UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-KFORM 10-K/A
Amendment No. 1
ANNUAL REPORT
PURSUANT TO SECTIONSSECTION 13 OR 15(d)
OF THE SECURITIES AND EXCHANGE ACT OF 1934
(Mark One)
(Mark One) | | |
Rx |
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) |
| OF THE SECURITIES EXCHANGE ACT OF 1934 For the Fiscal Year Ended December 31, 2006 |
OR
| | For the fiscal year ended December 31, 2006
| |
| o | Or
|
£ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) |
| OF THE SECURITIES EXCHANGE ACT OF 1934 |
| For the transition periodTransition Period from _____________ to ____________ |
Commission File Number000-26648
Commission file number: 000-26648eXegenics Inc.
eXegenics Inc.
(Exact name of registrant as specified in its charter)
Delaware
| 75-2402409
|
(State or other jurisdiction of
| (I.R.S. Employer
|
incorporation or organization)
| Identification No.)
|
| | |
1250 Pittsford-Victor Rd
Delaware | 14534
| 75-2402409 |
Pittsford, NY
(State or Other Jurisdiction of
Incorporation or Organization) | | (I.R.S. Employer Identification No.) |
(Zip Code)
| | |
4400 Biscayne Blvd, Suite 900 | | |
| Miami, Florida | | 33137 |
| (Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code:code(305) 575-6015
(585) 218-4375
Securities registered pursuant to Section 12(b) of the Act:
Title of each class
| |
|
| Title of Each Class | | Name of each exchangeEach Exchange on which registeredWhich Registered |
| N/A | | N/A |
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.01 Par Value Per Sharepar value $.01 per share
(Title of Class)
Indicate by checkmark ifcheck mark in the registrant is a well knownwell-known seasoned issuer, as defined in Rulerule 405 of the Securities Act. Yes o£ No Tx
Indicate by checkmark ifcheck mark in the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.act. Yes o£ No Tx
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes xT No £o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained herein, and will not be contained, to the best of registrant’sthe registrants knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. 10-K Yes o£
Indicate by checkmarkcheck mark whether the registrant is a large, accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act (Check One).of 1934.
Large accelerated filer Largeo Accelerated Filer o£ Non- Accelerated Filer £Non-accelerated Filer Tx
Indicate by check markcheckmark whether the registrant is a shell company (as defined in Exchange Act Rule 12b-2).12-b-2 of the Act) Yes oT No x£
The aggregate market value of the registrant’s voting common equity held by non-affiliates of the registrant on June 30, 2006 was $5,575,734 based on the last sale price as reported by OTC Bulletin Board.
As of March 15, 2007 the registrant had 36,550,369 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's definitive Proxy Statement relating to its 2007 Annual Meeting of Stockholders, which will be subsequently filed, with the Securities and Exchange Commission within 120 days after the end of the fiscal year to which this Report relates, are incorporated by reference into Part III of this Form 10-K where indicated.
EXEGENICS INC.EXPLANATORY STATEMENT
This Form 10-K/A is being filed as Amendment No. 1 to the Annual Report on Form 10-K
For of eXegenics Inc. (the “Company”) for the Fiscal Year Endedfiscal year ended December 31, 2006, for the purpose of adding information under Items 10, 11, 12, 13 and 14 of Part III.
TABLE OF CONTENTS
PART I:
| | Page
|
“Safe Harbor” Statement under the Private Securities Litigation Reform Act of 1995 | |
Item 1. | Business | 5-6 |
Item 1A. | Risk Factors | 6-9 |
Item 1B. | Unresolved Staff Comments | 9 |
Item 2. | Properties | 9 |
Item 3. | Legal Proceedings | 10 |
Item 4. | Submission of Matters to a Vote of Security Holders | 10 |
| | Executive Officers of the Registrant | 10-11 |
PART II:
| | | |
Item 5. | Market for Registrant's Common Equity, Related Stockholder | 11-13 |
| | Matters and Issuer Purchases of Equity Securities | |
Item 6. | Selected Financial Data | 14Page No. |
Item 7.PART III | Management's Discussion and Analysis of Financial Condition | 15-19 |
| | and Results of Operations | |
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 20 |
Item 8. | Financial Statements and Supplementary Data | 20 |
Item 9. | Changes in and Disagreements with Accountants on Accounting | 20 |
| | and Financial Disclosure | |
Item 9A. | Controls and Procedures | 20 |
Item 9B. | Other Information | 20 |
PART III:
| | |
Item 10. | | | | | | |
| Item 10 | | | 21 | | 1 | |
Item 11. | Executive Compensation | 21 | | | | |
Item 12.11 | | | | | 10 | |
| | | | | | |
| Item 12 | | | 21 | | | |
| | and Related Stockholder Matters | 21 | | | | |
Item 13.13 | | | 21 |
Item 14. | Principal Accountant Fees and Services | | 19 | |
PART IV:
| | | | | | |
Item 15.14 | | | | | 21 | |
| | | | | | |
| PART IV | | | | | | |
| | | | | | |
| Item 15 | | | 22-26 | | 22 | |
| EX-31.1 Section 302 CEO Certification |
| EX-31.2 Section 302 CAO Certification |
| EX-32.1 Section 906 CEO Certification |
| EX-32.2 Section 906 CAO Certification |
“SAFE HARBOR” STATEMENT UNDER THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995
This document contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. You can identify such forward-looking statements by the words “expects”, “intends,” “plans,” “projects,” “believes,” “estimates,” “likely,” “goal,” “assume” and similar expressions. In the normal course of business, eXegenics Inc. (“eXegenics” or the “Company”), in an effort to help keep its stockholders and the public informed about the Company may, from time to time, issue such forward-looking statements, either orally or in writing. Generally, these statements relate to business plans strategies or opportunities, and/or projected or anticipated benefits or other consequences of such plans, strategies, or opportunities, including anticipated revenues or earnings. eXegenics bases the forward-looking statements on its current expectations, estimates and projections. eXegenics cautions you that these statements are not guarantees of future performance and involve risks, uncertainties and assumptions that eXegenics cannot predict. In addition, eXegenics has based many of these forward-looking statements on assumptions about future events that may prove to be inaccurate. Therefore, the actual results of future events described in such forward-looking statements in this Annual Report, or elsewhere, could differ materially from those stated in such forward-looking statements. Among the factors that could cause actual results to differ materially are the risks and uncertainties discussed in this Annual Report, including, without limitation, the risk factors described in “Item 1A. Risk Factors” of this Report.
-i-
Item 10. Directors, Executive Officers and Corporate Governance.References in this Report on Form 10-K to “we,” “our”, “us”, “its”, the “Company” or “eXegenics” refer to eXegenics Inc., unless the context specifically requires otherwise.
Disclosures setThe following table sets forth in this Report on Form 10-K are qualified by the section captioned “Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995” and other cautionary statements set forth elsewhere in this Report.
PART I
Item 1. Business
General
eXegenics Inc., a Delaware corporation, currently has no business operations. Previously, eXegenics was engaged in the research, creation and development of drugs for the treatment and prevention of cancer and infectious diseases. It was formerly known as Cytoclonal Pharmaceutics, Inc. Historically, eXegenics operated as a drug discovery company, exploiting new enabling technologies to advance and shorten the new drug development cycle. Commencing in 2003, eXegenics began terminating its research and related activities. Since then, all ofinformation concerning our scientific staff and administrative positions have been eliminated and all of our research and development activities have been terminated. As such, eXegenics is a holding company with a portfolio of marketable securities and no operations. The principal offices of the Company are located at: 1250 Pittsford-Victor Road, Building 200, Suite 280, Pittsford, New York.
