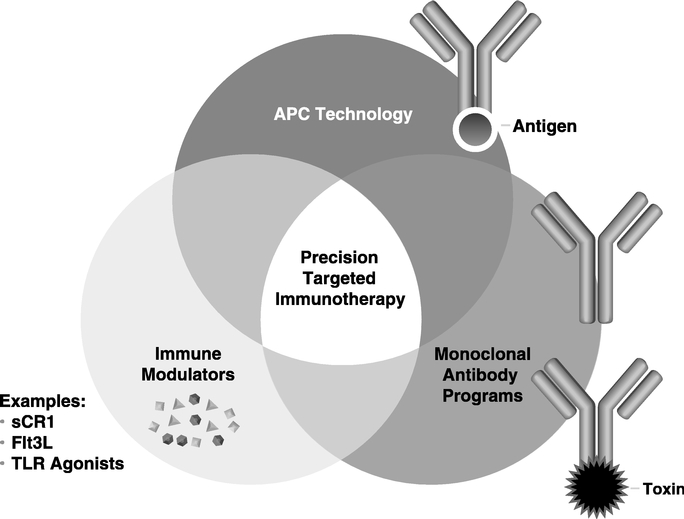
Precision Targeted Immunotherapy Platform:

Use these links to rapidly review the document
TABLE OF CONTENTS
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark one) | ||
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the fiscal year ended December 31, | ||
or | ||
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
Commission File Number 0-15006
CELLDEX THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 13-3191702 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
119 Fourth Avenue, Needham, Massachusetts 02494
(Address of principal executive offices) (Zip Code)
Registrant's telephone number, including area code:(781) 433-0771
Securities registered pursuant to Section 12(b) of the Act:
| Title of Class: | Name of Each Exchange on Which Registered: | |
|---|---|---|
| Common Stock, par value $.001 | NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act:None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this Chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer ý | Non-accelerated filer o (Do not check if a smaller reporting company) | Smaller Reporting Company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No ý
The aggregate market value of the voting and non-votingregistrant's common stock held by non-affiliates as of June 30, 20082009 was $103,792,910 (excludes shares held by directors and executive officers).$100.8 million. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the actions of the management or policies of the registrant, or that such person is controlled by or under common control with the registrant.
The number of shares of common stock outstanding at February 20, 200925, 2010 was 15,820,59331,711,124 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive Proxy Statement for our 2010 Annual Meeting of Stockholders are incorporated by reference into Part III of this Report.
CELLDEX THERAPEUTICS, INC.
ANNUAL REPORT ON FORM 10-K
YEAR ENDED DECEMBER 31, 2008
2009
| | | | Page | |||
|---|---|---|---|---|---|---|
Part I | ||||||
Item 1. | Business | 1 | ||||
Item 1A. | Risk Factors | |||||
Item 1B. | Unresolved Staff Comments | |||||
Item 2. | Properties | 37 | ||||
Item 3. | Legal Proceedings | 37 | ||||
Item 4. |
| 37 | ||||
Part II | ||||||
Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 38 | ||||
Item 6. | Selected Consolidated Financial Data | |||||
Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operations | |||||
Item 7A. | Quantitative and Qualitative Disclosure About Market Risk | |||||
Item 8. | Financial Statements and Supplementary Data | |||||
Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure | |||||
Item 9A. | Controls and Procedures | |||||
Item 9B. | Other information | |||||
Part III | ||||||
Item 10. | Directors, Executive Officers and Corporate Governance | |||||
Item 11. | Executive Compensation | |||||
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | |||||
Item 13. | Certain Relationships and Related Transactions, and Director Independence | |||||
Item 14. | Principal Accountant Fees and Services | |||||
Part IV | ||||||
Item 15. | Exhibits, Financial Statement Schedules | |||||
Signatures | ||||||
i
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995: This report on Form 10-K contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 under Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements include statements with respect to our beliefs, plans, objectives, goals, expectations, anticipations, assumptions, estimates, intentions and future performance, and involve known and unknown risks, uncertainties and other factors, which may be beyond our control, and which may cause our actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as "may," "will," "can," "anticipate," "assume," "should," "indicate," "would," "believe," "contemplate," "expect," "seek," "estimate," "continue," "plan," "point to," "project," "predict," "could," "intend," "target," "potential" and other similar words and expressions of the future.
There are a number of important factors that could cause the actual results to differ materially from those expressed in any forward-looking statement made by us. These factors include, but are not limited to:
ii
All forward-looking statements are expressly qualified in their entirety by this cautionary notice. You are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this report or the date of the document incorporated by reference into this report. We have no obligation, and expressly disclaim any obligation, to update, revise or correct any of the forward-looking statements, whether as a result of new information, future events or otherwise. We have expressed our expectations, beliefs and projections in good faith and we believe they have a reasonable basis. However, we cannot assure you that our expectations, beliefs or projections will result or be achieved or accomplished.
iii
A. General
As used herein, the terms "we," "us," "our," the "Company", or "Celldex" refer to Celldex Therapeutics, Inc., a Delaware corporation organized in 1983 (formerly known as AVANT Immunotherapeutics, Inc.) and its direct and indirect subsidiaries: Celldex Research Corporation ("Celldex Research"), and Celldex Therapeutics, Ltd. ("Celldex Ltd.") and Megan Health, Inc. ("Megan"). The Company'sOur principal activity since our inception has been research and product development conducted on our own behalf, as well as through joint development programs with several pharmaceutical companies and other collaborators. AVANT Immunotherapeutics, Inc. changed its name to Celldex Therapeutics, Inc. on October 1, 2008.
Celldex isWe are an integrated biopharmaceutical company that applies itsour comprehensive Precision Targeted Immunotherapy Platform to generate a pipeline of candidates to treat cancer and other difficult-to-treat diseases. Celldex'sOur immunotherapy platform includes a complementary portfolio of monoclonal antibodies, antibody-targeted vaccines, antibody-drug conjugates and immunomodulators to create novel disease-specific drug candidates.
Our strategy is to develop and demonstrate proof-of-concept for our product candidates before leveraging their value through partnerships or, in appropriate situations, continuing late stage development through commercialization ourselves. Demonstrating proof-of-concept for a product candidate generally involves bringing it through Phase 1 clinical trials and one or more Phase 2 clinical trials so that we are able to demonstrate, based on human trials, good safety data for the product candidate and some data indicating its effectiveness. We thus leverage the value of our technology portfolio through corporate, governmental and non-governmental partnerships. This approach allows us to maximize the overall value of our technology and product portfolio while best ensuring the expeditious development of each individual product.
Our current collaborations encompassinclude the commercialization of an oral human rotavirus vaccine and the development of oncology and infectious disease vaccines. Our product candidates address large market opportunities for which we believe current therapies are inadequate or non-existent.
AVANT Merger between AVANT and Celldex:
On March 7, 2008, we closed the merger (the "Merger") contemplated by the Agreement and Plan of Merger dated October 19, 2007 by and among Celldex (formerly AVANT Immunotherapeutics, Inc. ("AVANT"), Callisto Merger Corporation ("Merger Sub"), a wholly owned subsidiary of Celldex, and merged with Celldex Research (formerly known as Celldex Therapeutics, Inc.), a privately-held company, (the "Merger Agreement""AVANT Merger"). PursuantEffective October 1, 2008, we changed our name from AVANT Immunotherapeutics, Inc. to the terms of the Merger Agreement, Merger Sub merged with and into Celldex Research, with Celldex Research as the surviving company and a wholly-owned subsidiary of the Company. The total value of the transaction was approximately $75 million. Approximately 8.7 million shares were issued to the former Celldex Research shareholders in connection with the Merger. The Merger created a NASDAQ-listed, fully-integrated and diversified biopharmaceutical company with a deep pipeline of product candidates addressing high-value indications including oncology, infectious and inflammatory diseases. At the Merger, former Celldex and former AVANT shareholders owned 58% and 42% of the combined company on a fully diluted basis, respectively.Therapeutics, Inc.
Our board of directors approved a 1-for-12 reverse stock split of the Company's common stock, which became effective on March 7, 2008. As a result of the reverse stock split, each twelve shares of common stock were combined and reclassified into one share of common stock and the total number of shares outstanding was reduced from approximately 180 million shares (including the shares issued to former Celldex Research stockholders in the Merger) to approximately 15 million shares.
The AVANT Merger was accounted for using the purchase method of accounting and was treated as an acquisition by Celldex Research of Celldex (then AVANT), with Celldex Research being considered the accounting acquirer based on the application of criteria specified in Statement of Financial Accounting Standards "SFAS" No. 141,Business Combinations, ("SFAS 141"),AVANT even though Celldex (then AVANT)AVANT was the issuer of common stock and the surviving legal entity in the transaction. Under the purchase method of accounting, the deemed purchase price was allocated to AVANT's underlying tangible and identifiable intangible assets acquired and liabilities assumed based upon their respective fair values with any excess deemed purchase price allocated to goodwill. The valuation analysis conducted by the Company determined that the fair value of assets acquired and the fair value of liabilities assumed by Celldex Research exceeded the purchase price for AVANT, resulting in negative goodwill of approximately $6.0 million. In accordance with SFAS 141, the negative goodwill has been allocated to all of the acquired assets which were non-financial and non-current assets, including property and equipment, identifiable intangible assets, and in-process research and development. See Note 17 to the Company's consolidated financial statements for additional information.
Because Celldex Research was determined to be the acquirer for accounting purposes, the historical financial statements of Celldex Research became theour historical financial statementsas of the Company.closing of the AVANT Merger. Accordingly, theour financial statements of the Company prior to the AVANT Merger reflect the financial position, results of operations and cash flows of Celldex Research, which during the historical periods presented in the accompanying consolidated financial statements, was then majority-owned by Medarex, Inc. ("Medarex"). Following the AVANT Merger, the financial statements of the current period reflect the financial position, results of operation and cash flows of the Company.combined companies. The results of operations of AVANT are included in our results of operations beginning March 8, 2008.
Acquisition of CuraGen Corporation ("CuraGen")
On October 1, 2009, CuraGen, then a publicly-traded company, merged with a wholly-owned subsidiary of Celldex (the "CuraGen Merger"). In connection with the CuraGen Merger, effective
October 1, 2009, we (i) issued 15,722,713 shares of our common stock, or 0.2739 shares, in exchange for each share of outstanding CuraGen common stock, plus cash in lieu of fractional shares (the "CuraGen Exchange Ratio"), (ii) assumed all of the CuraGen stock options outstanding under the CuraGen 2007 Stock Plan (the "CuraGen 2007 Options"), and (iii) assumed the obligations of the $12.5 million in CuraGen 4% convertible subordinated debt due in February 2011 (the "CuraGen Debt"). The CuraGen 2007 Options are exercisable into 931,315 shares of our common stock after applying the CuraGen Exchange Ratio.
In connection with the consummation of the CuraGen Merger, effective October 1, 2009, Celldex, CuraGen, and The Bank of New York Mellon (formerly the Bank of New York) (the "Trustee") amended the CuraGen Debt to provide that the CuraGen Debt shall be convertible into 353,563 shares of Celldex common stock at the rate of 28.27823 shares of Celldex common stock per $1,000 principal amount of notes, or $35.36 per share.
Based on the closing price of our common stock on October 1, 2009 of $5.43, the fair value of the shares issued in the CuraGen Merger was $85.4 million. We have applied acquisition accounting as of October 1, 2009. Accordingly, the results of operations of the Company beginning March 8, 2008. Accordingly, except as otherwise discussed below, this report reflects the financial condition,CuraGen have been included in our results of operations and liquidity of the combined companies atbeginning October 1, 2009.
On December 31, 20082009, we completed the merger of our CuraGen subsidiary with and historicallyinto Celldex pursuant to a short-form merger effected under Delaware law. As a result, the separate corporate existence of Celldex Research on a stand-alone basis forCuraGen has ceased and we have succeeded to all periods prior to March 8, 2008. The financial condition, resultsrights, privileges, powers and franchises of operations and liquidity of the Company as of the years ended December 31, 2008, 2007 and 2006 may not be indicative of the Company's future performance or reflect what the Company's financial conditions, results of operations and liquidity would have been had the Merger been consummated as of January 1, 2006 or had the Company operated as a separate, stand-alone entity during the periods presented.CuraGen.
Celldex'sWe are a Delaware corporation organized in 1983. Our web site is located athttp://www.celldextherapeutics.com. On Celldex'sour web site, investors can obtain a copy of Celldex'sour annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and other reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act of 1934, as amended, as soon as reasonably practicable after Celldex fileswe file such material electronically with, or furnishes it to, the Securities and Exchange Commission.Commission ("SEC"). None of the information posted on our website is incorporated by reference into this Annual Report.
Research and Development Activities:Activities Our products are derived from a broad set of complementary technologies (collectively known as our Precision Targeted Immunotherapy Platform) which have the ability to utilize the human immune system and enable the creation of preventative and therapeutic agents. We are using our Precision Targeted Immunotherapy Platform to develop vaccines, therapeutic antibodies and other targeted immunotherapeutics that prevent or treat cancer, autoimmune disorders and disease caused by infectious organisms, and treatment vaccines that modify undesirable activity by the body's own proteins or cells. A number of our immunotherapeutic and vaccine product candidates are in various stages of clinical trials. We expect that a large percentage of
our research and development expenses will be incurred in support of our current and future clinical trial programs. Below is a table of our currently active programs:
CURRENT PROGRAMS AND PARTNERSHIPS
We currently have one product on the market and six products in clinical development. Our goal is to become a leading developer of innovative products that we call Precision Targeted Immunotherapeutics which are designed to address major unmet health care needs. Our success has depended and will continue to depend upon many factors, including our ability, and thatMost of our licenseesproducts are derived from a set of complementary technologies (collectively known as our Precision Targeted Immunotherapy Platform). This platform includes monoclonal antibodies, antibody-targeted vaccines, antibody-drug conjugates and collaborators,immunomodulators to successfullycreate novel disease-specific drugs. We are using our Precision Targeted Immunotherapy Platform to develop obtain regulatory approval fortargeted immunotherapies that prevent or treat specific forms of cancer, autoimmune disorders and commercialize our product candidates. To date, commercial sales have only been generated from Rotarix® and our former Megan poultry vaccines. We have had no commercial revenues from salesdisease caused by infectious organisms.
The following table includes the programs that we may not be ablecurrently believe are material to successfully develop, obtain regulatory approval for or commercialize our product candidates, and we are subject to a number of risks that you should be aware of before investing in Celldex. These risks are described more fully in "Item 1A. Risk Factors."business:
Product (generic) | Indication/Field | Partner | Status | ||||
|---|---|---|---|---|---|---|---|
CLINICAL | |||||||
CDX-110 | Glioblastoma multiforme | Pfizer (PF-4948568) | Phase 2b | ||||
CDX-011 | Metastatic melanoma and breast cancer | — | Phase 2 | ||||
CDX-1307 | Colorectal, bladder, pancreas, ovarian and breast tumors | — | Phase 1 | ||||
CDX-1401 | Multiple solid tumors | — | Phase 1/2 | ||||
CDX-1135 | Renal disease | — | Phase 1/2 | ||||
PRECLINICAL | |||||||
CDX-301 | Cancer, autoimmune disease and transplant | — | Preclinical | ||||
CDX-1127 | Immuno-modulation, multiple tumors | — | Preclinical | ||||
CDX-014 | Renal and ovarian cancer | — | Preclinical | ||||
CDX-1189 | Renal disease | — | Preclinical | ||||
MARKETED PRODUCTS | |||||||
Rotarix® | Rotavirus infection | GlaxoSmithKline | Marketed | ||||
Using our expertise in immunology, we are building business franchises in major disease areas: oncology, inflammatory and infectious diseases. Each of our business franchises addresses large market opportunities for which current therapies are inadequate or non-existent. We have pursued some of these opportunities independently in a highly focused manner. In other cases, we have leveraged the financial support and development capabilities of corporate and public sector partners to bring our development projects to fruition. The research we have pursued over the past several years has matured into what we believe is an exciting portfolio of product candidates.
Our success has depended and will continue to depend upon many factors, including our ability, and that of our licensees and collaborators, to successfully develop, obtain regulatory approval for and commercialize our product candidates. Commercial sales are currently only being generated from Rotarix®. We have had no commercial revenues from sales of our human therapeutic or other human vaccine products and we have had a history of operating losses. It is possible that we may not be able to successfully develop, obtain regulatory approval for or commercialize our product candidates, and we are subject to a number of risks that you should be aware of before investing in us. These risks are described more fully in "Item 1A. Risk Factors."
B. Development Strategy
Precision Targeted Immunotherapy Platform:


We believe there is tremendous untapped potential in immunotherapy that can be exploited through the right combination of therapeutic agents. Our industry has traditionally taken biologics that mediate effective cancer regression in mice and expected similar results in humans. There are many explanations why this strategy often does not succeed, but the most important is that immunotherapy has difficulties when following standard drug development. The mechanism of action is complex, activity is generally not dependent on highest tolerated dose, and patient response is highly variable. Our new understanding of the immune system, cancer's effect on immune mediated mechanisms, and the impact of conventional therapies on the immune system provides a new rationale for combining therapies that may lead to significant clinical responses. The concept of Precision Targeted Immunotherapy is to exploit this knowledge and the availability of good products that may not be sufficiently effective to be commercialized as a monotherapy, but which we believe may be very effective in combination approaches. Our goal is to develop products that maximize the efficacy of immunotherapy regimens through combinations of therapeutic agents. This includes:
TheTherapeutic Antibody Programs: These programs are based on the well validated approach to using antibodies that target to cancer and other diseases directly, or through interfering with critical interactions between the patient and the disease. Our antibody programs include antibody-drug conjugates (ADCs) that are designed to deliver potent cytotoxic molecules to cancer cells, and
traditional unmodified antibody approaches. Our current programs are based on fully human sequence antibodies to minimize patient reactivity against the drug. In addition, we have access through a Research and Commercialization Agreement with Medarex (now a subsidiary of Bristol-Myers Squibb) to the UltiMAb® Technology for generating fully human monoclonal antibodies. Under this agreement, we can exercise up to ten separate licenses to develop and commercialize therapeutic antibody products, either alone or through collaboration with our licensing partners.
Our APC technology:Targeting Technology™: This is a new class of vaccines based on our proprietary antibody-targeted vaccine technology that is used to generate an immune response against cancer or other diseases.
Our APC Targeting Technology™ uses human monoclonal antibodies or mAbs, linked to disease associated antigens to efficiently deliver the attached antigens to immune cells known as antigen presenting cells, or APCs. This technology has been designed to allow us to take advantage of many important characteristics of human monoclonal antibodies, including their long circulating half-life, well known safety profile, and standardized manufacturing procedures. We believe that our APC Targeting Technology™ provides significant manufacturing, regulatory and other practical advantages over patient specific and other immune-based treatments and can substantially reduce the dosage and cost currently required in conventional immunotherapies. Preclinical studies have demonstrated that APC Targeting Technology™ is more effective than conventional non-targeted vaccines. We have developed several proprietary monoclonal antibodies that can independently be developed to generate new product opportunities. We have initiatedOur CDX-1307 and CDX-1401 programs are in clinical development with the first APC technology product, called CDX-1307. In addition, CDX-1401 is completing its preclinical development and is expected to begin clinical testing in Phase 1/2 trials during 2009.
Therapeutic Antibody Programs:technology. These programs are based on the well validated approach to using antibodies that target to cancer and other diseases directly or through interfering with critical interactions between the patient and the disease. Celldex is in preclinical development for therapeutic human antibodies to molecules important in inflammation and cancer. In addition, Celldex has access through a Research and Commercialization Agreement with Medarex to the UltiMAb® Technology for
generating fully human monoclonal antibodies. Under this agreement, Celldex can exercise up to ten separate licenses to develop and commercialize therapeutic antibody products, either alone or through collaboration with Celldex licensing partners.
Immune System Modulators: Immune system modulators include drugs that activate or suppress specific parts of the immune system, including suchsystem. Currently we are combining our APC technology product candidates with molecules known as Toll-Like Receptor (TLR) agonists that can activate patients' innate and adaptive immunity,immunity. We are also developing an immune cell growth factor called FMS-like tyrosine kinase 3 ligand (FLT3-L or CDX-301) designed to expand immune cells and stem cells. In addition, we are investigating the activity of a complement inhibitor (CDX-1135) that suppresses inflammatory reactions. These agents further support our Precision Targeted Immunotherapy Platform.
Celldex'sOur strategy is to utilize our expertise to design and develop targeted immunotherapeutics that have significant and growing market potential; to establish governmental and corporate alliances to fund development; and to commercialize our products either through corporate partners or, in appropriate circumstances, through our own direct selling efforts. Our goal is to demonstrate clinical proof-of-concept for each product, and then seek partners to help see those products which we cannot develop ourselves through to commercialization. This approach allows us to maximize the overall value of our technology and product portfolios while best ensuring the expeditious development of each individual product. Implementation of this strategy is exemplified by our lead programs which are discussed in the following sections.
Factors that may significantly harm our commercial success, and ultimately the market price of our common stock, include but are not limited to, announcements of technological innovations or new commercial products by our competitors, disclosure of unsuccessful results of clinical testing or regulatory proceedings and governmental approvals, adverse developments in patent or other proprietary rights, public concern about the safety of products developed by Celldexus and general economic and market conditions. See "Item 1A. Risk Factors."
C. Cancer VaccineClinical Development Programs
CDX-110:CDX-110
Our lead clinical development program, CDX-110, is a peptide-based immunotherapy that targets the tumor specific molecule called EGFRvIII, a functional variant of the naturally expressed epidermal growth factor receptor ("EGFR"), a protein which has been well validated as a target for cancer
therapy. Unlike EGFR, EGFRvIII is not present in normal tissues, and has been shown to be a transforming oncogene that can directly contribute to the cancer cell growth.
EGFRvIII is commonly present in glioblastoma multiforme, or GBM, the most common and aggressive form of brain cancer, and has also been observed in various other cancers such as breast, ovarian, prostate, colorectal, and head & neck cancer. Our partner,
In April 2008, we and Pfizer Inc. ("Pfizer"), entered into a License and weDevelopment Agreement (the "Pfizer Agreement") under which Pfizer was granted an exclusive worldwide license to CDX-110. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Pfizer funds all development costs for these programs. We and Pfizer are currently pursuing the development of CDX-110 for GBM therapy and plan to expand the clinical development into other cancers through additional clinical studies. The Food and Drug Administration ("FDA") has granted orphan drug designation for CDX-110 for the treatment of EGFRvIII expressing GBM as well as fast track designation.
Initial clinical development of EGFRvIII immunotherapy was led by collaborating investigators at the Brain Center at Duke Comprehensive Cancer Center in Durham, North Carolina and at M.D. Anderson Cancer Center in Houston, Texas. The results from the Phase 1 (VICTORI) and Phase 2a (ACTIVATE) studies, which enrolled 16 and 21 patients, respectively, have demonstrated a significant increase in the time to disease progression (greater than 113%) in the patients who were vaccinated, and also in overall survival rates (greater than 100%), both relative to appropriately matched historical controls. An extension of the Phase 2a program (ACT II) at the same two institutions has enrolled 23 additional GBM patients treated in combination with temozolomide (the current standard of care). Preliminary results from this study (ACT II) currently estimates median overall survival to be 33.123.6 months, although the median has not yet been reached. Thereached, while the survival of a matched historical control group was 14.3 months and a subgroup treated with temozolomide (TMZ) of 15.215.0 months with a p value = 0.0078.0.0237. Overall time to progression for CDX-110in the ACT II study was 16.615.2 months compared with 6.46.3 months for the historical control group.
In May 2007, weWe initiated a Phase 2b/3 randomized study (ACT III) of CDX-110 combined with standard of care, temozolomide, versus standard of care alone in patients with GBM. We have opened a total ofGBM in over 30 sites inthroughout the United States for the study. The FDA has granted orphan drug designation for CDX-110 for the treatment of EGFRvIII expressing GBM as well as fast track designation.
States. In December 2008, we announced an amendment to convert the ACT III study to a single-arm Phase 2 clinical trial in which all patients will receive CDX-110 in combination with temozolomide and we will continue to enroll to approximately 60 patients.temozolomide. The decision, which followsfollowed the recommendation of the Independent Data Monitoring Committee, was based on the observation that the majority of patients randomized to the control (standard of care) arm withdrew from this open-label study after being randomized to the control arm. Patients currently participating on the control arm of the study will bewere offered the option to receive treatment with CDX-110. Under this amendment, the ACT III study will provideprovided for a multi-center, non-randomized dataset for CDX-110 in patients with newly diagnosed GBM. These data will provide important additional information that can be used to better design the future development of CDX-110. Enrollment in ACT III is complete with a total of over 60 patients enrolled and we expect to present updated results during 2010.
CDX-011
On April 16,CDX-011 (formerly CR011-vcMMAE) is an antibody-drug conjugate (ADC) that consists of a fully-human monoclonal antibody, CR011, linked to a potent cell-killing drug, monomethyl-auristatin E (MMAE). The CR011 antibody specifically targets glycoprotein NMB or (GPNMB) that is expressed in a variety of human cancers including breast cancer and melanoma. The ADC technology, comprised of MMAE and a stable linker system for attaching it to CR011, was licensed from Seattle Genetics, Inc. The ADC is designed to be stable in the bloodstream. Following intravenous administration, CDX-011 targets and binds to GPNMB, and upon internalization into the targeted cell, CDX-011 is designed to release MMAE from CR011 to produce a cell-killing effect. We acquired the rights to CDX-011 in connection with the CuraGen Merger.
Treatment of Breast Cancer: In June 2008, an open-label, multi-center Phase 1/2 study was initiated of CDX-011 administered intravenously once every three weeks to patients with locally advanced or metastatic breast cancer who have received prior therapy (median of seven prior regimens). The study began with a bridging phase to confirm the Companymaximum tolerated dose ("MTD") and Pfizer enteredhas expanded into a License and Development Agreement (the "Pfizer Agreement") under which Pfizer was granted an exclusive worldwide license to a therapeutic cancer vaccine candidate, CDX-110, in Phase 2 developmentopen-label, multi-center study.
The study confirmed the safety of CDX-011 at the pre-defined maximum dose level (1.88 mg/kg) in 6 patients. An additional 28 patients were enrolled as an expanded Phase 2 cohort (for a total of 34 treated patients at 1.88 mg/kg, the Phase 2 dose) to evaluate the progression-free survival ("PFS") rate at 12 weeks. As previously seen in melanoma patients, the 1.88 mg/kg dose was well tolerated in this patient population with the most common adverse events of rash, alopecia, and fatigue. The primary activity endpoint, which called for at least 5 of 25 (20%) patients in the Phase 2 study portion to be progression-free at twelve weeks, has been met. To date, 9 of 26 (35%) evaluable patients are without progression of disease at twelve weeks.
In addition, at the Phase 2 dose level, 4 of 32 (13%) evaluable patients achieved confirmed or unconfirmed Partial Responses ("PR") while 15 of 25 (60%) evaluable patients with measurable disease experienced some reduction in tumor size. GPNMB expression was identified in 10 of 14 (71%) of analyzed tumor samples and treatment with CDX-011 was associated with improved outcomes in all activity parameters in patients whose tumors expressed GPNMB. Notably, in patients who received the Phase 2 dose and whose tumors expressed GPNMB, 2 of 7 (29%) had confirmed PR, 5 of 7 (71%) had decreases in tumor size, and all 7 achieved at least stable disease with duration from 17.3 to 26.9 weeks. The median PFS in all patients was 9.1 weeks, but in patients whose tumors expressed GPNMB, median PFS was 18.3 weeks, compared to median PFS of 5.9 weeks for patients whose tumors did not express GPNMB. In patients with triple negative disease, 5 of 7 (71%) analyzed samples expressed GPNMB, 7 of 9 (78%) evaluable patients had tumor shrinkage, and the median PFS for these patients was 17.9 weeks.
We expect to initiate a randomized Phase 2b controlled study in patients with advanced breast cancer that express GPNMB in the second half of 2010.
Treatment of Metastatic Melanoma Cancer: In June 2006, a Phase 1/2 open-label, multi-center, dose escalation study was initiated to evaluate the safety, tolerability and pharmacokinetics of CDX-011 for patients with un-resectable Stage III or Stage IV melanoma who have failed no more than one prior line of cytotoxic therapy. During the Phase 1 portion of the study, doses of CDX-011 between 0.03 mg/kg to 2.63 mg/kg were evaluated and generally well tolerated, with rash and neutropenia emerging at higher doses. The MTD was determined to be 1.88 mg/kg administered intravenously (IV) once every three weeks.
In June 2009, CuraGen announced results for the treatment of glioblastoma multiforme. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the Pfizer Agreement, Pfizer made an upfront payment to the Company of $40 million and made a $10 million equity investment36 patients who were treated in the Company. Pfizer will fund all development costs for these programs.Phase 2 portion of the study. Of the patients enrolled, 94% had Stage IV disease of which two-thirds were classified as M1c, the poorest risk group. The Company is also eligiblestudy successfully met its primary activity endpoint, with 5 objective responses (1 unconfirmed) observed in 34 evaluable patients, and median duration of response of 5.3 months. The median overall PFS was 4.4 months. Tumor shrinkage was observed in 58% of patients, and 20 patients had best response of stable disease. Dermatologic adverse events consisting of rash, alopecia, and pruritus were the most common toxicities in this study. Other adverse events included fatigue, diarrhea, musculoskeletal pain, anorexia and nausea. Grade 3 or 4 neutropenia was observed in 5 patients. The absence of rash in the first cycle of treatment predicted a worse PFS. Additionally, in a subset of patients with tumor biopsies, high levels of tumor expression of GPNMB appeared to receive potential milestone payments exceeding $390 million forcorrelate with favorable outcome.
Enrollment has been completed in the successful developmentPhase 1 portion of the melanoma trial to evaluate more frequent dosing schedules of CDX-011, including a weekly and commercializationa two out of CDX-110every three-week regimen, to explore if more frequent administration can provide additional activity in patients with metastatic
melanoma. A dose of 1.0 mg/kg given once every week has been identified as the MTD in a weekly schedule, and additional EGFRvIII vaccine products, as well as royalties on any product sales. The Pfizer Agreement became effective after clearance undera dose of 1.5mg/kg was being explored in the Hart-Scott-Rodino Antitrust Improvements Acttwo out of 1976 (as amended) on May 19, 2008.three week schedule. Although median duration of follow-up was only 6 weeks, objective responses have thus far been observed in 3 of 11 evaluable patients treated with weekly CDX011 (1 confirmed) and 1 confirmed response in 8 evaluable patients treated with CDX-011 two out of every three weeks. We expect to present updated results during the first half of 2010.
CDX-1307:CDX-1307 The Company's
Our lead APC Targeting Technology™ product candidate, CDX-1307, is in development for the treatment of epithelial tumors such as colorectal, pancreatic, bladder, ovarian and breast cancers. CDX-1307 targets the beta chain of human chorionic gonadotropin, known as hCG-Beta, which is an antigen often found in epithelial tumors. The presence of hCG-Beta in these cancers correlates with a poor clinical outcome, suggesting that this molecule may contribute to tumor growth. Normal adult tissues have minimal expression of hCG-Beta; therefore, targeted immune responses are not expected to generate significant side effects.
CelldexEnrollment is completingcomplete in our two Phase 1 studies of CDX-1307 at multiple centers that are designed to explore safety and dose/effect relationships via two administration routes—intradermal (ID), a traditional vaccine route that allows efficient access to local dermal dendritic cells and intravenous (IV), a novel systemic approach to vaccination that might target a much larger population of dendritic cells. In bothThe Phase 1 studies there are dose escalationsinvestigated the safety and immunogenicity of CDX-1307 alone and CDX-1307in combination with the adjuvantadjuvants, including GM-CSF (known to increase mannose receptor expression on dendritic cells). At the highest dose levels, additional immune system modulators (Toll-Like and Toll-Like Receptor Agonists,("TLR") agonists (poly-ICLC or TLR agonists) have been added to determine what effect they have in augmenting an immune response.Hiltonol™ and R848 or resiquimod). Patients with an assortment of different tumor types that are known to express hCG-Beta are being accruedwere enrolled with retrospective analysis for hCG-Beta expression. AAn escalating four dose regimen iswas utilized with the possibility of retreatment if patients demonstrate tumor regression or stable disease.
Over Fifty (50)The Phase 1 studies enrolled over 80 patients with epithelial cancers have been treatedheavily pretreated, advanced-stage breast, colon, bladder and pancreatic cancer, with an average of 4.6 prior therapies across the treatment population. All patient cohorts demonstrated a favorable safety profile with no dose limiting toxicity to date. The combination of CDX-1307 with TLR agonists significantly enhanced immune responses against hCG-Beta, providing humoral responses in 88% of patients and cellular immune responses in 57% of patients analyzed to date. Immune responses occurred even in the Phase 1 clinical trials and more than half have evidencepresence of high circulating levels of hCG-Beta, expression by their tumor. The immunotherapy has been well tolerated with only minor adverse events observed (reddening atsuggesting that the injection site). Analysis of the initial cohorts with GM-CSF have revealed that severalCDX-1307 can overcome antigen tolerance in advanced and heavily pretreated cancers. Nine patients developed good humoral responses to
hCG-Beta, and some have demonstrated enhancement of circulating hCG-Beta-specific CD8 T cells. Thus, we are encouraged that CDX-1307 is providing similar results as predicted in the pre-clinical studies. In addition, one patient with pancreatic cancer had a 26% overall reduction in tumor burden and two breast cancer patients were stable for sixstudies experienced disease stabilization from 2.3 months during treatment. The investigators atto 11.4 months following the Duke Comprehensive Cancer Center were awarded a two year $500,000 grant from the Avon Foundation and the National Cancer Institute to support Phase 1 work in breast cancer. The safetyinitiation of CDX-1307 vaccination. Two of these patients have received multiple courses of CDX-1307 and continue treatment with stable disease at 6.4 and 11.4 months. These data provide the basis for advancing CDX-1307 into a front-line patient population selected for hCG-Beta expressing cancers.
We expect to initiate a randomized Phase 2b controlled study in combinationpatients with defined immune system modulators is now being evaluated with intent to enter Phase 2 clinical researchnewly diagnosed invasive bladder cancer in the second halfquarter of 2009.2010. Patient's whose bladder cancer expresses hCG-Beta are predicted to have more aggressive disease and shorter survival. In this study we plan to select only patients with confirmed hCG-Beta expression using a specific diagnostic assay.
CDX-1401:CDX-1401
CDX-1401 is a fusion protein consisting of a fully human monoclonal antibody with specificity for the dendritic cell receptor, DEC-205, linked to the NY-ESO-1 tumor antigen. In humans, NY-ESO-1 is one of the most immunogenic tumor antigens and has been detected in 20 - 30% of cancers, thus representing a broad opportunity. This product is intended to selectively deliver the NY-ESO-1 antigen to APCs for generating robust immune responses against cancer cells expressing NY-ESO-1. Unlike CDX-1307, which targets the mannose receptor
expressing dendritic cells, CDX-1401 is the first APC product targeting DEC-205 expressing dendritic cells. We are developing CDX-1401 for the treatment of malignant melanoma and a variety of solid tumors which express the proprietary cancer antigen NY-ESO-1, which the Companywe licensed from the Ludwig Institute for Cancer Research in 2006. The Company believesWe believe that preclinical studies have shown that CDX-1401 is effective for activation of human T-cell responses against NY-ESO-1.
In September 2009, we initiated enrollment in a dose-escalating Phase 1/2 clinical trial aimed at determining the optimal dose for further development based on the safety, tolerability, and immunogenicity of the CDX-1401 vaccine. The IND filing is planned fortrial will evaluate three different doses of the first halfvaccine in combination with resiquimod, an activator of 2009.TLR 7 and 8. We expect to be able to enter a Phase 1 studyenroll approximately 36 patients with a combination regimen, including TLRs, and will accruesolid tumor cancers at multiple tumors that express NY-ESO-1.clinical sites in the United States.
CDX-1135
CDX-1135 is a molecule that inhibits a part of the immune system called the complement system. The complement system is a series of proteins that are important initiators of the body's acute inflammatory response against disease, infection and injury. Excessive complement activation also plays a role in some persistent inflammatory conditions. CDX-1135 is a soluble form of naturally occurring Complement Receptor 1 that inhibits the activation of the complement cascade in animal models and in human clinical trials. We believe that regulating the complement system could have therapeutic and prophylactic applications in several acute and chronic conditions, including organ transplantation, multiple sclerosis, rheumatoid arthritis, age-related macular degeneration ("AMD"), atypical Hemolytic Uremic Syndrome ("aHUS"), Paroxysmal Nocturnal Hemaglobinuria ("PNH"), Dense Deposit Disease ("DDD") in kidneys, and myasthenia gravis. We are currently defining the most appropriate clinical development path for CDX-1135 and are focusing on rare disease conditions of unregulated complement activation as the fastest route to FDA approval.
Preclinical Development Programs
CDX-1127:CDX-301
CDX-301 is a FMS-like tyrosine kinase 3 ligand (Flt3L) that we licensed from Amgen in March 2009. CDX-301 is a growth factor for stem cells and immune cells called dendritic cells. Based on previous experience with this molecule, we believe that CDX-301 has considerable opportunity in various transplant settings as a stem cell mobilizing agent. In addition, CDX-301 is an immune modulating molecule that increases the numbers and activity of specific types of immune cells. We believe CDX-301 has significant opportunity for synergistic development in combination with proprietary molecules in our portfolio. We expect to file an Investigational New Drug ("IND") application for CDX-301 before the end of 2010.
Celldex hasCDX-1127
We have entered into a License Agreement with the University of Southampton, UK, to develop human antibodies to CD27, a potentially important target for immunotherapy of various cancers. In pre-clinicalpreclinical models, antibodies to CD27 alone have been shown to mediate anti-tumor effects, and may be particularly effective in combination with other immunotherapies. CD27 is a critical molecule in the activation pathway of lymphocytes. It is downstream from CD40, and may provide a novel way to regulate the immune responses. Engaging CD27 with the appropriate monoclonal antibody has proven highly effective at promoting anti-cancer immunity in mouse models. We are currently evaluating new human monoclonal antibodies in pre-clinicalpreclinical models.
D. Inflammatory Disease Development ProgramsTable of Contents
CDX-1135CDX-014
CDX-014 (formerly TP10):CR014-vcMMAE) is a fully-human monoclonal ADC that targets TIM-1, an immunomudulatory protein that appears to down regulate immune response to tumors. The antibody, CDX-014, is linked to a potent chemotherapeutic, monomethyl auristatin E (MMAE), using Seattle Genetics' proprietary technology. The ADC is designed to be stable in the bloodstream, but to release MMAE upon internalization into TIM-1-expressing tumor cells, resulting in a targeted cell-killing effect. CDX-014 has shown potent activity in preclinical models of ovarian and renal cancer. We acquired the rights to CDX-014 in connection with the CuraGen Merger.
CDX-1189
We have been developing immunotherapeutics that inhibit a part of the immune system called the complement system. The complement system is a series of proteins that are important initiators of the body's acute inflammatory response against disease, infection and injury. Excessive complement activation also plays a role in some persistent inflammatory conditions. Our lead compound, CDX-1135, a soluble form of naturally occurring Complement Receptor 1, has effectively shown to inhibit the activation of the complement cascade in animal models and in human clinical trials. We believe that regulating the complement system could have therapeutic and prophylactic applications in several acute and chronic conditions, including organ transplantation, multiple sclerosis, rheumatoid arthritis, age-related macular degeneration ("AMD"), atypical Hemolytic Uremic Syndrome and myasthenia gravis. We are currently defining the most appropriate clinical development path for CDX-1135 and are focusing on rare disease conditions of unregulated complement activation as potentially the fastest route to FDA approval.
CDX-1189: Celldex is developing therapeutic human antibodies to a signaling molecule known as CD89 or Fca receptor type I (FcaRI). CD89 is expressed by some white blood cells and leukemic cell lines, and has been shown to be important in controlling inflammation and tumor growth in animal models. Celldex hasWe have proprietary, fully human antibodies to CD89 in preclinical development. Depending upon the specific antibody used, anti-CD89 antibodies can either be activating and thus stimulate immune responses, or down-regulating and act as an anti-inflammatory agent.
Table of ContentsPartnerships
E. Infectious Disease Development Programs
CholeraGarde® Vaccine: CholeraGarde® is designed to be a safe, effective single-dose, oral cholera vaccine. Our partner, the International Vaccine Institute ("IVI"), has received $21 million in funding from the Bill & Melinda Gates Foundation for a Cholera Vaccine Initiative ("CHOVI"), which includes conducting further clinical trials of CholeraGarde®. The IVI is presently conducting a Phase 2 clinical trial of CholeraGarde in Bangladesh, with plans to sponsor additional Phase 2 studies in India and Thailand beginning in the first half of 2009, followed by Phase 3 field studies.
ETEC Vaccine: In November 2007, we We have entered into an agreementcollaborative partnership agreements with the Division of Microbiologypharmaceutical and Infectious Diseases of the NIAID, whereby NIAID is sponsoring a Phase 1 study of Celldex's investigational single-dose, oral vaccine designedother companies and organizations that provide financial and other resources, including capabilities in research, development, manufacturing, and sales and marketing, to offer combined protection against both enterotoxigenicEscherichia coli (ETEC) and cholera. In June 2008, NIAID initiated the Phase 1 trial of the ETEC vaccine candidate at Cincinnati Children's Hospital Medical Center.
In January 2009, we entered into an Exclusive License and Development Agreement with Vaccine Technologies, Inc. ("VTI"). Under the license agreement, Celldex has granted a worldwide fee- and royalty-bearing exclusive license to VTI to development and commercialize Celldex's CholeraGarde® and ETEC vaccine programs. Financial terms of the agreement with VTI include an upfront license fee, milestone payments and royalties on net sales of licensed products during the term of the agreement.
Ty800 Typhoid Fever Vaccine: The Company has developed an oral vaccine to offer rapid, single-dose protection againstSalmonella typhi, the cause of typhoid fever. Ty800 is targeted for both the travelers' market and global health needs. In 2006, the National Institute of Allergy and Infectious Disease ("NIAID") of the National Institutes of Health ("NIH") initiated a Phase 1/2 in-patient dose-ranging clinical trial aimed at demonstrating the safety and immunogenicity of the Ty800 typhoid fever vaccine. NIAID funded the production of Ty800 vaccine for clinical testing and completed the Phase 1/2 trial at a NIH-funded clinical site in 2007. Results showed the single-dose, oral vaccine to be well tolerated and immunogenic, with over 90% of vaccinated subjects generating immune responses. We initiatedsupport our own sponsored Phase 2 trial of Ty800 in July 2007. Preliminary results reported in April 2008 from the study showed that the single-dose, oral vaccine was well tolerated and immunogenic, demonstrating that the desired immune response was achieved. Incidence of reactogenicity symptoms and adverse events post-vaccination were similar to placebo. Importantly, immunogenic response was dose-dependent. Positive immune response or seroconversion (prospectively defined as a 4-fold increase in anti-LPS titers over pre-dose level) rates were 65.5% (36/55) and 80% (44/55) in the low and high dose groups, respectively, and was significantly (p<0.001) higher than placebo.
CDX-2401: The Company is also using its APC Targeting Technology™ to develop vaccines against infectious disease. The lead program is CDX-2401, an APC-Targeting prophylactic vaccine, aimed at providing protection from infection with HIV, the virus known to cause AIDS. This program is in a Bill & Melinda Gates Foundation funded partnership with collaborators at Rockefeller University in New York City, who have shown in model systems that protective immunity can be induced with such a vaccine. Preclinical studies and manufacturing development are in progress and the Company, with its collaborators, plans to file an IND for Phase 1 clinical studies in the first half of 2009.
F. Marketed Products
Rotavirus Vaccine: Rotavirus is a major cause of diarrhea and vomiting in infants and children. In 1997, we licensed our oral rotavirus strain to GlaxoSmithKline ("Glaxo"). All of the ongoing development for this program is being conducted and funded by Glaxo. Glaxo gained approval for its
rotavirus vaccine, Rotarix®, in Mexico in July 2004, which represented the first in a series of worldwide approvals and commercial launches for the product. Glaxo subsequently launched Rotarix® in additional Latin American and Asian Pacific countries during 2005 - 2007. Additionally, Glaxo filed for market approval with the European regulatory authorities in late 2004, which triggered a $2 million milestone payment to the Company. In February 2006, the European Commission granted approval of Rotarix® in the European Union, which triggered a $4 million milestone payment from Glaxo. On April 3, 2008, Rotarix® received approval from the FDA for the prevention of rotavirus gastroenteritis in infants. FDA approval triggered a $1.5 million milestone payment from Glaxo, of which $750,000 was retained by the Company. We licensed-in the rotavirus strain in 1995 and owe a license fee of 30% to Cincinnati Children's Hospital Medical Center ("CCH") on net royalties received from Glaxo. In May 2005, the Company entered into an agreement whereby an affiliate of Paul Royalty Fund ("PRF") purchased an interest in the net royalties the Company will receive on worldwide sales of Rotarix® (see Note 10 of our consolidated financial statements). The market launch of Rotarix® by Glaxo in the U.S. market during the quarter ended September 30, 2008 resulted in a $10 million milestone payment to the Company from PRF, which the Company received on October 1, 2008. We have received a total of $60 million in milestone payments under the PRF agreement. No additional milestone payments are due from PRF under the agreement.
In September 2006, we received notice from Glaxo that Glaxo would begin paying royalties on sales of Rotarix® vaccine at the lower of two royalty rates under their 1997 license agreement. Glaxo's decision to pay the lower royalty rate (which is 70% of the full rate) is based upon Glaxo's assertion that Rotarix® is not covered by the patents Glaxo licensed from the Company in Australia and certain European countries. If Glaxo's position stands, the royalties to which PRF is entitled will no longer be limited by a $27.5 million annual threshold, which the Company projected may have been reached in later years as sales of Rotarix® increased. Irrespective of Glaxo's position, we will still retain approximately 65% of the royalties on worldwide sales of Rotarix® once PRF receives 2.45 times the aggregate cash payments of $60 million it made to the Company, though the potential amount of such residual royalties will be lower if Glaxo's position stands.
Megan®Vac 1 and Megan®Egg Vaccines: On December 1, 2000, the Company acquired all of the outstanding capital stock of Megan. Megan has commercialized three veterinary vaccines; Argus™ SC, licensed by the United States Department of Agriculture ("USDA") in March 1998 and marketed by Intervet, Inc., and Megan®Vac 1 and Megan®Egg, licensed by the USDA in November 1998 and 2003, respectively, and marketed by Lohmann Animal Health International ("LAHI"). In January 2009, we sold the poultry vaccines business, consisting of Megan®Vac 1 and Megan®Egg, to LAHI for an upfront fee and potential milestone payments.
G. Product Development and Licensing Agreements
1. GlaxoSmithKline plc and Paul Royalty Fund II, L.P.
In 1997, the Company entered into an agreement with Glaxo to collaborate on the development and commercialization of our oral rotavirus strain and Glaxo assumed responsibility for all subsequent clinical trials and all other development activities. We licensed-in the rotavirus strain that was used to develop Glaxo's Rotarix® rotavirus vaccine in 1995 and owe a license fee of 30% to CCH on net royalties received from Glaxo. We are obligated to maintain a license with CCH with respect to the Glaxo agreement. All licensing fees are included in research and development expense. The term of the Glaxo agreement is through the expiration of the last of the relevant patents covered by the agreement, although Glaxo may terminate the agreement upon 90 days prior written notice.
In May 2005, the Company entered into an agreement whereby an affiliate of PRF purchased an interest in the net royalties the Company will receive on worldwide sales of Rotarix®. Under the PRF agreement, the Company will retain 50% of future Glaxo milestone payments beginning on the effective date of the agreement with PRF, with 70% of the remaining balance payable to PRF and 30% of the remaining balance payable to CCH, respectively. The Company's retained interests in Rotarix® net royalties which were not sold to PRF are recorded as product royalty revenue and a corresponding amount that is payable to CCH is recorded as royalty expense, which is included in research and development expense.
On April 3, 2008, Rotarix® received FDA market approval for the prevention of rotavirus gastroenteritis in infants, which triggered a $1.5 million milestone payment to the Company from Glaxo, $750,000 of which the Company retained under its agreement with PRF. In connection with the Company's purchase accounting for the Merger, the present value of the Company's retained amount, or $742,300, had been recorded as a current asset as of March 31, 2008. During the quarter ended June 30, 2008, the Company also recorded $225,000 in revenue and an offsetting amount in royalty expense for the payable due to CCH for its portion of the Glaxo milestone. The market launch of Rotarix® by Glaxo in the U.S. market during the quarter ended September 30, 2008 resulted in a $10 million milestone payment to the Company from PRF, which the Company received on October 1, 2008. As of March 31, 2008, the Company had recorded the expected present value of the $10 million milestone payment due from PRF of $9,053,200, the purchase accounting value assigned to the PRF milestone payment at the time of the Merger. During the quarter ended September 30, 2008, the Company recognized the balance of $946,800 as other income in the consolidated statement of operations. We have received $60 million in total milestone payments under the PRF agreement. No additional milestone payments are due from PRF under the agreement.
Royalty rates on Rotarix® escalate from 7% to 10% based on net product sales in countries that have valid patent protection. These royalty rates are discounted by 30% for "non-patent" countries (primarily international markets). In September 2006, the Company received notice from Glaxo that Glaxo would begin paying royalties on sales of Rotarix® vaccine at the lower of the two royalty rates under their 1997 license agreement. Glaxo's decision to pay the lower royalty rate (which is 70% of the full rate) is based upon Glaxo's assertion that Rotarix® is not covered by the patents Glaxo licensed from the Company in Australia and certain European countries. If Glaxo's position stands, the royalties to which PRF is entitled will no longer be limited by a $27.5 million annual threshold, which the Company projected may have been reached in later years as sales of Rotarix® increased. Irrespective of Glaxo's position, the Company will still retain approximately 65% of the royalties on worldwide sales of Rotarix® once PRF receives 2.45 times the aggregate cash payments of $60 million it made to Celldex, though the potential amount of such residual royalties will be lower if Glaxo's position stands.
2. GlaxoSmithKline plc and Corixa Corporation ("Corixa")
On December 21, 2005, Corixa, a wholly-owned subsidiary of Glaxo, and Celldex Ltd. (formerly Lorantis Limited), entered into a termination agreement of their collaboration of CDX-2101, or HepVax, for the development of a therapeutic vaccine for Hepatitis B (the "Termination Agreement"). Under the terms of the Termination Agreement, Glaxo paid the Company the sum of approximately $1,632,000. In addition, and subject to the terms and conditions of the Termination Agreement, Glaxo granted to the Company a worldwide, fully paid up, royalty-free, perpetual, nonexclusive license under the Corixa Patent Rights, Corixa Know-How Rights and Corixa Licensed Technology (each as defined in the Termination Agreement): (a) to use RC-529SE in products being developed and/or commercialized by Celldex Ltd or its Permitted Sublicensees in the Lorantis Field; and (b) to make or have made RC-529SE using RC-529 adjuvant for the limited use permitted by the license granted to reformulate Corixa's proprietary adjuvant.
3. Pfizer Inc.
Pfizer License and Development Agreement: On April 16, 2008, the Company and Pfizer entered into a License and Development Agreement (the "Pfizer Agreement") under which Pfizer was granted an exclusive worldwide license to a therapeutic cancer vaccine candidate, CDX-110, in Phase 2 development for the treatment of glioblastoma multiforme. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the Pfizer Agreement, Pfizer made an upfront payment to the Company of $40 million and made a $10 million equity investment in the Company. Pfizer will fund all development costs for these programs. The Company is also eligible to receive potential milestone payments exceeding $390 million for the successful development and commercialization of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales. The Pfizer Agreement became effective after clearance under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (as amended) on May 19, 2008.
On May 27, 2008, the Company received $10 million from Pfizer in exchange for 781,250 shares of the Company's common stock having a fair value of $10,867,188, or $13.91 per share, on that date. The $867,188 over the amount received from Pfizer was recorded as a reduction to deferred revenue of the $40 million upfront payment received from Pfizer on June 18, 2008.
The Company has applied the provisions of Emerging Issues Task Force (EITF) Issue No. 00-21 ("EITF 00-21"), Accounting for Revenue Arrangements with Multiple Deliverables, and determined that its performance obligations under this collaboration should be accounted for as a single unit of accounting. The Company's deliverables under this collaboration primarily include an exclusive license to its CDX-110 product candidate and its EGFRvIII technologies, research and development services as required under the collaboration and participation in the joint clinical development committee. The Company has estimated that its performance period under the collaboration will be 9.5 years based on an assessment of the period over which the Company will have met its performance obligations under the collaboration. Revenue, including research and development reimbursements, is being recognized on a straight-line basis over this period using the Contingency Adjusted Performance Model ("CAPM"). The $40,000,000 up-front payment was recorded as deferred revenue and this amount, less the $867,188 in excess fair value for the Company's common stock discussed above, is being amortized over the 9.5-year performance period at a rate of $1,029,810 per quarter.
The agreement also provides for reimbursement by Pfizer of all costs incurred by the Company in connection with the collaboration since the effective date. The Company invoices Pfizer monthly for its reimbursable costs and records the invoiced amount as deferred revenue. These deferred revenue amounts are amortized to revenue over the expected 9.5-year performance period on a straight-line basis using the CAPM model.
In connection with the initial deliverables under the Pfizer Agreement as discussed further in Note 11, the Company has paid a sublicense fee of $2,365,174 to each of two research universities, Duke University ("Duke") and Thomas Jefferson University ("TJU"), and paid TJU an additional license fee of $500,000. In October 2008, the Company paid an additional sublicense fee to TJU of $1,634,826. These payments were recorded as deferred costs in the "Other Assets" line item in the consolidated balance sheet and are being amortized over the 9.5-year performance period at a rate of $180,663 per quarter.
Pfizer Animal Health Agreement: The Company entered into a licensing agreement in December 2000 with Pfizer's Animal Health Division whereby Pfizer has licensed Megan's technology for the development of animal health and food safety vaccines. Under the agreement, the Company may receive additional milestone payments of up to $3 million based upon attainment of specified milestones. The Company may receive royalty payments on eventual product sales. The term of this agreement is through the expiration of the last of the patents covered by the agreement. The Company has no obligation to incur any research and development costs in connection with this agreement.
As of June 1, 2006, the Company entered into a Collaborative Research and Development Agreement with Pfizer aimed at the discovery and development of vaccines to protect animals. In 2007, further funded work at the Company on the joint research program was terminated by Pfizer after the Company provided two of four deliverables to Pfizer.
4. Rockefeller University ("Rockefeller")
The Company is developing a vaccine, CDX-2401, aimed at providing protection from infection with HIV, the virus known to cause AIDS. This program is in a Bill & Melinda Gates Foundation funded partnership with collaborators at Rockefeller and the Aaron Diamond AIDS Research Center, who have shown in model systems that protective immunity can be induced with such a vaccine. Preclinical studies and manufacturing development are in progress and payments to the Company are made on a time and materials basis.
5. Vaccine Technologies, Inc. ("VTI")
In January 2009, we entered into an Exclusive License and Development Agreement with VTI. Under the license agreement, Celldex has granted a worldwide fee- and royalty-bearing exclusive license to VTI to development and commercialize Celldex's CholeraGarde® and ETEC vaccine programs. Financial terms of the agreement with VTI include an upfront license fee, milestone payments and royalties on net sales of licensed products during the term of the agreement.
We depend on our collaborativethese relationships and may enter into more of them in the future. Some of the above referenced agreements give our collaboratorpartners have substantial responsibility to commercialize a product and to make decisions about the amount and timing of resources that are devoted to developing and commercializing a product. As a result, we do not have complete control over how resources are used toward some of our products.
In addition, someSome of theseour partnership agreements relate to products in the early stages of research and development. Others require Celldexus and our collaboratorcollaborators to jointly decide on the feasibility of developing a particular product using our technologies. In either case, these agreements may terminate without benefit to us if the underlying products are not fully developed. If we fail to meet our obligations under these agreements, they could terminate and we might need to enter into relationships with other collaborators and to spend additional time, money, and other valuable resources in the process.
Moreover, weWe cannot predict whether our collaborators will continue their development efforts or, if they do, whether their efforts will achieve success. Many of our collaborators face the same kinds of risks and uncertainties in their business that we face. A delay or setback to a collaborator will, at a minimum, delay the commercialization of any affected products, and may ultimately prevent it. Moreover, any collaborator could breach its agreement with us or otherwise not use best efforts to promote our products. A collaborator may choose to pursue alternative technologies or products that compete with our technologies or products. In either case, if a collaborator failed to successfully develop one of our products, we would need to find another collaborator. Our ability to do so would depend upon our legal right to do so at the time and whether the product remained commercially viable.
H. GlaxoSmithKline plc ("Glaxo") and Paul Royalty Fund II, L.P. ("PRF")
Rotavirus is a major cause of diarrhea and vomiting in infants and children. In 1997, we licensed our oral rotavirus strain to Glaxo and Glaxo assumed responsibility for all subsequent clinical trials and all other development activities. Glaxo gained approval for its rotavirus vaccine, Rotarix®, in Mexico in July 2004, which represented the first in a series of worldwide approvals and commercial launches for the product leading up to the approval in Europe in 2006 and in the U.S. in 2008. We licensed-in our
rotavirus strain in 1995 and owe a license fee of 30% to Cincinnati Children's Hospital Medical Center ("CCH") on net royalties received from Glaxo. We are obligated to maintain a license with CCH with respect to the Glaxo agreement. The term of the Glaxo agreement is through the expiration of the last of the relevant patents covered by the agreement, although Glaxo may terminate the agreement upon 90 days prior written notice.
In May 2005, we entered into an agreement whereby an affiliate of PRF purchased an interest in the milestone payments and net royalties that we will receive on the development and worldwide sales of Rotarix®. We have received a total of $60 million in milestone payments under the PRF agreement. No additional milestone payments are due from PRF under the agreement.
Royalty rates on Rotarix® escalate from 7% to 10% based on net product sales in countries that have valid patent protection. These royalty rates are discounted by 30% for "non-patent" countries (primarily international markets). In September 2006, we received notice from Glaxo that Glaxo would begin paying royalties on sales of Rotarix® vaccine at the lower of the two royalty rates under their 1997 license agreement. Glaxo's decision to pay the lower royalty rate (which is 70% of the full rate) is based upon Glaxo's assertion that Rotarix® is not covered by the patents Glaxo licensed from us in Australia and certain European countries. We are currently evaluating the basis for Glaxo's action and our potential remedies. If Glaxo's position stands, the royalties to which PRF is entitled will no longer be limited by a $27.5 million annual threshold, which we projected may have been reached in later years as sales of Rotarix® increased. Irrespective of Glaxo's position, we will still retain approximately 65% of the royalties on worldwide sales of Rotarix® once PRF receives 2.45 times the aggregate cash payments of $60 million it made to us, though the potential amount of such residual royalties will be lower if Glaxo's position stands.
Pfizer Inc.
Pfizer License and Development Agreement: In April 2008, we and Pfizer entered into the Pfizer Agreement under which Pfizer was granted an exclusive worldwide license to a therapeutic cancer vaccine candidate, CDX-110, in Phase 2 development for the treatment of glioblastoma multiforme. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the Pfizer Agreement, Pfizer made an upfront payment to us of $40 million and made a $10 million equity investment in us. Pfizer will fund all development costs for these programs. We are also eligible to receive potential milestone payments exceeding $390 million for the successful development and commercialization of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales. The Pfizer Agreement became effective after clearance under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (as amended) on May 19, 2008. In connection with the Pfizer Agreement, we paid a total of $6.9 million in sublicense fees to Duke University and Thomas Jefferson University.
Pfizer Animal Health Agreement: We entered into a licensing agreement in December 2000 with Pfizer's Animal Health Division whereby Pfizer has licensed our technology for the development of animal health and food safety vaccines. Under the agreement, we may receive additional milestone payments of up to $3 million based upon attainment of specified milestones. We may receive royalty payments on eventual product sales. The term of this agreement is through the expiration of the last of the patents covered by the agreement. We have no obligation to incur any research and development costs in connection with this agreement.
Rockefeller University ("Rockefeller")
We are providing research and development support to Rockefeller on the development of their vaccine, DCVax-001, which we refer to as CDX-2401, aimed at providing protection from infection with HIV, the virus known to cause AIDS. Rockefeller's program is in a Bill & Melinda Gates Foundation
funded partnership called the Grand Challenges initiative. Preclinical studies and manufacturing development are in progress and our collaborators plan to file an IND for Phase 1 clinical studies in the first half of 2010. Rockefeller pays us on a time and materials basis.
Vaccine Technologies, Inc. ("VTI")
In January 2009, we entered into a license agreement with VTI under which we granted a worldwide exclusive license to VTI to develop and commercialize our CholeraGarde® and ETEC vaccine programs. We may receive milestones payments and royalties with respect to development and commercialization of the technology licensed to VTI.
TopoTarget A/S ("TopoTarget")
In connection with the CuraGen Merger, we assumed the rights under the April 2008 agreement ("TopoTarget Agreement") between CuraGen and TopoTarget whereby we could receive up to $6 million in either potential commercial milestone payments related to future net sales of Belinostat or 10% of any sublicense income received by TopoTarget ("TopoTarget Payments"). Under the TopoTarget Agreement, CuraGen sold back its Belinostat rights to TopoTarget and received $25 million in cash, 5 million shares of TopoTarget common stock (sold by CuraGen in 2008 for net proceeds of $12 million) and the right to receive the TopoTarget Payments. In addition, TopoTarget assumed all financial and operational responsibility for the clinical development of Belinostat under the TopoTarget Agreement. In February 2010, TopoTarget entered into a co-development and commercialization agreement for Belinostat with Spectrum Pharmaceuticals, Inc. resulting in our receipt of $3 million of the TopoTarget Payments.
Research Collaboration and Licensing Agreements
Celldex hasWe have entered into licensing agreements with several universities and research organizations. Under the terms of these agreements, we have received licenses or options to license technology, specified patents or patent applications.
Table Our licensing and development collaboration agreements generally provide for royalty payments equal to specified percentages of Contentsproduct sales, annual license maintenance fees and continuing patent prosecution costs. In addition, we have committed to make potential future milestone payments to third parties of up to approximately $116 million as part of our various collaborations including licensing and development programs. Payments under these agreements generally become due and payable only upon achievement of certain developmental, regulatory and/or commercial milestones.
1. Medarex, Inc., a subsidiary of Bristol-Myers Squibb ("Medarex")
The CompanyWe and Medarex, a former related party, have entered into the following agreements, each of which was approved by a majority of its independent directors who did not have an interest in the transaction. These agreements include:
Under the terms of the Assignment and License Agreement and Research and Commercialization Agreement, with Medarex, Celldex willwe may be required to pay Medarex license feesmilestone and royalty payments to obtain commercial licenses for antibodies arising from research licenses granted by Medarex. Celldex will also be required to pay Medarex milestone payments with respect to the development of any products containing such licensed antibodies. These fees and milestones may total up to $7 to $10 million per licensed antibody if a product containing such licensed antibody receives approval from the FDA and/or equivalent foreign agencies. None
In addition, Celldex will be requiredOctober 2007, we and Medarex entered into a settlement and mutual release agreement which settled disputed amounts we owed Medarex. We issued to pay royaltiesMedarex 351,692 shares of our common stock equal in value to $3.0 million, based on any salesthe per share price of products containing licensed antibodies. The royalties will be payable$8.64 set on a country-by-country and product-by-product basis until the date which issecond trading day prior to the later of: (i) the expirationclosing date of the last-to-expire of theAVANT Merger and exchanged releases. At December 31, 2008, we owed Medarex patents covering the productan additional $3.0 million related to a Master Services Agreement, which we paid Medarex in such country or (ii) the tenth anniversary of the first commercial sale of a product in such country. Celldex will also be responsible for the payment of any royalties, license fees and milestone and other payments due to third parties if Celldex licenses any additional technology in order to commercialize such products.
To date, Celldex has not made any royalty payments on sales of any products and believes it is at least a number of years away from selling any products that would require Celldex to make any such royalty payments. Whether Celldex will be obligated to make milestone or royalty payments in the future is subject to the success of Celldex's product development efforts and, accordingly, is inherently uncertain.October 2009.
