “Patents, Proprietary Rights and Licenses.”
Our results of operations have varied significantly from year to year and quarter to quarter and may vary significantly in the future due to the occurrence of material recurring and nonrecurring events, including without limitation the receipt and payment of recurring and nonrecurring licensing payments, the initiation or termination of research collaborations, the on-going expenses to lease and maintain our facilities in Rhode Island and the increasing costs associated with our facility in California. To expand and provide high quality systems and support to our Research and Development programs, as well as to enhance our internal controls over financial reporting, we will need to hire more personnel, which will lead to higher operating expenses. Throughout 2006 and early 2007, we made a number of additions to our management team: In January, Maria Millan, M.D., FACS, joined us as Director, Liver Cell Transplant Program; in December, she was promoted to Vice President and Head of the Liver Program. In April, Elizabeth Leininger, Ph.D. was appointed Vice President, Regulatory Affairs and Quality Assurance. In December, Ann Tsukamoto, Ph.D., formerly Vice President, Research and Development, was promoted to the newly created position of Chief Operating Officer, and Rodney Young was promoted to Chief Financial Officer and Vice President, Finance and Administration. In January 2007, Stephen Huhn, M.D., F.A.C.S., F.A.A.P.Desmond H. O’Connell, Jr., joined us as Vice Presidentour Board of Directors. Currently an independent management consultant and Headprivate investor, Mr. O’Connell is a director of Abiomed, Inc. and was formerly a director, acting chief executive officer and chairman of the Neural Program.
Our Neural Program ranges from the preclinical stage,board of Serologicals Corporation until that company was sold in which we test human neural stem cells in small animal models of human diseases, both in-house and through external academic collaborators, through the development phase, in which we evaluate improvements to expansion methods and the toxicology of the cells, through the clinical development phase, with respect to the Phase I clinical trial in NCL mentioned above. In our Liver Program, we are engaged in evaluating our proprietary liver engrafting cell in variousin vivoassays, and are planning to advance our liver stem cell program into product development as rapidly as we can. Our pancreas program is still in the discovery stage and further evaluation of the therapeutic potential of the candidate human pancreatic stem/progenitor cell will be required.2006.
Critical Accounting Policies
We believe and the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our consolidated financial statements:
Use of Estimates
The accompanying discussion and analysis of our financial condition and results of operations are based on our Consolidated Financial Statements and the related disclosures, which have been prepared in accordance with U.S. generally accepted accounting principles (GAAP). The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of Americathese Consolidated Financial Statements requires our management to make estimates, assumptions, and judgments that affect the reported amounts in our Consolidated Financial Statements and accompanying notes. These estimates form the basis for making judgments about the carrying values of assets and liabilities. We base our estimates and judgments on historical experience and on various other assumptions that affect
37
we believe to be reasonable under the amounts reported incircumstances, and we have established internal controls related to the consolidated financial statements.preparation of these estimates. Actual results and the timing of the results could differ materially from these estimates. Significant estimates include the following:
| | |
| • | Accrued wind-down expenses (See Note 8). |
|
| • | The grant date fair value of share-based awards recognized as compensation expense in accordance with the provisions of Statement of Financial Accounting Standards (SFAS) No. 123 (Revised 2004) “Share Based Payment” (SFAS 123R). See “Stock-based Compensation” below. |
|
| • | Valuation allowance against net deferred tax assets (See Note 12). |
Marketable securitiesStock-Based Compensation
In accordance withOn January 1, 2006, we adopted Statement of Financial Accounting Standards 123 (revised 2004) (SFAS 123R),Share-Based Payment, which revises SFAS No. 115 “123,Accounting for Certain Investments in DebtStock-Based Compensation, and Equity Securities,” the Company has classified the Company’s short-term investments asavailable-for-sale marketable securities in the accompanying consolidated financial statements. The marketable securities are stated at fair market value, with unrealized gains and losses reported in other comprehensive income. Management reviews securities with unrealized lossessupersedes Accounting Principles Board Opinion 25,Accounting for other than temporary impairment. A decline inStock Issued to Employees. SFAS 123R requires us to recognize expense related to the fair value of securities that is deemed other than temporary is charged to earnings when so deemed. See Note 2.
Stock-Based Compensation
In December 2004, the Financial Accounting Standards Board (FASB) issued SFAS 123R. SFAS 123R requires all share-based payments to employees, or to non-employee directors as compensation for service on the Board of Directors, to be recognized as compensation expense in the consolidated financial statements based on the fair values of such payments. We maintain shareholder approved stock-based compensation plans, pursuant to which we granted stock-based compensation to our employees, and to non-employee directors for Board service. These grants are primarily in the form of options that allow a grantee to purchase a fixed number of shares of our common stock at a fixed exercise price equal to the market price of the shares at the date of the grant (“qualified stock option grants”). The options may vest on a single date or in tranches over a period of time, but normally they do not vest unless the grantee is still employed by or a director of the Company on the vesting date. The compensation expense for these grants will be recognized over the requisite service period which is typically the period over which the stock-based compensation awards, vest. We made no modifications to outstanding options with respect to vesting periods or exercise prices prior to adopting SFAS 123R. In March 2005, the Securities and Exchange Commission (SEC) issued Staff Accounting Bulletin No. 107 (SAB 107), which provides guidance on the implementation of SFAS 123R. We applied the principles of SAB 107 in conjunction with its adoption of SFAS 123R.
We adopted SFAS 123R effective January 1, 2006, using the modified-prospective transition method.including employee stock options. Under this transition method, compensation expense will be recognized based on the grant date fair value estimated in accordance with the provisions of SFAS 123R, for all new grants effective January 1, 2006, and for options granted prior to but not vested as of December 31, 2005. Prior periods were not restated to reflect the impact of adopting the new standard and therefore do not include fair value compensation expense related to stock option grants for those periods. In accordance with SFAS 123R, we recognized stock option related compensation expense of approximately $2,285,000 for the year ended December 31, 2006. Stock option related compensation expense was recognized on a straight line basis over the vesting period of each grant net of estimated forfeitures. We estimated forfeiture rates based on our historical experience within separate groups of employees. The estimated fair value of the options granted during 2006 and prior years was calculated using a Black Scholes Merton option pricing model
38
(Black Scholes model). The following summarizes the assumptions used in the Black Scholes model as applied by quarter for the year ended December 31, 2006:
| | | | | | | | | | | | | | | | | |
| | | First Quarter 2006 | | | Second Quarter 2006 | | | Third Quarter 2006 | | | Fourth Quarter 2006 | |
| |
| Risk — free interest rate (1) | | | 4.72 | % | | | 5.08 | % | | | 4.68 | % | | | 4.54 | % |
| Volatility (2) | | | 119.5 | % | | | 110.8 | % | | | 106.6 | % | | | 103.3 | % |
| Dividend yield (3) | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % |
| Expected term (years until exercise) (4) | | | 6.25 | | | | 6.25 | | | | 6.25 | | | | 6.25 | |
| | |
(1) | | The risk-free interest rate is based on US Treasury debt securities with maturities close to the expected term of the option. |
|
(2) | | Expected volatility is based on historical volatility of the Company’s stock factoring in daily share price observations. In computing expected volatility, the length of the historical period used is equal to the length of the expected term of the option. |
|
(3) | | No cash dividends have been declared on the Company’s common stock since the Company’s inception, and the Company currently does not anticipate paying cash dividends over the expected term of the option. |
|
(4) | | The expected term is equal to the average of the contractual life of the stock option and its vesting period. |
At December 31, 2006, approximately $5,775,000 of unrecognized compensation expense related to stock options is expected to be recognized over a weighted average period of approximately 1.6 years. The resulting effect on net loss and net loss per share attributable to common stockholders may not be representative of the effects in future periods, due to changes in forfeiture rates, additional grants and subsequent periods of vesting.
Prior to January 1, 2006, we accounted for ouremployee stock-based compensation plans under Accounting Principles Board Opinion No. 25 (APB 25), “Accounting for Stock Issued to Employees.’’ In accordance with APB 25, we generally recognized no compensation expense for qualified stock option grants, as the options were usually granted at fair market price of the underlying shares on the date of the grant. For options issued with an exercise price less than the fair market value of the sharesis estimated at the date of grant we recognizedbased on the difference between the exercise price and fair market value as compensation expense in accordance with APB 25. Prior to January 1, 2006, we provided pro forma disclosure amounts in accordance with Statement of Financial Accounting Standards No. 123“Accounting for Stock-Based Compensation,” (SFAS 123) as amended by Statement of Financial Accounting Standards No. 148“Accounting for Stock-Based Compensation — Transition and Disclosure,” (SFAS 148). Asaward’s fair value compensationusing the Black-Scholes-Merton (Black-Scholes) option-pricing model and is recognized as expense was disclosed but not recognized in periods prior to January 1, 2006, no cumulative adjustment for forfeitures was recorded in 2006. See table in “Stock-Based Compensation “under Note 1 below for an illustrationratably over the requisite service period. The Black-Scholes option-pricing model requires the use of certain assumptions, the most significant of which are our estimates of the effect on net loss and net loss per share if we had applied the fair value recognition provisionsexpected volatility of SFAS 123 to stock-based employee compensation in the prior years ended December 31, 2005 and 2004.
We account for stock options granted to non-employees in accordance with SFAS 123 and Emerging Issues Task Force (EITF)96-18 —“Accounting For Equity Instruments That Are Issued To Other Than Employees For Acquiring, Or In Conjunction With Selling, Goods Or Services,” and accordingly, recognize as expense the estimated fair value of such options as calculated using the Black Scholes model. The fair value is re-measured at each reporting date during the service period and is amortized over the vesting period of each option or the recipient’s contractual arrangement, if shorter. No stock options were issued to non-employees during the year ended December 31, 2006, other than options granted to non-employee members of the Board of Directors for service as Board members.
In July 2006, pursuant to the 2006 Equity Incentive Plan, we granted cash-settled Stock Appreciation Rights (SARs) to certain employees. The SARs give the holder the right, upon exercise, to the difference between the price per share of our common stock at the time of exercise and the exercise price of the SAR. The exercise price of the SARs is equal to the market price of our common sharesstock and the expected term of the award. Our estimate of the expected volatility is based on historical volatility. We currently use the simplified method to estimate the expected term of the option. Under this method, the expected term is the average of the contractual life of the option and its vesting period. As required under SFAS 123R, we review our valuation assumptions at the date of grant. The SARs will vest on the same schedule as our qualified options issued to employees, i.e., 25% on the first anniversary of theeach grant date and, then 1/48th every month thereafter. We will recognize compensation expense for the SARs over the requisite service period which is typically the period over which the awards vest. Since the vesting schedule of the SARs is identical
39
to the vesting schedule of options granted this period,as a result, our fair value calculation of the SARs issued using the Black Scholes model were based on the same assumptions used in calculating compensation expense for stock options granted this period. The fair value of the share-based compensation liability for the cost of the requisite service that has been rendered at the reporting date is re-measured at each reporting date through the date of settlement. The following table presents the activity of the Company’s SARs awards for the year ended December 31, 2006 and 2005.
| | | | | | | | | | | | | | | | | |
| | | 2006 | | | 2005 | |
| | | | | | Weighted Average
| | | | | | Weighted Average
| |
| | | SARs | | | Exercise Price | | | SARs | | | Exercise Price | |
| |
| Outstanding at January 1 | | | — | | | | — | | | | — | | | | — | |
| Granted | | | 1,564,599 | | | $ | 2.00 | | | | — | | | | — | |
| Exercised | | | — | | | | — | | | | — | | | | — | |
| Canceled | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Outstanding at September 30 | | | 1,564,599 | | | $ | 2.00 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| SARs exercisable at December 31 | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
future periods may change. For the year ended December 31, 2006, we recorded approximately $293,000 as2007, employee stock-based compensation expense was approximately $3,061,000. As of December 31, 2007, total compensation cost related to SARs granted. At December 31, 2006,unvested stock options not yet recognized was approximately $2,336,000 of unrecognized compensation expense related to SARs$6,956,000, which is expected to be recognized as expense over a weighted averageweighted-average period of approximately 1.91.6 years. The resulting effect on net loss and net loss per share attributable to common stockholders is not likely to be representative of the effects in future periods, due to changes in the fair value calculation which is dependent on the stock price, volatility, interest and forfeiture rates, additional grants and subsequent periods of vesting.
Long-Lived Assets
We routinely evaluate the carrying value of our long-lived assets. We record impairment losses on long-lived assets used in operations when events and circumstances indicate that assets may be impaired and the undiscounted cash flows estimated to be generated by the assets are less than the carrying amount of those assets. If an impairment exists, the charge to operations is measured as the excess of the carrying amount over the fair value of the assets.
Wind-down and Exit Costsexpenses
In connection with our wind-down of our research and manufacturing operations in Lincoln, Rhode Island, and the relocation of our corporate headquarters and remaining research and development activities and corporate headquarters, to California, in October 1999, we have provided our estimate of the exit cost obligation in accordance withEITF 94-3, “Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (Including Certain Costs Incurred in a Restructuring)”. On an ongoing basis we re-evaluate such estimate. For further discussion, see “Wind-down expenses” under “Results of Operations” and Note 8 to the consolidated financial statements.
40
RESULTS OF OPERATIONS
Years Ended December 31, 2006, 2005 and 2004
Revenues
Revenues totaled approximately $93,000, $206,000, and $141,000 for the years ended December 31, 2006, 2005 and 2004, respectively.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Change from
| |
| | | | | | | | | | | | Change from previous year: 2006
| | | previous year: 2005
| |
| | | | | | | | | | | | versus 2005 | | | versus 2004 | |
| | | 2006 | | | 2005 | | | 2004 | | | $ | | | % | | | $ | | | % | |
| |
| Revenue: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Revenue from licensing revenue | | $ | 55,299 | | | $ | 19,526 | | | $ | 22,206 | | | $ | 35,773 | | | | 183 | % | | $ | (2,680 | ) | | | (12 | )% |
| Revenue from grants | | | 37,551 | | | | 186,388 | | | | 118,828 | | | | (148,837 | ) | | | (80 | )% | | | 67,560 | | | | 57 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total revenue | | $ | 92,850 | | | $ | 205,914 | | | $ | 141,034 | | | $ | 113,064 | | | | (55 | )% | | $ | 64,880 | | | | 46 | % |
Revenues for 2006 include $38,000 that is part of a Small Business Technology Transfer (STTR) grant received in 2004 for approximately $464,000 over one and one half years for studies in Alzheimer’s disease. The STTR grant will support joint work with the McLaughlin Research Institute (MRI) in Great Falls, Montana. We retained $243,000 and the remaining $221,000 was disbursed to MRI. Revenues for 2006 also include licensing revenue of approximately $55,000 received from various licensees. Revenues for 2005 include $186,000 that was part of the STTR grant and approximately $20,000 in licensing revenue. Revenues for 2004 include $93,000 that completes the draw down of a one-year Small Business Innovation Research grant of $342,000 from the National Institute of Neurological Disease and Stroke (NINDS) received at the end of 2003, and $26,000 which is part of the STTR grant received in 2004. Total revenue for 2004 includes licensing revenue of $22,000.
Operating Expenses
Operating expenses totaled approximately $21,464,000, $16,594,000, and $15,541,000 for the years ended December 31, 2006, 2005 and 2004, respectively.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Change from
| | | Change from
| |
| | | | | | | | | | | | previous year: 2006
| | | previous year: 2005
| |
| | | | | | | | | | | | versus 2005 | | | versus 2004 | |
| | | 2006 | | | 2005 | | | 2004 | | | $ | | | % | | | $ | | | % | |
| |
| Operating Expenses | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research & development | | $ | 13,600,433 | | | $ | 8,226,734 | | | $ | 7,843,981 | | | $ | 5,373,699 | | | | 65 | % | | $ | 382,753 | | | | 5 | % |
| General & administrative | | | 7,154,042 | | | | 5,539,845 | | | | 4,870,014 | | | | 1,614,197 | | | | 29 | % | | | 669,831 | | | | 14 | % |
| Wind-down expenses | | | 709,209 | | | | 2,827,403 | | | | 2,826,879 | | | | (2,118,194 | ) | | | (75 | )% | | | 524 | | | | 0 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total expense | | $ | 21,463,684 | | | $ | 16,593,982 | | | $ | 15,540,874 | | | $ | 4,869,702 | | | | 29 | % | | $ | 1,053,108 | | | | 7 | % |
Research & development expenses
Research and development expenses totaled approximately $13,600,000 in 2006, as compared to $8,227,000 in 2005 and $7,844,000 in 2004.
2006 versus 2005. The increase of approximately $5,374,000 or 65% from 2005 to 2006 was primarily attributable to expansion of our operations in cell processing and clinical development, which consisted of an increase in personnel costs of approximately $2,669,000, an increase in external services of approximately $1,464,000, an increase in supplies and other expenses of $771,000 and the cost of additional space leased in 2006 allocated to research and development. Of the approximately $2,669,000 increase in personnel costs, approximately $1,344,000 was attributable to the expensing of stock-based compensation (which includes the grant of stock options, stock appreciation rights and share awards) as required by the new accounting
41
pronouncement SFAS 123R (see “Stock-Based Compensation” under Note 1), with the balance attributable to an increased head count which includes the hiring of key personnel necessary to facilitate the expansion of our cell processing and clinical operations. At December 31, 2006, we had thirty-five full-time employees working in research and development and laboratory support services as compared to thirty-three at December 31, 2005.
2005 versus 2004. The increase of $383,000 or 5% from 2004 to 2005 was primarily attributable to increased head count and related costs of $848,000 in 2005 as compared to 2004. At December 31, 2005, we had thirty-three full-time employees working in research and development and laboratory support services as compared to twenty-eight at December 31, 2004. This increase in 2005 was partially offset by a net decrease in expenses of $465,000 primarily related to external services. We required a high level of external services in 2004 for preclinical pharmacology and toxicology studies and other external services in preparation for submitting our first IND to the FDA. The decrease in expenses related to external services was also attributable to a decrease in valuation in 2005 of stock options granted as compensation to non-employees as compared to the valuation in 2004. The valuation — computed by the Black Scholes Method — is dependant on variable factors at the time of such valuation such as stock price, stock price volatility, interest rate and remaining life of the option. Our stock price at December 31, 2005 was $3.45 as compared to $4.23 at December 31, 2004.
General & administrative expenses
General and administrative expenses were approximately $7,154,000 in 2006, compared with $5,540,000 in 2005 and $4,870,000 in 2004.
2006 versus 2005. The increase of $1,614,000 or 29% from 2005 to 2006 was primarily attributable to the increase in personnel costs of approximately $1,826,000, of which approximately $1,423,000 was attributable to the expensing of stock-based compensation (which includes the grant of stock options and stock appreciation rights) as required by the new accounting pronouncement SFAS 123R (see “Stock-Based Compensation” under Note 1). The increase in personnel costs was partially offset by a net decrease in other costs primarily attributable to the expensing of the fair value of options granted to a consultant in 2005. No such options were granted in 2006.
2005 versus 2004. The increase of $670,000 or 14% from 2004 to 2005 was primarily attributable to expensing the fair value of stock options granted to our previous chief financial officer, which was approximately $457,000. The vesting of the options was accelerated as part of an agreement that retained our previous chief financial officer as a consultant for approximately six months following her employment termination date. The increase was also attributable to the increase in head count and related costs of approximately $394,000; increase in recruiting fees of approximately $145,000; and the increase in listing fees of approximately $114,000 incurred in 2005 for moving the listing of our shares from the Nasdaq Capital Market to the Nasdaq Global Market. The aforementioned increases were partially offset by a decrease in 2005 of approximately $186,000 for external services to evaluate and test our internal financial control systems so as to meet the requirements of and be in compliance with the Securities and Exchange Commission rules issued under Section 404 of the Sarbanes-Oxley Act, and a net decrease of approximately $254,000 in other expenses.
Wind-down expenses
In connection with our wind-down of our research and manufacturing operations in Lincoln, Rhode Island, and the relocation of our remaining research and development activities and corporate headquarters,laboratories to California in October 1999, we provided a reserve for our estimate of the exit cost obligation in accordance withEITF 94-3, “Liability Recognition for Certain Employee Termination Benefits and Other Costs to Exit an Activity (Including Certain Costs Incurred in a Restructuring.” The reserve reflects estimates of the ongoing costs of our former research and administrative facility in Lincoln, which we hold on a lease that terminates on June 30, 2013. We are seeking to sublease, assign, sell, or otherwise divest ourselves of our interest in the facility at the earliest possible time, but we cannot determine with certainty a fixed date by which such events will occur, if at all.
In determining the facility exit cost reserve amount, we are required to consider our lease payments through to the end of the lease term and estimate other relevant factors such as facility operating expenses, real estate market conditions in Rhode Island for similar facilities, occupancy rates, and sublease rental rates projected over the course of the leasehold. We re-evaluate the estimate each quarter, taking account of changes, if any, in each underlying
42
factor. The process is inherently subjective because it involves projections over time — from the date of the estimate through the end of the lease — and it is not possible to determine any of the factors except the lease payments with certainty over that period.
33
Management forms its best estimate on a quarterly basis, after considering actual sublease activity, reports from our broker/realtor about current and predicted real estate market conditions in Rhode Island, the likelihood of new subleases in the foreseeable future for the specific facility and significant changes in the actual or projected operating expenses of the property. We discount the projected net outflow over the term of the leasehold to arrive at the present value, and adjust the reserve to that figure. The estimated vacancy rate for the facility is an important assumption in determining the reserve because changes in this assumption have the greatest effect on estimated sublease income. In addition, the vacancy rate estimate is the variable most subject to change, while at the same time it involves the greatest judgment and uncertainty due to the absence of highly predictive information concerning the future of the local economy and future demand for specialized laboratory and office space in that area. The average vacancy rate of the facility forover the last five years 2001(2003 through 20062007) was approximately 67%73%, varying from 49%66% to 84%89%. As of December 31, 2006,2007, based on current information available to management, the vacancy rate is projected to be 91%80% for 2007,2008, and approximately 70% from 20082009 through the end of the lease. These estimates are based on actual occupancy as of December 31, 2006,2007, predicted lead time for acquiring new subtenants, historical vacancy rates for the area and assessments by our broker/realtor of future real estate market conditions. If the assumed vacancy rate for 20082009 to the end of the Leaselease had been five percentage points higher or lower at December 31, 2006,2007, then the reserve would have increased or decreased by approximately $239,000.$214,000. Similarly, a 5% increase or decrease in the operating expenses for the facility from 20062008 on would have increased or decreased the reserve by approximately $124,000,$122,000, and a 5% increase or decrease in the assumed average rental charge per square foot would have increased or decreased the reserve by approximately $66,000.$80,000. Management does not wait for specific events to change its estimate, but instead uses its best efforts to anticipate them on a quarterly basis.
The wind-down reserve at the end of December 31, 2005 was $6,098,000. For the year ended December 31, 2006,2007, we recorded actual expenses against this reserve of approximately $1,295,000.$1,420,000. Based on management’s evaluation of the factors mentioned above, and particularly the projected vacancy rates described above, we adjusted the reserve to $5,512,000$6,143,000 at December 31, 2007 by recording an additional $709,000$783,000 for the year ended December 31, 2006.2007.
Income Taxes
We account for income taxes in accordance with SFAS No. 109, Accounting for Income Taxes(SFAS 109) and FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes, an interpretation of FASB Statement No. 109,as amended by FASB Staff PositionNo. 48-1 (FIN 48). Under SFAS 109 and FIN 48, we must recognize deferred tax assets and liabilities for expected future tax consequences of temporary differences between the carrying amounts and tax bases of assets and liabilities. Income tax receivables and liabilities, and deferred tax assets and liabilities, are recognized based on the amounts that more likely than not would be sustained upon ultimate settlement with taxing authorities.
Developing our provision for income taxes and analyzing our tax positions requires significant judgment and knowledge of federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and, any valuation allowances that may be required for deferred tax assets.
We assess the likelihood of realizing our deferred tax assets to determine whether an income tax valuation allowance is required. Based on such evidence that can be objectively verified, we determine whether it is more likely than not that all or a portion of the deferred tax assets will be realized. The main factors that we consider include:
| | |
| • | cumulative losses in recent years; |
|
| • | income/losses expected in future years; and |
|
| • | the applicable statute of limitations. |
Tax benefits associated with uncertain tax positions are recognized in the period in which one of the following conditions is satisfied: (1) the more likely than not recognition threshold is satisfied; (2) the position is ultimately settled through negotiation or litigation; or (3) the statute of limitations for the taxing authority to examine and challenge the position has expired. Tax benefits associated with an uncertain tax position are reversed in the period in which the more likely than not recognition threshold is no longer satisfied.
34
We concluded that the realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance.
Contingencies
We are currently involved in certain legal proceedings. See Note 8, “Commitments and Contingencies,” in the Notes to Consolidated Financial Statements of Part II, Item 8 of thisForm 10-Kfor further information on these matters.
Results of Operations
Our results of operations have varied significantly from year to year and quarter to quarter and may vary significantly in the future due to the occurrence of material recurring and nonrecurring events, including without limitation the receipt and payment of recurring and nonrecurring licensing payments, the initiation or termination of research collaborations, the on-going expenses to lease and maintain our Rhode Island facilities, and the increasing costs associated with operating our California facility. To expand and provide high quality systems and support to our research and development programs, we will need to hire more personnel, which will lead to higher operating expenses.
Revenue
Revenue totaled approximately $57,000 in 2007, $93,000 in 2006, and $206,000 in 2005.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Change in
| | | Change in
| |
| | | | | | | | | | | | 2007
| | | 2006
| |
| | | | | | | | | | | | Versus 2006 | | | Versus 2005 | |
| | | 2007 | | | 2006 | | | 2005 | | | $ | | | % | | | $ | | | % | |
| |
| Revenue | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Licensing agreements and grants | | $ | 56,722 | | | $ | 92,850 | | | $ | 205,914 | | | $ | (36,128 | ) | | | (39 | )% | | $ | (113,064 | ) | | | (55 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The decrease in licensing and grant revenue in 2007 as compared to 2006 was primarily attributable to the completed draw down in 2006 of a breakdown$464,000 Small Business Technology Transfer Grant for studies in Alzheimer’s disease that was awarded in September 2004. The grant supported joint work with Dr. George A. Carlson of the McLaughlin Research Institute (MRI) in Great Falls, Montana. The decrease in licensing and grant revenue in 2006 as compared to 2005 was primarily attributable to lesser amounts drawn down in 2006 from the grant as compared to 2005. We received and recognized approximately $38,000 in 2006, $186,000 in 2005, and $26,000 in 2004 as grant revenue, the remainder was reimbursed to MRI.
35
Operating Expenses
Operating expense totaled approximately $28,648,000 in 2007, $21,464,000 in 2006, and $16,594,000 in 2005.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Change in
| | | Change in
| |
| | | | | | | | | | | | 2007
| | | 2006
| |
| | | | | | | | | | | | Versus 2006 | | | Versus 2005 | |
| | | 2007 | | | 2006 | | | 2005 | | | $ | | | % | | | $ | | | % | |
| |
| Operating Expenses | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Research & development | | $ | 19,937,426 | | | $ | 13,600,433 | | | $ | 8,226,734 | | | $ | 6,336,993 | | | | 47 | % | | $ | 5,373,699 | | | | 65 | % |
| General & administrative | | | 7,927,443 | | | | 7,154,042 | | | | 5,539,845 | | | | 773,401 | | | | 11 | % | | | 1,614,197 | | | | 29 | % |
| Wind-down expenses | | | 783,022 | | | | 709,209 | | | | 2,827,403 | | | | 73,813 | | | | 10 | % | | | (2,118,194 | ) | | | (75 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total expense | | $ | 28,647,891 | | | $ | 21,463,684 | | | $ | 16,593,982 | | | $ | 7,184,207 | | | | 33 | % | | $ | 4,869,702 | | | | 29 | % |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Research and Development Expenses
Our R&D expenses consist primarily of salaries and related personnel expenses, costs associated with clinical trials and regulatory submissions; costs associated with preclinical activities such as toxicology studies; certain patent-related costs such as licensing; facilities-related costs such as depreciation; lab equipment and supplies. Clinical trial expenses include payments to vendors such as clinical research organizations, contract manufacturers, clinical trial sites, laboratories for testing clinical samples and consultants. Cumulative R&D costs incurred since we refocused our activities on developing cell-based therapeutics (fiscal years 2000 through 2007) were approximately $75 million. Over this period, the majority of these figurescumulative costs were related to: (i) characterization of our proprietary HuCNS-SC cell, (ii) expenditures for toxicology and other preclinical studies, preparation and submission of our Investigational New Drug (IND) application for our Phase I trial for NCL to the FDA, and obtaining FDA clearance; and (iii) expenditures in connection with our HuCNS-SC Phase I clinical trial.
