(1) Consists of threetwo plans: The Nature’s Sunshine Products, Inc. 2012 Stock Incentive Plan (the “2012 Incentive Plan”), and the Nature’s Sunshine Products, Inc. 2009 Stock Incentive Plan (the “2009 Incentive Plan”) and the Nature’s Sunshine Products, Inc. 1995 Stock Option Plan (the “1995 Option Plan”). The 2012 Incentive Plan was approved by shareholders on August 1, 2012. The 2009 Incentive Plan was approved by shareholders on November 6, 2009. The terms of these plans are summarized in Note 12, “Capital Transactions”, of the Notes to Consolidated Financial Statements in Item 8, Part 2 of this report.
(2)During the year ended December 31, 2007, the Company issued nonqualified options to purchase shares of common stock outside of any shareholder approved stock incentive plan. The terms of these nonqualified options to purchase shares of common stock are summarized in Note 12,10, “Capital Transactions”, of the Notes to Consolidated Financial Statements in Item 8, Part 2 of this report.
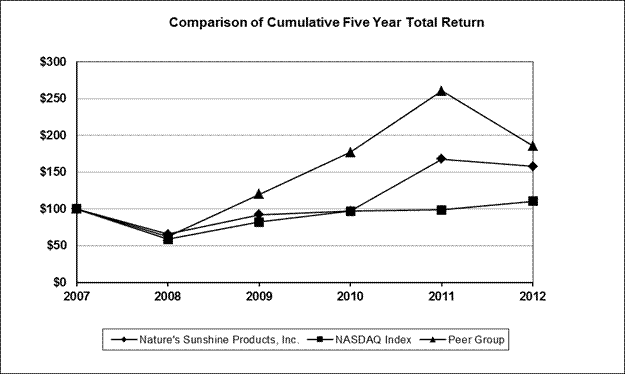
Performance Graph
The graph below depicts our common stock as an index, assuming $100.00 was invested on December 31, 2007,2008, along with the composite prices of companies listed on the NASDAQ Stock Market and our peer group. Standard & Poor’s Investment Services has provided us with this information. The comparisons in the graph are required by regulations of the SEC, and are not intended to forecast or be indicative of the possible future performance of our common stock. The publicly-traded companies in our peer group are Herbalife International, Inc.Ltd., NuSkin Enterprises, Inc. and USANA Health Sciences, Inc. We consider these companies to be our peer group as they have similar product lines and distribution techniques when compared to our business.

1723
Table of Contents
| | 12/31/2007 | | 12/31/2008 | | 12/31/2009 | | 12/31/2010 | | 12/31/2011 | | 12/31/2012 | | | 12/31/2008 | | 12/31/2009 | | 12/31/2010 | | 12/31/2011 | | 12/31/2012 | | 12/31/2013 | |
Nature’s Sunshine Products, Inc. | | $ | 100.00 | | $ | 65.95 | | $ | 92.33 | | $ | 97.09 | | $ | 167.80 | | $ | 158.10 | | | $ | 100.00 | | $ | 140.00 | | $ | 147.21 | | $ | 254.43 | | $ | 239.72 | | $ | 318.77 | |
NASDAQ Index | | 100.00 | | 59.03 | | 82.25 | | 97.32 | | 98.63 | | 110.78 | | | 100.00 | | 145.32 | | 171.50 | | 170.08 | | 199.76 | | 279.90 | |
Peer Group | | 100.00 | | 62.72 | | 120.22 | | 176.92 | | 260.52 | | 185.68 | | | 100.00 | | 191.68 | | 282.08 | | 415.38 | | 296.05 | | 861.14 | |
|
24
Table of Contents
Item 6. Selected Financial Data
The selected financial data presented below is summarized from our results of consolidated operations for each of the five years in the period ended December 31, 2012,2013, as well as selected consolidated balance sheet data as of December 31, 2013, 2012, 2011, 2010 2009 and 2008.
During the year ended December 31, 2012, the Company changed its method of accounting to present shipping and handling costs related to the sale of the Company’s products together with the cost of sales in the consolidated statement of operations. These shipping and handling costs have previously been presented by the Company as part of selling, general and administrative expenses. This change has been made retrospectively to all periods presented in the Company’s financial statements. In connection with the change in presentation, the Company also changed its definition of shipping and handling costs to include expenses of the Company’s distribution facilities to prepare products for shipment. Previously, the Company defined shipping and handling costs as costs incurred for a third-party shipper to transport products to the customer. This presentation is preferable as it better matches all the costs directly associated with generating revenue and therefore better presents gross margin, and this financial statement presentation will be more comparable to the Company’s peer group. The only impact on the Company’s financial statements was to increase cost of sales and decrease selling, general, and administrative in the consolidated statement of operations. The impact of this change in accounting principle was to increase cost of sales and decrease selling, general and administrative expenses by $21.6 million for the year ended December 31, 2012. The impact of this change in accounting principle on cost of sales and selling, general and administrative expenses for the four years ended December 31, 2011 was $20.0 million, $20.2 million, $19.6 million and $20.3 million, respectively.2009.
(Dollar and Share Amounts in Thousands, Except for Per Share Information and Other Information)
Consolidated Statement of Operations Data
| | Year Ended December 31, | | | Year Ended December 31, | |
| | 2012 | | 2011 | | 2010 | | 2009 | | 2008 | | | 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
| | | | | | | | | | | | | | | | | | | | | | |
Net sales revenue | | $ | 367,468 | | $ | 367,813 | | $ | 349,918 | | $ | 342,111 | | $ | 372,065 | | | $ | 378,096 | | $ | 367,468 | | $ | 367,813 | | $ | 349,918 | | $ | 342,111 | |
Cost of sales | | (93,324 | ) | (89,409 | ) | (89,264 | ) | (88,093 | ) | (91,915 | ) | | (94,814 | ) | (93,324 | ) | (89,409 | ) | (89,264 | ) | (88,093 | ) |
Gross profit | | 274,144 | | 278,404 | | 260,654 | | 254,018 | | 280,150 | | | 283,282 | | 274,144 | | 278,404 | | 260,654 | | 254,018 | |
| | | | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | | | |
Volume incentives | | 133,267 | | 133,883 | | 130,367 | | 126,105 | | 139,689 | | | 138,482 | | 133,267 | | 133,883 | | 130,367 | | 126,105 | |
Selling, general and administrative | | 106,861 | | 109,606 | | 119,024 | | 115,480 | | 133,638 | | | 120,743 | | 106,861 | | 109,606 | | 119,024 | | 115,480 | |
Contract termination costs | | — | | 14,750 | | — | | — | | — | | | — | | — | | 14,750 | | — | | — | |
| | 240,128 | | 258,239 | | 249,391 | | 241,585 | | 273,327 | | |
Operating income | | 34,016 | | 20,165 | | 11,263 | | 12,433 | | 6,823 | | | 24,057 | | 34,016 | | 20,165 | | 11,263 | | 12,433 | |
Other income, net | | 1,480 | | 1,847 | | 2,727 | | 2,331 | | 2,692 | | | 1,596 | | 1,480 | | 1,847 | | 2,727 | | 2,331 | |
Income before income taxes | | 35,496 | | 22,012 | | 13,990 | | 14,764 | | 9,515 | | | 25,653 | | 35,496 | | 22,012 | | 13,990 | | 14,764 | |
Provision for income taxes | | 10,116 | | 4,411 | | 5,521 | | 8,210 | | 8,306 | | | 8,044 | | 10,116 | | 4,411 | | 5,521 | | 8,210 | |
Net income from continuing operations | | 25,380 | | 17,601 | | 8,469 | | 6,554 | | 1,209 | | | 17,609 | | 25,380 | | 17,601 | | 8,469 | | 6,554 | |
Loss from discontinued operations | | — | | — | | (9,702 | ) | (439 | ) | (3,047 | ) | | — | | — | | — | | (9,702 | ) | (439 | ) |
Net income (loss) | | $ | 25,380 | | $ | 17,601 | | $ | (1,233 | ) | $ | 6,115 | | $ | (1,838 | ) | | $ | 17,609 | | $ | 25,380 | | $ | 17,601 | | $ | (1,233 | ) | $ | 6,115 | |
Consolidated Balance Sheet Data
| | December 31, | |
| | 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
| | | | | | | | | | | |
Cash and cash equivalents | | $ | 77,247 | | $ | 79,241 | | $ | 58,969 | | $ | 47,604 | | $ | 35,538 | |
Working capital | | 80,025 | | 83,943 | | 57,305 | | 41,370 | | 33,523 | |
Inventories | | 41,910 | | 43,280 | | 41,611 | | 36,235 | | 40,623 | |
Property, plant and equipment, net | | 32,022 | | 27,950 | | 25,137 | | 27,391 | | 28,757 | |
Total assets | | 199,612 | | 193,919 | | 175,811 | | 159,415 | | 156,139 | |
Long-term liabilities | | 25,784 | | 16,893 | | 20,575 | | 25,865 | | 31,175 | |
Total shareholders’ equity | | 105,259 | | 115,636 | | 87,438 | | 68,382 | | 57,095 | |
| | | | | | | | | | | | | | | | |
Summary Cash Flow Information
| | December 31, | |
| | 2012 | | 2011 | | 2010 | | 2009 | | 2008 | |
| | | | | | | | | | | |
Cash and cash equivalents | | $ | 79,241 | | $ | 58,969 | | $ | 47,604 | | $ | 35,538 | | $ | 34,853 | |
Working capital | | 83,943 | | 57,305 | | 41,370 | | 33,523 | | 30,200 | |
Current ratio | | 2.37 | | 1.85 | | 1.63 | | 1.49 | | 1.39 | |
Inventories | | 43,280 | | 41,611 | | 36,235 | | 40,623 | | 39,558 | |
Property, plant and equipment, net | | 27,950 | | 25,137 | | 27,391 | | 28,757 | | 30,224 | |
Total assets | | 193,919 | | 175,811 | | 159,415 | | 156,139 | | 164,276 | |
Long-term liabilities | | 16,893 | | 20,575 | | 25,865 | | 31,175 | | 32,679 | |
Total shareholders’ equity | | 115,636 | | 87,438 | | 68,382 | | 57,095 | | 53,677 | |
| | | | | | | | | | | | | | | | |
| | December 31, | |
| | 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
| | | | | | | | | | | |
Operating activities | | $ | 29,378 | | $ | 26,651 | | $ | 3,908 | | $ | 16,150 | | $ | 927 | |
Investing activities | | (8,564 | ) | (2,989 | ) | (1,679 | ) | (5,909 | ) | (297 | ) |
Financing activities | | (21,331 | ) | (3,133 | ) | 9,588 | | 132 | | (776 | ) |
| | | | | | | | | | | | | | | | |
1825
Table of Contents
Summary Cash Flow Information
| | December 31, | |
| | 2012 | | 2011 | | 2010 | | 2009 | | 2008 | |
| | | | | | | | | | | |
Operating activities | | $ | 26,651 | | $ | 3,908 | | $ | 16,150 | | $ | 927 | | $ | 772 | |
Investing activities | | (2,989 | ) | (1,679 | ) | (5,909 | ) | (297 | ) | (6,759 | ) |
Financing activities | | (3,133 | ) | 9,588 | | 132 | | (776 | ) | (3,102 | ) |
| | | | | | | | | | | | | | | | |
Common Share Summary
| | December 31, | | | December 31, | |
| | 2012 | | 2011 | | 2010 | | 2009 | | 2008 | | | 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
| | | | | | | | | | | | | | | | | | | | | | |
Cash dividend per share | | $ | 0.15 | | $ | — | | $ | — | | $ | 0.05 | | $ | 0.20 | | | $ | 1.90 | | $ | 0.15 | | $ | — | | $ | — | | $ | 0.05 | |
Basic and diluted earnings per share | | | | | | | | | | | | | | | | | | | | | | |
Basic weighted average number of shares | | 15,648 | | 15,550 | | 15,515 | | 15,510 | | 15,510 | | | 15,997 | | 15,648 | | 15,550 | | 15,515 | | 15,510 | |
Diluted weighted average number of shares | | 15,987 | | 15,695 | | 15,605 | | 15,512 | | 15,510 | | | 16,390 | | 15,987 | | 15,695 | | 15,605 | | 15,512 | |
Basic | | | | | | | | | | | | | | | | | | | | | | |
Net income from continuing operations | | $ | 1.62 | | $ | 1.13 | | $ | 0.55 | | $ | 0.42 | | $ | 0.08 | | | $ | 1.10 | | $ | 1.62 | | $ | 1.13 | | $ | 0.55 | | $ | 0.42 | |
Loss from discontinued operations | | $ | — | | $ | — | | $ | (0.63 | ) | $ | (0.03 | ) | $ | (0.20 | ) | | $ | — | | $ | — | | $ | — | | $ | (0.63 | ) | $ | (0.03 | ) |
Net income (loss) | | $ | 1.62 | | $ | 1.13 | | $ | (0.08 | ) | $ | 0.39 | | $ | (0.12 | ) | | $ | 1.10 | | $ | 1.62 | | $ | 1.13 | | $ | (0.08 | ) | $ | 0.39 | |
Diluted | | | | | | | | | | | | | | | | | | | | | | |
Net income from continuing operations | | $ | 1.59 | | $ | 1.12 | | $ | 0.54 | | $ | 0.42 | | $ | 0.08 | | | $ | 1.07 | | $ | 1.59 | | $ | 1.12 | | $ | 0.54 | | $ | 0.42 | |
Loss from discontinued operations | | $ | — | | $ | — | | $ | (0.62 | ) | $ | (0.03 | ) | $ | (0.20 | ) | | $ | — | | $ | — | | $ | — | | $ | (0.62 | ) | $ | (0.03 | ) |
Net income (loss) | | $ | 1.59 | | $ | 1.12 | | $ | (0.08 | ) | $ | 0.39 | | $ | (0.12 | ) | | $ | 1.07 | | $ | 1.59 | | $ | 1.12 | | $ | (0.08 | ) | $ | 0.39 | |
(1) — 2013 includes a one-time cash dividend of $1.50 per share paid on August 29, 2013.
Other Information
| | December 31, | | | December 31, | |
| | 2012 | | 2011 | | 2010 | | 2009 | | 2008 | | | 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
| | | | | | | | | | | | | | | | | | | | | | |
Square footage of property in use | | 768,513 | | 763,389 | | 750,390 | | 750,610 | | 731,277 | | | 771,439 | | 768,513 | | 763,389 | | 750,390 | | 750,610 | |
Number of employees | | 995 | | 1,003 | | 1,073 | | 1,191 | | 1,183 | | | 1,010 | | 995 | | 1,003 | | 1,073 | | 1,191 | |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion highlights the principal factors that have affected our financial condition, results of operations, liquidity and capital resources for the periods described. This discussion should be read in conjunction with our consolidated financial statements and the related notes in Item 8 of this report. This discussion contains forward-looking statements. Please see “Cautionary Note Regarding Forward-Looking Statements” for the risks, uncertainties and assumptions associated with these forward-looking statements.
During the year ended December 31, 2012, the Company changed its method of accounting to present shipping and handling costs related to the sale of the Company’s products together with the cost of sales in the consolidated statement of operations. These shipping and handling costs have previously been presented by the Company as part of selling, general and administrative expenses. This change has been made retrospectively to all periods presented in the Company’s financial statements. In connection with the change in presentation, the Company also changed its definition of shipping and handling costs to include expenses of the Company’s distribution facilities to prepare products for shipment. Previously, the Company defined shipping and handling costs as costs incurred for a third-party shipper to transport products to the customer. This presentation is preferable as it better matches all the costs directly associated with generating revenue and therefore better presents gross margin, and this financial statement presentation will be more comparable to the Company’s peer group. The only impact on the Company’s financial statements was to increase cost of sales and decrease selling, general, and administrative in the consolidated statement of operations. The impact of this change in accounting principle was to increase cost of sales and decrease selling, general and administrative expenses by $21.6 million for the year ended December 31, 2012. The impact of this change in accounting principle on cost of sales and selling, general and administrative expenses for the four years ended December 31, 2011 was $20.0 million, $20.2 million, $19.6 million and $20.3 million, respectively. The following discussion, including statements relating to the results of operations in fiscal years 2011 and 2010, reflect the change in accounting principle of our consolidated financial statement for prior years as noted above.
19
Table of Contents
OVERVIEW
Our Business, Industry and Target Market
Nature’s Sunshine Products, Inc., together with its subsidiaries, is a natural health and wellness company primarily engaged in the manufacturing and direct selling of nutritional and personal care products. The Company is a Utah corporation with its principal place of business in Lehi, Utah, and sells its products to a sales force of independent Managers and Distributors who use the products themselves or resell them to other Distributors or customers. The formulation, manufacturing, packaging, labeling, advertising, distribution and sale of each of our major product groups are subject to regulation by one or more governmental agencies.
The Company has three business segments that are divided based on the different characteristics of their Distributor bases, marketingselling and Distributor compensation plans and product formulations, as well as the internal organization of our officers and their responsibilities and business operations. Two business segments operate under the Nature’s Sunshine Products brand (NSP Americas, Asia Pacific and Europe and NSP Russia, Central and Eastern Europe), and one operates under the Synergy WorldWide brand.
We market our products in Australia, Austria, Belarus, Canada, Colombia, Costa Rica, the Czech Republic, Denmark, the Dominican Republic, Ecuador, El Salvador, Finland, Germany, Guatemala, Honduras, Hong Kong, Iceland, Indonesia, Ireland, Italy, Japan, Kazakhstan, Latvia, Lithuania, Malaysia, Mexico, Moldova, Mongolia, the Netherlands, Nicaragua, Norway, Panama, Peru, the Philippines, Poland, Russia, Singapore, Slovenia, South Korea, Spain, Sweden, Taiwan, Thailand, the Ukraine, the United Kingdom, the United States, Venezuela and Vietnam. We export our products to several other countries, including Argentina, Australia, Chile, Israel, New Zealand and Norway.
In 2012,2013, we experienced a decreasean increase in our consolidated net sales of 0.1 percent. Synergy WorldWide net sales increased approximately 7.22.9 percent compared to the same period in 2011 (or 10.63.7 percent in local currencies excluding the negative impact of foreign currency fluctuations). NSP Americas, Asia Pacific and Europe net sales decreased approximately 3.7 percent compared to the same period in 2011 (or a decrease in local currency net sales of 3.3 percent excluding the negative impact of foreign currency fluctuations)currencies). NSP Russia, Central and Eastern Europe net sales increased approximately 1.58.5 percent (or 8.4 percent in local currencies) compared to the
26
Table of Contents
same period in 2012. Synergy WorldWide net sales increased approximately 7.6 percent (or 8.0 percent in local currencies) compared to the same period in 2012. NSP Americas, Asia Pacific and Europe net sales decreased approximately 0.9 percent compared to the same period in 2011.2012. However, in local currencies, net sales in NSP Americas, Asia Pacific and Europe increased approximately 0.3 percent. Our most significant sales revenue growth was from our NSP Russia, Central and Eastern Europe, Mexico and Venezuela markets and in the Synergy businesses in Europe and South Korea markets during 2012.2013. Gains in these markets were partially offset principally by decreases in our NSP Mexico, NSP and Synergy Japan and NSP Peru and Synergy North American markets.
Net sales revenue excluding the impact of foreign currency exchange qualifies as a non-GAAP financial measure under U.S. GAAP. We believe that this non-GAAP financial measure more accurately represents the financial performance of the Company to management and investors comparing period over period results in a consistent manner.
Selling, general and administrative costs as a percentage of net sales revenue for 2012 decreased2013 increased to 29.131.8 percent from 29.829.1 percent in the prior year. year primarily as a result of the reduction of our royalty costs relatedCompany’s incremental investment in sales, marketing, science and product development personnel and programs to our Russian business as described below,stimulate sales growth and our successful efforts to reduce operating costs in all of our operating segments.
Primarily as a result of reductions of our royalty costs related to our Russian business and onetime contract termination costs of $14.7 million related to the NutriPlus settlement in 2011, our consolidated operating income for 2012 increased to 9.3 percent of net sales revenue from 5.5 percent in the prior year.drive profitability.
We distribute our products to consumers through an independent sales force comprised of independent Managers and Distributors.Distributors, some of whom also consume our products. Typically a person who joins our independent sales force begins as a Distributor. A Distributor interested in earning additional incomemay earn Manager status by committing more time and effort to selling our products, may earn Manager status, which is contingent uponrecruiting productive Distributors and attaining certain purchase levels, recruiting additional Distributors and demonstrating leadership abilities.product sales levels. On a worldwide basis, active Managers were approximately 16,60016,900 and 16,80016,600 and active Distributors and customers were approximately 333,400334,200 and 340,100333,400 at December 31, 2013 and 2012, and 2011, respectively. We anticipate that the number of Distributors and customers will increase if our existing business grows and we enter new international markets, and if current Managers and Distributors expand their networks and grow their businesses.
Critical Accounting Policies and Estimates
Our consolidated financial statements have been prepared in accordance with U.S. GAAP and form the basis for the following discussion and analysis on critical accounting policies and estimates. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On a regular basis, we evaluate our estimates and assumptions. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ from these estimates and those differences could have a material effect on our financial position and results of operations. Management has discussed the development, selection and disclosure of these estimates with the Board of Directors and its Audit Committee.
A summary of our significant accounting policies is provided in Note 1 of the Notes to Consolidated Financial Statements in Item 8 of this report. We believe the critical accounting policies and estimates described below reflect our more significant
20
Table of Contents
estimates and assumptions used in the preparation of our consolidated financial statements. The impact and any associated risks on our business that are related to these policies are also discussed throughout this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” where such policies affect reported and expected financial results.
Revenue Recognition
Net sales revenue and related volume incentive expenses are recorded when persuasive evidence of an arrangement exists, collectability is reasonably assured, the amount is fixed and determinable, and title and risk of loss have passed, generally, when the merchandise has been delivered.passed. The amount of the volume incentive is determined based upon the amount of qualifying purchases in a given month. It is necessary for usthe Company to make estimates about the timing of when merchandise has been delivered. These estimates are based upon ourthe Company’s historical experience related to time in transit, timing of when shipments occurred and shipping volumes. Amounts received for undelivered merchandise are recorded as deferred revenue.
From time to time, ourthe Company’s U.S. operations extend short-term credit associated with product promotions. In addition, for certain of ourthe Company’s international operations, we offerthe Company offers credit terms consistent with industry standards within the country of operation. Payments to Managers and Distributors for sales incentives or rebates related to individual purchases are recorded as a reduction of revenue. Payments for sales incentives and rebates are calculated monthly based upon qualifying sales. Membership fees are deferred and amortized as revenue over the life of the membership, primarily one year. Prepaid event registration fees are deferred and recognized as revenues when the related event is held.
A reserve for product returns is recorded based upon historical experience. We allowThe Company allows Managers or Distributors to return the unused portion of products within 90ninety days of purchase if they are not satisfied with the product. In some of ourthe Company’s markets, the requirements to return product are more restrictive. Sales returns for the years 2013, 2012 and 2011, and 2010, were approximately$1.5 million, $2.2 million, $0.6 million and $0.6 million, respectively. The increase in sales returns for year ended December 31, 2012 was due to unusally high
27
Table of Contents
product returns during the first quarter of 2012, related to a specific promotion in the Synergy Japan market. Product returns were not related to product quality and have since returned to normally lower return rates.
Accounts Receivable Allowances
Accounts receivable have been reduced by an allowance for amounts that may be uncollectible in the future. This estimated allowance is based primarily on the aging category, historical trends and management’s evaluation of the financial condition of the customer. This reserve is adjusted periodically as information about specific accounts becomes available.
Investments
Our available-for-sale investment portfolio is recorded at fair value and consists of various securities such as state and municipal obligations, U.S. government security funds, short-term deposits and various equity securities. These investments are valued using (a) quoted prices for identical assets in active markets or (b) from significant inputs that are observable or can be derived from or corroborated by observable market data for substantially the full term of the asset. Our trading portfolio is recorded at fair value and consists of various marketable securities that are valued using quoted prices in active markets.
For available-for-sale debt securities with unrealized losses, we perform an analysis to assess whether it intends to sell or whether it would be more likely than not required to sell the security before the expected recovery of the amortized cost basis. Where we intend to sell a security, or may be required to do so, the security’s decline in fair value is deemed to be other-than-temporary, and the full amount of the unrealized loss is recorded within earnings as an impairment loss.
For all other debt securities that experience a decline in fair value that is determined to be other-than-temporary and not related to credit loss, we record a loss, net of any tax, in accumulated other comprehensive income (loss). The credit loss is recorded within earnings as an impairment loss when sold. Management judgment is involved in evaluating whether a decline in an investment’s fair value is other-than-temporary.
Regardless of our intent to sell a security, we perform additional analysis on all securities with unrealized losses to evaluate losses associated with the creditworthiness of the security. Credit losses are identified where we do not expect to receive cash flows sufficient to recover the amortized cost basis of a security.
For equity securities, when assessing whether a decline in fair value below ourthe Company’s cost basis is other-than-temporary, we consider the fair market value of the security, the length of time and extent to which market value has been less than cost, the financial condition and near-term prospects of the issuer as well as specific events or circumstances that may influence the operations of the issuer, and our intent and ability to hold the investment for a sufficient time in order to enable recovery of our cost. New information and the passage of time can change these judgments. Where we have determined that we lack the intent and ability to hold an equity security to its expected recovery, the security’s decline in fair value is deemed to be other-than-temporary and is recorded within earnings as an impairment loss.
Inventories
Inventories are stated at the lower-of-cost-or-market, using the first-in, first-out method. The components of inventory cost include raw materials, labor and overhead. To estimate any necessary obsolescence or lower-of-cost-or-market adjustments,
21
Table of Contents
various assumptions are made in regard to excess or slow-moving inventories, non-conforming inventories, expiration dates, current and future product demand, production planning and market conditions.
Self-Insurance Liabilities
Similar to other manufacturers and Distributorsdistributors of products that are ingested, we face an inherent risk of exposure to product liability claims in the event that, among other things, the use of our products results in injury to consumers due to tampering by unauthorized third parties or product contamination. We have historically had a very limited number of product claims or reports from individuals who have asserted that they have suffered adverse consequences as a result of using our products. These matters have historically been settled to our satisfaction and have not resulted in material payments. We have established a wholly ownedwholly-owned captive insurance company to provide us with product liability insurance coverage, and have accrued a reserve that we believe is sufficient to cover probable and reasonable estimable liabilities related to product liability claims based upon our history. However, there can be no assurance that these estimates will prove to be sufficient, nor can there be any assurance that the ultimate outcome of any litigation for product liability will not have a material negative impact on our business prospects, financial position, results of operations or cash flows.
28
Table of Contents
We self-insure for certain employee medical benefits. The recorded liabilities for self-insured risks are calculated using actuarial methods, and are not discounted. The liabilities include amounts for actual claims and claims incurred but not reported. Actual experience, including claim frequency and severity as well as health care inflation, could result in actual liabilities being more or less than the amounts currently recorded.
Impairment of Long-Lived Assets
We review our long-lived assets, such as property, plant and equipment and intangible assets for impairment when events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. We use an estimate of future undiscounted net cash flows of the related assets or groups of assets over their remaining lives in measuring whether the assets are recoverable. An impairment loss is calculated by determining the difference between the carrying values and the fair values of these assets. We did not consider any of our long-lived assets to be impaired during the years ended December 31, 2013, 2012 2011 or 2010.2011.
Incentive Trip Accrual
We accrue for expenses associated with our direct sales marketing program, which rewards independent Managers and Distributors with paid attendance for incentive trips, including Company conventions and meetings. Expenses associated with incentive trips are accrued over qualification periods as they are earned. We specifically analyze incentive trip accruals based on historical and current sales trends as well as contractual obligations when evaluating the adequacy of the incentive trip accrual. Actual results could generate liabilities more or less than the amounts recorded. We have accrued incentive trip costs of approximately $4.6$5.8 million and $5.0$4.6 million at December 31, 20122013 and 2011,2012, respectively, which are included in accrued liabilities in the consolidated balance sheets.
Contingencies
We are involved in certain legal proceedings. When a loss is considered probable in connection with litigation or non-income tax contingencies and when such loss can be reasonably estimated with a range, we record our best estimate within the range related to the contingency. If there is no best estimate, we record the minimum of the range. As additional information becomes available, we assess the potential liability related to the contingency and revise the estimates. Revision in estimates of the potential liabilities could materially affect our results of operations in the period of adjustment. Our contingencies are discussed in further detail in Note 14,12, “Commitment and Contingencies”, of the Notes to Consolidated Financial Statements, in Item 8, Part 2 of this report.
Income Taxes
Our income tax expense, deferred tax assets and liabilities and contingent reserves reflect management’s best assessment of estimated future taxes to be paid. We are subject to income taxes in both the United States and numerous foreign jurisdictions. Significant judgments and estimates are required in determining the Company’s consolidated income tax expense.
Deferred income taxes arise from temporary differences between the tax and financial statement recognition of revenue and expense. In evaluating ourthe Company’s ability to recover ourits deferred tax assets, we considermanagement considers all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax planning strategies and recent financial operations. In projecting future taxable income, we developthe Company develops assumptions including the amount of future state, federal and foreign pretax operating income, the reversal of temporary differences, and the implementation of feasible and prudent tax planning strategies. These assumptions require significant judgment about the forecasts of future taxable income, and are consistent with the plans and estimates that we arethe Company is using to manage the underlying businesses. Valuation allowances are recorded as reserves against net deferred tax assets by the Company when it is determined that net deferred tax assets are not likely to be
22
Table of Contents
realized in the foreseeable future. As of December 31, 20122013 and 2011,2012, we had recorded valuation allowances of $8.1$11.3 million and $9.8$8.1 million, respectively, as offsets to our net deferred tax assets.
As of December 31, 2012,2013, we had foreign income tax net operating loss carryforwards of $4.2$5.6 million, which will expire at various dates from 2014 through 2023. As of December 31, 2013, through 2022. Thethe Company had approximately $4.4$4.1 million of foreign tax and withholding credits, most of which expire in 2020.
Changes in tax laws and rates could also affect recorded deferred tax assets and liabilities in the future. Management is not aware of any such changes that would have a material effect on the Company’s results of operations, cash flows or financial position.
The calculation of ourthe Company’s tax liabilities involves dealing with uncertainties in the application of complex tax laws and regulations in a multitude of jurisdictions across ourits global operations. Income tax positions must meet a more-likely-than-not recognition threshold to be recognized.
29
Table of Contents
Share-Based Compensation
We recognize all share-based payments to Directors and employees, including grants of stock options and restricted stock units, to be recognized in the statement of operations based on their grant-date fair values. We record compensation expense, net of an estimated forfeiture rate, over the vesting period of the stock options based on the fair value of the stock options on the date of grant. We estimated forfeiture rate is based upon historical experience.
Recent Accounting Pronouncements
In July 2013, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2013-11 Income Taxes (Topic 740): “Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists”. This update provides that an unrecognized tax benefit, or a portion thereof, should be presented in the financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, a similar tax loss, or a tax credit carryforward, except to the extent that a net operating loss carryforward, a similar tax loss, or a tax credit carryforward is not available at the reporting date to settle any additional income taxes that would result from disallowance of a tax position, or the tax law does not require the entity to use, the deferred tax asset for such purpose, then the unrecognized tax benefit should be presented as a liability. The amendments in this update are effective for interim and annual periods beginning after December 15, 2013. The adoption of this ASU is not expected to have a material impact on the Company’s consolidated financial statements.
PRESENTATION
Net sales revenue represents net sales including shipping and handling revenues offset by volume rebates given to Managers, Distributors and customers. Volume rebates as a percentage of retail sales may vary by country depending upon regulatory restrictions that limit or otherwise restrict rebates. We also offer reduced volume rebates with respect to certain products and promotions worldwide.
Our gross profit consists of net sales less cost of sales, which represents our manufacturing costs, the price we pay to our raw material suppliers and manufacturers of our products, and duties and tariffs, as well as shipping and handling costs related to product shipments and distribution to our Managers, Distributors and customers.
Volume incentives are a significant part of our direct sales program, and represent commission payments made to our Managers and Distributors. These payments are designed to provide incentives for reaching higher sales levels and for recruiting additional Distributors. Volume incentives vary slightly, on a percentage basis, by product due to our pricing policies and commission plans in place in our various operations.
Selling, general and administrative expensesrepresent our operating expenses, components of which include labor and benefits, sales events, professional fees, travel and entertainment, Distributor marketing, occupancy costs, communication costs, bank fees, depreciation and amortization, and other miscellaneous operating expenses.
Most of our sales to Distributors outside the United States are made in the respective local currencies. In preparing our financial statements, we translate revenues into U.S. dollars using average exchange rates. Additionally, the majority of our purchases from our suppliers generally are made in U.S. dollars. Consequently, a strengthening of the U.S. dollar versus a foreign currency can have a negative impact on our reported sales and contribution margins and can generate transaction losses on intercompany transactions.