Since the termination of operations, the board of directors of eXegenics and management have been focused on redeploying the remaining residual assets of eXegenics. The board established a committee - the Business Opportunities Search Committee - to study strategic direction and identify potential business opportunities. The objective of eXegenics is to redeploy its assets and actively pursue new business opportunities.
On February 9, 2007, eXegenics completed its sale of 19,440,491 shares of eXegenics common stock, constituting approximately 51% of the issued and outstanding shares of eXegenics capital stock, on a fully diluted basis, to a small group of investors. The stock sale was made pursuant to the terms of a previously announced stock purchase agreement dated August 14, 2006, as amended as of November 30, 2006. The investors paid eXegenics an aggregate purchase price of $8,613,000 at the closing, which is subject to adjustment based on eXegenics stockholders’ equity at the closing. The proceeds from the stock sale provide eXegenics with working capital that can be used to create future operational and business opportunities.
Insurance
In addition to rights to indemnification provided to eXegenics’executive officers and directors, under our certificate of incorporation, as amended, our bylaws and under the Delaware General Corporation Law (“DGCL”), we have entered into indemnification agreements with certain of our former officers and directors. The indemnification agreements provide such officers and directors with a specific contractual rights as toincluding their indemnification rights under our charter documents and the DGCL for indemnification and the advancement of expenses, and require eXegenics to maintain directors’ and officers’ liability insurance at the levels of coverage in placeages, as of the date the agreement(s) was entered into, for a period of six years after the individual ceases to be an officer or director of the Company. There can be no assurance that we will be able to maintain or increase our insurance coverage in the future on acceptable terms or that any claims against us will not exceed the amount of such coverage.
Patents and Trademarks
Historically, it was the Company’s practice to obtain protection, where possible, on its intellectual property and other proprietary rights, including protection of its products, processes and technologies, and to license such processes and technologies to generate royalties. During fiscal 2006, 2005 and 2004, eXegenics received no revenues under any of its licensing agreements, and it does not anticipate any royalty payments under these licensing agreements in the near future, including revenues from its Intellectual Property Assignment Agreement with NLC Pharma, Inc.
Research and Development
Since termination of its business operations, eXegenics has not sponsored research or development or new products or technologies.
Financial Information About Industry Segments
eXegenics has no business operations and did not have any significant business operations during its fiscal years ended December 31, 2006, 2005 and 2004. See eXegenics Statements of Operations contained in the Company’s financial statements contained in this Report on Form 10-K.
Liquidity and Capital Resources
At December 31, 2006 we had cash, cash equivalents and investments of approximately $8,596,000. Our future capital needs are uncertain. The Company may or may not need additional financing in the future to fund subsequently identified transactions and/or business opportunities. In the event it is determined that additional financing is necessary, there can be no assurances that such financing will be available or, if available, on favorable terms.
Employees
At December 31, 2006, the Company had no employees. Both our interim chief executive officer and our chief financial officer provide services to the Company as independent contractors.
Item 1A. Risk Factors
Risks that could adversely affect eXegenics’ financial condition, or its future business plans, strategies or opportunities, or its future operations are outlined below. Any of the risks in this Report or in eXegenics’ other filings with the Securities and Exchange Commission (the “SEC”) could materially adversely affect eXegenics’ financial condition, or its future business plans, strategies or opportunities, or its future operations. Additional risks and uncertainties not presently known to eXegenics or that are currently believed to be immaterial also may adversely affect eXegenics' financial condition, or its future business plans, strategies or opportunities, or its future operations.
eXegenics may not be able to successfully consummateproposed acquisitions or integrate acquired businesses.
The Business Opportunities Search Committee of the Board of Directors of eXegenics is charged with, among other things, identifying and evaluating business opportunities. If eXegenics were to pursue one or more strategic acquisitions, its failure to consummate or, if consummated, to successfully integrate and/or realize contemplated revenues, could adversely affect the Company’s financial condition.
eXegenics pursuit of business opportunities may subject it to numerous risks, including the following:
April 30, 2007:
· | the benefits of any potential business opportunity not materializing as planned or not materializing within the time periods or to the extent anticipated; | | | | | |
· | the possibility that eXegenics will pay more than the value it derives from any potential business opportunity; |
· | difficulties in the integration and assimilation of the operations, technologies, products and personnel of any acquired business; |
· | the diversion of management’s attention from other potential business opportunities; |
· | the availability of favorable acquisition financing; |
· | the potential loss of key employees and/or clients of any acquired business; |
· | the assumption of unknown liabilities, indemnities and potential disputes with the sellers; and |
· | risks of entering markets in which eXegenics has no or limited direct prior experience. |
Business opportunities pursued by eXegenics may involve a high degree of business and financial risk, which can result in substantial losses to eXegenics. There is generally going to be no publicly available information about the companies that eXegenics may evaluate and pursue, and eXegenics’ management will rely significantly on the diligence of eXegenics’ employees, agents and advisors to obtain and analyze information. If eXegenics is not able to identify all material information about these companies, among other factors, eXegenics may fail to receive the value it had anticipated. In addition, these businesses may have short operating histories, narrow product lines, small market shares and less experienced management than their competition and may be more vulnerable to customer preferences, market conditions, loss of key personnel, or economic downturns.
Further, acquisitions may require the use of significant amounts of cash, resulting in the inability to use those funds for other business opportunities or purposes. Acquisitions using our capital stock could have a dilutive effect, and could adversely affect the market price of our common stock.
No minimum guidelines have been established with respect to any particular industry that eXegenics may enter, or criteria with respect to any particular business that eXegenics may engage in a transaction, accordingly, we cannot provide you with risks that may be specific to a particular industry, transaction or business.
The Business Opportunities Search Committee has evaluated numerous potential acquisition or merger candidates for eXegenics, which included companies in a diverse group of industries, including medical devices, entertainment, banking and software development. Further, eXegenics has not established any criteria, including a specific length of operating history or a specified level of earnings, assets, and/or net worth, which it will require a company to have achieved, or without which eXegenics would not consider a transaction with such business entity. eXegenics may enter into a transaction with an entity engaged in a highly regulated business or in a business with unique operating risks; or, eXegenics may enter into a transaction with an entity having: no significant operating history, losses, limited or no potential for immediate earnings, limited assets, negative net worth or other negative characteristics. Our inability to advise you of particular risks associated with an industry or business opportunities, for example, environmental or health risks, competitive risks, or regulatory risks, and how they might impact our financial condition or future business opportunities or prospects is, itself, a risk.
eXegenics future success will depend, in part, in its ability to attract and retain highly skilled employees.
Our future success will depend, in part, on our ability to attract, retain and motivate highly skilled employees. Competition for highly skilled employees exists, and we may be unable to attract, integrate or retain the proper numbers of sufficiently qualified personnel that we may need in the future. To the extent that we are unable to hire and retain skilled employees in the future, our financial condition and our future business opportunities and operations will likely suffer.
Transactions may result in unfavorable tax treatment to eXegenics.
Federal and state tax consequences will, in all likelihood, be major considerations in any business opportunity we may pursue. Currently, such transactions may be structured so as to result in tax-free treatment to both companies, pursuant to various federal and state tax provisions. While eXegenics intends to structure any transaction so as to minimize the federal and state tax consequences to the Company, there can be no assurance that such business opportunity will meet the statutory requirements of a tax-free reorganization or that the parties will obtain the desired tax-free treatment. A non-qualifying reorganization could result in the imposition of both federal and state taxes which may have an adverse effect on both parties to the transaction and their shareholders.