We use and manage our R&D resources, including our employees and facilities, across various projects rather than on aproject-by-project basis for the following reasons. The allocations of time and resources change as the needs and priorities of individual projects and programs change, and many of our researchers are assigned to more than one project at any given time. Furthermore, we are exploring multiple possible uses for each of our proprietary cell types, so much of our R&D effort is complementary to and supportive of each of these projects. Lastly, much of our R&D effort is focused on manufacturing processes, which can result in process improvements useful across cell types. We also use external service providers to assist in the conduct of our clinical trials, to manufacture certain of our product candidates and to provide various other R&D related products and services. Many of these costs and expenses are complementary to and supportive of each of our programs. Because we do not have a development collaborator for any of our product programs, we are currently responsible for all costs incurred with respect to our product candidates.
R&D expense totaled approximately $19,937,000 in 2007, as compared to $13,600,000 in 2006 and $8,227,000 in 2005. At December 31, 2007, we had 49 full-time employees working in research and development and laboratory support services as compared to 35 at December 31, 2006 and 33 at December 31, 2005.
2007 versus 2006. The increase of approximately $6,337,000, or 47%, from 2006 to 2007 was primarily attributable to the continued expansion of our operations in cell processing and clinical development, including an increase in external services and clinical study costs of approximately $3,954,000, and an increase in personnel costs of approximately $1,442,000, of which approximately $206,000 was attributable to stock-based compensation expense. The remainder of the increase in 2007 was due to increases in supplies, rent, and other operating expenses.
2006 versus 2005. The increase of approximately $5,374,000, or 65%, from 2005 to 2006 was primarily attributable to expansion of our operations in cell processing and clinical development, which consisted of an increase in personnel costs of approximately $2,669,000, an increase in external services of approximately
36
$1,464,000, an increase in supplies and other expenses of $771,000 and the cost of additional space leased in 2006 allocated to research and development. Of the approximately $2,669,000 increase in personnel costs, approximately $1,344,000 was attributable to stock-based compensation expense. The remaining increase was primarily attributable to increased head count.
General and Administrative Expenses
General and administrative (G&A) expenses totaled approximately $7,927,000 in 2007, compared with $7,154,000 in 2006 and $5,540,000 in 2005.
2007 versus 2006. The increase of approximately $773,000, or 11%, from 2006 to 2007 was primarily attributable to an increase in external services of approximately $763,000, driven by quarter.an increase in legal fees related to patent prosecutions and litigation, and an increase in personnel costs of approximately $425,000, of which approximately $211,000 was attributable to an increase in stock-based compensation expense. These increases were partially offset by a decrease in other G&A expenses.
2006 versus 2005. The increase of approximately $1,614,000, or 29%, from 2005 to 2006 was primarily due to the increase in personnel costs of approximately $1,826,000, of which approximately $1,423,000 was attributable to stock-based compensation expense. The increase in personnel costs was partially offset by a net decrease in other costs primarily attributable to the expensing of the fair value of options granted to a consultant in 2005. No such options were granted in 2006.
Wind-down Expenses
In 1999, in connection with exiting our former research facility in Rhode Island, we created a reserve for the estimated lease payments and operating expenses related to it. The reserve has been re-evaluated and adjusted based on assumptions relevant to real estate market conditions and the estimated time until we could either fully sublease, assign or sell our remaining interests in the property. The reserve was approximately $6,145,000 at December 31, 2007 and $6,750,000 at December 31, 2006. Payments net of subtenant income were recorded against this reserve of $1,420,000 in 2007, $1,295,000 in 2006, and $1,079,000 in 2005. We re-evaluated the estimate and adjusted the reserve by recording in aggregate, additional wind-down expenses of $783,000 in 2007, $709,000 in 2006, and $2,827,000 in 2005. Expenses for this facility will fluctuate based on changes in tenant occupancy rates and other operating expenses related to the lease. Even though it is our intent to sublease, assign, sell, or otherwise divest ourselves of our interests in the facility at the earliest possible time, we cannot determine with certainty a fixed date by which such events will occur. In light of this uncertainty, based on estimates, we will periodically re-evaluate and adjust the reserve, as necessary. See Note 7 “Wind-down and exit costs,” in the Notes to Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information.
Other income (expense)Income (Expense)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Change from
| | | Change from
| |
| | | | | | | | | | | | previous year:
| | | previous year:
| |
| | | | | | | | | | | | 2006 versus 2005 | | | 2005 versus 2004 | |
| | | 2006 | | | 2005 | | | 2004 | | | $ | | | % | | | $ | | | % | |
| |
| Other income (expense): | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| License and settlement agreement | | $ | 103,359 | | | $ | 3,735,556 | | | | — | | | $ | (3,632,197 | ) | | | (97 | )% | | $ | 3,735,556 | | | * | N/M | |
| Interest income | | | 2,479,740 | | | | 1,122,963 | | | $ | 322,227 | | | | 1,356,777 | | | | (121 | )% | | | 800,736 | | | | 249 | % |
| Interest expense | | | (143,001 | ) | | | (171,909 | ) | | | (191,006 | ) | | | 28,908 | | | | (17 | )% | | | 19,097 | | | | (10 | )% |
| Other income (expense), net | | | (17,644 | ) | | | (36,892 | ) | | | (61,680 | ) | | | 19,248 | | | | (52 | )% | | | 24,788 | | | | (40 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total other income (expense) | | $ | 2,422,454 | | | $ | 4,649,718 | | | $ | (69,541 | ) | | $ | (2,227,264 | ) | | | (48 | )% | | $ | 4,580,177 | | | * | N/M | |
Other income totaled approximately $3,568,000 in 2007, compared with $2,422,000 in 2006 and $4,650,000 in 2005.
37
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Change in
| | | Change in
| |
| | | | | | | | | | | | 2007
| | | 2006
| |
| | | | | | | | | | | | Versus 2006 | | | Versus 2005 | |
| | | 2007 | | | 2006 | | | 2005 | | | $ | | | % | | | $ | | | % | |
| |
| Other income (expense): | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| License and settlement agreement | | $ | 550,467 | | | $ | 103,359 | | | $ | 3,735,556 | | | $ | 447,108 | | | | 433 | % | | $ | (3,632,197 | ) | | | (97 | )% |
| Interest income | | | 2,459,820 | | | | 2,479,740 | | | | 1,122,963 | | | | (19,920 | ) | | | (1 | )% | | | 1,356,777 | | | | 121 | % |
| Interest expense | | | (123,606 | ) | | | (143,001 | ) | | | (171,909 | ) | | | 19,395 | | | | (14 | )% | | | 28,908 | | | | (17 | )% |
| Gain on sale of investment | | | 715,584 | | | | — | | | | — | | | | 715,584 | | | | | *% | | | — | | | | | *% |
| Other expense, net | | | (33,899 | ) | | | (17,644 | ) | | | (36,892 | ) | | | (16,255 | ) | | | 92 | % | | | 19,248 | | | | (52 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total other income, net | | $ | 3,568,366 | | | $ | 2,422,454 | | | $ | 4,649,718 | | | $ | 1,145,912 | | | | 47 | % | | $ | (2,227,264 | ) | | | (48 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| * | | Non meaningfulCalculation can not be performed or is not meaningful. |
License and settlement agreementSettlement Agreement
In July 2005, we entered into an agreement with ReNeuron Limited, a wholly owned subsidiary of ReNeuron Group plc, a listed UK corporation (collectively referred to as “ReNeuron”). As part of the agreement, we granted ReNeuron a license that allows ReNeuron to exploit their“c-mycER” “c-mycER” conditionally immortalized adult human neural stem cell technology for therapy and other purposes. StemCellsWe received a 7.5% fully-diluted equity interest
43
in ReNeuron, subject to certain anti-dilution provisions, and a cross-license to the exclusive use of ReNeuron’s technology for certain diseases and conditions, including lysosomal storage diseases, spinal cord injury, cerebral palsy, and multiple sclerosis. The agreement also provides for full settlement of any potential claims that either we or ReNeuron might have had against the other in connection with any putative infringement of certain of each party’s patent rights prior to the effective date of the agreement. The agreement is Exhibit 10.71 to our Quarterly Report onForm 10-Q for the quarter ended June 30, 2005.
We recordedOther income from the license and settlement agreement totaled approximately $550,000 in 2007, $103,000 and $3,736,000 as other income in 2006, and $3,736,000 in 2005, respectively, which was the fair value of the ReNeuron shares we received under such agreement, net of legal fees and the value of the shares that waswere transferred to NeuroSpheres Ltd., an Alberta corporation from which we have licensed some of the patent rights that are the subject of the agreement with ReNeuron. See Note 2 “Financial Instruments — ReNeuron License Agreement” in the Notes to Consolidated Financial Statements of Part II, Item 8 of thisForm 10-Kfor more details onfurther information regarding this transaction.
Interest incomeIncome
Interest income for the years ended December 31, 2006, 2005 and 2004 totaled approximately $2,460,000 in 2007, $2,480,000 in 2006, and $1,123,000 and $322,000, respectively.in 2005. Interest income in 2007 was relatively flat compared to 2006, as a result of lower average bank balances offset by higher average yields. The increase in interest income from 20042005 to 2006 was primarily attributable to a higher average bank balance as a result of our financing transactions. (See “LiquiditySee “Cash Used in or Provided by Financing Activities,” in Liquidity and Capital Resources”Resources below for further detail on these transactions) and a higher yield on overnight and money market funds.information.
Interest expenseExpense
In 2006, interestInterest expense was approximately $124,000 in 2007, $143,000 in 2006, and $172,000 in 2005. The decreases in 2007 as compared to approximately $172,0002006 and in 2006 as compared to 2005 and approximately $191,000 in 2004. The decrease from 2004 to 2006 waswere attributable to lower outstanding debt and capital lease balances. See Note 8 “Commitment and Contingencies,” in the Notes to Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information.
38
Gain on Sale of Marketable Equity Securities
The gain on sale of marketable equity securities of approximately $716,000 in 2007 was attributable to sales of ReNeuron shares. See Note 2 “Financial Instruments,” in the Notes to Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information on this transaction.
Other income/expense,Expense, net
Other expensesexpense, net for 2007 were approximately $34,000, which included approximately $34,000 for state franchise taxes paid, partially offset by a gain of approximately $1,500 from the disposal of old equipment. Other expense, net for 2006 were approximately $18,000, which includeincluded approximately $20,000 for state franchise taxes paid, partially offset by a gain of approximately $2,000 from the disposal of old equipment. Other expensesexpense, net for 2005 were approximately $37,000, which includeincluded approximately $36,000 for state franchise taxes and approximately $1,000 from a write-off of obsolete equipment. Other expenses for 2004 were approximately $62,000, which include a loss of approximately $56,000 resulting from a write-off of obsolete lab equipment and approximately $6,000 for state franchise taxes.
Liquidity and Capital Resources
Since our inception, we have financed our operations through the sale of common and preferred stock, the issuance of long-term debt and capitalized lease obligations, revenuesrevenue from collaborative agreements, research grants, license fees, and interest income.
44
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Change in
| | | Change in
| |
| | | | | | | | | | | | 2007
| | | 2006
| |
| | | | | | | | | | | | Versus 2006 | | | Versus 2005 | |
| | | 2007 | | | 2006 | | | 2005 | | | $ | | | % | | | $ | | | % | |
| |
| At December 31: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and highly liquid investments(1) | | $ | 37,645,085 | | | $ | 51,795,529 | | | $ | 34,540,908 | | | $ | (14,150,444 | ) | | | (27 | )% | | $ | 17,254,621 | | | | 50 | % |
| Year ended December 31: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net cash used in operating activities | | $ | (20,856,746 | ) | | $ | (16,104,120 | ) | | $ | (11,870,568 | ) | | $ | (4,752,626 | ) | | | 29 | % | | $ | (4,233,552 | ) | | | 36 | % |
| Net cash used in investing activities | | | (27,155,656 | ) | | | (1,297,124 | ) | | | (847,505 | ) | | | (25,858,532 | ) | | | 1994 | % | | | (449,619 | ) | | | 53 | % |
| Net cash provided by financing activities | | | 5,976,042 | | | | 34,655,865 | | | | 6,199,449 | | | | (28,679,823 | ) | | | (83 | )% | | | 28,456,416 | | | | 459 | % |
We had
| | |
| (1) | | Cash and highly liquid investments include unrestricted cash, cash equivalents, and short-term and long-term marketable debt securities. Marketable equity securities, which are comprised of 4,821,924 ordinary shares of ReNeuron, are excluded from the amounts above. See Note 2, “Financial Instruments,” in the Notes to the Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information. |
Total cash and cash equivalents totalinghighly liquid investments were approximately $37,645,000 at December 31, 2007, compared with approximately $51,796,000 at December 31, 2006, and $34,541,000 at December 31, 2005.
2007 versus 2006. Cash equivalents are generally invested in U.S. Treasuries with maturities of less than 90 days. The table below summarizesdecrease in our cash flows for the respective fiscal years.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Change from
| | | Change from
| |
| | | | | | | | | | | | previous year:
| | | previous year:
| |
| | | | | | | | | | | | 2006 versus 2005 | | | 2005 versus 2004 | |
| | | 2006 | | | 2005 | | | 2004 | | | $ | | | % | | | $ | | | % | |
| |
| Net cash used in operating activities | | $ | (16,104,120 | ) | | $ | (11,870,568 | ) | | $ | (11,273,908 | ) | | $ | (4,233,552 | ) | | | 36 | % | | $ | (596,660 | ) | | | 5 | % |
| Net cash used in investing activities | | | (1,297,124 | ) | | | (847,505 | ) | | | (748,305 | ) | | | (449,619 | ) | | | 53 | % | | | (99,200 | ) | | | 13 | % |
| Net cash provided by financing activities | | | 34,655,865 | | | | 6,199,449 | | | | 40,000,042 | | | | 28,456,416 | | | | 459 | % | | | (33,800,592 | ) | | | (85 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Increase (decrease) in cash and cash equivalents | | $ | 17,254,621 | | | $ | (6,518,624 | ) | | $ | 27,977,829 | | | $ | 23,773,245 | | | | 365 | % | | $ | (34,496,452 | ) | | | (123 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
and highly liquid investments of approximately $14,150,000, or 27%, from December 31, 2006 to December 31, 2007 was primarily attributable to cash used in operating activities; partially offset by cash generated from financing activities.
We2006 versus 2005. The increase in our cash and highly liquid investments of approximately $17,255,000, or 50%, from December 31, 2005 to December 31, 2006 was primarily attributable to cash generated from financing activities; partially offset by cash used in operating activities.
Net Cash Used in Operating Activities
In our operating activities we used approximately $20,857,000 in cash in 2007, $16,104,000 $11,871,000, and $11,274,000 ofin cash in 2006, 2005 and 2004 respectively,$11,871,000 in our operating activities.cash in 2005. The increase in cash used in operating activities in 2007 as compared to 2006 and 2006 as compared to 2005 was primarily attributable to the expansion of our operations in cell processing and clinical development, including increases in 2006. headcount and headcount related expenses and external services.
39
Net Cash Used in Investing Activities
The increase infrom 2006 to 2007 of approximately $25,859,000 for net cash used in operatinginvesting activities was almost entirely due to the redeployment of cash held in money market funds (classified as cash equivalents) to marketable debt securities (classified as marketable securities). In February 2007, we sold 5,275,000 ordinary shares of ReNeuron for net proceeds of approximately $3,077,000. In addition, cash used in investing activities in 2007 includes a secured loan of $1,000,000 made to PCT in December 2007. See Note 2, “Financial Instruments,” in the Notes to the Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information on our investing activities. The increase from 2005 as compared to 20042006 of approximately $450,000 for net cash used in investing activities was primarily attributable to an increase in head count to strengthen our scientific and management team.capital expenditures.
Net Cash Provided by Financing Activities
The decrease from 2006 to 2007 of approximately $28,680,000 and the increase in 2006 from 2005 to 2006 of approximately $28,456,000 for net cash provided by financing activities was primarily attributable to the sale, on April 6, 2006, of 11,750,820 shares of our common stock to a limited number of institutional investors at a price of $3.05 per share. The CompanyWe received total proceeds, net of offering expenses and placement agency fees, of approximately $33,422,000.
Listed below are key financing transactions entered into by us in the last three years:
| | |
| • | In April 2007, a warrant issued as part of a June 16, 2004 financing arrangement, was exercised to purchase an aggregate of 575,658 shares of our common stock at $1.90 per share. We issued 575,658 shares of our common stock and received proceeds of approximately $1,094,000. |
|
| • | On December 29, 2006, we filed a Prospectus Supplement announcing the entry of a sales agreement with Cantor Fitzgerald & Co. under which up to 10,000,000 shares may be sold from time to time under a shelf registration statement. In 2007, we sold a total of 1,807,000 shares of our common stock under this agreement at an average price per share of $2.84 for gross proceeds of approximately $5,133,000. Cantor is paid compensation equal to 5.0% of the gross proceeds pursuant to the terms of the agreement. |
|
| | • | On April 6, 2006, we sold 11,750,820 shares of our common stock to a limited number of institutional investors at a price of $3.05 per share, for gross proceeds of approximately $35,840,000. The shares were offered as a registered direct offering under an effective shelf registration statement previously filed with and declared effective by the Securities and Exchange Commission. We received total proceeds, net of offering expenses and placement agency fees, of approximately $33,422,000. No warrants were issued as part of this financing transaction. |
| |
| | • | In March 2006, a warrant issued as part of a June 16, 2004 financing arrangement was exercised to purchase an aggregate of 526,400 shares of our common stock at $1.89 per share. We issued 526,400 shares of our common stock and received proceeds of approximately $995,000. |
|
| • | In 2005, warrants for the purchase of up to an aggregate of 2,958,348 warrantsshares were exercised. For the exercise of these warrants, we issued 2,842,625 shares of our common stock and received proceeds of approximately $5,939,000. |
|
| • | On October 26, 2004, we entered into an agreement with institutional investors with respect to the registered direct placement of 7,500,000 shares of our common stock at a purchase price of $3.00 per share, for gross proceeds of $22,500,000. C.E. Unterberg, Towbin LLC (Unterberg) and Shoreline Pacific, LLC (Shoreline) served as placement agents for the transaction. We sold these shares under a shelf registration statement previously filed with and declared effective by the U.S. Securities and Exchange Commission. For acting as our placement agent Unterberg and Shoreline received fees of approximately $1,350,000 and expense reimbursement of approximately $40,000. No warrants were issued as part of this financing transaction. |
|
| • | On June 16, 2004, we entered into an agreement with institutional and other accredited investors with respect to the private placement of approximately 13,160,000 shares of our common stock at a purchase price of $1.52 per share, for gross proceeds of approximately $20,000,000. Investors also received warrants exercisable for five years to purchase approximately 3,290,000 shares of common stock at an exercise price of $1.90 per share. During the period October 2004 to December 2005, part of these warrants were |
45
| | |
| | exercised to purchase an aggregate of 1,459,342 shares of our common stock at $1.90 per share. We received proceeds of $2,772,750 on issuance of the shares. Unterberg served as placement agent for the private placement. For acting as our placement agent, Unterberg received fees of approximately $1,200,000, expense reimbursement of approximately $25,000 and a five year warrant to purchase 526,400 shares of our common stock at an exercise price of $1.89 per share. |
We continue to have outstanding obligations in regard to our former facilities in Lincoln, Rhode Island. In 1997, we had entered into a fifteen-year lease for a scientific and administrative facility (the SAF) in a sale and leaseback arrangement. The lease includes escalating rent payments. For the year 2007, we expect to pay approximately $938,000 in operating lease payments and estimated operating expenses of approximately $490,000, before receipt ofsub-tenant income. In 1992 and 1994 we had undertaken direct financing transactions with the State of Rhode Island and received proceeds from the issuance of industrial revenue bonds totaling $5,000,000 to finance the construction of a pilot manufacturing facility and a related cell processing facility. The related leases are structured such that lease payments will fully fund all semiannual interest payments and annual principal payments through maturity in August 2014. For these related facilities we expect to pay approximately $365,000 in principal, interest and related expenses in 2007, before receipt ofsub-tenant income. We have subleased the pilot manufacturing facility and the cell processing facility, as well as approximately one-fourth of the SAF. For the year 2007, we expect to receive, in aggregate, approximately $573,000 insub-tenant rent for all of the Rhode Island facilities. As a result of the above transactions, our estimated cash outlay net ofsub-tenant rent for the Rhode Island facilities will be approximately $1,220,000 for 2007. We are actively seeking to sublease, assign or sell our remaining interests in these facilities. Failure to do so within a reasonable period of time will have a material adverse effect on our liquidity and capital resources.
The following table summarizes our future contractual cash obligations (including both Rhode Island and California leases, but excluding interest income andsub-lease income with respect to the Rhode Island properties):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | Payable in
| |
| | | Total
| | | | | | | | | | | | | | | | | | 2012
| |
| | | Obligations
| | | Payable in
| | | Payable in
| | | Payable in
| | | Payable in
| | | Payable in
| | | and
| |
| | | at12/31/06 | | | 2007 | | | 2008 | | | 2009 | | | 2010 | | | 2011 | | | Beyond | |
| |
Bonds Payable
(principal & interest) | | $ | 1,921,639 | | | $ | 332,545 | | | $ | 244,531 | | | $ | 244,572 | | | $ | 242,560 | | | $ | 242,321 | | | $ | 615,110 | |
| Operating lease payments | | | 15,014,498 | | | | 3,165,162 | | | | 3,469,017 | | | | 3,536,843 | | | | 1,767,304 | | | | 1,171,875 | | | | 1,904,297 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total contractual cash obligations | | $ | 16,936,137 | | | $ | 3,497,707 | | | $ | 3,713,548 | | | $ | 3,781,415 | | | $ | 2,009,864 | | | | 1,414,196 | | | $ | 2,519,407 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
We have incurred significant operating losses and negative cash flows since inception. We have not achieved profitability and may not be able to realize sufficient revenuesrevenue to achieve or sustain profitability in the future. We do not expect to be profitable in the next several years, but rather expect to incur additional operating losses. We have limited liquidity and capital resources and must obtain significant additional capital resources in order to sustain our product development efforts, for acquisition of technologies and intellectual property rights, for preclinical and clinical testing of our anticipated products, pursuit of regulatory approvals, acquisition of capital equipment, laboratory and office facilities, establishment of production capabilities, for general and administrative expenses and other working capital requirements. We rely on cash balances and proceeds from equity and debt offerings, proceeds from the transfer or sale of our intellectual property rights, equipment, facilities or investments, and government grants and funding from collaborative arrangements, if obtainable, to fund our operations.
40
We intend to pursue opportunities to obtain additional financing in the future through equity and debt financings, grants and collaborative research arrangements. We have a shelf registration statement which, as of December 31, 2006,2007, covered shares of our common stock up to a value of $64approximately $59 million that could be available for financings. On December 29, 2006, we filed a Prospectus Supplement announcing the entry of a sales agreement with Cantor Fitzgerald & Co. under which up to 10,000,000 shares may be sold from time to time under the shelf registration statement. The source, timing and availability of any future financing will depend principally upon market conditions, interest rates and, more specifically, on our progress in our exploratory, preclinical and future clinical development programs. Funding may not be available when needed — at all, or on terms acceptable to us. Lack of necessary funds may require us, among other things, to delay, scale back or eliminate some or all of our research and product
46
development programs, planned clinical trials,and/or our capital expenditures or to license our potential products or technologies to third parties.
Commitments
See Note 8, “Commitments and Contingencies” in the Notes to Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information.
Off-Balance Sheet Arrangements
We have certain contractual arrangements that create potential risk for us and are not recognized in our Consolidated Balance Sheets. Discussed below are those off-balance sheet arrangements that have or are reasonably likely to have a material current or future effect on our financial condition, changes in financial condition, revenue or expenses, results of operations, liquidity, capital expenditures or capital resources.
Operating Leases
We lease various real properties under operating leases that generally require us to pay taxes, insurance, maintenance, and minimum lease payments. Some of our leases have options to renew.
We entered into and amended a lease agreement for an approximately 68,000 square foot facility located at the Stanford Research Park in Palo Alto, California. At December 31, 2007, we had a space-sharing agreement covering approximately 10,451 square feet of this facility. We receive base payments plus a proportionate share of the operating expenses based on square footage over the term of the agreement. For the year 2008, we expect to receive, in aggregate, approximately $376,000 as part of the space-sharing agreement. As a result of the above transactions, our estimated net cash outlay for rent will be approximately $1,921,000 for 2008.
We continue to have outstanding obligations in regard to our former facilities in Lincoln, Rhode Island. In 1997, we had entered into a fifteen-year lease for a scientific and administrative facility (the SAF) in a sale and leaseback arrangement. The lease includes escalating rent payments. For the year 2008, we expect to pay approximately $1,172,000 in operating lease payments and estimated operating expenses of approximately $550,000, before receipt ofsub-tenant income. For the year 2008, we expect to receive, in aggregate, approximately $282,000 insub-tenant rent. As a result of the above transactions, our estimated cash outlay net ofsub-tenant rent for the SAF will be approximately $1,440,000 for 2008.
With the exception of operating leases for facilities,discussed above, we have not entered into any off balance sheet financial arrangements and have not established any special purpose entities. We have not guaranteed any debts or commitments of other entities or entered into any options on non-financial assets.
See Note 8, “Commitments and Contingencies,” in the Notes to Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information.
41
Contractual Obligations
In the table below, we set forth our legally binding and enforceable contractual cash obligations:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | Payable in
| |
| | | Total
| | | | | | | | | | | | | | | | | | 2013
| |
| | | Obligations
| | | Payable in
| | | Payable in
| | | Payable in
| | | Payable in
| | | Payable in
| | | and
| |
| | | at 12/31/07 | | | 2008 | | | 2009 | | | 2010 | | | 2011 | | | 2012 | | | Beyond | |
| |
| Operating lease payments(1) | | $ | 11,849,336 | | | $ | 3,469,017 | | | $ | 3,536,843 | | | $ | 1,767,304 | | | $ | 1,171,875 | | | $ | 1,171,875 | | | $ | 732,422 | |
| Capital lease (equipment) | | | 46,347 | | | | 19,862 | | | | 19,862 | | | | 6,623 | | | | — | | | | — | | | | — | |
| Bonds Payable (principal & interest)(2) | | | 1,589,093 | | | | 244,531 | | | | 244,572 | | | | 242,559 | | | | 242,321 | | | | 240,666 | | | | 374,444 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total contractual cash obligations | | $ | 13,484,776 | | | $ | 3,733,410 | | | $ | 3,801,277 | | | $ | 2,016,486 | | | $ | 1,414,196 | | | $ | 1,412,541 | | | $ | 1,106,866 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Operating lease payments exclude space-sharing andsub-lease income. See “Off-Balance Sheet Arrangements — Operating Leases” above for further information. |
|
| (2) | | See Note 8, “Commitments and Contingencies” in the Notes to Consolidated Financial Statements of Part II, Item 8 of thisForm 10-K for further information. |
We do not have any material unconditional purchase obligations or commercial commitments related to capital expenditures, clinical development, clinical manufacturing, or other external services contracts at December 31, 2007.
The above table excludes our obligation to lend up to an additional $2.8 million to PCT. This obligation is subject to the satisfaction of certain preconditions, which we do not expect to occur. See “Overview” above for further information.
Recent Accounting Pronouncements
In July 2006, the FASB issued Interpretation No. 48, “Accounting for Uncertainty in Income Taxes,” an interpretation of SFAS No. 109, “Accounting for Income Taxes” (SFAS 109). This Interpretation clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements in accordance with SFAS 109. This Interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. This Interpretation also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Interpretation is effective for fiscal years beginning after December 15, 2006. While our analysis of the impact of this Interpretation is not yet complete, we do not anticipate that it will have a material impact on its consolidated financial statements at the time of adoption.
In September 2006, the FASB issued Statement of Financial Accounting StandardsSFAS No. 157, “Fair Value Measurements” (SFAS(SFAS 157). This StandardSFAS 157 defines fair value, establishes a framework and gives guidance regarding the methods used for measuring fair value, in generally accepted accounting principles and expands disclosures about fair value measurements. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. The adoption of FASWe are currently assessing the impact that SFAS 157 is not expected tomay have a material impact on our consolidatedresults of operations and financial statements.position.
In September 2006,February 2007, the SecuritiesFASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Exchange Commission (SEC)Financial Liabilities — Including an amendment of FASB Statement No. 115(SFAS 159). SFAS 159 is expected to expand the use of fair value accounting but does not affect existing standards which require certain assets or liabilities to be carried at fair value. The objective of SFAS 159 is to improve financial reporting by providing companies with the opportunity to mitigate volatility in reported earnings caused by measuring related assets and liabilities differently without having to apply complex hedge accounting provisions. Under SFAS 159, a company may choose, at specified election dates, to measure eligible items at fair value and report unrealized gains and losses on items for which the fair value option has been elected in earnings at each subsequent reporting date. SFAS 159 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. We are currently assessing the impact that SFAS 159 may have on our results of operations and financial position.
