| (Mark One) | ||
ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the fiscal year ended December 31, | ||
OR | ||
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the transition period from to | ||
17, 2017: 43,186,484 ITEM 5: ITEM 10: ITEM 15: expect to initiate a first-in-human Phase 1 study of RAD140 in women with hormone receptor positive breast cancer in 2017. we hold worldwide commercialization rights, except for Japan. synthetic peptide analog that engages the parathyroid hormone receptor, or PTH1 receptor, and was selected for clinical development based on its potential for favorable bone building activity. with respect to time to peak concentration, or Tmax, half-life, or T1/2, and area under the curve, or AUC, was successfully modified to improve comparability to abaloparatide-SC. The results of this clinical evaluation will inform the design of a pivotal bioequivalence study that will be initiated following completion of activities related to manufacturing scale-up, production, and other activities required for the initiation of that study. - Dose-Escalation Study cancer in the United States to determine the recommended dose for a Phase 2 clinical trial and to make a preliminary evaluation of the potential anti-tumor effect of RAD1901. We continue to enroll patients in the EU Phase I FES-PET study. 2016. In 2017, we plan to initiate a first-in-human Phase 1 study of RAD140 in women with hormone receptor positive breast cancer. In of PTHrP. Abaloparatide is manufactured using organic chemistry techniques to create the 34 amino acid peptide. We Japan. to 86 received daily doses of one of the following: 80 µg of abaloparatide, a matching placebo, or the approved dose of 20 µg of Forteo for 18 months. data. Breast Cancer Our Investigational Drug—RAD1901 cancers; and We continue to enroll patients in the EU Phase I FES-PET study. The first three enrolled patients dosed at the 400-mg cohort had a tumor FES-PET signal intensity reduction ranging from 79% to 91% at day 14 compared to baseline. In December 2016, we reported positive results from the ongoing EU Phase 1 FES-PET study including 1 patient with a confirmed partial response by RECIST criteria with all three enrolled patients remained on study drug with mean duration of treatment of 5.64 cycles as of the October 7, 2016 data cut-off date. Adverse events reported to date have been grade 1 and 2 and manageable. This study will enroll 5 additional patients in the 400-mg daily oral cohort followed by 8 patients in the 200-mg daily oral cohort. (ribociclib), a CDK 4/6 inhibitor and BYL719 (alpelisib), an investigational phosphoinositide 3-kinase inhibitor. cancer. patented microneedle technology to administer drugs through the skin, as an alternative to subcutaneous injection. The API In 2016, five U.S. patents that we licensed from Ipsen expired. We own the federal trademark registration in the United States for Radius United States (not including any patent term extension under the Hatch-Waxman Act). The intended therapeutic formulation for abaloparatide-SC is covered by Patent No. 8,148,333 until 2027 in the United States (not including any patent term extension under the Hatch-Waxman Act). Related patents granted in Australia, China, Israel, Japan, South Korea, Mexico, New Zealand, Russia, Singapore, and Ukraine, and We have worldwide rights to commercialize abaloparatide-TD, including in Japan. will or may be asserted against us by third-parties. Should we need to defend ourselves and our partners against any such claims, substantial costs may be incurred. Furthermore, parties making such claims may be able to obtain injunctive or other equitable relief, which could effectively block our ability to develop or commercialize some or all Brisdelle The Agreement. In consideration for the rights to abaloparatide and in recognition of certain milestones having been met to date, we have paid to Ipsen an aggregate amount of from confidential Ipsen know-how, the agreement provides that we terminated in accordance with its terms. develop, manufacture and commercialize RAD1901 in Japan, or the Eisai Amendment. Specifically, we licensed the patent application that subsequently issued as Manufacturing Agreements the other party 18 months prior to the end of the then-current term. The certain force majeure events. NDA based on a determination that the drug is safe and effective for the proposed indication(s). the IND application. In such a case, the IND application sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. We cannot be sure that submission of an IND application will result in the FDA allowing clinical trials to begin. Detailed information about many clinical trials must be submitted to the National Institutes of Health, or NIH, for public disclosure on the government website ClinicalTrials.gov. Phase 3 trials typically are relied upon as the primary basis for approval because they provide the safety and effectiveness information needed to evaluate the overall benefit-risk relationship of the drug and to create the physician labeling. the application should be approved. The FDA is not bound by the recommendations of the advisory committee, but the Agency historically has tended to follow such recommendations. records related to safety reporting, distribution and/or manufacturing facilities; this latter effort includes assessment of ongoing compliance with cGMP regulations. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance. We have used and intend to continue to use third-party manufacturers to produce our products in clinical and commercial quantities, and future FDA inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of problems with a product after approval may result in restrictions on a product, including recall or withdrawal of the product from the market, labeling changes, imposition of REMS, or the requirement to conduct additional studies. In addition to the ANDA pathway, the Hatch-Waxman Act also established an abbreviated approval pathway under section 505(b)(2) of the FDCA for applications that contain full reports of investigations of safety and effectiveness, but where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. Section 505(b)(2) permits approval of applications other than those for duplicate products and permits reliance for such approvals on literature or on FDA’s finding of safety or effectiveness for an approved drug product. Under FDA’s “umbrella policy,” NCE exclusivity protects all drug products that contain the qualifying NCE, so if abaloparatide-TD is approved prior to the expiration to any NCE exclusivity granted to abaloparatide-SC, we would expect abaloparatide-TD to be protected by any remaining NCE exclusivity period. indication that has been approved for abaloparatide-TD. European Union. GMP, and product-specific European Union. The Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or MMA, established the Medicare Part D program to provide a voluntary prescription drug benefit to Medicare beneficiaries. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities to provide coverage of outpatient prescription drugs. Part D plans include both stand-alone prescription drug benefit plans and prescription drug coverage as a supplement to Medicare Advantage plans. Unlike Medicare Parts A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each Part D prescription drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, although not necessarily all of the drugs within each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee. We anticipate that a significant proportion of patients eligible for abaloparatide-SC will be Medicare beneficiaries and we expect that abaloparatide-SC, if approved, will be covered under Medicare Part D, although Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation's automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2025 unless additional Congressional action is taken. stringent restrictions on manufacturers' communications regarding off-label uses. contain ambiguities as to what is required to comply with the laws. Given the lack of clarity in laws and their implementation, our actions could be subject to the penalty provisions of the pertinent state authorities. the License Agreement and other declaratory judgment. We asserted, among other things, that Ipsen's claimed rights to co-promote abaloparatide in France and to a license from us with respect to Japan have permanently expired, and that Ipsen has breached the License Agreement by, among other things, allowing certain patents to expire and by purporting to license to a third party certain manufacturing and other rights that we contend Ipsen exclusively licensed to us. We are discontinue product development, reduce or forego sales and marketing efforts for any product candidate that is approved, forego attractive business opportunities or discontinue our operations entirely. Any additional sources of financing may not be available or may not be available on favorable terms and will likely involve the issuance of additional equity securities, which will have a dilutive effect on stockholders. Our future studies and the expenses associated with our commercialization efforts for abaloparatide-SC. approved, the past or which we expect for the future. Our financial results in some quarters may fall below expectations. Any of these events as well as the various risk factors listed in this "Risk Factors" section could adversely affect our financial results and cause our stock price to fall. research and clinical approaches will result in drugs that the FDA considers safe for humans and effective for proposed uses. Any collaboration arrangements that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize abaloparatide-SC, or any of our other product candidates. In addition, we, the FDA, or other equivalent regulatory authorities and ethics committees with jurisdiction over our studies may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the FDA or foreign regulatory authorities find deficiencies in our regulatory submissions or the conduct of these trials. Therefore, we cannot predict with any certainty the schedule for existing or future clinical trials. Any such unexpected expenses or delays in our clinical trials could increase our need for additional capital, which may not be available on favorable terms or at all. In addition, the FDA's policies may change and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature, or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would adversely affect our business. managed care providers and private insurance plans. Private insurers, such as health maintenance organizations and managed care providers, have implemented cost cutting and reimbursement initiatives and likely will continue to do so in the future. These include establishing formularies that govern the drugs and biologics that will be offered and also the on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. entities, some of whom may compete with us. If our collaborators assist competitors at our expense, our competitive position would be harmed. have a financial interest that affected the reliability of the data from the ACTIVE Clinical Trials, we could be subject to additional regulatory scrutiny and the utility of the ACTIVE Clinical Trials for purposes of our planned regulatory submissions could be compromised, which could have a material adverse effect on our business and the value of our common stock. business and financial condition would be materially harmed. Because the manufacturing process for abaloparatide-TD requires the use of 3M's proprietary technology, 3M is our sole source for finished clinical trial supplies of abaloparatide-TD. To date, we have not entered into a commercial supply agreement with 3M. If we product revenue. If we are unable to effectively establish and manage the distribution process, the commercial launch and sales of abaloparatide-SC will be delayed or severely compromised and our results of operations may be harmed. If we cannot compete successfully for market share against other drug companies, we may not achieve sufficient product revenues and our business will suffer. We may incur substantial liabilities and may be required to limit commercialization of our products in response to product liability lawsuits. October 3, 2027, absent any issued SPCs. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to patents issued or licensed to us, including interference proceedings before the USPTO. Third parties also may assert infringement claims against us. If we are found to infringe a third party's intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business. For example, we are aware of a applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned and licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions. An adverse determination in any such proceeding could reduce the scope of, or invalidate our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. assignment agreements with our employees and consultants. However, any of these parties may breach the agreements and disclose our proprietary information, and we may not be able to obtain adequate remedies for any breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to, or independently developed by a competitor, our competitive position would be harmed. Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities. conduct of various electronic healthcare transactions and protects the security and privacy of protected health information; affect our business, financial condition and results of operations. In addition, the federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous or radioactive materials and waste products may require us to incur substantial compliance costs that could materially adversely affect our business, financial condition and results of operations. manage our development efforts effectively; If we are not successful in completing acquisitions that we may pursue in the future, we would be required to reevaluate our business strategy and we may have incurred substantial expenses and devoted significant management time and resources in seeking to complete the acquisitions. In addition, we could use substantial portions of our available cash as all or a portion of the purchase price, or we could issue additional securities as consideration for these acquisitions, which could cause our stockholders to suffer significant dilution. and our business. Material weaknesses in our internal control over financial reporting could also reduce our ability to obtain financing or could increase the cost of any financing we obtain. We may be required to pay severance benefits to our employees who are terminated in connection with a change in control, which could harm our financial condition or results. Waltham, MA, USA Cambridge, MA, USA Quarter Ended June 30, 2014 (beginning June 6, 2014) Quarter Ended September 30, 2014 Quarter Ended December 31, 2014 On February 2016. Operating expenses: Research and development General and administrative Loss from operations Other (expense) income: Other (expense) income, net Interest (expense) income, net Net loss Other comprehensive loss, net of tax: Unrealized gain (loss) from available-for-sale securities Comprehensive loss Net (loss) earnings attributable to common stockholders Net (loss) earnings per share applicable to common stockholders—basic Net (loss) earnings per share applicable to common stockholders—diluted Weighted-average number of common shares used in net (loss) earnings per share applicable to common stockholders—basic Weighted-average number of common shares used in net (loss) earnings per share applicable to common stockholders—diluted Cash and cash equivalents Marketable securities Working capital Total assets Long-term liabilities Total liabilities Total convertible preferred stock and redeemable convertible preferred stock Total liabilities, convertible preferred stock, redeemable convertible preferred stock and stockholders' equity (deficit) expect to initiate a first-in-human Phase 1 study of RAD140 in women with hormone receptor positive breast cancer in 2017. with respect to Tmax, T1/2, and AUC was successfully modified so as to improve comparability to abaloparatide-SC. The results of this clinical evaluation will inform the design of a pivotal bioequivalence study that will be initiated following completion of activities related to manufacturing scale-up, production, and other activities required for the initiation of that study. - Dose-Escalation Study We continue to enroll patients in the EU Phase I FES-PET study. RAD140 in women with hormone receptor positive breast cancer in 2017. Abaloparatide-SC Abaloparatide-TD RAD1901 RAD140 Interest expense reflects interest due under our loan and security results of operations. and development expenses may be necessary in future periods. Historically, our estimated accrued clinical expenses have approximated actual expense incurred. Subsequent changes in estimates may result in a material change in our accruals. Performance Units Operating expenses: Research and development General and administrative Loss from operations Other (expense) income: Other (expense) income, net Loss on retirement of note payable Interest (expense) income, net Net loss Other (expense) income, net—For the year ended December 31, 2015, other expense, net of other income, was $35 thousand, as compared to Operating expenses: Research and development General and administrative Loss from operations Other (expense) income: Other (expense) income, net Loss on retirement of note payable Interest (expense) income, net Net loss Liquidity and Capital Resources opportunities will be available to us on favorable terms, and some could be dilutive to existing stockholders. Our future capital requirements will depend on many factors, including the scope and progress made in our research and development and commercialization activities, the results of our clinical trials, and the review and potential approval of our products by the Net cash (used in) provided by: Operating activities Investing activities Financing activities Net increase (decrease) in cash and cash equivalents 2015. 2015. offering costs, of approximately $281.5 million. Also, on July 28, 2015, the underwriters purchased an additional 608,108 shares by exercising an option to purchase additional shares that was granted to them in connection with the offering. As a result of the public offering and subsequent exercise of the underwriters' option, we received aggregate proceeds, net of underwriting discounts, commissions and estimated offering costs of approximately $323.8 million. Sales of Preferred Stock Series B redeemable convertible preferred stock(1) Series C redeemable convertible preferred stock(1) Series A-1 convertible preferred stock(1) Series A-5 convertible preferred stock(1) Series B convertible preferred stock Series B-2 convertible preferred stock Total On February 14, 2014, we entered into a Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement, or Purchase Agreement, pursuant to which we were able to raise up to approximately $40.2 million through the issuance of (1) up to 655,000 series B-2 Shares convertible preferred stock, or Series B-2, par value $.0001 per share, and (2) warrants to acquire up to 718,201 shares of our common stock, at an exercise price of $14.004 per share. On February 14, 2014, February 19, 2014, February 24, 2014, March 14, 2014 and March 28, 2014, we consummated closings under the Series B-2 Purchase Agreement, whereby, in exchange for aggregate proceeds to us of approximately $27.5 million, we issued an aggregate of 448,060 Series B-2 Shares and warrants to purchase up to a total of 491,293 shares of our common stock. The warrants issuable pursuant to the Purchase Agreement are exercisable for a period of five years from issuance. Debt Borrowings The following table summarizes our contractual obligations at December 31, Operating lease obligations As of December 31, Work Statement NB-3 Total Payments License Agreement Obligations France (as discussed above). into an amendment to the Eisai Agreement 2036. Off-Balance Sheet Arrangements ASSETS Current assets: Cash and cash equivalents Marketable securities Prepaid expenses and other current assets Total current assets Property and equipment, net Other assets Total assets LIABILITIES AND STOCKHOLDERS' EQUITY Current liabilities: Accounts payable Accrued expenses and other current liabilities Total current liabilities Note payable, net of current portion and discount Total liabilities Commitments and contingencies Stockholders' equity: Common stock, $.0001 par value; 200,000,000 shares authorized, 42,984,243 shares and 32,924,535 shares issued and outstanding at December 31, 2015 and 2014, respectively Additional paid-in-capital Accumulated other comprehensive income (loss) Accumulated deficit Total stockholders' equity Total liabilities and stockholders' equity OPERATING EXPENSES: Research and development General and administrative Loss from operations OTHER (EXPENSE) INCOME: Other (expense) income, net Loss on retirement of note payable Interest income Interest expense NET LOSS OTHER COMPREHENSIVE LOSS, NET OF TAX: Unrealized gain (loss) from available-for-sale securities COMPREHENSIVE LOSS LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS—BASIC AND DILUTED (Note 12) LOSS PER SHARE: Basic and diluted WEIGHTED AVERAGE SHARES: Basic and diluted Balance at December 31, 2012 Net loss Stock options exercised Issuance of preferred stock Accretion of dividends on preferred stock Stock-based compensation expense Balance at December 31, 2013 Net loss Unrealized loss from available-for-sale securities Issuance of preferred stock Accretion of dividends on preferred stock Issuance of warrants Exercise of warrants Stock options exercised Stock-based compensation expense Issuance of common stock, net Conversion of convertible preferred stock into common stock Reclassification of warrant liability to additional paid in capital Balance at December 31, 2014 Net loss Unrealized gain from available-for-sale securities Exercise of warrants Exercise of options Stock-based compensation expense Issuance of common stock, net Balance at December 31, 2015 Balance at December 31, 2012 Net loss Stock options exercised Issuance of preferred stock Accretion of dividends on preferred stock Stock-based compensation expense Balance at December 31, 2013 Net loss Unrealized loss from available-for-sale securities Issuance of preferred stock Accretion of dividends on preferred stock Issuance of warrants Exercise of warrants Stock options exercised Stock-based compensation expense Issuance of common stock, net Conversion of convertible preferred stock into common stock Reclassification of warrant liability to additional paid in capital Balance at December 31, 2014 Net loss Unrealized gain from available-for-sale securities Exercise of warrants Exercise of options Stock-based compensation expense Issuance of common stock, net Balance at December 31, 2015 CASH FLOWS USED IN OPERATING ACTIVITIES: Net loss Adjustments to reconcile net loss to net cash used in operating activities: Depreciation and amortization Amortization of premium (accretion of discount) marketable securities, net Stock-based compensation expense Research and development expense settled in stock Change in fair value of other current assets, warrant liability and other liability Non-cash interest Loss on retirement of note payable Changes in operating assets and liabilities: Prepaid expenses and other current assets Other long-term assets Accounts payable Accrued expenses and other current liabilities Net cash used in operating activities CASH FLOWS (USED IN) PROVIDED BY INVESTING ACTIVITIES: Purchases of property and equipment Purchases of marketable securities Sales and maturities of marketable securities Net cash (used in) provided by investing activities CASH FLOWS PROVIDED BY FINANCING ACTIVITIES: Proceeds from exercise of stock options Net proceeds from the issuance of preferred stock, net Proceeds from note payable, net Proceeds from issuance of common stock, net Deferred financing costs Payments on note payable Fee for early prepayment of note payable Net cash provided by financing activities NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR CASH AND CASH EQUIVALENTS AT END OF YEAR SUPPLEMENTAL DISCLOSURES: Cash paid for interest NON-CASH FINANCING ACTIVITIES: Accretion of dividends on preferred stock Reclassification of preferred stock to common stock Fair value of series A-6 convertible preferred stock issued as settlement of liability Fair value of warrants issued the treatment of breast cancer. Company's common stock. All shares and per share amounts in the financial statements and accompanying notes have been retroactively adjusted to give effect to the reverse stock split. marketable securities by maintaining its deposits and investments at high-quality financial institutions. The Company invests any excess cash in money market funds and other securities, and the management of these investments is not discretionary on the part of the financial institution. The Company has no significant off-balance-sheet risks such as foreign exchange contracts, option contracts, or other hedging arrangements. Income Taxes—The Company recognizes deferred tax assets and liabilities for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis, as well as operating loss and tax credit carryforwards. The Company measures deferred tax assets and liabilities using enacted tax rates expected to apply to taxable income in the years in which those temporary differences and carryforwards are expected to be recovered or settled. Deferred tax assets are reduced by a valuation allowance to reflect the uncertainty associated with their ultimate realization. The effect on deferred tax assets and liabilities as a result of a change in tax rates is recognized as income in the period that includes the enactment date. 2015. uses an expected dividend yield of zero. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of grant valuation for a period commensurate with the option's expected term. These assumptions are highly subjective and changes in them could significantly impact the value of the option and hence the related compensation expense. Comprehensive Income (Loss)—Comprehensive income (loss) refers to revenues, expenses, gains and losses that are excluded from net income (loss), as these amounts are recorded directly as an adjustment to stockholders' equity (deficit), net of tax. The Company's other comprehensive (loss) income is comprised of unrealized gains (losses) on its available-for-sale marketable securities. In January 2016, the FASB issued Cash and cash equivalents: Cash Money market Domestic corporate commercial paper Government-sponsored enterprise debt securities Domestic corporate debt securities Asset-backed securities Total Marketable securities: Domestic corporate debt securities Domestic corporate commercial paper Asset-backed securities Total Cash and cash equivalents: Cash Money market funds Domestic corporate debt securities Total Marketable securities: Domestic corporate debt securities Domestic corporate commercial paper Total 2015. Furniture and fixtures Computer equipment and software Manufacturing equipment Leasehold improvements Less accumulated depreciation and amortization Property and equipment, net as of December 31, 2016. Research costs—Nordic(1) Research costs—other Payroll and employee benefits Professional fees Accrued interest on notes payable Other Total accrued expenses and other current liabilties 6. Loan and Security Agreement NASDAQ Global Market on June 6, 2014. In connection with the offering, all outstanding shares of our convertible preferred stock converted into 19,465,132 shares of common stock and 2,862,654 shares of common stock were issued in satisfaction of accumulated dividends accrued on the preferred stock. In addition, all outstanding warrants to purchase shares of series A-1 convertible preferred stock and warrants to purchase shares of series B-2 convertible preferred stock were converted into the right to purchase 149,452 shares of common stock and the Company's warrant liability was reclassified to equity. On February 14, 2014, the Company entered into a Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement (the "Series B-2 Purchase Agreement"), pursuant to which the Company was able to raise up to approximately $40.2 million through the issuance of (1) up to 655,000 shares of its preferred stock (the "Series B-2"), and (2) warrants to acquire up to 718,201 shares of its common stock with an exercise price of $14.004 per share. In February and March 2014, the Company consummated closings under the Series B-2 Purchase Agreement, whereby, in exchange for aggregate gross proceeds to the Company of approximately $27.5 million, the Company issued an aggregate of 448,060 shares of Series B-2 and warrants to purchase up to a total of 491,293 shares of its common stock. The warrants can be exercised at any time prior to the fifth anniversary of their issuance. Upon completion of the Company's initial public offering, all outstanding warrants to purchase shares of series B-2 convertible preferred stock were converted into the right to purchase shares of common stock. Assets Cash and cash equivalents: Cash Money market funds(1) Domestic corporate commercial paper(2) Government-sponsored enterprise debt securities(2) Domestic corporate debt securities(2) Asset-backed securities(2) Total Marketable Securities Domestic corporate debt securities(2) Domestic corporate commercial paper(2) Asset-backed securities(2) Total Assets Cash and cash equivalents: Cash Money market funds(1) Domestic corporate debt securities(2) Total Marketable securities: Domestic corporate debt securities(2) Domestic corporate commercial paper(2) Total 9. License Agreements In consideration for these The date of the last to expire of the abaloparatide patents licensed from or co-owned with Ipsen, barring any extension thereof, is expected to be March 26, 2028. milestones. The Eisai Agreement, as amended, also grants the Company the right to grant sublicenses with prior written approval from Eisai. If the Company sublicenses the licensed technology to a third party, the Company will be obligated to pay Eisai, in addition to the milestones referenced above, a fixed low double digit percentage of certain fees received from such sublicensee and royalties in the low single digit range based on net sales of the sublicensee. The license agreement expires on a country-by-country basis on the later of (1) the date the last remaining valid claim in the licensed patents expires, lapses or is invalidated in that country, the product is not covered by data protection clauses, and the sales of The Company Payments in cash to be made to Nordic Plans approximately 2016. Expected term (years) Volatility Expected dividend yield Risk-free interest rates Options outstanding at December 31, 2014 Granted Exercised Cancelled Expired Options outstanding at December 31, 2015 Options exercisable at December 31, 2015 Options vested or expected to vest at December 31, 2015 unvested RSUs, which is expected to be recognized over a weighted-average period of approximately 3 years. Research and development General and administrative Share-based compensation expense included in operating expenses Numerator: Net loss Accretion of preferred stock Loss attributable to common stockholders—basic Effect of dilutive convertible preferred stock Loss attributable to common stockholders—diluted Denominator: Weighted-average number of common shares used in loss per share—diluted Loss per share—basic and diluted Convertible preferred stock Options to purchase common stock Warrants Performance units A reconciliation of income taxes computed using the U.S. federal statutory rate to that reflected in operations follows (in thousands): Income tax benefit using U.S. federal statutory rate State income taxes, net of federal benefit Stock-based compensation Research and development tax credits Change in the valuation allowance Permanent items Other Current assets: Accrued expenses Gross current deferred tax assets Valuation allowance Net current deferred tax assets Non-current assets: Net operating loss carryforwards Capitalized research and development Research and development credits Depreciation and amortization Accrued Expenses Stock-based compensation Other Gross non-current deferred tax assets Valuation allowance Net non-current deferred tax assets tax planning strategies to generate future taxable income. The Company is obligated to make monthly rent payments pursuant to these non-cancellable agreements as set forth below (in thousands): 2016 2017 2018 2019 2020 Total minimum lease payments 2015: Net loss Net loss applicable to common stock Net loss per share—basic and diluted Weighted-average common shares outstanding—basic and diluted 2014: Net loss Net loss applicable to common stock Net loss per share—basic and diluted Weighted-average common shares outstanding—basic and diluted 2016. 2016. Code of Business Conduct and Ethics 24, 2017
Title of each class Name of each exchange on which registered Common Stock, par value $0.0001 per share The NASDAQ Global Market 20152016 was $2.1$1.0 billion. For the purpose of the foregoing calculation only, all directors and executive officers of the registrant are assumed to be affiliates of the registrant.19, 2016: 43,014,243proxy statementProxy Statement for its 2016 annual meeting2017 Annual Meeting of stockholdersStockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.2015
2016 Currency and Conversions ITEM 1:Business3ITEM 1A:Risk Factors35ITEM 1B:Unresolved Staff Comments63ITEM 2:Properties63ITEM 3:Legal Proceedings63ITEM 4:Mine Safety Disclosures63 PART II Controls and Procedures120ITEM 9B:Other Information122 PART III Signatures130SPECIAL•the progress of, timing of and amount of expenses associated with our research, development and commercialization activities;•the success of our clinical studies for our investigational product candidates;•••••the timing of and our ability to commercialize abaloparatide following regulatory approval;•••••motivateretain key personnel; and• In this report, references to "dollar" or "$" are to the legal currency of the United States, and references to "euro" or "€" are to the single currency introduced on January 1, 1999 at the start of the third stage of European Economic and Monetary Union, pursuant to the Treaty establishing the European Communities, as amended by the Treaty on European Union and the Treaty of Amsterdam. Unless otherwise indicated, the financial information in this report has been expressed in U.S. dollars. Unless otherwise stated, the U.S. dollar equivalent information translating euros into U.S. dollars has been made, for convenience purposes, on the basis of the noon buying rate published by the Board of Governors of the Federal Reserve as of December 31, 2015, which was €1.00 = $1.0859. Such translations should not be construed as a representation that the euro has been, could have been or could be converted into U.S. dollars at the rate indicated, any particular rate or at all. Trademarks appearing in this report are the property of their respective holders. the investigational drug abaloparatide for subcutaneous injection, or abaloparatide-SC, has completed Phase 3 development for potential use in the reductiontreatment of fracture risk in postmenopausal women with osteoporosis andpostmenopausal osteoporosis. Our New Drug Application, or NDA, for abaloparatide-SC is currently under regulatory review by the U.S. Food and Drug Administration, or FDA, with a Prescription Drug User Fee Act, or PDUFA, date of March 30, 2017. Our European Marketing Authorisation Application, or MAA, for abaloparatide-SC is under review by the Committee for Medicinal Products for Human Use, or CHMP, of the European Medicines Agency, or EMA. We intend to commercialize abaloparatide-SC in Europe. the United States ourselves and our experienced commercial leaders are rapidly expanding the breadth of our capabilities and sales organization with highly skilled and tenured individuals.osteoporosisthe treatment of women with postmenopausal osteoporosis. We are focused on completing the manufacturing scale-up, production, and other activities required for the initiation of a pivotal bioequivalence study for abaloparatide-TD. In addition, we are evaluating our investigational drugproduct candidate, RAD1901, a selective estrogen receptor down-regulator/degrader, or SERD, for potential use in the treatment of hormone-driven and/or hormone-resistant breast cancer, andas well as for potential use in the treatment of vasomotor symptoms in postmenopausal women. We plan to complete our ongoing Phase 1 studies of RAD1901 in advanced metastatic breast cancer and our ongoing Phase 2b study of RAD1901 in postmenopausal vasomotor symptoms. In the first half of 2017, we plan to engage with regulatory agencies to gain alignment on defining the next steps for our RAD1901 breast cancer program, which would include the design of a Phase 2 trial. In the first half of 2017, we also expect to complete and report results from our ongoing Phase 2b vasomotor trial. Our preclinicalclinical pipeline also includes our internally developed investigational product candidate, RAD140, a non-steroidal selective androgen receptor modulator, or SARM, under investigation for potential applicationsuse in oncologythe treatment of breast cancer. In December 2016, we submitted an investigational new drug application, or IND, to the FDA and multiple conditions where androgen modulation may offer therapeutic benefit.proposedpotential indication and stage of development:
*submitted an MAA in the European Unionhold worldwide commercialization rights for all of these product candidates, excluding abaloparatide-SC, in November 2015,for which was validated in December 2015.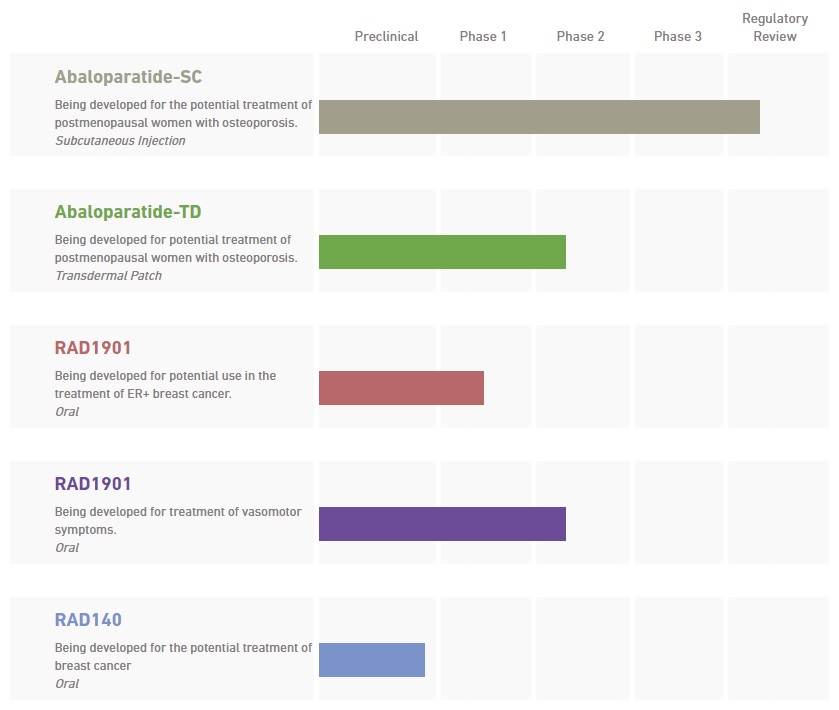
Abaloparatide was createda unique mechanismthe potential to provide the following advantages over other current standard of actioncare treatments for osteoporosis:the goallower vertebral and non-vertebral fracture risk;stimulating enhanced bone building activity including bone formation, increasing bone mineral density, restoring bone microarchitecturebone; augmenting bone strength. •Abaloparatide-SC—Abaloparatide abaloparatide-SC, an injectable subcutaneous formulation of abaloparatide, and abaloparatide-TD, a potential line extension of abaloparatide-SC in the form of a convenient, short-wear-time, transdermal patch.injection, which we refer to as abaloparatide-SC.injection. We hold worldwide commercialization rights to abaloparatide-SC, except for Japan. In December 2014, we announced the positive 18-month top-line data from our Phase 3 ACTIVE clinical trial of abaloparatide-SC. These results were published in which abaloparatide-SC met the primary endpoint with a statistically significant reductionJournal of the American Medical Association, or JAMA, in new vertebral fractures versus placebo, and inAugust 2016. In June 2015, we announced the positive top-line data from the first six months of theour 24-month ACTIVExtend clinical trial of abaloparatide-SC and the 24-month25-month combined fracture data from the ACTIVE and ACTIVExtend.ACTIVExtend clinical trials. These data were published in the Mayo Clinic Proceedings in February 2017. The combined 25-month fracture data from our Phase 3 clinical trial program for abaloparatide-SC formed the basis of our regulatory submissions in the United States and Europe. In November 2015, we submitted a marketing authorization application, oran MAA to the European Medicines Agency, or EMA, which was validated and is currently undergoing active regulatory reviewassessment by the EMA.CHMP. We anticipate that the CHMP may adopt an opinion regarding the MAA in 2017. In March 2016, we submitted an NDA to the FDA, which has been accepted for filing by the FDA with a PDUFA date of March 30, 2017. We intend to enter into one or more partnerships or collaborations for the potential commercialization of abaloparatide-SC prior to a commercial launch. We plan to submit a new drug application, or NDA, in the United States, at the end of the first quarter of 2016. Subject to regulatory review and a favorable regulatory outcome, we anticipate the first commercial sales of abaloparatide-SC willto take place in 2016.2017. We intend to commercialize abaloparatide-SC in the United States ourselves and our experienced commercial leaders are rapidly expanding the breadth of our capabilities and sales organization with highly skilled and tenured individuals. We expect to report the top-line results from our recently completed 24-month ACTIVExtend trial in the second quarter of 2017.•Abaloparatide-TD—abaloparatide-transdermal, which we refer to as abaloparatide-TD,abaloparatide- TD, based on 3M's3M’s patented Microstructured Transdermal System technology for potential use as a short wear-time transdermal patch. We hold worldwide commercialization rights to the abaloparatide-TD technology. During 2014, we reported progress towards the development of an optimized transdermal patch that may be capable of demonstrating comparability to abaloparatide-SC. In preliminary, nonhuman primate pharmacokinetic studies, we achieved a desirable pharmacokinetic profile, with comparable AUC, Cmax, Tmax and T1/2 relative to abaloparatide-SC. We believe that these results support continued clinical development ofare developing abaloparatide-TD toward future global regulatory submissions as ato build upon the potential post-approval line extensionsuccess of theour investigational drug abaloparatide-SC.product candidate, abaloparatide-SC, if approved. We commenced a human replicative clinical evaluation of the optimized abaloparatide-TD patch in December 2015, with the goal of achieving comparability to abaloparatide-SC. We expect to complete our clinicalIn September 2016, we presented results from this evaluation, which showed that the pharmacokinetic profile of thean optimized abaloparatide-TD patch during 2016.hasis being evaluated for potential for use as an oral non-steroidal treatment for hormone-driven and/or hormone-resistant, breast cancer. We hold worldwide commercialization rights to RAD1901. RAD1901 is currently being investigated in postmenopausal women with advanced estrogen receptor positive, or ER-positive, and human epidermal growth factor receptor 2-negative, or HER2-negative breast cancer, the most common form of the disease. TheStudies completed to date indicate that the compound has the potential for use as a single agent, or in combination with other therapies to overcome endocrine resistance infor the treatment of breast cancer. In September 2015, we announced results from We believe that, subject to successful development, regulatory review and approval, RAD1901 could have the potential to offer the following advantages compared to current standard of care treatments for ER-positive and HER2-negative breast cancer:maximum tolerated dose, or MTD, study of RAD1901 in 52 healthy volunteers. In the study, RAD1901 was administered to healthy postmenopausal women in doses ranging from 200mg to 1000mg, and the data showed that RAD1901 was well-tolerated and the overall safety was supportive of continued development. In addition, a subset of subjects that received 18F estradiol positron emission tomography, or FES-PET, imaging demonstrated suppression of the FES-PET signal to background levels after six days of dosing.two-part,multiple-part, dose-escalation study of RAD1901 in postmenopausal women with advanced ER-positive and HER2-negative advanced breastWe expectPart A of this Phase 1 study was designed to completeevaluate escalating doses of RAD1901. The Part B expansion cohort was initiated at 400 mg daily dosing in March 2016 to allow for an evaluation of additional safety, tolerability and preliminary efficacy. The patients enrolled in this study byare heavily pretreated ER-positive, HER2-negative advanced breast cancer patients who have received a median of 3 prior lines of therapy including fulvestrant and CDK4/6 inhibitors, and more than 50% of the middle ofpatients had ESR1 mutations. Dose escalation is currently ongoing with no dose limiting toxicities to date and we expect to initiate expansion cohorts in 2016.Pfizer'sPfizer’s palbociclib, a cyclin-dependent kinase, or CDK 4/6 inhibitor, or Novartis'Novartis’ everolimus, ana mTOR inhibitor, was effective in shrinking tumors. In preclinical patient-derived xenograft or PDx, breast cancer models with either wild type or mutant ESR1, treatment with RAD1901 resulted in marked tumor growth inhibition, and the combination of RAD1901 with either agent, palbociclib or everolimus, showed anti-tumor activity that was significantly greater than either agent alone. We believe that thisthese preclinical data suggestssuggest that RAD1901 has the potential to overcome endocrine resistance, is well-tolerated, and has a profile that is well suited for use in combination therapy.Novartis'Novartis’ investigational agent LEE011 (ribociclib), a CDK 4/6 inhibitor, and BYL719 (alpelisib), an investigational phosphoinositide 3-kinase inhibitor. as an estrogen receptor ligand for the potential relief of the frequency and severity of moderate to severe hot flashes in postmenopausal women with vasomotor symptoms. We commenced aexpect to report results from our Phase 2b clinical study of RAD1901 for the potential treatment of postmenopausal vasomotor symptoms in the first half of 2017.2015.other serious endocrine diseases. To achieve this goal, we plan to:•aour Phase 3 clinical trial of abaloparatide-SC, or the ACTIVE trial, and the first six months of anrecently completed our 24-month extension trial of abaloparatide-SC, or the ACTIVExtend trial, for the potential use in the reduction of fractures in postmenopausal osteoporosis. We submitted anOur NDA for abaloparatide-SC in the United States is undergoing regulatory review by the FDA with a PDUFA date of March 30, 2017 and our MAA in the European Union for abaloparatide-SC is under review by the CHMP with an opinion anticipated in November 2015, which was validated in December 2015, and plan2017. We are building a commercial organization to submit an NDA forsupport the potential commercialization of abaloparatide-SC in the United States atU.S. We intend to complete the endhiring of our U.S. sales force in the first quarter of 2016.2017. We expect to report the top-line results from our recently completed 24-month ACTIVExtend trial in the second quarter of 2017.