Our international operations have provided and willare expected to continue to provide a significant portion of our total net sales. As a result, total net sales will continue to be significantly affected by fluctuations in the U.S. dollar against foreign currencies. In order to provide a framework for assessing how our underlying businesses performed excluding the effect of foreign currency fluctuations, in addition to comparing the percent change in net sales from one period to another in U.S. dollars, we also compare the percentpercentage change in net sales from one period to another period by excluding the effects of foreign currency exchange as shown below. Net sales excluding the impact of foreign exchange fluctuations is not a U.S. GAAP financial measure. Net sales in local currency removes from net sales in U.S. dollars the impact of changes in exchange rates between the U.S. dollar and the functional currencies of our foreign subsidiaries, by translating the current period net sales into U.S. dollars using the same foreign currency exchange rates that were used to translate the net sales for the previous comparable period. We believe presenting the impact of foreign currency fluctuations is useful to investors because it allows a more meaningful comparison of net sales of our foreign operations from period to period. However, net sales excluding the impact of foreign currency fluctuations should not be considered in isolation or as an alternative to net sales in U.S. dollar measures that reflect current period exchange rates, or to other financial measures calculated and presented in accordance with U.S. GAAP.
Our gross profit consists of net sales less cost of sales, which represents our manufacturing costs, the price we pay to our raw material suppliers and manufacturers of our products and duties and tariffs as well as shipping and handling costs related to product shipments.
Volume incentives are a significant part of our direct sales marketing program, and represent commission payments made to our independent Managers and Distributors. These payments are designed to provide incentives for reaching higher sales levels and for recruiting additional Distributors. Volume incentives vary slightly, on a percentage basis, by product due to our pricing policies and commission plans in place in our various operations.
Selling, general and administrative expensesrepresent our operating expenses, components of which include labor and benefits, sales events, professional fees, travel and entertainment, Distributor marketing, occupancy costs, communication costs, bank fees, depreciation and amortization, and other miscellaneous operating expenses.
Most of our sales to Distributors outside the United States are made in the respective local currencies. In preparing our financial statements, we translate revenues into U.S. dollars using average exchange rates. Additionally, the majority of our purchases from our suppliers generally are made in U.S. dollars. Consequently, a strengthening of the U.S. dollar versus a foreign currency can have a negative impact on our reported sales and contribution margins and can generate transaction losses on intercompany transactions. Throughout the last five years, foreign currency exchange rates have fluctuated significantly. See Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
2330
Table of Contents
RESULTS OF OPERATIONS
The following table summarizes our consolidated operating results as a percentage of net sales revenue for the periods indicated:
| | Year Ended December 31, | | | Year Ended December 31, | |
| | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Net sales revenue | | 100.0 | % | 100.0 | % | 100.0 | % | | 100.0 | % | 100.0 | % | 100.0 | % |
Cost of sales | | (25.4 | ) | (24.3 | ) | (25.5 | ) | | (25.1 | ) | (25.4 | ) | (24.3 | ) |
Gross profit | | 74.6 | | 75.7 | | 74.5 | | | 74.9 | | 74.6 | | 75.7 | |
| | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | |
Volume incentives | | 36.2 | | 36.4 | | 37.3 | | | 36.6 | | 36.2 | | 36.4 | |
Selling, general and administrative | | 29.1 | | 29.8 | | 34.0 | | | 31.9 | | 29.1 | | 29.8 | |
Contract termination costs | | — | | 4.0 | | — | | | — | | — | | 4.0 | |
| | 65.3 | | 70.2 | | 71.3 | | |
| | | | | | | | | | | | | | |
Operating income | | 9.3 | | 5.5 | | 3.2 | | | 6.4 | | 9.3 | | 5.5 | |
| | | | | | | | | | | | | | |
Other income: | | | | | | | | | | | | | | |
Interest and other income, net | | 0.4 | | 0.4 | | 0.1 | | | 0.2 | | 0.4 | | 0.4 | |
Interest expense | | (0.1 | ) | — | | — | | | (0.1 | ) | (0.1 | ) | — | |
Foreign exchange gains, net | | 0.1 | | 0.1 | | 0.7 | | | 0.3 | | 0.1 | | 0.1 | |
| | 0.4 | | 0.5 | | 0.8 | | | 0.4 | | 0.4 | | 0.5 | |
| | | | | | | | | | | | | | |
Income before provision for income taxes | | 9.7 | | 6.0 | | 4.0 | | | 6.8 | | 9.7 | | 6.0 | |
Provision for income taxes | | 2.8 | | 1.2 | | 1.6 | | | 2.1 | | 2.8 | | 1.2 | |
| | | | | | | | | | | | | | |
Net income from continuing operations | | 6.9 | | 4.8 | | 2.4 | | |
Loss from discontinued operations, net of tax | | — | | — | | (2.8 | ) | |
| | | | | | | | |
Net income (loss) | | 6.9 | % | 4.8 | % | (0.4 | )% | |
Net income | | | 4.7 | % | 6.9 | % | 4.8 | % |
31
Table of Contents
Year Ended December 31, 2013, as Compared to the Year Ended December 31, 2012
Net Sales Revenue
The following table summarizes the changes in our net sales revenue by operating segment for the fiscal years ended December 31, 2013 and 2012.
| | Net Sales Revenue by Operating Segment | |
| | 2013 | | 2012 | | Percent
Change | | Impact of
Currency
Exchange | | Percent
Change
Excluding
Impact of
Currency | |
| | | | | | | | | | | |
NSP Americas, Asia Pacific and Europe: | | | | | | | | | | | |
NSP North America | | $ | 151,261 | | $ | 153,108 | | (1.2 | )% | $ | (397 | ) | (0.9 | )% |
NSP Latin America | | 48,525 | | 45,756 | | 6.1 | | (1,406 | ) | 9.1 | |
NSP Other | | 7,273 | | 10,081 | | (27.9 | ) | (789 | ) | (20.0 | ) |
| | 207,059 | | 208,945 | | (0.9 | ) | (2,592 | ) | 0.3 | |
| | | | | | | | | | | |
NSP Russia, Central and Eastern Europe | | $ | 62,747 | | $ | 57,853 | | 8.5 | % | $ | 18 | | 8.4 | % |
| | | | | | | | | | | |
Synergy WorldWide: | | | | | | | | | | | |
Synergy North America | | $ | 17,079 | | $ | 18,544 | | (7.9 | )% | $ | — | | (7.9 | )% |
Synergy Asia Pacific | | 59,605 | | 55,548 | | 7.3 | | (1,453 | ) | 9.9 | |
Synergy Europe | | 31,606 | | 26,578 | | 18.9 | | 1,002 | | 15.2 | |
| | 108,290 | | 100,670 | | 7.6 | | (451 | ) | 8.0 | |
| | | | | | | | | | | |
| | $ | 378,096 | | $ | 367,468 | | 2.9 | % | $ | (3,025 | ) | 3.7 | % |
Consolidated net sales revenue for the year ended December 31, 2013, was $378.1 million compared to $367.5 million in 2012, an increase of approximately 2.9 percent. We experienced a $3.0 million unfavorable impact in foreign currency exchange rate fluctuation in 2013, and our consolidated net sales revenue would have increased by 3.7 percent from 2012, but for such negative impact. The increase in net sales revenue for the year ended December 31, 2013 compared to the same period in 2012 is primarily due to an increase of net sales in our NSP Russia, Central and Eastern Europe and Synergy WorldWide segments and was partially offset by a decline of net sales in our NSP Americas, Asia Pacific and Europe segment.
NSP Americas, Asia Pacific and Europe
Net sales revenue related to NSP Americas, Asia Pacific and Europe for the year ended December 31, 2013, was $207.1 million compared to $208.9 million for the same period in 2012, a decrease of 0.9 percent. Fluctuation in foreign exchange rates had a $2.6 million unfavorable impact on net sales for the year ended December 31, 2013, and net sales revenue would have increased by 0.3 percent excluding this negative impact. Active Managers within NSP Americas, Asia Pacific and Europe totaled approximately 7,900 and 8,100 at December 31, 2013 and 2012, respectively. Active Distributors and customers within NSP Americas, Asia Pacific and Europe totaled approximately 150,600 and 153,000 at December 31, 2013 and 2012, respectively. Managers and Distributors within NSP Americas, Asia Pacific and Europe are predominantly practitioners, retailers and consumers of our products. Segment net sales revenue and the number of Distributors and customers decreased primarily due to lower sales in Japan and the United States and were partially offset by increased sales in Mexico and Venezuela. The Active Managers category includes Managers under our various compensation plans that have achieved and maintained certain product sales levels. As such, all Managers are considered to be active Managers. The Active Distributors and customers category includes our Distributors and customers who have purchased products directly from the Company for resale and/or personal consumption during the previous three months.
Notable activity in the following markets contributed to the results of NSP Americas, Asia Pacific and Europe:
In the United States, net sales revenues decreased approximately $1.0 million, or 0.8 percent, for the year ended December 31, 2013, compared to the same period in 2012. While we continue to see improvement in some key sequential business metrics, we have not yet returned to prior year levels. We continue efforts to stabilize the U.S. business through targeted investment in sales and marketing personnel, training, the launch of new products (including the strengthening of our weight management category), sales programs and incentive programs.
32
Table of Contents
In Venezuela, net sales revenues increased approximately $1.6 million, or 24.5 percent, for the year ended December 31, 2013, compared to the same period in 2012. In local currency, net sales increased 44.3 percent, compared to the same period in 2012. Due to Venezuela’s highly inflationary currency, fluctuations in foreign exchange rates had a $1.3 million unfavorable impact on net sales for the year ended December 31, 2013, respectively. The increase in local currency net sales is due to price increases designed to offset the inflationary environment in addition to an increased Manager and Distributor activity.
In Mexico, net sales revenues increased approximately $1.1 million, or 10.4 percent, for the year ended December 31, 2013, compared to the same period in 2012. In local currency, net sales increased 7.1 percent, compared to the same period in 2012. Fluctuations in foreign exchange rates had a $0.4 million favorable impact on net sales for the year ended December 31, 2013. Investment in local sales and marketing initiatives, including hiring a new General Manager for the market who started on April 1, 2013, resulted in a return to growth in net sales revenues in 2013. We opened a new sales center in Mexico City, launched a new energy drink and weight management program into the market in September and invested in sales incentives and promotions, all of which contributed to greater Manager and Distributor activity.
In Japan, net sales revenues decreased approximately $2.7 million, or 45.0 percent, for the year ended December 31, 2013, respectively, compared to the same period in 2012. In local currency, net sales decreased 32.8 percent compared to the same period in 2012. Fluctuations in foreign exchange rates had a $0.7 million unfavorable impact on net sales for the year ended December 31, 2013, respectively. Due to the continued challenges in returning the market to growth, we made the decision to merge the NSP business with the Synergy Japan business to create one unified approach with a common product offering and business opportunity model. The combined businesses began operating as Synergy Japan in January 2014, providing a greater opportunity for a return to profitable growth.
NSP Russia, Central and Eastern Europe
Net sales revenue related to NSP Russia, Central and Eastern Europe markets (primarily Russia, the Ukraine, and Belarus) for the year ended December 31, 2013, was $62.7 million, compared to $57.9 million for the same period in 2012, an increase of 8.5 percent. In local currency, net sales increased 8.4 percent compared to the same period in 2012. Fluctuations in foreign exchange rates had a nominally favorable impact on net sales for the year ended December 31, 2013. Active Managers within NSP Russia, Central and Eastern Europe totaled approximately 6,000 and 5,600 at December 31, 2013 and 2012, respectively. Active Distributors and customers within NSP Russia, Central and Eastern Europe totaled approximately 131,800 and 125,800 at December 31, 2013 and 2012, respectively. NSP Russia, Central and Eastern Europe’s business model is more oriented to a direct selling approach than that of NSP Americas, Asia Pacific and Europe. Net sales increased year-over-year for the fifth consecutive quarter as a result of improved recruiting, Distributor leadership engagement, Distributor recognition, promotion and training and the enhanced focus afforded by the corporate organizational realignment during 2012. In addition, we launched a new weight management program at our annual regional convention in September 2013.
Synergy WorldWide
Synergy WorldWide reported net sales revenue for the year ended December 31, 2013, of $108.3 million, compared to $100.7 million for the same period in 2012, an increase of 7.6 percent. Fluctuations in foreign exchange rates had a $0.5 million unfavorable impact on net sales for the year ended December 31, 2013, and net sales revenue would have increased by 8.0 percent from 2012 excluding the negative impact, respectively. Active Managers within Synergy WorldWide totaled approximately 3,000 and 2,900 at December 31, 2013 and 2012, respectively. Active Distributors and customers within Synergy WorldWide totaled approximately 51,800 and 54,600 at December 31, 2013 and 2012, respectively. Synergy WorldWide’s business model is operating under a traditional direct selling approach. Synergy WorldWide reported a growth of net sales revenue with the increase primarily due to performance in Europe and South Korea, partially offset by lower net sales in Japan and North America.
Notable activity in the following markets contributed to the results of Synergy WorldWide:
In South Korea, net sales revenues increased approximately $6.2 million, or 22.1 percent, for the year ended December 31, 2013, compared to the same period in 2012. In local currency, net sales increased 18.8 percent for the year ended December 31, 2013, compared to the same period in 2012. Fluctuations in foreign exchange rates had a $0.9 million favorable impact on net sales for the year ended December 31, 2013. During the year, promotions and incentives programs were implemented to support and enhance Distributor activity ahead of the Synergy WorldWide global summit held in South Korea in late September. During that event, the SLMsmart weight-management program was launched, which further contributed to the sustained growth through the end of the year.
In Europe, net sales revenues increased approximately $5.0 million, or 18.9 percent, for the year ended December 31, 2013, compared to the same period in 2012. In local currency, net sales increased 15.2 percent compared to the same period in 2012. Fluctuations in foreign exchange rates had a $1.0 million favorable impact on net sales for the year ended December 31, 2013. Strong
33
Table of Contents
Distributor leadership in recruiting and training efforts continues to effectively build our Distributor base thereby driving increased market penetration, supported by our investment in strengthening our sales and marketing organization across the region. In addition, the opening of the Poland market and the successful resolution of temporary shipping restrictions imposed by the Norwegian Food Authority, which had adversely impacted net sales in the fourth quarter of 2012, had a favorable impact on 2013 net sales.
In Japan, net sales revenues decreased approximately $2.1 million, or 19.6 percent, for the year ended December 31, 2013, compared to the same period in 2012. In local currency, net sales decreased 1.8 percent for the year ended December 31, 2013, compared to the same period in 2012. Fluctuations in foreign exchange rates had a $1.9 million unfavorable impact on net sales for the year ended December 31, 2013. New products and programs introduced and implemented to stimulate activity had a positive impact in the second half of 2013. In addition, as referenced above, in order to provide a more stable platform for growth, we made the decision to combine the NSP Japan and Synergy Japan businesses, and operate as a single entity from January 2014 forward.
In North America, net sales revenues decreased approximately $1.5 million, or 7.9 percent, for the year ended December 31, 2013, compared to the same period in 2012. The lack of recruiting, retention and training efforts have been the primary drivers for this decrease. Upcoming growth initiatives are being developed to effectively support recruiting, retention and training activity.
Further information related to our business segments is set forth in Note 13 of the Notes to Consolidated Financial Statements in Item 8 of this report.
Cost of Sales
Cost of sales as a percent of net sales revenue improved to 25.1 percent in 2013, compared to 25.4 percent in 2012.
Volume Incentives
Volume incentives are a significant part of our direct selling program, and represent commission payments made to our Managers and Distributors. These payments are designed to provide incentives for reaching higher product sales levels. Volume incentives vary slightly, on a percentage basis, by product due to our pricing policies and commission plans in place and the sales mix in our various markets. Volume incentives as a percent of net sales revenue increased to 36.6 percent in 2013, compared to 36.2 percent in 2012. The increase was primarily due to net sales increases in markets that pay a higher sales commission in our NSP Russia, Central and Eastern Europe and Synergy WorldWide segments.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased by approximately $13.9 million to $120.7 million for the year ended December 31, 2013. Selling, general and administrative expenses were 31.9 percent of net sales revenue for the year ended December 31, 2013, compared to 29.1 percent for the same period in 2012.
Significant increases to selling, general and administrative expenses during 2013 compared to the same period in 2012 included:
·$6.0 million of increased compensation and other benefit costs as a result of the Company’s incremental investment in sales, marketing, science and product development personnel and programs to stimulate sales growth and drive profitability globally; and
·$2.2 million of increased investments in Distributor conventions, meetings and incentive trips.
In addition, the increases in selling, general and administrative included the following one-time expenses:
·$1.4 million of one-time severance costs and the acceleration of stock option expense incurred related to the resignation of our former Chief Executive Officer;
·$1.3 million of one-time costs related to a five-year customs audit assessment in our Synergy South Korea market; and
·$1.1 million of one-time restructuring costs in certain markets.
Other Income, Net
Other income of $1.6 million, net for the year ended December 31, 2013, remained relatively consistent compared to the same period in 2012.
34
Table of Contents
Income Taxes
Our effective income tax rate was 31.4 percent for 2013, compared to 28.5 percent for 2012. The effective rate for 2013 differed from the federal statutory rate of 35.0 percent primarily due to the following:
(i) | | Adjustments relating to the U.S. tax impact of foreign operations decreased the effective tax rate by 15.9 percentage points in 2013. Included were adjustments for dividends received from foreign subsidiaries, adjustments for foreign tax credits, foreign tax rate differentials and adjustments relating to outside basis calculations under applicable U.S. GAAP. |
| | |
(ii) | | Adjustments to valuation allowances increased the effective rate by 9.3 percent in 2013. Included were increases to domestic valuation allowances on capital loss carryforwards, as well as the effect of valuation allowances of foreign deferred tax assets. |
| | |
(iii) | | Changes in the unrecognized tax benefits increased the effective tax rate by 8.4 percent in 2013. These net gains and losses were recorded for financial reporting purposes, but were excluded from the calculation of taxable income. |
| | |
(iv) | | A 4.4 percent rate reduction resulting from cumulative favorable adjustments related to foreign operations. These adjustments relate to items that are expensed for tax purposes but not for book purposes. |
Adjustments relating to the U.S. impact of foreign operations decreased the effective tax rate by 15.9 percentage points in 2013, and decreased the effective tax rate by 2.1 percentage points in 2012. Included were adjustments for dividends received from foreign subsidiaries, adjustments for foreign tax credits and adjustments relating to the unremitted earnings calculations under applicable U.S. GAAP. The components of this calculation were:
Components of U.S. tax impact of foreign operations | | 2013 | | 2012 | |
Dividends received from foreign subsidiaries | | 29.2 | % | 4.5 | % |
Foreign tax credits | | (34.1 | ) | (4.0 | ) |
Foreign tax rate differentials | | (10.6 | ) | (2.3 | ) |
Unremitted earnings | | (0.4 | ) | (0.3 | ) |
Total | | (15.9 | )% | (2.1 | )% |
From 2012 to 2013, the changes in components of the U.S. tax impact of foreign operations were significant. The primary reason the dividends received from foreign subsidiaries and the foreign tax credits changed by such a large amount was due to an increase in repatriation of foreign earnings to the U.S. from 2012 to 2013.
Changes to the effective rate due to dividends received from foreign subsidiaries, impact of foreign tax credits, foreign tax rate differentials and unremitted earnings calculation are expected to be recurring; however, depending on various factors, the changes may be favorable or unfavorable for a particular period. Given the large number of jurisdictions in which the Company does business and the number of factors that can impact effective tax rates in any given year, this rate is likely to reflect significant fluctuations from year-to-year.
35
Table of Contents
Year Ended December 31, 2012, as Compared to the Year Ended December 31, 2011
Net Sales Revenue
The following table summarizes the changes in our net sales revenue by operating segment for the fiscal years ended December 31, 2012 and 2011.
| | Net Sales Revenue by Operating Segment | |
| | 2012 | | 2011 | | Percent
Change | | Impact of
Currency
Exchange | | Percent
Change
Excluding
Impact of
Currency | |
| | | | | | | | | | | |
NSP Americas, Asia Pacific and Europe: | | | | | | | | | | | |
NSP North America | | $ | 153,108 | | $ | 155,847 | | (1.8 | )% | $ | (162 | ) | (1.7 | )% |
NSP Latin America | | 45,756 | | 49,563 | | (7.7 | ) | (586 | ) | (6.5 | ) |
NSP Other | | 10,081 | | 11,502 | | (12.4 | ) | (55 | ) | (11.9 | ) |
| | 208,945 | | 216,912 | | (3.7 | ) | (803 | ) | (3.3 | ) |
| | | | | | | | | | | |
NSP Russia, Central and Eastern Europe | | $ | 57,853 | | $ | 56,986 | | 1.5 | % | $ | (60 | ) | 1.6 | % |
| | | | | | | | | | | |
Synergy WorldWide: | | | | | | | | | | | |
Synergy North America | | $ | 18,544 | | $ | 21,234 | | (12.7 | )% | $ | — | | $ | (12.7 | )% |
Synergy Asia Pacific | | 55,548 | | 50,396 | | 10.2 | | (1,000 | ) | 12.2 | |
Synergy Europe | | 26,578 | | 22,285 | | 19.3 | | (2,197 | ) | 29.1 | |
| | 100,670 | | 93,915 | | 7.2 | | (3,197 | ) | 10.6 | |
| | | | | | | | | | | |
| | $ | 367,468 | | $ | 367,813 | | (0.1 | )% | $ | (4,060 | ) | $ | 1.0 | % |
24
Table of Contents
Consolidated net sales revenue for the year ended December 31, 2012, was $367.5 million compared to $367.8 million in 2011, a decrease of approximately 0.1 percent. We experienced a $4.1 million unfavorable impact in foreign currency exchange rate fluctuation in 2012, and our consolidated net sales revenue would have increased slightly from 2011 but for such negative impact. The decrease in net sales revenue for the year ended December 31, 2012, compared to the same period in 2011 is primarily due to a decline of net sales in our NSP Americas, Asia Pacific and Europe segment and was partially offset by improvements of net sales in our NSP Russia, Central and Eastern Europe and Synergy WorldWide segments.
NSP Americas, Asia Pacific and Europe
Net sales revenue related to NSP Americas, Asia Pacific and Europe for the year ended December 31, 2012, was $208.9 million compared to $216.9 million for the same period in 2011, a decrease of 3.7 percent. Fluctuation in foreign exchange rates had a $0.8 million unfavorable impact on net sales for the year ended December 31, 2012. Active Managers within NSP Americas, Asia Pacific and Europe totaled approximately 8,100 and 8,700 at December 31, 2012 and 2011, respectively. Active Distributors and customers within NSP Americas, Asia Pacific and Europe totaled approximately 153,000 and 165,600 at December 31, 2012 and 2011, respectively. Managers and Distributors within NSP Americas, Asia Pacific and Europe are predominantly practitioners of nutritional supplement therapies and retailers and consumers of our products. Segment net sales revenue and the number of Managers, Distributors and customers decreased primarily due to lower recruiting in the NSP United States and Mexico markets and a change in operating model in the NSP Peru market in response to changing local regulations.
Notable activity in the following markets contributed to the results of NSP Americas, Asia Pacific and Europe:
The United States market includes both English and Spanish language sales divisions, of which the English language division is approximately 80 percent of segment net sales revenue. Our English language division net sales revenue decreased $2.0 million for the year ended December 31, 2012, or 1.8 percent, compared to the same period in 2011. Our Spanish language division net sales revenue decreased $0.1 million, or 0.3 percent, for the year ended December 31, 2012, compared to the same period in 2011. We continue to be adversely affected by lower sales to Managers and lower recruiting in both divisions. We are continuing our efforts to return to growth through training, new products and incentive programs, while at the same time ensuring stability in sales to existing Distributors and customers.
36
Table of Contents
In Mexico, net sales revenues decreased approximately $1.4 million, or 11.4 percent, for the year ended December 31, 2012, compared to the same period in 2011. In local currency, net sales decreased 6.0 percent, compared to the same period in 2011. Fluctuations in foreign exchange rates had a $0.6 million unfavorable impact on net sales for the year ended December 31, 2012. The decrease in sales is due to lower Manager and Distributor activity. As a result, the local management team is currently being strengthened and a new business model is being developed.
In Peru, net sales revenues decreased approximately $2.4 million, or 63.0 percent, for the year ended December 31, 2012, compared to the same period in 2011. In local currency, net sales decreased 64.5 percent, respectively, compared to the same period in 2011. Fluctuations in foreign exchange rates had a $0.1 million favorable impact on net sales for the periods. The decrease in net sales is due to a change in local regulations that has restricted our ability to sell a majority of our key products in this market through our traditional direct selling business model. In response to this change in regulations, we are currently selling our products through a wholesale channel and continue to evaluate our product mix and selling model for Peru.
NSP Russia, Central and Eastern Europe
Net sales revenue related to NSP Russia, Central and Eastern Europe markets (Russia, the Ukraine, Belarus and several other Eastern European nations) for the year ended December 31, 2012, was $57.9 million compared to $57.0 million for the same period in 2011, an increase of 1.5 percent. In local currency, net sales increased 1.6 percent, respectively, compared to the same period in 2011. Fluctuations in foreign exchange rates had a $0.1 million unfavorable impact on net sales for the periods. The Russian markets continue to build on the momentum as a result of improved Manager and Distributor recruiting efforts and Distributor engagement. Active Managers within NSP Russia, Central and Eastern Europe totaled approximately 5,600 and 5,400 at December 31, 2012 and 2011, respectively. Active Distributors and customers within NSP Russia, Central and Eastern Europe totaled approximately 125,800 and 122,800 at December 31, 2012 and 2011, respectively. NSP Russia, Central and Eastern Europe’s business model is more oriented to a network marketingdirect selling approach.
Synergy WorldWide
Synergy WorldWide reported net sales revenue for the year ended December 31, 2012, of $100.7 million, compared to $93.9 million for the same period in 2011, an increase of 7.2 percent. In local currencies, net sales increased 10.6 percent for the year ended December 31, 2012, compared to the same period in 2011. Fluctuations in foreign exchange rates had a $3.2 million unfavorable impact on net sales for the year ended December 31, 2012. Active Managers within Synergy WorldWide totaled approximately 2,900 and 2,700 at December 31, 2012 and 2011, respectively. Active Distributors and customers within Synergy WorldWide totaled approximately 54,600 and 51,700 at December 31, 2012 and 2011, respectively. Synergy WorldWide’s business model is operating under a traditional network marketingdirect selling approach.
Notable activity in the following markets contributed to the results of Synergy WorldWide:
25
Table of Contents
In Europe, net sales revenues increased approximately $4.3 million, or 19.3 percent, for the year ended December 31, 2012, compared to the same period in 2011. In local currency, net sales increased 29.1 percent for the year ended December 31, 2012, compared to the same period in 2011. Fluctuations in foreign exchange rates had a $2.2 million unfavorable impact on net sales for the year ended December 31, 2012. Strong Distributor leadership in recruiting and training efforts continues to effectively build our Distributor base thereby driving increased market penetration. The sales increase was offset due to restrictions by the Norwegian food authority on the shipment of goods to Norwegian Distributors for a period beginning in late November 2012 and ending in late December 2012, when all issues were resolved with the Norwegian authorities. Shipments recommenced at the beginning of January 2013.
In Korea, net sales revenues increased approximately $7.7 million, or 38.2 percent, for the year ended December 31, 2012, compared to the same period in 2011. In local currency, net sales increased 40.8 percent for the year ended December 31, 2012, compared to the same period in 2011. Fluctuations in foreign exchange rates had a $0.5 million unfavorable impact on net sales for the year ended December 31, 2012. Net sales growth is due to continued collaboration between the Company and key Distributor leadership in developing sales groups with a strong selling system and a broad product line that is well accepted in Korea.
In Japan, net sales revenues decreased approximately $4.9 million, or 31.6 percent, for the year ended December 31, 2012, compared to the same period in 2011. Fluctuations in foreign exchange rates had a nominally unfavorable impact on net sales for the year ended December 31, 2012. The decrease in net sales is partially due to the challenging Japanese direct selling market. In addition, unusually high product returns during the first quarter of 2012 related to a specific promotion that contributed significantly to reduced net sales revenue for the year. Product returns were not related to product quality and have since returned to normal low return rates. Net sales have stabilized and maintained a consistent level since May 2012.
37
Table of Contents
In North America, net sales revenues decreased approximately $2.7 million, or 12.7 percent, for the year ended December 31, 2012, compared to the same period in 2011. The lack of recruiting, retention and training efforts has been the primary driver for this decrease. Upcoming growth initiatives are being modeled to effectively spark further recruiting, retention and training activity.
Further information related to our business segments is set forth in Note 1513 of the Notes to Consolidated Financial Statements in Item 8 of this report.
Cost of Sales
Cost of sales as a percent of net sales revenue increased to 25.4 percent in 2012, compared to 24.3 percent in 2011. These increases are primarily due to increased raw material costs, product and material write-offs, changes in product mix between markets and increased importation fees related to higher transfer prices within some of our markets. While the Company intends to seek continued cost reductions where possible, pricing pressure on raw materials, fuel costs and other factors could adversely affect our ability to reduce or maintain our current cost of sales rate in the future.
Volume Incentives
Volume incentives are a significant part of our direct sales marketing program, and represent commission payments made to our independent Managers and Distributors. These payments are designed to provide incentives for reaching higher product sales levels and for recruiting additional Distributors. Volume incentives vary slightly, on a percentage basis, by product due to our pricing policies and commission plans in place and the sales mix in our various markets. Volume incentives as a percent of net sales revenue decreased to 36.2 percent in 2012, compared to 36.4 percent in 2011.
Selling, General and Administrative Expenses
Selling, general and administrative expenses decreased to 29.1 percent of net sales revenue in 2012, compared to 29.8 percent in 2011, or by approximately $2.7 million to $106.9 million in 2012.
Significant increases to selling, general and administrative expenses during 2012 compared to the same period in 2011 included:
· $3.0 million of increased compensation related costs to the United States;
· $1.9 million of increased variable costs related to the sales growth of Synergy WorldWide in Europe and Korea; and
· $0.5 million of increased non-income tax reserves.
Significant decreases to selling, general and administrative expenses during 2012 compared to the same period in 2011 included:
26
Table of Contents
· $2.9 million of decreased royalty costs related to NSP Russia, Central and Eastern Europe as a result of the NutriPlus LLC settlement;
· $2.0 million decrease in variable costs related to lower sales in our NSP U.S. and Japan markets and Synergy Japan markets;
· $1.4 million of decreased professional fees related to our Russian business that were incurred in 2011 as a result of the NutriPlus LLC settlement;
· $0.9 million of decreased administrative costs due to U.S. convention, incentriveincentive trips and promotion costs;
· $0.6 million of decreased professional fees related to the United States; and
· $0.6 million of favorable currency fluctuations.
Contract Termination Costs
In 1999 and 2000, the Company and NutriPlus LLC (“NutriPlus”) entered into an Asset Purchase Agreement and subsequent Settlement Agreement (together the “Purchase Agreement”) under which the Company acquired certain assets in order to establish its Russian business, and NutriPlus acquired rights to receive certain royalty payments from the Company expressed as a percentage of the Company’s net sales in its Russian business.
On July 8, 2011, the Company and NutriPlus entered into a settlement agreement, in which the Company agreed to pay NutriPlus $21.7 million for the release of all past and future royalty obligations. Of the $21.7 million, the Company applied $7.0 million toward previously accrued and expensed but unpaid royalties, and $14.7 million in exchange for the contract termination and
38
Table of Contents
extinguishment of future royalty obligations. Due to the NutriPlus settlement in 2011, we eliminated an estimated $5.7 million in annual royalty costs in 2012.
Income Taxes
Our effective income tax rate was 28.5 percent for 2012, compared to 20.0 percent for 2011. The effective rate for 2012 differed from the federal statutory rate of 35.0 percent primarily due to the following:
(i) | | Adjustments to valuation allowances decreased the effective rate by 3.9 percent in 2012. Included were adjustments for the removal of domestic valuation allowances on foreign tax credits, as well as the effect of valuation allowances of foreign deferred tax assets. |
| | |
(ii) | | The impact of a decrease in the blended state rate between 2011 and 2012 led to a 2.7 percent increase to the effective rate in 2012. A decrease in the blended state rate causes a permanent difference relating to the state deferred tax assets. |
| | |
(iii) | | Foreign tax rate differentials that incrementally affect the federal statutory rate decreased the effective tax rate by approximately 2.3 percent in 2012. This is primarily due to differences between the lower statutory rates in foreign entities compared to the U.S. statutory rate of 35 percent. |
As a net result of these and other differences, the effective tax rate for 2012 was less than the statutory rate of 35.0 percent for the year ended December 31, 2012.