2. Rockefeller University ("Rockefeller")
OnIn November 1, 2005, the Companywe and Rockefeller entered into a license agreement for the exclusive worldwide rights to human DEC-205 receptor, with the right to sublicense the technology. The license grant is exclusive except that Rockefeller may use and permit other nonprofit organizations to use the human DEC-205 receptor patent rights for educational and research purposes. In addition,We may be required to pay milestone and royalty payments to Rockefeller with respect to development and commercialization of the Company acknowledges that Rockefeller has granted Howard Hughes Medical Institute ("HHMI") a paid-up, nonexclusive, irrevocable license to use the patent rights, biological materials, and technical information for HHMI's research purposes, but with no right to sublicense. The Companyhuman DEC-205 receptor. We may also be required to pay royalties on any product sales. The royalties will be payable on a country-by-country and licensed product-by-licensed product basis until the date which is the later of (i) the expiration of
the last to expire of the patents covering the licensed product in such country or (ii) ten years following the first commercial sale of a licensed product in such country.
The Company may be required to pay license fees and milestone payments to Rockefeller with respect to development of the human DEC-205 receptor. These fees and milestones may total up to $2 million to $4 million per product candidate that receives approval from the FDA and equivalent foreign agencies.
3. Duke University Brain Tumor Cancer Center ("Duke")
OnIn September 1, 2006, the Companywe and Duke University Brain Tumor Cancer Center of Duke University ("Duke") entered into a license agreement that gave the Companyus access and reference to the clinical data generated by Duke and its collaborators in order for the Companyus to generate itsour own filing with the FDA relating to itsthe CDX-110 product.
In exchange for referencing all the Duke data, the Company paid Duke a one-time upfront payment of $175,000 and issued to Duke 100,000 shares of the Company's common stock, which the Company recorded in 2006 as a licensing expense in research and development. The estimated aggregate fair value of the common shares issued was $330,000.
The Company We may be required to pay license feesmilestone and milestoneroyalty payments to Duke with respect to development and commercialization of the CDX-110 product. These fees and milestones may total up to $1.2 million if CDX-110 receives approval from the FDA and equivalent foreign agencies. The Company may also be required to pay royalties upon approval of CDX-110. The royalties will be payable on a country-by-country and licensed product-by-licensed product basis until the date of the expiration of the last to expire of the patents covering the licensed product in such country.
In connection with the Pfizer Agreement, discussed in Note 10, the Companywe determined that $2,365,174$2.4 million was payable to Duke as a sublicense fee. As agreed byprovided for under the Duke at the Company's option,license, we paid 50% of this amount was paid to Duke in the form of 81,512 shares of our common stock in October 2008.
4. Ludwig Institute for Cancer Research
On October 20, 2006, the Company and Ludwig Institute for Cancer Research ("Ludwig")
In October 2006, we and Ludwig entered into an agreement for the nonexclusive rights to six cancer tumor targets for use in combination with the Company'sour APC Targeting Technology. The term of the agreement is for ten years. As consideration for the nonexclusive license, the Company agreed to pay an annual license fee of $7,500 and $2,500 for each full-length antigen and partial-length antigen, respectively, until such antigens enter a randomized Phase 1 clinical trial.
As additional consideration for the nonexclusive license, the CompanyWe may be required to pay license feesmilestone and milestoneroyalty payments to Ludwig for the usewith respect to development and commercialization of the cancer targets in combination withtechnology licensed from Ludwig.
Alteris Therapeutics, Inc. ("Alteris")
In October 2005, we completed the Company's technology. The fees and milestonesacquisition of the assets of Alteris, including the EGFRvIII molecule that we licensed to Pfizer under the Pfizer Agreement. We may total up to $1.5 million to $2.5 million on a product candidate that receives approval from the FDA and equivalent foreign agencies. The Company may also be required to pay royaltiesAlteris up to $5.0 million upon obtaining the first approval for commercial sale of anya product candidate. The royalties will be payable on a country-by-country and licensed product-by-licensed product basis until the date of the expiration of the last to expire of the patents covering the licensed product in such country.containing EGFRvIII, including CDX-110.
5. Thomas Jefferson University ("TJU")
In February 2003, as part of its acquisition of the EGFR VIII technology from Alteris Therapeutics, Inc., the Companywe entered into three exclusive license agreements with Thomas Jefferson University ("TJU"). Under the license agreements, TJU has granted a worldwide fee-and royalty-bearing exclusive license.TJU. Under these licenses, the Company willwe may be obligatedrequired to pay milestone and royalty payments to TJU milestone payments which may total upwith respect to $3 million for the first licensed product developed during the term of
the license agreements, an annual license fee of $45,000, patentdevelopment and other expenses associated with licenses, as well as royalties on net sales of licensed products during the termcommercialization of the license agreements. The Company also issued 100,000 shares of its common stock totechnology licensed from TJU. In the event that TJU provides notice of default and the default is not cured within 60 days of such notice, TJU may terminate the license agreements. In connection with the Pfizer Agreement, the Companywe amended itsour licenses with TJU to add additional sublicensing rights and made a $500,000 one-time license paymentpaid $4.5 million in sublicense fees to TJU in June 2008.
As discussed in Note 10, the Company paid a sublicense fee of $2,365,174 to TJU during the quarter ended September 30, 2008 and paid an additional sublicense fee of $1,634,826 to TJU in October 2008.
6. Select Vaccines Limited3M Company
In February 2007, the Company entered into a research and development partnership with Select Vaccines Limited ("Select Vaccines"), a public Australian biotechnology company, focused on the use of Select Vaccines' virus-like particles ("VLPs") as a platform technology for the development of viral vaccines. Under the terms of the agreement, the Company made an upfront equity investment of $735,000 in Select Vaccines and agreed to fund influenza vaccine research and development for two years, as well as provide payments to Select Vaccines for the achievement of specific preclinical and clinical development milestones. On November 1, 2007, the Company notified Select Vaccines that, effective December 31, 2007, the Company was exercising its rights to terminate its Collaboration and License Agreement with Select Vaccines for strategic reasons. In AugustJune 2008, the Company sold its equity investment in Select Vaccine shares and recorded net proceeds of $250,882.
7. 3M Company
On June 11, 2008, the Companywe and 3M Company entered into a license agreement for the exclusive worldwide rights to access 3M Company's proprietary Immune Response Modifier, Resiquimod™, (and additional Toll-Like Receptor 7/8 agonists ("TLRs"TLR")) for clinical study with Celldex'sour proprietary APC Targeting Technology, for use as vaccine adjuvants, with the right to sublicense the technology.
The Company paid 3M Company a one-time upfront license fee which was charged to research and development expense in the quarter ended June 30, 2008. The Company We may be
required to pay annual license feesmilestone and milestoneroyalty payments to 3M Company with respect to development of Resiquimod™. The Company may also be required to pay royalties upon approval of any product candidate. The royalties will be payable on a country-by-country and licensed product-by-licensed product basis until the datecommercialization of the expiration of the last to expire of the patents covering thetechnology licensed product in such country.from 3M Company.
8. University of Southampton
In November 2008, the Company entered into an Exclusive Patent and Know-How License Agreement with the University of Southampton, UK ("Southampton")
In November 2008, we entered into a license agreement with Southampton to develop human antibodies towards CD27, a potentially important target for immunotherapy of various cancers. CD27 is a critical molecule in the activation pathway of lymphocytes, is downstream from CD40, and may provide a novel way to regulate the immune responses. In pre-clinicalpreclinical models, antibodies to CD27 have been shown to mediate anti-tumor effects alone, and may be particularly effective in combination with the Company'sour other immunotherapies.
The Company paid Southampton a one-time upfront license fee which was charged to research and development expense in the quarter ended December 31, 2008. The Company We may be required to pay annual license feesmilestone and milestoneroyalty payments to Southampton with respect to development and commercialization of CD27.the technology licensed from Southampton.
In March 2009, we entered into a license agreement with Amgen to expand our Precision Targeted Immunotherapy Platform by acquiring exclusive rights to CDX-301 and CD40 ligand (CD40L). CDX-301 and CD40L are immune modulating molecules that increase the numbers and activity of Contents
The Companyimmune cells that control immune responses. We may also be required to pay royalties upon approvalmilestone and royalty payments to Amgen with respect to development and commercialization of this technology licensed from Amgen.
Amgen Fremont (formerly Abgenix)
In connection with the CuraGen Merger, we assumed the license agreement between CuraGen and Amgen Fremont (successor in-interest to Abgenix) to develop fully-human monoclonal antibody therapeutics. In May 2009, an amendment to the license agreement ("Amgen Amendment") was entered into related to CuraGen's exclusive rights to develop and commercialize CDX-011 and 11 other licensed antigens. Under the Amgen Amendment, CuraGen and Amgen Fremont agreed to modify the terms of their existing cross-license of antigens whereby the amended license would be fully paid-up and royalty-free (except for any product candidate. The royalties willpotentially required payments by CuraGen to the original licensor of CDX-011).
Seattle Genetics, Inc. ("Seattle Genetics")
In connection with the CuraGen Merger, we assumed the license agreement between CuraGen and Seattle Genetics whereby CuraGen acquired the rights to proprietary antibody-drug conjugate ("ADC") technology for use with its their proprietary antibodies for the potential treatment of cancer. We may be payable on a country-by-countryrequired to pay milestone and licensed product-by-licensed product basis until the dateroyalty payments to Seattle Genetics with respect to development and commercialization of the expiration of the last to expire of the patents covering the licensed product in such country.
We depend on our collaborative relationships and may enter into more of them in the future. Some of the above referenced agreements give our collaborator substantial responsibility to make decisions about the amount and timing of resources that are devoted to developing a product. As a result, we do not have complete control over how resources are used toward some of our products.
Some of these agreements relate to products in the early stages of research and development and may require Celldex and our collaborator to jointly decide on the feasibility of developing a particular product using our technologies. In either case, these agreements may terminate without benefit to us if the underlying products are not fully developed. Moreover, once specific products are chosen for development, the agreements relating to them may require Celldex to meet specified milestones, to invest money and other resources in the development process or to negotiate additional licenses and other agreements, which may not be possible or advantageous. If we fail to meet our obligations under these agreements, they could terminate and we might need to enter into relationships with other collaborators and to spend additional time, money, and other valuable resources in the process.
Moreover, we cannot predict whether our collaborators will continue their development efforts or, if they do, whether their efforts will achieve success. Many of our collaborators face the same kinds of risks and uncertainties in their business that we face. A delay or setback to a collaborator will, at a minimum, delay the commercialization of any affected products, and may ultimately prevent it. Moreover, any collaborator could breach its agreement with us or otherwise not use best efforts to promote our products. A collaborator may choose to pursue alternative technologies or products that compete with our technologies or products. In either case, if a collaborator failed to successfully develop one of our products, we would need to find another collaborator. Our ability to do so would depend upon our legal right to do so at the time and whether the product remained commercially viable.ADC technology.
I. Competition
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. Many of the products that Celldex iswe are attempting to develop and commercialize will be competing with existing therapies. In addition, a number of companies are pursuing the development of pharmaceuticals that target the same diseases and conditions that Celldex iswe are targeting. Celldex facesWe face competition from pharmaceutical and biotechnology companies both in the United States and abroad. Celldex'sOur competitors may utilize discovery technologies and techniques or partner with collaborators in order to develop products more rapidly or successfully than Celldexus or itsour collaborators are able to do. Many of Celldex'sour competitors, particularly large pharmaceutical companies, have substantially greater financial, technical and human resources than Celldex does.we do. In addition, academic institutions,
government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies and may establish exclusive collaborative or licensing relationships with Celldex'sour competitors.
Table We face intense competition in our development activities. We face competition from many companies in the United States and abroad, including a number of Contents
Severallarge pharmaceutical companies, firms specialized in the development and biotechnology companies are actively engaged inproduction of vaccines, adjuvants and vaccine and immunotherapeutic delivery systems and major universities and research and development in areas related to therapeutic vaccines, includinginstitutions. These competitors include Alexion, Anadys, Antigenics, Baxter, BioSante, Crucell, Dendreon, Eli Lilly, Emergent, Genitope, GlaxoSmithKline, Idera, Intercell, Sanofi-Aventis,Immunogen, Maxygen, Merck, NeoPharm, Northwest Biotherapeutics, Novavax, Pfizer, Roche, Genitope, Northwest Biotherapeutics, Vical, Anadys, Idera,Sanofi-Aventis, Seattle Genetics, and Cell Genesys. Celldex isVical. We are aware that Genitope, Northwest Biotherapeutics and Dendreon areis in late stage clinical trials for therapeutic vaccines for the treatment of lymphoma, GBM, melanoma and prostate cancer respectively, which may compete with CDX-1307 CDX-110 and CDX-1401. In addition, companies such as ImClone, Inc.Eli Lilly with its approved product Erbitux™ for the treatment of colorectal cancer, and Genentech, Inc.Roche with its product Herceptin® for the treatment of metastatic breast cancer, have already commercialized antibody-based products that may compete with CDX-1307, CDX-1401 and CDX-110. Various other companies are developing or commercializing products in areas that Celldex haswe have targeted for product development. Some of these products use therapeutic approaches that may compete directly with Celldex'sour product candidates. Many of these companies and institutions, either alone or together with their partners, have substantially greater financial resources and larger research and development staffs than Celldex does.we do. These companies may succeed in obtaining approvals from the Food and Drug Administration ("FDA")FDA and foreign regulatory authorities for their products sooner than Celldex doeswe do for itsour products.
Celldex isWe are aware of a number of competitive products currently available in the marketplace or under development that are used for the prevention and treatment of the diseases that Celldex haswe have targeted for product development. Various companies are currently marketing or developing biopharmaceutical products that may compete with Celldex'sour product candidates that target colorectal cancer. Product candidates Celldexwe may develop are also subject to competition in the treatment of colorectal cancer from a number of products already approved and on the market, including the following chemotherapy products: AstraZeneca PLC's Tomudex®, Hoffman-LaRoche's Xeloda® (capecitabine), Immunex Corporation's Leucovorin® calcium, ImClone Systems' Erbitux™, Pfizer, Inc.'s Camptosar® (irinotecan) and Aduracil® (5-FU), Sanofi-Synthelabo Group's Eloxatin™ (oxaliplatin), Genentech's anti-VEGF antibody, Avastin™, GlaxoSmithKline's Eniluracil™, and Titan Pharmaceuticals' CeaVac™, in the treatment of patients with advanced-stage colorectal cancer. In addition, Celldex iswe are aware that other companies such as Cell Genesys and Dendreon may be developing additional cancer vaccines that could potentially compete with other Celldexof our product candidates. CelldexWe may also face competition from Medarex and Bristol-Myers Squibb, which are developing a therapeutic vaccine for the treatment of melanoma using Medarex's MDX-010 product candidate. CelldexWe also facesface competition from a number of companies working in the fields of anti-angiogenesis and specific active immunotherapy for the treatment of solid tumor cancers. Celldex expectsWe expect that competition among specific active immunotherapy and anti-angiogenesis products approved for sale will be based on various factors, including product efficacy, safety, reliability, availability, price and patent position.
CelldexWe are aware of specific companies that are developing antibody drug conjugates (ADCs) for use in the treatment of cancer. Trastuzumab-DM1 (T-DM1) is a first-in-class HER2 antibody drug conjugate comprised of Genentech's (a Member of the Roche Group) trastuzumab antibody linked to ImmunoGen's cell-killing agent, DM1. T-DM1 combines anti-HER2 activity and targeted intracellular delivery of the potent anti-microtubule agent, DM1 (a maytansine derivative). A Phase 3 clinical trial evaluating T-DM1 for second-line HER2-positive metastatic breast cancer is planned and may be competitive with our developmental program in the breast cancer indication. Other ADCs are in development by our collaborator of the MMAE technology, Seattle Genetics, using monomethylaurastatin derivatives as the cell-killing agent in hematologic cancers and other cancers.
Marketed products that are used in the treatment of melanoma include dacarbazine, temozolamide, and interleukin-2. In addition, several other pharmaceutical and biotechnology companies are engaged in research and development for the treatment of melanoma. Many more products are on the market or in development for the treatment of metastatic breast cancer. How CDX-011 will compete with these other commercial and development stage products in metastatic melanoma and breast cancer is not clear at this time.
We also facesface competition from pharmaceutical and biotechnology companies, academic institutions, government agencies and private research organizations in recruiting and retaining highly qualified scientific personnel and consultants and in the development and acquisition of technologies. Moreover, technology controlled by third parties that may be advantageous to Celldex'sour business may be acquired or licensed by Celldex'sour competitors, thereby preventing us from obtaining technology on commercially reasonable terms, if at all. CelldexWe will also compete for the services of third parties that may have already developed or acquired internal biotechnology capabilities or made commercial arrangements with other biopharmaceutical companies to target the diseases on which Celldex haswe have focused both in the U.S. and outside of the U.S.
Our competitive position will also depend upon our ability to attract and retain qualified personnel, obtain patent protection or otherwise develop proprietary products or processes and secure sufficient capital resources for the often lengthy period between technological conception and
commercial sales. We will require substantial capital resources to complete development of some or all of our products, obtain the necessary regulatory approvals and successfully manufacture and market our products. In order to secure capital resources, we anticipate having to sell additional capital stock, which would dilute existing stockholders. We may also attempt to obtain funds through research grants and agreements with commercial collaborators. However, these types of fundings are uncertain because they are at the discretion of the organizations and companies that control the funds. As a result, we may not receive any funds from grants or collaborations. Alternatively, we may borrow funds from commercial lenders, likely at high interest rates, which would increase the risk of any investment in Celldex.us.
J. Manufacturing
We have no experience in volumelarge scale manufacturing and we have relied upon collaborators or contractors to manufacture some of our proposed products for both clinical and commercial purposes to date. We have established our own manufacturing facility in Fall River, Massachusetts, to produce antibodies, vaccines and other products that we may develop at scale for clinical trials. In order for us to establish a commercial manufacturing facility, we will require substantial additional funds and will be required to hire and retain significant additional personnel and comply with the extensive cGMP regulations of the FDA applicable to such facility. The commercial manufacturing facility would also need to be licensed for the production of antibodies, vaccines and other products by the FDA. We intend to establish manufacturing arrangements with manufacturers that comply with the FDA's requirements and other regulatory standards, although there can be no assurance that we will be able to do so.
While we believe that there is currently sufficient capacity worldwide for the production of our potential products by our collaborators or through contract manufacturers, establishing long-term relationships with contract manufacturers and securing multiple sources for the necessary quantities of clinical and commercial materials required can be a challenge. Qualifying the initial source of clinical and ultimately commercial material is a time consuming and expensive process due to the highly regulated nature of the pharmaceutical/biotech industry. These costs are hopefully mitigated in the economies of scale realized in commercial manufacture and product sale. The key difficulty in qualifying more than one source for each product is the duplicated time and expense in doing so without the potential to mitigate these costs if the secondary source is never utilized.
In connection with the Pfizer Agreement, the manufacture of CDX-110 is the responsibility of Pfizer. To date, we have been arranging withutilized contract manufacturers for the manufacture of clinical trial supplies of CDX-110, CDX-1307CDX-011 and CDX-1135. Manufacture of the rotavirus vaccine is the responsibility of Glaxo, which has received from us a world-wide exclusive license to commercialize this vaccine.
Two The two clinical lots of CDX-1307 have been manufactured and released for clinical studies by Medarex. This material is being used in Celldex's currentour completed Phase 1 clinical trials of CDX-1307.CDX-1307 were manufactured by contract manufacturers. In 2009, Celldex expects towe completed the manufacture of additional quantities of CDX-1307 in itsour Fall River facility to meet futureplanned Phase 2 clinical material requirements. The Company is currently manufacturingWe have also manufactured in our Fall River facility CDX-1401 clinical materials for our Phase 1/2 clinical trial currently enrolling and CDX-2401 clinical materials for a Rockefeller-sponsored Phase 1 clinical trial expected to begin in the secondfirst half of 2009.
We contracted with Lonza Biologics plc for process development and scale-up of CDX-1135 for clinical trials and plan to manufacture CDX-1135 clinical materials in our Fall River facility in 2009. The Walter Reed Army Institute of Research ("WRAIR") has manufactured our bacterial vaccines under collaborative agreements with us. LAHI has manufactured the Megan®Vac 1 and Megan®Egg poultry vaccines.2010.
The manufacturing processes for our other vaccine and immunotherapeutic delivery systems and vaccines utilize known technologies. We believe that the products we currently have under development can be scaled up to permit manufacture in commercial quantities. However, there can be no assurance that we will not encounter difficulties in scaling up the manufacturing processes.
Use of third party manufacturers limits our control over and ability to monitor the manufacturing process. As a result, we may not be able to detect a variety of problems that may arise and may face additional costs in the process of interfacing with and monitoring the progress of our contract manufacturers. If third party manufacturers fail to meet our manufacturing needs in an acceptable manner, we would face delays and additional costs while we develop internal manufacturing capabilities or find alternative third party manufacturers. It may not be possible to have multiple third party manufacturers ready to supply us with needed material at all or without incurring significant costs.
K. Marketing
Under the terms of existing and future collaborativepartnership agreements, we rely and expect to continue to rely on the efforts of our collaborators, including Glaxo, Pfizer, VTI, and TopoTarget/Spectrum for the sale and marketing of our products. We have agreements with, among others, Glaxo, Pfizer, Biolipox (formerly Inflazyme and AdProTech) and VTI for the development and commercialization of some of our products. The relevant aspects of these relationships have been previously discussed under the heading "G. Product Development and Licensing Agreements." There can be no assurance that our collaborators will develop and market vaccine products incorporating our technologies, or, if marketed, that such efforts will be successful. The failure of our collaborators to successfully market products would harm our business.
We have retained, and in the future intend to retain, marketing rights to some of our product candidates, including vaccine and immunotherapeutic delivery systems and vaccine candidates, in selected geographic areas and for specified indications. We intend to seek marketing and distribution agreements and/or co-promotion agreements for the distribution of our products in these geographic areas and for these indications. We believe that these arrangements could enable us to generate greater financial return than might be obtained from early stage licensing and collaboration agreements. We have no marketing and sales staff and limited experience relating to marketing and distribution of commercial products, including vaccines. If we determine in the future to engage in direct marketing of our products, we will be required to recruit an experienced marketing group, develop a supporting distribution capability and incur significant additional expenditures. There can be no assurance that we will be able to establish a successful marketing force. We may choose or find it necessary to enter into strategic partnerships on uncertain, but potentially unfavorable, terms to sell, market and distribute our products. Any delay in the marketing or distribution of our products, whether it results from problems with internal capabilities or with a collaborative relationship, could harm the value of an investment in Celldex.us.
L. Patents, Licenses and Proprietary Rights
Celldex's policyIn general, our intellectual property strategy is to protect our technology by filing patent applications and obtaining patent rights covering our own technology, both in the United States and in foreign countries.countries that we consider important to our business. In addition, we have acquired and will seek to acquire as needed or desired, exclusive rights of others through assignment or license to complement our portfolio of patent rights. We also rely on trade secrets, unpatented know-how and technological expertise and innovation to develop and maintain our competitive position.
Patents:Patents
The successful development and marketing of products by Celldexus will depend in part on our ability to create and maintain intellectual property, including patent rights. We have established a proprietary patent position in the areas of complement inhibitor technology, cholesterol regulation technology, vaccine technologies, and preservation technologies, and we are the owner or exclusive licensee to proprietary patent positions in the areas of numerous patentsvaccine technologies, antibody technologies and pending applications around the world.complement inhibitor technology. Although we continue to pursue patent protection for our products, no assurance can be given that any pending application will issue as a patent, that any issued patent will have a scope that will be of commercial benefit, or that we will be able to successfully enforce our patent position against infringers. CelldexWe routinely reviews its
review our patent portfolio and adjusts its strategies for prosecution and maintenance of individual cases according to a number of factors including program priorities, stage of development, and patent term.
Celldex ownsWe own or licenseslicense rights under more than 500400 granted patents and national and regional patent applications around the world covering inventions relating to our business. Through Celldex's acquisition ofThe key patents owned by us or licensed to us that we consider important to our business include the assets of Alteris Therapeutics, Inc., Celldex has certain exclusive rights under nine issued nationalfollowing (the indicated and estimated patent expiry dates do not include any possible Patent Term Extensions or regional patents and three pending national patent applications relating toSupplementary Protection Certificates, if these may be secured in due course):
In the area of APC targeting, through Celldex's agreements with Medarex and Rockefeller, CelldexJapan is the owner or exclusive licensee of more than 20 issued patents and more than 70 pending national and regionalcurrently under appeal. We also have rights under patent applications worldwide. Through Celldex's agreement witharound the Ludwig Institute, Celldex has an optionworld relating to obtain certainuses of CDX-110 which are currently pending. If issued and maintained to full term in a form which covers commercial rightsuse of CDX-110, the latter filings could potentially provide additional patent protection for the relevant use in connection with Celldex's APC targeting technology under more than 100 national and regional patents andthe relevant territories to 2026.
In the area of complement inhibitor technology weused in CDX-1401 which, if issued in the main designated territories and maintained to full term in due course, would have rightsestimated patent expiry dates in 2028.
US.
We also have an exclusive license to 19 issued U.S. and foreign patents directed to a rotavirus strain that has been developed by Glaxo into a commercial rotavirus vaccine.
In December 2000, we acquired Megan
In January 2003, Celldex completed licensingtyphoid vaccines expire between 2013 and acquisition agreements2016. Our patent portfolio for ETEC includes pending patent applications around the world which, gave us ownership or exclusive rightsif issued and maintained to full term in certain defined fieldsdue course, would have estimated patent expiry dates in 2028.
There can be no assurance that patent applications owned by or licensed to Celldexus will result in granted patents or that, if granted, the resultant patents will afford protection against competitors with similar technology. It is also possible that third parties may obtain patents or other proprietary rights that may be necessary or useful to Celldex.us. In cases where third parties are first to invent a particular product or technology, it is possible that those parties will obtain patents that will be sufficiently broad to prevent us from using important technology or from further developing or commercializing important vaccine and immunotherapeutic systems and vaccine candidates. If licenses from third parties are necessary but cannot be obtained, commercialization of the covered products might be delayed or prevented. Even if these licenses can be obtained, they would probably require us to pay ongoing royalties and other costs, which could be substantial.
Although a patent has a statutory presumption of validity in the United States, the issuance of a patent is not conclusive as to validity or as to the enforceable scope of the patent claims. The validity or enforceability of a patent after its issuance by the Patent and Trademark Office can be challenged in litigation. As a business that uses a substantial amount of intellectual property, we face a heightened risk of intellectual property litigation. If the outcome of the litigation is adverse to the owner of the patent, third parties may then be able to use the invention covered by the patent without authorization or payment. There can be no assurance that our issued patents or any patents subsequently issued to or licensed by us will not be successfully challenged in the future. In addition, there can be no assurance that our patents will not be infringed or that the coverage of our patents will not be successfully avoided by competitors through design innovation.
We are aware that others, including universities and companies, have filed patent applications and have been granted patents in the United States and other countries which claim subject matter potentially useful or necessary to the commercialization of our products. The ultimate scope and validity of existing or future patents which have been or may be granted to third parties, and the availability and cost of acquiring rights in those patents necessary to the manufacture, use or sale of our products presently cannot be determined by Celldex.us.
Third parties may have or may obtain valid and enforceable patents or proprietary rights that could block Celldexus from developing products using Celldex'sour technology, including:
We use a mutatedVibrio cholerae in ourThe CholeraGarde® vaccine candidate and our VibrioVec® vaccine delivery system.system utilize mutatedVibrio cholerae strains. We are aware of an issued U.S. patent which claims a culture of mutatedVibrio cholerae. We believe that only one claim (the "Claim") of the patent may be pertinent to our CholeraGarde® and VibrioVec® products. The remaining claims of the patent cover other cultures, which we believe are not pertinent to the CholeraGarde® or VibrioVec® products. We have received an opinion of counsel from Fish & Richardson, P.C. that, based on the analysis set forth in their opinion and the facts known to them, the Claim is invalid. While a party challenging the validity of a patent has the burden of proving invalidity, the outcome of any litigation cannot be predicted with certainty. Accordingly, there can be no assurance that, if litigated, a court would conclude that the Claim is invalid.
In addition to the patents referred to in the previous paragraphs, there may be other patent applications and issued patents belonging to competitors that may require us to alter our vaccine candidates and vaccine and immunotherapeutic delivery systems, pay licensing fees or cease some of our activities. If our product candidates conflict with patents that have been or may be granted to competitors, universities or others, the patent owners could bring legal action against us claiming damages and seeking to enjoin manufacturing and marketing of the patented products. If any of these actions is successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to manufacture or market the affected products. There can be no assurance that we would prevail in any such action or that any license required under any such third party patent would be made available on acceptable terms or at all. We believe that there may be significant litigation in the biotechnology and vaccine industries regarding patent and other intellectual property rights. If we become involved in that litigation, we could consume substantial resources.
Licenses:Licenses
We have entered into several significant license agreements relating to technology that is being developed by Celldexus and/or its collaborators, including licenses from the following: Johns Hopkins University, Duke University and Thomas Jefferson University relating to technology used in or
with CDX-110; Medarex and GenPharm International relating to APC Targeting Technology and antibody technology; Rockefeller relating to APC Targeting Technology; Ludwig Institute relating to tumor antigens; 3M Company relating to Toll-Like Receptor (TLR) 7/8 agonist technology; Southampton relating to modulation of CD27 activity; Apovia and Celltech R&D relating to Hepatitis B core particle technology; Corixa relating to adjuvant formulations used with Celldex's product candidate CDX-2101; Harvard University and Massachusetts General Hospital relating to proprietary technology involving genetically alteredVibrio cholerea andSalmonella strains; and Cincinnati Children's Hospital involving proprietary rights and technologies relating to an attenuated rotavirus strain for a rotavirus vaccine.
our collaborators. In general, these institutions have granted us an exclusive worldwide license (with right to sublicense) to make, use and sell products embodying the licensed technology, subject to the reservation by the licensor of a non-exclusive right to use the technologies for non-commercial research purposes. Generally, the term of each license is through the expiration of
the last of the patents issued with respect to the technologies covered by the license. We have generally agreed to use reasonable efforts to develop and commercialize licensed products and to achieve specified milestones and pay license fees, milestone payments and royalties based on the net sales of the licensed products or to pay a percentage of sublicense income. If we breach our obligations, the licensor has the right to terminate the license, and, in some cases, convert the license to a non-exclusive license. Generally, we control and are responsible for the cost of defending the patent rights of the technologies that we license.
Proprietary Rights:Rights
We also rely on unpatented technology, trade secrets and confidential information, and no assurance can be given that others will not independently develop substantially equivalent information and techniques or otherwise gain access to our know-how and information, or that we can meaningfully protect our rights in such unpatented technology, trade secrets and information. We require each of our employees, consultants and advisors to execute a confidentiality agreement at the commencement of an employment or consulting relationship with Celldex.us. The agreements generally provide that all inventions conceived by the individual in the course of employment or in providing services to Celldexus and all confidential information developed by, or made known to, the individual during the term of the relationship shall be the exclusive property of Celldexus and shall be kept confidential and not disclosed to third parties except in limited specified circumstances. There can be no assurance, however, that these agreements will provide meaningful protection for our information in the event of unauthorized use or disclosure of such confidential information.
M. Government Regulation
Our activities and products are significantly regulated by a number of governmental entities, including the FDA in the United States and by comparable authorities in other countries and by the USDA with respect to products developed for animal health and food safety.countries. These entities regulate, among other things, the manufacture, testing, safety, effectiveness, labeling, documentation, advertising and sale of our products. We must obtain regulatory approval for a product in all of these areas before we can commercialize the product. Product development within this regulatory framework takes a number of years and involves the expenditure of substantial resources. Many products that initially appear promising ultimately do not reach the market because they are found to be unsafe or ineffective when tested. Our inability to commercialize a product would impair our ability to earn future revenues.
FDA Approval Process
In the United States, vaccines and immunotherapeutics for human use are subject to FDA approval as "biologics" under the Public Health Service Act and "drugs" under the Federal Food, Drug and Cosmetic Act. The steps required before a new product can be commercialized include: pre-clinicalpreclinical studies in animals, clinical trials in humans to determine safety and efficacy and FDA approval of the product for commercial sale.
Data obtained at any stage of testing is susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. Moreover, during the regulatory process, new or changed drug approval policies may cause unanticipated delays or rejection of our product. We may not obtain necessary regulatory approvals within a reasonable period of time, if at all, or avoid delays or other problems in testing our products. Moreover, even if we received regulatory approval for a product, the approval may require limitations on use, which could restrict the size of the potential market for the product.
The FDA provides that human clinical trials may begin thirty (30) days after receipt and review of an Investigational New Drug ("IND")IND application, unless the FDA requests additional information or changes to the study protocol
within that period. An IND must be sponsored and filed by Celldexus for each of our proposed products. Authorization to conduct a clinical trial in no way assures that the FDA will ultimately approve the product. Clinical trials are usually conducted in three sequential phases. In a Phase 1 trial, the product is given to a small number of healthy volunteers to test for safety (adverse effects). Phase 2 trials are conducted on a limited group of the target patient population; safety, optimal dosage and efficacy are studied. A Phase 3 trial is performed in a large patient population over a wide geographic area to provide evidence for the safety of the product and to prove and confirm efficacy. The FDA has ongoing oversight over all these trials and can order a temporary or permanent discontinuation if warranted. Such an action could materially harm Celldex.us. Clinical tests are critical to the success of our products but are subject to unforeseen and uncontrollable delay, including delay in enrollment of patients. Any delay in clinical trials could delay our commercialization of a product.
A product's safety and effectiveness in one test is not necessarily indicative of its safety and effectiveness in another test. Moreover, we may not discover all potential problems with a product even after completing testing on it. Some of our products and technologies have undergone only pre-clinicalpreclinical testing. As a result, we do not know whether they are safe or effective for humans. Also, regulatory authorities may decide, contrary to our findings, that a product is unsafe or not as effective in actual use as its test results indicated. This could prevent the product's widespread use, require its withdrawal from the market or expose us to liability.
The results of the clinical trials and all supporting data are submitted to the FDA for approval. A Biologics License Application ("BLA") is submitted for a biologic product; a New Drug Application ("NDA") for a drug product. The interval between IND filing and BLA/NDA filing is usually at least several years due to the length of the clinical trials, and the BLA/NDA review process can take over a year. During this time the FDA may request further testing or additional trials or may turn down the application. Even with approval, the FDA frequently requires post-marketing safety studies (known as Phase 4 trials) to be performed.
The FDA requires that the manufacturing facility that produces a licensed product meet specified standards, undergo an inspection and obtain an establishment license prior to commercial marketing. Subsequent discovery of previously unknown problems with a product or its manufacturing process may result in restrictions on the product or the manufacturer, including withdrawal of the product from the market. Failure to comply with the applicable regulatory requirements can result in fines, suspensions of regulatory approvals, product recalls, operating restrictions and criminal prosecution.
InExpedited Review and Approval
The FDA has various programs, including fast track, priority review, and accelerated approval, that are intended to expedite or simplify the process for reviewing drugs, and/or provide for approval on the basis of surrogate endpoints. Generally, drugs that may be eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs, and those that offer meaningful benefits over existing treatments, however, these programs do not affect the standards for approval. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform post-marketing clinical trials.
Orphan Drug
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, vaccinesor more than 200,000 individuals in the United States and for animal health and food safety use are subject to USDA approval. The steps required before a new product can be commercialized include: pre-clinical studies in animals; clinical trials in animals to determine safety and efficacy; and USDA approval of the product for commercial sale. The registration of these vaccines may be subject to numerous delays or possibly outright rejection of the product by the agency. Delays may occur at any stage of testing, clinical results may be subject to unfavorable interpretation by the agency and regulatory approval, if received, may require limitations on use which could restrict the size of the potential market for the product. The USDA requiresthere is no reasonable expectation that the manufacturing facility that producescost of developing and making available in the United States a licensed product meetdrug for this type of disease or condition will be recovered from sales in the United
specified standards, undergo an inspection and obtain an establishment license prior to commercial marketing. Under USDA regulations, this license is held byStates for that drug. If a product that has orphan drug designation subsequently receives the manufacturer offirst FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the developer ofsame drug for the product. Failure to comply with applicable USDA regulatory requirements can resultsame indication, except in fines, suspensions of regulatory approvals, product recalls, operating restrictions and criminal prosecution.very limited circumstances, for seven years.
The Advisory CommitteeOnce an approval is granted, the FDA may withdraw the approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on Immunization Practices ("ACIP")the results of the Centers for Disease Control ("CDC") has a role in setting the public marketthese post-marketing programs.
Foreign Regulation
In addition to regulations in the United States, for the vaccine products we intend to develop. The ACIP makes recommendations on the appropriate use of vaccines and related products and the CDC develops epidemiologic data relevant to vaccine requirements and usage.
Because we may market our products abroad, we will be subject to varyinga variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory requirements. Although international efforts are being madeauthorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to harmonize these requirements, applications must currently be made in each country. The data necessarycountry and the review time vary significantly from one country to another.may be longer or shorter than that required for FDA approval. Approval by the FDA does not ensure approval by the regulatory bodies of other countries.
Under European Union regulatory systems, we may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure, which is compulsory for medicines produced by biotechnology and optional for those which are highly innovative, provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure provides for mutual recognition of national approval decisions. Under the decentralized procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the applications and assessments report each member state must decide whether to recognize approval. If a member state does not recognize the marketing authorization, the disputed points are eventually referred to the European Commission, whose decision is binding on all member states. As in the United States, we may apply for designation of our products as orphan drug for the treatment of a specific indication in the European Union before the application for marketing authorization is made. Orphan drugs in Europe enjoy economic and marketing benefits, including a 10-year market exclusivity period for the approved indication, but not for the same drug, unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan-designated product.
Our collaborators are also subject to all of the above-described regulations in connection with the commercialization of products utilizing our technology.
N. Other Regulatory Processes
We are subject to a variety of financial disclosure and securities trading regulations as a public company in the U.S., including laws relating to the oversight activities of the SEC and the regulations of the NASDAQ Global Market, on which our shares are traded. We are also subject to regulation under other federal laws and regulation under state and local laws, including laws relating to occupational safety, laboratory practices, environmental regulations, and hazardous substance control.
Product Liability
The risk of product liability claims, product recalls and associated adverse publicity is inherent in the testing, manufacturing, marketing and sale of medical products. If and when we manufacture vaccines that are recommended for routine administration to children, we will be required to participate in the National Vaccine Injury Compensation Program. This program compensates children having adverse reactions to certain routine childhood immunizations with funds collected through an excise tax from the manufacturers of these vaccines.
We haveUnder our license agreements, we are required to maintain clinical trial liability insurance coverage in the amount of $5up to $14 million. However, there can be no assurance that such insurance coverage is or will continue to be adequate or available. We may choose or find it necessary under our collaborative agreements to increase our insurance coverage in the future. We may not be able to secure greater or broader product liability insurance coverage on acceptable terms or at reasonable costs when needed. Any liability for mandatory damages could exceed the amount of our coverage. A successful product liability claim against us could require us to pay a substantial monetary award. Moreover, a product recall could generate substantial negative publicity about our products and business and inhibit or prevent commercialization of other product candidates.
O. Employees; Scientific ConsultantsEmployees
As of February 20,December 31, 2009, we employed 7890 full time persons and 3 part time or temporary persons, 1114 of whom have doctoral degrees. Of these employees, 6377 were engaged in or directly support research and development activities. Celldex'sWe believe that our employee relations are good. We believe that our future success dependswill depend in large part upon itson our ability to attract and retain employees. Celldex faces competition for employees from other companies, researchexperienced and academic institutions, government agencies and other organizations. Celldex believes that its employee relations are good.skilled employees.
Item 1A. Risk FactorsRISK FACTORS
You should consider carefully these risk factors together with all of the information included or incorporated by reference in this Annual Report in addition to our financial statements and the notes to our financial statements. This section includes forward-looking statements.
The following is a discussion of the risk factors that we believe are material to Celldexus at this time. These risks and uncertainties are not the only ones facing Celldexus and there may be additional matters that we are unaware of or that we currently consider immaterial. All of these could adversely affect our business, results of operations, financial condition and cash flows.
Risks Related to Our Business
Our products and product candidates are subject to extensive regulatory scrutiny.
All of our products and product candidates are at various stages of development and commercialization and our activities, products and product candidates are significantly regulated by a number of governmental entities, including the FDA in the United States and by comparable authorities in other countries and by the USDA in the United States with respect to products developed for animal health and food safety.countries. These entities regulate, among other things, the manufacture, testing, safety, effectiveness, labeling, documentation, advertising and sale of our products and product candidates. We or our partners must obtain regulatory approval for a product candidate in all of these areas before we can commercialize the product candidate. Product development within this regulatory framework takes a number of years and involves the expenditure of substantial resources. This process typically requires extensive pre-clinicalpreclinical and clinical testing, which may take longer or cost more than we anticipate, and may prove unsuccessful due to numerous factors. Many product candidates that initially appear promising ultimately do not reach the market because they are found to be unsafe or ineffective when tested. Companies in the pharmaceutical, biotechnology and vaccines industries have suffered
significant setbacks in advanced clinical trials, even after obtaining promising results in earlier trials. Our inability to commercialize a product or product candidate would impair our ability to earn future revenues.
If our products do not pass required tests for safety and effectiveness, we will not be able to derive commercial revenue from them.
For CelldexIn order to succeed, we will need to derive commercial revenue from the products we have under development. The FDA has not approved our CDX-110 or CDX-011 product candidatecandidates or any of our other lead products for sale to date. Products in our vaccine programs are in various stages of pre-clinicalpreclinical and clinical testing. Pre-clinicalPreclinical tests are performed at an early stage of a product's development and provide information about a product's safety and effectiveness on laboratory animals. Pre-clinicalPreclinical tests can last years. If a product passes its pre-clinicalpreclinical tests satisfactorily, and we determine that further development is warranted, we would file an investigational new drugIND application for the product with the FDA, and if the FDA gives its approval we would begin phasePhase 1 clinical tests. Phase 1 testing generally lasts between 6 and 24 months. If phasePhase 1 test results are satisfactory and the FDA gives its approval, we can begin phasePhase 2 clinical tests. Phase 2 testing generally lasts between 6 and 36 months. If phasePhase 2 test results are satisfactory and the FDA gives its approval, we can begin phasePhase 3 pivotal studies. Phase 3 studies generally last between 12 and 48 months. Once clinical testing is completed and a new drug application is filed with the FDA, it may take more than a year to receive FDA approval.
In all cases we must show that a pharmaceutical product is both safe and effective before the FDA, or drug approval agencies of other countries where we intend to sell the product, will approve it for sale. Our research and testing programs must comply with drug approval requirements both in the United States and in other countries, since we are developing our lead products with companies, including Glaxo and Pfizer, which intend to or could later decide to commercialize them both in the U.S. and abroad. A product may fail for safety or effectiveness at any stage of the testing process. A major risk we face is the possibility that none of our products under development will come through the testing process to final approval for sale, with the result that we cannot derive any commercial revenue from them after investing significant amounts of capital in multiple stages of pre-clinicalpreclinical and clinical testing.
Product testing is critical to the success of our products but subject to delay or cancellation if we have difficulty enrolling patients.
As our portfolio of potential products moves from pre-clinicalpreclinical testing to clinical testing, and then through progressively larger and more complex clinical trials, we will need to enroll an increasing number of patients with the appropriate characteristics. At times we have experienced difficulty enrolling patients and we may experience more difficulty as the scale of our clinical testing program increases. The factors that affect our ability to enroll patients are largely uncontrollable and include principally the following:
If we cannot enroll patients as needed, our costs may increase or it could force us to delay or terminate testing for a product.
Any delay in obtaining regulatory approval would have an adverse impact on our ability to earn future revenues.
It is possible that none of the products or product candidates that we develop will obtain the regulatory approvals necessary for us to begin commercializing them. The time required to obtain FDA and other approvals is unpredictable but often can take years following the commencement of clinical trials, depending upon the nature of the product candidate. Any analysis we perform of data from clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. Any delay or failure in obtaining required approvals could have a material adverse effect on our ability to generate revenues from the particular product candidate. Furthermore, if we, or our partners, do not reach the market with our products before our competitors offer products for the same or similar uses, or if we, or our partners, are not effective in marketing our products, our revenues from product sales, if any, will be reduced.
We face intense competition in our development activities. We face competition from many companies in the United States and abroad, including a number of large pharmaceutical companies, firms specialized in the development and production of vaccines, adjuvants and vaccine and immunotherapeutic delivery systems and major universities and research institutions. These competitors include Anitgenics,Alexion, Anadys, Antigenics, Baxter, BioSante, Crucell, Dendreon, Eli Lilly, Emergent, Genitope, GlaxoSmithKline, Idera, Intercell, Sanofi-Aventis,Immunogen, Maxygen, Merck, NeoPharm, Northwest Biotherapeutics, Novavax, Pfizer, Roche, Genitope, Northwest Biotherapeutics, VicalSanofi-Aventis, Seattle Genetics, and Cell Genesys.Vical. Most of our competitors have substantially greater resources, more extensive experience in conducting pre-clinicalpreclinical studies and clinical testing and obtaining regulatory approvals for their products, greater operating experience, greater research and development and marketing capabilities and greater production capabilities than those of Celldex.ours. These companies might succeed in obtaining regulatory approval for competitive products more rapidly than we can for our products, especially if we experience any delay in obtaining required regulatory approvals.
Failure to comply with applicable regulatory requirements would adversely impact our operations.
Even after receiving regulatory approval, our products would ebbe subject to extensive regulatory requirements, and our failure to comply with applicable regulatory requirements will adversely impact our operations. In the United States, the FDA and USDA, as applicable, requirerequires that the
manufacturing facility that produces a product meet specified standards, undergo an inspection and obtain an establishment license prior to commercial marketing. Under USDA regulations, this license is held by the manufacturer of the product and not the developer of the product. Subsequent discovery of previously unknown problems with a product or its manufacturing process may result in restrictions on the product or the manufacturer, including withdrawal of the product from the market. Failure to comply with the applicable regulatory requirements can result in fines, suspensions of regulatory approvals, product recalls, operating restrictions and criminal prosecution.
We depend greatly on the intellectual capabilities and experience of our key executives and scientists and the loss of any of them could affect our ability to develop our products.
The loss of Anthony S. Marucci, our President and Chief Executive Officer, or other key members of our staff, including Avery W. Catlin, our Chief Financial Officer, Dr. Thomas Davis, our Chief Medical officer,Officer, or Dr. Tibor Keler, our Chief Scientific Officer, could harm us. We entered into employment agreements with Messrs. Marucci, Catlin, Davis and Keler. We also depend on our scientific and clinical collaborators and advisors, all of whom have outside commitments that may limit their availability to us. In addition, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled scientific, managerial and marketing personnel, particularly as we expand our activities in clinical trials, the regulatory approval process and sales and manufacturing. We routinely enter into consulting agreements with our scientific and clinical collaborators and advisors, opinion leaders and heads of academic departments in the ordinary course
of our business. We also enter into contractual agreements with physicians and institutions who recruit patients into our clinical trials on our behalf in the ordinary course of our business. Notwithstanding these arrangements, we face significant competition for this type of personnel from other companies, research and academic institutions, government entities and other organizations. We cannot predict our success in hiring or retaining the personnel we require for continued growth.
We rely on contract manufacturers. Should the cost, delivery and quality of clinical and commercial grade materials supplied by contract manufacturers vary to our disadvantage, our business operations could suffer significant harm.
Although we have small-lot manufacturing capability at our Fall River facility, we have in the past relied on, and expect to continue to rely on sourcing from third-party manufacturers for suitable quantities of some of our clinical and commercial grade materials essential to pre-clinicalpreclinical and clinical studies currently underway and to planned clinical trials in addition to those currently being conducted by third parties or us. The inability to have suitable quality and quantities of these essential materials produced in a timely manner would result in significant delays in the clinical development and commercialization of products, which could adversely affect our business, financial condition and results of operations. We mayalso rely on collaborators and contract manufacturers to manufacture proposed products in both clinical and commercial quantities in the future. Our leading vaccine candidates require specialized manufacturing capabilities and processes.
We have faced difficultiesmay face difficulty in securing commitments from U.S. and foreign contract manufacturers as these manufacturers have at times beencould be unwilling or unable to accommodate our needs. Relying on foreign manufacturers involves peculiar and increased risks, and on one occasion we had to terminate a contract with a foreign manufacturer and find a substitute source of material for planned clinical trials. These peculiar and increased risks include risksincluding the risk relating to the difficultiesdifficulty foreign manufacturers may face in complying with the FDA's Good Manufacturing Practices or GMP,("GMP") as a result of language barriers, lack of familiarity with GMP or the FDA regulatory process or other causes, economic or political instability in or affecting the home countries of our foreign manufacturers, shipping delays, potential changes in foreign regulatory laws governing the sales of our product supplies, fluctuations in foreign currency exchange rates and the imposition or application of trade restrictions.
There can be no assurances that we will be able to enter into long-term arrangements with third party manufacturers on acceptable terms, or at all. Further, contract manufacturers must also be able to meet our timetable and requirements, and must operate in compliance with GMP; failure to do so could result in, among other things, the disruption of product supplies. As noted above, non-U.S. contract manufacturers may face special challenges in complying with the FDA's GMP requirements, and although we are not currently dependent on non-U.S. collaborators or contract manufacturers, we may choose or be required to rely on non-U.S. sources in the future as we seek to develop stable supplies of increasing quantities of materials for ongoing clinical trials of larger scale. Our dependence upon third parties for the manufacture of our products may adversely affect our profit margins and our ability to develop and deliver products on a timely and competitive basis.
Prior to the establishment in 2008The significant third-parties who we currently rely on for sourcing of our own in-house antibody manufacturing capabilities at our Fall River facility, we had depended on third party suppliers and manufacturers, including Medarex, Biosyn Corporation, American Peptide Company, AmbioPharm, Inc., WRAIR, Lonza Biologics plc, Bioconcept, Inc., NeoMPS, Inc. and LAHI, to provide us with suitable quantities of some of our clinical and commercial grade materials necessaryinclude:
If these materialswe or our third-party manufacturers are not availableunable to produce drug material in suitable quantities of appropriate quality, in a timely manner, and at a feasible cost, our clinical tests will face delays.
We rely on third parties to plan, conduct and monitor our clinical tests, and their failure to perform as required would interfere with our product development.
We have reliedrely on third parties including, among others, Omnicare, Inc., Accelovance, the International Center for Diarrhoeal Disease Research, Bangladesh, the International Vaccines Institute, Cincinnati Children's Hospital Medical Center, The Cleveland Clinic, Radiant Research, Inc., Biobridges, LLC, Glaser Research Group, the NIH, Pfizer, Inc. and Glaxo to conduct thea significant majorityportion of our clinical research development activities. These activities can be characterized asinclude clinical patient recruitment and observation, clinical trial monitoring, clinical data management and analysis, safety monitoring and project management. We conduct approximately 75% of our project management and 50% of our medical and safety monitoring in-house for some of our programs and rely on third parties for the remainder of our clinical development activities. Our significant third-party clinical development providers include Pfizer for the development of our CDX-110 drug product.
If any of these third parties fails to perform as we expect or if their work fails to meet regulatory standards, our testing could be delayed, cancelled or rendered ineffective.
We depend greatly on third party collaborators to license, develop and commercialize some of our products, and they may not meet our expectations.
We have agreements with other companies, including Glaxo, Pfizer Biolipox and VTI for the licensing, development and ultimate commercialization of some of our products. Some of those agreements give substantial responsibility over the products to the collaborator. Some collaborators may be unable or unwilling to devote sufficient resources to develop our products as their agreements require. They often face business risks similar to ours, and this could interfere with their efforts. Also, collaborators may choose to devote their resources to products that compete with ours. If a collaborator does not successfully develop any one of our products, we will need to find another collaborator to do so. The success of our search for a new collaborator will depend on our legal right to do so at the time and whether the product remains commercially viable.
The success of our vaccine candidatesproducts depends in great part upon our and our collaborators' success in promoting them as superior to other treatment alternatives. We believe that vaccines like those under development by Celldexour products can be proven to offer disease prevention and treatment with notable advantages over drugs in terms of patient compliance and effectiveness. However, there can be no assurance that we will be able to prove these advantages or that the advantages will be sufficient to support the successful commercialization of our vaccines.
Table of Contentsproducts.
We may face delays, difficulties or unanticipated costs in establishing sales, distribution and manufacturing capabilities for our commercially ready products.
To date, we have chosen to retain, rather than license, all rights to some of our lead products, such as CDX-011 and our APC Targeting Technology programs. If we proceed with this strategy, we will have full responsibility for commercialization of these products if and when they are approved for sale. We currently lack the marketing, sales and distribution capabilities that we will need to carry out this strategy. To market any of our products directly, we must develop a substantial marketing and sales force with technical expertise and a supporting distribution capability. We have little expertise in this area, and we may not succeed. We may find it necessary to enter into strategic partnerships on uncertain but potentially unfavorable terms to sell, market and distribute our products when they are approved for sale.
Some of our products are difficult to manufacture, especially in large quantities, and we have not yet developed commercial scale manufacturing processes for any of our products. We do not currently plan to develop internal manufacturing capabilities to produce any of our products at commercial scale if they are approved for sale. To the extent that we choose to market and distribute these products ourselves, this strategy will make us dependent on other companies to produce our products in adequate quantities, in compliance with regulatory requirements, and at a competitive cost. We may not find third parties capable of meeting those manufacturing needs.
Certain factors could negatively affect the demand for and sales and profitability of Rotarix®, which would have a material adverse affect on our revenues.
Both the demand and ultimately the profitability of Rotarix® are components to our success. We have licensed a rotavirus strain to Glaxo for the purposes of Glaxo developing and commercializing their Rotarix® vaccine worldwide. Glaxo gained approval for Rotarix® in Mexico in July 2004, in the European Union in February 2006 and in the United States in April 2008. In May 2005, Celldexwe entered into an agreement whereby an affiliate of PRF purchased an interest in the net royalties we will receive on worldwide sales of Rotarix® (see Note 10 of our audited consolidated financial statements) and we retained 50% of Glaxo milestone payments, with the balance payable to PRF and CCH.. In addition, Celldex retains substantialwe retain upside participation in the worldwide net royalty streamroyalties from Rotarix® once, and if, worldwide net royalties once PRF receives an agreed upon return on capital invested (2.45 times PRF's aggregate cash payments to Celldexus of $60 million). The following are potential factors, among others, that may negatively affect the demand for Rotarix®:
Any of these factors could have a material adverse effect on the sales of Rotarix® and our results of operations.
Other factors could affect the demand for and sales and profitability of Rotarix® and any other of our current or future products.
In general, other factors that could affect the demand for and sales and profitability of our products include, but are not limited to:
Any of these factors could have a material adverse effect on Glaxo's sales of Rotarix® and on any other of our current or future products and results of operations.
We may be unable to manage multiple late stage clinical trials for a variety of product candidates simultaneously.
As our current clinical trials progress, we may need to manage multiple late stage clinical trials simultaneously in order to continue developing all of our current products. The management of late stage clinical trials is more complex and time consuming than early stage trials. Typically early stage trials involve several hundred patients in no more than 10-2010-30 clinical sites. Late stage (Phase 3) trials may involve up to several thousand patients in up to several hundred clinical sites and may require facilities in several countries. Therefore, the project management required to supervise and control such an extensive program is substantially larger than early stage programs. As the need for these resources is not known until some months before the trials begin it is necessary to recruit large numbers of experienced and talented individuals very quickly. If the labor market does not allow this team to be recruited quickly the sponsor is faced with a decision to delay the program or to initiate it with inadequate management resources. This may result in recruitment of inappropriate patients, inadequate monitoring of clinical investigators and inappropriate handling of data or data analysis. Consequently it is possible that conclusions of efficacy or safety may not be acceptable to permit filing of a BLA or NDA for any one of the above reasons or a combination of several.
We face the risk of product liability claims, which could exceed our insurance coverage, and produce recalls, each of which could deplete our cash resources.
As a participant in the pharmaceutical, biotechnology and vaccines industries, we are exposed to the risk of product liability claims alleging that use of our products or product candidates caused an
injury or harm. These claims can arise at any point in the development, testing, manufacture, marketing or sale of our products or product candidates and may be made directly by patients involved in clinical trials of our products, by consumers or healthcare providers or by individuals, organizations or companies selling our products. Product liability claims can be expensive to defend, even if the product or product candidate did not actually cause the alleged injury or harm.
Insurance covering product liability claims becomes increasingly expensive as a product candidate moves through the development pipeline to commercialization. We haveUnder our license agreements, we are required to maintain clinical trial liability insurance coverage in the amount of $5up to $14 million. However, there can be no assurance that such insurance coverage is or will continue to be adequate or available to us at a cost acceptable to us or at all. We may choose or find it necessary under our collaborative agreements to increase our insurance coverage in the future. We may not be able to secure greater or broader product liability insurance coverage on acceptable terms or at reasonable costs when needed. Any liability for damages resulting from a product liability claim could exceed the amount of our coverage, require us to pay a substantial monetary award from our own cash resources and have a material adverse effect on our business, financial condition and results of operations. Moreover, a product recall, if required, could generate substantial negative publicity about our products and business and inhibit or prevent commercialization of other products and product candidates.
In addition, some of our licensing and other agreements with third parties require or might require us to maintain product liability insurance. If we cannot maintain acceptable amounts of coverage on commercially reasonable terms in accordance with the terms set forth in these agreements, the corresponding agreements would be subject to termination, which could have a material adverse impact on our operations.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them.
Because we rely on third parties to develop our products, we must share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements will typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are typically controlled exclusively by us, although in some cases we may share these rights with other parties. We also conduct joint research and development programs which may require us to share trade secrets under the terms of research and development partnership or similar agreements. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. A competitor's discovery of our trade secrets would impair our competitive position.
We may not be able to successfully integrate newly-acquired technology with our existing technology or to modify our technologies to create new vaccines.
As part of our acquisition of technology assets from entities such as 3M Company and Amgen, we have acquired access to Resiquimod™ (a TLR7/ 7/8 agonists) agonist) and Flt3L, which may improve the immunogenicity of our vaccines. If we are able to integrate these licensed assets with our vaccine technologies, we believe these assets will give Celldex'sour vaccines a competitive advantage. However, if we are unable to successfully integrate licensed assets, or other technologies which we have acquired or may acquire in
the future, with our existing technologies and potential products currently under development, we may be unable to realize any benefit from our acquisition of these assets, or other technologies which we have acquired or may acquire in the future and may face the loss of our investment of financial resources and time in the integration process.
We believe that Celldex'sour vaccine technology portfolio may offer opportunities to develop vaccines that treat a variety of oncology, inflammatory and infectious diseases by stimulating a patient's immune system against those disease organisms. If our vaccine technology portfolio cannot be used to create effective vaccines against a variety of disease organisms, we may lose all or portions of our investment in development efforts for new vaccine candidates.
We license technology from other companies to develop products, and those companies could influence research and development or restrict our use of it.
Companies that license technologies to us that we use in our research and development programs may require us to achieve milestones or devote minimum amounts of resources to develop products using those technologies. They may also require us to make significant royalty and milestone payments, including a percentage of any sublicensing income, as well as payments to reimburse them for patent costs. The number and variety of our research and development programs require us to establish priorities and to allocate available resources among competing programs. From time to time we may choose to slow down or cease our efforts on particular products. If in doing so we fail to fully perform our obligations under a license, the licensor can terminate the licenses or permit our competitors to use the technology. Moreover, we may lose our right to market and sell any products based on the licensed technology.
We have many competitors in our field and they may develop technologies that make ours obsolete.
Biotechnology, pharmaceuticals and therapeutics are rapidly evolving fields in which scientific and technological developments are expected to continue at a rapid pace. We have many competitors in the U.S. and abroad, includingabroad. These competitors include Alexion, Anadys, Antigenics, Baxter, BioSante, Crucell, Dendreon, Eli Lilly, Emergent, BioSolutions,Genitope, GlaxoSmithKline, Idera, Intercell, Sanofi-Aventis,Immunogen, Maxygen, Merck, NeoPharm, Northwest Biotherapeutics, Novavax, Pfizer, Roche, Genitope, Northwest Biotherapeutics, Vical, Anadys, Idera,Sanofi-Aventis, Seattle Genetics, and Cell Genesys.Vical. Our success depends upon our ability to develop and maintain a competitive position in the product categories and technologies on which we focus. Many of our competitors have greater capabilities, experience and financial resources than we do. Competition is intense and is expected to increase as new products enter the market and new technologies become available. Our competitors may:
We rely on patents, patent applications and other intellectual property protections to protect our technology and trade secrets; which are expensive and may not provide sufficient protection.
Our success depends in part on our ability to obtain and maintain patent protection for technologies that we use. Biotechnology patents involve complex legal, scientific and factual questions and are highly uncertain. To date, there is no consistent policy regarding the breadth of claims allowed in biotechnology patents, particularly in regard to patents for technologies for human uses like those we use in our business. We cannot predict whether the patents we seek will issue. If they do issue, a competitor may challenge them and limit their scope. Moreover, our patents may not afford effective
protection against competitors with similar technology. A successful challenge to any one of our patents could result in a third party's ability to use the technology covered by the patent. We also face the risk that others will infringe, avoid or circumvent our patents. Technology that we license from others is subject to similar risks and this could harm our ability to use that technology. If we, or a company that licenses technology to us, were not the first creator of an invention that we use, our use of the underlying product or technology will face restrictions, including elimination.
If we must defend against suits brought against us or prosecute suits against others involving intellectual property rights, we will incur substantial costs. In addition to any potential liability for significant monetary damages, a decision against us may require us to obtain licenses to patents or other intellectual property rights of others on potentially unfavorable terms. If those licenses from third parties are necessary but we cannot acquire them, we would attempt to design around the relevant technology, which would cause higher development costs and delays, and may ultimately prove impracticable.
Our business requires us to use hazardous materials, which increases our exposure to dangerous and costly accidents.
Our research and development activities involve the use of hazardous chemicals, biological materials and radioactive compounds. Although we believe that our safety procedures for handling and disposing of hazardous materials comply with the standards prescribed by applicable laws and regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of an accident, an injured party will likely sue us for any resulting damages with
potentially significant liability. The ongoing cost of complying with environmental laws and regulations is significant and may increase in the future.
Health care reform and restrictions on reimbursement may limit our returns on potential products.
Because Celldex'sour strategy ultimately depends on the commercial success of our products, we assume, among other things, that end users of our products will be able to pay for them. In the United States and other countries, in most cases, the volume of sales of products like those we are developing depends on the availability of reimbursement from third-party payors, including national health care agencies, private health insurance plans and health maintenance organizations. Third-party payors increasingly challenge the prices charged for medical products and services. Accordingly, if we succeed in bringing products to market, and reimbursement is not available or is insufficient, we could be prevented from successfully commercializing our potential products.
The health care industry in the United States and in Europe is undergoing fundamental changes as a result of political, economic and regulatory influences. Reforms proposed from time to time include mandated basic health care benefits, controls on health care spending, the establishment of governmental controls over the cost of therapies, creation of large medical services and products purchasing groups and fundamental changes to the health care delivery system. We anticipate ongoing review and assessment of health care delivery systems and methods of payment in the United States and other countries. We cannot predict whether any particular reform initiatives will result or, if adopted, what their impact on us will be. However, we expect that adoption of any reform proposed will impair our ability to market products at acceptable prices.
Changes in laws affecting the health care industry could adversely affect our business.
In the U.S., there have been numerous proposals considered at the federal and state levels for comprehensive reforms of health care and its cost, and it is likely that federal and state legislatures and health agencies will continue to focus on health care reform in the future. Congress has considered legislation to reform the U.S. health care system by expanding health insurance coverage, reducing health care costs and making other changes. While health care reform may increase the number of patients who have insurance coverage for our products, it may also include cost containment measures that adversely affect reimbursement for our products. Congress has also considered legislation to change the Medicare reimbursement system for outpatient drugs, increase the amount of rebates that manufacturers pay for coverage of their drugs by Medicaid programs and facilitate the importation of lower-cost prescription drugs that are marketed outside the U.S. Some states are also considering legislation that would control the prices of drugs, and state Medicaid programs are increasingly requesting manufacturers to pay supplemental rebates and requiring prior authorization by the state program for use of any drug for which supplemental rebates are not being paid. Managed care organizations continue to seek price discounts and, in some cases, to impose restrictions on the coverage of particular drugs. Government efforts to reduce Medicaid expenses may lead to increased use of managed care organizations by Medicaid programs. This may result in managed care organizations influencing prescription decisions for a larger segment of the population and a corresponding constraint on prices and reimbursement for our products.