We are continuing to evaluate other underserved osteoporosis patient populations that might benefit from abaloparatide therapy. We may engage in additional clinical research with the goal of achieving additional labeling to treat these populations.•establishenter into one or more partnerships or collaborations for the potential commercialization of abaloparatide-SC prior to a commercial launch.•abaloparatide-TD and additional clinical research.abaloparatide-TD. We are developing abaloparatide-TD as a short-wear-time transdermal patch and we anticipate, pending successful development and a favorable regulatory outcome, commercial launch approximately two to three years after the approval and first commercial sale of abaloparatide-SC.abaloparatide-SC, if approved. We initiatedare currently working on the clinical evaluation of the optimized abaloparatide-TD patch in December 2015, with the goal of achieving pharmacokinetic equivalence to abaloparatide-SC. We expect to complete our clinical evaluation of the optimized abaloparatide-TD patch during2016. We believe abaloparatide-TD may be submitted for regulatory approval based upon a demonstration of bioequivalence to abaloparatide-SC. Upon completion of clinical evaluation of the optimized abaloparatide-TD patch, we will meet with regulatory agencies to discuss the regulatory pathmanufacturing scale-up, production, and other activities required for the abaloparatide-TD program.initiation of a pivotal bioequivalence study. If our clinical trials of abaloparatide-SC and abaloparatide-TD are successful, we expect to seek marketing approval of abaloparatide-TD as a line extension of abaloparatide-SC.We are continuing to evaluate other underserved osteoporosis patient populations that might benefit from abaloparatide therapy. We may engage in additional clinical research to achieve additional labeling to treat these populations.•cancer and vasomotor symptoms.cancer. During 2015,2016, we completed acontinued to enroll patients in our ongoing Phase 1 MTD studystudies of RAD1901 in healthy volunteers, and commenced Phase 1 studies in patients with metastatic breast cancer in the United States and the European Union. Preliminary results showfrom these studies suggest that RAD1901 hasmay have a favorable safety and tolerability profile and potential anti-tumor effect. We expectplan to initiate expansion cohorts in 2016.complete both of our ongoing RAD1901 Phase 1 breast cancer trials. In addition,the first half of 2017, we commencedintend to engage with regulatory agencies to gain alignment on defining the next steps for the program, which would include the design of a Phase 2b study2 trial.RAD1901RAD140 for the treatment of vasomotor symptomsbreast cancer. We submitted an IND to the FDA for RAD140 in December 2015.2016. In 2017, we plan to initiate a first-in-human Phase 1 study of RAD140 in women with hormone receptor positive breast cancer•according toper the National Osteoporosis Foundation, or NOF, is significantly under-recognized and under-treated in the population. While the aging of the population is a primary driver of an increase in cases, osteoporosis is also increasing from the use of drugs that induce bone loss, such as chronic use of glucocorticoids and aromatase inhibitors that are increasingly used for breast cancer and hormone therapies used for prostate cancer.2015,2016, total sales of branded osteoporosis drugs approximated $6.4$7.0 billion, worldwide, of which more than $3.0$4.0 billion was attributable to injectable therapies (Source: EvaluatePharma, February 2016, Evaluate Ltd, www.evaluate.com).therapies. There are two main types of osteoporosis drugs currently available in the United States, anti-resorptive agents and anabolic agents. Anti-resorptive agents act to prevent further bone loss by inhibiting the breakdown of bone, whereas anabolic agents stimulate bone formation to build new bone.hashave been associated with infrequent but serious adverse events, such as osteonecrosis of the jaw and atypical fractures, especially of long bones. These side effects, although uncommon, reportedly have created increasing concern with physicians and patients. ManyWe believe many physicians are seeking alternatives to bisphosphonates. Lilly's Forteo/ForsteoForsteo® (teriparatide) marketed by Eli Lilly and Amgen's ProliaCompany, or Lilly, and Prolia® (denosumab) marketed by Amgen Inc., or Amgen, are the two primary alternatives to bisphosphonates that are approved for the treatment of osteoporosis. Prolia has also been associated with infrequent but serious adverse events, such as osteonecrosis of the jaw and atypical fractures. In 2015,2016, Forteo/Forsteo had reported worldwide sales of approximately $1.3$1.5 billion, $0.6$0.8 billion in the U.S.United States and $0.7 billion outside of the U.S.,United States, and Prolia had reported sales of approximately $1.3$1.6 billion, $0.8$1.0 billion in the U.S.United States and $0.5$0.6 billion outside of the U.S.United States. Forteo, a 34 amino acid recombinant peptide of human parathyroid hormone, is the only anabolic drug approved in the United States for the treatment of osteoporosis. Today, the treatment of osteoporosis has no clear consensus goals for BMD, bone turnover biomarkers or fracture risk. Patients suffering from osteoporosis are generally treated with a bisphosphonate first, regardless of their initial BMD or fracture risk in order to preserve bone. Those patients that have already suffered from a fracture, lost BMD, or cannot tolerate or comply with bisphosphonate therapies may progress to other therapies such as a RANK-L inhibitor (denosumab) or anabolic therapy (such as teriparatide). Anabolic bone building agents have been reserved primarily for patients with the most severe BMD loss or who fail (i.e. fracture) on prior anti-resportives. The current anti-resorptive treatment paradigm means that the majority of patients only maintain their bone mass without having a sustained benefit in terms of fracture reduction. We believe there is substantive effort underway to update osteoporosis treatment guidelines towards goal directed therapy that could improve outcomes for both patients and payers. In this new potential goal directed paradigm, patients may be stratified by severity of BMD and fracture risk in order to create an individualized treatment plan based on achieving a target goal (either BMD or improved 10 year fracture risk reduction). Patients will then be offered therapies that reduce the near term, higher risk of fracture and then monitored periodically to ensure they remain at goal (i.e. the lowest possible achievable fracture risk score).highergreater awareness of the long termlong-term risk associated with osteoporotic fracture, and new, more effective therapies we believe osteoporosis treatment will expand and thuslikewise our potential commercial opportunity.(teriparatide)teriparatide) and parathyroid hormone-related protein, or PTHrP)PTHrP, represent a family of proteins and peptides that share regions of partial or complete amino acid sequence similarity. The first 34 amino acids of PTH analogs contain the binding site for engaging the PTH1 receptor. Abaloparatide is a unique 34 amino acid PTH analog that has 41% homology (i.e. amino acid similarity) to Forteo (teriparatide) and has 76% homology to the first 34 amino acidscreateddesigned to have a unique mechanism of action with the goal of stimulating enhanced bone building activity including bone formation, increasing bone mineral density, restoring bone microarchitecture and augmenting bone strength. We believe that abaloparatide is the most advanced PTH analog in clinical development for the treatment of osteoporosis and that, subject to regulatory review and approval, it could have the potential to provide the following advantages over other current standard of care treatments for osteoporosis:•••••are developing two formulations of abaloparatide: abaloparatide-SC, an injectable subcutaneous formulation of abaloparatide, andhave worldwide commercialization rights for abaloparatide-TD, a line extension of abaloparatide-SCincluding in the form of a convenient, short-wear-time, transdermal patch.believe that the resultspreviously announced positive 18-month top-line data from our Phase 3 ACTIVE clinical trial and positive top-line data from the first six months of our recently completed 24-month ACTIVExtend clinical trial, have demonstratedwhich included a one-month transition period for patients in the improved efficacyabaloparatide-SC and placebo groups from the ACTIVE clinical trial. The combined 25-month fracture data from our Phase 3 clinical trial program for abaloparatide-SC formed the basis of abaloparatide relative to teriparatide (Forteo/Forsteo)our regulatory submissions in treating osteoporosis, while still maintaining a well-tolerated long-term safety profile.the United States and Europe. In November 2015, we submitted an MAA in Europe, which was validated in December 2015, and are on trackwe submitted an NDA to submit an NDAthe FDA, which was accepted with a PDUFA date of March 30, 2017. We intend to lead the commercialization of abaloparatide-SC in the United States atand are currently completing the endbuild out of our commercialization capabilities and organization with highly experienced individuals. We expect to report the firsttop-line results from our recently completed 24-month ACTIVExtend trial in the second quarter of 2016. During 2014, we reported2017.towardson the development of an optimized, short-wear-time transdermal patch that may be capable of demonstrating comparability to abaloparatide-SC injection. In preliminary, nonhuman primate pharmacokinetic studies, we observed a favorable pharmacokinetic profile, with comparable AUC, Cmax, Tmax and T1/2 relative to abaloparatide-SC. We believe that these results support continued clinical development of abaloparatide-TD toward future global regulatory submissions as a potential post-approval line extension of the investigational drug abaloparatide-SC. We commenced the clinical evaluation of the optimized abaloparatide-TD patch at the end of 2015, with the goal of achieving comparability to abaloparatide-SC. In September 2016, we presented results from this evaluation, which showed that the pharmacokinetic profile of an optimized abaloparatide-TD patch with respect to Tmax, T1/2, and AUC, was successfully modified so as to improve comparability to abaloparatide-SC. The results of this clinical evaluation will inform the design of a pivotal bioequivalence study that will be initiated following completion of activities related to manufacturing scale-up, production, and other activities required for the initiation of that study. If our clinical trials of abaloparatide-SC and abaloparatide-TD are successful, we expect to seek marketing approval of abaloparatide-TD as a line extensionpotential expansion of abaloparatide-SC.our abaloparatide-SC franchise. We believe abaloparatide-TD maycould be submitted for regulatory approval based upon a demonstration of bioequivalence to abaloparatide-SC. Upon completion of clinical evaluation of the optimized abaloparatide-TD patch, we will meet with regulatory agencies to discuss the appropriate regulatory path for the abaloparatide-TD program. The FDA'sAny approval of abaloparatide-TD, and the timing of any such approval, is dependent upon the approval of abaloparatide-SC. current 18-month primary endpoint. Based upon our discussion with the FDA, we believe that the 18-month primary endpoint will be acceptable, provided that our NDA includes the 24-month fracture data derived from a 6-month extension of the abaloparatide 80 µg and placebo groups in our Phase 3 study during which patients received an approved alendronate (generic Fosamax) therapy for osteoporosis management. Accordingly, patients from the abaloparatide-SC and placebo groups from our ACTIVE trial were eligible to continue in ana 24-month extension study, or the ACTIVExtend trial, in which they received 70 mg once weekly of an approved alendronate therapy for osteoporosis management.management following completion of treatment in the ACTIVE trial and a one-month transition period during which they received no study treatments. We intend to submit thesubmitted our NDA for abaloparatide-SC with the 24-month25-month fracture data at the end of the first quarter of 2016.Abaloparatide-SC Phase 2 Clinical Trial In 2009, we completed a randomized, placebo-controlled, parallel group dose-finding Phase 2 study in the United States, Argentina, India and the United Kingdom. Data from our Phase 2 study showed The results also suggested that abaloparatide produced faster and greater BMD increases at the spine and the hip after six months and 12 months of treatment than did Forteo, which was a comparator in our study. Key findings were that the highest dose of abaloparatide tested of 80 µg increased mean lumbar spine BMD at six months and 12 months by 6.7% and 12.9% compared to the increases seen with Forteo trial arms of 5.5% and 8.6%, respectively. Abaloparatide also produced increases in mean femoral neck BMD at the hip at six months and 12 months of 3.1% and 4.1% compared to increases for Forteo of 1.1% and 2.2%, respectively. Abaloparatide was generally safe and well tolerated in this study,when administered once daily, with reported adverse events similar between abaloparatide, placebo and Forteo groups. In addition, the occurrence of hypercalcemia as a side effect for the 80 µg dose of abaloparatide was half that seen with Forteo.Abaloparatide-SC Phase 1 Clinical Trials We have completed seven Phase 1 clinical trials of abaloparatide-SC. Together with our Phase 2 and Phase 3 clinical trials, over 1,500 patients have received any dose/route of abaloparatide. The results of our Phase 1 clinical trials suggest that abaloparatide-SC is safe and well tolerated at doses of up to 240mg administered once daily.signseffects of anti-abaloparatide antibodies; and patient ratings of patch adhesion and local skin response to the transdermal patch technology were also acceptable.Abaloparatide-TD Phase 1 Clinical Trials We have completed three Phase 1 clinical trials that collectively evaluated the safety, PK, time course of delivery and dose ranging of abaloparatide-TD. Abaloparatide-TD was characterized by a rapid release of abaloparatide with a faster time to reach peak concentration as well as more rapid elimination in plasma compared to abaloparatide-SC. Peak transdermal drug levels were consistent with abaloparatide-SC. An optimal wear time of five minutes or less was identified as well as effective sites of application. Abaloparatide-TD showed an increase in the bone-formation marker P1NP in serum after seven days of exposure, consistent with bone-building activity, and was shown to be safe and well tolerated in all doses studied.Preclinical Pharmacology of Abaloparatide We have completed several preclinical studies of abaloparatide, and we observed the following:•abaloparatide was a potent selective agonist of the human PTH type 1 receptor (PTHR1), with binding selectivity for the RG vs R0 receptor conformation compared to PTH(1-34) and greater selectivity than PTHrP(1-34);•in models of calcium mobilization, abaloparatide has significantly less calcium mobilizing activity at higher doses than PTHrP(1-34), and less activity than PTH(1-34);•abaloparatide-SC stimulated the formation of normal, well-organized bone and restored BMD in ovariectomized, or OVX, osteopenic rats and primates; mechanical testing of bones from OVX rats after treatment with abaloparatide-SC revealed a significant increase in femur and vertebral bone strength; similar studies in rats with abaloparatide-TD showed comparable restoration of bone;•abaloparatide-SC was generally well tolerated over a wide range of doses in two species, rats and primates, for up to six months and nine months, respectively; and•safety pharmacology studies showed no respiratory, gastroenterologic, hematologic, renal or central nervous system effects. A two-year subcutaneous injection carcinogenicity study of abaloparatide in Fischer 344 albino rats was conducted to assess the carcinogenic potential of abaloparatide. The study was conducted accordingto the provisions set forth in Guidance ICH-S1A, ICH-S1B and ICH-S1C(R2), and the design was accepted under a Special Protocol Assessment by the FDA on July 15, 2009. This study evaluated three abaloparatide dose levels. The doses were selected based upon findings and tolerance in completed long-term rat toxicology studies and the anticipated tolerance over a two-year dosing period. Furthermore, the doses represent an exposure multiple over maximum clinical doses. The study included a cohort of rats being dosed with a daily subcutaneous injection of PTH(1-34) as a positive control, as it was anticipated that osteosarcomas would be observed with this treatment, as previously published for both rhPTH(1-34) and rhPTH(1-84) in similar two-year rat carcinogenicity studies. The positive control served to provide confirmation of the sensitivity of the model. The results of the study revealed osteosarcomas in our carcinogenicity study in both the abaloparatide and PTH(1-34) treated groups, with similar frequency between abaloparatide and PTH(1-34) when comparing comparable exposure multiples to the human therapeutic dose. We have also conducted one preclinical bone quality study in OVX rats with 12 months of daily abaloparatide-SC dosing and a second preclinical bone quality study in adult OVX monkeys for 16 months. The primary objective of these studies was to determine the long-term treatment effects of abaloparatide-SC on bone quality. Effects on bone mass, both cortical bone and cancellous bone, were assessed by BMD and peripheral quantitative CT, and bone strength was determined by biomechanical testing. The mechanisms by which abaloparatide affects bone were assessed by evaluation of biomarkers of bone turnover and histomorphometric indices of bone turnover. Data from the 12-month rat study showed marked, dose dependent increases in BMD following abaloparatide treatment, increases in bone formation markers, but not bone resorption, and an increase in bone strength. Results from the 16-month monkey OVX study have also shown significant BMD gains, together with increases in bone strength.between therapy levels.during the course of their treatment. About 5% of breast cancer patients have distant metastases at the time of diagnoses,diagnosis, and these patients have a five-year survival rate of only 25%, compared with a greater than 99% five-year survival rate for patients with only local disease.disease at diagnosis. Importantly, even patients without metastases at diagnosis are at risk for developing metastases over time.often acquire resistance to them by developing the ability to signal through the ER in a ligand-independent manner. In contrast, SERDs are a class of endocrine therapies that directly induce ER degradation. Therefore, SERDs should have the potential to treat ER-dependent tumors without allowing ligand-independent resistance to develop, and to act on AI- and SERM-resistant ER-positive tumors.ana daily oral non-steroidal treatment for hormone-driven and/or hormone-resistant, breast cancer. RAD1901 is currently being investigated in postmenopausal women with advanced ER-positive, HER2-negative advanced breast cancer, the most common form of the disease. The compound has the potential for use as a single agent or in combination with other therapies to overcome endocrine resistance in breast cancer. RAD1901 selectively binds to and degrades the estrogen receptor. In preclinical models of ER-positive breast cancer, RAD1901 has shown potent anti-tumor activity and complete degradation of the ER and progesterone receptor, an ER-regulated gene. RAD1901 has shown good tissue selectivity in preclinical models and does not appear to stimulate the uterine endometrium, while it appears to protect against bone loss in an ovariectomy-induced osteopenia rat model. When RAD1901 was used in combination with other approved breast cancer agents such as everolimus, an mTOR inhibitor, or the inhibitor palobciclib, a CDK 4/6 inhibitor, greater tumor shrinkage in PDxpatient-derived xenograft (PDx) animal models was achieved than with the agents alone. In addition, RAD1901 has been shown to effectively inhibit tumor growth in PDx animal models that harbor ER mutations, in the ER, a potential mechanism of endocrine therapy resistance. In aour phase 1 MTD healthy volunteer study, FES-PET imaging was used to assess how much RAD1901 has engaged in the ER, RAD1901 showed suppression of the FES-PET signal to background levels after six days of dosing. Studiesdosing in a subset of patients.•suppressdegrade estrogen receptor turnover;receptor;••oral administration; and•cancers.Studies—Studies—Breast Cancer In September 2015, we announced results from a Phase 1 maximum tolerated dose, or MTD, study of RAD1901 in 52 healthy volunteers. In the study, RAD1901 was administered to healthy postmenopausal women in doses ranging from 200mg to 1000mg, and the data showed that RAD1901 was well-tolerated and the overall safety was supportive of continued development. In addition, a subset of subjects that received 18F estradiol positron emission tomography, or FES-PET, imaging demonstrated suppression of the FES-PET signal to background levels after six days of dosing.two-part,multiple-part, dose-escalation study of RAD1901 in postmenopausal women with advanced ER-positive and HER2-negative advanced breast cancer in the United States to determine the recommended dose for a Phase 2 studiesclinical trial and to make a preliminary evaluation of the potential anti-tumor effect of RAD1901. We expectPart A of this Phase 1 study is designed to completeevaluate escalating doses of RAD1901 (n=13). The Part B expansion cohort (n=20) was initiated at 400 mg daily dosing in March 2016 to allow for an evaluation of additional safety, tolerability and preliminary efficacy. The patients enrolled in this study are heavily pretreated ER-positive, HER2-negative advanced breast cancer patients who have received a median of 3 prior lines of therapy. In December 2016, we reported positive results from the ongoing U.S. Phase 1 dose-escalation and expansion study that includes 2 patients with confirmed partial responses by RECIST criteria out of 19 patients with RECIST measurable disease. At the middledata cut-off date of 2016. Dose escalation is currently ongoingOctober 7, 2016, 14 patients had remained on study drug for greater than or equal to 4 months, 5 patients for greater than or equal to 6 months, and 7 patients were continuing on study drug as of the cut-off date. The results suggested that RAD1901 was well-tolerated with no dose limiting toxicities to date,the most commonly reported adverse events being low grade nausea and we expect to initiate expansion cohortsdyspepsia. A Part C tablet dosage form cohort (n=14) was initiated and enrollment completed in 2016.ERa,ERa, and to have both estrogen-like and estrogen antagonist effects in different tissues. RAD1901 has also been shown to have estrogen-like behavioral effects in an animal model of partner preference and to reduce vasomotor signs in an animal model of menopausal hot flashes.preference. In bone, RAD1901 protected against gonadectomy-inducedovariectomy-induced bone loss. RAD1901 does not stimulate the endometrium, as shown in short- and long-term animal models, where changes in uterine weight, uterine epithelial thickness, and C3 gene expression are measured, all of which are sensitive indicators. In studies in which an estrogen is used to stimulate the endometrium, RAD1901 antagonizes this estrogen-mediated stimulation of the endometrium. In cell culture, RAD1901 does not stimulate replication of breast cancer cells, and antagonizes the stimulating effects of estrogen on cell proliferation. Furthermore, in breast cancer cell lines a dose dependent down regulation of ERaERa is observed, a process we have shown to involve proteosomal-mediated degradation pathway. In a xenograft model of breast cancer, in which human breast cancer cells are implanted in mice and allowed to establish tumors in response to estrogen treatment, we observed that treatment with RAD1901 results in decreased tumor growth.Vasomotor symptoms Vasomotor symptoms, such as hot flashes, hot flushes and night sweats, are common during menopause, with up We submitted an IND for RAD140 in December 2016. In 2017, we plan to 85%initiate a first-in-human Phase 1 study of women experiencing them during the menopause transition, for a median duration of four years. An estimated two million women go through menopause every yearRAD140 in the United States, with a total population of 45 million postmenopausal women. In addition, most women receiving systemic therapy for breast cancer suffer hot flashes, often with more severe or prolonged symptoms than women experiencing natural menopause. These symptoms can disrupt sleep and interfere with quality of life. Historically hormone replacement therapy, or HRT, with estrogen and/or progesterone has been considered the most efficacious approach to relieving menopausal symptoms such as hot flashes. However, data from the Women's Health Initiative, or WHI, identified increased risks for malignancy and cardiovascular disease associated with estrogen therapy. Sales of HRT declined substantially after the release of the initial WHI data, but HRT remains the current standard of care for many women suffering from hot flashes. However, due to concerns about the potential long-term risks and contraindications associated with HRT, we believe that there is a significant need for new therapeutic options to treat vasomotor symptoms.Our Investigational Drug—RAD1901 RAD1901 is also being evaluated at low doses as an estrogen receptor ligand for the potential relief of the frequency and severity of moderate to severe hot flashes in postmenopausal women with vasomotor symptoms. We believe that the studies completed to date have demonstrated RAD1901's acceptable safety profile and potential to reduce or prevent hot flashes associated with menopause, while simultaneously providing a bone-protective effect, without stimulatinghormone receptor positive breast or uterine tissues. We commenced a Phase 2b clinical study of RAD1901 for the potential treatment of postmenopausal vasomotor symptoms in December 2015. We plan to enroll 300 healthy postmenopausal women between the ages of 40 and 65 years old with moderate to severe hot flashes in approximately sixty clinical sites across the United States.Phase 2 Study—Vasomotor Symptoms A Phase 2 proof-of-concept study was conducted in 100 healthy perimenopausal women using four doses of RAD1901 (10 mg, 25 mg, 50 mg and 100 mg) and placebo. The primary study outcome was reduction in the frequency and severity of moderate and severe hot flashes. While a classic dose-response effect was not demonstrated, efficacy was determined to occur at the 10 mg dose level which achieved a statistically significant reduction in the frequency of moderate and severe hot flashes both by linear trend test and by comparison to placebo and in overall (mild-moderate-severe) hot flashes at either the two-, three- or four-week time-points. A similar reduction in composite score (frequency × severity of hot flashes) was identified at all time-points, with a statistically significant difference from placebo achieved at the two-, three- or four-week time-points. Numerical reductions in mean severity and mean daily severity were observed, but did not reach statistical significance. We believe RAD1901 is an attractive candidate for advancement to Phase 3 development as a potential treatment for vasomotor symptoms. No serious adverse events, or SAEs, were reported during the course of the study. Overall, 69% of patients had an adverse event, generally mild or moderate in severity, with some evidence of dose dependency, and events were most commonly gastrointestinal symptoms and headaches. Three severe adverse events occurred, one in a placebo patient, none of which were considered treatment related. Two patients discontinued treatment due to an adverse event, neither in relation to the 10 mg dose.Phase 1 Study—Vasomotor Symptoms We have conducted Phase 1 safety, PK and bioavailability studies of RAD1901 in 80 healthy postmenopausal women over a range of doses. Bioavailability was determined to be approximately 10%. Food effect was also investigated and the presence of food was determined to increase absorption and delay clearance of RAD1901. RAD1901 was generally well tolerated at all dose levels tested. All study-related adverse events were of mild intensity, with some increase in frequency at the higher doses in the multiple dose group, most commonly gastrointestinal symptoms and headaches. There were no SAEs observed. or Lonza, using a solid phase peptide synthesis assembly process, and purification by high pressure liquid chromatography. Abaloparatide-SC is supplied as a liquid in a multi-dose cartridge for use in a pen delivery device. The pen delivery device is manufactured by Ypsomed AG.AG, or Ypsomed. The multi-dose cartridges and pen delivery device are filled, assembled and packaged by Vetter Pharma FertigungInternational GmbH, & Co. or Vetter.offor RAD1901 is manufactured for us on a contract basis by Patheon, Inc.2015,2016, we owned or co-owned 11 issued U.S. patents, as well as 2918 pending U.S. patent applications, and about 395 pending Patent Cooperation Treaty, or PCT, applications, 33 pending foreign patent applications in Europethe European Patent Office and 1514 other jurisdictions, and about 2232 granted foreign patents. As of December 31, 2015,2016, we had licenses to 83 U.S. patents related to compositions and related uses thereof, as well as numerous foreign counterparts to many of these patents and patent applications.iswas claimed in the United States (U.S. Patent No. 5,969,095), Europe, Australia, Canada, China, Hong Kong, South Korea, New Zealand, Poland, Russia, Singapore, Mexico, Hungary, and Taiwan. These patents have a statutory expiration date of 2016.and European Patent No. 0847278, which was included in the license from Ipsen and claimed the composition of matter of abaloparatide, lapsed due to Ipsen's failure to pay annuities. We are pursuing restoration of those patent rights. To date, the patent rights in Austria, Belgium, Denmark, Finland, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain, Sweden, and the United Kingdom have been restored. We believe that the data and market exclusivity provided in Europe for a new chemical entity, coupled with the need for a potential competitor to conduct clinical trials will likely provide a longer barrier to entry than the patent protection provided by the original European patent term, which will expireexpired in 2016. The Phase 3 clinical dosagesubcutaneous formulation of abaloparatide by the subcutaneous route for potential use in treating osteoporosis is covered by Patent No. 7,803,770 until the statutory term expires October 3, 2027, which we expect will be extended to March 26, 2028 (statutory term extended with 175 days of patent term adjustment due to delays in patent prosecution by the United States Patent and Trademark Office, or USPTO) in thecurrentlyadditional patent applications pending in Brazil, Canada, Europe, Hong Kong, India, South Korea, Norway, and Singapore,Norway, will have a patent expiration date of 2027, not taking into account extension under any applicable laws. A notice to grant the abaloparatide-SC osteoporosis treatment method has been received from the European Patent Office. When granted, this patent will have a normal expiry of October 3, 2027, not including any issued supplementary patent certificates, or SPC. Patent applications which covercovering various aspects of abaloparatide for microneedle application have been granted in Australia, Japan, and New Zealand, and additional patent applications are currently pending in the United States, Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, India, Japan, South Korea, Mexico, New Zealand, Russia, Singapore, and Ukraine. AnyThe issued patents and any patents that might issue from thesethe pending applications will have a statutory expiration date in 2032, not taking into account extension under any applicable laws.USU.S. Patent No. 7,612,114 (statutory term expires December 25, 2023 which we expect will be extended up to August 18, 2026 with 967 days of patent term adjustment not taking into account any Hatch-Waxman patent term extensions) covers RAD1901 as a composition of matter as well as the use of RAD1901 for treatment of estrogen-dependent osteoporosis or estrogen-dependent breast cancer. Corresponding patents issued in Australia, Canada, Japan, Poland, and Europe and pending in India will have a statutory expiration date in 2023, not taking into account extension under any applicable laws. Patent applications covering methods of using RAD1901 for the treatment of vasomotor symptoms are issued in the United States (US(U.S. Patent No. 8,933,130, statutory term expires June 22, 2027, which we expect will be extended up to October 19, 2031 with 1,580 days of patent term adjustment not taking into account any Hatch-Waxman patent term extensions), Canada and Europe; any issued patents will have a statutory expiration date in 2027. Patent applications covering a dosage form have been filedissued in Europe and are pending in the United States, Europe, Canada and Mexico, and any claims that mightissued or later issue from thesethe pending applications will have a statutory expiration date in 2031.USU.S. Patent No. 8,067,448 (statutory term expires February 19, 2029, which we expect will be extended to September 25, 2029, with 218 days of patent term adjustment due to delays by the USPTO, not taking into account any Hatch Waxman patent term extensions) and U.S. Patent No. 8,268,872 (statutory term expires February 19, 2029 which we expect will be extended to September 25, 2029 with patent term adjustment, subject to a terminal disclaimer of Patent Nos. 8,067,448 and 8,455,525). Related patents have been granted in Australia, Canada, Europe, Japan and Mexico and additional patent applications are pending in Brazil and India. Any patents issued from these filings will have a statutory expiration date in 2029.third partythird-party U.S. or foreign patent rights, or other proprietary rights, will be deemed infringed by the use of our technology. Nor can we predict with certainty which, if any, of these rights of our products in the United States and abroad, and could result in the award of substantial damages. In the event of a claim of infringement, we or our partners may be required to obtain one or more licenses from a third party. There can be no assurance that we can obtain a license on a reasonable basis should we deem it necessary to obtain rights to an alternative technology that meets our needs. The failure to obtain a license may have a material adverse effect on our business, results of operations and financial condition.Lilly's Forteo,Teriparatide, marketed by Lilly under the name Forteo/Forsteo, is the only anabolic drug approved in the United States and Europe for the treatment of osteoporosis. In addition, thereWe are otheraware of companies pursuing development of biosimilar and/or generic versions of teriparatide through various regulatory pathways, including Pfenex, Inc. in the United States; Teva Pharmaceutical Industries, Ltd., currently under EU and U.S. regulatory review; and STADA Arzneimittel AG and Gedeon Richter, approved in the European Union (with launch not expected until expiration of the applicable patents covering teriparatide, or if earlier, invalidation of such patents in connection with currently pending patent litigation and/or challenges).In April 2012,For example, Amgen and UCB and Amgen startedare co-developing romosozumab, a Phase 3 clinical trial program for their anti-sclerostinhumanized monoclonal antibody forthat inhibits the treatmentaction of osteoporosis. We are also aware of at least one biosimilar to Lilly's Forteo,sclerostin, which is currently under review by the EMA. FDA with a PDUFA date of July 19, 2017. PTH(1-34)PTH (1-34) that would compete with abaloparatide-TD.
