UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K10-K/A
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO
Commission File Number 001-32001
Aptose Biosciences Inc.
(Exact name of Registrant as specified in its Charter)
Canada | 98-1136802 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
251 Consumers Road, Suite 1105 Toronto, Ontario, Canada M2J 4R3 | M2J 4R3 |
(Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (647) 479-9828
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
| Trading Symbol(s) |
| Name of each exchange on which registered |
Common Shares, no par value |
| APTO |
| Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
|
|
|
| |||
Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
|
|
|
|
|
|
|
Emerging growth company |
| ☐ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting stock and nonvoting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked prices of such common equity, as of June 30, 2023 was $29,584,561.
As of March 26,April 29, 2024, the registrant had 15,717,70116,252,114 Common Shares outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of our Proxy Statement for our 2023 Annual Meeting of Stockholders (the “Proxy Statement”), are incorporated by reference in Part III.
Auditor Name: KPMG LLP Auditor Location: Vaughn, Canada Auditor Firm ID: 85
EXPLANATORY NOTE TO AMENDMENT NO. 1
Aptose Biosciences Inc., ("Aptose", the "Company," "we," or "our"), is filing this Amendment No. 1 on Form 10-K/A, or this Amendment No. 1, to its Annual Report on Form 10-K for the fiscal year ended December 31, 2023, which was filed with the Securities and Exchange Commission on March 26, 2024, (the "Original Form 10-K"), for the sole purpose of including the information required by Part III of Form 10-K. This information was previously omitted from the Original Form 10-K in reliance on General Instruction G(3) to Form 10-K, which permits the information required by Part III to be incorporated by reference from our definitive proxy statement if such statement is filed no later than 120 days after our fiscal year-end. We are filing this Amendment No. 1 to include Part III information in our Annual Report on Form 10-K because we will not file a definitive proxy statement containing this information within 120 days after the end of the fiscal year covered by the Original Form 10-K.
Pursuant to Rule 12b-15 under the Securities Exchange Act of 1934, as amended, or the Exchange Act, this Amendment No. 1 also contains certifications pursuant to Section 302 of the Sarbanes-Oxley Act of 2002, which are attached hereto. Because no financial statements have been included in this Amendment No. 1 and this Amendment No. 1 does not contain or amend any disclosure with respect to Items 307 and 308 of Regulation S-K, paragraphs 3, 4, and 5 of the certifications have been omitted.
Except as explicitly set forth herein, this Amendment No. 1 does not purport to modify or update the disclosures in, or exhibits to, the
Original Form 10-K or to update the Original Form 10-K to reflect events occurring after the date of such filing.
TABLE OF CONTENTS
2 | ||
|
| |
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
|
| |
|
| |
| ||
|
| |
| ||
| ||
|
| |
| ||
|
| |
|
| |
|
| |
| ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, |
|
|
| |
|
|
|
| ||
|
| |
| ||
| ||
i
This Annual Report on Form 10-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and is subject to the safe harbor created by those sections. For more information, see “Part I. Item 1. Business — Cautionary Note Regarding Forward-Looking Statements.”
As used in this report, the terms “Aptose,” “Aptose Biosciences,” the “Company,” “we,” “us,” “our” and similar references refer to Aptose Biosciences Inc. (formerly known as Lorus Therapeutics Inc.) and our consolidated subsidiaries, and the term “Common Shares” refers to our common shares, no par value.
Aptose had historically qualified as a “foreign private issuer” for purposes of reporting under the Exchange Act, and filing registration statements under the Securities Act of 1933, as amended. Effective December 31, 2018, however, Aptose ceased qualifying as a foreign private issuer and began filing reports with the United States Securities and Exchange Commission ("SEC") as a “domestic issuer.” As a result, Aptose changed the accounting standards by which it prepares its financial statements from International Financial Reporting Standards to generally accepted accounting principles in the United States, or “U.S. GAAP.” All financial statements contained in this Annual Report are presented in accordance with U.S. GAAP. This report contains the following trademark, trade name and service mark of ours: Aptose. This report also contains trademarks, trade names and service marks that are owned by other persons or entities.
PART I.
Item 1. Business
Overview
Aptose Biosciences Inc. is a science-driven, clinical-stage biotechnology company committed to precision medicines addressing unmet clinical needs in oncology, with an initial focus on hematology. The Company's small molecule cancer therapeutics pipeline includes products designed to provide single agent efficacy and to enhance the efficacy of other anti-cancer therapies and regimens without overlapping toxicities. The Company’s executive office is located in San Diego, California.III
Our ProgramsItem 10. Directors, Executive Officers and Corporate Governance
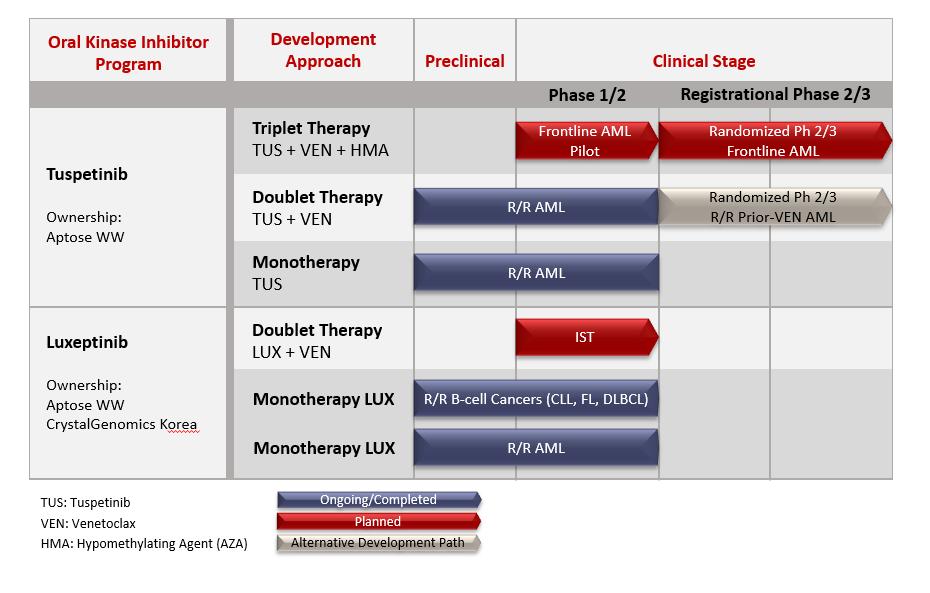
We are advancing oral targeted agents to treat life-threatening hematologic cancers that require immediate treatment. We have two clinical-stage oral kinase inhibitors under active development for the treatment of hematologic malignancies: tuspetinib (HM43239) and luxeptinib (CG-806). Tuspetinib and luxeptinib are being evaluated for safety, tolerability, pharmacokinetics and efficacy in Phase 1/2 clinical trials, and each molecule is described below. A third molecule (APTO-253) is not undergoing active clinical development and will not be discussed further.
Tuspetinib, Aptose’s lead asset, is being developed for frontline combination therapy in newly diagnosed AML patients to unlock the most significant patient impact and greatest commercial opportunity. Tuspetinib is a once-daily oral kinase inhibitor, targeting a select group of kinases operative in myeloid malignancies, such as acute myeloid leukemia ("AML") and the higher risk myelodysplastic syndromes ("hr-MDS"), and known to be involved in tumor proliferation, resistance to therapy, and differentiation. However, tuspetinib avoids kinases that typically cause toxicities associated with other kinase inhibitors and is consequently a well-tolerated antileukemic agent. The clinical development path for triplet combination therapy in newly diagnosed AML patients with tuspetinib-based triplet combination therapy (tuspetinib + the BCL-2 inhibitor venetoclax + hypomethylating agent; TUS+VEN+HMA) begins with a demonstration of safety and activity of tuspetinib as a single agent ("TUS") and then with the TUS+VEN doublet combination therapy in relapsed or refractory ("R/R") AML patients.
Tuspetinib monotherapy dose escalation and dose exploration activities have been completed as part of an international Phase 1/2 clinical trial designed to assess the safety, tolerability, pharmacokinetics, pharmacodynamic responses, and efficacy as a single agent in patients with R/R AML. Complete responses (“CRs”) without dose limiting toxicities were achieved at four dose levels across a broad diversity of mutationally-defined AML populations and with a highly favorable safety profile. Tuspetinib to date has demonstrated a favorable safety profile and has caused no drug-related QTc prolongations, liver or kidney toxicities, muscle damage, differentiation syndrome, and no
2
myelosuppression with continuous dosing of patients in remission. A recommended phase 2 dose ("RP2D") of 80 mg tuspetinib once daily as an oral tablet was selected and approved by the U.S. FDA for use as a single agent in patients with R/R AML. At the RP2D, tuspetinib demonstrated notable response rates in R/R AML patients that had never been treated with venetoclax (VEN-naive AML): CR/CRh=36% among all-comers, CR/CRh=50% among patients with mutated FLT3, and CR/CRh=25% in patients with wildtype FLT3.
Following completion of the single agent dose escalation and exploration trial, tuspetinib advanced into the APTIVATE expansion trial of the Phase 1/2 program in R/R AML patient populations treated with tuspetinib combined with the BCL-2 inhibitor venetoclax (TUS+VEN doublet), with the intent to position tuspetinib for triple combination studies in frontline therapy for newly diagnosed AML patients. The TUS+VEN doublet combination therapy (with both 40mg and 80mg TUS) maintained a favorable safety profile: no new or unexpected safety signals were observed, and there were no reported drug-related adverse events of QTc prolongation, differentiation syndrome, or deaths. Also, the TUS/VEN doublet combination (with 80mg TUS) achieved responses in heavily pretreated R/R AML patients, including those with wildtype or mutated FLT3, and those who failed prior therapy with venetoclax (Prior-VEN) or FLT3 inhibitors (Prior-FLT3i). Based on the safety and efficacy profile of tuspetinib, we believe that tuspetinib, if approved, can reach annual sales greater than $3 billion by 2035 because we believe tuspetinib could 1) become the preferred kinase inhibitor for inclusion in triplet combination for front line AML patients with FLT3 mutations and for patients with wild type FLT3, 2) become the preferred kinase inhibitor for inclusion in combination with venetoclax for second line AML patients, 3) serve as an effective agent for maintenance therapy to prevent relapse in patients who achieved a complete remission through a stem cell transplant or through drug-based therapy, 4) serve as an effective agent for the treatment of third line FLT3 mutated patients failed by prior therapy with other FLT3 inhibitors and 5) serve in front line triplet combinations, second line doublet combinations, and maintenance therapy for hr-MDS patients. These beliefs related to the potential commercial opportunity are based on management’s current assumptions and estimates, which are subject to change, and there can be no assurance that tuspetinib will ever be approved or successfully commercialized and, if approved and commercialized, that it will ever generate significant revenues. See our “Risk Factors – “We are an early-stage development company with no revenues from product sales.” and “We have a history of operating losses. We expect to incur net losses and we may never achieve or maintain profitability.” in this Annual Report on Form 10-K.
Luxeptinib, Aptose's second agent, is an oral, highly potent kinase inhibitor that selectively targets defined kinases operative in myeloid and lymphoid hematologic malignancies. This small molecule has been evaluated in a Phase 1a/b study for the treatment of patients having R/R B-cell leukemias and lymphomas (dose escalation from 150mg-900mg BID) and in a Phase 1a/b study for the treatment of patients with R/R AML or hr-MDS (dose escalation from 450 mg-900mg BID). These clinical studies demonstrated tumor shrinkage among B-cell cancer patients, including a complete response ("CR") in a DLBCL patient who received the original G1 formulation. Likewise, an MRD-negative CR in one R/R AML patient occurred with 450mg BID dosing of the original G1 formulation. Because absorption of the original G1 formulation hampered effectiveness of luxeptinib, a new G3 formulation was developed. Enrollment of patients in the B-cell malignancy trial and the AML trial have been completed, and the initial clinical evaluation of the G3 formulation with continuous BID dosing has been completed. The G3 formulation delivered superior plasma exposure levels relative to the original G1 formulation. Regarding potential next steps with luxeptinib, a molecularly defined subgroup of hematologic malignancy patients was recently identified that may benefit from treatment with luxeptinib in combination with venetoclax. An investigator-sponsored trial is being considered while non-clinical studies are underway to support the use of LUX+VEN for the treatment of these patients. In parallel, efforts are underway to identify sources of capital to support such a trial.
3
The following figure identifies the clinical stage agents in our pipeline and their respective stages of development.

Tuspetinib Program
Licensing Overview
On November 4, 2021, we entered into a licensing agreement (the "Tuspetinib Licensing Agreement") with the South Korean company Hanmi Pharmaceutical Co Ltd. (“Hanmi”) for the clinical and commercial development of tuspetinib (formerly HM43239). Under the terms of the Tuspetinib Licensing Agreement, Hanmi granted us exclusive worldwide rights to tuspetinib for all indications. Hanmi received an upfront payment of $12.5 million, including $5 million in cash and $7.5 million in Common Shares. Hanmi will also receive up to $407.5 million in future milestone payments contingent upon achieving certain clinical, regulatory and sales milestones across several potential indications, as well as tiered royalties on net sales. The term of the agreement will continue on a product-by-product and country-by-country basis until the expiration of the royalty period for such product in such country. The licenses to us will survive and become non-exclusive, perpetual, irrevocable and fully paid-up on a product-by-product and country-by-country basis, upon their natural expiration under the terms of the agreement.
Preclinical Profile
Tuspetinib is an oral, once-daily, highly potent myeloid kinome inhibitor designed to target key kinases operative in myeloid malignancies. In preclinical studies, tuspetinib demonstrated potent in vitro and in vivo activity against FLT3 ITD mutated as well as D835 and gatekeeper (F691) tyrosine kinase domain ("TKD") mutated AML that confer resistance to other agents. Additionally, tuspetinib inhibited phosphorylation of the SYK kinase, known to be highly activated in AML and associated with resistance to FLT3 targeted therapy. Tuspetinib also was designed to inhibit several kinases involved in tumor cell proliferation and/or differentiation, including mutant forms of c-KIT, JAK1, JAK2, and RSK, all with half maximal inhibitory concentration ("IC50") values < 10 nM.
Tuspetinib induced in vitro cytotoxicity in AML and Ba/F3 cell lines expressing FLT3 WT, ITD, and/or TKD point mutations. Tuspetinib showed greater inhibitory activity compared to quizartinib on Ba/F3 cells expressing
4
resistance-conferring ITD/TKD double mutations (ITD/F691L and ITD/D835Y). Thus, Tuspetinib may overcome clinically relevant ITD/TKD double mutations, which may result from sustained FLT3 inhibition. Moreover, target modulation was shown as tuspetinib inhibited FLT3 phosphorylation and downstream signaling molecules such as phospho-ERK and phospho-STAT5.
The in vivo anti-tumor efficacy of tuspetinib was demonstrated in murine xenograft models using MV-4-11 and MOLM-13 human AML cells having the ITD mutant form of FLT3 and using the MOLM-14 model having the ITD and F691L dual mutations of FLT3 with dosing regimens that match those currently under investigation. Tuspetinib exhibited dose-dependent tumor growth inhibition of models of FLT3 ITD mutant AML with complete tumor regression observed in some groups, and no change in body weight. Of note, tuspetinib produced greater tumor growth inhibition in the MOLM-14 FLT3-ITD/F691L model compared to gilteritinib, or entospletinib (SYK inhibitor) as single agents, and comparable activity to the gilteritinib plus entospletinib combination.
Latest Clinical Update and Program Status
On December 9, 2023, Aptose featured tuspetinib in an oral presentation at the 65th American Society of Hematology ("ASH") Annual Meeting and Exposition and announced that a growing body of clinical data for Aptose’s lead compound tuspetinib, demonstrates significant benefit as a single agent and in combination with venetoclax (VEN) in patients with R/R AML in the ongoing APTIVATE Phase 1/2 study. Data were presented in an oral presentation by lead investigator Naval G. Daver, M.D., Professor, Director Leukemia Research Alliance Program, Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX.
Dr. Daver reported data from more than 100 relapsed/refractory patients from multiple international clinical sites, who had failed prior therapy and then were treated with TUS as a single agent or the TUS+VEN doublet. Both TUS and the TUS+VEN doublet delivered multiple composite complete remissions ("CRc") in this very ill AML population, while maintaining a favorable safety profile across all treated patients. The data demonstrated tuspetinib as a single agent (TUS) is active and well tolerated in one of the most challenging and heterogeneous disease settings in oncology – relapsed and refractory AML. Tuspetinib demonstrated broad activity, including activity in patients with FLT3 wild-type AML (accounting for more than 70% of the AML population), FLT3 mutated AML, NPM1 mutated AML, as well as in patients with mutations historically associated with resistance to targeted therapy. Most notably, TUS targets VEN resistance mechanisms, enabling the TUS+VEN combination therapy to uniquely treat the very ill prior-VEN AML population, including both FLT3 mutant and FLT3 wildtype disease. From a broader perspective, the growing body of antileukemic activity, and continued favorable safety profile, support advancement of tuspetinib as the TUS+VEN+HMA triplet combination therapy for the treatment of frontline newly diagnosed AML patients.
Dr. Daver also pointed out that while patients on the TUS+VEN therapy are early in their treatment cycles, most patients achieving a response remained on treatment and that responses have begun to mature as dosing continues. Highlights of Dr. Daver’s ASH oral presentation include:
Name |
|
|
| ||
|
|
|
5
|
|
|
| ||
Dr. William G. Rice | 65 | President, Chief Executive Officer and Chairman | |||
Dr. Rafael Bejar | 53 | Chief Medical Officer | |||
Fletcher Payne | 61 | Chief Financial Officer and Chief Business Officer | |||
Philippe Ledru(*) | 57 | Chief Commercial Officer | |||
Non-Employee Directors | |||||
Carol G. Ashe | 66 | Director | |||
Dr. Denis Burger | 80 | Director | |||
Dr. Erich Platzer | 73 | Director | |||
Dr. Bernd R. Seizinger | 67 | Director | |||
Dr. Mark D. Vincent | 71 | Director | |||
Warren Whitehead | 71 | Director | |||
* Philippe Ledru departed the Corporation in | |||||
Clinical data from tuspetinib in AML were presented at the ASH Annual Meeting in December 2022 and during a Corporate Comprehensive Clinical Update Call held December 11, 2022. Data presented demonstrated that tuspetinib delivers single agent responses without prolonged myelosuppression or life-threatening toxicities in these very ill and heavily pretreated relapsed or refractory AML patients. Responses were observed in a broad range of mutationally-defined populations, including those with mutated forms of NPM1, MLL, TP53, NRAS, KRAS, DNMT3A, RUNX1, wild-type FLT3, ITD or TKD mutated FLT3, various splicing factors, and other genes As of October 6, 2022, 60 heavily pretreated R/R AML patients were enrolled at multiple centers and treated at doses escalating from 20 mg to 200 mg, with further dose exploration at the 40 mg, 80 mg, 120 mg and 160 mg dose levels. Tuspetinib delivered multiple CRs at 40 mg, 80 mg, 120 mg and 160 mg dose levels in which no DLTs were observed. Tuspetinib demonstrated clinically meaningful benefit in all responders, by either bridging successfully to HSCT or leading to a durable response, as well as a favorable safety profile. In addition to 5 CRcs and 1 PR reported at ASH 2021, 4 new CRcs and 3 new PR had been generated during 2022. New responses during 2022 were achieved with 160 mg, 120 mg, 80 mg, and 40 mg. Among efficacy-evaluable patients treated with 80 mg, 120 mg, or 160mg, response rates ranging from 19% to 75% were achieved in specific genotypic subpopulations of R/R AML patients. Significant bone marrow leukemic blast reductions were observed broadly in FLT3+ and FLT3 wildtype patients across multiple dose levels, comparable to reported gilteritinib data, except that the patients treated with tuspetinib were more heavily pre-treated relapsed and refractory AML patients than those treated with gilteritinib. Vignettes of patient experiences highlight the potency and breadth of tuspetinib to deliver complete remissions among several mutationally-defined populations with a diversity of adverse mutations. Tuspetinib continued to show a favorable safety profile with only mild AEs and no DLTs up to 160 mg per day, and no drug discontinuations from drug-related toxicity. No drug-related SAE, drug-related deaths, differentiation syndrome, AE of QT prolongation or DLT were observed through the 160 mg level. Tuspetinib avoids many of the typical toxicities observed with other tyrosine kinase inhibitors. We identified a safe therapeutic range with a broad therapeutic window, spanning the dose levels of 40, 80, 120 and 160 milligrams. We also announced that enrollment had been initiated in the APTIVATE expansion trial for monotherapy and drug combination therapy with tuspetinib. For the APTIVATE expansion trial, we selected 120 mg as the initiating single agent expansion dose and 80 mg as the initiating dose selected for combination with venetoclax.Information about our Executive Officers
As of January 30, 2023, we announced the dosing of patients in the APTIVATE Phase 1/2 clinical trial of tuspetinib, and that another clinical response has been achieved by a R/R AML patient receiving 40 mg tuspetinib once daily orally in the original dose exploration trial, the second response at the recently launched low-dose 40 mg cohort. In addition, we elucidated a rationale for the superior safety profile of tuspetinib. While several kinase inhibitors require high exposures that exert near complete suppression of a single target to elicit responses, those agents often cause additional toxicity because they also cause extensive inhibition of that target in normal cells. In contrast, tuspetinib simultaneously suppresses a small suite of kinase-driven pathways critical for leukemogenesis. Consequently, tuspetinib achieves clinical responses at lower exposures with less overall suppression of each pathway, thereby avoiding many toxicities observed with competing agents.
Concurrent with the European Hematology Association (EHA) Annual Congress held June 8-11, 2023, Aptose held an interim clinical update webcast on June 10, 2023, to present highlights from the ongoing clinical development of tuspetinib. Aptose reported completion of the tuspetinib dose escalation and dose exploration Phase 1/2 trial in 77 R/R AML patients, tuspetinib demonstrated a favorable safety profile, and tuspetinib delivered monotherapy responses across four dose levels with no dose-limiting toxicity in mutationally diverse and difficult to treat R/R AML populations, including patients with highly adverse mutations that typically do not respond to monotherapy or combination therapy: TP53-mutated patients with a CR/CRh = 20% and RAS-mutated patients with a CR/CRh = 22%. Aptose also reported completion of a successful End of Phase 1 Meeting with the US FDA for tuspetinib, that a monotherapy RP2D was selected as 80mg daily, and that all development paths remain open, including the single arm accelerated path. Following completion of the dose escalation and dose exploration phases of the Phase 1/2 clinical program, Aptose focused attention on the tuspetinib APTIVATE expansion trial. The APTIVATE trial sought to identify patient populations that may serve as development paths in R/R AML patients sensitive to the TUS+VEN doublet and can serve as development paths for accelerated and full approvals. We reported that patient enrollment in
6
the APTIVATE expansion trial has been brisk and preliminary CR activity had already been reported in patients receiving the TUS+VEN doublet who previously failed therapy with venetoclax.
On October 29, 2023, Aptose presented two posters related to the clinical and preclinical activity of tuspetinib at the European School of Haematology 6th International Conference: Acute Myeloid Leukemia "Molecular and Translational": Advances in Biology and Treatment, held October 29-31, 2023, in Estoril, Portugal. Clinical findings included 1) data from the APTO-TUS-HV01 clinical trial (the "Food Effect Study") evaluating the pharmacokinetic (PK) properties of tuspetinib in healthy human volunteers in which tuspetinib was administered with our without food, and 2) from an international Phase 1/2 study of tuspetinib as a single agent and in combination with venetoclax in patients with R/R AML from across clinical centers in the United States, South Korea, Spain, Australia and other sites. Data from the Food Effect Study in healthy human volunteers demonstrated tuspetinib can be administered with or without food and foresee no clinically meaningful difference in exposure. This is an important finding for patient convenience, as venetoclax is dosed with food and tuspetinib can now be simultaneously administered with the venetoclax rather than require staggered dosing. Findings from the Phase 1/2 clinical trial demonstrated tuspetinib as a single agent was well-tolerated and highly active among R/R AML patients with a diversity of adverse genotypes and delivered a 42% CR/CRh cross-evaluable venetoclax-naive patients at the 80mg daily RP2D. The TUS+VEN doublet has been well tolerated in the APTIVATE international Phase 1/2 expansion trial in R/R AML patients and achieved multiple responses in patients who previously failed venetoclax ("Prior-VEN failure AML"), including Prior-VEN failure patients who also previously failed FLT3 inhibitors, all of whom represent emerging populations of high unmet medical need. Notably, tuspetinib targets venetoclax resistance mechanisms that may re-sensitize Prior-VEN failure patients to venetoclax.
Separate from the clinical studies, the preclinical study (entitled: “Tuspetinib Oral Myeloid Kinase Inhibitor Creates Synthetic Lethal Vulnerability to Venetoclax”) presented by Aptose during the ESH Conference investigated the effects of tuspetinib on key elements of the phosphokinome and apoptotic proteome in both parental and TUS-resistant AML cells. In parental cells, tuspetinib inhibits key oncogenic signaling pathways and shifts the balance of pro- and anti-apoptotic proteins in favor of apoptosis, suggesting that it may generate vulnerability to venetoclax. Indeed, acquired resistance in the AML cells to tuspetinib generated a synthetic lethal vulnerability to venetoclax of unusually high magnitude. Concurrent administration of TUS+VEN therefore may discourage the emergence of resistance to tuspetinib during treatment. In conjunction with poster presentations at the ESH Conference, on October 30, 2023, Aptose held a “Clinical Update and KOL Data Review of AML Drug Tuspetinib” that was webcast and featured Dr. Naval Daver, MD, Professor, Director Leukemia Research Alliance Program, Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, Texas. Dr. Daver is the lead investigator on Aptose’s APTIVATE trial and is recognized for significant achievements in the development of novel AML treatments, including several combination therapies. Aptose presented data in 49 patients who received the TUS+VEN doublet, showing an overall response rate (ORR) of 48% among all patients that had achieved an evaluable stage, as well as a 44% ORR among Prior-VEN failure AML patients, including FLT3-unmutated (wildtype) patients (43% ORR) and FLT3-mutated patients (60% ORR), some of whom also had failed prior therapy with FLT3 inhibitors. The TUS+VEN doublet was well tolerated with no unexpected safety signals. The TUS/VEN doublet may serve the Prior-VEN failure R/R AML patients that represent a rapidly growing population that is highly refractory to any salvage therapy with response rates in the 4-15% range. The compelling data with the TUS+VEN doublet in R/R AML patients suggest a TUS+VEN+HMA triplet may serve the needs of frontline (1L) newly diagnosed AML patients.
On December 9, 2023, Aptose featured tuspetinib in an oral presentation at the 65th ASH Annual Meeting and Exposition and announced that a growing body of clinical data for Aptose’s lead compound tuspetinib, demonstrates significant benefit as a single agent and in combination with venetoclax in patients with R/R AML in the ongoing APTIVATE Phase 1/2 study. Data were presented in an oral presentation by lead investigator Naval G. Daver, M.D., Professor, Director Leukemia Research Alliance Program, Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX.
Luxeptinib Program
Licensing Overview
On May 7, 2018, we exercised an option by paying $2.0 million in cash to the South Korean company CrystalGenomics Invites Co. Ltd, formerly Crystal Genomics, Inc. (“CG”) to purchase an exclusive license to
7
research, develop and commercialize luxeptinib in all countries of the world except the Republic of Korea and China, for all fields of use (collectively, the “Rights”). Subsequently, on June 14, 2018, we announced that we entered into a license agreement with CG for us to gain a license for rights to CG-806 in China (including the People’s Republic of China, Hong Kong, and Macau) ) (the “China Rights”). Under the license agreement, we made an upfront payment to CG of $3.0 million for the China rights. CG is eligible for development, regulatory and commercial-based milestones, as well as single-digit royalties on product sales in China. The total deal value for the China Rights, including the upfront payment, is up to $125 million. Aptose now owns worldwide (excluding Korea) rights to luxeptinib, a first-in-class, highly potent oral small molecule being developed for AML, B-cell malignancies, and other hematologic malignancies. Future possible royalties that might be paid under these agreements are determined on a country-by-country and product-by-product basis, on net sales during the period of time beginning on the first commercial sale of such product in such country and continuing until the later of: (i) the expiration of the last-to-expire valid claim of the CG Patents in such country covering such product; and (ii) ten (10) years after the first commercial sale of such product in such country.
Preclinical Profile
Luxeptinib exhibits a picomolar IC50 toward FLT3 with the Internal Tandem Duplication (“FLT3-ITD”), potency against the wild type FLT3 and a host of mutant forms of FLT3, as well as single-digit nanomolar IC50’s against BTK and its C481S mutant (“BTK-C481S”). Further, luxeptinib suppresses a small group of other relevant oncogenic kinases/pathways (including CSF1R, PDGFRα, TRK, and the ERK, MYC, AKT/mTOR/S6K and AURK/H3S10 pathways) that are operative in AML and certain B cell malignancies, but does not inhibit the TEC, epidermal growth factor receptor (EGFR) and ErbB2/4 kinases that are responsible for safety concerns with certain other kinase inhibitors.
As a potent inhibitor of FLT3-ITD, luxeptinib may become an effective therapy in a high-risk subset of AML patients. This is because the FLT3-ITD mutation occurs in approximately 30% of patients with AML and is associated with a poor prognosis. In murine xenograft studies of human AML (FLT3-ITD), CG-806 administered orally resulted in tumor elimination without measurable toxicity. Importantly, luxeptinib targets other oncogenic kinases which may also be operative in FLT3-ITD AML, thereby potentially allowing the agent to become an important therapeutic option for a broader group of this difficult-to-treat AML patient population. The findings that luxeptinib targets all forms of FLT3 and several other key oncogenic pathways, and that luxeptinib was well tolerated from a safety perspective during efficacy and formal Good Laboratory Practice (“GLP”) toxicology studies, suggest that luxeptinib may also have applicability in treating patients, particularly those over the age of 65, who cannot tolerate other therapies.
Separate from the AML and FLT3 applications, luxeptinib may be a therapeutic option for patients with B cell malignancies. Overexpression of the BTK enzyme can drive oncogenic signaling of certain B cell malignancies, including CLL and certain NHL such as mantle cell lymphoma, follicular lymphoma, diffuse large cell B cell lymphoma, and others. Therapy of these patients with covalent, irreversible BTK inhibitors, such as ibrutinib, that target the active site cysteine (“Cys”) residue of BTK can be beneficial in many patients. However, therapy with covalent BTK inhibitors can select for BTK with a C481S mutation, thereby conferring resistance to covalent BTK inhibitors. Furthermore, approximately half of CLL patients have discontinued treatment with ibrutinib after 3.4 years of therapy. Discontinuation of ibrutinib is due to the development of drug resistance (in particular, patients have malignancies that developed the BTK-C481S mutation), or due to refractory disease (patient tumors did not respond to ibrutinib) or intolerance (side effects led to discontinuation of ibrutinib), according to a study performed at The Ohio State University. The C481S mutation is observed in 5-10% of the patients, while 40-45% of the patients were intolerant or refractory to ibrutinib. As a non-covalent, reversible inhibitor of BTK, luxeptinib does not rely on the Cysteine 481 residue for inhibition of the BTK enzyme. Indeed, recent X-ray crystallographic studies (with wild type and C481S BTK) demonstrated that luxeptinib binds productively to the BTK active site in a manner that is indifferent to the presence or absence of mutations at the 481 residue. Moreover, in vitro studies demonstrated that luxeptinib kills B cell malignancy cell lines on average approximately 1000 times more potently than ibrutinib and kills ibrutinib-resistance cancer cells, and that luxeptinib more potently killed primary malignant cells taken from the bone marrow of CLL and ALL B-cell cancer patients. Yet, luxeptinib demonstrated a high degree of safety in animal efficacy and GLP toxicology studies. Consequently, patients who are resistant, refractory or intolerant to ibrutinib or other commercially approved or development-stage BTK inhibitors with B cell malignancies may continue to be sensitive to luxeptinib therapy. This is particularly true since luxeptinib inhibits the wild type and mutant forms of BTK, as well as other kinases/pathways that drive the survival and proliferation of B cell malignancies.
8
Latest Clinical Update and Program Status
During 2023 and early 2024, clinical evaluation of the new G3 formulation of luxeptinib was performed and has now been completed. The G3 formulation was tested in a single dose bioavailability study in 20 patients, including both B-cell cancer and AML patients, and across 5 dose levels (10mg to 200mg). The G3 formulation then was evaluated in R/R AML patients with continuous dosing using two different dose levels (50mg BID and 200mg BID) in a total of 11 patients. Data show the G3 formulation dosed at 200mg twice daily can achieve 2-3uM steady state plasma levels, with approximately 10-fold better absorption, and interestingly even better tolerability, than the original G1 formulation. Thus, the G3 formulation achieved the desired plasma exposure benchmark and can serve as the formulation of choice for future studies with LUX. Aptose is exploring alternative development paths and collaborations to advance LUX as a single agent or in combination with VEN to treat defined R/R patient populations of high unmet need.
Luxeptinib was evaluated in a Phase 1 a/b trial in patients with relapsed or refractory B cell malignancies who have failed or are intolerant to standard therapies, and in a separate Phase 1 a/b trial in patients with relapsed or refractory AML or high-risk MDS. During 2022, a new G3 formulation was tested as a single dose in 20 patients during the ongoing Phase 1 a/b clinical program. Modeling of the PK properties of G3 predicted steady-state plasma exposure from continuous dosing with 50 mg of G3 (every 12 hours, Q12h) should be comparable to that of 900 mg of the original G1 formulation Q12h, representing a significant improvement in bioavailability with G3. On November 14, 2022, we announced dosing of the first AML patient to receive a continuous dosing regimen of the G3 formulation (50 mg G3 Q12h), with the protocol allowing for further dose escalation of G3 in subsequent patients. Clinical data from both studies were presented during a Corporate Comprehensive Clinical Update Call held December 11, 2022. During the Corporate Update Call, we announced a CR was achieved with a diffuse large B-cell lymphoma patient at the end of Cycle 22 with 900mg BID of the original G1 formulation. Previously, an MRD-negative CR was reported with a R/R AML patient receiving 450mg BID of the original G1 formulation.
Concurrent with the EHA Annual Congress held June 8-11, 2023, Aptose held an interim clinical update webcast on June 10, 2023. During the update, Aptose reviewed clinical findings with the new G3 formulation of luxeptinib. Aptose confirmed that continuous dosing with 50mg Q12h of the G3 formulation in multiple patients achieves roughly an equivalent pharmacokinetic profile as 900mg original G1 formulation, and that dose escalation with the G3 formulation was anticipated. Since completion of the 50mg G3 Q12h dose exploration, R/R AML patients have been dosed with 200mg Q12h G3.
Safety and PK data with continuous dosing of the G3 formulation have been completed and the 200mg dose of G3 luxeptinib achieved steady state exposure plasma levels of approximately 2uM. The amalgam of clinical safety, PK and activity data with all formulations of luxeptinib in B-cell cancer and AML patients are being collected and evaluated, and we plan to disclose the findings at a scientific presentation. In addition, a molecularly defined subgroup of CLL patients (harboring mutations in FLT3 receptor) has been identified as a potential target population for treatment with luxeptinib in combination with other agents, and a feasibility analysis for the potential development of luxeptinib for the target population is underway.
Competitive Conditions
The biotechnology and pharmaceutical industries are characterized by rapidly evolving technology and intense competition. There are numerous companies in these industries that are focusing their efforts on activities similar to ours. Some of these are companies with established positions in the pharmaceutical industry and may have substantially more financial and technical resources, more extensive research and development capabilities, and greater marketing, distribution, production, and human resources than us. In addition, we face competition from other companies for opportunities to enter partnerships with biotechnology and pharmaceutical companies and academic institutions.
Competition with our potential products may include chemotherapeutic agents, monoclonal antibodies, antisense therapies, small molecules, immunotherapies, vaccines, and other biologics with novel mechanisms of action. These drugs may kill cancer cells indiscriminately, or through a targeted approach, and some have the potential to be used in non-cancer indications. We also expect that we will experience competition from established and
9
emerging pharmaceutical and biotechnology companies that have other forms of treatment for the cancers that we target, including drugs currently in development for the treatment of cancer that employ a number of novel approaches for attacking these cancer targets. Cancer is a complex disease with more than 100 indications requiring drugs for treatment. The drugs in competition with our potential drugs have specific targets for attacking the disease, targets which are not necessarily the same as ours. These competitive drugs, however, could potentially also be used together in combination therapies with our drugs to manage the disease. Other factors that could render our potential products less competitive may include the stage of development, where competitors’ products may achieve earlier commercialization, as well as superior patent protection, better safety profiles, or a preferred cost-benefit profile.
Intellectual Property
We believe that our issued patents and pending applications are important in establishing and maintaining a competitive position with respect to our products and technology.
Tuspetinib (HM43239)
In November 2021, we licensed the exclusive rights to research, develop and commercialize tuspetinib (the "Tuspetinib Licensing Agreement"). Under the terms of the Tuspetinib Licensing Agreement, Hanmi granted Aptose exclusive worldwide rights to tuspetinib for all indications.Aptose is now the exclusive licensee of composition of matter and use patents covering tuspetinib, and tuspetinib analogs. Aptose believes that it now owns rights to a strong and defensive intellectual property position.