We and our collaborators and partners operate in a highly regulated industry. As a result, governmental actions may adversely affect our business, operations or financial condition, including:
The enactment in the U.S. of health care reform, possible legislation which could ease the entry of competing follow-on biologics in the marketplace, new legislation or implementation of existing statutory provisions on importation of lower-cost competing drugs from other jurisdictions, and legislation on comparative effectiveness research are examples of previously enacted and possible future changes in laws that could adversely affect our business. In addition, the Food and Drug Administration Amendments Act of 2007 included new authorization for the FDA to require post-market safety monitoring, along with an expanded clinical trials registry and clinical trials results database, and expanded authority for the FDA to impose civil monetary penalties on companies that fail to meet certain commitments.
If physicians, patients and third-party payors do not accept any future drugs that we may develop, we may be unable to generate significant revenue, if any.
Even if our drug candidates as well as any drug candidates that we may develop or acquire in the future obtain regulatory approval, they may not gain market acceptance among physicians, patients and health care payors. Physicians may elect not to recommend these drugs for a variety of reasons including:
If our approved drugs fail to achieve market acceptance, we would not be able to generate sufficient revenue from product sales to maintain or grow our business.
If we are not successful in integrating CuraGen's organization, we may not be able to operate efficiently after the CuraGen Merger, which may harm the value of our common stock.
Achieving the benefits of the CuraGen Merger will depend in part on the successful integration of CuraGen's clinical and preclinical programs and personnel in a timely and efficient manner. The integration process requires coordination of different development, regulatory, and manufacturing teams, and involves the integration of systems, applications, policies, procedures, business processes and operations. This may be difficult and unpredictable because of possible cultural conflicts and different opinions on scientific and regulatory matters. If we cannot successfully integrate CuraGen's programs and personnel, we may not realize the expected benefits of the CuraGen Merger.
Integrating CuraGen's programs may divert management's attention away from our operations.
The successful integration of CuraGen's programs and personnel may place a significant burden on our management and internal resources, including time that will be spent on winding down CuraGen's facility in Connecticut and transitioning certain CuraGen employees to our facilities. The diversion of management's attention and any difficulties encountered in the transition and integration process could result in delays in our clinical trial programs and could otherwise harm our business, financial condition and operating results.
Risks Related to Our Capital Stock
Our history of losses and uncertainty of future profitability make our common stock a highly speculative investment.
We have had no commercial revenues to date from sales of our human therapeutic or vaccine products and cannot predict when we will. We have an accumulated net operating lossesdeficit of approximately
$121.1$157.7 million as of December 31, 2008.2009. We expect to spend substantial funds to continue the research and productdevelopment testing of the followingour products that we have in the pre-clinicalpreclinical and clinical testing stages of development:development that have not been partnered.
| ||||
| ||||
| ||||
| ||||
| ||||
| ||||
| ||||
|
In anticipation of FDA approval of these products, we will need to make substantial investments to establish sales, marketing, quality control, and regulatory compliance capabilities. These investments will increase if and when any of these products receive FDA approval. We cannot predict how quickly our lead products will progress through the regulatory approval process. As a result, we may continue to lose money for several years.
We cannot be certain that the companywe will achieve or sustain profitability in the future. Failure to achieve profitability could diminish our ability to sustain operations, pay dividends on our common stock, obtain additional required funds and make required payments on our present or future indebtedness.
If we cannot sell capital stock to raise necessary funds, we may be forced to limit our research, development and testing programs.
We will need to raise more capital from investors to advance our leadclinical and preclinical products through clinical testing and to fund our operations until we receive final FDA approval and our products begin to generate revenues for us. However, based on our history of losses and the on-going uncertainty of the U.S. capital markets, we may have difficulty raising sufficient capital on terms that are acceptable to us, or at all. As of December 31, 2008,2009, we had cash, and cash equivalents and marketable securities of $44.3$82.5 million, which, at that time, we believed would support expected operations for more than 12 months.
We continue to seek partnerships with pharmaceutical and biotech companies and with other organizations to support the clinical development of our programs, in addition to funded research grants. This kind of funding is at the discretion of other organizations and companies which have limited funds and many companies compete with us for those funds. As a result, we may not receive any research grants or funds from collaborators. If we are unable to raise the necessary funds, we may have to delay or discontinue the clinical development of programs, license out programs earlier than expected, raise funds at significant discount or on other unfavorable terms, if at all, or evaluate a sale of all or part of our business.
Until the Company beginswe begin generating revenue, itwe may seek funding through the sale of equity, or securities convertible into equity, and further dilution to the then existing stockholders may result. If the Company raiseswe raise additional capital through the incurrence of debt, itsour business may be affected by the amount of leverage it incurs, and its borrowings may subject it to restrictive covenants.
Our share price has been and could remain volatile.
The market price of our common stock has historically experienced and may continue to experience significant volatility. From January 20082009 through December 2008,2009, the market price of our common stock has fluctuated from a high of $19.79$14.19 per share in the second quarter of 2008,2009, to a low of $4.24$4.16 per share in the fourth quarter of 2008.2009. Our progress in developing and commercializing our products, the impact of government regulations on our products and industry, the potential sale of a large volume of our common stock by selling stockholders, our quarterly operating results, changes in general conditions in the economy or the financial markets and other developments affecting us or our competitors could cause the market price of our common stock to fluctuate substantially with significant market losses. If our stockholders sell a substantial number of shares of common stock, especially if those sales are made during a short period of time, those sales could adversely affect the market losses occurring over the past year.price of our common stock and could impair our ability to raise capital. In addition, in recent years, the stock market has experienced significant price and volume fluctuations. This volatility has affected the market prices of securities issued by many companies for reasons unrelated to their operating performance and may adversely affect the price of our common stock. In addition, we could be subject to a securities class action litigation as a result of volatility in the price of our stock, which could result in substantial costs and diversion of management's attention and resources and could harm our stock price, business, prospects, results of operations and financial condition.
If our principal stockholders sell shares of common stock in large volumes, the trading price of our common stock could suffer.
If our principal stockholders sell a substantial number of shares of common stock, especially if those sales are made during a short period of time, those sales could adversely affect the market price of our common stock and could impair our ability to raise capital. As of February 20, 2009, Medarex, Inc. owned approximately 31.4% of our outstanding common stock, Apax WW Nominees Ltd. owned approximately 8.8%, and Pfizer Vaccines owned approximately 4.9%. Our officers and directors, and their affiliates, beneficially owned approximately 6.86% of our common stock as of February 20, 2009. Of our largest stockholders, only Medarex is subject to a "lock-up" agreement pursuant to which it has agreed not to sell shares of common stock, which, unless extended, expires on March 7, 2009. There can be no assurances that the Medarex lock-up will be extended.
Our principal stockholders, officers and directors own a large percentage of our voting stock and could exert significant influence over matters requiring stockholder approval.
As of February 20, 2009, Medarex, Inc., Apax WW Nominees Ltd., Pfizer Vaccines and our officers and directors, together beneficially owned approximately 52% of our common stock. Accordingly, these stockholders will be able to exert significant influence over matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other business combinations. This concentration could have the effect of delaying or preventing a change in control of the company.
The combined company's ability to use theour net operating loss carryforwards of the Company and its subsidiaries will be subject to limitation and, under certain circumstances, may be eliminated.
Generally, aUtilization of the net operating loss ("NOL") and R&D credit carryforwards may be subject to substantial annual limitation due to ownership change limitations that have occurred previously or that could occur in the future provided by Section 382 of more than 50% inthe Internal Revenue Code of 1986 ("Section 382"), as well as similar state provisions. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation's stock,corporation by value,more than 50 percentage points over a three-year period constitutes anperiod.
In October 2007, June 2009 and in December 2009, Celldex Research experienced a change in ownership change underas defined by Section 382 of the Internal Revenue Code. In general,Celldex Research, since its formation, has raised capital through the issuance of capital stock on several occasions which, combined with shareholders' subsequent disposition of those shares, has resulted in three changes of control, as defined by Section 382 imposes an annual limitation on a corporation's ability to use its net operating losses from taxable years or periods ending on or before the date of an ownership change to offset U.S. federal taxable income in any post-change year. The Company and its subsidiaries have experienced an ownership change as a result of the Merger, in which case the combined company may be subject to the limitation under Section 382 with respect to pre-change net operating losses of the Company and its subsidiaries. Section 382 imposes significant limitations of the use of net operating loss carryforwards.
Moreover, if a corporation experiences an ownership change and does not satisfy the requirement to continue the business enterprise of the corporation under Section 382(c)(1) (which generally requires that the corporation continue its historic business or use a significant portion of its historic business assets in a business for the two-year period beginning on the date of the ownership change), it cannot, subject to certain exceptions, use any net operating loss from a pre-change period to offset taxable income in post-change years.382. As a result of the rules described above,ownership change in October 2007, utilization of its Federal NOLs is subject to an annual limitation. Any unused annual limitation may be carried over to later years, and the extent (if any)amount of the limitation may, under certain circumstances, be subject to whichadjustment if the combined company willfair value of the our net assets are determined to be able to utilizebelow or in excess of the tax basis of such assets at the time of the ownership change, and such unrealized loss or gain is recognized during the five-year period after the ownership change. Subsequent ownership changes, as defined in Section 382, could further limit the amount of net operating losses from any pre-change periodloss carryforwards and research and development credits that can be utilized annually to offset future taxable income.
We have not undertaken a study to assess whether an ownership change or multiple ownership changes has occurred for (i) AVANT, (ii) CuraGen, (iii) Celldex Research on the state level, or (iv) R&D credits. If there has been an ownership change at any time since its formation, utilization of NOL or tax credit carryforwards would be subject to an annual limitation under Section 382.
Refer to Note 9, "Income Taxes," in the accompanying notes to the consolidated financial statements for additional discussion on income (and thus reduce tax liability) for post-change periods is uncertain.taxes.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
In November 2005, we entered into aWe lease amendment which extended our lease in Needham, Massachusetts through April, 2017. The lease amendment called for the complete renovation of the Needham facility by the landlord and Celldex, which was completed in 2007, and reduced our leased space to approximately 35,200 square feet of laboratory and office space. Under this lease amendment, we are obligated to pay an escalating base annual rent ranging from $879,700 to $1,161,200 during the extension term. Aggregate rental payments including common area maintenance costs for the years ended December 31, 2008 and 2007 for this facility were $1,437,040 and $1,911,088, respectively.
Celldex leases approximately 20,00081,600 square feet of office, manufacturing and laboratory space in Phillipsburg, New Jersey. The lease has an initial sixty-four month term which expires in August 2011. Under the lease agreement, wespace. Our major leased properties are obligated to pay an annual rentdescribed below:
Property Location | Approximate Square Feet | Use | Lease Expiration Date | ||||
|---|---|---|---|---|---|---|---|
| Needham, Massachusetts | 35,200 | Office Headquarters and Laboratory | April 2017 | ||||
| Fall River, Massachusetts | 23,400 | Manufacturing Facility | December 2010(1) | ||||
| Phillipsburg, New Jersey | 20,000 | Office and Laboratory | August 2011 | ||||
| New Haven, Connecticut | 3,000 | Office | January 2013 | ||||
We also lease a manufacturing facility of approximately 21,000 square feet in Fall River, Massachusetts. The lease has an initial seven-year term which expires in December 2010. Under the lease agreement and subsequent lease amendments, we are obligated to pay an annual rent of approximately $305,500 plus certain common area maintenance costs, subject to annual rent adjustments in the final two years. Aggregate rental payments including common area maintenance costs for the years ended December 31, 2008 and 2007 for this facility were $390,664 and $366,654, respectively.
The Company ceased operations at its Overland, Missouri facility near St. Louis and vacated the premises upon expiration of the lease term at September 30, 2007.
Following the announcement of the proposed acquisition by Celldex is not currentlyof CuraGen, a partyputative class action complaint,Margaret Capps v. Timothy Shannon, et al., was filed in the Connecticut Superior Court, Judicial District of New Haven, on June 9, 2009. A second putative class action complaint,Cheryl Smith v. CuraGen Corporation, et al., was filed in the Court of Chancery of the State of Delaware on June 15, 2009. Both lawsuits purported to any material legal proceedings.have been brought on behalf of all public stockholders of CuraGen, and named CuraGen, all of its former directors, Celldex, and Celldex's merger subsidiary as defendants. On July 21, 2009, the attorneys for the parties in the two actions executed a memorandum of understanding (the "MOU") pursuant to which such actions were subsequently dismissed with prejudice. CuraGen agreed to make certain revisions to the joint proxy statement/prospectus (which was prepared in connection with the approval by CuraGen's stockholders of the merger and by Celldex's stockholders of the stock issued to the former stockholders of CuraGen in the merger) as part of the agreement among the parties to settle the actions and agreed to pay attorneys' fees and expenses as awarded by the court, which had been expected to be $0.3 million but ultimately were reduced to $0.2 million. On August 27, 2009, a stipulation of settlement was submitted to the court for the action captionedCheryl Smith v. CuraGen Corporation, et al, pending in the Court of Chancery of the State of Delaware, and thereafter a fairness hearing was held on November 9, 2009, at which the court approved the settlement of the Delaware action and thereafter both the Delaware and Connecticut actions were dismissed.
CuraGen Corporation's former landlord filed a complaint in the Connecticut Superior Court, Judicial District of New Haven, on September 9, 2009, in case captionedT.K.J. Associates LLC v. CuraGen Corporation. The plaintiff alleges that CuraGen failed to pay rent and additional charges, and, upon vacating previously leased space, failed to restore the vacated space to the condition of initial occupancy, failed to pay utility charges, failed to remove personal property, and failed to comply with applicable environmental laws and regulations. In January 2010, CuraGen and its former landlord entered into a settlement and release agreement pursuant to which the complaint was dismissed with prejudice. In connection with the settlement, CuraGen paid disputed rent and utility charges totaling $0.1 million, restored previously vacated space to the condition of initial occupancy and made certain notices to comply with local environmental law and regulations.
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERSRESERVED
None.
Item 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock currently trades on The Nasdaq Global Market (the "NASDAQ") under the symbol "CLDX". Effective October 1, 2008, the Companywe changed itsour name from AVANT Immunotherapeutics, Inc. to Celldex Therapeutics, Inc. andPrior to October 1, 2008, our common stock began trading on the NASDAQ Global Market (the "NASDAQ") under the symbol "CLDX". Prior to that date and after August 24, 1998, we were traded on NASDAQ under the symbol "AVAN". Prior to the August 24, 1998 date, we were traded on NASDAQ under the symbol "TCEL". The following table sets forth for the periods indicated the high and low closing salessale prices per share for our common stock, as reported by NASDAQ. The numbers below reflect the 1-for-12 reverse stock split effected on March 7, 2008.
Fiscal Period | High | Low | ||||||
|---|---|---|---|---|---|---|---|---|
Year Ended December 31, 2007 | ||||||||
1Q (Jan. 1 - March 31, 2007) | $ | 18.60 | $ | 15.36 | ||||
2Q (April 1 - June 30, 2007) | 17.76 | 8.88 | ||||||
3Q (July 1 - Sept. 30, 2007) | 11.16 | 4.80 | ||||||
4Q (Oct. 1 - Dec. 31, 2007) | 9.48 | 4.80 | ||||||
Year Ended December 31, 2008 | ||||||||
1Q (Jan. 1 - March 31, 2008) | $ | 9.91 | $ | 5.64 | ||||
2Q (April 1 - June 30, 2008) | 19.79 | 9.55 | ||||||
3Q (July 1 - Sept. 30, 2008) | 16.98 | 9.67 | ||||||
4Q (Oct. 1 - Dec. 31, 2008) | 12.69 | 4.24 | ||||||
Fiscal Period | High | Low | ||||||
|---|---|---|---|---|---|---|---|---|
Year Ended December 31, 2009 | ||||||||
First Quarter | $ | 11.75 | $ | 5.13 | ||||
Second Quarter | 14.19 | 6.28 | ||||||
Third Quarter | 8.10 | 4.80 | ||||||
Fourth Quarter | 5.75 | 4.16 | ||||||
Year Ended December 31, 2008 | ||||||||
First Quarter | $ | 9.91 | $ | 5.64 | ||||
Second Quarter | 19.79 | 9.55 | ||||||
Third Quarter | 16.98 | 9.67 | ||||||
Fourth Quarter | 12.69 | 4.24 | ||||||
As of February 20, 2009,25, 2010, there were approximately 634792 shareholders of record of our common stock. TheOn February 25, 2010 the closing price of theour common stock, as reported by NASDAQ, was $7.95 as of the close of the market on February 20, 2009.$5.13 per share. We have not paid any dividends on our common stock since our inception and do not intend to pay any dividends in the foreseeable future. Declaration of dividends will depend, among other things, upon our operating and future earnings, our capital requirements and general business conditions.
Sales of Unregistered Equity Securities in the Quarter Ended December 31, 2008
In October 2008, the Company, in settlement of 50% of a sublicense fee due to Duke University in connection with the $40 million license fee received from Pfizer and as permitted under the license agreement with Duke, issued to Duke 81,512 shares of the Company's $.001 par value common stock having an aggregate market value of $1,182,587, or $14.51 per share.
CELLDEX THEAPEUTICS, INC., NASDAQ MARKET INDEX-U.S.INDEX—U.S. AND
PEER GROUP INDICES
The graph below compares the cumulative total stockholder return on the common stock for the period from December 31, 20032004 through December 31, 2008,2009, with the cumulative return on (i) NASDAQ Market Index—U.S. Companies and (ii) NASDAQ Pharmaceutical Index. The comparison assumes investment of $100 on December 31, 20032004 in our common stock and in each of the indices and, in each case, assumes reinvestment of all dividends. The points on the graph are as of December 31 of the year indicated.

| | 12/31/03 | 12/31/04 | 12/30/05 | 12/29/06 | 12/31/07 | 12/31/08 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Celldex Therapeutics, Inc. | $ | 100 | $ | 73 | $ | 69 | $ | 49 | $ | 18 | $ | 24 | |||||||
NASDAQ Stock Market (U.S.) Index | $ | 100 | $ | 109 | $ | 111 | $ | 122 | $ | 132 | $ | 64 | |||||||
NASDAQ Pharmaceutical Stock Index | $ | 100 | $ | 107 | $ | 117 | $ | 115 | $ | 121 | $ | 112 | |||||||
See Item 11 for information regarding our equity compensation plan.
| | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Celldex Therapeutics, Inc. | $ | 100 | $ | 94 | $ | 67 | $ | 25 | $ | 33 | $ | 19 | |||||||
NASDAQ Stock Market (U.S.) Index | $ | 100 | $ | 102 | $ | 112 | $ | 122 | $ | 59 | $ | 84 | |||||||
NASDAQ Pharmaceutical Stock Index | $ | 100 | $ | 110 | $ | 108 | $ | 113 | $ | 105 | $ | 119 | |||||||
Item 6. SELECTED CONSOLIDATED FINANCIAL DATA
SelectedThe following selected consolidated financial data is presented beloware derived from our financial statements. The consolidated statement of operations data for the years ended December 31, 2009, 2008 and 2007 2006, 2005, and 2004.the consolidated balance sheet data as of December 31, 2009 and 2008 have been derived from our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. This data should be read in conjunction with our audited consolidated financial statements and related notes which are included elsewhere in this Annual Report on Form 10-K, and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included in Item 7 below.
On October 1, 2009, the CuraGen Merger became effective. The CuraGen Merger was accounted for using the acquisition method of accounting and was treated as our acquisition of CuraGen. Accordingly, the financial information presented below for periods prior to October 1, 2009 reflects the financial position and the results of operations of us alone, and for periods from October 1, 2009 forward the combined financial position and combined results of operations of us and CuraGen.
On March 7, 2008, the merger between AVANT and CelldexMerger became effective. The mergerAVANT Merger was accounted for using the purchase method of accounting and was treated as theour acquisition of AVANT, a publicly registered company, by Celldex, a private company.AVANT. Accordingly, the financial information presented below for periods prior to March 8, 2008 reflects the financial position and the results of operations of Celldexus alone, and for periods from March 8, 2008 forward the
combined financial position and combined results of operations of AVANTus and Celldex. AVANT Immunotherapeutics, Inc. changed its name to Celldex Therapeutics, Inc. on October 1, 2008.AVANT. All amounts are in thousands except per share data.
CONSOLIDATED STATEMENTS OF OPERATIONS DATA
| | 2008 | 2007 | 2006(2) | 2005 | 2004 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
REVENUE: | |||||||||||||||||
Product Development and Licensing | $ | 3,716 | $ | 466 | $ | 466 | $ | 14 | $ | — | |||||||
Contracts and Grants | 533 | 940 | 433 | 57 | — | ||||||||||||
Product Sales and Royalty | 3,207 | — | — | — | — | ||||||||||||
Total Revenue | 7,456 | 1,406 | 899 | 71 | — | ||||||||||||
OPERATING EXPENSE: | |||||||||||||||||
Research and Development | 26,347 | 9,892 | 10,013 | 4,826 | 4,480 | ||||||||||||
Acquired In-Process Research and Development(3) | 14,756 | — | — | 8,447 | — | ||||||||||||
Other Operating Expense | 15,109 | 7,022 | 9,681 | 4,167 | 1,586 | ||||||||||||
Total Operating Expense | 56,212 | 16,914 | 19,694 | 17,440 | 6,066 | ||||||||||||
Investment and Other Income, Net | 1,255 | 435 | 960 | 290 | — | ||||||||||||
Net Loss | $ | (47,501 | ) | $ | (15,073 | ) | $ | (17,835 | ) | $ | (17,079 | ) | $ | (6,066 | ) | ||
Basic and Diluted Net Loss Per Common Share | $ | (3.34 | ) | $ | (1.81 | ) | $ | (2.15 | ) | $ | (3.00 | ) | $ | (1.22 | ) | ||
Weighted Average Common Shares Outstanding(1) | 14,217 | 8,309 | 8,279 | 5,699 | 4,961 | ||||||||||||
| | 2009 | 2008 | 2007 | 2006(2) | 2005 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
REVENUE: | |||||||||||||||||
Product Development and Licensing Agreements | $ | 5,662 | $ | 3,716 | $ | 466 | $ | 466 | $ | 14 | |||||||
Contracts and Grants | 1,802 | 533 | 940 | 433 | 57 | ||||||||||||
Product Royalties | 7,716 | 3,207 | — | — | — | ||||||||||||
Total Revenue | 15,180 | 7,456 | 1,406 | 899 | 71 | ||||||||||||
OPERATING EXPENSE: | |||||||||||||||||
Research and Development | 26,169 | 22,636 | 9,892 | 10,013 | 4,826 | ||||||||||||
Royalty Expense | 8,397 | 3,711 | — | — | — | ||||||||||||
Charge for In-Process Research and Development(3) | — | 14,756 | — | — | 8,447 | ||||||||||||
Other Operating Expense | 17,464 | 15,109 | 7,022 | 9,681 | 4,167 | ||||||||||||
Total Operating Expense | 52,030 | 56,212 | 16,914 | 19,694 | 17,440 | ||||||||||||
Operating Loss | (36,850 | ) | (48,756 | ) | (15,508 | ) | (18,795 | ) | (17,369 | ) | |||||||
Investment and Other Income, Net | 248 | 1,411 | 435 | 960 | 290 | ||||||||||||
Interest Expense | (452 | ) | (156 | ) | — | — | — | ||||||||||
Net Loss Before Income Taxes | (37,054 | ) | (47,501 | ) | (15,073 | ) | (17,835 | ) | (17,079 | ) | |||||||
Income Tax Benefit | 529 | — | — | — | — | ||||||||||||
Net Loss | $ | (36,525 | ) | $ | (47,501 | ) | $ | (15,073 | ) | $ | (17,835 | ) | $ | (17,079 | ) | ||
Basic and Diluted Net Loss Per Common Share | $ | (1.84 | ) | $ | (3.34 | ) | $ | (1.81 | ) | $ | (2.15 | ) | $ | (3.00 | ) | ||
Shares Used in Calculating Basic and Diluted Net Loss Per Common Share(1) | 19,823 | 14,217 | 8,309 | 8,279 | 5,699 | ||||||||||||
CONSOLIDATED BALANCE SHEET DATA
| | 2008 | 2007 | 2006 | 2005 | 2004 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Working Capital | $ | 32,975 | $ | (4,438 | ) | $ | 12,178 | $ | 24,852 | $ | (467 | ) | ||||
Total Assets | 69,793 | 9,375 | 22,163 | 33,133 | 1,283 | |||||||||||
Long Term Liabilities | 37,558 | 370 | 914 | 1,152 | — | |||||||||||
Accumulated Deficit | (121,149 | ) | (73,648 | ) | (58,575 | ) | (40,739 | ) | (23,660 | ) | ||||||
Total Stockholders' Equity (Deficit) | 18,134 | (1,132 | ) | 15,144 | 28,007 | 816 | ||||||||||
| | 2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Working Capital | $ | 69,569 | $ | 32,975 | $ | (4,438 | ) | $ | 12,178 | $ | 24,852 | |||||
Total Assets | 140,364 | 69,793 | 9,375 | 22,163 | 33,133 | |||||||||||
Long Term Liabilities | 52,190 | 37,558 | 370 | 914 | 1,152 | |||||||||||
Accumulated Deficit | (157,674 | ) | (121,149 | ) | (73,648 | ) | (58,575 | ) | (40,739 | ) | ||||||
Total Stockholders' Equity (Deficit) | 73,767 | 18,134 | (1,132 | ) | 15,144 | 28,007 | ||||||||||
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995: Statements contained in the following, Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations, that are not historical facts may be forward-looking statements that are subject to a variety of risks and uncertainties. Forward-looking statements include statements with respect to our beliefs, plans, objectives, goals, expectations, anticipations, assumptions, estimates, intentions and future performance, and involve known and unknown risks, uncertainties and other factors, which may be beyond our control, and which may cause our actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as "may," "will," "can," "anticipate," "assume," "should," "indicate," "would," "believe," "contemplate," "expect," "seek," "estimate," "continue," "plan," "point to," "project," "predict," "could," "intend," "target," "potential" and other similar words and expressions of the future.
There are a number of important factors that could cause the actual results to differ materially from those expressed in any forward-looking statement made by us. These factors include, but are not limited to:
All forward-looking statements are expressly qualified in their entirety by this cautionary notice. You are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this report or the date of the document incorporated by reference into this report. We have no obligation, and expressly disclaim any obligation, to update, revise or correct any of the forward-looking statements, whether as a result of new information, future events or otherwise. We have expressed our expectations, beliefs and projections in good faith and we believe they have a reasonable basis. However, we cannot assure you that our expectations, beliefs or projections will result or be achieved or accomplished.
Item 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
As used herein, the terms "we," "us," "our," the "Company", or "Celldex" refer to Celldex Therapeutics, Inc., a Delaware corporation organized in 1983 (formerly known as AVANT Immunotherapeutics, Inc.) and its subsidiaries: Celldex Research Corporation ("Celldex Research"), Celldex Therapeutics, Ltd. ("Celldex Ltd") and Megan Health, Inc. ("Megan"). The Company's principal activity since our inception has been research and product development conducted on our own behalf, as well as through joint development programs with several pharmaceutical companies and other collaborators. The Company changed its name from AVANT Immunotherapeutics, Inc. to Celldex Therapeutics, Inc. on October 1, 2008.
OVERVIEW
We are aan integrated biopharmaceutical company that uses novel applications of immunology to develop products for the prevention and treatment of diseases. Usingapplies our comprehensive Precision Targeted Immunotherapy Platform we are developingto generate a broadpipeline of candidates to treat cancer and other difficult-to-treat diseases. Our immunotherapy platform includes a complementary portfolio of vaccines, therapeutic antibodies and other targeted immunotherapeutics addressing a wide range of applications including oncology, inflammatory and infectious diseases. These include therapeutic cancer vaccines, monoclonal antibodies, single-dose, oralantibody-targeted vaccines, that protect against important disease-causing infectious agentsantibody-drug conjugates and a treatmentimmunomodulators to reduce complement-mediated tissue damage. We are advancing a robust pipeline of clinical and preclinical product candidates, the most advanced of which are for treatment of various cancers. Our lead programs are therapeutic cancer vaccines designed to instruct the patient's immune system to recognize and destroy cancer cells.create novel disease-specific drug candidates.
Our strategy is to develop and demonstrate proof-of-concept for our product candidates before leveraging their value through partnerships or, in appropriate situations, continuing late stage development through commercialization ourselves. Demonstrating proof-of-concept for a product candidate generally involves bringing it through Phase 1 clinical trials and one or more Phase 2 clinical trials so that we are able to demonstrate, based on human trials, good safety data for the product candidate and some data indicating its effectiveness. Our current collaborations encompass the commercialization of an oral human rotavirus vaccine, the development of oral cholera, typhoid fever, ETEC and HIV vaccines, and a therapeutic brain cancer vaccine. Our product candidates address large market opportunities for which we believe current therapies are inadequate or non-existent.
We are targeting our efforts where we can add the greatest value to the development of our products and technologies. Our goal is to demonstrate clinical proof-of-concept for each product, and then seek excellent partners to help see those products through to commercialization. We thus leverage the value of itsour technology portfolio through corporate, governmental and non-governmental partnerships. This approach allows us to maximize the overall value of our technology and product portfolio while best ensuring the expeditious development of each individual product.
Merger between AVANT Our current collaborations include the commercialization of an oral human rotavirus vaccine and Celldexthe development of oncology and infectious disease vaccines. Our product candidates address large market opportunities for which we believe current therapies are inadequate or non-existent.
Acquisition of CuraGen
On March 7, 2008,In connection with the CuraGen Merger, effective October 1, 2009, we closed(i) issued 15,722,713 shares of our common stock, or 0.2739 shares, in exchange for each share of outstanding CuraGen common stock, plus cash in lieu of fractional shares (the "CuraGen Exchange Ratio"), (ii) assumed all of the merger (the "Merger") contemplated byCuraGen Stock Options and (iii) assumed the AgreementCuraGen Debt. We acquired CuraGen to gain access to a pipeline of oncology-focused antibodies, including CDX-011 (formerly CR011) currently in Phase 2 clinical development for the treatment of breast and Planmelanoma cancer, and cash, cash equivalents and marketable securities of Merger dated$70.3 million.
The transaction is being accounted for under the acquisition method of accounting. All of the assets acquired and liabilities assumed in the transaction are recognized at their acquisition-date fair values, while transaction costs associated with the transaction are expensed as incurred.
Purchase Price
The purchase price for CuraGen is based on the acquisition-date fair value of the consideration transferred, which was calculated based on the closing price of our common stock of $5.43 per share on October 19, 2007 by and among Celldex (formerly AVANT Immunotherapeutics, Inc.)1, 2009. The acquisition-date fair value of the consideration transferred consisted of the following (in thousands):
Fair value of common stock issued | $ | 85,374 | ||
Fair value of CuraGen Stock Options | 2,868 | |||
Total consideration transferred | $ | 88,242 | ||
U.S. GAAP requires that the fair value of replacement awards attributable to precombination service be included in the consideration transferred. Of the CuraGen Stock Options assumed, all but 1%, Callisto Merger Corporation ("Merger Sub"), a wholly owned subsidiary of Celldex, and Celldex Research (formerly Celldex Therapeutics, Inc.) (the "Merger Agreement"). Pursuant towere immediately vested upon closing in accordance with the terms of the Merger Agreement, Merger Sub merged with and into Celldex Research, with Celldex Research as the surviving company and a wholly-owned subsidiary of the Company. The total value of the transaction was approximately $75 million. Approximately 8.7 million shares were issued to the former Celldex Research shareholders in connection with the Merger. The Merger created a NASDAQ-listed, fully-integrated and diversified biopharmaceutical company with a deep pipeline of product candidatesstock option agreements
addressing high-value indications including oncology, infectious and inflammatory diseases. Former Celldex Research and former AVANT shareholders owned 58% and 42%employment agreements. The fair value of the combined companyCuraGen Stock Options that has been attributed to precombination service is included in the consideration transferred.
Allocations of Assets and Liabilities
We have allocated the consideration transferred for CuraGen to net tangible assets, intangible assets, goodwill and a severance obligation. The difference between the aggregate consideration transferred and the fair value of assets acquired and liabilities assumed was allocated to goodwill. This goodwill relates to the potential synergies from the CuraGen Merger and a deferred tax liability related to acquired IPR&D intangible assets. None of the goodwill is expected to be deductible for income tax purposes. The following table summarizes the fair values of the assets acquired and liabilities assumed at the acquisition date (in thousands):
Cash and cash equivalents | $ | 51,654 | |||
Marketable securities | 18,638 | ||||
Identifiable intangible assets: | |||||
IPR&D | 11,800 | ||||
Amgen Amendment | 14,500 | ||||
TopoTarget Agreement | 2,400 | ||||
Other current and long-term assets | 756 | ||||
Goodwill | 8,965 | ||||
CuraGen Debt | (11,503 | ) | |||
Deferred tax liabilities, net | (5,190 | ) | |||
Other assumed liabilities | (3,778 | ) | |||
Total | $ | 88,242 | |||
The purchase price allocation has been prepared on a fully dilutedpreliminary basis respectively.
Our board of directors approved a 1-for-12 reverse stock splitand is subject to change as additional information becomes available concerning the fair value and tax basis of the Company's common stock, which became effective on March 7, 2008. As a result of the reverse stock split, each twelve shares of common stock were combinedacquired assets and reclassified into one share of common stock and the total number of shares outstanding was reduced from approximately 180 million shares (including the shares issuedliabilities. Any adjustments to former Celldex Research stockholders in the Merger) to approximately 15 million shares.
The Merger was accounted for using the purchase method of accounting and was treated as an acquisition by Celldex Research of Celldex (then AVANT), with Celldex Research being considered the accounting acquirer based on the application of criteria specified in Statement of Financial Accounting Standards "SFAS" No. 141,Business Combinations, ("SFAS 141"), even though Celldex (then AVANT) was the issuer of common stock and the surviving legal entity in the transaction. Under the purchase method of accounting, the purchase price was allocatedallocation will be made as soon as practicable but no later than one year from the acquisition date.
The estimated fair value attributed to the acquired tangible and identifiableIPR&D intangible assets and assumed liabilities, based upon theirrepresents an estimate of the fair value atof purchased in-process technology for CuraGen's research programs that, as of October 1, 2009, had not reached technological feasibility and have no alternative future use. Only those research programs that had advanced to a stage of development where we believed reasonable net future cash flow forecasts could be prepared and a reasonable likelihood of technical success existed were included in the date of acquisition, as follows:
Tangible assets acquired | $ | 34,959,482 | |||
Less: Liabilities assumed | (3,945,067 | ) | |||
Net tangible assets acquired | 31,014,415 | ||||
Intangible assets acquired: | |||||
Core Technology | 897,249 | ||||
Developed Technology | 273,796 | ||||
Strategic Partner Agreement | 629,499 | ||||
In-Process Research and Development ("IPR&D") | 14,755,908 | ||||
Total | $ | 47,570,867 | |||
The values assigned to the intangible assets acquired, includingestimated fair value. Accordingly, the IPR&D wereprograms primarily represent the estimated fair value of CDX-011. The estimated fair value of the IPR&D programs was determined based on fair market value using a risk adjusted discountedestimates of expected future net cash flow approach. Fair valuesflows. These expected future net cash flows included estimates for long-term tangiblerevenue and intangible assets andassociated costs for the IPR&D wereprograms based on (i) relevant industry factors, (ii) current and expected trends in the product development life cycle, (iii) the ability to engage a strategic partner, (iv) the ability to obtain regulatory approval, and (v) the ability to manufacture and commercialize the products. The probability-adjusted future net cash flows which reflect the different stages of development of each program are then reduced by $6,041,597 of negative goodwill. The Company is a biotechnology enterprise and its resources are substantially devoted to research and development at the datepresent valued utilizing an estimate of the Merger. Managementappropriate discount rate which is responsible for determiningconsistent with the uncertainties of the cash flows utilized. Finally, the expected future net cash flows were calculated assuming the Amgen Amendment (defined below) was not entered into because the fair value attributable to the Amgen Amendment is separated from the fair value of the acquired IPR&D.&D programs.
The values assigned to IPR&D relate to the development of a typhoid-ETEC-cholera combination travelers vaccine, a cholesterol management vaccine, and the CDX-1135 (formerly TP10) complement inhibitor in the amounts of $7.8 million, $0.9 million and $6 million, respectively. Each of these three significant research and development projects in-process were valued through detailed analysis of product development data concerning the stage of development, time and resources needed to complete the project, expected income-generating ability and associated risks. The value of IPR&D was determined by estimating the costs to develop the purchased in-process technology into commercially viable products, estimating the net cash flows from such projects and discounting the net cash flows back to their present values. The probability of success and discount rates used for each project take into account the uncertainty surrounding the successful development and commercialization of the purchased in-process technology. We expect to incur approximately $16.2 million to move these projects to the point of out-licensing them to third parties. The estimated revenues from the typhoid-ETEC-cholera vaccine, the cholesterol management vaccine, and CDX-1135 are expected to be generated beginning in 2014, 2015 and 2014, respectively. A discount rate of 29% was used to value these projects, which we believe to be commensurate with the stage of development and the uncertainties in the economic estimates described above. The resultingfuture net cash flows for these projectsCDX-011 were based on the expectation that a BLA for CDX-011 will be filed with the FDA by the end of 2015. We expect the commercial launch as promptly as commercially practicable after necessary regulatory approvals are received. Assuming a traditional
were based on management's best estimates of revenue, cost of sales, research and development costs, selling, general and administrative costs, and income taxestimeline for each project and the net cash flows reflect assumptions that would be used by market participants.
The significant assumptions underlying the valuations included potential revenues, costs of completion, the timing of product approvals and the selection of appropriate probability of success and discount rates. None of the Company's IPR&D projects have reached technological feasibility nor do they have any alternative future use. Consequently, in accordance with current U.S. GAAP, the fair value allocated to IPR&D was charged as an expense in the Company's consolidated financial statements as of the date of acquisition. The remaining acquired intangible assets arising from the acquisition are being amortized on a straight line basis over their estimated lives, which range from 4.5 to 8 years.
As of December 31, 2008, the technological feasibility of the projects had not yet been reached and no significant departures from the assumptions included in the valuation analysis had occurred. Substantial additional research and developmentregulatory review process, we expect CDX-011 will be required prior to reaching technological feasibility. In addition, each product needs to successfully complete a seriescommercially launched in 2016. These assumptions require various levels of in-house and external testing, clinical trials and to receiveapprovals from the FDA or comparable foreign regulatory authorities before CDX-011 could be commercialized in the U.S. or other regulatory approvalterritories. Drug development involves a high degree of risk and most products that make it into clinical development do not receive marketing approval. Numerous risks and uncertainties can delay or stop clinical development of a pharmaceutical product prior to commercialization. The Company is also dependent upon the activitiesreceipt of its collaborators in developing,marketing approval, including, but not limited to, results from clinical trials that do not support continuing development, issues related to manufacturing or intellectual property protection, and marketing its products. Thereother events or circumstances that cause unanticipated delays, technical problems or other difficulties. Given these risks and uncertainties, there can be no assurance that these projects will ever reach feasibility or develop into products that can be marketed profitably, nor can there be assurance that the Company and its collaboratorsdevelopment of CDX-011 will be ablesuccessfully completed. If the development of CDX-011 is not successful, in whole or in part, or completed in a timely manner, we may not realize the expected financial benefits from the development of CDX-011 or the transaction as a whole.
The estimated fair value attributed to the May 2009 amendment to the CuraGen and Amgen Fremont (successor in-interest to Abgenix) license agreement relates to CuraGen's exclusive rights to develop manufacture and commercialize these products beforeCDX-011 and 11 other licensed antigens ("Amgen Amendment"). Under the Company's competitors. If these products are not successfully developedAmgen Amendment, CuraGen and do not become commercially viable,Amgen Fremont agreed to modify the Company's financial conditionterms of their existing cross-license of antigens whereby the amended license would be fully paid-up and results of operations could be materially affected. See Note 17royalty-free (except for any potentially required payments by CuraGen to the Company's consolidated financial statements for additional information.
Because Celldex Research was determined to be the acquirer for accounting purposes, the historical financial statementsoriginal licensor of Celldex Research became the historical financial statementsCDX-011). The estimated fair value of the Company. Accordingly,Amgen Amendment was based on the financial statementsincrease in expected future net cash flows for the IPR&D programs related to CDX-011 after the Amgen Amendment was entered into as compared to the expected future net cash flows if the Amgen Amendment was not entered into. The estimated fair value attributed to the Amgen Amendment is being amortized through the date of the Company priorlast expiring patent covering CDX-011.
The estimated fair value attributed to TopoTarget Agreement between CuraGen and TopoTarget relates to CuraGen's rights under the Merger reflect the financial position, resultsTopoTarget Agreement to receive up to $6 million in either potential commercial milestone payments related to future net sales of operations and cash flowsBelinostat or 10% of Celldex Research, which, during the historical periods presented in the accompanying consolidated financial statements, was majority-ownedany sublicense income received by Medarex, Inc.TopoTarget ("Medarex"TopoTarget Payments"). Following the Merger, the financial statementsThe estimated fair value of the current period reflectTopoTarget Agreement was based on estimates of the financial position, results of operation andprobability-adjusted expected future net cash flows of the Company.TopoTarget Payments. The results of operations of AVANT are included inestimated fair value attributed to the results of operationsTopoTarget Agrement is being amortized through the date of the Company beginning March 8, 2008. Accordingly, except as otherwise discussed below, this report reflects the financial condition, results of operations and liquidityestimated receipt of the combined companies at December 31, 2008last payment under the TopoTarget Payments. In February 2010, TopoTarget entered into a co-development and historicallycommercialization agreement for Belinostat with Spectrum Pharmaceuticals, Inc. resulting in our receipt of Celldex Research on a stand-alone basis for all periods prior to March 8, 2008. The financial condition, results of operations and liquidity$3 million of the Company asTopoTarget Payments.
The deferred tax liability, net of the years ended December 31, 2008, 2007 and 2006 may not be indicative of the Company's future performance or reflect what the Company's financial conditions, results of operations and liquidity would have been had the Merger been consummated as of January 1, 2006, or had the Company operated as a separate, stand-alone entity during the periods presented.
Other Acquisitions
In October 2005, Celldex Research completed the acquisitions of Lorantis Limited ("Lorantis") and Alteris Therapeutics, Inc. ("Alteris"). Celldex Research issued approximately 2.8$5.2 million shares of its Class A common stock (valued at $34.0 million) in exchange for all of the issued and outstanding shares of capital stock of Lorantis. Net assets acquired included approximately $31.1 million in cash, $2.7 million of fixed assets, a working capital deficit of $723,000 and $870,000 of in-process research and development ("IPR&D"), which was expensed in 2005. In addition, Celldex Research incurred approximately $671,000 of costs relatedprimarily relates to the acquisition of Lorantis, which were expensed totemporary differences associated with the IPR&D
in 2005. As of December 31, 2008, none of the acquired research and development projects had reached technological feasibility.
The purchase price for the Alteris assets consisted of approximately 496,100 shares of Celldex Research common stock (valued at $6.0 million) and approximately $1.5 million in cash. Net assets acquired included approximately $6,000 of fixed assets, $1.3 million in acquired intangible assets, and $6.2 million of IPR&D, which was expensed in 2005. In addition, Celldex Research incurred approximately $708,000 of costs related to the acquisition of Alteris, which were expensed to IPR&D in 2005. Celldex may be required to pay Alteris up to $5.0 million upon obtaining the first approvalare not deductible for commercial sale of an EGFRvIII product. As of December 31, 2008, none of the acquired research and development projects had reached technological feasibility.tax purposes.
RESEARCHCURRENT PROGRAMS AND DEVELOPMENT ACTIVITIESPARTNERSHIPS
Our products are derived from a broad set of complementary technologies which have the ability to utilize the human immune system and enable the creation of preventative and therapeutic agents. We are using these technologies to develop vaccines and targeted immunotherapeutics that prevent or treat cancer and disease caused by infectious organisms, and treatment vaccines that modify undesirable activity by the body's own proteins or cells. A number of our immunotherapeutic and vaccine product candidates are in various stages of clinical trials. We expect that a large percentage of our research and development expenses will be incurred in support of our current and future clinical trial programs.
The following table includes the programs that we currently believe are material to our business:
Product (generic) | Indication/Field | Partner | Status | ||||
|---|---|---|---|---|---|---|---|
CLINICAL | |||||||
CDX-110 | Glioblastoma multiforme | Pfizer (PF-4948568) | Phase 2b | ||||
CDX-011 | Metastatic melanoma and breast cancer | — | Phase 2 | ||||
CDX-1307 | Colorectal, bladder, pancreas, ovarian and breast tumors | — | Phase 1 | ||||
CDX-1401 | Multiple solid tumors | — | Phase 1/2 | ||||
CDX-1135 | Renal disease | — | Phase 1/2 | ||||
PRECLINICAL | |||||||
CDX-301 | Cancer, autoimmune disease and transplant | — | Preclinical | ||||
CDX-1127 | Immuno-modulation, multiple tumors | — | Preclinical | ||||
CDX-014 | Renal and ovarian cancer | — | Preclinical | ||||
CDX-1189 | Renal disease | — | Preclinical | ||||
MARKETED PRODUCTS | |||||||
Rotarix® | Rotavirus infection | GlaxoSmithKline | Marketed | ||||
The expenditures that will be necessary to execute Celldex'sour business plan are subject to numerous uncertainties. Completion of clinical trials may take several years or more, and the length of time generally varies substantially according to the type, complexity, novelty and intended use of a product candidate. It is not unusual for the clinical development of these types of product candidates to each take five years or more, and for total development costs to exceed $100 million for each product candidate. CelldexOur estimates that clinical trials of the type we generally conduct are typically completed over the following timelines:
Clinical Phase | Estimated Completion Period | |
|---|---|---|
Phase 1 | 1 - 2 Years | |
Phase 2 | 1 - 5 Years | |
Phase 3 | 1 - 5 Years |
The duration and the cost of clinical trials may vary significantly over the life of a project as a result of differences arising during the clinical trial protocol, including, among others, the following:
Celldex testsWe test potential product candidates in numerous preclinical studies for safety, toxicology and immunogenicity. CelldexWe may then may conduct multiple clinical trials for each product candidate. As we obtain results from trials, we may elect to discontinue or delay clinical trials for certain product candidates in order to focus our resources on more promising product candidates.
An element of Celldex'sour business strategy is to pursue the research and development of a broad portfolio of product candidates. This is intended to allow Celldexus to diversify the risks associated with itsour research and development expenditures. As a result, Celldex believes itswe believe our future capital requirements
and itsour future financial success are not substantially dependent on any one product candidate. To the extent Celldex iswe are unable to maintain a broad range of product candidates, Celldex'sour dependence on the success of one or a few product candidates increases.
Celldex's product candidates also have not yet received FDA regulatoryRegulatory approval which is required before Celldexwe can market themour product candidates as therapeutic or vaccine products. In order to proceed to subsequent clinical trial stages and to ultimately achieve regulatory approval, the FDAregulatory agency must conclude that Celldex'sour clinical data establish safetyis safe and efficacy.effective. Historically, the results from preclinical testing and early clinical trials (through Phase 2) have often not been predictive of results obtained in later clinical trials. A number of new drugs, biologics and vaccines have shown promising results in early clinical trials, but subsequently failed to establish sufficient safety and efficacy data to obtain necessary regulatory approvals.
Furthermore, Celldex'sour business strategy includes the option of entering into collaborative arrangements with third parties to complete the development and commercialization of Celldex'sour product candidates. In the event that third parties take over the clinical trial process for one of Celldex'sour product candidates, the estimated completion date would largely be under control of that third party rather than Celldex. Celldexus. We cannot forecast with any degree of certainty which proprietary products, if any, will be subject to future collaborative arrangements, in whole or in part, and how such arrangements would affect Celldex'sour development plan or capital requirements. Celldex'sOur programs may also benefit from subsidies, grants, contracts or government or agency-sponsored studies that could reduce Celldex'sour development costs.
As a result of the uncertainties discussed above, among others, Celldex iswe are unable to estimate the duration and completion costs of itsour research and development projects or when, if ever, and to what extent it will receive cash inflows from the commercialization and sale of a product. Celldex'sOur inability to complete itsour research and development projects in a timely manner or itsour failure to enter into collaborative agreements, when appropriate, could significantly increase itsour capital requirements and could adversely impact itsour liquidity. These uncertainties could force Celldexus to seek additional, external sources of financing from time to time in order to continue with itsour business strategy. Celldex'sOur inability to raise additional capital, or to do so on terms reasonably acceptable to it,us, would jeopardize the future success of itsour business.
During the past five years through the end of 2008, CelldexDecember 31, 2009, we incurred an aggregate of $55.7$73.5 million in research and development costs. Duringexpenses. The following table indicates the yearamount incurred for each of our significant research programs and for other identified research and development activities during the years ended December 31, 2008, Celldex incurred an aggregate of $26.3 million2009 and 2008. The amounts disclosed in the following table reflect direct research and development costs.costs, license fees associated with the underlying technology and an allocation of indirect research and development costs to each program. Prior to 2008, the privately-held
CURRENT PROGRAMS AND PARTNERSHIPS
Celldex Research did not maintain records that allowed for quantification of research and development expenses by project.
| | Year Ended December 31, 2009 | Year Ended December 31, 2008 | ||||||
|---|---|---|---|---|---|---|---|---|
| | (In thousands) | |||||||
CLINICAL | ||||||||
CDX-110 | $ | 3,249 | $ | 7,621 | ||||
CDX-011 | 1,098 | — | ||||||
CDX-1307 | 6,510 | 3,446 | ||||||
CDX-1401 | 4,293 | 5,562 | ||||||
CDX-1135 | 473 | 159 | ||||||
PRECLINICAL | ||||||||
CDX-301 | 2,424 | — | ||||||
CDX-1127 | 1,308 | 1,040 | ||||||
CDX-014 | 8 | — | ||||||
CDX-1189 | 386 | 104 | ||||||
OTHER | ||||||||
Bacterial Vaccines (CholeraGarde®/ETEC/Ty800) | 124 | 1,481 | ||||||
CDX-2401 | 3,574 | 830 | ||||||
Other Programs | 2,722 | 2,393 | ||||||
Total R&D Expense | $ | 26,169 | $ | 22,636 | ||||
PROGRAM DEVELOPMENTS
A. Cancer VaccineClinical Development Programs
CDX-110:CDX-110
Our lead clinical development program, CDX-110, is a peptide-based immunotherapy that targets the tumor specific molecule called EGFRvIII, a functional variant of the naturally expressed epidermal growth factor receptor ("EGFR"), a protein which has been well validated as a target for cancer therapy. Unlike EGFR, EGFRvIII is not present in normal tissues, and has been shown to be a transforming oncogene that can directly contribute to the cancer cell growth.
EGFRvIII is commonly present in glioblastoma multiforme, or GBM, the most common and aggressive form of brain cancer, and has also been observed in various other cancers such as breast, ovarian, prostate, colorectal, and head & neck cancer. With our partner,
In April 2008, we and Pfizer Inc. ("Pfizer"), we entered into a License and Development Agreement (the "Pfizer Agreement") under which Pfizer was granted an exclusive worldwide license to CDX-110. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Pfizer funds all development costs for these programs. We and Pfizer are currently pursuing the development of CDX-110 for GBM therapy and plan to expand the clinical development into other cancers through additional clinical studies. The FDA has granted orphan drug designation for CDX-110 for the treatment of EGFRvIII expressing GBM as well as fast track designation.
Initial clinical development of EGFRvIII immunotherapy was led by collaborating investigators at the Brain Center at Duke Comprehensive Cancer Center in Durham, North Carolina and at M.D. Anderson Cancer Center in Houston, Texas. The results from the Phase 1 (VICTORI) and Phase 2a (ACTIVATE) studies, which enrolled 16 and 21 patients, respectively, have demonstrated a significant increase in the time to disease progression (greater than 113%) in the patients who were vaccinated, and also in overall survival rates (greater than 100%), both relative to appropriately matched historical controls. An extension of the Phase 2a program (ACT II) at the same two institutions has enrolled 23
additional GBM patients treated in combination with temozolomide (the current standard of care). Preliminary results from this study (ACT II) currently estimates median overall survival to be 33.123.6 months, although the median has not yet been reached. Thereached, while the survival of a matched historical control group was 14.3 months and a subgroup treated with temozolomide (TMZ) of 15.215.0 months with a p value = 0.0078.0.0237. Overall time to progression for CDX-110in the ACT II study was 16.615.2 months compared with 6.46.3 months for the historical control group.
In May 2007, weWe initiated a Phase 2b/3 randomized study (ACT III) of CDX-110 combined with standard of care, temozolomide, versus standard of care alone in patients with GBM. We intend to open a total ofGBM in over 30 sites inthroughout the United States for the study. The FDA has granted orphan drug designation for CDX-110 for the treatment of EGFRvIII expressing GBM as well as fast track designation.
States. In December 2008, we announced an amendment to convert the ACT III study to a single-arm Phase 2 clinical trial in which all patients will receive CDX-110 in combination with temozolomide and we will continue to enroll to approximately 60 patients.temozolomide. The decision, which followsfollowed the recommendation of the Independent Data Monitoring Committee, was based on the observation that the majority of patients randomized to the control (standard of care) arm withdrew from this open-label study after being randomized to the control arm. Patients currently participating on the control arm of the study will bewere offered the option to receive treatment with CDX-110. Under this amendment, the ACT III study will provideprovided for a multi-center, non-randomized dataset for CDX-110 in patients with newly diagnosed GBM. These data will provide important additional information that can be used to better design the future development of CDX-110. Enrollment in ACT III is complete with a total of over 60 patients enrolled and we expect to present updated results during 2010.
CDX-011
On April 16,CDX-011 (formerly CR011-vcMMAE) is an antibody-drug conjugate (ADC) that consists of a fully-human monoclonal antibody, CR011, linked to a potent cell-killing drug, monomethyl-auristatin E (MMAE). The CR011 antibody specifically targets glycoprotein NMB or (GPNMB) that is expressed in a variety of human cancers including breast cancer and melanoma. The ADC technology, comprised of MMAE and a stable linker system for attaching it to CR011, was licensed from Seattle Genetics, Inc. The ADC is designed to be stable in the bloodstream. Following intravenous administration, CDX-011 targets and binds to GPNMB and upon internalization into the targeted cell, CDX-011 is designed to release MMAE from CR011 to produce a cell-killing effect. We acquired the rights to CDX-011 in connection with the CuraGen Merger.
Treatment of Breast Cancer: In June 2008, an open-label, multi-center Phase 1/2 study was initiated of CDX-011 administered intravenously once every three weeks to patients with locally advanced or metastatic breast cancer who have received prior therapy (median of seven prior regimens). The study began with a bridging phase to confirm the Companymaximum tolerated dose ("MTD") and Pfizer enteredhas expanded into a LicensePhase 2 open-label, multi-center study.
The study confirmed the safety of CDX-011 at the pre-defined maximum dose level (1.88 mg/kg) in 6 patients. An additional 28 patients were enrolled as an expanded Phase 2 cohort (for a total of 34 treated patients at 1.88 mg/kg, the Phase 2 dose) to evaluate the progression-free survival ("PFS") rate at 12 weeks. As previously seen in melanoma patients, the 1.88 mg/kg dose was well tolerated in this patient population with the most common adverse events of rash, alopecia, and Development Agreement (the "Pfizer Agreement"fatigue. The primary activity endpoint, which called for at least 5 of 25 (20%) under which Pfizer was granted an exclusive worldwide license to CDX-110. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the Pfizer Agreement, Pfizer made an upfront payment to the Company of $40 million and made a $10 million equity investmentpatients in the Company. Pfizer will fundPhase 2 study portion to be progression-free at twelve weeks, has been met. To date, 9 of 26 (35%) evaluable patients are without progression of disease at twelve weeks.
In addition, at the Phase 2 dose level, 4 of 32 (13%) evaluable patients achieved confirmed or unconfirmed Partial Responses ("PR") while 15 of 25 (60%) evaluable patients with measurable disease experienced some reduction in tumor size. GPNMB expression was identified in 10 of 14 (71%) of analyzed tumor samples and treatment with CDX-011 was associated with improved outcomes in all development costs for these programs. The Company is also eligible to receive potential milestone payments exceeding $390 million foractivity parameters in patients whose tumors expressed GPNMB. Notably, in patients who received the successful developmentPhase 2 dose and commercializationwhose tumors expressed GPNMB, 2 of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales. The Pfizer Agreement7 (29%) had confirmed PR, 5 of 7 (71%) had
became effective after clearance underdecreases in tumor size, and all 7 achieved at least stable disease with duration from 17.3 to 26.9 weeks. The median PFS in all patients was 9.1 weeks, but in patients whose tumors expressed GPNMB, median PFS was 18.3 weeks, compared to median PFS of 5.9 weeks for patients whose tumors did not express GPNMB. In patients with triple negative disease, 5 of 7 (71%) analyzed samples expressed GPNMB, 7 of 9 (78%) evaluable patients had tumor shrinkage, and the Hart-Scott-Rodino Antitrust Improvements Actmedian PFS for these patients was 17.9 weeks.
We expect to initiate a randomized Phase 2b controlled study in patients with advanced breast cancer that express GPNMB in the second half of 1976 (as amended) on May 19, 2008.2010.
Treatment of Metastatic Melanoma Cancer: In June 2006, a Phase 1/2 open-label, multi-center, dose escalation study was initiated to evaluate the safety, tolerability and pharmacokinetics of CDX-011 for patients with un-resectable Stage III or Stage IV melanoma who have failed no more than one prior line of cytotoxic therapy. During the Phase 1 portion of the study, doses of CDX-011 between 0.03 mg/kg to 2.63 mg/kg were evaluated and generally well tolerated, with rash and neutropenia emerging at higher doses. The MTD was determined to be 1.88 mg/kg administered intravenously (IV) once every three weeks.
In June 2009, results were announced for the 36 patients who were treated in the Phase 2 portion of the study. Of the patients enrolled, 94% had Stage IV disease of which two-thirds were classified as M1c, the poorest risk group. The study successfully met its primary activity endpoint, with 5 objective responses (1 unconfirmed) observed in 34 evaluable patients, and median duration of response of 5.3 months. The median overall PFS was 4.4 months. Tumor shrinkage was observed in 58% of patients, and 20 patients had best response of stable disease. Dermatologic adverse events consisting of rash, alopecia, and pruritus were the most common toxicities in this study. Other adverse events included fatigue, diarrhea, musculoskeletal pain, anorexia and nausea. Grade 3 or 4 neutropenia was observed in 5 patients. The absence of rash in the first cycle of treatment predicted a worse PFS. Additionally, in a subset of patients with tumor biopsies, high levels of tumor expression of GPNMB appeared to correlate with favorable outcome.
Enrollment has been completed in the Phase 1 portion of the melanoma trial to evaluate more frequent dosing schedules of CDX-011, including a weekly and a two out of every three-week regimen, to explore if more frequent administration can provide additional activity in patients with metastatic melanoma. A dose of 1.0 mg/kg given once every week has been identified as the MTD in a weekly schedule, and a dose of 1.5mg/kg was being explored in the two out of three week schedule. Although median duration of follow-up was only 6 weeks, objective responses have thus far been observed in 3 of 11 evaluable patients treated with weekly CDX011 (1 confirmed) and 1 confirmed response in 8 evaluable patients treated with CDX-011 two out of every three weeks. We expect to present updated results during the first half of 2010.
CDX-1307:CDX-1307 The Company's
Our lead APC Targeting Technology™ product candidate, CDX-1307, is in development for the treatment of epithelial tumors such as colorectal, pancreatic, bladder, ovarian and breast cancers. CDX-1307 targets the beta chain of human chorionic gonadotropin, known as hCG-Beta, which is an antigen often found in epithelial tumors. The presence of hCG-Beta in these cancers correlates with a poor clinical outcome, suggesting that this molecule may contribute to tumor growth. Normal adult tissues have minimal expression of hCG-Beta; therefore, targeted immune responses are not expected to generate significant side effects.
CelldexEnrollment is completingcomplete in our two Phase 1 studies at multiple centers that are designed to explore safety and dose/effect relationships via two administration routes—intradermal (ID), a traditional vaccine route that allows efficient access to local dermal dendritic cells and intravenous (IV), a novel systemic approach to vaccination that might target a much larger population of dendritic cells. In both The Phase 1
studies there are dose escalationsinvestigated the safety and immunogenicity of CDX-1307 alone and CDX-1307in combination with the adjuvantadjuvants, including GM-CSF (known to increase mannose receptor expression on dendritic cells). At the highest dose levels planned, additional immune system modulators (Toll-Like and Toll-Like Receptor Agonists,("TLR") agonists (poly-ICLC or TLR agonists) have been added to determine what effect they have in augmenting an immune response.Hiltonol™ and R848 or resiquimod). Patients with an assortment of different tumor types that are known to express hCG-Beta are being accruedwere enrolled with retrospective analysis for hCG-Beta expression. AAn escalating four dose regimen iswas utilized with the possibility of retreatment if patients demonstrate tumor regression or stable disease.
Over fifty (50)The Phase 1 studies enrolled over 80 patients with epithelial cancers have been treatedheavily pretreated, advanced-stage breast, colon, bladder and pancreatic cancer, with an average of 4.6 prior therapies across the treatment population. All patient cohorts demonstrated a favorable safety profile with no dose limiting toxicity to date. The combination of CDX-1307 with TLR agonists significantly enhanced immune responses against hCG-Beta, providing strong humoral responses in 88% of patients and cellular immune responses in 57% of patients analyzed to date. Immune responses occurred even in the Phase 1 clinical trials and more than half have evidencepresence of high circulating levels of hCG-Beta, expression by their tumor. The immunotherapy has been well tolerated with only minor adverse events observed (reddening atsuggesting that the injection site). Analysis of the initial cohorts with GM-CSF have revealed that severalCDX-1307 can overcome antigen tolerance in advanced and heavily pretreated cancers. Nine patients developed good humoral responses to hCG-Beta, and some have demonstrated enhancement of circulating hCG-Beta-specific CD8 T cells. Thus, we are encouraged that CDX-1307 is providing similar results as predicted in the pre-clinical studies. In addition, one patient with pancreatic cancer had a 26% overall reduction in tumor burden and two breast cancer patients were stable for sixstudies experienced disease stabilization from 2.3 months during treatment. The investigators atto 11.4 months following the Duke Comprehensive Cancer Center were awarded a two year $500,000 grant from the Avon Foundation and the National Cancer Institute to support Phase 1 work in breast cancer. The safetyinitiation of CDX-1307 vaccination. Two of these patients have received multiple courses of CDX-1307 and continue treatment with stable disease at 6.4 and 11.4 months. These data provide the basis for advancing CDX-1307 into a front-line patient population selected for hCG-Beta expressing cancers.
We expect to initiate a randomized Phase 2b controlled study in combinationpatients with defined immune system modulators is now being evaluated with intent to enter Phase 2 clinical researchnewly diagnosed invasive bladder cancer in the second halfquarter of 2009.2010. Patient's whose bladder cancer expresses hCG-Beta are predicted to have more aggressive disease and shorter survival. In this study we plan to select only patients with confirmed hCG-Beta expression using a specific diagnostic assay.
CDX-1401:CDX-1401
CDX-1401 is a fusion protein consisting of a fully human monoclonal antibody with specificity for the dendritic cell receptor, DEC-205, linked to the NY-ESO-1 tumor antigen. In humans, NY-ESO-1 is one of the most immunogenic tumor antigens and has been detected in 20-30%20 - 30% of cancers, thus representing a broad opportunity. This product is intended to selectively deliver the NY-ESO-1 antigen to APCs for generating robust immune responses against cancer cells expressing NY-ESO-1. Unlike CDX-1307, which targets the mannose receptor expressing dendritic cells, CDX-1401 is the first APC product targeting DEC-205 expressing dendritic cells. We are developing CDX-1401 for the treatment of malignant melanoma and a variety of solid tumors which express the proprietary cancer antigen NY-ESO-1, which the Companywe licensed from the Ludwig Institute for Cancer Research in 2006. The Company believesWe believe that preclinical studies have shown that CDX-1401 is effective for activation of human T-cell responses against NY-ESO-1.
In September 2009, we initiated enrollment in a dose-escalating Phase 1/2 clinical trial aimed at determining the optimal dose for further development based on the safety, tolerability, and immunogenicity of the CDX-1401 vaccine. The IND filing is planned fortrial will evaluate three different doses of the first halfvaccine in combination with resiquimod, an activator of 2009.TLR 7 and 8. We expect to be ableenroll approximately 36 patients with solid tumor cancers at multiple clinical sites in the United States.