Adjustments relating to the U.S. impact of foreign operations increased the effective tax rate by 0.2 percentage points in 2012 and decreased the effective tax rate by 15.1 percentage points in 2011. Included were adjustments for dividends received from foreign subsidiaries, adjustments for foreign tax credits and adjustments relating to the unremitted earnings calculations under applicable U.S. GAAP. The components of this calculation were:
Components of U.S. tax impact of foreign operations | | 2012 | | 2011 | |
Dividends received from foreign subsidiaries | | 4.5 | % | 26.2 | % |
Foreign tax credits | | (4.0 | ) | (33.1 | ) |
Unremitted earnings | | (0.3 | ) | (8.2 | ) |
Total | | 0.2 | % | (15.1 | )% |
From 2011 to 2012, the changes in components of the U.S. tax impact of foreign operations were significant. The primary reason the dividends received from foreign subsidiaries, the foreign tax credits and the unremitted earnings decreased were due to a reduction in repatriation of foreign earnings to the U.S. from 2011 to 2012.
Changes to the effective rate due to dividends received from foreign subsidiaries, impact of foreign tax credits and unremitted earnings calculation are expected to be recurring; however, depending on various factors, the changes may be favorable or unfavorable for a particular period. Given the large number of jurisdictions in which the Company does business and the number of factors that can impact effective tax rates in any given year, this rate is likely to reflect significant fluctuations from year-to-year.
27
Table of Contents
Year Ended December 31, 2011, as Compared to the Year Ended December 31, 2010
Net Sales Revenue
The following table summarizes the changes in our net sales revenue by operating segment for the fiscal years ended December 31, 2011 and 2010.
| | Net Sales Revenue by Operating Segment | |
| | 2011 | | 2010 | | Percent
Change | | Impact of
Currency
Exchange | | Percent
Change
Excluding
Impact of
Currency | |
| | | | | | | | | | | |
NSP Americas, Asia Pacific and Europe: | | | | | | | | | | | |
NSP North America | | $ | 155,847 | | $ | 159,195 | | (2.1 | )% | $ | 599 | | (2.5 | )% |
NSP Latin America | | 49,563 | | 52,716 | | (6.0 | ) | (615 | ) | (4.8 | ) |
NSP Other | | 11,502 | | 13,302 | | (13.5 | ) | 814 | | (19.7 | ) |
| | 216,912 | | 225,213 | | (3.7 | ) | 798 | | (4.0 | ) |
| | | | | | | | | | | |
NSP Russia, Central and Eastern Europe | | $ | 56,986 | | $ | 56,141 | | 1.5 | % | $ | 12 | | 1.5 | % |
| | | | | | | | | | | |
Synergy WorldWide: | | | | | | | | | | | |
Synergy North America | | $ | 21,234 | | $ | 15,225 | | 39.5 | % | $ | — | | 39.5 | % |
Synergy Asia Pacific | | 50,396 | | 41,090 | | 22.6 | | 2,916 | | 15.6 | |
Synergy Europe | | 22,285 | | 12,249 | | 81.9 | | 1,038 | | 73.5 | |
| | 93,915 | | 68,564 | | 37.0 | | 3,954 | | 31.2 | |
| | | | | | | | | | | |
| | $ | 367,813 | | $ | 349,918 | | 5.1 | % | $ | 4,764 | | 3.8 | % |
Consolidated net sales revenue for the year ended December 31, 2011 was $367.8 million compared to $349.9 million in 2010, an increase of approximately 5.1 percent. Fluctuation in foreign exchange rates had a $4.8 million favorable impact on net sales for the year ended December 31, 2012. The increase in net sales revenue for the year ended December 31, 2011 compared to the same period in 2010, is primarily due to improvements in our NSP Russia, Central and Eastern Europe and Synergy WorldWide segments, and was partially offset by a decline in our NSP Americas, Asia Pacific and Europe segment.
NSP Americas, Asia Pacific and Europe
Net sales revenue related to NSP Americas, Asia Pacific and Europe for the year ended December 31, 2011 was $216.9 million compared to $225.2 million for the same period in 2010, or a decrease of 3.7 percent. In local currencies, net sales decreased 4.0 percent, compared to the same period in 2010. Fluctuation in foreign exchange rates had a $0.8 million favorable impact on net sales for the year ended December 31, 2011. Active Managers within NSP Americas, Asia Pacific and Europe totaled approximately 8,700 and 9,300 at December 31, 2011 and 2010, respectively. Active Distributors and customers within NSP Americas, Asia Pacific and Europe totaled approximately 165,600 and 181,600 at December 31, 2011 and 2010, respectively. NSP Americas, Asia Pacific and Europe Managers and Distributors are predominantly practitioners of nutritional supplement therapies, as well as retailers and consumers of our products, a segment that continued to be adversely affected by the economic downturn. Net sales revenue also decreased compared to the same period in the prior year due to the cancellation of some less profitable promotional programs.
Notable activity in the following markets contributed to the results of NSP Americas, Asia Pacific and Europe:
The United States market includes both English and Spanish language sales divisions, of which the English language division is approximately 80 percent of segment net sales revenue. Our English language division net sales revenue decreased $1.6 million for the year ended December 31, 2011, or 1.5 percent, compared to the same period in 2010. Our Spanish language division net sales revenue decreased $0.5 million, or 1.7 percent, for the year ended December 31, 2011, compared to the same period in 2010. While both the English and Spanish Divisions have been adversely affected by the poor economic environment, the cancellation of some less profitable promotional programs had a more significant impact on our Spanish Division.
In Japan, net sales revenues decreased approximately $0.7 million, or 8.9 percent, for the year ended December 31, 2011, respectively, compared to the same period in 2010. In local currency, net sales decreased 17.7 percent, respectively, compared to the same period in 2010. Fluctuations in foreign exchange rates had a $0.7 million favorable impact on net sales for the year ended December 31, 2011. The decrease in local currency sales was partially due to the earthquake, tsunami and subsequent nuclear disasters in the spring of 2011, which suppressed consumer confidence and spending and made it difficult to ship ordered products, as well as to retain active Distributors.
28
Table of Contents
In Mexico, net sales revenues decreased approximately $2.4 million, or 16.9 percent, for the year ended December 31, 2011, compared to the same periods in 2010. In local currency, net sales decreased 18.3 percent, compared to the same period in 2010. Fluctuations in foreign exchange rates had a $0.2 million favorable impact on net sales for the year ended December 31, 2011. The decrease in local currency net sales was principally due to the continuing effects of a tax law interpretation by the Mexican taxing authority requiring the collection of value-added tax on all of our products sold in Mexico and our compliance with this interpretation.
In the Dominican Republic, net sales revenues decreased approximately $1.4 million, or 35.9 percent, for the year ended December 31, 2011, respectively, compared to the same period in 2010. The decrease in sales was due in part to the elimination of promotional programs and events which historically had promoted sales growth, but did not produce profitable results.
NSP Russia, Central and Eastern Europe
Net sales revenue related to NSP Russia, Central and Eastern Europe markets (Russia, the Ukraine, Belarus and several other Eastern European nations), for the year ended December 31, 2011 was $57.0 million compared to $56.1 million for the same period in 2010, an increase of 1.5 percent. Excluding Belarus, net sales revenues increased approximately $3.1 million or 6.4 percent, for the year ended December 31, 2011. The Russian markets (excluding Belarus) continued to build on the momentum gained in the prior year as a result of improved Manager and Distributor recruiting efforts and strengthening economies in the region. However, this growth had been slowed by a growing debt crisis in Belarus, which had adversely affected NSP Russia, Central and Eastern Europe’s Belarusian Distributors’ ability to obtain foreign currency to purchase our products. Active Managers within NSP Russia, Central and Eastern Europe totaled approximately 5,400 and 5,400 at December 31, 2012 and 2011, respectively. Active Distributors and customers within NSP Russia, Central and Eastern Europe totaled approximately 122,800 and 123,400 at December 31, 2012 and 2011, respectively.
Synergy WorldWide
Synergy WorldWide reported net sales revenue for the year ended December 31, 2011 of $93.9 million, compared to $68.6 million for the same period in 2010, an increase of 37.0 percent. In local currencies, net sales increased 31.2 percent for the year ended December 31, 2011, compared to the same period in 2010. Fluctuations in foreign exchange rates had a $3.9 million favorable impact on net sales for the year ended December 31, 2011. The increase in net sales was primarily due to growth in Synergy WorldWide’s United States, Korean and European markets, offset by sales declines in its Japan and Indonesia markets. Active Managers within Synergy WorldWide totaled approximately 2,700 and 2,300 at December 31, 2011 and 2010, respectively. Active Distributors and customers within Synergy WorldWide totaled approximately 51,700 and 45,100 at December 31, 2011 and 2010, respectively. Synergy WorldWide’s business model is a traditional network marketing approach.
Notable activity in the following markets contributed to the results of Synergy WorldWide:
In North America, net sales revenues increased approximately $6.0 million, or 39.5 percent, for the year ended December 31, 2011, compared to the same period in 2010. Synergy WorldWide’s growth within the United States was due to effective Company and Manager sales and marketing efforts of a well-accepted product offering, which has resulted in the growth of its U.S. Distributor base through recruiting and retention.
In Europe, net sales revenues increased approximately $10.0 million, or 81.9 percent, for the year ended December 31, 2011, compared to the same period in 2010. In local currency, net sales increased 73.5 percent for the year ended December 31, 2011, compared to the same period in 2010. Fluctuations in foreign exchange rates had a $1.0 million favorable impact on net sales for the year ended December 31, 2011. Strong Distributor leadership and Distributor-driven marketing and development continued to drive increased market penetration primarily within the relatively new markets of Austria, Finland, Norway and Sweden.
In Korea, net sales revenues increased approximately $9.0 million, or 79.6 percent, for the year ended December 31, 2011, compared to the same period in 2010. In local currency, net sales increased 71.7 percent for the year ended December 31, 2011, compared to the same period in 2010. Fluctuations in foreign exchange rates had a $0.9 million favorable impact on net sales for the year ended December 31, 2011. Synergy WorldWide’s growth in Korea was due to productive joint Company and Manager efforts to develop sales groups as well as a broader product line that is well accepted in the market.
In Japan, net sales revenues decreased approximately $0.6 million, or 3.7 percent, for the year ended December 31, 2011, compared to the same period in 2010. In local currency, net sales decreased 12.4 percent for the year ended December 31, 2011, compared to the same period in 2010. Fluctuations in foreign exchange rates had a $1.4 million favorable impact on net sales for the year ended December 31, 2011. The decrease in local currency sales was partially due to the recent earthquake, tsunami and subsequent nuclear disasters in the spring of 2011, which suppressed consumer confidence and spending and made it difficult to ship ordered products, as well as to recruit and retain active Distributors.
29
Table of Contents
Further information related to our business segments is set forth in Note 15 of the Notes to Consolidated Financial Statements in Item 8 of this report.
Cost of Sales
Cost of sales as a percent of net sales revenue decreased to 24.3 percent in 2011, compared to 25.5 percent in 2010. These decreases were primarily due to reductions in product and material write-offs, changes in product mix between markets and lower raw material costs, partially offset by increased importation fees related to higher transfer prices within some of our foreign markets.
Volume Incentives
Volume incentives are a significant part of our direct sales marketing program, and represent commission payments made to our independent Managers and Distributors. These payments are designed to provide incentives for reaching higher sales levels and for recruiting additional Distributors. Volume incentives vary slightly, on a percentage basis, by product due to our pricing policies and commission plans in place and the sales mix in our various operations. Volume incentives as a percent of net sales revenue decreased to 36.4 percent in 2011, compared to 37.3 percent in 2010. The decreases were primarily due to reductions in volume incentive rates due to our lower Manager and Distributor base within NSP Americas, Asia Pacific and Europe and an increase in product purchases that pay a lower sales commission rate in Synergy WorldWide.
Selling, General and Administrative Expenses
Selling, general and administrative expenses decreased to 29.8 percent of net sales revenue in 2011, compared to 34.0 percent in 2010, or by approximately $9.4 million to $109.6 million in 2011.
Significant increases to selling, general and administrative expenses during the year ended December 31, 2011 compared to the same period in 2010 included:
·$3.7 million of increased variable costs related to the sales growth of Synergy WorldWide in Europe, Korea and the United States as well as the NSP Russia, Central and Eastern European markets;
·$2.9 million of performance-based stock compensation costs; and
·$1.5 million of unfavorable currency fluctuations.
Significant decreases to selling, general and administrative expenses during the year ended December 31, 2011, compared to the same period in 2010 included:
·$6.7 million of cost reductions in many of our foreign markets related to changes in leases and compensation costs;
·$2.6 million of decreased royalty costs related to our the NSP Russia, Central and Eastern Europe markets as a result of the NutriPlus settlement;
·$2.4 million related to our U.S. healthcare costs;
·$1.7 million of decreased U.S. compensation and other benefit related costs;
·$1.9 million of non-recurring severance costs related to changes in management and overall personnel reductions worldwide in the prior year;
·$1.1 million of decreased administrative costs in U.S. convention and promotion related costs due to the differences in event qualification periods between years; and
·$0.8 million of decreased non-income tax reserves between years.
Contract Termination Costs
On July 8, 2011, we entered into a settlement agreement with NutriPlus, from whom we acquired certain assets in 1999 in order to establish our Russian business, and to whom we agreed to pay a percentage of net sales in our Russian business in the form of a royalty payment, wherein both parties settled all claims in the arbitration, and bore their own costs associated with the arbitration. As a result of the settlement, the Company agreed to pay NutriPlus $21.7 million for the release of all past and future obligations. Of the $21.7 million, the Company applied $7.0 million toward previously accrued but unpaid royalties (which had been previously expensed), and $14.7 million in exchange for the contract termination and extinguishment of future royalty obligations in 2011.
For the year ended December 31, 2010, the Company recorded and expensed royalty payments to NutriPlus of approximately $5.6 million (included in selling, general and administrative expenses), which was approximately 10.0 percent of NSP Russia, Central and Eastern Europe’s revenue and 1.6 percent of the Company’s consolidated revenue. For the year ended December 31, 2011, the Company recorded and expensed royalty payments to NutriPlus of approximately $2.9 million, which was approximately 5.1 percent of NSP Russia, Central and Eastern Europe’s revenue and 0.8 percent of the Company’s consolidated revenue.
30
Table of Contents
As a result of the settlement, our operating costs were reduced and our consolidated operating income was increased by $2.6 million based on current sales volumes from our Russian business. This reduction in operating costs and corresponding increase in consolidated operating income will fluctuate based on future increases or decreases in sales from our Russian business.
Other Income, net
Other income, net for the year ended December 31, 2011, decreased $0.9 million, compared to the same period in 2010. The decrease was primarily due to prior year foreign exchange gains of $3.7 million in Venezuela as a result of the devaluation of the bolivar and the change to a highly inflationary economy that were non-recurring and recognized in 2010, offset by net foreign exchange gains and losses in certain of our other markets based on changes in exchange rates. The decrease in other net income in 2011 had been offset by an increase in interest and other income, net of $1.2 million due to the receipt of $0.7 in restricted cash in Venezuela that had been previously written-down.
Income Taxes
Our effective income tax rate was 20.0 percent for 2011, compared to 39.5 percent for 2010. The effective rate for 2011 differed from the federal statutory rate of 35.0 percent primarily due to the following:
(i)
| Adjustments relating to the U.S. tax impact of foreign operations decreased the effective tax rate by 15.1 percentage points in 2011. Included were adjustments for dividends received from foreign subsidiaries, adjustments for foreign tax credits and adjustments relating to outside basis calculations under applicable U.S. GAAP. Changes to the effective rate due to dividends received from foreign subsidiaries, adjustments for foreign tax credits and outside basis are expected to be recurring.
|
| |
(ii)
| Changes in the unrecognized tax benefits increased the effective tax rate by 8.2 percent in 2011. These net gains and losses were recorded for financial reporting purposes, but were excluded from the calculation of taxable income.
|
| |
(iii)
| Foreign tax rate differentials that incrementally affect the federal statutory rate decreased the effective tax rate by approximately 5.2 percent in 2011. This is primarily due to differences between the lower statutory rates in foreign entities compared to the U.S. statutory rate of 35 percent.
|
As a net result of these and other differences, the effective tax rate for 2011 was less than the statutory rate of 35.0 percent for the year ended December 31, 2011.
Adjustments relating to the U.S. tax impact of foreign operations decreased the effective tax rate by 15.1 percentage points in 2011 and increased the effective tax rate by 13.5 percentage points in 2010. Included were adjustments for dividends received from foreign subsidiaries, adjustments for foreign tax credits and adjustments relating to the unremitted earnings calculations under applicable U.S. GAAP. The components of this calculation were:
Components of U.S. tax impact of foreign operations | | 2011 | | 2010 | |
Dividends received from foreign subsidiaries | | 26.2 | % | 25.5 | % |
Foreign tax credits | | (33.1 | ) | (31.7 | ) |
Unremitted earnings | | (8.2 | ) | 19.7 | |
Total | | (15.1 | )% | 13.5 | % |
Between the years ended December 31, 2011 and 2010, the changes in the components of the U.S. tax impact of foreign operations were insignificant, except for the Company’s calculation of unremitted foreign earnings, which changed from an increase in the effective tax rate for 2010 to a decrease for 2011. The primary reason that the unremitted earnings caused an increase to the effective rate in 2010 and then a decrease in 2011 is that the company recorded adjustments in 2011 related to the settlement of IRS audits of the Company’s open tax years 2003-2008. As a result of these settlements, the Company’s historic foreign earnings in past years decreased, causing additional benefit in the Company’s unremitted earnings calculation, which in turn increased the net deferred tax asset related to unremitted earnings in 2011.
Changes to the effective rate due to dividends received from foreign subsidiaries, impact of foreign tax credits and the unremitted earnings calculation are expected to be recurring; however, depending on various factors, the changes may be favorable or unfavorable in a particular period. Given the large number of jurisdictions in which the Company does business and the number of factors that can impact effective tax rates in any given year, this rate is likely to reflect relatively significant fluctuations from year-to-year.
3139
Table of Contents
SUMMARY OF QUARTERLY OPERATIONS — UNAUDITED
During the year ended December 31, 2012, the Company changed its method of accounting to present shipping and handling costs related to the sale of the Company’s products together with the cost of sales in the consolidated statement of operations. These shipping and handling costs have previously been presented by the Company as part of selling, general and administrative expenses. This change has been made retrospectively to all periods presented in the Company’s financial statements. The impact of the change in accounting principle in the three months ended December 31, 2012 was to increase cost of sales and decrease selling, general and administrative expenses by $5.3 million. The total amount of shipping and handling costs reclassified by the Company from selling, general and administrative expense to cost of sales in its consolidated statements of operations for the three months ended September 30, 2012, June 30, 2012, and March 31, 2012 is $5.4 million, $5.5 million, and $5.4 million, respectively. The total amount of shipping and handling costs reclassified by the Company from selling, general and administrative expense to cost of sales in its consolidated statements of operations for the three months ended December 31, 2011, September 30, 2011, June 30, 2011, and March 31, 2011 is $5.1 million, $5.1 million, $4.9 million and $4.9 million, respectively.
The following tables presents the Company’s unaudited summary of quarterly operations during 20122013 and 20112012 for each of three month periods ended March 31, June 30, September 30, and December 31 (amounts in thousands).
| | For the Quarter Ended | |
| | March 31,
2013 | | June 30,
2013 | | September
30, 2013 | | December
31, 2013 | |
Net sales revenue | | $ | 96,479 | | $ | 93,675 | | $ | 92,458 | | $ | 95,484 | |
Cost of sales | | (24,445 | ) | (22,630 | ) | (23,655 | ) | (24,084 | ) |
Gross profit | | 72,034 | | 71,045 | | 68,803 | | 71,400 | |
| | | | | | | | | |
Volume incentives | | 34,975 | | 34,525 | | 33,920 | | 35,062 | |
Selling, general and administrative | | 30,117 | | 28,709 | | 28,170 | | 33,747 | |
Operating income | | 6,942 | | 7,811 | | 6,713 | | 2,591 | |
Other income (expense) | | 330 | | 1,482 | | (269 | ) | 53 | |
Income before income taxes | | 7,272 | | 9,293 | | 6,444 | | 2,644 | |
Tax provision | | 2,408 | | 3,241 | | 1,594 | | 801 | |
Net income | | $ | 4,864 | | $ | 6,052 | | $ | 4,850 | | $ | 1,843 | |
| | | | | | | | | |
Basic and diluted net income per common share | | | | | | | | | |
| | | | | | | | | |
Basic: | | | | | | | | | |
Net income per common share | | $ | 0.31 | | $ | 0.38 | | $ | 0.30 | | $ | 0.11 | |
| | | | | | | | | |
Diluted: | | | | | | | | | |
Net income per common share | | $ | 0.30 | | $ | 0.38 | | $ | 0.29 | | $ | 0.11 | |
| | | | | | | | | |
Dividends declared per common share | | $ | 0.10 | | $ | 0.10 | | $ | 1.60 | | $ | 0.10 | |
| | For the Quarter Ended | |
| | March 31,
2012 | | June 30,
2012 | | September
30, 2012 | | December
31, 2012 | |
Net sales revenue | | $ | 92,868 | | $ | 92,991 | | $ | 91,232 | | $ | 90,377 | |
Cost of sales | | (23,729 | ) | (22,610 | ) | (23,144 | ) | (23,841 | ) |
Gross profit | | 69,139 | | 70,381 | | 68,088 | | 66,536 | |
| | | | | | | | | |
Volume incentives | | 33,581 | | 33,540 | | 33,155 | | 32,991 | |
Selling, general and administrative | | 26,384 | | 26,530 | | 26,228 | | 27,719 | |
Operating income | | 9,174 | | 10,311 | | 8,705 | | 5,826 | |
Other income (expense) | | (110 | ) | 165 | | (214 | ) | 1,639 | |
Income before income taxes | | 9,064 | | 10,476 | | 8,491 | | 7,465 | |
Tax provision | | 1,836 | | 3,190 | | 2,121 | | 2,969 | |
Net income | | $ | 7,228 | | $ | 7,286 | | $ | 6,370 | | $ | 4,496 | |
| | | | | | | | | |
Basic and diluted net income per common share | | | | | | | | | |
| | | | | | | | | |
Basic: | | | | | | | | | |
Net income per common share | | $ | 0.46 | | $ | 0.47 | | $ | 0.41 | | $ | 0.28 | |
| | | | | | | | | |
Diluted: | | | | | | | | | |
Net income per common share | | $ | 0.46 | | $ | 0.46 | | $ | 0.40 | | $ | 0.28 | |
| | | | | | | | | |
Dividends declared per common share | | $ | — | | $ | 0.05 | | $ | 0.05 | | $ | 0.05 | |
3240
Table of Contents
| | For the Quarter Ended | |
| | March 31,
2011 | | June 30,
2011 | | September
30, 2011 | | December
31, 2011 | |
Net sales revenue | | $ | 92,844 | | $ | 91,811 | | $ | 91,102 | | $ | 92,056 | |
Cost of sales | | (23,501 | ) | (22,040 | ) | (21,957 | ) | (21,911 | ) |
Gross profit | | 69,343 | | 69,771 | | 69,145 | | 70,145 | |
| | | | | | | | | |
Volume incentives | | 34,298 | | 33,390 | | 32,733 | | 33,462 | |
Selling, general and administrative | | 27,424 | | 28,329 | | 26,767 | | 27,086 | |
Contract termination costs | | — | | — | | 14,750 | | — | |
Operating income (loss) | | 7,621 | | 8,052 | | (5,105 | ) | 9,597 | |
Other income (expense) | | 265 | | (420 | ) | 1,204 | | 798 | |
Income (loss) before income taxes | | 7,886 | | 7,632 | | (3,901 | ) | 10,395 | |
Tax provision (benefit) | | 1,264 | | 2,018 | | (1,645 | ) | 2,774 | |
Net income (loss) | | $ | 6,622 | | $ | 5,614 | | $ | (2,256 | ) | $ | 7,621 | |
| | | | | | | | | |
Basic and diluted net income (loss) per common share | | | | | | | | | |
| | | | | | | | | |
Basic: | | | | | | | | | |
Net income per common share | | $ | 0.43 | | $ | 0.36 | | $ | (0.14 | ) | $ | 0.49 | |
| | | | | | | | | |
Diluted: | | | | | | | | | |
Net income per common share | | $ | 0.43 | | $ | 0.36 | | $ | (0.14 | ) | $ | 0.48 | |
| | | | | | | | | |
Dividends declared per common share | | $ | — | | $ | — | | $ | — | | $ | — | |
Basic and diluted income (loss) per share is computed independently for each of the quarters presented. Therefore, the sum of the quarterly net lossincome per share may not equal the total computed for the year.
LIQUIDITY AND CAPITAL RESOURCES
Our principal use of cash is to pay for operating expenses, including volume incentives, inventory and raw material purchases, capital assets and funding of international expansion. As of December 31, 2012,2013, working capital was $83.9$80.0 million, compared to $56.9$83.9 million as of December 31, 2011.2012. At December 31, 2012,2013, we had $79.2$77.2 million in cash and cash equivalents, of which $49.8$63.0 million was held in our foreign markets and may be subject to various withholding taxes and other restrictions related to repatriation, and $2.1$2.0 million in unrestricted short-term investments, which were available to be used along with our normal cash flows from operations to fund any unanticipated shortfalls in future cash flows.
Our net consolidated cash inflows (outflows) are as follows (in thousands):
| | Year Ended December 31, | | | Year Ended December 31, | |
| | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Operating activities | | $ | 26,651 | | $ | 3,908 | | $ | 16,150 | | | $ | 29,378 | | $ | 26,651 | | $ | 3,908 | |
Investing activities | | (2,989 | ) | (1,679 | ) | (5,909 | ) | | (8,564 | ) | (2,989 | ) | (1,679 | ) |
Financing activities | | (3,133 | ) | 9,588 | | 132 | | | (21,331 | ) | (3,133 | ) | 9,588 | |
|
For the year ended December 31, 2013, operating activities provided cash in the amount of $29.4 million compared to $26.7 million for the same period in 2012. Operating cash flows increased due to the timing of payments and receipts for inventories, accrued volume incentives, accrued liabilities, income tax payable and the liability related to unrecognized tax benefits, and was partially offset by the timing of payments and receipts for accounts receivable, prepaid expenses, accounts payable, and deferred revenue as well as the decrease in our operating income.
For the year ended December 31, 2012, we generated cash from operating activities of $26.7 million compared to $3.9 million in 2011. Operating cash flow increased primarily due to the fact that we made payments of $21.7 million related to the NutriPlus settlement in 2011 that were not made in 2012.
For the year ended December 31, 2011, we generated cash from operating activities of $3.9 million compared to $16.2 million in 2010. Operating cash flow decreased primarily due to payments of $21.7 million related to the NutriPlus settlement in 2011 as noted above. Excluding the NutriPlus settlement payments made in 2011, operating cash flows increased $9.5 million primarily due to an increase in net income partially offset by the timing of payments of inventory and income tax liabilities.
Capital expenditures related to the purchase of equipment, computer systems and software for the years ended December 31, 2013, 2012 and 2011 and 2010 were $8.4 million, $6.6 million and $2.4 million, and $2.6 million, respectively. In 2013, the Company began to significantly reinvest in its information technology systems. Included within this plan is an Oracle ERP implementation program to provide the Company with a single integrated software solution that will integrate the Company’s business process on a worldwide basis. The increase in capital expenditures from 2011 to 2012 was related to the relocationCompany anticipates completion of our corporate headquarters, upgrade of our Spanish Fork production facility and the establishment of a new call center and the purchase of manufacturing and computer equipment.
33
Table of Contentsthis project by mid-2016.
During the years ended December 31, 2013, 2012 2011 and 2010,2011, we used cash to purchase available-for-sale investments of $0.4 million, $0.2 million $7.0 million and $3.4$7.0 million, respectively, and had cash proceeds of $0.2 million, $3.8 million and $7.7 million for 2013, 2012 and $0.1 million for 2012, 2011, and 2010, respectively, from the sale of such investments.
During the yearyears ended December 31, 2013 and 2012, we used cash to pay dividends in an aggregate amount of $30.4 million and $2.3 million, respectively. On August 8, 2013, the Company’s Board of Directors declared a special one-time cash dividend of $1.50 per share, for an aggregate special dividend of $24.0 million, in addition to its recurring quarterly cash dividend of $0.10 per share payable on August 29, 2013, to shareholders of record as of the close of business on August 19, 2013.
In addition, the Board of Directors authorized a $10 million share repurchase program to be implemented over two years. Such purchases may be made in the open market, through block trades, in privately negotiated transactions or otherwise. The timing and amount of any shares repurchased will be determined based on the Company’s evaluation of market conditions and other factors and the program may be discontinued or suspended at any time. The Company will fund the one-time special dividend and share repurchase program through available cash on hand, future cash flows from operations and borrowings under its revolving credit facility. During the year ended December 31, 2013, the Company repurchased 140,331 shares of its common stock under the share repurchase program for $2.5 million. At December 31, 2013, the remaining balance available for repurchases under the program was $7.5 million.
On August 9, 2011, the Company entered into a Revolving Creditrevolving credit agreement with Wells Fargo Bank, National Association that permits the Company to borrow up to $15 million through August 9, 2014, bearing interest at LIBOR plus 1.25 percent.percent (1.50 percent as of December 31, 2013). The Company must pay an annual commitment fee of 0.25 percent on the unused portion of the commitment. At
41
Table of Contents
On August 8, 2013, the Company renegotiated the revolving credit agreement with Wells Fargo Bank, N.A. to increase the borrowing limit to $25.0 million and extend the maturity to September 1, 2015. The Company will pay an annual commitment fee of 0.25 percent on the unused portion of the commitment and LIBOR plus 1.25 percent on any borrowings on the agreement (1.50 percent as of December 31, 2012, no amount was drawn2013). The renegotiation of the debt agreement will provide the Company with adequate financing to continue its cash generating operating activities. The Company borrowed $10.0 million under this credit line in August 2013 for general business purposes. The Company retains ample capital capacity to continue making long-term investments in its sales, marketing, science and product development initiatives and overall operations, as well as pursue strategic opportunities as they may arise. As of December 31, 2013, the outstanding balance under the facility.revolving credit agreement was $15.0 million.
In addition, on August 9, 2011, a term loan of $10.0 million was obtained in conjunction with the Revolving Creditrevolving credit agreement and has a maturity date of August 9, 2014 and a variable interest rate of LIBOR plus 1.25 percent (1.50 percent as of December 31, 2012)2013). The term loan is collateralized by the Company’s assets at the manufacturing facility in Spanish Fork, Utah. As of December 31, 2012,2013, the outstanding balance under this term loan was $5.6$2.3 million.
During the years ended December 31, 2013 and 2012, we used cash to make principal payments of $3.4 million and $3.6 million on our term loan, respectively. During the year ended December 31, 2013, we made a draw of $10.0 million on the revolving credit agreement. The term credit facility is secured by the Company’s manufacturing facility in Spanish Fork, Utah.
These loans contain restrictions on liquidity, leveraging, minimum net income and consecutive quarterly net losses. In addition, the agreements restrict capital expenditures, lease expenditures, other indebtedness, liens on assets, guaranties, loans and advances, and the merger, consolidation and the transfer of assets except in the ordinary course of business. As of December 31, 2012,2013, the Company was in compliance with these debt covenants.
We believe that, with this credit facility in place, our working capital requirements can be met for the foreseeable future with cash generated from operating activities, available cash and cash equivalents and draws on the credit facility. However, among other things, a prolonged economic downturn, a decrease in demand for our products, an unfavorable settlement of our unrecognized tax positions or non-income tax contingencies could adversely affect our long-term liquidity.
