 UNITED STATES
UNITED STATES
UNITED UNITED STATES
UNITED STATES
SECURITIESSECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORMFORM 10-K
[X]☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
ForFor the Fiscal Year Ended December 31, 20202023
[ ]☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OFOF 1934
For the transition period Forthetransitionperiodfrom _____ to ______to
CommissionCommission File Number: 001-36833
VOLITIONRX LIMITED
(Exact name of registrant as specified in its charter)
VOLITIONRX LIMITED |
(Exact name of registrant as specified in its charter) |
Delaware | 91-1949078 | |
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
1489 West Warm Springs Road, Suite 110
Henderson, Nevada 89014
(Address of principal executive offices)
+1 (646) 650–1351
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class: |
| Trading Symbol(s): | Name of Each Exchange on Which Registered: | |
Common Stock, par value $0.001 per share |
| VNRX | NYSE American, LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes [ ]☐ No [X].☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes [ ]☐ No [X].☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes [X]☒ No [ ].☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes [X]☒ No [ ].
☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
Large accelerated filer
| ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [ ]☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. [ ]☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes [ ]☐ No [X].☒
As of June 30, 2020,2023, the last trading day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the voting common stock held by non-affiliates of the registrant was $111,767,725$82,181,507 (based upon the $3.89$1.39 per share closing price for the registrant’s common stock as reported by the NYSE American on such date). This calculation does not reflect a determination that persons deemed to be affiliates for this purpose are affiliates for any other purpose.
As of March 10, 2021,15, 2024, there were 52,870,90782,068,442 shares of the registrant’s $0.001 par value common stock issued and outstanding.
|
PortionsTable of the registrant’s Proxy Statement for its 2021 Annual Meeting of Stockholders, to be filed on or before April 30, 2021 are incorporated by reference into Part III, Items 10-14 of this Annual Report on Form 10-K.
|
| Page | ||
|
|
| ||
|
|
| ||
|
|
| ||
|
|
| ||
|
|
| ||
|
|
| ||
|
|
| ||
|
| |||
3 | ||||
|
| |||
|
|
| ||
|
|
| ||
|
| |||
|
|
| ||
|
|
| ||
|
| |||
|
|
| ||
|
|
| ||
|
|
| ||
|
| 24 | ||
Item |
|
| 25 | |
|
| 26 | ||
26 | ||||
26 | ||||
|
|
| ||
PART |
|
| ||
|
|
| ||
|
|
| ||
|
|
| ||
|
|
| ||
| ||||
|
|
| ||
|
| 27 | ||
|
| 28 | ||
33 | ||||
F-34 - F-79 | ||||
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 80 | |||
80 | ||||
81 | ||||
DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS | 81 | |||
|
|
| ||
PART |
|
| ||
|
|
| ||
|
|
| ||
Item |
|
| 82 | |
92 | ||||
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 102 | |||
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 105 | |||
106 | ||||
|
|
| ||
|
|
| ||
107 | ||||
112 | ||||
113 | ||||
| 2 |
| Table of Contents |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K for the fiscal year ended December 31, 2020, which we refer to as2023 (this “Report”), and the information and documents incorporated by reference in this Report, containscontain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended or the Securities Act,(the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended or the Exchange Act,(the “Exchange Act”), which statements are subject to considerable risks and uncertainties. These forward-looking statements are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact included in this Report or incorporated by reference into this Report are forward-looking statements. Throughout this Report, westatements. We have attempted to identify forward-looking statements by using words such as “may,“aim,” “anticipate,” “believe,” “will,“continue,” “could,” “project,” “anticipate,“estimate(s),” “expect,” “estimate,“forecast(s),” “goal,” “intend,” “may,” “plan(s),” “potential,” “project,” “seek,” “should,” “continue,“strategy,” “potential,“will,” “plans,” “forecasts,” “goal,” “aim,” “seek,” “intend,”and other forms of these words or similar words or expressions or the negative thereof (although not all forward-looking statements contain these words). In particular, forward-looking statements contained in this Report, and the information and documents incorporated by reference within this Report, relate to, among other things, anyour predictions of earnings, revenues, expenses or other financial items; plans or expectations with respect to our development activities or business strategy, including regulatory approvals, commercialization and market acceptance; statements concerning industry trends and industry size; statements regarding anticipated demand for our products and market opportunity, or the products of our competitors; statements relating to manufacturing forecasts, and the potential impact of our relationshiprelationships with contract manufacturers, and original equipment manufacturers and distributors on our business; assumptions regarding the future cost and potential benefits of our research and development efforts; the effect of critical accounting policies; forecasts of our liquidity position or available cash resources;resource and financing plans; and statements relating to the assumptions underlying any of the foregoing. We caution you that the foregoing list may not include all of the forward-looking statements made in this Report and the information and documents incorporated by reference within this Report.
Some significant factors that may impact our estimates and uncertainties in greater detailforward-looking statements include, but are not limited to:
· | Our inability to generate any significant revenue or achieve profitability; | |
· | Our need to raise additional capital in the future; | |
· | Our expansion of our product development and sales and marketing capabilities could give rise to difficulties in managing our growth; | |
· | Our dependence on third-party distributors; | |
· | Our limited experience with sales and marketing; | |
· | The possibility that we may not be able to continue to operate, as indicated by the “going concern” opinion from our auditors; | |
· | Our ability to successfully develop, manufacture, market, and sell our future products; | |
· | Our ability to timely obtain necessary regulatory clearances or approvals to distribute and market our future products; | |
· | The acceptance by the marketplace of our future products; | |
· | The highly competitive and rapidly changing nature of the diagnostics market; | |
· | Protection of our patents, intellectual property and trade secrets; | |
· | Our reliance on third parties to manufacture and supply our intended products, and such manufacturers’ dependence on third-party suppliers; | |
· | The material weaknesses in our internal control over financial reporting that we have identified; | |
· | Pressures related to macroeconomic and geopolitical conditions; and | |
· | Other risks identified elsewhere in this Report, as well as in our other filings with the Securities and Exchange Commission (the “SEC”). |
For additional information, refer to the section entitled “Risk Factors” in Part I, Item 1A of this Report, and the other documents that we have filed with the U.S. Securities and Exchange Commission, or the SEC.SEC.
In addition, actual results may differ as a result of additional risks and uncertainties of which we are currently unaware or which we do not currently view as material to our business. For these reasons, readers are cautioned not to place undue reliance on any forward-looking statements.
You should read this Report in its entirety, including the documents that we file as exhibits to this Report and the documents that we incorporate by reference into this Report, with the understanding that our future results may be materially different from what we currently expect. The forward-looking statements we make speak only as of the date on which they are made. We expressly disclaim any intent or obligation to update any forward-looking statements after the date hereof to conform such statements to actual results or to changes in our opinions or expectations. If we do update or correct any forward-looking statements, readers should not conclude that we will make additional updates or corrections.
| 3 |
| Table of Contents |
ITEM 1.BUSINESS
Overview
VolitionRxImagine a world where diseases like cancer and sepsis can be diagnosed early and monitored easily using routine blood tests. That’s the world Volition is a multi-national epigenetics company that appliestrying to build by developing its NucleosomicsTM platform through its subsidiaries to developinnovative family of simple, easy to use, cost-effective blood tests.
Volition is a multi-national epigenetics company. It has patented technologies that use chromosomal structures, such as nucleosomes, and transcription factors as biomarkers in cancer and other diseases. The tests in the Company’s product portfolio detect certain characteristic changes that occur from the earliest stages of disease, enabling early detection and offering a better way to helpmonitor disease progression and a patient’s response to treatment.
The tests offered by Volition and its subsidiaries are designed to diagnose and monitor a range of life-altering diseases, including certain cancers and other diseases. We hope that through earlierdiseases associated with NETosis, such as sepsis and COVID-19. Early diagnosis we can help save and monitoring have the potential to not only prolong the life of patients but also improve thetheir quality of human and animals’ lives throughout the world.life.
We have several key pillars of focus:
· | Nu.Q® Vet - cost-effective, easy-to-use blood tests for dogs and other companion animals. The Nu.Q® Vet Cancer Test is commercially available as a cancer screening test in dogs. | |
· | Nu.Q® NETs - monitoring the immune system to save lives. | |
· | Nu.Q® Discover - a complete solution to profiling nucleosomes. | |
· | Nu.Q® Cancer - monitoring disease progression, response to treatment and Minimal Residual Disease (“MRD”). | |
· | Capture-PCRTM- isolating and capturing circulating tumor-derived DNA from plasma samples for early cancer detection. |
The Company has grown from a single two-meter lab bench at the University of Namur in Belgium to a purpose-built 17,000 square foot lab and 10,000 square foot production facility in Gembloux, Belgium, an Innovation Lab in California, and offices in California, London, Singapore and Nevada. In 2015, the Company’s common stock was listed on the New York Stock Exchange (VNRX). We now have a team of over 100 dedicated employees, spanning a wide range of disciplines; all united in our mission to improve outcomes for patients.
Cultivating successful, ongoing relationships with stakeholders worldwide has been fundamental to Volition’s development. We have fostered ties with leading academic institutions, clinical centers of excellence, multi-national pharmaceutical companies and financial institutions across the globe.
Volition’s Solution and the Science Behind It
We are dedicated to revolutionizing the diagnosis and monitoring of life-altering diseases by advancing the science of epigenetics.
Our assays are based on the science of NucleosomicsTM, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid, since changes in these parameters are an indication that disease is present.
Background to Genetics, Epigenetics and Cancer
Human genetics, the sequence of our DNA, is essentially a “recipe book” containing details of how to make each of the thousands of different proteins in the human body; simply put, there is a different gene (or recipe) for each protein. However, just because a recipe is in the book, does not mean you have to make it, and nobody makes all the proteins in their DNA. For example, men have all the genes necessary to make ovarian and uterine proteins but do not do so. Similarly, muscle cells do not make liver proteins or kidney proteins. This is because the genes for liver and kidney proteins are inactive or “switched off” in muscle cells. The mechanisms for the control of which genes are active or inactive in a cell are collectively known as epigenetics.
There are many different types of cancers, but generally, the primary cause of a cancer is a mutation within a cell of the DNA encoding or regulating the expression of one or more specific genes called oncogenes. While many mutations cause no consequence, some can lead to the uncontrolled expansion of the mutated cells and their dissemination to other parts of the body from the tissue of origin in a process called metastasis. Another consequence of these mutations is an alteration in the epigenetic regulation of many other genes and this, in turn, can create a unique epigenetic signature in the cancer cells.
Epigenetic control is therefore a critical factor in biology and medicine. A number of epigenetic cancer drugs have been in routine clinical useteam has worked tirelessly for more than a decade to evolve and master our understanding of the altered epigenetic signature seenrich, complex information encoded in cancer underpins Volition’s diagnostic approaches.circulating chromatin and in particular, in circulating nucleosomes and transcription factors. Our tests are platform agnostic and can be adapted to any workflow setting – manual, reference laboratory and point-of-care.
AWe believe that our focus on innovation and robust assay development, as well as our diverse intellectual property portfolio, positions us to become a significant player in this cutting-edge field of science.
Unlocking Epigenetics
We believe epigenetics is the most exciting field in disease detection and management today. Modern genetics – the study of genes and heredity – is underpinned by the linear sequences of molecular “letters” present in the DNA double helix of each living cell, many of which encode the genes. It has had an enormous impact on the practice of medicine, revolutionizing the way doctors identify people with inherited conditions, diagnose cancer, and, increasingly, design personalized treatment plans. However, there’s more to chromosomes than just the DNA sequence; at Volition, we focus on chromosomes’ second epigenetic code, which contains a wealth of additional information about the health and function of the body’s cells. You can think of the DNA sequence of each cell as the text of an instruction manual, and epigenetics as the formatting. Some parts of the manual are bolded, highlighted, or underlined, telling the cell to emphasize those sections, while others are struck out, telling the cell to ignore those genes. The cells of most bodily organs are continuously replaced by new ones. Epigenetic changes can be detected before the diseased cells themselves become abnormal enough to show up in traditional biopsies, and oftentimes before the first symptoms are felt. We aim to replace unpleasant, invasive, and often expensive screening and diagnostic tests with blood tests, helping to save lives and to reduce overall health care costs.
| 4 |
| Table of Contents |
We have two technologies:
· | Nucleosome Quantification (“Nu.Q®”) | |
· | Capture-PCRTM |
Chromosome, nucleosome and transcription factor structures represent a major mechanism for epigenetic control is mediated through chromosome and nucleosome structure.control. Each chromosome contains aone long, single molecule of DNA that is coated by a complex array of proteins, mostly in the form of nucleosomes, giving the stretched-out, unwound DNA/protein core, or chromatin, the appearance of “beads on a string.” Unwound chromatin is accessible for reading (or transcribing) and “unwound”unwound genes may be active. However, genes whose nucleosomes arewith coiled or supercoiled nucleosomes are inaccessible and inactive.
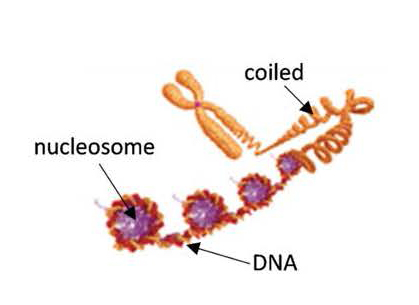
Figure 1 – A chromosome
Nu.Q®
Each nucleosome consists of a disc of eight histone proteins wrapped by a short length of DNA. Nucleosome structure has a dual role: first, it allows the compact storage and protection of the genetic material (or DNA), and second, it modulates the epigenetic regulation (transcription) of that DNA. This regulation is achieved through reversible chemical changes to both the DNA and protein components, as well as through the binding of specific regulatory proteins to the DNA.
Our patented Nucleosomics™ technology isolates circulating nucleosomes from the blood for quantification and analysis, to enable earlier diagnosis and monitoring of life-altering diseases.
Volition’s Epigenetic ApproachNu.Q® Product Overview
Volition’s approach is to investigate the epigenetic structure of chromatin and nucleosomes rather than investigating only the DNA sequence. We are continuously developing new technologies including:
·A suite of low cost Nu.Q® immunoassaysVet Cancer Test
Cancer is the most common cause of death in dogs over the age of 2 years in the US, and it is estimated that up to 50% of all dogs over the age of 10 will develop cancer in their lifetimes. There are an estimated 6 million pet dogs diagnosed with cancer each year. Earlier cancer detection can accurately measure nucleosomes containing numerous epigenetic signalsimprove outcomes, including the quality of life of the dog and its owner. Yet, as of today, there are few single assay cancer blood tests on the veterinary market. Currently, dogs are usually diagnosed when they are unwell or structure, now being developed onthere is a rangesuspicion of different enzyme-linked immunosorbent assay, or ELISA, platforms.
·cancer. Even then, dogs suspected of having cancer are required to undergo a variety of diagnostic tests that may be expensive, time consuming, and painful for the animal. We hope to change this with the introduction of the Nu.Q® Capture technology to isolate or enrich nucleosomes containing particular epigenetic signals or structures for a wide range of potential scientific and medical applications. For example, the enrichment of nucleosomes of tumor origin in blood samples taken from cancer patients.
·The production of synthetic (recombinant) nucleosomes, containing exact defined epigenetic signals and structures, is now in-house. These nucleosomes are used to ensure maximal accuracy of Nu.Q® immunoassay tests but also have many other applications including Research Use Only, or RUO, kits and as tools in epigenetic drug development.
Improving Outcomes forVet Cancer PatientsTest.
The prospects for cancer patients vary greatly depending on whether the diseaseNu Q® Vet Cancer Test is detected at an early localized stage when effective treatment options are available, or at an advanced stage when the disease may have spread,accessible and treatment is much more difficult. Unfortunately, most cancers are symptomless at early stage and most patients are not diagnosed until the disease has spreadaffordable screening test to other organsaid in the bodyearly detection of cancer in dogs. It’s a simple, cost effective, easy to use screening blood test recommended for older dogs (7 years and older) and those breeds at increased risk of developing cancer in their lifetimes (from 4 years).
Our test can be easily integrated into preventive care programs and used alongside other routine bloodwork during regular wellness visits. The Nu.Q® Vet Cancer Test is available to veterinarians in the likely outcomeUnited States, Europe, and Asia through our distributors, which include IDEXX Laboratories, Inc. (“IDEXX”), a global leader in pet healthcare innovation, and Heska Corporation (“Heska”), a leading global provider of advanced veterinary diagnostics, and now part of Mars Petcare, one of the largest pet health companies in the world.
Transfer of the Nu.Q® Vet Cancer Test onto Heska’s in-house diagnostic platform (the element i+) was completed in 2023.
We are currently conducting ongoing research regarding Nu.Q® Vet in pursuit of the following goals:
· | Broadening the range of cancers detected, | |
· | Differential diagnosis, | |
· | Pre-analytics for the use of Nu.Q® Vet in the feline population, | |
· | Use of the Nu.Q® platform in NETosis in canines, and | |
· | Use of Capture-PCRTM in canines. |
Nu.Q® NETs
Our Nu.Q® NETs assay is poor. Simple low-cost immunoassay blood testsa groundbreaking CE-marked diagnostic solution that clinicians can use to detect cancer at an early stage leading to earlier treatment would greatly improve patient outcomes.
The Limitations of DNA Sequencing in Cancer
The advent of next generation sequencing has revolutionized medical research and led to a host of medical and other innovations. For example, sequencing the DNA of tumor tissueremoved by surgery or biopsy uncovers cancer DNA mutations present in the tumor and isNETosis. Our assay can be used to direct patient treatment selection, but tissue biopsy cannot be used routinely for cancer detection.
However, small fragmentsidentify patients with clinically relevant elevated levels of cancer DNA from dead tumor cells are also found in the blood of cancer patients so it is possible to sequence circulating tumor DNA, or ctDNA, in a blood sample taken from a patient to test for any cancer DNA mutations (e.g., mutated P53, KRAS, EGFR). Unfortunately, these ctDNA blood tests, often called liquid biopsy tests, have thus far also proved ineffectual for early stage cancer detection.
The main reasons why ctDNA tests alone have not proved useful for early cancer detection include:
·The level of DNA fragments circulating in the blood is very low.
·Only a small proportion of the circulating DNA fragments are of tumor origin and the proportion is especially low in early stage cancer (usually less than 1%). The remaining “healthy” DNA fragments originate mainly from dead white blood cells.
·A DNA sequence mutation will occur on only one in several million (up to 20 million) of the circulating DNA fragments that do originate from cancer cells. This means that cancer mutations are found in one in millions of a small percentage of a very low level of circulating DNA fragments, with the result that ctDNA is undetectable in most early-stage cancer patients.
·Many cancer-like mutations have recently been found to be present in the blood of healthy elderly people through a process known as clonal hematopoiesis. Any DNA released from these cells could lead to false positive readings.
Volition’s Epigenetic Approach to Cancer, COVID-19 and Sepsis
Cancer is in essence a disease of genetic and epigenetic mis-regulation of oncogenes and tumor suppressor genes in the chromosomes of affected cells, leading to uncontrolled cell division and eventually to uncontrolled tumor growth and spread. Thus, the epigenetic signaling structures of chromosomes and nucleosomes are different in cancer cells and healthy cells of the same tissue.
When a cancer cell dies, its chromosomes are digested into nucleosomes as shown in the figure below. Most nucleosomes are metabolized, but some are released into the blood stream as circulating nucleosomes. The DNA attached to these nucleosomes is ctDNA. However, liquid biopsy companies extract only the DNA and discard the remainder of the nucleosome.
Volition analyzes whole circulating nucleosomes containing particular epigenetic signals and structures using our low cost, but highly accurate Nu.Q® nucleosome immunoassay tests.
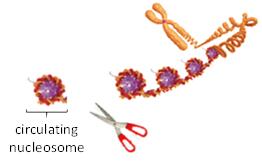
Figure 2 – Digestion of a chromosome into nucleosomes.
The epigenetic structure of nucleosomes of cancer origin are known to differ from that of nucleosomes from healthy cells. These epigenetic changes occur early and drive the development of cancer, for example by inappropriately activating oncogenes that promote cell division or inactivating tumor suppressor genes that repress cell division. However, the structural epigenetic changes that occur are not restricted to “1 in 20 million” nucleosomes or even to oncogenes and tumor suppressor genes, but are widely distributed, providing a larger cancer signal and enabling earlier detection of cancer. We use our Nu.Q® immunoassay tests to detect a variety of early stage cancers.
Circulating cancer nucleosomes also differ from nucleosomes of healthy origin in other ways. For example, the DNA fragments in cancer nucleosomes are approximately 20 base pairs (or about 14%) shorter than the DNA fragments in nucleosomes originating in healthy cells. This structural difference is used as the basis of one of Volition’s Nu.Q® Capture technologies to separate or enrich cancer nucleosomes by removing nucleosomes of healthy origin. Volition expects that Nu.Q® Capture technology will further increase the accuracy of its Nu.Q® immunoassay tests to detect early-stage cancers and will also be useful to ctDNA companies to decrease the cost and increase the accuracy of liquid biopsy tests.
In terms of background science, white blood cells help protect the body against infection. White cells engulf invading viruses and bacteria and produce antibodies against them. In addition, white cells also eject chromatin material out of the cell to form Neutrophil Extracellular Traps or(NETs) and enable physicians to rapidly treat these patients. Although NETs which catch and trap invading viruses.play a critical role in our normal immune response, elevated levels of NETs are a complicating factor in a wide variety of diseases including respiratory infections, SARS, and pneumonia as well as metabolic diseases, autoimmune conditions, inflammatory conditions, cancer, thrombosis, stroke and sepsis.
While cancer remains our core disease focus, given the prominence of infectious diseases as a result of the pandemic we are also researching the use of our proprietary technology in infectious diseases with particular regard to NETosis and the activation and release of NETs in disease.
In a respiratory infection, white cells migrate to the lungs to protect them from the virus. Elevated levels of NETs are a clinical complication of the novel coronavirus SARS-CoV-2, or COVID-19, associated with poor patient outcomes.
The ejected NETs material is made up of nucleosomes that can be detectedoutcomes in minute quantities using Volition’s Nu.Q® nucleosome assays. Indeed, we believe that we have the only available analytically validated quantitative nucleosome assay.
We believe the versatility of the Nu.Q® platformsepsis, cancer, and the range of applications for which these assays can be leveraged may help increase diagnostic power and monitor disease progression and potentially treatment response across a range of infectious diseases that involve the over production of NETs, such as COVID-19, pneumonia, influenza and sepsis.other diseases.
| 5 |
| Table of Contents |
While NETosis
Sepsis is stillthe number one cause of death in hospitals worldwide. It kills an estimated 11 million people a relatively new fieldyear, which is more than cancer or coronary disease. In 2017, there were an estimated 49 million cases worldwide, with over half of all cases occurring among children and accounting for Volition, given positive early results, we have formed2.9 million deaths in children under five years old. Just under half of all survivors are left with psychological and/or physical effects. Sepsis, also known as ‘blood poisoning’, is hard to identify. Initial symptoms of sepsis are difficult to distinguish from most infections and there is currently no test to diagnose it. Without prompt treatment, it can lead to multiple organ failure and death. Risk of death increases by 7.6% for every hour of treatment delay. Early detection and treatment of sepsis has the potential to improve survival – and improve the quality of life of survivors. Imagine if a Nu.Q® NETs teamsimple blood test could help diagnose sepsis and identify those patients more likely to provide increased focus and drive to the product development program.deteriorate.
Our COVID-19Nu.Q® NETs assay is the only analytically validated assay to quantify the level of NETs. It is platform agnostic so it can be adapted to any workflow/clinical setting – including central lab and point of care.
Nu.Q® Discover
Nu.Q® Discover is a complete solution to profiling nucleosomes which empowers drug developers and scientists, offering rapid epigenetic profiling in disease model development, preclinical testing, and clinical studies – from drug discovery to market launch. Nu.Q® Discover is a valuable research tool for R&D professionals working within the field of Pharmacoepigenetics, and studying the epigenetic basis for variation in 2020 showed nucleosome levels correlated positivelyresponse to drugs and can help to answer clinical questions, such as measuring treatment efficacy, or on-target and off-target effects in drug development. Drug developers and scientists can work with us, access our state-of-the-art proprietary assays and realize their longer-term, drug development needs. In this way, Nu.Q® Discover is able to unlock value from Volition’s IP portfolio by helping us to commercialize the areas we are not going to drive ourselves.
Our biomarkers support the entire drug discovery and development process from pre-clinical testing to market-readiness. We aim to assess disease severity, monitor treatment response, and enhance the understanding of disease pathology and treatments.
Nu.Q® Cancer
Our Nu.Q® Cancer pillar encapsulates a range of simple, cost effective blood-based assays. Cancer is a devastating disease that touches many peoples’ lives, accounting for approximately 10 million deaths worldwide each year. It is the second leading cause of death globally and exerts an enormous burden on families, communities, and health systems. Survival rates are improving in countries with strong significance:health systems, thanks to advances in cancer detection and treatment. However, access to timely diagnostics and therapies remains limited for cancer patients in low and middle-income countries.
·with disease severityNu.Q® Cancer can detect characteristic epigenetic changes in nucleosomes that occur during the earliest stages of cancer and has potential applications beyond cancer detection. Being able to use epigenetic information from the nucleosomes of tumor cells could help physicians:
· | Predict treatment response for each patient, | |
· | Monitor treatment response and disease progression, and | |
· | Promptly amend a patient’s cancer treatment regimen to achieve a better outcome. |
Nu.Q® Cancer could also play a pivotal role in MRD monitoring. The concept of MRD refers to the proportion of remaining cancer cells among otherwise normal bone marrow or, more rarely, among circulating blood cells after any given treatment of blood cancer. MRD monitoring has proven to be an independent prognostic factor and an important instrument for therapeutic decisions.
Capture-PCRTM
Based on over a decade of work on the chemistry of circulating chromatin fragments, we have also developed a transformational wet chemistry pathway that identifies and physically isolates chromatin fragments that we know are tumor-derived from background DNA of the same sequence, using Chromatin Immunoprecipitation (“ChIP”). Quantitative real-time PCR (“qPCR”) testing is undertaken to establish whether cancer is present.
This breakthrough method obviates expensive, time-consuming DNA sequencing and bioinformatics - allowing for rapid, cost-effective detection in a routine blood test. It may also be suitable for automation, enabling application in hospital laboratories.
In early-stage cancer, it is difficult to detect cancer-derived circulating tumor DNA (“ctDNA”) in the blood because it may comprise only 0.01% of the DNA present among a background of 99.99% normal DNA. Moreover, most of the cancer DNA has exactly the same sequence as normal DNA. Current ctDNA detection methods involve DNA extraction, sequencing of all (cancer and normal) circulating DNA and analysis of the sequencing data using sophisticated computer bioinformatics to tell them apart.
Our patented Capture-PCRTMis a novel method for liquid biopsy involving the first wave COVID-19 patients, reported physical isolation of a class of tumor-derived ctDNA fragments from blood. Cancer-derived ctDNA fragments are then extracted after removal of all normal background DNA of the same sequence for detection with a simple, low-cost PCR test.
· with differentiation
| 6 |
| Table of Contents |
Manufacturing Capabilities and Strategy
Our manufacturing facility in Belgium, known as Silver One, offers cutting edge, purpose-built manufacturing and processing facilities. We are currently focusing on manufacturing our key components such as the antibodies and positive controls at Silver One, as well as ELISA kits. We have also outsourced a portion of patients with mild disease from those admittedthe production of our ELISA kits to hospitals,a third-party manufacturer in the U.S. to facilitate logistics and to aim for large-scale production.
·between those whom survived or died.
Commercialization Strategy
We are now conducting studies of serial testing in second wave COVID-19 patientsguided by three underlying principles to determine how predictive our test is and hope to announce additional studies and data throughout 2021. We continue to research the use ofcommercialization strategy – ensuring our Nu.Q® technology for diagnostic and disease monitoring, and as a companion diagnostic to monitor treatment response in a variety of disease conditions.
products:
Research and Development
Volition is developing NucleosomicsTM technologies in a number of areas including:
·Adaptation and optimization of Nu.Q® immunoassay tests across multiple clinical platforms worldwide for the rapid quantification of epigenetic changes in blood and other biofluids. Volition’s Nu.Q® assays for use in clinical studies operate on a random-access immunoassay autoanalyzer approved by the Food and Drug Administration, or FDA, using a chemiluminescent magnetic particle-based assay format, a format which has enhanced analytical performance. Volition is pursuing both autoanalyzer and manual kit Nu.Q® platforms for use in its products, services and clinical studies.
·Nu.Q® assays (both the established plate format and the particle-based format mentioned above) are used for the development of Nu.Q® blood tests for the most prevalent cancers focusing initially on lung cancer, colorectal cancer and hematological cancers using our NucleosomicsTM biomarker discovery platform. Our development platform includes assays to be used for asymptomatic (screening) subjects, high-risk populations, symptomatic patients and to monitor disease progression and/or treatment response. We are developing blood based Nu.Q® assays to detect specific biomarkers that can be used individually or in combination to generate a profile which forms the basis of a product for a particular cancer or disease.
·Nu.Q® assays are used for the development of Nu.Q® blood tests for NETosis. We are developing blood based Nu.Q® assays to detect specific biomarkers that can be used individually or in combination to generate a profile which forms the basis of a product for a particular disease. Our development platform includes assays that may be used for diagnostic purposes and/or to monitor disease progression and/or treatment response.
·Nu.Q® Capture technology to isolate or enrich nucleosomes containing particular epigenetic signals or structures for complete analysis by mass spectrometry, DNA sequencing, immunoassays or other methods for a wide range of potential scientific and medical applications. For example, these applications include the enrichment of nucleosomes of tumor origin in blood samples taken from cancer patients for biomarker discovery, and widespread analysis of circulating chromatin fragments that include epigenetically active chromatin proteins.
The Company has also developed the use of the Nu.Q® technology in veterinary applications and launched its first product, the Nu.Q® Vet Cancer Screening Test, in the fourth quarter of 2020. We are in the process of developing additional veterinary products, including a treatment monitoring test, a disease recurrence test and a point-of-care platform. Our extensive intellectual property portfolio includes the coverage of veterinary applications.
Product Strategy Summary (Human and Canine)
| · | Result in low capital expenditures for licensors and end users and low operating expenses for Volition, |
| · | Are affordable, and |
| · | Are accessible worldwide. |
The principles above inform our overall commercialization strategy for our products, which is driven by the following:
| · | Conducting R&D in-house and through our research partners, | |||||
|
|
|
|
|
|
| kits and key components, and |
|
|
|
|
|
|
| in fragmented markets through regional companies. |
|
|
|
|
|
| ||
We aim to partner with established diagnostic companies to market, sell, and process our tests, leveraging their networks and expertise.
Commercialization Strategy
We aim to initially launch in Europe and Asia, and subsequentlyremain an IP powerhouse in the United States. We planepigenetic space and expect to workmonetize our IP and technologies through licensing and distribution contracts with partnerscompanies that have established distribution networks and expertise on a worldwide or regional basis, in both human and animal care across platforms (centralized labs and point-of-care / in-house diagnostics).
To this end, on March 28, 2022, Volition entered into a master license and product supply agreement with Heska. In exchange for granting Heska exclusive worldwide rights to commercialize Nu.Q® worldwide. Additionally, we are working on complete nucleosome analysis insell our Nu.Q® Capture technology. The goalVet Cancer Test at the point of this projectcare for companion animals, Volition received a $10.0 million upfront payment upon signing, received $13.0 million based upon the achievement of two milestones and is eligible to investigate waysreceive up to specifically target ctDNA. The abilityan additional $5.0 million based upon the achievement of a final milestone upon the earlier of the first commercial sale by or on behalf of Heska of a screening or monitoring test for lymphoma in felines, or the nine-month anniversary of the first peer reviewed paper evidencing clinical utility for the screening or monitoring of lymphoma in felines being published in any one of a number of periodicals identified by the parties. In addition, Volition has granted Heska non-exclusive rights to enrich ctDNA will allow us to use mass spectrometry to analyze histone and DNA modifications, and moreover to sequence the DNA present around the nucleosomes. This information might enable cancer diagnosis to identify the tissue of origin of that given cancer.
Commercialization will take multiple forms in various markets and opportunities including, but not limited to:
·Direct sales ofsell the Nu.Q® Vet Cancer Screening Test via the Gastrointestinal Laboratory at Texas A&M University, or TAMU.
·Salesin kit format for companion animals through Heska’s network of veterinary clinical products utilizing Nu.Q®Vet assays and/or Nu.Q® Capture reagents through distributor networks.
·Licensing of intellectual property, or IP, for clinical products utilizing Nu.Q® assays and/or Nu.Q® Capture reagents.
·Sales of clinical products utilizing Nu.Q® assays and/or Nu.Q® Capture reagents through distributor networks.
·Licensing of IP for RUO kit sales of Nu.Q® assays and/or Nu.Q® Capture reagents.
·Licensing of IP for laboratory developed patient testing services utilizing Nu.Q® assays and/or Nu.Q® Capture reagents.
·Provision of direct research services in the processing of samples using Nu.Q® RUO assays and/or Nu.Q® Capture. central reference laboratories.
IfWe also entered into a licensing and supply agreement with IDEXX in October 2022. This contract provides worldwide customer reach through IDEXX’s global reference laboratory network as we do not have enough fundscontinue to fully implementcommercialize our business plan, we will be forced to scale back our plan of operationstransformational Nu.Q® technology within the companion animal healthcare sector and our business activities, increase our anticipated timeframes to complete each milestone or seek additional funding. Incapitalize on the event that additional financing is delayed, we will prioritizesignificant opportunities available. IDEXX launched the maintenance of our research and development personnel and facilities, primarilyIDEXX Nu.Q® Canine Cancer Test in Belgium.January 2023.
Our Market Opportunity
Cancer is one ofIn November 2023, we launched the leading causes of death worldwide, accounting for around 9.5 million annual deaths globally. There are over 18 million new cases of cancer diagnosed each year and given the aging population this is expected to grow rapidly to over 29.5 million new cases annually by 2040. Currently, in the United States there are more than three new cases of cancer diagnosed and one person dies from a cancer-related cause every minute. Statistically, the chances of surviving cancer are greatly improved by early detection and treatment. However, there are currently very few blood tests for diagnosis of cancer in common clinical use.
Volition believes that early, non-invasive, accurate cancer diagnosis remains a significant unmet medical need and a significant commercial opportunity. For these reasons, cancer diagnostics is an active field of research and development both academically and commercially.
The global in vitro diagnostic medical device, or IVD, market was $64.5 billion in 2017 and is forecasted to reach $93.6 billion by 2025, registering a compound annual growth rate, or CAGR, of 4.8% from 2018 to 2025. The forecasted growth is due primarily to the increasing health care demands of an aging population.
The veterinary market for early stage cancer diagnostics is also large and growing, with approximately 77 million pet dogs in the United States alone. Cancer in dogs is widespread. It is the leading cause of death for dogs over the age of 10 years and there are over 6 million new dog cancer diagnoses each year. As cancer screening is not as commonplace in animal health as it is in human health, we believe blood tests like our Nu.Q® Vet Cancer Screening Test could transform how veterinarians manage cancer in companion animals.the UK and Ireland through our distributor, the Veterinary Pathology Group, and in the UK through Nationwide Laboratories.
WeOur Market Opportunity
Volition applies its technologies through its subsidiaries to develop simple, easy to use, cost-effective blood tests to help diagnose and monitor a range of life-altering diseases for both humans and animals including certain cancers and diseases associated with NETosis such as sepsis and COVID-19. Given the wide-ranging nature of our products in development we believe that our low-cost and easy-to-use ELISA based screening blood test for the early diagnosis of cancer will help streamline the screening process, and improve the treatment and quality of life for up to a third of dogs with malignancies, including common malignancies such as lymphoma and hemangiosarcoma. These two malignancies comprise approximately 2 million cases out of a total of 6 million annual dog cancer diagnoses in the United States.
The United Statesmarket opportunity is currently the largest veterinary market in the world and requires fewer and smaller clinical studies than the FDA process for human diagnostics. This generally allows for a much faster route to revenue for veterinary products as compared to human products.large.
We anticipate that because of their ease of use and cost efficiency of our tests they have the potential to become the first method of choice for cancer diagnostics, allowingdisease detection of a range of cancers at an earlier stage than typically occurs currently, and testing of animals who, for reasons such as time, cost or aversion to current methods, are not currently being tested. The initial veterinary focus is the United Statesmonitoring in both humans and canines but in time we plan to launch products for other cancers, other species, in other countries and other indications such as disease monitoring and even a point of care test.animals.
| 7 |
| Table of Contents |
Our Competition
Our Competition
Volition anticipates facingWe face competition primarily from other human-focused healthcare, pharmaceutical and diagnostic companies such as Exact Sciences Corporation, Guardant Health, GRAIL Inc., Freenome Holdings Inc, CellMax Life, Archer DX Inc., Foundation Medicine Inc., Oncocyte Corporation, OpKo Health Inc., MDNA Life Sciences Inc., Oncimmune Holdings Plc, Abbott Laboratories Inc., Cepheid Inc., Koninklijke Philips N.V.Hologic Corporation, Agilent Technologies Inc., GE Healthcare,Qiagen Inc., Thermo Fisher, Illumina, Becton Dickinson, BioMerieux, Siemens, Gen-Probe Incorporated, EpiGenomics AG, MDxHealth SA, Roche Diagnostics, EpiGenomics AG,Cytovale Inc., and Thrive Earlier Detection Corp,Immunexpress Inc., and from companies such as Mars Incorporated,PetDx, One Health Company (Fidocure) and IDEXX Laboratories, Inc.Vidium Animal Health focused on the veterinary space. There may also be other companies developing products competitive with ours of which we are unaware.
We predict that our future products will have a competitive edge compared to those offered by competitors on the basis that our tests are developed to be accurate, cost-effective, attractive from a government reimbursement perspective, easy to use, non-invasive, technologically advanced, and compatible with immunoassay systems, based on strong intellectual property and to be used for mass screenings.
Many of our competitors have substantially greater financial, technical, and other resources and larger, more established marketing, sales and distribution systems than we have. Many of our competitors also offer broad product lines outside of the diagnostic testing market and have brand recognition. Moreover, our competitors may make rapid technological developments that may result in our intended technologies and products becoming obsolete before we are able to enter the market, recover the expenses incurred to develop them or generate significant revenue. Our success will depend, in part, on our ability to develop our intended products in a timely manner, keep our future products current with advancing technologies, achieve market acceptance of our future products, gain name recognition and a positive reputation in the healthcare industry, and establish successful marketing, sales and distribution efforts.
Government Regulations
The healthcare industry, and thus our business, is subject to extensive federal, state, local and foreign regulation. Some of the pertinent laws pertinent to our businessand regulations have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations. In addition, these laws and their interpretations are subject to change.
Both United States federal and state governmental agencies continue to subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. As indicated by work plans and reports issued by these agencies, the federal government will continue to scrutinize, among other things, the marketing, labeling, promotion, manufacturing, and export of diagnostic health carehealthcare products. Our diagnostic products fall within the IVD medical device category and are subject to FDA clearance or approval in the United States.
The federal government also has increased funding in recent years to fight healthcare fraud, and various agencies, such as the United States Department of Justice, the Office of Inspector General of the Department of Health and Human Services, and state Medicaid fraud-controlfraud control units, are coordinating their enforcement efforts.
In Europe, medical devices are regulated by self-certification through the Conformité Européenne, or CE, mark system. Under the system, developers and manufacturers must operate a quality system and validate medical devices in a limited clinical trial to demonstrate the manufacturer has met analytical and clinical performance criteria. We have implemented an International Organization for Standardization standard, or ISO 13485, Medical Devices – Quality management systems – Requirements for regulatory purposes, or ISO 13485. The standard addresses managerial awareness of regulatory requirements, control systems, inspection and traceability, device design, risk and performance criteria, as well as verification for corrective and preventative measures for device failure. Medical device companies such as ours are subject to pre-market compliance assessments from notified bodies, a European Union certification organization which the national authority, or the competent authority, of a European Union member state designates to carry out one or more of the conformity assessment procedures. ISO 13485 certification establishes conformity to specific European Union directives related to medical devices and allows CE marking and sale of the device.
The European Commission’s In Vitro Diagnostic Regulation 2017/746, or the EU IVDR, became effective in May 2017, marking the start of a transition period for manufacturers selling IVD devices into Europe. The EU IVDR, which replaces IVD Directive 98/79/EC, has a transition period of five years, after which the EU IVDR will apply in full, and no new applications pursuant to the former directive will be accepted. Manufacturers have the duration of the five-year transition period to update their technical documentation and processes to meet the new, more stringent European Union regulatory requirements. We believe the most challenging changes under the EU IVDR will be those regarding the classification of products, which will bring almost all IVDs under the direct review and control of notified bodies, and the performance evaluation of IVDs, which will require extensive clinical and analytical performance studies but also demonstration of scientific validity. Additional requirements will apply to reinforce the safety of the products, such as extended responsibilities of the economic actors of the supply chain, increased post-marketing surveillance activities, unannounced audits from notified bodies, implementation of an improved traceability and transparency of the devices with, in particular, the introduction of the Unique Device Identification, or UDI, system and an expanded European Database on Medical Devices.
Notified bodies can begin auditing to the EU IVDR once they have been designated as a notified body under the EU IVDR by their competent authority. TÜV SÜD will be one such notified body. In practice, it will not be possible to CE mark a product according to the EU IVDR beforehand. We expect our devices will be deemed to be Class C devices under the EU IVDR, and the conformity assessment procedure for such devices will be a combination of quality management system audits and technical documentation assessments. The assessment time needed for a technical documentation assessment of a Class C device is expected to be between 2 to 6 months. We have commenced discussions with the TÜV SÜD to ensure compliance with the EU IVDR as soon as possible.
Regulatory Approach
Commercialization of our future products in the clinical IVDin vitro diagnostic (“IVD”) market (e.g.,(e.g. for patient diagnosis in hospitals, clinics, etc.) requires government approval (CE marking in Europe, FDA approval in the United States, and Chinese Food and Drug Administration or CFDA,(“CFDA”) approval in China).
In Our diagnostic products fall within the IVD medical device category and are subject to FDA clearance or approval in the United States, Volition anticipates that itsStates. We anticipate our tests will have to be cleared through the FDA’s premarket notification or (“510(k)”), process, or the FDA’sits premarket approval or PMA,(“PMA”) process. The determination of whether a 510(k) or a PMA is necessary will depend in part on the proposed indications for use and the FDA’s assessment of the risk associated with the use of the IVD for a particular indication. A similar system operates in China through the CFDA.
In Europe, IVD medical devices are regulated by the European Union,In Vitro Diagnostic Regulation 2017/746 (“EU IVDR”) which brings almost all IVDs under the direct review and control of designated assessment organizations (“Notified Bodies”), and the performance evaluation of IVDs, which requires extensive clinical and analytical performance studies in addition to a demonstration of scientific validity. Additional requirements are applied to reinforce the safety of the products such as extended responsibilities of the economic actors of the supply chain, increased post marketing surveillance activities, unannounced audits from Notified Bodies, implementation of an improved traceability and transparency of the devices with the introduction of the Unique Device Identification system and an expanded European Database on Medical Devices.
Tailored transitional periods have been introduced for on-market IVD devices that must undergo a conformity assessment involving Notified Bodies for the first time under the EU IVDR. The length of the transitional periods depends on the classification of device. The time needed for a Technical Documentation assessment of a device by our tests can be marketed afterNotified Body (“TÜV SÜD”) is expected to last for nine months at a declaration and marking that the test conformsminimum. Any new devices introduced to the essentialmarket will undergo EU IVDR assessment.
In practice, the conformity assessment procedure for our products requires a combination of Quality Management System (“QMS”) audits and Technical Documentation assessments. To support the conformity to the new IVDR, Belgian Volition has implemented a QMS, conforming to the internationally agreed standard ISO 13485 that sets out the QMS requirements ofspecific to the relevant European health,medical devices industry. We have completed inspections in 2023 with the TÜV SÜD and our QMS is in compliance with the EU IVDR. Belgian Volition has maintained its ISO certification since 2015.
| 8 |
| Table of Contents |
We will also be required to comply with numerous other federal, state, and local laws relating to matters such as safe working conditions, industrial safety, and environmental protection legislation, or CE marking. The CE mark is also recognizedlabor laws. We may incur significant costs to comply with such laws and regulations in certain Asian territories, including India, for the private payer market.future, and lack of compliance could have material adverse effects on our operations.
Intellectual Property
Volition is developing clinical products based on the enrichment and analysis of epigenetically modified circulating nucleosomeschromatin using immunoassay, mass spectrometry, DNA sequencing and other methods. We have used this position to build a growing, broad strong and exclusivestrong patent portfolio aroundcovering the ability to profile the epigenetic environment surrounding circulating chromosome fragments from diseased cells, including the epigenetic signaling status of nucleosomes, DNA, and other epigenetic chromatin proteins.
Our patent portfolio includes 2750 patent families (plus two in-licensed families) and a total 6479 patents granted related to our diagnostic tests (including veterinary applications), with 1012 patents granted in the United States, 1419 patents granted in Europe, and a further 4048 patents granted worldwide. Additionally, we have a total of 90132 patent applications currently pending, with 11 patent applications in the United States, 8 in Europe and a further 71 patent applications worldwide.
We intend to continue our development of the Nucleosomics™ technologies and will continue to apply for patents for future product developments. Our IP strategy is to protect the technologies and gain market exclusivity with patents in Europe and the United States and in other strategic countries. The patentspatent filings on the technologies underlying our products should provide broad coverage for each product, including protection through at least 2031.2043.
Employees
As of December 31, 2020,2023, we had 65110 full-time equivalentsequivalent (“FTE”) personnel compared to 50104 as of December 31, 2019.2022, reflecting the growth in our commercial and production activities. We continually assess employee turnover, recruitment initiatives, compensation and benefits programs, safety in performing critical laboratory work, diversity and other matters relevant to human capital management, and we review results with our board of directors on a periodic basis. We aim to offer competitive compensation (including salary, incentive bonus, and equity) and benefits packages into each of our locations and in each of our employee groups at each levelemployees around the globe as assessed with internal and external benchmarking data. We aim to build a pipeline for talent to create more opportunities for workplace diversity and to support greater representation within the Company.
Corporate History
The CompanyVolitionRx Limited is a Delaware corporation that was incorporated on September 24, 1998 in the State of Delaware under the name “Standard Capital Corporation.” On September 22, 2011, the Company filed a Certificate for Renewal and Revival of Charter with the Secretary of State of Delaware. Pursuant to Section 312 of Delaware General Corporation Law, the Company was revived under the new name of “VolitionRX Limited” (which name was subsequently amended to reflect “VolitionRx Limited”). The CompanyVolitionRx acquired its wholly owned operating subsidiary, Singapore Volition Pte. Limited, a Singapore registered company or (“Singapore Volition”) in October 2011. Volition on October 6, 2011.Global Services SRL, a Belgium private limited liability company (“Volition Global”), was formed in August 2021, which is a wholly owned operating subsidiary of VolitionRx. Singapore Volition has one subsidiary, Belgian Volition SRL, a Belgium private limited liability company or (“Belgian Volition,Volition”), which it acquired onin September 22, 2010. Belgian Volition has four subsidiaries, Volition Diagnostics UK Limited, a private limited company formed under the laws of England and Wales (“Volition Diagnostics”), which was formed onin November 13, 2015, Volition America, Inc., a Delaware corporation (“Volition America”), which was formed on in February 3, 2017, Volition Veterinary Diagnostics Development LLC, a Texas limited liability company (“Volition Vet”), which was formed onin June 3, 2019, and Volition Germany GmbH (formerly Octamer GmbH, or “Octamer” and now “Volition Germany”), a Munich, Germany-based epigenetic reagent company that it acquired onin January 10, 2020.
Our principal executive office is located at 13215 Bee Cave Parkway,1489 West Warm Springs Road, Suite 125, Galleria Oaks B, Austin, Texas 78738.110, Henderson, Nevada 89014. Our telephone number is +1 (646) 650-1351. Our website is located at www.volition.com. The information that can be accessed through our website is not incorporated by reference into this Report and should not be considered to be a part hereof.
Financial Information
See our consolidated financial statements and accompanying notes to the consolidated financial statements included in this Report.
| 9 |
| Table of Contents |
ITEM 1A. RISK FACTORS
WHERE YOU CAN GET ADDITIONAL INFORMATION
We file Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K pursuant to Section 13(a) or 15(d) of the Exchange Act electronically with the SEC. You can access these reports and other filings electronically on the SEC’s website, www.sec.gov.
ITEM 1A.RISK FACTORS
Our short and long-term success is subject to numerous risks and uncertainties, many of which involve factors that are difficult to predict or beyond our control. As a result, investing in our common stock involves substantial risk. Before deciding to purchase, hold or sell our common stock, stockholders, and potential stockholders should carefully consider the risks and uncertainties described below, in addition to the other information contained in or incorporated by reference into this Report, as well as the other information we file with the SEC. If any of these risks are realized, our business, financial condition, results of operations, and prospects could be materially and adversely affected. In that case, the value of our common stock could decline, and stockholders may lose all or part of their investment. Furthermore, additional risks and uncertainties of which we are currently unaware, or which we currently consider to be immaterial, could have a material adverse effect on our business.
Certain statements made in this section constitute “forward-looking statements,” which are subject to numerous risks and uncertainties including those described in this section. Refer to the section entitled “Cautionary Note Regarding Forward-Looking Statements” within this Report for additional information.
Risks Associated with Our Company
We operate in a rapidly changing environment that involves a number of risks that could materially affect our business, financial condition or future results, some of which are beyond our control. The summary below, as well as the discussion that follows the summary, highlights some of the risks that may affect future operating results. These are the risks and uncertainties we believe are most important for you to consider. We cannot be certain that we will successfully address these risks. If we are unable to address these risks, among other things, our business may not grow, our stock price may suffer, and we may be unable to stay in business. Additional risks and uncertainties not presently known to us, which we currently deem immaterial, or which are similar to those faced by other companies in our industry or business in general, may also impair our business operations.