In December 2007, the SEC issued Staff Accounting Bulletin (SAB) No. 108, “110,Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements”Share-Based Payment (SAB 108)110), which amends SAB 107,Share-Based Payment, to address diversitypermit public companies, under certain circumstances, to use the simplified method in practice in quantifying financial statement misstatements. SAB 108 requires that we quantify misstatements based on107 for employee option grants after December 31, 2007. Use of the simplified method after December 2007 is permitted only for companies whose historical data about their impact on eachemployees’ exercise behavior does not provide a reasonable basis for estimating the expected term of our financial statements and related disclosures.the options. We currently use the simplified method to estimate the expected term for employee option grants as adequate historical experience is not available to provide a reasonable estimate. SAB 108110 is effective for fiscal years endingemployee options granted after November 15, 2006. Our adoptionDecember 31, 2007. We intend to adopt SAB 110 effective January 1, 2008 and we are currently assessing our historical experience to evaluate if it can provide a reasonable estimate of this bulletin did not have a material impact on our consolidated financial statements.the expected term for employee option grants.
42
| |
| Item 7A. | Quantitative and Qualitative Disclosures About Market RiskQUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Interest Rate and Credit Risk
Our interest-bearing assets, or interest-bearing portfolio, consists of cash, cash equivalents, restricted cash, and marketable debt securities. The balance of our interest-bearing portfolio, was approximately $38,414,000, or 79%, of total assets at December 31, 2007 and $52,567,000, or 79%, of total assets at December 31, 2006. Interest income earned on these assets was approximately $2,460,000 in 2007 and $2,478,000 in 2006. Our interest income is sensitive to changes in the general level of interest rates, primarily U.S. interest rates. Our debt securities are primarily comprised of commercial paper, corporate debt and asset-backed securities. Generally, corporate obligations must have senior credit ratings of A2/A or the equivalent. See Note 1, “Summary of Significant Accounting Policies — Financial Instruments” and Note 2 “Financial Instruments” section in the Notes to Consolidated Financial Statements in Part II, Item 8 of thisForm 10-K for further information.
Our long-term debt is comprised of industrial revenue bonds issued by the State of Rhode Island to finance the construction of our pilot manufacturing facility in Rhode Island. See Note 8, “Commitments and Contingencies,” section in the Notes to Consolidated Financial Statements in Part II, Item 8 of thisForm 10-K for further information.
Equity Security and Foreign Exchange Risks
In July 2005, we entered into ana license and settlement agreement with ReNeuron.ReNeuron Limited, a wholly owned subsidiary of ReNeuron Group plc, a publicly listed UK corporation (collectively referred to as “ReNeuron”). As part of the agreement, we granted ReNeuron a license that allows ReNeuron to exploit itstheir “c-mycER” conditionally immortalized adult human neural stem cell technology for therapy and other purposes. In return for the license, we received a 7.5% fully-diluted equity interest in ReNeuron, subject to certain anti-dilution provisions, and a cross-license to the exclusive use of ReNeuron’s technology for certain diseases and conditions, including lysosomal storage diseases, spinal cord injury, cerebral palsy and multiple sclerosis. The agreement also provides for full settlement of any potential claims that either StemCellswe or ReNeuron might have had against the other in connection with any putative infringement of certain of each party’s patent rights prior to the effective date of the agreement. The agreement is Exhibit 10.71 to our Quarterly Report onForm 10-Q for the quarter ended June 30, 2005. On July 1, 2005,In February 2007, we were entitled to approximately 3,775,000sold 5,275,000 ordinary shares of ReNeuron representing 7.5% of its fully-diluted share capital. On August 12, 2005 ReNeuron listed its shares on the London Stock Exchange’s Alternative Investment Market (“AIM”), a market for smaller, growing companies. In accordance with the anti-dilution provisions of the agreement, the placement and listing of additional shares by ReNeuron resulted in StemCells’ receiving an additional 5,165,000 shares. Of the totalnet proceeds of approximately 8.9 million shares of ReNeuron received by StemCells on account of these events, 104,000 was transferred to NeuroSpheres LTD., an Alberta corporation from which StemCells has licensed some of the patent rights that are the subject of the agreement with ReNeuron. On June 29, 2006,$3,075,000. We recorded approximately $716,000 as realized gain for this transaction. In February 2007, ReNeuron issued additional shares of common stock as a consequence of which StemCells wascertain anti-dilution provisions in the agreement. We were entitled to approximately 439,000822,000 shares under the anti-dilution provisions of the agreement and net of approximately 5,60012,000 shares duewere transferred to Neurospheres Ltd. The CompanyNeuroSpheres. We recorded approximately $103,000$551,000 as other income for the additional shares due in 2006. The fair market value of the Company’s holdings in ReNeuron common stock as of December 31, 2005 (8,835,766 shares) and December 31,additional shares received.
47
2006 (9,274,837 shares) was approximately $3,721,000 and $7,266,000 respectively. Changes in market value as a result of changes in market price per share or the exchange rate between the USU.S. dollar and the British pound are accounted for under “other comprehensive income (loss)” if deemed temporary as in this case, and are not recorded as “other income or loss” until the shares are disposed of and a gain or loss realized. The unrealized gainloss as of December 31, 2006,2007 was approximately $3,188,000. A decline in the fair value of securities that is deemed other than temporary would be charged to earnings. In February 2007, ReNeuron issued additional shares of common stock; as a consequence of the anti-dilution provisions, StemCells was entitled to approximately 823,000 shares net of approximately 10,000 shares to be transferred to NeuroSpheres. These shares satisfy ReNeuron’s obligations under the anti-dilution provision of the agreement. As of February 26, 2007, StemCells had sold approximately 5,275,000 shares of ReNeuron, realizing net proceeds of approximately $3.1 million, and still held approximately 4.8 million shares.$249,000.
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | Share
| | | | | | | | |
| | | | | | | No. of
| | price at
| | Exchange
| | Market
| | | | |
| | | | | | | Shares
| | December 31,
| | Rate at
| | Value
| | | Expected
| |
Company/
| | | | | | at
| | 2006
| | December 31,
| | in USD at
| | | Future
| |
Stock
| | | | Associated
| | December
| | in
| | 2006
| | December 31,
| | | Cash
| |
Symbol | | Exchange | | Risks | | 31, 2006 | | GBP (£) | | 1 GBP = USD | | 2006 | | | Flows | |
| |
ReNeuron
Group
plc/RENE | | AIM (AIM is
the London Stock
Exchange’s Alternative
Investment Market) | | - Lower share
price
- Foreign
currency
translation
- Liquidity
- Bankruptcy | | 9,274,837 | | 0.40 | | 1.9586 | | $ | 7,266,278 | | | | (1) | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | Share
| | | | | | | | | |
| | | | | | | No. of
| | | price at
| | | Exchange
| | | Market
| | | |
| | | | | | | Shares
| | | December 31,
| | | Rate at
| | | Value
| | | Expected
|
| | | | | | | at
| | | 2007
| | | December 31,
| | | in USD at
| | | Future
|
| | | | | Associated
| | December 31,
| | | in
| | | 2007
| | | December 31,
| | | Cash
|
Company/Stock Symbol | | Exchange | | Risks | | 2007 | | | GBP(£) | | | 1 GBP = USD | | | 2007 | | | Flows |
| |
| ReNeuron Group plc/RENE | | AIM (AIM is the London Stock Exchange’s Alternative Investment Market) | | — Lower share price
— Foreign currency translation
— Liquidity
— Bankruptcy | | | 4,821,924 | | | | 0.205 | | | | 1.9843 | | | $ | 1,961,469 | | | (1) |
| | |
| (1) | | It is our intention to liquidate this investment when we can do so at prices acceptable to us. Although we are not legally restricted from selling the stock, the share price is subject to change and the volume traded has often been very small since the stock was listed on the AIM on August 12, 2005. The performance of ReNeuron Group plc stock since its listing does not predict its future value. |
4843
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Board of Directors and Stockholders of
StemCells, Inc.
We have audited management’s assessment, included in the accompanying Management’s Report on Internal Control Over Financial Reporting as of December 31, 2006, that StemCells, Inc. maintained effective’s (a Delaware corporation) internal control over financial reporting as of December 31, 2006,2007, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). StemCells, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting.reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on management’s assessment, and an opinion on the effectiveness of the Company’sStemCells, Inc.’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management’s assessment,assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management’s assessment that StemCells, Inc. maintained effective internal control over financial reporting as of December 31, 2006, is fairly stated, in all material respects, based on Internal Control — Integrated Framework issued by COSO. Furthermore, in our opinion, StemCells, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2006,2007 based on criteria established inInternal Control — Integrated Frameworkissued by COSO.
We also have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of StemCells, Inc. and subsidiary as of December 31, 20062007 and 2005,2006, and the related consolidated statements of operations, changes in stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 20062007 and our report dated March 1, 20077, 2008 expressed an unqualified opinion on those financial statements.thereon.
/s/ GRANT THORNTON LLP
San Jose, CAFrancisco, California
March 1, 20077, 2008
4944
| |
| Item 8. | Financial Statements and Supplementary DataFINANCIAL STATEMENTS AND SUPPLMENTARY DATA |
STEMCELLS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| | | | | |
| | | Page |
| |
| | | 5146 | |
| | | 5247 | |
| | | 5348 | |
| | | 5449 | |
| | | 5550 | |
| | | 5651 | |
5045
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
StemCells, Inc.
We have audited the accompanying consolidated balance sheets of StemCells, Inc. (a Delaware corporation) and subsidiary as of December 31, 20062007 and 2005,2006, and the related consolidated statements of operations, changes in stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2006.2007. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of StemCells, Inc. and subsidiary as of December 31, 20062007 and 2005,2006, and the results of itstheir operations and itstheir cash flows for each of the three years in the period ended December 31, 2006,2007, in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 1 to the consolidated financial statements, effective January 1, 2006 the Company adopted the provisions of Statement of Financial Accounting Standards No. 123(R),Share-Based Payment, applying the modified-prospective method.
We also have audited, in accordance with standards of the Public Company Accounting Oversight Board (United States), the effectiveness of StemCells, Inc.’s internal control over financial reporting as of December 31, 2006,2007, based on criteria established inInternal Control — Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 1, 20077, 2008 expressed an unqualified opinion on management’s assessment of, and an unqualified opinion on the effective operation of, internal control over financial reporting.thereon.
San Jose,Francisco, California
March 1, 20077, 2008
5146
StemCells, Inc.
| | | | | | | | | |
| | | December 31, | |
| | | 2006 | | | 2005 | |
| |
Assets | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 51,795,529 | | | $ | 34,540,908 | |
| Other receivables | | | 482,850 | | | | 201,919 | |
| Other current assets | | | 1,119,467 | | | | 386,966 | |
| Marketable securities | | | 4,132,646 | | | | — | |
| | | | | | | | | |
| Total current assets | | | 57,530,492 | | | | 35,129,793 | |
| Marketable securities | | | 3,133,632 | | | | 3,720,794 | |
| Property, plant and equipment, net | | | 3,596,150 | | | | 3,282,588 | |
| Other assets, net | | | 2,596,543 | | | | 2,705,513 | |
| | | | | | | | | |
| Total assets | | | 66,856,817 | | | $ | 44,838,688 | |
| | | | | | | | | |
| | | | | | | | | |
| |
Liabilities and Stockholders’ Equity |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 620,765 | | | $ | 637,122 | |
| Accrued expenses and other | | | 2,053,902 | | | | 1,483,300 | |
| Accrued wind-down expenses | | | 1,252,483 | | | | 1,118,796 | |
| Deferred revenue | | | 16,826 | | | | — | |
| Capital lease obligations, current portion | | | — | | | | 54,676 | |
| Bonds payable, current portion | | | 205,833 | | | | 254,167 | |
| | | | | | | | | |
| Total current liabilities | | | 4,149,809 | | | | 3,548,061 | |
| Bonds payable, less current maturities | | | 1,145,416 | | | | 1,351,250 | |
| Deposits and other long-term liabilities | | | 547,392 | | | | 522,866 | |
| Accrued wind-down expenses non current | | | 5,497,774 | | | | 6,186,930 | |
| Deferred rent | | | 959,732 | | | | 853,997 | |
| Deferred revenue, less current portion | | | 180,691 | | | | — | |
| | | | | | | | | |
| Total liabilities | | | 12,480,814 | | | | 12,463,104 | |
| Commitments (Note 6) | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Common stock, $.01 par value; 125,000,000 shares authorized; 78,046,304 and 65,396,022 shares issued and outstanding at December 31, 2006 and 2005, respectively | | | 780,462 | | | | 653,960 | |
| Additional paid-in capital | | | 255,299,508 | | | | 217,919,335 | |
| Accumulated deficit | | | (204,891,945 | ) | | | (185,943,564 | ) |
| Accumulated other comprehensive gain (loss) | | | 3,187,978 | | | | (254,147 | ) |
| | | | | | | | | |
| Total stockholders’ equity | | | 54,376,003 | | | | 32,375,584 | |
| | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 66,856,817 | | | $ | 44,838,688 | |
| | | | | | | | | |
See accompanying notes to consolidated financial statements.
52
StemCells, Inc.
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2006 | | | 2005 | | | 2004 | |
| |
| Revenue from collaborative and licensing agreements | | $ | 55,299 | | | $ | 19,526 | | | $ | 22,206 | |
| Revenue from grants | | | 37,551 | | | | 186,388 | | | | 118,828 | |
| | | | | | | | | | | | | |
| Total Revenues | | | 92,850 | | | | 205,914 | | | | 141,034 | |
| Operating Expenses | | | | | | | | | | | | |
| Research and development | | | 13,600,433 | | | | 8,226,734 | | | | 7,843,981 | |
| General and administrative | | | 7,154,042 | | | | 5,539,845 | | | | 4,870,014 | |
| Wind-down expenses | | | 709,209 | | | | 2,827,403 | | | | 2,826,879 | |
| | | | | | | | | | | | | |
| | | | 21,463,684 | | | | 16,593,982 | | | | 15,540,874 | |
| | | | | | | | | | | | | |
| Loss from operations | | | (21,370,834 | ) | | | (16,388,068 | ) | | | (15,399,840 | ) |
| Other Income (expense): | | | | | | | | | | | | |
| License and settlement agreement, net | | | 103,359 | | | | 3,735,556 | | | | — | |
| Interest income | | | 2,479,740 | | | | 1,122,963 | | | | 322,227 | |
| Interest expense | | | (143,001 | ) | | | (171,909 | ) | | | (191,006 | ) |
| Other income (expense) | | | (17,644 | ) | | | (36,892 | ) | | | (61,680 | ) |
| | | | | | | | | | | | | |
| | | | 2,422,454 | | | | 4,649,718 | | | | 69,541 | |
| | | | | | | | | | | | | |
| Net loss | | | (18,948,380 | ) | | | (11,738,350 | ) | | | (15,330,299 | ) |
| | | | | | | | | | | | | |
| Basic and diluted net loss per share | | $ | (0.25 | ) | | $ | (0.18 | ) | | $ | (0.31 | ) |
| | | | | | | | | | | | | |
| Weighted average shares used in basic and diluted loss per share calculations | | | 74,611,196 | | | | 63,643,176 | | | | 49,606,277 | |
| | | | | | | | | | | | | |
| | | | | | | | | |
| | | December 31, | |
| | | 2007 | | | 2006 | |
| |
ASSETS |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 9,759,169 | | | $ | 51,795,529 | |
| Marketable securities, current | | | 26,696,413 | | | | 4,132,646 | |
| Other receivables | | | 264,631 | | | | 482,850 | |
| Note receivable | | | 1,000,000 | | | | — | |
| Prepaid assets | | | 1,032,482 | | | | 1,119,467 | |
| | | | | | | | | |
| Total current assets | | | 38,752,695 | | | | 57,530,492 | |
| Marketable securities, non current | | | 3,150,971 | | | | 3,133,632 | |
| Property, plant and equipment, net | | | 3,905,404 | | | | 3,596,150 | |
| Other assets, non-current | | | 1,710,829 | | | | 1,720,361 | |
| Intangible assets, net | | | 762,667 | | | | 876,182 | |
| | | | | | | | | |
| Total assets | | $ | 48,282,566 | | | $ | 66,856,817 | |
| | | | | | | | | |
| |
LIABILITIES AND STOCKHOLDERS’ EQUITY |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 1,813,595 | | | $ | 620,765 | |
| Accrued expenses and other liabilities | | | 2,462,252 | | | | 2,053,902 | |
| Accrued wind-down expenses, current | | | 1,374,632 | | | | 1,252,483 | |
| Deferred revenue, current | | | 43,909 | | | | 16,826 | |
| Capital lease obligation, current | | | 17,530 | | | | — | |
| Bonds payable, current | | | 136,250 | | | | 205,833 | |
| | | | | | | | | |
| Total current liabilities | | | 5,848,168 | | | | 4,149,809 | |
| Capital lease obligation, non-current | | | 25,269 | | | | — | |
| Bonds payable, non-current | | | 1,009,166 | | | | 1,145,416 | |
| Deposits and other long-term liabilities | | | 527,804 | | | | 547,392 | |
| Accrued wind-down expenses, non-current | | | 4,768,859 | | | | 5,497,774 | |
| Deferred rent | | | 727,535 | | | | 959,732 | |
| Deferred revenue, non-current | | | 163,865 | | | | 180,691 | |
| | | | | | | | | |
| Total liabilities | | | 13,070,666 | | | | 12,480,814 | |
| Commitments and contingencies (Note 8) | | | | | | | | |
| Stockholders’ equity: | | | | | | | | |
| Common stock, $.01 par value; 125,000,000 shares authorized; issued and outstanding 80,681,087 at December 31, 2007 and 78,046,304 at December 31, 2006 | | | 806,810 | | | | 780,462 | |
| Additional paid-in capital | | | 264,603,711 | | | | 255,299,508 | |
| Accumulated deficit | | | (229,914,747 | ) | | | (204,891,945 | ) |
| Accumulated other comprehensive income (loss) | | | (283,874 | ) | | | 3,187,978 | |
| | | | | | | | | |
| Total stockholders’ equity | | | 35,211,900 | | | | 54,376,003 | |
| | | | | | | | | |
| Total liabilities and stockholders’ equity | | $ | 48,282,566 | | | $ | 66,856,817 | |
| | | | | | | | | |
See accompanying notesNotes to consolidated financial statements.Consolidated Financial Statements.
53
STEMCELLS, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Accumulated
| | | | | | | |
| | | | | | | | | Additional
| | | | | | Other
| | | | | | Total
| |
| | | Common Stock | | | Paid-in
| | | Accumulated
| | | Comprehensive
| | | Deferred
| | | Stockholders’
| |
| | | Shares | | | Amount | | | Capital | | | Deficit | | | Income (Loss) | | | Compensation | | | Equity | |
| |
| Balances, December 31, 2003 | | | 40,998,858 | | | $ | 409,989 | | | $ | 170,406,393 | | | $ | (158,874,916 | ) | | $ | — | | | $ | (977,908 | ) | | $ | 10,963,558 | |
| Issuance of common stock related to equity financing net of issuance cost of $2,863,021 | | | 20,660,000 | | | | 206,600 | | | | 39,433,578 | | | | — | | | | — | | | | — | | | | 39,640,178 | |
| Common stock issued for licensing agreements | | | 11,351 | | | | 114 | | | | 17,719 | | | | — | | | | — | | | | — | | | | 17,833 | |
| Common stock issued for external services | | | 41,050 | | | | 410 | | | | 72,640 | | | | — | | | | — | | | | — | | | | 73,050 | |
| Common stock issued pursuant to employee benefit plan | | | 48,707 | | | | 487 | | | | 93,526 | | | | — | | | | — | | | | — | | | | 94,013 | |
| Exercise of employee and consultant stock options | | | 62,916 | | | | 629 | | | | 44,750 | | | | — | | | | — | | | | — | | | | 45,379 | |
| Exercise of warrants | | | 306,525 | | | | 3,065 | | | | 579,333 | | | | — | | | | — | | | | — | | | | 582,398 | |
| Compensation expense from grant of options | | | — | | | | — | | | | 33,868 | | | | — | | | | — | | | | — | | | | 33,868 | |
| Deferred compensation | | | — | | | | — | | | | 737,493 | | | | — | | | | — | | | | (737,493 | ) | | | — | |
| Amortization of deferred compensation | | | — | | | | — | | | | — | | | | — | | | | — | | | | 829,569 | | | | 829,569 | |
| Net loss | | | — | | | | — | | | | — | | | | (15,330,299 | ) | | | — | | | | — | | | | (15,330,299 | ) |
| | | |
| | | |
| Balances, December 31, 2004 | | | 62,129,407 | | | | 621,294 | | | | 211,419,300 | | | | (174,205,215 | ) | | | — | | | | (885,832 | ) | | | 36,949,547 | |
| Expenses related to equity financing | | | — | | | | — | | | | (193,946 | ) | | | — | | | | — | | | | — | | | | (193,946 | ) |
| Common stock issued for external services | | | 2,022 | | | | 20 | | | | 8,310 | | | | — | | | | — | | | | — | | | | 8,330 | |
| Common stock issued pursuant to employee benefit plan | | | 28,459 | | | | 285 | | | | 110,772 | | | | — | | | | — | | | | — | | | | 111,057 | |
| Compensation expense from grant of options and stock (fair value) | | | — | | | | — | | | | 461,675 | | | | — | | | | — | | | | — | | | | 461,675 | |
| Exercise of employee and consultant stock options | | | 393,509 | | | | 3,935 | | | | 733,753 | | | | — | | | | — | | | | — | | | | 737,688 | |
| Exercise of warrants | | | 2,842,625 | | | | 28,426 | | | | 5,910,680 | | | | — | | | | — | | | | — | | | | 5,939,106 | |
| Deferred compensation | | | — | | | | — | | | | (531,208 | ) | | | — | | | | — | | | | 531,208 | | | | — | |
| Amortization of deferred compensation | | | — | | | | — | | | | — | | | | — | | | | — | | | | 354,624 | | | | 354,624 | |
| Unrealized loss on marketable securities | | | — | | | | — | | | | — | | | | — | | | | (254,147 | ) | | | — | | | | (254,147 | ) |
| Net loss | | | — | | | | — | | | | — | | | | (11,738,350 | ) | | | — | | | | — | | | | (11,738,350 | ) |
| Comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (11,992,497 | ) |
| | | |
| | | |
| Balances, December 31, 2005 | | | 65,396,022 | | | | 653,960 | | | | 217,919,336 | | | | (185,943,565 | ) | | | (254,147 | ) | | | — | | | | 32,375,584 | |
| Issuance of common stock related to equity financing net of issuance cost of $2,418,467 | | | 11,750,820 | | | | 117,508 | | | | 33,304,026 | | | | — | | | | — | | | | — | | | | 33,421,534 | |
| Common stock issued for licensing agreements | | | 3,848 | | | | 38 | | | | 9,962 | | | | — | | | | — | | | | — | | | | 10,000 | |
| Common stock issued pursuant to employee benefit plan | | | 50,120 | | | | 501 | | | | 121,955 | | | | — | | | | — | | | | — | | | | 122,456 | |
| Compensation expense from grant of options and stock (fair value) | | | — | | | | — | | | | 2,409,509 | | | | — | | | | — | | | | — | | | | 2,409,509 | |
| Exercise of employee and consultant stock options | | | 319,094 | | | | 3,191 | | | | 545,088 | | | | — | | | | — | | | | — | | | | 548,279 | |
| Exercise of warrants | | | 526,400 | | | | 5,264 | | | | 989,632 | | | | — | | | | — | | | | — | | | | 994,896 | |
| Unrealized gain on marketable securities | | | — | | | | — | | | | — | | | | — | | | | 3,442,125 | | | | — | | | | 3,442,125 | |
| Net loss | | | — | | | | — | | | | — | | | | (18,948,380 | ) | | | — | | | | — | | | | (18,948,380 | ) |
| Comprehensive loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (15,506,255 | ) |
| | | |
| | | |
| Balances, December 31, 2006 | | | 78,046,304 | | | $ | 780,462 | | | $ | 255,299,508 | | | $ | (204,891,945 | ) | | $ | 3,187,978 | | | | — | | | $ | 54,376,003 | |
| | | |
| | | |
See accompanying notes to consolidated financial statements.
5447
StemCells, Inc.
Consolidated Statements of Operations
| | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Revenue: | | | | | | | | | | | | |
| Revenue from licensing agreements and grants | | $ | 56,722 | | | $ | 92,850 | | | $ | 205,914 | |
| | | | | | | | | | | | | |
| Operating Expenses: | | | | | | | | | | | | |
| Research and development | | | 19,937,426 | | | | 13,600,433 | | | | 8,226,734 | |
| General and administrative | | | 7,927,443 | | | | 7,154,042 | | | | 5,539,845 | |
| Wind-down expenses | | | 783,022 | | | | 709,209 | | | | 2,827,403 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 28,647,891 | | | | 21,463,684 | | | | 16,593,982 | |
| | | | | | | | | | | | | |
| Operating losses | | | (28,591,169 | ) | | | (21,370,834 | ) | | | (16,388,068 | ) |
| Other Income (expense): | | | | | | | | | | | | |
| License and settlement agreement, net | | | 550,467 | | | | 103,359 | | | | 3,735,556 | |
| Realized gain on sale of marketable securities | | | 715,584 | | | | — | | | | — | |
| Interest income | | | 2,459,820 | | | | 2,479,740 | | | | 1,122,963 | |
| Interest expense | | | (123,606 | ) | | | (143,001 | ) | | | (171,909 | ) |
| Other income (expense) | | | (33,898 | ) | | | (17,644 | ) | | | (36,892 | ) |
| | | | | | | | | | | | | |
| Total other income, net | | | 3,568,367 | | | | 2,422,454 | | | | 4,649,718 | |
| | | | | | | | | | | | | |
| Net loss | | $ | (25,022,802 | ) | | $ | (18,948,380 | ) | | $ | (11,738,350 | ) |
| | | | | | | | | | | | | |
| Basic and diluted net loss per share | | $ | (0.31 | ) | | $ | (0.25 | ) | | $ | (0.18 | ) |
| | | | | | | | | | | | | |
| Shares used to compute basic and diluted loss per share | | | 79,772,351 | | | | 74,611,196 | | | | 63,643,176 | |
| | | | | | | | | | | | | |
See Notes to Consolidated Financial Statements.
48
StemCells, Inc.