CDX-1135
CDX-1135 is a molecule that inhibits a part of the immune system called the complement system. The complement system is a series of proteins that are important initiators of the body's acute inflammatory response against disease, infection and injury. Excessive complement activation also plays a role in some persistent inflammatory conditions. CDX-1135 is a soluble form of naturally occurring Complement Receptor 1 that inhibits the activation of the complement cascade in animal models and in human clinical trials. We believe that regulating the complement system could have therapeutic and
prophylactic applications in several acute and chronic conditions, including organ transplantation, multiple sclerosis, rheumatoid arthritis, age-related macular degeneration ("AMD"), atypical Hemolytic Uremic Syndrome ("aHUS"), Paroxysmal Nocturnal Hemaglobinuria ("PNH"), Dense Deposit Disease ("DDD") in kidneys, and myasthenia gravis. We are currently defining the most appropriate clinical development path for CDX-1135 and are focusing on rare disease conditions of unregulated complement activation as the fastest route to enter a Phase 1 study with a combination regimen, including TLRs, and will accrue multiple tumors that express NY-ESO-1.FDA approval.
Preclinical Development Programs
CDX-1127:CDX-301
CDX-301 is a FMS-like tyrosine kinase 3 ligand (Flt3L) that we licensed from Amgen in March 2009. CDX-301 is a growth factor for stem cells and immune cells called dendritic cells. Based on previous experience with this molecule, we believe that CDX-301 has considerable opportunity in various transplant settings as a stem cell mobilizing agent. In addition, CDX-301 is an immune modulating molecule that increases the numbers and activity of specific types of immune cells. We believe CDX-301 has significant opportunity for synergistic development in combination with proprietary molecules in our portfolio. We expect to file an IND application for CDX-301 before the end of 2010.
Celldex hasCDX-1127
We have entered into a License Agreement with the University of Southampton, UK, to develop human antibodies to CD27, a potentially important target for immunotherapy of various cancers. In pre-clinicalpreclinical models, antibodies to CD27 alone have been shown to mediate
anti-tumor effects, and may be particularly effective in combination with other immunotherapies. CD27 is a critical molecule in the activation pathway of lymphocytes. It is downstream from CD40, and may provide a novel way to regulate the immune responses. Engaging CD27 with the appropriate monoclonal antibody has proven highly effective at promoting anti-cancer immunity in mouse models. We are currently evaluating new human monoclonal antibodies in pre-clinicalpreclinical models.
B. Inflammatory Disease Development ProgramsCDX-014
CDX-014 (formerly CR014-vcMMAE) is a fully-human monoclonal ADC that targets TIM-1, an immunomudulatory protein that appears to down regulate immune response to tumors. The antibody, CDX-014, is linked to a potent chemotherapeutic, monomethyl auristatin E (MMAE), using Seattle Genetics' proprietary technology. The ADC is designed to be stable in the bloodstream, but to release MMAE upon internalization into TIM-1-expressing tumor cells, resulting in a targeted cell-killing effect. CDX-014 has shown potent activity in preclinical models of ovarian and renal cancer. We acquired the rights to CDX-014 in connection with the CuraGen Merger.
CDX-1189
CDX-1135 (formerly TP10): We have been developing immunotherapeutics that inhibit a part of the immune system called the complement system. The complement system is a series of proteins that are important initiators of the body's acute inflammatory response against disease, infection and injury. Excessive complement activation also plays a role in some persistent inflammatory conditions. Our lead compound, CDX-1135, a soluble form of naturally occurring Complement Receptor 1, has effectively shown to inhibit the activation of the complement cascade in animal models and in human clinical trials. We believe that regulating the complement system could have therapeutic and prophylactic applications in several acute and chronic conditions, including organ transplantation, multiple sclerosis, rheumatoid arthritis, age-related macular degeneration ("AMD"), atypical Hemolytic Uremic Syndrome and myasthenia gravis. We are currently defining the most appropriate clinical development path for CDX-1135 and are focusing on rare disease conditions of unregulated complement activation as the fastest route to FDA approval.
CDX-1189: Celldex is developing therapeutic human antibodies to a signaling molecule known as CD89 or Fca receptor type I (FcaRI). CD89 is expressed by some white blood cells and leukemic cell lines, and has been shown to be important in controlling inflammation and tumor growth in animal models. Celldex hasWe have proprietary, fully human antibodies to CD89 in preclinical development. Depending upon the specific antibody used, anti-CD89 antibodies can either be activating and thus stimulate immune responses, or down-regulating and act as an anti-inflammatory agent.
C. Infectious Disease Development Programs
CholeraGarde® Vaccine: CholeraGarde® is designed to be a safe, effective single-dose, oral cholera vaccine. Our partner, the International Vaccine Institute ("IVI"), has received $21 million in funding from the Bill & Melinda Gates Foundation for a Cholera Vaccine Initiative ("CHOVI"), includes conducting further clinical trials of CholeraGarde®. The IVI is presently conducting a Phase 2 clinical trial of CholeraGarde in Bangladesh, with plans to sponsor additional Phase 2 studies in India and Thailand beginning in the first half of 2009, followed by Phase 3 field studies.
ETEC Vaccine: In November 2007, we entered into an agreement with the Division of Microbiology and Infectious Diseases of the NIAID, whereby NIAID is sponsoring a Phase 1 study of Celldex's investigational single-dose, oral vaccine designed to offer combined protection against both enterotoxigenicEscherichia coli (ETEC) and cholera. In June 2008, NIAID initiated the Phase 1 trial of the ETEC vaccine candidate at Cincinnati Children's Hospital Medical Center.
In January 2009, we entered into an Exclusive License and Development Agreement with Vaccine Technologies, Inc. ("VTI"). Under the license agreement, Celldex has granted a worldwide fee- and royalty-bearing exclusive license to VTI to development and commercialize Celldex's CholeraGarde® and ETEC vaccine programs. Financial terms of the agreement with VTI include an upfront license fee, milestone payments and royalties on net sales of licensed products during the term of the agreement.
Ty800 Typhoid Fever Vaccine: The Company has developed an oral vaccine to offer rapid, single-dose protection againstSalmonella typhi, the cause of typhoid fever. Ty800 is targeted for both the travelers' market and global health needs. In 2006, the National Institute of Allergy and Infectious Disease ("NIAID") of the National Institutes of Health ("NIH") initiated a Phase 1/2 in-patient
dose-ranging clinical trial aimed at demonstrating the safety and immunogenicity of the Ty800 typhoid fever vaccine. NIAID funded the production of Ty800 vaccine for clinical testing and completed the Phase 1/2 trial at a NIH-funded clinical site in 2007. Results showed the single-dose, oral vaccine to be well tolerated and immunogenic, with over 90% of vaccinated subjects generating immune responses. We initiated our own sponsored Phase 2 trial of Ty800 in July 2007. Preliminary results reported in April 2008 from the study showed that the single-dose, oral vaccine was well tolerated and immunogenic, demonstrating that the desired immune response was achieved. Incidence of reactogenicity symptoms and adverse events post-vaccination were similar to placebo. Importantly, immunogenic response was dose-dependent. Positive immune response or seroconversion (prospectively defined as a 4-fold increase in anti-LPS titers over pre-dose level) rates were 65.5% (36/55) and 80% (44/55) in the low and high dose groups, respectively, and was significantly (p<0.001) higher than placebo.
CDX-2401: The Company is also using its APC Targeting Technology™ to develop vaccines against infectious disease. The lead program is CDX-2401, an APC-Targeting prophylactic vaccine, aimed at providing protection from infection with HIV, the virus known to cause AIDS. This program is in a Bill & Melinda Gates Foundation funded partnership with collaborators at Rockefeller University in New York City, who have shown in model systems that protective immunity can be induced with such a vaccine. Preclinical studies and manufacturing development are in progress and the Company, with its collaborators, plans to file an IND for Phase 1 clinical studies in the first half of 2009.
D. Marketed Products
Rotavirus Vaccine:Vaccine
Rotavirus is a major cause of diarrhea and vomiting in infants and children. In 1997, we licensed our oral rotavirus strain to GlaxoSmithKline ("Glaxo"). All of the ongoingGlaxo and Glaxo assumed responsibility for all subsequent clinical trials and all other development for this program is being conducted and funded by Glaxo.activities. Glaxo gained approval for its rotavirus vaccine, Rotarix®, in Mexico in July 2004, which represented the first in a series of worldwide approvals and commercial launches for the product. Glaxo subsequently launched Rotarix® in additional Latin American and Asian Pacific countries during 2005 - 2007. Additionally, Glaxo filed for market approval with the European regulatory authorities in late 2004, which triggered a $2 million milestone paymentproduct leading up to the Company. In Februaryapproval in Europe in 2006 the European Commission granted approval of Rotarix®and in the European Union, which triggered a $4 million milestone payment from Glaxo. On April 3, 2008, Rotarix® received approval from the FDA for the prevention of rotavirus gastroenteritisU.S. in infants. FDA approval triggered a $1.5 million milestone payment from Glaxo, of which $750,000 was retained by the Company.2008. We licensed-in theour rotavirus strain in 1995 and owe a license fee of 30% to Cincinnati Children's Hospital Medical Center ("CCH") on net royalties received from Glaxo. We are obligated to maintain a license with CCH with respect to the Glaxo agreement. The term of the Glaxo agreement is through the expiration of the last of the relevant patents covered by the agreement, although Glaxo may terminate the agreement upon 90 days prior written notice.
In May 2005, the Companywe entered into an agreement whereby an affiliate of Paul Royalty Fund ("PRF")PRF purchased an interest in the milestone payments and net royalties the Companythat we will receive on the development and worldwide sales of Rotarix® (see Note 10 of our consolidated financial statements). The market launch of Rotarix® by Glaxo in the U.S. market during the quarter ended September 30, 2008 resulted in a $10 million milestone payment to the Company from PRF, which the Company received on October 1, 2008. We have received a total of $60 million in milestone payments under the PRF agreement. No additional milestone payments are due from PRF under the agreement.
Royalty rates on Rotarix® escalate from 7% to 10% based on net product sales in countries that have valid patent protection. These royalty rates are discounted by 30% for "non-patent" countries (primarily international markets). In September 2006, we received notice from Glaxo that Glaxo would begin paying royalties on sales of Rotarix® vaccine at the lower of the two royalty rates under their 1997 license agreement. Glaxo's decision to pay the lower royalty rate (which is 70% of the full rate) is based upon Glaxo's assertion that Rotarix® is not covered by the patents Glaxo licensed from the Companyus in Australia and certain European countries. We are currently evaluating the basis for Glaxo's action and our potential remedies. If Glaxo's position stands, the royalties to which PRF is entitled will no longer be limited by a $27.5 million annual threshold, which the Companywe projected may have been reached in later years as sales of Rotarix® increased. Irrespective of Glaxo's position, we will still retain approximately 65% of the royalties on worldwide sales of Rotarix® once PRF receives 2.45 times the
aggregate cash payments of $60 million it made to the Company,us, though the potential amount of such residual royalties will be lower if Glaxo's position stands.
Megan®Vac 1 and Megan®Egg Vaccines: On December 1, 2000, the Company acquired all of the outstanding capital stock of Megan. Megan has commercialized three veterinary vaccines; Argus™ SC, licensed by the United States Department of Agriculture ("USDA") in March 1998 and marketed by Intervet, Inc., and Megan®Vac 1 and Megan®Egg, licensed by the USDA in November 1998 and 2003, respectively, and marketed by Lohmann Animal Health International ("LAHI"). In January 2009, we sold the poultry vaccines business, consisting of Megan®Vac 1 and Megan®Egg, to LAHI for an upfront fee and potential milestone payments.
TECHNOLOGY LICENSING
We have adopted a business strategy of out-licensing technology and programs that do not match our development focus or where we lack sufficient resources for the technology's or program's efficient development or where certain uses of the technology are outside of our focus. For example, when the Company acquired Megan, it entered into a licensing agreement in December 2000 with Pfizer's Animal Health Division to leverage the value of Megan's oral vaccine technology in a significant market opportunity (animal health and human food safety) outside of the Company's own focus on human health care. Under this Pfizer agreement, we may receive additional milestone payments of up to $3 million based upon attainment of specified milestones. We may receive royalty payments on eventual product sales. The term of this agreement is through the expiration of the last of the patents covered by the agreement.
Similarly, in January 2009, we sold our poultry vaccines business, consisting of Megan®Vac 1 and Megan®Egg, to LAHI and out-licensed our CholeraGarde® and ETEC vaccine programs to VTI.
CRITICAL ACCOUNTING POLICIES
Our significant accounting policies are described in Note 12 to the consolidated financial statements included in Item 8 of this Form 10-K. We believe our most critical accounting policies include accounting for business combinations, revenue recognition, for agreements entered into with various collaborators, accounting forproperty and equipment, impairment of long-lived assets, the amortization policy for acquired intangible assets and the estimates of costs incurred and assumptions made in recording accrued clinicalmarketable securities, research and contract manufacturing costsdevelopment expenses and assumptions made in calculating the fair value of stock-based compensation expense.
The methods, estimates and judgments we use in applying our most critical accounting policies have a significant impact on the results we report in our consolidated financial statements. We evaluate our estimates and judgments on an on-going basis. We base our estimates on historical experience and on assumptions that we believe to be reasonable under the circumstances. Our experience and assumptions form the basis for our judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may vary from what we anticipate and different assumptions or estimates about the future could materially change our reported results. We believe the following accounting policies are the most critical to us in that they are important to the portrayal of
our financial statements and they require our most difficult, subjective or complex judgments in the preparation of our consolidated financial statements:
Business Combinations
We account for business combinations that were completed after January 1, 2009 or will be completed in the future, including the CuraGen Merger, under the acquisition method of accounting. We assign the value of the consideration transferred to acquire a business to the tangible assets and identifiable intangible assets acquired and liabilities assumed on the basis of their fair values at the date of acquisition. We assess the fair value of assets, including intangible assets such as IPR&D, using a variety of methods including present-value models. Each asset is measured at fair value from the perspective of a market participant. The method used to estimate the fair values of IPR&D assets incorporates significant assumptions regarding the estimates a market participant would make in order to evaluate an asset, including a market participant's assumptions regarding the probability of completing IPR&D projects, which would require obtaining regulatory approval for marketing of the associated drug candidate; a market participant's estimates regarding the timing of and the expected costs to complete IPR&D projects; a market participant's estimates of future cash flows from potential product sales; and the appropriate discount rates for a market participant. Transaction costs and restructuring costs associated with the transaction are expensed as incurred.
IPR&D assets acquired in a business combination initially are recorded at fair value and accounted for as indefinite-lived intangible assets. These assets are maintained on our consolidated balance sheets until either the project underlying them is completed or the assets become impaired. If a project is completed, the carrying value of the related intangible asset is amortized over the remaining estimated life of the asset beginning in the period in which the project is completed. If a project becomes impaired or is abandoned, the carrying value of the related intangible asset is written down to its fair value and an impairment charge is taken in the period in which the impairment occurs. IPR&D assets will be tested for impairment on an annual basis during the third quarter, or earlier if impairment indicators are present.
Intangible assets acquired in a business combination with a finite life are recorded at fair value and amortized over the greater of economic consumption or on a straight-line basis over their estimated useful life.
The difference between the purchase price and the fair value of assets acquired and liabilities assumed in a business combination is allocated to goodwill. Goodwill will be evaluated for impairment on an annual basis during the third quarter, or earlier if impairment indicators are present.
For acquisitions completed prior to January 1, 2009, including the AVANT Merger, we expensed the fair value of IPR&D to research and development expense as of the acquisition date and included transaction costs associated with the business combination as part of the cost of the acquired company.
Revenue Recognition:Recognition The Company accounts
We account for revenue arrangements that include multiple deliverables in accordance with Emerging Issues Task Force ("EITF") No. 00-21,Accounting for Revenue Arrangements with Multiple Deliverables ("EITF 00-21"). EITF 00-21 addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting. In applying the guidance, revenue arrangements with multiple deliverables can only be considered as separate units of accounting if (i) the delivered item has value to the customer on a standalone basis, (ii) there is objective and reliable evidence of the fair value of the undelivered items
and (iii) if the right of return exists, delivery of the undelivered items is considered probable and substantially in the control of the vendor. If these criteria are not met, the revenue elements must beare considered a single unit of accounting for purposes of revenue recognition.
Payments received to fund certain research activities are recognized as revenue in the period in which the research activities are performed. Payments received in advance that are related to future performance are deferred and recognized as revenue when the research projects are performed.
The Company hasWe have entered into various license and development agreements with pharmaceutical and biotechnology companies. The terms of the agreements typically include non-refundable license fees, funding of research and development, payments based upon achievement of certain milestones and
royalties on net product sales. Non-refundable license fees are recognized as contract and license fee revenue when the Company haswe have a contractual right to receive such payments, provided that (i) a contractual arrangement exists, (ii) the contract price is fixed or determinable, (iii) the collection of the resulting receivable is reasonably assured and (iv) the Company haswe have no further performance obligations under the license agreement. Upfront non-refundable fees associated with license and development agreements where the Company haswe have continuing performance obligations under the terms of the agreement are recorded as deferred revenue and recognized as revenue over the estimated service period as the Company completes itswe complete our obligations. Where the Company'sour level of effort is relatively constant over the performance period or no other pattern is estimable, the revenue is recognized on a straight-line basis. Revenue is limited to the lesser of the cumulative amount of payments receiveddue or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date. If the estimated service period is subsequently modified, the period over which the upfront fee is recognized is modified accordingly on a prospective basis. The determination of the performance period involves judgment on management's part. Funding of research and development is recognized as revenue over the term of the applicable contract as costs are incurred related to that contract.
Milestone payments are recognized as revenue upon the achievement of mutually agreed milestones, provided that (i) the milestone event is substantive and its achievement is not reasonably assured at the inception of the agreement, and (ii) there is no continuing performance obligations associated with the milestone payment. Revenues from milestone payments related to arrangements under which the Company haswe have continuing performance obligations are recognized as revenue upon achievement of the milestone only if all of the following conditions are met: (i) the milestone payments are non-refundable; (ii) achievement of the milestone was not reasonably assured at the inception of the arrangement; (iii) substantive effort is involved in achieving the milestone; and, (iv) the amount of the milestone is reasonable in relation to the effort expended or the risk associated with achievement of the milestone. If any of these conditions are not met, the milestone payments are deferred and recognized as revenue over the term of the arrangement as Celldex completes itswe complete our performance obligations.
The Company hasWe have capitalized and deferred costs incurred in connection with the one-time signing and upfront payment (the initial deliverable) received with respect to a multiple deliverable arrangement. If there is deemed a single unit of accounting for such an arrangement, the capitalized deferred costs are amortized over the expected performance period of the arrangement.
Payments received to fund certain research activities are recognized as revenue in the period in which the research activities are performed. Revenue from contracts and grants including U.S. government grants under Small Business Innovation Research ("SBIR"), is recognized as the services are performed and recorded as effort is expended on the contracted work and billed to the government or our contractual partners.partner. Payments received in advance that are related to future performance are deferred and recognized as revenue when the research projects are performed.
Product royalty revenue consists of payments received from licensees for a portion of sales proceeds from products that utilize Celldex'sour licensed technologies and are recognized when the amount of and basis for such royalty payments are reported to us in accurate and appropriate form and in accordance with the related license agreement. Payments received in advance of activities being performed are recorded as deferred revenue. Any significant changes in the Company's estimates or assumptions could impact its revenue recognition.
Long-Lived Assets:Property and Equipment In the ordinary course of its business, the Company incurs substantial costs to construct property and equipment.
The treatment of costs to construct these assetsproperty and equipment depends on the nature of the costs and the stage of construction. Costs incurred in the project planning, and design, phase, and in the construction and installation phase,phases are capitalized as part of the cost of the asset. The Company stopsWe stop capitalizing these costs when the asset is substantially complete and ready for its intended use. Determining the appropriate period during which to capitalize costs, and assessing whether particular costs qualify for capitalization, requires us to make significant judgments. These judgments can have a material impact on our reported results.
For manufacturing property and equipment, the Companywe also capitalizes the cost of validating these assets for the underlying manufacturing process. Celldex completesWe complete the capitalization of validation costs when the asset is substantially complete and ready for its intended use. Costs capitalized include incremental labor and fringe benefits, and direct
consultancy services. We capitalize interest cost as part of the historical cost of acquiring certain assets during the period of time required to get the asset ready for its intended use.
Property and equipment is stated at cost and depreciated over the estimated useful lives of the related assets using the straight-line method. Laboratory equipment and office furniture and equipment are depreciated over a five-year periodfive years and computer equipment is depreciated over a three-year period.three years. Manufacturing equipment is amortized over a seven-seven to ten-year period.ten years. Leasehold improvements are amortized over the shorter of the estimated useful life or the non-cancelable term of the related lease, including any renewals that are reasonably assured of occurring. Property and equipment under construction is classified as construction in progress and is depreciated or amortized only after the asset is placed in service. Expenditures for maintenance and repairs are charged to expense whereas the costs of significant improvements which extend the life of the underlying asset are capitalized. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are eliminated and the related gainsany resulting gain or losses areloss is reflected in net income. Determining the economic livesour consolidated statements of property and equipment requires us to make significant judgments that can materially impact our operating results.operations.
AmortizationImpairment of Intangible Assets:Long-Lived Assets The Company has acquired intangible assets, which include core technology, developed technology and a strategic partner agreement, through the Merger and the acquisition of Lorantis Limited. These acquired intangible assets are being amortized on a straight-line basis over their estimated lives, which range from 4.5 to 11 years. The determination of the amortization period involves estimates and judgments on management's part. Any significant changes in the Company's estimates or assumptions could impact the carrying value of acquired intangible assets.
We evaluate the recoverability of these assets when facts and circumstances suggest the asset could be impaired in accordance with Statement of Financial Accounting Standards No. 144 ("SFAS 144"), "Accounting for the Impairment of Long-Lived Assets".
Accounting for the Impairment of Long-Lived Assets: The Company periodically evaluates itsour long-lived assets, primarilyincluding property and equipment, and intangible assets for potentialwhen circumstances indicate that an event of impairment under SFAS No. 144,Accounting formay have occurred. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the Impairmentuse of Long-Lived Assets, ("SFAS No. 144"). The Company performs these evaluations whenever events or changes in circumstances suggestthe asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of an asset or group ofthe assets, is not recoverable. Indicators of potential impairment include:
If the Company believes an indicator of potential impairment exists, the carrying values of the assets are evaluatedwritten-down to their estimated fair values.
Marketable Securities
We invest our excess cash balances in relationmarketable securities including municipal bond securities, U.S. government agency securities, and high-grade corporate bonds. We classify all of our marketable securities as current assets on the consolidated balance sheets because they are available-for-sale and available to the operating performance and future undiscounted cash flows of the underlying asset. The net book value of an asset is adjusted tofund current operations. Marketable securities are stated at fair value if its expected future undiscounted cash flowswith their unrealized gains and losses included as a component of accumulated other comprehensive income (loss), which is a separate component of stockholders' equity, until such gains and losses are less than its book value.realized. The Company charges impairments of the long-lived assets to operations if its evaluations indicate that the carryingfair value of these assetssecurities is not recoverable. We have identified no indicatorsbased on quoted prices for identical or similar assets. If a decline in the fair value is considered other-than-temporary, based on available evidence, the unrealized loss is transferred from other comprehensive income (loss) to the consolidated statements of impairment at December 31, 2008. When we determine thatoperations. Realized gains and losses are determined on the carrying value of intangible assets or long-lived assets is not recoverable, we may be required to record impairment charges for these assets that have not been previously recorded.specific identification method and are included in investment and other income, net.
Accrued Clinical Research and Contract Manufacturing Costs: The preparation of financial statements requires management to make estimates and assumptions that affect the reported amount of assets, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the period reported. Specifically, Celldex's management must make estimates of costs incurred to date, but not yet invoiced by external entities such as clinical research organizations ("CROs") and contract manufacturing organizations ("CMOs"). For CROs, management analyzes the progress of clinical trials, contract amendments for specific work, invoices received, and budgeted costs when evaluating the adequacy of the accrued liability. For CMOs, management analyzes the progress of process development and scale-up efforts and the production of clinical materials, contract amendments signed for specific work, invoices received, and budgeted costs when evaluating the adequacy of the accrued liability. The Company accrues these expenses based upon its assessment of the status of each study or manufacturing activity and the work completed, and upon information obtained from the CROs and CMOs. Significant management judgments and estimates must be made and used in connection with the accrued balance in any accounting period. Actual results may differ from the amount and timing of the accrued balance for any period.
In connection with certain clinical research agreements the Company makes payments in advance of the services performed. The Company accounts for these payments under the provisions of EITF Issue No. 07-3 (EITF 07-3),Accounting for Advance Payments for Goods or Services to Be Used in Future Research and Development ActivitiesExpenses. EITF 07-3 is limited to non-refundable advance payments for goods and services to be used or rendered in future research
Research and development activities pursuant to an executory contractual arrangement. In accordance with this pronouncement, the Company capitalizescosts, including internal and defers these advanced payments tocontract research costs, are expensed as incurred. Research and development expenses consist mainly of clinical research organizations, astrial costs, manufacturing of clinical material, toxicology and other current assets, until the goods have been delivered or the related services have been performed.studies, personnel costs, depreciation, license fees and funding of outside research.
Stock-Based Compensation Expense:Expense
We account forrecord stock-based awards under SFAS No. 123(R),Share-Based Payment, ("SFAS No. 123(R)"), which requires the measurement and recognition of compensation expense for all share-based paymentstock-based awards made to employees and directors including employee stock options and employee stock purchases related to the Employee Stock Purchase Plan ("employee stock purchases") based on the estimated fair values of the stock-based awards expected to vest at the grant date fair values.
and is adjusted, if necessary, to reflect actual forfeitures. Compensation expense for all share-based paymentstock-based awards to employees areand directors is recognized using the straight-line method over the term of vesting or performance. As stock-based compensation expense recognized in the Consolidated Statement of Operations is based on awards ultimately expected to vest, compensation expense has been reduced for estimated forfeitures. SFAS No. 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The Company estimates the fair value of share-based awards granted using the Black-Scholes option-pricing model ("Black-Scholes model"). The Company's determination of fair value of share-based payment awards on the date of grant using an option-pricing model is affected by the Company's stock price as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, the Company's expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors.
SFAS No. 123(R) did not change the accounting guidance We record stock-based compensation expense for how the Company accounts forstock options issued to non-employees. The Company accounts for options issuedgranted to non-employees in accordance with SFAS No. 123(R) and EITF Issue No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services. Thebased on the fair value of suchthe stock options which is periodically re-measured and income or expense is recognized duringover the vesting terms.
See Note 3 for additional information.term resulting in periodic adjustments to stock-based compensation expense.
RESULTS OF OPERATIONS
TheOur financial statements of the Company prior to the AVANT Merger reflect the financial position, results of operations and cash flows of Celldex Research (then Celldex).Research. Following the AVANT Merger but prior to the CuraGen Merger, our financial statements of the current period reflect the financial position, results of operation and cash flows of the combined companies. TheAVANT and Celldex Research. Following the CuraGen Merger, our financial statements reflect the financial position, results of operations of AVANT are included in the results of operations of the Company beginning March 8, 2008. The discussions of results of operations, liquidityoperation and capital resources below arecash flows of the combined companies for the period March 8, 2008 to(AVANT, Celldex Research and CuraGen).
Year Ended December 31, 2009 compared with Year Ended December 31, 2008
| | Year Ended December 31, | | | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Increase/ (Decrease) $ | Increase/ (Decrease) % | ||||||||||||
| | 2009 | 2008 | ||||||||||||
| | (In thousands) | |||||||||||||
Revenue: | ||||||||||||||
Product Development and Licensing Agreements | $ | 5,662 | $ | 3,716 | $ | 1,946 | 52 | % | ||||||
Contracts and Grants | 1,802 | 533 | 1,269 | 238 | % | |||||||||
Product Royalties | 7,716 | 3,207 | 4,509 | 141 | % | |||||||||
Total Revenue | $ | 15,180 | $ | 7,456 | $ | 7,724 | 104 | % | ||||||
Operating Expense: | ||||||||||||||
Research and Development | 26,169 | 22,636 | 3,533 | 16 | % | |||||||||
Royalty | 8,397 | 3,711 | 4,686 | 126 | % | |||||||||
Gain on Sale of Assets | (604 | ) | — | (604 | ) | n/a | ||||||||
Charge for In-Process Research and Development | — | 14,756 | (14,756 | ) | (100 | )% | ||||||||
General and Administrative | 17,119 | 14,748 | 2,371 | 16 | % | |||||||||
Amortization of Acquired Intangible Assets | 949 | 361 | 588 | 163 | % | |||||||||
Total Operating Expense | 52,030 | 56,212 | (4,182 | ) | (7 | )% | ||||||||
Operating Loss | (36,850 | ) | (48,756 | ) | (11,906 | ) | (24 | )% | ||||||
Investment and Other Income, Net | 248 | 1,411 | (1,163 | ) | (82 | )% | ||||||||
Interest Expense | (452 | ) | (156 | ) | 296 | 190 | % | |||||||
Net Loss Before Income Taxes | (37,054 | ) | (47,501 | ) | (10,447 | ) | (22 | )% | ||||||
Income Tax Benefit | 529 | — | 529 | n/a | ||||||||||
Net Loss | $ | (36,525 | ) | $ | (47,501 | ) | $ | (10,976 | ) | (23 | )% | |||
Net Loss
The $11.0 million decrease in net loss for the year ended December 31, 2009 compared to the year ended December 31, 2008 was primarily the result of a decrease in charges for acquired in-process research and historicallydevelopment combined with increased revenues, partially offset by increased research and development, royalty and general and administrative expenses.
Revenue
The $1.9 million increase in product development and licensing agreement revenue for the year ended December 31, 2009 was primarily due to an increase of $2.3 million in Pfizer related revenue. The $1.3 million increase in contract and grant revenue for the year ended December 31, 2009 was primarily due to an increase of $1.4 million in revenue related to our vaccine development work on Rockefeller's CDX-2401 program. The $4.5 million increase in product royalty revenue for the year ended December 31, 2009 was primarily related to our retained interests in Rotarix® net royalties which were not sold to PRF and which is equal to the amount payable to CCH and recognized in royalty expense by us.
Research and Development Expense
Research and development expenses consist primarily of (i) personnel expenses, (ii) laboratory supply expenses relating to the development of our technology, (iii) facility expenses, and (iv) product development expenses associated with our product candidates as follows:
| | Year Ended December 31, | | | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Increase/ (Decrease) $ | Increase/ (Decrease) % | |||||||||||
| | 2009 | 2008 | |||||||||||
| | (In thousands) | ||||||||||||
Personnel | $ | 11,108 | $ | 8,785 | $ | 2,323 | 26 | % | |||||
Laboratory Supplies | 2,517 | 2,179 | 338 | 16 | % | ||||||||
Facility | 4,782 | 4,180 | 602 | 14 | % | ||||||||
Product Development | 5,758 | 5,192 | 566 | 11 | % | ||||||||
Personnel expenses primarily include salary, benefits, stock-based compensation, payroll taxes and recruiting costs. The $2.3 million increase in personnel expenses for the year ended December 31, 2009 was primarily due to higher headcount and $0.9 million in severance expense related to the CuraGen Merger. We expect personnel expenses to remain relatively consistent over the next twelve months as the effect of our higher headcount will be offset by the reduction in severance expense.
Laboratory supply expenses include laboratory materials and supplies, services, and other related expenses incurred in the development of our technology. The $0.3 million increase in laboratory supply expenses for the year ended December 31, 2009 was primarily due to increased research, preclinical and manufacturing activities. We expect supply expenses to increase over the next twelve months as a result of increased research and development activities, although there may be fluctuations on a stand-alone basisquarterly basis.
Facility expenses include depreciation, amortization, utilities, rent, maintenance, and other related expenses incurred at our facilities. The $0.6 million increase in facility expenses for all periods priorthe year ended December 31, 2009 was primarily due to March 8, 2008.higher depreciation and amortization expenses. We expect facility expenses to increase over the next twelve months as a result of continued capital expansion, although there may be fluctuations on a quarterly basis.
Product development expenses include clinical investigator site fees, external trial monitoring costs, data accumulation costs, contracted research and outside clinical drug product manufacturing. The $0.6 million increase in product development expenses for the year ended December 31, 2009 was primarily due to an increase in preclinical work related to the CDX-1401 and CDX-2401 programs. The decrease in clinical expenses for CDX-110 for the year ended December 31, 2009 due to the transfer of clinical management of our CDX-110 program to Pfizer was primarily offset by the increase in clinical expenses related to our CDX-011 program acquired from CuraGen. We expect product development expenses to increase over the next twelve months due to the increase in clinical trial expenses related
to our CDX-011, CDX-1307, and CDX-1401 programs, although there may be fluctuations on a quarterly basis.
Royalty Expense
Royalty expenses include product royalty and sublicense royalty fees on our out-licensed programs. The $4.7 million increase in royalty expenses for the year ended December 31, 2009 was primarily due to an increase in Rotarix® related royalty fees. Our retained interests in Rotarix® net royalties which were not sold to PRF are recorded as product royalty revenue and a corresponding amount that is payable to CCH is recorded as royalty expense. We expect royalty expenses to increase over the next twelve months, although there may be fluctuations on a quarterly basis.
General and Administrative Expense
The $2.4 million increase in general and administrative expenses for the year ended December 31, 2009 was primarily due to (i) $3.3 million in severance expense related to the CuraGen Merger, (ii) an increase in consultant and legal expense of $0.6 million during the year ended December 31, 2009 primarily related to the CuraGen Merger and (iii) $0.7 million in severance expense, including related non-cash stock-based compensation expense, incurred during the year ended December 31, 2009 related to our former SVP, Business Development. The effect of these increases was partially offset by $1.4 million in severance expense and $1.3 million in stock-based compensation expense incurred during the year ended December 31, 2008 related to our former President and Chief Executive Officer. We expect general and administrative expense to decrease over the next twelve months primarily due to the lack of CuraGen Merger related expenses.
Amortization Expense
The $0.6 million increase in amortization expenses for the year ended December 31, 2009 was primarily due to intangible assets acquired in connection with the CuraGen Merger. We expect amortization expense of acquired intangible assets to increase over the next twelve months primarily due to the CuraGen Merger.
Investment and Other Income, Net
The $1.2 million decrease in investment and other income, net for the year ended December 31, 2009 was primarily due to other income of $0.9 million recorded for the year ended December 31, 2008 related to the $10 million milestone payment we received from PRF in connection with the U.S. launch of Rotarix®. We anticipate investment income to increase over the next twelve months due to the CuraGen Merger.
Interest Expense
The $0.3 million increase in interest expense for the year ended December 31, 2009 was primarily due to the CuraGen Debt we assumed in connection with the CuraGen Merger. We anticipate interest expense to increase over the next twelve months due to the CuraGen Debt.
Income Tax Benefit
The $0.5 million increase in income tax benefit for the year ended December 31, 2009 was due to non-cash tax consequences as a result of the CuraGen Merger.
Fiscal Year Ended December 31, 2008 compared with Fiscal Year endedEnded December 31, 2007
| | Year Ended December 31, | | | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Increase/ (Decrease) $ | Increase/ (Decrease) % | ||||||||||||
| | 2008 | 2007 | ||||||||||||
| | (In thousands) | |||||||||||||
Revenue: | ||||||||||||||
Product Development and Licensing Agreements | $ | 3,716 | $ | 466 | $ | 3,250 | 697 | % | ||||||
Contracts and Grants | 533 | 940 | (407 | ) | (43 | )% | ||||||||
Product Royalties | 3,207 | — | 3,207 | n/a | ||||||||||
Total Revenue | $ | 7,456 | $ | 1,406 | $ | 6,050 | 430 | % | ||||||
Operating Expense: | ||||||||||||||
Research and Development | 22,636 | 9,892 | 12,744 | 129 | % | |||||||||
Royalty | 3,711 | — | 3,711 | n/a | ||||||||||
Charge for In-Process Research and Development | 14,756 | — | 14,756 | n/a | ||||||||||
General and Administrative | 14,748 | 6,905 | 7,843 | 114 | % | |||||||||
Amortization of Acquired Intangible Assets | 361 | 117 | 244 | 209 | % | |||||||||
Total Operating Expense | 56,212 | 16,914 | 39,298 | 232 | % | |||||||||
Operating Loss | (48,756 | ) | (15,508 | ) | 33,248 | 214 | % | |||||||
Investment and Other Income, Net | 1,411 | 435 | 976 | 224 | % | |||||||||
Interest Expense | (156 | ) | — | (156 | ) | n/a | ||||||||
Net Loss | $ | (47,501 | ) | $ | (15,073 | ) | $ | 32,428 | 215 | % | ||||
Net Loss
The Company reported a consolidated net loss of $47,500,571, or $3.34 per share, for the year ended December 31, 2008, compared with a net loss of $15,073,050, or $1.81 per share, for the year ended December 31, 2007. The$32.4 million increase in net loss for the year ended December 31, 2008 includescompared to the combinedyear ended December 31, 2007 was primarily the result of increased operating expenses foras a result of the two companies andAVANT Merger including a one-time non-cash charge of $14,755,908$14.8 million for purchased in-process research and development related to the Merger which closed in March 2008.development. The weighted average common shares outstanding used to calculate net loss per common shareeffect of this increase was 14,217,388 in 2008 and 8,309,420 in 2007.offset by higher revenue.
Revenue
Total revenue increased to $7,455,507 for 2008 compared to $1,405,592 for 2007.
ProductThe $3.3 million increase in product development and licensing agreement revenue increased to $3,715,957 in 2008 from $466,156 in 2007 primarily due tofor the recognition of $2,870,359 of Pfizer deferred revenue and $225,000 of Glaxo milestone revenue payable to CCH. For the yearsyear ended December 31, 2008 and 2007, the Company recognized $466,156was primarily due to an increase of revenue under the Corixa termination agreement.
Contract$2.9 million in Pfizer related revenue. The $0.4 million decrease in contract and grant revenue decreased by $406,254 to $533,182 for work performed inthe year ended December 31, 2008 from $939,436 in 2007was primarily due to lower levels of vaccine development work billable to Rockefeller and Harvard in 2008.
Product The $3.2 million increase in product royalty revenue was $3,206,368 infor the year ended December 31, 2008 consisting of $3,034,565was related to the Company'sour retained interests in Rotarix® net royalties which were not sold to PRF and which is equal to the amount payable to CCH and recognized in research and development expense by the Company, and $171,803 related to royalties on Megan®Vac 1 and Megan®Egg product sales. There was no product royalty revenue in 2007.us.
OperatingResearch and Development Expense
Total operating expense increased to $56,211,495 for the year ended December 31, 2008 compared to $16,914,128 for the year ended December 31, 2007. Operating expense for 2008 includes a one-time non-cash charge of $14,755,908 for purchased in-process research and development related to the Merger in March 2008.
| | Year Ended December 31, | | | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Increase/ (Decrease) $ | Increase/ (Decrease) % | |||||||||||
| | 2008 | 2007 | |||||||||||
| | (In thousands) | ||||||||||||
Personnel | $ | 8,785 | $ | 3,250 | $ | 5,535 | 170 | % | |||||
Laboratory Supplies | 2,179 | 446 | 1,733 | 389 | % | ||||||||
Facility | 4,180 | 995 | 3,185 | 320 | % | ||||||||
Product Development | 5,192 | 2,455 | 2,737 | 111 | % | ||||||||
Research and development expenses consist primarily of (i)The $5.5 million increase in personnel expenses (ii) facilities and supply expenses relating to Celldex's technology, (iii) development costs associated with Celldex's product candidates, (iv) fees paid to third parties in conjunction with Celldex's clinical and preclinical development programs, and (v) license fees on in-licensed technologies and royalty fees on out-licensed programs. Research and development expense in 2008 increased by $16,455,480 to $26,347,189 from $9,891,709 in 2007. The changes relate primarily to the merger of the two companies and to costs associated with the following:
Royalty Expense
The $3.7 million increase in royalty expenses primarily related to clinical trials to increase in the future as it continues to develop its therapeutic product pipeline and bring forward new product candidates into clinical development.
General and development consultants toAdministrative Expense
The $7.8 million increase in the future as it enters into later stage clinical development.
GeneralChief Executive Officer and administrative expense increased $7,841,905 to $14,747,392 in 2008 compared to $6,905,487 in 2007 and was primarily attributed to(ii) increases in stock-based compensation expense of $1,985,593 for stock option awards, severance expense of $1,373,874, legal and patent expense of $1,963,399,$2.0 million, facility-related expenses of $1,075,448,$1.1 million, insurance expenses of $590,061$0.6 million and professional services of $564,935.$0.6 million, primarily due to the AVANT Merger.
Amortization Expense
The Company expects general and administrative expense to$0.2 million increase in 2009 asamortization expenses for the Company adds infrastructureyear ended December 31, 2008 was primarily due to support its therapeutic product pipeline and new product candidates.
Amortization expense of acquired intangible assets increased $244,074 to $361,006 in 2008 compared to $116,932 in 2007 and the increase was a result of the intangible assets acquired in
connection with the AVANT Merger. The Company expects amortization expense of acquired intangible assets to decrease in 2009 unless additional acquisitions are made.
Investment and Other Income, Net
InterestThe $1.0 million increase in our investment and other income, increased $819,931 to $1,255,417 innet for the year ended December 31, 2008 compared to $435,486 in 2007. The increase was primarily due to higher average cash balances inother income of $0.9 million recorded for the year ended December 31, 2008 compared to 2007 and the recognition of $946,800 in income from Paul Capital relatingrelated to the $10 million milestone payment forwe received from PRF in connection with the Rotarix® U.S. launch offset in part by interest expense of $155,972 in 2008 compared to none in 2007, lower interest rates in 2008 and the loss on sale of Select Vaccine shares.
Fiscal Year Ended December��31, 2007 compared with Fiscal Year ended December 31, 2006
Celldex reported a net loss of $15,073,050, or $1.81 per share, for the year ended December 31, 2007, a decrease of $2,762,212, or 15.5%, compared to a net loss of $17,835,262, or $2.15 per share, for the year ended December 31, 2006. The decrease in net loss between periods was due to decreased operating expenses, offset partially by increased revenues, investment and other income. The weighted average common shares outstanding used to calculate the net loss per common share was 8,309,420 in 2007 and 8,278,500 in 2006.
Revenue
Revenues totaled $1,405,592 and $899,184 for the years ended December 31, 2007 and 2006 respectively, an increase of $506,408, or 56.3%Rotarix®. Because revenues depend to a large extent on the grants and product development efforts of Celldex's collaborators, Celldex's period-to-period revenues can fluctuate significantly and are inherently difficult to predict.
Operating Expense
Research and development expenses consist primarily of (i) personnel expenses, (ii) facilities and supply expenses relating to Celldex's technology, (iii) development costs associated with Celldex's product candidates and (iv) fees paid to third parties in conjunction with Celldex's clinical and preclinical development programs. Research and development expenses decreased by $121,094, or 1.2%, from $10,012,803 to $9,891,709, during the year ended December 31, 2007, as compared to the year ended December 31, 2006. The changes relate primarily to costs associated with the following:
Celldex's general and administrative costs for the year ended December 31, 2007 were $6,905,487, a decrease of $1,490,214, or 17.7%, as compared to the year ended December 31, 2006. The decrease was primarily due to exiting of the U.K. facility and costs of operating the facility. This was partially offset by increased outside accounting and legal fees, stock-based compensation expense and Celldex's ongoing operations. General and administrative expenses, include salaries, benefits, stock based compensation, accounting, legal, business development and corporate administrative expense, including facility, travel, and other related expense.
Investment and Other Income, Net
Interest and other income decreased $524,200 to $435,486 in 2007 compared to $959,686 in 2006. The decrease was primarily due to lower cash balances in 2007 compared to 2006.
LIQUIDITY AND CAPITAL RESOURCES
At December 31, 2008, the Company's2009, our principal sources of liquidity consisted of cash, and cash equivalents and marketable securities of $44,257,286. The Company's$82.5 million. Our working capital at December 31, 2009 was $69.6 million. At December 31, 2009, we had 4% convertible subordinated debt due in February 2011 of $12.5 million. We incurred a loss of $36.5 million for the year ended December 31, 2009. Net cash used in operations for the year ended December 31, 2009 was $29.9 million. We believe that the cash inflows from existing grants and collaborations, interest income on invested funds and our current cash, cash equivalents and marketable securities at December 31, 2009 are sufficient to meet estimated working capital requirements and fund planned operations for at least the next twelve months.
Our cash equivalents are highly liquid investments with a maturity of three months or less at the date of purchase and consist primarily of time deposits and investments in money market mutual funds with commercial banks and financial institutions. Also, the Company maintainsWe maintain cash balances with financial institutions in excess of insured limits. The Company doesWe do not anticipate any losses with respect to such cash balances. We invest our excess cash balances in marketable securities including municipal bond securities, U.S. government agency securities, and high-grade corporate bonds that meet high credit quality standards, as specified in our investment policy. Our investment policy seeks to manage these assets to achieve our goals of preserving principal and maintaining adequate liquidity.
The use of the Company'sour cash flows for operations has primarily consisted of salaries and wages for itsour employees, facility and facility-related costs for itsour offices, laboratories and manufacturing facility, fees paid in connection with preclinical studies, clinical studies, contract manufacturing, laboratory supplies and services, consulting, legal and other professional fees. To date, and for the foreseeable future, the primary sources of cash flows from operations have been payments received from the Company'sour collaborative partners and from government entities. In general, the Company's sources of cash flows from operations for the foreseeable future will be upfront license payments, payments for the achievement of milestones, product royalty payments, payments under government contracts and grants and funded research and development under collaboration agreements that the Company may receive. The timing of any new collaboration agreements, government contracts or grants and any payments under these agreements, contracts or grants cannot be easily predicted and may vary significantly from quarter to quarter.
Cash Provided By or Used in Operating Activities
Net cash provided by operating activities was $18,280,766 for During the year ended December 31, 2008 comparednext twelve to cash used of $9,125,400 for the year ended December 31, 2007. The increase in net cash provided by operating activities was primarily attributed to Pfizer's one-time upfront payment to the Company of $40 million, PRF's one-time milestone payment of $10 million, a decrease in prepaid and other assets and an increase in accounts payable and accrued expenses, partially offset by increased net losses and an increase in accounts and other receivables. The Company expects that cash used in operations will increase in 2009 as the Company continues to develop its therapeutic product pipeline and bring forward new product candidates into clinical development.
Celldex has incurred and will continue to incur significant costs in the area of research and development, including preclinical and clinical trials, as its product candidates are developed. Celldex plans to spend significant amounts to progress its current product candidates through the clinical trial and commercialization process as well as to develop additional product candidates. As its product candidates progress through the clinical trial process, Celldextwenty-four months, we may be obligated to make significant milestone payments. Celldex also expects to incur future facility costs as a result of continued capital expansion, renovations and replacements. Celldex expect its general and administrative costs to increase as it expands its administrative and business development activities. Furthermore, Celldex expects
investment income to decrease as its funds future operations and capital expenditures from its cash reserves.
Cash Provided By or Used In Investing Activities
Cash provided by investing activities was $10,155,188 for the year ended December 31, 2008 compared to cash used in investing activities of $413,435 during 2007. The change in amounts between years primarily reflects the impact of the Merger and the result of increased expenditures on capital equipment in 2008, primarily for the conversion of the Company's Fall River facility into a cell culture manufacturing facility. The Company's investment in capital equipment is discretionary and it expects to spend less on capital expenditures in 2009.
Cash Provided by Financing Activities
Net cash provided by financing activities was $10,925,112 for the year ended December 31, 2008 compared to $264,339 for the year ended December 31, 2007. The increase in net cash provided by financing activities was primarily due to Pfizer's one-time $10 million equity investment in the Company and increases in the related party loan due to Medarex, offset in part by principal payments of long-term liabilities.
Other Liquidity Matters
On April 16, 2008, the Company and Pfizer entered into an agreement under which Pfizer was granted an exclusive worldwide license to a therapeutic cancer vaccine candidate, CDX-110, in Phase 2 development for the treatment of glioblastoma multiforme. The agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the licensing and development agreement, Pfizer made an upfront payment to the Company of $40 million and made a $10 million equity investment in the Company. Pfizer will fund all development costs for these programs. The Company is also eligible to receive potential milestone payments exceeding $390 million for the successful development and commercialization of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales. The Pfizer Agreement became effective after clearance under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (as amended) in May 2008.
On April 3, 2008, Rotarix® received FDA market approval for the prevention of rotavirus gastroenteritis in infants which triggered a $1.5 million milestone payment to the Company from Glaxo, $750,000 of which the Company has retained under its agreement with PRF. Rotarix® is now licensed in over 100 countries worldwide including the U.S. and the European Union. Glaxo's U.S. market launch of Rotarix® during the third quarter of 2008 resulted in a $10 million milestone payment from PRF, which the Company received in October 2008.
In 2009, the Company may take further steps to raise additional capital to meet our long-term liquidity needs including, but not limited to, the licensing of technology programs with existing or new collaborative partners, possible business combinations, or the issuance of common stock or other securities via private placements or public offerings. We believe that our current cash and cash equivalents are sufficient to fund planned operations for at least the next twelve months. While we may continue to seek capital through a number of means, there can be no assurance that additional financing will be available on acceptable terms, if at all, particularly in light of the recent disruptions in the financial markets and the Company'sour negotiating position in capital-raising efforts may worsen as existing resources are used. There is also no assurance that the Companywe will be able to enter into further collaborative relationships. Additional equity financing may be dilutive to the Company'sour stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict the Company'sour ability to operate as a business; and licensing or strategic collaborations may result in royalties or other terms which reduce the Company'sour economic potential from products under development. If the Company iswe are unable to raise the necessary
funds itnecessary to meet our long-term liquidity needs, we may have to delay or discontinue the development of programs, license out programs earlier than expected, raise funds at significant discount or on other unfavorable terms, if at all, or evaluate a sale ofsell all or part of the Company.us.
SUBSEQUENT EVENTSOperating Activities
In JanuaryNet cash used in operating activities was $29.9 million for the year ended December 31, 2009 compared to net cash provided by operating activities of $18.3 million for the Company enteredyear ended December 31, 2008. The increase in net cash used in operating activities was primarily due to Pfizer's up-front payment of $40 million received during the year ended December 31, 2008. We expect that cash used in operations will continue to increase in 2010 primarily related to the continued development of our therapeutic product pipeline, including bringing forward new product candidates into two transactions involvingclinical development.
We have incurred and will continue to incur significant costs in the salearea of its poultry vaccines businessresearch and development, including preclinical and clinical trials, as our product candidates are developed. We plan to spend significant amounts to progress our current product candidates through the clinical trial and commercialization process as well as to develop additional product candidates. As our product candidates progress through the clinical trial process, we may be obligated to make significant milestone payments.
Investing Activities
Net cash provided by investing activities was $45.1 million for the year ended December 31, 2009 compared to $10.2 million for the year ended December 31, 2008. The increase in net cash provided by investing activities was primarily due to the $51.7 million in cash acquired in the CuraGen Merger in 2009 offset by higher purchases of marketable securities in 2009 and the out-licensingcash acquired in the AVANT Merger in 2008. We expect to incur future facility cost as a result of its choleracontinued capital expansion, renovation and ETEC programs as more fully described below.replacements. Our investment in capital equipment is discretionary and there may be significant fluctuations on a quarterly basis.
(A) Lohmann Animal Health International ("LAHI")Financing Activities
On January 13,Net cash used in financing activities was $2.5 million for the year ended December 31, 2009 compared to net cash provided by financing activities of $10.9 million for the Company entered into a purchase agreementyear ended December 31, 2008. The increase in net cash used in financing activities was primarily due the $3.0 million payment to sell its poultry vaccines business to LAHI. Since 2002, LAHI has performed all manufacturing, marketingMedarex in 2009 and distribution activities for Celldex's marketed Megan®Vac 1 and Megan®Egg poultry vaccines and has paid Celldex product royalties. Financial terms ofPfizer's one-time $10 million equity investment during the transaction with LAHI includedyear ended December 31, 2008.
Other Liquidity Matters
Under the Pfizer Agreement, Pfizer made an upfront feepayment $40 million, an equity investment of $10.0 million and will fund all development costs for the licensed programs. We are also eligible to receive potential milestone payments.
(B) Vaccine Technologies, Inc. ("VTI")
On January 20, 2009,payments exceeding $390.0 million for the Company entered into an Exclusive License and Development Agreement with VTI. Under the license agreement, Celldex has granted a worldwide fee- and royalty-bearing exclusive license to VTI tosuccessful development and commercialize Celldex's CholeraGarde®commercialization of CDX-110 and ETECadditional EGFRvIII vaccine programs. Financial terms of the agreement with VTI include an upfront license fee, milestone payments andproducts, as well as royalties on net sales of licensed products during the term of the agreement.any product sales.
AGGREGATE CONTRACTUAL OBLIGATIONS
We have entered into licensing agreements with several universities, research organizations and other corporations. Under the terms of these agreements, we have received licenses or options to license technology, specified patents or patent applications. Our licensing and development collaboration agreements generally provide for royalty payments equal to specified percentages of product sales, annual license maintenance fees and continuing patent prosecution costs. In addition, we have committed to make potential future milestone payments to third parties of up to approximately $116 million as part of our various collaborations including licensing and development programs. Payments under these agreements generally become due and payable only upon achievement of certain developmental, regulatory and/or commercial milestones. Because the achievement of these milestones had not occurred as of December 31, 2009, such contingencies have not been recorded in our financial statements. We expect to incur approximately $0.9 million of milestone payments in 2010.
The following table summarizes Celldex'sour contractual obligations (not including contingent royalty and milestone payments as described above) at December 31, 20082009 and the effect such obligations and commercial commitments are expected to have on its liquidity and cash flow in future years. These obligations, commitments and supporting arrangements represent expected payments based on current operating forecasts, which are subject to change:
| | Total | 2009 | 2010 - 2012 | 2013 - 2014 | Thereafter | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Contractual obligations: | |||||||||||||||||
Operating lease obligations | $ | 19,989,900 | $ | 2,475,600 | $ | 7,374,200 | $ | 4,881,300 | $ | 5,258,800 | |||||||
Loan Payable* | 1,189,800 | 130,400 | 363,700 | 695,700 | |||||||||||||
Note Payable* | 401,700 | 177,200 | 224,500 | — | — | ||||||||||||
Licensing obligations | 3,630,000 | 510,000 | 1,755,000 | 830,000 | 535,000 | ||||||||||||
Severance obligations | 33,800 | 25,300 | 8,500 | — | — | ||||||||||||
Total contractual obligations | $ | 25,245,200 | $ | 3,318,500 | $ | 9,725,900 | $ | 6,407,000 | $ | 5,793,800 | |||||||
Commercial commitments: | |||||||||||||||||
Clinical development | $ | 5,502,600 | $ | 5,472,100 | $ | 30,500 | $ | — | $ | — | |||||||
Manufacturing development | 30,000 | 30,000 | |||||||||||||||
Total commercial commitments | $ | 5,532,600 | $ | 5,502,100 | $ | 30,500 | $ | — | $ | — | |||||||
| | Total | 2010 | 2011 - 2012 | 2013 - 2014 | Thereafter | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | (In thousands) | ||||||||||||||||
Contractual obligations: | |||||||||||||||||
Operating lease obligations(1) | $ | 17,963 | $ | 2,465 | $ | 5,063 | $ | 5,047 | $ | 5,388 | |||||||
Other contractual obligations(2) | 2,607 | 2,607 | — | — | — | ||||||||||||
Other long-term liabilities(3) | 810 | 180 | 154 | 118 | 358 | ||||||||||||
Convertible subordinated debt(4) | 12,503 | — | 12,503 | — | — | ||||||||||||
CuraGen Severance obligations | 2,623 | 1,918 | 705 | — | — | ||||||||||||
Total contractual obligations | $ | 36,506 | $ | 7,170 | $ | 18,425 | $ | 5,165 | $ | 5,746 | |||||||
InSuch amounts primarily consist of payments for our facility leases and assumes the future, weexercise of one five year renewal for our Fall River, MA facility.
RECENT ACCOUNTING PRONOUNCEMENTS
SFAS 141(R) and SFAS 160: In December 2007, the Financial Refer to Note 2, "Summary of Significant Accounting Standards Board ("FASB") issued SFAS No. 141(R),Business Combinations, ("SFAS No. 141(R)Policies,"), and SFAS No. 160,Noncontrolling Interests in Consolidated Financial Statements, an amendment of ARB No. 51, ("SFAS No. 160"), which introduce significant changes in the accounting for and reporting of business acquisitions and noncontrolling interests in a subsidiary. SFAS No. 141(R) isaccompanying notes to be applied prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. SFAS No. 160 is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption of both statements is prohibited. The adoption of SFAS No. 141(R) and SFAS No. 160 will only have an impact on the Company'sconsolidated financial statements if it is involved infor a business combination that occurs after January 1, 2009.
EITF 07-1: In December 2007, the EITF reached a consensus on Issue No. 07-1,Accounting for Collaborative Arrangements ("EITF 07-1"). The EITF concluded on the definitiondiscussion of a collaborative arrangement and that revenues and costs incurred with third parties in connection with collaborative arrangements would be presented gross or net based on the criteria in EITF 99-19,Reporting Revenue Gross as a Principal versus Net as an Agent, and otherrecent accounting literature. Based on the nature of the arrangement, payments to or from collaborators would be evaluated and its terms, the nature of the entity's business, and whether those payments are within the scope of other accounting literature would be presented. Companies are also required to disclose the nature and purpose of collaborative arrangements along with the accounting policies and the classification and amounts of significant financial statement amounts related to the arrangements. Activities in the arrangement conducted in a separate legal entity should be accounted for under other accounting literature; however, required disclosure under EITF 07-1 applies to the entire collaborative agreement. EITF 07-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years, and is to be applied retrospectively to all periods presented for all collaborative arrangements existing as of the effective date. The Company is currently evaluating the effect that the adoption of EITF 07-01 will have on its results of operations and financial condition.
FSP No. FAS 142-3: In April 2008, the FASB staff issued FASB Staff Position ("FSP") No. FAS 142-3,Determination of the Useful Life of Intangible Assets ("FSP No. FAS 142-3"). FSP No. FAS 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB No. 142. The intent of this FSP is to improve the consistency between the useful life of a recognized intangible under Statement 142 and the period of expected cash flows used to measure fair value of the asset under FASB No. 141 and other accounting principles generally accepted in the United States of America ("U.S.GAAP"). The FSP is effective for financial statements issued for fiscal years beginning after December 31, 2008, and interim periods within those fiscal years. Early adoption is prohibited. The adoption of FSP No. FAS 142-3 is not expected to have a material impact on Celldex's financial position and results of operations.
SFAS 162: In May 2008, FASB issued SFAS No. 162,"The Hierarchy of Generally Accepted Accounting Principles", or SFAS 162. SFAS 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements that are presented in conformity with generally accepted accounting principles in the United States. SFAS 162 is effective 60 days following the SEC's approval of the Public Company Accounting Oversight Board amendments to AU Section 411,"The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles." The Company does not expect SFAS 162 to have a material impact on its results of operations and financial condition.
EITF 03-6-1: In June 2008, FASB issued FASB Staff Position No. EITF 03-6-1,"Determining Whether Instruments Granted in Share-Based Payment Transactions Are Participating Securities", or FSP EITF 03-6-1. FSP EITF 03-6-1 addresses whether instruments granted in share-based payment transactions are participating securities prior to vesting and, therefore, need to be included in the earnings allocation in computing earnings per share (EPS) under the two-class method described in paragraphs 60 and 61 of FASB Statement No. 128,"Earnings per Share", or SFAS 128. The guidance applies to the calculation of EPS under SFAS 128 for share-based payment awards with rights to dividends or dividend equivalents. FSP EITF 03-6-1 clarifies that unvested share-based payment awards that contain nonforfeitable rights to dividends or dividend equivalents (whether paid or unpaid) are participating securities and shall be included in the computation of EPS pursuant to the two class method. FSP EITF 03-6-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those years. All prior-period EPS data presented shall be adjusted retrospectively (including interim financial statements, summaries of earnings and selected financial data) to conform with the provisions of this FSP. Early adoption is not permitted. The Company does not expect the adoption of FSP EITF 03-6-1 will have a material impact on its results of operations and financial condition.pronouncements.
OFF-BALANCE SHEET ARRANGEMENTS.
None.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We own financial instruments that are sensitive to market risk as part of our investment portfolio. Our investment portfolio is used to preserve our capital until it is used to fund operations, including our research and development activities. None of these market-risk sensitive instruments are held for trading purposes. We invest our cash primarily in money market mutual funds. These investments are evaluated quarterly to determine the fair value of the portfolio. From time to time, we invest our excess cash balances in marketable securities including municipal bond securities, U.S. government agency securities, and high-grade corporate bonds that meet high credit quality standards, as specified in our investment policy. Our investment portfolio includes only marketable securities with active secondary or resale marketspolicy seeks to help insuremanage these assets to achieve our goals of preserving principal and maintaining adequate liquidity. We have implemented investment policies regarding the amount and credit ratings of investments. Because of the short-term nature of these investments, we do not believe we have material exposure due to market risk. The impact to our financial position and results of operations from likely changes in interest rates is not material.
We do not utilize derivative financial instruments. See Note 1 to the Consolidated Financials Statements for a description of our use of other financial instruments. The carrying amounts reflected in the consolidated balance sheet of cash and cash equivalents, accounts receivables and accounts payable approximates fair value at December 31, 20082009 due to the short-term maturities of these instruments.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
| |||
| |||
| |||
| |||
| |||
| |||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To Thethe Board of Directors and Stockholders of
Celldex Therapeutics, Inc.:
In our opinion, the accompanying consolidated balance sheetsheets and the related consolidated statements of operations, and comprehensive loss, of stockholders' equity (deficit) and comprehensive loss, and of cash flows, present fairly, in all material respects, the financial position of Celldex Therapeutics, Inc. (formerly known as AVANT Immunotherapeutics, Inc.) and its subsidiaries at December 31, 2009 and December 31, 2008, and the results of their operations and their cash flows for each of the year thentwo years in the period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company did not maintain,maintained, in all material respects, effective internal control over financial reporting as of December 31, 2008,2009, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) because a material weakness in internal control over financial reporting existed related to the complement of the Company's accounting staff existing as of that date. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness referred to above is described in Management's Annual Report on Internal Control over Financial Reporting appearing under Item 9A. We considered this material weakness in determining the nature, timing, and extent of audit tests applied in our audit of the December 31, 2008 consolidated financial statements, and our opinion regarding the effectiveness of the Company's internal control over financial reporting does not affect our opinion on those consolidated financial statements.. The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in management's report referred to above.Management's Annual Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our integrated audit.audits. We conducted our auditaudits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the auditaudits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our auditaudits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our auditaudits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit providesaudits provide a reasonable basis for our opinions.
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for business combinations in 2009.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
March 2, 200912, 2010
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Celldex Therapeutics, Inc.
We have audited the accompanying consolidated balance sheetstatement of Celldex Research Corp. (formerly known as Celldex Therapeutics, Inc.) and subsidiary as of December 31, 2007, and the related consolidated statements of operations, and comprehensive loss, stockholders' equity (deficit) and comprehensive loss, and cash flowsflow of Celldex Therapeutics, Inc. and subsidiary for each of the two years in the periodyear ended December 31, 2007. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.audit.
