| Title of each class | Trading Symbol | Name of each exchange on which registered | ||
| American LLC | ||||
| American LLC |
Securities Registeredregistered pursuant to Section 12(g) of the Act: Common Stock, No Par ValueNone
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☑☒No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☑☒No
Indicate by check mark whether the registrant (i) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒Yes☐ Yes ☑No
Indicate by checkmark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ☒ Yes☐ Yes ☑ No
Indicate by checkmark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or an emerging growth company. See the definitions of "large“large accelerated filer," "accelerated filer"” “accelerated filer,” “smaller reporting company,” and "smaller reporting company"“emerging growth company,” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in rule 12b-2 of the Act). ☐ Yes ☑☒No
The aggregate market value of the voting and non-votingRegistrant’s common equity held by non-affiliates computed by reference to the price at which the common equity was last sold or the average bid and asked price of such common equity, as of the last business day of the registrant'sRegistrant’s most recently completed second fiscal quarter: $228,720quarter was $40,216,244.
On March 27, 2017, the registrant had 11,277,20029, 2024, there were shares of common stockCommon Stock issued and outstanding.
SPLASH BEVERAGE GROUP, INC.
FORM 10-K FOR THE YEAR ENDED DECEMBER 31, 2016
TABLE OF CONTENTS
i
PART I
Except as otherwise indicated, references to “we”, “us”, “our”, “Splash”, “SBG” and the “Company” refer to Splash Beverage Group, Inc. and its wholly owned subsidiaries.
This Annual Report on Form 10-K (this “Annual Report”) contains “forward-looking statements” Forward-looking statements reflect our current view about future events. When used in this Report, the words “anticipate,” “believe,” “estimate,” “expect,” “future,” “intend,” “plan,” or the negative of these terms and similar expressions, as they relate to us or our management, identify forward-looking statements. Such statements include, but are not limited to, statements contained in this Report relating to our business strategy, our future operating results and liquidity and capital resources outlook. Forward-looking statements are based on our current expectations and assumptions regarding our business, the economy and other future conditions. Because forward–looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward-looking statements. They are neither statements of historical fact nor guarantees of assurance of future performance. We caution you therefore against relying on any of these forward-looking statements. Important factors that could cause actual results to differ materially from those in the forward-looking statements include, without limitation, our ability to raise capital to fund continuing operations; our ability to protect our intellectual property rights; the impact of any infringement actions or other litigation brought against us; competition from other providers and products; our ability to develop and commercialize products and services; changes in government regulation; our ability to complete capital raising transactions; and other factors (including the risks contained in the section of this Annual Report entitled “Risk Factors”) relating to our industry, our operations and results of operations. Actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned.
Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them. We cannot guarantee future results, levels of activity, performance or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
Except as otherwise indicated, references to “we”, “us”, “our”, “Splash”, “SBG” and the “Company” refer to Splash Beverage Group, Inc. and its wholly owned subsidiaries.
Item 1. Business.
Company Overview
Splash is a portfolio company managing multiple brands across several growth segments within the consumer beverage industry. Splash has built organizational capabilities and an infrastructure enabling it to incubate and/or acquire brands with the intention of efficiently accelerating them to higher volume and sales revenue. The management team has proven capabilities in building consumer franchises and marketing and distributing multiple brands of beverages within the non-alcoholic and alcoholic segments. Manufacturing is typically outsourced to third party co-packers and distillers, or in select cases for a brand such as Copa DI Vino® wines, performed within our own facility in Oregon.
We believe the distribution landscape in the beverage industry is changing rapidly as tech-enabled e-commerce business models are thriving. Direct to consumer, office or home solutions are projected to continue to gain traction in the future. Recognizing this opportunity Splash continues to shape its operating model to be vertically integrated with our e-commerce platform, Qplash, which purchases local and regional brands for developing a direct line of sales to boutique retail stores and consumers.
Splash’s wholly owned subsidiary, Splash Beverage Group II, Inc. ("the Company," "it," "we," "us," or "our") was originally incorporated in the State of OhioNevada under the name TapouT Beverages, Inc. for the purpose of acquiring the rights under a license agreement with TapouT, LLC (Authentic Brands Group). Splash has license rights to the TapouT Performance brand in North America (Including US Territories and Military Bases), United Kingdom, Brazil, South Africa, Scandinavia, Peru, Colombia, Chile and Guatemala.
In December 2020, Splash Beverage Group Inc. purchased the key assets of the Copa DI Vino® single serve wine company. The operations and IP for Copa DI Vino® are wholly owned by Splash and incorporated in the state of Nevada under the name Copa DI Vino® Wine Group Inc.
In addition, Splash has a joint venture with SALT Naturally Flavored Tequila and Pulpoloco sangria that comes in a biodegradable can.
The Company’s leadership understands the importance of infusing beverage brands with strong popular culture and lifestyle elements that drive trial, belief and, most importantly, repeat purchases.
Our management team led by Robert Nistico has over 28 years of experience in all levels of the three-tier distribution system used in the beverage industry working with brands such as Red Bull and companies such as Gallo Winery and Republic National Distributing Company (RNDC Texas). Our President & CMO, Bill Meissner, has led major beverage brands including Sparkling Ice, Fuze, Sweet Leaf Tea and Jones Soda. Our CFO, Stacy McLaughlin, has over 15 years of experience in public company accounting and finance, with an emphasis on Septemberreporting, fundraising and mergers and acquisitions. Our Senior Vice President of Sales, James Allred, has over 25 years’ experience in the beverage industry, predominately with Anheuser-Busch.
Our Strategy
Our strategy is to combine the traditional approach of manufacturing, distributing, and marketing of beverages, with early-stage brands that have a reasonable level of pre-existing brand awareness and market presence, or have attributes that we believe to be purely innovative. We believe this allows us to break through the clutter of numerous brand introductions and dilute risk. We apply this philosophy regardless of whether the brand is 100% owned or a joint venture.
For acquisition or joint venture consideration, we prefer to work with brands that already have one or more of the following in place:
| ● | Some level of preexisting brand awareness. |
| ● | Regional presence that can be expanded. |
| ● | Licensing an existing brand name (TapouT for example). |
| ● | Add to an underdeveloped and/or growing category capitalizing on consumer trends. | |
| ● | Innovation to an existing attractive category (such as flavored tequila). | |
| ● | A near term clear path to profitability. |
We believe this platform model provides us with two paths to success: one, developing our wholly owned core brands and two, the ability to tap into high growth, early-stage brands ready to scale. This platform allows us to limit risk, and significantly reduce development expenses while simultaneously increasing efficiencies for all brands in our portfolio.
Our management team has over 120 years of combined experience in the beverage industry, including decades of successful brand introductions by our management team (Gallo, Red Bull, Bacardi, Diageo, Sparkling Ice, Coca-Cola, FUZE Beverage, NOS Energy, PepsiCo, SoBe Beverages, AB InBev, Muscle Milk, Marley Beverages), we believe our ability to break through the distribution and retail bottlenecks makes us an attractive joint venture partner to many new brand owners.
Splash has the ability to fully own a brand or be flexible to engage in business ventures structured with a revenue split, or an equity position.
The benefit to Splash in these shared brand ownerships is the ability to avoid the development costs for new products. This model spreads our risk over several brands, contributes to our economies of scale, improves our relationship with distributors and reduces the overall cost of infrastructure.
The Company also believes the distribution landscape in the beverage category is changing rapidly. Tech-enabled business models are thriving and direct to consumer, office and home solutions are projected to continue to gain traction as beverage alcohol regulations evolve. A core strategy for us is to optimize the early success we’re seeing with the Qplash online platform, our consumer-packaged goods retail division and our first entry point into the growing e-commerce channel.
Products
We currently produce, distribute and market SALT Naturally Flavored Tequila (“SALT”), a 100% agave 80 proof line of flavored tequilas, “TapouT Performance,” a line of performance beverages that complete in the hydration and energy categories, Copa DI Vino® single serve wine by the glass, and also import Pulpoloco Sangria in 3 1992.flavors.
The following is a description of these products.
SALT Flavored Tequila
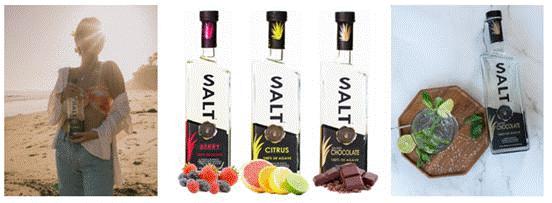
We oversee production, distribute, and market the following flavors under the brand name SALT Naturally Flavored Tequila:
| ● | Citrus flavor |
| ● | Berry flavor |
| ● | Chocolate flavor |
Vodka, rum, and brown spirits have experienced significant growth when flavors are introduced, and we expect this growth of flavors to continue, as the tequila category continues to rapidly expand.
SALT is currently being distributed by various Anheuser-Busch & Miller-Coors distributorships, and other distributors in multiple U.S. states. Additionally, SALT is for sale in Mexico. SALT has also launched in Guatemala and Japan and efforts continue to grow the brand’s international presence.
SALT is a business venture between the Company and SALT USA, LLC. All aspects of manufacturing, logistics, distribution and marketing are our responsibility.
TapouT Performance Isotonic Sports Drinks

We produce, market, sell and distribute the following sports beverages under the brand name TapouT:
| ● | TapouT Performance |
| ● | TapouT Energy |
TapouT Performance Beverages are a line of unique advanced performance beverages containing ingredients known for various functional benefits including, focus, cognition, energy, recuperative and cell regeneration which promotes better absorption of nutrients, increase hydration and cellular recovery. They are exclusively formulated with GRAS (FDA Designation “Generally Regarded as Safe”) ingredients versus controversial ingredients often used in many competitive products. TapouT Performance Beverages are all natural with highly innovative proprietary blends designed to enhance physical and or mental performance.
TapouT, formally associated with the UFC and mixed martial arts has been producing branded clothing and light exercise equipment for over 23 years and has a high level of aided and unaided brand awareness.
TapouT License Agreement
We have the rights under a License Agreement with ABG TapouT (the “License Agreement”) to produce, market, sell and distribute TapouT sports beverages in North America (including US Territories and Military Bases), United Kingdom, Brazil, South Africa, Australia, Scandinavia, Peru, Colombia, Chile and Guatemala. The beverages covered by the License Agreement include sports drinks, energy drinks, energy shots, electrolyte chews, energy bars, water, protein, and teas.
We pay a 6% royalty of net sales or a guaranteed minimum annual royalty of $660,000, whichever is greater. The License Agreement will expire on December 31, 2025, with a renewal option through December 31, 2028 at which time it will be reviewed and renegotiated if necessary.
We have the right to use the TapouT brand to market, advertise and promote for sale our TapouT beverages and branded products. As part of the alliance, Splash commits to investing 2% of sales in marketing to the TapouT Performance Brand. TapouT provides marketing collateral for advertising and promotion and has influential relationships with select celebrities and athletic talent. TapouT agrees to use reasonable efforts to request its retained celebrities and/or athletes be present at autograph signings, tradeshows and other similar events.
Copa DI Vino® Wine Group, Inc. (CdV) and Related Financing
On April 18, 2012 it changedDecember 24, 2020, the Company entered into an Asset Purchase Agreement with CdV, pursuant to which the Company purchased certain assets and assumed certain liabilities that comprise the CdV business for a total purchase price of $5,980,000, payable in the combination of $2,000,000 in cash, a $2,000,000 convertible promissory note to CdV and a variable number of shares of the Company’s common stock based on an attainment of revenue hurdles.
In conjunction with the acquisition, the Company also entered into a Revenue Loan and Security Agreement (the “Loan and Security Agreement”) by and among the Company, Robert Nistico, additional guarantor and each of the subsidiary guarantors from time-to-time party thereto (each a “Guarantor”, and, collectively, the “Guarantors”), and Decathlon Alpha IV, L.P. (the “Lender”). The Loan and Security Agreement provided for a revenue-based credit facility of $1,578,237 (the “Gross Amount”) with the Lender (the “Credit Facility”).
Copa DI Vino® Wine Group, Inc.

Copa DI Vino® is the leading producer of premium wine by the glass in the United States. The Copa DI Vino® product line is highly innovative as a ready to drink wine glass capable of going anywhere without the need for a bottle, corkscrew or glass. The company also has a growing keg wine business for on-premises restaurants and bars.
Through our acquisition of Copa DI Vino® Corporation, we are now able to offer nine varietals of wine: Pinot Grigio, Riesling, Merlot, Chardonnay, White Zinfandel, Moscato, Red Blend, Sauvignon Blanc and Cabernet Sauvignon. In addition to its domicilewine varietals, Copa DI Vino® also procures Pulpoloco, a sangria which is encased in an eco-friendly fiber based can from Spain. The rights to utilize this packaging for multiple categories were conveyed to SBG in conjunction with the distribution rights.

E-commerce
“Qplash” is a wholly owned division of Splash. It is our first entry point into the growing e-commerce channel. The division sells beverages online through www.qplash.com, and third-party storefronts such as Amazon.com. Inside of the division, there are two primary customer groups: business to business retailers, which in turn offer the products to their customers, and business to consumer, selling direct to end users. The business-to-business program allows businesses to control inventory, order with payment terms, and offer the convenience of delivery directly to each store.
Currently Qplash offers over 1,500 listings and has warehouses that ship from both California and Pennsylvania.
Our Competitive Strengths
We believe the following competitive strengths contribute to the Company’s success and differentiate us from our competitors:
| ● | An established distribution network through global sales channels; |
| ● | A hybrid distribution model that leverages multiple routes to market, including national chains, independent local markets, regional chains, and specialty food and C-Stores |
| ● | Long-term relationships with retailers and the establishment of chains; |
| ● | Premium customer service; |
| ● | Dynamic and sustainable product offerings of natural quality and freshness with health benefits; |
| ● | A highly experienced management team; |
| ● | Strategically selected, dedicated sales professionals; |
| ● | Qplash, our e-commerce platform, which provides us an integrated distribution platform for our non-alcoholic brands; |
| ● | Ability to execute and distribute across many geographies on behalf of our licensed brand portfolio; | |
| ● | Strong brand awareness through partnerships and acquisitions of brands with pre-existing brand awareness, or viewed as truly innovative; and | |
| ● | Celebrity and professional athlete endorsement of our brands. |
Manufacturing and Co-packing
We are responsible for the manufacturing of Copa DI Vino®, TapouT Performance and SALT. The Copa DI Vino® product line is bottled at our manufacturing facility in The Dalles, Oregon. Pulpoloco is imported from Spain as a finished product.
Although we are responsible for manufacturing TapouT Performance and SALT, we do not directly manufacture these products, but instead outsource such manufacturing to third party bottlers and contract packers and distillers.
Our TapouT Performance and SALT products are manufactured in the United States and Mexico, respectively under separate arrangements with each party. Our co-packaging arrangements are terminable upon request and do not obligate us to produce any minimum quantities of products within specified periods.
We purchase concentrates, flavors, dietary ingredients, cans, bottles, caps, labels, and other components and ingredients for our beverage products from our suppliers, which are delivered to our manufacturing operations and various third-party bottlers and co-packers. In some cases, certain common supplies may be purchased by our various third-party bottlers and co-packers. Depending on the product, the third-party bottlers or packers add filtered water and/or other ingredients (including dietary ingredients) for the manufacture and packaging of the finished products into our approved containers in accordance with our formulas.
Distribution
For our beverage-alcohol products, we operate within what is referred to as a “Three Tier Distribution System” where manufacturers are not permitted to sell directly to retailers, but instead contract for local and regional distribution with independent distributors. These distributors typically have geographic rights to distribute major beverage brands and call on every store in a given area such as major cities or regions. Our management team has extensive experience working within this channel and believes that we will be successful in building a strong network of these distributors.
In addition to working with these independent distributors, we also have distribution arrangements with national retail accounts to distribute some of our products directly through their warehouse operations. Most notably, SBG executed a distribution agreement with AB-InBev, for distribution with their own operations, AB ONE. This provides SBG very effective distribution capabilities.
Intellectual Property
During the fiscal year ended December 31, 2023, we were granted a trademark for Copa DI Vino®. The United States Patent and Trademark Office issued the trademark on March 12, 2024, providing our company exclusive rights to use the trademark in connection with the product categories specified in this Form 10-K.
Employees
We have 32 full-time employees, including non-officer employees and our executive officers. None of our employees are represented by a labor union. We have not experienced any work stoppages and consider our relations with our employees to be good.
Listing on the NYSE American
Our common stock and warrants are listed on the NYSE American exchange under the ticker symbols “SBEV” and “SBEV WT,” respectively.
Recent Developments
In January 2024, the Company entered into a convertible note with an individual in the amount of $250,000. The note has an eighteen-month term, accrues interest at 12% and is convertible into shares of common stock of the Company at $0.50 per share, which also includes 200% warrants at $0.25
In January 2024, the Company entered into a commercial loan in the amount of $500,000. The total cost of the loan is $250,000 and is paid in weekly increments of 6.97% of the current receivable balance.
In February 2024, the Company entered into a convertible note with an individual in the amount of $150,000. The note has an eighteen-month term, accrues interest at 12% and is convertible into shares of common stock of the Company at $0.40 per share, which also includes 250% warrants at $0.25.
In March 2024, the Company received a $109,000 cash advance from our chief executive officer, resulting in a related party payable. This note bears 0% interest.
Corporate Information
Splash was originally incorporated in the State of Colorado by mergingNevada under the name TapouT Beverages, Inc. for the purpose of acquiring the rights under a license agreement with TapouT, LLC (Authentic Brands Group) for the right to use the TapouT brand in connection with manufacturing and selling certain beverages.
Splash executed a reverse merger with a newly formed Colorado subsidiary.
On July 31, 2021, we changed the registered agent to himself. In March 2015, he fraudulently changed theour name of the Corporation from Canfield Medical Supply, Inc. to Business SolutionsSplash Beverage Group, Inc. In April 2015 he dissolved
On June 11, 2021, our common stock and warrants to purchase common stock began trading on the corporation.
On November 8, 2021, we changed our state of incorporation from Colorado to Nevada.
Our principal offices are located at 1314 E. Las Olas Blvd, Suite 221, Fort Lauderdale, Florida 33301. Our website address is www.splashbeveragegroup.com. We have not incorporated by reference into this Annual Report on Form 10-K the Corporationinformation that can be assessed through our website and you should not consider it to be part of this Annual Report on Form 10-K.
Available Information
We file annual, quarterly, and current reports, proxy statements and other information with the U.S. Securities Exchange Commission (the “SEC”). These filings are available to the public through the SEC’s website at http://www.sec.gov. All statements made in April 2016, and after contacting the Colorado Secretaryany of State, the Corporation was advised to file Articles of Reinstatement, which would have the result that the existenceour securities filings, including all forward-looking statements or information, are made as of the Corporation would be deemed for all purposes to have continued without interruption as if the dissolution never occurred. The Corporation was also advised that it would need to file Articles of Amendment to change its name back to Canfield Medical Supply, Inc. Immediately after receiving approval from the Majority shareholder and the Board of Directors, the Corporation filed the Articles of Reinstatement and the Articles of Amendment with the Colorado Secretary of State.
Item 1A. Risk Factors.
You should carefully consider the risks described below as well as other information provided to certain standards set by Medicare.
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Medicare | 27 | % | 13 | % | ||||
| Medicaid | 13 | % | 12 | % | ||||
| Private pay/private insurance | 47 | % | 63 | % | ||||
| Other | 13 | % | 12 | % | ||||
| Total | 100 | % | 100 | % | ||||
RISKS RELATED TO OUR BUSINESS
Risks Related to our customers. We do notBusiness
Our auditors have exclusive relationships with any of these suppliers/manufacturers. When the products we sell come with warranties, we are usually the person who the customer contacts when they have any kind of issue covered by a warranty. We then go to the manufacturer and order the part needed or otherwise take care of the problem. We do not warranty any products ourselves.
Rose, Snyder & Jacobs LLP, our capacity and we have roomindependent registered public accounting firm for substantial growth without needing to add any significant overhead.
We have sustained recurring losses and supplies we sell to patients for patient care under fee-for-service arrangements. Fee-for-service is a payment model where services are unbundled and paid for separately, and occurs when doctors and other health care providers receive a fee for each service, such as an office visit, test, or procedure. Since most of the manufacturers of the products we sell do not provide direct patient care, our services primarily involve providing in-home-delivery, set-up, and maintenance of home medical equipment. We do not have ongoing arrangements with patients or medical providers, other than rental agreements that we have for wheel chairshad working capital and hospital beds.
and regulatory initiatives in Congress and at CMS that affect or may affect Medicare reimbursement policies for products and services we provide. Specifically, a number of important legislative changes that affect our business were included in the Medicare Prescription Drug, Improvement and Modernization Act of 2003 ("MMA"); the Deficit Reduction Act of 2005 ("DRA"); MIPPA, which became law in 2008; and the comprehensive healthcare reform law signed in March 2010 ("the Reform Package"). These Acts and their implementing regulations and guidelines contain numerous provisions that are significant to us andwill continue to have, an impactadverse effect on our financial condition. In addition, continued operations today.
Management recognizes that it may be required to implementobtain additional resources via issuances of indebtedness or equity to successfully execute its business plans. No assurances can be given that management will be successful in raising additional capital, if needed, or on acceptable terms. These conditions raise substantial doubt about the DMEPOS competitive bidding program over time, with Round 1 of competition occurring in portions of 10 of the largest Metropolitan Statistical Areas ("MSAs") in 2007, launch of the program in 2008 and in 70 additional markets in 2009, and in additional markets after 2009.
We have experienced recurring losses from operations and negative cash flows from operating activities and anticipate that we will continue to incur significant operating losses in a defined region. CMS selects contract suppliers that agreethe future.
We have experienced recurring losses from operations and negative cash flows from operating activities. We expect to receive as paymentcontinue to incur significant expenses related to our ongoing operations and generate operating losses for the "single payment amount" calculated by CMS after bids are submitted. Bids are evaluated basedforeseeable future. The size of our losses will depend, in part, on the supplier meeting eligibilityrate of future expenditures, our ability to execute on our acquisition strategy and financial requirements, and contracts are awardedour ability to Medicare suppliers that offergenerate revenues. We incurred a net loss of $21.0 million for the best price and meet these standards. CMS determines a supplier's financial viability based on certain financial ratios and the supplier's credit report and score. Based on the information requested in the bid forms, we believe that the CMS may also consider other factors, suchyear ended December 31, 2023. Our accumulated deficit increased to $133.3 million as the volume which the bidder is offering to provide asof December 31, 2023, compared to the volume it previously provided, whether the bidder has the staff and facilities to handle the volume it is bidding for,prior year’s deficit of $112.3 million.
We may encounter unforeseen expenses, difficulties, complications, delays, and other miscellaneous items.
If we are not able to successfully execute on our future operating plans and objectives, our financial condition and results of operation may be reimbursed by Medicare for DMEPOS items supplied inmaterially adversely affected, and we may not be able to continue as a going concern.