As of December 31, 2023, Aptose owned rights in 46 issued patents, including 4 issued U.S. patents, and 23 patents validated in countries in Europe, that are in force and cover the tuspetinib compound, or analog compounds. These patents are expected to provide protection until 2038 through 2039. Patent applications are also pending in the United States and in contracting states to the Patent Cooperation Treaty for coverage of tuspetinib and analog compounds, with expected expiry dates between 2038 and 2042.
Luxeptinib (CG-806)
In May 2018 and June 2018, we licensed the Rights to CG-806, for all fields of use, in all territories outside of the Republic of Korea and China, by exercising an option we obtained through a June 2016 option-license agreement with CG that had granted us an exclusive option to research, develop and commercialize CG-806. In June 2018, we entered into a separate license agreement with CG for Aptose to gain a license for the China Rights. Aptose now owns worldwide Rights to CG-806, including an issued patent in China but excluding any Rights in Korea.
As of December 31, 2023, Aptose owned rights to 49 issued patents, including 3 issued U.S. patents, and 30 patents validated in countries in Europe, that are in force and cover numerous compounds, including the CG-806 compound, pharmaceutical compositions comprising the CG-806 compound, and methods of use for treating various diseases by administering various compounds, including the CG-806 compound. These patents are expected to provide protection until 2033-2038. Patent applications are also pending in the United States and in contracting states to the Patent Cooperation Treaty for coverage of CG-806, with expected expiry dates between 2038‑2039.
The Company’s research and development activities involve the controlled use of hazardous and radioactive materials and, accordingly, the Company is subject to federal, provincial and local laws and regulations in the United States and Canada governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. To the knowledge of the Company, compliance with such environmental laws and regulations does not and will not have any significant impact on its capital spending, profits or competitive position within the normal course of its operating activities. There can be no assurance, however, that the Company will not be required to incur significant costs to comply with environmental laws and regulations in the future or that its operations, business or assets will not be materially adversely affected by current or future environmental laws or regulations.
10
Employees
As of December 31, 2023, we employed 35 full-time persons and one part-time person in research and drug development and administration activities. Eleven of our employees hold Ph.D.s, two hold M.D.s, and numerous others hold degrees and designations such as M.Sc., B.Sc., C.P.A., C.M.A., M.Acc. and M.B.A. To encourage a focus on achieving long-term performance, employees and members of the board of directors of the Company (the “Board”) have the ability to acquire an ownership interest in the Company through Aptose’s share option and alternate compensation plans.
The business of the Company requires personnel with specialized skills and knowledge in oncology. Researchers must be able to design and implement studies to assess the efficacy of anticancer drugs. Specialized knowledge and skills relating to chemistry and formulation process development are also needed. Such knowledge and skills are needed to develop product specific analytical methods and formulation processes. The Company’s business also requires clinical and regulatory expertise and knowledge. The Company has trained scientists and personnel with broad experience in these fields.
None of our employees are unionized, and we consider our relations with our employees to be good.
Government Regulation
Overview
Our overall regulatory strategy is to work with the appropriate government departments which regulate the use and sale of therapeutic drug products. This includes the FDA in the United States, Health Canada in Canada, the European Medicines Agency (“EMA”) in Europe, and other local regulatory agencies with oversight of preclinical studies, clinical trials and marketing of therapeutic products. Where possible, we intend to take advantage of opportunities for accelerated development of drugs designed to treat rare and serious or life-threatening diseases. We also intend to pursue priority evaluation of any application for marketing approval filed in Canada, the United States or the European Union and to file additional drug applications in other markets where commercial opportunities exist. We may not be able to pursue these opportunities successfully.
Regulation(s) by government authorities in the United States, Canada, and the European Union are significant factors in guiding our current research and drug development activities. To clinically test, manufacture and market drug products for therapeutic use, we must be in compliance with guidance and regulations established by the regulatory agencies in the countries in which we currently operate or intend to operate.
The laws of most of these countries require the licensing of manufacturing facilities, carefully controlled research and the extensive testing of products. Biotechnology companies must establish the safety and efficacy of their new products in clinical trials; they must establish and comply with current good manufacturing practices ("cGMPs") for the manufacturing of the product and control over marketing activities before being allowed to market a product. The safety and efficacy of a new drug must be shown through human clinical trials of the drug carried out in accordance with the guidance and regulations established by local and federal regulatory agencies.
The process of completing clinical trials and obtaining regulatory approval for a new drug takes a number of years and requires the expenditure of substantial resources. Once a new drug or product license application is submitted, regulatory agencies may not review the application in a timely manner and may not approve the product. Even after a New Drug Application (“NDA”) submission has occurred and/or approval has been obtained, further studies, including post-marketing studies, may be required to provide additional data on the efficacy and safety necessary to confirm the approved indication or to gain approval for the use of the new drug as a treatment for clinical indications other than those for which the new drug was initially tested. Regulatory agencies also require post-marketing surveillance programs to monitor a new drug’s side effects, safety and long-term effects of the product. A serious safety or effectiveness problem involving an approved new drug may result in a regulatory agency mandating a withdrawal of the new drug from the market and possible civil action. It is possible that we could encounter such difficulties or excessive costs in our efforts to secure necessary approvals, which could delay or prevent us from manufacturing or marketing our products.
11
In addition to the regulatory product approval framework, biotechnology companies, including Aptose, are subject to regulation under local, provincial, state and federal law, including requirements regarding occupational safety, laboratory practices, environmental protection and hazardous substance control, and may be subject to other present and future local, provincial, state, federal and foreign regulation, including possible future regulation of the biotechnology industry.
Approval of New Drugs in Canada
In Canada, the manufacture and sale of new drugs are controlled by Health Canada. New drugs must pass through a number of testing stages, including pre-clinical testing and human clinical trials. Pre-clinical testing involves testing the new drug’s chemistry, pharmacology and toxicology in vitro and in vivo. Successful results (that is, potentially valuable pharmacological activity combined with an acceptable low level of toxicity) enable the developer of the new drug to file a clinical trial application to begin clinical trials involving humans.
To study a drug in Canadian patients, a clinical trial application submission must be filed with Health Canada. The clinical trial application submission must contain specified information, including the results of the pre-clinical tests completed at the time of the submission and any available information regarding use of the drug in humans. In addition, since the method of manufacture may affect the efficacy and safety of a new drug, information on manufacturing methods and standards and the stability of the drug substance and dosage form must be presented. Production methods and quality control procedures must be in place to ensure an acceptably pure product, essentially free of contamination, and to ensure uniformity with respect to all quality aspects.
In addition, all federally regulated trials must be approved and monitored by an independent committee of doctors, scientists, advocates and others to ensure safety and ethical standards, Institutional Review Boards (“IRBs”) or Ethics Review Boards (“ERBs”). The review boards study and approve all study-related documents before a clinical trial begins and also carefully monitor data to detect benefit or harm, and validity of results.
Provided Health Canada does not reject a clinical trial application submission and IRB or ERB approval has been obtained, clinical trials can begin. Clinical trials for product candidates in Canada, as in the United States, are generally carried out in three phases. Phase 1 involves studies to evaluate toxicity and ideal dose levels in healthy humans. The new drug is administered to human patients who have met the clinical trial entry criteria to determine pharmacokinetics, human tolerance and prevalence of any adverse side effects. Phases 2 and 3 involve therapeutic studies. In Phase 2, efficacy, dosage, side effects and safety are established in a small number of patients who have the disease or disorder that the new drug is intended to treat. In Phase 3, there are controlled clinical trials in which the new drug is administered to a large number of patients who are likely to receive benefit from the new drug. In Phase 3, the effectiveness of the new drug in patients is compared to that of standard accepted methods of treatment in order to provide sufficient data for the statistical proof of safety and efficacy for the new drug.
If clinical studies establish that a new drug has value, the manufacturer submits a new drug submission application to Health Canada for marketing approval. The new drug submission contains all known information known about the new drug, including the results of pre-clinical testing and clinical trials. Information about a substance contained in new drug submission includes its proper name, its chemical name, and details on its method of manufacturing and purification, and its biological, pharmacological and toxicological properties. The new drug submission also provides information about the dosage form of the new drug, including a quantitative listing of all ingredients used in its formulation, its method of manufacture, manufacturing facility information, packaging and labeling, the results of stability tests, and its diagnostic or therapeutic claims and side effects, as well as details of the clinical trials to support the safety and efficacy of the new drug. Furthermore, for biological products, an on-site evaluation is completed to assess the production process and manufacturing facility. It is required prior to the issuance of a notice of compliance. All aspects of the new drug submission are critically reviewed by Health Canada. If a new drug submission is found satisfactory, a notice of compliance is issued permitting the new drug to be sold for the approved use. In Canada, an establishment license must be obtained prior to marketing the product.
Health Canada has a policy of priority evaluation of new drug submissions for all drugs intended for serious or life-threatening diseases for which no drug product has received regulatory approval in Canada and for which there is reasonable scientific evidence to indicate that the proposed new drug is safe and may provide effective treatment.
12
An exception to the foregoing requirements relating to the manufacture and sale of a new drug is the limited authorization that may be available in respect of the sale of new drugs for emergency treatment. Under the special access program, Health Canada may authorize the sale of a quantity of a new drug for human use to a specific practitioner for the emergency treatment of a patient under the practitioner’s care. Prior to authorization, the practitioner must supply Health Canada with information concerning the medical emergency for which the new drug is required, such data as is in the possession of the practitioner with respect to the use, safety and efficacy of the new drug, the names of the institutions at which the new drug is to be used and such other information as may be requested by Health Canada. In addition, the practitioner must agree to report to both the drug manufacturer and Health Canada the results of the new drug’s use in the medical emergency, including information concerning adverse reactions, and must account to Health Canada for all quantities of the new drug made available.
The Canadian regulatory approval requirements for new drugs outlined above are similar to those of other major pharmaceutical markets. While the testing carried out in Canada is often acceptable for the purposes of regulatory submissions in other countries, individual regulatory authorities may request supplementary testing during their assessment of any submission. Therefore, the clinical testing conducted under Health Canada authorization or the approval of regulatory authorities of other countries may not be accepted by regulatory authorities outside Canada or other countries.
Approval of New Drugs in the United States
In the United States, the FDA controls and investigates the investigation, manufacturing, and sale of new drugs. New drugs require FDA approval of an NDA prior to commercial sale. In the case of certain biological products, a Biological License Application (“BLA”) must be obtained prior to marketing and batch releasing. As in Canada, to obtain marketing approval, data from adequate and well-controlled human clinical trials, demonstrating to the FDA’s satisfaction a new drug’s safety and effectiveness for its intended use, are required. Data are generated in studies conducted pursuant to an investigational new drug (“IND”) submission, similar to that required for a clinical trial application in Canada. Clinical trials with human subjects are characterized as Phase 1, Phase 2 and Phase 3 trials or a combination thereof. In a marketing application, the manufacturer must also demonstrate the identity, potency, quality and purity of the active ingredients of the new drug involved, and the stability of those ingredients. Further, the manufacturing facilities, equipment, processes and quality controls for the new drug must comply with the FDA’s current cGMP regulations for drugs both in a pre-licensing inspection before product licensing and in subsequent periodic inspections after licensing. An establishment license grants the sponsor permission to fabricate, package, label, distribute, import, wholesale or test the newly approved drug.
Federally regulated trials must be approved and monitored by an independent committee of doctors, scientists, advocates, and others to ensure safety and ethical standards, IRBs or ERBs. The review boards study and approve all study-related documents before a clinical trial begins and also carefully monitor data to detect benefit or harm, and validity of results.
Post-Approval Regulation
The monitoring of a new drug does not cease once it is on the market. For example, a manufacturer of a new drug must report any new information received concerning serious side effects, as well as the failure of the new drug to produce desired effects. If Health Canada determines it to be in the interest of public health, a notice of compliance for a new drug may be suspended and the new drug may be removed from the market.
A post surveillance program involves clinical trials conducted after a drug is marketed (referred to as Phase 4 studies in the United States) and is an important source of information on as yet undetected adverse outcomes, especially in populations that may not have been involved in the premarketing trials (e.g., children, the elderly, pregnant women) and the drug’s long-term morbidity and mortality profile. Regulatory authorities may require companies to conduct Phase 4 studies as a condition of market approval. Companies often conduct post-marketing studies in the absence of a regulatory mandate.
13
The foregoing description is a summary of the requirements for a new drug to be approved for marketing in North America. The EMA and Japanese Pharmaceuticals and Medical Devices Agency are also important regulatory authorities in drug development. Together with the FDA, they are the three International Conference on Harmonization parties which oversee the three largest markets for drug sales.
Information About Our Executive Officers
Aptose’s leadership team comprises accomplished industry, financial and clinical research professionals who are dedicated to building a comprehensive anticancer drug pipeline and clinical development programs focused on targeted therapeutics directed against dysregulated oncogenic processes in patients with life. Thelife-threatening hematologic malignancies. For the year ended December 31, 2023, the team includesincluded our Chairman, President Chairman and Chief Executive Officer, Dr. William G. Rice, our Senior Vice President, Chief Financial Officer and Chief Business Officer, Fletcher Payne; our Senior Vice President and Chief Medical Officer.Officer, Dr. Rafael Bejar and our Senior Vice President and Chief Commercial Officer, Philippe Ledru, who departed from the Corporation in 2024.
William G. Rice, Ph.D., age 64, joined Aptose65, serves as Chairman andthe President, Chief Executive Officer, and Chairman of the Board of Aptose and joined the company in October 2013. Dr. Rice brings 25 years of C-level experience in the biotech industry to Aptose. Prior to joining Aptose, Dr. Rice served as the President, Chief Executive Officer, and Chairman of the boardBoard of Directors of Cylene Pharmaceuticals, Inc. (“Cylene”), a private biotechnology company from 2003 to 2013. Prior to Cylene, Dr. Rice was the founder, President, Chief Executive Officer and Director of Achillion Pharmaceuticals, Inc. from 1998 to 2003. HeDr. Rice also served at the National Cancer Institute-Frederick National Laboratory for Cancer Research (FNLCR) as Senior Scientist and Head of the Drug Mechanism Laboratory at the National Cancer Institute-Frederick Cancer Research and Development Center from 1992 to 1998, andprior to which he served as a faculty member in the division of Pediatric Hematology and Oncology at the Emory University School of Medicine from 1989 to 1992. Dr. Rice performed his post-doctoral fellowship in the Department of Medicine, Division of Hematology and Oncology at the University of Michigan Medical Center from 1986 to 1989, prior to which he received his Ph.D. from the Emory University Department of Biochemistry.Biochemistry in 1986. Dr. Rice continues to serve as the Chairman of the Board of Directors of Cylene and was previously a member of the Board of Directors of Oncolytics Biotech Inc. (2015 to 2021)*. Dr. Rice makes valuable contributions to the Board of Directors based on his Ph.D. in Biochemistry, his extensive involvement in preclinical and clinical studies, his proven record of financings and licensing deals, and his more than 25 years of experience in the biotechnology industry as a senior executive and as a corporate director.
Fletcher Payne,, age 61, joined Aptose as Senior Vice President, and Chief Financial Officer (“CFO”) and Corporate Secretary in June 2022. Mr. Payne was appointed Chief Business Officer in October 2023. With a healthcare tenure of more than 25 years, Mr. Payne has held several CFO and senior management positions at biotech companies in addition to finance and accounting roles, and has overseen legal, corporate development and licensing functions. During his career, he has executed a wide array of business transactions totaling more than $3.7 billion, with healthcare focus in clinical testing, oncology, neurological, and orphan diseases indications. Mr. Payne most recently served as CFO of Syapse, where he completed several financing transactions and oversaw accounting, finance, corporate development, and legal functions. Prior, he served as CFO at Catalyst Bioscience, a publicly traded biotech company. He served in a CFO capacity and senior financial positions at CytomX Therapeutics, Plexxikon Inc., Rinat
2
Neuroscience Corporation, Dynavax Technologies Corporation, and Cell Genesys, among others. Mr. Payne holds a B.S. in Finance from the Haas School of Business, University of California, Berkeley.
Dr. Rafael Bejar, M.D, Ph.D.Ph.D., age 52,53, joined Aptose as Senior Vice President and Chief Medical Officer in January 2020. Dr. Bejar is an internationally recognized physician scientist with extensive research and clinical experience in the area of hematologic malignancies. Dr. Bejar joined Aptose from UC San Diego (“UCSD”) where he began working in 2012. He continues to serve at UCSD as an Associate Professor of Clinical Medicine, caring for patients and maintaining a research laboratory focused on translational studies of myeloid malignancies and also serves and is an independent consultant as a member of the Independent Data Monitoring Committee for other pharmaceutical companies. At UCSD, he founded the MDS Center of Excellence and led the Hematology Disease Team from 2017 to 2019. There he has directed several clinical studies and served as an advisor for numerous companies including Celgene, Takeda, AbbVie, Astex, Genoptix, Forty Seven, PersImmune, and Daiichi-Sankyo. Outside UCSD, Dr. Bejar sits on the Scientific Advisory Board for the MDS Foundation, is a prior member of the National Comprehensive Cancer Network Guidelines Committee, and has led projects for the International Working Group for MDS. He is frequently invited to speak at national and international meetings and has published articles in a variety of journals including The New England Journal of Medicine, Journal of Clinical Oncology, Leukemia, Blood, and Blood Advances. Dr. Bejar completed his fellowship at the Dana-Farber Cancer Institute and has been board certified in Hematology and Oncology. He completed his internship in Internal Medicine at the University of Chicago followed by his residency at the Brigham and Women’s Hospital in Boston where he later served a Medical Chief Resident and an Instructor in Hematology. He holds an M.D.MD degree and a Neuroscience Ph.D.PhD from UCSD and a B.S.BS in Physics from MIT.
Corporate Information
AptoseNon-Employee Directors
Carol G. Ashe, age 66, has been the Chief Business Officer at the New York Genome Center, an independent, non-profit academic research institution focused on genomic science, and its application to novel biomedical discoveries to advance the understanding of the genetic basis of neurodegenerative disease, neuropsychiatric disease, and cancer, since 2014. Previously, she served as Vice President of Corporate Development for Endo’s branded, generic and platform drug delivery pharmaceutical business units from 2011 to 2013; a Partner at SR One, the corporate venture capital fund of GlaxoSmithKline (“GSK”), from 2008 to 2010; and head of GSK’s US Corporate Legal Group supporting US-based mergers, acquisitions, and equity investments from 2007 to 2008. Prior to that, Ms. Ashe led GSK’s Global Business Development Transactions Legal Team supporting both the pharmaceutical and consumer healthcare business units from 1995 to 2007. In 2020, Ms. Ashe joined the Board of Elicio Therapeutics*, a clinical-stage biotechnology company developing a pipeline of novel immunotherapies, as an independent director and she is a publicly tradedmember of the Audit Committee, Nominating and Corporate Governance Committee and Chair of the Compensation Committee. Ms. Ashe received her BS degree in Biology from Pennsylvania State University, her law degree from Villanova University School of Law and is a registered patent attorney. Ms. Ashe makes valuable contributions to the Board based on over 25 years of experience in the pharmaceutical and biotechnology industry in business development and as legal counsel for business development transactions and patent matters.
Denis Burger, Ph.D., age 80, currently is the managing member of Paradigm Ventures LLC, a healthcare consulting and funding firm based in Portland, Oregon, and has been since 1986. Previously, he co-founded Trinity Biotech, PLC, a diagnostic biotechnology company governed bybased in Dublin, Ireland, where he was Chairman from 1992 to 1995 and served on its board of directors until 2020 and chaired its Audit Committee from 1996 to 2016. Dr. Burger served as the Canada Business Corporations Act ("CBCA")Chairman, Chief Executive Officer and a Director of AVI Biopharma Inc., an Oregon‑based biotechnology company, from 1996 to 2007. He was a co‑founder and Chairman of Epitope Inc. from 1981 to 1990. Dr. Burger was Vice Chairman and Chief Scientific Officer of CytoDyn Inc. from 2014 to 2018. Dr. Burger has served as President of Yamhill Valley Vineyards since 1983. In addition, Dr. Burger previously held a professorship in the Department of Microbiology and Immunology and Surgery (Surgical Oncology) at the Oregon Health Sciences University in Portland. Dr. Burger received his M.Sc. and Ph.D. in Microbiology and Immunology from the University of Arizona. Dr. Burger served on the board of directors of Epitope Inc (1986-1990)*, Trinity Biotech, PLC. (1992 to 2020)*, CytoDyn Inc. (2014 to 2018)* and AVI BioPharma Inc (1996-2007)*. Our headquarters are located at 251 Consumers Road, Suite 1105 Toronto, Ontario, Canada M2J 4R3 (telephone: 647-479-9828),Dr. Burger has served on the Board of Aptose since 2007 and ourwas Chair of the Audit Committee of Aptose from 2008 to 2015. Dr. Burger makes valuable contributions to the Board based on his Ph.D. in microbiology and immunology, and his more than 25 years of experience in the biotechnology industry as a senior executive offices are located at 12770 High Bluff Drive, Suite 120, San Diego, CA 92130 (telephone: 858-926-2730).and as a corporate director.
143
We file annual, quarterly, current reports, proxy statementsErich Platzer, M.D., Ph.D., age 72, served as a board-certified physician in internal medicine, hematology and medical oncology between 1979 and 1991. In 2001, Dr. Platzer co-founded HBM Healthcare Investments (formerly HBM BioVentures), a global leader in healthcare investing and served as their investment advisor until 2015. Previously, he served as the business director of oncology, as well as the global strategic marketing and therapeutic area head of oncology at Roche, Basel. He also served in various other information withleadership roles at Roche and was responsible for various strategic corporate partnerships. He has over 12 years of experience in academic medicine and research and was a key member of the SEC. The SEC maintains an Internet siteteam at MSKCC that contains our public filingspurified human G‑CSF in 1983 (recombinant form: Neupogen®). He earned his M.D. from the Medical School of the University of Erlangen, where he also received his “Dr. med. habil.” (M.D., Ph.D.). Dr. Platzer has served as a pharmaceutical industry expert on the board of directors of multiple biotech companies in both the U.S. and other information regardingEurope. Currently he serves as chairman of Vivoryon Therapeutics NV, as well as a director of Panavance Therapeutics Inc. and of MedTech Innovation Partners, MTIP, a Swiss VC firm focusing on MedTech and eHealth. He has also served as the Company, at www.sec.gov. We make these reports available freepresident of charge at our website http://www.aptose.com (underSwiss business angel group StartAngelsNetwork and remains a board member there. Dr. Platzer makes valuable contributions to the “Investors — Financial Information” caption).Board based on over twenty-five years’ experience in the biotechnology industry as a physician in hematology and medical oncology, as a corporate executive, and as a corporate director.
We are also a reporting issuer under the securities laws of every province of Canada.
Cautionary Note Regarding Forward-Looking StatementsBernd R. Seizinger, M.D., Ph.D. age 67, is an accomplished senior executive leader with more than 25 years of industry experience in both U.S. and Risk Factor SummaryEuropean biotechnology and pharmaceutical companies and multiple financial advisory positions. His current positions include: Chairman of the board of directors, Oxford BioTherapeutics (U.K. private company, since 2016); Co-founder, executive chairman of the board and acting CEO, CryptoMedix (U.S. private company, since 2015) and he is currently a member of the board of directors of the following publicly traded biotech companies: Aprea Therapeutics Inc. (U.S.; NASDAQ; since 2014)*; Oncolytics Biotech Inc. (Canada/U.S.; NASDAQ and TSX; since 2015)*; BioInvent International AB (Sweden; NASDAQ Stockholm; since 2018); Nykode Therapeutics ASA (Norway; Oslo Stock Exchange; since 2014). In addition, he is currently serving on the advisory board of biotech venture capital fund Pureos (Switzerland; since 2019) and is senior advisor to biotech venture fund Hadean (Sweden & Norway; since 2018). He previously served as VP for oncology drug discovery and VP for corporate and academic alliances at Bristol-Myers Squibb (U.S.). Subsequently, he served as executive vice president and CSO of U.S. biotech company Genome Therapeutics, followed by 12 years as CEO and President of German/U.S. biopharmaceutical company GPC Biotech (listed on Frankfurt Stock Exchange and NASDAQ). Prior to his corporate appointments, Dr. Seizinger held senior faculty positions at Harvard Medical School and Massachusetts General Hospital and was a Visiting Professor at Princeton University during his tenure at Bristol-Myers Squibb. Dr. Seizinger received his M.D. from Ludwig-Maximilians-Universität Munich, and his Ph.D. from Max-Planck-Institute of Psychiatry/Neurobiology in Munich.
This Annual Report
Mark. D. Vincent, M.D. age 71, has been a Professor of Oncology at the University of Western Ontario since 2008 and a staff medical oncologist at the London Regional Cancer Program since 1990. Dr. Vincent has also served as the co‑founder and Chief Executive Officer of Sarissa, Inc., a private company actively involved in the development of compounds which potentiate existing, approved targeted drugs including agents approved in leukemia, since 2000. Dr. Vincent holds multiple patents on Form 10-K contains forward-looking statements within the meaningpotentiation of cancer chemotherapy by the United States Private Securities Litigation Reform Actmanipulation of 1995drug resistance genes, sits on the advisory boards and “forward-looking information” withinspeakers panels of several major pharmaceutical companies, and is a frequent international lecturer on the meaningpositioning of applicable Canadian securities law. We refernew drugs in the complex evolving management of lung and gastro-intestinal cancer. Dr. Vincent completed his oncology training at the Royal Marsden Hospital in London, England, with a major focus on leukemia/lymphoma. Dr. Vincent makes valuable contributions to such forward-looking statements and forward-looking information collectively as “forward-looking statements”. These statements relate to future events or future performance and reflect our expectations and assumptions regarding our growth, results of operations, performance and business prospects and opportunities. Such forward-looking statements reflect our current beliefs and arethe Board based on information currently available to us. In some cases, forward-looking statements can be identified by terminology such as “may”, “would”, “could”, “will”, “should”, “expect”, “plan”, “intend”, “anticipate”, “believe”, “estimate”, “predict”, “potential”, “continue” or the negativeover 25 years of these terms or other similar expressions concerning matters that are not historical facts. The forward-looking statements in this Annual Report on Form 10-K include, among others, statements regarding our future operating results, economic performance and product development efforts and statements in respect of:
The forward-looking statements contained in this Annual Report on Form 10-K reflect our current views with respect to future events, are subject to significant risks and uncertainties, and are based upon a number of estimates and assumptions that, while considered reasonable by us, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies. Forward-looking statements contained in this Annual Report on Form 10-K are made as of the date of this Annual Report on Form 10-K.medical oncologist.
Except as required under applicable securities legislation, we undertake no obligation to publicly update or revise forward-looking statements, whether as a result of new information, future events or otherwise.
154
Risk Factor SummaryWarren Whitehead, age 71, serves as the Head of Corporate Strategy, previously Chief Financial Officer of Satellos Bioscience Inc. (“Satellos”), a TSX-listed regenerative medicine company aimed at developing therapeutics for degenerative muscle diseases, since August 2021. He previously served as the Chief Financial Officer of ProMIS Neurosciences Inc. (formerly Amorfix Life Sciences Ltd.), a TSX‑listed company targeting detection and effective treatment of Alzheimer’s disease and amyotrophic lateral sclerosis, from 2013 to 2015, after which he concentrated on his role on corporate boards until he joined Satellos in 2021. From 2006 to 2008, he was the Chief Financial Officer of Arius Research Inc., a TSX‑listed company developing anti‑cancer antibodies, where he provided financial guidance and leadership during the acquisition of Arius by Roche in 2008. He was also the former Chief Financial Officer of Labopharm Inc. from 2000 to 2006, where he completed a series of public equity financings, including a cross‑border Nasdaq offering. Other positions include Chief Financial Officer of Resolution Pharmaceuticals Inc., and a position in finance and business development at Glaxo Canada (now GlaxoSmithKline). Mr. Whitehead holds an MBA and BComm from the University of Windsor and a BA from the University of Western Ontario. Mr. Whitehead was the former Chairman and board member of Plantform Corporation until 2019 and a former Board Member of Telesta Therapeutics (TSX), which was acquired by Prometic Life Sciences in 2016.
Many factors could causeMr. Whitehead makes valuable contributions to the Board based on his financial expertise as a Chartered Professional Accountant (CPA) who has held chief financial officer roles at publicly traded pharmaceutical and biotechnology firms.
Family Relationships
There are no family relationships among our actual results, performance or achievementsdirectors and executive officers.
CORPORATE GOVERNANCE
Corporate governance relates to be materially different from any future results, performance, or achievementsthe activities of the Board, the members of which are elected by and are accountable to the Shareholders, and takes into account the role of the individual members of management who are appointed by the Board and who are charged with the day-to-day management of Aptose. The Board believes that may be expressed or implied by such forward-looking statements, including, among others:sound corporate governance practices are essential to contributing to the effective and efficient decision-making of management and the Board and to the enhancement of Shareholder value. The Board and management believe that Aptose has a sound governance structure in place for both management and the Board. Of particular note, Aptose has:
16
Should one or more of these risks or uncertainties materialize, or should the assumptions described in the Item 1A. Risk Factors in this Annual Report on Form 10-K underlying those forward-looking statements prove incorrect, actual results may vary materially from those described in the forward-looking statements.
Although we have attempted to identify factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements, there may be other factors that cause actions, events or results not anticipated, estimated or intended. Forward-looking statements are based upon our beliefs, estimatesEach of the committee charters and opinions at the time they are made and we undertake no obligation to update forward-looking statements if these beliefs, estimates and opinions or circumstances should change, except as required by applicable law. ThereCode of Ethics can be no assurance that forward-looking statements will prove to be accurate, as actual results and future events could differ materially from those anticipated in such statements. Accordingly, readers should not place undue reliancefound on forward-looking statements.the Corporation’s website at https://ir.aptose.com/corporate-governance.
We qualify all
Board Mandate
The Board has adopted a mandate in which it explicitly assumes responsibility for stewardship of the forward-looking statements containedCorporation. The Board is mandated to represent the Shareholders to ensure appropriate succession planning is in this Annual Report on Form 10-K byplace, select the foregoing cautionary statements.appropriate chief executive officer, assess and approve the strategic direction of the Corporation, ensure that appropriate processes for risk assessment, management and internal control
175
ITEM 1A. RISK FACTORSare in place, monitor management performance against agreed benchmarks, and assure the integrity of financial reports. A copy of the Board Mandate is attached hereto as APPENDIX A.
Risk Factors and Uncertainties
AnyComposition and Independence of the risks and uncertainties described below could significantly and negatively affect our business, prospects, financial condition, operating results, or credit ratings, which could cause the trading price of our Common Shares to decline. Additional risks and uncertainties not presently known to us, or risks that we currently consider immaterial, could also impair our business operations or financial condition. The following discussion of risk factors contains “forward-looking” statements, as discussed above.Board
Risks Related to our Business
ThereThe Corporation’s Board is substantial doubt that we can remaincurrently composed of sevendirectors, a going concern overmajority (six) of whom meet the next twelve months.
Asindependence standards under the listing standards of Nasdaq, the rules and regulations of the filing date, we have sufficient liquidity to supportSEC, and National Instrument 52-110 – Audit Committees(“NI 52-110”). Each year the Company's operations until August 2024. In order forBoard reviews the Company to meet its capital requirements,composition of the Board and continue to operate, additional financing will be necessary. The Companyassesses whether a Board member is evaluating strategies to obtain the required additional funding for future operations. These strategies may include, but are not limited to, obtaining equity financing, and restructuring of operations to decrease expenses. However, given the challenges in the U.S. and global financial markets, and the matter in Note 17 to the Financial Statements, Subsequent events, that may impact the Company's ability to raise financing in the capital markets, the Company may be unable to access further equity or when needed, if at all. As the Company is primarily pursuing one compound that is licensed from a related party with significant licensing payments who will have influence on the Company, other investors may not be willing to invest in the Company. As such, there can be no assurance that the Company will be able to obtain additional liquidity when needed or under acceptable terms, if at all. The consolidated financial statements do not reflect any adjustments to the carrying amounts and classification of assets, liabilities, and reported expenses that may be necessary if the Company were unable to continue as a going concern. Such adjustments may be material. See Note 2(a) to the Financial Statements, Going concern.
We are an early-stage development company with no revenues from product sales.
We are at an early stage of development. None of our potential products has obtained regulatory approval for commercial use and sale in any country and as such, no revenues have resulted from product sales. Significant additional investment will be necessary to complete the development of any of our product candidates. Preclinical and clinical trial work must be completed before our potential products could be ready for use within the markets that we have identified. We may fail to develop any products, obtain regulatory approvals, enter or complete clinical trials or commercialize any products. We do not know whether any of our potential product development efforts will prove to be effective, meet applicable regulatory standards, obtain the requisite regulatory approvals, be capable of being manufactured at a reasonable cost or be accepted in the marketplace.“independent”.
Director | Independence |
Carol Ashe Denis Burger | Yes Yes |
Erich Platzer | Yes |
William G. Rice | No |
Bernd R. Seizinger | Yes |
Mark Vincent | Yes |
Warren Whitehead | Yes |
Dr. William G. Rice, Ph.D., Chairman, President and Chief Executive Officer of the Corporation is not an independent director because of his role in the Corporation’s management team.
Directors Skills and Experience Matrix
The product candidates we arefollowing table provides summary information about the skills of the directors of the Corporation.
Carol Ashe | Denis Burger | Erich Platzer | William G. Rice | Bernd R. Seizinger | Mark Vincent | Warren Whitehead | |
Governance/Public Company Board Experience | ü | ü | ü | ü | ü | ü | ü |
Senior Leadership Experience | ü | ü | ü | ü | ü | ü | ü |
Financial and Accounting | ü | ü | ü | ü | ü | ü | |
Industry Experience | ü | ü | ü | ü | ü | ü | ü |
Global Business Experience | ü | ü | ü | ü | ü | ||
Business Development and M&A Experience | ü | ü | ü | ||||
Legal | ü | ||||||
Medicine & Science | ü | ü | ü | ü | ü |
Involvement of Directors with other Reporting Issuers
The following table outlines other reporting issuers where our directors serve on the Board:
Director | Reporting Issuer |
Carol G. Ashe | Elicio Therapeutics, Inc. |
Erich Platzer | Vivoryon Therapeutics NV |
6
Director | Reporting Issuer |
Bernd R. Seizinger | Aprea Therapeutics, Inc. Oncolytics Biotech Inc. Nykode Therapeutics ASA BioInvent International AB |
Board Leadership
The Corporation has a chair (the “Chair”) who is currently developing are not expectedalso the President and Chief Executive Officer of the Corporation, Dr. William G. Rice. As the Chair is an executive officer of the Corporation, the Corporation also has a Lead Director to be commercially viable for at leastensure that the next several yearsdirectors have an independent leadership contact and we may encounter unforeseen difficulties or delaysmaintain and enhance the quality of the Corporation’s corporate governance practices. Dr. Denis Burger, an independent director, is currently the Lead Director.
The Chair and the Lead Director together provide leadership to the Board in commercializing our product candidates.discharging its mandate and also assist the Board in discharging its stewardship functions, which include(i) satisfying themselves as to the integrity of the senior officers of the Corporation and that the senior officers create a culture of integrity throughout the organization; (ii) strategic planning; (iii) identifying and managing risks; (iv)succession planning; (v) adopting a disclosure policy; (vi) internal control and management information systems; and (vii) the Corporation’s approach to corporate governance. In addition, our potential products may not be effective or may cause undesirable side effects.the Lead Director must satisfy itself as to the integrity of the Chief Executive Officer and that the Chief Executive Officer creates a culture of integrity throughout the organization. The Lead Director also provides advice, counsel and mentorship to the Chief Executive Officer.
Our product candidates require
Board Oversight of Risk
With regard to risk management, the Board ensures that the business of the Corporation is conducted in compliance with applicable laws and regulations and according to the highest ethical standards; will identify and document the financial risks and other risks that the Corporation faces in the course of its business and ensure that such risks are appropriately managed; and will adopt a disclosure policy.
The Board as a whole has responsibility for risk oversight, with more in-depth reviews of certain areas of risk being conducted by the relevant Board committees that report on their deliberations to the full Board. The Board and its committees fulfill their oversight responsibilities with the support of management, whose reporting processes are designed to provide information to the Board about the identification, assessment and management of critical risks and management’s risk mitigation strategies. Areas of risk evaluated include regulatory, operational, financial (accounting, liquidity and tax), legal, cybersecurity compensation, competitive, health, safety and reputational risks.