Risk Factor Summary
Risks Related to Our Business and Business Strategy
· | We have incurred significant losses, and we may never achieve profitability. | |
· | We may need to raise additional capital in the future. If we are unable to secure adequate funds on terms acceptable to us, we may be unable to execute our plan of operations. | |
· | It is difficult to forecast our future performance, which may cause our financial results to fluctuate unpredictably. | |
· | The diagnostics market is highly competitive and subject to rapid technological change; accordingly, we will face fierce competition, including from companies with greater resources and experience than us, and our intended products may not achieve significant market penetration and/or may become obsolete. | |
· | Our management has broad discretion over the use of our available cash and might not allocate cash in ways that increase the value of your investment. | |
· | Our future success depends on our ability to retain our officers and directors, scientists, and other key employees and to attract, retain and motivate qualified personnel. | |
· | If any of our facilities or our laboratory equipment were damaged or destroyed, or if we experience a significant disruption in our operations for any reason, our ability to continue to operate our business could be materially harmed. | |
· | Failure in our information technology, storage systems or our clinical laboratory equipment could significantly disrupt our operations and our research and development efforts and subject us to liability. | |
· | Our business and reputation will suffer if we are unable to establish and comply with stringent quality standards to assure that the highest level of quality is observed in the performance of our tests. | |
· | Declining global economic or business conditions may have a negative impact on our business. | |
· | We may engage in acquisitions that are not successful and which could disrupt our business, cause dilution to our stockholders and reduce our financial resources. |
| 10 |
| Table of Contents |
Risks Related to Product Development, Commercialization and Sales of Our Products
· | If the marketplace does not accept the products in our development pipeline or any other diagnostic products we might develop, we may be unable to generate sufficient revenue to sustain and grow our business. | |
· | Our business is dependent on our ability to successfully develop and commercialize diagnostic products. If we fail to develop and commercialize diagnostic products, we may be unable to execute our plan of operations. | |
· | Failure to successfully develop, manufacture, market, and sell our future products will have a material adverse effect on our business, financial condition, and results of operations. | |
· | The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials which, in turn, could have a material adverse effect on our business. | |
· | Our research and development efforts will be hindered if we are not able to obtain samples, contract with third parties for access to samples or complete timely enrollment in future clinical trials. | |
· | If the third parties on which we increasingly rely to assist us with our current and anticipated pre-clinical development or clinical trials do not perform as expected, we may not be able to obtain regulatory clearance or approval or commercialize our products. | |
· | We expect to expand our product development, research and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations. | |
· | We have limited experience with sales and marketing and any failure to build and manage a sales and marketing team effectively, or to successfully engage third party providers for such services, could have a material adverse effect on our business. | |
· | We rely on third parties to manufacture and supply our intended products. Any problems experienced by these third parties could result in a delay or interruption in the supply of our intended products to our customers, which could have a material negative effect on our business. | |
· | We depend on third-party distributors to market and sell our products which will subject us to a number of risks. | |
· | The manufacturing operations of our third-party manufacturers will likely be dependent upon third-party suppliers, making us vulnerable to supply shortages and price fluctuations, which could harm our business. | |
· | Defects in our products may subject us to substantial damages which could materially harm our business or financial condition. |
Risks Related to Governmental Regulation and Reimbursement
· | Our failure to obtain necessary regulatory clearances or approvals on a timely basis would significantly impair our ability to distribute and market our future products on the clinical IVD market. | |
· | Reductions or changes in reimbursement policies could limit our ability to sell our products. | |
· | If we are found to have violated laws concerning the privacy and security of patient health information or other personal information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business. |
Risks Related to Our Intellectual Property
· | If the patents we rely on to protect our intellectual property prove to be inadequate, our ability to successfully commercialize our products will be harmed and we may never be able to operate our business profitably. | |
· | If third parties assert that we have infringed their patents and proprietary rights or challenge the validity of our patents and proprietary rights, we may become involved in intellectual property disputes and litigation that would be costly, time consuming, and delay or prevent the development or commercialization of our products. | |
· | If we are unable to protect our trade secrets, we may be unable to protect our interests in proprietary technology, processes and know-how that is not patentable or for which we have elected not to seek patent protection. |
Risks Related to Our Securities
· | The market prices and trading volume of our stock may be volatile. | |
· | We have identified material weaknesses in our internal control over financial reporting that have not yet been remediated, and although we are working to address such weaknesses, the failure to address these material weaknesses, or the identification of any others, could impact the reliability of our financial reporting and harm investors’ views of us, which could adversely impact our stock price. | |
· | We have a “going concern” opinion from our auditors, indicating the possibility that we may not be able to continue to operate. |
| 11 |
| Table of Contents |
· | Our Second Amended and Restated Certificate of Incorporation exculpates our officers and directors from certain liability to our Company and our stockholders. | |
· | Our corporate governance documents, and certain corporate laws applicable to us, and share ownership by executive officers and directors, could make a takeover attempt, which may be beneficial to our stockholders, more difficult. | |
· | We do not expect to pay dividends in the foreseeable future. | |
· | We may in the future issue additional shares of our common stock which would reduce investors’ ownership interests in the Company, and which may cause our stock price to decline. | |
· | Future sales of our common stock could depress the market price of our common stock. | |
· | If equity research analysts do not publish research or reports about our business, or if they do publish such reports but issue unfavorable commentary or downgrade our common stock, the price and trading volume of our common stock could decline. | |
· | If we fail to comply with the NYSE American’s continued listing requirements, our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted. | |
· | We are a smaller reporting company and a non-accelerated filer, and we cannot be certain if the reduced disclosure requirements applicable to our filing status, as well as the exemption from the requirement to provide an auditor’s attestation report regarding the effectiveness of our internal controls, will make our common stock less attractive to investors. |
Risks Related to Our Business and Business Strategy
We have incurred significant losses, and we may never achieve profitability.
We are a clinical stage company and have incurred losses since our formation. As of December 31, 2020,2023, we have an accumulated total deficit of approximately $110.2$202.6 million. As we continue the discovery and development of our future diagnostic products, we expect our expenses to increase significantly. Even as we begin to market and sell our intended products, we expect our losses to continue as a result of ongoing research and development expenses, as well as increased manufacturing, sales and marketing expenses. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders’ equity. Because of the numerous risks and uncertainties associated with our product development and commercialization efforts, we are unable to predict when or if we will become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to achieve and then maintain profitability, our business, financial condition and results of operations will be negatively affected, and the market value of our common stock will decline.
We may need to raise additional capital in the future. If we are unable to secure adequate funds on terms acceptable to us, we may be unable to execute our plan of operations.
We willmay require additional capital to fully fund our current strategic plan, which includes successfully commercializing our Nu.Q® cancer pipeline and developing future products. If we incur delays in commencing commercialization of our Nu.Q® cancer pipeline or other future products or in achieving significant product revenue, or if we encounter other unforeseen adverse business developments, we may exhaust our capital resources prior to the commencement of commercialization.
We cannot be certain that additional capital will be available when needed or that our actual cash requirements will not be greater than anticipated. Financing opportunities may not be available to us, or if available, may not be available on favorable terms. The availability of financing opportunities will depend on various factors, such as market conditions and our financial condition and outlook. In addition, if we raise additional funds through the issuance of equity or convertible debt securities, the percentage ownership of our stockholders could be significantly diluted, and these newly issued securities may have rights, preferences or privileges senior to those of existing stockholders. If we obtain debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations. If we are unable to obtain financing on terms favorable to us, we may be unable to execute our plan of operations and we may be required to cease or reduce development or commercialization of any future products, sell some or all of our technology or assets or merge with another entity.
| 12 |
| Table of Contents |
It is difficult to forecast our future performance, which may cause our financial results to fluctuate unpredictably.
Our limited operating history and the rapid evolution of the market for diagnostic products make it difficult for us to predict our future performance. A number of factors, many of which are outside of our control, may contribute to fluctuations in our financial results, such as:
· | our ability to develop or procure antibodies for clinical use in our future products; | |
· | our ability to translate preliminary clinical results to larger prospective symptomatic and screening populations; | |
· | the demand for our intended products; | |
· | our ability to obtain any necessary financing; | |
· | our ability to market and sell our future products; | |
· | market acceptance of our future products and technology; | |
· | performance of any future strategic business partners; | |
· | our ability to obtain regulatory clearances or approvals; | |
· | our success in collecting payments from third-party payors and customers; | |
· |
| |
· | competition with other diagnostics companies; and | |
· | adverse changes in the healthcare industry (human and canine). |
The diagnostics market is highly competitive and subject to rapid technological change; accordingly, we will face fierce competition, including from companies with greater resources and experience than us, and our intended products may not achieve significant market penetration and/or may become obsolete.
The diagnostics market is extremely competitive and characterized by rapidly evolving industry standards and new product enhancements. Our diagnostic tests are technologically innovative and require significant planning, design, development, and testing at the technological, product, and manufacturing process levels. These activities require significant capital commitments and investment. There can be no assurance that our intended products or proprietary technologies will remain competitive following the introduction of new products and technologies by competing companies within the industry. Furthermore, there can be no assurance that our competitors will not develop products that render our future products uncompetitiveobsolete or obsolete; that are more effective, accurate or can be produced at lower costs. There can be no assurance that we will be successful in the face of increasing competition from new technologies or products introduced by existing companies in the industry or by new companies entering the market.
·competition
The market for diagnostics is also significantly affected by new product introductions and other market activities of industry participants. Our competitors include large multinational corporations and their operating units, including Exact Sciences Corporation, Guardant Health, GRAIL Inc., Freenome Holdings Inc, CellMax Life, Archer DX Inc., Foundation Medicine Inc., Oncocyte Corporation, OpKo Health Inc., MDNA Life Sciences Inc., Abbott Laboratories Inc., Cepheid Inc., Hologic Corporation, Agilent Technologies Inc., Qiagen Inc., Thermo Fisher, Illumina, Becton Dickinson, BioMerieux, Siemens, Gen-Probe Incorporated, EpiGenomics AG, MDxHealth SA, Roche Diagnostics, Cytovale Inc. and Immunexpress Inc., and from companies such as PetDx, One Health Company (Fidocure) and Vidium Animal Health focused on the veterinary space. There may also be other companies developing products competitive with ours of which we are unaware. Successful commercialization of our services will require that we satisfactorily address the needs of various medical practitioners that constitute a target market to reach customers and to address potential resistance to recommendations for our services. If we are unable to continue to achieve significant market penetration, we will not be able to generate sufficient revenue to become profitable and our products may become obsolete.
Many of our competitors have greater resources and experience than us and may enjoy several competitive advantages, including:
· | significantly greater name recognition; | |
· | established relationships with healthcare professionals, companies and consumers; | |
· | additional lines of products, and the ability to offer rebates or bundle products to offer higher discounts or incentives to gain a competitive advantage; | |
· | established supply and distribution networks; and | |
· | greater resources for product development, sales and marketing, and intellectual property protection. |
Many of these other cancer diagnostics companies;companies have developed and
·adversewill continue to develop new products that will compete directly with our future products. In addition, many of our competitors spend significantly greater funds for the research, development, promotion, and sale of new and existing products. These resources may allow them to respond more quickly to new or emerging technologies and changes in consumer requirements. We also face competition in our search for third parties to assist us with sales and marketing of our product candidates, which may negatively impact our ability to enter into favorable sales and marketing arrangements. For all the healthcare industry (humanforegoing reasons, we may not be able to compete successfully against our competitors, which could jeopardize our ability to recoup research and canine)development expenditures, hurt our reputation and harm our business, results of operations and financial condition.
| 13 |
| Table of Contents |
Our management has broad discretion over the use of our available cash and might not allocate cash in ways that increase the value of your investment.
As of December 31, 2023, we had approximately $20.7 million in combined cash and cash equivalents compared to approximately $10.9 million as of December 31, 2022. Our management expects to deploy these resources primarily to expand our commercialization activities, to fund our product development efforts and for general corporate and working capital purposes. However, our management has broad discretion to pursue other objectives. Our management might not apply our cash in ways that increase or permit any return of your investment.
Our future success depends on our ability to retain our officers and directors, scientists, and other key employees and to attract, retain and motivate qualified personnel.
Our success depends on our ability to attract, retain and motivate highly qualified management and scientific personnel. In particular, we are highly dependent on Cameron Reynolds, our President and Chief Executive Officer, our other officers and directors, scientists and key employees. The loss of any of these persons or their expertise would be difficult to replace and could have a material adverse effect on our ability to achieve our business goals. In addition, the loss of the services of any one of these persons may impede the achievement of our research, development and commercialization objectives by diverting management’s attention to the identification of suitable replacements, if any. There can be no assurance that we will be successful in hiring or retaining qualified personnel and our failure to do so could have a material adverse effect on our business, financial condition and results of operations.
Recruiting and retaining qualified scientific personnel and, in the future, sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among pharmaceutical, biotechnology and diagnostic companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. We do not maintain “key person” insurance on any of our employees. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research, development and commercialization strategies. Our consultants and advisors, however, may have other commitments or employment that may limit their availability to us.
We expectIf any of our facilities or our laboratory equipment were damaged or destroyed, or if we experience a significant disruption in our operations for any reason, our ability to expandcontinue to operate our product development,business could be materially harmed.
If our present, or any future facilities, were to be damaged, destroyed or otherwise unable to operate, whether due to fire, floods, storms, tornadoes, earthquakes, other inclement weather events or natural disasters, employee malfeasance, terrorist acts, power outages, or otherwise, it may render it difficult or impossible for us to perform our research and salesdevelopment for some period of time and marketing capabilities,our business could be severely disrupted. The lead time from ordering to delivery of certain specialized equipment we use can be more than six months and difficult to substitute.
Failure in our information technology, storage systems or our clinical laboratory equipment could significantly disrupt our operations and our research and development efforts and subject us to liability.
Our ability to execute our business strategy depends, in part, on the continued and uninterrupted performance of our information technology systems, which support our operations including our research and development efforts. The integrity and protection of our own data, and that of our customers, clinical trial subjects and employees, is critical to our business. The regulatory environment governing information, security and privacy laws is increasingly demanding and continues to evolve. IT systems are vulnerable to damage from a variety of sources. High-profile security breaches at other companies and in government agencies have increased in recent years, and cyber-attacks are becoming more sophisticated and frequent, and in some cases have caused significant harm. Computer hackers and others routinely attempt to breach the security of technology products, services and systems, and to fraudulently induce employees, customers, or others to disclose information or unwittingly provide access to systems or data. While we devote significant resources to security measures to protect our systems and data, these measures cannot provide absolute security.
Any breach or interruption of our information technology systems could compromise our networks and the information stored therein could be accessed by unauthorized parties, publicly disclosed, lost or stolen. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our IT systems, unauthorized access, loss or disclosure could also disrupt our operations, including our ability to:
· | provide customer assistance services; | |
· | conduct research and development activities; | |
· | collect, process and prepare company financial information; | |
· | provide information about our tests and other patient and healthcare provider education and outreach efforts through our website; and | |
· | manage the administrative aspects of our business and damage to our reputation. |
| 14 |
| Table of Contents |
Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as the U.S. Health Insurance Portability and Accountability Act of 1996, similar U.S. state data protection regulations, including the California Consumer Privacy Act, the EU’s General Data Protection Regulation, and other regulations, the breach of which could result in significant penalties.
Failure to adequately protect and maintain the integrity of our information systems and data, including as a result weof a security breach, may encounter difficulties in managing our growth, which could disrupt our operations.
We are focused on developing our pipeline for future products. It is likely that our efforts will result in significant growth in the number of our consultants, advisors,losses and employees, in addition to the scope of our operations. In order to manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plan or disrupt our operations.
We have limited experience with direct sales and marketing and any failure to build and manage a direct sales and marketing team effectively, or to successfully engage third party providers for such services, could have a material adverse effect on our business.financial position, results of operations and cash flows.
Our productsbusiness and reputation will require several dynamicsuffer if we are unable to establish and evolving sales models tailoredcomply with stringent quality standards to different worldwide markets, users and products. Our sales strategyassure that the highest level of quality is initially focused onobserved in the clinical IVD market with the CE markingperformance of our first producttests.
Inherent risks are involved in Europe. Following CE markingproviding and marketing diagnostic and monitoring tests and related services. Patients and healthcare providers rely on us to provide accurate clinical and diagnostic information that may be used to make critical healthcare decisions. Consequently, users of our first producttests may have a greater sensitivity to errors than users of some other types of products and services. We must maintain high service standards and other quality controls. Performance or accuracy defects, incomplete or improper process controls, excessively slow turnaround times, unanticipated uses of our tests or mishandling of samples or test results (whether by us, patients, healthcare providers, courier delivery services, or others) can lead to adverse outcomes for patients and interruptions to our services. These events could lead to voluntary or legally mandated safety alerts relating to our tests or our laboratory facilities and could result in Europe we intendthe removal of our products and services from the market or the suspension of our laboratories’ operations. Insufficient quality controls and any resulting negative outcomes could result in significant costs and litigation, as well as negative publicity that could reduce demand for our tests and payers’ willingness to enter the European markets and, following the completion of any necessary regulatory clearances, certain Asian markets.cover our tests. Even if we receivemaintain adequate controls and procedures, damaging and costly errors may occur.
Declining global economic conditions may have a CE mark, we must still seek regulatory clearance in other jurisdictions. A failure to obtain these regulatory clearances in other jurisdictions could negatively affectnegative impact on our business. Pending completion
Concerns over U.S. healthcare reform legislation and energy costs, geopolitical issues, the availability and cost of our review of the regulatory environmentcredit and government stimulus programs in the United States includingand other countries, and global inflationary pressures may contribute to increased volatility and diminished expectations for the effect of recent pronouncements regarding Laboratory Developed Tests, or LDTs, byglobal economy. If the FDA, we may decide to enter the United States market through a Clinical Laboratory Improvement Amendments, or CLIA, certified laboratory located in the United States. We remain firmly committed to pursuing FDA approval as our primary objective. FDA approval can consist of PMA or 510(k) clearance depending on the test complexity and risk posed to patients. We intend to pursue the most appropriate approval pathway for each individual product developed. We intend to progressively grow to large volumes of tests sold to centralized laboratories and eventually reach the mass diagnostics testing market. The exact nature of the ideal sales strategy will evolve as we continue to develop our intended products and seek entry into the IVD markets. We also have limited experience with direct sales and marketing and we intend to engage a network of distributors to help commercialize our products worldwide. Any failure to build and manage a direct sales and marketing team effectively, or to successfully engage third-party providers for such services, could have a material adverse effect on our business.
There are significant risks involved in building and managing our sales and marketing organization, as well as identifying and negotiating deals with the right sales and distribution partners, including risks related to our ability to:
·identify appropriate partners;
·negotiate beneficial partnership and distribution agreements;
·hire qualified individuals as needed;
·generate sufficient leads within our targeted market for our sales force;
·provide adequate training for effective sales and marketing;
·protect intellectual property rights;
·retain and motivate our direct sales and marketing professionals; and
·effectively oversee geographically dispersed sales and marketing teams.
Our failure to adequately address these risks could have a material adverse effect on our ability to increase sales and use of our future products, which would cause our revenues to be lower than expected and harm our results of operations. Further, we are required to comply with numerous other federal, state, and local laws relating to matters such as safe working conditions, industrial safety, and labor laws. We may incur significant costs to comply with such laws and regulations in the future, and lack of compliance could have material adverse effects on our operations. We believe that we have structuredeconomic climate deteriorates, our business, operations to comply with applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise, which could have a material adverse impact onincluding our business.
We have identified material weaknesses in our internal control over financial reporting that have not yet been remediated, and the failure to address these material weaknesses, or the identification of any others, could impact the reliability of our financial reporting and harm investors’ views of us, which could adversely impact our stock price.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Exchange Act Rule 13a-15(f), internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
·pertainaccess to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of assets;
·provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being maderesearch use only, in accordance with authorizations of our management and/or directors; and
·provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
We have determined that we have material weaknesses in our internal control over financial reporting as of December 31, 2020. See Part II, Item 9A of this Reportclinical IVD markets for a complete discussion of these material weaknesses in our internal control over financial reporting and remediation efforts. Although we are undertaking steps to address these material weaknesses, the existence of a material weakness is an indication that there is more than a remote likelihood that a material misstatement of our financial statements will not be prevented or detected in the current or any future period. There can be no assurance that we will be able to fully implement our plans and controls, as further described in Item 9A, to address these material weaknesses, or that the plans and controls, if implemented, will be successful in fully remediating these material weaknesses. In addition, we may in the future identify further material weaknesses in our internal control over financial reporting that we have not discovered to date. If we fail to successfully remediate the identified material weaknesses, or we identify further material weaknesses in our internal controls, the market’s confidence in our financial statements could decline and the market price of our common stockdiagnostic tests, could be adversely impacted. Additionally, for so long as we remain asaffected, resulting in a smaller reporting company, under current rules our accounting firm will not be required to provide an opinion regarding our internal controls over financial reporting.
We have a “going concern” opinion from our auditors, indicating the possibility that we may not be able to continue to operate.
Our independent registered public accountants have expressed substantial doubt about our ability to continue as a going concern. This opinion could materially limit our ability to raise additional funds by issuing new debt or equity securities or otherwise. If we fail to raise sufficient capital when needed, we will not be able to complete our proposed business plan. As a result, we may have to liquidate our business and investors may lose their investments. Our ability to continue as a going concern is dependent upon our ability to successfully accomplish our plan of operations described herein, obtain financing and eventually attain profitable operations. Investors should consider our independent registered public accountant’s comments when deciding whether to invest in the Company.
Our management has broad discretion over the use of our available cash and might not spend available cash in ways that increase the value of your investment.
As of December 31, 2020, we had approximately $19.4 million in combined cash and cash equivalents compared to approximately $17.0 million as of December 31, 2019. Our management expects to deploy these resources primarily to expand our commercialization activities, to fund our product development efforts and for general corporate and working capital purposes. However, our management has broad discretion to pursue other objectives. Our management might not apply our cash in ways that increase or permit any return of your investment.
Risks Associated with Our Business
Failure to successfully develop, manufacture, market, and sell our future products will have a material adverse effectnegative impact on our business, financial condition and results of operations.
WeThe United Kingdom’s withdrawal from the European Union became effective in January 2021. Although it is known what the terms of this withdrawal were, it is still possible that greater restrictions on imports and exports between the European Union countries and the United Kingdom and increased regulatory complexities are in the process of developing a suite of diagnostic tests as well as additional products. The successful development and commercialization offorthcoming. These changes may adversely affect our intended products is critical to our future success. Our ability to successfully develop, manufacture, market and sell our future products is subject to a number of risks, many ofin the United Kingdom which are outside our control. There can be no assurance that we will be able to develop and manufacture products in commercial quantities at acceptable costs, successfully market any products, or generate revenues from the sale of any products. Failure to achieve any of the foregoing wouldcould have a materialan adverse effect on our business, financial condition, and results of operations.
In addition, following Russia’s military invasion of Ukraine in February 2022, NATO deployed additional military forces to Eastern Europe, and the United States, European Union, and other nations announced various sanctions against Russia. The invasion of Ukraine and the retaliatory measures that have been taken, and could be taken in future, by the U.S., NATO, and other countries have created global security concerns that could result in a regional conflict and otherwise have a lasting impact on regional and global economies, any or all of which could adversely affect our business.
We may engage in acquisitions that are not successful and which could disrupt our business, cause dilution to our stockholders and reduce our financial resources.
From time to time, we may consider opportunities to acquire or invest in other companies, products or technologies that may enhance our product platform or technology, expand the breadth of our markets or customer base, or otherwise advance our business strategies. Potential and completed acquisitions and investments involve numerous risks, including the following:
· | we may be unable to successfully integrate the acquired business(es) into our business; | |
· | we may be unable to realize the anticipated benefits of the acquisition; | |
· | the acquisition may not strengthen our competitive position; and | |
· | our future results may suffer if we do not effectively manage our expanded operations. |
We do not know if we will be able to identify future acquisitions or investments we deem suitable, whether we will be able to successfully complete any such acquisitions or investments on favorable terms or at all, or whether we will be able to successfully integrate any acquired products or technologies into our business. Our potential inability to integrate any acquired products or technologies effectively may adversely affect our business, operating results and financial condition.
| 15 |
| Table of Contents |
Risks Related to Product Development, Commercialization and Sales of Our Products
If the marketplace does not accept the products in our development pipeline or any other diagnostic products we might develop, we may be unable to generate sufficient revenue to sustain and grow our business.
Our intended products may never gain significant acceptance in the research or clinical marketplace and therefore may never generate substantial revenue or profits. Physicians, hospitals, clinical laboratories, researchers or others in the healthcare industry may not use our future products unless they determine that they are an effective and cost-efficient means of detecting and diagnosing cancer. If our research and studies do not satisfy providers, payors and others as to the reliability and effectiveness, we may experience reluctance or refusal on the part of the physician to use our future products. In addition, we will need to expend a significant amount of resources on marketing and educational efforts to create awareness of our future products and to encourage their acceptance and adoption. If the market for our future products does not develop sufficiently or the products are not accepted, our revenue potential will be harmed.
Our business is dependent on our ability to successfully develop and commercialize diagnostic products. If we fail to develop and commercialize diagnostic products, we may be unable to execute our plan of operations.
Our current business strategy focuses on discovering, developing and commercializing diagnostic products. The success of our business will depend on our ability to fully develop and commercialize the diagnostic products in our current development pipeline as well as continue the discovery and development of other diagnostics products.
Prior to commercializing the Nu.Q® tests and other diagnostic products, we will be required to undertake time-consuming and costly development activities with uncertain outcomes, including conducting clinical studies and obtaining regulatory clearance or approval in the United States, Asia and in Europe. Delays in obtaining approvals and clearances could have material adverse effects on us and our ability to fully carry out our plan of operations. We have limited experience in taking products through these processes and there are considerable risks involved in these activities. The science and methods that we are employing are innovative and complex, and it is possible that our development programs will ultimately not yield products suitable for commercialization or government approval. Products that appear promising in early development may fail to be validated in subsequent studies, and even if we achieve positive results, we may still fail to obtain the necessary regulatory clearances or approvals. Few research and development projects result in commercial products, and perceived viability in early clinical studies often is not replicated in later studies. At any point, we may abandon development of a product, or we may be required to expend considerable resources obtaining additional clinical and nonclinical data, which would adversely impact the timing for generating potential revenue from those products. Further, our ability to develop and launch diagnostic tests is dependent on our receipt of substantial additional funding. If our discovery and development programs yield fewer commercial products than we expect, we may be unable to execute our business plan, and our business, financial condition and results of operations may be adversely affected.
Failure to successfully develop, manufacture, market, and sell our future products will have a material adverse effect on our business, financial condition, and results of operations.
We are in the process of developing a suite of diagnostic tests as well as additional products. The successful development and commercialization of our intended products is critical to our future success. Our ability to successfully develop, manufacture, market, and sell our future products is subject to a number of risks, many of which are outside our control. There can be no assurance that we will be able to develop and manufacture products in commercial quantities at acceptable costs, successfully market any products, or generate revenues from the sale of any products. Failure to achieve any of the foregoing would have a material adverse effect on our business, financial condition, and results of operations.
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials which, in turn, could have a material adverse effect on our business.
As described above, weWe must conduct extensive testing of our product candidates and new indications of our marketed products before we can obtain regulatory approval to market and sell them. Success in pre-clinical studies or completed clinical trials does not ensure that later studies or trials, including continuing pre-clinical studies and large-scale clinical trials, will be successful nor does it necessarily predict future results. Favorable results in early studies or trials may not be repeated in later studies or trials, and product candidates in later stage trials may fail to show acceptable safety and efficacy despite having progressed through earlier trials. We may be required to demonstrate through large, long-term outcome trials that our product candidates are safe and effective for use in a broad population prior to obtaining regulatory approval. The failure of clinical trials to demonstrate the safety and effectiveness of our clinical candidates for the desired indication(s) would preclude the successful development of those candidates for such indication(s), in which event our business, prospects, results of operations and financial condition may be adversely affected.
| 16 |
| Table of Contents |
Our research and development efforts will be hindered if we are not able to obtain samples, contract with third parties for access to samples or complete timely enrollment in future clinical trials.
Access to human and animal sample types, such as blood is necessary for our research and product development. Acquiring samples from individuals / animals with clinical diagnoses or associated clinical outcomes through purchase or clinical studies is necessary. Lack of available samples can delay development timelines and increase costs of development. Generally, the agreements under which we gain access to human and animal samples are non-exclusive. Other companies may compete with us for access. If we are not able to negotiate access to clinical samples with research institutions, hospitals, clinical partners, pharmaceutical companies, or companies developing therapeutics and/or diagnostics on a timely basis, or at all, or if other laboratories or our competitors secure access to these samples before us, our ability to research, develop and commercialize future products will be limited or delayed. Equally, we may not be able to conduct or complete clinical studies in a timely manner if we are unable to enroll sufficient numbers of patients in such studies, which could consequently have an adverse effect on our research and development and product commercialization efforts.
If the third parties on which we increasingly rely to assist us with our current and anticipated pre-clinical development or clinical trials do not perform as expected, we may not be able to obtain regulatory clearance or approval or commercialize our products.
As our clinical infrastructure expands, we expect to increasingly rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct some of our current and anticipated pre-clinical investigations and clinical trials. For example, we currently rely on Diagnostic Oncology CRO, LLC (“DXOCRO”) to support development and clinical validation studies for our Nu.Q® product portfolio in the United States, including by conducting large-scale finding studies across multiple sites in the U.S. using our Nu.Q® NETs test to determine clinical utility in sepsis, which we hope to leverage in support of our U.S. commercialization strategy. However, if we are not able to maintain or reach mutually acceptable agreements with DXOCRO or other third parties on a timely basis, these third parties do not successfully carry out their commitments or regulatory obligations or meet expected deadlines, or the quality or accuracy of the data they obtain is compromised due to the failure to adhere to agreed-upon clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory clearance or approval for, or successfully commercialize, our products on a timely basis, if at all, and our business, operating results and prospects may be adversely affected.
We expect to expand our product development, research and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We are focused on developing our pipeline for current and future products. It is likely that our efforts will result in significant growth in the number of our consultants, advisors, and employees, in addition to the scope of our operations. For example, in connection with the anticipated commercialization of our products, we may add personnel to certain areas of our business including laboratory operations, quality assurance, and compliance. Further, as we build our commercialization efforts and expand research and development activities for new products, the scope and complexity of our operations is increasing significantly. As a result of our growth, our operating expenses and capital requirements have also increased, and we expect that they will continue to increase. Our ability to manage our growth effectively requires us to expend funds to implement and improve our managerial, operational and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plan or disrupt our operations.
We have limited experience with sales and marketing and any failure to build and manage a sales and marketing team effectively, or to successfully engage and maintain third party providers for such services, could have a material adverse effect on our business.
As an organization we have limited experience with direct sales however we are building a team of experienced individuals in terms of market intelligence, product management and account management in addition to building relationships with market-leading established distributors as commercial partners. For example, Heska has commenced pre-order sales of our Nu.Q® Vet Cancer Test for screening of cancer in canines to veterinarians worldwide at the point of care pursuant to our exclusive global supply and licensing agreement. We have also engaged IDEXX to make our Nu.Q® Vet Cancer Test available to reference laboratories in the United States. Although we are investing in direct marketing to support these commercial launches, we may rely on third party resources such as Heska’s global network of veterinarians and IDEXX’s reference laboratory network to successfully market this test and generate revenue. Any failure to build and manage a sales and marketing team effectively, or to successfully engage and maintain third-party providers for such services, could have a material adverse effect on our business.
| 17 |
| Table of Contents |
Our products will require several dynamic and evolving sales models tailored to different worldwide markets, users and products. Our clinical sales strategy is initially focused on the clinical IVD market with the CE marking of our first product in Europe, the Nu.Q® NETs test, in May 2022. With this CE marking of our first product in Europe we intend to enter the European markets and, following the completion of any necessary regulatory clearances, certain Asian markets. Even if we receive a CE mark for a certain product, we must still seek regulatory clearance in other jurisdictions. A failure to obtain these regulatory clearances in other jurisdictions could negatively affect our business. Pending completion of our review of the regulatory environment in the United States we may decide to enter the United States market through a Clinical Laboratory Improvement Amendments (“CLIA”), certified laboratory located in the United States. We remain firmly committed to pursuing FDA approval as our primary objective. FDA approval can consist of PMA or 510(k) clearance depending on the test complexity and risk posed to patients. We intend to pursue the most appropriate approval pathway for each individual product developed. We intend to progressively grow to large volumes of tests sold to centralized laboratories and eventually reach the mass diagnostics testing market. The exact nature of the ideal sales strategy will evolve as we continue to develop our intended products and seek entry into the IVD markets.
There are significant risks involved in building and managing our sales and marketing organization, as well as identifying and negotiating deals with the right sales and distribution partners, including risks related to our ability to:
· | identify appropriate partners; | |
· | negotiate beneficial partnership and distribution agreements; | |
· | hire qualified individuals as needed; | |
· | generate sufficient leads within our targeted market for our sales force; | |
· | provide adequate training for effective sales and marketing; | |
· | protect intellectual property rights; | |
· | retain and motivate our direct sales and marketing professionals; and | |
· | effectively oversee geographically dispersed sales and marketing teams. |
Our failure to adequately address these risks could have a material adverse effect on our ability to increase sales and use of our future products, which would cause our revenues to be lower than expected and harm our results of operations. Further, we are required to comply with numerous other federal, state, and local laws relating to matters such as safe working conditions, industrial safety, and labor laws. We may incur significant costs to comply with such laws and regulations in the future, and lack of compliance could have material adverse effects on our operations. We believe that we have structured our business operations to comply with applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise, which could have a material adverse impact on our business.
We rely on third parties to manufacture and supply our intended products. Any problems experienced by these third parties could result in a delay or interruption in the supply of our intended products to our customers, which could have a material negative effect on our business.
We rely on third parties to manufacture and supply our intended products. The manufacture of our intended diagnostic products requires specialized equipment and utilizes complicated production processes that would be difficult, time-consuming and costly to duplicate. If the operations of third-party manufacturers are interrupted or if they are unable to meet our delivery requirements due to capacity limitations or other constraints, we may be limited in our ability to fulfill our future sales orders. Any prolonged disruption in the operations of third-party manufacturers could have a significant negative impact on our ability to sell our future products, could harm our reputation and could cause us to seek other third-party manufacturing contracts, thereby increasing our anticipated development and commercialization costs. In addition, if we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards required by the FDA and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop products or receive approval of any products in a timely manner.
| 18 |
| Table of Contents |
We depend on third-party distributors to market and sell our products, which will subject us to a number of risks.
We depend, and expect to continue to depend, on third-party distributors to market, sell, and service our products in our intended markets. For example, Heska has commenced pre-order sales of our Nu.Q® Vet Cancer Test for screening of cancer in canines to veterinarians at the point of care and we engaged IDEXX to make our Nu.Q® Vet Cancer Test available to reference laboratories in the United States. Further, we have engaged with others including DNAtech, Portugal, and, through our agreement with Heska, with Scil Lab Europe, to launch the Nu.Q® Vet Cancer Test to customers in Europe. In November 2023, we launched the Nu.Q® Vet Cancer Test in the UK and Ireland through our distributor, the Veterinary Pathology Group, and in the UK through Nationwide Laboratories. We are subject to a number of risks associated with reliance upon these parties and other third-party distributors including the following:
· | lack of day-to-day control over the activities of third-party distributors; | |
· | third-party distributors may not commit the necessary resources to market and sell our products to our level of expectations; | |
· | third-party distributors may terminate their arrangements with us on limited or no notice or may change the terms of these arrangements in a manner unfavorable to us; and | |
· | disagreements with our distributors could result in costly and time-consuming litigation or arbitration which we could be required to conduct in jurisdictions with which we are not familiar. |
If we fail to establish and maintain satisfactory relationships with our third-party distributors, our revenues and market share may not grow as anticipated, and we could be subject to unexpected costs which could harm our results of operations and financial condition.
The manufacturing operations of our third-party manufacturers will likely be dependent upon third-party suppliers, making us vulnerable to supply shortages and price fluctuations, which could harm our business.
The operations of our future third-party manufacturers will likely be dependent upon third-party suppliers. A supply interruption or an increase in demand beyond a supplier’s capabilities could harm the ability of our future manufacturers to manufacture our intended products until new sources of supply are identified and qualified.
Reliance on these suppliers could subject us to a number of risks that could harm our business, including:
· | interruption of supply resulting from modifications to or discontinuation of a supplier’s operations; | |
· | delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s variation in a component; | |
· | a lack of long-term supply arrangements for key components with our suppliers; | |
· | inability to obtain adequate supply in a timely manner, or to obtain adequate supply on commercially reasonable terms; | |
· | difficulty and cost associated with locating and qualifying alternative suppliers for components in a timely manner; | |
· | production delays related to the evaluation and testing of products from alternative suppliers, and corresponding regulatory qualifications; | |
· | delay in delivery due to suppliers prioritizing other customer orders over ours; | |
· | damage to our brand reputation caused by defective components produced by the suppliers; and | |
· | fluctuation in delivery by the suppliers due to changes in demand from us or their other customers. |
We have implemented certain risk mitigation strategies including the diversification of suppliers by region and the internalization of certain production processes. However, any interruption in the supply of components of our future products or materials, or our inability to obtain substitute components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our future customers, which would have an adverse effect on our business.
Defects in our products may subject us to substantial damages which could materially harm our business or financial condition.
The products we develop could lead to product liability claims based on allegations that one or more of our products contained a design or manufacturing defect which resulted in the failure to detect the disease for which it was designed. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business or financial condition. We cannot assure you that our product liability insurance would protect our assets from the financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing insurance coverage in the future.
| 19 |
| Table of Contents |
Risks Related to Governmental Regulation and Reimbursement
Our failure to obtain necessary regulatory clearances or approvals on a timely basis would significantly impair our ability to distribute and market our future products on the clinical IVD market.
We are subject to regulation by the FDA in the United States, the CE in Europe, the CFDA in China, and other regulatory bodies in other countries where we intend to sell our future products. Before we are able to place our intended products in the clinical IVD markets in the United States, China and Europe, we will be required to obtain clearance or approval of our future products from the FDA and the CFDA with respect to the United States and China, respectively, and receive a CE mark with respect to Europe.
In Europe, since May 2022, IVD medical devices are regulated by the new EU IVDR. The European Union has recently adopted regulations that may imposemost challenging changes under the EU IVDR as compared to the previous Directive are those regarding the classification of products, which brings almost all IVDs under the direct review and control of Notified Bodies, and the performance evaluation of IVDs, which requires extensive clinical and analytical performance studies in addition to a demonstration of scientific validity. These changes and other additional requirements to obtain a CE mark, whichMark could result in delays and further expense, in terms of staff costs to us as compared to the current CE mark process. The new regulations will require each product submission to be thoroughly audited by notified bodies, instead ofprocess under the current self-certification process. The European Medical Device Regulations, or EU MDR, will be fully applicable in 2020 and the EU IVDR will be fully applicable in 2022.previous Directive.
Additionally, even if we receive the required government clearance or approval of our intended products, we are still subject to continuing regulation and oversight. Under the FDA, diagnostics are considered medical devices and are subject to ongoing controls and regulations, including inspections, compliance with established manufacturing practices, device-tracking, record-keeping, advertising, labeling, packaging, and compliance with other standards. The process of complying with such regulations with respect to current and new products can be costly and time-consuming. Failure to comply with these regulations could jeopardize our ability to sell our products and result in enforcement actions such as fines, civil penalties, injunctions, warning letters, recalls of products, delays in the introduction of products into the market, refusal of the FDA or other regulators to grant future clearances or approvals, delays by the FDA or other regulators in granting clearances or approvals, and the suspension or withdrawal of existing approvals by the FDA or other regulators, any of which could have a material adverse effect on our business, financial condition, and results of operations. Furthermore, any FDA regulations governing our future products are subject to change at any time, which may cause delays and have material adverse effects on our operations. In Europe, IVD companies are currently able to self-certify that they meet the appropriate regulatory requirements (which are subject to change with the EU MDR and the EU IVDR noted above) but are subject to inspection for enforcement. European national agencies, such as customs authorities and/or the Departments of Health, Industry and Labor, conduct market surveillance to ensure the applicable requirements have been met for products marketed within the European Union.
The cancer diagnostics market is highly competitive and subject to rapid technological change; accordingly, we will face fierce competition and our intended products may become obsolete.
The cancer diagnostics market is extremely competitive and characterized by evolving industry standards and new product enhancements. Cancer diagnostic tests are technologically innovative and require significant planning, design, development, and testing at the technological, product, and manufacturing process levels. These activities require significant capital commitments and investment. There can be no assurance that our intended products or proprietary technologies will remain competitive following the introduction of new products and technologies by competing companies within the industry. Furthermore, there can be no assurance that our competitors will not develop products that render our future products obsolete or that are more effective, accurate or can be produced at lower costs. There can be no assurance that we will be successful in the face of increasing competition from new technologies or products introduced by existing companies in the industry or by new companies entering the market.
Reductions or changes in reimbursement policies could limit our ability to sell our products.
Market acceptance and sales of our products will depend, in part, on reimbursement policies and may be affected by healthcare reform measures. Government authorities and third-party payers, such as private health insurers and health maintenance organizations, decide which products they will pay for and establish reimbursement levels for those products. To manage healthcare costs, many governments and third-party payers in the United States increasingly scrutinize the pricing of new products and require greater levels of evidence of favorable clinical outcomes and cost-effectiveness before extending coverage. We cannot be sure that reimbursement will be available for our products and, if reimbursement is available, the levelscope of such reimbursement. Reimbursement may impact the demand for, or the price of, our products. If reimbursement is not available or is available only at limited levels,amounts, we may not be able to successfully commercialize our future products.
If we are found to have violated laws concerning the privacy and security of patient health information or other personal information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
There are a number of U.S. and international laws protecting the privacy and security of personal information. These laws include the U.S. Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) and related regulations, U.S. state laws (such as the California Consumer Privacy Act (“CCPA”) and the California Privacy Rights Act (“CPRA”)), Canada’s Personal Information and Electronic Documents Act (“PIPEDA”) or the applicable provincial alternatives, the EU’s General Data Protection Regulation (“GDPR”), EU member states directives, or similar applicable laws. These laws place limits on how we may collect, use, share and store medical information and other personal information, and they impose obligations to protect that information against unauthorized access, use, loss, and disclosure.
If the marketplace does not accept the products in our development pipelinewe, or any other diagnostic products we might develop, we may be unable to generate sufficient revenue to sustain and growof our business.
Our intended products may never gain significant acceptance in the research or clinical marketplace and therefore may never generate substantial revenue or profits. Physicians, hospitals, clinical laboratories, researchers or others in the healthcare industry may not use our future products unless they determine that they are an effective and cost-efficient means of detecting and diagnosing cancer. If our research and studies do not satisfyservice providers payors and others aswho have access to the reliability and effectiveness, we may experience reluctance or refusal on the part of the physician to use our future products. In addition, we will need to expend a significant amount of resources on marketing and educational efforts to create awareness of our future products and to encourage their acceptance and adoption. If the marketpersonal data for our future products does not develop sufficiently or the products are not accepted, our revenue potential will be harmed.
We expect to face intense competition from companies with greater resources and experience than us, which may increase the difficulty for us to achieve significant market penetration.
The market for cancer diagnostics is intensely competitive, subject to rapid change, and significantly affected by new product introductions and other market activities of industry participants. Our competitors include large multinational corporations and their operating units, including Exact Sciences Corporation, Guardant Health, GRAIL Inc., Freenome Holdings Inc., CellMax Life, Archer DX Inc., Thrive Earlier Detection Corp., Foundation Medicine Inc., Oncocyte Corporation, OpKo Health Inc, MDNA Life Sciences Inc., Oncimmune Holdings Plc, Abbott Laboratories Inc., Cepheid Inc., Koninklijke Philips N.V., GE Healthcare, Siemens, Gen-Probe Incorporated, EpiGenomics AG, MDxHealth SA, Roche Diagnostics, Mars Incorporated, and IDEXX Laboratories, Inc. There may also be other companies developing products competitive with ours of which we are not aware. Manyresponsible, are found to be in violation of the privacy or security requirements of HIPAA, PIPEDA, GDPR, or applicable foreign, U.S. state and Canadian provincial laws, we could be subject to civil or criminal penalties, which could increase our competitors have greater resourcesliabilities, harm our reputation and experience than us and may enjoy several competitive advantages, including:
·significantly greater name recognition;
·established relationships with healthcare professionals, companies and consumers;
·additional lines of products, and the ability to offer rebates or bundle products to offer higher discounts or incentives to gain a competitive advantage;
·established supply and distribution networks; and
·greater resources for product development, sales and marketing, and intellectual property protection.
Many of these other companies have developed and will continue to develop new products that will compete directly with our future products. In addition, many of our competitors spend significantly greater funds for the research, development, promotion, and sale of new and existing products. These resources may allow them to respond more quickly to new or emerging technologies and changes in consumer requirements. We also face competition in our search for third parties to assist us with sales and marketing of our product candidates, which may negatively impact our ability to enter into favorable sales and marketing arrangements. For all the foregoing reasons, we may not be able to compete successfully against our competitors.
Declining global economic or business conditions may have a negative impact on our business.
Concerns over United States healthcare reform legislation and energy costs, geopolitical issues, the availability and cost of credit and government stimulus programs in the United States and other countries may contribute to increased volatility and diminished expectations for the global economy. If the economic climate deteriorates, our business, including our access to the RUO or clinical IVD markets for diagnostic tests, could be adversely affected, resulting in a negative impact on our business, financial condition and results of operations.
The United Kingdom’s withdrawal from the European Union became effective on January 1, 2021. Although it is known what the terms of this withdrawal are, it is still possible that greater restrictions on imports and exports between the European Union countries and the United Kingdom and increased regulatory complexities are forthcoming. These changes may adversely affect our ability to market our future products in the United Kingdom which could have anmaterial adverse effect on our business, financial condition and results of operations. We will rely on third parties to manufacture and supply our intended products. Any problems experienced by these third parties could result in a delay or interruptionoperating results. In addition, entities operating in the supplyhealthcare industry have increasingly become targets for hackers. Although we utilize a variety of our intended productsmeasures to our customers, which couldsecure the data that we control, even compliant entities can experience security breaches or have a material negative effect on our business.
inadvertent failures despite employing reasonable practices and safeguards.
| 20 |
|
| Table of Contents |
We will rely on third partiesmay also face new risks relating to manufacturedata privacy and supply our intended products. The manufacture of our intended diagnostic products will require specialized equipment and utilize complicated production processes that would be difficult, time-consuming and costly to duplicate. If the operations of third-party manufacturers are interrupted or if they are unable to meet our delivery requirements due to capacity limitations or other constraints, we may be limited in our ability to fulfill our future sales orders. Any prolonged disruption in the operations of third-party manufacturers could have a significant negative impact on our ability to sell our future products, could harm our reputation and could cause us to seek other third-party manufacturing contracts, thereby increasing our anticipated development and commercialization costs. In addition, if we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards required by the FDA and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop products or receive approval of any products in a timely manner.
The COVID-19 pandemic could adversely impact our business, including our clinical trials, development activities, business and operations.
In December 2019, a novel strain of coronavirus, COVID-19, was reported to have surfaced in Wuhan, China. Since then, the COVID-19 coronavirus has spread worldwide, includingsecurity as the United States, individual U.S. states or Canadian provinces, E.U. member states, and Europe. On March 11, 2020, the World Health Organization designated the outbreak of the novel strain of coronavirus knownother international jurisdictions adopt or implement new data privacy and security laws and regulations as COVID-19 as a global pandemic. Governments around the world have taken unprecedented actions to mitigate the spread of COVID-19, including stay-at-home orders, quarantine requirements, and limitations on travel, including the closing of national borders. As a result of these restrictions, most of our employees are working remotely where possible and we have limited employee travel.
As a result of the COVID-19 pandemic, we have experienced and may continue to experience disruptions that could severely impact our business and clinical trials, including:
·delays or difficulties in enrolling patients in clinical trials;
·delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff;
·diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as clinical trial sites and hospital staff supporting the conduct of our clinical trials;
·interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by governments, employers and others;
·limitations in employee resources that would otherwise be focused on the conduct of clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people;
·shutdowns or other business disruptions at our customers and collaborators;
·diversion of management resources to focus on mitigating the impacts of the COVID-19 pandemic; and
·impacts from prolonged remote work arrangements, such as strains on our business continuity plans and the inability of certain employees to perform their work remotely.
The global COVID-19 pandemic continues to rapidly evolve. The extent to which the COVID-19 pandemic may impact our business and clinical trials and development activities will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the ultimate duration and severity of the pandemic, travel restrictions and social distancing requirements in the countries where we conduct business, the effectiveness of actions taken to contain and treat the disease, and how quickly and to what extent more normalized economic and operating conditions can resume. If we or our customers experience prolonged shutdowns or other business disruptions beyond current expectations, our ability to conduct our business could be materially and adversely impacted, and our business, liquidity, and financial results may be adversely affected.
The continued spread of COVID-19 has led to disruption and volatility in the global capital markets, which increases the cost of, and adversely impacts access to, capital and increases economic uncertainty. This volatility and uncertainty may adversely affect our stock price. The actions that governments and individuals have taken in response to COVID-19 have led to a sharp contraction in many aspects of economies worldwide. The pandemic may cause an economic slowdown of potentially extended duration, and it is possible that it could cause a global recession. If this occurs, it could negatively impact our ability to develop and commercialize our products among other things. Even afterworldwide. For example, amendments to privacy and security laws (such as the COVID-19 pandemic has subsided,CCPA and the CPRA) may impose additional requirements on us and increase our regulatory and litigation risk. As we may continue to experience material adverse effects toexpand, our business as a result of the global economic impact of the pandemic.
will need to adapt to meet these and other similar legal requirements.
The manufacturing operations of our future third-party manufacturers will likely be dependent upon third-party suppliers, making us vulnerableRisks Related to supply shortages and price fluctuations, which could harm our business.Our Intellectual Property
The operations of our future third-party manufacturers will likely be dependent upon third-party suppliers. A supply interruption or an increase in demand beyond a supplier’s capabilities could harm the ability of our future manufacturers to manufacture our intended products until new sources of supply are identified and qualified.
Reliance on these suppliers could subject us to a number of risks that could harm our business, including:
·interruption of supply resulting from modifications to or discontinuation of a supplier’s operations;
·delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s variation in a component;
·a lack of long-term supply arrangements for key components with our suppliers;
·inability to obtain adequate supply in a timely manner, or to obtain adequate supply on commercially reasonable terms;
·difficulty and cost associated with locating and qualifying alternative suppliers for components in a timely manner;
·production delays related to the evaluation and testing of products from alternative suppliers, and corresponding regulatory qualifications;
·delay in delivery due to suppliers prioritizing other customer orders over ours;
·damage to our brand reputation caused by defective components produced by the suppliers; and
·fluctuation in delivery by the suppliers due to changes in demand from us or their other customers.
Any interruption in the supply of components of our future products or materials, or our inability to obtain substitute components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our future customers, which would have an adverse effect on our business.
We will depend on third-party distributors in the future to market and sell our future products which will subject us to a number of risks.
We will depend on third-party distributors to sell, market, and service our future products in our intended markets. We are subject to a number of risks associated with reliance upon third-party distributors including:
·lack of day-to-day control over the activities of third-party distributors;
·third-party distributors may not commit the necessary resources to market and sell our future products to our level of expectations;
·third-party distributors may terminate their arrangements with us on limited or no notice or may change the terms of these arrangements in a manner unfavorable to us; and
·disagreements with our future distributors could result in costly and time-consuming litigation or arbitration which we could be required to conduct in jurisdictions with which we are not familiar.
If we fail to establish and maintain satisfactory relationships with our future third-party distributors, our revenues and market share may not grow as anticipated, and we could be subject to unexpected costs which could harm our results of operations and financial condition.
If the patents we rely on to protect our intellectual property prove to be inadequate, our ability to successfully commercialize our future products will be harmed and we may never be able to operate our business profitably.
