SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
----------
FORM 10-K
|X|
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2004
2007
OR
|_|
FOR THE TRANSITION PERIOD FROM ___________________ TO _________.
COMMISSION FILE NUMBER
Commission File Number 0-26068
ACACIA RESEARCH CORPORATION
(Exact
(Exact name of registrant as specified in its charter)
DELAWARE 95-4405754
(State or other jurisdiction of (I.R.S. Employer
incorporation organization) Identification No.)
500 NEWPORT CENTER DRIVE, NEWPORT BEACH, CA 92660
(Address of principal executive offices) (Zip Code)
Registrant's
| DELAWARE | 95-4405754 |
| (State or other jurisdiction of | (I.R.S. Employer |
| incorporation organization) | Identification No.) |
| 500 NEWPORT CENTER DRIVE, NEWPORT BEACH, CA | 92660 |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: (949) 480-8300
Securities registered pursuant to Section 12(b) of the Act: NONE
| Title of Each Class | Name of Each Exchange on Which Registered |
| Acacia Research - Acacia Technologies Common Stock, $0.001 par value | The NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: ACACIA RESEARCH - ACACIA TECHNOLOGIES COMMON STOCK, $0.001 PAR VALUE
(TITLE OF CLASS)
ACACIA RESEARCH - COMBIMATRIX COMMON STOCK, $0.001 PAR VALUE
(TITLE OF CLASS)
----------
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to filing requirements for the past 90 days.
Yes |X|þ No [ ]
£
Indicate by check mark that disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant'sregistrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K |X|
10-K. þ
Indicate by check mark whether the Registrantregistrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ¨ | Accelerated filer þ | |
Non-accelerated filer ¨ (Do not check if a smaller reporting company) | Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes |X|¨ No [ ]
þ
The aggregate market value of the registrant'sregistrant’s Acacia Research - Acacia Technologies common stock and Acacia Research - CombiMatrix common stock held by non-affiliates of the registrant, computed by reference to the last sales prices of such stocks reported on The Nasdaq Stock Market, as of June 30, 2004,29, 2007, was approximately $123,737,135 and $133,494,318, respectively.$458,295,628. (All executive officers and directors of the registrant are considered affiliates.)
As of March 9, 2005, 27,212,769February 25, 2008, 30,132,922 shares of Acacia Research-Acacia Technologies common stock were issued and outstanding. As of March 9, 2005,
31,200,496 shares of Acacia Research-CombiMatrix common stock were issued and
outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant'sregistrant’s definitive proxy statement for its Annual Meeting of Stockholders to be filed with the Commission within 120 days after the close of its fiscal year are incorporated by reference into Part III.
================================================================================
ACACIA RESEARCH CORPORATION
FORM 10-K ANNUAL REPORT
FISCAL YEAR ENDED DECEMBER 31, 2004
ACACIA RESEARCH CORPORATION
ITEM PAGE
- ---- ----
2007
TABLE OF CONTENTS
| Item | Page | ||
| PART I | |||
| 1. | Business | 1 | |
| 1A. | Risk Factors | 6 | |
| 1B. | Unresolved Staff Comments | 13 | |
| 2. | Properties | 13 | |
| 3. | Legal Proceedings | 13 | |
| 4. | Submission of Matters to a Vote of Security Holders | 13 | |
| PART II | |||
| 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer | ||
| Purchases of Equity Securities | 14 | ||
| 6. | Selected Financial Data | 17 | |
| 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 19 | |
| 7A. | Quantitative and Qualitative Disclosures About Market Risk | 29 | |
| 8. | Financial Statements and Supplementary Data | 29 | |
| 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | 29 | |
| 9A. | Controls and Procedures | 29 | |
| 9B. | Other Information | 29 | |
| PART III | |||
| 10. | Directors, Executive Officers and Corporate Governance | 30 | |
| 11. | Executive Compensation | 30 | |
| 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 30 | |
| 13. | Certain Relationships and Related Transactions, and Director Independence | 30 | |
| 14. | Principal Accounting Fees and Services | 30 | |
| PART IV | |||
| 15. | Exhibits, Financial Statement Schedules | 31 | |
PART I
1. Business..................................................................1
2. Properties...............................................................22
3. Legal Proceedings.........................................................23
4. Submission of Matters to a Vote of Security Holders.......................23
PART II
5. Market for Registrant's Common Equity, Related Stockholder
Matters and Issuer Purchases of Equity Securities.........................24
6. Selected Financial Data...................................................26
7. Management's Discussion and Analysis of Financial Condition and
Results of Operations.....................................................30
7A. Quantitative and Qualitative Disclosures About Market Risk................53
8. Financial Statements and Supplementary Data...............................54
9. Changes in and Disagreements With Accountants on Accounting and
Financial Disclosure......................................................54
9A. Controls and Procedures...................................................54
9B. Other Information.........................................................54
PART III
10. Directors and Executive Officers of the Registrant........................55
11. Executive Compensation....................................................55
12. Security Ownership of Certain Beneficial Owners and Management............55
13. Certain Relationships and Related Transactions............................55
14. Principal Accounting Fees and Services....................................55
PART IV
15. Exhibits, Financial Statement Schedules...................................56
PART I
CAUTIONARY STATEMENT
This report contains forward-looking statements within the meaning of the "safe harbor"“safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Reference is made in particular to the description of our plans and objectives for future operations, assumptions underlying such plans and objectives, and other forward-looking statements included in this report. Such statements may be identified by the use of forward-looking terminology such as "may," "will," "expect," "believe," "estimate," "anticipate," "intend,"
"continue,"“may,” “will,” “expect,” “believe,” “estimate,” “anticipate,” “intend,” “continue,” or similar terms, variations of such terms or the negative of such terms. Such statements are based on management'smanagement’s current expectations and are subject to a number of factors and uncertainties, which could cause actual results to differ materially from those described in the forward-looking statements. Such statements address future events and conditions concerning product development, capital expenditures, earnings, litigation, regulatory matters, markets for products and services, liquidity and capital resources and accounting matters. Actual results in each case could differ materially from those anticipated in such statements by reason of factors such as future economic conditions, changes in consumer demand, legislative, regulatory and competitive developments in markets in which we and our subsidiaries operate, and other circumstances affecting anticipated revenues and costs.costs, as more fully disclosed in our discussion of risk factors incorporated by reference in Item 1A. of Part I of this report. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based. Additional factors that could cause such results to differ materially from those described in the forward-looking statements are set forth in connection with the forward-looking statements.
As used in this Form 10-K, "we," "us"“we,” “us” and "our"“our” refer to Acacia Research Corporation andand/or its subsidiary companies.
ITEM 1. BUSINESS
OVERVIEW
Acacia Research Corporation is comprised of two operating groups.
COMBIMATRIX GROUP
Our life sciences business, referred to as the "CombiMatrix group," is
comprised of our wholly owned subsidiary, CombiMatrix Corporation and
CombiMatrix Corporation's wholly owned subsidiary, CombiMatrix K.K. The
CombiMatrix group is seeking to become a broadly diversified biotechnology
company, through the development of proprietary technologies and products in the
areas of drug development, genetic analysis, nanotechnology research, defense
and homeland security markets, as well as other potential markets where its
products could be utilized. Among the technologies being developed by the
CombiMatrix group are a platform technology to rapidly produce customizable
arrays, which are semiconductor-based tools for use in identifying and
determining the roles of genes, gene mutations and proteins. This technology has
a wide range of potential applications in the areas of genomics, proteomics,
biosensors, drug discovery, drug development, diagnostics, combinatorial
chemistry, material sciences and nanotechnology. Other technologies include
proprietary molecular synthesis and screening methods for the discovery of
potential new drugs.
ACACIA TECHNOLOGIES GROUP
Ouroperating subsidiaries. All intellectual property acquisition, development, licensing business, referred to as the "Acacia
Technologies group," develops, acquires, and licenses patented technologies. The
Acacia Technologies group owns and out-licenses a portfolio of pioneering U.S.
and foreign patents covering digital audio and video transmission and receiving
systems, commonly known as audio-on-demand, video-on-demand, and audio/video
streaming. The Acacia Technologies group's patented proprietary digital media
transmission, or DMT(R), technology enables the digitization, encryption,
storage, transmission, receipt and playback of digital content via several means
including cable TV, which includes digital ad insertion and video on demand
programming, satellite TV programming, the Internet, which includes distance
learning and other Internet programming involving digital audio/video content,
wireless delivery of video content, fiber-optic delivery of video content and
hotel in-room digital delivery of programming.
The Acacia Technologies group owned, and out-licensed to consumer
electronics manufacturers, patented technology known as the V-chip. The V-chip
technology was protectedenforcement activities are conducted solely by U.S. Patent No. 4,554,584, which expired in July
2003. The Acacia Technologies group concluded its V-chip licensing program in
August 2004 as discussed below.
1
On January 28, 2005, Acacia Global Acquisition Corporation, a newly formed
wholly owned subsidiarycertain of Acacia Research Corporation, acquiredCorporation’s wholly owned operating subsidiaries.
Item 1. BUSINESS
OVERVIEW
Acacia Research Corporation’s operating subsidiaries acquire, develop, license and enforce patented technologies. Our operating subsidiaries generate license fee revenues and related cash flows from the assetsgranting of Global Patent Holdings, LLC,licenses for the use of patented technologies that our operating subsidiaries own or Global Patent Holdings,control. Our operating subsidiaries assist patent owners with the prosecution and development of their patent portfolios, the protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, if necessary, with the enforcement against unauthorized users of their patented technologies. Currently, on a privately held patent
holding company based in Northbrook, Illinois, which owned 11 patent licensing
companies. The acquisition givesconsolidated basis, our operating subsidiaries own or control the Acacia Technologies group ownership of
companies that control 27rights to 91 patent portfolios, which include 120 U.S. patents and certain foreign counterparts, and covercovering technologies used in a wide variety of industries, as set forth below.
The acquisition expandsindustries.
CombiMatrix Group Split-off Transaction and diversifiesRelated Discontinued Operations. In January 2006, Acacia Research Corporation’s board of directors approved a plan for its wholly owned subsidiary, CombiMatrix Corporation, the primary component of Acacia Technologies group's
revenue generating opportunitiesResearch Corporation’s CombiMatrix group, to become an independent public company. CombiMatrix Corporation’s registration statement on Form S-1 was declared effective by the Securities and acceleratesExchange Commission (“SEC”) on June 8, 2007. Following the executionredemption period required by Acacia Research Corporation’s Restated Certificate of Incorporation, on August 15, 2007 (the “Redemption Date”), CombiMatrix Corporation was split-off from Acacia Research Corporation through the redemption of all outstanding shares of Acacia Research-CombiMatrix common stock in exchange for the distribution of new shares of CombiMatrix Corporation common stock, on a pro-rata basis, to the holders of Acacia Research-CombiMatrix common stock on the Redemption Date (the “Split-off Transaction”). On the Redemption Date, every ten (10) shares of Acacia Research-CombiMatrix common stock outstanding on August 15, 2007, was redeemed for one (1) share of common stock of CombiMatrix Corporation. Subsequent to the Redemption Date, Acacia Research Corporation no longer owns any equity interests in CombiMatrix Corporation and the two companies operate independently of each other.
As a result of the Acacia
Technologies group's business strategySplit-off Transaction, we have disposed of acquiring, developing and licensing
patented technologies.our investment in CombiMatrix Corporation. Refer to Note 16 in10A to the accompanying Acacia Research Corporation consolidated financial statements, included elsewhere herein, for financial information relatedregarding presentation of the assets, liabilities, results of operations and cash flows for the CombiMatrix group as “Discontinued Operations,” for all periods presented, in accordance with guidance set forth in SFAS No. 144 “Accounting for the Impairment or Disposal of Long-Lived Assets” (“SFAS No. 144”).
1
Capital Structure
As a result of the Split-off Transaction, Acacia Research Corporation no longer owns any equity interests in CombiMatrix Corporation and the CombiMatrix group is no longer a business group of Acacia Research Corporation. Pursuant to ourthe terms of the Split-off Transaction, all outstanding shares of Acacia Research-CombiMatrix common stock were redeemed, and hence, all rights of holders of Acacia Research-CombiMatrix common stock ceased as of the Redemption Date, except for the right, upon the surrender to the exchange agent of shares of Acacia Research-CombiMatrix common stock, to receive new shares of CombiMatrix Corporation stock pursuant to the exchange ratio described above. Subsequent to the consummation of the Split-off Transaction, Acacia Research Corporation’s only class of common stock outstanding is its Acacia Research-Acacia Technologies common stock.
Prior to the Split-off Transaction, Acacia Research Corporation had two
segments.
RECAPITALIZATION AND MERGER TRANSACTIONS
On December 11, 2002, our stockholders voted in favor of a recapitalization
transaction, which became effective on December 13, 2002, whereby we created two
new classes of common stock called Acacia Research-CombiMatrix stock, or
AR-CombiMatrix stock, andoutstanding, its Acacia Research-Acacia Technologies common stock or(“AR-Acacia Technologies stock”) and its Acacia Research-CombiMatrix common stock (“AR-CombiMatrix stock”). AR-Acacia Technologies stock and divided our existing Acacia Research
Corporation common stock into shares of the two new classes of common stock.
AR-CombiMatrix stock iswas intended to reflect separately the performance of Acacia Research Corporation's CombiMatrixCorporation’s Acacia Technologies group. AR-Acacia TechnologiesAR-CombiMatrix stock iswas intended to reflect separately the performance of Acacia Research Corporation's
Acacia TechnologiesCorporation’s CombiMatrix group. Although the AR-CombiMatrix stock and the AR-Acacia Technologies stock areand the AR-CombiMatrix stock were intended to reflect the performance of our different business groups, they arewere both classes of common stock of Acacia Research Corporation and arewere not stock issued by the respective groups. As
Our Board of Directors has approved an amendment and restatement of our certificate of incorporation to remove reference to AR-CombiMatrix stock which was cancelled as a result holders of Acacia Research-Acaciathe redemption, to rename AR-Acacia Technologies stock as the only class of common stock, and Acacia
Research-CombiMatrixto provide that all 100,000,000 shares of AR-Acacia Technologies stock continuecurrently authorized may be issued as a single class of common stock. The amendment and restatement of our certificate of incorporation is subject to approval by the stockholders at the next annual meeting of stockholders, as further described in our proxy statement to be subject to all of the risks of an
investment in Acacia Research Corporation and all of its businesses, assets and
liabilities. The assets Acacia Research Corporation attributes to one group
could be subject to the liabilities of the other group. Included in the
CombiMatrix group and the Acacia Technologies group are certain wholly owned
subsidiaries that are not material, quantitatively or qualitatively, either
individually or in the aggregate, to either group, or to Acacia Research
Corporation as a whole.
On December 13, 2002, Acacia Research Corporation acquired the stockholder
interests in CombiMatrix Corporation not already owned by us (52% of the total
stockholder interests in CombiMatrix Corporation). The acquisition was
accomplished through a merger in which stockholders of CombiMatrix Corporation
other than Acacia Research Corporation received one share of the new
AR-CombiMatrix stock in exchange forfiled. If adopted, each share of CombiMatrix Corporation
common stock that they owned immediately priorwill be entitled to one vote and the merger.
OTHER
relative voting strength of the common stock will be equal notwithstanding the trading price of the common stock.
Other
Acacia Research Corporation, a Delaware corporation, was originally incorporated in California in January 1993 and reincorporated in Delaware in December 1999. Our website address is www.acaciaresearch.com.www.acaciaresearch.com. We make our filings with the Securities and Exchange Commission, or SEC, including our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports, available free of charge on our website as soon as reasonably practicable after we file these reports. In addition, we post the following information on our website:
o our corporate code of conduct, board of directors - code of conduct
and fraud policy;
o charters for our audit committee, nominating and corporate governance
committee, disclosure committee and compensation committee;
| · | our corporate code of conduct, our board of directors – code of conduct and our fraud policy; |
| · | charters for our audit committee, nominating and corporate governance committee, disclosure committee and compensation committee; |
The public may read and copy any materials that Acacia Research Corporation files with the SEC at the SEC'sSEC’s Public Reference Room at 450 Fifth Street N.W., Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Also, the SEC maintains an Internet website that contains reports, proxy and information statements, and other information regarding issuers, including the Acacia Research Corporation, that file electronically with the SEC. The public can obtain any documents that Acacia Research Corporation files with the SEC at http://www.sec.gov.
www.sec.gov.
2
BUSINESS GROUPS
COMBIMATRIX GROUP
(A DIVISION OF ACACIA RESEARCH CORPORATION)
BUSINESS
The CombiMatrix group is comprised
Intellectual Property Licensing Business
Acacia Research Corporation’s operating subsidiaries acquire, develop, license and enforce patented technologies. Our operating subsidiaries generate license fee revenues and related cash flows from the granting of licenses for the use of patented technologies that our operating subsidiaries own or control. Our operating subsidiaries assist patent owners with the prosecution and development of their patent portfolios, the protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, if necessary, with the enforcement against unauthorized users of their patented technologies. Currently, on a consolidated basis, our operating subsidiaries own or control the rights to 91 patent portfolios, which include U.S. patents and certain foreign counterparts, covering technologies used in a wide variety of industries. Our operating subsidiaries have established a track record of licensing success with more than 540 license agreements executed to date. Our professional staff includes in-house patent attorneys, licensing executives, engineers and business development executives.
Our clients are primarily individual inventors and small companies who have limited resources and/or expertise to effectively address the unauthorized use of their patented technologies, and also include large companies seeking to effectively and efficiently monetize their portfolio of patented technologies. In a typical client arrangement, our operating subsidiary will acquire a patent portfolio, or acquire rights to a patent portfolio, with our clients receiving an upfront payment for the purchase of the patent portfolio or patent portfolio rights, or receiving a percentage of our operating subsidiaries net recoveries from the licensing and enforcement of the patent portfolio, or a combination of the two.
In January 2005, our wholly owned subsidiary, CombiMatrix Corporation and CombiMatrix Corporation's wholly owned subsidiary,
CombiMatrix K.K. The CombiMatrix group is seeking to become a broadly
diversified biotechnology company, through the development of proprietary
technologies and products in the areas of drug development, genetic analysis,
nanotechnology research, defense and homeland security markets, as well as other
potential markets where its products could be utilized. Among the technologies
being developed by the CombiMatrix group are a platform technology to rapidly
produce customizable arrays, which are semiconductor-based tools for use in
identifying and determining the roles of genes, gene mutations and proteins.
This technology has a wide range of potential applications in the areas of
genomics, proteomics, biosensors, drug discovery, drug development, diagnostics,
combinatorial chemistry, material sciences and nanotechnology. Other
technologies include proprietary molecular synthesis and screening methods for
the discovery of potential new drugs.
TECHNOLOGIES
SEMICONDUCTOR BASED ARRAY
The CombiMatrix group's semiconductor based array technology enables the
rapid, parallel synthesis, immobilization and detection of molecules and
materials at discrete electrodes on a semiconductor chip. These chips, also
known as microelectrode arrays, are used in multiple applications in the areas
described above. The CombiMatrix group's technology integrates semiconductor
micro fabrication, proprietary software, chemistry and hardware into systems
that it believes will enable it, its customers and its partners to design and
fabricate arrays for biological, diagnostic, material sciences and
nanotechnology applications, typically within a few days. The CombiMatrix
group's system should enable researchers to conduct rapid, iterative experiments
in each of these fields.
For biological applications, the CombiMatrix group believes that its
customizable arrays will enable users to reduce the time and costs associated
with the discovery and development of pharmaceutical products. Although there
are numerous applications of the CombiMatrix group's arrays in life sciences
research, each depend on the synthesis, immobilization or detection of molecules
at discrete sites on the array. Some specific applications include studies of
genetic expression in cellular systems, genotyping and mutation analysis,
synthesis of nucleic acid drugs, and others.
Utilizing this array technology, the CombiMatrix group is engaged in four
strategic business areas:
o The development, manufacture and sale of research tools and services
to life sciences researchers
o The discovery of drugs based on the mechanism of ribonucleic acid
inhibition, or RNAi, and other approaches
o The development, manufacture and sale of biosensor systems and
technology for national defense and homeland security
o The development of tools for applications in nanotechnology and
materials science.
ELECTROCHEMICAL SYNTHESIS OF MOLECULES
The CombiMatrix group is utilizing its expertise in electrochemistry to
synthesize novel compounds, which can be screened in binding and cellular assays
to determine their potential as new drugs. The types of molecules that can be
synthesized electrochemically from precursors using various approaches,
proprietary to the CombiMatrix group, include organic compounds, nucleic acids,
peptides and others. These molecules can then be utilized in biochemical and
cellular screens to determine if they have appropriate potency to be considered
for downstream pre-clinical and clinical drug development.
3
POTENTIAL DRUG COMPOUNDS
Through its minority investment in Leuchemix, Inc., the CombiMatrix group
has access to proprietary compounds that have been shown to be cytotoxic towards
certain cancers in vitro and in vivo. Many of these compounds were discovered
through combinatorial chemistry, natural product chemistry and certain cellular
screening assays. Leuchemix, Inc. has access to state of the art laboratories
and equipment, which includes flow cytometry, molecular biology and cell culture
facilities. In addition, Leuchemix, Inc. has access to a bank of over 150
primary leukemia specimens and a panel of 15 leukemia and lymphoma cell lines as
well as several xenogenic animal model systems.
MARKET OVERVIEW
The markets for the CombiMatrix group's products include pharmaceutical and
biotechnology markets (also referred to as life sciences), national defense and
homeland security applications and the emerging markets for nanotechnology and
new materials. In the future, if the CombiMatrix group is successful in
developing approved drugs either internally or through its investments in
companies such as Leuchemix, Inc., the CombiMatrix group's market opportunities
will expand to include pharmacies, physicians, hospitals, patients and other
consumers of therapeutics. At this time, the majority of the CombiMatrix group's
efforts are focused on the life sciences and national defense markets.
GENERAL OVERVIEW OF LIFE SCIENCES AND PHARMACEUTICAL INDUSTRIES
The pharmaceutical and biotechnology industries are faced with increasing
costs and risks of failure in the drug discovery, development and
commercialization process. According to industry statistics, the time required
to commercialize a new drug averages 15 years. Declining research and
development productivity, rising costs of commercialization, increasing
reimbursement influence and shorter exclusivity periods have driven up the cost
to develop each successful compound to $1.7 billion according to recent industry
data. Only one compound now reaches the market for every thirteen discovered and
placed in preclinical trials, compared to one for every eight between 1995 and
2000. The pharmaceutical and biotechnology industries are working to reduce
their costs and risks of failure by turning to new technologies to help identify
deficiencies in drug candidates as early as possible in the process so that drug
discovery and development become more efficient and cost-effective.
Additionally, with vast amounts of genomic data becoming available for use in
the development of therapeutics and diagnostic tests, they are searching for
ways to expedite their analysis of available genomic data so that they can be
the first to bring new therapeutics and diagnostic tests to market.
DRUG DISCOVERY AND DEVELOPMENT
The discovery and development of new drugs for a particular disease
typically involves several steps. First, researchers identify a target for
therapeutic intervention, such as a protein or gene, that is either directly
involved in the disease or lies in a biochemical pathway leading to the disease.
The next step is to identify chemical compounds that interact with and modulate
the target's activity to inhibit or prevent the disease. Promising compounds
advance to subsequent stages, which include animal trials followed by human
trials.
Recent advances, including the sequencing of the human genome, have led to
the use of genomics in choosing and validating the targets for drug development.
This process begins with the discovery and identification of genes within the
genome and the functions of these genes in regulating biological processes and
disease. This information is used to assess the value of a particular gene or
its protein product as a target for drug discovery. According to industry
statistics, pharmaceutical and biotechnology companies worldwide spent
approximately $62.0 billion on drug research and development during 2003.
GENES AND PROTEINS
The human body is composed of billions of cells each containing DNA that
encodes the basic instructions for cellular function. The complete set of an
individual's DNA is called the genome, and is organized into 23 pairs of
chromosomes, which are further divided into smaller regions called genes. Each
gene is composed of a strand of four types of nucleotide bases, referred to as
A, C, G and T. The bases of one DNA strand bind to the bases of the other strand
in a specific fashion to form base pairs: the base A always binds with the base
T and the base G always binds with the base C.
The human genome has approximately 3.0 billion nucleotides and their
precise order is known as the DNA sequence. When a gene is turned on, or
expressed, the genetic information encoded in the DNA is copied to a specific
type of RNA, called messenger RNA, or mRNA. The mRNA provides instructions for
the synthesis of proteins. Proteins direct cellular function, the development of
individual traits and are involved in many diseases. Abnormal variations in the
sequence of a gene or in the level of gene expression can interfere with the
normal physiology of particular cells and lead to a disease, a predisposition to
a disease or an adverse response to drugs.
4
GENE EXPRESSION PROFILING
Gene expression profiling is the process of determining which genes are
active in a specific cell or group of cells and is accomplished by measuring
mRNA, the intermediary between genes and proteins. By comparing gene expression
patterns between cells from normal tissue and cells from diseased tissue,
researchers may identify specific genes or groups of genes that play a role in
the presence of disease. Studies of this type, used in drug discovery, require
monitoring thousands, and preferably tens of thousands, of mRNAs in large
numbers of samples. As the correlation between gene expression patterns and
specific diseases is determined, the CombiMatrix group believes that gene
expression profiling will have an increasingly important role as a diagnostic
tool. Diagnostic use of expression profiling tools is anticipated to grow
rapidly with the combination of the sequencing of various genomes and the
availability of more cost-effective technologies.
GENETIC VARIATION AND MUTATIONS
Genetic variation is also due to polymorphisms (mutations) in genomes,
although functional variations may also arise from differences in the way genes
are expressed in a given cell, as well as the timing and levels of their
expression.
The most common form of genetic variation occurs as a result of a
difference in a single nucleotide in the DNA sequence, commonly referred to as a
single nucleotide polymorphism, or SNP. The human genome is estimated to contain
between three and six million SNPs. By screening for polymorphisms, researchers
seek to correlate variability in the sequence of genes with a specific disease.
SNPs are believed to be associated with a large number of human diseases,
although most SNPs are believed to be benign and not to be associated with
disease. Determining which SNPs may be related to a disease is a complex process
requiring investigation of a vast number of SNPs. A SNP association study might
require testing for 200,000 possible SNPs in 1,000 patients. Although only a few
hundred of these SNPs might be clinically relevant, 200 million genotyping
tests, or assays, might be required to complete a study. Using currently
available technologies, this scale of SNP genotyping is both impractical and
prohibitively expensive.
While in some cases one SNP will be responsible for medically important
effects, it is now believed that the genetic component of most major diseases is
associated with a combination of SNPs. As a result, the scientific community has
recognized the importance of investigating combinations of many SNPs in an
attempt to discover medically valuable information. In order to understand how
genetic variation causes disease, researchers must compare gene sequence
polymorphisms, or conduct SNP genotyping, from healthy and diseased individuals.
Researchers may also compare gene expression patterns, or perform gene
expression profiling, from healthy and diseased tissues.
PROTEOMICS
Proteomics is the process of determining which proteins are present in
cells, how they interact with one another and how they are correlated with
genomic variation. This process is useful in drug discovery and diagnostics
because most drugs target proteins that play a role in the existence or
development of a disease.
CURRENT TECHNOLOGIES
Despite the recent sequencing of the human genome, scientists have a
limited understanding of the function of genes, how they interact with each
other, how they modulate disease, and how they correlate with protein
translation and function. Additionally, the role of specific mutations is poorly
understood.
Traditional technologies for analyzing genetic or protein variation and
function generally perform experiments individually, or serially, and often
require relatively large sample volumes, adding significantly to the cost of
conducting experiments. Arrays were developed to overcome the limitations of
traditional technologies and enable the parallel evaluation of large numbers of
genes.
An array is a collection of miniaturized test sites arranged in a manner
that permits many tests to be performed simultaneously, or in parallel, in order
to achieve higher throughput. The average size of test sites in an array and the
spacing between them defines the array's density. Higher density increases
parallel processing throughput. In addition to increasing the throughput, higher
density reduces the required volume for the sample being tested, and thereby
lowers costs. Currently, the principal commercially available ways to produce
arrays include mechanical deposition, bead immobilization, inkjet printing and
photolithography.
While current array technologies have revolutionized drug discovery and
development, the CombiMatrix group believes that it's advanced array technology
provides characteristics, including flexibility, superior cost metrics, and
performance which address certain needs of the life sciences market which are
not addressed by conventional arrays.
5
THE COMBIMATRIX SOLUTION
The CombiMatrix group believes that its system will have advantages over
other existing technologies because it is being designed to be a cost-effective,
fast, flexible, customizable alternative to existing analytical tools designed
for similar purposes. Researchers using the CombiMatrix group's system should be
able to design and order custom arrays, conduct their tests, analyze the
results, and reorder additional arrays incorporating modified test parameters,
all within a few days. The CombiMatrix group believes that its system will offer
several important advantages over competing products. These advantages arise
from a unique approach to fabricating the arrays utilizing a proprietary
electrochemical synthesis method on an array of microelectrodes that have been
fabricated on a silicon device.
GENETIC ANALYSIS PRODUCTS AND SERVICES
The CombiMatrix group's technology represents a significant advance over
existing array technologies and other platforms for combinatorial chemistry. The
first application of the technology that the CombiMatrix group is pursuing is in
the field of genomics, where it is developing an array for the analysis of DNA.
The CombiMatrix group believes that this technology may be applied to the fields
of genetic analysis and disease management.
CUSTOMARRAY(TM)
The CombiMatrix group's product for genetic studies is marketed under the
trade name CustomArray(TM). CustomArray(TM) is a highly flexible custom
oligonucleotide array that addresses researchers' specific requirements for
high-performance arrays that can interrogate small sets of target genes or whole
genomes at a low cost. CustomArrays currently come in two formats: the
lower-density CustomArray(TM) 902, and the medium-density CustomArray(TM) 12K.
The CustomArray(TM) 902 enables an analysis of roughly 1,000 genes, and the
CustomArray(TM) 12K enables analysis of up to 12,000 genes.
CustomArray(TM) is an advanced tool used to understand gene expression by
measuring mRNA activity within a cell type or groups of cells, enabling
researchers to understand disease, predisposition to disease, drug response and
drug development. CustomArray(TM) can also be used as a SNP genotyping tool
providing statistics on the effect of a SNP or groups of SNPs, giving rise to
data that is important in diagnostic testing. Because of the product's
flexibility, researchers have utilized and are evaluating the use of
CustomArrays for other applications such as gene assembly, sequencing, protein
translation and others.
ON-LINE ORDER PROCESSING AND SOFTWARE TOOLS
CustomArrays are designed and ordered through the CombiMatrix group's
on-line ordering process. Customers are able to utilize a number of tools to
design and order their arrays through an on-line interface via the World Wide
Web. Some of the tools available to the customers are referred to as the
CustomArray(TM) content software application suite of tools for designing and
ordering arrays.
The content software application provides a suite of sophisticated tools
that customers can use to design a custom array specific to their experimental
needs. This application allows the customer to submit a list of genes and/or
genomic sequences to the CombiMatrix group's probe design system. This design
process produces probe sequences optimal to the customer's requirements.
Customers also have the flexibility to re-design their array at anytime.
When the customer has finished designing their arrays using the CombiMatrix
group's proprietary software tools, the arrays may be ordered using the
e-commerce section of the CustomArray(TM) web site. Arrays are then manufactured
using our proprietary oligonucleotide synthesis technology to the specific
design requirements of the customer's order. The CombiMatrix group's proprietary
DNA synthesis technology enables product turnaround time of typically just a few
days. After production, each array is put through a rigorous quality control
process. To our knowledge, the CombiMatrix group is the only array company that
quality checks every single feature on each array produced prior to shipment.
DESIGN-ON-DEMAND(TM)
The CombiMatrix group has also launched a service known as
Design-on-Demand(TM) for its arrays. Through this service, customers can work
one-on-one with our staff of bioinformatic experts to assist them with designing
their arrays to meet their specific project goals. Customers can also access our
Design-on-Demand(TM) catalog of over 1,400 pre-designed genome arrays available
for ordering.
6
HOMELAND SECURITY AND DEFENSE APPLICATIONS
Through U.S. government funding, the CombiMatrix group's array technology
is being developed to simultaneously detect toxins, viruses, and bacteria using
either genomic analysis or antigen-antibody experiments, or assays. The ability
to conduct over 12,000 individual assays simultaneously means that the
CombiMatrix group's array can be configured to detect many biothreat agents of
interest to the U.S. Department of Defense and Department of Homeland Security
within hours and with a high degree of certainty that surpasses current
technologies. The CombiMatrix group's goal is that these systems will eventually
be portable and ultimately be completely automated.
The CombiMatrix group's technology can simultaneously identify hundreds of
different microbes (including viruses), determine their ability to cause
disease, and discover their characteristics, such as antibiotic resistance.
Working with academia, industry, and government laboratories, the CombiMatrix
group is developing assays, arrays and bioinformatics for quickly identifying
human, animal, and plant pathogens in a single-assay format. This format and
single test eliminates the need for a different test for each disease or threat
and eliminates the time lost in developing a new test for each new disease or
threat. For disease-control agencies, it simplifies the process, reduces costs,
and allows more rapid identification and reaction, all in an environment where
increased time can equate to increased illness and loss of lives.
This program is enabled by the characteristic of the CombiMatrix group's
array technology, which allows the binding reactions to be measured through
electrochemical means instead of optical methods. Though optical detection has
been successful in many applications and our other products utilize these
methods, we feel that electrochemical detection techniques have the potential to
be far superior. By eliminating the need for light sources, optical components,
their corresponding mechanical requirements as well as their power requirements,
we feel that we will be able to build detection systems that will be less
expensive, smaller, lighter and portable. In addition, certain technical
characteristics of electrochemical detection on the arrays may enable higher
sensitivity, better dynamic range and superior reproducibility in measurements.
Though the initial focus of our Government-funded development program is a
product for military and homeland security markets, the core technology being
developed will be applicable to products in the life sciences and human
healthcare markets as well.
DRUG DISCOVERY
The CombiMatrix group has initiated both internally focused and externally
focused programs to utilize its arrays to discover nucleic acid drugs, based on
the recently discovered mechanism known as RNAi (Ribonucleic Acid interference).
This field is often referred to as siRNA (small interfering Ribonucleic Acid) or
gene silencing.
The underlying principle in this field is that an appropriately
designed, double-stranded sequence of RNA can effectively shut down the
operation of a particular gene. If this inhibition cures a disease or alleviates
its symptoms, these RNA molecules can potentially become effective therapeutics.
The process of drug discovery utilizing the RNAi mechanism involves multiple
steps, the first of which is the design and synthesis of potential RNAi
sequences. The CombiMatrix group believes that its expertise in nucleic acid
design and synthesis on its semiconductor-based arrays provides a significant
advantage in discovery.
The CombiMatrix group has chosen to initially focus its integrated RNAi
discovery program on viral diseases for the following reasons:
o Viral infections affect millions of individuals throughout the world
each year
o There are relatively few effective anti-viral medications
o Most emerging diseases are viruses such as SARS and West Nile Virus
o The basis of infection is through transfer of viral genetic material
o Complete viral genomic sequences have recently been made available
o The CombiMatrix group's approach is suited to viral research because
it attempts to thwart a virus by building a cocktail of drugs to
target multiple genes or all the genes of a virus
o It is believed that an RNAi effect is already operating when the body
battles viral infections
7
NANOTECHNOLOGY
The CombiMatrix group has also entered into development programs to use its
arrays for the discovery of nano-structured materials. In analogy to the study
of genes and proteins in parallel using a highly-customizable array, the
CombiMatrix group will develop a system, which enables researchers to perform
combinatorial materials discovery work in a rapid, cost effective manner.
THE COMBIMATRIX GROUP'S STRATEGY
FOCUSING ON HIGH-GROWTH MARKETS
The CombiMatrix group's goal is to provide customers and partners with
tools in their discovery efforts as well as to perform discovery itself.
The CombiMatrix group will focus on markets that it believes are growing
rapidly and where it believes it has a competitive advantage. The first of these
markets are for gene expression, mutation analysis, and other applications for
the development of drugs and diagnostic products. Other markets include protein
analysis, homeland security and military applications, anti-viral drug
development nanotechnology and material sciences.
PARTNERING WITH MULTIPLE COMPANIES TO EXPAND MARKET OPPORTUNITY
The CombiMatrix group plans to pursue multiple relationships to facilitate
the expansion of its array technologies and to exploit large and diverse
markets. The CombiMatrix group expects to enter into relationships and
collaborations to gain access to complementary technologies, distribution
channels, manufacturing infrastructure and information content. The CombiMatrix
group intends to structure relationships that maximize its research and
development efforts with the strong distribution and manufacturing capabilities
of its customers and any entities with which the CombiMatrix group has joint
development efforts.
MAJOR STRATEGIC ALLIANCES
The CombiMatrix group intends to rapidly commercialize its array technology
for gene expression profiling through its own sales and marketing efforts. In
addition, the CombiMatrix group has had agreements with several strategic
partners, such as Roche Diagnostics GmbH, or Roche, to jointly develop its
technology. For example, Roche contributed extensive expertise in the areas of
instrument and reagent development, and offers a large and experienced worldwide
sales and marketing team. The CombiMatrix group believes that the combination of
its array technology with Roche's leadership position in the genetic analysis
and diagnostic markets will enable it to capture a significant portion of the
gene expression profiling and molecular diagnostic markets.
The CombiMatrix group has been awarded several U.S. government grants and
contracts to develop its electrochemical detection system for the detection of
biological threat agents. Though these programs initially focused on product
development for military and homeland security applications, the CombiMatrix
group believes that the core technology being developed will be applicable to
products in the life sciences and human healthcare markets as well.
The CombiMatrix group has also entered into a design, fabrication and
manufacturing relationship with Toppan Printing of Japan, or Toppan, and Furuno
Electronic Co., or Furuno, for the development and manufacture of new designs of
its electrochemical detection arrays and bench-top array synthesizers,
respectively.
In addition to these relationships, the CombiMatrix group has entered into
additional relationships and plans on establishing other relationships for
multiple applications of its technology.
EXPANDING TECHNOLOGIES INTO MULTIPLE PRODUCT LINES
The CombiMatrix group intends to utilize the flexibility of its
semiconductor based array technologies to develop multiple product lines. In
addition to providing new sources of revenue, it believes these product lines
will further its goal of establishing its array technology as the industry
standard for array-based analysis.
8
STRENGTHENING TECHNOLOGICAL LEADERSHIP
The CombiMatrix group plans to continue advancing its proprietary
technologies through its internal research efforts, collaborations with industry
leaders and strategic licensing. The CombiMatrix group may also pursue
acquisitions of complementary technologies and leverage its technologies into
other value-added businesses.
PROTECTING AND STRENGTHENING INTELLECTUAL PROPERTY
Through the CombiMatrix Corporation's four patents issued in the United
States and three corresponding patents granted in Europe, Australia and Taiwan,
its 59 patent applications pending in the United States, Europe and elsewhere
and its trade secrets, the CombiMatrix group believes it has suitable
intellectual property protection for its proprietary technologies in those
markets where it operates and where a market for its products and services
exists. The CombiMatrix group plans to build its intellectual property portfolio
through internal research efforts, collaborations with industry leaders,
strategic licensing and possible acquisitions of complementary technologies. The
CombiMatrix group also plans to pursue patent protection for downstream products
created using its proprietary products.
REGULATORY MATTERS
The CombiMatrix group sells array products to the pharmaceutical,
biotechnology and academic communities for research applications as well as
non-life sciences customers. In addition, its drug development efforts are early
stage. Therefore, its initial products do not require approval from, or be
regulated by, the FDA, as a manufacturer nor are they subject to the FDA's
current good manufacturing practice, or cGMP, regulations. Additionally, the
CombiMatrix group's initial products are not subject to certain reagent
regulations promulgated by the FDA. However, the manufacture, marketing and sale
of certain products and services for most clinical or diagnostic applications
will be subject to extensive government regulation as medical devices in the
United States by the FDA and in other countries by corresponding foreign
regulatory authorities.
SUBSIDIARIES
Prior to July 11, 2003, CombiMatrix K.K., a majority-owned subsidiary of
CombiMatrix Corporation, was operating under a joint venture agreement with
Marubeni Japan, or Marubeni, one of Japan's leading trading companies. The
primary purpose of the joint venture was to focus on development and licensing
opportunities for CombiMatrix Corporation's array technology with academic,
pharmaceutical and biotechnology organizations in the Japanese market. Marubeni
held a 10% minority interests in the joint venture. On July 11, 2003, Acacia
Research Corporation purchased the outstanding minority interests in CombiMatrix
K.K. from Marubeni. Acacia Research Corporation issued 200,000 shares of its
AR-CombiMatrix stock to Marubeni in exchange for Marubeni's 10% minority
interests in CombiMatrix K.K. This increase in ownership interest has been
attributed to the CombiMatrix group.
Prior to July 2, 2003, CombiMatrix Corporation owned 87% of Advanced
Material Sciences, which in turn holds an exclusive license for CombiMatrix
Corporation's array synthesis technology for the development and discovery of
advanced electronic materials for such purposes as fuel cell catalysts. In
consideration for this exclusive license, CombiMatrix Corporation will share in
the revenues earned by Advanced Material Sciences for commercialization of these
discoveries based on CombiMatrix Corporation's array technology. The term of
this arrangement is 20 years. On July 2, 2003, Acacia Research Corporation
increased its consolidated ownership interest in Advanced Material Sciences to
99% by acquiring 1,774,750 shares of Advanced Material Sciences common stock in
exchange for 295,790 shares of AR-CombiMatrix stock. This increased ownership
interest has been attributed to the CombiMatrix group.
MARKETING AND DISTRIBUTION
During 2004, the CombiMatrix group launched its CustomArray(TM) products
and is currently selling these products directly and through distributors to
customers in the United States, Australia, New Zealand and Japan. Where
appropriate, the CombiMatrix group will continue to market and sell its products
directly or through distribution arrangements and/or through other strategic
alliances.
In July 2001, CombiMatrix Corporation entered into non-exclusive worldwide
license, supply, research and development agreements with Roche. These
agreements were amended in September 2002, primarily to grant Roche
manufacturing rights with respect to the products under development in return
for additional cash consideration under the agreements. The revised agreements
also made minor modifications to terms of the agreements involving matters such
as milestones, payments and technical specifications, none of which were
considered to be material. Such minor modifications are a standard part of the
research and development process and in our experience are routinely made in
development agreements.
9
In March 2004, the agreements were modified to indicate that CombiMatrix
Corporation had completed all phases of its research and development commitments
to Roche.
Since the inception of our relationship with Roche, CombiMatrix Corporation
has engaged in a continuous process of monitoring and reevaluating the terms of
the agreements, and have amended the agreements in several respects to establish
more meaningful goals, milestones and timelines. The agreements are
non-exclusive with respect to CombiMatrix Corporation's core technology, meaning
that CombiMatrix Corporation remains free to license its core technology to
third parties for applications in the genomics, proteomics and other fields. The
agreements contain exclusivity or co-exclusivity provisions only with respect to
the specific products being co-developed for, and partially funded by, Roche
pursuant to the agreements.
Under the terms of the agreements, it is contemplated that Roche will
co-develop, use, manufacture, market and distribute CombiMatrix Corporation's
array and related technology for rapid production of customizable arrays. The
agreements provide for minimum payments by Roche to CombiMatrix Corporation over
the first three years after product launch, including milestone achievements,
payments for products, royalties and future research and development projects.
Nevertheless, because our agreements with Roche contain provisions that would
allow Roche to terminate the agreements, the future payments by Roche to
CombiMatrix Corporation might never be realized. Since July 2001, CombiMatrix
Corporation has received approximately $26.6 million in cash payments from Roche
from July 2001 through December 31, 2003. The CombiMatrix group has not received
any additional payments from Roche since December 31, 2003.
MANUFACTURING AND CUSTOMIZATION
The CombiMatrix group is developing automated, computer-directed
manufacturing processes for the synthesis of sequences of DNA, RNA, peptides or
small molecules on its arrays. Certain portions of its manufacturing, such as
semiconductor fabrication and processing, will be outsourced to subcontractors,
while the steps involving synthesis of biological materials and quality control
of its products will be conducted by the CombiMatrix group.
Substantially all of the components and raw materials used in the
manufacture of the CombiMatrix group's products, including semiconductors and
reagents, are currently provided from a limited number of sources or in some
cases from a single source. Although the CombiMatrix group believes that
alternative sources for those components and raw materials are available, any
supply interruption in a sole-sourced component or raw material might result in
up to a several-month production delay and materially harm the CombiMatrix
group's ability to manufacture products until a new source of supply, if any,
could be located and qualified. In addition, an uncorrected impurity or
supplier's variation in a raw material, either unknown to the CombiMatrix group
or incompatible with its manufacturing process, could have a material adverse
effect on its ability to manufacture products. The CombiMatrix group may be
unable to find a sufficient alternative supply channel in a reasonable time
period, or on commercially reasonable terms, if at all. The CombiMatrix group
utilizes non-standard semiconductor manufacturing processes to fabricate the
electrode array that is a key aspect of the array structure. Although the
CombiMatrix group has a supply agreement in place with the semiconductor wafer
manufacturer to ensure availability of the raw materials, it does not guarantee
a permanent supply. These non-standard processes are not widely available and it
may be difficult or expensive to obtain sufficient quantities of semiconductor
wafers if the current manufacturer changes or discontinues its manufacturing
production capability.
PATENTS AND LICENSES
CombiMatrix Corporation continues to build its intellectual property
portfolio to protect its product in those markets where it operates and where a
market for its products and services exists. In the United States, CombiMatrix
Corporation has been issued four United States patents. Three of the United
States patents (U.S. Patent No. 6,093,302 expiration date January 5, 2018; U.S.
Patent No. 6,280,595 expiration date September 10, 2019 and U.S. Patent No.
6,444,111 expiration date October 13, 2019) protect CombiMatrix Corporation's
core technology relating to methods for electrochemical synthesis of arrays. The
fourth United States Patent (U.S. Patent No. 6,456,942 expiration date January
25, 2020) describes and claims a network infrastructure for custom array
synthesis and analysis. Corresponding CombiMatrix Corporation core patents
describing and claiming methods for electrochemical synthesis of arrays have
been issued in Europe (entire EU), Australia and Taiwan and are pending in the
remaining major industrialized markets. In total, CombiMatrix Corporation has 59
patent applications pending in the Unites States, Europe and elsewhere.
The CombiMatrix group seeks to protect its corporate identity with
trademarks and service marks. In addition, its trademark strategy includes
protecting the identity and goodwill associated with its biological array
processor products. The CombiMatrix group purchases chemical reagents from
suppliers who are licensed under appropriate patent rights. It is the
CombiMatrix group's policy to obtain licenses from patent holders if needed to
practice its chemical processes.
10
The CombiMatrix group's success will depend, in part, upon its ability to
obtain patents and maintain adequate protection of its intellectual property in
the United States and other countries. If it does not protect its intellectual
property adequately, competitors may be able to use its technologies and thereby
erode any competitive advantage that the CombiMatrix group may have. The laws of
some foreign countries do not protect proprietary rights to the same extent as
the laws of the United States, and many companies have encountered significant
problems in protecting their proprietary rights abroad. These problems can be
caused by the absence of rules and methods for defending intellectual property
rights.
The patent positions of companies developing tools and drugs for the
biotechnology and pharmaceutical industries, including the CombiMatrix group's
patent position, generally are uncertain and involve complex legal and factual
questions. The CombiMatrix group will be able to protect its proprietary rights
from unauthorized use by third parties only to the extent that its proprietary
technologies are covered by valid and enforceable patents or are effectively
maintained as trade secrets. The CombiMatrix group's existing patent and any
future patents it obtains may not be sufficiently broad to prevent others from
practicing its technologies or from developing competing products. There also is
risk that others may independently develop similar or alternative technologies
or design around its patented technologies. In addition, others may challenge or
invalidate the CombiMatrix group's patents, or its patents may fail to provide
it with any competitive advantage. Enforcing its intellectual property rights
may be difficult, costly and time consuming, and ultimately may not be
successful.
The CombiMatrix group also relies upon trade secret protection for its
confidential and proprietary information. It seeks to protect its proprietary
information by entering into confidentiality and invention disclosure and
transfer agreements with employees, collaborators and consultants. These
measures, however, may not provide adequate protection for the CombiMatrix
group's trade secrets or other proprietary information. Employees, collaborators
or consultants may still disclose its proprietary information, and the
CombiMatrix group may not be able to meaningfully protect its trade secrets. In
addition, others may independently develop substantially equivalent proprietary
information or techniques or otherwise gain access to its trade secrets.
The CombiMatrix group cannot assure you that any of its patent applications
will result in the issuance of any additional patents, that its patent
applications will have priority of invention or filing date over similar rights
of others, or that, if issued, any of its patents will offer protection against
its competitors. Additionally, the CombiMatrix group cannot assure you that any
patent issued to it will not be challenged, invalidated or circumvented in the
future or that the intellectual property rights it has created will provide a
competitive advantage. Litigation may be necessary to enforce its intellectual
property rights or to determine the enforceability, scope of protection or
validity of the intellectual property rights of others.
COMPETITION
The CombiMatrix group expects to encounter competition in the area of
business opportunities from other entities having similar business objectives.
Many of these potential competitors possess greater financial, technical, human
and other resources than does the CombiMatrix group. The CombiMatrix group
anticipates that it will face increased competition in the future as new
companies enter the market and advanced technologies become available. In the
life sciences industry, many competitors have more experience in research and
development than the CombiMatrix group. Technological advances or entirely
different approaches developed by one or more of its competitors could render
the CombiMatrix group's processes obsolete or uneconomical. The existing
approaches of competitors or new approaches or technology developed by
competitors may be more effective than those developed by the CombiMatrix group.
The CombiMatrix group is aware of other companies or companies with
divisions that have, or are developing, technologies for the SNP genotyping,
gene expression profiling and proteomic markets. The CombiMatrix group believes
that its primary competitors will be Affymetrix, Inc., Agilent Technologies,
Inc., Becton, Dickinson and Company, Ciphergen Biosystems, Inc., Gene Logic
Inc., Illumina, Inc., Johnson & Johnson, Nanogen, Inc., Orchid Biosciences,
Inc., Applera Corporation, Roche Diagnostics GmbH and Sequenom, Inc. However,
the CombiMatrix group's market is rapidly changing, and the CombiMatrix group
expects to face additional competition from new market entrants, new product
developments and consolidation of its existing competitors. Many of the
CombiMatrix group's competitors have existing strategic relationships with major
pharmaceutical and biotechnology companies, greater commercial experience and
substantially greater financial and personnel resources than it does. The
CombiMatrix group expects new competitors to emerge and the intensity of
competition to increase in the future.
RESEARCH, DEVELOPMENT AND ENGINEERING
The CombiMatrix group's research and development expenses, excluding
non-cash stock compensation charges and acquired in-process research and
development charges, were $7.2 million (including Department of Defense related
research and development expenses of $2.0 million), $8.1 million and $18.2
million during 2004, 2003 and 2002, respectively. Research and development
11
related non-cash stock compensation charges were $91,000, $466,000 and $1.9
million during 2004, 2003 and 2002, respectively and acquired in-process
research and development charges were $17.2 million in 2002. The CombiMatrix
group intends to invest in its proprietary technologies through internal
development and, to the extent available, licensing of third-party technologies
to increase and improve other characteristics of its products. The CombiMatrix
group also plans to continue to invest in improving the cost-effectiveness of
its products through further automation and improved information technologies.
The CombiMatrix group's future research and development efforts may involve
research conducted by the CombiMatrix group, collaborations with other
researchers and the acquisition of chemistries and other technologies developed
by universities and other academic institutions.
The CombiMatrix group is developing a variety of life sciences and non-life
sciences products and services. Potential customers for these products operate
in industries characterized by rapid technological development. The CombiMatrix
group believes that its future success will depend in large part on its ability
to continue to enhance its existing products and services and to develop other
products and services, which complement existing ones. In order to respond to
rapidly changing competitive and technological conditions, the CombiMatrix group
expects to continue to incur significant research and development expenses
during the initial development phase of new products and services, as well as on
an ongoing basis.
GOVERNMENT GRANTS AND CONTRACTS
Government grants and contracts have allowed the CombiMatrix group to fund
certain internal scientific programs and exploratory research. The CombiMatrix
group retains ownership of all intellectual property and commercial rights
generated during these projects. The United States government, however, retains
a non-exclusive, non-transferable, paid-up license to practice the inventions
made with federal funds pursuant to applicable statutes and regulations. The
CombiMatrix group does not believe that the retained license will have any
impact on its ability to market its products. The CombiMatrix group does not
need government approval to enter into collaborations or other relationships
with third parties.
The CombiMatrix group has been awarded several grants from the federal
government in connection with its biological array processor technology since
it's inception. Most recently, in March of 2004, the CombiMatrix group was
awarded a two-year, $5.9 million contract with the Department of Defense to
further the development of the CombiMatrix group's array technology for the
detection of biological threat agents. Under the terms of the contract, the
CombiMatrix group will be reimbursed on a periodic basis for actual costs
incurred to perform its obligations, plus a fixed fee, of up to $5.9 million.
The CombiMatrix group will continue to pursue grants and contracts that
complement its research and development efforts.
RECENT ACTIVITIES
Significant milestones for the CombiMatrix group during 2004 include the
following:
GENETIC ANALYSIS PRODUCTS AND SERVICES
o In March 2004, the CombiMatrix group launched the CustomArray(TM) DNA
Microarray platform, its first commercially-available array product,
to the worldwide research marketplace. This platform offers
researchers the ability to order fully customizable arrays on demand
in any quantity they choose. CustomArrays(TM) are produced on a
standard slide format, a configuration that is most familiar to
researchers. The commercial launch of CustomArray(TM) also provides
the marketplace with full access to the CombiMatrix group's suite of
software applications, including array design and submission, online
ordering and extraction of experimental results.
o In June 2004, the CombiMatrix group entered into a co-marketing
relationship with Axon Instruments, Inc. and in July, the CombiMatrix
group entered into a co-marketing agreement with Strand Genomics.
o In July 2004, the CombiMatrix group launched the CustomArray(TM)12K
DNA expression array, offering researchers the ability to order a
fully customizable array with more than 12,000 sites.
CustomArray(TM)12K and related products enable researchers to study
any combination of genes from any genome on a single chip. Also in
July 2004, the CombiMatrix group made available to researchers new
CustomArrays(TM) for Human Drug Metabolism, Human Toxicology, and a
Core 67 Cancer Array.
o In August 2004, the CombiMatrix group entered into a multi-year
collaborative strategic alliance with Furuno Electric Company, Ltd.
("Furuno") to design, engineer and build CombiMatrix Corporation's
Bench-Top DNA Microarray Synthesizer for CustomArray(TM) formatted
arrays. Under the terms of the agreement, Furuno paid CombiMatrix
Corporation an upfront fee of $1,000,000 and will make additional
development and milestone payments in the future, in accordance with
the agreement.
12
o In December 2004, the CombiMatrix group launched Design-on-Demand(TM)
Arrays, which provides the marketplace with a comprehensive catalog of
microarray expression products for microbial, eukaryotic, and viral
genomes. These arrays offer scientists an affordable and flexible tool
to conduct whole-genome expression studies on a wide range of
organisms, including Influenza, HIV, Anthrax, and SARS coronavirus.
HOMELAND SECURITY AND DEFENSE APPLICATIONS
o In March 2004, the CombiMatrix group executed a two-year, $5.9 million
contract with the Department of Defense to further the development of
the CombiMatrix group's microarray technology for the detection of
biological threat agents. Additionally, In July 2004, the CombiMatrix
group announced that it will receive an additional $2.3 million from a
Department of Defense appropriations bill passed by Congress.
o In October 2004, the CombiMatrix group and Science Applications
International Corporation (SAIC) announced a collaboration to develop
arrays for the identification of multiple bio-threat organisms. The
intent of the collaboration is to leverage each company's respective
Department of Defense funding in order to expedite the development and
testing of new identification and diagnostic assays and products
against conventional bio-threat agents and emerging and genetically
engineered pathogens.
DRUG DISCOVERY
o In January 2004, the CombiMatrix group made the first commercially
available microarray designed for the H5N1 influenza A virus ("bird
flu"). The World Health Organization appealed on January 27, 2004 for
technical assistance and expert advice to help stop the threat to
humans and agriculture posed by bird flu virus. The CombiMatrix group
utilized its proprietary probe-design software and ability to rapidly
synthesize novel DNA arrays to respond within two days.
o During 2004, the CombiMatrix group entered into a number of
collaborations in the field of drug discovery and development,
including collaborations with: 1) Washington University in St. Louis
to develop a system for the synthesis of libraries of diverse,
non-nucleic acid molecules; 2) Professor Bonaventura Clotet, M.D.,
Ph.D., of the Retrovirology Laboratory irsiCaixa, to conduct the
initial efficacy screening of pooled siRNA compounds against the
hepatitis C virus; 3) Dr. Ulrich Melcher, Department of Biochemistry
and Molecular Biology and Dr. Alexander C. Lai, Department of
Microbiology and Molecular Genetics from Oklahoma State University to
utilize CombiMatrix group's `Bird Flu' CustomArray(TM) devices to
characterize Bird flu viruses at the genomic level; 4) St. Jude
Children's Research Hospital to study the genetic variation in the H9
variant of Bird Flu; and 5) Case Western Reserve University for work
in developing a novel diagnostic for Alzheimer's disease using the
CustomArray(TM) platform.
o In October 2004, the CombiMatrix group entered into an agreement to
acquire up to a one-third ownership interest in Leuchemix, Inc.
("Leuchemix"), a private drug development firm, which is developing
several compounds for the treatment of leukemia and other cancers. In
accordance with the terms of the purchase agreement, the CombiMatrix
group will purchase 3,137,500 shares of Series A Preferred Stock of
Leuchemix for a total purchase price of $4,000,000, to be paid
quarterly over the next two years. In accordance with the terms of the
purchase agreement, CombiMatrix Corporation's Chief Executive Officer
was named a director of Leuchemix.
NANOTECHNOLOGY
o In January 2004, the CombiMatrix group entered into collaboration with
Cyrano Sciences (which has been acquired by Smiths Detection) to
develop nanotechnology-based chemical sensors to be used for the
detection of biological agents in air and water.
o In September 2004, the CombiMatrix group and Intel Corporation entered
into an agreement to work together on the feasibility of various
projects utilizing the CombiMatrix group's core technology. The terms
and conditions of the agreement are confidential.
o During 2004, the CombiMatrix group's strategic partner, Nanomaterials
Discovery Corporation, was granted a U.S. patent entitled, "Electrode
Array for Development and Testing of Materials." In August 2004,
Nanomaterials Discovery Corporation received a $2.5 million Department
of Defense appropriation for the development of fuel cell technology,
which will utilize CombiMatrix group's NanoArray(TM) technology as a
13
component of this development. In December 2004, Nanomaterials
Discovery Corporation delivered its first prototype nanomaterials
workstation to the CombiMatrix group.
ADDITIONS TO THE COMBIMATRIX GROUP'S SCIENTIFIC ADVISORY BOARD
o In February 2004, the CombiMatrix group named F. Mark Modzelewski to
its Scientific Advisory Board. Mr. Modzelewski is currently a
principal of Lux Capital, a New York based venture capital firm, and
is also a member of the Nanotechnology Technical Advisory Group to
President Bush's Council of Advisors on Science and Technology
(PCAST). Mr. Modzelewski was recognized by Forbes magazine as one of
nanotech's five "powerbrokers" and he has testified before the U.S.
Senate on nanotechnology funding, investment, technology transfer and
global competition.
o In April 2004, the CombiMatrix group named Mark A. Kay, M.D., Ph.D.,
to its Scientific Advisory Board. Dr. Kay is a Professor, Departments
of Pediatrics and Genetics, and Director, Program in Human Gene
Therapy, at the Stanford University School of Medicine. Dr. Kay is one
of the founders of and is currently the Vice President of the American
Society of Gene Therapy and a leader and pioneer in areas including
RNAi, gene and nucleic-acid drug delivery, and gene therapy.
EMPLOYEES
As of December 31, 2004, the CombiMatrix group had 71 full-time employees,
14 of whom hold Ph.D. degrees and 40 of whom are engaged in full-time research
and development activities. The CombiMatrix group is not a party to any
collective bargaining agreement. The CombiMatrix group considers its employee
relations to be good.
ENVIRONMENTAL MATTERS
The operations of the CombiMatrix group involve the use, transportation,
storage and disposal of hazardous substances, and as a result, it is subject to
environmental and health and safety laws and regulations. The cost of complying
with these and any future environmental regulations could be substantial. In
addition, if the CombiMatrix group fails to comply with environmental laws and
regulations, or releases any hazardous substance into the environment, the
CombiMatrix group could be exposed to substantial liability in the form of
fines, penalties, remediation costs and other damages, or could suffer a
curtailment or shut down of its operations.
14
ACACIA TECHNOLOGIES GROUP
(A DIVISION OF ACACIA RESEARCH CORPORATION)
BUSINESS
The Acacia Technologies group, a division of Acacia Research Corporation,
including companies recently acquired from Global Patent Holdings as described
earlier, is currently comprised of certain of Acacia Research Corporation's
wholly owned subsidiaries and limited liability companies including: Acacia
Global Acquisition Corporation, Acacia Internet Access Corporation, Acacia Media
Technologies Corporation, Acacia Patent Acquisition Corporation, Acacia
Technologies Services Corporation, AV Technologies LLC, Broadcast Innovation
LLC, Data Innovation LLC, Financial Services Innovation LLC, Information
Technology Innovation LLC, InternetAd LLC, IP Innovation LLC, KY Data Systems
LLC, New Medium LLC, TechSearch LLC, VData LLC, Soundview Technologies, Inc.,
and Spreadsheet Automation Corporation and also includes all corporate assets,
liabilities, and related transactions of Acacia Research Corporation attributed
to the Acacia Research Corporation's intellectual property licensing business.
The Acacia Technologies group develops, acquires, licenses and enforces
patented technologies. From time to time, companies comprising the Acacia
Technologies group engage in litigation to enforce their patents. For a current
listing of pending patent enforcement litigation, see "Patent Enforcement
Litigation," below.
DIGITAL MEDIA TRANSMISSION TECHNOLOGY
The Acacia Technologies group owns and out-licenses a portfolio of
pioneering U.S. and foreign patents covering digital audio and video
transmission and receiving systems, commonly known as audio-on-demand,
video-on-demand, and audio/video streaming. The Acacia Technologies group's
patented proprietary DMT technology enables the digitization, encryption,
storage, transmission, receipt and playback of digital content via several means
including cable TV, which includes digital ad insertion and video on demand
programming, satellite TV programming, the Internet, which includes distance
learning and other Internet programming involving digital audio/video content,
wireless delivery of video content, fiber-optic delivery of video content and
hotel in-room digital delivery of programming. The Acacia Technologies group's
DMT technology is protected by five U.S. patents which expire in 2011and 31
foreign patents which expire in 2012.