The Audit Committee, Corporate Governance and Nominating Committee and Compensation Committee oversee risks associated with their respective principal areas of focus. The Audit Committee’s role includes a particular focus on the qualitative aspects of financial reporting to stockholders, on our processes for the management of business and financial risk, our financial reporting obligations and for compliance with significant fundingapplicable legal, ethical and regulatory requirements. The Audit Committee, along with management, is also responsible for developing and participating in a process for review of important financial and operating topics that present potential significant risk to reach regulatory approval assuming positive clinical results. We are currently conducting Phase 1 clinical trialsthe Corporation. The Compensation Committee is responsible for overseeing risks and exposures associated with our product candidates tuspetinibcompensation programs and luxeptinib. Significant additional capital will be necessaryarrangements, including our executive and director compensation programs and arrangements, and management succession planning. The Corporate Governance and Nominating Committee oversees risks relating to complete the Phase 1 clinical trials,our corporate governance matters and if required, Phase 2 or Phase 3 clinical trials. Such funding for our product candidates may be difficult, or impossible to raise in the public or private markets or through partnerships. If funding or partnerships are not readily attainable, the development of our product candidates may be significantly delayed or stopped altogether. The announcement of a delay or discontinuation of development of any of our product candidates could have a negative impact on our share price.policies and director succession planning.
187
We need to raise additional capital.
We haverecognize that a fundamental part of risk management is understanding not only the risks a company faces and what steps management is taking to manage those risks, but also understanding what level of risk is appropriate for that company. Through their involvement in setting our business strategy, the Board can assess management’s appetite for risk and also determine what constitutes an appropriate level of risk for the Corporation.
We believe our current Board leadership structure is appropriate and helps ensure proper risk oversight for the Corporation. The full Board conducts general risk oversight in connection with its role in reviewing our key long-term and short-term business strategies and monitoring on an ongoing needbasis the implementation of our key business strategies, while our standing Board committees conduct more specific risk oversight related to raise additional capital. Astheir responsibilities. The Chair ensures that there is sufficient time on the Board agenda for risk management discussions.
Nomination of Directors
Directors of the filing date, we have sufficient liquidityCorporation are expected to supportbring to the Company's operations until August 2024. To obtainBoard the necessary capital, we must rely on somebroadest possible knowledge and depth of experience from their chosen business or allprofession. Directors should evidence a demonstrated ability to deal with business, financial and social issues, both nationally and internationally. This implies a capacity to provide additional strength, diversity of views and up-to-date perceptions to the Board and its deliberations. It is the mandate of the following: additional share issues, debt issuances (including promissory notes), collaboration agreementsCorporate Governance and Nominating Committee to identify and recommend qualified candidates for the Board. In assessing whether identified candidates are suitable for the Board, the Corporate Governance and Nominating Committee considers: (i) the competencies and skills considered necessary for the Board as a whole; (ii) the competencies and skills that the existing directors possess and the competencies and skills nominees will bring to the Board; and (iii) whether nominees can devote sufficient time and resources to his or corporate partnerships and grants and tax credits to provide full or partial fundingher duties as a member of the Board. Potential candidates for our activities. Additional funding maymembership on the Board will not be available on terms that are acceptable to usdenied consideration by reason of race, sex, religion or in amounts thataffiliation with some special constituency group, nor will enable us to carry out our business plan.any candidate be selected solely for such reason.
Our need for capital
It is the Corporate Governance and Nominating Committee’s policy to consider director candidates recommended by our Shareholders in accordance with the provisions set forth in our Advance Notice By-Law, which may require us to:
In addition, sales of our Common Shares in the public markets, orInvestors section. Candidates recommended by the perception that such sales could occur, could depressCorporation’s Shareholders will be considered by the market price of our Common SharesCorporate Governance and impair our ability to raise capital through the sale of additional equity securities.
Our operations could be adversely affected by events outside of our control, suchNominating Committee and, as natural disasters, wars or health crises.
We may be impacted by business interruptions resulting from pandemics and public health emergencies, including war and terrorism or natural disasters including earthquakes, typhoons, floods and fires. Any such event, or a fear of the foregoing, could adversely impact us by causing operating, manufacturing, supply chain, clinical trial and project development delays and disruptions, labor shortages, travel and shipping disruption or shutdowns. We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results and financial condition.
We have a history of operating losses. We expect to incur net losses and we may never achieve or maintain profitability.
We have not been profitable since our inception in 1986. We reported net losses of $51.2 millionstated in the fiscal year ended December 31, 2023, $41.8 millionCorporate Governance and Nominating Committee Charter, such candidates shall be evaluated in the fiscal year ended December 31, 2022, $65.4 million in the fiscal year ended December 31, 2021, $55.2 million in the fiscal year ended December 31, 2020 , andsame manner as of December 31,all other director candidates. During 2023, we had an accumulated deficitreceived no recommendations of $515.5 million. We had negative shareholder's equity of $2.9 million as of December 31, 2023 (December 31, 2022, positive shareholder's equity of $37.7 million).director candidates from our Shareholders.
We have not generated any significant revenue to date
Diversity
The Corporate Governance and it is possible that we will never have sufficient product sales revenue (if any) to achieve profitability. We expect to continue to incur losses for at least the next several years as we or our collaboratorsNominating Committee takes diversity, including diversity of experience, perspective and licensees pursue clinical trials, research and development efforts. To become profitable, we, either alone or with our collaborators and licensees, must successfully develop, manufacture and market our current product candidates tuspetinib or luxeptinib,education, as well as continue to identify, develop, manufactureindividuals from other designated groups such as women, Aboriginal people, persons with disabilities and market new product candidates. It is possiblemembers of visible minorities (collectively, the “Designated Groups”), into consideration as part of its overall recruitment and selection process in respect of its Board and management. The Corporation does not have a formal policy on the representation of women or other members of the Designated Groups on the Board or management of the Corporation. The Board does not believe that wea formal policy will never have significant product sales revenue or receive royalties on our licensed product candidates. If funding is insufficient at any timenecessarily result in the future, we mayidentification or selection of the best candidates. As such, the Corporation does not be able to develop or commercialize our products, take advantagesee any meaningful value in adopting a formal policy in this respect at this time as it does not believe that it would further enhance diversity, including gender diversity, beyond the current recruitment and selection process carried out by the Corporate Governance and Nominating Committee. However, the Board is mindful of business opportunities or respond to competitive pressures.the benefit of diversity on the Board and management of the
198
We currently do not earn any revenues from our drug candidates and are therefore considered to be in the development stage. The continuation of our research and development activitiesCorporation and the commercializationneed to maximize the effectiveness of the targeted therapeutic products are dependent upon our ability to successfully financeBoard and complete our researchmanagement and development programs throughtheir respective decision-making abilities.
The Corporate Governance and Nominating Committee believes that having a combinationdiverse Board and management team offers a depth of equity financingperspective and payments from strategic partners. We have no current sourcesenhances Board and management operations. The Corporate Governance and Nominating Committee values diversity of significant payments from strategic partners. Asexperience, perspective, education and race, and considers the representation of women and other members of the filing date, we have sufficient liquidity to support the Company's operations until August 2024.Designated Groups, as part of its overall annual evaluation of director nominees for election or re-election as well as candidates for management positions.
We heavily rely
In addition, in searches for new directors or officers, the Corporate Governance and Nominating Committee will consider the level of representation of women and other members of the Designated Groups on the capabilitiesBoard and experiencein management and this will be one of our key executivesseveral factors used in its search process. This will be achieved through continuously monitoring the level of representation of women and scientistsother members of the Designated Groups on the Board and in management positions and, where appropriate, recruiting qualified candidates who are members of the Designated Groups as part of the Corporation’s overall recruitment and selection process to fill Board or management positions, as the need arises, through vacancies, growth or otherwise.
The Board has not adopted targets regarding the representation of women and other members of Designated Groups on the Board and in executive officer positions due to the small size of the Corporation and the lossneed to consider a balance of criteria in each individual appointment. It is important that each appointment to the Board or in executive officer positions be made, and be perceived as being made, on the merits of the individual and the needs of the Corporation at the relevant time. In addition, targets based on specific criteria such as gender or race, could limit the Board’s ability to ensure that the overall composition of the Board or management of the Corporation meets the needs of the Corporation. We are actively seeking additional candidates to join our Board and we strongly prioritize diverse candidates.
Currently, one out of seven (14%) members of the Board and one out of eight (13%) of the officers are women. One officer identifies as being of “Hispanic, Latinx or Spanish origin” and one as being of “Asian” origin, and one director identifies as being part of the LGBTQ+group, otherwise no members of the Board or officers of the Corporation who self-identify as being part of any of them could affect our ability to develop our products.the Designated Groups.
Board Diversity Matrix
The losstable below provides certain highlights of the composition of our executive officers could harm our operations and our ability to achieve strategic objectives. While we have employment agreements with our executive officers, such employment agreements do not guarantee their retention. We also depend on our scientific and clinical collaborators and advisors, allBoard members as of whom have outside commitments that may limit their availability to us. In addition, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled scientific, managerial, medical, clinical and regulatory personnel, particularly as we expand our activities and seek regulatory approvals for clinical trials. We routinely enter into consulting agreements with our scientific and clinical collaborators and advisors, key opinion leaders and academic partnersDecember 31, 2023. Each of the categories listed in the ordinary coursetable below has the meaning as it is used in Nasdaq Rule 5605(f).
Total Number of Directors | 7 | |||
Female | Male | Non-Binary | Did Not Disclose Gender | |
Part I: Gender Identity | ||||
Directors | 1 | 6 | - | - |
Part II: Demographic Background | ||||
African American or Black | - | - | - | - |
Alaskan Native or Native American | - | - | - | - |
Asian | - | - | - | - |
Hispanic or Latinx | - | - | - | - |
Native Hawaiian or Pacific Islander | - | - | - | - |
White | 1 | 6 | - | - |
9
Two or More Races or Ethnicities | - | - | - | - |
LGBTQ+ | 1 | |||
Did Not Disclose Demographic Background | - | |||
Director Term Limits and Other Mechanisms of our business. We also enter into contractual agreements with physicians and institutions who will recruit patients into our clinical trials on our behalf in the ordinary course of our business. Notwithstanding these arrangements, we face significant competitionBoard Renewal
The Board has not adopted term limits for these types of personnel from other companies, research and academic institutions, government entities and other organizations. We cannot predict our success in hiring or retaining the personnel we require for continued growth. The loss of the services of any of our executive officersdirectors or other key personnel could potentially harm our business, operating results or financial condition.
Our employees may engage in misconductmechanisms of board renewal at this time as it believes that the imposition of director term limits or other improper activities, including noncompliance with regulatory standardsmechanisms of board renewal on a board implicitly discounts the value of experience and requirements, which could have a material adverse effect on our business.
We are exposed tocontinuity amongst the Board members and runs the risk of employee fraudexcluding experienced and potentially valuable board members as a result of arbitrary determination. The Board believes that it can best strike a balance between continuity and fresh perspectives without mandated term limits or other misconduct. Misconductmechanisms of board renewal.
Position Descriptions
The Board has developed written position descriptions, which are reviewed annually, for the Chairman and the chairs of each of the committees. The Chief Executive Officer also has a written position description that has been approved by employees could include failuresthe Board and is reviewed annually.
Orientation and Continuing Education
It is the mandate of the Corporate Governance and Nominating Committee to comply with FDA/Health Canada regulations, provide accurate informationensure that a process is established for the orientation and education of new directors that addresses the nature and operation of the Corporation’s business and their responsibilities and duties as directors (including the contribution individual directors are expected to make and the commitment of time and resources that the Corporation expects from its directors).
The orientation includes an overview of the Corporation’s history and operations, a review of industry conditions and competition, an introduction to the FDA/Health Canada, comply with manufacturing standards we have established, comply with federal, stateCorporation’s management team and provincial health-care fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketingcorporate and business arrangementsinformation. Any further orientation is dependent on the needs of the new member and may include items such as formal training sessions and attendance at seminars.
With respect to the continuing education of directors, the Corporate Governance and Nominating Committee ensures that directors receive adequate information and continuing education opportunities on an ongoing basis to enable directors to maintain their skills and abilities as directors and to ensure their knowledge and understanding of the Corporation’s business remains current.
Assessments
It is the Board’s mandate, in conjunction with the Corporate Governance and Nominating Committee, to assess the participation, contributions and effectiveness of the Chair and the individual members of the Board on an annual basis. The Board also monitors the effectiveness of the Board and its committees and the actions of the Board as viewed by the individual directors and senior management.
The Board has developed a formal questionnaire to be completed by each director on an annual basis for the purpose of formally assessing the effectiveness of the Board as a whole, committees of the Board, and the contribution of individual directors. These questionnaires, and the issues arising therefrom, are intended to be reviewed and assessed by the Lead Director on an annual basis or more frequently from time to time as the need arises. The Lead Director takes appropriate action as required based on the results obtained.
Meeting Attendance
As stated in the healthcare industryBoard Mandate, all directors are subjectexpected to extensive lawsattend each meeting in person, by phone or by video conference depending on the format of the meeting, to the extent practicable. The Board of Directors held seven meetings during 2023, the Audit Committee met four times, the Corporate Governance and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in clinical trials, which could result in regulatory sanctions and serious harm to our reputation. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a substantial impact on our business and results of operations, including the imposition of substantial fines or other sanctions.
We have no sales, marketing or distribution experience and would have to invest significant financial and management resources to establish these capabilities.
We have no sales, marketing or distribution experience. We currently expect to rely heavily on third parties to launch and market our products, if they are approved. However, if we elect to develop internal sales, distribution and marketing capabilities, we will need to invest significant financial and management resources. For products where we decide to perform sales, marketing and distribution functions ourselves, we could face a number of additional risks, including:
2010
If we are unable to develop our own sales, marketing and distribution capabilities, we will not be able to successfully commercialize our products without reliance on third parties.
We may expand our business throughNominating Committee met four times, the acquisition of companies or businesses or by entering into collaborations or by in-licensing product candidates, each of which could disrupt our business and harm our financial condition.
We may seek to expand our pipeline and capabilities by acquiring one or more companies or businesses, entering into collaborations or in-licensing one or more product candidates. For example, in June 2016, we entered into a definitive agreement with CG, granting Aptose an exclusive option to research, develop and commercialize CG-806 in all countries of the world except the Republic of Korea, for all fields of use, and in November 2021 we entered into the Tuspetinib Licensing Agreement with Hanmi granting Aptose exclusive worldwide rights to develop and commercialize tuspetinib.
Acquisitions, collaborations and in-licenses involve numerous risks, including, but not limited to:
We have experience in entering collaborations and in-licensing product candidates; however, we cannot provide assurance that any acquisition, collaboration or in-license will result in any benefit to us. We may incorrectly judge the value or worth of an acquired company or business or in-licensed product candidate. In addition, our future success could depend in part on our ability to manage the rapid growth associated with some of these acquisitions, collaborations and in-licenses. We cannot assure you that we would be able to successfully combine our business with that of acquired businesses, manage a collaboration or integrate in-licensed product candidates. Furthermore, the development or expansion of our business may require a substantial capital investment by us.
Fluctuations in exchange rates can cause us to incur losses.
We may be exposed to fluctuations of the U.S. dollar against certain other currencies because we hold most of our cash and cash equivalents in U.S. dollars, while we incur some of our expenses in foreign currencies, primarily the Canadian dollar. Fluctuations in the value of currencies could cause us to incur currency exchange losses, and we do not currently employ a hedging strategy against exchange rate risk. As a result, changes in the exchange rate between the Canadian dollarCompensation Committee met four times and the U.S. dollar could materially impact our reported results of operationsResearch and distort period to period comparisons. In particular, to the extent that foreign currency-denominated (i.e., non-U.S. dollar) monetary assets do not equal the amount of our foreign currency denominated monetary liabilities, foreign currency gains or losses could arise and materially impact our financial statements. As a result of such foreign currency fluctuations, it could be more difficult to detect underlying trends in our business and results of operations. In addition, to the extent that fluctuations in currency exchange rates cause our results of operations to differ from our expectations or the expectations of our investors, the trading price of our Common Shares could be adversely affected.
21
Risks Related to Development Clinical Testing and Regulatory Approval of Our Product Candidates
Fast Track Designation by the FDA may not lead to a faster development or regulatory review or approval process.
We have obtained Fast Track Designation for tuspetinib for the treatment of patients with R/R AML and FLT3 mutation. We may seek Fast Track Designation for one or more of our other product candidates. If a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the product sponsor may apply for FDA Fast Track Designation. The FDA has broad discretion whether or not to grant this designation, so even if we believe a particular product candidate is eligible for this designation, we cannot assure you that the FDA would decide to grant it. Even if we do receive Fast Track Designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw Fast Track Designation if it believes that the designation is no longer supported by data from our clinical development program.
Clinical trials are long, expensive and uncertain processes and the FDA or Health Canada may ultimately not approve any of our product candidates. We may never develop any commercial drugs or other products that generate revenues.
None of our product candidates has received regulatory approval for commercial use and sale in North America. We cannot market a pharmaceutical product in any jurisdiction until it has completed thorough preclinical testing and clinical trials in addition to that jurisdiction’s extensive regulatory approval process. Approval in one country does not assure approval in another country. In general, significant research and development and clinical studies are required to demonstrate the safety and efficacy of our product candidates before we can submit any applications for regulatory approval.
Clinical trials are long, expensive and uncertain processes. Clinical trials may not start or be on schedule and the FDA, Health Canada or any other regulatory body may not ultimately approve our product candidates for commercial sale in the relevant territory. The clinical trials of any of our drug candidates could be unsuccessful, which would prevent us from advancing, commercializing or partnering the drug.
Even if the results of our preclinical studies or clinical trials are initially positive, it is possible that we will obtain different results in the later stages of drug development or that results seen in clinical trials will not continue with longer term treatment. Positive results in Phase 1 clinical trials may not necessarily repeat in larger Phase 2 or Phase 3 clinical trials.
Our preclinical studies and clinical trials may generate negative results that will not allow us to move towards the commercial use and sale of our product candidates. Furthermore, negative preclinical or clinical trial results may cause our business, financial condition, or results of operations to be materially adversely affected. Our tuspetinib and luxeptinib product candidates are currently being evaluated in Phase 1 studies, and are expected to undergo many years of testing and regulatory examinations prior to any potential regulatory approvals.Committee (the “R&D Committee”) met once.
Preparing, submitting and advancing applications for regulatory approval of products is complex, expensive and time intensive and entails significant uncertainty. A commitment of substantial resources to conduct time-consuming research, preclinical studies and clinical trials is required if we are to complete development of our products.
Clinical trials of our products require that we identify and enroll a large number of patients with the illness under investigation. We may not be able to enroll a sufficient number of appropriate patients to complete our clinical trials in a timely manner, particularly in smaller indications and indications where there is significant competition for patients. If we experience difficulty in enrolling a sufficient number of patients to conduct our clinical trials, we may need to delay or terminate ongoing clinical trials and will not accomplish objectives material to our success. Delays in planned patient enrollment or lower than anticipated event rates in our current clinical trials or future clinical trials also may result in increased costs, program delays, or both.
22
In addition, unacceptable toxicities or adverse side effects may occur at any time in the course of preclinical studies or human clinical trials or, if any product candidates are successfully developed and approved for marketing, during commercial use of any approved products. The appearance of any unacceptable toxicities or adverse side effects could interrupt, limit, delay or abort the development of any of our product candidates or, if previously approved, necessitate their withdrawal from the market. Furthermore, disease resistance or other unforeseen factors may limit the effectiveness of our potential products.
Our failure to develop safe and commercially viable drugs would substantially impair our ability to generate revenues and sustain our operations and would materially harm our business and adversely affect our share price.
We may choose to expend our limited resources on programs that do not yield successful product candidates as opposed to indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited resources and access to capital to fund our operations, our management must make strategic decisions as to which product candidates and indications to pursue and how much of our resources to allocate to each. Our management must also evaluate the benefits of developing in‑licensed or jointly owned technologies, which in some circumstances we may be contractually obligated to pursue, relative to developing other product candidates, indications or programs. Our management has broad discretion to suspend, scale down, or discontinue any or all of these development efforts, or to initiate new programs to treat other diseases. If we select and commit resources to opportunities that we are unable to successfully develop, or we forego more promising opportunities, our business, financial condition and results of operations will be adversely affected.
We may not achieve our projected development goals in the time frames we announce and expect.
We set goals for, and make public statements regarding, the expected timing of the accomplishment of objectives material to our success, such as the commencement and completion of clinical trials, the submission of a drug-regulatory application, and the expected costs to develop our product candidates. The actual timing and costs of these events can vary dramatically due to factors within and beyond our control, such as delays or failures in our IND submissions or clinical trials, issues related to the manufacturing of drug supply, uncertainties inherent in the regulatory approval process, market conditions and interest by partners in our product candidates, among other things. Our clinical trials may not be completed, we may not make regulatory submissions or receive regulatory approvals as planned; or we may not secure partnerships for any of our product candidates. Any failure to achieve one or more of these milestones as planned would have a material adverse effect on our business, financial condition and results of operations.
Delays in clinical testing could result in delays in commercializing our product candidates and our business may be substantially harmed.
We cannot predict whether any clinical trials will begin as planned, will need to be restructured or will be completed on schedule, if at all. Our product development costs will increase if we experience delays in clinical testing. Significant clinical trial delays could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before us, which would impair our ability to successfully commercialize our product candidates and may harm our financial condition, results of operations and prospects. The completion of clinical trials for our products, including the tuspetinib and luxeptinib clinical trials may be delayed for a number of reasons, including delays related, but not limited, to:
23
Our product development costs will increase if we experience delays in testing or approval or if we need to perform more or larger clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur, and we may need to amend study protocols to reflect these changes. Amendments may require us to resubmit our study protocols to regulatory authorities or IRBs or ethics committees or boards for re-examination, which may impact the cost, timing or successful completion of a trial. Delays or increased product development costs may have a material adverse effect on our business, financial condition and prospects.
We rely on contract manufacturers over whom we have limited control. If we are subject to quality, cost or delivery issues with the preclinical and clinical grade materials supplied by contract manufacturers, our business operations could suffer significant harm.
We rely on contract manufacturing organizations ("CMOs") to manufacture our product candidates for some preclinical studies and clinical trials. We rely on CMOs for manufacturing, filling, packaging, storing and shipping of drug product in compliance with cGMP regulations applicable to our products. The FDA and other regulatory agencies ensure the quality of drug products by carefully monitoring drug manufacturers’ compliance with cGMP regulations. The cGMP regulations for drugs contain minimum requirements for the methods, facilities and controls used in manufacturing, processing and packing of a drug product.
We contracted with multiple CMOs for the manufacture of tuspetinib and luxeptinib to supply the active ingredient and then drug product for our clinical trials. The synthesis of luxeptinib is challenging from a scale-up synthetic chemistry perspective. We pre-qualified CMOs to have the capacity, the systems and the experience to supply tuspetinib and luxeptinib for our clinical trials. We have qualified the manufacturing facilities and the FDA has also performed site audits for our selected CMOs. Despite the efforts to prequalify CMOs, delays and errors may occur, and any such manufacturing failures, delays or compliance issues could cause delays in the completion of our clinical trial programs.
24
There can be no assurances that CMOs will be able to meet our timetable and requirements. We have contracted with alternate suppliers in the event our current CMOs are unable to scale up production, or if our current CMOs otherwise experience any other significant problems in the manufacture of tuspetinib and luxeptinib. However, it is possible that all third-party manufacturing sources may experience failure or delays and may demand commercially unreasonable terms, which may lead to further delays in the development of our product candidates. Further, contract manufacturers must operate in compliance with cGMP and failure to do so could result in, among other things, the disruption of product supplies. Our dependence upon third parties for the manufacture of our products may adversely affect our profit margins and our ability to develop and deliver products on a timely and competitive basis.
Some components of our products are manufactured by third parties outside of the United States, and our business may be harmed by legal, regulatory, economic, political and public health risks associated with international trade and those markets.
We have third-party manufacturing partners in South Korea, Germany and the United Kingdom; in addition, some materials used by our third-party manufacturers are supplied by companies located in other countries, including China. Our reliance on suppliers and manufacturers in foreign markets creates risks inherent in doing business in foreign jurisdictions, including: (a) the burdens of complying with a variety of foreign laws and regulations, including laws relating to the importation and taxation of goods (b) public health crises, such as pandemics and epidemics, in the countries where our suppliers and manufacturers are located; (c) transportation interruptions or increases in transportation costs; and (d) foreign intellectual property infringement risks.
If we have difficulty enrolling patients in clinical trials, the completion of the trials may be delayed or canceled.
As our product candidates advance from preclinical testing to clinical testing, and then through progressively larger and more complex clinical trials, we will need to enroll an increasing number of patients that meet our eligibility criteria. There is significant competition for recruiting cancer patients in clinical trials, and we may be unable to enroll the patients we need to complete clinical trials for cancer indications on a timely basis or at all. Certain factors that affect enrollment of patients in our clinical trials are impacted by external forces that may be beyond our control. Such factors include, but are not limited to, the following:
If we are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience significant delays in doing so, we may not achieve marketing approval or realize the full commercial potential of our therapeutic product candidates.
We plan to develop companion diagnostics for our therapeutic product candidates. We expect that, at least in some cases, regulatory authorities may require the development and regulatory approval of a companion diagnostic as a condition to approving our therapeutic product candidates. We have limited experience and capabilities in developing or commercializing diagnostics and plan to rely in large part on third parties to perform these functions. We do not currently have any agreement in place with any third party to develop or commercialize companion diagnostics for any of our therapeutic product candidates.
25
Companion diagnostics are subject to regulation by the FDA, Health Canada and comparable foreign regulatory authorities as medical devices and may require separate regulatory approval or clearance prior to commercialization. If we, or any third parties that we engage to assist us, are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience delays in doing so, our business may be substantially harmed.
We rely and will continue to rely on third parties to conduct and monitor many of our preclinical studies and our clinical trials, and their failure to perform as required could cause substantial harm to our business.
We rely and will continue to rely on third parties to conduct a significant portion of our preclinical and clinical development activities. Preclinical activities include in vivo studies providing access to specific disease models, pharmacology and toxicology studies, and assay development. Clinical development activities include trial design, regulatory submissions, clinical patient recruitment, clinical trial monitoring, clinical data management and analysis, safety monitoring and project management, contract manufacturing and quality assurance. If there is any dispute or disruption in our relationship with third parties, or if they are unable to provide quality services in a timely manner and at a feasible cost, our active development programs will face delays. Further, if any of these third parties fails to perform as we expect or if their work fails to meet regulatory requirements, our testing could be delayed, canceled or rendered ineffective.
Negative results from clinical trials or studies of others and adverse safety events involving the targets of our products may have an adverse impact on our future commercialization efforts.
From time to time, studies or clinical trials on various aspects of biopharmaceutical products are conducted by academic researchers, competitors or others. The results of these studies or trials, when published, may have a significant effect on the market for the biopharmaceutical product that is the subject of the study. The publication of negative results of studies or clinical trials or adverse safety events related to our product candidates, or the therapeutic areas in which our product candidates compete, could adversely affect our share price and our ability to finance future development of our product candidates, and our business and financial results could be materially and adversely affected.
The design or our executionfollowing table illustrates the attendance record of clinical trials may not support regulatory approval.
The design or execution of a clinical trial can determine whether its results will support regulatory approvaleach director for all Board and flaws in the design or execution of a clinical trial may not become apparent until the clinical trial is well advanced. In some instances, there can be significant variability in safety or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. We do not know whether any Phase 2, Phase 3 or other clinical trials that we may conduct will demonstrate consistent or adequate efficacy and safety to obtain regulatory approval to market our product candidates.
Further, the FDA, Health Canada and comparable foreign regulatory authorities will have some discretion in the approval process and in determining when or whether regulatory approval will be obtained for any of our product candidates. Our product candidates may not be approved even if they achieve their primary endpoints in future Phase 3 clinical trials or registration trials. The FDA, Health Canada or other regulatory authorities may disagree with our trial design and our interpretation of data from preclinical studies and clinical trials. In addition, any of these regulatory authorities may change requirementscommittee meetings held for the approval of a product candidate even after reviewing and providing comments or advice on a protocol for a pivotal Phase 3 clinical trial that has the potential to result in approval by the FDA, Health Canada or another regulatory agency. In addition, any of these regulatory authorities may also approve a product candidate for fewer or more limited indications than we request or may grant approval contingent on the performance of costly post-marketing clinical trials. The FDA, Health Canada or other regulatory authorities may not approve the labeling claims that we believe would be necessary or desirable for the successful commercialization of our product candidates.
26
As a result of intense competition and technological change in the biotechnical and pharmaceutical industries, the marketplace may not accept our products or product candidates, and we may not be able to compete successfully against other companies in our industry and achieve profitability.
Many of our competitors have:
Consequently, our competitors may obtain FDA, Health Canada and other regulatory approvals for product candidates sooner and may be more successful in manufacturing and marketing their products than we or our collaborators are.
Our competitors’ existing and future products, therapies and technological approaches will compete directly with the products we seek to develop. Current and prospective competing products may be more effective than our existing and future products insofar as they may provide greater therapeutic benefits for a specific problem or may offer easier delivery or comparable performance at a lower cost.
For tuspetinib and luxeptinib in AML, examples of companies that have developed or are pursuing different therapies include Jazz (VYXEOS), Pfizer (MYLOTARG), Novartis (RYDAPT), Astellas (XOSPATA), AbbVie (VENCLEXTA), Daiichi Sankyo (quizartinib), Arog (crenolanib), Agios/Servier (TIBSOVO), Rigel (REZLIDHA), Celgene/BMS (IDHIFA), Kronos Bio (lanraplenib), Curis (emavusertib), Syndax (revumenib, SNDX-5613), and Kura (KO-539), among others.
For luxeptinib in B cell malignancies, examples of companies that have developed or are pursuing different approaches to BTK inhibition, both for the wild type and C481S-mutant forms, include AbbVie (IMBRUVICA), AstraZeneca (CALQUENCE), Beigene Co., Ltd. (Zanubrutinib), Merck (nemtabrutinib), and Eli Lilly (pirtobrutinib), among others.
Any product candidate that we develop and that obtains regulatory approval must then compete for market acceptance and market share. Our products may not gain market acceptance among physicians, patients, healthcare payers, insurers, the medical community and other stakeholders. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
Further, any products we develop may become obsolete or face generic entry before we recover any expenses we incurred in connection with the development of these products. As a result, we may never achieve profitability.
27
Risks Related to our Intellectual Property
We may be unable to obtain patents to protect our technologies from other companies with competitive products, and patents of other companies could prevent us from manufacturing, developing or marketing our products.
Patent protection
The patent positions of pharmaceutical and biotechnology companies are uncertain and involve complex legal and factual questions. The United States Patent and Trademark Office (“USPTO”) and many other patent offices in the world have not established a consistent policy regarding the breadth of claims that they will allow in biotechnology patents.
Our pending patent applications may not result in issued patents and our issued patents may not be held valid and enforceable if challenged. Competitors may be able to circumvent any such issued patents by adoption of a competitive, though non-infringing product or process. Interpretation and evaluation of pharmaceutical or biotechnology patent claims present complex and often novel legal and factual questions. Our business could be adversely affected by increased competition in the event that any patent granted to it is held to be invalid or unenforceable or is inadequate in scope to protect our operations.
Allowable patentable subject matter and the scope of patent protection obtainable may differ between jurisdictions. If a patent office allows broad claims, the number and cost of patent interference proceedings in the United States, or analogous proceedings in other jurisdictions and the risk of infringement litigation may increase. If it allows narrow claims, the risk of infringement may decrease, but the value of our rights under our patents, licenses and patent applications may also decrease.
The scope of the claims in a patent application can be significantly modified during prosecution before the patent is issued. Consequently, we cannot know whether our pending applications will result in the issuance of patents or, if any patents are issued, whether they will provide us with significant proprietary protection or will be circumvented, invalidated or found to be unenforceable.
Publication of discoveries in scientific or patent literature often lags behind actual discoveries. Patent applications filed in the United States generally will be published 18 months after the filing date unless the applicant certifies that the invention will not be the subject of a foreign patent application. In many other jurisdictions, such as Canada, patent applications are published 18 months from the priority date. We may not be aware of such literature. Accordingly, we cannot be certain that the named inventors of our products and processes were the first to invent that product or process or that we were the first to pursue patent coverage for our inventions.
In addition, United States patent laws may change which could prevent or limit us from filing patent applications or patent claims in the United States to protect our products and technologies or limit the exclusivity periods that are available to patent holders for United States patents. For example, the Leahy-Smith America Invents Act, (the “Leahy-Smith Act”) was signed into law in 2011 and includes a number of significant changes to United States patent law. These include changes to transition from a “first-to-invent” system to a “first-to-file” system and to the way issued patents are challenged. These changes may favor larger and more established companies that have more resources to devote to patent application filing and prosecution. It is not clear what, if any, impact the Leahy-Smith Act will ultimately have on the cost of prosecuting our patent applications in the United States, our ability to obtain patents in the United States based on our discoveries and our ability to enforce or defend our United States issued patents.
28
Until such time, if ever, that further patents are issued to us, we will rely upon the law of trade secrets to the extent possible given the publication requirements under international patent treaty laws and/or requirements under foreign patent laws to protect our technology and our products incorporating the technology. In this regard, we have adopted certain confidentiality procedures. These include: limiting access to confidential information to certain key personnel; requiring all directors, officers, employees and consultants and others who may have access to our intellectual property to enter into confidentiality agreements which prohibit the use of or disclosure of confidential information to third parties; and implementing physical security measures designed to restrict access to such confidential information and products. Our ability to maintain the confidentiality of our technology is crucial to our ultimate possible commercial success. The procedures adopted by us to protect the confidentiality of our technology may not be effective, third parties may gain access to our trade secrets or our trade secrets or those of our collaborators may be independently discovered by others. Our collaborators, employees and consultants and other parties may not comply with the terms of their agreements with us, and we might be unable to adequately enforce our rights or obtain adequate compensation for the damages caused by unauthorized disclosure or use of our trade secrets or know how. Further, by seeking patent protection in various countries, it is inevitable that a substantial portion of our technology will become available to our competitors, through publication of such patent applications.
Enforcement of intellectual property rights
Protection of the rights revealed in published patent applications can be complex, costly and uncertain. Our commercial success depends in part on our ability to maintain and enforce our proprietary rights. If third parties engage in activities that infringe our proprietary rights, our management’s focus will be diverted and we may incur significant costs in asserting our rights. We may not be successful in asserting our proprietary rights, which could result in our patents being held invalid or a court holding that the third party is not infringing, either of which would harm our competitive position.
Others may design around our patented technology. We may have to participate in interference proceedings declared by the USPTO, European opposition proceedings, or other analogous proceedings in other parts of the world to determine priority of invention and the validity of patent rights granted or applied for, which could result in substantial cost and delay, even if the eventual outcome is favorable to us. Our pending patent applications, even if issued, may not be held valid or enforceable.
Our products and product candidates may infringe the intellectual property rights of others, or others may infringe on our intellectual property rights which could increase our costs.
Our success also depends on avoiding infringement of the proprietary technologies of others. In particular, there may be certain issued patents and patent applications claiming subject matter which we or our collaborators may be required to license in order to research, develop or commercialize tuspetinib or luxeptinib. In addition, third parties may assert infringement or other intellectual property claims against us. An adverse outcome in these proceedings could subject us to significant liabilities to third parties, require disputed rights to be licensed from third-parties or require us to cease or modify our use of the technology. If we are required to license third-party technology, a license under such patents and patent applications may not be available on acceptable terms or at all. Further, we may incur substantial costs defending ourselves in lawsuits against charges of patent infringement or other unlawful use of another’s proprietary technology. We may also need to bring claims against others who we believe are infringing our rights in order to become or remain competitive and successful. Any such claims can be time consuming and expensive to pursue.
We may incur substantial cost in defending our intellectual property.
While we believe that our products and technology do not infringe proprietary rights of others, third parties may assert infringement claims in the future and such claims could be successful. Even if challenges are unsuccessful, we could incur substantial costs in defending ourselves against patent infringement claims brought by others or in prosecuting suits against others. In addition, others may obtain patents that we would need to license, which may not be available to us on reasonable terms. Whether we are able to obtain a necessary license would depend on the terms offered, the degree of risk of infringement and the need for the patent.
29
We have licensed important portions of our intellectual property from Hanmi and CG, and are subject to significant obligations under those license agreements.
The rights we hold under our license agreements with Hanmi and CG are critical to our business.
Our tuspetinib program is built around patents exclusively in-licensed from Hanmi, which permit us to research, develop and commercialize tuspetinib worldwide. Under the Tuspetinib Licensing Agreement, we are subject to significant obligations, including diligence obligations with respect to development and commercialization activities, payment obligations upon achievement of certain milestones and royalties on product sales, as well as other material obligations. Hanmi is eligible for payments upon the achievement of developmental, regulatory and commercial-based milestones, as well as tiered royalties on product sales.
Our luxeptinib program is built around patents exclusively in-licensed from CG, which permit us to research, develop and commercialize luxeptinib worldwide except for the Republic of Korea. Under our agreement with CG, we are subject to significant obligations, including diligence obligations with respect to development and commercialization activities, payment obligations upon achievement of certain milestones and royalties on product sales, as well as other material obligations. CG is eligible for payments upon the achievement of developmental, regulatory and commercial-based milestones, as well as low single-digit royalties on product sales.
