Delaware (State or other jurisdiction of incorporation or organization) | 27-0072226 (I.R.S. Employer Identification No.) | |
128 Sidney Street Cambridge, Massachusetts (Address of principal executive offices) | 02139 (Zip Code) | |
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Act. Yes o No ý $775 million. strategies. We own or have rights to trademarks, service marks and trade names that we use in connection with the operation of our business, including our corporate name, logos and website names. Other trademarks, service marks and trade names appearing in this report are the property of their respective owners. The trademarks that we own include ™, that is accelerating our discovery efforts. We have nominated an IntelliTrap™ molecule, ACE-2494, as a candidate for clinical development and will initiate IND-enabling activities in 2016. ACE-2494 is designed to treat systemic muscle disorders. We use our integrated platform of research, development and manufacturing technologies to rapidly and cost-effectively create, test and advance our novel mechanism. We are developing calcifications. We and Celgene are considering refocusing the sotatercept program on the treatment of patients with earlier, pre-dialysis kidney disease. We expect to meet with the FDA in the first half of 2016 to discuss the initiation of a clinical trial in pre-dialysis patients. inhibitors. We are patients. currently approved VEGF pathway inhibitors include Avastin® (bevacizumab), Cyramza® (ramucirumab), Inlyta® (axitinib), Nexavar® (sorafenib), Stivarga® (regorafenib), Sutent® (sunitinib), United States. In addition to Our Strategic Partnerships our terminated collaboration with Shire AG (Shire). Phase 3 clinical trials. Acceleron will manufacture clinical trials in the United States and Europe; MDS; and a CD95 ligand inhibitor, APG101, being studied by Apogenix in a Phase 1 study in transfusion dependent, lower risk MDS patients. diabetic nephropathy. carcinoma. pending applications with respect to our extensions. Dalantercept Patent Coverage licensed patents. The agreement terminates upon the expiration of the last valid claim of the licensed patent rights. We may terminate the agreement at any time by giving BIDMC advance written notice. The agreement may also be terminated by BIDMC in the event of a material breach by us or in the event we become subject to specified bankruptcy or similar circumstances. In any termination event, we retain our joint ownership of the patent rights and a worldwide non-exclusive license with right to sublicense. candidates and future products, are subject to extensive regulation by governmental authorities in the United States and other countries. In the United States, pharmaceutical products are regulated by the FDA under the Federal Food, Drug, and Cosmetic Act and other laws, including, in the case of biologics, the Public Health Service Act. We expect sotatercept, therapeutic candidate. listed in the protocol or investigator's brochure, or any findings from other studies or animal or in vitro testing that suggest a significant risk in humans exposed to the drug. An institutional review board, or IRB, at each institution participating in the clinical trial must review and approve the protocol before a clinical trial commences at that institution, approve the information regarding the trial and the consent form that must be provided to each research subject or the subject's legal representative, and monitor the study until completed. warning statements be included in the product labeling, may require that additional studies be conducted following approval as a condition of the approval, and may impose restrictions and conditions on product distribution, prescribing, or dispensing in the form of a Risk Evaluation and Mitigation Strategy, or REMS, or Legislation similar to the Orphan Drug Act has been enacted outside the U.S., including in the EU. The orphan legislation in the EU is available for therapies addressing chronic debilitating or life-threatening conditions that affect five or fewer out of 10,000 persons or are financially not viable to develop. The market exclusivity period is for ten years, although that period can be reduced to six years if, at the end of the fifth year, available evidence establishes that the product is sufficiently profitable not to justify maintenance of market exclusivity. The market exclusivity may be extended to 12 years if sponsors complete a pediatric investigation plan agreed upon with the relevant committee of the EMA. determines that information relating to the use of a drug in a pediatric population, or part of the pediatric population, may not produce health benefits in that population. Medicaid is a joint federal and state program that is administered by the states for low income and disabled beneficiaries. Under the Medicaid Drug Rebate Program, manufacturers are required to pay a rebate for each unit of product reimbursed by the state Medicaid programs. The amount of the rebate for each product is set by law and may be subject to an additional discount if certain pricing increases more than inflation. includes changes to the coverage and payment for drug products under government concerned member states. The reference member state prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. Within 90 days of receiving the reference member state's assessment report, each concerned member state must decide whether to approve the assessment report and related materials. If a member state does not recognize the marketing authorization, the disputed points are eventually referred to the European Commission, whose decision is binding on all member states. therapeutic candidates. Additional Capital To become and remain profitable, we or our partners must succeed in developing our acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate preclinical studies, clinical trials or other development activities for one or more of our we will have no rights to impose our development strategy on Celgene. Similarly, Celgene may decide to seek regulatory approval for, and limit commercialization of, either or both of sotatercept and More broadly, if Celgene elects to discontinue the development of both sotatercept and luspatercept, we may be unable to advance the products ourselves. monitoring and otherwise carrying out many of these trials. We and Celgene compete with many other companies for the resources of these third parties. The third parties on whom we and Celgene rely generally may terminate their engagements at any time, and having to enter into alternative arrangements would delay development and commercialization of our For Third-party claims of intellectual property infringement or misappropriation may prevent or delay our development and commercialization efforts. We may face a claim of misappropriation if a third party believes that we inappropriately obtained and used trade secrets of such third party. If we are found to have misappropriated a third party's trade secrets, we may be prevented from further using such trade secrets, limiting our ability to develop our technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, and outside scientific advisors, contractors and collaborators. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors, or outside scientific advisors might intentionally or inadvertently disclose our trade secret information to competitors. In addition, competitors may otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. business. medical providers. and establish reimbursement levels. Reimbursement by a third-party payer may depend upon a number of factors, including the third-party consider changes to existing In addition, the success of gene and/or cell therapy in beta-thalassemia patients could materially reduce the potential patient population for luspatercept, especially in transfusion dependent patients. research and marketing capabilities than we do and may also have products that have been approved or are in late stages of development, and have collaborative arrangements in our target markets with leading companies and research institutions. Established pharmaceutical companies may also invest heavily to accelerate discovery and development of novel compounds or to in-license novel compounds that could make the ultimately be unable to complete, the development and commercialization of our therapeutic candidates. these candidate therapeutics may show unacceptable toxicity or pharmacokinetic properties, or these therapeutic candidates may not be safe or effective in clinical trials. operations. therapeutic candidates. contamination from the use, manufacture, distribution, storage, handling, treatment or disposal of hazardous materials. In the event of contamination or injury, or failure to comply with environmental, occupational health and safety and export control laws and regulations, we could be held liable for any resulting damages and any such liability could exceed our assets and resources. We are uninsured for third-party contamination injury. price at which you purchased them. If we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common stock. return on their investment. As a result, investors seeking cash dividends should not purchase our common stock. the company may be unsuccessful. forums for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers or financial position. Stockholders Third quarter(1) Fourth quarter As of January 31, Graph The selected consolidated statements of operations Revenue: Collaboration revenue: License and milestone Cost-sharing, net Contract manufacturing Total revenue Costs and expenses: Research and development General and administrative Cost of contract manufacturing revenue Total costs and expenses Income (loss) from operations Total other expense, net Net (loss) income Comprehensive (loss) income Net (loss) income per share applicable to common stockholders(1) Basic Diluted Weighted-average number of common shares used in computing net (loss) income per share applicable to common stockholders Basic Diluted Balance Sheet Data: Cash and cash equivalents Total assets Total current liabilities Long term deferred revenue Long-term notes payable Warrants to purchase redeemable convertible preferred stock Warrants to purchase common stock Redeemable convertible preferred stock Total stockholder's equity (deficit) treat systemic muscle disorders. We expect to continue to incur significant expenses and increasing operating losses over at least the next several years. We expect our expenses will increase substantially in connection with our ongoing activities, as we: therapeutic candidates. Costs and Expenses the costs of We are also expensing the costs of a Phase 1 clinical trial for ACE-083. Sotatercept(1) ACE-536(1) Dalantercept ACE-083 ACE-031(2) Total direct research and development expenses Other expenses(3) Total research and development expenses director and officer insurance premiums, and investor relations costs associated with being a public company. We anticipate that our general and administrative expenses will increase in the future as we increase our headcount to support our continued research and development and potential commercialization of our therapeutic candidates. amounts not expected to be recognized as revenue within the 12 months following the balance sheet date are classified as deferred revenue, net of current portion. and incremental discount. Conversely, for arrangements under which an option is not considered substantive or if an option is priced at a significant and incremental discount, we would consider the item underlying the option to be a deliverable at the inception of the arrangement and a corresponding amount would be included in allocable arrangement consideration. Clinical Trial Accruals and Related Expenses We also estimate forfeitures at the time of grant, and revise those estimates in subsequent periods if actual forfeitures differ from estimates. We use historical data to estimate pre-vesting option forfeitures to the extent that actual forfeitures differ from our estimates, the difference is recorded as a cumulative adjustment in the period the estimates were revised. Stock-based compensation expense recognized in the consolidated financial statements is based on awards that are ultimately expected to vest. For the years ended December 31, 2015, 2014 and 2013, . stock and are now classified as a component of equity and are no longer subject to remeasurement. The exercise prices for each of these warrants remained unchanged. Revenue: Collaboration revenue: License and milestone Cost-sharing, net Total revenue Costs and expenses: Research and development General and administrative Total costs and expenses Income (loss) from operations Other expense, net Net loss Collaboration revenue: Celgene: License and milestone Cost-sharing, net Total Celgene Shire: License and milestone Cost-sharing, net Total Shire Total collaboration revenue Total revenue Revenue: Collaboration revenue: License and milestone Cost-sharing, net Contract manufacturing Total revenue Costs and operating expenses: Research and development General and administrative Cost of contract manufacturing revenue Total costs and expenses Income (loss) from operations Other expense, net Net (loss) income Collaboration revenue: Celgene: License and milestone Cost-sharing, net Total Celgene Shire: License and milestone Cost-sharing, net Total Shire Alkermes: License and milestone Cost-sharing, net Total Alkermes Total collaboration revenue Contract manufacturing revenue Total revenue As of December 31, Net cash provided by (used in): Operating activities Investing activities Financing activities Net increase (decrease) in cash and cash equivalents Celgene Collaboration. Other components of the change in operating assets and liabilities include a decrease in collaboration receivables of $0.2 million, a decrease in deferred rent of $0.5 million, a decrease in accounts payable of $0.2 million, and an increase in prepaid and other current assets of $0.3 million. purchase common stock. Operating Capital Requirements expect. Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to: Operating lease obligations(1) Less: sublease income(2) Venture debt facility(3) Total We also have obligations to make future payments to third party licensors that become due and payable on the achievement of certain development, regulatory and commercial milestones. We have not included these commitments on our consolidated balance sheet or in the table above because the achievement and timing of these milestones is not fixed or determinable. These commitments include the following: Item 7A. Quantitative and Qualitative Disclosures About Market Risks We have the ability to hold our investments until maturity, and therefore we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a change in market interest rates on our investments. Changes in Internal Control over Financial Reporting 2015. We are not filing any financial statement schedules as part of this Annual Report on Form 10-K because they are not applicable or the required information is included in the consolidated financial statements or notes thereto. Assets Current assets: Cash and cash equivalents Collaboration receivables (includes related party amounts of $3,616 and $1,840 at December 31, 2013 and 2012, respectively) Prepaid expenses and other current assets Total current assets Property and equipment, net Restricted cash Related party receivables Other assets Total assets Liabilities, redeemable convertible preferred stock and stockholders' equity (deficit) Current liabilities: Accounts payable Accrued expenses (includes related party amounts of $0 and $861at December 31, 2013 and 2012, respectively) Deferred revenue Deferred rent Notes payable, net of discount Total current liabilities Deferred revenue, net of current portion Deferred rent, net of current portion Notes payable, net of current portion and discount Warrants to purchase redeemable convertible preferred stock Warrants to purchase common stock Total liabilities Commitments and contingencies (Note 7) Redeemable convertible preferred stock (Note 8) Stockholders' equity (deficit): Undesignated preferred stock, $0.001 par value: 25,000,000 shares authorized and no shares issued or outstanding at December 31, 2013; No shares authorized, issued or outstanding at December 31, 2012 Common stock, $0.001 par value: 175,000,000 and 104,013,161 shares authorized at December 31, 2013 and 2012, respectively; 28,348,630 and 2,432,155, shares issued and outstanding at December 31, 2013 and 2012, Additional paid-in capital Accumulated deficit Total stockholders' equity (deficit) Total liabilities, redeemable convertible preferred stock and stockholders' equity (deficit) Loss Revenue: Collaboration revenue: License and milestone Cost-sharing, net Contract manufacturing Total revenue(1) Costs and expenses: Research and development General and administrative Cost of contract manufacturing revenue Total costs and expenses Income (loss) from operations Other (expense) income: Other expense, net Interest income Interest expense Total other expense, net Net (loss) income Comprehensive (loss) income Reconciliation of net (loss) income to net (loss) income applicable to common stockholders: Net (loss) income Accretion of dividends, interest, redemption value and issuance costs on redeemable convertible preferred stock Gain on extinguishment of redeemable convertible preferred stock Net (loss) income applicable to participating securities Net (loss) income applicable to common stockholders—basic Net (loss) income Accretion of dividends, interest, redemption value and issuance costs on redeemable convertible preferred stock Gain on extinguishment of redeemable convertible preferred stock Net (loss) income applicable to participating securities Net (loss) income applicable to common stockholders—diluted Net (loss) income per share applicable to common stockholders: (Note 2) Basic Diluted Weighted-average number of common shares used in computing net (loss) income per share applicable to common stockholders: Basic Diluted (1) Includes related party revenue (Note 14) Balance at December 31, 2010 Sale of Series F redeemable convertible preferred stock net of issuance costs of $92 Accretion of dividends, interest, redemption value and issuance costs related to redeemable convertible preferred stock Compensation expense associated with stock options Grant of stock options to nonemployees Exercise of stock options Exercise of common warrants Net loss Balance at December 31, 2011 Accretion of dividends, interest, redemption value and issuance costs related to redeemable convertible preferred stock Compensation expense associated with stock options Exercise of stock options Net loss Balance at December 31, 2012 Accretion of dividends, interest, redemption value and issuance costs related to redeemable convertible preferred stock Repurchase and retirement of redeemable convertible preferred stock Exercise of warrants to purchase convertible preferred stock Exercise of warrants to purchase common stock Compensation expense associated with stock options Exercise of stock options Conversion of redeemable convertible preferred stock into common stock Reclassification of warrants to purchase shares of redeemable convertible preferred stock into warrants to purchase common stock Issuance of common stock in connection with initial public offering and private placement, net of issuance costs of $2,692 Net loss Balance at December 31, 2013 Balance at December 31, 2010 Sale of Series F redeemable convertible preferred stock net of issuance costs of $92 Accretion of dividends, interest, redemption value and issuance costs related to redeemable convertible preferred stock Compensation expense associated with stock options Grant of stock options to nonemployees Exercise of stock options Exercise of common warrants Net income Balance at December 31, 2011 Accretion of dividends, interest, redemption value and issuance costs related to redeemable convertible preferred stock Compensation expense associated with stock options Exercise of stock options Net loss Balance at December 31, 2012 Accretion of dividends, interest, redemption value and issuance costs related to redeemable convertible preferred stock Repurchase and retirement of redeemable convertible preferred stock Exercise of warrants to purchase convertible preferred stock Exercise of warrants to purchase common stock Compensation expense associated with stock options Exercise of stock options Conversion of redeemable convertible preferred stock into common stock Reclassification of warrants to purchase shares of redeemable convertible preferred stock into warrants to purchase common stock Issuance of common stock in connection with initial public offering and private placement, net of issuance costs of $2,692 Net loss Balance at December 31, 2013 Operating Activities Net (loss) income Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: Depreciation and amortization Loss on disposition of property and equipment Stock-based compensation Amortization of debt discount Accretion of deferred interest Amortization of deferred debt issuance costs Change in fair value of warrants Gain on retirement of warrants Forgiveness of related party receivable Changes in assets and liabilities: Prepaid expenses and other current assets Collaboration receivables Related party receivable Accounts payable Accrued expenses Deferred revenue Deferred rent Restricted cash Net cash (used in) provided by operating activities Investing Activities Purchases of property and equipment Net cash used in investing activities Financing Activities Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs Proceeds from long-term debt, net of issuance costs Proceeds from issuance of common stock from initial public offering, net of issuance costs Proceeds from issuance of common stock from private placements Payments of long-term debt Payments made to repurchase redeemable convertible preferred stock, common stock and warrants to purchase common stock Proceeds from exercise of stock options and warrants to purchase common stock Net cash provided by financing activities Net increase (decrease) in cash and cash equivalents Cash and cash equivalents at beginning of period Cash and cash equivalents at end of period Supplemental Disclosure of Cash Flow Information: Cash paid for interest Supplemental Disclosure of Non-Cash Investing and Financing Activities: Accretion of dividends, interest, redemption value, and issuance costs on preferred stock Conversion of preferred stock into common stock Conversion of preferred stock warrants into common stock warrants Cashless exercise of warrants Capitalized follow-on public offering costs included in accrued expenses Purchase of property and equipment included in accounts payable and accrued expenses 2013 trials. contingent assets and liabilities at the date of the financial statements, and the reported amounts expensed during the reporting period. 2014. rare diseases. All material long-lived assets of the Company reside in the United States. The Company does use contract research organizations (CROs) and research institutions located outside the United States. Some of these expenses are subject to collaboration reimbursement which is presented as a component of cost sharing, net in the loss. and Restricted Cash collaboration receivables. inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company's assumptions about the inputs that market participants would use in pricing the asset or liability, and are developed based on the best information available in the circumstances. Assets: Money market funds Restricted cash Total assets Liabilities: Warrants to purchase common stock Total liabilities 2013 Assets: Money market funds Restricted cash Total assets Liabilities: Warrants to purchase redeemable convertible preferred stock Warrants to purchase common stock Total liabilities Beginning balance Change in fair value Exercises Repurchases Conversions Ending balance 2014. Company's 2014. Computer equipment and software Office and laboratory equipment Leasehold improvements 2013. therapeutic candidates. partner can use the other deliverable(s) for their intended purpose without the receipt of the remaining element(s), whether the value of the deliverable is dependent on the undelivered item(s) and whether there are other vendors that can provide the undelivered element(s). considered substantive or if an option is priced at a significant and incremental discount, the Company would consider the item underlying the option to be a deliverable at the inception of the arrangement and a corresponding amount would be included in allocable arrangement consideration. 2013 Stock-Based Compensation 2015. the tax position as well as consideration of the available facts and circumstances. As of December 31, losses incurred. Outstanding stock options Common stock warrants Preferred stock Preferred stock warrants been disclosed in the accompanying consolidated statements of operations and comprehensive consists entirely of unrealized holdings gains (losses) on investments as of December 31, 2015. 2013 Computer equipment and software Office equipment Laboratory equipment Leasehold improvements Construction in progress Total property and equipment Accumulated depreciation and amortization Property and equipment, net 2013 Collaboration expense Research and development related Employee compensation Professional services Other Warrant to purchase Series A Preferred Stock Warrants to purchase Series B Preferred Stock Warrants to purchase Series C-1 Preferred Stock Warrants to purchase Series D-1 Preferred Stock Warrants to purchase Common Stock Warrants to purchase Common Stock Warrants to purchase Common stock Warrants to purchase Common stock All warrants or net share settlement basis from the date of issuance. The warrant agreement contains a provision requiring an adjustment to the number of shares in the event the Company issues common stock, or securities convertible into or exercisable for common stock, at a price per share lower than the warrant exercise price. The Company concluded the anti-dilution feature required the warrants to be classified as liabilities under ASC Topic 815,Derivatives and Hedging—Contracts in Entity's Own Equity (ASC 815). The warrants are measured at fair value, with changes in fair value recognized as a gain or loss to other income (expense) in the consolidated statements of operations and comprehensive 2017. Fair value of underlying instrument Expected volatility Expected term (in years) Risk-free interest rate Expected dividend yield Fair value of underlying instrument Expected volatility Expected term (in years) Risk-free interest rate Expected dividend yield Fair value of underlying instrument Expected volatility Expected term (in years) Risk-free interest rate Expected dividend yield Fair value of underlying instrument Expected volatility Expected term (in years) Risk-free interest rate Expected dividend yield 2014 2015 2016 2017 2018 Total 2014 2015 Total On October 18, 2012, the Salk Institute for Biological Studies (Salk) filed a complaint in the Massachusetts Superior Court for Suffolk County, alleging that the Company breached one of the Company's two licensing agreements with Salk. The licensing agreement in dispute provides the Company with a license with respect to certain of Diagnostic Purposes, dated August 11, 2010 (the License Agreement). The License Agreement provides the Company with a license with respect to certain of Salk's U.S. patents related to the ActRIIB activin receptor proteins. On September 24, 2013, the Company completed its Series A Preferred Stock, $0.001 par value: 26,069,980 shares authorized, 6,410,976 shares issued and outstanding at December 31, 2012, at redemption value Series B Preferred Stock, $0.001 par value: 16,944,378 shares authorized, 4,204,185 shares issued and outstanding at December 31, 2012, at redemption value Series C Preferred Stock, $0.001 par value: 11,923,077 shares authorized, 2,978,062 shares issued, and outstanding at December 31, 2012, at redemption value Series C-1 Preferred Stock, $0.001 par value: 2,014,652 shares authorized, 457,875 issued, and outstanding at December 31, 2012, at redemption value Series D Preferred Stock, $0.001 par value: 955,414 shares authorized, 234,940 shares issued, and outstanding at December 31, 2012, at redemption value Series D-1 Preferred Stock, $0.001 par value: 2,802,548 shares authorized, 636,942 issued and outstanding at December 31, 2012, at redemption value Series E Preferred Stock, $0.001 par value: 3,662,422 shares authorized, 816,060 shares issued and outstanding at December 31, 2012, at redemption value Series F Preferred Stock, $0.001 par value: 9,704,756 shares authorized, 2,426,171 issued and outstanding at December 31, 2012, at redemption value Total redeemable convertible preferred stock from time to time in one or more series. The are issued or outstanding. Common Stock Reserved for Future Issuance Outstanding stock options to purchase common stock Shares available for future issuance under stock option plan Warrants to purchase common stock Shares available for future issuance under the employee stock purchase plan Total shares of authorized common stock reserved for future issuance research for a Per our agreement and concurrent with the IPO, Celgene purchased 666,667 shares of Common Stock at the IPO offer price of $15.00 per share for $10.0 million. a Phase 3 study in chronic kidney disease. sales generated from all geographies. Royalty payments are subject to certain reductions, including for entry of a generic product onto the market. 2013 of development expenses through December 31, 2012 and 100% thereafter). The Company determined its BESP for each of the undelivered elements under the arrangements as of the arrangement execution date as follows: Joint Development Committee. regulatory risks that must be overcome to achieve the milestones, the level of effort and investment required to achieve each milestone, and the monetary value attributed to each milestone. During the years ended December 31, respectively. accounting estimate with the remaining deferred revenue of $38.8 million at February 8, 2011, recognized prospectively over the new period of research and development and manufacturing services. In April 2013, the Company and Shire determined not to further pursue development of ACE-031 and Shire sent the Company a notice of termination for the ACE-031 collaboration. The collaboration terminated effective June 30, 2013. No amounts were recognized subsequent to the June 30, 2013 termination. During 2012, the Company executed a license agreement with a research institution for an exclusive, sublicensable, worldwide, royalty-bearing license. The Company is obligated to pay development milestones and commercial milestone fees totaling up to $1.0 million. Under the agreement, if the Company uses the inventors in the clinical research, the development milestones are waived and commercial milestones shall change to $0.8 million plus any waived milestones. The Company will also pay $25,000 annually upon first commercial sale as well as royalties of 1.5% of net sales on any products developed under the patents. During the years ended December 31, 2015, 2014 and 2013, paid and 2015. Additionally, on September 4, 2013, the respectively. Research and development General and administrative Expected volatility Expected term (in years) Risk-free interest rate Expected dividend yield Outstanding at December 31, 2012 Granted Exercised Canceled or forfeited Outstanding at December 31, 2013 Exercisable at December 31, 2013 Vested and expected to vest at December 31, 2013(2) exercise. compensation on a pretax basis. Savings Plan’s matching formula. All matching contributions and participant contributions vest immediately. Contributions totaled $0.3 million and $0.2 million for the year ended Deferred tax assets: U.S. and state net operating loss carryforwards Research and development credits Deferred revenue Accruals and other temporary differences Total deferred tax assets Less valuation allowance Net deferred tax assets A reconciliation of income tax expense computed at the statutory federal income tax rate to income taxes as reflected in the consolidated financial statements is as follows: Federal income tax expense at statutory rate State income tax, net of federal benefit Permanent differences Research and development credit Other Change in valuation allowance Effective income tax rate 2035. Included in the federal and state net operating loss carryforwards are approximately $38.9 million and $13.2 million, respectively, of deductions related to the exercise of stock options which represent an excess tax benefit which will be realized when it results in the reduction of cash income tax in accordance with ASC 718. 2030. amounts as disclosed are not subject to any material Section 382 limitations. For all years through December 31, On March 12, 2014, the Company License and milestone Cost sharing, net Related-Party Receivable disclosure: 2013: Total revenue Total costs and expenses Income (loss) from operations Net income (loss) Basic net income (loss) per share* Diluted net income (loss) per share* 2012: Total revenue Total costs and expenses Loss from operations Net loss Basic net loss per share* Diluted net loss per share*Title of Class: Name of Each Exchange on Which Registered Common Stock, $0.001 par value NASDAQ Global Market No ýoý ýo AsJune 30, 2013,the registrant’s voting and non-voting common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common stock was last sold (based on the closing share price as quoted on the NASDAQ Global Market) as of the last business day of the registrant'sregistrant’s most recently completed second fiscal quarter the registrant's common stock was not publicly traded. The registrant's common stock began trading on the NASDAQ Global Market on September 19, 2013. As of December 31, 2013, the aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $559 million, based on the closing price of the registrant's common stock on December 31, 2013.2014,2016, the registrant had 31,160,27437,096,412 shares of Common Stock, $0.001 par value per share, outstanding.ACCELERON PHARMA, INC.FORM 10-KINDEXPageterminology, including theterminology. The terms "believes," "estimates," "anticipates," "expects," "plans," "intends," "may," "could," "might," "will," "should," "approximately""anticipate", "believe", "contemplate", "continue", "could", "estimate", "expect", "forecast", "goal", "intend", "may", "plan", "potential", "predict", "project", "should", "strategy", "target", "will", "would", "vision", or, in each case, theirthe negative or other variations thereon or comparable terminology,other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. They appear a number of places throughout this Annual Report on Form 10-K and include, among other things, statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, things:trials, trials; make regulatory filings and obtain and maintain regulatory approvals for our product candidates, therapeutic candidates; our products,any approved therapeutic candidate, particularly in specific patient populations, expectationspopulations; clinical trial data, our results of operations, financial condition, liquidity, capital requirements, prospects, growth and strategies, the industry in which we operate and the trends that may affect the industry or us.TrademarksAcceleron®Acceleron Pharma® and IntelliTrap™. Solely for convenience, some of the trademarks, service marks and trade names referred to in this report are listed without the ® and ™ symbols, but we will assert, to the fullest extent under applicable law, our rights to our trademarks, service marks and trade names.protein therapeutics for cancer and rare diseases. Our research focusestherapeutic candidates that are based on the biology ofmechanisms that the Transforming Growth Factor-Beta (TGF-b) protein superfamily, a large and diverse group of molecules that are key regulators inhuman body uses to regulate the growth and repair of tissues throughoutits cells and tissues. Our research focuses on key natural regulators of cellular growth and repair, particularly the human body.Transforming Growth Factor-Beta, or TGF-beta, protein superfamily. We are leadersa leading company in understanding the biology of the TGF-b superfamilydiscovering and in targeting these pathways to develop important new medicines.developing therapeutic candidates that regulate cellular growth and repair. By couplingcombining our discovery and development expertise, including our proprietary knowledge of the TGF-bTGF-beta superfamily, withand our internal protein engineering and manufacturing capabilities, we have built a highly productive discovery and development platform that has generated innovative protein therapeutic candidates with novel mechanisms of action. These differentiated protein therapeutic candidates have the potential to significantly improve clinical outcomes for patients withacross many fields of medicine, and we have focused our discovery and development efforts on treatments for cancer and rare diseases.focusestimate that we have spent approximately $145.4 million on discoveringresearch and development for the three year period from 2013 through 2015.◦ Continue Phase 3 trials with Celgene for the treatment of myelodysplastic syndromes, the “MEDALIST” trial and beta-thalassemia, the “BELIEVE” trial; ◦ Report updated results from Phase 2 extension study trials in MDS and beta-thalassemia at major medical conferences during the year; and ◦ Announce initial preliminary Phase 2 data in ring sideroblast negative and erythropoiesis stimulating agent naïve MDS patients by year-end. ◦ Report additional ACE-083 data from Phase 1 trial; ◦ Initiate ACE-083 Phase 2 trial in facioscapulohumeral muscular dystrophy in the second half of 2016; and ◦ File an IND with the FDA for systemically-acting muscle agent, ACE-2494, by year-end. ◦ Announce top-line progression-free survival (PFS) results from Phase 2 DART study in renal cell carcinoma patients. ◦ Provide update on development strategy in pre-dialysis chronic kidney disease patients in the second half of 2016. protein therapeuticstherapeutic candidates that targetregulate cellular growth and repair. We have targeted a group of approximately 30 secreted proteins, or ligands, that are collectively referred to as the TGF-bTGF-beta superfamily. These ligands bind to subsets of 12 different receptors on the surface of cells, triggering intra-cellular changes in gene expression that guide cell growth and differentiation. The TGF-b superfamily ligands and their receptors represent an under-explored and diverse set of drug targets with the potential to yield therapeutics that modulate the growth and repair of diseased cells and tissues. We have three internally discovered protein therapeutic candidates that are currently being studied in numerous ongoing Phase 2 clinical trials, focused on cancer and rare diseases. Our two most advanced protein therapeutic candidates, sotatercept and ACE-536, promote red blood cell production through a novel mechanism. Together with our collaboration partner, Celgene Corporation (Celgene), we are developing sotatercept and ACE-536 to treat anemia and associated complications in patients withb-thalassemia and myelodysplastic syndromes (MDS). These red blood cell disorders are generally unresponsive to currently approved drugs. Our third clinical stage protein therapeutic candidate, dalantercept, is designed to inhibit blood vessel formation through a mechanism that is distinct from, and potentially synergistic with, the dominant class of cancer drugs that inhibit blood vessel formation, the vascular endothelial growth factor (VEGF) pathway inhibitors. We are developing dalantercept primarily for use in combination with these products to produce better outcomes for cancer patients. We estimate that we have spent approximately $152.3 million on research and development from 2010 through 2013. Sotatercept and ACE-536 have already shown promising biological activity in our initial clinical trials. We and Celgene have conducted six human clinical trials with sotatercept in over 160 healthy volunteers and cancer patients. We have conducted one clinical trial with ACE-536 in healthy volunteers. In these studies, both sotatercept and ACE-536 caused a dose-dependent increase in the number of red blood cells. Based on these results, we and Celgene have initiated Phase 2 clinical trials with each of these protein therapeutic candidates inb-thalassemia and MDS. In the ongoing trials of sotatercept and ACE-536 in patients withb-thalassemia, we have observed encouraging, dose-dependent increases in hemoglobin in non-transfusion dependent patients at the three dose levels tested. We and Celgene plan to initiate Phase 3 clinical trials for one or both of these protein therapeutic candidates in one or both ofb-thalassemia and MDS by the end of 2014 or early 2015. With respect to our third clinical stage protein therapeutic candidate, dalantercept, we have conducted a single agent Phase 1 clinical trial in patients with advanced solid tumors. Of the 29 evaluable patients treated in this clinical trial, one had a partial response and 13 had stable disease, according to RECIST criteria. Additionally, we have studied the single agent activity of dalantercept ina Phase 2 clinical trial in patients with advanced head and neck cancer. Our ongoing focus is on the use of dalantercept in combination with an approved VEGF pathway inhibitor where we have provided both a mechanistic rationale and supportive preclinical data demonstrating dalantercept in combination with a VEGF pathway inhibitor provides enhanced anti-tumor effects in mice bearing human renal cell carcinoma xenographs. In an ongoing Phase 2 clinical trial of dalantercept in combination with axitinib, an approved VEGF pathway inhibitor, in patients with advanced renal cell carcinoma we have completed the dose escalation stage and identified dose levels of dalantercept that are well tolerated in combination with the FDA approved dose level of axitinib. We have now initiated the dose expansion phase of this study and plan to start the randomized controlled part of the study at the end of Q1 or early Q2 2014. We also intend to initiate a Phase 2 clinical trial of dalantercept in combination with the VEGF pathway inhibitor sorafenib in patients with liver cancer in the first half of 2014. In addition to our clinical stage programs, we are developing a novel protein therapeutic candidate, ACE-083, for a first-in-human clinical trial that we expect to initiate by the end of 2014. ACE-083 has been designed to promote muscle growth in those muscles in which the drug is injected, with minimal systemic effect. We are focused on the development of ACE-083 for diseases in which increases in the size and function of specific muscles may provide a clinical benefit, including inclusion body myositis, facioscapulohumeral dystrophy (FSHD) and disuse atrophy. We are developing sotatercept and ACE-536 through our exclusive worldwide collaborations with Celgene. As of January 1, 2013, Celgene became responsible for paying 100% of worldwide development costs for both programs. We may receive up to an additional $560.0 million of potential development, regulatory and commercial milestone payments and, if these protein therapeutic candidates are commercialized, we will receive a royalty on net sales in the low-to-mid 20% range. We will co-promote sotatercept and ACE-536, if approved, in North America for which our commercialization costs will be entirely funded by Celgene. We have not entered into a partnership for dalantercept and retain worldwide rights to this program. As of December 31, 2013, our operations have been funded primarily by $105.1 million in equity investments from venture investors, $86.8 million from investors in our initial public offering, $49.2 million in equity investments from our collaboration partners Celgene and Alkermes, Inc. (Alkermes) and $203.6 million in upfront payments, milestones, and net research and development payments from our collaboration partners. We were incorporated in the state of Delaware in June 2003 as Phoenix Pharma, Inc., and we subsequently changed our name to Acceleron Pharma Inc. and commenced operations in February 2004. Our principal executive offices are located at 128 Sidney Street, Cambridge, Massachusetts 02139, and our telephone number is (617) 649-9200. Our Internet website iswww.acceleronpharma.com. The information on, or that can be accessed through, our website is not part of this annual report, and you should not rely on any such information in making the decision whether to purchase our common stock.Our Strategy Our goal is to be a leader in the discovery, development and commercialization of novel protein therapeutics for cancer and rare diseases. Key components of our strategy are:•Advance sotatercept and ACE-536 into Phase 3 trials in collaboration with Celgene. We and Celgene are jointly developing sotatercept and ACE-536. Assuming successful completion of the ongoing Phase 2 clinical trials inb-thalassemia and MDS, we plan to initiate Phase 3 clinical trials with Celgene for one or both protein therapeutics in one or both diseases by the end of 2014 or early 2015.•Explore new indications for sotatercept and ACE-536 with Celgene. We and Celgene are continuing our preclinical research to assess the opportunity for sotatercept and ACE-536 to treat certain red blood cell disorders known as hemoglobinopathies, which include diseases such as thalassemias and sickle cell disease. Based on our encouraging preclinical and clinical data inb-thalassemia and our emerging understanding of the mechanism of action of these protein therapeutic candidates, we believe there is a potential for activity for sotatercept and ACE-536 in sickle cell disease, and we continue to explore development of these protein therapeutic candidates for this disease.•Advance dalantercept into Phase 3-enabling clinical trials. Beyond our ongoing Phase 2 clinical trials, in 2014, we plan to initiate additional clinical trials of dalantercept in combination with either an approved anti-angiogenesis agent or chemotherapy in advanced solid tumors. One of these trials is expected to be in patients with liver cancer and other trials may be in patients with brain cancer, lung cancer or colon cancer.•Utilize our discovery and development platform to develop additional protein therapeutic candidates. In addition to sotatercept, ACE-536 and dalantercept, all of which were internally discovered using our research and development platform, we intend to continue to discover and develop other protein therapeutics that target and regulate various pathways in the TGF-b superfamily. We plan to bring an additional protein therapeutic candidate, ACE-083, into the clinic in 2014 targeting diseases involving muscle loss. We are also conducting pre-clinical development of ALK1 pathway inhibitors distinct from dalantercept for the treatment of diseases of the eye including age-related macular degeneration. In addition we are developing new protein therapeutic candidates for the treatment of cancer and diseases involving fibrosis.•Strategically leverage collaborations to advance our protein therapeutic candidates. As of December 31, 2013, we have received more than $250.0 million from our corporate partners, including Celgene. Our two collaborations with Celgene for sotatercept and ACE-536 provide us with significant funding and access to Celgene's considerable scientific, development, regulatory and commercial capabilities. We will continue to strategically evaluate possible collaborations where doing so could enhance the development or commercialization of other protein therapeutic candidates in our pipeline.•Establish commercialization and marketing capabilities in North America and potentially other markets. We have retained co-promotion rights in North America for sotatercept and ACE-536, which will be entirely funded by Celgene. We intend to build hematology, oncology and neuromuscular disorder focused specialty sales forces and marketing capability to commercialize our protein therapeutic candidates that receive regulatory approval.The Acceleron Discovery Platform: Novel Approaches to Potent Biology Since our founding, we have focused on developing protein therapeutics that target a group of approximately 30 secreted proteins, or ligands, that are collectively referred to as the TGF-b superfamily. These ligands bind to subsets of 12 different receptors on the surface of cells, triggering intra-cellular changes in gene expression that guide cell growth and differentiation. The TGF-bTGF-beta superfamily ligands and their receptors represent a diverse and underexplored set of drug targets with the potential to yield potent therapeutics for the growth and repair of diseased cells and tissues.TGF-bTGF-beta superfamily and its receptors, we have generated our novel IntelliTrap™ platform technology and a robust pipeline of innovative clinical and preclinical protein therapeutic candidates targeting key mechanisms underlying cancer and rare diseases.Our Focus—The TGF-b Superfamily On a daily basis, the human body must orchestrate the growth and differentiation of cells to maintain and repair its cells and organ systems. Stem cells and precursor cells are undifferentiated cell types that reside in most tissues of the body. When tissue growth or regeneration is required, these undifferentiated cells divide and, through a series of intermediate stages, give rise to new, fully differentiated cells that build or repair the affected tissue. Decades of research have identified the TGF-b superfamily and its associated receptors as key regulators of the growth and differentiation of stem and precursor cells. Until recently, regulation of the erythropoietin pathway was the primary therapeutic approach to stimulate red blood cell formation. Members of the TGF-b superfamily are now recognized as important regulators of red blood cell formation. We have shown that inhibition of members of the TGF-b superfamily ameliorates anemia in mouse models ofb-thalassemia and MDS. Based on our findings, Additionally, we are developing two proteinconducting a multi-target antibody discovery collaboration with Adimab LLC, or Adimab, a leading antibody discovery company, under which Adimab is generating human antibodies against undisclosed targets that we select. We expect that this collaboration will expand our biologics platform and provide us with enhanced access to antibody therapeutic candidates, sotatercept and ACE-536, each of which is currently in Phase 2 clinical trials to treat patients with these diseases.candidates. Members of the TGF-b superfamily also play a significant role in regulating blood vessel formation. We and our academic collaborators have shown that mice with a genetic defect in a particular receptor for members of the TGF-b superfamily are resistant to tumor growth due to reduced blood vessel formation in the tumor. We have used this insight to design our Phase 2 anti-angiogenic agent, dalantercept, for the treatment of cancer. Members of the family are also significant regulators of muscle development. A genetic defect in a TGF-b superfamily ligand, known as myostatin, causes profound increases in skeletal muscle. A naturally occurring mutation in myostatin has been identified in animals, such as "double-muscled" breeds of cattle and in the "bully whippet" offspring of whippet racing dogs, which have been selectively bred to have increased muscle mass or function. Furthermore, a mutation in myostatin has been identified in a human family, members of which exhibit exceptional musculature and strength. We are actively working on preclinical programs to increase muscle mass and strength. Ligands of the TGF-b superfamily cause these profound biological effects by altering gene expression in target cells. As shown in the illustration below, a ligand of the superfamily initiates intracellular signaling by binding to a receptor that is located on the surface of a target cell. Upon binding to the ligand, the receptor activates specific transcription factors inside the target cell, which are called Smad proteins. The activated Smad proteins regulate gene expression and guide cellular growth and differentiation.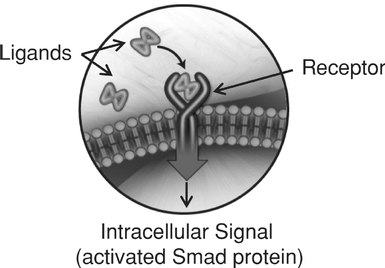