Our success depends, in large part, on our ability to protect proprietary methods, discoveries and technologies that we develop under the patents and intellectual property laws of the United States, Europe and other countries, so that we can seek to prevent others from unlawfully using our inventions and proprietary information. Our patent portfolio includes 2750 patent families (plus two in-licensed families) and a total 79 patents granted related to our diagnostic tests (including veterinary applications), with 1012 patents granted in the United States, 1419 patents granted in Europe and a further 4048 patents granted worldwide. Additionally, we have 90 patent applications pending, with 11 patent applications in the United States, 8 in Europe and a further 71132 patent applications pending, worldwide.
If we are not able to protect our proprietary technology and information, our competitors may use our inventions to develop competing products. We cannot assure you that any of the pending patent applications will result in patents being issued. In addition, due to technological changes that may affect our future products or judicial interpretation of the scope of our patents, our intended products might not, now or in the future, be adequately covered by our patents.
If third parties assert that we have infringed their patents and proprietary rights or challenge the validity of our patents and proprietary rights, we may become involved in intellectual property disputes and litigation that would be costly, time consuming, and delay or prevent the development or commercialization of our future products.
Our ability to commercialize our intended products depends on our ability to develop, manufacture, market and sell our future products without infringing the proprietary rights of third parties. Third parties may allege that our future products or our methods or discoveries infringe their intellectual property rights. Numerous United States and foreign patents and pending patent applications, which are owned by third parties, exist in fields that relate to our intended products and our underlying methodologies, discoveries and technologies. A third party may sue us for infringing its patent rights.
Our ability to successfully commercialize our intended products depends on our ability to protect our proprietary technology and information. Likewise, we may need to resort to litigation to enforce a patent issued or licensed to us or to determine the scope and validity of third-party proprietary rights. In addition, a third party may claim that we have improperly obtained or used its confidential or proprietary information. The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be substantial, and the litigation could divert our management’s attention from other aspects of our business. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation could limit our ability to continue our operations. Additionally, we cannot be certain of the level of protection, if any that will be provided by our patents if they are challenged in court, where our competitors may raise defenses such as invalidity, unenforceability or possession of a valid license.
If we are found to infringe upon intellectual property rights of third parties, we might be forced to pay damages, potentially including triple damages. In addition to any damages, we might have to pay, a court could require us to stop the infringing activity or obtain a license. Any license required under any patent may not be made available on commercially acceptable terms, if at all. In addition, such licenses are likely to be non-exclusive and, therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license and are unable to design around a patent, we may be unable to effectively market some or all of our future products, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations.
| 21 |
| Table of Contents |
If we are unable to protect our trade secrets, we may be unable to protect our interests in proprietary technology, processes and know-how that is not patentable or for which we have elected not to seek patent protection.
In addition to patented technology, we rely upon trade secret protection to protect our interests in proprietary know-how and for processes for which patents are difficult or impossible to obtain or enforce. We may not be able to protect our trade secrets adequately. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors and outside scientific advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. We rely, in part, on non-disclosure and confidentiality agreements with our employees, consultants and other parties to protect our trade secrets and other proprietary technology. These agreements may be breached, and we may not have adequate remedies for any breach. Moreover, others may independently develop equivalent proprietary information, and third parties may otherwise gain access to our trade secrets and proprietary knowledge. Any disclosure of confidential information into the public domain or to third parties could allow our competitors to learn our trade secrets and use the information in competition against us, which could adversely affect our competitive advantage.
Defects in our products may subject usRisks Related to substantial damages which could materially harm our business or financial condition.Our Securities
The products we develop could lead to product liability claims based on allegations that one or more of our products contained a design or manufacturing defect which resulted in the failure to detect the disease for which it was designed. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business or financial condition. We cannot assure you that our product liability insurance would protect our assets from the financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing insurance coverage in the future.
Risks Associated with Our Common Stock
The market prices and trading volume of our stock may be volatile.
The market price of our common stock is likely to be highly volatile and the trading volume may fluctuate and cause significant price variation to occur. We cannot assure you that the market prices of our common stock will not fluctuate or decline significantly in the future. Some of the factors that could negatively affect the prices of our shares or result in fluctuations in those prices or in trading volume of our common stock could include the following, many of which will be beyond our control:
| · | competition; |
· | comments by securities analysts regarding our business or prospects; | |
· | additions or departures of key personnel; | |
· | our ability to execute our business plan; | |
· | issuance of common stock or other securities; | |
· | operating results that fall below expectations; | |
· | loss of any strategic relationship; | |
· | industry developments; | |
· | economic and other external factors; and | |
· | period-to-period fluctuations in our financial results. |
In addition, the securities markets have from time-to-time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price and trading volume of our common stock.
Share ownership byWe have identified material weaknesses in our executive officersinternal control over financial reporting that have not yet been remediated, and directors make it more difficult for third partiesalthough we are working to acquireaddress such weaknesses, the failure to address these material weaknesses, or the identification of any others, could impact the reliability of our financial reporting and harm investors’ views of us, or effectuate a change of control that might be viewed favorably by other stockholders.which could adversely impact our stock price.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Exchange Act Rule 13a-15(f), internal control over financial reporting is a process designed by, or under the supervision of, March 10, 2021,the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
· | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of assets; | |
· | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and/or directors; and | |
· | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
| 22 |
| Table of Contents |
We have determined that we have material weaknesses in our executive officersinternal control over financial reporting as of December 31, 2023. See Part II, Item 9Aof this Reportfor a complete discussion of these material weaknesses in our internal control over financial reporting and directors beneficially owned,remediation efforts. Although we have taken and continue to take steps to address these material weaknesses, the existence of a material weakness is an indication that there is more than a remote likelihood that a material misstatement of our financial statements will not be prevented or detected in the aggregate, approximately 11.3%current or any future period. There can be no assurance that we will be able to fully implement our plans and controls, as further described in Item 9A, to address these material weaknesses, or that the plans and controls, if implemented, will be successful in fully remediating these material weaknesses. In addition, we may in the future identify further material weaknesses in our internal control over financial reporting that we have not discovered to date. If we fail to successfully remediate the identified material weaknesses, or we identify further material weaknesses in our internal controls, the market’s confidence in our financial statements could decline and the market price of our outstanding shares.common stock could be adversely impacted. Additionally, for so long as we remain as a smaller reporting company, under current rules our accounting firm will not be required to provide an opinion regarding our internal controls over financial reporting.
We have a “going concern” opinion from our auditors, indicating the possibility that we may not be able to continue to operate.
Our independent registered public accountants have expressed substantial doubt about our ability to continue as a going concern. This opinion could materially limit our ability to raise additional funds by issuing new debt or equity securities or otherwise. If we fail to raise sufficient capital when needed, we will not be able to complete our proposed business plan. As a result, if the executive officers and directors were to oppose a third party’s acquisition proposal for, or a change in control of, the Company, such officers and directorswe may have sufficient voting power to be ableliquidate our business and investors may lose their investments. Our ability to block or at least delay such an acquisition or changecontinue as a going concern is dependent upon our ability to successfully accomplish our plan of operations described herein, obtain financing and eventually attain profitable operations. Investors should consider our independent registered public accountant’s comments when deciding whether to invest in control from taking place, even if other stockholders would support such a sale or change of control.the Company.
Our Second Amended and Restated Certificate of Incorporation exculpates our officers and directors from certain liability to our Company and our stockholders.
Our Second Amended and Restated Certificate of Incorporation contains a provision limiting the liability of our officers and directors for their acts or failures to act, except for acts involving intentional misconduct, fraud or a knowing violation of law. This limitation on liability may reduce the likelihood of derivative litigation against our officers and directors and may discourage or deter our stockholders from suing our officers and directors based upon breaches of their duties to our Company.
Our corporate governance documents, and certain corporate laws applicable to us, and share ownership by executive officers and directors, could make a takeover attempt, which may be beneficial to our stockholders, more difficult.
Our Boardboard of Directors, or Board,directors has the power, under our charter documents to:
· |
| |
· | fill vacant directorships except for vacancies created by the removal of a director; | |
· | amend our bylaws without stockholder approval subject to certain exceptions; and | |
· | require compliance with an advance notice procedure with regard to business to be brought by a stockholder before an annual or special meeting of stockholders and with regard to the nomination by stockholders of candidates for election as directors. |
Further, our executive officers and directors beneficially own an amount of our outstanding shares of common stock without havingsuch that if they were collectively to obtain stockholder approvaloppose a third party’s acquisition proposal for, or a change in control of, the Company, such action;
·fill vacant directorships except for vacancies created by the removal of a director;
·amend our bylaws without stockholder approval subject to certain exceptions;officers and
·require compliance with an advance notice procedure with regard to businessdirectors may have sufficient voting power to be brought byable to influence whether such an acquisition or change in control takes place, even if other stockholders would support such a stockholder before an annualsale or special meetingchange of stockholders and with regard to the nomination by stockholders of candidates for election as directors. control.
These provisions and circumstances may discourage potential acquisition proposals and could delay or prevent a change of control, including under circumstances in which our stockholders might otherwise receive a premium over the market price of our common stock.
We do not expect to pay dividends in the foreseeable future.
We have never declared or paid cash dividends on our common stock. We do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest any future earnings in the development and growth of our business. Therefore, investors will not receive any funds unless they sell their common stock, and stockholders may be unable to sell their shares on favorable terms or at all. We cannot assure you of a positive return on investment or that you will not lose the entire amount of your investment in our common stock.
We may in the future issue additional shares of our common stock which would reduce investors’ ownership interests in the Company, and which may cause our stock price to decline.
Our Second Amended and Restated Certificate of Incorporation authorizes the issuance of 100,000,000 shares of common stock, par value $0.001 per share. The future issuance of all or part of our remaining authorized common stock may result in substantial dilution in the percentage of our common stock held by our then existing stockholders. We may value any common stock issued in the future on an arbitrary basis. The issuance of common stock for future services or acquisitions or other corporate actions may have the effect of diluting the percentage ownership of our stockholders and, depending upon the prices at which such shares are sold or issued, on their investment in our common stock and, therefore, could have an adverse effect on any trading market for our common stock.
| 23 |
| Table of Contents |
Future sales of our common stock could depress the market price of our common stock.
Sales of a substantial number of shares of our common stock in the public market or the perception that large sales of our shares could occur, could cause the market price of our common stock to decline or limit our future ability to raise capital through an offering of equity securities.
If equity research analysts do not publish research or reports about our business, or if they do publish such reports but issue unfavorable commentary or downgrade our common stock, the price and trading volume of our common stock could decline.
The trading market for our common stock could be affected by whether and to what extent equity research analysts publish research or reports about us and our business. If one or more equity analysts cover us and publish research reports about our common stock, the price of our stock could decline rapidly if one or more securities analysts downgrade our stock or if those analysts’ issue or offer unfavorable commentary or cease publishing reports about us. If any of these analysts ceases coverage of us, we could lose visibility in the market, which in turn could cause our common stock price or trading volume to decline and our common stock to be less liquid.
If we fail to comply with the NYSE American’s continued listing requirements, our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
Our common stock is listed on the NYSE American. The continued listing of our common stock on the NYSE American is subject to our continued compliance with certain listing requirements, including requirements related to corporate governance, our financial condition and operating results, the trading price of our common stock, number of stockholders and our market capitalization. If we fall out of compliance with the NYSE American’s listing standards and fail to regain compliance within the applicable cure periods, our common stock may be delisted from the NYSE American. The delisting of our common stock could materially reduce the liquidity of our common stock and result in a corresponding material reduction in the price of our common stock. In addition, delisting could harm our ability to raise capital on terms acceptable to us, or at all, reduce the amount of analyst coverage of our securities, result in the loss of confidence by investors and employees, and could lead to fewer business development opportunities, any of which could adversely affect our business.
We are a smaller reporting company and a non-accelerated filer and we cannot be certain if the reduced disclosure requirements applicable to our filing status, as well as the exemption from the requirement to provide an auditor’s attestation report regarding the effectiveness of our internal controls, will make our common stock less attractive to investors.
We are a “smaller reporting company,” meaning that we are not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and have a public float of less than $250 million measured as of the last business day of our most recently completed second fiscal quarter. “Smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. We are also a “non-accelerated filer,” meaning that although we havebased on our eligibility as a public float of more than $75 million measured“smaller reporting company” as of the last business day of our most recently completed second fiscal quarter, ourwell as having annual revenues areof less than $100 million.million in the most recent fiscal year for which audited financial statements are available. As a “non-accelerated filer,” we are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting. Decreased disclosures in our SEC filings due to our status as a “smaller reporting company” and as a “non-accelerated filer” may make it harder for investors to analyze our results of operations and financial prospects and may make our common stock a less attractive investment.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
| 24 |
| Table of Contents |
ITEM 1B.UNRESOLVED STAFF COMMENTS1C. CYBERSECURITY
None.We maintain an information security and cybersecurity program, as well as a cybersecurity governance framework, which are designed to protect our information systems against operational risks related to cybersecurity.
Cybersecurity Risk Management and Strategy
We recognize the importance of assessing, identifying, and managing material risks associated with cybersecurity threats which include, among other things, operational risks, intellectual property theft, fraud or extortion, harm to employees or customers, violation of privacy or security laws and related litigation and legal risk, and reputational risks.
We have developed and implemented a cybersecurity risk management program intended to protect the confidentiality, integrity, and availability of our critical systems and information, and detect and contain any cybersecurity incidents that impact us. The program is integrated into our overall risk management systems and processes, and includes a cybersecurity risk assessment process that routinely evaluates potential impacts of cybersecurity risks on our business, including our operations, financial stability, and reputation. These assessments inform our cybersecurity risk mitigation strategies. The results are regularly shared with management and the Audit Committee of our board of directors as part of the committee’s involvement in managing and overseeing cybersecurity risks.
Our cybersecurity risk management program also includes processes to triage, assess the severity of, escalate, contain, investigate, and remediate an incident, as well as to comply with potentially applicable legal obligations and mitigate brand and reputational damage. If a cybersecurity incident is determined to be a potentially material cybersecurity incident, our disclosure controls and procedures define the steps to determine materiality and disclose such a material cybersecurity incident.
While we do not believe that our business strategy, results of operations or financial condition have been materially adversely affected by any cybersecurity incidents, cybersecurity threats are pervasive and, similar to other global financial institutions, we, as well as our employees, customers, regulators, service providers, and other third parties have experienced a significant increase in information security and cybersecurity risk in recent years and will likely continue to be the target of cyber attacks. We continue to assess the risks and changes in the cyber environment, invest in enhancements to our cybersecurity capabilities, and engage in industry and government forums to promote advancements in our cybersecurity capabilities, as well as the broader financial services cybersecurity ecosystem. For more information on risks to us from cybersecurity threats, see the section entitled “Risk Factors — Failure in our information technology, storage systems or our clinical laboratory equipment could significantly disrupt our operations and our research and development efforts” included within this Report.
Cybersecurity Governance
Our board of directors is actively involved in overseeing risks from cybersecurity threats. At least once a year, the board of directors discusses our programs and policies related to cybersecurity and risk initiatives and considers them closely both from a risk management perspective and as part of our business strategy. Additionally, our board has delegated to our Audit Committee the authority to oversee and review the adequacy of our cybersecurity, information and technology security, and data privacy programs, procedures, and policies. Our Audit Committee is comprised entirely of independent directors who regularly evaluate cybersecurity risks.
The Audit Committee regularly receives updates from management with respect to the Company’s efforts to manage data protection, cybersecurity, and information and technology risks, and assesses the results of reviews from internal audits. Materials presented to our Audit Committee include updates on our data security posture, results from internal audit and third-party assessments, our incident response plan, and certain cybersecurity threat risks or incidents and developments, as well as the steps management has taken to respond to such risks. The committee also regularly engages with our Group IT Manager on technology risk-related topics.
Our processes also allow for our board of directors and the Audit Committee to be informed of key cybersecurity risks outside the regular reporting schedule. While the Audit Committee conducts meetings regularly, the committee is authorized to meet with management or individual directors at any time it deems appropriate to discuss matters relevant to the committee. The Company’s policy is for the board and the Audit Committee to receive prompt and timely information regarding any cybersecurity risk (including any incident) that meets reporting thresholds, as well as ongoing updates regarding any such risk, in accordance with our data breach reporting procedure and GDPR.
| 25 |
| Table of Contents |
ITEM 2.PROPERTIES
Listed below are our current facilities as of December 31, 2020:2023:
Location | Primary Function | Approx. Square Feet | Leased or Owned |
Namur, Belgium (1) | Research and development | 17,300 | Owned |
Namur, Belgium (2) | Manufacturing | 9,688 | Owned |
London, UK (3) | Sales and marketing | 323 | Leased, expiring |
Triple One, Singapore (4) | Sales and |
| Leased, expiring |
|
|
| Leased, expiring |
Carlsbad, California (6) | Research and development | 6,645 | Leased, expiring 2027 |
(1) | Belgian Volition purchased property located in Namur, Belgium, in October 2016, to be used as a laboratory facility for R&D. The purchase price for the property was €1.2 million, exclusive of any closing costs. | |
(2) | Belgian Volition purchased property located in Namur, Belgium, in December 2020, to be used as a manufacturing facility. The purchase price for the property was €0.6 million, exclusive of any closing costs. | |
(3) | Volition Diagnostics signed a new 24-month lease for this property located at 93-95 Gloucester Place, London, W1U 6JQ, United Kingdom, commencing February 1, 2022 until January 31, 2024, at an annual rent of £64,800 GBP. | |
(4) | Singapore Volition signed a one-year lease for this property, commencing July 1, 2023, located at 111 Somerset Road, Level 3, Triple One, Somerset, Singapore 238164, at an annual rent of SGD 77,580. | |
(5) | Volition America signed a one-year lease for this property, commencing on April 1, 2022, located at 1489 West Warm Springs Road, Suite 110, Henderson, Nevada 89014, at an annual rent of $14,868. Volition America entered into a new one-year lease for this property, commencing April 1, 2023, at an annual rent of $16,308. | |
(6) | Volition America signed a sixty-two month lease for this property, commencing on February 1, 2022, located at 6086 Corte Del Cedro, Carlsbad, California 92011 at an annual rent of $91,704. |
(1)Belgian Volition purchased property located in Namur, Belgium, in October 2016, to be used as a laboratory facility for R&D. The purchase price for the property was €1.2 million, exclusive of any closing costs.
(2)Belgian Volition purchased property located in Namur, Belgium, in December 2020, to be used as a manufacturing facility. The purchase price for the property was €0.6 million, exclusive of any closing costs.
(3)Volition Diagnostics UK signed a new 16-month lease for this property located at 93-95 Gloucester Place, London, W1U 6JQ, United Kingdom, commencing October 1, 2020 until January 31, 2022, at an annual rent of £64,800 GBP.
(4)Singapore Volition signed a one-year lease for this property, commencing July 1, 2020, located at 111 Somerset Road, Level 3, Triple One, Somerset, Singapore 238164, at an annual rent of $56,952 SGD.
(5)VolitionRx Limited signed a three-year lease for this property, commencing on June 1, 2019, located at 13215 Bee Cave Parkway, Suite 125, Galleria Oaks B, Austin, Texas 78738, at an annual rent of $34,384.
ITEM 3.LEGAL PROCEEDINGS
In the ordinary course of business, we may be subject to claims, counter claims, suits and other litigation of the type that generally arise from the conduct of our business. We are not aware of any threatened or pending litigation that we expect will have a material adverse effect on our business operations, financial condition or results of operations.
ITEM 4.MINE SAFETY DISCLOSURES
Not Applicable.applicable.
| 26 |
| Table of Contents |
PART II
PART II
ITEM 5.MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is traded on the NYSE American under the symbol “VNRX”.
As of March 10, 2021,15, 2024, there were 52,870,90782,068,442 shares of our common stock outstanding held by 136160 holders of record, based on information provided by our transfer agent. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees.
Dividends
We have not declared or paid any cash dividends on our common stock since inception and presently anticipate that all earnings, if any, will be retained for development of our business and that no dividends on our common stock will be declared in the foreseeable future. Any future dividends will be subject to the discretion of our board of directors and will depend upon, among other things, future earnings, operating and financial conditions, capital requirements, general business conditions and other pertinent facts. Therefore, there can be no assurance that any dividends on our common stock will be paid in the future.
Securities Authorized for Issuance Under Equity Compensation Plans
The information required under this item is incorporated by reference from our definitive proxy statement related to our 2021 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A, on or before April 30, 2021.
Recent Sales of Unregistered Securities
Effective January 1, 2021,On December 4, 2023, the Company issued a warrant to purchase up to 125,0003,205,431 shares of ourits common stock atto a non-U.S. investor for an exerciseaggregate purchase price of $3.95 per share, to an officer ofapproximately $2.67 million in a private placement transaction. Such shares were issued in reliance on the Company as an inducement to employment, which vests in full on January 1, 2022 (subject to continued employment through such date and accelerated vesting upon a change of control) and expires January 1, 2027.
Neither of the above issuances involved any underwriters, underwriting discounts or commissions, or any public offering and we believe were exemptexemption from the registration requirements ofunder Regulation S promulgated under the Securities Act by virtue of Section 4(a)(2) and/as the offer or Regulation D due to, among other things,sale was made in an offshore transaction and no directed selling efforts were made in the fact that there was no general solicitation or advertising, the transactions did not involve a public offering of securities, the representations of investment intentUnited States by the investors,issuer, a distributor, any of their respective affiliates, or any person acting on behalf of any of the foregoing. In addition, the investor certified that it is not a U.S. Person, as defined in Regulation S, and is not acquiring the securities for the account or benefit of any U.S. Person and agreed to resell such securities only in accordance with the provisions of Regulation S, pursuant to registration under the Securities Act, or pursuant to an available exemption from registration, and agreed not to engage in hedging transactions with regard to such securities unless in compliance with the Securities Act. The shares were restricted from further transfer as evidenced byissued in book entry form with a restrictive legend thereon.to such effect.
Repurchase of Equity Securities
No equity securities were repurchased during the fourth quarter of 2020.2023.
ITEM 6.SELECTED FINANCIAL DATA [RESERVED]
We are currently a smaller reporting company and are not required to disclose this information.
| 27 |
| Table of Contents |
ITEM 7.MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Company Overview
The following discussion and analysis of our financial condition and results of operations should be read in conjunctiontogether with theour consolidated financial statements and related notes thereto, which are included in Part II Item 8within this Report. This discussion includes an analysis of our financial condition and results of operations for the years ended December 31, 2023 and 2022 and year-over-year comparisons between those periods. Certain statements made in this section constitute “forward-looking statements,” which are subject to numerous risks and uncertainties including those described in this section. For additional information, refer to the section entitled “Cautionary Note Regarding Forward-Looking Statements” within this Report.
Company Overview
Volition is a multi-national epigenetics company. It has patented technologies that use chromosomal structures, such as nucleosomes, and transcription factors as biomarkers in cancer and other diseases. The tests in the Company’s product portfolio detect certain characteristic changes that occur from the earliest stages of disease, enabling early detection and offering a better way to monitor disease progression and a patient’s response to treatment.
The tests offered by Volition and its subsidiaries are designed to diagnose and monitor a range of life-altering diseases, including certain cancers and diseases associated with NETosis, such as sepsis and COVID-19. Early diagnosis and monitoring have the potential to not only prolong the life of patients but also improve their quality of life.
We have several key pillars of focus:
· | Nu.Q® Vet - cost-effective, easy-to-use blood tests for dogs and other companion animals. The Nu.Q® Vet Cancer Test is commercially available as a cancer screening test in dogs. | |
· | Nu.Q® NETs - monitoring the immune system to save lives. | |
· | Nu.Q® Discover - a complete solution to profiling nucleosomes. | |
· | Nu.Q® Cancer - monitoring disease progression, response to treatment and Minimal Residual Disease. | |
· | Capture-PCRTM- isolating and capturing circulating tumor derived DNA from plasma samples for early cancer detection. |
The Company has grown from a single two-meter lab bench at the University of Namur in Belgium to a purpose-built lab in Gembloux, Belgium, an Innovation Lab in California, and offices in California, London, Singapore and Nevada.
In 2015 the Company’s common stock was listed on the New York Stock Exchange (VNRX). We now have a team of over 100 dedicated employees, spanning a wide range of disciplines; all united in its mission to improve outcomes for patients.
We have identified the specific processes and resources required to achieve the near and medium-term objectives of our business plan, including personnel, facilities, equipment, research and testing materials including antibodies and clinical samples, and the protection of intellectual property. To date, operations have proceeded satisfactorily in relation to our business plan. However, it is possible that some resources will not readily become available in a suitable form or on a timely basis or at an acceptable cost. It is also possible that the results of some processes may not be as expected, and that modifications of procedures and materials may be required. Such events could result in delays to the achievement of the near and medium-term objectives of our business plan, in particular the progression of clinical validation studies and regulatory approval processes for the purpose of bringing products to the IVD market.
Our future as an operating business will depend on our ability to obtain sufficient capital contributions, financing and/or generate revenues as may be required to sustain our operations. Management plans to address the above as needed by: (a) securing additional grant funds; (b) obtaining additional financing through debt or equity or debt financing;transactions; (c) granting licenses to third parties in exchange for specified up-front and/or back endback-end payments; and (d) developing and commercializing our products on an accelerated timeline. Management continues to exercise tight cost controls to conserve cash.
Our ability to continue as a going concern is dependent upon our accomplishment of the plans described in the preceding paragraph and eventually to attain profitable operations. The accompanying consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern. If we are unable to obtain adequate capital, we could be forced to cease operations.
Developments—COVID-19 Pandemic
On March 11, 2020, the World Health Organization designated the outbreak of a novel strain of coronavirus known as COVID-19 as a global pandemic. Governments and businesses around the world have taken unprecedented actions to mitigate the spread of COVID-19, including but not limited to implementing shelter-in-place orders and significant restrictions on travel, as well as restrictions and guidelines that prohibit many employees from going to work. Uncertainty with respect to the economic effects of the pandemic has introduced significant volatility in the financial markets.
During the year ended December 31, 2020, we implemented contingency planning to protect the health and well-being of our employees, with the majority of our employees now working remotely where possible. We have implemented travel restrictions as well as protocols limiting visitor access to our facilities, and we are following social distancing practices. We did not observe significant impacts on our business or results of operations for the year ended December 31, 2020, due to the COVID-19 pandemic or the mitigation actions taken to slow its spread. To the extent the pandemic worsens, we cannot predict the effects it may have on our business, in particular with respect to demand for our services, our strategy, and our prospects, or the impact on our financial results.
Liquidity and Capital Resources
We have financed our operations since inception primarily through private placements and public offerings of our common stock. As of December 31, 2020,2023, we had cash and cash equivalents of approximately $19.4$20.7 million.
| 28 |
| Table of Contents |
Net cash used in operating activities was $16.5$18.1 million and $12.7$15.3 million for the years ended December 31, 20202023 and December 31, 2019,2022, respectively. The increase in cash used in operating activities during 20202023 when compared to 2022 was primarily due to increased researchhigher payroll costs and development activities together with increased personnel expenses.higher amounts paid to suppliers during the period.
Net cash used in investing activities was $1.6$1.1 million and $0.5$1.6 million for the years ended December 31, 20202023 and December 31, 2019,2022, respectively. The increasedecrease in cash used in investing activities during 20202023 was primarily a result of increaseddue to reduced purchases of laboratory equipment for our manufacturing facility in Belgium.as compared to 2022.
Net cash provided by financing activities after associated costs was $20.6$29.0 million and $16.9$6.9 million for the years ended December 31, 20202023 and December 31, 2019,2022, respectively.
The increase in net cash provided by financing activities during 2020for the 2023, when compared to 2022 was primarily due to more capital raisedthe following $8.0 million in net proceeds received from equity financing.the sale and issuance of common stock in a registered public offering in February 2023, before deducting offering expenses of $0.2 million, $17.6 million in net proceeds received from the sale and issuance of common stock in a registered public offering in June 2023, before deducting offering expenses of $0.1 million and $2.7 million (€2.5 million) in net proceeds received from the sale and issuance of common stock in a private placement in December 2023, also in June 2023 a $0.2 million loan was received from Namur Invest and in December 2023 a $1.6 million loan was received from Wallonie Entreprendre S.A.
This compares with $6.4 million in net cash received from the issuance of approximately 3.5 million shares of common stock in a registered public offering in August 2022, before deducting offering expenses of $0.2 million paid by the Company and a $1.0 million (€1.0 million) loan received in August 2022 from Namur Invest, a $0.5 million loan received in December 2022 from Namur Invest, and $0.8 million in cash received from the issuance of shares of common stock under our “at-the-market” facilities during the year, before deducting offering expenses of $0.2 million.
For additional information on our “at the market facilities,” refer to Note 7, Common Stock – Equity Distribution Agreements, of the Notes to consolidated financial statements included within this Report.
The following table summarizes our approximate contractual payments due by year as of December 31, 2020.2023.
Approximate Payments (Including Interest) Due by Years | ||||||||||||||||||||||||||||||||
Approximate Payments (Including Interest) Due by Year | Approximate Payments (Including Interest) Due by Year | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||
Total | 2021 | 2022 - 2025 | 2026 + |
| Total |
| 2024 |
| 2025 - 2028 |
|
| 2029 + | ||||||||||||||||||||
Description |
| $ |
| $ |
| $ |
| $ |
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||||||||||
Financing lease liabilities |
| 760,073 |
| 76,183 |
| 264,620 |
| 419,270 |
| 497,250 |
| 59,374 |
| 237,500 |
| 200,376 |
| |||||||||||||||
Operating lease liabilities and short-term lease |
| 367,267 |
| 209,570 |
| 157,697 |
| - |
| 646,662 |
| 256,768 |
| 389,894 |
| - |
| |||||||||||||||
Grants repayable |
| 328,821 |
| 69,218 |
| 156,030 |
| 103,573 |
| 478,562 |
| 55,855 |
| 186,218 |
| 236,489 |
| |||||||||||||||
Long-term debt |
| 3,919,701 |
| 991,070 |
| 2,198,823 |
| 729,808 |
| 5,791,541 |
| 1,481,023 |
| 3,986,716 |
| 323,802 |
| |||||||||||||||
Collaborative agreements obligations |
| 1,607,786 |
| 1,467,700 |
| 140,086 |
| - |
|
| 1,273,692 |
|
|
| 1,110,146 |
|
|
| 163,546 |
|
|
| - |
| ||||||||
Total |
| 6,983,648 |
| 2,813,741 |
| 2,917,256 |
| 1,252,651 |
|
| 8,687,707 |
|
|
| 2,963,166 |
|
|
| 4,963,874 |
|
|
| 760,667 |
| ||||||||
We intend to use our cash reserves to predominantly fund further research and development, and commercialization activities. We do not have any substantial source of revenues and expect to rely on additional future financing, through the sale of licensing or distribution rights, grant funding and the sale of equity or debt securities or the sale of licensing rights, to provide sufficient funding to execute our strategic plan. There is no assurance that we will be successful in raising further funds.
In the event additional financing is delayed, we will prioritize the maintenancecompletion of our research and development personnel and facilities, primarily in Belgium,clinical validation studies for the purpose of the sale of licensing or distribution rights, and the maintenance of our patent rights. In such instance, the completion of clinical validation studies and regulatory approval processes for the purpose of bringing products to the IVD market would be delayed. In the event of an ongoing lack of financing, it may be necessary to discontinue operations, which will adversely affect the value of our common stock.
We have not attained profitable operations and are dependent upon obtaining financing to pursue any extensive activities. For these reasons, our auditors included in their report on our audited financial statements for the fiscal year ended December 31, 20202023, an explanatory paragraph regarding factors that raise substantial doubt that we will be able to continue as a going concern.
| 29 |
| Table of Contents |
Results of Operations
Results of Operations
Comparison of the Years Ended December 31, 20202023 and December 31, 20192022
The following table sets forth our results of operations for the years ended on December 31, 20202023, and December 31, 2019,2022, respectively (expressed in United Stated Dollars, except outstanding share numbers and percentages).
|
|
| Increase |
| Percentage Increase |
|
|
| Increase |
| Percentage Increase |
| |||||||||||||||||
| 2020 |
| 2019 |
| (Decrease) |
| (Decrease) |
| 2023 |
| 2022 |
| (Decrease) |
| (Decrease) |
| |||||||||||||
| $ |
| $ |
| $ |
| % |
| $ |
|
| $ |
|
| $ |
|
| % |
| ||||||||||
Royalty |
| 1,369 |
| 2,911 |
| (1,542 | ) |
| (53 | %) | |||||||||||||||||||
Service | - |
| 16,204 |
| (16,204) |
| (100%) |
| 175,476 |
| 92,488 |
| 82,988 |
| 90 | % | |||||||||||||
Royalty | 2,112 |
| 892 |
| 1,220 |
| >100% | ||||||||||||||||||||||
Product | 11,321 |
| - |
| 11,321 |
| >100% |
|
| 598,457 |
|
|
| 210,993 |
|
|
| 387,464 |
|
| >100 | % | |||||||
|
|
|
|
|
|
|
| ||||||||||||||||||||||
Total Revenues | 13,433 |
| 17,096 |
| (3,663) |
| (21%) |
| 775,302 |
| 306,392 |
| 468,910 |
| >100 | % | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Research and development | 14,533,862 |
| 10,363,253 |
| 4,170,609 |
| 40% |
| 19,551,523 |
| 15,332,989 |
| 4,218,534 |
| 28 | % | |||||||||||||
General and administrative | 5,654,018 |
| 4,731,054 |
| 922,964 |
| 20% |
| 10,368,314 |
| 10,177,229 |
| 191,085 |
| 2 | % | |||||||||||||
Sales and marketing | 1,073,368 |
| 965,713 |
| 107,655 |
| 11% |
|
| 6,843,160 |
|
|
| 6,576,246 |
|
|
| 266,914 |
|
|
| 4 | % | ||||||
|
|
|
|
|
|
|
| ||||||||||||||||||||||
Total Operating Expenses | 21,261,248 |
| 16,060,020 |
| 5,201,228 |
| 32% |
|
| 36,762,997 |
|
|
| 32,086,464 |
|
|
| 4,676,533 |
|
|
| 15 | % | ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Grant income | 635,513 |
| 155,031 |
| 480,482 |
| >100% |
| 214,451 |
| 1,229,425 |
| (1,014,974 | ) |
| (83 | %) | ||||||||||||
Gain on disposal of fixed assets | 293,312 |
| - |
| 293,312 |
| >100% | ||||||||||||||||||||||
Loss on disposal of fixed assets |
| (15,843 | ) |
| - |
| (15,843 | ) |
| <(100 | %) | ||||||||||||||||||
Interest income | 49,495 |
| 112,367 |
| (62,872) |
| (56%) |
| 93,324 |
| 125,265 |
| (31,941 | ) |
| (25 | %) | ||||||||||||
Interest expense | (129,799) |
| (126,572) |
| 3,227 |
| 3% |
| (221,622 | ) |
| (173,087 | ) |
| 48,535 |
| 28 | % | |||||||||||
Other expenses | - |
| (196,957) |
| (196,957) |
| (>100%) | ||||||||||||||||||||||
|
|
|
|
|
|
|
| ||||||||||||||||||||||
Gain on change in fair value of warrant liability |
|
| 240,311 |
|
|
| - |
|
|
| 240,311 |
|
| >100 | % | ||||||||||||||
Total Other Income (Expenses) | 848,521 |
| (56,131) |
| 904,652 |
| (>100%) |
|
| 310,621 |
|
|
| 1,181,603 |
|
|
| (870,982 | ) |
| (74 | %) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Net Loss | (20,399,294) |
| (16,099,055) |
| 4,300,239 |
| 27% |
|
| (35,677,074 | ) |
|
| (30,598,469 | ) |
|
| 5,078,605 |
|
|
| 17 | % | ||||||
|
|
|
|
|
|
|
| ||||||||||||||||||||||
Revenues
Our operations are still predominantly in thetransitioning from a research and development stage and we had minimalto a commercialization stage. Revenue for the year ended December 31, 2023 was $775,302 compared with $306,392 for the year ended December 31, 2022. The main source of revenues of $13,433 and $17,096 during the years ended December 31, 20202023 and December 31, 2019, respectively.2022, was product sales of the Nu.Q® Vet Cancer Test and services revenue from our Nu.Q® Discover offering.
Operating Expenses
Total operating expenses increased to $21.3$36.8 million from $16.1$32.1 million for the years ended December 31, 20202023 and December 31, 2019,2022, respectively, as a result of the factors described below.
| 30 |
| Table of Contents |
Research and Development Expenses
Research and development expenses increased to $14.5$19.6 million from $10.4$15.3 million for the years ended December 31, 20202023 and December 31, 2019,2022, respectively. The increase in overall research and development expenditures during 20202023 was primarily related to higher antibody, sample, laboratory, personnel expenses and employee acquisition costs relatingclinical research costs. FTE personnel numbers within this division increased by three to Volition Germany.sixty six during 2023 compared to the prior year period.
| 2020 |
| 2019 |
| Change |
| 2023 |
| 2022 |
| Change |
| |||||
| $ |
| $ |
| $ |
| $ |
|
| $ |
|
| $ |
| |||
Personnel expenses | 5,171,967 |
| 3,833,289 |
| 1,338,678 |
| 9,207,822 |
| 7,125,017 |
| 2,082,805 |
| |||||
Stock based compensation | 340,075 |
| 410,178 |
| (70,103) | ||||||||||||
Stock-based compensation |
| 617,710 |
| 652,653 |
| (34,943 | ) | ||||||||||
Direct research and development expenses | 6,384,169 |
| 4,619,515 |
| 1,764,654 |
| 7,641,571 |
| 5,662,957 |
| 1,978,614 |
| |||||
Other research and development | 1,784,111 |
| 809,585 |
| 974,526 |
| 964,843 |
| 1,052,749 |
| (87,906 | ) | |||||
Depreciation and amortization | 853,540 |
| 690,686 |
| 162,854 |
|
| 1,119,577 |
|
|
| 839,613 |
|
|
| 279,964 |
|
Total research and development expenses | 14,533,862 |
| 10,363,253 |
| 4,170,609 |
|
| 19,551,523 |
|
|
| 15,332,989 |
|
|
| 4,218,534 |
|
General and Administrative Expenses
General and administrative expenses increased to $5.7$10.4 million from $4.7$10.2 million for the years ended December 31, 20202023 and December 31, 2019,2022, respectively. The increase in overall general and administrative expenditures during 20202023 was primarily due to higher personnel expenses and legal and professional fees offset by lower stock-based compensation. The FTE personnel number within this division remained at twenty two in connection with our capital raises and increased premiums for director and officer liability insurance.2023 compared to the prior year period.
| 2020 |
| 2019 |
| Change |
| 2023 |
| 2022 |
| Change |
| |||||
| $ |
| $ |
| $ |
| $ |
|
| $ |
|
| $ |
| |||
Personnel expenses | 2,135,578 |
| 2,185,349 |
| (49,771) |
| 5,492,705 |
| 5,047,242 |
| 445,463 |
| |||||
Stock-based compensation | 887,181 |
| 868,762 |
| 18,419 |
| 939,412 |
| 1,393,784 |
| (454,372 | ) | |||||
Legal and professional fees | 1,611,495 |
| 1,180,876 |
| 430,619 |
| 2,116,494 |
| 1,954,798 |
| 161,696 |
| |||||
Other general and administrative | 831,931 |
| 284,341 |
| 547,590 |
| 1,579,241 |
| 1,486,722 |
| 92,519 |
| |||||
Depreciation and amortization | 187,833 |
| 211,726 |
| (23,893) |
|
| 240,462 |
|
|
| 294,683 |
|
|
| (54,221 | ) |
Total general and administrative expenses | 5,654,018 |
| 4,731,054 |
| 922,964 |
|
| 10,368,314 |
|
|
| 10,177,229 |
|
|
| 191,085 |
|
|
| ||||||||||||||||
Sales and Marketing Expenses
Sales and marketing expenses increased to $1.1$6.8 million from $1.0$6.6 million for the years ended December 31, 20202023 and December 31, 2019,2022, respectively. The increase in overall sales and marketing expenditures was primarily due to increased direct marketing and professional fees partiallypersonnel expenses offset by lower stock-based compensation. The FTE personnel expenses.number within this division increased by three to twenty two in 2023 compared to the prior year period.
| 2020 |
| 2019 |
| Change |
| 2023 |
| 2022 |
| Change |
| |||||
| $ |
| $ |
| $ |
| $ |
|
| $ |
|
| $ |
| |||
Personnel expenses | 545,842 |
| 586,207 |
| (40,365) |
| 5,046,282 |
| 4,400,092 |
| 646,190 |
| |||||
Stock-based compensation | 164,236 |
| 188,173 |
| (23,937) |
| 732,422 |
| 1,068,222 |
| (335,800 | ) | |||||
Direct marketing and professional fees | 363,290 |
| 191,333 |
| 171,957 | ||||||||||||
Other sales and marketing expenses |
| 1,012,868 |
| 1,053,807 |
| (40,939 | ) | ||||||||||
Depreciation and amortization |
|
| 51,588 |
|
|
| 54,125 |
|
|
| (2,537 | ) | |||||
Total sales and marketing expenses | 1,073,368 |
| 965,713 |
| 107,655 |
|
| 6,843,160 |
|
|
| 6,576,246 |
|
|
| 266,914 |
|
| 31 |
| Table of Contents |
Other ExpensesIncome (Expenses)
For the year ended December 31, 2020,2023, other income increaseddecreased to $848,521approximately $0.3 million compared to other expensesincome of $56,131approximately $1.2 million for the year ended December 31, 2019.2022. This increasedecrease in other income was primarily due to reduced grant income received and the gain on disposal of fixed assets from the sale of equipmentapproximately $0.2 million during the year.2023 compared to $1.2 million in 2022.
Net Loss
For the year ended December 31, 2020,2023, the Company’s net loss was $20.4$35.7 million, an increase of approximately $4.3$5.1 million, in comparison to a net loss of $16.1$30.6 million for the year ended December 31, 2019.2022. The change was a result of the factors described above.
Going Concern
We have not attained profitable operations and are dependent upon obtaining external financing to continue to pursue our operational and strategic plans. For these reasons, management has determined that there is substantial doubt that the business will be able to continue as a going concern without further financing.
Off-Balance Sheet Arrangements
We have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
Future Equity or Debt Financings
We may seek to obtain additional capital through the sale of debt or equity securities if we deem it desirable or necessary. These sales may include the sale of equity securities from time to time through our “at the market offering program”facility” with Cantor Fitzgerald & Co. and Oppenheimer & Co. Inc.Jefferies LLC under the Equity Distribution Agreementan equity distribution agreement dated November 10, 2020May 20, 2022 (see Note 7, Common Stock – Equity Distribution Agreements, of the Notes to the consolidated financial statements). However, we may be unable to obtain such additional capital when needed, or on terms favorable to us or our stockholders, if at all. If we raise additional funds by issuing equity securities, the percentage ownership of our stockholders will be reduced, stockholders may experience additional dilution, or such equity securities may provide for rights, preferences or privileges senior to those of the holders of our common stock. If additional funds are raised through the issuance of debt securities, the terms of such securities may place restrictions on our ability to operate our business.
Critical Accounting Policies and Estimates
Our consolidated financial statements and accompanying notes have been prepared in accordance with United StatesU.S. generally accepted accounting principles or (“U.S. GAAP,GAAP”), applied on a consistent basis. The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods.
We also regularly evaluate estimates and assumptions related to deferred income tax asset valuation allowances, useful lives of property and equipment and intangible assets, borrowing rate used in operating lease right-of-use asset and liability valuations, impairment analysis of intangible assets and valuations of stock-based compensation.
We base our estimates and assumptions on current facts, historical experiences, information from third-party professionals and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. Actual results may differ materially and adversely from our estimates. To the extent there are material differences between the estimates and the actual results, future results of operations could be affected.
We regularly evaluate the accounting policies that we use to prepare our consolidated financial statements. A complete summary of these policies is included in the notesNotes to our consolidated financial statements.
We considerhave determined that for the periods reported in this Report the following accounting policies to be critical:are critical in understanding our financial condition and results of operations:
| 32 |
| Table of Contents |
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, “Compensation – Stock Compensation”. Under the provisions of ASC 718, stock-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized over the employee’s requisite service period, which is generally the vesting period. The fair value of our stock options and warrants is estimated using a Black-Scholes option valuation model. Restricted stock units are valued based on the closing stock price on the date of grant, (seerefer to Note 8 of the Notes to the consolidated financial statements).statements for further details.
Impairment of Long-Lived Assets
In accordance with ASC 360, “PropertyProperty Plant and Equipment”, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value. Impairment losses of $nil and $nil were recognized during the years ended December 31, 20202023 and December 31, 2019,2022, respectively.
Foreign Currency Translation
The Company has functional currencies in Euros, United StatesU.S. Dollars and British Pounds Sterling and its reporting currency is the United StatesU.S. Dollar. Management has adopted ASC 830-20, “Foreign Currency Matters – Foreign Currency Transactions.Transactions”. All assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. For revenues and expenses, the weighted average exchange rate for the period is used. Gains and losses arising on translation of foreign currency denominated transactions are included in Other Comprehensive Income.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company also regularly evaluates estimates and assumptions related to deferred income tax asset valuation allowances, useful lives of property and equipment and intangible assets, borrowing rate used in operating lease right-of-use asset and liability valuations, impairment analysis of intangible assets and valuations of stock-based compensation.
The Company bases its estimates and assumptions on current facts, historical experiences, information from third party professionals and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results of operations could be affected.
Recently Issued Accounting Pronouncements
The Company has implemented all applicable new accounting pronouncements that are in effect. The Company does not believe that there are any other applicable new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
ITEM 7A.QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are a smaller reporting company and are not required to disclose this information.
| 33 |
| Table of Contents |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
VOLITIONRX LIMITED
Consolidated Financial Statements
Consolidated Financial Statements
For the Years Ended December 31, 20202023 and 2019
2022
| Index | ||
|
| ||
Report of Independent Registered Public Accounting Firm (PCAOB ID: 3627) | F - | ||
F - | |||
Consolidated Statements of Operations and Comprehensive Loss | F - | ||
F - | |||
F - | |||
F - |
| F-34 |
| Table of Contents |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of VolitionRx Limited:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of VolitionRx Limited (“the Company”(the “Company”) as of December 31, 20202023 and 2019,2022, the related consolidated statements of operations and comprehensive loss, stockholders’ deficit, and cash flows for each of the years in the two-year period ended December 31, 20202023 and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 20202023 and 2019,2022, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2020,2023, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph Regarding Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred suffered recurring losses since inception, hasfrom operations, negative cash flows from operations and has minimal revenues which createsraises substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) related to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgements. The communication ofWe determined that there are no critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.matters.
Going Concern
As described further in Note 2 to the financial statements, the Company has incurred losses since inception, has negative cash flows from operations, and has generated minimal revenues. Accordingly, the Company has determined that these factors raise substantial doubt about its ability to continue as a going concern. The ability of the Company to continue as a going concern is dependent on its ability to obtain sufficient capital contributions, financing and/or generate revenues. Management plans to address the concerns as needed by, (a) securing additional grant funds, (b) obtaining additional financing through debt or equity transactions, (c) granting licenses to third parties in exchange for specified up-front and/or back end payments, (d) developing and commercializing its products on an accelerated timeline, and (e) continuing to exercise tight cost controls to conserve cash. Management has not concluded that these plans alleviate the substantial doubt related to its ability to continue as a going concern.
We determined the Company’s ability to continue as a going concern is a critical audit matter due to the estimation and uncertainty regarding the Company’s available capital and the risk of bias in management’s judgments and assumptions in their determination.
Our audit procedures related to the following:
·We performed testing procedures such as analytical procedures to identify conditions and events that indicate there could be substantial doubt about the entity's ability to continue as a going concern for a reasonable period of time.
·We reviewed and evaluated management's plans for dealing with adverse effect of these conditions and events.
·We inquired of Company management and reviewed company records to assess whether there are additional factors that contribute to the uncertainties disclosed.
·We assessed whether the Company’s determination that there is substantial doubt about its ability to continue as a going concern was adequately disclosed.
/s/ Sadler, Gibb & Associates, LLC
We have served as the Company’s auditor since 2011.