Certain of eXegenics’ charter provisions and Delaware law may prevent or deter potential business opportunities.
eXegenics certificate of incorporation, as amended, bylaws, stockholders rights agreement, and certain provisions of Delaware law contain provisions that may have the effect of discouraging, delaying or preventing eXegenics from pursuing potential business opportunities that a stockholder might consider favorable. The anti-takeover effect of these provisions may also have an adverse effect on the public trading price of eXegenics common stock.
The Sarbanes-Oxley Act of 2002
Since the enactment of the Sarbanes-Oxley Act of 2002 and the SEC’s implementing regulations of the same (collectively, the “Sarbanes-Oxley Act”), companies that have securities registered under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), including eXegenics, are subject to enhanced and more transparent corporate governance standards, disclosure requirements and accounting and financial reporting requirements. The Sarbanes-Oxley Act, among other things, (i) requires • a public company, with securities listed on an exchange (eXegenics securities are not currently listed on an exchange, but are quoted on the Over-the-Counter Bulletin Board), to establish and maintain audit committees, comprised solely of independent directors, which committee must be empowered to, among other things, engage, supervise and discharge the company’s auditors; • that a public company’s financial statements be certified by the principal executive and principal financial officers of such company; • increased and quicker public disclosure - real time -obligations by the company and its directors and executive officers, including disclosure of off-balance sheet transactions and accelerated reporting of transactions in company stock; (ii) prohibits personal loans to company directors and officers, except certain loans made by insured financial institutions on nonpreferential terms and in compliance with other bank regulatory requirements; and (iii) creates or provides for various new and increased civil and criminal penalties for violations of the securities laws.
Achieving and maintaining compliance with the Sarbanes-Oxley Act and the SEC’s implementing rules and regulations increases eXegenics use of outside legal and accounting advisors and, accordingly, increases eXegenics’ expenses related to such advisors, as well as increased time spent by eXegenics’ management in maintaining and evaluating continued compliance with the requirements of the Sarbanes-Oxley Act and SEC rules and regulations.
Our failure to adequately protect our intellectual property rights could result in our loss of such rights.
Prior to the termination of its research and development programs, eXegenics’ policy was to protect its intellectual property and other proprietary rights, including its technology. Subsequent to termination of its drug discovery operations, eXegenics has not been diligent in maintaining those protections and, as a result, some or all of our rights in our intellectual property, including our proprietary technology and other proprietary interests, may be subject to challenge, and we may lose our interests in or rights to certain intellectual property, including our proprietary technologies, that later turn out to be important to the Company in the future.
eXegenics common stock price may be volatile, which could result in substantial losses for our stockholders
The market price of shares of eXegenics common stock has been and is likely to continue to be highly volatile and may be significantly affected by factors such as the following:
· | announcements we make concerning new business development activities; |
· | actual or anticipated fluctuations in our financial condition and future operations and operating results; |
· | changes in the economic and political conditions in the United States and abroad;
|
· | terrorist attacks, war or the threat of terrorist attacks and war; |
· | regulatory developments in the United States and foreign countries; |
· | our common stock being quoted on the OTC Bulletin Board; and |
· | price and volume fluctuations in the stock market. |
A significant portion of our voting power is concentrated and, as a result, our other stockholders’ ability to influence corporate matters may be limited.
The Frost Group owns approximately 41% of our voting stock. Accordingly, the Frost Group will have significant influence over the management and affairs of eXegenics and over all matters requiring stockholder approval, including the election of directors and significant corporate transactions, such as a merger or other sale of eXegenics or its assets, for the foreseeable future. This concentrated control limits the ability of our other stockholders to influence corporate matters and, as a result, The Frost Group may take actions that eXegenics other stockholders do not view as beneficial.
We may have conflicts of interest with The Frost Group.
Conflicts of interest may arise between The Frost Group and us in a number of areas, including business combinations. The Frost Group has investments in a variety of companies and may present one or more of these companies as business opportunities to us. Three of the members of The Frost Group are members of, and represent a majority of, our board of directors. We cannot guarantee that any conflicts that may arise will be resolved in a matter that is favorable to us. Additionally, even if we do resolve such conflicts, the resolution may be less favorable to us than it would be if we were dealing with an unaffiliated third party.
We have a large number of authorized but unissued shares of common stock that may be issued in connection with business combinations or otherwise without stockholder approval.
As previously referenced, our Business Opportunities Search Committee, is charged with identifying and evaluating business opportunities. We are authorized to issue 225,000,000 shares of common stock, of which 36,550,369 shares are currently issued and outstanding and 1,568,240 shares are reserved for issuance upon conversion of our Series A preferred stock and upon exercise of outstanding options and warrants. In addition, we are authorized to issue up to 10,000,000 shares of preferred stock, of which 4,000,000 shares are designated Series A preferred stock, 983,240 of which are issued and outstanding, and an additional 30,000 shares are designated Series B preferred stock, none of which are issued and outstanding. Our authorized, but unissued and unreserved shares of capital stock are available to us for issuance from time to time for acquisitions or to raise capital. Whether or not any future issuance of shares would be submitted for stockholder vote depends upon whether stockholder approval would be required by applicable law and/or applicable stock exchange rules. The eXegenics board of directors intends to only seek approval of the eXegenics stockholders with respect to any future issuances of shares of eXegenics capital stock to the extent required by applicable law and/or applicable stock exchange rules. Any future issuances of shares of our capital stock would likely dilute the percentage ownership of the stockholders in eXegenics.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 2. Properties.
Our corporate offices are located at 1250 Pittsford-Victor Road, Building 200, Suite 280, Pittsford, New York 14534 and consist of approximately 500 square feet of office space. The Company sublets this office space from RFG Associates, a general partnership in which John A. Paganelli, our chairman and interim chief executive officer of the Company, is a partner. Monthly rent is $625 and the sublease may be terminated by either party upon thirty (30) days notice. eXegenics paid an aggregate of $10,000 in rent expenses in fiscal 2006.
Item 3. Legal Proceedings.
As previously reported by eXegenics in its quarterly report on Form 10-Q filed November 14, 2006, during the fourth quarter of fiscal 2006, the Company reached agreement with its former president and chief executive officer (Dr. Ronald Goode) in connection with a limited recourse note and pledge agreement entered into with Dr. Goode in May 2001 in connection with Dr. Goode’s subscription for shares of eXegenics common stock. The amount of the note was $300,000 plus 4.71% interest payable on a semi-annual basis. Dr. Goode had failed to make the semi-annual interest payments since May 2005, and failed to pay the principal due May 2006. On October 30, 2006, the Company reached agreement with Dr. Goode concerning the cancellation of the subscription agreement and note in consideration for the assignment to the Company of the 100,000 shares of eXegenics common stock underlying Dr. Goode’s subscription. As a result, the subscription receivable of $101,000 was eliminated and an offsetting amount was deducted from additional paid in capital.
Labidi Proceeding.
On October 5, 2005, in the matter brought by Abdel Hakim Labidi (one of our former employees) against the Company, a jury ruled in favor of Dr. Labidi determining that the Company converted certain biological research materials owned by Dr. Labidi, and the Company committed theft of biological materials owned by Dr. Labidi. The jury awarded Dr. Labidi a total of $600,000. The Company is reviewing this matter to determine the validity of appealing the decision of the jury. The final amount due by the Company to Dr. Labidi under such judgment is likely to be between $638,000 and $750,000; however the Company has recorded a provision of $638,000 in the financial statements.
Other than the Labidi matter, eXegenics is not currently aware of any other legal proceedings and no such proceedings are known to be contemplated by any governmental authorities. eXegenics maintains general liability insurance coverage in amounts deemed to be adequate by the Board of Directors.
Item 4. Submission of Matters to a Vote of Security Holders
No matters were submitted to a vote of security holders during the fourth quarter of fiscal 2006.