CONTRACTUAL OBLIGATIONS
The following table summarizes information about contractual obligations as of December 31, 20122013 (in thousands):
| | Total | | Less than 1 year | | 1-3 years | | 3-5 years | | After 5 years | | | Total | | Less than 1 year | | 1-3 years | | 3-5 years | | After 5 years | |
Operating lease obligations | | $ | 15,047 | | $ | 4,688 | | $ | 6,503 | | $ | 3,216 | | $ | 640 | | | $ | 15,434 | | $ | 4,841 | | $ | 6,105 | | $ | 4,035 | | $ | 453 | |
Purchase obligations(1) | | 8,344 | | 8,344 | | — | | — | | — | | | 43 | | 43 | | — | | — | | — | |
Self-insurance reserves(2) | | 2,990 | | 532 | | — | | — | | 2,458 | | | 526 | | 526 | | — | | — | | — | |
Other long-term liabilities reflected on the balance sheet(3) | | 1,276 | | — | | — | | — | | 1,276 | | | 971 | | — | | — | | — | | 971 | |
Unrecognized tax benefits(4) | | — | | — | | — | | — | | — | | | — | | — | | — | | — | | — | |
Long-term debt(5) | | 5,620 | | 3,350 | | 2,270 | | — | | — | | | 2,267 | | 2,267 | | — | | — | | — | |
Interest on long-term debt(6) | | 74 | | 61 | | 13 | | — | | — | | |
Revolving credit agreement(7) | | — | | — | | — | | — | | — | | |
Revolving credit facility(6) | | | 10,000 | | — | | 10,000 | | — | | — | |
Interest on long-term debt(7) | | | 167 | | 103 | | 64 | | — | | — | |
Capital commitments(8) | | | 6,478 | | 3,134 | | 3,344 | | — | | — | |
Total | | $ | 33,351 | | $ | 16,975 | | $ | 8,786 | | $ | 3,216 | | $ | 4,374 | | | $ | 35,886 | | $ | 10,914 | | $ | 19,513 | | $ | 4,035 | | $ | 1,424 | |
(1) Purchase obligations include non-cancelable purchase agreements for both botanical and non-botanical raw materials related to our forecasted 20132014 production estimates, as well as related packaging materials.
(2) At December 31, 2013, there were $2.8 million of liabilities. The Company retains a significant portion of the risks associated with certain employee medical benefits and product liability insurance. Recorded liabilities for self-insured risks are calculated using actuarial methods and are not discounted. Amounts for self-insurance obligations are included in accrued liabilities and long-term other liabilities on the Company’s consolidated balance sheet. Because of the high degree of uncertainty regarding the timing of future cash outflows associated with the product liability obligations, if any, the Company is unable to estimate the years in which cash settlement may occur with the respective tax authorities.
(3) The Company provides a nonqualified deferred compensation plan for its officers and certain key employees. Under this plan, participants may defer up to 100 percent of their annual salary and bonus (less the participant’s share of employment taxes). The deferrals become an obligation owed to the participant by the Company under the plan. Upon separation of the
42
Table of Contents
participant from the service of the Company, the obligation owed to the participant under the plan will be paid as a lump sum or over a period of either three or five years. As we cannot easily determine when our officers and key employees will separate from the Company, we have classified the obligation greater than five years for payment.
(4) At December 31, 2012,2013, there were $10.6$12.4 million of liabilities. Because of the high degree of uncertainty regarding the timing of future cash outflows associated with these liabilities, if any, the Company is unable to estimate the years in which cash settlement may occur with the respective tax authorities.
(5) The long-term debt is due in monthly installments of approximately $0.3 million, including interest, and is collateralized by the Company’s manufacturing facility in Spanish Fork, Utah and has a maturity date of August 9, 2014, and a variable interest rate of LIBOR plus 1.25 percent (1.50 percent as of December 31, 2012)2013).
34
Table of Contents
(6) The interest on the long-term debt is based upon LIBOR plus 1.25 percent (1.50 percent as of December 31, 2012).
(7)The Company entered into a Revolving Creditrevolving credit agreement with Wells Fargo Bank, National Association that permits the Company to borrow up to $15$25 million through August 9, 2014,September 1, 2015, bearing interest at LIBOR plus 1.25 percent. The Company must pay an annual commitment fee of 0.25 percent on the unused portion of the commitment. At December 31, 2012,2013, the Company had $15 million available under this facility.
(7)The interest on the long-term debt is based upon LIBOR plus 1.25 percent (1.50 percent as of December 31, 2013).
(8)In 2013, the Company began to significantly reinvest in its information technology systems. Included within this plan is an Oracle ERP implementation program to provide the Company with a single integrated software solution that will integrate the Company’s business process on a worldwide basis. The Company anticipates completion of this project by mid-2016.
The Company has entered into long-term agreements with third-parties in the ordinary course of business, in which it has agreed to pay a percentage of net sales in certain regions in which it operates, or royalties on certain products. In 2013, 2012 2011 and 2010,2011, the aggregate amounts of these payments were $1.5 million, $1.3 million $8.4 million and $3.0$8.4 million, respectively.
OFF-BALANCE SHEET ARRANGEMENTS
We have no off-balance sheet arrangements other than operating leases. We do not believe that these operating leases are material to our current or future financial position, results of operations, revenues or expenses, cash flows, capital expenditures or capital resources.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
We conduct business in several countries and intend to continue to grow our international operations. Net sales revenue, operating income and net income are affected by fluctuations in currency exchange rates, interest rates and other uncertainties inherent in doing business and selling product in more than one currency. In addition, our operations are exposed to risks associated with changes in social, political and economic conditions inherent in international operations, including changes in the laws and policies that govern international investment in countries where we have operations, as well as, to a lesser extent, changes in U. S.U.S. laws and regulations relating to international trade and investment.
Foreign Currency Risk
During the year ended December 31, 2012,2013, approximately 57.959.7 percent of our net sales revenue and approximately 55.556.7 percent of our operating expenses were realized outside of the United States. Inventory purchases are transacted primarily in U.S. dollars from vendors located in the United States. The local currency of each international subsidiary is generally the functional currency. We conduct business in twenty-threemultiple different currencies with exchange rates that are not on a one-to-one relationship with the U.S. dollar. All revenues and expenses are translated at average exchange rates for the periods reported. Therefore, our operating results will be positively or negatively affected by a weakening or strengthening of the U.S. dollar in relation to another fluctuating currency. Given the uncertainty and diversity of exchange rate fluctuations, we cannot estimate the effect of these fluctuations on our future business, product pricing, results of operations or financial condition, but we have provided consolidated sensitivity analyses below of functional currency/reporting currency exchange rate risks. Changes in various currency exchange rates affect the relative prices at which we sell our products. We regularly monitor our foreign currency risks and periodically take measures to reduce the risk of foreign exchange rate fluctuations on our operating results. We do not use derivative instruments for hedging, trading or speculating on foreign exchange rate fluctuations. Additional discussion of the impact on the effect of currency fluctuations has been included in our management’s discussion and analysis included in Part II, Item 7 of this report.
43
Table of Contents
The following table sets forth a composite sensitivity analysis of our net sales revenue, costs and expenses and operating income in connection with strengthening of the U.S. dollar (our reporting currency) by 10%, 15% and 25% against every other fluctuating functional currency in which we conduct business. We note that our individual net sales revenue, cost and expense components and our operating income were equally sensitive to increases in the strength of the U.S. dollar against every other fluctuating currency in which we conduct business.
35
Table of Contents
Exchange rate sensitivity for the year ended December 31, 20122013 (dollar amounts in thousands)
| | | | With Strengthening of U.S. Dollar by: | | | | | With Strengthening of U.S. Dollar by: | |
| | | | 10% | | 15% | | 25% | | | | | 10% | | 15% | | 25% | |
| | | | ($) | | (%) | | ($) | | (%) | | ($) | | %) | | | | | ($) | | (%) | | ($) | | (%) | | ($) | | %) | |
Net sales revenue | | $ | 367,468 | | $ | (12,821 | ) | (3.5 | )% | $ | (18,395 | ) | (5.0 | )% | $ | (28,206 | ) | (7.7 | )% | | $ | 378,096 | | $ | (13,528 | ) | (3.6 | )% | $ | (19,410 | ) | (5.1 | )% | $ | (29,761 | ) | (7.9 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost and expenses | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cost of sales | | 93,324 | | (3,333 | ) | (3.6 | )% | (4,782 | ) | (5.1 | )% | (7,333 | ) | (7.9 | )% | | 94,814 | | (3,719 | ) | (3.9 | )% | (5,336 | ) | (5.6 | )% | (8,181 | ) | (8.6 | )% |
Volume incentives | | 133,267 | | (4,751 | ) | (3.6 | )% | (6,817 | ) | (5.1 | )% | (10,453 | ) | (7.8 | )% | | 138,482 | | (5,021 | ) | (3.6 | )% | (7,204 | ) | (5.2 | )% | (11,047 | ) | (8.0 | )% |
Selling, general and administrative | | 106,861 | | (3,315 | ) | (3.1 | )% | (4,757 | ) | (4.4 | )% | (7,293 | ) | (6.8 | )% | | 120,743 | | (3,644 | ) | (3.0 | )% | (5,228 | ) | (4.3 | )% | (8,016 | ) | (6.7 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating income | | $ | 34,016 | | $ | (1,422 | ) | (4.2 | )% | $ | (2,039 | ) | (6.0 | )% | $ | (3,127 | ) | (9.2 | )% | | $ | 24,057 | | $ | (1,144 | ) | (4.8 | )% | $ | (1,642 | ) | (6.8 | )% | $ | (2,517 | ) | (10.5 | )% |
Certain of our operations, including Russia and the Ukraine, are served by a U.S. subsidiary through third-party entities, for which all business is conducted in U.S. dollars. Although changes in exchange rates between the U.S. dollar and the Russian ruble or the Ukrainian hryvnia do not result in currency fluctuations within our financial statements, a weakening or strengthening of the U.S. dollar in relation to these other currencies can significantly affect the prices of our products and the purchasing power of our independent Managers, Distributors and Distributorscustomers within these markets.
The following table sets forth a composite sensitivity analysis of our assets and liabilities by those balance sheet line items that are subject to exchange rate risk, together with the total gain or loss from the strengthening of the U.S. dollar in relation to our various fluctuating functional currencies. The sensitivity of our assets and liabilities, taken by balance sheet line items, is somewhat less than the sensitivity of our operating income to increases in the strength of the U.S. dollar in relation to other fluctuating currencies in which we conduct business.
Exchange Rate Sensitivity of Balance Sheet as of December 31, 20122013 (dollar amounts in thousands)
| | | | With Strengthening of U.S. Dollar by: | | | | | With Strengthening of U.S. Dollar by: |
| | | | 10% | | 15% | | 25% | | | | | 10% | | 15% | | 25% | |
| | | | (Loss) ($) | | (Loss) (%) | | (Loss) ($) | | (Loss) (%) | | (Loss) ($) | | (Loss) (%) | | | | | (Loss) ($) | | (Loss) (%) | | (Loss) ($) | | (Loss) (%) | | (Loss) ($) | | (Loss) (%) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Financial Instruments Included in Current Assets Subject to Exchange Rate Risk | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | $ | 79,241 | | $ | (4,582 | ) | (5.8 | )% | $ | (6,575 | ) | (8.3 | )% | $ | (10,081 | ) | (12.7 | )% | | $ | 77,247 | | $ | (2,101 | ) | (2.7 | )% | $ | (3,271 | ) | (4.2 | )% | $ | (5,331 | ) | (6.9 | )% |
Accounts receivable, net | | 9,614 | | (407 | ) | (4.2 | )% | (584 | ) | (6.1 | )% | (896 | ) | (9.3 | )% | | 10,206 | | (257 | ) | (2.5 | )% | (368 | ) | (3.6 | )% | (565 | ) | (5.5 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Financial Instruments Included in Current Liabilities Subject to Exchange Rate Risk | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Accounts payable | | 6,226 | | (65 | ) | (1.1 | )% | (94 | ) | (1.5 | )% | (143 | ) | (2.3 | )% | | 5,664 | | (122 | ) | (2.2 | )% | (175 | ) | (3.1 | )% | (268 | ) | (4.7 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net Financial Instruments Subject to Exchange Rate Risk | | $ | 82,629 | | (4,924 | ) | (6.0 | )% | (7,065 | ) | (8.6 | )% | (10,834 | ) | (13.2 | )% | | $ | 81,789 | | $ | (2,236 | ) | (2.8 | )% | $ | (3,464 | ) | (4.4 | )% | $ | (5,628 | ) | (7.1 | )% |
|
The following table sets forth the local currencies other than the U.S. dollar in which our assets that are subject to exchange rate risk were denominated as of December 31, 2012,2013, and exceeded $1 million upon translation into U.S. dollars. None of our liabilities that are denominated in a local currency other than the U.S. dollar and that are subject to exchange rate risk exceeded $1 million upon translation into U.S. dollars. We use the spot exchange rate for translating balance sheet items from local currencies into our reporting currency. The respective spot exchange rate for each such local currency meeting the foregoing thresholds is provided in the table as well.
3644
Table of Contents
Translation of Balance Sheet Amounts Denominated in Local Currency as of December 31, 20122013 (dollar amounts in thousands)
| | | | At Spot Exchange Rate per | | | | | At Spot Exchange Rate per | |
| | Translated into | | One U.S. Dollar as of | | | Translated into | | One U.S. Dollar as of | |
| | U.S. Dollars | | December 31, 2012 | | | U.S. Dollars | | December 31, 2013 | |
Cash and Cash Equivalents | | | | | | | | | | |
European Markets (Euro) | | | $ | 5,324 | | 0.7 | |
South Korea (Won) | | | 5,246 | | 1,063.3 | |
Venezuela (Bolivar) | | | 3,910 | | 6.3 | |
Japan (Yen) | | $ | 5,089 | | 85.9 | | | 2,519 | | 105.2 | |
Canada (Dollar) | | 3,787 | | 1.0 | | |
European Markets (Euro) | | 3,747 | | 0.8 | | |
Venezuela (Bolivar) | | 1,748 | | 5.3 | | |
United Kingdom (Pound Sterling) | | | 2,466 | | 0.6 | |
Colombia (Peso) | | 1,729 | | 1,770.0 | | | 1,705 | | 1,927.7 | |
Thailand (Baht) | | 1,547 | | 30.8 | | | 1,120 | | 32.9 | |
Indonesia (Rupiah) | | 1,322 | | 9,612.6 | | |
Mexico (Peso) | | 1,223 | | 13.0 | | |
Taiwan (Dollar) | | 1,198 | | 29.1 | | |
Canada (Dollar) | | | 1,087 | | 1.1 | |
Other | | 7,604 | | Varies | | | 6,283 | | Varies | |
Total foreign dominated cash and cash equivalents | | $ | 28,994 | | | | | $ | 29,660 | | | |
U.S. dollars held by foreign subsidiaries | | $ | 20,801 | | | | | $ | 33,352 | | | |
Total foreign cash and cash equivalents | | $ | 49,795 | | | | |
| | | | | | |
Accounts Receivable | | | | | | |
European Markets (Euro) | | $ | 1,900 | | 0.8 | | |
Other | | 2,578 | | Varies | | |
Total | | $ | 4,478 | | | | |
Total cash and cash equivalents held by foreign subsidiaries | | | $ | 63,012 | | | |
Finally, the following table sets forth the annual weighted average of fluctuating currency exchange rates of each of the local currencies per one U.S. dollar for each of the local currencies in which sales revenue exceeded $10.0 million during any of the three years presented. We use the annual average exchange rate for translating items from the statement of operations from local currencies into our reporting currency.
Year ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Canada (Dollar) | | 1.0 | | 1.0 | | 1.0 | | | 1.0 | | 1.0 | | 1.0 | |
European Markets (Euro) | | 0.8 | | 0.7 | | 0.8 | | | 0.8 | | 0.8 | | 0.7 | |
Japan (Yen) | | 79.8 | | 79.7 | | 87.7 | | | 97.4 | | 79.8 | | 79.7 | |
Korea (Won) | | 1,129.6 | | 1,108.6 | | 1,159.1 | | |
South Korea (Won) | | | 1,098.3 | | 1,129.6 | | 1,108.6 | |
Mexico (Peso) | | 13.2 | | 12.4 | | 12.6 | | | 12.8 | | 13.2 | | 12.4 | |
The local currency of the foreign subsidiaries is used as the functional currency, except for subsidiaries operating in highly inflationary economies or where the Company’s operations are served by a U.S. based subsidiary (for example, Russia and the Ukraine). The financial statements of foreign subsidiaries, where the local currency is the functional currency, are translated into U.S. dollars using exchange rates in effect at year-end for assets and liabilities and average exchange rates during each year for the results of operations. Adjustments resulting from translation of financial statements are reflected in accumulated other comprehensive loss, net of income taxes. Foreign currency transaction gains and losses are included in other income (expense) in the consolidated statements of operations.
The functional currency in highly inflationary economies is the U.S. dollar and transactions denominated in the local currency are re-measured as if the functional currency were the U.S. dollar. The re-measurement of local currencies into U.S. dollars creates translation adjustments, which are included in the consolidated statements of operations. A country is considered to have a highly inflationary economy if it has a cumulative inflation rate of approximately 100 percent or more over a three-year period as well as other qualitative factors including historical inflation rate trends (increasing and decreasing), the capital intensiveness of the operation and other pertinent economic factors. During 2010, Venezuela was considered to be highly inflationary, as noted above. During 2012, Belarus was also considered to be highly inflationary. During the periods ended December 31, 2013, 2012 2011 and 2010,2011, the Company’s Venezuelan subsidiary’s net sales revenue represented approximately 2.2 percent, 1.8 percent 1.4 percent and 1.71.4 percent of consolidated net sales revenue, respectively. During the periods ended December 31, 2013, 2012 2011 and 2010,2011, the Company’s Belarusian subsidiary’s net sales revenue represented approximately 2.1 percent, 1.8 percent 1.6 percent and 2.41.6 percent of consolidated net sales revenue, respectively. With the exceptions of Venezuela and Belarus, there were no other countries considered to have a highly inflationary economy during the periods ended December 31, 2013, 2012 2011 and 2010.2011.
Interest Rate Risk
The primary objectives of our investment activities are to preserve principal while maximizing yields without significantly increasing risk. These objectives are accomplished by purchasing investment grade securities. On December 31, 2012,2013, we had investments of $2.1$2.0 million of which $0.6$0.4 million were municipal obligations, which carry an average fixed interest rate of 5.1
37
Table of Contents
percent and mature over a three-yeartwo-year period. A hypothetical 1.0 percent change in interest rates would not have had a material effect on our liquidity, financial position or results of operations.
3845
Table of Contents
Item 8. Financial Statements and Supplementary Data
INDEX TO FINANCIAL STATEMENTS
3946
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Nature’s Sunshine Products, Inc.
We have audited the accompanying consolidated balance sheets of Nature’s Sunshine Products, Inc. and subsidiaries (the “Company”) as of December 31, 20122013 and 2011,2012, and the related consolidated statements of operations, comprehensive income, changes in shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2012.2013. Our audits also included the consolidated financial statement schedule listed in the Index at Item 15. These consolidated financial statements and consolidated financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on the consolidated financial statements and consolidated financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of Nature’s Sunshine Products, Inc. and subsidiaries as of December 31, 20122013 and 2011,2012, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2012,2013, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Note 2 to the consolidated financial statements, the Company changed its classification of shipping and handling costs from selling, general and administrative expenses to cost of sales in the consolidated statements of operations during the year ended December 31, 2012.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2012,2013, based on the criteria established in Internal Control — Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 6, 201317, 2014 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ Deloitte & Touche LLP | |
| |
Salt Lake City, Utah | |
March 6, 201317, 2014 | |
4047
Table of Contents
NATURE’S SUNSHINE PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(Amounts in thousands)
As of December 31, | | 2012 | | 2011 | | | 2013 | | 2012 | |
Assets | | | | | | | | | | |
Current assets: | | | | | | | | | | |
Cash and cash equivalents | | $ | 79,241 | | $ | 58,969 | | | $ | 77,247 | | $ | 79,241 | |
Accounts receivable, net of allowance for doubtful accounts of $631 and $647, respectively | | 9,614 | | 9,868 | | |
Accounts receivable, net of allowance for doubtful accounts of $1,087 and $631, respectively | | | 10,206 | | 9,614 | |
Investments available for sale | | 2,071 | | 5,677 | | | 2,006 | | 2,071 | |
Inventories | | 43,280 | | 41,611 | | | 41,910 | | 43,280 | |
Deferred income tax assets | | 5,307 | | 4,395 | | | 5,711 | | 5,307 | |
Prepaid expenses and other | | 5,820 | | 4,583 | | | 11,514 | | 5,820 | |
Total current assets | | 145,333 | | 125,103 | | | 148,594 | | 145,333 | |
| | | | | | | | | | |
Property, plant and equipment, net | | 27,950 | | 25,137 | | | 32,022 | | 27,950 | |
Investment securities | | 1,276 | | 1,429 | | |
Investment securities - trading | | | 971 | | 1,276 | |
Intangible assets, net | | 1,002 | | 1,151 | | | 853 | | 1,002 | |
Deferred income tax assets | | 11,516 | | 16,576 | | | 9,928 | | 11,516 | |
Other assets | | 6,842 | | 6,415 | | | 7,244 | | 6,842 | |
| | $ | 193,919 | | $ | 175,811 | | | $ | 199,612 | | $ | 193,919 | |
| | | | | | | | | | |
Liabilities and Shareholders’ Equity | | | | | | | | | | |
Current liabilities: | | | | | | | | | | |
Accounts payable | | $ | 6,226 | | $ | 5,980 | | | $ | 5,664 | | $ | 6,226 | |
Accrued volume incentives | | 18,130 | | 19,326 | | | 19,206 | | 18,130 | |
Accrued liabilities | | 27,302 | | 27,938 | | | 34,893 | | 27,302 | |
Deferred revenue | | 4,311 | | 2,603 | | | 4,173 | | 4,311 | |
Current installments of long-term debt | | 3,350 | | 3,296 | | |
Current installments of long-term debt and revolving credit facility | | | 2,267 | | 3,350 | |
Income taxes payable | | 2,071 | | 8,655 | | | 2,366 | | 2,071 | |
Total current liabilities | | 61,390 | | 67,798 | | | 68,569 | | 61,390 | |
| | | | | | | | | | |
Liability related to unrecognized tax benefits | | 10,571 | | 10,426 | | | 12,402 | | 10,571 | |
Long-term debt | | 2,270 | | 5,894 | | |
Long-term debt and revolving credit facility | | | 10,000 | | 2,270 | |
Deferred compensation payable | | 1,276 | | 1,429 | | | 971 | | 1,276 | |
Other liabilities | | 2,776 | | 2,826 | | | 2,411 | | 2,776 | |
Total long-term liabilities | | 16,893 | | 20,575 | | | 25,784 | | 16,893 | |
| | | | | | | | | | |
Commitments and Contingencies | | | | | | | | | | |
| | | | | | | | | | |
Shareholders’ equity: | | | | | | | | | | |
Common stock, no par value; 50,000 shares authorized, 15,810 and 15,569 shares issued and outstanding as of December 31, 2012 and 2011, respectively | | 77,292 | | 71,628 | | |
Common stock, no par value; 50,000 shares authorized, 16,179 and 15,810 shares issued and outstanding as of December 31, 2013 and 2012, respectively | | | 83,122 | | 77,292 | |
Retained earnings | | 48,910 | | 25,879 | | | 36,100 | | 48,910 | |
Accumulated other comprehensive loss | | (10,566 | ) | (10,069 | ) | | (13,963 | ) | (10,566 | ) |
Total shareholders’ equity | | 115,636 | | 87,438 | | | 105,259 | | 115,636 | |
| | $ | 193,919 | | $ | 175,811 | | | $ | 199,612 | | $ | 193,919 | |
See accompanying notes to consolidated financial statements.
4148
Table of Contents
NATURE’S SUNSHINE PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except per share information)
Year Ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Net sales revenue (net of the rebate portion of volume incentives of $43,121, $44,628, and $44,099, respectively) | | $ | 367,468 | | $ | 367,813 | | $ | 349,918 | | |
Net sales revenue | | | $ | 378,096 | | $ | 367,468 | | $ | 367,813 | |
Cost of sales | | (93,324 | ) | (89,409 | ) | (89,264 | ) | | (94,814 | ) | (93,324 | ) | (89,409 | ) |
Gross profit | | 274,144 | | 278,404 | | 260,654 | | | 283,282 | | 274,144 | | 278,404 | |
| | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | |
Volume incentives | | 133,267 | | 133,883 | | 130,367 | | | 138,482 | | 133,267 | | 133,883 | |
Selling, general and administrative | | 106,861 | | 109,606 | | 119,024 | | | 120,743 | | 106,861 | | 109,606 | |
Contract termination costs | | — | | 14,750 | | — | | | — | | — | | 14,750 | |
| | 240,128 | | 258,239 | | 249,391 | | |
Operating income | | 34,016 | | 20,165 | | 11,263 | | | 24,057 | | 34,016 | | 20,165 | |
Other income: | | | | | | | | | | | | | | |
Interest and other income, net | | 1,368 | | 1,470 | | 270 | | | 573 | | 1,368 | | 1,470 | |
Interest expense | | (178 | ) | (89 | ) | — | | | (231 | ) | (178 | ) | (89 | ) |
Foreign exchange gains, net | | 290 | | 466 | | 2,457 | | | 1,254 | | 290 | | 466 | |
| | 1,480 | | 1,847 | | 2,727 | | | 1,596 | | 1,480 | | 1,847 | |
Income before provision for income taxes | | 35,496 | | 22,012 | | 13,990 | | | 25,653 | | 35,496 | | 22,012 | |
Provision for income taxes | | 10,116 | | 4,411 | | 5,521 | | | 8,044 | | 10,116 | | 4,411 | |
Net income from continuing operations | | 25,380 | | 17,601 | | 8,469 | | |
Loss from discontinued operations | | — | | — | | (9,702 | ) | |
Net income (loss) | | $ | 25,380 | | $ | 17,601 | | $ | (1,233 | ) | |
Net income | | | $ | 17,609 | | $ | 25,380 | | $ | 17,601 | |
| | | | | | | | | | | | | | |
Basic and diluted net income (loss) per common share | | | | | | | | |
Basic and diluted net income per common share | | | | | | | | |
| | | | | | | | | | | | | | |
Basic: | | | | | | | | | | | | | | |
Net income from continuing operations | | $ | 1.62 | | $ | 1.13 | | $ | 0.55 | | |
Loss from discontinued operations | | $ | — | | $ | — | | $ | (0.63 | ) | |
Net income (loss) | | $ | 1.62 | | $ | 1.13 | | $ | (0.08 | ) | |
Net income | | | $ | 1.10 | | $ | 1.62 | | $ | 1.13 | |
| | | | | | | | | | | | | | |
Diluted: | | | | | | | | | | | | | | |
Net income from continuing operations | | $ | 1.59 | | $ | 1.12 | | $ | 0.54 | | |
Loss from discontinued operations | | $ | — | | $ | — | | $ | (0.62 | ) | |
Net income (loss) | | $ | 1.59 | | $ | 1.12 | | $ | (0.08 | ) | |
Net income | | | $ | 1.07 | | $ | 1.59 | | $ | 1.12 | |
| | | | | | | | | | | | | | |
Weighted average basic common shares outstanding | | 15,648 | | 15,550 | | 15,515 | | | 15,997 | | 15,648 | | 15,550 | |
Weighted average diluted common shares outstanding | | 15,987 | | 15,695 | | 15,605 | | | 16,390 | | 15,987 | | 15,695 | |
| | | | | | | | | | | | | | |
Dividends declared per common share | | $ | 0.15 | | $ | — | | $ | — | | | $ | 1.90 | | $ | 0.15 | | $ | — | |
See accompanying notes to consolidated financial statements.
NATURE’S SUNSHINE PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(Amounts in thousands)
Year Ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
| | | | | | | | | | | | | | |
Net income (loss) | | $ | 25,380 | | $ | 17,601 | | $ | (1,233 | ) | |
Foreign currency translation gain (loss) (net of tax) | | (522 | ) | (2,397 | ) | 4,591 | | |
Net income | | | $ | 17,609 | | $ | 25,380 | | $ | 17,601 | |
Foreign currency translation loss (net of tax) | | | (3,480 | ) | (522 | ) | (2,397 | ) |
Net unrealized gains (losses) on investment securities (net of tax) | | 25 | | (24 | ) | (4 | ) | | 83 | | 25 | | (24 | ) |
Write-off of Brazil cumulative translation adjustments | | — | | — | | 7,364 | | |
Total comprehensive income | | $ | 24,883 | | $ | 15,180 | | $ | 10,718 | | | $ | 14,212 | | $ | 24,883 | | $ | 15,180 | |
See accompanying notes to consolidated financial statements.
4249
Table of Contents
NATURE’S SUNSHINE PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
(Amounts in thousands, except per share data)
| | Common Stock | | Retained | | Accumulated
Other
Comprehensive | | | | | Common Stock | | Retained | | Accumulated
Other
Comprehensive | | | |
| | Shares | | Value | | Earnings | | Income (Loss) | | Total | | | Shares | | Value | | Earnings | | Income (Loss) | | Total | |
Balance at January 1, 2010 | | 15,510 | | $ | 67,183 | | $ | 9,511 | | $ | (19,599 | ) | $ | 57,095 | | |
Share-based compensation expense | | — | | 437 | | — | | — | | 437 | | |
Proceeds from the exercise of stock options | | 23 | | 132 | | — | | — | | 132 | | |
Net loss | | — | | — | | (1,233 | ) | — | | (1,233 | ) | |
Other comprehensive income | | — | | — | | — | | 11,951 | | 11,951 | | |
Balance at December 31, 2010 | | 15,533 | | 67,752 | | 8,278 | | (7,648 | ) | 68,382 | | |
Balance at January 1, 2011 | | | 15,533 | | $ | 67,752 | | $ | 8,278 | | $ | (7,648 | ) | $ | 68,382 | |
Share-based compensation expense | | — | | 3,478 | | — | | — | | 3,478 | | | — | | 3,478 | | — | | — | | 3,478 | |
Proceeds from the exercise of stock options | | 36 | | 398 | | — | | — | | 398 | | | 36 | | 398 | | — | | — | | 398 | |
Net income | | — | | — | | 17,601 | | — | | 17,601 | | | — | | — | | 17,601 | | — | | 17,601 | |
Other comprehensive loss | | — | | — | | — | | (2,421 | ) | (2,421 | ) | |
Other comprehensive income | | | — | | — | | — | | (2,421 | ) | (2,421 | ) |
Balance at December 31, 2011 | | 15,569 | | 71,628 | | 25,879 | | (10,069 | ) | 87,438 | | | 15,569 | | 71,628 | | 25,879 | | (10,069 | ) | 87,438 | |
Share-based compensation expense | | — | | 2,878 | | — | | — | | 2,878 | | | — | | 2,878 | | — | | — | | 2,878 | |
Tax benefit from exercise of stock options | | — | | 378 | | — | | — | | 378 | | | — | | 378 | | — | | — | | 378 | |
Proceeds from the exercise of stock options | | 241 | | 2,408 | | — | | — | | 2,408 | | | 241 | | 2,408 | | — | | — | | 2,408 | |
Cash dividends (0.15 per share) | | — | | — | | (2,349 | ) | — | | (2,349 | ) | | — | | — | | (2,349 | ) | — | | (2,349 | ) |
Net income | | — | | — | | 25,380 | | — | | 25,380 | | | — | | — | | 25,380 | | — | | 25,380 | |
Other comprehensive loss | | — | | — | | — | | (497 | ) | (497 | ) | | — | | — | | — | | (497 | ) | (497 | ) |
Balance at December 31, 2012 | | 15,810 | | $ | 77,292 | | $ | 48,910 | | $ | (10,566 | ) | $ | 115,636 | | | 15,810 | | 77,292 | | 48,910 | | (10,566 | ) | 115,636 | |
Share-based compensation expense | | | — | | 3,389 | | — | | — | | 3,389 | |
Tax benefit from exercise of stock options | | | — | | 653 | | — | | — | | 653 | |
Proceeds from the exercise of stock options | | | 509 | | 4,334 | | — | | — | | 4,334 | |
Repurchase of common stock | | | (140 | ) | (2,546 | ) | — | | — | | (2,546 | ) |
Cash dividends (1.90 per share) | | | — | | — | | (30,419 | ) | — | | (30,419 | ) |
Net income | | | — | | — | | 17,609 | | — | | 17,609 | |
Other comprehensive loss | | | — | | — | | — | | (3,397 | ) | (3,397 | ) |
Balance at December 31, 2013 | | | 16,179 | | $ | 83,122 | | $ | 36,100 | | $ | (13,963 | ) | $ | 105,259 | |
See accompanying notes to consolidated financial statements.