MARKET OVERVIEW
The Acacia Technologies group has launched an extensive DMT technology
licensing program. Potential licensees include cable TV companies, satellite TV
companies, hotel in-room entertainment companies, telecommunications companies,
wireless companies and online music, movie, adult entertainment, e-learning,
sports, news and information companies.
The use of DMT technology continues to grow both in the United States and
internationally. The transmission of digital content by cable TV companies
continues to increase with the use of video-on-demand and digital ad insertion
systems. Satellite TV companies are switching to hard drive based reception
systems to offer their content with on-demand functionality. Hotel in-room
entertainment companies are switching to electronic distribution systems and
digital storage systems to reduce costs and increase profitability.
Telecommunications companies have announced plans to deliver digital video via
fiber-optic and wireless companies have begun to deliver digital video content
with 3G delivery systems. Entertainment companies are making more digital
content available via the Internet in order to distribute content directly to
the consumer as opposed to using third party distributors and retail outlets.
MARKETING AND DISTRIBUTION
DMT Technology Licensing Program
Acacia Technologies group is currently licensing its DMT technology and has
entered into 294 DMT licensing agreements to date, including 107 cable TV
licenses, 182 licenses for online entertainment, movies, news, sports,
e-learning and corporate websites and licenses with 5 companies that provide
over 90% of video-on-demand TV entertainment to the hotel industry in the United
States. During 2004, we executed 170 DMT license agreements. Licensees include
Bloomberg L.P., Capella Education Company, Callaway Golf Company, B&C
Cablevision, Central Valley Cable TV, LLC, CinemaNow, Inc., Disney Enterprises,
Inc., General Dynamics Interactive Corporation, Grupo Pegaso, Harley-Davidson,
Inc., LodgeNet Entertainment Corporation, NXTV, Inc., On Command Corporation,
Oral Roberts University, Revlon Consumer Products Corporation, Seren
Innovations, Sonoco Products Company, The Travelers Indemnity Company, T. Rowe
Price Associates, Inc., 24/7 University, Inc., Wachovia Corporation, Wendy's
International, Inc., World Wrestling Entertainment, Inc. and Xerox Corporation.
15
In the first, second, third and fourth quarters of 2004, the Acacia
Technologies group recorded DMT license fee revenues of $599,000, $666,000,
$740,000 and $779,000, respectively, compared to DMT license fee revenues of
$6,000, $19,000, $186,000 and $481,000, respectively, in the comparable 2003
periods.
RECENT ACQUISITION
On January 28, 2005, Acacia Global Acquisition Corporation, acquired substantially all of the assets of Global Patent Holdings, LLC, a privately held patent holding company, which owned 11 patent licensing companies. Thecompanies (“GPH Acquisition”). In connection with the acquisition gives the Acacia Technologies groupwe acquired ownership of companies that own or control the rights to 27 patent portfolios, which includeincluded 120 U.S. patents and certain foreign counterparts, and covercovering technologies used in a wide variety of industries, including:
o AUDIO/VIDEO ENHANCEMENT AND SYNCHRONIZATION
-------------------------------------------
These patented technologies generally relateindustries. Refer to Note 7 to our consolidated financial statements included in this report for additional information regarding the GPH Acquisition. Subsequent to the useGPH Acquisition, we have continued to execute our business strategy in the area of a noise
reduction filtering system for digital video compression, and for
video and audio signals received by digital radios and video displays.
Other aspectspatent portfolio acquisitions, including the acquisition of, or the technologies generally relate to the
synchronization of audio/video signals. These technologies can be used
by broadcasters, broadcast equipment manufacturers, other electronics
manufacturers, and low frame rate video production, such as that used
on the Internet.
o BROADCAST DATA RETRIEVAL
------------------------
This patented technology generally relatesrights to, a system for
broadcasting and receiving programming content together with
supplemental data such as the titletotal of 59 additional patent portfolios covering a song, artist, content
description or a catalog number, which can be stored and recalled for
later viewing. This technology can be used in satellite radio, and
other broadcasting where data is transmitted along with the content.
o COMPUTER MEMORY CACHE COHERENCY
-------------------------------
This patented technology generally relates to interface circuits used
by intelligent peripheral devices with cache memory to communicate
with the main computer memory. By synchronizing main computer memory
and main cache memory, peripheral devices such as graphics processors
can operate at much higher speeds, without costs associated with their
own memory. This technology can be used in desktop, notebook, and
server computer systems.
o CREDIT CARD FRAUD PROTECTION
----------------------------
This patented technology generally relates to a computerized system
for protecting retailers and consumers engaged in credit card, check
card, and debit transactions. The system includes an electronic card
reader, and the generation and use of a transaction number, which
specifically identifies each transaction processed within the system.
As a result, the retailer does not necessarily have to print detailed
information concerning the cardholder's identity or account number on
the customer's receipt.
o DATA ENCRYPTION AND PRODUCT ACTIVATION
--------------------------------------
These patented technologies generally relate to accessing clear data,
and encrypted data via an identification label. Once decrypted, the
clear and decrypted data are combined to activate software programs,
and other files. Other aspects of the technologies generally relate to
the use of an operating system to transparently create an encrypted
file storage subsystem to fully secure user files from access by
anyone other than the user.
o DATABASE MANAGEMENT
-------------------
This patented technology generally relates to the improved
combination, display, and coordination of certain information from
data tables in a relational database software program. The user is
able to easily track the impact of a change to one table, on other
tables in the program through various tools including a graphical
representation.
o DIGITAL VIDEO PRODUCTION
------------------------
These patented technologies generally relate to features that can be
found in production video processing equipment. They cover improved
methods of equipment interconnection, aspects of graphical user
interface displays, and automation of video processing. These features
allow ease of equipment interconnection, clearer information display,
and automation of video production tasks previously performed
manually. Other aspects of the technologies generally relate to
automatic color correction, commonly used when transferring film to
video, and certain 3D effects, commonly used in video scene
transitions.
16
o DYNAMIC MANUFACTURING MODELING
------------------------------
This patented technology generally relates to a modeling and control
process used to decrease costs and increase production for factory
operations. Such simulation modeling can include awide variety of parameters such as products, fabrication sequences, collections of job
sets, scheduling rules,technologies and machine reliability standards. This
technology can be used for exacting manufacturing processes such as
semiconductor fabrication.
o ENHANCED INTERNET NAVIGATION
----------------------------with applications across several additional industries. These patented technologies generally relateand future patent portfolio acquisitions will continue to enhanced Internet
navigation by retrieving a page from a hyper-linked website for
retrieval offline on a personal computer. This enables certain website
content to be saved, retrieved,expand and accessed locally, without the need
for Internet connectivity. Other aspects of the technologies generally
relate to information distribution and processing via the use of a
linking reference to access sets of data. These technologies can be
used in email transmissions with links to websites, special offers,
and other information.
o IMAGE RESOLUTION ENHANCEMENT
----------------------------
This patented technology generally relates to the modification of a
video or printed display to improve the perceived image quality beyond
the basic pixel resolution of the display. The apparent improvement in
the resolution of an image occurs without requiring an increase in the
resolution of the signal or input. This technology can be used in
certain CRT, plasma and LCD televisions and displays, low resolution
cameras such as camera phones, and consumer and commercial printers.
o INTERACTIVE DATA SHARING
------------------------
This patented technology generally relates to the real time sharing of
changes to content, enabling users to interactively view, change and
add to the content from multiple remote terminals. This technology can
be used in certain types of conferencing such as web conferencing,
interactive gaming, and other forms of collaborative interactive
communication.
o INTERACTIVE TELEVISION
----------------------
These patented technologies generally relate to various aspects of
interactive television including receivers such as set-top boxes and
certain televisions used in digital satellite TV and digital cable TV
systems that permit television viewers to access interactive
television features supplied by their satellite TV and cable TV
providers as part of their digital programming packages. Data, which
is associated with the interactive television features and is
broadcast along with the video signal, is extracted and processed by
components within the receivers, and is then made available to viewers
who choose to access the interactive television features through their
remote control. Examples of such data include sports scores, weather
information, stock updates, interactive games, and movie listings.
Other aspects of the technologies generally relate to the scrambling
or encrypting of broadcast signals whereby the unscrambling or
decryption is accomplished through a removable card, commonly known as
a "smart card."
o INTERSTITIAL INTERNET ADVERTISING
---------------------------------
This patented technology generally relates to the display of certain
advertising, informational, and branding messages that appear between
or outside web pages when the user is conducting a search, by storing
the message prior to being displayed. This technology is most commonly
used by travel based and other reservation based websites.
o MICROPROCESSOR ENHANCEMENT
--------------------------
This patented technology generally relates to an architecture employed
in advanced pipeline microprocessors. This architecture allows for
conditional execution of microprocessor instructions, and a later
determination of whether the instructions executed should be written
back to memory. By conditionally executing instructions in this
architecture, significant improvements in microprocessor speed can be
achieved. Certain pipelined processor manufacturers are adopting this
method of processing to improve processor speed.
17
o MULTI-DIMENSIONAL BAR CODES
---------------------------
This patented technology generally relates to encoding and reading a
data matrix consisting of an array of data cells with a border. The
data matrix can contain a variety, amount, and depth of information
that would not fit on to an ordinary bar code. This patented
technology can have many applications in the manufacturing,
distribution, operations, accounting, and security industries such as
tracking the movement of products, collection of data, improved
production capabilities and anti-counterfeiting.
o NETWORK DATA BACK-UP
--------------------
This patented technology generally relates to a computer network
system for backing up data and program files listed by users from
networked work stations. User lists are stored locally, resulting in
increased speed and security. This technology can be used by network
software.
o RESOURCE SCHEDULING
-------------------
This patented technology generally relates to the creation and
maintenance of a schedule through the periodic management and
monitoring of interrelated and interdependent resources from a
database. These resource management tools can be part of scheduling
software used to plan and monitor the use of facilities, the
allocation of manpower, and the use and scheduling of other resources.
o ROTATIONAL VIDEO IMAGING
------------------------
This patented technology generally relates to a rotational video
imaging device for viewing the interior of a cavity or structure. This
technology can be used for medical devices such as endoscopes, and
non-medical devices capable of noninvasive surveillance and analysis.
o SPREADSHEET AUTOMATION
----------------------
This patented technology generally relates to automating the
production of worksheet files for use by electronic spreadsheet
programs. Specifically, the patented technology permits the efficient
retrieval of data from external databases by allowing the user to
select specific data from a database and import the specified data
into a spreadsheet program through uniquely streamlined spreadsheet
commands. The adaptive quality of the technology permits, among other
things, the user to retrieve updated information from an external
database without creating formatting issues in the user's spreadsheet
program.
The acquisition expands and diversifies the Acacia Technologies group'sdiversify our operating subsidiaries' revenue generating opportunities and acceleratesaccelerate the execution of the Acacia
Technologies group'sour overall business strategy, of acquiring, developingas we continue to build our leadership position in patent licensing.
Business Model and Strategy
The business model associated with the licensing patented technologies.
V-CHIP TECHNOLOGY
The Acacia Technologies group also owned and out-licensed to consumer
electronics manufacturers, patented technology known as the V-chip. The V-chip
technology was protectedenforcement activities conducted by U.S. Patent No. 4,554,584, which expired in July
2003. The V-chip was adopted by manufacturers of televisions soldour operating subsidiaries is summarized in the U.S. to
provide blocking of certain programming based upon its content rating code, in
compliance with the Telecommunications Act of 1996.
V-CHIP LICENSING PROGRAM
The V-chip patent expired in July 2003. The Acacia Technologies group has
licensed 13 major television manufacturers, representing approximately 75% of
the televisions sold in the United States, including Samsung Electronics,
Hitachi America, Ltd., LG Electronics, Inc., Funai Electric Co., Ltd., Daewoo
Electronics Corporation of America, Sanyo Manufacturing Corporation, Thomson
Multimedia, Inc., JVC Americas Corporation, Matsushita Electric Industrial Co.,
Ltd., Orion Electric Co. Ltd., Pioneer Electronics (USA) Incorporated, Philips
Electronics North America Corporationfollowing diagram:
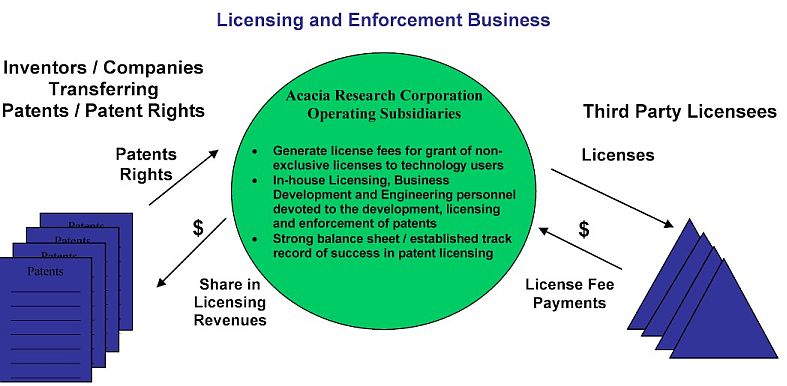
3
Our intellectual property acquisition, development, licensing and Loewe Opta Gmbh. To date, the Acacia
Technologies group has recognized $25.7 million in V-chip license fees,
including $1.5 million in previously deferred V-chip license fees in 2004. We
concluded the V-chip licensing program in August 2004 and do not expect to
receive any additional V-chip related revenues in future periods.
18
PATENT ENFORCEMENT LITIGATION
From time to time, companies comprising the Acacia Technologies group
engage in litigation to enforce their patents. A summary of patent enforcement
related litigation initiated by Acacia Technologies group companies is provided
below.
SOUNDVIEW TECHNOLOGIES
Litigation for patent infringement and anti-trust violations was pending in
the U.S. Court of Appeals for the Federal Circuit against Sony Corporation of
America, Mitsubishi Digital Electronics America, Inc., Sharp Electronics
Corporation and Toshiba America Consumer Products, Inc.
In August 2004, the U.S. Court of Appeals for the Federal Circuit affirmed
the September 2002 U.S. District Court for the District of Connecticut ruling
that the remaining television manufacturers named in the Acacia Technologies
group's V-chip patent infringement lawsuit do not infringe the Acacia
Technologies group's V-chip patent. Refer to Item 7. "Management's Discussion
and Analysis of Financial Condition and Results of Operations," for details of
the financial statement impact of the final ruling. The remaining non-Soundview
parties have a motion pending before the United States District Court for the
District of Connecticut seeking reimbursement of certain attorney's fees.
Management believes that the ultimate liability with respect to this claim, if
any, will not have a material effect on our financial position, results of
operations or cash flows.
ACACIA MEDIA TECHNOLOGIES CORPORATION
CABLE AND SATELLITE TV
In 2004, Acacia Media Technologies filed a Complaint in the District Court
for the Northern District of California alleging infringement of Acacia Media
Technologies' DMT patents against Comcast Corporation, Charter Communications,
Inc., The DirectTV Group, Inc., Echostar Communications Corporation, Boulder
Ridge Cable TV, Central Valley Cable TV, LLC, Seren Innovations, Inc., Cox
Communications, Inc., Hospitality Network, Inc. (a wholly owned subsidiary of
Cox that supplies hotel on-demand TV services) and Mediacom, LLC. As of December
31, 2004, Acacia Media Technologies has executed license and settlement
agreements with Boulder Ridge Cable TV, Central Valley Cable TV, and Seren
Innovations.
In September 2004, Acacia Media Technologies filed complaints in the U.S.
District Court for the District of Arizona, U.S. District Court for the District
of Minnesota and the U.S. District Court for the Northern District of Ohio -
Eastern Division, alleging infringement of Acacia Media Technologies' DMT
patents against certain cable and satellite companies located in Arizona,
Minnesota, and Ohio. Companies named in the lawsuits include Armstrong Group,
Arvig Communication Systems, Block Communications, Inc., Cable America
Corporation, Cable One, Inc., Cable System Services, Inc., Cannon Valley
Communications, Inc., East Cleveland Cable TV and Communications, LLC, Loretel
Cablevision, Massillon Cable TV, Inc., Mid-Continent Media, Inc., Nelsonville TV
Cable, Inc., NPG Cable, Inc., Precis Communications, Inc. San Carlos
Cablevision, LLC, Savage Communications, Inc., Sjoberg's Cablevision, Inc., US
Cable, and Wide Open West, LLC. As of December 31, 2004, Acacia Media
Technologies has executed license agreements with Precis Communications and
Cable System Services and dismissed the action against San Carlos Cablevision
and Nelsonville TV Cable.
INTERNET WEBSITES
In 2003, Acacia Media Technologies initiated DMT patent infringement
litigation in the Federal District Court for the Central District of California
(the "Court") against defendants who provide adult oriented digital content over
the Internet. As of December 31, 2004, New Destiny Internet Group, Inc., Audio
Communications Inc., VS Media, Ademia Multimedia, LLC, International Web
Innovations, Inc., Offendale Commercial BV, Ltd., Adult Entertainment Broadcast
Network, Cybertrend, Inc., Lightspeed Media Corp., Adult Revenue Services,
Innovative Ideas International, AskCS.com, Game Link, Inc., Club Jenna, Inc.,
Cybernet Ventures, Inc., ACMP, LLC, Global AVS, Inc. d/b/a DrewNet, ICS, Inc. /
AP Net Marketing, Inc., and National A-1 Advertising, remained in the
litigation.
HOTEL ON-DEMAND TV INDUSTRY
In November 2003, Acacia Media Technologies initiated a patent infringement
lawsuit in the Federal District Court for the Central District of California
against On Command Corporation, provider of interactive in-room entertainment,
information and business services to the lodging industry, regarding Acacia
Media Technologies' DMT technology. In June 2004, Acacia Media Technologies
entered into a license agreement for its DMT technology with On Command
Corporation settling all outstanding litigation between the parties.
19
PATENT ENFORCEMENT LITIGATION - RELATED TO ACQUIRED COMPANIES
Certain companies acquired as a result of the January 2005 acquisition of
the assets of Global Patent Holdings, as described above, have initiated patent
enforcement related litigation as follows:
IP INNOVATION, LLC
o IP Innovation, LLC et. al. v. Lexmark International, Inc., United
States District Court for the Northern District Of Illinois
o IP Innovation, LLC. v. Dell Computer, United States District Court for
the Northern District of Illinois
o IP Innovation, LLC v. WebCT., Digital Think, Inc., eCollege.com,
Docent Inc., United States District Court for the Southern District of
Texas, on appeal to the U.S. Court of Appeals for the Federal Circuit
IP INNOVATION, LLC AND NEW MEDIUM, LLC
o IP Innovation, LLC and New Medium, LLC et. al. v. Sony Electronics,
Inc., United States District Court for the Northern District of
Illinois
o IP Innovation, LLC and New Medium, LLC et. al. v. Matsushita Electric
Corporation of America, et. al., United States District Court for the
Northern District of Illinois
VDATA LLC
o VCode Holdings, Inc. et. al. v. Adidas America, AMD, Stamps.com,
Hitachi Global Storage Technologies Thailand, Ltd, et. al., United
States District Court for the District of Minnesota
INFORMATION TECHNOLOGY INNOVATION, LLC
o Information Technology Innovation, LLC et. al. v. Motorola, Inc.,
United Sates District Court for the Northern District of Illinois
BROADCAST INNOVATION, LLC
o Broadcast Innovation, LLC et. al. v. Echostar Communications
Corporation et. al., United States District Court for the District of
Colorado
o Broadcast Innovation, LLC et. al. v. Charter Communications Inc. et.
al., United States District Court for the District of Colorado, on
appeal to the U.S. Court of Appeals for the Federal Circuit
FINANCIAL SYSTEMS INNOVATION, LLC
o Ware et. al. v. H.E. Butt Grocery Company, Williams-Sonoma, Inc.,
Linens 'N Things, Inc., Petco Animal Supplies, Inc., Costco Wholesale
Corporation,, The Bombay Company , United Sates District court for the
Northern District of Texas
o Ware et. al. v. The Kroger Co. United States District court for the
Northern District of Georgia
o Financial Systems Innovation, LLC v. Via Technologies, Inc. et. al.,
CD, California
AV TECHNOLOGIES, LLC
o Technology Licensing Corporation et. al. v. Thomson, Inc., E.D.
California
20
THE ACACIA TECHNOLOGIES GROUP'S STRATEGY
The Acacia Technologies group's business strategy, conducted solely by our operating subsidiaries, includes the following:
IDENTIFY EMERGING GROWTH AREAS WHERE PATENTED TECHNOLOGIES WILL PLAY A
VITAL ROLE
following key elements:
· | Identify Emerging Growth Areas where Patented Technologies will Play a Vital Role |
The patent process breeds innovation and invention by granting a limited monopoly to the inventor in exchange for sharing the invention with the public. Certain technologies, such asincluding several of the technologies controlled by our DMT technology,operating subsidiaries, some of which are summarized below, become core technologies in the way products and services are manufactured, sold and delivered. The Acacia
Technologies group identifiesOur operating subsidiaries identify core, patented technologies that have been or are anticipated to be widely adopted by third parties in connection with the manufacture or sale of products and services.
CONTACT AND FORM ALLIANCES WITH OWNERS OF CORE, PATENTED TECHNOLOGIES
For years, many large companies have earned substantial revenue licensing
patented technologies to third parties. Other companies that do not have
internal licensing resources and expertise have continued to record the
estimated value of intellectual property on their financial statements without
deriving income from their intellectual property. Recent changes in securities
and financial reporting regulations require these companies to evaluate and
potentially reduce or write-off these intellectual property assets if they are
unable to substantiate these reported values.
The
| · | Contact and Form Alliances with Owners of Core, Patented Technologies |
| Often individual inventors and small companies have limited resources and/or expertise and are unable to effectively address the unauthorized use of their patented technologies. Individual inventors and small companies may lack sufficient capital resources and may also lack in-house personnel with patent licensing expertise and/or experience, which may make it difficult to effectively out-license and/or enforce their patented technologies. |
| For years, many large companies have earned substantial revenue licensing patented technologies to third parties. Other companies that do not have internal licensing resources and expertise have continued to record the capitalized carrying value of their intellectual property in their financial statements, without deriving income from their intellectual property or realizing the potential value of their intellectual property assets. Securities and financial reporting regulations require these companies to periodically evaluate and potentially reduce or write-off these intellectual property assets if they are unable to substantiate these reported carrying values. |
Acacia Technologies group seeksResearch Corporation’s subsidiaries seek to enter into business agreements with owners of intellectual property that do not have experience or expertise in the areas of intellectual property licensing and enforcement or that do not possess the in-house resources to devote to licensing and enforcement activities.
EFFECTIVELY AND EFFICIENTLY EVALUATE PATENTED TECHNOLOGIES FOR ACQUISITION,
LICENSING AND ENFORCEMENT
| · | Effectively and Efficiently Evaluate Patented Technologies for Acquisition, Licensing and Enforcement |
| Subtleties in the language of a patent, recorded interactions with the patent office, and the evaluation of prior art and literature can make a significant difference in the potential licensing and enforcement revenue derived from a patent or patent portfolio. Our specialists are trained and skilled in these areas. It is important to identify potential problem areas prior to acquisition and commercialization and determine whether potential problem areas can be overcome, before acquiring a patent portfolio or launching a licensing program. We have developed processes and procedures for identifying problem areas and evaluating the strength of a patent portfolio before the decision is made to allocate resources to an acquisition or a licensing and enforcement effort. |
| · | Purchase or Acquire the Rights to Patented Technologies |
| After evaluation, our operating subsidiaries may elect to purchase the patented technology, or become the exclusive licensing agent for the patented technology in all or in specific fields of use. In either case, the owner of the patent generally retains the rights to a portion of the net revenues generated from a patent’s licensing and enforcement program. Our operating subsidiaries generally control the licensing and enforcement process and utilize their experienced in-house personnel to reduce outside costs and to ensure that the necessary capital is allocated and deployed in an efficient and cost effective manner. |
| · | Successfully License and Enforce Patents with Significant Royalty Potential |
| As part of the patent evaluation process employed by our operating subsidiaries, significant consideration is also given to the identification of potential infringers, industries within which the potential infringers exist, longevity of the patented technology, and a variety of other factors that directly impact the magnitude and potential success of a licensing and enforcement program. Our specialists are trained in evaluating potentially infringing technologies and in presenting the claims of our patents and demonstrating how they apply to companies we believe are using our technologies in their products or services. These presentations generally take place in a non-adversarial business setting, but can also occur through the litigation process, if necessary. |
4
Patented Technologies
Currently, on a consolidated basis, our operating subsidiaries own or control the rights to 91 patent portfolios, with patent expiration dates ranging from 2008 to 2027, and covering technologies used in a wide variety of industries, including the following:
·Aligned Wafer Bonding | ·Embedded Broadcast Data | ·Portable Storage Devices With Links |
·Audio Communications Fraud Detection | ·Encrypted Media & Playback Devices | ·Product Activation |
·Audio Storage and Retrieval System | ·Enhanced Internet Navigation | ·Projector |
·Audio Video Enhancement & Synchronization | ·Facilities Operation Management System | ·Purifying Nucleic Acid |
·Authorized Spending Accounts | ·File Locking In Shared Storage Networks | ·Radio Communication With Graphics |
·Automated Notification of Tax Return Status | ·Flash Memory | ·Relational Database Access |
·Broadcast Data Retrieval | ·Fluid Flow Control And Monitoring | ·Remote Management Of Imaging Devices |
·Color Correction For Video Graphics Systems | ·Graphics Data Processing | ·Remote Video Camera |
·Compact Disk | ·Hearing Aid ECS | ·Resource Scheduling |
·Compiler | ·Heated Surgical Blades | ·Rule Based Monitoring |
·Computer Graphics | ·High Quality Image Processing | ·Software Feature Enablement |
·Computer Memory Cache Coherency | ·High Resolution Optics | ·Software License Management |
·Computer Simulations | ·Image Resolution Enhancement | ·Spreadsheet Automation |
·Computing Device Performance | ·Interactive Content In A Cable Distribution System | ·Storage Technology |
·Continuous TV Viewer Measuring | ·Interstitial Internet Advertising | ·Surgical Catheter |
·Copy Protection | ·Laptop Connectivity | ·Telematics |
·Credit Card Fraud Protection | ·Location Based Services | ·Television Data Display |
·Database Access | ·Medical Image Stabilization | ·Television Signal Scrambling |
·Database Management | ·Medical Monitoring | ·Text Auto-Completion |
·Data Encryption | ·Micromirror Digital Display | ·Vehicle Anti-Theft Parking Systems |
·Digital Newspaper Delivery | ·Microprocessor Enhancement | ·Vehicle Location |
·Digital Video Production | ·Multi-Dimensional Bar Codes | ·Vehicle Maintenance |
·DMT® | ·Multi-Dimensional Database Compression | ·Video Editing |
·Document Generation | ·Online Ad Tracking | ·Virtual Computer Workspaces |
·Document Retrieval Using Global Word Co-Occurrence Patterns | ·Parallel Processing With Shared Memory | ·Virtual Server |
·DRAM (Dynamic Random Access Memory) | ·Peer To Peer Communications | ·Web Personalization |
·Dynamic Manufacturing Modeling | ·Physical Access Control | ·Wireless Digital Messaging |
·Ecommerce Pricing | ·Picture Archiving & Communication Systems | ·Wireless Traffic Information |
·Electronic Address List Management | ·Pointing Device | ·Workspace With Moving Viewpoint |
·Electronic Message Advertising | ·Pop-Up Internet Advertising |
Patent Enforcement Litigation
Our operating subsidiaries are often required to engage in litigation to enforce their patents and patent rights. Certain of our operating subsidiaries are parties to ongoing patent enforcement related litigation, alleging infringement by third parties of certain of the patented technologies owned or controlled by our operating subsidiaries.
Competition
We expect to encounter increased competition in the area of patent acquisition and enforcement. This includes an increase in the number of competitors seeking to acquire the same or similar patents and technologies that we may seek to acquire. Companies such as British Technology Group, Rembrandt Management Group, and Intellectual Ventures LLC are already in the business of acquiring the rights to patents for the purpose of licensing and enforcement, and we expect more companies to enter the market. See, Risk Factors on page 6.
We also compete with venture capital firms and various industry leaders for technology licensing opportunities. Many of these competitors may have more financial and human resources than our operating subsidiaries. As we become more successful, we may find more companies entering the market for similar technology opportunities, which may reduce our market share in one or more technology industries that we currently rely upon to generate future revenue.
5
Other companies may develop competing technologies that offer better or less expensive alternatives to our patented technologies that we may acquire and/or out-license. Many potential competitors may have significantly greater resources than the resources that our operating subsidiaries possess. Technological advances or entirely different approaches developed by one or more of our competitors could render certain of the technologies owned or controlled by our operating subsidiaries obsolete and/or uneconomical.
Employees
As of December 31, 2007, on a consolidated basis, Acacia Research Corporation had 45 full-time employees. None of our subsidiaries are a party to any collective bargaining agreement. We consider our employee relations to be good.
An investment in our stock involves a number of risks. Before making a decision to purchase our securities, you should carefully consider all of the risks described in this annual report. If any of the risks discussed in this annual report actually occur, our business, financial condition and results of operations could be materially adversely affected. If this were to occur, the trading price of our securities could decline significantly and you may lose all or part of your investment. As used in this Form 10-K, “we,” “us” and “our” refer to Acacia Research Corporation and/or its wholly owned operating subsidiaries. All intellectual property acquisition, development, licensing and enforcement activities are conducted solely by certain of Acacia Research Corporation’s wholly owned operating subsidiaries.
GENERAL RISKS
WE HAVE A HISTORY OF LOSSES AND WILL PROBABLY INCUR ADDITIONAL LOSSES IN THE FUTURE.
We have sustained substantial losses since our inception resulting in a consolidated net accumulated deficit, as disclosed in the accompanying consolidated financial statements of Acacia Research Corporation included in Part IV Item 15 of this report. We may never become profitable, or if we do, we may never be able to sustain profitability. We expect to incur significant legal, marketing, general and administrative expenses. As a result, it is more likely than not that we will incur losses for the foreseeable future.
BECAUSE WE HAVE SUSTAINED LOSSES SINCE OUR INCEPTION, WE CANNOT ASSURE THAT OUR OPERATIONS WILL BE PROFITABLE.
We commenced operations in 1993 and, we have sustained substantial losses since our inception resulting in a net consolidated accumulated deficit, as disclosed in the accompanying consolidated financial statements of Acacia Research Corporation included in Part IV Item 15 of this report. If we continue to incur operating losses in future periods, we may not have enough capital to expand our business and our operating subsidiary companies’ businesses in the future.
IF WE, OR OUR SUBSIDIARIES, ENCOUNTER UNFORESEEN DIFFICULTIES AND CANNOT OBTAIN ADDITIONAL FUNDING ON FAVORABLE TERMS, OUR BUSINESS MAY SUFFER.
Acacia Research Corporation's consolidated cash and cash equivalents along with short-term investments totaled $51.4 million and $45.0 million at December 31, 2007 and 2006, respectively. To date, Acacia Research Corporation has relied primarily upon selling of equity securities and payments from our licensees to generate the funds needed to finance the operations of Acacia Research Corporation and our operating subsidiaries.
We cannot assure you that we will not encounter unforeseen difficulties, including the outside influences identified below, that may deplete our capital resources more rapidly than anticipated. As a result, our subsidiary companies may be required to obtain additional financing through bank borrowings, debt or equity financings or otherwise, which would require us to make additional investments or face a dilution of our equity interests. Any efforts to seek additional funds could be made through equity, debt or other external financings. Nevertheless, we cannot assure that additional funding will be available on favorable terms, if at all. If we fail to obtain additional funding when needed for our subsidiary companies and ourselves, we may not be able to execute our business plans and our business may suffer.
6
FAILURE TO EFFECTIVELY MANAGE OUR GROWTH COULD PLACE STRAINS ON OUR MANAGERIAL, OPERATIONAL AND FINANCIAL RESOURCES AND COULD ADVERSELY AFFECT OUR BUSINESS AND OPERATING RESULTS.
Our growth has placed, and is expected to continue to place, a strain on our managerial, operational and financial resources. Further, as our subsidiary companies’ businesses grow, we will be required to manage multiple relationships. Any further growth by us or our subsidiary companies or an increase in the number of our strategic relationships will increase this strain on our managerial, operational and financial resources. This strain may inhibit our ability to achieve the rapid execution necessary to successfully implement our business plan.
OUR FUTURE SUCCESS DEPENDS ON OUR ABILITY TO EXPAND OUR ORGANIZATION TO MATCH THE GROWTH OF OUR SUBSIDIARIES.
As our subsidiaries grow, the administrative demands upon Acacia Research Corporation and on our operating subsidiaries, will grow, and our success will depend upon our ability to meet those demands. These demands include increased accounting, management, legal services, staff support, and general office services. We may need to hire additional qualified personnel to meet these demands, the cost and quality of which is dependent in part upon market factors outside of our control. Further, we will need to effectively manage the training and growth of our staff to maintain an efficient and effective workforce, and our failure to do so could adversely affect our business and operating results.
THE AVAILABILITY OF SHARES FOR SALE IN THE FUTURE COULD REDUCE THE MARKET PRICE OF OUR COMMON STOCK.
In the future, we may issue securities to raise cash for acquisitions. We may also pay for interests in additional subsidiary companies by using a combination of cash and our common stock or just our common stock. We may also issue securities convertible into our common stock. Any of these events may dilute stockholders ownership interest in our company and have an adverse impact on the price of our common stock.
In addition, sales of a substantial amount of our common stock in the public market, or the perception that these sales may occur, could reduce the market price of our common stock. This could also impair our ability to raise additional capital through the sale of our securities.
DELAWARE LAW AND OUR CHARTER DOCUMENTS CONTAIN PROVISIONS THAT COULD DISCOURAGE OR PREVENT A POTENTIAL TAKEOVER OF ACACIA RESEARCH CORPORATION THAT MIGHT OTHERWISE RESULT IN OUR STOCKHOLDERS RECEIVING A PREMIUM OVER THE MARKET PRICE OF THEIR SHARES.
Provisions of Delaware law and our certificate of incorporation and bylaws could make the following more difficult: the acquisition of our company by means of a tender offer, proxy contest or otherwise, and the removal of incumbent officers and directors. These provisions include:
| · | section 203 of the Delaware General Corporation Law, which prohibits a merger with a 15%-or-greater stockholder, such as a party that has completed a successful tender offer, until three years after that party became a 15%-or-greater stockholder; |
| · | amendment of our bylaws by the stockholders requires a two-thirds approval of the outstanding shares; |
| · | the authorization in our certificate of incorporation of undesignated preferred stock, which could be issued without stockholder approval in a manner designed to prevent or discourage a takeover; |
| · | provisions in our bylaws eliminating stockholders’ rights to call a special meeting of stockholders, which could make it more difficult for stockholders to wage a proxy contest for control of our board of directors or to vote to repeal any of the anti-takeover provisions contained in our certificate of incorporation and bylaws; and |
| · | the division of our board of directors into three classes with staggered terms for each class, which could make it more difficult for an outsider to gain control of our board of directors. |
Such potential obstacles to a takeover could adversely affect the ability of our stockholders to receive a premium price for their stock in the event another company wants to acquire us.
7
AS A RESULT OF THE REDEMPTION OF AR-COMBIMATRIX STOCK FOR THE COMMON STOCK OF COMBIMATRIX CORPORATION, ACACIA RESEARCH CORPORATION MAY BE SUBJECT TO CERTAIN TAX LIABILITY UNDER THE INTERNAL REVENUE CODE.
Our distribution of the common stock of CombiMatrix Corporation in the redemption will be tax-free to Acacia Research Corporation if the distribution qualifies under Sections 368 and 355 of the Internal Revenue Code. If the split-off failed to qualify under Section 355 of the Internal Revenue Code, corporate tax would be payable by the consolidated group of which Acacia Research Corporation is the common parent, based upon the difference between the aggregate fair market value of the assets of CombiMatrix Corporation’s business and the adjusted tax bases of such business to Acacia Research Corporation prior to the redemption.
Acacia Research Corporation received a private letter ruling from the IRS to the effect that, among other things, the redemption would be tax free to Acacia Research Corporation and the holders of AR-Acacia Technologies stock and AR-CombiMatrix stock under Sections 368 and 355 of the Internal Revenue Code. The private letter ruling, while generally binding upon the IRS, was based upon factual representations and assumptions and commitments on our behalf with respect to future operations made in the ruling request. The IRS could modify or revoke the private letter ruling retroactively if the factual representations and assumptions in the request were materially incomplete or untrue, the facts upon which the private letter ruling was based were materially different from the facts at the time of the redemption, or if we do not meet certain commitments made.
If the split-off failed to qualify under Section 355 of the Internal Revenue Code, corporate tax, if any, would be payable by the consolidated group of which Acacia Research Corporation is the common parent, as described above. As such, the corporate level tax would be payable by Acacia Research Corporation. CombiMatrix Corporation has agreed however, to indemnify Acacia Research Corporation for this and certain other tax liabilities if they result from actions taken by CombiMatrix Corporation. Notwithstanding CombiMatrix Corporation’s agreement to indemnify us, under the Internal Revenue Code’s consolidated return regulations, each member of the Acacia Research Corporation consolidated group, including our company, will be severally liable for these tax liabilities. Further, we may be liable for additional taxes if Acacia Research Corporation takes certain actions within two years following the redemption, as more fully discussed in the immediately following risk factor. If we are found liable to the IRS for these liabilities, the resulting obligation could materially and adversely affect our financial condition, and we may be unable to recover on the indemnity from CombiMatrix Corporation.
FOLLOWING THE REDEMPTION OF AR-COMBIMATRIX STOCK FOR THE COMMON STOCK OF COMBIMATRIX CORPORATION, ACACIA RESEARCH CORPORATION MAY BE SUBJECT TO CERTAIN TAX LIABILITY UNDER THE INTERNAL REVENUE CODE FOR ACTIONS TAKEN BY EITHER OF THEM FOLLOWING THE REDEMPTION.
Even if the distribution qualifies under Section 368 and 355 of the Internal Revenue Code, it will be taxable to Acacia if Section 355(e) of the Internal Revenue Code applies to the distribution. Section 355(e) will apply if 50% or more of the AR-Acacia Technologies stock or CombiMatrix Corporation’s common stock, by vote or value, is acquired by one or more persons, other than the holders of AR-CombiMatrix stock who receive the common stock of CombiMatrix Corporation in the redemption, acting pursuant to a plan or a series of related transactions that includes the redemption. Any shares of the AR-Acacia Technologies stock, the AR-CombiMatrix stock or the common stock of CombiMatrix Corporation acquired directly or indirectly within two years before or after the redemption generally are presumed to be part of such a plan unless we can rebut that presumption. To prevent applicability of Section 355(e) or to otherwise prevent the distribution from failing to qualify under Section 355 of the Internal Revenue Code, CombiMatrix Corporation has agreed that, until two years after the redemption, it will not take any of the following actions unless prior to taking such action, it has obtained (and provided to us) a written opinion of tax counsel or a ruling from the Internal Revenue Service to the effect that such action will not cause the redemption to be taxable to us (collectively “Disqualifying Actions”):
merge or consolidate with another corporation;
liquidate or partially liquidate;
sell or transfer all or substantially all of its assets;
redeem or repurchase its stock (except in certain limited circumstances); or
take any other action which could reasonably be expected to cause Section 355(e) to apply to the distribution.
8
Further, if Acacia Research Corporation takes any Disqualifying Action, we may be subject to additional tax liability. Many of our competitors are not subject to similar restrictions and may issue their stock to complete acquisitions, expand their product offerings and speed the development of new technology. Therefore, these competitors may have a competitive advantage over us. Substantial uncertainty exists on the scope of Section 355(e), and we may have undertaken, may contemplate undertaking or may otherwise undertake in the future transactions which may cause Section 355(e) to apply to the redemption notwithstanding our desire or intent to avoid application of Section 355(e). Accordingly, we cannot provide you any assurance that we will not be liable for taxes if Section 355(e) applies to the redemption.
BECAUSE OUR BUSINESS OPERATIONS ARE SUBJECT TO MANY UNCONTROLLABLE OUTSIDE INFLUENCES, WE MAY NOT SUCCEED.
Our licensing and enforcement business operations are subject to numerous risks from outside influences, including the following:
· | New legislation, regulations or rules related to obtaining patents or enforcing patents could significantly increase our operating costs and decrease our revenue. |
Our operating subsidiaries acquire patents with enforcement opportunities and are spending a significant amount of resources to enforce those patents. If new legislation, regulations or rules are implemented either by Congress, the United States Patent and Trademark Office, or the courts that impact the patent application process, the patent enforcement process or the rights of patent holders, these changes could negatively affect our expenses and revenue. For example, new rules regarding the burden of proof in patent enforcement actions could significantly increase the cost of our enforcement actions, and new standards or limitations on liability for patent infringement could negatively impact our revenue derived from a patent or patent portfolio. The Acacia Technologies group's
specialistssuch enforcement actions. While we are trained and skillednot aware that any such changes are likely to occur in these areas. the foreseeable future, we cannot assure you that such changes will not occur.
| · | Trial judges and juries often find it difficult to understand complex patent enforcement litigation, and as a result, we may need to appeal adverse decisions by lower courts in order to successfully enforce our patents. |
It is importantdifficult to identify
potential problem areas priorpredict the outcome of patent enforcement litigation at the trial level. It is often difficult for juries and trial judges to commercializationunderstand complex, patented technologies, and determinate whether
potential problem areas can be overcome, before launchingas a licensing program.result, there is a higher rate of successful appeals in patent enforcement litigation than more standard business litigation. Such appeals are expensive and time consuming, resulting in increased costs and delayed revenue. Although we diligently pursue enforcement litigation, we cannot predict with significant reliability the decisions made by juries and trial courts.
| · | More patent applications are filed each year resulting in longer delays in getting patents issued by the United States Patent and Trademark Office. |
Certain of our operating subsidiaries hold and continue to acquire pending patents. We have identified a trend of increasing patent applications each year, which we believe is resulting in longer delays in obtaining approval of pending patent applications. The application delays could cause delays in recognizing revenue from these patents and could cause us to miss opportunities to license patents before other competing technologies are developed processesor introduced into the market. See the subheading “Competition is intense in the industries in which our subsidiaries do business and proceduresas a result, we may not be able to grow or maintain our market share for identifying problem areasour technologies and evaluatingpatents,” below.
| · | Federal courts are becoming more crowded, and as a result, patent enforcement litigation is taking longer. |
Our patent enforcement actions are almost exclusively prosecuted in federal court. Federal trial courts that hear our patent enforcement actions also hear criminal cases. Criminal cases always take priority over our actions. As a result, it is difficult to predict the strengthlength of time it will take to complete an enforcement action. Moreover, we believe there is a trend in increasing numbers of civil lawsuits and criminal proceedings before federal judges, and as a result, we believe that the risk of delays in our patent enforcement actions will have a greater affect on our business in the future unless this trend changes.
| · | Any reductions in the funding of the United States Patent and Trademark Office could have an adverse impact on the cost of processing pending patent applications and the value of those pending patent applications. |
The assets of our operating subsidiaries consists of patent portfolios, including pending patent applications before the decisionU.S. Patent and Trademark Office (USPTO). The value of our patent portfolios is madedependent upon the issuance of patents in a timely manner, and any reductions in the funding of the USPTO could negatively impact the value of our assets. Further, reductions in funding from Congress could result in higher patent application filing and maintenance fees charged by the USPTO, causing an unexpected increase in our expenses.
9
| · | Competition is intense in the industries in which our subsidiaries do business and as a result, we may not be able to grow or maintain our market share for our technologies and patents. |
We expect to allocate
resourcesencounter competition in the area of patent acquisition and enforcement as the number of companies entering this market is increasing. This includes competitors seeking to aacquire the same or similar patents and technologies that we may seek to acquire. Companies such as British Technology Group, Rembrandt Management Group, and Intellectual Ventures LLC are already in the business of acquiring the rights to patents for the purpose of licensing and enforcement, effort.
PURCHASE OR ACQUIRE THE RIGHTS TO PATENTED TECHNOLOGIES
After evaluation,and we expect more companies to enter the Acacia Technologies groupmarket. As new technological advances occur, many of our patented technologies may electbecome obsolete before they are completely monetized. If we are unable to purchasereplace obsolete technologies with more technologically advanced patented technologies, then this obsolescence could have a negative effect on our ability to generate future revenues.
Our licensing business also competes with venture capital firms and various industry leaders for technology licensing opportunities. Many of these competitors may have more financial and human resources than our company. As we become more successful, we may find more companies entering the patentedmarket for similar technology opportunities, which may reduce our market share in one or more technology industries that we currently rely upon to generate future revenue.
| · | Our patented technologies face uncertain market value. |
Our operating subsidiaries have acquired patents and technologies that are at early stages of adoption in the commercial and consumer markets. Demand for some of these technologies is untested and is subject to fluctuation based upon the rate at which our licensees will adopt our patents and technologies in their products and services. Refer to the related risk factor below.
| · | As patent enforcement litigation becomes more prevalent, it may become more difficult for us to voluntarily license our patents. |
We believe that the more prevalent patent enforcement actions become, the exclusive licensing agentmore difficult it will be for us to voluntarily license our patents. As a result, we may need to increase the patented
technology in all or in specific fields of use. In either case, the owner of the
patent generally retains the rights to a portion of the revenues generated from
a patent's licensing and enforcement program. The Acacia Technologies group
generally controls the licensing and enforcement process and utilizes its
experienced in-house personnel to reduce outside costs, and ensure that the
Acacia Technologies group's capital is allocated and utilized in an efficient
and cost effective manner.
SUCCESSFULLY LICENSE AND ENFORCE PATENTS WITH SIGNIFICANT ROYALTY POTENTIAL
As partnumber of our patent evaluation process, significant consideration is also
givenenforcement actions to cause infringing companies to license the identification of potential infringers, industries within whichpatent or pay damages for lost royalties. This may increase the potential infringers exist, longevityrisks associated with an investment in our company.
| · | The foregoing outside influences may affect other risk factors described in this annual report. |
Any one of the patented technology,foregoing outside influences may cause our company to need additional financing to meet the challenges presented or to compensate for a loss in revenue, and a varietywe may not be able to obtain the needed financing. See the heading “If we, or our subsidiaries, encounter unforeseen difficulties and cannot obtain additional funding on favorable terms, our business may suffer” above.
WE MAY FAIL TO MEET MARKET EXPECTATIONS BECAUSE OF FLUCTUATIONS IN QUARTERLY OPERATING RESULTS, WHICH COULD CAUSE THE PRICE OF ACACIA RESEARCH-ACACIA TECHNOLOGIES COMMON STOCK TO DECLINE.
Our reported revenues and operating results have fluctuated in the past and may continue to fluctuate significantly from quarter to quarter in the future. It is possible that in future periods revenues could fall below the expectations of othersecurities analysts or investors, which could cause the market price of our Acacia Research-Acacia Technologies common stock to decline. The following are among the factors that directly impact the magnitude and potential success of a
licensing and enforcement program. could cause our operating results to fluctuate significantly from period to period:
| · | the dollar amount of agreements executed each period, which is primarily driven by the nature and characteristics of the technology being licensed and the magnitude of infringement associated with a specific licensee; |
| · | the specific terms and conditions of agreements executed each period and the periods of infringement contemplated by the respective payments; |
| · | fluctuations in the total number of agreements executed; |
10
| · | fluctuations in the sales results or other royalty per unit activities of our licensees that impact the calculation of license fees due; |
| · | the timing of the receipt of periodic license fee payments and/or reports from licensees; |
| · | fluctuations in the net number of active licensees period to period; |
| · | costs related to acquisitions, alliances, licenses and other efforts to expand our operations; |
| · | the timing of payments under the terms of any customer or license agreements into which our operating subsidiaries may enter; and |
| · | expenses related to, and the results of, patent filings and other enforcement proceedings relating to intellectual property rights, as more fully described in this section. |
OUR REVENUES WILL BE UNPREDICTABLE, AND THIS MAY HARM OUR FINANCIAL CONDITION.
Acacia Technologies group's specialists are
trained in evaluating potentially infringing technologies and presenting the
application of patents to such technologies. These presentations generally take
place in a non-adversarial business setting, but can also occur through the
litigation process, if necessary.
PATENTS AND LICENSES
The Acacia Technologies group owns five issued U.S. patents relating to
audio and video transmission and receiving systems, commonly known as
audio-on-demand, video-on-demand and audio/video streaming, used for
distributing content via various methods as follows: U.S. Patent No. 5,132,992,
U.S. Patent No. 5,253,275, U.S. Patent No. 5,550,863, U.S. Patent No. 6,002,720
and U.S. Patent No. 6,144,702. In addition, the Acacia Technologies group owns
31 foreign patents also relating to audio and video transmission and receiving
systems technology. Foreign rights include an initial patent granted by the
European Patent Office covering Austria, Belgium, Denmark, Finland, France,
Germany, Greece, Italy, Luxembourg, Monaco, the Netherlands, Spain, Sweden,
21
Switzerland and the United Kingdom, and patents in Japan, Taiwan and Mexico. In
January 2004, the Acacia Technologies group was issued an additional European
patent for its DMT Technology. The new patent provides additional coverage in
the countries listed above. Acacia Technologies group's U.S. DMT patents expire
in 2011 and its foreign DMT patents expire in 2012.
In July 2004, the Acacia Technologies group acquired U.S. Patent No.
6,226,677 from LodgeNet Entertainment Corporation. The patent covers technology
and methods for redirecting users to a login page when accessing the Internet
and expires in 2019.
As a result of the January 28, 2005Global Acquisition Corporation's acquisition of the assets of Global Patent Holdings, LLC discussed above, the Acacia Technologies group acquiredin 2005, provided us with ownership of companies that control 27 patent portfolios, which include 120 U.S. patents and certain foreign counterparts, and covercounterparts. Rights to additional patent portfolios were acquired subsequent to the acquisition of the assets of Global Patent Holdings, bringing the current total number of patent portfolios controlled by Acacia Research Corporation’s operating subsidiaries to approximately 91, covering technologies used in a wide variety of industries. REGULATORY MATTERSThe acquisitions expand and diversify our revenue generating opportunities. We believe that our cash and cash equivalent balances, anticipated cash flow from operations and other external sources of available credit, will be sufficient to meet our cash requirements through at least March 2009, and for the foreseeable future. However, due to the nature of our licensing business and uncertainties regarding the amount and timing of the receipt of license fees from potential infringers, stemming primarily from uncertainties regarding the outcome of enforcement actions, rates of adoption of our patented technologies, the growth rates of our existing licensees and other factors, we cannot currently predict the amount and timing of the receipt of license fee revenues with a sufficient degree of precision.
As a result, our revenues may vary significantly from quarter to quarter, which could make our business difficult to manage and cause our quarterly results to be below market expectations. If this happens, the price of our Acacia Research-Acacia Technologies group's DMTcommon stock may decline significantly.
OUR OPERATING SUBSIDIARIES DEPEND UPON RELATIONSHIPS WITH OTHERS TO PROVIDE TECHNOLOGY-BASED OPPORTUNITIES THAT CAN DEVELOP INTO PROFITABLE ROYALTY-BEARING LICENSES, AND IF IT IS UNABLE TO MAINTAIN AND GENERATE NEW RELATIONSHIPS, THEN IT MAY NOT BE ABLE TO SUSTAIN EXISTING LEVELS OF REVENUE OR INCREASE REVENUE.
Our operating subsidiaries do not invent new technologies or products; they depend on acquiring new patents and inventions through their relationships with inventors, universities, research institutions, and others. If our operating subsidiaries are unable to maintain those relationships and continue to grow new relationships, then they may not be able to identify new technology-based opportunities for growth and sustainable revenue. Further, because we rely upon acquiring technology is utilizedfrom others, we cannot be certain that we will be able to obtain the volume and quality of available new technologies necessary to sustain our current or future growth. If we are unable to obtain the necessary volume and quality of new technologies, then we may need to reduce operations or revise our business model.
TECHNOLOGY COMPANY STOCK PRICES ARE ESPECIALLY VOLATILE, AND THIS VOLATILITY MAY DEPRESS THE PRICE OF OUR ACACIA RESEARCH-ACACIA TECHNOLOGIES COMMON STOCK.
The stock market has experienced significant price and volume fluctuations, and the market prices of technology companies have been highly volatile. We believe that various factors may cause the market price of our Acacia Research-Acacia Technologies common stock to fluctuate, perhaps substantially, including, among others, the following:
| · | announcements of developments in our patent enforcement actions; |
| · | developments or disputes concerning our patents; |
| · | our or our competitors’ technological innovations; |
| · | developments in relationships with licensees; |
11
| · | variations in our quarterly operating results; |
| · | our failure to meet or exceed securities analysts’ expectations of our financial results; or |
| · | a change in financial estimates or securities analysts’ recommendations; |
| · | changes in management’s or securities analysts’ estimates of our financial performance; |
| · | changes in market valuations of similar companies; |
| · | announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures, capital commitments, new technologies, or patents; and |
| · | failure to complete significant transactions. |
For example, the Nasdaq Computer Index had a range of $1,008.96 - $1,353.94 during the 52-weeks ended December 31, 2007 and the Nasdaq Composite Index had a range of $1,108.49 - $2,892.36 over the same period. Over the same period, our Acacia Research-Acacia Technologies common stock fluctuated within a range of $8.42 - $17.92, all of which was experienced in the fourth quarter. We believe fluctuations in our stock price during this period could have been impacted by cable TV, satellite TVcourt rulings in our patent enforcement actions. Court rulings in patent enforcement actions are often difficult to understand, even when favorable or neutral to the value of our patents and telecommunications systems. our overall business, and we believe that investors in the market may overreact, causing fluctuations in our stock prices that may not accurately reflect the impact of court rulings on our business operations and assets.
In the past, companies that have experienced volatility in the market price of their stock have been the objects of securities class action litigation. If our Acacia Research-Acacia Technologies common stock was the object of securities class action litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could materially harm our business and financial results.
THE MARKETS SERVED BY OUR OPERATING SUBSIDIARIES ARE SUBJECT TO RAPID TECHNOLOGICAL CHANGE, AND IF OUR OPERATING SUBSIDIARIES ARE UNABLE TO DEVELOP AND ACQUIRE NEW TECHNOLOGIES AND PATENTS, ITS REVENUES COULD STOP GROWING OR COULD DECLINE.
The cable TV, satellite
TVmarkets served by our operating subsidiaries’ licensees frequently undergo transitions in which products rapidly incorporate new features and telecommunications industriesperformance standards on an industry-wide basis. Products for communications applications, high-speed computing applications, as well as other applications covered by our operating subsidiaries’ intellectual property, are based on continually evolving industry standards. Our ability to compete in the future will, however, depend on our ability to identify and ensure compliance with evolving industry standards. This will require our continued efforts and success of acquiring new patent portfolios with licensing and enforcement opportunities. However, we expect to have sufficient liquidity and capital resources for the foreseeable future in order to maintain the level of acquisitions we believe we need to keep pace with these technological advances. However, outside influences may cause the need for greater liquidity and capital resources than expected, as described under the caption “Because our business operations are subject to federal regulation,
including FCCmany uncontrollable outside influences, we may not succeed” above.
THE SUCCESS OF OUR OPERATING SUBSIDIARIES DEPENDS IN PART UPON THEIR ABILITY TO RETAIN THE BEST LEGAL COUNSEL TO REPRESENT THEM IN PATENT ENFORCEMENT LITIGATION.
The success of our licensing business depends upon our operating subsidiaries’ ability to retain the best legal counsel to prosecute patent infringement litigation. As our operating subsidiaries’ patent enforcement actions increase, it will become more difficult to find the best legal counsel to handle all of our cases because many of the best law firms may have a conflict of interest that prevents its representation of our subsidiary companies.
OUR OPERATING SUBSIDIARIES, IN CERTAIN CIRCUMSTANCES, RELY ON REPRESENTATIONS, WARRANTIES AND OPINIONS MADE BY THIRD PARTIES, THAT IF DETERMINED TO BE FALSE OR INACCURATE, MAY EXPOSE CERTAIN OF OUR OPERATING SUBSIDIARIES TO CERTAIN LIABILITIES THAT COULD BE MATERIAL.
From time to time, our operating subsidiaries may rely upon representations and warranties made by third parties from whom certain of our operating subsidiaries acquired patents or the exclusive rights to license and enforce patents. We also may rely upon the opinions of purported experts. In certain instances, we may not have the opportunity to independently investigate and verify the facts upon which such representations, warranties, and opinions are made. By relying on these representations, warranties and opinions, our operating subsidiaries may be exposed to liabilities in connection with the licensing and other requirements. These industries are also often
subjectenforcement of certain patents and patent rights. It is difficult to extensive regulation bypredict the extent and nature of such liabilities which, in some instances, may be material.
12
IN CONNECTION WITH PATENT ENFORCEMENT ACTIONS CONDUCTED BY CERTAIN OF OUR SUBSIDIARIES, A COURT MAY RULE THAT OUR SUBSIDIARIES HAVE VIOLATED CERTAIN STATUTORY, REGULATORY, FEDERAL, LOCAL OR GOVERNING RULES OR STANDARDS, WHICH MAY EXPOSE US AND OUR OPERATING SUBSIDIARIES TO MATERIAL LIABILITIES, WHICH COULD MATERIALLY HARM OUR OPERATING RESULTS AND OUR FINANCIAL POSITION.
In connection with any of our patent enforcement actions, it is possible that a defendant may request and/or a court may rule that we have violated statutory authority, regulatory authority, federal rules, local and state authorities. While most cable
TV, satellite TV and telecommunication industry regulations do not apply
directlycourt rules, or governing standards relating to the Acacia Technologies group, they affect programming distributors,substantive or procedural aspects of such enforcement actions. In such event, a court may issue monetary sanctions against us or our operating subsidiaries or award attorney’s fees and/or expenses to a defendant(s), which could be material, and if required to be paid by us or our operating subsidiaries, could materially harm our operating results and our financial position.
OUR INVESTMENTS IN AUCTION RATE SECURITIES ARE SUBJECT TO RISKS, INCLUDING THE CONTINUED FAILURE OF FUTURE AUCTIONS, WHICH MAY CAUSE US TO INCUR LOSSES OR HAVE REDUCED LIQUIDITY.
We invest our cash balances in high-quality issuers and limit the amount of credit exposure to any one issuer other than the United States government and its agencies. At December 31, 2007, our investments in marketable securities consist of United States Government and Government Agency Bonds, and auction rate securities. Our auction rate securities are investment grade quality and are in compliance with our investment policy as of the large potential customers forend of 2007. We believe that the technologies coveredfair value of these investments continue to approximate their par value; however, we did experience failed auctions with certain of our issues in February 2008. Given the deteriorating credit markets, and the increased incidence of failure within the auction market in February 2008, there can be no assurance as to when we would be able to liquidate a particular issue. In such case of a failure, the auction rate securities continue to pay interest in accordance with their terms; however, we may not be able to access the par value of the invested funds until a future auction of these investments is successful, the security is called by the Acacia
Technologies group patent portfolio. The Acacia Technologies group monitors
pending legislation and administrative proceedingsissuer or a buyer is found outside of the auction process. Furthermore, if this situation were to ascertain relevance,
analyze impact and develop strategies regarding regulatory trends and
developments within these industries.
Federal law requires cable TV operatorspersist despite our ability to reserve up to one-third of a
system's channel capacity for local commercial television stations that have
elected must-carry status. In addition, a cable TV system is generallyhold such investments until maturity, we may be required to carry local non-commercial television stations. The FCC has also implemented
comparable rules for satellite TV carriers requiring that if a satellite TV
system carries one local broadcast stationrecord an impairment charge in a local market pursuantfuture period. The systemic failure of future auctions for auction rate securities may result in a loss of liquidity, substantial impairment to our investments, realization of substantial future losses, or a royalty-free license granted undercomplete loss of the Satellite Home Viewer Improvement Act of
1999, then it must carry all local broadcast stations in that market. To meet
these requirements, some cable TV and satellite TV systems must decide which
programming services to keep and which to remove in order to make space
available for local television stations. These must-carry requirements may
impact the Acacia Technologies group's information-on-demand and streaming media
business by causing cable TV and satellite TV systems operators to reduce the
number of channels on their systems that would have used technologies covered by
Acacia Technologies group's patent portfolio.
COMPETITION
The Acacia Technologies group expects to encounter competitioninvestment in the arealong-term which may have a material adverse effect on our business, results of business opportunities from other entities having similar business
objectives. Manyoperations, liquidity, and financial condition. See Note 2 of these potential competitors may possess financial,
technical, human and other resources greater than those of the Acacia
Technologies group. The Acacia Technologies group anticipates that it will face
increased competitionour Notes to Consolidated Financial Statements for additional information about our investments in the future as new companies enter the market.
Other companies may develop competing technologies that offer better or
less expensive alternatives to our DMT technology and/or other technologies that
we may acquire or out-license. Many potential competitors have significantly
greater resources. Technological advances or entirely different approaches
developed by one or more of its competitors could render Acacia Technologies
group's technologies obsolete or uneconomical.
EMPLOYEES
As of December 31, 2004, the Acacia Technologies group had 21 full-time
employees. None of the companies included in the Acacia Technologies group is a
party to any collective bargaining agreement. The Acacia Technologies group
considers its employee relations to be good.
ITEMmarketable securities.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
Item 2. PROPERTIES
Acacia Research Corporation leases approximately 9,14718,302 square feet of office space in Newport Beach, California, under a lease agreement that expires in February 2007. Subsequent to December 31, 2004, Acacia Research Corporation
executed an amendment to the Newport Beach, California location lease agreement
to rent an additional 2,993 square feet of office space. Our wholly owned
subsidiary, CombiMatrix Corporation, leases office and laboratory space totaling
approximately 90,111 square feet located north of Seattle, Washington, under a
lease agreement that expires in December 2008.2012. Presently, we are not seeking any additional facilities.
22
We are a guarantor under a lease agreement for office space in Hollywood,
California that expires in August 2005. The lease agreement was entered into by
Soundbreak.com Incorporated, or Soundbreak.com, which ceased operations in
February 2001. The leased premises is subleased through the remaining term of
the lease agreement.
ITEM
Item 3. LEGAL PROCEEDINGS
In the ordinary course of business, we are the subject of, or party to, various pending or threatened legal actions, including various counterclaims in connection with our intellectual property enforcement activities. We believe that any liability arising from these actions will not have a material adverse effect on our consolidated financial position, results of operations or cash flows.
From time to time, companies comprising the Acacia Technologies group
engage in litigation to enforce their patents. A summary of patent enforcement
related litigation is provided at
Item 1. "Business," under the caption "Patent
Enforcement Litigation."
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
None.
23
13
PART II
ITEM
Item 5. MARKET FOR REGISTRANT'SREGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHAESPURCHASES OF EQUITY SECURITIES
RECENT MARKET PRICES
Recent Market Prices
Acacia Research Corporation's two classes of common stock, Acacia
Research-CombiMatrix commonCorporation’s AR-Acacia Technologies stock and Acacia Research-Acacia Technologies commonAR-CombiMatrix stock commenced trading on the Nasdaq Stock Market on December 16, 2002. The2002, under the symbols “ACTG” and “CBMX,” respectively. Prior to December 16, 2002, Acacia Research Corporation’s only class of common stock began trading under the symbol “ACRI” on the NASDAQ National Market System on July 8, 1996.