Executive Officers of the Registrant
Set forth below are the names of the persons currently serving as a executive officers, their ages, their offices in the Company, if any, their principal occupations or employment for the past five years, and the names of other public companies in which such persons hold directorships.
| Name | | Age | | Position withTitle |
| Phillip Frost, M.D. | | | 70 | | | Chief Executive Officer and Chairman of the CompanyBoard |
| Dale Pfost, Ph.D. | | | 50 | | | President |
| Samuel J. Reich | | | 32 | | | Executive Vice President |
| Denis O’Shaughnessy, Ph.D. | | | 56 | | | Senior Vice President of Clinical Development |
| Adam Logal | | | 29 | | | Executive Director of Finance, Chief Accounting Officer, Treasurer & Secretary |
| John A. Paganelli | | | 72 | | | Director Chairman of the Board, Interim Chief Executive Officer, and Secretary |
Dr. David F. HostelleyA. Eichler | | 67 | 36 | | Chief Financial Officer | Director |
| Michael Reich | | | 63 | | | Director |
| Jane H. Hsiao, Ph.D., MBA | | | 59 | | | Director |
| Steven D. Rubin | | | 46 | | | Director |
| Robert Baron | | | 66 | | | Director |
| Richard A. Lerner, M.D. | | | 68 | | | Director |
| Melvin L. Rubin, M.D. | | | 75 | | | Director |
John A. Paganelli, Interim Chief Executive Officer, SecretaryPhillip Frost, M.D.Dr. Frost became the CEO and Chairman of our board of directors after consummation of the our acquisition of Acuity Pharmaceuticals Inc. and Froptix Corporation on March 27, 2007 (referred to as the “Acquisition”). Dr. Phillip Frost was named the Vice Chairman of the Board of DirectorsTeva Pharmaceutical Industries, Limited (“Teva”) in January 2006 when Teva acquired IVAX Corporation (“IVAX”). Dr. Frost had served as Chairman of the board of directors and Chief Executive Officer of IVAX Corporation since 1987. Dr. Frost was named Chairman of the Board of Ladenburg Thalmann & Co., Inc., an American Stock Exchange-listed investment banking and securities brokerage firm, in July 2006 and has been a director of Ladenburg Thalmann since March 2005. He serves on the Board of Regents of the Smithsonian Institution, a member of the Board of Trustees of the University of Miami, a Trustee of each of the Scripps Research Institutes, the Miami Jewish Home for the Aged, and the Mount Sinai Medical Center and is Vice Chairman of the Board of Governors of the American Stock Exchange. Dr. Frost is also a director of Protalix BioTherapeutics, Inc., an American Stock Exchange-listed biotech pharmaceutical company, Continucare Corporation, an American Stock Exchange-listed provider of outpatient healthcare and home healthcare services, Northrop Grumman Corp., a New York Stock Exchange-listed global defense and aerospace company, Castle Brands, Inc., an American Stock Exchange-listed developer and marketer of alcoholic beverages, and Cellular Technical Services, Inc., a public company with no ongoing operations.
Dale Pfost, Ph.D.Dr. Dale Pfost became our President after consummation of the Acquisition on March 27, 2007. Previously, Dr. Pfost served as the President, CEO and Chairman of Acuity Pharmaceuticals and was one of the members of the founding management team from 2003 through
-1-
March 2007. Dr. Pfost served as President, CEO and Chairman of Orchid BioSciences from 1996 through 2002 and was the founding CEO. From 1988 until 1996 Dr. Pfost served as President, CEO and Managing Director of Oxford GlycoSciences, where he was the founding CEO. Dr. Pfost was the founder and President of Infitek, Inc. from 1982 through 1984 until it was acquired by SmithKline Beckman where Dr. Pfost served in varying levels of increasing responsibilities through 1988.
Samuel J. Reich.Mr. Samuel Reich became our Executive Vice President after consummation of the Acquisition on March 27, 2007. Prior to joining us, Mr. Reich served as Executive Vice President, Research and Development and was a co-founder of Acuity. Mr. Reich co-founded Acuity in 2002 where he served in capacities of increasing responsibility from 2002 to March 2007. Prior to founding Acuity, Mr. Reich was a doctoral candidate at the University of Pennsylvania Medical School, where his doctoral research involved recognizing and pioneering the opportunity in RNAi therapeutics for treating ophthalmic diseases from 2001 until 2002.
John A. Paganelli. Paganelli.Mr. Paganelli has served as our Interim Chief Executive Officer and secretary sincefrom June 29, 2005 through the consummation of the Acquisition, and Chairman of the eXegenics Boardour board of Directors sincedirectors from December 2003.2003 through the consummation of the Acquisition. Mr., Paganelli served as President and Chief Executive Officer of Transamerica Life Insurance Company of New York from 1992 to 1997. Since 1987, Mr. Paganelli has been a partner in RFG Associates, a financial planning organization. Mr. Paganelli is the Managing Partner of Pharos Systems Partners, LLC, a company formed to raise capital to purchase the controlling interest in Pharos Systems International, a software development company. Mr. Paganelli is Chairman of the Board of Pharos Systems International. He was Vice President and Executive Vice President of PEG Capital Management, an investment advisory organization, from 1987 until 2000. From 1980 to January 2003, Mr. Paganelli was an officer and director-shareholder of Mike Barnard Chevrolet, Inc., an automobile dealership. Mr. Paganelli was on the Board of Directors of Mid Atlantic Medical Services, Inc. from 1999 until 2005. Mid Atlantic was listed on the New York Stock Exchange and through its wholly owned subsidiaries is in the business of selling various forms of health insurance. Mr. Paganelli was also on the Board of Directors of Mid Atlantic'sAtlantic’s subsidiary, MAMSI Life and Healthy Insurance Company. Mr. Paganelli holds an A.B. from Virginia Military Institute. In 2005 Mid Atlantic Medical Services, Inc. was acquired by UnitedHealth Group, Inc.
Denis O’Shaughnessy, Ph.D., Dr. Denis O’Shaughnessy became our Senior Vice President of Clinical Development upon consummation of the Acquisition on March 27, 2007. Prior to joining us, Dr. O’Shaughnessy served as Senior Vice President of Clinical Development for Acuity from October 2006 to March 2007. From 2005 to October 2006, Dr. O’Shaughnessy was an independent clinical research consultant. From 2000 to 2005, Dr. O’Shaughnessy was a founding member of Eyetech Pharmaceuticals and served as Senior Vice President of Clinical Development. From 1990 to 2000 Dr. O’Shaughnessy was employed by Hoffmann-La Roche with increasing levels of responsibility, most recently as Director of Clinical Operations. From 1980 through 1990, Dr. O’Shaughnessy served at several pharmaceutical companies in various roles of increasing responsibility most recently as Head of Clinical Research for Celltech Ltd.
Adam Logal. Mr. Logal became our Executive Director of Finance and Chief Accounting Officer after consummation of the Acquisition on March 27, 2007. Prior to joining the Company, Mr.
-2-
David F. Hostelley,Logal served in various finance capacities at Nabi Biopharmaceuticals, most recently as Sr. Director, Accounting and Reporting. From March 2006 to June 2006, Mr. Logal served as Interim Chief Financial Officer, Chief Accounting Officer and Treasurer and from November 2002 to June 2006 Mr. Logal served in various roles of increasing responsibility within the Finance Department. Prior to Nabi Biopharmaceuticals, Mr. Logal served from May 2001 to November 2002 as a tax accountant at Spherion Corporation, a recruiting and staffing company. From November 2000 to May 2001, Mr. Logal served as a staff accountant for Dunn & Co., CPAs PA, a public accounting firm.