If there is any conflict, dispute, disagreement or issue of non-performance between us and Hanmi or CG regarding our rights or obligations under the respective license agreements, including any conflict, dispute or disagreement arising from our failure to satisfy diligence or payment obligations under such agreements, Hanmi or CG may have a right to terminate the respective license. The loss of this license agreement could materially and adversely affect our ability to use intellectual property that could be critical to our drug discovery and development efforts, as well as our ability to enter into future collaboration, licensing and/or marketing agreements for one or more affected drug candidates or development programs.
Our business depends, in part, on our ability to use technology that we have licensed or will in the future license from third parties, including CG, and, if these licenses were terminated or if we were unable to license additional technology we may need in the future, our business will be adversely affected.
We currently hold licenses for certain technologies that are or may be critical to our current and subsequent product candidates. These include our exclusive license to research, develop and commercialize luxeptinib worldwide except for the Republic of Korea, and our exclusive license to develop and commercialize tuspetinib worldwide. Both licenses are subject to termination in the event of a breach by us of the license, if we fail to cure the breach following notice and the passage of a cure period. We may need to acquire additional licenses in the future to technologies developed by others. Furthermore, future license agreements may require us to make substantial milestone payments. We may also be obligated to make royalty payments on the sales, if any, of products resulting from the license. The termination of a license or the inability to license future technologies on acceptable terms may adversely affect our ability to develop or sell our products.
Legal and Regulatory Risk
Our ability to develop, produce and market our products is subject to extensive government regulation.
Government regulation is a significant factor in the development, production and marketing of our products. Research and development, testing, manufacture, marketing and sales of pharmaceutical products or related products are subject to extensive regulatory oversight, often in multiple jurisdictions, which may cause significant additional costs and/or delays in bringing products to market, and in turn, may cause significant losses to investors. The regulations applicable to our product candidates in a given jurisdiction may change. Even if granted, regulatory approvals may include significant limitations on the uses for which products can be marketed or may be conditioned on the conduct of post-marketing surveillance studies. Failure to comply with applicable regulatory requirements can, among other things, result in delay in approving or refusal to approve a product candidate, interruptions of clinical trials or manufacturing, suspension or withdrawal of regulatory approval, warning letters, the imposition of civil penalties or other monetary payments, product recall or seizure, operating restrictions, injunctions or criminal prosecution. In addition, regulatory agencies many not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates.
30
Requirements for regulatory approval vary widely from country to country. Regulatory authorities in other countries must approve a product prior to the commencement of marketing the product. The time required to obtain any such approval may be longer or shorter than in Canada or the United States. Approved drugs, as well as their manufacturers, are subject to continuing and ongoing review, and discovery of problems with these products or the failure to adhere to manufacturing or quality control requirements may result in regulatory restrictions being imposed.
Current and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and may adversely affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could, among other things, prevent or delay marketing approval of our product candidates, restrict or regulate post approval activities and affect our ability to profitably sell any products for which we obtain marketing approval.
For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or collectively the Affordable Care Act, was enacted to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Additionally, the Drug Supply Chain Security Act, enacted in 2013, imposed new obligations on manufacturers of pharmaceutical products related to product tracking and tracing.
Since its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the Affordable Care Act, and we expect there will be additional challenges and amendments to the Affordable Care Act in the future. On June 17, 2021, the United States Supreme Court dismissed the most recent judicial challenge to the Affordable Care Act without specifically ruling on the constitutionality of the Affordable Care Act. Prior to the Supreme Court’s decision, President Biden issued an executive order initiating a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the Affordable Care Act marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the Affordable Care Act. It is possible that the Affordable Care Act will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges and the healthcare reform measures of the Biden administration will impact the Affordable Care Act and our business.
We expect ongoing initiatives in the United States and internationally to increase pressure on drug pricing. Regulations that mandate price controls and limitations on patient access to products or establish prices paid by government entities or programs may impact product candidates that we may successfully develop. Pharmaceutical product pricing is subject to enhanced government and public scrutiny and calls for reform. Some U.S. states have implemented, and other U.S. states are considering, pharmaceutical price controls or patient access constraints under the Medicaid program, and some U.S. states are considering price-control regimes that would apply to broader segments of their populations that are not Medicaid eligible. Efforts by government officials or legislators to implement measures to regulate prices or payments for pharmaceutical products, including legislation on drug importation, could have an adverse effect on anticipated revenues from product candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop drug candidates.
Legislative and regulatory proposals have also been made to expand post approval requirements and restrict sales and promotional activities for pharmaceutical products in the US. Any healthcare reforms enacted in the future may, like the Affordable Care Act, be phased in over a number of years but, if enacted, could reduce our revenue, increase our costs, or require us to revise the ways in which we conduct business or put us at risk for loss of business. It is not clear whether additional legislative changes will be enacted, or whether the current regulations, guidance or interpretations will be changed, or what the impact of such changes on our business, if any, may be.
31
In Canada, the Patented Medicine Prices Review Board (the “PMPRB”) has jurisdiction to control prices of patented medicines that are considered excessive. Recent changes to the regulations governing the PMPRB are intended to lower the prices of patented medicines even further. The PMPRB’s jurisdiction could extend to any of our drug products that are approved in Canada and protected under Canadian patents, with an adverse effect on the prices that we would otherwise obtain for these drugs in the relevant market.
Coverage and adequate reimbursement may not be available for our product candidates, which could make it difficult for us to sell our products profitably.
Market acceptance and sales of any drug candidates that we develop will depend in part on the extent to which reimbursement for these products and related treatments will be available from third party payors, including government health administration authorities and private health insurers. Third party payors decide which drugs they will pay for and establish reimbursement levels. Third party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement to be provided for each of our drug candidates will be made on a plan-by-plan basis. One payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage, and adequate reimbursement, for the product. Additionally, a third-party payor’s decision to provide coverage for a drug does not imply that an adequate reimbursement rate will be approved. Each plan determines whether or not it will provide coverage for a drug, what amount it will pay the manufacturer for the drug, and on what tier of its formulary the drug will be placed. The position of a drug on a formulary generally determines the copayment that a patient will need to make to obtain the drug and can strongly influence the adoption of a drug by patients and physicians. Patients who are prescribed treatments for their conditions and providers performing the prescribed services generally rely on third party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products.
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Third party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. We cannot be sure that coverage and reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Inadequate coverage and reimbursement may impact the demand for, or the price of, any product for which we obtain marketing approval. If coverage and adequate reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize any drug candidates that we develop.
Additionally, there have been a number of legislative and regulatory proposals to change the healthcare system in the United States and in some foreign jurisdictions, including Canada, that could affect our ability to sell any future drugs profitably. These legislative and regulatory changes may negatively impact the reimbursement for any future drugs, following approval.
We are subject to U.S. and Canadian healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, fines, disgorgement, exclusion from participation in government healthcare programs, curtailment or restricting of our operations and diminished profits and future earnings.
Healthcare providers, physicians and others will play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our future arrangements with healthcare providers, patients and third-party payors could expose us to broadly applicable U.S. and Canadian laws and regulations relating to fraud abuse and healthcare more generally that may constrain the business or financial arrangements and collaborative partners through which we market, sell and distribute any products for which we obtain marketing approval.
32
Efforts to ensure that our collaborations with third parties, and our business generally, will comply with applicable U.S. and Canadian healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental laws and regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, contractual damages, reputational harm, disgorgement, curtailment or restricting of our operations, any of which could substantially disrupt our operations and diminish our profits and future earnings. If any of the physicians or other providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. The risk of the Company being found in violation of these laws is increased by the fact that many of them have not been fully interpreted regulatory authorities or courts, and their provisions are open to a variety of interpretations.
If product liability, clinical trial liability or environmental liability claims are brought against us or we are unable to obtain or maintain product liability, clinical trial or environmental liability insurance, we may incur substantial liabilities that could reduce our financial resources.
The clinical testing and commercial use of pharmaceutical products involves significant exposure to product liability, clinical trial liability, environmental liability and other risks that are inherent in the testing, manufacturing and marketing of our products. These liabilities, if realized, could have a material adverse effect on our business, results of operations and financial condition.
We have obtained limited product liability insurance coverage for our clinical trials on humans; however, our insurance coverage may be insufficient to protect us against all product liability damages. Regardless of merit or eventual outcome, liability claims may result in decreased demand for a future product, injury to reputation, withdrawal of clinical trial volunteers, loss of revenue, costs of litigation, distraction of management and substantial monetary awards to plaintiffs. Additionally, if we are required to pay a product liability claim, we may not have sufficient financial resources to complete development or commercialization of any of our product candidates and our business and results of operations will be adversely affected. In general, insurance will not protect us against some of our own actions, such as negligence.
As our development activities progress towards the commercialization of product candidates, our liability coverage may not be adequate, and we may not be able to obtain adequate product liability insurance coverage at a reasonable cost, if at all. Even if we obtain product liability insurance, our financial position may be materially adversely affected by a product liability claim. A product liability claim could also significantly harm our reputation and delay market acceptance of our product candidates. Additionally, product recalls may be issued at the direction of the FDA, other government agencies or other companies having regulatory control for pharmaceutical sales. If a product recall occurs in the future, such a recall could adversely affect our business, financial condition or reputation.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and radioactive and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
33
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
We may be unable to obtain partnerships for our product candidates, which could curtail future development and negatively affect our share price. In addition, our partners might not satisfy their contractual responsibilities or devote sufficient resources to our partnership.
Our strategy for the research, development and commercialization of our products requires entering into various arrangements with corporate collaborators, licensors, licensees and others, and our commercial success is dependent upon these outside parties performing their respective contractual responsibilities. The amount and timing of resources that such third parties will devote to these activities may not be within our control. These third parties may not perform their obligations as expected and our collaborators may not devote adequate resources to our programs. In addition, we could become involved in disputes with our collaborators, which could result in a delay or termination of the related development programs or result in litigation. We intend to seek additional collaborative arrangements to develop and commercialize some of our products. We may not be able to negotiate collaborative arrangements on favorable terms, or at all, in the future, and our current or future collaborative arrangements may not be successful.
If we cannot negotiate collaboration, license or partnering agreements, we may never achieve profitability and we may not be able to continue to develop our product candidates. Continuing Phase 1, and commencing Phase 2 and Phase 3 clinical trials for tuspetinib and luxeptinib would require significant amounts of funding and such funding may not be available to us.
Risks Related to Our Common Shares
Our share price has been and is likely to continue to be volatile and an investment in our Common Shares could suffer a decline in value.
You should consider an investment in our Common Shares as risky and invest only if you can withstand a significant loss and wide fluctuations in the market value of your investment. The market price of our Common Shares has been highly volatile and is likely to continue to be volatile. This leads to a heightened risk of securities litigation pertaining to such volatility. Factors affecting our Common Share price include but are not limited to:
34
Future sales of our Common Shares by us or by our existing shareholders could cause our share price to fall.
The issuance of Common Shares by the Company could result in significant dilution in the equity interest of existing shareholders and adversely affect the market price of our Common Shares. Sales by existing shareholders of a large number of our Common Shares in the public market and the issuance of Common Shares in connection with strategic alliances, or the perception that such additional sales could occur, could cause the market price of our Common Shares to decline and have an undesirable impact on our ability to raise capital.
We are susceptible to stress in the global economy and therefore, our business may be affected by the current and future global financial conditions.
If the increased level of volatility and market turmoil that have marked recent years continue, our operations, business, financial condition and the trading price of our Common Shares could be materially adversely affected. Furthermore, general economic conditions may have a great impact on us, including our ability to raise capital, our commercialization opportunities and our ability to establish and maintain arrangements with others for research, manufacturing, product development and sales.
Failure to meet the TSX's and the Nasdaq’s continued listing requirements could result in the delisting of our Common Shares, negatively impact the price of our Common Shares and negatively impact our ability to raise additional capital.
If we fail to satisfy the continued listing requirements of the Nasdaq Capital Market, such as the corporate governance requirements or the minimum closing bid price requirement, the exchange may take steps to delist our Common Shares. A delisting would likely have a negative effect on the price of our Common Shares and would impair your ability to sell or purchase our Common Shares when you wish to do so. In the event of a delisting notification, we anticipate that we would take actions to restore our compliance with applicable exchange requirements, such as stabilize our market price, improve the liquidity of our Common Shares, prevent our Common Shares from dropping below such exchange’s minimum bid price requirement, or prevent future non-compliance with such exchange’s listing requirements.
On February 29, 2024, the Company received a deficiency letter (the “2024 Deficiency Letter”) from the Nasdaq Listing Qualifications Department of The Nasdaq Stock Market LLC (“Nasdaq”) notifying the Company that the Company’s January 2024 private placement (the "Private Placement") of securities to Hanmi violated rule 5635(d) because the Company did not obtain shareholder approval prior to such issuance. Nasdaq stated that the Private Placement involved the issuance of greater than 20% of the issued and outstanding Common Shares of the Company at a discount to the Nasdaq official closing price on January 25, 2024, the date of the subscription agreement between the Company and Hanmi. The 2024 Deficiency Letter has no immediate effect on the listing of the Company’s Common Shares. In accordance with the Nasdaq Listing Rules, the Company has been given forty-five (45) calendar days, or until April 14, 2024, to submit a plan to regain compliance. If Nasdaq accepts the Company’s plan to regain compliance, Nasdaq can grant an extension of up to 180 calendar days from the date of the Deficiency Letter to evidence compliance. Although the Company believes that the Private Placement was completed in accordance with the Nasdaq Listing Rules, the Company respects Nasdaq’s query and intends to work with Nasdaq to resolve Nasdaq’s concerns and will consider available options to regain compliance. However, there can be no assurance that the Company will be able to regain compliance with the applicable Nasdaq Listing Rules.
Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act, new SEC regulations and Nasdaq rules, are creating uncertainty for companies such as ours. These laws, regulations and standards are subject to varying interpretations in some cases due to their lack of specificity, and as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We are committed to maintaining high standards of corporate governance and public disclosure. As a result, our efforts to comply with evolving laws, regulations and standards have resulted in, and are likely to continue to result in, increased general and administrative expenses and management time related to compliance
35
activities. If we fail to comply with these laws, regulations and standards, our reputation may be harmed and we might be subject to sanctions or investigation by regulatory authorities, such as the SEC. Any such action could adversely affect our financial results and the market price of our common stock.
Certain Canadian laws could delay or deter a change of control.
Limitations on the ability to acquire and hold our Common Shares may be imposed by the Competition Act in Canada. This legislation permits the Commissioner of Competition of Canada to review any acquisition of a significant interest in the Company. This legislation grants the Commissioner jurisdiction to challenge such an acquisition before the Canadian Competition Tribunal if the Commissioner believes that it would, or would be likely to, result in a substantial lessening or prevention of competition in any market in Canada. The Investment Canada Act subjects an acquisition of control of a company by a non-Canadian to government review if the value of our assets, as calculated pursuant to the legislation, exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant minister is satisfied that the investment is likely to result in a net benefit to Canada. Any of the foregoing could prevent or delay a change of control and may deprive or limit strategic opportunities for our shareholders to sell their shares.
The exercise of all or any number of outstanding stock options, the award of any additional options, restricted stock units or other stock-based awards or any issuance of shares to raise funds or acquire a business may dilute your Common Shares.
We have in the past and may in the future grant to some or all of our directors, officers and employees options to purchase our Common Shares and other stock-based awards as non-cash incentives to those persons. The issuance of any equity securities could, and the issuance of any additional shares will, cause our existing shareholders to experience dilution of their ownership interests.
Any additional issuance of shares or a decision to acquire other businesses through the sale of equity securities may dilute our investors’ interests, and investors may suffer dilution in their net book value per share depending on the price at which such securities are sold. Such issuance may reduce the proportionate ownership and voting power of all other shareholders. The dilution may result in a decline in the price of our Common Shares or a change in control.
We do not expect to pay dividends for the foreseeable future.
We have not paid any cash dividends to date and we do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest future earnings, if any, in the development and growth of our business. Therefore, investors will not receive any funds unless they sell their Common Shares, and shareholders may be unable to sell their shares on favorable terms or at all. We cannot assure you of a positive return on investment or that you will not lose the entire amount of your investment in our Common Shares. Prospective investors seeking or needing dividend income or liquidity should not purchase our Common Shares.
36
General Risks
It may be difficult for non-Canadian investors to obtain and enforce judgments against us because of our Canadian incorporation and presence.
We are a corporation existing under the laws of Canada. Some of our directors and some of the experts named or unnamed in this Annual Report on Form 10-K, are residents of Canada, and all or a substantial portion of their assets, and a substantial portion of our assets, are located outside the United States. Consequently, although we have appointed an agent for service of process in the United States, it may be difficult for holders of our shares who reside in the United States to effect service within the United States upon our directors and officers and experts who are not residents of the United States. It may also be difficult for holders of our shares who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. Investors should not assume that Canadian courts (i) would enforce judgments of United States courts obtained in actions against us or our directors, officers or experts predicated upon the civil liability provisions of the United States federal securities laws or the securities or “blue sky” laws of any state within the United States or (ii) would enforce, in original actions, liabilities against us or our directors, officers or experts predicated upon the United States federal securities laws or any such state securities or “blue sky” laws. In addition, we have been advised by our Canadian counsel that in normal circumstances, only civil judgments and not other rights arising from United States securities legislation are enforceable in Canada and that the protections afforded by Canadian securities laws may not be available to investors in the United States.
We are likely a “passive foreign investment company” which may have adverse United States federal income tax consequences for United States shareholders.
United States investors in our Common Shares should be aware that we believe we are classified as a passive foreign investment company (“PFIC”) during the tax year ended December 31, 2022,2023.
Director | Meetings Attended |
Audit Committee | Corporate Governance and Nominating Committee | Compensation Committee | R&D Committee | Board | |
Carol G. Ashe | - | 4 of 4 | 4 of 4 | - | 7 of 7 |
Denis Burger | 4 of 4 | 4 of 4 | 4 of 4 | - | 7 of 7 |
Erich Platzer | - | - | 3 of 4 | 1 of 1 | 7 of 7 |
William G. Rice | 4 of 4(1) | 4 of 4(1) | - | 1 of 1 | 7 of 7 |
Bernd R. Seizinger | 4 of 4 | - | - | 1 of 1 | 6 of 7 |
Mark Vincent | - | 4 of 4 | - | 1 of 1 | 7 of 7 |
Warren Whitehead | 4 of 4 | - | - | - | 7 of 7 |
Any failure to maintain an effective system of internal controls may result in material misstatements of our consolidated financial statements or cause us to fail to meet our reporting obligations or fail to prevent fraud; and in that case, our shareholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our Common Shares.
Section 404(a) of the Sarbanes-Oxley Act of 2002 requires that our management assess and report annually on the effectiveness of our internal control over financial reporting and identify any material weaknesses in our internal control over financial reporting.
37
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we fail to maintain an effective system of internal controls, we might not be able to report our financial results accurately or prevent fraud; and in that case, our shareholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our Common Shares. While we believe that we have sufficient personnel and review procedures to allow us to maintain an effective system of internal controls, we cannot assure you that we will not experience potential material weaknesses in our internal control. Even if we conclude that our internal control over financial reporting provides reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with US GAAP, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our future reporting obligations.
If we fail to timely achieve and maintain the adequacy of our internal control over financial reporting, we may not be able to produce reliable financial reports or help prevent fraud. Our failure to achieve and maintain effective internal control over financial reporting could prevent us from complying with our reporting obligations on a timely basis, which could result in the loss of investor confidence in the reliability of our consolidated financial statements, harm our business and negatively impact the trading price of our Common Shares.
Data security incidents and privacy breaches could result in important remediation costs, increased cyber security costs, litigation and reputational harm.
Cyber security incidents can result from deliberate attacks or unintentional events. Cyber-attacks and security breaches could include unauthorized attempts to access, disable, improperly modify or degrade the Company’s information, systems and networks, the introduction of computer viruses and other malicious codes and fraudulent “phishing” emails that seek to misappropriate data and information or install malware onto users’ computers. Cyber-attacks in particular vary in technique and sources, are persistent, frequently change and are increasingly more targeted and difficult to detect and prevent against. Our network security and data recovery measures and those of third parties with which we contract, may not be adequate to protect against cyber-attacks.
Disruptions due to cyber security incidents could adversely affect our business. In particular, a cyber security incident could result in the loss or corruption of data from our research and development activities, including clinical trials, which may cause significant delays to some or all of our clinical programs. Also, our trade secrets, including unpatented know how, technology and other proprietary information could be disclosed to competitors further to a breach, which would harm our business and competitive position. We expect that risks and exposures related to cyber security attacks will remain high for the foreseeable future due to the rapidly evolving nature and sophistication of these threats. While we have invested in the protection of data and information technology, there can be no assurance that our efforts to implement adequate security measures would be sufficient to protect us against cyber-attacks.
We must successfully upgrade and maintain our information technology systems.
We rely on various information technology systems to manage our operations. There are inherent costs and risks associated with maintaining, modifying and/or changing these systems and implementing new systems, including potential disruption of our internal control structure, substantial capital expenditures, additional administration and operating expenses, retention of sufficiently skilled personnel to implement and operate its systems, demands on management time and other risks and costs of delays or difficulties in transitioning to new systems or of integrating new systems into our current systems. In addition, our information technology system implementations may not result in productivity improvements at a level that outweighs the costs of implementation, or at all. The implementation of new information technology systems may also cause disruptions in our business operations and have an adverse effect on our business, prospects, financial condition and operating results.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
38
ITEM 1C. CYBERSECURITY
Cybersecurity Risk Management and Strategy
We have developed and maintain a cybersecurity program designed to assess, identify, and manage risks from cybersecurity threats. As part of this program, we conduct periodic assessments of our IT Systems to evaluate the effectiveness of applicable security controls. These assessments follow industry-standard frameworks and include a review of our information security controls to assess cybersecurity capabilities and maturity. The results of these assessments are reported to the Audit Committee of the Board of Directors..
In general, we seek to address cybersecurity risks through a cross-functional approach that is focused on preserving the confidentiality, integrity, and availability of the information that we collect and store by identifying, preventing, and mitigating cybersecurity threats and effectively responding to cybersecurity incidents when they occur.
We have established a cybersecurity policy that outlines the governance processes for identifying and managing material risks to privacy and cybersecurity. In addition, our cybersecurity policy describes our capabilities and processes for the detection, response, analysis, mitigation, recovery, and reporting of cybersecurity incidents. We also manage and maintain business continuity and disaster recovery capabilities to help ensure the availability of business-critical technology resources.
Governance Related to Cybersecurity Risks
Management is responsible for the day-to-day management of risks we face, while our board of directors,invitation as a whole and through committees, has responsibility formember of management.
All director nominees are expected to attend the oversight of risk management. Our Audit Committee oversees the management of risks from cybersecurity threats. In addition, the full board reviews our major risk exposures, their potential impact on us, and the steps we take to manage them.
Our Chief Information Officer ("CIO") is responsible for developing, implementing, and maintaining our cybersecurity risk management policies and procedures. The individual currently serving in the role of CIO has over thirty-five years of experience in cybersecurity, information security, data protection, regulatory compliance, and risk management within complex and international business verticals such as pharmaceutical/biotech, technology, and logistics. The CIO provides regular cybersecurity updates to our board of directors.
Our Information Technology Steering Committee ("ITSC") oversees matters regarding the Company’s Information Technology strategy, priorities, and governance, including cybersecurity threats and risk assessments, through periodic meetings and frequent communications. ITSC members include representatives from the Finance, Regulatory Affairs, Operations, and Information Technology departments. The ITSC has a charter that is reviewed internally to ensure it is aligned with our business strategy. As outlined in its charter, and relative to cybersecurity, the ITSC is responsible for identifying and assessing material cybersecurity risks across the Company, including escalating to our Audit Committee and Executive Management where appropriate.
39
ITEM 2. PROPERTIES
We lease 7,556 square feet of office space in San Diego, California and 2,078 square feet of office space in Toronto, Canada. The lease for the San Diego office space is scheduled to expire on May 31, 2026. Aptose previously leased 2,618 square feet of laboratory space in San Diego. We exited this laboratory space prior to the expiration of the lease on February 28, 2023. The lease for the Toronto office space is scheduled to expire on June 30, 2024. We believe that our facilities are sufficient to meet our needs and that suitable additional space will be available as and when needed.
ITEM 3. LEGAL PROCEEDINGS
We know of no material pending legal proceedings to which our company or subsidiaries is a party or of which any of our properties, or the properties of our subsidiaries, is the subject. However, from time to time, we may be subject to various pending or threatened legal actions and proceedings, including those that arise in the ordinary course of our business. Such matters are subject to many uncertainties and to outcomes that are not predictable with assurance and that may not be known for extended periods of time.
ITEM 4. MINE SAFETY DISCLOSURES
None.
40
PART II.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our Common Shares are currently traded on The Nasdaq Capital Market under the symbol “APTO” and the Toronto Stock Exchange under the symbol “APS.”
As of March 26, 2024, there were approximately 30 shareholders of record of our Common Shares, which included Cede & Co., a nominee for Depository Trust Company, or DTC, and CDS & Co., a nominee for The Canadian Depository for Securities Ltd., ("CDS"). Common shares that are held by financial institutions as nominees for beneficial owners are deposited into participant accounts at either DTC or CDS, and are considered to be held of record by Cede & Co. or CDS & Co., each as one shareholder.
We currently intend to retain all future earnings, if any, for the operation and expansion of our business and, therefore, do not anticipate declaring or paying cash dividends on our Common Shares in the foreseeable future.
Repurchases of Equity Securities
There were no repurchases of equity securities during the fourth quarter of 2023.
ITEM 6. RESERVED
41
ITEM 7 - MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This discussion contains forward-looking statements that involve risks and uncertainties. When reviewing the discussion below, you should keep in mind the substantial risks and uncertainties that impact our business. In particular, we encourage you to review the risks and uncertainties described in “Risk Factors” in Part I, Item 1A in this Annual Report on Form 10-K. These risks and uncertainties could cause actual results to differ materially from those projected or implied by our forward-looking statements contained in this report. These forward-looking statements are made as of the date of this management’s discussion and analysis, and we do not intend, and do not assume any obligation, to update these forward-looking statements, except as required by law.Meeting. All amounts are expressed in United States dollars unless otherwise stated.
Aptose Biosciences Inc. is a science-driven clinical stage biotechnology company committed to precision medicines addressing the unmet clinical needs in oncology, with an initial focus on hematology. The Company's small molecule cancer therapeutics pipeline includes products designed to provide single agent efficacyWilliam G. Rice, Denis Burger, Carol G. Ashe, Erich Platzer, Bernd Seizinger, Mark Vincent and to enhance the efficacyWarren Whitehead attended last year’s Annual and Special Meeting of other anti-cancer therapies and regimens without overlapping toxicities. The Company’s executive office is in San Diego, California, and our head office is in Toronto, Canada.
Aptose Programs
We are advancing oral targeted agents to treat life-threatening hematologic cancers that require immediate treatment. We have two clinical-stage oral kinase inhibitors under active development for the treatment of hematologic malignancies: tuspetinib ("HM43239" or "TUS") and luxeptinib ("CG-806" or "LUX"). Tuspetinib and luxeptinib are being evaluated for safety, tolerability, pharmacokinetics and efficacy in Phase 1/2 clinical trials, and each molecule is described below. A third molecule (APTO-253) is not under clinical development and will not be discussed further.
Tuspetinib, Aptose’s lead asset, is being developed for frontline combination therapy in newly diagnosed AML patients to unlock the most significant patient impact and greatest commercial opportunity. Tuspetinib is a once-daily oral kinase inhibitor, targeting a select group of kinases operative in myeloid malignancies, such as acute myeloid leukemia ("AML") and the higher risk myelodysplastic syndromes ("hr-MDS"), and known to be involved in tumor proliferation, resistance to therapy, and differentiation. However, tuspetinib avoids kinases that typically cause toxicities associated with other kinase inhibitors and is consequently a well-tolerated antileukemic agent. The clinical development path for triplet combination therapy in newly diagnosed AML patients with tuspetinib-based triplet combination therapy (TUS + VEN + hypomethylating agent; (TUS+VEN+HMA), the TUS+VEN doublet in relapsed or refractory R/R AML patients.
Tuspetinib has completed the dose escalation and dose exploration stages of an international Phase 1/2 clinical trial designed to assess the safety, tolerability, pharmacokinetics, pharmacodynamic responses, and efficacy as a single agent in patients with R/R AML. Complete responses (“CRs”) without dose limiting toxicities were achieved at four dose levels across a broad diversity of mutationally-defined AML populations and with a highly favorable safety profile. Tuspetinib to date has demonstrated a favorable safety profile and has caused no drug-related QTc prolongations, liver or kidney toxicities, muscle damage, differentiation syndrome, and no myelosuppression with continuous dosing of patients in remission. A recommended phase 2 dose (RP2D) of 80 mg tuspetinib once daily as an oral tablet was selected and approved by the U.S. FDA for use as a single agent in patients with R/R AML. At the RP2D, tuspetinib demonstrated notable response rates in R/R AML patients that had never been treated with venetoclax (VEN-naive AML): CR/CRh=36% among all-comers, CR/CRh=50% among patients with mutated FLT3, and CR/CRh=25% in patients with wildtype FLT3.Shareholders.
Following completion of the single agent dose escalation and exploration trial, tuspetinib advanced into the APTIVATE expansion trial of the Phase 1/2 program in R/R AML patient populations treated with the TUS+VEN doublet, with the intent to position tuspetinib for triple combination studies in frontline therapy. The TUS+VEN doublet combination therapy maintained a favorable safety profile: no new or unexpected safety signals were observed, and there were no reported drug-related adverse events of QTc prolongation, differentiation syndrome, or deaths. The TUS+VEN doublet combination also achieved responses in heavily pretreated R/R AML patients, including those with wildtype or mutated FLT3, and those who failed prior therapy with venetoclax ("Prior-VEN") or FLT3 inhibitors ("Prior-FLT3i"). Based on the safety and efficacy profile of tuspetinib, we believe that tuspetinib, if
42
approved, can reach annual sales greater than $3 billion by 2035 because we believe tuspetinib could 1) become the preferred kinase inhibitor for inclusion in triplet combination for front line AML patients with FLT3 mutations and for patients with wild type FLT3, 2) become the preferred kinase inhibitor for inclusion in combination with venetoclax for second line AML patients, 3) serve as an effective agent for maintenance therapy to prevent relapse in patients who achieved a complete remission through a stem cell transplant or through drug-based therapy, 4) serve as an effective agent for the treatment of third line FLT3 mutated patients failed by prior therapy with other FLT3 inhibitors and 5) serve in front line triplet combinations, second line doublet combinations, and maintenance therapy for hr-MDS patients. These beliefs related to the potential commercial opportunity are based on management’s current assumptions and estimates, which are subject to change, and there can be no assurance that tuspetinib will ever be approved or successfully commercialized and, if approved and commercialized, that it will ever generate significant revenues. See our “Risk Factors – “We are an early-stage development company with no revenues from product sales.” and “We have a history of operating losses. We expect to incur net losses and we may never achieve or maintain profitability.” in this Annual Report on Form 10-K.Executive Sessions
Luxeptinib is an oral, highly potent kinase inhibitor that selectively targets defined kinases operative in myeloid and lymphoid hematologic malignancies. This small molecule has been evaluated in a Phase 1a/b study forThe independent directors meet regularly without the treatment of patients having R/R B-cell leukemias and lymphomas and in a Phase 1a/b study for the treatment of patients with R/R AML or hr-MDS. These clinical studies demonstrated tumor shrinkage among B-cell cancer patients, including a CR in a diffuse large B-cell lymphoma patient that was determined via biopsy analysis at the end of Cycle 22 with 900mg BID dosingpresence of the original G1 formulation. Likewise, an MRD-negative CR in one R/R AML patient occurred with 450mg BID dosingnon-independent directors and members of the original G1 formulation. Because absorption of the original G1 formulation hampered effectiveness of luxeptinib, a new G3 formulation was developed. Enrollment of patients in the B-cell malignancy trial and the AML trial have been completed, and clinical evaluation of the G3 formulation has been completed. The G3 formulation was determined to deliver superior plasma exposure levels relative to the original G1 formulation, and any future trial with luxeptinib should use the G3 formulation. Regarding potential next steps with luxeptinib, recent therapeutic strategies with CLL B-cell cancer patients typically involve therapy with certain BTK inhibitors in combination with venetoclax (VEN). Drug resistance has begun to emerge in a molecularly defined subgroup of these patients, and the drug resistance has been correlated with mutations in the FLT3 receptor. Although FLT3 mutations are typically associated with AML patients, these R/R CLL prior-BTKi/Prior-VEN/FLT3-mutated patients are difficult to treat and represent a potential commercial market of approximately $200 million by 2039. The Dana Farber Cancer Institute identified this emerging patient population and has requested luxeptinib be tested as part of an investigator sponsored trial in combination with VEN in the R/R CLL prior-BTKi/Prior-VEN/FLT3-mutated patients. Non-clinical studies are underway to position LUX+VEN for the treatment of these patients, and efforts are underway to identify sources of capital to support such a trial to develop LUX for a molecularly defined CLL subpopulation with a high unmet medical need.
PROGRAM UPDATES
Tuspetinib
Tuspetinib is an oral, highly potent, small molecule inhibitor of kinases operative in myeloid malignancies and known to be involved in tumor proliferation, resistance to therapy and differentiation. Preclinical in vitro and in vivo studies suggest that Tuspetinib may be an effective monotherapy and combination therapy in patients with hematologic malignancies including AML. An international Phase 1/2 clinical trial in patients with relapsed or refractory AML is ongoing. The dose escalation portion of this study to date has observed evidence of robust clinical activity, including multiple complete responses in R/R AML patients with various disease genotypes, and no toxicity trends that should prevent further dose escalation.
The FDA granted orphan drug designation to tuspetinib for the treatment of patients with AML in October 2018. Orphan drug designation is granted by the FDA to encourage companies to develop therapies for the treatment of diseases that affect fewer than 200,000 individuals in the United States. Orphan drug status provides research and development tax credits, an opportunity to obtain grant funding, exemption from FDA application fees and other benefits. The orphan drug designation also provides us with seven additional years of marketing exclusivity in this indication.
43
Manufacturing:
Following the Tuspetinib licensing agreement between Aptose and Hanmi on November 4, 2021 (the "Tuspetinib Licensing Agreement"), Aptose received from Hanmi an existing inventory of drug product expected to support continuation of the current Phase 1/2 study. The Company and Hanmi also entered into a separate supply agreement in 2022 for additional production of new drug substance and drug product to support further clinical development. Additional batches of API and drug product have been produced by other companies during 2022 and 2023.
Program Updates at Recent Scientific Forums:
On December 9, 2023, Aptose featured tuspetinib in an oral presentation at the 65th American Society of Hematology (ASH) Annual Meeting and Exposition and announced that a growing body of clinical data for Aptose’s lead compound tuspetinib, demonstrates significant benefit as a single agent and in combination with venetoclax in patients with R/R AML in the ongoing APTIVATE Phase 1/2 study. Data were presented in an oral presentation by lead investigator Naval G. Daver, M.D., Professor, Director Leukemia Research Alliance Program, Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX.
Dr. Daver reported data from more than 100 relapsed/refractory patients from multiple international clinical sites, who had failed prior therapy and then were treated with TUS as a single agent or TUS+VEN. Both TUS and TUS+VEN delivered multiple composite complete remissions (CRc) in this very ill AML population, while maintaining a favorable safety profile across all treated patients. The data demonstrated tuspetinib is active and well tolerated in one of the most challenging and heterogeneous disease settings in oncology – relapsed and refractory AML. Tuspetinib demonstrated broad activity, including activity in patients with FLT3 wild-type AML (accounting for more than 70% of the AML population), FLT3 mutated AML, NPM1 mutated AML, as well as in patients with mutations historically associated with resistance to targeted therapy. Most notably, TUS targets VEN resistance mechanisms, enabling TUS+VEN uniquely to treat the very ill prior-VEN AML population, including both FLT3 mutant and FLT3 wildtype disease. From a broader perspective, the growing body of antileukemic activity, and continued favorable safety profile, support advancement of tuspetinib in a TUS+VEN+HMA triplet for the treatment of frontline newly diagnosed AML patients.”
Dr. Daver also pointed out that while patients on the TUS+VEN therapy are early in their treatment cycles, most achieving a response remained on treatment and that responses have begun to mature as dosing continues. Highlights of Dr. Daver’s ASH oral presentation include:
|
|
|
|
44
On October 29, 2023, Aptose presented two posters related to the clinical and preclinical activity of tuspetinib at the European School of Haematology 6th International Conference: Acute Myeloid Leukemia "Molecular and Translational": Advances in Biology and Treatment, held October 29-31, 2023, in Estoril, Portugal. Clinical findings included 1) data from the APTO-TUS-HV01 clinical trial (the "Food Effect Study") evaluating the pharmacokinetic (PK) properties of tuspetinib in healthy human volunteers in which tuspetinib was administered with our without food, and 2) from an international Phase 1/2 study of tuspetinib as a single agent (TUS) and in combination with venetoclax in patients with R/R AML from across clinical centers in the United States, South Korea, Spain, Australia and other sites. Data from the Food Effect Study in healthy human volunteers demonstrated tuspetinib can be administered with or without food and foresee no clinically meaningful difference in exposure. This is an important finding for patient convenience, as venetoclax is dosed with food and tuspetinib can now be co-administered with venetoclax rather than in staggered dosing. Findings from the Phase 1/2 clinical trial demonstrated tuspetinib as a single agent was well-tolerated and highly active among R/R AML patients with a diversity of adverse genotypes and delivered a 42% CR/CRh cross-evaluable venetoclax (VEN) naive patients at the 80mg daily RP2D. The TUS+VEN doublet has been well tolerated in the APTIVATE international Phase 1/2 expansion trial in R/R AML patients and achieved multiple responses in patients who previously failed venetoclax ("Prior-VEN failure AML"), including Prior-VEN failure patients who also previously failed FLT3 inhibitors, all of whom represent emerging populations of high unmet medical need. Notably, tuspetinib targets venetoclax resistance mechanisms that may re-sensitize Prior-VEN failure patients to venetoclax.