As a result of the Split-off Transaction described above, all outstanding shares of AR-CombiMatrix stock were redeemed, and hence, all rights of holders of AR-CombiMatrix stock ceased as of the Redemption Date (August 15, 2007), except for the right, upon the surrender to the exchange agent of shares of AR-CombiMatrix stock, to receive new shares of CombiMatrix Corporation stock pursuant to the exchange ratio described above. Subsequent to the consummation of the Split-off Transaction, Acacia Research Corporation’s only class of common stock outstanding is its AR-Acacia Technologies stock.
Prior to the Split-off Transaction, Acacia Research Corporation had two classes of common stock were created as a resultoutstanding, its AR-Acacia Technologies stock and its AR-CombiMatrix stock. AR-Acacia Technologies stock was intended to reflect separately the performance of Acacia Research Corporation's recapitalization thatCorporation’s “Acacia Technologies group.” AR-CombiMatrix stock was approved byintended to reflect separately the performance of Acacia Research Corporation's stockholders on December 11, 2002. The two classes of stock
replaced Acacia Research Corporation's common stock formerly traded onCorporation’s “CombiMatrix group.” Although the Nasdaq stock market under the symbol ACRI. Acacia Research-AcaciaAR-Acacia Technologies
common stock and Acacia Research-CombiMatrix commonthe AR-CombiMatrix stock are listed on the
Nasdaq National Market System under the symbols "ACTG" and "CBMX," respectively.
Acacia Research-CombiMatrix stock iswere intended to reflect the performance of Acacia Research Corporation's CombiMatrix group, and Acacia Research-Acacia
Technologiesour different business groups, they were both classes of common stock is intended to reflect the performance of Acacia Research
Corporation's Acacia Technologies group.
Holders of Acacia Research-Acacia Technologies stock and Acacia
Research-CombiMatrix stock are stockholders of Acacia Research Corporation. As a
result, holders of Acacia Research-Acacia Technologies stock and Acacia
Research-CombiMatrix stock continue to be subject to all of the risks of an
investment in Acacia Research Corporation and allwere not stock issued by the respective groups. As a result of its businesses, assets and
liabilities. The assetsthe Split-off Transaction, the CombiMatrix group is no longer a business group of Acacia Research Corporation attributes to one group
could be subject to the liabilities of the other group.
Corporation.
The markets for securities such as the two classes of our common stock have historically experienced extremesignificant price and volume fluctuations during certain periods. These broad market fluctuations and other factors, such as new product developments and trends in our industry and the investment markets generally, as well as economic conditions and quarterly variations in our results of operations, may adversely affect the market price of our two classes of common stock.
The high and low bid prices for our two classes of common stock as reported by NASDAQ for the periods indicated are as follows.shown in the table below. Such prices are inter-dealer prices without retail markups, markdowns or commissions and may not necessarily represent actual transactions.
2007 | 2006 | |||||||||||||||||||||||||||||||
Fourth Quarter | Third Quarter | Second Quarter | First Quarter | Fourth Quarter | Third Quarter | Second Quarter | First Quarter | |||||||||||||||||||||||||
| Acacia Research-Acacia Technologies stock: | ||||||||||||||||||||||||||||||||
| High | $ | 17.92 | $ | 16.75 | $ | 16.84 | $ | 16.56 | $ | 15.58 | $ | 14.95 | $ | 14.65 | $ | 9.00 | ||||||||||||||||
| Low | $ | 8.42 | $ | 10.87 | $ | 12.76 | $ | 12.23 | $ | 11.05 | $ | 9.31 | $ | 8.85 | $ | 6.65 | ||||||||||||||||
Acacia Research-CombiMatrix stock(1): | ||||||||||||||||||||||||||||||||
| High | N/A | $ | 0.74 | $ | 0.81 | $ | 1.85 | $ | 1.07 | $ | 1.68 | $ | 2.75 | $ | 2.90 | |||||||||||||||||
| Low | N/A | $ | 0.57 | $ | 0.50 | $ | 0.58 | $ | 0.70 | $ | 0.96 | $ | 1.45 | $ | 1.34 | |||||||||||||||||
1) Reflects prices of AR-CombiMatrix stock through close of trading on August 14, 2007, immediately prior to the Redemption.
14
STOCK PERFORMANCE GRAPH
This performance graph shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities under that Section and shall not be deemed to be incorporated by reference into any filing of Acacia Research Corporation under the Securities Act of 1933, as amended.
The Stock Performance Graph depicted below compares the yearly change in Acacia Research Corporation’s cumulative total stockholder return for the last five fiscal years with the cumulative total return of the Nasdaq Stock Market (U.S.) Composite Index and the Nasdaq Biotech Index.
| 2003 | 2004 | 2005 | 2006 | 2007 | ||||||||||||||||
| AR-Acacia Technologies stock | $ | 226 | $ | 220 | $ | 286 | $ | 555 | $ | 373 | ||||||||||
| AR-CombiMatrix stock | $ | 92 | $ | 109 | $ | 38 | $ | 22 | $ | 16 | * | |||||||||
| Nasdaq Composite Index | $ | 150 | $ | 163 | $ | 165 | $ | 181 | $ | 199 | ||||||||||
| Nasdaq Biotech Index | $ | 146 | $ | 155 | $ | 159 | $ | 161 | $ | 168 | ||||||||||
____________________________
*Based on the closing trading price of AR-CombiMatrix stock on August 14, 2007, immediately prior to the Redemption.
The graph covers the period from December 31, 2002 to December 31, 2007, except the share price of AR-CombiMatrix stock in 2007 is measured as of August 14, 2007. Cumulative total returns are calculated assuming that $100 was invested on December 31, 2002, in each class of Acacia Research Corporation’s common stock outstanding as of December 31, 2002, and in each index, and that all dividends, if any, were reinvested. Refer to Acacia Research Corporation’s Dividend Policy below. Stockholder returns over the indicated period should not be considered indicative of future stock prices or shareholder returns.
On March 9, 2005,February 25, 2008, there were approximately 171114 owners of record of Acacia Research-Acacia Technologies stock and 162 owners of record of Acacia
Research-CombiMatrixResearch Corporation stock. The majority of the outstanding shares of Acacia Research-Acacia Technologies stock and Acacia Research-CombiMatrixResearch Corporation stock are held by a nominee holder on behalf of an indeterminable number of ultimate beneficial owners.
DIVIDEND POLICY
Dividend Policy
To date, we have not declared or paid any cash dividends with respect to our capital stock, and the current policy of the board of directors is to retain earnings, if any, to provide for the growth of Acacia Research Corporation.Corporation and our operating subsidiaries. Consequently, we do not expect to pay any cash dividends in the foreseeable future. Further, there can be no assurance that our proposed operations will generate revenues and cash flow needed to declare a cash dividend or that we will have legally available funds to pay dividends.
24
USE OF PROCEEDS
In April 2004, Acacia Research Corporation raised net proceeds of
approximately $13,715,000 through the sale of three million shares of Acacia
Research - CombiMatrix common stock in a registered direct offering. The net
proceeds from this offering were attributed to the CombiMatrix group. The net
proceeds are being utilized to provide working capital for the CombiMatrix
group's business.
All of the shares of Acacia Research-CombiMatrix common stock were offered
pursuant to an effective registration statement previously filed with the
Securities and Exchange Commission.
EQUITY COMPENSATION PLAN INFORMATION
15
Equity Compensation Plan Information
The following table provides information as of December 31, 2004 with respect to ourAcacia Research Corporation’s common shares issuable under our equity compensation plans:
plans as of December 31, 2007:
Plan Category | (a) Number of securities to be issued upon exercise of outstanding options | (b) Weighted-Average exercise price of outstanding options | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||
| Equity compensation plans approved by security holders | ||||||
2002 Acacia Technologies Stock Incentive Plan(1) | 4,919,000 | $8.45 | 18,000 | |||
2007 Acacia Technologies Stock Incentive Plan(2) | 50,000 | $16.01 | 171,000 | |||
| Subtotal | 4,969,000 | $8.52 | 189,000 | |||
| Equity compensation plans not approved by security holders(3) | N/A | N/A | N/A | |||
| Total | 4,969,000 | $8.52 | 189,000 |
_________________________
| (1) | Our 2002 Acacia Technologies Stock Incentive |
| (2) | Our 2007 Acacia Technologies Stock Incentive Plan, or the 2007 Plan, allows for the granting of stock options and other awards to eligible individuals, which generally includes directors, officers, employees and consultants, and was approved by security holders on May 15, 2007. The 2007 Plan does not segregate the number of securities remaining available for future issuance among stock options and other awards. The shares authorized for future issuance represents the total number of shares available through any combination of stock options or other awards. The initial share reserve under the 2007 Plan was 560,000 shares of our Acacia Research-Acacia Technologies stock. The share reserve under the 2007 Plan automatically increases on January 1, 2008 and 2009, by an amount equal to two percent (2%) of the total number of shares of our Acacia Research-Acacia Technologies stock outstanding on the last trading day of December in the prior calendar year, except that the automatic increase in the share reserve will be three percent (3%) of our outstanding common stock on such January 1, if our common stock has appreciated by at least thirty percent (30%) in the prior calendar year. After January 1, 2009, no new additional shares will be added to the 2007 Plan without security holder approval (except for shares subject to outstanding awards that are forfeited or otherwise returned to the 2007 Plan). Column (a) excludes 339,000 in nonvested restricted stock awards outstanding at December 31, 2007. Refer to Note 11 to our consolidated financial statements included elsewhere herein. |
| (3) | We have not authorized the issuance of equity securities under any plan not approved by security holders. |
16
Item 6. SELECTED FINANCIAL DATA
The consolidatingconsolidated selected balance sheet data as of December 31, 20042007 and 20032006 and the consolidatingconsolidated selected statementstatements of operations data for the years ended December 31, 2004, 20032007, 2006 and 20022005 set forth below have been derived from our audited consolidated financial statements included elsewhere herein, and should be read in conjunction with those financial statements (including notes thereto). The consolidatingconsolidated selected balance sheet data as of December 31, 2002,
20012005, 2004 and 20002003 and the consolidatingconsolidated selected statementstatements of operations data for the years ended December 31, 20012004 and 20002003 have been derived from auditedunaudited consolidated financial statements not included herein, but which were previously filed with the SEC.
Acacia Research Corporation derived the Acacia Technologies group and
CombiMatrix group balance sheet data and statement of operations data from the
separate audited financial statements of the Acacia Technologies group and the
CombiMatrix group for the years ended December 31, 2004, 2003 and 2002 included
elsewhere herein, and the table should be read in conjunction with those
financial statements and related notes.
The AR-Acacia Technologies stock and the AR-CombiMatrix stock are intended
to reflect the separate performance of the respective divisions of Acacia
Research Corporation, rather than the performance of Acacia Research Corporation
as a whole. The chief mechanisms intended to cause the AR-Acacia Technologies
stock and the AR-CombiMatrix stock to reflect the financial performance of the
respective groups are provisions in our restated certificate of incorporation
and common stock policies governing dividends and distributions to each class of
stock, which specifically require the allocation of earnings to each class based
upon the performance of the two groups determined in accordance with generally
accepted accounting principles. Under these provisions, Acacia Research
Corporation factors the assets and liabilities and income or losses attributable
to the respective groups, determined as described under Item 7 "Management's
Discussion and Analysis of Financial Condition and Results
Consolidating Statements of Operations -
Critical Accounting Policies," into the determination of the amounts available
to pay dividends, if any, on the shares issued for the respective groups and
require Acacia Research Corporation to exchange, redeem or distribute a dividend
on the stock of a group if all or substantially all of the assets allocated to
the respective group are sold to a third party.
The Acacia Technologies group and the CombiMatrix group are not separate
legal entities. Holders of AR-Acacia Technologies stock and AR-CombiMatrix stock
are stockholders of Acacia Research Corporation. As a result, stockholders
continue to be subject to all of the risks of an investment in Acacia Research
Corporation and all of its businesses, assets and liabilities. The assets that
Acacia Research Corporation attributes to one group could be subject to the
liabilities of the other group.
26
- -------------------
(1) On February 13, 2001, the board of directors of Soundbreak.com, one of our
majority-owned subsidiaries, resolved to cease operations as of February
15, 2001 and liquidate the remaining assets and liabilities of
Soundbreak.com. See Note 11 to the Acacia Research Corporation consolidated
financial statements.
(2) Certain potential common shares for the periods shown above have been
excluded from the per share calculations because the effect of their
inclusion would be anti-dilutive. Data
(In addition, allthousands, except share and per share information has been adjusted as appropriate for all periods presented to
reflect a ten percent (10%) stock dividend distributed on December 5, 2001
for stockholders of record as of November 21, 2001.
(3) Effective January 1, 2001, we changed our accounting policy for balance
sheet classification of employee stock-based compensation resulting from
awards in consolidated subsidiaries. As a result, effective January 1,
2001, amortized non-cash stock compensation charges related to subsidiary
stock options are included in minority interests in our consolidated
balance sheet. Prior to the change in accounting policy, amortized non-cash
stock compensation charges related to subsidiary stock options were
reflected as "accrued stock compensation" in consolidated liabilities.
There is no impact on previous consolidated statements of operations as a
result of this change in accounting policy.
Total liabilities at December 31, 2001 include a capital lease obligation
totaling $2.8 million which was paid in full in October 2002.
(4)data)
| For the Years Ended December 31, | ||||||||||||||||||||
| 2007 | 2006 | 2005 | 2004 | 2003 | ||||||||||||||||
| License fee revenues | $ | 52,597 | $ | 34,825 | $ | 19,574 | $ | 4,284 | $ | 692 | ||||||||||
Marketing, general and administrative expenses (including non-cash stock compensation charges) | 20,042 | 14,123 | 8,097 | 5,043 | 4,300 | |||||||||||||||
| Inventor royalties and contingent legal fees expense - patents | 29,224 | 17,159 | 11,331 | - | - | |||||||||||||||
| Legal expenses - patents | 7,024 | 4,780 | 2,468 | 3,133 | 1,886 | |||||||||||||||
| Amortization of patents | 5,583 | 5,313 | 4,922 | 501 | 502 | |||||||||||||||
| Operating loss | (9,511 | ) | (6,847 | ) | (7,244 | ) | (6,055 | ) | (6,013 | ) | ||||||||||
| Other income, net | 2,359 | 1,524 | 1,071 | 471 | 408 | |||||||||||||||
| Loss from continuing operations before minority interests | (7,152 | ) | (5,323 | ) | (6,173 | ) | (5,445 | ) | (5,468 | ) | ||||||||||
| Loss from continuing operations | (7,359 | ) | (5,363 | ) | (6,038 | ) | (5,439 | ) | (5,451 | ) | ||||||||||
| Discontinued operations - Split-off of CombiMatrix Corporation and other | (8,086 | ) | (20,093 | ) | (12,638 | ) | 606 | (18,969 | ) | |||||||||||
| Net loss | $ | (15,445 | ) | $ | (25,456 | ) | $ | (18,676 | ) | $ | (4,833 | ) | $ | (24,420 | ) | |||||
| Loss per common share - basic and diluted: | ||||||||||||||||||||
| Loss from continuing operations | ||||||||||||||||||||
| Acacia Research - Acacia Technologies stock | $ | (0.26 | ) | $ | (0.19 | ) | $ | (0.23 | ) | $ | (0.27 | ) | $ | (0.28 | ) | |||||
| Discontinued operations - Split-off of CombiMatrix Corporation | ||||||||||||||||||||
| Acacia Research - CombiMatrix stock | $ | (0.14 | ) | $ | (0.49 | ) | $ | (0.37 | ) | $ | 0.02 | $ | (0.76 | ) | ||||||
| Weighted average number of common and potential common shares used in computation of income (loss) per common share: | ||||||||||||||||||||
| Acacia Research - Acacia Technologies stock: | ||||||||||||||||||||
| Basic and diluted | 28,503,314 | 27,547,651 | 26,630,732 | 19,784,883 | 19,661,655 | |||||||||||||||
| Acacia Research - CombiMatrix stock: | ||||||||||||||||||||
| Basic | 55,862,707 | 40,605,038 | 33,678,603 | 29,962,596 | 24,827,819 | |||||||||||||||
| Diluted | 55,862,707 | 40,605,038 | 33,678,603 | 30,995,663 | 24,827,819 | |||||||||||||||
Consolidating Balance Sheet Data
(In thousands)
| At December 31, | ||||||||||||||||||||
| 2007 | 2006 | 2005 | 2004 | 2003 | ||||||||||||||||
| Total assets: | ||||||||||||||||||||
| Acacia Research Corporation | $ | 71,051 | $ | 65,770 | $ | 68,893 | $ | 33,058 | $ | 39,978 | ||||||||||
| Discontinued operations - Split-off of CombiMatrix Corporation | - | 44,214 | 52,541 | 55,388 | 50,161 | |||||||||||||||
| Eliminations | - | (380 | ) | - | (119 | ) | (99 | ) | ||||||||||||
| Total | $ | 71,051 | $ | 109,604 | $ | 121,434 | $ | 88,327 | $ | 90,040 | ||||||||||
| Total liabilities: | ||||||||||||||||||||
| Acacia Research Corporation | $ | 6,247 | $ | 4,276 | $ | 6,647 | $ | 3,472 | $ | 4,188 | ||||||||||
| Discontinued operations - Split-off of CombiMatrix Corporation | - | 11,399 | 7,443 | 8,560 | 24,424 | |||||||||||||||
| Eliminations | - | (380 | ) | - | (119 | ) | (99 | ) | ||||||||||||
| Total | $ | 6,247 | $ | 15,295 | $ | 14,090 | $ | 11,913 | $ | 28,513 | ||||||||||
| Minority interests: | ||||||||||||||||||||
| Acacia Research Corporation | $ | - | $ | - | $ | 443 | $ | 778 | $ | 1,127 | ||||||||||
| Discontinued operations - Split-off of CombiMatrix Corporation | - | - | 4 | - | - | |||||||||||||||
| Total | $ | - | $ | - | $ | 447 | $ | 778 | $ | 1,127 | ||||||||||
| Stockholders' equity: | ||||||||||||||||||||
| Acacia Research Corporation | $ | 64,804 | $ | 61,494 | $ | 61,803 | $ | 28,808 | $ | 34,663 | ||||||||||
| Discontinued operations - Split-off of CombiMatrix Corporation | - | 32,815 | 45,094 | 46,828 | 25,737 | |||||||||||||||
| Total | $ | 64,804 | $ | 94,309 | $ | 106,897 | $ | 75,636 | $ | 60,400 | ||||||||||
17
Factors Affecting Comparability:
| · | Effective January 1, 2006, Acacia Research Corporation adopted the provisions of Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123R”), which sets forth the accounting requirements for “share-based” compensation payments to employees and non-employee directors and requires that compensation cost relating to share-based payment transactions be recognized in the statement of operations. Refer to Note 2 and Note 11 to the Acacia Research Corporation consolidated financial statements included elsewhere herein, for additional information and a description of the impact of SFAS No. 123R on our consolidated statements of operations data presented above. Non-cash stock compensation charges included in Marketing, General and Administrative expense were $5,908,000, $3,946,000 and $356,000 in 2007, 2006 and 2005, respectively. |
| · | In September 2007, we recorded a non-cash impairment charge of $235,000, related to the write-off of a patent-related intangible asset. In June 2006, we recorded a non-cash charge of $297,000, related to the write-off of a patent-related intangible asset. In June 2003, we recorded an impairment charge of $207,000 for an other-than-temporary decline in the fair value of a cost method investment. |
| · | As a result of the conclusion of the V-chip patent litigation, our subsidiary, Soundview Technologies Inc., recognized $1,500,000 of V-chip related deferred license fee revenues, $668,000 of V-chip related deferred legal costs, and a non-cash V-chip related goodwill impairment charge of $1.6 million in the third quarter of 2004. |
| · | In January 2005, Acacia Global Acquisition Corporation consummated the GPH Acquisition as described above. The aggregate purchase consideration was approximately $25.1 million, including $5.0 million of cash, the issuance of 3,938,832 shares of Acacia Research-Acacia Technologies common stock, or AR-Acacia Technologies stock, valued at $19.3 million (net of estimated common stock registration costs of $212,000) and acquisition costs, including registration costs, of $796,000. $25.1 million of the purchase price was allocated to patent related intangible assets acquired, which are being amortized on a straight-line basis over a weighted-average estimated economic useful life of six years. As a result of the GPH Acquisition and subsequent patent acquisitions through December 31, 2007, amortization expense totaled $5.6 million, $5.3 million and $4.9 million in 2007, 2006 and 2005, respectively, as compared to approximately $501,000, and $502,000, in 2004 and 2003, respectively. |
| · | As a result of the Split-off Transaction, as discussed above, we have disposed of our investment in CombiMatrix Corporation. Refer to Note 10A to the Acacia Research Corporation consolidated financial statements, included elsewhere herein, for information regarding presentation of the assets, liabilities, results of operations and cash flows for the CombiMatrix group as “Discontinued Operations,” for all periods presented, in accordance with guidance set forth in SFAS No. 144 “Accounting for the Impairment or Disposal of Long-Lived Assets” (“SFAS No. 144”). |
18
Item 7. "Management's Discussion and Analysis of Financial
Condition - Critical Accounting Policies" for a description of allocation
policies applied in preparation of the separate group financial statements.
(5) The 2002 share and per share information gives effect to the
recapitalization transaction described elsewhere herein as of January 1,
2002. Historical share and per share information for the Acacia
Research-Acacia Technologies stock and Acacia Research-CombiMatrix stock is
not presented as these classes of securities were not part of Acacia
Research Corporation's capital structure during 2001 and prior periods.
FACTORS AFFECTING COMPARABILITY:
o In the fourth quarter of 2000, Acacia Research Corporation recorded
$1.0 million in write-offs of other early stage investments and $2.6
million in write-offs of equity investments, attributed to the Acacia
Technologies group.
28
o During the year ended December 31, 2000, CombiMatrix Corporation
recorded deferred non-cash stock compensation charges aggregating
approximately $53.8 million in connection with the granting of stock
options. Deferred non-cash stock compensation charges are being
amortized by the CombiMatrix group over the respective option grant
vesting periods, which ranged from one to four years. Amortization of
deferred non-cash stock compensation charges totaled $606,000, $1.5
million, $6.4 million and $20.0 million in 2004, 2003, 2002 and 2001,
respectively. Non-cash stock compensation charges were not significant
in periods prior to 2001. Deferred non-cash stock compensation charges
were fully amortized as of December 31, 2004.
o In connection with Acacia Research Corporation's increased focus on
the media technologies and life sciences sectors, certain of Acacia
Research Corporation's businesses allocated to the Acacia Technologies
group ceased operations and certain investments were written off in
2000. As a result, marketing, general and administrative costs related
to salaries, benefits, consulting, legal and other professional costs
were significantly reduced in 2001.
o In June 2003 and September 2002, Acacia Research Corporation recorded
impairment charges of $207,000 and $2.7 million, respectively, for an
other-than-temporary decline in the fair value of a cost method
investment, attributed to the Acacia Technologies group.
o On December 13, 2002, Acacia Research Corporation increased its
consolidated ownership interest in CombiMatrix Corporation from 48% to
100% as discussed at Item 7. "Critical Accounting Policies -
Accounting for Business Combinations." $17.2 million of the total
purchase price of $46.0 million was attributed to acquired in-process
research and development, or IPR&D, and was charged to expense in the
consolidated statement of operations and comprehensive loss for the
year ended December 31, 2002. Amounts allocated to IPR&D have been
attributed to the CombiMatrix group.
o As of December 31, 2002, Acacia Research Corporation owned 100% of its
significant subsidiaries, including Acacia Media Technologies
Corporation, Soundview Technologies Corporation and CombiMatrix
Corporation. As such, Minority Interests amounts and balances
reflected in the statement of operations and balance sheet,
respectively, decreased significantly subsequent to December 31, 2002.
o On September 30, 2002, CombiMatrix Corporation and Dr. Donald
Montgomery, an officer and stockholder of CombiMatrix Corporation,
entered into a settlement agreement with Nanogen to settle all pending
litigation between the parties. In addition to other terms of the
settlement agreement as described elsewhere herein, CombiMatrix
Corporation agreed to pay Nanogen $1.0 million and issued 4,016,346
shares, or 17.5% of its outstanding shares post issuance, to Nanogen.
The $1.0 million in payments have been expensed in the consolidated
statement of operations for the year ended December 31, 2002 under
"legal settlement charges." The issuance of the CombiMatrix
Corporation common shares in settlement of the litigation with Nanogen
has been accounted for as a nonmonetary transaction. Accordingly,
included in "legal settlement charges" in the consolidated statements
of operations for the year ended December 31, 2002 is a charge in the
amount of $17.5 million based on the fair value of the CombiMatrix
Corporation common shares issued to Nanogen. Amounts related to the
settlement have been attributed to the CombiMatrix group.
o In March 2004, the CombiMatrix group completed all phases of its
research and development agreement with Roche Diagnostics, GmbH
("Roche"). As a result of completing all of its obligations under this
agreement and in accordance with the CombiMatrix group's revenue
recognition policies for multiple-element arrangements, the
CombiMatrix group recognized all previously deferred Roche related
contract revenues totaling $17,302,000 during the first quarter of
2004.
o As a result of the conclusion of the V-chip patent litigation, the
Acacia Technologies group recognized $1,500,000 of V-chip related
deferred license fee revenues and $668,000 of V-chip related deferred
legal costs in the third quarter of 2004. The Acacia Technologies
group recognized $43,000 and $24.1 million in V-chip license fees in
2002 and 2001, respectively. See Item 3. "Legal Proceedings."
29
ITEM 7. MANAGEMENT'SMANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
THE FOLLOWING DISCUSSION SHOULD BE READ IN CONJUNCTION WITH OUR FINANCIAL
STATEMENTS INCLUDED ELSEWHERE IN THIS FORM
The following discussion should be read in conjunction with our consolidated financial statements included elsewhere in this Form 10-K. THIS DISCUSSION CONTAINS
FORWARD-LOOKING STATEMENTS THAT INVOLVE RISKS AND UNCERTAINTIES. OUR ACTUAL
RESULTS COULD DIFFER MATERIALLY FROM THOSE ANTICIPATED IN THESE FORWARD-LOOKING
STATEMENTS AS A RESULT OF VARIOUS FACTORS INCLUDING THOSE SET FORTH UNDER "RISK
FACTORS" HEREIN.
GENERALThis discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of various factors including those set forth under item 1A. “Risk Factors” elsewhere herein.
General
Acacia Research Corporation’s operating subsidiaries acquire, develop, license and enforce patented technologies. Our operating subsidiaries generate license fee revenues and related cash flows from the granting of licenses for the use of patented technologies that our operating subsidiaries own or control. Our operating subsidiaries assist patent owners with the prosecution and development of their patent portfolios, the protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, if necessary, with the enforcement against unauthorized users of their patented technologies. Currently, on a consolidated basis, our operating subsidiaries own or control the rights to 91 patent portfolios, which include U.S. patents and certain foreign counterparts, covering technologies used in a wide variety of industries.
As used in this Form 10-K, “we,” “us” and “our” refer to Acacia Research Corporation is comprisedand/or its wholly owned operating subsidiaries. All intellectual property acquisition, development, licensing and enforcement activities are conducted solely by certain of twoAcacia Research Corporation’s wholly owned operating groups,subsidiaries.
CombiMatrix Group Split-off Transaction and Related Discontinued Operations. As discussed below under the caption “Discontinued Operations – Split-off of CombiMatrix Corporation,” the CombiMatrix group, andwhich was previously presented as a separate reportable segment, was split-off from Acacia Research Corporation (the “Split-off Transaction”), effective August 15, 2007 (the “Redemption Date”). As such, the Acacia Technologies group.
Our life sciences business, referred to asresults of operations for the "CombiMatrixCombiMatrix group" is
seeking to become a broadly diversified biotechnology company, through the
development of proprietary technologies and products in the areasaccompanying consolidated financial statements are presented as part of drug
development, genetic analysis, nanotechnology research, defense and homeland
security markets, as well as other potential markets where its products could be
utilized.
OurAcacia Research Corporation’s results from discontinued operations in accordance with SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets,” or SFAS No. 144. Accordingly, the CombiMatrix group’s results of operations in prior periods have been reclassified to discontinued operations to conform to the current period presentation.
The intellectual property licensing business, referred to as the "Acacia
Technologies group," is responsible for theacquisition, development, acquisition, licensing and enforcement of patented technologies.
The CombiMatrix group and thebusiness conducted by Acacia Technologies group's businesses areResearch Corporation’s operating subsidiaries, is described more fully in Item 1. "Business,"“Business,” of this report.
OVERVIEW
COMBIMATRIX GROUP
During 2004, the CombiMatrix group's
Overview
Our operating activities were highlighted
by the completion of its researchduring 2007, 2006 and development agreement with Roche, the
execution of a two-year, $5.9 million contract with the Department of Defense to
further the development of the CombiMatrix group's microarray technology for the
detection of biological threat agents, execution of a multi-year collaboration
agreement with Furuno Electric Co. to develop a bench-top DNA array synthesizer
and the launch of CustomArray(TM), its first commercially available array
platform. The CombiMatrix group also continued its efforts in the area of drug
discovery by executing a research and development agreement in the area of siRNA
with irsiCiaxa Foundation and by making a minority investment in Leuchemix,
which is developing potential therapies to treat leukemia. As a result of
completing its research and development agreement with Roche, the CombiMatrix
group's research and development programs shifted to a number of internally
funded programs that support the activities summarized above. With the
completion of its obligations under the Roche agreements, research and
development expenses continued to decrease in 2004, as compared to 2003, as
efforts shifted to internally funded research and development programs. The
decrease in research and development expenses was partially offset by an
increase in marketing and sales expenses related to the launch of the
CombiMatrix group's CustomArray(TM) 902 DNA array platform in March 2004 and its
CustomArray(TM) 12K DNA expression array in July 2004.
Historically, the CombiMatrix group was substantially dependent on its
research and development arrangements with Roche, relying on payments by Roche
to fund the majority of its research and development activities and related
resources engaged in fulfilling its contractual obligations to Roche. Roche's
primary service to the CombiMatrix group is to distribute its technology
platform. If the CombiMatrix group were to lose its relationship with Roche, the
CombiMatrix group would continue to distribute its technology platform itself or
be required to establish a distribution agreement with other partners. This
could prove difficult, time-consuming and expensive, and the CombiMatrix group
may not be successful in achieving this objective.
During 2003, the CombiMatrix group's operating activities were highlighted
by the receipt of significant payments from its strategic partners and
licensees, including $9.8 million related to the completion of certain
milestones and delivery of prototype products and services pursuant to its
agreements with Roche and an up-front payment and a milestone payment totaling
$2.4 million pursuant to its agreement with Toppan. The CombiMatrix group also
completed several Roche related research and development projects during the
third and fourth quarters of 2003, resulting in a decrease in related research
and development expenses during 2003, as compared to 2002. The CombiMatrix group
continued its efforts with its existing partners and continued to focus on
identifying new strategic relationships with the goal of maximizing the
opportunities in the life sciences sector that will be created by
commercializing its array system.
In 2002, the CombiMatrix's group's operating activities were highlighted by
the receipt of $11.5 million in milestone payments pursuant to its license,
research and development agreements with Roche, which were recorded as deferred
revenues. In addition, the CombiMatrix group recognized $533,000 in grant and
contract revenues, including $274,000 in grant revenue resulting from completion
of its Phase II SBIR Department of Defense contract, $141,000 in one-time
contract research and development revenues, $100,000 in revenue related to
performance and completion of its Phase I National Institutes of Health grant
and $306,000 from the sale of a genomics array synthesizer system and related
array products to two institutions in Japan.
30
ACACIA TECHNOLOGIES GROUP
In 2004 and 2003, the Acacia Technologies group's operating activities2005, were principally focused on the continued development, licensing and commercializationenforcement of its
DMTthe patent portfolio.portfolios owned or controlled by our operating subsidiaries, including the continued pursuit of multiple ongoing technology licensing and enforcement programs and the commencement of new technology licensing and enforcement programs. In addition, we continued our focus on business development, including the acquisition of several additional patent portfolios by certain of our operating subsidiaries and the continued pursuit of additional opportunities to partner with patent owners and provide our unique intellectual property licensing, development and enforcement services.
License fee revenues recognized in 2007 totaled $52.6 million, representing a 51% increase over revenues recognized in 2006, which totaled $34.8 million, and a greater than 100% increase over revenues recognized in 2005, which totaled $19.6 million. The Acacia Technologies group began to recognize DMTincrease in license fee revenues in 20032007 and continued its focus on commercializing its DMT2006 reflects the impact of the increase in patent portfolios owned or controlled by our operating subsidiaries, and the increase in the number of patent licensing and enforcement programs launched and generating revenues since the end of 2005.
Revenues for 2007 included license fees from 91 new licensing agreements covering 16 of our technology licensing and enforcement programs, as compared to 72 new licensing agreements covering 14 of our technology licensing and enforcement programs in 2006 and 83 new licensing agreements covering 12 of our technology licensing and enforcement programs in 2005. On a consolidated basis, our operating subsidiaries generated licensing revenues from 8 new technology licensing and enforcement programs during 2004.2007, as compared to 7 new programs in 2006 and 11 new programs in 2005. To date, on a consolidated basis, we have generated revenues from 28 technology licensing and enforcement programs.
License fee revenues for 2007 included fees from the Acacia Technologies group has entered into
294 DMT licensing agreements, including 107 cable TV licenses, 182 licensesof our Color Correction for online entertainment, movies, news, sports, e-learningVideo Graphics Systems technology, DMT® technology, Audio/Video Enhancement and corporate websitesSynchronization technology, Audio Communications Fraud Detection technology, Credit Card Fraud Protection technology, Electronic Address List Management technology, Image Resolution Enhancement technology, Pop-Up Internet Advertising technology, Portable Storage Devices with Links technology, Multi-dimensional Bar Codes technology, Product Activation technology, Rule Based Monitoring technology, Spreadsheet Automation technology, Telematics technology, Vehicle Magnetic Braking technology and licenses with 5 companies that provide over 90%Virtual Computer Workspaces technology licensing programs.
19
Management measures and assesses the performance and growth of video-on-demand TV
entertainment to the hotel industry in the United States. During 2004, we
executed 170 DMT license agreements. In the first, second, thirdpatent licensing and fourth
quarters of 2004, the Acacia Technologies group recorded DMTenforcement business conducted by our operating subsidiaries based on consolidated license fee revenues recognized across all of $599,000, $666,000, $740,000our technology licensing and $779,000, respectively, compared to
$6,000, $19,000, $186,000 and $481,000, respectively, in the comparable 2003
periods.
The Acacia Technologies group's continued development, and
commercializationenforcement programs on a trailing twelve-month basis. Trailing twelve-month revenues totaled $52.6 million as of the DMT patent portfolio included increased marketing,
general and administrative expenses in 2004,December 31, 2007, as compared to 2003$47.9 million as of September 30, 2007, $46.8 million as of June 30, 2007, $55.3 million as of March 31, 2007, $34.8 million as of December 31, 2006 and 2002,
related$19.6 million as of December 31, 2005.
Operating expenses increased during 2007 and 2006, as compared to 2005, due primarily to the hiring of additional patent licensing, and business development and engineering personnel, and an increase in patent related legal, research and consulting and marketing expenses.
Patent related legal expenses excluding V-chip related legal fees, also
increased due to an increase in costs incurred in connection with the Acacia
Technologies group's ongoing DMT patent commercializationcontinued growth and expansion of our technology licensing and enforcement programs, including increased legal costs related to new patent claimsbusiness and the
identification of additional potential licensees of our DMT technologyan increase in corporate, general and increased patent enforcementadministrative costs related to ongoing DMToperations. Inventor royalties expenses and contingent legal fees expenses increased in 2007 and 2006, as compared to 2005, primarily due to the related increase in license fee revenues, as discussed above, and the impact of the varying economic terms related to inventor agreements and contingent legal fee arrangements associated with the revenue generating patent related
litigation as summarizedportfolios in Item 1. "Business," elsewhere herein.
In 2004, the Acacia Technologies group alsoeach period.
During 2007, certain of our operating subsidiaries continued to execute itsour business strategy in the area of patent portfolio acquisitions. In July 2004,acquisitions, including, in the Acacia Technologies groupfourth quarter of 2007, the acquisition of, or the acquisition of the rights to, patent portfolios in the Authorized Spending Accounts, Compiler, Copy Protection, Projector, and Virtual Server technology areas. During 2007, we acquired U.S. Patent No. 6,226,677 from LodgeNet
Entertainment Corporation. Thea total of 31 new patent coversportfolios with applications over a wide range of technology areas, as compared to 20 new patent portfolios in 2006 and methods for
redirecting users to a login page when accessing the Internet. The8 new patent purchase agreement with LodgeNet Entertainment Corporation includes terms by
which the companies will divide any future revenues received by the Acacia
Technologies group from licensing the patent. In December 2004, the Acacia
Technologies group executed a binding letter of intentportfolios in 2005 (excluding portfolios acquired in connection with the 2005 GPH Acquisition).
In January 2005, acquisitionAcacia Global Acquisition Corporation acquired substantially all of the assets of Global Patent Holdings, as discussed
earlier. TheLLC, a privately held patent holding company, which owned 11 patent licensing companies (“GPH Acquisition”). In connection with the acquisition gives the Acacia Technologies groupwe acquired ownership of companies that own or control the rights to 27 patent portfolios, which includeincluded 120 U.S. patents and certain foreign counterparts, and covercovering technologies used in a wide variety of industries as discussed earlier. Theseindustries.
Refer to “Liquidity and Capital Resources” below for information regarding the impact of patent and patent rights acquisitions expand and diversify theon Acacia Technologies group's revenue generating opportunities and accelerate the
execution of the Acacia Technologies group's business strategy of acquiring,
developing and licensing patented technologies.
As a result of the August 2004 final ruling of non-infringement in the
Acacia Technologies group's V-chip litigation, operating results in 2004 include
$1.5 million of previously deferred V-chip license fee revenues, $668,000 of
previously deferred V-chip related legal costs and an impairment charge of $1.6
million associated with the write-off of 100% of V-chip related goodwill. As a
result of the final ruling, the Acacia Technologies group concluded its V-chip
licensing program and does not expect to recognize any additional V-chip
technology related revenues in future periods.
During 2002, the Acacia Technologies group's operating activities were
highlighted by the continued building of its executive management team,
including the hiring of key media technology and intellectual property industry
experts that are responsible for the development, licensing and protection of
the Acacia Technologies group's intellectual property and proprietary
technologies, as well as the pursuit of additional licensing and strategic
business alliances with leading companies in the intellectual property licensing
industry. In addition, in 2002, the Acacia Technologies group increased legal
and third-party consulting activities related to its ongoing DMT patent
marketing and commercialization efforts, including patent claims construction,
patent prosecution and related research and engineering costs.
CRITICAL ACCOUNTING POLICIES
OurResearch Corporation’s consolidated financial statements andfor the separate groupperiods presented.
As of December 31, 2007, we had several option agreements with third-party patent portfolio owners regarding the potential acquisition of additional patent portfolios.
Critical Accounting Policies
Our consolidated financial statements are prepared in conformity with accounting principles generally accepted in the United States of America. In preparing these financial statements, we make assumptions, judgments and estimates that can have a significant impact on amounts reported in our financial statements. We base our assumptions, judgments and estimates on historical experience and various other factors that we believe to be reasonable under the circumstances. Actual results could differ materially from these estimates under different assumptions or conditions. On a regular basis we evaluate our assumptions, judgments and estimates and make changes accordingly.
We believe that, of the significant accounting policies discussed in Note 2 to our consolidated and separate group financial statements, the following accounting policies require our most difficult, subjective or complex judgments:
o revenue recognition;
o research and development expenses;
o accounting for income taxes;
o valuation of long-lived and intangible assets and goodwill;
o accounting for business combinations, and;
o management and allocation policies relating to AR-Acacia Technologies
stock and AR-CombiMatrix stock.
| · | revenue recognition; |
| · | stock-based compensation expense; |
| · | valuation of long-lived and intangible assets; and |
| · | impairment of marketable securities; |
We discuss below the critical accounting assumptions, judgments and estimates associated with these policies. Historically, our assumptions, judgments and estimates relative to our critical accounting policies have not differed materially from actual results. For further information on our critical accounting policies, seerefer to Note 2 to the consolidated financial statements and
separate group statements included herein.
REVENUE RECOGNITION
20
Revenue Recognition
As described below, significant management judgments must be made and used in connection with the revenue recognized in any accounting period. Material differences may result in the amount and timing of revenue recognized or deferred for any period, if management made different judgments.
Revenue is recognized, in accordance with Staff Accounting Bulletin No. 104, “Revenue Recognition,” (“SAB No. 104”), when (i) persuasive evidence of an arrangement exists, (ii) all obligations have been performed pursuant to the terms of the agreement, (iii) amounts are fixed or determinable and (iv) collectibility of amounts is reasonably assured.
31
ACACIA TECHNOLOGIES GROUP
We make estimates and judgments when determining whether the collectibility of license fees receivable from licensees is reasonably assured. The Acacia
Technologies group assessesWe assess the collectibility of accrued license fees receivable based on a number of factors, including past transaction history with licensees and the credit-worthiness of licensees. If it is determined that collection is not reasonably assured, the fee is recognized when collectibility becomes reasonably assured, assuming all other revenue recognition criteria have been met, which is generally upon receipt of cash. Management estimates regarding collectibility impact the actual revenues recognized each period and the timing of the recognition of revenues. Our assumptions and judgments regarding future collectibility could differ from actual events, thus materially impacting our financial position and results of operations.
The Acacia Technologies group recognizes
Certain license agreements provide for the payment of contractually determined paid-up license fees to our operating subsidiaries in consideration for the grant of a non-exclusive, retroactive and future license to manufacture and/or sell products covered by patented technologies owned or controlled by our operating subsidiaries. Generally, the execution of these license agreements also provide for the release of the licensee from certain claims and the dismissal of any pending litigation. Pursuant to the terms of these agreements, our operating subsidiaries have no further obligation with respect to the grant of the non-exclusive retroactive and future license and related releases, including no express or implied obligation on our operating subsidiaries’ part to maintain or upgrade the technology, or provide future support or services. Generally, the agreements provide for the grant of the license and releases upon execution of the agreement. As such, the earnings process is generally complete upon the execution of the agreement, and revenue is recognized upon execution of the agreement, when collectibility is reasonably assured, and all other revenue recognition criteria have been met.
For those arrangements where royalties cannot be reasonably estimated, our operating subsidiaries recognize revenue upon the receipt of cash or license fee statements from their licensees, as described at Note 2 to our consolidated financial statements contained elsewhere herein. Our operating subsidiaries recognize certain license fee revenues when earned over the term of the license agreement in exchange for the grant of non-exclusive licenses to use certain technologies for which weour operating subsidiaries own or control patents. WeOur operating subsidiaries recognize revenue for estimates of license fees earned during the applicable period, based on historical activities of licensees, historical sales or per unit growth rates of licensees and other relevant available information regarding licensee activities that factor into the calculation of periodic license fees due. Revisions, if any, are made for actual licensee fees received in the following quarter. Historically, these revisions have not been material to our consolidated financial statements. For those arrangements where royalties cannot
be reasonably estimated, we recognize revenue upon the receipt of cash or
license fee statements from our licensees as described at Note 2 to our
consolidated financial statements contained elsewhere herein. Our estimatesEstimates of periodic license fees due could differ from actual events, thus materially impacting our financial position and results of operations.
The Acacia Technologies group is
Our operating subsidiaries are responsible for the licensing and enforcement of itstheir respective patented technologies and pursuespursue third parties that are utilizing itstheir intellectual property without a license or who have under-reported the amount of royalties owed under a license agreement with the Acacia
Technologies group.agreement. As a result of these activities, from time to time, weour operating subsidiaries may recognize royalty revenues in a current period that relate to infringements by our licensees that occurred in prior periods. These royalty recoveries may cause revenues to be higher than expected during a particular reporting period and may not occur in subsequent periods. Differences between amounts initially recognized and amounts subsequently audited or reported as an adjustment to those amounts, will beare recognized in the period such adjustment is determined as a change in accounting estimate.
COMBIMATRIX GROUP
Significant estimates, judgments
Stock-based Compensation Expense
Effective January 1, 2006, Acacia Research Corporation adopted the provisions of Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123R”), which is a revision of SFAS No. 123, “Accounting for Stock-Based Compensation.” SFAS No. 123R supersedes Accounting Principles Board (“APB”) Opinion No. 25, Accounting for Stock Issued to Employees, and assumptions are requiredamends SFAS No. 95, “Statement of Cash Flows.” SFAS No. 123R sets forth the accounting requirements for “share-based” compensation payments to employees and non-employee directors and requires all share based-payments to be recognized as expense in connection
with the CombiMatrix group's accounting for multiple-element arrangements with
strategic partners and licensees.
The CombiMatrix group accounts for revenues under multiple-element
arrangements in accordance withstatement of operations. In March 2005, the SEC published Staff Accounting Bulletin No. 104, "Revenue
Recognition," or 107 (“SAB No. 104107”), which requires stock-based compensation to be classified in the same expense line items as cash compensation (i.e. marketing, general and Emerging Issues Task Force Consensus, or EITF,
Issue 00-21, "Revenue Arrangements with Multiple Deliverables," and related
pronouncements. Arrangements with multiple elements or deliverables must be
segmented into individual units of accountingadministrative expenses). The compensation cost for all stock-based awards is measured at the grant date, based on the separate deliverables
only if there is objective and verifiable evidence of fair value to allocate the
consideration received to the deliverables. Accordingly, revenues from
multiple-element arrangements involving license fees, up-front payments and
milestone payments, which are received and/or billable in connection with other
rights and services that represent continuing obligations of the CombiMatrix
group, are deferred until all of the multiple elements have been delivered or
until objective and verifiable evidence of the fair value of the undelivered
elements has been established. Upon establishing objectiveaward (determined using a Black-Scholes option pricing model), and verifiable
evidenceis recognized as an expense over the employee’s requisite service period (generally the vesting period of the equity award). Determining the fair value of stock-based awards at the elements in multiple-element arrangements, the
fair value is allocated to each element of the arrangement, such as license fees
or research and development projects, based on the relative fair values of the
elements. The CombiMatrix group determines the fair value of each element in
multiple-element arrangements based on objective and verifiable evidence of fair
value, which is determined for each element based on the price charged when the
same element is sold separately to a third party. If objective and verifiable
evidence of fair value of all undelivered elements exists but objective and
verifiable evidence of fair value does not exist for one or more delivered
elements, then revenue is recognized using the residual method. Under the
residual method, the revenues from delivered elements are not recognized until
the fair value of the undelivered element(s) have been determined.
In March 2004, the CombiMatrix group completed all phases of its research
and development agreement with Roche. As a result of completing all obligations
under this agreement and in accordance with the CombiMatrix group's revenue
recognition policies for multiple-element arrangements, the CombiMatrix group
recognized all previously deferred Roche related contract revenues during the
first quarter of 2004. All payments received togrant date from Furuno and Toppan,
totaling $3.4 million, have been classified as deferred revenues in the
CombiMatrix group's consolidated balance sheets due to the determination that
objective and verifiable evidence of fair value does not currently exist for the
remaining undelivered elements. Pursuant to EITF 00-21, SAB No. 104 and related
guidance, the elements associated with the amounts received to date and
additional milestone payments will be treated as one accounting unit. The
up-front fees and cash payments received upon the accomplishment of the
contractual milestones will be deferred. Revenue will be recognized when all of
the related elements, for which objective and reliable evidence does not exist,
have been delivered and there is objective and reliable evidence to support the
fair value for all of the undelivered elements.
32
RESEARCH AND DEVELOPMENT EXPENSES
The CombiMatrix group accounts for research and development expenses
pursuant to Statement of Financial Accounting Standards, or SFAS, No. 2,
"Accounting for Research and Development Costs," which requires that all
research and development costs be charged to expense as incurred, including
costs incurred to acquire technologies, which are utilized in research and
development and which have no alternative future use. Also, costs related to
filing and pursuing patent applications are expensed as incurred, as
recoverability of such expenditures is uncertain. Research and development
refers to a plan or design for a new product or process or for a significant
improvement to an existing product or process whether intended for sale or use.
Significant management estimates and judgments, are required with respect toincluding estimating the determinationmarket price volatility of which costs relate to plans or designs for a new product or
process or for a significant improvement to an existing product. Had the
CombiMatrix group determined that certain costs incurred were not related to
researchour classes of common stock and development activities, different accounting treatment for such
costs may have been required.
The costs of software developed or obtained for internal use is expensed as
incurred until certain capitalization criteria have been met, at which time such
costs are capitalized and reported as a component of property and equipment. To
date, these costs have been classified as research and development expenses.
Significant management estimates are required with respect to the determination
of when certain capitalization criteria have been met. Typically this occurs
upon completion of a prototype and design phase and a functioning model exists.
Thereafter, all software program costs are requiredemployee stock option exercise behavior.
21
SFAS No. 123R also requires stock-based compensation expense to be capitalized and
amortized over the remainingrecorded only for those awards expected to vest using an estimated useful life of the software. Had
management made differing judgments regarding the capitalization criteria,
different accounting treatment of costs of software developed for internal use
may have been required.
ACCOUNTING FOR INCOME TAXESpre-vesting forfeiture rate. As part of the process of preparing our consolidated financial statements,
we are requiredsuch, SFAS No. 123R requires Acacia Research Corporation to estimate pre-vesting option forfeitures at the time of grant and reflect the impact of estimated pre-vesting option forfeitures on compensation expense recognized. Estimates of pre-vesting forfeitures must be periodically revised in subsequent periods if actual forfeitures differ from those estimates. We consider several factors in connection with our income taxes in eachestimate of the jurisdictions in
which we operate. This process involves the estimatingpre-vesting forfeitures including types of our actual current tax
exposure together with assessing temporary differences resulting from differing
treatmentawards, employee class, and historical pre-vesting forfeiture data. The estimation of items, such as deferred revenue, amortization of intangibles and
asset depreciation for tax and accounting purposes. These differences result in
deferred tax assets and liabilities, which are included within our consolidated
balance sheet. We must then assess the likelihoodstock awards that our deferred tax assets
will be recovered from future taxable incomeultimately vest requires judgment, and to the extent we believe that
recovery is not likely, we must establish a valuation allowance. To the extent
we establish a valuation allowance or increase this allowance in a period, we
must include an expense within the tax provision in the consolidated statement
of operations and comprehensive loss.
Significant management judgment is required in determining our provision
for income taxes, our deferred tax assets and liabilities and our valuation
allowance. We have recorded a full valuation allowance against our deferred tax
assets of $94.1 million as of December 31, 2004, due to uncertainties related to
our ability to utilize our deferred tax assets, primarily consisting of certain
net operating losses carried forward, before they expire. In assessing the need
for a valuation allowance, we have considered our estimates of future taxable
income, the period over which our deferred tax assets may be recoverable, our
history of losses and our assessment of the probability of continuing losses in
the foreseeable future. In management's estimate, any positive indicators,
including forecasts of potential future profitability of our businesses, are
outweighed by the uncertainties surrounding our estimates and judgments of
potential future taxable income. In the event that actual results differ from theseour estimates, or we adjust these estimates should we believe we wouldsuch amounts will be able
to realize these deferred tax assets in the future, an adjustment to the
valuation allowance would increase incomerecorded as cumulative adjustments in the period such determination was
made. Any changes in the valuation allowance could materially impactestimates are revised. If actual results differ significantly from these estimates, stock-based compensation expense and our
financial position and results of operations.
VALUATION OF LONG-LIVED AND INTANGIBLE ASSETS AND GOODWILL
Goodwill is evaluated for impairment using a fair value approach at the
reporting unit level annually, or earlier if an event occurs or circumstances
change that would more likely than not reduce the fair value of a reporting unit
below its carrying amount. A reporting unit canoperations could be an operating segment or a
business if discrete financial information is preparedmaterially impacted.
Refer to Notes 2 and reviewed by
management. Our reporting units are: 1)11 to the Acacia Technologies groupResearch Corporation consolidated financial statements included in Part IV, Item 15 of this report for more information.
Valuation of Long-lived and 2) the
CombiMatrix group. Under the impairment test, if a reporting unit's carrying
amount exceeds its estimated fair value, goodwill impairment is recognized to
the extent that the reporting unit's carrying amount of goodwill exceeds the
implied fair value of the goodwill. The fair value of Acacia Research
Corporation's reporting units are estimated using discounted cash flows and
other valuation techniques. Significant judgments and estimates are required in
determining forecasted cash inflows and outflows, the timing of cash flows and
discount rates commensurate with the risks involved.
Intangible Assets
We review long-lived assets, including patent related intangibles, for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Factors we consider important, which could trigger an impairment review include the following:
o significant underperformance relative to expected historical or
projected future operating results;
o significant changes in the manner of our use of the acquired assets or
the strategy for our overall business;
33
o significant negative industry or economic trends;
o significant adverse changes in legal factors or in the business
climate, including adverse regulatory actions or assessments; and
o significant decline in our stock price for a sustained period.
| · | significant underperformance relative to expected historical or projected future operating results; |
| · | significant changes in the manner of our use of the acquired assets or the strategy for our overall business; |
| · | significant negative industry or economic trends; |
| · | significant adverse changes in legal factors or in the business climate, including adverse regulatory actions or assessments; and |
| · | significant decline in our stock price for a sustained period. |
We calculate estimated future undiscounted cash flows, before interest and taxes, resulting from the use of the asset and its estimated value at disposal and compare it to its carrying value in determining whether impairment potentially exists. If a potential impairment exists, a calculation is performed to determine the fair value of the long-lived asset. This calculation is based on a valuation model and discount rate commensurate with the risks involved. Third party appraised values may also be used in determining whether impairment potentially exists.
As described above, in assessing the recoverability of goodwill and other
intangible assets, estimates of market values, estimates of the amount and projections regarding
estimatedtiming of future cash flows, and estimates of other factors are used to determine the fair value of the respective assets. If these estimates or related projections change in future periods, future goodwillintangible asset impairment tests may result in a chargecharges to earnings. ACCOUNTING FOR BUSINESS COMBINATIONS
The cost of an acquired business is assignedRefer to Note 6 to the tangible and
identifiable intangible assets acquired and liabilities assumedconsolidated financial statements, included elsewhere herein, for information on impairment charges recorded during the basisperiods presented.
Impairment of their fair values at the date of acquisition. We assess fair value using a
variety of methods, including the use of present value models, independent
appraisers, and estimation of current selling prices and replacement values.
Amounts recorded as intangible assets, including acquired in-process research
and development, or IPR&D, are based on assumptions and estimates regarding the
amount and timing of projected revenues and costs, appropriate risk-adjusted
discount rates, as well as assessing the competition's ability to commercialize
products before we can. Also, upon acquisition, we determine the estimated
economic lives of the acquired intangible assets for amortization purposes.
Actual results may vary from projected results.
On December 13, 2002, Acacia Research Corporation increased its
consolidated ownership interest in CombiMatrix Corporation from 48% to 100% by
acquiring from existing stockholders of CombiMatrix Corporation 11,987,052
shares of CombiMatrix Corporation common stock in exchange for 11,987,052 shares
of AR-CombiMatrix stock with a fair value of $46.0 million. The transaction was
accounted for as a step acquisition using the purchase method of accounting. Marketable Securities
The fair value of the AR-CombiMatrix stock issued in the transaction wasour investments is determined by quoted market prices. Realized and unrealized gains and losses are recorded based on the quoted market pricespecific identification method. We review impairments associated with our investments in marketable securities in accordance with Emerging Issues Task Force (“EITF”) 03-1 and FSP SFAS 115-1 and 124-1, “The Meaning of AR-CombiMatrix stock averaged over a five-day period
(from December 16, 2002,Other-Than-Temporary-Impairment and Its Application to Certain Investments,” to determine the first dayclassification of trading for the AR-CombiMatrix stock
through December 20, 2002). The total purchase price of $46.8 million, including
acquisition costs of $0.8 million, was allocated to the fair value of assets
acquired and liabilities assumed, including acquired in-process research and
development,any impairment as “temporary” or IPR&D. The amount attributable to CombiMatrix Corporation's core
technology and related patents was $5.3 million, which is being amortized using
the straight-line method over the estimated economic useful life of 7 years. The
amount attributable to IPR&D of $17.2 million was charged to expense and is
included“other-than-temporary.” For investments classified as available-for-sale, unrealized losses that are other than temporary are recognized in the consolidated statement of operations and comprehensive lossincome (loss) (hereinafter “consolidated statement of operations”). An impairment is deemed other than temporary unless (a) we have the ability and intent to hold an investment for a period of time sufficient for recovery of its carrying amount and (b) positive evidence indicating that the year ended December 31, 2002. Theinvestment's carrying amount attributable to goodwill was $16.0
million. Amounts allocated to patents, IPR&D and goodwill have been attributedis recoverable within a reasonable period of time outweighs any evidence to the CombiMatrix group.
As described above, $17.2 millioncontrary. All available evidence, both positive and negative, is considered to determine whether, based on the weight of that evidence, the carrying amount of the purchase price was allocatedinvestment is recoverable within a reasonable period of time.
We believe that our investments in certain auction rate securities continue to IPR&D projects that had not yet reached technological feasibility and had no
alternativeapproximate their par value; however, such risks, including the systemic failure of future use. Accordingly, this amount was immediately expensedauctions for auction rate securities, may result in a loss of liquidity, substantial impairment to our investments, realization of substantial future losses, or a complete loss of the investment in the 2002 consolidated statement of operations and comprehensive loss as of the
acquisition date. Management was responsible for determining the fair value of
the tangible and identifiable intangible assets acquired and liabilities
assumed, including IPR&D, at the date of acquisition. Management considered a
number of factors, including reference to independent valuations. Management
utilized an income approach to determine the fair values of tangible and
identifiable intangible assets acquired and liabilities assumed. The in-process
technologies were valued using a discounted cash flow model on a
project-by-project basis,long-term which estimated the cash flows expected to result from
each project once it has reached technological feasibility. A discount rate
consistent with the risks of each project was used to estimate the present value
of future cash flows. In estimating future cash flows, management considered the
contribution of its core technology (for which a United States patent was
obtained in July 2000) that would be required for successful exploitation of
purchased in-process technology, in order to value the core and in-process
technologies discretely. As a result, future cash flows relating to each
purchased IPR&D project were reduced in order to reflect the contribution of
core technology to each IPR&D project. The cash flows from these projects
attributable to core technology were then separately valued to determine the
intangible asset value of purchased core technology. In determining the
contribution of core technology to in-process projects, management used a profit
split approach which considered the estimated profit split between a licensor
and licensee of the core technology and management's assessment of how critical
the core technology was to the IPR&D projects.
34
The nature of the efforts to develop the purchased IPR&D into commercially
viable products principally relates to the completion and/or acceleration of
existing development programs. These efforts include testing current and
alternative materials used in array design, testing of existing and alternative
methods for array synthesis, developing prototype machinery (including operating
software) to synthesize, hybridize and read individual arrays, and to perform
numerous experiments, or assays, with actual target samples in order to
determine customer protocols and procedures for using the CombiMatrix group's
array system. The costs of these effortsmay have been included in the CombiMatrix
group's projections to successfully launch the purchased IPR&D projects. The
resulting net cash flows from such projects are based on management's estimates
of revenues, cost of sales, research and development expenses, sales and
marketing expenses, general and administrative expenses, the anticipated effect
of income taxes, and required returns on working capital, fixed assets and other
assets necessary to support the generation of these cash flows.
The discounting of net cash flows relating to core technology to their
present value is based on CombiMatrix Corporation's weighted average cost of
capital, or WACC. The WACC calculation produces the average required rate of
return of an investment in an operating enterprise, based on various required
rates of return from investments in various areas of that enterprise. The WACC
for CombiMatrix Corporation was approximately 20% at the time of the merger and
is the rate used in discounting the net cash flows attributable to purchased
core technology. Due to the additional inherent risks associated with the
purchased IPR&D projects, including if and when the technologies will ultimately
become commercially viable, market acceptance risks, and threats from competing
technologies, higher discount rates were used to value the projects. The
discount rates used for each project are described below.
The forecast data employed in the valuation analyses was based upon product
level forecast information obtained by Acacia Research Corporation from numerous
internal and external resources. These resources included publicly available
databases, external market research consultants, company-sponsored focus groups
and internal market experts. Management reviewed and challenged the forecast
data and related assumptions and utilized the information in analyzing IPR&D.
The forecast data and assumptions are inherently uncertain and unpredictable.
However, based upon the information available at this time, Acacia Research
Corporation management believes the forecast data and assumptions to be
reasonable. These assumptions may be incomplete or inaccurate and no assurance
can be given that unanticipated events and circumstances will not occur.
Accordingly, actual results may vary from the forecasted results. Any such
variance may result in a material adverse effect on Acacia Research
Corporation's financial condition andour business, results of operations.
In the allocationoperations, liquidity, and financial condition. See Note 2 of purchase priceour Notes to the IPR&D, the concept of
alternative future use was specifically consideredConsolidated Financial Statements for each of the programs
under development. The acquired IPR&D consists of CombiMatrix Corporation's work
to complete each of the identified programs. The programs are very specific to
research market for which they are intended. There are no alternative uses for
the in-process programsadditional details about our investments in the event that the programs fail in their continued
development or are otherwise not feasible. The development effort for the
acquired IPR&D does not possess an alternative future use for auction rate securities and other marketable securities.
22
Acacia Research Corporation as defined by generally accepted accounting principles. Following is
a brief description
Results of each IPR&D project.
GENOMICS BIOLOGICAL ARRAY SYSTEM: CombiMatrix Corporation's genomics
biological array processor system is being developed to discretely immobilize
sequences of DNA or RNA within individual test sites on a modified semiconductor
chip coated with a three-dimensional layer of porous material. The system also
includes proprietary hardware units and related software applications to be able
to synthesize materials onto the chips, apply target samples of genetic
materials and interpret the results. The application of this system will be in
gene expression profiling and SNP genotyping, which could lead to the better
understanding of gene function and ultimately therapeutic discovery to fight
disease. CombiMatrix Corporation's projected cash flow models from
commercializing this system include servicing CombiMatrix Corporation's existing
relationship with Roche as well as other strategic partners, including
pharmaceutical, biotech and academic institutions. The fair value assigned to
the genomics biological array system IPR&D project was $14.0 million. A
risk-adjusted discount rate of 32% was applied to the project's estimated cash
flows. The CombiMatrix group is substantially complete with the Genomics IPR&D
project contemplated at the acquisition date and launched its CustomArray(TM)
DNA Microarray platform in March 2004.