Draper, UT
March 22, 202125, 2024
| F-35 |
| Table of Contents |
VOLITIONRXVOLITIONRX LIMITED
Consolidated Balance Sheets
(Expressed in United States Dollars, except share numbers)
| December 31, |
| December 31, |
| 2020 |
| 2019 |
| $ |
| $ |
ASSETS |
|
|
|
Current Assets |
|
|
|
Cash and cash equivalents | 19,444,737 |
| 16,966,168 |
Accounts Receivable | 7,118 |
| - |
Prepaid expenses | 303,178 |
| 267,518 |
Other current assets | 576,660 |
| 322,593 |
|
|
|
|
Total Current Assets | 20,331,693 |
| 17,556,279 |
|
|
|
|
Property and equipment, net | 5,171,134 |
| 2,981,225 |
Operating lease right-of-use assets | 326,085 |
| 381,483 |
Intangible assets, net | 321,641 |
| 372,305 |
|
|
|
|
Total Assets | 26,150,553 |
| 21,291,292 |
|
|
|
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
| ||
Current Liabilities |
|
|
|
Accounts payable | 1,539,547 |
| 627,253 |
Accrued liabilities | 3,491,740 |
| 2,168,588 |
Management and directors’ fees payable | 55,174 |
| 21,979 |
Current portion of long-term debt | 841,319 |
| 647,569 |
Current portion of financing lease liabilities | 59,930 |
| 97,946 |
Current portion of operating lease liabilities | 179,624 |
| 257,244 |
Current portion of grant repayable | 69,218 |
| 39,295 |
|
|
|
|
Total Current Liabilities | 6,236,552 |
| 3,859,874 |
|
|
|
|
Long-term debt, net of current portion | 2,606,885 |
| 2,195,278 |
Finance lease liabilities, net of current portion | 601,967 |
| 607,708 |
Operating lease liabilities, net of current portion | 151,828 |
| 131,875 |
Grant repayable, net of current portion | 259,603 |
| 297,991 |
|
|
|
|
Total Liabilities | 9,856,835 |
| 7,092,726 |
|
|
|
|
STOCKHOLDERS’ EQUITY |
| ||
Common Stock |
|
|
|
Authorized: 100,000,000 shares of common stock, at $0.001 par value |
|
|
|
Issued and outstanding: 48,607,017 shares and 41,125,303 shares, respectively | 48,607 |
| 41,125 |
Additional paid-in capital | 126,526,239 |
| 103,853,627 |
Accumulated other comprehensive income (loss) | (59,978) |
| 125,670 |
Accumulated deficit | (110,173,971) |
| (89,821,856) |
Total VolitionRx Limited Stockholders' Equity | 16,340,897 |
| 14,198,566 |
Non-controlling interest | (47,179) |
| - |
Total Stockholders’ Equity | 16,293,718 |
| 14,198,566 |
|
|
|
|
Total Liabilities and Stockholders’ Equity | 26,150,553 |
| 21,291,292 |
|
|
|
|
(The accompanying notes are an integral part of these consolidated financial statements) | |||
|
|
|
|
|
| December 31, |
|
| December 31, |
| ||
|
| 2023 |
|
| 2022 |
| ||
|
| $ |
|
| $ |
| ||
ASSETS |
|
|
|
|
|
| ||
Current Assets |
|
|
|
|
|
| ||
Cash and cash equivalents |
|
| 20,729,983 |
|
|
| 10,867,050 |
|
Accounts receivable |
|
| 242,617 |
|
|
| 72,609 |
|
Prepaid expenses |
|
| 521,370 |
|
|
| 784,920 |
|
Other current assets |
|
| 360,125 |
|
|
| 447,566 |
|
Total Current Assets |
|
| 21,854,095 |
|
|
| 12,172,145 |
|
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
| 5,523,013 |
|
|
| 5,393,012 |
|
Operating lease right-of-use assets |
|
| 549,504 |
|
|
| 619,392 |
|
Intangible assets, net |
|
| 23,886 |
|
|
| 110,505 |
|
Total Assets |
|
| 27,950,498 |
|
|
| 18,295,054 |
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS' DEFICIT |
|
| ||||||
Current Liabilities |
|
|
|
|
|
|
|
|
Accounts payable |
|
| 3,211,287 |
|
|
| 3,043,008 |
|
Accrued liabilities |
|
| 3,928,761 |
|
|
| 2,872,247 |
|
Deferred revenue |
|
| 23,000,000 |
|
|
| 10,000,000 |
|
Management and directors’ fees payable |
|
| 59,625 |
|
|
| 71,119 |
|
Current portion of long-term debt |
|
| 1,207,007 |
|
|
| 1,066,700 |
|
Warrant liability |
|
| 126,649 |
|
|
| - |
|
Current portion of financing lease liabilities |
|
| 48,570 |
|
|
| 46,014 |
|
Current portion of operating lease liabilities |
|
| 199,323 |
|
|
| 245,163 |
|
Current portion of grant repayable |
|
| 55,855 |
|
|
| 41,836 |
|
Total Current Liabilities |
|
| 31,837,077 |
|
|
| 17,386,087 |
|
|
|
|
|
|
|
|
|
|
Long-term debt, net of current portion |
|
| 3,624,860 |
|
|
| 2,779,240 |
|
Finance lease liabilities, net of current portion |
|
| 400,022 |
|
|
| 436,132 |
|
Operating lease liabilities, net of current portion |
|
| 378,054 |
|
|
| 400,091 |
|
Grant repayable, net of current portion |
|
| 422,707 |
|
|
| 420,466 |
|
Total Liabilities |
|
| 36,662,720 |
|
|
| 21,422,016 |
|
|
|
|
|
|
|
|
|
|
Stockholders' Deficit |
|
| ||||||
Common stock |
|
|
|
|
|
|
|
|
Authorized: 100,000,000 shares of common stock, at $0.001 par value |
|
|
|
|
|
|
|
|
Issued and outstanding: 81,898,321 shares and 57,873,379 shares, respectively |
|
| 81,898 |
|
|
| 57,873 |
|
Additional paid-in capital |
|
| 194,448,414 |
|
|
| 164,397,468 |
|
Accumulated other comprehensive income |
|
| 243,940 |
|
|
| 227,097 |
|
Accumulated deficit |
|
| (202,576,507 | ) |
|
| (167,257,429 | ) |
Total VolitionRx Limited Stockholders' Deficit |
|
| (7,802,255 | ) |
|
| (2,574,991 | ) |
Non-controlling interest |
|
| (909,967 | ) |
|
| (551,971 | ) |
Total Stockholders’ Deficit |
|
| (8,712,222 | ) |
|
| (3,126,962 | ) |
|
|
|
|
|
|
|
|
|
Total Liabilities and Stockholders’ Deficit |
|
| 27,950,498 |
|
|
| 18,295,054 |
|
|
|
|
|
|
|
|
|
|
(The accompanying notes are an integral part of these consolidated financial statements) | ||||||||
| F-36 |
| Table of Contents |
VOLITIONRXVOLITIONRX LIMITED
Consolidated Statements of Operations and Comprehensive Loss
(Expressed in United States Dollars, except share numbers)
| For the year ended | ||
December 31, 2020 |
| December 31, 2019 | |
$ |
| $ | |
Revenues |
|
|
|
Service | - |
| 16,204 |
Royalty | 2,112 |
| 892 |
Product | 11,321 |
| - |
|
|
|
|
Total Revenues | 13,433 |
| 17,096 |
|
|
|
|
Operating Expenses |
|
|
|
|
|
|
|
Research and development | 14,533,862 |
| 10,363,253 |
General and administrative | 5,654,018 |
| 4,731,054 |
Sales and marketing | 1,073,368 |
| 965,713 |
|
|
|
|
Total Operating Expenses | 21,261,248 |
| 16,060,020 |
|
|
|
|
Operating Loss | (21,247,815) |
| (16,042,924) |
|
|
|
|
Other Income (Expenses) |
|
|
|
Grant income | 635,513 |
| 155,031 |
Gain on disposal of fixed assets | 293,312 |
| - |
Interest income | 49,495 |
| 112,367 |
Interest expense | (129,799) |
| (126,572) |
Other expenses | - |
| (196,957) |
|
|
|
|
Total Other Income (Expenses) | 848,521 |
| (56,131) |
|
|
|
|
Net Loss | (20,399,294) |
| (16,099,055) |
Net Loss attributable to Non-Controlling Interest | 47,179 |
| - |
Net Loss attributable to VolitionRx Limited Stockholders | (20,352,115) |
| (16,099,055) |
|
|
|
|
Other Comprehensive Income (Loss) |
|
|
|
Foreign currency translation adjustments | (185,648) |
| (97,981) |
|
|
|
|
Net Comprehensive Loss | (20,584,942) |
| (16,197,036) |
|
|
|
|
Net Loss Per Share – Basic and Diluted | (0.45) |
| (0.41) |
|
|
|
|
Weighted Average Shares Outstanding |
|
|
|
– Basic and Diluted | 45,278,847 |
| 39,180,369 |
|
|
|
|
|
|
|
|
(The accompanying notes are an integral part of these consolidated financial statements) | |||
|
| For the Years Ended |
| |||||
|
| December 31, 2023 |
|
| December 31, 2022 |
| ||
|
|
|
|
| $ |
| ||
Revenues |
|
|
|
|
|
| ||
Royalty |
|
| 1,369 |
|
|
| 2,911 |
|
Service |
|
| 175,476 |
|
|
| 92,488 |
|
Product |
|
| 598,457 |
|
|
| 210,993 |
|
Total Revenues |
|
| 775,302 |
|
|
| 306,392 |
|
|
|
|
|
|
|
|
|
|
Operating Expenses |
|
|
|
|
|
|
|
|
Research and development |
|
| 19,551,523 |
|
|
| 15,332,989 |
|
General and administrative |
|
| 10,368,314 |
|
|
| 10,177,229 |
|
Sales and marketing |
|
| 6,843,160 |
|
|
| 6,576,246 |
|
Total Operating Expenses |
|
| 36,762,997 |
|
|
| 32,086,464 |
|
|
|
|
|
|
|
|
|
|
Operating Loss |
|
| (35,987,695 | ) |
|
| (31,780,072 | ) |
|
|
|
|
|
|
|
|
|
Other Income (Expenses) |
|
|
|
|
|
|
|
|
Grant income |
|
| 214,451 |
|
|
| 1,229,425 |
|
Loss on disposal of fixed assets |
|
| (15,843 | ) |
|
| - |
|
Interest income |
|
| 93,324 |
|
|
| 125,265 |
|
Interest expense |
|
| (221,622 | ) |
|
| (173,087 | ) |
Gain on change in fair value of warrant liability |
|
| 240,311 |
|
|
| - |
|
Total Other Income |
|
| 310,621 |
|
|
| 1,181,603 |
|
|
|
|
|
|
|
|
|
|
Net Loss |
|
| (35,677,074 | ) |
|
| (30,598,469 | ) |
Net Loss attributable to Non-Controlling Interest |
|
| 357,996 |
|
|
| 329,676 |
|
Net Loss attributable to VolitionRx Limited Stockholders |
|
| (35,319,078 | ) |
|
| (30,268,793 | ) |
|
|
|
|
|
|
|
|
|
Other Comprehensive Income (Loss) |
|
|
|
|
|
|
|
|
Foreign currency translation adjustments |
|
| 16,843 |
|
|
| 78,771 |
|
Net Comprehensive Loss |
|
| (35,660,231 | ) |
|
| (30,519,698 | ) |
|
|
|
|
|
|
|
|
|
Net Loss Per Share – Basic and Diluted attributable to VolitionRx Limited Stockholders |
|
| (0.50 | ) |
|
| (0.55 | ) |
|
|
|
|
|
|
|
|
|
Weighted Average Shares Outstanding |
|
|
|
|
|
|
|
|
– Basic and Diluted |
|
| 71,234,565 |
|
|
| 55,350,401 |
|
|
|
|
|
|
|
|
|
|
(The accompanying notes are an integral part of these consolidated financial statements) | ||||||||
| F-37 |
| Table of Contents |
VOLITIONRXVOLITIONRX LIMITED
Consolidated Statement of Stockholders’ EquityDeficit
For the Years Ended December 31, 2023 and 2022 | ||||||||||||||||||||||||||||
(Expressed in United States Dollars, except share numbers)
| ||||||||||||||||||||||||||||
|
|
|
|
|
|
|
| Additional |
|
| Accumulated Other |
|
|
|
|
| Non |
|
|
|
| |||||||
|
| Common Stock |
|
| Paid-in |
|
| Comprehensive |
|
| Accumulated |
|
| Controlling |
|
|
|
| ||||||||||
|
| Shares |
|
| Amount |
|
| Capital |
|
| Income (Loss) |
|
| Deficit |
|
| Interest |
|
| Total |
| |||||||
|
| # |
|
| $ |
|
| $ |
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| |||||||
Balance, December 31, 2021 |
|
| 53,772,261 |
|
|
| 53,772 |
|
|
| 154,730,938 |
|
|
| 148,326 |
|
|
| (136,988,636 | ) |
|
| (222,295 | ) |
|
| 17,722,105 |
|
Common stock issued for settlement of RSUs |
|
| 297,289 |
|
|
| 297 |
|
|
| (297 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
Common stock issued in public offerings, net |
|
| 3,803,829 |
|
|
| 3,804 |
|
|
| 6,732,640 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 6,736,444 |
|
Tax withholdings paid related to stock-based compensation |
|
| - |
|
|
| - |
|
|
| (180,472 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (180,472 | ) |
Stock-based compensation |
|
| - |
|
|
| - |
|
|
| 3,114,659 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 3,114,659 |
|
Foreign currency translation |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 78,771 |
|
|
| - |
|
|
| - |
|
|
| 78,771 |
|
Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (30,268,793 | ) |
|
| (329,676 | ) |
|
| (30,598,469 | ) |
Balance, December 31, 2022 |
|
| 57,873,379 |
|
|
| 57,873 |
|
|
| 164,397,468 |
|
|
| 227,097 |
|
|
| (167,257,429 | ) |
|
| (551,971 | ) |
|
| (3,126,962 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for settlement of RSUs |
|
| 658,102 |
|
|
| 658 |
|
|
| (658 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
Common stock issued for cash, net of issuance costs and allocation to warrant liability |
|
| 23,380,134 |
|
|
| 23,380 |
|
|
| 27,997,696 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 28,021,076 |
|
Common stock repurchased and retired |
|
| (13,294 | ) |
|
| (13 | ) |
|
| (31,759 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
| (31,772 | ) | |
Tax withholdings paid related to stock-based compensation |
|
| - |
|
|
| - |
|
|
| (203,878 | ) |
|
| - |
|
|
| - |
|
|
| - |
|
|
| (203,878 | ) |
Stock-based compensation |
|
| - |
|
|
| - |
|
|
| 2,289,545 |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| 2,289,545 |
|
Foreign currency translation |
|
| - |
|
|
| - |
|
|
| - |
|
|
| 16,843 |
|
|
| - |
|
|
| - |
|
|
| 16,843 |
|
Net loss |
|
| - |
|
|
| - |
|
|
| - |
|
|
| - |
|
|
| (35,319,078 | ) |
|
| (357,996 | ) |
|
| (35,677,074 | ) |
Balance, December 31, 2023 |
|
| 81,898,321 |
|
|
| 81,898 |
|
|
| 194,448,414 |
|
|
| 243,940 |
|
|
| (202,576,507 | ) |
|
| (909,967 | ) |
|
| (8,712,222 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(The accompanying notes are an integral part of these consolidated financial statements) | ||||||||||||||||||||||||||||
For the Years Ended December 31, 2020 and 2019 | |||||||||||||
(Expressed in United States Dollars, except share numbers) | |||||||||||||
|
|
|
|
| Additional |
|
Accumulated Other |
|
|
| Non |
|
|
| Common Stock |
| Paid-in |
| Comprehensive |
| Accumulated |
| Controlling |
|
| ||
| Shares |
| Amount |
| Capital |
| Income (Loss) |
| Deficit |
| Interest |
| Total |
# |
| $ |
| $ |
| $ |
| $ |
| $ |
| $ | |
Balance, December 31, 2018 | 35,335,378 |
| 35,335 |
| 85,604,271 |
| 223,651 |
| (73,722,801) |
| - |
| 12,140,456 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued in exercise of stock options | 2,858 |
| 3 |
| (3) |
| - |
| - |
| - |
| - |
Common stock issued in exercise of warrants | 5,783,867 |
| 5,784 |
| 16,568,745 |
| - |
| - |
| - |
| 16,574,529 |
Common stock issued in public offering, net | 3,200 |
| 3 |
| 16,544 |
| - |
| - |
| - |
| 16,547 |
Stock-based compensation | - |
| - |
| 1,467,113 |
| - |
| - |
| - |
| 1,467,113 |
Modification of financing warrants | - |
| - |
| 196,957 |
| - |
| - |
| - |
| 196,957 |
Foreign currency translation | - |
| - |
| - |
| (97,981) |
| - |
| - |
| (97,981) |
Net loss for the year | - |
| - |
| - |
| - |
| (16,099,055) |
| - |
| (16,099,055) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2019 | 41,125,303 |
| 41,125 |
| 103,853,627 |
| 125,670 |
| (89,821,856) |
| - |
| 14,198,566 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for Director compensation in Volition Germany | 73,263 |
| 73 |
| 333,896 |
| - |
| - |
| - |
| 333,969 |
Common stock repurchase and retirement | (11,364) |
| (11) |
| (54,423) |
| - |
| - |
| - |
| (54,434) |
Common stock issued in exercise of stock options | 147,268 |
| 147 |
| 82,353 |
| - |
| - |
| - |
| 82,500 |
Common stock issued for exercise of warrants | 25,000 |
| 25 |
| 61,725 |
| - |
| - |
| - |
| 61,750 |
Common stock issued in public offering, net | 7,247,547 |
| 7,248 |
| 21,045,034 |
| - |
| - |
| - |
| 21,052,282 |
Tax withholdings paid related to Stock-based compensation | - |
| - |
| (187,465) |
| - |
| - |
| - |
| (187,465) |
Stock-based compensation | - |
| - |
| 1,391,492 |
| - |
| - |
| - |
| 1,391,492 |
Foreign currency translation | - |
| - |
| - |
| (185,648) |
| - |
| - |
| (185,648) |
Net loss for the Year | - |
| - |
| - |
| - |
| (20,352,115) |
| (47,179) |
| (20,399,294) |
Balance, December 31, 2020 | 48,607,017 |
| 48,607 |
| 126,526,239 |
| (59,978) |
| (110,173,971) |
| (47,179) |
| 16,293,718 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(The accompanying notes are an integral part of these consolidated financial statements) | |||||||||||||
| F-38 |
| Table of Contents |
VOLITIONRXVOLITIONRX LIMITED
Consolidated Statements of Cash Flows
(Expressed in United States Dollars)
| For the year ended |
| For the Years Ended |
| |||||||
December 31, 2020 |
| December 31, 2019 |
| December 31, 2023 |
| December 31, 2022 | December 31, 2022 |
| |||
$ |
| $ |
| $ |
| $ | $ |
| |||
Operating Activities: |
|
|
|
|
|
|
|
| |||
Net loss | (20,399,294) |
| (16,099,055) |
| (35,677,074 | ) |
| (30,598,469 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
| |||
Depreciation and amortization | 716,181 |
| 676,815 |
| 1,152,534 |
| 936,084 |
| |||
Amortization of operating lease right-of-use assets | 325,192 |
| 225,597 |
| 260,743 |
| 253,864 |
| |||
Gain on disposal of fixed assets | (293,312) |
| - | ||||||||
Stock based compensation | 1,391,492 |
| 1,467,113 | ||||||||
Common stock issued for Director compensation in Volition Germany | 333,969 |
| - | ||||||||
Financing costs for warrants modified | - |
| 196,957 | ||||||||
Loss on disposal of fixed assets |
| 15,843 |
| - |
| ||||||
Stock-based compensation |
| 2,289,545 |
| 3,114,659 |
| ||||||
Gain on change in fair value of warrant liability |
| (240,311 | ) |
| - |
| |||||
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
| |||
Accounts receivable |
| (169,666 | ) |
| (72,609 | ) | |||||
Prepaid expenses | (35,660) |
| (22,080) |
| 263,550 |
| 433,995 |
| |||
Accounts receivable | (7,118) |
| - | ||||||||
Other current assets | (254,062) |
| (92,838) |
| 87,441 |
| 351,514 |
| |||
Accounts payable and accrued liabilities | 2,052,753 |
| 1,105,211 |
| 1,224,743 |
| 548,611 |
| |||
Deferred revenue |
| 13,000,000 |
| 9,987,488 |
| ||||||
Management and directors’ fees payable | 33,195 |
| 20,779 |
| (11,494 | ) |
| (184 | ) | ||
Right-of-use assets operating leases liabilities | (327,580) |
| (217,954) | ||||||||
Operating lease liabilities |
|
| (258,853 | ) |
|
| (232,695 | ) | |||
Net Cash Used In Operating Activities | (16,464,244) |
| (12,739,455) |
|
| (18,062,999 | ) |
|
| (15,277,742 | ) |
|
|
|
|
|
|
|
|
| |||
Investing Activities: |
|
|
| ||||||||
Purchases of property and equipment | (1,941,060) |
| (511,266) |
|
| (1,083,749 | ) |
|
| (1,570,182 | ) |
Proceeds from sales of property and equipment | 293,312 |
| - | ||||||||
Net Cash Used In Investing Activities | (1,647,748) |
| (511,266) |
|
| (1,083,749 | ) |
|
| (1,570,182 | ) |
Financing Activities: |
|
|
|
|
|
|
|
| |||
Net proceeds from issuance of common shares | 21,196,532 |
| 16,591,076 |
| 28,388,036 |
| 6,736,444 |
| |||
Tax withholdings paid related to stock-based compensation | (187,465) |
| - |
| (203,878 | ) |
| (180,472 | ) | ||
Common stock repurchased | (54,434) |
| - |
| (31,772 | ) |
| - |
| ||
Proceeds from grants repayable | 3,802 |
| 32,795 |
| 25,315 |
| 218,445 |
| |||
Proceeds from long-term debt | 346,465 |
| 838,039 |
| 1,854,877 |
| 1,523,098 |
| |||
Payments on long-term debt | (545,389) |
| (351,009) |
| (981,291 | ) |
| (1,268,386 | ) | ||
Payments on grants repayable | (41,257) |
| (39,335) |
| (22,096 | ) |
| (45,664 | ) | ||
Payments on financing leases | (97,417) |
| (142,039) |
|
| (46,506 | ) |
|
| (45,433 | ) |
Net Cash Provided By Financing Activities | 20,620,837 |
| 16,929,527 |
|
| 28,982,685 |
|
|
| 6,938,032 |
|
Effect of foreign exchange on cash and cash equivalents | (30,276) |
| (139,860) |
|
| 26,996 |
|
|
| 195,629 |
|
|
|
|
|
|
|
|
|
| |||
Net Change in Cash and Cash Equivalents | 2,478,569 |
| 3,538,946 |
| 9,862,933 |
| (9,714,263 | ) | |||
Cash and Cash Equivalents – Beginning of Year | 16,966,168 |
| 13,427,222 |
|
| 10,867,050 |
|
|
| 20,581,313 |
|
Cash and Cash Equivalents – End of Year | 19,444,737 |
| 16,966,168 |
|
| 20,729,983 |
|
|
| 10,867,050 |
|
|
|
|
|
|
|
|
|
| |||
Supplemental Disclosures of Cash Flow Information: |
|
|
|
|
|
|
|
| |||
Interest paid | 129,799 |
| 126,847 |
| 221,622 |
| 173,110 |
| |||
Income tax paid | - |
| - | ||||||||
|
|
|
|
|
|
|
|
| |||
Non - Cash Financing Activities: |
|
|
| ||||||||
Common Stock issued on cashless exercises of stock options and warrants | 118 |
| 3 | ||||||||
Loan payable for purchase of manufacturing building | 584,449 |
| - | ||||||||
Non-Cash Financing Activities: |
|
|
|
|
| ||||||
Common Stock issued for settlement of vested RSUs |
| 658 |
| 297 |
| ||||||
Offering costs from issuance of common stock | 1,250,848 |
| - |
| 392,822 |
| 427,443 |
| |||
Fair value of warrants issued in connection with public offering at issuance |
| 366,960 |
| - |
| ||||||
Non-cash note payable |
| 356,258 |
| 620,549 |
| ||||||
|
|
|
|
|
|
|
|
| |||
(The accompanying notes are an integral part of these consolidated financial statements) | (The accompanying notes are an integral part of these consolidated financial statements) | (The accompanying notes are an integral part of these consolidated financial statements) | |||||||||
| F-39 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 20202023 and 20192022
($ expressed in United States Dollars)
Note 1 – Organization and Nature of Operations
The Company was incorporated under the laws of the State of Delaware on September 24, 1998. On September 22, 2011, the Company filed a Certificate for Renewal and Revival of Charter with the Secretary of State of Delaware. Pursuant to Section 312(1) of the Delaware General Corporation Law, the Company was revived under the new name of “VolitionRX Limited” and the name change became effective on October 11, 2011. On October 7, 2016, the Company amended its Certificate of Incorporation to reflect a name change to “VolitionRx Limited.”
On October 6, 2011, the Company entered into a share exchange agreement with Singapore Volition Pte. Limited, a Singapore corporation incorporated on August 5, 2010 (“Singapore Volition”), and the shareholders of Singapore Volition. Pursuant to the terms of the share exchange agreement, the former shareholders of Singapore Volition held 85% of the issued and outstanding common shares of the Company. The issuance was deemed to be a reverse acquisition for accounting purposes and as such, Singapore Volition is regarded as the predecessor of the Company. The number of shares outstanding and per share amounts of the Company have been restated to recognize the foregoing recapitalization.
The Company’s principal business objective through its subsidiaries is to develop and bring to market simple, easy to use, cost effective blood tests designed to help diagnose and monitor a range of life-altering diseases, including certain cancers and other diseases.diseases associated with NETosis such as sepsis and COVID-19. The tests are based on the science of NucleosomicsTM, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid – an indication that disease is present. The Company has onetwo wholly owned subsidiary,subsidiaries, Volition Global Services SRL (“Volition Global”) which was formed in August 2021 and Singapore Volition. Singapore Volition has one wholly owned subsidiary, Belgian Volition SRL, a Belgium private limited liability company formerly known as ValiBioSA (“Belgian Volition”), which it acquired as ofin September 22, 2010. Belgian Volition has four subsidiaries, Volition Diagnostics UK Limited (“Volition Diagnostics”), which was formed as ofin November 13, 2015, Volition America, Inc. (“Volition America”), which was formed as ofin February 3, 2017, Volition Germany GmbH (“Volition Germany”), which was acquired as ofin January 10, 2020, as well as its majority-owned subsidiary Volition Veterinary Diagnostics Development LLC (“Volition Vet”), which was formed as ofin June 3, 2019. Following the acquisition of Singapore Volition in 2011, the Company’s fiscal year end was changed from August 31 to December 31.
NoteNote 2 - Going– Liquidity and Going Concern Assessment
The Company'sManagement assesses liquidity and going concern uncertainty in the Company’s consolidated financial statements to determine whether there is sufficient cash on hand and working capital, including available borrowings on loans, to operate for a period of at least one year from the date the consolidated financial statements are prepared using accounting principles generally acceptedissued or available to be issued, which is referred to as the “look-forward period”, as defined in GAAP. As part of this assessment, based on conditions that are known and reasonably knowable to management, management will consider various scenarios, forecasts, projections, estimates and will make certain key assumptions, including the timing and nature of projected cash expenditures or programs, its ability to delay or curtail expenditures or programs and its ability to raise additional capital, if necessary, among other factors. Based on this assessment, as necessary or applicable, management makes certain assumptions around implementing curtailments or delays in the United Statesnature and timing of America, or U.S. GAAP, applicableprograms and expenditures to a going concern which contemplates the realization of assetsextent it deems probable those implementations can be achieved and liquidation of liabilities inmanagement has the normal course of business. proper authority to execute them within the look-forward period.
The Company has incurred substantial losses since its inception of $110.2$202.6 million, has negative cash flows from operations, and has minimal revenues which createsand expects to continue to incur operating losses in the near-term. These factors raise substantial doubt about its ability to continue as a going concern forconcern. The Company believes that it has access to capital resources through possible public or private equity offerings, debt financings, corporate collaborations, related party funding, or other means to continue as a period at least one year from the date of issuance of these consolidated financial statements.going concern.
The future of the Company as an operating business will depend on its ability to obtain sufficient capital contributions, financing and/or generate revenues as may be required to sustain its operations. Management plans to address the above as needed by, (a) securing additional grant funds, (b) obtaining additional financing through debt or equity transactions; (c) granting licenses and/or distribution rights to third parties in exchange for specified up-front and/or back endback-end payments, and (d) developing and commercializing its products onin an accelerated timeline.efficient manner. Management continues to exercise tight cost controls to conserve cash.
The ability of the Company to continue as a going concern is dependent upon its ability to successfully accomplish the plans described in the preceding paragraph and to eventually attain profitable operations. The accompanying consolidated financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern. If the Company is unable to obtain adequate capital, it could be forced to cease operations.
| F-40 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 20202023 and 20192022
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies
Basis of Presentation
The consolidated financial statements of the Company have been prepared in accordance with U.S. GAAP and are expressed in US dollars. The Company’s fiscal year end is December 31.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company also regularly evaluates estimates and assumptions related to deferred income tax asset valuation allowances, useful lives of property and equipment and intangible assets, borrowing rate used in operating lease right-of-use asset and liability valuations, impairment analysis of intangible assets and valuations of stock-based compensation.
The Company bases its estimates and assumptions on current facts, historical experiences and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results of operations could be affected.
Principles of Consolidation
The accompanying consolidated financial statements for the year ended December 31, 20202023 include the accounts of the Company and its subsidiaries. The Company has two wholly owned subsidiaries, Singapore Volition Pte. Limited and Volition Global Services SRL. Singapore Volition has one wholly owned subsidiary, Belgian Volition SRL. Belgian Volition has four subsidiaries, Volition Diagnostics UK Limited, Volition America, Inc, Volition Germany GmbH, and its one majority owned subsidiary Volition America, and Volition Vet.Veterinary Diagnostics Development LLC. See Note 10(f), Commitments and Contingencies – Other Commitments, for more information regarding Volition Vet, Volition Germany and Volition Germany.America. All intercompany balances and transactions have been eliminated in consolidation.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with a maturity of three months or less at the time of issuance to be cash equivalents. As of December 31, 2020,2023, and December 31, 2019,2022, the Company had $19,444,737$20,729,983 and $16,966,168,$10,867,050, respectively, in cash and cash equivalents. As of December 31, 2020,2023, and December 31, 2019,2022, the Company had $18,592,210$15,220,237 and $16,499,679,$7,925,876, respectively, in its domestic accounts in excess of Federal Deposit insured limits. As of December 31, 2020,2023, and December 31, 2019,2022, the Company had $831,110$4,227,147 and $2,887,483,$1,725,981, respectively, in its foreign accounts in excess of the Belgian Deposit insured limits. As of December 31, 2020,2023, and December 31, 2019,2022, the Company had $282,137$107,349 and $170,387,$100,601, respectively, in its foreign accounts in excess of the Singapore Deposit insured limits. As of December 31, 2020,2023, and December 31, 2019,2022, the Company had $186,168$320,124 and $777,432,$326,631, respectively, in its foreign accounts in excess of the UK Deposit insured limits.
Accounts Receivable
Trade accountsAccounts receivable are stated atconsist of trade receivables in the amount the Company expects to collect.normal course of business. Due to the nature of the accounts receivable balance, the Company believes the risk of doubtful accounts is minimal and therefore no allowance is recorded. If the financial condition of the Company’s customers were to deteriorate, adversely affecting their ability to make payments, additional allowances would be required. The Company may provide for estimated uncollectible amounts through a charge to earnings and a credit to a valuation allowance. Balances that remain outstanding after the Company has used reasonable collection efforts are written off through a charge to the valuation allowance and a credit to accounts receivable. As of December 31, 2020,2023, the accounts receivable balance was $7,118$242,617 and the allowance for doubtful accounts was $nil.
| F-41 |
|
| Table of Contents |
Property and equipment are stated at historical cost and depreciated over the useful life of the asset using the straight-line method. Useful lives are assigned to assets depending on their category. For details regarding property and equipment, refer to Note 4.
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 20202023 and 20192022
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
Property and Equipment
Property and equipment is stated at historical cost less accumulated depreciation. Leasehold improvements are amortized over the lesser of the base term of the lease or estimated life of the leasehold improvements. Depreciation is calculated using the straight-line method over the estimated useful lives as follows:
Useful Life | |
Computer hardware and software | 3 years |
Laboratory equipment | 5 years |
Office furniture and equipment | 5 years |
Buildings | 30 years |
Building improvements | 5-15 years |
Land | Not amortized |
Basic and Diluted Net Loss Per Share
The Company computes net loss per share in accordance with Accounting Standards Codification (“ASC”) 260, “Earnings Per Share,” which requires presentation of both basic and diluted earnings per share (“EPS”) on the face of the income statement. Basic EPS is computedcalculated by dividing net loss availableincome (loss) attributable to common stockholders (numerator)Volition shareholders by the weighted average number of shares of common stock outstanding (denominator)during each period. Diluted earnings (loss) per share is calculated by adjusting the weighted average number of shares of common stock outstanding for the dilutive effect, if any, of common stock equivalents. Common stock equivalents whose effect would be antidilutive are not included in diluted earnings (loss) per share. The Company uses the treasury stock method to determine the dilutive effect, which assumes that all common stock equivalents have been exercised at the beginning of the period and that the funds obtained from those exercises were used to repurchase shares of common stock of the Company at the average closing market price during the period. Diluted EPS gives effect to all dilutiveThere were 9,197,021 and 7,787,013 potential common shares outstanding during the period using the treasury stock method. In computing diluted EPS, the average stock price for the period is used in determining the number of shares assumed to be purchased from the exercise of stock options or warrants. As of December 31, 2020, and December 31, 2019, 4,556,669 and 4,359,301, respectively, of potential common shares equivalents from stock options, RSUs and warrants were excluded from the diluted EPS calculations as their effect is anti-dilutive.anti-dilutive for the years ended December 31, 2023, and December 31, 2022, respectively.
Foreign Currency Translation
The Company has functional currencies in Euros, US Dollars and British Pounds Sterling and its reporting currency is the US Dollar. Management has adopted ASC 830-20, “Foreign Currency Matters – Foreign Currency Transactions”. All assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. For revenues and expenses, the weighted average exchange rate for the period is used. Gains and losses arising on translation of foreign currency denominated transactions are included in other comprehensive income (loss).
Other Comprehensive Income (Loss)
ASC 220, “Other Comprehensive Income/(Loss)”, establishes standards for the reporting and display of other comprehensive loss and its components in the financial statements. As of December 31, 2023, the Company had $243,940 of accumulated other comprehensive income, relating to foreign currency translation.
| F-42 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2023 and 2022
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
Financial Instruments
Pursuant to ASC 820, “Fair Value Measurements and Disclosures,” an entity is required to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the assets or liabilities such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The Company’s financial instruments consist principally of cash, accounts payable, accrued liabilities, notes payable, and amounts due to related parties. Pursuant to ASC 820, the fair value of cash is determined based on “Level 1” inputs, which consists of quoted prices in active markets for identical assets. The Company believes that the recorded values of all of our other financial instruments approximate their current fair values because of their nature and respective maturity dates or durations.
Included in the following table are the Company’s major categories of assets and liabilities measured at fair value on a recurring basis as of December 31, 2023.
Fair Value Measurements at December 31, 2023 | ||||||||||||||||
Description | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
$ | $ | $ | $ | |||||||||||||
Liabilities | ||||||||||||||||
Warrant liability | - | 126,649 | - | 126,649 | ||||||||||||
As of December 31, 2022, there was no warrant liability. The following table provides a roll-forward of the warrant liability measured at fair value on a recurring basis for the year ended December 31, 2023, as follows:
Warrant Liability | ||||
$ | ||||
Balance at December 31, 2022 | - | |||
Fair value of warrant liability, at issuance | 366,960 | |||
Gain on change in fair value of warrant liability | (240,311 | ) | ||
Balance at December 31, 2023 | 126,649 | |||
| F-43 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2023 and 2022
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
Income Taxes
Potential benefits of income tax losses are not recognized in the accounts until realization is more likely than not. The Company has adopted ASC 740, “Accounting for Income Taxes,”Taxes” as of its inception. Pursuant to ASC 740, the Company is required to compute tax asset benefits for net operating losses carried forward. The potential benefits of net operating losses have not been recognized in these consolidated financial statements because the Company cannot be assured it is more likely than not it will utilize the net operating losses carried forward in future years. Refer to Note 9 for further details.
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
Other Comprehensive Income (Loss)
ASC 220, “Other Comprehensive Income/(Loss)”, establishes standards for the reporting and display of other comprehensive loss and its components in the financial statements. As of December 31, 2020, the Company had $59,978 of accumulated other comprehensive income, relating to foreign currency translation.
Revenue Recognition
The Company adopted ASC 606, “Revenue from Contracts with Customers,” effective January 1, 2019. Under ASC 606, the Company recognizes revenues when the customer obtains control of promised goods or services, in an amount that reflects the consideration which the Company expects to receive in exchange for those goods or services. The Company recognizes revenues following the five-step model prescribed under ASC 606: (i) identify contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenues when (or as) the Company satisfies the performance obligation(s).
The Company generates revenueproduct revenues from its license agreement with Active Motif, Inc. (“Active Motif”) for the sale of ROU kitsits Nu.Q® Vet Cancer Test, from which the Company receives royalties.sale of nucleosomes, and from the sale of research use only kits. In addition, revenue is received from external third parties for services the Company performs for them in its laboratory. The Company also generates product revenues from the sale of its Nu.Q® Vet Cancer Screening Test and from the sale of nucleosomes.
Revenues, and their respective treatment for financial reporting purposes under ASC 606, are as follows:
Royalty
The Company receives royalty revenues on the net sales recognized during the period in which the revenue is earned, and the amount is determinable from the licensee. These are presented under “Royalty” under the consolidated statements of operations. The Company does not have future performance obligations under this revenue stream. In accordance with ASC 606, the Company records these revenues based on estimates of the net sales that occurred during the relevant period from the licensee. The relevant period estimates of these royalties are based on preliminary gross sales data provided by Active Motif and analysis of historical gross-to-net adjustments. Differences between actual and estimated royalty revenues are adjusted for in the period in which they become known.
Product
The Company includes revenue from product sales recognized during the period in which goods are shipped to third parties, and the amount is deemed collectable from the third parties. These are presented in “Product” in the consolidated statements of operations and comprehensive loss.
Service
The Company includes revenue recognized from laboratory services performed in the Company’s laboratory on behalf of third parties under “Service” under the consolidated statements of operations.
For each development and/or commercialization agreement that results in revenues, the Company identifies all performance obligations, aside from those that are immaterial, which may include a license to intellectual property and know-how, development activities and/or transition activities. In order to determine the transaction price, in addition to any upfront payment, the Company estimates the amount of variable consideration at the outset of the contract either utilizing the expected value or most likely amount method, depending on the facts and circumstances relative to the contract. The Company constrains the estimates of variable consideration such that it is probable that a significant reversal of previously recognized revenue will not occur throughout the life of the contract. When determining if variable consideration should be constrained, management considers whether there are factors outside the Company’s control that could result in a significant reversal of revenue. In making these assessments, the Company considers the likelihood and magnitude of a potential reversal of revenue. These estimates are re-assessed each reporting period as required.
| F-44 |
| Table of Contents |
VOLITIONRX LIMITED
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 20202023 and 20192022
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
Revenue Recognition (continued)
Licensing
The Company includes revenue recognized from the licensing of certain rights to third parties in “Licensing” in the consolidated statements of operations and comprehensive loss. For each licensing, development and/or commercialization agreement that results in revenues, the Company identifies all performance obligations, aside from those that are immaterial, which may include a license to intellectual property and know-how, development activities and/or transition activities. In order to determine the transaction price, in addition to any upfront payment, the Company estimates the amount of variable consideration at the outset of the contract either utilizing the expected value or most likely amount method, depending on the facts and circumstances relative to the contract. The Company constrains (reduces) the estimates of variable consideration such that it is probable that a significant reversal of previously recognized revenue will not occur throughout the life of the contract. When determining if variable consideration should be constrained, management considers whether there are factors outside the Company’s control that could result in a significant reversal of revenue. In making these assessments, the Company considers the likelihood and magnitude of a potential reversal of revenue. These estimates are re-assessed each reporting period as required.
Revenue from Heska Agreement
On March 28, 2022, Belgian Volition entered into a Master License and Supply Agreement (the “License Agreement”) with Heska Corporation (“Heska”), a leading global provider of advanced veterinary diagnostics, pursuant to which Belgian Volition granted Heska worldwide exclusive rights to sell the Nu.Q® Vet Cancer Test at the point of care (“POC”) initially for the screening of lymphoma and hemangiosarcoma in dogs (“Canine Lymphoma & HSA”), and non-exclusive rights to sell its Nu.Q® Vet Cancer Test in kit format (“Kits”) through Heska’s network of central reference laboratories (“Central Lab”) initially for Canine Lymphoma & HSA.
Under and subject to the terms of the License Agreement, Belgian Volition received an upfront payment of $10.0 million in 2022, and received further milestone payments in 2023 of (i) $6.5 million upon the first commercial sale by or on behalf of Heska of a POC screening test for Canine Lymphoma & HSA and (ii) $6.5 million upon the first commercial sale by or on behalf of Heska of a POC monitoring test for the same conditions. A further milestone payment of $5.0 million will be payable to Volition pursuant to the Agreement upon the earlier of (a) the first commercial sale by or on behalf of Heska of a screening or monitoring test for lymphoma in felines, or (b) the 9-month anniversary of the first peer reviewed paper evidencing clinical utility for the screening or monitoring of lymphoma in felines being published in any one of a number of periodicals identified by the parties. Any further expansion of the License Agreement to cover the use of the Nu.Q® Vet Cancer Test for other cancer and non-cancer indications is subject to negotiation between the parties.
Pursuant to the terms of the License Agreement, Belgian Volition will also supply Central Lab Kits and will receive a pre-agreed price per test, adjusted annually for inflation. The price per test for POC key components (“Key Components”) is also discounted to reflect the lower cost to Belgian Volition and additional assembly costs for Heska, as well as consideration for Heska’s upfront and milestone payments. Heska will assemble the Key Components for use at the POC, and is additionally responsible for marketing and distribution efforts and related costs.
| F-45 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 3 - Summary of Significant Accounting Policies (continued)
Revenue Recognition (continued)
The License Agreement may be terminated by either party for a material breach by the other party, subject to notice and cure provisions, or in the event of the other party’s insolvency. Heska also has the option to terminate if it is unable to adapt the Key Components for use on a POC platform. Unless earlier terminated, the License Agreement will continue in effect for an initial term of 22 years for POC and 5 years for Central Lab, with the Central Lab term then continuing on a rolling one-year basis for the POC term.
According to ASC Topic 606, “Revenue from Contracts with Customers”, a performance obligation is a commitment to provide a distinct good or service or a series of distinct goods or services. Goods and services that are not distinct are bundled with other goods or services in the contract until a bundle of goods or services that is distinct is created. A good or service promised to a customer is distinct if the customer can benefit from the good or service either on its own or together with other resources that are readily available to the customer and the entity’s promise to transfer the good or service to the customer is separately identifiable from other promises in the contract.
In conjunction with the License Agreement, the Company evaluated whether or not the performance obligations granted under the License Agreement were distinct and concluded that they were not distinct as Heska could not benefit from the license without the supply (manufacturing) services. The supply services are highly specialized and are dependent on the supply of the product from the Company. As such, the performance obligations granted under the License Agreement were combined to constitute a single performance obligation and the Company accounts for them as a single contract.
During the first quarter of 2022, the Company received a $10.0 million upfront payment under the License Agreement and further upfront payments totaling $13.0 million in the fourth quarter of 2023, which is included as deferred revenue on the accompanying consolidated balance sheet as of $23.0 million. The Company allocated the milestone payments that were not constrained to the single performance obligation in the contract. The Company expects to recognize the total $28.0 million of milestone amounts under the License Agreement over time using an output method based on Key Components and Kits supplied to Heska.
In determining the transaction price, the Company analyzed the variable consideration and whether or not such variable consideration was constrained. The Company will reassess this variable consideration at each reporting period and adjust the transaction price, if necessary. The total Key Components and Kits that the Company expects to manufacture for Heska over the life of the contract will be a significant judgment in recognizing revenue once the Company begins to supply product to Heska.
Sales to the Company’s three largest customers represented over 61% of total sales for the year ended December 31, 2023.
Deferred Revenue (Contract Liabilities) and Contract Assets
Deferred revenue consists of amounts for which the Company has an unconditional right to bill, and/or amounts for which payment has been received (including non-refundable amounts) but have not been recognized as revenue because the related performance obligations are deemed incomplete. As of December 31, 2023, the Company recorded $23.0 million as deferred revenue in respect of a non-refundable payment received in relation to a licensing and product supply agreement with Heska Corporation. As of December 31, 2022, the Company recorded $10.0 million as deferred revenue.
Contract assets include costs and services incurred on contracts with open performance obligations. These contract assets were immaterial as of December 31, 2023.
Research and Development
In accordance with ASC 730, the Company follows the policy of expensing its research and development costs in the period in which they are incurred. The Company incurred research and development expenses of $14.5$19.6 million and $10.4$15.3 million during the years ended December 31, 20202023 and 2019,2022, respectively.
| F-46 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 3 - Summary of Significant Accounting Policies (continued)
Impairment of Long-Lived Assets
In accordance with ASC 360, “Property Plant and Equipment”, the Company testsreviews the carrying value of long-lived assets or asset groupssuch as intangible assets with definite lives, property and equipment, and right-of-use (“ROU”) assets for recoverability whenimpairment whenever events or changes in circumstances indicate that theirthe carrying amount mayof the assets might not be recoverable. Circumstances which could trigger a reviewThese events and circumstances may include but are not limited to: significant decreases in the market price of an asset or asset group, significant changes in the asset;extent or manner in which an asset or asset group is being used by the Company or in its physical condition, a significant change in legal factors or in the business climate, a history or forecast of future operating or cash flow losses, significant disposal activity, a significant decline in the Company’s share price, a significant decline in revenue or adverse changes in the business climate or legal factors; accumulationeconomic environment. If such facts indicate a potential impairment, the Company would assess the recoverability of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that thean asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life. Recoverability is assessed based ongroup by determining if the carrying amountvalue of the asset and its fair value which is generally determined based ongroup exceeds the sum of the projected undiscounted cash flows expected to result from the use and eventual disposition of the eventual disposalassets over the remaining economic life of the primary asset in the asset group. If the recoverability test indicates that the carrying value of the asset as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amountgroup is not recoverable, the Company will estimate the fair value of the asset group using appropriate valuation methodologies, which would typically include an estimate of discounted cash flows. Any impairment would be measured as the difference between the asset group’s carrying amount and exceedsits estimated fair value. Impairment losses of $nil and $nil were recognized during the years ended December 31, 20202023, and December 31, 2019,2022, respectively.
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, “Compensation – Stock Compensation”. Under the provisions of ASC 718, stock-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized over the employee’s requisite service period, which is generally the vesting period. The fair value of our stock options and warrants is estimated using a Black-Scholes option valuation model. Restricted stock units (“RSUs)” are valued based on the closing stock price on the date of grant.grant (intrinsic value method). The fair value of RSUs that include a market vesting condition will be measured on the grant date using a Monte Carlo Simulation of a Geometric Brownian Motion stock path model and incorporating the probability of vesting occurring, with the estimated fair value of these awards will be recognized over the derived service period (as determined by the valuation model), with such recognition occurring regardless of whether the market condition is met. The Company has elected to recognize forfeitures as they occur. Refer to Note 8 for further details.
Operating Leases
The Company adopted FASB issued Accounting Standards Update No. 2016-02 – Leases (“Topic 842”) as of January 1, 2019, that requires lessees to recordaccounts for leases in accordance with ASC 842, “Leases.” The Company determines whether a contract is a lease at contract inception or for a modified contract at the present value of operating lease payments asmodification date. At inception or modification, the Company recognizes right-of-use assets (“ROU”) and related lease liabilities on the balance sheet. See Note 10(b)sheet for discussionall leases greater than one year in duration. Lease liabilities and their corresponding ROU assets are initially measured at the present value of the guidanceunpaid lease payments as of the lease commencement date. If the lease contains a renewal and/or termination option, the exercise of the option is included in the term of the lease if the Company is reasonably certain that a renewal or termination option will be exercised. As the Company’s leases do not provide an implicit rate, the Company uses an estimated incremental borrowing rate (“IBR”) based on the information available at the commencement date of the respective lease to determine the present value of future payments. The IBR is determined by estimating what it would cost the Company to borrow a collateralized amount equal to the total lease payments over the lease term based on the contractual terms of the lease and the Company’s accounting policy.location of the leased asset.
Operating lease payments are recognized as an expense on a straight-line basis over the lease term in equal amounts of rent expense attributed to each period during the term of the lease, regardless of when actual payments are made. This generally results in rent expense in excess of cash payments during the early years of a lease and rent expense less than cash payments in later years. The difference between rent expense recognized and actual rental payments is typically represented as the spread between the ROU asset and lease liability.
| F-47 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 $ expressed in United States Dollars) |
Note 3 - Summary of Significant Accounting Policies (continued)
Operating Leases (continued)
When calculating the present value of minimum lease payments, we account for leases as one single lease component if a lease has both lease and non-lease fixed cost components. Variable lease and non-lease cost components are expensed as incurred.
We do not recognize ROU assets and lease liabilities for short-term leases that have an initial lease term of 12 months or less. We recognize the lease payments associated with short-term leases as an expense on a straight-line basis over the lease term.
Grant Income
The Company receives funding from public bodies for a proportion of the costs of specific projects. Funds are received in line with claims submitted for the agreed expenditure. The Company recognizes grant income once claims submitted are approved and funds are received. General working capital funding received at the commencement of a project is treated as deferred income and is recorded in accrued liabilities until it has been utilized for the expenditure claimed. Funding received that is repayable is shown as a liability.
Reclassifications
Certain reclassifications within operating expenses have been made to prior period’s consolidated financial statements to conform to the current period financial statement presentation. There is no impact in total to the results of operations and cash flows in all periods presented.
Concentration of Credit Risk
Financial instruments that potentially subject the company to concentration of credit risk consist primarily of accounts receivable.
The company performs ongoing credit evaluations of its customers and maintains allowances for potential credit losses. Management does not believe significant credit risks exist at December 31, 2023.
As at December 31, 2023 the two largest customer balances represented over 65% of the total outstanding accounts receivable balance.
Recent Accounting Pronouncements
The Company considers the applicability and impact of all Accounting Standard Updates “ASUs” issued by the Financial Accounting Standards Board (“FASB”). The Company has implementedevaluated all newrecent accounting pronouncements and determined that are in effect. Thethe adoption of pronouncements applicable to the Company doeshas not believe that there are any other new accounting pronouncements that have been issued that mighthad or is not expected to have a material impact on itsthe Company’s consolidated financial position or results of operations.
statements.
VOLITIONRX LIMITED
NotesIn June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, or ASU 2016-13, which requires the measurement and recognition of expected credit losses for financial assets held at amortized cost. ASU 2016-13 replaces the existing incurred loss impairment model with an expected loss model. It also eliminates the concept of other-than-temporary impairment and requires credit losses related to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressedavailable-for-sale debt securities to be recorded through an allowance for credit losses rather than as a reduction in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
COVID-19 Pandemic Impact
On March 11, 2020, the World Health Organization designated the outbreakamortized cost basis of the novel strainsecurities. These changes may result in earlier recognition of coronavirus known as COVID-19 as a global pandemic. Governments and businesses around the world have taken unprecedented actions to mitigate the spread of COVID-19, including, but not limited to, shelter-in-place orders, quarantines, significant restrictions on travel, as well as restrictions that prohibit many employees from going to work. Uncertainty with respect to the economic impacts of the pandemic has introduced significant volatility in the financial markets.credit losses. The Company adopted ASU 2016-13 on January 1, 2023. The adoption of ASU 2016-13 did not observe significant impacts on its business or results of operations for the twelve months ended December 31, 2020 due to the global emergence of COVID-19. While the extent to which COVID-19 impacts the Company’s future results will depend on future developments, the pandemic and associated economic impacts could result inhave a material impact toon the Company’s futureconsolidated financial condition, results of operationsstatements and cash flows.related disclosures.
| F-48 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
NoteNote 4 - Property and Equipment
The Company’s property and equipment consist of the following amounts as of December 31, 20202023 and December 31, 2019:2022:
|
|
|
|
|
|
| December 31, 2020 |
|
|
|
|
| December 31, 2023 |
| |||||||||
|
|
|
|
| Accumulated |
| Net Carrying |
|
|
| Accumulated |
| Net Carrying |
| |||||||||
|
|
| Cost |
| Depreciation |
| Value |
| Cost |
| Depreciation |
| Value |
| |||||||||
| Useful Life |
| $ |
| $ |
| $ |
| $ |
|
| $ |
|
| $ |
| |||||||
Computer hardware and software | 3 years |
| 550,254 |
| 412,805 |
| 137,449 |
| 724,534 |
| 610,577 |
| 113,957 |
| |||||||||
Laboratory equipment | 5 years |
| 2,586,997 |
| 1,060,153 |
| 1,526,844 |
| 4,753,253 |
| 2,491,149 |
| 2,262,104 |
| |||||||||
Office furniture and equipment | 5 years |
| 271,656 |
| 171,247 |
| 100,409 |
| 378,800 |
| 280,396 |
| 98,404 |
| |||||||||
Buildings | 30 years |
| 2,366,236 |
| 207,111 |
| 2,159,125 |
| 2,113,031 |
| 377,328 |
| 1,735,703 |
| |||||||||
Building improvements | 5-15 years |
| 1,285,383 |
| 184,813 |
| 1,100,570 |
| 1,610,016 |
| 429,639 |
| 1,180,377 |
| |||||||||
Land | Not amortized |
| 146,737 |
| - |
| 146,737 |
|
| 132,468 |
|
|
| - |
|
|
| 132,468 |
| ||||
|
|
|
|
|
|
|
|
|
| 9,712,102 |
|
|
| 4,189,089 |
|
|
| 5,523,013 |
| ||||
|
|
| 7,207,263 |
| 2,036,129 |
| 5,171,134 |
|
|
|
|
|
|
| |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| December 31, 2022 |
| |||||||||
|
|
|
|
|
|
| December 31, 2019 |
|
|
| Accumulated |
| Net Carrying |
| |||||||||
|
|
|
|
| Accumulated |
| Net Carrying |
| Cost |
| Depreciation |
| Value |
| |||||||||
|
|
| Cost |
| Depreciation |
| Value |
|
| $ |
|
| $ |
|
|
| $ |
| |||||
| Useful Life |
| $ |
| $ |
| $ | ||||||||||||||||
Computer hardware and software | 3 years |
| 426,461 |
| 280,554 |
| 145,907 |
| 656,759 |
| 497,306 |
|
| 159,453 |
| ||||||||
Laboratory equipment | 5 years |
| 2,052,348 |
| 1,256,637 |
| 795,711 |
| 4,190,289 |
| 1,951,387 |
| 2,238,902 |
| |||||||||
Office furniture and equipment | 5 years |
| 217,545 |
| 114,242 |
| 103,303 |
| 358,575 |
| 239,436 |
| 119,139 |
| |||||||||
Buildings | 30 years |
| 1,472,211 |
| 139,021 |
| 1,333,190 |
| 2,054,332 |
| 298,397 |
| 1,755,935 |
| |||||||||
Building improvements | 5-15 years |
| 630,824 |
| 117,526 |
| 513,298 |
| 1,317,132 |
| 326,337 |
| 990,795 |
| |||||||||
Land | Not amortized |
| 89,816 |
| - |
| 89,816 |
|
| 128,788 |
|
|
| - |
|
|
| 128,788 |
| ||||
|
|
|
|
|
|
|
|
|
| 8,705,875 |
|
|
| 3,312,863 |
|
|
| 5,393,012 |
| ||||
|
|
| 4,889,205 |
| 1,907,980 |
| 2,981,225 | ||||||||||||||||
TheDuring the years ended December 31, 2023 and December 31, 2022, the total capital expenditure was $1.1 million and $1.6 million, respectively, the majority of capital expenditures in 2020 are related to purchasing a new manufacturing facilitywhich was from purchases of $0.8 million, manufacturing building improvements of $0.6 million, and laboratory equipment of $1.0 million.equipment.