Consolidated Statements of Stockholder’s Equity
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Accumulated
| | | | | | | |
| | | | | | | | | Additional
| | | | | | Other
| | | | | | Total
| |
| | | Common Stock | | | Paid-in
| | | Accumulated
| | | Comprehensive
| | | Deferred
| | | Stockholders’
| |
| | | Shares | | | Amount | | | Capital | | | Deficit | | | Income (Loss) | | | Compensation | | | Equity | |
| |
Balances, December 31, 2004 | | | 62,129,407 | | | $ | 621,294 | | | $ | 211,419,300 | | | $ | (174,205,215 | ) | | $ | — | | | $ | (885,832 | ) | | $ | 36,949,547 | |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | (11,738,350 | ) | | | — | | | | — | | | | (11,738,350 | ) |
| Change in unrealized loss on securities available-for-sale | | | — | | | | — | | | | — | | | | — | | | | (254,147 | ) | | | — | | | | (254,147 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | (11,992,497 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Expenses related to equity financing | | | — | | | | — | | | | (193,946 | ) | | | — | | | | — | | | | — | | | | (193,946 | ) |
| Common stock issued for external services | | | 2,022 | | | | 20 | | | | 8,310 | | | | — | | | | — | | | | — | | | | 8,330 | |
| Common stock issued pursuant to employee benefit plan | | | 28,459 | | | | 285 | | | | 110,772 | | | | — | | | | — | | | | — | | | | 111,057 | |
| Compensation expense from grant of options and stock (fair value) | | | — | | | | — | | | | 461,675 | | | | — | | | | — | | | | — | | | | 461,675 | |
| Exercise of employee and consultant stock options | | | 393,509 | | | | 3,935 | | | | 733,753 | | | | — | | | | — | | | | — | | | | 737,688 | |
| Exercise of warrants | | | 2,842,625 | | | | 28,426 | | | | 5,910,680 | | | | — | | | | — | | | | — | | | | 5,939,106 | |
| Deferred compensation | | | — | | | | — | | | | (531,208 | ) | | | — | | | | — | | | | 531,208 | | | | — | |
| Amortization of deferred compensation | | | — | | | | — | | | | — | | | | — | | | | — | | | | 354,624 | | | | 354,624 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balances, December 31, 2005 | | | 65,396,022 | | | | 653,960 | | | | 217,919,336 | | | | (185,943,565 | ) | | | (254,147 | ) | | | — | | | | 32,375,584 | |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | (18,948,380 | ) | | | — | | | | — | | | | (18,948,380 | ) |
| Change in unrealized gain on securities available-for-sale | | | — | | | | — | | | | — | | | | — | | | | 3,442,125 | | | | — | | | | 3,442,125 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | (15,506,255 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock related to equity financing net of issuance cost of $2,418,467 | | | 11,750,820 | | | | 117,508 | | | | 33,304,026 | | | | — | | | | — | | | | — | | | | 33,421,534 | |
| Common stock issued for licensing agreements | | | 3,848 | | | | 38 | | | | 9,962 | | | | — | | | | — | | | | — | | | | 10,000 | |
| Common stock issued pursuant to employee benefit plan | | | 50,120 | | | | 501 | | | | 121,955 | | | | — | | | | — | | | | — | | | | 122,456 | |
| Compensation expense from grant of options and stock (fair value) | | | — | | | | — | | | | 2,409,509 | | | | — | | | | — | | | | — | | | | 2,409,509 | |
| Exercise of employee and consultant stock options | | | 319,094 | | | | 3,191 | | | | 545,088 | | | | — | | | | — | | | | — | | | | 548,279 | |
| Exercise of warrants | | | 526,400 | | | | 5,264 | | | | 989,632 | | | | — | | | | — | | | | — | | | | 994,896 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balances, December 31, 2006 | | | 78,046,304 | | | | 780,462 | | | | 255,299,508 | | | | (204,891,945 | ) | | | 3,187,978 | | | | — | | | | 54,376,003 | |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | — | | | | — | | | | — | | | | (25,022,802 | ) | | | — | | | | — | | | | (25,022,802 | ) |
| Change in unrealized loss on securities available-for-sale | | | — | | | | — | | | | — | | | | — | | | | (3,471,852 | ) | | | — | | | | (3,471,852 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | (28,494,654 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock related to equity financing net of issuance cost of $297,465 | | | 1,807,000 | | | | 18,070 | | | | 4,816,983 | | | | — | | | | — | | | | — | | | | 4,835,053 | |
| Common stock issued for licensing agreements | | | 3,865 | | | | 39 | | | | 9,961 | | | | — | | | | — | | | | — | | | | 10,000 | |
| Common stock issued pursuant to employee benefit plan | | | 73,074 | | | | 731 | | | | 172,429 | | | | — | | | | — | | | | — | | | | 173,160 | |
| Compensation expense from grant of options and stock (fair value) | | | — | | | | — | | | | 3,008,315 | | | | — | | | | — | | | | — | | | | 3,008,315 | |
| Exercise of employee stock options | | | 175,186 | | | | 1,752 | | | | 208,521 | | | | — | | | | — | | | | — | | | | 210,273 | |
| Exercise of warrants | | | 575,658 | | | | 5,756 | | | | 1,087,994 | | | | — | | | | — | | | | — | | | | 1,093,750 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balances, December 31, 2007 | | | 80,681,087 | | | $ | 806,810 | | | $ | 264,603,711 | | | $ | (229,914,747 | ) | | $ | (283,874 | ) | | $ | — | | | $ | 35,211,900 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See Notes to Consolidated Financial Statements.
49
StemCells, Inc.
Consolidated Statements of Cash Flows
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, | | | Year Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | | | 2007 | | 2006 | | 2005 | |
| |
Cash flows from operating activities: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (18,948,380 | ) | | $ | (11,738,350 | ) | | $ | (15,330,299 | ) | | $ | (25,022,802 | ) | | $ | (18,948,380 | ) | | $ | (11,738,350 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | | | | | | | | | | | | |
| Depreciation and amortization | | | 1,044,688 | | | | 1,082,793 | | | | 1,037,719 | | | | 1,174,510 | | | | 1,044,688 | | | | 1,082,793 | |
| Amortization of deferred compensation | | | — | | | | 354,624 | | | | 829,569 | | | | — | | | | — | | | | 354,624 | |
| Issue of shares and options in exchange for services | | | 2,531,966 | | | | 581,062 | | | | 200,931 | | | | 3,181,475 | | | | 2,531,966 | | | | 581,062 | |
| Loss on disposal of fixed assets | | | 1,573 | | | | 1,377 | | | | 54,644 | | |
| (Gain) loss on disposal of fixed assets | | | | (1,500 | ) | | | 1,573 | | | | 1,377 | |
| Gain on sale of marketable securities | | | | (715,584 | ) | | | — | | | | — | |
| Non-cash income from license and settlement agreement, net | | | (103,359 | ) | | | (3,974,941 | ) | | | — | | | | (550,467 | ) | | | (103,359 | ) | | | (3,974,941 | ) |
| Changes in operating assets and liabilities: | | | | | | | | | | | | | | | | | | | | | | | | |
| Accrued interest receivable | | | (112,542 | ) | | | (47,928 | ) | | | (61,660 | ) | |
| Other receivables | | | (168,389 | ) | | | 26,972 | | | | 26,160 | | |
| Other current assets | | | (732,501 | ) | | | (177,892 | ) | | | (29,026 | ) | |
| Other assets, net | | | 56,270 | | | | (47,053 | ) | | | — | | |
| Accrued interest and other receivables | | | | 218,219 | | | | (280,931 | ) | | | (20,956 | ) |
| Prepaid assets | | | | 86,985 | | | | (732,501 | ) | | | (177,892 | ) |
| Intangible and other assets, net | | | | 19,532 | | | | 56,270 | | | | (47,053 | ) |
| Accounts payable and accrued expenses | | | 554,245 | | | | 48,135 | | | | 665,409 | | | | 1,601,180 | | | | 554,245 | | | | 48,135 | |
| Accrued wind-down expenses | | | (555,469 | ) | | | 1,777,697 | | | | 1,705,045 | | | | (606,766 | ) | | | (555,469 | ) | | | 1,777,697 | |
| Deferred revenue | | | 197,517 | | | | — | | | | — | | | | 10,257 | | | | 197,517 | | | | — | |
| Deferred rent | | | 105,735 | | | | 330,196 | | | | (372,400 | ) | | | (232,198 | ) | | | 105,735 | | | | 330,196 | |
| Deposits and other long-term liabilities | | | 24,526 | | | | (87,260 | ) | | | — | | | | (19,587 | ) | | | 24,526 | | | | (87,260 | ) |
| | | | | | | | | | | | | | | |
| Net cash used in operating activities | | | (16,104,120 | ) | | | (11,870,568 | ) | | | (11,273,908 | ) | | | (20,856,746 | ) | | | (16,104,120 | ) | | | (11,870,568 | ) |
| | | | | | | | | |
Cash flows from investing activities: | | | | | | | | | | | | | | | | | | | | | | | | |
| Purchases of property, plant and equipment | | | (1,258,749 | ) | | | (817,505 | ) | | | (676,138 | ) | |
| Acquisition of other assets | | | (38,375 | ) | | | (30,000 | ) | | | (72,167 | ) | |
| Purchase of marketable securities | | | | (27,861,561 | ) | | | — | | | | — | |
| Proceeds from sale of marketable securities | | | | 3,074,654 | | | | — | | | | — | |
| Advance made under note receivable | | | | (1,000,000 | ) | | | — | | | | — | |
| Purchases of property, plant and equipment, net | | | | (1,319,374 | ) | | | (1,258,749 | ) | | | (817,505 | ) |
| Purchase of other assets | | | | (49,375 | ) | | | (38,375 | ) | | | (30,000 | ) |
| | | | | | | | | | | | | | | |
| Net cash used in investing activities | | | (1,297,124 | ) | | | (847,505 | ) | | | (748,305 | ) | | | (27,155,656 | ) | | | (1,297,124 | ) | | | (847,505 | ) |
| | | | | | | | | |
Cash flows from financing activities: | | | | | | | | | | | | | | | | | | | | | | | | |
| Proceeds (expense) from issuance of common stock, net | | | 33,421,534 | | | | (193,946 | ) | | | 39,640,178 | | | | 4,835,053 | | | | 33,421,534 | | | | (193,946 | ) |
| Proceeds from the exercise of stock options | | | 548,279 | | | | 737,688 | | | | 45,379 | | | | 210,273 | | | | 548,279 | | | | 737,688 | |
| Proceeds from the exercise of warrants | | | 994,896 | | | | 5,939,106 | | | | 582,398 | | | | 1,093,750 | | | | 994,896 | | | | 5,939,106 | |
| Repayments of capital lease obligations | | | (54,676 | ) | | | (39,232 | ) | | | (30,830 | ) | |
| Proceeds (repayments) of capital lease obligations | | | | 42,799 | | | | (54,676 | ) | | | (39,232 | ) |
| Repayments of debt obligations | | | (254,168 | ) | | | (244,167 | ) | | | (237,083 | ) | | | (205,833 | ) | | | (254,168 | ) | | | (244,167 | ) |
| | | | | | | | | | | | | | | |
| Net cash provided by financing activities | | | 34,655,865 | | | | 6,199,449 | | | | 40,000,042 | | | | 5,976,042 | | | | 34,655,865 | | | | 6,199,449 | |
| | | | | | | | | | | | | | | |
| Increase (decrease) in cash and cash equivalents | | | 17,254,621 | | | | (6,518,624 | ) | | | 27,977,829 | | | | (42,036,360 | ) | | | 17,254,621 | | | | (6,518,624 | ) |
| Cash and cash equivalents at beginning of year | | | 34,540,908 | | | | 41,059,532 | | | | 13,081,703 | | | | 51,795,529 | | | | 34,540,908 | | | | 41,059,532 | |
| | | | | | | | | | | | | | | |
| Cash and cash equivalents at end of the year | | $ | 51,795,529 | | | $ | 34,540,908 | | | $ | 41,059,532 | | | $ | 9,759,169 | | | $ | 51,795,529 | | | $ | 34,540,908 | |
| | | | | | | | | | | | | | | |
| Supplemental disclosure of cash flow information: | | | | | | | | | | | | | | | | | | | | | | | | |
| Interest paid | | $ | 143,001 | | | $ | 171,909 | | | $ | 191,006 | | | $ | 123,606 | | | $ | 143,001 | | | $ | 171,909 | |
| | | | | | | | | |
| Supplemental schedule of non-cash investing and financing activities: | | | | | | | | | | | | | | | | | | | | | | | | |
| Stock issued for licensing agreements | | $ | 10,000 | (1) | | | — | | | $ | 17,833 | (2) | |
Stock issued for licensing agreements(1) | | | $ | 10,000 | | | $ | 10,000 | | | | — | |
| | | | | | | | | |
| | |
| (1) | | Under terms of a license agreement with the California Institute of Technology (Cal Tech), annual fees of $5,000 were due on each of two patents to which StemCells holds a license from Cal Tech, payable in cash or stock at the Company’sour choice. The CompanyWe elected to pay the fees in stock and issued shares of 3,865 in 2007 and 3,848 sharesin 2006 to Cal Tech. |
|
(2) | | Under the terms of a license agreement with Cal Tech, fees of $10,000 and $5,000 were due on the issuance of two patents to which StemCells holds a license from Cal Tech, payable in cash or stock at the Company’s choice. The Company elected to pay the fees in stock and issued 9,535 unregistered shares to Cal Tech. The Company also paid $2,833 in stock (1,816 shares) as part of an option agreement with the Board of Trustees of the Leland Stanford Junior University to acquire an exclusive license to an invention. |
See accompanying notesNotes to consolidated financial statements.Consolidated Financial Statements.
5550
StemCells, Inc.
Notes to Consolidated Financial Statements
December 31, 20062007
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
| |
| Note 1. | Summary of Significant Accounting Policies |
Nature of Business
StemCells, Inc., a Delaware corporation, (the Company) is a biopharmaceutical company that operates in one segment, the development of novel cell-based therapeutics designed to treat human diseases and disorders.
The accompanying consolidated financial statements have been prepared on the basis that the Companywe will continue as a going concern. Since inception, the Company haswe have incurred annual losses and negative cash flows from operations and has an accumulated deficit of approximately $204.9$230 million at December 31, 2006. The Company has2007. We have not derived revenuesrevenue from the sale of products, and doesdo not expect to receive revenuesrevenue from product sales for at least several years. ItWe may notnever be able to realize sufficient revenuesrevenue to achieve or sustain profitability in the future.
StemCells expectsWe expect to incur additional operating losses over the next several years. The Company has veryforeseeable future. We have limited liquidity and capital resources and must obtain significant additional capital and other resources in order to sustain itsour product development efforts, to provide funding for the acquisition of technologies and intellectual property rights, for preclinical and clinical testing of our anticipated products, pursuit of regulatory approvals, acquisition of capital equipment, laboratory and office facilities, establishment of production capabilities, for general and administrative expenses and other working capital requirements. StemCells reliesWe rely on our cash reserves, and proceeds from equity and debt offerings, proceeds from the transfer or sale of intellectual property rights, equipment, facilities or investments, and government grants and funding from collaborative arrangements, if obtainable, to fund itsour operations. If the Company exhausts itswe exhaust our cash reserves and isare unable to realizeobtain adequate financing, itwe may be unable to meet our operating obligations and we may be required to initiate bankruptcy proceedings. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
Principles of Consolidation
The consolidated financial statements include the accounts of the CompanyStemCells, Inc., and our wholly owned subsidiary, StemCells California, Inc., a wholly owned subsidiary. All significant inter-company balances Material intercompany accounts and transactions have been eliminated on consolidation.eliminated.
Use of Estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principles generally accepted in the United States of America requires our management to make estimatesjudgments, assumptions and assumptionsestimates that affect the amounts reported in theour consolidated financial statements.statements and accompanying notes. Actual results could differ materially from thesethose estimates.
Significant estimates include the following:
| | |
| | • | Accrued wind-down expenses (See Note 8)7). |
| |
| | • | The grant date fair value of share-based awards recognized as compensation expense in accordance with the provisions of Statement of Financial Accounting Standards (SFAS) No. 123 (Revised 2004) “Share“Share Based Payment”Payment” (SFAS 123R). (See Note 10)6). |
| |
| | • | Valuation allowance against net deferred tax assets (See Note 12). |
Marketable securitiesFinancial Instruments
In accordanceCash Equivalents and Marketable Securities
All money market and highly liquid investments with SFAS No. 115 “Accounting for Certain Investments in Debt and Equity Securities,”a maturity of 90 days or less at the Company hasdate of purchase are classified itsas cash equivalents. Highly liquid investments with maturities of 365 days or less not previously classified asavailable-for-sale cash equivalents are classified as marketable securities, in the accompanying consolidated financial statements. The marketable securities are stated at fair market value,current. Investments with unrealized gains and losses reported in other comprehensive income. Management reviews securities with unrealized losses for othermaturities greater than 365 days
5651
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
than temporary impairment. A decline
are classified as marketable securities, non-current. Our marketable debt and equity securities have been classified and accounted for as available-for-sale. Management determines the appropriate classification of its investments in marketable debt and equity securities at the time of purchase and reevaluates the available-for-sale designations as of each balance sheet date. These securities are carried at fair value (see Note 2, “Financial Instruments,” below), with the unrealized gains and losses reported as a component of stockholders’ equity. The cost of securities sold is based upon the specific identification method.
If the estimated fair value of a security is below its carrying value, we evaluate whether we have the intent and ability to retain our investment for a period of time sufficient to allow for any anticipated recovery to the cost of the investment, and whether evidence indicating that the cost of the investment is recoverable within a reasonable period of time outweighs evidence to the contrary. Other-than-temporary declines in estimated fair value of all marketable securities that is deemed other than temporary isare charged to earnings when so deemed (See Note 2).
Reclassification
Certain reclassifications of prior year amounts have been made to conform to current year presentation. Patent related expenses of approximately $703,000 and $916,000 for prior“other income (expense), net.” No such impairment was recognized during the years ended December 31, 20052007, 2006 and 2004 respectively have been reclassified2005.
Other Receivables
Our non-trade receivables generally consist of interest income on our financial instruments, revenue from researchlicensing agreements and development expense to general and administrative expense on the consolidated statements of operations for that period to conform with the current year presentation. The reclassifications had no effect on total assets, liabilities, equity, or net loss previously reported.rent from our sub-lease tenants.
Cash and Cash Equivalents
The Company considers cash equivalents to be only those investments that are highly liquid, readily convertible to cash and which mature within three months from the date of purchase.
Estimated Fair Value of Financial Instruments
The carryingestimated fair value of cash and cash equivalents, other receivables, accounts payable and the current portion of the bonds payable approximates their estimated faircarrying values due to the short maturities of these instruments. The carryingestimated fair value of long-termour marketable debt securities approximates its faircarrying value based on current rates available to the Companyus for similar debt.debt securities.
Property, Plant and Equipment
Property, plant, and equipment, including thatthose held under capital lease, obligations, isare stated at cost and depreciated usingcost. Depreciation is computed by use of the straight-line method over the estimated lifeuseful lives of the respective asset,assets, or the lease term if shorter, as follows:
| | | | | | | | | |
| Building and improvements | | | | | | | 3 - 20 years | |
| Machinery and equipment | | | | | | | 3 - 10 years | |
| Furniture and fixtures | | | | | | | 3 - 10 years | |
Leasehold improvementsRepairs and maintenance costs are amortized on a straight-line basis over the shorter of their estimated useful lives or lease terms.expensed as incurred.
PatentIntangible Assets (Patent and License CostsCosts)
Prior to fiscal year 2001, the Companywe capitalized certain patent costs, related to patent applications. Accumulated costs werewhich are being amortized over the estimated economic life of the patents, not to exceed 17 years, using the straight-line method, commencing at the time the patent was issued. Costs related to patent applications are charged to expenseand would be expensed at the time such patents are deemed to have no continuing value. Since 2001, the Company’s policy has been to expense all patent costs are expensed as incurred. At December 31, 2006 and 2005, total costs capitalized amounted to approximately $980,000 and the related accumulated amortization was approximately $404,000 and $348,000, respectively. Patent related expenses totaled approximately $852,000, $703,000, and $753,000 in 2006, 2005 and 2004, respectively. License costs are capitalized as incurred and amortized over the periodestimated life of the license agreement.
Stock-Based CompensationImpairment of Long-Lived Assets
In December 2004, theWe review property, plant, and equipment and certain identifiable intangibles for impairment in accordance with Financial Accounting Standards Board (FASB) issued SFAS 123R. SFAS 123R requires all share-based paymentsStatement of Financial Accounting Standards (SFAS) No. 144,Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to employees,Be Disposed Of. Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Recoverability of these assets is measured by comparing the carrying amount to non-employee directors as compensation for service onfuture undiscounted cash flows the Board of Directors,assets are expected to be recognized as compensation expense in the consolidated financial statements based on the fair values of such payments. The Company maintains shareholder approved stock-based compensation plans,generate. If property, plant, and equipment and patents are
5752
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
pursuant to which it granted stock-based compensation to its employees, and to non-employee directors for Board service. These grants are primarily in the form of options that allow a grantee to purchase a fixed number of shares of the Company’s common stock at a fixed exercise price equal to the market price of the shares at the date of the grant (“qualified stock option grants”). The options may vest on a single date or in tranches over a period of time, but normally they do not vest unless the grantee is still employed by or a director of the Company on the vesting date. The compensation expense for these grants will be recognized over the requisite service period which is typically the period over which the stock-based compensation awards vest. In March 2005, the Securities and Exchange Commission (SEC) issued Staff Accounting Bulletin No. 107 (SAB 107), which provides guidance on the implementation of SFAS 123R. The Company applied the principles of SAB 107 in conjunction with its adoption of SFAS 123R.
The Company adopted SFAS 123R effective January 1, 2006, usingconsidered to be impaired, the modified-prospective transition method. Under this transition method, compensation expense will be recognized based on the grant date fair value estimated in accordance with the provisions of SFAS 123R for all new grants effective January 1, 2006, and for options granted prior to but not vested as of December 31, 2005. Prior periods were not restated to reflect the impact of adopting the new standard and therefore do not include fair value compensation expense related to stock option grants for those periods. In accordance with SFAS 123R, the Company recognized stock option related compensation expense of approximately $2,285,000 for the year ended December 31, 2006. Stock option related compensation expense was recognized on a straight line basis over the vesting period of each grant net of estimated forfeitures. The Company estimated forfeiture rates based on its historical experience within separate groups of employees. The estimated fair value of the options granted during 2006 and prior years was calculated using a Black Scholes Merton option pricing model (Black Scholes model). The following summarizes the assumptions used in the Black Scholes model as applied by quarter for the year ended December 31, 2006:
| | | | | | | | | | | | | | | | | |
| | | First Quarter
| | | Second Quarter
| | | Third Quarter
| | | Fourth Quarter
| |
| | | 2006 | | | 2006 | | | 2006 | | | 2006 | |
| |
| Risk — free interest rate(1) | | | 4.72 | % | | | 5.08 | % | | | 4.68 | % | | | 4.54 | % |
| Volatility(2) | | | 119.5 | % | | | 110.8 | % | | | 106.6 | % | | | 103.3 | % |
| Dividend yield(3) | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % |
| Expected term (years until exercise)(4) | | | 6.25 | | | | 6.25 | | | | 6.25 | | | | 6.25 | |
| | |
(1) | | The risk-free interest rate is based on US Treasury debt securities with maturities close to the expected term of the option. |
|
(2) | | Expected volatility is based on historical volatility of the Company’s stock factoring in daily share price observations. In computing expected volatility, the length of the historical period used is equal to the length of the expected term of the option. |
|
(3) | | No cash dividends have been declared on the Company’s common stock since the Company’s inception, and the Company currently does not anticipate paying cash dividends over the expected term of the option. |
|
(4) | | The expected term is equal to the average of the contractual life of the stock option and its vesting period. |
The adoption of SFAS 123R had the following impact on our consolidated statement of operations for the year ended December 31, 2006:
| | | | | |
| Increase in net loss | | $ | 2,285,000 | |
| Increase in basic and diluted net loss per share | | $ | 0.03 | |
At December 31, 2006, approximately $5,775,000 of unrecognized compensation expense related to stock options is expectedimpairment to be recognized over a weighted average period of approximately 1.6 years. The resulting effect on net loss and net loss per share attributable to common stockholders may not be representative ofequals the effects in future periods, due to changes in forfeiture rates, additional grants and subsequent periods of vesting.
58
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
Prior to January 1, 2006, the Company accounted for its stock-based compensation plans under Accounting Principles Board Opinion No. 25 (APB 25), “Accounting for Stock Issued to Employees.” In accordance with APB 25, the Company generally recognized no compensation expense for qualified stock option grants, as the options were usually granted at fair market price of the underlying shares on the date of the grant. For options issued with an exercise price less than the fair market value of the shares at the date of grant, the Company recognized the difference between the exercise price and fair market value as compensation expense in accordance with APB 25. Prior to January 1, 2006, the Company provided pro forma disclosure amounts in accordance with Statement of Financial Accounting Standards No. 123 “Accounting for Stock-Based Compensation,” (SFAS 123) as amendedamount by Statement of Financial Accounting Standards No. 148 “Accounting for Stock-Based Compensation — Transition and Disclosure,” (SFAS 148). As fair value compensation expense was disclosed but not recognized in periods prior to January 1, 2006, no cumulative adjustment for forfeitures was recorded in 2006. See table below for an illustration of the effect on net loss and net loss per share if we had applied the fair value recognition provisions of SFAS 123 to stock-based employee compensation in the prior years ended December 31, 2005 and 2004.
| | | | | | | | | |
| | | 2005 | | | 2004 | |
| |
| Net loss applicable to common stockholders — as reported | | $ | (11,738,350 | ) | | $ | (15,330,299 | ) |
| Add: Stock-based employee/ director compensation expense included in reported net loss under the intrinsic value method | | | — | | | | 33,868 | |
| Deduct: Total stock-based employee/director compensation expense under the fair value based method for all awards | | | (1,019,120 | ) | | | (819,317 | ) |
| | | | | | | | | |
| Net loss pro forma | | $ | (12,757,470 | ) | | $ | (16,115,748 | ) |
| | | | | | | | | |
| Basic and diluted net loss per share as reported | | $ | (0.18 | ) | | $ | (0.31 | ) |
| Basic and diluted net loss per share pro forma | | $ | (0.20 | ) | | $ | (0.32 | ) |
| Shares used in computing basic and diluted loss per share amounts | | | 63,643,176 | | | | 49,606,277 | |
The Company accounts for stock options granted to non-employees in accordance with SFAS 123 and Emerging Issues Task Force (EITF)96-18 —“Accounting For Equity Instruments That Are Issued To Other Than Employees For Acquiring, Or In Conjunction With Selling, Goods Or Services,”and accordingly, recognizes as expense the estimated fair value of such options as calculated using the Black Scholes model. The fair value is re-measured at each reporting date during the service period and is amortized over the vesting period of each option or the recipient’s contractual arrangement, if shorter. No stock options were issued to non-employees during the year ended December 31, 2006 other than options granted to non-employee members of the Board of Directors for service as Board members.
In July 2006, pursuant to the 2006 Equity Incentive Plan, the Company granted cash-settled Stock Appreciation Rights (SARs) to certain employees. The SARs give the holder the right, upon exercise, to the difference between the price per share of our common stock at the time of exercise and the exercise price of the SAR. The exercise price of the SARs is equal to the market price of our common shares at the date of grant. The SARs will vest on the same schedule as our qualified options issued to employees, i.e., 25% on the first anniversary of the grant date and then 1/48th every month thereafter. The maximum contractual term for the SARs granted is ten years. We will recognize compensation expense for the SARs over the requisite service period which is typically the period over which the awards vest. Since the vesting schedule of the SARs is identical to the vesting schedule of options granted this period, our fair value calculation of the SARs issued using the Black Scholes model were based on the same assumptions used in calculating compensation expense for stock options granted this period. The fair value of the share-based compensation liability for the cost of the requisite service that has been rendered at the reporting date is
59
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
re-measured at each reporting date through the date of settlement. The following table presents the activity of the Company’s SARs awards for the year ended December 31, 2006 and 2005.
| | | | | | | | | | | | | | | | | |
| | | 2006 | | | 2005 | |
| | | | | | Weighted
| | | | | | Weighted
| |
| | | | | | Average Exercise
| | | | | | Average Exercise
| |
| | | SARs | | | Price | | | SARs | | | Price | |
| |
| Outstanding at January 1 | | | — | | | | — | | | | — | | | | — | |
| Granted | | | 1,564,599 | | | $ | 2.00 | | | | — | | | | — | |
| Exercised | | | — | | | | — | | | | — | | | | — | |
| Canceled | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Outstanding at September 30 | | | 1,564,599 | | | $ | 2.00 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| SARs exercisable at December 31 | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
For the year ended December 31, 2006, the Company recorded approximately $294,000 as compensation expense related to SARs granted. At December 31, 2006, approximately $2,336,000 of unrecognized compensation expense related to SARs is expected to be recognized over a weighted average period of approximately 1.9 years. The resulting effect on net loss and net loss per share attributable to common stockholders is not likely to be representative of the effects in future periods, due to changes in the fair value calculation which is dependent on the stock price, volatility, interest and forfeiture rates, additional grants and subsequent periods of vesting.
Long-Lived Assets
The Company routinely evaluates the carrying value of its long-lived assets. The Company records impairment losses on long-lived assets used in operations when events and circumstances indicate that assets may be impaired and the undiscounted cash flows estimated to be generated by the assets are less than the carrying amount of those assets. If an impairment exists, the charge to operations is measured as the excess of the carrying amount over theexceeds its estimated fair value of the assets.market value. No such impairment was recognized during the years ended December 31, 2007, 2006 2005 and 2004.2005.
Income TaxesRevenue Recognition
The liability method is usedWe currently recognize revenue resulting from the licensing and use of our technology and intellectual property. Such licensing agreements may contain multiple elements, such as upfront fees, payments related to accountthe achievement of particular milestones and royalties. Revenue from upfront fees for income taxes. Deferred tax assetslicensing agreements that contain multiple elements are generally deferred and liabilities are determined basedrecognized on differences between financial reporting and income tax basesa straight-line basis over the term of assets and liabilities as well as net operating loss carry forwards and tax credits carryforwards and are measured using the enacted tax rates and laws that are expected to be in effect when the differences reverse. Deferred tax assets may be reduced by a valuation allowance to reflect the uncertaintyagreement. Fees associated with their ultimate realization.
substantive at risk performance-based milestones are recognized as revenue upon completion of the scientific or regulatory event specified in the agreement, and royalties received are recognized as earned. Revenue Recognition
Revenues from collaborative agreements and grants are recognized as earned upon either the incurring of reimbursable expenses directly related to the particular research plan or the completion of certain development milestones as defined within the terms of the relevant collaborative agreement. Payments receivedagreement or grant.
Research and Development Costs
Our research and development expenses consist primarily of salaries and related personnel expenses, costs associated with clinical trials and regulatory submissions; costs associated with preclinical activities such as toxicology studies; certain patent-related costs such as licensing; facilities-related costs such as depreciation; lab equipment and supplies. Clinical trial expenses include payments to vendors such as clinical research organizations, contract manufacturers, clinical trial sites, laboratories for testing clinical samples and consultants. All research and development costs are expensed as incurred.