The TGF-b superfamily ligands are divided into subgroups termed the activins, the Growth and Differentiation Factors (GDFs), the Bone Morphogenetic Proteins (BMPs) and the TGF-b subgroup (for which the superfamily is named). Our clinical stage protein therapeutic candidates focus on the activin, GDF and BMP subgroups. We believe that, by employing our proprietary discovery and development platform, we can design protein therapeutic candidates that alter TGF-b superfamily signaling and unlock the therapeutic potential of this group of proteins.Acceleron Approach By combining the powerful biology of the TGF-b superfamily with our discovery and development expertise and our internal protein engineering and manufacturing capabilities, we have built a robust clinical and preclinical pipeline of protein therapeutic candidates targeting key mechanisms underlying cancer and rare diseases. We have taken a comprehensive, receptor-focused approach to access the biology of the TGF-b superfamily. We recognized that the 12 receptors for the superfamily act as control points for the ligands and therefore represent an attractive approach for pharmacological intervention. We have in-licensed patent rights for nine of the 12 receptors and systematically evaluated interactions between each receptor and a comprehensive panel of ligands. In the body, these ligands are naturally regulated by trap proteins that bind to the ligands thereby blocking ligand-receptor interactions and diminishing signaling in the cell. To mimic this natural regulatory approach, we have built our protein therapeutic candidates using the ligand-binding part of the receptors, depicted in the upper part of the figure below, as traps that capture the relevant groups of ligands in each biological process. We link the ligand-binding portion, the extracellular domain, of these receptors to the portion of a human antibody known as the Fc domain, depicted in the lower part of the figure below, which confers favorable pharmaceutical properties. The resulting "fused" proteins can be administered by simple intravenous or subcutaneous injection and reside in the blood for sufficient periods of time to permit dosing on a weekly or monthly basis.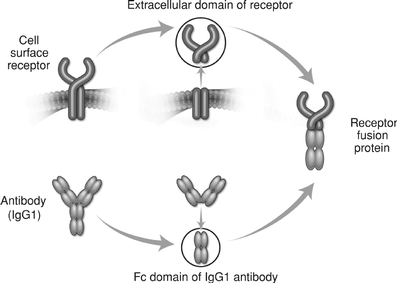
Protein therapeutics constructed this way are referred to as "receptor fusion proteins" or "ligand traps". Some of the most successful protein therapeutics on the market belong to this category including Enbrel® (etanercept), Eylea® (aflibercept) and Orencia® (abatacept). As shown in the figure below, our receptor fusion proteins act as ligand traps by binding to ligands of the TGF-b superfamily, preventing those ligands from binding to the cell surface receptors, and thereby preventing activation of Smad proteins in the target cell.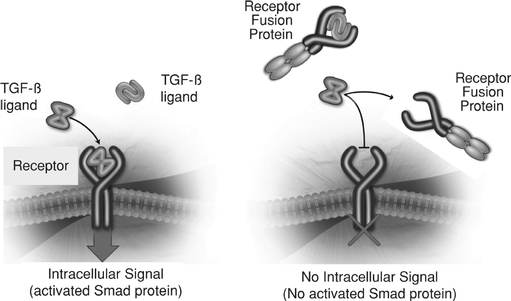
To take full advantage of our proprietary discovery and development platform, we have developed an integrated set of technologies and capabilities to rapidly and cost-effectively create, test and advance multiple protein therapeutic candidates. Our protein engineering expertise allows us to create and optimize our receptor fusion proteins. We have developed the capability to generate recombinant cell lines that produce our protein therapeutic candidates, and assess the activity of these molecules in animals using our internal animal pharmacology facility or the capabilities of our academic collaborators. We have also invested in infrastructure to manufacture Phase 1 and Phase 2 clinical material quickly and flexibly using our internal current good manufacturing practices, or cGMP, compliant protein production facility to support clinical development of our protein therapeutic candidates. protein therapeutic candidates. Our robust clinical and preclinical pipeline is focused on areas of high-unmet medical need, particularly in the areas of cancer and rare diseases.Our Product Pipeline We have four development stage protein therapeutic candidates, of which three are currently in numerous ongoing clinical trials and the fourth we expect to begin human clinical trials by the end of 2014. Celgene is currently conducting four Phase 2 clinical trials and overseeing three investigator-sponsored trials with sotatercept. We are conducting two Phase 2 clinical trials with ACE-536, two Phase 2 clinical trials with dalantercept and overseeing a collaborative group-sponsored Phase 2 clinical trial of dalantercept. We expect to initiate a Phase 2 clinical trial with dalantercept in patients with hepatocellular carcinoma in the first half of 2014 and to initiate a Phase 1 clinical trial with ACE-083 by the end of 2014.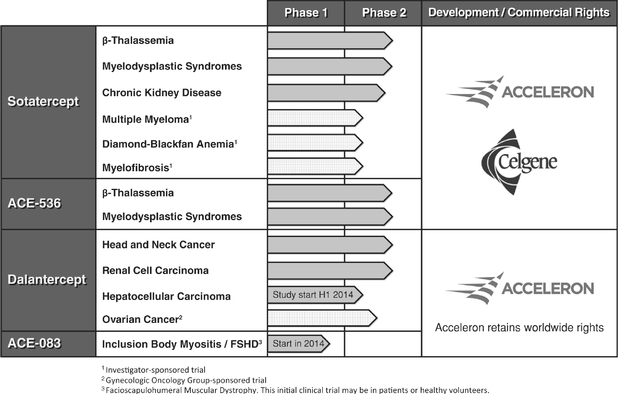
Sotatercept and ACE-536Anemia in Patients with ß-thalassemia and MDS Erythropoiesis, the process by which precursor cells proliferate and differentiate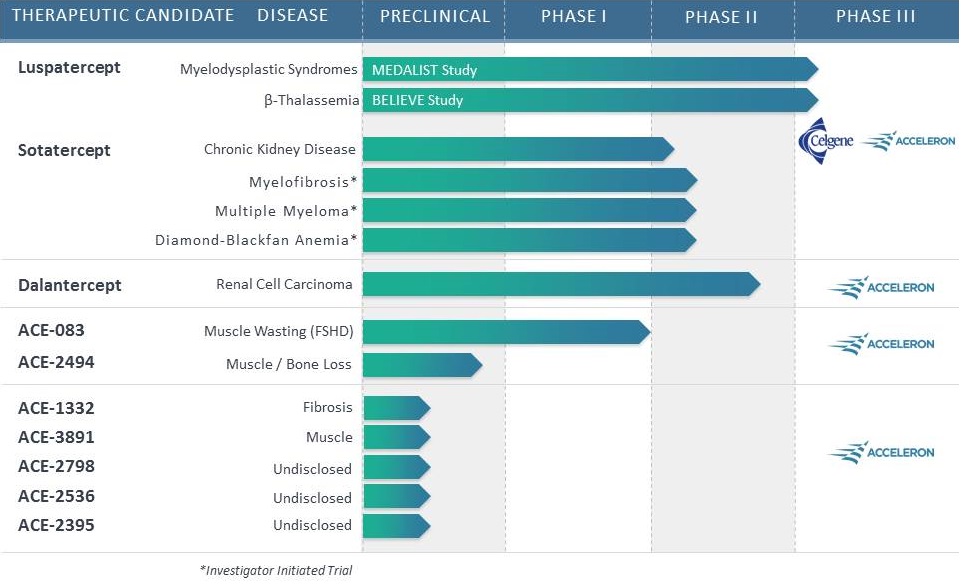
give rise to red blood cells, is one of the most important and active processes in human biology. The primary role of red blood cells is to carry and deliver oxygen to other cells throughout the body. At any given time, there are approximately 25 trillion red blood cells in normal adult circulation which account for roughly 25% of the body's total number of cells. The human body produces 2.4 million new red blood cells each second. Red blood cell formation starts in the bone marrow with cells referred to as red blood cell precursors. These precursor cells go through many rounds of cellular proliferation, combined with cellular differentiation, to become more specialized cells to carry out their role as mature, functional red blood cells. We believe this highly active process ofpromote red blood cell production is normally tightly controlled by positive and negative regulators of the erythropoietic process. Erythropoietin isthrough a positive regulator that stimulates proliferation of early red blood cell precursor cells, the BFU-E and CFU-E cells depicted in the figure below. Based on our research, it is now recognized that certain ligands in the TGF-b superfamily are negative regulators of red blood cell precursors, starting with the Pro-E cells and those that follow, as depicted in the figure below. These members of the TGF-b superfamily restrain the maturation of these precursors into later stage precursors and ultimately into functional red blood cells (RBCs).Depiction of Normal Erythropoiesis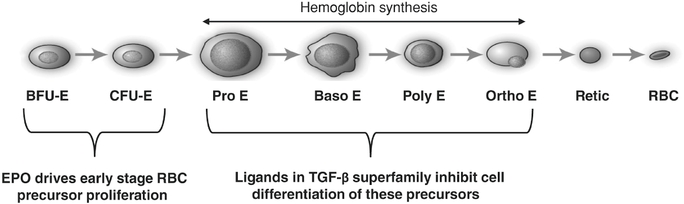
In certain diseases, the highly active process of red blood cell production does not function properly, leading to a reduction in the number of functional red blood cells, a condition known as anemia. Anemia in some disease settings is currently treated by the use of erythropoiesis stimulating agents, such as recombinant erythropoietin, that stimulate proliferation of early stage precursors of red blood cells. However, in certain diseases, such asb-thalassemia and MDS, anemia is caused by defects in the production of late stage red blood cell precursors, which is known as ineffective erythropoiesis. Anemias caused by ineffective erythropoiesis are not well-treated by current therapies. As shown in the illustration below, ineffective erythropoiesis is characterized by an over-abundance of early stage red blood cell precursors and a decreased ability of late stage precursor cells to properly differentiate into healthy, functional red blood cells. The resulting anemia stimulates the body's overproduction of erythropoietin, which exacerbates the over-abundance of early stage precursors. Because the defective step in ineffective erythropoiesis lies downstream of the early stage precursors, the increase in the number of these cells fails to resolve the anemia.Depiction of Ineffective Erythropoiesis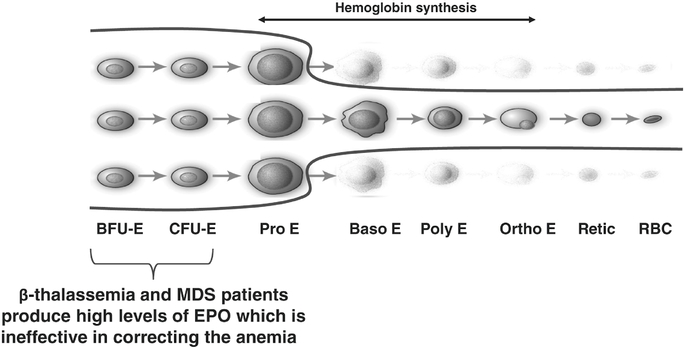
Based on our preclinical research, we believe that TGF-b superfamily ligands function as negative regulators of erythropoiesis by inhibiting the maturation of these early stage red blood cell precursors. Both sotatercept and ACE-536 are ligand traps designed to inhibit these negative regulators of late stage red blood cell precursors and promote their maturation into functional red blood cells.sotatercept and ACE-536,luspatercept, through our collaborations with Celgene, as treatmentsa treatment for anemia and associated complications in diseases in which erythropoiesis-stimulating agents are either not approved or are not well-suited to treat the underlying anemia. Inanemia, such as beta-thalassemia and MDS.such asb-thalassemiacharacterized by abnormal proliferation and MDSdifferentiation of blood precursor cells, including red blood cell precursors, in which anemia is caused by ineffective erythropoiesis, we believe both sotatercept and ACE-536 may help correct this defective process. Although similarthe bone marrow. This leads to peripheral reductions in terms of their effects on red blood cells, thereoften accompanied by decreases in white blood cells and platelets, as well as a risk of disease progression to acute myeloid leukemia. Although MDS patients may have varying forms of the disease, anemia is present in the vast majority of MDS patients at the time of diagnosis. MDS is primarily a disease of the elderly, with 88% of cases diagnosed in individuals 60 years of age or older. Cancer surveillance databases estimate the annual incidence of MDS in the United States at 10,000 to 15,000 cases and the overall U.S./EU prevalence at approximately 125,000 patients.differencesclassified as lower risk and 30% are classified as higher risk. High risk patients are typically treated with inhibitors of DNA methyltransferase such as Vidaza® or Dacogen®, or generic versions that are now available in howsome countries. The patients categorized as low risk typically receive erythropoiesis stimulating agents as first-line therapy, though erythropoiesis stimulating agents are not approved by the FDA or the EMA for the treatment of anemia in MDS patients. Our internal market research estimates that erythropoiesis stimulating agents generate $500 to $700 million in annual U.S. sales from their use in this disease. After failure on erythropoiesis stimulating agents, patients are treated with red blood cell transfusion and/or Revlimid®, Vidaza® or Dacogen®. Across the disease, approximately 15% of patients have a specific chromosomal mutation and are treated with Revlimid® (2015 U.S. sales of $152 million for MDS).two protein therapeutic candidates bindMDS patients is not a consequence of erythropoietin deficiency. The ineffective erythropoiesis of MDS may be caused by excess signaling by members of the TGF-beta superfamily, which signaling inhibits red blood cell maturation. For this reason we believe that blocking this excess signaling by luspatercept may reverse this inhibition. Approximately 50% of MDS patients are unresponsive to the administration of recombinant erythropoietin and inhibit ligands. Unlike ACE-536, sotatercept bindsinstead require red blood cell transfusions, which can increase the risk of infection and iron-overload related toxicities. Treatment-resistant anemia resulting from ineffective erythropoiesis is a major cause of morbidity in MDS patients.inhibits activin A,ineffective erythropoiesis. Treatment with a TGF-b superfamily ligand,mouse version of luspatercept, referred to as RAP-536, reduced P-SMAD2/3 levels and has been showncaused statistically significant increases in red blood cell count, hemoglobin levels and hematocrit compared to increasecontrols. Additionally, RAP-536 reduced the ineffective erythropoiesis as evidenced by the improvement in the ratio of red blood cell precursors to other cells in the bone mass and biomarkers of bone formation inmarrow.trials. Given its effects on bone, sotatercept is being studiedtrial with luspatercept in patients with chronic kidney disease, where itvery low, low and intermediate risk MDS per the Revised International Prognostic Scoring System, the "MEDALIST" trial. The MEDALIST trial targets patients with very low, low or intermediate risk MDS with ring sideroblasts who require RBC transfusions. The trial is double-blinded, placebo-controlled and will enroll an estimated 210 patients randomized 2:1, luspatercept versus placebo. In order to enroll in the trial, patients must be: refractory / intolerant to prior erythropoiesis stimulating agents (ESA) or ESA ineligible, ring sideroblast positive, receive a transfusion of at least 2 units of RBCs every 8 weeks confirmed for a minimum of 16 weeks with no consecutive 8-week period free from transfusion, and no prior lenalidomide, hypomethylating agents or immunosuppressive therapy. Patients are excluded from the study if they have del(5q) or secondary MDS. The primary endpoint for efficacy analysis will be the proportion of patients who become RBC-transfusion independent for a period of at least 8 weeks during the first 24 weeks of treatment.the potentialbeen designed to treatfocus on patients with this particular cellular morphology. The most common adverse events observed in this extension study, which may be related to luspatercept, were bone pain, headache, hypotonia, myalgia and nausea. There were no drug-related serious adverse events.mineral and bone disorder. In addition, in preclinical studies, sotatercept inhibits the growthalleviate other disease complications, such as leg ulcers.thalassemiasbeta-thalassemias comprise a heterogeneous group of disorders arising from defects in the genes that encode the proteins that comprise hemoglobin. Hemoglobin is a four-subunit protein complex formed of twoa-subunits alpha-subunits and twob-subunits, beta-subunits, each with an iron-containing heme group that binds to and carries oxygen molecules within red blood cells. There are two main classifications of thalassemia,a-thalassemia alpha-thalassemia andb-thalassemia, beta-thalassemia, depending on whether the genetic defect lies in the gene encoding thea-subunit alpha-subunit or theb-subunit.b-thalassemia beta-subunit. Beta-thalassemia is particularly prevalent throughout the Mediterranean region, Middle East, and Southeast Asia, and, due to migration and immigration, is now a global disease. The Thalassaemia International Federation estimates that there are approximately 300,000 patients worldwide withb-thalassemia, beta-thalassemia, approximately 20,000 of which are in the United States and Europe, who are dependent on frequent blood transfusions. We estimate that there are at least as manyb-thalassemia beta-thalassemia patients in the same regions who are not transfusion dependent and not included in these estimates. Many of these patients have hemoglobin levels that are approximately half that of normal individuals and experience significant complications of the disease. Anemia ofb-thalassemia Beta-thalassemia is treated primarily a result of ineffective erythropoiesis. The genetic defect leads to decreased production of theb-subunits of hemoglobin resulting in an excess amount of thea-subunits. In normal erythropoiesis, excess unpaireda-subunits are eliminated by a cellular component called the proteasome. The proteasome is normally required for effective red blood cell maturation to selectively remove cellular components and organelles such as mitochondria which are replaced by hemoglobin, which constitutes 90% of the protein in a mature red blood cell. In thalassemia, the proteasome becomes saturated with the abnormally high levels of unpaireda-subunits and is unable to remove other cellular components and participate in the maturation process; this causes the block in maturation. Moreover, those freea-subunits that are not eliminated by the proteasome form aggregates, called hemichromes, which damage the maturing red blood cells. These hemichromes, along with the saturation of the proteasome by unpaireda-subunits, contribute to the ineffective erythropoiesis ofb-thalassemia. The damaged red blood cells are filtered out by the spleen and have a reduced life span, resulting in anemia and enlargement of the spleen. Patients with the most severe form ofb-thalassemia produce few, if any,b-subunits, resulting in an increased amount of freea-subunits and consequently a high number of hemichromes. These patients typically present with life-threatening anemia within the first year of life and require regular and lifelong red blood cell transfusions usually every 2that, over time, cause a toxic accumulation of iron in the body. A central challenge for managing patients with beta-thalassemia is to 4 weeks. Becauserestore the red blood cells contain significant amounts ofcell levels while avoiding iron this intensive transfusion regimen contributes to a condition known as ironoverload, which is the principal cause of mortality. Consequently, therapy to reduce iron overload, called iron chelation therapy, is also part of standard treatment in these patients and typically begins after patients have received approximately 20 units of blood from transfusions during their lifetime.overload. Iron chelation therapy alone costs between $25,000$40,000 and $40,000$60,000 per year in countries such as the United States and Italy and yet does not treat the underlying anemia. The course of the disease depends largely on whether patients are maintained on an adequate transfusion and iron chelation regimen. Poor compliance with transfusion and/or iron chelation is associated with a poor prognosis and shortened survival. However, even with the standard of care, patients are at risk of infection from transfusions as well as toxicities related to iron chelation therapy. Patients with an intermediate form ofb-thalassemia, who are not necessarily dependent on frequent transfusions early in life, nevertheless suffer from a wide range of debilitating conditions. The ongoing ineffective erythropoiesis leads to various complications affecting a wide range of organ systems. By the second decade of life, most of these patients' hemoglobin levels have declined to the 6-8 g/dL range, or approximately half that of normal individuals. In an attempt to correct this chronic anemia, the body produces high levels of erythropoietin resulting in a continued stimulation of the early red blood cell precursors in the bone marrow. The number of these precursors grows to such an extent in the bone marrow that it leads to skeletal deformities, porosity of the long bones, and bone fractures. Splenomegaly, or enlargement of the spleen, is the result in part of continuous clearance by the spleen of the malformed red blood cells damaged by hemichromes. This commonly leads patients to require removal of their spleen, which in turn leads to worsening of other complications, such as blood clots. Iron overload is another significant complication even in the absence of red blood cell transfusions. This is due to increased intestinal iron absorption as a result of the ongoing ineffective erythropoiesis. Patients also suffer from various endocrine disorders due, in large part, to the accumulation of iron in the endocrine glands. Importantly, iron can also accumulate in the liver and heart, leading to severe complications such as liver fibrosis and heart failure.b-thalassemia. beta-thalassemia. Hematopoietic stem cell transplantation is viewed as the only curative approach forb-thalassemia, beta-thalassemia, although this option is limited by the availability of appropriate donors and by risks, including death, associated with the bone marrow transplant procedure. Consequently this treatment is used only in the most severely affected patients.Myelodysplastic Syndromes Myelodysplastic syndromes, or MDS, are a group of heterogeneous hematologic diseases characterized by abnormal proliferation and differentiation of blood precursor cells, including red blood cell precursors, inGiven the bone marrow. This leads to peripheral reductions in red blood cells, often accompanied by decreases in white blood cells and platelets, as well as a riskeffects of disease progression to acute myeloid leukemia. Anemia is present in the vast majority of MDS patients at the time of diagnosis. MDS is primarily a disease of the elderly, with 88% of cases diagnosed in individuals 60 years of age or older. Cancer surveillance databases estimate the annual incidence of MDS in the United States at 10,000 to 15,000 cases and the overall U.S. prevalence at approximately 30,000 to 60,000 patients. Hematopoietic stem cell transplantation represents the only treatment modality with curative potential, although the relatively high morbidity and mortality of this approach limits its use. Approximately 23% of MDS patients are categorized as intermediate-2 to high risk. These patients are typically treated with inhibitors of DNA methyltransferase such as Vidaza® (2012 U.S. sales of $324.0 million for MDS) or Dacogen® (2012 U.S. sales of $233 million for MDS). Of the remaining 77% of patients categorized as low to intermediate-1 risk, approximately 10% have a specific chromosomal mutation and are typically treated with Revlimid® (2012 U.S. sales of $257.0 million for MDS). The remaining 67% of patients typically receive red blood cell transfusions or erythropoiesis stimulating agents, though erythropoiesis stimulating agents are not approved by the FDA or the EMAfor the treatment of anemia in MDS patients. Our internal market research estimates that erythropoiesis stimulating agents generate $500 to $700 million in annual U.S. sales from their use in this disease. The anemia in MDS is primarily due to ineffective erythropoiesis, and a significant number of MDS patients have serum erythropoietin levels substantially above the normal range, indicating that the anemia in these MDS patients is not a consequence of erythropoietin deficiency. The ineffective erythropoiesis of MDS may be caused by excess signaling by members of the TGF-bTGF-beta superfamily which signaling inhibits red blood cell maturation. For this reasonligands on late-stage erythropoiesis, we believe that blocking this excess signaling by sotatercept or ACE-536 may reverse this inhibition. Approximately 50% of MDS patients are unresponsive to the administration of recombinant erythropoietin and instead require red blood cell transfusions, which can increase the risk of infection and iron-overload related toxicities. Treatment-resistant anemia resulting from ineffective erythropoiesis is a major cause of morbidity in MDS patients.Chronic Kidney Disease Anemia is a common complication of chronic kidney disease. Because erythropoietin is produced primarily in the kidney and to a lesser extent in the liver, patients with chronic kidney disease produce sub-optimal amounts of erythropoietin, which leads to anemia. Additional serious complications of chronic kidney disease include a condition known as chronic kidney disease—mineral and bone disorder that occurs when the diseased kidneys fail to maintain proper levels of calcium and phosphorous in the blood, leading to abnormal bone hormone levels, weakened bones and vascular calcification. Bone and vascular disorders are common complications in people with chronic kidney disease and bone disorders affect almost all patients receiving dialysis. According to the United States Renal Data System, there are over 400,000 chronic kidney disease patients receiving dialysis in the United States. Erythropoiesis stimulating agents have been approved for this indication for over twenty years. Sotatercept has the potential to differentiate itself from erythropoiesis stimulating agents in this patient population because of its positive effects on bone metabolism observed following the administration of sotatercept in preclinical models, healthy volunteers and cancer patients. Additionally,investigated our candidate therapeutics in mouse models of vascular calcification, sotatercept causedthis disease. We evaluated the effects of RAP-536 in a reductionseries of calcified depositsstudies using a mouse model of beta-thalassemia. These mice carry deletion mutations in the aorta.Sotatercept Clinicalbeta-globin genes, resulting in a deficiency of beta-globin protein and Preclinical Developmenthematologic abnormalities very similar to those seen in human beta-thalassemia patients, including severe anemia and the formation of hemichromes resulting in ineffective erythropoiesis. These mice also exhibit severe complications common in patients with thalassemia, such as an enlarged spleen, bone loss and iron overload. As reported in our 2014 publication in the journal Sotatercept isBlood, RAP-536 treatment improved numerous hematologic parameters in these mice, including a soluble receptor fusion protein consistingdecrease in hemoglobin aggregates, significant increases in red blood cell count, hemoglobin levels, and hematocrit, decreased serum erythropoietin, normalized red blood cell size, and reduced red blood cell breakdown, as measured by serum bilirubin. Importantly, RAP-536 decreased the elevated levels of the extracellular domainactivated transcription factor, P-SMAD2/3, restored iron homeostasis and improved the maturation of the activin receptor type IIA (ActRIIA) linked to the Fc domain of human IgG1. Sotatercept acts as a protein trap for TGF-b superfamily ligands that signal through the ActRIIA receptor. Sotatercept has increasedlater-stage red blood cellscell precursor populations, in multiple clinical trials.the bone marrow and spleen, with concomitant reductions in the earlier-stage red blood cell precursor populations.Ongoing Phase 2 Clinical Trials of Sotatercept Our collaboration partner,is currently conducting four Phase 2 clinical trials of sotatercept in patients withb-thalassemia, MDS and chronic kidney disease. The FDA has granted orphan designation for sotatercept for the treatment ofb-thalassemia. We understand that Celgene plans to submit an application for orphan drug designation of sotatercept for treatment of MDS. Through collaborations with leading academic institutions, Celgene is also overseeing three investigator-sponsored trials.Celgene-Sponsored Clinical Trials ß-thalassemia. Celgene is conductinginitiated a Phase 23 clinical trial of sotatercept designed aswith luspatercept in regularly transfused patients with beta-thalassemia, the "BELIEVE" trial. The BELIEVE trial targets adult beta-thalassemia patients who are regularly transfused. The trial is double-blinded and placebo-controlled and will enroll an ascending dose studyestimated 300 patients randomized 2:1, luspatercept versus placebo. In order to determine the safety and efficacy of sotatercept in adults withb-thalassemia.The dose levels to be studied are 0.1, 0.3, 0.5 mg/kg and 0.75 mg, with the possibility of further dose escalation up to 1.0 and 1.5 mg/kg, given subcutaneously once every three weeks for a period of 6 cycles with continued treatment at the discretion of the investigator for up to 22 months. Each cohort includes six or more patients during the dose escalation phase, followed by an expansion phase at a selected dose level in up to ten additional patients. The first patientenroll in the trial, was first dosed in November 2012. Celgene has completed enrollingpatients must receive 6-20 units RBC transfused over the 0.1, 0.3,prior 24 weeks and 0.5 mg/kg cohorts and is now enrolling patients in the 0.75 mg/kg cohort.have no transfusion-free period ≥ 35 days. Patients will be monitored for a 12-week prospective pre-treatment period to calculate baseline transfusion burden. The primary outcome measureendpoint for efficacy analysis will be the proportion of patients with at least a 33% reduction in transfusion burden during weeks 13 to 24 of the trial is to identify a safe dose level and to measure efficacy (1) in transfusion dependent patients by a reduction of transfusion burden by³ 20% compared to the pretreatment transfusion burden for each patient and (2) in non-transfusion dependent patients by an increase in hemoglobin level by³1 g/dL compared12 weeks preceding treatment.baseline hemoglobin, sustained for 12 weeks. This trial will also evaluate as exploratory endpoints the effects of sotatercept on iron overload, which is an important cause of morbidity and mortality associated withb-thalassemia, and bone metabolism. The trial is being conducted in six sites in Italy, France, Greece, and the United Kingdom and may enroll approximately 65 patients. Sotatercept has generated encouraging preliminary data in the ongoing Phase 23 clinical trial, of sotatercept inb-thalassemia patients. As shown in the figure below, sotatercept has generated encouraging dose-dependent increases in hemoglobin levels in patients in the Phase 2 clinical trial whowe are non-transfusion dependent based on preliminary data from the three lowest dose levels in that trial.Mean Change in Hemoglobin Level From Baseline by Dose Cohort inNon-Transfusion Dependentb-Thalassemia Patients Treated With Sotatercept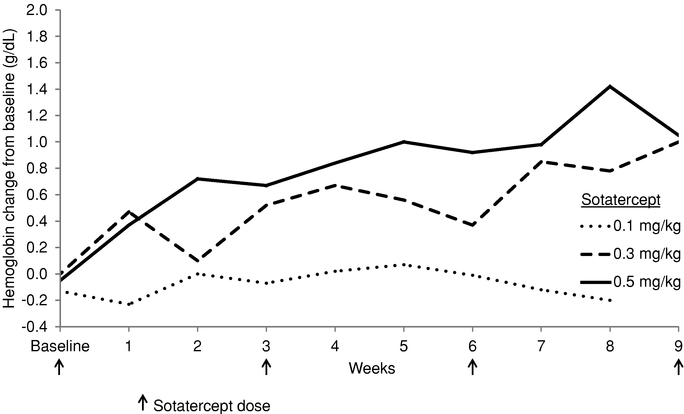
Another analysis of the data from this trial also shows dose-dependent increases in hemoglobin levels. In the analysis shown below, within the first two months of receiving the first dose of sotatercept:•84% of non-transfusion dependent patients in each of the 0.5 and 0.3 mg/kg dose levels achieved at least a 1 g/dL increase in hemoglobin, while none of the non-transfusion dependent patients at the lowest dose level (0.1 mg/kg) achieved this threshold.•33%, 16% and 0% of non-transfusion dependent patients achieved a hemoglobin increase of at least 2 g/dL in the 0.5, 0.3, and 0.1 mg/kg dose levels, respectively.Maximum Change in Hemoglobin Level From Baseline in Non-Transfusion Dependentb-Thalassemia Patients During the First 3 Cycles (Day 64) of Sotatercept Treatment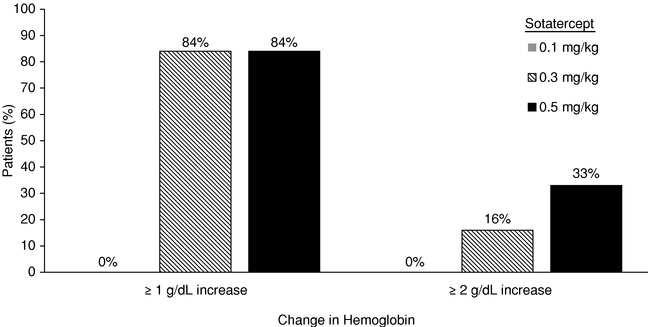
The figure below shows that there is a statistically significant relationship (p<0.001) between drug exposure and the maximum increase in hemoglobin during the first three cycles across the three lowest dose levels of sotatercept. The x-axis shows the levels of sotatercept in patients' serum and the y-axis shows the patients' maximum change in hemoglobin. The figure illustrates that as the drug exposure in patients increases, so does the maximum increase in hemoglobin.Relationship Between Drug Exposure and Hemoglobin Level in Non-Transfusion Dependentb-Thalassemia Patients Through the First 3 Cycles (Day 64) of Sotatercept Treatment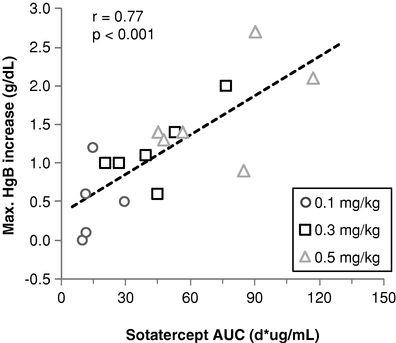
Only patients completing the first 3 planned treatment cycles are included.(AUC) area under the curve; HgB, hemoglobin We expect Celgene to establish a range of recommended sotatercept dose levels, based on these data and additional data to be gathered as the clinical trials continue. We expect that in future clinical trials, patients will begin treatment at a recommended starting dose level and to undergo individualized dose titration based on hemoglobin response and tolerability to achieve and maintain an appropriate hemoglobin level. We expect Celgene to continue to dose escalate in this trial with the objective to determine the dose range for evaluation in the expansion stage of this trial. If this activity is confirmed with an acceptable safety profile, we and Celgene plan to initiate pivotal trial(s) inb-thalassemia by the end of 2014 or early 2015. At the dose levels that have been studied to date, we have not yet observed an effect in the transfusion dependent patients. Based on currently projected timelines, which are subject to change, we expect additional data from this clinical trial to become available as follows: data from additional dose levels and extended treatment in patients from the dose escalation portion of the clinical trial in the second quarter of 2014, and additional data in the fourth quarter of 2014. MDS. Celgene is conducting a Phase 2 clinical trial of sotatercept for the treatment of anemia in patients with low-or intermediate-1 risk MDS. The dose levels to be studied are 0.1, 0.3, 0.5 and 1.0 and up to 2.0 mg/kg given subcutaneously once every three weeks for five cycles, and up to three additional cycles for late responders, with continued treatment at the discretion of the investigator. Each cohort may include up to 20 patients receiving a single dose level during the dose escalation phase, followed by an expansion phase at a selected dose level in up to 15 additional patients. The first patient in the trial was first dosed in December 2012. Celgene has currently completed the 0.1, 0.3 and 0.5 mg/kg cohorts and is now enrolling patients in the 1.0 mg/kg cohort. Dose escalation may go up to 2.0 mg/kg. The primary outcome measure is erythroid hematological improvement (HI-E). For patients who require transfusions of <4 units of red blood cells in the eight weeks prior to dosing, HI-E is an increase in hemoglobin of³1.5 g/dL sustained over a period³8 weeks in the absence of red blood cell transfusions. For subjects that require transfusions of³4 units of red blood cells in the eight weeks prior to dosing, HI-E is a decrease of³4 units of red blood cells transfused over a period of eightweeks compared to the number of units transfused in the eight weeks prior to treatment. This trial will also evaluate the effects of sotatercept on iron overload and bone metabolism. The trial is being conducted at up to 23 sites in the United States and France and may enroll up to 115 patients. Based on currently projected timelines, which are subject to change, we expect additional data from this clinical trial to become available as follows: data from the dose escalation portion of the clinical trial in the second quarter of 2014, and additional data in the fourth quarter of 2014.Chronic Kidney Disease. Celgene is conducting two Phase 2 clinical trials with sotaterceptof luspatercept in patients with chronic kidney disease.beta-thalassemia. The first is a Phase 2 clinical trial with sotatercept designed as a randomized, placebo-controlled dose escalation study to evaluate the pharmacokinetics, safety, efficacy, tolerability and pharmacodynamics of sotatercept for the correction of anemia in patients with chronic kidney disease on hemodialysis. The first patient in the trial was first dosed in August 2010. The first dose level was 0.1 mg/kg administered subcutaneously as a single dose. Subsequent dose levels to be studied are 0.3, 0.5 and 0.7 mg/kg administered subcutaneously once every four weeks for up to eight cycles. Each cohort will include up to 12 (nine sotatercept-treated and three placebo-treated) patients receiving a single dose level during the dose escalation phase, followed by an additional cohort at a selected dose level. Celgene has completed enrollment in the 0.1, 0.3 and 0.5 mg/kg cohorts and is now enrolling patients in the 0.7 mg/kg cohort. The primary endpoints are pharmacokinetics and safety. Other endpoints include effects on hemoglobin and serum markers of bone metabolism. The trial is being conducted at up to 21 sites in the United States and may enroll up to 56 patients. Early data from this trial are encouraging. An interim analysis from this clinical trial indicates that sotatercept produces dose dependent increases in hemoglobin in end stage renal disease patients on hemodialysis. The data will be presented at the National Kidney Foundation Spring Clinical Meeting in April 2014. Based in part on these interim data, and previously observed effects of sotatercept on bone biomarkers, Celgene has initiated a second phase 2 clinical trial in Europe with sotatercept in patients with end stage renal disease (ESRD) who are on hemodialysis. The first patient in this trial was first dosed in December 2013. The study is designed as a two-part studytrial, with an ascending dose part to assessevaluate the safety and efficacy of sotatercept asluspatercept in patients with beta-thalassemia, and an expansion part in which additional patients are enrolled at a therapy to treat anemiaselected dose level (3-month base study). We have currently completed enrollment and to control the adverse manifestationstreatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Patients in both partsall of the study must first be ondose escalation cohorts as well as the expansion cohort of the trial. Patients enrolled in the initial 3-month trial are eligible to enroll in a stable dosesecond Phase 2 trial (extension study) that permits dosing with luspatercept for up to an additional two years. This trial is currently being conducted at sites in Italy and Greece.an erythropoiesis stimulating agent (ESA) to maintain hemoglobin levelsSeptember 25, 2015, at the 57th ASH Annual Meeting and after an ESA treatment free periodExposition in December 2015. As of approximately five days, will then be switched to treatment with sotatercept. The first part isthe cut-off date, a dose-escalation studytotal of intravenous and subcutaneous routes of administration of sotatercept in approximately 6064 patients to evaluate pharmacokinetics, safety and tolerability. Patientswere treated in the dose escalation partand expansion cohorts of thethis study, will be given sotaterceptin which luspatercept was administered subcutaneously, once every two weeks up to a3 weeks. A total of eight doses59 patients were evaluable for efficacy (5 patients were ongoing with <12 weeks treatment). Of these 59 patients, 31 were non-transfusion dependent and followed for approximately four months after their last dose. The first part28 were transfusion dependent. Specifically, 22 of the study is designed to inform the dosing regimens to be tested28 (79%) transfusion dependent patients had a ≥20% reduction in transfusion burden, 21 of 28 (75%) had a ≥33% reduction, and 16 of 28 (57%) had a ≥50% reduction over a 12-week period.second partmajority of non-transfusion dependent patients with or without iron chelation therapy, and in the clinical trial.majority of transfusion dependent patients receiving iron chelation therapy. Improvement in quality of life in non-transfusion dependent patients correlated with increase in hemoglobin. Rapid healing of leg ulcers, a serious complication of beta-thalassemia, was observed in 3 patients, with 2 additional patients experiencing partial healing. The second part will bemost common related adverse events were bone pain, myalgia, headache, arthralgia, musculoskeletal pain, asthenia, injection site pain, back pain and pain in jaw. There were no drug-related serious adverse events. 6 of 59 (10%) patients discontinued early with an associated adverse event: bone pain (2 patients) and arthralgia, asthenia, cerebrovascular accident and headache (1 patient each).randomized, controlled study of approximately 230 patients to evaluate the efficacydisorder characterized by anemia and safety of sotatercept versus an erythropoiesis stimulating agent. Efficacy measures for part two of the study include the change in mean hemoglobin concentration from baseline and the ability of sotatercept to maintain patients' hemoglobin levels within a target range after switching from an ESA to sotatercept. Measures of biomarkers for bone formationmineral and bone resorptiondisorder that leads to bone loss and forcardiovascular disease. The mineral metabolism also will be studied, alongand bone disorder in these patients is not well-managed with imagingcurrent therapies. Studies in mice show that sotatercept may have beneficial effects on fibrotic damage to the kidney and on the development of calcified deposits that may contribute to the elevated risk of heart vascular disease in CKD patients. Data from our ongoing Phase 2 clinical trial in patients with end-stage kidney disease shows that sotatercept may have beneficial effects on the mineral and bone disorder in these patients and may decrease the accumulation of vascular calcification.Investigator Sponsored Trials Through collaborations with leading academic institutions, Celgene is overseeing investigator-sponsoredalso being studied through investigator-initiated clinical trials in multiple myeloma, Diamond-Blackfan anemia and myelofibrosis.••adultsadult patients with Diamond-Blackfan anemia who are red blood cell transfusion-dependent.•Completed Clinical Trials Six humantrials of sotatercept, including Phase 1 clinical trials in healthy volunteers and Phase 2 clinical trials of patients with multiple myeloma, breast cancer, and non-small cell lung cancer, collectively involving over 160 patients have been conductedstage therapeutic candidate, dalantercept, is designed to date. In healthy volunteers, we observed increases in red blood cells and hemoglobin. The mean change in hemoglobin for the patients who received a single dose of 1.0 mg/kg was almost 3 g/dL, which is similar to receiving a transfusion of three units of blood. We have also shown that in a randomized, placebo-controlled trial in patients with multiple myeloma receiving melphalan, prednisolone and thalidomide, sotatercept produced dose-dependent increases in hemoglobin. In the placebo and 0.1 mg/kg sotatercept cohorts, none of the patients achieved at least a 1.5 g/dL increase in hemoglobin at day 29 of the trial compared to their baseline levels. In the 0.3 and 0.5 mg/kg sotatercept cohorts, 13% and 38% of the patients, respectively, achieved at least a 1.5 g/dL increase in hemoglobin at day 29 of the trial compared to their baseline levels. In a randomized, placebo-controlled clinical trial in breast cancer patients who had anemia due to myelosuppressive chemotherapy, sotatercept produced dose-dependent increases in hemoglobin levels. In both the placebo and 0.1 mg/kg sotatercept cohorts, 20% of the patients had their hemoglobin levels increase to at least 11 g/dL maintained for 28 days in the absence of a red blood cell transfusion or use of an erythropoiesis stimulating agent. In the 0.3 mg/kg cohort, 22% of the patients achieved this outcome and in the 0.5 mg/kg cohort, 75% of the patients achieved this threshold. In a randomized, dose-ranging Phase 2 trial of sotatercept in patients with metastatic non-small cell lung cancer, sotatercept, administered at a fixed dose of 15 or 30 mg given subcutaneously every six weeks, produced increases in hemoglobin. In patients who did not receive red blood cell transfusions within the first four weeks, the change from baseline was at least 1 g/dL of hemoglobin for 40% of patients at week 2 and 16% of patients at week four. Given the results of these trials, we and Celgene may decide to pursue further clinical development in the future in one or more of these indications.Safety Across the completed clinical trials, sotatercept has been generally well-tolerated. In studies with healthy volunteers, the only treatment-related serious adverse event was a report of persistent, progressive high blood pressure in one subject. While the precise cause of elevated blood pressure cannot be determined, it was an expected consequence of elevated red blood cell levels that occurred in this subject. Commonly observed adverse events included headache, infection, dizziness, hypertension, hot flush, tingling, muscle spasms, limb injury, fatigue and asthenia. In three studies of patients withcancer (myeloma, breast and lung cancer), one sudden death was reported in a myeloma patient. The event was evaluated as probably related to the concurrent anti-myeloma therapy of melphalan, prednisolone and thalidomide and possibly related to sotatercept. One patient with advanced breast cancer experienced serious adverse events of perforated gastric ulcer and peptic ulcer disease that were evaluated as possibly related to sotatercept. One patient with advanced lung cancer experienced a serious adverse event of a cerebrovascular accident (blockage of atreat cancers by inhibiting blood vessel information by inhibiting signaling through the brain) that was suspected as related to treatment. AmongALK1 receptor. This mechanism is distinct from, and potentially synergistic with, the ongoing clinical trials managed by Celgene, no treatment-related serious adverse events have been reported in the MDS trial. In theb-thalassemia trial, two patients have exhibited serious adverse events that were suspected as related to sotatercept: bone pain and superficial thrombophlebitis (an inflamed blood clot in a superficial vein) . One patient at the 0.5 mg/kg dose level had a treatment-related Grade 3 adverse event of ventricular extrasystoles (ventricular heart contractions) and discontinued treatment.Sotatercept Investigational New Drug (IND) Applications Sotatercept is the subject of three separate company-sponsored U.S. IND applications. We submitted the first IND to the FDA on March 13, 2006 for the treatment of postmenopausal osteoporosis. There are currently no studies being conducted under this IND. We submitted the second IND to the FDA on March 27, 2009 to assess the use of sotatercept for the treatment of anemia in various cancer-related indications. We transferred sponsorship of both INDs to Celgene on January 19, 2010. Under the second IND, sotatercept is currently being studied in patients with lower-risk MDS. A third IND was submitted by Celgene to the FDA on January 25, 2010 to assess sotatercept for the treatment of anemia in patients with end-stage renal disease. In addition, sotatercept is being studied in Europe under three separate Clinical Trial Applications (CTAs). The first CTA is for a Phase 2 study for the treatment of anemia in adult patients withb-thalassemia, submitted to France on December 28, 2011, to the United Kingdom on July 26, 2012, to Italy on July 27, 2012, and to Greece on November 23, 2012. The second CTA is for a Phase 2 study for the treatment of anemia in patients with lower-risk MDS, submitted to France on October 10, 2012. The third CTA is for a Phase 2 study for the treatment of anemia in patients with chronic kidney disease, with Belgium, Germany, Portugal, Spain, and the UK joining under the Voluntary Harmonization Procedure on June 17, 2013. Sotatercept is also being studied in the United States under three investigator-sponsored INDs.Preclinical Studies In preclinical studies, RAP-011 (the mouse equivalent of sotatercept) was evaluated in a broad range of animal pharmacology studies to assess its biological effects. RAP-011 has been shown to increase red blood cell counts in mice, rats, and monkeys. RAP-011 showed increased hemoglobin and red blood cell counts in mouse models ofb-thalassemia and MDS, demonstrating decreased ineffective erythropoiesis in these models. RAP-011 was also able to prevent chemotherapy-induced anemia in a mouse model of this condition and was able to correct anemia in a mouse model of chronic kidney disease. RAP-011 increased bone mineral density in ovariectomized mice and has demonstrated positive effects in mice on bone lesions and bone metastases in a numberdominant class of cancer models including models of multiple myeloma. The preclinical activity of sotatercept is also being evaluated in a mouse model of sickle cell disease.ACE-536 Clinical and Preclinical Development ACE-536 is a soluble receptor fusion protein consisting of a modified extracellular domain ofdrugs that inhibit blood vessel formation, the activin receptor type IIB (ActRIIB) linked to the Fc domain of human IgG1.Ongoing Phase 2 Clinical Trials of ACE-536 We are conducting Phase 2 clinical trials of ACE-536 in patients withb-thalassemia and in patients with MDS. The FDA has granted orphan designation for ACE-536 for the treatment ofb-thalassemia and for the treatment of MDS.