RAD1901RAD1901Brisdelle.Nordic Bioscience Abaloparatide-SC Phase 3 Clinical Trial—We have entered into agreements with Nordic Bioscience Clinical Development VII A/S, or Nordic, to conduct the ACTIVE trial. On March 29, 2011, we entered into a Clinical Trial Services Agreement, or the Clinical Trial Services Agreement. On the same date, we also entered into Work Statement NB-1, as amended on December 9, 2011, June 18, 2012, March 28, 2014, May 19, 2014, July 22, 2014, August 15, 2014 and March 12, 2015, or Work Statement NB-1, and the Stock Issuance Agreement, as amended and restated on May 16, 2011, and as further amended on February 21, 2013, March 28, 2014, and May 19, 2014, or the Stock Issuance Agreement. Abaloparatide-SC Phase 3 Clinical Extension Study—On February 21, 2013, we entered into the Work Statement NB-3, as amended on February 28, 2014, March 23, 2015, July 8, 2015 and October 21, 2015, or the Work Statement NB-3. Pursuant to the Work Statement NB-3, Nordic performed the ACTIVExtend trial following the completion of the ACTIVExtend, and, upon completion of the ACTIVExtend trial, an additional period of 18 months of standard-of-care osteoporosis management, or the Second Extension. In April 2015, we entered into an amendment to the Work Statement NB-3, or the NB-3 Amendment. The NB-3 Amendment was effective as of March 23, 2015 and provides that Nordic will perform additional services, including monitoring of patients enrolled in the Second Extension. Payments in cash to be made to Nordic under the NB-3 Amendment are denominated in euros and total up to approximately €4.1 million ($4.5 million). Payments in cash to be made to Nordic under the Work Statement NB-3, are denominated in both euros and U.S. dollars and total up to €11.9 million ($12.9 million) and $1.1 million, respectively. In addition, payments are due to Nordic in connection with the Work Statement NB-3 pursuant to the Stock Issuance Agreement, as discussed below. Stock Issuance Agreement—Pursuant to the Stock Issuance Agreement, Nordic agreed to purchase 6,443 shares of our Series A-5 convertible preferred stock, which provided them with the right to receive quarterly stock dividends, payable in shares of our Series A-6 convertible preferred stock, for services rendered under Work Statement NB-1 and Work Statement NB-3. The Stock Issuance Agreement was later amended to provide that in the event an initial public offering of our common stock occurred prior to June 30, 2014, any rights to receive stock dividends in relation to Work Statement NB-1 and Work Statement NB-3, for all periods of time after 2014, would be changed from the right to receive stock to the right to receive a total cash payment of $4.3 million, payable in ten equal monthly installments of $430,000 beginning on March 31, 2015. The amendment also stipulated
3Mthat all consideration to be paid to Nordic pursuant to the Stock Issuance Agreement at any time after the consummation of an initial public offering be payable in cash. As we completed an initial public offering on June 11, 2014, Nordic no longer has the right to receive stock and has been paid in cash for all periods after June 11, 2014.3MDevelopment and Clinical Supplies3M Agreement. In December 2012, we entered into an amendment to the Development and Clinical Supplies3M Agreement in which 3M agreed to develop and manufacture clinical and toxicology supplies for the Phase 3 abaloparatide-TD clinical study. In addition, 3M agreed that it will not use jointly owned intellectual property developed during and resulting from its work with us on abaloparatide-TD in relation to any other PTH or PTHrP analogue or derivative. We hold exclusive worldwide rights to this use of the 3M transdermal technology. We pay 3M for services delivered pursuant to the Development and Clinical Supplies Agreement on a fee-for-service or a fee-for-deliverable basis as specified in the Development and Clinical Supplies Agreement. We have paid 3M approximately $16.7 million, in the aggregate, through December 31, 2015 in respect to services and deliverables delivered pursuant to the Development and Clinical Supplies Agreement.Development and Clinical Supplies Agreement, as amended,agreement provides for services through December 31, 2017, unless it is sooner terminated. Either party may terminate the Development and Clinical Supplies Agreement upon aagreement in the event of an uncured material breach by the other party unless such other party curesparty.alleged breach within the notice periodagreement on a fee-for-service or a fee-for-deliverable basis as specified in the Development and Clinical Supplies Agreement. The Development and Clinical Supplies Agreement contains customary risk allocation clauses withagreement. We have paid 3M indemnifying usapproximately $19.3 million, in respect of third-party claims arising from any personal injury to the extent that such claim results from 3M's breach of warrantyaggregate, through December 31, 2016 with respect to abaloparatide-TD meeting applicable specifications;services and us indemnifyingdeliverables delivered pursuant to the 3M in respect of third-party claims arising from our or our agent's use, testing or clinical studies of abaloparatide-TD. The Development and Clinical Supplies Agreement contains other customary clauses and terms as are common in similar agreements in the industry. on September 12, 2007 and May 11, 2011, or the License Agreement, under which we exclusively licensed certain Ipsen compound technology and related patents covering abaloparatide to research, develop, manufacture and commercialize certain compounds and related products in all countries, except Japan (where we do not hold abaloparatide-SC development and commercialization rights) and France (where our commercialization rights arewere subject to certain co-marketing and co-promotion rights exercisable by Ipsen, provided that certain conditions included in the License Agreement were met). We believe that Ipsen's co-marketing and co-promotion rights in France have been met).permanently expired. Ipsen also granted us an exclusive right and license under the Ipsen compound technology and related patents to make and have made compounds or product in Japan. Ipsen alsofurther granted us an exclusive right and license under certain Ipsen formulation technology and related patents solely for purposes of enabling us to develop, manufacture and commercialize compounds and products covered by the compound technology license in all countries, except Japan (where we do not hold abaloparatide-SC development and commercialization rights) and France (where our commercialization rights are subject to certain co-marketing and co-promotion rights exercisable by Ipsen, provided that certain conditions included in the License Agreement have been met)(as discussed above). With respect to France, if Ipsen exercises its co-marketing andco-promotion rights, then Ipsen may elect to receive a percentage of the net sales of the product by both parties in France (subject to a mid-double digit percentage cap), and Ipsen shall bear a corresponding percentage of the costs and expenses incurred by both parties with respect to such marketing and promotion efforts in France; Ipsen shall also pay us a mid-single digit royalty on Ipsen's allocable portion of net sales of the product by both parties in France. Specifically, we licensed US Patent No. 5,969,095 (statutory term expires March 29, 2016), entitled "Analogs of Parathyroid Hormone," US Patent No. 6,544,949 (statutory term ends March 29, 2016), entitled "Analogs of Parathyroid Hormone," and the corresponding foreign patents and continuing patent applications. European Patent No. 0847278, which was included in the license from Ipsen and claimed the composition of matter of abaloparatide, lapsed due to Ipsen's failure to pay annuities. We are pursuing restoration of those rights. To date, the patent rights in Austria, Belgium, Denmark, Finland, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain, Sweden, and the United Kingdom have been restored. We believe that the data and market exclusivity provided in Europe for a new chemical entity, coupled with the need for a potential competitor to conduct clinical trials, will likely provide a longer barrier to entry than the patent protection provided by the original European patent term, which will expire in 2016. We also have rights to joint intellectual property related to abaloparatide, including rights to the jointly derived intellectual property contained in US Patent No. 7,803,770 (statutory term expires October 3, 2027 which we expect will be extended to March 26, 2028 with 175 days of patent term adjustment due to delays in patent prosecution by the USPTO), US Patent No. 8,148,333 (statutory term expires October 3, 2027 which we expect will be extended to November 8, 2027 with 36 days of patent term adjustment due to delays in patent prosecution by the USPTO) and related patents and patent applications both in the United States and worldwide that cover the method of treating osteoporosis using the ACTIVE trial dosage strength and form. Two corresponding European applications are pending with claims to the intended therapeutic formulation for abaloparatide-SC. Examination has been requested, and substantive examination has commenced for one application and has not yet commenced for the other one. Upon grant, these patents could be validated in any designated contracting or extension states and potentially could be considered for a Supplemental Protection Certificate depending upon the timing of its grant. Related cases granted in Australia, China, Israel, Japan, South Korea, Mexico, New Zealand, Russia, Singapore, and Ukraine, and currently pending in Brazil, Canada, Europe, Hong Kong, India, South Korea, Norway, and Singapore, will have a patent expiration date of 2027. Patent applications which cover various aspects of abaloparatide for microneedle application are pending in the United States, Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, India, Japan, South Korea, Mexico, New Zealand, Russia, Singapore, and Ukraine. Any patents that might issue from these applications will have a statutory expiration date in 2032, not taking into account extension under any applicable laws.$1.0$4.3 million. The license agreement further requires us to make payments upon the achievement of certain future clinical and regulatory milestones. The range ofTotal additional milestone payments that could be paidpayable under the agreement is €10.0are €32.0 million to €36.0 million ($10.9 million to $39.133.6 million). Should abaloparatide be approved and subsequently become commercialized, the agreement provides that we or our sublicensees will be obligated towould pay to Ipsen a fixed five percent royalty based onare obligated towould pay a percentage of certain payments received from such sublicensee (in lieu of milestone payments not achieved at the time of such sublicense). The applicable percentage is in the low double digit range. In addition, if we or our sublicensees commercialize a product that includes a compound discovered by us based on or derivedwill be obligated towould pay to Ipsen a fixed low single digit royalty on net sales of such product on a country-by-country basis until the later of the last to expire of our patents that cover such product or for a period of 10 years after the first commercial sale of such product in such country. The license agreement contains other customary clauses and terms as are common in similar agreements in the industry.country;country, or (2) a period of 10 years after the first commercial sale of the licensed products in such country, unless it is sooner terminated.seeking to havechallenge any Ipsen patent right declared invalid or unenforceable.patent. The License Agreement can also be terminated by Ipsen if we fail to use reasonable commercial efforts to develop the licensed product for sale and commercialization in those countries within the territory where it is commercially reasonable to do so as contemplated by the License Agreement, or fail to use reasonable commercial efforts to perform our obligations under the latest revised version of the development plan approved by the joint steering committee, or fail to use reasonable commercial efforts to launch and sell one licensed product in those countries within the territory where it is commercially reasonable to do so. Either party may also terminate the License Agreement upon aan uncured material breach by the other party unless such other party cures the alleged breach within the notice period specified in the license agreement.party. Ipsen may terminate the License Agreement in the event thatif the License Agreement is assigned or sublicensed, or in the event thatif a third party acquires us, or in the event thatif we acquire control over a PTH or a PTHrP compound that is in clinical development or is commercially available in the territory, and that,if following such assignment, sublicense, acquisition, or acquisition of control by us, such assignee, sublicensee, acquirer or we, fail to meet the timetable under the latest revised version of the development plan approved by the joint steering committee under the License Agreement. Any failure to meet such timetable for purposes of such termination clause is deemed a material breach by us. The License Agreement contains customary risk allocation clauses with each party indemnifying the other in respect of third-party claims arising out of or resulting from: (1) the gross negligence or willful misconduct of such party, its affiliates, licensees, distributors or contractors; (2) any breach by such party of its representations and warranties or any other provision of the License Agreement or any related agreement; (3) the manufacture on behalf of such party of any licensed product or compound; (4) (in the case of Ipsen) the use, development, handling or commercialization of any licensed compound, licensed product or the Ipsen formulation technology by or on behalf of Ipsen or any of its affiliates, licensees, distributors or contractors; and (5) (in our case) the making, use, development, handling or commercialization of any licensed compound or any licensed product by or on our behalf or any of our affiliates, licensees or contractors. The license agreement contains other customary clauses and terms as are common in similar agreements in the industry.USU.S. Patent No. 7,612,114 (statutory term expires December 25, 2023 which we expect will be extended to August 18, 2026 with 967 days of patent term adjustment due to delays by the USPTO), entitled "Selective Estrogen Receptor Modulator," the corresponding foreign patent applications and continuing patent applications. AsIn consideration for the worldwide rights to RAD1901 and in recognition of certain milestones having been met to date, we have paid to Eisai an initial license feeaggregate amount of $0.5$1.9 million. We have also agreed to pay Eisai certainadditional fees in the range of $1.0up to $22.3 million, to $20.0 million (inclusive of the $0.5 million initial license fee), payable upon the achievement of certain clinical and regulatory milestones. In consideration for the rights to RAD1901 in Japan, we paid Eisai an initial license fee of $0.4 million upon execution ofAmendment. The Eisai Amendment also provides for additional payments, payable upon the achievement of certain clinical and regulatory milestones in Japan. Under the license with Eisai,Agreement as amended, by the Eisai Amendment, or the Eisai Agreement, should a product covered by the licensed technology be commercialized, we will be obligated to pay to Eisai royalties in a variable mid-single digit range based on net sales of the product on a country-by-country basis. The royalty rate will be reduced, on a country-by-country basis, at such time as the last remaining valid claim in the licensed patents expires, lapses or is invalidated and the product is not covered by data protection clauses. In addition, the royalty rate will be reduced, on a country-by-country basis, if, in addition to the conditions specified in the previous sentence, lawful generic versions of such product account for more than a specified minimum percentage of the total sales of all products that contain the licensed compound during a calendar quarter. The latest licensed patent is expected to expire, barring any extension thereof, is expected on August 18, 2026.andin addition to royalties in the low single digit range based on net sales of the sublicensee.versionversions of the product account for more than a specified percentage of the total sales of all pharmaceutical products containing the licensed compound in that country;country, or (2) a period of 10 years after the first commercial sale of the licensed products in such country, unless it is sooner terminated. The Eisai Agreement may be terminated by us The can also be terminated by Eisai on a country-by-country basis, at any time prior to the date on which we have submitted for either an NDA approval or EMA marketing approval with respect to a licensed product, upon prior written notice to us, if Eisai makes a good faith determination, in accordance with certain provisions specified in the agreement, that we have not used commercially reasonable efforts to develop the licensed product in the territory having reference to prevailing principles and time scales associated with the development, clinical testing and government approval of products of a like nature to such licensed product, unless such default is cured within the period specified in the Eisai Agreement or if not capable of being cured within such period we commence efforts to cure and make diligent efforts to do so.territory. Either party may also terminate the Eisai Agreementagreement upon aan uncured material breach by the other party unless such other party cures the alleged breach within the notice period specified in the Eisai Agreement. Either party may also terminate the Eisai Agreementor upon the bankruptcy or insolvency of the other party. Eisai may also terminate the Eisai Agreementlicense agreement, with prior written notice, if we are acquired by, or if we transfer all of our pharmaceutical business assets (or an essential part of such assets) or more than 50% of our voting stock to, any third party person or organization, or otherwise come under the control of, such a person or organization,whether resulting from merger, acquisition, consolidation or otherwise in the event thatof certain changes of control involving us, if Eisai reasonably determines that the person or organizationentity assuming control of us is not able to perform under the Eisai Agreementlicense agreement with the same degree of skill and diligence that we would use, such determination being made with reference to the following criteria with respect to the person or organization assuming control of us: (1) whether such person or organization has the financial resources to assume our obligations with respect to development and commercialization of products; (2) whether such person or organization has personnel with skill and experience adequate to assume our obligations with respect to development and commercialization of products at the stage of development and commercialization as of the date of such change; and (3) whether such person or organization expressly assumes all obligations imposed on us by the Eisai Agreement and agrees to dedicate personnel and financial resources to the development and commercialization of the licensed product that are at least as great as those provided by us.use. Eisai shall further have the right to terminate if the acquiring person or organization: (a)entity has any material and active litigations with Eisai;Eisai, or (b) is a hostile takeover bidder against us which has not been approved by our board of directors as constituted immediately prior to such change of control. The Eisai Agreement contains customary risk allocation. We agreed to indemnify Eisai in respect of third-party claims arising out of or resulting from: (1) negligence, recklessness or intentional acts or omissions by us, our affiliatesus.licensees; (2) any breach by us of a representation, warranty or covenant; and (3) any personal injury arising out of the labeling, packaging, package insert, other materials or promotional claims with respect to any licensed product by us, our affiliates, licensees or distributors in the territory. Eisai agreed to indemnify us for (1) negligence, recklessness or intentional acts or omissions by Eisai or its affiliates and licensees and (2) any breach by Eisai of a representation, warranty or covenant. The license agreement contains other customary clauses and terms as are common in similar agreements in the industry.LonzaOctober 2007,June 2016, we entered into a Development and Manufacturing ServicesSupply Agreement with Lonza as amended in May 2011, January 2014 and December 2015,Ypsomed, or the Development and Manufacturing Service Agreement. We and Lonza have entered into a series of Work Orders pursuant to the Development and Manufacturing ServicesYpsomed Supply Agreement, pursuant to which LonzaYpsomed agreed to supply commercial and clinical supplies of a disposable pen injection device customized for subcutaneous injection of abaloparatide, or the Device. We agreed to purchase a minimum number of Devices at prices per Device that decrease with an increase in quantity supplied. In addition, we agreed to make milestone payments for Ypsomed’s capital developments in connection with the initiation of the commercial supply of the Device and to pay a one-time capacity fee. All costs and payments under the Ypsomed Supply Agreement are delineated in Swiss Francs.performed pharmaceutical development and manufacturing servicesan initial term of three years from the earlier of the date of delivery of the first commercial batch of Devices after regulatory approval or June 1, 2017, after which, it automatically renews for our abaloparatide product.two-year terms until terminated. We pay Lonza for services rendered and deliverables delivered pursuant to these work orders on a fee for service basis as specified inor Ypsomed may terminate the applicable work statement. The Development and Manufacturing Services Agreement will expire on March 31, 2016 unless it is sooner terminated, and is subject to renewalagreement at any time by us for successive multiple-year terms withproviding notice to Lonza.Development and Manufacturing Services Agreement or any Work Orderagreement may also be terminated by either party upon a material breach byof the agreement, due to a party’s bankruptcy, insolvency, or dissolution, or due to a change of control of either party under certain circumstances. We may terminate the agreement in the event that Ypsomed is unable to obtain regulatory or other approval for the manufacture and sale of Devices or if such approval is revoked. During the initial term of the agreement, we estimate that we will be obligated to make total minimum payments to Ypsomed of approximately CHF 3.9 million ($4.0 million) in the aggregate, including the milestone payments and one-time capacity fee.with respect totwo years before the Development and Manufacturing Services Agreement unless such other party cures the alleged breach within the notice period specified in the Development and Manufacturing Services Agreement. Either party may also terminate a Work Order if force majeure conditions have prevented performance by the other party for more than a specified period of time with respect to such Work Order. Termination of any Work Order for force majeure shall not result in terminationend of the Development and Manufacturing Services Agreement or any other Work Orders, which shall remain in force until terminated. Either partythen-current term that it does not intend to renew. also terminate the DevelopmentVetter Supply Agreement effective upon written notice to us if we fail to maintain certain insurance required under the agreement, or breach provisions regarding ethical business practices, laws, and Manufacturing Services Agreement upon the bankruptcy or insolvency of the other party.regulations. We may also terminate the Development and Manufacturing Services Agreement or any Work Order with prioragreement effective upon written notice to Lonza for convenience. We may also terminate the Development and Manufacturing Services Agreement or any Work Order if we reasonably determine that Lonza is or will be unable to perform the applicable services in accordance with the agreed upontimeframe and budget set forth in the applicable Work Order, or if LonzaVetter if: (1) Vetter fails to obtain or maintain any material governmental licenses or approvals, required(2) Vetter has breached provisions regarding ethical business practices, laws, and regulations, or (3) we fail to obtain certain regulatory approvals. Either party may terminate the agreement due to: (1) the other party’s bankruptcy or insolvency, (2) the other party’s uncured breach of the agreement, (3) a continuing force majeure event, or (4) a failure to reach mutual agreement on a change in connection with such services. The Development andthe scope of work or services that Vetter reasonably believes it cannot perform because the change is in violation of applicable law.contains customary risk allocation clausesor the Manufacturing Agreement, with each party indemnifying the other in respect of third-party claims arising out of or resulting from: (i) the negligence or willful misconduct of such party, its affiliates and their respective officers, directors, employees and agents in performing its obligations under the Developing and Manufacturing Services Agreement; and (ii) any breach by such party of its representations and warranties under the Development and Manufacturing Services Agreement. We havePPL, as successor-in-interest to Lonza, pursuant to which PPL has agreed to indemnify Lonzamanufacture the commercial and clinical supplies of the API for abaloparatide. We agreed to purchase the API in respectbatches at a price per gram in euros, subject to an annual increase by PPL. We are also required to purchase a minimum number of third-party claims arisingbatches annually. The Manufacturing Agreement has an initial term of six years, after which, it automatically renews for three-year terms unless either party provides notice of non-renewal 24 months before the end of the then-current term.relatingdissolution, or due to the use of our product.RAD1901RAD140 in the United States.•••••••NDA.dosage;dosage, (2) identify possible adverse effects and safety risks;risks, and (3) evaluate preliminarily the efficacy of the drug for specific target indications. Several different doses of the drug may be looked at in Phase 2 to see which dose has the desired effects. Patients are monitored for side effects and for any improvement in their illness, symptoms, or both.or in some cases,and, if not, the agency may issue a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for a specific indication(s). A Complete Response Letter indicates that the review cycle of the application is complete and the application is not ready for approval. Such a letter usually describes all of the deficiencies that the FDA has identified in an NDA that must be satisfactorily addressed before it can be approved. A Complete Response Letter may require additional clinical data and/or an additional pivotal Phase 3 clinical trial(s), and/or other significant, expensive and time-consuming requirements related to clinical trials, preclinical studies or manufacturing. Even if such additional information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. The FDA could also require, as a condition of NDA approval, post-marketing testing and surveillance to monitor the drug's safety or efficacy, or impose other conditions. Approval may also be contingent on a Risk Evaluation and Mitigation Strategy, or REMS, that limits the labeling, distribution or promotion of a drug product. The FDA also may condition approval on, among other things, changes to proposed labeling, development of adequate controls and specifications, or a commitment to conduct one or more post-marketing studies or clinical trials. Once issued, the FDA may withdraw product approval if ongoing regulatory requirements are not met or if safety problems occur after the product reaches the market.to:to, among other requirements: (1) report certain adverse reactions to the FDA within specific time frames, (2) comply with certain requirements concerning advertising and promotional labeling for their products, and (3) continue to have quality control and manufacturing procedures conform to cGMP regulations after approval.approval, (4) make periodic reports to FDA about the approved product, and (5) comply with requirements regarding distribution of the drug product. The FDA periodically inspects the sponsor'smarket.—Under-Under the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act, Congress created an abbreviated FDA review process for generic versions of pioneer (brand name) drug products.products under section 505(j) of the FDCA. Section 505(j) provides for approval of an abbreviated new drug application, or Abbreviated New Drug Application, or ANDA applicant demonstrate, among other things, that the proposed generic drug product's active ingredient is the same as that of the reference product, that the proposed generic is bioequivalent to the reference product, that any impurities in the proposed product do not affect the product's safety or effectiveness, and that its manufacturing processes and methods ensure the consistent potency and purity of its proposed product.duringor active moiety for five years after initial approval of the five-year period.NCE. A drug is a NCE if the FDA has not previously approved an NDA for another drug that contains the same active moiety, which FDA defines to mean the molecule or ion (excluding certain specified appended portions) responsible for the physiological or pharmacological action of the drug substance. We believe abaloparatide qualifies as an NCE and thus expect to be eligible for five years of dataNCE exclusivity following any FDA approval of abaloparatide-SC.usesversions or conditions of use of previously approved drug products, such as new indications, delivery mechanisms, dosage forms, strengths, or other conditions of use. For example, if abaloparatide-SC is approved for commercialization and we are successful in performing a clinical trial of abaloparatide-TD that provides a new basis for approval (a different delivery mechanism), and that FDA considers essential to approval of the drug, it is possible that we may become eligible for a three yearthree-year period of market exclusivity which protectsfor approval of an NDA for abaloparatide-TD. Any such three-year exclusivity period would protect against the approval (but not the filing) of ANDAs and 505(b)(2) applications referencing abaloparatide-TD for the protected use but willtransdermal route of administration. Such exclusivity period for abaloparatide-TD would generally not, however, prohibit the FDA from accepting or approving ANDAs or 505(b)(2) applications referencing only abaloparatide-SC or 505(b)(2) applications that reference abaloparatide-TD but that seek approval for a different route of administration or for a use other products containingthan for the same active ingredient.providesubmit certain information about patents related to the drugtheir drugs for listing in the FDA's list of Approved Drug Products with Therapeutic Equivalence Evaluations (commonly known as the Orange Book). ANDA and 505(b)(2) applicants generally must then certifysubmit a certification or statement regarding each of the patents listed with the FDA for the reference product. A certification that a listed patent is invalid and/or will not be infringed by the marketing of the ANDA or 505(b)(2) applicant's product is called a "Paragraph IV certification." If the sponsor of an ANDA or 505(b)(2) applicantapplication that references a drug with unexpired exclusivity provides such a notification ofParagraph IV certification for a patent invalidity or non-infringement,for a reference product that is protected by NCE exclusivity, then the FDA may accept the ANDA or 505(b)(2) application beginning four years after approval of the NDA.reference product's NDA (rather than five years). If an ANDA or 505(b)(2) application containing a Paragraph IV certification is submitted to the FDA and accepted as a reviewable filing by the Agency, the ANDA or 505(b)(2) applicant then must provide, within 20 days of FDA acceptance, notice to the NDA holder and patent owner stating that the application has been submitted and providing the factual and legal basis for the applicant's opinion that the patent is invalid and/or not infringed. The NDA holder or patent owner then may file suit against the ANDA or 505(b)(2) applicant for patent infringement. If this is done within 45 days of receiving notice of the Paragraph IV certification, a one-time 30-month stay of the FDA's ability to approve the ANDA or 505(b)(2) application is triggered. The 30-month stay begins at the end of the NDA holder's data exclusivity period, or, if data exclusivity has expired, on the date that the patent holder is notifiedof receipt of the submissionParagraph IV notice and, in the case where an ANDA or 505(b)(2) application is submitted before a reference product's NCE exclusivity expires (i.e., four years after approval of the ANDA. Thereference product), the 30-month period is extended to ensure that approval of the ANDA or 505(b)(2) application cannot be granted for 7 1/2 years after initial approval of the reference product. Nevertheless, the FDA may approve the proposed product before the expiration of the 30-month stay (or 7 1/2 year period) if a court finds the patent invalid and/or not infringed or if the court shortens the period because the parties have failed to cooperate in expediting the litigation.EU,European Union, medicinal products are authorized following a similar demanding process as that required in the United States and applications are based on the ICH Common Technical Document. In the European Economic Area, or EEA (comprised of 28 EU Member States plus Iceland, Liechtenstein and Norway), medicines can be authorized by using either the centralized authorization procedure or national authorization procedures.followinga single marketing authorization application is submitted to the opinionEMA. The CHMP then carries out a scientific assessment of the EMA's Committee for Medicinal Products for Human Use (CHMP),application and issues an opinion as to whether or not the medicine should be marketed. Following the issuance of the CHMP's opinion, the European Commission issuesdecides whether or not to grant a single marketing authorization valid across the EEA. The centralized procedure is compulsory for human medicines derived from certain biotechnology processes, advanced therapy medicinal products (such as gene therapy, somatic cell therapy and tissue engineered products), medicines containing a new active substance and which are indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, or neurodegenerative disorders, diabetes, autoimmune diseases and other immune dysfunctions, viral diseases, and officially designated orphan medicines. ForThe centralized procedure is optional for applicants seeking marketing authorizations for medicines that do not fall within these categories, an applicant has the option of submitting an application for a centralized marketing authorization to the EMA, as long as the medicine concerned containswhich contain a new active substance not yet authorized in the EEA, isor constitute a significant therapeutic, scientific or technical innovation, or if its authorization via the centralized procedure would be in the interest of public health in the EEA. In November 2015, we submitted an MAA for abaloparatide-SC to the EMA under the centralized procedure. The MAA was validated in December 2015 and is currently undergoing regulatory review by the EMA.•any EU countrythe European Union and that does not fall within the mandatory scope of the centralized procedure. The applicant selects on of the countries in which it is seeking a marketing authorization to act as a Reference Member State.