35
PROTEOMICS BIOLOGICAL ARRAY SYSTEM: CombiMatrix Corporation's proteomics
biological array processor system is being developed to discretely immobilize
proteins and other small molecules within individual test sites on a modified
semiconductor chip in a similar fashion as described above for the genomics
biological array system. However, the porous reaction layer coating used in
synthesis and manner in which the software used to design probes for protein
immobilization are significantly different from what is currently being
developed for the genomics application. Further, the proteomics biological array
system primarily uses electrochemical methods for detection of assay results,
which contrasts with the optical means that are the primary method used with the
CombiMatrix Corporation's genomics products. These differences arise largely due
to the inherent biological differences between DNA molecules and protein
molecules and how they interact with the CombiMatrix group's proprietary
technology. The proteomics biological array system is used for detection and
identification of bio-threat agents in CombiMatrix Corporation's biological and
chemical threat agent detector development programs that are currently in
process. Although ongoing research and development efforts in commercializing
this system have been positive, there can be no assurance that the system will
be successfully launched and accepted by the government, pharmaceutical, biotech
and academic research fields. The fair value assigned to the proteomics
biological array system IPR&D project was $3.2 million. A risk- adjusted
discount rate of 60% was applied to the project's estimated cash flows. The
Proteomics IPR&D project is ongoing as projected and the expected completion of
the project coincides with the expected completion of the CombiMatrix group's
obligations under its $5.9 million Department of Defense contract to further the
development of the CombiMatrix group's microarray technology for the detection
of biological threat agents. The Department of Defense contract was
approximately 34% complete as of December 31, 2004. Based on actual costs
incurred through December 31, 2004, the CombiMatrix group expects to incur
approximately $2.2 million and $819,000 in research and development costs during
2005 and 2006, respectively, to complete its obligations to the Department of
Defense under this contract. The CombiMatrix group has also contracted with
strategic partners such as Toppan and Furuno to further develop aspects of its
genomics array platform and continues to invest internally to develop and launch
new products. Such activities are consistent with the original projections.
MANAGEMENT AND ALLOCATION POLICIES RELATING TO AR-ACACIA TECHNOLOGIES STOCK AND
AR-COMBIMATRIX STOCK
The management and allocation policies applicable to the preparation of the
divisional financial statements of the CombiMatrix group and the Acacia
Technologies group (collectively, the "groups") may be modified or rescinded, or
additional policies may be adopted, at the sole discretion of the Acacia
Research Corporation board of directors at any time without approval of the
stockholders. The group divisional financial statements reflect the application
of the management and allocation policies adopted by the Acacia Research
Corporation board of directors to various corporate activities, as described
below. The group's divisional financial statements should be read in conjunction
with Acacia Research Corporation's consolidated financial statements and related
notes.
CORPORATE GENERAL AND ADMINISTRATIVE SERVICES AND FACILITIES
Acacia Research Corporation allocates the cost of corporate general and
administrative services and facilities between the groups generally based upon
utilization. Where determinations based on utilization alone are impracticable,
Acacia Research Corporation uses other methods and criteria, which require the
use of judgments and estimates, that management believes to be equitable and to
provide a reasonable estimate of the cost attributable to each group. Except as
otherwise determined by management, the allocated costs of providing such
services and facilities include, without limitation, all costs and expenses of
personnel employed in connection with such services and facilities, including,
without limitation, all direct costs of such personnel, such as payroll, payroll
taxes and fringe benefit costs (calculated at the appropriate annual composite
rate therefore) and all overhead costs and expenses directly related to such
personnel and the services or facilities provided by them. The corporate general
and administrative services and facilities allocated between the groups include,
without limitation, legal services, accounting services (tax and financial),
insurance and deductibles payable in connection therewith, employee benefit
plans and administration thereof, investor relations, stockholder services and
services relating to the Acacia Research Corporation board of directors.
Refer to Note 2 in the consolidated financial statements for details on
allocation methodologies used to allocate costs between the two groups.
36
ACACIA RESEARCH CORPORATION CONSOLIDATED
RESULTS OF OPERATIONS
NET LOSS (IN THOUSANDS)
Operations
Net Loss (In thousands)
| 2007 | 2006 | 2005 | ||||||||||
| Loss from continuing operations | $ | (7,359 | ) | $ | (5,363 | ) | $ | (6,038 | ) | |||
| Loss from discontinued operations - Split-off of CombiMatrix Corporation and other | (8,086 | ) | (20,093 | ) | (12,638 | ) | ||||||
| Net loss | (15,445 | ) | (25,456 | ) | (18,676 | ) | ||||||
The changes in consolidated net loss were primarily due to operating results and activities, as discussed below.
Revenues (In thousands)
| 2007 | 2006 | 2005 | ||||||||||
| License fees | $ | 52,597 | $ | 34,825 | $ | 19,574 | ||||||
License Fees. Revenues for 2007 included license fees from 91 new licensing agreements covering 16 of its researchour technology licensing and development agreement with Roche. As a
resultenforcement programs, as compared to 72 new licensing agreements covering 14 of completing all obligations under this agreementour technology licensing and enforcement programs in accordance with
the CombiMatrix group's revenue recognition policies for multiple-element
arrangements, the CombiMatrix group recognized $17.3 million2006, and 83 new licensing agreements covering 12 of researchour technology licensing and development contract revenues during the first quarter of 2004, all of which
were previously deferred.enforcement programs in 2005. The majority of research and development efforts under
the Roche agreement were incurred prior to 2004.
LICENSE FEES. Licenseincrease in license fee revenues are comprisedin 2007 and 2006, as compared to 2005, reflects the impact of DMT technology license
feesthe increase in patent portfolios owned or controlled by our operating subsidiaries since 2005, and previously deferred V-chip technology license fees recognized by the Acacia Technologies group. DMT technology license fees were $2.8 million and
$692,000 in 2004 and 2003, respectively. Therelated increase was primarily due to the
significant growth in the number of DMT technology license agreements executedpatent licensing and enforcement programs developed, launched and generating revenues since March2005. On a consolidated basis, as of December 31, 2003. During 2004, we executed 170 DMT license agreements.2007, 28 of our licensing programs had begun generating licensing revenues, up from 20 as of December 31, 2006 and 13 as of December 31, 2005. License fee revenues willrecognized by our operating subsidiaries fluctuate from period to period primarily based on the increase
in license agreements executed, fluctuations in the sales results or other
royalty per unit activities of our licensees that impact the calculation of
license fees due, the timing of the receipt of periodic license fee statements
and or payments from licensees, and other factors. Periodic license fee revenues
may include amounts that relate to prior license periods or prior periods of
infringement, which are recognized as revenues in the period received. DMT
license fees related to prior periods of infringement for the periods presented
above were not significant. following factors:
| · | the dollar amount of agreements executed each period, which is primarily driven by the nature and characteristics of the technology being licensed and the magnitude of infringement associated with a specific licensee; |
| · | the specific terms and conditions of agreements executed each period and the periods of infringement contemplated by the respective payments; |
| · | fluctuations in the total number of agreements executed; |
| · | fluctuations in the sales results or other royalty per unit activities of our licensees that impact the calculation of license fees due; |
| · | the timing of the receipt of periodic license fee payments and/or reports from licensees; and |
| · | fluctuations in the net number of active licensees period to period. |
Costs incurred in connection with the Acacia
Technologies group's ongoingour operating subsidiaries licensing and enforcement activities, other than inventor royalties expense, contingent legal fees expense and patent-related legal expenses, are included in marketing, general and administrative expenses.
License
Operating Expenses (In thousands)
| 2007 | 2006 | 2005 | ||||||||||
| Marketing, general and administrative expenses (including non-cash stock compensation | ||||||||||||
| expense of $5,908 for 2007, $3,946 for 2006 and $356 for 2005) | $ | 20,042 | $ | 14,123 | $ | 8,097 | ||||||
| Inventor royalties and contingent legal fees expense - patents | 29,224 | 17,159 | 11,331 | |||||||||
| Legal expenses - patents | 7,024 | 4,780 | 2,468 | |||||||||
| Write-off of patent-related intangible asset | 235 | 297 | - | |||||||||
| Amortization of patents | 5,583 | 5,313 | 4,922 | |||||||||
Marketing, General and Administrative Expenses. Marketing, general and administrative expenses consist of compensation and related personnel costs including non-cash stock compensation expenses, office and facilities costs, legal and accounting professional fees, public relations, marketing, stock administration and other corporate costs, and patent related development, commercialization, research, consulting and maintenance costs.
Excluding the impact of non-cash stock compensation charges discussed below, the net increase for the periods presented was due primarily to the addition of licensing, business development and engineering personnel and an increase in other personnel related expenses in each comparable period, a one-time severance charge for an employee separation under the Acacia Research Corporation Executive Severance Plan in the first quarter of 2007, an increase in patent acquisition and business development related research and consulting costs and an increase in corporate, general and administrative costs, including an increase in accounting fees related to the CombiMatrix Split-off Transaction. The overall increase in marketing, general and administrative expenses is reflective of the continued growth and expansion of our intellectual property acquisition, licensing and enforcement business conducted by our operating subsidiaries and related ongoing operations. In 2007, as compared to 2006, these increases were partially offset by a decrease in consulting expenses due to the expiration of the consulting agreement with the former CEO of Global Patent Holdings, LLC in January 2007. The increases in 2006, as compared to 2005, were partially offset by a reduction in Acacia Research Corporation’s Sarbanes-Oxley compliance costs.
23
Non-cash stock compensation charges increased during 2007, as compared to 2006, due to the issuance of equity based incentive awards to new and existing employees during 2007. In addition, the increase in non-cash stock compensation expense also reflects the increase in the AR-Acacia Technologies stock price during 2007, as compared to 2006, as illustrated at Item 5. “Recent Market Prices” above. The weighted average fair value of stock options granted during 2007 and 2006 was $9.07 and $5.35, respectively. The weighted average fair value of nonvested restricted shares granted during 2007 and 2006 was $14.04 and $11.87, respectively (see Note 11 to the consolidated financial statements for information on the determination of fair value for share based awards under SFAS No. 123R). The increase in non-cash stock compensation charges in 2006, as compared to 2005, reflects our adoption of SFAS No. 123R, effective January 1, 2006, which required public companies to measure all employee stock-based compensation awards using a fair-value method and record such expense in their consolidated financial statements, as described under “Critical Accounting Estimates.”
A summary of the main drivers of the change in marketing, general and administrative expenses, including the impact of non-cash stock compensation charges, for the periods presented, is as follows (in thousands):
| 2007 vs. 2006 | 2006 vs. 2005 | |||||||
| Increase in personnel expenses | $ | 2,767 | $ | 1,247 | ||||
| Increase (decrease) in GPH Acquisition related consulting expenses | (925 | ) | 96 | |||||
| One-time severance charge for employee separation | 360 | - | ||||||
| Increase in foreign taxes paid on licensing fees | 125 | 6 | ||||||
| Increase in accounting and other professional fees | 141 | 392 | ||||||
| Increase in patent development / commercialization and other | 1,021 | 92 | ||||||
| Increase in office and facilities and other marketing, general and administrative costs | 468 | 603 | ||||||
| Increase in non-cash stock compensation expense | 1,962 | 3,590 | ||||||
Inventor Royalties and Contingent Legal Fees Expense. Inventor royalties expense totaled $12.1 million and $9.6 million in 2007 and 2006, respectively, and contingent legal fees expense totaled $17.2 million and $7.5 million in 2007 and 2006, respectively. Inventor royalties expenses and contingent legal fees expenses for the periods presented were incurred in connection with the recognition of the related license fee revenues, summarized above. The majority of the patent portfolios owned or controlled by our operating subsidiaries are subject to patent and patent rights agreements with inventors containing provisions granting to the original patent owner the right to receive inventor royalties based on future net revenues, as defined in the respective agreements, and may also be subject to contingent legal fee arrangements with external law firms engaged on a contingent fee basis. The economic terms of the inventor and contingent fee arrangements, if any, vary across our patent portfolios. As such, inventor royalties and contingent legal fee expenses fluctuate period to period based on the amount of revenues recognized each period and the mix of specific patent portfolios, with varying economic terms, generating revenues each period.
The increase in inventor royalties expense and contingent legal fees expense in 2007 was due in part to the increase in license fee revenues recognized in 2007, as compared to 2006, as discussed above, and also reflects the impact of the mix of patent portfolios with varying economic terms generating the revenues during the respective periods. A portion of 2006 revenues were comprised of license fees from patent portfolios that were not subject to a contingent legal fee arrangement, whereas the majority of patent portfolios generating revenues in 2007 were subject to contingent legal fee arrangements. As a result, in 2006, no contingent legal fees expense was incurred on a portion of revenues, resulting in the 128% increase in contingent legal fees expense in 2007, as compared to 2006, as compared to 51% increase in license fee revenues for 2004 include $1.5 millionthe same periods.
Results in previously deferred2005 included $225,000 of V-chip license fees (originally deferred in 2001)related inventor royalties expense recognized as a result of the conclusion of all V-chip related litigation activities in August 2004, as described at Item 3.
"Legal Proceedings." We concluded our V-chipOctober of 2005.
Legal Expense – Patents. Patent-related legal expenses include patent-related prosecution and enforcement costs incurred by outside law firms engaged on an hourly basis and the out-of-pocket expenses incurred by outside law firms engaged on a contingent fee basis. Patent-related legal expenses fluctuate from period to period based on patent enforcement and prosecution activity associated with ongoing licensing program in August 2004
and do not expect to receive any additional V-chip related revenues in future
periods.
GOVERNMENT CONTRACT AND COST OF GOVERNMENT CONTRACT REVENUES. In March
2004,enforcement programs and the CombiMatrix group executed a two-year $5.9 million research and
development contract with the Department of Defense to further the developmenttiming of the CombiMatrix group's array technologycommencement of new licensing and enforcement programs in each period. Patent-related legal expenses include case related costs billed by outside counsel for discovery, depositions, economic analyses, damages assessments, expert witnesses and other consultants, case related audio/video presentations for the detectioncourt, and other litigation support and administrative costs.
24
The increase in patent related legal expenses in 2007, as compared to 2006, is primarily due to a net increase in ongoing patent enforcement litigation and an increase in litigation support related out of biological
threat agents. Under the terms of the contract, the CombiMatrix group is
reimbursed on a periodic basis for actual costs incurred to perform its
obligations, plus a fixed fee. Revenues are recognized under the
percentage-of-completion method of accounting, using the cost-to-cost approach
to measure completeness at the end of each reporting period. Cost of government
contract revenues reflect researchpocket expenses, third party technical consulting expenses and developmentprofessional expert expenses incurred in connection with certain of our patent portfolios that are further along in the CombiMatrix group's commitments under its biowarfare
detection contractprosecution of the related litigation and certain of our patent portfolios that have proceeded to trial and concluded. The increase in patent related legal expenses in 2006, as compared to 2005, is primarily due to a net increase in ongoing patent enforcement litigation in each respective period, and to a lesser extent, an increase in the number of portfolios where we have engaged outside law firms on an hourly or discounted hourly basis.
We expect patent-related legal expenses to continue to fluctuate period to period based on the factors summarized above, in connection with current and future patent commercialization and enforcement programs.
Amortization of Patents. The increase in 2007, as compared to 2006, was due to additional patent amortization charges related to certain patent portfolios acquired by our operating subsidiaries in late 2006 and throughout 2007. Patent amortization charges will continue to be significant in future periods as we continue to amortize the Departmentacquired patent related costs over a weighted-average remaining economic useful life of Defense, whichapproximately four years.
The increase in 2006, as compared to 2005, was approximately 34%
completedue to twelve full months of patent amortization expense resulting from the GPH Acquisition in 2006, as compared to 11 months of December 31, 2004. Based on actualamortization in 2005. Patent amortization expense related to the GPH Acquisition was $4.7 million and $4.4 million in 2006 and 2005, respectively. In addition, patent amortization expense in 2006 and 2005 includes additional patent amortization charges related to certain of the patent portfolios acquired by our operating subsidiaries subsequent to the GPH Acquisition.
Refer to “Liquidity and Capital Resources” below for patent portfolio acquisition costs incurred through
December 31, 2004,during the CombiMatrix group expects to incur approximately $2.2
million and $819,000 in research and development costs during 2005 and 2006,
respectively, to complete its obligationsperiods presented.
Write-off of Patent-related Intangible Asset. In September 2007, we recorded a non-cash impairment charge of $235,000, related to the Departmentwrite-off of Defense under
this contract.
37
Government contract revenues in 2002 included amounts earned from the
CombiMatrix group's performance under its Phase I SBIR Department of Defense
contract, Phase I NIH grant and one-time contract research and development
revenues.a patent-related intangible asset. The SBIR Department of Defense and NIH grants wererelated licensing program was completed during the third quarter of 2002.
SERVICE CONTRACTS. Service contract revenues include maintenance and
service contract fees recognized by CombiMatrix K.K. from existing array
customers2007 resulting in Japan. Asthe write-off of December 31, 2004,the remaining carrying value of the patent-related intangible asset as of September 30, 2007. In June 2006, we recorded a non-cash impairment charge of $297,000, related to the write-off of a patent-related intangible asset. During the second quarter of 2006, pursuant to the terms of these contracts had
expired.
PRODUCT REVENUES AND COST OF PRODUCT SALES. Product revenuesthe respective license agreement, management elected to terminate its rights to exclusively license and costs of
product sales during 2004 relate to domestic and international salesenforce the patent, resulting in the write-off of the remaining carrying value of the patent-related intangible asset as of June 30, 2006.
Discontinued Operations – Split-off of CombiMatrix group's array products. TheCorporation
In January 2006, Acacia Research Corporation’s board of directors approved a plan for its wholly owned subsidiary, CombiMatrix group launched its
CustomArray(TM) 902 DNA array platformCorporation, to become an independent public company. CombiMatrix Corporation’s registration statement on Form S-1 was declared effective by the SEC on June 8, 2007. Following the redemption period required by Acacia Research Corporation’s Restated Certificate of Incorporation, on August 15, 2007 (the “Redemption Date”), CombiMatrix Corporation was split-off from Acacia Research Corporation through the redemption of all outstanding shares of AR-CombiMatrix common stock in March 2004 and its CustomArray(TM) 12K
DNA expression array in July 2004. Product revenues and costexchange for the distribution of product sales
during 2003 and 2002 were recognized bynew shares of CombiMatrix K.K. from sales of DNA array
synthesizers and related array products and services to Japanese research
institutions.
RESEARCH AND DEVELOPMENT EXPENSES (IN THOUSANDS)
RESEARCH AND DEVELOPMENT EXPENSES. The decrease in research and development
expenses in 2004, as compared to 2003 and 2002, was primarily dueCorporation, on a pro-rata basis, to the CombiMatrix group's completionholders of several Roche related research and development
projects during the third and fourth quarters of 2003, and final completionAR-CombiMatrix stock as of the research and development agreement with Roche inRedemption Date (the “Split-off Transaction”). On the first quarterRedemption Date, every ten (10) shares of 2004.
During 2003 and 2002,AR-CombiMatrix stock outstanding on August 15, 2007, was redeemed for one (1) share of common stock of CombiMatrix Corporation. Subsequent to the CombiMatrix group's research and development
activities were driven primarily by ongoing performance obligations under the
product commercialization phase of its license and research and development
agreements with Roche. These activities include costs associated with direct
labor, supplies and materials, development of prototype arrays and instruments
and the use of outside consultants for certain engineering efforts.
With the completion of the research and development agreement with Roche,
year-to-date and future research and development expenses were and will continue
to be incurred in connection with the CombiMatrix group's commitments under its
collaboration and supply agreements with various strategic partners including
Furuno and Toppan, as well as ongoing internal research and development efforts
in the areas of genomics, drug discovery and development and material sciences.
The CombiMatrix group expects its research and development expenses to continue
to be volatile and such expenses could increase in future periods as additional
contract and/or internal research and development agreements are undertaken.
CHARGE FOR ACQUIRED IN-PROCESS RESEARCH AND DEVELOPMENT. Operating expenses
in 2002 include a non-cash charge for acquired in-process research and
development of $17.2 million, related toRedemption Date, Acacia Research Corporation's purchase
of the stockholderCorporation no longer owns any equity interests in CombiMatrix Corporation and the two companies operate independently of each other.
As a result of the Split-off Transaction, the assets, liabilities, results of operations and cash flows of CombiMatrix Corporation have been eliminated from the continuing operations of Acacia Research Corporation and Acacia Research Corporation does not have any continuing involvement in the operations of CombiMatrix Corporation. As a result of the Split-off Transaction, we have disposed of our investment in CombiMatrix Corporation, and therefore, in accordance with guidance set forth in SFAS No. 144, Acacia Research Corporation’s accompanying consolidated balance sheets, statements of operations and statements of cash flows for the current period presented reflect the assets, liabilities, results of operations and cash flows for CombiMatrix Corporation as discontinued operations. Consolidated financial statements presented for the comparable prior year periods have been restated to conform to this presentation. CombiMatrix Corporation was previously presented as a separate operating segment of Acacia Research Corporation under SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information.”
25
The Split-off Transaction was accounted for by Acacia Research Corporation at historical cost. Accordingly, no gain or loss on disposal was recognized in the accompanying consolidated statements of operations. Included in the current period consolidated balance sheet is a charge to consolidated shareholders’ equity totaling $35,444,000, reflecting the distribution of our investment in the net assets of CombiMatrix Corporation to holders of AR-CombiMatrix stock, as of the Redemption Date, as described above. We received a private letter ruling from the IRS with regard to the U.S. federal income tax consequences of the Split-off Transaction to the effect that we did not already
own in December 2002. See "Critical Accounting policies" abovethe Split-Transaction will be treated as a tax-free exchange under Sections 368 and 355 of the Internal Revenue Code of 1986, as amended.
Refer to Note 810A to the Acacia Research Corporation consolidated financial statements included elsewhere herein for information regarding the allocationcarrying amount(s) of the purchase pricemajor classes of assets, liabilities, revenues and pretax loss included in discontinued operations for the accountingperiods presented.
Other Discontinued Operations. Results for acquired in-process research and development.
MARKETING, GENERAL AND ADMINISTRATIVE EXPENSES AND LEGAL SETTLEMENT CHARGES (IN
THOUSANDS)
MARKETING, GENERAL AND ADMINISTRATIVE EXPENSES. The change in 2004, as
compared to 2003, was due primarily to an increase in personnel costs2005 include charges, net of minority interests, of $237,000 related to the addition of patent licensing and business development personnel for the
Acacia Technologies group of $418,000, an increase in the Acacia Technologies
group's patent related commercialization expenses of $110,000, an increase in
corporate professional fees relatedestimated additional costs to ongoing Sarbanes-Oxley compliance
projects at both operating groups of $621,000 and an increase in marketing and
sales costs of $447,000 related to the launch of the CombiMatrix group's
CustomArray(TM) DNA array platform beginning in March 2004 and the overall
expansion of the CombiMatrix group's sales and marketing division.
The change in 2003, as compared to 2002, was due primarily to a decrease in
the CombiMatrix group's corporate legal expenses of approximately $827,000,
primarily related to the settlement of litigation with Nanogen, a decrease in
legal, accounting and other professional fees related to Acacia Research
Corporation's recapitalization and merger transactions completed in December
2002 of $2.0 million, a decrease in marketing and sales personnel costs,
primarily for the CombiMatrix group, and general and administrative personnel
38
costs for both groups of $1.2 million and a decrease in other general and
administrative costs of $578,000. This decrease was partially offset by an
increase in the CombiMatrix group's rent and utilities expenses of $473,000, as
a result of the increase in occupancy of its corporate headquarters in Mukilteo,
Washington during 2003, and an increase in costs related to the Acacia
Technologies group's ongoing DMT patent commercialization and enforcement
efforts of $219,000, including increased consulting and engineering costs
related to new patent claims, enforcement and the identification of additional
potential licensees of our DMT technology.
LEGAL EXPENSE - PATENTS (ACACIA TECHNOLOGIES GROUP ONLY). Patent related
legal expenses in 2004 included $668,000 in deferred V-chip related legal fees
recognized as a result of the conclusion of V-chip related litigation in August
2004, as described at Item 3. "Legal Proceedings." The Acacia Technologies
group's patent related legal expenses, excluding V-chip related legal fees,
increased to $2.5 million during 2004, as compared to $1.9 million, and $1.4
million in the comparable 2003 and 2002 periods, due to an increase in costsbe incurred in connection with the Acacia Technologies group's ongoing DMT patent
commercializationdiscontinued operations of Soundbreak.com (originally ceased operations in February 2001), related primarily to certain noncancellable lease obligations and enforcement programs, including increased legal costsa reduction in estimated amounts recoverable from existing sublease arrangements. The related to new patent claims and the identification of additional potential
licensees of our DMT technology and increased patent enforcement costs related
to ongoing DMT patent related litigation. See Item 3. "Legal Proceedings" for a
description of ongoing DMT related litigation. We expect patent related legal
expenses to continue to fluctuate based on actual outside patent counsel fees
incurred in connection with the Acacia Technologies group's ongoing DMT and
other patent commercialization and enforcement programs. DMT related legal fees
to outside attorneys are charged based on actual time and out-of-pocket expenses
incurredlease obligations, which were guaranteed by external counsel and are not incurred on a contingent fee basis.
In connection with the January 2005 acquisition as described above, we
acquired 27 additional patent portfolios. Although most litigation with respect
to those portfolios is likely to be handled on a contingency basis where
attorneys fees are paid out of license fee revenues collected, certain other
costs and expenses in connection with our maintenance, licensing, and
enforcement of patents are likely to increase as a result of the acquisition
including patent filing fees, patent development costs, travel costs, expert and
consulting fees, and other third-party expenses.
LEGAL SETTLEMENT CHARGES. In 2002, in connection with the September 2002
settlement agreement between CombiMatrix Corporation, Dr. Donald Montgomery, and
Nanogen (see Note 13 to the Acacia Research Corporation, consolidated financial
statements included elsewhere herein), the CombiMatrix group expensed a $1.0
million payment to Nanogen and recorded a non-cash chargeexpired in the amount of $17.5
million related to the fair value of CombiMatrix Corporation common shares
issued to Nanogen. In addition, the CombiMatrix group recorded a net non-cash
charge totaling $812,000 during 2004, which reflects the fair value of
AR-CombiMatrix common stock issued and potentially issuable to Nanogen, Inc.
during the period in connection with certain anti-dilution provisions of the
settlement agreement. Periodic charges and the related liability are estimated
based on the number of shares issuable and or potentially issuable and the
AR-CombiMatrix stock price at the end of the respective reporting period. The
anti-dilution provisions of the settlement agreement expire in SeptemberAugust 2005.
NON-CASH STOCK COMPENSATION AMORTIZATION (IN THOUSANDS)
The decrease in the CombiMatrix group's non-cash stock compensation
amortization was primarily due to the accelerated method of stock compensation
amortization utilized, which results in higher amounts of amortization in the
earlier vesting periods and the impact of non-cash stock compensation expense
reversals related to the forfeiture of certain unvested stock options during the
respective periods. Non-cash stock compensation expense reversals totaled
$185,000 in 2004 and $1.2 million in both 2003 and 2002. Non-cash deferred stock
compensation amounts were fully amortized as of December 31, 2004.
IMPAIRMENT CHARGES (IN THOUSANDS)
39
GOODWILL IMPAIRMENT CHARGE. In August 2004, as a result of the adverse
ruling in the Soundview Technologies litigation described at Item 3. "Legal
Proceedings," the Acacia Technologies group recorded a non-cash impairment
charge, included in operating expenses, totaling $1.6 million associated with
the write-down of 100% of the goodwill related to the V-chip.
IMPAIRMENT OF COST METHOD INVESTMENT. In 2003, we recorded an impairment
charge of $207,000 for an other-than-temporary decline in the fair value of our
cost method investment. Impairment indicators included a continued decline in
the working capital of the entity and reference to a recent equity transaction
and related valuation indicating an other-than-temporary decline in fair value
of the investment. In 2002, we recorded an impairment charge of $2.7 million for
an other-than-temporary decline in the fair value of the same cost method
investment. Impairment indicators included recurring losses, a decline in
working capital and the completion of a recent equity transaction at an amount
below our carrying value.
AMORTIZATION OF PATENTS (IN THOUSANDS)
The decrease in 2003, as compared to 2002, was due to a reduction in patent
amortization expense due to V-chip technology related patent costs and other
intangibles that were fully amortized during 2002. With the acquisition of the
assets of Global Patent Holdings, as described above, we expect that a
significant portion of the approximated $25.0 million purchase price will be
allocated to the patents acquired, which will be amortized over the estimated
economic useful lives of the respective patents, resulting in increased
amortization charges in 2005 and future periods. See Note 15 to the Acacia
Research Corporation consolidated financial statements for additional
information.
REALIZED AND UNREALIZED GAINS/LOSSES (IN THOUSANDS)
The decrease in the Acacia Technologies group's realized and unrealized
gains/losses on short-term investments was due to no investments classified as
trading securities held during 2004 and 2003 and the sale of the balance the
Acacia Technologies group's trading securities during 2002.
INCOME TAXES (IN THOUSANDS)
The 2004, 2003 and 2002 income tax benefits primarily reflect the impact of
the reversal of deferred tax liabilities related to the amortization of
identifiable intangible assets related to certain of Acacia Research
Corporation's step acquisitions in 2002, 2001 and 2000. $569,000 of the 2002
income tax benefit also reflects the impact of differences between the 2001
income tax provision and Acacia Research Corporation's final 2001 consolidated
tax return filed in September 2002, and is the result of additional deductions
against Soundview Technologies taxable income.
MINORITY INTERESTS (IN THOUSANDS)
The decrease is due to Acacia Research Corporation's acquisition of the
remaining ownership interests in Soundview and Acacia Media Technologies in
2001, CombiMatrix Corporation in December 2002, and CombiMatrix K.K. in July
2003 and approximately 99% of the remaining ownership interests in Advanced
Material Sciences in July 2003.
INFLATION
Inflation
Inflation has not had a significant impact on Acacia Research Corporation or any of our subsidiaries in the current or prior periods.
40
LIQUIDITY AND CAPITAL RESOURCES
Liquidity and Capital Resources
Acacia Research Corporation'sCorporation’s consolidated cash and cash equivalents along
withand short-term investments totaled $52.4$51.4 million at December 31, 2004,2007, compared to $50.5$45.0 million at December 31, 2003.2006. Working capital, related to continuing operations, at December 31, 20042007 was $49.2$48.1 million, compared to $31.1$42.2 million at December 31, 2003. At December 31,
2003 working capital included $17.7 million in deferred revenues primarily
related to the CombiMatrix group's agreements with Roche and Toppan, which are
not subject to any refund or repayment obligations and do not represent payment
obligations of Acacia Research Corporation. 2006.
The increase in working capital in
2004 was due primarily to the recognition of $17.3 million in deferred Roche
related contract revenues in the first quarter of 2004 and the impact of net cash flow activities as discussed below.
The changeschange in cash and cash equivalents and short-term investments related to continuing operations for 2004, 20032007, 2006 and 2002 were2005 was comprised of the following (in thousands):
The
| 2007 | 2006 | 2005 | ||||||||||
| Net cash provided by (used in) continuing operations: | ||||||||||||
| Operating activities | $ | 5,166 | $ | 6,608 | $ | (2,608 | ) | |||||
| Investing activities | (2,145 | ) | 10,513 | (13,094 | ) | |||||||
| Financing activities | 5,014 | 1,475 | 19,836 | |||||||||
Operating Activities. License fee payments received from licensees increased to $51.4 million in 2007, compared to $38.6 million in 2006, reflecting the increase in net cash outflows from operations for the CombiMatrix grouplicense fee revenues recognized in 2004,2007, as compared to 2003,2006, as discussed above. The decrease in consolidated net cash inflows from operations in 2007, as compared to 2006, was primarily due to a decreasethe increases in operating cash
receipts from customers, which totaled $3.0 million in 2004, comprised of $1.7
million from the Department of Defense, $1.0 million from Furunopatent-related legal expenses, personnel expenses, and $265,000
from the sale of array productsother corporate, general and related services, compared to $12.8 million
in the comparable 2003 period, consisting primarily of $9.8 million related to
the completion of certain milestones and delivery of prototype products and
services pursuant to its agreements with Roche and an up-front payment of $1.0
million and a $1.4 million milestone payment pursuant to its agreement with
Toppan. The decrease in payments from customers was partially offset by the
decrease in the CombiMatrix group's operatingadministrative expenses, as described above, and the impact of the timing of the receipt of payments from customers and payments to inventors, attorneys and other vendors. Consolidated accounts receivable increased to $1.4 million at December 31, 2007, compared to $269,000 at December 31, 2006. The majority of accounts receivable from licensees at December 31, 2007 was collected in January 2008, in accordance with the terms of the related underlying license agreements.
The change to consolidated net cash inflows from operations in 2006, as compared to net cash outflows from operations for the Acacia Technologies groupin 2005, was primarily due to anthe increase in DMT license fee payments received from licensees, which totaled $3.1$38.6 million in 2004, as2006, compared to $665,000,$15.6 million in 2003,
which2005. The increase in license fee revenues in 2006 was partially offset by the increaseincreases in marketing,inventor royalties expenses, contingent legal fees expenses, patent-related legal expenses, personnel expenses, and other corporate, general and administrative and patent related research, commercialization and legal expenses, as described above, and the impact of the timing of vendor payments.
The decrease in net cash outflows from operations in 2003, as comparedpayments to 2002, was primarily attributableinventors, attorneys and other vendors. Consolidated accounts receivable decreased to the reduction in research and development
expenses incurred by the CombiMatrix group and the decrease in consolidated
marketing, general and administrative expenses, which were partially offset by
the impact of the timing of vendor payments. The change was also due to an
increase in operating cash receipts during 2003 from Roche and Toppan, as
discussed above. Toppan related cash payments are included in deferred revenues$269,000 at December 31, 2004 and 2003. In 2002,2006, compared to $4.4 million at December 31, 2005, due to the operating cash receipts were
primarily from Roche, totaling $11.6 million.
collection of license fees receivable at December 31, 2005, during the first quarter of 2006, in accordance with the terms of the related underlying license agreements.
Investing Activities. The change in net cash flows used in investing activities was due primarily
to Acacia Research Corporation'sfor the periods presented reflects fluctuations in net purchases and sales of available-for-sale investments in connection with ongoing short-term cash management activities. Short-term investments represent capital available to fund current operations and fund capital expenditures. Net cash outflows from investing activities for 2005 also included the impact of cash consideration and changes in short term investmentsrelated acquisition and registration costs, totaling $5.8 million, paid by Acacia Global Acquisition Corporation, in connection with the GPH Acquisition in the first quarter of 2005. In addition, certain financing
activities discussed below. Fixed asset purchases were $891,000, $86,000of our operating subsidiaries incurred patent acquisition costs of $3.8 million, $1.0 million and $1.1 million$445,000 in 2004, 20032007, 2006 and 2002, respectively.
The change in2005, respectively, related to the acquisition of additional patent portfolios, as described earlier.
26
Financing Activities. Consolidated net cash flows provided byinflows from financing activities in 2004, as
compared to 2003, was due to the completion of an equity financing which raised2005 included net proceeds of approximately $13.7$19.5 million, throughrelated to the sale of 3.5 million shares of AR–Acacia Research
- - CombiMatrix commonTechnologies stock during 2004, compared to equity financing net
proceeds of $4.9 million during the comparable 2003 period. Financing activities
in 2004 also included proceeds, primarilyFebruary 2005. Net cash inflows from the exercise of Acacia Research -
CombiMatrix common stock warrants and stock options, totaling $5.2 million,
compared to $1.1 million in the comparable 2003 period. Net proceeds from the
sale of Acacia Research-CombiMatrix common stock, or AR-CombiMatrix stock, were
attributed to the CombiMatrix group. Cash used in financing activities in 2002
was comprised primarily of payments on the CombiMatrix group's capital lease
obligation totaling $2.8 million (the capital lease obligation was paid in full
in November 2002)2007, 2006 and net capital distributions to minority shareholders of
subsidiaries of $318,000, which were partially offset by proceeds from2005 also included stock option exercises of $242,000.
In February 2005, Acacia Research Corporation raised grossexercise proceeds of $19.6$5.0 million, through the sale of 3,500,000 shares of Acacia Research - Acacia
Technologies common stock at a price of $5.60 per share in a registered direct
offering. Net proceeds raised of approximately $19.58 million, which are net of
related issuance costs, were attributed to the Acacia Technologies group. All of
the shares of Acacia Research-Acacia Technologies common stock were offered
pursuant to an effective shelf registration statement previously filed with the
Securities and Exchange Commission. Total maximum proceeds of the shelf
registration are $50 million, of which $15.4 million remains available
subsequent to the February 2005 direct offering described above.
41
We have sustained losses since our inception contributing to an accumulated
deficit of $188.2 million on a consolidated basis, which includes net losses of
$4.8 million, $24.4$1.5 million, and $59.0 million 2004, 2003 and 2002,$304,000, respectively.
Net losses include significant non-cash acquired in-process research and
development, litigation and stock compensation charges as reflected in the
accompanying Acacia Research Corporation consolidated results of operations data
for 2004, 2003 and 2002. The consolidated accumulated deficit includes a
non-cash increase related to a reclassification of accumulated deficit in the
amount of $21.7 million to permanent capital representing the fair value of the
ten percent (10%) stock dividend distributed to stockholders in 2001.
There can be no assurance that we will become profitable. If we do, we may
never be able to sustain profitability. We expect to incur losses for the
foreseeable future. We continue to closely monitor and manage operating expenses
and capital expenditures and may take steps to raise additional capital.
Management believes that ourAcacia Research Corporation’s consolidated cash and cash equivalent balances, including
the net proceeds of the February 2005 equity financing and related availability
under the shelf registration statement described above, anticipated cash flow from operations and other external sources of available credit, will be sufficient to meet ourits cash requirements through at least March 2009 and for the next twelve
months. There can be no assurances that we will notforeseeable future. Acacia Research Corporation and its subsidiaries may however encounter unforeseen difficulties that may deplete our capital resources more rapidly than anticipated.anticipated, including those set forth in Item 1A. “Risk Factors” included elsewhere herein. Any efforts to seek additional funding could be made through equity, debt or other external financing and there can be no assurance that additional funding will be available on favorable terms, if at all. If weAcacia Research Corporation and its subsidiaries fail to obtain additional funding when needed, they may not be able to execute their business plans and their businesses may suffer. Refer to the “Liquidity and Risks” discussion included in Note 1 to the Acacia Research Corporation consolidated financial statements included elsewhere herein for additional information.
As of March 5, 2008, we held $6.3 million of investment grade securities with an auction reset feature (“auction rate securities”). Our auction rate securities consist of securities issued by closed-end investment companies with portfolio asset coverage of at least 200%, and auction rate investments backed by student loans, issued under programs such as the Federal Family Education Loan Program, all of which had credit ratings of AAA when purchased. The Dutch auction process that resets the applicable interest rate at predetermined calendar intervals is intended to provide liquidity to the holder of auction rate securities by matching buyers and sellers within a market context enabling the holder to gain immediate liquidity by selling such interests at par or rolling over their investment. If there is an imbalance between buyers and sellers the risk of a failed auction exists.
We have not experienced a failed auction for any of our securities as of December 31, 2007. However, we did experience failed auctions with certain of our issues in February 2008. Given the deteriorating credit markets, and the increased incidence of failure within the auction market in February 2008, there can be no assurance as to when we would be able to liquidate a particular issue. In such case of a failure, the auction rate securities continue to pay interest in accordance with their terms, however, we may not be able to executeaccess the par value of the invested funds until a future auction of these investments is successful, the security is called by the issuer or a buyer is found outside of the auction process. Furthermore, if this situation were to persist despite our business plansability to hold such investments until maturity, we may be required to record an impairment charge in a future period.
Management believes that the fair value of our investments in auction rate securities continue to approximate their par value and that the underlying credit quality of the assets backing its auction rate securities investments have not been impacted by the reduced liquidity of these investments subsequent to December 31, 2007. We will continue to monitor and evaluate our business may suffer.investments in auction rate securities for any further reduction in liquidity and potential impairment in future periods. See the CombiMatrix group and the
Acacia Technologies group discussion and analysisNote 2 of our Notes to Consolidated Financial Statements for additional factors
impacting the adequacy ofdetails about our available funds.
OFF-BALANCE SHEET ARRANGEMENTS
investments in auction rate securities and other marketable securities.
Off-Balance Sheet Arrangements
We have not entered into off-balance sheet financing arrangements, other than operating leases. We have no significant commitments for capital expenditures in 2005. Other than as set forth below, we2008. We have no committed lines of credit or other committed funding or long-term debt. The following table lists Acacia Research Corporation'sCorporation’s material known future cash commitments as of December 31, 2004,2007, and any material known commitments arising from events subsequent to year end:
- ----------
(1) Refer
| Payments Due by Period (In thousands) | ||||||||||||||||||||||||||||
| Contractual Obligations | Total | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 and Thereafter | |||||||||||||||||||||
| Operating leases | $ | 3,850 | $ | 868 | $ | 903 | $ | 939 | $ | 977 | $ | 163 | $ | - | ||||||||||||||
| Total contractual obligations | $ | 3,850 | $ | 868 | $ | 903 | $ | 939 | $ | 977 | $ | 163 | $ | - | ||||||||||||||
27
FIN 48 Liability. Effective January 1, 2007, we adopted the provisions of FASB Interpretation No. 48 (“FIN 48”), “Accounting for Uncertainty in Income Taxes,” (see Note 2 to Note 13 to the Acacia Research Corporation consolidated financial
statements for a description of the September 30, 2002 settlement agreement
between CombiMatrix Corporation and Dr. Donald Montgomery and Nanogen.
(2) See Note 6 to the Acacia Research Corporation consolidated financial statements included elsewhere hereinherein). As of December 31, 2007, the liability for additional information regarding
the October 2004 Leuchemix transaction.
(3) Excludes any potential future payments contingent upon the completionuncertain tax positions, associated primarily with state income taxes, was $115,000, of certain milestones in accordance with the agreement.
(4) Reflects $2.0 million consulting contract commitment, including
reimbursable expenses,which none is expected to be paid over two yearswithin one year. The liability for uncertain tax positions is recorded in connection with the
Acacia Technologies group's purchase of the assets of Global Patent
Holdings, LLC in January 2005, as described above.
In connection with the purchase of the outstanding ownership interests in
Acacia Media Technologies in November 2001, Acacia Media Technologies also
executed related assignment agreements which granted to the former owners of
Acacia Media Technologies' current patent portfolio the right to receive a
royalty of 15% of future net revenues, as defined in the agreements, generated
by Acacia Media Technologies' current patent portfolio, which includes its DMT
patents. No royalty obligation has been incurred as of December 31, 2004. Any
royalties paid pursuant to the agreements will be expensedother long-term liabilities in the consolidated statement of operations.
42
RECENT ACCOUNTING PRONOUNCEMENTS
balance sheet.
Recent Accounting Pronouncements
Refer to Note 2 to the Acacia Research Corporation consolidated financial statements included elsewhere herein.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our exposure to market risk is limited primarily to interest income
sensitivity, which is affected by changes in the general level of United States
interest rates, particularly because a significant portion of our investments
are in short-term debt securities issued by the U.S. government, U.S.
corporations, institutional money market funds and other money market
instruments. The primary objective of our investment activities is to preserve
principal while at the same time maximizing the income received without
significantly increasing risk. To minimize risk, we maintain a portfolio of
cash, cash equivalents and short-term investments in a variety of
investment-grade securities and with a variety of issuers, including corporate
notes, commercial paper and money market instruments. Due to the nature of our
short-term investments, we believe that we are not subject to any material
market risk exposure. We do not have any derivative financial instruments.
43
DISCUSSION OF SEGMENTS' OPERATIONS, FINANCIAL RESOURCES AND LIQUIDITY
COMBIMATRIX GROUP MANAGEMENT'S DISCUSSION AND ANALYSIS
(A DIVISION OF ACACIA RESEARCH CORPORATION)
YOU SHOULD READ THIS DISCUSSION IN CONJUNCTION WITH THE COMBIMATRIX GROUP,
A DIVISION OF ACACIA RESEARCH CORPORATION, FINANCIAL STATEMENTS AND RELATED
NOTES AND THE ACACIA RESEARCH CORPORATION CONSOLIDATED FINANCIAL STATEMENTS AND
RELATED NOTES, BOTH INCLUDED ELSEWHERE HEREIN. HISTORICAL RESULTS AND PERCENTAGE
RELATIONSHIPS ARE NOT NECESSARILY INDICATIVE OF OPERATING RESULTS FOR ANY FUTURE
PERIODS.
See
28
Item 1. "Business," for a general overview of the CombiMatrix group's
business. Although AR-CombiMatrix stock is intended to reflect the separate
performance of the CombiMatrix group, rather than the performance of Acacia
Research Corporation as a whole, the CombiMatrix group is not a separate legal
entity. Holders of AR-CombiMatrix stock are stockholders of Acacia Research
Corporation. As a result, they continue to be subject to all of the risks of an
investment in Acacia Research Corporation and all of its businesses, assets and
liabilities. The assets Acacia Research Corporation attributes to the
CombiMatrix group could be subject to the liabilities of the Acacia Technologies
group.
COMBIMATRIX GROUP
(A DIVISION OF ACACIA RESEARCH CORPORATION)
RESULTS OF OPERATIONS
DIVISION NET INCOME (LOSS) (IN THOUSANDS)
The changes in net income (loss) were primarily due to operating results
and activities as discussed below.
REVENUES AND COST OF REVENUES (IN THOUSANDS)
RESEARCH AND DEVELOPMENT CONTRACT. In March 2004, the CombiMatrix group
completed all phases of its research and development agreement with Roche. As a
result of completing all obligations under this agreement and in accordance with
the CombiMatrix group's revenue recognition policies for multiple-element
arrangements, the CombiMatrix group recognized $17.3 million of research and
development contract revenues during the first quarter of 2004, all of which
were previously deferred. The majority of research and development efforts under
the Roche agreement were incurred prior to 2004.
GOVERNMENT CONTRACT AND COST OF GOVERNMENT CONTRACT REVENUES. In March
2004, the CombiMatrix group executed a two-year $5.9 million research and
development contract with the Department of Defense to further the development
of the CombiMatrix group's array technology for the detection of biological
threat agents. Under the terms of the contract, the CombiMatrix group is
reimbursed on a periodic basis for actual costs incurred to perform its
obligations, plus a fixed fee. Revenues are recognized under the
percentage-of-completion method of accounting, using the cost-to-cost approach
to measure completeness at the end of each reporting period. Cost of government
contract revenues reflect research and development expenses incurred in
connection with the CombiMatrix group's commitments under its biowarfare
detection contract with the Department of Defense, which was approximately 34%
complete as of December 31, 2004. Based on actual costs incurred through
December 31, 2004, the CombiMatrix group expects to incur approximately $2.2
million and $819,000 in research and development costs during 2005 and 2006,
respectively, to complete its obligations to the Department of Defense under
this contract.
44
Government contract revenues in 2002 included amounts earned from the
CombiMatrix group's performance under its Phase I SBIR Department of Defense
contract, Phase I NIH grant and one-time contract research and development
revenues. The SBIR Department of Defense and NIH grants were completed during
the third quarter of 2002.
SERVICE CONTRACTS. Service contract revenues include maintenance and
service contract fees recognized by CombiMatrix K.K. from existing array
customers in Japan. As of December 31, 2004, the terms of these contracts had
expired.
PRODUCT REVENUES AND COST OF PRODUCT SALES. Product revenues and costs of
product sales during 2004 relate to domestic and international sales of the
CombiMatrix group's array products. The CombiMatrix group launched its
CustomArray(TM) DNA array platform in March 2004 and its CustomArray(TM) 12K DNA
expression array in July 2004. Product revenues and cost of product sales during
2003 and 2002 were recognized by CombiMatrix K.K. from sales of DNA array
synthesizers and related array products and services to Japanese research
institutions.
RESEARCH AND DEVELOPMENT EXPENSES (IN THOUSANDS)
RESEARCH AND DEVELOPMENT EXPENSES. The decrease in research and development
expenses in 2004, as compared to 2003 and 2002, was primarily due to the
CombiMatrix group's completion of several Roche related research and development
projects during the third and fourth quarters of 2003, and final completion of
the research and development agreement with Roche in the first quarter of 2004.
During 2003 and 2002, the CombiMatrix group's research and development
activities were driven primarily by ongoing performance obligations under the
product commercialization phase of its license and research and development
agreements with Roche. These activities include costs associated with direct
labor, supplies and materials, development of prototype arrays and instruments
and the use of outside consultants for certain engineering efforts.
With the completion of the research and development agreement with Roche,
year-to-date and future research and development expenses were and will continue
to be incurred in connection with the CombiMatrix group's commitments under its
collaboration and supply agreements with various strategic partners including
Furuno and Toppan, as well as ongoing internal research and development efforts
in the areas of genomics, drug discovery and development and material sciences.
The CombiMatrix group expects its research and development expenses to continue
to be volatile and such expenses could increase in future periods as additional
contract and/or internal research and development agreements are undertaken.
CHARGE FOR ACQUIRED IN-PROCESS RESEARCH AND DEVELOPMENT. Operating expenses
in 2002 include a non-cash charge for acquired in-process research and
development of $17.2 million, related to Acacia Research Corporation's purchase
of the stockholder interests in CombiMatrix Corporation that we did not already
own.
MARKETING, GENERAL AND ADMINISTRATIVE EXPENSES AND LEGAL SETTLEMENT CHARGES (IN
THOUSANDS)
MARKETING, GENERAL AND ADMINISTRATIVE EXPENSES. The increase in 2004, as
compared to 2003, was due primarily to an increase in corporate professional
fees related to the CombiMatrix group's Sarbanes-Oxley compliance projects of
approximately $303,000 and an increase in marketing and sales costs of
approximately $447,000 related to the launch of the CombiMatrix group's
CustomArray(TM) DNA array platform beginning in March 2004 and overall expansion
of the CombiMatrix group's sales and marketing division.
The decrease in 2003, as compared to 2002, was due primarily to a decrease
in the CombiMatrix group's corporate legal expenses of approximately $827,000,
primarily related to the settlement of litigation with Nanogen, a decrease in
legal, accounting and other professional fees of approximately $704,000 related
to Acacia Research Corporation's recapitalization and merger transactions
completed in December 2002 and a decrease in overhead of approximately $383,000
related primarily to reduced general marketing and sales and administrative
personnel. This decrease was partially offset by an increase in the CombiMatrix
group's rent and utilities expenses of approximately $473,000 as a result of the
increase in occupancy of its corporate headquarters in Mukilteo, Washington
during 2003.
45
Included in marketing, general and administrative expenses are allocated
corporate charges of $689,000 in 2004, $894,000 in 2003 and $1.2 million in
2002. See "Critical Accounting Policies" for a description of the management
allocation policies implemented.
LEGAL SETTLEMENT CHARGES. In 2002, in connection with the September 2002
settlement agreement between CombiMatrix Corporation, Dr. Donald Montgomery, and
Nanogen the CombiMatrix group expensed a $1.0 million payment to Nanogen and
recorded a non-cash charge in the amount of $17.5 million related to the fair
value of CombiMatrix Corporation common shares issued to Nanogen. In addition,
the CombiMatrix group recorded a net non-cash charge totaling $812,000 during
2004, which reflects the fair value of AR-CombiMatrix common stock issued and
potentially issuable to Nanogen, Inc. during the period in connection with
certain anti-dilution provisions of the settlement agreement. Periodic charges
and the related liability are estimated based on the number of shares issuable
and or potentially issuable and the AR-CombiMatrix stock price at the end of the
respective reporting period. The anti-dilution provisions of the settlement
agreement expire in September 2005.
NON-CASH STOCK COMPENSATION AMORTIZATION (IN THOUSANDS)
The decrease was primarily due to the accelerated method of stock
compensation amortization utilized which results in higher amounts of
amortization in the earlier vesting periods and the impact of non-cash stock
compensation expense reversals related to the forfeiture of certain unvested
stock options during the respective periods. Non-cash stock compensation expense
reversals totaled $185,000 in 2004 and $1.2 million in both 2003 and 2002.
Non-cash deferred stock compensation amounts were fully amortized as of December
31, 2004.
MINORITY INTERESTS (IN THOUSANDS)
The decrease was primarily due to Acacia Research Corporation's acquisition
of the remaining ownership interests in CombiMatrix Corporation in December 2002
and CombiMatrix K.K. in July 2003. Acacia Research Corporation's interests in
CombiMatrix K.K. have been attributed to the CombiMatrix group.
INFLATION
Inflation has not had a significant impact on the CombiMatrix group in the
current or prior periods.
LIQUIDITY AND CAPITAL RESOURCES
At December 31, 2004, cash and cash equivalents and short-term investments
totaled $23.7 million, compared to $17.3 million at December 31, 2003. Working
capital at December 31, 2004 was $22.1 million, compared to a working capital
deficit at December 31, 2003 of $1.6 million. At December 31, 2003 working
capital included $17.6 million in deferred revenues primarily related to the
CombiMatrix group's agreements with Roche and Toppan, which are not subject to
any refund or repayment obligations and do not represent payment obligations of
the CombiMatrix group. Working capital increased in 2004 due primarily to the
recognition of $17.3 million in Roche related deferred contract revenues in the
first quarter of 2004 and the impact of net cash flow activities as discussed
below.
The change in cash and cash equivalents for the years ended December 31,
2004, 2003 and 2002 was comprised of the following (in thousands):
46
The increase in net cash outflows from operations for the CombiMatrix group
in 2004, compared to 2003, was primarily due to a decrease in operating cash
receipts from customers, which totaled $3.0 million in 2004, comprised of $1.7
million from the Department of Defense, $1.0 million from Furuno and $265,000
from the sale of array products and related services, compared to $12.8 million
in the comparable 2003 period, consisting primarily of $9.8 million related to
the completion of certain milestones and delivery of prototype products and
services pursuant to its agreements with Roche and an up-front payment of $1.0
million and a $1.4 million milestone payment pursuant to its agreement with
Toppan. The decrease in payments from customers was partially offset by the
decrease in operating expenses and the impact of the timing of the receipt of
payments from customers and payments to vendors.
The decrease in net cash outflows from operations in 2003, as compared to
2002, was primarily attributable to the reduction in research and development
expenses incurred by the CombiMatrix group and the decrease in marketing,
general and administrative expenses as discussed above. The change was also due
to an increase in operating cash receipts during 2003, from Roche and Toppan, as
discussed above. Toppan related cash payments are included in deferred revenues
at December 31, 2004 and 2003. In 2002, the CombiMatrix group's negative cash
flows from operations was due primarily to the continued expansion of the
group's research and development activities including its efforts under its
Roche and NASA agreements executed in 2001. Losses from operations in 2002 were
partially offset by the receipt of milestone and advance payments of $11.6
million primarily from Roche.
The change in net cash flows used in investing activities was due primarily
to the CombiMatrix group's ongoing short term cash management activities and
changes in short term investments in connection with certain financing
activities discussed below. Fixed asset purchases were $810,000, $83,000 and
$1.0 million in 2004, 2003 and 2002, respectively.
The change in net cash inflows attributed to the CombiMatrix group from
financing activities in 2004, compared to 2003, was due to the completion of an
equity financing which raised net proceeds of approximately $13.7 million
through the sale of Acacia Research - CombiMatrix common stock during 2004,
compared to equity financing net proceeds of $4.9 million during the comparable
2003 period. Financing activities in 2004 also included proceeds from the
exercise of Acacia Research - CombiMatrix common stock warrants and stock
options, totaling $5.1 million, compared to $1.0 million in the comparable 2003
period. Cash used in financing activities in 2002 was comprised primarily of
payments on the CombiMatrix group's capital lease obligation totaling $2.8
million (the capital lease obligation was paid in full in November 2002), which
were partially offset by proceeds from stock option exercises of $106,000.
To date, the CombiMatrix group has relied primarily upon selling equity
securities as well as payments from strategic partners to generate the funds
needed to finance the implementation of the CombiMatrix group's business
strategies. The CombiMatrix group cannot assure that it will not encounter
unforeseen difficulties that may deplete capital resources more rapidly than
anticipated. Any efforts to seek additional funds could be made through equity,
debt or other external financings, however the CombiMatrix group cannot assure
that additional funding will be available on favorable terms, if at all. If the
CombiMatrix group fails to obtain additional funding when needed, the
CombiMatrix group may not be able to execute its business strategies and its
business may suffer.
The CombiMatrix group's long-term capital requirements will be substantial
and the adequacy of its available funds will depend upon many factors,
including:
o the costs of commercialization activities, including sales and
marketing, manufacturing and capital equipment;
o the CombiMatrix group's continued progress in research and development
programs;
o the costs involved in filing, prosecuting, enforcing and defending any
patents claims, should they arise;
o the CombiMatrix group's ability to license technology;
o competing technological developments;
o the creation and formation of strategic partnerships;
47
o the costs associated with leasing and improving our headquarters in
Mukilteo, Washington; and
o other factors that may not be within the CombiMatrix group's control.
The CombiMatrix group believes that its cash and cash equivalent and
short-term investment balances, anticipated cash flow from operations and other
external sources of available credit will be sufficient to meet its cash
requirements through at least the next twelve months. However, changes may occur
that would cause the CombiMatrix group's available capital resources to be
consumed significantly sooner than it currently expects.
The CombiMatrix group may be unable to raise sufficient additional capital
on favorable terms or at all. If it fails to do so, it may have to curtail or
cease operations or enter into agreements requiring it to relinquish rights to
certain technologies, products or markets because it will not have the capital
necessary to exploit them.
OFF-BALANCE SHEET ARRANGEMENTS
The CombiMatrix group has not entered into off-balance sheet financing
arrangements, other than operating leases. The CombiMatrix group has no
significant commitments for capital expenditures in 2005. Other than as set
forth below, the CombiMatrix group has no committed lines of credit or other
committed funding or long-term debt. The following table lists the CombiMatrix
group's material known future cash commitments as of December 31, 2004:
- ----------
(1) Refer to Note 13 to the Acacia Research Corporation consolidated financial
statements for a description of the September 30, 2002 settlement agreement
between CombiMatrix Corporation and Dr. Donald Montgomery and Nanogen.
(2) Excludes any allocated rent expense in connection with Acacia Research
Corporation's management allocation policies.
(3) See Note 5 to the CombiMatrix group financial statements for additional
information regarding the October 2004 Leuchemix transaction.
(4) Excludes any potential future payments contingent upon the completion of
certain milestones in accordance with the agreement.
RECENT ACCOUNTING PRONOUNCEMENTS
Refer to Note 2 to the Acacia Research Corporation consolidated financial
statements included elsewhere herein.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The CombiMatrix group's exposure to market risk is limited to interest
income sensitivity, which is affected by changes in the general level of United
States interest rates, particularly because the majority of the group's
investments are in short-term debt securities issued by the U.S. treasury and by
U.S. corporations. The primary objective of the group's investment activities is
to preserve principal while at the same time maximizing the income the
CombiMatrix group receives without significantly increasing risk. To minimize
risk, the CombiMatrix group maintains its portfolio of cash, cash equivalents
and short-term investments in a variety of investment-grade securities and with
a variety of issuers, including corporate notes, commercial paper, government
securities and money market funds. Due to the nature of its short-term
investments, the CombiMatrix group believes that it is not subject to any
material market risk exposure.
At December 31, 2004, the CombiMatrix group had certain assets and
liabilities denominated in Japanese Yen as a result of forming CombiMatrix K.K.
However, due to the relative insignificance of those amounts, the CombiMatrix
group does not believe that it has significant exposure to foreign currency
exchange rate risks. The CombiMatrix group currently does not use derivative
financial instruments to mitigate this exposure. The CombiMatrix group continues
to review this and may begin hedging certain foreign exchange risks through the
use of currency forwards or options in future periods.
48
ACACIA TECHNOLOGIES GROUP MANAGEMENT'S DISCUSSION AND ANALYSIS
(A DIVISION OF ACACIA RESEARCH CORPORATION)
YOU SHOULD READ THIS DISCUSSION IN CONJUNCTION WITH THE ACACIA TECHNOLOGIES
GROUP, A DIVISION OF ACACIA RESEARCH CORPORATION, FINANCIAL STATEMENTS AND
RELATED NOTES AND THE ACACIA RESEARCH CORPORATION CONSOLIDATED FINANCIAL
STATEMENTS AND RELATED NOTES, BOTH INCLUDED ELSEWHERE HEREIN. HISTORICAL RESULTS
AND PERCENTAGE RELATIONSHIPS ARE NOT NECESSARILY INDICATIVE OF OPERATING RESULTS
FOR ANY FUTURE PERIODS.
GENERAL
See Item 1. "Business," for a description of the Acacia Technologies
group's business. Although the AR-Acacia Technologies stock is intended to
reflect the separate performance of the Acacia Technologies group, rather than
the performance of Acacia Research Corporation as a whole, the Acacia
Technologies group is not a separate legal entity. Holders of the AR-Acacia
Technologies stock are stockholders of Acacia Research Corporation. As a result,
they continue to be subject to all of the risks of an investment in Acacia
Research Corporation and all of Acacia Research Corporation's businesses, assets
and liabilities. The assets Acacia Research Corporation attributes to the Acacia
Technologies group could be subject to the liabilities of the CombiMatrix group.
ACACIA TECHNOLOGIES GROUP
(A DIVISION OF ACACIA RESEARCH CORPORATION)
RESULTS OF OPERATIONS
DIVISION NET LOSS (IN THOUSANDS)
The changes in net loss were primarily due to operating results and
activities as discussed below.
REVENUES (IN THOUSANDS)
LICENSE FEES. License fee revenues are comprised of DMT technology license
fees and previously deferred V-chip technology license fees recognized by the
Acacia Technologies group. DMT technology license fees were $2.8 million and
$692,000 in 2004 and 2003, respectively. The increase was primarily due to the
significant growth in the number of DMT technology license agreements executed
since March 31, 2003. During 2004, we executed 170 DMT license agreements.
License fee revenues will fluctuate from period to period based on the increase
in license agreements executed, fluctuations in the sales results or other
royalty per unit activities of our licensees that impact the calculation of
license fees due, the timing of the receipt of periodic license fee statements
and or payments from licensees, and other factors. Periodic license fee revenues
may include amounts that relate to prior license periods or prior periods of
infringement, which are recognized as revenues in the period received. DMT
license fees related to prior periods of infringement for the periods presented
above were not significant. Costs incurred in connection with the Acacia
Technologies group's ongoing licensing activities are included in marketing,
general and administrative expenses and do not expect to receive any additional
V-chip related revenues in future periods.
License fee revenues for 2004 include $1.5 million in previously deferred
V-chip license fees (originally deferred in 2001) recognized as a result of the
conclusion of V-chip related litigation in August 2004, as described at Item 3.
"Legal Proceedings." We concluded our V-chip licensing program in August 2004.
MARKETING, GENERAL AND ADMINISTRATIVE EXPENSE (IN THOUSANDS)
49
MARKETING, GENERAL AND ADMINISTRATIVE EXPENSES. The change in 2004, as
compared to 2003, was due primarily to an increase in costs related to the
addition of licensing and business development personnel of $418,000, an
increase in patent related commercialization costs of $110,000, and an increase
in corporate professional fees related to the group's Sarbanes-Oxley compliance
projects of $318,000.