Stock-Based Compensation
On January 1, 2006, we adopted SFAS No. 123 (revised 2004) (SFAS 123R),Share-Based Payment, which revises SFAS 123,Accounting for Stock-Based Compensation, and supersedes Accounting Principles Board Opinion 25 (APB 25),Accounting for Stock Issued to Employees. SFAS 123R requires us to expense the fair value of our stock-based compensation awards to employees. We elected to use the modified prospective transition method as permitted by SFAS 123R and therefore have not restated our financial results for prior periods. Under this transition method, we apply SFAS 123R to new awards, as well as to awards that vest, are modified, repurchased, or cancelled after January 1, 2006. The compensation cost we record for these awards are based on their grant-date fair value as calculated and amortized over their vesting period. See Note 6, “Stock-Based Compensation” for further information.
Prior to the adoption of SFAS 123R, we accounted for stock-based compensation awards using the intrinsic value method of APB 25. Accordingly, we did not recognize compensation expense in advanceour Consolidated Statement of research performed are designatedOperations for options we granted that had an exercise price equal to the market value of the underlying common stock on the date of grant. As required by SFAS 123, we also provided certain pro forma disclosures for stock-based awards as deferred revenue. The Company recognizes non-refundable upfront license feesif the fair-value-based approach of SFAS 123 had been applied.
We account for stock options granted to non-employees in accordance with SFAS 123 and certain other related fees on a straight-line basisEmerging Issues Task Force (EITF)96-18Accounting For Equity Instruments That Are Issued To Other Than Employees For Acquiring, Or In Conjunction With Selling, Goods Or Services, and accordingly, expense the estimated fair value of such options as calculated using the Black-Scholes model over the developmentservice period. Fees associated with substantiveThe estimated fair value is re-measured at risk, performance based milestones are recognized as revenue upon their completion, as defined ineach reporting date and is amortized over the respective agreements. Incidental assignment of technology rights is recognized as revenue at time of receipt.remaining service period.
6053
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
Research and Development CostsIncome Taxes
We account for income taxes in accordance with SFAS No. 109, Accounting for Income Taxes(SFAS 109) and FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes, an interpretation of FASB Statement No. 109,as amended by FASB Staff PositionNo. 48-1 (FIN 48). This approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities. Income tax receivables and liabilities and deferred tax assets and liabilities are recognized based on the amounts that more likely than not will be sustained upon ultimate settlement with taxing authorities.
Developing our provision for income taxes and analyzing our uncertain tax positions requires significant judgment and knowledge of federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and, any valuation allowances that may be required for deferred tax assets.
We assess the realization of our deferred tax assets to determine whether an income tax valuation allowance is required. Based on such evidence that can be objectively verified, we determine whether it is more likely than not that all or a portion of the deferred tax assets will be realized. The Company expenses all researchmain factors that we consider include:
| | |
| • | Cumulative losses in recent years; |
|
| • | Income/losses expected in future years; |
|
| • | The applicable statute of limitations. |
Tax benefits associated with uncertain tax positions are recognized in the period in which one of the following conditions is satisfied: (1) the more likely than not recognition threshold is satisfied; (2) the position is ultimately settled through negotiation or litigation; or (3) the statute of limitations for the taxing authority to examine and development costs as incurred. Researchchallenge the position has expired. Tax benefits associated with an uncertain tax position are derecognized in the period in which the more likely than not recognition threshold is no longer satisfied.
We concluded that the realization of deferred tax assets is dependent upon future earnings, if any, the timing and development costs include costsamount of personnel, external services, supplies, facilities and miscellaneous other costs.which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance.
Net Loss per Share
Basic and diluted net loss per share have beenis computed usingbased on the weighted-average number of shares of our common stock outstanding during the period. Basic earningsDiluted net loss per share excludes anyis computed based on the weighted-average number of shares of our common stock and other dilutive effects of options, shares subject to repurchase, warrants and convertible securities. Diluted earnings per share includes the impact of potentially dilutive securities if dilutive.
| | | | | | | | | | | | | |
| | | Years Ended December 31, | |
| | | 2006 | | | 2005 | | | 2004 | |
| |
| Net loss | | $ | (18,948,380 | ) | | $ | (11,738,350 | ) | | $ | (15,330,299 | ) |
| Weighted average shares used in computing basic and diluted net loss per share amounts | | | 74,611,196 | | | | 63,643,176 | | | | 49,606,277 | |
| Basic and diluted net loss per share | | $ | (0.25 | ) | | $ | (0.18 | ) | | $ | (0.31 | ) |
The Company has excluded outstanding stockfollowing are the basic and dilutive net loss per share computations for the last three fiscal years:
| | | | | | | | | | | | | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Net loss | | $ | (25,022,802 | ) | | $ | (18,948,380 | ) | | $ | (11,738,350 | ) |
| Weighted average shares outstanding used to compute basic and diluted net loss per share | | | 79,772,351 | | | | 74,611,196 | | | | 63,643,176 | |
| Basic and diluted net loss per share | | $ | (0.31 | ) | | $ | (0.25 | ) | | $ | (0.18 | ) |
54
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
Outstanding options and warrants to purchase shares of our common stock were excluded from the calculationcomputation of diluted net loss per common share because all such securities arethe effect would have been anti-dilutive for all applicable periods presented. These outstanding securities consist of the following potential common shares:presented below:
| | | | | | | | | | | | | | |
| | | Years Ended December 31, | | | | | | | | | | | | | |
| | | 2006 | | 2005 | | 2004 | | | 2007 | | 2006 | | 2005 | |
| |
| Outstanding options | | | 8,501,503 | | | | 6,608,109 | | | | 6,682,201 | | | | 9,028,810 | | | | 8,501,503 | | | | 6,608,109 | |
| Outstanding warrants | | | 1,930,658 | | | | 2,521,400 | | | | 5,490,285 | | | | 1,355,000 | | | | 1,930,658 | | | | 2,521,400 | |
| | | | | | | | | |
| Total | | | | 10,383,810 | | | | 10,432,161 | | | | 9,129,509 | |
| | | | | | | | | |
Comprehensive Income (Loss)
Comprehensive income (loss) is comprised of net income (loss)losses and other comprehensive income (loss)(or “OCI”). The only component of other comprehensive income (loss) is anOCI includes certain changes in stockholders’ equity that are excluded from net losses. Specifically, we include in OCI changes in unrealized gain of $3,187,978 related togains and losses on our marketable securities. Comprehensive income for the years ended December 31, 2007, 2006 and 2005 has been reflected in the Consolidated Statements of Stockholders’ Equity.
The activity in OCI is as follows:
| | | | | | | | | | | | | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| (Decrease) increase in unrealized gains/(losses) on marketable securities | | $ | (2,756,268 | ) | | $ | 3,442,125 | | | $ | (254,147 | ) |
| Reclassification adjustment for gains on marketable securities included in net income | | | (715,584 | ) | | | — | | | | — | |
| | | | | | | | | | | | | |
| Other comprehensive income (loss) | | $ | (3,471,852 | ) | | $ | 3,442,125 | | | $ | (254,147 | ) |
| | | | | | | | | | | | | |
Recent Accounting Pronouncements
In July 2006, the FASB issued Interpretation No. 48,“Accounting for Uncertainty in Income Taxes”, an interpretation of SFAS No. 109,“Accounting for Income Taxes” (SFAS 109). This Interpretation clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements in accordance with SFAS 109. This Interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. This Interpretation also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Interpretation is effective for fiscal years beginning after December 15, 2006. While the Company’s analysis of the impact of this Interpretation is not yet complete, it does not anticipate that it will have a material impact on its consolidated financial statements at the time of adoption.
In September 2006, the FASB issued Statement of Financial Accounting StandardsSFAS No. 157,“Fair Value Measurements”Measurements(SFAS 157). This StandardSFAS 157 defines fair value, establishes a framework and gives guidance regarding the methods used for measuring fair value, in generally accepted accounting principles and expands disclosures about fair value measurements. SFAS 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. We are currently assessing the impact that SFAS 157 may have on our results of operations and financial position.
In February 2007, the FASB issued SFAS No. 159,The adoptionFair Value Option for Financial Assets and Financial Liabilities — Including an amendment of FAS 157FASB Statement No. 115(SFAS 159). SFAS 159 is expected to expand the use of fair value accounting but does not affect existing standards which require certain assets or liabilities to be carried at fair value. The objective of SFAS 159 is to improve financial reporting by providing companies with the opportunity to mitigate volatility in reported earnings caused by measuring related assets and liabilities differently without having to apply complex hedge accounting provisions. Under SFAS 159, a company may choose, at specified election dates, to measure eligible items at fair value and report unrealized gains and losses on items for which the fair value option has been elected in earnings at each subsequent reporting date. SFAS 159 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. We are currently assessing the impact that SFAS 159 may have on our results of operations and financial position.
In December 2007, the SEC issued Staff Accounting Bulletin 110,Share-Based Payment (SAB 110), which amends SAB 107,Share-Based Payment, to permit public companies, under certain circumstances, to use the simplified method in SAB 107 for employee option grants after December 31, 2007. Use of the simplified method after December 2007 is permitted only for companies whose historical data about their employees’ exercise behavior does not provide a reasonable basis for estimating the expected term of the options. We currently use the simplified method to estimate the expected term for employee option grants as adequate historical experience is not expected to have a material impact on the Company’s consolidated financial statements.
6155
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
In September 2006, the Securities and Exchange Commission (SEC) issued Staff Accounting Bulletin (SAB) No. 108,“Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements”(available to provide a reasonable estimate. SAB 108), to address diversity in practice in quantifying financial statement misstatements. SAB 108 requires that the Company quantify misstatements based on their impact on each of its financial statements and related disclosures. SAB 108110 is effective for fiscal years endingemployee options granted after November 15, 2006. The Company’s adoptionDecember 31, 2007. We intend to adopt SAB 110 effective January 1, 2008 and we are currently assessing our historical experience to evaluate if it can provide a reasonable estimate of this bulletin did not have a material impact on its consolidated financial statements.the expected term for employee option grants.
| |
| Note 2. | ReNeuron License AgreementFinancial Instruments |
Cash, cash equivalents and marketable securities
The following table summarizes the fair value of our cash, cash equivalents and available-for-sale securities held in our investment portfolio:
| | | | | | | | | | | | | | | | | |
| | | | | | Gross Unrealized
| | | Gross Unrealized
| | | | |
| | | Amortized Cost | | | Gains | | | Losses | | | Fair Value | |
| |
December 31, 2007 | | | | | | | | | | | | |
| Cash | | $ | 549,544 | | | $ | — | | | $ | — | | | $ | 549,544 | |
| | | | | | | | | | | | | | | | | |
| Money market accounts | | | 5,079,564 | | | | — | | | | — | | | | 5,079,564 | |
| Marketable debt securities (maturity within 90 days) | | | 4,130,404 | | | | — | | | | (343 | ) | | | 4,130,061 | |
| | | | | | | | | | | | | | | | | |
| Total cash equivalents | | | 9,209,968 | | | | — | | | | (343 | ) | | | 9,209,625 | |
| Marketable debt securities (maturity within 1 year) | | | 26,680,824 | | | | 19,137 | | | | (3,548 | ) | | | 26,696,413 | |
| | | | | | | | | | | | | | | | | |
| Total marketable securities, current | | | 26,680,824 | | | | 19,137 | | | | (3,548 | ) | | | 26,696,413 | |
| Marketable debt securities | | | 1,180,394 | | | | 9,109 | | | | — | | | | 1,189,503 | |
| Marketable equity securities | | | 2,269,697 | | | | — | | | | (308,229 | ) | | | 1,961,468 | |
| | | | | | | | | | | | | | | | | |
| Total marketable securities, non-current | | | 3,450,091 | | | | 9,109 | | | | (308,229 | ) | | | 3,150,971 | |
| | | | | | | | | | | | | | | | | |
| Total cash, cash equivalents, and marketable securities | | $ | 39,890,427 | | | $ | 28,246 | | | $ | (312,120 | ) | | $ | 39,606,553 | |
| | | | | | | | | | | | | | | | | |
December 31, 2006 | | | | | | | | | | | | | | | | |
| Cash | | $ | 162,685 | | | $ | — | | | $ | — | | | $ | 162,685 | |
| Money market accounts | | | 51,632,844 | | | | — | | | | — | | | | 51,632,844 | |
| | | | | | | | | | | | | | | | | |
| Total cash and cash equivalents | | | 51,795,529 | | | | — | | | | — | | | | 51,795,529 | |
| Marketable equity securities, current | | | 2,319,505 | | | | 1,813,141 | | | | — | | | | 4,132,646 | |
| Marketable equity securities, non-current | | | 1,758,795 | | | | 1,374,837 | | | | — | | | | 3,133,632 | |
| | | | | | | | | | | | | | | | | |
| Total cash, cash equivalents, and marketable securities | | $ | 55,873,829 | | | $ | 3,187,978 | | | $ | — | | | $ | 59,061,807 | |
| | | | | | | | | | | | | | | | | |
Our investment in marketable debt securities consist primarily of commercial paper, corporate debt securities, and asset-backed securities. Our marketable debt securities classified as a non-current investment consists of a single security which has an effective maturity date in February 2009.
Our investment in marketable equity securities consists of shares in ReNeuron Group plc, a publicly listed UK corporation. In July 2005, the Companywe entered into a license and settlement agreement with ReNeuron Limited, a wholly owned subsidiary of ReNeuron Group plc, a publicly listed UK corporation (collectively referred to as “ReNeuron”). As part of the agreement, the Companywe granted ReNeuron a license that allows ReNeuron to exploit their “c-mycER” conditionally immortalized adult
56
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
human neural stem cell technology for therapy and other purposes. In return for the license, StemCellswe received a 7.5% fully-diluted equity interest in ReNeuron, subject to certain anti-dilution provisions, and a cross-license to the exclusive use of ReNeuron’s technology for certain diseases and conditions, including lysosomal storage diseases, spinal cord injury, cerebral palsy and multiple sclerosis. The agreement also provides for full settlement of any potential claims that either StemCellswe or ReNeuron might have had against the other in connection with any putative infringement of certain of each party’s patent rights prior to the effective date of the agreement. The agreement is Exhibit 10.71 to the Company’s Quarterly Report onForm 10-QIn February 2007, we sold 5,275,000 ordinary shares of ReNeuron for the quarter ended June 30, 2005. An amendment tonet proceeds of approximately $3,075,000. We recognized approximately $716,000 as realized gain from this transaction. In February 2007, as a consequence of certain anti-dilution provisions in the agreement, was entered on April 3, 2006, a copy of which was attached as Exhibit 10.1 to the Company’s Quarterly Report onForm 10-Q for the quarter ended March 31, 2006. On June 29, 2006, ReNeuron issued us an additional 822,000 shares of common stock of which StemCells was entitled to 439,071 shares because of the anti-dilution provisions within the agreement and net of approximately 12,000 shares duewhich were transferred to NeurospheresNeuroSpheres Ltd., (NeuroSpheres) an Alberta corporation from which StemCells haswe have licensed some of the patent rights that are the subject ofto the agreement with ReNeuron. The CompanyWe recorded approximately $103,000$550,000 as other income for the additional shares. The fair market valueWe owned 4,821,924 ordinary shares of the Company’s holdings in ReNeuron common stock as ofat December 31, 2005 (8,835,766 shares)2007 and 9,274,837 at December 31, 2006 (9,274,837 shares) was approximately $3,721,000 and $7,266,000 respectively. 2006.
Changes in market value as a result of changes in market price per share or the exchange rate between the US dollar and the British pound are accounted for under “other comprehensive income (loss)” if deemed temporary as in this case, and are not recorded as “other income or loss” until the shares are disposed of and a gain or loss realized.
In accordance with FASB Staff PositionFAS 115-1 andFAS 124-1,The Meaning of Other-Than-Temporary Impairment and Its Application to Certain Investments, the following table shows the gross unrealized gainlosses and fair value for those investments that were in an unrealized loss position as of December 31, 2006 is approximately $3,188,000. A decline2007, aggregated by investment category and the length of time that individual securities have been in the fair value of securities that is deemed other than temporary would be charged to earnings. See also Note 15 “Subsequent Events”.a continuous loss position:
| |
Note 3. | Property, Plant and Equipment, Net |
Property, plant and equipment consist of the following:
| | | | | | | | | |
| | | December 31, | |
| | | 2006 | | | 2005 | |
| |
| Building and improvements | | $ | 3,369,775 | | | $ | 3,359,417 | |
| Machinery and equipment | | | 4,712,950 | | | | 3,489,695 | |
| Furniture and fixtures | | | 366,053 | | | | 348,963 | |
| | | | | | | | | |
| | | | 8,448,778 | | | | 7,198,075 | |
| Less accumulated depreciation and amortization | | | (4,852,628 | ) | | | (3,915,487 | ) |
| | | | | | | | | |
| | | $ | 3,596,150 | | | $ | 3,282,588 | |
| | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Less than 12 Months | | | 12 Months of Greater | | | Total | |
| | | Fair Value | | | Unrealized Loss | | | Fair Value | | | Unrealized Loss | | | Fair Value | | | Unrealized Loss | |
| |
December 31, 2007 | | | | | | | | | | | | | | | | | | | | | | | | |
| Marketable debt securities | | $ | 9,418,713 | | | $ | (3,891 | ) | | $ | — | | | $ | (— | ) | | $ | 9,418,713 | | | $ | (3,891 | ) |
| Marketable equity securities | | | 1,961,468 | | | | (308,229 | ) | | | | | | | | | | | 1,961,468 | | | | (308,229 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 11,380,181 | | | $ | (312,120 | ) | | $ | — | | | $ | (— | ) | | $ | 11,380,181 | | | $ | (312,120 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Depreciation expense was approximately $944,000, $958,000,Unrealized losses in our marketable debt securities portfolio are due to eight U.S. corporate debt securities primarily consisting of commercial paper. For these securities, the unrealized losses are primarily due to a change in interest rates. Because we have the ability and $933,000 for the years endedintent to hold these investments until a forecasted recovery of carrying value, which may be maturity or call date, we do not consider these investments to be other-than-temporarily impaired as of December 31, 2006, 20052007. See Note 1, “Summary of Significant Accounting Policies — Cash Equivalents and 2004, respectively.Marketable Securities,” for further discussion of the criteria used to determine impairment of our marketable securities.
Note Receivable
In December 2007, we committed to make a secured loan of up to $3.8 million to Progenitor Cell Therapy, LLC (PCT) in return for a period of exclusivity to allow for due diligence and negotiation of a possible acquisition transaction. Of this amount, $1.0 million was lent and outstanding at December 31, 2007 with the maturity date within twelve months from the effective date of the loan. In March 2008, we terminated discussions to acquire PCT. We anticipate the $1.0 million loan will be repaid in accordance with its terms.
6257
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
| |
| Note 3. | Property, Plant and Equipment |
Property, plant and equipment balances at December 31 are summarized below:
| | | | | | | | | |
| | | 2007 | | | 2006 | |
| |
| Building and improvements | | $ | 3,397,639 | | | $ | 3,369,775 | |
| Machinery and equipment | | | 6,002,945 | | | | 4,712,950 | |
| Furniture and fixtures | | | 369,068 | | | | 366,053 | |
| | | | | | | | | |
| | | | 9,769,652 | | | | 8,448,778 | |
| Less accumulated depreciation and amortization | | | (5,864,248 | ) | | | (4,852,628 | ) |
| | | | | | | | | |
| Net property, plant and equipment, net | | $ | 3,905,404 | | | $ | 3,596,150 | |
| | | | | | | | | |
Depreciation expense was approximately $1,012,000 in 2007, $944,000 in 2006, and $958,000 in 2005.
| |
| Note 4. | Intangible and Other Assets Net |
OtherThe components of our intangible assets at December 31 are as follows:summarized below:
| | | | | | | | | |
| | | December 31, | |
| | | 2006 | | | 2005 | |
| |
| Patents, net | | $ | 575,962 | | | $ | 631,764 | |
| License agreements, net | | | 300,220 | | | | 437,117 | |
| Security deposit — building lease | | | 942,282 | | | | 752,500 | |
| Restricted Cash-(Letter of Credit) | | | 778,079 | | | | 884,132 | |
| | | | | | | | | |
| | | $ | 2,596,543 | | | $ | 2,705,513 | |
| | | | | | | | | |
| | | | | | | | | | | | | |
| | | Gross Carrying
| | | Accumulated
| | | | |
Intangible Asset Class | | amount | | | Amortization | | | Net Carrying Amount | |
| |
2007 | | | | | | | | | | | | |
| Patents | | $ | 979,612 | | | $ | (459,452 | ) | | $ | 520,160 | |
| License agreements | | | 1,761,623 | | | | (1,519,116 | ) | | | 242,507 | |
| | | | | | | | | | | | | |
| Total intangible assets | | $ | 2,741,235 | | | $ | (1,978,568 | ) | | $ | 762,667 | |
| | | | | | | | | | | | | |
2006 | | | | | | | | | | | | |
| Patents | | $ | 979,612 | | | $ | (403,650 | ) | | $ | 575,962 | |
| License agreements | | | 1,712,248 | | | | (1,412,028 | ) | | | 300,220 | |
| | | | | | | | | | | | | |
| Total intangible assets | | $ | 2,691,860 | | | $ | (1,815,678 | ) | | $ | 876,182 | |
| | | | | | | | | | | | | |
At December 31, 2006 and 2005, accumulated amortization was approximately $1,816,000 and $1,715,000, respectively, for patents and license agreements. Amortization expense was approximately $163,000 in 2007, $101,000 in 2006, and $125,000 and $104,000 for the years ended December 31, 2006, 2005, and 2004, respectively. Over the next five years, the estimated aggregatein 2005.
The expected future annual amortization expense based on current balances for patents and license agreementsof our intangible assets is expected to be approximately $116,000.as follows:
| | | | | | | | | |
For the year ending December 31: | | | | | | | | |
| 2008 | | | | | | $ | 107,249 | |
| 2009 | | | | | | $ | 107,249 | |
| 2010 | | | | | | $ | 107,249 | |
| 2011 | | | | | | $ | 69,468 | |
| 2012 | | | | | | $ | 68,295 | |
Other assets at December 31 are summarized below:
| | | | | | | | | |
| | | 2007 | | | 2006 | |
| |
| Prepaid royalties | | $ | 180,250 | | | $ | 189,782 | |
| Security deposit (building lease) | | | 752,500 | | | | 752,500 | |
| Restricted cash (letter of credit) | | | 778,079 | | | | 778,079 | |
| | | | | | | | | |
| Total other long-term assets | | $ | 1,710,829 | | | $ | 1,720,361 | |
| | | | | | | | | |
58
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
| |
| Note 5. | Accrued Expenses and Other |
Accrued expenses and other current liabilitiesat December 31 are as follows:summarized below:
| | | | | | | | | | |
| | | December 31, | | | | | | | | | |
| | | 2006 | | 2005 | | | 2007 | | 2006 | |
| |
| External services | | $ | 323,162 | | | $ | 404,875 | | | $ | 360,340 | | | $ | 323,162 | |
| Employee compensation | | | 1,570,915 | | | | 972,906 | | | | 1,885,249 | | | | 1,570,915 | |
| Other | | | 159,825 | | | | 105,519 | | | | 216,663 | | | | 159,825 | |
| | | | | | | | | | | |
| Total other accrued liabilities | | | $ | 2,462,252 | | | $ | 2,053,902 | |
| | | $ | 2,053,902 | | | $ | 1,483,300 | | | | | | |
| | | | | | | |
| |
| Note 6. | LeasesStock-Based Compensation |
We currently grant options under three equity incentive plans and as of December 31, 2007, we had 12,000,000 shares authorized under these three plans. However, at our annual stockholders meeting held on June 12, 2007, our stockholders approved an amendment to our 2006 Equity Incentive Plan to provide for an annual increase in the number of shares of common stock available for issuance under the plan each January 1 (beginning January 1, 2008) equal to 4% of the outstanding common shares as of that date. The Company has undertakenamendment further provided an aggregate limit of 30,000,000 shares issuable pursuant to incentive stock options under the plan. Under these three plans we may grant incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock, and performance-based shares to our employees, directors and consultants, at prices determined by our Board of Directors. Incentive stock options may only be granted to employees under these plans with a grant price not less than the fair market value on the date of grant.
Generally, stock options granted to employees have a maximum term of ten years, and vest over a four year period from the date of grant; 25% vest at the end of one year, and 75% vest monthly over the remaining three-year service period. We may grant options with different vesting terms from time to time. Upon employee termination of service, any unexercised vested option will be forfeited three months following termination or the expiration of the option, whichever is earlier.
Our compensation expense for stock options issued from our equity incentive plans for the last two fiscal years was as follows:
| | | | | | | | | |
| | | 2007 | | | 2006 | |
| |
| Research and development expense | | $ | 1,347,239 | | | $ | 1,048,697 | |
| General and administrative expense | | | 1,558,056 | | | | 1,236,334 | |
| | | | | | | | | |
| Total employee stock-based compensation expense and effect on net loss | | $ | 2,905,295 | | | $ | 2,285,031 | |
| | | | | | | | | |
| Effect on basic and diluted net loss per common share | | $ | (0.04 | ) | | $ | (0.03 | ) |
| | | | | | | | | |
As of December 31, 2007, we have approximately $6,956,000 of total unrecognized compensation expense related to unvested awards granted under our various share-based plans that we expect to recognize over a weighted-average period of 1.6 years.
59
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
The following table illustrates the effect of net loss and net loss per common share as if we had applied the fair value recognition provisions of SFAS 123 to stock-based compensation for the year ended December 31, 2005:
| | | | | |
| | | 2005 | |
| |
| Net loss: | | | | |
| Net loss, as reported | | $ | (11,738,350 | ) |
| Deduct: Stock-based employee compensation expense determined under fair value-based method for all awards | | | (1,019,120 | ) |
| | | | | |
| Pro forma net loss | | $ | (12,757,470 | ) |
| | | | | |
| Basic and diluted earnings per common share: | | | | |
| As reported | | $ | (0.18 | ) |
| | | | | |
| Pro forma | | $ | (0.20 | ) |
| | | | | |
| Shares used in computing basic and diluted loss per share | | | 63,643,176 | |
| | | | | |
The fair value of options granted is estimated as of the date of grant using the Black-Scholes option pricing model, which requires certain assumptions as of the date of grant. The weighted-average assumptions used for the last three fiscal years are as follows:
| | | | | | | | | | | | | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Expected life (years)(1) | | | 6.25 | | | | 6.25 | | | | 5.00 | |
| Risk-free interest rate(2) | | | 4.36 | % | | | 4.72 | % | | | 4.14 | % |
| Expected volatility(3) | | | 95.2 | % | | | 109.0 | % | | | 100.7 | % |
| Expected dividend yield(4) | | | 0 | % | | | 0 | % | | | 0 | % |
| | |
| (1) | | The expected term in 2007 and 2006 is equal to the average of the contractual life of the stock option and its vesting period as of the date of grant. In 2005 we assumed a reasonable term based on information available at the time. |
|
| (2) | | The risk-free interest rate is based on U.S. Treasury debt securities with maturities close to the expected term of the option as of the date of grant. |
|
| (3) | | Expected volatility is based on historical volatility over the most recent historical period equal to the length of the expected term of the option as of the date of grant. |
|
| (4) | | We have neither declared nor paid dividends on any share of common stock and we do not expect to do so in the foreseeable future. |
At the end of each reporting period we estimate forfeiture rates based on our historical experience within separate groups of employees and adjust the stock-based compensation expense accordingly.