• In lightfactproduct to the other countries where national authorizations are being sought (Concerned Members States). The Concerned Member States review the evaluation and reach consensus as to whether or not to approve the application. If the application is successful, then each country issues its own national authorization for the product.developed their own, often varying, approaches.approaches and policies such a price control, profit control, international price comparisons, and reference pricing. In many EU Member States, pricing negotiations must take place between the holder of the marketing authorization and the competent national authorities before the cost of the product is sold in their market with thecan be funded within national healthcare schemes. The holder of the marketing authorization is usually required, in order to get support for reimbursement under national health schemes and, therefore, practical access to the market, to provide evidence demonstrating the pharmaco-economic superioritycost-effectiveness or otherwise added value benefit of its product in comparison with directly and indirectly competing products. products, a procedure commonly referred to as a health technology assessment.EU.products priorproducts. Prior to approvingthe CHMP adopting an opinion with respect to approvability of an application for marketing authorization, the EMA, acting upon the advice of the CHMP, may decide to coordinate an inspection of the proposed manufacturing site in order to verify the manufacturer's compliance with EU GMP principles and guidelines or to investigate a product.specific matter arising from the assessment of the application. If after receiving clearance from regulatory agencies, a company makesthere is a material change in manufacturing equipment,required.required from the relevant competent regulatory authority. Once we or our partners commercialize products, we will be required to comply with cGMP,regulationsrequirements as set out in the terms of the marketing authorization enforced by, the European Commission, the EMA and the competent authorities of EU Member States following product approval.the grant of marketing authorization. Also like the FDA, the EMA, the competent authorities of the EU Member States and other regulatory agencies also conduct regular, periodic visits to re-inspect equipment, facilities, and processes following the initial approval of a product. If as a result of these inspections, it is determined that our or our partners'the equipment, facilities, or processes used to manufacture our product do not comply with applicable regulations and conditions of product approval, regulatory agencies may seek civil, criminal or administrative sanctions, or enforcement actions and/or remedies against us, including the suspension of our manufacturing operations or the withdrawal of our product from the market.EU.European Union. Abridged applications for the authorization of generic versions of drugs authorized by EMA can be submitted to the EMA through the centralized procedure referencing the innovator's data and demonstrating bioequivalence to the reference product, among other things.therapies; this system is usually referred to as "8+2+1".therapies. We expect to be eligible for at least ten years of exclusivity (8 years of data exclusivity + 2 years of market exclusivity) following any approval of abaloparatide-SC. At this time, we do not believe that there are orphan or pediatric applications for abaloparatide that would be likely to result in a grant of exclusivity or supplemental protection certificate in the EU.United StatesU.S. or EU trials may be submitted in support of a marketing authorization application, additional clinical trials conducted in the host territory, or studying people of the ethnicity of the host territory, may be required prior to the filing or approval of marketing applications within the country.OnIn January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or the ATRA, was enacted, which among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers. There is no legislation at the EU level governing theEU. AsEuropean Union are based upon national rules subject to the control of the Transparency Directive, which aims to ensure the transparency of measures established by EU countries to control the pricing and reimbursement of medicinal products. It defines a result,series of procedural requirements designed to verify that national pricing and reimbursement decisions do not create obstacles to the pharmaceutical trade within the EU's Internal Market. The competent authorities of each of the 28 EU Member States have adopted individual strategies regulating the pricing and reimbursement of medicinal products in their territory. These strategies often vary widely in nature, scope and application. However, a major element that they have in common is an increased move towardstoward reduction in the reimbursement price of medicinal products, a reduction in the number and type of products selected for reimbursement, and an increased preference for generic products over innovative products. These efforts have mostly been executed through these countries' existing price-control methodologies, including price cuts, mandatory rebates, value-based pricing, and reference pricing (i.e., referencing prices in other countries and using those reference prices to set a price). The government of the UK announced the phase-out of its established Pharmaceutical Pricing Reimbursement Scheme approach in January 2014 and the adoption of a new value-based pricing approach, at least for new product introductions. Under this approach, in a complete departure from established methodologies, reimbursement levels of each drug will be explicitly based on an assessment of value, looking at the benefits for the patient, unmet need, therapeutic innovation, and benefit to society as a whole. It is increasingly common in many EU Member States for Marketing Authorization Holders to be required, in order to get support for reimbursement under national health schemes and, therefore, access to the market, to demonstrate the pharmaco-economic superioritycost effectiveness or otherwise added value benefit of their products as compared to products already subject to pricing and reimbursement in specific countries. In order for drugs to be evaluated positively under such criteria, pharmaceutical companies may need to re-examine, and considerAGenerally, a company can make only those claims relating to safety and efficacy that are approved by the FDA following review and approval of an NDA. Physicians may prescribe legally available drugs for uses that are not described in the drug's labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties, and often reflect a physician's belief that the off-label use is the best treatment for the patients. The FDA does not regulate the behavior of physicians in their choice of treatments, but FDA regulations and enforcement policies do imposeFailureIn addition, the FDA also regulates communications about investigational drugs, including with respect to the pre-approval promotion of investigational drugs. Recent case law suggests that pharmaceutical companies may have a First Amendment right to provide truthful and non-misleading information about off-label uses of their products to physicians and others, but the scope of this right remains unclear. Accordingly, failure to comply with applicable FDA requirements may subject a company to adverse publicity, enforcement action by the FDA, corrective advertising, consent decrees and the full range of civil and criminal penalties available to the FDA.2015,2016, we employed 73229 full-time employees and 28 part-time employees, 2039 of whom held Ph.D. or M.D. degrees. Forty-eight107 of our employees were engaged in research and development activities and 27130 were engaged in support administration, including business development and finance. Of our 130 employees engaged in support administration, 69 are part of the organization we are building to support the potential commercialization of abaloparatide-SC in the United States. We use and intend to usecontinue using CROs and other third parties to perform our clinical studies and manufacturing.merger transaction,Merger, the Former Operating Company was merged with and into us and we assumed the business of the Former Operating Company and changed our name to Radius Health, Inc.TableContentsLegal Proceedingsnot currently involved in any material legalseeking dismissal of Ipsen’s claims, as well as declaratory relief, compliance with the License Agreement, and other damages, costs and fees to be determined by the Arbitral Tribunal.$62.5 million, and $60.7$62.5 million for the years ended December 31, 2016, 2015, 2014, and 2013,2014, respectively. As of December 31, 2015,2016, we had an accumulated deficit of $445.8$628.6 million. Until we succeed in developing and then commercializing one or more of our product candidates, we expect to incur substantial losses and may never achieve or maintain profitability. We also expect to continue to incur significant operating and capital expenditures and anticipate that our expenses will increase substantially as we:•••implement additionalinfrastructure;hiring additional personnel; and•hire additional personnel.maygenerate revenues and become profitable, we expect that we will need to raise additional capital, which may not be available on favorable terms, if at all, in order to continue operating our business.ishave been approved by the FDA or foreign regulatory authorities for sale. In March 2016, we submitted an NDA to the FDA for abaloparatide-SC, which was accepted for filing by the FDA with a PDUFA date of March 30, 2017. Even if our NDA for abaloparatide-SC is approved, we would still expect to incur significant expenses and net losses as we begin our first commercialization efforts for abaloparatide-SC and continue development and commercialization efforts for our other product candidates. Therefore, for the foreseeable future, we will have to fund our operations and capital expenditures with our existing cash and cash equivalents and short and long-term marketable securities, or through strategic financing opportunities, that could include, but are not limited to partnering or other collaboration agreements, future offerings of our equity, royalty-based financing arrangements, and/or the incurrence of debt.2015,2016, we believe that, prior to the consideration of revenue from the potential future sales of any of our investigational products that may receive regulatory approval, we have sufficient capital to fund our development plans, U.S. commercial scale-up and other operational activities into 2018. We have based this estimate on assumptions that may prove to be wrong, and we could use up our available capital resources sooner than we currently expect. If we fail to obtain additional capital, we may be unable to complete our planned preclinical and clinical trials and obtain approval of any product candidates from the FDA and foreign regulatory authorities. In addition, we could be forced tostudies.•continuing to undertake preclinical development and clinical trials;•participating in regulatory approval processes;•formulating and manufacturing products; and•approved.OurParticularly over the near term as we continue to build our commercial capabilities and, if approved, commercialize abaloparatide-SC, our revenues, if any, may fluctuate from quarter to quarter and our future quarterly and annual expenses as a percentage of our revenues may be significantly different from those we have recorded ina new drug application, oran NDA from the FDA, or in any foreign countries unless and until we receive the requisite approval from regulatory authorities in those foreign countries. In addition, the approval of abaloparatide-TD as a potential line extension to abaloparatide-SC is dependent on the earlier approval of abaloparatide-SC. Obtaining approval of a product candidate is an extensive, lengthy, expensive and uncertain process, and any approval of abaloparatide-SC may be delayed, limited or denied for many reasons, including:•postmenopausal women with postmenopausal osteoporosis to the satisfaction of the FDA or foreign regulatory authorities;••••••the FDA or foreign regulatory authorities may not accept data generated at our clinical study sites;••••monthsmonths' extension of the abaloparatide 80 µg and placebo groups in our Phase 3 study, which groups received an approved alendronate (generic Fosamax) therapy for osteoporosis management. The NDA that we plan to submitsubmitted to the FDA in March 2016 for abaloparatide-SC as a proposed treatment for osteoporosis will includeincluded the 24-month fracture data. We cannot be certain that the FDA will be supportive of this plan, will not change this approval policy again or will not adopt other approval policies or regulations that adversely affect any NDA that we may submit, the occurrence of any of which may further delay FDA approval.•••We expect thatIn March 2016, we submitted our NDA would be submittedfor abaloparatide-SC to the CenterFDA which was accepted for Drug Evaluation and Research and be reviewed with support fromfiling by the FDA Officewith a PDUFA date of Combination Products and the FDA Center for Devices and Radiological Health for the device aspects of the abaloparatide-SC product candidate.March 30, 2017. In addition, there are device-related manufacturing and other regulatory requirements (e.g., current good manufacturing practices, or cGMPs, and adverse event reporting) to which we may be subject by virtue of the product's status as a drug/device combination product. As a result of these factors, we may experience delays in the product development and regulatory review and approval process in seeking a drug/device combination product approval under an NDA.if we comply with all FDA requests,though our NDA for abaloparatide-SC was accepted for filing, the FDA may ultimately reject oneretains complete discretion in deciding whether or morenot to approve an NDA and there is no guarantee that abaloparatide-SC will be approved for the treatment of our NDAs.women with postmenopausal osteoporosis or any other indication. We may never obtain regulatory approval for abaloparatide-SC, or any of our other product candidates. Failure to obtain FDA approval of abaloparatide-SC, or any of our other product candidates will severely undermine our business by leaving us without a saleable product, and therefore without any source of revenues, until another product candidate can be developed. There is no guarantee that we will ever be able to develop or acquire any other product candidate.••••••••ourthese other product candidates or whether any such NDA or equivalent application would be accepted for filing by the FDA or foreign regulatory authorities or approved if filed.•••••continualcontinuing requirements of and review by the FDA and foreign regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, and requirements regarding the distribution of drug products, including drug samples to physicians and recordkeeping. Even if we obtain marketing approval of a product candidate, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety and/or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs••••••••••••••••••••out of pocketout-of-pocket obligations of member patients for such products. In addition, particularly in the United States and increasingly in other countries, we may be required to provide discounts and pay rebates to state and federal governments and agencies in connection with purchases of our products that are reimbursed by such entities. It is possible that future legislation in the United States and other jurisdictions could be enacted which could potentially impact the reimbursement rates for the products we are developing and may develop in the future and also could further impact the levels of discounts and rebates paid to federal and state government entities. Any legislation that impacts these areas, including the ongoing consideration of the repeal and replacement of the ACA and other legislation focused on drug pricing, could impact, in a significant way, our ability to generate revenues from sales of products that, if successfully developed, we bring to market. There is no legislation atlevel governingcountries to control the pricing and reimbursement of medicinal products inproducts. The Transparency Directive defines a series of procedural requirements designed to verify that national pricing and reimbursement decisions do not create obstacles to the EU. As a result,pharmaceutical trade within the EU's Internal Market. The competent authorities of each of the 28 EU Member States have adopted individual strategiespolicies and rules regulating the pricing and reimbursement of medicinal products in their territory. These strategies often vary widely in nature, scope and application. However, a major element that they have in common is an increased move towardstoward reduction in the reimbursement price of medicinal products, a reduction in the number and type of products selected for reimbursement, and an increased preference for generic products over innovative products. These efforts have mostly been executed through these countries' existing price control methodologies. These efforts have mostly been executed through these countries' existing price-control methodologies, including price cuts, mandatory rebates, value-based pricing, and reference pricing (i.e., referencing prices in other countries and using those reference prices to set a price). The government of the UK announced the phase out of its established Pharmaceutical Pricing Reimbursement Scheme approach in January 2014 and the adoption of a new value based pricing approach, at least for new product introductions. Under this approach, in a complete departure from established methodologies, reimbursement levels of each drug will be explicitly based on an assessment of value, looking at the benefits for the patient, unmet need, therapeutic innovation, and benefit to society as a whole. It is increasingly common in many EU Member States for Marketing Authorization Holders to be required, in order to get support for reimbursement under national health schemes and, therefore, practical access to the market to demonstrate the pharmaco economic superioritycost-effectiveness or added value benefit of their products as compared to products already subject to pricing and reimbursement in specific countries. In order for drugs to be evaluated positively under such criteria, pharmaceutical companies may need to re-examine, and consider altering, a number of traditional functions relating to the selection, study, and management of drugs, whether currently marketed, under development, or being evaluated as candidates for research and/or development.third partythird-party researchers, investigators and collaborators are required to comply with good clinical practice, or GCP, requirements, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area, or EEA, and comparable foreign regulatory authorities for all of our products in clinical development. Regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, EMA or other comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials complies with GCP regulations. In addition, our clinical trials must be conducted with product produced under cGMP regulations. Our failure to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process. In addition, these third parties may not assign as great a priority to our programs or pursue them as diligently as we would if we were undertaking such programs ourselves. If outside collaborators fail to devote sufficient time and resources to our drug-development programs, or if their performance is substandard, the approval of our FDA or foreign regulatory authority applications, if any, and our introduction of new drugs, if any, will be delayed. These collaborators may also have relationships with other commercialof abaloparatide-SC are beingwhich we refer to as the ACTIVExtend clinical trials, have been managed by Nordic Bioscience Clinical Development VII A/S, or Nordic, at certain clinical sites operated by the Center for Clinical and Basic Research, or CCBR, a leading global CRO with extensive experience in global osteoporosis registration studies. Nordic controls, and holds an ownership interest in,after we submitfollowing our NDA.regulatory approval submissions. We do not control the facilities or manufacturing process, and are completely dependent on, our contract manufacturing partners for compliance with cGMPs for manufacture of both active drug substances and finished drug products. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or other regulatory authorities, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or a comparableLonza Group Ltd., or Lonza,PPL, which produceshas agreed to produce supplies of bulk drug product of abaloparatide API to support the abaloparatide-SC and abaloparatide-TD clinical studies and any potential commercial launch. We also depend on Vetter Pharma Fertigung GmbH & Co, or Vetter, and Ypsomed AG, or Ypsomed for the production of finished drug product clinical and commercial supplies of abaloparatide-SC and we depend on 3M Co. and 3M Innovative Properties Co., or, together 3M, for the production of abaloparatide-TD. Because of our dependence on Vetter for the "fill and finish" part of the manufacturing process for abaloparatide-SC, we are subject to the risk that Vetter may not have the capacity from time to time to produce sufficient quantities of abaloparatide to meet the needs of our clinical studies or be able to scale to commercial production of abaloparatide. While we are currently in discussions, to date, we have not entered into a long-term agreement with any of Lonza, Vetter or Ypsomed, each of whom currently produces abaloparatide or related components on a purchase order basis for us. Accordingly, Lonza, Vetter and Ypsomed could terminate their relationship with us at any time and for any reason. We may not be able to negotiate long-term agreements on acceptable terms, or at all. If our relationship with any of these contract manufacturers is terminated, or if they are unable to produce abaloparatide or related components in required quantities, on a timely basis or at all, and/or if we are forced to accept unfavorablein compliance with the terms forof our future relationship,agreements, ourwereare not able to negotiate commercial supply terms with 3M, as we depend on 3M for production of abaloparatide-TD, we would be unable to commercialize this product if it were to be approved. Or, if we are forced to accept unfavorable terms for our future relationship with 3M, our business and financial condition would be materially harmed. If abaloparatide-SC or any of our other current product candidates or any product candidates we may develop or acquire in the future receive FDA or foreign regulatory authority approval, we will rely on one or more third-party contractors to manufacture our drugs or related components. Our anticipated future reliance on a limited number of third-party manufacturers exposes us to the following risks:•••••have limitedare building our commercial and medical affairs capabilities and no sales capabilities and we have no experience commercializing a pharmaceutical product. We intend to build an internal sales force to market and sell our products to specialists within the target indications, if approved, and also to pursue collaborative arrangements to market and sell our products more broadly within the target indications if approved. Therefore, our future success depends, in part, on our ability to enter into and maintain collaborative relationships for such capabilities, the collaborators' strategic interest in the products under development and such collaborators' ability to successfully market and sell any such products.•••••osteoporosis inwomen with postmenopausal women.osteoporosis. In March 2016, we submitted an NDA for the FDA for abaloparatide-SC, which has been accepted for filing by the FDA with a PDUFA date of March 30, 2017. We expect to compete against well-known treatment options, including Lilly's Forteo.teriparatide, marketed by Lilly as Forteo/Forsteo. We may also face competition from generic or biosimilar versions teriparatide. For example, a biosimilar version of teriparatide was recently approved in the European Union, although the product is not expected to be launched until the expiration or invalidation of applicable patents covering teriparatide. We are also aware of other companies pursuing development of biosimilar and/or generic versions of teriparatide in the U.S. and EU through various regulatory pathways. The availability of a generic or biosimilar teriparatide on the market would likely exert pricing pressure on theIn April 2012,For example, UCB and Amgen started a Phase 3 clinical trial program for theirare co-developing an anti-sclerostin anabolic monoclonal antibody for the treatment of osteoporosis. In addition, thereosteoporosis, which is at least one biosimilar to Lilly's Forteocurrently under review by the EMA which, if approved, could exert pricing pressure on the anabolic class in which abaloparatide-SC would compete.FDA with a PDUFA date of July 19, 2017. In order to compete successfully in this market, we will have to demonstrate to physicianphysicians and payors that the treatment of osteoporosis with abaloparatide-SC is worthwhile and is a better alternative to existing or new therapies.•••••(US(U.S. Patent No. 5,969,095) and several additional countries. Because the abaloparatide composition of matter patent was filed in 1996, it is expected to have an expirationexpired in 2016 in the United States, and additional countries where it hashad issued. Prior to its expiration, European Patent No. 0847278, which was included in the license from Ipsen Pharma SAS, or Ipsen and claimed the composition of matter of abaloparatide, lapsed due to Ipsen's failure to pay annuities. We are pursuingPrior to expiration, we pursued restoration of those patent rights. To date, the patent rights in Austria, Belgium, Denmark, Finland, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain, Sweden and the United Kingdom have been restored.various countries. As a result of the lapse and expiration of patent rights, we believe that some of Ipsen's rights under our license agreement with Ipsen have terminated. We are currently involved in discussionsa pending arbitration proceeding with Ipsen regarding these Ipsen rights and related terms of our license agreement. If we fail to reach agreement, we or Ipsen may determine to pursue available remedies, including formal dispute resolution. We believe that the data and market exclusivity provided in Europe for a new chemical entity, coupled with the need for a potential competitor to conduct clinical trials, will likely provide a longer barrier to entry than the patent protection provided by the original European patent term, which will expire in 2016.USU.S. Patent No. 7,803,770 that we believe provides exclusivity until October 3, 2027 and may be extended to March 26, 2028 in the United States (not including any Hatch-Waxman patent term extension) for the method of treating osteoporosis with the intended therapeutic dose for abaloparatide-SC.USU.S. Patent No. 8,148,333 that we believe provides exclusivity until 2027 in the United States (not including any Hatch-Waxman patent term extension) for the intended therapeutic formulation for abaloparatide-SC.(US(U.S. Patent No. 8,933,130) for treating vasomotor disturbances or hot flashes on January 13, 2015 (statutory term expires on June 22, 2027, and may be extended to October 19, 2031 with 1,580 days of patent term adjustment due to delays in patent prosecution by the USPTO).filed.issued in Canada, Europe, and Mexico, and are pending in Canada and Europe. Pending patent applications in the United States and elsewhere may not issue since the interpretation of the legal requirements of patentability in view of any claimed invention before a patent office are not always predictable. As a result, we could encounter challenges or difficulties in building, maintaining and/or defending our intellectual property both in the United States and abroad.provisionalpatent issued in the United States claiming the use of RAD1901 for an indication we are not pursuing and a patent application filed with the USPTO that could be relevant to the use of RAD1901 to treat indications for which we are developing RAD1901. If a patent issues from this patent application with claims covering the use of RAD1901 to treat indications for which we are developing RAD1901, we may need to license the patent in order to commercialize RAD1901 specifically for the treatment of such indications even if RAD1901 were successfully developed and approved. We cannot assure you that we will be able to secure a license on reasonable terms, if at all. If we need a license of such patent in order to commercialize RAD1901 and are unable to secure one on reasonable terms, our business would be materially harmed. and/or Ipsen to comply with any required fee payment, documentary and/or procedural requirements as they might relate to any patents we have licensed from them. Failure to meet a required fee payment, document production or procedural requirement can result in the abandonment of a pending patent application or the lapse of an issued patent. In some instances, the defect can be cured through late compliance but there are situations where the failure to meet the required event cannot be cured. Any failures could compromise the intellectual property protection around our preclinical or clinical candidates and possibly weaken or eliminate our ability to protect our eventual market share for that product.•••••• President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or ACA.ACA, was enacted. ACA includes a number of provisions affecting the pharmaceutical industry, including annual, non-deductible fees on any entity that manufactures or imports some types of branded prescription drugs and biologics and increases in Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program. In addition, among other things, ACA also establishes a new Patient-Centered Outcomes Research Institute to oversee, identify priorities and conduct comparative clinical effectiveness research. In addition, other legislative changes have been proposed and adopted since ACA was enacted, which also may impact our business. On August 2, 2011, the President signed into law the Budget Control Act of 2011, or BCA, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint Select Committee did not achieve its targeted deficit reduction of at least $1.2 trillion for the years 2013OnIn January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or ATRA, was enacted, which among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers. The full impact on our business of these new laws is uncertain. We cannot predict whether other legislative changes will be adopted, if any, or how such changes would affect the pharmaceutical industry generally or our business in particular.••••••As we advance our product candidates through the development process, weWe are expanding, and will needcontinue to expand, our development, regulatory, manufacturing, quality, distribution, sales and marketing capabilities or contract with other organizations to provide these capabilities for us.capabilities. As our operations expand,part of this expansion, we expect that we will need to manage additional relationships with various collaborators, suppliers and other organizations. Our ability to manage our operations and growth requires us to continue to improve our operational, financial and management controls, reporting systems and procedures. For example, some jurisdictions, such as the District of Columbia, have imposed licensing requirements for sales representatives. In addition, the District of Columbia and the Commonwealth of Massachusetts, as well as the federal government by way of the Sunshine Act, have established reporting requirements that would require public reporting of compensation and other "transfers of value" paid to health care professionals and teaching hospitals, as well as ownership and investment interests held by such professionals and their immediate family members. Because the reporting requirements vary in each jurisdiction, compliance will be complex and expensive and may create barriers to entering the commercialization phase. The need to build new systems as part of our growth could place a strain on our administrative and operational infrastructure. We may not be able to make improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls. Such requirements may also impact our opportunities to collaborate with physicians at academic research centers as new restrictions on academic-industry relationships are put in place. In the past, collaborations between academia and industry have led to important new innovations, but the new laws may have an effect on these activities. While we cannot predict whether any legislative or regulatory changes will have negative or positive effects, they could have a material adverse effect on our business, financial condition and potential profitability.••••••••••••••••••7.46.2 million shares of our common stock as of December 31, 2015.2016. These stockholders, acting together, have the ability to significantly influence the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these stockholders, acting together, have the ability to significantly influence the management and affairs of our company. Accordingly, this concentration of ownership might harm the market price of our common stock by:•••6,159,5109,860,000 shares of our common stock for issuance under our equity incentive plans as of December 31, 2015,2016, which includes 4,408,3692,960,000 shares of common stock issuable upon the exercise of options outstanding as of December 31, 2015, and2016, 25,000 shares of common stock issuable upon the vesting of performance stock units, and approximately 57,000 restricted stock units, each of which will become eligible for sale in the public market in the future, subject to certain legal and contractual limitations. In addition, as of December 31, 2015,2016, warrants to purchase 631,5880 shares of our common stock were outstanding. Pursuant to our employee stock purchase plan, eligible employees may participate in an employee stock purchase plan sponsored by us. The current plan allows for the issuance of 1,290,954 shares of common stock to eligible employees. As of December 31, 2016, there were 1,290,594 shares available for future sale to employees under this plan. Shares of our common stock issued upon exercise of these warrants may be sold in the public market, subject to prior registration or under an exemption from registration.•••••••2015,2016, we had $419.5$526.7 million of federal and $323.0$385.3 million of state net operating loss carryforwards available to offset future taxable income. Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, if a corporation undergoes an "ownership change" (generally defined as a greater than 50% change (by value) in its equity ownership over a three year period), the corporation's ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. We are in the process of conducting a detailed analysishave completed studies through December 31, 2015, to determine whether anany ownership change has occurred since our formation and have determined that transactions have resulted in two ownership changes, as defined under Section 382382. There could be additional ownership changes in the future that could further limit the amount of the Code has previously occurred. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss and tax credit carryforwards to offset U.S. federal taxable income may become subject to limitations, which could potentially result in increased future tax liability to us.that we can utilize.20152016, are provided below:Location Function LocationFunctionSize (approximate Corporate Headquarters 27,640 24,880 Leased Pasippany,Parsippany, NJ, USAOffice space 10,530 10,530 Leased Laboratory and office space 4,600 4,600Subleased Wayne, PA, USA Office space 14,000 Subleased not currently involved in any material legalseeking dismissal of Ipsen’s claims, as well as declaratory relief, compliance with the License Agreement, and other damages, costs and fees to be determined by the Arbitral Tribunal. 2016 High Low Quarter Ended March 31, 2016 $ 62.61 $ 24.75 Quarter Ended June 30, 2016 40.91 29.27 Quarter Ended September 30, 2016 59.88 36.45 Quarter Ended December 31, 2016 55.97 24.75 2015 High Low Quarter Ended March 31, 2015 $ 51.22 $ 35.02 Quarter Ended June 30, 2015 69.16 34.76 Quarter Ended September 30, 2015 84.64 52.50 Quarter Ended December 31, 2015 77.10 45.89 High Low $ 14.60 $ 7.46 24.28 8.09 42.57 16.55 19, 2016,17, 2017, the closing price of our common stock was $27.00$45.65 per share as reported on The NASDAQ Global Market.2015,2016, with the cumulative total return of (a) the Nasdaq Biotechnology Index and (b) the Nasdaq Composite Index, over the same period. This graph assumes the investment of $100 on June 6, 2014 in our common stock, the Nasdaq Biotechnology Index and the Nasdaq Composite Index and assumes the reinvestment of dividends, if any. The graph assumes our closing sales price on June 6, 2014 of $8.01 per share as the initial value of our common stock and not the initial offering price to the public of $8.00 per share.