ß-thalassemia. We are conducting a Phase 2 clinical trial of ACE-536, designed as an ascending dose trial to evaluate the safety and efficacy in patients withb-thalassemia. The dose levels to be studied are 0.2, 0.4, 0.6, 0.8 and 1.0 mg/kg given subcutaneously once every three weeks for up to 85 days. Each cohort will include three to six patients receiving a single dose level during the dose escalation phase. This will be followed by an expansion phase at a selected dose level in up to 20 patients. We are in the process of amending the protocol to include additional types of transfusion-dependent patients, to increase the size of the expansion cohort of the trial and to potentially study higher dose levels of ACE-536. The first patient in the trial was first dosed in March 2013. We have completed enrollment of the 0.2, 0.4, 0.6 and 0.8 mg/kg cohorts and are currently enrolling patients in the 1.0 mg/kg cohort. The primary outcome measure is the proportion of patients who have an increase in hemoglobin of³1.5 g/dL from baseline for³14 days (in the absence of red blood cell transfusions) in non-transfusion dependent patients or a³20% reduction in red blood cell transfusion burden compared to the pretreatment transfusion burden in transfusion dependent patients. This trial will also examine the effects of ACE-536 on iron overload, an important cause of morbidity and mortality inb-thalassemia patients. Secondary endpoints include markers of serum iron and hemolysis. The trial is being conducted at up to seven sites in Italy and Greece, and we plan to include additional sites in Europe and may enroll up to 72 patients. Initial data from this clinical trial is encouraging. As of January 3, 2014, preliminary data after three cycles (approximately two months) of treatment with ACE-536 show that non-transfusion dependentb-thalassemia patients in the 0.6 mg/kg dose group achieved a mean increase in hemoglobin of approximately 1.5 g/dL, while patients in the 0.2 and 0.4 mg/kg dose groups achieved a mean increase in hemoglobin of approximately 0.0 and 0.8 g/dL, respectively. Acceleron and Celgene intend to announce interim data in June, 2014 at the annual congress of the European Hematology Association (EHA). For sotatercept and ACE-536 inb-thalassemia, we and Celgene expect to present additional interim data from non-transfusion dependent patients as well as data from transfusion dependent patients. In transfusion dependentb-thalassemia patients treated with ACE-536, preliminary data show a decrease in the frequency of red blood cell transfusions. Based on these data and additional data to be gathered during the clinical trial, we expect to establish a range of recommended ACE-536 dose levels. We expect that in future clinical trials, patients will begin treatment at a recommended starting dose level and to undergo individualized dose titration based on hemoglobin response and tolerability to achieve and maintain an appropriate hemoglobin level. Based on currently projected timelines, which are subject to change, we expect data from this clinical trial to become available as follows: data from the dose escalation portion of the clinical trial during the second quarter of 2014, and additional data in the fourth quarter of 2014. MDS. We are conducting a Phase 2 clinical trial of ACE-536 designed as an ascending dose trial in patients with low or intermediate-1 risk MDS. The dose levels to be studied are 0.125, 0.25, 0.5, 0.75, 1.0, 1.33 and 1.75 mg/kg given subcutaneously once every three weeks for up to 85 days. Each cohort will include three to six patients receiving a single dose level during the dose escalation phase. This will be followed by an expansion phase at a selected dose level in up to 30 patients. The first patient in the trial was first dosed in January 2013. We have currently completed enrollment in the 0.125, 0.25, 0.5, 0.75 and 1.0 mg/kg cohorts and are now enrolling patients in the 1.33 mg/kg cohort. The primaryoutcome measure is the proportion of patients who have an increase of hemoglobin³ 1.5 g/dL from baseline for 14 days in the absence of red blood cell transfusions in non-transfusion dependent patients or a³50% or³4 unit reduction of red blood cell transfusions over a period of eight weeks compared to pretreatment transfusion burden in transfusion-dependent patients. This trial will also examine the effects of ACE-536 on iron overload. The trial is being conducted at up to nine sites in Germany and may enroll up to 72 patients. For MDS patients treated with ACE-536, interim data indicate that there is a positive effect on hemoglobin levels in these patients, and we expect to present data from both transfusion dependent and non-transfusion dependent MDS patients at EHA in June, 2014. Based on currently projected timelines, which are subject to change, we expect additional data from this clinical trial to become available as follows: data from the dose escalation portion of the clinical trial during the second quarter of 2014, and additional data in the fourth quarter of 2014.Completed Phase 1 Clinical Trial ACE-536 was studied in a double-blind, placebo-controlled, randomized, ascending dose Phase 1 clinical trial in 32 healthy volunteers. ACE-536 produced dose-dependent increases in hemoglobin and red blood cells. The proportion of subjects with a hemoglobin increase of³1.0 g/dL increased on a dose-dependent basis, with approximately 80% of subjects in the 0.25 mg/kg dose level achieving this threshold.Safety In the completed Phase 1 clinical trial in healthy volunteers, ACE-536 was well-tolerated. No ACE-536 related serious adverse events were reported in the completed Phase 1 clinical trial. Commonly observed possibly or probably treatment-related adverse events included injection site bruising, injection site blemish, dry skin, numbness, muscle spasms, muscle pain, generalized itchiness and raised rash. In the ongoing Phase 2 clinical trials, there have been no ACE-536 related serious adverse events. One patient in the ACE-536 thalassemia trial who was treated at the 0.8 mg/kg dose level had dose-limiting toxicity of worsening bone pain. The patient had a dose reduction to 0.6 mg/kg for the second cycle and subsequently withdrew from the study.ACE-536 Investigational New Drug (IND) Applications ACE-536 is being studied in the United States under an IND that we submitted to the FDA on June 14, 2011. The indication identified in the IND is for the treatment of anemia in patients with MDS. No studies are being conducted under this IND at this time. In addition, ACE-536 is being studied in Europe under two separate Clinical Trial Applications (CTAs). The first is for a Phase 2 study for the treatment of anemia in adult patients withb-thalassemia, submitted to Italy on August 29, 2012, to Turkey on June 14, 2013, and to Greece on July 2, 2013. The second is for a Phase 2 study for the treatment of anemia in patients with low- or intermediate-1 risk MDS, submitted to Germany on August 21, 2012.Preclinical Studies A number of preclinical pharmacology studies have been conducted with ACE-536 or its mouse version, RAP-536, that demonstrate its effects on red blood cells, hemoglobin and hematocrit. Collectively ACE-536 and RAP-536 have shown activity in mouse models ofb-thalassemia, MDS, chemotherapy-induced anemia, acute blood loss and renal anemia. ß-thalassemia. RAP-536 has been evaluated in a series of studies using a mouse model ofb-thalassemia. These mice carry deletion mutations in theb-globin genes, resulting in a deficiency ofb-globin protein and hematologic abnormalities very similar to those seen in humanb-thalassemia patients, including severe anemia and the formation of hemichromes resulting in ineffective erythropoiesis. These mice also exhibit severe complications common in patients with thalassemia, suchas an enlarged spleen, bone loss and iron overload. In these mice, RAP-536 treatment improved numerous hematologic parameters, including significant increases in red blood cell count, hemoglobin levels, and hematocrit, decreased serum erythropoietin, normalized red blood cell size, and reduced red blood cell breakdown, as measured by serum bilirubin. Representative blood smears were taken from theb-thalassemia mouse studies for both the placebo-treated animals and the RAP-536 treated animals. As shown in the image below, RAP-536 improved red blood cell morphology by reducing the number of poorly formed and damaged red blood cells, and reducing the amount of cellular debris that results from dying red blood cells.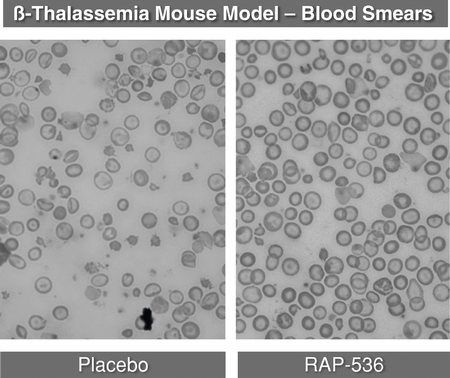
Importantly, RAP-536 improved the maturation of later stage red blood cell precursor populations, in the bone marrow and spleen, with concomitant reductions in the earlier-stage red blood cell precursor populations. We believe RAP-536 reduced the ineffective erythropoiesis by decreasing the formation of harmful hemichromes. It appears that RAP-536 may achieve this effect in part by stimulating the proteasome, thus promoting the removal of unpaireda-hemoglobin and stimulating red blood cell maturation. This reduction in ineffective erythropoiesis reduced severe and common complications of the disease in mice, evidenced by reduced iron deposition in organs, reduced spleen weights and normalized bone density. Based on the numerous beneficial effects of RAP-536 in this mouse model ofb-thalassemia, we believe that it is modifying the disease and has the potential to do so in human patients. MDS. In a mouse model of MDS, RAP-536 treated animals had statistically significant increases in red blood cell count, hemoglobin levels and hematocrit compared to controls. Additionally, RAP-536 reduced the ineffective erythropoiesis as evidenced by the improvement in the ratio of red blood cell precursors to other cells in the bone marrow. Sickle Cell Disease. We and Celgene are exploring the preclinical activity of ACE-536 in a mouse model of sickle cell disease. Taken together, our clinical and preclinical results suggest that ACE-536 could be a meaningful novel therapy to treat anemia.DalanterceptInhibiting Angiogenesis to Limit Tumor Growth Angiogenesis is a process by which new blood vessels are formed. Angiogenesis can be simplified to two major stages—the proliferative stage followed by the maturation stage. During the proliferative stage, vascular endothelial cells, the cells lining the inside of the blood vessels, multiply in number and migrate to the site where a new vessel will be formed. This proliferative stage is followed by the maturation stage during which the endothelial cells coalesce to form tubes which are then stabilized through the recruitment of perivascular cells that form an outer layer of the blood vessels resulting in fully formed, functional vessels. Tumors depend on angiogenesis to form new blood vessels to supply nutrients and oxygen to feed the rapidly growing malignant cells. The principal molecule driving the proliferative stage of angiogenesis in tumors is a protein called vascular endothelial growth factor, (VEGF). Inhibiting VEGF-driven angiogenesis to control tumor growth has become an important and widely-used approach to cancer treatment. There are several FDA-approved cancer drugs that inhibit theor VEGF, pathway with over $8 billion in aggregate worldwide sales. Despite the success of these drugs, many patients fail to respond or develop resistance to VEGF pathway inhibitor therapy, resulting in an unmet need for new therapies to inhibit angiogenesis by a different mechanism.using our knowledge of the TGF-b superfamily to developdeveloping dalantercept a novel protein therapeutic candidate targeting the maturation stage of angiogenesis. Recently, the activin receptor-like kinase 1 (ALK1) has been recognized as a key regulator of the maturation stage of angiogenesis. ALK1 is one of the 12 receptorsprimarily for ligands in the TGF-b superfamily and is found primarily on endothelial cells. The importance of the ALK1 pathway in angiogenesis was discovered, in part, through research into the genetic basis of the disease hereditary hemorrhagic telangiectasia 2 (HHT-2) in which patients manifest vascular defects including reduced ability to form capillary beds, which are the networks of small blood vessels that connect arteries to veins and are necessary for nutrient and waste exchange in tissues. This research revealed that these patients have only one of two functional copies of the ALK1 gene. We reasoned that leveraging the biology of the ALK1 pathway to inhibit maturation of blood vessels could impair the growth of tumors by limiting the development of capillary beds within the tumor. To test this hypothesis, mice with a predisposition to develop tumors were bred to have only one, rather than two copies, of the ALK1 gene. In response to the loss of half of the ALK1 genes, tumor growth and size and blood vessel density in the tumor were reduced by half. These results and additional research in the field have established the ALK1 signaling pathway as a promising target for developing a new class of anti-angiogenesis agents—ALK1 pathway inhibitors. We believe one promising opportunity for dalantercept will be its use in combination with VEGF pathway inhibitors because these agents target distinct sequential steps in angiogenesis. Moreover, we and others have hypothesized that agents, such as dalantercept, that inhibit vessel maturation are able to sensitize the tumor vasculature to the anticancer effects of VEGF pathway inhibition. We believe that newly formed blood vessels become more resistant to VEGF pathway inhibitors as they mature. Therefore we believe that by preventing blood vessel maturation, dalantercept may maintain newly formed vessels in an immature state that increases their susceptibility to VEGF pathway inhibitors. We and our academic collaborators have also shown in two mouseproduce better outcomes for cancer models that treatment with dalantercept decreases metastases. This is in contrast to VEGF pathway inhibitors that increase metastases in mouse cancer models.Inlyta® (axitinib)Votrient® (pazopanib), Caprelsa® (vandetanib), and Votrient® (pazopanib)Zaltrap® (ziv-aflibercept). FourThree large markets for which these drugs have been approved are non-small cell lung cancer, colorectal cancer and renal cell carcinoma and liver cancer.•will be 228,190were 221,200 new cases of lung cancer in the United States in 20132015 with 159,480158,040 deaths. In 2012,2015, sales of Avastin® in NSCLC were $1.2an estimated $1.1 billion in the United States and $1.7$1.8 billion worldwide.•will be 142,820were 132,700 new cases of colon cancer or rectal cancer in the United States in 20132015 with 50,83049,700 deaths. In 2012,2015, sales of Avastin® for colorectal cancer were $1.2an estimated $1.3 billion in the United States and $3.6 billion worldwide.•will be 65,150were 61,560 new cases of kidney and renal cell carcinomapelvis cancer in the United States in 20132015 with 13,68014,080 deaths. In 2012,2015, U.S. sales of drugs for renal cell carcinoma were $1.2$1.4 billion, of which $800 million$1.0 billion were anti-angiogenesis drugs that target the VEGF pathway, principallyNexavar®Inlyta®, Votrient® and Avastin®. Worldwide sales in 20122015 of drugs for renal cell carcinoma were $3.1$3.5 billion, of which $2.3$2.6 billion were drugs that target the VEGF pathway.•Liver Cancer. The National Cancer Institute estimates there will be 30,640 new cases of liver cancer in the United States in 2013 with 21,670 deaths. The only drug approved in the United States for the treatment of liver cancer is the VEGF pathway inhibitor Nexavar®. In 2012, sales of Nexavar® for liver cancer were $189 million in the United States and $756 million worldwide.••Developing Indications. It is believed that angiogenesis is importantthe growth and spread ofcombination with axitinib, a number of additional highly-vascularized cancers, including endometrial cancer (cancertyrosine kinase inhibitor of the uterus), ovarian cancer, and head and neck cancer. While no anti-angiogenesis agents are approved in the U.S. for these indications, Avastin® is approved in EuropeVEGF pathway, for the treatment of ovarian cancer. The firstrenal cell carcinoma in the DART trial, a two cancerspart Phase 2 clinical trial. In Part 1 of the DART trial, dalantercept plus axitinib produced clinical outcomes that exceed historical results with axitinib alone. We have reported a median progression-free survival (PFS) for dalantercept plus axitinib of 8.3 months. There was no control arm in Part 1 of the DART trial, however published data from a prior phase 3 trial of axitinib alone (the AXIS trial) in a similar patient subgroup show a median PFS of 4.8 months. We are currently conducting Part 2 of the DART trial, which is a double-blind, placebo-controlled trial, in which an estimated 130 patients are randomized to dalantercept plus axitinib or placebo plus axitinib. We expect to report on progression free survival from Part 2 of the DART trial by the end of 2016. In the open-label Part 1 and blinded Part 2 of the DART trial, the following serious adverse events have been reported as related to dalantercept, dalantercept or placebo (blinded Part 2), or both dalantercept and axitinib: fluid overload, dyspnea, epistaxis, renal injury, acute renal failure and hyponatremia. Non-serious adverse events associated with axitinib did not generally occur with higher than expected frequency or severity. In addition to the DART trial, we are studyingconducting a clinical trial to evaluate the treatment of patients with advanced hepatocellular cancer (HCC) with a combination of dalantercept plus sorafenib, another tyrosine kinase inhibitor of the VEGF pathway. A total of 21 patients have been enrolled as of December 30, 2015. The preliminary data indicate a VEGF pathway inhibitor are renal cell carcinoma and liver cancer. In renal cell cancer, sunitinib and axitinib aregeneral lack of efficacy for the most prescribed VEGF pathway inhibitors for first and second line patients, respectively. In the first line setting, sunitinib results in progression-free survival rates of approximately 11 months. In the second line setting, for patients whose disease had progressed despite receiving sunitinibdalantercept plus sorafenib combination in the first line setting, axitinib produced a progression-free survival ratetreatment of approximately 4.8 months. Weadvanced HCC, and therefore we believe the combination of dalantercept plus axitinibthat it is unlikely that additional patients will be enrolled in the second line settingHCC trial.the potential to increase the rate of progression-free survival greater than axitinib alone. In liver cancer, the VEGF pathway inhibitor, sorafenib, is approved in the first line setting yet the unmet medical need remains significant. In the first line setting, sorafenib results in time to progression of approximately 5.5 months.Dalantercept Clinical and Preclinical Development Dalantercept is comprised of the extracellular domain of the ALK1 receptor linked to the Fc domain of IgG1. Dalantercept actsbeen tested as a ligand trap for ligandssingle-agent therapy in the TGF-b superfamily that signal through the ALK1 receptor. We havefour completed clinical trials: a Phase 1 clinical trial of dalantercept and are pursuing a program of ongoing and planned Phase 2 trials seeking to demonstrate single agent activity of dalantercept for advanced solid tumors and activity of dalantercept in combination with approved VEGF pathway inhibitors or chemotherapy in advanced solid tumors.Ongoing and Planned Phase 2 Clinical Trials of Dalantercept We are currently conducting two Phase 2 clinical trials of dalantercept in head and neck cancer, ovarian cancer and renal cell carcinoma. Additionally, through collaborations with a National Cancer Institute funded collaborative research group, the Gynecologic Oncology Group, we are overseeing an additional Phase 2 clinical trial in ovarianendometrial cancer. We plan to initiate a trialIn these studies of dalantercept in hepatocellular carcinoma in the first half of 2014. We planas monotherapy, there was not sufficient activity to submit applications for orphan designationwarrant further development of dalantercept for those indications or subsets of indications that meet FDA requirements for orphan status.Acceleron Sponsored Clinical Trials Squamous Cell Carcinoma of the Head and Neck. We are conducting a single agent Phase 2 clinical trial of dalanterceptas monotherapy in an ascending dose trial in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. The first patient in the trial was first dosed in October 2011. After an initial cohort of two patients treated at a fixed dose level of 80 mg, we amended the trial and began recruitment of patients under the amended protocol in the first quarter of 2012 to study the dose level of 0.6 mg/kg given subcutaneously once every three weeks. The protocol was subsequently amended to increase the dose level of dalantercept to 1.2 mg/kg. Patients continue to receive dalantercept until there is disease progression (either clinically or as measured by analysis of radiographic imaging according to RECIST criteria) or dalantercept is no longer tolerated. The primary outcome measure is objective response rate as measured by RECIST criteria, and there are a number of secondary outcome measures ofthese tumor response. The trial is being conducted in 12 centers in the United States, and we completed enrollment in July 2013 with a total of 46 patients, including two patients treated at the 80 mg dose, 13 at the 0.6 mg/kg dose, and 31 at the 1.2 mg/kg dose. Of these 46 patients, 41 patients (one patient at 80 mg, 13 patients at 0.6 mg/kg, and 27 patients at 1.2 mg/kg) were evaluable for radiological response according to RECIST criteria as of December 20, 2013. The preliminary data for these 41 patients are as follows: no patients at 80 mg, three patients (23%) at 0.6 mg/kg and ten patients (37%) at 1.2 mg/kg achieved stable disease as their best response at the beginning of their third cycle and, at the 1.2 mg/kg dose level, one patient (2.4%) achieved a partial response. None of these patients achieved a complete response. We intend to present additional data from this trial at the American Society of Clinical Oncology annual meeting in late May or early June 2014. These preliminary data suggest dalantercept has dose dependent but modest single agent activity in patients with advanced squamous cell carcinoma of the head and neck.types. We believe the greatest potential for dalantercept will be in combination with VEGF pathway inhibitors or in combination with cytotoxic chemotherapy. Renal Cell Carcinoma. We are conductingtwo-part Phase 21 clinical trial of dalanterceptwith ACE-083 in combination with axitinib, an approved VEGF pathway inhibitor, in patients with advanced renal cell carcinoma. Part one of thishealthy volunteers. ACE-083 has been well tolerated and no serious adverse events have been reported. Initial data from the Phase 1 trial is designed as a single-arm dose escalation and expansion stage withshowed that, at the primary endpoint of evaluating the safety and tolerability of various dose levels of dalantercept in combination with axitinib to select ahighest dose level tested, ACE-083 generated a mean increase in muscle volume of dalantercept (in combination with axitinib) for further study if merited. The dose levels of dalantercept studiedapproximately 14.5% in the dose escalation stage were 0.6, 0.9, and 1.2 mg/kg given subcutaneously once every three weeks. The number of patients enrolled attreated muscle. We have completed enrollment for the 0.6, 0.9 and 1.2 mg/kg dose levels were four, four and five patients, respectively. The dose level of 1.2 mg/kg was selected for use in the expansion phaseACE-083 Phase 1 clinical trial, and we are now enrolling up to 20 additional patients in this stage of the study. The first patient in the trial was first dosed in January 2013. Patients continue to receive dalantercept and axitinib until there is disease progression (either clinically or as measured by RECIST criteria) or the combination is no longer tolerated. Up to a total of 44 patients may be enrolled in the dose escalation and expansion part of the trial. Part two of the trial will be a randomized comparison of the selected dose of dalantercept in combination with axitinib versus axitinibalone with a total of 112 patients. The primary endpoint of part two of the trial will be progression-free survival. The trial is currently being conducted in 7 sites in the United States. We believe that early preliminary data from this trial are encouraging. No dose-limiting toxicities have been observed during the 29 day assessment period for each dose cohort. Of the 13 patients enrolled in the dose escalation part of the trial, seven (54%) had received one prior therapy and six (46%) had received³ 2 prior therapies, including two patients with³ 2 prior VEGF-pathway inhibitors. As of December 24, 2013, six of eleven (55%) evaluable patients completed³ 6 cycles (approximately 4.5 months) of treatment, including two of four patients at the 0.6 mg/kg dose level, two of four patients at the 0.9 mg/kg dose level and two of three patients at the 1.2 mg/kg dose level. These data provide preliminary evidence that dalantercept can be safely combined with the VEGF-pathway inhibitor axitinib in patients with renal cell carcinoma. As of the most recent analysis (August 28, 2013) of tumor response according to RECIST criteria in the first two cohorts, one patient had achieved a partial response and one patient had achieved stable disease at the 0.6 mg/kg dose level, and one patient had achieved a partial response and three patients had achieved stable disease at the 0.9 mg/kg dose level. Data from patients at the 1.2 mg/kg dose level have not yet been analyzed. We intend to present additional data from this trial at the American Society of Clinical Oncology annual meeting in late May or early June 2014. Based on currently projected timelines, which are subject to change, we expect to initiate part two of this trial by the end of the first quarter or the beginning of the second quarter of 2014, which, if successful, we expect would enable a Phase 3 trial. We expect additional dose escalation data to be available when we initiate part two of the trial.Hepatocellular Carcinoma In the first half of 2014, we plan to initiate a Phase 2 single-arm dose escalation and expansion clinical trial of dalantercept in combination with sorafenib, an approved VEGF pathway inhibitor,ACE-083 in patients with hepatocellular carcinoma. The primary endpointfacioscapulohumeral dystrophy, or FSHD, in the second half of 2016.be to evaluate the safetysuffer from disability, pain, and tolerability of various dose levels of dalantercept in combination with sorafenib. Secondary endpoints are expected to include time to progression, progression-free survival, disease control rate, and overall survival. The dose levels planned are 0.9 mg/kg of dalantercept given subcutaneously once every three weeks in combination with 400 mg sorafenib given once daily, 1.2 mg/kg of dalantercept given subcutaneously once every three weeks in combination with 400 mg sorafenib given once daily, and 1.2 mg/kg of dalantercept given subcutaneously once every three weeks in combination with 400 mg sorafenib given twice daily. Each cohort will include up to six patients receiving a single dose level during the dose escalation phase, followed by an expansion phase in up to 20 additional patients. Patients will continue to receive dalantercept and sorafenib until theredepression. FSHD is disease progression (either clinically or as measured by RECIST criteria) or the combination is no longer tolerated.Gynecologic Oncology Group (GOG) Sponsored Trials The Gynecologic Oncology Group, one of the National Cancer Institute's funded collaborative cancer research groups, sponsored two Phase 2 clinical trials to study the activitymost prevalent forms of dalantercept at a dose level of 1.2 mg/kg given as a single agent via subcutaneous injection every three weeks. The first trial was in patients with recurrent or persistent endometrial cancer and the second trial is in patients with recurrent or persistent ovarian cancer. Both of these clinical trials were designed as two part studies to assess the activity of dalantercept based on either of two endpoints: RECIST-defined response rate or progression free survival at 6 months. If there is sufficient activity in the first part of the trial, additional patients will be enrolled in the second, expanded part of the trial. The endometrial cancer study enrolled 28 patients in part one. Of the 28 patients, 16 (57.1%) achieved stable disease and 5 (17.9%) were alive and progression free for at least 6 months. Of these 5 patients, 3 were alive and progression free without receiving non-protocol therapy for at least 6 months. Fatigue, anemia,constipation, and edema were the most commonly reported toxicities regardless of attribution. The GOG has notified us that there was not sufficient activity to enroll additionalmuscular dystrophy affecting roughly 19,000 patients in the second part of the endometrial cancer trial. The GOG ovarian cancer study enrolled 30 patients to part 1. Because this part 1 of the ovarian cancer study is fully enrolled, at this time the study is closed to further enrollment. We anticipate that in the middle of 2014, we may receive notification from the GOG if there is sufficient activity to enroll additional patients in the second part of the ovarian cancer trial.Phase 1 Trial Results A Phase 1 ascending dose trial evaluated the safety, tolerability, pharmacokinetics and anti-tumor activity of dalantercept in patients with advanced solid tumors. Dalantercept was given subcutaneously approximately once every three weeks until disease progression. Thirty-seven patients were enrolled in dose groups at 0.1, 0.2, 0.4, 0.8, 1.6, 3.2 and 4.8 mg/kg. In this trial, dalantercept demonstrated anti-tumor activity based on decreases or stabilization of tumor size. As shown in the figure below, out of the 29 evaluable patients treated, one (3%) had a partial response and 13 (45%) had stable disease according to RECIST criteria. Of the 13 who experienced stable disease, eight experienced stable disease for at least three months. Treatment continued until the patient experienced progressive disease. The figure below displays each patient's best overall response by the maximum percent change decrease in target lesion size. The dose level each patient received is shown below their bar.Best Overall Response by the Maximum Percent Change Decrease in Target Lesion Size According to RECIST v1.1 Criteria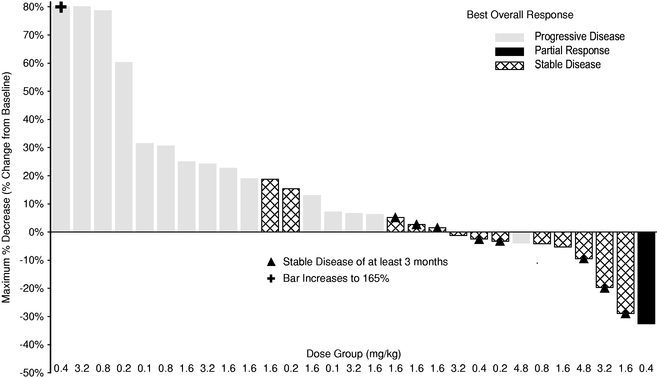
these effectsour clinical development activities, we are expanding our research capabilities in order to increase the rate at which our highly productive research group can identify and advance new, internally discovered, therapeutic candidates for clinical development. Our discovery efforts are primarily focused on tumor size, dalantercept demonstrated likely anti-angiogenic activity evidenced by a reduction of tumor metabolic activity as well as decreases in tumor blood flow. Lastly, some patients were observedidentifying new protein therapeutic candidates from our IntelliTrap™ platform and identifying novel antibodies. We have selected our first IntelliTrap™ therapeutic candidate, ACE-2494, for pre-clinical evaluation and advancement to have dilated blood vessels in the skin, similar to those in HHT-2 patients, suggesting ALK1 pathway inhibition.Safety In clinical trials to date, dalantercept has been generally well-tolerated. Inby the initial Phase 1 clinical trial in advanced cancer patients, five patients outend of 37 experienced serious adverse events deemed treatment-related that were reported2016. We are also evaluating ACE-3891 as left ventricular dysfunction, fatigue, fluid overload, and congestive heart failure. Two of these patients had prior coronary artery disease. In subsequent trials fluid overload has been successfully managed with diuretics. The following treatment related adverse events have been observed in our ongoing clinical trials. Two patients in the head and neck cancer clinical trial have experienced serious adverse events of fluid accumulation around the lungs that were determined to be possibly related to dalantercept. Another patient in the head and neck trial has experienced serious adverse events of tracheal obstruction and pulmonary edema that were determined to be possibly related to dalantercept. In the clinical trial of patients with endometrial cancer, seven patients have experienced treatment-related serious adverse events reported as fluid accumulation in the abdominal cavity, fluid accumulation around the lungs, rectal fistula, gastric bleeding, vomiting, anemia, and shortness of breath. In the clinical trial of patients with ovarian cancer, one patient has experienced treatment related serious adverse events reported as hypokalemia (decreased potassium), anorexia, dehydration and increased creatinine and another patient experienced a treatment related serious adverse event of shortness of breath. In the renal cell carcinoma trial, there has been one dalantercept-related serious adverse events of fluid overload and shortness of breath. Adverse events associated with axitinib did not occur with higher than expected frequency or severity.Dalantercept Investigational New Drug (IND) Applications Dalantercept is being studied in the United States under an IND that we submitted to the FDA on July 29, 2009candidate therapeutic for the treatment of patients with advanced solid tumors or multiple myeloma. Dalantercept is also being studied in the United States under two INDs sponsored by the Gynecologic Oncology Group: the first was submitted on August 2, 2012muscle disease, ACE-1332, a selective TGF-beta antagonist, for the treatment of recurrent/persistent endometrial carcinoma and the second submitted on September 25, 2012 for the treatment of recurrent/persistent ovarian carcinoma.Preclinical Studies We have demonstrated that dalantercept as a single agent inhibits tumor growth and angiogenesis in a variety of mouse models of cancer. Importantly, we have shown that dalantercept is a potent inhibitor of the maturation stage of angiogenesis. This is in contrast with VEGF pathway inhibitors that target the proliferative stage of angiogenesis. We also demonstrated that dalantercept in combinationdisorders with a VEGF pathway inhibitor provides enhanced anti-tumor effects. In mice bearing human renal cell carcinoma xenografts, wefibrotic component, and our academic collaborators have shown that simultaneous administration of both dalanterceptACE-2798, ACE-2536 and sunitinib, a VEGF-receptor tyrosine kinase inhibitor, had substantially greater efficacy than either agent alone. In another mouse model of human renal cell carcinoma that develops resistance to sunitinib, tumorTable of ContentsACE-2395 for undisclosed therapeutic areas.growth was blocked by the simultaneous administration of dalantercept. The figures below summarize those results.Dalantercept/Sunitinib CombinationExceeds Activity of Either Alone(Mouse Model of Renal Cell Carcinoma (A498))Dalantercept/Sunitinib CombinationSlows Tumor Growth in a Sunitinib Resistant Model(Mouse Model of Renal Cell Carcinoma (786O))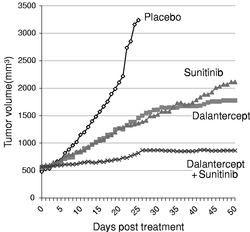
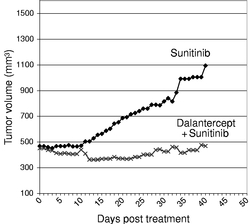
Collaboration with Drs. Wang, Bhatt, Mier, Atkins; Beth Israel Deaconess Medical Center, BostonDevelopment Objectives For sotatercept and ACE-536, our development strategy, determined in collaboration with Celgene, for bothb-thalassemia and MDS is to conduct similar clinical trials with each protein therapeutic candidate in each disease essentially in parallel. For each disease, we and Celgene will review the data from both studies and determine which, if either, protein therapeutic candidate to move forward into subsequent, pivotal studies. It is our goal to initiate the Phase 3 clinical trials for one or both protein therapeutic candidates in one or both of these diseases by the end of 2014 or early 2015. In addition, we and Celgene are performing preclinical research to assess the opportunity for sotatercept and ACE-536 to treat additional red blood cell disorders known as hemoglobinopathies, which include diseases such as thalassemias and sickle cell disease. Based on our encouraging preclinical and clinical data inb-thalassemia and our emerging understanding of the mechanism of action of these protein therapeutic candidates, we believe there is a potential for activity in other diseases such as sickle cell disease and preclinical studies are currently underway. For dalantercept, our development strategy is to continue the renal cell carcinoma trial and to initiate during 2014 part two of the trial that compares the combination of dalantercept and axitinib to axitinib alone. We plan to initiate the hepatocellular carcinoma trial in the first half of 2014. We will also work toward completion of the ongoing single agent trial in head and neck cancer. We expect to initiate during the second half of 2014, at least one additional trial of dalantercept in cancer patients in combination with an approved VEGF pathway inhibitor. We are currently considering trials of dalantercept in combination with bevacizumab in glioblastoma (a form of brain cancer), and in colorectal cancer or lung cancer in combination with chemotherapy and/or a VEGF pathway inhibitor.Our Preclinical Pipeline We are using our discovery platform and knowledge of the TGF-b superfamily to design and evaluate promising new protein therapeutic candidates that inhibit ligands of the TGF-b superfamily.We have preclinical stage protein therapeutic candidates in our pipeline that have shown promising activity in animal models such as:•inhibition of liver fibrosis in mouse models of this condition;•improvement of cardiovascular function in a mouse model of a fibrotic disorder of the lungs; and•improvement in diseases of the eye such as in a mouse laser-induced neovascularization model of age-related macular degeneration (AMD).ACE-083 We are developing a novel protein therapeutic candidate, ACE-083, for a first-in-human clinical trial that we expect to initiate by the end of 2014. ACE-083 acts as a trap for ligands in the TGF-b superfamily that are known to be involved in the regulation of muscle mass. By inhibiting these ligands, ACE-083 can increase muscle mass, as we have demonstrated in animal studies. ACE-083 has been designed to affect those muscles in which the drug is injected. In preclinical animal studies, ACE-083 has shown dose dependent increases in muscle mass in the injected muscles but no systemic increases in muscle mass. We are focused on the development of ACE-083 for diseases in which increases in the size and function of specific muscles may provide a clinical benefit, including inclusion body myositis, facioscapulohumeral dystrophy (FSHD) and disuse atrophy.ACE-083 Selectively Doubles Muscle Mass inInjected Muscle with One Month of Treatment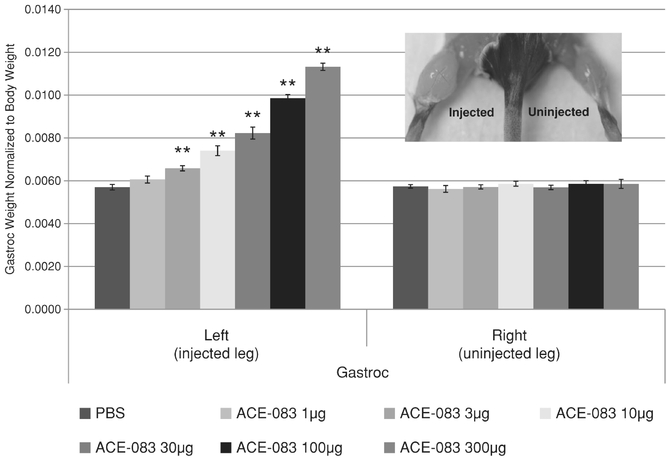
**p<0.05 vs. PBS (placebo) & vs. non-injected legALK1 Pathway Inhibitors for the Treatment of Diseases of the Eye Although VEGF pathway inhibitors are the standard of care for age-related macular degeneration, or AMD, there is a need for improved therapies. Perivascular cell coverage may protect endothelial cells and limit the effectiveness of VEGF pathway inhibitors in AMD. By reducing the number of perivascular cells, the activity of VEGF pathway inhibitors on unprotected endothelial cells may be potentially enhanced. Human genetic evidence indicates that patients with hereditary hemorrhagic telangiectasia (HHT-2) who lack expression of a single ALK1 gene results in defective vasculature with reduced perivascular cell coverage. We are evaluating ALK1 pathway inhibitors, distinct from dalantercept, for use in the treatment of diseases of the eye. The combination of a VEGF pathway inhibitor and an ALK1 pathway inhibitor may lead to more effective treatment of neoangiogenesis diseases of the eye including AMD.$250.0$297.7 million from our collaborations with Celgene, Alkermes plc (Alkermes) and Shire.ACE-536,luspatercept, which we refer to as the ACE-536Luspatercept Agreement under which we granted to Celgene worldwide rights to ACE-536luspatercept and also amended certain terms of the Sotatercept Agreement. These agreements provide Celgene exclusive licenses for these protein therapeutic candidates in all indications, as well as exclusive rights to obtain a license to certain future compounds.2013,2015, we have received $41.6$43.0 million in research and development funding and milestone payments for the sotatercept program.b-thalassemia, beta-thalassemia, MDS and chronic kidney disease and will be responsible for any future clinical trials for sotatercept as well as for all future manufacture of sotatercept. We are eligible to receive future development, regulatory and commercial milestones of up to $360.0 million for the sotatercept program and up to an additional $348.0 million for each of the three discovery stage programs. None of the three discovery stage programs has advanced to the stage to achieve payment of a milestone, nor do we expect any such milestone payments in the near future. ACE-536Luspatercept Agreement. Under the terms of the ACE-536Luspatercept Agreement, we and Celgene are collaborating in the development and commercialization of ACE-536.luspatercept. We also granted Celgene an option to license products for which Acceleron files an investigational new drug application for the treatment of anemia. Celgene paid $25.0 million to us upon execution of the ACE-536Luspatercept Agreement in August 2011 and, as of December 31, 2013,2015, we have received $31.9$60.3 million in research and development funding and milestone payments for the ACE-536luspatercept program.b-thalassemia, beta-thalassemia, as well as manufacturing the clinical supplies for these studies. Celgene will conduct subsequent Phase 2 andACE-536luspatercept for all Phase 1 and Phase 2 clinical trials, and Celgene will have responsibility for the manufacture of ACE-536luspatercept for Phase 3 clinical trials and commercial supplies. We are eligible to receive future development, regulatory and commercial milestones of up to $200.0 million for the ACE-536luspatercept program.910 to the financial statements in this Annual Report on Form 10-K for the revenue recognition accounting, including changes in estimates, pursuant to the revenue recognition accounting literature.ACE-536Luspatercept Agreements. As of January 1, 2013, Celgene became responsible for paying 100% of worldwide development costs for both programs. Celgene will be responsible for all commercialization costs worldwide. We are obligated to co-promote sotatercept, ACE-536luspatercept and future products, in each case if approved, under both agreements in North America, and Celgene will pay all costs related thereto. We will receive tiered royalties in the low-to-mid 20% range on net sales of sotatercept and ACE-536.luspatercept. The royalty schedules for sotatercept and ACE-536luspatercept are the same. Celgene is obligated to use commercially reasonable efforts to develop and commercialize sotatercept and ACE-536.luspatercept. Celgene may determine that it is commercially reasonable to develop and commercialize only sotaterceptluspatercept or ACE-536sotatercept and discontinue the development or commercialization of the other protein therapeutic candidate, or Celgene may determine that it is not commercially reasonable to continue development of one or both of sotatercept and ACE-536.luspatercept. In the event of any such decision, we may be unable to progress the discontinued candidate or candidates ourselves. The agreements are terminable by either party upon a breach that is uncured and continuing or by Celgene for convenience on a country by country or product by product basis, or in its entirety. Celgene may also terminate the agreement, in its entirety or on a product by product basis, for failure of a product to meet a development or clinical trial endpoint. Termination for cause by us or termination by Celgene for convenience or failure to meet an endpoint will have the effect of terminating the applicable license, while termination for cause by Celgene will have the effect of reducing remaining royalties by a certain percentage.TGF-bTGF-beta superfamily in return for an upfront license payment. We are entitled to future development, regulatory and sales milestones and mid-single digit royalties on product sales for each drug developed and commercialized by Alkermes using this technology. To our knowledge, Alkermes is not currently developing any products usingpursuing technology licensed under this technology.collaboration. Rights pertaining to a lead program, referred to ACE-771, were returned pursuant to the terms of the agreement, and we are not pursuing development of ACE-771 at this point in time. protein therapeutic candidate. We granted Shire an exclusive license in markets outside of North America. Under the terms of the agreement, Shire made an upfront cash payment of $45.0 million. We received $9.0 million in research and development payments from Shire during the term of the agreement. In April 2013, we and Shire determined not to further advance the development of ACE-031, and Shire terminated our collaboration agreement, effective as of June 30, 2013 and all rights reverted to us. We currently have no plans to continue the development of ACE-031.