We conducted our auditsaudit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purposes of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provideaudit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial positionresults of Celldex Research Corp. (formerly known as Celldex Therapeutics, Inc.) attheir operations and their cash flow for the year ended December 31, 2007 and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2007,Celldex Therapeutics, Inc. in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
MetroPark, New Jersey
May 7, 2008
CELLDEX THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
| | December 31, 2008 | December 31, 2007 | |||||||
|---|---|---|---|---|---|---|---|---|---|
ASSETS | |||||||||
Current Assets: | |||||||||
Cash and Cash Equivalents | $ | 44,257,286 | $ | 4,909,530 | |||||
Accounts and Other Receivables | 1,826,685 | 132,496 | |||||||
Prepaid and Other Current Assets | 992,473 | 656,347 | |||||||
Total Current Assets | 47,076,444 | 5,698,373 | |||||||
Property and Equipment, Net | 13,567,180 | 1,918,036 | |||||||
Intangible Assets, Net | 2,472,440 | 1,032,903 | |||||||
Other Assets | 6,677,171 | 725,193 | |||||||
Total Assets | $ | 69,793,235 | $ | 9,374,505 | |||||
LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) | |||||||||
Current Liabilities: | |||||||||
Accounts Payable | $ | 2,153,393 | $ | 749,867 | |||||
Accrued Expenses | 3,841,159 | 2,519,419 | |||||||
Payable Due Medarex | 2,957,248 | 5,835,552 | |||||||
Current Portion of Deferred Revenue | 4,931,327 | 974,156 | |||||||
Current Portion of Long-Term Liabilities | 218,459 | 57,447 | |||||||
Total Current Liabilities | 14,101,586 | 10,136,441 | |||||||
Deferred Revenue | 36,488,713 | 219,754 | |||||||
Other Long-Term Liabilities | 1,069,257 | 150,207 | |||||||
Commitments and Contingent Liabilities (Note 15) | |||||||||
Stockholders' Equity (Deficit): | |||||||||
Convertible Preferred Stock, 3,000,000 Shares Authorized; None Issued and Outstanding at December 31, 2008 | — | ||||||||
Convertible Preferred Stock, $1.00 Par Value; 1,000,000 Shares Authorized; None Issued and Outstanding at December 31, 2007 | — | ||||||||
Common Stock, $.001 Par Value; 300,000,000 Shares Authorized; 15,789,756 Issued and Outstanding at December 31, 2008 | 15,790 | ||||||||
Class A Common Stock, $.01 Par Value; 6,800,000 Shares Authorized, Issued and Outstanding at December 31, 2007 (2,811,147 shares issued and outstanding after adjustments to reflect the Merger and a reverse stock split of 1-for-12 effective March 7, 2008) | 2,811 | ||||||||
Common Stock, $.01 Par Value; 50,000,000 Shares Authorized; 13,300,000 Issued and Outstanding at December 31, 2007(5,498,273 shares issued and outstanding after adjustments to reflect the Merger and a reverse stock split of 1-for-12 effective March 7, 2008) | 5,498 | ||||||||
Additional Paid-In Capital | 136,661,181 | 69,889,205 | |||||||
Accumulated Other Comprehensive Income | 2,605,726 | 2,619,036 | |||||||
Accumulated Deficit | (121,149,018 | ) | (73,648,447 | ) | |||||
Total Stockholders' Equity (Deficit) | 18,133,679 | (1,131,897 | ) | ||||||
Total Liabilities and Stockholders' Equity (Deficit) | $ | 69,793,235 | $ | 9,374,505 | |||||
The accompanying notes are an integral part of the consolidated financial statements.
CELLDEX THERAPEUTICS, INC.CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS(In thousands, except share and per share amounts)
| | Year Ended December 31, 2008 | Year Ended December 31, 2007 | Year Ended December 31, 2006 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
REVENUE: | |||||||||||
Product Development and Licensing Agreements | $ | 3,715,957 | $ | 466,156 | $ | 466,156 | |||||
Contracts and Grants | 533,182 | 939,436 | 433,028 | ||||||||
Product Royalties | 3,206,368 | — | — | ||||||||
Total Revenue | 7,455,507 | 1,405,592 | 899,184 | ||||||||
OPERATING EXPENSE: | |||||||||||
Research and Development | 26,347,189 | 9,891,709 | 10,012,803 | ||||||||
General and Administrative | 14,747,392 | 6,905,487 | 8,395,701 | ||||||||
Charge for In-Process Research and Development | 14,755,908 | — | — | ||||||||
U.K Facility Exit Costs | — | — | 1,168,696 | ||||||||
Amortization of Acquired Intangible Assets | 361,006 | 116,932 | 116,932 | ||||||||
Total Operating Expense | 56,211,495 | 16,914,128 | 19,694,132 | ||||||||
Operating Loss | (48,755,988 | ) | (15,508,536 | ) | (18,794,948 | ) | |||||
Investment and Other Income, Net | 1,255,417 | 435,486 | 959,686 | ||||||||
Net Loss | $ | (47,500,571 | ) | $ | (15,073,050 | ) | $ | (17,835,262 | ) | ||
Basic and Diluted Net Loss Per Common Share (See Note 1) | $ | (3.34 | ) | $ | (1.81 | ) | $ | (2.15 | ) | ||
Shares Used in Calculating Basic and Diluted Net Loss per Share (See Note 1) | 14,217,388 | 8,309,420 | 8,278,500 | ||||||||
COMPREHENSIVE LOSS: | |||||||||||
Net Loss | $ | (47,500,571 | ) | $ | (15,073,050 | ) | $ | (17,835,262 | ) | ||
Unrealized Gain/(Loss) on Foreign Exchange Translation | (13,310 | ) | 230,840 | 2,882,403 | |||||||
Comprehensive Loss | $ | (47,513,881 | ) | $ | (14,842,210 | ) | $ | (14,952,859 | ) | ||
| | December 31, 2009 | December 31, 2008 | |||||||
|---|---|---|---|---|---|---|---|---|---|
ASSETS | |||||||||
Current Assets: | |||||||||
Cash and Cash Equivalents | $ | 57,002 | $ | 44,257 | |||||
Marketable Securities | 25,451 | — | |||||||
Accounts and Other Receivables | 544 | 1,827 | |||||||
Prepaid and Other Current Assets | 979 | 992 | |||||||
Total Current Assets | 83,976 | 47,076 | |||||||
Property and Equipment, Net | 11,489 | 13,567 | |||||||
Intangible Assets, Net | 29,979 | 2,473 | |||||||
Goodwill | 8,965 | — | |||||||
Other Assets | 5,955 | 6,677 | |||||||
Total Assets | $ | 140,364 | $ | 69,793 | |||||
LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||||
Current Liabilities: | |||||||||
Accounts Payable | $ | 1,445 | $ | 2,154 | |||||
Accrued Expenses | 5,615 | 3,841 | |||||||
Payable Due Medarex | — | 2,957 | |||||||
Current Portion of Deferred Revenue | 5,191 | 4,931 | |||||||
Current Portion of Long-Term Liabilities | 2,156 | 218 | |||||||
Total Current Liabilities | 14,407 | 14,101 | |||||||
Deferred Revenue | 34,191 | 36,489 | |||||||
Convertible Subordinated Debt | 11,684 | — | |||||||
Other Long-Term Liabilities | 6,315 | 1,069 | |||||||
Total Liabilities | 66,597 | 51,659 | |||||||
Commitments and Contingent Liabilities (Notes 12 and 15) | |||||||||
Stockholders' Equity: | |||||||||
Convertible Preferred Stock, $.01 Par Value; 3,000,000 Shares Authorized; No Shares Issued and Outstanding at December 31, 2009 and 2008 | — | — | |||||||
Common Stock, $.001 Par Value; 297,000,000 Shares Authorized; 31,685,061 and 15,789,756 Shares Issued and Outstanding at December 31, 2009 and 2008, respectively | 32 | 16 | |||||||
Additional Paid-In Capital | 228,863 | 136,661 | |||||||
Accumulated Other Comprehensive Income | 2,546 | 2,606 | |||||||
Accumulated Deficit | (157,674 | ) | (121,149 | ) | |||||
Total Stockholders' Equity | 73,767 | 18,134 | |||||||
Total Liabilities and Stockholders' Equity | $ | 140,364 | $ | 69,793 | |||||
The accompanying notes are an integral part of the consolidated financial statements.
CELLDEX THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| | Year Ended December 31, 2009 | Year Ended December 31, 2008 | Year Ended December 31, 2007 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
REVENUE: | |||||||||||
Product Development and Licensing Agreements | $ | 5,662 | $ | 3,716 | $ | 466 | |||||
Contracts and Grants | 1,802 | 533 | 940 | ||||||||
Product Royalties | 7,716 | 3,207 | — | ||||||||
Total Revenue | 15,180 | 7,456 | 1,406 | ||||||||
OPERATING EXPENSE: | |||||||||||
Research and Development | 26,169 | 22,636 | 9,892 | ||||||||
Royalty | 8,397 | 3,711 | — | ||||||||
Gain on Sale of Assets | (604 | ) | — | — | |||||||
Charge for In-Process Research and Development | — | 14,756 | — | ||||||||
General and Administrative | 17,119 | 14,748 | 6,905 | ||||||||
Amortization of Acquired Intangible Assets | 949 | 361 | 117 | ||||||||
Total Operating Expense | 52,030 | 56,212 | 16,914 | ||||||||
Operating Loss | (36,850 | ) | (48,756 | ) | (15,508 | ) | |||||
Investment and Other Income, Net | 248 | 1,411 | 435 | ||||||||
Interest Expense | (452 | ) | (156 | ) | — | ||||||
Net Loss Before Income Taxes | (37,054 | ) | (47,501 | ) | (15,073 | ) | |||||
Income Tax Benefit | 529 | — | — | ||||||||
Net Loss | $ | (36,525 | ) | $ | (47,501 | ) | $ | (15,073 | ) | ||
Basic and Diluted Net Loss Per Common Share (See Note 2) | $ | (1.84 | ) | $ | (3.34 | ) | $ | (1.81 | ) | ||
Shares Used in Calculating Basic and Diluted Net Loss per Share (See Note 2) | 19,823 | 14,217 | 8,309 | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
CELLDEX THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (DEFICIT)FOR THE YEARS ENDED DECEMBER 31, 2008, 2007 AND 2006
COMPREHENSIVE LOSS
(In thousands, except share amounts)
| | Common Stock Shares(1) | Common Stock Par Value(1) | Class A Common Stock Shares(1) | Class A Common Stock Par Value(1) | Additional Paid-In Capital | Accumulated Other Comprehensive Income | Accumulated Deficit | Total Stockholders' Equity (Deficit) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at December 31, 2005 | 5,456,933 | $ | 5,457 | 2,811,147 | $ | 2,811 | $ | 69,232,776 | $ | (494,207 | ) | $ | (40,740,135 | ) | $ | 28,006,702 | ||||||||||
Share-Based Compensation | — | — | — | — | 1,760,165 | — | — | 1,760,165 | ||||||||||||||||||
Shares Issued to Duke University in Connection with Licensing Agreement | 41,340 | 41 | — | — | 329,959 | — | — | 330,000 | ||||||||||||||||||
Comprehensive Income (Loss): | ||||||||||||||||||||||||||
Net Loss | — | — | — | — | — | — | (17,835,262 | ) | (17,835,262 | |||||||||||||||||
Other Comprehensive Income | — | — | — | — | — | 2,882,403 | — | 2,882,403 | ||||||||||||||||||
Total Comprehensive Loss | (14,952,859 | ) | ||||||||||||||||||||||||
Balance at December 31, 2006 | 5,498,273 | $ | 5,498 | 2,811,147 | $ | 2,811 | $ | 71,322,900 | $ | 2,388,196 | $ | (58,575,397 | ) | $ | 15,144,008 | |||||||||||
Share-Based Compensation | — | — | — | — | 1,604,922 | — | — | 1,604,922 | ||||||||||||||||||
Medarex Return of Capital | — | — | — | — | (3,038,617 | ) | — | — | (3,038,617 | ) | ||||||||||||||||
Comprehensive Income (Loss): | ||||||||||||||||||||||||||
Net Loss | — | — | — | — | — | — | (15,073,050 | ) | (15,073,050 | |||||||||||||||||
Other Comprehensive Income | — | — | — | — | — | 230,840 | — | 230,840 | ||||||||||||||||||
Total Comprehensive Loss | (14,842,210 | ) | ||||||||||||||||||||||||
Balance at December 31, 2007 | 5,498,273 | $ | 5,498 | 2,811,147 | $ | 2,811 | $ | 69,889,205 | $ | 2,619,036 | $ | (73,648,447 | ) | $ | (1,131,897 | ) | ||||||||||
Exchange of Class A for Common Stock | 2,811,147 | 2,811 | (2,811,147 | ) | (2,811 | ) | — | — | — | — | ||||||||||||||||
Shares Issued to Medarex in Settlement of a Payable | 351,692 | 352 | — | — | 3,038,265 | — | — | 3,038,617 | ||||||||||||||||||
Shares Received in Exchange in the Merger | 6,265,889 | 6,266 | — | — | 46,869,106 | — | — | 46,875,372 | ||||||||||||||||||
Cash Paid for Fractional Shares in Connection with the Merger | (7 | ) | — | — | — | — | — | — | — | |||||||||||||||||
Shares Issued to Pfizer in connection with the CDX-110 Licensing Agreement | 781,250 | 781 | 10,866,407 | 10,867,188 | ||||||||||||||||||||||
Shares Issued to Duke University in Settlement of a Payable | 81,512 | 82 | 1,182,505 | 1,182,587 | ||||||||||||||||||||||
Share-Based Compensation | — | — | — | — | 4,815,693 | — | — | 4,815,693 | ||||||||||||||||||
Comprehensive Loss: | ||||||||||||||||||||||||||
Net Loss | — | — | — | — | — | — | (47,500,571 | ) | (47,500,571 | ) | ||||||||||||||||
Other Comprehensive Loss | — | — | — | — | — | (13,310 | ) | — | (13,310 | ) | ||||||||||||||||
Total Comprehensive Loss | (47,513,881 | ) | ||||||||||||||||||||||||
Balance at December 31, 2008 | 15,789,756 | $ | 15,790 | — | $ | — | $ | 136,661,181 | $ | 2,605,726 | $ | (121,149,018 | ) | $ | 18,133,679 | |||||||||||
(1) Adjusted to reflect the Merger exchange ratio and a reverse stock split of 1-for-12 effective March 7, 2008.
| | Common Stock Shares | Common Stock Par Value | Class A Common Stock Shares | Class A Common Stock Par Value | Additional Paid-In Capital | Accumulated Other Comprehensive Income | Accumulated Deficit | Total Stockholders' Equity (Deficit) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at December 31, 2006 | 5,498,273 | $ | 5 | 2,811,147 | $ | 3 | $ | 71,323 | $ | 2,388 | $ | (58,575 | ) | $ | 15,144 | |||||||||||
Share-Based Compensation | — | — | — | — | 1,605 | — | — | 1,605 | ||||||||||||||||||
Medarex Return of Capital | — | — | — | — | (3,039 | ) | — | — | (3,039 | ) | ||||||||||||||||
Comprehensive Income (Loss): | ||||||||||||||||||||||||||
Net Loss | — | — | — | — | — | — | (15,073 | ) | (15,073 | ) | ||||||||||||||||
Translation Adjustments | — | — | — | — | — | 231 | — | 231 | ||||||||||||||||||
Total Comprehensive Loss | (14,842 | ) | ||||||||||||||||||||||||
Balance at December 31, 2007 | 5,498,273 | $ | 5 | 2,811,147 | $ | 3 | $ | 69,889 | $ | 2,619 | $ | (73,648 | ) | $ | (1,132 | ) | ||||||||||
Exchange of Class A for Common Stock | 2,811,147 | 3 | (2,811,147 | ) | (3 | ) | — | — | — | — | ||||||||||||||||
Shares Issued to Medarex in Settlement of a Payable | 351,692 | 1 | — | — | 3,038 | — | — | 3,039 | ||||||||||||||||||
Shares Received in Connection with the AVANT Merger | 6,265,882 | 6 | — | — | 46,869 | — | — | 46,875 | ||||||||||||||||||
Shares Issued to Pfizer in connection with the CDX-110 Licensing Agreement | 781,250 | 1 | 10,866 | 10,867 | ||||||||||||||||||||||
Shares Issued to Duke University in Settlement of a Payable | 81,512 | — | 1,183 | 1,183 | ||||||||||||||||||||||
Share-Based Compensation | — | — | — | — | 4,816 | — | — | 4,816 | ||||||||||||||||||
Comprehensive Loss: | ||||||||||||||||||||||||||
Net Loss | — | — | — | — | — | — | (47,501 | ) | (47,501 | ) | ||||||||||||||||
Translation Adjustments | — | — | — | — | — | (13 | ) | — | (13 | ) | ||||||||||||||||
Total Comprehensive Loss | (47,514 | ) | ||||||||||||||||||||||||
Balance at December 31, 2008 | 15,789,756 | $ | 16 | — | $ | — | $ | 136,661 | $ | 2,606 | $ | (121,149 | ) | $ | 18,134 | |||||||||||
Shares Issued in Connection with the CuraGen Merger | 15,722,713 | 16 | — | — | 88,227 | — | — | 88,243 | ||||||||||||||||||
Shares Issued under Stock Option and Employee Stock Purchase Plans | 172,592 | — | — | — | 917 | — | — | 917 | ||||||||||||||||||
Share-Based Compensation | — | — | — | — | 3,058 | — | — | 3,058 | ||||||||||||||||||
Comprehensive Loss: | ||||||||||||||||||||||||||
Net Loss | — | — | — | — | — | — | (36,525 | ) | (36,525 | ) | ||||||||||||||||
Translation Adjustments | — | — | — | — | — | (12 | ) | — | (12 | ) | ||||||||||||||||
Net Change in Unrealized Loss on Marketable Securities | — | — | — | — | — | (48 | ) | — | (48 | ) | ||||||||||||||||
Total Comprehensive Loss | (36,585 | ) | ||||||||||||||||||||||||
Balance at December 31, 2009 | 31,685,061 | $ | 32 | — | $ | — | $ | 228,863 | $ | 2,546 | $ | (157,674 | ) | $ | 73,767 | |||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
CELLDEX THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | Year Ended December 31, 2008 | Year Ended December 31, 2007 | Year Ended December 31, 2006 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Cash Flows From Operating Activities: | ||||||||||||
Net Loss | $ | (47,500,571 | ) | $ | (15,073,050 | ) | $ | (17,835,816 | ) | |||
Adjustments to Reconcile Net Loss to Cash Provided by (Used in) Operating Activities: | ||||||||||||
Depreciation and Amortization | 2,176,427 | 710,156 | 769,520 | |||||||||
Amortization of Intangible Assets | 361,006 | 116,932 | 116,932 | |||||||||
Impairment of Investment in Select Vaccines Limited | 297,146 | — | — | |||||||||
Loss (Gain) on Impairment and Disposal of Assets | 33,795 | — | (136,161 | ) | ||||||||
U.K. Facilities Exit Costs | — | — | 1,101,603 | |||||||||
Non-Cash License Fees Paid with Stock | — | — | 330,000 | |||||||||
In-Process Research and Development | 14,755,908 | — | — | |||||||||
Stock-Based Compensation Expense | 4,815,693 | 1,604,924 | 1,760,165 | |||||||||
Changes in Assets and Liabilities | ||||||||||||
Accounts and Other Receivables | (1,655,600 | ) | 4,167,335 | 940,000 | ||||||||
Prepaid and Other Current Assets | 9,979,807 | (587,077 | ) | 794,000 | ||||||||
Other Assets—Deferred Costs | (6,413,770 | ) | — | — | ||||||||
Accounts Payable and Accrued Expenses | 1,221,278 | 28,116 | (1,300,000 | ) | ||||||||
Deferred Revenue | 40,116,130 | 42,304 | (466,157 | ) | ||||||||
Other Long-Term Liabilities—Deferred Rent | 93,517 | (78,808 | ) | 286,462 | ||||||||
Net Cash Provided by (Used in) Operating Activities | 18,280,766 | (9,125,400 | ) | (13,639,452 | ) | |||||||
Cash Flows From Investing Activities: | ||||||||||||
Cash Acquired in the Acquisition of AVANT, Net of Transaction Costs | 10,750,255 | — | — | |||||||||
Other Non Current Assets | — | (335,054 | ) | — | ||||||||
Restricted Cash Deposits | (1,737 | ) | (3,070 | ) | 168,000 | |||||||
Acquisition of Property and Equipment | (1,304,706 | ) | (75,311 | ) | (2,478,719 | ) | ||||||
Proceeds from Disposal or Sale of Assets | 460,494 | — | 144,000 | |||||||||
Proceeds from Sale of Shares of Select Vaccines Limited | 250,882 | — | — | |||||||||
Net Cash Provided by (Used in) Investing Activities | 10,155,188 | (413,435 | ) | (2,166,719 | ) | |||||||
Cash Flows From Financing Activities: | ||||||||||||
Net Proceeds from Stock Issuance | 10,867,188 | — | — | |||||||||
Related Party Loan Due to Medarex | 160,313 | 264,339 | 2,077,573 | |||||||||
Payment of Loans and Note Payable | (102,389 | ) | — | — | ||||||||
Net Cash Provided by Financing Activities | 10,925,112 | 264,339 | 2,077,573 | |||||||||
Effect of Exchange Rate Changes on Cash and Cash Equivalents | (13,310 | ) | 183,840 | 2,516,000 | ||||||||
Net Increase (Decrease) in Cash and Cash Equivalents | 39,347,756 | (9,090,656 | ) | (11,212,598 | ) | |||||||
Cash and Cash Equivalents at Beginning of Period | 4,909,530 | 14,000,186 | 25,212,784 | |||||||||
Cash and Cash Equivalents at End of Period | $ | 44,257,286 | $ | 4,909,530 | $ | 14,000,186 | ||||||
Supplemental Disclosure of Non-Cash Flow Information | ||||||||||||
Shares Received in Exchange in the Merger | $ | 46,251,952 | $ | — | $ | — | ||||||
Shares Issued to Medarex in Settlement of a Payable | $ | 3,038,617 | — | $ | — | |||||||
Shares Issued to Duke University in Settlement of a Payable | $ | 1,182,587 | $ | — | $ | — | ||||||
Supplemental Disclosure of Cash Flow Information | ||||||||||||
Cash Paid for Interest | $ | 142,210 | $ | — | $ | — | ||||||
| | Year Ended December 31, 2009 | Year Ended December 31, 2008 | Year Ended December 31, 2007 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Cash Flows From Operating Activities: | ||||||||||||
Net Loss | $ | (36,525 | ) | $ | (47,501 | ) | $ | (15,073 | ) | |||
Adjustments to Reconcile Net Loss to Cash Provided by (Used in) Operating Activities: | ||||||||||||
Depreciation and Amortization | 2,583 | 2,176 | 710 | |||||||||
Amortization of Intangible Assets | 949 | 361 | 117 | |||||||||
Realized Loss on Sales and Maturities of Marketable Securities | 24 | — | — | |||||||||
(Gain) Loss on Sale or Disposal of Assets | (556 | ) | 331 | — | ||||||||
Stock-Based Compensation Expense | 3,058 | 4,816 | 1,605 | |||||||||
Non-Cash Interest Expense | 181 | — | — | |||||||||
Non-Cash Tax Benefit | (529 | ) | — | — | ||||||||
In-Process Research and Development | — | 14,756 | — | |||||||||
Changes in Operating Assets and Liabilities, Net of Acquisitions | ||||||||||||
Accounts and Other Receivables | 1,283 | (1,656 | ) | 4,167 | ||||||||
Prepaid and Other Current Assets | 743 | 9,980 | (587 | ) | ||||||||
Other Assets | 722 | (6,414 | ) | — | ||||||||
Accounts Payable and Accrued Expenses | (2,463 | ) | 1,221 | (28 | ) | |||||||
Deferred Revenue | (2,038 | ) | 40,116 | 42 | ||||||||
Other Long-Term Liabilities | 2,699 | 94 | (79 | ) | ||||||||
Net Cash (Used in) Provided by Operating Activities | (29,869 | ) | 18,280 | (9,126 | ) | |||||||
Cash Flows From Investing Activities: | ||||||||||||
Cash Acquired in AVANT Merger, Net of Transaction Costs | — | 10,750 | — | |||||||||
Cash Acquired in CuraGen Merger | 51,654 | — | — | |||||||||
Sales and Maturities of Marketable Securities | 2,674 | — | — | |||||||||
Purchases of Marketable Securities | (9,559 | ) | — | — | ||||||||
Other Non Current Assets | — | — | (335 | ) | ||||||||
Restricted Cash Deposits | — | (2 | ) | (3 | ) | |||||||
Acquisition of Property and Equipment | (528 | ) | (1,305 | ) | (75 | ) | ||||||
Proceeds from Sale or Disposal of Assets | 850 | 712 | — | |||||||||
Net Cash Provided by (Used in) Investing Activities | 45,091 | 10,155 | (413 | ) | ||||||||
Cash Flows From Financing Activities: | ||||||||||||
Net Proceeds from Stock Issuances | 668 | 10,867 | — | |||||||||
Related Party Loan Due to Medarex | (2,957 | ) | 160 | 265 | ||||||||
Payment of Other Long-Term Liabilities | (176 | ) | (102 | ) | — | |||||||
Net Cash (Used in) Provided by Financing Activities | (2,465 | ) | 10,925 | 265 | ||||||||
Effect of Exchange Rate Changes on Cash and Cash Equivalents | (12 | ) | (13 | ) | 184 | |||||||
Net Increase (Decrease) in Cash and Cash Equivalents | 12,745 | 39,347 | (9,090 | ) | ||||||||
Cash and Cash Equivalents at Beginning of Period | 44,257 | 4,910 | 14,000 | |||||||||
Cash and Cash Equivalents at End of Period | $ | 57,002 | $ | 44,257 | $ | 4,910 | ||||||
Supplemental Disclosure of Non-Cash Flow Information | ||||||||||||
Shares Received in Exchange in the Merger | $ | — | $ | 46,252 | $ | — | ||||||
Shares Issued to Medarex in Settlement of a Payable | $ | — | $ | 3,039 | $ | — | ||||||
Shares Issued to Duke University in Settlement of a Payable | $ | — | $ | 1,183 | $ | — | ||||||
Shares Issued to Executive Officers | $ | 250 | $ | — | $ | — | ||||||
Supplemental Disclosure of Cash Flow Information | ||||||||||||
Cash Paid for Interest | $ | 157 | $ | 142 | $ | — | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTSYEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1) SUMMARYNATURE OF SIGNIFICANT ACCOUNTING POLICIESBUSINESS AND OVERVIEW
Nature of Business and Overview
Celldex Therapeutics, Inc. (formerly known as AVANT Immunotherapeutics, Inc.) (the "Company" or "Celldex") is engaged in the discovery, development and commercialization of productsan integrated biopharmaceutical company that harness the human immune systemapplies its comprehensive Precision Targeted Immunotherapy Platform to prevent and treat disease. The Company is developing a portfolio of vaccines and targeted immunotherapeutics addressing a wide range of applications including oncology, infectious and inflammatory diseases. The portfolio includesgenerate a pipeline of therapeuticcandidates to treat cancer vaccines,and other difficult-to-treat diseases. The Company's immunotherapy platform includes a complementary portfolio of monoclonal antibodies, single-dose, oralantibody-targeted vaccines aimed at protecting travelers and people in regions where infectious diseases are endemic and a treatmentimmunomodulators to reduce complement-mediated tissue damage. The Company is advancing a pipeline of clinical and preclinical product candidates, the most advanced of which are for treatment of various cancers.create novel disease-specific drug candidates. The Company's lead programs are therapeutic cancer vaccines designed to instruct the patient's immune system to recognize and destroy cancer cells. The Company further leverages the value of its technology portfolio through corporate, governmental and non-governmental partnerships. One successful collaboration with GlaxoSmithKline ("Glaxo") resulted in our licensethe commercialization of a rotavirus strain to GlaxoSmithKline that was used in the development ofRotarix®, an oral human rotavirus vaccine. Current collaborations encompass the development of vaccines addressed to cancer therapies, global health, human food safety and animal health. The Company's product candidates address large market opportunities for which the Company believes current therapies are inadequate or non-existent.
Merger between AVANT and Celldex: On March 7, 2008, Celldex (formerly known as AVANT Immunotherapeutics, Inc.) completed the merger of Callisto Merger Corporation ("Merger Sub"), a wholly owned subsidiary of Celldex, with and into Celldex Research Corporation (formerly known as Celldex Therapeutics, Inc.) ("Celldex Research"), a privately-held company, (the "Merger"). Effective October 1, 2008, the Company changed its name from AVANT Immunotherapeutics, Inc. to Celldex Therapeutics, Inc.
At the special meeting of the Company's shareholders held on March 6, 2008 in connection with the Merger, stockholders approved four proposals: (i) the issuance of shares of the Company's common stock pursuant to the Merger Agreement in the amount necessary to result in the former Celldex Research stockholders owning 58% of the Company's common stock on a fully diluted basis, (ii) an amendment to the Company's Third Restated Certificate of Incorporation to increase the number of authorized shares to 300,000,000, (iii) an amendment to the Company's Third Restated Certificate of Incorporation to effect a reverse stock split in a ratio ranging from one-for-twelve to one-for-twenty of all the issued and outstanding shares of the Company's common stock, the final ratio to be determined by the Company's board of directors and (iv) adoption of the 2008 Stock Option and Incentive Plan.
Also, pursuant to the terms of the Merger Agreement, former Celldex Research shareholders received 4.96 shares of the Company's common stock in exchange for each share of Celldex Research common stock and Class A common stock they owned at the effective time of the Merger, plus cash in lieu of fractional shares. The Company also assumed all of Celldex Research's stock options outstanding at the effective time of the Merger.
The Company's board of directors approved a 1-for-12 reverse stock split of the Company's common stock, which became effective on March 7, 2008. As a result of the reverse stock split, each twelve shares of common stock were combined and reclassified into one share of common stock and the total number of shares outstanding was reduced from approximately 180 million shares (including
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
the shares issued to former Celldex Research stockholders in the Merger) to approximately 15 million shares.
The Merger was accounted for using the purchase method of accounting and was treated as an acquisition by Celldex Research of Celldex (then AVANT), with Celldex Research being considered the accounting acquirer based on the application of criteria specified in Statement of Financial Accounting Standards ("SFAS") No. 141,Business Combination, ("SFAS 141"), even though Celldex (then AVANT) was the issuer of common stock and the surviving legal entity in the transaction. Under the purchase method of accounting, the deemed purchase price was allocated to AVANT's underlying tangible and identifiable intangible assets acquired and liabilities assumed based upon the respective fair value of each with any excess deemed purchase price allocated to goodwill. The valuation analysis conducted by the Company determined that the fair value of assets acquired and the fair value of liabilities assumed by Celldex Research exceeded the purchase price for AVANT, resulting in negative goodwill of approximately $6.0 million. In accordance with SFAS 141, the negative goodwill has been allocated to all of the acquired assets that were non-financial and non-current assets, including property and equipment, identifiable intangible assets, and in-process research and development. See Note 17 to the Company's consolidated financial statements for additional information.
Because Celldex Research was determined to be the acquirer for accounting purposes, the historical financial statements of Celldex Research became the historical financial statements of the Company as of the closing of the Merger. Accordingly, the financial statements of the Company prior to the Merger reflect the financial position, results of operations and cash flows of Celldex Research, which during the historical periods presented in the accompanying consolidated financial statements, was then majority-owned by Medarex, Inc. ("Medarex"). Following the Merger, the financial statements of the current period reflect the financial position, results of operation and cash flows of the Company. The results of operations of AVANT are included in the results of operations of the Company beginning March 8, 2008. Accordingly, except as otherwise discussed below, this report reflects the financial condition, results of operations and liquidity of the combined companies at December 31, 2008 and historically of Celldex Research on a stand-alone basis for all periods prior to March 8, 2008.
The Company's cash and cash equivalents at December 31, 2008 were $44,257,286. Its working capital at December 31, 2008 was $32,974,858. The Company incurred a loss of $47,500,571 for the year ended December 31, 2008. Net cash provided by operations for the year ended December 31, 2008 was $18,280,766. The Company believes that cash inflows from existing grants and collaborations, interest income on invested funds and its current cash and cash equivalents will be sufficient to meet estimated working capital requirements and fund operations beyond December 31, 2009. The working capital requirements of the Company are dependent on several factors including, but not limited to, the costs associated with research and development programs, pre-clinical and clinical studies, manufacture of clinical materials and the scope of collaborative arrangements.
During 2009, Celldex may take steps to raise additional capital including, but not limited to, the licensing of technology programs with existing or new collaborative partners, possible business combinations, or the issuance of common stock via private placements or public offerings. The Company believes that its current cash and cash equivalents are sufficient to fund planned operations for at least the next twelve months. While the Company continues to seek capital through a number of means, there can be no assurance that additional financing will be available on acceptable terms, if at
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
all, particularly in light of the recent disruptions in the financial markets and the Company's negotiating position in capital-raising efforts may worsen as existing resources are used. There is also no assurance that the Company will be able to enter into further collaborative relationships. Additional equity financing may be dilutive to the Company's stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict the Company's ability to operate as a business; and licensing or strategic collaborations may result in royalties or other terms that reduce the Company's economic potential from products under development. If the Company is unable to raise the necessary funds, it may have to delay or discontinue the development of programs, license out programs earlier than expected, raise funds at significant discount or on other unfavorable terms, if at all, or evaluate a sale of all or a part of the Company.
On April 16, 2008, the Company and Pfizer Inc. ("Pfizer") entered into a License and Development Agreement (the "Pfizer Agreement") under which Pfizer will bewas granted an exclusive worldwide license to a therapeutic cancer vaccine candidate, CDX-110, in Phase 2 development for the treatment of glioblastoma multiforme ("GBM"). The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the Pfizer Agreement, Pfizer made an upfront payment to the Company of $40 million and made a $10 million equity investment in the Company. Pfizer will fund all development costs for these programs. The Company is also eligible to receive potential milestone payments exceeding $390 million for the successful development and commercialization of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales.
On April 3, 2008, Rotarix® received Food The Company's other clinical and Drug Administration ("FDA")preclinical product candidates address large market approvalopportunities for the prevention of rotavirus gastroenteritis in infants. FDA approval triggered a $1.5 million milestone payment to the Company from GlaxoSmithKline plc ("Glaxo"), $750,000 of which the Company has retained underbelieves current therapies are inadequate or non-existent.
AVANT Merger
On March 7, 2008, the Company's agreementCompany (formerly known as AVANT Immunotherapeutics, Inc.) ("AVANT") merged with Paul Royalty FundCelldex Research Corporation (formerly known as Celldex Therapeutics, Inc.) ("PRF"Celldex Research"), a privately-held company, (the "AVANT Merger"). Rotarix® is now licensed in over 100 countries worldwide includingEffective October 1, 2008, the U.S.Company changed its name from AVANT Immunotherapeutics, Inc. to Celldex Therapeutics, Inc.
The AVANT Merger was accounted for using the former purchase method of accounting and was treated as an acquisition by Celldex Research of AVANT, with Celldex Research being considered the accounting acquirer even though AVANT was the issuer of common stock and the European Union. Glaxo initiated its U.S. launchsurviving legal entity in the transaction. Under the former purchase method of Rotarix®accounting, the deemed purchase price was allocated to AVANT's underlying tangible and identifiable intangible assets acquired and liabilities assumed based upon the respective fair value of each with any excess deemed purchase price allocated to goodwill. The valuation analysis conducted by the Company determined that the fair value of assets acquired and the fair value of liabilities assumed by Celldex Research exceeded the purchase price for AVANT, resulting in negative goodwill of approximately $6.0 million. The negative goodwill was allocated to all of the acquired assets that were non-financial and non-current assets, including property and equipment, identifiable intangible assets, and in-process research and development ("IPR&D").
Because Celldex Research was determined to be the acquirer for accounting purposes, the historical financial statements of Celldex Research became the historical financial statements of the Company as of the closing of the AVANT Merger. Accordingly, the financial statements of the Company prior to the AVANT Merger reflect the financial position, results of operations and cash flows of Celldex Research, which during the third quarter of 2008 which resultedhistorical periods presented in the Company receiving a $10 million milestone payment from PRF in October 2008.
Basis of Presentation
Theaccompanying consolidated financial statements, includewas then majority-owned by Medarex, Inc. ("Medarex"). Following the accountsAVANT Merger, the financial statements reflect the financial position, results of Celldex Therapeutics, Inc. and its direct and indirect wholly-owned subsidiaries: Celldex Research, Celldex Therapeutics, Ltd. ("Celldex Ltd.") and Megan Health, Inc. ("Megan"). All intercompany transactions have been eliminated.
Cash and Cash Equivalents
Cashoperation and cash equivalents consistflows of cashthe combined companies. Accordingly, this report reflects the financial condition, results of operations and short-term investments with original maturitiesliquidity of three months or less. Cash and cash equivalents are statedthe combined companies at cost, which approximates fair value. At December 31, 2009 and 2008 investments were primarily in money market mutual funds.
Celldex may invest its cash in debt instruments of financial institutions, government entities and corporations, and mutual funds. The Company has established guidelines relative to credit ratings, diversification and maturities to mitigate risk and maintain liquidity. Financial instruments, which potentially subjectfor the Company to concentrations of credit risk, consist principally of cash and cashperiod from the AVANT Merger through December 31, 2009.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006(1) NATURE OF BUSINESS AND OVERVIEW (Continued)
(1)Acquisition of CuraGen Corporation ("CuraGen")
As more fully discussed in Note 19, on October 1, 2009, CuraGen, a former publicly-traded company, merged with a wholly-owned subsidiary ("Merger Sub") of the Company (the "CuraGen Merger") in accordance with a definitive merger agreement dated May 28, 2009 (the "CuraGen Merger Agreement"). Following the CuraGen Merger, the financial statements reflect the financial position, results of operation and cash flows of the combined companies. Accordingly, this report reflects the financial condition, results of operations and liquidity of the combined companies at December 31, 2009 and for the period from the CuraGen Merger through December 31, 2009.
On December 31, 2009, the Company completed the merger of the Merger Sub with and into Celldex pursuant to a short-form merger effected under Delaware law. As a result, the separate corporate existence of CuraGen has ceased and the Company has succeeded to all rights, privileges, powers and franchises of CuraGen.
Capital Requirements
At December 31, 2009, the Company had cash, cash equivalents and marketable securities of $82.5 million; working capital of $69.6 million; and 4% convertible subordinated debt ("CuraGen Debt") due in February 2011 of $12.5 million. The Company incurred a loss of $36.5 million for the year ended December 31, 2009. Net cash used in operations for the year ended December 31, 2009 was $29.9 million. The Company believes that the cash, cash equivalents and marketable securities at December 31, 2009 in addition to the cash inflows from existing grants and collaborations and interest income on invested funds will be sufficient to meet estimated working capital requirements and fund planned operations for at least the next twelve months. The working capital requirements of the Company are dependent on several factors including, but not limited to, the costs associated with research and development programs, preclinical and clinical studies, manufacture of clinical materials, the scope of collaborative arrangements.
During the next twelve to twenty-four months, the Company may take steps to raise additional capital to meet its long-term liquidity needs including, but not limited to, the licensing of technology programs with existing or new collaborative partners, possible business combinations, or the issuance of common stock via private placements or public offerings. While the Company continues to seek capital through a number of means, there can be no assurance that additional financing will be available on acceptable terms, if at all, particularly in light of the recent disruptions in the financial markets. The Company's negotiating position in capital-raising efforts may worsen as existing resources are used. There is also no assurance that the Company will be able to enter into further collaborative relationships. Additional equity financing may be dilutive to the Company's stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict the Company's ability to operate as a business; and licensing or strategic collaborations may result in royalties or other terms that reduce the Company's economic potential from products under development. If the Company is unable to raise the funds necessary to meet its long-term liquidity needs, it may have to (i) delay or discontinue the development of programs, (ii) license out programs earlier than expected, (iii) raise funds at significant discount or on other unfavorable terms, or (iv) sell all or a part of the Company.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The consolidated financial statements reflect the operations of the Company and its wholly-owned subsidiaries. All intercompany transactions have been eliminated. The Company operates in one segment, which is the business of development, manufacturing and commercialization of novel therapeutics for human health care.
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America ("U.S. GAAP") requires management to make estimates and use assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the dates of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with a maturity date of 90 days or less at the date of purchase to be cash equivalents. Cash equivalents consist principally of money market funds and debt securities.
Marketable Securities
The Company invests its excess cash balances in marketable securities including municipal bond securities, U.S. government agency securities, and high-grade corporate bonds. The Company classifies all of its marketable securities as current assets on the consolidated balance sheets because they are available-for-sale and available to fund current operations. Marketable securities are stated at fair value with their unrealized gains and losses included as a component of accumulated other comprehensive income (loss), which is a separate component of stockholders' equity, until such gains and losses are realized. The fair value of these securities is based on quoted prices for identical or similar assets. If a decline in the fair value is considered other-than-temporary, based on available evidence, the unrealized loss is transferred from other comprehensive income (loss) to the consolidated statements of operations. Realized gains and losses are determined on the specific identification method and are included in investment and other income, net.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk primarily consist of cash, cash equivalents, marketable securities and accounts receivable. The Company invests its cash, cash equivalents and marketable securities in debt instruments and interest bearing accounts at major financial institutions in excess of insured limits. The Company mitigates credit risk by limiting the investment type and maturity to securities that preserve capital, maintain liquidity and have a high credit quality.
The Company has not historically experienced credit losses from its accounts receivable and therefore has not established an allowance for doubtful accounts. Receivables of $0.2 million and $1.4 million were due from Pfizer at December 31, 2009 and 2008, respectively.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
equivalents Revenue from Glaxo and accounts receivable. CashPfizer represented 52% and cash equivalents consist of cash34% for the year ended December 31, 2009 and money market funds which are all held with three financial institutions in50% and 38% for the U.S. and one financial institution in the United Kingdom.
Investment in Securities
In August 2008, the Company sold its equity investment in Select Vaccines Limited ("Select Vaccines") shares for net proceeds of $250,882 and recorded a loss of $297,129. The Company had classified its equity investment in Select Vaccines shares as available-for-sale securities under SFAS 115,Accounting for Certain Investments in Debt and Equity Securities, ("FAS 115").
Restricted Cash
Restricted cash of $182,130 and $180,139 atyear ended December 31, 2008, andof total Company revenue, respectively. For the year ended December 31, 2007, respectively, represents security deposits forcertain other customers represented more than 10% of total Company revenue and these customers are not expected to represent 10% or more of total Company revenue in the Company's facilities in Phillipsburg, New Jersey, of which the Company took occupancy in 2006.future.
Fair Value of Financial Instruments
The Company enters into various types of financial instruments in the normal course of business. The carrying amounts of the Company's cash and cash equivalents, accounts receivable, accounts payable and accrued expenses approximate their fair values due to the short-term nature of these financial instruments. Receivables are concentrated in the pharmaceutical industryitems. See Note 3 for additional information.
Property and from United Kingdom Inland Revenue. Management considers the likelihood of market credit risk to be remote.
Accounts Receivable and Significant CustomersEquipment
Trade accounts receivable are recorded at the invoiced amount and do not bear interest. The Company has not historically experienced credit losses from its accounts receivable and therefore has not established an allowance for doubtful accounts. The Company does not have any off-balance-sheet credit exposure related to its customers.
Accounts and other receivables consist of the following:
| | December 31, 2008 | December 31, 2007 | |||||
|---|---|---|---|---|---|---|---|
Trade | $ | 1,690,029 | $ | — | |||
Other | 136,656 | 132,496 | |||||
| $ | 1,826,685 | $ | 132,496 | ||||
At December 31, 2008, trade receivables primarily consist of $1,431,382 due from Pfizer (see Note 10).
Other receivables primarily consist of money market interest receivable, an employee loan receivable and research and development tax credit receivable from United Kingdom Inland Revenue.
For the year ended December 31, 2008, revenue from Glaxo and Pfizer represented 50% and 38%, respectively, of total Company revenue. For the years ended December 31, 2007 and 2006, certain
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
customers represented more than 10% of total Company revenue. This was due to low levels of revenue in such years, and these customers in future years are not expected to represent 10% or more of total Company revenue.
Long-Lived Assets:
In the ordinary course of its business, the Company incurs substantial costs to construct property and equipment. The treatment of costs to construct these assetsproperty and equipment depends on the nature of the costs and the stage of construction. Costs incurred in the project planning, and design, phase, and in the construction and installation phase,phases are capitalized as part of the cost of the asset. The Company stops capitalizing these costs when the asset is substantially complete and ready for its intended use.
For manufacturing property and equipment, the Company also capitalizes the cost of validating these assets for the underlying manufacturing process. CelldexThe Company completes the capitalization of validation costs when the asset is substantially complete and ready for its intended use. Costs capitalized include incremental labor and fringe benefits, and direct consultancy services. The Company capitalizes interest cost as part of the historical cost of acquiring certain assets during the period of time required to get the asset ready for its intended use.
Property and equipment is stated at cost and depreciated over the estimated useful lives of the related assets using the straight-line method. Laboratory equipment and office furniture and equipment are depreciated over a five-year periodfive years and computer equipment is depreciated over a three-year period.three years. Manufacturing equipment is amortized over a seven-seven to ten-year period.ten years. Leasehold improvements are amortized over the shorter of the estimated useful life or the non-cancelable term of the related lease, including any renewals that are reasonably assured of occurring. Property and equipment under construction is classified as construction in progress and is depreciated or amortized only after the asset is placed in service. Expenditures for maintenance and repairs are charged to expense whereas the costs of significant improvements which extend the life of the underlying asset are capitalized. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are eliminated and the related gainsany resulting gain or losses areloss is reflected in net income.the Company's consolidated statements of operations.
AccountingBusiness Combinations
On January 1, 2009, the Company adopted the new acquisition method of accounting for business combinations that are completed after January 1, 2009, including the Impairment of Long-Lived Assets:
CuraGen Merger. The Company periodically evaluates its long-livedassigns the value of the consideration transferred to acquire a business to the tangible assets primarily property and equipment andidentifiable intangible assets for potential impairment under SFAS No. 144,Accounting foracquired and liabilities assumed on the Impairmentbasis of Long-Lived Assets, ("SFAS No. 144").their fair values at the date of acquisition. The Company performs these evaluations whenever events or changes in circumstances suggest thatassesses the carrying amount of an asset or groupfair value of assets, is not recoverable. Indicatorsincluding intangible assets such as IPR&D, using a variety of potential impairment include:
If the Company believes an indicator of potential impairment exists, the carryingfair values of the assets are evaluated in relation to the operating performance and future undiscounted cash flows of the underlying asset. The net book value of an asset is adjusted to fair value if its expected future
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1)(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
undiscountedIPR&D assets incorporates significant assumptions regarding the estimates a market participant would make in order to evaluate an asset, including a market participant's assumptions regarding the probability of completing IPR&D projects, which would require obtaining regulatory approval for marketing of the associated drug candidate; a market participant's estimates regarding the timing of and the expected costs to complete IPR&D projects; a market participant's estimates of future cash flows from potential product sales; and the appropriate discount rates for a market participant. Transaction costs and restructuring costs associated with the transaction are less than its book value. Theexpensed as incurred.
For acquisitions completed prior to January 1, 2009, including the AVANT Merger, the Company charges impairmentsexpensed the fair value of IPR&D of $14.8 million to research and development expense as of the long-livedacquisition date and included transaction costs associated with the business combination as part of the cost of the acquired company. See Notes 18 and 19 for discussion of the AVANT and CuraGen Merger, respectively.
Intangible Assets
IPR&D assets to operations if its evaluations indicate thatacquired in a business combination initially are recorded at fair value and accounted for as indefinite-lived intangible assets. These assets are maintained on the Company's consolidated balance sheets until either the project underlying them is completed or the assets become impaired. If a project is completed, the carrying value of these assetsthe related intangible asset is not recoverable. Management had identified no indicatorsamortized over the remaining estimated life of impairment at December 31, 2008. When we determine thatthe asset beginning in the period in which the project is completed. If a project becomes impaired or is abandoned, the carrying value of the related intangible asset is written down to its fair value and an impairment charge is taken in the period in which the impairment occurs. IPR&D assets will be tested for impairment on an annual basis during the third quarter, or long-livedearlier if impairment indicators are present.
Intangible assets is not recoverable, we may be required to record impairment charges for these assets that have not been previously recorded.
Accounting for Patent Costs:
Patent costs are expensed as incurred. Certain patent costs are reimbursed by the Company's product development and licensing partners. Any reimbursed patent costsacquired in a business combination with a finite life are recorded as product development and licensing agreement revenues in the Company's financial statements.
Interest Capitalization
The Company capitalizes interest cost as part of the historical cost of acquiring certain assets during the period of time required to get the asset ready for its intended use. The amount of capitalized interest is limited to the amount of interest incurred by the Company and has not been significant to the Company's financial position or results of operations.
Operating Leases
The Company presently has three facilities that are located at Phillipsburg, New Jersey, and Needham and Fall River, Massachusetts, under non-cancellable operating lease agreements for office, laboratory and manufacturing space. The rent payments for the three locations escalate over the lease term. Rent expense is recorded on a straight-line basis over the terms of the leases, including any renewals that are reasonably assured of occurring. The difference between rent expense and amounts paid under the lease agreements is recorded as deferred rent liability in the accompanying consolidated balance sheets. Tenant improvements paid by the landlord are capitalized as leasehold improvementsfair value and amortized over the shortergreater of their estimated useful liveseconomic consumption or the remaining lease term.
Intangible Assets
The Company has acquired intangible assets, which include core technology, developed technology and a strategic partner agreement, through the Merger and the acquisition of Lorantis Limited ("Lorantis"). These acquired intangible assets are being amortized on a straight-line basis over their estimated lives, which range from 4.5 to 11 years.useful life.
Goodwill
The determination ofdifference between the amortization period involves estimatespurchase price and judgments on management's part. Any significant changes in the Company's estimates or assumptions could impact the carryingfair value of assets acquired intangible assets.and liabilities assumed in a business combination is allocated to goodwill. Goodwill will be evaluated for impairment on an annual basis during the third quarter, or earlier if impairment indicators are present.
Impairment of Long-Lived Assets
The Company evaluates the recoverability of theseits long-lived assets, including property and equipment, and intangible assets when facts and circumstances suggestindicate that an event of impairment may have occurred. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset couldand its eventual disposition. In the event that such cash flows are not expected to be impaired in accordance with SFAS No. 144.
Revenue Recognition
The Company accounts for revenue arrangements that include multiple deliverables in accordance with Emerging Issues Task Force ("EITF") No. 00-21,Accounting for Revenue Arrangements with Multiple Deliverables ("EITF 00-21"). EITF 00-21 addresses howsufficient to determine whether an arrangement involving multiple deliverables contains more than one unitrecover the carrying amount of accounting. In applying the guidance,assets, the assets are written-down to their estimated fair values.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1)(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Revenue Recognition
The Company accounts for revenue arrangements withthat include multiple deliverables can only be considered as separate units of accounting if (i) the delivered item has value to the customer on a standalone basis, (ii) there is objective and reliable evidence of the fair value of the undelivered items and (iii) if the right of return exists, delivery of the undelivered items is considered probable and substantially in the control of the vendor. If these criteria are not met, the revenue elements must beare considered a single unit of accounting for purposes of revenue recognition.
Payments received to fund certain research activities are recognized as revenue in the period in which the research activities are performed. Payments received in advance that are related to future performance are deferred and recognized as revenue when the research projects are performed.
The Company has entered into various license and development agreements with pharmaceutical and biotechnology companies. The terms of the agreements typically include non-refundable license fees, funding of research and development, payments based upon achievement of certain milestones and royalties on net product sales. Non-refundable license fees are recognized as contract and license fee revenue when the Company has a contractual right to receive such payments, provided that (i) a contractual arrangement exists, (ii) the contract price is fixed or determinable, (iii) the collection of the resulting receivable is reasonably assured and (iv) the Company has no further performance obligations under the license agreement. Upfront non-refundable fees associated with license and development agreements where the Company has continuing performance obligations under the terms of the agreement are recorded as deferred revenue and recognized as revenue over the estimated service period as the Company completes its obligations. Where the Company's level of effort is relatively constant over the performance period or no other pattern is estimable, the revenue is recognized on a straight-line basis. Revenue is limited to the lesser of the cumulative amount of payments receiveddue or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date. If the estimated service period is subsequently modified, the period over which the upfront fee is recognized is modified accordingly on a prospective basis. The determination of the performance period involves judgment on management's part. Funding of research and development is recognized as revenue over the term of the applicable contract as costs are incurred related to that contract.
Milestone payments are recognized as revenue upon the achievement of mutually agreed milestones, provided that (i) the milestone event is substantive and its achievement is not reasonably assured at the inception of the agreement, and (ii) there is no continuing performance obligations associated with the milestone payment. Revenues from milestone payments related to arrangements under which the Company has continuing performance obligations are recognized as revenue upon achievement of the milestone only if all of the following conditions are met: (i) the milestone payments are non-refundable; (ii) achievement of the milestone was not reasonably assured at the inception of the arrangement; (iii) substantive effort is involved in achieving the milestone; and, (iv) the amount of the milestone is reasonable in relation to the effort expended or the risk associated with achievement of the milestone. If any of these conditions are not met, the milestone payments are deferred and recognized as revenue over the term of the arrangement as Celldexthe Company completes its performance obligations.
The Company has capitalized and deferred costs incurred in connection with the one-time signing and upfront payment (the initial deliverable) received with respect to a multiple deliverable
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
arrangement. If there is deemed a single unit of accounting for such an arrangement, the capitalized deferred costs are amortized over the expected performance period of the arrangement.
Payments received to fund certain research activities are recognized as revenue in the period in which the research activities are performed. Revenue from contracts and grants including U.S. government grants under Small Business Innovation Research ("SBIR"), is recognized as the services are performed and recorded as effort is expended on the contracted work and billed to the
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
government or ourthe Company's contractual partner. Payments received in advance that are related to future performance are deferred and recognized as revenue when the research projects are performed.
Product royalty revenue consists of payments received from licensees for a portion of sales proceeds from products that utilize Celldex'sthe Company's licensed technologies and are recognized when the amount of and basis for such royalty payments are reported to us in accurate and appropriate form and in accordance with the related license agreement. Payments received in advance of activities being performed are recorded as deferred revenue. Any significant changes in the Company's estimates or assumptions could impact its revenue recognition.
Research and Development CostsExpenses
Research and development costs, including internal and contract research costs, are expensed as incurred. Research and development expenses consist mainly of clinical trial costs, manufacturing of clinical material, toxicology and other studies, salaries,personnel costs, depreciation, technology accesslicense fees royalty fees, including the cost of Rotarix® royalty revenues retained by the Company, and funding of outside research.
Patent Costs to acquire technologies that are utilized in research and development that have no alternative future use
Patent costs are expensed as incurred. Certain patent costs are reimbursed by the Company's product development and licensing partners. Any reimbursed patent costs are recorded as product development and licensing agreement revenues in the Company's financial statements.
Acquired In-Process ResearchStock-Based Compensation
The Company records stock-based compensation expense for all stock-based awards made to employees and Development
Acquired In-Process Research and Development ('IPR&D") representsdirectors based on the estimated fair value assignedvalues of the stock-based awards expected to research and development projects that we acquire that have not been completedvest at the grant date and is adjusted, if necessary, to reflect actual forfeitures. Compensation expense for all stock-based awards to employees and directors is recognized using the straight-line method over the term of acquisition and which have no future alternative use. Accordingly,vesting or performance.
The Company records stock-based compensation expense for stock options granted to non-employees based on the fair value of such projectsthe stock options which is recorded asre-measured over the vesting term resulting in process research and development expense asperiodic adjustments to stock-based compensation expense.
Foreign Currency Translation
The functional currency of the acquisition date.
The value assigned to acquired IPR&DCompany's foreign subsidiary is determined by estimating the costs to develop the acquired technology into commercially viable products, estimating the resulting net cash flows from the projects,local currency. All assets and discounting the net cash flows to present value. The revenue and costs projections used to value IPR&D were, as applicable, reduced based on the probability of developing a new drug. Additionally, the projections considered the relevant market sizes and growth factors, expected trends in technology, and the nature and expected timing of new product introductions by us and our competitors. The resulting net cash flows from such projects are based on management's estimates of revenues, cost of sales, operating expenses, and income taxes from such projects. The rates utilized to discount the net cash flows to their present value were commensurate with the stage of developmentliabilities of the projects and uncertaintiesforeign subsidiary are re-measured into U.S. dollars at rates of exchange in effect at the economic estimates used in the projections described above.
If these projects are not successfully developed, the operationsend of the company may be adversely affectedyear. Revenue and expense amounts are re-measured using the average exchange rates for the period. Net unrealized gains and losses resulting from foreign currency translation are included in future periods. Additionally, the valueother comprehensive income (loss). At December 31, 2009 and December 31, 2008, accumulated other comprehensive income includes a net unrealized gain related to foreign currency translation of other acquired intangible assets may become impaired. We believe that the assumptions used in the Company's IPR&D analysis were reasonable at the time of the respective acquisition. No assurance can be given, however, that the underlying assumptions used to estimate expected project revenues, development costs or profitability, or the events associated with such projects, will transpire as estimated.$2.6 million.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1)(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Clinical Research and Contract Manufacturing Accruals
Most of the Company's clinical trials are performed by third-party contract research organizations ("CROs") and certain clinical supplies are manufactured by contract manufacturing organizations ("CMOs"). Invoicing from these third parties may be monthly based upon services performed or based upon milestones achieved. The Company accrues these expenses based upon its assessment of the status of each study or manufacturing activity and the work completed, and upon information obtained from the CROs and CMOs.
Foreign Currency Translation
The financial statements of Celldex Ltd have been translated into U.S. dollars in accordance with SFAS No. 52,Foreign Currency Translation. All asset and liability accounts have been translated using the exchange rates in effect at the balance sheet date. Revenues and expenses have been translated using the average exchange rate for the period. Translated gains and losses resulting from the changes in exchange rates have been reported in other comprehensive income (loss). As of December 31, 2008 and December 31, 2007, the accumulated unrealized foreign exchange translation gains (losses) included in accumulated other comprehensive income were ($2,605,726) and $2,619,036, respectively.
Income Taxes
The Company accounts for income taxes in accordance with the provisions of SFAS No. 109,Accounting For Income Taxes. The Company uses the asset and liability method to account for income taxes, including the recognition of deferred tax assets and deferred tax liabilities for the anticipated future tax consequences attributable to differences between financial statement amounts and their respective tax bases.basis. Quarterly, the Company reviews its deferred tax assets for recovery. A valuation allowance is established when the Company believes that it is more likely than not that its deferred tax assets will not be realized. Changes in valuation allowances from period to period are included in the Company's tax provision in the period of change.
The Company records uncertain tax positions in the financial statements only if it is more likely than not that the uncertain tax position will be sustained upon examination by the taxing authorities. The Company records interest and penalties related to uncertain tax positions in income tax expense.
Comprehensive Loss
Comprehensive loss is comprised of net loss and certain changes in stockholders' equity that are excluded from net loss. The Company includes foreign currency translation adjustments for subsidiaries in which the functional currency is not the U.S. dollar and unrealized gains and losses on marketable securities in other comprehensive loss. The consolidated statements of stockholders' equity (deficit) and comprehensive loss reflect total comprehensive loss for the years ended December 31, 2009, 2008 and 2007.
Net Loss Per Share
The Company computes and reports earnings per share in accordance with the provisions of SFAS No. 128,Earnings Per Share. The computations of basic and diluted Basic net loss per common share areis based upon the weighted averageweighted-average number of common shares outstanding andduring the period, excluding restricted stock that has been issued but is not yet vested. Diluted net loss per common share is based upon the weighted-average number of common shares outstanding during the period plus additional weighted-average potentially dilutive securities. Potentiallycommon shares outstanding during the period when the effect is dilutive. The potentially dilutive securities include stock options and warrants. Options to purchase 2,070,993, 787,440 and 1,058,659common shares of common stock werethat have not been included in the December 31, 2008, 2007 and 2006 computation of diluted net loss per common share respectively,calculations because inclusion of such sharesthe effect would have anbeen anti-dilutive effect on net loss per share. Share amounts shownare as follows:
| | Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | 2009 | 2008 | 2007 | |||||||
Stock options | 3,576,159 | 2,070,993 | 787,440 | |||||||
Convertible debt | 353,563 | — | — | |||||||
Restricted stock | 16,000 | — | — | |||||||
| 3,945,722 | 2,070,993 | 787,440 | ||||||||
Recent Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board ("FASB") or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, the Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on the consolidated balance sheets and share amounts and basic and diluted net loss per share amounts shown on the consolidated statementsCompany's financial position or results of operations and comprehensive loss have been adjusted to reflect the Merger exchange ratio and a reverse stock split of 1-for-12 effective March 7, 2008.
Comprehensive Loss
SFAS No. 130,Reporting Comprehensive Income, ("SFAS No. 130") established the standards for reporting and displaying comprehensive income (loss) in financial statements. Comprehensive incomeupon adoption.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1)(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
(loss) In October 2009, the FASB issued a new U.S. GAAP accounting standard which amends existing revenue recognition accounting guidance to provide accounting principles and application guidance on whether multiple deliverables exist, how the arrangement should be separated, and the consideration allocated. This guidance eliminates the requirement to establish objective evidence of fair value of undelivered products and services and instead provides for separate revenue recognition based upon management's estimate of the selling price for an undelivered item when there is definedno other means to include all changes in stockholders' equity (deficit) during the period other than those changes that result from investments by and distributions to stockholders. During the years ended December 31, 2008, 2007 and 2006, the Company reported other comprehensive income (loss) of ($13,310), $230,840 and $2,882,403, respectively, related to unrealized foreign exchange translation gains.
Stock-Based Compensation
The Company accounts for stock-based awards under SFAS No. 123 (revised 2004),Share-Based Payment, ("SFAS No. 123(R)"), which requires the measurement and recognition of compensation expense for all share-based payment awards made to employees and directors including employee stock options and employee stock purchases related to the Employee Stock Purchase Plan ("employee stock purchases") based on estimated grant date fair values.
Compensation expense for all share-based payment awards to employees are recognized using the straight-line method over the term of vesting or performance. As stock-based compensation expense recognized in the Consolidated Statement of Operations is based on awards ultimately expected to vest, compensation expense has been reduced for estimated forfeitures. SFAS No. 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The Company estimatesdetermine the fair value of share-based awards granted using the Black-Scholes option-pricing model ("Black-Scholes model").that undelivered item. The Company's determination of fair value of share-based payment awards on the date of grant using an option-pricing model is affected by the Company's stock price as well as assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, the Company's expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors.
SFAS No. 123(R) did not change the accountingexisting guidance for how the Company accounts for options issued to non-employees. The Company accounts for options issued to non-employees in accordance with SFAS No. 123(R) and EITF Issue No. 96-18,Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services. The value of such options is periodically re-measured and income or expense is recognized during the vesting terms.
See Note 3 for additional information.
Use of Estimates
The preparation of the financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect reported amounts and disclosures. Actual results could differ from those estimates.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Segment Information
SFAS No. 131,Disclosures about Segments of an Enterprise and Related Information, establishes standards for reporting information on operating segments in interim and annual financial statements. The Company has determined that it is engaged in one industry segment, which is the business of development, manufacturing and commercialization of novel therapeutics for human health care. Management uses consolidated financial information in determining how to allocate resources and assess performance and reviews our operating results on an aggregate basis and manages our operations as a single operating segment.
Recent Accounting Pronouncements
SFAS 141(R) and SFAS 160: In December 2007, the Financial Accounting Standards Board ("FASB") issued SFAS No. 141(R),Business Combinations, ("SFAS No. 141(R)"), and SFAS No. 160,Noncontrolling Interests in Consolidated Financial Statements, an amendment of ARB No. 51, ("SFAS No. 160"), which introduce significant changes in the accounting for and reporting of business acquisitions and noncontrolling interests in a subsidiary. SFAS No. 141(R) is to be applied prospectively to business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. SFAS No. 160 is effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2008. Earlier adoption of both statements is prohibited. The adoption of SFAS No. 141(R) and SFAS No. 160 will only have an impact on the Company's financial statements if it is involved in a business combination that occurs after January 1, 2009.
EITF 07-1: In December 2007, the EITF reached a consensus on Issue No. 07-1,Accounting for Collaborative Arrangements ("EITF 07-1"). The EITF concluded on the definition of a collaborative arrangement and that revenues and costs incurred with third parties in connection with collaborative arrangements would be presented gross or net based on the criteria in EITF 99-19,Reporting Revenue Gross as a Principal versus Net as an Agent, and other accounting literature. Based on the nature of the arrangement, payments to or from collaborators would be evaluated and its terms, the nature of the entity's business, and whether those payments are within the scope of other accounting literature would be presented. Companies are alsopreviously required to disclose the nature and purpose of collaborative arrangements along with the accounting policies and the classification and amounts of significant financial statement amounts related to the arrangements. Activities in the arrangement conducted in a separate legal entity should be accounted for under other accounting literature; however, required disclosure under EITF 07-1 applies to the entire collaborative agreement. EITF 07-1 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years, and is to be applied retrospectively to all periods presented for all collaborative arrangements existing as of the effective date. The Company is currently evaluating the effect that the adoption of EITF 07-01 will have on its results of operations and financial condition.