4350
Table of Contents
NATURE’S SUNSHINE PRODUCTS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts Inin Thousands)
Year Ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | | | | | | | |
Net income (loss) | | $ | 25,380 | | $ | 17,601 | | $ | (1,233 | ) | |
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | | | | | | | | |
Write-off of cumulative translation adjustments | | — | | — | | 7,364 | | |
Net income | | | $ | 17,609 | | $ | 25,380 | | $ | 17,601 | |
Adjustments to reconcile net income to net cash provided by operating activities: | | | | | | | | |
Provision for doubtful accounts | | 45 | | (133 | ) | 213 | | | 535 | | 45 | | (133 | ) |
Depreciation and amortization | | 4,078 | | 4,362 | | 4,254 | | | 4,466 | | 4,078 | | 4,362 | |
Tax benefit from stock option exercise | | (378 | ) | — | | — | | | (653 | ) | (378 | ) | — | |
Share-based compensation expense | | 2,878 | | 3,478 | | 437 | | | 3,389 | | 2,878 | | 3,478 | |
Loss on sale of property and equipment | | 85 | | 224 | | 138 | | |
(Gain) loss on sale of property and equipment | | | (128 | ) | 85 | | 224 | |
Deferred income taxes | | 4,270 | | (5,073 | ) | 2,128 | | | 1,092 | | 4,270 | | (5,073 | ) |
Loss on restricted cash | | — | | — | | 747 | | |
Amortization of bond discount | | 9 | | 9 | | 22 | | | 1 | | 9 | | 9 | |
Purchase of trading investment securities | | (92 | ) | (102 | ) | (177 | ) | | (88 | ) | (92 | ) | (102 | ) |
Proceeds from sale of trading investment securities | | 354 | | 438 | | 313 | | | 510 | | 354 | | 438 | |
Realized and unrealized gains | | (90 | ) | (44 | ) | (165 | ) | |
Amortization of prepaid taxes related to gain on intercompany sales | | — | | — | | 1,353 | | |
Realized and unrealized gains on investments | | | (122 | ) | (90 | ) | (44 | ) |
Foreign exchange gains | | (290 | ) | (466 | ) | (3,005 | ) | | (1,254 | ) | (290 | ) | (466 | ) |
Changes in assets and liabilities: | | | | | | | | | | | | | | |
Accounts receivable | | 266 | | (3,742 | ) | 2,181 | | | (1,358 | ) | 266 | | (3,742 | ) |
Inventories | | (1,466 | ) | (5,566 | ) | 4,732 | | | 838 | | (1,466 | ) | (5,566 | ) |
Prepaid expenses and other | | (1,155 | ) | 1,032 | | (238 | ) | | (5,728 | ) | (1,155 | ) | 1,032 | |
Other assets | | (193 | ) | 2,986 | | (80 | ) | | (303 | ) | (193 | ) | 2,986 | |
Accounts payable | | 77 | | 1,316 | | 588 | | | (552 | ) | 77 | | 1,316 | |
Accrued volume incentives | | (1,279 | ) | 805 | | 1,163 | | | 1,286 | | (1,279 | ) | 805 | |
Accrued current and other long-term liabilities | | (1,289 | ) | (6,152 | ) | 619 | | |
Accrued liabilities | | | 7,379 | | (1,289 | ) | (6,152 | ) |
Deferred revenue | | 1,708 | | (782 | ) | (1,128 | ) | | (138 | ) | 1,708 | | (782 | ) |
Income taxes payable | | (6,259 | ) | 5,009 | | (3,679 | ) | | 1,071 | | (6,259 | ) | 5,009 | |
Liability related to unrecognized tax positions | | 145 | | (10,943 | ) | (423 | ) | | 1,831 | | 145 | | (10,943 | ) |
Deferred compensation payable | | (153 | ) | (349 | ) | 26 | | | (305 | ) | (153 | ) | (349 | ) |
Net cash provided by operating activities | | 26,651 | | 3,908 | | 16,150 | | | 29,378 | | 26,651 | | 3,908 | |
CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | | | | | | | |
Purchases of property, plant and equipment | | (6,629 | ) | (2,419 | ) | (2,595 | ) | | (8,570 | ) | (6,629 | ) | (2,419 | ) |
Purchases of investments available for sale | | (174 | ) | (6,968 | ) | (3,439 | ) | | (442 | ) | (174 | ) | (6,968 | ) |
Proceeds from sale/maturities of investments available for sale | | 3,789 | | 7,697 | | 125 | | | 200 | | 3,789 | | 7,697 | |
Proceeds from sale of property, plant and equipment | | 25 | | 11 | | — | | | 248 | | 25 | | 11 | |
Net cash used in investing activities | | (2,989 | ) | (1,679 | ) | (5,909 | ) | | (8,564 | ) | (2,989 | ) | (1,679 | ) |
CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | | | | | | | |
Payments of cash dividends | | (2,349 | ) | — | | — | | | (30,419 | ) | (2,349 | ) | — | |
Proceeds from issuance of long-term debt | | — | | 10,000 | | — | | |
Principal payments of long-term debt | | (3,570 | ) | (810 | ) | — | | |
Borrowings on long-term debt and revolving credit facility | | | 10,000 | | — | | 10,000 | |
Principal payments of long-term debt and revolving credit facility | | | (3,353 | ) | (3,570 | ) | (810 | ) |
Tax benefit from stock option exercise | | 378 | | — | | — | | | 653 | | 378 | | — | |
Proceeds from exercise of stock options | | 2,408 | | 398 | | 132 | | | 4,334 | | 2,408 | | 398 | |
Repurchase of common stock | | | (2,546 | ) | — | | — | |
Net cash provided by (used in) financing activities | | (3,133 | ) | 9,588 | | 132 | | | (21,331 | ) | (3,133 | ) | 9,588 | |
Effect of exchange rates on cash and cash equivalents | | (257 | ) | (452 | ) | 1,693 | | | (1,477 | ) | (257 | ) | (452 | ) |
Net increase in cash and cash equivalents | | 20,272 | | 11,365 | | 12,066 | | |
Net increase (decrease) in cash and cash equivalents | | | (1,994 | ) | 20,272 | | 11,365 | |
Cash and cash equivalents at beginning of the year | | 58,969 | | 47,604 | | 35,538 | | | 79,241 | | 58,969 | | 47,604 | |
Cash and cash equivalents at end of the year | | $ | 79,241 | | $ | 58,969 | | $ | 47,604 | | | $ | 77,247 | | $ | 79,241 | | $ | 58,969 | |
4451
Table of Contents
Year Ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Supplemental disclosure of cash flow information: | | | | | | | | | | | | | | |
Cash paid for income taxes | | $ | 12,960 | | $ | 6,570 | | $ | 5,627 | | | $ | 10,278 | | $ | 12,960 | | $ | 6,570 | |
Cash paid for interest | | 128 | | 36 | | — | | | 128 | | 128 | | 36 | |
Supplemental disclosure of noncash investing and financing activities: | | | | | | | | | | | | | | |
Purchases of property, plant and equipment included in accounts payable | | $ | 169 | | $ | 19 | | $ | 196 | | | $ | 155 | | $ | 169 | | $ | 19 | |
See accompanying notes to consolidated financial statements.
4552
Table of Contents
NATURE’S SUNSHINE PRODUCTS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in thousands, except per share information)
NOTE 1: NATURE OF OPERATIONS AND SIGNIFICANT ACCOUNTING POLICIES
Nature of Operations
Nature’s Sunshine Products, Inc., together with its subsidiaries (hereinafter referred to collectively as the “Company”), is a natural health and wellness company primarily engaged in the manufacturing and direct selling of nutritional and personal care products. Nature’s Sunshine Products, Inc.The Company is a Utah corporation with its principal place of business in Lehi, Utah. The CompanyUtah and sells its products to a sales force of independent Managers and Distributors who use the products themselves or resell them to other Distributors or consumers. The formulation, manufacturing, packaging, labeling, advertising, distribution and sale of each of the Company’s major product groups are subject to regulation by one or more governmental agencies.
The Company markets its products in Australia, Austria, Belarus, Canada, Colombia, Costa Rica, the Czech Republic, Denmark, the Dominican Republic, Ecuador, El Salvador, Finland, Germany, Guatemala, Honduras, Hong Kong, Iceland, Indonesia, Ireland, Italy, Japan, Kazakhstan, Latvia, Lithuania, Malaysia, Mexico, Moldova, Mongolia, the Netherlands, Nicaragua, Norway, Panama, Peru, the Philippines, Poland, Russia, Singapore, Slovenia, South Korea, Spain, Sweden, Taiwan, Thailand, the Ukraine, the United Kingdom, the United States, Venezuela and Vietnam. The Company also exports its products to several other countries, including Argentina, Australia, Chile, Israel, New Zealand and Norway.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts and transactions of the Company and its subsidiaries. At December 31, 20122013 and 2011,2012, substantially all of the Company’s subsidiaries were wholly owned. The Company operates a limited number of markets in jurisdictions where local laws require the formation of a partnership with an entity domiciled in that market. These partners have no rights to participate in the sharing of revenues, profits, losses or distribution of assets upon liquidation of these partnerships.
Intercompany balances and transactions have been eliminated in consolidation.
Discontinued Operations
As more fully described in Note 3, “Discontinued Operations,” the Company ceased the operations of its Brazilian subsidiary in the third quarter of 2010, and the results of its operations are classified as discontinued operations in the Company’s statements of operations and accompanying notes for all periods presented.
Use of Estimates
The preparation of consolidated financial statements in accordance with accounting principles generally accepted in the United States (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities, in these financial statements and accompanying notes. Actual results could differ from these estimates and those differences could have a material effect on the Company’s financial position and results of operations.
The significant accounting estimates inherent in the preparation of the Company’s financial statements include estimates associated with its evaluation of impairment of long-lived assets, the determination of liabilities related to Manager and Distributor incentives, the determination of income tax assets and liabilities, certain other non-income tax and value-added tax contingencies, legal contingencies, share-based compensation and the valuation of investments. In addition, significant estimates form the basis for allowances with respect to the collection of accounts receivable, inventory valuations and self-insurance liabilities associated with product liability and medical claims. Various assumptions and other factors enter into the determination of these significant estimates. The process of determining significant estimates takes into account historical experience and current and expected economic conditions.
53
Table of Contents
Classification of Venezuela as a Highly Inflationary Economy and Devaluation of Its Currency
Since January 1, 2010, Venezuela has been designated as a highly inflationary economy. Accordingly, the U.S. dollar became the functional currency for the Company’s subsidiary in Venezuela. All gains and losses resulting from the re-measurement of its financial statements are determined using official rates.
46
Table On February 11, 2013, the Venezuelan government announced the further devaluation of Contentsthe bolivar to 6.3 bolivars per U.S. dollar.
Currency restrictions enacted by the government of Venezuela require approval from the government’s currency control agency organization(“CADIVI”) in order for the Company’s subsidiary in Venezuela to obtain U.S. dollars at the official exchange rate to pay for imported products or to repatriate dividends back to the Company. The government of Venezuela enacted additional currency restrictions, which effectively replacedPrior to January 1, 2010, the market rate, withwhich is substantially lower than the official rate, was available to obtain U.S. dollars or other currencies without approval of the CADIVI. In 2013, the CADIVI established a new official exchange rate of 6.3 bolivars per U.S. dollar, and mandated that entities domiciled in Venezuela must formerly apply and be approved by the CADIVI to obtain U.S. dollars or U.S. dollar denominated securities through banking institutions approved by the government at the official CADIVI exchange rate of 6.3. On a daily basis, the CADIVI will determine how many U.S. dollars will be sold and which previously approved companies are authorized to buy. Subsequently, the Venezuela Central Bank will pay the foreign entities directly to limit the amount of U.S. dollars available within Venezuela.
The Company re-measures its results in Venezuela at the official CADIVI rate, which was approximately 6.3 bolivars per U.S. dollar as of December 31, 2013. However, since the further devaluation of the bolivar to 6.3, the Company has been unsuccessful in obtaining U.S. dollars at the official CADIVI exchange rate and it remains uncertain when the Company’s applications will be approved to complete exchange transactions in the future.
In addition to the further devaluation of the bolivar in 2013, the CADIVI enacted a new currency exchange mechanism, the Complementary System for Foreign Currency Administration (“SICAD”), to replace the System for Foreign Currency Denominated Securities (“SITME”), which is administeredwas enacted in 2010 to end the trading of currency at the market rate. Under SICAD, the Company will be able to obtain U.S. dollars at a higher unofficial exchange rate determined by the Venezuela Central Bank. Under SITME, entities domiciled in Venezuela can obtain U.S. dollar denominated securities in limited quantities each month through banking institutions approved byCADIVI. The Company is currently monitoring the government.SICAD exchange mechanism and the potential for exchange transactions should the Company’s applications to exchange at the official CADIVI rate of 6.3 be unsuccessful.
During 2013, 2012 and 2011, the Company’s Venezuelan subsidiary’s net sales revenue represented approximately 2.2 percent, 1.8 percent and 1.4 percent of consolidated net sales revenue, respectively. As of December 31, 2013 and 2012, the Company’s Venezuelan subsidiary held cash and cash equivalents of $3,922 and $1,748, respectively. At this time, the Company is not able to reasonably estimate the future state of exchange controls in Venezuela and its availability of U.S. dollars at the official CADIVI exchange rate or at the SITME rate. However, the Company has been successful so far repatriating funds from Venezuela for imported products using the SITME rate. The Company re-measures its results in Venezuela at the SITME rate, which was approximately 5.3 bolivars per U.S. dollar as of December 31, 2011. On February 12, 2013, the Venezuelan government announced the devaluation of the bolivar official rate to 6.3.
During 2012, 2011 and 2010, the Company’s Venezuelan subsidiary’s net sales revenue represented approximately 1.8 percent, 1.4 percent and 1.7 percent of consolidated net sales revenue, respectively. The Company’s Venezuelan subsidiary held total assets of $8,306 and $8,101 (which includes an intercompany receivables denominated in U.S. dollars of $2,251 and $2,110) and net assets of $5,633 and $5,020 at December 31, 2012 and 2011, respectively.
Due to the rising uncertainty in the Company’s ability to recover certain cash balances in Venezuela, the Company recorded a charge of $747 during the year ended December 31, 2010, to write-off the remaining cash balance, which is included in other expense. However, this cash balance of $747 was recovered during the year ended December 31, 2011 and a gain was recorded in other income and expense.
Classification of Belarus as a Highly Inflationary Economy and Devaluation of Its Currency
As ofSince June 30, 2012, Belarus washas been designated as a highly inflationary economy. Historically, theThe U.S. dollar has beenis the Company’s functional currency for this market. As a result, there were no resulting gains or losses from a re-measurement of the financial statements using official rates of the Company’s Belarusian subsidiary. However, as a result of the weakening of the Belarusian ruble, the purchasing power of ourthe Company’s Distributors in this market has become diminished. During the periods ended December 31, 2013, 2012 2011 and 2010,2011, the Company’s Belarusian subsidiary’s net sales revenue represented approximately 2.1 percent, 1.8 percent 1.6 percent and 2.41.6 percent of consolidated net sales revenue, respectively. The Company’s total and net assets related to Belarus as of December 31, 2012 and 2011 were insignificant.
Cash and Cash Equivalents
The Company considers all highly liquid short-term investments with original maturities of three months or less to be cash equivalents. Substantially all of the Company’s cash deposits either exceed the United States federally insured limit or are located in countries that do not have government insured accounts or are subject to tax withholdings when repatriating earnings.
Accounts Receivable
Accounts receivable consist principally of receivables from credit card companies, arising from the sale of products to the Company’s Distributors, and receivables from Distributors in foreign markets. Accounts receivable have been reduced by an allowance for amounts that may be uncollectible in the future. However, due to the geographic dispersion of credit card and Distributor receivables, the collection risk is not considered to be significant. Substantially all of the receivables from credit card companies were current as of December 31, 20122013 and 2011.2012. Although receivables from Distributors can be significant, the Company performs ongoing credit evaluations of its importers and maintains an allowance for potential credit losses. This estimated allowance
54
Table of Contents
is based primarily on the aging category, historical trends and management’s evaluation of the financial condition of the customer. This reserve is adjusted periodically as information about specific accounts becomes available.
Investment Securities
The Company’s available-for-sale investment portfolio is recorded at fair value and consists of various securities such as state and municipal obligations, U.S. government security funds, short-term deposits and various equity securities. These investments are valued using (a) quoted prices for identical assets in active markets or (b) from significant inputs that are observable or can be derived from or corroborated by observable market data for substantially the full term of the asset. The Company’s trading portfolio is recorded at fair value and consists of various marketable securities that are valued using quoted prices in active markets.
For available-for-sale debt securities with unrealized losses, the Company performs an analysis to assess whether it intends to sell or whether it would be more likely than not required to sell the security before the expected recovery of the amortized cost basis. Where the Company intends to sell a security, or may be required to do so, the security’s decline in fair value is deemed to be other-than-temporary, and the full amount of the unrealized loss is recorded within earnings as an impairment loss.
47
Table of Contents
For all other debt securities that experience a decline in fair value that is determined to be other-than-temporary and not related to credit loss, the Company records a loss, net of any tax, in accumulated other comprehensive income (loss). The credit loss is recorded within earnings as an impairment loss when sold. Management judgment is involved in evaluating whether a decline in an investment’s fair value is other-than-temporary.
Regardless of the Company’s intent to sell a security, the Company performs additional analysis on all securities with unrealized losses to evaluate losses associated with the creditworthiness of the security. Credit losses are identified where we dothe Company does not expect to receive cash flows sufficient to recover the amortized cost basis of a security.
For equity securities, when assessing whether a decline in fair value below ourthe Company’s cost basis is other-than-temporary, the Company considers the fair market value of the security, the length of time and extent to which market value has been less than cost, the financial condition and near-term prospects of the issuer as well as specific events or circumstances that may influence the operations of the issuer, and ourthe Company’s intent and ability to hold the investment for a sufficient time in order to enable recovery of ourthe cost. New information and the passage of time can change these judgments. Where the Company has determined that the Companyit lacks the intent and ability to hold an equity security to its expected recovery, the security’s decline in fair value is deemed to be other-than-temporary and is recorded within earnings as an impairment loss.
The Company also has certain investment securities classified as trading securities. The Company maintains its trading securities portfolio to generate returns that are offset by corresponding changes in certain liabilities related to the Company’s deferred compensation plans (see Note 13)11). The trading securities portfolio consists of marketable securities, which are recorded at fair value and are included in long-term investment securities on the consolidated balance sheets because they remain assets of the Company until they are actually paid out to the participants. These investment securities are not available to the Company to fund its operations as they are restricted for the payment of the deferred compensation payable. The Company has established a rabbi trust to finance obligations under the plan. Both realized and unrealized gains and losses on trading securities are included in interest and other income.
Fair Value of Financial Instruments
The Company’s financial instruments consist primarily of cash and cash equivalents, accounts receivable, investments, accounts payable and long-term debt. Other than investments, which are carried at fair value, and long-term debt, the carrying values of these financial instruments approximate their fair values due to their short-term nature. The carrying amount reflected in the consolidated balance sheet for long-term debt approximates fair value due to the interest rate on the debt being variable based on current market rates. During the year ended December 31, 20122013 and 2011,2012, the Company did not have any write-offs related to the re-measurement of non-financial assets at fair value on a nonrecurring basis subsequent to their initial recognition.
Inventories
Inventories are stated at the lower-of-cost-or-market, using the first-in, first-out method. The components of inventory cost include raw materials, labor and overhead. To estimate any necessary obsolescence or lower-of-cost-or-market adjustments, various assumptions are made in regard to excess or slow-moving inventories, non-conforming inventories, expiration dates, current and future product demand, production planning and market conditions.
55
Table of Contents
Property, Plant and Equipment
Property, plant and equipment are recorded at cost less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the related assets. Estimated useful lives for buildings range from 20 to 50 years; building improvements range from 7 to 10 years; machinery and equipment range from 2 to 10 years; computer software and hardware range from 3 to 10 years; and furniture and fixtures range from 2 to 5 years. Leasehold improvements are amortized over the shorter of the lease term or the estimated useful lives of the related assets. Maintenance and repairs are expensed as incurred and major improvements are capitalized.
Intangible Assets
Intangible assets consist of purchased product formulations. Such intangible assets are amortized using the straight-line method over the estimated economic lives of the assets of 9 to 15 years. Intangible assets, net of accumulated amortization, totaled $1,002$853 and $1,151$1,002 at December 31, 20122013 and 2011,2012, respectively.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets, such as property, plant and equipment and intangible assets for impairment when events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. The Company uses an estimate of future undiscounted net cash flows of the related assets or groups of assets over their remaining lives in measuring whether the assets are recoverable. An impairment loss is calculated by determining the difference between the carrying values and the fair values of these assets. The Company did not consider any of its long-lived assets to be impaired during the years ended December 31, 2013, 2012 2011 or 2010.
48
Table of Contents2011.
Incentive Trip Accrual
The Company accrues expenses for incentive tripsexpenses associated with its direct sales marketing program, which rewards independent Managers and Distributors with paid attendance at itsfor incentive trips, including Company conventions and meetings. Expenses associated with incentive trips are accrued over qualification periods as they are earned. The Company specifically analyzes incentive trip accruals based on historical and current sales trends as well as contractual obligations when evaluating the adequacy of the incentive trip accrual. Actual results could result ingenerate liabilities being more or less than the amounts recorded. The Company has accrued convention and meeting costs of $4,624$5,784 and $5,047$4,624 at December 31, 20122013 and 2011,2012, respectively, which are included in accrued liabilities in the consolidated balance sheets.
Foreign Currency Translation
The local currency of the foreign subsidiaries is used as the functional currency, except for subsidiaries operating in highly inflationary economies or where the Company’s operations are served by a U.S. based subsidiary (for example Belarus, Russia and the Ukraine). The financial statements of foreign subsidiaries where the local currency is the functional currency are translated into U.S. dollars using exchange rates in effect at year end for assets and liabilities and average exchange rates during each year for the results of operations. Adjustments resulting from translation of financial statements are reflected in accumulated other comprehensive loss, net of income taxes. Foreign currency transaction gains and losses are included in other income (expense) in the consolidated statements of operations.
The functional currency in highly inflationary economies is the U.S. dollar and transactions denominated in the local currency are re-measured as if the functional currency were the U.S. dollar. The re-measurement of local currencies into U.S. dollars creates translation adjustments, which are included in the consolidated statements of operations. A country is considered to have a highly inflationary economy if it has a cumulative inflation rate of approximately 100 percent or more over a three year period as well as other qualitative factors including historical inflation rate trends (increasing and decreasing), the capital intensiveness of the operation, and other pertinent economic factors. During 2012 and 2010, Belarus and Venezuela respectively, were considered to be highly inflationary as noted above. With the exception of Belarus and Venezuela, there were no countries considered to have a highly inflationary economy during 2013, 2012 2011 or 2010.2011.
Revenue Recognition
Net sales revenue and related volume incentive expenses are recorded when persuasive evidence of an arrangement exists, collectability is reasonably assured, the amount is fixed and determinable, and title and risk of loss have passed, generally when the merchandise has been delivered.passed. The amount of the volume incentive is determined based upon the amount of qualifying purchases in a given month. It is necessary for the Company to make estimates about the timing of when merchandise has been delivered. These estimates are based upon the Company’s historical
56
Table of Contents
experience related to time in transit, timing of when shipments occurred and shipping volumes. Amounts received for undelivered merchandise are recorded as deferred revenue.
From time to time, the Company’s U.S. operations extend short-term credit associated with product promotions. In addition for certain of the Company’s international operations, the Company offers credit terms consistent with industry standards within the country of operation. Payments to Managers and Distributors for sales incentives or rebates are recorded as a reduction of revenue. Payments for sales incentives and rebates are calculated monthly based upon qualifying sales. Membership fees are deferred and amortized as revenue over the life of the membership, primarily one year. Prepaid event registration fees are deferred and recognized as revenues when the related event is held.
A reserve for product returns is recorded based upon historical experience. The Company allows Managers or Distributors to return the unused portion of products within ninety days of purchase if they are not satisfied with the product. In some of ourthe Company’s markets, the requirements to return product are more restrictive. Sales returns for the years 2013, 2012 and 2011, were $1,454, $2,249 and 2010, were $2,249, $606, and $605, respectively. The increase in sales returns for year ended December 31, 2012 was due to unusallyunusually high product returns during the first quarter of 2012 related to a specific promotion in the Synergy Japan market. Product returns were not related to product quality and have since returned to normal lowlower return rates.
Amounts billed to customers for shipping and handling are reported as a component of net sales revenue. Shipping and handling revenues of approximately $10,868, $11,264 $11,615 and $12,165$11,615 were reported as net sales revenue for the years ended December 31, 2013, 2012, 2011, and 2010,2011, respectively.
Taxes that have been assessed by governmental authorities and that are directly imposed on revenue-producing transactions between the Company and its customers, including sales, use, value-added, and some excise taxes, are presented on a net basis (excluded from net sales).
49
Table of Contents
Advertising Costs
Advertising costs are expensed as incurred and classified in selling, general and administrative expenses. Advertising expense incurred for the years ended December 31, 2013, 2012 2011 and 20102011 totaled approximately $2,194, $1,418 $1,191 and $1,318,$1,191, respectively.
Research and Development
All research and development costs are expensed as incurred and classified in selling, general and administrative expense. Total research and development expenses were approximately $2,039, $1,464 and $1,552 in 2013, 2012 and $2,020 in 2012, 2011, and 2010, respectively.
Contingencies
The Company is involved in certain legal proceedings. When a loss is considered probable in connection with litigation or non-income tax contingencies and when such loss can be reasonably estimated with a range, the Company records its best estimate within the range related to the contingency. If there is no best estimate, the Company records the minimum of the range. As additional information becomes available, it assesses the potential liability related to the contingency and revises the estimates. The Company’s contingencies are discussed in further detail in Note 14.12.
Income Taxes
The Company’s income tax expense, deferred tax assets and liabilities and contingent reserves reflect management’s best assessment of estimated future taxes to be paid. The Company is subject to income taxes in both the U.S. and numerous foreign jurisdictions. Significant judgments and estimates are required in determining the consolidated income tax expense.
Deferred income taxes arise from temporary differences between the tax and financial statement recognition of revenue and expense. In evaluating the Company’s ability to recover its deferred tax assets, management considers all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax planning strategies and recent financial operations. In projecting future taxable income, the Company develops assumptions including the amount of future state, federal and foreign pretax operating income, the reversal of temporary differences, and the implementation of feasible and prudent tax planning strategies. These assumptions require significant judgment about the forecasts of future taxable income, and are consistent with the plans and estimates that the Company is using to manage the underlying businesses.
Changes in tax laws and rates could also affect recorded deferred tax assets and liabilities in the future. Management is not aware of any such changes that would have a material effect on the Company’s results of operations, cash flows or financial position.
57
Table of Contents
The calculation of the Company’s tax liabilities involves dealing with uncertainties in the application of complex tax laws and regulations in a multitude of jurisdictions across ourits global operations. Income tax positions must meet a more-likely-than-not recognition threshold to be recognized.
Net Income (Loss) Per Common Share
Basic net income (loss) per common share (“Basic EPS”) is computed by dividing net income (loss) by the weighted average number of common shares outstanding during the period. Diluted net income (loss) per common share (“Diluted EPS”) reflects the potential dilution that could occur if stock options or other contracts to issue common stock were exercised or converted into common stock. The computation of Diluted EPS does not assume exercise or conversion of securities that would have an anti-dilutive effect on net lossincome per common share.
50
Table of Contents
Following is a reconciliation of the numerator and denominator of Basic EPS to the numerator and denominator of Diluted EPS for all years:
| | 2012 | | 2011 | | 2010 | |
Net income (loss) available to common stockholders | | | | | | | |
Net income from continuing operations | | $ | 25,380 | | $ | 17,601 | | $ | 8,469 | |
Loss from discontinued operations | | — | | — | | (9,702 | ) |
Net income (loss) | | $ | 25,380 | | $ | 17,601 | | $ | (1,233 | ) |
| | | | | | | |
Basic weighted-average shares outstanding | | 15,648 | | 15,550 | | 15,515 | |
| | | | | | | |
Basic net income (loss) per common share: | | | | | | | |
Net income from continuing operations | | $ | 1.62 | | $ | 1.13 | | $ | 0.55 | |
Loss from discontinued operations | | $ | — | | $ | — | | $ | (0.63 | ) |
Net income (loss) | | $ | 1.62 | | $ | 1.13 | | $ | (0.08 | ) |
| | | | | | | |
Diluted Shares Outstanding | | | | | | | |
Basic weighted-average shares outstanding | | 15,648 | | 15,550 | | 15,515 | |
Stock options | | 339 | | 145 | | 90 | |
Diluted weighted-average shares outstanding | | 15,987 | | 15,695 | | 15,605 | |
| | | | | | | |
Diluted net income (loss) per common share: | | | | | | | |
Net income from continuing operations | | $ | 1.59 | | $ | 1.12 | | $ | 0.54 | |
Loss from discontinued operations | | $ | — | | $ | — | | $ | (0.62 | ) |
Net income (loss) | | $ | 1.59 | | $ | 1.12 | | $ | (0.08 | ) |
| | | | | | | |
Potentially dilutive shares excluded from diluted-per-share amounts: | | | | | | | |
Stock options | | 88 | | 219 | | 50 | |
| | | | | | | |
Potentially anti-dilutive shares excluded from diluted-per-share amounts: | | | | | | | |
Stock options | | 254 | | 200 | | 419 | |
| | 2013 | | 2012 | | 2011 | |
Net income available to common stockholders | | | | | | | |
Net income | | $ | 17,609 | | $ | 25,380 | | $ | 17,601 | |
| | | | | | | |
Basic weighted-average shares outstanding | | 15,997 | | 15,648 | | 15,550 | |
| | | | | | | |
Basic net income per common share: | | | | | | | |
Net income | | $ | 1.10 | | $ | 1.62 | | $ | 1.13 | |
| | | | | | | |
Diluted Shares Outstanding | | | | | | | |
Basic weighted-average shares outstanding | | 15,997 | | 15,648 | | 15,550 | |
Stock-based awards | | 393 | | 339 | | 145 | |
Diluted weighted-average shares outstanding | | 16,390 | | 15,987 | | 15,695 | |
| | | | | | | |
Diluted net income per common share: | | | | | | | |
Net income | | $ | 1.07 | | $ | 1.59 | | $ | 1.12 | |
| | | | | | | |
Potentially dilutive shares excluded from diluted-per-share amounts: | | | | | | | |
Stock options | | 135 | | 88 | | 219 | |
| | | | | | | |
Potentially anti-dilutive shares excluded from diluted-per-share amounts: | | | | | | | |
Stock options | | 210 | | 254 | | 200 | |
Potentially dilutive shares excluded from diluted-per-share amounts include performance basedperformance-based options to purchase shares of common stock for which certain earnings metrics have not been achieved. Potentially anti-dilutive shares excluded from diluted-per-share amounts include both non-qualified stock options and unearned performance-based options to purchase shares of common stock with exercise prices greater than the weighted-average share price during the period and shares that would be anti-dilutive to the computation of diluted net income (loss) per share for each of the years presented.
Share-Based Compensation
The Company’s outstanding stock options include time-based stock options which vest over differing periods ranging from the date of issuance up to 48 months from the option grant date, performance-based stock options which vestalready vested upon achieving operating income margins of six, eight and ten percent as reported in four of five consecutive quarters over the term of the options, and performance-based stock options which vest upon achieving cumulative annual net sales revenue growth targets over a rolling two-year period subject to the Company maintaining at least an eight percent operating income margin during the applicable period, and performance-based stock options which vest upon achieving annual net sales targets over a rolling one-year period.
The Company recognizes all share-based payments to Directors and employees, including grants of employee stock options and restricted stock units, to be recognized in the statement of operations based on their grant-date fair values. The Company recordsWe record compensation expense, net
58
Table of Contents
of an estimated forfeitures,forfeiture rate, over the vesting period of the stock options based on the fair value of the stock options on the date of grant. Management considers several factors when estimating forfeitures, including types of awards, employee class, andThe Company’s estimated forfeiture rate is based upon historical experience.
Comprehensive Income (Loss)
Comprehensive income (loss) includes all changes in shareholders’ equity except those resulting from investments by, and distributions to, shareholders. Accordingly, the Company’s comprehensive income (loss) includes net income (loss), net unrealized gains (losses) on investment securities, reclassifications of realized gains, and foreign currency adjustments that arise from the translation of the financial statements of the Company’s foreign subsidiaries.
Recent Accounting Pronouncements
In June 2011,July 2013, the FASBFinancial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2011-05 for Comprehensive2013-11 Income Taxes (Topic 220)740): “Presentation“Presentation of Comprehensive Income”an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists”. ASU 2011-05 eliminates the option to report other comprehensive income and its
51
Table of Contents
componentsThis update provides that an unrecognized tax benefit, or a portion thereof, should be presented in the consolidated statementfinancial statements as a reduction to a deferred tax asset for a net operating loss carryforward, a similar tax loss, or a tax credit carryforward, except to the extent that a net operating loss carryforward, a similar tax loss, or a tax credit carryforward is not available at the reporting date to settle any additional income taxes that would result from disallowance of shareholders’ equity and comprehensive income and requires ana tax position, or the tax law does not require the entity to presentuse, the total of comprehensive income,deferred tax asset for such purpose, then the components of net incomeunrecognized tax benefit should be presented as a liability. The amendments in this update are effective for interim and the components of other comprehensive income either in a single continuous statement or in two separate but consecutive statements. Effective January 1, 2012, the Company adopted the two separate but consecutive statements.annual periods beginning after December 15, 2013. The adoption of this ASU 2011-05 didis not expected to have a material impact on the Company’s consolidated statements of operations and financial condition.statements.