Board of Directors
David F. Hostelley. Mr. HostelleyJane H. Hsiao, Ph.D., MBA.Dr. Hsiao has served as our chief financial officera director of the Company since July 1, 2005.February 2007. Dr. HostelleyHsiao served as the Vice Chairman-Technical Affairs of IVAX from 1995 to January 2006, when Teva acquired IVAX. Dr. Hsiao served as IVAX’s Chief Technical Officer since 1996, and as Chairman, Chief Executive Officer and President of IVAX Animal Health, IVAX’s veterinary products subsidiary, since 1998. From 1992 until 1995, Dr. Hsiao served as IVAX’s Chief Regulatory Officer and Assistant to the Chairman. Dr. Hsiao served as Chairman and President of DVM Pharmaceuticals from 1998 through 2006 and is also a Certified Public Accountant licenseddirector of Protalix BioTherapeutics, Inc., an American Stock Exchange-listed biotech pharmaceutical company, and Cellular Technical Services Company, Inc., a public company with no ongoing operations.
Steven D. Rubin.Mr. Rubin has served as a director of the Company since February 2007. Mr. Rubin served as the Senior Vice President, General Counsel and Secretary of IVAX from August 2001 until September 2006. Prior to joining IVAX, Mr. Rubin was Senior Vice President, General Counsel and Secretary with privately held Telergy, Inc., a provider of business telecommunications and diverse optical network solutions, from early 2000 to August 2001. In addition, he was with the Miami law firm of Stearns Weaver Miller Weissler Alhadeff & Sitterson from 1986 to 2000, in the statesCorporate and Securities Department. Mr. Rubin had been a shareholder of Ohiothat firm since 1991 and New York. In 1984 he earned his Ph.D. in management while a lecturer in the MBA Program of Baldwin-Wallace College. He currently lectures in Accounting and Management for Myers University, Cleveland, Ohio.
He has structured numerous acquisitions in the fields of printing, oil and gas development, private schools, insurance agencies, hotels, manufacturing, debit card issuance, health clubs and service entities. In his capacity of trainer in the field of Project Management, Dr. Hostelley has taught the executives of: Ford Motor Company, Westinghouse, National Fuel Gas, General Electric, Stromberg-Carlson, Doehler-Jarvis, Marvin Windows, Progressive Insurance, EDI Engineering, Sun Exploration, Tennessee Valley Authority, SPX Corporation, The Venezuelan Oil Ministry, Ford Museum in Greenfield Village, and Trans Ohio Savings and Loan. He has lectured in South Africa, Venezuela, Canada, and the United States.
He is now serving as interim president and board member of Organetix, Inc., (Symbol OGTX.OB); and as a board member and CFO of DSI Direct Sales, Inc. (Symbol DSDI.STET).director since 1998. Mr. HostelleyRubin currently serves on the Executive Committeeboard of directors of Dreams, Inc., a vertically integrated sports licensing and products company.
David A. Eichler. Mr. Eichler became a director of the Cleveland ChapterCompany after the consummation of the Muscular Dystrophy Association.Acquisition on March 27, 2007. Mr. Eichler is a Managing Director of Psilos Group, a New York-based venture capital firm specializing in healthcare investments. Mr. Eichler joined Psilos in 1999 and focuses on investments in the specialty pharmaceutical, medical technology, healthcare services and healthcare IT sectors. Mr. Eichler has served on the board of directors of several Psilos portfolio companies, and has extensive experience as an advisor to senior management on M&A, financial restructuring and capital raising transactions. Mr. Eichler has been a director of Acuity since 2004 and also currently serves as Chairman of Caregiver Services, Inc., a leading provider of in-home care services. Prior to joining Psilos, Mr. Eichler was an investment banker at Wasserstein Perella & Co. where he was a member of the firm’s Healthcare Group.
Michael Reich. Mr. Michael Reich became a director of the Company after the consummation of the Acquisition on March 27, 2007. Mr. Michael Reich is a proprietor of a commercial property development company. Previously, Mr. Reich was chief executive officer of Cosrich, Inc., a manufacturer of popularly priced cosmetics and toiletries, including numerous well known brands.
-3-
Mr. Reich’s area of expertise is in operations, finance and marketing. Prior to the Acquisition, Mr. Reich had been a board member of Acuity since 2003. Michael Reich is the uncle of Samuel Reich, the Company’s Executive Vice President.
PART II
Item 5. MarketRobert A. Baron.Mr. Baron has served on our board of directors since 2003. Previously, Mr. Baron served as the President of Cash City, Inc. from 1999 to 2003. Cash City is a payday advance and check cashing business. From 1997 to 1999 Mr. Baron was the President of East Coast Operations for Registrant’s Common Equity, Related Stockholder MattersCSS/TSC, Inc., a distributor of blank t-shirts and Issuer Repurchasesfleece and accessories and a subsidiary of Equity Securities
Common Stock
eXegenicsTultex, Inc., a publicly held company. From 1986 to 1997, Mr. Baron was the chairman of T shirt City, Inc., a privately held company. From 1993 to 1997, Mr. Baron was a member of the board of directors of Suburban Bank Corp. When Mr. Baron was on Suburban’s board, its common stock was traded on NASDAQ. Mr. Baron is quotedalso a director of Hemobiotech, Inc., a development stage biopharmaceutical company, and Andover Medical, Inc., a medical equipment distributor.
Richard A. Lerner, M.D.Dr. Lerner became a director of the Company after the consummation of the Acquisition on March 27, 2007. Dr. Lerner has been President of The Scripps Research Institute, a private, non-profit biomedical research organization, since 1986. Dr. Lerner is a member of numerous scientific associations, including the National Academy of Science and the Royal Swedish Academy of Sciences. Dr. Lerner serves as director of Kraft Foods, Inc. He is also on the Over-the-Counter Bulletinboard of directors for Xencor and Intra-Collular Therapies, two privately held biotechnology companies, and serves on the scientific advisory board of Dyadic, a biotechnology company.
Melvin L. Rubin, M.D.Dr. Rubin became a director of the Company after the consummation of the Acquisition on March 27, 2007. Dr. Rubin is member of the faculty at the University of Florida Department of Ophthalmology where he holds the titles of Professor and Chairman Emeritus of Ophthalmology and Shaler Richardson Eminent Scholar Emeritus. He has been a member of the University of Florida Department of Ophthalmology faculty since 1963. He has served national ophthalmology on the board of directors and as president of the American Academy of Ophthalmology (“AAO”) and later, president of the Foundation of the AAO. He has also been trustee and president of the Association for Research in Vision and Ophthalmology, and on the board of directors and chairman of the American Board of Ophthalmology.
CORPORATE GOVERNANCE
Corporate Governance Policy
We will regularly monitor developments in the area of corporate governance and review our processes and procedures in light of such developments. In those efforts, we review Federal laws affecting corporate governance, such as the Sarbanes-Oxley Act of 2002. In addition, in anticipation of our application to list our shares of common stock on the American Stock Exchange (the “AMEX”), we also review applicable rules of the AMEX.
Board of Directors Meetings
Our business, property and affairs are managed under the trading symbol EXEG.direction of our board of directors. Members of our board of directors are kept informed of our business through discussions with our Chairman and Chief Executive Officer, President, Chief Accounting Officer and other officers and
-4-
employees, by reviewing materials provided to them and by participating in meetings of our board of directors and its committees.
Our board of directors met 14 times during 2006: 4 of which were at regularly scheduled meetings and 10 of which were at special meetings. During 2006, the committees of our board of directors held a total of 4 meetings. Each director attended at least 75% of the total number of meetings of the board of directors and each committee of the board on which such director served.