The decrease in 2003, as compared to 2002, was due primarily to a decrease
in legal, accounting and other professional fees related to Acacia Research
Corporation's recapitalization and merger transactions completed in December
2002 of $1.3 million, a decrease in overhead related to a reduction in general
and administrative personnel of $837,000 and a decrease in general and
administrative expenses of $546,000. This decrease was partially offset by an
increase in costs related to the Acacia Technologies group's ongoing DMT patent
commercialization and enforcement efforts, including increased consulting and
engineering costs related to new patent claims, enforcement and the
identification of additional potential licensees of our DMT technology of
$219,000.
LEGAL EXPENSE - PATENTS. Patent related legal expenses in 2004 included
$668,000 in deferred V-chip related legal fees recognized as a result of the
conclusion of V-chip related litigation in August 2004, as described at Item 3.
"Legal Proceedings." The Acacia Technologies group's patent related legal
expenses, excluding V-chip related legal fees, increased to $2.5 million during
2004, as compared to $1.9 million, and $1.4 million in the comparable 2003 and
2002 periods, due to an increase in costs incurred in connection with the Acacia
Technologies group's ongoing DMT patent commercialization and enforcement
programs, including increased legal costs related to new patent claims and the
identification of additional potential licensees of our DMT technology and
increased patent enforcement costs related to ongoing DMT patent related
litigation. See Item 3. "Legal Proceedings" for a description of ongoing DMT
related litigation. We expect patent related legal expenses to continue to
fluctuate based on actual outside patent counsel fees incurred in connection
with the Acacia Technologies group's ongoing DMT and other patent
commercialization and enforcement programs. DMT related legal fees to outside
attorneys are charged based on actual time and out-of-pocket expenses incurred
by external counsel and are not incurred on a contingent fee basis.
In connection with the January 2005 acquisition as described above, the
Acacia Technologies group acquired 27 additional patent portfolios. Although
most litigation with respect to those portfolios is likely to be handled on a
contingency basis where attorneys fees are paid out of license fee revenues
collected, certain other costs and expenses in connection with the Acacia
Technologies group's maintenance, licensing, and enforcement of patents are
likely to increase as a result of the acquisition including patent filing fees,
patent development costs, travel costs, expert and consulting fees, and other
third-party expenses.
IMPAIRMENT CHARGES (IN THOUSANDS)
GOODWILL IMPAIRMENT CHARGE. In August 2004, as a result of the adverse
ruling in the Soundview Technologies litigation described at Item 3. "Legal
Proceedings," the Acacia Technologies group recorded a non-cash impairment
charge, included in operating expenses, totaling $1.6 million associated with
the write-down of 100% of the goodwill related to the V-chip.
IMPAIRMENT OF COST METHOD INVESTMENT. In 2003, we recorded an impairment
charge of $207,000 for an other-than-temporary decline in the fair value of our
cost method investment. Impairment indicators included a continued decline in
the working capital of the entity and reference to a recent equity transaction
and related valuation indicating an other-than-temporary decline in fair value
of the investment. In 2002, we recorded an impairment charge of $2.7 million for
an other-than-temporary decline in the fair value of the same cost method
investment. Impairment indicators included recurring losses, a decline in
working capital and the completion of a recent equity transaction at an amount
below our carrying value.
AMORTIZATION OF PATENTS (IN THOUSANDS)
The decrease in 2003, as compared to 2002, was due to a reduction in patent
amortization expense due to V-chip technology related patent costs and other
intangibles that were fully amortized during 2002. With the acquisition of the
assets of Global Patent Holdings, as described above, we expect that a
significant portion of the approximated $25.0 million purchase price will be
allocated to the patents acquired, which will be amortized over the economic
useful lives of the respective patents, resulting in increased amortization
charges in 2005 and future periods. See Note 15 to the Acacia Research
Corporation consolidated financial statements for additional information.
50
REALIZED AND UNREALIZED GAINS/LOSSES (IN THOUSANDS)
The decrease is due to no investments classified as trading securities held
during 2004 and 2003 and the sale of the balance of our trading securities
during 2002.
INCOME TAXES (IN THOUSANDS)
The 2004, 2003 and 2002 income tax benefits primarily reflect the impact of
the reversal of deferred tax liabilities related to the amortization of
identifiable intangible assets related to certain of Acacia Research
Corporation's step acquisitions in 2002, 2001 and 2000. $569,000 of the 2002
income tax benefit also reflects the impact of differences between the 2001
income tax provision and Acacia Research Corporation's final 2001 consolidated
tax return filed in September 2002, and is the result of additional deductions
against Soundview Technologies taxable income.
INFLATION
Inflation has not had a significant impact on the Acacia Technologies group
in the current or previous periods.
LIQUIDITY AND CAPITAL RESOURCES
The Acacia Technologies group's cash and cash equivalents and short-term
investments totaled $28.6 million at December 31, 2004, compared to $33.2
million at December 31, 2003. Working capital at December 31, 2004 was $27.1
million, compared to $32.7 million at December 31, 2003.
The changes in cash and cash equivalents for 2004, 2003 and 2002 were
comprised of the following (in thousands):
The change in net cash outflows from operations for the Acacia Technologies
group was primarily due to an increase in DMT license fee payments received from
licensees which totaled $3.1 million in 2004, compared to $665,000, in the
comparable 2003 period, which was partially offset by the increase in marketing,
general and administrative expenses as discussed above and the impact of the
timing of vendor payments.
The increase in net cash outflows from operations in 2003, as compared to
2002, was primarily due to an increase in costs incurred in connection with the
launch of the Acacia Technologies group's ongoing DMT patent commercialization
and enforcement programs, including increased legal costs related to new patent
claims and the identification of additional potential licensees of our DMT
technology and the timing of vendor payments, primarily related to professional
fees incurred in connection with Acacia Research Corporation's merger and
recapitalization transaction completed 2002. The increase in costs was partially
offset by payments received from licensees in 2003 totaling $665,000 in
connection with the launch of the Acacia Technologies group's DMT licensing
program.
The change in net cash flows provided by (used in) continuing investing was
primarily due to the purchases and sales of short-term investments in connection
with the Acacia Technologies group's ongoing short-term cash management
activities.
51
Net cash outflows attributed to the Acacia Technologies group from
financing activities in 2004, 2003 and 2002 were comprised of corporate and
acquisition costs allocated to the CombiMatrix group of $396,000, $620,000 and
$1.9 million, respectively, partially offset by AR-Acacia Technologies sock
option exercise proceeds of $90,000, $190,000 and $136,000, respectively.
In February 2005, Acacia Research Corporation raised gross proceeds of
$19,600,000 through the sale of 3,500,000 shares of Acacia Research - Acacia
Technologies common stock at a price of $5.60 per share in a registered direct
offering. Net proceeds raised of approximately $19,575,000, which are net of
related issuance costs, were attributed to the Acacia Technologies group. All of
the shares of Acacia Research-Acacia Technologies common stock were offered
pursuant to an effective registration statement previously filed with the
Securities and Exchange Commission.
The Acacia Technologies group believes that its cash and cash equivalent
balances, including the proceeds from the February 2005 equity financing
described above, anticipated cash flow from operations and other external
sources of available credit will be sufficient to meet its cash requirements
through at least the next twelve months.
To date, the Acacia Technologies group has relied primarily upon selling of
Acacia Research Corporation equity securities and payments from our V-chip
licensees (primarily in 2001) and DMT licensees (2003 to current) to generate
the funds needed to finance the operations of the Acacia Technologies group. As
discussed earlier, the V-chip patent expired in July 2003, and the Judge
affirmed the ruling of non-infringement as discussed above. In 2003, the Acacia
Technologies group began to commercially license its DMT technology recognizing
approximately $3.5 million in DMT license fee revenues to date, and intends to
acquire and develop additional intellectual property. In July 2004, the Acacia
Technologies group acquired U.S. Patent No. 6,226,677 from LodgeNet
Entertainment Corporation, which covers technology and methods for redirecting
users to a login page when accessing the Internet, and launched its licensing
and enforcement program for this patent in the third quarter of 2004. Acacia
Global Acquisition Corporation's acquisition of the assets of Global Patent
Holdings, LLC as discussed above, provides the Acacia Technologies group with
ownership of companies that control 27 patent portfolios, which include 120 U.S.
patents and certain foreign counterparts, and cover technologies used in a wide
variety of industries. The acquisitions expand and diversify the Acacia
Technologies group's revenue generating opportunities.
However, there can be no assurance that the Acacia Technologies group will
be able to implement its future plans. Failure by management to achieve its
plans would have a material adverse effect on the Acacia Technologies group and
on Acacia Research Corporation's ability to achieve its intended business
objectives. The Acacia Technologies group's success also depends on its ability
to protect its intellectual property.
The timing of the receipt of revenues by the Acacia Technologies group's
business operations are subject to certain risks and uncertainties, including:
o market acceptance of our technologies and services;
o business activities and financial results of our licensees;
o technological advances that may make our technologies obsolete or less
competitive;
o increases in operating costs, including costs for legal services,
engineering and research and personnel;
o the availability and cost of capital;
o general economic conditions; and
o governmental regulation that may restrict the Acacia Technologies
group's business.
OFF-BALANCE SHEET ARRANGEMENTS
The Acacia Technologies group has not entered into off-balance sheet
financing arrangements, other than operating leases. The Acacia Technologies
group has no significant commitments for capital expenditures in 2005. Other
than as set forth below, the Acacia Technologies group has no committed lines of
credit or other committed funding or long-term debt. The following table lists
the Acacia Technologies group's material known future cash commitments as of
December 31, 2004, and material known commitments arising from events subsequent
to year end:
52
- -------------
(1) Excludes any allocated rent expense in connection with Acacia Research
Corporation's management allocation policies.
(2) Reflects $2.0 million consulting contract commitment, including
reimbursable expenses, to be paid over two years in connection with the
Acacia Technologies group's purchase of the assets of Global Patent
Holdings, LLC in January 2005, as described above.
In connection with the purchase of the outstanding ownership interests in
Acacia Media Technologies in November 2001, Acacia Media Technologies also
executed related assignment agreements which granted to the former owners of
Acacia Media Technologies' current patent portfolio the right to receive a
royalty of 15% of future net revenues, as defined in the agreements, generated
by Acacia Media Technologies' current patent portfolio, which includes its DMT
patents. No royalty obligation has been incurred as of December 31, 2004. Any
royalties paid pursuant to the agreements will be expensed in the statement of
operations.
RECENT ACCOUNTING PRONOUNCEMENTS
Refer to Note 2 to the Acacia Research Corporation consolidated financial
statements included elsewhere herein.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The Acacia Technologies group's exposure to market risk is limited
primarily to interest income sensitivity, which is affected by changes in the
general level of United States interest rates, particularly because a
significant portion of our investments are in short-term debt securities issued
by United States corporations, institutional money market funds and other money
market instruments. The primary objective of our investment activities is to
preserve principal while at the same time maximizing the income received without
significantly increasing risk. To minimize risk, we maintain a portfolio of
cash, cash equivalents and short-term investments in a variety of
investment-grade securities and with a variety of issuers, including U.S.
government and corporate notes and bonds, commercial paper and money market
instruments. Due to the nature of our short-term investments, we believe that we
are not subject to any material market risk exposure. We do not have any
derivative financial instruments.
RISK FACTORS
An investment in our stock involves a number of risks. Before making a
decision to purchase our securities, you should carefully consider all of the
risks described in this annual report. If any of the risks incorporated by
reference into this annual report actually occur, our business, financial
condition and results of operations could be materially adversely affected. If
this were to occur, the trading price of our securities could decline
significantly and you may lose all or part of your investment. You should
carefully review the "Risk Factors" set forth on pages 3 through 21 of our Form
S-3 Registration Statement filed on February 1, 2005, incorporated herein by
reference.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Refer to the caption "Quantitative and Qualitative Disclosures About Market
Risk" for Acacia Research Corporation, the CombiMatrix group and the Acacia
Technologies group under Item 7. "Management's Discussion and Analysis of
Financial Condition and Results of Operations."
The primary objective of our investment activities is to preserve principal while concurrently maximizing the income we receive from our investments without significantly increasing risk. Some of the securities that we may invest in may be subject to market risk. This means that a change in prevailing interest rates may cause the principal amount of the investment to fluctuate. For example, if we hold a security that was issued with a fixed interest rate at the then-prevailing rate and the prevailing interest rate later rises, the current value of the principal amount of our investment will decline. To minimize this risk in the future, we intend to maintain our portfolio of cash equivalents and short-term investments in a variety of securities, including commercial paper, money market funds, high-grade corporate bonds, government and non-government debt securities and certificates of deposit. In general, money market funds are not subject to market risk because the interest paid on such funds fluctuates with the prevailing interest rate. As of December 31, 2004,2007, all of our investments were in money market funds, high-grade corporate bonds, auction rate securities, certificates of deposit and U.S. government debt securities. A hypothetical 100 basis point increase in interest rates would not have a material impact on the fair value of our available-for-sale securities as of December 31, 2004. See Note2007. Refer to Item 1A. “Risk Factors,” Item 7. “Liquidity and Capital Resources,” and Notes 2 and 3 to the Acacia Research Corporation consolidated financial statements.
53
ITEMstatements included in this report for additional information.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and related financial information required to be filed hereunder are indexed under Item 15 of this report and are incorporated herein by reference.
ITEM
Item 9. CHANGES IN AND DISAGREEMENTS WITH INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM
Item 9A. CONTROLS AND PROCEDURES
CONCLUSION REGARDING THE EFFECTIVENESS OF DISCLOSURE CONTROLS AND PROCEDURES
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended. Based on this evaluation, our principal executive officer and our principal financial officer concluded that, as of the end of the period covered by this annual report, our disclosure controls and procedures were effective to ensure that the information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934 is accumulated and communicated to management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure, and that such information is recorded, processed, summarized and reported within the time periods prescribed by the SEC.
MANAGEMENT'S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control - Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2004.
Our management's assessment of2007.
Grant Thornton, LLP, the effectiveness of ourindependent registered public accounting firm who audited the Company’s consolidated financial statements included in this Form 10-K, has issued a report on the Company’s internal control over financial reporting, as of December 31, 2004 has been audited by
PricewaterhouseCoopers LLP, an independent registered public accounting firm, as
stated in their report which is included herein.
ITEM
Item 9B. OTHER INFORMATION
None 54
29
PART III
ITEM
Item 10. DIRECTORS, AND EXECUTIVE OFFICERS OF THE REGISTRANT
AND CORPORATE GOVERNANCE
Except as provided below, the information required by this Item is incorporated by reference from the information under the captions entitled "Election“Election of Directors-Nominees," "Executive Officers"” “Executive Officers” and "Section“Section 16(a) Beneficial Ownership Reporting Compliance"Compliance” in our definitive proxy statement to be filed with the SEC no later than April 30, 2005.
CODE OF CONDUCT.
29, 2008.
Code of Conduct.
Acacia Research Corporation has adopted a Code of Conduct that applies to all of its employees, including its chief executive officer, chief financial and accounting officer, president and any persons performing similar functions. Our Code of Conduct is provided on our internet website at www.acaciaresearch.com.
ITEMwww.acaciaresearch.com.
Item 11. EXECUTIVE COMPENSATION
The information required by this Item is incorporated by reference from the information under the caption entitled "Executive“Executive Officer Compensation and Other Information"Information” in our definitive proxy statement to be filed with the SEC no later than April 30, 2005.
ITEM29, 2008.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The information required by this Item is incorporated by reference from the information under the caption entitled "Security“Security Ownership of Certain Beneficial Owners and Management"Management” in our definitive proxy statement to be filed with the SEC no later than April 30, 2005.
ITEM29, 2008.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item is incorporated by reference from the information under the caption entitled "Certain Transactions"“Certain Transactions” in our definitive proxy statement to be filed with the SEC no later than April 30, 2005.
ITEM29, 2008.
Item 14. PRINICPALPRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this Item is incorporated by reference from the information under the caption entitled "Audit“Audit Committee Report"Report” in our definitive proxy statement to be filed with the SEC no later than April 29, 2008.
30
2005.
55
PART IV
ITEM
Item 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as part of this report.
(1) Financial Statements
Acacia Research Corporation Consolidated Financial Statements PAGE
----
Report of Independent Registered Public Accounting Firm................F-1
Consolidated Balance Sheets as of December 31, 2004 and 2003...........F-2
Consolidated Statements of Operations and Comprehensive Loss
for the Years Ended December 31, 2004, 2003 and 2002................F-3
Consolidated Statements of Stockholders' Equity for the Years
Ended December 31, 2004, 2003 and 2002..............................F-4
Consolidated Statements of Cash Flows for the Years Ended
December 31, 2004, 2003 and 2002....................................F-5
Notes to Consolidated Financial Statements.............................F-6
*CombiMatrix Group Financial Statements PAGE
(A Division of Acacia Research Corporation) ----
Report of Independent Registered Public Accounting Firm................F-43
Balance Sheets as of December 31, 2004 and 2003........................F-44
Statements of Operations
for the Years Ended December 31, 2004, 2003 and 2002................F-45
Statements of Allocated Net Worth for the Years
Ended December 31, 2004, 2003 and 2002..............................F-46
Statements of Cash Flows for the Years Ended
December 31, 2004, 2003 and 2002....................................F-47
Notes to Financial Statements..........................................F-48
*Acacia Technologies Group Financial Statements PAGE
(A Division of Acacia Research Corporation) ----
Report of Independent Registered Public Accounting Firm................F-64
Balance Sheets as of December 31, 2004 and 2003........................F-65
Statements of Operations
for the Years Ended December 31, 2004, 2003 and 2002................F-66
Statements of Allocated Net Worth for the Years
Ended December 31, 2004, 2003 and 2001..............................F-67
Statements of Cash Flows for the Years Ended
December 31, 2004, 2003 and 2002....................................F-68
Notes to Financial Statements..........................................F-69
*NOTE: We are presenting, the Acacia Research Corporation consolidated financial
statements and the separate financial statements for the CombiMatrix group and
the Acacia Technologies group. The separate financial statements and
accompanying notes of the two groups are being provided as additional disclosure
regarding the financial performance of the two divisions and to provide
investors with information regarding the potential value and operating results
of the respective businesses, which may affect the respective share values. The
separate financial statements should be reviewed in conjunction with Acacia
Research Corporation's consolidated financial statements and accompanying notes.
The presentation of separate financial statements is not intended to indicate
that we have changed the title to any of our assets or changed the
responsibility for any of our liabilities, nor is it intended to indicate that
the rights of our creditors have been changed. Acacia Research Corporation, and
not the individual groups, is the issuer of the securities. Holders of the two
securities are stockholders of Acacia Research Corporation and do not have a
separate and exclusive interest in the respective groups.
56
| Page | |
| Acacia Research Corporation Consolidated Financial Statements | |
| Reports of Independent Registered Public Accounting Firm – Grant Thornton LLP | F-1 |
| Report of Independent Registered Public Accounting Firm – PricewaterhouseCoopers LLP | F-3 |
| Consolidated Balance Sheets as of December 31, 2007 and 2006 | F-4 |
| Consolidated Statements of Operations and Comprehensive Loss for the Years Ended December 31, 2007, 2006 and 2005 | F-5 |
| Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2007, 2006 and 2005 | F-6 |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2007, 2006 and 2005 | F-7 |
| Notes to Consolidated Financial Statements | F-8 |
(2) Financial Statement Schedules
Financial statement schedules are omitted because they are not applicable or the required information is shown in the Financial Statements or the Notes thereto.
(3) Exhibits
See
Refer to Item 15(b) below
below.
(b) Exhibits. The following exhibits are either filed herewith or incorporated herein by reference:
EXHIBIT
NUMBER DESCRIPTION
- ------ -----------
2.1
Exhibit Number | Description |
| 2.1 | Agreement and Plan of Merger of Acacia Research Corporation, a California corporation, and Acacia Research Corporation, a Delaware corporation, dated as of |
| 2.2 | Agreement and Plan of Reorganization by and among Acacia Research Corporation, Combi Acquisition Corp. and CombiMatrix Corporation dated as of March 20, 2002 (2) |
| 3.1 | Restated Certificate of Incorporation as amended(3) |
| 3.2 | Amended and Restated Bylaws |
| 10.1* | Acacia Research Corporation 1996 Stock Option Plan, as amended (4) |
| 10.2* | Form of Option Agreement constituting the Acacia Research Corporation 1996 Executive Stock Bonus Plan (5) |
| 10.3* | 2002 Acacia Technologies Stock Incentive Plan (6) |
| 10.4* | 2007 Acacia Technologies Stock Incentive Plan (7) |
| 10.5* | Form of Acacia Technologies Stock Option Agreement for the 2007 Acacia Technologies Stock Incentive Plan (8) |
| 10.6* | Form of Acacia Technologies Stock Issuance Agreement for the 2002 Acacia Technologies Stock Incentive Plan (8) |
| 10.7* | Form of Acacia Technologies Stock Issuance Agreement for the 2007 Acacia Technologies Stock Incentive Plan (8) |
| 10.8 | Lease Agreement dated January 28, 2002, between Acacia Research Corporation and The Irvine Company (9) |
| 10.9 | Settlement Agreement dated September 30, 2002, by and among Acacia Research Corporation, CombiMatrix Corporation, Donald D. Montgomery, Ph.D. and Nanogen, Inc.(10) |
| 10.10 | Form of Indemnification Agreement (11) |
| 10.11 | Form of Subscription Agreement between Acacia Research Corporation and certain investors (12) |
| 10.12 | Third Amendment to lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (13) |
31 2001 filed on March 27, 2002
(SEC File No. 000-26068).
(11) Incorporated by reference from Acacia Research Corporation's Annual Report
on Form 10-K for the year ended December 31, 2002 filed on March 27, 2003
(SEC File No. 000-26068)
(12) Incorporated by reference from Acacia Research Corporation's Quarterly
Report on Form 10-Q filed on November 5, 2004 (SEC File No. 000-26068).
58
Exhibit Number | Description |
| 10.13 | Standby Equity Distribution Agreement dated June 14, 2006 between Acacia Research Corporation and Cornell Capital Partners, L.P. (14) |
| 10.14 | Amendment to Standby Equity Distribution Agreement dated June 14, 2006 between Acacia Research Corporation and Cornell Capital Partners, L.P. (15) |
| 10.15 | Manufacturing and Supply Agreement between Acacia Research Corporation and Furuno Electric Company, Ltd. Effective July 1, 2006 (16) |
| 10.16 | Placement Agency Agreement between Acacia Research Corporation and Oppenheimer & Co., dated December 7, 2006 (17) |
| 10.17 | Form of Subscription Agreement (17) |
| 10.18 | Form of Investors Warrant (17) |
| 10.19* | Employment Agreement, dated January 28, 2005, by and between Acacia Technologies Services Corporation, and Dooyong Lee, as amended |
| 10.20* | Employment Agreement, dated April 12, 2004, by and between Acacia Media Technologies Corporation and Edward Treska. |
| 10.21 | Fourth Amendment to lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company |
| 10.22 | Fifth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company |
| 21.1 | List of Subsidiaries |
| 23.1 | Consent of Independent Registered Public Accounting Firm - Grant Thornton LLP |
| 23.2 | Consent of Independent Registered Public Accounting Firm - PricewaterhouseCoopers LLP |
| 31.1 | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 | Certification of the Chief Executive Officer provided pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 32.2 | Certification of the Chief Financial Officer provided pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
___________________________
| * | The referenced exhibit is a management contract, compensatory plan or arrangement. |
| † | Portions of this exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission. |
| (1) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on December 30, 1999 (SEC File No. 000-26068). |
| (2) | Incorporated by reference as Appendix A to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| (3) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report Amendment No. 1 on Form 10-Q/A for the period ended June 30, 2006, filed on June 5, 2007. |
| (4) | Incorporated by reference as Appendix A to the Definitive Proxy Statement on Schedule 14A filed on April 10, 2000 (SEC File No. 000-26068). |
| (5) | Incorporated by reference from Acacia Research Corporation’s Definitive Proxy as Appendix A Statement on Schedule 14A filed on April 26, 1996 (SEC File No. 000-26068). |
| (6) | Incorporated by reference as Appendix E to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
32
| (7) | Incorporated by reference to Acacia Research Corporation’s Registration Statement on Form S-8 (SEC File No. 333-144754) which became effective on July 20, 2007. |
| (8) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended September 30, 2007. |
| (9) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2001 filed on March 27, 2002 (SEC File No. 000-26068). |
| (10) | Incorporated by reference as Appendix D to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| (11) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year ended December 31, 2002 filed on March 27, 2003 (SEC File No. 000-26068). |
| (12) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on September 19, 2005 (SEC File No. 000-26068). |
| (13) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on May 10, 2006 (SEC File No. 000-26068). |
| (14) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on June 15, 2006 (SEC File No. 000-26068). |
| (15) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on June 22, 2006 (SEC File No. 000-26068). |
| (16) | Incorporated by reference from Acacia Research Corporation’s Quarterly Report on Form 10-Q filed on November 9, 2006 (SEC File No. 000-26068). |
| (17) | Incorporated by reference from Acacia Research Corporation’s Report on Form 8-K filed on December 13, 2006 (SEC File No. 000-26068). |
33
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
DATED: March 15, 2005 ACACIA RESEARCH CORPORATION
/s/ Paul R. Ryan
----------------------------
Paul R. Ryan
CHAIRMAN OF THE BOARD
AND CHIEF EXECUTIVE OFFICER
(AUTHORIZED SIGNATORY)
| Dated: March 14, 2008 | ACACIA RESEARCH CORPORATION /s/ Paul R. Ryan Paul R. Ryan Chairman of the Board and Chief Executive Officer (Authorized Signatory) |
Pursuant to the requirements of the Securities and Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and the capacities and on the dates indicated.
SIGNATURE TITLE DATE
--------- ----- ----
/s/ Paul R. Ryan Chairman of the Board and March 15, 2005
- ---------------------------- Chief Executive Officer
Paul R. Ryan (Principal Chief Executive)
/s/ Robert L. Harris, II Director and President March 15, 2005
- ----------------------------
Robert L. Harris, II
/s/ Clayton J. Haynes Chief Financial Officer March 15, 2005
- ---------------------------- (Principal Financial Officer)
Clayton J. Haynes
/s/ Thomas B. Akin Director March 15, 2005
- ----------------------------
Thomas B. Akin
/s/ Fred A. de Boom Director March 15, 2005
- ----------------------------
Fred A. de Boom
/s/ Edward W. Frykman Director March 15, 2005
- ----------------------------
Edward W. Frykman
/s/ G. Louis Graziadio, III Director March 15, 2005
- ----------------------------
G. Louis Graziadio, III
/s/ Amit Kumar, Ph.D. Director March 15, 2005
- ----------------------------
Amit Kumar, Ph.D.
/s/ Rigdon Currie Director March 15, 2005
- ----------------------------
Rigdon Currie
59
| Signature | Title | Date |
| /s/ Paul R. Ryan | Chairman of the Board and | March 14, 2008 |
| Paul R. Ryan | Chief Executive Officer | |
| (Principal Chief Executive) | ||
| /s/ Robert L. Harris, II | Director and President | March 14, 2008 |
| Robert L. Harris, II | ||
| /s/ Clayton J. Haynes | Chief Financial Officer and Treasurer | March 14, 2008 |
| Clayton J. Haynes | (Principal Financial Officer) | |
| /s/ Fred A. de Boom | Director | March 14, 2008 |
| Fred A. de Boom | ||
| /s/ Edward W. Frykman | Director | March 14, 2008 |
| Edward W. Frykman | ||
| /s/ G. Louis Graziadio, III | Director | March 14, 2008 |
| G. Louis Graziadio, III | ||
| /s/ William S. Anderson | Director | March 14, 2008 |
| William S. Anderson |
34
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To The
Board of Directors and Shareholders
Stockholders of
Acacia Research Corporation
We have completed an integrated auditaudited the accompanying consolidated balance sheet of Acacia Research Corporation's December
31, 2004 consolidated financial statements and of its internal control over
financial reportingCorporation (a Delaware corporation) as of December 31, 20042007, and auditsthe related consolidated statements of its December 31, 2003operations and December 31, 2002 consolidatedcomprehensive loss, stockholders’ equity, and cash flows for the year then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Our
opinions, basedThose standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits, are presented below.
Consolidated Financial statements
- ---------------------------------
audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements listedreferred to above present fairly, in all material respects, the financial position of Acacia Research Corporation as of December 31, 2007, and the results of its operations and its cash flows for the year then ended in conformity with accounting principles generally accepted in the index
appearing under Item 15(a)United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Acacia Research Corporation’s internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 12, 2008 expressed an unqualified opinion thereon.
/s/ GRANT THORNTON LLP
Irvine, California
March 12, 2008
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders of
Acacia Research Corporation
We have audited Acacia Research Corporation’s internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Acacia Research Corporation’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on Acacia Research Corporation’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on page 56the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Acacia Research Corporation maintained, in all material respects, effective internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control—Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet of Acacia Research Corporation as of December 31, 2007 and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for the year then ended and our report dated March 12, 2008, expressed an unqualified opinion on these financial statements.
/s/ GRANT THORNTON LLP
Irvine, California
March 12, 2008
F-2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
of Acacia Research Corporation:
In our opinion, the consolidated balance sheet as of December 31, 2006 and the related consolidated statements of operations and comprehensive loss, shareholders' equity and cash flows for each of the two years in the period ended December 31, 2006, present fairly, in all material respects, the financial position of Acacia Research Corporation and its subsidiaries at December 31, 2004 and December 31, 2003,2006, and the results of their operations and their cash flows for each of the threetwo years in the period ended December 31, 20042006, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit of financial
statements includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
Internal control over
As discussed in Note 2 to the consolidated financial reporting
- -----------------------------------------
Also, in our opinion, management's assessment, included in the accompanying
Management's Report on Internal Control over Financial Reporting, thatstatements, the Company maintained effective internal control over financial reportingchanged the manner in which it accounts for share-based compensation in 2006.
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
Orange County, California
March 12, 2007, except for the last paragraph
in Note 9, and Note 10A, as to which the
date is March 10, 2008
F-3
ACACIA RESEARCH CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
As of December 31, 2004 based on2007 and 2006
(In thousands, except share and per share information)
| December 31, | December 31, | |||||||
| 2007 | 2006 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 40,467 | $ | 32,215 | ||||
| Short-term investments | 10,966 | 12,783 | ||||||
| Accounts receivable | 1,409 | 269 | ||||||
| Prepaid expenses and other current assets | 1,356 | 1,187 | ||||||
| Total current assets related to discontinued operations - Split-off of CombiMatrix Corporation | - | 15,552 | ||||||
| Total current assets | 54,198 | 62,006 | ||||||
| Property and equipment, net of accumulated depreciation | 323 | 221 | ||||||
| Patents, net of accumulated amortization | 16,307 | 18,515 | ||||||
| Other assets | 223 | 200 | ||||||
| Total non-current assets related to discontinued operations - Split-off of CombiMatrix Corporation | - | 28,662 | ||||||
| $ | 71,051 | $ | 109,604 | |||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued expenses | $ | 3,462 | $ | 2,201 | ||||
| Royalties and contingent legal fees payable | 2,343 | 1,684 | ||||||
| Deferred revenues | 321 | 360 | ||||||
| Total current liabilities related to discontinued operations - Split-off of CombiMatrix Corporation | - | 3,211 | ||||||
| Total current liabilities | 6,126 | 7,456 | ||||||
| Other liabilities | 121 | 31 | ||||||
| Total non-current liabilities related to discontinued operations - Split-off of CombiMatrix Corporation | - | 7,808 | ||||||
| Total liabilities | 6,247 | 15,295 | ||||||
| Commitments and contingencies (Note 12) | ||||||||
| Redeemable stockholders' equity: | ||||||||
| Preferred stock | ||||||||
| Acacia Research Corporation, par value $0.001 per share; 10,000,000 shares authorized; | ||||||||
| no shares issued or outstanding | - | - | ||||||
| Common stock | ||||||||
| Acacia Research - Acacia Technologies stock, par value $0.001 per share; 100,000,000 | ||||||||
| shares authorized; 28,231,701 shares issued and outstanding as of December 31, 2006 | - | 28 | ||||||
| Acacia Research - CombiMatrix stock, par value $0.001 per share; 100,000,000 shares | ||||||||
| authorized; 50,365,810 shares issued and outstanding as of December 31, 2006 | - | 50 | ||||||
| Additional paid-in capital | 326,599 | |||||||
| Accumulated comprehensive income | 2 | |||||||
| Accumulated deficit | (232,370 | ) | ||||||
| Stockholders' equity: | ||||||||
| Preferred stock | ||||||||
| Acacia Research Corporation, par value $0.001 per share; 10,000,000 shares authorized; | ||||||||
| no shares issued or outstanding | - | - | ||||||
| Common stock | ||||||||
| Acacia Research - Acacia Technologies stock, par value $0.001 per share; 100,000,000 | ||||||||
| shares authorized; 30,102,482 shares issued and outstanding as of December 31, 2007 | 30 | - | ||||||
| Additional paid-in capital | 159,972 | - | ||||||
| Accumulated comprehensive income | (3 | ) | - | |||||
| Accumulated deficit | (95,195 | ) | - | |||||
| Total stockholders' equity | 64,804 | 94,309 | ||||||
| $ | 71,051 | $ | 109,604 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
For the criteria established in Internal Control -
Integrated Framework issued by the Committee of Sponsoring Organizations of the
Treadway Commission (COSO), is fairly stated, in all material respects, based on
those criteria. Furthermore, in our opinion, the Company maintained, in all
material respects, effective internal control over financial reporting as ofYears Ended December 31, 2004, based on the criteria established in Internal Control -
Integrated Framework issued by the COSO.
2007, 2006 and 2005
(In thousands, except share and per share information)
| 2007 | 2006 | 2005 | |||||||||||
| License fee revenues | $ | 52,597 | $ | 34,825 | $ | 19,574 | |||||||
| Operating expenses: | |||||||||||||
| Marketing, general and administrative expenses (including non-cash stock compensation | |||||||||||||
| expense of $5,908 for 2007, $3,946 for 2006 and $356 for 2005) | 20,042 | 14,123 | 8,097 | ||||||||||
| Inventor royalties and contingent legal fees expense - patents | 29,224 | 17,159 | 11,331 | ||||||||||
| Legal expenses - patents | 7,024 | 4,780 | 2,468 | ||||||||||
| Write-off of patent-related intangible asset | 235 | 297 | - | ||||||||||
| Amortization of patents | 5,583 | 5,313 | 4,922 | ||||||||||
| Total operating expenses | 62,108 | 41,672 | 26,818 | ||||||||||
| Operating loss | (9,511 | ) | (6,847 | ) | (7,244 | ) | |||||||
| Interest and investment income | 2,359 | 1,524 | 1,071 | ||||||||||
| Loss from continuing operations before income taxes | (7,152 | ) | (5,323 | ) | (6,173 | ) | |||||||
| (Provision) benefit for income taxes | (207 | ) | (40 | ) | 135 | ||||||||
| Loss from continuing operations | (7,359 | ) | (5,363 | ) | (6,038 | ) | |||||||
| Discontinued operations: | |||||||||||||
| Loss from discontinued operations - Split-off of CombiMatrix Corporation | (8,086 | ) | (20,093 | ) | (12,401 | ) | |||||||
| Loss on disposal of discontinued operations - Soundbreak.com | - | - | (237 | ) | |||||||||
| Total loss from discontinued operations | (8,086 | ) | (20,093 | ) | (12,638 | ) | |||||||
| Net loss | (15,445 | ) | (25,456 | ) | (18,676 | ) | |||||||
| Unrealized gains (losses) on short-term investments | (21 | ) | 59 | (36 | ) | ||||||||
| Unrealized gains (losses) from discontinued operations - Split-off of CombiMatrix Corporation | 16 | (55 | ) | 111 | |||||||||
| Comprehensive loss | $ | (15,450 | ) | $ | (25,452 | ) | $ | (18,601 | ) | ||||
| Loss per common share: | |||||||||||||
| Acacia Research - Acacia Technologies stock: | |||||||||||||
| Loss from continuing operations | $ | (7,359 | ) | $ | (5,363 | ) | $ | (6,038 | ) | ||||
| Basic and diluted loss per share | (0.26 | ) | (0.19 | ) | (0.23 | ) | |||||||
| Loss on disposal of discontinued operations | $ | - | $ | - | $ | (237 | ) | ||||||
| Basic and diluted loss per share | - | - | (0.01 | ) | |||||||||
| Net loss | $ | (7,359 | ) | $ | (5,363 | ) | $ | (6,275 | ) | ||||
| Basic and diluted loss per share | (0.26 | ) | (0.19 | ) | (0.24 | ) | |||||||
| Acacia Research - CombiMatrix stock - Discontinued Operations - Split-off of CombiMatrix Corporation: | |||||||||||||
| Loss from discontinued operations - Split-off of CombiMatrix Corporation | $ | (8,086 | ) | $ | (20,093 | ) | $ | (12,401 | ) | ||||
| Basic and diluted loss per share | (0.14 | ) | (0.49 | ) | (0.37 | ) | |||||||
| Weighted average shares: | |||||||||||||
| Acacia Research - Acacia Technologies stock: | |||||||||||||
| Basic and diluted | 28,503,314 | 27,547,651 | 26,630,732 | ||||||||||
| Acacia Research - CombiMatrix stock: | |||||||||||||
| Basic and diluted | 55,862,707 | 40,605,038 | 33,678,603 | ||||||||||
The Company's management is responsible for maintaining effective internal
control over financial reporting and for its assessmentaccompanying notes are an integral part of the effectiveness of
internal control over financial reporting. Our responsibility is to express
opinions on management's assessment and on the effectiveness of the Company's
internal control over financial reporting based on our audit. We conducted our
audit of internal control over financial reporting in accordance with the
standards of the Public Company Accounting Oversight Board (United States).
Those standards require that we plan and perform the audit to obtain reasonable
assurance about whether effective internal control over financial reporting was
maintained in all material respects. An audit of internal control over financial
reporting includes obtaining an understanding of internal control over financial
reporting, evaluating management's assessment, testing and evaluating the design
and operating effectiveness of internal control, and performing such other
procedures as we consider necessary in the circumstances. We believe that our
audit provides a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to
provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance
with generally accepted accounting principles. A company's internal control over
financial reporting includes those policies and procedures that (i) pertain to
the maintenance of records that, in reasonable detail, accurately and fairly
reflect the transactions and dispositions of the assets of the company; (ii)
provide reasonable assurance that transactions are recorded as necessary to
permit preparation of financial statements in accordance with generally accepted
accounting principles, and that receipts and expenditures of the company are
being made only in accordance with authorizations of management and directors of
the company; and (iii) provide reasonable assurance regarding prevention or
timely detection of unauthorized acquisition, use, or disposition of the
company's assets that could have a material effect on thethese consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting
may not prevent or detect misstatements. Also, projections of any evaluation of
effectiveness to future periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree of compliance
with the policies or procedures may deteriorate
/s/PricewaterhouseCoopers LLP
Los Angeles, California
March 14, 2005
F-1
F-5
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
For the Years Ended December 31, 2007, 2006 and 2005
(In thousands, except share information)
| AR-Acacia | AR- | AR-Acacia | AR- | |||||||||||||||||||||||||||||||||
| Technologies | CombiMatrix | Technologies | CombiMatrix | |||||||||||||||||||||||||||||||||
| Redeemable | Redeemable | Redeemable | Redeemable | Additional | Deferred | Other | ||||||||||||||||||||||||||||||
| Common | Common | Common | Common | Paid-in | Stock | Comprehensive | Accumulated | |||||||||||||||||||||||||||||
| Shares | Shares | Stock | Stock | Capital | Compensation | Income (Loss) | Deficit | Total | ||||||||||||||||||||||||||||
| 2005 | ||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2004 | 19,811,524 | 31,200,496 | $ | 20 | $ | 31 | $ | 263,900 | $ | - | $ | (77 | ) | $ | (188,238 | ) | $ | 75,636 | ||||||||||||||||||
| Net loss | (18,676 | ) | (18,676 | ) | ||||||||||||||||||||||||||||||||
| Stock options exercised | 133,986 | 5,555 | 315 | 315 | ||||||||||||||||||||||||||||||||
| Stock issued for the acquisition of Global Patent Holdings, net of registration costs (Note 7) | 3,938,832 | 4 | 19,289 | 19,293 | ||||||||||||||||||||||||||||||||
| Units issued in direct offering, net offering costs | 3,500,000 | 7,786,351 | 4 | 8 | 32,244 | 32,256 | ||||||||||||||||||||||||||||||
| Warrant liability | (2,194 | ) | (2,194 | ) | ||||||||||||||||||||||||||||||||
| Deferred stock compensation | 337,900 | 1,713 | (1,713 | ) | - | |||||||||||||||||||||||||||||||
| Compensation expense relating to stock options | (121 | ) | 313 | 192 | ||||||||||||||||||||||||||||||||
| Unrealized gain on short-term investments | 2 | 2 | ||||||||||||||||||||||||||||||||||
| Unrealized gain on foreign currency translation | 73 | 73 | ||||||||||||||||||||||||||||||||||
| Balance at December 31, 2005 | 27,722,242 | 38,992,402 | $ | 28 | $ | 39 | $ | 315,146 | $ | (1,400 | ) | $ | (2 | ) | $ | (206,914 | ) | $ | 106,897 | |||||||||||||||||
| 2006 | ||||||||||||||||||||||||||||||||||||
| Net loss | (25,456 | ) | (25,456 | ) | ||||||||||||||||||||||||||||||||
| Stock options exercised | 389,959 | 1,475 | 1,475 | |||||||||||||||||||||||||||||||||
| Units issued in direct offering, net offering costs | 11,323,408 | 11 | 12,098 | 12,109 | ||||||||||||||||||||||||||||||||
| Warrant liability | (7,104 | ) | (7,104 | ) | ||||||||||||||||||||||||||||||||
| Reclassification of deferred stock compensation (see Note 2) | (1,400 | ) | 1,400 | - | ||||||||||||||||||||||||||||||||
| Stock issued to consultant | 50,000 | 94 | 94 | |||||||||||||||||||||||||||||||||
| Compensation expense relating to stock options and restricted stock awards | 119,500 | 6,306 | 6,306 | |||||||||||||||||||||||||||||||||
| Unrealized gain on short-term investments | 61 | 61 | ||||||||||||||||||||||||||||||||||
| Unrealized loss on foreign currency translation | (57 | ) | (57 | ) | ||||||||||||||||||||||||||||||||
| Other | - | - | (16 | ) | (16 | ) | ||||||||||||||||||||||||||||||
| Balance at December 31, 2006 | 28,231,701 | 50,365,810 | $ | 28 | $ | 50 | $ | 326,599 | $ | - | $ | 2 | $ | (232,370 | ) | $ | 94,309 | |||||||||||||||||||
(continued below)
F-6a
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (CONTINUED)
For the Years Ended December 31, 2007, 2006 and 2005
(In thousands, except share information)
AR-Acacia Technologies Common Shares | AR- CombiMatrix Common Shares | AR-Acacia Technologies Common Stock | AR- CombiMatrix Common Stock | Additional Paid-in Capital | Deferred Stock Compensation | Other Comprehensive Income (Loss) | Accumulated Deficit | Total | ||||||||||||||||||||||||||||
| 2007 | ||||||||||||||||||||||||||||||||||||
| Activities related to continuing operations: | ||||||||||||||||||||||||||||||||||||
| Net loss from continuing operations | (7,359 | ) | (7,359 | ) | ||||||||||||||||||||||||||||||||
| Stock options exercised | 1,062,513 | 1 | 5,013 | 5,014 | ||||||||||||||||||||||||||||||||
| Compensation expense relating to stock options and restricted stock awards | 808,268 | 1 | 5,908 | 5,909 | ||||||||||||||||||||||||||||||||
| Unrealized loss on short-term investments | (21 | ) | (21 | ) | ||||||||||||||||||||||||||||||||
| Other | (55 | ) | (55 | ) | ||||||||||||||||||||||||||||||||
| Activities related to discontinued operations - Split-off of CombiMatrix Corporation: | ||||||||||||||||||||||||||||||||||||
| Loss from discontinued operations - Split-off of CombiMatrix Corporation | (8,086 | ) | (8,086 | ) | ||||||||||||||||||||||||||||||||
| Stock options and warrants exercised and units issued in direct offering, net offering costs | 9,203,959 | 10 | 480 | 490 | ||||||||||||||||||||||||||||||||
| Compensation expense relating to stock options | 726 | 726 | ||||||||||||||||||||||||||||||||||
| Warrant liability | 9,089 | 9,089 | ||||||||||||||||||||||||||||||||||
| Stock issued to consultant | 306,000 | 208 | 208 | |||||||||||||||||||||||||||||||||
| Unrealized gain on short-term investments | 13 | 13 | ||||||||||||||||||||||||||||||||||
| Other | 11 | 11 | ||||||||||||||||||||||||||||||||||
| Discontinued operations - Split-off of CombiMatrix Corporation | (59,875,769 | ) | (60 | ) | (188,062 | ) | 3 | 152,675 | (35,444 | ) | ||||||||||||||||||||||||||
| Balance at December 31, 2007 | 30,102,482 | - | $ | 30 | $ | - | $ | 159,972 | $ | - | $ | (3 | ) | $ | (95,195 | ) | $ | 64,804 | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6b
ACACIA RESEARCH CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the Years Ended December 31, 2007, 2006 and 2005
(In thousands)
| 2007 | 2006 | 2005 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | (15,445 | ) | $ | (25,456 | ) | $ | (18,676 | ) | |||
| Discontinued operations - Split-off of CombiMatrix Corporation | 8,086 | 20,093 | 12,401 | |||||||||
| Adjustments to reconcile net loss to net cash provided by (used in) operating | ||||||||||||
| activities from continuing operations: | ||||||||||||
| Depreciation and amortization | 5,702 | 5,392 | 4,981 | |||||||||
| Minority interests | - | - | (2 | ) | ||||||||
| Non-cash stock compensation | 5,908 | 3,946 | 356 | |||||||||
| Deferred income taxes | - | (36 | ) | (143 | ) | |||||||
| Loss on disposal of discontinued operations - Soundbreak.com | - | - | 237 | |||||||||
| Write-off of patent-related intangible asset | 235 | 297 | - | |||||||||
| Other | 112 | (96 | ) | - | ||||||||
| Changes in assets and liabilities: | ||||||||||||
| Accounts receivable | (1,140 | ) | 4,152 | (4,228 | ) | |||||||
| Prepaid expenses and other assets | (193 | ) | (150 | ) | (774 | ) | ||||||
| Accounts payable and accrued expenses | 1,281 | 819 | (729 | ) | ||||||||
| Royalties and contingent legal fees payable | 659 | (2,074 | ) | 3,758 | ||||||||
| Deferred revenues | (39 | ) | (279 | ) | 211 | |||||||
| Net cash provided by (used in) operating activities from continuing operations | 5,166 | 6,608 | (2,608 | ) | ||||||||
| Net cash used in operating activities from discontinued operations | (7,782 | ) | (15,261 | ) | (14,078 | ) | ||||||
| Net cash used in operating activities | (2,616 | ) | (8,653 | ) | (16,686 | ) | ||||||
| Cash flows from investing activities: | ||||||||||||
| Purchase of property and equipment | (223 | ) | (179 | ) | (75 | ) | ||||||
| Purchase of available-for-sale investments | (13,035 | ) | (16,409 | ) | (39,919 | ) | ||||||
| Sale of available-for-sale investments | 14,873 | 28,147 | 33,141 | |||||||||
| Business acquisition | - | (16 | ) | (5,796 | ) | |||||||
| Patent acquisition costs | (3,760 | ) | (1,030 | ) | (445 | ) | ||||||
| Net cash provided by (used in) investing activities from continuing operations | (2,145 | ) | 10,513 | (13,094 | ) | |||||||
| Net cash provided by (used in) investing activities from discontinued operations | (5,199 | ) | 4,628 | 3,390 | ||||||||
| Net cash provided by (used in) investing activities | (7,344 | ) | 15,141 | (9,704 | ) | |||||||
| Cash flows from financing activities: | ||||||||||||
| Proceeds from the sale of common stock, net of issuance costs | - | - | 19,532 | |||||||||
| Proceeds from the exercise of stock options | 5,014 | 1,475 | 304 | |||||||||
| Net cash provided by financing activities from continuing operations | 5,014 | 1,475 | 19,836 | |||||||||
| Net cash provided by financing activities from discontinued operations | 5,369 | 11,917 | 12,735 | |||||||||
| Net cash provided by financing activities | 10,383 | 13,392 | 32,571 | |||||||||
| Effect of exchange rate on cash related to discontinued operations - Split-off of | ||||||||||||
| CombiMatrix Corporation | - | - | 73 | |||||||||
| Increase in cash and cash equivalents | 423 | 19,880 | 6,254 | |||||||||
| Cash and cash equivalents, beginning (including cash and cash equivalents related to discontinued | ||||||||||||
| operations - split-off of CombiMatrix Corporation of $7,829, $5,666 and $2,985, respectively) | 40,044 | 20,164 | 13,910 | |||||||||
| Cash and cash equivalents, ending | 40,467 | 40,044 | 20,164 | |||||||||
| Less: Cash and cash equivalents of discontinued operations, ending | - | (7,829 | ) | (5,666 | ) | |||||||
| Cash and cash equivalents of continuing operations, ending | $ | 40,467 | $ | 32,215 | $ | 14,498 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS
BUSINESSES
Description of Business. Acacia Research Corporation ("we," "us" and "our") is comprised of two
operating groups.
Our life sciences business, referred to as the "CombiMatrix group," is
comprised of our wholly owned subsidiary, CombiMatrix Corporation and
CombiMatrix Corporation's wholly owned subsidiary, CombiMatrix K.K.
The CombiMatrix group is seeking to become a broadly diversified
biotechnology company, through the development of proprietary technologies and
products in the areas of drug development, genetic analysis, nanotechnology
research, defense and homeland security markets, as well as other potential
markets where its products could be utilized. Among the technologies being
developed by the CombiMatrix group are a platform technology to rapidly produce
customizable arrays, which are semiconductor-based tools for use in identifying
and determining the roles of genes, gene mutations and proteins. This technology
has a wide range of potential applications in the areas of genomics, proteomics,
biosensors, drug discovery, drug development, diagnostics, combinatorial
chemistry, material sciences and nanotechnology. Other technologies include
proprietary molecular synthesis and screening methods for the discovery of
potential new drugs. CombiMatrix K.K., a wholly owned Japanese corporation
located in Tokyo, is exploring opportunities for CombiMatrix Corporation's array
system with pharmaceutical and biotechnology companies in the Asian market.
Our intellectual property licensing business, referred to as the "the
Acacia Technologies group," is primarily comprised of our interests in three
wholly owned subsidiaries: (1) Acacia Media Technologies Corporation, ("Acacia
Media Technologies") a Delaware corporation, (2) Soundview Technologies, Inc.,
("Soundview Technologies") a Delaware corporation, and (3) Acacia Internet
Access Corporation, a Delaware corporation, and also includes all corporate
assets, liabilities, and related transactions of Acacia Research Corporation, attributedand its wholly owned operating subsidiaries. As used herein, “we,” “us” and “our” refer to the Acacia Research Corporation'sCorporation and/or its wholly owned operating subsidiaries. All intellectual property acquisition, development, licensing business.
Theand enforcement activities are conducted solely by certain of Acacia Technologies group develops, acquires,Research Corporation’s wholly owned operating subsidiaries.
Acacia Research Corporation’s operating subsidiaries acquire, develop, license and licensesenforce patented technologies. IncludingOur operating subsidiaries generate license fee revenues and related cash flows from the impactgranting of licenses for the January 28, 2005 acquisitionuse of patented technologies that our operating subsidiaries own or control. Our operating subsidiaries assist patent owners with the assetsprosecution and development of Global Patent Holdings, LLC ("Global Patent Holdings") discussed at
Note 15,their patent portfolios, the Acacia Technologies group controls 29protection of their patented inventions from unauthorized use, the generation of licensing revenue from users of their patented technologies and, if necessary, with the enforcement against unauthorized users of their patented technologies. Currently, on a consolidated basis, our operating subsidiaries own or control the rights to 91 patent portfolios, which include 126 U.S. patents and certain foreign counterparts, covering technologies used in a wide variety of industries including
audio/video-on-demand, digital ad insertion, interactive television, broadcast
equipment, data transmission, cache coherency, data file synchronization, data
matrix bar codes, dynamic manufacturing models, product activation, encryption,
image resolutionindustries.
CombiMatrix Group Split-off Transaction and enhancement, scheduling software, interstitial Internet
advertising, interactive simulation systems, peerRelated Discontinued Operations. In January 2006, Acacia Research Corporation’s board of directors approved a plan for its wholly owned subsidiary, CombiMatrix Corporation, the primary component of Acacia Research Corporation’s CombiMatrix group, to peer network
communications, spreadsheet programs, endoscopic cameras, video noise reduction,become an independent public company. CombiMatrix Corporation’s registration statement on Form S-1 was declared effective by the Securities and audio/video synchronization.
Exchange Commission (“SEC”) on June 8, 2007. Following the redemption period required by Acacia Research Corporation’s Restated Certificate of Incorporation, on August 15, 2007 (the “Redemption Date”), CombiMatrix Corporation was split-off from Acacia Research Corporation through the redemption of all outstanding shares of Acacia Research-CombiMatrix common stock in exchange for the distribution of new shares of CombiMatrix Corporation common stock, on a pro-rata basis, to the holders of Acacia Research-CombiMatrix common stock on the Redemption Date (the “Split-off Transaction”). On the Redemption Date, every ten (10) shares of Acacia Research-CombiMatrix common stock outstanding on August 15, 2007, was redeemed for one (1) share of common stock of CombiMatrix Corporation. Subsequent to the Redemption Date, Acacia Research Corporation no longer owns any equity interests in CombiMatrix Corporation and the two companies operate independently of each other.
As a result of the Split-off Transaction, we have disposed of our investment in CombiMatrix Corporation. Refer to Note 10A for information regarding presentation of the assets, liabilities, results of operations and cash flows for the CombiMatrix group as discontinued operations in the accompanying consolidated financial statements for all periods presented, in accordance with guidance set forth in SFAS No. 144 “Accounting for the Impairment or Disposal of Long-Lived Assets” (“SFAS No. 144”).
Capital Structure. As a result of the Split-off Transaction, the CombiMatrix group is no longer a business group of Acacia Research Corporation. Pursuant to the Split-off Transaction, all outstanding shares of Acacia Research-CombiMatrix common stock were redeemed, and hence, all rights of holders of Acacia Research-CombiMatrix common stock ceased as of the Redemption Date, except for the right, upon the surrender to the exchange agent of shares of Acacia Research-CombiMatrix common stock, to receive new shares of CombiMatrix Corporation stock pursuant to the exchange ratio described above. Subsequent to the consummation of the Split-off Transaction, Acacia Research Corporation’s only class of common stock outstanding is its Acacia Research-Acacia Technologies common stock.
Prior to the Split-off Transaction, Acacia Research Corporation had two classes of common stock outstanding, its Acacia Research-Acacia Technologies common stock (“AR-Acacia Technologies stock”) and its Acacia Research-CombiMatrix common stock ("AR-CombiMatrix stock"). AR-Acacia Technologies stock was intended to reflect separately the performance of Acacia Research Corporation’s Acacia Technologies group. AR-CombiMatrix stock was intended to reflect separately the performance of Acacia Research Corporation’s CombiMatrix group. Although the AR-Acacia Technologies stock and the AR-CombiMatrix stock were intended to reflect the performance of our different business groups, they were both classes of common stock of Acacia Research Corporation and were not stock issued by the respective groups.
We were incorporated on January 25, 1993 under the laws of the State of California. In December 1999, we changed our state of incorporation from California to Delaware.
LIQUIDITY AND RISKS
To date, we
F-8
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Liquidity and our subsidiaries have relied primarily upon selling equity
securities and payments from our strategic partners and licensees to generate
the funds needed to finance the implementation of our plans of operation for our
subsidiaries. Risks
Management believes that ourAcacia Research Corporation’s consolidated cash and cash equivalent and short-term investment balances, anticipated cash flow from operations and other external sources of available credit will be sufficient to meet ourAcacia Research Corporation’s cash requirements, on a consolidated basis, through at least March 2009. To date, we and our subsidiaries have relied primarily upon selling equity securities and payments from our licensees to generate the next twelve
months.funds needed to finance the implementation of our plans of operation for our subsidiaries.
There can be no assurance that Acacia Research Corporation will be able to implement its future plans. Failure by management to achieve its plans would have a material adverse effect on Acacia Research Corporation’s ability to achieve its intended business objectives. We may be required to obtain additional financing. We cannot assureThere can be no assurance that additional funding will be available on favorable terms, if at all. If we fail to obtain additional funding when needed, we may not be able to execute our business plans and our businesses may suffer. Our business operations
The timing of the receipt of revenues by Acacia Research Corporation’s operating subsidiaries are also subject to certain risks and uncertainties, including:
F-6
o market acceptance of products and services;
o technological advances that may make our products and services
obsolete or less competitive;
o increases in operating costs, including costs for supplies, personnel
and equipment;
o the availability and cost of capital;
o general economic conditions; and
o governmental regulation that may restrict our subsidiaries'
businesses.
The CombiMatrix group is deploying unproven
| · | market acceptance of our operating subsidiaries’ patented technologies |
| · | business activities and financial results of our licensees; |
| · | technological advances that may make our patented technologies obsolete or less competitive; |
| · | increases in operating costs, including costs for legal services, engineering and research and personnel; |
| · | the availability and cost of capital; and |
| · | governmental regulation that may restrict Acacia Research Corporation’s business. |
Acacia Research Corporation's ability to achieve its
intended business objectives. The CombiMatrix group'sCorporation’s success also depends on its operating subsidiaries’ ability to protect itstheir intellectual property. In addition, the CombiMatrix group cannot assure that it will not encounter
unforeseen difficulties that may deplete capital resources more rapidly than
anticipated. Any efforts to seek additional funds could be made through equity,
debt or other external financings; however, the CombiMatrix group cannot assure
that additional funding will be availableOur operating subsidiaries rely on favorable terms, if at all. If the
CombiMatrix group fails to obtain additional funding when needed, the
CombiMatrix group may not be able to execute its business strategies and its
business may suffer.
The CombiMatrix group's business depends on issued and pending patents, and
the loss of any patents or the group's failure to secure the issuance of patents
covering elements of its business processes would materially harm its business
and financial condition. The patents covering the CombiMatrix group's core
technology begin to expire January 5, 2018.
To date, the Acacia Technologies group has relied primarily upon selling of
Acacia Research Corporation equity securities and payments from our V-chip
licensees (primarily in 2001) and Digital Media Transmission ("DMT(R)")
licensees (2003 to current) to generate the funds needed to finance the
operations of the Acacia Technologies group. The V-chip patent expired in July
2003. The V-chip licensing program was concluded in August 2004 and we do not
expect to collect any additional V-chip related license fee revenues in future
periods. The Acacia Technologies group began to commercially license its DMT
technology in 2003, recognizing approximately $3.5 million in DMT license fee
revenues to date, and intends to acquire and develop additional intellectual
property. Acacia Global Acquisition Corporation's acquisition of the assets of
Global Patent Holdings, LLC as discussed at Note 15, provides the Acacia
Technologies group with ownership of companies that control 27 patent
portfolios, which include 120 U.S. patents and certain foreign counterparts, and
cover technologies used in a wide variety of industries. The acquisition expands
and diversifies the Acacia Technologies group's revenue generating
opportunities.
However, there can be no assurance that the Acacia Technologies group will
be able to implement its future plans. Failure by management to achieve its
plans would have a material adverse effect on the Acacia Technologies group and
on Acacia Research Corporation's ability to achieve its intended business
objectives. The Acacia Technologies group's success also depends on its ability
to protect its intellectual property.
The Acacia Technologies group relies on itstheir proprietary rights and their protection. Although reasonable efforts will be taken to protect the Acacia
Technologies group'sour operating subsidiaries’ proprietary rights, the complexity of international trade secret, copyright, trademark and patent law, and common law, coupled with limited resources and the demands of quick delivery of technologies to market, create risk that these efforts will prove inadequate. Accordingly, if weour operating subsidiaries are unsuccessful with litigation to protect ourtheir intellectual property rights, the future consolidated revenues of the Acacia Technologies groupResearch Corporation could be adversely affected.
The Acacia Technologies group's U.S. DMT patents expire in 2011 and its foreign
DMT patents expire in 2012.
F-7
RECAPITALIZATION TRANSACTION
On December 11, 2002, our stockholders voted in favor of a recapitalization
transaction, which became effective on December 13, 2002, whereby we created two
new classes of common stock called Acacia Research-CombiMatrix stock
("AR-CombiMatrix stock") and Acacia Research-Acacia Technologies stock
("AR-Acacia Technologies stock"), and divided our existing Acacia Research
Corporation common stock into shares of the two new classes of common stock.
AR-CombiMatrix stock is intended to reflect separately the performance of Acacia
Research Corporation's CombiMatrix group. AR-Acacia Technologies stock is
intended to reflect separately the performance of Acacia Research Corporation's
Acacia Technologies group. Although the AR-CombiMatrix stock and the AR-Acacia
Technologies stock are intended to reflect the performance of our different
business groups, they are both classes of common stock of Acacia Research
Corporation and are not stock issued by the respective groups.
All share and per share information in the consolidated financial
statements and accompanying notes to the consolidated financial statements,
unless otherwise noted, give effect to the recapitalization as of January 1,
2002.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
ACCOUNTING PRINCIPLES AND FISCAL YEAR END.
Accounting Principles and Fiscal Year End. The consolidated financial statements and accompanying notes are prepared on the accrual basis of accounting in accordance with generally accepted accounting principles in the United States of America. We have a December 31 year end.
PRINCIPLES OF CONSOLIDATION.
Principles of Consolidation. The accompanying consolidated financial statements include the accounts of Acacia Research Corporation and its wholly owned and majority-owned subsidiaries. Investments for which Acacia Research
Corporation possesses the power to direct or cause the direction of the
management and policies, either through majority ownership or other means, are
accounted for under the consolidation method. Material intercompany transactions and balances have been eliminated in consolidation. Investments in companies in
which we maintain an ownership interest of 20% to 50% or exercise significant
influence over operating and financial policies are accounted for under the
equity method. The cost method is used where we maintain ownership interests of less than 20% and do not exercise significant influence over the investee.
REVISION IN THE CLASSIFICATION OF CERTAIN SECURITIES. In connection with
the preparation of this report, we concluded that it was appropriate to classify
our auction rate municipal bonds and variable rate municipal demand notes as
current investments. Previously, such investments had been classified as cash
and cash equivalents. Accordingly, we have revised our prior classification to
report these securities as current investments in our Consolidated Balance Sheet
as of December 31, 2003. We have also made corresponding adjustments to our
Consolidated Statement of Cash Flows for the year ended December 31, 2002, to
reflect the gross purchases and sales of these securities as investing
activities rather than as a component of cash and cash equivalents. This change
in classification does not affect previously reported cash flows from operations
or from financing activities in our previously reported Consolidated Statements
of Cash Flows, or our previously reported Consolidated Statements of Income for
any period.