CompetitionCompetitionprotein therapeuticstherapeutic candidates we may develop or commercialize in the future from pharmaceutical and biotechnology companies worldwide. Many of the entities developing and marketing potentially competing products have significantly greater financial resources and expertise than we do in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize products that are more effective, have fewer side effects, are more convenient or are less expensive than any products that we may develop.protein therapeuticstherapeutic candidates are approved, they will compete with currently marketed drugs and therapies used for treatment of the following indications, and potentially with drug candidates currently in development for the same indications:ß-thalassemiasotaterceptluspatercept or ACE-536 is approved for the treatment of patients withb-thalassemia, it would compete with:•Red blood cell transfusions and iron chelation therapy, such as Novartis's oral iron chelating agent, Exjade®. We are also aware that Shire is studying a new oral iron chelator in clinical trials.•Fetal hemoglobin stimulating agents, such as hydroxyurea, which are primarily used to treat patients with anemia from sickle cell disease, are sometimes used to treat patients withb-thalassemia. In addition, HQK-1001, a fetal hemoglobin stimulating agent being developed by HemaQuest Pharmaceuticals, Inc., has completed a Phase 1/2 clinical trial and an investigator sponsored Phase 2 clinical trial in patients withb-thalassemia.•Hematopoietic stem cell transplant treatment is given to a small percentage of patients withb-thalassemia, since it requires a sufficiently well-matched source of donor cells. Certain academic centers around the world are seeking to develop improvements to this approach.•Other therapies in development, including gene therapy are being developed by several different groups, including bluebird bio, Inc., Memorial Sloan Kettering Cancer Center, GlaxoSmithKline plc, and Sangamo BioSciences Inc. in collaboration with Biogen Idec.MDS If either sotatercept or ACE-536 is approved for the treatment of patients with MDS, it would compete with the following:•Amgen'sAmgen is currently studying erythropoiesis stimulating agent, Aranesp®, and Janssen Pharmaceuticals is currentlystudying erythropoiesis stimulating agent Eprex® in Phase 3 clinical trials in Europe for treatment of anemia in patients with lower risk MDS.••• and an anti-cancer therapy being developed by Onconova to treat patients with MDS.Chronic Kidney Diseaseeither sotatercept or ACE-536luspatercept is approved for the treatment of patients with beta-thalassemia, it would compete with:it would compete primarily with erythropoiesis stimulating agents thatand have been approvedshown to treat these patients for over 20 years. In 2011,lower blood pressure, reduce proteinuria, and slow the Centers for Medicare and Medicaid Services (CMS) changed the reimbursement practice for erythropoiesis stimulating agentsprogression of kidney disease. There are also a number of drugs in chroniclate-stage clinical trials to slow progression of diabetic nephropathy, a form of kidney disease patients on dialysis, which has ledin diabetic patients. Janssen Pharmaceuticals. NephroGenex, Abbvie, and Bayer have programs in ongoing Phase 3 clinical trials, and Mitsubishi Pharma, Lilly, and Vascular Pharmaceuticals, have programs in ongoing or completed Phase 2 clinical trials, to changes in the way erythropoiesis stimulating agents are used in clinical practice, including decreasing the numberslow progression of patients treated with erythropoiesis stimulating agents as well as decreasing the average dose and duration of therapy. These changes and the anticipated future introduction of biosimilar erythropoiesis stimulating agents are expected to generate additional price pressure in this market. Additionally, we are aware that Astellas Pharma and Fibrogen are developing oral, small molecule treatments that increase the production of erythropoietin to treat patients with anemia.with:• Pfizer and Tracon are each developing non-VEGF angiogenesis inhibitors.•Pfizer's fully human monoclonal antibody to the ALK1 receptor, which is in Phase 2 trials in malignant pleural mesothelioma.carcinoma, head and neck, endometrial and ovarian cancer.the process of moving our lead pre-clinical protein therapeutic candidate, ACE-083, into its initiala Phase 1 clinical trial. Wetrial in healthy volunteers and plan to develop ACE-083 for the treatment of neuromuscular disorders and other diseases characterized by a loss of muscle function. function including facioscapulohumeral muscular dystrophy (FSHD). One potential competitor of ACE-083 in FSHD is Resolaris® (ATYR1940), which is an investigational protein in Phase 1b/2 studies to treat adult patients with FSHD being developed by aTyr Pharma.other approaches to treatingtreat other muscle loss diseases that are in clinical trials. Novartis is developing bimagrumab (BYM338), a monoclonal antibody targeting the activin receptor type IIB (ActRIIB), in various phasePhase 2 clinical trials to treat pathological muscle loss and weakness.weakness and in Phase a 2/3 clinical trial to treat patients with sporadic inclusion body myositis (sIBM). Regeneron is developing a myostatin monoclonal antibody, REGN1033 (SAR391786), which has completed a Phase 2 clinical trial for treatment of sarcopenia. Pfizer is conducting a Phase 2 clinical study for PF-06252616, a myostatin antibody, in patients with Duchenne muscular dystrophy (DMD). Nationwide Children's Hospital, in collaboration with The Myositis Association, and Parent Project Muscular Dystrophy isand Milo Biotechnology, are conducting a phasePhase 1 clinical trial of a gene therapy delivery of follistatin (FS344) to muscle in patients with Becker muscular dystrophy (BMD) and sporadic inclusion body myositis (sIBM). Eli Lilly is developing, LY2495655, a myostatin monoclonal antibody in phase 2 clinical trials for disuse muscle atrophy and cancer-related cachexia. Regeneron and Sanofi are developing a myostatin monoclonal antibody, REGN1033 (SAR391786), which is in phase 1 clinical development for treatment of sarcopenia. Biogen Idec is conducting a phase 1 clinical trial of BIIB023, a monoclonal antibody targeting the tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK), to evaluate its effects on muscle atrophy in healthy male volunteers. Atara Bio is developing PINTA 745, a myostatin inhibiting peptibody to treat protein energy wasting in patients with end stage renal disease who are on dialysis.
CommercializationCommercializationACE-536luspatercept in North America, and under the terms of our agreements with Celgene, our commercialization costs will be entirely funded by Celgene. We also currently retain worldwide commercialization rights for our oncology protein therapeutic candidate, dalantercept, and our muscle protein therapeutic candidate, ACE-083. We intend to build hematology oncology and neuromuscular disorder focused, specialty sales forces in North America and, possibly, other markets to effectively support the commercialization of these and future products. We believe that a specialty sales force will be sufficient to target key prescribing physicians in these areas. We currently do not have any sales or marketing capabilities or experience. We will establish the required capabilities within an appropriate time frame ahead of any product approval and commercialization to support a product launch. If we are not able to establish sales and marketing capabilities or are not successful in commercializing our future products, either on our own or through collaborations with Celgene, any future product revenue will be materially adversely affected.protein therapeutics,therapeutic candidates, novel biological discoveries, screening and drug development technology, to operate without infringing on the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also rely on trade secrets, know-how, continuing technological innovation and potential in-licensing opportunities to develop and maintain our proprietary position. Additionally, we expect to benefit from a variety of statutory frameworks in the United States, Europe and other countries that relate to the regulation of biosimilar molecules and orphan drug status. These statutory frameworks provide periods of non-patent-based exclusivity for qualifying molecules. See "Government Regulations".protein therapeutics.therapeutic candidates. We seek composition-of-matter and method-of-treatment patents for each such protein in key therapeutic areas. We also seek patent protection with respect to companion diagnostic methods and compositions and treatments for targeted patient populations. We have sought patent protection alone or jointly with our collaborators, as dictated by our collaboration agreements.75160 issued patents and approximately 300390 pending patent applications, with pending and issued claims relating to all of our current clinical stage protein therapeutic candidates, sotatercept, ACE-536luspatercept, dalantercept and dalantercept. Of these, approximately 20 issued patents cover the nine receptors for the TGF-b superfamily that we have selected as the core focus of our discovery approach.ACE-083. These figures include in-licensed patents and patent applications to which we hold exclusive commercial rights.with respect to our receptor-focused platform will expire on dates ranging from 2013 to 2018, and our issued patents and protein therapeutic candidates will expire on dates ranging from 2026 to 2033,2035, exclusive of possible patent term extensions, However, the actual protection afforded by a patent varies on a product by product basis, from country to country and depends upon many factors, including the type of patent, the scope of its coverage, the availability of extensions of patent term, the availability of legal remedies in a particular country and the validity and enforceability of the patent.protein therapeuticstherapeutic candidates remain highly unsettled. No consistent policy regarding the patent-eligibility or the breadth of claims allowed in such patents has emerged to date in the United States, Europe or other countries. Changes in either the patent laws or in interpretations of patent laws in the United States and other countries can diminish our ability to protect our inventions and enforce our intellectual property rights. Accordingly, we cannot predict the breadth or enforceability of claims that may be granted in our patents or in third-party patents. The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights. Our ability to maintain and solidify our proprietary position for our drugs and technology will depend on our success in obtaining effective claims and enforcing those claims once granted. We do not know whether any of the patent applications that we may file or license from third parties will result in the issuance of any patents. The issued patents that we own or may receive in the future, may be challenged, invalidated or circumvented, and the rights granted under any issued patents may not provide us with sufficient protection or competitive advantages against competitors with similar technology. Furthermore, our competitors may be able to independently develop and commercialize similar drugs or duplicate our technology, business model or strategy without infringing our patents. Because of the extensive time required for clinical development and regulatory review of a drug we may develop, it is possible that, before any of our drugs can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of any such patent. The patent positions for our most advanced programs are summarized below:plus any extensionsexclusive of possible patent term available under national law.twothree issued patents covering the treatment of anemia by administration of sotatercept in the United States and similar patents issued or pending in many other major jurisdictions worldwide, including Europe, Australia, Canada, Japan, China, South Korea, Brazil, Mexico, Russia, Israel and India. The expected expiration date for these compositionmethod of mattertreatment patents is 2027, exclusive of possible patent term extensions.reductiontreatment of tumor cell burden in multiple myeloma.ACE-536 Patent Coverage We hold two issued patents covering the ACE-536 composition of matter in the United States,myeloma and additional patents issued or pending in many other major jurisdictions worldwide, including Europe, Japan, China, South Korea, Brazil, Mexico, Russia and India. The expected expiration dates for these composition of matter patents are 2028 and 2029, exclusive of possible patent term extensions. We hold one issued patent covering the treatment of anemia by administration of ACE-536bone loss in the United States and similar patents issued or pending in other major jurisdictions worldwide, includingEurope, Japan, China, South Korea, Brazil, Mexico, Russia and India. The expected expiration date for these method of treatment patents is 2029 exclusive of possible patent term extensions.claiming the dalantercept composition of matter, if issued, are either 2027 or 2029, exclusive of possible patent term extensions. InACE-536luspatercept and the discontinued program ACE-031.ACE-031, which we refer to as the ActRIIB Agreement. The licenses granted are exclusive as to the therapeutic products that are covered by the patents and non-exclusive as to diagnostic products and other products that are developed using the Salk patent rights. If we sublicense the Salk patent rights, we will owe Salk a percentage of sublicensing revenue, excluding payments based on sales. Under the agreements, Salk retained rights, on behalf of itself and other non-profit academic institutions, to practice under the licensed rights for non-profit purposes. We agreed to pay Salk specified development milestone payments totaling up to $2.0 million for sotatercept and $0.7 million for ACE-536.luspatercept. In addition, we are required to pay Salk royalties in the low single-digits on worldwide net product sales by us or our sublicensees of products claimed in the licensed patents, or derived from use of the licensed patent rights, with royalty obligations continuing at a reduced rate for a period of time after patent expiration. The agreements terminate upon the expiration of royalty obligations. We may terminate either agreement at any time by giving Salk advance written notice. Either agreement may also be terminated by Salk in the event of a material breach by us or in the event we become subject to bankruptcy or similar circumstances.protein therapeuticACE-536,luspatercept, dalantercept, ACE-083 and dalanterceptACE-2494 to be regulated by the FDA as biologics and to be reviewed by the Center for Drug Evaluation and Research (CDER) as proteins intended for therapeutic use. Protein therapeuticsTherapeutic candidates require the submission of a Biologics License Application, or BLA, and approval by the FDA prior to being marketed in the U.S. Manufacturers of protein therapeuticstherapeutic candidates may also be subject to state regulation. Failure to comply with FDA requirements, both before and after product approval, may subject us or our partners, contract manufacturers, and suppliers to administrative or judicial sanctions, including FDA refusal to approve applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, fines and/or criminal prosecution.••••••protein therapeutic.involves trials ininvolve a larger, but still limited, patient population to evaluate preliminarily the efficacy of the drug candidate for specific, targeted indications to determine dosage tolerance and optimal dosage and to identify possible short-term adverse effects and safety risks. protein therapeutic candidates. Results from one trial are not necessarily predictive of results from later trials. Furthermore, the FDA or the sponsor may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB's requirements or if the drug candidate has been associated with unexpected serious harm to patients.2013,2016, is almost $2.0approximately $2.4 million, subject to certain limited deferrals, waivers, and reductions that may be available. The fees typically increase each year. Each BLA submitted to the FDA for approval is reviewed for administrative completeness and reviewability within 60 days following receipt by the FDA of the application. If the BLA is found complete, the FDA will file the BLA, triggering a full review of the application. The FDA may refuse to file any BLA that it deems incomplete or not properly reviewable at the time of submission. The FDA's established goal is to review 90% of priority BLA applications within six months after the application is accepted for filing and 90% of standard BLA applications within 10 months of the acceptance date, whereupon a review decision is to be made. The FDA, however, may not approve a drug candidate within these established goals and its review goals are subject to change from time to time. Further, the outcome of the review, even if generally favorable, may not be an actual approval but a "complete response letter" that describes additional work that must be done before the application can be approved. Before approving a BLA, the FDA may inspect the facility or facilities at which the product is manufactured and will not approve the product unless the facility complies with cGMPs. The FDA may deny approval of a BLA if applicable statutory or regulatory criteria are not satisfied, or may require additional testing or information, which can extend the review process. FDA approval of any application may include many delays or never be granted. If a product is approved, the approval may impose limitations on the uses for which the product may be marketed, may require thatprotein therapeutic candidates under development. recently-enacted Patient Protection and Affordable Care Act of 2010, under the subtitle of Biologics Price Competition and Innovation Act of 2009, or the BPCI, a statutory pathway has been created for licensure, or approval, of biological products that are biosimilar to, and possibly interchangeable with, earlier biological products licensed under the Public Health Service Act. Also under the BPCI, innovator manufacturers of original reference biological products are granted 12 years of exclusivity before biosimilars can be approved for marketing in the United States. The objectives of the BPCI are conceptually similar to those of the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the "Hatch-Waxman Act", which established abbreviated pathways for the approval of drug products. The implementation of an abbreviated approval pathway for biological products is under the direction of the FDA and is currently being developed. In late 2010, the FDA held a hearing to receive comments from a broad group of stakeholders regarding the implementation of the BPCI. Since that hearing in 2010, the FDA, in February 2012 and February 2013, has issued several draft guidances for industry related to the BPCI, addressing scientific, quality and procedural issues relevant to an abbreviated application for a biosimilar product. The approval of a biologic product biosimilar to one of our products could have a material adverse impact on our business as it may be significantly less costly to bring to market and may be priced significantly lower than our products.ACE-536Luspatercept has orphan drug designation in the United States for the treatment ofb-thalassemia beta-thalassemia and for the treatment of MDS. The FDA has granted orphan designation for sotatercept for the treatment ofb-thalassemia.The law required the FDA to issue related draft guidance within a year after the law's enactment and also promulgate confirming regulatory changes. In June 2013,May 2014, the FDA published a draft Guidance for Industry entitled, "Expedited Programs for Serious Conditions—Drugs and Biologics" which provides guidance on FDA programs that are intended to facilitate and expedite development and review of new drugs as well as threshold criteria generally applicable to concluding that a drug is a candidate for these expedited development and review programs. In addition to the Fast Track, accelerated approval and priority review programs discussed above, the FDA also provided guidance on a new program for Breakthrough Therapy designation. A request for Breakthrough Therapy designation should be submitted concurrently with, or as an amendment to an IND. FDA has already granted this designation to over 30100 new drugs and recentlyhas approved a couple ofmore than 25 Breakthrough Therapy designated drugs.payors,payers, such as government health programs, commercial insurance and managed healthcare organizations. These third-party payorspayers are increasingly challenging the prices charged for medical products and services and imposing controls to manage costs. The containment of healthcare costs has become a priority of federal and state governments and the prices of drugs have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. In addition, there is significant uncertainty regarding the reimbursement status of newly approved healthcare products. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. If third-party payorspayers do not consider our products to be cost-effective compared to other therapies, the payorspayers may not cover our protein therapeutic candidates, we may seek approval and coverage for those products under Medicaid, Medicare and the Public Health Service (PHS) pharmaceutical pricing program and also seek to sell the products to federal agencies.FFSFSS participation is required for a drug product to be covered and paid for by certain federal agencies and for coverage under Medicaid, Medicare Part B and the PHS pharmaceutical pricing program. FSS pricing is negotiated periodically with the Department of Veterans Affairs. FSS pricing is intended to not exceed the price that a manufacturer charges its most-favored non-federal customer for its product. In addition, prices for drugs purchased by the Veterans Administration, Department of Defense (including drugs purchased by military personnel and dependents through the TRICARE retail pharmacy program), Coast Guard, and PHS are subject to a cap on pricing (known as the "federal ceiling price") and may be subject to an additional discount if pricing increases more than inflation.payors,payers, it is not clear what effect, if any, the research will have on the sales of any product, if any such product or the condition that it is intended to treat is the subject of a study. It is also possible that comparative effectiveness research demonstrating benefits in a competitor's product could adversely affect the sales of any of our approved protein therapeutics.therapeutic candidates. If third-party payorspayers do not consider our products to be cost-effective compared to other available therapies, they may not cover our products as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.health carehealthcare programs. Adoption of other new legislation at the federal or state level could further limit reimbursement for pharmaceuticals.protein therapeutic candidates or products to other available therapies. The conduct of such a clinical trial could be expensive and result in delays in our commercialization efforts. Third-party payorspayers are challenging the prices charged for medical products and services, and many third-party payorspayers limit reimbursement for newly-approved health carehealthcare products. Recent budgetary pressures in many European Union countries are also causing governments toprotein therapeutic candidates. Whether or not we obtain FDA approval for a protein therapeutic candidate, we must obtain approval from the comparable regulatory authorities of foreign countries or economic areas, such as the European Union, before we may commence clinical trials or market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
ManufacturingManufacturingACE-536luspatercept, dalantercept, ACE-083 and dalantercept.ACE-2494. We manufacture material compliant to U.S. and European cGMP at our 12,000 square foot multi-product facility located at our corporate headquarters in Cambridge, Massachusetts. We have the capabilities to manufacture receptor fusion proteins, monoclonal antibodies, and other protein therapeutics.20 fulltime22 full time employees focus on our process development and manufacturing activities. We believe that our strategic investment in manufacturing capabilities allows us to advance our protein therapeutic candidates at a more rapid pace and provides us with more portfolio flexibility than if we used a contract manufacturer. The facility produces drug substance in a cost-effective manner while allowing us to retain control over the process and provides an ability to balance the requirements of multiple programs and avoid costly commitments of funds before clinical data are available.researchpreclinical stage lead molecules into manufacturing. We have designed our manufacturing facility and processes to provide maximum flexibility and rapid change over for the manufacture of different protein therapeutic candidates. We outsource fill-finish, packaging, labeling, shipping, and distribution. protein therapeutic candidates using readily available raw materials and well established manufacturing procedures based on a standardized process modified for each of our protein therapeutic candidates. We produce our proteins in bioreactors using Chinese hamster ovary cells that have been genetically engineered to produce our specific protein therapeutic candidates. We then purify the proteins using industry standard methods, which include affinity chromatography and ultrafiltration operations. Processes developed within our facility have been successfully transferred to commercial facilities based on stainless steel bioreactors. We have conducted comparability characterization on sotatercept between our Phase 2 material and material made at a commercial manufacturer and found them to be comparable.protein therapeutic candidates. For our early phase protein therapeutic candidates, we intend to continue to manufacture drug substance for preclinical testing and Phase 1 and Phase 2 clinical development at our current facilities. As ACE-536luspatercept progresses to Phase 3 clinical trials, we intend to transfer the process for Phase 3 production to Celgene, under the terms of our collaboration agreements. We have already successfully transferred the manufacturing process for sotatercept to Celgene, and we expect Celgene will use a contract manufacturer for Phase 3 and commercial supply of sotatercept and ACE-536.luspatercept. We intend to contract with a third party manufacturer for the supply of dalantercept and ACE-083 for Phase 3 clinical trials.2013,2015, we had 80102 full-time employees, 6376 of whom are involved in research, development or manufacturing, and 2027 of whom have Ph.D. or M.D. degrees. We have no collective bargaining agreements with our employees and we have not experienced any work stoppages. We consider our relations with our employees to be good.
Corporate InformationTableWe were incorporated in the state of ContentsDelaware in June 2003 as Phoenix Pharma, Inc., and we subsequently changed our name to Acceleron Pharma Inc. and commenced operations in February 2004. Our principal executive offices are located at 128 Sidney Street, Cambridge, Massachusetts 02139, and our telephone number is (617) 649-9200. Our Internet address isInformation Available on the InternetOur internet addressis www.acceleronpharma.com. The contents of our website are not part of this Annual Report on Form 10-K and our internet address is included in this document as an inactive textual reference.10-K. We make available free of charge through our web sitewebsite our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to these reports filed or furnished pursuant to Section 12(a)13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, as soon as reasonably practicable after we electronically file or furnish such materials to the Securities and Exchange Commission. You may read and copy any materials filed with the Securities and Exchange Commission at the Securities and Exchange Commission's Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operationwww.sec.gov. The reference to these website addresses does not constitute incorporation by reference of the information contained on the websites and should not be considered part of this document.relatedRelated to our financial positionOur Financial Position and needNeed for additional capital2013,2015, we had an accumulated deficit of $192.3$307.5 million. We do not know whether or when we will become profitable. To date, we have not commercialized any products or generated any revenues from the sale of products, and we do not expect to generate any product revenues in the foreseeable future. Our losses have resulted principally from costs incurred in our discovery and development activities. protein therapeutic candidates. If we do not receive the anticipated milestone payments or do not enter into partnerships for dalantercept, ACE-083 or other protein therapeutic candidates on acceptable terms, our operating losses will substantially increase over the next several years as we execute our plan to expand our discovery, research, development, manufacturing and commercialization activities. There can be no assurance that we will enter into a new collaboration or achieve milestones and, therefore, no assurance our losses will not increase prohibitively in the future. protein therapeutic candidates, obtaining regulatory approval for them, and manufacturing, marketing and selling those products for which we or our partners may obtain regulatory approval. We or they may not succeed in these activities, and we may never generate revenue from product sales that is significant enough to achieve profitability. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our failure to become or remain profitable would depress our market value and could impair our ability to raise capital, expand our business, discover or develop other protein therapeutic candidates or continue our operations. A decline in the value of our company could cause you to lose all or part of your investment.2013,2015, our cash, and cash equivalents and investments were $113.2$136.0 million. We believe that we will continue to expend substantial resources for the foreseeable future developing dalantercept, ACE-083 and new protein therapeutic candidates. These expenditures will include costs associated with research and development, potentially acquiring new technologies, conducting preclinical studies and clinical trials, potentially obtaining regulatory approvals and manufacturing products, as well as marketing and selling products approved for sale, if any. In addition, other unanticipated costs may arise. Because the outcome of our planned and anticipated clinical trials is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our protein therapeutic candidates.ACE-536.luspatercept. Other than those costs, our future capital requirements depend on many factors, including:•protein therapeutic candidates, and conducting preclinical studies and clinical trials;•protein therapeutic candidates if clinical trials are successful;•protein therapeutics,therapeutic candidates, if any of these protein therapeuticstherapeutic candidates is approved for sale, including marketing, sales and distribution costs;• protein therapeutic candidates for clinical trials in preparation for regulatory approval and in preparation for commercialization;•••initial public offering and the concurrent private placement, and the follow-on public offering together within January 2016 and receipt of anticipated milestone payments, and our existing cash and cash equivalents will be sufficient to fund our projected operating requirements into the second half of 2017.2019. However, our operating plan may change as a result of many factors currently unknown to us, and we may need additional funds sooner than planned. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe that we have sufficient funds for our current or future operating plans. Additional funds may not be available when we need them on terms that areprotein therapeutic candidates or delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our protein therapeutic candidates.protein therapeuticstherapeutic candidates on unfavorable terms to us.protein therapeutics,therapeutic candidates, or grant licenses on terms that are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts for dalantercept, ACE-083 or any protein therapeuticstherapeutic candidates other than sotaterceptluspatercept or ACE-536,sotatercept, or grant rights to develop and market protein therapeuticstherapeutic candidates that we would otherwise prefer to develop and market ourselves.Protein Therapeutic Candidatesprotein therapeutics,therapeutic candidates, our business will be adversely affected. protein therapeutic candidates will be subject to extensive governmental regulations relating to, among other things, development, clinical trials, manufacturing and commercialization. In order to obtain regulatory approval for the commercial sale of any protein therapeutic candidates, we or our partners must demonstrate through extensive preclinical studies and clinical trials that the protein therapeutic candidate is safe and effective for use in each target indication. Clinical testing is expensive, time-consuming and uncertain as to outcome. We or our partners may gain regulatory approval for sotatercept, ACE-536,luspatercept, dalantercept, ACE-083 or any other protein therapeutic candidate in some but not all of the territories available or some but not all of the target indications or may receive approval with limited labeling or boxed warnings, resulting in limited commercial opportunity for the approved protein therapeutics,therapeutic candidates, or we or they may never obtain regulatory approval for these protein therapeutic candidates. protein therapeutic candidates could result in increased costs to us as well as a delay or failure in obtaining regulatory approval, or prevent us from commercializing our protein therapeutic candidates on a timely basis, or at all.••••••••protein therapeutic candidates to the clinical sites;•••protein therapeutic candidates that are viewed to outweigh its potential benefits; or•protein therapeutic candidates.Clinical may occur at any stage of clinical development, and because our protein therapeutic candidates are in an early stage of development, there is a high risk of failure, and we may never succeed in developing marketable products or generating product revenue. early encouraging preclinical and clinical results to date for sotatercept, ACE-536luspatercept, dalantercept and dalanterceptACE-083 are not necessarily predictive of the results of our ongoing or future clinical trials. Promising results in preclinical studies of a drug candidate may not be predictive of similar results in humans during clinical trials, and successful results from early clinical trials of a drug candidate may not be replicated in later and larger clinical trials or in clinical trials for different indications. If the results of our or our current or future partners' ongoing or future clinical trials are inconclusive with respect to the efficacy of our protein therapeutic candidates or if we or they do not meet the clinical endpoints with statistical significance or if there are safety concerns or adverse events associated with our protein therapeutic candidates, we or our partner may be prevented or delayed in obtaining marketing approval for our protein therapeutic candidates. In addition, data obtained from trials and studies are susceptible to varying interpretations, and regulators may not interpret our data as favorably as we do, which may delay or prevent regulatory approval. Alternatively, even if we or our partners obtain regulatory approval, that approval may be for indications or patient populations that are not as broad as intended or desired or may require labeling that includes significant use or distribution restrictions or safety warnings. We or our partners may also be required to perform additional or unanticipated clinical trials to obtain approval or be subject to additional post-marketing testing requirements to maintain regulatory approval. In addition, regulatory authorities may withdraw their approval of a product or impose restrictions on its distribution, such as in the form of a modified risk evaluation and mitigation strategy.protein therapeutics,therapeutic candidates, if approved, we or they may be subject to disciplinary action by the FDA's Office of Prescription Drug Promotion (OPDP) or other regulatory authorities.If we or our partners fail to obtain regulatory approval in jurisdictions outside the United States, we and they will not be able to market our products in those jurisdictions. We and our partners intend to market our protein therapeutic candidates, if approved, in international markets. Such marketing will require separate regulatory approvals in each market and compliance with numerous and varying regulatory requirements. The approval procedures vary from country-to-country and may require additional testing. Moreover, the time required to obtain approval may differ from that required to obtain FDA approval. In addition, in many countries outside the United States, a protein therapeutic must be approved for reimbursement before it can be approved for sale in that country. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We or our partners may not obtain foreign regulatory approvals on a timely basis, if at all. We or our partners may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market.Even if we or our partners receive regulatory approval for our protein therapeutic candidates, such products will be subject to ongoing regulatory review, which may result in significant additional expense. Additionally, our protein therapeutic candidates, if approved, could be subject to labeling and other restrictions, and we or our partners may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our products. Any regulatory approvals that we or our partners receive for our protein therapeutic candidates may also be subject to limitations on the approved indicated uses for which the product may be marketed or to conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 clinical trials, and surveillance to monitor safety and efficacy. In addition, if the FDA approves any of our protein therapeutic candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping for the product will be subject to extensive and ongoing regulatory requirements. These requirementsinclude submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with current good manufacturing practice, or cGMP, and GCP, for any clinical trials that we or our partners conduct post-approval. Later discovery of previously unknown problems with an approved protein therapeutic, including adverse events of unanticipated severity or frequency, or with manufacturing operations or processes, or failure to comply with regulatory requirements, may result in, among other things:•restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls;•fines, warning letters, or holds on clinical trials;•refusal by the FDA to approve pending applications or supplements to approved applications filed by us or our partners, or suspension or revocation of product license approvals;•product seizure or detention, or refusal to permit the import or export of products; and•injunctions or the imposition of civil or criminal penalties. The FDA's policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our protein therapeutic candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we or our partners are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or not able to maintain regulatory compliance, we or our partners may lose any marketing approval that may have been obtained and we may not achieve or sustain profitability, which would adversely affect our business.two of our three clinical stage protein therapeutic candidates, sotatercept and ACE-536.our most advanced therapeutic candidate, luspatercept. If Celgene does not devote sufficient resources to the development of these candidates, discontinues development of these candidates, is unsuccessful in its efforts, or chooses to terminate its agreements with us, our business will be materially harmed.ACE-536.luspatercept. Pursuant to the sotatercept agreement, responsibility for all clinical and other product development activities and for manufacturing sotatercept has been transferred to Celgene. For ACE-536,luspatercept, we are responsible for conducting the two ongoing Phase 2 clinical trials in MDS, and we are also responsible for manufacturing supplies for Phase 1 and Phase 2 studies. Celgene will be responsible for all clinical development and manufacturing activities after such studies are completed. As of January 1, 2013, Celgene became responsible for paying 100% of worldwide development costs for sotatercept and ACE-536.luspatercept. We will co-promote sotatercept and ACE-536,luspatercept, if approved by the FDA and its counterparties, in North America. Celgene will be responsible for all commercialization costs, including the cost of our promotion activities.ACE-536.luspatercept. Celgene may determine that it is commercially reasonable to develop and commercialize only sotaterceptluspatercept or ACE-536sotatercept and discontinue the development or commercialization of the other protein therapeutic candidate, or Celgene may determine that it is not commercially reasonable to continue development of one or both of sotatercept and ACE-536.luspatercept. For example, Celgene may elect not to undertake the development of sotatercept for the treatment of pre-dialysis patients. This may occur for many reasons, including internal business reasons or because of unfavorable regulatory feedback. Further, on review of the safety and efficacy data available to date, the FDA may impose requirements on the clinical trial program that render such a program commercially nonviable. In the event of any such decision, we maywould be unable to progress the discontinued candidatesotatercept for this or candidatesother indications ourselves. In addition, under our collaboration agreements, once Celgene takes over development activities of a protein therapeutic candidate, it may determine the development plan and activities for that protein therapeutic candidate. We may disagree with Celgene about the development strategy it employs, butACE-536luspatercept to narrower indications than we would pursue. We would be prevented from developing or commercializing a candidate in an indication that Celgene has chosen not to pursue.•protein therapeutic candidates by Celgene.• protein therapeutic candidates that are the subject of its partnerships with us. For example, Celgene is currently commercializing and/or developing certain of its existing products, lenalidomide and azacitidine, for certain MDS patients for which sotatercept and ACE-536 areluspatercept is also being developed.•• protein therapeutic candidates in such a way as to elicit litigation that could jeopardize or invalidate our intellectual property rights or expose us to potential liability.••protein therapeutic candidates. If we were to terminate an agreement with Celgene due to Celgene's breach or Celgene terminated the agreement without cause, the development and commercialization of sotatercept and ACE-536luspatercept could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continueown.own if we choose not to, or are unable to, enter into a new collaboration for these candidates.• protein therapeutic candidates, and if they do not properly and successfully perform their obligations to us, we may not be able to obtain regulatory approvals for our protein therapeutic candidates. protein therapeutic candidates, and we will continue to work with Celgene on trials for sotatercept and ACE-536.luspatercept. However, we and Celgene rely on CROs and other third parties to assist in managing,protein therapeutic candidates.protein therapeutic candidates may not meet regulatory requirements.requirements or may be delayed. If clinical trials do not meet regulatory requirements or if these third parties need to be replaced, preclinical development activities or clinical trials may be extended, delayed, suspended or terminated. If any of these events occur, we or Celgene may not be able to obtain regulatory approval of our protein therapeutic candidates on a timely basis or at all. and Celgene intend to rely on third-party manufacturers to make our protein therapeutics,therapeutic candidates, and any failure by a third-party manufacturer may delay or impair our and Celgene's ability to complete clinical trials or commercialize our protein therapeutic candidates.ACE-536luspatercept, dalantercept, ACE-083 and dalantercept.ACE-2494. For Phase 3 and commercial supply of our products that we have not partnered, we expect to use contract manufacturing organizations. Successfully transferring complicated manufacturing techniques to contract manufacturing organizations and scaling up these techniques for commercial quantities will be time consuming and we may not be able to achieve such transfer. Moreover, the market for contract manufacturing services for protein therapeuticstherapeutic candidates is highly cyclical, with periods of relatively abundant capacity alternating with periods in which there is little available capacity. If any need we or Celgene have for contract manufacturing services increases during a period of industry-wide tight capacity, we or Celgene may not be able to access the required capacity on a timely basis or on commercially viable terms. and Celgene's contractors' ability to operate or lead to delays in our clinical development programs. We believe that our current fill & finish contractors are operating in accordance with cGMP, but we can give no assurance that FDA or other regulatory agencies will not conclude that a lack of compliance exists. In addition, any delay in contracting for fill & finish services, or failure of the contract manufacturer to perform the services as needed, may delay clinical trials, registration and launches. Any such issues may have a substantial negative effect on our business. our two lead products, sotatercept and ACE-536,our most advanced therapeutic candidate, luspatercept, we rely on our collaboration partner Celgene to produce, or contract for the production of, bulk drug substance and finished drug product for late stage clinical trials and for commercial supplies of any approved candidates. Any failure by Celgene or by third-parties with which Celgene contracts may delay or impair the ability to complete late stage clinical trials or commercialize either or both of sotatercept and ACE-536,luspatercept, if approved. We produced drug substance for preclinical and Phase 1 and 2 clinical trials for sotatercept and ACE-536. will be responsible for manufacturing ACE-536luspatercept for future late-stage clinical trials. Celgene generally does not perform the manufacture of the drug substance or drug product for either sotatercept or ACE-536luspatercept itself. Celgene has used and may continue to use contract manufacturers for the manufacture of drug substance and drug product for sotatercept and we have no expectation that Celgene plans to perform the manufacture of bulk drug substance or drug product for either sotatercept or ACE-536luspatercept in the future. However, Celgene would have the right to manufacture sotatercept or ACE-536,luspatercept, itself or through the use of contract manufacturers. We understand that they have entered into a manufacturing arrangementarrangements for Phase 2clinical and commercial supplies of sotatercept and luspatercept bulk drug substance with contract manufacturers with considerable biotherapeutics manufacturing experience, including manufacturing monoclonal antibodies through processes similar to those used for sotatercept. To date Celgene has not entered into an arrangement with a third party to manufacture suppliesIf any of sotaterceptthese manufacturers is unwilling or ACE-536 for commercial sales. If they are unable to contract at the appropriate time with a manufacturer willing and able to manufacture sufficient quantities of sotatercept and ACE-536and/or luspatercept to meet clinical or commercial demand, either for technical or business reasons, the development and commercialization of sotatercept and ACE-536and/or luspatercept may be delayed.a part of our strategy is to strategically evaluate and as deemed appropriate, enter into additional partnerships in the future for our other product candidates when strategically attractive, including potentially with major biotechnology or pharmaceutical companies. We face significant competition in seeking appropriate partners for our protein therapeutic candidates, and the negotiation process is time-consuming and complex. In order for us to successfully partner our protein therapeutic candidates, potential partners must view these protein therapeutic candidates as economically valuable in markets they determine to be attractive in light of the terms that we are seeking and other available products for licensing by other companies. Even if we are successful in our efforts to establish new strategic partnerships, the terms that we agree upon may not be favorable to us, and we may not be able to maintain such strategic partnerships if, for example, development or approval of a protein therapeutic candidate is delayed or sales of an approved product are disappointing. Any delay in entering into new strategic partnership agreements related to our proteinother therapeutic candidates could delay the development and commercialization of our proteinthese therapeutic candidates and reduce their competitiveness even if they reach the market.our proteindalantercept, ACE-083 or other therapeutic candidates, we will bear all of the risk and costs related to the development of any such protein therapeutic candidate, and we may need to seek additional financing, hire additional employees and otherwise develop expertise for which we have not budgeted. This could negatively affect the development of any unpartnered protein therapeutic candidate.protein therapeutic candidates, we may not be able to compete effectively. protein therapeutic candidates. The patent position of biotechnology companies is generally uncertain because it involves complex legal and factual considerations. The standards applied by the United States Patent and Trademark Office, or USPTO, and foreign patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in biotechnology patents. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our protein therapeutic candidates in the United States or in other countries. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found. We may be unaware of prior art that could be used to invalidate an issued patent or prevent our pending patent applications from issuing as patents. Even if patents do protein therapeutic candidates, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our protein therapeutic candidates or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business. protein therapeutic candidates fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity for our protein therapeutic candidates, it could dissuade companies from collaborating with us. Several patent applications covering our protein therapeutic candidates have been filed recently. We cannot offer any assurances about which, if any, patents will issue, the breadth of any such patents or whether any issued patents will be found invalid and unenforceable or will be threatened by third parties. Any successful challenge to these patents or any other patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any protein therapeutic candidate that we or our current or future partners may develop. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we were the first to file any patent application related to a protein therapeutic candidate. Furthermore, if third parties have filed such patent applications, an interference proceeding in the United States can be initiated by the USPTO or a third party to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. In addition, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. Various extensions may be available; however, the life of a patent and the protection it affords is limited. If we encounter delays in obtaining regulatory approvals, the period of time during which we could market a protein therapeutic candidate under patent protection could be reduced. Even if patents covering our protein therapeutic candidates are obtained, once the patent life has expired for a product, we may be open to competition from biosimilar products.partnercurrent or future partners may be unable to prevent competitors from entering the market with a product that is similar to or the same as our protein therapeutics.therapeutic candidates. In addition, the royalty we would receive under our collaboration agreements with Celgene for sotatercept and ACE-536luspatercept would be reduced by 50% if such product ceases to be covered by a valid claim of our patents even if no competitor with a similar product has entered the market. protein therapeutic candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our protein therapeutic candidates may be subject to claims of infringement of the patent rights of third parties. protein therapeutic candidates, that we failed to identify. For example, applications filed before November 29, 2000 and certain applications filed after that date that will not be filed outside the United States remain confidential until issued as patents. Except for the preceding exceptions, patent applications in the United States and elsewhere are generally published only after a waiting period of approximately 18 months after the earliest filing. Therefore, patent applications covering our platform technology or our protein therapeutic candidates could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our platform technologies, our protein therapeutic candidates or the use or manufacture of our protein therapeutic candidates. protein therapeutic candidate until such patent expired or unless we or our partners obtain a license. These licenses may not be available on acceptable terms, if at all. Even if we or our partners were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we or our partners could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we or our partners are unable to enter into licenses on acceptable terms. If Celgene is required to enter a license agreement with a third party in order to import, develop,ACE-536,luspatercept, the royalty rate and sales milestone payments that we could receive may be reduced by up to 50%. This could harm our business significantly. protein therapeutic candidates. Defending against claims of patent infringement or misappropriation of trade secrets could be costly and time consuming, regardless of the outcome. Thus, even if we were to ultimately prevail, or to settle at an early stage, such litigation could burden us with substantial unanticipated costs. In addition, litigation or threatened litigation could result in significant demands on the time and attention of our management team, distracting them from the pursuit of other company business. In the event of a successful claim of infringement against us or our partners, we may have to pay substantial damages, including treble damages and attorneys' fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.protein therapeutic candidates, and we may be required to pay damages.protein therapeutics,therapeutic candidates, programs, or intellectual property could be diminished. Accordingly, the market price of our common stock maycould decline.protein therapeutic candidate, may be adversely affected. For example, the Salk Institute for Biological Studies recently filed a lawsuit against us alleging under one of our license agreements with them, which pertains to ActRIIB, its entitlement to a further share of certain payments received by us under our now-terminated agreement with Shire AG and a share of certain payments received by us under our on-going collaboration agreement with Celgene in connection with ACE-536. Although we and Salk have agreed that ACE-536 is not covered by any patent rights licensed from Salk, an unfavorable outcome in this litigation may lead to us owing significant damages to Salk and higher-than-anticipated future payments under this license in connection with development milestone payments that we may receive from Celgene. It is possible that Salk may seek to terminate the license covering the receptor. We do not believe that such a termination would have a material impact on our business or the development of any of our products. The patents under this license covered only one of our protein therapeutic candidates, ACE-031, the development of which has been discontinued. See "Business—Legal Proceedings" for a description of this proceeding.businessProtein Therapeutic Candidatesprotein therapeutic candidates, if approved, among physicians, patients, health carehealthcare payers and in cancer markets, acceptance by the operators of major cancer clinics.ACE-536,luspatercept, dalantercept, ACE-083 or any other protein therapeuticstherapeutic candidates that we may develop or acquire in the future, the product may not gain market acceptance among physicians, health care payors,healthcare payers, patients and the medical community. Market acceptance of any approved products depends on a number of factors, including:••••••••• protein therapeutic candidate, if approved and commercialized, may be accepted in only limited capacities or not at all. If any approved products are not accepted by the market to the extent that we expect, we may not be able to generate significant revenue and our business would suffer. protein therapeutic candidates, which could make it difficult for us to sell our products profitably.protein therapeuticstherapeutic candidates will depend significantly on the availability of adequate coverage and reimbursement from third-party payers and may be affected by existing and future health carehealthcare reform measures. Government authorities and third-party payors,payers, such as private health insurers and health maintenance organizations, decide which drugs they will pay forpayor'spayer's determination that use of a product is:••••• protein therapeutic candidates. Also, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our products. If reimbursement is not available or is available only to limited levels, we may not be able to commercialize certain of our products. In addition in the United States, third-party payers are increasingly attempting to contain health carehealthcare costs by limiting both coverage and the level of reimbursement of new drugs. As a result, significant uncertainty exists as to whether and how much third-party payers will reimburse patients for their use of newly approved drugs, which in turn will put pressure on the pricing of drugs. protein therapeutic candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-party payers or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be adversely affected.The impact of recenton us is currently unknown, and may adversely affect our business model.health carehealthcare availability, the method of delivery or payment for health carehealthcare products and services could negatively impact our business, operations and financial condition. There is significant interest in promoting health carehealthcare reform, as evidenced by the enactment in the United States of the Patient Protection and Affordable Care Act and the Health Care and Education Reconciliation Act in 2010. It is likely that federal and state legislatures within the United States and foreign governments will continue tohealth carehealthcare legislation. We cannot predict the reform initiatives that may be adopted in the future or whether initiatives that have been adopted will be repealed or modified. The continuing efforts of the government, insurance companies, managed care organizations and other payers of healthcare services to contain or reduce costs of healthcare may adversely affect:•••••protein therapeutic candidates. Our objective is to design, develop and commercialize new products with superior efficacy, convenience, tolerability and safety. In many cases, the protein therapeuticstherapeutic candidates that we commercialize with our strategic partners or on our own will compete with existing, market-leading products.ACE-536Luspatercept or sotatercept, if approved, will compete with these therapies. In addition, one or more products not currently approved for the treatment of anemia in MDS may in the future be granted marketing approval for the treatment of anemia in MDS or other conditions for which ACE-536luspatercept or sotatercept might be approved, including Aranesp®, being developed by Amgen, which is in Phase 3 trials. While there are currently no drug products approved for the treatment of anemia inb-thalassemia, beta-thalassemia, red blood cell transfusions are extensively used and sotatercept or ACE-536,luspatercept, if approved, would compete with this therapy. In addition, HQK-1001, a fetal hemoglobin stimulating agent being developed by HemaQuest Pharmaceuticals, has completed a Phase1/2 clinical trial and an investigator sponsored Phase 2 clinical trial in patients withb-thalassemia. Further, the future approval, in one or more regions, of a biosimilar product to one of our products could create substantial competition and have a material impact on our business.ACE-536,luspatercept, if approved for the treatment of chemotherapy-induced anemia or anemia of chronic kidney disease, would compete with erythropoiesis-stimulating agents, such as Epogen® and Aranesp®, marketed by Amgen, and Procrit®, marketed by Johnson & Johnson, that are currently approved for the treatment of chemotherapy-induced anemia or anemia of chronic kidney disease and other therapies in development including oral, small molecule treatments being developed by Astellas Pharma and Fibrogen designed to increase the body's production of erythropoietin.protein therapeuticstherapeutic candidates that we develop obsolete. As a result of all of these factors, our competitors may succeed in obtaining patent protection and/or FDA approval or discovering, developing and commercializing protein therapeuticstherapeutic candidates before we do. In addition, any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and to be commercially successful. If we are not able to compete effectively against potential competitors, our business will not grow and our financial condition and operations will suffer.Our protein therapeuticscause undesirable side effectsincur additional costs or have other properties that delayexperience delays in completing, or prevent their regulatory approval or limit their commercial potential.protein therapeuticstherapeutic candidates could cause us, Celgene or regulatory authorities to interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities and potential products liability claims. We and Celgene are currently conducting a number of Phase 2clinical trials for our clinical stage protein therapeutic candidates. Serious adverse events deemed to be caused by our protein therapeuticstherapeutic candidates could have a material adverse effect on the development of our protein therapeutic candidates and our business as a whole. The most common adverse event to dateFor a more complete description of the safety profile for our therapeutic candidates, see the description of each of our therapeutic candidates in the clinical trials evaluating the safety and efficacy"Business" section of our protein therapeutic candidates has been hypertension in our clinical trials for sotatercept and fluid retention in our clinical trials for dalantercept. this Annual Report on Form 10-K. protein therapeutic candidates and these events may change as we gather more information, and additional unexpected adverse events may occur. There can be no assurance that additional adverse events associated with our protein therapeutic candidates will not be observed. Recently, we discontinued the development of ACE-031, another of our clinical stage protein therapeutics that binds to ligands of the TGF-b superfamily. Clinical development of ACE-031 was put on clinical hold in 2011 due to preliminary safety observations in patients. After gathering further information from animal toxicology studies, we and our ACE-031 partner, Shire AG, determined that the risk-benefit profile of ACE-031 was not appropriate for the intended patient population, boys aged four and older with a genetic muscle wasting disease, and we discontinued development of this protein therapeutic candidate. As is typical in drug development, we have a program of ongoing toxicology studies in animals for our other clinical stage protein therapeuticstherapeutic candidates and cannot provide assurance that the findings from such studies or any ongoing or future clinical trials will not adversely affect our clinical development activities.protein therapeutic candidates either before or after receipt of marketing approval, then a number of potentially significant negative consequences could result, including:•our clinical trials may be put on hold;•maymay:regulatorymarketing approval for our protein therapeutic candidates;•regulatory authorities may withdraw approvals of our protein therapeutic candidates;•regulatory authorities may require additional warnings on the label;•patients may be required;patients;•we could and•our reputation may suffer.these events could prevent us from achieving or maintaining market acceptancewhich may harm our business and results of operations.proteintherapeutic candidates will be safe or effective, or receive regulatory approval.and could substantially increase commercialization costs.scientific personnel, we may be unable to successfully develop our protein therapeutics,therapeutic candidates, conduct our clinical trials and commercialize our protein therapeutic candidates.including our scientific co-founders, may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading. We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations, to provide accurate information to the FDA, to comply with manufacturing standards we have established, to comply with federal and state healthcare fraud and abuse laws and regulations, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.growthorganizational changes and expandingsuccessfully adjusting our operations successfully. protein therapeutic candidates through clinical trials and commercialization, we will need to expand our development, regulatory, manufacturing, marketing and sales capabilities or contract with third parties to provide these capabilities for us. As our operations expand, we expect that we will need to manage additional relationships with various strategic partners, suppliers and other third parties. Future growth will impose significant added responsibilities on members of management. Our future financial performance and our ability to commercialize ourprotein therapeutic candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train and integrate additional management, administrative and, if necessary, sales and marketing personnel. We may not be able to accomplish these tasks, and our failure to accomplish any of them could prevent us from successfully growing our company.protein therapeutics. protein therapeutic candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our protein therapeutic candidates. Even a successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:••••••••protein therapeutic candidates; and• use hazardous chemicals and radioactive and biological materials in certain aspects of our business and are subject to a variety of federal, state and local laws and regulations governing the use, generation, manufacture, distribution, storage, handling, treatment and disposal of these materials.materials that we use in our manufacturing process. Although we believe our safety procedures for handling and disposing of these materials and waste products comply with these laws and regulations, we cannot eliminate the risk of accidental injury orWe are eligible to be treated as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012, and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors. We are an "emerging growth company", as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, which we refer to as the Sarbanes-Oxley Act, (2) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and (3) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if thevalueprice of our common stock held by non-affiliates exceeds $700.0 million as of any June 30 before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in non-convertible debt during any three-year period before that time, we would cease to be an emerging growth company immediately. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.Our stock price could be highly volatile, and as a result you may not be able to resell your shares at or above the public offering price.•protein therapeutic candidates, including sotatercept, ACE-536luspatercept, dalantercept and dalantercept;ACE-083;••••••••••••••••••Our principal stockholders and management own a significant percentage of our stock and will be able to exercise significant influence over matters subject to stockholder approval. As of December 31, 2013, our executive officers, directors and principal stockholders, together with their respective affiliates, beneficially owned approximately 66.6% of our common stock, including shares subject to outstanding options and warrants that are exercisable within 60 days after such date, Accordingly, these stockholders will be able to exert a significant degree of influence over our management and affairs and over matters requiring stockholder approval, including the election of our board of directors and approval of significant corporate transactions. This concentration of ownership could have the effect of entrenching our management and/or the board of directors, delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material and adverse effect on the fair market value of our common stock.Sales of a substantial number of shares of our common stock in the public market could cause our stock pricefall. As of January 31, 2014, we have 31,160,274 shares of common stock outstanding. Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. Of these outstanding shares, 14,544,551 are currently held by directors, executive officers and other parties that may be deemed to be their or our affiliates and are available for sale subject to volume limitations, other restrictions under the securities laws and, in some cases, vesting schedules. We also have registered shares of common stock that we may issue under our equity compensation plans. These shares can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and, in certain cases, lock-up agreements that we expect to expire on April 22, 2014We are incurringincur significant increased costs as a result of operating as a public company and complying with the Sarbanes-Oxley Act, especially now that we are a large accelerated filer and are no longer an ""emerging growth company,"" and our management iscontinues to be required to devote substantial time to new compliance initiatives. newly public company, we are incurringincur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act, and rules of the SEC and those of The NASDAQ Global Market, or NASDAQ have imposed various requirements on public companies including requiring establishment and maintenance of effective disclosure and financial controls. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations have increased and will continue to increase our legal and financial compliance costs and will make some activities more time-consuming and costly.Act, beginning with our annual report on Form 10-K for the fiscal year ended December 31, 2013.Act. In addition, because we will beare no longer an emerging growth company under the JOBS Act, we are required to have our independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting beginning with our annual report on Form 10-K following the date on which we are no longer an emerging growth company.reporting. Our compliance with Section 404 of the Sarbanes-Oxley Act will requirerequires that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we willmay need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. If we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by NASDAQ, the SEC or other regulatory authorities, which would require additional financial and management resources.Commencing with our annual report on Form 10-K for the year ending December 31, 2014, we will beWe are required, under Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. This assessment will need tomust include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a control deficiency, or combination of control deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. Section 404 of the Sarbanes-Oxley Act also generally requires an attestation from our independent registered public accounting firm on the effectiveness of our internal control over financial reporting. However, forNow that we no longer qualify as long as we remain an emerging growth company as defined in the JOBS Act, we intend to take advantage ofare no longer exempted from certain exemptions from various reporting requirements, that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply withsuch as the independent registered public accounting firm attestation requirement. Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge, and compile the system and process documentation necessary to perform the evaluation needed to comply with Section 404. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. attestation.once that firm begin its Section 404 reviews, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by the NASDAQ, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.our ability to pay cash dividends is currently prohibited by the terms of our debt financing arrangement, and any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any••••••••••because we are incorporated in the state of Delaware, we are governed bysubject to the provisions of Section 203 of the Delaware General Corporation Law, which limitsprohibits a publicly-held Delaware corporation from engaging in a "business combination" with an "interested stockholder" for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. As a result, you may lose your ability of stockholders owningto sell your stock for a price in excess of 15%the prevailing market price due to these protective measures and efforts by stockholders to change the direction or management of our outstanding voting stock to merge or combine with us.Properties.
Properties94,50088,000 square feet of office and laboratory space in three adjacent buildings with aggregate monthly net-rentrent expense of approximately $0.3 million. We have subletmillion, which will increase to approximately 20,000 square feet$0.4 million in April 2016. Two of space in one of our leased buildings. Two leases expire in September 2018 and one lease expires in May 2015. March 2021.Proceedings.
Proceedings On October 18, 2012, the Salk Institute for Biological Studies, whichreferare not currently a party to as Salk, filed a complaintany material legal proceedings, we could become subject to legal proceedings in the Massachusetts Superior Court for Suffolk County, alleging that we breached oneordinary course of business. We do not expect any such potential items to have a significant impact on our two licensing agreements with Salk. The licensing agreement in dispute provides us with a license with respect to certain of Salk's U.S. patents related to the ActRIIB activin receptor proteins. Salk contends that, under the licensing agreement, we owed Salk a greater share of the upfront payment that we received under our now-terminated agreement with Shire AG regarding ACE-031 and a share of the upfront payment and development milestone payments that we have received under our ongoing collaboration agreement with Celgene regarding ACE-536. Salk is seeking a total of approximately $10.5 million plus interest in payment and a 15% share of future development milestone payments received under our agreement with Celgene regarding ACE-536. We contend that no additional amounts are due to Salk and that we have complied with all of our payment obligations under the applicable Salk license agreement. We moved to dismiss the complaint on December 3, 2012. The Court denied our motion on February 28, 2013. On March 14, 2013, Acceleron answered the complaint and asserted patent invalidity counterclaims. On the basis of those counterclaims, Acceleron removed the action on March 28, 2013 to the United States District Court for the District of Massachusetts. The parties have since reached an agreement on a stipulation as to certain patent issues raised in the action, and Acceleron has dismissed its counterclaims. The Court held an initial scheduling conference on May 30, 2013, and the fact discovery is now closed. The case is currently scheduled for trial in September 2014. We intend to defend our position vigorously.Disclosures.
DisclosuresSecurities.
SecuritiesHolders Common Stock Price 2015 2014 High Low High Low First Quarter $ 43.00 $ 35.11 $ 57.89 $ 33.81 Second Quarter $ 37.90 $ 26.94 $ 42.24 $ 28.53 Third Quarter $ 36.31 $ 20.00 $ 35.00 $ 23.61 Fourth Quarter $ 50.86 $ 21.93 $ 48.50 $ 27.64 High Low $ 23.41 $ 18.50 $ 40.02 $ 16.78 (1)Represents the period from September 19, 2013, the date on which our common stock first began to trade on The NASDAQ Global Market after the pricing of our initial public offering, through September 30, 2013, the end of our third fiscal quarter.2014,2016, there were approximately 173105 holders of record of our common stock.our ability to pay cash dividends is currently prohibited by the terms of our debt financing arrangements, and any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Any future determination to pay dividends will be made at the discretion of our board of directors.Graph(1)20132015 of cumulative total return on assumed investment of $100.00 in cash in our common stock, the NASDAQ Composite Index and the NASDAQ Biotechnology Index. Such returns are based on historical results and are not intended to suggest future performance. Data for the NASDAQ Composite Index and the NASDAQ Biotechnology Index assume reinvestment of dividends.327 MONTH CUMULATIVE TOTAL RETURN*
RETURN(1)(2)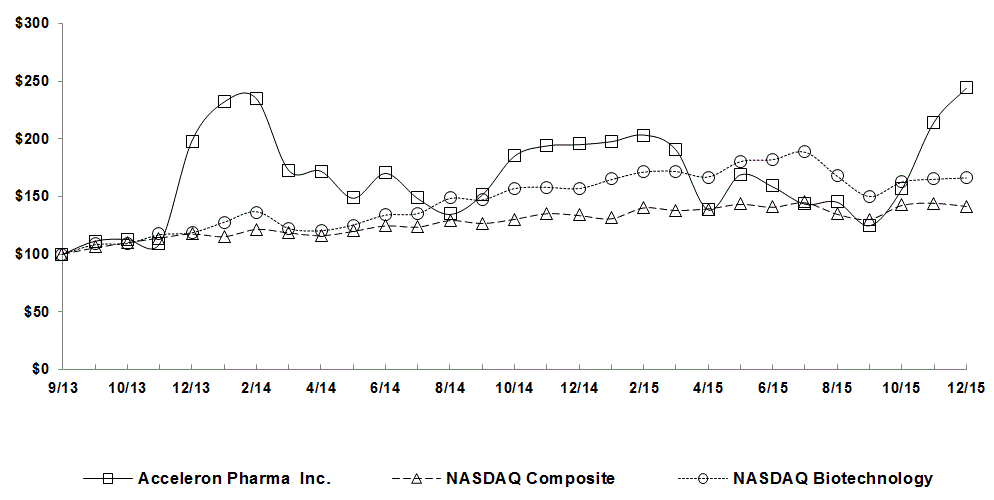
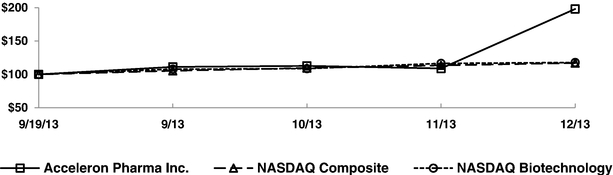
*$100 invested on 9/19/13 in stock or 8/31/13 in index, including reinvestment of dividends.Fiscal year ending December 31.(1) This performance graph shall not be deemed "soliciting material" or to be "filed" with the SEC for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or otherwise subject to the liabilities under that Section, and shall not be deemed incorporated by reference into any filing of Acceleron Pharma Inc. under the Securities Act of 1933, as amended. (2) $100 invested on September 19, 2013 in stock, or on August 31, 2013 in each index, including reinvestment of dividends. During the year ended December 31, 2013, we have issued the following securities that were not registered under the Securities Act. The following share numbers reflect the 1-for-4 reverse split of our common stock and preferred stock effected on September 5, 2013.Sales of Capital Stock On September 24, 2013, we issued 666,667 shares of common stock for total consideration of $10,000,000. This issuance of common stock was exempt pursuant to Rule 506 and Section 4(a)(2) of the Securities Act of 1933.Conversion of Preferred Stock On September 24, 2013, we issued 18,516,993 shares of common stock upon conversion of all of our outstanding shares of preferred stock. The issuance of the common stock upon conversion of the preferred stock was exempt pursuant to Section 3(a)(9) of the Securities Act of 1933.(1)This performance graph shall not be deemed "soliciting material" or to be "filed" with the SEC for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or otherwise subject to the liabilities under that Section, and shall not be deemed incorporated by reference into any filing of Acceleron Pharma, Inc. under the Securities Act of 1933, as amended.
None.Grants and Exercises of Stock Options From January 1, 2013 through December 31, 2013, we granted options to purchase a total of 552,750 shares of our common stock to employees at a weighted-average exercise price of $23.88 per share. During the same period, we issued 45,900 shares of common stock upon the exercise of options to purchase such shares of common stock at a weighted average price of $1.44 per share. Option grants and the issuances of common stock upon exercise of such options were exempt pursuant to Rule 701 and Section 4(2) of the Securities Act of 1933.Use of Proceeds from Initial Public Offering of Common Stock On September 24, 2013, we completed the initial public offering (IPO) of our common stock pursuant to a registration statement on Form S-1(File No. 333-190417) and issued and sold 6,417,000 shares of our common stock, including 837,000 shares of common stock sold pursuant to the underwriters' full exercise of their option to purchase additional shares at a public offering price of $15.00 per share, for aggregate gross proceeds of $96.3 million. All of the shares issued and sold in the IPO were registered under the Securities Act pursuant to a Registration Statement on Form S-1 (File No. 333-190417), which was declared effective by the SEC on September 18, 2013, and a registration statement filed pursuant to Rule 462(b) of the Securities Act. Citigroup Global Markets Inc. Leerink Swann LLC acted as joint book-running managers of the offering and as representatives of the underwriters. JMP Securities LLC and Piper Jaffray & Co. acted as co-managers for the offering. The net proceeds to us, after deducting underwriting discounts of $6.8 million and offering expenses totaling $2.7 million, were approximately $86.8 million. No offering expenses were paid directly or indirectly to any of our directors or officers (or their associates) or persons owning 10.0% or more of any class of our equity securities or to any other affiliates. As of December 31, 2013, we had used approximately $10.3 million of the net proceeds from our initial public offering to fund operations, capital expenditures, working capital and other general corporate purposes. On March 12, 2014, the Company paid off the remaining principal outstanding under the Loan Agreement, the deferred fees and early repayment fees, totalling $17.8 million. None of the net proceeds have been paid directly or indirectly to any of our directors or officers (or their associates) or persons owning ten percent or more of any class of our equity securities or to any other affiliates. We are holding the remaining balance of the net proceeds from the offering in prime money market funds. There has been no material change in our planned use of the balance of the net proceeds from our initial public offering as described in our final prospectus filed with the SEC on September 19, 2013 pursuant to Rule 424(b) under the Securities Act. There were no repurchases of shares of common stock made during the year ended December 31, 2013.Data.
DataSELECTED FINANCIAL DATA and comprehensive (loss) income data for the years ended December 31, 2013, 20122015, 2014 and 20112013 and the consolidated balance sheet data as of December 31, 20132015 and 20122014 have been derived from our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. We derived the selected consolidated financial data for the years ended December 31, 2012 and 2011 and as of December 31, 2013, 2012 and 2011 from audited financial statements which are not included in this Annual Report on Form 10-K. Year Ended December 31, 2013 2012 2011 $ 43,948 $ 9,696 $ 74,406 13,282 5,558 4,760 — — 1,745 57,230 15,254 80,911 36,051 35,319 32,713 14,227 8,824 8,142 — — 1,500 50,278 44,143 42,355 6,952 (28,889 ) 38,556 (28,850 ) (3,693 ) (2,290 ) $ (21,898 ) $ (32,582 ) $ 36,266 $ (21,898 ) $ (32,582 ) $ 36,266 $ (4.15 ) $ (24.84 ) $ 0.80 $ (4.15 ) $ (24.84 ) $ 0.78 9,407 2,401 2,328 9,407 2,401 2,716 December 31, 2013 2012 2011 $ 113,163 $ 39,611 $ 65,037 123,732 49,212 73,789 18,162 38,802 23,853 5,620 6,760 33,350 9,048 16,525 — — 1,422 1,046 30,753 5,229 3,347 — 268,610 241,549 57,812 (290,973 ) (232,691 ) Year Ended December 31, (in thousands, except per share data) 2015 2014 2013 2012 2011 Consolidated Statements of Operations Data: Revenue: Collaboration revenue: License and milestone $ 1,184 $ 1,673 $ 43,948 $ 9,696 $ 74,406 Cost-sharing, net 16,913 12,959 13,282 5,558 4,760 Contract manufacturing — — — — 1,745 Total revenue 18,097 14,632 57,230 15,254 80,911 Costs and expenses: Research and development 58,404 50,897 36,051 35,319 32,713 Litigation Settlement — 5,000 — — — General and administrative 20,572 14,199 14,227 8,824 8,142 Cost of contract manufacturing revenue — — — — 1,500 Total costs and expenses 78,976 70,096 50,278 44,143 42,355 (Loss) income from operations (60,879 ) (55,464 ) 6,952 (28,889 ) 38,556 Total other (expense) income, net (3,015 ) 4,205 (28,850 ) (3,693 ) (2,290 ) Net (loss) income $ (63,894 ) $ (51,259 ) $ (21,898 ) $ (32,582 ) $ 36,266 Net (loss) income per share applicable to common stockholders(1) Basic $ (1.92 ) $ (1.63 ) $ (4.15 ) $ (24.84 ) $ 0.80 Diluted $ (1.92 ) $ (1.63 ) $ (4.15 ) $ (24.84 ) $ 0.78 Weighted-average number of common shares used in computing net (loss) income per share applicable to common stockholders Basic 33,303 31,515 9,407 2,401 2,328 Diluted 33,303 31,515 9,407 2,401 2,716 (1)See Note 2 within the notes to our consolidated financial statements appearing elsewhere in this Annual Report on Form 10-K for a description of the method used to calculate basic and diluted net (loss) income per common share. As of December 31, (in thousands) 2015 2014 2013 2012 2011 Consolidated Balance Sheet Data: Cash and cash equivalents $ 27,783 $ 176,460 $ 113,163 $ 39,611 $ 65,037 Short and long-term investments 108,198 — — — — Total assets 146,337 186,296 123,732 49,212 73,789 Total current liabilities 14,491 9,253 18,162 38,802 23,853 Long-term deferred revenue 4,239 4,816 5,620 6,760 33,350 Long-term deferred rent 1,157 1,818 2,337 2,837 3,335 Long-term notes payable — — 9,048 16,525 — Warrants to purchase redeemable convertible preferred stock — — — 1,422 1,046 Warrants to purchase common stock 17,187 14,124 30,753 5,229 3,347 Redeemable convertible preferred stock — — — 268,610 241,549 Total stockholder's equity (deficit) 109,263 156,285 57,812 (290,973 ) (232,691 ) (1) See Note 2 within the notes to our consolidated financial statements appearing elsewhere in this Annual Report on Form 10-K for a description of the method used to calculate basic and diluted net (loss) income per common share. protein therapeutics for cancer and rare diseases. Our research focusestherapeutic candidates that are based on the biology ofmechanisms that the Transforming Growth Factor-Beta (TGF-b) protein superfamily, a large and diverse group of molecules that are key regulators inhuman body uses to regulate the growth and repair of tissues throughoutits cells and tissues. Our research focuses on key natural regulators of cellular growth and repair, particularly the human body.Transforming Growth Factor-Beta, or TGF-beta, protein superfamily. We are leadersa leading company in understanding the biology of the TGF-b superfamilydiscovering and in targeting these pathways to develop important new medicines.developing therapeutic candidates that regulate cellular growth and repair. By couplingcombining our discovery and development expertise, including our proprietary knowledge of the TGF-bTGF-beta superfamily, withand our internal protein engineering and manufacturing capabilities, we have built a highly productive research &discovery and development platform that has generated innovative protein therapeutic candidates with novel mechanisms of action. These differentiated protein therapeutic candidates have the potential to significantly improve clinical outcomes for patients withacross many fields of medicine, and we have focused our discovery and development efforts on treatments for cancer and rare diseases.threefour internally discovered protein therapeutic candidates that are currently being studied in numerous ongoing Phase 2 clinical trials, focused on cancertrials: luspatercept, sotatercept, dalantercept and rare diseases. Our two most advanced protein therapeutic candidates,ACE-083.and ACE-536,are partnered with Celgene Corporation, or Celgene. Luspatercept is designed to promote red blood cell production through a novel mechanism. Together with our collaboration partner, Celgene Corporation, which we refer to as Celgene,mechanism, and we are developing sotatercept and ACE-536luspatercept with Celgene to treat anemia and associated complications in patients withb-thalassemia and myelodysplastic syndromes (MDS), red blood cell disorders that are generally unresponsive to currently approved drugs. Our third and beta-thalassemia. In 2015, Celgene initiated two Phase 3 clinical stage protein therapeutic candidate, dalantercept, is designed to inhibit blood vessel formation through a mechanism that is distinct from,trials for luspatercept for the treatment of MDS and potentially synergistic with, the dominant class of cancer drugs that inhibit blood vessel formation, the vascular endothelial growth factor (VEGF) pathway inhibitors.beta-thalassemia. We are developing dalantercept primarily for use in combination with these successful products to produce better outcomes for cancer patients. Weand Celgene are developing sotatercept to treat patients with chronic kidney disease. Sotatercept has the potential to treat several complications of chronic kidney disease including mineral-bone disorder, vascular calcification and ACE-536 through our exclusive worldwide collaborations with Celgene. As of January 1, 2013,anemia. Celgene becameis responsible for paying 100% of worldwidethe development costs for both programs.all clinical trials for luspatercept and sotatercept, including our ongoing earlier stage clinical trials for these therapeutic candidates. We may receive up to an additional $560.0 million of potential development, regulatory and commercial milestone payments still outstanding and, if these protein therapeutic candidates are commercialized, we will receive a royalty on net sales in the low-to-mid 20% range. We also will co-promote luspatercept and sotatercept, and ACE-536if approved, in North America if approved, for which our commercialization costs will be entirely funded by Celgene.not entered intonominated an IntelliTrap™ molecule, ACE-2494, as a partnershipcandidate for dalanterceptclinical development and retain worldwide rightswill initiate IND-enabling activities in 2016. ACE-2494 is designed to this program.2013,2015, our operations have been funded primarily funded by $105.1 million in equity investments from venture investors, $86.8$219.3 million in net proceeds from our initial public offering, $49.2investors, $64.2 million in equity investments from our collaboration partners and $203.6$233.5 million in upfront payments, milestones, and net research and development payments from our strategiccollaboration partners. We estimate that we have spent approximately $145.4 million on research and development for the three year period from 2013 through 2015.•dalantercept;•protein therapeutic candidates;•protein therapeutics;•protein therapeuticstherapeutic candidates for our preclinical studies and clinical trials;•protein therapeutics;therapeutic candidates; and•protein therapeutic candidates, which wecandidates. We expect that this will take a number of years and is subject to significant uncertainty. All current and future development and commercialization costs for sotatercept and ACE-536luspatercept are paid by Celgene. If we obtain regulatory approval for dalantercept, ACE-083 or any future protein therapeutic candidate, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution to the extent that such costs are not paid by future partners. We will seek to fund our operations through the sale of equity, debt financings or other sources, including potential additional collaborations. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms, or at all. If we fail to raise capital or enter into such other arrangements as, and when, needed, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our protein therapeutics.protein therapeuticstherapeutic candidates and potentially begin to commercialize any approved products. For a description of the numerous risks and uncertainties associated with product development, see "Risk Factors".protein therapeutics.therapeutic candidates. Cost sharing revenue represents amounts reimbursed by our collaboration partners for expenses incurred by us for research and development activities and, potentially, co-promotion activities, under our collaboration agreements. Cost sharing revenue is recognized in the period that the related activities are performed. To the extent that we reimburse collaborators for costs incurred in connection with activities performed by them, we record these costs as a reduction of cost-sharing revenue.Contract Manufacturing Revenue We have generated contract manufacturing revenue in the past but have no current contract manufacturing arrangements. Contract manufacturing revenue consists of revenue received for producing bulk drug substance for third parties other than our partners.protein therapeutic candidates, which include:•••••• protein therapeutic candidates or if, when, or to what extent we will generate revenues from the commercialization and sale of any of our protein therapeutic candidates for which we or any partner obtain regulatory approval. We or our partners may never succeed in achieving regulatory approval for any of our protein therapeutic candidates. The duration, costs and timing of clinical trials and development of our protein therapeutic candidates will depend on a variety of factors, including:•••• protein therapeutic candidate could mean a significant change in the costs and timing associated with the development of that protein therapeutic candidate. For example, if the FDA, or another regulatory authority were to require us to conduct clinical trials beyond those that we currently anticipate will be required for the completion of the clinical development of protein therapeutics,therapeutic candidates, or if we experience significant delays in the enrollment in any clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development.2013,2015, we have incurred $287.2$396.5 million in research and development expenses. We plan to increase our research and development expenses for the foreseeable future as we continue the development of our TGF-bTGF-beta platform protein therapeutics,therapeutic candidates, the discovery and development of preclinical protein therapeutics, including ACE-083,therapeutic candidates, and the development of sotatercept, ACE-536luspatercept, dalantercept and dalantercept. BeginningACE-083. As of January 1, 2013, expenses associated with sotatercept and ACE-536luspatercept are reimbursed 100% by Celgene. These reimbursements are recorded as revenue. Of the Phase 2 clinical trials that are underway for sotatercept, ACE-536luspatercept and dalantercept, we are expensingsixseven clinical trials of ACE-536luspatercept and dalantercept, of which the twofour for ACE-536luspatercept are reimbursed by Celgene. protein therapeutic candidates, and preclinical animal toxicology studies through third-party CROs. The only costs we track by each protein therapeutic candidate are external costs such as services provided to us by CROs, manufacturing of preclinical and clinical drug substance,product, and other outsourced research and development expenses. We do not assign or allocate to individual development programs internal costs such as salaries and benefits, facilities costs, lab supplies and the costs of preclinical research and studies. Our external research and development expenses for sotatercept, ACE-536,luspatercept, dalantercept, ACE-031 (for which development was suspended in April 2013) and ACE-083 (for which development commenced in the fourth quarter of 2013) during the years ended December 31, 2013, 20122015, 2014 and 2011,2013, are as follows: Year ended December 31, (in thousands) 2015 2014 2013 Sotatercept(1) $ — $ — $ 2 Luspatercept(1) 9,744 7,944 5,081 Dalantercept 8,864 7,526 4,636 ACE-083 3,293 5,111 105 ACE-031(2) 3 8 1,023 Total direct research and development expenses 21,904 20,589 10,847 Other expenses(3) 36,500 30,308 25,204 Total research and development expenses $ 58,404 $ 50,897 $ 36,051 Year ended December 31, 2013 2012 2011 $ 2 $ 6 $ 974 5,081 2,885 681 4,636 3,422 1,323 105 — — 1,023 3,453 4,240 10,847 9,766 7,218 25,204 25,553 25,495 $ 36,051 $ 35,319 $ 32,713 (1) As of January 1, 2013, expenses associated with sotatercept and luspatercept are reimbursed 100% by Celgene. These reimbursements are recorded as revenue and are presented as cost-sharing, net. In the periods presented, Celgene conducted most of the development activities for sotatercept, and we do not incur and are not reimbursed for expenses related to development activities directly conducted by Celgene. (2) In April 2013, we and Shire AG, or Shire, determined not to further advance the development of ACE-031, and Shire terminated our collaboration agreement, effective as of June 30, 2013. (1)Beginning January 1, 2013, expenses associated with sotatercept and ACE-536 are reimbursed 100% by Celgene. These reimbursements are recorded as revenue and are presented as cost-sharing, net.