During the years ended December 31, 20202023 and December 31, 2019,2022, the Company recognized $627,555$1,080,475 and $589,532,$865,262, respectively, in depreciation expense.
| F-49 |
| Table of Contents |
During the year ended December 31, 2020, the Company sold laboratory equipment for cash proceeds of $293,312, resulting in a gain on disposal of equipment of $293,312.
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 5 - Intangible Assets
The Company’s intangible assets consist of patents, mainly acquired in the acquisition of Belgian Volition. The patents are being amortized over the assets’ estimated useful lives, which range from 8 to 20 years.
|
|
|
|
| December 31, 2020 | ||||||||||||||||
|
|
| Accumulated |
| Net Carrying |
|
|
|
|
| December 31, 2023 |
| |||||||||
| Cost |
| Amortization |
| Value |
|
|
| Accumulated |
| Net Carrying |
| |||||||||
| $ |
| $ |
| $ |
| Cost |
| Amortization |
| Value |
| |||||||||
|
|
|
|
|
|
| $ |
| $ |
| $ |
| |||||||||
Patents | 1,256,064 |
| 934,423 |
| 321,641 |
|
| 1,130,936 |
|
|
| 1,107,050 |
|
|
| 23,886 |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||
|
|
|
|
| December 31, 2019 |
|
|
|
|
| December 31, 2022 |
| |||||||||
|
|
| Accumulated |
| Net Carrying |
|
|
| Accumulated |
| Net Carrying |
| |||||||||
| Cost |
| Amortization |
| Value |
| Cost |
| Amortization |
| Value |
| |||||||||
| $ |
| $ |
| $ |
| $ |
| $ |
| $ |
| |||||||||
|
|
|
|
|
| ||||||||||||||||
Patents | 1,147,391 |
| 775,086 |
| 372,305 |
|
| 1,104,103 |
|
|
| 993,598 |
|
|
| 110,505 |
| ||||
During the years ended December 31, 20202023 and December 31, 2019,2022, the Company recognized $88,626$84,910 and $87,285,$75,558, respectively, in amortization expense.
The Company amortizes the long-lived assets on a straight-line basis with terms ranging from 8 to 20 years. The annual estimated amortization schedule over the next five years is as follows:
2021 | $ | 94,278 |
2022 | $ | 94,278 |
2023 | $ | 94,278 |
2024 | $ | 38,807 |
Total Intangible Assets | $ | 321,641 |
2024 |
| $ | 23,886 |
|
Total Intangible Assets |
| $ | 23,886 |
|
The Company periodically reviews its long-lived assets to ensure that their carrying value does not exceed their fair market value. The Company carried out such a review in accordance with ASC 360 as of December 31, 2020.2023. The result of this review confirmed that the ongoing value of the patents was not impaired as of December 31, 2020.2023.
Note 6 - Related Party Transactions
| F-50 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
As of December 31, 2020,2023, the Company was authorized to issue 100 million shares of common stock par value $0.001 per share, of which 48,607,01781,898,321 and 41,125,30357,873,379 shares were issued as of December 31, 20202023 and December 31, 2019,2022, respectively.
20202023
Issuances Upon Warrant and Option Exercises
From January 7, 2020 to August 17, 2020, 97,500 stock options were exercised to purchase shares of common stock at $2.50 per share in cashless exercises that resulted in the issuance of 30,033 shares of common stock.
From January 7, 2020 to August 17, 2020, 97,500 stock options were exercised to purchase shares of common stock at $3.00 per share in cashless exercises that resulted in the issuance of 16,539 shares of common stock.Equity Capital Raises
On January 7, 2020, 35,000 stock options were exercised to purchase shares of common stock at $4.00 per share in cashless exercises that resulted in the issuance of 6,486 shares of common stock.
From February 24, 2020 to September 2, 2020, 11,599 stock options were exercised to purchase shares of common stock at $2.35 per share in cashless exercises that resulted in the issuance of 2,752 shares of common stock.
From July 16, 2020 to August 10, 2020, 210,000 stock options were exercised to purchase shares of common stock at $2.50 per share in cashless exercises and withholding of shares for taxes that resulted in the issuance of 39,197 shares of common stock.
From July 21, 2020 to August 12, 2020, 210,000 stock options were exercised to purchase shares of common stock at $3.00 per share in cashless exercises and withholding of shares for taxes that resulted in the issuance of 22,261 shares of common stock.
On August 12, 2020, 15,000 stock options were exercised to purchase shares of common stock at $2.50 per share that resulted in the issuance of 15,000 shares of common stock for proceeds to the Company of $37,500.
On August 12, 2020, 15,000 stock options were exercised to purchase shares of common stock at $3.00 per share that resulted in the issuance of 15,000 shares of common stock for proceeds to the Company of $45,000.
On September 18, 2020, 25,000 warrants were exercised to purchase shares of common stock at $2.47 per share that resulted in the issuance of 25,000 shares of common stock for proceeds to the Company of $61,750.
Stock Issuance for Services
On January 9, 2020, 73,263 shares were issued as fully paid shares of common stock valued at $333,969 as compensation to a managing director of Volition Germany (see Note 10(f)).
Stock Repurchase
On January 12, 2020, the Company purchased from its Chief Medical Officer 11,364 shares of our common stock at $4.79 per share, for a total cost to the Company of $54,434. These shares were subsequently retired.
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 7 - Common Stock (continued)
Equity Capital Raise
On May 20, 2020,17, 2023, the Company entered into an underwriting agreement with NationalNewbridge Securities Corporation acting on its own behalf and as representative of the several underwriters,(“Newbridge”) in connection with thean underwritten public offering issuance and sale by the Company of 4,365,0004,945,000 shares of the Company’s common stock, atwhich includes Newbridge’s exercise in full of its overallotment option, pursuant to the Company’s “shelf” registration statement on Form S-3 (declared effective by the SEC on November 8, 2021, File No. 333-259783) (as amended and supplemented from time to time, the “2021 Form S-3”). The public offering price was $1.75 per share. The underwriter purchased the shares from the Company at a price of $2.75$1.6275 per share lesson February 22, 2023, after taking into account the underwriting discounts and commissions. UnderThe net proceeds received by the termsCompany for the sale and issuance of the agreement,shares were approximately $8.0 million, before deducting offering expenses of $0.2 million paid by the Company.
On June 1, 2023, the Company grantedentered into an underwriting agreement with Prime Executions, Inc. dba Freedom Capital Markets (“Freedom”) acting as the underwriters an option, exercisable for 30 days from the datebook-running manager of the agreement, to purchase up to 654,750 additionaloffering and Bancroft Capital, LLC acting as co-manager in connection with an underwritten public offering of 14,950,000 shares of the Company’s common stock, which includes Freedom’s exercise in full of its overallotment option, pursuant to cover overallotments, if any, at the 2021 Form S-3. The underwriter purchased 13,000,000 shares and 1,950,000 shares from the Company on June 5 and June 23, 2023 respectively. The public offering price was $1.27 per share. The underwriter purchased the shares from the Company at a price of $2.75$1.1811 per share, less underwriting discountsshare. The net proceeds received by the Company for the sale and commissions. On May 21, 2020, the underwriters exercised the overallotment option in full. As a resultissuance of the equity capital raise,shares were approximately $17.6 million, before deducting offering expenses of $0.1 million paid by the Company. In addition, the Company issued warrants to purchase an aggregate of 448,500 shares of Company common stock to Freedom, at an exercise price of $2.00 per share.
The Company evaluated the warrants as either equity-classified or liability-classified instruments based on an assessment of the specific terms of the warrants and applicable authoritative guidance in ASC 480 and ASC 815-40. The Company determined the warrants issued in the Freedom offering failed the indexation guidance under ASC 815-40, specifically, the warrants provide for a totalBlack-Scholes value calculation in the event of certain transactions (“Fundamental Transactions”), which includes a floor on volatility utilized in the value calculation at 100% or greater. The Company has determined that this provision introduces leverage to the holders of the warrants that could result in a value that would be greater than the settlement amount of a fixed-for-fixed option on the Company’s own equity shares. Accordingly, pursuant to ASC 815-40, the Company has classified the fair value of the warrants as a liability upon issuance and marked to market each reporting period in the Company’s consolidated statement of operations until their exercise or expiration.
| F-51 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 7 - Common Stock (continued)
Equity Capital Raises (continued)
The fair value of the warrants as of June 1, 2023, the issuance date, and December 31, 2023, were $366,960 and $126,649, respectively. The warrant liability was estimated using the Black-Scholes pricing model with the following assumptions.
|
|
|
| (At Issuance) |
| |||
|
| December 31, 2023 |
|
| June 1, 2023 |
| ||
|
|
|
|
|
|
| ||
Risk-free interest rate |
|
| 3.89 | % |
|
| 3.70 | % |
Expected volatility |
|
| 76.30 | % |
|
| 71.56 | % |
Expected life (years) |
|
| 4.44 |
|
|
| 5.03 |
|
Expected dividend yield |
|
| - |
|
|
| - |
|
Total fair value |
| $ | 126,649 |
|
| $ | 366,960 |
|
On December 5, 2023 the Company issued 3,205,431 shares of its common stock in a private placement to Wallonie Entreprendre S.A (W.E.) at a purchase price of $0.8337 per share, or an aggregate purchase price of approximately 5$2.7 million (€2.5 million). The shares for aggregate gross proceeds of $13.8 million. Additionally,common stock will not be registered under the Securities Act of 1933, as amended (the “Securities Act”) or any state securities laws and unless so registered may not be offered or sold in connection with thisthe United States except pursuant to an exemption from, or in a transaction $1.1 million was incurred in fees relatingnot subject to, the equity offering, resulting in net proceedsregistration requirements of $12.7 million.the Securities Act and applicable state securities laws.
Equity Distribution AgreementsCapital Raises
2022
On November 10, 2020,July 29, 2022, the Company entered into an underwriting agreement with Newbridge in connection with an underwritten public offering of 3,450,000 shares of the Company’s common stock, which includes Newbridge’s exercise in full of its overallotment option, pursuant to the “2021 Form S-3. Newbridge purchased the shares from the Company at a price of $1.87 per share. The offering closed on August 2, 2022. The Company received net proceeds of approximately $6.4 million from the offering before deducting offering expenses of $0.2 million paid by the Company.
| F-52 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 7 - Common Stock (continued)
EquityDistribution AgreementAgreements
2023
During the year ended December 31, 2023, the Company raised aggregate net proceeds (net of broker commissions and fees) of approximately $0.7 million under the 2022 EDA (as defined below) through the sale of 279,703 shares of its common stock. As of December 31, 2023, the Company has raised aggregate net proceeds (net of broker commissions and fees) of approximately $1.5 million under the 2022 EDA through the sale of 630,532 shares of its common stock. See Note 11 for additional details regarding sales under the 2022 EDA subsequent to December 31, 2023.
2022
On May 20, 2022, the Company entered into an equity distribution agreement (the “2020“2022 EDA”) with Cantor Fitzgerald & Co.Jefferies LLC (“Cantor”Jefferies”) and Oppenheimer & Co. Inc. (“Oppenheimer”), to sell shares of itsthe Company’s common stock, havingwith an aggregate offering price of up to $25,000,000$25.0 million, from time-to-time,time to time through an “at the marketmarket” offering program” pursuant to the Company’s effective “shelf” registration statement on2021 Form S-3 (File No. 333-227248) and related prospectuses, through Cantor and Oppenheimer eachJefferies acting as the Company’s agent and/or principal. The Company is not obligated to sell any shares under the 20202022 EDA. As of December 31, 2020, the Company had made no sales of common stock under the 2020 EDA. See Note 11 for details regarding additional sales of common stock under the 2020 EDA after December 31, 2020.
On September 7, 2018, the Company entered into an equity distribution agreement (as amended, the “2018 Equity Distribution Agreement”) with Oppenheimer, which agreement allows it to offer and sell shares of common stock having an aggregate offering price of up to $10.0 million from time-to-time through an “at the market offering program,” pursuant to a shelf registration statement on Form S-3 (declared effective by the SEC on September 28, 2018, File No.333-227248) and related prospectuses, through Oppenheimer acting as the Company’s agent and/or principal. From inception through December 31, 2020,2022, the Company raised aggregate net proceeds (net of broker’sbroker commissions and fees) of approximately $8.5$0.8 million under the 2018 Equity Distribution Agreement2022 EDA through the sale of 2,230,997350,829 shares of its common stock.
For the year ended December 31, 2020,From January 1, 2022 through May 7, 2022, the Company raised aggregate net proceeds (net of broker’sbroker commissions and fees) of approximately $8.5 million$9,500 under the 2018 Equity Distribution Agreementits equity distribution agreement with Cantor Fitzgerald & Co. and Oppenheimer & Co. Inc. (the “2021 EDA”) through the sale of 2,227,7973,000 shares of its common stock. Additionally, in connection with this transaction $126,492 was incurred in fees relating toThe Company terminated the Equity Distribution Agreement. See Note 11 for details regarding additional sales of common stock under the 2018 Equity Distribution Agreement after December 31, 2020.2021 EDA effective May 7, 2022.
20192023 and 2022
Issuances Upon Warrant and Option Exercises
On August 10, 2018,For the Company issued to Cotterford Company Limited (“Cotterford”) 5.0 millionyears ended December 31, 2023 and December 31, 2022 no warrants were exercised.
2023 and 2022
Stock Option Exercises
During the year ended December 31, 2023 and December 31, 2022 no shares of common stock at a pricewere issued pursuant to the exercise of $1.80 per share in a private placement offering, for aggregate gross proceeds of $9.0 million. In connection with the transaction, approximately $0.1 million was incurred for legal and other fees resulting in net proceeds of approximately $8.9 million. Additionally, the Company issued to Cotterford a warrant to purchase up to an additional 5.0 million shares of common stock at an exercise price of $3.00 per share payable in cash. This transaction resulted in Cotterford becoming a significant stockholder and therefore a related party in accordance with U.S. GAAP. The shares of common stock (including the shares underlying the warrant) were subsequently registered for resale on Form S-3 (declared effective by the SEC on October 15, 2018, File No. 333-227731).options.
| F-53 |
| Table of Contents |
From January 30, 2019 to February 26, 2019, warrants to purchase 754,475 shares of our common stock were exercised at a price of $2.20 per share, for gross proceeds to the Company of approximately $1.66 million.
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
On March 8, 2019, Cotterford partially exercised its warrant and purchased 1,724,138 shares of our common stock at a price of $2.90 per share, for gross proceeds to the Company of $5.0 million.
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 7 - Common Stock (continued)
2019 (continued)Stock Options Expired / Cancelled
Issuances Upon Warrant and Option Exercises (continued)
On May 3, 2019, Cotterford partially exercised its warrant and purchased 1,666,667 shares of our common stock at a price of $3.00 per share, for gross proceeds toThe table below summarizes the Company of $5.0 million.
On July 24, 2019, Cotterford exercised the remainder of its warrant and purchased 1,609,195 shares of our common stock at a price of $3.00 per share, for gross proceeds to the Company of approximately $4.8 million.
From August 20, 2019 to September 20, 2019, 6,166 stock options granted under the Company’s 2015 Stock Incentive Plan (the “2015 Plan”) or the 2011 Equity Incentive Plan (the “2011 Plan”), as indicated, that expired or were exercised to purchase shares of our common stock at $2.35 per share in a cashless exercise that resulted in the issuance of 2,487 shares of our common stock.
On November 15, 2019, 4,167 stock options were exercised to purchase shares of our common stock at $5.00 per share in a cashless exercise that resulted in the issuance of 371 shares of our common stock.
From November 25, 2019 to November 27, 2019, warrants to purchase 29,392 shares of our common stock were exercised at a price of $2.40 per share, for gross proceeds to the Company of $70,541.
Equity Distribution Agreements
Forcancelled during the year ended December 31, 2019, The Company raised aggregate net proceeds (net2023.
Equity Incentive Plan |
| Options (#) |
|
| Grant Date |
| Options Cancelled (#) |
|
| Grant Price ($) |
|
| Cancellation Date | ||||
2015 |
|
| 25,000 |
|
| Apr 15, 2016 |
|
| 25,000 |
|
|
| 4.00 |
|
| Feb 18, 2023 | |
2015 |
|
| 55,000 |
|
| Apr 13, 2020 |
|
| 55,000 |
|
|
| 3.60 |
|
| Feb 18, 2023 | |
2015 |
|
| 50,000 |
|
| Mar 30, 2017 |
|
| 50,000 |
|
|
| 5.00 |
|
| Feb 18, 2023 | |
2015 |
|
| 50,000 |
|
| Feb 11, 2019 |
|
| 50,000 |
|
|
| 3.25 |
|
| Feb 18, 2023 | |
2015 |
|
| 50,000 |
|
| Jan 23, 2018 |
|
| 50,000 |
|
|
| 4.00 |
|
| Feb 18, 2023 | |
2015 |
|
| 32,383 |
|
| Aug 3, 2021 |
|
| 32,383 |
|
|
| 3.40 |
|
| Feb 18, 2023 | |
2011 |
|
| 5,267 |
|
| Mar 20, 2013 |
|
| 5,267 |
|
|
| 4.35 |
|
| Mar 20, 2023 | |
2011 |
|
| 1,100 |
|
| Mar 20, 2013 |
|
| 1,100 |
|
|
| 4.35 |
|
| Mar 20, 2023 | |
2015 |
|
| 4,317 |
|
| Aug 3, 2021 |
|
| 4,317 |
|
|
| 3.40 |
|
| Jun 28, 2023 | |
2011 |
|
| 550 |
|
| Sep 2, 2013 |
|
| 550 |
|
|
| 3.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 550 |
|
| Sep 2, 2013 |
|
| 550 |
|
|
| 4.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 550 |
|
| Sep 2, 2013 |
|
| 550 |
|
|
| 4.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 2,167 |
|
| Sep 2, 2013 |
|
| 2,167 |
|
|
| 3.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 2,167 |
|
| Sep 2, 2013 |
|
| 2,167 |
|
|
| 4.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 2,167 |
|
| Sep 2, 2013 |
|
| 2,167 |
|
|
| 4.35 |
|
| Sep 2, 2023 | |
2015 |
|
| 4,318 |
|
| Aug 3, 2021 |
|
| 4,318 |
|
|
| 3.40 |
|
| Sep 28, 2023 | |
|
|
| 285,536 |
|
|
|
|
| 285,536 |
|
|
|
|
|
|
| |
| F-54 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 7 - Common Stock (continued)
RSU Settlements
2023
During the year ended December 31, 2023 we issued a total of broker’s commissions and fees) of $16,547 under the 2018 Equity Distribution Agreement through the sale of 3,200658,102 shares of its common stock.stock from the settlement of RSUs, as follows:
Equity Incentive Plan |
| RSUs # |
|
| Vest Date |
| Shares Issued |
|
| Shares Withheld for Taxes |
| |||
2015 |
|
| 4,000 |
|
| Feb 8, 2023 |
|
| 2,369 |
|
|
| 1,631 |
|
2015 |
|
| 15,000 |
|
| Mar 1, 2023 |
|
| 9,609 |
|
|
| 5,391 |
|
2015 |
|
| 15,000 |
|
| Mar 25, 2023 |
|
| 15,000 |
|
|
| - |
|
2015 |
|
| 2,500 |
|
| Apr 4, 2023 |
|
| 1,759 |
|
|
| 741 |
|
2015 |
|
| 13,500 |
|
| Apr 4, 2023 |
|
| 7,995 |
|
|
| 5,505 |
|
2015 |
|
| 35,000 |
|
| Apr 4, 2023 |
|
| 22,610 |
|
|
| 12,390 |
|
2015 |
|
| 50,000 |
|
| May 1, 2023 |
|
| 35,707 |
|
|
| 14,293 |
|
2015 |
|
| 4,000 |
|
| Jun 1, 2023 |
|
| 2,270 |
|
|
| 1,730 |
|
2015 |
|
| 7,500 |
|
| Jun 1, 2023 |
|
| 4,257 |
|
|
| 3,243 |
|
2015 |
|
| 208,809 |
|
| Aug 3, 2023 |
|
| 167,809 |
|
|
| 41,000 |
|
2015 |
|
| 34,102 |
|
| Aug 15, 2023 |
|
| 23,764 |
|
|
| 10,338 |
|
2015 |
|
| 12,000 |
|
| Sep 7, 2023 |
|
| 7,046 |
|
|
| 4,954 |
|
2015 |
|
| 12,500 |
|
| Sep 21, 2023 |
|
| 7,434 |
|
|
| 5,066 |
|
2015 |
|
| 357,346 |
|
| Oct 4, 2023 |
|
| 298,738 |
|
|
| 58,608 |
|
2015 |
|
| 19,904 |
|
| Oct 4, 2023 |
|
| 6,883 |
|
|
| 13,021 |
|
2015 |
|
| 21,583 |
|
| Oct 13, 2023 |
|
| 21,583 |
|
|
| - |
|
2015 |
|
| 21,750 |
|
| Nov 1, 2023 |
|
| 21,750 |
|
|
| - |
|
2015 |
|
| 334 |
|
| Nov 29, 2023 |
|
| 334 |
|
|
| - |
|
2015 |
|
| 2,000 |
|
| Dec 15, 2023 |
|
| 1,185 |
|
|
| 815 |
|
|
|
| 836,828 |
|
|
|
|
| 658,102 |
|
|
| 178,726 |
|
| F-55 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 7 - Common Stock (continued)
Note 8 – Stock-Based CompensationRSU Settlements (continued)
a)2022
During the year ended December 31, 2022 we issued a total of 297,289 shares of common stock from the settlement of RSUs, as follows:
Equity Incentive Plan |
| RSUs # |
|
| Vest Date |
| Shares Issued |
|
| Shares Withheld for Taxes |
| |||
2015 |
|
| 15,000 |
|
| Mar 25, 2022 |
|
| 15,000 |
|
|
| - |
|
2015 |
|
| 26,250 |
|
| Apr 13, 2022 |
|
| 21,712 |
|
|
| 4,538 |
|
2015 |
|
| 50,000 |
|
| May 1, 2022 |
|
| 35,000 |
|
|
| 15,000 |
|
2015 |
|
| 230,102 |
|
| Aug 3, 2022 |
|
| 191,992 |
|
|
| 38,110 |
|
2015 |
|
| 12,000 |
|
| Sep 7, 2022 |
|
| 7,038 |
|
|
| 4,962 |
|
2015 |
|
| 19,905 |
|
| Oct 4, 2022 |
|
| 13,022 |
|
|
| 6,883 |
|
2015 |
|
| 21,750 |
|
| Nov 1, 2022 |
|
| 12,344 |
|
|
| 9,406 |
|
2015 |
|
| 2,000 |
|
| Dec 15, 2022 |
|
| 1,181 |
|
|
| 819 |
|
|
|
| 377,007 |
|
|
|
|
| 297,289 |
|
|
| 79,718 |
|
| F-56 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 - Stock-based Compensation
a) Warrants
The following table summarizes the changes in warrants outstanding of the Company during the yearsyear ended December 31, 20202023 and December 31, 2019:2022:
|
|
| Weighted Average |
| Number of |
| Exercise Price |
| Warrants |
| ($) |
Outstanding at December 31, 2018 | 6,107,617 |
| 2.88 |
Granted | - |
| - |
Exercised | (5,783,867) |
| 2.86 |
Expired | (133,750) |
| 2.20 |
Outstanding at December 31, 2019 | 190,000 |
| 2.90 |
Granted | 50,000 |
| 3.45 |
Exercised | (25,000) |
| 2.47 |
Expired | (40,000) |
| 4.53 |
Outstanding at December 31, 2020 | 175,000 |
| 2.75 |
|
|
|
|
Exercisable at December 31, 2020 | 125,000 |
| 2.47 |
|
|
|
| Weighted Average |
| |||
|
| Number of |
|
| Exercise Price |
| ||
|
| Warrants |
|
| $ |
| ||
Outstanding at December 31, 2021 |
|
| 485,000 |
|
|
| 3.88 |
|
Granted |
|
| 54,000 |
|
|
| 3.05 |
|
Outstanding at December 31, 2022 |
|
| 539,000 |
|
|
| 3.80 |
|
Granted |
|
| 448,500 |
|
|
| 2.00 |
|
Expired |
|
| (125,000 | ) |
|
| 2.47 |
|
Outstanding at December 31, 2023 |
|
| 862,500 |
|
|
| 3.05 |
|
|
|
|
|
|
|
|
|
|
Exercisable at December 31, 2023 |
|
| 835,500 |
|
|
| 3.05 |
|
Warrants Granted
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)2023
Note 8 – Stock-Based Compensation (continued)The Company issued warrants to purchase an aggregate of 448,500 shares of Company common stock to Freedom, at an exercise price of $2.00 per share.
a) Warrants (continued)The Company evaluated the warrants as either equity-classified or liability-classified instruments based on an assessment of the specific terms of the warrants and applicable authoritative guidance in ASC 480 and ASC 815-40. The Company determined the warrants issued in the Freedom offering failed the indexation guidance under ASC 815-40, specifically, the warrants provide for a Black-Scholes value calculation in the event of certain transactions (“Fundamental Transactions”), which includes a floor on volatility utilized in the value calculation at 100% or greater. The Company has determined that this provision introduces leverage to the holders of the warrants that could result in a value that would be greater than the settlement amount of a fixed-for-fixed option on the Company’s own equity shares. Accordingly, pursuant to ASC 815-40, the Company has classified the fair value of the warrants as a liability upon issuance and marked to market each reporting period in the Company’s consolidated statement of operations until their exercise or expiration.
20202022
Effective February 26, 2020, the vesting criteria of the remaining installment of a warrant originally granted March 20, 2013 to an officer of the Company, and previously amended, was deemed met pursuant to the approval of the Compensation Committee, resulting in the vesting of the Warrant as to 125,000 shares effective February 26, 2020, with an expiration date of February 26, 2023.
Effective March 1, 2020,April 4, 2022, the Company granted warrantsa warrant to purchase 50,00054,000 shares of common stock to a Company employee for services to the Company. These warrantsCompany and/or its subsidiaries. This warrant shall vest on September 1, 2021 (subjectin two equal installments at 12 months and 24 months from the grant date, subject to continued employment through such date)service and expire on March 1, 2026,April 4, 2028 and April 4, 2029, respectively, with an exercise price of $3.45$3.05 per share. The Company has calculated the estimated fair market value of these warrantsthis warrant at $86,771,$80,901, using the Black-Scholes model and the following assumptions: term 3.753.5 years, stock price $3.44,$2.95, exercise price $3.45, 69.03%$3.05, 71.07% volatility, 0.95% risk free2.53% risk-free rate, and no forfeiture rate.
| F-57 |
| Table of Contents |
2019
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 - Stock-based Compensation (continued)
a) Warrants (continued)
Warrant Expiration
2023
Effective March 5, 2019, the Company entered into an amendment to an outstanding warrant to purchase up to an aggregate of 5.0 million shares of our common stock, originally issued to Cotterford, a significant stockholder, in connection with an equity financing completed on or about August 10, 2018. The amendment temporarily reduced the exercise price of such warrant from $3.00 per share to $2.90 per share through the close of business on March 8, 2019. As a result of this amendment, $196,957 of financing costs were recorded in other expenses.
On March 8, 2019, Cotterford partially exercised its warrant and purchased 1,724,138 shares of our common stock at $2.90 per share resulting in gross proceeds to the Company of $5.0 million.
On May 3, 2019, Cotterford partially exercised its warrant and purchased 1,666,667 shares of our common stock at $3.00 per share resulting in gross proceeds of $5.0 million to the Company.
On July 1, 2019, the Company modified the performance criteria for certain vesting milestones onFebruary 26, 2023, a warrant agreement held by an officer of the Company and as a result the Company re-measured warrants held by the officer, to purchase 125,000 shares of common stock at an exercise price of $2.47 per share, resulting in $11,829 of additional warrant expense to be recorded over the vesting period. These warrants vest on achievement of certain business objectives and expire 3 years from the date of vesting.expired unexercised.
On July 24, 2019, Cotterford exercised the remainder of its warrant and purchased 1,609,195 shares of our common stock at $3.00 per share resulting in gross proceeds of $4.8 million to the Company.2022
During the year 2019, warrants to purchase an aggregate of 5,783,067 shares of our common stock were exercised (including the exercises by Cotterford referenced above) for gross cash proceeds to the Company of approximately of $16.6 million.
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Endedended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 8 – Stock-based Compensation (continued)
a) Warrants (continued)2022, no warrants expired unexercised.
Below is a table summarizing the warrants issued and outstanding as of December 31, 2020, which2023. The warrants outstanding have a weighted average exercise price of $2.47$3.05 per share and an aggregate weighted average remaining contractual life of 3.013.83 years. The warrants exercisable have a weighted average price of $3.05 per share.
|
|
|
|
|
| Weighted Average |
| Proceeds to |
|
|
|
| Exercise |
| Remaining | Company if | |
Number |
| Number |
| Price |
| Contractual | Exercised | |
Outstanding |
| Exercisable |
| ($) |
| Life (Years) |
| ($) |
125,000 |
| 125,000 |
| 2.47 |
| 2.15 |
| 308,750 |
50,000 |
| 0 |
| 3.45 |
| 5.17 |
| 172,500 |
175,000 |
| 125,000 |
|
|
|
|
| 481,250 |
|
|
|
|
|
|
| Weighted Average |
|
| Proceeds to |
| |||||||
|
|
|
|
| Exercise |
|
| Remaining |
|
| Company if |
| ||||||
Number |
|
| Number |
|
| Price |
|
| Contractual |
|
| Exercised |
| |||||
Outstanding |
|
| Exercisable |
|
| ($) |
|
| Life (Years) |
|
| $ |
| |||||
| 448,500 |
|
|
| 448,500 |
|
|
| 2.00 |
|
|
| 4.46 |
|
|
| 897,000 |
|
| 54,000 |
|
|
| 27,000 |
|
|
| 3.05 |
|
|
| 4.76 |
|
|
| 164,700 |
|
| 50,000 |
|
|
| 50,000 |
|
|
| 3.45 |
|
|
| 2.17 |
|
|
| 172,500 |
|
| 125,000 |
|
|
| 125,000 |
|
|
| 3.95 |
|
|
| 3.01 |
|
|
| 493,750 |
|
| 185,000 |
|
|
| 185,000 |
|
|
| 4.90 |
|
|
| 3.09 |
|
|
| 906,500 |
|
| 862,500 |
|
|
| 835,500 |
|
|
|
|
|
|
|
|
|
|
| 2,634,450 |
|
Stock-based compensation expense related to warrants of $68,541$30,574 and $8,506$84,102 was recorded for the years ended December 31, 2020,2023, and December 31, 2019,2022, respectively. Total remaining unrecognized compensation cost related to non-vested warrants is approximately $38,565$5,238 and is expected to be recognized over a period of 0.670.26 years. As of December 31, 2020,2023, the total intrinsic value of warrants was $199,500.$nil.
| F-58 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 - Stock-based Compensation (continued)
b) Options
The Company currently has options outstanding under both its 2011 Equity Incentive Plan (the “2011 Plan”) (for option issuances prior to 2016)2016,) and its 2015 Plan (for option issuances commencing in 2016). Effective as of January 1, 2016, no additional awards were or may be made under the 2011 Plan.
The 2015 Plan was adopted by the Board of Directors on August 18, 2015 and approved by the stockholders at an annual meeting held on October 30, 2015. On August 5, 2016, the Board of Directors adopted an amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such Plan by 750,000 shares to an aggregate maximum of 1,750,000 shares, which amendment was approved by the stockholders at an annual meeting held on October 7, 2016. On June 13, 2017, the Board of Directors adopted a subsequent amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such Plan by 750,000 shares to an aggregate maximum of 2,500,000 shares, which amendment was approved by the stockholders at an annual meeting held on September 8, 2017. On June 15, 2018, the Board of Directors adopted a subsequent amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such Plan by 750,000 shares to an aggregate maximum of 3,250,000 shares, which amendment was approved by the stockholders at an annual meeting held on September 7, 2018.
On March 27, 2019, the Board of Directors adopted a subsequent amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under thesuch Plan by 1,000,000 shares to an aggregate maximum of 4,250,000 shares, which amendment was approved by the stockholders at an annual meeting held on June 14, 2019. On March 31, 2021, the Board of Directors adopted a subsequent amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such Plan by 1,750,000 shares to an aggregate maximum of 6,000,000 shares, which amendment was approved by the stockholders at an annual meeting held on June 17, 2021.
On April 4, 2022, the Board of Directors adopted a subsequent amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such plan by 1,750,000 shares to an aggregate maximum of 7,750,000 shares, which amendment was approved by the stockholders at an annual meeting held on June 13, 2022.
On April 17, 2023, the Board of Directors adopted a subsequent amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such plan by 1,950,000 shares to an aggregate maximum of 9,700,000 shares, which amendment was approved by the stockholders at an annual meeting held on June 28, 2023.
The 2015 Plan permits the grant of incentive stock options, non-statutory stock options, restricted stock awards, stock bonus awards, stock appreciation rights, restricted stock units and performance awards. The primary purpose of the 2015 Plan is to enhance the Company’s ability to attract and retain the services of qualified employees, officers, directors, consultants and other service providers upon whose judgment, initiative and efforts the successful conduct and development of the Company’s business largely depends, and to provide additional incentives to such persons or entities to devote their utmost effort and skill to the advancement and betterment of the Company, by providing them an opportunity to participate in the ownership of the Company that is tied to the Company’s performance, thereby giving them an interest in the success and increased value of the Company. The 2015 Plan is administered by the Compensation Committee comprised solely of members of the Board of Directors or by the Board of Directors as a whole.
| F-59 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 -Stock-based Compensation (continued)
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 8 – Stock-based Compensation (continued)
The following table summarizes the changes in options outstanding of the Company during the years ended December 31, 20202023 and December 31, 2019:2022.
|
|
|
| Weighted Average |
|
| Number of |
| Exercise Price |
|
| Options |
| ($) |
Outstanding at December 31, 2018 |
| 3,498,801 |
| 4.00 |
Granted |
| 730,000 |
| 3.25 |
Exercised |
| (10,333) |
| 3.42 |
Expired/Cancelled |
| (49,167) |
| 3.31 |
Outstanding at December 31, 2019 |
| 4,169,301 |
| 3.88 |
Granted |
| 845,000 |
| 3.60 |
Exercised |
| (691,599) |
| 2.81 |
Expired/Cancelled |
| (44,083) |
| 4.21 |
Outstanding at December 31, 2020 |
| 4,278,619 |
| 4.00 |
|
|
|
|
|
Exercisable at December 31, 2020 |
| 3,448,619 |
| 4.10 |
|
|
|
| Weighted Average |
| |||
|
| Number of |
|
| Exercise Price |
| ||
|
| Options |
|
| $ |
| ||
Outstanding at December 31, 2021 |
|
| 5,027,518 |
|
|
| 3.87 |
|
Expired/Cancelled |
|
| (42,413 | ) |
|
| 3.43 |
|
Outstanding at December 31, 2022 |
|
| 4,985,105 |
|
|
| 3.87 |
|
Expired/Cancelled |
|
| (285,536 | ) |
|
| 3.89 |
|
Outstanding at December 31, 2023 |
|
| 4,699,569 |
|
|
| 3.87 |
|
|
|
|
|
|
|
|
|
|
Exercisable at December 31, 2023 |
|
| 4,699,569 |
|
|
| 3.87 |
|
20202023
Effective April 13, 2020,During the Company granted stockyear ended December 31, 2023, no options to purchase 835,000 shares of common stock to various Company personnel (including directors, executives, members of management and employees) in exchange for services providedwere granted.
| F-60 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 - Stock-based Compensation(continued)
b) Options (continued)
2023
During the Company. These options vest on April 13, 2021 and expire 5 years after the vesting date, with an exercise price of $3.60 per share. The Company has calculated the estimated fair market value of these options at $1,481,709, using the Black-Scholes model andyear ended December 31, 2023, the following assumptions: term 3.5 years, stock price $3.52, exercise price $3.60, 72.94% volatility, 0.54% risk free rate, and no forfeiture rate.table summarizes the options cancelled.
Equity Incentive Plan |
| Options (#) |
|
| Grant Date |
| Options Cancelled (#) |
|
| Grant Price ($) |
|
| Cancellation Date | ||||
2015 |
|
| 25,000 |
|
| Apr 15, 2016 |
|
| 25,000 |
|
|
| 4.00 |
|
| Feb 18, 2023 | |
2015 |
|
| 55,000 |
|
| Apr 13, 2020 |
|
| 55,000 |
|
|
| 3.60 |
|
| Feb 18, 2023 | |
2015 |
|
| 50,000 |
|
| Mar 30, 2017 |
|
| 50,000 |
|
|
| 5.00 |
|
| Feb 18, 2023 | |
2015 |
|
| 50,000 |
|
| Feb 11, 2019 |
|
| 50,000 |
|
|
| 3.25 |
|
| Feb 18, 2023 | |
2015 |
|
| 50,000 |
|
| Jan 23, 2018 |
|
| 50,000 |
|
|
| 4.00 |
|
| Feb 18, 2023 | |
2015 |
|
| 32,383 |
|
| Aug 3, 2021 |
|
| 32,383 |
|
|
| 3.40 |
|
| Feb 18, 2023 | |
2011 |
|
| 5,267 |
|
| Mar 20, 2013 |
|
| 5,267 |
|
|
| 4.35 |
|
| Mar 20, 2023 | |
2011 |
|
| 1,100 |
|
| Mar 20, 2013 |
|
| 1,100 |
|
|
| 4.35 |
|
| Mar 20, 2023 | |
2015 |
|
| 4,317 |
|
| Aug 3, 2021 |
|
| 4,317 |
|
|
| 3.40 |
|
| Jun 28, 2023 | |
2011 |
|
| 550 |
|
| Sep 2, 2013 |
|
| 550 |
|
|
| 3.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 550 |
|
| Sep 2, 2013 |
|
| 550 |
|
|
| 4.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 550 |
|
| Sep 2, 2013 |
|
| 550 |
|
|
| 4.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 2,167 |
|
| Sep 2, 2013 |
|
| 2,167 |
|
|
| 3.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 2,167 |
|
| Sep 2, 2013 |
|
| 2,167 |
|
|
| 4.35 |
|
| Sep 2, 2023 | |
2011 |
|
| 2,167 |
|
| Sep 2, 2013 |
|
| 2,167 |
|
|
| 4.35 |
|
| Sep 2, 2023 | |
2015 |
|
| 4,318 |
|
| Aug 3, 2021 |
|
| 4,318 |
|
|
| 3.40 |
|
| Sep 28, 2023 | |
|
|
| 285,536 |
|
|
|
|
| 285,536 |
|
|
|
|
|
|
| |
2022
During the year ended December 31, 2022, the following table summarizes the options cancelled.
Equity Incentive Plan |
| Options (#) |
|
| Grant Date |
| Options Cancelled (#) |
|
| Grant Price ($) |
|
| Cancellation Date | ||||
2015 |
|
| 2,515 |
|
| Aug 3, 2021 |
|
| 2,515 |
|
|
| 3.40 |
|
| Aug 18, 2022 | |
2015 |
|
| 5,000 |
|
| Apr 13, 2020 |
|
| 5,000 |
|
|
| 3.60 |
|
| Nov 18, 2022 | |
2015 |
|
| 2,515 |
|
| Aug 3, 2021 |
|
| 2,515 |
|
|
| 3.40 |
|
| Nov 18, 2022 | |
2015 |
|
| 32,383 |
|
| Aug 3, 2021 |
|
| 32,383 |
|
|
| 3.40 |
|
| Nov 18, 2022 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
| 42,413 |
|
|
|
|
| 42,413 |
|
|
|
|
|
|
| |
| F-61 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 - Stock-based Compensation(continued)
Effective December 1, 2020, the Company granted stock options to purchase 10,000 shares of common stock to a Company employee for services to the Company. These options vest on December 1, 2021 and expire 5 years after the vesting date, with an exercise price of $3.40 per share. The Company has calculated the estimated fair market value of these options at $16,315 using the Black-Scholes model and the following assumptions: term 3.5 years, stock price $3.30, exercise price $3.40, 71.60% volatility, 0.55% risk free rate, and no forfeiture rate.
2019
Effective February 11, 2019, the Company granted stock options to purchase 730,000 shares of our common stock to various Company personnel (including directors, executives, members of management and employees) for services to the Company. These options vested on February 11, 2020 and expire 5 years after the vesting date, with an exercise price of $3.25 per share. The Company has calculated the estimated fair market value of these options at $1,569,816, using the Black-Scholes model and the following assumptions: term 6 years, stock price $3.16, exercise price $3.25, 77.86% volatility, 2.52% risk free rate, and no forfeiture rate. Subsequent to the February 2019 grant, stock options to purchase 45,000 shares of common stock subject to the grant were forfeited.
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 8 – Stock-Based Compensation (continued)
b)b) Options (continued)
Below is a table summarizing the options issued and outstanding as of December 31, 2020,2023, all of which were issued pursuant to the 2011 Plan (for option issuances prior to 2016) or the 2015 Plan (for option issuances commencing in 2016)and which have a weighted average exercise price of $4.00$ 3.87 per share and an aggregate weighted average remaining contractual life of 2.98 years.4.23 years.
As of December 31, 2020, an aggregate of 251,867 shares of common stock remained available for future issuance under the 2015 Plan.
|
|
|
|
|
| Weighted Average |
| Proceeds to |
|
|
|
| Exercise |
| Remaining |
| Company if |
Number |
| Number |
| Price |
| Contractual Life |
| Exercised |
Outstanding |
| Exercisable |
| ($) |
| (Years) |
| ($) |
685,000 |
| 685,000 |
| 3.25 |
| 4.12 |
| 2,226,250 |
10,351 |
| 10,351 |
| 3.35 |
| 0.33 |
| 34,676 |
10,000 |
| - |
| 3.40 |
| 5.92 |
| 34,000 |
820,000 |
| - |
| 3.60 |
| 5.28 |
| 2,952,000 |
20,000 |
| 20,000 |
| 3.80 |
| 0.38 |
| 76,000 |
1,782,837 |
| 1,782,837 |
| 4.00 |
| 1.81 |
| 7,131,348 |
15,268 |
| 15,268 |
| 4.35 |
| 1.15 |
| 66,416 |
89,163 |
| 89,163 |
| 4.38 |
| 3.06 |
| 390,534 |
50,000 |
| 50,000 |
| 4.80 |
| 2.00 |
| 240,000 |
796,000 |
| 796,000 |
| 5.00 |
| 2.24 |
| 3,980,000 |
4,278,619 |
| 3,448,619 |
|
|
|
|
| 17,131,224 |
|
|
|
|
|
|
| Weighted Average |
|
| Proceeds to |
| |||||||
|
|
|
|
| Exercise |
|
| Remaining |
|
| Company if |
| ||||||
Number |
|
| Number |
|
| Price |
|
| Contractual Life |
|
| Exercised |
| |||||
Outstanding |
|
| Exercisable |
|
| ($) |
|
| (Years) |
|
| $ |
| |||||
| 585,000 |
|
|
| 585,000 |
|
|
| 3.25 |
|
|
| 1.12 |
|
|
| 1,901,250 |
|
| 981,569 |
|
|
| 981,569 |
|
|
| 3.40 |
|
|
| 7.60 |
|
|
| 3,337,335 |
|
| 740,000 |
|
|
| 740,000 |
|
|
| 3.60 |
|
|
| 6.36 |
|
|
| 2,664,000 |
|
| 1,607,837 |
|
|
| 1,607,837 |
|
|
| 4.00 |
|
|
| 2.73 |
|
|
| 6,431,348 |
|
| 89,163 |
|
|
| 89,163 |
|
|
| 4.38 |
|
|
| 4.07 |
|
|
| 390,534 |
|
| 50,000 |
|
|
| 50,000 |
|
|
| 4.80 |
|
|
| 3.01 |
|
|
| 240,000 |
|
| 646,000 |
|
|
| 646,000 |
|
|
| 5.00 |
|
|
| 3.24 |
|
|
| 3,230,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4,699,569 |
|
|
| 4,699,569 |
|
|
|
|
|
|
|
|
|
|
| 18,194,467 |
|
Stock-based compensation expense related to stock options of $1,220,165$287,363 and $1,458,607 were$1,127,502 was recorded for the yearsyear ended December 31, 20202023 and December 31, 20192022 respectively. Total remaining unrecognized compensation cost related to non-vested stock options is approximately $416,706$nil and is expected to be recognized over a period of 0.92nil years. As of December 31, 2020,2023, the total intrinsic value of stock options was $688,489.$nil.
c)As of December 31, 2023, an aggregate of 608,190 shares of common stock remained available for future issuance under the 2015 Plan.
| F-62 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 -Stock-based Compensation(continued)
c) Restricted Stock Units (RSUs)
Below is a table summarizing the RSUs issued and outstanding as of December 31, 2020,2023, all of which were issued pursuant to the 2015 Stock Incentive Plan.
|
|
|
| Weighted Average |
| |||
|
| Number of |
|
| Exercise Price |
| ||
|
| RSUs |
|
| $ |
| ||
Outstanding at December 31, 2021 |
|
| 810,750 |
|
|
| 3.33 |
|
Granted |
|
| 1,892,102 |
|
|
| 1.64 |
|
Vested |
|
| (377,007 | ) |
|
| 3.33 |
|
Cancelled |
|
| (62,937 | ) |
|
| 2.88 |
|
Outstanding at December 31, 2022 |
|
| 2,262,908 |
|
|
| 2.05 |
|
Granted |
|
| 2,317,882 |
|
|
| 0.79 |
|
Vested |
|
| (836,828 | ) |
|
| 2.35 |
|
Cancelled |
|
| (109,010 | ) |
|
| 1.77 |
|
Outstanding at December 31, 2023 |
|
| 3,634,952 |
|
|
| 1.01 |
|
Effective April 13, 2020,
2023
Below is a table summarizing the CompanyRSUs granted RSUsduring the year ended December 31, 2023, all of 52,500 shares of common stock to various Company personnel (including a director and an employee) in exchange for services providedwhich were issued pursuant to the Company.2015 Plan. These RSUs vest equally over 2 years, with 50% vestingperiods stated on each of April 13, 2021 and April 13, 2022the dates noted, subject to continued service, and will result in totalthe compensation expense of $184,800.stated. The exception to this is specified in note (iv) which is described in detail below.
|
| Equity |
|
|
|
|
| First |
| Second |
|
| Third |
|
| RSU |
| |||||||
|
| Incentive |
| RSUs # |
|
| Grant |
| Vesting |
| Vesting |
| Vesting |
|
| Vesting |
|
| Expense |
| ||||
Note |
| Plan |
|
|
| Date |
| Period |
| Date |
| Date |
|
| Date |
|
| $ |
| |||||
|
| 2015 |
|
| 57,000 |
|
| Mar 27, 2023 |
| 36 Months |
| Mar 27, 2024 |
| Mar 27, 2025 |
|
| Mar 27, 2026 |
|
|
| 98,040 |
| ||
|
| 2015 |
|
| 50,000 |
|
| Mar 27, 2023 |
| 24 Months |
| Mar 27, 2024 |
| Mar 27, 2025 |
|
|
| N/A |
|
|
| 86,000 |
| |
|
| 2015 |
|
| 5,325 |
|
| Mar 27, 2023 |
| 12 Months |
| Mar 27, 2024 |
| N/A |
|
|
| N/A |
|
|
| 9,159 |
| |
|
| 2015 |
|
| 47,000 |
|
| Jun 15, 2023 |
| 36 Months |
| Jun 15, 2024 |
| Jun 15, 2025 |
|
| Jun 15, 2026 |
|
|
| 74,260 |
| ||
|
| 2015 |
|
| 8,392 |
|
| Jun 15, 2023 |
| 12 Months |
| Jun 15, 2024 |
|
| N/A |
|
|
| N/A |
|
|
| 13,260 |
|
|
| 2015 |
|
| 43,165 |
|
| Jul 13, 2023 |
| 6 Months |
| Oct 13, 2023 |
| Jan 13, 2024 |
|
|
| N/A |
|
|
| 56,978 |
| |
|
| 2015 |
|
| 14,000 |
|
| Jul 13, 2023 |
| 36 Months |
| Jul 13, 2024 |
| Jul 13, 2025 |
|
| Jul 13, 2026 |
|
|
| 18,479 |
| ||
|
| 2015 |
|
| 34,000 |
|
| Sep 11, 2023 |
| 36 Months |
| Sep 11, 2024 |
| Sep 11, 2025 |
|
| Sep 11, 2026 |
|
|
| 44,540 |
| ||
|
| 2015 |
|
| 1,569,000 |
|
| Sep 28, 2023 |
| 36 Months |
| Sep 28, 2024 |
| Sep 28, 2025 |
|
| Sep 28, 2026 |
|
|
| 1,098,300 |
| ||
(i) |
| 2015 |
|
| 450,000 |
|
| Oct 19, 2023 |
| Up to 42 Months |
| Variable |
| Variable |
|
| Variable |
|
|
| 306,000 |
| ||
|
| 2015 |
|
| 40,000 |
|
| Dec 11, 2023 |
| 36 Months |
| Dec 11, 2024 |
| Dec 11, 2025 |
|
| Dec 11, 2026 |
|
|
| 23,200 |
| ||
|
|
|
|
| 2,317,882 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1,828,216 |
|
| F-63 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 - Stock-based Compensation (continued)
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 8 – Stock-Based Compensation (continued)
c)c) Restricted Stock Units (RSUs) (continued)
(i) | These RSUs were granted by the Compensation Committee of the Board of Directors in September 2023, with an effective date of October 19, 2023 and vest upon the share price closing above $5.00 per share for a minimum of thirty consecutive trading days within a period of three years from the date of grant, with further time-based vesting in a single installment six months after the timely achievement of the target, if at all, and subject to continued service. The estimated fair value of the RSUs that include a market vesting condition will be measured on the grant date using a Monte Carlo Simulation of a Geometric Brownian Motion stock path model and incorporating the probability of vesting occurring. The estimated fair value of these awards will be recognized over the derived service period (as determined by the valuation model), with such recognition occurring regardless of whether the market condition is met. |
2022
EffectiveBelow is a table summarizing the RSUs granted during the year ended December 1, 2020, the Company granted RSUs31, 2022, all of 15,000 shares of common stock to a non-executive director of the Company in exchange for services providedwhich were issued pursuant to the Company.2015 Plan. These RSUs vest equally over 2 years, with 50% vestingperiods stated on each of December 1, 2021 and December 1, 2022the dates noted, subject to continued service, and will result in totalthe compensation expense of $49,500.stated. The exception to this is specified in note (iv) which is described in detail below.
|
|
| Weighted Average |
| Number of |
| Exercise Price |
| RSUs |
| ($) |
Outstanding at December 31, 2019 | - |
| - |
Granted | 67,500 |
| 3.47 |
Vested | - |
| - |
Cancelled | - |
| - |
Outstanding at December 31, 2020 | 67,500 |
| 3.47 |
|
| Equity |
|
|
|
|
| First |
| Second |
| Third |
|
| RSU |
| ||||||
|
| Incentive |
| RSUs |
|
| Grant |
| Vesting |
| Vesting |
| Vesting |
| Vesting |
|
| Expense |
| |||
Note |
| Plan |
| # |
|
| Date |
| Period |
| Date |
| Date |
| Date |
|
| $ |
| |||
|
| 2015 |
|
| 8,000 |
|
| Feb 8, 2022 |
| 24 Months |
| Feb 8, 2023 |
| Feb 8, 2024 |
|
| N/A |
|
|
| 22,640 |
|
|
| 2015 |
|
| 30,000 |
|
| Mar 1, 2022 |
| 24 Months |
| Mar 1, 2023 |
| Mar 1, 2024 |
|
| N/A |
|
|
| 84,300 |
|
|
| 2015 |
|
| 32,000 |
|
| Apr 4, 2022 |
| 24 Months |
| Apr 4, 2023 |
| Apr 4, 2024 |
|
| N/A |
|
|
| 94,400 |
|
|
| 2015 |
|
| 104,000 |
|
| Apr 4, 2022 |
| 36 Months |
| Apr 4, 2023 |
| Apr 4, 2024 |
| Apr 4, 2025 |
|
|
| 306,800 |
| |
|
| 2015 |
|
| 33,000 |
|
| Jun 1, 2022 |
| 24 Months |
| Jun 1, 2023 |
| Jun 1, 2024 |
|
| N/A |
|
|
| 80,850 |
|
|
| 2015 |
|
| 63,102 |
|
| Aug 15, 2022 |
| 24 Months |
| Aug 15, 2023 |
| Aug 15, 2024 |
|
| N/A |
|
|
| 126,835 |
|
|
| 2015 |
|
| 25,000 |
|
| Sep 21, 2022 |
| 24 Months |
| Sep 21, 2023 |
| Sep 21, 2024 |
|
| N/A |
|
|
| 42,250 |
|
(ii) |
| 2015 |
|
| 1,144,000 |
|
| Oct 4, 2022 |
| 36 Months |
| Oct 4, 2023 |
| Oct 4, 2024 |
| Oct 4, 2025 |
|
|
| 1,670,240 |
| |
(iii) |
| 2015 |
|
| 450,000 |
|
| Oct 4, 2022 |
| Up to 42 Months |
| Variable |
| Variable |
| Variable |
|
|
| 321,078 |
| |
(iv) |
| 2015 |
|
| 3,000 |
|
| Nov 29, 2022 |
| 36 Months |
| Nov 29, 2023 |
| Nov 29, 2024 |
| Nov 29, 2025 |
|
|
| 6,450 |
| |
|
|
|
|
| 1,892,102 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2,755,843 |
|
(ii) | These RSUs vest upon the achievement of corporate goals focused around product development and commercialization with further time-based vesting, subject to continued service of the award recipient to the Company through the applicable vesting dates. On October 13, 2022, the Compensation Committee of the Board of Directors approved the satisfactory achievement of certain corporate goals previously established by the Compensation Committee, which resulted in the vesting of the rights with respect to an aggregate of 198,275 RSUs. The RSUs are further subject to a three-year time-based vesting schedule, vesting in three equal installments on the dates set forth in the table above, and conditioned upon the recipient’s continued service through the applicable vesting date. On January 12, 2023, the Compensation Committee of the Board of Directors approved the satisfactory achievement of certain additional corporate goals, which resulted in the vesting of the rights with respect to an aggregate of an additional 424,875 RSUs, subject to the foregoing time-based vesting and conditioned upon the recipient’s continued service through the applicable vesting date. |
| F-64 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 - Stock-based Compensation (continued)
c) Restricted Stock Units (RSUs) (continued)
(iii) | These RSUs vest upon the share price closing above $5.00 per share for a minimum of ten consecutive trading days within a period of three years from the date of grant, with further time-based vesting in a single installment six months after the timely achievement of the target, if at all, and subject to continued service. The estimated fair value of the RSUs that include a market vesting condition will be measured on the grant date using a Monte Carlo Simulation of a Geometric Brownian Motion stock path model and incorporating the probability of vesting occurring. The estimated fair value of these awards will be recognized over the derived service period (as determined by the valuation model), with such recognition occurring regardless of whether the market condition is met. |
(iv) | The Company granted an aggregate of 3,000 RSUs on November 29, 2022 as an employment inducement award. These RSUs are subject to time-based vesting and subject to the continued service of each recipient. |
Below is a table summarizing the RSUs vested during the year ended December 31, 2023, all of which were issued pursuant to the 2015 Plan.