60
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
A summary of our stock option activity and related information for the last three fiscal years is as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | Outstanding Options | |
| | | | | | | | | Weighted-
| | | Weighted-Average
| | | Aggregate
| |
| | | Shares Available
| | | Number
| | | Average
| | | remaining
| | | Intrinsic
| |
| | | for Grant | | | of Shares | | | Exercise Price | | | contractual term | | | Value(1) | |
| |
Balance at December 31, 2004 | | | 6,339,406 | | | | 6,682,201 | | | $ | 2.67 | | | | | | | | | |
| Granted | | | (1,075,481 | ) | | | 1,075,481 | | | $ | 4.75 | | | | | | | | | |
| Exercised | | | | | | | (423,989 | ) | | $ | 1.76 | | | | | | | | | |
| Cancelled (forfeited and expired) | | | 725,584 | | | | (725,584 | ) | | $ | 3.11 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2005 | | | 5,989,509 | | | | 6,608,109 | | | $ | 3.02 | | | | | | | | | |
| Granted | | | (2,818,684 | ) | | | 2,818,684 | | | $ | 2.38 | | | | | | | | | |
| Exercised | | | | | | | (369,214 | ) | | $ | 1.82 | | | | | | | | | |
| Cancelled (forfeited and expired) | | | 556,076 | | | | (556,076 | ) | | $ | 2.82 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2006 | | | 3,726,901 | | | | 8,501,503 | | | $ | 2.88 | | | | | | | | | |
| Granted | | | (2,484,100 | ) | | | 2,484,100 | | | $ | 2.33 | | | | | | | | | |
| Exercised | | | | | | | (175,186 | ) | | $ | 1.20 | | | | | | | | | |
| Cancelled (forfeited and expired) | | | 1,781,607 | | | | (1,781,607 | ) | | $ | 4.91 | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2007 | | | 3,024,408 | | | | 9,028,810 | | | $ | 2.36 | | | | 7.26 | | | $ | 826,558 | |
| | | | | | | | | | | | | | | | | | | | | |
| Exercisable at December 31, 2007 | | | | | | | 4,600,618 | | | $ | 2.24 | | | | 5.68 | | | $ | 826,558 | |
| | | | | | | | | | | | | | | | | | | | | |
| Vested and expected to vest(2) | | | | | | | 8,337,077 | | | $ | 2.34 | | | | 7.34 | | | $ | 826,558 | |
| | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Aggregate intrinsic value represents the value of the closing price per share of our common stock on the last trading day of the fiscal period in excess of the exercise price multiplied by the number of options outstanding or exercisable. |
|
| (2) | | Shares include options vested and those expected to vest net of estimated forfeitures. |
The estimated weighted average fair value per share of options granted was approximately $1.85 in 2007, $2.37 in 2006, and $3.75 in 2005, based on the assumptions in the Black-Scholes model discussed above. Total intrinsic value of options exercised at time of exercise was approximately $396,700 in 2007, $453,000 in 2006, and $519,000 in 2005.
The following is a summary of changes in unvested options:
| | | | | | | | | |
| | | | | | Weighted average
| |
| | | | | | grant date fair
| |
Unvested Options | | Number of options | | | value | |
| |
| Unvested options at December 31, 2006 | | | 3,598,784 | | | $ | 2.16 | |
| Granted | | | 2,484,100 | | | | 1.85 | |
| Vested | | | (1,485,573 | ) | | | 2.14 | |
| Cancelled | | | (169,119 | ) | | | 1.88 | |
| | | | | | | | | |
| Unvested options at December 31, 2007 | | | 4,428,192 | | | $ | 2.00 | |
| | | | | | | | | |
The estimated fair value of options vested were approximately $3,173,000 in 2007 and $2,292,000 in 2006.
61
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
The following table presents weighted average exercise price and term information about significant option groups outstanding at December 31, 2007:
| | | | | | | | | | | | | | | | | |
| Options outstanding at December 31, 2007 | |
| | | | | | Weighted Average
| | | Weighted Average
| | | | |
Range of
| | | | | Remaining
| | | Exercise
| | | | |
Exercise Prices | | Number Outstanding | | | Term (Yrs.) | | | Price | | | Aggregate Intrinsic Value | |
| |
| Less than $2.00 | | | 2,383,594 | | | | 5.4 | | | $ | 1.19 | | | $ | 826,558 | |
| $2.00 — $3.99 | | | 5,882,631 | | | | 8.1 | | | $ | 2.45 | | | | — | |
| $4.00 — $5.99 | | | 762,585 | | | | 7.0 | | | $ | 5.23 | | | | — | |
| | | | | | | | | | | | | | | | | |
| | | | 9,028,810 | | | | | | | | | | | $ | 826,558 | |
| | | | | | | | | | | | | | | | | |
| Options vested at December 31, 2007 | |
| | | | | | Weighted Average
| | | Weighted Average
| | | | |
Range of
| | | | | Remaining
| | | Exercise
| | | | |
Exercise Prices | | Number Outstanding | | | Term (Yrs.) | | | Price | | | Aggregate Intrinsic Value | |
| |
| Less than $2.00 | | | 2,100,174 | | | | 5.1 | | | $ | 1.14 | | | $ | 826,558 | |
| $2.00 — $3.99 | | | 1,999,056 | | | | 6.1 | | | $ | 2.65 | | | | — | |
| $4.00 — $5.99 | | | 501,388 | | | | 6.7 | | | $ | 5.17 | | | | — | |
| | | | | | | | | | | | | | | | | |
| | | | 4,600,618 | | | | | | | | | | | $ | 826,558 | |
| | | | | | | | | | | | | | | | | |
| Options expected to vest after at December 31, 2007 | |
| | | | | | Weighted Average
| | | Weighted Average
| | | | |
Range of
| | | | | Remaining
| | | Exercise
| | | | |
Exercise Prices | | Number Outstanding | | | Term (Yrs.) | | | Price | | | Aggregate Intrinsic Value | |
| |
| Less than $2.00 | | | 232,886 | | | | 7.5 | | | $ | 1.61 | | | $ | — | |
| $2.00 — $3.99 | | | 3,278,971 | | | | 9.1 | | | $ | 2.35 | | | | — | |
| $4.00 — $5.99 | | | 224,602 | | | | 7.7 | | | $ | 5.34 | | | | — | |
| | | | | | | | | | | | | | | | | |
| | | | 3,736,459 | | | | | | | | | | | $ | — | |
| | | | | | | | | | | | | | | | | |
Stock Appreciation Rights
In July 2006, we granted cash-settled Stock Appreciation Rights (SARs) to certain employees under the 2006 Equity Incentive Plan. The SARs give the holder the right, upon exercise, to the difference between the price per share of our common stock at the time of exercise and the exercise price of the SAR. The exercise price of the SAR is equal to the market price of our common stock at the date of grant. The SARs vest 25% on the first anniversary of the grant date and 75% vest monthly over the remaining three-year service period. Compensation expense is based on the fair value of SARs which is calculated using the Black-Scholes option pricing model. The share-based compensation expenses and liability are re-measured at each reporting date through the date of settlement.
62
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
The following is a summary of the changes in non-vested SARs for the last two fiscal years:
| | | | | | | | | | | | | | | | | |
| | | 2007 | | | 2006 | |
| | | | | | Weighted
| | | | | | Weighted
| |
| | | | | | Average Exercise
| | | | | | Average Exercise
| |
| | | Number | | | Price | | | Number | | | Price | |
| |
| Outstanding at January 1 | | | 1,564,599 | | | $ | 2.00 | | | | — | | | | — | |
| Granted | | | — | | | | — | | | | 1,564,599 | | | $ | 2.00 | |
| Exercised | | | — | | | | — | | | | — | | | | — | |
| Forfeited and expired | | | (86,380 | ) | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Outstanding SARs at December 31 | | | 1,478,219 | | | $ | 2.00 | | | | 1,564,599 | | | $ | 2.00 | |
| | | | | | | | | | | | | | | | | |
| SARs exercisable at December 31 | | | 47,370 | | | $ | 2.00 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
The total compensation expense related to SARs was approximately $135,000 in 2007 and $294,000 in 2006. At December 31, 2007, approximately $752,000 of unrecognized compensation expense related to SARs is expected to be recognized over a weighted average period of approximately 1.5 years. The resulting effect on net loss and net loss per share attributable to common stockholders is not likely to be representative of the effects in future periods, due to changes in the fair value calculation which is dependent on the stock price, volatility, interest and forfeiture rates, additional grants and subsequent periods of vesting.
| |
| Note 7. | Wind-down and exit costs |
In October 1999, we relocated to California from Rhode Island and established a wind down reserve for the estimated lease payments and operating costs of the Rhode Island facilities through an expected disposal date of June 30, 2000. We did not fully sublet the Rhode Island facilities in 2000. Even though we intend to dispose of the facility at the earliest possible time, we cannot determine with certainty a fixed date by which such disposal will occur. In light of this uncertainty, we periodically re-evaluate and adjust the reserve. We consider various factors such as our lease payments through to the end of the lease, operating expenses, the current real estate market in Rhode Island, and estimated subtenant income based on actual and projected occupancy.
The components of our wind-down reserve at December 31 are as follows:
| | | | | | | | | |
| | | 2007 | | | 2006 | |
| |
| Accrued wind-down reserve at beginning of period | | $ | 5,512,000 | | | $ | 6,098,000 | |
| Less actual expenses recorded against estimated reserve during the period | | | (1,420,000 | ) | | | (1,295,000 | ) |
| Additional expense recorded to revise estimated reserve at period-end | | | 783,000 | | | | 709,000 | |
| | | | | | | | | |
| Revised reserve at period-end | | | 4,875,000 | | | | 5,512,000 | |
| Add deferred rent at period end | | | 1,268,000 | | | | 1,238,000 | |
| | | | | | | | | |
| Total accrued wind-down expenses at period-end (current and non current) | | $ | 6,143,000 | | | $ | 6,750,000 | |
| | | | | | | | | |
| Accrued wind-down expenses, current | | $ | 1,374,000 | | | $ | 1,252,000 | |
| Non current | | | 4,769,000 | | | | 5,498,000 | |
| | | | | | | | | |
| Total accrued wind-down expenses | | $ | 6,143,000 | | | $ | 6,750,000 | |
| | | | | | | | | |
63
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
| |
| Note 8. | Commitments and Contingencies |
Leases
Capital leases
We entered into direct financing transactions with the State of Rhode Island and received proceeds from the issuance of industrial revenue bonds totaling $5,000,000 to finance the construction of itsRhode Island’s pilot manufacturing facility. The related leaseslease agreements are structured such that lease payments will fully fund all semiannual interest payments and annual principal payments through maturity in August 2014. Interest rates vary with the respective bonds’ maturities, ranging from 8.2% to 9.5%. The bonds contain certain restrictive covenants which limit, among other things, the payment of cash dividends and the sale of the related assets. The Company
Operating leases
We entered into a fifteen-year lease agreement for a laboratory facility in Rhode Island in connection with a sale and leaseback arrangement in 1997. The lease hasterm expires June 30, 2013. The lease contains escalating rent payments, and accordingly, the Company is recognizing rent expensewhich we recognize on a straight-line basis. At December 31, 2006, the Company had $1,238,000 in2007, deferred rent expense was approximately $1,268,000 for this facility whichand is presentedincluded as part of the wind-down accrual.accrual on the accompanying Consolidated Balance Sheet.
As of February 1, 2001, the CompanyWe entered into and amended a5-year lease agreement for 40,000 square feet of an approximately 68,000 square foot facility located inat the Stanford Research Park in Palo Alto, CA.California. The facility includes space for animals, laboratories, offices, and a GMP (Good Manufacturing Practices) suite. GMP facilities can be used to manufacture materials for clinical trials. On December 19, 2002The lease term expires March 31, 2010. Under the Company negotiated an amendment to the lease, which resulted in reducing the average annual rent over the remaining term of the lease from approximately $3.7 millionagreement we were required to $2.0 million. As part of the amendment the Company issuedprovide a letter of credit on January 2, 2003 for $503,079,a total of approximately $778,000, which was an addition to the letter of credit in the amount of $275,000 issued at commencement of the lease, to serveserves as a security deposit for the duration of the lease.lease term. The Company negotiated an amendment to the lease
63
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
effective April 1, 2005, which extends the termletter of the lease through March 31, 2010, includes an immediate reduction in the rent per square foot, and provides for an expansioncredit issued by our financial institution is collateralized by a certificate of the leased premises by approximately 28,000 additional square feet effective July 1, 2006. In addition, the Company has sublet some of the additional spacedeposit for the period from April 1, 2005 through June 30, 2006. The average annual rent for the period commencing April 1, 2005 to March 31, 2010 will be approximately $2 million before subtenant income.same amount, which is reflected as restricted cash in other assets, non-current on our Consolidated Balance Sheets. The lease hascontains escalating rent payments, which the Company is recognizingwe recognize on a straight-line basis. At December 31, 2006, the Company had2007, deferred rent liability for this facility of $960,000.was approximately $728,000, and is reflected as deferred rent on our Consolidated Balance Sheet. At December 31, 2006 the Company has2007, we had a space-sharing agreementsagreement covering in total approximately 11,00010,451 square feet of this facility. The Company receives the amount ofWe receive base rentpayments plus thea proportionate share of the operating expenses that it pays for such spacebased on square footage over the term of these agreements.the agreement.
AsThe table below summarizes the components of December 31, 2006, future minimum lease payments and sublease income under operating and capital leases arerent expense at fiscal year end as follows:
| | | | | | | | | | | | | |
| | | Bonds
| | | Operating
| | | Sublease
| |
| | | Payable | | | Leases | | | Income | |
| |
| 2007 | | $ | 332,545 | | | $ | 3,165,162 | | | $ | 909,846 | |
| 2008 | | | 244,531 | | | | 3,469,017 | | | | 407,389 | |
| 2009 | | | 244,572 | | | | 3,536,843 | | | | 387,210 | |
| 2010 | | | 242,560 | | | | 1,767,304 | | | | 97,508 | |
| 2011 | | | 242,321 | | | | 1,171,875 | | | | — | |
| Thereafter | | | 615,110 | | | | 1,904,297 | | | | — | |
| | | | | | | | | | | | | |
| Total minimum lease payments | | | 1,921,639 | | | $ | 15,014,498 | | | $ | 1,801,953 | |
| | | | | | | | | | | | | |
| Less amounts representing interest | | | 570,390 | | | | | | | | | |
| | | | | | | | | | | | | |
| Present value of bonds payable payments | | | 1,351,249 | | | | | | | | | |
| Less current maturities | | | 205,833 | | | | | | | | | |
| | | | | | | | | | | | | |
| Bonds payable, less current maturities | | $ | 1,145,416 | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Rent expense | | $ | 2,963,339 | | | $ | 2,967,911 | | | $ | 2,983,879 | |
| Sublease income | | | (492,306 | ) | | | (616,600 | ) | | | (962,757 | ) |
| | | | | | | | | | | | | |
| Rent expense, net | | $ | 2,471,033 | | | $ | 2,351,311 | | | $ | 2,021,122 | |
| | | | | | | | | | | | | |
Rent expense for the years ended December 31, 2006, 2005 and 2004, was $1,868,000, $1,611,000 and $1,109,000 respectively.
In September 2003 the Company was awarded a one year, $342,000 Small Business Innovation Research grant from the National Institute of Neurological Disease and Stroke (NINDS), to further its work in the treatment of spinal cord injuries. For this award, the Company has recognized approximately $143,000 and $93,000 as grant revenue for 2003 and 2004, respectively. The remaining $106,000 was reimbursed to a subcontractor.
In September 2004 the Company was awarded a Small Business Technology Transfer (STTR) grant for approximately $464,000 over one and one half years for studies in Alzheimer’s disease. The grant supports joint work with Dr. George A. Carlson of the McLaughlin Research Institute (MRI) in Great Falls, Montana. The Company received and recognized approximately $26,000, $186,000 and $38,000 as grant revenue for 2004, 2005 and 2006 respectively. The remainder was reimbursed to MRI.
| |
Note 8. | Wind-down of Encapsulated Cell Technology Research and Development Program |
In October 1999, the Company relocated to California from Rhode Island and established a wind down reserve for the estimated lease payments and operating costs of the Rhode Island facilities through an expected disposal date of June 30, 2000. The Company did not fully sublet the Rhode Island facilities in 2000. Even though the
64
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
Company intends to dispose of the facility at the earliest possible time, the Company’s management cannot determine with certainty a fixed date by which such disposal will occur. In light of this uncertainty, the Company periodically re-evaluates and adjusts the reserve. The Company considers various factors such as the Company’s
Future minimum lease payments through to the end of the lease, operating expenses, the current real estate market in Rhode Island, and estimated subtenant income based on actual and projected occupancy. Atunder all leases at December 31, 2005 the reserve was approximately $6,098,000. In 2006, the company recorded $1,295,000 in operating expenses against the reserve. The Company re-valued the reserve to $5,970,000, $5,847,000, $5,647,000 and $5,512,000 at March 31, 2006, June 30, 2006, September 30, 2006, and December 31, 2006, respectively, by recording an additional $156,000, $175,000, $168,000 and $210,000 respectively2007 are as wind-down expenses. At December 31, 2006, the adjusted reserve was $5,512,000. Even though it is the intent of the Company to dispose of the facility at the earliest possible time, it cannot determine with certainty a fixed date by which such disposal will occur. In light of this uncertainty, based on estimates, the Company will periodically re-evaluate and adjust the reserve.follows:
| | | | | | | | | | | | | | | | | |
| | | Bonds
| | | Capital
| | | Operating
| | | Sublease
| |
| | | Payable | | | Leases | | | Leases | | | Income | |
| |
| 2008 | | $ | 244,531 | | | $ | 19,862 | | | $ | 3,469,017 | | | $ | 388,989 | |
| 2009 | | | 244,572 | | | | 19,862 | | | | 3,536,843 | | | | 387,210 | |
| 2010 | | | 242,559 | | | | 6,623 | | | | 1,767,304 | | | | 97,508 | |
| 2011 | | | 242,321 | | | | — | | | | 1,171,875 | | | | — | |
| 2012 | | | 240,666 | | | | — | | | | 1,171,875 | | | | — | |
| Thereafter | | | 374,444 | | | | — | | | | 732,422 | | | | — | |
| | | | | | | | | | | | | | | | | |
| Total minimum lease payments | | | 1,589,093 | | | | 46,347 | | | $ | 11,849,336 | | | $ | 873,707 | |
| | | | | | | | | | | | | | | | | |
| Less amounts representing interest | | | 443,677 | | | | 3,548 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Present value of bonds payable payments | | | 1,145,416 | | | | 42,799 | | | | | | | | | |
| Less current maturities | | | 136,250 | | | | 17,530 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Bonds payable, less current maturities | | $ | 1,009,166 | | | $ | 25,269 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Wind-down reserveCommitments
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | January to
| | | April to
| | | July to
| | | October to
| | | January to
| | | January to
| |
| | | March 31,
| | | June 30,
| | | September 30,
| | | December 31,
| | | December 31,
| | | December 31,
| |
| | | 2006 | | | 2006 | | | 2006 | | | 2006 | | | 2006 | | | 2005 | |
| |
| Accrued wind-down reserve at beginning of period | | $ | 6,098,000 | | | | 5,970,000 | | | | 5,847,000 | | | | 5,647,000 | | | $ | 6,098,000 | | | $ | 4,350,000 | |
| Less actual expenses recorded against estimated reserve during the period | | | (284,000 | ) | | | (298,000 | ) | | | (368,000 | ) | | | (345,000 | ) | | | (1,295,000 | ) | | | (1,079,000 | ) |
| Additional expense recorded to revise estimated reserve at period-end | | | 156,000 | | | | 175,000 | | | | 168,000 | | | | 210,000 | | | | 709,000 | | | | 2,827,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Revised reserve at period-end | | | 5,970,000 | | | | 5,847,000 | | | | 5,647,000 | | | | 5,512,000 | | | | 5,512,000 | | | | 6,098,000 | |
| Add deferred rent at period end | | | 1,215,000 | | | | 1,223,000 | | | | 1,230,000 | | | | 1,238,000 | | | | 1,238,000 | | | | 1,208,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total accrued wind-down expenses at period-end (current and non current portion) | | $ | 7,185,000 | | | $ | 7,070,000 | | | $ | 6,877,000 | | | $ | 6,750,000 | | | $ | 6,750,000 | | | $ | 7,306,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Accrued wind-down expenses | | | | | | | | | | | | | | | | | | | | | | | | |
| Current portion | | $ | 1,182,000 | | | $ | 1,255,000 | | | $ | 950,000 | | | $ | 1,252,000 | | | $ | 1,252,000 | | | $ | 1,119,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Non current portion | | | 6,003,000 | | | | 5,815,000 | | | | 5,927,000 | | | | 5,498,000 | | | | 5,498,000 | | | | 6,187,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total Accrued wind-down expenses | | $ | 7,185,000 | | | $ | 7,070,000 | | | $ | 6,877,000 | | | $ | 6,750,000 | | | $ | 6,750,000 | | | $ | 7,306,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| |
Note 9. | Consulting Arrangements |
Consulting Arrangements
In September 1997, the Companywe entered into consulting arrangements with the principal scientific founders of StemCells California, Dr. Irving Weissman, Dr. Fred H. Gage, and Dr. David Anderson and with Dr. Richard M. Rose, then President and CEO of StemCells California. To attract and retain Drs. Rose, Weissman, Gage, and Anderson, and to expedite the progress of the Company’sour stem cell program, the Companywe awarded these individuals options to acquire a total of approximately 1.6 million shares of the Company’sour common stock, vesting
65
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
over a period of eight years and at an exercise price of $5.25 per share, the quoted market price at the grant date. Based on the fair value of these options and their respective vesting schedules, the Companywe recorded an expense of $0,approximately $355,000 in 2006 and $830,000 for the years 2006,in 2005, and 2004, respectively.no expense was recorded in 2007. The fair value was determined using the Black Scholes method. As of December 31, 2005, these options have beenwere fully vested and expensed.
Other
In December 2007, we committed to make a secured loan of up to $3.8 million to Progenitor Cell Therapy, LLC (PCT) in return for a period of exclusivity to allow for due diligence and negotiation of a possible acquisition transaction. Of this amount, $1.0 million was lent and outstanding at December 31, 2007 with the maturity date within twelve months from the effective date of the loan. In March 2008, we terminated discussions to acquire PCT. We anticipate the $1.0 million loan will be repaid in accordance with its terms.
Contingencies
In July 2006, we filed suit against Neuralstem, Inc., in the Federal District Court for the District of Maryland, alleging that its Neuralstem’s activities violate claims in four of the patents we exclusively licensed from NeuroSpheres. Neuralstem has filed a motion for dismissal or summary judgment in the alternative, citing Title 35, Section 271(e)(1) of the United States Code, which says that it is not an act of patent infringement to make, use or sell a patented invention “solely for uses reasonably related to the development and submission of information” to the FDA. Neuralstem argues that because it does not have any therapeutic products on the market yet, the activities complained of fall within the protection of Section 271(e)(1) — that is, basically, that the suit is premature. This issue will be decided after discovery is complete. Subsequent to filing its motion to dismiss, in December 2006, Neuralstem petitioned the U.S. Patent and Trademark Office (PTO) to reexamine two of the patents in our infringement action against Neuralstem, namely U.S. Patent No. 6,294,346 (claiming the use of human neural stem
65
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
cells for drug screening) and U.S. Patent No. 7,101,709 (claiming the use of human neural stem cells for screening biological agents). In April 2007, Neuralstem petitioned the PTO to reexamine the remaining two patents in the suit, namely U.S. Patent No. 5,851,832 (claiming methods for proliferating human neural stem cells) and U.S. Patent No. 6,497,872 (claiming methods for transplanting human neural stem cells). These requests were granted by the PTO and, in June 2007, the parties voluntarily agreed to stay the pending litigation while the PTO considers these reexamination requests. In October 2007, Neuralstem petitioned the PTO to reexamine a fifth patent, namely U.S. Patent No. 6,103,530, which claims a culture medium for proliferating mammalian neural stem cells. In September 2007, the PTO issued first office actions in each of the first four reexaminations. The Company has since filed its first responses to each of these, and expects all four patents to re-issue in 2008.
| |
Note 10.9. | Stockholders’ EquityCommon Stock |
We have neither declared nor paid dividends on any share of common stock and do not expect to do so in the foreseeable future.
Sale of Common Stockcommon stock
Listed below are key financingMajor transactions entered into by the Company through the sale of itsinvolving our common stock duringfor the lastprevious three years:years include the following:
| | |
| | • | In April 2007, a warrant issued as part of a June 16, 2004 financing arrangement, was exercised to purchase an aggregate of 575,658 shares of our common stock at $1.90 per share. We issued 575,658 shares of our common stock and received proceeds of approximately $1,094,000. |
|
| • | On December 29, 2006, we filed a Prospectus Supplement announcing the entry of a sales agreement with Cantor under which up to 10,000,000 shares may be sold from time to time under a shelf registration statement. In 2007, we sold a total of 1,807,000 shares of our common stock under this agreement at an average price per share of $2.84 for gross proceeds of approximately $5,133,000. Cantor is paid compensation equal to 5.0% of the gross proceeds pursuant to the terms of the agreement. |
|
| • | On April 6, 2006, the Companywe sold 11,750,820 shares of our common stock to a limited number of institutional investors at a price of $3.05 per share, for gross proceeds of approximately $35,840,000. The shares were offered as a registered direct offering under an effective shelf registration statement previously filed with and declared effective by the Securities and Exchange Commission. The CompanyWe received total proceeds, net of offering expenses and placement agency fees, of approximately $33,422,000. No warrants were issued as part of this financing transaction. |
| |
| | • | OnIn March 2006, a warrant issued as part of a June 16, 2004 the Company entered into an agreement with institutional and other accredited investors with respect to the private placement of approximately 13,160,000 shares of the Company’s common stock at a purchase price of $1.52 per share, for gross proceeds of approximately $20,000,000. Investors also received warrants exercisable for five years to purchase approximately 3,290,000 shares of the Company’s common stock at an exercise price of $1.90 per share. During the period October 2004 to December 2005, part of these warrants werefinancing arrangement was exercised to purchase an aggregate of 1,459,342526,400 shares of the Company’sour common stock at $1.90$1.89 per share. The CompanyWe issued 526,400 shares of our common stock and received proceeds of $2,772,750 on issuance of the shares. C.E. Unterberg, Towbin LLC (Unterberg) served as placement agent for the private placement. For acting as the Company’s placement agent, Unterberg received fees of approximately $1,200,000, expense reimbursement of approximately $25,000 and a five year warrant to purchase 526,400 shares of the Company’s common stock at an exercise price of $1.89 per share.$995,000. |
| |
| | • | In October 2004,2005, an aggregate of 2,958,348 warrants were exercised. For the Company entered into agreements with institutional investors with respect to the registered direct placementexercise of 7,500,000these warrants, we issued 2,842,625 shares of itsour common stock at a purchase price of $3.00 per share, for grossand received proceeds of $22,500,000. Unterberg and Shoreline Pacific, LLC (Shoreline) served as placement agents for the transaction. For acting as the Company’s placement agent, Unterberg and Shoreline received fees totaling $1,350,000 and expense reimbursement of approximately $40,000.$5,939,000. |
Stock Issued For Technology Licenses
Under License Agreementslicense agreements with NeuroSpheres, Ltd., the Companywe obtained an exclusive patent license covering all uses of certain neural stem cell technology. The CompanyWe made up-front payments to NeuroSpheres of 65,000 shares of itsour common stock and $50,000, and will make additional cash payments whenas stated milestones are achieved. Effective in 2004, the Companywe began making annual $50,000 payments, creditable against certain royalties.
Pursuant to the terms of a license agreement with the California Institute of Technology (Cal Tech) and the Company’sour acquisition of its wholly owned subsidiary, StemCells California, StemCellswe issued 14,513 shares of common stock to Cal Tech. The CompanyWe issued an additional 12,800 shares of common stock to Cal Tech with a market value of approximately $40,000 in May 2000, upon execution of an amendment adding four families of patent applications to the license agreement. In August 2002 the Company acquired an additional license from Cal Tech to a different technology, pursuant to which the Company issued 27,535 shares of its common stock with a market value of approximately $35,000. The Company also issued 9,535 shares(market value of approximately $15,000)and 3,848 shares (market value of approximately $10,000) of its common stock in 2004 and 2006
66
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
respectively
agreement. In August 2002, we acquired an additional license from Cal Tech for a different technology, pursuant to which we issued 27,535 shares of our common stock with a market value of approximately $35,000. We also issued 3,865 shares (market value of approximately $10,000) in 2007, 3,848 shares (market value of approximately $10,000) in 2006, and 9,535 shares (market value of approximately $15,000) in 2004 of our common stock to Cal Tech for the issuance and annual license fees of two patents covered under this additional license.
In December 2004, the Company made part payment of $2,833 in stock (1,816 shares) as part of an option agreement with the Board of Trustees of the Leland Stanford Junior University to acquire an exclusive license to an invention. The remainder of the option fee ($7,167) was paid in cash. The Company did not exercise this option but retains a non-exclusive license to the invention.
Stock Option Plans
The Company has adopted several stock plans that provide for the issuance of incentive and nonqualified stock options, various stock and performance awards and stock appreciation rights, at prices to be determined by the Board of Directors. In the case of incentive stock options, such price will not be less than the fair market value on the date of grant. Options granted to employees generally vest ratably over four years and are exercisable for ten years from the date of grant or within three months of termination. Prior to April, 2004, the Company sometimes paid its directors and some of its consultants in below-market options or in stock awards from its stock plans.
The Company has four active stock option plans that were authorized to award 14,000,000 shares in aggregate. 5,320,935 shares were available for grant from these four plans at December 31, 2006.”