(2)In April 2013, we and Shire AG, which we refer to as Shire, determined not to further advance the development of ACE-031, and Shire terminated our collaboration agreement, effective as of June 30, 2013.(3)Other expenses include unallocated employee and contractor-related expenses, facility expenses, lab supplies and miscellaneous expenses.Contract Manufacturing Expenses Contract manufacturing expenses consist primarily of costs incurred for the production of bulk drug substance for third parties other than our partners. The costs generally include employee-related expenses including salary and benefits, direct materials and overhead costs including rent, depreciation, utilities, facility maintenance and insurance. We do not have any current contract manufacturing arrangements.(3) Other expenses include unallocated employee and contractor-related expenses, facility expenses, lab supplies and miscellaneous expenses. protein therapeutics.therapeutic candidates. Additionally, if and when we believe regulatory approval of a protein therapeutic candidate appears likely, to the extent that we are undertaking commercialization of such protein therapeutic candidate ourselves, we anticipate an increase in payroll and related expenses as a result of our preparation for commercial operations.protein therapeutics.2015,2016, the expected completion date of the proof-of-concept trials for ACE-536luspatercept under the Celgene collaboration. Another instance relates to our arrangement with Shire AG, where in April 2013, we and Shire determined not to further advance the development of ACE-031 or back-up compounds and Shire terminated our collaboration agreement effective as of June 30, 2013.and modifications to existing stock options, and restricted stock unit awards, to be recognized in the statements of operations and comprehensive income (loss) based on their fair values. We recognize the compensation cost of awards subject to service-based vesting conditions over the requisite service period, which is generally equal to the vesting term. For awards subject to both performance and service-based vesting conditions, we recognize compensation cost using an accelerated recognition method when it is probable that the performance condition will be achieved. If achievement of the performance condition is not probable, but the award will vest based on the service condition, we recognize the expense over the requisite service period. We account for stock-based awards to non-employees using the fair value method. Stock options granted to non-employees are subject to periodic revaluation over their vesting terms and stock-based compensation cost is recognized using an accelerated recognition method.We will continueDuring 2015 we began to apply this process untilestimate our volatility by using a sufficient amountblend of our stock price history, for the length of time we have market data for our stock and the historical information regarding the volatility of our own stock price becomes available.similar public companies for the expected term of each grant. We have estimated the expected life of our employee stock options using the "simplified" method, whereby, the expected life equals the average of the vesting term and the original contractual term of the option. The risk-free interest rates for periods within the expected life of the option are based on the U.S. Treasury yield curve in effect during the period the options were granted.2012 and 2011, we used arespectively, the Company applied an estimated forfeiture rate of approximately 4%, 5% and 5%, respectively.$2.2$12.1 million, $1.2$4.8 million and $1.4$2.2 million for the years ended December 31, 2015, 2014 and 2013, 2012 and 2011, respectively. As of December 31, 2013, we had $10.1 million of unrecognized compensation expense, net of related forfeiture estimates, which is expected to be recognized over a weighted-average remaining vesting period of approximately 2.66 years. We expect the impact of our stock-based compensation expense for stock-based awards granted to employees and non-employees to grow in future periods due to the potential increases in the value of our common stock and headcount.2013,2015, we had warrants outstanding to purchase 979,699398,011 shares of common stock, of which warrants to purchase 857,586392,805 shares of our common stock contain a provision requiring an adjustment to the number of shares in the event we issue common stock, or securities convertible into or exercisable for common stock, at a price per share lower than the warrant exercise price. The anti-dilution feature requires the warrants to be classified as liabilities and measured at fair value, with changes in fair value recognized as a component of other income (expense). The fair value of the warrants to purchase common stock on the date of issuance and on each re-measurement date for those warrants to purchase common stock are classified as liabilities and arewas estimated using either the Monte Carlo simulation framework, up until September 30, 2013, which incorporated threeincorporates future financing events over the remaining life of the warrants to purchase common stock. Duestock, or for certain re-measurement dates including December 31, 2015, 2014 and 2013, due to the warrants being deeply in the money, as of December 31, 2013, the valuation method to estimate the warrants to purchase common stock was changed to the Black-Scholes option pricing model.model was used. At each reporting period the company evaluates the best valuation methodology. Any modifications to the warrant liabilities are recorded in earnings during the period of the modification. The significant assumptions used in estimating the fair value of our warrant liabilities include the exercise price, volatility of the stock underlying the warrant, risk-free interest rate, estimated fair value of the stock underlying the warrant, and the estimated life of the warrant. The common stock warrant liability was $17.2 million, $14.1 million and $30.8 millions and $5.2 millionsmillion as of December 31, 2015, 2014 and 2013, and 2012, respectively. The remeasurementAt the end of each reporting period, the Company remeasured the fair value of the outstanding warrants, using current assumptions, resultedresulting in an increase (decrease) in fair value of $26.9$3.5 million, $1.9$(5.0) million and $0.3$26.9 million, respectively, which was recorded in other expenseincome (expense), net in the accompanying consolidated statements of operations and comprehensive (loss) incomeloss for the years ended December 31, 2013, 20122015, 2014 and 2011 respectively.2013. The changes in value of the warrant liability were primarily due to increasesfluctuations in the estimated value during 2011 and 2012 and in market price during 2013, of our common stock.Emerging Growth Company Status The Jumpstart our Business Startups Act of 2012, or the JOBS Act, permits an "emerging growth company" such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to "opt out" of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.20132015 and 20122014 Year Ended
December 31, Year Ended
December 31, Increase
(Decrease) Increase
(Decrease) 2013 2012 2015 2014 $ 43,948 $ 9,696 $ 34,252 $ 1,184 $ 1,673 $ (489 ) 13,282 5,558 7,724 16,913 12,959 3,954 57,230 15,254 41,976 18,097 14,632 3,465 36,051 35,319 732 58,404 50,897 7,507 Litigation settlement — 5,000 (5,000 ) 14,227 8,824 5,403 20,572 14,199 6,373 50,278 44,143 6,135 78,976 70,096 8,880 6,952 (28,889 ) 35,841 (28,850 ) (3,693 ) (25,157 ) (Loss) income from operations (60,879 ) (55,464 ) (5,415 ) Other (expense) income, net (3,015 ) 4,205 (7,220 ) $ (21,898 ) $ (32,582 ) $ 10,684 $ (63,894 ) $ (51,259 ) $ (12,635 ) $57.2$18.1 million in the year ended December 31, 2013,2015, compared to $15.3$14.6 million in year ended December 31, 2012. The $42.02014. All of the revenue in both periods was derived from the Celgene agreements. This $3.5 million$10.0year ended December 31, 2015 were zero compared to $5.0 million in the same period in 2014. This decrease was due to the settlement of litigation with the Salk Institute in July 2014. (in thousands) 2014 2013 Revenue: Collaboration revenue: License and milestone $ 1,673 $ 43,948 $ (42,275 ) Cost-sharing, net 12,959 13,282 (323 ) Total revenue 14,632 57,230 (42,598 ) Costs and expenses: Research and development 50,897 36,051 14,846 Litigation settlement 5,000 — 5,000 General and administrative 14,199 14,227 (28 ) Total costs and expenses 70,096 50,278 19,818 (Loss) income from operations (55,464 ) 6,952 (62,416 ) Other income (expense), net 4,205 (28,850 ) 33,055 Net loss $ (51,259 ) $ (21,898 ) $ (29,361 ) paymentpayments received during 2013 in connection with our Celgene collaboration for the first patient dosed in a Phase 2 trial in ACE-536, $7.0 million milestone payment received in connection with our Celgene collaboration forluspatercept and initiation of a phasePhase 2b study of sotatercept in end-stage renal disease patients, and recognizing an additional $16.7$24.3 million of deferred revenue becauserelated to Shire. Shire ended our collaboration as of June 30, 2013. The remaining increase of $8.32013 and there is no Shire related revenue during 2014. Celgene deferred revenue also decreased $1.0 million was primarilyduring 2014 as we complete our deliverables under the collaboration agreement. Net cost sharing revenue decreased $0.3 million due to an increase in net cost-sharingCelgene cost sharing revenue from Celgene of $9.8$0.3 million due to Celgene assuming 100% of the costs of development for these protein therapeutic candidates as of January 1, 2013, and recognition of $0.6 million deferred revenue from Celgene, offset by a $0.6 million decrease in net cost-sharing revenue from Shire of $2.1 million due to the end of the collaboration as of June 30, 2013.Shire. Year Ended
December 31, Year Ended
December 31, Increase
(Decrease) Increase
(Decrease) 2013 2012 2014 2013 $ 19,626 $ 2,035 $ 17,591 $ 1,673 $ 19,626 $ (17,953 ) 12,658 2,879 9,779 12,959 12,658 301 32,284 4,914 27,370 14,632 32,284 (17,652 ) 24,322 7,661 16,661 — 24,322 (24,322 ) 624 2,679 (2,055 ) — 624 (624 ) 24,946 10,340 14,606 — 24,946 (24,946 ) 57,230 15,254 41,976 14,632 57,230 (42,598 ) $ 57,230 $ 15,254 $ 41,976 $ 14,632 $ 57,230 $ (42,598 ) 2013, compared to $35.3 million in the year ended December 31, 2012.2013. This $0.7$14.8 million increase was primarily due to an increase in expenses associated with clinical activity totaling $3.1$9.6 million, an increase inhigher personnel related expenses associated with clinical drug supplyof $3.9 million, higher legal expenses of $1.0 million higher spend for in-licensing of $0.3 million, higher compensation related expense of $0.3 million, and a $0.7 million increase in miscellaneous research and development expenses primarily due to an antibody collaboration agreement, executed in 2014, offset by a reduction of $0.5 million because of a royalty owedpayment due to the Salk Institute for a milestone achieved during 2013.$0.5 million, partially offset by a reductionlitigation with the Salk Institute in preclinical animal studies totaling $3.0 million, legal spend for patent related matters of $0.8 million, and depreciation of $0.4 million.July 2014.2013,2014, compared to $8.8$14.2 million in the year ended December 31, 2012. This $5.42013. During 2014, increases of $0.7 million increase was primarilyfor higher personnel related expenses and $2.0 million related to higher professional fees and insurance were primarily offset by a decrease of $2.8 million for legal services in connection with our litigation withexpenses due to the Salk Institute and for increased professional fees and financial consulting services in connection with business development activities totaling $3.3 million, $0.2 million for higher insurance expenses, and higher total compensation expenses totaling $1.8 million.settlement of ongoing litigation.Expense,Income (Expense), Net. Other expense,income, net was $4.2 million in the year ended December 31, 2014, compared to expense of $28.9 million in the year ended December 31, 2013, compared to $3.7 million in the year ended December 31, 2012.2013. This $25.2$33.1 million increase was primarily due to highera $30.7 million difference in the effect of marking the common stock warrant liability to market in each period. The additional increase was a $1.2 million decrease in interest expense associated withbecause the increase in fair value of the liability for warrants of $24.5 millionoutstanding long term debt was retired during March 2014 and an increase in interest expense of $0.6 million due to a higher average outstanding debt balance in the year ended December 31, 2013.Comparison of Years Ended December 31, 2012 and 2011 Year Ended
December 31, Increase
(Decrease) 2012 2011 $ 9,696 $ 74,406 $ (64,710 ) 5,558 4,760 798 — 1,745 (1,745 ) 15,254 80,911 (65,657 ) 35,319 32,713 2,606 8,824 8,142 682 — 1,500 (1,500 ) 44,143 42,355 1,788 (28,889 ) 38,556 (67,445 ) (3,693 ) (2,290 ) (1,403 ) $ (32,582 ) $ 36,266 $ (68,848 ) Revenue. We recognized revenue of $15.3$1.3 million for the year ended December 31, 2012, compared to $80.9 million for the year ended December 31, 2011. The $65.6 million decrease in revenue in 2012 was primarily due to a decreaseeffect of $64.7 million in license and milestone revenue, because during 2011, upon signing the ACE-536 Celgene collaboration and amending the sotatercept agreement, we recognized upfront payments and deferred revenue totaling $54.8 million. During 2011, we also recognized the remaining $2.4 million of deferred revenue from the Alkermes collaboration. Also, in 2012 we did not recognize any milestone payments compared to $7.0 million during 2011. The decrease in license and milestone revenue was offset by higher 2012 cost-sharing revenue due primarily to lower reimbursements paid to Shire for ACE-031. We also recognized $1.7 million for a contract manufacturing project during 2011. The following table shows revenue from all sources for the periods presented. Year Ended
December 31, Increase (Decrease) 2012 2011 $ 2,035 $ 63,607 $ (61,572 ) 2,879 (121 ) 3,000 4,914 63,486 (58,572 ) 7,661 8,392 (731 ) 2,679 4,148 (1,469 ) 10,340 12,540 (2,200 ) — 2,407 (2,407 ) — 733 (733 ) — 3,140 (3,140 ) 15,254 79,166 (63,912 ) — 1,745 (1,745 ) $ 15,254 $ 80,911 $ (65,657 ) Research and Development Expenses. Research and development expenses were $35.3 million in the year ended December 31, 2012, compared to $32.7 million for the year ended December 31, 2011. This $2.6 million increase was primarily due to increases in expenses related to preclinical animal toxicology studies of $2.6 million, patent-related legal services of $0.9 million, external testing of $0.6 million, clinical trial activities of $0.5 million, contract labor of $0.5 million, outsourced research of $0.3 million and management bonuses of $0.3 million partially offset by decreases in expenses related to depreciation of $1.3 million, contract manufacturing of $0.7 million, supplies of $0.4 million, and in-licensing of $0.5 million. General and Administrative Expenses. General and administrative expenses were $8.8 million in the year ended December 31, 2012, compared to $8.1 million for the year ended December 31, 2011. This $0.7 million increase was primarily related to higher professional fees for legal costs of $0.4 million in connection with litigation activities and higher compensation costs of $0.3 million. Cost of Contract Manufacturing Revenue. There was no cost of contract manufacturing revenue for the year ended December 31, 2012, compared to $1.5 million for the year ended December 31, 2011. This decrease was due to there being no contract manufacturing services provided during 2012. Other Expense, Net. Other expense, net was $3.7 million in the year ended December 31, 2012, compared to $2.3 million for the year ended December 31, 2011. The increase was primarily due to a $1.8 million increase in fair value of the liability formarking preferred stock warrants to purchase redeemable convertible preferred stock and common stock.market prior to their conversion during the initial public offering.2013,2015, we had an accumulated deficit of $192.3$307.5 million. We anticipate that we will continue to incur losses for at least the next several years. We expect that our research and development and general and administrative expenses will continue to increase and, as a result, we will need additional capital to fund our operations, which we may raise through a combination of the sale of equity, debt financings or other sources, including potential additional collaborations.2013,2015, our operations have been primarily funded by $105.1 million in equity investments from venture investors $49.2prior to the IPO, $219.3 million from public investors, $64.2 million in equity investments from our partners and $203.6$233.5 million in upfront payments, milestones, and net research and development payments from our collaboration partners. In As$129.0$129.2 million, after deducting underwriting discounts and offering expenses. Year Ended December 31, 2013 2012 2011 $ (19,650 ) $ (38,884 ) $ 9,056 (307 ) (441 ) (27 ) 93,509 13,899 21,092 $ 73,552 $ (25,426 ) $ 30,121 Year Ended December 31, (in thousands) 2015 2014 2013 Net cash (used in) provided by: Operating activities $ (44,211 ) $ (53,220 ) $ (19,650 ) Investing activities (108,971 ) (514 ) (307 ) Financing activities 4,505 117,031 93,509 Net (decrease) increase in cash and cash equivalents $ (148,677 ) $ 63,297 $ 73,552 The significant decrease in net2013, compared to the year ended December 31, 2012, is2015, and consisted primarily of a net loss of $63.9 million adjusted for non-cash items including an increase in fair value of warrants of $3.5 million, stock-based compensation expense of $12.1 million, depreciation and amortization of $1.2 million, and a net increase due to the receiptchanges in operating assets and liabilities of a $10.0 million milestone payment from Celgene$3.3 million. The significant items in the first quarterchange in operating assets and liabilities include a decrease in deferred revenue of 2013,$1.2 million for the Celgene Collaboration. Other components of the change in operating assets and $7.0liabilities include a decrease in deferred rent of $0.5 million in the fourth quarter, and an increase in proceeds from cost-sharing revenue, net. The significant decreaseTableaccrued expenses of Contentsin$5.0 million.provided byused in operating activities was $53.2 million for the year ended December 31, 2012, compared to the year ended December 31, 2011, is2014, and consisted primarily of a net loss of $51.3 million adjusted for non-cash items including a decrease in fair value of warrants of $5.0 million, stock-based compensation expense of $4.8 million, depreciation and amortization of $1.1 million, payments of deferred interest of $0.5 million, and a net decrease due to changes in operating assets and liabilities of $2.3 million. The significant items in the upfrontchange in operating assets and milestone paymentsliabilities include a decrease in deferred revenue of $32.5$1.7 million related tofor the ACE-536 Agreement received during 2011. $0.7operatinginvesting activities was $38.9$109.0 million, $0.5 million and $0.3 million for the years ended December 31, 2015, 2014 and 2013, respectively. The significant increase during 2015 was due to the purchase of investments. During 2014 and 2013 cash used in investing activities consisted of purchases of property and equipment.20122015 and iswas primarily due to a net loss$4.6 million in proceeds received from the employee stock purchase plan, and the exercise of $32.6 million adjusted for non-cash items including an increase in the fair value ofstock options and warrants of $2.3 million, stock-based compensation of $1.2 million, depreciation and amortization of $1.3 million, and accretion of deferred interest of $0.3 million and a net decrease in operating assets and liabilities of $11.5 million. The significant items in the change in operating assets and liabilities include a decrease in deferred revenue of $9.7 million due to the ongoing recognition of revenue deferred in connection with up-front payments for the Celgene and Shire collaboration agreements, a decrease in accounts payable of $1.3 million and an increase in collaboration receivables of $1.1 million, offset in part by an increase in accrued expenses of $1.6 million. Other components of the change in operating assets and liabilities include an increase in prepaid expenses and other current assets of $0.6 million and a decrease in deferred rent of $0.5 million.operatingfinancing activities was $9.1$117.0 million for the year ended December 31, 20112014 and is primarily due toconsisted of $129.2 million in net incomeproceeds received from our follow on public offering and $4.2 million from the exercise of $36.3 million, which was impacted by non-cash items including depreciationstock options and amortization of $3.1 million, stock-based compensation of $1.4 million, an increase in the fair value of warrants, of $0.5 million, accretion of deferred interest of $0.3 million and amortization of debt discount of $0.2 million and a net decrease in operating assets and liabilities of $32.8 million. The significant items in the change in operating assets and liabilities include a decrease in deferred revenue of $35.1 million due primarily to the acceleration of deferred revenue associated with the Celgene collaboration upfront payments as a result of the modification of the collaboration agreement, as well as a decrease in accrued expenses of $2.8 million, offset in part by a decrease in prepaid expenses and other current assets$16.3 million of $2.6 million and a decrease in collaboration receivables of $1.8 million. Other components of the change in operating assets and liabilities include an increase in accounts payable of $0.3 million and an increase in deferred rent of $0.2 million. Investing Activities. Net cash used in investing activities was $0.3 million for the year ended December 31, 2013, $0.4 million for the year ended December 31, 2012 and $27,000 for the year ended December 31, 2011 and consisted of purchases of property and equipment. Financing Activities.principal repayments to pay off our venture debt credit facility. line,credit facility, and $0.3 million paid to repurchase and retire redeemable convertible preferred stock, common stock and warrants to purchase common stock. Net cash provided by financing activities was $13.9 million for the year ended December 31, 2012 and consisted of $19.9 million in net proceeds received from the drawdown of our new venture debt line in June 2012, as well as $0.2 million received from the exercise of stock options and warrants to purchase common stock, offset by $6.2 million of principal payments made to pay down our previous venture debt line. Net cash provided by financing activities was $21.1 million for the year ended December 31, 2011 and consisted primarily of $30.4 million of net proceeds received from the sale of 9,704,756 shares of our Series F preferred stock, as well as $0.2 million received from the exercise of stock options and warrants to purchase common stock, offset in part by $9.5 million of principal payments made to pay down a previous venture debt facility.protein therapeutics.therapeutic candidates. We anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of, and seek and obtain regulatory approvals for, dalantercept, ACE-083 and any future protein therapeutics,therapeutic candidates, and begin to commercialize any approved products. We are subject to all of the risks incident in the development of protein therapeutics,therapeutic candidates, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. Since the closing of our initial public offering, we have incurred, and expect to continue to incur, additional costs associated with operating as a public company. We anticipate that we will need additional funding in connection with our continuing operations.we receive from our January 2016 public offerings, together with receipt of anticipated milestone payments and our existing cash and cash equivalentsoffering, will be sufficient to fund our projected operating requirements into the second half of 2017.2019. However, we will require additional capital for the further development of our existing protein therapeutic candidates and may also need to raise additional funds sooner to pursue other development activities related to additional protein therapeutic candidates. or debt financings or other sources including potential additional collaborations. Additional capital may not be available on favorable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our protein therapeutic candidates. If we raise additional funds through the issuance of additional debt or equity securities, it could result in dilution to our existing stockholders and increased fixed payment obligations, and these securities may have rights senior to those of our common stock. If we incur indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We may not be able to enter into new collaboration arrangements for any of our proprietary protein therapeutic candidates. Any of these events could significantly harm our business, financial condition and prospects.•••protein therapeutic candidates and potential protein therapeutic candidates;•protein therapeutic candidates that we pursue;••••••protein therapeutic candidates;••property including our litigation with the Salk Institute. See "Business—Litigation".
property.2013.2015.(in thousands) Total 2016 2017 through 2018 2019 through 2020 After 2020 Operating lease obligations(1) $ 13,971 $ 4,386 $ 8,124 $ 1,296 $ 165 Total $ 13,971 $ 4,386 $ 8,124 $ 1,296 $ 165 Total 1 year 2 - 3 years 4 - 5 years $ 19,457 $ 4,522 $ 8,044 $ 6,891 (1,084 ) (832 ) (252 ) — 18,194 18,194 — — $ 36,567 $ 21,884 $ 7,792 $ 6,891 (1) We lease office and lab space at 128 Sidney Street and 149 Sidney Street in Cambridge, Massachusetts under noncancelable operating leases that expire in September 2018. We also lease space at 125 Sidney Street under a noncancelable operating lease that expires in March 2021. Our leasehold improvements are being amortized over 2-10 years which represent the shorter of their useful life or remaining lease term. (1)We lease office space at 128 Sidney Street and 149 Sidney Street in Cambridge, Massachusetts under noncancelable operating leases that expire in September 2018, and at 12 Emily Street in Cambridge, Massachusetts under a noncancelable operating lease that expires in May 2015. Our leasehold improvements are being amortized over 3-10 years which represent the shorter of their useful life or remaining lease term.(2)In February 2011, we entered into a sublease for 14,214 square feet of office space at 12 Emily Street in Cambridge, Massachusetts, that expires in May 2015. On December 31, 2013 the sublease was expanded to the remaining space in that facility. The expansion portion sublease expires in December 2014.(3)In June 2012, we entered into a $20.0 million venture debt facility to provide working capital to fund operating activities. The loans under this debt facility are secured by our assets and are being repaid over 42 months beginning with a 12 month interest only period. Interest rates were fixed at the time of drawdown, with an effective rate of 11.8%. On March 12, 2014, the Company paid off the remaining principal outstanding under the Loan Agreement. The cash obligation balance includes the principal, the interest through the date of repayment, deferred fees and early repayment fees.••also agreed to pay Salk specified development milestone payments totaling up to $2.0 million for sotatercept. Under the other agreement, we also agreed to pay Salk specified development milestone payments of up to $0.7 million for ACE-536.luspatercept. In addition, under both agreements, we are required to pay Salk royalties in the low single-digits on worldwide net product sales by us or our sublicensees under the licensed patent rights of products claimed in the licensed patents, or products derived from use of the licensed patent rights, with royalty obligations for sotatercept continuing at a reduced rate for a period of time after patent expiration.$66.1$109.8 million as of December 31, 2013,2015, which have been fully offset by a valuation allowance due to uncertainties surrounding our ability to realize these tax benefits. The deferred tax assets are primarily composed of federal and state tax net operating loss, or NOL, carryforwards, research and development tax credit carryforwards, and deferred revenue, accruals and other temporary differences. As of December 31, 2013,2015, we had federal NOL carryforwards of approximately $141.0$276.1 million and state NOL carryforwards of $122.9$229.8 million available to reduce future taxable income, if any. These federal NOL carryforwards expire at various times through 2033 and the state NOL carryforwards expire at various times through 2033.2035. In general, if we experience a greater than 50 percent aggregate change in ownership of certain significant stockholders over a three-year period, or a Section 382 ownership change, utilization of our pre-change NOL carryforwards are subject to an annual limitation under Section 382 of the Internal Revenue Code of 1986, as amended, and similar state laws. Such limitations may result in expiration of a portion of the NOL carryforwards before utilization and may be substantial. If we experience a Section 382 ownership change in connection with our public offerings or as a result of future changes in our stock ownership, some of which changes are outside our control, the tax benefits related to the NOL carryforwards may be limited or lost. For additional information about our taxes, see Note 1213 to the financial statements in this Annual Report on Form 10-K.CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS The following is a description of transactions, since January 1, 2012, to which we have been a party, in which the amount involved exceeded or will exceed $120,000, and in which any related person had a direct or indirect material interest.Indemnification Agreements We have entered into indemnification agreements with each of our directors and executive officers. These agreements will require us to indemnify these individuals and, in certain cases, affiliates of suchTable of Contentsindividuals, to the fullest extent permissible under Delaware law against liabilities that may arise by reason of their service to us or at our direction, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified.Registration Rights Agreement In connection with our Series F preferred stock financing, on December 22, 2011, we entered into an amended and restated registration rights agreement with the holders of all of our then-outstanding shares of preferred stock including certain of our named executive officers and entities with which certain of our directors are affiliated. The agreement provides that these holders, for so long as they may hold registrable securities, as defined in the agreement, have the right to demand that we file a registration statement with respect to the common stock issued upon conversion of our preferred stock. These holders may also request that shares of common stock held by them be included in certain registration statements that we are otherwise filing. See "Description of Capital Stock—Registration Rights".Right of First Refusal and Co-Sale Agreement In connection with our Series F preferred stock financing, on December 22, 2011, we entered into an amended and restated right of first refusal and co-sale agreement with the holders of all of our then-outstanding shares of preferred stock including certain of our named executive officers and entities with which certain of our directors are affiliated. Pursuant to the terms of this agreement, in the event of a proposed sale of shares of our common or preferred stock, the seller was required to first offer such shares to the company and to the other investors, subject to certain conditions and restrictions. This agreement terminated upon the completion of our initial public offering on September 24, 2013.Voting Agreement In connection with our Series F preferred stock financing on December 22, 2011, we entered into an amended and restated voting agreement with the holders of all of our then-outstanding shares of preferred stock including certain of our named executive officers and entities with which certain of our directors are affiliated, with respect to the election of directors and certain other matters. All of our current directors were elected pursuant to the terms of this agreement. This agreement terminated upon the completion of our initial public offering on September 24, 2013.Investor Rights Agreement In connection with our Series F preferred stock financing, on December 22, 2011, we entered into an amended and restated investor rights agreement with the holders of all of our then-outstanding shares of preferred stock including certain of our named executive officers and entities with which certain of our directors are affiliated. Pursuant to the terms of this agreement, we granted our investors certain information rights as well as the right to participate pro rata in any future private financing rounds. This agreement terminated upon the completion of our initial public offering on September 24, 2013.Transactions with Our Executive Officers, Directors and 5% Stockholders On March 13, 2013, we repurchased shares of our common and preferred stock, as well as warrants to purchase shares of our common stock, from UBS Juniper Crossover Fund, LLC, an affiliate of OrbiMed Advisors, for $300,000 in aggregate. On January 28, 2008, we entered into a secured promissory note with our chief executive officer, which was amended on November 13, 2012, with a principal balance of $200,000 and an interest rate of3.11% per annum. The note was secured by a pledge of shares of the company's stock under a pledge agreement dated January 28, 2008. The terms of the note provided that it would mature on January 28, 2014, or be forgiven upon the occurrence of certain corporate events, including the filing of a registration statement with the SEC. The outstanding principal balance and accrued and unpaid interest on the note was forgiven on August 7, 2013. See "Executive Compensation—Executive Loan". For additional Related Party transactions disclosure, please see Note 14 to the financial statement in this Annual Report on Form 10-K.2013,2015 and December 31, 2014, we had cash, and cash equivalents and investments of $113.2 million.$136.0 million and $176.5 million, respectively. Our cash equivalents are invested primarily in bank deposits and money market mutual funds consisting of U.S. government-backed securities.funds. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because ourrates. Our investments are subject to interest rate risk and could fall in short-term securities.value if market interest rates increase. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, we do not believe an immediate 100 basis point change in interest rates would not have a material effect on the fair market value of our portfolio.Data.DataDisclosure.DisclosureProcedures.Procedures2013,2015, management, with the participation of our Chief Executive Officer and Chief Financial Officer, performed an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act. Our disclosure controls and procedures are designed to ensure that information required to be disclosed in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to our management, including the Chief Executive Officer and the Chief Financial Officer, to allow timely decisions regarding required disclosures. Any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objective. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2013,2015, the design and operation of our disclosure controls and procedures were effective. This Annual Report on Form 10-K does not include a reportattestation reportevaluation of the effectiveness of our internal control over financial reporting as of December 31, 2015 based upon the Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on this evaluation, management, including our Chief Executive Officer and Chief Financial Officer, has concluded that our internal control over financial reporting was effective as of December 31, 2015.due to the transition period established by rules of the SEC for newly public companies.as stated in their report which is included herein.quarteryear ended December 31, 2013,2015, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. None./s/ Ernst & Young LLP 20142016 annual meeting of stockholders, pursuant to Regulation 14A of the Securities Exchange Act of 1934, as amended, also referred to in this Form 10-K as our 20142016 Proxy Statement, which we expect to file with the SEC no later than April 30, 2014.20142016 Proxy Statement.and Director Compensation20142016 Proxy Statement.20142016 Proxy Statement.Independence.
Independence20142016 Proxy Statement.20142016 Proxy Statement.Schedules.
Schedules(a)The following documents are filed as part of this report:(1)Financial Statements.(a) The following documents are filed as part of this report: (1) Financial Statements. (2) Financial Statement Schedules. (2)Financial Statement Schedules(3)Exhibits(3) Exhibits. (b)(b) The list of Exhibits filed as a part of this Annual Report on Form 10-K is set forth on the Exhibit Index immediately preceding such Exhibits and is incorporated herein by this reference. (c) None. (c)None.(the Company) as of December 31, 20132015 and 2012,2014, and the related consolidated statements of operations and comprehensive (loss) income,loss, redeemable convertible preferred stock and stockholders'stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2013.2015. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements,statements. An audit also includes assessing the accounting principles used and significant estimates made by management, andas well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.20132015 and 2012,2014, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2013,2015, in conformity with U.S. generally accepted accounting principles. /s/ Ernst & Young LLP March 17, 2014 December 31, 2013 2012 $ 113,163 $ 39,611 3,616 2,776 2,243 1,474 119,022 43,861 3,705 4,059 913 913 — 233 92 146 $ 123,732 $ 49,212 $ 885 $ 642 6,927 6,153 2,031 27,840 499 499 16,868 3,668 27,210 38,802 5,620 6,760 2,337 2,837 — 16,525 — 1,422 30,753 5,229 65,920 71,575 — 268,610 — —
respectively 29 3 250,107 — (192,324 ) (290,976 ) 57,812 (290,973 ) $ 123,732 $ 49,212 December 31, 2015 2014 Assets Current assets: Cash and cash equivalents $ 27,783 $ 176,460 Short-term investments 77,064 — Collaboration receivables (all amounts are with a related party) 3,628 3,367 Prepaid expenses and other current assets 2,458 2,480 Total current assets 110,933 182,307 Property and equipment, net 3,106 3,087 Long-term investments 31,134 — Restricted cash 796 902 Other assets 368 — Total assets $ 146,337 $ 186,296 Liabilities and stockholders' equity Current liabilities: Accounts payable $ 875 $ 724 Accrued expenses 12,400 6,848 Deferred revenue 555 1,162 Deferred rent 661 519 Total current liabilities 14,491 9,253 Deferred revenue, net of current portion 4,239 4,816 Deferred rent, net of current portion 1,157 1,818 Warrants to purchase common stock 17,187 14,124 Total liabilities 37,074 30,011 Commitments and contingencies (Note 8) — — Stockholders' equity: Undesignated preferred stock, $0.001 par value: 25,000,000 shares authorized and no shares issued or outstanding — — Common stock, $0.001 par value: 175,000,000 shares authorized; 33,313,355 and 32,432,025, shares issued and outstanding at December 31, 2015 and 2014, respectively 34 33 Additional paid-in capital 416,926 399,835 Accumulated deficit (307,477 ) (243,583 ) Accumulated other comprehensive loss (220 ) — Total stockholders' equity 109,263 156,285 Total liabilities and stockholders' equity $ 146,337 $ 186,296 (Loss) Income Year Ended December 31, 2015 2014 2013 Revenue: Collaboration revenue: License and milestone $ 1,184 $ 1,673 $ 43,948 Cost-sharing, net 16,913 12,959 13,282 Total revenue(1) 18,097 14,632 57,230 Costs and expenses: Research and development 58,404 50,897 36,051 Litigation settlement — 5,000 — General and administrative 20,572 14,199 14,227 Total costs and expenses 78,976 70,096 50,278 (Loss) income from operations (60,879 ) (55,464 ) 6,952 Other (expense) income: Other (expense) income, net (3,527 ) 5,044 (26,797 ) Interest income 512 83 39 Interest expense — (922 ) (2,092 ) Total other (expense) income, net (3,015 ) 4,205 (28,850 ) Net loss $ (63,894 ) $ (51,259 ) $ (21,898 ) Other comprehensive loss: Net unrealized holding (losses) on short-term and long-term investments during the period $ (220 ) $ — $ — Comprehensive loss $ (64,114 ) $ (51,259 ) $ (21,898 ) Reconciliation of net loss to net loss applicable to common stockholders: Net loss $ (63,894 ) $ (51,259 ) $ (21,898 ) Accretion of dividends, interest, redemption value and issuance costs on redeemable convertible preferred stock — — (19,870 ) Gain on extinguishment of redeemable convertible preferred stock — — 2,765 Net loss applicable to common stockholders—basic and diluted $ (63,894 ) $ (51,259 ) $ (39,003 ) Net loss per share applicable to common stockholders-basic and diluted $ (1.92 ) $ (1.63 ) $ (4.15 ) Weighted-average number of common shares used in computing net loss per share applicable to common stockholders-basic and diluted 33,303 31,515 9,407 ___________________________________________________________ (1) Includes related party revenue (Note 15) $ 18,097 $ 14,632 $ 32,284 Year Ended December 31, 2013 2012 2011 $ 43,948 $ 9,696 $ 74,406 13,282 5,558 4,760 — — 1,745 57,230 15,254 80,911 36,051 35,319 32,713 14,227 8,824 8,142 — — 1,500 50,278 44,143 42,355 6,952 (28,889 ) 38,556 (26,797 ) (2,255 ) (485 ) 39 91 17 (2,092 ) (1,529 ) (1,822 ) (28,850 ) (3,693 ) (2,290 ) $ (21,898 ) $ (32,582 ) $ 36,266 $ (21,898 ) $ (32,582 ) $ 36,266 $ (21,898 ) $ (32,582 ) $ 36,266 (19,870 ) (27,061 ) (21,757 ) 2,765 — — — — (12,645 ) $ (39,003 ) $ (59,643 ) $ 1,864 $ (21,898 ) $ (32,582 ) $ 36,266 (19,870 ) (27,061 ) (21,757 ) 2,765 — — — — (12,395 ) $ (39,003 ) $ (59,643 ) $ 2,114 $ (4.15 ) $ (24.84 ) $ 0.80 $ (4.15 ) $ (24.84 ) $ 0.78 9,407 2,401 2,328 9,407 2,401 2,716 $ 32,284 $ 4,914 $ 64,220 Series A Redeemable
Convertible
Preferred Stock Series B Redeemable
Convertible
Preferred Stock Series C Redeemable
Convertible
Preferred Stock Series C-1
Redeemable
Convertible
Preferred Stock Series D
Redeemable
Convertible
Preferred Stock Series D-1
Redeemable
Convertible
Preferred Stock Number of
Shares Value Number of
Shares Value Number of
Shares Value Number of
Shares Value Number of
Shares Value Number of
Shares Value 6,410,976 $ 57,433 4,204,185 $ 55,880 2,978,062 $ 48,726 457,875 $ 7,571 234,940 $ 2,989 636,942 $ 8,392 — — — — — — — — — — — — — 4,616 — 5,584 — 5,594 — 908 — 668 — 1,736 — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — Value Value Value Value Value Value 6,410,976 62,049 4,204,185 61,464 2,978,062 54,320 457,875 8,479 234,940 3,657 636,942 10,128 — 4,616 — 5,580 — 5,589 — 908 — 668 — 1,736 — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — 6,410,976 66,665 4,204,185 67,044 2,978,062 59,909 457,875 9,387 234,940 4,325 636,942 11,864 December 31, 2012 6,410,976 $ 66,665 4,204,185 $ 67,044 2,978,062 $ 59,909 457,875 $ 9,387 234,940 $ 4,325 636,942 $ 11,864 — 3,408 — 4,138 — 4,068 — 661 — 487 — 1,273 — 3,408 — 4,138 — 4,068 — 661 — 487 — 1,273 — — (139,741 ) (2,267 ) (21,744 ) (445 ) — — (2,906 ) (54 ) — — — — (139,741 ) (2,267 ) (21,744 ) (445 ) — — (2,906 ) (54 ) — — 46,668 678 — — — — — — — — — — 46,668 678 — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — (6,457,644 ) (70,751 ) (4,064,444 ) (68,915 ) (2,956,318 ) (63,532 ) (457,875 ) (10,048 ) (232,034 ) (4,758 ) (636,942 ) (13,137 ) (6,457,644 ) (70,751 ) (4,064,444 ) (68,915 ) (2,956,318 ) (63,532 ) (457,875 ) (10,048 ) (232,034 ) (4,758 ) (636,942 ) (13,137 ) — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — $ — — $ — — $ — — $ — — $ — — $ — December 31, 2013 — — — — — — — — — — — — Issuance of common stock net of expenses of $554 — — — — — — — — — — — — Stock-based compensation — — — — — — — — — — — — Exercise of stock options — — — — — — — — — — — — Net exercise of warrants to purchase common stock — — — — — — — — — — — — Exercise of warrants to purchase common stock — — — — — — — — — — — — Net loss — — — — — — — — — — — — Balance at December 31, 2014 — — — — — — — — — — — — Stock-based compensation — — — — — — — — — — — — Exercise of stock options — — — — — — — — — — — — Issuance of common stock related to ESPP — — — — — — — — — — — — Net exercise of warrants to purchase common stock — — — — — — — — — — — — Unrealized loss on available-for-sale securities — — — — — — — — — — — — Net loss — — — — — — — — — — — — Balance at December 31, 2015 — $ — — $ — — $ — — $ — — $ — — $ — Series E Redeemable
Convertible
Preferred Stock Series F Redeemable
Convertible
Preferred Stock Total
Redeemable
Convertible
Preferred
Stock Common Stock Number of
Shares Value Number of
Shares Value Number of
Shares $0.001 Par
Value Additional
Paid-In
Capital Accumulated
Deficit Total
Stockholders'