As of December 31, 2003, before this revision in classification, $7,750,000
of these current investments were classified as cash and cash equivalents on our
Consolidated Balance Sheet. For the fiscal year ended December 31, 2002, before
this revision in classification, net cash used in investing activities related
to these current investments of $7,750,000 were included in cash and cash
equivalents in our Consolidated Statement of Cash Flows.
REVENUE RECOGNITION.
Revenue Recognition. We recognize revenue in accordance with Staff Accounting Bulletin No. 104, "Revenue Recognition" ("“Revenue Recognition” (“SAB No. 104"104”) and related authoritative pronouncements. Revenues from multiple-element arrangements are
accounted for in accordance with Emerging Issues Task Force ("EITF") Issue
00-21, "Revenue Arrangements with Multiple Deliverables." Revenue is recognized when (i) persuasive evidence of an arrangement exists, (ii) all obligations have been substantially performed pursuant to the terms of the license agreement, (iii) amounts are fixed or determinable and (iv) collectibility of amounts is reasonably assured.
F-8
COMBIMATRIX GROUP
Revenues from multiple-element arrangements involving license fees,
up-front payments and milestone payments, which are received and/or billable by
us in connection with other rights and services that represent continuing
obligations of ours, are deferred until all of the elements have been delivered
or until we have established objective and verifiable evidence of the fair value
of the undelivered elements.
Revenues from government grants and contracts are recognized in accordance
with Accounting Research Bulletin ("ARB") No. 43, "Government Contracts," and
related pronouncements. Accordingly, revenues are recognized under the
percentage-of-completion method of accounting, using the cost-to-cost approach
to measure completeness at each reporting period. Under the
percentage-of-completion method of accounting, contract revenues and expenses
are recognized in the period that work is performed based on the percentage of
actual incurred costs to estimated total contract costs. Actual contract costs
and cost estimates include direct charges for labor and materials and indirect
charges for labor, overhead and certain general and administrative charges.
Contract change orders and claims are included when they can be reliably
estimated and are considered probable. For contracts that extend over a one-year
period, revisions in contract cost estimates, if they occur, have the effect of
adjusting current period earnings applicable to performance in prior periods.
Should current contract estimates indicate an overall future loss to be
incurred, a provision is made for the total anticipated loss in the current
period.
Revenue from the sale of products and services, including shipping and
handling fees, are recognized when delivery has occurred or services have been
rendered.
Deferred revenues arise from payments received in advance of the
culmination of the earnings process. Deferred revenues expected to be recognized
within the next twelve months are classified within current liabilities.
Deferred revenues will be recognized as revenue in future periods when the
applicable revenue recognition criteria as described above are met.
F-9
ACACIA TECHNOLOGIES GROUP
RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Under the terms of our DMT license agreements, the Acacia Technologies
group grantsour operating subsidiaries grant non-exclusive licenses for the use of its patented DMT technology.
Pursuanttechnologies, which they own or control. In general, pursuant to the terms of our DMT licensethe agreements once executed,with licensees, upon the Acacia
Technologies group hasgrant of the licenses, we have no further obligations with respect to the grant of the
licenses.licenses granted. License fees paid to and recognized as revenue by the Acacia
Technologies groupour subsidiaries are non-refundable.
Revenue
Revenues generated from license agreements are generally accrued and recognized as revenue in the period earned, provided that amounts are fixed or determinable and collectibility is reasonably assured.
Certain license agreements provide for the payment of contractually determined paid-up license fees in consideration for the grant of a non-exclusive, retroactive and future license to manufacture and/or sell products covered by patented technologies owned or controlled by our operating subsidiaries. Certain of the agreements also provide for future royalties or additional required payments based on future activities. Generally, the execution of these license agreements also provide for the release of the licensee from certain claims and the dismissal of any pending litigation. Pursuant to the terms of these agreements, our operating subsidiaries have no further obligation with respect to the grant of the non-exclusive retroactive and future license and related releases, including no express or implied obligation on our operating subsidiaries’ part to maintain or upgrade the technology, or provide future support or services. Generally, the agreements provide for the grant of the license and releases upon execution of the agreement. As such, the earnings process is complete upon the execution of the agreement, and revenue is recognized upon execution of the agreement, when collectibility is reasonably assured, and all other revenue recognition criteria have been met. Refer to Note 12 for information on inventor royalties and contingent legal fees.
Certain license agreements provide for the calculation of license fees based on a licensee'slicensee’s actual quarterly sales or actual per unit activity, applied to a contractual royalty rate. Licensees that pay license fees on a quarterly basis generally report actual quarterly sales or actual per unit activity information and related quarterly license fees due to the Acacia
Technologies group within 30 to 45 days after the end of the quarter in which such sales or activity takes place. The amount of license fees due under these license agreements each quarter cannot be reasonably estimated by management. Consequently, the Acacia Technologies group
recognizesour operating subsidiaries recognize revenue from these licensing agreements on a three-month lag basis, in the quarter following the quarter of sales or per unit activity, provided amounts are fixed or determinable and collectibility is reasonably assured. The lag method described above allows for the receipt of licensee royalty reports prior to the recognition of revenue.
Certain license agreements provide for the payment of a minimum upfront annual license fee at the inception of each annual license term. Minimum upfront annual license fees are generally determined based on a licensee'slicensee’s estimated annual sales or a licensee'slicensee’s base level of per unit activity. These minimum upfront annual license fee payments are deferred and amortized to revenue on a straight-line basis over the annual license term. To the extent actual annual royalties, determined and reported in accordance with the terms of the respective agreements, exceed the minimum upfront annual license fees paid, the additional royalties are recognized in revenue in the quarter following the quarter in which the base per unit activity was exceeded or the quarter following the annual license term, depending on the terms of the respective agreement, provided that amounts are fixed or determinable and collectibility is reasonably assured. F-9
Amounts of additional royalties due under these license agreements cannot be reasonably estimated by management.
License fee payments received by the Acacia Technologies group that do not meet the revenue recognition criteria described above are deferred until the revenue recognition criteria are met. The Acacia Technologies group assesses
collectionWe assess the collectibility of accrued license fees receivable based on a number of factors, including past transaction history and credit-worthiness.credit-worthiness of licensees. If it is determined that collection is not reasonably assured, the fee is recognized when collectibility becomes reasonably assured, assuming all other revenue recognition criteria have been met, which is generally upon receipt of cash.
As a result of our licensing
Cash and any related intellectual property
enforcement activities that we choose to conduct, we may recognize royalty
revenues that relate to prior period infringements by licensees. Differences
between amounts initially recognized and amounts subsequently audited or
reported as an adjustment to those amounts will be recognized in the period the
adjustment is determined as a change in accounting estimate.
CASH AND CASH EQUIVALENTS. Cash Equivalents. We consider all highly liquid, short-term investments with original maturities of three months or less when purchased to be cash equivalents.
SHORT-TERM INVESTMENTS.
F-10
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Short-term Investments. Our short-term investments are held in a variety of interest bearing instruments including U.S. government debt securities, high-grade corporate bonds, commercial paper, auction rate securities, money market accounts, certificates of deposit and other high-credit quality marketable securities. Investments in securities with original maturities of greater than three months and less than one year and other investments representing amounts that are available for current operations are classified as short-term investments. Investments are classified into categories in accordance with the provisions of Statement of Financial Accounting Standards ("SFAS"(“SFAS”) No. 115, "Accounting“Accounting for Certain Investments in Debt and Equity Securities" ("Securities” (“SFAS No. 115"115”). At December 31, 20042007 and 2003,2006, all of our investments are classified as available-for-sale, which are reported at fair value with related unrealized gains and losses in the value of such securities recorded as a separate component of comprehensive income (loss) in stockholders'stockholders’ equity until realized.
During 2002, certain of our investments were classified as trading securities.
Realized and unrealized gains and losses in the value of trading securities are
included in net loss in the consolidated statements of operations and
comprehensive loss.
The fair value of our investments is determined by quoted market prices. Realized and unrealized gains and losses are recorded based on the specific identification method. For investments classified as available-for-sale, unrealized losses that are other than temporary are recognized in net loss.
the consolidated statement of operations and comprehensive income (loss) (hereinafter “consolidated statement of operations”). An impairment is deemed other than temporary unless (a) we have the ability and intent to hold an investment for a period of time sufficient for recovery of its carrying amount and (b) positive evidence indicating that the investment's carrying amount is recoverable within a reasonable period of time outweighs any evidence to the contrary. All available evidence, both positive and negative, is considered to determine whether, based on the weight of that evidence, the carrying amount of the investment is recoverable within a reasonable period of time.
The cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization is included in interest income (expense). Interest and dividends on all securities are included in interest income.
At December 31, 20042007 and 2003,2006, we held $11,900,000$10,660,000 and $7,750,000,$7,288,000, respectively of short-term investments, whichinvestment grade securities with an auction reset feature (“auction rate securities”). At December 31, 2007, our auction rate securities consist of securities issued by closed-end investment companies with portfolio asset coverage of at least 200%, and auction rate municipal
bonds and variableinvestments backed by student loans, issued under programs such as the Federal Family Education Loan Program, all of which had credit ratings of AAA when purchased. As of March 5, 2008, we held $6,250,000 in auction rate municipal demand notes classified as available-for-sale
securities. securities investments.
Our investments in these securities are recorded at cost, which approximates fair market value due to their variable interest rates, which typically reset every 7 to 35 days, and, despite the long-term nature of their stated contractual maturities, we have the ability to quickly liquidate these
securities.maturities. As a result, we had no cumulative gross unrealized holding gains (losses) or gross realized gains (losses) from our current investments.these investments for the periods presented. All income generated from these current investments was recorded as interest income.
CONCENTRATION OF CREDIT RISKS.
The Dutch auction process that resets the applicable interest rate at predetermined calendar intervals is intended to provide liquidity to the holder of auction rate securities by matching buyers and sellers within a market context enabling the holder to gain immediate liquidity by selling such interests at par or rolling over their investment. If there is an imbalance between buyers and sellers the risk of a failed auction exists.
We have not experienced a failed auction for any of our securities as of December 31, 2007. However, we did experience failed auctions with certain of our issues in February 2008. Given the deteriorating credit markets, and the increased incidence of failure within the auction market in February 2008, there can be no assurance as to when we would be able to liquidate a particular issue. In such case of a failure, the auction rate securities continue to pay interest in accordance with their terms, however, we may not be able to access the par value of the invested funds until a future auction of these investments is successful, the security is called by the issuer or a buyer is found outside of the auction process. Furthermore, if this situation were to persist despite our ability to hold such investments until maturity, we may be required to record an impairment charge in a future period.
Management believes that the fair value of our investments in auction rate securities continue to approximate their par value and that the underlying credit quality of the assets backing its auction rate securities investments have not been impacted by the reduced liquidity of these investments subsequent to December 31, 2007. We will continue to monitor and evaluate our investments in auction rate securities for any further reduction in liquidity and potential impairment in future periods.
F-11
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Concentration of Credit Risks. Financial instruments that potentially subject Acacia Research Corporation to concentrations of credit risk are cash equivalents and short-term investments. We place our cash equivalents and short-term investments primarily in investment grade, short-term debt instruments. Cash equivalents are also invested in deposits with certain financial institutions and may, at times, exceed federally insured limits. We have not experienced any significant losses on our deposits of cash and cash equivalents.
Two of the Acacia Technologies group's licensees accounted for approximately 27%19% and 12% of Acacia Research Corporation's DMTthe license fee revenues recognized during the year ended December 31, 2004, and one licensee represents
approximately 25% of accounts receivable at December 31, 2004.2007. One licensee accounted for approximately 28%represented 14% of the Acacia Research Corporation's license fee revenues recognized during the year ended December 31, 2003,2006 and alsothree licensees accounted for 19%, 15% and 15% of license fee revenues recognized during the year ended December 31, 2005. One licensee represented approximately 31%89% of accounts receivable at December 31, 2003.
Research2007. Three licensees represented approximately 37%, 24% and development contract revenues recognized by the CombiMatrix
group for the year ended13% of accounts receivable at December 31, 2004 relate to its research and
development agreement with Roche. Government contract revenues recognized by the
CombiMatrix group for the year ended December 31, 2004 relate to its two-year,
F-10
$5.9 million contract with the Department of Defense awarded in March 2004. At
December 31, 2004, accounts receivable related to the CombiMatrix group included
$248,000 due from the Department of Defense. In 2004, 2003 and 2002, 45%, 100%
and 38% of the CombiMatrix group's array product and service sales were recorded
by CombiMatrix K.K.
2006.
Acacia Research Corporation performs regular credit evaluations of its significant licensees and customers and has not experienced any significant credit losses.
Substantially all of the components
Property and raw materials used in the
manufacture of the CombiMatrix group's products, including semiconductors and
reagents, are currently provided from a limited number of sources or in some
cases from a single source. Although the CombiMatrix group believes that
alternative sources for those components and raw materials are available, any
supply interruption in a sole-sourced component or raw material might result in
up to a several-month production delay and materially harm the CombiMatrix
group's ability to manufacture products until a new source of supply, if any,
could be located and qualified. The CombiMatrix group utilizes non-standard
semiconductor manufacturing processes to fabricate the electrode array that is a
key aspect of the array structure. Although the CombiMatrix group has a supply
agreement in place with a semiconductor wafer manufacturer to ensure
availability of the raw materials, it does not guarantee a permanent supply.
INVENTORY. Inventory, which consists primarily of raw materials to be used
in the production of our array products, is stated at the lower of cost or
market using the first-in, first-out method.
PROPERTY AND EQUIPMENT. Equipment. Property and equipment are recorded at cost. Major additions and improvements that materially extend useful lives of property and equipment are capitalized. Maintenance and repairs are charged against the results of operations as incurred. When these assets are sold or otherwise disposed of, the asset and related depreciation are relieved, and any gain or loss is included in the consolidated statement of operations and comprehensive loss for the period of sale or disposal. Depreciation is computed on a straight-line basis over the following estimated useful lives of the assets:
Machine shop and laboratory equipment.......
| Furniture and fixtures | 3 to 5 years |
| Computer hardware and software | 3 to 5 years |
| Leasehold improvements | 2 to 5 years (Lesser of lease term or useful life of improvement) |
Rental payments on operating leases are charged to 5 years
Furniture and fixtures...................... 3 to 7 years
Computer hardware and software.............. 3 to 5 years
Leasehold improvements...................... Lesserexpense in the consolidated statement of operations on a straight-line basis over the lease term or useful
life of improvement
Construction in progress includes direct costs incurred related to
internally constructed assets which are depreciated once the asset is placed
into service.
ORGANIZATION COSTS. term.
Organization Costs. Costs of start-up activities, including organization costs, are expensed as incurred.
PATENTS AND GOODWILL. Goodwill and identifiable intangibles, including
patents, are recorded when the consideration paid for acquisitions exceeds the
fair value of the net tangible assets acquired.
Patents. Patents, once issued or purchased, are amortized on the straight-line method over their remaining economic useful lives, ranging from threetwo to twentyseven years. Goodwill is not
amortized.
IMPAIRMENT OF LONG-LIVED ASSETS AND GOODWILL.
Impairment of Long-lived Assets. We review long-lived assets and intangible assets for potential impairment annually and when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. In the event the sum of the expected undiscounted future cash flows resulting from the use of the asset is less than the carrying amount of the asset, an impairment loss equal to the excess of the asset'sasset’s carrying value over its fair value is recorded. If an asset is determined to be impaired, the loss is measured based on quoted market prices in active markets, if available. If quoted market prices are not available, the estimate of fair value is based on various valuation techniques, including a discounted value of estimated future cash flows.
Goodwill is evaluated for impairment in accordance with SFAS No. 142,
"Goodwill and Other Intangible Assets" ("SFAS No. 142") and is subject to a
periodic review for potential impairment at a reporting unit level. Reviews for
potential impairment must occur at least annually and may be performed earlier,
if circumstances indicate that an impairment may have occurred. Acacia Research
Corporation has elected to perform its annual tests for indications
Fair Value of goodwill
F-11
impairment as of December 31 of each year. Our two reporting units as of
December 31, 2004 are: 1) the Acacia Technologies group and 2) the CombiMatrix
group. The fair values of our reporting units are estimated using a discounted
cash flow analysis and by reference to quoted market prices of Acacia Research
Corporation's classes of stock.
SFAS No. 142 requires us to compare the fair value of our reporting units
to their carrying amounts on an annual basis to determine if there is potential
goodwill impairment. If the fair value of a reporting unit is less than its
carrying value, an impairment loss is recorded to the extent that the fair value
of the goodwill within the reporting unit is less than its carrying value. There
can be no assurance that future goodwill impairment tests will not result in a
charge to earnings.
As a result of the August 2004 adverse ruling in Soundview Technologies'
V-chip related litigation described at Note 13, as of September 30, 2004,
Soundview Technologies was no longer considered a reporting unit of the Acacia
Technologies group.
FAIR VALUE OF FINANCIAL INSTRUMENTS. Financial Instruments. FOREIGN CURRENCY TRANSLATION. The functional currency of our foreign entity
is the local currency (Japanese Yen). Foreign currency translation is reported
pursuantRefer to SFAS No. 52, "Foreign Currency Translation" ("SFAS No. 52"). Assets
and liabilities recorded in foreign currencies are translated at the exchange
rate“Short-term Investments” above for information on the balance sheet date. Translation adjustments resulting from this
process are charged or credited to other comprehensive income. Revenue and
expenses are translated at average ratesfair value of exchange prevailing during the year.
STOCK-BASED COMPENSATION. At December 31, 2004,short-term investments.
Stock-Based Compensation. Effective January 1, 2006, Acacia Research Corporation hasadopted the provisions of Statement of Financial Accounting Standards No. 123 (revised 2004), “Share-Based Payment” (“SFAS No. 123R”), which sets forth the accounting requirements for “share-based” compensation payments to employees and non-employee directors and requires that compensation cost relating to share-based payment transactions be recognized in the statement of operations. The compensation cost for all stock-based awards is measured at the grant date, based on the fair value of the award, and is recognized as an expense, on a straight-line basis, over the employee’s requisite service period (generally the vesting period of the equity award) which is generally two to four years.
F-12
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The fair value of each option award is estimated on the date of grant using a Black-Scholes option valuation model. The fair value of restricted stock awards is determined by the product of the number of shares granted and the grant date market price of the underlying common stock.
SFAS No. 123R requires stock-based employee compensation plans, whichexpense to be recorded only for those awards expected to vest using an estimated forfeiture rate. Acacia Research Corporation estimates pre-vesting option forfeitures at the time of grant and reflects the impact of estimated pre-vesting option forfeitures on compensation expense recognized. To the extent that actual results differ from our estimates, such amounts will be recorded as cumulative adjustments in the period the estimates are described more fullyrevised.
We adopted SFAS No. 123R using the modified prospective transition method. Under this transition method, compensation cost recognized for the periods presented includes: (i) compensation cost for all stock-based awards granted prior to, but not yet vested as of January 1, 2006 (based on the grant-date fair value estimated in Note 12. Compensationaccordance with the original provisions of SFAS No. 123 and previously presented in the pro forma footnote disclosures), and (ii) compensation cost for all stock-based awards granted subsequent to January 1, 2006 (based on the grant-date fair value estimated in accordance with the new provisions of SFAS No. 123R).
The fair value of stock options issuedgranted during the years ended December 31, 2007 and 2006 were estimated using the Black-Scholes option-pricing model, based on the following weighted-average assumptions:
| For the Year Ended | |||
| December 31, | |||
| 2007 | 2006 | ||
| Risk Free Interest Rate | 4.64% | 4.30% | |
| Term | 5.71 years | 6 years | |
| Volatility | 68% | 75% | |
Option awards granted prior to employees isAcacia Research Corporation’s implementation of SFAS No. 123R were accounted for in accordance withusing the intrinsic value method pursuant to the recognition and measurement principles of Accounting Principles Board ("APB") Opinion No. 25, "Accounting“Accounting for Stock Issued to Employees" ("Employees” (“APB No. 25"25”), and related interpretations. CompensationAccordingly, no stock-based employee compensation cost attributablerelated to suchoption awards was reflected in the accompanying consolidated statements of operations for the year ended December 31, 2005, because all options is recognized
based ongranted under Acacia Research Corporation’s plans had exercise prices equal to the difference, if any, betweenmarket value of the closing market price of theunderlying common stock on the date of grant andgrant. Stock-based compensation expense reflected in the exercise priceaccompanying consolidated statement of operations for the option. Compensation cost is
generally deferred and amortized on an accelerated basis over the vesting period
of the individual optionyear ended December 31, 2005 related to restricted stock awards using the amortization method prescribedoriginally granted in Financial Accounting Standards Board ("FASB") Interpretation No. 28, "Accounting
for Stock Appreciation Rights and Other Variable Stock Option or Award Plans"
("FIN No. 28"). We have adopted the disclosure only requirements of SFAS No.
123, "Accounting for Stock-Based Compensation" ("SFAS No. 123"), as amended by
SFAS No. 148 "Accounting for Stock-Based Compensation--Transition and
Disclosure--an amendment of SFAS No. 123" ("SFAS No. 148"), with respect to
options issued to employees. Compensation cost of stock options and warrants
issued to non-employee service providers is accounted for under the fair value
method required by SFAS No. 123 and related interpretations.
2005.
The following table illustrates the pro forma effect on net income (loss)loss from continuing operations and earningsloss per share from continuing operations for the year ended December 31, 2005, if Acacia Research Corporationwe had applied the fair value recognition provisions of SFAS No. 123 to share-based awards, prior to the adoption of SFAS No. 123R (in thousands, except per share data):
| Loss from continuing operations, as reported | $ | (6,038 | ) | |
| Add: Stock-based compensation, intrinsic value method reported in net loss | 356 | |||
| Deduct: Pro forma stock-based compensation fair value method | (2,103 | ) | ||
| Loss from continuing operations, pro forma | $ | (7,785 | ) | |
| Basic and diluted loss per share from continuing operations, as reported | $ | (0.23 | ) | |
| Basic and diluted loss per share from continuing operations, pro forma | $ | (0.29 | ) | |
Weighted Average Assumptions used(1): | ||||
| Risk free interest rate | 3.90% | |||
| Volatility | 91% | |||
| Expected term | 5 years | |||
__________________________
| (1) | The fair value |
F-13
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SFAS No. 123R does not require the recording of deferred stock compensation charges in stockholder’s equity on the grant date of a stock-based award. As such, all deferred stock compensation information above gives effect to the
recapitalization as of January 1, 2002. As a result, stock-based
compensation informationcharges previously recorded under APB No. 25, totaling $1,400,000 at December 31, 2005, related to restricted stock awards, were reversed upon adoption of SFAS No. 123R, with a corresponding reduction being recorded in consolidated additional paid-in capital.
Acacia Research Corporation common
stockadopted the alternative transition method provided in 2002 has been omitted fromFASB Staff Position No. 123(R)-3, “Transition Election Related to Accounting for Tax Effects of Share-Based Payment Awards.” The alternative transition method includes a simplified method to establish the table above.
(2) The fair valuebeginning balance of stock options was determined using the Black-Scholes
option-pricing model. The fair value calculations assume no expected
dividends.
RESEARCH AND DEVELOPMENT EXPENSES. Research and development expenses
consist of costs incurred for direct and overhead-related research expenses and
are expensed as incurred. Costs to acquire technologies, which are utilized in
research and development and which have no alternative future use are expensed
when incurred. Costsadditional paid-in-capital pool (“APIC pool”) related to filing and pursuing patent applications are
expensed as incurred, as recoverabilitythe tax effects of such expendituresemployee stock-based compensation which is uncertain.
Software developed for use in our products is expensed as incurred until both
(i) technological feasibility for the software has been established and (ii) all
research and development activities for the other components of the system have
been completed. We believe these criteria are met after we have received
evaluations from third-party test sites and completed any resulting
modificationsavailable to absorb tax deficiencies recognized subsequent to the products. Expenditures to date have been classified as
research and development expense.
ACQUIRED IN-PROCESS RESEARCH AND DEVELOPMENT. The value assigned to
acquired in-process research and development ("IPR&D") is determined by
identifying acquired specific in-process research and development projects that
would be continued and for which (a) technological feasibility has not been
established at the acquisition date, (b) there is no alternative future use and
(c) the fair value is estimable with reasonable reliability, upon consummationadoption of a business combination.
ADVERTISING. Costs associated with marketing and advertising of the
CombiMatrix group's products and services are expensed as incurred. For the
years ended December 31, 2004, 2003 and 2002, marketing and advertising expenses
incurred by the CombiMatrix group were $314,000, $26,000 and $62,000,
respectively.
INCOME TAXES. SFAS 123(R).
Income Taxes. Income taxes are accounted for using an asset and liability approach that requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in Acacia Research Corporation'sCorporation’s consolidated financial statements or consolidated tax returns. A valuation allowance is established to reduce deferred tax assets if all, or some portion, of such assets will more than likely not be realized.
ACCOUNTING FOR SALES OF STOCK BY A SUBSIDIARY. Gains
Effective January 1, 2007, Acacia Research Corporation adopted FASB Interpretation No. 48 (“FIN 48”), “Accounting for Uncertainty in Income Taxes,” which clarifies the accounting for uncertainty in income taxes recognized in the financial statements in accordance with FASB Statement No. 109, “Accounting for Income Taxes.” FIN 48 provides guidance on the financial statement recognition and measurement of a tax position taken or losses resultingexpected to be taken in a tax return. FIN 48 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosures, and transition. In accordance with FIN 48, a tax position is a position in a previously filed tax return or a position expected to be taken in a future tax filing that is reflected in measuring current or deferred income tax assets and liabilities. Tax positions shall be recognized only when it is more likely than not (likelihood of greater than 50%), based on technical merits, that the position will be sustained upon examination. Tax positions that meet the more likely than not threshold should be measured using a probability weighted approach as the largest amount of tax benefit that is greater than 50% likely of being realized upon settlement. The adoption of FIN 48 did not have a material impact on our consolidated financial position, results of operations or cash flows.
The total amount of unrecognized tax benefits as of January 1, 2007 and December 31, 2007 was $56,000 and $115,000, respectively, all of which, if recognized, would affect the effective tax rate.
Acacia Research Corporation recognizes interest and penalties with respect to unrecognized tax benefits in income tax expense. We have identified no uncertain tax position for which it is reasonably possible that the total amount of unrecognized tax benefits will significantly increase or decrease within 12 months.
Acacia Research Corporation is subject to taxation in the U.S. and various state jurisdictions. With no material exceptions, Acacia Research Corporation is no longer subject to U.S. federal or state examinations by tax authorities for years before 2001.
At December 31, 2007, Acacia Research Corporation had U.S. federal and state income tax net operating loss carryforwards as summarized at Note 9. Due to uncertainties surrounding our ability to generate future taxable income to realize these assets, a full valuation allowance has been established to offset our net deferred tax assets. All NOLs and tax credits generated by the continuing operations of Acacia Research Corporation and its operating subsidiaries have been retained by Acacia Research Corporation subsequent to the Split-off Transaction. Subsequent to the Split-off Transaction, all NOLs and tax credits generated by CombiMatrix Corporation and its subsidiaries have been retained by CombiMatrix Corporation and are not available to Acacia Research Corporation.
F-14
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Utilization of the NOL and R&D credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations that may have occurred or that could occur in the future, as required by Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”), as well as similar state provisions. These ownership changes may limit the amount of NOL and R&D credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an “ownership change” as defined by Section 382 of the Code results from a subsidiary's saletransaction or series of transactions over a three-year period resulting in an ownership change of more than 50 percentage points of the outstanding stock of a company by certain stockholders or public groups. Since Acacia Research Corporation’s formation, we have raised capital through the issuance of capital stock on several occasions (both before and after its public offering) which, combined with the purchasing stockholders’ subsequent disposition of those shares, may have resulted in such an ownership change, or could result in an ownership change in the future upon subsequent disposition.
We have not completed a study to third partiesassess whether an ownership change has occurred or whether there have been multiple ownership changes since our formation due to the complexity and cost associated with such a study, and the fact that there may be additional such ownership changes in the future. If we have experienced an ownership change at a price per share in
excessany time since our formation, utilization of the NOL or belowR&D credit carryforwards would be subject to an annual limitation under Section 382 of the Code, which is determined by first multiplying the value of Acacia Research Corporation's average carrying amount per
sharestock at the time of the ownership change by the applicable long-term, tax-exempt rate, and then could be subject to additional adjustments, as required. Any limitation may result in expiration of a portion of the NOL or R&D credit carryforwards before utilization. Further, until a study is completed and any limitation known, no amounts are generally reflectedbeing considered as an uncertain tax position or disclosed as an unrecognized tax benefit under FIN 48. Due to the existence of a full valuation allowance, future changes in stockholders' equityour unrecognized tax benefits will not impact our effective tax rate. Any carryforwards that will expire prior to utilization as a direct increase or
decrease to capital in excessresult of par or stated value.
COMPREHENSIVE (LOSS) INCOME. such limitations will be removed from deferred tax assets with a corresponding reduction of the valuation allowance.
Comprehensive Income (Loss). Comprehensive income (loss) income is the change in equity from transactions and other events and circumstances other than those resulting from investments by owners and distributions to owners.
SEGMENT REPORTING.
Segment Reporting. We use the management approach, which designates the internal organization that is used by management for making operating decisions and assessing performance as the basis of Acacia Research Corporation'sCorporation’s reportable segments.
USE OF ESTIMATES.
Use of Estimates. The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make estimates and assumptions that affect the reported amount of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates. EARNINGS PER SHARE. We believe that, of the significant accounting policies described herein, the accounting policies associated with revenue recognition, stock-based compensation expense, valuation of long-lived and intangible assets and impairment of marketable securities, require our most difficult, subjective or complex judgments.
Earnings (Loss) Per Share.Basic earnings per share for each class of common stock is computed by dividing the income or loss allocated to each class of common stock by the weighted averageweighted-average number of outstanding shares of that class of common stock. Diluted earnings per share is computed by dividing the income or loss allocated to each class of common stock by the weighted averageweighted-average number of outstanding shares of that class of common stock, including the dilutive effect of common stock equivalents. Potentially dilutive common stock equivalents primarily consist of employee stock options, unvested restricted stock, restricted stock unit grants and common stock purchase warrants.
F-13
warrants (AR-CombiMatrix stock only).
The earnings or losses allocated to each class of common stock are determined by Acacia Research Corporation'sCorporation’s board of directors. This determination is generally based on the net income or loss amounts of the corresponding group determined in accordance with accounting principles generally accepted in the United States of America, consistently applied. Acacia
Research Corporation believesWe believe this method of allocation isto be systematic and reasonable. The Acacia Research Corporation board
As a result of directors can, at its
discretion, change the method of allocatingSplit-off Transaction, earnings or losses allocated to eachthe CombiMatrix group are presented as discontinued operations in the accompanying consolidated financial statements. Subsequent to the Split-off Transaction, Acacia Research Corporation’s only class of common stock at any time.
outstanding is its AR-Acacia Technologies stock.
F-15
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The following table presents a reconciliationthe weighted-average number of common shares outstanding used in basic and diluted loss per share:
SEPARATE GROUP PRESENTATION. AR-CombiMatrix stock
| For the Year Ended December 31, | ||||||||||||
| 2007 | 2006 | 2005 | ||||||||||
| Acacia Research - Acacia Technologies stock | ||||||||||||
| Basic and diluted weighted average number of common shares outstanding | 28,503,314 | 27,547,651 | 26,630,732 | |||||||||
| All outstanding stock options and nonvested restricted stock excluded from the computation of | ||||||||||||
| diluted loss per share because the effect of inclusion would have been anti-dilutive | 5,884,934 | 6,385,810 | 6,315,000 | |||||||||
Acacia Research - CombiMatrix stock - Discontinued Operations - Split-off of CombiMatrix Corporation (1) | ||||||||||||
| Basic and diluted weighted average number of common shares outstanding | 55,862,707 | 40,605,038 | 33,678,603 | |||||||||
| Outstanding stock options excluded from the computation of diluted loss per share | ||||||||||||
| because the effect of inclusion would have been anti-dilutive | 7,003,390 | 8,068,139 | 6,925,000 | |||||||||
| Warrants excluded from the computation of diluted loss per share because | ||||||||||||
| the option exercise price was greater than the average market price of the common shares | 23,838,648 | 14,090,279 | 1,879,888 | |||||||||
____________
(1) Reflects activity and AR-Acacia
Technologies stock are intended to reflect the separate performanceamounts outstanding as of the respective division of Acacia Research Corporation. The CombiMatrix group and
the Acacia Technologies group are not separate legal entities. Holders of
AR-CombiMatrix stock and AR-Acacia Technologies stock are stockholders of Acacia
Research Corporation. As a result, holders of AR-CombiMatrix stock and AR-Acacia
Technologies stock continue to be subject to all of the risks of an investment
in Acacia Research Corporation and all of its businesses, assets and
liabilities. The assets Acacia Research Corporation attributes to one of the
groups could be subject to the liabilities of the other group. The group
financial statements have been prepared in accordance with generally accepted
accounting principles in the United States of America, and taken together,
comprise all the accounts included in the corresponding consolidated financial
statements of Acacia Research Corporation. The financial statements of the
groups reflect the financial condition, results of operations, and cash flows of
the businesses included therein. The financial statements of the groups include
the accounts or assets of Acacia Research Corporation specifically attributed to
the groups and were prepared using amounts included in Acacia Research
Corporation's consolidated financial statements.
Minority interests represent participation of other stockholders in
the net equity and in the division earnings and losses of the groups and are
reflected in the caption "Minority interests" in the group financial statements.
Minority interests adjust group net results of operations to reflect only the
group's share of the division earnings or losses of non-wholly owned investees.
Financial effects arising from one group that affect Acacia Research
Corporation's results of operations or financial condition could, if
significant, affect the results of operations or financial condition of the
other group and the market price of the class of common stock relating to the
other group. Any division net losses of the CombiMatrix group or of the Acacia
Technologies group, and dividends or distributions on, or repurchases of,
AR-CombiMatrix stock or AR-Acacia Technologies stock, will reduce the assets of
Acacia Research Corporation legally available for payment of dividends on
AR-CombiMatrix stock or AR-Acacia Technologies stock.
MANAGEMENT ALLOCATION POLICIES. The management and allocation policies
applicable to the preparation of the financial statements of the CombiMatrix
group and the Acacia Technologies group may be modified or rescinded, or
additional policies may be adopted, at the sole discretion of the Acacia
Research Corporation board of directors at any time without approval of the
stockholders. The group's financial statements reflect the application of the
management and allocation policies adopted by the Acacia Research Corporation
board of directors to various corporate activities, as described below.
F-14
Management has no plans to change allocation methods or the composition of the
groups. The group financial statements should be read in conjunction with the
Acacia Research Corporation consolidated financial statements and related notes.
TREASURY AND CASH MANAGEMENT POLICIES. Cash and cash equivalents and
short-term investments are attributed to the groups based on the respective cash
and cash equivalents and short term investments balances of the entities
comprising each group. Acacia Research Corporation's cash and the cash held by
its intellectual property licensing businesses, including all cash raised
through Acacia Research Corporation's previous offerings, have been attributed
to the Acacia Technologies group as these funds are intended to support the
intellectual property licensing businesses of Acacia Research Corporation. All
cash raised by CombiMatrix Corporation and Advanced Material Sciences have been
attributed to the CombiMatrix group. Acacia Research Corporation manages most
treasury and cash management activities on a decentralized basis, with each
group separately managing its own treasury activities. Pursuant to treasury and
cash management policies adopted by the Acacia Research Corporation board of
directors, the following applies:
o Acacia Research Corporation will attribute each future issuance of
AR-Acacia Technologies stock (and the proceeds thereof) to the Acacia
Technologies group and will attribute each future issuance of
AR-CombiMatrix stock (and the proceeds thereof) to the CombiMatrix
group;
o Acacia Research Corporation will attribute each future incurrence or
issuance of external debt or preferred stock (and the proceeds
thereof), if any, between the groups or entirely to one group as
determined by the Acacia Research Corporation board of directors,
based on the extent to which Acacia Research Corporation incurs or
issues the debt or preferred stock for the benefit of the CombiMatrix
group or the Acacia Technologies group;
o Dividends, if any, on AR-Acacia Technologies stock will be charged
against the Acacia Technologies group, and dividends, if any on
AR-CombiMatrix stock will be charged against the CombiMatrix group;
o Repurchases of AR-Acacia Technologies stock will be charged against
the Acacia Technologies group and repurchases of AR-CombiMatrix stock
will be charged against the CombiMatrix group;
o Acacia Research Corporation accounts for any cash transfers from
Acacia Research Corporation to or for the account of a group, from a
group to or for the account of Acacia Research Corporation, or from
one group to or for the account of the other group (other than
transfers in return for assets or services rendered) as short-term
loans unless (i) the Acacia Research Corporation board of directors
determines that a given transfer (or type of transfer) should be
accounted for as a long-term loan, (ii) the Acacia Research
Corporation board of directors determines that a given transfer (or
type of transfer) should be accounted for as a capital contribution or
(iii) the Acacia Research Corporation board of directors determines
that a given transfer (or type of transfer) should be accounted for as
a return of capital. There are no specific criteria to determine when
Acacia Research Corporation will account for a cash transfer as a
long-term loan, a capital contribution or a return of capital rather
than an inter-group revolving credit advance; provided, however, that
cash advances from Acacia Research Corporation to the Acacia
Technologies group or to the CombiMatrix group up to $25.0 million on
a cumulative basis shall be accounted for as short-term or long-term
loans at interest rates at which Acacia Research Corporation could
borrow such funds and shall not be accounted for as a capital
contribution. The Acacia Research Corporation board of directors will
make such a determination in the exercise of its business judgment at
the time of such transfer based upon all relevant circumstances.
Factors the Acacia Research Corporation board of directors may
consider include, without limitation, the current and projected
capital structure of each group; the financing needs and objectives of
the recipient group; the availability, cost and time associated with
alternative financing sources; and prevailing interest rates and
general economic conditions; and
F-15
o Any cash transfers accounted for as short-term loans will bear
interest at the rate at which Acacia Research Corporation could borrow
such funds. In addition, any cash transfers accounted for as a
long-term loan will have interest rates, amortization, maturity,
redemption and other terms that reflect the then-prevailing terms on
which Acacia Research Corporation could borrow such funds.
ASSETS AND LIABILITIES. Acacia Research Corporation's assets and
liabilities have been attributed to the Acacia Technologies group and the
CombiMatrix group based on the respective asset and liabilities of the business
comprising each group. Net intangible assets recorded at the Acacia Research
Corporation level, primarily consisting of acquired patents and goodwill
balances, have been attributed to the respective businesses comprising each
group to which the intangibles and goodwill relate.
CORPORATE GENERAL AND ADMINISTRATIVE SERVICES AND FACILITIES. Acacia
Research Corporation allocates the cost of corporate general and administrative
services and facilities between the groups generally based upon utilization.
Where determinations based on utilization alone are impracticable, Acacia
Research Corporation utilizes other methods and criteria that management
believes to be equitable and to provide a reasonable estimate of the cost
attributable to each group. Except as otherwise determined by management, the
allocated costs of providing such services and facilities include, without
limitation, all costs and expenses of personnel employed in connection with such
services and facilities, including, without limitation, all direct costs of such
personnel, such as payroll, payroll taxes and fringe benefit costs (calculated
at the appropriate annual composite rate therefore) and all overhead costs and
expenses directly related to such personnel and the services or facilities
provided by them. In addition, allocated costs include all materials used in
connection with such services or facilities, billed at their net cost to the
provider of the services or facilities plus all overhead costs and expenses
related to such materials. Except as may otherwise be specifically provided
pursuant to the terms of any agreements among Acacia Research Corporation and
the groups or any resolutions of the Acacia Research Corporation board of
directors, the corporate general and administrative services and facilities to
be allocated between the groups include, without limitation, legal services,
accounting services (tax and financial), insurance and deductibles payable in
connection therewith, employee benefit plans and administration thereof,
investor relations, stockholder services, and services relating to the board of
directors.
Direct salaries, payroll taxes and fringe benefits are allocated to
the groups based on the percentage of actual time incurred by specific employees
to total annual time available and direct costs including, postage, insurance,
legal fees, accounting and tax and other are allocated to the groups based on
specific identification of costs incurred on behalf of each group. Other direct
costs, including direct depreciation expense, computer costs, general office
supplies and rent are allocated to the groups based on the ratio of direct
salaries to total salaries. Indirect costs, including indirect salaries and
benefits, investor relations, rent, general office supplies and indirect
depreciation are allocated to the groups based on the ratio of direct salaries
for each group to total direct salaries. Included in marketing, general and
administrative expenses of the Acacia Technologies group are allocated corporate
charges of $3,395,000, $2,864,000 and $4,906,000 relating to the periods ending
December 31, 2004, 2003 and 2002, respectively. Included in marketing, general
and administrative expenses of the CombiMatrix group are allocated corporate
charges of $689,000, $894,000 and $1,161,000 relating to the periods ending
December 31, 2004, 2003 and 2002, respectively.
Management believes that the methods and criteria used to allocate
costs are equitable and provide a reasonable estimate of the cost attributable
to the groups. Based on the allocation methods used, Acacia Research Corporation
believes that the allocation of expenses as presented in the accompanying
consolidating financial information reflects a reasonable estimation of expenses
that would be recognized if the groups were separate stand-alone registrants.
ALLOCATION OF FEDERAL AND STATE INCOME TAXES. Acacia Research
Corporation determines its federal income taxes and the federal income taxes of
its subsidiaries that own assets allocated between the groups on a consolidated
basis. Acacia Research Corporation allocates consolidated federal income tax
provisions and related tax payments or refunds between the Acacia Technologies'
group and CombiMatrix group based principally on the taxable income and tax
credits directly attributable to each group. Such allocations reflect each
group's contribution, whether positive or negative, to Acacia Research
Corporation's consolidated federal taxable income and consolidated federal tax
liability and tax credit position. Acacia Research Corporation will credit tax
benefits that cannot be used by the group generating those benefits but can be
used on a consolidated tax return basis to the group that generated such
benefits.
F-16
Inter-group transactions are treated as taxed as if each group was a
stand-alone company. Depending on the tax laws of the respective jurisdictions,
state and local income taxes are calculated on either a consolidated or combined
basis between the groups based on their respective contribution to such
consolidated or combined state taxable incomes. State and local income tax
provisions and related tax payments or refunds which are determined on a
separate corporation basis are allocated between the groups in a manner designed
to reflect the respective contributions of the groups to Acacia Research
Corporation's separate or local taxable income.
RECENT ACCOUNTING PRONOUNCEMENTS.
Redemption Date.
Recent Accounting Pronouncements. In December 2004,2007, the FASB issued SFAS No. 123141 (revised 2004)2007), "Share-Based
Payments," that addressesBusiness Combinations (“SFAS No. 141R”). SFAS 141R establishes principles and requirements for how an acquirer recognizes and measures in its financial statements the accounting for share-based payment transactionsidentifiable assets acquired, the liabilities assumed, any noncontrolling interest in which an enterprise receives employee services in exchange for (a) equity
instrumentsthe acquiree and the goodwill acquired. SFAS No. 141R also establishes disclosure requirements to enable the evaluation of the enterprise or (b) liabilities that are based on the fair
valuenature and financial effects of the enterprise's equity instruments or that may be settled by the
issuance of such equity instruments.business combinations. SFAS No. 123(R) will require141R is effective for Acacia Research Corporation to measure all employee stock-based compensation awards
using a fair-value method and record such expense in its consolidated and
separate group financial statements. The adoptionas of SFAS No. 123(R) will
require additional accounting related toJanuary 1, 2009. We are currently evaluating the income tax effects and additional
disclosure regarding the cash flow effects resulting from share-based payment
arrangements. SFAS No. 123(R) is effective beginning in the quarter ending
September 30, 2005. The effectpotential impact of the adoption of SFAS No. 123(R) is expected to
be comparable to the effect disclosed141R on a pro forma basis resulting from the
application of the current fair-value recognition provisions of SFAS No. 123, as
shown in Note 2 above.
In December 2004, the FASB issued SFAS No. 153 "Exchanges of Nonmonetary
Assets, an amendment of APBO 29" to address the accounting for nonmonetary
exchanges of productive assets. SFAS No. 153 amends APBO 29, "Accounting for
Nonmonetary Exchanges," which established a narrow exception from fair-value
measurement for nonmonetary exchanges of similar productive assets. SFAS No. 153
eliminates that exception and replaces it with an exception for exchanges that
do not have commercial substance. Under SFAS No. 153 nonmonetary exchanges are
required to be accounted for at fair value, recognizing any gains or losses, if
their fair value is determinable within reasonable limits and the transaction
has commercial substance. SFAS No. 153 specifies that a nonmonetary exchange has
commercial substance if future cash flows of the entity are expected to change
significantly as a result of the exchange. The provisions of SFAS No. 153 apply
to nonmonetary asset exchanges in fiscal periods beginning after June 15, 2005.
Adoption of SFAS No. 153 is not expected to have a material impact on Acacia
Research Corporation's, the CombiMatrix group's or the Acacia Technologies
group'sour consolidated financial position, results of operations or cash flows.
In November 2004,December 2007, the FASB issued SFAS No. 151, "Inventory Costs - 160, Noncontrolling Interests in Consolidated Financial Statements—an amendment of ARBAccounting Research Bulletin No. 43, chapter 4." 51 (“SFAS No. 151 requires that "abnormal"
amounts160”). SFAS 160 establishes accounting and reporting standards for ownership interests in subsidiaries held by parties other than the parent, the amount of idle facility expense, freight, handling costs, and wasted material
are to be recognized as current-period charges rather than as components of
inventory. The statement also requires that allocation of fixed production
overheadsconsolidated net income attributable to the costsparent and to the noncontrolling interest, changes in a parent’s ownership interest, and the valuation of conversion be based onretained noncontrolling equity investments when a subsidiary is deconsolidated. SFAS No. 160 also establishes disclosure requirements that clearly identify and distinguish between the normal capacityinterests of the production facilities.parent and the interests of the noncontrolling owners. SFAS No. 160 is effective for Acacia Research Corporation adopted SFAS No. 151 duringbeginning January 1, 2009. We are currently evaluating the fourth quarterpotential impact of 2004. The implementationthe adoption of SFAS No. 151 did not have a
material impact160 on Acacia Research Corporation's, the CombiMatrix group's or the
Acacia Technologies group'sour consolidated financial position, results of operations or cash flows.
In June 2004,December 2007, the FASB issuedratified the Emerging Issues Task Force consensus on EITF Issue No. 02-14, "Whether07-1, Accounting for Collaborative Arrangements (EITF Issue No. 07-1”) that discusses how parties to certain collaborative arrangements should account for various activities. The consensus indicates that costs incurred and revenues generated from transactions with third parties should be reported by the collaborators on the respective line items in their income statements pursuant to EITF Issue No. 99-19, “Reporting Revenue Gross as a Principal Versus Net as an Investor
Should ApplyAgent.” Additionally, the Equity Methodconsensus provides that income statement characterization of Accountingpayments between the participants in a collaborative arrangement should be based upon existing authoritative pronouncements; analogy to Investments Other Than Common
Stock."such pronouncements if not within their scope; or a reasonable, rational, and consistently applied accounting policy election. EITF 02-14 addresses whether the equity method of accounting applies
when an investor does not have an investment in voting common stock of an
investee but exercises significant influence through other means. EITF 02-14
states that an investor should only apply the equity method of accounting when
it has investments in either common stock or in-substance common stock of a
corporation, provided that the investor has the abilityIssue No. 07-1 is effective for Acacia Research Corporation beginning January 1, 2009 and is to exercise significant
influence over the operating and financial policiesbe applied retrospectively to all periods presented for collaborative arrangements existing as of the investee. The
accounting provisionsdate of adoption. We do not expect EITF 02-14 are effective for reporting periods
beginning after September 15, 2004. The adoption of EITF 02-14 did notIssue No. 07-1 to have a material impact on Acacia Research Corporation's, the CombiMatrix group's or the
Acacia Technologies group'sour consolidated financial position, results of operations or cash flows.
F-17
In February 2007, the FASB issued SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities Including an Amendment of FASB Statement No. 115 (“SFAS No. 159”). SFAS No. 159 permits entities to choose to measure many financial instruments and certain other items at fair value, with unrealized gains and losses related to these financial instruments reported in earnings at each subsequent reporting date. SFAS No. 159 is effective for Acacia Research Corporation beginning January 1, 2008. We do not expect the adoption of SFAS No. 159 to have a material impact, if any, on our consolidated financial position, results of operations and cash flows.
F-16
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In September 2006, the FASB issued SFAS No. 157, Fair Value Measurements (“SFAS No. 157”). SFAS No. 157 establishes a common definition for fair value to be applied to U.S. generally accepted accounting principles guidance requiring use of fair value, establishes a framework for measuring fair value, and expands disclosure about such fair value measurements. SFAS No. 157 is effective for fiscal years beginning after November 15, 2007. In February 2008, the FASB issued FSP SFAS No. 157–1 which amends SFAS No. 157 to exclude FASB Statement No. 13, Accounting for Leases, and other accounting pronouncements that address fair value measurements for purposes of lease classification or measurement under Statement 13. In February 2008, the FASB issued FSP SFAS No. 157-2 (“FSP 157-2”) which would delay the effective date of SFAS No. 157 for all nonfinancial assets and nonfinancial liabilities, except those that are recognized or disclosed at fair value in the financial statements on a recurring basis (at least annually). FSP 157-2 partially defers the effective date of SFAS No. 157 to fiscal years beginning after November 15, 2008, and interim periods within those fiscal years for items within the scope of FSP 157-2. We do not expect the adoption of SFAS No. 157 to have a material impact, if any, on our consolidated financial position, results of operations and cash flows.
3. SHORT-TERM INVESTMENTS
Short-term investments consistconsists of the following at December 31, 20042007 and 20032006 (in thousands):
2007 | 2006 | |||||||||||||||
| Amortized | Fair | Amortized | Fair | |||||||||||||
Cost | Value | Cost | Value | |||||||||||||
| Available-for-sale securities: | ||||||||||||||||
| Corporate and municipal bonds and notes | $ | - | $ | - | $ | 975 | $ | 1,000 | ||||||||
| Auction rate securities | 10,660 | 10,660 | 7,288 | 7,288 | ||||||||||||
| U.S. government securities | 300 | 300 | 4,502 | 4,495 | ||||||||||||
| Corporate securities | 9 | 6 | - | - | ||||||||||||
| $ | 10,969 | $ | 10,966 | $ | 12,765 | $ | 12,783 | |||||||||
Gross unrealized gains and losses related to available-for-sale securities were not material for 2004, 2003 and 2002. Allthe periods presented. Except for investments in debtauction rate securities, all investments classified as available-for-sale as ofat December 31, 20042007 and 2006 have contractual maturities of one year or less. For auction rate securities, contractual maturity dates range up to thirty-five years, or are due within one
year.
perpetual, with reset dates every 7 to 63 days. Refer to Note 2 for more information.
4. PROPERTY AND EQUIPMENT
Property and equipment consists of the following at December 31, 20042007 and 20032006 (in thousands):
2007 | 2006 | |||||||
| Furniture and fixtures | $ | 309 | $ | 256 | ||||
| Computer hardware and software | 401 | 298 | ||||||
| Leasehold improvements | 141 | 78 | ||||||
| 851 | 632 | |||||||
| Less: accumulated depreciation | (528 | ) | (411 | ) | ||||
| $ | 323 | $ | 221 | |||||
Depreciation expense was $1,154,000, $1,428,000$119,000, $79,000 and $1,573,000$59,000 for the years ended December 31, 2004, 20032007, 2006 and 2002,2005, respectively. Amortization of
assets held under capital lease included in depreciation expense was $590,000
for the year ended December 31, 2002. The capital lease obligation was paid in
full in November 2002. Fully depreciated assets of $937,000 were written off in
2004.
F-17
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
5. BALANCE SHEET COMPONENTS
ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses and other consists of the following at December 31, 20042007 and 20032006 (in thousands):
2004 2003
--------- ---------
Accounts payable .................................. $ 498 $ 409
Payroll and other employee benefits ............... 436 501
Accrued vacation .................................. 538 437
Accrued liabilities of discontinued operations .... 272 388
Accrued legal expenses ............................ 1,195 495
Accrued consulting and other professional fees .... 596 478
Deferred rent ..................................... 340 284
Other accrued liabilities ......................... 264 252
--------- ---------
$ 4,139 $ 3,244
========= =========
F-18
Deferred revenues consist of the following at December 31, 2004 and 2003
(in thousands):
2004 2003
---------- ----------
Milestone and up-front payments .... $ 3,959 $ 20,405
License fee payments ............... 428 1,604
---------- ----------
4,387 22,009
Less: current portion .............. (494) (17,670)
---------- ----------
$ 3,893 $ 4,339
========== ==========
In March 2004, the CombiMatrix group completed all phases of its research
and development agreement with Roche. As a result of completing all of its
obligations under this agreement and in accordance with the CombiMatrix group's
revenue recognition policies for multiple-element arrangements, the CombiMatrix
group recognized all previously deferred Roche related contract revenues
totaling $17,302,000 during the first quarter of 2004.
In August 2004, the CombiMatrix group received a $1,000,000 upfront payment
from Furuno Electric Co., LTD ("Furuno") as part of a multi-year collaboration
agreement to develop a bench-top array synthesizer for commercial applications.
In 2003, the CombiMatrix group received upfront and milestone payments from
Toppan Printing Co., LTD. ("Toppan") totaling $2,400,000, pursuant to a
multi-year collaboration and supply agreement to develop and manufacture arrays
using the CombiMatrix group's proprietary electrochemical detection approach.
The payments received from Furuno and Toppan are included in deferred revenues
at December 31, 2004, in accordance with the CombiMatrix group's revenue
recognition policies for multiple-element arrangements.
As a result of the final ruling in the Acacia Technologies group's V-chip
litigation described at Note 13, the Acacia Technologies group recognized
$1,500,000 of V-chip related deferred license fee revenues and $668,000 of
V-chip related deferred legal costs in the third quarter of 2004.
2007 | 2006 | |||||||
| Accounts payable | $ | 361 | $ | 150 | ||||
| Payroll and other employee benefits | 371 | 223 | ||||||
| Accrued vacation | 365 | 286 | ||||||
| Accrued legal expenses - patent | 2,082 | 1,131 | ||||||
| Accrued consulting and other professional fees | 108 | 371 | ||||||
| Other accrued liabilities | 175 | 40 | ||||||
| $ | 3,462 | $ | 2,201 | |||||
6. INVESTMENTS
In October 2004 (the "Investment Date"), the CombiMatrix group entered into
an agreement to acquire up to a one-third ownership interest in Leuchemix, Inc.
("Leuchemix"), a private drug development firm, which is developing several
compounds for the treatment of leukemia and other cancers. In accordance with
the terms of the purchase agreement, the CombiMatrix group will purchase
3,137,500 shares of Series A Preferred Stock of Leuchemix for a total purchase
price of $4,000,000. The ownership interest will be acquired and paid for
quarterly, beginning with the fourth quarter of 2004 and continuing through the
third quarter of 2006. As of December 31, 2004, the CombiMatrix group has
initially invested $250,000 for a 3% interest in the total outstanding voting
securities of Leuchemix. In accordance with the terms of the purchase agreement,
CombiMatrix Corporation's CEO was named a director of Leuchemix. Although the
CombiMatrix group's investment in Leuchemix only represented approximately 3% of
Leuchemix's total outstanding voting securities as of the Investment Date, the
CombiMatrix group's investment is being accounted for under the equity method as
the CombiMatrix group has the ability to exercise significant influence over
Leuchemix, primarily due to CombiMatrix Corporation's representation on
Leuchemix's board of directors.
The CombiMatrix group's 3% interest in the equity in loss of Leuchemix,
including its share of the amortization expense related to the excess purchase
consideration over the book value of Leuchemix was not material for the
year-ended December 31, 2004. Future investments in Leuchemix will be accounted
for as step acquisitions. Summary financial information for Leuchemix was not
significant as of December 31, 2004.
F-19
In the second quarter of 2003, the Acacia Technologies group recorded an
impairment charge of $207,000 for an other-than-temporary decline in the fair
value of its cost method investment in Advanced Data Exchange ("ADX").
Impairment indicators included a continued decline in the working capital of the
entity and reference to a recent equity transaction and related valuation
indicating an other-than-temporary decline in fair value of the investment. In
September 2002, we recorded an impairment charge of $2,748,000 for an
other-than-temporary decline in the fair value of ADX. Impairment indicators
included recurring losses, a decline in working capital and the completion of a
recent equity transaction with a shareholder at an amount below our carrying
value. The fair value of our investment in ADX was determined by reference to
available financial and market information.
On April 25, 2002, CombiMatrix Corporation purchased our interest in
Advanced Material Sciences. CombiMatrix Corporation issued 180,982 shares of its
common stock in exchange for our 58% interest in Advanced Material Sciences. As
a result of the sale of our interest in Advanced Material Sciences, as of
December 31, 2002 CombiMatrix Corporation owned 87% of Advanced Material
Sciences and the remaining interests are owned by unaffiliated entities. The
purchase was accounted for pursuant to APB Opinion No. 16, "Business
Combinations," and related interpretations and EITF 90-5, "Exchanges of
Ownership Interests between Entities under Common Control." Accordingly, the
transaction was accounted for using PATENTS
Acacia Research Corporation's basis in the
net assets of Advanced Material Sciences and as a result, Acacia Research
Corporation's 2002 consolidated financial statements reflect the assets and
liabilities of Advanced Material Sciences at historical cost.
7. INTANGIBLES
The Acacia Technologies group had $121,000 and $1,776,000 of goodwill at
December 31, 2004 and 2003, respectively. The CombiMatrix group had $19,424,000
of goodwill at December 31, 2004 and 2003.
In August 2004, as a result of the adverse ruling in Acacia Technologies
group's V-chip patent infringement lawsuit described at Note 13, the Acacia
Technologies group recorded an impairment charge totaling $1,616,000 in
connection with the write-down of 100% of the goodwill related to the V-chip.
Refer to Note 8, "Step Acquisitions," for additions to goodwill during 2003
and 2002.
Acacia Research Corporation'sCorporation’s only identifiable intangible assets at
December 31, 2004are patents and 2003 are patents.patent rights, with estimated remaining economic useful lives up to seven years. The gross carrying amounts and accumulated amortization related to acquired intangible assets as of December 31, 20042007 and 2003 and amortization
expense for 2004, 2003 and 2002, related to patents, by segment,2006 are as follows (in thousands):
- ----------
(1) Excludes gross cost and accumulated amortization as of December 31, 2003
totaling $6,045,000 related to the write off of V-chip related intangibles
in 2004, in connection with the conclusion of V-chip litigation as
discussed at Note 13.
2007 | 2006 | |||||||
| Gross carrying amount – patents | $ | 33,607 | $ | 30,317 | ||||
| Accumulated amortization | (17,300 | ) | (11,802 | ) | ||||
| Patents, net | $ | 16,307 | $ | 18,515 | ||||
The Acacia Technologies group and the CombiMatrix group's patents are being
amortized overweighted-average remaining estimated economic useful liveslife of approximately 9 yearsAcacia Research Corporation’s patents is four years. Aggregate patent amortization expense was $5,583,000, $5,313,000 and 11 years,$4,922,000 in 2007, 2006 and 2005, respectively. AggregateAnnual aggregate amortization expense for each of the next five years through December 31, 20092012 is estimated to be $1,595,000 per year ($500,000$4,403,000 in 2008, $3,885,000 in 2009, $3,676,000 in 2010, $2,766,000 in 2011 and $755,000 in 2012.
For the years ended December 31, 2007 and 2006, our operating subsidiaries incurred and capitalized patent acquisition costs totaling $3,760,000 and $1,030,000, respectively, in connection with the acquisition of the rights to several additional patent portfolios. The patents have estimated economic useful lives ranging from five to seven years and are being amortized over a weighted-average economic useful life of seven years for the Acacia Technologies group2007 acquisitions and $1,095,000six years for the CombiMatrix group).2006 acquisitions. At December 31, 20042007 and 2003,2006, all of ourAcacia Research Corporation’s acquired intangible assets other than
goodwill were subject to amortization.
F-20
8. STEP
In September 2007, we recorded a non-cash impairment charge of $235,000, related to the write-off of a patent-related intangible asset. The related licensing program was completed during the third quarter of 2007 resulting in the write-off of the remaining carrying value of the patent-related intangible asset as of September 30, 2007. In June 2006, we recorded a non-cash impairment charge of $297,000, related to the write-off of a patent-related intangible asset. During the second quarter of 2006, pursuant to the terms of the respective license agreement, management elected to terminate its rights to exclusively license and enforce the patent, resulting in the write-off of the remaining carrying value of the patent-related intangible asset as of June 30, 2006.
F-18
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
7. ACQUISITIONS
On July 11, 2003,January 28, 2005, Acacia Global Acquisition Corporation, a wholly owned subsidiary of Acacia Research Corporation, purchasedacquired substantially all of the outstanding
minority interestsassets of Global Patent Holdings, LLC. The acquisition provided us with sole ownership of 11 patent licensing companies that own or control the rights to 27 patent portfolios, which include 120 U.S. patents and certain foreign counterparts, and cover technologies used in its consolidated subsidiary CombiMatrix K.K. from Marubeni
Corporation ("Marubeni"). Acacia Research Corporation issued 200,000 sharesa wide variety of its AR-CombiMatrix stock to Marubeni in exchange for Marubeni's 10% minority
interests (120 shares) in CombiMatrix K.K. industries.
The transactionacquisition was accounted for as
a step acquisition using the purchase method of accounting. The fair value of
the AR-CombiMatrix stock issued in the transaction was based on the quoted
market price of AR-CombiMatrix stock on the exchange date. The total purchase
price of $450,000 was allocated to the fair value of assets acquired and
liabilities assumed. The amount attributable to goodwill was $393,000.
On July 2, 2003, Acacia Research Corporation increased its consolidated
ownership interest in Advanced Material Sciences from 87% to 99% by acquiring
1,774,750 shares of Advanced Material Sciences common stock in exchange for
295,790 shares of AR-CombiMatrix stock. The transaction was accounted for as a
step acquisition usingUnder the purchase method of accounting.accounting, the purchase consideration is allocated to the assets acquired, including tangible assets, patents and other identifiable intangibles and liabilities assumed, based on their estimated fair market values at the date of acquisition. The fairstatement of operations for the year ended December 31, 2005 includes the results of the acquired companies beginning on January 28, 2005, the date of acquisition. The aggregate purchase consideration was approximately $25,105,000, including $5.0 million of cash, the issuance of 3,938,832 shares of AR-Acacia Technologies stock valued at $19,293,000 (net of estimated common stock registration costs of $228,000) and other acquisition costs, including registration costs, totaling $812,000. The value of the Acacia Researchcommon shares issued in the transaction was determined based on the quotedaverage market price of AR-CombiMatrixAR-Acacia Technologies stock, as reported on NASDAQ, over the exchange date. The total purchase price of
$769,000 was allocated to5-day period before and after the fair value of assets acquired and liabilities
assumed. The amount attributable to goodwill was $172,000.