Separate from the clinical studies, the preclinical study (entitled: “Tuspetinib Oral Myeloid Kinase Inhibitor Creates Synthetic Lethal Vulnerability to Venetoclax”) presented by Aptose during the ESH Conference investigated the effects of tuspetinib on key elements of the phosphokinome and apoptotic proteome in both parental and TUS-resistant AML cells. In parental cells, tuspetinib inhibits key oncogenic signaling pathways and shifts the balance of pro- and anti-apoptotic proteins in favor of apoptosis, suggesting that it may generate vulnerability to venetoclax. In addition, acquired resistance in the AML cells to tuspetinib generated a synthetic lethal vulnerability to venetoclax of unusually high magnitude. Concurrent administration of TUS+VEN therefore may discourage the emergence of resistance to tuspetinib during treatment.
In conjunction with poster presentations at the ESH Conference, on October 30, 2023, Aptose held a “Clinical Update and KOL Data Review of AML Drug Tuspetinib” that was webcast and featured Dr. Naval Daver, MD, Professor, Director Leukemia Research Alliance Program, Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, Texas. Dr. Daver is the lead investigator on Aptose’s APTIVATE trial and is recognized for significant achievements in the development of novel AML treatments, including several combination therapies. Aptose presented data in 49 patients who received the TUS+VEN doublet, showing an overall response rate ("ORR") of 48% among all patients that had achieved an evaluable stage, as well as a 44% ORR among Prior-VEN failure AML patients, including FLT3-unmutated ("wildtype") patients (43% ORR) and FLT3-mutated patients (60% ORR), some of whom also had failed prior therapy with FLT3 inhibitors. The TUS+VEN doublet was well tolerated with no unexpected safety signals. The TUS+VEN doublet may serve the Prior-VEN failure R/R AML patients that represent a rapidly growing population that is highly refractory to any salvage therapy. The compelling data with the TUS+VEN doublet in R/R AML patients suggest a TUS+VEN+HMA triplet may also serve the needs of frontline (1L) newly diagnosed AML patients.
Concurrent with the European Hematology Association (EHA) Annual Congress held June 8-11, 2023, Aptose held an interim clinical update webcast on June 10, 2023, to present highlights from the ongoing clinical development of tuspetinib. Aptose reported completion of the tuspetinib dose escalation and dose exploration Phase 1/2 trial in 77 R/R AML patients, tuspetinib demonstrated a favorable safety profile, and tuspetinib delivered monotherapy responses across four dose levels with no dose-limiting toxicity in mutationally diverse and difficult to treat R/R AML populations, including patients with highly adverse mutations that typically do not respond to monotherapy or combination therapy: TP53-mutated patients with a CR/CRh = 20% and RAS-mutated patients with a CR/CRh = 22%. Aptose also reported completion of a successful End of Phase 1 Meeting with the US FDA for tuspetinib, that a monotherapy RP2D was selected as 80mg daily, and that all development paths remain open, including the single arm accelerated path. Following completion of the dose escalation and dose exploration phases of the Phase 1/2 clinical program, Aptose focused attention on the tuspetinib APTIVATE expansion trial. The APTIVATE trial is designed to identify patient populations sensitive to tuspetinib monotherapy that may serve as development paths for single arm accelerated approval and to use the TUS+VEN doublet in R/R AML patients and identify patient populations of unmet need that are sensitive to the TUS+VEN doublet and can serve as development paths for accelerated and full approvals.
45
We reported that patient enrollment in the APTIVATE expansion trial has been brisk and preliminary CR activity had already been reported in patients receiving the TUS+VEN doublet who previously failed therapy with venetoclax. During the interim clinical update webcast Aptose also reviewed clinical findings with the new G3 formulation of luxeptinib. Aptose disclosed that continuous dosing with 50mg of the G3 formulation achieves roughly an equivalent pharmacokinetic profile as 900mg original G1 formulation, and that dose escalation with the G3 formulation was anticipated.
On March 23, 2023, Aptose announced the APTIVATE Phase 1/2 expansion trial with tuspetinib had been initiated and already had treated several R/R AML patients in the monotherapy arm, and that patient enrollment had been initiated in the doublet combination treatment arm of the APTIVATE trial with the TUS+VEN doublet. Since then, patients have continued to enroll and receive tuspetinib on the monotherapy arm. Plus, enrollment and dosing of patients on the TUS+VEN doublet arm have been brisk. Clinical investigator interest for tuspetinib is evident, and early signs of antileukemic activity during the APTIVATE trial have fueled the level of excitement for the trial.
Clinical responses to monotherapy with tuspetinib have been observed in a broad range of mutationally defined populations, including those with mutated forms of NPM1, MLL, TP53, DNMT3A, RUNX1, wild-type FLT3, ITD or TKD mutated FLT3, various splicing factors, and other genes. In the March 23, 2023, announcement, Aptose also highlighted an unexpected observation of a 29% CR/CRh response rate with tuspetinib monotherapy in R/R AML patients having mutations in the RAS gene or other genes in the RAS pathway. Responses in RAS-mutated patients are important because the RAS pathway is often mutated in response to therapy by other agents as the AML cells mutate toward resistance to those other agents. Collectively, these observations of broad clinical activity of tuspetinib, along with its favorable safety profile, position tuspetinib for potential accelerated development paths, as well as for doublet, triplet and maintenance therapy indications.
On January 30, 2023, Aptose announced dosing of patients in the APTIVATE Phase 1/2 clinical trial of tuspetinib, and that another clinical response has been achieved by a R/R AML patient receiving 40 mg tuspetinib once daily orally in the original dose exploration trial, the second response at the recently launched low-dose 40 mg cohort. In addition, Aptose elucidated a rationale for the superior safety profile of tuspetinib. While several kinase inhibitors require high exposures that exert near complete suppression of a single target to elicit responses, those agents often cause additional toxicity because they also cause extensive inhibition of that target in normal cells. In contrast, tuspetinib simultaneously suppresses a small suite of kinase-driven pathways critical for leukemogenesis. Consequently, tuspetinib achieves clinical responses at lower exposures with less overall suppression of each pathway, thereby avoiding many of the toxicities observed with competing agents.
46
Tuspetinib has completed the dose escalation and dose exploration phases of an international Phase 1/2 study in patients with relapsed or refractory AML across clinical centers in the United States and South Korea. Clinical data from tuspetinib in AML were presented at the ASH Annual Meeting in December 2022 and presented during a Corporate Comprehensive Clinical Update Call held December 11, 2022. Data presented demonstrated that tuspetinib delivers single agent responses without prolonged myelosuppression or life-threatening toxicities in these very ill and heavily pretreated relapsed or refractory AML patients. Responses were observed in a broad range of mutationally-defined populations, including those with mutated forms of NPM1, MLL, TP53, NRAS, KRAS, DNMT3A, RUNX1, wild-type FLT3, ITD or TKD mutated FLT3, various splicing factors, and other genes As of October 6, 2022, 60 heavily pretreated R/R AML patients were enrolled at multiple centers and treated at doses escalating from 20 mg to 200 mg, with further dose exploration at the 40 mg, 80 mg, 120 mg and 160 mg dose levels. Tuspetinib delivered multiple CRs at 40 mg, 80 mg, 120 mg and 160 mg dose levels in which no DLTs were observed. Tuspetinib demonstrated clinically meaningful benefit in all responders, by either bridging successfully to HSCT or leading to a durable response, as well as a favorable safety profile. In addition to 5 CRc and 1 PR reported at ASH 2021, 4 new CRc and 3 new PR had been generated during 2022. New responses during 2022 were achieved with 160 mg,120 mg, 80 mg, and 40 mg. Among efficacy-evaluable patients treated with 80 mg, 120 mg, or 160mg, the following response rates ranging from 19% to 75% were achieved in specific genotypic subpopulations of r/r AML patients. Significant bone marrow leukemic blast reductions were observed broadly in FLT3+ and FLT3 wildtype patients across multiple dose levels, comparable to reported gilteritinib data, but in more heavily pre-treated relapsed and refractory AML patients. Vignettes of patient experiences highlight the potency and breadth of tuspetinib to deliver complete remissions among several mutationally-defined populations with a diversity of adverse mutations. Tuspetinib continued to show a favorable safety profile with only mild AEs and no DLTs up to 160 mg per day, and no drug discontinuations from drug-related toxicity. No drug-related SAE, drug-related deaths, differentiation syndrome, AE of QT prolongation or DLT were observed through the 160 mg level. Tuspetinib avoids many of the typical toxicities observed with other tyrosine kinase inhibitors. Aptose identified a safe therapeutic range with a broad therapeutic window, spanning the dose levels of 40, 80, 120 and 160 milligrams. Aptose also announced that enrollment had been initiated in the APTIVATE expansion trial for monotherapy and drug combination therapy with tuspetinib. For the APTIVATE expansion trial, Aptose selected 120 mg as the initiating single agent expansion dose and 80 mg as the initiating dose selected for combination with venetoclax.
At the European Hematology Association Annual Congress 2022 held June 9-12, 2022, Aptose presented preclinical data from tuspetinib in a poster entitled “Myeloid Kinome Inhibitor HM43239 Overcomes Acquired Resistance in Acute Myeloid Leukemia Models.” Oral HM43239 potently inhibits kinases that drive AML, including SYK, diverse forms of the FLT3, JAK1 and JAK2, and mutant forms of the c-KIT kinases. The SYK and JAK1/2 intracellular kinases and the FLT3 (mutated and wildtype) and cKIT (mutated) receptor kinases mediate oncogenic signaling pathways in AML that can drive malignant proliferation and promote drug resistance to certain drugs. Tuspetinib was developed to overcome shortcomings of other drugs, such as simple SYK inhibitors and approved inhibitors of FLT3. These preclinical findings support the continued clinical development of tuspetinib for the treatment of multiple AML populations, particularly those who have failed other therapies.
Major conclusions include
47
Luxeptinib
Indication and Clinical Trials:
Luxeptinib is currently being evaluated in a Phase 1 a/b trial in patients with relapsed or refractory B cell malignancies who have failed or are intolerant to standard therapies, and in a separate Phase 1 a/b trial in patients with relapsed or refractory AML or high-risk MDS. During 2022, a new G3 formulation was tested as a single dose in 20 patients during the ongoing Phase 1 a/b clinical program. Modeling of the PK properties of G3 predicted steady-state plasma exposure from continuous dosing with 50 mg of G3 (every 12 hours, Q12h) should be comparable to that of 900 mg of the original G1 formulation Q12h, representing a significant improvement in bioavailability with G3. On November 14, 2022, Aptose announced dosing of the first AML patient to receive a continuous dosing regimen of the G3 formulation (50 mg G3 Q12h), with the protocol allowing for further dose escalation of G3 in subsequent patients. Clinical data from both studies were presented during a Corporate Comprehensive Clinical Update Call held December 11, 2022. During the Corporate Update Call, Aptose announced a CR was achieved with a DLBCL patient at the end of Cycle 22 with 900mg BID of the original G1 formulation. Previously, an MRD-negative CR was reported with a R/R AML patient receiving 450mg BID of the original G1 formulation. Aptose expects that 9-15 patients will determine if G3 is safe and achieves desired exposures to deliver clinical responses.
Manufacturing:
During fiscal years 2017 and 2018, we created a scalable chemical synthetic route for the manufacture of luxeptinib drug substance and have scaled the manufacture of API to multi-kg levels, we completed the manufacture of a multi-kg batch of API under GMP conditions as our API supply for our first-in-human clinical trials, and we manufactured under GMP conditions two dosage strengths of capsules to serve as our clinical supply in those human studies. During fiscal 2019 and 2020, we completed successful manufacture of multiple batches of API and drug product, and planned numerous GMP production campaigns to supply the ongoing trial and planned trials into the future. To date we have been able to manufacture API and capsules to support clinical supplies under GMP conditions. In fiscal 2021 and 2022 we continued our manufacturing campaigns and scale-up and tech transfer activities to support additional manufacturing capacity for the ongoing and planned clinical trials of luxeptinib. Additional research and development funds were utilized to support the development of the G3 formulation of luxeptinib. Now that the G3 formulation has been successfully manufactured and has demonstrated encouraging PK properties, the manufacture of additional batches of the first and second generation formulation of luxeptinib are discontinued, and the amount of drug substance manufacturing is reduced.
Publication of Peer-Reviewed Research Articles Related to Luxeptinib:
During the first quarter of 2023, Aptose and its scientific collaborators published a peer reviewed research article, entitled “Luxeptinib interferes with LYN-mediated activation of SYK and modulates BCR signaling in lymphoma,” in the online journal PLOS ONE. The article elucidates the mechanism by which luxeptinib suppresses the B-cell receptor and compares it to BTK inhibitor ibrutinib. Results showed that both luxeptinib and ibrutinib are potent inhibitors of recombinant forms of BTK but have different efficacy profiles and effects on BTK activity. Luxeptinib was more effective than ibrutinib at reducing both steady state and anti-IgM-induced phosphorylation of the LYN and SYK kinases upstream of BTK where IB has little or no effect.
During the first quarter of 2022, three separate peer-reviewed research articles were published that presented preclinical data related to the application of luxeptinib to the treatment of AML, certain B-cell lymphomas and inflammation. These publications contribute to the body of preclinical data demonstrating luxeptinib’s activity as a lymphoid and myeloid kinome inhibitor, and now as an inflammation kinome inhibitor, and support its continued clinical development in several therapeutic areas.
Program Updates at Recent Scientific Forums:
On December 11, 2021, we presented clinical updates from luxeptinib in patients with R/R B-cell malignancies and R/R AML in two virtual poster presentations at the 63rd ASH Annual Meeting (A Phase 1 a/b Dose Escalation Study of the Mutation Agnostic BTK/FLT3 Inhibitor Luxeptinib (CG-806) in Patients with Relapsed or Refractory B-Cell Malignancies; A Phase 1 a/b Dose Escalation Study of the Mutation Agnostic FLT3/BTK Inhibitor Luxeptinib
48
(CG-806) in Patients with Relapsed or Refractory Acute Myeloid Leukemia. The presentations highlighted that in both of these Phase 1/2 studies luxeptinib has been generally well tolerated at dose levels of 450, 600 and 750 mg BID over multiple cycles, and that patients already were being dosed at the 900 mg level. Target engagement of BTK and FLT3, and anti-tumor activity, including dose- and exposure-dependent tumor reductions, have been observed in multiple patients collectively between the studies, including in patients with follicular lymphoma, diffuse large B-cell lymphoma, CLL/SLL, and AML.
We have completed several non-clinical and clinical studies that demonstrate the highly differentiated profile of luxeptinib. Key studies that have been presented at scientific forums are as follows:
49
Other corporate matters
Nasdaq Notice and Reverse Stock Split
On July 18, 2022, we received a letter from the Nasdaq Stock Market, LLC (“Nasdaq”) indicating that, for the last 30 consecutive business days, the bid price for our Common Shares had closed below the minimum $1.00 per share required for continued inclusion on the Nasdaq Capital Market under the Nasdaq Listing Rules. The notice had no effect on the listing of our Common Shares.
On May 23, 2023, our shareholders voted to approve special resolutions providing for an amendment to our articles of incorporation to effect the Reverse Stock Split at a ratio in the range of 1-for-10 to 1-for-20. Our Board of Directors then approved a ratio of 1-for-15 on May 23, 2023. On May 24, 2023, we filed articles of amendment under the CBCA to give effect to the Reverse Stock Split (consolidation) of our Common Shares on the basis of one post-consolidation Common Share for each 15 pre-consolidation Common Shares. The Common Shares commenced trading on a post-Reverse Stock Split basis at market open on Tuesday, June 6, 2023. The Reverse Share Split was primarily intended to bring us into compliance with the minimum bid price requirement for maintaining the listing of the Common Shares on Nasdaq and to make the bid price more attractive to investors. On June 21, 2023, Nasdaq confirmed that we had regained compliance with the minimum bid price requirement. All references in this report to historical Common Share prices, numbers of Common Shares, and earnings per share calculations have been presented to reflect the effect of the Reverse Stock Split.
On February 29, 2024, we received a deficiency letter (the “2024 Deficiency Letter”) from the Nasdaq Listing Qualifications Department of The Nasdaq Stock Market LLC (“Nasdaq”) notifying the Company that the Company’s January 2024 Private Placement of securities to Hanmi violated 5635(d) because the Company did not obtain shareholder approval prior to such issuance. Nasdaq stated that the Private Placement involved the issuance of greater than 20% of the issued and outstanding Common Shares of the Company at a discount to the Nasdaq official closing price on January 25, 2024, the date of the subscription agreement between the Company and Hanmi. The 2024 Deficiency Letter has no immediate effect on the listing of the Company’s Common Shares. In accordance with the Nasdaq Listing Rules, the Company has been given forty-five (45) calendar days, or until April 14, 2024, to submit a plan to regain compliance. If Nasdaq accepts the Company’s plan to regain compliance, Nasdaq can grant an extension of up to 180 calendar days from the date of the Deficiency Letter to evidence compliance. Although the Company believes that the Private Placement was completed in accordance with the Nasdaq Listing Rules, the Company respects Nasdaq’s query and intends to work with Nasdaq to resolve Nasdaq’s concerns and will consider available options to regain compliance. However, there can be no assurance that the Company will be able to regain compliance with the applicable Nasdaq Listing Rules.
50
LIQUIDITY AND CAPITAL RESOURCES
We are an early-stage development company and we currently do not earn any revenues from our drug candidates. The continuation of our research and development activities and the commercialization of the targeted therapeutic products are dependent upon our ability to successfully finance and complete our research and development programs through a combination of equity financing and payments from strategic partners. We have no current sources of significant payments from strategic partners. As of the filing date, we have sufficient liquidity to support the Company's operations until August 2024.
Sources of liquidity:
The following table presents our cash and cash equivalents, investments and working capital as of December 31, 2023 and 2022.
(in thousands) |
| Balances at December 31, 2023 |
|
| Balances at December 31, 2022 |
| ||
Cash and cash equivalents |
| $ | 9,252 |
|
| $ | 36,970 |
|
Investments |
|
| — |
|
|
| 9,989 |
|
Total |
| $ | 9,252 |
|
| $ | 46,959 |
|
Working capital |
| $ | (3,375 | ) |
| $ | 37,235 |
|
Working capital is a non-GAAP measure and represents cash, cash equivalents, investments, prepaid expenses and other current assets less current liabilities.
As of December 31, 2023, we reported negative shareholder's equity of $2.9 million (December 31, 2022, positive shareholder's equity of $37.7 million). In order for the Company to meet its capital requirements, and continue to operate, additional financing will be necessary. The Company is evaluating strategies to obtain the required additional funding for future operations. These strategies may include, but are not limited to, obtaining equity financing, and restructuring of operations to decrease expenses. However, given the challenges in the U.S. and global financial markets, and the matter in Note 17 to the Financial Statements, Subsequent events, that may impact the Company's ability to raise financing in the capital markets, the Company may be unable to access further equity or when needed, if at all. As the Company is primarily pursuing one compound that is licensed from a related party with significant licensing payments who will have influence on the Company, other investors may not be willing to invest in the Company. As such, there can be no assurance that the Company will be able to obtain additional liquidity when needed or under acceptable terms, if at all. The consolidated financial statements do not reflect any adjustments to the carrying amounts and classification of assets, liabilities, and reported expenses that may be necessary if the Company were unable to continue as a going concern. Such adjustments may be material. If necessary funds are not available, we may have to delay, reduce the scope of, or eliminate some of our development programs, potentially delaying the time to market for any of our product candidates.
Our cash needs for the next twelve months include estimates of the number of patients and rate of enrollment of our clinical trials, the amount of drug product that we will require to support our clinical trials, and our general corporate overhead costs to support our operations, and our reliance on our manufacturers. We have based these estimates on assumptions and plans which may change and which could impact the magnitude and/or timing of operating expenses and our cash runway.
Since our inception, we have financed our operations and technology acquisitions primarily from equity financing, proceeds from the exercise of warrants and stock options, and interest income on funds held for future investment.
51
Hanmi Investment September 2023
On August 10, 2023, the Company entered into a binding term sheet with Hanmi whereby Hanmi agreed to invest, at their sole discretion, up to a maximum of $7 million in Aptose up to a total ownership of 19.99 percent of Aptose by Hanmi. On September 6, 2023, the Company entered into a subscription agreement with Hanmi, pursuant to which the Company sold 668,449 Common Shares to Hanmi for gross proceeds of $3 million. Hanmi held 884,152 Common Shares of Aptose as of December 31, 2023. Hanmi subsequently invested $4 million in Aptose as noted below. See below, "January 2024 Public Offering Financing and Concurrent Hanmi Investment." In addition, see Note 17 to the Financial Statements, Subsequent events.
2023 Committed Equity Facility
On May 25, 2023, the Company and Keystone Capital Partners, LLC ("Keystone") entered into a committed equity facility, (the "2023 Committed Equity Facility"), which provides that subject to the terms and conditions set forth therein, we may sell to Keystone up to the lesser of (i) $25.0 million of the Common Shares and (ii) a number of Common Shares equal to 19.99% of the Common Shares outstanding immediately prior to the execution of the 2023 Committed Equity Facility Agreement with Keystone which respect to the 2023 Committed Equity Facility (subject to certain exceptions) (the “Total Commitment”), from time to time during the 24-month term of the 2023 Committed Equity Facility. Additionally, on May 25, 2023, the Company entered into a Registration Rights Agreement with Keystone, pursuant to which the Company agreed to file a registration statement with the SEC covering the resale of Common Shares that are issued to Keystone under the 2023 Committed Equity Facility. This registration statement became effective on June 30, 2023 and the 2023 Committed Equity Facility commencement date was July 12, 2023 (the "Commencement Date").
Upon entering into the 2023 Committed Equity Facility, the Company agreed to issue to Keystone an aggregate of 25,156 Common Shares (the “Commitment Shares”) as consideration for Keystone’s commitment to purchase Common Shares upon the Company’s direction under the 2023 Committed Equity Facility. The Company issued 7,547 Common Shares, or 30% of the Commitment Shares, on the date of the 2023 Committed Equity Facility Agreement (the “Initial Commitment Shares”). An additional 7,547 Common Shares, or 30% of the Commitment Shares, were issued to Keystone 90 days following the Commencement Date (the “First Back-End Commitment Shares”). The remaining 10,062 Common Shares, or 40% of the Commitment Shares, were issued to Keystone subsequent to December 31, 2023 (the “Second Back-End Commitment Shares”). See Note 17 to the Financial Statements, Subsequent events.
In the year ended December 31, 2023, the Company's sales of Common Shares to Keystone comprised 720,494 Common shares sold to Keystone at an average price of $2.91 per Common share for gross cash proceeds of $2.1 million. In addition, 15,094 Commitment Shares were issued in 2023 and the remaining 10,062 Commitment Shares were issued in January 2024.
January 2024 Public Offering Financing and Concurrent Hanmi Investment
On January 31, 2024, the Company announced the closing of a $9.7 million public offering (the “Public Offering”) and a $4 million private placement (the “Private Placement”) with Hanmi Pharmaceutical. The Public Offering comprised 5,649,122 Common Shares and warrants at a combined offering price of US $1.71 per share. This included 736,842 Common Shares and warrants pursuant to a full exercise by the underwriter of its over-allotment option (the “Over-Allotment Option”). The Private Placement with Hanmi comprised 2,105,263 Common Shares of the Company sold at a price of $1.90, representing an 11% premium over the price of the Common Shares issued as part of the Public Offering. Financing costs included underwriting costs of 7% and approximately $400,000 in professional fees. The Company also issued Hanmi warrants to purchase Common Shares at an exercise price of $1.71 per Warrant Share. The total number of Common Shares outstanding after the closing of the Public Offering, including the Over-Allotment Option, and Private Placement was 15,706,810 and warrants outstanding were 8,332,163. As of the filing date, the total Common Share ownership by Hanmi was 2,989,414, representing ownership of 19.03% of the outstanding Common Shares of the Company. Hanmi also owns 2,339,181 warrants to purchase Common Shares at an exchange price of $1.71 per Warrant Share.
52
At-The-Market Facilities
On December 9, 2022, the Company entered into an equity distribution agreement pursuant to which the Company may, from time to time, sell Common Shares having an aggregate offering value of up to $50 million through Jones Trading Institutional Services LLC ("Jones Trading") on Nasdaq (the "2022 ATM Facility"). During the year ended December 31, 2022, the Company issued 4,836 Common Shares under this 2022 ATM Facility at an average price of $10.81 per Common Share for gross proceeds of $52 thousand ($51 thousand net of share issuance costs).management. During the year ended December 31, 2023, independent directors met six times without the Company issued 336,690 Common Shares under this 2022 ATM Facility at an average price of $5.62 for gross proceeds of $1.9 million ($1.8 million net of share issuance costs). As of December 31, 2023, the Company had raised a total of $1.9 million gross proceeds ($1.9 million net of share issuance costs) under the 2022 ATM Facility. Costs associated with the proceeds consisted of 3% cash commission.
On May 5, 2020, the Company entered an “at-the-market” equity distribution agreement with Piper Sandler & Co. (“Piper Sandler”) and Canaccord Genuity LLC (“Canaccord Genuity”) acting as co-agents (the “2020 ATM Facility”). Under the termspresence of the 2020 ATM Facility, the Company could, from time to time, sell Common Shares having an aggregate offering value of up to $75 million through Piper Sandler and Canaccord Genuity on Nasdaq. During the period from January 1, 2022 to October 31, 2022, the date the Agreement was terminated, the company issued During the year ended December 31, 2022, the Company issued 3,646 Common Shares under the 2020 ATM Facility at an average price of $14.25 per Common Share for gross proceeds of $52 thousand ($50 thousand net of share issuance costs). As of October 31, 2022, the date the 2020 ATM Facility was terminated, the Company had raised a total of $89 thousand gross proceeds ($86 thousand net of share issuance costs) under the 2020 ATM Facility. Costs associated with the proceeds consisted of a 3% cash commission.
2022 Base Shelf
In October 2022, we filed a short form base shelf prospectus (the 2022 “Base Shelf”) that allows us to distribute, upon the filing of prospectus supplements, up to $200,000,000 of Common Shares, warrants, or units comprising any combination of Common Shares and warrants. The Base Shelf was declared effective by the SEC on October 21, 2022 and expires on October 7, 2025.
Our ability to raise additional funds could be affected by adverse market conditions, the status of our product pipeline, and various other factors and we may be unable to raise capital when needed, or on terms favorable to us. If the necessary funds are not available, we may need to delay, reduce the scope of, or eliminate some of our development programs, potentially delaying the time to market for any of our product candidates. As of the filing date, we have sufficient liquidity to support the Company's operations until August 2024.
Cash flows:
The following table presents a summary of our cash flows for the years ended December 31, 2023 and 2022:
| For the Years Ended, |
| ||||||
(in thousands) |
| December 31, 2023 |
|
| December 31, 2022 |
| ||
Net cash provided by (used in): |
|
|
|
|
|
| ||
Operating activities |
| $ | (44,590 | ) |
| $ | (32,322 | ) |
Investing activities |
|
| 9,960 |
|
|
| 30,066 |
|
Financing activities |
|
| 6,910 |
|
|
| 116 |
|
Effect of exchange rates changes on cash and |
|
| 2 |
|
|
| (4 | ) |
Net (decrease)/increase in cash and |
| $ | (27,718 | ) |
| $ | (2,144 | ) |
53
Cash used in operating activities:
Our cash used in operating activities for the years ended December 31, 2023 and 2022 was approximately $44.6 million and $32.3 million, respectively. Our uses of cash for operating activities for both years consisted primarily of salaries and wages for our employees, facility and facility-related costs for our offices and laboratories, fees paid in connection with preclinical and clinical studies, drug manufacturing costs, laboratory supplies and materials, and professional fees. Net cash used in operating activities was higher in the year ended December 31, 2023 as compared with the year ended December 31, 2022 resulting mostly from higher operating expenses, as discussed further below. See “Results of Operations.”
We do not expect to generate positive cash flow from operations for the foreseeable future due to additional research and development costs, including costs related to drug discovery, preclinical testing, clinical trials, and manufacturing, as well as operating expenses associated with supporting these activities, and potential milestone payment to our collaborators. It is expected that negative cash flow will continue until such time, if ever, that we receive regulatory approval to commercialize any of our products under development and/or royalty or milestone revenue from any such products exceeds expenses.
Cash flow from investing activities:
Our cash provided by investing activities for the years ended December 31, 2023 and December 31, 2022 was $10.0 million and $30.1 million, consisting mainly of proceeds from the maturity of investments.
The composition and mix of cash, cash equivalents and investments is based on our evaluation of conditions in financial markets and our near-term liquidity needs. We have exposure to credit risk, liquidity risk and market risk related to our investments. The Company manages credit risk associated with its cash and cash equivalents and investments by maintaining minimum standards of R1‑low or A‑low investments. The Company invests only in highly rated corporations and treasury bills which are capable of prompt liquidation. The Company manages its liquidity risk by continuously monitoring forecasts and actual cash flows. The Company is subject to interest rate risk on its cash and cash equivalents and investments. The Company does not believe that the results of operations or cash flows would be affected to any significant degree by a sudden change in market interest rates relative to interest rates on the investments, owing to the relative short‑term nature of the investments.
Cash flow from financing activities:
Our cash flow from financing activities for the year ended December 31, 2023 was approximately $6.9 million, and consisted of $3.0 million in proceeds from shares issued to Hanmi, $2.1 million in proceeds from the Committed Equity Facility, $1.8 million from shares issued from the 2022 ATM facility, and $29 thousand from the issuance of shares under the ESPP plan.
Our cash flow from financing activities for the year ended December 31, 2022 was approximately $116 thousand, and consisted of proceeds from the from shares issued from the 2022 ATM Facility of approximately $51 thousand, proceeds from shares issued from the 2020 ATM Facility of $50 thousand, and proceeds from the exercise of stock options of $15 thousand.
Contractual Obligations and Off-Balance Sheet Financing
As of December 31, 2023, we have not entered into any off-balance sheet arrangements.
The Company enters into research, development and license agreements in the ordinary course of business where the Company receives research services and rights to proprietary technologies. Milestone and royalty payments that may become due under various agreements are dependent on, among other factors, clinical trials, regulatory approvals and ultimately the successful development of a new drug, the outcome and timing of which is uncertain.
54
On November 4, 2021, the Company entered into the Tuspetinib Licensing Agreement with Hanmi for exclusive global rights to its compound named tuspetinib. Under the Tuspetinib Licensing Agreement, the Company has maximum obligations for clinical development and global regulatory milestones totaling $64.5 million for the first potential clinical indication of tuspetinib, $34 million for the second indication, and $29 million for the third indication. The Company has maximum obligations for tiered global sales-based milestones totaling $280 million. The Company also has an obligation for tiered royalty payments on global sales of commercialized product. The timing of any milestone or royalty payments that may become due is not yet determinable.
Under the license agreement with CG with regards to the Rights (other than the China Rights), the Company has obligations for development milestones of $16 million related to the initiation of Phase 2 and pivotal clinical trials, and regulatory milestones totaling $44 million. The Company also has an obligation to pay royalty payments on sales of commercialized product. The timing of any milestone or royalty payments that may become due is not yet determinable.
Under the license agreement with CG with regards to the China Rights, we entered into a license agreement with CG to gain an exclusive license to luxeptinib in China (including the People’s Republic of China, Hong Kong and Macau). The Company has future obligations of development milestones of $6 million related to approval of an IND and to the initiation of Phase 2 and pivotal clinical trials, and regulatory milestones totaling $20 million. The Company also has an obligation to pay sales milestones and royalty payments on sales of commercialized product. The timing of any milestone or royalty payments that may become due is not yet determinable.
RESULTS OF OPERATIONS
A summary of the results of operations for the years ended December 31, 2023 and 2022 is presented below:
| Year ended December 31, |
| ||||||
(in thousands except per Common Share data) |
| 2023 |
|
| 2022 |
| ||
|
|
|
|
|
|
| ||
Revenues |
| $ | - |
|
| $ | - |
|
Research and development expenses |
|
| 36,765 |
|
|
| 28,088 |
|
General and administrative expenses |
|
| 15,591 |
|
|
| 14,514 |
|
Net finance income |
|
| 1,149 |
|
|
| 779 |
|
Net loss |
| $ | (51,207 | ) |
| $ | (41,823 | ) |
Unrealized gain/(loss) on securities available-for-sale |
|
| 2 |
|
|
| (2 | ) |
Total comprehensive loss |
| $ | (51,205 | ) |
| $ | (41,825 | ) |
Basic and diluted loss per Common Share |
| $ | (7.58 | ) |
| $ | (6.80 | ) |
Net loss of $51.2 million for the year ended December 31, 2023 increased by approximately $9.4 million as compared with $41.8 million for the year ended December 31, 2022, primarily as of a result of an increase in research and development program costs and personnel expenses of $8.7 million, and a $1.1 million increase in general and administrative costs.
Research and Development Expenses
Research and development expenses consist primarily of costs incurred related to the research and development of our product candidates. Costs include the following:
55
We have ongoing Phase 1 clinical trials for our product candidates tuspetinib and luxeptinib. Tuspetinib was licensed into Aptose in November 2021 and we assumed sponsorship, and the related costs, of the tuspetinib study effective January 1, 2022. In December 2021, we discontinued the APTO-253 program.
We expect our research and development expenses to be higher for the foreseeable future as we continue to advance tuspetinib into larger clinical trials.
The research and development (“R&D”) expenses for the years ended December 31, 2023 and 2022 were as follows:
| Year ended December 31, |
| ||||||
(in thousands) |
| 2023 |
|
| 2022 |
| ||
Program costs – Tuspetinib |
| $ | 24,925 |
|
| $ | 10,083 |
|
Program costs – Luxeptinib |
|
| 3,510 |
|
|
| 8,426 |
|
Program costs – APTO-253 |
|
| 40 |
|
|
| 141 |
|
Personnel expenses |
|
| 6,878 |
|
|
| 7,181 |
|
Stock-based compensation |
|
| 1,373 |
|
|
| 2,218 |
|
Depreciation of equipment |
|
| 39 |
|
|
| 39 |
|
| $ | 36,765 |
|
| $ | 28,088 |
| |
R&D expenses increased by $8.7 million to $36.8 million for the year ended December 31, 2023 as compared with $28.1 million for the comparative period in 2022. Changes to the components of our R&D expenses presented in the table above are primarily as a result of the following activities:
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits and travel, including stock-based compensation for our executive, finance, business development, human resource, and support functions. Other general and administrative expenses and professional fees for auditing, and legal services, investor relations and other consultants, insurance and facility related expenses.
We expect that our general and administrative expenses will increase for the foreseeable future as we incur additional costs associated with being a publicly traded company and to support our expanding pipeline of activities. We also expect our intellectual property related legal expenses to increase as our intellectual property portfolio expands.
56
The general and administrative expenses for the years ended December 31, 2023 and 2022 are as follows:
| Year ended December 31, |
| ||||||
(in thousands) |
| 2023 |
|
| 2022 |
| ||
General and administrative, excluding items below: |
| $ | 13,262 |
|
| $ | 11,444 |
|
Stock-based compensation |
|
| 2,280 |
|
|
| 2,989 |
|
Depreciation of equipment |
|
| 49 |
|
|
| 81 |
|
| $ | 15,591 |
|
| $ | 14,514 |
| |
General and administrative expenses for the year ended December 31, 2023 were approximately $15.6 million as compared with $14.5 million for the comparative period in 2022, an increase of approximately $1.1 million. The increase was primarily as a result of higher salaries expenses, higher travel expenses, and higher professional fees, partly offset by a decrease in stock-based compensation costs of $709 thousand.
Stock-based compensation decreased by approximately $709 thousand mostly as a result of a lower number of options granted in the year ended December 31, 2023, with those options having a lower grant date fair value as compared with the options granted in the comparative period, and additional compensation recognized in the comparative period for modifications made to then vested and unvested stock options for one former company officer,management non-independent director as part of a separation and release agreement.