NOTE 2: ACCOUNTING CHANGE FOR SHIPPING AND HANDLING COSTS
During the year ended December 31, 2012, the Company changed its method of accounting to present shipping and handling costs related to the sale of the Company’s products together with the cost of sales in the consolidated statement of operations. These shipping and handling costs have previously been presented by the Company as part of selling, general and administrative expenses. This change has been made retrospectively to all periods presented in the Company’s financial statements. In connection with the change in presentation, the Company also changed its definition of shipping and handling costs to include expenses of our distribution facilities to prepare products for shipment. Previously, the Company defined shipping and handling costs as costs incurred for a third-party shipper to transport products to the customer. The amount of shipping and handling costs under the previous definition were $12,231, $11,245, and $10,310 for the three years ended December 31, 2012. The amount of additional costs included in shipping and handling costs under the new definition increased the retrospective adjustment for shipping and handling costs by $9,374, $8,754, and $9,914 for the three years ended December 31, 2012. This presentation is preferable as it better matches all the costs directly associated with generating revenue and therefore better presents gross margin, and this financial statement presentation will be more comparable to the Company’s peer group. The only impact on the Company’s financial statements was to increase cost of sales and decrease selling, general, and administrative expenses in the consolidated statement of operations. The impact of this change in accounting principle was to increase cost of sales and decrease selling, general and administrative expenses by $21,605 for the year ended December 31, 2012. The impact of this change in accounting principle on cost of sales and selling, general and administrative expenses for the years ended December 31, 2011 and 2010 was $19,999 and $20,224, respectively.
Year Ended December 31, | | Under Previous
Method | | As Reported | | Difference | |
2012 | | | | | | | |
Cost of sales | | 71,719 | | 93,324 | | 21,605 | |
Selling, general and administrative | | 128,466 | | 106,861 | | (21,605 | ) |
Year Ended December 31, | | As Previously
Reported | | As Adjusted | | Difference | |
2011 | | | | | | | |
Cost of sales | | $ | 69,410 | | $ | 89,409 | | $ | 19,999 | |
Selling, general and administrative | | 129,605 | | 109,606 | | (19,999 | ) |
| | | | | | | |
2010 | | | | | | | |
Cost of sales | | $ | 69,040 | | $ | 89,264 | | $ | 20,224 | |
Selling, general and administrative | | 139,248 | | 119,024 | | (20,224 | ) |
NOTE 3: DISCONTINUED OPERATIONS
During 2010, the Company ceased its operations in Brazil as a result of declining sales and increased regulatory restrictions, which made it difficult for the Company to register and sell key products in that market. This market was part of the Company’s NSP Americas, Asia Pacific and Europe segment.
The following table summarizes the operating results of the Company’s discontinued operations for the years:
Year Ended December 31, | | 2012 | | 2011 | | 2010 | |
Net sales revenue | | $ | — | | $ | — | | $ | 682 | |
| | | | | | | |
Loss before income tax provision | | $ | — | | $ | — | | $ | (9,702 | ) |
Income tax provision | | — | | — | | — | |
Loss from discontinued operations | | $ | — | | $ | — | | $ | (9,702 | ) |
The loss before income taxes related to the discontinued operations for the year ended December 31, 2010, includes a charge of $8,236 related to exiting Brazil, of which $7,364 was a non-cash write-off of accumulated translation adjustments that were previously included in shareholders’ equity.
The cash flows from our discontinued operations are included in the Company’s 2010 operating cash flow, but were insignificant.
NOTE 4: INVENTORIES
The composition of inventories is as follows:
As of December 31, | | 2012 | | 2011 | |
Raw materials | | $ | 13,287 | | $ | 12,992 | |
Work in process | | 742 | | 1,230 | |
Finished goods | | 29,251 | | 27,389 | |
Total inventory | | $ | 43,280 | | $ | 41,611 | |
52
Table of Contents
As of December 31, | | 2013 | | 2012 | |
Raw materials | | $ | 10,848 | | $ | 13,287 | |
Work in process | | 740 | | 742 | |
Finished goods | | 30,322 | | 29,251 | |
Total inventory | | $ | 41,910 | | $ | 43,280 | |
NOTE 5:3: PROPERTY, PLANT AND EQUIPMENT
The composition of property, plant and equipment is as follows:
As of December 31, | | 2012 | | 2011 | | | 2013 | | 2012 | |
Land and improvements | | $ | 3,800 | | $ | 3,800 | | | $ | 3,800 | | $ | 3,800 | |
Buildings and improvements | | 33,309 | | 31,233 | | | 33,655 | | 33,309 | |
Machinery and equipment | | 17,919 | | 17,395 | | | 18,209 | | 17,919 | |
Furniture and fixtures | | 21,182 | | 22,197 | | | 17,884 | | 16,956 | |
Computer software and hardware | | | 8,255 | | 4,226 | |
| | 76,210 | | 74,625 | | | 81,803 | | 76,210 | |
Accumulated depreciation and amortization | | (48,260 | ) | (49,488 | ) | | (49,781 | ) | (48,260 | ) |
Total property, plant and equipment | | $ | 27,950 | | $ | 25,137 | | | $ | 32,022 | | $ | 27,950 | |
Depreciation expense was $4,317, $3,929 $4,210 and $4,136$4,210 for the years ended December 31, 2013, 2012 and 2011, and 2010, respectively.
NOTE 6:4: INTANGIBLE ASSETS
At December 31, 20122013 and 2011,2012, intangibles for product formulations had a gross carrying amount of $1,763 and $1,763, accumulated amortization of $761$910 and $612,$761, and a net amount of $1,002$853 and $1,151,$1,002, respectively. The estimated useful lives of the product formulations range from 9 to 15 years.
59
Table of Contents
Amortization expense for intangible assets for the years ended December 31, 2013, 2012 2011 and 20102011 was $149, $152$149 and $118,$152, respectively. Estimated amortization expense for the five succeeding fiscal years and thereafter is as follows:
Year Ending December 31, | | Estimated
Amortization
Expense | | | | |
2013 | | $ | 149 | | |
2014 | | 149 | | | $ | 149 | |
2015 | | 149 | | | 149 | |
2016 | | 91 | | | 91 | |
2017 | | 91 | | | 91 | |
2018 | | | 91 | |
Thereafter | | 373 | | | 282 | |
Total | | $ | 1,002 | | | $ | 853 | |
NOTE 7:5: INVESTMENT SECURITIES
The amortized cost and estimated fair values of available-for-sale securities by balance sheet classification are as follows:
As of December 31, 2012 | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Value | | |
As of December 31, 2013 | | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Value | |
Municipal obligations | | $ | 608 | | $ | 30 | | $ | — | | $ | 638 | | | $ | 403 | | $ | 12 | | $ | — | | $ | 415 | |
U.S. government securities funds | | 995 | | — | | (9 | ) | 986 | | | 997 | | — | | (14 | ) | 983 | |
Equity securities | | 227 | | 228 | | (8 | ) | 447 | | | 227 | | 386 | | (5 | ) | 608 | |
Total short-term investment securities | | $ | 1,830 | | $ | 258 | | $ | (17 | ) | $ | 2,071 | | | $ | 1,627 | | $ | 398 | | $ | (19 | ) | $ | 2,006 | |
As of December 31, 2011 | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Value | | |
As of December 31, 2012 | | | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair
Value | |
Municipal obligations | | $ | 1,158 | | $ | 51 | | $ | — | | $ | 1,209 | | | $ | 608 | | $ | 30 | | $ | — | | $ | 638 | |
U.S. government securities funds | | 988 | | — | | (5 | ) | 983 | | | 995 | | — | | (9 | ) | 986 | |
Short-term deposits | | 3,104 | | — | | — | | 3,104 | | |
Equity securities | | 227 | | 159 | | (5 | ) | 381 | | | 227 | | 228 | | (8 | ) | 447 | |
Total short-term investment securities | | $ | 5,477 | | $ | 210 | | $ | (10 | ) | $ | 5,677 | | | $ | 1,830 | | $ | 258 | | $ | (17 | ) | $ | 2,071 | |
The municipal obligations held at fair value of $638$415 at December 31, 2012,2013, all mature in less than threetwo years.
During 2013, 2012 2011 and 2010,2011, the proceeds from the sales of available-for-sale securities were $200, $3,789 $7,697 and $125,$7,697, respectively. There were no gross realized gains (losses) on sales of available-for-sale securities (net of tax) for the years ended December 31, 2013, 2012 and 2011, and 2010, respectively.
53
Table of Contents
The Company’s trading securities portfolio totaled $1,276$971 and $1,429$1,276 at December 31, 20122013 and 2011,2012, respectively, and generated gains of $122, $116 $16 and $158,$16, for the years ended December 31, 2013, 2012 2011 and 2010,2011, respectively.
As of December 31, 20122013 and 2011,2012, the Company had unrealized losses of $8$14 and $5,$9, respectively, in its municipal obligations, short-term deposits and equityU.S. government securities investments.funds. These losses are due to the interest rate sensitivity of the municipal obligations and the performance of the overall stock market for the equity securities.
NOTE 8:6: ACCRUED LIABILITIES
The composition of accrued liabilities is as follows:
As of December 31, | | 2012 | | 2011 | | | 2013 | | 2012 | |
Foreign non-income tax contingencies (See Note 14) | | $ | 5,358 | | $ | 6,801 | | |
Sales, use and property tax (See Note 14) | | 3,690 | | 3,426 | | |
Foreign non-income tax contingencies (See Note 12) | | | $ | 5,363 | | $ | 5,358 | |
Sales, use and property tax (See Note 12) | | | 4,498 | | 3,690 | |
Salaries and employee benefits | | 8,217 | | 6,611 | | | 11,749 | | 8,217 | |
Convention and meeting costs | | 4,624 | | 5,047 | | | 5,784 | | 4,624 | |
Other | | 5,413 | | 6,053 | | | 7,499 | | 5,413 | |
Total | | $ | 27,302 | | $ | 27,938 | | | $ | 34,893 | | $ | 27,302 | |
60
Table of Contents
NOTE 9:7: LONG-TERM DEBT AND REVOLVING CREDIT FACILITY
On August 9, 2011, the Company entered into a Revolving Creditrevolving credit agreement with Wells Fargo Bank, N.A. that permits the Company to borrow up to $15,000 through August 9, 2014, bearing interest at LIBOR plus 1.25 percent.percent (1.50 percent as of December 31, 2013). The Company must pay an annual commitment fee of 0.25 percent on the unused portion of the commitment.
On August 8, 2013, the Company renegotiated the revolving credit agreement with Wells Fargo Bank, N.A. to increase to the borrowing limit to $25,000 and extend the maturity to September 1, 2015. At December 31, 2012,2013, the Company had $15,000 availableoutstanding balance under this facility.the revolving credit agreement was $10,000.
A term loan of $10,000 was obtained in conjunction with the Revolving Creditrevolving credit agreement with Wells Fargo Bank, N.A. and has a maturity date of August 9, 2014 and a variable interest rate of LIBOR plus 1.25 percent (1.50 percent as of December 31, 20122013 and 2011)2012). As of December 31, 2013, the outstanding balance under the term loan was $2,267. The term loan is collateralized by the Company’s manufacturing facility in Spanish Fork, Utah.
Long-term debt consists of the following:
As of December 31, | | 2012 | | 2011 | | | 2013 | | 2012 | |
Term loan due in monthly installments of approximately $284, including interest, secured by real estate | | $ | 5,620 | | $ | 9,190 | | |
Term loan in monthly installments of approximately $284, including interest | | | $ | 2,267 | | $ | 5,620 | |
Less current installments | | (3,350 | ) | (3,296 | ) | | (2,267 | ) | (3,350 | ) |
| | | — | | 2,270 | |
| | | | | | |
Revolving credit agreement | | | 10,000 | | — | |
| | | | | | |
Long-term debt less current installments | | $ | 2,270 | | $ | 5,894 | | | $ | 10,000 | | $ | 2,270 | |
The various debt agreements contain restrictions on liquidity, leveraging, minimum net income and consecutive quarterly net losses. In addition, the agreements restrict capital expenditures, lease expenditures, other indebtedness, liens on assets, guaranties, loans and advances, and the merger, consolidation and the transfer of assets except in the ordinary course of business. The Company is in compliance with these debt covenants as of December 31, 2012.
The aggregate maturities of long-term debt are as follows: $3,350 in 2013 and $2,270 in 2014.2013.
NOTE 10:8: ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS)
The components of accumulated other comprehensive income (loss), net of tax, are as follows:
| | Foreign Currency
Translation
Adjustments | | Net Unrealized
Gains (Losses) On
Available-For-Sale
Securities | | Total
Accumulated Other
Comprehensive Loss | | | Foreign Currency
Translation
Adjustments | | Net Unrealized
Gains (Losses) On
Available-For-Sale
Securities | | Total
Accumulated Other
Comprehensive Loss | |
Balance as of January 1, 2010 | | $ | (19,749 | ) | $ | 150 | | $ | (19,599 | ) | |
Activity, net of tax | | 11,955 | | (4 | ) | 11,951 | | |
Balance as of December 31, 2010 | | (7,794 | ) | 146 | | (7,648 | ) | |
Balance as of January 1, 2011 | | | $ | (7,794 | ) | $ | 146 | | $ | (7,648 | ) |
Activity, net of tax | | (2,397 | ) | (24 | ) | (2,421 | ) | | (2,397 | ) | (24 | ) | (2,421 | ) |
Balance as of December 31, 2011 | | (10,191 | ) | 122 | | (10,069 | ) | | (10,191 | ) | 122 | | (10,069 | ) |
Activity, net of tax | | (522 | ) | 25 | | (497 | ) | | (522 | ) | 25 | | (497 | ) |
Balance as of December 31, 2012 | | $ | (10,713 | ) | $ | 147 | | $ | (10,566 | ) | | (10,713 | ) | 147 | | (10,566 | ) |
Activity, net of tax | | | (3,480 | ) | 83 | | (3,397 | ) |
Balance as of December 31, 2013 | | | $ | (14,193 | ) | 230 | | $ | (13,963 | ) |
|
NOTE 11:9: INCOME TAXES
Income from continuing operations before provision (benefit) for income taxes are taxed under the following jurisdictions:
Year Ended December 31, | | 2013 | | 2012 | | 2011 | |
Domestic | | $ | 6,111 | | $ | 17,625 | | $ | 2,338 | |
Foreign | | 19,542 | | 17,871 | | 19,674 | |
Total | | $ | 25,653 | | $ | 35,496 | | $ | 22,012 | |
5461
Table of Contents
Year Ended December 31, | | 2012 | | 2011 | | 2010 | |
Domestic | | $ | 17,625 | | $ | 2,338 | | $ | 4,453 | |
Foreign | | 17,871 | | 19,674 | | 9,537 | |
Total | | $ | 35,496 | | $ | 22,012 | | $ | 13,990 | |
Components of the provision (benefit) for income taxes from continuing operations for each of the three years in the period ended December 31, 20122013 are as follows:
Year Ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Current: | | | | | | | | | | | | | | |
Federal | | $ | 1,901 | | $ | 4,094 | | $ | 3,330 | | | $ | (773 | ) | $ | 1,901 | | $ | 4,094 | |
State | | 248 | | 961 | | (306 | ) | | 399 | | 248 | | 961 | |
Foreign | | 3,835 | | 4,429 | | 369 | | | 7,326 | | 3,835 | | 4,429 | |
Subtotal | | 5,984 | | 9,484 | | 3,393 | | | 6,952 | | 5,984 | | 9,484 | |
Deferred: | | | | | | | | | | | | | | |
Federal | | 2,303 | | (4,408 | ) | 2,149 | | | 1,654 | | 2,303 | | (4,408 | ) |
State | | 1,207 | | (719 | ) | 1,186 | | | 186 | | 1,207 | | (719 | ) |
Foreign | | 622 | | 54 | | (1,207 | ) | | (748 | ) | 622 | | 54 | |
Subtotal | | 4,132 | | (5,073 | ) | 2,128 | | | 1,092 | | 4,132 | | (5,073 | ) |
Total provision for income taxes | | $ | 10,116 | | $ | 4,411 | | $ | 5,521 | | | $ | 8,044 | | $ | 10,116 | | $ | 4,411 | |
The provision for income taxes, from continuing operations, as a percentage of income before provision for income taxes, differs from the statutory U.S. federal income tax rate due to the following:
Year Ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Statutory U.S. federal income tax rate | | 35.0 | % | 35.0 | % | 35.0 | % | | 35.0 | % | 35.0 | % | 35.0 | % |
State income taxes, net of U.S. federal income tax benefit | | 2.7 | | 0.7 | | 4.1 | | | 1.4 | | 2.7 | | 0.7 | |
U.S. tax impact of foreign operations | | 0.2 | | (15.1 | ) | 13.5 | | | (15.9 | ) | (2.1 | ) | (20.3 | ) |
Valuation allowance change | | (3.9 | ) | (0.3 | ) | 20.9 | | | 9.3 | | (3.9 | ) | (0.3 | ) |
Tax contingencies | | (1.0 | ) | (1.4 | ) | 1.8 | | |
Foreign exchange gains (losses) | | — | | — | | (10.1 | ) | |
Gain on sale of intercompany assets | | — | | — | | 10.6 | | |
Charitable contributions | | — | | (0.3 | ) | (0.2 | ) | |
Foreign tax rate differential | | (2.3 | ) | (5.2 | ) | (5.2 | ) | |
Unrecognized tax benefits | | 0.4 | | 8.2 | | 4.1 | | | 8.4 | | 0.4 | | 8.2 | |
Meals and entertainment | | 0.2 | | 0.3 | | 0.4 | | |
Tax adjustment for inflation | | — | | — | | (0.3 | ) | |
Domestic manufacturing deduction | | (0.4 | ) | (1.1 | ) | — | | | (1.3 | ) | (0.4 | ) | (1.1 | ) |
One-time bad debt and worthless stock deduction | | — | | — | | (33.0 | ) | |
Nondeductible foreign expenses | | (0.9 | ) | (1.3 | ) | (0.1 | ) | | (4.4 | ) | (0.9 | ) | (1.3 | ) |
Other | | (1.5 | ) | 0.5 | | (2.0 | ) | | (1.1 | ) | (2.3 | ) | (0.9 | ) |
Effective income tax rate | | 28.5 | % | 20.0 | % | 39.5 | % | | 31.4 | % | 28.5 | % | 20.0 | % |
Pretax earnings of a foreign subsidiary or affiliate are subject to U.S. taxation when effectively repatriated. The Company does not intend to reinvest undistributed earnings indefinitely in the Company’s foreign subsidiaries.
Adjustments relating to the U.S. impact of foreign operations increaseddecreased the effective tax rate by 0.215.9 percentage points in 2013, decreased the effective tax rate by 2.1 percentage points in 2012 and decreased the effective tax rate by 15.120.3 percentage points in 2011. Included were adjustments for dividends received from foreign subsidiaries, adjustments for foreign tax credits, and adjustments relating to the unremitted earnings calculations under applicable U.S. GAAP. Adjustments relating to the U.S. tax impact of foreign operations decreased the effective tax rate by 15.1 percentage points in 2011 and increased the effective tax rate by 13.5 percentage points in 2010. Included were adjustments for dividends received from foreign subsidiaries, adjustments for foreign tax creditsdifferentials and adjustments relating to the unremitted earnings calculations under applicable U.S. GAAP. The components of this calculation were:
Components of U.S. tax impact of foreign operations | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Dividends received from foreign subsidiaries | | 4.5 | % | 26.2 | % | 25.5 | % | | 29.2 | % | 4.5 | % | 26.2 | % |
Foreign tax credits | | (4.0 | ) | (33.1 | ) | (31.7 | ) | | (34.1 | ) | (4.0 | ) | (33.1 | ) |
Foreign tax rate differentials | | | (10.6 | ) | (2.3 | ) | (5.2 | ) |
Unremitted earnings | | (0.3 | ) | (8.2 | ) | 19.7 | | | (0.4 | ) | (0.3 | ) | (8.2 | ) |
Total | | 0.2 | % | (15.1 | )% | 13.5 | % | | (15.9 | )% | (2.1 | )% | (20.3 | )% |
The significant components of the deferred tax assets (liabilities) are as follows:
5562
Table of Contents
As of December 31, | | 2012 | | 2011 | | | 2013 | | 2012 | |
Inventory | | $ | 1,718 | | $ | 1,635 | | | $ | 1,502 | | $ | 1,718 | |
Accrued liabilities | | 3,464 | | 3,269 | | | 4,380 | | 3,464 | |
Impaired investments | | 727 | | 765 | | | — | | 727 | |
Deferred compensation | | 3,075 | | 2,514 | | | 364 | | 483 | |
Equity-based compensation | | | 2,993 | | 2,592 | |
Intangibles assets | | 2,308 | | 8,084 | | | 389 | | 2,308 | |
Bad debts | | 49 | | 59 | | | $ | 225 | | 49 | |
Net operating losses | | 4,152 | | 4,547 | | | 5,593 | | 4,152 | |
Foreign tax and withholding credits | | 4,406 | | 5,675 | | | 4,066 | | 4,406 | |
Non-income tax accruals | | 452 | | 170 | | | 397 | | 452 | |
Health insurance accruals | | 186 | | 171 | | | 184 | | 186 | |
Undistributed foreign earnings | | 2,704 | | 2,425 | | | 4,008 | | 2,704 | |
Other deferred tax assets | | 2,357 | | 2,265 | | | 2,260 | | 2,357 | |
Capital loss carryforward | | | 721 | | — | |
Valuation allowance | | (8,149 | ) | (9,836 | ) | | (11,340 | ) | (8,149 | ) |
Total deferred tax assets | | 17,449 | | 21,743 | | | $ | 15,742 | | 17,449 | |
Other deferred tax liabilities | | (720 | ) | (868 | ) | | (231 | ) | (720 | ) |
Total deferred tax liabilities | | (720 | ) | (868 | ) | | (231 | ) | (720 | ) |
Total deferred taxes, net | | $ | 16,729 | | $ | 20,875 | | | $ | 15,511 | | $ | 16,729 | |
The components of deferred tax assets (liabilities), net are as follows:
As of December 31, | | 2012 | | 2011 | | | 2013 | | 2012 | |
Net current deferred tax assets | | $ | 5,307 | | $ | 3,945 | | | $ | 5,711 | | $ | 5,307 | |
Net non-current deferred tax assets | | 11,516 | | 17,026 | | | 9,928 | | 11,516 | |
Total net deferred tax assets | | 16,823 | | 20,971 | | | 15,639 | | 16,823 | |
| | | | | | | | | | |
Net current deferred tax liabilities | | (1 | ) | (1 | ) | | (2 | ) | (1 | ) |
Net non-current deferred tax liabilities | | (93 | ) | (95 | ) | | (126 | ) | (93 | ) |
Total net deferred tax liabilities | | (94 | ) | (96 | ) | | (128 | ) | (94 | ) |
| | | | | | | | | | |
Total deferred taxes, net | | $ | 16,729 | | $ | 20,875 | | | $ | 15,511 | | $ | 16,729 | |
Net current deferred tax liabilities are included in accrued liabilities and net non-current deferred tax liabilities are included in other liabilities in the consolidated balance sheets.
Management has provided a valuation allowance of $8,149$11,340 and $9,836$8,149 as of December 31, 20122013 and 2011,2012, respectively, for certain deferred tax assets, including foreign net operating losses, for which management cannot conclude it is more likely than not that they will be realized. The Company reviewed its tax positions and decreasedincreased its valuation allowance by approximately $1,687$3,191 in 20122013 primarily due to a domestic decreaseincrease of $2,079 partially offset by$1,965 and a foreign increase of $392.$1,226.
At December 31, 2012,2013, foreign subsidiaries had unused operating loss carryovers for tax purposes of approximately $4,152.$5,593. The net operating losses will expire at various dates from 20132014 through 2022.2023. For financial reporting purposes, the release of these valuation allowances would reduce income tax expenses. At December 31, 2012,2013, the Company had approximately $4,406$4,066 of foreign tax credit,and withholding credits, most of which expire in 2020.
The Company is subject to regular audits by federal, state and foreign tax authorities. These audits may result in additional tax liabilities. The Company believes it has appropriately provided for income taxes for all years. Several factors drive the calculation of its tax reserves. Some of these factors include: (i) the expiration of various statutes of limitations; (ii) changes in tax law and regulations; (iii) the issuance of tax rulings; and (iv) settlements with tax authorities. Changes in any of these factors may result in adjustments to the Company’s reserves, which would impact its reported financial results.
The Company’s U.S. federal income tax returns for 2009 through 20112012 are open to examination for federal tax purposes. The Company has several foreign tax jurisdictions that have open tax years from 20042006 through 2011.2012. The Internal Revenue Service (“IRS”) is currently conducting an audit of the Company’s U.S. federal income tax returns for the 2009 and 2010through 2011 tax years. The IRS has also indicated that it intends to include 2011 in the aforementioned audit. The Company is currently unable to determine the precise outcome of these matters and their related impact if any, on the Company’s financial condition, results of operations or cash flows.
In October 2009, the IRS issued an examination report formally proposing adjustments with respect to the 2003 through 2005 taxable years, which primarily relate to the prices that were charged in intra-group transfers of property and the disallowance of related deductions. The Company challenged the IRS proposals, and took the matter before the Office of Appeals of the Internal Revenue Service. A settlement was reached with IRS Appeals and a proposed settlement agreement was signed by NSP in January 2012. The Company has made all required payments as of June 30, 2012 related to this matter and the examination period for the 2003 through 2005 taxable years closed on December 31, 2012.
5663
Table of Contents
During 2011, the IRS issued an examination report formally proposing adjustments with respect to the 2006 through 2008 taxable years. As with the previous examination cycle discussed above, the adjustments relate primarily to the prices that were charged in intra-group transfers of property and the disallowance of related deductions. The Company reached a settlement of the issues with the IRS examination team and an agreement was signed by the Company in January 2012. The Company has made all required payments as of December 31, 2012 related to this matter and the examination period for the 2006 through 2007 taxable years closed on September 15, 2012. The examination period for the 2008 taxable year previously closed on March 15, 2012.
The total outstanding balance for liabilities related to unrecognized tax benefits at December 31, 2013 and 2012 was $12,402 and 2011 was $10,571, and $10,426, respectively, all of which would favorably impact the effective tax rate if recognized. Included in these amounts is approximately $1,052$1,352 and $1,460,$1,052, respectively, of interest and penalties. The Company increased interest and penalties approximately $300 and decreased interest and penalties approximately $408 and $321 for the years ended December 31, 20122013 and 2011,2012, respectively. The Company accounts for interest expense and penalties for unrecognized tax benefits as part of its income tax provision.
During the years ended December 31, 2013, 2012 2011 and 2010,2011, the Company added approximately $2,656, $3,471 $2,379 and $3,682,$2,379, respectively, to its liability for unrecognized tax benefits. Included in these amounts are approximately $300, $339 $491 and $1,250$491 for the years ended December 31, 2013, 2012 2011 and 2010,2011, respectively, related to interest expense and penalties. In addition, the Company recorded a benefit related to the lapse of applicable statute of limitations of approximately $323, $2,815 $1,728 and $3,132$1,728 for the years ended December 31, 2013, 2012 2011 and 2010,2011, respectively, all of which favorably impacted the Company’s effective tax rate.
A reconciliation of the beginning and ending amount of liabilities associated with uncertain tax benefits, excluding interest and penalties, is as follows for the years:
Year Ended December 31, | | 2012 | | 2011 | | 2010 | |
| | | | | | | |
Unrecognized tax benefits, opening balance | | $ | 8,966 | | $ | 15,058 | | $ | 19,687 | |
Settlement of liability reclassified as income tax payable | | — | | (4,479 | ) | — | |
Payments on liability | | (15 | ) | (2,590 | ) | — | |
Tax positions taken in a prior period | | | | | | | |
Gross increases | | 1,120 | | 541 | | 2,432 | |
Gross decreases | | (504 | ) | — | | (980 | ) |
Tax positions taken in the current period | | | | | | | |
Gross increases | | 2,011 | | 1,347 | | — | |
Gross decreases | | — | | — | | — | |
Lapse of applicable statute of limitations | | (2,068 | ) | (914 | ) | (1,566 | ) |
Currency translation adjustments | | 9 | | 3 | | (4,515 | ) |
Unrecognized tax benefits, ending balance | | $ | 9,519 | | $ | 8,966 | | $ | 15,058 | |
Based upon the Company’s negotiations with the IRS in prior audit cycles, it is not likely the Company would seek competent authority related to its current unrecognized tax reserves.
Year Ended December 31, | | 2013 | | 2012 | | 2011 | |
| | | | | | | |
Unrecognized tax benefits, opening balance | | $ | 9,519 | | $ | 8,966 | | $ | 15,058 | |
Settlement of liability reclassified as income tax payable | | (10 | ) | — | | (4,479 | ) |
Payments on liability | | — | | (15 | ) | (2,590 | ) |
Tax positions taken in a prior period | | | | | | | |
Gross increases | | — | | 1,120 | | 541 | |
Gross decreases | | (184 | ) | (504 | ) | — | |
Tax positions taken in the current period | | | | | | | |
Gross increases | | 2,356 | | 2,011 | | 1,347 | |
Gross decreases | | — | | — | | — | |
Lapse of applicable statute of limitations | | (323 | ) | (2,068 | ) | (914 | ) |
Currency translation adjustments | | (308 | ) | 9 | | 3 | |
Unrecognized tax benefits, ending balance | | $ | 11,050 | | $ | 9,519 | | $ | 8,966 | |
The Company anticipates that unrecognized tax benefits will increase approximately $1,000$1,200 to $1,500$1,700 within the next twelve months due to additional transactions related to commissions and transfer pricing.
The Company believes that it is reasonably possible that unrecognized tax benefits will decrease approximately $1,500$0 to $2,000$500 within the next twelve months due to the close of audits or the expiration of statutes of limitations in various foreign jurisdictions.
Although the Company believes its estimates are reasonable, the Company can make no assurance that the final tax outcome of these matters will not be different from that which it has reflected in its historical income tax provisions and accruals. Such differences could have a material impact on the Company’s income tax provision and operating results in the period in which the Company makes such determination.
In September 2013, the U.S. Department of the Treasury and the Internal Revenue Service (IRS) issued final regulations addressing the acquisition, production and improvement of tangible property, and also proposed regulations addressing the disposition of property. These regulations replace previously issued temporary regulations and are effective for tax years beginning January 1, 2014. The Company is in the process of analyzing the impact of these new regulations but does not believe they will have a material impact on the Company’s consolidated financial statements.
NOTE 12:10: CAPITAL TRANSACTIONS
Dividends
The declaration of future dividends is subject to the discretion of the Company’s Board of Directors and will depend upon various factors, including the Company’s earnings, financial condition, restrictions imposed by any indebtedness that may be outstanding, cash requirements, future prospects and other factors deemed relevant by its Board of Directors.
On May 7, 2012,March 6, 2013, the Company announced that itsCompany’s Board of Directors approveddeclared a cash dividend of $0.05$0.10 per common share in an aggregate amount of $780$1,583 that was paid on May 29, 2012March 28, 2013, to shareholders of record on March 18, 2013. On May 18, 2012. On August 1, 2012,8, 2013, the Company’s
5764
Table of Contents
the Company announced that its Board of Directors approveddeclared a cash dividend of $0.05$0.10 per common share in an aggregate amount of $780$1,587 that was paid on May 30, 2013, to shareholders of record on May 20, 2013. On August 8, 2013, the Company’s Board of Directors declared a special one-time cash dividend of $1.50 per common share in addition to the Company’s recurring quarterly dividend of $0.10 per common share that was paid on August 23, 201229, 2013, to shareholders of record on August 13, 2012.19, 2013. The amount of the cash dividend paid to shareholders was $25,627. On November 2, 2012,6, 2013, the Company announced that itsCompany’s Board of Directors approveddeclared a cash dividend of $0.05$0.10 per common share in an aggregate amount of $789$1,622 that was paid on November 26, 201229, 2013, to shareholders of record on November 15, 2012.19, 2013.
Share Repurchase Program
On August 8, 2013, the Board of Directors authorized a $10,000 share repurchase program to be implemented over two years. Such purchases may be made in the open market, through block trades, in privately negotiated transactions or otherwise. The timing and amount of any shares repurchased will be determined based on the Company’s evaluation of market conditions and other factors and the program may be discontinued or suspended at any time.