It is important that CBA for the time period covered by the competitive bidding program unless the supplier meets certain exceptions or acquires a winning bidder. Because the applicable statutes mandatewe meet our sales goals and increase sales going forward as our operating plan already reflects prior significant cost containment measures and may make it difficult to achieve top-line growth if further significant reductions become necessary. If we do not meet our sales goals, our available cash and working capital will decrease and our financial savings from the competitive bidding program, a winning contract suppliercondition will receive lower Medicare payment rates under competitive bidding than the otherwise applicable DMEPOS fee schedule rates. As competitive bidding is phased in across the country under the revised MIPPA and Reform Package implementation schedule,be negatively impacted.
In order to be successful, we believe that we will experience a reduction in reimbursement. In addition, there is an increasing risk that the competitive bidding prices will become a benchmark for reimbursement from other payors, as evidenced by the Administration's fiscal budget proposal which would cap state Medicaid reimbursement levels at competitive bid rates using an as-yet-undetermined methodology. Neither MIPPA nor the Reform Package prevents CMS from adjusting prices for DMEPOS items in non-bid areas; however, before using its authority to adjust prices in non-bid areas, MIPPA requires that CMS issue a regulation that specifies the methodology to be used and consider how prices through competitive bidding compare to costs for those items and services in the non-bid areas.
| ● | increase the sales volume and gross margins for our products and those that we will acquire; | |
| ● | maintain efficiencies in operations; | |
| ● | manage our operating expenses to sufficiently support operating activities; | |
| ● | maintain fixed costs at or near current levels; and | |
| ● | avoid significant increases in variable costs relating to production, marketing and distribution. |
We cannot predict whether these or other efforts to repeal or amend the program will be successful, or their potential impact on us.
and introducing new brands, products or product extensions to the Medicare program maintain a surety bondmarket. Our ability to successfully enter new distribution areas and obtain national accounts will, in turn, depend on various factors, many of $50,000 per National Provider Identifier ("NPI") number which Medicare has approved for billing privileges. We obtainedare beyond our control, including, but not limited to, the required surety bondcontinued demand for our location beforebrands and products in target markets, the October 2009 deadline,ability to price our products at competitive levels, available positions within the retailer’s planograms, the ability to establish and it is automatically renewed annually on August 1.
Demand for our products may be adversely affected by |
Our beverage portfolio is comprised of a number of unique brands with reputations and consumer imagery that have been enactedbuilt over time. Our investments in marketing as well as our strong commitment to dateproduct quality are reflectedintended to have a favorable impact on brand image and consumer preferences. If we do not adequately anticipate and react to changing demographics, consumer and economic trends, health concerns and product preferences, our financial results could be adversely affected.
Additionally, failure to introduce new brands, products or product extensions into the marketplace as current ones mature and to meet the changing preferences of consumers could prevent us from gaining market share and achieving long-term profitability. Product lifecycles can vary and consumer preferences and loyalties change over time. Although we try to anticipate these shifts and innovate new products to introduce to our consumers, we may not succeed. Consumer preferences also are affected by factors other than taste, such as health and nutrition considerations and obesity concerns, shifting consumer needs, changes in consumer lifestyles, increased consumer information and competitive product and pricing pressures. Sales of our products may be adversely affected by negative publicity associated with these issues. If we do not adequately anticipate or adjust to respond to these and other changes in consumer preferences, we may not be able to maintain and grow our brand image and our sales may be adversely affected.
Volatility in the price or availability of the inputs we depend on, including raw materials, packaging, energy and labor, could adversely impact our financial results.
The principal raw materials we use include glass bottles, aluminum cans, PET, fiber-board, labels and cardboard cartons, flavorings and sweeteners. These component and ingredient costs are subject to fluctuation. If there were to be substantial increases in the prices of our ingredients, raw materials and packaging materials, to the extent that they cannot be recouped through increases in the prices of finished beverage products, would increase our operating costs and could reduce our profitability. If our supply of these raw materials is impaired or if prices increase significantly, it could affect the affordability of our products and reduce sales.
If we are unable to secure sufficient ingredients or raw materials including glass, sugar, and other key supplies, we might not be able to satisfy demand on a short-term basis.
Changes in government regulation or failure to comply with existing regulations could adversely affect our business, financial condition and results of operations foroperations.
Our business and properties are subject to various federal, state and local laws and regulations, including those governing the applicable periodsproduction, packaging, quality, labeling and distribution of beverage products. In addition, various governmental agencies have enacted or are considering additional taxes on soft drinks and other sweetened beverages. Changes in existing laws or regulations could require material expenses and negatively affect our financial results through December 31, 2016. lower sales or higher costs.
We cannot estimatecompete in an industry that is brand-conscious, so brand name recognition and acceptance of our products are critical to our success.
Our business is dependent upon awareness and market acceptance of our products and brands by our target markets. In addition, our business depends on acceptance by our independent distributors and retailers of our brands as beverage brands that have the combined possible impactpotential to provide incremental sales growth. If we are not successful in the revitalization and growth of all legislative, regulatoryour brand and contemplated reimbursement changes thatproduct offerings, we may not achieve and maintain satisfactory levels of acceptance by independent distributors and retail consumers. Any failure of our brand to maintain or increase acceptance or market penetration would likely have a material adverse effect on our revenues and financial results.
Our brands and brand images are keys to our business and any inability to maintain a positive brand image could have a material adverse effect on our results of operations, cash flow,operations.
Our success depends on our ability to maintain brand image for our existing products and capital resources. Moreover,effectively build up brand image for new products and brand extensions. We cannot predict whether our estimatesadvertising, marketing and promotional programs will have the desired impact on our products’ branding and on consumer preferences. In addition, negative public relations and product quality issues, whether real or imagined, could tarnish our reputation and image of the affected brands and could cause consumers to choose other products. Our brand image can also be adversely affected by unfavorable reports, studies and articles, litigation, or regulatory or other governmental action, whether involving our products or those of our competitors.
Competition from traditional and large, well-financed non-alcoholic and alcoholic beverage manufacturers may adversely affect our distribution relationships and may hinder development of our existing markets, as well as prevent us from expanding our markets.
The beverage industry is highly competitive. We compete with other beverage companies not only for consumer acceptance but also for shelf space in retail outlets and for marketing focus by our distributors, all of whom also distribute other beverage brands. Our products compete with all non-alcoholic and alcoholic beverages, most of which are marketed by companies with substantially greater financial resources than ours. Some of these competitors are placing severe pressure on independent distributors not to carry competitive brands such as ours. We also compete with regional beverage producers and “private label” brands.
Increased competitor consolidations, market-place competition, particularly among branded beverage products, and competitive product and pricing pressures could impact our earnings, market share and volume growth. If, due to such pressure or other competitive threats, we are unable to sufficiently maintain or develop our distribution channels, we may be unable to achieve our current revenue and financial targets. Competition, particularly from companies with greater financial and marketing resources than ours, could have a material adverse effect on our existing markets, as well as on our ability to expand the market for our products.
Legislative or regulatory changes that affect our products, including new taxes, could reduce demand for products or increase our costs.
Taxes imposed on the sale of certain of these changes appearingour products by federal, state and local governments in the United States, or other countries in which we operate could cause consumers to shift away from purchasing our beverages. Several municipalities in the United States have implemented or are considering implementing taxes on the sale of certain “sugared” beverages, including non-diet soft drinks, fruit drinks, teas and flavored waters to help fund various initiatives. These taxes could materially affect our business and financial results.
Our reliance on distributors, retailers and brokers could affect our ability to efficiently and profitably distribute and market our products, maintain our existing markets and expand our business into other geographic markets.
Our ability to maintain and expand our existing markets for our products, and to establish markets in new geographic distribution areas, is dependent on our ability to establish and maintain successful relationships with reliable distributors, retailers and brokers strategically positioned to serve those areas. Most of our distributors, retailers and brokers sell and distribute competing products, including non-alcoholic and alcoholic beverages, and our products may represent a small portion of their businesses. The success of this "Government Regulation" sectionnetwork will depend on the performance of the distributors, retailers and brokers of this network. There is a risk that the mentioned entities may not adequately perform their functions within the network by, without limitation, failing to distribute to sufficient retailers or positioning our products in localities that may not be receptive to our product. Our ability to incentivize and motivate distributors to manage and sell our products is affected by competition from other beverage companies, some of which may have greater resources than we do. To the extent that our distributors, retailers and brokers are baseddistracted from selling our products or do not employ sufficient efforts in managing and selling our products, including re-stocking the retail shelves with our products, our sales and results of operations could be adversely affected. Furthermore, such third-parties’ financial position or market share may deteriorate, which could adversely affect our distribution, marketing and sales activities.
Our ability to maintain and expand our distribution network and attract additional distributors, retailers and brokers will depend on a number of assumptions andfactors, some of which are subject to uncertainties and there can be no assurance that the actual impact was not or will not be different fromoutside our estimates. However, given the recent significant increases in industry audit volume and the increasing regulatory burdens associated with responding to those audits, it is likely that the negative pressures from legislative and regulatory changes will continue and accelerate.
| ● | the level of demand for our brands and products in a particular distribution area; |
| ● | our ability to price our products at levels competitive with those of competing products; and |
| ● | our ability to deliver products in the quantity and at the time ordered by distributors, retailers and brokers. |
We may not be similarable to thosesuccessfully manage all or any of these factors in any of our current or prospective geographic areas of distribution. Our inability to achieve success with regards to any of these factors in a geographic distribution area will have a material adverse effect on our relationships in that particular geographic area, thus limiting our ability to maintain or expand our market, which will likely adversely affect our revenues and financial results.
It is difficult to predict the timing and amount of our sales because our distributors are not required to place minimum orders with us.
Our independent distributors and national accounts are not required to place minimum monthly or annual orders for our products. In order to reduce their inventory costs, independent distributors typically order products from us on a “just in time” basis in quantities and at such times based on the demand for the products in a particular distribution area. Accordingly, we cannot predict the timing or quantity of purchases by any of our independent distributors or whether any of our distributors will continue to purchase products from us in the same frequencies and volumes as they may have done in the past. Additionally, our larger distributors and national partners may make orders that are larger than we have historically been required to fill. Shortages in inventory levels, supply of raw materials or other key supplies could negatively affect us.
If we do not adequately manage our inventory levels, our operating results could be adversely affected.
We need to maintain adequate inventory levels to be able to deliver products to distributors on a timely basis. Our inventory supply depends on our ability to correctly estimate demand for our products. Our ability to estimate demand for our products is imprecise, particularly for new products, seasonal promotions and new markets. If we materially underestimate demand for our products or are unable to maintain sufficient inventory of raw materials, we might not be able to satisfy demand on a short-term basis. If we overestimate distributor or retailer demand for our products, we may end up with too much inventory, resulting in higher storage costs, increased trade spend and the risk of inventory spoilage. If we fail to manage our inventory to meet demand, we could damage our relationships with our distributors and retailers and could delay or lose sales opportunities, which would unfavorably impact our future sales and adversely affect our operating results. In addition, if the inventory of our products held by our distributors and retailers is too high, they will not place orders for additional products, which would also unfavorably impact our sales and adversely affect our operating results.
If we fail to maintain relationships with our independent contract manufacturers, our business could be harmed.
We do not manufacture SALT Tequila, Pulpoloco Sangria or TapouT performance drinks but instead outsource the manufacturing process to third-party bottlers and independent contract manufacturers (co-packers). We do not own the plants or the majority of the Medicare program. Budget pressures onequipment required to manufacture and package these state programs often resultbrands. Our ability to maintain effective relationships with contract manufacturers and other third parties for the production and delivery of our beverage products in pricinga particular geographic distribution area is important to the success of our operations within each distribution area. We may not be able to maintain our relationships with current contract manufacturers or establish satisfactory relationships with new or replacement contract manufacturers, whether in existing or new geographic distribution areas. The failure to establish and coverage changesmaintain effective relationships with contract manufacturers for a distribution area could increase our manufacturing costs and extended payment practicesthereby materially reduce gross profits from the sale of our products in that area. Poor relations with any of our contract manufacturers could adversely affect the amount and timing of product delivered to our distributors for resale, which would in turn adversely affect our revenues and financial condition. In addition, our agreements with our contract manufacturers are terminable at any time, and any such termination could disrupt our ability to deliver products to our customers.
The volatility of energy and increased regulations may have a detrimentalan adverse impact on our operations and/gross margin.
Over the past few years, volatility in the global oil markets has resulted in variable fuel prices, which many shipping companies have passed on to their customers by way of higher base pricing and increased fuel surcharges. If fuel prices increase, we expect to experience higher shipping rates and fuel surcharges, as well as energy surcharges on our raw materials. It is hard to predict what will happen in the fuel markets in 2024 and beyond. Due to the price sensitivity of our products, we may not always be able to pass such increases on to our customers.
Disruption within our supply chain, contract manufacturing or distribution channels could have an adverse effect on our business, financial performance. States sometimes have interposed intermediariescondition and results of operations.
Our ability, through our suppliers, business partners, contract manufacturers, independent distributors and retailers, to administer their Medicaid programs,make, move and sell products is critical to our success. Damage or have adopted alternative pricing methodologies for certain drugs, biologicals,disruption to our suppliers or to manufacturing or distribution capabilities due to weather, natural disaster, fire or explosion, terrorism, pandemics such as influenza COVID-19, labor strikes or other reasons, could impair the manufacture, distribution and home medical equipment under their Medicaid programs that reducesale of our products. Many of these events are outside of our control. Failure to take adequate steps to protect against or mitigate the levellikelihood or potential impact of reimbursement received by us without a corresponding offsetsuch events, or increase to compensate for the service costs incurred. effectively manage such events if they occur, could adversely affect our business, financial condition and results of operations.
We periodically evaluate the possibility of stopping or reducingrely upon our Medicaid business in any stateongoing relationships with reimbursement or administrative policies that make it difficult for usour key flavor suppliers. If we are unable to safely care for patients or conduct operations profitably. Moreover, the Reform Package increases Medicaid enrollment over a number of years and imposes additional requirementssource our flavors on states which, combined with the current economic environment and state deficits,acceptable terms from our key suppliers, we could further strain state budgets and therefore result in additional policy changes or rate reductions. The President's most recent budget proposal, would limit the amount state Medicaid programs pay for DMEPOS to be no higher than Medicare payment levels, including those impacted by Medicare competitive bidding. We cannot currently predict the adverse impact, if any, that any such change to or reductionsuffer disruptions in our Medicaid business mightbusiness.
We currently purchase our flavor concentrate from various flavor concentrate suppliers, and continually develop other sources of flavor concentrate for each of our products. Generally, flavor suppliers hold the proprietary rights to their flavor-specific ingredients. Although we have the exclusive rights to flavor concentrates developed with our current flavor concentrate suppliers, and while we have the rights to the ingredients for our products, we do not have the list of ingredients for our flavor extracts and concentrates. Consequently, we may be unable to obtain these exact flavors or concentrates from alternative suppliers on short notice. If we have to replace a flavor supplier, we could experience disruptions in our operations, cash flow and capital resources, but such impactability to deliver products to our customers, which could be material. In addition, we cannot predict whether states will consider similar or other reimbursement reductions, whether or how healthcare reform provisions pertaining to Medicaid will ultimately be implemented or whether any such changes would have a material adverse effect on our results of operations.
If we are unable to attract and retain key personnel, our efficiency and operations cash flowwould be adversely affected; in addition, management turnover causes uncertainties and capital resources.
Our success depends on our ability to attract and Accountability Act of 1996 ("HIPAA") is comprised of a number of components pertainingretain highly qualified employees in such areas as finance, sales, marketing and product development. We compete to the privacyhire new employees, and, security of certain protected health information ("PHI"), as well as the standard formatting of certain electronic health transactions. Many states have similar, butin some cases, must train them and develop their skills and competencies. We may not identical, restrictions. Existingbe able to provide our employees with competitive salaries, and any new lawsour operating results could be adversely affected by increased costs due to increased competition for employees, higher employee turnover or regulations have a significant effect on the manner in which we handle healthcare related data and communicate with payors. Among other provisions, the HITECH Act of the American Recovery and Reinvestment Act of 2009 ("ARRA") includes additional requirements relatedincreased employee benefit costs.
Changes to the privacy and security of PHI, clarifies and increases penalties of HIPAA and provides State Attorneys General with HIPAA enforcement authority. We have adopted a number ofoperations, policies and procedures, which can often occur with the appointment of new personnel, can create uncertainty, may negatively impact our ability to conform to HIPAA requirements,execute quickly and effectively, and may ultimately be unsuccessful. In addition, management transition periods are often difficult as modified by the HITECH Actnew employees gain detailed knowledge of ARRA, throughout our operations, and friction can result from changes in strategy and management style. Management turnover inherently causes some loss of institutional knowledge, which can negatively affect strategy and execution.
Further, to the extent we have educatedexperience additional management turnover, our employees about theseoperations, financial condition and employee morale could be negatively impacted. In addition, competition for top management is high and it may take months to find a candidate that meets our requirements. If we are unable to attract and retain qualified management personnel, our business could suffer.
If we fail to protect our trademarks and trade secrets, we may be unable to successfully market our products and compete effectively.
We cannot, however, guarantee that we will not haverely on a HIPAA privacycombination of trademark and trade secrecy laws, confidentiality procedures and contractual provisions to protect our intellectual property rights. Failure to protect our intellectual property could harm our brand and our reputation, and adversely affect our ability to compete effectively. Further, enforcing or data security concerndefending our intellectual property rights, including our trademarks,
copyrights, licenses and trade secrets, could result in the future.expenditure of significant financial and managerial resources. We face potential administrative, civilregard our intellectual property, particularly our trademarks and possible criminal sanctions iftrade secrets to be of considerable value and importance to our business and our success, and we doactively pursue the registration of our trademarks in the United States and internationally. However, the steps taken by us to protect these proprietary rights may not comply with the existingbe adequate and may not prevent third parties from infringing or new lawsmisappropriating our trademarks, trade secrets or similar proprietary rights. In addition, other parties may seek to assert infringement claims against us, and regulations dealing with the privacy and securitywe may have to pursue litigation against other parties to assert our rights. Any such claim or litigation could be costly. In addition, any event that would jeopardize our proprietary rights or any claims of PHI. Imposition of any such sanctionsinfringement by third parties could have a material adverse effect on our operations.
As part of Healthcare Fraudthe licensing strategy of our brands, we enter into licensing agreements under which we grant our licensing partners certain rights to use our trademarks and Abuse Laws
We may be required in the Reform Packagefuture to record a significant charge to earnings if our goodwill or intangible assets become impaired.
Under United States Generally Accepted Accounting Principles (“U.S. GAAP”), we are required to review our intangible assets for impairment at least annually or when events or changes in circumstances indicate the carrying value may not be recoverable. Factors that are designedmay be considered a change in circumstances indicating that the carrying value of our intangible assets may not be recoverable include, declining or slower than anticipated growth rates for certain of our existing products, a decline in stock price and market capitalization, and slower growth rates in our industry.
We may be required in the future to reduce healthcare fraudrecord a significant charge to earnings during the period in which we determine that our intangible assets have been impaired. Any such charge would adversely impact our results of operations. As of December 31, 2023, our intangible assets totaled approximately $4.71 million.
If we encounter product recalls or other product quality issues, our business may suffer.
Product quality issues, real or imagined, or allegations of product contamination, even when false or unfounded, could tarnish our image and abuse.could cause consumers to choose other products. In addition, private insurers and various state enforcement agencies have increased their levelbecause of scrutinychanging government regulations or implementation thereof, or allegations of healthcare claims in an effort to identify and prosecute fraudulent and abusive practices in the healthcare area. Fromproduct contamination, we may be required from time to time to recall products entirely or from specific markets. Product recalls could affect our profitability and could negatively affect brand image.
Our business is subject to many regulations and noncompliance is costly.
The production, marketing and sale of our beverages, including contents, labels, caps and containers, are subject to the rules and regulations of various federal, provincial, state and local health agencies. If a regulatory authority finds that a current or future product or production batch or “run” is not in compliance with any of these regulations, we may be fined, or production may be stopped, which would adversely affect our financial condition and results of operations. Similarly, any adverse publicity associated with any noncompliance may damage our reputation and our ability to successfully market our products. Furthermore, the rules and regulations are subject to change from time to time and while we closely monitor developments in this area, we cannot anticipate whether changes in these rules and regulations will impact our business adversely. Additional or revised regulatory requirements, whether labeling, environmental, tax or otherwise, could have a material adverse effect on our financial condition and results of investigationsoperations.
Significant additional labeling or warning requirements may inhibit sales of affected products.
Various jurisdictions may seek to adopt significant additional product labeling or warning requirements relating to the chemical content or perceived adverse health consequences of certain of our products. These types of requirements, if they become applicable to one or more of our products under current or future environmental or health laws or regulations, may inhibit sales of such products. In California, a law requires that a specific warning appear on any product that contains a component listed by the state as having been found to cause cancer or birth defects. This law recognizes no generally applicable quantitative thresholds below which a warning is not required. If a component found in one of our products is added to the list, or if the increasing sensitivity of detection methodology that may become available under this law and related regulations as they currently exist, or as they may be amended, results in the detection of an infinitesimal quantity of a listed substance in one of our beverages produced for sale in California, the resulting warning requirements or adverse publicity could affect our sales.
Litigation or legal could expose us to significant liabilities and damage our reputation.
We may become party to litigation claims and legal proceedings. Litigation involves significant risks, uncertainties and costs, including distraction of management attention away from our business operations. We evaluate litigation claims and legal proceedings to assess the likelihood of unfavorable outcomes and to estimate, if possible, the amount of potential losses. Based on these assessments and estimates, we establish reserves and disclose the relevant litigation claims or legal proceedings, as appropriate. These assessments and estimates are based on the information available to management at the time and involve a significant amount of management judgment. Actual outcomes or losses may differ materially from those envisioned by our current assessments and estimates. Our policies and procedures require strict compliance by our employees and agents with all U.S. and local laws and regulations applicable to our business operations, including those prohibiting improper payments to government officials. Nonetheless, our policies and procedures may not ensure full compliance by our employees and agents with all applicable legal requirements. Improper conduct by our employees or agents could damage our reputation or lead to litigation or legal proceedings that could result in civil or criminal penalties, including substantial monetary fines, as well as disgorgement of profits.
Additionally, there has been public attention directed at the beverage alcohol industry, which we believe is due to concern over problems related to harmful use of alcohol, including drinking and driving, underage drinking and health consequences from the misuse of alcohol. We could be exposed to lawsuits relating to product liability or marketing or sales practices with respect to our alcoholic products. Adverse developments in lawsuits concerning these types of matters or a party to additional litigation which alleges violationssignificant decline in the social acceptability of law. If any of those matters were successfully asserted against us, therebeverage alcohol products that may result from lawsuits could behave a material adverse effect on our business, liquidity, financial position,condition and results of operations.