Equity (deficit) Common Stock 816,060 $ 8,423 — $ — $ 189,414 2,261,461 $ 3 $ — $ (248,820 ) $ (248,817 ) — — 2,426,171 30,378 30,378 — — — — — — 2,511 — 140 21,757 — — (1,617 ) (20,140 ) (21,757 ) — — — — — — — 1,212 — 1,212 — — — — — — — 215 — 215 — — — — — 94,748 — 190 — 190 — — — — — 37,249 — (0 ) — — — — — — — — — — 36,266 36,266 Value Value Comprehensive Income (Loss) 816,060 10,934 2,426,171 30,518 241,549 2,393,458 3 — (232,694 ) (232,691 ) — 2,459 — 5,505 27,061 — — (1,361 ) (25,700 ) (27,061 ) — — — — — — — 1,206 — 1,206 — — — — — 38,697 — 155 — 155 — — — — — — — — (32,582 ) (32,582 ) 816,060 13,393 2,426,171 36,023 268,610 2,432,155 3 — (290,976 ) (290,973 ) 816,060 $ 13,393 2,426,171 $ 36,023 $ 268,610 2,432,155 $ 3 $ — $ (290,976 ) $ — $ (290,973 ) — 1,802 — 4,033 19,870 — — (4,230 ) (15,640 ) (19,870 ) — 1,802 — 4,033 19,870 — — (4,230 ) (15,640 ) — (19,870 ) (13,103 ) (224 ) (4,825 ) (74 ) (3,064 ) (722 ) — 2,773 — 2,773 (13,103 ) (224 ) (4,825 ) (74 ) (3,064 ) (722 ) — 2,773 — — 2,773 — — — — 678 — — — — — — — — — 678 — — — — — — — — — — — 23,735 — — — — — — — — — 23,735 — — — — — — — — — — — — 2,196 — 2,196 — — — — — — — 2,196 — — 2,196 — — — — — 292,802 — 653 — 653 — — — — — 292,802 — 653 — — 653 (802,957 ) (14,971 ) (2,421,346 ) (39,982 ) (286,094 ) 18,516,993 19 149,885 136,190 286,094 (802,957 ) (14,971 ) (2,421,346 ) (39,982 ) (286,094 ) 18,516,993 19 149,885 136,190 — 286,094 — — — — — — — 2,012 — 2,012 — — — — — — — 2,012 — — 2,012 — — — — — 7,083,667 7 96,818 — 96,825 — — — — — 7,083,667 7 96,818 — — 96,825 — — — — — — — — (21,898 ) (21,898 ) — — — — — — — — (21,898 ) — (21,898 ) — $ — — $ — $ — 28,348,630 $ 29 $ 250,107 $ (192,324 ) $ 57,812 — — — — — 28,348,630 29 250,107 (192,324 ) — 57,812 Issuance of common stock net of expenses of $554 — — — — — 2,760,000 3 129,171 — — 129,174 Stock-based compensation — — — — — — — 4,778 — — 4,778 Exercise of stock options — — — — — 853,507 1 3,207 — — 3,208 Net exercise of warrants to purchase common stock — — — — — 303,204 — 7,422 — — 7,422 Exercise of warrants to purchase common stock — — — — — 166,684 — 5,150 — — 5,150 Net loss — — — — — — — — (51,259 ) — (51,259 ) Balance at December 31, 2014 — — — — — 32,432,025 33 399,835 (243,583 ) — 156,285 Stock-based compensation — — — — — — — 12,075 — — 12,075 Exercise of stock options — — — — — 837,361 1 3,962 — — 3,963 Issuance of common stock related to ESPP — — — — — 23,787 — 589 — — 589 Net exercise of warrants to purchase common stock — — — — — 20,182 — 465 — — 465 Unrealized loss on available-for-sale securities — — — — — — — — — (220 ) (220 ) Net loss — — — — — — — — (63,894 ) — (63,894 ) Balance at December 31, 2015 — $ — — $ — $ — 33,313,355 $ 34 $ 416,926 $ (307,477 ) $ (220 ) $ 109,263 Year Ended December 31, Year Ended December 31, 2013 2012 2011 2015 2014 2013 $ (21,898 ) $ (32,582 ) $ 36,266 Net loss $ (63,894 ) $ (51,259 ) $ (21,898 ) Adjustments to reconcile net loss to net cash used in operating activities: 915 1,293 3,134 1,176 1,118 915 32 — — 34 25 32 2,196 1,206 1,427 12,075 4,778 2,196 — 51 162 342 335 271 — (536 ) 342 186 84 79 — 36 186 26,875 2,258 481 3,528 (5,037 ) 26,875 (76 ) — — — — (76 ) 237 — — — — 237 Net amortization of premium on investments (406 ) — — (823 ) (594 ) 2,564 7 (255 ) (823 ) (840 ) (1,116 ) 1,836 (261 ) 249 (840 ) (4 ) (8 ) (6 ) — — (4 ) (53 ) (1,272 ) 334 151 (161 ) (53 ) 709 1,640 (2,773 ) 4,976 (17 ) 709 (26,949 ) (9,696 ) (35,130 ) (1,184 ) (1,673 ) (26,949 ) (499 ) (482 ) 211 (519 ) (499 ) (499 ) — (1 ) 200 106 11 — (19,650 ) (38,884 ) 9,056 Net cash used in operating activities (44,211 ) (53,220 ) (19,650 ) Purchase of investments (134,697 ) — — Proceeds from maturities investments 26,685 — — (307 ) (441 ) (27 ) (959 ) (514 ) (307 ) (307 ) (441 ) (27 ) (108,971 ) (514 ) (307 ) — — 30,378 — 19,935 — 86,825 — — Proceeds from issuance of common stock from public offering, net of issuance costs (47 ) 129,174 86,825 10,000 — — — — 10,000 (3,669 ) (6,191 ) (9,476 ) — (16,331 ) (3,669 ) (300 ) — — — — (300 ) Proceeds from issuances of common stock related to employee stock purchase plan 589 — — 653 155 190 3,963 4,188 653 93,509 13,899 21,092 4,505 117,031 93,509 73,552 (25,426 ) 30,121 39,611 65,037 34,916 $ 113,163 $ 39,611 $ 65,037 Net (decrease) increase in cash and cash equivalents (148,677 ) 63,297 73,552 Cash and cash equivalents at beginning of year 176,460 113,163 39,611 Cash and cash equivalents at end of year $ 27,783 $ 176,460 $ 113,163 $ 1,657 $ 1,065 $ 1,461 $ — $ 1,574 $ 1,657 $ 19,870 $ 27,061 $ 21,757 $ — $ — $ 19,870 $ 286,094 $ — $ — $ — $ — $ 286,094 $ 2,012 $ — $ — $ — $ — $ 2,012 $ 678 $ — $ — Reclassification of warrant liability to additional paid-in capital $ 465 $ 11,592 $ 678 $ 74 $ — $ — $ 306 $ — $ 74 $ 297 $ — $ — $ 270 $ 11 $ 297 2013, 20122015, 2014 and 2011 was incorporated in the state of Delaware on June 13, 2003, as Phoenix Pharma, Inc. The Company subsequently changed its name to Acceleron Pharma Inc. and commenced operations in February 2004. The Company is a Cambridge, Massachusetts-based biopharmaceutical company focused on the discovery, development and commercialization of novel protein therapeutics for cancer and rare diseases. The Company's research focusestherapeutic candidates that are based on the biology ofmechanisms that the Transforming Growth Factor-Beta (TGF-b) protein superfamily, a large and diverse group of molecules thathuman body uses to regulate the growth and repair of tissues throughoutits cells and tissues. The Company's research focuses on key natural regulators of cellular growth and repair, particularly the human body.Transforming Growth Factor-Beta (TGF-beta) protein superfamily. By couplingcombining its discovery and development expertise, including its proprietary knowledge of the TGF-bTGF-beta superfamily, withand its internal protein engineering and manufacturing capabilities, the Company has built a highly productive researchdiscovery and development platform that has generated numerous innovative protein therapeuticstherapeutic candidates with novel mechanisms of action. The Company has four internally discovered three protein therapeuticstherapeutic candidates that are currently being studied in 12 ongoing Phase 2 clinical trials, focused on the areas of cancer and rare diseases.Pharma Security Corporation.Securities Corp. All significant intercompany balances and transactions have been eliminated in consolidation.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20112. Summary of Significant Accounting Policies (Continued) Actual results could materially differ from those estimates.IPO.initial public offering (IPO). The Company's board of directors (the Board) determined the estimated fair20132015 or 2012.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20112. Summary of Significant Accounting Policies (Continued)statementconsolidated statements of operations and comprehensive income (loss).accounts receivable.collaboration receivables. The Company maintains its cash and cash equivalent balances in the form of money market accounts with financial institutions that management believes are creditworthy. The Company's investment policy includes guidelines on the quality of the institutions and financial instruments and defines allowable investments that the Company believes minimizes the exposure to concentrationconcentrations of credit risk.accounts receivable. and notes payable, approximated their fair values at December 31, 2013 and 2012,2015 or 2014 due to the short-term nature of these instruments, and for the notes payable, the interest rates the Company believes it could obtain for borrowings with similar terms.instruments. See discussion below on the determination of the fair value of the Company's preferred and common stock warrants.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20112. Summary of Significant Accounting Policies (Continued)Topic 820 establishes a three-tier fair value hierarchy that distinguishes between the following:•••6)7). During the periods presented, the Company has not changed the manner in which it values assets and liabilities that are measured at fair value using Level 3 inputs.20132015 and 20122014 and (in thousands): December 31, 2013 December 31, 2015 Quoted Prices
in Active Markets
for Identical Items
(Level 1) Significant Other
Observable
Inputs
(Level 2) Significant
Unobservable
Inputs
(Level 3) Total Quoted Prices
in Active Markets
for Identical Items
(Level 1) Significant Other
Observable
Inputs
(Level 2) Significant
Unobservable
Inputs
(Level 3) Total $ 101,394 $ — $ — $ 101,394 $ 24,811 $ — $ — $ 24,811 Corporate obligations — 67,706 — 67,706 U.S. Treasury securities — 10,991 — 10,991 Certificates of deposit — 16,776 — 16,776 Mortgage and other asset backed securities — 13,228 — 13,228 913 — — 913 796 — — 796 $ 102,307 $ — $ — $ 102,307 $ 25,607 $ 108,701 $ — $ 134,308 $ — $ — $ 30,753 $ 30,753 $ — $ — $ 17,187 $ 17,187 $ — $ — $ 30,753 $ 30,753 $ — $ — $ 17,187 17,187 2013, 20122015, 2014 and 20112. Summary of Significant Accounting Policies (Continued) December 31, 2012 Quoted Prices
in Active Markets
for Identical Items
(Level 1) Significant other
Observable
Inputs
(Level 2) Significant
Unobservable
Inputs
(Level 3) Total $ 36,847 $ — $ — $ 36,847 913 — — 913 $ 37,760 $ — $ — $ 37,760 $ — $ — $ 1,422 $ 1,422 — — 5,229 5,229 $ — $ — $ 6,651 $ 6,651 December 31, 2014 Quoted Prices
in Active Markets
for Identical Items
(Level 1) Significant Other
Observable
Inputs
(Level 2) Significant
Unobservable
Inputs
(Level 3) Total Assets: Money market funds $ 169,679 $ — $ — $ 169,679 Restricted cash 902 — — 902 Total assets $ 170,581 $ — $ — $ 170,581 Liabilities: Warrants to purchase common stock $ — $ — $ 14,124 $ 14,124 Total liabilities $ — $ — $ 14,124 $ 14,124 Year Ended
December 31, 2013 2012 2015 2014 $ 6,651 $ 4,393 $ 14,124 $ 30,753 26,875 2,258 3,528 (5,037 ) (678 ) — (465 ) (11,592 ) (83 ) — — — (2,012 ) — — — $ 30,753 $ 6,651 $ 17,187 $ 14,124 2013 or 2012 except for the transfer out of the warrants to purchase redeemable convertible preferred stock as described below. During the year 2013, as a result of the closing of the IPO, the warrants to purchase preferred stock were converted to warrants to purchase common stock. The resulting warrants to purchase common stock meet the criteria to be classified as permanent equity2015 and are no longer required to be measured at fair value at each reporting period.preferredcommon stock that were classified as liabilities was estimated using the Black-Scholes option pricing model aton the date of issuance and on each re-measurement date. Thisdate for those warrants to purchase common stock classified as liabilities was estimated using either the Monte Carlo simulation framework, which incorporates future financing events over the remaining life of the warrants to purchase common stock, or for certain re-measurement dates including December 31, 2015 and 2014, due to the warrants being deeply in the money, the Black-Scholes option pricing model was used. The Black-Scholes method of valuation involves using inputs such as the fair value of theAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20112. Summary of Significant Accounting Policies (Continued) various classes of preferred stock, stock price volatility, the contractual term of the warrants, risk free interest rates, and dividend yields. At each reporting period the company evaluates the best valuation methodology. Due to the nature of these inputs, the valuation of the warrants is considered a Level 3 measurement.67 for further discussions of the accounting for the warrants, as well as for a summary of the significant inputs and assumptions used to determine the fair value of the warrants. The fair value of warrants to purchase common stock that are classified as liabilities was estimated using a Monte Carlo model up until September 30, 2013. This method of valuation involves using inputs such as the fair value of a share of common stock, stock price volatility, and the contractual term of the warrants. Due to the warrants being deeply in the money as of December 31, 2013, the valuation method to estimate the warrants to purchase common stock was changed to the Black-Scholes option pricing model. Due to the nature of these inputs, the valuation of the warrants is considered a Level 3 measurement.20132015 or 2012.Asset Estimated Useful Life 3 years 3 years Shorter of the useful life or remaining lease term 2013, 2012 or 2011.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 20122015, 2014 and 20112. Summary of Significant Accounting Policies (Continued)protein therapeutics.9.10. The arrangements generally contain multiple elements or deliverables, which may include (1) licenses, or options to obtain licenses, to the Company's technology, (2) research and development activities performed for the collaboration partner, (3) participation on Joint Development Committees, and (4) manufacturing clinical or preclinical material. Payments pursuant to these arrangements typically include non-refundable, up-front payments, milestone payments upon achieving significant development events, research and development reimbursements, sales milestones, and royalties on future product sales.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20112. Summary of Significant Accounting Policies (Continued)income (loss).loss. To the extent that the Company reimburses the collaborator for costs incurred, the Company records these costs as a reduction of cost-sharing revenue.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20112. Summary of Significant Accounting Policies (Continued)Contract Manufacturing Revenue Contract manufacturing revenue is recognized upon deliveryrisk of loss occurs.9.10.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20112. Summary of Significant Accounting Policies (Continued)2013,2015, the Company had two stock-based compensation plans, which are more fully described in Note 10.11. The Company accounts for stock-based compensation in accordance with the provisions of ASC Topic 718,Compensation—Stock Compensation (ASC 718), which requires the recognition of expense related to the fair value of stock-based compensation awards in the consolidated statements of operations and comprehensive income (loss).loss.1011 for a discussion of the assumptions used by the Company in determining the grant date fair value of options granted under the Black-Scholes option pricing model, as well as a summary of the stock option activity under the Company's stock-based compensation planplans for the year ended December 31, 2013. of the tax position as2013, 20122015, 2014 and 20112. Summary 2013Significant Accounting Policies (Continued)2013 and 2012,2015 or 2014, the Company does not have any significant uncertain tax positions.20132015, 2014 and 2012,2013, the Company has excluded the effects of all potentially dilutive shares, which include redeemable convertible preferred stock, warrants for redeemable convertible preferred stock, warrants for common stock and outstanding common stock options, from the weighted-average number of common shares outstanding as their inclusion in the computation for these two years would be anti-dilutive due to net losses.income (loss)loss per share for the periods indicated because including them would have had an anti-dilutive effect (in thousands): Year Ended December 31, Year Ended December 31, 2013 2012 2011 2015 2014 2013 3,709 3,730 — 3,191 3,230 3,709 908 884 874 398 422 908 Shares issuable under employee stock purchase plan 15 14 — Restricted stock units 521 — — 13,218 18,166 — — — 13,218 114 248 — — — 114 4,125 3,666 17,949 17,949 23,028 874 income (loss)loss consists of net income (loss)loss and other comprehensive income (loss),loss, which includes certain changes in equity that are excluded from net income (loss).loss. Comprehensive income (loss)loss hasAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20112. Summary of Significant Accounting Policies (Continued)(loss) incomeloss. Accumulated other comprehensive loss is presented separately on the consolidated balance sheets and equals the Company's net income (loss) for all periods presented.15.Application16.not take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards,Consolidated Financial Statements (Continued)as a result, will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. December 31, December 31, 2013 2012 2015 2014 $ 921 $ 919 $ 1,163 $ 956 205 179 260 213 12,334 11,815 11,458 11,311 9,930 10,088 9,990 9,930 — 8 390 11 23,390 23,009 23,261 22,421 (19,685 ) (18,950 ) (20,155 ) (19,334 ) $ 3,705 $ 4,059 $ 3,106 $ 3,087 $0.9$1.2 million, $1.3$1.1 million and $3.1$0.9 million for the years ending December 31, 2015, 2014 and 2013 2012 and 2011, respectively.2013, 20122015, 2014 and 201120132015, and 2012,2014, the Company maintained letters of credit totaling $0.8 million and $0.9 million respectively, held in the form of a money market accountcertificates of deposit as collateral for the Company's facility lease obligations and its credit cards.5.The Company determines the appropriate classification of marketable securities at the time of purchase and reevaluates such designation at each balance sheet date. The Company has classified all of its marketable securities at December 31, 2015 as “available-for-sale” pursuant to ASC 320, Investments – Debt and Equity Securities. The Company records available-for-sale securities at fair value, with the unrealized gains and losses included in accumulated other comprehensive income (loss) in stockholders’ equity. There were no realized gains or losses on marketable securities for the years ended December 31, 2015, 2014 and 2013. December 31, 2015 Amortized Cost Gross Unrealized Gains Gross Unrealized Losses Estimated Fair Value Cash and cash equivalents due in 90 days or less $ 27,783 $ — $ — $ 27,783 Available-for-sale securities: Corporate obligations due in one year or less 53,243 — (81 ) 53,162 Corporate obligations due in more than one year 14,112 — (72 ) 14,040 U.S. Treasury securities due in one year or less 6,016 — (4 ) 6,012 U.S. Treasury securities due in more than one year 4,995 — (15 ) 4,980 Certificates of deposit due in one year or less 11,890 — — 11,890 Certificates of deposit due in more than one year 4,886 — — 4,886 Mortgage and other asset backed securities due in one year or less 6,010 — (10 ) 6,000 Mortgage and other asset backed securities due in more than one year 7,266 — (38 ) 7,228 Total available-for-sale securities $ 108,418 $ — $ (220 ) $ 108,198 Total cash, cash equivalents and available-for-sale securities $ 136,201 $ — $ (220 ) $ 135,981 December 31, 2014 Amortized Cost Gross Unrealized Gains Gross Unrealized Losses Estimated Fair Value Cash and cash equivalents due in 90 days or less $ 176,460 $ — $ — $ 176,460 Total cash and cash equivalents $ 176,460 $ — $ — $ 176,460 December 31, 2013 2012 December 31, $ — $ 1,000 2015 2014 1,330 1,282 $ 4,938 $ 2,291 2,930 2,448 3,551 3,050 1,017 607 1,198 426 1,650 816 2,713 1,081 $ 12,400 $ 6,848 $ 6,927 $ 6,153 6. Warrants as of December 31, 2015 December 31, 2014 Expiration December 31, 2015 December 31, 2014 Warrants to purchase common stock 393 409 5.88 June 10, 2020 - July 9, 2020 Liability(2) Liability(2) Warrants to purchase common stock 5 13 4.00 - 7.40 March 31, 2016 - December 31, 2017 Equity(1) (3) (4) Equity(1) (3) (4) All warrants 398 422 $ 5.88 Warrants as of Balance Sheet
Classification Weighted-
Average
Exercise
Price Per
Share December 31, December 31,
2013 December 31,
2012 2013 2012 Expiration — 107 $ 4.00 February 28, 2013 N/A(1) Liability — 32 7.40 December 21, 2013 N/A(2)(4) Liability — 46 10.92 June 25, 2019 N/A(2) Liability — 64 12.56 March 18, 2020 N/A(2) Liability 46 — 10.92 June 25, 2019 Equity(2) N/A 64 — 12.56 March 18, 2020 Equity(2) N/A 857 872 5.88 June 10, 2020 - July 9, 2020 Liability Liability 13 13 4.00 - 7.40 March 31, 2015 - December 31, 2017 Equity(3) Equity 980 1,134 $ 6.53 (1)On February 6, 2013, the warrant holder exercised a warrant to purchase 107 shares of Series A Preferred Stock on a net basis, resulting in the issuance of 47 shares of Series A Preferred Stock.(2)Warrants to purchase Series B Preferred Stock, Series C-1 Preferred Stock, and Series D-1 Preferred Stock were converted to warrants to purchase common stock at the closing of the IPO on September 24, 2013.(3)Warrants to purchase common stock were issued in connection with various debt financing transactions that were consummated in periods prior to December 31, 2012. See discussion below for further details.(4)On December 19 and 20, 2013, the warrants holders exercised warrants to purchase 32 shares of Common Stock on a net basis, resulting in the issuance of 24 shares of Common Stock.(1) (2) In May 2015, the warrant holders exercised warrants to purchase 16,665 shares of Common Stock on a net basis, resulting in the issuance of 13,588 shares of Common Stock. Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20116. Warrants (Continued)(3) (4) 2012,2014, the Company issued warrants for the purchase of up to 106,500 shares of the Company's Series A redeemable convertible preferred stock (Series A Preferred Stock), 31,891 shares of the Company's Series B redeemable convertible preferred stock (Series B Preferred Stock), 45,786 shares of the Company's Series C-1 redeemable convertible preferred stock (Series C-1 Preferred Stock), and 63,693 shares of the Company's Series D-1 redeemable convertible preferred stock (Series D-1 Preferred Stock). Each warrant was immediately exercisable. The warrants to purchase Series A and Series B Preferred Stock expire seven years from the original date of issuance, while the warrants to purchase Series C-1 and Series D-1 Preferred Stock expire ten years from the original date of issuance. The warrants to purchase shares of the Company's preferred stock have an exercise price equal to the original issuance price of the underlying instrument. Each warrant is exercisable on either a physical settlement or net share settlement basis and the redemption provisions are outside the control of the Company. In connection with the closing of the Company's IPO on September 24, 2013, the outstanding warrants to purchase Series B Preferred Stock, Series C-1 Preferred Stock, and Series D-1 Preferred Stock were converted into warrants to purchase common stockstock. The exercise prices for each of these warrants remained unchanged.2013, 20122015 and 2011,2014, the Company remeasured the fair value of all of its outstanding warrants to purchase shares of the Company's preferred stock up until the conversion of such warrants on September 24, 2013, using current assumptions, resulting in an increase in fair value of $1.3 million, $0.4 million,zero, zero and $0.1$1.3 million, respectively, which was recorded in other expense net in the accompanying consolidated statementsstatement of operations and comprehensive (loss) income.loss. As a result of the closing of the IPO and the resulting conversion of the warrants to purchase preferred shares into warrants to purchase common stock, the fair value of the warrant liability at September 24, 2013 was reclassified to permanent equity and therefore, is no longer subject to remeasurement.incomeloss for the year ended December 31, 2013.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20116. Warrants (Continued)income (loss)loss for each reporting period thereafter. The fair value of the common stock warrants were recorded as a discount to the preferred stock issued of $3.0 million, and the preferred stock were being accreted to the redemption value. At the end of each reporting period, the Company remeasured the fair value of the outstanding warrants, using current assumptions, resulting in an increase (decrease) in fair value of $26.9$3.5 million, $1.9$(5.0) million and $0.3$26.9 million, respectively, which was recorded in other expenseincome (expense), net in the accompanying consolidated statements of operations and comprehensive (loss) incomeloss for the years ended December 31, 2013, 20122015, 2014 and 2011.2013. The Company will continue to re-measure the fair value of the liability associated with the warrants to purchase common stock at the end of each reporting period until the earlier of the exercise or the expiration of the applicable warrants. On March 31, 2013, the Company retired 13,994 warrants to purchase common stock as a consequence of a repurchase of shares from an investor. All remaining outstanding warrants were fully vested and exercisable as of December 31, 2013.2015.2012,2015 the Company issued warrants to purchase up to 12,634 shares of common stock. The awards of warrants to purchase shares of common stock are accounted for as equity instruments. The warrants are exercisable at any time through their respective expiration dates. The fair value at issuance was calculated using the Black-Scholes option-pricing model, and was charged to interest expense during the periods the related debt was outstanding. The Company issued warrants to purchase up to 41,388 shares As of common stock in periods prior to December 31, 2012 in exchange for consulting services provided by2015, 5,206 warrants remain outstanding and expire a third party pursuant to stand-alone award agreements that are independent of an equity incentive plan. The warrants vested upon achievement of four milestones and were outstanding for approximately seven years from the date of issuance. During the year endedvarious dates through December 31, 2011, the holder exercised 41,388 warrants to purchase common stock on a net basis resulting in the issuance of 37,249 shares of common stock. There were no exercises, cancellations, or expirations of warrants during the years ended December 31, 2013 or 2012.up until September 30, 2013, whichAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20116. Warrants (Continued)incorporated three incorporates future financing events over the remaining life of the warrants to purchase common stock. Duestock, or for certain re-measurement dates including December 31, 2015 and 2014, due to the warrants being deeply in the money, as of December 31, 2013, the valuation method to estimate the warrants to purchase common stock was changed to the Black-Scholes option pricing model. Due to the nature of these inputs and the valuation techniques utilized, the valuation of the warrants to purchase preferred stock and common stock are considered a Level 3 measurement (Note 2). The fair value of each warrant to purchase shares of the Company's Series A Preferred Stock was estimated using the Black-Scholes option pricing model withwas used. At each reporting period the following assumptions: Year Ended December 31, 2013(2) 2012(1) 2011 n/a $ 9.24 $ 6.76 n/a 69.1 % 66.0 % n/a 0.16 1.16 n/a 0.04 % 0.12 % n/a — % — % (1)During December 2012, the expiration date of the warrant to purchase Series A Preferred Stock was extended from December 21, 2012 to February 28, 2013.(2)The warrant to purchase Series A Preferred Stock was exercised during the three months ended March 31, 2013. The fair value of each warrant to purchase shares of the Company's Series B Preferred Stock was estimated using the Black-Scholes option pricing model with the following assumptions: Year Ended December 31, 2013(1) 2012 2011 $ 20.03 $ 9.96 $ 7.56 71.2 % 69.1 % 66.0 % 0.24 0.97 1.98 0.04 % 0.16 % 0.25 % — % — % — % (1)Warrants to purchase Series B Preferred Stock were converted to warrants to purchase common stock at the closing of the IPO on September 24, 2013.2013, 20122015, 2014 and 20116. Warrants (Continued) The fair value of each warrant to purchase shares of the Company's Series C-1 Preferred Stock was estimated using the Black-Scholes option pricing model with the following assumptions: Year Ended December 31, 2013(1) 2012 2011 $ 20.03 $ 11.04 $ 8.84 71.2 % 69.1 % 66.0 % 5.73 6.46 7.46 1.75 % 0.95 % 1.35 % — % — % — % (1)Warrants to purchase Series C-1 Preferred Stock were converted to warrants to purchase common stock at the closing of the IPO on September 24, 2013. The fair value of each warrant to purchase shares of the Company's Series D-1 Preferred Stock was estimated using the Black-Scholes option pricing model with the following assumptions: Year Ended December 31, 2013(1) 2012 2011 $ 20.03 $ 10.52 $ 8.84 71.2 % 69.1 % 66.0 % 6.48 7.22 8.22 1.75 % 1.18 % 1.62 % — % — % — % (1)Warrants to purchase Series D-1 Preferred Stock were converted to warrants to purchase common stock at the closing of the IPO on September 24, 2013.Fair Value of Underlying Instrument The Company estimated the fair value of its shares of Series A Preferred Stock, Series B-1 Preferred Stock, Series C-1 Preferred Stock and Series D-1 Preferred Stock as of December 31, 2012 and 2011, and through the exercise or conversion of its Preferred stock warrants during 2013, using the PWERM (probability-weighted expected return method).Expected Volatility The Company estimated the expected volatility based on actual historical volatility of the stock price of similar companies with publicly-traded equity securities. The Company calculated the historical volatility of the selected companies by using daily closing prices over a period of the expected term of the associated award. The companies were selected based on their enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected term of the associated award. A decrease in the selected volatility would decrease the fair value of the underlying instrument.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20116. Warrants (Continued)Expected Term The Company based the expected term on the actual remaining contractual term of each respective warrant. A decrease in the expected term would decrease the fair value of the underlying instrument.Risk-Free Interest Rate The Company estimated the risk-free interest rate in reference to the yield on U.S. Treasury securities with a maturity date commensurate with the expected term of the associated award. A decrease in the selected risk-free rate would decrease the fair value of the underlying instrument.Expected Dividend Yield The Company estimated the expected dividend yield based on consideration of its historical dividend experience and future dividend expectations. The Company has not historically declared or paid dividends to stockholders. Moreover, it does not intend to pay dividends in the future, but instead expects to retain any earnings to invest in the continued growth of the business. Accordingly, the Company assumed an expected dividend yield of 0.0%.7.May 2018.March 2021. All of the Company's leases contain escalating rent clauses, which require higher rent payments in future years. The Company expenses rent on a straight-line basis over the term of the lease, including any rent-free periods. In addition, the Company received certain leasehold improvementlease incentives, and recorded these incentives as deferred rent, which is amortized as a reduction of rent expense over the life of the lease. Rent expense of approximately $3.5$3.4 million, $3.5$3.3 million and $3.6$3.5 million were incurred during the years ended December 31, 2015, 2014 and 2013, 2012 and 2011, respectively.2013,2015, are as follows (in thousands): $ 4,522 4,106 3,938 3,938 2,953 $ 19,457 2016 4,386 2017 4,547 2018 3,577 2019 640 2020 656 2021 165 Total $ 13,971 In February 2011,subleasefive-year building lease for approximately 12,200 square feet of space located at 125 Sidney Street, Cambridge, Massachusetts. The lease commenced on January 1, 2016 when the lessor delivered the building to the Company. Rent will commence on April 1, 2016 and is approximately $0.6 million for the first year with annual rent escalations thereafter. The total operating lease obligation of for the term of this agreement foris $3.1 million. In addition, the lease provides a portioncontribution from the landlord towards the initial build-out of onethe space of up to $0.5 million. The Company has the option to extend this lease by an additional five years. In accordance with the lease, the Company will enter into a cash-collateralized irrevocable standby letter of credit in the amount of $0.2 million, naming the landlord as beneficiary. facility leases.business. The tenant will pay rent onCompany was not subject to any material legal proceedings during the lease from February 28, 2011 until May 30, 2015. Onyears ended December 31, 2015, 2014 and 2013, and, to the sublease agreement was expanded to include the remaining spacebest of thatAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20117. Commitments and Contingencies (Continued)facility. The expansion portion will be leased for one year, through December 31, 2014. The Company will continue to utilize the expansion portion of the leased property, once the sublease expires. Future annual minimum sublease proceeds as of December 31, 2013,its knowledge, no material legal proceedings are as follows (in thousands): $ 832 252 $ 1,084 Legal ProceedingsSalk'sSalk’s U.S. patents related to the ActRIIB activin receptor proteins. Salk contendscontended that, under the licensing agreement, the Company owed Salk a greater share of the upfront payment that it received under its now-terminated agreement with Shire AG regarding ACE-031 and a share of the upfront payment and development milestone payments that the Company has received under its ongoing collaboration agreement with Celgene regarding ACE-536.luspatercept. Salk iswas seeking a total of approximately $10.5 million plus interest in payment and a 15% share of future development milestone payments received under the agreement with Celgene regarding ACE-536.luspatercept. The Company contendscontended that no additional amounts arewere due to Salk and that it hashad complied with all of its payment obligations under the applicable Salk license agreement.Company'sCompany’s motion on February 28, 2013. On March 14, 2013, Acceleron answered the complaint and asserted patent invalidity counterclaims. On the basis of those counterclaims, Acceleron removed the action on March 28, 2013 to the United States District Court for the District of Massachusetts. The parties have since reached an agreement on a stipulation as to certain patent issues raised in the action, and Acceleron hasthe Company dismissed its counterclaims. The Court held an initial scheduling conference on May 30, 2013, and the fact discovery is nowwas closed. The case is currently scheduled for trial in September 2014. The intends to defend its position vigorously. The Company evaluated the suit under ASC Topic 450,Contingencies, as a loss contingency. The estimated loss from a loss contingency shall be accrued if information available before the financial statements are issued indicates that it is probable a liability had been incurred at the date of the financial statements, and the amount of loss can be reasonably estimated. BecauseSalk Institute for Biological Studies entered into an amendment (the Amendment) to that certain Exclusive License Agreement between Salk and the Company believes that the potential for an unfavorable outcome is not probable, it has not established a reserve with respect to the dispute as of December 31, 2013 or 2012. The Company's estimates can be affected by various factors. As of December 31, 2013regarding Activin Receptors (Type IIB) and 2012, management has determined a loss is reasonably possible. Although the Company believes it would successfully defend the lawsuit, the Company has in the past participated in settlement discussions with Salk. Accordingly, the Company has estimated the range of possible losses as of December 31, 2013 and 2012 to be between $0 and $10.5 million plus interest.2013, 20122015, 2014 and 20117. Commitments2013Contingencies (Continued)20132015 and 2012,2014, or royalties on future sales of specified products. No milestone or royalty payments under these agreements are expected to be payable in the immediate future. See Note 910 for discussion of these arrangements.8. Redeemable Convertible Preferred Stock and As of December 31, 2012, the authorized capital stock of the Company was 104,013,161 shares of common stock, par value $0.001 per share and 74,077,227 shares of preferred stock, par value $0.001 per share, of which: (1) 26,069,980 shares have been designated as Series A Preferred Stock, (2) 16,944,378 shares have been designated as Series B Preferred Stock, (3) 11,923,077 shares have been designated as Series C redeemable convertible (Series C Preferred Stock), (4) 2,014,652 shares have been designated as Series C-1 Preferred Stock, (5) 955,414 shares have been designated as Series D redeemable convertible preferred stock (Series D Preferred Stock), (6) 2,802,548 shares have been designated as Series D-1 Preferred Stock, (7) 3,662,422 shares have been designated as Series E Preferred Stock, and (8) 9,704,756 shares have been designated as Series F redeemable convertible preferred stock (Series F Preferred Stock, and all collectively the Preferred Stock). On September 4, 2013, the Board and the stockholders of the Company approved a one-for-four reverse stock split of the Company's outstanding common stock, which was effected on September 5, 2013. Stockholders entitled to fractional shares as a result of the reverse stock split received a cash payment in lieu of receiving fractional shares. The Company's historical share and per share information have been retroactively adjusted to give effect to this reverse stock split. Shares of common stock underlying outstanding stock options and other equity instruments were proportionately reduced and the respective exercise prices, if applicable, were proportionately increased in accordance with the terms of the agreements governing such securities. On September 4, 2013, the Board also approved for filing immediately following the effectiveness of the Company's registration statement in connection with its IPO, the Restated Certificate of Incorporation to increase the authorized number of shares of common stock from 104,013,161 to 175,000,000, to authorize 25,000,000 shares of undesignated preferred stock, par value $0.001 per share, and to eliminate all references to the previously designated Series Preferred Stock. This Restated Certificate of Incorporation was approved by the stockholders on September 4, 2013.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20118. Redeemable Convertible Preferred Stock and Stockholders' Equity (Continued)initial public offering (IPO)IPO whereby the Company sold 6,417,000 shares of common stock (including 837,000 shares of common stock sold by the Company pursuant to the full exercise of an overallotment option by the underwriters in connection with the offering) at a price of $15.00 per share. The shares began trading on The Nasdaq Global Market on September 19, 2013. The aggregate net proceeds received by the Company from the offering were $86.8 million, net of underwriting discounts and commissions and estimated offering expenses payable by the Company. Upon the closing of the IPO, all outstanding shares of convertible preferred stock converted into 18,516,993 shares of common stock and warrants exercisable for convertible preferred stock were automatically converted into warrants exercisable for 141,370 shares of common stock, resulting in the reclassification of the related convertible preferred stock warrant liability of $2.0 million to additional paid-in capital.2013, 20122015, 2014 and 20118. Redeemable Convertible Preferred Stock and Stockholders' Equity (Continued)Redeemable Convertible Preferred Stock Prior2013the closing of the initial public offering, at which time allissue 25,000,000 shares of Preferred Stock were converted into shares of commonpreferred stock the Company's Preferred Stock consisted of the following (in thousands, except share and per share data): December 31,
2012 $ 66,665 67,044 59,909 9,387 4,325 11,864 13,393 36,023 $ 268,610 holders of the Company's Preferred Stock had certain voting, dividend, and redemption rights, as well as liquidation preferences and conversion privileges. All rights, preferences, restrictions, qualifications and privileges associated withlimitations of such stock are determined by the Preferred Stock were terminated at the timeboard of the Company's IPO in conjunction with the conversiondirectors. As of all outstandingDecember 31, 2015 no shares of Preferred Stock into shares of common stock.2013.2015.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20118. Redeemable Convertible Preferred Stock and Stockholders' Equity (Continued)2013,2015, the Company has reserved for future issuance the following number of shares of common stock (in thousands):December 31, 2013 2015 3,191 3,9421,890 956398 980251 Additional shares reserved for unissued, but designated, Preferred Stock 25,000 30,730 30,8789.ACE-536luspatercept (the ACE-536Luspatercept Agreement), and also amended certain terms of the Sotatercept Agreement. These agreements provide Celgene exclusive licenses for Sotaterceptsotatercept and ACE-536luspatercept in all indications, as well as exclusive rights to obtain a license to certain future compounds. Celgene is a global biopharmaceutical company primarily engaged in the discovery, development and commercialization of innovative therapies designed to treat cancer and immune-inflammatory related diseases.b-thalassemia beta-thalassemia and will be responsible for any Phase 3 clinical trials, as well as additional Phase 2 clinical trials, and will be responsible for overseeing the manufacture of Phase 3 and commercial supplies by third-party contract manufacturing organizations. Under the agreement, the Company was eligible to receive clinical milestones of up to $88.0 million, regulatory milestones of up to $272.0 million, and commercial milestones of up to $150.0 million for sotatercept. Clinical milestone payments are triggered upon initiation of a defined phase of clinicalAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20119. Significant Agreements (Continued)producttherapeutic candidate. Regulatory milestone payments are triggered upon the acceptance of the marketing application and upon the approval to market a producttherapeutic candidate by the Food and Drug Administrationproducttherapeutic candidate. Regulatory milestone payments are triggered upon the acceptance of the marketing application and upon the approval to market a producttherapeutic candidate by the FDA or other global regulatory authorities. Commercial milestone payments are triggered when an approved pharmaceutical product reaches certain defined levels of net sales by Celgene in countries outside of North America. Option fee payments are triggered upon license of any of the option programs by Celgene. In addition, to the extent an option compound is commercialized, the Company would be entitled to receive tiered royalty payments in the low-to-mid twenty percent range of net sales from sales generated from all geographies. Royalty payments are subject to certain reductions, including for entry of a generic product onto the market. None of the three discovery stage programs has advanced to the stage to achieve payment of a milestone.ACE-536Luspatercept Agreement described below, the Company and Celgene agreed to modify the terms of the Sotatercept Agreement. The modified terms included: (1) a change to the responsibility for development costs to align with the ACE-536Luspatercept Agreement, with Celgene responsible for more than half of the worldwide costs through December 31, 2012, and 100% of the development costs thereafter, (2) future contingent development milestones for sotatercept were amended to a two-category (oncology and non-oncology) structure with potential future clinical milestones of $27.0 million and regulatory milestones of $190.0 million from a four-category (various cancer indications) structure and, (3) future contingent development milestones for option compounds were amended to a two-category (oncology and non-oncology) structure with potential future clinical milestones of $25.5 million and regulatory milestones of $142.5 million from a four-category (various cancer indications) structure, and (4) an option to buy down tiered royalty payments on both Sotaterceptsotatercept and ACE-536luspatercept with a one-time $25.0 million payment on or prior to January 1, 2013. The potential commercial milestones remained unchanged. As of December 31, 2013,To date, the Company has received $41.6$43.0 million in research and development funding and milestone payments for sotatercept under the original and modified agreements, of which $7.0 million is a clinical milestone payment earned in December 2013 that resulted from Celgene's start of a Phase 2b clinical trial in chronic kidney disease.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20119. Significant Agreements (Continued)ACE-536ACE-536Luspatercept Agreement, the Company and Celgene collaborate worldwide for the joint development and commercialization of ACE-536.luspatercept. The Company also granted Celgene an option for future products for which Acceleron files an Investigational New Drug application for the treatment of anemia. Celgene paid $25.0 million on the closing of the ACE-536Luspatercept Agreement in August, 2011. Under the terms of the ACE-536 Agreement theACE-536luspatercept for the Phase 1 and Phase 2 clinical trials and Celgene will be responsible for overseeing the manufacture of Phase 3 and commercial supplies by third party contract manufacturing organizations. The Company is eligible to receive clinical milestones of up to $32.5 million, regulatory milestones of up to $105.0 million and commercial milestones of up to $80.0 million for ACE-536.luspatercept. The Company will receive additional, lower development, regulatory, and commercial milestones for any additional products for the treatment of anemia on which Celgene exercises an option. Clinical milestone payments are triggered upon initiation of a defined phase of clinical research for a producttherapeutic candidate. Regulatory milestone payments are triggered upon the acceptance of the marketing application and upon approval to market a protein therapeutic candidate by the FDA or other global regulatory authorities. Commercial milestone payments are triggered when an approved pharmaceutical product reaches certain defined levels of net sales by Celgene in countries outside of North America. In addition, to the extent ACE-536luspatercept is commercialized, the Company would be entitled to receive tiered royalty payments in the low-to-mid twenty percent range of net sales fromAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20119. Significant Agreements (Continued)2013,2015, the Company has received $31.9$60.3 million in research and development funding and milestone payments for ACE-536.luspatercept. The next likely clinical milestone payment would be $15.0 million and result from theCelgene's start of a Phase 3 study in MDS orb-thalassemia. beta-thalassemia. The Company has not yet identified additional compounds for the treatment of anemia. Accordingly, there is no assurance that the Company will generate future value from additional programs.ACE-536Luspatercept Agreement will expire on a country-by-country basis on the occurrence of both of the following: (1) the expiration of the royalty term with respect to all license products in such country, and (2) the end of the option term. The royalty term for each licensed product in each country outside North America is the period commencing with first commercial sale of the applicable licensed product in the applicable country and ending on the latest of expiration of specified patent coverage or a specified period of years. The royalty term for each licensed product in North America is the period commencing with the first commercial sale in North America and ending, on a licensed product and country-by-country basis on the date which commercialization of such licensed product has ceased. The option term runs until the later of (1) the date on which no development or commercialization activities are ongoing or are expected to commence for any licensed products under the ACE-536Luspatercept Agreement; (2) the date on which no development or commercialization activities are ongoing or are expected to commence for any licensed products under the Sotatercept Agreement and all option rights under the Sotatercept Agreement have been forfeited with respect to each option compound where Celgene has made a payment with respect to such compound; and (3) the royalty term for all licensed products under the ACE-536Luspatercept Agreement and the Sotatercept Agreement has ended; provided that if at the time the option term would otherwise end any option compounds under the ACE-536Luspatercept Agreement are in clinical development the option term shall continue until Celgene's rights to such compound are either exercised or forfeited.ACE-536Luspatercept Agreement with respect to one or more licensed targets or in its entirety, upon 180 days' notice (or 45 days' notice if the licensed product has failed to meet certain end point criteria with respect to clinical trials or other development activities), provided that Celgene may not terminate the ACE-536Luspatercept Agreement prior to the completion of the on-going ACE-536b -thalassemialuspatercept beta-thalassemia and ACE-536luspatercept MDS Phase 2 clinical trials, except under certain conditions. The agreement may also be terminated in its entirety by either Celgene or the Company in the event of a material breach by the other party or in the event of a bankruptcy filing of the other party. There are no cancellation, termination or refund provisions in this arrangement that contain material financial consequences to the Company.ACE-536Luspatercept Agreements through December 31, 2012. As of January 1, 2013, Celgene ishas been responsible for paying 100% of the worldwide development costs under both agreements. Celgene will be responsible for all commercialization costs worldwide. The Company has the right to co-promote sotatercept, ACE-536luspatercept and future products under both agreements in North America. Celgene's option to buy down royalty rates for sotatercept and ACE-536luspatercept expired unexercised and, therefore, the Company will receive tiered2013, 20122015, 2014 and 20119. Significant Agreements (Continued)ACE-536.luspatercept. The royalty schedules for sotatercept and ACE-536luspatercept are the same.605- 25,605-25, applicable to the arrangement, since the Company could not establish VSOEvendor-specific objective evidence (VSOE) for the undelivered elements, the Company was required to recognize the initial consideration, consisting of the $45.0 million of nonrefundable upfront license and option payments, over the period the undelivered elements were to be delivered, which was initially estimated to be 15 years. As of the date of the modification of the agreement, there was approximately $34.7 million of deferred revenue under the arrangement.ACE-536Luspatercept Agreement and the amendment to the Sotatercept Agreement were negotiated in contemplation of each other, and the Company had not yet completed all of its obligations pursuant to the Sotatercept Agreement, the agreements were considered one arrangement for accounting purposes. The deliverables under the combined arrangement include: (1) licenses to develop and commercialize sotatercept and ACE-536,luspatercept, (2) performance of research and development services, (3) participation on the joint development committees, and (4) the performance of manufacturing services to provide clinical material to Celgene. The Company has determined the option to future products related to the treatment of anemia represents a substantive option. The Company is under no obligation to discover, develop or deliver any new compounds that modulate anemia and Celgene is not contractually obligated to exercise the option. As a result, the Company is at risk as to whether Celgene will exercise the option.ACE-536Luspatercept Agreement and amended Sotatercept Agreement consisted of (1) the $25.0 million up-front payment for the license of ACE-536,luspatercept, (2) the remaining deferred revenue from the Sotatercept Agreement of $34.7 million, and (3) estimated payments for development activities and manufacturing services of $18.0 million. The Company used its BESPbest estimate of selling price (BESP) for each of the undelivered elements as the Company did not have VSOE or (third-party evidence) TPE of selling price for each deliverable. The Company's BESP considered its development plan for the compounds, expected manufacturing services, and reimbursement from Celgene (reimbursement of more than halfAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20119. Significant Agreements (Continued)••••joint development committees.ACE-536Luspatercept Agreement and amended Sotatercept Agreement. At the end of each reporting period, the Company reassesses the estimated payments to be received related to these services and the BESPACE-536Luspatercept Agreement, related to the dosing of the first patient in a multiple-dose clinical trial. The Company evaluated the milestone and determined that it was not substantive, as there was no significant uncertainty to achieving the milestone upon execution of the ACE-536Luspatercept Agreement. As such, the Company allocated the $7.5 million payment based on the allocation of arrangement consideration determined at the execution date of the ACE-536Luspatercept Agreement and amended Sotatercept Agreement. Based on this allocation, the Company recognized $4.8 million of the payment upon achievement, with the remaining $2.7 million recognized as revenue as the undelivered elements are delivered, consistent with the treatment of the up-front payment. During 2011, the Company achieved a $7.0 million clinical milestone under its Sotatercept Agreement, related to the dosing of the first patient for a Phase 2b clinical trial. The Company evaluated the milestone and deemed it to be substantive and consistent with the definition of a milestone included in ASU 2010-17 and, accordingly, recognized the $7.0 million payment in revenue during the year ended in December 31, 2011. During January 2013, the Company achieved a $10.0 million clinical milestone under its ACE-536Luspatercept Agreement, related to the dosing of the first patient for a Phase 2 clinical trial. The Company evaluated the milestone and deemed it to be substantive and consistent with the definition of a milestone included in ASU 2010-17 and, accordingly, recognized the $10.0 million payment in revenue during the year ended December 31, 2013. During December 2013, the Company achieved a $7.0 million clinical milestone under its Sotatercept Agreement, related to Celgene's start of a Phase 2b clinical trial in chronic kidney disease. The Company evaluated the milestone and deemed it to be substantive and consistent with the definition of a milestone included in ASU 2010-17 and, accordingly, recognized the $7.0 million payment in revenue during the year ended December 31, 2013.The remaining development milestones under the ACE-536Luspatercept and Sotatercept Agreements were deemed to be substantive and consistent with the definition of a milestone included in ASU 2010-17 and, accordingly, the Company will recognize payments related to the achievement of such milestones, if any, when such milestone is achieved. Factors considered in this determination included scientific andAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20119. Significant Agreements (Continued)2013 20122015, 2014 and 2011,2013, the Company recognized $2.6$1.2 million, $2$1.7 million and $54.8$2.6 million, respectively, of the total deferred revenue as license and milestone revenue in the accompanying consolidated statements of operations and comprehensive loss.ACE-536Luspatercept Agreement the Company retains responsibility for certain research and development activities through the completion of Phase 1 and initial Phase 2 clinical trials, as well as manufacturing the clinical supplies for these studies. Celgene is responsible for the conduct of subsequent Phase 2 and Phase 3 clinical studies. In November, 2013, the Company agreed to conduct additional activities for the benefit of the ACE-536luspatercept program including certain clinical and non-clinical services such as multiple toxicology studies and associated assay development and sample testing, clinical extension studies, and market development work. These activities will be reimbursed under the same terms and rates of the existing Agreements. The Company evaluated the additional services to be provided and determined that as the Company is under no obligation to conduct these additional activities, these services do not represent a deliverable under or modification to the ACE-536Luspatercept Agreement, but rather, represent a separate services arrangement which should be accounted for as the services are delivered.ACE-536luspatercept until December 31, 2012 and 100% of the costs from January 1, 2013 and thereafter. Payments from Celgene with respect to research and development costs incurred by the Company are recorded as cost-sharing revenue. Payments by the Company to Celgene for research and development costs incurred by Celgene are recorded as a reduction to cost-sharing revenue. During the years ended December 2013, 201231, 2015, 2014 and 2011,2013, the Company recorded net cost-sharing revenue of $16.9 million, $13.0 million and $12.7 million, $2.9 million and $(0.1) million, respectively, which includes payments to Celgene of $0, $2.8 million and $2.8 million, respectively, which were recorded as contra-revenue.