$100 $100 invested on June 6, 2014 in stock or index19, 2016,17, 2017, there were 6934 holders of record of our common stock. The actual number of stockholders is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.2015.2015.2016.2013, 2014, 2015 and 20152016 and the balance sheetssheet data as of December 31, 20142015 and 20152016 from the audited consolidated financial statements contained in Item 8 of Part II of this Form 10-K. The selected balance sheet data as of December 31, 2011, 2012, 2013 and 20132014 and the statement of operations data for the years ended December 31, 20112012 and 20122013 has been derived from the audited financial statements for such years not included in this Form 10-K.20112012 has been recast to reflect the adoption of Accounting Standards Update No. 2011-05,Presentation of Comprehensive Income. Year Ended December 31,
Data 2015 2014 2013 2012 2011 (in thousands) $ 68,280 $ 45,719 $ 60,536 $ 54,961 $ 36,179 30,797 13,674 6,829 9,469 5,330 (99,077 ) (59,393 ) (67,365 ) (64,430 ) (41,509 ) (1,607 ) (713 ) 9,085 (2,095 ) (236 ) (842 ) (2,373 ) (2,410 ) (2,603 ) (731 ) (101,526 ) (62,479 ) (60,690 ) (69,128 ) (42,476 ) 26 (21 ) — (5 ) 8 $ (101,500 ) $ (62,500 ) $ (60,690 ) $ (69,133 ) $ (42,468 ) $ (101,526 ) $ (71,479 ) $ (78,161 ) $ (83,120 ) $ 113 $ (2.56 ) $ (4.04 ) $ (203.91 ) $ (225.71 ) $ 0.51 $ (2.56 ) $ (4.04 ) $ (203.91 ) $ (225.71 ) $ 0.06 39,643,099 17,699,487 383,310 368,261 219,254 39,643,099 17,699,487 383,310 368,261 1,774,935 Year Ended December 31, 2016 2015 2014 2013 2012 (in thousands) Operating expenses: Research and development $ 107,406 $ 68,280 $ 45,719 $ 60,536 $ 54,961 General and administrative 77,542 30,797 13,674 6,829 9,469 Loss from operations (184,948 ) (99,077 ) (59,393 ) (67,365 ) (64,430 ) Other (expense) income: Other (expense) income, net (293 ) (1,607 ) (713 ) 9,085 (2,095 ) Interest (expense) income, net 2,437 (842 ) (2,373 ) (2,410 ) (2,603 ) Net loss (182,804 ) (101,526 ) (62,479 ) (60,690 ) (69,128 ) Other comprehensive loss, net of tax: Unrealized gain (loss) from available-for-sale securities 66 26 (21 ) — (5 ) Comprehensive loss $ (182,738 ) $ (101,500 ) $ (62,500 ) $ (60,690 ) $ (69,133 ) Net (loss) earnings attributable to common stockholders $ (182,804 ) $ (101,526 ) $ (71,479 ) $ (78,161 ) $ (83,120 ) Net (loss) earnings per share applicable to common stockholders—basic $ (4.24 ) $ (2.56 ) $ (4.04 ) $ (203.91 ) $ (225.71 ) Net (loss) earnings per share applicable to common stockholders—diluted $ (4.24 ) $ (2.56 ) $ (4.04 ) $ (203.91 ) $ (225.71 ) Weighted-average number of common shares used in net (loss) earnings per share applicable to common stockholders—basic 43,067,952 39,643,099 17,699,487 383,310 368,261 Weighted-average number of common shares used in net (loss) earnings per share applicable to common stockholders—diluted 43,067,952 39,643,099 17,699,487 383,310 368,261 As of December 31, As of December 31, 2015 2014 2013 2012 2011 2016 2015 2014 2013 2012 (in thousands) (in thousands) $ 159,678 $ 28,518 $ 12,303 $ 18,653 $ 25,128 $ 258,567 $ 159,678 $ 28,518 $ 12,303 $ 18,653 313,661 76,758 — 4,000 31,580 73,880 313,661 76,758 — 4,000 459,128 86,774 (22,675 ) 8,026 56,607 302,084 459,128 86,774 (22,675 ) 8,026 482,465 108,417 12,758 25,300 63,637 340,282 482,465 108,417 12,758 25,300 — 24,394 1,945 38,222 19,806 379 — 24,394 1,945 38,222 21,180 44,953 37,257 55,312 26,589 33,104 21,180 44,953 37,257 55,312 — — 252,802 170,649 156,658 — — — 252,802 170,649 482,465 108,417 12,758 25,300 63,637 340,282 482,465 108,417 12,758 25,300 the investigational drug abaloparatide for subcutaneous injection, or abaloparatide-SC, has completed Phase 3 development for potential use in the reductiontreatment of fracture risk in postmenopausal women with osteoporosis andpostmenopausal osteoporosis. Our New Drug Application, or NDA, for abaloparatide-SC is currently under regulatory review by the U.S. Food and Drug Administration, or FDA, with a Prescription Drug User Fee Act, or PDUFA, date of March 30, 2017. Our European Marketing Authorisation Application for abaloparatide-SC is under review by the Committee for Medicinal Products for Human Use, or CHMP, of the European Medicines Agency, or EMA. We intend to commercialize abaloparatide-SC in Europe. the United States and our experienced commercial leaders are rapidly expanding the breadth of our capabilities and sales organization with highly skilled and tenured individuals.investigational abaloparatide transdermal patch, or abaloparatide-TD, for potential use in osteoporosisthe treatment of women with postmenopausal osteoporosis. We are focused on completing the manufacturing scale-up, production, and other activities required for the initiation of a pivotal bioequivalence study for abaloparatide-TD. In addition, we are evaluating our investigational drugproduct candidate, RAD1901, a selective estrogen receptor down-regulator/degrader, for potential use in the treatment of hormone-driven and/or hormone-resistant breast cancer, andas well as for potential use in the treatment of vasomotor symptoms in postmenopausal women. We plan to complete our ongoing Phase 1 studies of RAD1901 in advanced metastatic breast cancer and our ongoing Phase 2b study of RAD1901 in postmenopausal vasomotor symptoms. In the first half of 2017, we intend to engage with regulatory agencies to gain alignment on defining the next steps for our RAD1901 breast cancer program, which would include the design of a Phase 2 trial. In the first half of 2017, we also expect to complete and report results from our RAD1901 Phase 2b vasomotor trial.preclinicalclinical pipeline also includes our internally developed investigational product candidate, RAD140, a non-steroidal selective androgen receptor modulator, or SARM, under investigation for potential applicationsuse in oncologythe treatment of breast cancer. In December 2016, we submitted an investigational new drug application, or IND, to the FDA and multiple conditions where androgen modulation may offer therapeutic benefit.createddesigned to have a unique mechanism of action with the goal of stimulating enhanced bone building activity including bone formation, increasing bone mineral density, restoring bone microarchitecture and augmenting bone strength. We are developing two formulations of abaloparatide:•Abaloparatide-SCAbaloparatide-SC——AbaloparatideAbaloparatide-SC has completed Phase 3 development for potential use as a daily self-administered injection, which we refer to as abaloparatide-SC.injection. We hold worldwide commercialization rights to abaloparatide-SC, except for Japan. In December 2014, we announced the positive 18-month top-line data from our Phase 3 ACTIVE clinical trial of abaloparatide-SC. These results were published in which abaloparatide-SC met the primary endpoint with a statistically significant reductionJournal of the American Medical Association, or JAMA, in new vertebral fractures versus placebo, and inAugust 2016. In June 2015, we announced the positive top-line data from the first six months of theour 24-month ACTIVExtend clinical trial of abaloparatide-SC and the 24-month25-month combined fracture data from the ACTIVE and ACTIVExtend.ACTIVExtend clinical trials. These data were published in the Mayo Clinic Proceedings in February 2017. The combined 25-month fracture data from our Phase 3 clinical trial program for abaloparatide-SC formed the basis of our regulatory submissions in the United States and Europe. In November 2015, we submitted a marketing authorization application, oran MAA to the European Medicines Agency, or EMA, which was validated and is currently undergoing active regulatory reviewassessment by the EMA.CHMP. We anticipate that the CHMP may adopt an opinion regarding the MAA in 2017. In March 2016, we submitted an NDA to the FDA, which has been accepted for filing by the FDA with a PDUFA date of March 30, 2017. We intend to enter into one or more partnerships or collaborations for the potential commercialization of abaloparatide-SC prior to a commercial launch. We plan to submit a new drug application, or NDA, in the United States, at the end of the first quarter of 2016. Subject to regulatory review and a favorable regulatory outcome, we anticipate the first commercial sales of abaloparatide-SC will take place in 2016.2017. We intend to commercialize abaloparatide-SC in the United States ourselves and our experienced commercial leaders are rapidly expanding the breadth of our capabilities and sales organization with highly skilled and tenured individuals. We expect to report the top-line results from our recently completed 24-month ACTIVExtend trial in the second quarter of 2017.•Abaloparatide-TD—We are also developing abaloparatide-transdermal, which we refer to as abaloparatide-TD, based on 3M's3M’s patented Microstructured Transdermal System technology for potential use as a short wear-time transdermal patch. We hold worldwide commercialization rights to the abaloparatide-TD technology. During 2014, we reported progress towards the development of an optimized transdermal patch that may be capable of demonstrating comparability to abaloparatide-SC. In preliminary, nonhuman primate pharmacokinetic studies, we achieved a desirable pharmacokinetic profile, with comparable AUC, Cmax, Tmax and T1/2relative to abaloparatide-SC. We believe that these results support continued clinical development ofare developing abaloparatide-TD toward future global regulatory submissions as ato build upon the potential post-approval line extensionsuccess of theour investigational drug abaloparatide-SC.product candidate, abaloparatide-SC, if approved. We commenced a human replicative clinical evaluation of the optimized abaloparatide-TD patch in December 2015, with the goal of achieving comparability to abaloparatide-SC. We expect to complete our clinicalIn September 2016, we presented results from this evaluation, which showed that the pharmacokinetic profile of thean optimized abaloparatide-TD patch during 2016.has ais being evaluated for potential for use as an oral non-steroidal treatment for hormone-driven and/or hormone-resistant, breast cancer. We hold worldwide commercialization rights to RAD1901. RAD1901 is currently being investigated in postmenopausal women with advanced estrogen receptor positive, or ER-positive, HER2-negative breast cancer, the most common form of the disease. TheStudies completed to date indicate that the compound has the potential for use as a single agent or in combination with other therapies to overcome endocrine resistance infor the treatment of breast cancer. In September 2015, we announced results from a maximum tolerated dose, or MTD, study of RAD1901 in 52 healthy volunteers. In the study, RAD1901 was administered to healthy postmenopausal women in doses ranging from 200mg to 1000mg, and the data showed that RAD1901 was well-tolerated and the overall safety was supportive of continued development. In addition, a subset of subjects that received 18F estradiol positron emission tomography, or FES-PET, imaging demonstrated suppression of the FES-PET signal to background levels after six days of dosing.two-part,multiple-part, dose-escalation study of RAD1901 in postmenopausal women with advanced ER-positive and HER2-negative advanced breast cancer in the United States to determine the recommended dose for a Phase 2 clinical trial and to make a preliminary evaluation of the potential anti-tumor effect of RAD1901. We expectPart A of this Phase 1 study was designed to completeevaluate escalating doses of RAD1901. The Part B expansion cohort was initiated at 400-mg daily dosing in March 2016 to allow for an evaluation of additional safety, tolerability and preliminary efficacy. The patients enrolled in this study byare heavily pretreated ER-positive, HER2-negative advanced breast cancer patients who have received a median of 3 prior lines of therapy including fulvestrant and CDK4/6 inhibitors, and more than 50% of the middle ofpatients had ESR1 mutations. Dose escalation is currently ongoing with no dose limiting toxicities to date and we expect to initiate expansion cohorts in 2016.Pfizer'sPfizer’s palbociclib, a cyclin-dependent kinase, or CDK 4/6 inhibitor, or Novartis'Novartis’ everolimus, an mTOR inhibitor, was effective in shrinking tumors. In preclinical patient-derived xenograft or PDx, breast cancer models with either wild type or mutant ESR1, treatment with RAD1901 resulted in marked tumor growth inhibition, and the combination of RAD1901 with either agent, palbociclib or everolimus, showed anti-tumor activity that was significantly greater than either agent alone. We believe that this preclinical data suggest that RAD1901 has the potential to overcome endocrine resistance, is well-tolerated, and has a profile that is well suited for use in combination therapy.Novartis'Novartis’ investigational agent LEE011 (ribociclib), a CDK 4/6 inhibitor, and BYL719 (alpelisib), an investigational phosphoinositide 3-kinase inhibitor.commenced aexpect to report results from our Phase 2b clinical study of RAD1901 for the potential treatment of postmenopausal vasomotor symptoms in the first half of 2017.2015.Table2016 and plan to initiate a first-in-human Phase 1 study of Contents20152016 were approximately $195.9$212.9 million. We began tracking program expenses for abaloparatide-TD in 2007, and program expenses from inception to December 31, 20152016 were approximately $33.7$39.1 million. We began tracking program expenses for RAD1901 in 2006, and program expenses from inception to December 31, 20152016 were approximately $27.7$55.5 million. We began tracking program expenses for RAD140 in 2008, and program expenses from inception to December 31, 20152016 were approximately $5.7$8.9 million. These expenses relate primarily to external costs associated with manufacturing, preclinical studies, and clinical trial costs.2014 and 20132014 (in thousands): Year Ended December 31, Year Ended December 31, 2015 2014 2013 2016 2015 2014 $ 19,870 $ 32,044 $ 45,977 $ 17,016 $ 19,870 $ 32,044 2,585 1,493 11,459 5,394 2,585 1,493 9,926 2,250 — 27,751 9,926 2,250 495 — — 3,181 495 — as a result ofrelated to the issuance of stock option grants, restricted stock units, and performance unit grants to employees, directors, and consultants. The stock-based compensation expense is included in the respective categories of expense in the statement of operations (research(i.e., research and development andor general and administrative expenses). We expect to record additional non-cash compensation expense in the future, which may be significant.agreement,agreements In May 2014, we entered into on May 23, 2011 with General Electric Capital Corporation, or GECC, as agent and lender, and Oxford Finance, as a lender, or the Original Credit Facility, and our loan and security agreement, entered into on May 30,or the 2014 Credit Facility, with Solar Capital Ltd., or Solar, as agent and lender, and Oxford Finance, as lender, or the New Credit Facility. Under the Original Credit Facility, we drew $12.5 million under an initial and second term loan during the year ended December 31, 2011 and an additional $12.5 million under a third term loan during the year ended December 31, 2012. Under the New Credit Facility,lender. In May 2014, we drew $21.0 million under an initial term loan on May 30, 2014. Onunder this facility, of which we used approximately $9.3 million to repay all the amounts owed under a previous credit facility we had entered into with other financial institutions. 10, 2014, we entered into a first amendment to the New2014 Credit Facility, or the First Amendment. PursuantAmendment, pursuant to the terms of the First Amendment,which we drew a second term loan of $4.0 million was drawn on July 10, 2014. On May 30, 2014, we used approximately $9.3 million of the New Credit Facility to repay all the amounts owed under the Original Credit Facility. Onmillion. In August 4, 2015, we prepaid all amounts owed under the 2014 Credit Facility, and the First Amendment.as amended. After consideration of relevant fees required under the Credit Facility and the First Amendment, the total payment amounted to $26.5 million.20142015 and 2013,2014, other income (expense) primarily reflects changes in the fair value of our warrant liability and the series A-6 convertible preferred stock liability and stock asset outstanding prior to our initial public offering from the date of the initial accrual to the reporting date.ourthese consolidated financial statements requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenues and expenses during the reportedreporting periods. We evaluate our estimates and judgments on an ongoing basis. Estimates include useful lives with respect to long-lived assets and intangible assets, accounting for stock-based compensation, contingencies, intangible assets, tax valuation reserves, and accrued expenses. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Our actual results may differ from these estimates under different assumptions or conditions."critical" because they require usthe most critical to make judgmentsaid you in fully understanding and estimates about matters that are uncertain at the time we make the estimate,evaluating our financial condition and different estimates, which would have been reasonable, could have been used, which would have resulted in different financial results.•••20152016 and 2014,2015, we held financial assets that were measured using Level 1 and Level 2 inputs. Assets measured using Level 1 inputs include money market funds, which are valued using quoted market prices with no valuation adjustments applied. Assets measured using Level 2 inputs include marketable securities that consist primarily of domestic corporate debt securities (direct issuance bonds, corporate bonds, etc.) and are valued using third-party pricing resources, which generally use interest rates and yield curves observable at commonly quoted intervals of similar assets as observable inputs for pricing.20152016 and 2014,2015, we held no Level 3 assets or liabilities. Years Ended December 31, Change 2016 2015 $ % (in thousands) Operating expenses: Research and development $ 107,406 $ 68,280 $ 39,126 57 % General and administrative 77,542 30,797 46,745 152 % Loss from operations (184,948 ) (99,077 ) (85,871 ) 87 % Other (expense) income: Other (expense), net (293 ) (35 ) (258 ) 737 % Loss on retirement of note payable — (1,572 ) 1,572 (100 )% Interest income (expense), net 2,437 (842 ) 3,279 (389 )% Net loss $ (182,804 ) $ (101,526 ) (81,278 ) 80 % TableResearch and development expenses—For the year ended December 31, 2016, research and development expense was $107.4 million, as compared to $68.3 million for the year ended December 31, 2015, an increase of Contents$39.1 million, or 57%. This increase was primarily a result of increased compensation expense, including an increase of $3.3 million of non-cash stock-based compensation expense, due to growth in headcount from 48 research and development employees as of December 31, 2015, to 107 research and development employees as of December 31, 2016. This increase in spend was also driven by higher contract service costs associated with the development of our investigational product candidate RAD1901 as a result of the increased clinical and manufacturing activities in 2016, as compared to 2015. These amounts were partially offset by a decrease in the total professional contract service costs associated with the development of abaloparatide-SC as more patients completed study protocol activities associated with the 24-month ACTIVExtend clinical trial in 2016, as compared to 2015. Years Ended
December 31, Change Years Ended December 31, Change 2015 2014 $ % 2015 2014 $ % (in thousands) (in thousands) $ 68,280 $ 45,719 $ 22,561 49 % $ 68,280 $ 45,719 $ 22,561 49 % 30,797 13,674 17,123 125 % 30,797 13,674 17,123 125 % (99,077 ) (59,393 ) 39,684 67 % (99,077 ) (59,393 ) (39,684 ) 67 % (35 ) (510 ) (475 ) –93 % (35 ) (510 ) 475 (93 )% (1,572 ) (203 ) 1,369 674 % (1,572 ) (203 ) (1,369 ) 674 % (842 ) (2,373 ) (1,531 ) –65 % (842 ) (2,373 ) 1,531 (65 )% $ (101,526 ) $ (62,479 ) 39,047 62 % $ (101,526 ) $ (62,479 ) (39,047 ) 62 % planned NDA submissions for our investigational product candidate abaloparatide-SC, and an increase in contract service costs associated with the development of our investigational product candidate RAD1901 as a result of the initiation of various preclinical and manufacturing activities in late 2014. These amounts were partially offset by a decrease in the total professional contract service costs associated with the development of abaloparatide-SC resulting from the completion of theour Phase 3 18-month fracture study in October 2014 and the first six months of theour ACTIVExtend clinical trial. We expect that costs associated with the development of abaloparatide-SC will continue to decrease over the course of the ACTIVExtend clinical trial as patients complete treatment. We expect that the costs associated with the development of abaloparatide-TD will increase as we begin to advance an optimized abaloparatide-TD product in additional clinical studies. We expect that the costs associated with the development of RAD1901 will increase as we begin to advance RAD1901 through various preclinical and clinical studies, including a Phase 1 study in metastatic breast cancer, which commenced in late 2014, and a Phase 2b study in vasomotor symptoms, which commenced in December 2015.$0.5 millionother income, net of expense during the year ended December 31, 2014.2014 of $0.5 million. Other expense, net of other income, for the year ended December 31, 2015 consisted primarily of state taxes. The $0.5 million of other expense, net of income, for the year ended December 31, 2014 was primarily due to an increase in the fair value of our warrant liability as a result of an overall increase in the fair value of the underlying common stock from December 31, 2013 to June 6, 2014. Following our initial public offering on June 6, 2014, the carrying value of our warrant liability was reclassified to equity.New2014 Credit Facility, as amended, on August 4, 2015. For the year ended December 31, 2014, loss on retirement of note payable wasa previous credit facility $0.2 million. This loss was a result of themillion due to prepayment of our Original Credit Facility onin May 30, 2014.Years Ended December 31, 2014 and December 31, 2013 Years Ended
December 31, Change 2014 2013 $ % (in thousands) $ 45,719 $ 60,536 $ (14,817 ) –24 % 13,674 6,829 6,845 100 % (59,393 ) (67,365 ) (7,972 ) –12 % (510 ) 9,085 9,595 106 % (203 ) — 203 100 % (2,373 ) (2,410 ) (37 ) –2 % $ (62,479 ) $ (60,690 ) 1,789 3 % Research and development expenses—For the year ended December 31, 2014, research and development expense was $45.7 million compared to $60.5 million for the year ended December 31, 2013, a decrease of $14.8 million, or 24%. This decrease is primarily a result of a decrease in the total professional contract service costs associated with the development of abaloparatide-SC and abaloparatide-TD, partially offset by an increase in professional contract services costs associated with the development of RAD1901. During the year ended December 31, 2014, we incurred professional contract service costs associated with the development of abaloparatide-SC, abaloparatide-TD and RAD1901 of $32.0 million, $1.5 million and $2.3 million, respectively, compared to $46.0 million, $11.5 million and zero, respectively, for the year ended December 31, 2013. The decrease in contract service costs associated with the development of abaloparatide-SC is primarily a result of the completion of the Phase 3 18-month fracture study in October 2014. Additionally, fewer patients were enrolled in the 6-month extension study as of December 31, 2014, as compared to the year ended December 31, 2013, as certain patients completed treatment. In addition, there will be variability from quarter to quarter in the costs for abaloparatide-SC, driven primarily by the euro/dollar exchange rate,which is more fully described below under "Research and Development Agreements." The decrease in contract service costs associated with the development of abaloparatide-TD is a result of the completion of the Phase 2 clinical trial (which began dosing patients in September 2012) in September 2013. The increase in contract service costs associated with the development of RAD1901 is a result of the initiation of various preclinical, clinical, and manufacturing activities in 2014. General and administrative expenses—For the year ended December 31, 2014, general and administrative expense was $13.7 million compared to $6.8 million for the year ended December 31, 2013, an increase of $6.8 million, or 100%. This increase was primarily due to an increase in compensation costs of $4.5 million, including an increase of $3.9 million in non-cash stock-based compensation expense as a result of the issuance of new option awards during 2014, as well as the acceleration of vesting for a portion of our Chief Executive Officer's outstanding option awards, in accordance with his employment agreement, upon completion of our initial public offering. This increase can also be attributed to higher legal fees and consulting support costs of approximately $1.7 million during the year ended December 31, 2014. Other (expense) income, net—For the year ended December 31, 2014, other expense, net of other income, was $0.5 million, as compared to other income, net of expense during the year ended December 31, 2013 of $9.1 million. Other expense, net of other income, primarily reflects changes in the fair value of the stock asset, stock liability, other liability and warrant liability. The $0.5 million of other expense, net of income, for the year ended December 31, 2014 was primarily due to an increase in the fair value of our warrant liability as a result of an overall increase in the fair value of the underlying common stock from December 31, 2013 to June 6, 2014. Following our initial public offering on June 6, 2014, our warrant liability was reclassified to equity. The $9.1 million of other income, net of expense, as of December 31, 2013 was primarily due to a decrease in the fair value of our stock liability and other liability as a result of an overall decline in the fair value of the underlying convertible preferred stock from December 31, 2012 to December 31, 2013. Loss on retirement of note payable—For the year ended December 31, 2014, loss on retirement of note payable was $0.2 million. This loss was a result of the prepayment of our Original Credit Facility on May 30, 2014. Interest (expense) income—For the year ended December 31, 2014, interest expense, net of interest income, was $2.4 million, consistent with $2.4 million for the year ended December 31, 2013.2015,2016, we have incurred an accumulated deficit of $445.8$628.6 million, primarily as a result of expenses incurred through a combination of research and development activities related to our various investigational product20152016 was $473.3$332.4 million. We have financed our operations since inception primarily through the public offerings of our common stock, private sale of preferred stock, borrowing under credit facilities and the receipt of $5.0 million in fees associated with an option agreement.partnershippartnering and/or collaboration activities, we have sufficient capital to fund our development plans, U.S. commercial scale-up and other operational activities, for not less than twelve months from the date of this filing and into 2018. We expect to finance the future development costs of our clinical product portfolio with our existing cash, cash equivalents and marketable securities, or through strategic financing opportunities, that could include, but are not limited to, partnering or other collaboration agreements, future offerings of equity, royalty-based financing arrangements, or the incurrence of debt. However, there is no guarantee that any of these strategic financingU.S. Food and Drug Administration, or FDA and the European Medicines Agency.foreign regulatory authorities. The successful development of our investigational product candidates is subject to numerous risks and uncertainties associated with developing drugs, which could have a significant impact on the cost and timing associated with the development of our product candidates. If we fail to obtain additional future capital, we may be unable to complete our planned preclinical and clinical trials and obtain approval of any investigational product candidates from the FDA and foreign regulatory authorities. Years ended December 31, 2015 2014 2013 $ (87,103 ) $ (48,345 ) $ (45,017 ) (239,822 ) (78,065 ) 3,971 458,085 142,625 34,696 $ 131,160 $ 16,215 $ (6,350 ) Years ended December 31, 2016 2015 2014 Net cash (used in) provided by: Operating activities $ (139,804 ) $ (87,103 ) $ (48,345 ) Investing activities 236,120 (239,822 ) (78,065 ) Financing activities 2,573 458,085 142,625 Net increase in cash and cash equivalents $ 98,889 $ 131,160 $ 16,215 ongoing Phase 3 clinical trial of abaloparatide-SC. The $11.2 million net non-cash adjustments to reconcile net loss to net cash used in operations included stock-based compensation expense of $7.1 million, $2.7 million of research and development expenses settled in stock, and a $0.5 million increase in the fair value of our warrant liability and stock liability as a result of an increase in the fair value of the underlying convertible preferred stock and common stock from December 31, 2013 to June 6, 2014. Net cash used in operating activities for the year ended December 31, 2013 was $45.0 million, which was primarily the result of a net loss of $60.7 million, partially offset by net changes in working capital of $9.7 million and $6.0 million net non-cash adjustments to reconcile net loss to net cash used in operations. The $60.7 million net loss was primarily due to expenses incurred in connection with our Phase 3 clinical trial of abaloparatide-SC and our Phase 2 clinical study of abaloparatide-TD, whichfinished dosing patients during the three months ended September 30, 2013. The $6.0 million net non-cash adjustments to reconcile net loss to net cash used in operations included $13.1 million of research and development expenses settled in stock and stock-based compensation expense of $1.5 million, and was partially offset by a $9.1 million reduction in the fair value of our warrant liability, stock liability and other liability as a result of a decline in the fair value of the underlying convertible preferred stock from December 31, 2012 to December 31, 2013.used inprovided by investing activities for the year ended December 31, 20152016 was $239.8$236.1 million, as compared to net cash used in investing activities of $78.1$239.8 million for the year ended December 31, 2014. The net cash used in investing activities during the year ended December 31, 2014 was primarily a result of $97.7 million in purchases of marketable securities and $0.9 million of purchases of property and equipment, partially offset by $20.5 million of net proceeds received from the sale or maturity of marketable securities. The net cash provided by investing activities during the year ended December 31, 2013 was primarily a result of $21.0 million net proceeds received from the sale or maturity of marketable securities, partially offset by $17.1 million in purchases of marketable securities.20152016 was $458.1$2.6 million, as compared to $142.6$458.1 million of net cash provided by financing activities for the year ended December 31, 2014.New2014 Credit Facility.New2014 Credit Facility, partially offset by payments under our Original Credit Facilitya previous credit facility arrangement of $13.2 million. Net cash provided by financing activities for the year ended December 31, 2013 consisted of $42.9 million of net proceeds from the issuance of our series B convertible preferred stock in April and May of 2013, partially offset by payments under our Credit Facility of $8.2 million.an additionala public offering whereby we sold 2,750,000 shares of common stock at a price of $18.25 per share, for aggregate proceeds, net of underwriting discounts, commissions and offering costs, of approximately $46.9 million. On October 7, 2014, the underwriters purchased an additional 378,524 shares in the aggregate by exercising a portion of the over-allotment option granted to them in connection with the offering. As a result of the public offering and subsequent exercise of the over-allotment option, we received aggregate proceeds, net of underwriting discounts, commissions and offering costs of approximately $53.4 million.2015,2016, we had received aggregate net cash proceeds of $238.2 million from the sale of shares of our preferred stock as follows:Issue Year No. Shares Series B redeemable convertible preferred stock (1) 2003, 2004, 2005 1,599,997 $ 23,775 Series C redeemable convertible preferred stock (1) 2006, 2007, 2008 10,146,629 82,096 Series A-1 convertible preferred stock (1) 2011 9,223,041 61,591 Series A-5 convertible preferred stock (1) 2011 64,430 525 Series B convertible preferred stock 2013 701,235 42,870 Series B-2 convertible preferred stock 2014 448,060 27,368 Total 22,183,392 $ 238,225 Year No. Shares Net Proceeds
(in thousands) 2003, 2004, 2005 1,599,997 $ 23,775 2006, 2007, 2008 10,146,629 82,096 2011 9,223,041 61,591 2011 64,430 525 2013 701,235 42,870 2014 448,060 27,368 22,183,392 $ 238,225 (1) Share amounts stated in pre-Merger shares, which converted into the rights to one-tenth of one share pursuant to the Merger. (1)Share amounts stated in pre-Merger shares, which converted into the rights to one-tenth of one share pursuant to the Merger. On April 23, 2013, we entered into a Series B Convertible Preferred Stock and Warrant Purchase Agreement, or the Series B Purchase Agreement, pursuant to which we could raise, at any time on or prior to May 10, 2013, up to approximately $60.0 million through the issuance of (1) up to 980,000 shares of its Series B preferred stock , or the Series B, and (2) warrants to acquire up to approximately 1,075,000 shares of its common stock with an exercise price of $14.004 per share. On April 23, 2013, we consummated a first closing under the Series B Purchase Agreement, whereby in exchange for aggregate proceeds of approximately $43.0 million, we issued 700,098 shares of Series B and warrants to purchase up to a total of 767,651 shares of our common stock. On May 10, 2013, we consummated a second closing under the Series B Purchase Agreement, whereby in exchange for aggregate proceeds of approximately $0.1 million, we issued 1,137 shares of Series B and warrants to purchase up to a total of 1,246 shares of our common stock. The warrants can be exercised at any time prior to the fifth anniversary of their issuance. On 30, 2014, we entered into our New2014 Credit Facility with Solar and Oxford Finance, pursuant to which Solar and Oxford agreed to make available to us $30.0 million in the aggregate subject to certain conditions to funding. An initial term loan was made onin May 30, 2014 in an aggregate principal amount equal to $21.0 million, or the Initial Term Loan. As security for its obligations under the New Credit Facility, we granted a security interest in substantially all of our existing and after-acquired assets except for our intellectual property and certain other customary exclusions. On 10, 2014, we entered into a first amendment to the New2014 Credit Facility, or the First Amendment. Pursuant to the terms of the First Amendment, a second term loan of $4.0 million was drawn onin July 10, 2014. On 4, 2015, the Company prepaid all amounts owed under the 2014 Credit Facility and the First Amendment. After consideration of relevant fees required under the 2014 Credit Facility and the First Amendment, the total payment amounted to $26.5 million.partnering or other collaboration agreements, future offerings of our equity, royalty-based financing arrangements, or the incurrence of debt. We anticipate that we will make determinations as to which additional programs to pursue and how much funding to direct to eachdevelopment, regulatory approval and potential commercialization. We submitted an MAA to the EMA in November 2015 and plan to submitsubmitted an NDA to the FDA at the endin March 2016 with a PDUFA date of the first quarter of 2016.March 30, 2017. Obtaining approval of an investigational product candidate is an extensive, lengthy, expensive, and uncertain process, and any approval of abaloparatide-SC may be delayed, limited or denied for many reasons, including:•postmenopausal women with postmenopausal osteoporosis to the satisfaction of the FDA or other foreign regulatory authorities;••••••••••(suchsuch as the start of a clinical trial, filing of an NDA, approval by the FDA, or product launch).launch. The table below excludes these potential payments we may be required to make under our agreements because the timing of payments and actual amounts paid under those agreements may be different depending on the timing of receipt of goods or services or changes to agreed-upon terms or amounts for some obligations, and those agreements are cancelable upon written notice by us and therefore, not long-term liabilities. Additionally, the expected timing of payment of the obligations presented below is estimated based on current information.2015: Total Less than
1 Year 1 to 3 Years 3 to 5 Years More than