We are subject to risks inherent in sales of products in international markets.
Our operations outside of the United States, contribute to our revenue and profitability, and we believe that developing and emerging markets could present future growth opportunities for us. However, there can be no assurance that existing or new products that we manufacture, distribute or sell will be accepted or be successful in any particular foreign market, due to local or global competition, product price, cultural differences, and consumer preferences or otherwise. There are many factors that could adversely affect demand for our products in foreign markets, including our inability to attract and maintain key distributors in these markets; volatility in the economic growth of certain of these markets; changes in economic, political or social conditions, the status and renegotiations of the North American Free Trade Agreement, imposition of new or increased labeling, product or production requirements, or other legal restrictions; restrictions on the import or export of our products or ingredients or substances used in our products; inflationary currency, devaluation or fluctuation; increased costs of doing business due to compliance with complex foreign and U.S. laws and regulations. If we are unable to effectively operate or manage the risks associated with operating in international markets, our business, financial condition or results of operations or prospects.
Water scarcity and poor quality could negatively impact our costs and capacity.
Water is a providermain ingredient in substantially all of services underour products, is vital to the Medicare and Medicaid programs, we must comply with a provisionproduction of the federal Social Security Act, commonly knownagricultural ingredients on which our business relies and is needed in our manufacturing process. It also is critical to the prosperity of the communities we serve. Water is a limited resource in many parts of the world, facing unprecedented challenges from overexploitation, increasing demand for food and other consumer and industrial products whose manufacturing processes require water, increasing pollution and emerging awareness of potential contaminants, poor management, lack of physical or financial access to water, sociopolitical tensions due to lack of public infrastructure in certain areas of the world and the effects of climate change. As the demand for water continues to increase around the world, and as water becomes scarcer and the "federal anti-kickback statute." The federal anti-kickback statute prohibitsquality of available water deteriorates, we may incur higher costs or face capacity constraints and the offerpossibility of reputational damage, which could adversely affect our profitability or receiptnet operating revenues in the long run.
Fluctuations in quantity and quality of grape supply could adversely affect our business.
A shortage in the supply of quality grapes may result from a variety of factors that determine the quality and quantity of our grape supply, including weather conditions, pruning methods, diseases and pests, the ability to buy grapes on long and short-term contracts and the number of vines producing grapes. Any shortage in grape production could cause a reduction in the amount of wine we are able to produce, which could reduce sales and adversely impact our results from operations. Factors that reduce the quantity of our grapes may also reduce their quality, which in turn could reduce the quality or amount of wine we produce. Deterioration in the quality of our wines could harm our brand name, reduce sales and adversely impact our business and results of operations.
Contamination of our wines could harm our business.
We are subject to certain hazards and product liability risks, such as potential contamination, through tampering or otherwise, of ingredients or products. Contamination of any bribe, kickback,of our wines could force us to destroy wine held in inventory and could cause the need for a product recall, which could significantly damage our reputation for product quality. We maintain insurance against certain of these kinds of risks, and others, under various insurance policies. However, the insurance may not be adequate or rebate in return for the referralmay not continue to be available at a price or arranging for the referral of patients, products or services covered by federal healthcare programs. Federal healthcare programs have been definedon terms that are satisfactory to include plansus and programs that provide health benefits funded by the United States Government, including Medicare, Medicaid, and TRICARE (formerly known as the Civilian Health and Medical Program of the Uniformed Services or CHAMPUS), among others. Some courts and the OIG interpret the statutethis insurance may not be adequate to cover any arrangement where even one purposeresulting liability.
Our business and operations would be adversely impacted in the event of a failure or interruption of our information technology infrastructure or as a result of a cybersecurity attack.
The proper functioning of our own information technology (IT) infrastructure is critical to the efficient operation and management of our business. We may not have the necessary financial resources to update and maintain our IT infrastructure, and any failure or interruption of our IT system could adversely impact our operations. In addition, our IT is vulnerable to cyberattacks, computer viruses, worms and other malicious software programs, physical and electronic break-ins, sabotage and similar disruptions from unauthorized tampering with our computer systems. We believe that we have adopted appropriate measures to mitigate potential risks to our technology infrastructure and our operations from these IT-related and other potential disruptions. However, given the unpredictability of the remuneration is to influence referrals. Violationstiming, nature and scope of the federal anti-kickback statute may result in civil and criminal penalties and exclusion from participation in federal healthcare programs.
If we fail to comply with personal data protection and privacy laws, we could be subject to adverse publicity, government enforcement actions and/or private litigation, which could negatively affect our business and operating results.
In the ordinary course of our business, we receive, process, transmit and store information relating to identifiable individuals (“personal data”), primarily employees, former employees and consumers with whom we interact. As a result, we are subject to various U.S. federal and state and foreign laws and regulations relating to personal data. These laws have been subject to frequent changes, and new legislation in this area may be enacted in other jurisdictions at any time. These laws impose operational requirements for companies receiving or processing personal data, and many provide for significant penalties for noncompliance. These requirements with respect to personal data have subjected and may continue in the future to subject the Company to, among other things, additional costs and expenses and have required and may in the future require costly changes to our business practices and information security systems, policies, procedures and practices. Our security controls over personal data, the training of employees and vendors on data privacy and data security, and the policies, procedures and practices we implemented or may implement in the future may not prevent the improper disclosure of personal data by us or the third-party service providers and vendors whose technology, systems and services we use in connection with the receipt, storage and transmission of personal data. Unauthorized access or improper disclosure of personal data in violation of personal data protection or privacy laws could harm our reputation, cause loss of consumer confidence, subject us to regulatory enforcement actions (including fines), and result in private litigation against us, which could result in loss of revenue, increased costs, liability for monetary damages, fines and/or criminal prosecution, all of which could negatively affect our business and operating results.
If our third-party service providers and business partners do not satisfactorily fulfill their commitments and responsibilities, our financial results could suffer.
In the conduct of our business, we rely on relationships with third parties, including cloud data storage and other information technology service providers, suppliers, distributors, contractors, joint venture partners and other external business partners, for certain functions or for services in support of key portions of our operations. These third-party service providers and business partners are subject to similar risks as we are relating to cybersecurity, privacy violations, business interruption, and systems and employee failures, and are subject to legal, regulatory and market risks of their own. Our third-party service providers and business partners may not fulfill their respective commitments and responsibilities in a timely manner and in accordance with the agreed-upon terms. In addition, while we have procedures in place for selecting and managing our relationships with third-party service providers and other business partners, we do not have control over their business operations or governance and compliance systems, practices and procedures, which increases our financial, legal, reputational and operational risk. If we are unable to effectively manage our third-party relationships, or for any reason our third-party service providers or business partners fail to satisfactorily fulfill their commitments and responsibilities, our financial results could suffer.
Our results of operations cash flow, capitalmay fluctuate from quarter to quarter for many reasons, including seasonality.
Our sales are seasonal, and we experience fluctuations in quarterly results as a result of many factors. Companies similar to ours have historically generated a greater percentage of our revenues during the warm weather months of April through September. Timing of customer purchases will vary each year and sales can be expected to shift from one quarter to another. As a result, management believes that period-to-period comparisons of results of operations are not necessarily meaningful and should not be relied upon as any indication of future performance or results expected for the fiscal year.
Changes in accounting standards and subjective assumptions, estimates and judgments by management related to complex accounting matters could significantly affect our financial results.
The U.S. GAAP and related pronouncements, implementation guidelines and interpretations with regard to a wide variety of matters that are relevant to our business, such as, but not limited to, stock-based compensation, trade spend and promotions, and income taxes are highly complex and involve many subjective assumptions, estimates and judgments by our management. Changes to these rules or their interpretation or changes in underlying assumptions, estimates or judgments by our management could significantly change our reported results.
If we are unable to maintain effective disclosure controls and procedures and internal control over financial reporting, our stock price and investor confidence could be materially and adversely affected.
We are required to maintain both disclosure controls and procedures and internal control over financial reporting that are effective. Because of their inherent limitations, internal control over financial reporting, however well designed and operated, can only provide reasonable, and not absolute, assurance that the controls will prevent or detect misstatements. Because of these and other inherent limitations of control systems, there is only the reasonable assurance that our controls will succeed in achieving their goals under all potential future conditions. The failure of controls by design deficiencies or absence of adequate controls could result in a material adverse effect on our business and financial results, which could also negatively impact our stock price and investor confidence.
We are dependent on a distiller in Mexico to provide us with our finished SALT tequila product. Failure to obtain satisfactory performance from them or a loss of their services could cause us to lose sales, incur additional costs, and lose credibility in the marketplace.
We depend on a distiller in Mexico, a company in Jalisco, for the production, bottling, labeling, capping and packaging of our finished tequila product. We do not have a written agreement with our distiller in Mexico obligating it to produce our product. The termination of our relationship with our distiller in Mexico distiller or an adverse change in the terms of its services could have a negative impact on our business. If our distiller in Mexico increases its prices, we may not have alternative sources of supply at comparable prices and may not be able to raise the prices of our products to cover all, or even a portion, of the increased costs. In addition, if our distiller in Mexico fails to perform satisfactorily, fails to handle increased orders, or the loss of the services of our distiller in Mexico, along with delays in shipments of products, could cause us to fail to meet orders, lose sales, incur additional costs, and/or expose us to product quality issues. In turn, this could cause us to lose credibility in the marketplace and damage our relationships with our customers and consumers, ultimately leading to a decline in our business and results of operations.
Regulatory decisions and changes in the legal, regulatory and tax environment where our tequila is produced and where we operate could limit our business activities or increase our operating costs and reduce our margins.
Our business is subject to extensive regulation regarding production, distribution, marketing, advertising and labeling of beverage alcohol products in the U.S. and in Mexico, where our tequila is produced. We are required to comply with these regulations and maintain various permits and licenses. We are also required to conduct business only with holders of licenses to import, warehouse, transport, distribute, and sell spirits. We cannot assure you that these and other governmental regulations, applicable to our industry, will not change or become more stringent. Moreover, because these laws and regulations are subject to interpretation, we may not be able to predict when, and to what extent, liability may arise. Additionally, due to increasing public concern over alcohol-related societal problems, including driving while intoxicated, underage drinking, alcoholism and health consequences from the abuse of alcohol, various levels of government may seek to impose additional restrictions or limits on advertising or other marketing activities promoting beverage alcohol products. Failure to comply with any of the current or future regulations and requirements relating to our industry and products, could result in monetary penalties, suspension or even revocation of our licenses and permits. Costs of compliance with changes in regulations could be significant and could harm our business, as we may find it necessary to raise our prices in order to maintain profit margins, which could lower the demand for our products and reduce our sales and profit potential.
In addition, the distribution of beverage alcohol products is subject to extensive taxation both in the United States and internationally (and, in the United States, at both the federal and state government levels), and beverage alcohol products themselves are the subject of national import and excise duties in most countries around the world. An increase in taxation or in import or excise duties could also significantly harm our sales revenue and margins, both through the reduction of overall consumption and by encouraging consumers to switch to lower-taxed categories of beverage alcohol.
We face substantial competition in the alcoholic and non-alcoholic beverage industry, and we may not be able to effectively compete.
Consolidation among spirits producers, distributors, wholesalers, or retailers could create a more challenging competitive landscape for our products. Consolidation at any level could hinder the distribution and sale of our products as a result of reduced attention and resources allocated to our brands, both during and liquidity.
Our competitors may respond to industry and economic conditions more rapidly or effectively than we do. Our competitors offer products that compete directly with ours for shelf space, promotional displays, and consumer purchases. Pricing, (including price promotions, discounting, couponing, and free goods), marketing, new product introductions, entry into our distribution networks, and other competitive behavior by our competitors could adversely affect our sales margins, and profitability.
Our business operations may be adversely affected by social, political and economic conditions affecting market risks and the demand for and pricing of our products. These risks include:
| ● | Unfavorable economic conditions and related low consumer confidence, high unemployment, weak credit or capital markets, sovereign debt defaults, sequestrations, austerity measures, higher interest rates, political instability, higher inflation, deflation, lower returns on pension assets, or lower discount rates for pension obligations; |
| ● | Changes in laws, regulations, or policies – especially those that affect the production, importation, marketing, sale, or consumption of our beverage alcohol products; |
| ● | Tax rate changes (including excise, sales, tariffs, duties, corporate, individual income, dividends, capital gains), or changes in related reserves, changes in tax rules or accounting standards, and the unpredictability and suddenness with which they can occur; |
| ● | Dependence upon the continued growth of brand names; |
| ● | Changes in consumer preferences, consumption, or purchase patterns – particularly away from tequila, and our ability to anticipate and react to them; bar, restaurant, travel, or other on-premise declines; |
| ● | Unfavorable consumer reaction to our products, package changes, product reformulations, or other product innovation; |
| ● | Decline in the social acceptability of beverage alcohol products in our markets; |
| ● | Production facility or supply chain disruption; |
| ● | Imprecision in supply/demand forecasting; |
| ● | Higher costs, lower quality, or unavailability of energy, input materials, labor, or finished goods; |
| ● | Route-to-consumer changes that affect the timing of our sales, temporarily disrupt the marketing or sale of our products, or result in higher implementation related or fixed costs; |
| ● | Inventory fluctuations in our products by distributors, wholesalers, or retailers; Competitors’ consolidation or other competitive activities, such as pricing actions (including price reductions, promotions, discounting, couponing, or free goods), marketing, category expansion, product introductions, or entry or expansion in our geographic markets; |
| ● | Insufficient protection of our intellectual property rights; |
| ● | Product recalls or other product liability claims; product counterfeiting, tampering, or product quality issues; |
| ● | Significant legal disputes and proceedings; government investigations (particularly of industry or company business, trade or marketing practices); |
| ● | Failure or breach of key information technology systems; |
| ● | Negative publicity related to our company, brands, marketing, personnel, operations, business performance or prospects; and |
| ● | Business disruption, decline, or costs related to organizational changes, reductions in workforce, or other cost-cutting measures, or our failure to attract or retain key executive or employee talent. |
Uncertainty in the financial markets and other adverse changes in general economic or political conditions in any of the major countries in which we do business could adversely affect our industry, business and results of operations.
Global economic uncertainties, including foreign currency exchange rates, affect businesses such as ours in a number of ways, making it difficult to accurately forecast and plan our future business activities. There can be no assurance that economic improvements will occur, or that they would be sustainable, or that they would enhance conditions in markets relevant to us.
Our limited operating history makes it difficult to forecast our future results, making any investment in us highly speculative.
We have a limited operating history, and our historical financial and operating information is of limited value in predicting our future operating results. We may not accurately forecast customer behavior and recognize or respond to emerging trends, changing preferences or competitive factors facing us, and, therefore, we may fail to make accurate financial forecasts. Our current and future expense levels are based largely on our investment plans and estimates of future revenue. As a result, we may be unable to adjust our spending in a timely manner to compensate for any unexpected revenue shortfall, which could then force us to curtail or cease our business operations.
Risks Related to Our Securities
An investment in our common stock is speculative and there can be no assurance of any return on any such investment.
An investment in our common stock is speculative and there is no assurance that investors will obtain any return on their investment. Investors will be subject to substantial risks involved in an investment in the Company, including the risk of losing their entire investment.
Future sales of common stock, or the perception of such future sales, by some of our existing stockholders could cause our stock price to decline.
The market price of our common stock could decline as a result of sales of a large number of shares of our common stock in the market or the perception that these sales may occur. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell shares in the future at a time and at a price that we deem appropriate.
From time to time, certain of our stockholders may be eligible to sell all or some of their common shares by means of ordinary brokerage transactions in the open market pursuant to Rule 144 promulgated under the Securities Act of 1933, as amended (the “Securities Act”), subject to certain limitations. In general, pursuant to Rule 144, non-affiliate stockholders may sell freely after six months subject only to the current public information requirement. Affiliates may sell after six months subject to the Rule 144 volume, manner of sale (for equity securities), and current public information and notice requirements.
Our Board of Directors may issue and fix the terms of shares of our Preferred Stock without stockholder approval, which could adversely affect the voting power of holders of our Common Stock or any change in control of our Company.
Our Articles of Incorporation authorize the issuance of up to 5,000,000 shares of “blank check” preferred stock, with par value $0.001 per share, with such designation rights and preferences as may be determined from time to time by the Board of Directors. Our Board of Directors is empowered, without shareholder approval, to issue shares of preferred stock with dividend, liquidation, conversion, voting or other rights which could adversely affect the voting power or other rights of the holders of our common stock. In the event of such issuances, the preferred stock could be used, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of our company. Any such issuance would be subject to terms and conditions of any current offering that may disallow any such issuance.
Because certain principal stockholders own a large percentage of our voting stock, other stockholders’ voting power may be limited.
As of December 31, 2016, we had eight full-time and two part-time employees. None2023, our ten (10) largest shareholders own or controlled approximately 21.2% of our employees were represented byoutstanding common stock. If those stockholders act together, they would have the ability to have a labor unionsubstantial influence on matters submitted to our stockholders for approval, including the election and removal of directors and the approval of any merger, consolidation or sale of all or substantially all of our assets. As a result, our other stockholders may have little or no influence over matters submitted for shareholder approval. In addition, the ownership of such stockholders could preclude any unsolicited acquisition of us, and consequently, adversely affect the price of our common stock. These stockholders may make decisions that are adverse to your interests.
We do not expect to pay dividends and investors should not buy our Common Stock expecting to receive dividends.
We do not anticipate that we will declare or pay any dividends in the foreseeable future. Consequently, you will only realize an economic gain on your investment in our common stock if the price appreciates. You should not purchase our common stock expecting to receive cash dividends. Therefore, our failure to pay dividends may cause you to not see any return on your investment even if we are successful in our business operations.
There can be no assurances that our common stock will not be subject to potential delisting if we do not continue to maintain the listing requirements of the NYSE American.
Since June 11, 2021, our common stock has been listed on the NYSE American, under the symbol “SBEV”. The NYSE American has rules for continued listing, including, without limitation, minimum market capitalization and other requirements. Failure to maintain our listing (i.e., being de-listed from the NYSE American), would make it more difficult for shareholders to sell our common stock and more difficult to obtain accurate price quotations on our common stock. This could have an adverse effect on the price of our common stock. Our ability to issue additional securities for financing or other labor organization.
On October 6, 2023, the NYSE American notified the Company that we were not in compliance with Section 1003(a)(i) of the continued listing standards set forth in the NYSE American Company Guide (the “Company Guide”), requiring a listed company to have stockholders’ equity of (i) at least $2.0 million if it has reported losses from continuing operations or net losses in two of its three most recent fiscal years. The notice had no immediate impact on the listing of our common stock, subject to our compliance with the other continued listing requirements. In accordance with applicable NYSE American procedures, we submitted a plan of compliance (the “Plan”) advising of the definitive action(s) the Company has taken, is taking, or would take, that would bring us into compliance with the continued listing standards within the 18 months of receipt of the notice. The NYSE American reviewed and accepted the Plan as a reasonable demonstration of an ability to conform to the relevant standards in the 18-month period. On December 20, 2023, we received a notification (the “Plan Letter”), with NYSE American acceptance of the proposed plan and further deficiency notice. In the Plan Letter the NYSE American indicated that in addition to Section 1003(a)(i), the Company was also not in compliance with Section 1003(a)(ii) of the Company Guide, requiring a listed company to have stockholders’ equity of at least $4.0 million if it has reported losses from continuing operations or net losses in three of its four most recent fiscal years.
Our common stock will continue to be listed and traded on the NYSE American during the 18-month period, subject to the Company’s compliance with the other continued listing standards of the NYSE American and continued periodic review by the NYSE American of the Company’s progress with respect to its Plan. There can be no assurance that the Company will be able to meet its goals set forth in the Plan. If we are unable to satisfy the NYSE American rules and listing standards, or are unable to make progress on our Plan, our securities could be subject to delisting.
If the NYSE American were to delist our securities from trading, we could face significant consequences, including, but not requiredlimited to, provide the information required by this item.
| ● | a limited availability for market quotations for our securities; |
| ● | reduced liquidity with respect to our securities; |
| ● | a determination that our common stock is a “penny stock,” which will require brokers trading in our common stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our common stock; |
| ● | limited amount of news and analyst coverage; and |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
Our common stock could be further diluted as the result of the issuance of additional common stock, convertible securities, warrants or options.
Our issuance of additional common stock, convertible securities, options and warrants could affect the rights of our stockholders, result in a reduction in the overall percentage holdings of our stockholders, could put downward pressure on the market price of our common stock, could result in adjustments to conversion and exercise prices of outstanding notes and warrants, and could obligate us to issue additional common stock to certain of our stockholders.
Item 1B. Unresolved Staff Comments.
None.
Item 1C. Cybersecurity
We have established policies and processes for assessing, identifying, and managing material risk from cybersecurity threats, and have integrated these processes into our overall risk management systems and processes. We routinely assess material risks from cybersecurity threats, including any potential unauthorized occurrence on or conducted through our information systems that may result in adverse effects on the confidentiality, integrity, or availability of our information systems or any information residing therein.
We conduct periodic risk assessments to identify cybersecurity threats, as well as assessments in the event of a material change in our business practices that may affect information systems that are vulnerable to such cybersecurity threats. These risk assessments include identification of reasonably foreseeable internal and external risks, the likelihood and potential damage that could result from such risks, and the sufficiency of existing policies, procedures, systems, and safeguards in place to manage such risks.
Following these risk assessments, we re-design, implement, and maintain reasonable safeguards to minimize identified risks; reasonably address any identified gaps in existing safeguards; and regularly monitor the effectiveness of our safeguards. Primary responsibility for assessing, monitoring and managing our cybersecurity risks rests with the President who reports to our Chief Executive Officer, to manage the risk assessment and mitigation process.
We engage consultants, or other third parties in connection with our risk assessment processes. These service providers assist us to design and implement our cybersecurity policies and procedures, as well as to monitor and test our safeguards. We require each third-party service provider to certify that it has the ability to implement and maintain appropriate security measures, consistent with all applicable laws, to implement and maintain reasonable security measures in connection with their work with us, and to promptly report any suspected breach of its security measures that may affect our company.
We have not encountered cybersecurity challenges that have materially impaired our operations or financial standing.
Governance
Our board of directors addresses the Company’s cybersecurity risk management as part of its general oversight function. The board of directors’ audit committee is responsible for overseeing Company’s cybersecurity risk management processes, including oversight and mitigation of risks from cybersecurity threats.
Our cybersecurity risk assessment and management processes are implemented and maintained by certain Company management, including the information technology team at the direction of our President. Our executive team including our Chief Executive Officer, and Chief Financial Officer are responsible for hiring appropriate personnel, helping to integrate cybersecurity risk considerations into the Company’s overall risk management strategy, and communicating key priorities to relevant personnel. This executive team is responsible for approving budgets, helping prepare for cybersecurity incidents, approving cybersecurity processes, and reviewing security assessments and other security-related reports.