The following table presents the combined activity of the Company’s stock option plans for the years ended December 31:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2006 | | | 2005 | | | 2004 | |
| | | | | | Weighted
| | | | | | Weighted
| | | | | | Weighted
| |
| | | | | | Average
| | | | | | Average
| | | | | | Average
| |
| | | Options | | | Exercise Price | | | Options | | | Exercise Price | | | Options | | | Exercise Price | |
| |
| Outstanding at January 1 | | | 6,608,109 | | | $ | 3.02 | | | | 6,682,201 | | | $ | 2.67 | | | | 5,025,374 | | | $ | 2.91 | |
| Granted | | | 2,818,684 | | | | 2.38 | | | | 1,075,481 | | | | 4.75 | | | | 1,932,772 | | | | 1.92 | |
| Exercised | | | (369,214 | ) | | | 1.82 | | | | (423,989 | ) | | | 1.76 | | | | (152,673 | ) | | | 0.30 | |
| Canceled | | | (556,076 | ) | | | 2.82 | | | | (725,584 | ) | | | 3.11 | | | | (123,272 | ) | | | 3.49 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Outstanding at December 31 | | | 8,501,503 | | | $ | 2.88 | | | | 6,608,109 | | | $ | 3.02 | | | | 6,682,201 | | | $ | 2.67 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Options exercisable at December 31 | | | 4,862,717 | | | $ | 3.07 | | | | 4,265,713 | | | $ | 3.03 | | | | 3,687,243 | | | $ | 2.98 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
The aggregate intrinsic value of options exercised during the year ended December 31, 2006 was approximately $453,000. Cash received from stock option exercises during the year ended December 31, 2006 was approximately $548,000. The aggregate intrinsic value of the options outstanding as at December 31, 2006 was approximately $5,028,000.
67
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
The maximum contractual term for stock options is ten years. The following table presents weighted average price and life information about significant option groups outstanding at December 31, 2006:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding at December 31, 2006 | | | Options vested as at December 31, 2006 | | | Options expected to vest after December 31, 2006 | |
| | | | | | Weighted
| | | | | | | | | | | | Weighted
| | | | | | | | | | | | Weighted
| | | | | | | |
| | | | | | Average
| | | | | | | | | | | | Average
| | | | | | | | | | | | Average
| | | | | | | |
| | | | | | Remaining
| | | Weighted
| | | | | | | | | Remaining
| | | Weighted
| | | | | | | | | Remaining
| | | Weighted
| | | | |
Range of
| | | | | Contractual
| | | Average
| | | Aggregate
| | | | | | Contractual
| | | Average
| | | Aggregate
| | | | | | Contractual
| | | Average
| | | Aggregate
| |
Exercise
| | Number
| | | Life
| | | Exercise
| | | Intrinsic
| | | Number
| | | Life
| | | Exercise
| | | Intrinsic
| | | Number
| | | Life
| | | Exercise
| | | Intrinsic
| |
| Prices | | Outstanding | | | (years) | | | Price | | | Value | | | Exercisable | | | (years) | | | Price | | | Value | | | Exercisable | | | (years) | | | Price | | | Value | |
| | | | | | | | |
| |
Less than
$2.00 | | | 2,479,787 | | | | 6.24 | | | $ | 1.16 | | | $ | 3,688,645 | | | | 1,936,757 | | | | 5.85 | | | $ | 1.06 | | | $ | 3,081,834 | | | | 446,877 | | | | 7.66 | | | $ | 1.53 | | | $ | 500,148 | |
$2.00 -
$3.99 | | | 3,637,611 | | | | 8.10 | | | $ | 2.51 | | | | 1,339,625 | | | | 966,711 | | | | 4.43 | | | $ | 2.80 | | | | 109,381 | | | | 2,221,054 | | | | 9.43 | | | $ | 2.40 | | | | 1,027,682 | |
$4.00 -
$5.99 | | | 2,384,105 | | | | 3.43 | | | $ | 5.20 | | | | — | | | | 1,959,248 | | | | 2.30 | | | $ | 5.17 | | | | — | | | | 360,504 | | | | 8.67 | | | $ | 5.31 | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total | | | 8,501,503 | | | | | | | | | | | $ | 5,028,270 | | | | 4,862,716 | | | | | | | | | | | $ | 3,191,215 | | | | 3,028,435 | | | | | | | | | | | $ | 1,527,830 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The weighted average fair value per share of options granted during 2006, 2005 and 2004 was $2.37, $3.75 and $1.64, respectively. The fair value of options at the date of grant were estimated using the Black Scholes model with the following weighted average assumptions:
| | | | | | | | | | | | | |
| | | Options | |
| | | 2006 | | | 2005 | | | 2004 | |
| |
| Expected life (years) | | | 6.25 | | | | 5 | | | | 5 | |
| Risk-free interest rate | | | 4.72 | % | | | 4.14 | % | | | 3.60 | % |
| Expected volatility | | | 109.0 | % | | | 100.7 | % | | | 111.6 | % |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % |
The Company has neither declared nor paid dividends on any share of its common stock and does not expect to do so in the foreseeable future.
Common Stock Reserved
The Company hasWe reserved the following shares of common stock reserved for the exercise of options, warrants and other contingent issuances of common stock, as of December 31, 2006:2007:
| | | | | |
| Shares reserved for exercise of stock options | | | 13,822,43813,574,178 | |
| Shares reserved for warrants related to financing transactions | | | 1,830,6581,255,000 | |
| Shares reserved for compensation related to external services | | | 100,000 | |
| Shares reserved for warrants related to previously converted 3% convertible preferred stock | | | 514,072 | |
| Shares reserved for license agreements | | | 96,15292,287 | |
Shelf reserve for possible future issuances of shares(1)shares | | | 4,721,1429,264,962 | |
| | | | | |
| Total | | | 21,084,46224,800,499 | |
| | | | | |
In September 2004, we were awarded a Small Business Technology Transfer (STTR) grant for approximately $464,000 for studies in Alzheimer’s disease conducted over an 18 month period. The grant supported joint work with Dr. George A. Carlson of the McLaughlin Research Institute (MRI) in Great Falls, Montana. We received and recognized approximately $26,000 in 2006, $186,000 in 2005, and $38,000 in 2004 as grant revenue, the remainder was reimbursed to MRI.
Our 401(k) Plan covers substantially all of our employees. Participants in the plan are permitted to contribute a fixed percentage of their total annual cash compensation to the plan (subject to the maximum employee contribution defined by law). We match 50% of employee contributions, up to a maximum of 6% of each employee’s eligible compensation in the form of shares of common stock. We recorded expense of $179,000 in 2007, $157,000 in 2006, and $111,000 in 2005 for our contributions under our 401(k) Plan.
| |
(1)Note 12. | | On October 4, 2005, the Company filed a shelf registration statement providing for the sale of up to $100,000,000 of its common stock (see “Liquidity and Capital Resources” and Note 15 “Subsequent Events” for details of shares sold under this registration); no specific number of shares has been reserved for this purpose.Income Taxes |
In July 2006, the FASB issued FIN 48 which clarifies the accounting for uncertainty in income taxes by prescribing the recognition threshold a tax position is required to meet before being recognized in the financial statements. We adopted FIN 48 effective January 1, 2007. The adoption of FIN 48 did not impact our consolidated financial condition, results of operations or cash flows. At the adoption date of January 1, 2007 and as of December 31, 2007, we have not recorded any unrecognized tax benefits. Deferred income taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting
6867
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
| |
Note 11. | Research Agreements |
The Company has entered various research agreements and collaborations with academic institutions. Under such arrangements, the Company is typically granted rights to the related intellectual property or an option to obtain such rights on terms to be agreed, in exchange for research funding and specified royalties on any resulting product revenue. In addition, StemCells occasionally makes grants to academic institutions to support research of interest to the Company without requesting any intellectual property interests in return.
Under a November 1997 Research Funding and Option Agreement with The Scripps Research Institute (Scripps), StemCells funded certain research in the total amount of approximately $931,000 and issued 4,837 shares of the Company’s common stock and a stock option to purchase 9,674 shares of the Company’s Common Stock with an exercise price of $.01 per share upon the achievement of specified milestones. As a result of the agreement, StemCells acquired an exclusive license to certain inventions resulting from the sponsored research, subject to the payment of royalties and certain other amounts, including payments totaling $425,000 on the achievement of certain milestones. The Company has also entered Sponsored Research Agreements and License Agreements with Oregon Health & Science University (OHSU) under which it paid OHSU approximately $295,000, 4,838 shares of the Company’s common stock and issued an option to OHSU to purchase an additional 62,888 shares with an exercise price of $.01 per share. The option has vested as to 9,675 shares for which shares were issued on March 31, 2002; the remaining option was terminated and the Company issued 4,000 shares of its common stock, with a market value of approximately $3,900, to OHSU in January 2003, pursuant to an amendment to the license agreement.
In 2006, 2005 and 2004, the Company made research grants and gifts to support relevant research totaling approximately $25,000, $200,000 and $61,000 respectively to academic institutions.
Deferred income taxes reflect net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’sour deferred tax assets and liabilities at December 31 are as follows:
| | | | | | | | | | |
| | | December 31, | | | | | | | | | |
| | | 2006 | | 2005 | | | 2007 | | 2006 | |
| |
| Deferred tax assets: | | | | | | | | | | | | | | | | |
| Capitalized research and development costs | | $ | 25,058,000 | | | $ | 19,618,000 | | | $ | 31,779,000 | | | $ | 25,058,000 | |
| Net operating losses | | | 41,015,000 | | | | 39,894,000 | | | | 42,730,000 | | | | 41,015,000 | |
| Research and development credits | | | 5,671,000 | | | | 5,130,000 | | | | 6,103,000 | | | | 5,671,000 | |
| Accrued wind down cost | | | 2,205,000 | | | | 2,439,000 | | | | 1,950,000 | | | | 2,205,000 | |
| Stock-based compensation | | | 180,000 | | | | — | | | | 245,000 | | | | 180,000 | |
| Other | | | 361,000 | | | | 305,000 | | | | 315,000 | | | | 361,000 | |
| | | | | | | | | | | |
| | | | 74,490,000 | | | | 67,386,000 | | | | 83,122,000 | | | | 74,490,000 | |
| Valuation allowance | | | (74,490,000 | ) | | | (67,386,000 | ) | | | (83,122,000 | ) | | | (74,490,000 | ) |
| | | | | | | | | | | |
| Net deferred tax assets | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | |
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by approximately $8,632,000 in 2007, $7,105,000 in 2006, and $5,027,000 and $1,974,000 during 2006, 2005, and 2004 respectivelyin 2005.
69
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
As of December 31, 2006, the Company2007, we had the following:
| | |
| | • | Net operating loss carry forwards for federal income tax purposes of approximately $118,560,000$122,230,000 which expire in the years 20072008 through 2026.2027. |
| |
| | • | Federal research and development tax credits of approximately $4,624,000$4,696,000 which expire in the years 20072008 through 2026.2027. |
| |
| | • | Net operating loss carry forwards for state income tax purposes of approximately $11,747,000$19,309,000 which expire in the years 2009 through 2016.2017. |
| |
| | • | State research and development tax credits of approximately $1,586,000$2,132,000 ($1,407,000 net of federal tax effect) which do not expire. |
The effective tax rate as a percentage of income before income taxes differs from the statutory federal income tax rate (when applied to income before income taxes) for the years ended December 31 as follows:
| | | | | | | | | | | | | |
| | | 2006 | | | 2005 | | | 2004 | |
| |
| Statutory federal income tax (benefit) rate | | | (34 | )% | | | (34 | )% | | | (34 | )% |
| Increase (decrease) resulting from: | | | | | | | | | | | | |
| Expenses not deductible for taxes | | | 4.5 | | | | 2.4 | | | | 1.9 | |
| Increase in valuation allowance | | | 29.5 | | | | 31.6 | | | | 32.1 | |
| | | | | | | | | | | | | |
| Effective tax (benefit) rate | | | 0 | % | | | 0 | % | | | 0 | % |
| | | | | | | | | | | | | |
| |
Note 13. | Employee Retirement Plan |
The Company has a qualified defined contribution plan covering substantially all employees. Participants are allowed to contribute a fixed percentage of their total annual cash compensation to the plan (subject to the maximums defined by law) and the Company matches 50% of employee contributions, up to a maximum of 6% of the employee’s compensation, with the Company’s common stock. The related expense was $157,000, $111,000, and $78,000 for the years ended December 31, 2006, 2004 and 2003, respectively.
| |
Note 14. | Quarterly Financial Information (unaudited) |
| | | | | | | | | | | | | | | | | |
| | | Quarter | |
Year ended December 31, 2006: | | First | | | Second | | | Third | | | Fourth | |
| | | (In thousands, except per share data) | |
| |
| Total revenue | | $ | 42 | | | $ | 21 | | | $ | 18 | | | $ | 12 | |
| Operating expenses | | | 4,525 | (1) | | | 4,775 | (1) | | | 5,581 | (1) | | | 6,583 | (1) |
| Other income (expense) | | | 291 | | | | 765 | | | | 699 | | | | 668 | |
| Net loss | | | (4,193 | ) | | | (3,989 | ) | | | (4,863 | ) | | | (5,903 | ) |
| Basic and diluted (loss) per share | | $ | (0.06 | ) | | $ | (0.05 | ) | | $ | (0.06 | ) | | $ | (0.08 | ) |
| Year ended December 31, 2005: | | | | | | | | | | | | | | | | |
| Total revenue | | $ | 35 | | | $ | 37 | | | $ | 91 | | | $ | 43 | |
| Operating expenses | | | 3,645 | (1) | | | 4,121 | (1) | | | 4,183 | (1) | | | 4,645 | (1) |
| Other income (expense) | | | 161 | | | | 216 | | | | 3,998 | (2) | | | 275 | |
| Net loss | | | (3,449 | ) | | | (3,868 | ) | | | (94 | ) | | | (4,327 | ) |
| Basic and diluted (loss) per share | | $ | (0.06 | ) | | $ | (0.06 | ) | | $ | (0.00 | ) | | $ | (0.07 | ) |
| | | | | | | | | | | | | |
| | | 2007 | | | 2006 | | | 2005 | |
| |
| Statutory federal income tax (benefit) rate | | | (34 | )% | | | (34 | )% | | | (34 | )% |
| State income tax (benefit) rate | | | (6 | ) | | | (6 | ) | | | (6 | ) |
| Increase (decrease) resulting from: | | | | | | | | | | | | |
| Expenses not deductible for taxes | | | 4.9 | | | | 5.3 | | | | 2.8 | |
| Increase in valuation allowance | | | 35.1 | | | | 34.7 | | | | 37.2 | |
| | | | | | | | | | | | | |
| Effective tax (benefit) rate | | | 0 | % | | | 0 | % | | | 0 | % |
| | | | | | | | | | | | | |
Our policy is to recognize interest and penalties related to income tax matters in income tax expense. Because we have no tax liabilities, no tax-related interest and penalties have been expensed in our consolidated statements of operations during 2007 or accrued as a liability in our consolidated balance sheets at December 31, 2007. We do not anticipate any significant changes to total unrecognized tax benefits as a result of settlement of audits or the expiration of statute of limitations within the next twelve months.
| | |
(1) | | Includes adjustment of wind-down accrual — see Note 8. |
|
(2) | | Includes income recognized on receipt of shares received from ReNeuron — see Note 2. |
7068
StemCells, Inc.
Notes to Consolidated Financial Statements — (Continued)
We file U.S. federal income tax returns, as well as tax returns with the State of California and the State of Rhode Island. Due to the carry forward of unutilized net operating losses and research and development credits, our federal tax returns from 1993 forward remain subject to examination by the Internal Revenue Service, and our State of California tax returns from 1999 forward and our State of Rhode Island tax returns from 2002 forward remain subject to examination by the respective state tax authorities.
*****
QUARTERLY FINANCIAL DATA (unaudited)
(in thousands, except per share amounts)
| | | | | | | | | | | | | | | | | |
| | | 2007 Quarter Ended | |
| | | December 31 | | | September 30 | | | June 30 | | | March 31 | |
| |
| Total revenue | | $ | 30 | | | $ | 13 | | | $ | 8 | | | $ | 6 | |
| Operating expenses(1) | | | 8,353 | | | | 7,749 | | | | 6,041 | | | | 6,505 | |
| Other income, net | | | 497 | | | | 582 | | | | 609 | | | | 1,880 | |
| Net loss | | | (7,825 | ) | | | (7,154 | ) | | | (5,424 | ) | | | (4,620 | ) |
| Basic and diluted loss per share | | $ | (0.10 | ) | | $ | (0.09 | ) | | $ | (0.07 | ) | | $ | (0.06 | ) |
| | | | | | | | | | | | | | | | | |
| | | 2006 Quarter Ended | |
| | | December 31 | | | September 30 | | | June 30 | | | March 31 | |
| |
| Total revenue | | $ | 12 | | | $ | 18 | | | $ | 21 | | | $ | 42 | |
| Operating expenses(1) | | | 6,583 | | | | 5,581 | | | | 4,775 | | | | 4,525 | |
| Other income, net | | | 668 | | | | 699 | | | | 765 | | | | 291 | |
| Net loss | | | (5,903 | ) | | | (4,863 | ) | | | (3,989 | ) | | | (4,193 | ) |
| Basic and diluted (loss) per share | | $ | (0.08 | ) | | $ | (0.06 | ) | | $ | (0.05 | ) | | $ | (0.06 | ) |
| | |
| (1) | | Includes adjustment of wind-down accrual — see Note 15. | Subsequent Events7. |
On December 29, 2006, the Company entered into a sales agreement with Cantor Fitzgerald & Co. (“Cantor”) for the purpose of selling up to 10,000,000 shares of its common stock shares of the Company’s common stock inat-the-market offerings or negotiated transactions from time to time. Cantor will be paid compensation equal to 5.0% of the gross proceeds from the sales of common stock pursuant to the terms of the agreement. A copy of the agreement is attached as Exhibit 10.1 to ourform 8-K dated December 29, 2006. In January 2007, the Company sold a total of 397,000 shares at an average price of $3.51 per share and received net proceeds of approximately $1,325,000 under this agreement.
In February 2007, the Company sold approximately 5,275,000 ordinary shares of ReNeuron for net proceeds of approximately $3.1 million. In February 2007, ReNeuron issued additional shares of common stock; as a consequence of the anti-dilution provisions. StemCells was entitled to approximately 823,000 shares net of approximately 10,000 shares to be transferred to Neurosphere. As of February 26, 2007, the Company owned approximately 4.8 million ordinary shares of ReNeuron.
7169
| |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial DisclosureCHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| |
| Item 9A. | Controls and ProceduresCONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
The Company’s management, with the participation of its chief executive officer and chief financial officer, evaluated the effectiveness of the design and operation of its disclosure controls and procedures (as defined inRules 13a-15(e) and15d-15(e) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this annual report. Based on this evaluation, the Company’s principal executive officer and principal financial officer concluded that these disclosure controls and procedures are effective to ensure that the information required to be disclosed in our reports filed or submitted under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the requisite time periods, and to provide reasonable assurance that information required to be disclosed by the Company in such reports is accumulated and communicated to the Company’s management, including its chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Controls
There have been no changes in the Company’s internal control over financial reporting during the quarter ended December 31, 2006,2007, that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting. The Company’s management, including its principal executive officer and principal financial officer, assessed the effectiveness of its internal control over financial reporting based on the framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The evaluation of the design and operating effectiveness of internal controls over financial reporting include among others those policies and procedures that:
| | |
| | • | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| |
| | • | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and |
| |
| | • | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
During the fiscal year 2006,2007, the Company periodically tested the design and operating effectiveness of its internal controls. Among other matters, the Company sought in its evaluation to determine whether there were any “significant deficiencies” or “material weakness” in its internal control over financial reporting, or whether it had identified any acts of fraud involving management or other employees.
72
Based on the above evaluation, the Company’s chief executive officer and chief financial officer have assessedconcluded that as of December 31, 2006,2007, the Company’s internal controls over financial reporting were effective. Nonetheless, it is important to acknowledge that due to its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
70
Management’s assessment of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2006 has been audited by Grant Thornton LLP, an independent registered public accounting firm, as stated in their report which appears herein.
| |
| Item 9B. | Other Information |
None
73
PART III
| |
| Item 10. | Directors and Executive Officers of the RegistrantDIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT |
TheExecutive Officers
Below are the name, age and principal occupations for the last five years of each executive officer of StemCells, Inc., as of February 29, 2008. All such persons have been elected to serve until their successors are elected and qualified or until their earlier resignation or removal.
| | | | | | |
Martin M. McGlynn,
President and Chief
Executive Officer | | | 61 | | | Martin M. McGlynn joined the company on January 2001, when he was appointed President and Chief Executive Officer of the company and of its wholly-owned subsidiary, StemCells California, Inc. He was elected to the Board of Directors in February 2001. |
Ann Tsukamoto, PhD
Chief Operating Officer | | | 55 | | | Ann Tsukamoto, Ph.D., joined the company in November 1997 as Senior Director of Scientific Operations; was appointed Vice President, Scientific Operations in June 1998 and Vice President, Research and Development in February 2002. In November 2006, she was promoted to Chief Operating Officer, in which role she retains responsibility for the company’s research and development efforts. |
Rodney K.B. Young,
Chief Financial Officer
and Vice President,
Finance and Administration | | | 45 | | | Rodney K.B. Young joined the company in September 2005 as Chief Financial Officer and Vice President, Finance. In November 2006 he became CFO and Vice President, Finance and Administration, with responsibilities for administrative functions including Human Resources and Information Technology in addition to Finance. From 2003 to 2005, Mr. Young was Chief Financial Officer and a director of Extropy Pharmaceuticals, Inc., a private biopharmaceutical company focused on developing drugs for pediatric indications. |
Directors
Below are the name, age and principal occupations for the last five years of each Director of StemCells, Inc., as of February 29, 2008. Directors are elected to staggered three year terms.
| | | | | | |
| Eric H. Bjerkholt | | | 48 | | | Eric H. Bjerkholt was elected to the Board of Directors in March 2004. Mr. Bjerkholt joined Sunesis Pharmaceuticals, Inc., in 2004 as Senior Vice President and Chief Financial Officer. Since February 2007, he has served as Senior Vice President, Corporate Development and Finance, and Chief Financial Officer. From 2002 to 2004, Mr. Bjerkholt was Senior Vice President and Chief Financial Officer at IntraBiotics Pharmaceuticals, Inc. |
| Ricardo B. Levy, Ph.D. | | | 63 | | | Ricardo B. Levy, Ph.D. was elected to the Board of Directors in September 2001. He currently serves on several boards of directors. |
| Martin M. McGlynn | | | 61 | | | Martin M. McGlynn was elected to the Board of Directors in February 2001. He is President and Chief Executive Officer of the Company, a position he has held since January 2001. |
| Desmond H. O’Connell, Jr. | | | 72 | | | Desmond H. O’Connell, Jr. was elected to the Board of Directors in January 2007. He has been an independent management consultant and private investor since 1990. |
71
| | | | | | |
| Roger Perlmutter, M.D., Ph.D. | | | 55 | | | Roger M. Perlmutter, M.D., Ph.D., was elected to the Board of Directors in December 2000. He is Executive Vice President, Research and Development, of Amgen, Inc., a position he has held since January 2001. |
| John J. Schwartz, Ph.D. | | | 73 | | | John J. Schwartz, Ph.D., was elected to the Board of Directors in December 1998 and was elected Chairman of the Board at the same time. He is currently President of Quantum Strategies Management Company. |
| Irving Weissman, M.D. | | | 68 | | | Irving L. Weissman, M.D., was elected to the Board of Directors in September 1997. He is the Virginia and Daniel K. Ludwig Professor of Cancer Research, Professor of Pathology and Professor of Developmental Biology at Stanford. |
Certain other information required by this Item regarding our officers, Directors, and corporate governance is incorporated herein by reference fromto the information appearing under the headings “Information About Our Directors” and “Information About Ownership of Our Common Stock” in our definitive proxy statement to be filed with the Securities and Exchange Commission within 120 days of December 31, 2007 (the “2008 Proxy Statement for the 2007 Annual Meeting of Shareholders.Statement”).
| |
| Item 11. | Executive CompensationEXECUTIVE COMPENSATION |
The information required by this Item is incorporated by reference from Item 5 of this Annual Report onForm 10-K and our Proxy Statement for the 20072008 Annual Meeting of Shareholders.Stockholders.
| |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder MattersSECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item is incorporated by reference from Item 5 of this Annual Report onForm 10-K and from our Proxy Statement for the 2008 Annual Meeting of Stockholders.
| |
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS |
The information required by this Item is incorporated by reference from our Proxy Statement for the 20072008 Annual Meeting of Shareholders.Stockholders.
| |
Item 13.14. | Certain Relationships and Related TransactionsPRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this Item is incorporated by reference from our Proxy Statement for the 20072008 Annual Meeting of Shareholders.
| |
Item 14. | Principal Accounting Fees and Services |
The information required by this Item is incorporated by reference from our Proxy Statement for the 2007 Annual Meeting of Shareholders.Stockholders.
PART IV
| |
| Item 15. | Exhibits and Financial Statement SchedulesEXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) DOCUMENTS FILED AS PART OF THISThe following documents are included as part of this Annual Report onFORMForm 10-K.
(1) Financial Statements:
The financial statements filed as part of this Report are listed and indexed under Item 8 above.
(2) Financial Statement Schedules:
Schedules are not included herein because they are not applicable or the required information appears in the Financial Statements or Notes thereto.