Equity Incentive Plan |
| RSUs # |
|
| Vest Date |
| Shares Issued |
|
| Shares Withheld for Taxes |
| |||
2015 |
|
| 4,000 |
|
| Feb 8, 2023 |
|
| 2,369 |
|
|
| 1,631 |
|
2015 |
|
| 15,000 |
|
| Mar 1, 2023 |
|
| 9,609 |
|
|
| 5,391 |
|
2015 |
|
| 15,000 |
|
| Mar 25, 2023 |
|
| 15,000 |
|
|
| - |
|
2015 |
|
| 2,500 |
|
| Apr 4, 2023 |
|
| 1,759 |
|
|
| 741 |
|
2015 |
|
| 13,500 |
|
| Apr 4, 2023 |
|
| 7,995 |
|
|
| 5,505 |
|
2015 |
|
| 35,000 |
|
| Apr 4, 2023 |
|
| 22,610 |
|
|
| 12,390 |
|
2015 |
|
| 50,000 |
|
| May 1, 2023 |
|
| 35,707 |
|
|
| 14,293 |
|
2015 |
|
| 4,000 |
|
| Jun 1, 2023 |
|
| 2,270 |
|
|
| 1,730 |
|
2015 |
|
| 7,500 |
|
| Jun 1, 2023 |
|
| 4,257 |
|
|
| 3,243 |
|
2015 |
|
| 208,809 |
|
| Aug 3, 2023 |
|
| 167,809 |
|
|
| 41,000 |
|
2015 |
|
| 34,102 |
|
| Aug 15, 2023 |
|
| 23,764 |
|
|
| 10,338 |
|
2015 |
|
| 12,000 |
|
| Sep 7, 2023 |
|
| 7,046 |
|
|
| 4,954 |
|
2015 |
|
| 12,500 |
|
| Sep 21, 2023 |
|
| 7,434 |
|
|
| 5,066 |
|
2015 |
|
| 357,346 |
|
| Oct 4, 2023 |
|
| 298,738 |
|
|
| 58,608 |
|
2015 |
|
| 19,904 |
|
| Oct 4, 2023 |
|
| 6,883 |
|
|
| 13,021 |
|
2015 |
|
| 21,583 |
|
| Oct 13, 2023 |
|
| 21,583 |
|
|
| - |
|
2015 |
|
| 21,750 |
|
| Nov 1, 2023 |
|
| 21,750 |
|
|
| - |
|
2015 |
|
| 334 |
|
| Nov 29, 2023 |
|
| 334 |
|
|
| - |
|
2015 |
|
| 2,000 |
|
| Dec 15, 2023 |
|
| 1,185 |
|
|
| 815 |
|
|
|
| 836,828 |
|
|
|
|
| 658,102 |
|
|
| 178,726 |
|
| F-65 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 - Stock-based Compensation (continued)
c) Restricted Stock Units (RSUs) (continued)
Below is a table summarizing the RSUs vested during the year ended December 31, 2022, all of which were issued pursuant to the 2015 Plan.
Equity Incentive Plan |
| RSUs # |
|
| Vest Date |
| Shares Issued |
|
| Shares Withheld for Taxes |
| |||
2015 |
|
| 15,000 |
|
| Mar 25, 2022 |
|
| 15,000 |
|
|
| - |
|
2015 |
|
| 26,250 |
|
| Apr 13, 2022 |
|
| 21,712 |
|
|
| 4,538 |
|
2015 |
|
| 50,000 |
|
| May 1, 2022 |
|
| 35,000 |
|
|
| 15,000 |
|
2015 |
|
| 230,102 |
|
| Aug 3, 2022 |
|
| 191,992 |
|
|
| 38,110 |
|
2015 |
|
| 12,000 |
|
| Sep 7, 2022 |
|
| 7,038 |
|
|
| 4,962 |
|
2015 |
|
| 19,905 |
|
| Oct 4, 2022 |
|
| 13,022 |
|
|
| 6,883 |
|
2015 |
|
| 21,750 |
|
| Nov 1, 2022 |
|
| 12,344 |
|
|
| 9,406 |
|
2015 |
|
| 2,000 |
|
| Dec 15, 2022 |
|
| 1,181 |
|
|
| 819 |
|
|
|
| 377,007 |
|
|
|
|
| 297,289 |
|
|
| 79,718 |
|
Below is a table summarizing the RSUs cancelled during the year ended December 31, 2023, all of which were originally issued pursuant to the 2015 Plan.
Equity Incentive Plan |
| RSUs # |
|
| Cancellation Date |
| RSUs Cancelled |
| ||
2015 |
|
| 23,000 |
|
| Apr 30, 2023 |
|
| 23,000 |
|
2015 |
|
| 21,000 |
|
| May 5, 2023 |
|
| 21,000 |
|
2015 |
|
| 2,000 |
|
| Jun 15, 2023 |
|
| 2,000 |
|
2015 |
|
| 17,343 |
|
| Jun 28, 2023 |
|
| 17,343 |
|
2015 |
|
| 14,000 |
|
| Jul 28, 2023 |
|
| 14,000 |
|
2015 |
|
| 10,000 |
|
| Sep 22, 2023 |
|
| 10,000 |
|
2015 |
|
| 2,667 |
|
| Oct 4, 2024 |
|
| 2,667 |
|
2015 |
|
| 19,000 |
|
| Oct 20, 2024 |
|
| 19,000 |
|
|
|
| 109,010 |
|
|
|
|
| 109,010 |
|
Below is a table summarizing the RSUs cancelled during the year ended December 31, 2022, all of which were originally issued pursuant to the 2015 Plan.
Equity Incentive Plan |
| RSUs # |
|
| Cancellation Date |
| RSUs Cancelled |
| ||
2015 |
|
| 33,000 |
|
| May 31, 2022 |
|
| 33,000 |
|
2015 |
|
| 1,365 |
|
| Aug 18, 2022 |
|
| 1,365 |
|
2015 |
|
| 17,572 |
|
| Nov 18, 2022 |
|
| 17,572 |
|
2015 |
|
| 11,000 |
|
| Nov 21, 2022 |
|
| 11,000 |
|
|
|
| 62,937 |
|
|
|
|
| 62,937 |
|
| F-66 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 8 - Stock-based Compensation (continued)
c) Restricted Stock Units (RSUs) (continued)
Below is a table summarizing the RSUs issued and outstanding as of December 31, 2020 and2023 of which the last to vest have an aggregate weighted averagea remaining contractual life of 0.922.87 years.
|
|
|
| Weighted Average |
|
|
|
| Remaining |
Number |
| Share Price |
| Contractual Life |
Outstanding |
| ($) |
| (Years) |
15,000 |
| 3.30 |
| 1.42 |
52,500 |
| 3.52 |
| 0.78 |
67,500 |
|
|
| 0.92 |
|
|
| Weighted Average |
|
| Weighted Average |
| |||
|
|
| Grant date |
|
| Remaining |
| |||
Number |
|
| Fair Value |
|
| Contractual Life |
| |||
Outstanding |
|
| $ |
|
| (Years) |
| |||
| 40,000 |
|
|
| 0.58 |
|
|
| 1.87 |
|
| 450,000 |
|
|
| 0.68 |
|
|
| 3.30 |
|
| 450,000 |
|
|
| 0.69 |
|
|
| 2.26 |
|
| 1,569,000 |
|
|
| 0.70 |
|
|
| 1.75 |
|
| 34,000 |
|
|
| 1.31 |
|
|
| 1.70 |
|
| 35,582 |
|
|
| 1.32 |
|
|
| 1.16 |
|
| 719,987 |
|
|
| 1.46 |
|
|
| 0.84 |
|
| 36,392 |
|
|
| 1.58 |
|
|
| 1.02 |
|
| 12,500 |
|
|
| 1.69 |
|
|
| 0.73 |
|
| 102,325 |
|
|
| 1.72 |
|
|
| 1.00 |
|
| 29,000 |
|
|
| 2.01 |
|
|
| 0.62 |
|
| 666 |
|
|
| 2.15 |
|
|
| 0.94 |
|
| 11,500 |
|
|
| 2.45 |
|
|
| 0.42 |
|
| 9,000 |
|
|
| 2.81 |
|
|
| 0.17 |
|
| 85,000 |
|
|
| 2.95 |
|
|
| 0.51 |
|
| 50,000 |
|
|
| 3.31 |
|
|
| 0.33 |
|
|
|
|
|
|
|
|
|
|
|
|
| 3,634,952 |
|
|
|
|
|
|
|
|
|
Stock-based compensation expense related to RSUs of $102,786$1,971,607 and $nil$1,903,054 was recorded in the yearyears ended December 31, 2020,2023, and December 31, 2019,2022, respectively. Total remaining unrecognized compensation cost related to non-vested RSUs is $131,514. As of December 31, 2020, the total intrinsic value of RSUs was $262,575.$1,925,613.
| F-67 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 9 - Income Taxes
The Company has estimated net operating losses for the years ended December 31, 20202023 and 20192022 of $24.0$33.1 million and $17.3$28.6 million, respectively, available to offset taxable income in future years.
The significant components of deferred income taxes and assets as of December 31, 20202023 and December 31, 20192022 are as follows:
Net Deferred Tax Liability |
| December 31, 2020 |
| December 31, 2019 |
$ | $ | |||
Excess of tax over book depreciation and amortization |
| (966) |
| (3,901) |
ROU Asset |
| (69,407) |
| (41,250) |
Lease Liability |
| 73,407 |
| 43,896 |
Prepaid expenses |
| - |
| - |
Allowance for doubtful accounts |
| - |
| - |
Accrued expenses |
| 1,154 |
| 1,154 |
Stock-based compensation |
| 21,533 |
| - |
Net Operating Losses carry-forward |
| 24,011,113 |
| 17,326,179 |
Research and development tax credits |
| 390,666 |
| 231,243 |
Gross deferred tax assets |
| 24,427,500 |
| 17,557,321 |
Valuation allowance |
| (24,427,500) |
| (17,557,321) |
Net deferred tax asset |
| - |
| - |
|
|
|
|
|
Change in Valuation Allowance |
| (6,870,179) |
|
|
|
|
|
|
|
Summary Rate Reconciliation |
| December 31, 2020 |
| December 31, 2019 |
% | % | |||
Federal statutory rate |
| 21.0 |
| 21.0 |
State income taxes, net of federal benefit |
| - |
| - |
Permanent Differences |
| 6.1 |
| 4.1 |
Stock based compensation |
| (1.3) |
| (2.4) |
Federal Research & Development Credits |
| 0.5 |
| 0.6 |
Foreign taxes |
| 7.4 |
| 6.7 |
Federal Deferred Rate Decrease |
| - |
| (0.2) |
Change in Valuation Allowance |
| (33.7) |
| (29.8) |
Total |
| - |
| - |
|
|
|
|
|
Disclosure Amounts |
| December 31, 2020 |
|
|
|
|
|
|
|
Net Operating Losses - United States |
| 21,963,567 |
|
|
Net Operating Losses - Foreign |
| 69,344,065 |
|
|
Credit Carryforward - United States |
| - |
|
|
Credit Carryforward - Foreign |
| 390,666 |
|
|
|
|
|
|
|
Increase in Valuation Allowance |
| 6,870,179 |
|
|
Net Deferred Tax Liability |
| December 31, 2023 |
|
| December 31, 2022 |
| ||
| $ |
| $ |
| ||||
Excess of tax over book depreciation and amortization |
|
| (71,391 | ) |
|
| (46,001 | ) |
ROU Asset |
|
| (108,326 | ) |
|
| (117,134 | ) |
Lease Liability |
|
| 113,834 |
|
|
| 122,279 |
|
Accrued expenses |
|
| 8,933 |
|
|
| 5,655 |
|
Capitalized research expenses |
|
| 2,723,982 |
|
|
| 1,237,122 |
|
Stock-based compensation |
|
| 322,177 |
|
|
| 321,956 |
|
Net Operating Losses carry-forward |
|
| 33,092,721 |
|
|
| 28,556,992 |
|
Research and development tax credits |
|
| 1,033,416 |
|
|
| 769,317 |
|
Gross deferred tax assets |
|
| 37,115,346 |
|
|
| 30,850,186 |
|
Valuation allowance |
|
| (37,115,346 | ) |
|
| (30,850,186 | ) |
|
|
|
|
|
|
|
|
|
Net deferred tax asset |
|
| - |
|
|
| - |
|
|
|
|
|
|
|
|
|
|
Change in Valuation Allowance |
|
| (6,265,160 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Summary Rate Reconciliation |
| December 31, 2023 |
|
| December 31, 2022 |
| ||
| % |
| % |
| ||||
Federal statutory rate |
|
| 21.0 |
|
|
| 21.0 |
|
Permanent Differences |
|
| (3.3 | ) |
|
| (0.6 | ) |
Stock-based compensation |
|
| (0.8 | ) |
|
| (0.3 | ) |
Federal Research & Development Credits |
|
| 0.7 |
|
|
| 0.7 |
|
Foreign taxes |
|
| (0.2 | ) |
|
| (0.1 | ) |
Federal Deferred Rate Decrease |
|
| - |
|
|
| 0.5 |
|
Change in Valuation Allowance |
|
| (17.5 | ) |
|
| (21.2 | ) |
Total |
|
| (0.1 | ) |
|
| - |
|
|
|
|
|
|
|
|
|
|
Disclosure Amounts |
| December 31, 2023 |
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Net Operating Losses - United States |
|
| 41,344,440 |
|
|
|
|
|
Net Operating Losses - Foreign |
|
| 103,744,933 |
|
|
|
|
|
Credit Carryforward - United States |
|
| - |
|
|
|
|
|
Credit Carryforward - Foreign |
|
| 1,033,416 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase in Valuation Allowance |
|
| 6,265,160 |
|
|
|
|
|
| F-68 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 10 - Commitments and Contingencies
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies
a) Finance Lease Obligations
In 2015, the Company entered into an equipment finance lease to purchase three Tecan machines (automated liquid handling robots) for €550,454, maturing May 2020. As of December 31, 2020, the balance payable was $nil.
In 2016, the Company entered into a real estate capital lease with ING Asset Finance Belgium S.A. (“ING”) to purchase a property located in Belgium for €1.12 million, maturing May 2031, with implicit interest of 2.62%. As of December 31, 2020,2023, the balance payable was $650,209.$448,592.
In 2018, the Company entered into a capital lease with BNP Paribas leasing solutions to purchase a freezer for the Belgium facility for €25,000, maturing January 2022, with implicit interest of 1.35%. The leased equipment is amortized on a straight-line basis over 5 years. As of December 31, 2020,2023, the balance payable was $11,688.
$0. The following is a schedule showing the future minimum lease payments under financing leases by years and the present value of the minimum payments as of December 31, 2020. 2023.
2021 | $ | 76,183 | ||||
2022 | $ | 67,308 | ||||
2023 | $ | 65,772 | ||||
2024 | $ | 65,770 |
| $ | 59,374 |
|
2025 | $ | 65,770 |
| $ | 59,374 |
|
2026 |
| $ | 59,376 |
| ||
2027 |
| $ | 59,375 |
| ||
2028 |
| $ | 59,375 |
| ||
Greater than 5 years | $ | 419,270 |
| $ | 200,376 |
|
Total | $ | 760,073 |
| $ | 497,250 |
|
Less: Amount representing interest | $ | (98,176) |
| $ | (48,658 | ) |
Present value of minimum lease payments | $ | 661,897 |
| $ | 448,592 |
|
b) Operating Lease Right-of-Use Liabilities
The Company adopted Topic 842 on January 1, 2019. The Company elected to adopt this standard using the optional modified retrospective transition method and recognized a cumulative-effect adjustment to the consolidated balance sheet on the date of adoption. Comparative periods have not been restated. With the adoption of Topic 842, the Company’s consolidated balance sheet now contains the following line items: Operating lease right-of-use assets, current portion of operating lease liabilities and operating lease liabilities, net of current portion.
As all the existing leases subject to the new lease standard were previously classified as operating leases by the Company, they were similarly classified as operating leases under the new standard. The Company has determined that the identified operating leases did not contain non-lease components and require no further allocation of the total lease cost. Additionally, the agreements in place did not contain information to determine the rate implicit in the leases, so we used our incremental borrowing rate as the discount rate. Our weighted average discount rate is 2.87% and the weighted average remaining lease term is 39 months.
As of December 31, 2020,2023, operating lease right-of-use assets and liabilities arising from operating leases were $326,085$549,504 and $331,452,$577,377, respectively. During the year ended December 31, 2020,2023, cash paid for amounts included for the measurement of lease liabilities was $230,627$259,098 and the Company recorded operating lease expense of $231,343.
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020$261,005. Our weighted average discount rate is 2.38% and 2019
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (continued)
b) Operating Lease Right-of-Use Liabilities (continued)the weighted average remaining lease term is 25 months.
The following is a schedule showing the future minimum lease payments under operating leases by years and the present value of the minimum payments as of December 31, 2020.2023.
2021 | $ | 187,848 | ||||
2022 | $ | 78,469 | ||||
2023 | $ | 53,231 | ||||
2024 | $ | 25,997 |
| $ | 223,398 |
|
2025 |
| $ | 170,676 |
| ||
2026 |
| $ | 143,176 |
| ||
2027 |
| $ | 70,784 |
| ||
2028 |
| $ | 5,258 |
| ||
Total Operating Lease Obligations | $ | 345,545 |
| $ | 613,292 |
|
Less: Amount representing interest | $ | (14,093) |
| $ | (35,915 | ) |
Present Value of minimum lease payments | $ | 331,452 |
| $ | 577,377 |
|
| F-69 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 10 – Commitments and Contingencies (continued)
b) Operating Lease Right-of-Use Liabilities (continued)
The Company’s office space leases are short term, and the Company has elected under the short-term recognition exemption not to recognize them on the balance sheet. During the year ended December 31, 2020, $30,1172023, $72,542 was recognized in short-term lease costs associated with the office space leaseleases in Singapore.Singapore and Nevada. The annual payments remaining for such short-term office leases as of December 31, 2023, were as follows:
2021 | $ | 21,722 | ||||
2024 |
| $ | 33,370 |
| ||
Total Operating Lease Liabilities | $ | 21,722 |
| $ | 33,370 |
|
c) Grants Repayable
In 2010, the Company entered into an agreement with the Walloon Region government in Belgium for a colorectal cancer research grant for €1.05 million.€1,048,020. Per the terms of the agreement, €314,406 of the grant is to be repaid by installments over the period from June 30, 2014 to June 30, 2023. The Company has recorded the balance of €733,614 to other income in previous years as there is no obligation to repay this amount. In the event that the Company receives revenue from products or services as defined in the agreement, it is due to pay a 6% royalty on such revenue to the Walloon Region. The maximum amount payable to the Walloon Region, in respect of the aggregate of the amount repayable of €314,406 and the 6% royalty on revenue, is twice the amount of funding received. As of December 31, 2020,2023, the grant balance repayable was $106,881.$27,597.
In 2018, the Company entered into an agreement with the Walloon Region government in Belgium for a colorectal cancer research grant for €605,000. Per the terms of the agreement, €181,500 of the grant is to be repaid by instalments over 12 years commencing in 2020. 2020. In the event that the Company receives revenue from products or services as defined in the agreement, it is due to pay a 3.53% royalty on such revenue to the Walloon Region. The maximum amount payable to the Walloon Region, in respect of the aggregate of the amount repayable of €181,500 and the 3.53% royalty on revenue, is equal to the amount of funding received. As of December 31, 2020,2023, the grant balance repayable was $221,940.$102,128.
In 2020, the Company entered into an agreement with the Walloon Region government in Belgium for a research grant for €495,000. Per the terms of the agreement, €148,500 of the grant is to be repaid by installments over 10 years commencing in 2023. In the event that the Company receives revenue from products or services as defined in the agreement, it is due to pay a 2.89% royalty on such revenue to the Walloon Region. The maximum amount payable to the Walloon Region, in respect of the aggregate of the amount repayable of €148,500 and the 2.89% royalty on revenue, is equal to the amount of funding received. As of December 31, 2023, the grant balance repayable was $94,600.
In 2020, the Company entered into an agreement with the Walloon Region government in Belgium for a research grant for €929,433. Per the terms of the agreement, €278,830 of the grant is to be repaid by instalments over 15 years commencing in 2022. In the event that the Company receives revenue from products or services as defined in the agreement, it is due to pay a 4.34% royalty on such revenue to the Walloon Region. The maximum amount payable to the Walloon Region, in respect of the aggregate of the amount repayable of €278,830 and the 4.34% royalty on revenue, is equal to the amount of funding received. As of December 31, 2023, the grant balance repayable was $254,237.
| F-70 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 10 – Commitments and Contingencies (continued)
c) Grants Repayable (continued)
As of December 31, 2020,2023, the balance repayable was $328,821$478,562 and the annual payments remaining were as follows:
2021 | $ | 69,218 |
2022 | $ | 51,480 |
2023 | $ | 52,764 |
2024 | $ | 22,194 |
2025 | $ | 29,592 |
Greater than 5 years | $ | 103,573 |
Total Grants Repayable | $ | 328,821 |
2024 |
| $ | 55,855 |
|
2025 |
| $ | 37,436 |
|
2026 |
| $ | 45,120 |
|
2027 |
| $ | 50,093 |
|
2028 |
| $ | 53,569 |
|
Greater than 5 years |
| $ | 236,489 |
|
Total Grants Repayable |
| $ | 478,562 |
|
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (continued)
d) Long-Term Debt
In 2016, the Company entered into a 7-year loan agreement with Namur Invest for €440,000 with a fixed interest rate of 4.85%, maturing December 2023. As of December 31, 2020,2023, the principal balance payable was $269,400.$0.
In 2016, the Company entered into a 15-year loan agreement with ING for €270,000 with a fixed interest rate of 2.62%, maturing December 2031. As of December 31, 2020,2023, the principal balance payable was $255,725.
In 2017, the Company entered into a 4-year loan agreement with Namur Invest for €350,000 with a fixed interest rate of 4.00%, maturing June 2021. As of December 31, 2020, the principal balance payable was $64,863.$175,055.
In 2017, the Company entered into a 7-year loan agreement with SOFINEX for up to €1 million with a fixed interest rate of 4.50%, maturing September 2024. As of December 31, 2020,2023, €1 million has been drawn down under this agreement and the principal balance payable was $1,039,390.
In 2018, the Company entered into a 4-year loan agreement with Namur Innovation and Growth for €500,000 with fixed interest rate of 4.00%, maturing June 2022. As of December 31, 2020, the principal balance payable was $272,524.$275,974.
In 2019, the Company entered into a 4-year loan agreement with Namur Innovation and Growth for €500,000 with fixed interest rate of 4.80%, maturing September 2024. As of December 31, 2020,2023, the principal balance payable was $611,406.$126,186.
On October 13,In 2020, the Company entered into a 10-year loan agreement with Namur Invest for a maximum of €830,000 with fixed interest rate of 4.00%, maturing March 2021.2031. As of December 31, 2020,2023, the amount that has been drawn down under this agreement was €764,547,€633,719, representing a principal balance payable of $934,896.$699,560.
On November 23, 2021, the Company entered into a 3 ½ year loan agreement with SOFINEX for a maximum of €450,000 with fixed interest rate of 5.00%, maturing June 2025. As of December 31, 2023, the amount that has been drawn down under this agreement was €450,000, representing a principal balance payable of $289,773.
On August 16, 2022, the Company entered into a 4-year loan agreement with Namur Invest for a maximum of €1,000,000 with fixed interest rate of 6.00%, maturing July 2026. As of December 31, 2023, the amount that has been drawn down under this agreement was €1,000,000, representing a principal balance payable of $836,745.
| F-71 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 10 – Commitments and Contingencies (continued)
d) Long-Term Debt (continued)
On November 18, 2022, the Company entered into a 4-year loan agreement with Namur Invest for a maximum of €500,000 with fixed interest rate of 5.45%, maturing December 2027. As of December 31, 2023, the amount that has been drawn down under this agreement was €500,000, representing a principal balance payable of $551,949.
In June 2023, the Company entered into a 4-year loan agreement with Namur Invest for a maximum of €400,000 with fixed interest rate of 7.00%, maturing June 2027. As of December 31, 2023, €200,000 had been drawn down under this agreement and the principal balance payable was $220,780.
On December 1, 2023, the Company entered into a 5-year loan agreement with Wallonie Entreprendre S.A. for a maximum of €2.5 million with fixed interest rate of 7.68%, maturing December 2028. As of December 31, 2023, €1,500,000 had been drawn down under this agreement and the principal balance payable was $1,655,845.
As of December 31, 2020,2023, the total balance for long-term debt payable was $3,448,204$4,831,867 and the payments remaining were as follows:
2021 | $ | 991,070 |
2022 | $ | 804,373 |
2023 | $ | 699,623 |
2024 | $ | 544,437 |
2025 | $ | 150,390 |
Greater than 5 years | $ | 729,808 |
Total | $ | 3,919,701 |
Less: Amount representing interest | $ | (471,497) |
Total Long-Term Debt | $ | 3,448,204 |
2024 |
| $ | 1,481,023 |
|
2025 |
| $ | 922,911 |
|
2026 |
| $ | 692,557 |
|
2027 |
| $ | 452,470 |
|
2028 |
| $ | 1,918,778 |
|
Greater than 5 years |
| $ | 323,802 |
|
Total |
| $ | 5,791,541 |
|
Less: Amount representing interest |
| $ | (959,674 | ) |
Total Long-Term Debt |
| $ | 4,831,867 |
|
| F-72 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
NoteNote 10 – Commitments and Contingencies (continued)
e) Collaborative Agreement Obligations
In 2015, the Company entered into a research sponsorship agreement with the German Cancer Research Center, in Germany for a 3-year period for €338,984. As of December 31, 2020, $nil is still to be paid by the Company under this agreement.
In 2016, the Company entered into a research co-operation agreement with DKFZ, in Germany for a 5-year period for €400,000. As of December 31, 2020, $244,562 is still to be paid by the Company under this agreement.
In 2017, the Company entered into a collaborative research agreement with Munich University, in Germany for a 3-year period for €360,000. As of December 31, 2020, $nil is still to be paid by the Company under this agreement.
In 2017, the Company entered into a clinical study research agreement with the University of Michigan for a 3-year period for up to $3 million. This agreement was amended in February 2020 to redefine a new clinical study. Pursuant to the terms of the amendment, the parties acknowledged that, although not fully completed, the requirements of the original clinical study had been satisfied, including any and all payment obligations by Volition America. Further, the Amendment provided that a new clinical study would be undertaken at no additional cost to Volition America. As of December 31, 2020, $nil is still to be paid by the Company under this agreement.
In 2018, the Company entered into a research collaboration agreement with the University of Taiwan for a 3-year period for a cost to the Company of up to $2.55 million payable over such period. As of December 31, 2020, $892,5002023, $510,000 is still to be paid by the Company under this agreement.
In 2019,2022, the Company entered into a sponsored research collaboration agreement with theThe University of TaiwanTexas MD Anderson Cancer Center to collect a totalevaluate the role of 1,200 samples for a 2-year periodneutrophil extracellular traps ("NETs") in cancer patients with sepsis for a cost to the Company of up to $320,000 payable over such period.$449,406. As of December 31, 2020, $96,0002023, $449,406 is still to be paid by the Company under this agreement.
In 2019,August 2023, the Company entered into a funded sponsoredproject research agreement with Guy’s and St Thomas’ NHS Foundation Trust to evaluate the Texas A&M University (“TAMU”)practical clinical utility of the Nu.Q® H3.1 nucleosome levels in consideration for the license grantedadult patients with sepsis to the Company for a 5-year periodfacilitate early diagnosis and prognostication for a cost to the Company of up to $400,000 payable over such period.£162,338. As of December 31, 2020, $329,9862023, $206,697 is still to be paid by the Company under this agreement and as of December 31, 2023, $41,339 is due by the Company under this agreement.
In 2019,July 2023, the Company entered into a lyophilization studyresearch agreement with Xenetic Biosciences Inc and CLS Therapeutics Ltd to evaluate the anti-tumoral effects of Nu.Q® CAR T cells for a CE marking project including GMP validation and documentation with Biomerica Inc. for $160,000.cost to the Company of $107,589. As of December 31, 2020, $nil2023, $107,589 is still to be paid by the Company under this agreement.
On September 16, 2020, the Company entered into a research agreement for the bioinformatic analysis of cell-free DNA fragments from whole-genome sequencing with the Hebrew University of Jerusalem for 6 months for a cost to the Company of €54,879. Asand as of December 31, 2020, $44,7382023, $26,142 is still to be paiddue by the Company under this agreement.
As of December 31, 2020,2023, the total amount to be paid for future research and collaboration commitments was approximately $1.6 million$ 1,273,692 and the annual payments remaining were as follows:
2021 | $ | 1,467,700 |
2022 | $ | 140,086 |
Total Collaborative Agreement Obligations | $ | 1,607,786 |
2024 |
| $ | 1,110,146 |
|
2025 - 2028 |
| $ | 163,546 |
|
Total Collaborative Agreement Obligations |
| $ | 1,273,692 |
|
| F-73 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
NoteNote 10 – Commitments and Contingencies (continued)
f) Other Commitments
Belgian Volition
On October 1, 2020, Belgian Volition entered into an agreement with Gaetan Michel to serve as Chief Executive Officer for an indefinite period, which employment may be terminated without compensation or notice on grounds of serious misconduct by either party. In exchange for his services, Mr. Michel shall receive, among other things (i) €10,000 per month, and (ii) the equivalent of one-half of his salary for the 12-month non-competition period following termination of the agreement, subject to adjustments.
Volition Vet
On November 4, 2020, the Company terminated a consulting services agreement with Novis Animal Solutions LLC to provide chief commercial officer services for Volition Vet. The termination was effective immediately and the compensation payable to Novis for the required two-month notice period and a general release of any claims was $19,000. As of December 31, 2020, Novis Animal Solutions LLC has no equity interest in Volition Vet.
On October 25, 2019, the Company entered into an agreement with TAMU for provision of in kindin-kind services of personnel, animal samples and laboratory equipment in exchange for a non-controlling interest of 7.5% in Volition Vet with an additional 5%, vesting in a year from the date of the agreement, giving TAMU in aggregate, a 12.5% equity interest as of such date. As of December 31, 2020,2023, TAMU has a 12.5 %12.5% equity interest in Volition Vet.
Volition Germany
On January 10, 2020, the Company, through its wholly-owned subsidiary Belgian Volition, acquired an epigenetic reagent company, Octamer GmbH (“Octamer”), based in Munich, Germany, and hired its founder for his expertise and knowledge to be passed to Company personnel. On March 9, 2020, Octamer was renamed to Volition Germany GmbH (or “Volition Germany”).
Upon considering the definition of a business, as defined in ASC 805 “Business Combinations,” paragraph 805-10-20, which is an integrated set of activities and assets that is capable of being conducted and managed for the purpose of providing a return, the Company has determined that this did not constitute a business. This is primarily due to the fact that additional inputs are needed in the form of training personnel further to produce outputs. Accordingly, the Company has treated this transaction as the hiring of a member of management, described below, rather than accounting for the transaction as a business combination.
The Company agreed to terms of the transaction on December 13, 2019 and closed on January 10, 2020. Pursuant to the transaction agreement, the Company purchased all outstanding shares of Octamer. In exchange, the Company agreed to issue 73,263 newly-issued restricted shares of Company common stock valued at $333,969 (based on the $4.56 per share volume weighted trading price for the five days prior to December 13, 2019), committed to pay approximately €350,000, subject to adjustments, and agreed to pay off certain Octamer expenses leading up to the agreement (representing net liabilities of $6,535). At closing, the Company issued 73,263 restricted shares of Company common stock, paid an adjusted amount of approximately $357,000 (€321,736) and recorded a holdback liability of $55,404 (€50,000) to be paid after the holdback period of 9 months following the closing (subject to offset for breaches of representations and warranties).
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020 and 2019
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (continued)
f) Other Commitments (continued)
Volition Germany (continued)
In connection with the transaction agreement, the Company also entered into a 2-year Managing Director’s agreement with the founder of Octamer to continue to manage Volition Germany for a payment of €288,000 payable in equal monthly installments over such 2-year period and a royalty agreement with the founder providing for the payment of royalties in the amount of 6% of net sales of Volition Germany’s nucleosomes as reagents to pharmaceutical companies for use in the development, manufacture and screening of molecules for use as therapeutic drugs for a period of 5five years post-closing.
The Company recorded approximately $753,000 in compensation expense as a result of cash paid, holdback liability, stock issued and assumption of expenses. As of December 31, 2020, $176,085 is still to be paid by the Company under the Managing Director’s agreement, $2392023, $216 is payable under the 6% royalty agreement on sales to date (towardstowards the Company’s aggregate minimum royalty obligation of $134,217), and $52,581 is still to be paid under the holdback liability. The Company has no further financial obligations under the transaction agreement. $121,429.
Volition America
On November 3, 2020, the Company entered into a professional services master agreement (the “Master Agreement”) with Diagnostic Oncology CRO, LLC (“DXOCRO”) to conduct a pivotal clinical trial and provide regulatory submission and reimbursement related services. UnderOn August 8, 2022, the termsCompany and DXOCRO amended and restated the Master Agreement to expand the scope of DXOCRO’s consultant services provided thereunder (the “A&R Master Agreement”). The A&R Master Agreement requires DXOCRO to support development and clinical validation studies for the Company’s Nu.Q® product portfolio in the United States, including by conducting large-scale finding studies across multiple sites in the U.S. using Nu.Q® NETs and Nu.Q® Cancer tests to determine clinical utility in sepsis and non-Hodgkin’s lymphoma. The Company anticipates DXOCRO’s services under this agreement will be completed by the end of the agreement Diagnostic Oncology CRO, LLC will provide ad hoc consulting assistance onfirst quarter 2024 at a project-by-project basis relatedtotal cost to the review and assessment of existing data and information to prepare recommended intended use claims and coverage/reimbursement plans to support the preparation of FDA pre-submissions, clinical trial protocol development and study administration, and potential 510k regulatory marketing submissions of the Company’s diagnostic tests, including those proposed for use as an adjunct diagnostic tool for common and aggressive forms of Non-Hodgkin’s Lymphoma. The initial projects contemplated by the agreement relating to Non-Hodgkin’s Lymphoma obligate the Company to pay in aggregate of up to $2.9 million over a period of 22 months. Such$4.2 million. The Company’s payment obligations are on a project-by-project basis as deliverables are executed and subject to certain terms and conditions. Additionally,accrue upon delivery of projects under the agreement. The Company may terminate the agreement or any project with or without causethereunder upon at least 30 days’ prior written notice. Unless earlier terminated, the termA&R Master Agreement terminates on the later of the agreement is until December 31, 2025 or such laterthe date as whenupon which all projectsservices have been completed. As of December 31, 2020, $nil2023, $90,862 is to be paidpayable under the A&R Master Agreement, and up $208,320 maybe payable by Company under this agreement.in future periods for services rendered. See Note 11 for additional details regarding the A&R Master Agreement subsequent to December 31, 2023.
Singapore Volition
On November 10, 2020, the Company entered into a consulting services agreement through a related party transaction between its wholly owned subsidiary, Singapore Volition and PB Commodities Pte Ltd (“PB Commodities”). This agreement is effective December 1, 2020 and provides for consultancy services to be rendered by Cameron Reynolds through PB Commodities to Singapore Volition. Singapore Volition will also make available the services of Mr. Reynolds, as Group Chief Executive Officer, to the Company and its subsidiaries, pursuant to services agreements entered into by and between Singapore Volition and the Company or its subsidiaries. The term of the agreement is perpetual, commencing on December 1, 2020 until terminated upon six months’ prior notice. The agreement includes a six-month non-compete following termination of the agreement. PB Commodities will receive a monthly fee of $35,650 in exchange for the services provided by Mr. Reynolds.
g) Legal Proceedings
There are no legal proceedings which the Company believes will have a material adverse effect on its financial position.
| F-74 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 10 – Commitments and Contingencies (continued)
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2020h) Commitments in Respect of Corporate Goals and 2019
($ expressed in United States Dollars)Performance-Based Awards
Note 11 - Subsequent EventsIn August 2021 and October 2021 the Compensation Committee of the Board of Directors approved the granting of equity-based awards under the 2015 Plan as well as cash bonuses, each of which vests upon achievement of certain corporate goals focused around product development and commercialization, to various personnel including directors, executives, members of management, consultants and employees of the Company and/or its subsidiaries.
Employment AgreementsOn June 23, 2022, the Compensation Committee of the Board of Directors approved the achievement of all of the remaining outstanding corporate goals related to the August 2021 and October 2021 awards, resulting in the payment of the cash bonus awards and the vesting of the remaining rights to the equity-based awards, which equity-based awards remain subject to time-based vesting in equal installments on each of August 3, 2022 and August 3, 2023 (with the exception of October 4, 2022 and October 4, 2023 for one award) and the continuous service of the award recipient through the applicable vesting date.
Group Chief Commercial Officer
Effective January 1, 2021, Gael Forterre entered into an employment agreement with Volition AmericaIn October 2022, the Compensation Committee of the Board of Directors approved the granting of RSUs under the 2015 Plan to serve as Group Chief Commercial Officer. Mr. Forterre’s employment agreement continues until terminated by either party providing not less than three months’ prior notice. In exchange for his services, Mr. Forterre shall receive, among other things (i) $15,000 per month; and (ii) a lump sum severance payment if terminated by Volition America without cause (as per the agreement) equal to the salary that he would have received between the date of termination and the completion of a three-month notice period. This employment agreement superseded and replaced in its entirety that certain employment agreement dated December 30, 2020 between Mr. Forterre and Volition America for services as Vice President of Sales of Volition America.
Effective January 1, 2021, the Company granted warrants to purchase 125,000 shares of common stock to Gael Forterrevarious employees in exchange for services provided to the Company as Group Chief Commercial Officer.Company. These warrantsRSUs vest on January 1, 2022upon the achievement of certain corporate goals focused around product development and expire 5commercialization with further time based vesting over three years, after the vesting date, with an exercise price of $3.95 per share.and subject to continued service.
Effective January 1, 2021,In October 2022, the Company grantedCompensation Committee of the Board of Directors approved the granting of RSUs of 5,000 shares of common stockunder the 2015 Plan to a Gael Forterrevarious employees in exchange for services provided to the Company as Group Chief Commercial Officer.Company. These RSUs vested immediately onvest upon the share price closing above $5.00 per share for a minimum of ten consecutive trading days within a period of three years from the date of grant, with further time-based vesting in a single installment six months after the timely achievement of the target, if at all, and subject to continued service.
In October 2022 the Compensation Committee of the Board of Directors approved the granting of cash bonuses, payable upon achievement of various corporate goals focused around product development, manufacturing, financing and commercialization, to various personnel including directors, executives, members of management, consultants and employees of the Company and/or its subsidiaries. Conditional upon the achievement by January 1, 20212023 and resultedJuly 1, 2023 of all specified corporate goals as set forth in the issuanceminutes of 3,000 sharesthe Compensation Committee, as well as continued service by the award recipients, the Company at the sole discretion of common stock, net of withholding shares for taxes.
Groupthe Chief FinancialExecutive Officer
Effective February 1, 2021, Terig Hughes entered into an employment agreement with Singapore Volition as Group and the Chief Financial Officer paid a cash bonus to such award recipients.
An aggregate of 1,144,000 RSUs were issued under the 2015 Plan in connection with the October 2022 grants and Treasurer. Mr. Hughes’ employment agreement continues until terminated by either party providing not less than three months’ prior notice. In exchange for his services, Mr. Hughes shall receive, among other things (i) $30,000 SGD per month (approximately $22,500);an aggregate of 1,000,000 stock options and (ii) a lump sum severance payment if terminated by Singapore Volition without cause (as per500,000 RSUs were issued under the agreement) equal2015 Plan in connection with the August 2021 and October 2021 grants.
As of December 31, 2023, the Company has recognized compensation expense of $901,410 in relation to the salaryoptions from the 2021 grants that he wouldvested in 2023. The Company has no unrecognized compensation expense in relation to such stock options, based on the outcomes related to the prescribed performance targets on the outstanding awards.
Total | Amortized |
| Amortized |
| Amortized | Un-Amortized |
| ||
Award | 2023 |
| 2022 |
| 2021 | 2023 |
| ||
$ | $ |
| $ | $ | $ | ||||
969,593 |
| - |
| 580,412 |
| 389,181 |
| - |
|
901,410 |
| 270,547 |
| 450,090 |
| 180,773 |
| - |
|
| F-75 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 10 – Commitments and Contingencies (continued)
h) Commitments in Respect of Corporate Goals and Performance-Based Awards (continued)
As of December 31, 2023, the Company has recognized compensation expense of $759,039 in relation to RSUs from the 2021 grants that have received betweenvested in 2023. The Company has no unrecognized compensation expense in relation to such RSUs, based on the dateoutcomes related to the prescribed performance targets on the outstanding awards.
Total |
| Amortized | Amortized | Amortized | Un-Amortized |
| |||
Award |
| 2023 | 2022 | 2021 | 2023 |
| |||
$ |
| $ | $ | $ | $ |
| |||
822,149 |
| - |
| 493,207 |
| 328,942 |
| - |
|
759,039 |
| 228,109 |
| 379,191 |
| 151,739 |
| - |
|
As of terminationDecember 31, 2023, the Company has recognized total compensation expense of $1,077,417 of which $527,940 is in relation to RSUs from the 2022 grants that have vested in 2023, $325,207 is in relation to RSUs from such grants that will vest in 2024, and $224,270 is in relation to RSUs from such grants that will vest in 2025. The Company has unrecognized compensation expense of $507,679 in relation to such RSUs, based on the outcomes related to the prescribed performance targets on the outstanding awards.
Total |
| Vesting |
| Amortized |
| Amortized |
|
| |
Award |
| Year |
| 2023 |
| 2022 |
| Un-Amortized |
|
$ |
|
|
| $ |
| $ |
| $ |
|
527,940 |
| 2023 |
| 393,853 |
| 134,087 |
| - |
|
521,493 |
| 2024 |
| 260,119 |
| 65,088 |
| 196,286 |
|
535,663 |
| 2025 |
| 177,584 |
| 46,686 |
| 311,393 |
|
1,585,096 |
|
|
| 831,556 |
| 245,861 |
| 507,679 |
|
In September 2023, the Compensation Committee of the Board of Directors approved the granting of cash bonuses, payable upon achievement of various corporate goals focused around revenue, operations and regulatory, to various personnel including directors, executives, members of management, consultants and employees of the Company and/or its subsidiaries. Conditional upon the achievement by December 31, 2023, and June 30, 2024 of specified corporate goals as set forth in the minutes of the Compensation Committee, as well as continued service by the award recipients, the Company at the sole discretion of the Chief Executive Officer and the completionChief Financial Officer would pay a cash bonus to such award recipients. As of a three-month notice period. December 31, 2023, the Company has accrued compensation expense of $1,071,198 in relation to the cash bonuses to be paid upon achievement of the specified corporate goals based on the expected outcomes related to the prescribed performance targets.
Effective February 1, 2021,In September 2023, the Compensation Committee of the Board of Directors approved the granting of an aggregate of 1,569,000 RSUs under the 2015 Plan to various personnel including directors, executives, members of management, consultants and employees of the Company granted warrants to purchase 185,000 shares of common stock to Terig Hughesand/or its subsidiaries in exchange for services provided to the Company. These RSUs vest upon the achievement of certain corporate goals focused around revenue, operations and regulatory targets as of December 31, 2023, and June 30, 2024, as set forth in the minutes of the Compensation Committee, with further time based vesting over three years, and subject to continued service by the award recipient. The achievement of the corporate goals is to be determined by the Compensation Committee in its sole discretion.
| F-76 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 10 – Commitments and Contingencies (continued)
h) Commitments in Respect of Corporate Goals and Performance-Based Awards (continued)
In September 2023, the Compensation Committee of the Board of Directors approved the granting of an aggregate of 450,000 RSUs under the 2015 Plan to various employees in exchange for services provided to the Company. These RSUs have an effective date of grant of October 19, 2023, vest upon the share price closing above $5.00 per share for a minimum of 30 consecutive trading days within a period of three years from the effective date of grant and ending October 19, 2026, with further time-based vesting in a single installment six months after the timely achievement of the target, if at all, and subject to continued service by the award recipients. The estimated fair value of the RSUs that include a market vesting condition will be measured on the grant date using a Monte Carlo Simulation of a Geometric Brownian Motion stock path model and incorporating the probability of vesting occurring. The estimated fair value of these awards will be recognized over the derived service period (as determined by the valuation model), with such recognition occurring regardless of whether the market condition is met.