Our cybersecurity incident response and vulnerability management policies are designed to escalate certain cybersecurity incidents to members of management depending on the circumstances, including our Chief Executive Officer, and Chief Financial Officer. In addition, the Company’s incident response and vulnerability management policies include reporting to the audit committee of the board of directors for certain cybersecurity incidents including significant breaches to the Company’s networks or systems. The audit committee receives regular reports from the information technology team concerning the Company’s significant cybersecurity threats and risk and the processes the Company has implemented to address them. The audit committee also has access to various reports, summaries or presentations related to cybersecurity threats, risk and mitigation.
Item 2. Properties.
Splash’s physical offices are located at 4120 Boardman-Canfield Road, Canfield, Ohio 44406. We rent1500 Cordova Rd; Fort Lauderdale, FL 33316 and 1491 2nd Street, Sarasota FL 34236 while our offices pursuant to a three-year lease extension which expires in June 2017. Our monthly rentbusiness office is approximately $2,700, plus costs.
Item 3. Legal Proceedings.
We are not currently a party to any pending legal proceedings are currently pendingthat we believe will have a material adverse effect on our business or threatenedfinancial conditions. We may, however, be subject to various claims and legal actions arising in the bestordinary course of our knowledge.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant'sRegistrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
The Company’s Common Stock
Aggregate Number of Holders of Common Stock
As of March 29, 2024, there were 45,129,687 shares of Common Stock issued and outstanding. As of March 29, 2024, at our transfer agent owners totaled approximately 273 holders of record holders of our common stock on March 21, 2017 was approximately 120.
Dividends
We have not paiddeclared any cash dividends on our common stock since inception and do not anticipate paying anysuch dividends in the foreseeable future. Management's current policy isWe plan to retain any future earnings if any, for use in our operationsbusiness operations. Any decisions as to future payment of cash dividends will depend on our earnings and for expansionfinancial position and such other factors as the Board of the business.
Securities Authorized for Issuance under Equity Compensation Plans
None.
Equity Compensation Plan Information
The information required by this item with respect to securities authorized for issuance under equity compensation plans oris set forth in Part III, Item 12 of this Annual Report on Form 10-K, and is incorporated herein by reference.
Purchases of Equity Securities by the Issuer.
There were no repurchases of our common stock option plans.
Use of Unregistered Securities
On June 7, 2021, our Registration Statement, as amended, and originally filed on Form S-1 (File No. 333-255091) was declared effective by the last three monthsSEC for our initial public offering of 20167,500,000 units, including 3,750,000 additional shares of common stock and the first month3,750,000 warrants to purchase shares of 2017, we sold a total of 750,000 shares to one accredited investor, who was alreadycommon stock, each at an existing shareholder, at aoffering price of $.10$4.00 per share, for $75,000. We reliedaggregate gross proceeds of approximately $15.0 million. After deducting underwriting discounts and commissions and other estimated offering expenses incurred by us of approximately $1.2 million, the net proceeds from the offering were approximately $13.8 million. Kingswood Capital Markets, a division of Benchmark Investments, LLC acted as sole book-running manager and the representative of the underwriters of the underwritten public offering. No offering costs were paid or are payable, directly, or indirectly, to our directors or officers, to persons owning 10% or more of any class of our equity securities, or to any of our affiliates. Our common stock and warrants are traded on Nasdaq under the exemption provided by Rule 506 under Regulation D.
There has been no material change in the expected use of the net proceeds from our underwritten public offering as described in our final prospectus filed with the SEC on June 14, 2021. Upon receipt, the net proceeds from our underwritten public offering were held in cash and cash equivalents. As of December 31, 2023, we have used approximately all of the net proceeds from the underwritten public offering, primarily on working capital and general corporate purposes.
Item 6. Selected Financial Data.
Item 7. Management'sManagement’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis should be read in conjunction with the Audited Consolidated Financial Statements and Notes to Audited Consolidated Financial Statements filed herein.
Business Overview
Canfield Medical Supply, Inc. (“CMS”) a company’s whose common stock was quoted on the OTCQB entered into an Agreement and Plan of Merger with SBG Acquisition Inc. (“Merger Sub”), a Nevada Corporation wholly-owned by Canfield, and Splash Beverage Group, II Inc.. a Nevada corporation (“Splash”) pursuant to which Merger Sub merged with and into Splash (the “Merger”) with Splash as the surviving company and a wholly-owned subsidiary of Canfield. The Merger was consummated on March 31, 2020.
As the owners and management of Splash had voting and operating control of CMS following the Merger, the Merger transaction was accounted for as a reverse acquisition (that is with Splash as the acquiring entity), followed by a physician asrecapitalization.
On July 31, 2020, CMS changed its name to Splash Beverage Group, Inc. (“SBG”). On June 11, 2021, SBG’s common stock and warrant to purchase common stock began trading on the NYSE American under the symbols “SBEV” and SBEV WT,” respectively.
On November 8, 2021, SBG reincorporated into the State of Nevada and became a Nevada corporation.
Our principal offices are located at 1314 E. Las Olas Blvd, Suite 221, Fort Lauderdale, Florida 33301. Our website address is www.splashbeveragegroup.com. We have not incorporated by reference into this Annual Report on Form 10-K the information that can be assessed through our website and you should not consider it to be part of an overall care plan.
Results of OperationOperations for the year endedYear Ended December 31, 2016 as2023, compared to the year endedYear Ended December 31, 2015
Revenue
Revenues for the year ended December 31, 20162023 were $1,102,291 as$18.9 million compared to the revenues of $897,341$18.1 million for the year ended December 31, 2015.2022. The 23% increase in sales is primarilywas mainly due to winning the Medicare competitive bidding for wheel chairs and a few other itemsan increase in our local market as well as a shiftE-commerce segment of $0.4 million and an increase in customer focus away from Medicare and Medicaid towards private pay/private insurance customers due to continually decreasing Medicare and Medicaid reimbursement rates.
Cost of Goods Sold
Cost of goods sold for the year ended December 31, 20162023 were $484,908 as$13.3 million compared to costscost of goods sold for the year ended December 31, 20152022 of $426,980.$12.2 million. The 14%$1.1 million increase in the latest yearcost of goods sold was due to the increase in theour increased sales volume, combined with the fact that Medicare has reduced the amount it is paying the Company for its products.
Operating Expenses
Operating expenses for the year ended December 31, 20162023 were $577,384 as$20.9 million compared to $451,462$27.3 million for the year ended December 31, 2015.2022. Non cash-operating expenses related to share issuance was $1.2 million as of December 31, 2023 compared to $7.4 million in December 31, 2022. The 28% increase in the latest one year periodremaining operating expense decrease of $0.2 million was primarily due to a $53,096decreases in sales and marketing expense and other general and administrative expenses of $1.0 million, which were offset by an increase of $0.8 million in salariessalary and wages resulting from the hiring of a sales representative, and a $47,628 increase in professional fees resulting from the completion of our 2014 and 2015 audits and 10K and 10Q filings, as well as our 2016 10Q filings, to bring our filings current with the SEC.
Other Income/(Expense)
Other expense for the year ended December 31, 2016 was $48,846 as2023 were $5.7 million compared to a net income of $19,153$0.2 million for the year ended December 31, 2015.2022. The primary reasons for the change from a profitother expense increase of $19,153 in 2015 to a $48,846 loss in 2016 were the$5.5 million is mainly driven by an increase in salariesamortization of debt discount of $3.8 million and wages and thea $1.9 million increase in professional fees as explained above.
LIQUIDITY AND CAPITAL RESOURCES
Liquidity is the ability of a company to generate funds to support its current and Capital Resources
As of December 31, 2016,2023, we had working capitaltotal cash of $2,784$379,978, as compared to negative working capital of $(24,069) as ofwith $4,431,745 at December 31, 2015.
Net cash provided byused for continuing operating activities during the year ended December 31, 20162023, was $32,113$10.2 million as compared to the net cash providedused by continuing operating activities infor the year ended December 31, 20152022, of $13,075.$14.0 million. The primary reason for the improvementchange in net cash used was thedue to an increase of $57,022$3.8 million in gross profit due primarily to $114,950amortization of debt and a decrease of $0.6 million in increased revenues, which was only partiallylosses of the business, offset by a decrease of $16.5 million in working capital. Net cash used for discontinued operating activities during the increased salaries and professional fees. Although we experienced a $25,997 increase in depreciation in 2016 overyear ended December 31, 2023, was $0 as compared to $0.03 million for the 2015 period, this is non-cash operating expense and thus does not affect cash flows from operations..
Net cash used for investing activities during the year ended December 31, 20162023, was $52,076 which represented $58,424 used for the purchase of equipment which was offset by $6,348 received from the sale of equipment$0.01 as compared to $26,880the net cash used for the purchase of equipment and $6,295 received from the sale of equipmentinvesting activities during the year ended December 31, 2015.
Net cash provided by (used for) financing activities during the year ended December 31, 20162023, was $74,279 as$6.1 million compared to $10,055 used for$14.4 million provided from financing activities infor the year ended December 31, 2015. The Company sold shares of its common stock during2022. During the year ended December 31, 20162023, we received $0 from the issuance of common stock compared to $11.4 million during the year ending December 31, 2022. We received $6.6 million and $4.0 million proceeds from the issuance of debt in years ending December 31, 2023 and 2022, respectively. In the year ending December 31, 2023, $0.2 million was received from a shareholder advance and a $0.4 million shareholder advance was repaid in the year ending December 31, 2022. Principal repayment of debt of $1.0 million and $0.6 million were made in years ending December 31, 2023 and 2022 respectively. In the year ending December 31, 2023 a cash advance from related party of $0.4 million was received.
In order to have sufficient cash to fund our operations, we will need to raise $90,000additional equity or debt capital. There can be no assurance that additional funds will be available when needed from any source or, if available, will be available on terms that are acceptable to help payus. We will be required to pursue sources of additional capital through various means, including debt or equity financings. Future financings through equity investments are likely to be dilutive to existing stockholders. Also, the terms of securities we may issue in future capital transactions may be more favorable for new investors. Newly issued securities may include preferences, superior voting rights, the issuance of warrants or other derivative securities, and the issuance of incentive awards under equity employee incentive plans, which may have additional dilutive effects. Further, we may incur substantial costs associatedin pursuing future capital and/or financing, including investment banking fees, legal fees, accounting fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with being a public company. Othercertain securities we may issue, such as convertible notes and warrants, which will adversely impact our financial condition. Our ability to obtain needed financing may be impaired by such factors as the capital markets and our history of losses, which could impact the availability or cost of future financings. If the amount of capital we are able to raise from financing activities consisted of principal payments on long-term debt of $8,844 and $7,053 during 2016 and 2015, respectively, and net payments ontogether with our line of credit of $6,877 and $3,002 during 2016 and 2015, respectively.
Critical Accounting Policies and Estimates
The preparation of our consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, and liabilities andexpenses, as well as the disclosure of contingent assets and liabilities atliabilities. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the date of the financial statements and the reported amounts of revenues and expenses during the reporting period.circumstances. Actual results could differ from those estimates.
Revenue
The Company faces significant judgment in revenue recognition due to the complexities of the beverage industry’s competitive landscape and diverse distribution channels. Determining the timing of revenue recognition involves assessing factors such as control transfer, returns, allowances, trade promotions, and distributor sell-through data. Historical analysis, market trends assessment, and contractual term evaluations inform revenue recognition judgments. However, inherent uncertainties persist, underscoring the critical nature of revenue recognition as it significantly impacts financial statements and performance evaluation.
Allowance for Doubtful Accounts
The allowance for doubtful accounts is established based on historical experience, current economic conditions, and specific customer collection issues. Management evaluates the collectability of accounts receivable on an ongoing basis and adjusts the allowance as necessary. Changes in economic conditions or customer creditworthiness could result in adjustments to the allowance for doubtful accounts, impacting our reported financial results.
Inventory Valuation
We value inventory at the lower of cost or net realizable value. Estimating the net realizable value of inventory involves significant judgment, particularly when market conditions change rapidly or when excess or obsolete inventory exists. Management regularly assesses inventory quantities on hand, future demand forecasts, and market conditions to determine whether write-downs to inventory are necessary.
Fair Value Measurements
We measure certain financial assets and liabilities at fair value on a recurring basis. Fair value measurements involve significant judgment and estimation, particularly when observable inputs are limited or not available. Management utilizes valuation techniques such as discounted cash flow models, market comparables, and third-party appraisals to determine fair values.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
Not applicable for smaller reporting companies.
Item 8. Financial Statements and Supplementary Data.
| Financial Statements | Page | ||
| F-2 | |||
| F-3 | |||
| Consolidated Balance Sheets December 31, 2023 and December 31, 2022 | F-4 | ||
| Consolidated Statements of Operations For the Years Ended December 31, 2023 and December 31 2022 | F-5 | ||
| Consolidated Statements of Changes in | F-6 | ||
| Consolidated Statements of Cash Flows For the Year Ended December 30, 2023 and 2022 | F-7 | ||
| Notes to the Consolidated Financial Statements | F-8 | ||
Report of Independent Registered Public Accounting Firm
To the Board of Directors
Splash Beverage Group, Inc.
Fort Lauderdale, Florida
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheetssheet of Canfield Medical Supply,Splash Beverage Group, Inc. (the Company) as of“Company”) at December 31, 2016 and 2015,2022, and the related consolidated statements of operations, stockholders'changes in stockholders’ equity (deficit), and cash flows for the years then ended. The Company's management is responsibleyear ended December 31, 2022, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2022, and the results of its operations and its cash flows for thesethe year ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements.statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on thesethe Company’s financial statements based on our audits.
We conducted our auditsaudit in accordance with the standards of the Public Company Accounting Oversight Board (United States).PCAOB. Those standards require that we plan and perform the auditsaudit to obtain reasonable assurance about whether the financial statements are free of material misstatement.misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included considerationAs part of our audit, we are required to obtain an understanding of internal control over financial reporting, as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company'sCompany’s internal control over financial reporting. Accordingly, we express no such opinion. An
Our audit includesincluded performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence supportingregarding the amounts and disclosures in the financial statements, assessingstatements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statement presentation.statements. We believe that our audits provideaudit provides a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the December 31, 2022 audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which they relate.
Intangible Assets Impairment Assessments
As described in Note 2 to the consolidated financial statements, the Company has intangible assets of approximately $4.9 million at December 31, 2022. In most cases, no directly observable market inputs are available to measure the fair value to determine if the asset is impaired. Therefore, an estimate is derived indirectly and is based on valuation techniques utilizing undiscounted and discounted after-tax cash flows and discount rates. The estimates that management used in calculating the net present values depend on assumptions specific to the nature of the management service activities with regard to the amount and timing of projected future cashflows; long-term forecasts; actions of competitors (competing services), future tax and discount rates.
The principal considerations for our determination that performing procedures relating to the intangible assets impairment assessment is a critical audit matter are the significant judgment by management when developing the net present value of the intangible assets. This in turn led to a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions related to the amount and timing of projected future cash flows and the discount rate. In addition, the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements These procedures included testing management’s process for developing the fair value estimate; evaluating the appropriateness of the net present value techniques; testing the completeness and accuracy of underlying data used in the model; and evaluating the significant assumptions used by management, including the amount and timing of projected future cash flows and the discount rate. Evaluating management’s assumptions related to the amount and timing of projected future cash flows and the discount rate involved evaluating whether the assumptions used by management reasonable considering the current and past performance of the intangible assets, the consistency with external market and industry data, and whether these assumptions were consistent with evidence obtained in other areas of the audit.
/s/ Daszkal Bolton LLP
Daszkal Bolton LLP
Fort Lauderdale, Florida
March 31, 2023
We served as the Company’s auditor from 2020 to March 2023.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
Splash Beverage Group, Inc.
Fort Lauderdale, Florida
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Splash Beverage Group, Inc. at December 31, 2023, and the related consolidated statements of operations, changes in stockholders’ equity and cash flows for the year ended December 31, 2023, and the related notes (collectively referred to as the financial statements). In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as ofat December 31, 2016 and 2015,2023, and the results of its operations and its cash flows for the years thenyear ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 73 to the consolidated financial statements, the Company has incurredsuffered recurring losses since its inception,from operations and has an accumulated deficit and a working capital deficit, and has not yet established profitable operations. These factorsdeficiency that raise substantial doubt about theits ability of the Company to continue as a going concern. Management'sManagement’s plans in regards toregarding these matters are also described in Note 7. These3. The consolidated financial statements do not include any adjustments that might result from the outcome of these uncertainties.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
Rose, Snyder & Jacobs LLP
We have served as the Company’s auditor since 2023
Encino, CA
March 29, 2024
| CANFIELD MEDICAL SUPPLY, INC. | ||||||||
| BALANCE SHEETS | ||||||||
| December 31, | December 31, | |||||||
| ASSETS | 2016 | 2015 | ||||||
| Current Assets | ||||||||
| Cash | $ | 61,659 | $ | 7,343 | ||||
| Accounts receivable, net | 206,254 | 167,063 | ||||||
| Inventory | 25,231 | 21,589 | ||||||
| Total Current Assets | 293,144 | 195,995 | ||||||
| Equipment, net of accumulated depreciation of $76,197 and $40,771 | 62,190 | 43,753 | ||||||
| Total Assets | $ | 355,334 | $ | 239,748 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) | ||||||||
| Current Liabilities | ||||||||
| Accounts payable and accrued liabilities | $ | 209,069 | $ | 135,211 | ||||
| Line of credit | 70,373 | 77,250 | ||||||
| Current portion of long-term debt | 10,918 | 7,603 | ||||||
| Total Current Liabilities | 290,360 | 220,064 | ||||||
| Long-term debt | 25,305 | 21,169 | ||||||
| Total Liabilities | 315,665 | 241,233 | ||||||
| Stockholders' Equity (Deficit) | ||||||||
| Preferred stock, no par value; 5,000,000 shares authorized; no shares | ||||||||
| issued and outstanding | - | - | ||||||
| Common stock, no par value; 100,000,000 shares authorized; | ||||||||
| 10,927,200 and 10,027,200 shares issued and outstanding, respectively | 208,515 | 118,515 | ||||||
| Accumulated deficit | (168,846 | ) | (120,000 | ) | ||||
| Total Stockholders' Equity (Deficit) | 39,669 | (1,485 | ) | |||||
| Total Liabilities and Stockholders' Equity (Deficit) | $ | 355,334 | $ | 239,748 | ||||
| The accompanying footnotes are an integral part of these financial statements. | ||||||||
| CANFIELD MEDICAL SUPPLY, INC. | ||||||||
| STATEMENTS OF OPERATIONS | ||||||||
| Year Ended | Year Ended | |||||||
| December 31, 2016 | December 31, 2015 | |||||||
| Sales (net of returns) | $ | 1,012,291 | $ | 897,341 | ||||
| Cost of goods sold | 484,908 | 426,980 | ||||||
| Gross profit | 527,383 | 470,361 | ||||||
| Operating expenses: | ||||||||
| Salaries and wages | 298,368 | 245,272 | ||||||
| Professional fees | 87,198 | 39,570 | ||||||
| Depreciation | 55,760 | 29,763 | ||||||
| Other selling, general and administrative | 136,058 | 136,857 | ||||||
| Total operating expenses | 577,384 | 451,462 | ||||||
| Income (loss) from operations | (50,001 | ) | 18,899 | |||||
| Other income (expense): | ||||||||
| Interest expense | (4,671 | ) | (5,368 | ) | ||||
| Gain on sale of fixed assets | 5,826 | 5,622 | ||||||
| 1,155 | 254 | |||||||
| Income (loss) before provision for income taxes | (48,846 | ) | 19,153 | |||||
| Provision for income tax | - | - | ||||||
| Net income (loss) | $ | (48,846 | ) | $ | 19,153 | |||
| Net income (loss) per share (basic and fully diluted) | $ | (0.00 | ) | $ | 0.00 | |||
| Weighted average number of common shares outstanding | 10,484,113 | 10,027,200 | ||||||
| Splash Beverage Group, Inc. |
| Consolidated Balance Sheets |
| December 31, 2023 and December 31, 2022 |
| December 31, 2023 | December 31, 2022 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 379,978 | $ | 4,431,745 | ||||
| Accounts Receivable, net | 890,631 | 1,812,110 | ||||||
| Prepaid Expenses | 220,320 | 348,036 | ||||||
| Inventory | 2,252,469 | 3,721,307 | ||||||
| Other receivables | 233,850 | 344,376 | ||||||
| Total current assets | 3,977,248 | 10,657,574 | ||||||
| Non-current assets: | ||||||||
| Deposit | 49,446 | 49,290 | ||||||
| Goodwill | 256,823 | 256,823 | ||||||
| Intangibles assets, net | 4,459,309 | 4,851,377 | ||||||
| Investment in Salt Tequila USA, LLC | 250,000 | 250,000 | ||||||
| Right of use assets | 556,140 | 750,042 | ||||||
| Property and equipment, net | 349,802 | 489,597 | ||||||
| Total non-current assets | 5,921,520 | 6,647,129 | ||||||
| Total assets | $ | 9,898,768 | $ | 17,304,703 | ||||
| Liabilities and Stockholders’ Equity | ||||||||
| Liabilities: | ||||||||
| Current liabilities | ||||||||
| Accounts payable and accrued expenses | $ | 4,444,286 | $ | 3,383,187 | ||||
| Right of use liability, current portion | 262,860 | 268,749 | ||||||
| Related party notes payable | 380,000 | — | ||||||
| Notes payable, net of discounts | 7,748,518 | 1,080,257 | ||||||
| Liability to issue shares | — | 91,800 | ||||||
| Shareholder advances | 200,000 | — | ||||||
| Accrued interest payable | 1,714,646 | 141,591 | ||||||
| Total current liabilities | 14,750,310 | 4,965,584 | ||||||
| Long-term Liabilities: | ||||||||
| Notes payable, net of discounts | 457,656 | 2,536,319 | ||||||
| Right of use liability, net of current portion | 296,128 | 480,666 | ||||||
| Total long-term liabilities | 753,784 | 3,016,985 | ||||||
| Total liabilities | $ | 15,504,094 | $ | 7,982,569 | ||||
| Stockholders’ equity: | ||||||||
| Preferred stock, $ par value, shares authorized, shares issued | — | — | ||||||
| Common Stock, $ par, shares authorized, and shares issued and outstanding, at December 31, 2023 and December 31, 2022, respectively | 44,330 | 41,086 | ||||||
| Additional paid in capital | 127,701,710 | 121,632,547 | ||||||
| Accumulated Other Comprehensive Income | (16,583 | ) | (20,472 | ) | ||||
| Accumulated deficit | (133,334,783 | ) | (112,331,027 | ) | ||||
| Total stockholders’ equity | (5,605,326 | ) | 9,322,134 | |||||
| Total liabilities and stockholders’ equity | $ | 9,898,768 | $ | 17,304,703 | ||||
The accompanying footnotesnotes are an integral part of these consolidated financial statements.