(b)(3) Exhibits.
| | |
EXHIBIT NO.The documents set forth below are filed herewith or incorporated by reference to the location indicated. | | TITLE OR DESCRIPTION
|
|
3.1 | | Restated Certificate of Incorporation of the Registrant |
3.2+ | | Amended and Restated By-Laws of the Registrant |
4.1^^ | | Specimen Common Stock Certificate |
4.2++ | | Form of Warrant Certificate issued to a certain purchaser of the Registrant’s Common Stock in April 1995 |
4.3X | | Common Stock Purchase Warrant |
4.4X | | Callable Warrant |
4.5XXX | | Callable Warrant, dated June 21, 2001, issued to Millennium Partners, L.P. |
4.6XXX | | Common Stock Purchase Warrant, Class A, dated June 21, 2001, issued to Millennium Partners, L.P. |
7472
| | |
EXHIBIT NO.
| | TITLE OR DESCRIPTION
|
|
4.7{*} | | Certificate of Designations of the Powers, Preferences and Relative, Participating, Optional and other Special Rights of Preferred Stock and Qualifications, Limitations and Restrictions Thereof of 3% Cumulative Convertible Preferred Stock for StemCells, Inc. |
4.8{*} | | Warrant to Purchase Common Stock — Riverview Group, LLC |
4.9XXXX | | Warrant to Purchase Common Stock — Cantor Fitzgerald & Co. |
4.10&& | | Warrant to Purchase Common Stock — Riverview Group, LLC |
10.1* | | Form of at-will Employment Agreement between the Registrant and most of its employees |
10.2* | | Form of Agreement for Consulting Services between the Registrant and members of its Scientific Advisory Board |
10.3* | | Form of Nondisclosure Agreement between the Registrant and its Contractors |
10.4* | | 1992 Equity Incentive Plan |
10.5* | | 1992 Stock Option Plan for Non-Employee Directors |
10.7+ | | Research Agreement dated as of March 16, 1994 between NeuroSpheres, Ltd. and Registrant |
10.8+ | | Lease Agreement between the Registrant and Rhode Island Industrial Facilities Corporation, dated as of August 1, 1992 |
10.9+ | | First Amendment to Lease Agreement between Registrant and The Rhode Island Industrial Facilities Corporation dated as of September 15, 1994 |
10.10# | | Lease Agreement dated as of November 21, 1997 by and between Hub RI Properties Trust, as Landlord, and CytoTherapeutics, Inc., as Tenant |
10.11!! | | CTI individual stockholders option agreement dated as of July 10, 1996 among the Company and the individuals listed therein |
10.12! | | Consulting Agreement dated as of September 25, 1997 between Dr. Irving Weissman and the Registrant |
10.13!!! | | StemCells, Inc. 1996 Stock Option Plan |
10.14!!! | | 1997 StemCells Research Stock Option Plan (the “1997 Plan”) |
10.15!!! | | Form of Performance-Based Incentive Option Agreement issued under the 1997 Plan |
10.16$** | | License Agreement dated as of October 27, 1998 between The Scripps Research Institute and the Registrant |
10.17$** | | License Agreement dated as of October 27, 1998 between The Scripps Research Institute and the Registrant |
10.18$** | | License Agreement dated as of November 20, 1998 between The Scripps Research Institute and the Registrant |
10.19++** | | License Agreement dated as of June 1999 between The Scripps Research Institute and the Registrant |
10.20++** | | License Agreement dated as of June 1999 between The Scripps Research Institute and the Registrant |
10.21XX | | License Agreement, dated as of October 30, 2000, between the Registrant and NeuroSpheres Ltd. |
10.22XX | | Letter Agreement, dated January 2, 2001, between the Registrant and Martin McGlynn |
10.23XX | | Lease, dated February 1, 2001, between the Board of Trustees of Stanford University and the Registrant |
10.24$$ | | $2001 Equity Incentive Plan |
10.25& | | Agreement, dated as of April 9, 2003, between the Registrant and Riverview Group, L.L.C. |
10.26&& | | Form of Registration Rights Agreement between the Registrant and Riverview Group, L.L.C. |
10.27&&& | | Securities Purchase Agreement, dated as of May 7, 2003, between the Registrant and Riverview Group, L.L.C. |
10.28% | | Securities Purchase Agreement dated as of December 9, 2003, between the Registrant and Riverview Group, L.L.C. |
| | | | | |
Exhibit No. | | Title or Description |
| |
| | 3 | .1- | | Restated Certificate of Incorporation of the Registrant |
| | 3 | .2-- | | Amended and Restated By-Laws of the Registrant |
| | 4 | .1^ ^ | | Specimen common stock Certificate |
| | 4 | .2++ | | Form of Warrant Certificate issued to a certain purchaser of the Registrant’s common stock in April 1995 |
| | 4 | .3X | | common stock Purchase Warrant |
| | 4 | .4X | | Callable Warrant |
| | 4 | .5XXX | | Callable Warrant, dated June 21, 2001, issued to Millennium Partners, L.P. |
| | 4 | .6XXX | | Common stock Purchase Warrant, Class A, dated June 21, 2001, issued to Millennium Partners, L.P. |
| | 4 | .7{*} | | Certificate of Designations of the Powers, Preferences and Relative, Participating, Optional and other Special Rights of Preferred Stock and Qualifications, Limitations and Restrictions Thereof of 3% Cumulative Convertible Preferred Stock for StemCells, Inc. |
| | 4 | .8{*} | | Warrant to Purchase common stock — Riverview Group, LLC |
| | 4 | .9XXXX | | Warrant to Purchase common stock — Cantor Fitzgerald & Co. |
| | 4 | .10&& | | Warrant to Purchase common stock — Riverview Group, LLC |
| | 10 | .1* | | Form of at-will Employment Agreement between the Registrant and most of its employees |
| | 10 | .2* | | Form of Agreement for Consulting Services between the Registrant and members of its Scientific Advisory Board |
| | 10 | .3* | | Form of Nondisclosure Agreement between the Registrant and its Contractors |
| | 10 | .4* | | 1992 Equity Incentive Plan |
| | 10 | .5* | | 1992 Stock Option Plan for Non-Employee Directors |
| | 10 | .7+ | | Research Agreement, dated as of March 16, 1994, between NeuroSpheres, Ltd. and Registrant |
| | 10 | .8+ | | Lease Agreement between the Registrant and Rhode Island Industrial Facilities Corporation, dated as of August 1, 1992 |
| | 10 | .9+ | | First Amendment to Lease Agreement between Registrant and The Rhode Island Industrial Facilities Corporation dated as of September 15, 1994 |
| | 10 | .10# | | Lease Agreement, dated as of November 21, 1997, by and between Hub RI Properties Trust, as Landlord, and CytoTherapeutics, Inc., as Tenant |
| | 10 | .11!! | | CTI individual stockholders option agreement, dated as of July 10, 1996, among the Company and the individuals listed therein |
| | 10 | .12! | | Consulting Agreement, dated as of September 25, 1997, between Dr. Irving Weissman and the Registrant |
| | 10 | .13!!! | | StemCells, Inc. 1996 Stock Option Plan |
| | 10 | .14!!! | | 1997 StemCells Research Stock Option Plan (the “1997 Plan”) |
| | 10 | .15!!! | | Form of Performance-Based Incentive Option Agreement issued under the 1997 Plan |
| | 10 | .16$** | | License Agreement, dated as of October 27, 1998, between The Scripps Research Institute and the Registrant |
| | 10 | .17$** | | License Agreement, dated as of October 27, 1998, between The Scripps Research Institute and the Registrant |
| | 10 | .18$** | | License Agreement, dated as of November 20, 1998, between The Scripps Research Institute and the Registrant |
| | 10 | .19++** | | License Agreement, dated as of June 199,9 between The Scripps Research Institute and the Registrant |
| | 10 | .20++** | | License Agreement, dated as of June 1999, between The Scripps Research Institute and the Registrant |
| | 10 | .21XX | | License Agreement, dated as of October 30, 2000, between the Registrant and NeuroSpheres Ltd. |
| | 10 | .22XX | | Letter Agreement, dated January 2, 2001, between the Registrant and Martin McGlynn |
| | 10 | .23XX | | Lease, dated February 1, 2001, between the Board of Trustees of Stanford University and the Registrant |
| | 10 | .24$$ | | 2001 Equity Incentive Plan |
| | 10 | .25& | | Agreement, dated as of April 9, 2003, between the Registrant and Riverview Group, L.L.C. |
| | 10 | .26&& | | Form of Registration Rights Agreement between the Registrant and Riverview Group, L.L.C. |
7573
| | |
EXHIBIT NO.
| | TITLE OR DESCRIPTION
|
|
10.29^^^ | | Form of Securities Purchase Agreement dated as of June 16, 2004 between the Registrant and certain Purchasers parties thereto |
10.30^^^ | | Form of Warrant |
10.31^^^^ | | Amended and Restated 2004 Equity Incentive Plan of the Registrant |
10.32§ | | License Agreement dated as of July 1, 2005 between the Registrant and ReNeuron Limited |
10.33§§ | | Letter Agreement effective as of September 6, 2005, between the Registrant and Rodney K.B. Young |
10.34§§ | | Consulting Agreement effective as of September 6, 2005 between the Registrant and Judi R. Lum |
10.35^ | | Sales Agreement with Cantor Fitzgerald |
10.35XX | | Side Letter, dated March 17, 2001, between the Company and Oleh S. Hnatiuk regarding NeuroSpheres License Agreement, dated October 30, 2000 |
14.1%% | | Code of Ethics |
21X | | Subsidiaries of the Registrant |
23.1 | | Consent of Grant Thornton, LLP , Independent Registered Public Accounting Firm |
31.1 | | Certification Pursuant to Securities Exchange ActRule 13(a)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Martin McGlynn, Chief Executive Officer) |
31.2 | | Certification Pursuant to Securities Exchange ActRule 13(a)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Rodney K.B. Young, Chief Financial Officer) |
32.1 | | Certification Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Martin McGlynn, Chief Executive Officer) |
32.2 | | Certification Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Rodney K.B. Young, Chief Financial Officer) |
| | | | | |
Exhibit No. | | Title or Description |
| |
| | 10 | .27&&& | | Securities Purchase Agreement, dated as of May 7, 2003, between the Registrant and Riverview Group, L.L.C. |
| | 10 | .28% | | Securities Purchase Agreement, dated as of December 9, 2003, between the Registrant and Riverview Group, L.L.C. |
| | 10 | .29^ ^ ^ | | Form of Securities Purchase Agreement, dated as of June 16, 2004, between the Registrant and certain Purchasers parties thereto |
| | 10 | .30^ ^ ^ | | Form of Warrant |
| | 10 | .31^ ^ ^ ^ | | Amended and Restated 2004 Equity Incentive Plan of the Registrant |
| | 10 | .32§ | | License Agreement, dated as of July 1, 2005, between the Registrant and ReNeuron Limited |
| | 10 | .33§§ | | Letter Agreement, effective as of September 6, 2005, between the Registrant and Rodney K.B. Young |
| | 10 | .34§§ | | Consulting Agreement, effective as of September 6, 2005, between the Registrant and Judi R. Lum |
| | 10 | .35^ | | Sales Agreement with Cantor Fitzgerald |
| | 10 | .36XX | | Side Letter, dated March 17, 2001, between the Company and Oleh S. Hnatiuk regarding NeuroSpheres License Agreement, dated October 30, 2000 |
| | 10 | .37 | | Note Purchase Agreement with Progenitor Cell Therapy, LLC, dated December 3, 2007. |
| | 10 | .38@ | | License Agreement, dated April 1, 1997, by and among Registrant, NeuroSpheres Ltd. and NeuroSpheres Holdings Ltd. |
| | 14 | .1%% | | Code of Ethics |
| | 21 | X | | Subsidiaries of the Registrant |
| | 23 | .1 | | Consent of Grant Thornton, LLP , Independent Registered Public Accounting Firm |
| | 31 | .1 | | Certification Pursuant to Securities Exchange ActRule 13(a)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Martin McGlynn, Chief Executive Officer) |
| | 31 | .2 | | Certification Pursuant to Securities Exchange ActRule 13(a)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Rodney K.B. Young, Chief Financial Officer) |
| | 32 | .1 | | Certification Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Martin McGlynn, Chief Executive Officer) |
| | 32 | .2 | | Certification Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Rodney K.B. Young, Chief Financial Officer) |
| | |
| ! | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, the Registrant’s Quarterly Report on FormForm 10-Q for the quarter ended September 30, 1997 and filed on November 14, 1997. |
| |
| !! | | Previously filed with the Commission as an Exhibit to and incorporated by reference to, the Registrant’s Quarterly Report onForm 10-Q for the quarter ended September 30, 1996. |
| |
| !!! | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, the Registrant’s Registration Statement onForm S-8, FileNo. 333-37313. |
| |
| $ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s annual report onForm 10-K for the fiscal year ended December 31, 1998 and filed on March 31, 1999. |
| |
| $$ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s definitive proxy statement filed May 1, 2001. |
| |
| % | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on December 10, 2003. |
| |
| %% | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Annual Report onForm 10-K for the fiscal year ended December 31, 2003 |
| |
| & | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on April 15, 2003. |
| |
| && | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on May 13, 2003. |
74
| | |
| &&& | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on May 15, 2003. |
| |
| * | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, Registration Statement onForm S-1, File No. 33-45739. |
76
| | |
| ** | | Confidential treatment requested as to certain portions. The term “confidential treatment” and the mark “**” as used throughout the indicated Exhibits mean that material has been omitted and separately filed with the Commission. |
| |
| ^ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on December 29, 2006. |
| |
| ^^ | | Previously filed with the Commission as an Exhibit to, and incorporated by reference to, the Registrant’s Registration Statement onFormS-3, FileNo. 333-117360. |
| |
| ^^^ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K filed on June 17, 2004. |
| |
| ^^^^ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrants Registration Statement onForm S-8, FileNo. 333-118263. |
| |
| {*} | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K filed on December 7, 2001. |
| |
| + | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, the Registrant’s Registration Statement onForm S-1, FileNo. 33-85494. |
| |
| ++ | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, the Registrant’s Registration Statement onForm S-1, FileNo. 33-91228. |
| |
| - | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Annual Report onForm 10-K for the fiscal year ended December 31, 2006 and filed on March 15, 2007. |
|
| -- | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on May 7, 2007. |
|
| § | | Previously filed with the Commission as an Exhibit to and incorporated herein by reference to, the Registrant’s Quarterly Report onForm10-Q for the quarter ended June 30, 2005. |
| |
| §§ | | Previously filed with the Commission as an Exhibit to and incorporated herein by reference to, the Registrant’s current report onForm 8-K filed on September 7, 2005. |
| |
| X | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Registration Statement onForm S-1, FileNo. 333-45496. |
| |
| XX | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Annual Report onForm 10-K for the fiscal year ended December 31, 2000 and filed on April 2, 2001. |
| |
| XXX | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Registration Statement filed onForm S-1 as amended toForm S-3, File No.No. 333-61726. |
| |
| XXXX | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Registration Statement filed onForm S-3, FileNo. 333-75806. |
|
| @ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Annual Report onForm 10-K/A for the fiscal year ended December 31, 2006 and filed on April 1, 1997. |
|
| # | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s annual report onForm 10-K for the fiscal year ended December 31, 1997 and filed on March 30, 1998. |
7775
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
STEMCELLS, INC.
Martin McGlynn
PRESIDENT AND CHIEF
EXECUTIVE OFFICER
Dated: March 9, 200713, 2008
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | | | | |
Signature | | Capacity | | Date |
| |
| | | | |
/s/ MARTIN McGLYNNMartin McGlynn
Martin McGlynn | | President and Chief Executive Officer
and Director (principal executive officer) | | March 9, 200713, 2008 |
| | | | | |
/s/ RODNEYRodney K.B. YOUNGYoung
Rodney K.B. Young | | Chief Financial Officer
(principal financial officer) | | March 9, 200713, 2008 |
| | | | | |
/s/ GEORGE KOSHYGeorge Koshy
George Koshy | | Chief Accounting Officer
(principal accounting officer) | | March 9, 200713, 2008 |
| | | | | |
/s/ ERIC BJERKHOLTEric Bjerkholt
Eric Bjerkholt | | Director | | March 7, 200711, 2008 |
| | | | | |
/s/ RICARDORicardo B. LEVY PH.D.Levy, Ph.D.
Ricardo B. Levy, Ph.D. | | Director | | March 10, 20079, 2008 |
| | | | | |
/s/ DESMONDDesmond H. O’CONNELL, JR.O’Connell, Jr.
Desmond H. O’Connell, Jr. | | Director | | March 7, 200710, 2008 |
| | | | | |
/s/ ROGERRoger M. PERLMUTTER,Perlmutter, M.D.
Roger M. Perlmutter, M.D. | | Director | | March 7, 200710, 2008 |
| | | | | |
/s/ JOHNJohn J. SCHWARTZ, PH.D.Schwartz, Ph.D.
John J. Schwartz, Ph.D. | | Director, Chairman of the Board | | March 9, 200711, 2008 |
| | | | | |
/s/ IRVINGIrving L. WEISSMAN,Weissman, M.D.
Irving L. Weissman, M.D. | | Director | | March 12, 200711, 2008 |
7876
EXHIBIT INDEXExhibit Index
| | |
EXHIBIT NO.
| | TITLE OR DESCRIPTION
|
|
3.1 | | Restated Certificate of Incorporation of the Registrant |
3.2+ | | Amended and Restated By-Laws of the Registrant |
4.1^^ | | Specimen Common Stock Certificate |
4.2++ | | Form of Warrant Certificate issued to a certain purchaser of the Registrant’s Common Stock in April 1995 |
4.3X | | Common Stock Purchase Warrant |
4.4X | | Callable Warrant |
4.5XXX | | Callable Warrant, dated June 21, 2001, issued to Millennium Partners, L.P. |
4.6XXX | | Common Stock Purchase Warrant, Class A, dated June 21, 2001, issued to Millennium Partners, L.P. |
4.7{*} | | Certificate of Designations of the Powers, Preferences and Relative, Participating, Optional and other Special Rights of Preferred Stock and Qualifications, Limitations and Restrictions Thereof of 3% Cumulative Convertible Preferred Stock for StemCells, Inc. |
4.8{*} | | Warrant to Purchase Common Stock — Riverview Group, LLC |
4.9XXXX | | Warrant to Purchase Common Stock — Cantor Fitzgerald & Co. |
4.10&& | | Warrant to Purchase Common Stock — Riverview Group, LLC |
10.1* | | Form of at-will Employment Agreement between the Registrant and most of its employees |
10.2* | | Form of Agreement for Consulting Services between the Registrant and members of its Scientific Advisory Board |
10.3* | | Form of Nondisclosure Agreement between the Registrant and its Contractors |
10.4* | | 1992 Equity Incentive Plan |
10.5* | | 1992 Stock Option Plan for Non-Employee Directors |
10.7+ | | Research Agreement dated as of March 16, 1994 between NeuroSpheres, Ltd. and Registrant |
10.8+ | | Lease Agreement between the Registrant and Rhode Island Industrial Facilities Corporation, dated as of August 1, 1992 |
10.9+ | | First Amendment to Lease Agreement between Registrant and The Rhode Island Industrial Facilities Corporation dated as of September 15, 1994 |
10.10# | | Lease Agreement dated as of November 21, 1997 by and between Hub RI Properties Trust, as Landlord, and CytoTherapeutics, Inc., as Tenant |
10.11!! | | CTI individual stockholders option agreement dated as of July 10, 1996 among the Company and the individuals listed therein |
10.12! | | Consulting Agreement dated as of September 25, 1997 between Dr. Irving Weissman and the Registrant |
10.13!!! | | StemCells, Inc. 1996 Stock Option Plan |
10.14!!! | | 1997 StemCells Research Stock Option Plan (the “1997 Plan”) |
10.15!!! | | Form of Performance-Based Incentive Option Agreement issued under the 1997 Plan |
10.16$** | | License Agreement dated as of October 27, 1998 between The Scripps Research Institute and the Registrant |
10.17$** | | License Agreement dated as of October 27, 1998 between The Scripps Research Institute and the Registrant |
10.18$** | | License Agreement dated as of November 20, 1998 between The Scripps Research Institute and the Registrant |
10.19++** | | License Agreement dated as of June 1999 between The Scripps Research Institute and the Registrant |
10.20++** | | License Agreement dated as of June 1999 between The Scripps Research Institute and the Registrant |
10.21XX | | License Agreement, dated as of October 30, 2000, between the Registrant and NeuroSpheres Ltd. |
| | | | | |
Exhibit No. | | Title or Description |
| |
| | 3 | .1- | | Restated Certificate of Incorporation of the Registrant |
| | 3 | .2-- | | Amended and Restated By-Laws of the Registrant |
| | 4 | .1^ ^ | | Specimen common stock Certificate |
| | 4 | .2++ | | Form of Warrant Certificate issued to a certain purchaser of the Registrant’s common stock in April 1995 |
| | 4 | .3X | | common stock Purchase Warrant |
| | 4 | .4X | | Callable Warrant |
| | 4 | .5XXX | | Callable Warrant, dated June 21, 2001, issued to Millennium Partners, L.P. |
| | 4 | .6XXX | | Common stock Purchase Warrant, Class A, dated June 21, 2001, issued to Millennium Partners, L.P. |
| | 4 | .7{*} | | Certificate of Designations of the Powers, Preferences and Relative, Participating, Optional and other Special Rights of Preferred Stock and Qualifications, Limitations and Restrictions Thereof of 3% Cumulative Convertible Preferred Stock for StemCells, Inc. |
| | 4 | .8{*} | | Warrant to Purchase common stock — Riverview Group, LLC |
| | 4 | .9XXXX | | Warrant to Purchase common stock — Cantor Fitzgerald & Co. |
| | 4 | .10&& | | Warrant to Purchase common stock — Riverview Group, LLC |
| | 10 | .1* | | Form of at-will Employment Agreement between the Registrant and most of its employees |
| | 10 | .2* | | Form of Agreement for Consulting Services between the Registrant and members of its Scientific Advisory Board |
| | 10 | .3* | | Form of Nondisclosure Agreement between the Registrant and its Contractors |
| | 10 | .4* | | 1992 Equity Incentive Plan |
| | 10 | .5* | | 1992 Stock Option Plan for Non-Employee Directors |
| | 10 | .7+ | | Research Agreement, dated as of March 16, 1994, between NeuroSpheres, Ltd. and Registrant |
| | 10 | .8+ | | Lease Agreement between the Registrant and Rhode Island Industrial Facilities Corporation, dated as of August 1, 1992 |
| | 10 | .9+ | | First Amendment to Lease Agreement between Registrant and The Rhode Island Industrial Facilities Corporation dated as of September 15, 1994 |
| | 10 | .10# | | Lease Agreement, dated as of November 21, 1997, by and between Hub RI Properties Trust, as Landlord, and CytoTherapeutics, Inc., as Tenant |
| | 10 | .11!! | | CTI individual stockholders option agreement, dated as of July 10, 1996, among the Company and the individuals listed therein |
| | 10 | .12! | | Consulting Agreement, dated as of September 25, 1997, between Dr. Irving Weissman and the Registrant |
| | 10 | .13!!! | | StemCells, Inc. 1996 Stock Option Plan |
| | 10 | .14!!! | | 1997 StemCells Research Stock Option Plan (the “1997 Plan”) |
| | 10 | .15!!! | | Form of Performance-Based Incentive Option Agreement issued under the 1997 Plan |
| | 10 | .16$** | | License Agreement, dated as of October 27, 1998, between The Scripps Research Institute and the Registrant |
| | 10 | .17$** | | License Agreement, dated as of October 27, 1998, between The Scripps Research Institute and the Registrant |
| | 10 | .18$** | | License Agreement, dated as of November 20, 1998, between The Scripps Research Institute and the Registrant |
| | 10 | .19++** | | License Agreement, dated as of June 199,9 between The Scripps Research Institute and the Registrant |
| | 10 | .20++** | | License Agreement, dated as of June 1999, between The Scripps Research Institute and the Registrant |
| | 10 | .21XX | | License Agreement, dated as of October 30, 2000, between the Registrant and NeuroSpheres Ltd. |
| | 10 | .22XX | | Letter Agreement, dated January 2, 2001, between the Registrant and Martin McGlynn |
| | 10 | .23XX | | Lease, dated February 1, 2001, between the Board of Trustees of Stanford University and the Registrant |
| | 10 | .24$$ | | 2001 Equity Incentive Plan |
| | 10 | .25& | | Agreement, dated as of April 9, 2003, between the Registrant and Riverview Group, L.L.C. |
7977
| | |
EXHIBIT NO.
| | TITLE OR DESCRIPTION
|
|
10.22XX | | Letter Agreement, dated January 2, 2001, between the Registrant and Martin McGlynn |
10.23XX | | Lease, dated February 1, 2001, between the Board of Trustees of Stanford University and the Registrant |
10.24$$ | | 2001 Equity Incentive Plan |
10.25& | | Agreement, dated as of April 9, 2003, between the Registrant and Riverview Group, L.L.C. |
10.26&& | | Form of Registration Rights Agreement between the Registrant and Riverview Group, L.L.C. |
10.27&&& | | Securities Purchase Agreement, dated as of May 7, 2003, between the Registrant and Riverview Group, L.L.C. |
10.28% | | Securities Purchase Agreement dated as of December 9, 2003, between the Registrant and Riverview Group, L.L.C. |
10.29^^^ | | Form of Securities Purchase Agreement dated as of June 16, 2004 between the Registrant and certain Purchasers parties thereto |
10.30^^^ | | Form of Warrant |
10.31^^^^ | | Amended and Restated 2004 Equity Incentive Plan of the Registrant |
10.32§ | | License Agreement dated as of July 1, 2005 between the Registrant and ReNeuron Limited |
10.33§§ | | Letter Agreement effective as of September 6, 2005, between the Registrant and Rodney K.B. Young |
10.34§§ | | Consulting Agreement effective as of September 6, 2005 between the Registrant and Judi R. Lum |
10.35^ | | Sales Agreement with Cantor Fitzgerald |
10.35XX | | Side Letter dated March 17, 2001, between the Company and Oleh S. Hnatiuk regarding NeuroSpheres License Agreement dated October 30, 2000. |
14.1%% | | Code of Ethics |
21X | | Subsidiaries of the Registrant |
23.1 | | Consent of Grant Thornton, LLP , Independent Registered Public Accounting Firm |
31.1 | | Certification Pursuant to Securities Exchange ActRule 13(a)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Martin McGlynn, Chief Executive Officer) |
31.2 | | Certification Pursuant to Securities Exchange ActRule 13(a)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Rodney K.B. Young, Chief Financial Officer) |
32.1 | | Certification Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Martin McGlynn, Chief Executive Officer) |
32.2 | | Certification Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Rodney K.B. Young, Chief Financial Officer) |
| | | | | |
Exhibit No. | | Title or Description |
| |
| | 10 | .26&& | | Form of Registration Rights Agreement between the Registrant and Riverview Group, L.L.C. |
| | 10 | .27&&& | | Securities Purchase Agreement, dated as of May 7, 2003, between the Registrant and Riverview Group, L.L.C. |
| | 10 | .28% | | Securities Purchase Agreement, dated as of December 9, 2003, between the Registrant and Riverview Group, L.L.C. |
| | 10 | .29^ ^ ^ | | Form of Securities Purchase Agreement, dated as of June 16, 2004, between the Registrant and certain Purchasers parties thereto |
| | 10 | .30^ ^ ^ | | Form of Warrant |
| | 10 | .31^ ^ ^ ^ | | Amended and Restated 2004 Equity Incentive Plan of the Registrant |
| | 10 | .32§ | | License Agreement, dated as of July 1, 2005, between the Registrant and ReNeuron Limited |
| | 10 | .33§§ | | Letter Agreement, effective as of September 6, 2005, between the Registrant and Rodney K.B. Young |
| | 10 | .34§§ | | Consulting Agreement, effective as of September 6, 2005, between the Registrant and Judi R. Lum |
| | 10 | .35^ | | Sales Agreement with Cantor Fitzgerald |
| | 10 | .36XX | | Side Letter, dated March 17, 2001, between the Company and Oleh S. Hnatiuk regarding NeuroSpheres License Agreement, dated October 30, 2000 |
| | 10 | .37 | | Note Purchase Agreement with Progenitor Cell Therapy, LLC, dated December 3, 2007. |
| | 10 | .38@ | | License Agreement, dated April 1, 1997, by and among Registrant, NeuroSpheres Ltd. and NeuroSpheres Holdings Ltd. |
| | 14 | .1%% | | Code of Ethics |
| | 21 | X | | Subsidiaries of the Registrant |
| | 23 | .1 | | Consent of Grant Thornton, LLP , Independent Registered Public Accounting Firm |
| | 31 | .1 | | Certification Pursuant to Securities Exchange ActRule 13(a)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Martin McGlynn, Chief Executive Officer) |
| | 31 | .2 | | Certification Pursuant to Securities Exchange ActRule 13(a)-14(a), as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (Rodney K.B. Young, Chief Financial Officer) |
| | 32 | .1 | | Certification Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Martin McGlynn, Chief Executive Officer) |
| | 32 | .2 | | Certification Pursuant to 18 U.S.C. Section 1350, As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Rodney K.B. Young, Chief Financial Officer) |
| | |
| ! | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, the Registrant’s Quarterly Report on FormForm 10-Q for the quarter ended September 30, 1997 and filed on November 14, 1997. |
| |
| !! | | Previously filed with the Commission as an Exhibit to and incorporated by reference to, the Registrant’s Quarterly Report onForm 10-Q for the quarter ended September 30, 1996. |
| |
| !!! | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, the Registrant’s Registration Statement onForm S-8, FileNo. 333-37313. |
| |
| $ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s annual report onForm 10-K for the fiscal year ended December 31, 1998 and filed on March 31, 1999. |
| |
| $$ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s definitive proxy statement filed May 1, 2001. |
| |
| % | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on December 10, 2003. |
80
| | |
| %% | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Annual Report onForm 10-K for the fiscal year ended December 31, 2003 |
| |
| & | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on April 15, 2003. |
78
| | |
| && | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on May 13, 2003. |
| |
| &&& | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on May 15, 2003. |
| |
| * | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, Registration Statement onForm S-1, File No. 33-45739. |
| |
| ** | | Confidential treatment requested as to certain portions. The term “confidential treatment” and the mark “**” as used throughout the indicated Exhibits mean that material has been omitted and separately filed with the Commission. |
| |
| ^ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on December 29, 2006. |
| |
| ^^ | | Previously filed with the Commission as an Exhibit to, and incorporated by reference to, the Registrant’s Registration Statement onFormS-3, FileNo. 333-117360. |
| |
| ^^^ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K filed on June 17, 2004. |
| |
| ^^^^ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrants Registration Statement onForm S-8, FileNo. 333-118263. |
| |
| {*} | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K filed on December 7, 2001. |
| |
| + | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, the Registrant’s Registration Statement onForm S-1, FileNo. 33-85494. |
| |
| ++ | | Previously filed with the Commission as Exhibits to, and incorporated herein by reference to, the Registrant’s Registration Statement onForm S-1, FileNo. 33-91228. |
| |
| - | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Annual Report onForm 10-K for the fiscal year ended December 31, 2006 and filed on March 15, 2007. |
|
| -- | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s current report onForm 8-K on May 7, 2007. |
|
| § | | Previously filed with the Commission as an Exhibit to and incorporated herein by reference to, the Registrant’s Quarterly Report onForm10-Q for the quarter ended June 30, 2005. |
| |
| §§ | | Previously filed with the Commission as an Exhibit to and incorporated herein by reference to, the Registrant’s current report onForm 8-K filed on September 7, 2005. |
| |
| X | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Registration Statement onForm S-1, FileNo. 333-45496. |
| |
| XX | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Annual Report onForm 10-K for the fiscal year ended December 31, 2000 and filed on April 2, 2001. |
| |
| XXX | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Registration Statement filed onForm S-1 as amended toForm S-3, File No.No. 333-61726. |
| |
| XXXX | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Registration Statement filed onForm S-3, FileNo. 333-75806. |
|
| @ | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s Annual Report onForm 10-K/A for the fiscal year ended December 31, 2006 and filed on April 1, 1997. |
|
| # | | Previously filed with the Commission as an Exhibit to, and incorporated herein by reference to, the Registrant’s annual report onForm 10-K for the fiscal year ended December 31, 1997 and filed on March 30, 1998. |
8179