As of December 31, 2023, the Company had recognized total compensation expense of $173,986. The Company has unrecognized compensation expense of $924,313 in relation to the RSUs from the 2023 grants, of which $271,342 in relation to RSUs that will vest in 2024, $318,588 in relation to RSUs that will vest in 2025, and $334,383 in relation to RSUs that will vest in 2026. based on the outcomes related to the prescribed performance targets on the outstanding awards.
Total |
|
| Vesting |
| Amortized |
|
|
| ||||
Award |
|
| Year |
| 2023 |
|
| Un-Amortized |
| |||
$ |
|
|
|
| $ |
|
| $ |
| |||
| 366,112 |
|
| 2024 |
|
| 94,770 |
|
|
| 271,342 |
|
| 366,101 |
|
| 2025 |
|
| 47,513 |
|
|
| 318,588 |
|
| 366,086 |
|
| 2026 |
|
| 31,703 |
|
|
| 334,383 |
|
| 1,098,299 |
|
|
|
|
| 173,986 |
|
|
| 924,313 |
|
Conditional upon the achievement by January 1, 2023 and July 1, 2023 of all specified corporate goals as Groupset forth in the minutes of the Compensation Committee, as well as continued service by the award recipients, the Company at the sole discretion of the Chief Executive Officer and the Chief Financial Officer paid a cash bonus to such award recipients.
As of December 31, 2023, the Company has paid compensation expense of $1.1 million in relation to the July 1, 2023 specified corporate goals based on the actual outcomes related to the prescribed performance targets.
| F-77 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 11 - Subsequent Events
Equity Distribution Agreement
During the period from January 1, 2024 through March 15, 2024, the Company sold 13,350 shares of common stock for aggregate proceeds (net of broker commissions and fees) of approximately $15,733 under the 2022 EDA.
RSUs Vesting
On January 13, 2024, 21,582 RSUs previously granted to a contractor vested and resulted in the issuance of 21,582 shares of common stock.
On March 1, 2024, 9,000 RSUs previously granted to employees vested and resulted in the issuance of 6,057 shares of common stock. An aggregate of 2,943 shares of common stock were withheld as taxes and returned to the 2015 Plan.
RSUs Granted
Effective February 22, 2024, the Company granted RSUs of 14,000 shares of common stock to an employee of the Company in exchange for services provided to the Company. These RSUs vest over 3 years, with one-third vesting on each of February 22, 2025, February 22, 2026 and February 22, 2027, subject to continued service by the employee, and will result in total compensation expense of $13,590.
Issuance of Shares
See the reference below under “License Agreement” regarding the issuance of 129,132 shares of restricted common stock to EpiCypher.
RSUs Cancellations
On January 16, 2024, 36,000 RSUs previously granted to an employee were cancelled and returned as authorized shares under the 2015 Plan upon cessation of employment of such employee prior to vesting.
On February 9, 2024, 2,000 RSUs previously granted to an employee were cancelled and returned as authorized shares under the 2015 Plan upon the cessation of employment of such employee prior to vesting.
Cash Bonus Payments
On January 24, 2024, the Compensation Committee of the Board of Directors approved the achievement of certain of the corporate milestones related to the September 28, 2023 authorization for the payment of cash bonuses to various personnel, including directors, executives, members of management, consultants and employees of the Company and/or its subsidiaries, upon the achievement of certain corporate goals focused around revenue, operations and regulatory, resulting in the vesting and payment of 85% of the cash bonuses.
License Agreement
On January 18. 2024, Belgian Volition entered into a License Agreement with EpiCypher, Inc., pursuant to which the Company may research, develop, manufacture and commercialize products and services using method covered by certain patents of EpiCypher. In addition to license fees and royalty payments, the Company issued shares of its restricted common stock to EpiCypher effective March 12, 2024, in a private placement.
| F-78 |
| Table of Contents |
VOLITIONRX LIMITED Notes to Consolidated Financial Statements For Years Ended December 31, 2023 and 2022 ($ expressed in United States Dollars) |
Note 11 - Subsequent Events (continued)
Diagnostic Oncology CRO, LLC Second A&R Master Agreement
Effective February 10, 2024 the Company and DXOCRO further amended and restated the A&R Master Agreement to expand the scope of DXOCRO’s consultant services provided thereunder (the “Second A&R Master Agreement”). The Second A&R Master Agreement requires DXOCRO to conduct a prospective optimization/range finding study of Volition’s Nu.Q® H3.1 in vitro diagnostic (IVD) test proposed for use in sepsis. The study is an extension of the sepsis monitoring clinical trial that was previously covered under a separate exhibit. The Company anticipates DXOCRO’s additional services under this Agreement will be completed by the end of the third quarter of 2024 at a total additional cost to the Company of up to $0.7 million. The Company’s payment obligations accrue upon delivery of projects under the Agreement. The Company may terminate the Agreement or any project thereunder upon at least 30 days’ prior written notice. Unless earlier terminated, the Second A&R Master Agreement terminates on the later of December 31, 2025 or the date upon which all services have been completed.
Employment Agreement
On March 19, 2024, Volition Diagnostics entered into a Contract of Employment with Dr. Andrew Retter pursuant to which Dr. Retter will serve as Chief Medical Officer commencing April 1, 2024. The term of the Contract of Employment is perpetual until terminated upon at least 12 weeks’ prior notice (or such greater period as required by statute) and provides for an annual base salary of £180,000 payable monthly in arrears as well as contributions towards Dr. Retter’s pension.
END NOTES TO FINANCIALS
| F-79 |
| Table of Contents |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Under the supervision and with the participation of management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures as of December 31, 2023, as such term is defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act. As a result of this evaluation, our principal executive officer and principal financial officer have concluded that, as of December 31, 2023, our disclosure controls and procedures were not effective due to the material weaknesses in internal control over financial reporting described below. Notwithstanding the identified material weaknesses, management, including our principal executive officer and principal financial officer, believes the consolidated financial statements included in this Annual Report on Form 10-K fairly represent, in all material respects, our financial condition, results of operations and cash flows as of and for the periods presented in accordance with U.S. GAAP.
Disclosure controls and procedures are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Management has evaluated the effectiveness of our internal control over financial reporting based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. As a result of this assessment, management has concluded that, as of December 31, 2023, our internal control over financial reporting was not effective due to the material weaknesses in internal control over financial reporting described below. As a smaller reporting company, we are not required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm in this Annual Report on Form 10-K.
The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with U.S. GAAP. The Company’s internal control over financial reporting includes policies and procedures that:
· | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; | |
· | provide reasonable assurance that transactions are recorded as necessary to permit preparation of the financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and our Board of Directors; and | |
· | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements. |
Material Weaknesses in Internal Control over Financial Reporting
We identified material weaknesses in our internal controls over financial reporting. A material weakness is defined as a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses identified include:
· | we do not have sufficient written documentation of our internal control policies and procedures, including written policies and procedures to ensure the correct application of accounting and financial reporting with respect to the current requirements of U.S. GAAP and SEC disclosure requirements. |
Notwithstanding the material weakness, we believe that our financial statements contained in this Annual Report on Form 10-K fairly present our financial position, results of operations and cash flows for the periods covered by this report in all material respects.
Our management, with the oversight of our audit committee, has initiated steps and plans to take additional measures to remediate the underlying causes of the material weakness, which we currently believe will be primarily through revising precision level management review controls and gaining additional assurance regarding our outside service providers’ quality control procedures. It is possible that we may determine that additional remediation steps will be necessary in the future.
| 80 |
| Table of Contents |
Planned Remediation of Material Weaknesses
Our management has been actively engaged in developing and implementing remediation plans to address material weaknesses described above. These remediation efforts are ongoing and include or are expected to include:
· | engaging internal control consultants to assist us in performing a financial reporting risk assessment as well as identifying and designing our system of internal controls necessary to mitigate the risks identified; | |
· | preparation of written documentation of our internal control policies and procedures; | |
· | increasing personnel resources and technical accounting expertise within the accounting function to replace our outside service providers; and | |
· | until we have sufficient technical accounting resources, we have engaged external consultants to provide support and to assist us in our evaluation of more complex applications of GAAP. |
We continue to enhance corporate oversight over process-level controls and structures to ensure that there is appropriate assignment of authority, responsibility, and accountability to enable remediation of our material weaknesses. We believe that our remediation plan will be sufficient to remediate the identified material weaknesses and strengthen our internal control over financial reporting. As we continue to evaluate, and work to improve, our internal control over financial reporting, management may determine that additional measures to address control deficiencies or modifications to the remediation plan are necessary.
Changes in Internal Control Over Financial Reporting
Other than changes described under “Planned Remediation of Material Weaknesses” above, there were no changes in our internal control over financial reporting during the year ended December 31, 2023, which were identified in connection with management’s evaluation required by paragraph (b) of Rules 13a-15 and 15d-15 under the Exchange Act, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
The Audit Committee of the Board of Directors meets regularly with our financial management, and with the independent registered public accounting firm engaged by us. Internal accounting controls and the quality of financial reporting are discussed during these meetings. The Audit Committee has discussed with the independent registered public accounting firm matters required to be discussed by the auditing standards adopted or established by the Public Company Accounting Oversight Board (“PCAOB”). In addition, the Audit Committee and the independent registered public accounting firm have discussed the independent registered public accounting firm’s independence from the Company and its management, including the matters in the written disclosures required by PCAOB Rule 3526 “Communicating with Audit Committees Concerning Independence.”
The Company is not required to include, and does not include an auditor’s attestation report under SEC Rules. Consequently, the Company’s registered public accounting firm has not attested to management’s reports on the Company’s internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable
| 81 |
| Table of Contents |
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Identification of Directors
The following table sets forth the names and ages of the Company’s Directors as of December 31, 2023.
Name |
| Notes |
|
| Age |
|
| Position with the Company |
| Officer/Director Since | |||
Cameron Reynolds |
|
|
|
|
| 52 |
|
| President |
| October 6, 2011 | ||
|
|
|
|
|
|
|
|
| Chief Executive Officer |
| October 6, 2011 | ||
|
|
|
|
|
|
|
|
| Director |
| October 6, 2011 | ||
Dr. Martin Faulkes |
|
|
|
|
| 79 |
|
| Executive Chairman |
| October 6, 2011 | ||
|
|
|
|
|
|
|
|
| Director |
| October 6, 2011 | ||
Dr. Phillip Barnes |
|
| (1)(2) |
|
| 62 |
|
| Director |
| October 9, 2019 | ||
Dr. Alan Colman |
|
| (1) |
|
| 75 |
|
| Director |
| October 6, 2011 | ||
Dr. Edward Futcher |
|
| (1)(3) |
|
| 69 |
|
| Director |
| June 23, 2016 | ||
Mickie Henshall |
|
| (2)(3) |
|
| 52 |
|
| Director |
| August 15, 2022 | ||
Guy Innes |
|
| (1)(3) |
|
| 67 |
|
| Director |
| October 6, 2011 | ||
Kim Nguyen |
|
| (2)(3) |
|
| 46 |
|
| Director |
| March 25, 2021 | ||
(1) | Member of the Audit Committee |
(2) | Member of the Compensation Committee |
(3) | Member of the Nominations and Governance Committee |
Term of Office
Each Director serves for a term of one year and until his or her successor is elected at the Annual Stockholders Meeting and is qualified, subject to removal by the stockholders.
Background and Business Experience of Directors
The business experience during the past five years of the directors is as follows:
CAMERON REYNOLDS serves as our President and Chief Executive Officer and as a director. He has served as a director of Belgian Volition since October 27, 2010, as the director and Chief Executive Officer of Volition Diagnostics since November 13, 2015, as a director of Volition America since February 3, 2017 and a manager of Volition Vet since August 7, 2019. Prior to completion of the transactions under the Share Exchange Agreement, Mr. Reynolds was the Chief Executive Officer of Singapore Volition, a position he held from August 5, 2010 to June 1, 2018, and a director of Singapore Volition from August 5, 2010 to September 1, 2021. He also served as the Managing Director of Belgian Volition between January 18, 2012 and July 24, 2015. Since February 2017, Mr. Reynolds has served as a non-executive director of Pathify Holdings, Inc. (formerly Ucroo Incorporated), a SaaS EdTech company, and since December 2023 has served as a non-executive director of GlycanAge Ltd, which manufactures and provides wellness tests which measure biological age. Further, from July 2018 and December 2021, he served as a non-executive director of Wellfully Limited (ASX: WFL) (formerly OBJ Limited), a developer of magnetic, micro-array drug and ingredient delivery technologies for use in pharmaceutical, cosmetic and skincare, and consumer healthcare industries. Between 2005 and 2011, Mr. Reynolds held a number of board directorships including Atlantic Mining PLC, Carbon Mining PLC, Magellan Copper and Gold PLC (Carbon Mining and Magellan Copper and Gold both became part of Solfotara Mining and Copper Development Corp.), KAL Energy Inc. (OTC: KALG), Iofina Natural Gas PLC (AIM: IOF), Canyon Copper Corp. (TSX-V: CNC, OTCBB: CNYC), and Hunter Bay Resources (TSX-V: HBY). From 2004 until 2011, Mr. Reynolds founded and served as Managing Director and Director of Mining House Ltd., a company providing consultancy and office support services, where he was responsible for identifying potential mining projects, coordinating the preliminary evaluations and securing the financing with a view to listing the company on the AIM, the TSX and the U.S. OTC. From 1998 until 2001, Mr. Reynolds served as the commercialization director for Probio, Inc., a company that commercialized intellectual property in the animal biotechnology fields including transgenesis and cloning research from the University of Hawaii, where his duties and responsibilities included managing legal and contract issues with the University of Hawaii, implementing patenting strategy, managing stockholder issues including a merger and its legal and contractual documentation, overseeing office management, monitoring budgetary concerns, and team building and recruitment. Furthermore, Mr. Reynolds held a junior management position in 1996 at Integrated Coffee Technologies, a genetically modified coffee company where he was responsible for business plan creation, office management, recruitment, and business development. From 1994 to 1995, Mr. Reynolds worked for Southern China Group, a mineral exploration company, where, as regional manager, he established operations in Hong Kong and Yunnan. Mr. Reynolds received a Bachelor of Commerce and an M.B.A. from the University of Western Australia. Our board of directors believes Mr. Reynolds brings the Company strong experience in management, structuring and strategic planning based on his over 30 years of entrepreneurial executive experience in the mining and biotechnology sectors and is qualified to serve as a director of the Company.
| 82 |
| Table of Contents |
DR. MARTIN FAULKES serves as Executive Chairman of our board of directors. Dr. Faulkes has also served as a Manager of Volition Vet since August 7, 2019. Prior to completion of the transactions under the Share Exchange Agreement, Dr. Faulkes served as a Director of Singapore Volition from August 18, 2010 to December 15, 2015, and as Executive Chairman of the board of directors of Singapore Volition from March 22, 2011, to December 15, 2015. Dr. Faulkes also served as President of Volition Vet from August 7, 2019 to May 1, 2021 and as Director of Belgian Volition from August 10, 2011 to March 31, 2016. Since 1998, Dr. Faulkes has focused on charitable activities as the founder and sole benefactor of The Dill Faulkes Educational Trust, a U.K. registered charity, or DFET, where he serves as the Chairman. Prior to founding DFET, Dr. Faulkes founded Triad Plc., a computer software development company that provides systems and consultants to the business community, where he was a Director from 1987 to 1998, and responsible for controlling the company financially. From 1985 to 1987, he became Managing Director of System Programming Ltd., a company that provides computer programming for systems in businesses such as airlines, utility companies, banks, and insurance companies, where he was responsible for all aspects of the business. Prior to working for System Programming Ltd., Dr. Faulkes served from 1979 to 1984 as founder, President and Chief Executive Officer for Logica Inc., a company providing bespoke software to all industries but mainly banks and communications companies. Dr. Faulkes was responsible for all aspects of the business, including sales, finance, recruitment, staff management and project control. Dr. Faulkes has over 40 years of entrepreneurial and managerial experience as the founder and Chief Executive Officer of several software companies within the United Kingdom and the United States. Dr. Faulkes received a B.Sc. in Mathematics from Hull University and his Ph.D. in Mathematics from Queen Elizabeth College, University of London. Our board of directors believes that Dr. Faulkes is qualified to serve as a director of the Company based on his extensive experience in business development and management.
DR. PHILLIP BARNES serves as a director. Dr. Barnes is currently retired. Between 2009 and 2016, he served on the Board of Directors of a number of United Kingdom National Health Service, or NHS, hospitals both as Chief Medical Officer and Chief Executive Officer. These warrants vestDr. Barnes was also involved in a number of national and regional advisory groups for the NHS and pharmaceutical industry. Prior to his career as a physician executive, Dr. Barnes was Consultant Neurologist at King’s College Hospital, London between 1995 and 2009, and Hon. Senior Lecturer in Neurology at King’s College London between 1999 and 2009. He served as Clinical Director for Neurology, a managerial and administrative role, between 1995 and 1998, and from 1998 until 2008 was Chief of Service of the King’s Neurosciences Centre, the United Kingdom’s largest Regional Neuroscience Centre. Dr. Barnes received a B.Sc. in Basic Medical Sciences, a Ph.D. in Anatomy and Neuroendocrinology from the University of London and a clinical medical degree (B.M. B.Ch.) from the University of Oxford. Our board of directors believes that Dr. Barnes is qualified to serve as a director of the Company based on Februaryhis extensive experience both as a clinician and Board member within the United Kingdom’s NHS and related academic institutions.
DR. ALAN COLMAN serves as a director. Prior to completion of the transactions under the Share Exchange Agreement, Dr. Colman served as a Director of Singapore Volition from April 1, 2011 to December 15, 2015. Dr. Colman currently serves as chairman of the Scientific Advisory Board of Belgian Volition, a position he has held since April 5, 2011. Dr. Colman is currently an Associate at the Harvard University Department of Stem Cell and Regenerative Biology, and is also a director of Gaibian Associates Pte. Ltd., a consultancy company in Singapore. Until its acquisition by Vertex Pharmaceuticals, a biopharmaceuticals company, in October 2019, he served on the Scientific Advisory Board of Semma Therapeutics, Inc., a stem cell therapy company based in Cambridge, Massachusetts, a position he held since December 2014. From 2007 to 2013, Dr. Colman served as the Executive Director of the A*STAR Singapore Stem Cell Consortium, or A*STAR. Concurrently, Dr. Colman was Professor of Regenerative Medicine at King’s College, London, United Kingdom, from 2008 to 2009. Prior to joining the A*STAR, Dr. Colman was Chief Scientific Officer and then Chief Executive Officer for ES Cell International, a Singaporean human embryonic stem cell company, from 2002 to 2007. Dr. Colman was the research director at PPL Therapeutics in Edinburgh, United Kingdom., or PPL, from the late 1980s until 2002, where he was responsible for leading PPL’s research program strategy, also playing a role in PPL’s financing rounds, culminating in its listing on the London Stock Exchange in 1996. PPL attracted considerable media attention because of its participation in the technique of somatic nuclear transfer that led to the world’s first sheep cloned from an adult cell, Dolly, in 1996. Dr. Colman had a successful university career in the Universities of Oxford, Warwick, Birmingham, where he was Professor of Biochemistry, and London, as described above. Dr. Colman’s main current interest is the development of human disease models using induced pluripotent stem cells. Dr. Colman received a B.A., M.A. and Ph.D. from Oxford University. Dr. Colman has extensive experience in the molecular biology field where he has worked in the production of transgenic livestock, somatic nuclear transfer, and human disease models. Our board of directors believes that Dr. Colman is qualified to serve as a director of the Company and a member of the Scientific Advisory Board based on his extensive experience in biochemistry, stem cell research and pathology
| 83 |
| Table of Contents |
DR. EDWARD FUTCHER serves as a director. Since 1997, Dr. Futcher has held non-executive directorships with a variety of private companies. He co-founded Azima, Inc. in 2003, a company that provides advanced machine diagnosis to large industrial facilities and, from 2003 to 2008, served as its Vice President of Engineering with responsibility for the engineering, information technology and customer support groups. Prior to that, from 1997 to 2003, Dr. Futcher served as Vice President of Technology of interWAVE Communications International, Ltd., a company providing GSM and CDMA cellular infrastructure equipment, where he was responsible for operational management of acquisitions and interim management of the worldwide research and development organization. From 1997 to 1999, Dr. Futcher served as Vice President of Engineering of interWAVE Communications. From 1994 to 1997, Dr. Futcher was Director of Engineering at Tellabs, Inc., a telecommunications equipment supplier. Dr. Futcher received a B.S. and Ph.D. in Physics from the University of London, as well as a post-graduate certificate in international securities, investment and banking from the ICMA Centre, University of Reading. Our board of directors believes that Dr. Futcher is qualified to serve as a director of the Company based on his extensive engineering, commercial and management experience in dynamic and fast-growing high technology companies, and his ability to provide strategic counsel in connection with the commercialization of the Nu.Q® blood-based diagnostic platform.
MICKIE HENSHALL serves as a director. Ms. Henshall currently serves as Chief Marketing Officer of REALM IDx, Inc. (formerly Konica Minolta Precision Medicine), the parent company of Ambry Genetics Corporation and Invicro, LLC, which is focused on pioneering developments in the field of integrated diagnostics by uniting genomics, imaging, radiology and pathology with advanced artificial intelligence to develop innovative healthcare solutions. In her current role, which she has held since October 2020, Ms. Henshall has been responsible for the corporate name change and rebranding, restructuring the marketing organization, supporting the company’s commercial expansion and implementing formal product development processes. Prior to joining REALM IDx, from April 2020 to October 2020, Ms. Henshall served as Chief Marketing Officer of Genomic Life, Inc., a health services company focused on expanding access to precision medicine. She was responsible for corporate strategy and marketing of the company’s paid benefit program, Cancer Guardian, which provides genetic risk insights and comprehensive genomic profiling with support services for those diagnosed with cancer. Prior to that, from 2017 to April 2020, Ms. Henshall was Vice President of Marketing at Agena Bioscience (now part of Mesa Laboratories, Inc. (NASDAQ:MLAB)), a molecular diagnostics company delivering instrument systems and assays for targeted analysis of genetic disease and variant profiling. Ms. Henshall led Agena’s Corporate Marketing, Product Management, Scientific Affairs, Custom Assay Services, and the regional marketing teams in China, Australia, and Germany. Between 2014 and 2017, Ms. Henshall served as Vice President of Marketing at Accriva Diagnostics, Inc., or Accriva, a hospital-focused, point-of-care cardiovascular diagnostics company, where she established a new marketing team, developed re-branding of Accriva’s point-of-care diagnostics products, generated a five-year portfolio roadmap and provided support in preparing Accriva for sale to Werfen Life. From 2010 to 2014, Ms. Henshall was Vice President of Marketing at Biotix, Inc, a supplier of laboratory consumables, where she managed all aspects of global marketing initiatives, including corporate branding, channel marketing, public relations, and sales training. From 2005 to 2010, Ms. Henshall had increasing levels of leadership roles at Illumina, Inc. (NASDAQ:ILMN), a global leader in genomic technologies, where she supported the company through many firsts, including developing its first diagnostic portfolio strategy, convening its first Diagnostic Advisory Board, onboarding its first diagnostics marketing team, and development and launch of its first FDA cleared system. Ms. Henshall is a marketing executive with over 20 years’ experience in developing and implementing marketing and sales strategies for the IVD, clinical and life science markets. Ms. Henshall holds a B.S. in Integrative Biology from the University of California, Berkeley. Our board of directors believes that Ms. Henshall is qualified to serve as a director of the Company based on her extensive marketing and commercial experience.
GUY INNES serves as a director. Prior to completion of the transactions under the Share Exchange Agreement, Mr. Innes served as a Director of Singapore Volition, a position he held from August 18, 2010 to December 15, 2015. Mr. Innes has served as a non-executive Director on the boards of mineral exploration companies such as Carbon Mining PLC and Magellan Copper & Gold PLC, both from 2007 to 2010, and on the board of ProBio Inc., a company that commercialized intellectual property in the animal biotechnology fields including transgenesis and cloning research from the University of Hawaii, from 2000 to 2006. Mr. Innes had a long career in banking and private equity, including advisory roles with Quartz Capital Partners Limited, or Quartz, a small London based investment bank specializing in new technology companies, from 1997 to 2000, where Mr. Innes served as Head of Corporate Finance and was responsible for managing the corporate finance department and leading the transactions undertaken by Quartz including initial public offerings, private placements and mergers and acquisitions; Baring Private Equity Partners Limited, a private equity investment firm, in London and Singapore from 1995 to 1997, where he was involved in the setting up, recruiting of managers and capital raising for an Asian media and communications private equity fund; and Baring Brothers & Co. Limited, a London based international investment bank, in London and Paris from 1984 to 1995, where he was involved in executing and advising on national and international mergers and acquisitions, as well as initial public offerings and capital raising. Mr. Innes is a chartered accountant and a member of the Institute of Chartered Accountants in England and Wales. Mr. Innes has extensive experience in financing and managing technology companies. Mr. Innes received a B.Sc. in Geography from Bristol University. Our board of directors believes Mr. Innes is qualified to serve as a director of the Company based on his extensive technical, financial and managerial background.
| 84 |
| Table of Contents |
KIM NGUYEN serves as a director. Ms. Nguyen is currently Group Director, Asia Pacific Human Resources, or APAC HR, for the London Stock Exchange Group, a position she has held since November 2023. Between January 2022 and expire 5November 2023, Ms. Nguyen served as Vice President of International HR of Binance, a blockchain company. From September 2017 and January 2022, Ms. Nguyen served as Head of HR for Google Asia Pacific Pte. Ltd. Prior to that, Ms Nguyen worked with Google UK Limited from April 2007 to September 2017, where she held a number of positions working across the Technology and Sales divisions across Europe and Latin America. Ms. Nguyen's HR career spans over 20 years afterin Technology, FinTech and Financial Services businesses. She has built and led large teams driving business transformation, corporate reorganization, workforce planning, crisis management, mergers and acquisitions, as well as driving HR strategy for new market expansion. She has worked across all international markets and has helped companies navigate culture and talent needs in a global setting, while meeting local labour law requirements. With experience in both multinational and start up environments she can competently operate in more established environments or build structures and programs from the vesting date, with an exercise priceground up. Ms. Nguyen received a B.S. in Psychology Honors from the University of $4.90 per share.New South Wales, Sydney, Australia. Our board of directors believes that Ms. Nguyen is qualified to serve as a director of the Company based on her extensive experience within international labor law and practice, leadership and talent management development, organizational design and HR analytics.
Identification of Executive Officers
The following table sets forth the names and ages of the Company’s executive officers as of December 31, 2023.
Name | Age | Position with the Company | Role Held Since | ||||||
Cameron Reynolds | 52 | President | October 6, 2011 | ||||||
Chief Executive Officer | October 6, 2011 | ||||||||
Director | October 6, 2011 | ||||||||
Dr. Martin Faulkes | 79 | Executive Chairman | October 6, 2011 | ||||||
Director | October 6, 2011 | ||||||||
Terig Hughes | 53 | Chief Financial Officer | February 1, 2021 | ||||||
Treasurer | February 1, 2021 | ||||||||
Dr. Gaetan Michel | 51 | Chief Operating Officer |
| February 1, 2021 | |||||
Dr. Jacob Micallef | 67 | Chief Scientific Officer | January 1, 2015 | ||||||
Gael Forterre | 43 | Chief Commercial Officer | February 1, 2021 | ||||||
Nicholas Plummer | 53 | Group General Counsel | November 1, 2021 | ||||||
Louise Batchelor | 53 | Group Chief Marketing & Communications Officer | September 12, 2022 | ||||||
Rodney Rootsaert | 52 | Corporate Secretary | October 6, 2011 | ||||||
Dr. Salvatore Thomas Butera | 73 | Chief Executive Officer, Volition Vet | May 1, 2021 | ||||||
Dr. Jasmine Kway | 52 | Chief Executive Officer, Singapore Volition | June 1, 2018 | ||||||
| 85 |
| Table of Contents |
Term of Office
Each officer serves for such term as determined by their employment agreement as approved by the board of directors or Compensation Committee.See “Item 11. Executive Compensation – Employment and Consulting Agreements.”
Background and Business Experience of Executive Officers
The business experience during the past five years of the executive officers is as follows:
CAMERON REYNOLDS serves as our President and Chief Executive Officer and is a Director of the Company. Additional information regarding Mr. Reynolds is provided under “Item 10. — Directors, Executive Officers and Corporate Governance – Background and Business Experience of Directors” of this Report.
DR. MARTIN FAULKES serves as our Executive Chairman of our Board of directors. Additional information regarding Dr. Faulkes is provided under “Item 10. — Directors, Executive Officers and Corporate Governance - Background and Business Experience of Directors” of this Report.
TERIG HUGHES serves as our Chief Financial Officer and Treasurer. He has also served as Chief Financial Officer and Treasurer of both Volition America since December 1, 2022, and of Volition Vet since March 17, 2023, as well as a Director of Volition Global Services since September 2, 2021. Between September 1, 2021 and August 8, 2022, Mr. Hughes served as a Director of Singapore Volition. Mr. Hughes joined the Company from AUM Biosciences Pte. Ltd., a fast-growing biotechnology company focused on developing novel drugs for cancer treatment, where he was the Chief Financial Officer based in Singapore, and oversaw all aspects of business and finance, from initial start-up in 2018 through to first revenue in 2020. Prior to 2018, Mr. Hughes held a number of senior finance and business leadership positions at Elsevier, a division of RELX Group plc (formerly Reed Elsevier), an FTSE 100 company. From 2014 to 2017, Mr. Hughes was the regional Managing Director of RELX Group plc for India and Southeast Asia, overseeing all aspects of the business including sales, marketing, and product development. From 2006 to 2014, he served as the company’s Finance Director for Asia Pacific, during which he managed and oversaw accounting and finance functions, including financial planning and analysis. During this period, he also oversaw a successful finance transformation project, which included systems implementation, transition to a shared-services model, and outsourcing of various work streams, as well as a number of mergers and acquisitions projects in China. From 2003 to 2006, Mr. Hughes was the Vice President Finance of Elsevier’s US Journals and Pharma Communications Division where he provided commercial support to senior management, oversaw implementation of internal control standards as required by the Sarbanes-Oxley Act of 2002, and managed the annual pricing process. Mr. Hughes has over 25 years of accounting, finance and business management experience gained through an international career spanning the United States, Europe and Asia. Mr. Hughes received a B.Sc. in Accounting and Law from De Montfort University, Leicester in the United Kingdom.
DR. GAETAN MICHEL serves as our Chief Operating Officer. In addition, Dr. Michel has served as Director and Chief Executive Officer of Volition America since November 16, 2021, as Chief Executive Officer and Manager of Volition Global Services since September 15, 2021 and September 1, 2021, respectively, as Manager and President of Volition Veterinary since August 7, 2019 and May 1, 2021, respectively, and as Chief Executive Officer and Manager of Belgian Volition since November 15, 2022. In addition, from June 22, 2015 to November 4, 2021, Dr. Michel served as Manager of Belgian Volition, from July 1, 2015 to September 14, 2021 as Chief Executive Officer of Belgian Volition, from August 14, 2020 to May 1, 2021 as Chief Executive Officer of Volition Veterinary, and from January 10, 2020 to October 23, 2020, as a Managing Director of Volition Germany. Dr. Michel also previously served as Belgian Volition’s Chief Operations Officer from July 2014 to June 2018. Dr. Michel has over ten years of experience in production management. Prior to joining Belgian Volition, from 2010 to 2014 Dr. Michel worked as production director for Bone Therapeutics SA (Euronext Brussels and Paris: BOTHE), a bone cell therapy-based pharmaceutical company, where his responsibilities included establishing two new production plants to commence manufacturing for two phase III clinical trials, developing quality systems for new products in negotiation with the Belgian health authorities, and establishing a product plant for an injectable medical device. From 2007 to 2010, Dr. Michel worked for KitoZyme, a global manufacturer of biopolymers of fungal origin with its core business in weight management, digestive and cardiovascular health. During this period, Dr. Michel established both the production and process development departments and oversaw the commencement of the company’s industrial phase culminating in the roll out of first products. Prior to joining KitoZyme, following the completion of his Ph.D. in 2002, Dr. Michel joined Advanced Array Technologies, or AAT, a University of Namur spin-off company as project manager in proteomics. AAT later became Eppendorf Array Technologies, part of the German Eppendorf biotech company, where Dr. Michel became production manager and was involved in establishing production processes and equipment. Dr. Michel received a Ph.D. in Biochemistry from the University of Namur, Belgium.
| 86 |
| Table of Contents |
DR. JACOB MICALLEF serves as our Chief Scientific Officer. Dr. Micallef previously served as a Director of Belgian Volition between August 10, 2011 and March 31, 2016. Prior to the Share Exchange Agreement, he served as Chief Scientific Officer of Belgian Volition from October 11, 2010 to December 31, 2014, but was not otherwise involved with Singapore Volition. Dr. Micallef joined Cronos Therapeutics Limited, or Cronos, a company developing oncology drugs, in 2004, and in 2006, Cronos was listed in the United Kingdom on the Alternative Investment Market, or AIM, becoming ValiRx plc, or ValiRx (AIM:VAL). Dr. Micallef continued to work as Technical Officer for ValiRx, where he in-licensed the NucleosomicsTM technology and co-founded ValiBio SA, which is now Belgian Volition. From 2004 to 2007, he taught “science and enterprise” to science research workers from four universities at CASS Business School before joining Cronos. In 2001, Dr. Micallef co-founded Gene Expression Technologies, a company developing oncology drugs, where he successfully led the development of the chemistry of the GeneICE technology and implemented the manufacture of GeneICE molecules. He also played a major role in business development and procured a GeneICE contract with Bayer AG. Over a 15-year period, starting in 1985, Dr. Micallef worked for the World Health Organization, or WHO. While working for WHO, Dr. Micallef developed new diagnostic products in the areas of reproductive health and cancer. In 1990, he commenced development of a new diagnostic technology platform for WHO which was launched in 1992 and supported 13 tests. Dr. Micallef also initiated and implemented in-house manufacture, previously outsourced to Abbott Diagnostics Inc., and world-wide distribution of these products for WHO. Also, in 1990, he started a “not-for-profit” WHO company, Immunometrics Ltd., which marketed and distributed those diagnostic products worldwide. Dr. Micallef has over 20 years of experience in research and development and in the management of early-stage biotechnical companies, including the manufacture of biotechnology products and the establishment of manufacturing operations. Dr. Micallef received his M.B.A. from Imperial College, University of London and his Ph.D. from King’s College, University of London.
GAEL FORTERRE serves as our Chief Commercial Officer. He has almost twenty years of experience investing in and scaling fast growing companies. Since October 2013, Mr. Forterre has served as the Managing Partner of Armori Capital Management, LLC, or Armori, an investment advisory firm. Mr. Forterre launched Armori in October 2013, for which he conducted a series of investments on behalf of family offices and institutional investors, and actively supported the growth of its portfolio companies. From December 2021 to February, 2024, Mr. Forterre served as a non-executive director for Integrated Wellness Acquisition Corp., a special purpose acquisition company. Mr. Forterre also served as the Chief Executive Officer of Ucroo Incorporated, or Ucroo (now Pathify Holdings, Inc.), a SaaS EdTech company, between January 2019 and December 2020, during which period he supervised the launch of its product suite and tripled sales two years in a row; he also served as its Chief Financial Officer between January 2018 and December 2018 and acted as its consultant from August 2017 to December 2017. Mr. Forterre also served as a board member of Ucroo between August 2019 and March 2021, and as a board member of ARTICLE22, a design-focused social enterprise that he co-founded in 2013, from July 2013 to June 2021. Between 2005 and 2012, Mr. Forterre worked in various positions including as a structurer, an analyst and a trader for BNP Paribas in the corporate and investment banking division, in both New York and Paris, where he was selected to join the top talent program. Mr. Forterre received an M.S. in finance from Sorbonne Paris I and a double M.B.A. from both Columbia Business School and the London Business School.
NICHOLAS PLUMMER serves as our Group General Counsel. Mr. Plummer is a solicitor qualified in England. From 1995 to 2004, Mr. Plummer worked at the United Kingdom and international law firm Ashurst as a corporate lawyer, before moving into his first in-house role as General Counsel and Company Secretary of Ark Therapeutics Group PLC, a UK-listed biotech company, from 2004 to 2008. From 2013 to 2021, Mr. Plummer served as the EU Managing Counsel at Patheon, subsequently Pharma Services Group of Thermo Fisher Scientific, a contract drug developer and manufacturer. Mr. Plummer has over twenty-five years of legal experience in private practice and in-house roles, primarily in the healthcare sector. Mr. Plummer holds a LL.B. law degree (Hons) from Reading University and was admitted as a Solicitor to the Supreme Court of England and Wales in 1997.
LOUISE BATCHELOR serves as our Group Chief Marketing and Communications Officer. Ms. Batchelor joined the Company in April 2016 from ACULD Limited, a strategic marketing consultancy specializing in healthcare where she served as director and owner since August 2011. From April 2006 to January 2009, Ms. Batchelor was the global brand marketing manager for Reckitt Benckiser Plc, a British, multinational consumer goods company, where she led the development of the global brand for the Lysol® germ protection range. From 2001 to 2009, Ms. Batchelor also served as the European business unit director for Reckitt Benckiser, based in Paris, France, where she was responsible for general management of the European business and led the European regulatory strategy and launch of multiple products. Prior to joining Reckitt Benckiser, Ms. Batchelor was a product manager, marketing executive, primary care field sales manager and a senior market research executive at Zeneca Pharmaceuticals Ltd in the United Kingdom, from August 1993 to October 2000. Ms. Batchelor has thirty years of global marketing, sales and leadership experience gained through an international career spanning the United States, Europe and the United Kingdom. Ms. Batchelor received a B.A. in business studies from Sheffield Hallam University.
| 87 |
| Table of Contents |
RODNEY ROOTSAERT serves as our Corporate Secretary. Prior to the completion of the transactions under the Share Exchange Agreement, he was the Administration and Legal Officer of Singapore Volition, a position he held since August 6, 2010. Mr. Rootsaert became a Director of Singapore Volition on December 15, 2015. He has been a Director and Secretary of Belgian Volition since October 4, 2010, a Director of Volition Diagnostics since November 13, 2015, Secretary of Volition Vet since August 7, 2019, and Secretary of Volition America since November 16, 2021. Between August 7, 2019 and March 17, 2023, Mr. Rootsaert served as Treasurer of Volition Vet. Mr. Rootsaert served as director and corporate secretary of Mining House Ltd., a company providing consultancy and office support services, between 2007 and 2018. His responsibilities included ensuring compliance with all relevant statutory and regulatory requirements. From 2007 until 2011, Mr. Rootsaert served as corporate secretary for Magellan Copper and Gold Plc., a mineral exploration company, where his duties included maintaining and preparing company documents, accounts and contracts. Mr. Rootsaert has over 15 years of experience in providing corporate, legal and administrative services. Mr. Rootsaert holds an LL.B. degree from the University of Western Australia.
DR. SALVATORE THOMAS BUTERAserves as the Chief Executive Officer of Volition Veterinary. Dr. Butera previously served as a Director of the Company between December 1, 2020 and March 25, 2021, before resigning in order to prepare to assume the role of Chief Executive Officer of Volition Veterinary, effective May 1, 2021. Between 2016 and April 2021, Dr. Butera served as Business Development Director at Veterinary Centers of America, or VCA, which is in the practice and business of veterinary medicine, part of Mars Veterinary Health, or (“Mars”), and was actively involved in business development roles with Mars, leading divisions such as Pet Partners, LLC, or PPL, Banfield Pet Hospitals and VCA, and serving as Co-Founder, Board Member and Chief Medical Officer of PPL prior to its acquisition by Mars. Dr. Butera received his B.A. from Fairfield University and his D.V.M. from the University of Missouri Veterinary School.
DR. JASMINE KWAY serves as the Chief Executive Officer of Singapore Volition. Dr. Kway previously served as Singapore Volition’s Vice President of Asia from January 2017 until June 2018. Prior to joining Singapore Volition, during 2016 Dr. Kway served as Chief Executive Officer of intellectual property practice at RHT i-Assets Advisory, or RHTiAA, one of the leading companies of the RHT Group of Companies and RHT Holdings, a Singapore-headquartered integrated leading professional services company providing professional services in Asia. While at RHTiAA, Dr. Kway was focused on managing buy-side and sell-side clients globally in technology and intellectual property transactions, business development, and fund raising. Prior to RHTiAA, Dr. Kway worked in both the private and public sectors, including as Executive Vice President, Business Development at Transpacific IP Group Limited, a full-service intellectual property company, from 2010 to 2015, and as Director of Industry Liaison of the National University of Singapore from 2005 to 2010. In these positions, Dr. Kway formulated and implemented national intellectual property policies, corporate intellectual property strategies and management, intellectual property and technology development, commercialization, fundraising, and investment. Dr. Kway received her Bachelor of Engineering (Honors) and Doctorate degrees from the National University of Singapore.
CORPORATE GOVERNANCE
Family Relationships
We currently do not have any family relationships between any officers, directors, or director nominees of our Company.
Involvement in Certain Legal Proceedings
During the past ten years no director, executive officer or director nominee of VolitionRx has been involved in any legal proceedings required to be disclosed pursuant to Item 401(f) of Regulation S-K. Additionally, no director, executive officer or director nominee of VolitionRx is party to, or has any material interests in, any material legal proceedings that are adverse to the Company or its subsidiaries.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires our directors, executive officers and stockholders who own more than ten percent of any registered class of our equity securities registered pursuant to Section 12 of the Exchange Act, (“Reporting Persons”), to file with reports of ownership and reports of changes in ownership of securities with the SEC. Based solely on our review of the reports that have been filed by or on behalf of such Reporting Persons in this regard, and the representations made by our directors and executive officers to us, we believe that there has been compliance with all Section 16(a) filing requirements applicable to such Reporting Persons with respect to the fiscal year ended December 31, 2023, except for one late Form 4 reporting a transaction for Mickie Henshall.
| 88 |
| Table of Contents |
Code of Ethics
We have adopted a Code of Ethics that applies to our directors, officers and employees, including our Chief Executive Officer and Chief Financial Officer. A copy of our Code of Ethics is available on our Company website at https://ir.volition.com/governance-documents. Amendments to our Code of Ethics that apply to our principal executive officer, principal financial officer, principal accounting officer, controller or persons performing similar functions, if any, will be posted on our website at https://ir.volition.com/governance-documents. We will disclose any waivers of provisions of our Code of Ethics that apply to such persons by disclosing such information on a Current Report on Form 8-K.
Committees of the Board of Directors
Our board of directors has established an Audit Committee, a Compensation Committee and a Nominations and Governance Committee. The Committees operate pursuant to written charters adopted by the board of directors, copies of which are available on our website at https://ir.volition.com/committee-charters. In addition, from time to time, our board of directors may establish special committees when necessary to address specific issues. The composition and functions of each of our Audit, Compensation and Nominations and Governance Committees are described below. Members serve on these committees until their resignation subject to each committee member’s annual re-election to our board of directors, or until otherwise determined by our board of directors.
Audit Committee
Our Audit Committee currently consists of four members, Mr. Innes (Chair), and Drs. Barnes, Colman and Futcher, each of whom has been determined to be an independent director under applicable SEC rules and the NYSE American Company Guide. The Audit Committee shall at all times be composed exclusively of directors who are, in the opinion of our board of directors, free from any relationship which would interfere with the exercise of independent judgment as a committee member and who possess an understanding of financial statements and generally accepted accounting principles.
The Audit Committee is responsible for, among other things:
· | appointing, terminating, compensating and overseeing the work of any independent auditor engaged to prepare or issue an audit report or other audit, review or attest services; | |
· | reviewing all audit and non-audit services to be performed by the independent auditor, taking into consideration whether the independent auditor’s provision of non-audit services to us is compatible with maintaining the independent auditor’s independence; | |
· | reviewing and discussing the adequacy and effectiveness of our accounting and financial reporting processes and internal controls and the audits of our financial statements; | |
· | establishing and overseeing procedures for the receipt, retention and treatment of complaints received by us regarding accounting, internal accounting controls or auditing matters, including procedures for the confidential, anonymous submission by our employees regarding questionable accounting or auditing matters; | |
· | investigating any matter brought to its attention within the scope of its duties and engaging independent counsel and other advisors as the Audit Committee deems necessary; | |
· | determining compensation of the independent auditors and of advisors hired by the Audit Committee and ordinary administrative expenses; | |
· | reviewing and discussing with management and the independent auditor the annual and quarterly financial statements prior to their release; | |
· | monitoring and evaluating the independent auditor’s qualifications, performance and independence on an ongoing basis; | |
· | reviewing reports to management prepared by the internal audit function, as well as management’s response; | |
· | reviewing and assessing the adequacy of the Audit Committee’s formal written charter on an annual basis, | |
· | reviewing risks relating to financial statements, the auditing and financial reporting process, liquidity risks and market risks; | |
· | overseeing and reviewing the adequacy of the Company’s cybersecurity, information and technology security, and data privacy programs, procedures, and policies; | |
· | reviewing and approving transactions with related persons for potential conflict of interest situations on an ongoing basis; and | |
· | overseeing such other matters that are specifically delegated to the Audit Committee by our board of directors from time to time. |
The board of directors has affirmatively determined that Mr. Innes is designated as an “Audit Committee financial expert.”
| 89 |
| Table of Contents |
Compensation Committee
Our compensation committee consists of three members, Mss. Nguyen (Chair) and Henshall, and Dr. Barnes, each of whom has been determined to be an independent director under the NYSE American Company Guide.
The compensation committee is responsible for, among other things:
· | developing, reviewing, and approving our overall compensation programs for directors and officers, and regularly reporting to the full board of directors regarding the adoption of such programs; | |
· | developing, reviewing and approving our cash and equity incentive plans, including approving individual grants or awards thereunder; | |
· | reviewing and approving individual and company performance goals and objectives that may be relevant to the compensation of executive officers and other key employees; | |
· | reviewing and discussing with management the tables and narrative discussion regarding executive officer and director compensation to be included in the annual proxy statement; | |
· | reviewing and assessing periodically, the adequacy of the Compensation Committee’s formal written charter; | |
· | preparing the annual Compensation Committee Report; | |
· | approving the adoption of, and revisions to, or the termination of our Clawback and Forfeiture Policy; and | |
· | overseeing such other matters that are specifically delegated to the Compensation Committee by our board of directors from time to time. |
In fulfilling its responsibilities, the compensation committee has the authority to delegate any or all of its responsibilities to a subcommittee of the compensation committee.
Nominations and Governance Committee
Our nominations and governance committee consists of four members, Dr. Futcher (Chair), Mss. Nguyen and Henshall and Mr. Innes, each of whom has been determined to be an independent director under the NYSE American Company Guide.
The nominations and governance committee is responsible for, among other things:
· | identifying and screening candidates for our board of directors, and recommending nominees for election as directors; | |
· | developing policies and procedures for identifying, evaluating, and recommending director candidates, including consideration of nominees recommended by stockholders; | |
· | developing and recommending to the Board criteria for Board membership, which shall include specific minimum qualifications; | |
· | assessing, on an annual basis, the performance of the board of directors and any committee thereof; | |
· | reviewing the structure of the board of directors’ committees and recommending to the board of directors, for its approval, directors to serve as members of each committee, including each committee’s respective chair, if applicable; | |
· | establishing and periodically review the Company’s succession plans for the Board and senior management positions; | |
· | reviewing and assessing, on an annual basis, the adequacy of the Nominations and Governance Committee’s formal written charter; | |
· | evaluating whether any position held or proposed to be held by any new or existing director would represent a conflict of interest with such director’s membership on the Board or any committee; | |
· | assisting the Board in providing oversight and guidance with regard to sustainability and ESG matters, including by reviewing and evaluating the Company’s programs, practices, and reporting related to ESG issues and impacts to support the sustainable growth of the Company’s business; and | |
· | generally advising our board of directors on corporate governance and related matters including, without limitation, with respect to the Company’s Certificate of Incorporation, Bylaws and charters of other committees. |
| 90 |
| Table of Contents |
Nominating Procedures
The Nominations and Governance Committee considers candidates for the board of directors from any reasonable source, including stockholder recommendations. The committee will not evaluate candidates differently based on who has made the proposal. The committee has the authority under its charter to hire and pay a fee to consultants or search firms to assist in the process of identifying and evaluating candidates. The Nominations and Governance Committee, and our board of directors, believe that directors should possess the highest personal and professional ethics, integrity and values, and be committed to representing the long-term interests of the Company’s stockholders. Each director must also be able to dedicate the time and resources sufficient to ensure the diligent performance of his or her duties. Further, our board of directors is intended to encompass a range of talents, experience, skills, backgrounds, and expertise sufficient to provide sound and prudent guidance with respect to the operations and interests of the Company and its stockholders. The Company values diversity and seeks to achieve a diversity of professional experiences, personal backgrounds, and personal characteristics, including race, ethnicity, age, gender and sexual orientation, on our board of directors, but no specific policy regarding board diversity has been adopted. We have adopted the Rooney Rule to increase diversity of board candidates for future elections.