| CANFIELD MEDICAL SUPPLY, INC. | ||||||||||||||||
| STATEMENTS OF STOCKHOLDERS' EQUITY (DEFICIT) | ||||||||||||||||
| Common Stock (No Par) | Accumulated | Stockholders' | ||||||||||||||
| Shares | Amount | Deficit | Equity (Deficit) | |||||||||||||
| Balances at December 31, 2014 | 10,027,200 | $ | 118,515 | $ | (139,153 | ) | $ | (20,638 | ) | |||||||
| Net income for the year | - | - | 19,153 | 19,153 | ||||||||||||
| Balances at December 31, 2015 | 10,027,200 | 118,515 | (120,000 | ) | (1,485 | ) | ||||||||||
| Sales of common stock | 900,000 | 90,000 | - | 90,000 | ||||||||||||
| Net (loss) for the year | - | - | (48,846 | ) | (48,846 | ) | ||||||||||
| Balances at December 31, 2016 | 10,927,200 | $ | 208,515 | $ | (168,846 | ) | $ | 39,669 | ||||||||
| Splash Beverage Group, Inc. |
| Consolidated Statements of Operations |
| For the Years Ended December 31, 2023 and December 31, 2022 |
| 2023 | 2022 | |||||||
| Net revenues | $ | 18,850,152 | $ | 18,087,486 | ||||
| Cost of goods sold | (13,281,457 | ) | (12,168,621 | ) | ||||
| Gross margin | 5,568,695 | 5,918,865 | ||||||
| Operating expenses: | ||||||||
| Contracted services | 1,402,572 | 1,505,788 | ||||||
| Salary and wages | 5,003,392 | 4,179,403 | ||||||
| Non-cash share-based compensation | 1,169,858 | 7,409,884 | ||||||
| Other general and administrative | 10,786,011 | 11,411,535 | ||||||
| Sales and marketing | 2,493,520 | 2,806,888 | ||||||
| Total operating expenses | 20,855,353 | 27,313,498 | ||||||
| Loss from continuing operations | (15,286,658 | ) | (21,394,633 | ) | ||||
| Other income/(expense): | ||||||||
| Other Income/expense | (30,328 | ) | — | |||||
| Interest income | 2,634 | 6,068 | ||||||
| Interest expense | (1,856,777 | ) | (251,497 | ) | ||||
| Amortization of debt discount | (3,832,628 | ) | — | |||||
| Total other expense | (5,717,099 | ) | (245,429 | ) | ||||
| Provision for income taxes | — | — | ||||||
| Net (loss) from continuing operations, net of tax | (21,003,757 | ) | (21,640,062 | ) | ||||
| Net (loss) income from discontinued operations, net of tax | — | (199,154 | ) | |||||
| Gain on discontinued operations | — | 148,747 | ||||||
| Net income (loss) from discontinued operations, net of tax | — | (50,407 | ) | |||||
| Net loss | $ | (21,003,757 | ) | $ | (21,690,469 | ) | ||
| Other comprehensive loss | ||||||||
| Foreign currency translation gain (loss) | 3,889 | (20,472 | ) | |||||
| Total comprehensive loss | (20,999,868 | ) | (21,710,941 | ) | ||||
| Loss per share - continuing operations | ||||||||
| Basic and Diluted | ) | ) | ||||||
| Weighted average number of common shares outstanding - continuing operations | ||||||||
| Basic and Diluted | ||||||||
| Income (loss) per share - discontinued operations | ||||||||
| Basic and Diluted | ) | ) | ||||||
| Weighted average number of common shares outstanding - discontinued operations | ||||||||
| Basic and Diluted | ||||||||
The accompanying footnotesnotes are an integral part of these consolidated financial statements.
| CANFIELD MEDICAL SUPPLY, INC. | ||||||||
| STATEMENTS OF CASH FLOWS | ||||||||
| December 31, | December 31, | |||||||
| 2016 | 2015 | |||||||
| Cash Flows From Operating Activities: | ||||||||
| Net income (loss) | $ | (48,846 | ) | $ | 19,153 | |||
| Adjustments to reconcile net loss to net cash provided by operating activities: | ||||||||
| Gain on sale of fixed assets | (5,826 | ) | (5,622 | ) | ||||
| Depreciation | 55,760 | 29,763 | ||||||
| Changes in current assets and liabilities | ||||||||
| Increase in accounts receivable | (39,191 | ) | (86,880 | ) | ||||
| Increase in inventory | (3,642 | ) | (7,275 | ) | ||||
| Increase in accounts payable and accrued liabilities | 73,858 | 63,936 | ||||||
| Net cash provided by operating activities | 32,113 | 13,075 | ||||||
| Cash Flows From Investing Activities: | ||||||||
| Proceeds from sale of fixed assets | 6,348 | 6,295 | ||||||
| Purchases of property and equipment | (58,424 | ) | (26,880 | ) | ||||
| Net cash (used for) investing activities | (52,076 | ) | (20,585 | ) | ||||
| Cash Flows From Financing Activities: | ||||||||
| Net payments on line of credit | (6,877 | ) | (3,002 | ) | ||||
| Payments on long-term debt | (8,844 | ) | (7,053 | ) | ||||
| Proceeds from sales of common stock. | 90,000 | - | ||||||
| Net cash provided by (used for) financing activities | 74,279 | (10,055 | ) | |||||
| Net Increase (Decrease) in Cash | 54,316 | (17,565 | ) | |||||
| Cash At The Beginning Of The Period | 7,343 | 24,908 | ||||||
| Cash At The End Of The Period | $ | 61,659 | $ | 7,343 | ||||
| Schedule Of Non-Cash Investing And Financing Activities | $ | - | ||||||
| Purchase of equipment with long-term debt | $ | 16,295 | $ | 19,446 | ||||
| Supplemental Disclosure | ||||||||
| Cash paid for interest | $ | 4,671 | $ | 5,460 | ||||
| Cash paid for income taxes | $ | - | $ | - | ||||
| Splash Beverage Group, Inc. |
| Consolidated Statements of Changes in Stockholders’ Equity |
| For the Years ended December 31, 2023 and 2022 |
| Common stock | Additional Paid-in | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ Equity | ||||||||||||||||||||
| Shares | Amount | Capital | Income | Deficit | (Deficit) | |||||||||||||||||||
| Balances at December 31, 2021 | 33,596,232 | 33,596 | 99,480,188 | — | (90,640,557 | ) | 8,873,227 | |||||||||||||||||
| Issuance of common stock on convertible instruments | 377,796 | 378 | 1,514,533 | — | — | 1,514,911 | ||||||||||||||||||
| Issuance of warrants for services | — | — | 3,849,144 | — | 3,849,144 | |||||||||||||||||||
| Issuance of warrants on convertible instruments | — | — | 1,898,265 | — | — | 1,898,265 | ||||||||||||||||||
| Issuance of common stock for services | 2,215,363 | 2,215 | 3,466,722 | — | — | 3,468,937 | ||||||||||||||||||
| Issuance of common stock and warrants for cash | 4,896,129 | 4,896 | 11,423,695 | — | 11,428,591 | |||||||||||||||||||
| Accumulated Comprehensive Income - Translation | — | — | — | (20,472 | ) | — | (20,472 | ) | ||||||||||||||||
| Net loss | — | (21,690,469 | ) | (21,690,469 | ) | |||||||||||||||||||
| Balances at December 31, 2022 | 41,085,520 | 41,086 | 121,632,547 | (20,472 | ) | (112,331,026 | ) | 9,322,134 | ||||||||||||||||
| Note discount created from issuance of common stock and warrants on convertible instruments | 2,275,000 | 2,275 | 4,585,975 | — | — | 4,588,250 | ||||||||||||||||||
| Share based compensation | — | — | 840,817 | — | — | 840,817 | ||||||||||||||||||
| Conversion of notes payable to common stock | 452,914 | 453 | 229,891 | — | — | 230,344 | ||||||||||||||||||
| Issuance of common stock for services | 516,665 | 516 | 412,481 | — | — | 412,997 | ||||||||||||||||||
| Accumulated Comprehensive Income - Translation | — | — | — | 3,889 | — | 3,889 | ||||||||||||||||||
| Net loss | — | — | — | — | (21,003,757 | ) | (21,003,757 | ) | ||||||||||||||||
| Balances at December 31, 2023 | 44,330,099 | 44,330 | 127,701,710 | (16,583 | ) | (133,334,783 | ) | (5,605,326 | ) | |||||||||||||||
The accompanying footnotesnotes are an integral part of these consolidated financial statements
| Splash Beverage Group, Inc. |
| Consolidated Statements Cash Flows |
| For the Year Ended December 30, 2023 and 2022 |
| 2023 | 2022 | |||||||
| Net loss | $ | (21,003,757 | ) | $ | (21,690,469 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 545,977 | 936,020 | ||||||
| ROU assets, net | 3,474 | 4,093 | ||||||
| Amortization of debt discount | 3,832,628 | — | ||||||
| Gain from sale of discontinued operation | — | 84,375 | ||||||
| Non-cash share based compensation | 1,169,858 | 7,318,081 | ||||||
| Changes in working capital items: | ||||||||
| Accounts receivable, net | 921,479 | (697,658 | ) | |||||
| Inventory, net | 1,468,838 | (1,797,828 | ) | |||||
| Prepaid expenses and other current assets | 238,241 | (43,294 | ) | |||||
| Deposits | (157 | ) | 281,596 | |||||
| Accounts payable and accrued expenses | 1,061,101 | 1,594,300 | ||||||
| Accrued Interest payable | 1,573,055 | (29,861 | ) | |||||
| Net cash used in operating activities - continuing operations | (10,189,263 | ) | (14,040,644 | ) | ||||
| Net cash used in operating activities - discontinued operations | — | (32,774 | ) | |||||
| Cash Flows from Investing Activities: | ||||||||
| Capital Expenditures | (14,113 | ) | (102,698 | ) | ||||
| Net cash used in investing activities -– continuing operations | (14,113 | ) | (102,698 | ) | ||||
| Net cash used in investing activities - discontinued operations | — | — | ||||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds from issuance of Common stock | — | 11,428,591 | ||||||
| Cash advance (repayment) from shareholder | 200,000 | (390,500 | ) | |||||
| Related party cash advance | 380,000 | — | ||||||
| Proceeds from issuance of debt | 6,610,681 | 4,045,420 | ||||||
| Principal repayment of debt | (1,042,961 | ) | (636,560 | ) | ||||
| Net cash provided by financing activities - continuing operations | 6,147,720 | 14,446,951 | ||||||
| Net cash provided by financing activities - discontinued operations | — | — | ||||||
| Net cash effect of exchange rate changes on cash | 3,889 | (20,472 | ) | |||||
| Net Change in Cash and Cash Equivalents | (4,051,767 | ) | 250,362 | |||||
| Cash and Cash Equivalents, beginning of year | 4,431,745 | 4,181,383 | ||||||
| Cash and Cash Equivalents, end of year | $ | 379,978 | $ | 4,431,745 | ||||
| Supplemental Disclosure of Cash Flow Information: | ||||||||
| Cash paid for Interest | $ | 243,087 | $ | 204,594 | ||||
| Supplemental Disclosure of Non-Cash Investing and Financing Activities | ||||||||
| Convertible notes payable and accrued interest converted to common stock (377,796 shares) | $ | — | $ | 1,514,911 | ||||
| Convertible notes payable and accrued interest converted to common stock (452,914 shares) | $ | 230,000 | $ | — | ||||
The accompanying notes are an integral part of these consolidated financial statements.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 1 – Business Organization and 2015
Splash Beverage Group (“SBG” or “Splash”), formally Canfield Medical Supply, Inc. (the "Company"(“CMS”), was incorporated in the State of Ohio on September 3, 1992, and changed domicile to Colorado on April 18, 2012. The Company isCMS was in the business of home health services, primarily the selling of durable medical equipment and medical supplies to the public, nursing homes, hospitals and other end users.
On December 31, 2019, CMS entered into an Agreement and Plan of Merger (the “Merger Agreement”) with SBG Acquisition Inc. (“Merger Sub”), a Nevada Corporation wholly owned by CMS, and Splash Beverage Group, Inc. a Nevada corporation (“Splash”) pursuant to which Merger Sub merged with and into Splash (the “Merger”) with Splash as the surviving company and a wholly-owned subsidiary of CMS. The Merger was consummated on March 31, 2020.
As the owners and management of Splash have voting and operating control of CMS following the Merger, the Merger transaction was accounted for as a reverse acquisition (that is with Splash as the acquiring entity), followed by a recapitalization.
As part of the recapitalization, previously issued shares of SBG preferred stock have been reflected as shares of common stock that were received in the Merger. These common shares have been retrospectively presented as outstanding for all periods.
Splash specializes in the manufacturing process, distribution, and sales & marketing of various beverages across multiple channels. Splash operates in both the non-alcoholic and alcoholic beverage segments. Additionally, Splash operates its own vertically integrated B-to-B and B-to-C E-commerce distribution platform called Qplash, further expanding its distribution abilities and visibility.
In July 2020 the Company filed a Certificate of Amendment of Articles of Incorporation of CMS with the Secretary of State of the State of Colorado, pursuant to which the Company changed its name from CMS. to Splash Beverage Group, Inc. On July 31, 2020, we received approval from FINRA to change the Company’s name from CMS to Splash Beverage Group, Inc. Our new ticker symbol is SBEV.
On December 24, 2020, SBG consummated an Asset Purchase Agreement (the “Copa APA”) with Copa DI Vino® Corporation (“CdV”), to purchase certain assets and assume certain liabilities that comprise the Copa DI Vino® business for a total purchase price of $5,980,000, payable in the combination of $2,000,000 in cash (“Cash Consideration”), $2,000,000 convertible promissory note (the “Convertible Note”) to Seller and a variable number of shares of the Company’s common stock based on a attainment of revenue hurdles. CdV is one of the leading producers of premium wine by the glass in the United States with its primary offices and facilities in The Dalles, Oregon.
On February 2021, Management initiated a plan to divest its CMS business. As a result, the assets and operations of CMS have been retrospectively reflected as discontinued operations. On November 12, 2021 the Company changed its state of Domicile from Colorado to Nevada.
In coordination with up listing to the NYSE on June 11, 2021 the Company consummated a 1.0 for 3.0 reverse stock split. All common stock shares stated herein have been adjusted to reflect the split.
Note 2 – Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
These consolidated financial statements include the accounts of Splash and its wholly owned subsidiaries, Holdings and Splash Mex, CMS (as discontinued operations), and CdV. All intercompany balances have been eliminated in consolidation.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 2 – Summary of Significant Accounting Policies, continued
Our investment in Salt Tequila USA, LLC is accounted for at cost, as the company does not have the ability to exercise significant influence.
Our accounting and reporting policies conform to accounting principles generally accepted in the United States of America (GAAP).
Certain reclassifications have been made to the prior period financial statements to conform to the current period classifications. These reclassifications had no impact on net loss.
Use of Estimates
The preparation of consolidated financial statements in conformity with generally accepted accounting principlesGAAP requires our management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosuredisclosures of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash Equivalents and cash equivalents
We consider all highly liquid investmentssecurities with an original maturity of three months or less asto be cash equivalents.
Our cash in bank deposit accounts, at times, may exceed federally insured limits of $250,000. At December 31, 2023, the Company’s cash on deposit with financial institutions, at times, had not exceed federally insured limits of $250,000. The Company had approximately $3.8 million over the federally insured limits in 2022. Our cash in uninsured foreign bank accounts was $0 and 2015 no allowance$1,941 at December 31, 2023 and December 31, 2022, respectively.
Accounts Receivable and Allowance for bad debtsDoubtful Accounts
Accounts receivables are carried at their estimated collectible amounts and are periodically evaluated for collectability based on past credit history with clients and other factors. We establish provisions for losses on accounts receivable on the basis of loss experience, known and inherent risk in the account balance, and current economic conditions. At December 31, 2023 and December 31, 2022, our accounts receivable amounts are reflected net of allowances of $183,089 and $13,683, respectively.
Inventory
Inventory is stated at the lower of cost or net realizable value, accounted for using the weighted average cost method. The inventory balances at December 31, 2023 and December 31, 2022 consisted of raw materials, work-in-process, and finished goods held for distribution. The cost elements of inventory consist of purchase of products, transportation, and warehousing. We establish provisions for excess or inventory near expiration based on management’s estimates of forecast turnover of inventories on hand and under contract. A significant change in the timing or level of demand for certain products as compared to forecast amounts may result in recording additional provisions for excess or expired inventory in the future. Provisions for excess inventory are included in cost of goods sold and have historically been adequate to provide for losses on inventory. We manage inventory levels and purchase commitments in an effort to maximize utilization of inventory on hand and under commitments. The amount of our reserve was necessary.
Property and Equipment
We record property and equipment
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 2 – Summary of Significant Accounting Policies, continued
Depreciation expense totaled $153,908 and $182,886 for the years ended December 31, 2023 and 2022 respectively. Property and equipment are recorded at cost and depreciated under straight line methods over each item's estimated useful life.
| Schedule of property and equipment | ||||||||
| 2023 | 2022 | |||||||
| Auto | 45,420 | 45,420 | ||||||
| Machinery & equipment | 1,160,578 | 1,108,870 | ||||||
| Buildings & Tanks | 233,323 | 282,988 | ||||||
| Leasehold improvements | 723,638 | 713,068 | ||||||
| Computer Software | 5,979 | — | ||||||
| Office furniture & equipment | 9,157 | 13,636 | ||||||
| Total cost | 2,178,095 | 2,163,983 | ||||||
| Accumulated depreciation | (1,828,293 | ) | (1,674,385 | ) | ||||
| Property, plant & equipment, net | 349,802 | 489,597 | ||||||
Excise taxes
The Company carries inventorypays alcohol excise taxes based on product sales to both the Oregon Liquor Control Commission and to the U.S. Department of durable medical equipmentthe Treasury, Alcohol and medical suppliesTobacco Tax and Trade Bureau (TTB). The Company also pays taxes to the State of Florida – Division of Alcoholic Beverages and Tobacco. The Company is liable for resale. Inventory is accounted forthe taxes upon the removal of product from the Company’s warehouse on a first–per gallon basis. The federal tax rate is affected by a small winery tax credit provision which decreases based upon the number of gallons of wine production in first-out basis.
Fair Value of Financial Instruments
Financial Accounting Standards (“FASB”) guidance specifies a hierarchy of valuation techniques based on whether the inputs to conformthose valuation techniques are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to current year presentation. Formerly, proceeds received fromunadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the sale of fixed assets have been lumped together with fixed asset purchases in the investing sectionlowest priority to unobservable inputs (Level 3 measurement). The three levels of the Statement of Cashflows, but have been segregatedfair value hierarchy are as follows:
| Level 1 - | Unadjusted quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities. |
| Level 2 - | Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly (e.g., quoted prices of similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active). |
| Level 3 - | Unobservable inputs for the asset or liability. Financial instruments are considered Level 3 when their fair values are determined using pricing models, discounted cash flows or similar techniques and at least one significant model assumption or input is unobservable. |
The liabilities and indebtedness presented on the current year to more clearly represent the flow of cash in relation to property and equipment.
Revenue recognition
We recognize revenue under ASC 606, Revenue from product sales is recognized subsequentContracts with Customers (Topic 606). This guidance sets forth a five-step model which depicts the recognition of revenue in an amount that reflects what we expect to a patient (customer) ordering a product at an agreed upon price, andreceive in exchange for the transfer of goods or services to customers.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 2 – Summary of Significant Accounting Policies, continued
We recognize revenue when delivery has occurred, and collectability is reasonably assured. A purchase arrangement is evidenced byour performance obligations under the terms of a written order, with delivery considered as made after physical customer acceptance. Although rare, defective products may be returned, with other return issues considered on a case by case basis. Services such as periodic scheduled deliveries are contracted in writing, and generally billed monthly. Any service revenue earned by the Company for services such as safety and set up consulting or claims processing is recorded after the service is performed. Rental of durable home medical equipment is evidenced by written contract with revenue recognized when rentthe customer are satisfied. Product sales occur once control of our products is earned.
Distribution expenses to transport our products, and warehousing expense after manufacture are accounted for in Other General and Administrative cost.
Cost of Goods Sold
Cost of goods sold include the costs of products, packaging, transportation, warehousing, and costs associated with valuation allowances for expired, damaged or impaired inventory. The cost of transportation from production site to other 3rd party warehouses or customer is included in Other General and Administrative cost.
Other General and Administrative Expenses
Other General and Administrative expenses include Amazon selling fees, royalty cost for selling TapouT, cost of transportation from production site to other 3rd party warehouses or customers, insurance cost, consulting cost, legal and audit fees, investor relations expenses, travel & entertainment expenses, occupancy cost and other cost.
Stock-Based Compensation
We account for stock-based compensation in accordance with ASC 718,”Compensation - Stock Compensation”. Under the fair value recognition provisions, cost is measured at the grant date based on the fair value of the award and is recognized as expense ratably over the requisite service period, which is generally the option vesting period. We use the Black-Scholes option pricing model to determine the fair value of stock options. We early adopted ASU 2018-07, “Improvements to Nonemployee Share-Based Payment Accounting”, which aligns accounting treatment for such awards to non-employees with the existing guidance on employee share-based compensation in ASC 718.
We measure stock-based awards at the grant-date fair value for employees, directors and consultants and recognize compensation expense on a straight-line basis over the vesting period of the award. Determining the appropriate fair value of stock-based awards requires the input of subjective assumptions, including the fair value of our common stock, and for stock options and warrants, the expected life of the option and warrant, and expected stock price volatility and exercise price. We used the Black-Scholes option pricing model to value its stock-based awards. The assumptions used in calculating the fair value of stock-based awards represent management’s best estimates and involve inherent uncertainties and the application of management’s judgment. As a result, if factors change and management uses different assumptions, stock-based compensation expense could be materially different for future awards. The expected life of stock options/warrants were estimated using the “simplified method,” which calculates the expected term as the midpoint between the weighted average time to vesting and the contractual maturity, we have limited historical information to develop reasonable expectations about future exercise patterns. The simplified method is based on the average of the vesting tranches and the contractual life of each classificationgrant. For stock price volatility, we use comparable public companies as a percentbasis for its expected volatility to calculate the fair value of total revenuesaward. The risk-free interest rate is based on U.S. Treasury notes with a term approximating the expected life of the award. The estimation of the number of awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from the Company’s current estimates, such amounts are recognized as follows:
| December 31, | ||||
| 2016 | 2015 | |||
| Medicare | 27% | 13% | ||
| Medicaid | 13% | 12% | ||
| Private pay/private insurance | 47% | 63% | ||
| Other | 13% | 12% | ||
| Total | 100% | 100% | ||
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 2 – Summary of $4,912 and $2,676, respectively.