CRITICAL ACCOUNTING POLICIES
Critical Accounting Policies and Estimates
We periodically review our financial reporting and disclosure practices and accounting policies to ensure that they provide accurate and transparent information relative to the current economic and business environment. As part of this process, we have reviewed our selection, application and communication of critical accounting policies and financial disclosures. A “critical accounting policy” is one which is both important to the portrayal of our financial condition and results and requires management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Management has discussed the development and selection of the critical accounting policies with the Audit Committeemeetings of the Board, and members of the Audit Committee has reviewedand Corporate Governance and Nominating Committee each met four times without the disclosure relating to critical accounting policiespresence of management as part of meetings of their committees. All meetings of the Compensation Committee in this MD&A. For additional information, please see2023 were held without the discussionpresence of our significant accounting policies in Note 2 tomanagement (including the Financial Statementsnon-independent director).
Significant accounting judgments and estimates
Management’s assessment of our ability to continue as a going concern involves making a judgment, at a particular point in time, about inherently uncertain future outcomes and events or conditions. Please see the “Liquidity and Capital Resources” section in this document for a discussion of the factors considered by management in arriving at its assessment. The critical accounting policies, judgments and estimates made by management are the estimates related to prepaid and accrued R&D activities.
Research and Development Activities:
R&D costs are expensed as incurred. R&D costs consist primarily of salaries and benefits, stock-based compensation, manufacturing, contract services, clinical trials, and research related overhead. Non-refundable advance payments for goods and services that will be used in future research are recorded in prepaid and other assets and are expensed when the services are performed.
57
The Company records expenses for research and development activities based on Management’s estimates of services received and efforts expended pursuant to contracts with vendors that conduct research and development on the Company’s behalf. The financial terms vary from contract to contract and may result in uneven payment flows as compared with services performed or products delivered. As a result, the Company is required to estimate research and development expenses incurred during the period, which impacts the amount of accrued expenses and prepaid balances related to such costs as of each balance sheet date. Management estimates the amount of work completed through discussions with internal personnel and the contract research and contract manufacturing organizations as to the progress or stage of completion of the services. The Company’s estimates are based on a number of factors, including the Company’s knowledge of the status of each of the research and development project milestones, and contract terms together with related executed change orders. Management makes significant judgments and estimates in determining the accrued balance at the end of each reporting period.
Although Management does not expect our estimates to be materially different from amounts actually incurred, if the estimates of the status and timing of services performed differ from the actual status and timing of services performed, it could result in the Company reporting amounts that are too high or too low in any particular period. As of December 31, 2023, the Company has recorded approximately $0.7 million in prepaid expenses and approximately $6.5 million in accrued liabilities related to its research and development activities. If the estimates are too high or too low by a factor of 10% this would mean that prepaid expenses would be over or understated by approximately $70 thousand, and accrued liabilities would be over or understated by approximately $650 thousand. On a combined basis, this could mean an increase or decrease in research and development expenses by approximately $720 thousand. To date, there have been no material differences between the estimates of such expenses and the amounts actually incurred.
Updated share information
As of March 26, 2024, we had 15,717,701 Common Shares issued and outstanding. In addition, there were 1,504,636 Common Shares issuable upon the exercise of outstanding stock options.
ITEM 7A. QUALITATIVE AND QUANTITATIVE DISCLOSURES ABOUT MARKET RISK
Under SEC rules and regulations, as a smaller reporting company, we are not required to provide this information.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and supplementary data required pursuant to this items are included in Item 15 of this Annual Report and are presented beginning on page F-1 of this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
As of the end of our fiscal year ended December 31, 2023, an evaluation of the effectiveness of our “disclosure controls and procedures” (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) was carried out by our management, with the participation of our principal executive officer and principal financial officer. Based upon that evaluation, our principal executive officer and principal financial officer have concluded that as of the end of that fiscal year, our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in SEC rules and forms and (ii) accumulated and communicated to our management, including our principal executive officer and principal financial officers, to allow timely decisions regarding required disclosure.
58
It should be noted that while our principal executive officer and principal financial officer believe that our disclosure controls and procedures provide a reasonable level of assurance that they are effective, they do not expect that our disclosure controls and procedures or internal control over financial reporting will prevent all errors or fraud. A control system, no matter how well conceived or operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as such term is defined in Exchange Act Rule 13a-15(f). Internal control over financial reporting is a process designed under the supervision and with the participation of our management, including our principal executive and financial officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
As of December 31, 2023, our management assessed the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013 Framework). Based on this assessment, our management concluded that, as of December 31, 2023, our internal control over financial reporting was effective based on those criteria. We are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K under the Securities Act. For as long as we continue to be a smaller reporting company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not smaller reporting companies.
CHANGES IN INTERNAL CONTROL OVER FINANCIAL REPORTING
There were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) under the 1934 Act) during our fiscal quarter ended December 31, 2023, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
59
PART III.
Certain information required by Part III of this Annual Report on Form 10-K is omitted from this report because we are incorporating by reference to the definitive Proxy Statement for our 2024 Annual Meeting of Shareholders, referred to as the Proxy Statement, which will be filed with the SEC within 120 days of the 2023 fiscal year-end.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “Election of Directors,” “Nomination of Directors,” and “Corporate Governance – Board Committees,” except for the information required with respect to our executive officers, which has been included under the heading “Executive Officers” in Item 1, Part I of this Form 10-K, and is incorporated herein by reference, and except for information on our code of ethics.Ethical Business Conduct
We have adopted a code of ethics for directors, officers (including our principal executive officer, principal financial officer and principal accounting officer) and employees, known as the Code of Business Conduct and Ethics. The Code of Business Conduct and Ethics is available on our website at http://www.aptose.com under the Corporate Governance section of our Investor RelationsInvestors page. We will promptly disclose on our website (i) the nature of any amendment to the policy that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and (ii) the nature of any waiver, including an implicit waiver, from a provision of the policy that is granted to one of these specified individuals that is required to be disclosed pursuant to SEC rules and regulations, the name of such person who is granted the waiver and the date of the waiver.
The Corporate Governance and Nominating Committee regularly monitors compliance with the Code through communications with management and reports through the Disclosure and Insider Trading Policy (as described below) and ensures that management of the Corporation encourages and promotes a culture
11
of ethical business conduct. A copy of the Code may be found by accessing the SEC’s EDGAR filing database at www.sec.gov, on SEDAR+ at www.sedarplus.ca and on our website at www.aptose.com.
The Corporation has developed a Disclosure and Insider Trading Policy that covers “whistle blowing” and provides an anonymous means for employees and officers to report violations of the Code or any other corporate policies, in addition to providing guidelines on employee trading in the Corporation’s securities.
The Board has not granted any waiver of the Code in favor of a director or officer of the Corporation. No material change reports have been filed since the beginning of the Corporation’s most recently completed fiscal year that pertain to any conduct of a director or executive officer that constitutes a departure from the Code.
Conflicts of Interest
The Corporate Governance and Nominating Committee monitors the disclosure of conflicts of interest by directors and ensures that no director will vote or participate in a discussion on a matter in respect of which such director has a material interest.
Shareholder Communications with the Board
Shareholders may communicate with the Board or any one particular director by sending correspondence, addressed to Mr. Fletcher Payne, Senior Vice President, Chief Financial Officer, Chief Business Officer and Corporate Secretary, 12770 High Bluff Drive, San Diego, California, 92130, with an instruction to forward the communication to the Board or one or more particular directors. He will forward promptly all such shareholder communications to the Board, or the one or more particular directors, after ascertaining whether the communications are appropriate to the duties and responsibilities of the Board.
Board Committees
The Corporation has a standing Audit Committee, a Corporate Governance and Nominating Committee and a Compensation Committee, each of which are composed entirely of independent directors. In 2023, the Corporation established the R&D Committee. Each current member of the R&D Committee, except for Dr. Rice, qualifies as “independent” under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110.
Audit Committee
Membership. The current members of the Audit Committee are Denis Burger, Bernd R. Seizinger and Warren Whitehead. Mr. Whitehead is the Chair of the Audit Committee.The Board has determined that all members of the Audit Committee qualify as financial experts under the listing standards of Nasdaq.
In addition, each current member of the Audit Committee qualifies as “independent” for purposes of membership on audit committees under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110. Meetings. The Audit Committee met four times during the period from January 1, 2023 until December 31, 2023.
Committee Mandate. Among its responsibilities, the Audit Committee:
• serves as an independent and objective party to monitor the integrity of our financial reporting process and systems of internal controls regarding finance, accounting, and legal compliance, including the review of our consolidated financial statements, MD&A and annual and interim results; |
• identifies and monitors the management of the principal risksthat could impact our financial reporting; |
12
• monitors the independence and performance of our independent auditors, including the pre-approval of all audit fees and all permitted non-audit services in accordance with federal securities laws and the rules and regulations of the SEC; |
• provides an avenue of communication among the independent auditors, management, and the Board; and |
• encourages continuous improvement of, and foster adherence to, our policies, procedures and practices at all levels. |
The Audit Committee is also responsible for implementing and overseeing our whistle-blowing procedures and reviewing Aptose’s plans to mitigate cybersecurity risks and respond to data breaches.
Corporate Governance and Nominating Committee
Membership. The current members of the Corporate Governance and Nominating Committee are Mark Vincent, Carol Ashe and Denis Burger. Dr. Vincent is the Chair of the Corporate Governance and Nominating Committee. Each current member of the Committee qualifies as “independent” under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110.
Meetings. The Corporate Governance and Nominating Committee met four times during the period from January 1, 2023 until December 31, 2023. In addition, governance matters were discussed and considered at the Board level.
Committee Mandate. Among its responsibilities, the Corporate Governance and Nominating Committee:
· identifies qualified individuals to become Board members, consistent with criteria approved by the Board; |
• determines the composition of the Board and its committees; |
• selects the director nominees for the next annual meeting of shareholders; |
• monitors a process to assess Board, committee and management effectiveness; |
• aids and monitors management succession planning; and |
· develops, recommends to the Board, implements and monitors policies and processes related to the Corporation’s corporate governance guidelines |
Compensation Committee
Membership. The Compensation Committee is currently comprised of Carol Ashe, Denis Burger and Erich Platzer. Dr. Burger is the Chair of the Compensation Committee. Each current member of the Compensation Committee qualifies as “independent” for purposes of membership on compensation committees under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110, and as a “non-employee director” within the meaning of Rule 16b-3 under the Exchange Act. Meetings. The Compensation Committee met four times during the period from January 1, 2023 until December 31, 2023. In addition, compensation matters were discussed and considered at the Board level.
Committee Mandate. Among its responsibilities, the Compensation Committee:
• reviews and makes recommendations to the Board regarding the corporate goals and objectives, performance and compensation of the Chief Executive Officer and other senior executive officers on an annual basis; |
13
• evaluates the performance of the Chief Executive Officer and other senior executive officers; |
• makes recommendations to the Board with respect to the compensation policies for the non-employee directors; |
• makes recommendations regarding annual bonus policies for employees, the incentive-compensation plans and equity-based plans for the Corporation; and |
• reviews executive compensation disclosure before the Corporation publicly discloses this information. |
As part of its process to make recommendations to the Board with respect of the compensation for the non-employee directors and other employees of the Corporation, the Compensation Committee consults with the President and Chief Executive Officer and other officers of the Corporation to obtain recommendations as it deems necessary.
R&D Committee
Membership. The current members of the R&D Committee are Erich Platzer, William Rice, Bernd R. Seizinger and Mark Vincent. Dr. Seizinger is the Chair of the R&D Committee. Each current member of the R&D Committee, except for Dr. Rice, qualifies as “independent” under the listing standards of Nasdaq, the rules and regulations of the SEC and NI 52-110.
Meetings. The R&D Committee met once during the period from January 1, 2023 until December 31, 2023.
Committee Mandate. The R&D Committee’s responsibilities include:
• serving in an advisory role and interacts with both management and external advisors to develop insights and recommendations regarding the Corporation’s approach to product development and technical innovation; |
• assisting management in the identification, evaluation and oversight of appropriate technology and product development investments; |
• overseeing the innovation strategy of the Corporation, including periodic reviews of the Corporation’s research and development portfolio and its overall competitiveness, the science and technology underlying major research and development initiatives, the competitive environment and disruptive technology impacts; |
• providing feedback and input regarding the Corporation’s development of innovative business models, strategies and tactics; and |
• assisting the Board with the interpretation of scientific and clinical development data. |
Item 11 Executive Compensation
Our leadership team comprises accomplished industry, financial and clinical research professionals who are dedicated to building a comprehensive anticancer drug pipeline and clinical development programs focused on targeted therapeutics directed against dysregulated oncogenic processes in patients with life-threatening hematologic malignancies. For the year ended December 31, 2023, the leadership team included our Chairman, President and Chief Executive Officer, Dr. William G. Rice, our Senior Vice President, Chief Financial Officer and Chief Business Officer, Fletcher Payne; our Senior Vice President and Chief
14
Medical Officer, Dr. Rafael Bejar and our Senior Vice President and Chief Commercial Officer, Philippe Ledru, who departed the Corporation in 2024.
The following discussion covers the compensation arrangements for Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru, prior to his departure in 2024, (each, an “NEO” and, collectively the “Named Executive Officers”).
Compensation Philosophy
The Compensation Committee’s mandate is to review and advise the Board on the recruitment, appointment, performance, compensation, benefits and termination of executive officers. The Compensation Committee also administers and reviews procedures and policies with respect to equity-based compensation plans, employee benefit programs, pay equity and employment equity and reviews executive compensation disclosure where it is publicly disclosed.
Aptose’s executive compensation program is designed to:
These objectives are achieved by offering executives and employees a compensation package that is competitive and rewards the achievement of both our short-term and long-term objectives. As such, our compensation package consists of three key elements:
The Compensation Committee reviews each of these items on a stand-alone basis and also reviews compensation as a total package. Adjustments to compensation are made as appropriate following a review of the compensation package as a whole.
Independent Advice
In 2023, the Compensation Committee retained Radford (an Aon Consulting Company), to provide independent advice to the Compensation Committee and to conduct a detailed assessment of the executive compensation program of the Corporation. Radford was retained to identify a peer group of companies as well as perform an assessment of the competitiveness of the Corporation’s executive compensation process. Radford did not provide any services to management. The Compensation Committee has sole authority to retain and terminate any compensation consultant to be used to assist it in the evaluation of executive officer compensation. The Compensation Committee has sole authority to approve such consultants’ fees and retention terms and to obtain advice and assistance from internal or external legal, accounting or other advisors.
The Compensation Committee conducted an independence assessment for Radford in accordance with the Compensation Committee’s charter and Nasdaq listing standards, considering factors included in the listing
15
standards. The Compensation Committee determined, based on an analysis of these factors, that the work of Radford as the independent compensation consultant did not create any conflict of interest. Radford concluded, in December 2023, that the executive compensation program was positioned at the market’s 50thpercentile in the aggregate (50th to 75th percentile for the long‑term incentives pre‑commercial portion of the compensation package, with the potential for actual compensation being higher or lower based on performance).
Comparator Group
In 2023, the Compensation Committee and the Board, with advice from Radford, its independent compensation consultant, reviewed the compensation of its executive officers against that of its compensation peer group. The comparator group considers direct competitors for talent, especially for industry-specific roles. The comparator group is comprised of 17 publicly traded biopharmaceutical companies with less than 100 employees and market values below $100 million (as of October 2023). The companies comprising the comparator group are as follows:
Comparator Group Companies | |
Atreca, Inc. | Kronos Bio, Inc. |
Bolt Biotherapeutics, Inc. | Leap Therapeutics, Inc. |
Candel Therapeutics, Inc. | Mustang Bio, Inc. |
Cardiff Oncology, Inc. | Syros Pharmaceuticals, Inc. |
Curis, Inc. | Rain Oncology, Inc. |
Cyteir Therapeutics, Inc. | Vincerx Parma, Inc. |
eFFECTOR Therapeutics, Inc | TRACON Pharmaceuticals, Inc. |
Harpoon Therapeutics, Inc. | |
Pay Positioning
The Corporation endeavors to target total cash compensation (salary and short-term incentive) somewhat above the 50th percentile of the comparator group, and generally provides long-term incentive opportunities in the 50th to 75th percentile of the comparator group. The Compensation Committee believes this approach aligns executive compensation with the long-term interests of Shareholders and with the Corporation’s strategy, particularly when relatively few executives are performing multiple executive roles. In 2023, Radford provided detailed information to the Compensation Committee relating to compensation values, pay mix and incentive vehicles at the comparator group companies. In addition, the Compensation Committee considered compensation in relation to executives located in Central and Southern California which are regions of relatively higher cost of living and highly competitive for recruitment and retention. Based on this information and also taking into account experience in the role, scope of the role, performance and retention risk, as further explained below, the Compensation Committee suggested compensation goals for the executives for 2024 and the following years aligned with the target pay positioning set out above.
Although the Compensation Committee considers Radford’s recommendations in its review of executive compensation, the Compensation Committee ultimately makes its own decisions about compensation matters. The Compensation Committee realizes that using a peer benchmark such as the one provided by Radford is neither the only means for gathering and validating market data nor the only criteria for establishing executive compensation. In instances where an executive is uniquely critical to our success, the Compensation Committee may provide compensation in excess of the benchmark of the comparator group companies. Upward or downward variations for base salary and long-term incentives may also occur as a result of the individual’s experience level, the balance of the individual’s different elements of compensation, market factors and other strategic considerations. The Compensation Committee believes that, given the competitiveness of our industry and our company culture, our base compensation, cash incentives and equity programs must remain flexible, reward the achievement of clearly defined corporate
16
goals. In addition, the Compensation Committee believes that such programs must be sufficient to retain our existing executive officers and to hire new executive officers, when necessary, and that unnecessary turnover at the executive level can have expensive consequences from the perspectives of time lost and capital required.
In 2023, achievements that were considered by the Compensation Committee when making compensation recommendations included, (i) for the oral, myeloid kinome inhibitor tuspetinib, the completion of dose escalation/dose exploration and the APTIVATE expansion trial to include single agent and drug combination in AML patient population; and (ii) for the oral, dual lymphoid and myeloid kinome inhibitor luxeptinib, dose escalation and evaluation with respect to third generation drug substance, and the manufacture of sufficient third generation drug substance to support clinical needs. The rigorous cash management and the management of the business relationships with strategic partners were also taken into account. In addition, the exceptional market environment for the hiring and retention of talent was an important factor for the Compensation Committee and the Board when making compensation decisions.
Base Salary
In establishing base salaries, the objective of the Compensation Committee is to establish levels that will enable Aptose to attract and retain executive officers that can effectively contribute to the long-term success of the Corporation. Base salary for each executive officer is determined by the individual’s skills, abilities, experience, past performance and anticipated future contribution to our success. The members of the Compensation Committee use their knowledge of the industry and of industry trends as well as independent third-party consultants to assist with the determination of an appropriate compensation package for each executive officer.
Short-Term Compensation Incentives
Short-term compensation incentives motivate our executive officers to achieve specified performance objectives and to reward them for their achievement in the event that those objectives are met. Each year, the Compensation Committee approves the annual corporate objectives encompassing scientific, clinical, regulatory, business and corporate development and financial criteria. The annual cash incentive for the executive officers is based, at least in part, on the level of achievement of these annual objectives, assuming these objectives are still relevant at the time of evaluation.
All corporate and executive officer objectives and short-term incentives are reviewed by the Compensation Committee and approved by the Board.
The annual cash incentives for executive officers for the year ended December 31, 2023 ranged from 24% to 41% of base salary. Payment of cash bonuses for the Chief Executive Officer and the Chief Financial Officer was voluntarily deferred until the Corporation meets certain financing goals during 2024, and a portion of the potential cash bonus was paid to the Chief Medical Officer, with any remainder of the bonus deferred until the Corporation meets certain financing goals during 2024.
Cash incentives are determined as soon as practicable after the end of the fiscal year and, for the Named Executive Officers (as defined hereinafter), are included in the Summary Compensation Table in the year in respect of which they are earned.
Short-Term Compensation Incentives - Performance Metrics
The performance of the Named Executive Officers for the period ended December 31, 2023 was measured with respect to the following objectives:
17
1) | Achievement of certain milestones for the clinical development of the tuspetinib and luxeptinib programs; | |
2) | Achievement of certain milestones related to finance, financing and accounting; and | |
3) | Achievement of certain milestones related to corporate and business development. |
Each of the above objectives is weighted at 50%, 30% and 20%, respectively, in relation to assessment of satisfaction of overall corporate objectives and determination of any general corporate bonuses and additional unanticipated accomplishments during 2023 were also considered.
Long-Term Incentive Plans
Long-term compensation incentives at Aptose reward an executive’s contribution to the attainment of Aptose’s long-term objectives, align an executive’s performance with the long-term performance of Aptose and to provide an additional incentive for an executive to enhance shareholder value. Long-term incentive compensation for directors, officers, employees and consultants is reviewed annually and may be accomplished through the grant of share options and of stock-based awards under the 2021 Stock Incentive Plan.
In certain cases, executive officers may be granted share options on the commencement of employment with Aptose in accordance with the responsibility delegated to each executive officer for achieving corporate objectives and enhancing shareholder value in accordance with those objectives.
The number of options granted for certain executives of Aptose for the year ended December 31, 2023 was based on achievement of both corporate and executive officer objectives. The Compensation Committee recommends the allocation of options, and options are priced using the closing market price of the Shares on the Toronto Stock Exchange (“TSX”) or on Nasdaq, as applicable, on the last trading day prior to the grant. Options to purchase Shares granted under the 2021 Stock Incentive Plan expire ten years from the date of grant and vest over a term recommended by the Compensation Committee and approved by the Board of Directors. During 2023, options granted to Aptose Named Executive Officers vested over four years. The Compensation Committee and the Board consider previous grants of options when considering new grants of options.
Stock and option awards may be subject to accelerated vesting in the event of termination or change of control, see “Termination and Change of Control Benefits.”
Other Benefits
In certain cases, the Compensation Committee may recommend inclusion of automobile allowances, and the payment of certain professional dues as a component of a competitive remuneration package for executives.
Hedge or Offset Instruments
Pursuant to Aptose’s Disclosure and Insider Trading Policy, no officer, director or other member of management of the Corporation may engage in short sales, transactions in put or call options, hedging transactions, margin accounts, pledges or other inherently speculative transactions with respect to the Corporation’s stock at any time.
Clawback Policy
The Board has adopted an incentive compensation recovery policy (the “Clawback Policy”) which provides for the recovery of erroneously awarded incentive compensation in the event that the Corporation is
18
required to prepare an accounting restatement due to material noncompliance of the Corporation with any financial reporting requirements under the federal securities laws.
Employment Agreements
Aptose entered into an employment agreement with Dr. Rice on October 25, 2013 upon his commencement as Chairman, President, and Chief Executive Officer. This agreement was amended and restated on August 19, 2014. Pursuant to the employment agreement, Dr. Rice is entitled to an annual base salary of $624,000, which amount is reviewed annually by the Board and increased at the Board’s discretion, upon the advice of the Compensation Committee. Dr. Rice is also eligible for an annual discretionary bonus of up to 55% of his current base salary. The annual bonus is based on the Corporation’s and Dr. Rice’s achievement of objectives and milestones to be determined on an annual basis by the Board. Dr. Rice is entitled to receive termination benefits described under “Termination and Change of Control Benefits” below. Dr. Rice also receives employee benefits including, without limitation, participation in our 401(k) plan with a 3% non-elective company contribution, participation in Aptose’s group health coverage plan and life insurance plan for US employees, 25 days of paid vacation time annually, and an annual automobile allowance of $18,000. Dr. Rice is subject to certain non-compete restrictions. Dr. Rice receives no remuneration for his service as Chairman of the Board, director or as a member of the R&D Committee of the Board.
Aptose entered into an employment agreement with Mr. Payne upon his commencement as Chief Financial Officer, effective June 27, 2022. Mr. Payne was promoted to Chief Financial Officer and Chief Business Officer in November 2023. Pursuant to the employment agreement, Mr. Payne is entitled to an annual base salary of $447,000 which amount is reviewed annually by the Board and increased at the Board’s discretion, upon the advice of the Compensation Committee. Mr. Payne is also eligible for an annual discretionary bonus of up to 40% of his current base salary. The annual bonus is based on the Corporation’s and Mr. Payne’s achievement of objectives and milestones to be determined on an annual basis by the Board. Mr. Payne is entitled to receive termination benefits described under “Termination and Change of Control Benefits” below and receives employee benefits, including, without limitation, participation in any 401(k) plan with a 3% non-elective company contribution, participation in other benefits provided by us to our U.S.-based executive officers and other employees, which consist to date of life insurance and health benefits, and 20 days of paid vacation time annually. Mr. Payne is subject to certain non-compete restrictions.
Aptose entered into an employment agreement with Dr. Bejar upon his commencement as Chief Medical Officer, effective January 1, 2020. Pursuant to the employment agreement, Dr. Bejar is entitled to an annual base salary of $490,000 which amount is reviewed annually by the Board and increased at the Board’s discretion, upon the advice of the Compensation Committee. Dr. Bejar is also eligible for an annual discretionary bonus of up to 40% of his current base salary. The annual bonus is based on the Corporation’s and Dr. Bejar’s achievement of objectives and milestones to be determined on an annual basis by the Board. Dr. Bejar is entitled to receive termination benefits described under “Termination and Change of Control Benefits” below and receives employee benefits, including, without limitation, participation in any 401(k) plan with a 3% non-elective company contribution, participation in other benefits provided by us to our U.S.-based executive officers and other employees, which consist to date of life insurance and health benefits, and 20 days of paid vacation time annually. Dr. Bejar is subject to certain non-compete restrictions.
Aptose entered into an employment agreement with Mr. Ledru upon his commencement as Chief Commercial Officer, effective April 6, 2022. Pursuant to the employment agreement, Mr. Ledru was entitled to an annual base salary of $410,000 which amount was reviewed annually by the Board and increased at the Board’s discretion, upon the advice of the Compensation Committee. Mr. Ledru was also eligible for an annual discretionary bonus of up to 40% of his current base salary. The annual bonus is based on the Corporation’s and Mr. Ledru’s achievement of objectives and milestones to be determined on an
19
annual basis by the Board. Mr. Ledru was entitled to receive termination benefits described under “Termination and Change of Control Benefits,” below and received employee benefits, including, without limitation, participation in any 401(k) plan with a 3% non-elective company contribution, participation in other benefits provided by us to our U.S. based executive officers and other employees, which consist to date of life insurance and health benefits, and 20 days of paid vacation time annually. Mr. Ledru was subject to certain non-compete restrictions. Mr. Ledru departed from the Corporation in 2024.
Summary Compensation Table
The following table details the compensation information for the fiscal years ended December 31, 2022 and December 31, 2023 of the Corporation for the Named Executive Officers. All amounts presented in the following tables are as recorded in US dollars.
Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock awards(1) | Option awards(2) ($) | All other compensation(3) ($) | Total compensation ($) |
Dr. William G. Rice Chairman, President and Chief Executive Officer | 2023 2022 | 623,077 599,289 | - 294,360 | 99,000 - | 174,691 942,890 | 27,900 27,150 | 924,668 1,863,689 |
Fletcher Payne Senior Vice President, Chief Financial Officer and Chief Business Officer | 2023 2022 | 448,131 223,269 | - 125,000 | 65,993 - | 87,345 564,961 | 9,900 9,150 | 611,370 922,380 |
Dr. Rafael Bejar Senior Vice President and Chief Medical Officer | 2023 2022 | 488,846 459,231 | 98,000 200,000 | 65,993 - | 87,345 532,691 | 9,900 9,150 | 750,085 1,201,072 |
Philippe Ledru Senior Vice President and Chief Commercial Officer(4) | 2023 2022 | 425,769 299,615 | - 226,417 | 65,993 - | 87,345 502,307 | 9,900 9,150 | 589,008 1,037,489 |
20
Share options are subject to the executives’ continued employment with the Corporation and have a maximum term of 10 years. All share option grants issued to Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru may be subject to accelerated vesting following termination of employment. See “Termination and Change of Control Benefits” below.
Outstanding Equity Awards at Fiscal Year-End
Option-based Awards | Share-based awards | |||||
Name and Principal Position | Number of securities underlying unexercised options (#) exercisable | Number of securities underlying unexercised options (#) unexercisable | Option exercise price ($) | Option expiration date | Number shares or units of stock that have not vested (#) | Market value of shares or units of stock that have not vested ($) |
Dr. William G. Rice Chairman, President and Chief Executive Officer | 3,333 | Nil | 15.45 | 6-Jun-27 | Nil | Nil |
6,666 | Nil | 17.21(1) | 28-Mar-27 | |||
23,332 | 23,334(2) | 20.10 | 17-Jan-32 | |||
26,666 | Nil | 28.65 | 2-Jan-29 | |||
20,000 | Nil | 42.00 | 19-Jan-28 | |||
4,000 | Nil | 43.26(1) | 30-Mar-26 | |||
26,666 | Nil | 46.05 | 22-Jan-28 | |||
26,408 | Nil | 64.55(1) | 15-Jun-24 | |||
15,244 | 7,622(3) | 65.55 | 4-Jan-31 | |||
9,333 | Nil | 67.95(1)(4) | 9-Apr-24 | |||
352 | Nil | 78.82(1)(4) | 28-Jan-24 | |||
8,000 | Nil | 78.82(1) | 9-Jun-25 | |||
111,111 | 22,222(5) | 103.65 | 30-Jan-30 | |||
Nil | 40,000(6) | 12.15 | 5-Jul-32 | |||
Nil | 26,666(7) | 9.90 | 18-Jan-33 | |||
Fletcher Payne Senior Vice President, Chief Financial Officer and Chief Business Officer | 33,333 | 33,333(8) | 12.72 | 26-Jun-32 | Nil | Nil |
Nil | 13,333(7) | 9.90 | 18-Jan-33 | |||
Dr. Rafael Bejar Senior Vice President and Chief Medical Officer | 22,222 | 4,444(9) | 85.05 | 1-Jan-30 | Nil | Nil |
11,111 | 2,222(5) | 103.65 | 30-Jan-30 | |||
15,244 | 7,622(3) | 65.55 | 4-Jan-31 | |||
23,333 | Nil | 35.25 | 18-Aug-31 | |||
20,000 | 20,000(2) | 20.10 | 17-Jan-32 | |||
Nil | 13,333(7) | 9.90 | 18-Jan-33 | |||
Philippe Ledru Senior Vice President and Chief Commercial Officer(11) | 19,999 | 20,001(10) | 18.75 | 12-Apr-32 | Nil | Nil |
Nil | 13,333(7) | 9.90 | 18-Jan-33 | |||
1. Converted from the Canadian exercise price at the rate of 0.7913 Canadian dollars per U.S. dollar.
2. Unexercisable options vest as follows: 33.33% vested on January 17, 2024, 33.33% vest on January 17, 2025, and 33.33% vest on January 17, 2026.
21
3. Unexercisable options vest as follows: 50% vested on January 4, 2024, and 50% vest on January 4, 2025.
4. Options expiring before the date of the Meeting.
5. Unexercisable options vested on January 30, 2024.
6. Unexercisable options vest upon reaching certain performance triggers as determined by the Board.
7. Unexercisable options vest as follows: 50% vested on January 19, 2024. 16.67% vest on January 19, 2025, 16.67% vest on January 19, 2026 and 16.67 vest on January 19, 2027.
8. Unexercisable options vest as follows: 33.33% vest on June 26, 2024, 33.33% vest on June 26, 2025, and 33.33% vest on June 26, 2026.
9. Unexercisable options vested on January 1, 2024.
10. Unexercisable options vest as follows: 33.33% vested on April 12, 2024, 33.33% vest on April 12, 2025, and 33.33% vest on April 12, 2026.
11. EXECUTIVEMr. Ledru departed from the Corporation in 2024.
Retirement Benefits
The Corporation maintains a 401(k) plan in which eligible employees of the Corporation may choose to participate, including the Named Executive Officers. The Corporation makes non-elective contributions of 3% of compensation for all eligible employees, subject to the maximum allowed by the Internal Revenue Code Section 401(k).
Termination and Change of Control Benefits
The employment agreements of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru, prior to his departure, provide that if their employment is terminated by the Corporation other than for “cause”, or if the Named Executive Officer resigns for “good reason” each of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru shall be entitled to a payment equivalent to 12 months of their respective annual base salaries at the time of termination (Dr. Rice’s December 31, 2023 annual base salary represented $624,000, Mr. Payne’s annual base salary represented $447,000, Dr. Bejar’s base salary represented $490,000 and Mr. Ledru’s base salary represented $410,000), plus an amount equal to the average bonus remuneration received from the Corporation during the last three years of employment completed prior to the termination date, prorated based on the number of days the executive worked during the year of the termination. In addition, the employment agreements of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru provide that certain payments related to health benefits will continue to be made for a period of 12 months following termination of their employment.
The employment agreements of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru provide that, in the event their employment with the Corporation is terminated within three months immediately preceding or 12 months immediately following the consummation of a “change of control” (defined as the consummation of any of the following: (a) the acquisition of the Corporation by another entity by means of any transaction or series of related transactions to which the Corporation is a party, (b) a sale, lease or other conveyance of all or substantially all of the assets of the Corporation, or (c) liquidation, dissolution or winding up of the Corporation, whether voluntary of involuntary), each of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru would be eligible, subject to certain conditions, to receive a payment equivalent to 18 months of their annual base salaries at the time of termination, plus an amount equal to 150% of the average bonus remuneration received from the Corporation during the last three years of employment completed prior to the termination date, prorated based on the number of days the executive worked during the year of the termination, as well as continuation of the payments related to health benefits for a period of 12 months following the termination following a change of control.
The employment agreements of Dr. Rice, Mr. Payne, Dr. Bejar and Mr. Ledru provide that in the event of their termination, other than for cause, the vesting and exercisability of all then outstanding unvested share options, RSUs or other equity awards then held by such NEO become immediately vested and exercisable and shall remain exercisable as set forth in the applicable award documents.
22
Pay versus Performance
As required by Section 953(b) of the Dodd-Frank Wall Street Reform and Consumer Protection Act, and Item 402(4) of Regulation S-K, we are providing the following information about the relationship of the annual total compensation of our NEOs and our performance.
Pay versus Performance Table
Year | Summary Compensation Table total for CEO | CEO Compensation Actually Paid(1) | Average Summary Compensation Table total for other NEOs(2) | Average Compensation Actually Paid to other NEOs(1)(2) | Value of initial fixed $100 investment based on TSR | Net loss | |
(a) | (b) | (c) | (d) | (e) | (f) | (g) | |
2022 | $1,863,689 | $5,219,075 | $1,053,647 | $1,642,392 | $38 | $(41,823) | |
2023 | $924,668 | $3,666,103 | $650,154 | $1,436,252 | $11 | $(51,207) |
Year | Fair Value of covered year Unvested Equity Awards | Fair Value of covered year Vested Equity Awards | Change in Fair value of covered year Unvested Equity Awards | Change in Fair Value of covered year Vested Awards | Amounts reported in the Summary Compensation Table for equity awards | Equity Value Included in Compensation Actually Paid | |
(a) | (b) | (c) | (d) | (e) | (f) = (a) + (b) + (c) + (d) – (e) | ||
2022 | $3,172,676 | $1,707,132 | $(553,710) | $(27,822) | $942,890 | $3,355,386 | |
2023 | $1,765,374 | $1,465,542 | $(291,609) | $(23,181) | $174,691 | $2,741,435 |
Narrative
The relationship between compensation actually paid and the pay of our NEOs is described below:
The chart below shows the relationship between the compensation actually paid to the CEO and the average compensation actually paid to our other NEOs, on one hand, and the Corporation’s cumulative
23
TSR (total shareholder return, based on an initial investment of $100 on December 31, 2021) over the two most recently completed financial years.
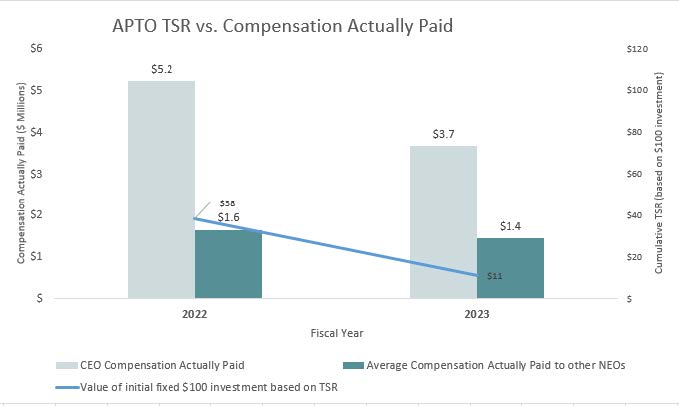
24
The chart below shows the relationship between the compensation actually paid to the CEO and the average compensation actually paid to our other NEOs, on one hand, and the Corporation’s net loss over the two most recently completed financial years.
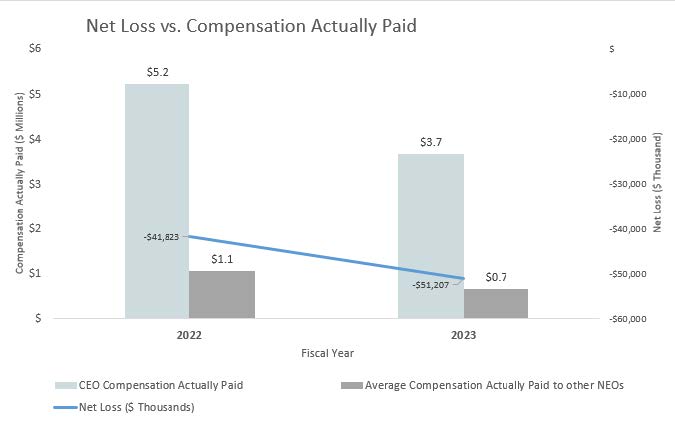
DIRECTOR COMPENSATION
Overview
The information required by this item is incorporated herein by referenceCompensation Committee makes recommendations regarding compensation payable to our non-employee directors to the informationentire Board, which then makes final decisions regarding such compensation.