Stockholders who wish to suggest qualified candidates should write to the Chair of the Nominations and Governance Committee c/o VolitionRx Limited, 1489 West Warm Springs Road, Suite 110, Henderson, Nevada 89014, in accordance with the time periods and information requirements set forth in the Bylaws, specifying the name of the candidates and stating in detail the qualifications of such persons for consideration by the committee. A written statement from the candidate consenting to be named as a candidate and, if nominated and elected, to serve as a director should accompany any such recommendation.
| 91 |
| Table of Contents |
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth the principal positions of the named executive officers of the Company and the compensation awarded to, earned by or paid to such persons for all services rendered in all capacities to the Company and its subsidiaries, for the fiscal years ended December 31, 2023, and 2022.
|
| Notes |
|
| Year |
| Salary |
|
| Bonus |
|
| Fees |
|
| Notes |
|
| Option Awards |
|
| Stock Awards |
|
| All Other Compensation |
|
| Notes |
|
| Total |
| ||||||||||
Name and Principal Position |
|
|
|
| ($) |
|
| ($) |
|
| ($) |
|
|
|
| ($)(1) |
|
| ($)(2) |
|
| ($) |
|
|
|
| ($) |
| ||||||||||||||
Cameron Reynolds |
|
| (3) |
| 2023 |
|
| 391,011 |
|
|
| 39,495 |
|
|
| 115,219 |
|
|
| (4)(5) |
|
| 26,297 |
|
|
| 174,202 |
|
|
| 26,709 |
|
|
|
|
|
| 772,933 |
| |||
President and Chief Executive Officer |
|
|
|
|
| 2022 |
|
| - |
|
|
| - |
|
|
| 509,824 |
|
|
| (4)(5) |
|
| 97,165 |
|
|
| 124,782 |
|
|
| 28,840 |
|
|
| (4) |
|
| 760,611 |
| ||
Dr. Gaetan Michel |
|
| (6) |
| 2023 |
|
| 394,689 |
|
|
| 58,940 |
|
|
| 35,436 |
|
|
| (7)(8) |
|
| 24,253 |
|
|
| 125,406 |
|
|
| 29,084 |
|
|
|
|
|
|
| 667,808 |
| ||
Chief Operating Officer |
|
|
|
|
| 2022 |
|
| 331,201 |
|
|
| 54,734 |
|
|
| 31,981 |
|
|
| (7)(8) |
|
| 80,397 |
|
|
| 100,157 |
|
|
| 27,143 |
|
|
|
|
|
|
| 625,613 |
| |
Dr. Salvatore Thomas Butera |
|
| (9) |
| 2023 |
|
| 332,753 |
|
|
| 128,600 |
|
|
|
|
|
|
|
|
|
|
| 15,062 |
|
|
| 127,474 |
|
|
| 15,900 |
|
|
|
|
|
|
| 619,789 |
| |
Chief Executive Office, Volition Vet |
|
|
|
|
| 2022 |
|
| 297,667 |
|
|
| 100,000 |
|
|
|
|
|
|
|
|
|
|
| 55,653 |
|
|
| 206,305 |
|
|
| 16,467 |
|
|
|
|
|
|
| 676,092 |
|
(1) | All option and warrant award amounts have been calculated based upon the aggregate grant date fair value computed in accordance with FASB ASC Topic 718. Information regarding assumptions made in valuing the option and warrant awards can be found in Note 8 of the “Notes to the Consolidated Financial Statements” included in Item 8 of this Report. The amounts disclosed do not necessarily reflect the dollar amounts of compensation actually realized, or that may be realized, by our named executive officers with respect to the options and warrants. |
(2) | Amounts listed include the value of granted performance-based restricted stock units not yet deemed earned. |
(3) | Mr. Reynolds’salary for the year ended December 31, 2023, was determined pursuant to the Reynolds Employment Agreement (for the period and as described in the section entitled Employment and Consulting Agreements). The amount disclosed under Bonus reflects one month’s salary paid to Mr. Reynolds as a bonus for the year ended December 31, 2023, payable upon the achievement of certain specified corporate goals. On |
| 92 |
| Table of Contents |
(4) | These amounts were paid in Singapore Dollars at an average exchange rate of $0.74 USD to $1 SGD and of $0.73 USD to $1 SGD for the years ended December 31, 2023 and 2022, respectively. |
(5) | This amount was paid to PB Commodities Pte. Ltd., or PB Commodities, for the provision of the services |
(6) | Dr. Michel’s salary for the year ended December 31, 2023 and 2022, was determined pursuant to the Michel Employment Agreement (as described in the section entitled Employment and Consulting Agreements). The amount disclosed under Bonus reflects two month’s salary paid to Dr. Michel as a bonus, upon the achievement of certain specified corporate goals, for each of the years ended December 31, 2023 and 2022, respectively. On October 4, 2022, Dr. Michel was granted a restricted stock unit award for 74,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Dr. Michel’s continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. On October 4, 2022, Dr. Michel was also granted a restricted stock unit award for 100,000 shares of common stock of VolitionRx under the 2015 Plan, vesting upon the achievement of a closing stock price target above $5.00 per share of the Company’s common stock for a minimum of ten consecutive trading days prior to October 4, 2025, and also subject to time-based vesting in a single installment six months after the timely achievement of the closing stock price target, if at all. On September 28, 2023, Dr. Michel was granted a restricted stock unit award for 101,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Dr. Michel’s continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. On October 19, 2023, Dr. Michel was also granted a restricted stock unit award for 100,000 shares of common stock of VolitionRx under the 2015 Plan, vesting upon the achievement of a closing stock price target above $5.00 per share of the Company’s common stock for a minimum of thirty consecutive trading days prior to October 19, 2026, and also subject to time-based vesting in a single installment six months after the timely achievement of the closing stock price target, if at all. The amounts disclosed under All Other Compensation consist of (i) $19,184 paid by Volition |
(7) | These amounts were paid in Euros at an average exchange rate of $1.08 USD to €1 EUR and of $1.05 USD to €1 EUR for the years ended December 31, 2023 and 2022, respectively. |
(8) | This amount was paid to 3F Management SPRL, |
(9) | Dr. Butera’s salary for the years ended December 31, 2023 and 2022, was determined pursuant to the Butera Employment Agreement (as described in the section entitled Employment and Consulting Agreements). The amount disclosed under Bonus reflects an annual cash bonus of up to 40% of his base salary. On October 4, 2022, Dr. Butera was granted a restricted stock unit award for 64,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Dr. Butera’s continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. On September 28, 2023, Dr. Butera was granted a restricted stock unit award for 85,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Dr. Butera’s continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. The amounts disclosed under All Other Compensation consist of (i) $6,000 paid by Volition Vet for the premiums of a health insurance policy for Dr. Butera and his dependents, plus $9,900 in contributions made by Volition Vet to Dr. Butera’s 401(k) pension plan during the year ended December 31, 2023, and (ii) $7,317 paid by Volition Vet for the premiums of a health insurance policy for Dr. Butera and his dependents, plus $9,150 in contributions made by Volition Vet to Dr. Butera’s 401(k) pension plan during the year ended December 31, 2022. |
| 93 |
| Table of Contents |
Employment and Consulting Agreements
Cameron Reynolds
Effective December 1, 2020, Singapore Volition and PB Commodities, a Singapore corporation, entered into a Consulting Services Agreement, or the Reynolds Consulting Agreement. Pursuant to the terms of the Reynolds Consulting Agreement, Singapore Volition will make available the services of Mr. Reynolds, as Group Chief Executive Officer, to VolitionRx and its subsidiaries, pursuant to the services agreements entered into by and between Singapore Volition and VolitionRx or its subsidiaries. The term of the Reynolds Consulting Agreement is perpetual until terminated upon six months’ prior notice. The agreement includes a six-month non-compete following termination of the agreement. PB Commodities received a monthly fee of $50,994 SGD (increased from $48,450 SGD on May 1, 2022) in exchange for the services provided by Mr. Reynolds, subject to annual review and adjustment. Effective March 1, 2023, Mr. Reynolds entered into an Employment Agreement with Singapore Volition, or the Reynolds Employment Agreement, which superseded and replaced the Reynolds Consulting Agreement. Pursuant to the terms of the Reynolds Employment Agreement, Mr. Reynolds shall serve as Group Chief Executive Officer of Singapore Volition. Singapore Volition will also make available the services of Mr. Reynolds, as Group Chief Executive Officer, to VolitionRx and its other subsidiaries, pursuant to services agreements entered into by and between Singapore Volition and VolitionRx or its subsidiaries. In exchange for his services, Mr. Reynolds shall receive, among other things (i) $39,495 per month (increased from $37,525 on May 1, 2023) from Singapore Volition (subject to annual review and adjustment), (ii) payment of up to $1,500 per month towards the premiums of a health insurance policy for Mr. Reynolds and his dependents, (iii) payment of 3% of the aggregate of Mr. Reynolds’ salary and any cash bonus awards towards Mr. Reynolds’ personal pension scheme and (iv) a lump sum severance payment if terminated by Singapore Volition without cause (as per the agreement) equal to the salary that he would have received between the date of termination and the completion of a six-month notice period. The foregoing description of each of the Reynolds Consulting Agreement and the Reynolds Employment Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 21, 2020, and Exhibit 10.27 to the Company’s Annual Report on Form 10-K filed with the SEC on March 15, 2023.
Dr. Gaetan Michel
On September 15, 2021, Dr. Michel entered into an Employment Agreement with Volition America, or the Michel Employment Agreement. Pursuant to the terms of the Michel Employment Agreement, Dr. Michel shall serve as Chief Operating Officer of Volition America. Volition America will also make available the services of Dr. Michel, as Chief Operating Officer, to VolitionRx and its other subsidiaries, pursuant to services agreements entered into by and between Volition America and VolitionRx or its subsidiaries. In exchange for his services, Dr. Michel shall receive (i) $30,873 per month (increased from $28,067 on May 1, 2023 and subject to annual review and adjustment); and (ii) a lump sum severance payment if terminated by Volition America without cause (as per the agreement) equal to the salary that he would have received between the date of termination and the completion of a three-month notice period. On September 15, 2021, Volition Global Services and 3F Management entered into a Consulting Services Agreement, or the Michel Consulting Agreement. Pursuant to the terms of the Michel Consulting Agreement, 3F Management will make available the services of Dr. Michel as Chief Executive Officer of Volition Global Services. 3F Management receives a monthly fee of €2,430 (increased from €2,210 on May 1, 2023) in exchange for the services provided by Dr. Michel, subject to annual review and adjustment. The foregoing description of each of the Michel Employment Agreement, and the Michel Consulting Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 10, 2021, and Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on November 10, 2021.
Dr. Salvatore Thomas Butera
Effective May 1, 2021, Dr. Butera entered into an Employment Agreement with Volition Vet, or the Butera Employment Agreement. Pursuant to the terms of the Butera Employment Agreement, Dr. Butera shall serve as Chief Executive Officer of Volition Vet. In exchange for his services, Dr. Butera shall receive (i) $28,198 per month (increased from $26,792 on May 1, 2023 and subject to annual review and adjustment); (ii) an annual cash bonus of up to 40% of his base salary unless raised by the VolitionRx Board of Directors, its Compensation Committee or the Company’s Board of Managers, as applicable, and (iii) a lump sum severance payment if terminated by Volition Vet without cause (as per the agreement) equal to the salary that he would have received between the date of termination and the completion of a two-month notice period. The foregoing description of the Butera Employment Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.6 to the Company’s Quarterly Report on Form 10-Q filed with the SEC on May 11, 2021.
| 94 |
| Table of Contents |
Outstanding Equity Awards
The following table summarizes the outstanding restricted stock unit awards for our named executive officers as of the fiscal year ended December 31, 2023.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
Equity Incentive Plan Awards:
Restricted Stock Units (RSUs)
Name |
| Grant Date |
| # of Shares, units or other rights unvested |
|
| Notes |
|
| Market value of shares, units or other rights unvested |
| |||
|
|
|
|
|
|
|
|
|
|
| (1) | |||
Cameron Reynolds |
| October 04, 2022 |
|
| 59,333 |
|
|
| (2) |
| $ | 42,542 |
| |
|
| October 04, 2022 |
|
| 200,000 |
|
|
| (3) |
|
| $ | 143,400 |
|
|
| September 28, 2023 |
|
| 118,000 |
|
|
| (4) |
| $ | 84,606 |
| |
|
| October 19, 2023 |
|
| 200,000 |
|
|
| (5) |
| $ | 143,400 |
| |
Dr. Gaetan Michel |
| October 04, 2022 |
|
| 49,333 |
|
|
| (6) |
| $ | 35,372 |
| |
|
| October 04, 2022 |
|
| 100,000 |
|
|
| (7) |
| $ | 71,700 |
| |
|
| September 28, 2023 |
|
| 101,000 |
|
|
| (8) |
| $ | 72,417 |
| |
|
| October 19, 2023 |
|
| 100,000 |
|
|
| (9) |
| $ | 71,700 |
| |
Dr. Salvatore Thomas Butera |
| May 01, 2021 |
|
| 50,000 |
|
|
| (10) |
| $ | 35,850 |
| |
|
| October 04, 2022 |
|
| 42,666 |
|
|
| (11) |
| $ | 30,592 |
| |
|
| September 28, 2023 |
|
| 85,000 |
|
|
| (12) |
| $ | 60,945 |
| |
(1) | The market value of unvested restricted stock unit awards as of December 31, 2023, is calculated by multiplying the number of shares subject to such awards by the closing price of our common stock on December 29, 2023, the last trading day of the year, which was $0.717 per share. |
(2) | These restricted stock units were awarded on October 4, 2022, subject to vesting upon achievement of certain corporate performance goals on or prior to December 31, 2022, and June 30, 2023, and also subject to time-based vesting. The performance goals were deemed met on October 13, 2022, January 12, 2023 and July 5, 2023, resulting in an aggregate of 100% of the rights to the referenced restricted stock units vesting. Upon meeting the performance goals, 29,667 of the restricted stock units vested on October 4, 2023. The balance of 59,333 restricted stock units shall vest in two installments of 29,667 and 29,666 units on each of October 4, 2024 and 2025. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
(3) | These restricted stock units were awarded on October 4, 2022, subject to vesting upon achievement of a closing stock price target above $5.00 per share of the Company’s common stock for a minimum of ten consecutive trading days prior to October 4, 2025. Upon meeting the closing stock price target, if at all, the restricted stock units shall vest in a single installment six months after the timely achievement of the closing stock price target. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
(4) | These restricted stock units were awarded on September 28, 2023 subject to vesting upon achievement of certain corporate performance goals on or prior to December 31, 2023 and June 30, 2024, and also subject to time-based vesting. On January 24, 2024 certain of the performance goals were deemed to have been timely met resulting in vesting of rights with respect to 50,150 restricted stock units. Upon meeting the performance goals, the restricted stock units are further subject to a 3-year time-based vesting schedule, vesting in three installments of approximately one-third on each of September 28, 2024, 2025 and 2026, respectively. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
| 95 |
| Table of Contents |
(5) | These restricted stock units were awarded on October 19, 2023, subject to vesting upon achievement of a closing stock price target above $5.00 per share of the Company’s common stock for a minimum of thirty consecutive trading days prior to October 19, 2026. Upon meeting the closing stock price target, if at all, the restricted stock units shall vest in a single installment six months after the timely achievement of the closing stock price target. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
(6) | These restricted stock units were awarded on October 4, 2022, subject to vesting upon achievement of certain corporate performance goals on or prior to December 31, 2022 and June 30, 2023, and also subject to time-based vesting. The performance goals were deemed met on October 13, 2022, January 12, 2023 and July 5, 2023, resulting in an aggregate of 100% of the rights to the referenced restricted stock units vesting. Upon meeting the performance goals, 24,667 of the restricted stock units vested on October 4, 2023. The balance of 49,333 restricted stock units shall vest in two installments of 24,667 and 24,666 units on each of October 4, 2024 and 2025. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
(7) | These restricted stock units were awarded on October 4, 2022, subject to vesting upon achievement of a closing stock price target above $5.00 per share of the Company’s common stock for a minimum of ten consecutive trading days prior to October 4, 2025. Upon meeting the closing stock price target, if at all, the restricted stock units shall vest in a single installment six months after the timely achievement of the closing stock price target. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
(8) | These restricted stock units were awarded on September 28, 2023 subject to vesting upon achievement of certain corporate performance goals on or prior to December 31, 2023 and June 30, 2024, and also subject to time-based vesting. On January 24, 2024, certain of the December 2023 performance goals were deemed to have been timely met, resulting in the vesting of rights with respect to 42,925 restricted stock units. Upon meeting the performance goals, the restricted stock units are further subject to a 3-year time-based vesting schedule, vesting in three installments of approximately one-third on each of September 28, 2024, 2025 and 2026, respectively. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
(9) | These restricted stock units were awarded on October 19, 2023, subject to vesting upon achievement of a closing stock price target above $5.00 per share of the Company’s common stock for a minimum of thirty consecutive trading days prior to October 19, 2026. Upon meeting the closing stock price target, if at all, the restricted stock units shall vest in a single installment six months after the timely achievement of the closing stock price target. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
(10) | These restricted stock units were awarded on May 1, 2021, subject to a 3-year time-based vesting schedule, vesting in three installments of approximately 50,000 restricted stock units on each of May 1, 2022, 2023 and 2024, respectively. 50,000 of the restricted stock units vested on each of May 1, 2022 and 2023, respectively. The balance of 50,000 restricted stock units shall vest on May 1, 2024. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
(11) | These restricted stock units were awarded on October 4, 2022, subject to vesting upon achievement of certain corporate performance goals on or prior to December 31, 2022 and June 30, 2023, and also subject to time-based vesting. The performance goals were deemed met on October 13, 2022, January 12, 2023 and July 5, 2023, resulting in an aggregate of 100% of the rights to the referenced restricted stock units vesting. Upon meeting the performance goals, 21,334 of the restricted stock units vested on October 4, 2023. The balance of 42,666 restricted stock units shall vest in two equal installments of on each of October 4, 2024 and 2025. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
(12) | These restricted stock units were awarded on September 28, 2023 subject to vesting upon achievement of certain corporate performance goals on or prior to December 31, 2023 and June 30, 2024, and also subject to time-based vesting. On January 24, 2024 certain of the December 2023 performance goals were deemed to have been timely met, resulting in the vesting of rights with respect to 36,125 restricted stock units. Upon meeting the performance goals, the restricted stock units are further subject to a 3-year time-based vesting schedule, vesting in three installments of approximately one-third on each of September 28, 2024, 2025 and 2026, respectively. Upon vesting and settlement, the holder will receive a number of shares of common stock equal to the number of restricted stock units that have vested. |
| 96 |
| Table of Contents |
The following table sets forth the outstanding stock option awards for our named executive officers as of the fiscal year ended December 31, 2023.
Stock Options
Name |
| Grant Date |
| Options Exercisable (#) |
|
| Notes |
|
| Options Expiration Date | |||
Cameron Reynolds |
| July 23, 2015 |
|
| 55,000 |
|
|
| (1) |
| July 23, 2025 | ||
|
| April 15, 2016 |
|
| 125,000 |
|
|
| (2) |
|
| April 15, 2026 | |
|
| March 30, 2017 |
|
| 100,000 |
|
|
| (3) |
| March 30, 2027 | ||
|
| January 23, 2018 |
|
| 75,000 |
|
|
| (4) |
| January 23, 2028 | ||
|
| February 11, 2019 |
|
| 50,000 |
|
|
| (5) |
| February 11, 2025 | ||
|
| April 13, 2020 |
|
| 85,000 |
|
|
| (6) |
| April 13, 2030 | ||
|
| August 03, 2021 |
|
| 91,486 |
|
|
| (7) |
| August 03, 2031 | ||
Dr. Gaetan Michel |
| July 23, 2015 |
|
| 2,000 |
|
|
| (8) |
| July 23, 2025 | ||
|
| October 04, 2021 |
|
| 73,360 |
|
|
| (9) |
| October 04, 2031 | ||
Dr. Salvatore Thomas Butera |
| August 03, 2021 |
|
| 52,400 |
|
|
| (10) |
| August 03, 2031 | ||
(1) | On July 23, 2015, Mr. Reynolds was granted an option to purchase 55,000 shares of common stock of VolitionRx under the 2011 Equity Incentive Plan, or the 2011 Plan, vesting in full on the six-month anniversary of the date of grant. In December 2018, the board of directors amended the terms of the option to extend the expiration date of the vested installment from four years from the vesting date to five years and six months from the vesting date, or July 23, 2021. In July 2021, the board of directors further amended the terms of the option to extend the expiration date of the vested installment from six years to ten years from grant (or July 23, 2025). |
(2) | On April 15, 2016, Mr. Reynolds was granted an option to purchase 125,000 shares of common stock of VolitionRx under the 2015 Plan, vesting in full on the 12-month anniversary of the date of grant. In November 2021, the Compensation Committee amended the terms of the option to extend the expiration date of the vested installment from six years to ten years from grant (or April 15, 2026). |
(3) | On March 30, 2017, Mr. Reynolds was granted an option to purchase 100,000 shares of common stock of VolitionRx under the 2015 Plan, vesting in full on the 12-month anniversary of the date of grant. In November 2021, the Compensation Committee amended the terms of the option to extend the expiration date of the vested installment from six years to ten years from grant (or March 30, 2027). |
(4) | On January 23, 2018, Mr. Reynolds was granted an option to purchase 75,000 shares of common stock of VolitionRx under the 2015 Plan, vesting in full on the 12-month anniversary of the date of grant. In November 2021, the Compensation Committee amended the terms of the option to extend the expiration date of the vested installment from six years to ten years from grant (or January 23, 2028). |
(5) | On February 11, 2019, Mr. Reynolds was granted an option to purchase 50,000 shares of common stock of VolitionRx under the 2015 Plan, vesting in full on the 12-month anniversary of the date of grant. This option expires six years from the date of grant (or February 11, 2025). |
(6) | On April 13, 2020, Mr. Reynolds was granted an option to purchase 85,000 shares of common stock of VolitionRx under the 2015 Plan, vesting in full on the 12-month anniversary of the date of grant. In December 2021, the Compensation Committee amended the terms of the option to extend the expiration date of the vested installment from six years to ten years from grant (or April 13, 2030). |
| 97 |
| Table of Contents |
(7) | On August 3, 2021, Mr. Reynolds was granted an option to purchase 91,486 shares of common stock of VolitionRx under the 2015 Plan, vesting upon achievement of certain corporate performance goals on or prior to July 1, 2022, and also subject to a 2-year time-based vesting schedule, vesting in two installments at 12 months and at 24 months from the date of grant. The performance goals were deemed met on April 7, 2022, and June 23, 2022 respectively, resulting in the rights to the referenced options vesting. Upon meeting the performance goals, 50% of the options vested on August 3, 2022, and August 3, 2023, respectively. |
(8) | On July 23, 2015, Dr. Michel was granted an option to purchase 2,000 shares of common stock of VolitionRx under the 2011 Plan, vesting in full on the six-month anniversary of the date of grant. In December 2018, the board of directors amended the terms of the option to extend the expiration date of the vested installment from four years from the vesting date to five years and six months from the vesting date, or July 23, 2021. In July 2021, the board of directors further amended the terms of the option to extend the expiration date of the vested installment from six years to ten years from grant (or July 23, 2025). |
(9) | On October 4, 2021, Dr. Michel was granted an option to purchase 73,360 shares of common stock of VolitionRx under the 2015 Plan, vesting upon achievement of certain corporate performance goals on or prior to July 1, 2022, and also subject to a 2-year time-based vesting schedule, vesting in two installments at 12 months and at 24 months from the date of grant. The performance goals were deemed met on April 7, 2022, and June 23, 2022 respectively, resulting in the rights to the referenced options vesting. Upon meeting the performance goals, 50% of the options vested on October 4, 2022, and October 4, 2023, respectively. |
(10) | On August 3, 2021, Dr. Butera was granted an option to purchase 52,400 shares of common stock of VolitionRx under the 2015 Plan, vesting upon achievement of certain corporate performance goals on or prior to July 1, 2022, and also subject to a 2-year time-based vesting schedule, vesting in two installments at 12 months and at 24 months from the date of grant. The performance goals were deemed met on April 7, 2022, and June 23, 2022 respectively, resulting in the rights to the referenced options vesting. Upon meeting the performance goals, 50% of the options vested on August 3, 2022, and August 3, 2023, respectively. |
Long-Term Incentive Plans
Since July 1, 2021, we have offered to our eligible U.S.-based salaried employees, including our U.S.-based named executive officers, a customary, tax-qualified defined contribution retirement (401(k)) plan. For 2022, we provided a company match on employee contributions of 100% on the first 3% of an employee’s pay, which we believe to be in line with prevailing practices for major U.S. corporations.
Volition Diagnostics operates a Group Personal Pension Plan, or the Pension Plan, and makes defined monthly contributions into a separate fund on behalf of its eligible United Kingdom employees, as required by the Pensions Act 2008 (UK). Certain of the Company’s executive officers who are based in the United Kingdom are eligible to participate in the Pension Plan. Volition Diagnostics contributes five percent of the gross salary paid to those of its eligible employees to the Pension Plan. Those eligible employees are also required to contribute to the Pension Plan. All risks associated with this type of plan are assumed by the employees. The Pension Plan was effective commencing April 6, 2017.
Other than the foregoing, there are no arrangements or plans in which VolitionRx or its direct or indirect subsidiaries provides pension, retirement or similar benefits for directors or executive officers.
Severance and Change of Control Benefits
In the event of a termination of employment under certain circumstances, the named executive officers are entitled to severance payments as detailed in the section of this Proxy Statement entitled Employment and Consulting Agreements.
Additionally, under certain circumstances involving a change in control, merger, sale of all or substantially all of our assets or other similar corporate transaction, where the successor or acquiring corporation (if any) refuses to assume, convert, replace or substitute awards, then the vesting of unvested awards will accelerate pursuant to the terms of the 2015 Plan.
| 98 |
| Table of Contents |
Compensation of Directors
The following table sets forth the compensation paid to the directors of VolitionRx for the fiscal year ended December 31, 2023 other than directors who also served as named executive officers. No executive officer is paid compensation for his role as a director. There are no employment agreements by and between the Company and the non-employee directors.
Director Compensation Table
Name |
| Notes |
|
| Fees Earned or Paid In Cash |
|
| Stock Awards |
|
| Option Awards |
|
| All Other Compensation |
|
| Total |
| ||||||
|
|
|
|
|
|
|
| (1) |
| (2) |
|
|
|
|
|
| ||||||||
|
|
|
|
| $ |
|
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||||
Dr. Martin Faulkes |
|
| (3) |
|
| 205,126 |
|
|
| 65,026 |
|
|
| 12,779 |
|
|
| 34,381 |
|
|
| 317,312 |
| |
Dr. Phillip Barnes |
|
| (4) |
|
| 43,363 |
|
|
| 16,093 |
|
|
| 2,482 |
|
|
| - |
|
|
| 61,938 |
| |
Richard Brudnick |
|
| (5) |
|
| 21,682 |
|
|
| 6,662 |
|
|
| 2,482 |
|
|
| - |
|
|
| 30,826 |
| |
Dr. Alan Colman |
|
| (6) |
|
| 65,045 |
|
|
| 17,233 |
|
|
| 3,724 |
|
|
| - |
|
|
| 86,002 |
| |
Dr. Edward Futcher |
|
| (7) |
|
| 43,363 |
|
|
| 16,093 |
|
|
| 2,482 |
|
|
| - |
|
|
| 61,938 |
| |
Mickie Henshall |
|
| (8) |
|
| - |
|
|
| 2,218 |
|
|
| - |
|
|
| - |
|
|
| 2,218 |
| |
Guy Innes |
|
| (9) |
|
| 43,363 |
|
|
| 16,093 |
|
|
| 2,482 |
|
|
| - |
|
|
| 61,938 |
| |
Kim Nguyen |
|
| (10) |
|
| 43,363 |
|
|
| 16,093 |
|
|
| 2,482 |
|
|
| - |
|
|
| 61,938 |
| |
(1) | Amounts listed include the value of granted performance-based restricted stock units not yet deemed earned. The restricted stock units granted to all directors (except Mr. Brudnick) on September 28, 2023, had share prices of $0.70. |
(2) | All option awards have been calculated based upon the aggregate grant date fair value computed in accordance with FASB ASC Topic 718. The Company has calculated the estimated fair market value of these options granted on August 3, 2021, using the Black-Scholes model and the following assumptions: term 5.5 years; stock price: $3.31; exercise price: $3.40; 69.13% volatility; 1.19% risk-free rate, and no forfeiture rate. The amounts disclosed do not necessarily reflect the dollar amounts of compensation actually realized, or that may be realized, by our directors with respect to the options. |
(3) | On March 7, 2017, Dr. Faulkes entered into an Employment Agreement with Volition Diagnostics, or the Faulkes Employment Agreement, which took effect on April 1, 2017. Volition Diagnostics agreed to make available to VolitionRx the services of Dr. Faulkes as Executive Chairman of the board of directors of VolitionRx, pursuant to a services agreement entered into by and between Volition |
On September 28, 2023, Dr. Faulkes was granted a
| |
The amount disclosed under All Other Compensation reflects two months’ salary paid to Dr. Faulkes as a bonus for the year ended December 31, 2023, payable upon the achievement of certain specified corporate goals. |
| 99 |
| Table of Contents |
(4) | On October 9, 2019, Dr. Barnes entered into an Independent Director Agreement with VolitionRx, or the Barnes Independent Director Agreement, pursuant to which Dr. Barnes will continue to serve as a member of the Board of VolitionRx subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRx’s governing documents. In exchange for his services, Dr. Barnes received $10,840 per calendar quarter. The foregoing description of the Barnes Independent Director Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.33 to the Company’s Quarterly Report on Form 10-Q filed May 12, 2015. |
On September 28, 2023, Dr. Barnes was granted a restricted stock unit award for 20,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Dr. Barnes’ continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. | |
(5) | On March 25, 2021, Mr. Brudnick entered into an Independent Director Agreement with VolitionRx, or the Brudnick Independent Director Agreement, pursuant to which Mr. Brudnick will continue to serve as a member of the Board of VolitionRx subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRx’s governing documents. In exchange for his services, Mr. Brudnick received $10,840 per calendar quarter, pro-rated for length of service. The foregoing description of the Brudnick Independent Director Agreement does not purport to summary all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.33 to the Company’s Quarterly Report on Form 10-Q filed May 12, 2015. |
Effective June 28, 2023, Mr. Brudnick resigned from the Board of VolitionRx. Mr. Brudnick’s (i) options to purchase 4,317 shares of common stock and 17,343 restricted stock units expired unvested upon his resignation; and (ii) further options to purchase 4,317 shares of common stock expired on September 28, 2023. | |
(6) | On March 31, 2015, Dr. Colman entered into an Independent Director Agreement with VolitionRx, or the Colman Independent Director Agreement, pursuant to which Dr. Colman will continue to serve as a member of the Board of VolitionRx subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRx’s governing documents. In exchange for his services, Dr. Colman received $16,261 per calendar quarter. The foregoing description of the Colman Independent Director Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.33 to the Company’s Quarterly Report on Form 10-Q filed May 12, 2015. |
On September 28, 2023, Dr. Colman was granted a restricted stock unit award for 20,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Dr. Colman’s continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. | |
(7) | On June 23, 2016, Dr. Futcher entered into an Independent Director Agreement with VolitionRx, or the Futcher Independent Director Agreement, pursuant to which Dr. Futcher will continue to serve as a member of the Board of VolitionRx subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRx’s governing documents. In exchange for his services, Dr. Futcher received $10,840 per calendar quarter. The foregoing description of the Futcher Independent Director Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.33 to the Company’s Quarterly Report on Form 10-Q filed May 12, 2015. |
On September 28, 2023, Dr. Futcher was granted a restricted stock unit award for 20,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Dr. Futcher’s continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. | |
(8) | On August 15, 2022, Ms. Henshall entered into an Independent Director Agreement with VolitionRx, or the Henshall Independent Director Agreement, pursuant to which Ms. Henshall will continue to serve as a member of the Board of VolitionRx subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRx’s governing documents. At this time, Ms. Henshall has elected to not receive any cash compensation in exchange for her services. The foregoing description of the Henshall Independent Director Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.33 to the Company’s Quarterly Report on Form 10-Q filed May 12, 2015. |
| 100 |
| Table of Contents |
On September 28, 2023, Ms. Henshall was granted a restricted stock unit award for 20,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Ms. Henshall’s continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. | |
(9) | On March 31, 2015, Mr. Innes entered into an Independent Director Agreement with VolitionRx, or the Innes Independent Director Agreement, pursuant to which Mr. Innes will continue to serve as a member of the Board of VolitionRx subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRx’s governing documents. In exchange for his services, Mr. Innes received $10,840 per calendar quarter. The foregoing description of the Innes Independent Director Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.33 to the Company’s Quarterly Report on Form 10-Q filed May 12, 2015. |
On September 28, 2023, Mr. Innes was granted a restricted stock unit award for 20,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Mr. Innes’ continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. | |
(10) | On March 25, 2021, Ms. Nguyen entered into an Independent Director Agreement with VolitionRx, or the Nguyen Independent Director Agreement, pursuant to which Ms. Nguyen will continue to serve as a member of the Board of VolitionRx subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRx’s governing documents. In exchange for her services, Ms. Nguyen received $10,840 per calendar quarter. The foregoing description of the Nguyen Independent Director Agreement does not purport to summary all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.33 to the Company’s Quarterly Report on Form 10-Q filed May 12, 2015. |
On September 28, 2023, Ms. Nguyen was granted a restricted stock unit award for 20,000 shares of common stock of VolitionRx under the 2015 Plan, vesting, subject to the achievement of certain corporate goals and Ms. Nguyen’s continued service, over 3-years in three equal installments at 12 months, at 24 months, and at 36 months from the date of grant. |
Stockholder Recommendations to the Board of Directors
Stockholders can direct communications to our Secretary, Rodney Rootsaert, at our executive offices, located at 1489 West Warm Springs Road, Suite 110, Henderson, Nevada 89014. However, while we appreciate all comments from stockholders, we may not be able to individually respond to all communications. We attempt to address stockholder questions and concerns in our press releases and documents filed with the SEC so that all stockholders have access to information about us at the same time. Mr. Rootsaert collects and evaluates all stockholder communications. All communications addressed to our directors and executive officers will be reviewed by those parties unless the communication is clearly frivolous.
| 101 |
|
| Table of Contents |
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Security Ownership of Management
The following table sets forth certain information concerning the number of shares of our common stock owned beneficially as of March 15, 2024, by: (i) each of our directors and director nominees, (ii) each of our named executive officers; (iii) all of our directors and director nominees, and executive officers as a group; and (iv) each person or group known by us to beneficially own more than 5% of our outstanding shares of common stock.
We have determined beneficial ownership in accordance with the rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under the rules of the SEC, a person is deemed to be a beneficial owner of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of such security, or investment power, which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which the person has a right to acquire beneficial ownership within sixty (60) days. Under these rules more than one person may be deemed a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest.
Unless otherwise indicated below, to the best of our knowledge (i) each beneficial owner named in the table has the sole voting and sole investment power with respect to all shares beneficially owned, subject to community property laws where applicable, and (ii) the address of such beneficial owner is 1489 West Warm Springs Road, Suite 110, Henderson, Nevada 89014.
Name and Address of Beneficial Owner |
| Notes |
|
| Amount and Nature of Beneficial Ownership |
|
| Percent of Class (1) |
| |||
Directors and Named Executive Officers: |
|
|
|
| (#) |
|
| (%) |
| |||
Dr. Phillip Barnes |
|
| (2 | ) |
|
| 38,321 |
|
| * |
| |
Dr. Salvatore Thomas Butera |
|
| (3 | ) |
|
| 287,957 |
|
| * |
| |
Dr. Alan Colman |
|
| (4 | ) |
|
| 316,232 |
|
| * |
| |
Dr. Martin Faulkes |
|
| (5 | ) |
|
| 2,367,139 |
|
|
| 2.9% | |
Dr. Edward Futcher |
|
| (6 | ) |
|
| 481,571 |
|
| * |
| |
Mickie Henshall |
|
| (7 | ) |
|
| 11,206 |
|
| * |
| |
Guy Innes |
|
| (8 | ) |
|
| 2,734,810 |
|
|
| 3.3% | |
Dr. Gaetan Michel |
|
| (9 | ) |
|
| 123,612 |
|
| * |
| |
Thi Kim-Lien Nguyen |
|
| (10 | ) |
|
| 66,377 |
|
| * |
| |
Cameron Reynolds |
|
| (11 | ) |
|
| 2,868,465 |
|
|
| 3.5% | |
All Executive Officers and Directors as a Group (17 Persons) |
|
| (12 | ) |
|
| 11,061,057 |
|
|
| 12.9% | |
5% Stockholders: |
|
|
|
|
|
|
|
|
|
|
|
|
Eight Corporation Limited |
|
| (13 | ) |
|
| 12,005,332 |
|
|
| 14.6% | |
c/o Crowe Morgan |
|
|
|
|
|
|
|
|
|
|
|
|
8 St. George's Street |
|
|
|
|
|
|
|
|
|
|
|
|
Douglas, Isle of Man IM1 1 AF |
|
|
|
|
|
|
|
|
|
|
|
|
United Kingdom |
|
|
|
|
|
|
|
|
|
|
|
|
| 102 |
| Table of Contents |
(1) | For purposes of the table, the percent of class is based upon 82,068,442 shares of our common stock
|
(2) | Dr. Barnes’ beneficial ownership includes direct ownership of (i) 14,686 shares of common stock |
(3) | Dr. Butera’s beneficial ownership includes direct ownership of (i) 185,557 shares of common stock, (ii) options to purchase 52,400 shares of common stock that are exercisable within 60 days and (iii) restricted stock units for |
(4) | Dr. Colman’s beneficial ownership includes direct ownership of (i) 168,279 shares of common stock and (ii) options to purchase 147,953 shares of common stock that are exercisable within 60 days. |
(5) | Dr. Faulkes’ beneficial ownership includes direct ownership of (i) 1,591,682 shares of common stock and (ii) options to purchase 419,457 shares of common stock that are exercisable within 60 days. Dr. Faulkes’ beneficial ownership also includes indirect ownership of 356,000 shares of common stock held directly by The Dill Faulkes Educational Trust Limited, or DFET. Dr. Faulkes serves as the |
(6) | Dr. Futcher’s beneficial ownership includes direct ownership of (i) 61,936 shares of common stock and (ii) options to purchase 63,635 shares of common stock that are exercisable within 60 days. Dr. Futcher’s beneficial ownership also includes indirect ownership of 356,000 shares of common stock held directly by DFET. Dr. Futcher serves as a director and a trustee of DFET and shares voting and dispositive control over such shares. |
(7) | Ms. Henshall’s beneficial ownership consists of direct ownership of 11,206 shares of common stock. |
(8) |
|
(9) | Dr. Michel’s beneficial ownership includes direct ownership of (i) 48,252 shares of common stock and (ii) options to purchase 75,360 shares of common stock that are exercisable within 60 days. |
(10) | Ms. Nguyen’s beneficial ownership includes direct ownership of (i) 27,186 shares of common stock and (ii) options to purchase 8,635 shares of common stock that are exercisable within 60 days. Ms. Nguyen’s beneficial ownership also includes indirect ownership of 30,556 shares of common stock held directly by Ms. Nguyen’s spouse. |
(11) | Mr. Reynolds’ beneficial ownership includes direct ownership of (i) 1,245,185 shares of common stock and (ii) options to purchase 581,486 shares of common stock that are exercisable within 60 days. Mr. Reynolds’ beneficial ownership also includes indirect ownership of (x) 34,076 shares of common stock held directly by Mr. Reynolds’ spouse and (y) 1,007,718 shares of common stock held directly by Concord International, Inc., of which Mr. Reynolds is the majority stockholder and shares voting and dispositive control over such shares. |
(12) | The number of executive officers, directors as a group includes 2 executive officers of the Company’s subsidiaries. The amount beneficially owned by the executive officers, directors as a group consists of an aggregate of (i) 7,703,955 shares of common stock, (ii) options to purchase 2,997,102 shares of common stock that are exercisable within 60 days, (iii) restricted stock units for |
(13) | Based on the information contained in the |
| 103 |
|
| Table of Contents |
Changes in Control
We are not aware of any arrangements that have resulted, or may at a subsequent date result, in a change in control of the Company.
Securities Authorized for Issuance Under Equity Compensation Plans
Under the 2015 Plan, we may grant incentive awards, including options, restricted stock, stock bonuses, stock appreciation rights, restricted stock units or performance awards, to any qualified employee, officer, director, consultant or other service provider that provides services to us or any of our affiliates. An aggregate of 9,700,000 shares of our common stock are reserved for issuance under the 2015 Plan. The purpose of the 2015 Plan is to provide additional incentives to eligible participants to devote their utmost effort and skill to the advancement and betterment of the registrant, by providing them an opportunity to participate in the ownership of the registrant and thereby have an interest in the success and increased value of the Company. The 2015 Plan replaced the 2011 Plan which was approved by the stockholders. No further grants will be made under the 2011 Plan. The following table sets forth information about the securities authorized for issuance under our equity compensation plans as of December 31, 2023.
Plan Category |
| Number of Securities to be issued upon exercise of outstanding options, warrants and rights |
|
| Weighted-average exercise price of outstanding options, warrants and rights |
|
| Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column |
| |||
| (a) |
|
| (b) (1) |
|
| (a)) |
| ||||
Equity compensation plans approved by security holders: |
|
|
|
|
|
|
|
|
| |||
2011 Equity Incentive Plan |
|
| 292,000 |
|
| $ | 4.00 |
|
|
| - |
|
2015 Equity Incentive Plan |
|
| 8,042,521 |
|
| $ | 3.86 |
|
|
| 608,190 |
|
Equity compensation plans not approved by security holders (2) |
|
| 862,500 |
|
| $ | 3.05 |
|
|
| - |
|
Total |
|
| 9,197,021 |
|
|
| - |
|
|
| 608,190 |
|
1. | The weighted-average exercise price does not take into account 3,634,952 shares of common stock |
2. | Consists of warrants to purchase (i) 414,000 shares
|
| 104 |
| Table of |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
See Item 11. Executive Compensation – Employment and Consulting Agreements of this Report.
We provide indemnification to our directors and officers so that they will be free from undue concern about personal liability in connection with their service to the Company. Under our Bylaws and Certificate of Incorporation, we are required to indemnify our directors and officers to the extent permitted by Delaware law. Additionally, as part of the engagement letters and/or Independent Director Agreements with each of our directors and/or executive officers, certain indemnification provisions may require us, among other things, to indemnify our directors and executive officers for expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or officer in any action or proceeding arising out of his or her service as one of our directors or officers.
Other than the foregoing, we are not aware of any transaction to which we are a party that has occurred during the past two fiscal years, or in any currently proposed transaction, involving our directors, nominees for directors, executive officers, or any person who owned of record or was known to own beneficially more than 5% of our outstanding shares of common stock, or any associate or affiliate of such persons or companies, where such person or entity has any material interest, direct or indirect, in such transaction and that requires disclosure under Item 404(a) of Regulation S-K.
Director Independence
For purposes of determining director independence, the board of directors reviews a summary of the relationships of each director, including any of his or her family members or related parties, with the Company or management and other facts relevant to the analysis of whether the directors qualify as “independent directors” under the NYSE American Company Guide §803(A)(2). No director qualifies as independent unless the board of directors affirmatively determines that the director does not have a relationship that would interfere with the exercise of his or her independent judgment in carrying out his or her responsibilities as a director. In addition, the NYSE American Company Guide provides a non-exclusive list of persons who may not be considered independent.
The board of directors has affirmatively determined that each of Drs. Barnes, Colman and Futcher, Mss. Henshall and Nguyen, and Mr. Innes, is an independent director under the NYSE American Company Guide. In addition, the members of the Audit Committee are independent directors pursuant to the heightened independence criteria for members of Audit Committees set forth in the applicable SEC rules.
Our independent directors meet as often as is necessary to fulfill their responsibilities but at least annually in executive session without management or non-independent directors in accordance with the requirements of NYSE American Company Guide §802(c).
Policy on the Review, Approval or Ratification of Transactions with Related Persons
The Company has not adopted a separate written policy for the approval or ratification of all transactions with related parties that are required to be reported under Item 404(a) of Regulation S-K. Rather, at this time and pursuant to its existing charter, and unless otherwise provided by the board of directors, the Audit Committee of the board of directors reviews the material facts of all such transactions and either ratifies, approves or disapproves of the entry into the transaction.
No director is allowed to participate in the approval of a transaction for which he or she is a related party and the director has to provide all material information concerning the transaction to the Audit Committee.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
| Years Ended |
| |||||
|
| December 31, |
| |||||
|
| 2023 |
|
| 2022 |
| ||
|
| $ |
|
| $ |
| ||
Audit fees |
|
| 79,000 |
|
|
| 75,500 |
|
Audit-Related fees |
|
| - |
|
|
| - |
|
Tax fees |
|
| - |
|
|
| - |
|
All other fees |
|
| 12,500 |
|
|
| 42,407 |
|
Total |
|
| 91,500 |
|
|
| 117,907 |
|
Audit Fees
Represents the aggregate fees billed to us for each of the last two fiscal years for professional services rendered by the principal accountants for the audit of our annual financial statements and review of financial statements included in our Form 10-Q filings or services that are normally provided by the accountants in connection with statutory and regulatory filings or engagement for those fiscal years.
Audit-Related Fees
Represents the aggregate fees billed to us in each of the last two fiscal years for assurance and related services by the principal accountants that are reasonably related to the performance of the audit or review of our financial statements that are not already reported in Audit Fees. These services include accounting consultations and attestation services that are not required by statute.
Tax Fees
Represents the aggregate fees billed to us in each of the last two fiscal years for professional services rendered by the principal accountants for tax compliance, tax advice, and tax planning.
All Other Fees
Represents the aggregate fees billed in each of the last two fiscal years for products and services provided by the principal accountants to us, excluding those enumerated above.
Policy on Audit Committee Pre-approval of Audit and Permissible Non-audit Services of Independent Auditor
All audit and non-audit services by our independent registered public accounting firm are pre-approved by our Audit Committee. For audit services, the independent registered public accounting firm provides the Audit Committee with an audit plan, including proposed fees in advance of the annual audit. The Audit Committee approves the plan and fees for the audit.
Pursuant to its charter, the Audit Committee may establish pre-approval policies and procedures, subject to SEC and NYSE American rules and regulations, to approve audit and non-audit services; however, it has not yet done so.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
·segregation of duties in some areas of Finance;
·oversight in the area of Information Technology (”IT”), where certain processes may affect the internal controls over financial reporting; and
·monitoring of review controls with respect to accounting for complex transactions.
|
|
|
| Incorporated by Reference |
| ||||||||
Exhibit Number |
Exhibit Description |
| Form | File No. | Exhibit |
| Filing Date |
| Filed Herewith | ||||
|
| 8-K/A |
| 000-30402 |
| 2.1 |
| 5/8/12 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K/A |
| 000-30402 |
| 10.15 |
| 1/11/12 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 000-30402 |
| 2.1 |
| 9/29/11 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K/A |
| 000-30402 |
| 10.28 |
| 4/5/12 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Second Amended and Restated Certificate of Incorporation, as currently in effect. |
| 8-K |
| 001-36833 |
| 3.1 |
| 10/11/16 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-8 |
| 333-208512 |
| 4.2 |
| 12/11/15 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-36833 |
| 4.1 |
| 2/20/20 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K/A |
| 000-30402 |
| 10.6 |
| 2/24/12 |
|
|
| ||
| 107 |
| Table of Contents |
|
|
|
| Incorporated by Reference |
|
|
| ||||||
Exhibit Number |
| Exhibit Description |
| Form |
| File No. |
| Exhibit |
| Filing Date |
| Filed Herewith |
|
| VolitionRx Limited 2011 Equity Incentive Plan dated November 17, 2011. |
| 8-K |
| 000-30402 |
| 4.1 |
| 11/18/11 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| VoitionRx Limited 2011 Equity Incentive Plan Form of Stock Option Agreement. |
| 8-K |
| 000-30402 |
| 4.2 |
| 11/18/11 |
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| VolitionRx Limited 2011 Equity Incentive Plan Form of Stock Award Agreement for Restricted Stock. |
| 8-K |
| 000-30402 |
| 4.3 |
| 11/18/11 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| VolitionRx Limited 2015 Stock Incentive Plan, as amended March 27, 2019. |
| 8-K |
| 001-36833 |
| 10.1 |
| 6/30/23 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-8 |
| 333-214118 |
| 10.2 |
| 10/14/16 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-8 |
| 333-214118 |
| 10.3 |
| 10/14/16 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-8 |
| 333-214118 |
| 10.4 |
| 10/14/16 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-8 |
| 333-214118 |
| 10.5 |
| 10/14/16 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-8 |
| 333-214118 |
| 10.6 |
| 10/14/16 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-8 |
| 333-214118 |
| 10.7 |
| 10/14/16 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-Q |
| 001-36833 |
| 10.33 |
| 5/12/15 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-36833 |
| 10.1 |
| 10/31/16 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-36833 |
| 10.28 |
| 3/10/17 |
|
|
| ||
| 108 |
| Table of Contents |
|
|
|
| Incorporated by Reference |
|
|
| |||||||
Exhibit Number |
| Exhibit Description |
| Form |
| File No. |
| Exhibit |
| Filing Date |
| Filed Herewith |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| 10-K |
| 001-36833 |
| 10.30 |
| 3/10/17 |
|
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| 8-K |
| 001-36833 |
| 10.1 |
| 9/21/17 |
|
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| 10-Q |
| 001-36833 |
| 10.1 |
| 11/09/17 |
|
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| 10-K |
| 001-36833 |
| 10.22 |
| 2/20/20 |
|
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| 10-Q |
| 001-36833 |
| 1.1 |
| 11/12/20 |
|
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| 10-Q |
| 001-36833 |
| 10.1 |
| 11/12/20 |
|
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
| Common Stock Warrant issued by VolitionRx Limited to Gael Forterre, dated January 1, 2021. |
| 10-K |
| 001-36833 |
| 10.18 |
| 03/22/21 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| 10-K |
| 001-36833 |
| 10.19 |
| 3/22/21 |
|
|
| |||
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
| 10-K |
| 001-36833 |
| 10.20 |
| 3/22/21 |
|
|
| |||
| 109 |
| Table of Contents |
|
|
|
| Incorporated by Reference |
| ||||||||
Exhibit Number |
| Exhibit Description |
| Form |
| File No. |
|
Exhibit |
| Filing Date |
| Filed Herewith |
|
|
| 10-Q |
| 001-36833 |
| 10.6 |
| 5/11/21 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-3 |
| 333-259783 |
| 1.2 |
| 9/24/21 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-Q |
| 001-36833 |
| 10.1 |
| 11/10/21 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-Q |
| 001-36833 |
| 10.2 |
| 11/10/21 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-Q |
| 001-36833 |
| 10.3 |
| 11/10/21 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-Q |
| 001-36833 |
| 10.1 |
| 5/11/22 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-36833 |
| 1.1 |
| 5/20/22 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-36833 |
| 1.1 |
| 8/2/22 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-36833 |
| 1.1 |
| 2/21/23 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
001-36833 | 10.27 |
3/15/23 |
|
| |||||||
| 110 |
| Table of Contents |
|
|
|
| Incorporated by Reference | |||||||||
Exhibit Number |
| Exhibit Description |
| Form |
| File No. |
| Exhibit |
| Filing Date |
| Filed Herewith |
|
|
| 8-K |
| 001-36833 |
| 1.1 |
| 6/5/23 |
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Form of Underwriter Warrant issued to Prime Executions, Inc. dba Freedom Capital Markets. |
| 8-K |
| 001-36833 |
| 4.1 |
| 6/5/23 |
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| X
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| X |
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| X |
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Power of Attorney (included on the signature page of this Report). |
|
|
|
|
|
|
|
|
| X |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| X |
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| X |
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| X |
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| X |
| ||
| 111 |
| Table of Contents |
Incorporated by Reference | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Exhibit Number |
|
ITEM 16. FORM 10-K SUMMARY None.
SIGNATURES Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
POWER OF ATTORNEY KNOW ALL PERSONS BY THESE PRESENTS that each individual whose signature appears below constitutes and appoints Cameron Reynolds and Rodney Rootsaert, and each or either of them, acting individually, his or her true and lawful attorney-in-fact and agent, with full power of substitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or his, her or their substitute or substitutes, may lawfully do or cause to be done or by virtue hereof. Pursuant to the requirements of the Securities Exchange Act of 1934, this Report on Form 10-K has been signed below by the following persons in the capacities and on the date indicated.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||