Independence Determination
Our board of directors will observe all applicable criteria for independence established by the AMEX and other governing laws and applicable regulations. No director is deemed to be independent unless our board of directors determines that the director has no relationship which would interfere with the exercise of independent judgment in carrying out his or her responsibilities as a director. Our board of directors has determined that the following directors are independent as determined by listing standards of the AMEX and other applicable regulations: Robert Baron, David A. Eichler, Richard A. Lerner, M.D., Michael Reich and Melvin L. Rubin, M.D.
In making this determination, the board of directors considered all relevant factors, including the fact that Michael Reich is the uncle of Samuel Reich, the Company’s Executive Vice President.
Standing Committees of the Board of Directors
Our board of directors maintains several standing committees, including an audit committee established in accordance with section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended (Exchange Act), a compensation committee, and a nominating and governance committee. These committees and their functions are described below. Our board of directors may also establish various other committees to assist it in its responsibilities.
Our board of directors has adopted a written charter for each of its standing committees. The full text of each charter is available on our website at http://www.opko.com.
The following table shows the high and low bid quotations for eXegenics common stock, on a per share basis, duringcurrent members (indicated by an “X” or “Chair”) of each full quarterly period within the two most recent fiscal years, as quoted on the OTCBB. Such prices reflect inter-dealer quotations, without adjustment for any retail markup, markdown or commission and may not necessarily represent actual transactions.
| | | High | | Low | |
2006: | | | | | |
| First Quarter | | $ | 0.46 | | $ | 0.39 | |
| Second Quarter | | | 0.45 | | | 0.38 | |
| Third Quarter | | | 1.09 | | | 0.38 | |
| Fourth Quarter | | | 0.99 | | | 0.72 | |
2005: | | | | | | | |
| First Quarter | | $ | 0.45 | | $ | 0.32 | |
| Second Quarter | | | 0.47 | | | 0.35 | |
| Third Quarter | | | 0.44 | | | 0.36 | |
| Fourth Quarter | | | 0.46 | | | 0.39 | |
On March 15, 2007 the last sale price of our common stock was $2.40.standing board committees:
| | | | | | |
| | | | | | Corporate |
| | | | | | Governance and |
| | Audit | | Compensation | | Nominating |
| Phillip Frost, M.D. | | — | | — | | — |
| Jane H. Hsiao, Ph.D., MBA | | — | | — | | — |
| Robert Baron | | X | | — | | X |
| David A. Eichler | | Chair | | X | | — |
| Richard A. Lerner, M.D. | | | | Chair | | X |
| John A. Paganelli | | — | | — | | — |
| Michael Reich | | X | | — | | — |
| Melvin L. Rubin, M.D. | | — | | X | | Chair |
| Steven D. Rubin | | — | | — | | — |
-5-
Audit Committee
StockholdersOur audit committee oversees our corporate accounting and financial reporting processes. Our audit committee:
| • | | evaluates the qualifications, independence and performance of our registered independent public accounting firm; |
|
| • | | determines the engagement of our registered independent public accounting firm; |
|
| • | | approves the retention of our registered independent public accounting firm to perform any proposed permissible non-audit services; |
|
| • | | ensures the rotation of the partners of our registered independent public accounting firm on our engagement team as required by law; |
|
| • | | reviews our systems of internal controls established for finance, accounting, legal compliance and ethics; |
|
| • | | reviews our accounting and financial reporting processes; |
|
| • | | provides for effective communication between our board of directors, our senior and financial management and our independent auditors; |
|
| • | | discusses with management and our independent auditors the results of our annual audit and the review of our quarterly financial statements; |
|
| • | | reviews the audits of our financial statements; |
|
| • | | implements a pre-approval policy for certain audit and non-audit services performed by our registered independent public accounting firm; and |
|
| • | | reviews and approves any related party transactions that we are involved in. |
At March 15 2007, there were approximately 153 holdersOur audit committee is comprised of recordMessrs. Eichler, Reich and Baron. Our board of directors has determined that Mr. Eichler is an “audit committee financial expert” as currently defined under the SEC’s rules implementing Section 407 of the Sarbanes-Oxley Act of 2002. We believe that the composition and functioning of our common stock.
Dividends
We have never declared nor paid any cash dividends on our common stock. We currently anticipate that we will retain any future earnings to fund future business opportunities. Accordingly, we do not anticipate paying any cash dividends on our common stock in the foreseeable future.
Recent Sales of Unregistered Securities
As previously reported by eXegenics in its current report on Form 8-K dated February 9, 2007, and subsequent to the end of fiscal 2006, on February 9, 2007, the Company sold 19,440,491 shares of its common stock, constituting approximately 51%audit committee complies with all applicable requirements of the issued and outstanding sharesSarbanes-Oxley Act of eXegenics capital stock, on a fully diluted basis, to a small group of investors. The stock sale was made pursuant to2002, the terms of a previously announced stock purchase agreement dated August 14, 2006, as amended as of November 30, 2006. The investors paid eXegenics an aggregate purchase price of $8.6 million at the closing. The purchase price is subject to a downward adjustment based upon the stockholders equity of eXegenics on the date of closing. In addition, on February 9, 2007, the Company issued 50,000 shares of eXegenics common stock to each of John A. Paganelli, our Interim Chief Executive Officer, Secretary and Chairman of the Board of Directors of eXegenics, and Robert Baron, a director of eXegenics in consideration of their service to the Business Opportunities Search Committee of the eXegenics Board. The shares were issued pursuant to stock grants approved by the stockholders of eXegenics. The shares of eXegenics common stock were offered and sold in reliance upon an exemption from registration under Section 4(2) of the Securities Act for “transactions by an issuer not involving a public offering” and Rule 506 or Regulation D of the Securities Act.
Issuer Purchases of Equity Securities.
As previously reported by eXegenics in its quarterly report on Form 10-Q filed November 14, 2006, during the fourth quarter of fiscal 2006, the Company reached agreement with its former president and chief executive officer (Dr. Ronald Goode) in connection with a limited recourse note and pledge agreement entered into with Dr. Goode in May 2001 in connection with Dr. Goode’s subscription for shares of eXegenics common stock. The amount of the note was $300,000 plus 4.71% interest payable on a semi-annual basis. Dr. Goode failed to make the semi-annual interest payments since May 2005, and principal due May 2006. On October 30, 2006, the Company reached agreement with Dr. Goode concerning the cancellation of the subscription agreement and note in consideration for the assignment to the Company of the 100,000 shares of eXegenics common stock underlying Dr. Goode’s subscription. As a result, the subscription receivable of $101,000 was eliminated and an offsetting amount was deducted from additional paid in capital.
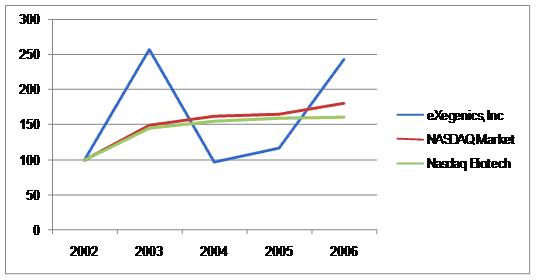
PERFORMANCE GRAPH(1)
The following table compares the annual percentage change in our cumulative total stockholder return on our common stock during a period commencing on December 31, 2002 and ending on December 31, 2006 (as measured by dividing (A) the sum of the cumulative amount of dividends for the measurement period, assuming dividend reinvestment,AMEX and the difference between our share price atSEC’s rules and regulations, including those regarding the end and the beginning of the measurement period; by (B) our share price at the beginning of the measurement period) with the cumulative total return of the Nasdaq Stock Market (US) Index and a peer group, the Nasdaq Biotech Index, during such period. We have not paid any dividends on our common stock, and we do not include dividends in the representationindependence of our performance. The stock price performance shown on the graph below is not necessarily indicative ofaudit committee members. We intend to comply with future price performance and only reflects eXegenics’ relative stock price for the period from December 31, 2002 through December 31, 2006.