FSP No. FAS 142-3: In April 2008, the FASB staff issued FASB Staff Position ("FSP") No. FAS 142-3,Determination of the Useful Life of Intangible Assets ("FSP No. FAS 142-3"). FSP No. FAS 142-3 amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB No. 142.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(1) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
The intent of this FSP is to improve the consistency between the useful life of a recognized intangible under Statement 142 and the period of expected cash flows used to measure fair value of the asset under FASB No. 141 and other accounting principles generally acceptedundelivered item be the price of the item either sold in a separate transaction between unrelated third parties or the price charged for each item when the item is sold separately by the vendor. This was difficult to determine when the product was not individually sold because of its unique features. Under the existing guidance if the fair value of all of the elements in the United States of America ("U.S.GAAP"). The FSP is effective for financial statements issued for fiscal years beginning after December 31, 2008, and interim periods within those fiscal years. Early adoption is prohibited. The adoption of FSP No. FAS 142-3 isarrangement was not expected to have a material impact on Celldex's financial position and results of operations.
SFAS 162: In May 2008, FASB issued SFAS No. 162,"The Hierarchy of Generally Accepted Accounting Principles", or SFAS 162. SFAS 162 identifies the sources of accounting principles and the framework for selecting the principles to be used in the preparation of financial statements that are presented in conformity with generally accepted accounting principles in the United States. SFAS 162 is effective 60 days following the SEC's approvaldeterminable, then revenue was deferred until all of the Publicitems were delivered or fair value was determined. The Company Accounting Oversight Board amendmentsexpects to AU Section 411,"The Meaning of Present Fairly in Conformity with Generally Accepted Accounting Principles." Theadopt the standard on January 1, 2010 on a prospective basis. While the Company does not expect SFAS 162the adoption of this standard to have a material impact on its financial position and results of operations, and financial condition.this standard may impact the Company in the event it completes future transactions or modifies existing collaborative relationships.
EITF 03-6-1: In June 2008,January 2010, the FASB issued FASB Staff Position No. EITF 03-6-1,"Determining Whether Instruments Grantedupdated guidance to amend the disclosure requirements related to recurring and nonrecurring fair value measurements. This update requires new disclosures on significant transfers of assets and liabilities between Level 1 and Level 2 of the fair value hierarchy (including the reasons for these transfers) and the reasons for any transfers in Share-Based Payment Transactions Are Participating Securities", or FSP EITF 03-6-1. FSP EITF 03-6-1 addresses whether instruments granted in share-based payment transactions are participating securities priorout of Level 3. This update also requires a reconciliation of recurring Level 3 measurements about purchases, sales, issuances and settlements on a gross basis. In addition to vesting and, therefore, need to be included in the earnings allocation in computing earnings per share (EPS) under the two-class method described in paragraphs 60 and 61 of FASB Statement No. 128,"Earnings per Share", or SFAS 128. The guidance applies to the calculation of EPS under SFAS 128 for share-based payment awards with rights to dividends or dividend equivalents. FSP EITF 03-6-1these new disclosure requirements, this update clarifies certain existing disclosure requirements. For example, this update clarifies that unvested share-based payment awards that contain nonforfeitable rightsreporting entities are required to dividends or dividend equivalents (whether paid or unpaid) are participating securitiesprovide fair value measurement disclosures for each class of assets and shall be includedliabilities rather than each major category of assets and liabilities. This update also clarifies the requirement for entities to disclose information about both the valuation techniques and inputs used in the computation of EPS pursuant to the two class method. FSP EITF 03-6-1 isestimating Level 2 and Level 3 fair value measurements. This update will become effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those years. All prior-period EPS data presented shall be adjusted retrospectively (including interim financial statements, summaries of earnings and selected financial data) to conformthe Company with the provisionsinterim and annual reporting period beginning January 1, 2010, except for the requirement to provide the Level 3 activity of purchases, sales, issuances, and settlements on a gross basis, which will become effective for the Company with the interim and annual reporting period beginning January 1, 2011. The Company will not be required to provide the amended disclosures for any previous periods presented for comparative purposes. Other than requiring additional disclosures, adoption of this FSP. Early adoption isupdate will not permitted. The Company does not expect the adoption of FSP EITF 03-6-1 will have a material impacteffect on its results of operations andthe Company's consolidated financial condition.statements.
(2)(3) FAIR VALUE MEASUREMENTS
On January 1, 2008, The fair value of the Company adopted SFAS No. 157,Fair Value Measurements, ("SFAS No. 157"), and SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities Including an amendment of FASB Statement No. 115, ("SFAS No. 159"), for its financialCompany's assets and liabilities. The adoption of SFAS No. 157 did not have a material impact onliabilities reflects the Company's financial position or resultsestimate of operations. As permitted by FASB Staff Position No. FAS 157-2,Effective Date of FASB Statement No. 157, the Company elected to defer the adoption of SFAS No. 157 for all nonfinancial assets and nonfinancial liabilities, except those that are recognized or disclosed at fair value in the financial statements on a recurring basis, until January 1, 2009. SFAS No. 159 permits entities to choose to measure many financial instruments and certain other items at fair value that are
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(2) FAIR VALUE MEASUREMENTS (Continued)
not currently required to be measured at fair value. The Company did not elect to adopt the fair value option for eligible financial instruments under SFAS No. 159.
SFAS No. 157 provides a framework for measuring fair value under U.S. GAAP and requires expanded disclosures regarding fair value measurements. SFAS No. 157 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Market participants are buyers and sellers in the principal market that are (i) independent, (ii) knowledgeable, (iii) able to transact, and (iv) willing to transact.
SFAS No. 157 requiresIn connection with measuring the usefair value of valuation techniques that are consistent with the market approach, the income approach and/or the cost approach. The market approach uses prices and other relevant information generated by market transactions involving identical or comparableits assets and liabilities. The income approach uses valuation techniques to convert future amounts, such as cash flows or earnings, to a single present amount on a discounted basis. The cost approach is based onliabilities, the amount that currently would be required to replace the service capacity of an asset (replacement cost). Valuation techniques should be consistently applied. SFAS No. 157 also establishes a fair value hierarchy which requires an entityCompany seeks to maximize the use of observable inputs where available,(market data obtained from sources independent from the Company) and to minimize the use of unobservable inputs when measuring(the Company's assumptions about how
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(3) FAIR VALUE MEASUREMENTS (Continued)
market participants would price assets and liabilities). The following fair value. The standard describes three levels of inputs that may bevalue hierarchy is used to measure fair value:classify assets and liabilities based on the observable inputs and unobservable inputs used in order to value the assets and liabilities:
Level | ||
|
| |
Level 3: | Unobservable inputs | |
|
|
The following tables set forth the Company's financial instruments consist mainlyassets subject to fair value measurements:
| | As of December 31, 2009 | Level 1 | Level 2 | Level 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | (In thousands) | ||||||||||||
Cash equivalents | $ | 53,780 | $ | 53,780 | — | — | |||||||
Marketable securities | $ | 25,451 | — | $ | 25,451 | — | |||||||
| $ | 79,231 | $ | 53,780 | $ | 25,451 | — | |||||||
| | As of December 31, 2008 | Level 1 | Level 2 | Level 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | (In thousands) | ||||||||||||
Cash equivalents | $ | 43,457 | $ | 43,457 | — | — | |||||||
| $ | 43,457 | $ | 43,457 | — | — | ||||||||
Intangible assets acquired in connection with the CuraGen Merger were accounted for as described in Note 19. The estimated fair value of these nonfinancial assets was based on Level 3 inputs.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) MARKETABLE SECURITIES
A summary of cash, and cash equivalents short-term accounts receivable, accounts payable and debt obligations. Short-term accounts receivable and accounts payable are reflected in the accompanying consolidated financial statements at cost, which approximates fair value due to the short-term naturemarketable securities as of these instruments.December 31, 2009 is shown below:
(3) STOCK-BASED COMPENSATION
| | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | (In thousands) | |||||||||||||||
Cash and cash equivalents | ||||||||||||||||
Cash and money market funds | $ | 57,002 | $ | — | $ | — | $ | 57,002 | ||||||||
Total cash and cash equivalents | $ | 57,002 | $ | — | $ | — | $ | 57,002 | ||||||||
Marketable securities | ||||||||||||||||
U.S. government obligations | ||||||||||||||||
Maturing in one year or less | $ | 9,698 | $ | 5 | $ | — | $ | 9,703 | ||||||||
Maturing after one year through two years | 7,129 | 6 | 22 | 7,113 | ||||||||||||
Total U.S. government obligations | $ | 16,827 | $ | 11 | $ | 22 | $ | 16,816 | ||||||||
Corporate debt securities | ||||||||||||||||
Maturing in one year or less | $ | — | $ | — | $ | — | $ | — | ||||||||
Maturing after one year through two years | 8,672 | — | 37 | 8,635 | ||||||||||||
Total corporate debt securities | $ | 8,672 | $ | — | $ | 37 | $ | 8,635 | ||||||||
Total marketable securities | $ | 25,499 | $ | 11 | $ | 59 | $ | 25,451 | ||||||||
Total cash, cash equivalents and marketable securities | $ | 82,501 | $ | 11 | $ | 59 | $ | 82,453 | ||||||||
As of December 31, 2008,2009, unrealized losses in the portfolio were primarily due to increases in interest rates. The marketable securities held by the Company were high investment grade and there were no marketable securities that have been in a continuous unrealized loss position for more than 12 months at December 31, 2009. The Company did not consider these investments to be other-than-temporarily impaired as of December 31, 2009.
(5) STOCK-BASED COMPENSATION
At December 31, 2009, the Company had two shareholder approved, share-basedstock-based compensation plans: the 2004 Employee Stock Purchase Plan (the "2004 ESPP Plan") and the 2008 Stock Option and Incentive Plan (the "2008 Plan").
Employee Stock Purchase Plan
At December 31, 2009, a total of 62,500 shares of common stock are reserved for issuance under the 2004 ESPP Plan. Under the 2004 ESPP Plan, each participating employee may purchase up to 250 shares of common stock per year, through payroll deductions, at a purchase price equal to 85% of the lower of the fair market value of the common stock at either the beginning of the offering period or the applicable exercise date. During the year ended December 31, 2009, the Company issued 2,979 shares under the 2004 ESPP Plan. During the year ended December 31, 2008, the Company issued no shares under the 2004 ESPP Plan. At December 31, 2009, 56,906 shares were available for issuance under the 2004 ESPP Plan.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(3)(5) STOCK-BASED COMPENSATION (Continued)
Employee Stock Purchase Plan
The 2004 ESPP Plan was adopted on May 13, 2004 and assumed by the Company in connection with the Merger. All full time employees of the Company are eligible to participate in the 2004 ESPP Plan. A total of 12,500 shares of common stock are reserved for issuance under the 2004 ESPP Plan. Under the 2004 ESPP Plan, each participating employee may contribute up to 15% of his or her compensation to purchase up to 100 shares of common stock per year in any six-month offering period and may withdraw from the offering at any time before stock is purchased. Participation terminates automatically upon termination of employment. The purchase price per share of common stock in an offering is 85% of the lower of its fair market value at either the beginning of the offering period or the applicable exercise date. At December 31, 2008, 9,885 shares were available for issuance under the 2004 ESPP Plan.
As a consequence of the Merger, no purchase period was offered beginning on January 1, 2008. The last purchase period began on July 1, 2008 and ended on December 31, 2008.
Employee Stock Option and Incentive Plan
Stock Option Plan Description
On March 6, 2008, the Company's 2008 Plan was adopted at a special meeting of its shareholders. The 2008 Plan replaced the 1999 Stock Option and Incentive Plan (the "1999 Plan") and the Amended and Restated 1991 Stock Compensation Plan, which was an amendment and restatement of the Company's 1985 Incentive Option Plan. The 2008 Plan permits the granting of incentive stock options (intended to qualify as such under Section 422A of the Internal Revenue Code of 1986, as amended), non-qualified stock options, stock appreciation rights, performance share units, restricted stock and other awards of restricted stock in lieu of cash bonuses to employees, consultants and outside directors.
TheAt December 31, 2009, the 2008 Plan allowsallowed for a maximum of 1,500,0003,900,000 shares of common stock to be issued prior to October 19, 2017. The Company's board of directors determines the term of each option, option price, and number of shares for which each option is granted and the rate at which each option vests. Options generally vest over a period not to exceed four years. The term of each option cannot exceed ten years (five years for options granted to holders of more than 10% of the voting stock of the Company) and the exercise price of stock options cannot be less than the fair market value of the common stock at the date of grant (110% of fair market value for incentive stock options granted to holders of more than 10% of the voting stock of the Company). The 2008 Plan also provides for discretionary grants of non-qualified stock options to non-employee directors. Vesting of all employee and non-employee director stock option awards is accelerated upon a change in control as defined in the 2008 Plan.
In connection with the AVANT Merger, the Company assumed the obligations of Celldex Research under Celldex Research's 2005 Equity Incentive Plan (the "Celldex Research 2005 Plan") and each outstanding option to purchase Celldex Research common stock (a "Celldex Research Stock Option") granted under the Celldex Research 2005 Plan. Each Celldex Research Stock Option assumed by the Company is deemed to constitute an option to acquire, on the same terms and conditions as were applicable under the Celldex Research 2005 Plan, shares of the Company's common stock that have been adjusted consistent with the ratio at which the Company's common stock was issued in exchange for Celldex Research's common stock in the AVANT Merger. As of March 7, 2008, the Company assumed
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(3) STOCK-BASED COMPENSATION (Continued)
options to acquire 1,446,913 shares of its common stock at a weighted average exercise price of $8.35. The Celldex Research Stock Options generally vest over a two-to four-year period and the term of each option cannot exceed ten years from the date of grant. No additional awards will be issued under the Celldex Research 2005 Plan.
General Option Information
A summary In connection with the CuraGen Merger, the Company assumed the obligations of CuraGen under CuraGen's 2007 Stock Plan (the "CuraGen 2007 Plan") and each outstanding option to purchase CuraGen common stock option activity(a "CuraGen Stock Option") granted under the 2008CuraGen 2007 Plan. Each CuraGen Stock Option assumed by the Company is deemed to constitute an option to acquire, on the same terms and conditions as were applicable under the CuraGen 2007 Plan, andshares of the Celldex Research 2005 PlanCompany's common stock that have been adjusted consistent with the ratio at which the Company's common stock was issued in exchange for CuraGen's common stock in the CuraGen Merger. As of October 1, 2009, the Company assumed options to acquire 931,315 shares of its common stock with a weighted average exercise price of $3.17. As of October 1, 2009, all of the CuraGen Stock Options were fully vested except for 8,993 shares which generally vest over a two year ended December 31, 2008, adjusted to reflectperiod. No additional awards will be issued under the Merger exchange ratio and a reverse stock split of 1-for-12 effective March 7, 2008, is as follows:
| | Shares | Weighted Average Exercise Price Per Share | Weighted Average Remaining Contractual Term (In Years) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Options Outstanding at January 1, 2008 | 787,440 | $ | 12.70 | 5.81 | ||||||
Granted | 2,738,545 | 8.36 | ||||||||
Exercised | — | — | ||||||||
Canceled/forfeited | (1,450,707 | ) | 10.62 | |||||||
Expired | (4,285 | ) | 22.71 | |||||||
Options Outstanding at December 31, 2008 | 2,070,993 | $ | 8.39 | 8.69 | ||||||
Options Vested and Expected to Vest at December 31, 2008 | 1,878,642 | $ | 8.40 | 8.68 | ||||||
Options Exercisable at December 31, 2008 | 1,154,473 | $ | 8.46 | 8.94 | ||||||
Options Available for Grant | 875,506 | |||||||||
Weighted Average Fair Value of Options Granted during the year | $ | 4.37 | ||||||||
CuraGen 2007 Plan. The aggregate intrinsicfair value of options outstanding at December 31, 2008the CuraGen Stock Options that were attributed to precombination service was $80,478.
Non-Employee Grants
The Company has historically granted stock options to consultants for services. These options were issued at or above their fair market value onincluded in the date of grant and generally have four-year vesting terms from date of grant. Should the Company or the consultant terminate the consulting agreements, any unvested options will be cancelled. Options issued to non-employees are marked-to-market, which means that as the Company's stock price fluctuates, the related expense either increases or decreases. The Company recognized expense of $409,229, $85,515 and $41,638 related to non-employee consultant stock options for the years ended December 31, 2008, 2007 and 2006, respectively.CuraGen Merger consideration.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(3)(5) STOCK-BASED COMPENSATION (Continued)
A summary of stock option activity under the 2008 Plan, CuraGen 2007 Plan and Celldex Research 2005 Plan for the year ended December 31, 2009 is as follows:
| | Shares | Weighted Average Exercise Price Per Share | Weighted Average Remaining Contractual Term (In Years) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Options Outstanding at January 1, 2009 | 2,070,993 | $ | 8.39 | 8.7 | ||||||
Granted | 757,579 | 8.27 | ||||||||
CuraGen Stock Options assumed | 931,315 | 3.17 | ||||||||
Exercised | (124,273 | ) | 5.21 | |||||||
Canceled/forfeited | (34,519 | ) | 8.73 | |||||||
Expired | (24,936 | ) | 9.79 | |||||||
Options Outstanding at December 31, 2009 | 3,576,159 | $ | 7.10 | 6.6 | ||||||
Options Vested and Expected to Vest at December 31, 2009 | 3,362,775 | $ | 7.03 | 6.5 | ||||||
Options Exercisable at December 31, 2009 | 2,506,684 | $ | 6.63 | 5.9 | ||||||
Shares Available for Grant under the 2008 Plan | 2,494,595 | |||||||||
The total intrinsic value of stock options exercised during the year ended December 31, 2009 was $0.3 million. No stock options were exercised during the year ended December 31, 2008. The weighted average grant-date fair value of stock options granted during the years ended December 31, 2009 and 2008 was $5.29 and $4.37, respectively. The total fair value of stock options vested during the year ended December 31, 2009 and 2008 was $2.6 million and $2.8 million, respectively.
The aggregate intrinsic value of stock options outstanding at December 31, 2009 was $1.5 million. The aggregate intrinsic value of stock options vested and expected to vest at December 31, 2009 was $1.5 million. As of December 31, 2009, total compensation cost related to non-vested employee and non-employee director stock options not yet recognized was approximately $3.5 million, net of estimated forfeitures, which is expected to be recognized as expense over a weighted average period of 2.6 years.
Shares Issued to Executive Officers
In January 2009, the Company granted 29,340 shares of common stock from the 2008 Plan to its executive officers. The value of these shares of $0.3 million was expensed during the year ended December 31, 2008.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(5) STOCK-BASED COMPENSATION (Continued)
Restricted Stock
In December 2009, the Company granted 2,000 shares of restricted stock to each of its non-employee directors. A summary of restricted stock activity under the 2008 Plan for the year ended December 31, 2009 is as follows:
| | Shares | Weighted Average Grant Date Fair Value (per share) | |||||
|---|---|---|---|---|---|---|---|
Outstanding and unvested at December 31, 2008 | — | — | |||||
Granted | 16,000 | $ | 4.48 | ||||
Vested | — | — | |||||
Canceled | — | — | |||||
Outstanding and unvested at December 31, 2009 | 16,000 | $ | 4.48 | ||||
Valuation and Expenses Information
The following table summarizes stock-basedStock-based compensation expense related to employee and non-employee stock options, restricted stock and employee stock purchases for the years ended December 31, 2009, 2008 and 2007 and 2006, respectively, which was allocatedrecorded as follows:
| | Year Ended December 31, 2008 | Year Ended December 31, 2007 | Year Ended December 31, 2006 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Research and development | $ | 1,648,997 | $ | 423,819 | $ | 671,525 | ||||
General and administrative | 3,166,696 | 1,181,103 | 1,088,640 | |||||||
Total stock-based compensation expense | $ | 4,815,693 | $ | 1,604,922 | $ | 1,760,165 | ||||
| | 2009 | 2008 | 2007 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | (In thousands) | |||||||||
Research and development | $ | 1,383 | $ | 2,035 | $ | 424 | ||||
General and administrative | 1,675 | 2,781 | 1,181 | |||||||
Total stock-based compensation expense | $ | 3,058 | $ | 4,816 | $ | 1,605 | ||||
Based on basic and diluted weighted average common shares outstanding of 14,217,388, 8,309,420 and 8,278,500,In connection with the effect of stock-based compensation expense recorded for the years ended December 31, 2008, 2007 and 2006 had a $0.34 per share, $0.19 per share and $0.21 per share negative impact on basic and diluted net loss per common share, respectively.
During the quarter ended March 31, 2008,AVANT Merger, the Company entered into an Option Cancellation Agreement concurrent with a Stock Option Grant Agreement with Celldex Research employees. The Option Cancellation Agreement providedaccounted for the cancellation of all previously granted options under the Celldex Research 2005 Plan while the Stock Option Grant Agreement provided for the re-grant of stock options pursuant to the Option Cancellation Agreement. In addition, at the consumption of the Merger, all options to purchase former Celldex Research common stock then outstanding under the Celldex Research 2005 Plan were assumed by the Company and converted into options to purchase shares of the Company's common stock. The number of shares subject to the outstanding awards and related exercise price was proportionately adjusted by the same exchange ratio as former Celldex Research shareholders received in accordance with the provisions of the Celldex Research 2005 Plan.
The Company considered both the re-grant of stock options and exchange of Celldex Research optionsStock Options into options to acquire shares of the Company's common stock as a modification under the provisions of SFAS No. 123(R).modification. The modification affected a total of 15 employees, including members of the Celldex Research board of directors. The total incremental compensation cost resulting from the modifications amounted to approximatelywas $2.6 million, of which $0.9 million was related to vested awards and was recognized immediately as stock basedstock-based compensation induring the quarterthree months ended March 31, 2008.
In accordance with Dr. Ryan's Severance Agreement (which is discussed further in Note 16), the Company granted Dr. Ryan fully vested stock options for 153,125 shares as of July 16, 2008, the effective date of the Severance Agreement, and recorded $1.3 million of stock-based compensation in general and administrative expense during the quarter ended September 30, 2008.
As of December 31, 2008, total compensation cost related to non-vested employee and non-employee director stock options not yet recognized was approximately $3.2 million, net of estimated forfeitures, which is expected to be recognized as expense over a weighted average period of 2.01 years. The total fair value of employee and non-employee director stock options vested, including
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(3)(5) STOCK-BASED COMPENSATION (Continued)
the incremental fair value for options vested that were modified, during the twelve months ended December 31, 2008 was $2,785,995.
The fair values of employee and non-employee director stock options and employee stock purchases granted during the years ended December 31, 2009, 2008 2007 and 20062007 were valued using the Black-Scholes option-pricing model with the following assumptions:
| | Year Ended December 31, 2008 | Year Ended December 31, 2007(1) | Year Ended December 31, 2006(1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Expected stock price volatility (employees) | 55 - 67 | % | 79.57 | % | 67.1 | % | ||||
Expected stock price volatility (non-employee directors) | 57 - 67 | % | 79.5 | % | 67.1 | % | ||||
Expected option term (employees) | 3 - 6.25 Years | 5 Years | 5.2 Years | |||||||
Expected option term (non-employee directors) | 4 - 6 Years | 5 Years | 5.2 Years | |||||||
Risk-free interest rate | 1.75 - 3.27 | % | 3.85 | % | 4.52 | % | ||||
Expected dividend yield | None | None | None | |||||||
| | Year Ended December 31, 2009 | Year Ended December 31, 2008 | Year Ended December 31, 2007(1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Expected stock price volatility (employees) | 65 - 68 | % | 55 - 67 | % | 80 | % | ||||
Expected stock price volatility (non-employee directors) | 67 | % | 57 - 67 | % | 80 | % | ||||
Expected stock price volatility (2004 ESPP) | 90 - 98 | % | 98 | % | n/a | |||||
Expected option term (employees) | 6.1 - 6.3 Years | 3 - 6.3 Years | 5 Years | |||||||
Expected option term (non-employee directors) | 5.5 Years | 4 - 6 Years | 5 Years | |||||||
Expected option term (2004 ESPP) | .5 Years | .5 Years | n/a | |||||||
Risk-free interest rate (options) | 1.8 - 3.4 | % | 1.8 - 3.3 | % | 3.9 | % | ||||
Risk-free interest rate (2004 ESPP) | 0.3 | % | 0.3 | % | n/a | |||||
Expected dividend yield | None | None | None | |||||||
In 2008, the Company used its daily historical stock price volatility consistent with the expected term of grant as the basis for its expected volatility assumption in accordance with SFAS No. 123(R) and SAB 107 for its employee and non-employee director stock options and employee stock purchases. The Company has concluded that its historical volatility is representative of expected future stock price trends. The expected volatility used by Celldex Research in 2007 and 2006 was based on the average volatility of a group of companies that Celldex Research believed would be considered a peer group had it been a publicly-held company.
U.S. GAAP allows companies to continue to utilize the simplified method in developing an estimate of the expected term of "plain vanilla" stock options when there have been structural changes in a Company's business such that historical exercise data does not provide a reasonable basis upon which to estimate expected term. Due to the AVANT Merger and the CuraGen Merger, historical exercise patterns do not provide a reasonable basis to estimate expected term of current option grants. Accordingly, the Company utilized the simplified method to estimate expected term of stock options granted during the years ended December 31, 2009 and 2008. The simplified method estimates the expected term as the mid-point between the vesting date and the expiration date. In 2008 and 2009, the Company used its daily historical stock price volatility consistent with the expected term of grant as the basis for its expected volatility assumption. The risk-free interest rate assumption is based upon observed interest rates appropriate forthe yield of U.S. Treasury securities consistent with the expected term of the Company's employee and non-employee director stock options and employee stock purchases.option. The dividend yield assumption is based on the Company's history of zero dividend payouts and expectation that no dividends will be paid in the foreseeable future.
The expected term of employee and non-employee director stock options represents For the weighted-average period the stock options are expected to remain outstanding. SAB 110 provides for a simplified method for estimating expected term for "plain-vanilla" options. The simplified method is based on the vesting period and the contractual term for each grant or for each vesting tranche for awards with graded vesting. The mid-point between the vesting date and the expiration date is used as the expected term under this method. In December 2007, the Securities and Exchange Commission released SAB 110, which extended the use of the simplified method if a company met certain criteria. The Company has concluded that the Merger represents a significant structural change in its business and in the terms of its share option grants such that its historical exercise data may no longer provide a reasonable basis upon which to estimate expected term. The Company has elected to follow the guidance of SAB 110 and has adopted the simplified method in determining expected term for all of its
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(3) STOCK-BASED COMPENSATION (Continued)
stock option awards. There were 205,703 stock options granted to non-employee directors during the yearyears ended December 31, 2008.
Forfeitures2009 and 2008, forfeitures were estimated based on historical experience by applying an eleven and zero percent forfeiture rate to employee and non-employee director stock option awards, granted during the years ended December 31, 2008, respectively.
The Company has not recognized any tax benefits or deductions related to the tax effects of employee stock-based compensation as the Company carries a full deferred tax asset valuation allowance and has significant net operating loss carryforwards available.
(4) RETIREMENT SAVINGS PLAN
The Company's 401(k) Plan (the "401(k) Plan") is intended to be a tax-qualified plan covering substantially all employees. Under the terms of the 401(k) Plan, employees may elect to contribute up to 15% of their compensation, or the statutory prescribed limits. The Company may make 50% matching contributions on up to 4% of a participant's annual salary. Benefit expense for the 401(k) Plan was approximately $74,269, $39,899 and $21,133 for the years ended December 31, 2008, 2007 and 2006, respectively.
(5) PROPERTY AND EQUIPMENT
Property and equipment include the following:
| | December 31, 2008 | December 31, 2007 | ||||||
|---|---|---|---|---|---|---|---|---|
Laboratory Equipment | $ | 2,448,848 | $ | 1,551,896 | ||||
Manufacturing Equipment | 1,507,806 | — | ||||||
Office Furniture and Equipment | 1,085,549 | 405,581 | ||||||
Leasehold Improvements | 12,564,529 | 2,046,663 | ||||||
Construction in Progress | 70,796 | — | ||||||
Total Property and Equipment | 17,677,528 | 4,004,140 | ||||||
Less Accumulated Depreciation and Amortization | (4,110,348 | ) | (2,086,104 | ) | ||||
| $ | 13,567,180 | $ | 1,918,036 | |||||
A portion of the purchase price in the Merger totaling $15,170,702 has been allocated and recorded to acquired property and equipment above and was then reduced by approximately $2,606,649 of negative goodwill.
As a result of the Merger, the Company has converted its Fall River manufacturing facility to provide mammalian cell culture production capabilities and classified certain manufacturing-related equipment having a fair value of $451,100 as long-lived assets to be disposed of by sale. The fair value was established based on quoted market prices by an equipment re-seller less estimated costs to remove and sell the equipment. During the year ended December 31, 2008, the Company sold six of seven and wrote off one of the long-lived assets held-for-sale for $460,494 and recorded a gain of $9,394 on disposal of assets. During the year ended December 31, 2008, the Company disposed, by sale or
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(5) PROPERTY AND EQUIPMENT (Continued)
abandonment, assets and assets held-for-sale having a net book value of $646,472 and recorded a net loss of $33,795 to research and development expense.
Depreciation expense related to equipment and leasehold improvements was $2,176,427, $710,156 and $769,520 for the years ended December 31, 2008, 2007 and 2006, respectively.
In December 2006, in connection with the assignment of the Company's United Kingdom lease (see Note 16) to a third party, the Company sold certain leasehold improvements, laboratory equipment, and furniture and fixtures for $2,207,854. As a result, the Company recorded a gain on sale of fixed assets in its consolidated statement of operations of $136,161 for the year ended December 31, 2006.
(6) INTANGIBLE AND OTHER ASSETS
Intangible assets include the following:
| | December 31, 2008 | December 31, 2007 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Estimated Lives | Gross Intangible Assets | Accumulated Amortization | Net Intangible Assets | Gross Intangible Assets | Accumulated Amortization | Net Intangible Assets | |||||||||||||||
Intangible Assets: | ||||||||||||||||||||||
Core Technology | 4.5 - 11 years | $ | 2,193,249 | $ | (530,778 | ) | $ | 1,662,471 | $ | 1,296,000 | $ | (263,097 | ) | $ | 1,032,903 | |||||||
Strategic Partner Agreement | 8 years | 629,499 | (65,038 | ) | 564,461 | — | — | — | ||||||||||||||
Asset Held for Sale—Developed Technology | 8 years | 273,796 | (28,288 | ) | 245,508 | — | — | — | ||||||||||||||
Total Intangible Assets | $ | 3,096,544 | $ | (624,104 | ) | $ | 2,472,440 | $ | 1,296,000 | $ | (263,097 | ) | $ | 1,032,903 | ||||||||
On March 7, 2008, the Merger was completed. Under the purchase method of accounting, the Company determined the identifiable intangible assets acquired based upon the respective fair values of certain technology and intellectual property acquired and license agreement assumed. The Company has determined that these technologies had alternative future uses and will be incorporated into a number of the Company's vaccine programs. A portion of the purchase price in the transaction totaling $2,174,100 was allocated and recorded to acquired intangible assets above and then was reduced by approximately $373,556 of negative goodwill.
At December 31, 2008, the Company classified the intangible asset—"developed technology"—as a long-lived asset to be disposed of by sale due to the Company's negotiations with LAHI at year-end and the subsequent sale of the Megan poultry vaccines business related to the developed technology intangible asset to LAHI in January 2009.
All of the Company's intangible assets are amortized over their estimated useful lives. Total amortization expense for intangible assets was $361,006, $116,932 and $116,932 for the years ended December 31, 2008, 2007 and 2006, respectively.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(6) INTANGIBLEPROPERTY AND OTHER ASSETS (Continued)EQUIPMENT
The estimated future amortization expense of intangible assets as of December 31, 2008Property and the five succeeding years and thereafter is as follows:
Year ending December 31, | Estimated Amortization Expense | |||
|---|---|---|---|---|
2009 | $ | 381,236 | ||
2010 | 381,236 | |||
2011 | 381,236 | |||
2012 | 305,653 | |||
2013 | 230,071 | |||
2014 and thereafter | 547,500 | |||
At December 31, 2008, the balance of other assets includes the net unamortized balance of $6,413,515 of sublicense income royalty fees paid to Duke and TJU in connection with the Pfizer Agreement. As more fully discussed in Note 10, the Company is recognizing the $40 million upfront license fee received from Pfizer on a straight-line basis over the Company's estimated period of performance of 9.5 years. The Company paid these two research universities a total of $6,865,173 in sublicense income royalty fees directly related to the Pfizer Agreement. The sublicense income royalty fees have been deferred and are being amortized to royalty expense over the same 9.5-year performance period at the rate of $180,663 per quarter.
(7) ACCRUED EXPENSES
Accrued expenses are comprised of amounts owed to employees, vendors, and suppliers for work performed on behalf of us. The Company evaluates the accrued expense balance related to these activities based upon information received from the supplier and estimated progress toward completion of objectives to ensure that the balance is appropriately stated. Such estimates are subject to changes as additional information becomes available. Accrued expensesequipment include the following:
| | December 31, 2008 | December 31, 2007 | |||||
|---|---|---|---|---|---|---|---|
Accrued License Fees | $ | 672,507 | $ | — | |||
Accrued Payroll and Employee Benefits | 1,953,336 | 511,038 | |||||
Accrued Clinical Trials | 119,523 | 424,916 | |||||
Accrued Manufacturing Expenses | — | 97,738 | |||||
Accrued Professional Fees | 432,010 | 407,212 | |||||
Accrued Restructuring Expenses | — | 1,011,732 | |||||
Other Accrued Expenses | 663,783 | 66,783 | |||||
| $ | 3,841,159 | $ | 2,519,419 | ||||
| | December 31, 2009 | December 31, 2008 | ||||||
|---|---|---|---|---|---|---|---|---|
| | (In thousands) | |||||||
Laboratory Equipment | $ | 2,643 | $ | 2,449 | ||||
Manufacturing Equipment | 1,622 | 1,508 | ||||||
Office Furniture and Equipment | 1,165 | 1,085 | ||||||
Leasehold Improvements | 12,601 | 12,564 | ||||||
Construction in Progress | 180 | 71 | ||||||
Total Property and Equipment | 18,211 | 17,677 | ||||||
Less Accumulated Depreciation and Amortization | (6,722 | ) | (4,110 | ) | ||||
| $ | 11,489 | $ | 13,567 | |||||
Depreciation and amortization expense related to property and equipment was $2.6 million, $2.2 million and $0.7 million for the years ended December 31, 2009, 2008 and 2007, respectively.
(7) INTANGIBLE ASSETS AND GOODWILL
Intangible assets, net of accumulated amortization, and goodwill are as follows:
| | December 31, 2009 | December 31, 2008 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Estimated Life | Cost | Accumulated Amortization | Net | Cost | Accumulated Amortization | Net | |||||||||||||||
| | | (In thousands) | ||||||||||||||||||||
Intangible Assets: | ||||||||||||||||||||||
IPR&D | Indefinite | $ | 11,800 | — | $ | 11,800 | — | — | — | |||||||||||||
Amgen Amendment | 16 years | 14,500 | $ | (224 | ) | 14,276 | — | — | — | |||||||||||||
TopoTarget Agreement | 1.75 years | 2,400 | (343 | ) | 2,057 | — | — | — | ||||||||||||||
Core Technology | 4.5 - 11 years | 2,193 | (832 | ) | 1,361 | $ | 2,193 | $ | (531 | ) | $ | 1,662 | ||||||||||
Strategic Partner Agreement | 8 years | 630 | (145 | ) | 485 | 630 | (65 | ) | 565 | |||||||||||||
Asset Held for Sale | — | — | — | — | 274 | (28 | ) | 246 | ||||||||||||||
Total Intangible Assets | $ | 31,523 | $ | (1,544 | ) | $ | 29,979 | $ | 3,097 | $ | (624 | ) | $ | 2,473 | ||||||||
Goodwill | Indefinite | $ | 8,965 | — | $ | 8,965 | — | — | — | |||||||||||||
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER(7) INTANGIBLE ASSETS AND GOODWILL (Continued)
At December 31, 2008, the Company had recorded $0.2 million in intangible assets related to its poultry vaccines as a long-lived asset to be disposed of by sale due to the Company's negotiations with Lohmann Animal Health International ("LAHI"). In January 2009, the Company entered into a purchase agreement ("LAHI Agreement") to sell its poultry vaccines assets to LAHI. Under the LAHI Agreement, LAHI paid an upfront fee of $0.8 million and agreed to pay potential milestone payments. The Company recorded a gain of $0.6 million related to the LAHI Agreement based on the upfront fee less the net book value of the related asset.
Amortization expense for intangible assets was $0.9 million, $0.4 million and $0.1 million for the years ended December 31, 2009, 2008 and 2007, and 2006respectively.
The estimated future amortization expense of intangible assets for the next five years is as follows:
Year ending December 31, | Estimated Amortization Expense | |||
|---|---|---|---|---|
| | (In thousands) | |||
2010 | $ | 2,650 | ||
2011 | 1,964 | |||
2012 | 1,203 | |||
2013 | 1,127 | |||
2014 | 1,127 | |||
(8) ACCRUED EXPENSES
Accrued expenses include the following:
| | December 31, 2009 | December 31, 2008 | |||||
|---|---|---|---|---|---|---|---|
| | (In thousands) | ||||||
Accrued Royalty and License Fees | $ | 1,323 | $ | 672 | |||
Accrued Payroll and Employee Benefits | 1,915 | 1,953 | |||||
Accrued Research and Development Contract Costs | 918 | 120 | |||||
Accrued Professional Fees | 512 | 432 | |||||
Other Accrued Expenses | 947 | 664 | |||||
| $ | 5,615 | $ | 3,841 | ||||
(8)
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(9) INCOME TAXES
During the first quarterThe components of 2008 the Company underwent a merger in which Celldex Therapeutics, Inc. (then AVANT) and Celldex Research became a combined group forincome tax reporting purposes. The merger was treated as a purchase under SFAS 141 with Celldex Research being the accounting acquirer. Together they form a combined group and report income taxes as such with Celldex as the parent company and Celldex Research as the subsidiary. As a result of this merger, allexpense attributable to continuing operations consist of the prior tax attributes of both Celldex and Celldex Research will carry forward for potential future use subject to potential limitations. These tax attributes are includedfollowing:
| | Year Ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2009 | 2008 | 2007 | ||||||||
| | (In thousands) | ||||||||||
Income tax benefit (provision): | |||||||||||
Federal | $ | 12,750 | $ | 10,198 | $ | 4,545 | |||||
State | (1,757 | ) | 6,958 | 711 | |||||||
Foreign | 126 | 193 | 844 | ||||||||
Expiration of Net Operating Losses and Research & Development Tax Credits | (3,992 | ) | (1,306 | ) | — | ||||||
| 7,127 | 16,043 | 6,100 | |||||||||
Deferred tax valuation allowance | (6,598 | ) | (16,043 | ) | (6,100 | ) | |||||
| $ | 529 | $ | — | $ | — | ||||||
Included in the Company's incomestate tax provision.provision above for the year ended December 31, 2009 is the effect of a rate decrease on the deferred tax asset and liabilities offset by a $0.5 million tax benefit due to non-cash tax consequences of the CuraGen Merger.
| | Year Ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| | 2008 | 2007 | 2006 | ||||||||
Income tax benefit (provision): | |||||||||||
Federal | $ | 10,198,100 | $ | 4,544,800 | $ | 2,899,100 | |||||
State | 6,958,100 | 711,000 | 512,600 | ||||||||
Foreign | 193,000 | 844,500 | 2,602,000 | ||||||||
| 17,349,200 | 6,100,300 | 6,013,700 | |||||||||
Deferred tax valuation allowance | (17,349,200 | ) | (6,100,300 | ) | (6,013,700 | ) | |||||
| $ | — | $ | — | $ | — | ||||||
A reconciliation between the amount of reported income tax and the amount computed using the U.S. Statutory rate of 34% follows:
| | 2008 | 2007 | 2006 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Pre-tax book income (loss) | $ | (47,500,572 | ) | $ | (15,073,050 | ) | $ | (17,835,262 | ) | |
Loss at Statutory Rates | (16,108,800 | ) | (4,943,800 | ) | (5,506,600 | ) | ||||
Research and Development Credits | (1,325,000 | ) | (306,000 | ) | (276,000 | ) | ||||
State Taxes | (6,958,100 | ) | (711,100 | ) | (512,600 | ) | ||||
Other | 85,300 | (139,400 | ) | 281,500 | ||||||
In-Process R&D | 6,957,400 | — | — | |||||||
Expiration of Net Operating Losses and Research & Development Tax Credits | (7,830,000 | ) | — | — | ||||||
Change in Valuation Allowance | 25,179,200 | 6,100,300 | 6,013,700 | |||||||
| $ | — | $ | — | $ | — | |||||
| | 2009 | 2008 | 2007 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | (In thousands) | |||||||||
Pre-tax book income (loss) | $ | (37,054 | ) | $ | (47,501 | ) | $ | (15,073 | ) | |
Loss at Statutory Rates | (12,571 | ) | (16,109 | ) | (4,944 | ) | ||||
Research and Development Credits | (1,456 | ) | (1,325 | ) | (306 | ) | ||||
State Taxes | 1,757 | (6,958 | ) | (711 | ) | |||||
Other | 1,151 | 85 | (139 | ) | ||||||
IPR&D | — | 6,958 | — | |||||||
Expiration of Net Operating Losses and Research & Development Tax Credits | 3,992 | 1,306 | — | |||||||
Change in Valuation Allowance | 6,598 | 16,043 | 6,100 | |||||||
Income tax (benefit) provision | $ | (529 | ) | $ | — | $ | — | |||
Deferred tax assets and liabilities are recognized based on temporary differences between the financial reporting and tax basis of assets and liabilities using future expected enacted rates. A valuation allowance is recorded against deferred tax assets if it is more likely than not that some or all of the deferred tax assets will not be realized.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(8)(9) INCOME TAXES (Continued)
The principal components of the deferred tax assets and liabilities at December 31, 20082009 and 2007,2008, respectively, are as follows:
| | December 31, 2008 | December 31, 2007 | ||||||
|---|---|---|---|---|---|---|---|---|
Gross Deferred Tax Assets | ||||||||
Net Operating Loss Carryforwards | $ | 94,406,000 | $ | 20,960,000 | ||||
Tax Credit Carryforwards | 15,026,000 | 1,347,000 | ||||||
Deferred Expenses | 16,835,000 | 2,267,000 | ||||||
Stock-based Compensation | 2,049,000 | 1,148,000 | ||||||
Fixed Assets | 1,458,000 | 571,000 | ||||||
Accrued Expenses and Other | 474,000 | 324,000 | ||||||
Deferred Revenue | 2,094,000 | 477,000 | ||||||
| 132,342,000 | 27,094,000 | |||||||
Gross Deferred Tax Liabilities | ||||||||
Acquired Intangibles | (101,000 | ) | — | |||||
Deferred Tax Assets Valuation Allowance | (132,241,000 | ) | (27,094,000 | ) | ||||
Net Deferred Tax Asset (Liability) | $ | — | $ | — | ||||
| | December 31, 2009 | December 31, 2008 | ||||||
|---|---|---|---|---|---|---|---|---|
| | (In thousands) | |||||||
Gross Deferred Tax Assets | ||||||||
Net Operating Loss Carryforwards | $ | 55,715 | $ | 94,406 | ||||
Tax Credit Carryforwards | 16,735 | 15,026 | ||||||
Deferred Expenses | 17,300 | 19,600 | ||||||
Stock-based Compensation | 3,795 | 2,049 | ||||||
Fixed Assets | 2,088 | 1,458 | ||||||
Accrued Expenses and Other | 1,122 | 474 | ||||||
Deferred Revenue | 15,535 | 2,094 | ||||||
| 112,290 | 135,107 | |||||||
Gross Deferred Tax Liabilities | ||||||||
Other Acquired Intangibles | (6,500 | ) | (101 | ) | ||||
IPR&D Intangibles | (4,661 | ) | — | |||||
Deferred License Costs and Other | (2,651 | ) | (2,765 | ) | ||||
| (13,812 | ) | (2,866 | ) | |||||
Total Deferred Tax Assets and Liabilities | 98,478 | 132,241 | ||||||
Deferred Tax Assets Valuation Allowance | (103,139 | ) | (132,241 | ) | ||||
Net Deferred Tax Asset (Liability) | $ | (4,661 | ) | $ | — | |||
As of December 31, 2008,2009, the Company had the following federal and state net operating loss ("NOL") carryforwards of approximately $227,164,000 and $82,685,000, respectively, and federal and state research and development ("R&D") credit carryforwards of approximately $10,425,000 and $6,972,000, respectively. The federal and state net operating loss and R&D credit carryforwards relate primarilycarryforwards:
In general, an ownership change, as defined by Section 382 of the Internal Revenue Code, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. SinceSuch ownership changes can significantly limit the amount of NOL carryforwards that may be utilized in future periods. The Company currently expects that it is remote that the CuraGen loss carryforwards may be utilized and, as such, no related asset has been recorded for such losses. The Company has not completed an analysis of losses generated by AVANT, however, the Company believes it is remote that $105 million of the AVANT loss carryforwards may be utilized in future periods and there may be substantial limitations on the Company's formation,ability to use the remaining losses of $40.2 million. Following the merger of Celldex and AVANT, the Company has raised capital and completed acquisitions through the issuance of capital stock on several occasions which may have resultedexperienced changes in one or more changes of control,ownership as defined by Section 382 or could result in a change of control in the future upon subsequent disposition.
The Company has not currently completed a study to assess whether an ownership change has occurred, or whether there have been multiple ownership changes since our formation, due to the significant complexity and related costs associated with such study. If the Company has experienced a change of control at any time since its formation, utilization of its NOL or tax credit carryforwards
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(8)(9) INCOME TAXES (Continued)
would beJune 2009 and December 2009. Further, prior to the merger of AVANT and Celldex, Celldex Research as a stand alone company experienced a change in ownership in October 2007. As a result of the ownership change in October 2007, utilization of the Celldex Research Federal NOLs is subject to an annual limitation of $4.5 million on $28.3 million of NOLs generated before that date. As a result of the company ownership changes in June 2009 and December 2009, there is an annual limitation amount of $6.0 million on $65.1 million NOLs. Any unused annual limitation may be carried over to later years, and the amount of the limitation may, under Section 382.certain circumstances, be subject to adjustment if the fair value of the Company's net assets are determined to be below or in excess of the tax basis of such assets at the time of the ownership change, and such unrealized loss or gain is recognized during the five-year period after the ownership change.
Similar to the AVANT and CuraGen NOL carryforwards above, the Company believes that it is remote that federal and state research and development ("R&D") credits of $20.6 million and $14.4 million, respectively, will be utilized in the future periods. Further, untilthe Company's ability to use the state NOL carryforwards of approximately $76.2 million and the remaining federal and state R&D credit carryforwards of approximately $11.3 million and $7.7��million, respectively, may be substantially limited. These state NOLs and federal and state credits expire at various dates starting in 2010 going through 2029. The Company has not yet completed a study of these credits to substantiate the amounts. Until a study is completed, and any limitations known, no amounts are being presented as an uncertain tax position under FIN 48.position.
In addition to uncertainties surroundingSubsequent ownership changes, as defined in Section 382, could further limit the useamount of NOLnet operating loss carryforwards in a change of control, the Company has identified orphan drug and research and development credits as material componentsthat can be utilized annually to offset future taxable income.
As of its deferred tax asset. The uncertainties in these components arise from judgmentsDecember 31, 2009, the Company also has foreign NOL carryforwards of approximately $34.9 million in the allocation of costs utilized to calculate these credits. TheUK which expire over various periods. These NOLs may be limited in the foreign jurisdiction and the Company has not conducted studiesyet undertaken any study to analyzeassess the usability of these credits to substantiate the amounts due to the significant complexity and cost associated with such study. Any limitation may result in expiration of a portion of the NOL or tax credits carryforwards before utilization. Further, until a study is completed and any limitation known, no amounts are being presented as an uncertain tax position under FIN 48.NOLs.
Massachusetts, New Jersey and MissouriConnecticut are the three states in which the Company primarily operates or has operated and has income tax nexus. Open federal and state return years subject to examination by major tax jurisdictions include the tax years ended December 31, 2005, 2006 and 2007. Carryforward attributes that were generated prior to 2005 may still be adjusted upon examination by the Internal Revenue Service if they either have been or will be used in a future period. The Company is currently under examination for sales and use taxes by the State of Connecticut for the period from July 1, 2006 through June 30, 2009. The Company is not currently under examination by the Internal Revenue Service or any other jurisdictions for any tax years.
On January 1, 2007, the Company adopted FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement 109 ("FIN 48"). FIN 48 prescribes a comprehensive model for recognizing, measuring, presenting and disclosing in the financial statements tax positions taken or expected to be taken on a tax return, including a decision whether to file or not to file in a particular jurisdiction. As a result of the implementation of FIN 48, Celldex recognized no material adjustment in the liability for unrecognized income tax benefits. As a result of the adoption of FIN 48 there is no material impact of unrecognized income tax benefits.year.
The Company's policy is to recognize interest and penalties related to uncertain tax positions in income tax expense. There have been no interest or penalties recognized in the consolidated statement of operations and on the consolidated balance sheet as a result of FIN 48 calculations. The Company has not recorded any interest and penalties on any unrecognized tax benefits since its inception.
As required by Statement of Financial Accounting Standards No. 109, management has evaluated the positive and negative evidence bearing upon the realizability of its net deferred tax assets, which are comprised principally of net operating loss carryforwards, capitalized R&D expenditures and R&D tax credit carryforwards. ManagementThe Company has determined that it is more likely than not that Celldexit will not recognize the benefits of federal and state deferred tax assets and, as a result, a full valuation allowance of approximately $132,241,000 has been establishedwas maintained at December 31, 2008. The2009 against the Company's net increase in the valuation allowance for 2008 is primarily due to the acquisition of AVANT.deferred tax assets.
(9)(10) STOCKHOLDERS' EQUITY
(A) Public and PrivateCommon Stock Offerings
The Company implemented a 1-for-12 reverse stock split of the Company's common stock on March 7, 2008, as approved by the Company's stockholders. The Company has a shelf registration statement filed withretroactively applied the Securitiesreverse stock split to all the share and Exchange Commission to registerper share amounts for sale any combination of securities describedall periods presented in the filing up to a dollarthese consolidated financial statements.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(9)(10) STOCKHOLDERS' EQUITY (Continued)
amount of $40 million. At December 31, 2008, no securities had been sold by the Company from this shelf registration.
(B) Convertible Preferred Stock
At December 31, 2008,2009, the Company had authorized preferred stock comprised of 96,925 shares of convertible Class B and 3,000,000 shares of convertiblepreferred stock all of which have been designated Class C of whichPreferred Stock including 350,000 shares haswhich have been designated as ClassSeries C-1 Junior Participating Cumulative the terms of which are to be determined by our Board of Directors. There was no preferred stock outstanding at December 31, 2008.Preferred Stock (the "Series C-1 Preferred Stock")."
(C) Shareholder Rights Plan
The Company's Board has adopted a Shareholder Rights Plan, as set forth in the Shareholder Rights Agreement, as amended, between the Company and Computer Investor Services, LLC (formerly EquiServeComputershare Trust Company, N.A.), as Rights Agent (the "Rights Agreement"). Pursuant to the terms of the Rights Agreement, the Board declared a dividend distribution of one Preferred Stock Purchase Right ("Right") for each outstanding share of the Company's common stock. These rights,Each Right, which expireexpires in November 2014, entitleentitles their holdersholder to purchase from the Company one ten-thousandth of a share (a "Unit") of Series C-1 Junior Participating Cumulative Preferred Stock par value $0.01 per share, ("C-1 Preferred Stock") at a cash exercise price of $35.00 per Unit, subject to adjustment. The rightsRights will trade separately from the common stock and will become exercisable only when a person or group has acquired 15% or more of the outstanding common stock or upon the commencement by a person or group of a tender offer that would result in such person or group acquiring 15% or more of the outstanding common stock other than as a result of repurchases of stock by the Company or certain inadvertent actions by a shareholder. In the event a person or group acquires 15% or more of the outstanding common stock each holder of a rightRight (except for any such person or group) would be entitled to receive upon exercise sufficient Units of Series C-1 Preferred Stock to equal a value of two times the exercise price of the purchase right.Right. In the event the Company is acquired in a merger or other business combination transaction or if 50% or more of the Company's assets or earning power is sold, each holder of a rightRight (except for any such person or group described above) would receive upon exercise common stock of the acquiring company with a value equal to two times the exercise price of the right.Right.
(11) SIGNIFICANT REVENUE ARRANGEMENTS
As of December 31, 2008, the Company has authorized the issuance of 350,000 shares of C-1 Preferred Stock for use in connection with the shareholder rights plan.
(D) Merger with Celldex
At the special meetingA summary of the Company's shareholders held on March 6, 2008 in connection with the Merger (as described in Note 1), stockholders approved four proposals: (i) the issuance of shares of the Company's common stock pursuant to the Merger Agreement in the amount necessary to result in the former Celldex Research stockholders owning 58% of the Company's common stock on a fully diluted basis, (ii) an amendment to the Company's Third Restated Certificate of Incorporation to increase the number of authorized shares to 300,000,000, (iii) an amendment to the Company's Third Restated Certificate of Incorporation to effect a reverse stock split in a ratio ranging from one-for-twelve to one-for-twenty of all the issued and outstanding shares of the Company's common
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(9) STOCKHOLDERS' EQUITY (Continued)
stock, the final ratio to be determined within the discretion of the Company's board of directors and (iv) adoption of the 2008 Stock Option and Incentive Plan.
The Company's board of directors approved a 1-for-12 reverse stock split of its common stock, which became effective on, March 7, 2008. As a result of the reverse stock split, each twelve shares of common stock were combined and reclassified into one share of common stock and the total number of shares outstanding was reduced from approximately 180 million shares (including the shares issued to former Celldex Research stockholders in the merger) to approximately 15 million shares.
Also, pursuant to the terms of the Merger Agreement, former Celldex Research shareholders received 4.96 shares of common stock in exchange for each share of Celldex common stock and Class A common stock they own. The Company's stockholders retained 42% of, and the former Celldex Research stockholders owned 58% of, the outstanding shares of the Company's common stock on a fully-diluted basis. The Company also assumed all of Celldex Research's stock options outstanding at the time of the Merger.
(10) PRODUCT DEVELOPMENT AND LICENSING AGREEMENTS
Oursignificant revenue from product development and licensing agreements was received pursuant to contracts and arrangements with different organizations. Total revenue recognized by us in connection with these contracts for the years ended December 31, 2008, 2007 and 2006 were $7,455,507, $1,405,592 and $899,184, respectively. A summary of these contracts follows:
(A) GlaxoSmithKline plc ("Glaxo") and Paul Royalty Fund II, L.P. ("PRF")
In 1997, the Company entered into an agreement with Glaxo to collaborate on the development and commercialization of the Company's oral rotavirus strain and Glaxo assumed responsibility for all subsequent clinical trials and all other development activities. The Company's licensed-in the rotavirus strain that was used to develop Glaxo's Rotarix® rotavirus vaccine in 1995 and owes a license fee of 30% to Cincinnati Children's Hospital Medical Center ("CCH") on net royalties received from Glaxo. The Company is obligated to maintain a license with CCH with respect to the Glaxo agreement. All licensing fees are included in research and development expense. The term of the Glaxo agreement is through the expiration of the last of the relevant patents covered by the agreement, although Glaxo may terminate the agreement upon 90 days prior written notice.
In May 2005, the Company entered into an agreement whereby an affiliate of PRF purchased an interest in the net royalties the Company will receive on worldwide sales of Rotarix®. Under the PRF agreement, the Company will retain 50% of future Glaxo milestone payments beginning on the effective date of the agreement with PRF, with 70% of the remaining balance payable to PRF and 30% of the remaining balance payable to CCH, respectively. The Company's retained interests in Rotarix® net royalties which were not sold to PRF are recorded as product royalty revenue and a corresponding amount that is payable to CCH is recorded as royalty expense, which is included in researchexpense. Product royalty revenue and development expense. For the year ended December 31, 2008, the Company recognized revenue of $3,259,565, including $225,000royalty expense related to the GSK milestone payment discussed below, related to itsCompany's retained interestsinterest in Rotarix®, respectively, which is payable to CCH. was
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER(11) SIGNIFICANT REVENUE ARRANGEMENTS (Continued)
7.7 million and $3.0 million for the years ended December 31, 2009 and 2008, 2007respectively. Under the PRF agreement, the Company also retained 50% of Glaxo milestone payments beginning on the effective date of the agreement with PRF, with 70% and 2006
(10) PRODUCT DEVELOPMENT AND LICENSING AGREEMENTS (Continued)
On30% of the remaining balance payable to PRF and CCH, respectively. In April 3, 2008, Rotarix® received FDAFood and Drug Administration ("FDA") market approval for the prevention of rotavirus gastroenteritis in infants, which triggered a $1.5 million milestone payment to the Company from Glaxo, $750,000 of which the Company has retained under its agreement with PRF. In connection with the Company's purchase accounting for the Merger, the present value of the Company's retained amount, or $742,300, had been recorded as a current asset as of March 31, 2008.Glaxo. During the quarterthree months ended June 30, 2008, the Company also recorded $225,000$0.2 million in product development and licensing agreement revenue and an offsettinga corresponding amount inpayable to CCH as royalty expense for the payable duerelated to CCH for its portion of the Glaxo milestone. The market launch of Rotarix® by Glaxo in
On October 1, 2008, the U.S. market during the quarter ended September 30, 2008 resulted inCompany received a $10 million milestone payment from PRF related to the Company from PRF, whichmarket launch of Rotarix® in the Company received on October 1, 2008.U.S. As of March 31, 2008, the Company recorded the expected presentestimated fair value of the $10 million milestone payment due from PRF of $9,053,200,$9.1 million in connection with the purchase accounting value assigned tofor the PRF milestone payment at the time of theAVANT Merger. During the quarterthree months ended September 30, 2008, the Company recognized the balance of $946,800$0.9 million as other income in the consolidated statement of operations. The Company has received $60 million in total milestone payments under the PRF agreement. No additional milestone payments are due from PRF under the agreement.
Royalty rates on Rotarix® escalate from 7% to 10% based on net product sales in countries that have valid patent protection. These royalty rates are discounted by 30% for "non-patent" countries (primarily international markets). In September 2006, the Company received notice from Glaxo that Glaxo would begin paying royalties on sales of Rotarix® vaccine at the lower of the two royalty rates under their 1997 license agreement. Glaxo's decision to pay the lower royalty rate (which is 70% of the full rate) is based upon Glaxo's assertion that Rotarix® is not covered by the patents Glaxo licensed from the Company in Australia and certain European countries. If Glaxo's position stands, the royalties to which PRF is entitled will no longer be limited by a $27.5 million annual threshold, which the Company projected may have been reached in later years as sales of Rotarix® increased. Irrespective of Glaxo's position, the Company will still retain approximately 65% of the royalties on worldwide sales of Rotarix® once PRF receives 2.45 times the aggregate cash payments of $60 million it made to Celldex,the Company, though the potential amount of such residual royalties will be lower if Glaxo's position stands.
(B) Glaxo and Corixa Corporation ("Corixa")
OnIn December 21, 2005, the Company and Corixa, a wholly-owned subsidiary of Glaxo, and Celldex Ltd (formerly Lorantis), entered into a termination agreement of their collaboration of CDX-2101, or HepVax, for the development of a therapeutic vaccine for Hepatitis B (the "Termination Agreement"). Under the terms of the Termination Agreement, Glaxo paid the Company the sum of approximately $1,632,000. In addition, and subject to the terms and conditions of the Termination Agreement, Glaxo granted to$1.6 million for which the Company a worldwide, fully paid up, royalty-free, perpetual, nonexclusive license under the Corixa Patent Rights, Corixa Know-How Rightsrecorded product development and Corixa Licensed Technology (each as defined in the Termination Agreement): (a) to use RC-529SE in products being developed and/or commercialized by Celldex Ltd or its Permitted Sublicensees in the Lorantis Field;licensing agreement revenue of $0.2 million, $0.5 million and (b) to make or have made RC-529SE using RC-529 adjuvant for the limited use permitted by the license granted to reformulate Corixa's proprietary adjuvant.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(10) PRODUCT DEVELOPMENT AND LICENSING AGREEMENTS (Continued)
The Company has concluded that because the original collaboration between Corixa and Lorantis contained multiple deliverables, EITF 00-21 applies. For$0.5 million during the years ended December 31, 2009, 2008 and 2007, and 2006, the Company recognized $466,156 of revenue under the Termination Agreement.respectively.
(C) Pfizer Inc ("Pfizer")
(1) Pfizer License and Development Agreement
On April 16, 2008, the Company and Pfizer entered into a License and Development Agreement (the "Pfizer Agreement") under which Pfizer was granted an exclusive worldwide license to a therapeutic cancer vaccine candidate, CDX-110, in Phase 2 development for the treatment of glioblastoma multiforme. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the Pfizer Agreement, Pfizer made an upfront payment to the Company of $40 million and made a $10 million equity investment in the Company.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(11) SIGNIFICANT REVENUE ARRANGEMENTS (Continued)
Pfizer will fund all development costs for these programs. The Company is also eligible to receive potential milestone payments exceeding $390 million for the successful development and commercialization of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales. The Pfizer Agreement became effective after clearance under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (as amended) on May 19, 2008.
On May 27, 2008, the Company received $10 million from Pfizer in exchange for 781,250 shares of the Company's common stock having a fair value of $10,867,188,$10.9 million, or $13.91 per share, on that date. The $867,188$0.9 million over the amount received from Pfizer was recorded as a reduction to deferred revenue of the $40 million upfront payment received from Pfizer on June 18, 2008.
The Company has applied the provisions of Emerging Issues Task Force (EITF) Issue No. 00-21 ("EITF 00-21"), Accounting for Revenue Arrangements with Multiple Deliverables, and determined that its performance obligations under this collaboration should be accounted for as a single unit of accounting. The Company's deliverables under this collaboration primarily include an exclusive license to its CDX-110 product candidate and its EGFRvIII technologies, research and development services as required under the collaboration and participation in the joint clinical development committee. The Company has estimated that its performance period under the collaboration will be 9.5 years based on an assessment of the period over which the Company will have met its performance obligations under the collaboration. Revenue, including research and development reimbursements, is being recognized on a straight-line basis over this period using the Contingency Adjusted Performance Model ("CAPM"). The $40,000,000$40 million up-front payment, was recorded as deferred revenue and this amount, less the $867,188$0.9 million in excess fair value for the Company's common stock discussed above, was recorded as deferred revenue and is being amortized over the 9.5-year performance period at a rate of $1,029,810$1.0 million per quarter.
The agreement also provides for reimbursement by Pfizer of all costs incurred by the Company in connection with the collaboration since the effective date. The Company invoices Pfizer monthly for its reimbursable costs and records the invoiced amount as deferred revenue. These deferred revenue amounts are amortized to revenue over the expectedestimated 9.5-year performance period on a straight-line basis using the CAPM model. For the year ended December 31, 2008, theThe Company had incurred and invoiced Pfizer $4,856,735$3.2 million and $4.9 million in reimbursable costs related to the Pfizer collaboration.collaboration for the years ended December 31, 2009 and 2008, respectively.
The Company recorded product development and licensing agreement revenue under the Pfizer Agreement as follows:
| | Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2009 | 2008 | |||||
| | (In thousands) | ||||||
Up-front portion | $ | 4,119 | $ | 2,551 | |||
Reimbursable costs portion | �� | 1,063 | 319 | ||||
| $ | 5,182 | $ | 2,870 | ||||
In connection with the Pfizer Agreement, the Company paid a total of $6.9 million in sublicense fees to Duke University and Thomas Jefferson University. The Company recorded these deferred sublicense fees to other assets in the consolidated balance sheets and is amortizing them to royalty expense over the 9.5-year performance period. The Company recorded $0.7 million and $0.5 million in royalty expense related to these deferred sublicense fees during the years ended December 31, 2009
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31,(11) SIGNIFICANT REVENUE ARRANGEMENTS (Continued)
and 2008, 2007 and 2006
(10) PRODUCT DEVELOPMENT AND LICENSING AGREEMENTS (Continued)
For the year endedrespectively. At December 31, 2009 and 2008, the Company recorded revenue under this collaboration of $2,870,359 which is included in Product Development and Licensing Agreements Revenue. Of this amount, $2,551,639 was attributed to the amortization of the $40 million upfront payment and $318,720 was attributed to the $4,856,735 reimbursable costs incurred by the Company for which Pfizer is obligated to reimburse the Company.