Acacia Research Corporation's interests in Advanced Material Sciences and
CombiMatrix K.K. have been attributed to the CombiMatrix group.
On December 13, 2002, Acacia Research Corporation increased its
consolidated ownership interest in CombiMatrix Corporation from 48% to 100% by
acquiring from existing stockholders of CombiMatrix Corporation 11,987,274
shares of CombiMatrix Corporation common stock in exchange for 11,987,274 shares
of AR-CombiMatrix stock with a fair value of $46,007,000. The merger was
designed to consolidate our ownership of CombiMatrix Corporation and permit us
to effectuate the recapitalization transaction described elsewhere herein, by
creating the CombiMatrix group.
The transaction was accounted for as a step acquisition using the purchase
method of accounting. The fair valueterms of the AR-CombiMatrix stock issued in the
transaction was based on the quoted market price of AR-CombiMatrix stock
averaged over a five-day period (from December 16, 2002, the first day of
trading for the AR-CombiMatrix stock, through December 20, 2002).
acquisition were agreed to and announced.
The following table summarizes the total purchase consideration and the allocation of the consideration paid to the estimated fair valuesvalue of the assets acquired and liabilities assumed and the allocation of the purchase price at the
date of acquisition (in thousands):
Acquisition costs:
Exchange of AR-CombiMatrix stock for CombiMatrix
Corporation common stock .............................. $ 46,007
Acquisition expenses ...................................... 834
-----------
Total acquisition cost .................................... $ 46,841
===========
Purchase price allocation:
Fair value of 52% of CombiMatrix
Corporation net tangible assets at December 13, 2002 .. 8,313
Intangible assets acquired:
Core technology/patent ................................ 5,283
Acquired in-process research and development .......... 17,237
Goodwill (non-deductible for tax purposes) ............ 16,008
-----------
Total ..................................................... $ 46,841
===========
The total purchase price of $46,841,000
| Purchase Consideration: | ||||
| Cash paid | $ | 5,000 | ||
| Fair value of AR-Acacia Technologies stock issued | 19,293 | |||
| Acquisition and registration costs | 812 | |||
| Total purchase consideration | $ | 25,105 | ||
| Purchase Price Allocation: | ||||
| Fair value of net tangible assets acquired at January 28, 2005 | $ | (26 | ) | |
| Intangible assets acquired - patents and patent rights | 25,131 | |||
| Total | $ | 25,105 | ||
Management was allocated to the fair value of
assets acquired and liabilities assumed, including acquired IPR&D, as shown in
the table above. The amount attributable to CombiMatrix Corporation's core
technology and related patents is being amortized using the straight-line method
over the estimated economic useful life of 7 years. Amounts allocated to
patents, IPR&D and goodwill have been attributed to the CombiMatrix group.
F-21
In conjunction with the allocation of the purchase price, Acacia Research
Corporation was required to adjust CombiMatrix Corporation's assets and
liabilities to fair value. Deferred revenue, primarily consisting of milestone
payments and other cash receipts from Roche and NASA, was reduced by $8,425,000
to reflect the fair value of the continuing obligation related to the 52%
interest in CombiMatrix Corporation acquired by Acacia Research Corporation.
The amount attributable to IPR&D projects (comprised of two projects:
Genomics and Proteomics biological array systems) that had not yet reached
technological feasibility and had no alternative future use of $17,237,000 was
charged to expense on the acquisition date and is included in the consolidated
statement of operations and comprehensive loss for the year ended December 31,
2002.
Management was responsible for determining the fair value of the tangible and identifiable intangible assets acquired and liabilities assumed including
IPR&D, at the date of acquisition. Management considered a number of factors, including reference to an independent valuations.valuation. The in-process technologiespatents and patent rights acquired were valued using a discounted cash flow model on a project-by-projectpatent portfolio by portfolio basis, which estimated the future net cash flows expected to result from the licensing of each project once it has
reached technological feasibility.portfolio, taking into account potential infringers of the patents, usage of the underlying technologies, estimated license fee revenues, contingent legal fee arrangements, inventor royalties due to former patent holders, other estimated costs, tax implications and other factors. A discount rate consistent with the risks of
each projectassociated with achieving the estimated net cash flows was used to estimate the present value of future estimated net cash flows. In
estimating future cash flows, management considered the contribution of its core
technology (for which a United States patent was obtainedManagement’s valuation resulted in July 2000) that
would be required for successful exploitation of purchased in-process
technology, in order to value the core and in-process technologies discretely.
As a result, future cash flows relating to each purchased IPR&D project were
reduced in order to reflect the contribution of core technology to each IPR&D
project. The cash flows from these projects attributable to core technology were
then separately valued to determine the intangible assetan estimated fair value of purchased core
technology. In determiningpatent related assets acquired of approximately $27,000,000, resulting in approximately $1,900,000 of excess fair value over the contributioncost of core technology to in-process
projects, management usednet assets acquired, which has been allocated as a profit split approach which considered the estimated
profit split between a licensor and licensee of the core technology and
management's assessment of how critical the core technology waspro rata reduction to the IPR&D
projects.
The nature of the efforts to develop the purchased IPR&D into commercially
viable products principally relates to the completion and/or acceleration of
existing development programs. These efforts include testing current and
alternative materials used in array design, testing of existing and alternative
methods for array synthesis, developing prototype machinery (including operating
software) to synthesize, hybridize and read individual arrays, and to perform
numerous experiments, or assays, with actual target samples in order to
determine customer protocols and procedures for using the CombiMatrix group's
array system. Following is a brief description of the two IPR&D projects
identified.
Genomics Biological Array System: CombiMatrix Corporation's genomics
biological array processor system is being developed to discretely immobilize
sequences of DNA or RNA within individual test sites on a modified semiconductor
chip coated with a three-dimensional layer of porous material. The system also
includes proprietary hardware units and related software applications to be able
to synthesize materials onto the chips, apply target samples of genetic
materials and interpret the results. The fair valueamounts that otherwise would have been assigned to the genomics
biological array system IPR&D project was $13,978,000. A risk-adjusted discount
rateassets acquired, in accordance with the purchase method of 32% was appliedaccounting.
In connection with the acquisition described above, Acacia Global Acquisition Corporation entered into a consulting agreement with the former CEO of Global Patent Holdings, LLC. The agreement required the payment of $2,000,000 in consulting fees over a two-year period, and certain reimbursable consulting related expenses, commencing on the date of acquisition. Marketing, general and administrative expenses for the years ended December 31, 2007, 2006 and 2005 include $103,000, $1,087,000 and $1,009,000, respectively, in expenses related to the project's estimated cash flows.
Proteomics Biological Array System: CombiMatrix Corporation's proteomics
biological array processor system is being developed to discretely immobilize
proteinsconsulting agreement. The consulting agreement expired in January 2007.
The acquisition was treated for tax purposes as a taxable asset acquisition and, other small molecules within individual test sites on a modified
semiconductor chipas such, there were no book/tax basis differences associated with the acquisition. As such, Acacia Research Corporation did not record any deferred income taxes in a similar fashion as described above forconnection with the genomics
biological array system. The proteomics biological array system is used for
detection and identificationapplication of bio-threat agents in CombiMatrix Corporation's
biological and chemical threat agent detector development programs that are
currently in process. The fair value assigned to the proteomics biological array
system IPR&D project was $3,259,000. A risk-adjusted discount ratepurchase method of 60% was
applied toaccounting.
F-19
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
8. STOCKHOLDERS’ EQUITY
Capital Stock
As of December 31, 2007, the project's estimated cash flows.
F-22
9. STOCKHOLDERS' EQUITY
REDEEMABLE CAPITAL STOCK
The authorized capital stock of Acacia Research Corporation consists of 110,000,000210,000,000 shares, of which 50,000,000100,000,000 shares is a class of common stock designated as "AR-CombiMatrix“AR–Acacia Technologies stock,"” having a par value of $0.001 per share, 50,000,000100,000,000 shares is a class of common stock designated as "AR-Acacia
Technologies“AR–CombiMatrix stock,"” having a par value of $0.001 per share, and 10,000,000 is a class of preferred stock having a par value of $0.001 per share (the "Preferred
Stock"“Preferred Stock”) and issuable in one or more series as determined by the board of directors pursuant to Acacia Research Corporation'sCorporation’s restated certificate of incorporation.
Holders of AR-CombiMatrix stock and AR-Acacia Technologiesour classes of common stock vote together as a single class (except in certain limited circumstances). Each
shareSubsequent to the consummation of AR-CombiMatrixthe Split-off Transaction on August 15, 2007, as described above at Note 1, Acacia Research Corporation’s only class of common stock entitles the holder to one vote. Each share ofoutstanding is its AR-Acacia Technologies stock entitles the holder, for any particular vote, to a
number of votes equal to the average market value of a share of AR-Acacia
Technologies stock divided by the average market value of a share of
AR-CombiMatrix stock over a specified 20-trading day period ending on the tenth
trading day prior to the record date for determining the stockholders entitled
to vote.stock. Holders of each class of common stock are entitled to receive ratably such dividends, if any, as may be declared by the board of directors out of funds legally available therefore.
Under our restated certificate of incorporation, in the event of our dissolution, liquidation or winding up, after payment or provision for payment of the debts and other liabilities and full preferential amounts to which holders of any preferred stock are entitled, regardless of the group to which
such shares of preferred stock were attributed, the holders of AR-CombiMatrix
stock and AR-Acacia Technologiesour common stock will be entitled to receive our assets remaining for distribution to holders of common stock on a per share basis in proportion to the liquidation units per share of such class. Each share of
AR-CombiMatrix stock will have one liquidation unit. Each share of AR-Acacia
Technologies stock will have a number of liquidation units equal to the quotient
of the average market value of a share of AR-Acacia Technologies stock over the
20-trading day period ending on the 40th trading day after the effective date of
the recapitalization, divided by the average market value of a share of
AR-CombiMatrix stock over the same period.
Holders of each class of common stock have no preemptive, subscription, redemption or conversion rights. Management, at its discretion may, at any time,
convert each share of AR-CombiMatrix stock into a number of shares of AR-Acacia
Technologies stock at a 10% premium over the average market price.
Each class of stock is designed to reflect the financial performance of the
respective group, rather than the performance of
Acacia Research Corporation as
a whole. The chief mechanisms intended to cause the AR-CombiMatrix stock and the
AR-Acacia Technologies stock to reflect the financial performance of the
respective group are provisions in Acacia Research Corporation's restated
certificate of incorporation governing dividends and distributions. Under these
provisions, Acacia Research Corporation will:
o factor the assets and liabilities and income or losses attributable to
the respective group into the determination of the amount available to
pay dividends on the shares issued for the respective group; and
o require Acacia Research Corporation to exchange, redeem or distribute
a dividend on the stock of a group if all or substantially all of the
assets allocated to the respective group are sold to a third party.
Management of Acacia Research Corporation cannot assure the holders of
AR-CombiMatrix stock or AR-Acacia Technologies stock that the market values of
the two share classes will in fact reflect the separate performance of each
class of stock. Holders of AR-CombiMatrix stock and AR-Acacia Technologies stock
are stockholders of Acacia Research Corporation and as a result, are subject to
all of the risks of an investment in Acacia Research Corporation and all of its
businesses, assets and liabilities. Financial effects from one group that affect
Acacia Research Corporation's consolidated results of operations or financial
condition could, if significant, affect the results of operations or financial
condition of the other group.
F-23
Acacia Research Corporation'sCorporation’s board of directors, subject to state laws and limits in our restated certificate of incorporation, including those discussed above, will beare able to declare dividends on AR-CombiMatrix stock and AR-Acacia
Technologiesour common stock in its discretion. To date, Acacia Research Corporation has never paid or declared cash dividends on shares of our stock, nor do we anticipate paying cash dividends on either of the two classes of stock in the foreseeable future.
The allocation
In connection with the consummation of corporate expenses is generally basedthe Split-off Transaction on utilization and
is in accordance withAugust 15, 2007, the Redemption Date, CombiMatrix Corporation was split-off from Acacia Research Corporation'sCorporation through the redemption of all outstanding shares of AR-CombiMatrix common stock in exchange for the distribution of new shares of CombiMatrix Corporation common stock. Subsequent to the consummation of the Split-off Transaction, Acacia Research Corporation’s only class of common stock outstanding is its AR-Acacia Technologies common stock. Pursuant to Acacia Research Corporation’s amended and restated certificate of incorporation, neither class of common stock is redeemable as of and subsequent to the Redemption Date. As such, we no longer label the common stock in the accompanying consolidated balance sheet as of December 31, 2007 and statement of stockholders’ equity for the purposeyear ended December 31, 2007 as “redeemable.”
In February 2008, our Board of measuring earnings availableDirectors approved an amendment and restatement of our certificate of incorporation to stockholders
ofremove reference to AR-CombiMatrix stock andwhich was cancelled as a result of the redemption described above, to rename AR-Acacia Technologies stock as the only class of common stock, and does not
necessarily reflectto provide that all 100,000,000 shares of AR-Acacia Technologies stock currently authorized may be issued as a single class of common stock. The amendment and restatement of our certificate of incorporation is subject to approval by the financial condition, cash flowsstockholders at the next annual meeting of stockholders. If adopted, each share of common stock will be entitled to one vote and operating results of
each division as if it were a stand-alone entity. The management and allocation
policies applicable to the determinationrelative voting strength of the assets and liabilities and
income or losses attributable tocommon stock will be equal notwithstanding the respective group may be modified or
rescinded, or additional policies may be adopted, at the sole discretion of
Acacia Research Corporation's board of directors at any time without approvaltrading price of the stockholders. Acacia Research Corporation's management and board of
directors have the ability to: transfer funds between the groups at the
discretion of management and the board of directors; allocate financing costs
between groups that may not reflect the separate borrowing costs of the groups;
and charge a greater or lesser portion of the total corporate tax liability to
the groups than that which would have been charged if the groups were
stand-alone entities. Acacia Research Corporation's management and board of
directors do not presently intend to modify or rescind the methodologies and
assumptions underlying the allocations in the pro forma financial statements.
See Note 2 for a description of applicable management allocation policies.
OTHER
common stock.
Other
In April 2004,February 2005, Acacia Research Corporation raised gross proceeds of $15,000,000$19,600,000 through the sale of 3,000,0003,500,000 shares of Acacia Research -
CombiMatrix commonAR-Acacia Technologies stock at a price of $5.00$5.60 per share in a registered direct offering. Net proceedsProceeds raised, of approximately $13,715,000, which are net of related issuance costs, were attributed to the CombiMatrix group.
During 2004 and 2003, proceeds of $2,093,000 and $450,000 were received
from the issuance of 761,205 and 164,000 shares, respectively, of AR-CombiMatrix
stock related to the exercise of certain warrants issued in connection with the
May 2003 private equity financing described below. The proceeds from the
warrants exercised were attributed to the CombiMatrix group.
In May 2003, totaled approximately $19,532,000.
F-20
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
9. INCOME TAXES
Acacia Research Corporation completed a private equity
financing, raising gross proceeds of $5,247,000 through the issuance of
2,385,000 units. Each unit consists of one share of AR-CombiMatrix common stock
and one-half, five-year callable common stock purchase warrant. Each full common
stock purchase warrant entitles the holder to purchase a share of AR-CombiMatrix
stock at a price of $2.75 per share and is callable by Acacia Research
Corporation beginning in May 2004 once the daily average of the high and low
prices of Acacia Research Corporation's AR-CombiMatrix stock on the Nasdaq
SmallCap Market is equal to or above $4.50 for 20 consecutive trading days.
Acacia Research Corporation issued an additional 31,502 shares of AR-CombiMatrix
stock in lieu of cash payments in conjunction with the private placement for
finder's fees. Net proceeds raised from the private equity financing of
$4,862,000 were attributed to the CombiMatrix group.
In September 2002, CombiMatrix Corporation issued 4,016,346 shares of its
common stock to Nanogen, Inc. ("Nanogen") in settlement of all outstanding
litigation between the parties (see Note 13). As a result of the transaction,
Acacia Research Corporation's equity ownership in CombiMatrix Corporation
decreased from 58% to 48%. A loss totaling $550,000, resulting from CombiMatrix
Corporation's issuance of stock to a third party at a value per share below our
carrying amount per share has been reflected as a direct reduction to additional
paid-in capital in consolidated stockholders' equity.
F-24
10. INCOME TAXES
The benefitCorporation’s provision (benefit) for income taxes consists of the following (in thousands):
2004 2003 2002
-------- -------- --------
Current:
U.S. Federal tax ......... $ - $ (2) $ (572)
State taxes .............. 4 9 4
-------- -------- --------
4 7 (568)
-------- -------- --------
Deferred:
U.S. Federal tax ......... (279) (280) (289)
State taxes .............. - - -
-------- -------- --------
(279) (280) (289)
-------- -------- --------
$ (275) $ (273) $ (857)
======== ======== ========
2007 | 2006 | 2005 | ||||||||||
| Current: | ||||||||||||
| U.S. Federal tax | $ | - | $ | - | $ | - | ||||||
| State taxes | 207 | 76 | 8 | |||||||||
| 207 | 76 | 8 | ||||||||||
| Deferred: | ||||||||||||
| U.S. Federal tax | - | (36 | ) | (143 | ) | |||||||
| State taxes | - | - | - | |||||||||
| - | (36 | ) | (143 | ) | ||||||||
| $ | 207 | $ | 40 | $ | (135 | ) | ||||||
The tax effects of temporary differences and carryforwards that give rise to significant portions of deferred assets and liabilities consist of the following at December 31, 20042007 and 20032006 (in thousands):
2007 | 2006 | |||||||
| Deferred tax assets: | ||||||||
| Basis in affiliates | $ | 495 | $ | 495 | ||||
| Depreciation and amortization | 3,632 | 2,292 | ||||||
| State taxes | 3 | - | ||||||
| Deferred revenue | 127 | 142 | ||||||
| Stock compensation | 2,451 | 1,672 | ||||||
| Accrued liabilities and other | 153 | 140 | ||||||
| Write-off of investments | 1,344 | 1,250 | ||||||
| Net operating loss and capital loss carryforwards and credits | 22,938 | 22,075 | ||||||
| Total deferred tax assets | 31,143 | 28,066 | ||||||
| Less: valuation allowance | (30,816 | ) | (27,562 | ) | ||||
| Net deferred tax assets, net of valuation allowance | 327 | 504 | ||||||
| Deferred tax liabilities: | ||||||||
| Intangibles | (327 | ) | (504 | ) | ||||
| Net deferred tax liability | $ | - | $ | - |
A reconciliation of the federal statutory income tax rate and the effective income tax rate is as follows:
F-25
2007 | 2006 | 2005 | ||||||||||
| Statutory federal tax rate | (34 | %) | (34 | %) | (34 | %) | ||||||
| State income taxes, net of federal tax effect | 3 | % | 1 | % | - | |||||||
| Equity compensation | 1 | % | 6 | % | - | |||||||
| Non deductible permanent items | 1 | % | - | 1 | % | |||||||
| Capital loss carryforwards | 6 | % | - | - | ||||||||
| Valuation allowance | 26 | % | 27 | % | 31 | % | ||||||
| 3 | % | - | (2 | %) | ||||||||
At December 31, 2004, Acacia Research Corporation has2007, we had established a full valuation allowance against our net deferred tax assets, totaling approximately $94,118,000, which are fully offset by a valuation
allowance due to management'smanagement’s determination that the criteria for recognition have not been met.
In
F-21
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
At December 2002,31, 2007, Acacia Research Corporation increased its ownership
interest in CombiMatrix Corporation from 48% to 100%. As a result of the
increase in ownership, Acacia Research Corporation files a consolidatedhad U.S. federal
income tax returns that includes the Acacia Technologies group (excluding
discontinued operations) and the CombiMatrix group.
At December 31, 2004, consolidated U.S. Federal and state income tax net operating loss carry forwards ("NOLs"(“NOLs”), excluding NOLs related to subsidiaries
for which we do not file a consolidated tax return, weretotaling approximately $138,835,000$58,258,000 and $38,659,000,$41,961,000, expiring between 20052010 and 2024.2027, and 2008 and 2017, respectively. In addition, weAcacia Research Corporation had consolidated tax credit carryforwards of approximately $2,931,000. The
amount of the CombiMatrix Corporation NOLs and tax credits acquired, totaling
approximately $90,131,000 (expiring between 2011 and 2024) and $2,869,000,
respectively, that can be utilized annually to offset future taxable income or
tax liability has been limited under the Internal Revenue Code due to the
ownership change resulting from our December 2002 increase in ownership interest
in CombiMatrix Corporation to 100%.
$40,000.
As of December 31, 2004, the aggregate tax NOLs at other subsidiaries for
which we do not file a consolidated tax return are2007, approximately $20,252,000 for
federal income tax purposes, expiring between 2018 and 2024. However, the use of
these NOLs is limited to the separate earnings of the respective subsidiaries.
In addition, ownership changes may also restrict the use of NOLs and tax
credits.
Had the Acacia Technologies group and the CombiMatrix group each filed
separate tax returns, the provision (benefit) for income taxes and division net
income (loss) would not have differed from the amounts reported in Acacia
Research Corporation's statement of operations for the years ended December 31,
2004, 2003, and 2002.
As of December 31, 2004, approximately $9,844,000$11,762,000 of the valuation allowance related to the tax benefits of stock option deductions included in Acacia Research Corporation'sCorporation’s NOLs. At such time as the valuation allowance is released, the benefit will be credited to additional paid-in capital. 11. DISCONTINUED OPERATIONS
Income taxes paid during the periods presented were not material.
During the year ended December 31, 2007, we determined that certain of our deferred tax assets, principally “Basis in affiliates,” and the related full valuation allowance were overstated for the period since December 31, 1996 through December 31, 2006. The Basis in affiliates amount of approximately $28 million was frozen prior to 2004 since we had acquired the remaining minority interest of the affiliate. We retroactively corrected our deferred tax asset balances and the related valuation allowance in the deferred tax asset table by approximately $30 million. The correction had no impact on the consolidated balance sheet, statements of operations, stockholders’ equity or cash flows for any period and there was no other impact to the consolidated financial statements. Management concluded this correction was not material to the consolidated financial statements.
10. ACCOUNTING FOR THE SPLIT-OFF OF COMBIMATRIX CORPORATION
In September 2004January 2006, Acacia Research Corporation’s board of directors approved a plan for its wholly owned subsidiary, CombiMatrix Corporation, to become an independent public company. CombiMatrix Corporation’s registration statement on Form S-1 was declared effective by the SEC on June 8, 2007. Following the redemption period required by Acacia Research Corporation’s Restated Certificate of Incorporation, on August 15, 2007 (the “Redemption Date”), CombiMatrix Corporation was split-off from Acacia Research Corporation through the redemption of all outstanding shares of AR-CombiMatrix stock in exchange for the distribution of new shares of CombiMatrix Corporation common stock, on a pro-rata basis, to the holders of AR-CombiMatrix stock as of the Redemption Date (the “Split-off Transaction”). On the Redemption Date, every ten (10) shares of AR-CombiMatrix stock outstanding on August 15, 2007, was redeemed for one (1) share of common stock of CombiMatrix Corporation. Subsequent to the Redemption Date, Acacia Research Corporation no longer owns any equity interests in CombiMatrix Corporation and 2002, we accrued an additional $104,000the two companies operate independently of each other.
As a result of the Split-off Transaction, the CombiMatrix group is no longer a business group of Acacia Research Corporation. As a result of the Split-off Transaction, all outstanding shares of AR-CombiMatrix stock were redeemed, and $200,000
(nethence, all rights of minority interests), respectively,holders of AR-CombiMatrix stock ceased as of the Redemption Date, except for the right, upon the surrender to the exchange agent of shares of AR-CombiMatrix stock, to receive new shares of CombiMatrix Corporation stock pursuant to the exchange ratio described above.
The Split-off Transaction was accounted for by Acacia Research Corporation at historical cost. Accordingly, no gain or loss on disposal was recognized in estimatedthe accompanying consolidated statements of operations. Included in the current period consolidated balance sheet is a charge to consolidated shareholders’ equity totaling $35,444,000, reflecting the distribution of our investment in the net assets of CombiMatrix Corporation to holders of AR-CombiMatrix stock, as of the Redemption Date, as described above. We received a private letter ruling from the IRS with regard to the U.S. federal income tax consequences of the Split-off Transaction to the effect that the Split-Transaction will be treated as a tax-free exchange under Sections 368 and 355 of the Internal Revenue Code of 1986, as amended.
For the period from January 1, 2007 through August 15, 2007, revenues and pre-tax loss related to CombiMatrix Corporation included in discontinued operations were $2,968,000 and $8,086,000, respectively. Net loss from discontinued operations for the year ended December 31, 2007, includes direct costs to be incurred in connection with the discontinuedSplit-off Transaction, originally included in Acacia Research Corporation corporate accounts, totaling $136,000 for the year ended December 31, 2007.
F-22
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
10A. DISCONTINUED OPERATIONS – SPLIT-OFF OF COMBIMATRIX CORPORATION
In January 2006, Acacia Research Corporation’s board of directors approved a plan for its wholly owned subsidiary, CombiMatrix Corporation, to become an independent public company. CombiMatrix Corporation’s registration statement on Form S-1 was declared effective by the SEC on June 8, 2007.
As a result of the Split-off Transaction, the CombiMatrix group is no longer a business group of Acacia Research Corporation. As a result of the consummation of the Split-off Transaction, the assets, liabilities, results of operations and cash flows of CombiMatrix Corporation have been eliminated from the continuing operations of Soundbreak.com (originally ceasedAcacia Research Corporation and Acacia Research Corporation does not have any continuing involvement in the operations of CombiMatrix Corporation. As a result of the Split-off Transaction, we have disposed of our investment in February 2001). CombiMatrix Corporation, and therefore, in accordance with guidance set forth in SFAS No. 144, Acacia Research Corporation’s accompanying consolidated balance sheets, statements of operations and statements of cash flows for the current period presented reflect the assets, liabilities, results of operations and cash flows for CombiMatrix Corporation as discontinued operations. Consolidated financial statements presented for the comparable prior year periods have been restated to conform to this presentation. CombiMatrix Corporation was previously presented as a separate operating segment of Acacia Research Corporation under SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information.”
The additional accruals relate primarily to
certain noncancellable lease obligations andcarrying amounts of the inability to sublease the
related office space at rates commensurate with our existing obligations.
Themajor classes of assets and liabilities of theand revenues and pretax loss included in discontinued operations atfor the following periods were as follows (in thousands):
| December 31, 2006 | ||||
| Cash and available-for-sale investments | 14,342 | |||
| Accounts receivable, inventory and other asset | 1,210 | |||
| Property and equipment, net of accumulated depreciation | 1,785 | |||
| Intangible assets and goodwill | 24,210 | |||
| Other assets | 2,667 | |||
| Accounts payable and accrued expenses | (2,846 | ) | ||
| Deferred revenues | (1,441 | ) | ||
| Warrant liability | (6,732 | ) | ||
| Revenues | Pre-tax Loss | |||||||
| For the year ended December 31, 2006 | 5,740 | (20,127 | ) | |||||
| For the year ended December 31, 2005 | 8,033 | (12,568 | ) | |||||
Net loss from discontinued operations related to CombiMatrix Corporation includes direct costs incurred in connection with the Split-off Transaction, originally included in Acacia Research Corporation corporate accounts, totaling $133,000 for the year ended December 31, 2004 and 2003 consist primarily of $889,000 and $1,953,000 of cash and cash
equivalents and $275,000 and $388,000 of accounts payable and accrued expenses,
respectively.
12. STOCK OPTIONS AND WARRANTS
2006.
11. STOCK-BASED INCENTIVE PLANS
The 2002 Acacia Technologies Stock Incentive Plan (the "AR-Acacia
Technologies Group Plan"(“2002 Plan”) and the 2002 CombiMatrix2007 Acacia Technologies Stock Incentive Plan (the
"AR-CombiMatrix Group Plan"(“2007 Plan”) (collectively, the “Plans”) were approved by the stockholders of Acacia Research Corporation in December 2002 (see Note 1). The AR-Acacia Technologies
Group Plan authorizesand May 2007, respectively. Both Plans allow grants of stock options, stock awards and performance shares with respect to AR-Acacia Technologies stock. The AR-CombiMatrix Group
Plan authorizes grants of stock options, stock awardsto eligible individuals, which generally includes directors, officers, employees and performance shares
with respect to AR-CombiMatrix stock. Directors and certain officers and key
employees with responsibilities involving both the Acacia Technologies group and
the CombiMatrix group may be granted awards under both incentive plans in a
manner which reflects their responsibilities. The board of directors believes
that granting participants awards tied to performance of the group in which the
participants work and, in certain cases the other group, is in the best interest
of the Acacia Research Corporation and its stockholders.
F-26
As a result of the recapitalization of Acacia Research Corporation in
December 2002 (see Note 1), each outstanding option and warrant to acquire a
share of Acacia Research Corporation common stock under the existing stock
option plans or warrants was converted into separately exercisable options or
warrants,consultants. Except as the case may be, to acquire 0.5582 of a share of AR-CombiMatrix
stock and one share of AR-Acacia Technologies stock. The conversion ratio for
shares of AR-CombiMatrix stock is equal to the quotient obtained by dividing (a)
the number of shares of CombiMatrix Corporation common stock owned by Acacia
Research Corporation immediately prior to the effective time of the merger by
(b) the total number of shares of Acacia Research Corporation common stock
issued and outstanding immediately prior to the effective time. The exercise
price for the resulting AR-Acacia Technologies stock options and warrants and
AR-CombiMatrix stock options and warrants was calculated by multiplying the
exercise price under such existing stock option or warrant by a fraction, the
numerator of which is the result obtained by multiplying the opening price of
the applicable class of common stock underlying such option on the first date
such stocks are traded after the recapitalization times the applicable
conversion ratio and the denominator of which is the sum of such amounts for the
AR-CombiMatrix stock and the AR-Acacia Technologies stock. However, the
aggregate intrinsic value of the options was not increased, and the ratio of the
exercise price per option to the market value per share was not reduced. The
converted options continue to be governed bynoted below, the terms and conditionsprovisions of the original option plans.
As a result of the merger transaction with CombiMatrix Corporation,Plans are identical in December 2002 (see Note 8), each outstanding option to purchase shares of
CombiMatrix Corporation common stock under CombiMatrix Corporation's 1995 Stock
Option Plan, 1998 Stock Option Plan and 2000 Stock Awards Plan, whether or not
exercisable, was assumed by all material respects.
F-23
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Acacia Research Corporation. Each assumed option
continues to be governed by the same terms and conditions that governed it under
the applicable CombiMatrix Corporation plan immediately before the effective
time of the merger except that the option is exercisable for shares of
AR-CombiMatrix stock rather than CombiMatrix Corporation common stock. The
number of shares of AR-CombiMatrix stock issuable upon exercise of the assumed
option, as well as the exercise price, is the same as the number of shares of
CombiMatrix Corporation common stock issuable and exercise price prior to the
merger. The exchange of AR-CombiMatrix stock options for CombiMatrix Corporation
common stock options is considered a modification (or settlement) of a
stock-based compensation arrangement resulting in a new measurement date for the
respective awards. The new measurement date for the award modifications was
December 13, 2002, the effective date of the merger, and resulted in additional
stock-based compensation of $116,000, which was allocated to the CombiMatrix
group.
STOCK OPTION PLANS
The terms of the AR-Acacia Technologies Group Plan and the AR-CombiMatrix
Group Plan are identical except that AR-Acacia Technologies stock may be issued
only under the AR-Acacia Technologies Group Plan and AR-CombiMatrix stock may be
issued only under the AR-CombiMatrix Group Plan.
Acacia Research Corporation'sCorporation’s compensation committee administers the discretionary option grant and stock issuance programs. ThisThe compensation committee determines which eligible individuals are to receive option grants or stock issuances under those programs, the time or times when the grants or issuances are to be made, the number of shares subject to each grant or issuance, the status of any granted option as either an incentive stock option or a non-statutory stock option under the federal tax laws, the vesting schedule to be in effect for the option grant or stock issuance and the maximum term for which any granted option is to remain outstanding. PROGRAMS
Each of the incentive plans has four separate programs:
o DISCRETIONARY OPTION GRANT PROGRAM. Under the discretionary option
grant program, our compensation committee may grant (1) non-statutory
options to purchase shares of AR-Acacia Technologies stock and
AR-CombiMatrix stock, as applicable, to eligible individuals in the
employ or service of Acacia Research Corporation or our subsidiaries
(including employees, non-employee board members and consultants) at
anThe exercise price not less than 85% of the fair market value of those
shares on the grant date and (2) incentive stock options is generally equal to purchase
F-27
shares of AR-Acacia Technologies stock and AR-CombiMatrix stock, as
applicable, to eligible employees at an exercise price not less than
100% of the fair market value of those shares on the grant date (not
less than 110% of fair market value if such employee actually or
constructively owns more than 10% of our voting stock or the voting
stock of any of our subsidiaries).
o STOCK ISSUANCE PROGRAM. Under the stock issuance program, eligible
individuals may be issued shares of AR-Acacia Technologies stock and
AR-CombiMatrix stock, as applicable, directly, upon the attainment of
performance milestones or the completion of a specified period of
service or as a bonus for past services. Under this program, the
purchase price for the shares shall not be less than 100% of the fair market value of the sharesAR-Acacia Technologies stock on the date of issuance,grant. Options generally begin to be exercisable six months to one year after grant and payment may begenerally expire ten years after grant. Stock options generally vest over two to three years and restricted shares generally vest in full after two to three years (generally represents the form of cash or past services rendered.
o AUTOMATIC OPTION GRANT PROGRAM.requisite service period in accordance with SFAS No. 123R).
Programs
The Plans provide for the following separate programs:
| · | Discretionary Option Grant Program. Under the discretionary option grant program, our compensation committee may grant (1) non-statutory options to purchase shares of AR-Acacia Technologies stock to eligible individuals in the employ or service of Acacia Research Corporation or our subsidiaries (including employees, non-employee board members and consultants) at an exercise price not less than 85% of the fair market value of those shares on the grant date and (2) incentive stock options to purchase shares of AR-Acacia Technologies stock to eligible employees at an exercise price not less than 100% of the fair market value of those shares on the grant date (not less than 110% of fair market value if such employee actually or constructively owns more than 10% of our voting stock or the voting stock of any of our subsidiaries). |
| · | Stock Issuance Program. Under the stock issuance program, eligible individuals may be issued shares of AR-Acacia Technologies stock directly, upon the attainment of performance milestones or the completion of a specified period of service or as a bonus for past services. Under this program, the purchase price for the shares shall not be less than 100% of the fair market value of the shares on the date of issuance, and payment may be in the form of cash or past services rendered. |
| · | Automatic Option Grant Program (2002 Plan only). Under the automatic option grant program, option grants will automatically be made at periodic intervals to eligible non-employee members of our board of directors to purchase shares of AR-Acacia Technologies stock |
Commencing in fiscal 2008, in lieu of the option grants described above, each non-employee director will receive restricted stock units for the number of shares determined by dividing the annual retainer by the closing price of the common stock on the grant date. Eachdate, provided that such individual who
first becomeshas served as a non-employee board memberdirector for at any time afterleast 6 months. In addition, as of May 2007, each new non-employee director will receive restricted stock units for the datenumber of shares determined by dividing the annual Board of Directors retainer by the closing price of the adoption of the incentive plans by our board of directors will
automatically receive an option to purchase 20,000 shares of AR-Acacia
Technologies stock and 20,000 shares of AR-CombiMatrixcommon stock on the datecommencement date.
The number of shares of common stock available for issuance under the individual joins the board of directors. In addition,2002 Plan automatically increases on the first businesstrading day inof January each calendar year followingduring the adoptionterm of the incentive plansPlan by our boardan amount equal to three percent (3%) of directors, each non-employee board
member thenthe total number of shares of common stock outstanding on the last trading day in office, including eachDecember of the immediately preceding calendar year, but in no event shall any such annual increase exceed 500,000 shares. The aggregate number of shares of common stock available for issuance under the 2002 Plan shall not exceed 20,000,000 shares. At December 31, 2007, there were 18,000 shares available for grant under the 2002 Plan.
F-24
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The initial share reserve under the 2007 Plan was 560,000 shares. The number of shares of common stock available for issuance under the 2007 Plan automatically increases on January 1, 2008 and 2009, by an amount equal to two percent (2%) of the total number of shares of our current non-employee
board members who is thencommon stock outstanding on the last trading day of December in office, will automatically be granted an
option to purchase 15,000 shares of AR-Acacia Technologies stock and
15,000 shares of AR-CombiMatrix stock, providedthe prior calendar year, except that the individualautomatic increase in the share reserve will be three percent (3%) of our outstanding common stock on such January 1, if our common stock has served on the board of directors forappreciated by at least six months.
o DIRECTOR FEE OPTION GRANT PROGRAM. If this program is put into effectthirty percent (30%) in the future, itprior calendar year. After January 1, 2009, no new additional shares will allow non-employee members of our board of
directors the opportunity to apply a portion of any retainer fee
otherwise payable to them in cash each yearbe added to the acquisition of
special below-market option grants.
Limited stock appreciation rights will automatically be included as part of
each2007 Plan without stockholder approval (except for shares subject to outstanding awards that are forfeited or otherwise returned to the 2007 Plan). At December 31, 2007, there were 171,000 shares available for grant made under the automatic and director fee option grant programs, and
these rights may also be granted to one or more of our officers as part of their
option grants under the discretionary option grant program.
2007 Plan.
The AR-Acacia Technologies Group Plan and the AR-CombiMatrix Group PlanPlans do not segregate the number of securities remaining available for future issuance among stock options and other awards. The shares authorized for future issuance represents the total number of shares available through any combination of stock options or other awards. Upon the exercise of stock options or the granting of restricted stock, it is Acacia Research Corporation’s policy to issue new shares of the respective class of common stock.
Our board of directors may amend or modify the incentive plansPlans at any time, subject to any required stockholder approval. The incentive plansPlans will terminate no later than the tenth anniversary of the approval of the incentive plans by our stockholders. Options are generally exercisable six months to one
The following table summarizes stock option activity for the Plans for the year after grant and
expire five years after grant for directors or up to ten years after grant for
key employees. The authorized number of shares of common stock subject to the
AR-Acacia Technologies Group Plan is 6,208,000 shares. The authorized number of
shares of common stock subject to the AR-CombiMatrix Group Plan is 9,710,000
shares. Atended December 31, 2004, shares available for2007:
| Weighted-Average | |||||||
| Remaining | Aggregate | ||||||
Options | Exercise Price | Contractual Term | Intrinsic Value | ||||
| Outstanding at December 31, 2006 | 5,958,000 | $7.93 | |||||
| Granted | 300,000 | $14.20 | |||||
| Exercised | (1,063,000) | $4.72 | |||||
| Forfeited | (226,000) | $18.20 | |||||
| Outstanding at December 31, 2007 | 4,969,000 | $8.52 | 4.8 years | $14,528,000 | |||
| Vested and expected to vest at December 31, 2007 | 4,956,000 | $8.52 | 4.8 years | $14,518,000 | |||
| Exercisable at December 31, 2007 | 4,577,000 | $8.32 | 4.5 years | $14,197,000 | |||
The weighted-average grant are 228,000date fair value of stock options granted during the years ended December 31, 2007, 2006 and 2,166,000 under the AR-Acacia Technologies Group Plan2005 was $9.07, $5.35 and the AR-CombiMatrix
Group Plan,$4.17, respectively. The following is a summarytotal intrinsic value of commonoptions exercised during the years ended December 31, 2007, 2006 and 2005 was $10,812,000, $3,463,000 and $464,000, respectively. The fair value of options vested during the years ended December 31, 2007, 2006 and 2005 was $3,520,000, $3,960,000, and $1,455,000, respectively. As of December 31, 2007, the total unrecognized compensation expense related to nonvested stock option activities:
F-28
awards was $2,435,000, which is expected to be recognized over a weighted-average term of approximately 2 years.
The following table summarizes nonvested restricted share activity for the year ended December 31, 2007:
Nonvested Restricted Shares | Weighted Average Grant Date Fair Value | |||||||
| Nonvested restricted stock at December 31, 2006 | 428,000 | $7.10 | ||||||
| Granted | 846,000 | $14.04 | ||||||
| Vested | (324,000 | ) | $6.04 | |||||
| Forfeited | (35,000 | ) | $6.22 | |||||
| Nonvested restricted stock at December 31, 2007 | 915,000 | $13.92 | ||||||
F-25
ACACIA RESEARCH CORPORATION COMMON STOCK (THROUGH DECEMBER 13, 2002):
AR-ACACIA TECHNOLOGIES STOCK (FROM DECEMBER 13, 2002):
AR-COMBIMATRIX STOCK (FROM DECEMBER 13, 2002):
F-29
Options outstanding at
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The weighted-average grant date fair value of nonvested restricted stock granted during the years ended December 31, 2004 are summarized as follows:
AR-ACACIA TECHNOLOGIES STOCK:
AR-COMBIMATRIX STOCK:
Information2007, 2006 and 2005 was $14.04, $11.87 and $5.07, respectively. The fair value of nonvested restricted stock vested during the years ended December 31, 2007, 2006 and 2005 was $1,954,000, $215,000, and $0, respectively. As of December 31, 2007, the total unrecognized compensation expense related to options granted for the periods presentednonvested restricted stock awards was $9,422,000, which is as
follows:
- ----------
(1) From January 1, 2002 through December 13, 2002.
At December 31, 2004 and 2003, there were 283,000 and 1,045,000 warrants
outstanding, respectively, issued in connection with the May 2003 equity
financing discussed elsewhere herein, representing rights to purchase
AR-CombiMatrix common stock at a per share exercise price2007, 6,073,000 shares of $2.75, which are
exercisable through May 2008.
At December 31, 2003, there were 1,240,000 warrants outstanding
representing rights to purchase AR-Acacia Technologies common stock at a per
share exercise price of $13.23 and 692,000 warrants outstanding representing
rights to purchase AR-CombiMatrix common stock at a per share exercise price of
$10.50. These warrants expired unexercised in January 2004.
F-30
We have adoptedare reserved for issuance under the disclosure only requirements of SFAS No. 123 with
respect to options issued to employees. Refer to Note 2 for additional SFAS No.
123 disclosures.
13.Plans.
12. COMMITMENTS AND CONTINGENCIES
OPERATING LEASES
We lease
Operating Leases
Acacia Research Corporation leases certain office furniture and equipment and laboratory and office space under various operating lease agreements expiring over the next 4 years.in 2012. Minimum annual rental commitments onfor operating leases of continuing operations having initial or remaining non-cancelablenoncancellable lease terms in excess of one year are as follows (in thousands):
YEAR
----
2005................................................. $ 2,271
2006................................................. 2,226
2007................................................. 1,986
2008................................................. 1,615
Thereafter........................................... -
---------
Total minimum lease payments......................... $ 8,098
=========
| Year | ||||
| 2008 | $ | 868 | ||
| 2009 | 903 | |||
| 2010 | 939 | |||
| 2011 | 977 | |||
| 2012 | 163 | |||
| Total minimum lease payments | $ | 3,850 | ||
Rent expense related to continuing operations for the years ended December 31, 2007, 2006 and 2005 approximated $749,000, $565,000 and $453,000, respectively. Rental payments are expensed in 2004, 2003the statement of operations in the period to which they relate. Scheduled rent increases are amortized on a straight-line basis over the lease term.
Inventor Royalties and 2002 approximated $2,241,000, $2,473,000Contingent Legal Expenses
In connection with the acquisition of certain patents and $2,063,000, respectively.
LITIGATION
patent rights, certain operating subsidiaries of Acacia Research Corporation executed related agreements which grant to the former owners of the respective patents or patent rights, the right to receive inventor royalties based on future net license fee revenues (as defined in the respective agreements) generated as a result of licensing the respective patents or patent portfolios. Inventor royalties paid pursuant to the agreements are expensed in the consolidated statement of operations in the period that the related license fee revenues are recognized. In certain instances, pursuant to the terms of the underlying inventor agreements, costs paid by us to acquire patents are recoverable from future net revenues. Patent acquisition costs that are recoverable from future net revenues are amortized over the estimated economic useful life of the related patents, or as the prepaid royalties are earned by the inventor, as appropriate, and the related expense is included in amortization expense in the consolidated statement of operations. Any unamortized patent acquisition costs recovered from net revenues are expensed in the period recovered, and included in inventor royalties and contingent legal fees – patents in the consolidated statement of operations.
In connection with the licensing and enforcement activities of our operating subsidiaries, they may retain the services of law firms that specialize in intellectual property licensing and enforcement and patent law. These law firms may be retained on a contingent fee basis in which the law firms are paid on a scaled percentage of any negotiated license fees, settlements or judgments awarded based on how and when the license fees, settlements or judgments are obtained. In instances where there are no recoveries from potential infringers (ie. license fees), no contingent legal fees are paid; however, our operating subsidiaries may be liable for certain out of pocket legal costs incurred pursuant to the underlying legal services agreement. Legal fees advanced by contingent law firms that are required to be paid in the event that no license recoveries are obtained are expensed as incurred and included in liabilities in the consolidated balance sheet.
F-26
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Patent Enforcement and Other Litigation
Acacia Research Corporation is subject to claims, counterclaims and legal actions that arise in the ordinary course of business. Management believes that the ultimate liability with respect to these claims and legal actions, if any, will not have a material effect on our consolidated financial position, results of operations or cash flows. From timeOperating subsidiaries of Acacia Research Corporation are often required to time, companies comprising the Acacia Technologies
group engage in litigation to enforce their patents.
COMBIMATRIX CORPORATION
On November 28, 2000, Nanogen filed a complaint in the United States
District Court for the Southern District of California against CombiMatrix
Corporationpatents and Donald D. Montgomery, Ph.D., an officer, directorpatent rights.
Guarantees and stockholder of CombiMatrix Corporation. Dr. Montgomery was employed by Nanogen
as a senior research scientist between May 1994 and August 1995. The Nanogen
complaint alleged, among other things, breach of contract, trade secret
misappropriation and that U.S. Patent No. 6,093,302 and other proprietary
information belonging to CombiMatrix Corporation are instead the property of
Nanogen. The complaint sought, among other things, correction of inventorship on
the patent, the assignment of rights in the patent and pending patent
applications to Nanogen, an injunction preventing disclosure of trade secrets,
damages for trade secret misappropriation and the imposition of a constructive
trust.
F-31
On September 30, 2002, CombiMatrix Corporation and Dr. Donald Montgomery
entered into a settlement agreement with Nanogen to settle all pending
litigation between the parties. Pursuant to the terms of the settlement
agreement, Nanogen dismissed with prejudice its lawsuit against CombiMatrix
Corporation and Dr. Montgomery. In return, CombiMatrix Corporation agreed to pay
Nanogen $500,000 within 30 days of the settlement and an additional $500,000,
which was paid in September 2003. CombiMatrix Corporation also agreed to make
quarterly payments to Nanogen equal to 12.5% of payments to CombiMatrix
Corporation from sales of products developed by CombiMatrix Corporation and its
affiliates and based on the patents that had been in dispute in the litigation,
up to an annual maximum of $1,500,000. The minimum quarterly payments under the
settlement agreement will be $37,500 per quarter for the period from October 1,
2003 through October 1, 2004, and $25,000 per quarter thereafter until the
patents expire in 2018. Also, pursuant to the settlement agreement, CombiMatrix
Corporation issued to Nanogen 4,016,346 shares, or 17.5% of its outstanding
shares post-issuance, subject to an anti-dilution provision related to the
exercise of CombiMatrix Corporation options and warrants that were outstanding
on the effective date of the agreement, for a period of up to three years.
The issuance of the CombiMatrix Corporation common shares in settlement of
the litigation with Nanogen was accounted for as a nonmonetary transaction.
Accordingly, CombiMatrix Corporation recorded a non-cash litigation settlement
charge in the consolidated statements of operations for the year ended December
31, 2002 of approximately $17,471,000, which was based on the fair value of the
CombiMatrix Corporation common shares issued to Nanogen. Management was
responsible for determining the fair value of the common shares issued to
Nanogen on the settlement date and considered a number of factors, including
reference to an independent third-party valuation. Management utilized an income
approach to estimate the value of the common shares issued, based on the present
value of CombiMatrix Corporation's future estimated cash flows. Future estimated
cash flows included management's estimates of revenues, cost of sales, research
and development expenses, sales and marketing expenses, general and
administrative expenses, the anticipated effect of income taxes, and required
returns on working capital, fixed assets and other assets necessary to support
the generation of these cash flows. Future estimated cash flows were discounted
to the present value using a discount rate of 25%, which reflected a required
rate of return, comprised of an estimated weighted-average cost of capital,
which was further increased to reflect the risk profile of the company's
business.
Total legal settlement charges recorded in the consolidated statement of
operations for the year ended December 31, 2002 include the fair value of the
common shares issued to Nanogen in the amount of $17,471,000 and a charge in the
amount of $1,000,000 related to the cash payments due to Nanogen discussed
above.
During the year ended December 31, 2004, the CombiMatrix group recorded a
net non-cash charge totaling $812,000 in connection with the anti-dilution
provisions of the settlement agreement. The non-cash charge reflects
management's estimate of the fair value of AR-CombiMatrix stock issued to
Nanogen, Inc. as a result of certain options and warrants exercised during 2004
and the fair value of AR-CombiMatrix stock potentially issuable to Nanogen, Inc.
as of December 31, 2004. The liability is adjusted at each balance sheet date
for changes in the market value of the AR-CombiMatrix stock and is reflected as
a long-term liability until settled in equity. The anti-dilution provisions of
the settlement agreement expire in September 2005.
PATENT ENFORCEMENT LITIGATION
SOUNDVIEW TECHNOLOGIES
In September 2002, the United States District Court for the District of
Connecticut granted a motion for summary judgment filed by the defendants in
Soundview Technologies pending patent infringement and antitrust lawsuit against
Sony Corporation of America, the Consumer Electronics Manufacturers Association
and the Electronics Industries Alliance d/b/a Consumer Electronics Association
in the United States District Court for the Eastern District of Virginia (filed
on April 5, 2000), alleging that television sets utilizing certain content
blocking technology (commonly known as the "V-chip") and sold in the United
States infringe Soundview Technologies' U.S. Patent No. 4,554,584. In granting
the motion, the court ruled that the defendants have not infringed on Soundview
Technologies' patent.
In September 2003, a motion for summary judgment filed by the remaining
defendants was granted by the United States District Court for the District of
Connecticut on Soundview Technologies' anti-trust claims due to the Court's
previous ruling of non-infringement as described above.
In August 2004, the U.S. Court of Appeals for the Federal Circuit affirmed
the September 2002 U.S. District Court for the District of Connecticut ruling
that the remaining television manufacturers named in the Acacia Technologies
group's V-chip patent infringement lawsuit do not infringe the Acacia
Technologies group's V-chip patent. As a result of the ruling, the Acacia
Technologies group recorded an impairment charge of $1,616,000 in connection
with the write-off of goodwill related to the V-chip. In addition, as a result
of the conclusion of the V-chip patent litigation, the Acacia Technologies group
recognized $1,500,000 of previously deferred V-chip license fee revenues and
$668,000 of previously deferred V-chip related legal costs in the third quarter
of 2004. The remaining Non-Soundview parties have a motion pending before the
United States District Court for the District of Connecticut seeking
reimbursement of certain attorney's fees. Management believes that the ultimate
liability with respect to these claims and legal actions, if any, will not have
a material effect on our financial position, results of operations or cash
flows.
F-32
The final ruling in the V-chip litigation has no impact on the revenues
that we have recognized to date from licenseesIndemnifications
Certain of our V-chip technology.
ACACIA MEDIA TECHNOLOGIES CORPORATION
CABLE AND SATELLITE TV
In 2004, Acacia Media Technologies filed a Complaint in the District Court
for the Northern District of California alleging infringement of Acacia Media
Technologies' DMT patents against Comcast Corporation, Charter Communications,
Inc., The DirectTV Group, Inc., Echostar Communications Corporation, Boulder
Ridge Cable TV, Central Valley Cable TV, LLC, Seren Innovations, Inc., Cox
Communications, Inc., Hospitality Network, Inc. (a wholly owned subsidiary of
Cox that supplies hotel on-demand TV services) and Mediacom, LLC. As of December
31, 2004, Acacia Media Technologies has executed license and settlement
agreements with Boulder Ridge Cable TV, Central Valley Cable TV, and Seren
Innovations.
In September 2004, Acacia Media Technologies filed complaints in the U.S.
District Court for the District of Arizona, U.S. District Court for the District
of Minnesota and the U.S. District Court for the Northern District of Ohio -
Eastern Division, alleging infringement of Acacia Media Technologies' DMT
patents against certain cable and satellite companies located in Arizona,
Minnesota, and Ohio. Companies named in the lawsuits include Armstrong Group,
Arvig Communication Systems, Block Communications, Inc., Cable America
Corporation, Cable One, Inc., Cable System Services, Inc., Cannon Valley
Communications, Inc., East Cleveland Cable TV and Communications, LLC, Loretel
Cablevision, Massillon Cable TV, Inc., Mid-Continent Media, Inc., Nelsonville TV
Cable, Inc., NPG Cable, Inc., Precis Communications, Inc. San Carlos
Cablevision, LLC, Savage Communications, Inc., Sjoberg's Cablevision, Inc., US
Cable, and Wide Open West, LLC. As of December 31, 2004, Acacia Media
Technologies has executed license agreements with Precis Communications and
Cable System Services and dismissed the action against San Carlos Cablevision
and Nelsonville TV Cable.
INTERNET WEBSITES
In 2003, Acacia Media Technologies initiated DMT patent infringement
litigation in the Federal District Court for the Central District of California
(the "Court") against defendants who provide adult oriented digital content over
the Internet. As of December 31, 2004, New Destiny Internet Group, Inc., Audio
Communications Inc., VS Media, Ademia Multimedia, LLC, International Web
Innovations, Inc., Offendale Commercial BV, Ltd., Adult Entertainment Broadcast
Network, Cybertrend, Inc., Lightspeed Media Corp., Adult Revenue Services,
Innovative Ideas International, AskCS.com, Game Link, Inc., Club Jenna, Inc.,
Cybernet Ventures, Inc., ACMP, LLC, Global AVS, Inc. d/b/a DrewNet, ICS, Inc. /
AP Net Marketing, Inc., and National A-1 Advertising, remained in the
litigation.
HOTEL ON-DEMAND TV INDUSTRY
In November 2003, Acacia Media Technologies initiated a patent infringement
lawsuit in the Federal District Court for the Central District of California
against On Command Corporation, provider of interactive in-room entertainment,
information and business services to the lodging industry, regarding Acacia
Media Technologies' DMT technology. In June 2004, Acacia Media Technologies
entered into a license agreement for its DMT technology with On Command
Corporation settling all outstanding litigation between the parties.
GUARANTEES AND INDEMNIFICATIONS
Acacia Research Corporation hasoperating subsidiaries have made guarantees and indemnities under which itthey may be required to make payments to a guaranteed or indemnified party, in relation to certain transactions, including revenue transactions in the ordinary course of business. In connection with certain facility leases Acacia Research Corporation hasand certain of its operating subsidiaries have indemnified its lessors for certain claims arising from the facilityfacilities or the lease.leases. Acacia Research Corporation indemnifies its directors and officers to the maximum extent permitted under the laws of the State of Delaware. However, Acacia Research Corporation has a directors and officers insurance policy that may reduce its exposure in certain circumstances and may enable it to recover a portion of future amounts that may be payable, if any. The duration of the guarantees and indemnities varies and, in many cases is indefinite but subject to statute of limitations. The majority of guarantees and F-33
indemnities do not provide any limitations of the maximum potential future payments Acacia Research Corporationthat we could be obligated to make. To date, we have made no payments related to these guarantees and indemnities. Acacia
Research Corporation estimatesWe estimate the fair value of itsour indemnification obligations asto be insignificant based on this history and insurance coverage and hashave therefore, not recorded any liability for these guarantees and indemnities in the accompanying consolidated balance sheets.
13. RETIREMENT SAVINGS PLAN AND EXECUTIVE SEVERANCE POLICY
Retirement Savings Plan. Acacia Research Corporation is a guarantor under a lease agreement for
office space that expires in August 2005. The lease agreement was entered into
by Soundbreak.com, which ceased operations in February 2001. The leased premises
is subleased through the remaining term of the lease agreement. Refer to Note 11
for additional information regarding discontinued operations.
OTHER
COMBIMATRIX GROUP
In July 2001, CombiMatrix Corporation entered into a non-exclusive
worldwide license, supply, research and development agreement with Roche. Under
the terms of the agreement, Roche will purchase, use and resell CombiMatrix
Corporation's array and related technologies for production of customizable
arrays. Additionally, CombiMatrix Corporation and Roche will develop a platform
technology, providing a range of standardized arrays for use in research
applications. The agreement has a 15-year term and provides for minimum payments
by Roche to CombiMatrix Corporation over the first three years, including
payments upon the achievement of certain milestone and payments for products,
royalties and research and development projects. During 2003 and 2002, the
CombiMatrix group's research and development activities were driven primarily by
ongoing performance obligations under the product commercialization phase of its
license and research and development agreements with Roche. These activities
include costs associated with direct labor, supplies and materials, development
of prototype arrays and instruments and the use of outside consultants for
certain engineering efforts. As previously discussed in Note 5, the CombiMatrix
group completed all phases of its research and development agreement with Roche
in March 2004.
As previously disclosed in Note 6, the CombiMatrix group has entered into
an agreement with Leuchemix to purchase a total of $4,000,000 of Series A
Preferred Stock of Leuchemix over a two-year period. Future quarterly cash
investments by the CombiMatrix group in Leuchemix are $1,600,000 in 2005 and
$2,150,000 in 2006.
In March 2004, the CombiMatrix group was awarded a two-year, $5.9 million
contract with the Department of Defense to further the development of the
CombiMatrix group's array technology for the detection of biological threat
agents. Under the terms of the contract, the CombiMatrix group will perform
research and development activities as described under the contract and will be
reimbursed on a periodic basis for actual costs incurred to perform its
obligations, plus a fixed fee, of up to $5.9 million. Based on actual costs
incurred through December 31, 2004, the CombiMatrix group expects to incur
approximately $2.2 million and $819,000 in research and development costs during
2005 and 2006, respectively, to complete its obligations to the Department of
Defense under this contract. As of December 31, 2004, the biowarfare detection
contract with the Department of Defense was approximately 34% complete.
In July 2004, the CombiMatrix group and collaborator irsiCaixa Foundation
("IRSI") entered into a three-year research, development and licensing agreement
to develop certain siRNA compounds for pre-clinical drug development against the
HIV virus. Pursuant to the terms of the agreement, the CombiMatrix group will
make quarterly research and development funding payments to IRSI totaling
$450,000 over a period of three years, which began in July 2004. In addition,
the CombiMatrix group may make future contingent milestone payments for
compounds that are developed, in accordance with the terms of the agreement. In
consideration for receiving rights to commercialize the compounds under
development, the CombiMatrix group will pay royalties to IRSI based on
commercial sales of related products, in accordance with the agreement.
HUMAN RESOURCES
The CombiMatrix group provides certain severance benefits such that if an
executive who is a vice president or higher is terminated for other than cause,
death or disability, the executive will receive payments equal to three months'
base salary and other medical and dental benefits on a bi-weekly basis over a
three-month period. If termination occurs as a result of a change in control
F-34
transaction, these benefits will be extended by three months. The CombiMatrix
group also offers a general severance plan providing all employees with certain
benefits upon their termination of employment due to lack of work. Under this
plan, terminated employees will be provided with either four-weeks notice or
four-weeks' salary in lieu of notice, and paid a lump-sum amount based on the
employee's length of service, plus accrued benefits. The terminated employees
will also be provided continuing medical and dental benefits, as well as
continuation of life insurance, for a period ranging from two to 26 weeks
subsequent to the date of termination, depending upon the employee's length of
service.
ACACIA TECHNOLOGIES GROUP
In connection with the purchase of the outstanding ownership interests in
Acacia Media Technologies in November 2001, Acacia Media Technologies also
executed related assignment agreements which granted to the former owners of
Acacia Media Technologies' current patent portfolio the right to receive a
royalty of 15% of future net revenues, as defined in the agreements, generated
by Acacia Media Technologies' current patent portfolio, which includes its DMT
patents. No royalty obligation has been incurred as of December 31, 2004. Any
royalties paid pursuant to the agreements will be expensed in the consolidated
statement of operations.
14. RETIREMENT SAVINGS PLANS
The Acacia Technologies group and the CombiMatrix group have separate employee savings and retirement plansplan under section 401(k) of the Internal Revenue Code (the "Plans"“Plan”). The Plans arePlan is a defined contribution plansplan in which eligible employees may elect to have a percentage of their compensation contributed to the Plans,Plan, subject to certain guidelines issued by the Internal Revenue Service. The Acacia Technologies group and the CombiMatrix groupResearch Corporation may contribute to the PlansPlan at the discretion of Acacia Research Corporation'sthe board of directors. There were no contributions made by the Acacia Technologies group
or by the CombiMatrix groupResearch Corporation during the years ended December 31, 2004, 20032007, 2006 and 2002.
2005.
Executive Severance Policy. Under Acacia Research Corporation’s Executive Severance Policy, full-time employees with the title of Senior Vice President and higher (“Officer”) are entitled to receive certain benefits upon termination of employment. If employment of an Officer is terminated for other than cause or other than on account of death or disability, Acacia Research Corporation will (i) promptly pay to the Officer a lump sum amount equal to the aggregate of (a) accrued obligations ( i.e., the Officer’s annual base salary through the date of termination to the extent not theretofore paid and any compensation previously deferred by the Officer (together with any accrued interest or earnings thereon) and any accrued vacation pay, and reimbursable expenses, in each case to the extent not theretofore paid) and (b) three (3) months of the Officer’s base salary for each full year that the Officer was employed by the Company (the "Severance Period"), up to a maximum of twelve (12) months of the Officer's base salary and (ii) provide to the Officer, Acacia Research Corporation paid COBRA coverage for the medical and dental benefits selected by the Officer in the year in which the termination occurs, for the duration of the Severance Period.
14. SUPPLEMENTAL CASH FLOW INFORMATION
Cash paid by Acacia Research Corporation for income taxes was not material for the periods presented. Refer to Note 7 for a summary of the non cash investing activities in connection with the 2005 GPH Acquisition. Refer to Note 6 for impairment charges, and other non-cash reductions in intangibles during the periods presented. Refer to Notes 10 and 10A for information regarding the Split-off of CombiMatrix Corporation.
F-27
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
15. SUBSEQUENT EVENTS
EQUITY FINANCING
DISCONTINUED OPERATIONS - OTHER
In February 2005, Acacia Research Corporation raised gross proceedsaccrued an additional $237,000 (net of $19,600,000 through the sale of 3,500,000 shares of Acacia Research - Acacia
Technologies common stock at a price of $5.60 per shareminority interests), respectively, in a registered direct
offering. Net proceeds raised of approximately $19,575,000, which are net of
related issuanceestimated costs were attributed to the Acacia Technologies group. All of
the shares of Acacia Research-Acacia Technologies common stock were offered
pursuant to an effective registration statement previously filed with the
Securities and Exchange Commission.