5 Years (in thousands) $ 8,273 $ 1,944 $ 3,827 $ 2,502 $ — Total 1 to 3 Years 3 to 5 Years (in thousands) Operating lease obligations $ 7,914 $ 3,020 $ 3,702 $ 1,192 $ — have entered into agreements with Nordic Bioscience Clinical Development VII A/S, or Nordic, to conduct our Phase 3 clinical trial of abaloparatide-SC, or the Phase 3 Clinical Trial. On March 29, 2011, we entered into a Clinical Trial Services Agreement, or the Clinical Trial Services Agreement. On the same date, we also entered into Work Statement NB-1, as amended on December 9, 2011, June 18, 2012, March 28, 2014, May 19, 2014, July 22, 2014, August 15, 2014 and March 12, 2015, or Work Statement NB-1, and the Stock Issuance Agreement, as amended and restated on May 16, 2011, and as further amended onIn February 21, 2013, March 28, 2014, and May 19, 2014, or the Stock Issuance Agreement. We recognized research and development expense for the amounts due to Nordic under the Work Statement NB-1 ratably over the estimated per patient treatment period beginning upon enrollment in the Phase 3 Clinical Trial, or a twenty-month period, except for the amounts due under the fourth amendment to the Work Statement NB-1, which we recognized on a per patient basis when the end-of-study visit and all other required procedures were completed. We recorded no expense, $8.2 million, and $31.6 million of research and development expense during the years ended December 31, 2015, 2014, and 2013, respectively for per patient costs incurred for patients that had enrolled in the Phase 3 Clinical Study. As of December 31, 2015, all obligations due to Nordic under Work Statement NB-1 had been paid. Abaloparatide-SC Phase 3 Clinical Extension Study—On February 21, 2013, we entered into the Work Statement NB-3, as amended on February 28, 2014, March 23, 2015, July 8, 2015 and October 21, 2015, or the Work Statement NB-3. Pursuantcontracted with Nordic for it to the Work Statement NB-3, Nordic performedperform an extension study to evaluate six months of standard-of-care osteoporosis management following the completion of the Phase 3 clinical trial of abaloparatide-SC, or the Extension Study,Clinical Trial (the "Extension Study"), and, upon completion of this initial six months, an additional period of 18 months of standard-of-care osteoporosis management or the Second Extension.(the "Second Extension").entered into an amendmentcontracted with Nordic to the Work Statement NB-3, or the NB-3 Amendment. The NB-3 Amendment was effective as of March 23, 2015 and provides that Nordic will perform additional services, including additional monitoring of patients enrolled in the Second Extension. Payments in cash to be made to Nordic under the NB-3 Amendmentfor these additional services are denominated in euroseuro and total up to approximately €4.1 million ($4.54.3 million).underfor the Work Statement NB-3services related to the Extension Study and the Second Extension are denominated in both euros and U.S. dollars and total up to €11.9 million ($12.912.5 million) and $1.1 million, respectively. In addition, payments are due to Nordic in connection with the Work Statement NB-3 pursuant to the Stock Issuance Agreement, as discussed below. As of December 31, 2015, services related to2016, the last patient last visit in the Second Extension are ongoinghad occurred and all obligations due to Nordic in relation to the Extension Study have been paid.WeThe Company recorded $2.5 million, $5.4 million, and $9.6 million, and $4.5 million of research and development expense duringfor the years ended December 31, 2016, 2015, 2014, and 2013, respectively,2014 respectively, for per patient costs incurred.2015,2016, we had a liability of $2.9$1.2 million reflected in accrued expenses and other current liabilities on the balance sheet resulting from services provided by Nordic under the Second Extension, which are payable in cash. Stock Issuance Agreement—Pursuant to the Stock Issuance Agreement, Nordic agreed to purchase 6,443 shares of our Series A-5 convertible preferred stock, which provided them with the right to receive quarterly stock dividends, payable in shares of our Series A-6 convertible preferred stock, for services rendered under Work Statement NB-1 and Work Statement NB-3. The Stock Issuance Agreement was later amended to provide that in the event an initial public offering of our common stock occurred prior to June 30, 2014, any rights to receive stock dividends in relation to Work Statement NB-1 and Work Statement NB-3, for all periods of time after 2014, would be changed from the right to receive stock to the right to receive a total cash payment of $4.3 million, payable in ten equal monthly installments of $430,000 beginning on March 31, 2015. The amendment also stipulated that all consideration to be paid to Nordic pursuant to the Stock Issuance Agreement at any time after the consummation of an initial public offering be payable in cash. As we completed an initial public offering on June 11, 2014, Nordic no longer has the right to receive stock and has been paid in cash for all periods after June 11, 2014.$1.3$1.1 million, and $3.9$1.3 million for pass through costs during years ended December 31, 2016, 2015, and 2014, and 2013, respectively.relation to Work Statement NB-3connection with the Second Extension will approximate the following as of December 31, 2015 (in thousands): TOTAL(1) LESS THAN 1 YEAR(1) 1 - 3 YEARS(1) EURO DENOMINATED
PAYMENTS EURO DENOMINATED
PAYMENTS EURO DENOMINATED
PAYMENTS EURO USD
EQUIVALENT(2) USD
DENOMINATED
PAYMENTS EURO USD
EQUIVALENT(2) USD
DENOMINATED
PAYMENTS EURO USD
EQUIVALENT(2) USD
DENOMINATED
PAYMENTS € 4,748 $ 5,156 $ 430 € 4,349 $ 4,723 $ 430 € 399 $ 433 $ — € 4,748 $ 5,156 $ 430 € 4,349 $ 4,723 $ 430 € 399 $ 433 $ —
$1.3 million, excluding pass through costs, payable within 1 year.(1)The amounts above exclude pass-through costs and, in accordance with work statement NB-3, may be adjusted from time to time and at the end of the study to reflect actual study activities completed by the study subjects.(2)USD equivalent is based upon the noon buying rate published by the Board of Governors of the Federal Reserve on December 31, 2015.exclusively licensed the worldwide rights (except for development and commercial rights in Japan) to abaloparatide and analogs from an affiliate ofentered into a license agreement with Ipsen Pharma SAS, or Ipsen, including US Patent No. 5,969,095 (statutory term expires March 29, 2016) entitled "Analogs of Parathyroid Hormone"as amended, or the License Agreement, under which we exclusively licensed certain Ipsen compound technology and related patents covering abaloparatide to research, develop, manufacture and commercialize certain compounds and related products in all countries, except Japan (where we do not hold abaloparatide-SC development and commercialization rights) and France (where our commercialization rights were subject to certain co-marketing and co-promotion rights exercisable by Ipsen, provided that claims abaloparatide and US Patent No. 6,544,949, (statutory term expires March 29, 2016) entitled "Analogs of Parathyroid Hormone" that claims abaloparatide and US Patent No. 6,544,949, (effective filing date March 29, 1996, statutory term expires March 29, 2016), entitled "Analogs of Parathyroid Hormone" that claims methods of treating osteoporosis using abaloparatide and pharmaceutical compositions comprising abaloparatide, and the corresponding foreign patents and continuing patent applications. European Patent No. 0847278, which wascertain conditions included in the license from Ipsen and claimed the composition of matter of abaloparatide, lapsed due to Ipsen's failure to pay annuities. We are pursuing restoration of those rights. To date, the patent rights in Austria, Belgium, Denmark, Finland, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain, Sweden, and United Kingdom have been restored.License Agreement were met). We believe that Ipsen's co-marketing and co-promotion rights in France have permanently expired. Ipsen also granted us an exclusive right and license under the data and market exclusivity provided in Europe for a new chemical entity, coupled with the need for a potential competitor to conduct clinicaltrials will likely provide a longer barrier to entry than the patent protection provided by the original European patent term, which will expire in 2016. We also have rights to joint intellectual property related to abaloparatide, including rights to the jointly derived intellectual property contained in US Patent No. 7,803,770 (statutory term expires October 3, 2027, and may be extended to March 26, 2028 with 175 days of patent term adjustment due to delays in patent prosecution by the United States Patent and Trademark Office, or USPTO), US Patent No. 8,148,333 (statutory term expires October 3, 2027 and may be extended to November 8, 2027 with 36 days of patent term adjustment due to delays in patent prosecution by the USPTO)Ipsen compound technology and related patents to make and patent applications bothhave made compounds or product in Japan. Ipsen further granted us an exclusive right and license under certain Ipsen formulation technology and related patents solely for purposes of enabling us to develop, manufacture and commercialize compounds and products covered by the United Statescompound technology license in all countries, except Japan and worldwide that cover the method of treating osteoporosis using the Phase 3 Clinical Trial dosage strength and form. A corresponding European application is pending with claims to the intended therapeutic formulation for abaloparatide-SC. Examination has been requested, but substantive examination has not yet commenced. Upon grant, this patent could be validated in any designated contracting or extension states and potentially could be considered for a Supplemental Protection Certificate depending upon the timing of its grant. Related cases granted in China, Australia, Singapore, Japan, Israel, Mexico, New Zealand, Russia and Ukraine, and currently pending in Europe, Canada, Brazil, Singapore, South Korea, India, Norway, and Hong Kong will have a patent expiration date of 2027. Patent applications which cover various aspects of abaloparatide for microneedle application are pending in the United States, Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, India, Japan, Korea, Mexico, New Zealand, Russia, Singapore, and Ukraine. Any patents that might issue from these applications will have an expiration date in 2032, not taking into account extension under applicable laws.$1.0$4.3 million. The license agreement further requires us to make payments upon the achievement of certain future regulatory and commercial milestones, including upon acceptance of an NDA submission for review by the FDA. The range ofmilestones. Total additional milestone payments that could be paidpayable under the agreement is €10.0are €32.0 million to €36.0 million ($10.9 million to $39.133.6 million). Should abaloparatide be approved and subsequently commercialized, the agreement provides that we will be obligated towould pay to Ipsen a fixed five percent royalty based on net sales of the product by us or our sublicensees on a country-by-country basis until the later of the last to expire of the licensed patents or for a period of 10 years after the first commercial sale in such country. The date of the last to expire of the abaloparatide patents licensed from or co-owned with Ipsen, barring any extension thereof, is expected to be March 26, 2028. In the event that we sublicense abaloparatide to a third party, the agreement provides that we are obligated towould pay a percentage of certain payments received from such sublicensee (in lieu of milestone payments not achieved at the time of such sublicense). The applicable percentage is in the low double digit range. In addition, if we or our sublicensees commercialize a product that includes a compound discovered by us based on or derived from confidential Ipsen know-how, the agreement provides that we will be obligated towould pay to Ipsen a fixed low single digit royalty on net sales of such product on a country-by-country basis until the later of the last to expire of licensed patents that cover such product or for a period of 10 years after the first commercial sale of such product in such country. The license agreement contains other customary clauses and terms as are common in similar agreements in the industry.onin March 9, 2015, we enteredinunder which Eisai granted us an exclusive right and license to research, develop, manufacture and commercialize RAD1901 in Japan. In consideration for the rights to RAD1901 in Japan, we paid Eisai an initial license fee of $0.4 million upon execution of the amendment, which was expensed during the three months ended March 31, 2015.The range ofTotal additional milestone payments that could be paidpayable under the Eisai agreement, is $1.0as amended, are $22.3 million, to $20.0 million. The license agreement further requires us to make paymentspayable upon the achievement of certain future clinical and regulatory milestones. Should RAD1901 be approved and subsequently become commercialized, we will be obligated to pay to Eisai a royalty in a variable mid-single digit range based on net sales of the product on a country-by-country basis for a period that expires on the later of (1) the date the last remaining valid claim in the licensed patents expires, lapses or is invalidated in that country, the product is not covered by data protection clauses, and the sales of lawful generic version of the product account for more than a specified percentage of the total sales of all pharmaceutical products containing the licensed compound in thatvalid claimlicensed patent is expected to expire, barring any extension thereof, on August 18, 2026. The royalty rate shall then be subject to reduction and the royalty obligation will expire at such time as sales of lawful generic versionversions of such product account for more than a specified minimum percentage of the total sales of all products that contain the licensed compound. We were also granted the right to grant sublicenses with prior written approval from Eisai. If we sublicense RAD1901 to a third party, we will be obligated to pay Eisai, in addition to the milestones referenced above, a fixed low double digit percentage of certain fees we receive from such sublicensee and royalties in a variable mid-single digit range based on net sales of the sublicensee. The license agreement contains other customary clauses and terms as are common in similar agreements in the industry.2015,2016, we had federal and state net operating loss carryforwards of approximately $419.5$526.7 million and $323.0$385.3 million, respectively, the use of which may be limited, as described below. If not utilized, the net operating loss carryforwards will expire at various dates through 2035.are in the process of completing a studyhave completed studies through December 31, 2015, to assessdetermine whether anany ownership change has occurred or whether theresince our formation and have been multipledetermined that transactions have resulted in two ownership changes, since our inception. as defined under Section 382. There could be additional ownership changes in the future that could further limit the amount of net operating loss and tax credit carryforwards that we can utilize.noteNote 2 to our consolidated financial statements included in this Annual Report.2015,2016, we had cash, cash equivalents and short-term marketable securities of $473.3$332.4 million, consisting of cash, money market funds, domestic corporate debt securities, domestic corporate commercial paper, and asset-backed securities. This exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our investments are in marketable securities. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate 10% change in interest rates would not have a material effect on the fair market value of our portfolio. We generally have the ability to hold our investments until maturity, and therefore we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a change in market interest rates on our investments. We carry our investments based on publicly available information. As of December 31, 2015,2016, we do not have any hard to value investment securities or securities for which a market is not readily available or active.PAGEPAGE 20152016 and 2014,2015, and the related consolidated statements of operations and comprehensive loss, convertible preferred stock, redeemable convertible preferred stock and stockholders' equity (deficit), and cash flows for each of the three years in the period ended December 31, 2015.2016. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.20152016 and 2014,2015, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2015,2016, in conformity with U.S. generally accepted accounting principles.2015,2016, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 25, 201624, 2017 expressed an unqualified opinion thereon. /s/ Ernst & Young LLP 25, 201624, 2017 December 31,
2015 December 31,
2014 December 31, 2016 December 31, 2015 $ 159,678 $ 28,518 $ 258,567 $ 159,678 Restricted cash 47 — 313,661 76,758 73,880 313,661 6,969 2,057 2,315 6,969 480,308 107,333 334,809 480,308 1,897 842 4,922 1,897 260 242 551 260 $ 482,465 $ 108,417 $ 340,282 $ 482,465 $ 6,228 $ 2,292 $ 6,128 $ 6,228 14,952 18,267 26,597 14,952 21,180 20,559 32,725 21,180
—
24,394 Other non-current liabilities 379 — $ 21,180 $ 44,953 $ 33,104 $ 21,180
4
3 Common stock, $.0001 par value; 200,000,000 shares authorized, 43,141,134 shares and 42,984,243 shares issued and outstanding at December 31, 2016 and 2015, respectively 4 4 907,040 407,720 935,671 907,040 5 (21 ) Accumulated other comprehensive income 71 5 (445,764 ) (344,238 ) (628,568 ) (445,764 ) 461,285 63,464 307,178 461,285 $ 482,465 $ 108,417 $ 340,282 $ 482,465 December 31, December 31, 2015 2014 2013 2016 2015 2014 $ 68,280 $ 45,719 $ 60,536 $ 107,406 $ 68,280 $ 45,719 30,797 13,674 6,829 77,542 30,797 13,674 (99,077 ) (59,393 ) (67,365 ) (184,948 ) (99,077 ) (59,393 ) (35 ) (510 ) 9,085 Other (expense), net (293 ) (35 ) (510 ) (1,572 ) (203 ) — — (1,572 ) (203 ) 1,043 94 30 2,437 1,043 94 (1,885 ) (2,467 ) (2,440 ) — (1,885 ) (2,467 ) $ (101,526 ) $ (62,479 ) $ (60,690 ) $ (182,804 ) $ (101,526 ) $ (62,479 ) 26 (21 ) — 66 26 (21 ) $ (101,500 ) $ (62,500 ) $ (60,690 ) $ (182,738 ) $ (101,500 ) $ (62,500 ) $ (101,526 ) $ (71,479 ) $ (78,161 ) $ (182,804 ) $ (101,526 ) $ (71,479 ) $ (2.56 ) $ (4.04 ) $ (203.91 ) $ (4.24 ) $ (2.56 ) $ (4.04 ) 39,643,099 17,699,487 383,310 43,067,952 39,643,099 17,699,487 Convertible Preferred Stock Series B-2 Series B Series A-1 Series A-2 Series A-3 Series A-4 Series A-5 Series A-6 Convertible Preferred Stock Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Series B-2 Series B Series A-1 Series A-2 Series A-3 Series A-4 Series A-5 Series A-6 — — — — 939,612 $ 71,957 983,208 $ 86,714 142,227 $ 11,182 3,998 $ 271 6,443 $ 525 — $ — 701,235 41,514 496,111 23,168 2,378 6,780 7,263 1,050 Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount — $ — 701,235 $ 43,892 939,612 $ 78,737 983,208 $ 93,977 142,227 $ 12,232 3,998 $ 271 6,443 $ 525 496,111 $ 23,168 — $ — 701,235 $ 43,892 939,612 $ 78,737 983,208 $ 93,977 142,227 $ 12,232 3,998 $ 271 6,443 $ 525 496,111 $ 23,168 448,060 26,152 186,847 10,109 448,060 26,152 186,847 10,109 685 1,515 3,084 3,246 470 685 1,515 3,084 3,246 470 (448,060 ) (26,837 ) (701,235 ) $ (45,407 ) (939,612 ) (81,821 ) (983,208 ) (97,223 ) (142,227 ) (12,702 ) (3,998 ) (271 ) (6,443 ) (525 ) (682,958 ) (33,277 ) (448,060 ) (26,837 ) (701,235 ) (45,407 ) (939,612 ) (81,821 ) (983,208 ) (97,223 ) (142,227 ) (12,702 ) (3,998 ) (271 ) (6,443 ) (525 ) (682,958 ) (33,277 ) — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — Convertible Preferred Stock Series B-2 Series B Series A-1 Series A-2 Series A-3 Series A-4 Series A-5 Series A-6 Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Shares Amount Balance at December 31, 2014 — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — Net loss Unrealized gain from available-for-sale securities Exercise of warrants Exercise of options Stock-based compensation expense Issuance of common stock, net Balance at December 31, 2015 — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — Net loss Unrealized gain from available-for-sale securities Exercise of warrants Exercise of options Stock-based compensation expense Issuance of common stock, net Balance at December 31, 2016 — $ — — $ — — $ — — $ — — $ — — $ — — $ — — $ — Stockholders' Equity (Deficit) Common Stock Additional
Paid-In-Capital Accumulated Other
Comprehensive Income
(Loss) Accumulated
Deficit Total Stockholders'
(Deficit) Equity Stockholders' Equity (Deficit) Shares Amount Amount Amount Amount Amount Common Stock Additional Paid-In Capital Amount
Comprehensive Income
(Loss)
Deficit
(Deficit) Equity 380,328 $ — $ — $ — $ (200,661 ) $ (200,661 ) (60,690 ) (60,690 ) 5,336 13 13 — (1,521 ) (15,950 ) (17,471 ) 1,508 1,508 Shares Amount Amount Amount Amount Amount 385,664 $ — $ — $ — $ (277,301 ) $ (277,301 ) 385,664 $ — $ — $ — $ (277,301 ) $ (277,301 ) (62,479 ) (62,479 ) (62,479 ) (62,479 ) (21 ) (21 ) (21 ) (21 ) — — (4,542 ) (4,458 ) (9,000 ) (4,542 ) (4,458 ) (9,000 ) 41 41 41 41 20,435 — 20,435 — 49,382 170 170 49,382 170 170 7,070 7,070 Share-based compensation expense 7,070 7,070 10,141,268 1 103,803 103,804 10,141,268 1 103,803 103,804 22,327,786 2 298,061 298,063 22,327,786 2 298,061 298,063 3,117 3,117 3,117 3,117 32,924,535 $ 3 $ 407,720 $ (21 ) $ (344,238 ) $ 63,464 December 31, 2014 32,924,535 $ 3 $ 407,720 $ (21 ) $ (344,238 ) $ 63,464 (101,526 ) (101,526 ) (101,526 ) (101,526 ) 26 26 26 26 529,862 — 529,862 — 267,684 2,337 2,337 267,684 2,337 2,337 14,734 14,734 9,262,162 1 482,249 482,250 42,984,243 $ 4 $ 907,040 $ 5 $ (445,764 ) $ 461,285 Share-based compensation expense 14,734 14,734 Balance at Issuance of common stock, net 9,262,162 1 482,249 482,250 December 31, 2015 42,984,243 $ 4 $ 907,040 $ 5 $ (445,764 ) $ 461,285 Net loss (182,804 ) (182,804 ) Unrealized gain from available-for-sale securities 66 66 Exercise of warrants 19,172 — Exercise of options 137,719 2,573 2,573 Share-based compensation expense 26,058 26,058 Balance at Issuance of common stock, net — Balance at December 31, 2016 43,141,134 $ 4 $ 935,671 $ 71 $ (628,568 ) $ 307,178 Year Ended December 31, Year Ended December 31, 2015 2014 2013 2016 2015 2014 $ (101,526 ) $ (62,479 ) $ (60,690 ) $ (182,804 ) $ (101,526 ) $ (62,479 ) 176 77 27 586 176 77 1,714 429 27 791 1,714 429 14,734 7,070 1,508 26,058 14,734 7,070 — 2,717 13,118 — — 2,717 — 505 (9,087 ) — — 505 183 295 387 — 183 295 1,572 57 — — 1,572 57 (4,914 ) (1,639 ) 1,721 4,607 (4,914 ) (1,639 ) (108 ) (105 ) — (291 ) (108 ) (105 ) 3,936 1,991 (250 ) (100 ) 3,936 1,991 (2,870 ) 2,737 8,222 11,349 (2,870 ) 2,737 (87,103 ) (48,345 ) (45,017 ) (139,804 ) (87,103 ) (48,345 ) (1,231 ) (857 ) (2 ) (2,936 ) (1,231 ) (857 ) (579,088 ) (97,678 ) (17,070 ) (260,547 ) (579,088 ) (97,678 ) 340,497 20,470 21,043 499,603 340,497 20,470 (239,822 ) (78,065 ) 3,971 236,120 (239,822 ) (78,065 ) 2,337 170 13 2,573 2,337 170 — 27,368 42,870 — — 27,368 — 24,555 — — — 24,555 482,250 103,804 — — 482,250 103,804 — (116 ) — — — (116 ) (25,000 ) (13,156 ) (8,187 ) — (25,000 ) (13,156 ) (1,502 ) — — — (1,502 ) — 458,085 142,625 34,696 2,573 458,085 142,625 131,160 16,215 (6,350 ) NET INCREASE IN CASH AND CASH EQUIVALENTS 98,889 131,160 16,215 28,518 12,303 18,653 159,678 28,518 12,303 $ 159,678 $ 28,518 $ 12,303 $ 258,567 $ 159,678 $ 28,518 $ 1,490 $ 1,971 $ 1,796 $ — $ 1,490 $ 1,971 Property and equipment purchases in accrued expense at period end $ 675 $ — $ — $ — $ 9,000 $ 17,471 $ — $ — $ 9,000 $ — $ 298,063 $ — $ — $ — $ 298,063 $ — $ 10,109 $ 23,168 $ — $ — $ 10,109 $ — $ 1,552 $ 1,356 $ — $ — $ 1,552 NatureNature of Businesspostmenopausal women with osteoporosis andpostmenopausal osteoporosis. Radius' New Drug Application ("NDA"), for abaloparatide-SC is currently under regulatory review in Europe.by the U.S. Food and Drug Administration ("FDA"), with a Prescription Drug User Fee Act ("PDUFA") date of March 30, 2017. Radius’ European Marketing Authorisation Application ("MAA") for abaloparatide-SC is under review by the Committee for Medicinal Products for Human Use of the EMA ("CHMP"). The Company'sRadius clinical pipeline also includes an investigational abaloparatide transdermal patch ("abaloparatide-TD") for potential use in the treatment of women with postmenopausal osteoporosis and the investigational drug RAD1901 for potential use in the treatment of hormone-driven and/or hormone-resistant breast cancer, andas well as for potential use in the treatment of vasomotor symptoms in postmenopausal women. The Company's preclinicalRadius' clinical pipeline includes RAD140, a non-steroidal selective androgen receptor modulator, under investigation for potential applicationsuse in oncology and multiple conditions where androgen modulation may offer therapeutic benefit.approvalapprovals to market its investigational product candidates, market acceptance of the Company's investigational product candidates following receipt of regulatory approval, competition for its investigational product candidates following receipt of regulatory approval, and the continued ability to obtain adequate financing to fund the Company's future operations. The Company has incurred losses and expects to continue to incur additional losses for the foreseeable future. As of December 31, 2015,2016, the Company had an accumulated deficit of $445.8$628.6 million, and total cash, cash equivalents and marketable securities of $473.3$332.4 million.2015,2016, the Company believes that, prior to the consideration of revenue from the potential future sales of any of its investigational products that may receive regulatory approval or proceeds from partnershippartnering and/or collaboration activities, it has sufficient capital to fund its development plans, U.S. commercial scale-up and other operational activities, for not less than twelve months from the date of this filing and into 2018. The Company expects to finance the future development costs of its clinical product portfolio with its existing cash and cash equivalents and marketable securities, or through strategic financing opportunities that could include, but are not limited to partnering or other collaboration agreements, future offerings of its equity, or the incurrence of debt. However, there is no guarantee that any of these strategic or financing opportunities will be executed or executed on favorable terms, and some could be dilutive to existing stockholders. If the Company fails to obtain additional future capital, it may be unable to complete its planned preclinical studies and clinical trials and obtain approval of certain investigational product candidates from the U.S. Food and Drug AdministrationFDA or foreign regulatory authorities.subsidiary, Radius Health Securities Corporation.subsidiaries. All material intercompany balances and transactions have been eliminated in consolidation.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)2. Summary of Significant Accounting Policies (Continued)equivalentequivalents balance at December 31, 20152016 and 2014.2015.20152016 and 2014.2015.Level 1 Quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. Level 2
Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3
Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)2. Summary of Significant Accounting Policies (Continued)An assessment of inventoryInventory capitalization begins either on or after receiving approval for the date a New Drug Application is accepted for filing byfiled with the U.S. Food and Drug Administration, or an international equivalent. Determining whether or not to continue to record the commercial supply costs related to a product candidate as research and development expenses, or to capitalize these costs as inventory, involves significant judgment. There were no capitalized inventories as of December 31, 20152016 and 2014.2015.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)2. Summary of Significant Accounting Policies (Continued)20152016 and 2014.June 2014 initial public offering in June 2014, the Company uses the simplified method described in the SEC's Staff Accounting Bulletin No. 107,Share-Based Payment, to determine the expected life of the option grants. The estimate of expected volatility is based on a review of the historical volatility of similar publicly held companies in the biotechnology field over a period commensurate with the option's expected term. The Company has never declared or paid any cash dividends on its common stock and does not expect to do so in the foreseeable future. Accordingly, itRadius Health, Inc.Notes to Consolidated Financial Statements (Continued)2. Summary of Significant Accounting Policies (Continued) the two-class method, which is an earnings allocation formula that determines net loss per share for the holders of the Company's common shares and participating securities. Prior to the initial public offering, all of the Company's series of preferred stock contained participation rights in any dividend paid by the Company and were deemed to be participating securities. Net income available to common shareholders and participating preferred shares was allocated to each share on an as-converted basis as if all of the earnings for the period had been distributed. The participating securities do not include a contractual obligation to share in losses of the Company and are not included in the calculation of net loss per share in the periods that have a net loss.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)2. Summary of Significant Accounting Policies (Continued)AugustMay 2014, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update (ASU) No. 2014-09, Revenue from Contracts with Customers (Topic 606), which supersedes all existing revenue recognition requirements, including most industry-specific guidance. The new standard requires a company to recognize revenue when it transfers goods or services to customers in an amount that reflects the consideration that the company expects to receive for those goods or services. The standard will be effective for the year ending December 31, 2018. To date, we have no contracts with customers. We expect to have FDA approval in 2017 and will evaluate our accounting policy at the time.interim and annual fiscal periods beginningperiod ending after December 15, 2016 and interim periods thereafter, with early adoption permitted. The Company does not expect the adoption of ASU 2014-15 todid not have a material impact on itsthe Company's results of operations, financial position or cash flows. In January 2015, the FASB issued Accounting Standards Update No. 2015-01,Income Statement—Extraordinary and Unusual Items (Subtopics 225-20)("ASU 2015-01"). ASU 2015-01 eliminates the concept of extraordinary items from GAAP. The amendments under ASU 2015-01 are effective for interim and annual fiscal periods beginning after December 15, 2015, with early adoption permitted. The Company does not expect the adoption of ASU 2015-01 to have a material impact on its results of operations, financial position or cash flows. In April 2015, the FASB issued Accounting Standards Update No. 2015-03,Interest—Imputation of Interest (Subtopic 835-30)("ASU 2015-03"). ASU 2015-03 requires that, instead of presentation as an asset, debt issuance costs be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The amendments under ASU 2015-03 are effective for interim and annual fiscal periods beginning after December 15, 2015, with early adoption permitted, and should be applied on a retrospective basis. The Company does not expect the adoption of ASU 2015-03 to have a material impact on its results of operations, financial position or cash flows. In April 2015, the FASB issued Accounting Standards Update No. 2015-05,Intangibles-Goodwill and Other-Internal-Use Software (Subtopic 350-40)("ASU 2015-05"). ASU 2015-05 updates guidance regarding accounting for cloud computing arrangements. The amendments under ASU 2015-05 are effective for interim and annual fiscal periods beginning after December 15, 2015, with early adoption permitted. The Company does not expect the adoption of ASU 2015-05 to have a material impact on its results of operations, financial position or cash flows. In November 2015, the FASB issued Accounting Standards Update No. 2015-17,Income Taxes (Topic 740)("ASU 2015-17"). ASU 2015-17 requires deferred tax liabilities and assets to be classified as noncurrent in a classified statement of financial position, instead of separating deferred income tax liabilities and assets into current and noncurrent amounts. The amendments under ASU 2015-17 apply to all entities that present a classified statement of financial position and are effective, for public entities, for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods, with early adoption permitted for all entities as of the beginning of an interim or annual reporting period. The Company elected to early adopt ASU 2015-17 effective December 31, 2015 on a prospective basis. Adoption of this ASU resulted in a reclassification of our net current deferred tax asset to the net non-current deferred tax asset in our Consolidated Balance Sheet as of December 31, 2015. No prior periods were retrospectively adjusted.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)2. Summary of Significant Accounting Policies (Continued)Accounting Standards UpdateASU No. 2016-01,Financial Statements—Overall (Subtopics 825-10)("ASU 2016-01"). ASU 2016-01 provides updated guidance on the recognition and measurement of financial assets and financial liabilities that will supersede most current guidance. ASU 2016-01 primarily affects the accounting for equity investments, financial liabilities under the fair value option, and the presentation and disclosure requirements for financial instruments. The amendments in ASU 2016-01 supersede the guidance to classify equity securities with readily determinable fair values into different categories and require equity securities to be measured at fair value with changes in the fair value recognized through net income.earnings. The amendments under ASU 2016-01 are effective, for public business entities, for periods beginning after December 15, 2017, including interim periods within those fiscal years, and with early adoption permitted. The Company does not expect the adoption of ASU 2016-01 to have a material impact on its results of operations, financial position or cash flows. December 31, 2015 December 31, 2016 Amortized
Cost Value Gross
Unrealized
Gains Gross
Unrealized
Losses Fair
Value
Cost Value
Unrealized
Gains
Unrealized
Losses
Value $ 2,934 $ — $ — $ 2,934 $ 77,443 $ — $ — $ 77,443 83,257 — — 83,257 173,631 — — 173,631 39,984 — — 39,984 5,487 — — 5,487 15,996 — — 15,996 10,007 — — 10,007 2,006 — — 2,006 7,500 — — 7,500 $ 159,678 $ — $ — $ 159,678 $ 258,567 $ — $ — $ 258,567 $ 173,142 $ — $ (107 ) $ 173,035 $ 19,317 $ — $ (2 ) $ 19,315 84,004 154 — 84,158 31,852 78 — 31,930 56,510 1 (43 ) 56,468 22,639 — (4 ) 22,635 $ 313,656 $ 155 $ (150 ) $ 313,661 $ 73,808 $ 78 $ (6 ) $ 73,880 Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)3. Marketable Securities (Continued) December 31, 2014 December 31, 2015 Amortized
Cost Value Gross
Unrealized
Gains Gross
Unrealized
Losses Fair
Value
Cost Value
Unrealized
Gains
Unrealized
Losses
Value $ 1,519 $ — $ — $ 1,519 $ 2,934 $ — $ — $ 2,934 23,994 — — 23,994 83,257 — — 83,257 Domestic corporate commercial paper 39,984 — — 39,984 Government-sponsored enterprise debt securities 15,996 $ — $ — 15,996 3,005 — — 3,005 10,007 $ — $ — 10,007 Asset-backed securities 7,500 $ — $ — 7,500 $ 28,518 $ — $ — $ 28,518 $ 159,678 $ — $ — $ 159,678 69,542 — (33 ) 69,509 $ 173,142 — $ (107 ) $ 173,035 7,237 12 — 7,249 84,004 154 — 84,158 Asset-backed securities 56,510 $ 1 $ (43 ) 56,468 $ 76,779 $ 12 $ (33 ) $ 76,758 $ 313,656 $ 155 $ (150 ) $ 313,661 20152016 or December 31, 2014.2015. There were 5713 debt securities in an unrealized loss position for less than 12 months at December 31, 20152016 and there were 3457 debt securities that had been in an unrealized loss position for less than 12 months at December 31, 2014.2015. The aggregate unrealized loss on these securities as of December 31, 2016 and 2015 and 2014 was less than $150approximately $6 thousand and $34$150 thousand, respectively, and the fair value was $225.7$35.7 million and $68.9$225.7 million, respectively. The Company considered the decline in market value for these securities to be primarily attributable to current economic conditions. As it was not more likely than not that the Company would be required to sell these securities before the recovery of their amortized cost basis, which may be maturity, the Company did not consider these investments to be other-than-temporarily impaired as of December 31, 20152016 and 2014.20152016 and 2014,2015, marketable securities solely consisted of investments that mature within one year.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued) December 31, Estimated Useful