Income tax
We use the liability method of accounting for income taxes pursuant toas set forth in ASC 740.740,”Income Taxes”. Under ASC 740,the liability method, deferred taxes are provided for usingdetermined based on the liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss carryforwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amountsfinancial statement and tax basis of assets and liabilities and theirusing tax bases. Deferred tax assets are reduced byrates expected to be in effect during the years in which the basis differences reverse. We record a valuation allowance when init is not more likely than not that the opiniondeferred tax assets will be realized.
Company management assesses its income tax positions and records tax benefits for all years subject to examination based upon its evaluation of management, itthe facts, circumstances and information available at the reporting date. In accordance with ASC 740-10, for those tax positions where there is a greater than 50% likelihood that a tax benefit will be sustained, our policy is to record the largest amount of tax benefit that is more likely than not to be realized upon ultimate settlement with a taxing authority that some portion orhas full knowledge of all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment.
For those income tax purposes, and thereforepositions where there is less than 50% likelihood that a pass-through entity payingtax benefit will be sustained, no income tax atbenefit will be recognized in the corporate level. Thefinancial statements. Company hadmanagement has determined that there are no material loss carryforwards as of December 31, 2011. Included in the Company's accumulated deficit from February 2012 forward is approximately $99,000 in undistributed S-Corporation losses. At December 31, 2016 and 2015 the Company had net operating loss carryforwards (NOL's) of approximately $70,000 and $21,000 respectively, which may be applied against future taxable income and which expire beginning in 2034. However, if certain substantial changes in the Company's ownership should occur, there could be an annual limitation on the amount of net operating loss carryforwards that can be utilized. The amount of and ultimate realization of the benefits from the operating loss carryforwards for incomeuncertain tax purposes is dependent, in part, upon the tax laws in effect, the future earnings of the Company, and other future events, the effects of which cannot be determined. Because of the uncertainty surrounding the realization of the loss carryforwards, the Company has established a valuation allowance equal to the tax effect (35% - 30% federal and 5% state) of the loss carryforwards of approximately $24,500 and $7,350positions at December 31, 20162023 and 2015, respectively, and therefore, no deferred tax asset has been recognized for the loss carryforwards. The change in valuation allowance is approximately $17,150 and $6,650 for the periods ended December 31, 2016 and 2015, respectively. The tax effect of remaining NOL's and resulting deferred tax assets of $24,500 remain fully reserved by valuation allowance, due to continued uncertainty as to their utilization.
The net income (loss) per share is computed by dividing the net income (loss) by the weighted average number of shares of common outstanding. Warrants, stock options, and common stock issuable upon the conversion of the Company'sCompany’s convertible debt or preferred stock (if any), are not included in the computation if the effect would be anti-dilutive.
Weighted average number of shares outstanding excludes anti-dilutive common stock equivalents, including warrants to purchase shares of common stock and would increasewarrants granted by our Board that have not been exercised totaling .
Advertising
We conduct advertising for the earningspromotion of our products. In accordance with ASC 720-35, advertising costs are charged to operations when incurred. We recorded advertising expense of $1,721,547 and $732,618 for the years ended December 30, 2023 and 2022, respectively.
Goodwill and other intangibles
Goodwill represents the excess of acquisition cost over the fair value of the net assets acquired and is not subject to amortization. The Company reviews goodwill annually in the fourth quarter for impairment or decrease loss per share.
The gross amounts and accumulated amortization of the Company’s acquired identifiable intangible assets with finite useful lives, included in other intangible assets, net in the accompanying consolidated balance sheets, were as follows:
| Schedule of identifiable intangible assets | ||||||||||||
| December 31, 2023 | ||||||||||||
| Gross Amount | Accumulated Amortization | Amortization Period | ||||||||||
| Finite: | (in years) | |||||||||||
| Brands | $ | 4,459,000 | $ | 891,803 | 15 | |||||||
| Customer Relationships | 957,000 | 191,400 | 15 | |||||||||
| License | 360,000 | 233,488 | 11 | |||||||||
| Total Intangible Assets | $ | 5,776,000 | $ | 1,316,691 | ||||||||
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 2 – Summary of Significant Accounting Policies, continued
At the time of acquisition, the Company estimates the fair value of the acquired identifiable intangible assets based upon the facts and circumstances related to the particular intangible asset. Inherent in such estimates are judgments and estimates of future revenue, profitability, cash flows and appropriate discount rates for any present value calculations. The Company preliminarily estimates the value of the acquired identifiable intangible assets and then finalizes the estimated fair values during the twelvepurchase allocation period, which does not extend beyond 12 months ended December 31, 2016from the date of acquisition. The Company’s amortization expense for acquired identifiable intangible assets with finite useful lives was $392,068 for fiscal years 2023 and 2022. Estimated amortization expense for acquired identifiable intangible assets for fiscal year 2024 and the succeeding years is as follows:
| Schedule of future intangible asset amortization expense useful lives | |||||
| Future Intangible Asset Amortization Expense | |||||
| Fiscal Year: | |||||
| 2024 | $ | 392,068 | |||
| 2025 | 392,068 | ||||
| 2026 | 392,068 | ||||
| 2027 | 392,068 | ||||
| 2028 | 363,580 | ||||
| Thereafter | 2,527,457 | ||||
| Total | $ | 4,459,309 | |||
Long-lived assets
The Company evaluates long-lived assets for impairment on an annual basis, when relocating or 2015.
Foreign Currency Gain/Losses
Foreign subsidiaries’ functional currency is the local currency of operations and the net assets of foreign operations are translated into U.S. dollars using current exchange rates. Gain or losses from these translation adjustments are included in the accompanying balance sheets, approximates fair value.
Recent Accounting Pronouncements
Adoption of FASB ASU 2020-06
In August 2020, the Financial Accounting Standards Board (FASB) issued ASU No. 2020-06, “Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity.” ASU 2020-06 simplifies the accounting for convertible instruments and contracts by removing certain models that were previously required to be applied. The amendments are effective for the fiscal years beginning after December 15, 2023, with early adoption permitted. The Company is currently evaluating the impact this update will have on its consolidated financial Statements.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 3 – Liquidity, Capital Resources and Going Concern Considerations
During 2023, the Company received 40% and 25%, respectively,$6.6 million from the issuance of its net revenues from Medicare and Medicaid. debt. This event served to mitigate the conditions that previously raised substantial doubt about the Company’s ability to continue as a going concern.
The Company’s consolidated financial statements have been prepared on the basis of US GAAP for a going concern, on the premise that the Company is able to obtain reimbursements through competitive bidding processes,meet its obligations as they come due in the normal course of business. The Company sustained a net loss of approximately $.0 million and negative cash flows from operating activities of approximately $10.2 million for the year ended December 31, 2023. To date the Company has generated cash flows from issuances of equity and indebtedness.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As of March 29, 2024, the Company has incurred significant losses from operations and has experienced negative cash flows from operating activities. Additionally, the Company’s current liabilities exceed its current assets, and it has a working capital deficit.
Management’s plans in regard to these matters include actions to sustain the Company’s operations, such as seeking additional funding to meet its obligations and implement its business plan. However, there is no guaranteeassurance that the Company will be selected as a winning contract supplier under future bidding rounds.
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Rent | $ | 27,492 | $ | 27,492 | ||||
| Office expenses | 43,107 | 44,829 | ||||||
| Other SG&A | 65,459 | 64,536 | ||||||
| Total | $ | 136,058 | $ | 136,857 | ||||
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| Office equipment | $ | 2,934 | $ | 2,934 | ||||
| Vehicles | 58,577 | 42,282 | ||||||
| Wheelchair/hospital bed rental pool | 76,876 | 39,308 | ||||||
| Total property and equipment | 138,387 | 84,524 | ||||||
| Accumulated depreciation | (76,197 | ) | (40,771 | ) | ||||
| Net property and equipment | $ | 62,190 | $ | 43,753 | ||||
| December 31, | ||||||||
| 2016 | 2015 | |||||||
| 3.53% installment note payable $352 monthly, including interest, through July 2019, collateralized by vehicle with carrying value of $7,503. | $ | 10,426 | $ | 14,213 | ||||
| 2.99% installment note payable $350 monthly, including interest, through August 2019, collateralized by vehicle with carrying value of $9,723 | 10,745 | 14,559 | ||||||
| 3.79% installment note payable $299 monthly, including interest, through July 2021, collateralized by vehicle with carrying value of $14,665 | 15,052 | 0 | ||||||
| 36,223 | 28,772 | |||||||
| Less principal due within one year | (10,918 | ) | (7,603 | ) | ||||
TOTAL LONG-TERM DEBT | $ | 25,305 | $ | 21,169 | ||||
| Principal payments due on long-term debt subsequent to December 31, 2016, are as follows: | ||||
| 2017 | $ | 10,918 | ||
| 2018 | 11,296 | |||
| 2019 | 8,511 | |||
| 2020 | 3,434 | |||
| 2021 | 2,064 | |||
| TOTAL | $ | 36,223 | ||
The Company may raise additional capital throughfinancial statements do not include any adjustments that might result from the saleoutcome of its equity securities, through an offering of debt securities, or through borrowings from financial institutions or related parties. By doing so,this uncertainty. If the Company hopes to generate sufficient capital to execute its business plan of selling medical supplies on an ongoing basis. Management believes that actions presently being taken to obtain additional funding provide the opportunity for the Companyis unable to continue as a going concern.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 4 – Notes Payable, Related Party Notes Payable, and Revenue Financing Arrangements
Notes payable are generally nonrecourse and secured by all Company owned assets.
| Schedule of notes payable | ||||||||||||
| Interest Rate | December 31, 2023 | December 31, 2022 | ||||||||||
| Notes Payable | ||||||||||||
| In March 2014, the Company entered into a short-term loan agreement with an entity in the amount of $200,000. The note included warrants for 272,584 shares of common stock at $ per share. The warrants expired unexercised on February 28, 2017. The loan and interest was paid off in February 2023 | 8 | % | — | 200,000 | ||||||||
| In December 2020, the Company entered into a 56- month loan with a company in the amount of $1,578,237. The loan requires payments of 3.75% through November 2022 and 4.00% through September 2025 of the previous month’s revenue. Note is due September 2025. Note is guaranteed by a related party see note 6. | 17 | % | 371,693 | 1,044,445 | ||||||||
| In April 2021, the Company entered into various six-month loans with individuals totaling in the amount of $168,000. The loans had an original maturity of October 2021 with principal and interest due at maturity with conversion price of $3.30 per share. The loans were extended to March 31, 2024. | 7 | % | 168,000 | 168,000 | ||||||||
| In May 2021, the Company entered into various six-month loans with individuals totaling in the amount of $60,000. The loans had an original maturity of October 2021 with principal and interest due at maturity with conversion price of $3.30 per share. The loans were extended to March 31, 2024. | 7 | % | 60,000 | 60,000 | ||||||||
| In August 2022, we entered into a 56-months auto loan in the amount of $45,420. | 2.35 | % | 32,996 | 42,396 | ||||||||
| In December 2022, the Company entered into various eighteen-month loans with individuals totaling in the amount of $4,000,000. The notes included 100% warrant coverage. The loans mature in June 2024 with principal and interest due at maturity with conversion price of $1.00 per share. | 12 | % | 4,000,000 | 4,000,000 | ||||||||
| In February 2023, the Company entered into a twelve-month loan with an entity in the amount of $2,000,000. The convertible note included the issuance of shares of common stock . The loan matures in February 2024 with conversion price of $0.85 per share and is non-interest bearing | — | % | 1,769,656 | — | ||||||||
| In May 2023, the Company entered into various eighteen-month loans with individuals totaling in the amount of $800,000. The notes included 50% warrant coverage. The loans mature in November 2024 with principal and interest due at maturity with conversion price of $1.00 per share. | 12 | % | 800,000 | — | ||||||||
| In June 2023, the Company entered into various eighteen-month loans with individuals totaling in the amount of $350,000. The notes included 50% warrant coverage. The loans mature in December 2024 with principal and interest due at maturity with conversion price of $1.00 per share. | 12 | % | 350,000 | — | ||||||||
| In July 2023, the Company entered into a twelve-month loan with an individual in the amount of $750,000. The note included 50% warrant coverage. The loan matures in July 2024 with principal and interest due at maturity with conversion price of $1.00 per share. | 12 | % | 750,000 | — | ||||||||
| In July 2023, the Company entered into a twelve-month loan with an individual in the amount of $100,000. The note included 50% warrant coverage. The loan matures in January 2025 with principal and interest due at maturity with conversion price of $1.00 per share. | 12 | % | 100,000 | — | ||||||||
| In August 2023, the Company entered into a twelve-month loan with an individual in the amount of $300,000. The convertible note included the issuance of shares of common stocks. The loan matures in August 2024 with principal and interest due at maturity with conversion price of $0.85 per share and is non-interest bearing. | — | 300,000 | — | |||||||||
| In October 2023, the Company entered into a three-month loan with an individual in the amount of $500,000. The loan matures in January 2024 with principal and interest due at maturity. The loan was extended to March 2024. | 10 | % | 500,000 | — | ||||||||
| In October 2023, the Company entered into a loan with an individual in the amount of $196,725 The loan matures in March 2024. Note is guaranteed by a related party. | — | 91,785 | — | |||||||||
| In October 2023, the Company entered into a loan with an individual in the amount of $130,000. The loan requires payment of 17% of daily Shopify sales. | — | 88,431 | — | |||||||||
| In October 2023, the Company entered into a eighteen-month loan with individuals totaling in the amount of $1,250,000. The note included 100% warrant coverage. The loan matures in April 2025 with principal and interest due at maturity with conversion price of $1.00 per share | 12 | % | 1,250,000 | — | ||||||||
| In December 2023, we entered into a 2.5-month loan with an individual in the amount of $450,000. The loan had a maturity of March 2024 with principal and interest due at maturity. | 10 | % | 450,000 | — | ||||||||
| Total notes payable | $ | 11,082,561 | $ | 5,514,841 | ||||||||
| Less notes discount | (2,876,387 | ) | (1,898,265 | ) | ||||||||
| Less current portion | (7,748,518 | ) | (1,080,257 | ) | ||||||||
| Long-term notes payable | $ | 457,656 | $ | 2,536,319 | ||||||||
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 4 – Notes Payable, Shareholder Notes Payable, and Revenue Financing Arrangements, continued
Interest expense on notes payable was $1,836,377 and $246,090 for the years ended December 31, 2023 and 2022, respectively. Accrued interest was $1,714,646 and $141,591 at December 31, 2023 and December 31, 2022, respectively. The Company’s effective interest rate was 60.17% for the year ended December 31, 2023.
As of December 31, 2023, the Company’s convertible note balances are convertible into shares of common stock.
Notes discount of $2,876,387 and $1,898,265 for the year ending December 31, 2023 and 2022 respectively is related to the discounted warrants and common shares issued in connection with the notes.
| Schedule of notes payable | ||||||||||
| Interest Rate | December 31, 2023 | December 31, 2022 | ||||||||
| Shareholder Notes Payable | ||||||||||
| In February 2023, we entered into a loan with an individual in the amount of $200,000. The annual interest rate is 12% | 12% | 200,000 | — | |||||||
| Less current portion | (200,000 | ) | — | |||||||
| Long-term notes payable | $ | — | $ | — | ||||||
Interest expense on related party notes payable was $20,400 and $5,407 for the years ended December 31, 2023 and 2022, respectively.
Note 5 – Licensing Agreement and Royalty Payable
We have a licensing agreement with ABG TapouT, LLC (“TapouT”), providing us with licensing rights to the brand “TapouT” on (i) energy drinks, (ii) energy bars, (iii) coconut water, (iv) electrolyte gum/chews, (v) energy shakes, (vi) powdered drink mix, (viii) water (including enhanced water), (vii) energy shots, (viii) teas, and (ix) sports drinks sold in the North America (including US Territories and Military Bases), United Kingdom, Brazil, South Africa, Australia, Scandinavia, Peru, Colombia, Chile and Guatemala. Under the terms of the agreement, we are required to pay a 6% royalty on net sales, as defined. In January 2017,2023 and 2022, we are required to make monthly payments of $55,000 and $54,450, respectively.
There were no unpaid royalties at December 31, 2023 and 2022. We paid the guaranteed minimum royalty payments of $660,000 and $653,400 for the years ended December 31, 2023 and 2022, which is included in general and administrative expenses.
In connection with the Copa Asset Purchase Agreement, we acquired the license to certain patents from 1/4 Vin SARL (“1/4 Vin”) On February 16, 2018, the Copa DI Vino® entered into three separate license agreements with 1/4 Vin SARL, (1/4 Vin). 1/4 Vin has the right to license certain patents and patent applications relating to inventions, systems, and methods used in the Company’s manufacturing process. In exchange for notes payable, 1/4 Vin granted the Company sold 350,000a nonexclusive, royalty-bearing, non-assignable, nontransferable, terminable license which would continue until the subject equipment is no longer in service or the patents expire. Amortization is approximately $31,000 annually until the license agreement is fully amortized. The asset is being amortized over a 10-year useful life.
Note 6 – Stockholders’ Equity
Common Stock
During the twelve-months ended December 31, 2022, we issued shares of common stock as part of the public offerings, shares in exchange for services, shares in connection with the purchase of Copa DI Vino®, shares on conversion of convertible instruments, and shares for cash.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 6 – Stockholders’ Equity, continued
Private Placement Memorandum (PPM)
In July 2022, we issued shares of common stock of the Company, at a purchase price of $ per share. In December 2022, we issued shares of common stock of the Company, at a purchase price of $ per share this placement included 100% warrant coverage.
In December 2022, we issued Convertible Notes for shares at $ per share with warrants to purchase 4,000,000 shares of common stock at $ per share.
Stock Plans
A summary of the Company’s stock option plan and changes during the year ended is as follows:
| Schedule of stock option plan | ||||||||||||
| Plan Category | No. of Shares to be Issued Upon Exercise or Vesting of Outstanding Stock Options | Weighted Average Exercise Price of Outstanding Stock Options | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities | |||||||||
| Equity compensation plan approved by board of directors | 4,259,008 | 1.13 | 2,846,068 | |||||||||
| Total | 4,259,008 | 1.13 | 2,846,068 | |||||||||
In August 2020, the Board adopted the 2020 Stock Incentive Plan (the “2020 Plan”), which provides for the grant of Options, Restricted Stock Awards, Stock Appreciation Rights, Performance Units and Performance Bonuses to consultants and eligible recipients.
The 2020 Plan has an “evergreen” feature, which provides for the annual increase in the number of shares issuable under the plan by an amount equal to 5% of the number of issued and outstanding common shares at year end, unless otherwise adjusted by the Board of Directors. At January 1, 2023 and 2022, the number of shares issuable under the 2020 plan increased by and shares, respectively.
In October 2023, the shareholders voted to increase the number of shares issuable under the Plan to 7.5%.
At December 31, 2023 the number of shares authorized under the 2020 plan is .
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 6 – Stockholders’ Equity, continued
The following is a summary of the Company’s stock option activity:
| Schedule of stock option activity | |||||||||||||||||
| Options | December 31, 2023 | December 31, 2022 | |||||||||||||||
| Number of Options | Weighted Average Exercise Price | Number of Options | Weighted Average Exercise Price | ||||||||||||||
| Balance - January 1, 2023* | 1,151,000 | $ | 1.12 | 1,065,000 | 2.60 | ||||||||||||
| Granted | 3,441,008 | 1.13 | 146,000 | $ | 2.31 | ||||||||||||
| Exercises | — | — | — | — | |||||||||||||
| Cancelled | 333,000 | 1.18 | 60,000 | 2.60 | |||||||||||||
| Balance – December 31, 2023 | 4,259,008 | $ | 1.13 | 1,151,000 | $ | 2.56 | |||||||||||
| Exercisable – December 31, 2023 | 3,910,787 | $ | 1.12 | 732,746 | $ | 2.58 | |||||||||||
| * | These prices are reflective of the price modification made on April 24, 2023. |
In May 2022, we granted options to purchase common stock to employees and consultants, these options vest between one and four years and were valued at $ on the grant date. ..
In the three months ending June 30, 2023, the Company granted options to employees and directors at weighted average strike price of $, weighted average expected life of years, weighted average volatility of %, weighted average risk-free rate of % and no dividend. On April 24, 2023, the Company modified the price of options to $ from a weighted average price of $. The options have a weighted average expected life of years, weighted average volatility of %, weighted average risk-free rate of % and no dividend. Following ASC Topic 718 the Company recognized an incremental expense from the modification of the option pricing resulting in an expense of $7,348 that was reflected in the quarter.
The Company determined the grant date fair value of the options granted using the Black Scholes Method using the following assumptions:
| Schedule of stock option assumption | ||||||||
| December 31, 2023 | December 31, 2022 | |||||||
| Risk-free interest rates | % | % | ||||||
| Exercise price | $ | 1.08 – 1.36 | $ | 2.60 | ||||
| Expected life | years | years | ||||||
| Expected volatility | % | % | ||||||
| Expected dividends | ||||||||
During the year ended December 31, 2023, the fair value of options granted amounted to $. As of December 31, 2023, the intrinsic value of stock options outstanding and exercisable was $. Stock compensation expense for the years ended December 31, 2023 and 2022 was $ and $, respectively.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 6 – Stockholders’ Equity, continued
At December 31, 2023, there was approximately $300,000 of unrecognized compensation costs related to stock options which will be recognized over the weighted average remaining years of .
The following is a summary of the Company’s Warrant activity
| Schedule of warrant activity | ||||||||||||||||
| Warrants | December 31, 2023 | December 31, 2022 | ||||||||||||||
| Number of Warrants | Weighted Average Exercise Price | Number of Warrants | Weighted Average Exercise Price | |||||||||||||
| Balance – beginning of the year | 14,343,896 | $ | 1.85 | 10,143,896 | $ | 2.51 | ||||||||||
| Granted | 2,250,000 | 0.58 | 4,200,000 | 0.25 | ||||||||||||
| Exercises | 68,146 | 2.19 | — | — | ||||||||||||
| Cancelled | 2,345,677 | 2.32 | — | — | ||||||||||||
| Balance - end of the year | 14,180,073 | $ | 1.56 | 14,343,896 | $ | 1.85 | ||||||||||
The fair value of warrants recognized in the period has been estimated using the Black-Scholes option pricing model with the following assumptions.
| Schedule of assumptions used in Black-Scholes option pricing model | ||||||||
| December 31, 2023 | December 31, 2022 | |||||||
| Risk-free interest rates | % | % | ||||||
| Exercise price | $ | 0.55 | $ | 0.96 | ||||
| Expected life | years | years | ||||||
| Expected volatility | % | % | ||||||
| Expected dividends | ||||||||
Note 7 – Related Parties
During the normal course of business, we incurred expenses related to services provided by our CEO or Company expenses paid by our CEO, resulting in related party payables. In conjunction with the acquisition of Copa DI Vino®, the Company also entered into a Revenue Loan and Security Agreement (the “Loan and Security Agreement”) by and among the Company, Robert Nistico, additional Guarantor and each of the subsidiary guarantors from time-to-time party thereto (each a “Guarantor”, and, collectively, the “Guarantors”), and Decathlon Alpha IV, L.P. (the “Lender”). The Loan and Security Agreement provided for a revenue-based credit facility of $1,578,237 (the “Gross Amount”) with the Lender (the “Credit Facility”). There was $371,693 outstanding and $989,702 accrued interest under this agreement as of December 31, 2023. Additionally, the Company is subject to $757,554 of penalties associated with this agreement as of December 31, 2023. The lender has agreed to waive the penalties in the event the Company repays the loan obligation in full prior to maturity. The Company intends to pay off the obligation prior to maturity.