In connection with the initial deliverables under the Pfizer Agreement as discussed further in Note 11, the Company has paid a sublicense fee of $2,365,174 to each of two research universities, Duke University ("Duke") and Thomas Jefferson University ("TJU"), and paid TJU an additional license fee of $500,000. The Company paid an additional sublicense fee to TJU of $1,634,826 in October 2008. The Company has capitalized a total of $6,865,173 of deferred costs in the "Other Assets" line item in the consolidated balance sheet. These deferred costs are being amortized over the 9.5-year performance period at a rate of $180,663 per quarter. The Company has recognized $451,657 of these costs as royalty expense during the year ended December 31, 2008. The unamortized balance of deferred costs at December 31, 2008 was $6,413,516.$5.7 million and $6.4 million, respectively.
(2) Pfizer Animal Health Agreement
The Company entered into a licensing agreement in December 2000 with Pfizer's Animal Health Division whereby Pfizer has licensed Megan's technology for the development of animal health and food safety vaccines. Under the agreement, the Company may receive additional milestone payments of up to $3 million based upon attainment of specified milestones. The Company may receive royalty payments on eventual product sales. The term of this agreement is through the expiration of the last of the patents covered by the agreement. The Company has no obligation to incur any research and development costs in connection with this agreement.
As of June 1, 2006, the Company entered into a Collaborative Research and Development Agreement with Pfizer aimed at the discovery and development of vaccines to protect animals. In 2007, further funded work at the Company on the joint research program was terminated by Pfizer after the Company provided two of four deliverables to Pfizer.
(D) Rockefeller University ("Rockefeller") and Gates Grand Challenge Award
The Company is developing aproviding research and development support to Rockefeller on the development of their vaccine, DCVax-001, which the Company refers to as CDX-2401, aimed at providing protection from infection with HIV, the virus known to cause AIDS. This program is in a Bill & Melinda Gates Foundation funded partnership with collaborators at Rockefeller andcalled the Aaron Diamond AIDS Research Center, who have shown in model systems that protective immunity can be induced with such a vaccine.Grand Challenges initiative. Preclinical studies and manufacturing development are in progress and payments to the Company are made on a time and materials basis. ForThe Company recorded grant revenue from Rockefeller of $1.8 million, $0.4 million and $0.8 million for the years ended December 31, 2009, 2008 2007 and 2006, the Company recognized grant revenue from Rockefeller of $428,569, $829,610 and $252,457,2007, respectively.
(11) RESEARCH(12) COLLABORATION AND LICENSING AGREEMENTS
CelldexThe Company has entered into licensing agreements with several universities and research organizations. Under the terms of these agreements, we havethe Company has received licenses or options to license technology, specified patents or patent applications. CelldexThe Company's licensing and development collaboration agreements generally provide for royalty payments equal to specified percentages of product sales, annual license maintenance fees and continuing patent prosecution costs. In addition, the Company has expensed nonrefundablecommitted to make potential future milestone payments to third parties of up to approximately $116 million as part of the Company's various collaborations including licensing and development programs. Payments under these agreements generally become due and payable only upon achievement of certain developmental, regulatory and/or commercial milestones. Nonrefundable license feesfee expense was $0.7 million, $0.8 million and $0.2 million for the years ended December 31, 2009, 2008 and 2007, respectively.
Medarex, Inc., a subsidiary of Bristol-Myers Squibb ("Medarex")
Medarex, a former related party, and the Company have entered into the following agreements, each of which was approved by a majority of its independent directors who did not have an interest in the transaction. These agreements include:
Under the terms of the Assignment and License Agreement and Research and Commercialization Agreement, the Company may be required to pay milestone and royalty payments to Medarex with respect to the development of any products containing such licensed antibodies.
In October 2007, the Company and Medarex entered into a settlement and mutual release agreement with settled disputed amounts the Company owed Medarex. The Company issued to Medarex 351,692 shares of the Company's common stock equal in value to $3.0 million, based on the per share price of $8.64 set on the second trading day prior to the closing date of the AVANT Merger and exchanged releases. At December 31, 2008, the Company owed Medarex an additional $3.0 million related to a Master Services Agreement which the Company paid Medarex in October 2009.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(11) RESEARCH(12) COLLABORATION AND LICENSING AGREEMENTS (Continued)
approximately $752,642, $220,000 and $325,000 in the years ended December 31, 2008, 2007 and 2006, respectively.
(A) Medarex, Inc.Rockefeller University ("Rockefeller")
The Company and Medarex have entered into an Assignment and License Agreement that provides for the assignment of certain patent and other intellectual property rights and a license to certain Medarex technology and a Research and Commercialization Agreement which provides the Company with certain rights to obtain exclusive commercial licenses to proprietary monoclonal antibodies raised against certain antigens. Under these agreements with Medarex, Celldex may be obligated to pay license fees, milestone payments and royalties relating to the development and regulatory approval of certain of its technologies.
Under the terms of the Research and Commercialization Agreement with Medarex, Celldex will be required to pay Medarex license fees to obtain commercial licenses for antibodies arising from research licenses granted by Medarex. Celldex will also be required to pay Medarex milestone payments with respect to the development of any products containing such licensed antibodies. These fees and milestones may total up to $7 to $10 million per licensed antibody if a product containing such licensed antibody receives approval from the FDA and/or equivalent foreign agencies. None of Celldex's product candidates currently under development trigger such milestone payments. In general, potential milestone payments for Celldex's antibody product candidates may or may not be triggered and may vary in size depending on a number of variables, almost all of which are currently uncertain. Typically, the events that trigger these payments per product candidate include:
In addition, Celldex will be required to pay royalties on any sales of products containing licensed antibodies. The royalties will be payable on a country-by-country and product-by-product basis until the date which is the later of: (i) the expiration of the last-to-expire of the Medarex patents covering the product in such country or (ii) the tenth anniversary of the first commercial sale of a product in such country. Celldex will also be responsible for the payment of any royalties, license fees and milestone and other payments due to third parties if Celldex licenses any additional technology in order to commercialize such products.
To date, Celldex has not made any royalty payments on sales of any products and believes it is at least a number of years away from selling any products that would require Celldex to make any such royalty payments. Whether Celldex will be obligated to make milestone or royalty payments in the future is subject to the success of Celldex's product development efforts and, accordingly, is inherently uncertain.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(11) RESEARCH COLLABORATION AND LICENSING AGREEMENTS (Continued)
(B) Rockefeller University
On November 1, 2005, the Company and Rockefeller University ("Rockefeller") entered into a license agreement for the exclusive worldwide rights to human DEC-205 receptor, with the right to sublicense the technology. The license grant is exclusive except that Rockefeller may use and permit other nonprofit organizations to use the human DEC-205 receptor patent rights for educational and research purposes. In addition,The Company may be required to pay milestone and royalty payments to Rockefeller with respect to development and commercialization of the Company acknowledges that Rockefeller has granted Howard Hughes Medical Institute ("HHMI") a paid-up, nonexclusive, irrevocable license to use the patent rights, biological materials, and technical information for HHMI's research purposes, but with no right to sublicense.human DEC-205 receptor. The Company may also be required to pay royalties on any product sales. The royalties will be payable on a country-by-country and licensed product-by-licensed product basis until the date which is the later of (i) the expiration of the last to expire of the patents covering the licensed product in such country or (ii) ten years following the first commercial sale of a licensed product in such country.
The Company may be required to pay license fees and milestone payments to Rockefeller with respect to development of the human DEC-205 receptor. These fees and milestones may total up to $2 million to $4 million per product candidate that receives approval from the FDA and equivalent foreign agencies.
(C) Duke University Brain Tumor Cancer Center
On September 1, 2006, the Company and Duke University Brain Tumor Cancer Center of Duke University ("Duke")
In September 2006, the Company and Duke entered into a license agreement that gave the Company access and reference to the clinical data generated by Duke and its collaborators in order for the Company to generate its own filing with the FDA relating to its CDX-110 product.
In exchange for referencing all the Duke data, the Company paid Duke a one-time upfront payment of $175,000 and issued to Duke 100,000 shares of the Company's common stock, which the Company recorded in 2006 as a licensing expense in research and development. The estimated aggregate fair value of the common shares issued was $330,000.
The Company may be required to pay license feesmilestone and milestoneroyalty payments to Duke with respect to development and commercialization of the CDX-110 product. These fees and milestones may total up to $1.2 million if CDX-110 receives approval from the FDA and equivalent foreign agencies. The Company may also be required to pay royalties upon approval of CDX-110. The royalties will be payable on a country-by-country and licensed product-by-licensed product basis until the date of the expiration of the last to expire of the patents covering the licensed product in such country.
In connection with the Pfizer Agreement, discussed in Note 10, the Company determined that $2,365,174$2.4 million was payable to Duke as a sublicense fee. As agreed byprovided for under the Duke atlicense, the Company's option,Company paid 50% of this amount was paid to Duke in the form of 81,512 shares of the Company's common stock in October 2008.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(11) RESEARCH COLLABORATION AND LICENSING AGREEMENTS (Continued)
(D) Ludwig Institute for Cancer Research
On October 20, 2006, the Company and Ludwig Institute for Cancer Research ("Ludwig")
In October 2006, the Company and Ludwig entered into an agreement for the nonexclusive rights to six cancer tumor targets for use in combination with the Company's APC Targeting Technology. The term of the agreement is for ten years. As consideration for the nonexclusive license, the Company agreed to pay an annual license fee of $7,500 and $2,500 for each full-length antigen and partial-length antigen, respectively, until such antigens enter a randomized Phase 1 clinical trial.
As additional consideration for the nonexclusive license, theThe Company may be required to pay license feesmilestone and milestoneroyalty payments to Ludwig for the usewith respect to development and commercialization of the cancer targets in combination withtechnology licensed from Ludwig.
Alteris Therapeutics, Inc. ("Alteris")
In October 2005, the Company's technology. The fees and milestones may total upCompany completed the acquisition of the assets of Alteris, including the EGFRvIII molecule that the Company licensed to $1.5 million to $2.5 million on a product candidate that receives approval fromPfizer under the FDA and equivalent foreign agencies.Pfizer Agreement. The Company may also be required to pay royaltiesAlteris up to $5.0 million upon obtaining the first approval for commercial sale of anya product candidate. The royalties will be payable on a country-by-country and licensed product-by-licensed product basis until the date of the expiration of the last to expire of the patents covering the licensed product in such country.containing EGFRvIII, including CDX-110.
(E) Thomas Jefferson University ("TJU")
In February 2003, the Company entered into three exclusive license agreements with Thomas Jefferson University ("TJU"). Under the license agreements, TJU has granted a worldwide fee-and royalty-bearing exclusive license.TJU. Under these licenses, the Company willmay be obligatedrequired to pay milestone and royalty payments to TJU milestone payments which may total upwith respect to $3 million for the first licensed product developed during the termdevelopment and commercialization of the license agreements, an annual license fee of $45,000, patent and other expenses associated with licenses, as well as royalties on net sales oftechnology licensed products during the term of the license agreements. The Company also issued 100,000 shares of its common stock tofrom TJU. In the event that TJU provides notice of default and the default is not cured within 60 days of such notice, TJU may terminate the license agreements. In connection with the Pfizer Agreement, the Company amended its licenses with TJU to add additional sublicensing rights and made a $500,000 one-time license paymentpaid $4.5 million in sublicense fees to TJU in June 2008.
As discussed in Note 10, the3M Company paid a sublicense fee of $2,365,174 to TJU during the quarter ended September 30, 2008 and paid an additional sublicense fee of $1,634,826 to TJU in October 2008.
(F) Select Vaccines Limited ("Select Vaccines")
In February 2007, the Company entered into a research and development partnership with Select Vaccines, a public Australian biotechnology company, focused on the use of Select Vaccines' virus-like particles ("VLPs") as a platform technology for the development of viral vaccines. Under the terms of the agreement, the Company made an upfront equity investment of $735,000 in Select Vaccines and agreed to fund influenza vaccine research and development for two years, as well as provide payments to Select Vaccines for the achievement of specific preclinical and clinical development milestones. On November 1, 2007, the Company notified Select Vaccines that, effective December 31, 2007, the Company was exercising its rights to terminate its Collaboration and License Agreement with Select Vaccines for strategic reasons.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(11) RESEARCH COLLABORATION AND LICENSING AGREEMENTS (Continued)
In August 2008, the Company sold its equity investment in Select Vaccine shares for net proceeds of $250,882 and recorded a loss of $297,129. The Company had classified its equity investment in Select Vaccine shares as available-for-sale securities under SFAS 115,Accounting for Certain Investments in Debt and Equity Securities, ("FAS 115").
(G) 3M Company ("3M Company")
On June 11, 2008, the Company and 3M Company entered into a license agreement for the exclusive worldwide rights to access 3M Company's proprietary Immune Response Modifier, Resiquimod™, (and
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(12) COLLABORATION AGREEMENTS (Continued)
additional Toll-Like Receptor7/ 7/8 agonists ("TLR")) for clinical study with Celldex'sthe Company's proprietary APC Targeting Technology, for use as vaccine adjuvants, with the right to sublicense the technology.
The Company paid 3M Company a one-time upfront license fee which was charged to research and development expense in the quarter ended June 30, 2008. The Company may be required to pay annual license feesmilestone and milestoneroyalty payments to 3M Company with respect to development of Resiquimod™. The Company may also be required to pay royalties upon approval of any product candidate. The royalties will be payable on a country-by-country and licensed product-by-licensed product basis until the datecommercialization of the expiration of the last to expire of the patents covering thetechnology licensed product in such country.from 3M Company.
(H) University of Southampton, UK ("Southampton")
In November 2008, the Company entered into an Exclusive Patent and Know-How License Agreementa license agreement with the University of Southampton UK, to develop human antibodies towards CD27, a potentially important target for immunotherapy of various cancers. CD27 is a critical molecule in the activation pathway of lymphocytes, is downstream from CD40, and may provide a novel way to regulate the immune responses. In pre-clinicalpreclinical models, antibodies to CD27 have been shown to mediate anti-tumor effects alone, and may be particularly effective in combination with the Company's other immunotherapies.
The Company paid Southampton a one-time upfront license fee which was charged to research and development expense in the quarter ended December 31, 2008. The Company may be required to pay annual license feesmilestone and milestoneroyalty payments to Southampton with respect to development and commercialization of CD27.the technology licensed from Southampton.
Amgen Inc. ("Amgen")
In March 2009, the Company entered into a license agreement with Amgen to expand its Precision Targeted Immunotherapy Platform by acquiring exclusive rights to FMS-like tyrosine kinase 3 ligand (Flt3L) and CD40 ligand (CD40L). Flt3L and CD40L are immune modulating molecules that increase the numbers and activity of immune cells that control immune responses. The Company may also be required to pay royalties upon approvalmilestone and royalty payments to Amgen with respect to development and commercialization of any product candidate.technology licensed from Amgen.
Seattle Genetics, Inc. ("Seattle Genetics")
In connection with the CuraGen Merger, the Company assumed the license agreement between CuraGen and Seattle Genetics whereby CuraGen acquired the rights to proprietary antibody-drug conjugate ("ADC") technology for use with the Company's proprietary antibodies for the potential treatment of cancer. The royalties willCompany may be payable on a country-by-countryrequired to pay milestone and licensed product-by-licensed product basis until the dateroyalty payments to Seattle Genetics with respect to development and commercialization of the expiration of the last to expire of the patents covering the licensed product in such country.ADC technology.
(12) RELATED PARTY TRANSACTIONS(13) 4% CONVERTIBLE DEBT DUE 2011
Medarex is a major shareholderIn connection with the CuraGen Merger, the Company assumed $12.5 million in 4% convertible subordinated debt due February 15, 2011 (the "CuraGen Debt"). In addition, effective October 1, 2009, Celldex, CuraGen, and The Bank of Celldex, owning approximately 31.4%New York Mellon (the "Trustee") amended the CuraGen Debt to provide that the CuraGen Debt shall be convertible into 353,563 shares of the Company's outstandingCelldex common stock at December 31, 2008.the rate of 28.27823 shares of Celldex common stock per $1,000 principal amount of notes, or $35.36 per share. The Company has the right to redeem the notes at a redemption price equal to 100.571% of the principal amount of the notes plus accrued and Medarex have entered intounpaid interest, if any. The CuraGen Debt is convertible by the holders of the CuraGen Debt at any time prior to maturity.
As of October 1, 2009, the Company recorded the CuraGen Debt at its estimated fair value of $11.5 million as part of the acquisition accounting related to the CuraGen Merger. The initial carrying value of the CuraGen Debt is being accreted ratably, over the term of the CuraGen Debt, to $12.5 million due at maturity. Interest expense on the CuraGen Debt was $0.3 million for the three
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(12) RELATED PARTY TRANSACTIONS(13) 4% CONVERTIBLE DEBT DUE 2011 (Continued)
following agreements, each of which was approved by a majority of its independent directors who did not have anmonths ended December 31, 2009 and included $0.2 million in discount accretion. At December 31, 2009, the carrying value and the accrued interest inon the transaction. These agreements include:
The Company may be required to pay license fees and milestone payments to Medarex with respect to any antibodies developed using its HuMab-Mouse technology. These fees and milestones may total up to $7outstanding $12.5 million to $10in CuraGen Debt was approximately $11.9 million, per antibody that receives approval from the FDA and equivalent foreign agencies.
The Company may also be required to pay royalties on any product sales. The royalties will be payable on a country-by-country and licensed product-by-licensed product basis until the date which is the later of (i) the expiration of the last to expire of the patents covering the licensed product in such country or (ii) ten years following the first commercial sale of a licensed product in such country.
The Company and Medarex entered into a settlement and mutual release agreement on October 19, 2007, whereby the parties agreed to a settlement with respect to a disputed return of capital related to certain unsuccessful initial public offering costs that were funded by Medarex on behalf of the Company in prior years. The Company agreed to issue to Medarex 351,692 of the Company's shares equal in value to $3,038,617, based on the per share price of $8.64 set on the second trading day prior to the closing date of the Merger. Medarex has agreed to amend certain terms of the existing Research and Commercialization Agreement and Assignment and License Agreement. Both parties have agreed to mutual releases under the settlement and mutual release agreement.
The Company has recorded a payable due Medarex of $2,957,248 at December 31, 2008.
(13) DEFERRED REVENUE
At December 31, 2008, deferred revenue associated with the Pfizer Agreement represented $41,119,189 of the total current and long-term deferred revenue of $41,420,040 at that date. As more fully discussed in Note 10, Pfizer made a $40 million upfront license payment, made a $10 million equity investment and agreed to reimburse the Company monthly for all costs incurred in connection with the collaborative effort on CDX-110. Through December 31, 2008, the Company has incurred and
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(13) DEFERRED REVENUE (Continued)
invoiced Pfizer for reimbursable costs in the amount of $4,856,735. Under applicable accounting literature, the Company has determined that its performance obligations under the Pfizer Agreement should be accounted for as a single unit of accounting over an estimated 9.5-year period of expected performance by the Company under the Agreement. Accordingly, the $40 million upfront license payment, less $867,188 allocated to the fair value of Pfizer equity investment, and the $4,856,735 for reimbursable costs have been deferred and are being recognized as revenue over the 9.5-year period on a straight-line basis utilizing the Contingency Adjusted Performance Model.
Expected future recognition of the deferred revenue balance at December 31, 2008 for each of the next five years and thereafter is as follows; 2009—$4,931,327, years 2010 through 2013 per year—$4,630,476, and thereafter—$17,966,809.quoted market prices.
(14) OTHER LONG-TERM LIABILITIES
Other long-term liabilities include the following:
| | December 31, 2008 | December 31, 2007 | ||||||
|---|---|---|---|---|---|---|---|---|
Deferred Rent | $ | 301,171 | $ | 207,654 | ||||
Loan Payable | 686,254 | — | ||||||
Note Payable | 300,291 | — | ||||||
Total | 1,287,716 | 207,654 | ||||||
Less Current Portion | ||||||||
Deferred Rent | 57,451 | 57,447 | ||||||
Loan Payable | 49,954 | — | ||||||
Note Payable | 111,054 | — | ||||||
| 218,459 | 57,447 | |||||||
| �� | ||||||||
Long-Term Portion | $ | 1,069,257 | $ | 150,207 | ||||
| | December 31, 2009 | December 31, 2008 | ||||||
|---|---|---|---|---|---|---|---|---|
| | (In thousands) | |||||||
Deferred Rent | $ | 377 | $ | 301 | ||||
CuraGen Severance (See Note 16) | 2,623 | — | ||||||
Deferred Tax Liabilities (See Note 19) | 4,661 | — | ||||||
Loan Payable | 632 | 686 | ||||||
Note Payable | 178 | 300 | ||||||
Total | 8,471 | 1,287 | ||||||
Less Current Portion | (2,156 | ) | (218 | ) | ||||
Long-Term Portion | $ | 6,315 | $ | 1,069 | ||||
In December 2003, the Company entered into a Lease Agreement (the "Lease Agreement"), a Secured Promissory Note: Equipment Loan (the "Secured Promissory Note") and a Security Agreement with the Massachusetts Development Finance Agency ("MassDevelopment"), an economic development entity for the Commonwealth of Massachusetts, for the Company to occupy and build-out a manufacturing facility in Fall River, Massachusetts.
(A) Loan Payable
Under the Lease Agreement, the Company received a Specialized Tenant Improvement loan ("Loan of $1,227,800Payable") that accrues interest at an interesta rate of 5.5% per annum to finance the build-out of its Fall River facility which was recorded as leasehold improvements. The Company is amortizing the leasehold improvements over the remaining expected lease term.facility. Principal and interest payments on the loanLoan Payable are due monthly using an amortization period of 15 years.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(14) OTHER LONG-TERM LIABILITIES (Continued)
In connection with the Merger, the Company recorded $722,683 as the fair value of the loan payable based on current market interest rates available to the Company for long-term liabilities with similar terms and maturities. At December 31, 2008, the Company has recorded a loan payable of $686,254 to MassDevelopment, of which $49,954 was classified as current and $636,300 as long-term. Based on current market interest rates available to Celldex for long-term liabilities with similar terms and maturities, the fair value of the loan payable is approximately $685,900 at December 31, 2008.
(B) Note Payable
Under the Secured Promissory Note, the Company received $903,657 fromissued a note payable to MassDevelopment ("Note Payable") that accrues interest at an interesta rate of 5.5% per annum to finance the purchases of manufacturing and laboratory equipment to be placed in its Fall River facility (the "Loan").facility. The LoanNote Payable has a term of 84 months. The Loanmonths and is collateralized by all of the equipment purchased with the principal amount. Thea net book value of these collateralized assets at December 31, 2008 was $359,635.2009 of $0.3 million.
In connection with the Merger, the Company recorded $366,251 as the fair value of the note payable basedBased on current market interest rates available to the Company for long-term liabilities with similar terms and maturities. At December 31, 2008,maturities, the balance of the note payable to MassDevelopment was $300,291, of which $111,054 was classified as current and $189,237 as long-term. Based on current market interest rates available to Celldex for long-term liabilities with similar terms and maturities,Company believes the fair value of the note payable is approximately $358,400Loan Payable and Note Payable approximates their carrying value at December 31, 2008.2009.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(14) OTHER LONG-TERM LIABILITIES (Continued)
The Company is obligated to repay the following table summarizesprincipal amounts for the Company's approximate contractual obligations to MassDevelopment with respect toLoan Payable and Note Payable over the loannext five years and note payable:thereafter:
| | Loan Payable | Note Payable | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | Principal | Interest | Total | Principal | Interest | Total | |||||||||||||
2009 | $ | 50,000 | $ | 80,400 | $ | 130,400 | $ | 111,100 | $ | 66,100 | $ | 177,200 | |||||||
2010 | 51,500 | 74,300 | 125,800 | 144,000 | 33,200 | 177,200 | |||||||||||||
2011 | 53,300 | 67,900 | 121,200 | 45,200 | 2,100 | 47,300 | |||||||||||||
2012 | 55,200 | 61,500 | 116,700 | — | — | — | |||||||||||||
2013 | 57,600 | 54,500 | 112,100 | — | — | — | |||||||||||||
Thereafter | 418,700 | 164,900 | 583,600 | — | — | — | |||||||||||||
Total Obligation | $ | 686,300 | $ | 503,500 | $ | 1,189,800 | $ | 300,300 | $ | 101,400 | $ | 401,700 | |||||||
Less: Current Portion | 50,000 | 111,100 | |||||||||||||||||
Total Long-Term Portion | $ | 636,300 | $ | 189,200 | |||||||||||||||
| | Loan Payable | Note Payable | |||||
|---|---|---|---|---|---|---|---|
| | (In thousands) | ||||||
2010 | $ | 47 | $ | 133 | |||
2011 | 54 | 45 | |||||
2012 | 55 | — | |||||
2013 | 58 | — | |||||
2014 | 60 | — | |||||
Thereafter | 358 | — | |||||
Total | $ | 632 | $ | 178 | |||
(15) COMMITMENTS AND CONTINGENCIES
(A) Commitments for the Needham, Massachusetts Facility
In November 2005, the Company entered into a lease amendment that extended its lease of laboratory and office space in Needham, Massachusetts through April, 2017 and reduced the Company's leased space to approximately 35,200 square feet.2017. Under this lease amendment, the Company is obligated to pay an escalating base annual rent ranging from $879,700 to $1,161,200 during
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(15) COMMITMENTS AND CONTINGENCIES (Continued)
the remaining lease term. Aggregate rental payments including common area maintenance costs for("CAM") during the year ended December 31, 2008 for this facility was $1,437,040.remaining lease term.
(B) Commitments for the Fall River, Massachusetts Facility
In December 2003, the Company entered into a lease, as amended, with MassDevelopment to occupy and build-out an 11,800 square foota manufacturing facility in Fall River, Massachusetts. The lease has an initial seven-year term that expires in December 2010 and two renewal options of five years each. Management has determined that it is reasonably assured that the Company will exercise one five-year renewal option. Therefore, the Company is amortizing leasehold improvements made to the Fall River facility over the remaining original lease term plus one five-year renewal term. In November 2005, December 2006 and October 2008, the Company amended the MassDevelopment lease to increase the rentable space to approximately 14,300, 16,200 and 21,000 square feet, respectively, at the Fall River facility. Aggregate rental payments including common area maintenance costs for the year ended December 31, 2008 for this facility was $390,664.
(C) Commitments for the Philipsburg, New Jersey Facility
The Company leases approximately 20,000 square feet of office and laboratory space in Phillipsburg, New Jersey. The lease has an initial five-year term which expires in August 2011. Under the lease agreement, the Company is obligated to pay an annual rent of approximately $347,700 plus certain common area maintenance costs. Aggregate rental payments including common area maintenance costs for the years ended December 31, 2008 and 2007 for this facility were $370,652 and $347,652, respectively.
As an incentive to enter into athe lease agreement, with the Phillipsburg landlord, the Company received four months of rent-free occupancy of the facility, andfree rent which the Company is amortizing this over the original five-year term of the lease. In addition, the landlord provided the Company with an allowance on future rent payments towards tenant improvements that the Company made to the facility and that credit is included in deferred rent and is being amortized over the lease term. Construction of the tenant improvements was completed in August 2006.
The Company entered into a letter of credit facility with a national U.S. financial institution for $177,000, which is collateralized by a security deposit for the leased facility in Phillipsburg, New Jersey. The total amount of theCompany recorded restricted cash related to this security deposit is recordedof $0.2 million to other assets in Other Assets on the Company's consolidated balance sheets.
(D) Commitments to Licensors under Certain Intellectual Property License Agreements
The Company has certain obligations to pay licensors based on payments received by the Company from its licensees. The Company believes that it has in the past,sheets at December 31, 2009 and is continuing to satisfy its payment obligations to its licensors based on the Company's interpretation of its license agreements with those licensors. If a licensor was to disagree with the calculation of payments made by the Company pursuant to the license agreements, then the Company may be required to make additional license payments to one or more licensors. There can be no assurances that a licensor will not dispute the Company's interpretation of those license agreements or the Company's calculation of payments due. Accordingly,December 31, 2008.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(15) COMMITMENTS AND CONTINGENCIES (Continued)
the Company may have a contingent liability, in an amount which it cannot determine with precision, based on the risk that such additional payments may have to be made. There can be no assurances that a license payment, once made, will not be the subject of a later dispute by either the licensor or the Company.
(E) Commitments for Operating Leases
Obligations for base rent and common area maintenanceCAM costs (CAM) under facility and other non-cancelable operating leases as of December 31, 20082009, assuming the exercise of one five year renewal option for the Company's Fall River, MA facility, are approximately as follows:
Year ending December 31, | | |||
|---|---|---|---|---|
2009 | $ | 2,475,600 | ||
2010 | 2,542,800 | |||
2011 | 2,506,200 | |||
2012 | 2,325,200 | |||
2013 and thereafter | 10,140,100 | |||
Total minimum lease payments | $ | 19,989,900 | ||
Year ending December 31, | | |||
|---|---|---|---|---|
| (In thousands) | | |||
2010 | $ | 2,465 | ||
2011 | 2,613 | |||
2012 | 2,450 | |||
2013 | 2,495 | |||
2014 | 2,552 | |||
2015 and thereafter | 5,388 | |||
Total minimum lease payments | $ | 17,963 | ||
The Company's total rent and CAM expense for all facility leases was $2,198,356$2.5 million, $2.2 million and $347,652$0.3 million for the years ended December 31, 2009, 2008 and 2007, respectively.
(16) SEVERANCE ARRANGEMENTS
Dr. Una S. Ryan:Ronald C. Newbold, former Senior Vice President, Business Development, resigned from his position effective March 1, 2009 pursuant to the provision of his employment agreement that deems a resignation within the year following a change of control (in this case, the AVANT Merger) as a termination resulting from a change of control. In May 2008,accordance with Dr. Newbold's employment agreement, the Company recorded severance expense during the three months ended March 31, 2009 of $0.7 million including non-cash stock-based compensation expense related to the acceleration of vesting of options to purchase 107,485 shares of Company common stock as provided for under Dr. Newbold's employment agreement.
The Company and Dr. Una S. Ryan, who had been theformer President and Chief Executive Officer of the Company, informed the Company's Board of her intention to depart from the Company pending negotiation of the terms of her separation. The Company and Dr. Ryan executed a separation agreement effective July 16, 2008 (the "Separation Agreement") setting forth such terms regarding Dr. Ryan's separation from the Company. Pursuant to the Separation Agreement, the Company recorded severance expense during the three months ended June 30, 2008 of $1.4 million. The Separation Agreement also provided among other things, for: (i) a lump sum cash payment of $1,323,203, plus interest infor the amount of $10,784, which was paid on November 8, 2008; (ii) a mutual general release; (iii) payment of insurance premiums under COBRA for 18 months; (iv) reimbursement of attorneys' fees up to $30,000 and (v) vesting of options to purchase 153,125 shares of Company common stock (of the options to purchase 612,500 shares of Company common stock which had been granted to Dr. Ryan on March 7, 2008). The remainder of Dr. Ryan's options terminated as of July 16, 2008. The Separation Agreement also provided for Dr. Ryan's resignation, effective July 16, 2008, from her position as a director of the Company and each of its subsidiaries. At December 31, 2008, the Company has accrued the present value of the expected remaining COBRA benefits due Dr. Ryan totaling $24,542. During the year ended December 31, 2008, the Company paid the lump sum cash payment of $1,323,203, plus interest in the amount of $10,784, reimbursable attorney fees of $30,000 and insurance premiums under COBRA of $6,786.
With respect to Dr. Ryan's options, the Company recorded stock-based compensation expense of $1.3 million chargedrelated to general and administrative expense, for the fully vestedacceleration of vesting of options granted to Dr. Ryan in connection with the Separation Agreement was appropriately recorded in July 2008, when the criteria for establishing a grant date under SFAS 123(R) were met.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(16) SEVERANCE ARRANGEMENTS (Continued)
Dr. Robert F. Burns: The Company and Dr. Robert F. Burns, formerly theformer President and Chief Executive Officer of Celldex Research, entered into a separation and mutual release agreement dated as of October 19, 2007, under which (i) Dr. Burns' employment was terminated effective as of February 15, 2008. Until such date, Dr. Burns had no obligation to render services to2008, (ii) the Company although he wasagreed to hold himself available to consult with the Company by telephone at reasonable times. Asnine months of severance the Company was obligated to pay to Dr. Burns the monthly sum of £33,333 for nine consecutive months, commencing with the first payment on March 15, 2008,payments and a payment of £100,000 on December 15, 2008, in each case less applicable withholdings and other customary payroll deductions. Dr. Burns is also entitled to the continuation of benefits untilthrough February 15, 2010. All2010, (iii) all of Dr. Burns' stock options became fully vested and exercisable onthrough February 15, 2008,2011, and he may exercise them for up to three years following that date.(iv) Dr. Burns and the Company provided one another with mutual releases under such separation and mutual release agreement.
As Dr. Burns has not provided substantive service to the Company since October 19, 2007, these severance benefits, which in the aggregate equal $1,014,017, were accrued in the consolidated financial statements as of December 31, 2007. In addition, stock-based compensation was adjusted for the modification of Dr. Burns' stock option awards in accordance with SFAS No. 123(R).
The following table sets forth an analysis of the severance costs, which are included in accrued liabilities in the consolidated balance sheet as of December 31, 2008 and 2007:
| | Balance at December 31, 2007 | Charges | Paid Cash | Balance at December 31, 2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Severance and benefits | $ | 1,014,017 | $ | 1,384,658 | $ | (2,364,827 | ) | $ | 33,848 | ||||
Exit Activities in the U.K.: In December 2006, the Company adopted a plan to reduce operating expenses, following its decision to assign its leased facility in Cambridge, United Kingdom, to a third party. The plan included a reduction of 18 full-time employees in both research and development and general and administrative areas of the Company. As a result of staffing reductions, the Company recorded severance benefits expense of $477,508 as of December 31, 2006.
In December 2006, the Company entered into an agreement with a third party to assign the lease entered into by Celldex Ltd. (formerly Lorantis) in June 2003. Under the assignment, the assignee assumed all costs and expenses associated with the leased facility. As part of the agreement of assignment, the Company agreed to a six-month free rent period to the assignee as an incentive to enter into the lease assignment, whereby the Company paid the rent for the period this period of $691,187. This amount is reflected in the 2006 consolidated statement of operations (see Note 5 for additional information).
(17) MERGER OF CELLDEX AND CELLDEX RESEARCH
On March 7, 2008, Celldex (formerly AVANT Immunotherapeutics, Inc.) completed the Merger with Celldex Research (formerly Celldex Therapeutics Inc.) with Celldex Research considered the accounting acquirer, even though Celldex (then AVANT) issued common stock and was the surviving legal entity in the transaction.releases. The Company issued 8,309,420 shares of its common stock in exchangerecorded
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(17) MERGER OF CELLDEX AND CELLDEX RESEARCH(16) SEVERANCE ARRANGEMENTS (Continued)
$1.0 million in severance expense related to the separation and mutual release agreement during the three months ended December 31, 2007.
CuraGen employees who did not receive offers of employment were terminated upon the consummation of the CuraGen Merger. These employees were eligible for severance payments ("CuraGen Severance") upon termination of employment under certain circumstances, including following the CuraGen Merger. U.S. GAAP requires severance obligations that are incurred by the acquiree for the benefit of the acquirer to be recognized as an expense in the post-combination period. Because the offer of employment was at the option of the Company, the Company has deemed the CuraGen Severance to be at its benefit. On October 1, 2009, the Company recorded severance expense of $3.3 million and $0.9 million to general and administrative and research and development, respectively, related to the CuraGen Severance
The following table sets forth an analysis of the CuraGen Severance costs included in long-term liabilities at December 31, 2009:
| | Balance at December 31, 2008 | Charges | Paid Cash | Balance at December 31, 2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | (In thousands) | ||||||||||||
CuraGen Severance | — | $ | 4,246 | $ | (1,623 | ) | $ | 2,623 | |||||
(17) RETIREMENT SAVINGS PLAN
The Company's 401(k) Plan (the "401(k) Plan") is intended to be a tax-qualified plan covering substantially all employees. Under the terms of the 401(k) Plan, employees may elect to contribute up to 15% of their compensation, or the statutory prescribed limits. The Company may make 50% matching contributions on up to 4% of a participant's annual salary. Benefit expense for the 401(k) Plan was $0.1 million for the years ended December 31, 2009 and 2008.
(18) AVANT MERGER
In connection with the AVANT Merger, effective March 7, 2008, the Company issued 8,309,420 shares of its common stock in exchange for all of the outstanding capital stock of Celldex Research, on the basis of 4.65 shares of Celldex (then AVANT) common stock for each share of Celldex Research common stock such that Celldex Research shareholders owned 58% of the Company's common stock on a fully diluted basis and CelldexAVANT shareholders retained 42%. The Company also issued 351,692 shares having a value of $3,038,617 in settlement of a payable due Medarex. The purchase price of $47,570,867$47.6 million represents the shares attributable to former AVANT shareholders and consisted of (i) the 6,265,8896,265,882 shares outstanding of Celldex (then AVANT)AVANT common stock on the effective date of the Merger valued at $46,875,372$46.9 million and (ii) estimated transaction costs totaling $695,495.$0.7 million.
The acquisition has been accounted for as a purchase with Celldex Research the accounting acquirer. Consequently, the operating results of Celldex (then AVANT) since March 8, 2008 have been included in the consolidated results of operations.
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(18) AVANT MERGER (Continued)
The purchase price was allocated to the acquired tangible and identifiable intangible assets and assumed liabilities, based upon their fair value at the date of acquisition, as follows:follows (in thousands):
Tangible assets acquired | $ | 34,959,482 | |||
Less: Liabilities assumed | (3,945,067 | ) | |||
Net tangible assets acquired | 31,014,415 | ||||
Intangible assets acquired: | |||||
Core Technology | 897,249 | ||||
Developed Technology | 273,796 | ||||
Strategic Partner Agreement | 629,499 | ||||
In-Process Research and Development ("IPR&D") | 14,755,908 | ||||
Total | $ | 47,570,867 | |||
Tangible assets acquired | $ | 34,960 | |||
Less: Liabilities assumed | (3,945 | ) | |||
Net tangible assets acquired | 31,015 | ||||
Intangible assets acquired: | |||||
Core Technology | 897 | ||||
Developed Technology | 274 | ||||
Strategic Partner Agreement | 629 | ||||
In-Process Research and Development ("IPR&D") | 14,756 | ||||
Total | $ | 47,571 | |||
The values assigned to the intangible assets acquired, including the IPR&D, were determined based on fair market value using a risk adjusted discounted cash flow approach. Fair values for long-term tangible and intangible assets and for IPR&D were then reduced by $6,041,597$6.0 million of negative goodwill. The Company is a biotechnology enterprise and its resources are substantially devoted to research and development at the date of the Merger. Management is responsible for determining the fair value of the acquired IPR&D.
The values assigned to IPR&D relateprimarily related to the development of a typhoid-ETEC-cholera combination travelers vaccine a cholesterol management vaccine, and the CDX-1135 (formerly TP10) complement inhibitor in the amounts of $7.8 million $0.9 million and $6 million, respectively. Each of these three significant research and development projects in-process were valued through detailed analysis of product development data concerning the stage of development, time and resources needed to complete the project, expected income-generating ability and associated risks. The value of IPR&D was determined by estimating the costs to develop the purchased in-process technology into commercially viable products, estimating the net cash flows from such projects and discounting the net cash flows back to their present values. The probability of success and discount rates used for each project take into account the uncertainty surrounding the successful development and commercialization of the purchased in-process technology. We expect to incur approximately $16.2 million to move these projects to the point of out-licensing them to third parties. The estimated revenues from the
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007 and 2006
(17) MERGER OF CELLDEX AND CELLDEX RESEARCH (Continued)
typhoid-ETEC-cholera vaccine, the cholesterol management vaccine, and CDX-1135 are expected to be generated beginning in 2014, 2015 and 2014, respectively. A discount rate of 29% was used to value these projects, which we believe to be commensurate with the stage of development and the uncertainties in the economic estimates described above. The resulting net cash flows for these projects were based on management's best estimates of revenue, cost of sales, research and development costs, selling, general and administrative costs, and income taxes for each project and the net cash flows reflect assumptions that would be used by market participants.
The significant assumptions underlying the valuations included potential revenues, costs of completion, the timing of product approvals and the selection of appropriate probability of success and discount rates. None of the Company's IPR&D projects havehad reached technological feasibility nor dodid they have any alternative future use. Consequently, in accordance with current U.S. GAAP,the former purchase method of accounting, the fair value allocated to IPR&D was charged as an expense in the Company's consolidated financial statements as of the date of acquisition. The remaining acquired intangible assets arising from the acquisition are being amortized on a straight line basis over their estimated lives,lives.
In January 2009, the Company entered into a license agreement with Vaccine Technologies, Inc. ("VTI") under which range from 4.5it granted a worldwide exclusive license to 8 years.
As of December 31, 2008, the technological feasibilityVTI to develop and commercialize our CholeraGarde® and ETEC vaccine programs. The Company may receive milestones payments and royalties with respect to development and commercialization of the projects had not yet been reachedtechnology licensed to VTI and no longer expects to incur significant departurescosts on these projects. The Company is continuing the development of CDX-1135 and expects to incur approximately $6.3 million to move this project to the point of potentially out-licensing it to a third party. Estimated revenues from CDX-1135 are expected to be generated beginning in 2014.
(19) CURAGEN MERGER
In connection with the assumptionsCuraGen Merger, effective October 1, 2009, the Company (i) issued 15,722,713 shares of common stock of the Company, or 0.2739 shares, in exchange for each share of outstanding CuraGen common stock, plus cash in lieu of fractional shares (the "CuraGen Exchange Ratio"), (ii) assumed all of the CuraGen Stock Options and (iii) assumed the CuraGen Debt. The Company acquired CuraGen to gain access to a pipeline of oncology-focused antibodies, including
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(19) CURAGEN MERGER (Continued)
CDX-011 (formerly CR011) currently in Phase 2 clinical development for the treatment of breast and melanoma cancer, and cash, cash equivalents and marketable securities of $70.3 million.
The transaction is being accounted for under the acquisition method of accounting. All of the assets acquired and liabilities assumed in the transaction are recognized at their acquisition-date fair values, while transaction costs associated with the transaction are expensed as incurred.
Purchase Price
The purchase price for CuraGen is based on the acquisition-date fair value of the consideration transferred, which was calculated based on the closing price of the Company's common stock of $5.43 per share on October 1, 2009. The acquisition-date fair value of the consideration transferred consisted of the following (in thousands):
Fair value of common stock issued | $ | 85,374 | ||
Fair value of CuraGen Stock Options | 2,868 | |||
Total consideration transferred | $ | 88,242 | ||
U.S. GAAP requires that the fair value of replacement awards attributable to precombination service be included in the valuation analysis had occurred. Substantial additional researchconsideration transferred. Of the CuraGen Stock Options assumed, all but 1%, were immediately vested upon closing in accordance with the terms of the stock option agreements and development will be required prioremployment agreements. The fair value of the CuraGen Stock Options that has been attributed to reaching technological feasibility. In addition, each product needs to successfully complete a seriesprecombination service is included in the consideration transferred.
Allocations of clinical trialsAssets and to receive FDA or other regulatory approval prior to commercialization.Liabilities
The Company has allocated the consideration transferred for CuraGen to net tangible assets, intangible assets, goodwill and a severance obligation. The difference between the aggregate consideration transferred and the fair value of assets acquired and liabilities assumed was allocated to goodwill. This goodwill relates to the potential synergies from the CuraGen Merger and a deferred tax liability related to acquired IPR&D intangible assets. None of the goodwill is also dependent upon the activities of its collaborators in developing, manufacturing and marketing its products. There canexpected to be no assurance that these projects will ever reach feasibility or develop into products that can be marketed profitably, nor can there be assurance that the Company and its collaborators will be able to develop, manufacture and commercialize these products before the Company's competitors. If these products are not successfully developed and do not become commercially viable, the Company's financial condition and results of operations could be materially affected.
deductible for income tax purposes. The following unaudited pro forma financial summary is presented as iftable summarizes the operations of Celldex and Celldex Research were combined as of January 1, 2006. The unaudited pro forma combined results are not necessarily indicativefair values of the actual results that would have occurred hadassets acquired and liabilities assumed at the acquisition been consummated at that date or of the future operations of the combined entities. The following pro forma financial summary includes charges for in-process research and development of $14,755,908 and $14,440,009 for the years ended December 31, 2008 and 2007, respectively, which are material non-recurring charges.(in thousands):
Years Ended December 31, | 2008 | 2007 | |||||
|---|---|---|---|---|---|---|---|
Revenue | $ | 9,016,365 | $ | 4,174,140 | |||
Net loss | (52,512,300 | ) | (52,298,522 | ) | |||
Basic and diluted net loss per share | (3.51 | ) | (3.52 | ) | |||
Cash and cash equivalents | $ | 51,654 | |||
Marketable securities | 18,638 | ||||
Identifiable intangible assets: | |||||
IPR&D | 11,800 | ||||
Amgen Amendment | 14,500 | ||||
TopoTarget Agreement | 2,400 | ||||
Other current and long-term assets | 756 | ||||
Goodwill | 8,965 | ||||
CuraGen Debt | (11,503 | ) | |||
Deferred tax liabilities, net | (5,190 | ) | |||
Other assumed liabilities | (3,778 | ) | |||
Total | $ | 88,242 | |||
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2008, 2007(19) CURAGEN MERGER (Continued)
The purchase price allocation has been prepared on a preliminary basis and 2006is subject to change as additional information becomes available concerning the fair value and tax basis of the acquired assets and liabilities. Any adjustments to the purchase price allocation will be made as soon as practicable but no later than one year from the acquisition date.
The estimated fair value attributed to IPR&D intangible assets represents an estimate of the fair value of purchased in-process technology for CuraGen's research programs that, as of October 1, 2009, had not reached technological feasibility and have no alternative future use. Only those research programs that had advanced to a stage of development where the Company believed reasonable net future cash flow forecasts could be prepared and a reasonable likelihood of technical success existed were included in the estimated fair value. Accordingly, the IPR&D programs primarily represent the estimated fair value of CDX-011. The estimated fair value of the IPR&D programs was determined based on estimates of expected future net cash flows. These expected future net cash flows included estimates for revenue and associated costs for the IPR&D programs based on (i) relevant industry factors, (ii) current and expected trends in the product development life cycle, (iii) the ability to engage a strategic partner, (iv) the ability to obtain regulatory approval, and (v) the ability to manufacture and commercialize the products. The probability-adjusted future net cash flows which reflect the different stages of development of each program are then present valued utilizing an estimate of the appropriate discount rate which is consistent with the uncertainties of the cash flows utilized. Finally, the expected future net cash flows were calculated assuming the Amgen Amendment (defined below) was not entered into because the fair value attributable to the Amgen Amendment is separated from the fair value of the IPR&D programs.
The expected future net cash flows for CDX-011 were based on the expectation that a Biologics License Application ("BLA") for CDX-011 will be filed with the FDA by the end of 2015. The Company expects the commercial launch as promptly as commercially practicable after necessary regulatory approvals are received. Assuming a traditional timeline for the regulatory review process, the Company expects CDX-011 will be commercially launched in 2016. These assumptions require various levels of in-house and external testing, clinical trials and approvals from the FDA or comparable foreign regulatory authorities before CDX-011 could be commercialized in the U.S. or other territories. Drug development involves a high degree of risk and most products that make it into clinical development do not receive marketing approval. Numerous risks and uncertainties can delay or stop clinical development of a pharmaceutical product prior to the receipt of marketing approval, including, but not limited to, results from clinical trials that do not support continuing development, issues related to manufacturing or intellectual property protection, and other events or circumstances that cause unanticipated delays, technical problems or other difficulties. Given these risks and uncertainties, there can be no assurance that the development of CDX-011 will be successfully completed. If the development of CDX-011 is not successful, in whole or in part, or completed in a timely manner, the Company may not realize the expected financial benefits from the development of CDX-011 or the transaction as a whole.
The estimated fair value attributed to the May 2009 amendment to the CuraGen and Amgen Fremont (successor in-interest to Abgenix) license agreement relates to CuraGen's exclusive rights to develop and commercialize CDX-011 and 11 other licensed antigens ("Amgen Amendment"). Under the Amgen Amendment, CuraGen and Amgen Fremont agreed to modify the terms of their existing cross-license of antigens whereby the amended license would be fully paid-up and royalty-free (except for any potentially required payments by CuraGen to the original licensor of CDX-011). The estimated
(18)
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(19) CURAGEN MERGER (Continued)
fair value of the Amgen Amendment was based on the increase in expected future net cash flows for the IPR&D programs related to CDX-011 after the Amgen Amendment was entered into as compared to the expected future net cash flows if the Amgen Amendment was not entered into. The estimated fair value attributed to the Amgen Amendment is being amortized through the date of the last expiring patent covering CDX-011.
The estimated fair value attributed to the April 2008 agreement ("TopoTarget Agreement") between CuraGen and TopoTarget A/S ("TopoTarget") relates to CuraGen's rights under the TopoTarget Agreement to receive up to $6 million in either potential commercial milestone payments related to future net sales of Belinostat or 10% of any sublicense income received by TopoTarget ("TopoTarget Payments"). Under the TopoTarget Agreement, CuraGen sold back its Belinostat rights to TopoTarget and received $25 million in cash, 5 million shares of TopoTarget common stock (sold by CuraGen in 2008 for net proceeds of $12 million) and the right to receive the TopoTarget Payments. In addition, TopoTarget assumed all financial and operational responsibility for the clinical development of Belinostat under the TopoTarget Agreement. The estimated fair value of the TopoTarget Agreement was based on estimates of the probability-adjusted expected future net cash flows of the TopoTarget Payments. The estimated fair value attributed to the TopoTarget Agrement is being amortized through the date of the estimated receipt of the last payment under the TopoTarget Payments. In February 2010, TopoTarget entered into a co-development and commercialization agreement for Belinostat with Spectrum Pharmaceuticals, Inc. resulting in the Company's receipt of $3 million of the TopoTarget Payments.
The deferred tax liability, net of $5.2 million primarily relates to the temporary differences associated with the IPR&D intangible assets, which are not deductible for tax purposes.
Acquisition-Related Expenses, Including Severance
The Company incurred $2.9 million in acquisition-related expenses in the consolidated statements of operations for the year ended December 31, 2009. These costs include fees for investment banking services, legal, accounting, due diligence, tax, valuation, printing and other various services necessary to complete the transaction. In addition, the Company recorded $3.3 million and $0.9 million in CuraGen Severance expenses to general and administrative and research and development, respectively, in the consolidated statements of operations for the year ended December 31, 2009.
Pro Forma Financial Information
The operating results of CuraGen, which include approximately $2.3 million of research and development expense and $3.7 million in general and administrative expense, have been included in the accompanying consolidated financial statements from October 1, 2009, to December 31, 2009. CuraGen had no revenues from October 1, 2009 through December 31, 2009. The following unaudited pro forma financial summary is presented as if the operations of the Company and CuraGen were combined as of January 1, 2008. The unaudited pro forma combined results are not necessarily indicative of the actual
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(19) CURAGEN MERGER (Continued)
results that would have occurred had the acquisition been consummated at that date or of the future operations of the combined entities.
| | Years Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| | 2009 | 2008 | |||||
| | (In thousands) | ||||||
Revenue | $ | 15,180 | $ | 8,630 | |||
Net loss | (40,262 | ) | (25,786 | ) | |||
(20) SELECTED QUARTERLY FINANCIAL DATA (Unaudited)
2008 | Q1 2008 | Q2 2008 | Q3 2008 | Q4 2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Total revenue | $ | 147,398 | $ | 1,961,611 | $ | 2,358,136 | $ | 2,988,362 | |||||
Net loss | (22,130,682 | ) | (10,260,510 | ) | (7,656,158 | ) | (7,453,221 | ) | |||||
Basic and diluted net loss per common share | (2.19 | ) | (0.67 | ) | (0.49 | ) | (0.47 | ) | |||||
2009 | Q1 2009 | Q2 2009 | Q3 2009 | Q4 2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | (In thousands, except per share amounts) | ||||||||||||
Total revenue | $ | 3,732 | $ | 2,685 | $ | 4,030 | $ | 4,733 | |||||
Net loss | (7,703 | ) | (8,705 | ) | (7,174 | ) | (12,943 | ) | |||||
Basic and diluted net loss per common share | (0.49 | ) | (0.55 | ) | (0.45 | ) | (0.41 | ) | |||||
2007 | Q1 2007 | Q2 2007 | Q3 2007 | Q4 2007 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Total revenue | $ | 144,040 | $ | 609,184 | $ | 268,974 | $ | 383,394 | |||||
Net loss | (4,032,403 | ) | (2,755,137 | ) | (4,057,018 | ) | (4,228,492 | ) | |||||
Basic and diluted net loss per common share(1) | (0.49 | ) | (0.33 | ) | (0.49 | ) | (0.51 | ) | |||||
(19) SUBSEQUENT EVENTS
In January 2009, the Company entered into two transactions involving the sale of its poultry vaccines business and the out-licensing of its cholera and ETEC programs as more fully described below.
(A) Lohmann Animal Health International ("LAHI")
On January 13, 2009, the Company entered into a purchase agreement to sell its poultry vaccines business to LAHI. Since 2002, LAHI has performed all manufacturing, marketing and distribution activities for Celldex's marketed Megan®Vac 1 and Megan®Egg poultry vaccines and has paid Celldex product royalties. Financial terms of the transaction with LAHI included an upfront fee and potential milestone payments.
(B) Vaccine Technologies, Inc. ("VTI")
On January 20, 2009, the Company entered into an Exclusive License and Development Agreement with VTI. Under the license agreement, Celldex has granted a worldwide fee- and royalty-bearing exclusive license to VTI to development and commercialize Celldex's CholeraGarde® and ETEC vaccine programs. Financial terms of the agreement with VTI include an upfront license fee, milestone payments and royalties on net sales of licensed products during the term of the agreement.
2008 | Q1 2008 | Q2 2008 | Q3 2008 | Q4 2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | (In thousands, except per share amounts) | ||||||||||||
Total revenue | $ | 147 | $ | 1,962 | $ | 2,358 | $ | 2,988 | |||||
Net loss | (22,131 | ) | (10,260 | ) | (7,656 | ) | (7,454 | ) | |||||
Basic and diluted net loss per common share | (2.19 | ) | (0.67 | ) | (0.49 | ) | (0.47 | ) | |||||
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.Not applicable.
Item 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures.Procedures
The Company,As of December 31, 2009, we evaluated, with the registrant, maintainsparticipation of our Chief Executive Officer and Chief Financial Officer, the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the "Exchange Act")). Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2009. Our disclosure controls and procedures are designed to provide reasonable assurance that information required to be disclosed byin the Company in its reports that it files and submitswe file or submit under the Securities Exchange Act of 1934, as amended (the "Exchange Act"), is recorded, processed, summarized and reported within time periods specified by the SEC's rules and forms, and that such information is accumulated and communicated to itsour management, including itsour Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
As required by Rule 13a 15 under the Exchange Act, as of December 31, 2008, we carried out an evaluation under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of the year ended December 31, 2008.
Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of December 31 2008, as a result of the material weakness discussed below, our disclosure controls and procedures were not effective as of December 31, 2008. Notwithstanding the material weakness discussed below, our management has concluded that the consolidated financial statements included in this Annual Report on Form 10-K fairly present in all material respects our financial condition, results of operations and cash flows for the periods presented in conformity with generally accepted accounting principles.
Management's Annual Report on Internal Control Over Financial Reporting.Reporting
ManagementOur management is responsible for establishing and maintaining adequate internal control over our financial reporting as defined in Rules 13a-15(f) of the Securities Exchange Act of 1934, as amended.reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act as the process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projectionsProjections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies andor procedures may deteriorate.
The Company hasUnder the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted itsan evaluation of the effectiveness of itsour internal control over financial reporting based uponon the framework provided inInternal Control-IntegratedControl—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
A material weakness is a deficiency, or a combination of deficiencies, in Based on this evaluation, our management concluded that our internal control over financial reporting such that there is a reasonable possibility that a material misstatementwas effective as of the Company's annual or interim financial statements will not be prevented or detected on a timely basis.December 31, 2009.
The following material weakness existed in the Company'seffectiveness of our internal control over financial reporting as of December 31, 2008
The Company did not maintain a sufficient complement of personnel with the appropriate skills, training and experience as of December 31, 2008. Specifically, the quantity and level of experience of the Company's accounting staff did not adequately evolve with the increased roles, responsibilities and
complexity of the Company's operations as a result of the Merger. Additionally, this material weakness could result in misstatements of financial statement accounts and disclosures that would results in a material misstatement of the consolidated financial statements that would not be prevented or detected.
Because of this material weakness, management has concluded that our internal control over financial reporting was not effective at December 31, 2008.
The effectiveness of the Company's internal control over financial reporting as of December 31, 20082009 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their attestation report, which is included in Item 8 of this Form 10-K.herein.
Changes in Internal Control Over Financial Reporting.Reporting
There have beenwere no changes in our internal control over financial reporting during the fourth quarter of 2008three months ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Remediation of Material Weakness
In the fourth quarter of 2008, management supplemented the Company's accounting staff with a dedicated, part-time senior consultant. Although, improvement was made to the operating effectiveness of our internal control over financial reporting as of December 31, 2008, the material weakness will not be considered remediated until the existing or new additional resources and additional improvement to the operating of our internal control over financial reporting are in place for a sufficient period of time and are tested.
None.Submission of Matters to a Vote of Security Holders
On December 16, 2009, we held our Annual Meeting of Stockholders at which the stockholders (i) elected nine directors to our Board of Directors; (ii) ratified the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm for the year ending December 31, 2009; (iii) approved an amendment to our 2008 Stock Option and Incentive Plan to increase the shares reserved for issuance thereunder to 3,900,000 and (iv) approved an amendment to our 2004 Employee Stock Purchase Plan to increase the shares reserved for issuance thereunder to 62,500.
At the Annual Meeting of Stockholders, the following votes were tabulated for the proposals before our Stockholders:
Election of Directors:
| | Number of Shares/Votes | ||||||
|---|---|---|---|---|---|---|---|
| | For | Authority Withheld | |||||
Larry Ellberger | 22,320,214 | 1,334,572 | |||||
Anthony S. Marucci | 22,231,618 | 1,423,168 | |||||
Herbert J. Conrad | 22,314,720 | 1,340,066 | |||||
George O. Elston | 22,290,130 | 1,354,256 | |||||
Karen Shoos Lipton | 22,325,111 | 1,329,675 | |||||
Rajesh B. Parekh, Ph.D. | 19,731,867 | 3,922,919 | |||||
Harry H. Penner, Jr. | 22,211,760 | 1,443,026 | |||||
Charles R. Schaller | 22,221,826 | 1,432,960 | |||||
Timothy M. Shannon | 22,281,701 | 1,373,085 | |||||
Ratification of the appointment of PricewaterhouseCoopers LLP:
| FOR | AGAINST | ABSTAIN | BROKER NON-VOTES | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 22,824,672 | 703,037 | 127,077 | — | ||||||||
Approval of an amendment to our 2008 Stock Option and Incentive Plan to increase the shares reserved for issuance thereunder to 3,900,000:
| FOR | AGAINST | ABSTAIN | BROKER NON-VOTES | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11,780,331 | 1,142,425 | 51,787 | 10,658,145 | ||||||||
Approval of an amendment to our 2004 Employee Stock Purchase Plan to increase the shares reserved for issuance thereunder to 62,500:
| FOR | AGAINST | ABSTAIN | BROKER NON-VOTES | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11,936,847 | 989,380 | 48,316 | 10,658,145 | ||||||||
The number of shares issued, outstanding and eligible to vote as of the record date of November 2, 2009 was 31,602,188. A quorum was present with 23,654,786 shares represented by proxies or 74.9% of the eligible voting shares.
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information The information required by this Item 10 will be included in the definitive Proxy Statement for our 2010 Annual Meeting of Stockholders, or the 2010 Proxy Statement, under "Information Regarding the Current Directors and Executive Officers of Celldex,
The following table sets forth the members of the Board of Directors of Celldex, their ages as of December 31, 2008 and the year in which each first became a director.
Directors | Age | Year First Became Director | |||||
|---|---|---|---|---|---|---|---|
Charles R. Schaller | 72 | 2008 | |||||
Herbert J. Conrad | 75 | 2008 | |||||
Larry Ellberger | 60 | 2003 | |||||
George O. Elston | 43 | 2008 | |||||
Karen Shoos Lipton | 55 | 2001 | |||||
Dr. Rajesh B. Parekh | 47 | 2008 | |||||
Harry H. Penner, Jr. | 63 | 1997 | |||||
Anthony S. Marucci | 46 | 2008 | |||||
The following biographical descriptions set forth certain information with respect to the directors and the executive officers who are not directors, based on information furnished to Celldex by each director and executive officer.
Directors
Charles R. Schaller became the Chairman of the Board of Directors of Celldex upon consummation of the Merger. Mr. Schaller had been a director of Celldex Research since November 2006. Mr. Schaller also has been a Director of Medarex, Inc., an affiliate of Celldex, since 1987, and was Chairman of the Medarex Board of Directors from 1987 to 1997. Since 1989, Mr. Schaller has been a chemical industry management consultant and, until June 2002, he served as a director of AstroPower, Inc., a publicly traded U.S. manufacturer of photo-voltaic (PV) products until being acquired by General Electric. Mr. Schaller is a graduate of Yale University and is a graduate of the program in management development at Harvard Business School.
Herbert J. Conrad became a director of Celldex upon consummation of the Merger. Mr. Conrad had been a director of Celldex Research since March 2004. Mr. Conrad is currently a Director of Pharmasset, Inc., Savient Pharmaceuticals and Symphony Evolution and serves on the Medical Advisory Board of Henry Schein. He served as chairman of the board of directors of GenVec, Inc. from 1996 to 2003, where he was the Chief Executive Officer from September 1996 to December 1996. From 1960 to 1993 Mr. Conrad was with Hoffmann La Roche where he was President of the U.S. Pharmaceuticals Division from 1982 through 1993. Mr. Conrad earned his undergraduate and graduate degrees from the Brooklyn College of Pharmacy. He also received a Doctorate in Humane Letters (honorary) from Long Island University. He received B.S. and M.S. degrees from Brooklyn College of Pharmacy and an honorary Doctorate in Humane Letters from Long Island University.
Larry Ellberger. Mr. Ellberger has been a director of Celldex since August 2003. He is a Founder and Principal of Healthcare Ventures Associates, Inc., a consulting firm for the pharmaceutical, biotechnology and medical device industries. He was most recently Interim Chief Executive Officer of PDI, Inc., a diversified sales and marketing services provider to the biopharmaceutical, medical device and diagnostic industries. From 2000 to 2003, he was Senior Vice President of Powderject plc. He also served as a director of Powderject. Previously, Mr. Ellberger was an employee of W.R. Grace & Co. from 1995 to 1999, serving as Chief Financial Officer from 1996 and Senior Vice President, Strategic Planning and Development from 1995 and Acting Chief Executive Officer in 1997. From 1975 to 1995, Mr. Ellberger held numerous senior executive positions at American Cyanamid Company, serving the
last four years as Vice President, Corporate Development. Mr. Ellberger currently serves on the Board of Directors of Transpharma, Ltd. and The Jewish Children's Museum and was formerly Chairman of the Board of Omrix BioPharmaceuticals, Inc. until its acquisition by Johnson & Johnson.
George O. Elston became a director of Celldex upon consummation of the Merger. Mr. Elston had been a director of Celldex Research since March 2004 and is currently Chief Financial Officer of Optherion, Inc., a privately held biopharmaceutical company located in New Haven, CT. Mr. Elston has more than 20 years of financial and business expertise in the biotechnology and medical device industries. Before joining Optherion, Mr. Elston was with Elusys Therapeutics where he raised significant funding from government and private sources, completed multiple strategic collaborations with large pharmaceutical and biotechnology firms, and oversaw collaborations with the U.S. Department of Defense and the National Institutes of Health. Before joining Elusys, Mr. Elston was Chief Financial Officer of Trillium USA, Inc., where he established the financial and administrative functions for the Company's multi-national operations. Previously, Mr. Elston was with C.R. Bard, Inc., an international manufacturer and distributor of medical devices, where he directed financial operations for multiple manufacturing facilities in several countries and successfully integrated strategic acquisitions; and with Price Waterhouse. Mr. Elston received his BBA in Public Accounting from Pace University and is a Certified Public Accountant.
Karen Shoos Lipton. Ms. Lipton has been a director of Celldex since May 2001. Ms. Lipton was appointed Chief Executive Officer of the American Association of Blood Banks (dba AABB) in October 1994. Previously she has held senior positions at the American Red Cross since 1984, including Acting Senior Vice President, Biomedical Services (1993-1994) and Secretary and General Counsel (1990-1993). Prior to the American Red Cross, Ms. Lipton was a lawyer in private practice.