Life (In Years) 2015 2014 5 $ 314 $ 167 3 479 230 10 1,127 598 Shorter of useful life or remaining lease term 322 16 2,242 1,011 (345 ) (169 ) $ 1,897 $ 842 December 31, 2016 2015 Furniture and fixtures, lab and office equipment 5 $ 901 $ 314 Computer equipment and software 3 1,412 479 Manufacturing equipment 10 1,209 1,127 Leasehold improvements Shorter of useful life or remaining lease term 1,253 322 Construction in progress - 1,078 — 5,853 2,242 Less accumulated depreciation and amortization (931 ) (345 ) Property and equipment, net $ 4,922 $ 1,897 Duringyear ended December 31, 2014,assets displayed indicators of impairment that would trigger the Company retired $0.7 million of property and equipment. The retirement was primarily due to the disposal of leasehold improvements and other property asneed for further analysis. As a result of the Company's office relocation. Allqualitative assessment, the Company concluded that there were no indicators of impairment for any property and equipment assets were fully depreciated prior to retirement. December 31, 2016 2015 Research costs—Nordic (1) $ 1,228 $ 2,898 Research costs—other 8,404 5,178 Payroll and employee benefits 9,338 3,330 Professional fees 7,532 3,546 Other current liabilities 95 — Total accrued expenses and other current liabilities $ 26,597 $ 14,952 December 31, 2015 2014 $ 2,898 $ 11,536 5,178 3,336 3,330 1,659 3,546 1,304 — 234 — 198 $ 14,952 $ 18,267 (1) Includes amounts accrued ratably over the estimated per patient treatment period for the services provided by Nordic under the Second Extension. Amounts do not include pass-through costs which are expensed as incurred or upon delivery. See Note 10 for additional information. (1)Includes amounts accrued ratably over the estimated per patient treatment period under the Nordic Work Statement NB-1 and Work Statement NB-3. Amounts do not include pass-through costs which are expensed as incurred or upon delivery. See note 10 for additional information. On 23, 2011, the Company entered into a loan and security agreement with Oxford Finance LLC ("Oxford") and General Electric Capital Corporation ("GECC") pursuant to which Oxford and GECC agreed to lend the Company up to $25.0 million. Upon entering into the loan andRadius Health, Inc.Notes to Consolidated Financial Statements (Continued)6. Loan and Security Agreement (Continued)security agreement, the Company borrowed $6.3 million on May 23, 2011("Term Loan A"), $6.3 million on November 21, 2011 ("Term Loan B") and an additional $12.5 million on May 29, 2012 ("Term Loan C"). Interest on the outstanding Term Loan A was payable on a monthly basis through and including December 1, 2011. Principal and interest payments on Term Loan A was payable in 36 equal monthly installments beginning December 1, 2011 through November 1, 2014, with a final balloon payment of $0.6 million due upon maturity on November 22, 2014. Interest was payable on Term Loan A at an annual interest rate of 10.16%. Interest on the outstanding Term Loan B was payable on a monthly basis through and including June 1, 2012. Principal and interest payments on Term Loan B was payable in 30 equal monthly installments beginning June 1, 2012, through November 1, 2014, with a final balloon payment of $0.6 million due upon maturity on November 22, 2014. Interest was payable on Term Loan B at an annual interest rate of 10%. Interest on Term Loan C was payable on a monthly basis through, and including, November 1, 2012. Principal and interest payments on Term Loan C was payable in 24 monthly installments beginning December 1, 2012, through November 1, 2014 with a final balloon payment of $1.3 million upon maturity on November 22, 2014. Interest was payable on Term Loan C at an annual interest rate of 10%. On May 30, 2014, the Company entered into a loan and security agreement (the "Credit Facility)"2014 Credit Facility"), with Solar Capital Ltd. ("Solar"), as collateral agent and a lender, and Oxford, as a lender (the "Lenders"), pursuant to which Solar and Oxford agreed to make available to the Company $30.0 million in the aggregate subject to certain conditions to funding. An initial term loan was made onin May 30, 2014 in an aggregate principal amount equal to $21.0 million (the "Initial Term Loan"). The Company used approximately $9.3 million of the Initial Term Loan to repay all amounts owed under itsa previous loan and security agreement with GECC and Oxford. Onother financial institutions. 10, 2014, the Company entered into a first amendment to the 2014 Credit Facility (the "First Amendment"). The terms of the First Amendment, among other things, provided the Company with, subject to certain customary funding conditions, additional term loans in an aggregate principal amount of $4.0 million upon the closing of the First Amendment. The Company borrowed the additional $4.0 million on July��10,in July 2014.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)7. Stockholders' Equity and Convertible Preferred Stock (Continued)whereby it soldof 2,750,000 shares of common stock at a price of $18.25 per share, for aggregate proceeds, net of underwriting discounts, commissions, and offering costs, of approximately $46.9 million. On October 7, 2014, the underwriters purchased an additional 378,524 shares, in the aggregate, by exercising a portion of the over-allotment option granted to them in connection with the offering. As a result of the public On April 23, 2013, the Company entered into a Series B Convertible Preferred Stock and Warrant Purchase Agreement (the "Series B Purchase Agreement"), pursuant to which the Company could raise, at any time on or prior to May 10, 2013, up to approximately $60.0 million through the issuance of (1) up to 980,000 shares of its Series B preferred stock (the "Series B") and (2) warrants to acquire up to approximately 1,075,000 shares of its common stock with an exercise price of $14.004 per share. On April 23, 2013, the Company consummated a first closing under the Series B Purchase Agreement, whereby in exchange for aggregate proceeds of approximately $43.0 million, it issued 700,098 shares ofRadius Health, Inc.Notes to Consolidated Financial Statements (Continued)7. Stockholders' Equity and Convertible Preferred Stock (Continued)Series B and warrants to purchase up to a total of 767,651 shares of its common stock. On May 10, 2013, the Company consummated a second closing under the Series B Purchase Agreement, whereby in exchange for aggregate proceeds of approximately $0.1 million, it issued 1,137 shares of Series B and warrants to purchase up to a total of 1,246 shares of its common stock. The warrants can be exercised at any time prior to the fifth anniversary of their issuance.20152016 and December 31, 20142015 (in thousands): As of December 31, 2015 Level 1 Level 2 Level 3 Total $ 2,934 $ — $ — $ 2,934 83,257 — — 83,257 — 39,984 — 39,984 — 15,996 — 15,996 — 10,007 — 10,007 — 7,500 — 7,500 $ 86,191 $ 73,487 $ — $ 159,678 $ — $ 173,035 $ — $ 173,035 — 84,158 — 84,158 — 56,468 — 56,468 $ — $ 313,661 $ — $ 313,661 As of December 31, 2016 Level 1 Level 2 Level 3 Total Assets Cash and cash equivalents: Cash $ 77,443 $ — $ — $ 77,443 Money market funds (1) 173,631 — — 173,631 Domestic corporate commercial paper (2) — 5,487 — 5,487 Domestic corporate debt securities (2) — 2,006 — 2,006 Total $ 251,074 $ 7,493 $ — $ 258,567 Marketable Securities Domestic corporate debt securities (2) $ — $ 19,315 $ — $ 19,315 Domestic corporate commercial paper (2) — 31,930 — 31,930 Asset-backed securities (2) — 22,635 — 22,635 Total $ — $ 73,880 $ — $ 73,880 Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)8. Fair Value Measurements (Continued) As of December 31, 2015 Level 1 Level 2 Level 3 Total Assets Cash and cash equivalents: Cash $ 2,934 $ — $ — $ 2,934 Money market funds (1) 83,257 — — 83,257 Domestic corporate commercial paper (2) — 39,984 — 39,984 Government-sponsored enterprise debt securities (2) — 15,996 — 15,996 Domestic corporate debt securities (2) — 10,007 — 10,007 Asset-backed securities (2) — 7,500 — 7,500 Total $ 86,191 $ 73,487 $ — $ 159,678 Marketable securities: Domestic corporate debt securities (2) $ — $ 173,035 $ — $ 173,035 Domestic corporate commercial paper (2) — 84,158 — 84,158 Asset-backed securities (2) — 56,468 — 56,468 Total $ — $ 313,661 $ — $ 313,661 As of December 31, 2014 Level 1 Level 2 Level 3 Total $ 1,519 $ — $ — $ 1,519 23,994 — — 23,994 — 3,005 — 3,005 $ 25,513 $ 3,005 $ — $ 28,518 $ — $ 69,509 $ — $ 69,509 — 7,249 — 7,249 $ — $ 76,758 $ — $ 76,758 (1) Fair value is based upon quoted market prices. (2) Fair value is based upon quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active and model-based valuation techniques for which all significant assumptions are observable in the market or can be corroborated by observable market data for substantially the full term of the assets. Inputs are obtained from various sources, including market participants, dealers and brokers. (1)Fair value is based upon quoted market prices.(2)Fair value is based upon quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active and model-based valuation techniques for which all significant assumptions are observable in the market or can be corroborated by observable market data for substantially the full term of the assets. Inputs are obtained from various sources, including market participants, dealers and brokers. On 27, 2005, the Company entered into a license agreement (the "Ipsen"License Agreement"), as amended, with SCRAS S.A.S, a French corporation on behalfan affiliate of itself and its affiliates (collectively, "Ipsen"). Under the Ipsen Agreement, Ipsen granted toPharma SAS ("Ipsen") under which the Company an exclusive right and license underexclusively licensed certain Ipsen compound technology and related patents covering abaloparatide to research, develop, manufacture and commercialize certain compounds and related products in all countries, except Japan (where the Company does not hold abaloparatide-SC development and commercialization rights) and France (where the Company'sCompany’s commercialization rights arewere subject to certain co-marketing and co-promotion rights exercisable by Ipsen, provided that certain conditions included in the IpsenLicense Agreement have beenwere met). With respect to France, if Ipsen exercises itsWe believe that Ipsen's co-marketing and co-promotion rights then Ipsen may elect to receive a percentage of the net sales of the products by both parties in France (subject to a mid-double digit percentage cap), and Ipsen shall bear a corresponding percentage of the costs and expenses incurred by both parties with respect to such marketing and promotion efforts in France. Ipsen shall also pay the Company a mid-single digit royalty on Ipsen's allocable portion of net sales of the product by both parties in France. Abaloparatide is subject to the Ipsen Agreement.have permanently expired. Ipsen also granted the Company an exclusive right and license under the Ipsen compound technology and related patents to make and have made compounds or product in Japan. Ipsen alsofurther granted the Company an exclusive right and license under certain Ipsen formulation technology and related patents solely for purposes of enabling the Companyus to develop, manufacture and commercialize compounds and products covered by the compound technology license in all countries, except Japan (where the Company does not hold commercialization rights) and France (where the Company's commercialization rights are subject to(as discussed above).Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)9. License Agreements (Continued)certain co-marketing and co-promotion rights exercisable by Ipsen, provided that certain conditions included in the Ipsen Agreement have been met).licenses,rights, the Company made a nonrefundable, non-creditable paymentpayments in aggregate of $0.25$4.3 million to Ipsen, which was expensed during 2005.Ipsen. The IpsenLicense Agreement provides for further payments upon the achievement of certain future regulatory and commercial milestones, including upon acceptance of a new drug application submission for review by the U.S. Food and Drug Administration. The range ofmilestones. Total additional milestone payments that could be paidpayable under the agreement is €10.0€32.0 million to €36.0 million ($10.9 million to $39.1(approximately $33.6 million). Should abaloparatide be approved and subsequently commercialized, the agreement provides that the Company will be obligated towould pay to Ipsen a fixed five percent royalty based on net sales of the product by the Company or its sublicensees on a country-by-country basis until the later of the last to expire of the licensed patents or for a period of 10 years after the first commercial sale in such country.the rights licensed from Ipsen,abaloparatide to a third party, then the agreement provides that the Company will also be required towould pay Ipsen a percentage of certain payments received from such sublicensee (in lieu of milestone payments not achieved at the time of such sublicense). The applicable percentage is in the low double digit range. In addition, if the Company or its sublicensees commercialize a product that includes a compound discovered by it based on or derived from confidential Ipsen know-how, it will be obligated tothen the agreement provides that the Company would pay to Ipsen a fixed low single digit royalty on net sales of such product on a country-by-country basis until the later of the last to expire of licensed patents that cover such product or for a period of 10 years after the first commercial sale of such product in such country.The Eisai Agreement provides for further payments in the range of $1.0 million to $20.0 million (inclusive of the $0.5 million initial license fee), payable upon the achievement of certain clinical and regulatory milestones. OnIn March 9, 2015, the Company entered into an amendment to the Eisai Agreement (the "Eisai Amendment") in which Eisai granted to the Company the exclusive right and license to research, develop, manufacture and commercialize RAD1901 in Japan. In consideration for the rights to RAD1901 in Japan, the Company paid Eisai an initiala license fee of $0.4 million upon execution of the Eisai Amendment, which was recognized as research and development expense in 2015. The Eisai Amendment, as amended, also provides for additional payments of up to $22.3 million, payable upon the achievement of certain clinical and regulatory milestones in Japan.valid claimlicensed patent is expected to expire, barring any extension thereof, is expected on August 18, 2026.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)9. License Agreements (Continued)a lawful generic versionversions of the product account for more than a specified percentage of the total sales of all pharmaceutical products containing the licensed compound in that country; or (2) a period of 10 years after the first commercial sale of the licensed products in such country, unless it is sooner terminated.On March 29, 2011, The Company contracted with Nordic Bioscience Clinical Development VII A/S ("Nordic") to conduct the Company and Nordic entered into a Clinical Trial Services Agreement (the "Clinical Trial Services Agreement"), a Work Statement NB-1, as amended on December 9, 2011, June 18, 2012, March 28, 2014, May 19, 2014, July 22, 2014, August 15, 2014 and March 12, 2015 (the "Work Statement NB-1") and a Stock Issuance Agreement, as amended and restated on May 16, 2011, and as further amended on February 21, 2013, March 28, 2014, and May 19, 2014 (the "Stock Issuance Agreement"). Pursuant to the Work Statement NB-1, Nordic managed theCompany's Phase 3 clinical trial of abaloparatide-SC (the "Phase 3 Clinical Trial").recognized research and development expensealso contracted with Nordic for the amounts dueNordic to Nordic under the Work Statement NB-1 ratably over the estimated per patient treatment period beginning upon enrollment in the Phase 3 Clinical Trial, or a twenty-month period. The Company recognized research and development expense for the amounts due to Nordic under the fourth amendment to the Work Statement NB-1, which was recognized on a per patient basis when the end-of-study visit and all other required procedures were completed. The Company recorded no expense, $8.2 million, and $31.6 million during the years ended December 31, 2015, 2014, and 2013, respectively, for per patient costs incurred for patients that had enrolled in the Phase 3 Clinical Study. As of December 31, 2015, all obligations due to Nordic under Work Statement NB-1 had been paid. Abaloparatide-SC Phase 3 Clinical Extension Study—On February 21, 2013, the Company entered into a Work Statement NB-3, as amended on February 28, 2014, March 23, 2015, July 8, 2015 and October 21, 2015 (the "Work Statement NB-3"). Pursuant to the Work Statement NB-3, Nordic performedperform an extension study to evaluate six months of standard-of-care osteoporosis management following the completion of the Phase 3 Clinical Trial (the "Extension Study"), and, upon completion of this initial six months, an additional period of 18 months of standard-of-care osteoporosis management (the "Second Extension").entered into an amendmentcontracted with Nordic to the Work Statement NB-3 (the "NB-3 Amendment"). The NB-3 Amendment was effective as of March 23, 2015 and provides that Nordic will perform additional services, including additional monitoring of patients enrolled in the Second Extension. Payments in cash to be made to Nordic under the NB-3 Amendmentfor these additional services are denominated in euros and total up to approximately €4.1 million ($4.5(approximately $4.3 million).Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)10. Research Agreements (Continued)underfor the Work Statement NB-3services related to the Extension Study and Second Extension are denominated in both euros and U.S. dollars and total up to €11.9 million ($12.9(approximately $12.5 million) and $1.1 million, respectively. In addition, payments are due to Nordic in connection with the Work Statement NB-3 pursuant to the Stock Issuance Agreement, as discussed below. As of December 31, 2015, services related to2016, the last patient's final visit in the Second Extension are ongoinghad occurred and all obligations due to Nordic in relation to the Extension Study have been paid.$9.6 million, and $4.5$9.6 million, for the years ended December 31, 2016, 2015, 2014, and 20132014 respectively, for per patient costs incurred.2015,2016, the Company had a liability of $2.9$1.2 million reflected in accrued expenses and other current liabilities on the consolidated balance sheet resulting from services provided by Nordic under the Second Extension, which are payable in cash.Issuance Agreement—Pursuant to the Stock Issuance Agreement, Nordic agreed to purchase 6,443 sharesBenefit Plansthe Company's Series A-5 convertible preferred stock, which provided Nordic with the right to receive quarterly stock dividends, payable in shares of the Company's Series A-6 convertible preferred stock ("Series A-6") for services rendered under Work Statement NB-1 and Work Statement NB-3. The Stock Issuance Agreement was later amended to provide that in the event an initial public offering of the Company's common stock occurred prior to June 30, 2014, any rights to receive stock dividends in relation to Work Statement NB-1 and Work Statement NB-3, for all periods of time after 2014, would be changed from the right to receive stock to the right to receive a total cash payment from the Company of $4.3 million, payable in ten equal monthly installments of $430,000 beginning on March 31, 2015. The amendment also stipulated that all consideration to be paid to Nordic pursuant to the Stock Issuance Agreement at any time after the consummation of an initial public offering be payable in cash. As the Company completed an initial public offering on June 11, 2014, Nordic no longer has the right to receive stock from the Company and has been paid in cash for all periods after June 11, 2014. Prior to the issuance of shares of stock to Nordic in satisfaction of quarterly dividends earned under Work Statement NB-1 and Work Statement NB-3, the liability to issue shares of stock was being accounted for as a liability in the Company's balance sheet, based upon the fair value of the Series A-6. Changes in the fair value from the date of accrual to the date of issuance of the Series A-6 shares were recorded as a gain or loss in other (expense) income in the statement of operations.11. Stock-based Compensation2015,2016, under which equity awards have been granted to employees, directors and consultants:••2015,2016, an aggregate ofRadius Health, Inc.Notes to Consolidated Financial Statements (Continued)11. Stock-based Compensation (Continued)6,160,0009,860,000 shares have been authorized for issuance under the Company's stock-based compensation plans, with approximately 4,408,0006,374,000 options outstanding. The number of common shares available for granting of future awards under these plans was approximately 1,100,0002,960,000 at December 31, 2015."Incentive"2003 Plan") provides for the granting of incentive stock options and nonqualified options to key employees, directors and consultants of the Company. The exercise price of the incentive stock options, as determined by the Company's board of directors, must be at least 100% (110% in the case of incentive stock options granted to a stockholder owning in excess of 10% of the Company's common stock) of the common stock fair value as of the date of the grant. The provisions of the Incentive2003 Plan limit the exercise of incentive stock options, but in no case may the exercise period extend beyond ten years from the date of grant (five years in the case of incentive stock options granted to a stockholder owning in excess of 10% of the Company's common stock). Stock options generally vest over a four-year period. Certain options contain explicit performance conditions. The Company authorized approximately 884,000 shares of common stock for issuance under the Incentive2003 Plan."Equity"2011 Plan") provides for the granting of incentive stock options and nonqualified options to key employees, directors and consultants of the Company. The exercise price of the incentive stock options, as determined by the Company's board of directors, must be at least 100% (110% in the case of incentive stock options granted to a stockholder owning in excess of 10% of the Company's common stock) of the common stock fair value as of the date of the grant. The provisions of the Equity2011 Plan limit the exercise of incentive stock options, but in no case may the exercise period extend beyond ten years from the date of grant (five years in the case of incentive stock options granted to a stockholder owning in excess of 10% of the Company's common stock). Stock options generally vest over a four-year period.period, subject to continued employment with, or services to, the Company. During 2015, the Company also issued stock options to certain members of its board of directors which vested immediately. Certain options contain explicit performance conditions. The Company has authorized approximately 5,276,0008,976,000 shares of common stock for issuance under the Equity2011 Plan. In addition, the shares remaining available for issuance under the Incentive2003 Plan were assumed as shares authorized under the Equity2011 Plan.hashad historically determined, with input from management, the estimated fair value of the Company's common stock on the date of grant based on a number of objective and subjective factors, including:••••Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)11. Stock-based Compensation (Continued)• Subsequent toEquity2011 Plan.2013 was $30.52, $8.26 and $4.67 respectively. The weighted-average assumptions used in the Black-Scholes option-pricing model were as follows: Years Ended
December 31, 2015 2014 2013 2016 2015 2014 6.08 6.06 6.25 5.95 6.08 6.06 55 % 59 % 62 % 55 % 55 % 59 % 0 % 0 % 0 % 0 % 0 % 0 % 1.72 % 2.06 % 2.45 % 1.91 % 1.72 % 2.06 % 20152016 is as follows (in thousands, except for per share and weighted-average contractual life amounts): Shares Weighted-Average
Exercise Price
(in dollars
per share) Weighted-Average
Contractual Life
(In Years) Aggregate
Intrinsic
Value 3,220 $ 13.58 1,487 57.75 (268 ) 8.73 (31 ) 17.31 — — 4,408 $ 28.75 8.24 $ 151,544 1,738 $ 12.21 6.96 $ 85,747 4,316 $ 28.43 8.22 $ 149,570 Shares
Exercise Price
(in dollars
per share)
Contractual Life
(In Years) Options outstanding at December 31, 2015 4,408 $ 28.75 Granted 2,259 37.08 Exercised (138 ) 18.69 Cancelled (155 ) 41.41 Expired — — Options outstanding at December 31, 2016 6,374 $ 31.60 7.89 $ 76,364 Options exercisable at December 31, 2016 2,687 $ 21.28 6.59 $ 54,659 Options vested or expected to vest at December 31, 2016 6,269 $ 31.43 7.87 $ 75,884 2014 was $14.7 million, respectively.$0.7 million, respectively.when the RSU vests. The RSUs vest in four substantially equal installments on each of the first four anniversaries of the vesting commencement date, subject to the employee's continued employment with, or services to, the Company on such vesting date. Compensation expense is recognized on a straight line basis.TableContentsRadius Health, Inc.NotesRSU activity during the nine months ended December 31, 2016 is as follows (in thousands, except for per share amounts): RSUs RSUs Outstanding at December 31, 2015 — $ — Granted 59 33.03 Vested — — Forfeited (2 ) 33.03 RSUs Outstanding at December 31, 2016 57 $ 33.03 Consolidated Financial Statements (Continued)11. Stock-based Compensation (Continued) Years Ended December 31, 2015 2014 2013 $ 7,864 $ 1,953 $ 302 6,870 5,117 1,206 $ 14,734 $ 7,070 $ 1,508 Years Ended December 31, 2016 2015 2014 Research and development $ 11,190 $ 7,864 $ 1,953 General and administrative 14,868 6,870 5,117 Share-based compensation expense included in operating expenses $ 26,058 $ 14,734 $ 7,070 analysis.analysis performed by an independent valuation firm. This valuation methodology utilizes several key assumptions including the forecasted stock price, stock price volatility, risk-free rate as of valuation date, stock price as of grant date, and the trigger for the performance condition to be met.2015,2016, there was approximately $51.3$0.4 million of total unrecognized compensation expense related to unvested share-based compensation arrangements,PUs, which is expected to be recognized over a weighted-average period of approximately 32 years.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued) Year Ended December 31, 2015 2014 2013 $ (101,526 ) $ (62,479 ) $ (60,690 ) — (9,000 ) (17,471 ) (101,526 ) (71,479 ) (78,161 ) — — — $ (101,526 ) $ (71,479 ) $ (78,161 ) 39,643,099 17,699,487 383,310 $ (2.56 ) $ (4.04 ) $ (203.91 ) Year Ended December 31, 2016 2015 2014 Numerator: Net loss $ (182,804 ) $ (101,526 ) $ (62,479 ) Accretion of preferred stock — — (9,000 ) Loss attributable to common stockholders—basic (182,804 ) (101,526 ) (71,479 ) Effect of dilutive convertible preferred stock — — — Loss attributable to common stockholders—diluted $ (182,804 ) $ (101,526 ) $ (71,479 ) Denominator: Weighted-average number of common shares used in loss per share— basic and diluted 43,067,952 39,643,099 17,699,487 Loss per share—basic and diluted $ (4.24 ) $ (2.56 ) $ (4.04 ) 2014, and 20132014 all of the Company's classes of convertible preferred stock, options to purchase common stock, warrants and performance units outstanding were assumed to be anti-dilutive as earnings attributable to common stockholders was in a loss position. Year Ended December 31 Year Ended December 31 2015 2014 2013 2016 2015 2014 — 3,857,664 6,617,686 — — 3,857,664 3,903,051 2,466,492 1,743,890 5,815,168 3,903,051 2,466,492 822,726 1,271,520 545,797 630,444 822,726 1,271,520 — — — Restricted Stock Units 42,363 — — As ofCompany had federal and stateCompany’s net operating loss ("NOL") carryforwards of approximately $419.5 millionlosses (NOLs) and $323.0 million, respectively, which may be used to offset future taxable income. The Company also had federal and state tax credits of $5.8 million and $0.8 million, respectively, to offset future tax liabilities. The NOL and tax credit carryforwards will expire at various dates through 2035, and are subject to review and possible adjustment by federal and state tax authorities. The Internal Revenue Code contains provision that may limit the NOL and tax credit carryforwards available to be used in any given year in the event of certain changes in the ownership interests of significant stockholders under Section 382 of the Internal Revenue Code.full valuation allowance.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)13. Income Taxes (Continued)Approximately $14.1 million of the federal and state NOL carryforwards are attributable to excess tax benefits which will be recorded as an increase to additional paid-in capital when realized. Year Ended December 31, Year Ended December 31, 2015 2014 2013 2016 2015 2014 $ (34,391 ) $ (21,243 ) $ (20,635 ) $ (62,141 ) $ (34,391 ) $ (21,243 ) (4,434 ) (2,494 ) (2,255 ) (5,236 ) (4,434 ) (2,494 ) 752 149 92 1,585 752 149 (1,469 ) (499 ) (1,277 ) (2,794 ) (1,469 ) (499 ) 39,291 23,186 27,194 48,096 39,291 23,186 26 910 (3,085 ) 53 26 910 225 (9 ) (34 ) 1,371 225 (9 ) $ — $ — $ — Expiring NOLs and credits - 382 Limitation $ 19,066 — — Income tax expense $ — $ — $ — December 31, 2015 2014 $ — $ 671 — 671 — (671 ) $ — $ — $ 154,239 $ 121,278 263 356 6,313 4,844 (119 ) (47 ) 1,073 — 7,753 3,158 29 — 169,551 129,589 (169,551 ) (129,589 ) $ — $ — December 31, 2016 2015 Non-current assets: NOL carryforwards $ 193,436 $ 154,239 Capitalized research and development 1,970 263 Research and development credits 4,525 6,313 Depreciation and amortization (173 ) (119 ) Accrued expenses 3,109 1,073 Stock-based compensation 14,903 7,753 Other 55 29 Gross non-current deferred tax assets 217,825 169,551 Valuation allowance (217,825 ) (169,551 ) Net non-current deferred tax assets $ — $ — Effective December 31, 2015, the Company early adopted ASU 2015-17 on a prospective basis. ASU 2015-17 requires deferred tax liabilities and assets to be classified as noncurrent in a classifiedTable of ContentsFASB ASC 740Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)13. —Income Taxes (Continued)statement of financial position, instead of separating deferred income tax liabilities and assets into current and noncurrent amounts. Adoption of this ASU resulted inrequires that a reclassification of our net currentvaluation allowance be established to reduce a deferred tax asset to the net non-currentits realizable value when it is more likely than not that all or a portion of a deferred tax asset will not be realized. A review of all available positive and negative evidence needs to be considered, including the utilization of past tax credits and length of carry-back and carry-forward periods, reversal of temporary differences, tax planning strategies, our current and past performance, the market environment in our Consolidated Balance Sheet aswhich we operate, and the evaluation of December 31, 2015. No prior periods were retrospectively adjusted.20152016 primarily relates to the net loss incurred by the Company.hashad no material unrecognized tax benefits or related interest and penalties accrued.uncertain tax positions. The Company has not, as yet, conducted a study of its research and development credit carryforwards. In addition, the Company is in the process of conducting an Internal Revenue Code Section 382Such a study which may impact its ability to utilize available NOL and tax credit carryforwards. These studies may result in adjustmentsan adjustment to the Company'sCompany’s research and development credit carryforwards and NOL carryforwards; however, until a study is completed and any adjustment is known, no amounts areamount is being presented as an uncertain tax position. A full valuation allowance has been provided against the Company'sCompany’s research and development credits and net operating loss carryforward and, if an adjustment is required, this adjustment would be offset by an adjustment to the valuation allowance. Thus, there would be no impact to the consolidated balance sheet or consolidated statement of operations if an adjustment were required. The Company would recognize both accrued interest and penalties related to unrecognized benefits in income tax expense. The Company has not recorded anyrecognizes interest or penalties on any unrecognized benefits since inception.20122013 through 2015.2016. The Company files income tax returns in the United States Colorado, Connecticut, Florida, Pennsylvania, New Jersey, New York, and Massachusetts.several states. There are currently no federal or state audits in progress.and New Jersey and Pennsylvania under non-cancellable operating leases that expire over various terms through the end of 2020.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)14. Commitments and Contingencies (Continued) Future Lease
Commitments
Commitments $ 1,944 2,214 $ 3,020 1,613 2,203 1,327 1,499 1,175 1,192 $ 8,273 $ 7,914 and 2013 was $0.6$2.8 million, $0.2$0.6 million and $0.2 million, respectively.15. Related Party TransactionsManufacturing Agreements On July 24, 2013,—In June 2016, the Company entered into a ConsultingSupply Agreement with Morana Jovan-Embiricos, Ph.D.Ypsomed AG (“Ypsomed”), pursuant to which Ypsomed agreed to supply commercial and clinical supplies of a disposable pen injection device (the "Consulting Agreement""Device"), customized for subcutaneous injection of abaloparatide. The Company has agreed to purchase a memberminimum number of Devices at prices per Device that decrease with an increase in quantity supplied. In addition, the Company has agreed to make milestone payments for Ypsomed’s capital developments in connection with the initiation of the Company's boardcommercial supply of directors. Pursuantthe Device and to pay a one-time capacity fee. All costs and payments under the Consulting Agreement, Dr. Jovan-Embiricos agreedagreement are delineated in Swiss Francs. The agreement has an initial term of three years from the earlier of the date of delivery of the first commercial batch of Devices after regulatory approval and June 1, 2017, after which, it automatically renews for two-year terms until terminated. The Company will purchase the Device subject to provide financial and strategic consulting services as may be requested byminimum annual quantity requirements over the initial three-year term of the agreement. During the initial term of the agreement, the Company estimates that it will be obligated to make total minimum payments to Ypsomed of approximately CHF 3.9 million ($4.0 million) in the aggregate, including the milestone payments and such other consulting services as may be reasonably requested by the Company, from time to time from July 1, 2013 untilone-time capacity fee.30, 2014. The Company agreed to pay Dr. Jovan-Embiricos an aggregate consulting fee in cash of $160,000, of which $80,000 was paid on July 30, 2013 and the remaining $80,000 was paid on October 2, 2013. As of December 31, 2015, no amounts were due to or from Dr. Jovan-Embiricos. On January 23, 2014,2016, the Company entered into a consulting agreementCommercial Supply Agreement with Orbit Advisors Limited (the "Orbit Agreement"Vetter Pharma International GmbH (“Vetter”), a Swiss company ("Orbit"), and Morana Jovan-Embiricos, Ph.D. and an agreement terminating the Consulting Agreement dated July 24, 2013. The Orbit Agreement was effective as of January 22, 2014 and would continue in effect until December 31, 2014 or until the earlier termination thereof in accordance with its terms (the "Term"). Pursuantpursuant to the Orbit Agreement, Orbit hadwhich Vetter has agreed to provide financialformulate the finished abaloparatide-SC drug product containing the active pharmaceutical ingredient (“API”) of abaloparatide, to fill cartridges with the drug product, to assemble the pen delivery device, and strategic consultingto package the pen for commercial distribution. The Company has agreed to purchase the cartridges and pens in specified batch sizes at a price per unit. For labeling and packaging services, as may be requested by the Company and such other consulting services as may have been reasonably requested by the Company, from time to time during the Term. The Companyhas agreed to pay Orbita per unit price dependent upon the number of pens loaded with cartridges that are labeled and packaged. These prices are subject to an aggregate consulting fee in cash of $400,000 in four equal installments of $100,000 on each of January 31, 2014, June 30, 2014, September 30, 2014 and December 31, 2014.annual price adjustment. The Orbit Agreement contained customary provisions, applicableCompany will purchase these services subject to both Orbit and Dr. Jovan-Embiricos, as Orbit's representative underminimum annual quantity requirements over the Orbit Agreement, regarding the treatmentinitial five-year term of the Company's confidential information and assignmentagreement. The agreement has an initial term of inventions, as well as an obligationfive years, which began on January 1, 2016, after which, it automatically renews for two-year terms unless either party notifies the other party two years before the end of Orbit and Dr. Jovan-Embiricosthe then-current term that it does not intend to not solicit, during the Term and for a period of one year thereafter, any person or entity engaged by the Company as an employee, customer or supplier of, or consultant or advisor to, the Company to terminate such party's relationship with the Company. On February 27, 2014,renew.letterManufacturing Services Agreement with Polypeptide Laboratories Holding AB ("PPL"), as successor-in-interest to Lonza Group Ltd., pursuant to which PPL has agreed to manufacture the commercial and clinical supplies of the API for abaloparatide. The Company has agreed to purchase the API in batches at a price per gram in euros, subject to an annual increase by PPL. The Company is also required to purchase a minimum number of batches annually. The agreement terminatinghas an initial term of a six years, after which, it automatically renews for three-year terms unless either party provides notice of non-renewal 24 months before the Orbit Agreement. Asend of December 31, 2015, no amounts were due to or from Orbit Advisors Limited.the then-current term.Radius Health, Inc.Notes to Consolidated Financial Statements (Continued)16.20152016 and 20142015 is as follows (in thousands, except for share and per share data): Three Months Ended Three Months Ended March 31, June 30, September 30, December 31, 2016: Net loss $ (40,463 ) $ (43,435 ) $ (46,186 ) $ (52,720 ) Net loss applicable to common stock (40,463 ) (43,435 ) (46,186 ) (52,720 ) Net loss per share—basic and diluted (0.94 ) (1.01 ) (1.07 ) (1.22 ) Weighted-average common shares outstanding—basic and diluted 43,012,924 43,042,883 43,092,921 43,122,210 March 31, June 30, September 30, December 31, $ (17,057 ) (22,965 ) (28,264 ) $ (33,240 ) $ (17,057 ) $ (22,965 ) $ (28,264 ) $ (33,240 ) (17,057 ) (22,965 ) (28,264 ) (33,240 ) (17,057 ) (22,965 ) (28,264 ) (33,240 ) (0.47 ) (0.61 ) (0.68 ) (0.77 ) (0.47 ) (0.61 ) (0.68 ) (0.77 ) 36,268,975 37,895,651 41,331,612 42,924,137 36,268,975 37,895,651 41,331,612 42,924,137
$ (14,488 ) $ (12,609 ) $ (17,420 ) $ (17,962 ) (19,457 ) (16,640 ) (17,420 ) (17,962 ) (50.45 ) (2.22 ) (0.59 ) (0.55 ) 385,664 7,500,148 29,746,426 32,678,459 2015.2015,2016, based on the criteria set forth in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on that assessment, our management concluded that our internal control over financial reporting was effective as of December 31, 2015.20152016 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which is contained in Item 9A of this Annual Report on Form 10-K.20152016 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.2015,2016, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Radius Health, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.2015,2016, based on the COSO criteria.20152016 and 2014,2015, and the related consolidated statements of operations and comprehensive loss, convertible preferred stock, redeemable convertible preferred stock and stockholders' equity (deficit) and cash flows for each of the three years in the period ended December 31, 20152016 of Radius Health, Inc. and our report dated February 25, 201624, 2017 expressed an unqualified opinion thereon. /s/ Ernst & Young LLP 25, 201624, 2017following table setsinformation required with respect to this item will be set forth the name, age and positionin our definitive Proxy Statement to be delivered to our stockholders in connection with our Annual Meeting of each of our executive officers and directors:NamePositionRobert E. Ward58President, Chief Executive Officer and DirectorLorraine Fitzpatrick, M.D. 61Chief Medical OfficerB. Nicholas Harvey55Senior Vice President, Chief Financial Officer, Treasurer and SecretaryGary Hattersley, Ph.D. 49Senior Vice President, Chief Scientific OfficerBrent Hatzis-Schoch. 51Senior Vice President, General CounselDinesh Purandare52Senior Vice President, Head of Global OncologyDavid Snow54Chief Commercial OfficerGregory Williams, Ph.D. 56Chief Development OfficerAlan H. Auerbach(3)(4)46DirectorWillard H. Dere, M.D(1)(2). 62DirectorCatherine Friedman(1)(3)55DirectorAnsbert K. Gadicke, M.D.(2)(3)57DirectorJean-Pierre Garnier(3)68DirectorKurt C. Graves(2)(3)(4)48Chairman of the BoardOwen Hughes(1)41DirectorAnthony Rosenberg(4)62DirectorDebasish Roychowdhury(2)54Director