On September 29, 2023, the Company also entered into a Purchase and Sales Future Receivables Agreement (the “Loan and Security Agreement”) by and among the Company, Robert Nistico, additional Guarantor and each of the subsidiary guarantors from time-to-time party thereto (each a “Guarantor”, and, collectively, the “Guarantors”), and Knightsbridge Funding LLC (the “Lender”). The Loan and Security Agreement provided a loan of $165,000, with the gross and interest amount of $241,725 with the Lender (the “Credit Facility”). There was $99,185 outstanding under this agreement as of December 31, 2023.
There were related party advances from our chief executive officer in the amount of $0.4 million outstanding as of December 31, 2023 and a shareholder note payable outstanding in the amount of $ as of December 31, 2023.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 8 – Investment in Salt Tequila USA, LLC
The Company has a marketing and distribution agreement with SALT in Mexico for the manufacturing of our Tequila product line.
The Company has a 22.5% percentage interest in SALT Tequila USA, LLC (“SALT”), and has the right to increase its ownership to 37.5%. This investment is accounted for at cost.
Note 9 – Lease
We have various operating lease agreements primarily related to real estate and office space. Our real estate leases represent a majority of our lease liability. Our lease payments are mainly fixed. Any variable lease payments, including utilities and common area maintenance are expensed during the period incurred. Variable lease costs were immaterial for the years ended December 31, 2023 and 2022. A majority of our real estate leases include options to extend the lease. We review all options to extend at the inception of the lease and account for these options when they are reasonably certain of being exercised.
Operating lease expense is recognized on a straight-line basis over the lease term and is included in operating expense on our consolidated statement of operations. Operating lease cost was $363,890 and $315,980 during the years ended December 31, 2023 and 2022, respectively.
The following table sets for the maturities of our operating lease liabilities and reconciles the respective undiscounted payments to the operating lease liabilities in the consolidated balance sheet at December 31, 2023:
| Schedule of operating lease liabilities | ||||
| Undiscounted Future Minimum Lease Payments | Operating Lease | |||
| 2024 | $ | 286,168 | ||
| 2025 | 287,193 | |||
| 2026 | 17,857 | |||
| Total | 591,218 | |||
| Amount representing imputed interest | (32,230 | ) | ||
| Total operating lease liability | 558,988 | |||
| Current portion of operating lease liability | (262,860 | ) | ||
| Operating lease liability, non-current | $ | 296,128 | ||
The table below presents information for lease costs related to our operating leases at December 31, 2023:
| Schedule of lease costs | ||||
| Operating lease cost: | ||||
| Amortization of leased assets | $ | 330,728 | ||
| Interest of lease liabilities | 33,162 | |||
| Total operating lease cost | $ | 363,890 |
The table below presents lease- related terms and discount rates at December 31, 2023:
| Schedule of lease- related terms and discount rates | ||||
| Remaining term on leases | 30 months | |||
| Incremented borrowing rate | 5.0 | % |
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 10 – Segment Reporting
We have two reportable operating segments: (1) the manufacture and distribution of non-alcoholic and alcoholic beverages, and (2) the retail sale of beverages and groceries online. These operating segments are managed separately and each segment’s major customers have different characteristics. Segment Reporting is evaluated by our chief operating decision maker, which continues to be our chief executive officer.
| Schedule of segment reporting information | ||||||||
| Revenue | For the Year Ended, December 31, 2023 | For the Year Ended, December 31, 2022 | ||||||
| Splash Beverage Group | $ | 5,072,479 | $ | 4,759,586 | ||||
| E-Commerce | 13,777,673 | 13,327,900 | ||||||
| Total Revenues continuing operations | $ | 18,850,152 | $ | 18,087,486 | ||||
| Total Revenues discontinuing operations | $ | — | $ | 385,174 | ||||
| Contribution after Marketing expenses | 2023 | 2022 | ||||||
| Splash Beverage Group | $ | (1,749,163 | ) | $ | (2,202,790 | ) | ||
| E-Commerce | 4,824,338 | 5,314,767 | ||||||
| Total Contribution after Marketing expenses continuing operations | 3,075,175 | 3,111,977 | ||||||
| Contracted services | 1,402,572 | 1,505,788 | ||||||
| Salary and wages | 5,003,392 | 4,179,403 | ||||||
| Non-cash share-based compensation | 1,169,858 | 7,409,884 | ||||||
| Other general and administrative | 10,786,011 | 11,411,535 | ||||||
| Loss from continuing operations | $ | (15,286,658 | ) | $ | (21,394,633 | ) | ||
| Total Assets | December 31, 2023 | December 31, 2022 | ||||||
| Splash Beverage Group | $ | 9,188,213 | $ | 14,723,553 | ||||
| E-Commerce | 710,555 | 2,581,150 | ||||||
| Total Assets | $ | 9,898,768 | $ | 17,304,703 | ||||
Splash Beverage Group revenue increased for the year ending December 31, 2023 versus December 31, 2022 by $0.3 million or 7% with the main contribution from the increase in revenue coming from TapouT and Pulpoloco. The contribution after marketing expenses increased by $0.04 million for the year ending December 31, 2023 versus December 31, 2022 due to increased sales partially offset by cost increases.
E-Commerce revenue increased for the year ending December 31, 2023 versus December 31, 2022 by $0.4 million driven by expanded territory coverage, new products being sold and increased cart size when customers checking out. Contribution after Marketing expenses declined by $0.4 million due to increase by cost.
Note 11 – Commitment and Contingencies
We are a party to asserted claims and are subject to regulatory actions in the ordinary course of business. The results of such proceedings cannot be predicted with certainty, but we do not anticipate that the outcome, if any, arising out of any such matter will have a material adverse effect on its business, financial condition or results of operations.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 12 – Registration Statement
Underwriting Agreement
On June 10, 2021, we entered into an underwriting agreement (“Underwriting Agreement”) relating to an unaffiliated investor at $.10/underwritten public offering (the “Offering”) of common stock, (the “Common Stock”) and warrants to purchase one share of Common Stock (the “Warrants”). Pursuant to the Offering, we sold 3,750,000 shares of Common Stock and 4,312,500 Warrants, which include 562,500 Warrants sold upon the partial exercise of the Underwriters’ over-allotment, for total gross proceeds of $35,000.
On February 17, 2022, we entered into an underwriting agreement (“Underwriting Agreement”) relating to an underwritten public offering (the “Offering”) of common stock, (the “Common Stock”) to purchase one share of Common Stock. Pursuant to the Offering, we sold 2,300,000 shares of Common Stock for total gross proceeds of approximately $9.2 million. After deducting the underwriting commissions, discounts, and offering expenses payable by we, we received net proceeds of approximately $7.9 million.
On September 22, 2022, we entered into an underwriting agreement (“Underwriting Agreement”) relating to an underwritten public offering (the “Offering”) of common stock, (the “Common Stock”) to purchase one share of Common Stock. Pursuant to the Offering, we sold shares of Common Stock for total gross proceeds of approximately $3.6 million. After deducting the underwriting commissions, discounts, and offering expenses, we received net proceeds of approximately $3.1 million.
Representative’s Warrants
On June 15, 2021, pursuant to the Underwriting Agreement, the Company issued Representative’s Warrants to purchase up to an aggregate of 150,000 shares of Common Stock. The Representative’s Warrants may be exercised beginning on December 10, 2021 until June 10, 2026. The initial exercise price of each Representative Warrant is $4.60 per share, which represents 115% of the Offering Price.
Note 13 – Tax Provision
The Company has evaluated subsequent events through the date these financial statements werepositive and negative evidence in assessing the realizability of its deferred tax assets. This assessment included the evaluation of scheduled reversals of deferred tax liabilities, estimates of projected future taxable income and tax planning strategies to determine which deferred tax assets are more likely than not to be realized in the future. Due to uncertainty about the Company’s ability to utilize its deferred tax assets, the Company has recorded a full valuation allowance against its deferred tax assets.
At December 31, 2023, the Company’s net operating loss carryforward for Federal income tax purposes was $108,922,763, which will be available to offset future taxable income. If not used, these carry forwards will begin to expire in 2032, except for the net operating losses generated January 1, 2018 and after, which amounted to $90,921,071, which can be issuedcarried forward indefinitely.
There was no income tax expense or benefit for the years ended December 31, 2023 and determined that there are no other reportable subsequent events.
Splash Beverage Group, Inc.
Notes to the Consolidated Financial Statements
Note 13 – Tax Provision, continued
The reconciliation of the income tax benefit is computed at the U.S. federal statutory rate as follows:
| Schedule of effective income tax rate reconciliation | ||||||||
| 2023 | 2022 | |||||||
| Federal Statutory Tax Rate | 21.00 | % | 21.00 | % | ||||
| Permanent Differences | (0.89 | )% | (3.80 | )% | ||||
| Change in Valuation Allowance | (20.11 | )% | (17.20 | )% | ||||
| Net deferred tax asset | — | — | ||||||
The tax effects of temporary differences which give rise to the significant portions of deferred tax assets or liabilities at December 31 are as follows:
| Schedule of deferred tax assets or liabilities | ||||||||
| 2023 | 2022 | |||||||
| Deferred Tax Assets: | ||||||||
| Net Operating Losses | $ | 27,606,474 | $ | 22,758,336 | ||||
| Deferred Rent | — | 380 | ||||||
| Accrued Interest/Interest Expense Limitation | 1,518,618 | 1,263,639 | ||||||
| Total deferred tax assets | 29,125,092 | 24,022,355 | ||||||
| Deferred Tax Liabilities: | ||||||||
| Depreciation | (120,502 | ) | (93,476 | ) | ||||
| Total deferred tax liabilities | (120,502 | ) | (93,476 | ) | ||||
| Less: Valuation allowance | (29,004,590 | ) | (23,928,879 | ) | ||||
| Total Net Deferred Tax Assets | $ | — | $ | — | ||||
The Company continually evaluates expiring statutes of limitations, audits, proposed settlements, changes in tax law and new authoritative rulings. The open tax years subject to examination with respect to the Company’s operations are 2015 through 2023.
Note 14 – Subsequent Events
In January 2024, the Company entered into a convertible note with an individual in the amount of $250,000. The note has an eighteen-month 18 term, accrues interest at 12% and is convertible into shares of common stock of the Company at $0.50 per share, which also includes 200% warrants at $0.25
In January 2024, the Company entered into a commercial loan in the amount of $500,000. The total cost of the loan is $250,000 and is paid in weekly increments of 6.97% of the current receivable balance.
In February 2024, the Company entered into a convertible note with an individual in the amount of $150,000. The note has an eighteen-month 18 term, accrues interest at 12% and is convertible into shares of common stock of the Company at $0.40 per share, which also includes 250% warrants at $0.25.
In March 2024, the Company received a $ cash advance from our chief executive officer, resulting in a related party payable. This note bears 0% interest.
We have notes that expire in 2024 that we plan to extend or payoff.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
(1) Evaluation of Disclosure Controls and Procedures.
We have adopted and maintain disclosure controls and procedures (as such term is defined in Exchange Act Rules 13a-15(e) and 15d-15(e) under the Exchange Act), that are designed to ensure that information required to be disclosed in our reports under the Exchange Act, is recorded, processed, summarized and reported within the time periods required under the SEC’s rules and forms and that the information is gathered and communicated to our management, including our Chief Executive Officer (Principal Executive Officer) and Chief Financial Officer (Principal Financial Officer), to allow for timely decisions regarding required disclosure.
As required by Exchange Act Rule 13a-15, our Chief Executive Officer and PrincipalChief Financial Officer have evaluatedcarried out an evaluation of the effectiveness of the design and operationsoperation of our disclosure controls and procedures pursuant to Exchange Act Rule 13a-15 as of the end of the period covered by this quarterly report,report. Based on the foregoing evaluation, our Chief Executive Officer and haveChief Financial Officer concluded that due to our limited resources our disclosure controls and procedures are adequate.
(2) Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting for our company. Our internal control system was designed to, in general, provide reasonable assurance to our management and board regarding the preparation and fair presentation of published financial statements, but because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2023. Based on that assessment, our management has determined that as of December 31, 2023, our internal control over financial reporting was not effective due to material weaknesses related to a limited segregation of duties due to our limited resources and the small number of employees. Management has determined that this control deficiency constitutes a material weakness which could result in material misstatements of significant accounts and disclosures that could result in a material misstatement to our interim or annual financial statements that would not be prevented or detected. In addition, due to limited staffing, we are not always able to detect minor errors or omissions in reporting.
This Annual Report does not include an attestation report of our independent registered public accounting firm regarding management’s assessment of our internal control over financial reporting pursuant to temporary rules of the SEC.
(3) Changes in Internal Control over Financial Reporting.
There has been no change in our internal control over financial reporting (as definedother than items highlighted above, identified in connection with the evaluation required by paragraph (d) of Rules 13a-15(f) and 15d-15(f)13a-15 or 15d-15 under the Securities Exchange Act)Act of 1934 that occurred during the period covered by this reportour most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The following table sets forth our directors is elected by the stockholders to a term of one year and serves until his successor is elected and qualified. Each of our officers is elected by the board of directors to a term of one year and serves until his or her successor is duly elected and qualified, or until he or she is removed from office. The board of directors has no committees.
| Name | Age | |||
| Chief Financial Officer | ||||
| William Meissner | 57 | President, Chief Marketing Officer | ||
| Justin Yorke | 57 | Director | ||
| John Paglia | 56 | Director | ||
| Bill Caple | 65 | Director |
Directors are expected toelected annually and hold said offices/positionsoffice until the next annual meeting of our stockholders. These officersthe stockholders of the Company and directorsuntil their successors are our only officers, directors, promoterselected. Officers are elected annually by the Board of Directors (the “Board”) and control persons.
Robert Nistico, age 60, on March 31, 2020 became the Chief Executive Officer and Directors
Stacy McLaughlin, age 42, became the Chief Financial Officer on January 24, 2024. Prior to serving as our Chief Financial Officer, Ms. McLaughlin was the Chief Financial Officer of Material Technologies, Corp. from 2022 to 2023. From 2013 to 2021, Ms. McLaughlin was the Vice President and Chief Financial Officer of Willdan Group, Inc. (Willdan), and prior to that, she was their Compliance Manager from 2010 to 2013. During her tenure at Willdan, she was responsible for accounting and finance functions, SEC reporting, investor relations, treasury, and managed a follow-on equity offering. Prior to Willdan, Ms. McLaughlin was, from 2009 to 2010, Senior Associate at Windes & McClaughry Accountancy Corporation and, from 2004 when heto 2009, Senior Audit Associate at the public accounting firm KPMG LLP. Ms. McLaughlin has a Masters in Accounting from the University of Southern California and BS from the University of Arizona. Ms. McLaughlin is a Certified Public Accountant (CPA).
William Meissner, age 57, became the President and sole Director. He also founded Medical Billing Assistance, Inc. ("Medical Billing")Chief Marketing Officer of the Company in 1994. Medical Billing was involved in electronic billingMay of medical claims to Medicare. Medical Billing completed an acquisition of FCID Medical, Inc. in December 2010 and2020. Mr. West resigned from all positionsMeissner is a proven leader with Medical Billing at that time. Mr. West received a Bachelor's of Arts Degree in Biology from Wittenberg University in 1977. He plans to continue devoting his full time to our affairs. We believe that Mr. Michael West's 24more than twenty years of experience serving as either our President or Vice President enables himsuccess in growing consumer brand companies with both large multinational and medium sized entrepreneurial organizations. Meissner has held several other leadership and board director roles. Prior to make valuable contributionsSplash Meissner was a board director and CEO in a beverage vertical organized by a mid-cap PE firm designed to our Board of Directors.
Justin Yorke, age 57, became a member of the Board of the Company on March 31, 2020. Since March 31, 2020, Mr. Yorke has also served as the Company’s Secretary. Mr. Yorke has over 25 years of experience in finance. Based in Hong Kong for over 10 years, he managed funds for a private Swiss Bank, Darier Henstch from 1997 to 2000. Prior to that, from 1995 to 1997, Mr. Yorke managed funds for Peregrine Investments and from 1990 to 1995 Unifund, Asia, Ltd, Hong Kong, a high net-worth family office headquartered Geneva, Switzerland. From 2000 to 2004, he was a partner at Asiatic Investment Management, based in San Francisco. Since 2004, Mr. Yorke has been a partner in San Gabriel Advisors, LLC and Arroyo Capital Management, LLC and is the manager of the San Gabriel Fund, JMW Fund and Richland Fund. The funds are highly diversified in focus with investment holdings, public, private equity and debt investments and real estate investments. He has a B.A. degree from UCLA. Mr. Yorke is the principal of WesBev LLC, which prior to the merger between CMS and our Company was the majority shareholder of the Company. He also is an acting director and audit committee chair of Processa Pharmaceuticals, (Nasdaq: PCSA). Mr. Yorke served as non-executive Chairman of Jed Oil and a Director/CEO at JMG Exploration.
Dr. Paglia, age 56, became a member of the Board of the Company as an independent director on February 26, 2024. He is currently an independent director, Audit Committee Chair and a member of the Nominating & Corporate Governance and Compensation Committee of Simulations Plus, Inc., from 2014 to present. Mr. Paglia is also an independent director, Audit Committee Chair and a member of the Nominating & Corporate Governance and Compensation Committee of Aeluma, Inc., from 2021 to present. Additionally, Dr. Paglia is currently on the Advisory Board of multiple companies, including SUM Ventures, Axxes Capital Inc., VitaNav Inc., and DigiLife Fund, among others. Dr. Paglia, a Professor of Finance, currently works at Pepperdine University in various positions, which have included Senior Associate Dean and Executive Director, since 2000-present. Dr. Paglia has a Doctor of Philosophy in Business Administration, from the University of Kentucky, a Master of Business Administration from Gannon University, a Bachelor of Science from Gannon University, and is also a Certified Public Accountant and Charted Financial Analyst.
Bill Caple, age 65, has served as an independent director of the Company since May 3, 2023. Over the past five years, Mr. Caple has primarily served as a consultant on corporate strategies, business development, corporate finance, and M&A. Mr. Caple is currently a board member of Covax Data, Inc. (“Covax”), where he also assists with establishing sales channels and business development for Covax’s cyber security AI blockchain product and assisting the company raise growth capital. Mr. Caple also founded and runs Caple Advisory, an international management consulting practice and investment banking firm, with a BBAconcentration in 1978. He plansAsia. Previously, Mr. Caple served as a board member and C-suite executive of multiple hi-tech businesses, netting successful exits and public offerings of his companies (e.g. OTG Software NASDAQ: OTGS, now part of Dell EMC and OpenText). The Company believes that Mr. Caple is an asset to devote approximately 5the Company because of his wealth of experience and success in corporate finance strategies, M&A, and business development to 10 hours per monthround out the Board’s top-tier level of expertise in key subjects.
Family Relationships
There are no family relationships among and between the issuer’s directors, officers, persons nominated or chosen by the issuer to become directors or officers, or beneficial owners of more than ten percent of any class of the issuer’s equity securities.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act requires that our directors and executive officers and persons who beneficially own more than 10% of our common stock (referred to herein as the “reporting persons”) file with the SEC various reports as to their ownership of and activities relating to our affairs. Wecommon stock. Such reporting persons are required by the SEC regulations to furnish us with copies of all Section 16(a) reports they file. Based solely on our review of copies of the reports filed with the SEC and the written representations of our directors and executive officers, we believe that Mr. Stephen West's 38 yearsall reporting requirements for fiscal year 2023 were complied with by each person who at any time during the 2023 fiscal year was a director or an executive officer or held more than 10% of salesour common stock, except for the following: Bill Caple, Fatima Dhalla (interim CFO at the time), and Stacy McLaughlin each filed a late Form 3 report at the time of their appointments and on becoming insiders of the Company; Ron Wall filed a late Form 4 report on January 31, 2023 related to the grant of options to purchase our common stock on May 2, 2022; Justin Yorke, Candance Crawford and Peter McDonough each filed a late Form 4 report on May 15, 2023 related to the grant of options to purchase our common stock on April 24, 2023; Bill Caple filed a late Form 4 report on May 19, 2023 related to the grant of options to purchase our common stock on May 1, 2023; and Ron Wall filed a late Form 4 report on August 3, 2023 related to the grant of options to purchase our common stock on May 2, 2023.
Committees of the Board of Directors
Audit Committee
We have separately designated an Audit Committee. The Audit Committee is responsible for, among other things, the appointment, compensation, removal and oversight of the work of the Company’s independent registered public accounting firm, overseeing the accounting and financial reporting process of the Company, and reviewing related person transactions. Our Audit Committee is comprised of John Paglia and Bill Caple. Under NYSE listing standards and applicable SEC rules, all the directors on the audit committee must be independent. Also, as a smaller reporting company, we are only required to maintain an audit committee of two independent directors. Our Board has determined that John Paglia and Bill Caple are independent under NYSE listing standards and applicable SEC rules. John Paglia is the Chairperson of the audit committee. Each member of the audit committee is financially literate and our Board has determined that John Paglia qualifies as an “audit committee financial expert” as defined in applicable SEC rules. The Audit Committee operates under a written charter adopted by the Board of Directors, which can be found on our website at www.splashbeveragegroup.com. During 2023, the Audit Committee held four meetings in person or through conference calls.
Compensation and Management Resources Committee
We have established a Compensation and Management Resources Committee of our Board of Directors. The purpose of the Compensation and Management Resources Committee is to assist the Board in discharging its responsibilities relating to executive experiencecompensation, succession planning for the Company’s executive team, and to review and make recommendations to the Board regarding employee benefit policies and programs, incentive compensation plans and equity-based plans.
The members of our Compensation and Management Resources Committee are Bill Caple, John Paglia and Justin Yorke. Bill Caple is the chairperson of the Compensation and Management Resources Committee.
Under NYSE listing standards, we are required to have at least two members of the compensation committee, all of whom must be independent directors. Our board of directors has determined that each of John Paglia and Bill Caple is independent under NYSE listing standards. The Compensation and Management Resources Committee is responsible for, among other things, (a) reviewing all compensation arrangements for the executive officers of the Company and (b) administering the Company’s stock option plans. The Compensation and Management Resource Committee operates under a written charter adopted by the Board of Directors, which can be found on our website at www.splashbeveragegroup.com within the “Investor Information” section.