Dr. Rajesh B. Parekh became a director of Celldex upon consummation of the Merger. Dr. Parekh had been a director of Celldex Research since March 2004 and has been a General Partner at Advent Venture Partners (UK) since 2006. Prior to joining Advent, Dr. Parekh was an Entrepreneur-in-Residence at Abingworth Management Limited (UK) from 2003-2005. Dr. Parekh has also been a Visiting Professor at the University of Oxford. He was a co-founder and served as Chief Scientific Officer and Senior Vice President of Research and Development of Oxford GlycoSciences, plc (UK) from 1988 to 2003. Dr. Parekh was also chairman of Galapagos NV (Belgium) since 2004 and currently serves on the boards of directors of seven companies including private companies in the United States and Europe and one public European company. He received his B.A. and D. Phil. degrees in Biochemistry and Molecular Medicine from the University of Oxford.
Harry H. Penner, Jr. Mr. Penner has been a director of Celldex since January 1997 and was Chairman of Celldex from 2007 until the Merger. He is Chairman and CEO of Nascent BioScience, LLC, a firm which has been instrumental in the founding and development of a number of new biotechnology companies, including New Haven Pharmaceuticals, Inc. (of which he is currently Chairman and CEO), Rib-X Pharmaceuticals, Inc., Marinus Pharmaceuticals, Inc., RHEI Pharmaceuticals, Inc., RxGen Inc., and MAK Scientific. He has served as BioScience Advisor to the Governor of the State of Connecticut, and as Chair of the Connecticut Board of Governors of Higher Education, CURE, the Connecticut BioScience Cluster, and the Connecticut Technology Council. From 1993 to 2001, Mr. Penner was President, CEO and a director of Neurogen Corporation. Previously, he served as Executive Vice President of Novo Nordisk A/S and President of Novo Nordisk of North America, Inc. from 1988 to 1993. He serves on the boards of Altus Pharmaceuticals, Inc., Ikonisys, Inc. Rib-X, Marinus, Rhei, and RxGen. Mr. Penner holds degrees from the University of Virginia (BA), Fordham University (JD), and New York University (LLM).
Anthony S. Marucci was appointed as permanent President and Chief Executive Officer of Celldex in October 2008 and as a director of the Company in December 2008. Mr. Marucci had been Executive Vice President, Corporate Development of Celldex upon consummation of the Merger. Mr. Marucci
had been Celldex Research's Acting Chief Executive Officer since October 2007 and its Vice President, Chief Financial Officer, Treasurer and Secretary since May 2003. In addition, he was Treasurer of Medarex from December 1998 to March 2004. Mr. Marucci held a series of senior financial positions at Medarex from December 1998 to May 2003. Mr. Marucci is a member of the Board of Trustees of BioNJ and also serves as its Treasurer. Mr. Marucci received his M.B.A. from Columbia University.
Executive Officers
The following persons are currently executive officers who are not directors of Celldex(1)(2). Officers are elected annually by the Board of Directors until their successors are duly elected and qualified.
| ||||
| ||||
| ||||
|
Anthony S. Marucci was appointed as permanent President and Chief Executive Officer of Celldex in September 2008 and as a director of the Company in December 2008. See Mr. Marucci's biography underDirectors above.
Avery W. Catlin. Mr. Catlin joined Celldex in January 2000. Prior to joining Celldex, he served as Vice President, Operations and Finance, and Chief Financial Officer of Endogen, Inc., a public life science research products company, from 1996 to 1999. From 1992 to 1996, Mr. Catlin held various financial positions at Repligen Corporation, a public biopharmaceutical company, serving the last two years as Chief Financial Officer. Earlier in his career, Mr. Catlin held the position of Chief Financial Officer at MediSense, Inc., a Massachusetts-based medical device company. Mr. Catlin received his B.A. degree from the University of Virginia and M.B.A. from Babson College and is a Certified Public Accountant.
Dr. Tibor Keler became Senior Vice President and Chief Scientific Officer of Celldex upon consummation of the Merger. Dr. Keler had been Celldex Research's Vice President, Research and Discovery and Chief Scientific Officer since May 2003. In addition, he was Senior Director of Preclinical Development and Principal Scientist at Medarex, Inc. from September 1993 to March 2004. While at Medarex, he was responsible for the development of Celldex's technology and products, as well as for the preclinical development and testing of numerous Medarex products now in Phase II clinical trials. Dr. Keler received his Ph.D. in Microbiology from the University of Pennsylvania.
Thomas Davis, MD became Senior Vice President and Chief Medical Officer of Celldex upon consummation of the Merger. Dr. Davis was Vice President of Clinical Development and Chief Medical Officer of Celldex Research. Dr. Davis was formerly Chief Medical Officer at GenVec, and Senior Director of Clinical Science at Medarex. He has supervised clinical efforts in adult hematologic malignancies and marrow transplantation and therapeutic antibodies at the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI) and worked with Dr. Ron Levy on the development of rituximab and idiotype vaccines at Stanford University. Dr. Davis received his B.A.
degree in Biophysics from Johns Hopkins University, M.S. degree in Physiology from Georgetown University and his M.D. from Georgetown University School of Medicine.
Section" "Section 16(a) Beneficial Ownership Reporting Compliance,
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires Celldex's directors and executive, officers, and persons who are beneficial owners of more than 10% of a registered class of our equity securities, to file reports of ownership and changes in ownership with the Securities and Exchange Commission (the "SEC"). These persons are required by SEC regulations to furnish the Company with copies of all Section 16(a) forms they file. To our knowledge, based solely on a review of the copies of such reports furnished to us, and written representations that no other reports were required during the fiscal year ended December 31, 2008, all Section 16(a) filing requirements applicable to such persons were satisfied, except for the filing of a Form 4 on September 23, 2008, which reported three transactions by an entity of which Dr. Rajesh Parekh was a related party, which took place between September 8 - 10, 2008. Dr. Parekh disclaimed beneficial ownership of those shares.
Code" "Code of Business Conduct and Ethics
We have adopted a Code of Business ConductEthics" and Ethics that applies to our directors, officers, and employees. The purpose of the Code of Business Conduct and Ethics is to deter wrongdoing and to promote, among other things, honest and ethical conduct and to ensure to the extent possible that our business is conducted in a consistently legal and ethical manner. Our Code of Business Conduct and Ethics is publicly available on our website atwww.celldextherapeutics.com. If we make any substantive amendments to the Code of Business Conduct and Ethics or grant any waiver, including any implicit waiver from a provision of the Code of Business Conduct and Ethics to our directors or executive officers, we will disclose the nature of such amendments or waiver on our website or in a current report on Form 8-K.
The"The Board of Directors and Its Committees
Board of Directors. CelldexCommittees" and is currently managedincorporated herein by an eight member Board of Directors, a majority of whom are "independent" as that termreference. If the 2010 Proxy Statement is defined innot filed with the applicable NASDAQ listing standards. Our Board of Directors met nine times in 2008. Each ofSEC within 120 days after the directors attended at least 75% of the aggregate of (i) the total number of meetingsend of our Boardmost recent fiscal year, we will provide such information by means of Directors (held during the period for which such directors servedan amendment to this Annual Report on the Board of Directors) and (ii) the total number of meetings of all committees of our Board of Directors on which the Director served (during the periods for which the director served on such committee or committees). Our annual meeting of stockholders is generally held to coincide with one of the Board's regularly scheduled meetings. Celldex does not have a formal policy requiring members of the Board of Directors to attend our annual meetings, although our directors typically attend the annual meeting. Each of the then current directors attended the 2008 annual meeting of stockholders.Form 10-K.
Audit Committee. The Board of Directors has established an Audit Committee currently consisting of Larry Ellberger, Chairman, Harry H. Penner, Jr., and George O. Elston. Karen Shoos Lipton was also a member of the Audit Committee during a portion of 2008. The Audit Committee makes recommendations concerning the engagement of independent public accountants, reviews with the independent public accountants the scope and results of the audit engagement, approves professional services provided by the independent public accountants, reviews the independence of the independent public accountants, considers the range of audit and non-audit fees, and reviews the adequacy of our internal accounting controls. Each member of the Audit Committee is "independent" as that term is defined in the rules of the Securities and Exchange Commission and the applicable NASDAQ listing standards. The Board has determined that each Audit Committee member has sufficient knowledge in financial and auditing matters to serve on the Committee. The Board has designated George O. Elston as an "audit committee financial expert," as defined under the applicable
rules of the Securities and Exchange Commission and the applicable NASDAQ listing standards. The Audit Committee met eight times during 2008. Our Board has adopted an Audit Committee Charter, which is available for viewing at www.celldextherapeutics.com.
Compensation Committee. The Board of Directors has established a Compensation Committee currently consisting of Dr. Rajesh B. Parekh, Chairman, Harry H. Penner, Jr. and Charles R. Schaller. During 2008, Karen Shoos Lipton and Larry Ellberger also were members of the Compensation Committee during a portion of 2008. The primary function of the Compensation Committee is to assist the Board in the establishment of compensation for the Chief Executive Officer and, upon his recommendation, to approve the compensation of other officers and senior employees and to approve certain other personnel and employee benefit matters. The Compensation Committee met four times during 2008. Our Board has adopted a Compensation Committee Charter, which is available for viewing atwww.celldextherapeutics.com. Each member of the Compensation Committee is "independent" as that term is defined in the rules of the Securities and Exchange Commission and the applicable NASDAQ listing standards.
Nominating and Corporate Governance Committee. The Board of Directors has established a Nominating and Corporate Governance Committee consisting of Herbert J. Conrad, Chairman, Karen Shoos Lipton and Charles R. Schaller. The primary function of the Nominating and Corporate Governance Committee is to assist the Board in reviewing, investigating and addressing issues regarding Board composition, policy and structure; membership on Board committees; and other matters regarding the governance of Celldex. The Nominating and Corporate Governance Committee met once during 2008. Our Board has adopted a Nominating and Corporate Governance Charter, which is available for viewing atwww.celldextherapeutics.com. Each member of the Nominating and Corporate Governance Committee is "independent" as that term is defined in the rules of the Securities and Exchange Commission and the applicable NASDAQ listing standards.
The process followed by the Nominating and Corporate Governance Committee to identify and evaluate candidates includes (i) the review of requests from Board members, management, members of the Nominating and Corporate Governance Committee, stockholders and other external sources; (ii) meetings from time to time to evaluate biographical information and background material relating to potential candidates to the Board; and (iii) interviews of selected candidates by members of the Committee and the Board. All nominees must have, at a minimum, high personal and professional integrity, exceptional ability and judgment, and be effective in collectively serving the long-term interests of all stockholders. Other qualifications that may be considered by the Committee are described in the Nominating and Corporate Governance Charter.
Stockholders may recommend individuals to the Nominating and Corporate Governance Committee for consideration as potential director candidates by submitting their names and background to the Secretary of Celldex at the address set forth below under "Stockholder Communications." All such recommendations will be forwarded to the Nominating and Corporate Governance Committee, which will review and consider only such recommendations if appropriate biographical and other information is provided, as described below, on a timely basis. All securityholder recommendations for director candidates must be submitted to Celldex not less than 120 calendar days prior to the date on which Celldex's proxy statement is released to stockholders in connection with the Company's annual meeting, and must include the following information:
Assuming that appropriate information is provided for candidates recommended by stockholders, the Nominating and Corporate Governance Committee will evaluate those candidates by following substantially the same process, and applying substantially the same criteria, as for candidates submitted by Board members or other persons, as described above and as set forth in its written charter.
Item 11. EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
Overview
We believe that the compensation of our executive officers should focus executive behavior on the achievement of near-term corporate targets as well as long-term business objectives and strategies. We reviewed the data reported The information required by this Item 11 will be included in the 2007 executive compensation survey of over 400 biotechnology companies independently prepared by Aon-Radford but did not tie any aspect of compensation to any survey of peers. We believe that pay-for-performance compensation programs, which reward our executives when they achieve certain financial and business goals, create stockholder value and thus have emphasized company and individual performance in setting compensation. We use a combination of base salary, annual cash incentive compensation programs, a long-term equity incentive compensation program and a broad based benefits program to create a competitive compensation package for our executive management team. We describe below our compensation philosophy, policies and practices with respect to our Chief Executive Officer, Chief Financial Officer and our other executive officers, who are collectively referred to as our Named Executive Officers.
Administration and Objectives of Our Executive2010 Proxy Statement under "Executive Compensation, Program
The Compensation Committee of the Board of Directors, which is comprised of non-employee directors, is responsible for establishing and administering the policies governing the compensation of Celldex's employees, including salary, bonus and stock option grants. The policy of the Compensation Committee is to compensate our employees with competitive salaries based on their level of experience and job performance. All permanent employees, including executive officers, are eligible for annual bonus awards based on achievement of Celldex's strategic corporate goals, and participation in our stock option program. The bonus awards and stock option grants are made in accordance with the Celldex Performance Incentive Plan and 2008 Stock Option and Incentive Plan. The Compensation Committee is also responsible for the administration of our 2004 Employee Stock Purchase Plan, in which employees participate on a voluntary basis.
Our compensation committee has designed our overall executive compensation program to achieve the following objectives:
We use a mix of short-term compensation (base salaries and cash incentive bonuses) and long-term compensation (equity incentive compensation) to provide a total compensation structure that is designed to achieve these objectives. We determine the percentage mix of compensation structures that we think is appropriate for each of our executive officers. In general, the Compensation Committee believes that a substantial percentage of the compensation of our executive officers should be performance based. The Compensation Committee uses its judgment and experience and the recommendations of the chief executive officer (except for his own compensation) to determine the appropriate mix of compensation for each individual.
In determining whether to adjust the compensation of any one of our executive officers, including our Named Executive Officers, we annually take into account the changes, if any, in the following:
In addition, with respect to new executive officers, we take into account their prior base salary and annual cash incentives, their expected contribution and our business needs. We believe that our executive officers should be fairly compensated each year relative to market pay levels within our industry and that there should also be internal equity among our executive officers.
Executive Compensation Components
In order to both attract and retain experienced and qualified executives to manage Celldex, the Compensation Committee's policy on executive compensation is to (i) pay salaries which are competitive with the salaries of executives in comparable positions in the biotechnology industry, and (ii) allow for additional compensation upon achievement of goals under the Performance Incentive Plan and through the appreciation of stock-based incentive awards. This policy is designed to have a significant portion of each executive's total compensation be tied to Celldex's progress in order to incentivize the executive to fully dedicate himself or herself to achievement of corporate goals, and to align the executive's interest with those of our stockholders through equity incentive compensation.
Our executive compensation program is primarily composed of base salary, annual incentive cash compensation payable on an annual basis and equity compensation. In addition, we provide our executives with benefits that are generally available to our salaried employees, including medical,
dental, group life and accidental death and dismemberment insurance, short- and long-term disability coverage and our 401(k) plan. Within the context of the overall objectives of our compensation programs, we determined the specific amounts of compensation to be paid to each of our executives in 2008 based on a number of factors including:
We discuss each of the primary elements of our executive compensation in detail below. While we have identified particular compensation objectives that each element of executive compensation serves, our compensation programs complement each other and collectively serve all of our executive compensation objectives described above. Accordingly, whether or not specifically mentioned below, we believe that, as a part of our overall executive compensation, each element to a greater or lesser extent serves each of our objectives.
Base salary. Each executive officer (except the chief executive officer whose performance is reviewed by the Compensation Committee) has an annual performance review with the chief executive officer who makes recommendations on salary increases, promotions and stock option grants to the Compensation Committee. We have historically established base salaries for each of our executives based on many factors, including average salary increases expected in the biotechnology industry in the Boston, Massachusetts and central New Jersey areas, competition in the marketplace to hire and retain executives, experiences of our Board members and leadership team with respect to salaries and compensation of executives in similarly situated companies in our industry and other similar industries, as well as additional factors which we believe enables us to hire and retain our leadership team in an extremely competitive environment. Our compensation committee annually reviews salary ranges and individual salaries for our executive officers.
Performance Incentive Plan. We have designed our performance plan program to reward our executive officers upon the achievement of certain annual revenue, cash flow, research, clinical development, regulatory and business development goals, as approved in advance by our compensation committee and the board of directors. The bonus award is based on achievement of Celldex's corporate goals which are set at the beginning of each fiscal year and measured against performance at the end of the year by Celldex in accordance with the Performance Incentive Plan. The corporate goals were allocated between specific product and financial performance targets. Our performance plan emphasizes pay-for-performance and is intended to closely align executive compensation with achievement of certain operating results and an increase in stockholder value. The compensation committee and the board of directors communicate the bonus criteria to employees, including the named executive officers, at the beginning of the fiscal year. The performance goals and bonus criteria established by the compensation committee under the Performance Incentive Plan are designed to require significant effort and operational success on the part of our executives and Celldex for achievement. We measure such bonus criteria against actual operating results on an annual basis.
Following the Merger, the Compensation Committee worked with management to set bonus goals for the Company for 2008. Among those goals were: post-Merger integration of programs, personnel and budgets; finalizing the license and development agreement transaction with Pfizer or obtaining financing to ensure sufficient cash reserves through the end of 2010; developing a 2008 and 2009 operational budget and obtaining Board approval; initiating at least two new INDs in cancer and infectious disease; development, and Board approval, of a plan to build a clinical pipeline of novel
monoclonal antibodies; and announce clinical activities on three lead immunotherapy products. Following the end of 2008, the Compensation Committee determined that the Company had achieved at least 85% of its stated bonus objectives. In addition, the Compensation Committee determined that several additional achievements, namely the agreement in principle to out-license the CholeraGarde and ETEC programs, the agreement in principle to sell the poultry vaccine business, the sale of the Company's former interest in Select Vaccines, the acquisition of TLR assets from 3M Company and the on-going clinical development of those assets within three months of licensing-in resiquimod, as well as the progress of clinical development of CDX-110 with Pfizer, resulted in the Compensation Committee's determination that the Company's 2008 bonus pool should be be determined as if 100% of the goals for 2008 had been achieved.
Equity Compensation. We also use stock options and equity-based incentive programs to attract, retain, motivate and reward our executive officers. Through our equity-based grants, we seek to align the interests of our executive officers with our stockholders, reward and motivate both near-term and long-term executive performance and provide an incentive for retention. Our decisions regarding the amount and type of equity incentive compensation and relative weighting of these awards among total executive compensation have been based on our understanding of market practices of similarly situated companies and our negotiations with our executives in connection with their initial employment or promotion.
Our recent practice has been to grant equity-based awards to our executive officers, if any at all, on an annual basis. All such grants are subject to approval by the Compensation Committee at a regularly scheduled meeting during the year. The date of grant and the fair market value of the award are based upon the date of the Compensation Committee meeting approving such grant. When granting stock options, the Compensation Committee considers a number of factors in determining the amount of equity incentive awards, if any, to grant to our executives, including:
Equity compensation awards to our Named Executive Officers primarily consist of stock option awards. Stock option awards provide our executive officers with the right to purchase shares of our common stock at a fixed exercise price typically for a period of up to ten years, subject to continued employment with Celldex. Stock options are earned on the basis of continued service to us and generally vest over four years, beginning with 25% vesting one year after the date of grant, then pro-rata vesting primarily quarterly or monthly thereafter. All historical option grants were made at what our Compensation Committee and Board of Directors determined to be the fair market value of our shares of our common stock on the respective grant dates.
On January 6, 2009, the Board of Directors awarded 5,868 shares of restricted stock having a fair value of $50,000 to each of Messrs. Marucci, Catlin, Keler, Davis and Newbold based on the degree of success in 2008 of integrating the businesses of Celldex and AVANT following the Merger.
In April 2007, we adopted an equity grant policy that formalizes how we grant equity awards by setting a regular schedule for grants, outlining grant approval requirements and specifying how awards are priced. We believe that this policy will enable us to avoid any option backdating issues or concerns that our awards were timed to precede or follow our release or withholding of material non-public information.
Other Benefits
We believe that establishing competitive benefit packages for our employees is an important factor in attracting and retaining highly qualified personnel. Executive officers are eligible to participate in all of our employee benefit plans, such as medical, dental, group life and accidental death and dismemberment insurance, short- and long-term disability coverage and our 401(k) plan, in each case on the same basis as other employees. We provide a matching contribution under our 401(k) plan.
Summary Compensation Table
The following summary compensation table reflects certain information concerning compensation for services in all capacities awarded to, earned by or paid during the years ended December 31, 2008, 2007 and 2006 to each person who served as Celldex's Chief Executive Officer, Chief Financial Officer, the three other most highly compensated executive officers employed by the Company as of December 31, 2008 and up to two additional executive officers who would have been among the most highly compensated executive officers had they been employed as of December 31, 2008 (collectively, the "Named Executive Officers").
Name and Principal Position | Years | Salary ($) | Bonus ($)(1) | Stock Awards ($) | Option Awards ($)(2) | Non-Equity Incentive Plan Compensation ($) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($)(3) | Total ($) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Anthony S. Marucci(4) | 2008 | 302,800 | 137,400 | 50,000 | (5) | 675,884 | — | — | 4,783 | 1,170,867 | |||||||||||||||||||
President and Chief | 2007 | — | — | — | — | — | — | — | — | ||||||||||||||||||||
Executive Officer | 2006 | — | — | — | — | — | — | — | — | ||||||||||||||||||||
Una S. Ryan, Ph.D.(6) | 2008 | 235,358 | — | — | 1,309,096 | — | — | 1,336,368 | 2,880,822 | ||||||||||||||||||||
Former President and Chief | 2007 | 440,000 | 123,200 | — | 12,266 | — | — | 2,700 | 578,166 | ||||||||||||||||||||
Executive Officer | 2006 | 415,000 | 73,040 | 1,225,000 | (7) | 26,250 | — | — | 2,700 | 1,741,990 | |||||||||||||||||||
Avery W. Catlin | 2008 | 262,170 | 57,700 | 50,000 | (5) | 156,570 | — | — | 3,483 | 529,923 | |||||||||||||||||||
Senior Vice President and | 2007 | 251,121 | 35,818 | — | 15,615 | — | — | 2,700 | 305,254 | ||||||||||||||||||||
Chief Financial Officer | 2006 | 241,462 | 21,249 | — | 12,088 | — | — | 2,680 | 277,479 | ||||||||||||||||||||
Tibor Keler., Ph.D.(8) | 2008 | 250,000 | 96,600 | 50,000 | (5) | 675,884 | — | — | 3,822 | 1,076,306 | |||||||||||||||||||
Senior Vice President and | 2007 | — | — | — | — | — | — | — | — | ||||||||||||||||||||
Chief Scientific Officer | 2006 | — | — | — | — | — | — | — | — | ||||||||||||||||||||
Thomas Davis, M.D.(9) | 2008 | 300,000 | 96,600 | 50,000 | (5) | 266,926 | — | — | 3,886 | 717,412 | |||||||||||||||||||
Senior Vice President and | 2007 | — | — | — | — | — | — | — | — | ||||||||||||||||||||
Chief Medical Officer | 2006 | — | — | — | — | — | — | — | |||||||||||||||||||||
Ronald C. Newbold, Ph.D.(10) | 2008 | 250,000 | 62,500 | 50,000 | (5) | 172,237 | — | — | 4,134 | 538,871 | |||||||||||||||||||
Senior Vice President, | 2007 | — | — | — | — | — | — | — | — | ||||||||||||||||||||
Business Development | 2006 | — | — | — | — | — | — | — | — | ||||||||||||||||||||
Grants of Plan-Based Awards
The following table provides information on stock options, restricted stock units and performance stock units granted in 2008, 2007 and 2006 to each of Celldex's Named Executive Officers. The numbers below reflect the 1-for-12 reverse stock split effected on March 7, 2008.
| | | Estimated Future Payouts Under Equity Incentive Plan Awards | | | | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name | Grant Date | Threshold (#) | Target (#) | Maximum (#) | All Other Stock Awards: Number of Shares or Units (#) | All Other Option Awards: Number of Securities Underlying Options (#) | Exercise or Base Price of Option Awards ($/Sh)(1) | Market Price on Date of Grant (#)(1) | Grant Date Fair Value of Stock and Option Awards ($)(2) | |||||||||||||||||||
Anthony S. Marucci | 03/06/08 | 254,243 | 8.16 | 7.56 | 1,022,057 | |||||||||||||||||||||||
Una S. Ryan, Ph.D. | 07/16/08 | 153,125 | 8.16 | 14.95 | 1,309,096 | |||||||||||||||||||||||
Avery W. Catlin | 03/07/08 | 183,333 | 8.16 | 7.56 | 762,317 | |||||||||||||||||||||||
Tibor Keler, Ph.D. | 03/06/08 | 254,243 | 8.16 | 7.56 | 1,022,057 | |||||||||||||||||||||||
Thomas Davis, M.D. | 03/06/08 | 148,825 | 8.16 | 7.56 | 636,971 | |||||||||||||||||||||||
Ronald C. Newbold, Ph.D.(3) | 03/06/08 | 107,485 | 8.16 | 7.56 | 457,886 | |||||||||||||||||||||||
Outstanding Equity Awards
The following table sets forth certain information regarding the stock option grants and stock awards to the Named Executive Officers of Celldex at the end of fiscal 2008. The numbers below reflect the 1-for-12 reverse stock split effected on March 7, 2008.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END—DECEMBER 31, 2008
| | Option Awards | Stock Awards | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) | Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (#) | |||||||||||||||||||
Anthony S. Marucci(1) | 193,525 | 60,718 | 8.16 | 03/06/2018 | ||||||||||||||||||||||||
Una S. Ryan, Ph.D. | 153,125 | — | 8.16 | 03/07/2011 | ||||||||||||||||||||||||
Avery W. Catlin(2) | — | 183,333 | 8.16 | 03/07/2015 | ||||||||||||||||||||||||
Tibor Keler, Ph.D(3) | 193,525 | 60,718 | 8.16 | 03/06/2018 | ||||||||||||||||||||||||
Thomas Davis, M.D.(4) | 34,454 | 114,371 | 8.16 | 03/06/2018 | ||||||||||||||||||||||||
Ronald C. Newbold, Ph.D.(5) | 20,025 | 87,460 | 8.16 | 03/06/2018 | ||||||||||||||||||||||||
Option Exercises and Stock Vested
The following table sets forth certain information regarding the number of option exercises in fiscal 2008 and the number of shares of restricted stock issued under the Celldex 2008 Stock Option and Incentive Plan that vested in fiscal 2008 and the corresponding amounts realized by the Named Executive Officers of Celldex. The numbers below reflect the 1-for-12 reverse stock split effected on March 7, 2008.
OPTION EXERCISES AND STOCK VESTED
| | Option Awards | Stock Awards | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name | Number of Shares Acquired on Exercise (#) | Value Realized on Exercise ($) | Number of Shares Acquired on Vesting (#)(1) | Value Realized on Vesting ($) | |||||||||
Anthony S. Marucci | — | — | 5,868 | 50,000 | |||||||||
Avery W. Catlin | — | — | 5,868 | 50,000 | |||||||||
Tibor Keller, Ph.D. | — | — | 5,868 | 50,000 | |||||||||
Thomas Davis, M.D. | — | — | 5,868 | 50,000 | |||||||||
Ronald C. Newbold, Ph.D. | — | — | 5,868 | 50,000 | |||||||||
Employment Agreements
The Company became the sole shareholder of Celldex Research as the result of the Merger. Celldex Research was a party to employment agreements with each of Mr. Anthony Marucci, Dr. Tibor Keler, Dr. Thomas Davis and Dr. Ronald Newbold. Each of the employment agreements' initial terms had expired, but the agreements renewed automatically on a year-to-year basis absent notice of termination. Mr. Marucci had served as Celldex's Executive Vice President, Corporate Development, until his appointment as interim Chief Executive Officer in July 2008 and his appointment as CEO and President in September 2008 as further described below. While serving as Executive Vice President, Corporate Development, Mr. Marucci received an annual base salary of $250,000, subject to annual review and a bonus of up to 30% of base salary, plus a special weekly bonus upon his appointment as interim CEO. His salary was increased to $458,000 upon becoming CEO and President. Dr Keler serves as Celldex's Senior Vice President and Chief Scientific Officer and receives an annual base salary of $250,000, subject to annual review and a bonus of up to 30% of base salary. Dr. Davis serves as Celldex's Senior Vice President and Chief Medical Officer and receives an annual base salary of $300,000, subject to annual review and a bonus of up to 25% of base salary. In 2008, Dr. Newbold served as Celldex's Senior Vice President, Business Development, and received an annual salary of $250,000, subject to annual review and a bonus of up to 25% of base salary.
Each of the Celldex Research employment agreements provided for the payment of severance benefits in connection with certain terminations of service. In the event the employee's service is terminated as a result of Celldex Research's non-renewal of the agreement, by the employee for "good reason" or otherwise by Celldex Research without cause, the employee would have been entitled to one year's severance pay, subject to reduction (in the case of a non-renewal termination only) if such employee finds alternative employment during that period. Each of the agreements also included a change of control termination right in favor of the employee that would have allowed the employee to receive benefits, including a lump-sum payment of one full year's salary, continued medical benefits for
two years and the acceleration of options, if such employee terminated his employment within one year following the consummation of the merger.
The agreements included customary non-competition and non-solicitation provisions that apply during the term of employment and for a period of one year thereafter in the case of a resignation by the employee without cause or a "for cause" termination of the employee by Celldex.
The prior employment agreements that Celldex Research had with Messrs. Davis, Keler and Marucci have been terminated and are no longer in effect. Dr. Newbold gave notice that he is terminating his employment with Celldex Research effective March 1, 2009.
In 2008, Mr. Catlin had an agreement with the Company under which he was eligible for a severance payment of twelve months' base salary, continuation of health insurance benefits for twelve months and 100% vesting of all stock option grants in the event of his termination following a change-of-control, as defined in the Company's Stock Option and Incentive Plan.
As of October 19, 2007, Celldex Research and Dr. Robert F. Burns, Celldex Research's former CEO, entered into a separation and mutual release agreement under which Dr. Burns' employment was terminated, effective as of February 15, 2008. As severance, Celldex Research was obligated to pay to Dr. Burns the sum of GBP 33,333.33 per month for nine consecutive months, commencing with the first payment on March 15, 2008, and a payment of GBP 100,000.00 on December 15, 2008, in each case less applicable withholdings and other customary payroll deductions. Dr. Burns is also entitled to the continuation of benefits until February 15, 2010. All of Dr. Burn's stock options became fully exercisable on February 15, 2008, and he may exercise them for up to three years following that date. Dr. Burns and Celldex Research provided one another with mutual releases under the separation and mutual release agreement.
Effective May 7, 2008, Dr. Una S. Ryan resigned from her position as the Company's President and Chief Executive Officer and we entered into a Separation and General Release Agreement with Dr. Ryan effective July 16, 2008 ("Ryan Separation Agreement"). The Ryan Separation Agreement provides, among other things, for: (i) a lump sum cash payment of $1,323,203, plus interest in the amount of $10,784.10, which is payable on November 8, 2008; (ii) a mutual general release; (iii) payment of insurance premiums under COBRA for 18 months; (iv) reimbursement of attorneys' fees up to $30,000 and (v) vesting of options to purchase 153,125 shares of Company common stock (of the options to purchase 612,500 shares of Company common stock which had been granted to Dr. Ryan on March 7, 2008). The remainder of Dr. Ryan's options terminated as of July 16, 2008, the date of the Ryan Separation Agreement and the date of Dr. Ryan's resignation from our Board of Directors.
Prior to her resignation, the terms of Dr. Ryan's compensation were governed by the following employment agreement, which is no longer in effect. Dr. Ryan entered into an employment agreement with the Company (the "agreement"), which was amended and restated as of August 20, 1998, amended as of December 23, 2002, September 18, 2003 and again as of October 19, 2007. The term of the agreement would have been for 13 months from the effective date of the Merger, with rolling automatic one-year extensions. If prior to a change in control (as defined in the Company's Stock Option and Incentive Plan), Dr. Ryan's employment had been terminated by the Company without cause (as defined in the agreement), Dr. Ryan would have been eligible to receive a lump sum amount equal to one year's salary, at the rate then in effect, and continuation of group health plan benefits for a period of up to twelve (12) months. If within a year after a change in control, Dr. Ryan's employment had been terminated by the Company without cause or by Dr. Ryan for good reason (as defined in the agreement), or if a change in control had occurred within one (1) year after Dr. Ryan is terminated without cause by the Company, Dr. Ryan would have been entitled to receive a lump sum amount equal to three (3) times the base amount (as defined in Section 280G(b)(3) of the Internal Revenue Code of 1986, as amended) applicable to Dr. Ryan, less one dollar ($1.00). Such severance may have
been further reduced to the extent necessary to preserve the Company's tax deduction. Further, if Dr. Ryan's employment had been terminated by the Company without cause or by Dr. Ryan for good reason at any time after the Merger, or Dr. Ryan resigned or was terminated by the Company or after the first anniversary of the Merger for any reason, the Company would have been required to pay Dr. Ryan a special retirement payment of $1,323,203.
On July 23, 2008, the Company entered into an employment agreement ("Employment Agreement") with Anthony S. Marucci (the "Executive"). Mr. Marucci served as the Company's Executive Vice President, Corporate Development from March 7, 2008 and served as its Chief Executive Officer and President on an interim basis until September 25, 2008 when he was appointed as the Company's permanent Chief Executive Officer. The Employment Agreement provides, among other things, for: (i) an annual base salary of $250,000 (increased to $458,000 on September 25, 2008); (ii) an annual cash bonus in an amount established by the Company's Board of Directors; (iii) a weekly bonus of $3,992.31 during the period in which the Executive serves as interim Chief Executive Officer and President (which ended on September 25, 2008); (iv) a lump sum severance payment equal to 200% of the Executive's then-base salary (not including bonus) in the event that his employment is terminated without cause or he resigns "for good reason" (as defined in the Employment Agreement); and (v) accelerated vesting of any unvested Equity Awards (as defined in the Employment Agreement) and a lump sum cash payment equal to twenty four (24) times Executive's highest monthly base compensation (not including bonus) during the twenty-four month period prior to the date of termination plus the average of the annual discretionary bonuses (but not the bonuses received for serving as interim Chief Executive Officer) received by the Executive during the two full fiscal years prior to the date of termination in the event of termination without cause or resignation "for good reason" by the Executive within one year immediately following a Change in Control (as defined in the Employment Agreement). The Employment Agreement has an initial term through July 30, 2011 and shall automatically renew for additional one year terms unless either party gives ninety (90) days prior written notice of its intent not to renew.
On January 6, 2009, the Company entered into employment agreements with Mr. Catlin and Drs. Davis, MD and Keler.
The employment agreements between the Company and Messrs. Catlin, Davis and Keler provide, among other things, for: (i) annual base salary ($288,250 in the case of Mr. Catlin, $362,400 in the case of Dr. Davis, and $342,000 in the case of Dr. Keler); (ii) an annual discretionary bonus in an amount established by the Company's Board of Directors or the Compensation Committee thereof; (iii) a lump sum severance payment equal to 200% of the executive's then-base salary n the event that his employment is terminated without cause or he resigns "for good reason" (as defined in the employment agreement); and (iv) accelerated vesting of any unvested equity awards (as defined in the employment agreement) and a lump sum cash payment equal to twelve (12) times the executive's highest monthly base compensation (not including bonus) during the twenty-four month period prior to the date of termination plus the average of the annual discretionary bonuses received during the two full fiscal years prior to the date of termination in the event of termination without cause or resignation "for good reason" by the executive within one year immediately following a change in control (as defined in the employment agreement).
The employment agreements have an initial term through December 31, 2011 and shall automatically renew for additional one year terms unless either party gives ninety (90) days prior written notice of its intent not to renew. The Company may terminate the employment agreements without cause, on 90-days' prior notice, or for cause, subject to a 30-day cure period in certain circumstances.
On January 6, 2009, the Company also entered into an amended and restated employment agreement with Anthony S. Marucci, President and Chief Executive Officer, which removed references
to his being "interim" Chief Executive Officer and President and conformed the initial term and other provisions so that they are coordinated with the employment agreements entered into and between the Company and Messrs. Catlin, Davis and Keler.
Pension Benefits
None of our Named Executive Officers participate in qualified or nonqualified defined benefit plans sponsored by Celldex.
Nonqualified Deferred Compensation
None of our Named Executive Officers are covered by a defined contribution or other plan that provides for the deferral of compensation on a basis that is not tax-qualified.
Potential Payments Upon Termination of Employment or Change in Control
Certain of our Named Executive Officers have and had provisions in their employment agreements regarding severance upon certain termination events or acceleration of stock options in the event of a change of control of Celldex or termination following a change of control. These severance and acceleration provisions are described in "Employment Agreements," and certain estimates of these change of control benefits are provided in the tables below.
Anthony S. Marucci
The following table describes the potential payments and benefits upon employment termination for Anthony S. Marucci, president and chief executive officer, as if his employment had terminated as of December 31, 2008, the last business day of our latest fiscal year.
Executive benefits and payments upon termination | Voluntary resignation for no good reason | Voluntary resignation for good reason | Termination by Celldex not for cause | Termination by Celldex for cause | Voluntary termination by the executive for good reason or termination by Celldex without cause in connection with or following change of control(1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Base salary | $ | — | $ | 916,000 | $ | 916,000 | $ | — | $ | 916,000 | ||||||
Bonus | — | — | — | — | 106,200 | |||||||||||
Equity Awards Acceleration | — | — | — | — | — | |||||||||||
Continuation of Health Benefits | — | 23,550 | 23,550 | — | 23,550 | |||||||||||
Total | $ | — | $ | 939,550 | $ | 939,550 | $ | — | $ | 1,045,750 | ||||||
Avery W. Catlin
The following table describes the potential payments and benefits upon employment termination for Avery W. Catlin, chief financial officer, as if his employment had terminated as of December 31, 2008, the last business day of our latest fiscal year.
Executive benefits and payments upon termination | Voluntary resignation for no good reason | Voluntary resignation for good reason | Termination by Celldex not for cause | Termination by Celldex for cause | Voluntary termination by the executive for good reason or termination by Celldex without cause in connection with or following change of control(1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Base salary | $ | — | $ | 576,500 | $ | 576,500 | $ | — | $ | 288,250 | ||||||
Bonus | — | — | 46,759 | |||||||||||||
Equity Awards Acceleration | — | — | — | — | — | |||||||||||
Continuation of Health Benefits | — | 16,838 | 16,838 | — | 16,838 | |||||||||||
Total | $ | — | $ | 593,338 | $ | 593,338 | $ | — | $ | 351,847 | ||||||
Tibor Keler, Ph.D.
The following table describes the potential payments and benefits upon employment termination for Tibor Keler, Ph.D., chief scientific officer, as if his employment had terminated as of December 31, 2008, the last business day of our latest fiscal year.
Executive benefits and payments upon termination | Voluntary resignation for no good reason | Voluntary resignation for good reason | Termination by Celldex not for cause | Termination by Celldex for cause | Voluntary termination by the executive for good reason or termination by Celldex without cause in connection with or following change of control(1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Base salary | $ | — | $ | 684,000 | $ | 684,000 | $ | — | $ | 342,000 | ||||||
Bonus | — | — | — | — | 85,800 | |||||||||||
Equity Awards Acceleration | — | — | — | — | — | |||||||||||
Continuation of Health Benefits | — | 23,550 | 23,550 | — | 23,550 | |||||||||||
Total | $ | — | $ | 707,550 | $ | 707,550 | $ | — | $ | 451,350 | ||||||
Thomas Davis, M.D.
The following table describes the potential payments and benefits upon employment termination for Thomas Davis, M.D., chief medical officer, as if his employment had terminated as of December 31, 2008, the last business day of our latest fiscal year.
Executive benefits and payments upon termination | Voluntary resignation for no good reason | Voluntary resignation for good reason | Termination by Celldex not for cause | Termination by Celldex for cause | Voluntary termination by the executive for good reason or termination by Celldex without cause in connection with or following change of control(1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Base salary | $ | — | $ | 724,800 | $ | 724,800 | $ | — | $ | 362,400 | ||||||
Bonus | — | — | — | — | 85,800 | |||||||||||
Equity Awards Acceleration | — | — | — | — | — | |||||||||||
Continuation of Health Benefits | — | 23,718 | 23,718 | — | 23,718 | |||||||||||
Total | $ | — | $ | 748,518 | $ | 748,518 | $ | — | $ | 471,918 | ||||||
Ronald C. Newbold, Ph.D.
The following table describes the potential payments and benefits upon employment termination for Ronald C. Newbold, Ph.D., vice president, business development, as if his employment had terminated as of December 31, 2008, the last business day of our latest fiscal year.
Executive benefits and payments upon termination | Voluntary resignation for no good reason | Voluntary resignation for good reason | Termination by Celldex not for cause | Termination by Celldex for cause | Voluntary termination by the executive for good reason or termination by Celldex without cause in connection with or following change of control(1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Base salary | $ | — | $ | 250,000 | $ | 250,000 | $ | — | $ | 250,000 | ||||||
Bonus | — | — | — | — | 59,688 | |||||||||||
Equity Awards Acceleration | — | — | — | — | — | |||||||||||
Continuation of Health Benefits | — | 23,718 | 23,718 | — | 23,718 | |||||||||||
Total | $ | — | $ | 273,718 | $ | 273,718 | $ | — | $ | 333,406 | ||||||
Director Compensation
Effective March 8, 2008, the following director non-equity compensation policy was adopted. Directors who are not employees of Celldex are each entitled to receive a retainer fee of $50,000 each fiscal year ("Annual Retainer"). The Chairman of the Board is entitled to receive an annual retainer fee of $40,000 in addition to his or her Annual Retainer and any retainer for committee service. The Chairperson of each committee of the Board of Directors is entitled to receive an annual retainer fee of $30,000 in addition to his or her Annual Retainer. Each committee member (other than the Chairperson of a committee) will receive an annual retainer of $20,000 in addition to his or her Annual
Retainer. Each Director who resides outside the United States shall receive an additional stipend of $20,000. Stipends and retainers are paid in advance on a quarterly basis. The Directors shall be reimbursed for necessary travel and business expenses as incurred but will not receive any additional fees for attending meetings or calls of the Board of Directors. As of February 20, 2009, the current independent directors had the following stock options outstanding: Charles R. Schaller—26,882, Herbert J. Conrad—29,879, Larry Ellberger—29,728, George O. Elston—29,879, Karen Shoos Lipton—29,728, Rajesh B. Parekh—29,879 and Harry H. Penner, Jr.—29,728.
This table summarizes the annual cash compensation for Celldex's non-employee directors during 2008.
Name | Fees Earned or Paid in Cash ($) | Stock Award ($) | Option Awards ($)(1) | Non-Equity Incentive Plan Compensation ($) | Change in Pension Value and Nonqualified Deferred Compensation Earnings | All Other Compensation ($) | Total ($) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Charles R. Schaller | 139,000 | — | 79,496 | — | — | — | 218,496 | |||||||||||||||
Herbert J. Conrad | 88,750 | — | 46,231 | — | — | — | 134,981 | |||||||||||||||
Larry Ellberger | 83,000 | — | 75,089 | — | — | — | 158,089 | |||||||||||||||
George O. Elston | 78,750 | — | 46,231 | — | — | — | 124,981 | |||||||||||||||
Karen Shoos Lipton | 73,000 | — | 75,089 | — | — | — | 148,089 | |||||||||||||||
Dr. Rajesh B. Parekh | 81,750 | — | 46,231 | — | — | — | 127,981 | |||||||||||||||
Harry H. Penner, Jr. | 93,000 | — | 75,089 | — | — | — | 168,089 | |||||||||||||||
Compensation"Compensation Committee Interlocks and Insider Participation,
The Compensation Committee" and is incorporated herein by reference. If the 2010 Proxy Statement is not filed with the SEC within 120 days after the end of the Boardour most recent fiscal year, we will provide such information by means of Directors was composed at various times during the year by the following five non-employee directors: Messrs. Rajesh B. Parekh, Harry H. Penner, Jr., Larry Ellberger and Charles R. Schaller and Ms. Karen Shoos Lipton. None of these Compensation Committee members was an officer or employee of Celldex during the year. No Compensation Committee interlocks between Celldex and another entity existed.
COMPENSATION COMMITTEE REPORT*
The Compensation Committee of Celldex has reviewed the Compensation Discussion and Analysis with management and based on a review of the Compensation Discussion Analysis, the Compensation Committee recommendedamendment to the Board that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Equity Compensation Plan Information
The following table provides information as of December 31, 2008 regarding shares of common stock of Celldex that mayrequired by this Item 12 will be issuedincluded in the 2010 Proxy Statement under our existing equity compensation plans, including Celldex's 2008 Stock Option and Incentive Plan (the "2008 Plan") and Celldex's 2004 Employee Stock Purchase Plan (the "2004 Plan"). Footnote (4) to the table sets forth the total number of shares of common stock of Celldex issuable upon the exercise of assumed options as of December 31, 2008, and of assumed options and warrants as of March 7, 2008, and the weighted average exercise price of these options and warrants.
| | Equity Compensation Plan Information | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| | Number of securities to be issued upon exercise of outstanding options, warrants and rights(1) | Weighted Average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plan (excluding securities referenced in column (a)) | |||||||
| | (a) | (b) | (c) | |||||||
Equity compensation plans approved by security holders(2) | 2,070,993 | (3) | $ | 8.39 | 885,391 | (4) | ||||
Security"Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information as of February 20, 2009 with respect toManagement" and "Equity Compensation Plan Information" and is incorporated herein by reference. If the beneficial ownership of common stock of the Company by the following: (i) each of the Company's current directors; (ii) each of the Named Executive Officers; (iii) the current executive officers; (iv) all of the executive officers and directors as a group; and (v) each person known by the Company to own beneficially more than five percent (5%) of the outstanding shares of the Company's common stock.
For purposes of the following table, beneficial ownership2010 Proxy Statement is determined in accordancenot filed with the applicable SEC rules and the information is not necessarily indicative of beneficial ownership for any other purpose. Except as otherwise noted in the footnotes to the table, we believe that each person or entity named in the table has sole voting and investment power with respect to all shares of the Company's common stock shown as beneficially owned by that person or entity (or shares such power with his or her spouse). Under the SEC's rules, shares of the Company's common stock issuable under options that are exercisable on or within 60120 days after February 20, 2009 ("Presently Exercisable Options") are deemed outstanding and therefore included in the numberend of shares reported as beneficially owned by a person or entity named in the table and are used to compute the percentage of the common stock beneficially owned by that person or entity. These shares are not, however, deemed outstanding for computing the percentage of the common stock beneficially owned by any other person or entity.
The percentage of the common stock beneficially owned by each person or entity named in the following table is based on 15,820,593 shares of common stock outstanding as of February 20, 2009 plus any shares issuable upon exercise of Presently Exercisable Options held by such person or entity and reflects the 1-for-12 reverse stock split effected on March 7, 2008:
Name and Business Address of Beneficial Owners* | Amount and Nature of Beneficial Ownership(1) | Percentage of Common Stock(2) | |||||
|---|---|---|---|---|---|---|---|
Medarex, Inc.(3) | 4,960,848 | (4) | 31.4 | % | |||
Apax WW Nominees Ltd. | 1,384,663 | 8.8 | |||||
Pfizer Vaccines | 781,250 | 4.9 | |||||
Directors and Executive Officers | |||||||
Charles R. Schaller | 23,882 | (5) | ** | ||||
Herbert J. Conrad | 23,879 | (6) | ** | ||||
Larry Ellberger | 23,728 | (7) | ** | ||||
George O. Elston | 23,879 | (8) | ** | ||||
Karen Shoos Lipton | 24,061 | (9) | ** | ||||
Dr. Rajesh B. Parekh | 23,879 | (10) | ** | ||||
Harry H. Penner, Jr. | 23,728 | (11) | ** | ||||
Anthony S. Marucci | 215,585 | (12) | 1.3 | ||||
Una S. Ryan, Ph.D. | 314,058 | (13) | 2.0 | ||||
Avery W. Catlin | 53,592 | (14) | ** | ||||
Tibor Keler, Ph.D. | 215,585 | (15) | 1.3 | ||||
Thomas Davis M.D. | 70,941 | (16) | ** | ||||
Ronald C. Newbold, Ph.D. | 113,353 | (17) | ** | ||||
All Directors and Executive Officers as a group (Consisting of 12 persons) | 1,150,150 | (18) | 6.86 | % | |||
2009. Does not include 4,960,848 shares of common stock owned by Medarex. Mr. Schaller is a director of Medarex and to the extent that by virtue of his role as director Mr. Schaller may be deemed to share the power to vote (and direct the vote of) or dispose of (or direct the disposition of) such shares of common stock owned of record by Medarex, Mr. Schaller disclaims beneficial ownership of the shares of common stock owned by Medarex.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
It is our policy that all employees and directors, as well as their family members, must avoid any activity that is or has the appearance of conflicting with Celldex's business interest. This policy isThe information required by this Item 13 will be included in our Codethe 2010 Proxy Statement under "Election of Business ConductDirectors" and Ethics. All directors"Approval of Related Person Transactions and officers of Celldex complete a directorsTransactions with Related Persons" and officers questionnaire atis incorporated herein by reference. If the beginning of each year, in which they are asked to disclose family relationships and other related party transactions. Our Audit Committee must review and approve all related party transactions, as defined in Item 404 of Regulation S-K. Our Audit Committee's procedures for reviewing related party transactions are2010 Proxy Statement is not in writing. In fiscal 2008, there were no related party transactions. Charles Schaller is an independent director of Medarex, Inc. which is a principal shareholder of the Company. Medarex also has contractual relationshipsfiled with the Company. Mr. Schaller does participate inSEC
within 120 days after the review or approvalend of any matter in which Medarex has a material interest.
Director Independence
Forour most recent fiscal year, we will provide such information by means of an amendment to this Annual Report on director independence, please see Item 10 above under the caption "The Board of Directors and Its Committees."Form 10-K.
Item 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The Audit Committee approved the engagement of PricewaterhouseCoopers LLP as Celldex's independent registered public accounting firm for fiscal 2008 and the Company's stockholders ratified the appointment of PricewaterhouseCoopers LLP at the Annual Meeting of Stockholders on September 25, 2008.
Audit Fees
Represents fees for professional services provided in connection with the audit of Celldex's annual audited financial statements and reviews of Celldex's quarterly financial statements, advice on accounting matters directly related to the audit and audit services provided in connection with other statutory or regulatory filings. Fees, including out of pocket expenses, for the fiscal year 2008 audit, including assurance services provided in connection with the assessment and testing of internal controls pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, quarterly reviews of Forms 10-Q during fiscal year 2008 and in connection with the Merger completed in 2008 were $645,913.
Audit-Related Fees
Audit-related fees are for assurance and other activities not explicitly related to the audit of Celldex's financial statements, and consisted principally of fees for consultations concerning financial accounting and reporting standards. There were no audit-related fees billed by PricewaterhouseCoopers LLP for fiscal 2008.
Tax Fees
Tax fees are associated with tax compliance, tax advice, tax planning and tax preparation services. In 2008 and 2007, we engaged another public accounting firm to perform these services.
All Other Fees
Other fees of $1,500 were billed by PricewaterhouseCoopers LLP in fiscal years 2008.
The Audit Committee is responsible for appointing, setting compensation and overseeing the work of the independent auditors. The Audit Committee has established a policy regarding pre-approval of all auditing services and the terms thereof and non-audit services (other than non-audit services prohibited under Section 10A(g) of the Exchange Act or the applicable rules of the SEC or the Public Company Accounting Oversight Board) to be provided to Celldex by the independent auditor. However, the pre-approval requirement may be waived with respect to the provision of non-audit services for Celldex if the "de minimus" provisions of Section 10A(i)(1)(B) of the Exchange Act are satisfied.
The Audit Committee has considered whether the provision of Audit-Related Fees, Tax Fees, and all other fees as described above is compatible with maintaining PricewaterhouseCoopers, LLP's independence and has determined that such services for fiscal years 2008, 2007 and 2006 were compatible. All such services were approved by the Audit Committee pursuant to Rule 2-01 of Regulation S-X under the Exchange Act to the extent that rule was applicable.
The Audit Committee is responsible for reviewing and discussing the audit financial statements with management, discussing with the independent registered public accountants the matters required in Auditing Standards No. 61, receiving written disclosures from the independent registered public accountantsinformation required by the applicable requirements of the Public Company Accounting Oversight Board regarding the independent registered public accountants' communications with the Audit Committee concerning independence and discussing with the independent registered public accountants their independence, and recommending to the board of directors that the audit financial statementsthis Item 14 will be included in the company's annual report2010 Proxy Statement under "Independent Registered Public Accounting Firm" and is incorporated herein by reference. If the 2010 Proxy Statement is not filed with the SEC within 120 days after the end of our most recent fiscal year, we will provide such information by means of an amendment to this Annual Report on Form 10-K.
Item 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
See "Index to Consolidated Financial Statements" at Item 8.
Schedules are omitted since the required information is not applicable or is not present in amounts sufficient to require submission of the schedule, or because the information required is included in the Consolidated Financial Statements or Notes thereto.
| | | Incorporated by Reference to | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | Form and SEC File No. | Exhibit No. | SEC Filing Date | |||||
| Plan of Acquisition, Reorganization, Arrangement, Liquidation or Succession | |||||||||
| 2.1 | Agreement and Plan of Merger, dated as of October 19, 2007, by and among AVANT, Celldex Merger Corporation, and Celldex Therapeutics, Inc. | 8-K (000-15006) | 2.1 | 10/22/07 | |||||
2.2 | Agreement and Plan of Merger, dated as of May 28, 2009, by and among Celldex Therapeutics, Inc., CuraGen Corporation and Cottrell Merger Sub, Inc. | 8-K (000-15006) | 2.1 | 5/29/09 | |||||
Articles of Incorporation and By-Laws | |||||||||
| 3.1 | Third Restated Certificate of Incorporation | S-4 (333-59215) | 3.1 | 7/16/98 | |||||
3.2 | Certificate of Amendment of Third Restated Certificate of Incorporation | S-4 (333-59215) | 3.1 | 7/16/98 | |||||
3.3 | Second Certificate of Amendment of Third Restated Certificate of Incorporation | S-4 (333-59215) | 3.2 | 7/16/98 | |||||
3.4 | Third Certificate of Amendment of Third Restated Certificate of Incorporation | 10-Q (000-15006) | 3.1 | 5/10/02 | |||||
3.5 | Fourth Certificate of Amendment of Third Restated Certificate of Incorporation | 8-K (000-15006) | 3.1 | 3/11/08 | |||||
3.6 | Fifth Certificate of Amendment of Third Restated Certificate of Incorporation | 8-K (000-15006) | 3.2 | 3/11/08 | |||||
3.7 | Amended and Restated By-Laws as of March 14, 2007 | 10-K (000-15006) | 3.5 | 3/18/08 | |||||
Instruments Defining the Rights of Security Holders | |||||||||
| 4.1 | Shareholder Rights Agreement dated November 5, 2004 | 8-A (000-15006) | 4.1 | 11/8/04 | |||||
4.2 | Amendment No. 1 to Shareholder Rights Agreement dated October 19, 2007 | 8-A/A (000-15006) | 10.1 | 10/22/07 | |||||
| | | Incorporated by Reference to | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | Form and SEC File No. | Exhibit No. | SEC Filing Date | |||||
| 4.3 | Amendment No. 2 to Shareholder Rights Agreement dated March 7, 2008 | 8-A/A (000-15006) | 10.1 | 3/7/08 | |||||
4.4 | Certificate of Designations, Preferences and Rights of a Series of Preferred Stock classifying and designating the Series C-1 Junior Participating Cumulative Preferred Stock | 8-A (000-15006) | 3.1 | 11/8/04 | |||||
4.5 | Indenture, dated February 17, 2004 between CuraGen and Trustee | Filed herewith | |||||||
4.6 | Supplemental Indenture, dated September 30, 2009, by and among Celldex, CuraGen, Merger Sub, and Trustee. | 8-K (000-15006) | 4.1 | 10/2/09 | |||||
4.7 | Second Supplemental Indenture, dated December 31, 2009, by and among Celldex, CuraGen, and Trustee. | 8-K (000-15006) | 4.1 | 12/31/09 | |||||
Material Contracts—Leases | |||||||||
| 10.1 | Commercial Lease Agreement of May 1, 1996 between the Company and Fourth Avenue Ventures Limited Partnership | 10-Q/A (000-15006) | 10.11 | 8/23/96 | |||||
10.2 | Extension of Lease Agreement of May 1, 1997 between the Company and DIV Needham 53 LLC (successor in interest to Fourth Avenue Ventures Limited Partnership) dated as of August 23, 2001 | 10-K (000-15006) | 10.9 | 3/27/02 | |||||
10.3 | First Amendment to Lease by and between the Company and DIV Needham 53 LLC dated November 29, 2005 | 10-K (000-15006) | 10.40 | 3/16/06 | |||||
*10.4 | Lease Agreement, by and between the Company and the Massachusetts Development Finance Agency, dated as of December 22, 2003 | 10-Q (000-15006) | 10.1 | 4/30/04 | |||||
10.5 | Second Amendment to Lease by and between the Company and the Massachusetts Development Finance Agency dated as of November 4, 2005 | 10-K (000-15006) | 10.41 | 3/16/06 | |||||
10.6 | Lease Agreement dated as of October 21, 2005 by and between Phillipsburg Associates, L.P. and the Company. | S-4 (333-148291) | 10.10 | 1/18/08 | |||||
Material Contracts—License, Collaboration, Supply and Distribution Agreements | |||||||||
| *10.7 | License and Royalty Agreement by and between Pfizer Inc and the Company dated as of December 1, 2000 | 10-K (000-15006) | 10.13 | 3/27/01 | |||||
*10.8 | Amendment to License and Royalty Agreement by and between Pfizer Inc and the Company dated as of December 1, 2000 | 10-K (000-15006) | 10.14 | 3/27/01 | |||||
*10.9 | Collaborative Research and Development Agreement by and between Pfizer Inc. and the Company dated as of December 1, 2000 | 10-K (000-15006) | 10.15 | 3/27/01 | |||||
*10.10 | License Agreement between the Company and SmithKline Beecham PLC dated as of December 1, 1997 | 10-K (000-15006) | 10.20 | 3/28/00 | |||||
| | | Incorporated by Reference to | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | Form and SEC File No. | Exhibit No. | SEC Filing Date | |||||
| 10.11 | Amendment Agreement, dated January 9, 2003, between the Company and SmithKline Beecham PLC | 10-K/A (000-15006) | 10.21 | 9/12/03 | |||||
10.12 | License Agreement, dated as of January 31, 2003, by and between the Company and Elan Drug Delivery Limited | 10-K/A (000-15006) | 10.22 | 9/12/03 | |||||
10.13 | License and Clinical Trials Agreement, effective as of February 27, 1995, between the Company and the James N. Gamble Institute of Medical Research | 10-K/A (000-15006) | 10.23 | 9/12/03 | |||||
10.14 | License Agreement, dated as of November 25, 1988, by and among The Johns Hopkins University, Brigham and Women's Hospital and the Company | 10-K/A (000-15006) | 10.28 | 9/12/03 | |||||
10.15 | Purchase Agreement, dated as of May 16, 2005, by and between the Company and PRF Vaccine Holdings LLC | 8-K (000-15006) | 10.1 | 5/18/05 | |||||
10.16 | Amendment Agreement to Purchase Agreement between the Company and PRF Vaccine Holdings LLC, dated as of March 14, 2006 | 8-K (000-15006) | 10.1 | 3/15/06 | |||||
*10.17 | Exclusive License Agreement dated February 1, 2003 by and between Thomas Jefferson University ("TJU") and the Company | S-4 (333-148291) | 10.1 | 1/18/08 | |||||
*10.18 | License Agreement dated as of November 1, 2005 by and between The Rockefeller University and the Company | S-4 (333-148291) | 10.2 | 1/18/08 | |||||
*10.19 | License Agreement dated September 1, 2006 by and between Duke University and the Company | S-4 (333-148291) | 10.3 | 1/18/08 | |||||
*10.20 | Assignment and License Agreement, as amended, dated April 6, 2004 by and among Medarex, Inc., GenPharm International, Inc. and the Company | S-4 (333-148291) | 10.4 | 1/18/08 | |||||
*10.21 | Research and Commercialization Agreement, as amended, dated as of April 6, 2004 by and among Medarex, Inc., GenPharm International, Inc. and the Company | S-4 (333-148291) | 10.5 | 1/18/08 | |||||
*10.22 | Supply Agreement dated August 18, 2006 by and between the Company and Biosyn | S-4 (333-148291) | 10.9 | 1/18/08 | |||||
10.23 | License and Development Agreement dated as of April 16, 2008 between the Company and Pfizer Vaccines, LLC | 10-Q (000-15006) | 10.1 | 5/19/08 | |||||
*10.24 | Research Collaboration and Commercialization Agreement effective October 20, 2006 between the Company and the Ludwig Institute for Cancer Research | 10-K (000-15006) | 10.45 | 3/2/09 | |||||
*10.25 | Vaccine Adjuvant License and Collaboration Agreement dated on May 30, 2008 between the Company and 3M Innovation Properties Company | 10-K (000-15006) | 10.46 | 3/2/09 | |||||
| Incorporated by Reference to | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | SEC File No. | Exhibit No. | SEC Filing Date | |||||
(000-15006) | 10.47 | 3/2/09 | |||||||
Collaboration Agreement dated June 18, 2004 between Seattle Genetics and | |||||||||
Filed herewith | |||||||||
| 10-Q | 10.2 | 4/30/04 | |||||||
Secured Promissory Note: Equipment Loan, by and between | (000-15006) | 10.3 | 4/30/04 | ||||||
*10.33 | |||||
Common Stock Purchase Agreement dated as of April 16, 2008 between | (000-15006) | 10.2 | 8/11/08 | ||||||
| 2008 Stock Option and | |||||||||
† | 2004 Employee Stock Purchase Plan, as amended and restated | Filed herewith | |||||||
†10.36 | Employment Agreement, dated January 6, 2009, by and between | (000-15006) | 10.1 | 1/8/09 | |||||
† | Employment Agreement, dated January 6, 2009, by and between | (000-15006) | 10.2 | 1/8/09 | |||||
† | Employment Agreement, dated January 6, 2009, by and between | (000-15006) | 10.3 | 1/8/09 | |||||
† | Amended and Restated Employment Agreement, dated January 6, 2009, by and between | (000-15006) | 10.4 | 1/8/09 | |||||
(000-15006) | 10.1 | 1/25/10 | |||||||
| Incorporated by Reference to | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | SEC File No. | Exhibit No. | SEC Filing Date | |||||
| Filed herewith | |||||||||
Filed herewith | |||||||||
21.0 | List of Subsidiaries | Filed herewith | |||||||
23.1 | Consent of PricewaterhouseCoopers LLP Independent Registered Public Accounting Firm of Celldex Therapeutics, Inc. (formerly known as AVANT Immunotherapeutics, Inc.) | Filed herewith | |||||||
23.2 | Consent of Ernst & Young LLP Independent Registered Public Accounting Firm of Celldex Research Corporation (formerly known as Celldex Therapeutics, Inc.) | Filed herewith | |||||||
31.1 | Certification of President and Chief Executive Officer | Filed herewith | |||||||
31.2 | Certification of Senior Vice President and Chief Financial Officer | Filed herewith | |||||||
32 | Section 1350 Certifications | Furnished herewith | |||||||
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CELLDEX THERAPEUTICS, INC. | ||||
By: | /s/ ANTHONY S. MARUCCI | |||
Date | Anthony S. Marucci | |||
March 12, 2010 | President and Chief Executive Officer | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title | Date | ||
|---|---|---|---|---|
| /s/ ANTHONY S. MARUCCI Anthony S. Marucci | President, Chief Executive Officer, and Director (Principal Executive Officer) | |||
/s/ AVERY W. CATLIN Avery W. Catlin | Senior Vice President, Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) | |||
/s/ LARRY ELLBERGER Larry Ellberger | Director, Chairman of the Board of Directors | |||
/s/ HERBERT J. CONRAD Herbert J. Conrad | Director | March 12, 2010 | ||
/s/ GEORGE O. ELSTON George O. Elston | Director | |||
/s/ KAREN SHOOS LIPTON Karen Shoos Lipton | Director | |||
/s/ DR. RAJESH B. PAREKH Dr. Rajesh B. Parekh | Director | |||
/s/ HARRY H. PENNER, JR. Harry H. Penner, Jr. | Director | |||
/s/ CHARLES R. SCHALLER Charles R. Schaller | Director | March 12, 2010 | ||
/s/ TIMOTHY M. SHANNON, M.D. Timothy M. Shannon, M.D. | Director | March 12, 2010 |