Dr. Rice receives no remuneration for his service as Chairman of the Board and director or as a member of the R&D Committee of the Board.
Cash Compensation
Non-employee directors are entitled to an annual fee of $60,000 with no per meeting fees. The Lead Director is entitled to an additional annual fee of $40,000. The chair of each committee is entitled to an additional annual fee of $15,000, with the exception of the chair of the Audit Committee who is entitled to an additional annual fee of $20,000. Each committee member is entitled to receive an annual fee of $10,000 per committee, and members of the Audit Committee are entitled to an additional annual fee of $3,500. All fees are paid in quarterly installments.
Non-employee directors are reimbursed for any out-of-pocket travel expenses incurred in order to attend meetings. Executive directors are not entitled to directors’ compensation.
Option Awards
Upon appointment to the Board a non-employee director will be entitled to an initial option grant under the 2021 Stock Incentive Plan and each year thereafter non-employee directors are eligible for an additional grant at the beginning of the fiscal year. The options vest 50% after one year, and 25% for each of the
25
second and third years. If a director resigns, the director will have 90 days from the Proxy Statement underdate of resignation to exercise all vested and unexercised options.
The maximum compensation (cash and equity awards) that may be received by any director during a financial year has been set to $500,000.
The following table details the sections entitled “Executivecompensation earned by each non-employee director for the year ended December 31, 2023:
Name | Fees earned or paid in cash ($) | Option awards(1)(2) ($) | Total ($) |
Carol G. Ashe | 80,000(3) | 43,669 | 123,669 |
Dr. Denis Burger | 128,500(5) | 43,669 | 172,169 |
Dr. Mark Vincent | 85,000(6) | 42,682 | 127,682 |
Mr. Warren Whitehead | 80,000(7) | 42,682 | 122,682 |
Dr. Erich Platzer | 80,000(8) | 43,669 | 123,669 |
Dr. Bernd R. Seizinger | 88,500(9) | 43,669 | 132,169 |
During the year ended December 31, 2023, the following share options were granted to Aptose directors: 3,333 share options for Ms. Ashe, 3,333 share options for Dr. Burger, 3,333 share options for Dr. Vincent, 3,333 share options for Mr. Whitehead, 3,333 share options for Dr. Platzer and 3,333 share options for Dr. Seizinger. All options granted will vest over three years.
26
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the sections entitled “ShareSecurity Ownership of Certain Beneficial Owners and Management and Directors” and “EquityRelated Shareholder Matters
Equity Compensation Plan Information.”Information
The following table sets forth certain details as at the end of the year ended December 31, 2023 with respect to compensation plans pursuant to which equity securities of the Corporation are authorized for issuance.
Plan Category |
| Number of Shares to be |
|
| Weighted- |
|
| Number of Shares remaining | ||||||||
Equity compensation plans approved by security holders |
| 1,183,947 |
| $44.78 | 382,408 |
| ||||||||||
Equity compensation plans not approved by security holders | - | - | - | |||||||||||||
Total | 1,183,947 | $44.78 | 382,408 | |||||||||||||
[1] Canadian exercise prices have been converted to U.S. dollars based on an exchange rate as of April 15, 2024 of US$1.00 equals C$1.38.
Security Ownership of Certain Beneficial Owners and Management
The table below sets forth information known to us regarding the beneficial ownership of our Shares as of April 29, 2024 for:
The number of Shares beneficially owned by a person includes shares subject to options held by that person that are currently exercisable or that become exercisable within 60 days of April 15, 2024. Percentage calculations assume, for each person and group, that all Shares that may be acquired by such person or group pursuant to options currently exercisable or that become exercisable within 60 days of April 15, 2024 are outstanding for the purpose of computing the percentage of Shares owned by such person or group. However, such unissued Shares described above are not deemed to be outstanding for calculating the percentage of Shares owned by any other person.
27
Except as otherwise indicated, the persons in the table below have sole voting and investment power with respect to all Shares shown as beneficially owned by them, subject to community property laws where applicable and subject to the information contained in the notes to the table.
Name of Beneficial Owner | Amount and | Percent of | ||||||
Named Executive Officers and Directors | ||||||||
Carol G. Ashe | 22,764 | * | ||||||
Dr. Rafael Bejar | 123,053 | * | ||||||
Dr. Denis Burger | 34,427 | * | ||||||
Philippe Ledru | 40,033 | * | ||||||
Fletcher Payne | 47,332 | * | ||||||
Dr. Erich Platzer | 64,161 | * | ||||||
Dr. William G. Rice | 326,379 | 2.0% | ||||||
Dr. Bernd R. Seizinger | 21,999 | * | ||||||
Mark D. Vincent | 32,994 | * | ||||||
Warren Whitehead | 30,662 | * | ||||||
All Executive Officers and Directors as a Group (10 persons) | 743,804 | 4.6% | ||||||
Beneficial Owners of More Than 5% | ||||||||
Hanmi Pharmaceuticals Co., Ltd.(2) | 2,989,415 | 19.99% | ||||||
*Does not exceed one percent of Shares outstanding | ||||||||
1. Includes for the persons listed below the following Shares subject to options held by such persons that are currently exercisable or become exercisable within 60 days of April 29, 2024: Ms. Carol G. Ashe: 21,163; Dr. Rafael Bejar: 115,720; Dr. Denis Burger: 32,983; Mr. Philippe Ledru: 33,333; Mr. Fletcher Payne: 40,000; Dr. Erich Platzer: 30,495; Dr. William G. Rice: 292,163; Dr. Bernd R. Seizinger: 4,999; Dr. Mark Vincent: 32,561; and Mr. Warren Whitehead: 29,662. | ||||||||
2. Based on information contained in a schedule 13D/A filed with the SEC on February 5, 2024 by Hanmi. Hanmi also owns common share purchase warrants which, when exercised, will increase the number of Shares beneficially owned by Hanmi. | ||||||||
3. Hanmi's ownership gives effect to the 19.99% ownership blocker restriction contained on certain of its securities. Hanmi currently holds warrants that if we did not give effect to the Blocker their ownership interest in the Corporation would be 22.17%. | ||||||||
28
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AN DIRECTOR INDEPENDENCECertain Relationships and Related Transactions, and Director Independence
The information required
INTEREST OF CERTAIN PERSONS IN MATTERS TO BE ACTED UPON
None of the directors or executive officers of the Corporation, none of the persons who have been directors or executive officers of the Corporation at any time since January 1, 2023, none of the proposed nominees for election as a director of the Corporation and none of the associates or affiliates of any of the foregoing has any material interest, direct or indirect, by this item is incorporated herein by referenceway of beneficial ownership of securities or otherwise, in any matter scheduled to be acted upon at the Meeting other than the election of directors.
INTEREST OF RELATED PERSONS IN TRANSACTIONS
For the last two completed fiscal years, no director, proposed director, executive officer, or immediate family member of a director, proposed director or executive officer nor, to the information from the Proxy Statement under the sections entitled “Corporate Governance - Independenceknowledge of our directors or executive officers, after having made reasonable inquiry, any person or company who beneficially owns, directly or indirectly, Shares carrying more than 5% of the Board” and “Interestvoting rights attached to all Shares outstanding at the date hereof, or any immediate family member thereof, had any material interest, direct or indirect, in any transaction or proposed transaction of Related Persons in Transactions.”the Corporation which involves an amount exceeding the lesser of $120,000 or one percent of the average of the Corporation’s total assets at year-end for the last two completed fiscal years.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item is incorporated herein by reference to the information from the Proxy Statement under the section entitled “Audit,
Item 14 Principal Accountant Fees and Services
Audit, Audit-Related, Tax and Other Fees”Fees
The tables below present fees for professional services rendered by KPMG LLP for the fiscal years ended December 31, 2023 and “Pre-Approval Policies and Procedures.”2022, respectively.
Aggregate Amount Billed(3) | ||||||||
2023 | 2022 | |||||||
Audit Fees(1) | $ | 467,098 | $ | 390,265 | ||||
Tax Fees(2) | 30,129 | 33,098 | ||||||
Total | $ | 497,227 | $ | 423,363 | ||||
(1) Audit fees consisted of the audit of our annual financial statements for the fiscal years ended December 31, 2023 and 2022, respectively, and interim reviews. In addition, audit fees consist of the aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit or review of the issuer’s financials and include the provision of comfort letters and consents and the review of documents filed with regulatory authorities. (2) Tax fees include fees billed for assistance in the preparation of corporate tax returns and related filings and general tax advisory services. (3) All fees by KPMG are invoiced and paid in Canadian dollars. Fees for 2023 have been translated to US dollars at the Bank of Canada average annual exchange rate of 0.7410 and 2022 have been translated to US dollars at the Bank of Canada average annual exchange rate of 0.7685. | ||||||||
6029
PART IV.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
| |
| |
| |
| |
| |
| |
|
All schedules are omitted because they are not applicable or the required information is shown in the Financial Statements or notes thereto.(3) Exhibits
Exhibit Number |
| Description of Document |
|
|
|
3.1 |
| |
|
|
|
3.2 |
| |
|
|
|
3.3 |
| |
|
|
|
4.1* |
| |
|
|
|
10.1 |
| |
|
|
|
10.2+ |
| |
|
|
|
10.3+ |
| |
|
|
|
10.4+ |
| |
|
|
|
10.5+ |
| |
|
|
|
10.6^ |
| |
|
|
|
61
10.7^ |
| |
|
|
|
10.8^ |
| |
|
|
|
10.9^ |
| |
|
|
|
10.10^ |
| |
|
|
|
10.11^ |
| |
|
|
|
30
10.12^ |
| |
|
|
|
10.13+ |
| |
|
|
|
10.14+ |
| |
|
|
|
10.15^ |
| |
|
|
|
10.16 |
| |
|
|
|
10.17 |
| |
|
|
|
10.18 |
| |
|
|
|
10.19 |
| |
|
|
|
10.20 |
| |
|
|
|
10.21 |
| |
|
|
|
10.22 |
|
62
|
|
|
21.1* |
| |
|
|
|
23.1* |
| |
|
|
|
24.1* |
| |
|
|
|
31.1* |
| |
|
|
|
31.2* |
| |
|
|
|
32.1* |
| |
|
|
|
31
32.2* |
| |
|
|
|
97.1* |
| |
|
|
|
101.INS* |
| Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
101.SCH |
| Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents |
104 |
| Cover Page Interactive Data File (embedded within the Inline XBRL document) |
+ Indicates management contract or compensatory plan.
* Filed herewith.
Confidential treatment has been sought with respect to certain portions of this exhibit.
Confidential treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission.
** In accordance with Rule 406T of Regulation S-T, the Inline XBRL related information in Exhibit 101 to this Annual Report on Form 10-K is deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act, is deemed not filed for purposes of Section 18 of the Exchange Act, and otherwise is not subject to liability under these sections.
ITEM 16. form 10-k summary
None.
6332
SIGNATURES
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on the 26th29th day of March,April, 2024.
Aptose Biosciences Inc. | /s/ William G. Rice |
By: | William G. Rice, Ph.D. |
| President, Chief Executive Officer and Chairman of the Board of Directors |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Dr. William G. Rice and Mr. Fletcher Payne, and each of them, his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or their or his substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature |
| Title |
|
|
|
/s/ William G. Rice |
|
|
William G. Rice, Ph.D. |
| President, Chief Executive Officer and Chairman of the Board of Directors (Principal Executive Officer) |
|
|
|
/s/ Fletcher Payne |
|
|
Fletcher Payne |
| Senior Vice President and Chief Financial Officer (Principal Financial Officer and Accounting Officer)
|
|
|
|
/s/ Denis R. Burger |
|
|
Denis R. Burger, Ph.D. |
| Director, Lead Independent |
|
|
|
/s/ Carol G. Ashe |
|
|
Carol G. Ashe |
| Director |
|
|
|
/s/ Erich M. Platzer |
|
|
Erich M. Platzer, M.D., Ph.D. |
| Director |
|
|
|
/s/ Bernd R. Seizinger |
|
|
Bernd R. Seizinger, M.D., Ph.D. |
| Director |
|
|
|
/s/ Mark D. Vincent |
|
|
Mark |
| Director |
|
|
|
/s/ Warren Whitehead |
|
|
Warren Whitehead |
| Director |
6433

Consolidated Financial Statements
APTOSE BIOSCIENCES INC.
Years ended December 31, 2023 and 2022
F-1
|
KPMG LLP
100 New Park Place, Suite 1400
Vaughan, ON L4K 0J3
Tel 905-265 5900
Fax 905-265 6390
www.kpmg.ca
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors
Aptose Biosciences Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of Aptose Biosciences Inc. and subsidiaries (the Company) as of December 31, 2023 and 2022, the related consolidated statements of loss and comprehensive loss, changes in shareholders’ equity, and cash flows for the years then ended, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Notes 1 and 2(a) to the consolidated financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Notes 1 and 2(a). The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but
|
F-2
|
|
not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Research and Development Prepaid and Accrued Costs
As discussed in Notes 2(i), 4 and 9 to the consolidated financial statements, the Company records expenses for research and development activities based on Management’s estimates of services received and efforts expended pursuant to contracts with vendors that conduct research and development on the Company’s behalf. The financial terms vary from contract to contract and may result in uneven payment flows as compared with services performed or products delivered. As a result, the Company is required to estimate research and development expenses incurred during the period, which impacts the amount of accrued expenses and prepaid balances related to such costs as of each balance sheet date. Management estimates the amount of work completed through discussions with internal personnel and the contract research and contract manufacturing organizations as to the progress or stage of completion of the services. The Company’s estimates are based on a number of factors, including the Company’s knowledge of the status of each of the research and development project milestones, and contract terms together with related executed change orders. Management makes significant judgments and estimates in determining the accrued balance at the end of each reporting period.
We identified the evaluation of research and development prepaid and accrued costs as a critical audit matter. Higher degree of auditor judgment was required in evaluating the results of our audit procedures because of the subjectivity and estimation uncertainty associated with this estimate.
F-3
|
|
The following are the primary procedures we performed to address this critical audit matter. For a selection of prepaid and accrued costs amounts for research and development projects, we assessed the Company’s estimates by:
•inquiring with Company personnel responsible for overseeing the research and development activities to understand progress of the activities including project milestones, and contract terms together with related executed change orders
•inspecting the terms of the contracts, including related executed change orders, between the Company and the respective contract research and contract manufacturing organizations, the correspondence between the Company and these organizations as to the completion status, invoices received by the Company subsequent to period end, and using this information to arrive at an independent estimate of the prepaid or accrual amounts and comparing it to the amounts recorded by the Company
We have served as the Company’s auditor since 1994.
/s/KPMG LLP
Chartered Professional Accountants, Licensed Public Accountants
Vaughan, Canada
March 26, 2024
F-4
APTOSE BIOSCIENCES INC.
Consolidated Statements of Financial Position
(Expressed in thousands of US dollars)
| December 31, |
|
| December 31, |
| |||
Assets |
|
|
|
|
|
| ||
Current assets: |
|
|
|
|
|
| ||
Cash and cash equivalents |
| $ | 9,252 |
|
| $ | 36,970 |
|
Investments |
|
| — |
|
|
| 9,989 |
|
Prepaid expenses |
|
| 2,042 |
|
|
| 2,303 |
|
Other current assets |
|
| 600 |
|
|
| 257 |
|
Total current assets |
|
| 11,894 |
|
|
| 49,519 |
|
Non-current assets: |
|
|
|
|
|
| ||
Property and equipment |
|
| 152 |
|
|
| 211 |
|
Right-of-use assets, operating leases |
|
| 943 |
|
|
| 1,297 |
|
Total non-current assets |
|
| 1,095 |
|
|
| 1,508 |
|
Total assets |
| $ | 12,989 |
|
| $ | 51,027 |
|
Liabilities and Shareholders’ Equity |
|
|
|
|
|
| ||
Current liabilities: |
|
|
|
|
|
| ||
Accounts payable to related party |
| $ | 2,554 |
|
| $ | 2,984 |
|
Accounts payable |
|
| 3,492 |
|
|
| 3,342 |
|
Accrued liabilities to related party |
|
| — |
|
|
| 572 |
|
Accrued liabilities |
|
| 8,829 |
|
|
| 5,085 |
|
Current portion of lease liability, operating leases |
|
| 394 |
|
|
| 301 |
|
Total current liabilities |
|
| 15,269 |
|
|
| 12,284 |
|
Non-current liabilities: |
|
|
|
|
|
| ||
Lease liability, operating leases |
|
| 621 |
|
|
| 1,002 |
|
Total liabilities |
|
| 15,890 |
|
|
| 13,286 |
|
Shareholders’ equity: |
|
|
|
|
|
| ||
Share capital: |
|
|
|
|
|
| ||
Common shares, no par value, unlimited authorized shares, |
|
| 444,806 |
|
|
| 437,520 |
|
Additional paid-in capital |
|
| 72,146 |
|
|
| 68,869 |
|
Accumulated other comprehensive loss |
|
| (4,316 | ) |
|
| (4,318 | ) |
Deficit |
|
| (515,537 | ) |
|
| (464,330 | ) |
Total shareholders’ equity |
|
| (2,901 | ) |
|
| 37,741 |
|
Total liabilities and shareholders’ equity |
| $ | 12,989 |
|
| $ | 51,027 |
|
See accompanying notes to consolidated financial statements.
Going concern, see Note 2(a).
Commitments, see Note 10.
Subsequent events, see Note 17.
F-5
APTOSE BIOSCIENCES INC.
Consolidated Statements of Loss and Comprehensive Loss
(Expressed in thousands of US dollars, except for per common share data)
| Year ended December 31, 2023 |
|
| Year ended December 31, 2022 |
| |||
Revenue |
| $ | — |
|
| $ | — |
|
Expenses: |
|
|
|
|
|
| ||
Research and development, related party |
|
| 3,492 |
|
|
| 3,556 |
|
Research and development |
|
| 33,273 |
|
|
| 24,532 |
|
General and administrative |
|
| 15,591 |
|
|
| 14,514 |
|
Operating expenses |
|
| 52,356 |
|
|
| 42,602 |
|
Other income: |
|
|
|
|
|
| ||
Interest income |
|
| 1,151 |
|
|
| 788 |
|
Foreign exchange loss |
|
| (2 | ) |
|
| (9 | ) |
Total other income |
|
| 1,149 |
|
|
| 779 |
|
Net loss |
|
| (51,207 | ) |
|
| (41,823 | ) |
Other comprehensive loss: |
|
|
|
|
|
| ||
Unrealized gain (loss) on securities available-for-sale securities |
|
| 2 |
|
|
| (2 | ) |
Total comprehensive loss |
| $ | (51,205 | ) |
| $ | (41,825 | ) |
Basic and diluted loss per common share |
| $ | (7.58 | ) |
| $ | (6.80 | ) |
Weighted average number of common shares outstanding used in the |
|
| 6,755 |
|
|
| 6,151 |
|
See accompanying notes to consolidated financial statements.
F-6
APTOSE BIOSCIENCES INC.
(Expressed in thousands of US dollars, except for per common share data)
| Common Shares |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||
| Shares |
|
| Amount |
|
| Additional |
|
| Accumulated other |
|
| Deficit |
|
| Total |
| |||||||
Balance, December 31, 2022 |
|
| 6,158 |
|
| $ | 437,520 |
|
| $ | 68,869 |
|
| $ | (4,318 | ) |
| $ | (464,330 | ) |
| $ | 37,741 |
|
Common shares issued under |
|
| 668 |
|
|
| 2,989 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 2,989 |
|
Common shares issued in exchange for RSUs |
|
| 38 |
|
|
| 376 |
|
|
| (376 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
Common shares issued under |
|
| 337 |
|
|
| 1,809 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 1,809 |
|
Common shares issued under |
|
| 735 |
|
|
| 2,083 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 2,083 |
|
Stock-based compensation |
|
| — |
|
|
| — |
|
|
| 3,653 |
|
|
| — |
|
|
| — |
|
|
| 3,653 |
|
Common shares issued under the ESPP plan |
|
| 6 |
|
|
| 29 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 29 |
|
Other comprehensive gain / loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 2 |
|
|
| — |
|
|
| 2 |
|
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (51,207 | ) |
|
| (51,207 | ) |
Balance, December 31, 2023 |
|
| 7,942 |
|
| $ | 444,806 |
|
| $ | 72,146 |
|
| $ | (4,316 | ) |
| $ | (515,537 | ) |
| $ | (2,901 | ) |
Balance, December 31, 2021 |
|
| 6,147 |
|
| $ | 437,386 |
|
| $ | 63,673 |
|
| $ | (4,316 | ) |
| $ | (422,507 | ) |
| $ | 74,236 |
|
Common shares issued under |
|
| 5 |
|
|
| 51 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 51 |
|
Common shares issued under the May 2020 ATM |
|
| 4 |
|
|
| 50 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 50 |
|
Common shares issued upon exercise of stock options |
|
| 1 |
|
|
| 26 |
|
|
| (11 | ) |
|
| — |
|
|
| — |
|
|
| 15 |
|
Stock-based compensation |
|
| — |
|
|
| — |
|
|
| 5,207 |
|
|
| — |
|
|
| — |
|
|
| 5,207 |
|
Common shares issued under the ESPP plan |
|
| 1 |
|
|
| 7 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 7 |
|
Other comprehensive loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (2 | ) |
|
| — |
|
|
| (2 | ) |
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (41,823 | ) |
|
| (41,823 | ) |
Balance, December 31, 2022 |
|
| 6,158 |
|
| $ | 437,520 |
|
| $ | 68,869 |
|
| $ | (4,318 | ) |
| $ | (464,330 | ) |
| $ | 37,741 |
|
See accompanying notes to consolidated financial statements.
F-7
APTOSE BIOSCIENCES INC.
Consolidated Statements of Cash Flows
(Expressed in thousands of US dollars)
| Year ended December 31, 2023 |
|
| Year ended December 31, 2022 |
| |||
Cash flows from operating activities: |
|
|
|
|
|
| ||
Net loss for the year |
| $ | (51,207 | ) |
| $ | (41,823 | ) |
Items not involving cash: |
|
|
|
|
|
| ||
Stock-based compensation |
|
| 3,653 |
|
|
| 5,207 |
|
Depreciation and amortization |
|
| 88 |
|
|
| 120 |
|
Disposal of property and equipment |
|
| — |
|
|
| 16 |
|
Amortization of right-of-use assets |
|
| 378 |
|
|
| 408 |
|
Interest on lease liabilities |
|
| 93 |
|
|
| 35 |
|
Unrealized foreign exchange gain/(loss) |
|
| — |
|
|
| 4 |
|
Accrued interest on investments |
|
| — |
|
|
| (60 | ) |
Change in operating working capital: |
|
|
|
|
|
| ||
Prepaid expenses |
|
| 261 |
|
|
| 173 |
|
Operating lease payments |
|
| (405 | ) |
|
| (546 | ) |
Other assets |
|
| (343 | ) |
|
| (124 | ) |
Accounts payable, related party |
|
| (430 | ) |
|
| 2,984 |
|
Accounts payable |
|
| 150 |
|
|
| 4,627 |
|
Accrued liabilities, related party |
|
| (572 | ) |
|
| 572 |
|
Accrued liabilities |
|
| 3,744 |
|
|
| (3,915 | ) |
Cash used in operating activities |
|
| (44,590 | ) |
|
| (32,322 | ) |
Cash flows from financing activities: |
|
|
|
|
|
| ||
Issuance of common shares to Hanmi |
|
| 2,989 |
|
|
| — |
|
Issuance of common shares under Committed Equity Facility |
|
| 2,083 |
|
|
| — |
|
Issuance of common shares under 2022 ATM |
|
| 1,809 |
|
|
| 51 |
|
Issuance of common shares under 2020 ATM |
|
| — |
|
|
| 50 |
|
Issuance of commons shares under the ESPP share purchase |
|
| 29 |
|
|
| — |
|
Issuance of common shares pursuant to exercise of stock options |
|
| — |
|
|
| 15 |
|
Cash provided by financing activities |
|
| 6,910 |
|
|
| 116 |
|
Cash flows from (used in) investing activities: |
|
|
|
|
|
| ||
Maturity (acquisition) of investments, net |
|
| 9,989 |
|
|
| 30,090 |
|
Purchase of property and equipment |
|
| (29 | ) |
|
| (24 | ) |
Cash provided by (used in) investing activities |
|
| 9,960 |
|
|
| 30,066 |
|
Effect of exchange rate fluctuations on cash and cash equivalents held |
|
| 2 |
|
|
| (4 | ) |
Decrease in cash and cash equivalents |
|
| (27,718 | ) |
|
| (2,144 | ) |
Cash and cash equivalents, beginning of year |
|
| 36,970 |
|
|
| 39,114 |
|
Cash and cash equivalents, end of year |
| $ | 9,252 |
|
| $ | 36,970 |
|
See accompanying notes to consolidated financial statements.
F-8
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
1. Reporting entity:
Aptose Biosciences Inc. (“Aptose,” “the Company,” “we,” “us,” or “our”) is a science-driven, clinical-stage biotechnology company committed to the development and commercialization of precision medicines addressing unmet clinical needs in oncology, with an initial focus on hematology. The Company's small molecule cancer therapeutics pipeline includes products designed to provide single agent efficacy and to enhance the efficacy of other anti-cancer therapies and regimens without overlapping toxicities. The Company’s executive office is located in San Diego, California, and our head office is located in Toronto, Canada.
We are advancing targeted agents to treat life-threatening hematologic cancers that, in most cases, are not elective for patients and require immediate treatment. We have two clinical-stage investigational products for hematological malignancies: tuspetinib, an oral, potent myeloid kinase inhibitor, and luxeptinib, an oral, dual lymphoid and myeloid kinase inhibitor.
Since our inception, we have financed our operations and technology acquisitions primarily from equity financing, proceeds from the exercise of warrants and stock options, and interest income on funds held for future investment. Our uses of cash for operating activities have primarily consisted of salaries and wages for our employees, facility and facility-related costs for our offices and laboratories, fees paid in connection with preclinical and clinical studies, licensing fees, drug manufacturing costs, laboratory supplies and materials, and professional fees.
Management recognizes that in order for us to meet our capital requirements, and continue to operate, additional financing will be necessary. We plan to raise additional funds to fund our business operations but there is no assurance that such additional funds will be available for us to finance our operations on acceptable terms, if at all. These conditions raise substantial doubt about the Company’s ability to continue as a going concern, see Note 2(a), Going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
In order for the Company to meet its capital requirements, and continue to operate, additional financing will be necessary. The Company is evaluating strategies to obtain the required additional funding for future operations. These strategies may include, but are not limited to, obtaining equity financing, and restructuring of operations to decrease expenses. However, given the impact of the economic downturn on the U.S. and global financial markets, the Company may be unable to access further equity or when needed. As such, there can be no assurance that the Company will be able to obtain additional liquidity when needed or under acceptable terms, if at all. The consolidated financial statements do not reflect any adjustments to the carrying amounts and classification of assets, liabilities, and reported expenses that may be necessary if the Company were unable to continue as a going concern. Such adjustments may be material.
Our ability to raise additional funds could be affected by adverse market conditions, the status of our product pipeline, possible delays in enrollment in our trial, and various other factors and we may be unable to raise capital when needed, or on terms favorable to us. If necessary funds are not available, we may have to delay, reduce the scope of, or eliminate some of our development programs, potentially delaying the time to market for any of our product candidates.
We do not expect to generate positive cash flow from operations for the foreseeable future due to the early stage of our clinical trials. It is expected that negative cash flow will continue until such time, if ever, that we receive regulatory approval to commercialize any of our products under development and/or royalty or milestone revenue from any such products exceeds expenses.
Our cash needs for the next twelve months include estimates of the number of patients and rate of enrollment of our clinical trials, the amount of drug product that we will require to support our clinical trials, and our general corporate overhead costs to support our operations, and our reliance on our manufacturers. We have based these estimates on assumptions and plans which may change and which could impact the magnitude and/or timing of operating expenses and our cash runway, See Note 2(a), Going concern.
F-9
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
The Company's financial statements are prepared using generally accepted accounting principles in the United States of America applicable to a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business. As of December 31, 2023, the Company had an accumulated deficit of approximately $515.5 million (December 31, 2022, $464.3 million); cash and cash equivalents and investment balances of approximately $9.3 million (December 31, 2022, $47.0 million); negative working capital of approximately $3.4 million (December 31, 2022, positive working capital of $37.2 million); and negative shareholder's equity of $2.9 million (December 31, 2022, positive shareholder's equity of $37.7 million). Management recognizes that in order to meet the capital requirements, and continue to operate, additional financing will be necessary. The Company plans to raise additional funds to fund our business operations through equity financing under the 2022 ATM Facility, the 2023 Committed Equity Facility and other facilities, as further described in Note 12. Management continues considering other options for raising capital including debt, equity, collaborations, and reorganization to reduce operational expenses. However, given the impact of the financial markets, and the matter in Note 17, Subsequent events, that may impact the Company's ability to raise financing in the capital markets, the Company may be unable to access financing when needed, if at all. As such, there can be no assurance that the Company will be able to obtain additional liquidity when needed or under acceptable terms, if at all. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. For information about financing events and the regulatory matter that may impact the Company's ability to raise financing in the capital markets which arose subsequent to December 31, 2023, see Note 17, Subsequent events.
On May 23, 2023, during the Aptose Annual and Special Meeting of Shareholders, our shareholders voted to approve special resolutions providing for an amendment to our articles of incorporation to effect a reverse share split of our outstanding Common Shares, at a ratio in the range of 1-for-10 to 1-for-20. Our Board of Directors then approved a ratio of 1-for-15 on May 23, 2023. On May 24, 2023, we filed articles of amendment under the Canada Business Corporations Act ("CBCA") to give effect to the reverse stock split (consolidation) of our Common Shares on the basis of one post-consolidation Common Share for each 15 pre-consolidation Common Shares (the “Reverse Stock Split”). The Common Shares commenced trading on a post-Reverse Stock Split basis at market open on Tuesday, June 6, 2023. All references in this report to historical Common Share prices, numbers of Common Shares, and earnings per share calculations have been presented to reflect the effect of the Reverse Stock Split.
These consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States, or GAAP and the rules and regulations of the Securities and Exchange Commission, or SEC, related to annual reports filed on Form 10-K, assuming the Company will continue as a going concern. The going concern assumption contemplates the realization of assets and satisfaction of liabilities in the normal course of business. However, substantial doubt about the Company's ability to continue as a going concern exists. As of the filing date, we have sufficient liquidity to support the Company's operations until August 2024.
In order for the Company to meet its capital requirements, and continue to operate, additional financing will be necessary. The Company is evaluating strategies to obtain the required additional funding for future operations. These strategies may include, but are not limited to, obtaining equity financing, and restructuring of operations to decrease expenses. However, given the challenges in the U.S. and global financial markets, and the matter in Note 17, Subsequent events, that may impact the Company's ability to raise financing in the capital markets, the Company may be unable to access further equity or when needed, if at all. As the Company is primarily pursuing one compound that is licensed from a related party with significant licensing payments who will have influence on the Company, other investors may not be willing to invest in the Company. As such, there can be no assurance that the Company will be able to obtain additional liquidity when needed or under acceptable terms, if at all. The consolidated financial statements do not reflect any adjustments to the carrying amounts and classification of assets, liabilities, and reported expenses that may be necessary if the Company were unable to continue as a going concern. Such adjustments may be material.
The functional and presentation currency of the Company is the US dollar.
F-10
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
These consolidated financial statements include the accounts of its subsidiaries. All intercompany transactions, balances, revenue, and expenses are eliminated on consolidation.
The preparation of the consolidated financial statements requires management to make judgments, estimates and assumptions that affect the application of accounting policies and reported amounts of assets and liabilities at the date of the consolidated financial statements and reported amounts of revenue and expenses during the reporting period. Actual outcomes could differ from those estimates. The consolidated financial statements contain estimates, which by their nature, are uncertain.
The impacts of such estimates are pervasive throughout the consolidated financial statements and mayrequire accounting adjustments based on future occurrences.
The estimates and underlying assumptions are reviewed on a regular basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised and in any future periods affected.
The Company’s operating leases of tangible property with terms greater than twelve months are recognized as right of use assets, which represents the lessee’s right to use, or control the use of, a specified asset for the lease term, and a corresponding lease liability, which represents the lessee’s obligation to make lease payments under a lease, measured on a discounted basis. Landlord inducements in the form of free rent periods are netted against lease payments to the landlord in measuring right-of-use assets and lease liabilities.
(e) Cash and cash equivalents:
Cash and cash equivalents are short-term highly liquid investments with original maturities of 90 days or less as of the date of purchase. Cash equivalents are accounted for an amortized cost basis, which approximates their fair value due to their short-term maturities.
Investments consist of term deposits with original maturities greater than 90 days and are classified by management as securities available-for-sale. These available-for-sale securities are recorded at estimated fair values. Unrealized gains and losses on these investments are recorded in accumulated other comprehensive income (AOCI) in shareholder’s equity. Realized gains and losses and declines in value that are judged to be other than temporary are included in interest income.
The Company is subject to credit risk from the Company’s cash and cash equivalents and investments. The carrying amount of the financial assets represents the maximum credit exposure. The Company manages credit risk associated with its cash and cash equivalents and investments by maintaining minimum standards of R1‑low or A‑low investments and the Company invests only in highly rated corporations and treasury bills, which are capable of prompt liquidation.
F-11
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
The Company has cash accounts in Canada and the US. The Canada Deposit Insurance Corporation (CDIC) and the US Federal Deposit Insurance Corporation (FDIC) provide insurance to protect depositors against the loss of their deposits in case of a bank failure. However, the maximum amount of coverage varies by jurisdiction and account type. In Canada, the CDIC insures eligible deposits up to $100,000 (CAD) per depositor, per insured category, per member institution. In the United States, the FDIC insures deposits up to $250,000 per depositor, per insured bank, for each account ownership category. It is important to note that not all deposits are eligible for insurance coverage. For example, deposits in foreign currency, deposits held in trust, and investments such as mutual funds, stocks, and bonds are not insured by either the FDIC or CDIC.
The Company is subject to intermediary risk associated with the actions of financial intermediaries, such as banks or investment managers, who act on behalf of clients to buy and sell assets. The Company has diversified the investments with two large financial institutions to reduce the concentration of risk in any one institution, and spread the risk. This measure reduces the likelihood of being significantly impacted by the failure of a single financial institution.
The Company has reduced the exposure to individual investment vehicles to minimize the risk of loss in case of adverse events. The Company has diversified the investment portfolio across different asset classes and investment vehicles to achieve this goal.
(h) Property and equipment:
Property and equipment is measured at cost less accumulated depreciation and accumulated impairment losses. Cost includes expenditures that are directly attributable to the acquisition of the asset. The Company records depreciation at rates that charge operations with the cost of the assets over their estimated useful lives on a straight‑line basis as follows:
|
| |
|
| |
|
| |
|
| |
|
|
The residual value, useful life and methods of depreciation of the assets are reviewed at each reporting period and adjusted prospectively if appropriate.
Research and development (R&D) costs are expensed as incurred. R&D costs consist primarily of salaries and benefits, stock-based compensation, manufacturing, contract services, clinical trials and research related overhead. Non-refundable advance payments for goods and services that will be used in future research are recorded in prepaid and other assets and are expensed when the services are performed.
The Company records expenses for research and development activities based on Management’s estimates of services received and efforts expended pursuant to contracts with vendors that conduct research and development on the Company’s behalf. The financial terms vary from contract to contract and may result in uneven payment flows as compared with services performed or products delivered. As a result, the Company is required to estimate research and development expenses incurred during the period, which impacts the amount of accrued expenses and prepaid balances related to such costs as of each balance sheet date. Management estimates the amount of work completed through discussions with internal personnel and the contract research and contract manufacturing organizations as to the progress or stage of completion of the services. The Company’s estimates are based on a number of factors, including the Company’s knowledge of the status of each of the research and development project milestones, and contract terms together with related executed change orders. Management makes significant judgments and estimates in determining the accrued balance at the end of each reporting period.
F-12
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
The Company measures its financial assets and liabilities at fair value. The carrying amounts for the Company’s financial instruments, including cash and cash equivalents, accounts payable and accrued liabilities approximate their fair value due to their short maturities. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date.
The Company has a stock‑based compensation plan (the “Plan”) available to officers, directors, employees, and consultants with grants under the Plan approved by the Company’s Board of Directors. Under the Plan, the exercise price of each option equals the closing trading price of the Company’s stock on the day prior to the grant if the grant is made during the trading day or the closing trading price on the day of the grant if the grant is issued after markets have closed. Vesting is provided for at the discretion of the Board of Directors and the expiration of options is to be no greater than 10 years from the date of grant.