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20119. Significant Agreements (Continued)At December 31, 2012, the Company had classified the remaining deferred revenue as current in the consolidated balance sheet. Upon the effectiveness of the termination of the Shire Agreement in the second quarter of 2013, the Company accelerated the recognition of $22.4 million of remaining deferred revenue from upfront non-refundable payments received under the Shire Agreement as it had no further obligation for deliverables under the Shire Agreement. During the yearsyear ended December 31, 2013, 2012 and 2011, the Company recognized $24.3 million $7.7 million and $8.4 million, respectively of the up-front, non-refundable payments as license and milestone revenue in the accompanying consolidated statements of operations and comprehensive loss.yearsyear ended December 31, 2013, 2012 and 2011, the Company recorded net cost-sharing revenue of $0.6 million, $2.7 million and $4.1 million, respectively, which includes payments to Shire of $0.2 million, $0.7 million and $2.0 million, respectively, which are recorded as contra-revenue in the accompanying consolidated statements of operations and comprehensive loss.Alkermes License In December 2009, the Company entered into a Collaboration and License Agreement with Alkermes Inc. (Alkermes) relating to a proprietary technology platform for extending the circulating half-life of certain proteins. Under the terms of the agreement, Alkermes paid the Company an up-front cash payment of $2.0 million in December 2009, which was deferred and No amounts we recognized as license revenue ratably over the estimated research and development term. In addition, Alkermes purchased 636,942 shares of Series D-1 Preferred Stock at a per share price of $12.56, resulting in gross proceedssubsequent to the Company of $8.0 million. The Company determined thatJune 30, 2013 termination. price of $12.56 paid by Alkermes included a premium of $2.32 per share over the fair value of the Series D-1 Preferred Stock of $10.24 as calculated by the Company in its contemporaneous stock valuation. Accordingly, the Company has recognized the premium of $1.5 million as additional license revenue on a straight-line basis over theAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20119. Significant Agreements (Continued)term discussed above. In October 2011, Alkermes discontinued development of the compounds being investigated under the license agreement, and as a result, the Company recognized the remaining $2.4 million of the up-front payment as revenue, as it had no further obligations under the arrangement, though the license continues. As the principal in the collaboration, Acceleron recognized cost-sharing revenue for reimbursement payments from Alkermes. During the year ended December 31, 2011, the Company recognized net cost-sharing revenue of $0.7 million. No amounts were recognized in subsequent periods.ImmunoGen Services Agreement In October 2010, the Company entered into a Biopharmaceutical Services Agreement with ImmunoGen, Inc. Acceleron agreed to develop and manufacture an ImmunoGen product. The Company determined the arrangement should be accounted for as a service arrangement, using the proportional performance method. Accordingly, the Company recognized revenue as the underlying performance criteria were met. The costs associated with the services were charged to operations as incurred. As of December 31, 2011, the work was completed and the Company recorded revenue of $1.7 million for the year ended December 31, 2011.Other The Company entered into a license agreement with a non-profit institution for an exclusive, sublicensable, worldwide, royalty-bearing license to certain patents developed by the institution (Primary Licensed Products). In addition, the Company was granted a non-exclusive, non-sub- licensablenon-sub-licensable license for Secondary Licensed Products. As compensation for the licenses, the Company issued 62,500 shares of its common stock to the institution, the fair value of which was $25,000, and was expensed during 2004, to research and development expense. The Company also agreed to pay specified development milestone payments totaling up to $2.0 million for sotatercept and $0.7 million for ACE-536.luspatercept. In addition, the Company is obligated to pay milestone fees based on the Company's research and development progress, and U.S. sublicensing revenue ranging from 10%-25%, as well as a royalty ranging from 1.0%-3.5% of net sales on any products developed under the licenses. During the years ended December 31, 2013, 20122015, 2014 and 2011,2013, the Company paid and expensed milestones and fees defined under the agreement totaling $0.5$0.1 million, $0$0.1 million and $0.5 million, respectively. Therespectively, which is recorded as research and development expense.WeThe Company agreed to pay specified development and sales milestone payments aggregating up to $1.0 million relating to the development and commercialization of dalantercept. In addition, we arethe Company is required to pay royalties in the low single-digits on worldwide net product sales of dalantercept, with royalty obligations continuing at a 50% reduced rate for a period of time after patent expiration. If we sublicense ourthe Company sublicenses its patent rights, weit will owe a percentage of sublicensing revenue, excluding payments based on the level of sales, profits or other levels of commercialization. During the years ended December 31, 2013, 20122015, 2014 and 2011,2013, the Company did not reach any milestones defined under the agreement and, therefore, no amounts have been paid or expensed.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 20119. Significant Agreements (Continued)2012, the Company did not reach anyexpensed milestones and fees defined under the agreement totaling $0.0 million, zero and therefore, no amounts have beenzero, respectively, which is recorded as research and development expense.or expensed.10.an upfront and research fee of $0.3 million upon execution of the agreement and the Company is obligated to pay additional research fees of approximately $0.6 million over approximately the next year, depending on the success of the research program. The Company also received an option to obtain a commercial license to the molecules developed during the collaboration. During the year ended December 31, 2015 and December 31, 2014 the Company expensed milestones and fees of $1.4 million and $0.6 million, which is recorded as research and development expense.2013,2015, the Company had two stock-based compensations plans, which are more fully described below.956,0001,889,730 at December 31, 2013.andor the 2013 Plan since its inception. Stock options carry an exercise price equal to the estimated fair value of the Company's common stock on the date of grant. Options generally expire ten10 years following the date of grant. Stock options and restricted stock awards typically vest over four4 years, but vesting provisions can vary based on the discretion of the Board.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 201110. Stock-Based Compensation (Continued)Company's board of directorsBoard and stockholders approved the adoption of the 2013 Employee Stock Purchase Plan (the 2013 ESPP). Under the 2013 ESPP, 275,000 shares of the Company's common stock will be available for issuance andto eligible employeesemployees. The per-share purchase price at the end of the Company may purchase shares of common stock during pre-specified purchase periods at a priceeach offering period is equal to the lesser of 85% of the fair market valueclosing price of aone share of itsthe Company’s common stock at the beginning of the purchase period or 85% of the fair market value of a share of its common stock at the end of the purchase period.offering period, whichever is lower, subject to Internal Revenue Service limits. The 2013 ESPP will terminate on September 4, 2023, the tenth anniversary of the initial adoption of the plan. The Board has not determined the dateinitial offering period commenced on whichSeptember 16, 2014 and the initial purchase period will commence underoccur on the 2013 ESPP. As6 month anniversary. The Company recorded $0.3 million, $0.1 million and zero of December 31, 2013, the initial purchase period under the 2013 ESPP has not yet commenced. During 2010, the Company modified the awards of three employees that left the Company. The modifications all related to the term of vested options post termination. The changes ranged from 3.5 years to the remaining life of the option. Awards were reviewed under ASC 718, and the fair value of the unvested options that were modified will be re-measured and thestock-based compensation expense adjusted at each reporting period. During theduring years ended December 31, 2015, 2014 and 2013, 2012 and 2011 non-employee stock compensation expense of $3,000, $36,000, and $0.2 million respectively, was recorded.$2.2$12.1 million, $1.2$4.8 million and $1.4$2.2 million during the years ended December 31, 2015, 2014 and 2013, 2012 and 2011, respectively.(loss) incomeloss is as follows (in thousands): Year Ended December 31, 2013 2012 2011 $ 659 $ 514 $ 686 1,537 692 741 $ 2,196 $ 1,206 $ 1,427 Year Ended December 31, 2015 2014 2013 Research and development $ 4,852 $ 2,065 $ 659 General and administrative 7,223 2,713 1,537 $ 12,075 $ 4,778 $ 2,196 Year Ended December 31, Year Ended December 31, 2013 2012 2011 2015 2014 2013 71.5 % 69.0 % 66.0 % 67.0 % 70.9 % 71.5 % 6.0 6.0 6.0 6.0 6.0 6.0 1.85 % 0.9 % 1.1 % 1.67 % 1.82 % 1.85 % — % — % — % — % — % — % Fair ValueUnderlying Instrument Thean active market for the Company’s common stock prior to the completion of the Company’s initial public offering (IPO) on September 19, 2013, the Board, the members of which the Company estimatesbelieves have extensive business, finance, and venture capital experience, were required to estimate the fair value of itsthe Company’s common stock at the time of each option grant. The Board considered numerous objective and subjective factors in determining the value of the Company’s common stock at each option grant date, including the following factors: (1) prices for the Company’s preferred stock, which the Company had sold to outside investors in arm’s-length transactions, and the rights, preferences, and privileges of the Company’s preferred stock and common stock; (2) valuations performed by an independent valuation specialist; (3) the Company’s stage of development and revenue growth; (4) the fact that the option grants involved illiquid securities in a private company; and (5) the likelihood of achieving a liquidity event for the shares of common stock underlying the options, such as an initial public offering or sale of the Company, given prevailing market conditions. The Company believes this to have been a reasonable methodology based upon the Company’s internal peer company analyses, and based on several arm’s-length transactions involving the Company’s preferred stock, supportive of the results produced by this valuation methodology. Prior to the Company’s common stock being actively traded, the determination of fair value involved assumptions, judgments and estimates. If different assumptions were made, stock-based awards to employeescompensation expense, net loss and consolidated net loss per share could have been significantly different.option pricingoption-pricing model. As there was no public market for its common stock prior to September 19, 2013, the effective date of the Company’s IPO, and as the trading history of the Company’s common stock was limited through December 31, 2014, the Company determined the volatility for options granted based on an analysis of reported data for a peer group of companies that issued options with substantially similar terms. The expected volatility of options granted has been determined using an average of the historical volatility measures of this peer group of companies. During 2015 we began to estimate our volatility by using a blend of our stock price history, for the length of time we have market data for our stock and the historical volatility of similar public companies for the expected term of each grant. The expected life of options has been determined utilizing the “simplified method”. The simplified method is based on the average of the vesting tranches and the contractual life of each grant. The risk-free interest rate is based on a treasury instrument whose term is consistent with the expected life of the stock options. The Company has not paid, and does not anticipate paying, cash dividends on its common stock; therefore, the expected dividend yield is assumed to be zero. In addition, based on an analysis of the historical actual forfeitures, the Company applied an estimated forfeiture rate of approximately 4% for each of the years ended December 31, 2015, 2014 and 2013, respectively, in determining the expense recorded in the accompanying consolidated statements of operations and comprehensive loss.2013, 20122015, 2014 and 201110. Stock-Based Compensation (Continued)Expected Volatility The Company estimated the expected volatility based on actual historical volatility of the stock price of similar companies with publicly-traded equity securities. The Company calculated the historical volatility of the selected companies by using daily closing prices over a period of the expected term of the associated award. The companies were selected based on their enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected term of the associated award. A decrease in the selected volatility would decrease the fair value of the underlying instrument.Expected Term The Company estimates the expected life of its employee stock options using the "simplified" method, as prescribed in Staff Accounting Bulletin (SAB) No. 107, whereby, the expected life equals the arithmetic average of the vesting term and the original contractual term of the option due to its lack of sufficient historical data.Risk-Free Interest Rate The Company estimated the risk-free interest rate in reference to the yield on U.S. Treasury securities with a maturity date commensurate with the expected term of the associated award. A decrease in the selected risk-free rate would decrease the fair value of the underlying instrument.Expected Dividend Yield The Company estimated the expected dividend yield based on consideration of its historical dividend experience and future dividend expectations. The Company has not historically declared or paid dividends to stockholders. Moreover, it does not intend to pay dividends in the future, but instead expects to retain any earnings to invest in the continued growth of the business. Accordingly, the Company assumed an expected dividend yield of 0.0%.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 201110. Stock-Based Compensation (Continued)20132015 (in thousands, except per share amounts and years): Number
of Grants Weighted-
Average
Exercise
Price Per
Share
Average
Contractual
Life Aggregate
Intrinsic
Value(1)Outstanding at December 31, 2014 3,230 $ 9.77 Granted 870 $ 40.01 Exercised (837 ) $ 4.73 Canceled or forfeited (72 ) $ 31.72 Outstanding at December 31, 2015 3,191 $ 18.85 6.31 $ 95,479,304 Exercisable at December 31, 2015 1,997 $ 10.79 4.98 $ 75,853,297 Vested and expected to vest at December 31, 2015(2) 3,135 $ 18.56 6.26 $ 94,709,640 Number
of Grants Weighted-
Average
Exercise
Price Per
Share Weighted-
Average
Contractual
Life (in years) Aggregate
Intrinsic
Value(1) 3,730 $ 4.16 6.62 $ 13,946 553 $ 23.88 (293 ) $ 2.24 (48 ) $ 4.39 3,942 $ 7.05 6.43 $ 128,334 2,544 $ 4.07 5.13 $ 90,401 3,860 $ 6.89 6.37 $ 126,258 (1)(1) The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying options and the estimated fair value of the common stock for the options that were in the money at December 31, 2015 and 2014. (2) This represents the number of vested options at December 31, 2015, plus the number of unvested options expected to vest at December 31, 2015, based on the unvested options outstanding at December 31, 2015, adjusted for the estimated forfeiture rate. that were in the money at December 31, 2013 and 2012.(2)This represents the number of vested options at December 31, 2013, plus the number of unvested options expected to vest at December 31, 2013, based on the unvested options outstanding at December 31, 2013, adjusted for the estimated forfeiture rate. During the years ended December 31, 2013, 2012 and 2011, the Company granted stock options to purchase an aggregaterespective date of 552,750, 722,000 and 334,175 shares of its common stock, respectively, with a weighted-average grant date fair values of options granted of $15.27, $7.20, $5.12, respectively. During the years ended December 31, 2013, 2012 and 2011, current and former employees of the Company exercised a total of 292,802, 38,697 and 94,748 options, respectively, resulting in total proceeds of $0.7 million, $0.2 million, and $0.2 million, respectively. The aggregate intrinsic value of options exercised during the years ended December 31, 2013, 2012 and 2011, under the Company's stock option plans, was $7 million, $47,000 and $ 186,000, respectively.2013,2015, there was $10.1$19.5 million of unrecognized compensation expense related to unvested stock options, under the Company's stock option plans, that is expected to be recognized over a weighted-average period of 2.662.31 years.11. Number
of Grants Weighted-
Average
Grant Date Fair ValueUnvested balance at December 31, 2014 — $ — Granted 527 $ 31.67 Vested — $ — Forfeited (6 ) $ 41.20 Unvested balance at December 31, 2015 521 $ 31.57 Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 201111. 401(k) Savings Plan (Continued)The Company has not made anyFor the years 2015 and 2014 the Board approved matching contributions of up to $5,000 and $2,500 respectively, per eligible participant pursuant to the 401(k) Plan to date.12.December 31, 2015 and 2014 have been recorded in the consolidated statement of operations and comprehensive loss.2013, 20122015, 2014 and 2011,2013, the Company did not record a current or deferred income tax expense or benefit.(loss) incomeloss before income taxes was $(21.9)$(63.9) million, $(32.6)$(51.3) million and $36.3$(21.9) million for the years ended December 31, 2013, 20122015, 2014 and 2011,2013, respectively, and was generated entirely in the United States. Year Ended December 31, Year Ended December 31, 2013 2012 2015 2014 $ 51,886 $ 35,584 $ 90,732 $ 75,334 5,519 5,384 7,864 6,704 3,005 21,882 1,883 2,348 5,675 5,333 9,319 5,416 66,085 68,183 109,798 89,802 (66,085 ) (68,183 ) (109,798 ) (89,802 ) $ — $ — $ — $ — 20132015 and 2012.2014. The valuation allowance decreasedincreased by $2.1$20.0 million during the year ended December 31, 2013, due primarily to the decrease in deferred revenue during the period. The valuation allowance increased by $11.2 million during the year ended December 31, 2012,2015, due primarily to the generation of net operating losses during the period.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 201112. Income Taxes (Continued) Year Ended December 31, Year Ended December 31, 2013 2012 2011 2015 2014 2013 34.0 % 34.0 % 34.0 % 34.0 % 34.0 % 34.0 % (1.5 )% 4.2 % 5.0 % 4.6 % 4.1 % (1.5 )% (43.1 )% (3.4 )% 1.5 % (8.3 )% 2.0 % (43.1 )% 0.7 % — % (1.0 )% 1.2 % 1.6 % 0.7 % 0.0 % (0.4 )% — % — % 4.6 % — % 9.9 % (34.4 )% (39.5 )% (31.5 )% (46.3 )% 9.9 % 0.0 % 0.0 % 0.0 % — % — % — % 20132015 and 2012,2014, the Company had U.S. federal net operating loss carryforwards of $141.0$276.1 million and $93.3$211.2 million, respectively, which may be available to offset future income tax liabilities and expire at various dates through 2033.2035. As of December 31, 20132015 and 2012,2014, the Company also had U.S. state net operating loss carryforwards of $122.9$229.8 million and $75.4$165.0 million, respectively, which may be available to offset future income tax liabilities and expire at various dates through 2033.20132015 and 2012,2014, the Company had federal research and development tax credit carryforwards of $3.9$5.5 million and $3.8$4.8 million, respectively, available to reduce future tax liabilities which expire at various dates through 2033.2035. As of December 31, 20132015 and 2012,2014, the Company had state research and development tax credit carryforwards of approximately $2.4$3.5 million and $2.4$2.9 million, respectively, available to reduce future tax liabilities which expire at various dates through 2028.three-yearthree‑year period in excess of 50 percent, as defined under Sections 382 and 383 of the Internal Revenue Code, respectively, as well as similar state provisions. This could limit the amount of tax attributes that can be utilized annually to offset future taxable income or tax liabilities. The amount of the annual limitation is determined based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation in future years. The Company has completed several financings since its inception which may have resulted in a change in control as defined by Sections 382 and 383 of the Internal Revenue Code, or could result in a change in control in theTheThrough June 2014, the Company has not, as of yet, conducted a studycompleted an assessment to determine if any such changeswhether there may have occurredbeen a Section 382 ownership change and determined that could limit its ability to useit is more-likely-than-not that the Company’s net operating loss and tax credit carryforwards.20132015 and 2012,2014, the Company had no accrued interest or penalties related to uncertain tax positions and no amounts have been recognized in the Company's consolidated statements of operations and comprehensive (loss) income.loss.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 201112. Income Taxes (Continued)2013,2015, the Company generated research credits but has not conducted a study to document the qualified activities. This study may result in an adjustment to the Company'sCompany’s research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position for these two years.the years ended December 31, 2015 and 2014. A full valuation allowance has been provided against the Company'sCompany’s research and development credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the deferred tax asset established for the research and development credit carryforwards and the valuation allowance.20102012 through December 31, 2013.2015. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service, state or foreign tax authorities to the extent utilized in a future period.13.iswas required to repay the aggregate principal balance under the Loan Agreement in 42 months. The first 12 payments arewere interest only and the remaining 30 payments arewere equal monthly installments of principal plus interest. The Loan Agreement provided that the interest only period could be extended under certain circumstances. The Company did not trigger the requirements and began paying principal in July 2013.iswas payable at the 8.5%. The Loan Agreement also included a closing fee of $0.2 million. The Company is amortizingamortized the cost over the 42 months of loan. The Loan Agreement iswas also subject to an additional deferred payment of $1.2 million due with the final payment. The Company is recordingrecorded the deferred payment to interest expense over the term of the Loan Agreement. The resulting effective interest rate iswas approximately 11.8%. The Loan Agreement iswas secured by a lien on all of the Company's personal property as of, or acquired after, the date of the Loan Agreement, except for intellectual property. The Loan Agreement defines events of default, including the occurrence of an event that results in a material adverse effect upon the Company's business operations, properties, assets or condition (financial or otherwise), its ability to perform its obligations under and in accordance with the terms of the Loan Agreement, or upon the ability of the lenders to enforce any of their rights or remedies with respect to such obligations, or upon the collateral under the Loan Agreement or upon the liens of the lenders on such collateral or upon the priority of such liens. The lenders also received a right, to purchase at fair value, up to $2.0 million of equity of the Company sold in any sale by the Company to third parties of equity securities resulting in at least $5.0 million in net cash proceeds to the Company, subject to certain exceptions. On December 30, 2013, in conjunction with the establishment of the Company's wholly owned subsidiary, Acceleron Security Corporation Inc., the Loan Agreement was modified to add a debt covenant that requires the Company to maintain a cash balance equal to 150% of the outstanding principal debt balance in a separate bank account, at all times. That bank account can be subject to the lender's exclusive control in the event of a default. As of December 31, 2013 andAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 201113. Long-Term Debt (Continued)2012, there have been no events of default under the loan and the Company was in compliance with all financial and nonfinancial covenants at December 31, 2013. As of December 31, 2013 and 2012, the principal balance outstanding was $16.3 million and $20.0 million, respectively.paid offrepaid the remaining principal outstanding underbalance of the Loan Agreement. In accordance with ASC 470-10-45Intent and Ability to Refinance on A Long Term Basis,At the time of repayment the Company has classifiedrecognized interest expense related to the outstanding debt balanceremaining $0.6 million of the $1.2 million deferred payment due with the final payment. The Company also recognized $0.3 million in prepayment fees as a current liability as of December 31, 2013.14.additional expense9.7%12.3% and 9.9%12.8% of the Company's fully diluted equity as of December 31, 20132015 and 2012,2014, respectively. Refer to Note 910 for additional information regarding this collaboration agreement.yearyears ended December 31, 2015, 2014 and 2013, the Company recognized $18.1 million, $14.6 million and $32.3 million, respectively, in collaboration revenue under the Celgene collaboration arrangement and, asarrangement. As of December 31, 2013,2015 and 2014, the Company had $7.7$4.8 million and $6.0 million, respectively, of deferred revenue related to the Celgene collaboration arrangement. During the year ended December 31, 2012, the Company recognized $4.9 million in collaboration revenue under the Celgene collaboration arrangement and, as of December 31, 2012, had $10.3 million of deferred revenue related to the Celgene collaboration arrangement. During the year ended December 31, 2011, the Company recognized $63.5 million in collaboration revenue under the Celgene collaboration arrangement.2013, 20122015, 2014 and 20112013 as follows (in thousands): Year Ended December 31, 2013 2012 2011 $ 19,626 $ 2,035 $ 63,607 12,658 2,879 (121 ) $ 32,284 $ 4,914 $ 63,486 Year Ended December 31, 2015 2014 2013 License and milestone $ 1,184 $ 1,673 $ 19,626 Cost sharing, net 16,913 12,959 12,658 $ 18,097 $ 14,632 $ 32,284 Alkermes One of the Company's directors is also the Chairman, President, and Chief Executive Officer of Alkermes plc, the parent company of Alkermes, with which the Company entered into a collaboration agreement during 2009.Acceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 201114. Related Party Transactions (Continued) As of December 31, 2013, Alkermes held 718,655 common shares of the Company's (upon conversion of its investment in the Company's Preferred stock) and warrants to purchase 42,624 shares of common stock. As of December 31, 2012, Alkermes held 695,250 shares of the Company's Preferred Stock and warrants to purchase 42,624 shares of common stock. Upon the closing of the IPO on September 24, 2013, all of the shares of the Company's preferred stock held by Alkermes were converted into 718,655 shares of common stock. For the year ended December 31, 2011, Alkermes paid the Company $0.7 million for research services. No such fees were paid to the Company during 2012 or 2013. The Company evaluated the forgiveness provisions and determined that forgiveness was not probable as of December 31, 2012, and as such, continued to record the Note Receivable as an asset at December 31, 2012. As a result of the Company's filing of a registration statement with the SEC on August 6, 2013 which triggered the forgiveness of the Note Receivable, the Company expensed the unpaid principal and interest expense totaling $0.2 million as compensation expense during the year ended December 31, 2013.15.disclosure. The Company has evaluated all subsequent events and determined that there are no material recognized or unrecognized subsequent events requiring disclosure, other the following:28, 2014,11, 2016, the Company completed a follow-onits underwritten public offering of its common stock and issued and sold 2,760,000 shares of our common stock, including 360,0003,750,000 shares of common stock sold pursuant to the underwriters' full exercise of their option to purchase additional shares at a public offering price of $50.00$40.00 per share, forshare. The aggregate net proceeds received by the Company, were $129.0 million, after deducting underwriting discounts of $8.3 million and commissions and other estimated offering expenses, totaling $0.7were approximately $140.4 million. All of the shares issued and sold in this follow-on public offering were registered under the Securities Act pursuant to a Registration Statement on Form S-1 (File No. 333-193252), which wasAcceleron Pharma Inc.Notes to Consolidated Financial Statements (Continued)Years Ended December 31, 2013, 2012 and 201115. Subsequent Events (Continued)declared effective by the SEC on January 22, 2014, and a registration statement filed pursuant to Rule 462(b) of the Securities Act. On March 12, 2014, the Company paid off the remaining principal outstanding under the Loan Agreement (see Note 13 Long-term Debt). Accordingly, the Company has classified the outstanding debt balance as a current liability as of December 31, 2013.16.2013.2015. This information has been prepared on the same basis as the audited financial statements and includes all adjustments (consisting only of normal recurring adjustments) necessary to present fairly the unaudited quarterly results of operations set forth herein. Net income (loss) per share for all periods presented have been retroactively adjusted to reflect the 1-for-4 reverse stock split effected on September 5, 2013. For the Three Months Ended(1) March 31 June 30 September 30 December 31 (in thousands except per share data) $ 15,012 $ 26,427 $ 4,270 $ 11,521 (11,876 ) (12,276 ) (11,154 ) (14,972 ) 3,136 14,151 (6,884 ) (3,451 ) 1,647 13,078 (18,513 ) (18,110 ) $ (0.24 ) $ 0.30 $ (5.62 ) $ (0.64 ) $ (0.24 ) $ 0.28 $ (5.62 ) $ (0.64 ) $ 3,324 $ 4,040 $ 3,905 $ 3,985 (10,257 ) (10,944 ) (10,763 ) (12,179 ) (6,933 ) (6,904 ) (6,858 ) (8,194 ) (7,588 ) (7,400 ) (7,215 ) (10,379 ) $ (1.50 ) $ (5.93 ) $ (5.82 ) $ (7.10 ) $ (1.50 ) $ (5.93 ) $ (5.82 ) $ (7.10 ) (1)The amounts were computed independently for each quarter, and the sum of the quarters may not total the annual amounts.*Applicable to common stockholders For the Three Months Ended(1) March 31 June 30 September 30 December 31 (in thousands except per share data) 2015 Total revenue $ 4,420 $ 5,717 $ 4,155 $ 3,804 Total costs and expenses 19,479 18,811 18,768 21,919 Loss from operations (15,059 ) (13,094 ) (14,613 ) (18,115 ) Net loss (14,574 ) (10,383 ) (11,858 ) (27,082 ) Basic net loss per share* $ (0.45 ) $ (0.32 ) $ (0.36 ) $ (0.81 ) Diluted net loss per share* $ (0.45 ) $ (0.32 ) $ (0.36 ) $ (0.81 ) 2014 Total revenue $ 3,307 $ 4,078 $ 3,508 $ 3,739 Total costs and expenses 15,515 21,389 14,899 18,293 Loss from operations (12,208 ) (17,311 ) (11,391 ) (14,554 ) Net loss (9,120 ) (16,550 ) (7,972 ) (17,617 ) Basic net loss per share* $ (0.30 ) $ (0.52 ) $ (0.25 ) $ (0.55 ) Diluted net loss per share* $ (0.30 ) $ (0.52 ) $ (0.25 ) $ (0.55 ) Exhibits(1) The amounts were computed independently for each quarter, and the sum of the quarters may not total the annual amounts. * Applicable to common stockholders numberDescription of exhibit Exhibit Description Filing Date 3.1 Restated Certificate of Incorporation (incorporated by reference to exhibit 3.1 to the Company's Current Report on Form 8-K (001-36065), filed on September 24, 2013)(Exhibit 3.1)9/24/2013 001-36065
3.23.2
Amended and Restated By-laws (incorporated by reference to exhibit 3.2 to the Company's Current Report on Form 8-K (001-36065), filed on September 24, 2013)(Exhibit 3.2)9/24/2013 001-36065
4.14.1
Form of Common Stock Certificate (incorporated by reference to exhibit 4.1 to the Company's Registration Statement on Form S-1/A (333-190417), filed on September 6, 2013)S-1 (Exhibit 4.1)8/7/2013 333-190417
4.24.2
Form of Amended and Restated Registration Rights Agreement (incorporated by reference to exhibit 4.2 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 4.2)8/7/2013 333-190417
4.34.3
Form of Warrant to Purchase Stock, issued to Series E Investors as of June 10, 2010 and July 9, 2010 (incorporated by reference to exhibit 4.3 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 4.3)8/7/2013 333-190417
4.44.4
Form of Common Stock Warrant Certificate, issued to General Electric Capital Corporation as of March 28, 2007 (incorporated by reference to exhibit 4.4 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 4.4)8/7/2013 333-190417
10.1*10.1
Form of Director Indemnification Agreement (incorporated by reference to exhibit 10.1 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 10.1)8/7/2013 333-190417
10.2+10.2Form of Amended and Restated Voting Agreement (incorporated by reference to exhibit 10.2 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)10.3Amended and Restated Right of First Refusal and Co-sale Agreement, dated as of December 22, 2011 (incorporated by reference to exhibit 10.3 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)10.4Amended and Restated Investor Rights Agreement, dated as of December 22, 2011 (incorporated by reference to exhibit 10.4 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)10.5Loan and Security Agreement, dated as of June 7, 2012, among Oxford Finance LLC, the Lenders listed on Schedule 1.1, Silicon Valley Bank, MidCap Financial SBIC, LP, and Acceleron Pharma Inc. (incorporated by reference to exhibit 10.5 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)10.6+
Collaboration, License and Option Agreement between Acceleron Pharma Inc. and Celgene Corporation, dated as of February 20, 2008, and amended as of August 2, 2011 (incorporated by reference to exhibit 10.6 to the Company's Registration Statement on Form S-1/A (333-190417), filed on September 6, 2013)(Exhibit 10.6)9/6/2013 333-190417
10.310.7+
Amended and Restated License Agreement between Acceleron Pharma Inc. and Ludwig Institute for Cancer Research Ltd., dated as of August 6, 2010 (incorporated by reference to exhibit 10.7 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 10.7)8/7/2013 333-190417
10.4+10.8+
Exclusive License Agreement between Beth Israel Deaconess Medical Center and Acceleron Pharma Inc., dated as of June 21, 2012 (incorporated by reference to exhibit 10.8 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 10.8)8/7/2013 333-190417
10.5+10.9+
Collaboration, License and Option Agreement between Acceleron Pharma Inc. and Celgene Corporation, dated as of August 2, 2011 (incorporated by reference to exhibit 10.9 to the Company's Registration Statement on Form S-1/A (333-190417), filed on September 6, 2013)(Exhibit 10.9)ExhibitnumberDescription of exhibit 10.109/6/2013333-190417 10.6 Exclusive License Agreement between Salk Institute for Biological Studies and Acceleron Pharma Inc., dated as of August 10, 2010 (incorporated by reference to exhibit 10.10 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 10.10)8/7/2013 333-190417
10.710.11
Amended and Restated License Agreement between Salk Institute for Biological Studies and Acceleron Pharma Inc., dated as of August 11, 2010 (incorporated by reference to exhibit 10.11 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 10.11)8/7/2013 333-190417
10.810.12
Amendment, dated as of July 25, 2014, to Amended and Restated License Agreement between Salk Institute for Biological Studies and Acceleron Pharma Inc. dated as of August 11, 2010Form 10-Q (Exhibit 10.1) 8/12/2014 001-36065 10.9 Indenture of Lease between Massachusetts Institute of Technology and Acceleron Pharma Inc., dated as of May 20, 2008 (incorporated by reference to exhibit 10.12 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 10.12)8/7/2013 333-190417
10.10*10.13
Acceleron Pharma Inc. 2013 Equity Incentive Plan (incorporated by reference to exhibit 4.4 to the Company's Registration Statement on Form S-8 (333-192789), filed on December 12, 2013)(Exhibit 4.4)12/12/2013 333-192789
10.11*10.14
Form of Acceleron Pharma Inc. Cash Incentive Plan (incorporated by reference to exhibit 10.14 to the Company's Registration Statement on Form S-1/A (333-190417), filed on August 19, 2013)(Exhibit 10.14)8/19/2013 333-190417
10.12*10.15
Acceleron Pharma Inc. 2003 Stock Option and Restricted Stock Plan (incorporated by reference to exhibit 10.15 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)(Exhibit 10.15)8/7/2013 333-190417
10.13*10.16Promissory Note by and between Acceleron Pharma Inc. and John Knopf, dated as of January 28, 2008, and amended November 13, 2012 (incorporated by reference to exhibit 10.16 to the Company's Registration Statement on Form S-1 (333-190417), filed on August 7, 2013)10.17Form of Amended and Restated Employment Agreement between John Knopf and Acceleron Pharma Inc. (incorporated by reference to exhibit 10.17 to the Company's Registration Statement on , dated as of August 26, 2013S-1/A (333-190417), filed on August 19, 2013)10-K3/2/2015 001-36065
10.14*10.18Form of Amended and Restated Employment Agreement between Matthew L. Sherman and Acceleron Pharma Inc. (incorporated by reference to exhibit 10.18 to the Company's Registration Statement on , dated as of August 26, 2013S-1/A (333-190417), filed on August 19, 2013)10-K3/2/2015 001-36065 10.19Exhibit Description of orFiling Date 10.15* Amended and Restated Employment Agreement between John D. Quisel and Acceleron Pharma Inc. (incorporated by reference to exhibit 10.19 to the Company's Registration Statement on , dated as of August 26, 2013S-1/A (333-190417), filed on August 19, 2013)10-K3/2/2015 001-36065
10.16*10.20
Amended and Restated Employment Agreement between Kevin F. McLaughlin and Acceleron Pharma Inc. dated as of January 31, 20143/2/2015 001-36065 10.16.1* First Amendment to Amended and Restated Employment Agreement, by and between Kevin F. McLaughlin and Acceleron Pharma Inc., dated as of September 9, 2015 9/11/2015 001-36065 10.17* Amended and Restated Employment Agreement between Steven D. Ertel and Acceleron Pharma Inc. dated as of January 31, 2014 3/2/2015 001-36065 10.17.1* First Amendment to Amended and Restated Employment Agreement, by and between Steven D. Ertel and Acceleron Pharma Inc., dated as of September 9, 2015 9/11/2015 001-36065 10.18* Employee Stock Purchase Plan (incorporated by reference to exhibit 10.20 to the Company's Registration Statement on Form S-1/A (333-190417), filed on September 6, 2013)(Exhibit 10.20)9/6/2013 333-190417
10.19*10.21
Form of Non-statutoryNon-Statutory Stock Option Agreement under the 2013 Equity Incentive Plan (incorporated by reference to exhibit 10.21 to the Company's Registration Statement on Form S-1/A (333-193252), filed on January 9, 2013)S-1 (Exhibit 10.21)1/9/2014 333-193252
10.20*10.22
Form of Incentive Stock Option Agreement under the 2013 Equity Incentive Plan (incorporated by reference to exhibit 10.22 to the Company's Registration Statement on Form S-1/A (333-193252), filed on January 9, 2013)S-1 (Exhibit 10.22)1/9/2014 333-193252
10.21*21.1
Form of Restricted Stock Unit Award Agreement under the 2013 Equity Incentive Plan (2016)X 10.22* Form of Restricted Stock Unit Award Agreement under the 2013 Equity Incentive Plan (Executives) 9/11/2015 001-36065 21.1 List of Subsidiaries X
23.123.1
Consent of Ernst & Young LLP, Independent Registered Public Accounting FirmX
31.131.1
Certification of Principal Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of Sarbanes Oxleythe Sarbanes-Oxley Act of 2002 by Chief Executive OfficerX
31.231.2
Certification of Principal Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of Sarbanes Oxleythe Sarbanes-Oxley Act of 2002 by Chief Financial OfficerX ExhibitnumberDescription of exhibit32.1 Certification of periodic financial reportPrincipal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of Sarbanes Oxleythe Sarbanes-Oxley Act of 2002
32.2X
Certification of periodic financial report pursuant to Section 906 of Sarbanes Oxley Act of 2002
101101
The following financial information from the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 20132015 formatted in ExtensibleXBRL (eXtensible Business Reporting Language,Language): (i) the Consolidated Balance Sheets as of December 31, 2015 and December 31, 2014, (ii) the Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2015, 2014 and 2013, (iii) the Consolidated Statements of Comprehensive Income for the years ended December 31, 2015, 2014 and 2013, (iv) the Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders' Equity (Deficit), for the years ended December 31, 2015, 2014 and 2013, (v) the Consolidated Statements of Cash Flows for the years ended December 31, 2015, 2014 and 2013, and (vi) the Notes to the Consolidated Financial StatementsX +Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment and this exhibit has been submitted separately.+ Confidential treatment has been granted by, or is being requested from, the Securities and Exchange Commission as to certain portions of this Exhibit, which portions have been omitted and filed separately with the Securities and Exchange Commission as part of an application for confidential treatment pursuant to the Securities Act of 1933, as amended, and the Securities Exchange Act of 1934, as amended, as applicable. * Management contract or compensatory plan or arrangement. ACCELERON PHARMA INC.
Date: March 17, 2014February 25, 2016
By:By:
/s/ JOHN L. KNOPF, PH.D.Signature TitleDate
Title
Date/s/ JOHN L. KNOPF, PH.D. John L. Knopf, Ph.D. Chief Executive Officer, President and PresidentDirector (Principal Executive Officer) March 17, 2014
John L. Knopf, Ph.D./s/ KEVIN F. MCLAUGHLIN Kevin F. McLaughlin
Senior Vice President, Chief Financial Officer and Treasurer (Principal Financial Officer and Principal Accounting Officer)
March 17, 2014Kevin F. McLaughlin /s/ FRANCOIS NADER, M.D. Chair of the Board of Directors Francois Nader, M.D. �� /s/ JEAN M. GEORGE Director Jean M. George
DirectorMarch 17, 2014/s/ GEORGE GOLUMBESKI, PH.D. Director George Golumbeski, Ph.D.
DirectorMarch 17, 2014/s/ EDWIN M. KANIA, JR.Edwin M. Kania, Jr.
Director
March 17, 2014/s/ TERRENCE C. KEARNEY Director Terrence C. Kearney /s/ TOM MANIATIS, PH.D. Director Tom Maniatis, Ph.D.
DirectorMarch 17, 2014/s/ TERRANCE G. MCGUIRE Director Terrance G. McGuire
DirectorMarch 17, 2014/s/ RICHARD F. POPS Director Richard F. Pops
DirectorMarch 17, 2014/s/ JOSEPH S. ZAKRZEWSKI Director Joseph S. Zakrzewski
DirectorMarch 17, 2014