The following is a summary of the Company’s repurchases of common shares during the year ended December 31, 2013:
Period | | Number of
Shares | | Average
Price Paid per Share | | Program Balance Used
for Repurchases | |
July 1 — September 30, 2013 | | 108 | | $ | 18.06 | | $ | 1,945 | |
October 1 — December 31, 2013 | | 32 | | 18.44 | | 601 | |
| | 140 | | $ | 18.14 | | $ | 2,546 | |
Share-Based Compensation
During the year ended December 31, 2012, the Company’s shareholders adopted and approved the Nature’s Sunshine Products, Inc. 2012 Stock Incentive Plan (the “2012 Incentive Plan”). The 2012 Incentive Plan provides for the grant of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock, restricted stock units, dividend equivalent rights, performance awards, stock awards and other stock-based awards. The Compensation Committee of the Board of Directors has authority and discretion to determine the type of award as well as the amount, terms and conditions of each award under the 2012 Incentive Plan, subject to the limitations of the 2012 Incentive Plan. A total of 1,500 shares of the Company’s common stock were authorized for the granting of awards under the 2012 Incentive Plan. The number of shares available for awards, as well as the terms of outstanding awards, are subject to adjustment as provided in the 2012 Incentive Plan for stock splits, stock dividends, recapitalizations and other similar events.
The Company also maintains a stock incentive plan, which was approved by shareholders in 2009 (the “2009 Incentive Plan”). The 2009 Incentive Plan also provided for the grant of incentive stock options, non-statutory stock options, stock appreciation rights, restricted stock, restricted stock units, dividend equivalent rights, performance awards, stock awards and other stock-based awards. Under the 2012 Incentive Plan, any shares subject to award, or awards forfeited or reacquired by the Company issued under the 2009 Incentive Plan are available for award up to a maximum of 400 shares.
The Company also maintained a stock option plan, which was approved by shareholders in 1995 and expired in 2005 (“the 1995 Stock Plan”). The 1995 Stock Plan provided for the granting or awarding of certain nonqualified stock options to officers, directors and other employees. The term, not to exceed 10 years, and the vesting and exercise period of each stock option awarded under the 1995 Stock Plan were determined by the Company’s Board of Directors.
Stock option activity for 2013, 2012 2011 and 20102011 consisted of the following:
| | Number of
Shares (in
thousands) | | Weighted Average Exercise
Price Per Share | | | Number of
Shares (in
thousands) | | Weighted Average Exercise
Price Per Share | |
Options outstanding at January 1, 2010 | | 455 | | $ | 8.25 | | |
Granted | | 475 | | 10.03 | | |
Forfeited or canceled | | (48 | ) | 9.98 | | |
Exercised | | (23 | ) | 5.81 | | |
Options outstanding at December 31, 2010 | | 859 | | 9.20 | | |
Options outstanding at January 1, 2011 | | | 859 | | $ | 9.20 | |
Granted | | 630 | | 10.84 | | | 630 | | 10.84 | |
Forfeited or canceled | | (79 | ) | 9.64 | | | (79 | ) | 9.64 | |
Exercised | | (36 | ) | 11.08 | | | (36 | ) | 11.08 | |
Options outstanding at December 31, 2011 | | 1,374 | | 9.88 | | | 1,374 | | 9.88 | |
Granted | | 686 | | 15.11 | | | 686 | | 15.11 | |
Forfeited or canceled | | (35 | ) | 13.60 | | | (35 | ) | 13.60 | |
Exercised | | (241 | ) | 9.95 | | | (241 | ) | 9.95 | |
Options outstanding at December 31, 2012 | | 1,784 | | $ | 11.81 | | | 1,784 | | 11.81 | |
Granted | | | 832 | | 15.85 | |
Forfeited or canceled | | | (184 | ) | 13.65 | |
Exercised | | | (506 | ) | 8.56 | |
Options outstanding at December 31, 2013 | | | 1,926 | | $ | 12.54 | |
65
Table of Contents
On August 29, 2013, the Company paid a special one-time cash dividend of $1.50 per common share. In accordance with the provisions of the Company’s stock incentive plans, the exercise price of all outstanding stock options on the ex-dividend date were decreased by $1.50 per share in order to prevent a dilution of benefits or potential benefits intended to be made available to the stock option holders. Because this modification was required by the provisions of the Company’s stock incentive plans, no additional share-based compensation expense was recorded.
The Company’s outstanding stock options include time-based stock options which vest over differing periods ranging from the date of issuance up to 48 months from the option grant date, performance-based stock options which vestalready vested upon achieving operating income margins of six, eight and ten percent as reported in four of five consecutive quarters over the term of the options, and performance-based stock options which vest upon achieving cumulative annual net sales revenue growth targets over a rolling two-year period subject to the Company maintaining at least an eight percent operating income margin during the applicable period, and performance-based stock options which vest upon achieving annual net sales targets over a rolling one-year period.
During the year ended December 31, 2013, the Company issued options to purchase 832 shares of common stock under the 2012 Stock Incentive Plan to the Company’s executive officers and other employees, which are composed of both time-based stock options and net sales revenue performance-based stock options. These options were issued with a weighted-average exercise price of $15.85 per share and a weighted-average grant date fair value of $6.55 per share. All of the options issued have an option termination date of ten years from the option grant date.
During the year ended December 31, 2012, the Company issued time-based options to purchase 217 shares of common stock under the 2009 Incentive Plan to the Company’s new senior executives. These options were issued with a weighted average exercise price of $15.65 per share and a weighted average grant date fair value of $7.66 per share. All of the options issued have an option termination date of ten years from the option grant date.
Also, during the year ended December 31, 2012, the Company issued options to purchase 469 shares of common stock under the 2012 Incentive Plan to the Company’s executive officers and other employees, which are composed of both time-based stock options and net sales revenue performance-based stock options. These options were issued with a weighted average exercise price of $14.86 per share and a weighted average grant date fair value of $7.00 per share. All of the options issued have an option termination date of ten years from the option grant date.
58
Table of Contents
During the year ended December 31, 2011, the Company issued options to purchase 630 shares of common stock under the 2009 Incentive Plan to the Company’s executive officers, other employees and to new members of its Board of Directors. These options were issued with a weighted-average exercise price of $10.84 per share, and a weighted-average grant date fair value of $5.13 per share. All of the options issued have an option termination date of ten years from the option grant date.
During the year ended December 31, 2010, the Company issued options to purchase 475 shares of common stock under the 2009 Incentive Plan, issued to the Company’s executive officers and other employees and to a new member of the Board of Directors, of which 300 were subject to achieving certain operating income metrics. These options were issued with a weighted average exercise price of $10.03 per share, and a weighted-average grant date fair value of $4.27 per share. All of the 475 options issued have an option termination date of ten years from the option grant date.
For the years ended December 31, 2013, 2012 2011 and 2010,2011, the Company issued 506, 241 36 and 2336 shares of common stock upon the exercise of stock options at an average exercise price of $8.56, $9.95 $11.08 and $5.81$11.08 per share, respectively. The aggregate intrinsic values of options exercised during the years ended December 31, 2013, 2012 2011and 2010and 2011 was $4,576, $1,427 $211 and $82,$211, respectively. For the yearyears ended December 31, 2013 and 2012, the Company recognized $653 and $378 of tax benefits from the exercise of stock options during the period.period, respectively.
The fair value of each option grant was estimated on the date of the grant using the Black-Scholes option-pricing model with the following weighted average assumptions for the years ended December 31, 2013, 2012 2011 and 2010:2011:
| | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Weighted average grant date fair value of grants | | $ | 7.21 | | $ | 5.13 | | $ | 4.27 | | | $ | 6.55 | | $ | 7.21 | | $ | 5.13 | |
Expected life (in years) | | 4.0 to 6.0 | | 3.0 to 4.0 | | 4.0 | | | 5.0 to 6.0 | | 4.0 to 6.0 | | 3.0 to 4.0 | |
Risk-free interest rate | | 0.3 to 0.9 | | 0.7 to 1.2 | | 0.6 to 1.5 | | | 0.6 to 1.5 | | 0.3 to 0.9 | | 0.7 to 1.2 | |
Expected volatility | | 58.5 to 66.0 | | 49.0 to 67.7 | | 51.3 to 65.8 | | | 55.9 to 58.2 | | 58.5 to 66.0 | | 49.0 to 67.7 | |
Dividend yield | | 0.0 to 1.3 | | 0.0 | | 0.0 | | | 2.1 to 2.7 | | 0.0 to 1.3 | | 0.0 | |
|
Expected option lives and volatilities are based on historical data of the Company. The risk free interest rate is calculated as the average U.S. Treasury bill rate that corresponds with the option life. The dividend yield is based on the Company’s historical and expected amount of dividend payouts, at the time of grant. On August 29, 2013, the Company paid a special one-time cash dividend of $1.50 per common share. The Company has excluded this special one-time cash dividend from the dividend yield used in the Black-Scholes option-pricing model calculations as it is not representative of future dividends declared by the Company.
Share-based compensation expense from time-based stock options for the years ended December 31, 2013, 2012 and 2011 was $3,166, $2,101 and 2010 was $2,101, $557, and $437, respectively; the related tax benefit was approximately $1,251, $850 $222 and $173,$222, respectively. As of December 31, 2013, 2012 2011 and 2010,2011, the unrecognized share-based compensation cost related to grants described above was $2,715,$3,294,
66
Table of Contents
$2,715 and $607, and $451, respectively. As of December 31, 2012,2013, the remaining compensation cost is expected to be recognized over the weighted-average period of approximately 1.81.9 years.
Shared-based compensation expense from operating income performance-based stock options for the years ended December 31, 2013, 2012 and 2011 was $0, $653 and 2010 was $653, $2,921, and $0, respectively; the related tax benefit of approximately $0, $255 $1,168 and $0,$1,168, respectively. As of December 31, 2012, there iswas no remaining compensation expense to be recognized for the operating income performance-based stock options.
The Company has not recognized any share-based compensation expense related to the net sales revenue performance-based stock options for the year ended December 31, 2012.2013. Should the Company attain all of the net sales revenue metrics related to the net sales revenue performance-based stock option grants, the Company would recognize up to $600$800 of potential share-based compensation expense.
The following table summarizes information about options outstanding and exercisable at December 31, 2012.2013.
| | Options Outstanding | | Options Exercisable | |
Range of Option
Prices Per Share | | Options
Outstanding | | Weighted-Avg.
Remaining
Contractual Life | | Weighted-Avg.
Exercise Price
Per Share | | Options
Exercisable | | Weighted-Avg.
Remaining
Contractual Life | | Weighted-Avg.
Exercise Price
Per Share | |
$5.35 to $9.99 | | 740 | | 7.5 | | $ | 8.08 | | 690 | | 7.5 | | $ | 8.05 | |
$10.00 to $11.99 | | 188 | | 7.3 | | 11.38 | | 188 | | 7.3 | | 11.38 | |
$12.00 to $16.12 | | 856 | | 9.0 | | 15.12 | | 133 | | 8.5 | | 15.05 | |
| | 1,784 | | 8.2 | | $ | 11.81 | | 1,011 | | 7.6 | | $ | 9.59 | |
| | Options Outstanding | | Options Exercisable | |
Range of Option
Prices Per Share | | Options
Outstanding | | Weighted-Avg.
Remaining
Contractual Life | | Weighted-Avg.
Exercise Price
Per Share | | Options
Exercisable | | Weighted-Avg.
Remaining
Contractual Life | | Weighted-Avg.
Exercise Price
Per Share | |
$3.85 to $9.99 | | 456 | | 6.5 | | $ | 6.93 | | 456 | | 6.5 | | $ | 6.93 | |
$10.00 to $13.99 | | 986 | | 8.6 | | 13.41 | | 285 | | 7.8 | | 13.39 | |
$14.00 to $19.20 | | 484 | | 9.0 | | 16.06 | | 97 | | 6.0 | | 15.26 | |
| | 1,926 | | 8.2 | | $ | 12.59 | | 838 | | 7.2 | | $ | 10.09 | |
At December 31, 2013, the aggregate intrinsic value of outstanding options to purchase 1,926 shares of common stock, the exercisable options to purchase 838 shares of common stock, and options to purchase 905 shares of common stock expected to vest was $9,415, $6,069 and $3,179, respectively. At December 31, 2012, the aggregate intrinsic value of outstanding options to purchase 1,784 shares of common stock, the exercisable options to purchase 1,011 shares of common stock, and options to purchase 644 shares of common stock expected to vest was $5,315, $5,016 and $281, respectively. At
Restricted stock unit activity for the period ended December 31, 2011,2013 and 2012, is as follows:
| | Number of
Shares | | Weighted Average
Grant Date
Fair Value | |
Units outstanding at January 1, 2012 | | — | | $ | — | |
Granted | | 18 | | 12.07 | |
Issued | | — | | — | |
Forfeited | | — | | — | |
Units outstanding at December 31, 2012 | | 18 | | 12.07 | |
Granted | | 17 | | 12.90 | |
Issued | | (3 | ) | 12.07 | |
Forfeited | | — | | — | |
Units outstanding at December 31, 2013 | | 32 | | 12.47 | |
| | | | | | |
During the aggregate intrinsic value of outstanding options to purchase 1,374 sharesperiod ended December 31, 2013, the Company issued 17 restricted stock units (RSUs) of common stock under the exercisable options2012 Incentive Plan to purchase 772 sharesthe Board of common stock,Directors. The RSUs were issued with a weighted average grant date fair value of $12.90 per share and options to purchase 602 shares of common stock expected to vest was $7,757, $4,819 and $2,732, respectively.
59
Table of Contentsin 12 monthly installments over a one year period from the grant date.
During the period ended December 31, 2012, the Company issued 18 restricted stock units (RSUs) of common stock under the 2012 Incentive Plan to the Board of Directors. The RSUs were issued with a weighted average grant date fair value of $12.07 per share and vest in 12 monthly installments over a one year period from the grant date.
Restricted stock unit activity for the period ended December 31, 2012 is as follows:
| | Number of
Shares | | Weighted Average
Grant Date
Fair Value | |
Units outstanding at December 31, 2011 | | — | | $ | — | |
Granted | | 18 | | 12.07 | |
Issued | | — | | — | |
Forfeited | | — | | — | |
Units outstanding at December 31, 2012 | | 18 | | 12.07 | |
| | | | | | |
RSUs are valued at the market value on the date of grant. Due to post-vesting restrictions, a Finnerty Model was utilized to calculate a valuation discount from the market value of common shares reflecting the restriction embedded in the RSUs preventing the sale of the underlying shares over a certain period of time. The Finnerty Model proposes to estimate a discount for lack of marketability such as transfer restrictions by using an option pricing theory. This model has gained recognition through its ability to address the magnitude of the discount by considering the volatility of a company’s stock price and the length of restriction. The concept underpinning the Finnerty Model is that restricted stock cannot be sold over a certain period of time. Using assumptions
67
Table of Contents
previously determined for the application of the option pricing model at the valuation date, the Finnerty Model discount for lack of marketability is approximately 17.5 percent for a common share.
Share-based compensation expense from RSUs for the period ended December 31, 2013 and 2012 was approximately $223 and $124, respectively; and the related tax benefit was approximately $49.$88 and $49, respectively. As of December 31, 2013 and 2012, the unrecognized share-based compensation expense related to the grants described above was $99.$62 and $99, respectively. As of December 30, 2012,2013, the remaining compensation expense is expected to be recognized over the weighted average period of approximately 0.4 years.
NOTE 13:11: EMPLOYEE BENEFIT PLANS
Deferred Compensation Plans
The Company sponsors a qualified deferred compensation plan which qualifies under Section 401(k) of the Internal Revenue Code. During 2012,2013, the Company made matching contributions of 5060 percent of employee contributions up to a maximum of five percent of the employee’s compensation (the match was increased from 50 percent to 60 percent of employee contributions up to a maximum of five percent beginning in fiscal year 2013). The Company’s contributions to the plan vest after a period of three years. During 2013, 2012 2011 and 2010,2011, the Company contributed to the plan approximately $832, $551 $438 and $1,065,$438, respectively.
The Company provides a nonqualified deferred compensation plan for its officers and certain key employees. Under this plan, participants may defer up to 100 percent of their annual salary and bonus. Although participants direct the investment of these funds, they are classified as trading securities and are included in long-term investment securities on the consolidated balance sheets because they remain assets of the Company until they are actually paid out to the participants. The Company has established a trust to finance obligations under the plan. At the end of each year and at other times provided under the plan, the Company adjusts its obligation to a participant by the investment return or loss on the funds selected by the participant under rules established in the plan. Upon separation of employment of the participant with the Company, the obligation owed to the participant under the plan will be paid as a lump sum or over a period of either three or five years (and will continue to be adjusted by the applicable investment return or loss during the period of pay-out). The Company had deferred compensation plan assets of approximately $1,276$971 and $1,429$1,276 as of December 31, 20122013 and 2011,2012, respectively. The change in the liability associated with the deferred compensation plan is recorded in the deferred compensation payable.
NOTE 14:12: COMMITMENTS AND CONTINGENCIES
Contractual Obligations
The Company leases certain facilities and equipment used in its operations and accounts for leases with escalating payments using the straight-line method. The Company incurred expenses of approximately $6,125, $6,096 $6,177 and $6,174$6,177 in connection with operating leases during 2013, 2012 2011 and 2010,2011, respectively. The approximate aggregate commitments under non-cancelable operating leases in effect at December 31, 20122013 were as follows:
Year Ending December 31, | | | |
2013 | | $ | 4,688 | |
2014 | | 3,961 | |
2015 | | 2,542 | |
2016 | | 1,618 | |
2017 | | 1,598 | |
Thereafter | | 640 | |
Total | | $ | 15,047 | |
60
Table of Contents
Year Ending December 31, | | | |
2014 | | $ | 4,841 | |
2015 | | 3,427 | |
2016 | | 2,678 | |
2017 | | 2,575 | |
2018 | | 1,460 | |
Thereafter | | 453 | |
Total | | $ | 15,434 | |
The Company enters into contracts with suppliers to ensure the availability of both botanical and non-botanical raw materials, as well as packing materials, in advance of its annual requirements. As of December 31, 2012,2013, the Company has entered into non-cancelable purchase agreements for $8,344$43 related to fiscal year 20132014 production needs.
The Company has entered into long-term agreements with third-parties in the ordinary course of business, in which it has agreed to pay a percentage of net sales in certain regions in which it operates, or royalties on certain products. In 2013, 2012 2011 and 2010,2011, the aggregate amounts of these payments were $1,468, $1,270 and $8,360, respectively.
In 2013, the Company began to significantly reinvest in its information technology systems. Included within this plan is an Oracle ERP implementation program to provide the Company with a single integrated software solution that will integrate the
68
Table of Contents
Company’s business process on a worldwide basis. The Company has committed to invest an additional $6,478 over the course of the project and $2,987,anticipates completion of this project by mid-2016. This amount is expected to be paid in future years as follows: $3,134 in 2014, $3,001 in 2015, and $343 in 2016, respectively.
Legal Proceedings
The Company is party to various legal proceedings, including those noted below.proceedings. Management cannot predict the ultimate outcome of these proceedings, individually or in the aggregate, or their resulting effect on the Company’s business, financial position, results of operations or cash flows as litigation and related matters are subject to inherent uncertainties, and unfavorable rulings could occur. Were an unfavorable outcome to occur, there exists the possibility of a material adverse impact on the business, financial position, results of operations, or cash flows for the period in which the ruling occurs and/or future periods. The Company maintains product liability, general liability and excess liability insurance coverage. However, no assurances can be given that such insurance will continue to be available at an acceptable cost to the Company, that such coverage will be sufficient to cover one or more large claims, or that the insurers will not successfully disclaim coverage as to a pending or future claim.
Since late 2007, the Company has administered its sales in Belarus, Georgia, Kazakhstan, Moldova, Mongolia, Russia and Ukraine (the “Territories”) through an International Reseller Agreement (“Reseller Agreement”) with a third party general dealer (the “General Dealer”) based in Russia. The General Dealer administers the marketing and distribution of the Company’s products in the Territories. The Reseller Agreement has a one-year term, which has been renewed annually by the parties, and is set to expire on November 1, 2014. As a part of its services, the General Dealer provides certain discounts (the “Discounts”) to its network of dealers related to the costs associated with transporting the Company’s products from the General Dealer to the dealers. In July 2013, the General Dealer began to withhold the amount of these Discounts from the funds remitted each month to the Company for the sale of the products, claiming that it is entitled to reimbursement for these costs under the Reseller Agreement. These withholdings have averaged approximately $330 per month and totaled approximately $2,000 at December 31, 2013. The Company disagrees with the General Dealer’s interpretation of the Reseller Agreement and disputes any and all of the General Dealer’s reimbursement claims. While the parties have attempted to negotiate a resolution to the dispute, they have been unable to resolve the matter and the Company now plans to submit a claim for payment of the withheld funds to binding arbitration in the United Kingdom, pursuant to the terms of the Reseller Agreement. Notwithstanding the annual renewal of the Reseller Agreement, the General Dealer may seek reimbursement of Discounts with respect to the periods prior to July 2013, which would total approximately $22,000. The Company believes the General Dealer’s claims are meritless and the Company intends to pursue its claims against the General Dealer.
The General Dealer is the Company’s sole general dealer in the Territories and the Company currently does not have in place any other third party or Company infrastructure capable of immediately assuming the General Dealer’s marketing and distribution obligations. Although the Company plans to continue to operate under the Reseller Agreement through the expiration of its term and is currently negotiating an extension, if the Company’s relationship with the General Dealer deteriorates or if the General Dealer ceases to provide such services in the Territories, the Company will need to identify, engage and train a new general dealer, establish its own marketing and distribution operations in the Territories, or create a hybrid model. Any such cessation of service and subsequent transition to another third party provider, or the establishment of its own distribution operations, could result in disruption to the Company’s business in the Territories, which could have an adverse effect on the Company’s business and results of operations. The Company is committed to its Managers and Distributors in the Territories and is actively working to ensure that they will supported going forward.
Other Litigation
The Company is party to various other legal proceedings in several foreign jurisdictions related to VATvalue-added tax assessments and other civil litigation. While there is a reasonable possibility that a material loss may be incurred, either the losses are not considered to be probable or the Company cannot at this time estimate the loss, if any; therefore, no provision for losses has been provided. The Company believes future payments related to these matters could range from $0 to approximately $1,100.$900.
Non-Income Tax Contingencies
The Company has reserved for certain state sales and use tax and foreign non-income tax contingencies based on the likelihood of an obligation in accordance with accounting guidance for probable loss contingencies. Loss contingency provisions are recorded for probable losses at management’s best estimate of a loss, or when a best estimate cannot be made, a minimum loss contingency amount is recorded. The Company provides provisions for potential payments of tax to various tax authorities for contingencies related to non-income tax matters, including value addedvalue-added taxes and sales tax. The Company provides provisions for U.S. state sales taxes in each of the states where the Company has nexus. As of December 31, 20122013 and 2011,2012, accrued liabilities include $6,207$6,312 and $6,921,$6,207, respectively, related to non-income tax contingencies. While management believes that the assumptions and estimates used to determine this liability are reasonable, the ultimate outcome of those matters cannot presently be determined. The Company is not able at this time to predict the ultimate outcomes of those matters or to estimate the effect of the ultimate outcomes, if greater than the amounts accrued, would have on the financial condition, results of operations or cash flows of the Company.
69
Table of Contents
Self-Insurance Liabilities
Similar to other manufacturers and Distributors of products that are ingested, the Company faces an inherent risk of exposure to product liability claims in the event that, among other things, the use of its products results in injury. The Company carries insurance in the types and amounts it considers reasonably adequate to cover the risks associated with its business. The Company has a wholly owned captive insurance company to provide it with product liability insurance coverage. The Company has accrued an amount that it believes is sufficient to cover probable and reasonably estimable liabilities related to product liability claims based on the Company’s history of such claims. However, there can be no assurance that these estimates will prove to be sufficient, nor can there be any assurance that the ultimate outcome of any litigation for product liability will not have a material negative impact on the Company’s business prospects, financial position, results of operations or cash flows.
The Company self-insures for certain employee medical benefits. The recorded liabilities for self-insured risks are calculated using actuarial methods and are not discounted. The liabilities include amounts for actual claims and claims incurred but not reported. Actual experience, including claim frequency and severity as well as health care inflation, could result in actual liabilities being more or less than the amounts currently recorded.
The Company reviews its self-insurance accruals on a quarterly basis and determines, based upon a review of its recent claims history and other factors, which portions of its self-insurance accruals should be considered short-term and long-term. The Company has accrued $2,990$2,811 and $2,892$2,990 for product liability and employee medical claims at December 31, 2013 and 2012, and 2011,
61
Table of Contents
respectively, of which $532$526 and $489$532 was classified as short-term. Such amounts are included in accrued liabilities and other long-term liabilities on the Company’s consolidated balance sheets.
Government Regulations
The Company is subject to governmental regulations pertaining to product formulation, labeling and packaging, product claims and advertising, and to the Company’s direct selling system. The Company is also subject to the jurisdiction of numerous foreign tax and customs authorities. Any assertions or determinations that either the Company or the Company’s Distributors are not in compliance with existing statutes, laws, rules or regulations could potentially have a material adverse effect on the Company’s operations. In addition, in any country or jurisdiction, the adoption of new statutes, laws, rules or regulations, or changes in the interpretation of existing statutes, laws, rules or regulations could have a material adverse effect on the Company and its operations. Although management believes that the Company is in compliance, in all material respects, with the statutes, laws, rules and regulations of every jurisdiction in which it operates, no assurance can be given that the Company’s compliance with applicable statutes, laws, rules and regulations will not be challenged by foreign authorities or that such challenges will not have a material adverse effect on the Company’s financial position, results of operations or cash flows.
NOTE 15:13: OPERATING BUSINESS SEGMENT AND INTERNATIONAL OPERATION INFORMATION
The Company has three business segments. These business segments are components of the Company for which separate information is available that is evaluated regularly by the chief executive officer in deciding how to allocate resources and in assessing relative performance.
The Company has two business segments that operate under the Nature’s Sunshine Products brand and are divided based on the characteristics of their Distributor base, similarities in compensation plans, as well as the internal organization of NSP’s officers and their responsibilities (NSP Americas, Asia Pacific and Europe; and NSP Russia, Central and Eastern Europe). The Company’s third business segment operates under the Synergy WorldWide brand, which distributes its products through different marketingselling and Distributor compensation plans and products with formulations that are sufficiently different from those of the NSP Americas, Asia Pacific and Europe; and NSP Russia, Central and Eastern Europe to warrant accounting for these operations as a separate business segment. Net sales revenues for each segment have been reduced by intercompany sales as they are not included in the measure of segment profit or loss reviewed by the chief executive officer. The Company evaluates performance based on contribution margin (loss) by segment before consideration of certain inter-segment transfers and expenses.
Historically, the Company had reported its two business segments that operate under the Nature’s Sunshine Products brand based upon their geographic operations in the United States (NSP United States) and in the countries outside the United States (NSP International). This was how the Company was regularly evaluated by the chief executive officer and executive team.
During 2012, the Company engaged in a reorganization process in which the business segments, the roles of upper management responsible for the operating segments, and the information provided to the chief operating decision maker were reevaluated. As a result of the reorganization process, the two historical NSP segments (NSP United States and NSP International), which were previously separated based on geographical operations, were divided into two different identifiable segments (NSP Americas, Asia Pacific and Europe; and NSP Russia, Central and Eastern Europe) based on the nature of the business activities of each segment, the existence of Managers responsible for them, and information presented to the chief operating decision maker. The two new segments distribute products in differing ways in that NSP Americas, Asia Pacific and Europe sells products through a mixture of retailing, practitioners and direct selling while NSP Russia, Central and Eastern Europe’s business model is more oriented to a network marketing approach. The chief operating decision maker now reviews the operating results of the two new NSP segments. The new NSP segments also conform to the revised internal management structure. There was no change to the Synergy WorldWide segment. The presentation of the comparative information has been revised to conform to the 2012 presentation.
62
Table of Contents
Reportable business segment information for the years ended December 31, 2013, 2012 2011 and 20102011 is as follows:
Year Ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Net sales revenue: | | | | | | | | | | | | | | |
NSP Americas, Asia Pacific and Europe | | $ | 208,945 | | $ | 216,912 | | $ | 225,213 | | | $ | 207,059 | | $ | 208,945 | | $ | 216,912 | |
NSP Russia, Central and Eastern Europe | | 57,853 | | 56,986 | | 56,141 | | | 62,747 | | 57,853 | | 56,986 | |
Synergy WorldWide | | 100,670 | | 93,915 | | 68,564 | | | 108,290 | | 100,670 | | 93,915 | |
Total net sales revenue | | 367,468 | | 367,813 | | 349,918 | | | 378,096 | | 367,468 | | 367,813 | |
Contribution margin (1): | | | | | | | | | | | | | | |
NSP Americas, Asia Pacific and Europe | | 82,778 | | 86,004 | | 86,570 | | | 84,247 | | 82,778 | | 86,004 | |
NSP Russia, Central and Eastern Europe | | 21,957 | | 22,374 | | 20,755 | | | 22,542 | | 21,957 | | 22,374 | |
Synergy WorldWide | | 36,142 | | 36,143 | | 22,962 | | | 38,011 | | 36,142 | | 36,143 | |
Total contribution margin | | 140,877 | | 144,521 | | 130,287 | | | 144,800 | | 140,877 | | 144,521 | |
| | | | | | | | | | | | | | |
Selling, general and administrative | | 106,861 | | 109,606 | | 119,024 | | | 120,743 | | 106,861 | | 109,606 | |
Contract termination costs | | — | | 14,750 | | — | | | — | | — | | 14,750 | |
Operating income | | 34,016 | | 20,165 | | 11,263 | | | 24,057 | | 34,016 | | 20,165 | |
| | | | | | | | | | | | | | |
Other income, net | | 1,480 | | 1,847 | | 2,727 | | | 1,596 | | 1,480 | | 1,847 | |
Income before provision for income taxes | | $ | 35,496 | | $ | 22,012 | | $ | 13,990 | | | $ | 25,653 | | $ | 35,496 | | $ | 22,012 | |
(1) Contribution margin consists of net sales revenue less cost of sales and volume incentives expense.