The Company uses the fair value based method of accounting for employee awards granted under the Plan. The Company calculates the fair value of each stock option grant using the Black‑Scholes option pricing model at the grant date. The stock‑based compensation cost of the options is recognized as stock‑based compensation expense over the relevant vesting period of the stock options using an estimate of the number of options that will eventually vest.
Stock options awarded to non‑employees are measured at the grant-date fair value of the equity instruments issued in accordance with FASB accounting standards update No 2018-07, Topic 718.
The Company has a stock incentive plan pursuant to which the Board maygrant equity settled stock‑based awards comprised of restricted stock units or dividend equivalents to employees, officers, consultants, independent contractors, advisors and non‑employee directors of the Company. Compensation cost for restricted share units is measured at fair value at the date of grant, which is the market price of the underlying security, and is expensed over the award’s vesting period on a straight-line basis using an estimate of the number of awards that will eventually vest.
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker, or CODM. The Company’s Chief Executive Officer serves as its CODM. The Company views its operations and manages its business as one segment, which is the discovery and development of personalized therapies addressing unmet medical needs in oncology. The Company operates primarily in the U.S.
Basic loss per share is computed by dividing the net loss available to common shareholders by the weighted average number of shares outstanding during the year. Diluted loss per share is computed similarly to basic loss per share except that the weighted average share outstanding is increased to include additional shares for the assumed exercise of stock options and warrants, if dilutive. The number of additional shares is calculated by assuming that outstanding stock options and warrants were exercised and that the proceeds from such exercises were used to acquire common stock at the average market price during the year. The inclusion of the Company’s stock options and warrants in the computation of diluted loss per share has an anti‑dilutive effect on the loss per share and, therefore, they have been excluded from the calculation of diluted loss per share.
F-13
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
The Company accounts for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted rates in effect for the year in which these temporary differences are expected to be recovered or settled. Valuation allowances are provided if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company provides reserves for potential payments of tax to various tax authorities related to uncertain tax positions and other issues. Reserves are based on a determination of whether and how much of a tax benefit taken by the Company in its tax filing is more likely than not to be realized following resolution of any potential contingencies present related to the tax benefit. Potential interest and penalties associated with such uncertain tax positions are recorded as components of income tax expense. As of December 31, 2023 and December 31, 2022, the Company has not recorded any reserves for potential payments as the Company has a history of losses and does not have any revenue from operations.
(o) Recent Accounting Pronouncements
There were various accounting standards and interpretations issued recently, none of which are expected to have a material impact on our financial position, operations, or cash flows.
Cash and cash equivalents consists of cash of $2,764 thousand (December 31, 2022 ‑ $596 thousand) and deposits in high interest savings accounts, money market funds and accounts with original maturities less than 90 days totaling $6,488 thousand (December 31, 2022 ‑ $36,374 thousand).
| December 31, |
|
| December 31, |
| |||
Prepaid research and development expenses |
| $ | 720 |
|
| $ | 1,271 |
|
Prepaid insurance |
|
| 882 |
|
|
| 893 |
|
Other prepaid expenses |
|
| 440 |
|
|
| 139 |
|
Total |
| $ | 2,042 |
|
| $ | 2,303 |
|
F-14
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
December 31, 2023 |
| Cost |
|
| Accumulated |
|
| Net book value |
| |||
Laboratory equipment |
| $ | 197 |
|
| $ | 87 |
|
| $ | 110 |
|
Computer hardware |
|
| 29 |
|
|
| 25 |
|
|
| 4 |
|
Computer software |
|
| 222 |
|
|
| 222 |
|
|
| 0 |
|
Office furniture |
|
| 140 |
|
|
| 132 |
|
|
| 8 |
|
Leasehold improvements |
|
| 213 |
|
|
| 183 |
|
|
| 30 |
|
Total |
| $ | 801 |
|
| $ | 649 |
|
| $ | 152 |
|
|
|
|
|
|
|
|
|
|
| |||
December 31, 2022 |
| Cost |
|
| Accumulated |
|
| Net book value |
| |||
Laboratory equipment |
| $ | 197 |
|
| $ | 48 |
|
| $ | 149 |
|
Computer hardware |
|
| 198 |
|
|
| 177 |
|
|
| 21 |
|
Computer software |
|
| 222 |
|
|
| 222 |
|
|
| — |
|
Office furniture |
|
| 140 |
|
|
| 117 |
|
|
| 23 |
|
Leasehold improvements |
|
| 184 |
|
|
| 166 |
|
|
| 18 |
|
Total |
| $ | 941 |
|
| $ | 730 |
|
| $ | 211 |
|
In the year ended December 31, 2022, the company recorded a loss on disposition of fixed assets of $16 thousand, with these assets having had an original cost of $196 thousand and accumulated depreciation of $180 thousand. There was no loss on disposition of fixed assets in the year ended December 31, 2023. In the years ended December 31, 2023 and December 31, 2022, the Company had additions to fixed assets of $29 thousand and $24 thousand, respectively.
| Year ended December 31, 2023 |
|
| Year ended December 31, 2022 |
| |||
|
|
|
|
|
|
| ||
Right-of-use assets, beginning of year |
| $ | 3,100 |
|
| $ | 1,860 |
|
Additions to right-of-use assets |
|
| 24 |
|
|
| 1,240 |
|
Right-of-use assets, end of year |
|
| 3,124 |
|
|
| 3,100 |
|
Accumulated amortization |
|
| (2,181 | ) |
|
| (1,803 | ) |
Right-of use assets, NBV |
| $ | 943 |
|
| $ | 1,297 |
|
Investments consisted of the following as of December 31, 2023 and December 31, 2022:
| ||||||||||||
|
|
| ||||||||||
|
|
|
|
|
|
| ||||||
|
|
|
|
|
|
| ||||||
| December 31, 2022 |
| ||||||||||
| Cost |
|
| Unrealized gain |
|
| Market value |
| ||||
United States Treasury Bills |
| $ | 9,991 |
|
| $ | (2 | ) |
| $ | 9,989 |
|
Total |
| $ | 9,991 |
|
| $ | (2 | ) |
| $ | 9,989 |
|
F-15
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
The fair value hierarchy establishes three levels to classify the inputs to valuation techniques used to measure fair value.
Level 1 ‑ inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 ‑ inputs are quoted prices in markets that are not active, quoted prices for similar assets or liabilities in active markets, inputs other than quoted prices that are observable for the asset or liability, or inputs that are derived principally from or corroborated by observable market data or other means; and
Level 3 ‑ inputs are unobservable (supported by little or no market activity).
The fair value hierarchy gives the highest priority to Level 1 inputs and the lowest priority to Level 3 inputs.
The following table presents the fair value of the Company’s financial instruments for the periods presented:
| December 31, |
|
| Level 1 |
|
| Level 2 |
|
| Level 3 |
| |||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
| ||||
High interest savings accounts |
| $ | 2,002 |
|
| $ | — |
|
| $ | 2,002 |
|
| $ | — |
|
United States Treasury Bills |
|
| 4,486 |
|
|
| — |
|
|
| 4,486 |
|
|
| — |
|
Total |
| $ | 6,488 |
|
| $ | — |
|
| $ | 6,488 |
|
| $ | — |
|
| December 31, |
|
| Level 1 |
|
| Level 2 |
|
| Level 3 |
| |||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
| ||||
Money Market accounts |
| $ | 165 |
|
| $ | — |
|
| $ | 165 |
|
| $ | — |
|
Money Market Funds |
|
| 22,343 |
|
|
| — |
|
|
| 22,343 |
|
|
| — |
|
High interest savings accounts |
|
| 13,866 |
|
|
| — |
|
|
| 13,866 |
|
|
| — |
|
United States Treasury Bills |
|
| 9,989 |
|
|
| — |
|
|
| 9,989 |
|
|
| — |
|
Total |
| $ | 46,363 |
|
| $ | — |
|
| $ | 46,363 |
|
| $ | — |
|
Accrued liabilities as of December 31, 2023 and December 31, 2022 consisted of the following:
| December 31, |
|
| December 31, |
| |||
Accrued personnel-related costs |
| $ | 1,989 |
|
| $ | 2,302 |
|
Accrued research and development expenses |
|
| 6,527 |
|
|
| 2,550 |
|
Other accrued expenses |
|
| 313 |
|
|
| 233 |
|
Total |
| $ | 8,829 |
|
| $ | 5,085 |
|
Aptose leases office space in San Diego, California and Toronto, Canada. The lease for the San Diego office space is scheduled to expire in May 31, 2026. Aptose previously leased lab space in San Diego, which we exited prior to the expiration of the lease on February 28, 2023. The costs incurred in exiting this laboratory space were not material. We lease office space in Toronto, Ontario, Canada, with this lease scheduled to expire on June 30, 2024. The Company has not included any extension periods in calculating its right-to-use assets and lease liabilities. The Company also enters into leases for small office equipment.
F-16
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
Minimum payments, undiscounted, under our operating leases are as follows:
Years ending December 31, |
|
|
| |
2024 |
|
| 459 |
|
2025 |
|
| 462 |
|
2026 |
|
| 198 |
|
Total |
| $ | 1,119 |
|
To calculate the lease liability, the lease payments in the table above were discounted over the remaining term of the leases using the Company’s incremental borrowing rate as of January 1, 2019 for existing leases at the time of adopting ASC 842, and for new leases after the date adoption, as of the date of the execution date of the new lease. The following table presents the weighted average remaining term of the leases and the weighted average discount rate:
| December 31, |
|
| December 31, |
| |||
Weighted-average remaining term – operating |
|
| 2.4 |
|
|
| 3.3 |
|
Weighted-average discount rate – operating |
|
| 7.38 | % |
|
| 6.62 | % |
Lease liability, current portion |
| $ | 394 |
|
| $ | 301 |
|
Lease liability, long term portion |
|
| 621 |
|
|
| 1,002 |
|
Lease liability, total |
| $ | 1,015 |
|
| $ | 1,303 |
|
Operating lease costs and operating cash flows from our operating leases are as follows:
| Year ended |
|
| Year ended |
| |||
|
|
|
|
|
|
| ||
Operating lease cost |
| $ | 471 |
|
| $ | 443 |
|
Operating cash flows from operating leases |
| $ | 405 |
|
| $ | 546 |
|
Hanmi Pharmaceutical Co. Ltd.
On November 4, 2021, Aptose entered into a licensing agreement (the "Tuspetinib Licensing Agreement") with the South Korean company Hanmi. Under the terms of the Tuspetinib Licensing Agreement, Hanmi granted Aptose exclusive worldwide rights to tuspetinib for all indications. Hanmi received an upfront payment of $12.5 million, including $5 million in cash and $7.5 million in Common Shares. Aptose issued Hanmi 215,703 Common Shares in this upfront licensing payment. Hanmi will also receive up to $407.5 million in future milestone payments contingent upon achieving certain clinical, regulatory and sales milestones across several potential indications, as well as tiered royalties on net sales. The term of the agreement will continue on a product-by-product and country-by-country basis until the expiration of the royalty period for such product in such country. The licenses to Aptose will survive and become non-exclusive, perpetual, irrevocable and fully paid-up on a product-by-product and country-by-country basis, upon their natural expiration under the terms of the agreement.
In 2022, the Company and Hanmi also entered into a separate supply agreement for additional production of new drug substance ("API") and drug product to support further tuspetinib clinical development, for which the Company pays Hanmi per batch of production. Expenses related to this supply agreement have been recognized by the Company, amounting to $3.5 million and approximately $3.6 million, for the years ended December 31, 2023 and 2022, respectively. Since inception to December 31, 2023, $7.1 million has been recognized for the period under the supply agreement.
The Company paid supply costs to Hanmi of $4.5 million and nil in the years ended December 31, 2023 and 2022, respectively. Since inception to December 31, 2023, payments of $4.5 million have been made under the supply
F-17
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
agreement. At December 31, 2023, there was $2.6 million in accounts payable and nil in accrued liabilities to Hanmi related to the supply agreement. (At December 31, 2022, there was $3.0 million in accounts payable and $0.6 million in accrued liabilities). Transactions involving related parties cannot be presumed to be carried out on an arm's-length basis, as the requisite conditions of competitive, free-market dealings may not exist.
On August 10, 2023, the Company entered into a binding term sheet with Hanmi whereby Hanmi agreed at their sole discretion to invest, up to a maximum of $7 million in Aptose up to a total ownership of 19.99 percent of Aptose by Hanmi. On September 6, 2023, the Company entered into a subscription agreement with Hanmi, pursuant to which the Company sold 668,449 Common Shares to Hanmi for gross proceeds of $3 million. The issuance of shares, in exchange for gross proceeds of $3 million, is recorded in Common Shares on the balance sheet as of December 31, 2023. For information regarding Hanmi's investment subsequent to December 31, 2023, see Note 17, Subsequent events.
Hanmi held 884,152 Common Shares of Aptose as of December 31, 2023. As of the filing date, the total common share ownership by Hanmi was 2,989,414, representing ownership of 19.03% of the outstanding Common Shares of the Company. Hanmi also owns 2,339,181 warrants to purchase Common Shares at an exchange price of $1.71 per Warrant Share. See Note 17, Subsequent events for information about shares sold to Hanmi subsequent to December 31, 2023 and the Nasdaq Deficiency Letter.
The Company has authorized share capital of an unlimited number of common voting shares.
(i) Hanmi
On August 10, 2023, the Company entered into a binding term sheet with Hanmi whereby Hanmi agreed at their sole discretion to invest, up to a maximum of $7 million in Aptose up to a total ownership of 19.99 percent of Aptose by Hanmi. On September 6, 2023, the Company entered into a subscription agreement with Hanmi, pursuant to which the Company sold 668,449 Common Shares to Hanmi for proceeds of $3 million.
Hanmi held 884,152 Common Shares of Aptose as of December 31, 2023 See Note 17, Subsequent events.
(ii) 2023 Committed Equity Facility
On May 25, 2023, the Company and Keystone Capital Partners, LLC ("Keystone") entered into a committed equity facility, (the "2023 Committed Equity Facility"), which provides that subject to the terms and conditions set forth therein, we may sell to Keystone up to the lesser of (i) $25.0 million of the Common Shares and (ii) a number of Common Shares equal to 19.99% of the Common Shares outstanding immediately prior to the execution of the 2023 Committed Equity Facility Agreement with Keystone which respect to the 2023 Committed Equity Facility (subject to certain exceptions) (the “Total Commitment”), from time to time during the 24-month term of the 2023 Committed Equity Facility. Additionally, on May 25, 2023, the Company entered into a Registration Rights Agreement with Keystone, pursuant to which the Company agreed to file a registration statement with the SEC covering the resale of Common Shares that are issued to Keystone under the 2023 Committed Equity Facility. This registration statement became effective on June 30, 2023 and the 2023 Committed Equity Facility commencement date was July 12, 2023 (the "Commencement Date").
Upon entering into the 2023 Committed Equity Facility, the Company agreed to issue to Keystone an aggregate of 25,156 Common Shares (the “Commitment Shares”) as consideration for Keystone’s commitment to purchase Common Shares upon the Company’s direction under the 2023 Committed Equity Facility. The Company issued 7,547 Common Shares, or 30% of the Commitment Shares, on the date of the 2023 Committed Equity Facility Agreement (the “Initial Commitment Shares”). An additional 7,547
F-18
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
Common Shares, or 30% of the Commitment Shares, were issued to Keystone 90 days following the Commencement Date (the “First Back-End Commitment Shares”). The remaining 10,062 Common Shares, or 40% of the Commitment Shares, shall be issued to Keystone 180 days following the Commencement Date (the “Second Back-End Commitment Shares”, together with the First Back-End Commitment Shares, the “Back-End Commitment Shares”). See Note 17, Subsequent events.
In the year ended December 31, 2023, the Company's issuance of Common Shares to Keystone comprised 720,494 Common shares sold to Keystone at an average price of $2.91 per Common share for cash proceeds of $2.1 million and the 15,094 Commitment Shares. See Note 17, Subsequent events.
(iii) 2022 At-The-Market Facility
On December 9, 2022, the Company entered into an equity distribution agreement pursuant to which the Company may, from time to time, sell Common Shares having an aggregate offering value of up to $50 million through Jones Trading Institutional Services LLC ("Jones Trading") on Nasdaq (the "2022 ATM Facility"). During the year ended December 31, 2022, the Company issued 4,836 Common Shares under this 2022 ATM Facility at an average price of $10.81 per Common Share for gross proceeds of $52 thousand ($51 thousand net of share issuance costs). During the year ended December 31, 2023, the Company issued 336,690 Common Shares under this 2022 ATM Facility at an average price of $5.62 for gross proceeds of $1.9 million ($1.8 million net of share issuance costs). Since inception to December 31, 2023, the Company had raised a total of $1.9 million gross proceeds ($1.9 million net of share issuance costs) under the 2022 ATM Facility. Costs associated with the proceeds consisted of 3% cash commission.
(iv) 2020 At-The-Market Facility
On May 5, 2020, the Company entered an “at-the-market” equity distribution agreement with Piper Sandler & Co. (“Piper Sandler”) and Canaccord Genuity LLC (“Canaccord Genuity”) acting as co-agents (the “2020 ATM Facility”). Under the terms of the 2020 ATM Facility, the Company could, from time to time, sell Common Shares having an aggregate offering value of up to $75 million through Piper Sandler and Canaccord Genuity on Nasdaq. During the period from January 1, 2022 to October 31, 2022, the date the Agreement was terminated, the company issued During the year ended December 31, 2022, the Company issued 3,646 Common Shares under the 2020 ATM Facility at an average price of $14.25 per Common Share for gross proceeds of $52 thousand ($50 thousand net of share issuance costs). As of October 31, 2022, the date the 2020 ATM Facility was terminated, the Company had raised a total of $89 thousand gross proceeds ($86 thousand net of share issuance costs) under the 2020 ATM Facility. Costs associated with the proceeds consisted of a 3% cash commission.
For information regarding recent equity issuances subsequent to December 31, 2023, see Note 17, Subsequent events.
Loss per common share is calculated using the weighted average number of Common Shares outstanding and is presented in the table below:
(in thousands) |
| Year ended December 31, 2023 |
|
| Year ended December 31, 2022 |
| ||
Net loss |
| $ | (51,207 | ) |
| $ | (41,823 | ) |
Weighted-average common shares – basic |
|
| 6,755 |
|
|
| 6,151 |
|
Net loss per share – basic and diluted |
| $ | (7.58 | ) |
| $ | (6.80 | ) |
The effect of any potential exercise of the Company’s stock options outstanding during the years ended December 31, 2023 and December 31, 2022 has been excluded from the calculation of diluted loss per common share as it would be anti‑dilutive.
F-19
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
Effective June 1, 2021,the Company adopted a new stock incentive plan (New Incentive Plan) and an employee stock purchase plan (ESPP).
The New Incentive Plan authorizes the Board of Directors to administer the New Incentive Plan to provide equity‑based compensation in the form of stock options, stock appreciation rights., restricted stock, restricted stock units and Dividend Equivalents.
The Corporation currently maintains its existing Share Option Plan and 2015 Stock Incentive Plan (2015 SIP). Effective June 1, 2021 no further grants will be made under the Share Option Plan or 2015 SIP, though existing grants under the Share Option Plan will remain in effect in accordance with their terms.
The aggregate number of our common shares, no par value, that may be issued under all awards under the New Incentive Plan is (i) 691,400, plus (ii) any of our Common Shares subject to any outstanding award under our prior plans that, after June 1, 2021, are not purchased or are forfeited or reacquired by us, or otherwise not delivered to the participant due to termination, cancellation or cash settlement of such award subject to the share counting provisions of the New Incentive Plan.
Under both the Share Option Plan and the New Incentive Plan, the exercise price of each option equals the closing trading price of the Company’s stock on the day prior to the grant if the grant is made during the trading day or the closing trading price on the day of grant if the grant is issued after markets have closed. Vesting is provided for at the discretion of the Board of Directors and the expiration of options is to be no greater than 10 years from the date of grant.
The Company uses the fair value-based method of accounting for employee awards granted under both plans. The Company calculates the fair value of each stock option grant using the Black‑Scholes option pricing model at the grant date. The stock‑based compensation cost of the options is recognized as stock‑based compensation expense over the relevant vesting period of the stock options using an estimate of the number of options that will eventually vest.
The ESPP, which is administered by the Board of Directors, allows eligible employees of the Company with an opportunity to purchase Common Shares through accumulated payroll deductions up to a maximum 15% of eligible compensation. The ESPP will be implemented by consecutive offering periods with a new offering period commencing on the first trading day on or after February 1 and August 1 each year, or on such other date as the Board of Directors will determine, and continuing thereafter until terminated in accordance with the Plan. Unless the Board of Directors provides otherwise, the purchase price will be equal to eighty‑five percent (85%) of the fair market value of a Common Share on the offering date or the exercise date, whichever is lower.
The maximum number of Common Shares which will be made available for sale under the ESPP will be 113,333 Common Shares.
The first six-month offering period began on February 1, 2022 and ended on August 1, 2022. The second six-month period began on August 1, 2022 and ended on February 1, 2023. The third six-month period began on February 2, 2023, and ended on August 1, 2023. There were 5,891 and 724 Common Shares issued under the ESPP in the years ended December 31, 2023 and 2022, respectively.
F-20
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
Stock option transactions for the year ended December 31, 2023 and December 31, 2022, are summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| ||||
| Options |
|
| Weighted average |
|
| Weighted |
|
| Aggregate |
| |||||
|
| (in thousands) |
|
|
|
|
|
|
|
|
|
| ||||
Outstanding, December 31, 2021 |
|
| 1,007 |
|
| $ | 69.15 |
|
|
|
|
|
|
| ||
Granted |
|
| 420 |
|
|
| 17.85 |
|
|
|
|
|
|
| ||
Exercised |
|
| (1 | ) |
|
| 16.20 |
|
|
|
|
|
|
| ||
Forfeited |
|
| (326 | ) |
|
| 57.60 |
|
|
|
|
|
|
| ||
Outstanding, December 31, 2022 |
|
| 1,100 |
|
| $ | 52.20 |
|
|
|
|
|
|
| ||
Granted |
|
| 217 |
|
|
| 9.87 |
|
|
|
|
|
|
| ||
Exercised |
|
| 0 |
|
|
|
|
|
|
|
|
|
| |||
Forfeited |
|
| (133 | ) |
|
| 50.87 |
|
|
|
|
|
|
| ||
Outstanding, December 31, 2023 |
|
| 1,184 |
|
| $ | 44.78 |
|
|
| 6.9 |
|
| $ | — |
|
Exercisable, December 31, 2023 |
|
| 714 |
|
| $ | 59.00 |
|
|
| 5.9 |
|
| $ | — |
|
Vested and expected to vest, December 31, 2023 |
|
| 1,099 |
|
| $ | 46.46 |
|
|
| 6.8 |
|
| $ | — |
|
Aggregate intrinsic value represents the excess of the value of the closing stock price on the previous trading day of the respective balance sheet dates over the exercise price of the stock options. Total intrinsic value of options exercised was nil for 2023 (2022 – $3 thousand).
As of December 31, 2023, there was $1.2 million of total unrecognized compensation cost related to non-vested stock options, which is expected to be recognized over an estimated weighted-average period of 1.7 years.
The following table presents the weighted average assumptions that were used in the Black‑Scholes option pricing model to determine the fair value of stock options granted during the period, and the resultant weighted average fair values:
| Year ended December 31, 2023 |
|
| Year ended December 31, 2022 |
| |||
Risk-free interest rate |
|
| 3.42 | % |
|
| 2.17 | % |
Expected dividend yield |
|
| — |
|
|
| — |
|
Expected volatility |
|
| 80.3 | % |
|
| 82.7 | % |
Expected life of options (years) |
|
| 5 |
|
|
| 5 |
|
Grant date fair value |
| $ | 6.53 |
|
| $ | 11.85 |
|
The Company uses historical data to estimate the expected dividend yield and expected volatility of its Common Shares in determining the fair value of stock options. The expected life of the options represents the estimated length of time the options are expected to remain outstanding.
The following table presents the vesting terms of options granted in the period:
|
| Year ended December 31, 2023 |
|
| Year ended December 31, 2022 |
| ||
|
| Options |
|
| Options |
| ||
| (in thousands) |
|
| (in thousands) |
| |||
3-year vesting (50%-25%-25%) |
|
| 48 |
|
|
| 28 |
|
4-year vesting (50%-16 2/3%-16 2/3%-16 2/3%) |
|
| 169 |
|
|
| 352 |
|
Earlier of performance criteria or 4 years |
|
| — |
|
|
| 40 |
|
Total stock options granted in the year |
|
| 217 |
|
|
| 420 |
|
F-21
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
During the year ended December 31, 2022, the option agreements of one Company officer were modified as part of a separation and release agreement. Vested options of 56,737, with exercise prices ranging from $20.1 to $103.65, were allowed to continue to be exercisable for an additional 12-month period, and also 31,810 options that would have expired unvested, were allowed to continue to vest for a 12-month period. As there was no service requirement, during the year ended December 31, 2022, the company recorded $67 thousand in additional compensation related to these modifications.
The Company has a stock incentive plan (SIP) pursuant to which the Board may grant stock-based awards comprised of restricted stock units or dividend equivalents to employees, officers, consultants, independent contractors, advisors and non-employee directors of the Company. Each restricted stock unit ("RSU") is automatically redeemed for one Common Share of the Company upon vesting. During the year ended December 31, 2023, the Company granted 38,000 RSUs with immediate vesting and an exercise price of $9.90 (the "2023 RSU Grant"). On February 6, 2023, all of these RSUs were redeemed for 38,000 Common Shares. The following table presents the vesting and redemption of the RSUs granted in the year ended December 31, 2023. No RSUs were granted in the year ended December 31, 2022.
Year ended | Year ended | |||||||||||
Number of options | Weighted average grant date fair value | Number of options | Weighted average grant date fair value | |||||||||
Outstanding, beginning of period | - | $ | - | - | $ | - | ||||||
Granted | 38 | 9.90 | - | - | ||||||||
Vested and redeemed | (38 | ) | (9.90 | ) | - | - | ||||||
Outstanding, ending of period | - | $ | - | - | $ | - | ||||||
The Company recorded share-based payment expense related to stock options and RSUs as follows:
| Year ended December 31, 2023 |
|
| Year ended December 31, 2022 |
| |||
Research and development |
| $ | 1,373 |
|
| $ | 2,218 |
|
General and administrative |
|
| 2,280 |
|
|
| 2,989 |
|
Total |
| $ | 3,653 |
|
| $ | 5,207 |
|
The Company enters into research, development and license agreements in the ordinary course of business where the Company receives research services and rights to proprietary technologies. Milestone and royalty payments that may become due under various agreements are dependent on, among other factors, clinical trials, regulatory approvals and ultimately the successful development of a new drug, the outcome and timing of which is uncertain.
On November 4, 2021, the Effective Date, the Company entered into the Tuspetinib Licensing Agreement with Hanmi for global rights to tuspetinib. In consideration of the license and other rights granted, Aptose made an upfront payment to Hanmi in the amount of $12.5 million, including $5.0 million in cash and $7.5 million in Aptose Common Shares (the “Shares”). The number of Shares issued was determined using the average market closing price of the Common Shares on the NASDAQ stock market over the five (5) trading day period ending on the Effective Date. Accordingly, Aptose issued 215,703 shares to Hanmi.
F-22
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
Under the Company’s license agreement with Hanmi, the Company has maximum obligations for clinical development and global regulatory milestones totaling $64.5 million for the first potential clinical indication of tuspetinib, $34 million for the second indication, and $29 million for the third indication. The Company has maximum obligations for tiered global sales-based milestones totaling $280 million. The Company also has an obligation for tiered royalty payments on global sales of commercialized product. The timing of any milestone or royalty payments that may become due is not yet determinable.
On March 21, 2016, the Company entered into a license agreement with CrystalGenomics Invites Co. Ltd. ("CG"), formerly CrystalGenomics, Inc.) for rights to luxeptinib, in all territories outside of the Republic of Korea and China, the Company has obligations for development milestones of $16 million related to the initiation of Phase 2 and pivotal clinical trials, and regulatory milestones totaling $44 million. The Company also has an obligation to pay royalty payments on sales of commercialized product. The timing of any milestone or royalty payments that may become due is not yet determinable.
On June 13, 2018, the Company entered into a license agreement with CG to gain an exclusive license to luxeptinib in China. The Company has potential future obligations of development milestones of $6 million related to approval of an Investigational New Drug (“IND”) and to the initiation of Phase 2 and pivotal clinical trials, and regulatory milestones totaling $20 million. The Company also has an obligation to pay sales milestones and royalty payments on sales of commercialized product. The timing or likelihood of any milestone or royalty payments that may become due is not yet determinable.
For the years ended December 31, 2023 and 2022, the total comprehensive loss is as follows:
| December 31, |
|
| December 31, |
| |||
Loss attributed to US foreign operations |
| $ | (45,652 | ) |
| $ | (36,615 | ) |
Loss attributed to Canadian operations |
|
| (5,555 | ) |
|
| (5,208 | ) |
Loss before income taxes |
| $ | (51,207 | ) |
| $ | (41,823 | ) |
Major items causing the Company’s income tax rate to differ from the statutory rate of approximately 26.5% (December 31, 2022 – 26.5%) are as follows:
| Year ended December 31, 2023 |
|
| Year ended December 31, 2022 |
| |||
Net loss |
| $ | (51,207 | ) |
| $ | (41,823 | ) |
Statutory Canadian corporate tax rate |
|
| 26.5 | % |
|
| 26.5 | % |
Computed expected tax recovery |
| $ | (13,570 | ) |
| $ | (11,083 | ) |
Non-deductible permanent differences |
|
| 873 |
|
|
| 1,376 |
|
Change in valuation allowance |
|
| 13,059 |
|
|
| 10,821 |
|
Foreign tax rate differential |
|
| (677 | ) |
|
| (466 | ) |
Prior year true-up adjustments |
|
| 355 |
|
|
| (703 | ) |
Other |
|
| (40 | ) |
|
| 55 |
|
| $ | — |
|
| $ | — |
| |
F-23
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
The tax effects of temporary differences that give rise to significant portions of the unrecognized deferred tax assets are presented below:
| December 31, |
|
| December 31, |
| |||
Net operating losses carried forward |
| $ | 73,552 |
|
| $ | 60,092 |
|
Research and development expenditures |
|
| 5,025 |
|
|
| 5,023 |
|
Property, equipment, and other intangible assets |
|
| 7,321 |
|
|
| 7,264 |
|
Research and development tax credits |
|
| 4,930 |
|
|
| 4,968 |
|
Financing costs |
|
| 431 |
|
|
| 873 |
|
Right-of-use assets |
|
| 19 |
|
|
| 2 |
|
Total deferred tax assets |
|
| 91,278 |
|
|
| 78,222 |
|
Valuation allowance |
|
| (91,278 | ) |
|
| (78,222 | ) |
Net deferred tax asset |
| $ | — |
|
| $ | — |
|
The valuation allowance at December 31, 2023 was primarily related to net operating loss carryforwards that, in the judgment of management, are not more-likely than-not to be realized. In assessing the realizability of deferred tax assets, management considers whether it is more-likely-than-not that all or some portion of the deferred assets will not be realized. This ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the period in which those deductible temporary difference become deductible. Based on the history of losses and projections for future taxable income, management believes that it is not more-likely than-not that the Company will realize the benefits of these deductible temporary differences (e.g. deferred tax assets).
The Company has certain deductible Canadian research and development expenditures that have not been deducted for tax purposes, totaling $19.0 million, that can be carried forward indefinitely. The Company also has Canadian non‑refundable federal and provincial investment tax credits of approximately $2.4 million which are available to reduce future federal taxes payable and begin to expire in 2024, as well as non‑refundable US research and development tax credits of approximately $3.1 million which are available to reduce future US taxes payable and begin to expire in 2038.
In addition, the Company has Canadian non-capital loss carryforwards of $266.2 million. To the extent that the non-capital loss carryforwards are not used, they begin to expire in 2026. The Company also has a US non-capital loss carryforward of $1.1 million. To the extent that the non-capital loss carryforwards are not used, they begin to expire in 2034.
The Company files income tax returns with Canada and its provinces and territories. Generally, we are subject to routine examinations by the Canada Revenue Agency ("CRA"). Income tax returns filed with various provincial jurisdictions are generally open to examination for periods of four to five years subsequent to the filing of the respective return.
The Company also files income tax returns for our U.S. operations and subsidiary with the U.S. federal and state tax jurisdictions. Generally, we are subject to routine examination by taxing authorities in the U.S. jurisdictions. There are presently no examination of our U.S. federal and U.S. state returns. We believe that our tax positions comply with the applicable tax law.
Selected financial data (unaudited) for the periods presented was as follows:
| March 31, |
|
| June 30, |
|
| September 30, |
|
| December 31, |
| |||||
Revenue |
| $ | — |
|
| $ | — |
|
| $ | — |
|
| $ | — |
|
Net loss |
| $ | (13,676 | ) |
| $ | (14,129 | ) |
| $ | (11,447 | ) |
| $ | (11,955 | ) |
Basic and diluted loss per common share |
| $ | (2.25 | ) |
| $ | (2.27 | ) |
| $ | (1.76 | ) |
| $ | (1.57 | ) |
F-24
APTOSE BIOSCIENCES INC.
Notes to Consolidated Financial Statements Years ended December 31, 2023 and 2022
(Tabular amounts in thousands of United States dollars, except as otherwise noted)
| March 31, |
|
| June 30, |
|
| September 30, |
|
| December 31, |
| |||||
Revenue |
| $ | — |
|
| $ | — |
|
| $ | — |
|
| $ | — |
|
Net loss |
| $ | (11,481 | ) |
| $ | (10,565 | ) |
| $ | (9,777 | ) |
| $ | (10,000 | ) |
Basic and diluted loss per common share |
| $ | (1.81 | ) |
| $ | (1.66 | ) |
| $ | (1.66 | ) |
| $ | (1.67 | ) |
Subsequent to the year end, the Company issued 408,168 stock options to directors, officers, employees and consultants with an average exercise price of $2.00. 388,170 stock options vest 50% after one year and 16.67% on each of the next three anniversaries, and 19,998 options vest 50% after one year and 25% on each of the next two anniversaries. The future expense associated with this option grant is $402 thousand.
In January 2024, the Company issued 10,062 Common Shares to Keystone under the 2023 Committed Equity Facility, as the Second Back-End Commitment Shares.
On January 31, 2024, the Company announced the closing of a $9.7 million public offering (the “Public Offering”) and a $4 million private placement (the “Private Placement”) with Hanmi Pharmaceutical. The Public Offering comprised 5,649,122 common shares of the Company (the “Common Shares”) and warrants at a combined offering price of US $1.71 per share. This included 736,842 Common Shares and warrants pursuant to a full exercise by the underwriter of its over-allotment option (the “Over-Allotment Option”). The Private Placement comprised 2,105,263 Common Shares of the Company sold at a price of $1.90, representing an 11% premium over the price of the Common Shares issued as part of the Public Offering. Nasdaq subsequently issued a letter to the Company regarding the value and the date of the Private Placement, as discussed in this note, below. Financing costs of approximately $1.4 million included underwriting costs of 7% and approximately $0.4 million in professional fees. The Company also issued Hanmi warrants to purchase Common Shares at an exercise price of US $1.71 per Warrant Share. The total number of Common Shares outstanding after the closing of the Public Offering, including the Over-Allotment Option, and Private Placement was 15,706,810 and warrants outstanding were 8,332,163. As of the date of this report, the total common share ownership by Hanmi was 2,989,414. Hanmi also owns 2,339,181 warrants to purchase Common Shares at an exchange price of $1.71 per Warrant Share.
On February 29, 2024, the Company received a deficiency letter (the “2024 Deficiency Letter”) from the Nasdaq Listing Qualifications Department of The Nasdaq Stock Market LLC (“Nasdaq”) notifying the Company that the Company’s January 2024 Private Placement of securities to Hanmi violated 5635(d) because the Company did not obtain shareholder approval prior to such issuance. Nasdaq stated that the Private Placement involved the issuance of greater than 20% of the issued and outstanding Common Shares of the Company at a discount to the Nasdaq official closing price on January 25, 2024, the date of the subscription agreement between the Company and Hanmi. The 2024 Deficiency Letter has no immediate effect on the listing of the Company’s Common Shares. In accordance with the Nasdaq Listing Rules, the Company has been given forty-five (45) calendar days, or until April 14, 2024, to submit a plan to regain compliance. If Nasdaq accepts the Company’s plan to regain compliance, Nasdaq can grant an extension of up to 180 calendar days from the date of the Deficiency Letter to evidence compliance. Although the Company believes that the Private Placement was completed in accordance with the Nasdaq Listing Rules, the Company respects Nasdaq’s query and intends to work with Nasdaq to resolve Nasdaq’s concerns and will consider available options to regain compliance. However, there can be no assurance that the Company will be able to regain compliance with the applicable Nasdaq Listing Rules.
On February 5, 2024, the Company issued 10,891 Common Shares under the ESPP.
F-25