The duties and responsibilities of the Compensation and Management Resources Committee in accordance with its charter are to review and discuss with management and the Board the objectives, philosophy, structure, cost and administration of the Company’s executive compensation and employee benefit policies and programs; no less than annually, review and approve, with respect to the Chief Executive Officer and the other executive officers (a) all elements of compensation, (b) incentive targets, (c) any employment agreements, severance agreements and change in control agreements or provisions, in each case as, when and if appropriate, and (d) any special or supplemental benefits; make recommendations to the Board with respect to the Company’s major long-term incentive plans applicable to directors, executives and/or non-executive employees of the Company and approve (a) individual annual or periodic equity-based awards for the Chief Executive Officer and other executive officers and (b) an annual pool of awards for other employees with guidelines for the administration and allocation of such awards; recommend to the Board for its approval a succession plan for the Chief Executive Officer, addressing the policies and principles for selecting a successor to the Chief Executive Officer, both in an emergency situation and in the technology industryordinary course of business; review programs created and his knowledgemaintained by management for the development and succession of other executive officers and any other individuals identified by management or the Compensation and Management Resources Committee; review the establishment, amendment and termination of employee benefits plans, review employee benefit plan operations and administration; and any other duties or responsibilities expressly delegated to the Compensation and Management Resources Committee by the Board from time to time relating to the Committee’s purpose.
The Compensation and Management Resources Committee may request any officer or employee of the Company or the Company’s outside counsel to attend a meeting of the Compensation and Management Resources Committee or to meet with any members of, or consultants to, the Compensation and Management Resources Committee. The Company’s Chief Executive Officer does not attend any portion of a meeting where the Chief Executive Officer’s performance or compensation is discussed, unless specifically invited by the Compensation and Management Resources Committee.
The Compensation and Management Resources Committee has the sole authority to retain and terminate any compensation consultant to be used to assist in the evaluation of director, Chief Executive Officer or other executive officer compensation or employee benefit plans and has sole authority to approve the consultant’s fees and other retention terms. The Compensation and Management Resources Committee also has the authority to obtain advice and assistance from internal or external legal, accounting or other experts, advisors and consultants to assist in carrying out its duties and responsibilities and has the authority to retain and approve the fees and other retention terms for any external experts, advisors or consultants.
During 2023, the Compensation Management Resources Committee held four meetings in person or through conference calls.
Nominating and Corporate Governance Committee
The Nominating and Corporate Governance Committee is responsible for overseeing the appropriate and effective governance of the Company, including, among other things, (a) nominations to the Board of Directors and making recommendations regarding the size and composition of the Board of Directors and (b) the development and recommendation of appropriate corporate governance principles. The Nominating and Corporate Governance Committee consists of John Paglia and Bill Caple, each of whom is an independent director (as defined under Section 803 of the NYSE American LLC Company Guide). The Chairperson of the committee is Bill Caple. The Nominating and Corporate Governance Committee operates under a written charter adopted by the Board of Directors, which can be found on our Company's history qualify himwebsite at www.splashbeveragegroup.com within the “Investor Information” section.
The Nominating and Corporate Governance Committee adheres to servethe Company’s bylaws provisions and Securities and Exchange Commission rules relating to proposals by stockholders when considering director candidates that might be recommended by stockholders, along with the requirements set forth in the committee’s Policy with Regard to Consideration of Candidates Recommended for Election to the Board of Directors, also available on our website. The Nominating and Corporate Governance Committee of the Board of Directors is responsible for identifying and selecting qualified candidates for election to the Board of Directors prior to each annual meeting of the Company’s stockholders. In identifying and evaluating nominees for director, the Committee considers each candidate’s qualities, experience, background and skills, as awell as other factors, such as the individual’s ethics, integrity and values which the candidate may bring to the Board of Directors.
During 2023, the Nominating and Corporate Governance Committee held two meetings in person or through conference calls.
Meetings of the Board of Directors same as above
During 2023, the Board of Directors held five meetings. During 2023, each member of our Board of Directors.
The Board of Directors also approved certain actions by unanimous written consent.
Director Independence
The NYSE listing standards require that a majority of our Board be independent. Our Board has determined that John Paglia and Bill Caple are “independent directors” as defined in the NYSE listing standards. Our independent directors will have regularly scheduled meetings at which only independent directors are present.
Involvement in Certain Legal Proceedings
Our Directors and Executive Officers have not been involved in any of the following events during the past ten years:
| 1. | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| 2. | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| 3. | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; |
| 4. | being found by a court of competent jurisdiction in a civil action, the Securities and Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| 5. | being subject of, or a party to, any federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| 6. | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
| 7. | Such person was the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of: |
i. Any federal or state securities or commodities law or regulation; or
ii. Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a reviewtemporary or permanent injunction, order of Forms 3disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
iii. Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
| 8. | Such person was the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Board leadership structure and 4role in risk oversight
The Board of Directors oversees our business and amendments thereto furnishedaffairs and monitors the performance of management. In accordance with corporate governance principles, the Board of Directors does not involve itself in day-to-day operations. The directors keep themselves informed through discussions with the Chief Executive Officer and other key executives, visits to the Company duringCompany’s facilities, by reading the fiscal year ended December 31, 2016,reports and certain written representations, no persons who were either a director, executive officerother materials that we send them and by participating in Board and committee meetings. Each director’s term will continue until the election and qualification of his or beneficial owner of more than 10% of the Company's Common Stock, failed to file on a timely basis reports requiredher successor, or his or her earlier death, resignation or removal. The information set forth in Item 1C is incorporated herein by Section 16(a) of the Exchange Act during the fiscal year ended December 31, 2016.
Code of Ethics
We have adopted a code of business conduct and ethics that applies to itsour directors, officers (including our Chief Executive Officer, Chief Financial Officer and any person performing similar functions) and employees. Our Code of Ethics is available at our website at www.splashbeveragegroup.com.
Clawback Policy
On September 20, 2023, the Board adopted the Splash Beverage Group Clawback Policy (the “Clawback Policy”), effective September 20, 2023, providing for the recovery of certain incentive-based compensation from current and former executive officers because it only has two executive officers who are also board members.
Item 11. Executive Compensation.
The following table sets forth information for our two most recently completed fiscal years ending December 31, 2023 and December 31, 2022 concerning all of the compensation awarded to, earned by or paid to the executive officers named below. No other employees earned a salary over $100,000 in the last two completed fiscal years.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards($) | Option Awards($) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings($) | All Other Compensation ($) | Total($) | ||||||||||||||||||||||||
| Michael West | 2016 | $ | 86,750 | - | - | - | - | - | - | $ | 86,750 | ||||||||||||||||||||||
| 2015 | $ | 67,750 | - | - | - | - | - | - | $ | 67,500 | |||||||||||||||||||||||
| Steve West | 2016 | $ | - | - | - | - | - | - | - | $ | - | ||||||||||||||||||||||
| 2015 | $ | - | - | - | - | - | - | - | $ | - | |||||||||||||||||||||||
| Name and Principal Position | Year | Salary | Bonus | Other | Stock Awards | Option Awards | Nonequity Incentive Plan Compensation | Nonqualified Deferred Compensation Earnings | Total | |||||||||||||||||||||||||||
| Robert Nistico, CEO | 2023 | 333,125 | — | 14,400 | — | — | — | — | 347,525 | |||||||||||||||||||||||||||
| 2022 | 325,000 | 100,000 | 14,400 | — | — | — | — | 439,000 | ||||||||||||||||||||||||||||
| William Meissner, President and CMO | 2023 | 333,125 | — | — | — | — | — | — | 333,125 | |||||||||||||||||||||||||||
| 2022 | 325,000 | 90,000 | — | — | 260,000 | — | — | 415,000 | ||||||||||||||||||||||||||||
| Ronald Wall, CFO(1) | 2023 | 249,438 | — | — | — | — | — | — | 249,438 | |||||||||||||||||||||||||||
| 2022 | 217,708 | 60,000 | 28,429 | — | 254,100 | — | — | 560,237 | ||||||||||||||||||||||||||||
| Fatima Dhalla, Interim CFO(2) | 2023 | 55,950 | — | — | — | — | — | — | 55,950 | |||||||||||||||||||||||||||
(1) On September 26, 2023, Ronald Wall resigned as Chief Financial Officer is not paid a salary. We doof the Company.
(2) Effective January 19, 2024, Fatima Dhalla, resigned as the Interim Chief Financial Officer of the Company.
Employment Agreements
Except as described below, the Company does not have any employment agreements in place with eitherany of its executive officers. The board of directors reserves the right to increase the salary of our executive officers.
Robert Nistico – CEO and Director
On March 12, 2012, the Company entered into an employment agreement with Robert Nistico, pursuant to which Mr. Nistico serves as Chief Executive Officer of the Company. Pursuant to Mr. Nistico’s employment agreement, the Company pays Mr. Nistico an annual salary of $275,000. Mr. Nistico is also eligible to receive an annual bonus of 50% of his annual salary, and was granted an option to purchase 350,000 shares of common stock. In the event Mr. Nistico terminates his employment with the Company he shall provide the Company a minimum of 45 days of written notice.
On December 9, 2019, the board of directors of the Company extended Mr. Nistico’s employment agreement beginning December 1, 2019, and ending on November 30, 2024. Pursuant to the amendment, the Company increased Mr. Nistico’s base salary from $275,000 to $325,000.
Stacy McLaughlin - CFO
Pursuant to the terms of an employment agreement dated January 22, 2024, the Company employs Ms. Stacy McLaughlin as its Chief Financial Officer on a full-time basis. Effective January 24, 2024, Ms. McLaughlin’s annual salary is $325,000. She is also entitled to an annual performance bonus of up to $162,500, upon achieving certain targets that are to be defined on an annual basis. Ms. McLaughlin is also entitled to participate in all qualified plans, holidays and other employee benefits which the Company, in its sole discretion, may maintain from time to time for the benefit of its employees in general. On March 5, 2024, pursuant to her employment agreement, Ms. McLaughlin was granted 600,000 restricted shares of Common Stock. These shares will vest in tranches of 50,000 per quarter, until exhausted, with the first tranche vesting upon the completion of the first quarter of 2024. Continued vesting of these shares is subject to Ms. McLaughlin’s employment remaining in good standing with the Company. In the event that the company is acquired within the two years of January 24, 2024, the vesting schedule that the shares are subject to will accelerate, contingent on Ms. McLaughlin’s employment being in good standing to the date on which the acquisition closes.
William Meissner – CMO and President
On May 4, 2020, the Company entered into an employment agreement with William Meissner, pursuant to which Mr. Meissner serves as President and Chief Marketing Officer of Company. Pursuant to Mr. Meissner’s employment agreement, the Company pays Mr. Meissner an annual base salary of $325,000 and includes annual increases based on cost of living adjustments and performance at the discretion of the Company’s Chief Executive Officer. Mr. Meissner is also eligible for a discretionary bonus, as determined by the Company’s Chief Executive Officer, of up to 50% of Mr. Meissner’s base salary. Mr. Meissner also received a grant of an option to purchase 666,667 shares of common stock under the Company’s equity incentive plan. The employment agreement with Mr. Meissner’s does not have a fixed termination date and permits the Company to terminate Mr. Meissner upon twenty days prior written notice and grants Mr. Meissner the right to resign upon twenty days prior written notice.
Directors Compensation
During the fiscal year ended December 31, 2023, our directors have not beenwere paid any compensation in cash for serving as Directors of the Company and thereCompany.
| Name | Year | Fees Earned or Paid in Cash | All Other Compensation | Stock Awards | Option Awards | Total Compensation | ||||||||||||||||||
| Candace Crawford | 2023 | 76,000 | — | — | 125,000 | 76,000 | ||||||||||||||||||
| Peter McDonough | 2023 | 70,996 | — | — | 125,000 | 70,996 | ||||||||||||||||||
| Justin Yorke | 2023 | — | — | — | 125,000 | — | ||||||||||||||||||
| Bill Caple | 2023 | 46,664 | — | — | 125,000 | — | ||||||||||||||||||
| John Paglia | 2023 | — | — | — | — | — | ||||||||||||||||||
Pension, Retirement or Similar Benefit Plans
There are no presentarrangements or plans in which we provide pension, retirement or understandings with respectsimilar benefits for directors or executive officers. We have no material bonus or profit sharing plans pursuant to future compensation.
Indebtedness of Directors, Senior Officers, Executive Officers and Other Management
None of our directors, executive officers or any associate or affiliate of our Company during the last two fiscal years is or has been indebted to our Company by way of guarantee, support agreement, letter of credit or other similar agreement or understanding currently outstanding.
Equity Compensation Plan
On May 21, 2020, the Board adopted the 2020 Long-Term Incentive Compensation Plan (the “2020 Plan”), which provides for the grant of Options, Restricted Stock Awards, Stock Appreciation Rights, Performance Units and Performance Bonuses to consultants and other eligible recipients. The Plan has been in effect since July 1, 2020, for a period of ten years thereafter. The Plan continues to remain in effect until all matters relating to the payment of Awards and administration of the Plan have been settled.
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes the total outstanding equity awards onas of December 31, 2016.
| Name | Grant Date | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Un-Exercisable | Plan Awards: Number of Securities Underlying Unexercised Unearned Options | Option Exercise Price | Option Expiration Date | ||||||||||||
| Robert Nistico | 2/28/2020 | 159,008 | — | — | $ | 1.12 | 2/21/2025 | |||||||||||
| Robert Nistico | 10/16/2020 | 1,000,000 | — | — | $ | 1.12 | 10/15/2025 | |||||||||||
| Robert Nistico | 9/16/2021 | 530,000 | — | — | $ | 1.12 | 9/16/2031 | |||||||||||
| William Meissner | 10/16/2020 | 416,667 | — | — | $ | 1.12 | 10/16/2025 | |||||||||||
| William Meissner | 9/16/2021 | 66,666 | — | 33,334 | $ | 1.12 | 9/16/2031 | |||||||||||
| (1) | Unless otherwise noted, the business address of each of the following individuals is 1314 East Las Olas Blvd, Suite 221 Fort Lauderdale, Florida 33301 |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of March 27, 2017,29, 2024, for:
| ● | each of our current directors and executive officers; |
| ● | all of our current directors and executive officers as a group; and |
| ● | each person, or group of affiliated persons, who beneficially owned more than 5% of our common stock. |
Except as indicated by (i) each person or entity who is known bythe footnotes below, we believe, based on information furnished to us, that the persons and entities named in the table below have sole voting and sole investment power with respect to own beneficially more than 5% of the outstandingall shares of common stock based on total outstanding shares of 11,277,200, (ii) each of our Directors, (iii)that they beneficially, subject to applicable community property laws. Unless otherwise specified, the address for each of the Executive Officerspersons named in the Summarytable is 1314 E Las Olas Blvd. Suite 221, Fort Lauderdale, Florida 33301.
Our calculation of the percentage of beneficial ownership is based on 45,129,687 shares of common stock outstanding as of March 29, 2024. We have determined beneficial ownership in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under Rule 13d-3 of the Exchange Act of 1934, as amended (the “Exchange Act”), a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise has or shares: (i) voting power, which includes the power to vote or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person or persons, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person or persons (and only such person or persons) by reason of these acquisition rights.
| Name | Shares of Common Stock | Percentage of Common Stock | ||||||
| Executive Officers and Directors | ||||||||
| Robert Nistico | 1,410,000 | 3.1 | % | |||||
| Justin Yorke(1) | 5,486,109 | 12.2 | % | |||||
| John Paglia | — | — | ||||||
| William Meissner | — | — | ||||||
| Stacy McLaughlin | — | — | ||||||
| Officers and Directors as a Group (6 individuals) | 6,896,109 | 15.3 | % | |||||
| 5% or greater owners: | ||||||||
| LK Family Partnership | 2,992,014 | 6.6 | % | |||||
| Total | 9,888,123 | 21.9 | % | |||||
| (1) | Of which 3,297,243 shares are held by Richland Fund LLC, 1,398,012 shares are held by JMW Fund LLC and 790,854 shares are held by San Gabriel LLC. All funds are managed by Mr. Yorke. |
Securities Authorized for Issuance under our Equity Compensation Table,Plan
The following table gives information as of December 31, 2023, the end of the most recently completed fiscal year, about shares of common stock that have been issued under our Splash Beverage Group, Inc. 2020 Incentive Plan. Under the 2020 Incentive Plan we have 4,259,008 options outstanding as of December 31, 2023. See Note 6. On October 6, 2023, at our 2023 annual meeting of stockholders our stockholders approved an amendment to the 2020 Incentive Plan to: (1) increase the aggregate number of shares of common stock available by 1,500,000 shares to a total of 1,807,415 shares and (iv) all(2) increase the automatic annual increase in the number of our Officers and Directorsshares under the 2020 Incentive Plan from 5% to 7.5% of the total number of shares of common stock outstanding as a Group:
| Name and Address of Beneficial Owner | Beneficial Ownership | Approximate Percent Owned | ||
Michael J. West 4120 Boardman-Canfield Road Canfield, OH 44406 | 8,344,000 | 74.0% | ||
Stephen H. West 16325 East Dorado Avenue Centennial, CO 80015 | 300,000 | 2.7% | ||
All Officers and Directors as a group (2 persons) | 8,644,000 | 76.7% |
Plan Category | No. of Shares to be Issued Upon Exercise or Vesting of Outstanding Stock Options | Weighted Average Exercise Price of Outstanding Stock Options | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans | |||||||||
| Equity compensation plan approved by board of directors | 4,259,008 | 1.13 | 2,846,068 | |||||||||
| Total | 4,259,008 | 1.13 | 2,846,068 | |||||||||
Item 13. Certain Relationships and Related Transactions and Director Independence.
The following is a description of the transactions and series of similar transactions, since December 31, 2023, that we were a participant or will be a participant in, which:
| ● | the amount involved exceeds the lesser of $120,000 or one percent of the average of the smaller reporting company’s total assets at year-end for the last two completed fiscal years; and |
| ● | any of our directors, executive officers, holders of more than 5% of our capital stock (which we refer to as “5% stockholders”) or any member of their immediate family had or will have a direct or indirect material interest, other than compensation arrangements with directors and executive officers. |
During the normal course of business, we incurred expenses related to services provided by our CEO or Company expenses paid by our CEO, resulting in related party payables. In conjunction with the acquisition of Copa DI Vino®, the Company also entered into a Revenue Loan and Security Agreement (the “Loan and Security Agreement”) by and among the Company, Robert Nistico, additional Guarantor and each of the subsidiary guarantors from time-to-time party thereto (each a “Guarantor”, and, collectively, the “Guarantors”), and Decathlon Alpha IV, L.P. (the “Lender”). The Loan and Security Agreement provided for a revenue-based credit facility of $1,578,237 (the “Gross Amount”) with the Lender (the “Credit Facility”). There was $371,693 outstanding and $989,702 accrued interest under this agreement as of December 31, 2023.
On September 29, 2023, the Company also entered into a Purchase and Sales Future Receivables Agreement (the “Loan and Security Agreement”) by and among the Company, Robert Nistico, additional Guarantor and each of the subsidiary guarantors from time-to-time party thereto (each a “Guarantor”, and, collectively, the “Guarantors”), and Knightsbridge Funding LLC (the “Lender”). The Loan and Security Agreement provided a loan of $165,000, with the gross and interest amount of $241,725 with the Lender (the “Credit Facility”). There was $99,185 outstanding under this agreement as of December 31, 2023.
There were related party advances from our chief executive officer in the amount of $0.4 million outstanding as of December 31, 2023 and a shareholder note payable outstanding in the amount of $200,000 as of December 31, 2023.
Item 14. Principal Accounting Fees and Services.
December 31, 2016 for professional services rendered by our principal accounting firm, Pritchett, Siler & Hardy, P.C., for the audit of our financial statements included in Form 10-K, and for the review of our interim condensed financial statements included in Form 10-Q, were approximately $54,000. The aggregate fees billed during the fiscal year ended 2023
| Audit – Rose, Snyder & Jacobs LLP | $ | 40,000 | ||
| Audit – Daszkal Bolton, LLP and CohnReznick LLP | $ | 10,000 | ||
| Audit related | — | |||
| Tax | 29,000 | |||
| Total | $ | 79,000 |
December 31, 2015 for professional services rendered by our former principal accounting firm, Cutler & Co., LLC, for the audit of our financial statements included in Form 10-K, and for the review of our interim condensed financial statements included in Form 10-Q, were approximately $10,000.
| Audit | $ | 193,000 | ||
| Audit related | — | |||
| Tax | 19,725 | |||
| Total | $ | 212,725 |
PART IV
Item 15. Exhibits and Financial Statement Schedules.
The following documents are filed as part of this Annual Report on Form 10-K:
1. Financial Statements. See the Financial Statements starting on page 18.
2. Exhibits. The exhibits listed in the Exhibit Index, which appears immediately following the signature page and is incorporated herein by reference, and filed as part of this Annual Report on Form 10-K.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
(Registrant) | |||
| Date: March 29, 2024 | By: | /s/ Robert Nistico | |
Pursuant to the requirements of the Securities Act of 1934 this Annual Report on Form 10-K was signed by the following persons on behalf of the Registrant and in the capacities and on the dates stated:
| Title | Date | |||
| /s/ Robert Nistico | Chief Executive Officer and Director | March 29, 2024 | ||
| Robert Nistico | (Principle Executive Officer) | |||
| /s/ Stacy McLaughlin | Chief Financial Officer, Treasurer | March 29, 2024 | ||
| Stacy McLaughlin | (Principal Financial and Accounting Officer) | |||
| /s/ Justin Yorke | Director, Secretary | March 29, 2024 | ||
| Justin Yorke | ||||
| /s/John Paglia | Director | March 29, 2024 | ||
| John Paglia | ||||
| /s/ Bill Caple | ||||
| Director | March 29, 2024 | |||
EXHIBIT INDEX
| Exhibit No. | Description of Exhibit | ||
| 1.1 |
| 31.1 | Rule 13a-14(a)/ 15d-14(a) Certification of Principal Executive Officer* | |
| 31.2 | Rule 13a-14(a)/ 15d-14(a) Certification of Principal Financial Officer* | |
| 32.1 | Certification of CEO pursuant to 18. U.S.C. Section 1350 as adopted, pursuant to Section 906 of Sarbanes-Oxley Act of | |
| 32.2 | Certification of CFO pursuant to 18. U.S.C. Section 1350 as adopted, pursuant to Section 906 of Sarbanes-Oxley Act of | |
| 97.1 | Clawback Policy of the Company* | |
| *101.INS | Inline XBRL Instance Document (filed herewith) | |
| *101.SCH | Inline XBRL Taxonomy Extension Schema (filed herewith) | |
| *101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase (filed herewith) | |
| *101.LAB | Inline XBRL Taxonomy Extension Label Linkbase (filed herewith) | |
| *101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase (filed herewith) | |
| *101.DEF | Inline XBRL Taxonomy Definition Linkbase (filed herewith) | |
| *104 | Cover Page Interactive Data File (embedded within the Inline XBRL document filed as Exhibit 101) |
| * | Filed herewith | |
42