Year Ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Capital expenditures: | | | | | | | | | | | | | | |
NSP Americas, Asia Pacific and Europe | | $ | 5,684 | | $ | 993 | | $ | 1,898 | | | $ | 8,018 | | $ | 5,684 | | $ | 993 | |
NSP Russia, Central and Eastern Europe | | 44 | | 66 | | — | | | 4 | | 44 | | 66 | |
Synergy WorldWide | | 1,051 | | 1,183 | | 779 | | | 534 | | 1,051 | | 1,183 | |
Total capital expenditures | | $ | 6,779 | | $ | 2,242 | | $ | 2,677 | | | $ | 8,556 | | $ | 6,779 | | $ | 2,242 | |
| | | | | | | | | | | | | | |
Depreciation and amortization: | | | | | | | | | | | | | | |
NSP Americas, Asia Pacific and Europe | | $ | 3,339 | | $ | 3,827 | | $ | 3,721 | | | $ | 3,568 | | $ | 3,339 | | $ | 3,827 | |
NSP Russia, Central and Eastern Europe | | 36 | | 43 | | 23 | | | 27 | | 36 | | 43 | |
Synergy WorldWide | | 703 | | 492 | | 510 | | | 871 | | 703 | | 492 | |
Total depreciation and amortization | | $ | 4,078 | | $ | 4,362 | | $ | 4,254 | | | $ | 4,466 | | $ | 4,078 | | $ | 4,362 | |
As of December 31, | | 2012 | | 2011 | | | 2013 | | 2012 | |
Assets: | | | | | | | | | | |
NSP Americas, Asia Pacific and Europe | | $ | 136,237 | | $ | 124,483 | | | $ | 148,278 | | $ | 136,237 | |
NSP Russia, Central and Eastern Europe | | 8,558 | | 9,030 | | | 11,233 | | 8,558 | |
Synergy WorldWide | | 49,124 | | 42,298 | | | 40,101 | | 49,124 | |
Total assets | | $ | 193,919 | | $ | 175,811 | | | $ | 199,612 | | $ | 193,919 | |
From an individual country perspective, only the United States comprises approximately 10 percent or more of consolidated net sales revenue for any of the years ended December 31, 2013, 2012 2011 and 20102011 as follows:
Year Ended December 31, | | 2012 | | 2011 | | 2010 | | | 2013 | | 2012 | | 2011 | |
Net sales revenue: | | | | | | | | | | | | | | |
United States | | $ | 154,716 | | $ | 159,471 | | $ | 156,767 | | | $ | 152,209 | | $ | 154,716 | | $ | 159,471 | |
Other | | 212,752 | | 208,342 | | 193,151 | | | 225,887 | | 212,752 | | 208,342 | |
Total net sales revenue | | $ | 367,468 | | $ | 367,813 | | $ | 349,918 | | | $ | 378,096 | | $ | 367,468 | | $ | 367,813 | |
Revenue generated by each of the Company’s product lines is set forth below (U.S. dollars in thousands):
Year Ended December 31, | | 2013 | | 2012 | | 2011 | |
NSP Americas, Asia Pacific and Europe: | | | | | | | |
General health | | $ | 88,239 | | $ | 91,994 | | $ | 95,342 | |
Immunity | | 25,187 | | 25,331 | | 26,099 | |
Cardiovascular | | 14,004 | | 13,897 | | 15,095 | |
Digestive | | 59,877 | | 58,127 | | 62,175 | |
Personal care | | 5,543 | | 6,400 | | 5,908 | |
Weight management | | 14,209 | | 13,196 | | 12,293 | |
| | 207,059 | | 208,945 | | 216,912 | |
NSP Russia, Central and Eastern Europe: | | | | | | | |
General health | | $ | 22,690 | | $ | 20,540 | | $ | 19,448 | |
Immunity | | 7,902 | | 7,365 | | 7,558 | |
Cardiovascular | | 4,324 | | 4,367 | | 5,342 | |
Digestive | | 15,693 | | 14,501 | | 14,857 | |
Personal care | | 8,817 | | 8,908 | | 7,848 | |
Weight management | | 3,321 | | 2,172 | | 1,933 | |
| | 62,747 | | 57,853 | | 56,986 | |
Synergy WorldWide: | | | | | | | |
General health | | $ | 36,723 | | $ | 33,969 | | $ | 29,592 | |
Immunity | | 1,394 | | 1,104 | | 802 | |
Cardiovascular | | 42,154 | | 42,696 | | 41,163 | |
Digestive | | 16,897 | | 14,904 | | 13,606 | |
Personal care | | 7,097 | | 5,631 | | 6,674 | |
Weight management | | 4,025 | | 2,366 | | 2,078 | |
| | 108,290 | | 100,670 | | 93,915 | |
Total net sales revenue | | $ | 378,096 | | $ | 367,468 | | $ | 367,813 | |
6371
Table of Contents
Year Ended December 31, | | 2012 | | 2011 | | 2010 | |
NSP Americas, Asia Pacific and Europe: | | | | | | | |
Herbal Products | | $ | 118,211 | | $ | 121,907 | | $ | 118,531 | |
Vitamins and Mineral and Other Nutritional Supplements | | 80,567 | | 81,974 | | 91,852 | |
Personal Care Products | | 6,150 | | 7,503 | | 7,924 | |
Other Products | | 4,017 | | 5,528 | | 6,906 | |
| | 208,945 | | 216,912 | | 225,213 | |
NSP Russia, Central and Eastern Europe: | | | | | | | |
Herbal Products | | $ | 25,311 | | $ | 25,120 | | $ | 26,867 | |
Vitamins and Mineral and Other Nutritional Supplements | | 26,147 | | 24,202 | | 24,416 | |
Personal Care Products | | 6,203 | | 7,479 | | 4,638 | |
Other Products | | 192 | | 185 | | 220 | |
| | 57,853 | | 56,986 | | 56,141 | |
Synergy WorldWide: | | | | | | | |
Herbal Products | | $ | 39,607 | | $ | 33,563 | | $ | 28,045 | |
Vitamins and Mineral and Other Nutritional Supplements | | 54,128 | | 50,936 | | 32,296 | |
Personal Care Products | | 5,350 | | 7,526 | | 6,542 | |
Other Products | | 1,585 | | 1,890 | | 1,681 | |
| | 100,670 | | 93,915 | | 68,564 | |
Total net sales revenue | | $ | 367,468 | | $ | 367,813 | | $ | 349,918 | |
From an individual country perspective, only the United States and Venezuela comprise 10 percent or more of consolidated property, plant and equipment as follows:
As of December 31 | | 2012 | | 2011 | | | 2013 | | 2012 | |
Property, plant and equipment | | | | | | | | | | |
United States | | $ | 20,923 | | $ | 18,119 | | | $ | 25,713 | | $ | 20,923 | |
Venezuela | | 3,535 | | 3,939 | | | 3,207 | | 3,535 | |
Other | | 3,492 | | 3,079 | | | 3,102 | | 3,492 | |
Total property, plant and equipment | | $ | 27,950 | | $ | 25,137 | | | $ | 32,022 | | $ | 27,950 | |
NOTE 16:14: RELATED PARTY TRANSACTIONS
The Company maintains split-dollar life insurance policies on certain executives. The cash surrender value of $49$48 and $51$49 related to such policies is recorded in other assets as of December 31, 20122013 and 2011,2012, respectively.
Mr. Eugene Hughes, a former member of the Company’s Board of Directors and a shareholder, retired as an employee of the Company effective as of December 22, 2008. Prior to his retirement, the Company and Mr. Hughes entered into a Retirement and Consulting Agreement, dated as of December 9, 2008, pursuant to which Mr. Hughes provides consulting services to the Company for an initial term of eight years following his retirement. In exchange for such consulting services, Mr. Hughes will receive (i) annual compensation of $215 for the first two years of service, (ii) annual compensation of $100 for the remainder of the initial term, (iii) annual compensation of $50 after the initial term, and (iv) certain medical and life insurance benefits.
NOTE 17:15: FAIR VALUE
The fair value of a financial instrument is the amount that could be received upon the sale of an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. Financial assets are marked to bid prices and financial liabilities are marked to offer prices. Fair value measurements do not include transaction costs. A fair value hierarchy is used to prioritize the quality and reliability of the information used to determine fair values of each financial instrument. Categorization within the fair value hierarchy is based on the lowest level of input that is significant to the fair value measurement. The fair value hierarchy is defined into the following three categories:
Level 1: Quoted market prices in active markets for identical assets or liabilities.
Level 2: Observable market based inputs or unobservable inputs that are corroborated by market data.
Level 3: Unobservable inputs that are not corroborated by market datadata.
The following table presents the Company’s hierarchy for its asset measured at fair value on a recurring basis as of December 31, 2013:
| | Level 1 | | Level 2 | | Level 3 | | | |
| | Quoted Prices
in Active
Markets for
Identical Assets | | Significant
Other
Observable
Inputs | | Significant
Unobservable
Inputs | | Total | |
Investments available-for-sale | | | | | | | | | |
Municipal obligations | | $ | — | | $ | 415 | | $ | — | | $ | 415 | |
U.S. government security funds | | 983 | | — | | — | | 983 | |
Equity securities | | 608 | | — | | — | | 608 | |
Investment securities | | 971 | | — | | — | | 971 | |
Total assets measured at fair value on a recurring basis | | $ | 2,562 | | $ | 415 | | $ | — | | $ | 2,977 | |
6472
Table of Contents
The following table presents the Company’s hierarchy for its asset measured at fair value on a recurring basis as of December 31, 2012:
| | Level 1 | | Level 2 | | Level 3 | | | |
| | Quoted Prices
in Active
Markets for
Identical Assets | | Significant
Other
Observable
Inputs | | Significant
Unobservable
Inputs | | Total | |
Investments available-for-sale | | | | | | | | | |
Municipal obligations | | $ | — | | $ | 638 | | $ | — | | $ | 638 | |
U.S. government security funds | | 986 | | — | | — | | 986 | |
Equity securities | | 447 | | — | | — | | 447 | |
Investment securities | | 1,276 | | — | | — | | 1,276 | |
Total assets measured at fair value on a recurring basis | | $ | 2,709 | | $ | 638 | | $ | — | | $ | 3,347 | |
The following table presents the Company’s hierarchy for its assets measured at fair value on a recurring basis as of December 31, 2011:
| | Level 1 | | Level 2 | | Level 3 | | | |
| | Quoted Prices
in Active
Markets for
Identical Assets | | Significant
Other
Observable
Inputs | | Significant
Unobservable
Inputs | | Total | |
Investments available-for-sale | | | | | | | | | |
Municipal obligations | | $ | — | | $ | 1,209 | | $ | — | | $ | 1,209 | |
U.S. government security funds | | 983 | | — | | — | | 983 | |
Short-term deposits | | — | | 3,104 | | — | | 3,104 | |
Equity securities | | 381 | | — | | — | | 381 | |
Investment securities | | 1,429 | | — | | — | | 1,429 | |
Total assets measured at fair value on a recurring basis | | $ | 2,793 | | $ | 4,313 | | $ | — | | $ | 7,106 | |
Investments available-for-sale — The majority of the Company’s investment portfolio consist of various securities such as state and municipal obligations, U.S. government security funds, short-term deposits and various equity securities. The Level 1 securities are valued using quoted prices for identical assets in active markets including equity securities and U.S. government treasuries. The Level 2 securities include investments in state and municipal obligations whereby all significant inputs are observable or can be derived from or corroborated by observable market data for substantially the full term of the asset.
Investment securities — The majority of the Company’s trading portfolio consists of various marketable securities that are valued using quoted prices in active markets.
For the years ended December 31, 20122013 and 2011,2012, there were no fair value measurements using significant unobservable inputs (Level 3).
NOTE 18:16: SUMMARY OF QUARTERLY OPERATIONS — UNAUDITED
As discussed in Note 2, during the year ended December 31, 2012, the Company changed its method of accounting to present shipping and handling costs related to the sale of the Company’s products together with the cost of sales in the consolidated statement of operations. These shipping and handling costs have previously been presented by the Company as part of selling, general and administrative expenses. This change has been made retrospectively to all periods presented in the Company’s financial statements. The impact of the change in accounting principle in the three months ended December 31, 2012 was to increase cost of sales and decrease selling, general and administrative expenses by $5,281. The total amount of shipping and handling costs reclassified by the Company from selling, general and administrative expense to cost of sales in its consolidated statements of operations for the three months ended September 30, 2012, June 30, 2012, and March 31, 2012 is $5,431, $5,524, and $5,369, respectively. The total amount of shipping and handling costs reclassified by the Company from selling, general and administrative expense to cost of sales in its consolidated statements of operations for the three months ended December 31, 2011, September 30, 2011, June 30, 2011, and March 31, 2011 is $5,061, $5,078, $4,911 and $4,949, respectively.
The following tables presents the Company’s unaudited summary of quarterly operations during 20122013 and 20112012 for each of three month periods ended March 31, June 30, September 30, and December 31.
| | For the Quarter Ended | |
| | March 31,
2013 | | June 30,
2013 | | September
30, 2013 | | December
31, 2013 | |
Net sales revenue | | $ | 96,479 | | $ | 93,675 | | $ | 92,458 | | $ | 95,484 | |
Cost of sales | | (24,445 | ) | (22,630 | ) | (23,655 | ) | (24,084 | ) |
Gross profit | | 72,034 | | 71,045 | | 68,803 | | 71,400 | |
| | | | | | | | | |
Volume incentives | | 34,975 | | 34,525 | | 33,920 | | 35,062 | |
Selling, general and administrative | | 30,117 | | 28,709 | | 28,170 | | 33,747 | |
Operating income | | 6,942 | | 7,811 | | 6,713 | | 2,591 | |
Other income (expense) | | 330 | | 1,482 | | (269 | ) | 53 | |
Income before income taxes | | 7,272 | | 9,293 | | 6,444 | | 2,644 | |
Tax provision | | 2,408 | | 3,241 | | 1,594 | | 801 | |
Net income | | $ | 4,864 | | $ | 6,052 | | $ | 4,850 | | $ | 1,843 | |
| | | | | | | | | |
Basic and diluted net income per common share | | | | | | | | | |
| | | | | | | | | |
Basic: | | | | | | | | | |
Net income per common share | | $ | 0.31 | | $ | 0.38 | | $ | 0.30 | | $ | 0.11 | |
| | | | | | | | | |
Diluted: | | | | | | | | | |
Net income per common share | | $ | 0.30 | | $ | 0.38 | | $ | 0.29 | | $ | 0.11 | |
| | | | | | | | | |
Dividends declared per common share | | $ | 0.10 | | $ | 0.10 | | $ | 1.60 | | $ | 0.10 | |
6573
Table of Contents
| | For the Quarter Ended | |
| | March 31,
2012 | | June 30,
2012 | | September
30, 2012 | | December
31, 2012 | |
Net sales revenue | | $ | 92,868 | | $ | 92,991 | | $ | 91,232 | | $ | 90,377 | |
Cost of sales | | (23,729 | ) | (22,610 | ) | (23,144 | ) | (23,841 | ) |
Gross profit | | 69,139 | | 70,381 | | 68,088 | | 66,536 | |
| | | | | | | | | |
Volume incentives | | 33,581 | | 33,540 | | 33,155 | | 32,991 | |
Selling, general and administrative | | 26,384 | | 26,530 | | 26,228 | | 27,719 | |
Operating income | | 9,174 | | 10,311 | | 8,705 | | 5,826 | |
Other income (expense) | | (110 | ) | 165 | | (214 | ) | 1,639 | |
Income before income taxes | | 9,064 | | 10,476 | | 8,491 | | 7,465 | |
Tax provision | | 1,836 | | 3,190 | | 2,121 | | 2,969 | |
Net income | | $ | 7,228 | | $ | 7,286 | | $ | 6,370 | | $ | 4,496 | |
Basic and diluted net income per common share | | | | | | | | | |
| | | | | | | | | |
Basic: | | | | | | | | | |
Net income per common share | | $ | 0.46 | | $ | 0.47 | | $ | 0.41 | | $ | 0.28 | |
| | | | | | | | | |
Diluted: | | | | | | | | | |
Net income per common share | | $ | 0.46 | | $ | 0.46 | | $ | 0.40 | | $ | 0.28 | |
| | | | | | | | | |
Dividends declared per common share | | $ | — | | $ | 0.05 | | $ | 0.05 | | $ | 0.05 | |
| | For the Quarter Ended | |
| | March 31,
2011 | | June 30,
2011 | | September
30, 2011 | | December
31, 2011 | |
Net sales revenue | | $ | 92,844 | | $ | 91,811 | | $ | 91,102 | | $ | 92,056 | |
Cost of sales | | (23,501 | ) | (22,040 | ) | (21,957 | ) | (21,911 | ) |
Gross profit | | 69,343 | | 69,771 | | 69,145 | | 70,145 | |
| | | | | | | | | |
Volume incentives | | 34,298 | | 33,390 | | 32,733 | | 33,462 | |
Selling, general and administrative | | 27,424 | | 28,329 | | 26,767 | | 27,086 | |
Contract termination costs | | — | | — | | 14,750 | | — | |
Operating income (loss) | | 7,621 | | 8,052 | | (5,105 | ) | 9,597 | |
Other income (expense) | | 265 | | (420 | ) | 1,204 | | 798 | |
Income (loss) before income taxes | | 7,886 | | 7,632 | | (3,901 | ) | 10,395 | |
Tax provision (benefit) | | 1,264 | | 2,018 | | (1,645 | ) | 2,774 | |
Net income (loss) | | $ | 6,622 | | $ | 5,614 | | $ | (2,256 | ) | $ | 7,621 | |
| | | | | | | | | |
Basic and diluted net income (loss) per common share | | | | | | | | | |
| | | | | | | | | |
Basic: | | | | | | | | | |
Net income per common share | | $ | 0.43 | | $ | 0.36 | | $ | (0.14 | ) | $ | 0.49 | |
| | | | | | | | | |
Diluted: | | | | | | | | | |
Net income per common share | | $ | 0.43 | | $ | 0.36 | | $ | (0.14 | ) | $ | 0.48 | |
| | | | | | | | | |
Dividends declared per common share | | $ | — | | $ | — | | $ | — | | $ | — | |
Basic and diluted income (loss) per share is computed independently for each of the quarters presented. Therefore, the sum of the quarterly net loss per share may not equal the total computed for the year.
Item 9. Change In and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
This report includes the certifications of our Chief Executive Officer and Chief Financial Officer required by Rule 13a-14 of the Securities Exchange Act of 1934 (the “Exchange Act”). See Exhibits 31.1 and 31.2. This Item 9A includes information concerning the controls and control evaluations referred to in those certifications.
66
Table of Contents
Overview
Management is responsible for establishing and maintaining adequate internal controls over financial reporting for the Company.
The following discussion sets forth a summary of management’s evaluation of our disclosure controls and procedures as of December 31, 2012.2013. In addition, this item provides a discussion of management’s evaluation of internal control over financial reporting.
Our independent registered public accountants have also issued an audit report on our internal control over financial reporting. This report appears below.
74
Table of Contents
Evaluation of Disclosure Controls and Procedures
Our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) are designed to ensure that information required to be disclosed in reports filed or submitted under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in rules and forms adopted by the SEC, and that such information is accumulated and communicated to management, including the Chief Executive Officer and the Chief Financial Officer, to allow timely decisions regarding required disclosures.
In connection with the preparation of our Annual Report as of December 31, 2012,2013, our management, under the supervision and with the participation of the Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2012.2013. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures were effective as of December 31, 2012.2013.
Management’s Report on Internal Control over Financial Reporting
Management, with the participation of our Chief Executive Officer and Chief Financial Officer, has conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework set forth in “Internal“Internal Control—Integrated Framework”Framework (1992)” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on management’s assessment under this framework, management has concluded that our internal control over financial reporting was effective as of December 31, 2012.2013. The effectiveness of our internal control over financial reporting as of December 31, 20122013 has been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report which is included herein.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) under the Exchange Act) that occurred during the fourth quarter ended December 31, 20122013, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
6775
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Nature’s Sunshine Products, Inc.:
We have audited the internal control over financial reporting of Nature’s Sunshine Products, Inc. and subsidiaries (the “Company”) as of December 31, 2012,2013, based on criteria established in Internal Control — Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2012,2013, based on the criteria established in Internal Control — Integrated Framework (1992)issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements and consolidated financial statement schedule as of and for the year ended December 31, 20122013 of the Company and our report dated March 6, 201317, 2014 expressed an unqualified opinion on those consolidated financial statements and consolidated financial statement schedule, and included an explanatory paragraph regarding the Company’s change in accounting method for presentation of shipping and handling costs.schedule.
/s/ Deloitte & Touche LLP | |
| |
Salt Lake City, Utah | |
March 6, 201317, 2014 | |
6876
Table of Contents
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2012,2013, except that the information required with respect to our executive officers is set forth under Item 1. “Business”, of this Annual Report on Form 10-K, and is incorporated herein by reference.
Item 11. Executive Compensation
The information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2012.2013.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2012.2013.
Item 13. Certain Relationships and Related Transactions and Director Independence
The information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2012.2013.
Item 14. Principal Accounting Fees and Services.
The information required by this Item is incorporated herein by reference to our definitive proxy statement to be filed with the SEC no later than 120 days after the close of our fiscal year ended December 31, 2012.2013.
77
Table of Contents
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a)(1) | | List of Financial Statements |
| | |
| | The following are filed as part of this report: |
| | |
| | Report of Independent Registered Public Accounting Firm |
| | |
| | Consolidated balance sheets as of December 31, 20122013 and 20112012 |
| | |
| | Consolidated statements of operations for the years ended December 31, 2013, 2012, 2011, and 20102011 |
| | |
| | Consolidated statements of comprehensive income for the years ended December 31, 2013, 2012, 2011, and 20102011 |
| | |
| | Consolidated statements of changes in shareholders’ equity for the years ended December 31, 2013, 2012, 2011, and 20102011 |
| | |
| | Consolidated statements of cash flows for the years ended December 31, 2013, 2012, 2011, and 20102011 |
| | |
| | Notes to consolidated financial statements |
| | |
(a)(2) | | List of Financial Statement Schedules |
| | |
| | Schedule II - Valuation and Qualifying Accounts. |
| | |
| | Financial statement schedules other than the one listed are omitted for the reason that they are not required or are not applicable, or the required information is shown in the financial statements or notes thereto, or contained elsewhere in this report. |
| | |
(a)(3) | | List of Exhibits |
| | |
| | Exhibit Index as seen below |
6978
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Nature’s Sunshine Products, Inc.
Date: March 6, 201317, 2014 | By: | /s/ Michael D. DeanGregory L. Probert |
| | |
| | Michael D. Dean,Gregory L. Probert,
|
| | Chief Executive Officer and Chairman of the Board |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | |
/s/ Gregory L. Probert | | Chief Executive Officer and Chairman of the Board and Director | | March 6, 201317, 2014 |
Gregory L. Probert | | | | |
| | | | |
/s/ Michael D. Dean
| | Chief Executive Officer and Director
| | March 6, 2013
|
Michael D. Dean
| | | | |
| | | | |
/s/ Kristine F. Hughes | | Vice Chair of the Board and Director | | March 6, 201317, 2014 |
Kristine F. Hughes | | | | |
| | | | |
/s/ Stephen M.Steve Bunker | | Executive Vice President, | | March 6, 201317, 2014 |
Stephen M.Steve Bunker
| | Chief Financial Officer and Treasurer, Chief Accounting | | |
| | Officer | | |
| | | | |
/s/ Albert R. Dowden | | Director | | March 6, 201317, 2014 |
Albert R. Dowden | | | | |
| | | | |
/s/Mark R. Genender
| | Director
| | March 6, 2013
|
Mark R. Genender
| | | | |
| | | | |
/s/ Robert B. Mercer | | Director | | March 6, 201317, 2014 |
Robert B. Mercer | | | | |
| | | | |
/s/ Willem Mesdag | | Director | | March 6, 201317, 2014 |
Willem Mesdag | | | | |
| | | | |
/s/ Beth Springer | | Director | | March 17, 2014 |
Beth Springer | | | | |
| | | | |
/s/ Jeffrey D. Watkins | | Director | | March 6, 201317, 2014 |
Jeffrey D. Watkins | | | | |
7079
Table of Contents
NATURE’S SUNSHINE PRODUCTS, INC.
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
FOR THE YEARS ENDED DECEMBER 31, 2013, 2012, 2011, AND 20102011
(Amounts in thousands)
Description | | Balance at
Beginning
of Year | | Provisions | | Amounts
Written Off | | Amounts
Recovered | | Effect of
Currency
Translation | | Balance at
End of Year | | | Balance at
Beginning
of Year | | Provisions | | Amounts
Written Off | | Amounts
Recovered | | Effect of
Currency
Translation | | Balance at
End of Year | |
| | | | | | | | | | | | | | |
Year ended December 31, 2013 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Allowance for doubtful accounts receivable | | | $ | 631 | | $ | 535 | | $ | (18 | ) | $ | 1 | | $ | (62 | ) | $ | 1,087 | |
| | | | | | | | | | | | | | |
Allowance for sales returns | | | 154 | | 1,435 | | (1,454 | ) | — | | — | | 135 | |
| | | | | | | | | | | | | | |
Allowance for obsolete inventory | | | 2,254 | | 1,600 | | (1,577 | ) | 41 | | 89 | | 2,407 | |
| | | | | | | | | | | | | | |
Tax valuation allowance | | | 8,149 | | 3,191 | | — | | — | | — | | 11,340 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Year ended December 31, 2012 | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Allowance for doubtful accounts receivable | | $ | 647 | | $ | 45 | | $ | (86 | ) | $ | (1 | ) | $ | 26 | | $ | 631 | | | $ | 647 | | $ | 45 | | $ | (86 | ) | $ | (1 | ) | $ | 26 | | $ | 631 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Allowance for sales returns | | 109 | | 2,296 | | (2,249 | ) | — | | (2 | ) | 154 | | | 109 | | 2,296 | | (2,249 | ) | — | | (2 | ) | 154 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Allowance for obsolete inventory | | 2,083 | | 985 | | (809 | ) | — | | (5 | ) | 2,254 | | | 2,083 | | 985 | | (809 | ) | — | | (5 | ) | 2,254 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Tax valuation allowance | | 9,836 | | (1,687 | ) | — | | — | | — | | 8,149 | | | 9,836 | | (1,687 | ) | — | | — | | — | | 8,149 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Year ended December 31, 2011 | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Allowance for doubtful accounts receivable | | $ | 918 | | $ | (133 | ) | $ | (174 | ) | $ | 12 | | $ | 24 | | $ | 647 | | | $ | 918 | | $ | (133 | ) | $ | (174 | ) | $ | 12 | | $ | 24 | | $ | 647 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Allowance for sales returns | | 90 | | 625 | | (606 | ) | — | | — | | 109 | | | 90 | | 625 | | (606 | ) | — | | — | | 109 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Allowance for obsolete inventory | | 2,397 | | 732 | | (1,057 | ) | 10 | | 1 | | 2,083 | | | 2,397 | | 732 | | (1,057 | ) | 10 | | 1 | | 2,083 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Tax valuation allowance | | 12,282 | | (2,390 | ) | (56 | ) | — | | — | | 9,836 | | | 12,282 | | (2,390 | ) | (56 | ) | — | | — | | 9,836 | |
| | | | | | | | | | | | | | |
Year ended December 31, 2010 | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Allowance for doubtful accounts receivable | | $ | 1,840 | | $ | 213 | | $ | (1,148 | ) | $ | — | | $ | 13 | | $ | 918 | | |
| | | | | | | | | | | | | | |
Allowance for sales returns | | 55 | | 640 | | (605 | ) | — | | — | | 90 | | |
| | | | | | | | | | | | | | |
Allowance for obsolete inventory | | 2,833 | | 1,196 | | (1,605 | ) | — | | (27 | ) | 2,397 | | |
| | | | | | | | | | | | | | |
Tax valuation allowance | | 18,662 | | 302 | | (6,706 | ) | — | | 24 | | 12,282 | | |
7180
Table of Contents
LIST OF EXHIBITS
Item No. | | Exhibit |
| | |
3.1(1) | | Adjusted and Adjusted Articles of Incorporation. |
3.2(1) | | Adjusted and Adjusted By-laws. |
10.1(1)* | | Nature’s Sunshine Products, Inc. Tax Deferred Retirement Plan, as Adjusted as of March 1, 2008. |
10.2(1)* | | Nature’s Sunshine Products, Inc. Supplemental Elective Deferral Plan, as Adjusted effective as of January 1, 2008. |
10.3(1)*
| | 1995 Stock Option Plan, as Adjusted.
|
10.4(1)*
| | Form of Stock Option Agreement (1995 Stock Option Plan).
|
10.6(3)* | | Employment Agreement, dated as of December 21, 2007, between Nature’s Sunshine Products, Inc. and Stephen M.Steve Bunker. |
10.7(2)* | | Amendment to Employment Agreement, dated as of December 30, 2008, by and between Nature’s Sunshine Products, Inc. and Stephen M.Steve Bunker. |
10.11(4)* | | Retirement and Consulting Agreement, dated as of December 9, 2008, by and between Nature’s Sunshine Products, Inc. and Eugene Hughes. |
10.12(5)*
| | Employment Agreement, dated as of December 21, 2007, by and between Nature’s Sunshine Products, Inc. and Jamon Jarvis.
|
10.13(5)*
| | Amendment to Employment Agreement, dated as of December 30, 2008, by and between Nature’s Sunshine Products, Inc. and Jamon Jarvis.
|
10.14(6)10.14(5)
| | 2009 Stock Incentive Plan |
10.15(9)* | | Form of Award Agreement (2009 Stock Incentive Plan) |
10.16(7)
| | First Amendment to Employment Agreement and Form of Consulting Agreement, dated March 12, 2011, by and between the Company and Douglas Faggioli
|
10.17(7)
| | Employment Agreement, dated March 12, 2010, by and between the Company and Michael D. Dean
|
10.18(7)
| | Stock Option Agreement, dated March 12, 2010, by and between the Company and Michael D. Dean
|
10.19(8)10.19(7)*
| | Employment Agreement, dated June 17, 2011, by and between the Company and Gregory L. Probert |
10.20(8)10.20(7)*
| | Stock Option Agreement, dated June 17, 2011, by and between the Company and Gregory L. Probert |
10.21(9)10.21(8)*
| | Employment Agreement, dated January 25, 2012, by and between the Company and D. Wynne Roberts |
10.22(9)10.22(8)*
| | Stock Option Agreement, dated February 6, 2012, by and between the Company and D.Wynne Roberts |
10.23(12)10.23(11)
| | 2012 Stock Incentive Plan |
10.24(12)10.24(11)*
| | Form of Award Agreement (2012 Stock Incentive Plan) |
10.25(9)* | | Amendment to Employment Agreement, dated March 4, 2013, by and between the Company and Gregory L. Probert |
10.26(9)* | | Consulting Agreement, dated March 4, 2013, by and between the Company and Michael D. Dean |
10.27(9)* | | Amendment to Employment Agreement, dated March 4, 2013, by and between the Company and Michael D. Dean |
10.28(12)* | | Amendment to Employment Agreement, dated October 1, 2013, by and between the Company and Gregory L. Probert |
10.29(10)* | | Employment Agreement, dated October 2, 2013, by and between the Company and Richard D. Strulson |
10.30(10)* | | Stock Option Agreement, dated November 4, 2013, by and between the Company and Richard D. Strulson |
10.31(10)* | | Employment Agreement, dated April 16, 2013, by and between the Company and Matthew L. Tripp |
10.32(10)* | | Stock Option Agreement, dated May 6, 2013, by and between the Company and Matthew L. Tripp |
14(1) | | Nature’s Sunshine Products, Inc. Code of Conduct. |
18(10)
| | Letter from Independent Registered Public Accounting Firm regarding change in accounting pricnciple
|
21(10) | | List of Subsidiaries of Registrant. |
23.1(10) | | Consent of Independent Registered Public Accounting Firm. |
31.1(10) | | Certification of Chief Executive Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as Adjusted. |
31.2(10) | | Certification of Chief Financial Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as Adjusted. |
32.1(11)32.1(10)
| | Certification of Chief Executive Officer pursuant to 18 U.S.C. § 1350. |
32.2(11)32.2(10)
| | Certification of Chief Financial Officer pursuant to 18 U.S.C. § 1350. |
101.INS** | | XBRL Instance Document |
101.SCH** | | XBRL Taxonomy Extension Schema Document |
101.CAL** | | XBRL Taxonomy Extension Calculation Linkbase Document |
101.LAB** | | XBRL Taxonomy Extension Label Linkbase Document |
101.PRE** | | XBRL Taxonomy Extension Presentation Linkbase Document |
101.DEF** | | XBRL Taxonomy Extension Definition Linkbase Document |
7281
Table of Contents
** | | Attached as Exhibit 101 to this report are the following documents formatted in XBRL (Extensible Business Reporting Language): (i) the Consolidated Statement of Operations for the years ended December 31, 2013, 2012 2011 and 2010,2011, (ii) the Consolidated Balance Sheets as of December 31, 20122013 and 2011,2012, the (iii)Consolidated Statements of Changes in Shareholders’ Equity and Comprehensive Income (Loss) for the years ended December 31, 2013, 2012 2011 and 20102009 and (iv) the Consolidated Statement of Cash Flows for the years ended December 31, 2013, 2012 2011 and 2010.2011. Users of this data are advised pursuant to Rule 406T of Regulation S-T that this interactive data file is deemed not filed or part of a registration statement or prospectus for purposes of sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of section 18 of the Securities and Exchange Act of 1934, and otherwise is not subject to liability under these sections. |
(1) | | Previously filed with the SEC on November 9, 2009 as an exhibit to the Quarterly Report on Form 10-Q for the quarter ended September 30, 2010 and is incorporated herein by reference. |
(2) | | Previously filed with the SEC on January 12, 2009 as an exhibit to the Current Report on Form 8-K and is incorporated herein by reference. |
(3) | | Previously filed with the SEC on December 31, 2007 as an exhibit to the Current Report on Form 8-K and is incorporated herein by reference. |
(4) | | Previously filed with the SEC on February 12, 2009 as an exhibit to the registration statement on Form 10 and is incorporated herein by reference. |
(5) | | Previously filed with the SEC on March 20, 2009 as an exhibit to the Annual Report on Form 10-K for the year ended December 31, 2008 and is incorporated herein by reference. |
(6)
| | Previously filed with the SEC on October 19, 200+ as Appendix C, an exhibit to the Registrant’s Proxy Statement and is incorporated herein by reference.
|
(7)(6)
| | Filed with the SEC on March 16, 2010 as an exhibit to the Current Report on Form 8-K and is incorporated herein by reference. |
(8)(7)
| | Filed with the SEC on June 22, 2011 as an exhibit to the Current Report on Form 8-K and is incorporated herein by reference. |
(9)(8)
| | Filed with the SEC on February 23, 2011 as an exhibit to the Current Report on Form 8-K and is incorporated herein by reference. |
(9) | | Filed with the SEC on March 8, 2013 as an exhibit to the Current Report on Form 8-K and is incorporated herein by reference. |
(10) | | Filed herewith. |
(11) | | Furnished herewith.Filed with the SEC on August 3, 2012 as an exhibit to the Current Report on Form 8-K and is incorporated herein by reference.
|
(12) | | Filed with the SEC on August 3, 2012October 4, 2013 as an exhibit to the Current Report on Form 8-K and is incorporated herein by reference. |
| | |
* | | Management contract or compensatory plan. |
7382