ACQUISITION
On January 28, 2005, Acacia Global Acquisition Corporation, a newly formed
corporation, acquired the assets of Global Patent Holdings, LLC, a privately
held patent holding company based in Northbrook, Illinois, which owned 11 patent
licensing companies. The acquisition gives the Acacia Technologies group 100%
ownership of companies that control 27 patent portfolios, which include 120 U.S.
patents and certain foreign counterparts, and cover technologies used in a wide
variety of industries, as set forth below. The acquisition expands and
diversifies the Acacia Technologies group's revenue generating opportunities and
accelerates the execution of the Acacia Technologies group's business strategy
of acquiring, developing and licensing patented technologies.
The acquisition is being accounted for by the purchase method of accounting
and, accordingly, the consolidated statement of operations will include the
results of the acquired companies beginning on January 28, 2005, the date of
acquisition. The aggregate purchase consideration was approximately $24,605,000,
including $5.0 million of cash, the issuance of 3,938,832 shares of Acacia
Research--Acacia Technologies common stock valued at $19,505,000 and estimated
acquisition costs of $100,000. The value of the common shares issued was
determined based on the
F-35
average market price of AR-Acacia Technologies stock, as reported on NASDAQ,
over the 5-day period (December 13 - December 17, 2004) before and after the
terms of the acquisition were agreed to and announced.
The following table summarizes the estimated preliminary total purchase
consideration (in thousands):
Estimated Purchase Consideration:
Cash paid ............................................. $5,000
Fair value of AR-Acacia Technologies stock issued ..... 19,505
Estimated Acquisition costs .......................... 100
----------
$24,605
==========
Other:
Consulting contract .................................. $2,000
==========
Management's determination of the fair value of net assets acquired from
Global Patent Holdings and the related purchase price allocation is ongoing and
is anticipated to be completed by the end of the first quarter of 2005. The
purchase price will be allocated to the assets acquired and liabilities assumed
based on their estimated fair market values at the date of acquisition,
including, net tangible assets, patents and other identifiable intangibles. Any
additional excess purchase price after the initial allocation to identifiable
net tangible and identifiable intangible assets will be assigned to goodwill.
Amounts attributable to patents will be amortized using the straight-line method
over the estimated economic useful life of the underlying patents.
The Acacia Technologies group executed a consulting agreementincurred in connection with the acquisition described above, which requiresdiscontinued operations of Soundbreak.com (originally ceased operations in February 2001). The additional accruals relate primarily to certain noncancellable lease obligations, the payment of $2.0 million
in consulting fees over a two-year period,inability to sublease the related office space at rates commensurate with our existing obligations and certain reimbursable consultinglease termination costs. The related expenses, commencing on the date of acquisition.
16. CONSOLIDATING SEGMENT INFORMATIONlease obligations, which were guaranteed by Acacia Research Corporation, has adoptedexpired in August 2005. The assets and liabilities related to the provisions of SFAS No. 131,
"Disclosures about Segments of an Enterprise and Related Information." Our chief
operating decision maker is considered to be Acacia Research Corporation's CEO.
The CEO reviews and evaluates financial information presented on a group basis
as described below. Management evaluates performance based on the profit or loss
from continuing operations and financial position of its segments. Acacia
Research Corporation has two reportable segments as described in Note 1.
Material intercompany transactions and transfers have been eliminated in
consolidation. The accounting policies of the segments are the same as those
described in the summary of significant accounting policies.
F-36
Presented below is consolidating financial information for our reportable
segments reflecting the businesses of the CombiMatrix group and the Acacia
Technologies group. Earnings attributable to each group have been determined in
accordance with accounting principles generally accepted in the United States.
CONSOLIDATING BALANCE SHEETS (IN THOUSANDS)
NOTE: Segment information for the Acacia Technologies group includes discontinued operations related to Soundbreak.com. Total assets related to
discontinued operations totaled $1,443,000 and $2,150,000of Soundbreak.com at December 31, 20042007 and December 31, 2003, respectively. Total liabilities related to discontinued
operations totaled $275,000 and $395,000 at December 31, 2004 and December 31,
2003, respectively.
F-37c
CONSOLIDATING STATEMENTS OF OPERATIONS (IN THOUSANDS)
F-38c
CONSOLIDATING STATEMENTS OF CASH FLOWS (IN THOUSANDS)
F-39c
17. SUPPLEMENTAL CASH FLOW INFORMATION (IN THOUSANDS)
F-40
18.2006 were not material.
16. QUARTERLY FINANCIAL DATA (UNAUDITED)
(unaudited)
The following table sets forth unaudited consolidated statementstatements of operations data for the eight quarters in the period ended December 31, 2004.2007. This information has been derived from our unaudited condensed consolidated financial statements that have been prepared on the same basis as the audited consolidated financial statements and, in the opinion of management, include all adjustments, consisting of normal recurring adjustments, necessary for a fair presentationstatement of the information when read in conjunction with the audited consolidated financial statements and related notes thereto. Our quarterly results have been in the past and may in the future be subject to significant fluctuations. As a result, we believe that results of operations for interim periods should not be relied upon as any indication of the results to be expected in any future periods.
F-41
F-42b
COMBIMATRIX GROUP
(A DIVISION OF ACACIA RESEARCH CORPORATION)
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Acacia Research Corporation
In our opinion, the financial statements listed in the index appearing
under Item 15(a)(1) on page 56 present fairly, in all material respects, the
financial position of CombiMatrix Group (a division of Acacia Research
Corporation as described in Note 1) at December 31, 2004 and December 31, 2003,
and the results of their operations and their cash flows for each
As a result of the three
yearsSplit-off Transaction (refer to Notes 10 and 10A), we have disposed of our investment in CombiMatrix Corporation, and therefore, in accordance with guidance set forth in SFAS No. 144, the statement of operations data for the four quarters in the period ended December 31, 2004, in conformity with accounting
principles generally accepted in the United States of America. These financial
statements are the responsibility of Acacia Research Corporation's management;
our responsibility is to express an opinion on these financial statements based
on our audits. We conducted our audits of these statements in accordance with
the standards of the Public Company Accounting Oversight Board (United States),
those standards require that we plan and perform the audit to obtain reasonable
assurance about whether the financial statements are free of material
misstatement. An audit includes examining, on a test basis, evidence supporting
the amounts and disclosures in the financial statements, assessing the
accounting principles used and significant estimates made by management, and
evaluating the overall financial statement presentation. We believe that our
audits provide a reasonable basis for our opinion.
As more fully described in Note 1 to the financial statements, CombiMatrix
Group is a division of Acacia Research Corporation; accordingly, the financial
statements of CombiMatrix group should be read in conjunction with the
consolidated financial statements of Acacia Research Corporation.
/s/ PricewaterhouseCoopers LLP
Los Angeles, California
March 14, 2005
F-43
COMBIMATRIX GROUP
(A DIVISION OF ACACIA RESEARCH CORPORATION)
STATEMENTS OF ALLOCATED NET WORTH
(IN THOUSANDS)
Balance at December 31, 2001 .................................... $ 3,723
Net assets attributed to the CombiMatrix group .................. 77,813
Division net income (loss) ...................................... (46,219)
-----------
Balance at December 31, 2002 .................................... 35,317
Net assets attributed to the CombiMatrix group .................. 9,389
Division net income (loss) ...................................... (18,969)
-----------
Balance at December 31, 2003 .................................... 25,737
Net assets attributed to the CombiMatrix group .................. 20,381
Division net income (loss) ...................................... 710
-----------
Balance at December 31, 2004 .................................... $ 46,828
===========
THE ACCOMPANYING NOTES ARE AN INTEGRAL PART OF THESE FINANCIAL STATEMENTS.
F-46
COMBIMATRIX GROUP
(A DIVISION OF ACACIA RESEARCH CORPORATION)
NOTES TO FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS
Acacia Research Corporation is comprised of two separate divisions: the
CombiMatrix group and the Acacia Technologies group (the "groups").
The CombiMatrix group, a division of Acacia Research Corporation, is
comprised of Acacia Research Corporation's wholly owned subsidiary, CombiMatrix
Corporation and CombiMatrix Corporation's wholly owned subsidiary, CombiMatrix
K.K. The CombiMatrix group is seeking to become a broadly diversified
biotechnology company, through the development of proprietary technologies and
products in the areas of drug development, genetic analysis, nanotechnology
research, defense and homeland security markets, as well as other potential
markets where its products could be utilized. Among the technologies being
developed by the CombiMatrix group are a platform technology to rapidly produce
customizable arrays, which are semiconductor-based tools for use in identifying
and determining the roles of genes, gene mutations and proteins. This technology
has a wide range of potential applications in the areas of genomics, proteomics,
biosensors, drug discovery, drug development, diagnostics, combinatorial
chemistry, material sciences and nanotechnology. Other technologies include
proprietary molecular synthesis and screening methods for the discovery of
potential new drugs. CombiMatrix K.K., a wholly owned Japanese corporation
located in Tokyo, is exploring opportunities for CombiMatrix Corporation's array
system with pharmaceutical and biotechnology companies in the Asian market.
LIQUIDITY AND RISKS
The CombiMatrix group is deploying new and unproven technologies and
continues to develop its commercial products. The CombiMatrix group has several
ongoing long-term development projects that involve experimental technology and
may require several years and substantial expenditures to complete. Management
believes that existing cash and cash equivalents and short-term investments are
adequate to fund operations through the next twelve months. However, the ability
to meet business objectives is dependent upon the CombiMatrix group's ability to
raise additional financing, substantiate its technology and ultimately to fund
itself from continuing operations. There can be no assurance that such funding
will be available at acceptable terms or at all.
The CombiMatrix group's business operations are also subject to certain
risks and uncertainties, including:
o market acceptance of products and services;
o technological advances that may make its products and services
obsolete or less competitive;
o increases in operating costs, including costs for supplies, personnel
and equipment;
o the availability and cost of capital;
o general economic conditions; and
o governmental regulation that may restrict its business.
The CombiMatrix group has historically been substantially dependent on its
existing arrangements with strategic partners including Roche Diagnostics GmbH
("Roche"), and has relied upon payments by Roche and other partners for a
majority of its future revenues. The CombiMatrix group intends to enter into
additional strategic partnerships to develop and commercialize future products.
However, there can be no assurance that the CombiMatrix group will be able to
implement its future plans. Failure by management to achieve its plans would
have a material adverse effect on the CombiMatrix group's ability to achieve its
intended business objectives. The CombiMatrix group's success also depends on
its ability to protect its intellectual property.
The CombiMatrix group's business depends on issued and pending patents, and
the loss of any patents or the group's failure to secure the issuance of patents
covering elements of its business processes would materially harm its business
and financial condition. The patents covering the CombiMatrix group's core
technology begin to expire January 5, 2018.
F-48
RECAPITALIZATION TRANSACTION
On December 11, 2002, Acacia Research Corporation's stockholders voted in
favor of a recapitalization transaction, which became effective on December 13,
2002, whereby Acacia Research Corporation created two new classes of common
stock called Acacia Research-CombiMatrix stock ("AR-CombiMatrix stock") and
Acacia Research-Acacia Technologies stock ("AR-Acacia Technologies stock"), and
divided the existing Acacia Research Corporation common stock into shares of the
two new classes of common stock. AR-CombiMatrix stock is intended to2007, presented below, reflect
separately the performance of Acacia Research Corporation's CombiMatrix group.
AR-Acacia Technologies stock is intended to reflect separately the performance
of Acacia Research Corporation's Acacia Technologies group. Although the
AR-CombiMatrix stock and the AR-Acacia Technologies stock are intended to
reflect the performance of Acacia Research Corporation's different business
groups, they are both classes of common stock of Acacia Research Corporation and
are not stock issued by the respective groups.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF PRESENTATION. AR-CombiMatrix stock is intended to reflect the
separate performance of the respective division of Acacia Research Corporation.
The CombiMatrix group is not a separate legal entity. Holders of AR-CombiMatrix
stock are stockholders of Acacia Research Corporation. As a result, holders of
AR-CombiMatrix stock are subject to all of the risks of an investment in Acacia
Research Corporation and all of its businesses, assets and liabilities. The
assets Acacia Research Corporation attributes to the CombiMatrix group could be
subject to the liabilities of the Acacia Technologies group.
The CombiMatrix group financial statements have been prepared in accordance
with generally accepted accounting principles in the United States of America,
and taken together with the Acacia Technologies group financial statements,
comprise all the accounts included in the corresponding consolidated financial
statements of Acacia Research Corporation. The financial statements of
CombiMatrix group reflect the financial condition, results of operations, and
cash flows of the businesses included therein. The financial statements of the
CombiMatrix group include the accounts or assets of Acacia Research Corporation
specifically attributed to the CombiMatrix group and were prepared using amounts
included in Acacia Research Corporation's consolidated financial statements.
Minority interests represent participation of other stockholders in the
allocated net assets and in the division earnings and losses of the CombiMatrix
group and are reflected in the caption minority interests in CombiMatrix group's
financial statements. Minority interests adjust CombiMatrix group's net results
of operations to reflect only CombiMatrix group's share of the division earnings
or losses of non-wholly owned investees of Acacia Research Corporation that have
been attributed to the CombiMatrix group.
Financial effects arising from one group that affect Acacia Research
Corporation's results of operations or financial condition could, if
significant, affect the results of operations or financial condition of the
other group and the market price of the class of common stock relating to the
other group. Any division net losses of thefor CombiMatrix group or the Acacia
Technologies group and dividends or distributions on, or repurchases of,
AR-CombiMatrix stock or AR-Acacia Technologies stock or repurchases of preferred
stock of Acacia Research Corporation will reduce the assets of Acacia Research
Corporation legally available for payment of dividends on AR-CombiMatrix stock
or AR-Acacia Technologies stock.
Refer to Note 2 to the Acacia Research Corporationas discontinued operations. The unaudited consolidated financial
statements included elsewhere herein for the Acacia Research Corporation
principles of consolidation, the management allocation policies, treasury and
cash management policies, asset and liability attribution policies, corporate,
general and administrative services and facilities allocation policies and
federal and state income tax allocation policies, utilized in the preparation of
the separate CombiMatrix group financial statements.
REVENUE RECOGNITION. The CombiMatrix group recognizes revenue in accordance
with Staff Accounting Bulletin No. 104, "Revenue Recognition" ("SAB No. 104")
and related authoritative pronouncements. Revenues from multiple-element
arrangements are accounted for in accordance with Emerging Issues Task Force
("EITF") Issue 00-21, "Revenue Arrangements with Multiple Deliverables." Revenue
is recognized when (i) persuasive evidence of an arrangement exists, (ii) all
obligations have been performed pursuant to the terms of the license agreement,
(iii) amounts are fixed or determinable and (iv) collectibility of amounts is
reasonably assured.
F-49
Revenues from multiple-element arrangements involving license fees,
up-front payments and milestone payments, which are received and/or billable by
us in connection with other rights and services that represent continuing
obligations of ours, are deferred until all of the elements have been delivered
or until the CombiMatrix group has established objective and verifiable evidence
of the fair value of the undelivered elements.
Revenues from government grants and contracts are recognized in accordance
with Accounting Research Bulletin ("ARB") No. 43, "Government Contracts," and
related pronouncements. Accordingly, revenues are recognized under the
percentage-of-completion method of accounting, using the cost-to-cost approach
to measure completeness at each reporting period. Under the
percentage-of-completion method of accounting, contract revenues and expenses
are recognized in the period that work is performed based on the percentage of
actual incurred costs to estimated total contract costs. Actual contract costs
and cost estimates include direct charges for labor and materials and indirect
charges for labor, overhead and certain general and administrative charges.
Contract change orders and claims are included when they can be reliably
estimated and are considered probable. For contracts that extend over a one-year
period, revisions in contract cost estimates, if they occur, have the effect of
adjusting current period earnings applicable to performance in prior periods.
Should current contract estimates indicate an overall future loss to be
incurred, a provision is made for the total anticipated loss in the current
period.
Revenue from the sale of products and services, including shipping and
handling fees, are recognized when delivery has occurred or services have been
rendered.
Deferred revenues arise from payments received in advance of the
culmination of the earnings process. Deferred revenues expected to be recognized
within the next twelve months are classified within current liabilities.
Deferred revenues will be recognized as revenue in future periods when the
applicable revenue recognition criteria as described above are met.
CASH AND CASH EQUIVALENTS. The CombiMatrix group considers all highly
liquid, short-term investments with original maturities of three months or less
when purchased to be cash equivalents.
SHORT-TERM INVESTMENTS. The CombiMatrix group's short-term investments are
held in a variety of interest bearing instruments including high-grade corporate
bonds, money market accounts and other high-credit quality marketable
securities. Investments in securities with original maturities of greater than
three months and less than one year are classified as short-term investments.
Investments are classified in accordance with the provisions of Statement of
Financial Accounting Standards No. 115, "Accounting for Certain Investments in
Debt and Equity Securities" ("SFAS No. 115"). Investments are classified as
available-for-sale, which are reported at fair value with related unrealized
gains and losses in the value of such securities recorded as a component of
allocated net worth until realized.
The fair value of the CombiMatrix group's investments is determined by
quoted market prices. Realized and unrealized gains and losses are recorded
based on the specific identification method. For investments classified as
available-for-sale, unrealized losses that are other than temporary are
recognized in division net loss.
The cost of debt securities is adjusted for amortization of premiums and
accretion of discounts to maturity. Such amortization is included in interest
income (expense). Interest and dividends on all securities are included in
interest income.
CONCENTRATION OF CREDIT RISKS. Financial instruments that potentially
subject the CombiMatrix group to concentrations of credit risk are cash
equivalents and short-term investments. The CombiMatrix group places its cash
equivalents and short-term investments primarily in investment grade, short-term
debt instruments. Cash equivalents are invested in deposits with certain
financial institutions and may, at times, exceed federally insured limits. The
CombiMatrix group has not experienced any significant losses on its deposits of
cash and cash equivalents.
Research and development contract revenues recognized by the CombiMatrix
group for the year ended December 31, 2004 relate to its research and
development agreement with Roche. Government contract revenues recognized by the
CombiMatrix group for the year ended December 31, 2004 relate to its two-year,
F-50
$5.9 million contract with the Department of Defense awarded in March 2004. At
December 31, 2004, accounts receivable included $248,000 due from the Department
of Defense. In 2004, 2003 and 2002, 45%, 100% and 38% of the CombiMatrix group's
array product and service sales were recorded by CombiMatrix K.K.
Substantially all of the components and raw materials used in the
manufacture of the CombiMatrix group's products, including semiconductors and
reagents, are currently provided from a limited number of sources or in some
cases from a single source. Although the CombiMatrix group believes that
alternative sources for those components and raw materials are available, any
supply interruption in a sole-sourced component or raw material might result in
up to a several-month production delay and materially harm the CombiMatrix
group's ability to manufacture products until a new source of supply, if any,
could be located and qualified. The CombiMatrix group utilizes non-standard
semiconductor manufacturing processes to fabricate the electrode array that is a
key aspect of the array structure. Although the CombiMatrix group has a supply
agreement in place with a semiconductor wafer manufacturer to ensure
availability of the raw materials, it does not guarantee a permanent supply.
INVENTORY. Inventory, which consists primarily of raw materials to be used
in the production of the CombiMatrix group's array products, is stated at the
lower of cost or market using the first-in, first-out method.
PROPERTY AND EQUIPMENT. Property and equipment is recorded at cost.
Additions and improvements that increase the value or extend the life of an
asset are capitalized. Maintenance and repairs are expensed as incurred.
Disposals are removed at cost less accumulated depreciation or amortization and
any gain or loss from disposition is reflected in the statement of operations in
the period of disposition. Depreciation is computed on a straight-line basis
over the following estimated useful lives of the assets:
Machine shop and laboratory equipment............ 3 to 5 years
Furniture and fixtures........................... 5 to 7 years
Computer hardware and software................... 3 years
Leasehold improvements........................... Lesser of lease term or
useful life of improvement
Construction in progress includes direct costs incurred related to
internally constructed assets which are depreciated once the asset is placed
into service. Certain leasehold improvements, furniture and equipment held under
capital leases are classified as property and equipment and are amortized over
their useful lives using the straight-line method. Lease amortization is
included in depreciation expense.
ORGANIZATION COSTS. Costs of start-up activities, including organization
costs, are expensed as incurred.
PATENTS AND GOODWILL. Goodwill and identifiable intangibles, including
patents, are recorded when the consideration paid for acquisitions exceeds the
fair value of the net tangible assets acquired. Patents, once issued or
purchased, are amortized on the straight-line method over their economic
remaining useful lives, ranging from seven to twenty years. Goodwill is not
amortized.
IMPAIRMENT OF LONG-LIVED ASSETS AND GOODWILL. Long-lived assets and
intangible assets are reviewed for potential impairment when events or changes
in circumstances indicate the carrying amount of an asset may not be
recoverable. In the event the sum of the expected undiscounted future cash flows
resulting from the use of the asset is less than the carrying amount of the
asset, an impairment loss equal to the excess of the asset's carrying value over
its fair value is recorded. If an asset is determined to be impaired, the loss
is measured based on quoted market prices in active markets, if available. If
quoted market prices are not available, the estimate of fair value is based on
various valuation techniques, including a discounted value of estimated future
cash flows.
Goodwill is evaluated for impairment in accordance with SFAS No. 142,
"Goodwill and Other Intangible Assets" ("SFAS No. 142") and is subject to a
periodic review for potential impairment at a reporting unit level. Reviews for
potential impairment must occur at least annually and may be performed earlier,
if circumstances indicate that an impairment may have occurred. The CombiMatrix
group has elected to perform its annual tests for indications of goodwill
impairment as of December 31 of each year. The CombiMatrix group has one
reporting unit. The fair value of the CombiMatrix group reporting unit is
estimated using discounted cash flow analysis and by reference to quoted market
prices of AR-CombiMatrix stock.
F-51
SFAS No. 142 requires the CombiMatrix group to compare the fair value of
its reporting unit to its carrying amount on an annual basis to determine if
there is potential goodwill impairment. If the fair value of the reporting unit
is less than its carrying value, an impairment loss is recorded to the extent
that the fair value of the goodwill within the reporting unit is less than its
carrying value. There can be no assurance that future goodwill impairment tests
will not result in a charge to earnings.
FAIR VALUE OF FINANCIAL INSTRUMENTS. The carrying value of cash and cash
equivalents, accounts receivables, accounts payable and accrued expenses
approximate fair value due to their short-term maturity.
FOREIGN CURRENCY TRANSLATION. The functional currency of CombiMatrix K.K.
is the local currency (Japanese Yen). Foreign currency translation is reported
pursuant to SFAS No. 52, "Foreign Currency Translation" ("SFAS No. 52"). Assets
and liabilities recorded in foreign currencies are translated at the exchange
rate on the balance sheet date. Translation adjustments resulting from this
process are charged or credited to allocated net worth. Revenue and expenses are
translated at average rates of exchange prevailing during the year. Foreign
currency transactions gains and losses were insignificantdata for the years ended
December 31, 2004, 2003 and 2002.
STOCK-BASED COMPENSATION. Refer to Note 2 to the Acacia Research
Corporation consolidated financial statements included elsewhere herein.
Stock option and related option plan information is omitted from the
CombiMatrix group footnotes because AR-CombiMatrix stock is part of the capital
structure of Acacia Research Corporation. The CombiMatrix group is not a
separate legal entity. Holders of AR-CombiMatrix stock continue to be
stockholders of Acacia Research Corporation. This presentation reflects the fact
that the CombiMatrix group does not have legally issued common or preferred
stock, nor are warrant issuances or employee stock transactions legal
transactions of the CombiMatrix group. Refer to the Acacia Research Corporation
consolidated financial statements for disclosures regarding Acacia Research
Corporation's stock option plans.
RESEARCH AND DEVELOPMENT EXPENSES. Research and development expenses
consist of costs incurred for direct and overhead-related research expenses and
are expensed as incurred. Costs to acquire technologies which are utilized in
research and development and which have no alternative future use are expensed
when incurred. Costs related to filing and pursuing patent applications are
expensed as incurred, as recoverability of such expenditures is uncertain.
Software developed for use in the CombiMatrix group's products is expensed as
incurred until both (i) technological feasibility for the software has been
established and (ii) all research and development activities for the other
components of the system have been completed. Management believes these criteria
are met after the CombiMatrix group has received evaluations from third-party
test sites and completed any resulting modifications to the products.
Expenditures to date have been classified as research and development expense.
ACQUIRED IN-PROCESS RESEARCH AND DEVELOPMENT. The value assigned to
acquired in-process research and development ("IPR&D") is determined by
identifying acquired specific in-process research and development projects that
would be continued and for which (a) technological feasibility has not been
established at the acquisition date, (b) there is no alternative future use and
(c) the fair value is estimable with reasonable reliability, upon consummation
of a business combination.
ADVERTISING. Costs associated with marketing and advertising of the
CombiMatrix group's products and services are expensed as incurred. For the
years ended December 31, 2004, 2003 and 2002, marketing and advertising expenses
incurred by the CombiMatrix group were $314,000, $26,000 and $62,000,
respectively.
INCOME TAXES. Income taxes are accounted for using an asset and liability
approach that requires the recognition of deferred tax assets and liabilities
for the expected future tax consequences of events that have been recognized in
the CombiMatrix group's financial statements or tax returns. A valuation
allowance is established to reduce deferred tax assets if all, or some portion,
of such assets will more than likely not be realized.
SEGMENTS. The CombiMatrix group follows SFAS No. 131, "Disclosure about
Segments of an Enterprise and Related Information" ("SFAS No. 131"), which
establishes annual and interim reporting standards for an enterprise's operating
segments and related disclosures about its products, services, geographic areas
and major customers. Management has determined that the CombiMatrix group
operates in one segment.
F-52
USE OF ESTIMATES. The preparation of financial statements in conformity
with generally accepted accounting principles in the United States of America
requires management to make estimates and assumptions that affect the reported
amount of assets and liabilities and disclosure of contingent assets and
liabilities at the date of the combined financial statements and the reported
amounts of revenues and expenses during the reporting period. Actual results
could differ from these estimates.
EARNINGS PER SHARE. Earnings per share information is omitted from the
CombiMatrix group statements of operations because AR-CombiMatrix stock is part
of the capital structure of Acacia Research Corporation. The CombiMatrix group
is not a separate legal entity. Holders of AR-CombiMatrix stock continue to be
stockholders of Acacia Research Corporation. This presentation reflects the fact
that the CombiMatrix group does not have legally issued common or preferred
stock, nor are warrant issuances or employee stock transactions legal
transactions of the CombiMatrix group. Refer to the Acacia Research Corporation
consolidated financial statements for earnings per share information for Acacia
Research Corporation's classes of stock.
CERTAIN RISKS AND UNCERTAINTIES. The CombiMatrix group's products and
services will be concentrated in a highly competitive market that is
characterized by rapid technological advances, frequent changes in customer
requirements and evolving regulatory requirements and industry standards.
Failure to anticipate or respond adequately to technological advances, changes
in customer requirements, changes in regulatory requirements or industry
standards, or any significant delays in the development or introduction of
planned products or services, could have a material adverse effect on the
CombiMatrix group's business and operating results.
RECENT ACCOUNTING PRONOUNCEMENTS. Refer to Note 2 to the Acacia Research
Corporation consolidated financial statements included elsewhere herein.
3. SHORT-TERM INVESTMENTS
Short-term investments consist of the following at December 31, 2004 and
2003 (in thousands):
Gross unrealized gains and losses related to available-for-sale securities
were not material for the periods presented. All investments in debt securities
classified as available-for-sale at December 31, 2004 have contractual
maturities of one year or less.
4. PROPERTY AND EQUIPMENT
Property and equipment consists of the following at December 31, 2004 and
2003 (in thousands):
F-53
Depreciation and amortization expense was $1,105,000, $1,314,000 and
$1,364,000 for the years ended December 31, 2004, 2003 and 2002. Amortization of
assets held under capital lease included in depreciation and amortization
expense was $590,000 for the year ended December 31, 2002. In November 2002, the
capital lease obligation was repaid and title to the assets previously under
capital lease was transferred back to the CombiMatrix group. Fully depreciated
assets of $663,000 were written off in 2004.
5. INVESTMENTS
In October 2004 (the "Investment Date"), the CombiMatrix group entered into
an agreement to acquire up to a one-third ownership interest in Leuchemix, Inc.
("Leuchemix"), a private drug development firm, which is developing several
compounds for the treatment of leukemia and other cancers. In accordance with
the terms of the purchase agreement, the CombiMatrix group will purchase
3,137,500 shares of Series A Preferred Stock of Leuchemix for a total purchase
price of $4,000,000. The ownership interest will be acquired and paid for
quarterly, beginning with the fourth quarter of 2004 and continuing through the
third quarter of 2006. As of December 31, 2004, the CombiMatrix group has
initially invested $250,000 for a 3% interest in the total outstanding voting
securities of Leuchemix. In accordance with the terms of the purchase agreement,
CombiMatrix Corporation's CEO was named a director of Leuchemix. Although the
CombiMatrix group's investment in Leuchemix only represented approximately 3% of
Leuchemix's total outstanding voting securities as of the Investment Date, the
CombiMatrix group's investment is being accounted for under the equity method as
the CombiMatrix group has the ability to exercise significant influence over
Leuchemix, primarily due to CombiMatrix Corporation's representation on
Leuchemix's board of directors.
The CombiMatrix group's 3% interest in the equity in loss of Leuchemix,
including its share of the amortization expense related to the excess purchase
consideration over the book value of Leuchemix was not material for the
year-ended December 31, 2004. Future investments in Leuchemix will be accounted
for as step acquisitions. Summary financial information for Leuchemix was not
significant as of December 31, 2004.
6. INTANGIBLES
The CombiMatrix group has $19,424,000 of goodwill at December 31, 2004 and
2003.
The CombiMatrix group's only identifiable intangible assets are patents,
which are being amortized over an economic useful life of approximately 11
years. The gross carrying amounts and accumulated amortization related to
acquired intangible assets, all related to patents, as of December 31, 2004 and
2003, are as follows (in thousands):
2004 2003
----------- -----------
Gross carrying amount - patents ..... $ 12,095 $ 12,095
Accumulated amortization ............ (3,074) (1,978)
----------- -----------
Patents, net ........................ $ 9,021 $ 10,117
=========== ===========
Aggregate patent amortization expense was $1,096,000, $1,095,000 and
$399,000 in 2004, 2003 and 2002, respectively. Annual aggregate amortization
expense for each of the next five years through December 31, 2009 is estimated
to be $1,095,000 per year. Refer to Note 12, "Step Acquisitions Allocated to the
CombiMatrix Group," for additions to intangibles and goodwill during 2004 and
2003.
F-54
7. BALANCE SHEET COMPONENTS
Accounts payable, accrued expenses and other consists of the
following at December 31, 2004 and December 31, 2003 (in thousands):
2004 2003
----------- -----------
Accounts payable ............................. $ 410 $ 305
Payroll and other employee benefits .......... 317 304
Accrued vacation ............................. 355 287
Deferred rent ................................ 340 284
Accrued consulting and other professional
fees ....................................... 299 242
Other accrued liabilities .................... 243 250
----------- -----------
$ 1,964 $ 1,672
=========== ===========
Deferred revenues consist of the following at December 31, 2004 and 2003
(in thousands):
2004 2003
----------- -----------
Milestone and up-front payments .............. $ 3,959 $ 20,405
Less: current portion ....................... (66) (17,566)
----------- -----------
$ 3,893 $ 2,839
=========== ===========
In March 2004, the CombiMatrix group completed all phases of its research
and development agreement with Roche. As a result of completing all of its
obligations under this agreement and in accordance with the CombiMatrix group's
revenue recognition policies for multiple-element arrangements, the CombiMatrix
group recognized all previously deferred Roche related contract revenues
totaling $17,302,000 during the first quarter of 2004.
In August 2004, the CombiMatrix group received a $1,000,000 upfront payment
from Furuno Electric Co., LTD ("Furuno") as part of a multi-year collaboration
agreement to develop a bench-top array synthesizer for commercial applications.
In 2003, the CombiMatrix group received upfront and milestone payments from
Toppan Printing Co., LTD. ("Toppan") totaling $2,400,000, pursuant to a
multi-year collaboration and supply agreement to develop and manufacture arrays
using the CombiMatrix group's proprietary electrochemical detection approach.
The payments received from Furuno and Toppan are included in deferred revenues
at December 31, 2004 in accordance with the CombiMatrix group's revenue
recognition policies for multiple-element arrangements.
8. INCOME TAXES
CombiMatrix group's allocated benefit for income taxes consists of the
following (in thousands):
2004 2003 2002
----------- ----------- -----------
Current:
U.S. Federal tax ....... $ - $ - $ -
State taxes ............ - - -
----------- ----------- -----------
- - -
----------- ----------- -----------
Deferred:
U.S. Federal tax ....... (136) (136) (147)
State taxes ............ - - -
----------- ----------- -----------
(136) (136) (147)
----------- ----------- -----------
$ (136) $ (136) $ (147)
=========== =========== ===========
The tax effects of temporary differences and carryforwards that give rise
to significant portions of deferred assets and liabilities consist of the
following at December 31, 2004 and 2003 (in thousands):
F-55
A reconciliation of the federal statutory income tax rate and the effective
income tax rate is as follows:
At December 31, 2004, the CombiMatrix group has deferred tax assets
totaling approximately $40,794,000, which are fully offset by a valuation
allowance due to management's determination that the criteria for asset
recognition have not been met.
Acacia Research Corporation files a consolidated federal income tax return
that includes the Acacia Technologies group (excluding discontinued operations)
and the CombiMatrix group.
At December 31, 2004, the CombiMatrix group had federal net operating loss
carryforwards of approximately $90,131,000, which will begin to expire in 2012
through 2024. In addition, the CombiMatrix group has tax credit carryforwards of
approximately $2,869,000. Utilization of net operating loss carryforwards and
tax credit carryforwards are subject to the "change of ownership" provisions
under Section 382 of the Internal Revenue Code. The amount of such limitations
has not been determined.
Had the CombiMatrix group filed separate tax returns, the benefit for
income taxes and division net loss would not have differed from the amounts
reported in the CombiMatrix group's statements of operations for the years ended
December 31, 2004, 2003, and 2002.
F-56
9. COMMITMENTS AND CONTINGENCIES
OPERATING LEASES
In October 2000, CombiMatrix Corporation entered into a non-cancelable
operating lease for office space. A security deposit in the form of a $783,000
letter of credit was issued November 1, 2000, which was increased to $1,200,000
during 2001 and to $1,500,000 during 2002. Future minimum operating lease
payments as of December 31, 2004 are as follows (in thousands):
YEAR
----
2005 ....................................... $ 1,923
2006 ....................................... 1,836
2007 ....................................... 1,937
2008 ....................................... 1,615
Thereafter ................................. -
-----------
Total minimum lease payments ............... $ 7,311
===========
Rent expense for the years ended December 31, 2004, 2003 and 2002 was
$1,933,000, $2,006,000 and $1,618,000, respectively.
COLLABORATIVE AND RESEARCH AGREEMENTS
In July 2001, CombiMatrix Corporation entered into a non-exclusive
worldwide license, supply, research and development agreement with Roche. Under
the terms of the agreement, Roche will purchase, use and resell CombiMatrix
Corporation's array and related technologies for production of customizable
arrays. Additionally, CombiMatrix Corporation and Roche will develop a platform
technology, providing a range of standardized arrays for use in research
applications. The agreement has a 15-year term and provides for minimum payments
by Roche to CombiMatrix Corporation over the first three years, including
payments upon the achievement of certain milestone and payments for products,
royalties and research and development projects. During 2003 and 2002, the
CombiMatrix group's research and development activities were driven primarily by
ongoing performance obligations under the product commercialization phase of its
license and research and development agreements with Roche. These activities
include costs associated with direct labor, supplies and materials, development
of prototype arrays and instruments and the use of outside consultants for
certain engineering efforts. As previously discussed in Note 7, the CombiMatrix
group completed all phases of its research and development agreement with Roche
in March 2004.
As previously disclosed in Note 5, the CombiMatrix group has entered into
an agreement with Leuchemix to purchase a total of $4,000,000 of Series A
Preferred Stock of Leuchemix over a two-year period. Future quarterly cash
investments by the CombiMatrix group in Leuchemix are $1,600,000 in 2005 and
$2,150,000 in 2006.
In March 2004, the CombiMatrix group was awarded a two-year, $5.9 million
contract with the Department of Defense to further the development of the
CombiMatrix group's array technology for the detection of biological threat
agents. Under the terms of the contract, the CombiMatrix group will perform
research and development activities as described under the contract and will be
reimbursed on a periodic basis for actual costs incurred to perform its
obligations, plus a fixed fee, of up to $5.9 million. Based on actual costs
incurred through December 31, 2004, the CombiMatrix group expects to incur
approximately $2.2 million and $819,000 in research and development costs during
2005 and 2006, respectively, to complete its obligations to the Department of
Defense under this contract. As of December 31, 2004, the biowarfare detection
contract with the Department of Defense was approximately 34% complete.
F-57
In July 2004, the CombiMatrix group and collaborator irsiCaixa Foundation
("IRSI") entered into a three-year research, development and licensing agreement
to develop certain siRNA compounds for pre-clinical drug development against the
HIV virus. Pursuant to the terms of the agreement, the CombiMatrix group will
make quarterly research and development funding payments to IRSI totaling
$450,000 over a period of three years, which began in July 2004. In addition,
the CombiMatrix group may make future contingent milestone payments for
compounds that are developed, in accordance with the terms of the agreement. In
consideration for receiving rights to commercialize the compounds under
development, the CombiMatrix group will pay royalties to IRSI based on
commercial sales of related products, in accordance with the agreement.
HUMAN RESOURCES
The CombiMatrix group provides certain severance benefits such that if an
executive who is a vice president or higher is terminated for other than cause,
death or disability, the executive will receive payments equal to three months'
base salary and other medical and dental benefits on a bi-weekly basis over a
three-month period. If termination occurs as a result of a change in control
transaction, these benefits will be extended by three months. The CombiMatrix
group also offers a general severance plan providing all employees with certain
benefits upon their termination of employment due to lack of work. Under this
plan, terminated employees will be provided with either four-weeks notice or
four-weeks' salary in lieu of notice, and paid a lump-sum amount based on the
employee's length of service, plus accrued benefits. The terminated employees
will also be provided continuing medical and dental benefits, as well as
continuation of life insurance, for a period ranging from two to 26 weeks
subsequent to the date of termination, depending upon the employee's length of
service.
LITIGATION
On November 28, 2000, Nanogen, Inc. ("Nanogen") filed suit against
CombiMatrix Corporation and Dr. Donald Montgomery, an officer, director and
stockholder of CombiMatrix Corporation. The Nanogen suit alleged, among other
things, that CombiMatrix Corporation's issued patent and certain pending patent
applications, trade secrets and related technologies that were inappropriately
obtained by CombiMatrix Corporation and that Nanogen was the legal owner of the
patents, trade secrets and related technologies. The suit sought, among other
things, correction of inventorship on CombiMatrix Corporation's issued patent,
the assignment of rights in the issued patent and pending patent applications to
Nanogen, an injunction preventing disclosure of trade secrets, damages for trade
secret misappropriation and the imposition of a constructive trust.
On September 30, 2002, CombiMatrix Corporation and Dr. Donald Montgomery
entered into a settlement agreement with Nanogen, Inc. to settle all pending
litigation between the parties. Pursuant to the terms of the settlement
agreement, CombiMatrix Corporation agreed to pay Nanogen $500,000 within 30 days
of the settlement, which was paid, and an additional $500,000 within one year of
the settlement (paid in September 2003). CombiMatrix Corporation also agreed to
make quarterly payments to Nanogen equal to 12.5% of total sales of products
developed by CombiMatrix Corporation and its affiliates and based on the patents
that had been in dispute in the litigation, up to an annual maximum of
$1,500,000. The minimum quarterly payments under the settlement agreement will
be $37,500 per quarter for the period from October 1, 2003 through October 1,
2004, and $25,000 per quarter thereafter until the patents expire. Also,
pursuant to the settlement agreement, CombiMatrix Corporation issued to Nanogen
4,016,346 shares, or 17.5% of its outstanding shares post-issuance, subject to
an anti-dilution provision related to the exercise of CombiMatrix Corporation
options and warrants that were outstanding on the effective date of the
agreement, for a period of up to three years.
The issuance of the CombiMatrix Corporation common shares in settlement of
the litigation with Nanogen was accounted for as a nonmonetary transaction.
Accordingly, CombiMatrix Corporation recorded a non-cash litigation settlement
charge in the consolidated statements of operations for the year ended December
31, 2002 of approximately $17,471,000, which was based on the fair value of the
CombiMatrix Corporation common shares issued to Nanogen. Management was
responsible for determining the fair value of the common shares issued to
Nanogen on the settlement date and considered a number of factors, including
reference to an independent third-party valuation. Management utilized an income
approach to estimate the value of the common shares issued, based on the present
value of CombiMatrix Corporation's future estimated cash flows. Future estimated
cash flows included management's estimates of revenues, cost of sales, research
and development expenses, sales and marketing expenses, general and
administrative expenses, the anticipated effect of income taxes, and required
returns on working capital, fixed assets and other assets necessary to support
the generation of these cash flows. Future estimated cash flows were discounted
to the present value using a discount rate of 25%, which reflected a required
rate of return, comprised of an estimated weighted-average cost of capital,
which was further increased to reflect the risk profile of the company's
business.
F-58
Total legal settlement charges recorded in the CombiMatrix group statement
of operations for the year ended December 31, 2002 include the fair value of the
common shares issued to Nanogen in the amount of $17,471,000 and a charge in the
amount of $1,000,000 related to the cash payments due to Nanogen discussed
above.
During the year ended December 31, 2004, the CombiMatrix group recorded a
net non-cash charge totaling $812,000 in connection with the anti-dilution
provisions of the settlement agreement. The non-cash charge reflects
management's estimate of the fair value of AR-CombiMatrix stock issued to
Nanogen, Inc. as a result of certain options and warrants exercised during 2004
and the fair value of AR-CombiMatrix stock potentially issuable to Nanogen, Inc.
as of December 31, 2004. The liability is adjusted at each balance sheet date
for changes in the market value of the AR-CombiMatrix stock and is reflected as
a long-term liability until settled in equity. The anti-dilution provisions of
the settlement agreement expire in September 2005.
The CombiMatrix group is subject to other claims and legal actions that
arise in the ordinary course of business. Management believes that the ultimate
liability with respect to these claims and legal actions, if any, will not have
a material effect on CombiMatrix group's financial position, results of
operations or cash flows.
10. RETIREMENT SAVINGS PLAN
The CombiMatrix group has an employee savings and retirement plan under
section 401(k) of the Internal Revenue Code (the "Plan"). The Plan is a defined
contribution plan in which eligible employees may elect to have a percentage of
their compensation contributed to the Plan, subject to certain guidelines issued
by the Internal Revenue Service. The CombiMatrix group may contribute to the
Plan at the discretion of Acacia Research Corporation's board of directors.
There were no contributions made by the CombiMatrix group during the years ended
December 31, 2004, 2003 and 2002.
11. ALLOCATED NET WORTH
The CombiMatrix group's statements of allocated net worth present the
equity transactions of Acacia Research Corporation, which are attributed to the
CombiMatrix group as "Net assets attributed to the CombiMatrix group." This
presentation reflects the fact that the CombiMatrix group does not have legally
issued common or preferred stock, nor are warrant issuances or employee stock
option transactions legal transactions of the CombiMatrix group. Presented below
is a detail of the equity transactions of Acacia Research Corporation which
relate to the businesses of the CombiMatrix group and which therefore comprise
the balances reflected in the group's net assets attributed to CombiMatrix group
(in thousands):
F-59
EQUITY FINANCINGS
In April 2004, Acacia Research Corporation raised net proceeds of
approximately $13,715,000 through the sale of 3,000,000 shares of Acacia
Research - CombiMatrix common stock in a registered direct offering. The net
proceeds from this offering were attributed to the CombiMatrix group.
In May 2003, Acacia Research Corporation completed a private equity
financing, raising gross proceeds of $5,247,000 through the issuance of
2,385,000 units. Each unit consists of one share of AR-CombiMatrix common stock
and one-half five-year callable common stock purchase warrant. Each full common
stock purchase warrant entitles the holder to purchase a share of AR-CombiMatrix
stock at a price of $2.75 per share and is callable by Acacia Research
Corporation once the daily average of the high and low prices of Acacia Research
Corporation's AR-CombiMatrix stock on the Nasdaq SmallCap Market is equal to or
above $4.50 for 20 consecutive trading days. Acacia Research Corporation issued
an additional 31,502 shares of AR-CombiMatrix stock in lieu of cash payments in
conjunction with the private placement for finder's fees. Net proceeds raised
from the private equity financing of $4,862,000 have been attributed to the
CombiMatrix group.
WARRANTS
During 2004 and 2003, proceeds of $2,093,000 and $450,000 were received
from the issuance of 761,205 and 164,000 shares, respectively, of AR-CombiMatrix
stock related to the exercise of certain warrants issued in connection with the
May 2003 private equity financing described below. The proceeds from the
warrants exercised were attributed to the CombiMatrix group.
12. STEP ACQUISITIONS ALLOCATED TO THE COMBIMATRIX GROUP
On July 11, 2003, Acacia Research Corporation purchased the outstanding
minority interests in its consolidated subsidiary CombiMatrix K.K. from
Marubeni. Acacia Research Corporation issued 200,000 shares of its
AR-CombiMatrix stock to Marubeni in exchange for Marubeni's 10% minority
interests (120 shares) in CombiMatrix K.K. The transaction was accounted for as
a step acquisition using the purchase method of accounting. The fair value of
F-60
the AR-CombiMatrix stock issued in the transaction was based on the quoted
market price of AR-CombiMatrix stock on the exchange date. The total purchase
price of $450,000 was allocated to the fair value of assets acquired and
liabilities assumed. The amount attributable to goodwill was $393,000.
In April 2002, CombiMatrix Corporation purchased Acacia Research
Corporation's majority interest in Advanced Material Sciences. CombiMatrix
Corporation issued 180,982 shares of its common stock to Acacia Research
Corporation in exchange for Acacia Research Corporation's 58% interest in
Advanced Material Sciences. As a result of this transaction, CombiMatrix
Corporation owned 87% of Advanced Material Sciences as of December 31, 2002,
with the remaining interests owned by unaffiliated parties. The April 2002
transaction was accounted for using Acacia Research Corporation's basis in the
net assets of Advanced Material Sciences and as a result, the CombiMatrix
group's 2002 financial statements reflected the assets and liabilities of
Advanced Material Sciences at historical cost.
On July 2, 2003, Acacia Research Corporation increased its consolidated
ownership interest in Advanced Material Sciences to 99% by acquiring 1,774,750
shares of Advanced Material Sciences common stock in exchange for 295,790 shares
of AR-CombiMatrix stock. The transaction was accounted for as a step acquisition
using the purchase method of accounting. The fair value of the Acacia Research
shares issued in the transaction was based on the quoted market price of
AR-CombiMatrix stock on the exchange date. The total purchase price of $769,000
was allocated to the fair value of assets acquired and liabilities assumed. The
amount attributable to goodwill was $172,000.
Acacia Research Corporation's interests in Advanced Material Sciences and
CombiMatrix K.K. have been attributed to the CombiMatrix group.
On December 13, 2002, Acacia Research Corporation increased its
consolidated ownership interest in CombiMatrix Corporation from 48% to 100% by
acquiring from existing stockholders of CombiMatrix Corporation 11,987,052
shares of CombiMatrix Corporation common stock in exchange for 11,987,052 shares
of AR-CombiMatrix stock with a fair value of $46,007,000. The merger was
designed to consolidate Acacia Research Corporation's ownership of CombiMatrix
Corporation and permit Acacia Research Corporation to effectuate the
recapitalization transaction described elsewhere herein, by creating the
CombiMatrix group.
The transaction was accounted for as a step acquisition using the purchase
method of accounting. The fair value of the AR-CombiMatrix stock issued in the
transaction was based on the quoted market price of AR-CombiMatrix stock
averaged over a five-day period (from December 16, 2002, the first day of
trading for the AR-CombiMatrix stock, through December 20, 2002).
The following table summarizes the estimated fair values of the assets
acquired and liabilities assumed and the allocation of the purchase price at the
date of acquisition (in thousands):
F-61
The total purchase price of $46,841,000 was allocated to the fair value of
assets acquired and liabilities assumed, including acquired IPR&D, as shown
above. The amount attributable to CombiMatrix Corporation's core technology and
related patents is being amortized using the straight-line method over the
estimated economic useful life of 7 years. Amounts allocated to patents, IPR&D
and goodwill have been attributed to the CombiMatrix group.
In conjunction with the allocation of the purchase price, Acacia Research
Corporation was required to adjust CombiMatrix Corporation's assets and
liabilities to fair value. Deferred revenue, primarily consisting of milestone
payments and other cash receipts from Roche and NASA, was reduced by $8,425,000
to reflect the fair value of the continuing obligation related to the 52%
interest in CombiMatrix Corporation acquired by Acacia Research Corporation.
The amount attributable to IPR&D projects (comprised of two projects:
Genomics and Proteomics biological array systems) that had not yet reached
technological feasibility and had no alternative future use of $17,237,000 was
charged to expense on the acquisition date and is included in the accompanying
statement of operations for the year ended December 31, 2002.
Management was responsible for determining the fair value of the tangible
and identifiable intangible assets acquired and liabilities assumed, including
IPR&D, at the date of acquisition. Management considered a number of factors,
including reference to independent valuations. The in-process technologies were
valued using a discounted cash flow model on a project-by-project basis, which
estimated the cash flows expected to result from each project once it has
reached technological feasibility. A discount rate consistent with the risks of
each project was used to estimate the present value of future cash flows. In
estimating future cash flows, management considered the contribution of its core
technology (for which a United States patent was obtained in July 2000) that
would be required for successful exploitation of purchased in-process
technology, in order to value the core and in-process technologies discretely.
As a result, future cash flows relating to each purchased IPR&D project were
reduced in order to reflect the contribution of core technology to each IPR&D
project. The cash flows from these projects attributable to core technology were
then separately valued to determine the intangible asset value of purchased core
technology. In determining the contribution of core technology to in-process
projects, management used a profit split approach which considered the estimated
profit split between a licensor and licensee of the core technology and
management's assessment of how critical the core technology was to the IPR&D
projects.
The nature of the efforts to develop the purchased IPR&D into commercially
viable products principally relates to the completion and/or acceleration of
existing development programs. These efforts include testing current and
alternative materials used in array design, testing of existing and alternative
methods for array synthesis, developing prototype machinery (including operating
software) to synthesize, hybridize and read individual arrays, and to perform
numerous experiments, or assays, with actual target samples in order to
determine customer protocols and procedures for using the CombiMatrix group's
array system. Following is a brief description of the two IPR&D projects
identified.
Genomics Biological Array System: CombiMatrix Corporation's genomics
biological array processor system is being developed to discretely immobilize
sequences of DNA or RNA within individual test sites on a modified semiconductor
chip coated with a three-dimensional layer of porous material. The system also
includes proprietary hardware units and related software applications to be able
to synthesize materials onto the chips, apply target samples of genetic
materials and interpret the results. The value assigned to the genomics
biological array system IPR&D project was $13,978,000. A risk-adjusted discount
rate of 32% was applied to the project's estimated cash flows.
Proteomics Biological Array System: CombiMatrix Corporation's proteomics
biological array processor system is being developed to discretely immobilize
proteins and other small molecules within individual test sites on a modified
semiconductor chip in a similar fashion as described above for the genomics
biological array system. The proteomics biological array system is used for
detection and identification of bio-threat agents in CombiMatrix Corporation's
biological and chemical threat agent detector development programs that are
currently in process. The value assigned to the proteomics biological array
system IPR&D project was $3,259,000. A risk-adjusted discount rate of 60% was
applied to the project's estimated cash flows.
F-62
13. SUPPLEMENTAL CASH FLOW INFORMATION (IN THOUSANDS)
F-63
ACACIA TECHNOLOGIES GROUP
(A DIVISION OF ACACIA RESEARCH CORPORATION)
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Acacia Research Corporation
In our opinion, the financial statements listed in the index appearing
under Item 15(a)(1) on page 56 present fairly, in all material respects, the
financial position of Acacia Technologies Group (a division of Acacia Research
Corporation as described in Note 1) at December 31, 2004 and December 31, 2003,
and the results of their operations and their cash flows for each of the three
yearsfour quarters in the period ended December 31, 2004, in conformity with accounting
principles generally accepted in the United States of America. These financial
statements are the responsibility of Acacia Research Corporation's management;
our responsibility is2006, presented below, have been reclassified to express an opinion on these financial statements based
on our audits. We conducted our audits of these statements in accordance with
the standards of the Public Company Accounting Oversight Board (United States),
those standards require that we plan and perform the auditconform to obtain reasonable
assurance about whether the financial statements are free of material
misstatement. An audit includes examining, on a test basis, evidence supporting
the amounts and disclosures in the financial statements, assessing the
accounting principles used and significant estimates made by management, and
evaluating the overall financial statementthis presentation. We believe that our
audits provide a reasonable basis for our opinion.
As more fully described in Note 1 to the financial statements, Acacia
Technologies group is a division of Acacia Research Corporation; accordingly,
the financial statements of Acacia Technologies group should be read in
conjunction with the consolidated financial statements of Acacia Research
Corporation.
/s/ PricewaterhouseCoopers LLP
Los Angeles, California
March 14, 2005
F-64
F-28
ACACIA RESEARCH CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Quarter Ended | ||||||||||||||||||||||||||||||||
| Mar. 31, | Jun. 30, | Sep. 30, | Dec. 31, | Mar. 31, | Jun. 30, | Sep. 30, | Dec. 31, | |||||||||||||||||||||||||
| 2007 | 2007 | 2007 | 2007 | 2006 | 2006 | 2006 | 2006 | |||||||||||||||||||||||||
(In thousands, except share and per share information) | ||||||||||||||||||||||||||||||||
(Unaudited) | ||||||||||||||||||||||||||||||||
| License fees | $ | 25,185 | $ | 5,865 | $ | 9,544 | $ | 12,003 | $ | 4,717 | $ | 14,371 | $ | 8,424 | $ | 7,313 | ||||||||||||||||
| Operating expenses | 21,133 | 9,979 | 14,836 | 16,160 | 7,487 | 13,537 | 9,859 | 10,789 | ||||||||||||||||||||||||
| Operating income (loss) | 4,052 | (4,114 | ) | (5,292 | ) | (4,157 | ) | (2,770 | ) | 834 | (1,435 | ) | (3,476 | ) | ||||||||||||||||||
| Interest and investment income | 407 | 650 | 647 | 655 | 359 | 394 | 390 | 381 | ||||||||||||||||||||||||
| Income (loss) from continuing operations before | ||||||||||||||||||||||||||||||||
| income taxes and minority interests | 4,459 | (3,464 | ) | (4,645 | ) | (3,502 | ) | (2,411 | ) | 1,228 | (1,045 | ) | (3,095 | ) | ||||||||||||||||||
| Benefit (provision) for income taxes | (24 | ) | (124 | ) | (29 | ) | (30 | ) | 32 | (70 | ) | (2 | ) | - | ||||||||||||||||||
| Income (loss) from continuing operations | 4,435 | (3,588 | ) | (4,674 | ) | (3,532 | ) | (2,379 | ) | 1,158 | (1,047 | ) | (3,095 | ) | ||||||||||||||||||
| Loss from discontinued operations - Split-off of CombiMatrix Corporation | (2,133 | ) | (3,667 | ) | (2,286 | ) | - | (7,719 | ) | (3,501 | ) | (4,329 | ) | (4,544 | ) | |||||||||||||||||
| Net income (loss) | $ | 2,302 | $ | (7,255 | ) | $ | (6,960 | ) | $ | (3,532 | ) | $ | (10,098 | ) | $ | (2,343 | ) | $ | (5,376 | ) | $ | (7,639 | ) | |||||||||
| Earnings (loss) per common share: | ||||||||||||||||||||||||||||||||
| Acacia Research - Acacia Technologies stock: | ||||||||||||||||||||||||||||||||
| Earnings (loss) from continuing operations | $ | 4,435 | $ | (3,588 | ) | $ | (4,674 | ) | $ | (3,532 | ) | $ | (2,379 | ) | $ | 1,158 | $ | (1,047 | ) | $ | (3,095 | ) | ||||||||||
| Basic earnings (loss) per share | 0.16 | (0.13 | ) | (0.16 | ) | (0.12 | ) | (0.09 | ) | 0.04 | (0.04 | ) | (0.11 | ) | ||||||||||||||||||
| Diluted earnings (loss) per share | 0.14 | (0.13 | ) | (0.16 | ) | (0.12 | ) | (0.09 | ) | 0.04 | (0.04 | ) | (0.11 | ) | ||||||||||||||||||
| Acacia Research - CombiMatrix stock - Discontinued | ||||||||||||||||||||||||||||||||
| Operations - Split-off of CombiMatrix Corporation: | ||||||||||||||||||||||||||||||||
| Net loss | $ | (2,133 | ) | $ | (3,667 | ) | $ | (2,286 | ) | $ | - | $ | (7,719 | ) | $ | (3,501 | ) | $ | (4,329 | ) | $ | (4,544 | ) | |||||||||
| Basic and diluted loss per share | (0.04 | ) | (0.06 | ) | (0.04 | ) | - | (0.20 | ) | (0.09 | ) | (0.11 | ) | (0.10 | ) | |||||||||||||||||
| Weighted average shares: | ||||||||||||||||||||||||||||||||
| Acacia Research - Acacia Technologies stock: | ||||||||||||||||||||||||||||||||
| Basic | 27,841,286 | 28,298,328 | 28,739,499 | 29,117,523 | 27,400,857 | 27,507,024 | 27,567,848 | 27,708,902 | ||||||||||||||||||||||||
| Diluted | 30,969,991 | 28,298,328 | 28,739,499 | 29,117,523 | 27,400,857 | 30,324,732 | 27,567,848 | 27,708,902 | ||||||||||||||||||||||||
| Acacia Research - CombiMatrix stock: | ||||||||||||||||||||||||||||||||
| Basic and diluted | 52,516,220 | 57,143,839 | 59,875,769 | - | 38,992,402 | 39,018,844 | 40,209,640 | 44,120,736 | ||||||||||||||||||||||||
F-29
Exhibit Number | Description |
| 2.1 | Agreement and Plan of Merger of Acacia Research |
| 2.2 | Agreement and Plan of Reorganization by and among Acacia Research Corporation, Combi Acquisition Corp. and CombiMatrix Corporation dated as of March 20, 2002 (2) |
| 3.1 | Restated Certificate of Incorporation as amended(3) |
| 3.2 | Amended and Restated Bylaws |
| 10.1* | Acacia Research Corporation 1996 Stock Option Plan, as amended (4) |
| 10.2* | Form of Option Agreement constituting the Acacia Research Corporation 1996 Executive Stock Bonus Plan (5) |
| 10.3* | 2002 Acacia Technologies Stock Incentive Plan (6) |
| 10.4* | 2007 Acacia Technologies Stock Incentive Plan (7) |
| 10.5* | Form of Acacia Technologies Stock Option Agreement for the 2007 Acacia Technologies Stock Incentive Plan (8) |
| 10.6* | Form of Acacia Technologies Stock Issuance Agreement for the 2002 Acacia Technologies Stock Incentive Plan (8) |
| 10.7* | Form of Acacia Technologies Stock Issuance Agreement for the 2007 Acacia Technologies Stock Incentive Plan (8) |
| 10.8 | Lease Agreement dated January 28, 2002, between Acacia Research Corporation and The Irvine Company (9) |
| 10.9 | Settlement Agreement dated September 30, 2002, by and among Acacia Research Corporation, CombiMatrix Corporation, Donald D. Montgomery, Ph.D. and Nanogen, Inc.(10) |
| 10.10 | Form of Indemnification Agreement (11) |
| 10.11 | Form of Subscription Agreement between Acacia Research Corporation and certain investors (12) |
| 10.12 | Third Amendment to lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company (13) |
| 10.13 | Standby Equity Distribution Agreement dated June 14, 2006 between Acacia Research Corporation and Cornell Capital Partners, L.P. (14) |
| 10.14 | Amendment to Standby Equity Distribution Agreement dated June 14, 2006 between Acacia Research Corporation and Cornell Capital Partners, L.P. (15) |
| 10.15 | Manufacturing and Supply Agreement between Acacia Research Corporation and Furuno Electric Company, Ltd. Effective July 1, 2006 (16) |
| 10.16 | Placement Agency Agreement between Acacia Research Corporation and Oppenheimer & Co., dated December 7, 2006 (17) |
| 10.17 | Form of Subscription Agreement (17) |
| 10.18 | Form of Investors Warrant (17) |
| 10.19* | Employment Agreement, dated January 28, 2005, by and between Acacia Technologies Services Corporation, and Dooyong Lee, as amended |
| 10.20* | Employment Agreement, dated April 12, 2004, |
| 10.21 | Fourth Amendment to lease dated January 28, 2002 |
| 10.22 | Fifth Amendment to Lease dated January 28, 2002 between Acacia Research Corporation and the Irvine Company |
| 21.1 | List of Subsidiaries |
| 23.1 | Consent of Independent Registered Public Accounting Firm - |
| 23.2 | Consent of Independent Registered Public Accounting Firm - PricewaterhouseCoopers LLP |
| 31.1 | Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 | Certification of the Chief Executive Officer provided pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 32.2 | Certification of the Chief Financial Officer provided pursuant to 18 U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
III-1
______________
| * | The referenced exhibit is a management contract, compensatory plan or arrangement. |
| † | Portions of this exhibit have been omitted pursuant to a request for confidential treatment and |
| (1) | Incorporated by reference from Acacia Research Corporation’s Report on |
| (2) | Incorporated by |
| (3) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report Amendment No. 1 on Form 10-Q/A for the period ended June 30, 2006, filed on June 5, 2007. |
| (4) | Incorporated by reference as Appendix A to the Definitive Proxy Statement on Schedule 14A filed on April 10, 2000 (SEC File No. 000-26068). |
| (5) | Incorporated by reference from Acacia Research Corporation’s Definitive Proxy as Appendix A Statement on Schedule 14A filed on April 26, 1996 (SEC File No. 000-26068). |
| (6) | Incorporated by reference as Appendix E to the Proxy Statement/Prospectus which formed part of Acacia Research Corporation’s Registration Statement on Form S-4 (SEC File No. 333-87654) which became effective on November 8, 2002. |
| (7) | Incorporated by reference to Acacia Research Corporation’s Registration Statement on Form S-8 (SEC File No. 333-144754) which became effective on July 20, 2007. |
| (8) | Incorporated by reference to Acacia Research Corporation’s Quarterly Report on Form 10-Q for the period ended September 30, 2007. |
| (9) | Incorporated by reference from Acacia Research Corporation’s Annual Report on Form 10-K for the year
III-2 |