Stockholders, which currently is expected to be held on June 7, 2017. Such information is incorporated herein by reference.(1)Member of the audit committee.(2)Member of the nominating and corporate governance committee.(3)Member of the compensation committee.(4)Member of the strategy committee.Robert E. Ward has served as our President and Chief Executive Officer and as a member of ourOur Board of Directors since December 2013. Prioradopted a Code of Conduct and Ethics applicable to joining Radius, Mr. Ward was Vice President for Strategy and External Alliances for the New Opportunities iMed of AstraZeneca, a biopharmaceutical company, from 2011 to 2013. In addition, he served as Co-Chair of the Joint Development Committees in Astra Zeneca's drug development partnerships with Alcon and Galderma. Prior to AstraZeneca, from 2010 to 2011, Mr. Ward was the Managing Director of Harriman Biopartners, LLC, a biopharmaceutical company, and from 2006 to 2010 he was the Vice President of Corporate Development for NPS Pharmaceuticals, a pharmaceutical company. Mr. Ward received a B.A. in Biology and a B.S. in Physiological Psychology, both from the University of California, Santa Barbara; an M.S. in Management from the New Jersey Institute of Technology; and an M.A. in Immunology from The Johns Hopkins University School of Medicine. We believe Mr. Ward is qualified to serve as a member of our Board of Directors because of his role with us and his extensive operational knowledge of, and executive level management experience in, the global biopharmaceutical industry.Lorraine Fitzpatrick, M.D., has served as our Chief Medical Officer since July 2015. Prior to joining Radius, Dr. Fitzpatrick was a Medicine Development Leader and Group Director at GlaxoSmithKline, a pharmaceutical company, from August 2006 to July 2015. Prior to GlaxoSmithKline, she was an Executive Director at Amgen Inc., a biopharmaceutical company, focusingon osteoporosis and oncology from 2004 to 2006. She has served as Chair of the General Clinical Research Center study section of the National Center for Research Resources, National Institute of Health, or NIH; on the Advocacy Committee of the American Society of Bone and Mineral Research, or ASBMR; and as Chair of the Public Communications Committee and the Media Relations Steering Committee of The Endocrine Society, TES. She has also been a member of the Publications Committee of the ASBMR and TES and on the Advisory Committee for the Office of Research on Women's Health at the NIH. Dr. Fitzpatrick has served on the National Committee for Quality Assurance Technical Subgroup on Osteoporosis, the Clinical Guidelines Committee of TES, the Scientific Program Committees of North American Menopause Society and the ASBMR, and as Associate Editor for The Mayo Clinic Proceedings and the American Medical Association Scientific Advisory Board: Osteoporosis Guidelines. Dr. Fitzpatrick received a B.S. in Molecular Biology from Wellesley College and received her medical degree from the Pritzker School of Medicine at the University of Chicago.B. Nicholas Harvey has served as our Senior Vice President, Chief Financial Officer, Treasurer and Secretary since November 2010, and served as a member of our Board of Directors from November 2010 until the consummation of the merger of our predecessor company with us in May 2011, or the Merger. Prior to that, Mr. Harvey served as the Chief Financial Officer and Senior Vice President of our predecessor company from December 2006 until the Merger. Mr. Harvey received a Bachelor of Economics degree and a Bachelor of Laws degree with first-class honors from the Australian National University and an M.B.A. from the Harvard Business School.Gary Hattersley, Ph.D., served as Chief Scientific Officer since January 2014. Prior to his current role, Dr. Hattersly served as our Senior Vice President of Preclinical Development from December 2011 to December 2013, and President of Biology from May 2011 to December 2011. From 2003 until the Merger, Dr. Hattersly served in various roles in our predecessor company, including as Vice President of Biology, Senior Director of Research and Director of Disease Biology & Pharmacology. Dr. Hattersley received a Ph.D. in Experimental Pathology from St. George's Hospital Medical School.Brent Hatzis-Schoch has served as our Senior Vice President, General Counsel, since April 2015. Prior to joining Radius, from July 2013 to April 2015. Mr. Hatzis-Schoch was Senior Vice President and Chief Legal Counsel of Merz Pharma in Frankfurt, Germany. Prior to Merz, Mr. Hatzis-Schoch served for five years as General Counsel to Agennix AG, a publicly-traded development stage biopharmaceutical company. He has held senior legal positions in the U.S. and internationally, including as European legal counsel for Baxter International, Associate General Counsel of Pharmacia Corporation, and General Counsel of GPC Biotech AG. Mr. Hatzis-Schoch holds a J.D. from George Washington University and a B.A. from the University of Delaware.Dinesh Purandare has served as our Senior Vice President, Head of Global Oncology since March 2015. Prior to joining Radius, Mr. Purandare spent five years at Sanofi Oncology, a pharmaceutical company. He held the role of Vice President and Head of Marketing from March 2010 to September 2012, and Vice President and Project Head, from October 2012 to March 2015. He also co-chaired the Joint Development Committee (Oncology) of Sanofi and Regeneron Pharmaceuticals and was a member of the Oncology Management Team at Sanofi. Prior to Sanofi, he served as Vice President and Head of Oncology Center of Excellence at GlaxoSmithKline headquarters in the UK and held other senior positions at Pharmacia /Pfizer and Farmitalia Carlo Erba (Milan, Italy). Mr. Purandare received degrees in Business Management and Organic Chemistry from the University of Bombay. He also received a Diploma in Advanced Marketing Management from the Chartered Institute of Marketing, U.K.David Snow has served as our Chief Commercial Officer since September 2015. Prior to joining Radius, Mr. Snow was President of the biopharmaceutical company, AstraZeneca's China business from January 2012 to December 2014. He was also the first global commercialization Vice President forAstraZeneca's prescription medication Brilinta and head of U.S. Commercial Operations from March 2010 to December 2011. Before joining AstraZeneca, Mr. Snow held numerous global and US commercial leadership roles for Bristol-Myers Squibb, Searle and Hoechst-Roussel. He served on the Research and Development based Pharmaceutical Association Committee industry association board in China for several years. Mr. Snow received his B.S. in Business Administration from Auburn University, and an M.B.A from New York University—Leonard N. Stern School of Business.Gregory Williams, Ph.D., has served as our Chief Development Officer since January 2014. Prior to joining Radius, Dr. Williams was Vice President of Regulatory Affairs, Global Product and Clinical Development, and Program Management with The Medicines Company, a biopharmaceutical company, from 2006 to 2013. He was Vice President of Regulatory Affairs, Regulatory Compliance and Program Management for NPS Pharmaceuticals, a pharmaceutical company, from 2004 to 2006. Dr. Williams has a Ph.D. in Biopharmaceutics from Rutgers University and an M.B.A. from Cornell University.Alan H. Auerbach has served on our Board of Directors since May 2011 and served as a member of the Board of Directors, our predecessor company from October 2010 until the Merger. Mr. Auerbach is currently the Founder, Chief Executive Officer, PresidentChief Financial Officer, other officers of Radius and Chairmanall other employees of the BoardRadius. The Code of Puma Biotechnology, Inc., a company dedicated to in-licensingConduct and developing drugs for the treatment of cancer and founded in 2010. Previously, Mr. Auerbach founded Cougar Biotechnology, or Cougar, in May 2003 and served as the company's Chief Executive Officer, President and as a member of its Board of Directors until July 2009. From July 2009 until January 2010, Mr. Auerbach served as the Co-Chairman of the Integration Steering Committee at Cougar after its acquisition by Johnson & Johnson. Mr. Auerbach received a B.S. in Biomedical Engineering from Boston University and an M.S. in Biomedical Engineering from the University of Southern California. We believe Mr. AuerbachEthics is qualified to serve as a member of our Board of Directors because of his business and professional experience, including his leadership of Cougar in drug development, private and public financings and a successful sale of the business. Willard H. Dere, M.D. has servedavailable on our Board of Directors since November 2014. Dr. Dere has been Executive Director of Personalized Health at the University of Utah Health Sciences Center, and a Professor of Medicine in the School of Medicine since November 2014. Priorwebsite, http://radiuspharm.com/.that, he served at Amgen Inc., a biopharmaceutical company, as the Senior Vice President, Global Development from December 2004 to June 2007, and from April 2014 to October 2014, and as International Chief Medical Officer from January 2007 to April 2014. Before he joined Amgen in 2003, Dr. Dere served as Vice President of Endocrine, Bone and General Medicine Research and Development at Eli Lilly and Company, a biopharmaceutical company, where he also held various other roles in clinical pharmacology, regulatory affairs, and both early-stage translational, and late-stage clinical research. Dr. Dere received B.A. degrees in history and zoology and a M.D. degree from the University of California, Davis. We believe Mr. Dere is qualified to serve as a member of our Board of Directors because of his strong medical background and extensive experience in the pharmaceutical industry.Jean-Pierre Garnier has serveddisclose on our Boardwebsite any amendments to, or waivers from, our Code of Directors since December 2015. Mr. Garnier is currently Chairman of the Board of Actelion Ltd.,Conduct and was previously Chief Executive Officer of GlaxoSmithKline plc from 2000Ethics that are required to 2008. In addition, Mr. Garnier is also a member of the Board of Directors of United Technologies Corporation and of Renault S.A., and an Operating Partner at Advent International, a global private equity firm. Mr. Garnier previously served as Chief Executive Officer of Pierre Fabre S.A. from 2008be disclosed pursuant to 2010, as Chief Executive Officer and Executive Member of the Board of Directors of GlaxoSmithKline plc from 2000 to 2008 and as Chief Executive Officer of SmithKline Beecham plc in 2000 and as Chief Operating Officer and Executive Member of the Board of Directors of SmithKline Beecham plc from 1996 to 2000. Mr. Garnier was previously Chairman of Cerenis from 2010 to 2011, and a board member of the Stanford Advisory Council on Interdisciplinary Biosciences, Weill Cornell Medical College and the Dubai International Capital Advisory Board. He is also a member of the Advisory Board of the Newman's Own Foundation. We believe Dr. Garnier isqualified to serve as a member of our Board of Directors because of his significant business and professional experience, including his extensive experience in the life sciences industry, membership on various boards of directors and his previous leadership and management roles.Catherine Friedman has served on our Board of Directors since August 2015. Previously, Ms. Friedman held the position of Managing Director at Morgan Stanley from 1997 to 2006 and head of West Coast Healthcare and co-head of the Biotechnology Practice at Morgan Stanley from 1993 to 2006. Since 2007, Ms. Friedman has been a director of XenoPort Inc., where she serves on the Audit and Nominating and Governance Committees, and Enteromedics, where she serves as Chair of the Audit Committee; in June 2014, she joined the Board of Innoviva (formerly known as Theravance), where she serves on the Audit and Compensation Committees; and in May 2013 she joined the Board of GSV Capital, a publicly traded investment fund, where she serves as Chair of the Audit Committee and on the Valuation Committee. Ms. Friedman is a member of the Board of Trustees for Sacred Heart Schools in Atherton. She is a graduate of Harvard University and received an MBA from the University of Virginia Darden School of Business, where she is currently a Darden School Foundation Board of Trustees member. We believe Ms. Friedman is qualified to serve as a member of our Board of Directors due to her extensive experience as a member on various boards of directors, her educational background and her previous leadership and management roles. Ansbert K. Gadicke, M.D. has served on our Board of Directors since May 2011 and served as a member of the board of directors of our predecessor company from November 2003 until the Merger. Dr. Gadicke has been the Co-Founder and Managing Director of MPM Capital, a venture capital firm, since August 1996. Dr. Gadicke received an M.D. from J.W. Goethe University in Frankfurt. Dr. Gadicke is a director of Chiasma, Inc., OSS Healthcare, Inc., Sideris Pharmaceuticals, Inc., RWHD, Inc. and Mitokyne, Inc. He served on the board of directors of Idenix Pharmaceuticals, Inc. from 1998 to 2005, BioMarin Pharmaceuticals, Inc. from 1997 to 2001, Verastem, Inc. from 2010 to 2012, Pharmasset, Inc. from 1999 to 2007 and PharmAthene, Inc. from 2004 to 2007. We believe Dr. Gadicke is qualified to serve as a member of our Board of Directors because of his business and professional experience, including his experience in the venture capital industry and his years of analyzing development opportunities in the life sciences sector.Kurt C. Graves has served on our Board of Directors since May 2011 and as Chairman of our Board of Directors since November 2011. Mr. Graves has been the Chairman, President and Chief Executive Officer of Intarcia Therapeutics, a biotechnology company, since April 2012. Mr. Graves served as Executive Chairman of Biolex Therapeutics, a biotechnology company, from November 2010 to March 2012, and served as Executive Chairman of Intarcia Therapeutics from August 2010 to April 2012. Previously, he served as Executive Vice President, Chief Commercial Officer and Head of Strategic Development at Vertex Pharmaceuticals Inc. from July 2007 to October 2009. Prior to joining Vertex, Mr. Graves held various leadership positions at Novartis pharmaceuticals from 1999 to June 2007. He was also the first Chief Marketing Officer for the Pharmaceuticals division from September 2003 to June 2007. He currently serves as a director of Intarcia Therapeutics, Pulmatrix Therapeutics and Achillion Pharmaceuticals. He served on the board of directors of Biolex Therapeutics and Springleaf Therapeutics from 2010 to 2012. Mr. Graves received a B.S. in Biology from Hillsdale College. We believe Mr. Graves is qualified to serve as a member of our Board of Directors because of his extensive experience in the life sciences industry, membership on various boards of directors and his leadership and management experience.Owen Hughes has served on our Board of Directors since April 2013. He has served as the Chief Business Officer and Head of Corporate Development at Intarcia Therapeutics, Inc., a biotechnology company, since February 2013. Prior to Intarcia, he served as a Director at Brookside Capital, a hedge fund under the Bain Capital umbrella, managing public and private healthcare investments from March 2008 to January 2013. Mr. Hughes has served as a Senior Portfolio Manager at Pyramis Global Advisors from 2006 to 2008, co-founder and partner at Triathlon Fund Management from 2003 to 2006,an Investment Associate at Ziff Brothers Investments from 2001 to 2003, and an Assistant Vice President at Morgan Stanley/Merrill Lynch from 1998 to 2001. Mr.��Hughes is a director of Malin PLC. He earned a bachelor of arts from Dartmouth College. We believe Mr. Hughes is qualified to serve as a member of our Board of Directors because of his extensive business and professional experience, including his experience in the venture capital industry and years of analyzing development opportunities in the life sciences sector.Anthony Rosenberg has served on our Board of Directors since March 2015. From January 2013 to February 2015, Mr. Rosenberg served as Corporate Head of M&A and Licensing at Novartis International, a pharmaceutical company. From March 2005 to December 2012, he served as Global Head of Business Development and Licensing at Novartis Pharmaceuticals. Prior to that, Mr. Rosenberg was Global Head of the Transplant and Immunology Business Unit at Novartis Pharmaceuticals from 2000 to 2005. Mr. Rosenberg initially joined Sandoz, a predecessor to Novartis, in 1980. Mr. Rosenberg served as a director of Idenix Pharmaceuticals, Inc. from June 2009 to March 2012 and from December 2012 to March 2013. Mr. Rosenberg holds a B.Sc from the University of Leicester and an M.Sc in physiology from the University of London. We believe Mr. Rosenberg is qualified to serve as a member of our Board of Directors due to his extensive experience in mergers and acquisitions and licensing in the pharmaceutical sector.Debasish Roychowdhury has served on our Board of Directors since July 2015. Dr. Roychowdhury has been President of Nirvan Consultants, LLC since December 2013, where he advises biotechnology companies and institutions. He was one of the founding members of Seragon Pharmaceutical's Clinical and Scientific Advisory Board and was Seragon's Chief Medical Officer, prior to its acquisition by Roche Pharma, from March 2014 to August 2014. Prior to Seragon, Dr. Roychowdhury was the Senior Vice President and Head of the Global Oncology Division at Sanofi from August 2009 to November 2013. Prior to that, he served as the Vice President for Clinical Development at GlaxoSmithKline, from 2005 to 2009, and directed the Oncology Global Regulatory group at Eli Lilly and Company, a pharmaceutical company, from 1999 to 2005. Prior to his role in industry, Dr. Roychowdhury served as faculty member at the University of Cincinnati. He received his M.D from the All India Institute of Medical Sciences. He is a member of the Board of Directors for Celvad S.A. and Lytix Biopharma AS. We believe Dr. Roychowdury is qualified to serve as a member of our Board of Directors because of his strong medical background, specifically related to oncology, and extensive experience in the pharmaceutical industry.www.radiuspharm.com.http://radiuspharm.com/. Any amendments to the code, or any waivers offrom its requirements, will be disclosed on our website. Information contained on or accessible through our website is not incorporated by reference into this report, and you should not consider information contained on or accessible through our website to be part of this report.is containedwill be set forth in our definitive Proxy Statement for our 20162017 Annual Meeting of Stockholders and is incorporated herein by reference.is containedwill be set forth in our definitive Proxy Statement for our 20162017 Annual Meeting of Stockholders and is incorporated herein by reference.is containedwill be set forth in our definitive Proxy Statement for our 20162017 Annual Meeting of Stockholders and is incorporated herein by reference.is containedwill be set forth in our definitive Proxy Statement for our 20162017 Annual Meeting of Stockholders and is incorporated herein by reference.is containedwill be set forth in our definitive Proxy Statement for our 20162017 Annual Meeting of Stockholders and is incorporated herein by reference.(a)Financial Statements(a) Financial Statements (b)Financial Statement Schedules(b) Financial Statement Schedules (c)Exhibits(c) Exhibits RADIUS HEALTH, INC.
By:By:
/s/ ROBERT E. WARD25, 2016Signature TitleDate
Title
Date/s/ ROBERT E. WARD Robert E. Ward Chief Executive Officer and Director (Principal Executive Officer) February 25, 201624, 2017
Robert E. Ward/s/ B. NICHOLAS HARVEY B. Nicholas Harvey
Chief Financial Officer (Principal Accounting and Financial Officer)February 25, 2016/s/ ALAN H. AUERBACHAlan H. AuerbachDirectorFebruary 25, 2016/s/ WILLARD H. DEREWillard H. DereDirectorFebruary 25, 2016/s/ CATHERINE FRIEDMANCatherine FriedmanDirectorFebruary 25, 2016/s/ ANSBERT K. GADICKEAnsbert K. GadickeDirectorFebruary 25, 2016/s/ JEAN-PIERRE GARNIERJean-Pierre GarnierDirectorFebruary 25, 2016SignatureTitleDate/s/ KURT C. GRAVESKurt C. GravesDirector February 25, 201624, 2017/s/ OWEN HUGHESOwen HughesDirectorFebruary 25, 2016/s/ ANTHONY ROSENBERGAnthony RosenbergDirectorFebruary 25, 2016/s/ DEBASISH ROYCHOWDHURYDebasish RoychowdhuryDirectorFebruary 25, 2016Exhibit
Number Exhibit Description Form File No. Exhibit Filing
Date Filed/
Furnished
Herewith 3.1 Restated Certificate of Incorporation, filed on June 11, 2014 8-K 001-35726 3.1 6/13/14
3.2
Amended and Restated By-Laws
8-K
001-35726
3.2
6/13/14
4.1
Fifth Amended and Restated Stockholders' Agreement, dated April 24, 2014, by and among the Company and the stockholders party thereto
S-1/A
333-194150
4.2
4/25/14
10.1
Form of Warrant to Purchase Shares of Common Stock in connection with the Series B Convertible Preferred Stock and Warrant Purchase Agreement, issued by the Company to certain investors and attached schedule with details
8-K
001-35726
10.2
4/25/13
10.2
Form of Warrant to Purchase Shares of Common Stock in connection with the Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement, issued by the Company to certain investors and attached schedule with details
8-K
001-35726
10.2
2/21/14
10.3
Form of Warrant to Purchase Shares of Series A-1 Convertible Preferred Stock issued by the Company to GE Capital Equity Investments
10-K
001-35726
10.5
3/10/15
10.4
^
Clinical Trial Services Agreement, dated March 29, 2011, by and between the Company, as successor to Radius Health, Inc., and Nordic BioScience Clinical Development VII A/S
8-K/A
000-53173
10.1
10/24/11
Exhibit
Number Exhibit Description Form File No. Exhibit Filing
Date Filed/
Furnished
Herewith 10.5 ^ Work Statement NB-1, dated March 29, 2011, by and between the Company and Nordic Bioscience Clinical Development VII A/S, as amended on December 9, 2011, June 18, 2012, November 6, 2013, March 28, 2014, May 19, 2014 and July 22, 2014 10-K 001-35726 10.11 3/10/15
10.5
(a)
Amendment No. 8 to Work Statement NB-1, effective as of August 15, 2014, by and between the Company and Nordic Bioscience Clinical Development VII A/S
*
10.5
(b)
Amendment No. 9 to Work Statement NB-1, effective as of March 12, 2015, by and between the Company and Nordic Bioscience Clinical Development VII A/S
10-Q
001-35726
10.4
5/6/15
10.6
^
Work Statement NB-2, dated February 21, 2013, by and between the Company and Nordic Bioscience Clinical Development VII A/S, as amended on November 6, 2013
10-K
001-35726
10.12
3/10/15
10.7
^
Work Statement NB-3, dated February 21, 2013, by and between the Company and Nordic Bioscience Clinical Development VII A/S, as amended on February 28, 2014
10-K
001-35726
10.13
3/10/15
10.7
(a)
Amendment No. 2 to Work Statement NB-3, effective as of March 23, 2015, by and between the Company and Nordic Bioscience Clinical Development VII A/S
10-Q
001-35726
10.5
5/6/15
10.7
(b)
Amendment No. 3 to Work Statement NB-1, effective as of July 8, 2015, by and between the Company and Nordic Bioscience Clinical Development VII A/S
10-Q
001-35726
10.8
8/6/15
Exhibit
Number Exhibit Description Form File No. Exhibit Filing
Date Filed/
Furnished
Herewith 10.7 (c) Amendment No. 4 to Work Statement NB-3, effective as of October 21, 2015, by and between the Company and Nordic Bioscience Clinical Development VII A/S *
10.7
(d)
Amendment No. 5 to Work Statement NB-3, effective as of January 15, 2016, by and between the Company and Nordic Bioscience Clinical Development VII A/S
*
10.8
Amended and Restated Stock Issuance Agreement, dated May 16, 2011, by and between the Company, as successor to Radius Health, Inc., and Nordic BioScience Clinical Development VII A/S, as amended on February 21, 2013, March 28, 2014 and May 19, 2014
10-K
001-35726
10.14
3/10/15
10.9
^
License Agreement, dated September 27, 2005, by and between the Company, as successor to Nuvios, Inc., and Ipsen Pharma SAS (f/k/a SCRAS S.A.S.) on behalf of itself and its affiliates, as amended on September 12, 2007 and May 11, 2011
10-K
001-35726
10.15
3/10/15
10.10
^
Pharmaceutical Development Agreement, dated January 2, 2006, by and between the Company, as successor to Radius Health, Inc., and Beaufour Ipsen Industrie SAS, as amended on January 1, 2007, January 1, 2009, June 16, 2010 and December 15, 2011
10-K
001-35726
10.16
3/10/15
10.10
(a)
Amendment No. 6, dated August 14, 2015, to the Pharmaceutical Development Agreement, dated January 2, 2006, by and between the Company and Beaufour Ipsen Industrie SAS, as amended
10-Q
001-35726
10.4
11/5/15
Exhibit
Number Exhibit Description Form File No. Exhibit Filing
Date Filed/
Furnished
Herewith 10.11 ^ Development and Manufacturing Services Agreement, dated October 16, 2007, by and between the Company, as successor to Radius Health, Inc., and LONZA Sales Ltd., as amended on May 19, 2011 and January 30, 2014, and Work Orders thereunder through March 9, 2014 10-K 001-35726 10.17 3/10/15
10.11
(a)
Amendment No. 3 to Development and Manufacturing Services Agreement, dated December 31, 2015, by and between the Company and LONZA Sales Ltd.
*
10.11
(b)
Work Order #7, dated February 24, 2015, to the Development and Manufacturing Agreement, dated October 16, 2007, by and between the Company, as successor to Radius Health, Inc., and LONZA Sales Ltd.
10-Q
001-35726
10.7
5/6/15
10.11
(c)^
Work Order #8, dated March 27, 2015, to the Development and Manufacturing Services Agreement, dated October 16, 2007, by and between the Company, as successor to Radius Health, Inc., and LONZA Sales Ltd., as amended
10-Q
001-35726
10.4
8/6/15
10.11
(d)^
Work Order #9, dated May 7, 2015, to the Development and Manufacturing Services Agreement, dated October 16, 2007, by and between the Company, as successor to Radius Health, Inc., and LONZA Sales Ltd., as amended
10-Q
001-35726
10.5
8/6/15
10.11
(e)^
Work Order #10, dated May 22, 2015, to the Development and Manufacturing Services Agreement, dated October 16, 2007, by and between the Company, as successor to Radius Health, Inc., and LONZA Sales Ltd., as amended
10-Q
001-35726
10.6
8/6/15
Exhibit
Number Exhibit Description Form File No. Exhibit Filing
Date Filed/
Furnished
Herewith 10.11 (f)^ Work Order No. 11, dated August 11, 2015, to the Development and Manufacturing Services Agreement, dated October 16, 2007, by and between the Company and LONZA Sales Ltd., as amended 10-Q 001-35726 10.2 11/5/15
10.11
(g)^
Work Order No. 12, dated August 24, 2015, to the Development and Manufacturing Services Agreement, dated October 16, 2007, by and between the Company and LONZA Sales Ltd., as amended
10-Q
001-35726
10.3
11/5/15
10.11
(h)^
Work Order No.13, dated September 28, 2015, to the Development and Manufacturing Services Agreement, dated October 16, 2007, by and between the Company and LONZA Sales Ltd., as amended
10-Q
001-35726
10.7
11/5/15
10.12
^
Development and Clinical Supplies Agreement, dated June 19, 2009, by and among the Company, as successor to Radius Health, Inc., and 3M Co. and 3M Innovative Properties Co., as amended on December 31, 2009, September 16, 2010, September 29, 2010, March 2, 2011 and November 30, 2012 and Change Order Forms thereunder through March 9, 2014
10-K
001-35726
10.18
3/10/15
10.12
(a)
Change Order Form #22, dated March 2, 2015, to the Development and Clinical Supplies Agreement, dated June 19, 2009, by and among the Company and 3M Co. and 3M Innovative Properties Co., as amended
10-Q
001-35726
10.6
5/6/15
Exhibit
Number Exhibit Description Form File No. Exhibit Filing
Date Filed/
Furnished
Herewith 10.12 (b)^ Change Order Form #23, dated August 26, 2015, to Fifth Amendment to Development and Clinical Supplies Agreement, effective as of November 30, 2012, by and among the Company and 3M Co. and 3M Innovative Properties Co. 10-Q 001-35726 10.5 11/5/15
10.12
(b)^
Change Order Form #26, dated May 13, 2015, to Fifth Amendment to Development and Clinical Supplies Agreement, effective as of November 30, 2012, by and among the Company and 3M Co. and 3M Innovative Properties Co.
10-Q
001-35726
10.3
8/6/15
10.13
^
License Agreement, dated June 29, 2006, by and between the Company and Eisai Co., Ltd.
8-K/A
000-53173
10.25
10/24/11
10.13
(a)^
License Agreement Amendment No. 1, dated March 9, 2015, by and between the Company and Eisai Co. Ltd.
10-Q
001-35726
10.3
5/6/15
10.14
†
Radius Health, Inc. 2003 Long-Term Incentive Plan (as amended)
10-K
001-35726
10.20
3/10/15
10.15
†
Radius Health, Inc. 2003 Long-Term Incentive Plan Form of Stock Option Agreement
8-K
000-53173
10.32
5/23/11
10.16
†
Radius Health, Inc. 2011 Equity Incentive Plan (as amended and restated)
8-K
001-35726
10.1
5/11/15
10.17
†
Form of Radius Health, Inc. 2011 Equity Incentive Plan Stock Option Agreement
S-1/A
333-175091
10.83
11/7/11
10.18
†
Radius Health, Inc. Non-Employee Director Compensation Program
10-K
001-35726
10.24
3/10/15
10.19
†
Employment Letter Agreement, dated November 14, 2003, by and between the Company, as successor to Nuvios, Inc., and Gary Hattersley
8-K
000-53173
10.49
5/23/11
Exhibit
Number Exhibit Description Form File No. Exhibit Filing
Date Filed/
Furnished
Herewith 10.20 † Employment Letter Agreement, dated November 15, 2006, by and between the Company, as successor to Radius Health, Inc., and B. Nicholas Harvey 8-K 000-53173 10.51 5/23/11
10.21
†
Executive Employment Agreement, dated as of December 12, 2013, by and between the Company and Robert Ward
8-K
001-35726
10.1
12/17/13
10.22
†
Employment Letter Agreement, dated January 3, 2014, by and between the Company and Greg Williams
S-1/A
333-194150
10.141
4/3/14
10.23
†
Form of Indemnification Agreement by and between the Company and the individuals listed on Schedule A thereto
10-K
001-35716
10.30
3/10/15
10.24
Indenture of Lease, dated May 14, 2014, by and between the Company and BP Bay Colony LLC
8-K
001-35726
10.1
5/20/14
10.24
(a)
First Amendment, dated September 9, 2015, to Lease, dated May 14, 2014, by and between the Company and BP Bay Colony LLC
10-Q
001-35726
10.6
11/5/15
21.1
Subsidiary of the Company
*
23.1
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm
*
31.1
Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer
*
31.2
Rule 13a-14(a)/15d-14(a) Certification of Chief Financial Officer
*
32.1
Section 1350 Certification of Chief Executive Officer
**
32.2
Section 1350 Certification of Chief Financial Officer
**
101.INS
XBRL Instance Document
*
101.SCH
XBRL Taxonomy Extension Schema Document
*ExhibitNumberExhibit DescriptionFormFile No.ExhibitFilingDateFiled/FurnishedHerewith101.CALB. Nicholas Harvey XBRL Taxonomy Extension Calculation Linkbase Document /s/ ALAN H. AUERBACH Director February 24, 2017 Alan H. Auerbach *
/s/ WILLARD H. DERE101.LAB
DirectorXBRL Taxonomy Extension Label Linkbase Document*February 24, 2017
Willard H. Dere101.PRE
XBRL Taxonomy Extension Presentation Linkbase Document*
101.DEF
XBRL Taxonomy Extension Definition Linkbase Document
/s/ CATHERINE FRIEDMAN
Director
February 24, 2017Catherine Friedman
*/s/ ANSBERT K. GADICKE Director February 24, 2017 Ansbert K. Gadicke /s/ JEAN-PIERRE GARNIER Director February 24, 2017 Jean-Pierre Garnier /s/ KURT C. GRAVES Director February 24, 2017 Kurt C. Graves /s/ OWEN HUGHES Director February 24, 2017 Owen Hughes /s/ ANTHONY ROSENBERG Director February 24, 2017 Anthony Rosenberg /s/ DEBASISH ROYCHOWDHURY Director February 24, 2017 Debasish Roychowdhury ^Confidential treatment has been granted with respectredacted portions of this exhibit. Redacted portions of this exhibit have beenpreviously filed separatelyExhibits refer to the Company’s filings with the SEC.†A management contractSecurities and Exchange Commission, or compensatory plan or arrangement required to be filed as an exhibit pursuant to Item 15(a)(3) of Form 10-K.*Filed herewith.**Furnished herewith. Exhibit Description Form File No. Exhibit 3.1 Restated Certificate of Incorporation 8-K 3.1 6/13/2014 3.2 Amended and Restated By-Laws 8-K 3.2 6/13/2014 4.1 Fifth Amended and Restated Stockholders' Agreement, dated April 24, 2014, between the Company and the stockholders party thereto S-1/A 333-194150 4.2 4/25/2014 Management Contracts and Compensatory Plans 10.1 Radius Health, Inc. 2003 Long-Term Incentive Plan (as amended) 10-K 10.20 3/10/2015 10.1(a) Radius Health, Inc. 2003 Long-Term Incentive Plan Form of Stock Option Agreement 8-K 000-53173 10.32 5/23/2011 10.2 Radius Health, Inc. 2011 Equity Incentive Plan (as amended and restated) 8-K 10.1 5/27/2016 10.2(a) Form of Radius Health, Inc. 2011 Equity Incentive Plan Stock Option Agreement for Incentive Stock Options * 10.2(b) Form of Radius Health, Inc. 2011 Equity Incentive Plan Stock Option Agreement for Non-Incentive Stock Options * 10.2(c) Form of Radius Health, Inc. 2011 Equity Incentive Plan Restricted Stock Unit Grant Notice and Restricted Stock Unit Agreement, attached as Exhibit A thereto * 10.3 Radius Health, Inc. 2016 Employee Stock Purchase Plan 8-K 10.2 5/27/2016 10.4 Radius Health, Inc. Non-Employee Director Compensation Program (as amended) * 10.5 Employment Letter Agreement, dated November 14, 2003, between the Company, as successor to Nuvios, Inc., and Gary Hattersley 8-K 000-53173 10.49 5/23/2011 10.5(a) Executive Severance Agreement, dated July 1, 2015, between the Company and Gary Hattersley 8-K 10.2 7/10/2015 10.6 Employment Letter Agreement, dated November 15, 2006, between the Company, as successor to Radius Health, Inc., and B. Nicholas Harvey 8-K 000-53173 10.51 5/23/2011 10.6(a) Executive Severance Agreement, dated July 1, 2015, between the Company and B. Nicholas Harvey 8-K 10.1 7/10/2015 10.7 Executive Employment Agreement, dated December 12, 2013, between the Company and Robert Ward 8-K 10.1 12/17/2013 10.7(a) First Amendment, dated July 1, 2015, to Executive Employment Agreement, dated December 12, 2013, between the Company and Robert Ward 8-K 10.5 7/10/2015 10.8 Employment Letter Agreement, dated January 3, 2014, between the Company and Greg Williams S-1/A 333-194150 10.141 4/3/2014 10.8(a) Executive Severance Agreement, dated July 1, 2015, between the Company and Greg Williams 8-K 10.4 7/10/2015 10.9 Employment Letter Agreement, dated December 28, 2014, between the Company and Brent Hatzis-Schoch * 10.10 Employment Letter Agreement, dated February 20, 2015, between the Company and Dinesh Purandare * 10.11 Employment Letter Agreement, dated July 3, 2015, between the Company and Lorraine Fitzpatrick, M.D. * 10.12 Employment Letter Agreement, dated August 31, 2015, between the Company and David Snow * 10.13 Form of Executive Severance Agreement between the Company and David Snow, Lorraine Fitzpatrick, Dinesh Purandare and Brent Hatzis-Schoch * 10.14 Form of Indemnification Agreement between the Company and its directors 10-K 10.30 3/10/2015 Other Agreements 10.15 Form of Warrant to Purchase Shares of Common Stock in connection with the Series B Convertible Preferred Stock and Warrant Purchase Agreement, issued by the Company to certain investors and attached schedule with details 8-K 10.2 4/25/2013 10.16 Form of Warrant to Purchase Shares of Common Stock in connection with the Series B-2 Convertible Preferred Stock and Warrant Purchase Agreement, issued by the Company to certain investors and attached schedule with details 8-K 10.2 2/21/2014 10.17 Form of Warrant to Purchase Shares of Series A-1 Convertible Preferred Stock issued by the Company to GE Capital Equity Investments 10-K 10.5 3/10/2015 10.18^ License Agreement, dated September 27, 2005, between the Company, as successor to Nuvios, Inc., and Ipsen Pharma SAS (f/k/a SCRAS S.A.S.) on behalf of itself and its affiliates, as amended on September 12, 2007 and May 11, 2011 10-K 10.15 3/10/2015 10.19^ Development and Clinical Supplies Agreement, dated June 19, 2009, between the Company, as successor to Radius Health, Inc., 3M Co. and 3M Innovative Properties Co., as amended on December 31, 2009, September 16, 2010, September 29, 2010, March 2, 2011 and November 30, 2012 10-K 10.18 3/10/2015 10.20^ License Agreement, dated June 29, 2006, between the Company and Eisai Co., Ltd. 8-K/A 000-53173 10.25 10/24/2011 10.20(a) License Agreement Amendment No. 1, dated March 9, 2015, between the Company and Eisai Co., Ltd. 10-Q 10.3 5/6/2015 10.21^ Supply Agreement, dated June 23, 2016, between the Company and Ypsomed AG 10-Q 10.1 8/4/2016 10.22^ Commercial Supply Agreement, dated June 28, 2016, between the Company and Vetter Pharma International GmbH 10-Q 10.2 8/4/2016 10.23^ Manufacturing Services Agreement, dated July 13, 2016, between the Company and Polypeptide Laboratories Holding (PPL) AB, as successor to Lonza Sales Ltd 10-Q 10.1 11/3/2016 10.24 Indenture of Lease, dated May 14, 2014, between the Company and BP Bay Colony LLC 8-K 10.1 5/20/2014 10.24(a) First Amendment, dated September 9, 2015, to Lease, dated May 14, 2014, between the Company and BP Bay Colony LLC 10-Q 10.6 11/5/2015 21.1 Subsidiaries of the Company * 23.1 Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm * 31.1 Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer * 31.2 Rule 13a-14(a)/15d-14(a) Certification of Chief Financial Officer * 32.1 Section 1350 Certification of Chief Executive Officer ** 32.2 Section 1350 Certification of Chief Financial Officer ** 101.INS XBRL Instance Document * 101.SCH XBRL Taxonomy Extension Schema Document * 101.CAL XBRL Taxonomy Extension Calculation Linkbase Document * 101.LAB XBRL Taxonomy Extension Label Linkbase Document * 101.PRE XBRL Taxonomy Extension Presentation Linkbase Document * 101.DEF XBRL Taxonomy Extension Definition Linkbase Document * ^ Confidential treatment has been granted with respect to redacted portions of this exhibit. Redacted portions of this exhibit have been filed separately with the SEC. * Filed herewith. ** Furnished herewith.