PERFORMANCE GRAPH
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
AMONG EXEGENICS INC.,
THE NASDAQ STOCK MARKET (U.S.) INDEX
AND THE NASDAQ BIOTECH INDEX, OUR PEER GROUP
| | | 2002 | | 2003 | | 2004 | | 2005 | | 2006 | |
| EXEGENICS INC | | $ | 100.00* | | $ | 257.14 | | $ | 97.14 | | $ | 117.14 | | $ | 242.86 | |
| NASDAQ Market Index (US) | | $ | 100.00* | | $ | 150.01 | | $ | 162.89 | | $ | 165.13 | | $ | 180.85 | |
| Nasdaq Biotech Index | | $ | 100.00* | | $ | 145.75 | | $ | 154.68 | | $ | 159.06 | | $ | 160.69 | |
* | | $100 invested on 12/31/02 in stock or index-including reinvestment of dividends. Fiscal year ending December 31. |
(1) The information contained in this section - Performance Graph - will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended (the “Securities Act”) or the Exchange Act, exceptrequirements to the extent that eXegenics specifically incorporates it by reference. Further,they become applicable to us.
On April 25, 2007, the information contained in this section Performance Graph - shall not be deemed to be “soliciting material”board of directors amended and restated the Company’s Audit Committee Charter.
Compensation Committee
Our compensation committee administers the compensation program for our executive officers. Our compensation committee reviews and either approves, on behalf of the board of directors, or to be “filed” with the Securities and Exchange Commission or subject to Regulation 14A or 14C orrecommends to the liabilitiesboard of section 18directors for approval, (i) annual salaries, bonuses, and other compensation for our executive officers, and (ii) individual equity awards for our employees and executive officers. Our compensation committee also oversees our compensation policies and practices.
-6-
Our compensation committee also performs the following functions related to executive compensation:
| • | | coordinates the board of directors’ role in establishing performance criteria for executive officers; |
|
| • | | annually evaluates each of our executive officers’ performance; |
|
| • | | reviews and approves the annual salary, bonus, stock options and other benefits, direct and indirect, of our executive officers, including our Chief Executive Officer; |
|
| • | | reviews and recommends new executive compensation programs; · annually reviews the operation and efficacy of our executive compensation programs; |
|
| • | | periodically reviews that executive compensation programs comport with the compensation committee’s stated compensation philosophy; |
|
| • | | establishes and periodically reviews policies in the area of senior management perquisites; |
|
| • | | reviews and recommends to the board of directors the appropriate structure and amount of compensation for our directors; |
|
| • | | reviews and approves material changes in our employee benefit plans; |
|
| • | | administers our equity compensation and employee stock purchase plans; and |
|
| • | | reviews the adequacy of the compensation committee and its charter and recommends any proposed changes to the board of directors not less than annually. |
In deciding upon the appropriate level of compensation for our executive officers, the compensation committee regularly reviews our compensation programs relative to our strategic objectives and emerging market practice and other changing business and market conditions. In addition, the compensation committee also takes into consideration the recommendations of our Chief Executive Officer concerning compensation actions for our other executive officers and any recommendations of compensation consultants. The primary role of consultants is to provide objective data, analysis and advice to the compensation committee. In providing data and recommendations to the compensation committee, our consultants work with our Chief Executive Officer and management to obtain information needed to carry out its assignments.
Our compensation committee is comprised of Dr. Rubin, Dr. Lerner and Mr. Eichler. We believe that the composition and functioning of our compensation committee complies with all applicable requirements of the ExchangeSarbanes-Oxley Act exceptof 2002, the AMEX and the SEC’s rules and regulations, including those regarding the independence of our compensation committee members. We intend to comply with future requirements to the extent that eXegenics specifically requests that such information be treated as soliciting material or specifically incorporates it by reference into a filing under the Securities Act or the Exchange Act.they become applicable to us.
Item 6. Selected Financial Data
The following selected financial data of eXegenics should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Continuing Operations” and the Company’s financial statements and the notes to those statements and other financial information appearing elsewhere in this Report on Form 10-K.
eXegenics Inc.
SELECTED FINANCIAL DATA
| | | Year Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | | 2003 | | 2002 | |
| Statement of Operations Data | | | | | | | | | | | |
| Revenue | | $ | — | | $ | — | | $ | — | | $ | 13,000 | | $ | 562,000 | |
| Research and development | | | — | | | — | | | — | | | 154,000 | | | 3,948,000 | |
| General and administrative expenses | | | 1,117000 | | | 1,438,000 | | | 2,051,000 | | | 2,938,000 | | | 4,770,000 | |
| Expenses related to strategic redirection | | | — | | | — | | | — | | | 653,000 | | | 864,000 | |
| Merger, tender offers and consent solicitation expenses | | | — | | | — | | | — | | | 2,233,000 | | | 2,010,000 | |
| Operating loss | | | (1,117,000 | ) | | (1,438,000 | ) | | (2,051,000 | ) | | (5,965,000 | ) | | (11,030,000 | ) |
| Gain on disposition | | | — | | | — | | | — | | | — | | | 4,000 | |
| Gain on sale of investments (net) | | | — | | | 1,064,000 | | | — | | | — | | | — | |
| Interest income | | | 469,000 | | | 190,000 | | | 127,000 | | | 174,000 | | | 686,000 | |
| Interest expense | | | — | | | (2,000 | ) | | (2,000 | ) | | (2,000 | ) | | (18,000 | ) |
| Loss before tax benefit and cumulative effect of a change in accounting principle | | | (648,000 | ) | | (186,000 | ) | | (1,926,000 | ) | | (5,793,000 | ) | | (10,358,000 | ) |
| Tax benefit | | | — | | | — | | | — | | | — | | | — | |
| Net Loss | | | (648,000 | ) | | (186,000 | ) | | (1,926,000 | ) | | (5,793,000 | ) | | (10,358,000 | ) |
| Preferred Stock | | | | | | | | | | | | | | | | |
| Dividend | | | (238,000 | ) | | (234,000 | ) | | (223,000 | ) | | (207,000 | ) | | (169,000 | ) |
| Net loss attributable to common stockholders | | $ | (886,000 | ) | $ | (420,000 | ) | $ | (2,149,000 | ) | $ | (6,000,000 | ) | $ | (10,527,000 | ) |
| Basic and diluted loss per common share | | $ | (0.04 | ) | $ | (0.03 | ) | $ | (0.13 | ) | $ | (0.38 | ) | $ | (0.67 | ) |
| | | December 31, | |
| | | 2006 | | 2005 | | 2004 | | 2003 | | 2002 | |
| Balance Sheet Data | | | | | | | | | | | |
| Total assets | | $ | 8,752,000 | | $ | 9,000,000 | | $ | 10,071,000 | | $ | 11,342,000 | | $ | 17,515,000 | |
| Working capital | | | 8,079,000 | | | 8,723,000 | | | 9,829,000 | | | 10,296,000 | | | 15,924,000 | |
| Stockholders’ equity | | $ | 8,079,000 | | $ | 8,723,000 | | $ | 9,832,000 | | $ | 10,304,000 | | $ | 16,074,000 | |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read in conjunction with, and is qualified in its entirety by, the financial statements and the notes thereto included with this Report on Form 10-K. This “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this Report contains certain forward-looking statements as that term is defined in the Private Securities Litigation Reform of 1995. Such statements are based on management’s current expectations and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. When used herein, the words “anticipate,” “believe,” “estimate,” “expect” and similar expressions as they relate to our management or us are intended to identify such forward-looking statements. Our actual results, performance or achievements could differ materially from those expressed in, or implied by, these forward-looking statements. Historical operating results are not necessarily indicative of the trends in operating results for any future period.
The discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates, including those related to investments, intangible assets, income taxes, contingencies and litigation. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Overview
eXegenics currently has no business operations. eXegenics was formerly known as Cytoclonal Pharmaceutics, Inc. and was involved in the research, creation and development of drugs for the treatment and prevention of cancer and infectious diseases. Historically, eXegenics operated as a drug discovery company, exploiting new enabling technologies to advance and shorten the new drug development cycle. Commencing in 2003, eXegenics began terminating its research and related activities. Since then, all of our scientific staff and administrative positions have been eliminated and all of our research and development activities have been terminated. As such, eXegenics is a holding company with a portfolio of marketable securities and no operations.
Since the termination of operations,April 25, 2007, the board of directors amended and restated the Company’s Compensation Committee Charter.
Corporate Governance and Nominating Committee
Our corporate governance and nominating committee’s responsibilities include the selection of eXegenicspotential candidates for our board of directors. It also makes recommendations to our board of
-7-
directors concerning the structure and management have been focused on redeploying the remaining residual assets of eXegenics. The board established a committee - the Business Opportunities Search Committee - to study strategic direction and identify potential business opportunities. The objective of eXegenics is to redeploy its assets and actively pursue new business opportunities.
On February 9, 2007, eXegenics completed its sale of 19,440,491 shares of eXegenics common stock, constituting approximately 51%membership of the issuedother board committees and outstanding sharesconsiders director candidates recommended by others, including our Chief Executive Officer, other board members, third parties and shareholders. Our nominating and governance committee is comprised of eXegenics capital stock, on a fully diluted basis, to a small group of investors. The stock sale was made pursuant to the terms of a previously announced stock purchase agreement dated August 14, 2006, as amended as of November 30, 2006. The investors paid eXegenics an aggregate purchase price of $8,613,000 at the closing, which is subject to adjustment based on eXegenics stockholders’ equity at the closing. The proceeds from the stock sale provide eXegenics with working capital that can be used to create future operationalDr. Rubin, Dr. Lerner and business opportunities.
Critical Accounting Policies
We believe the following critical accounting policies affect management’s more significant judgments and estimates used in the preparation of our financial statements.
eXegenics considers all non-restrictive, highly liquid short-term investments purchased with an original maturity of three months or less to be cash equivalents. Investments consist of equity securities and are classified as available for sale and reported at their fair values. The realized gains and losses from these investments are reported in current earnings. Unrealized gains and losses from these securities are reported as a separate component of stockholders’ equity and excluded from current earnings.
In May 2005, the FASB issued Statement of Financial Statement Accounting Standards No. 154, “Accounting Changes and Error Corrections-a replacement of APB Opinion No. 20 and FASB Statement No. 3” (“SFAS 154”). This Statement replaces APB Opinion No. 20, “Accounting Changes,” and FASB Statement No. 3, “Reporting Accounting Changes in Interim Financial Statements.” SFAS 154 requires retrospective application to prior periods’ financial statements for changes in accounting principle, unless it is impractical to determine either the period-specific effects or the cumulative effect of the change. SFAS 154 also requires that a change in depreciation, amortization, or depletion method for long, non-financial assets be accounted for as a change in accounting estimate effected by a change in accounting principle. SFAS 154 is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The Company believes that adoption of the provisions of SFAS 154 will not have a material effect on the Company’s financial condition.
In July 2006, the Financial Accounting Standards Board (FASB) issued FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes - an interpretation of FASB Statement No. 109” (FIN 48), which clarifies the accounting and disclosure for uncertainty in tax positions, as defined. FIN 48 seeks to reduce the diversity in practice associated with certain aspects of the recognition and measurement related to accounting for income taxes. The Company does not expect the interpretation will have a material impact on its financial condition.
In September 2006, the FASB issued statement No. 157, “Fair Value Measurements”, (SFAS 157). SFAS 157 defines fair value, establishes a framework for measuring fair value in accordance with accounting principles generally accepted in the United States, and expands disclosures about fair value measurements. SFAS 157 is effective for fiscal years beginning after November 15, 2007, with earlier application encouraged. Any amounts recognized upon adoption as a cumulative effect adjustment will be recorded to the opening balance of retained earnings in the year of adoption. The Company has not yet determined the impact of this Statement on its financial condition.
We record a valuation allowance to reduce our deferred tax assets to the amount that is more likely than not to be realized. While we have considered future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for the valuation allowance, in the event we were to determine that we would be able to realize deferred tax assets in the future in excess of its net recorded amount, an adjustment to the net deferred tax asset would increase income in the period such determination was made. Likewise, should we determine that we would not be able to realize all or part of our net deferred tax asset in the future, an adjustment to the net deferred tax asset would be charged to income in the period such determination was made.
Results of Operations
Fiscal Year Ended December 31, 2006 Compared to Fiscal Year Ended December 31, 2005
Revenues
We recognized $0 from license, research and development revenues during fiscal 2006 and 2005. There was no license, research and development revenue as a result of eXegenics exit from the drug discovery business and termination of related research and development activities. There were no operations in 2006.
Research and Development Expenses
We incurred research and development expenses of $0 during fiscal 2006 and fiscal 2005. This was a result of eXegenics exist from the drug discovery business and termination of related research and development activities.
General and Administrative Expenses
General and administrative expenses for fiscal 2006 were $1,117,000 compared to $1,438,000 for fiscal 2005, a decrease of $321,000 or 22%. General and administrative expenses decreased primarily as a result of the reduction in payroll and related expenses. Significant variances in fiscal 2006, compared to fiscal 2005, were as follows: headcount related expenses, primarily salaries, travel and entertainment, health insurance, employee relations and office expenses declined by $288,000; investor and public relations expense declined by $5,000; insurance, primarily directors and officers liability insurance expense declined by $78,000; audit fees declined by $49,000; leased equipment expenses declined by $46,000; board of director travel expenses declined by $4,000 and miscellaneous expenses declined $86,000. The decrease in general and administrative expenses was partially offset by the following: a $180,000 increase in legal expenses (primarily attributable to the increase in the reserve for on ongoing litigation with Dr. Labidi), an increase in professional consulting fees of $25,000 and a $30,000 increase in board of director compensation.
Merger, Tender Offers and Consent Solicitation Expenses
In 2006 and 2005, we incurred no expenses related to failed merger, tender offers and consent solicitation activities. In 2006, in anticipation of the transactions completed by the Stock Purchase Agreement previously discussed, eXegenics incurred approximately $56,000 in legal, accounting and other related costs.
Expenses Related to Terminating the Drug Discovery Operations
As a result of our decision to terminate our drug discovery operations, in fiscal 2006 and 2005 we incurred no costs associated with expenses from terminated operations. No expenses were recognized in 2006 or 2005 for eXegenics’ strategic redirection.
Interest Income
Interest income for fiscal 2006 was $469,000 as compared to $190,000 for fiscal 2005, an increase of $279,000 or 68%. The increase in interest income was due to higher interest rates.
Other Income and Expenses
Other Income and expenses was $0 during fiscal 2006 and a profit of $1,062,000 during fiscal 2005. The decrease was due to the appreciation and sale, by eXegenics of Javelin Pharmaceuticals, Inc. common stock in 2005.
Net Loss
We incurred net losses of $648,000 during fiscal 2006 and $186,000 during fiscal 2005. The increase in net loss of $462,000 or 60% is a result of the aforementioned sale of investments in 2005. Net loss per common share for fiscal 2006 was $0.04 and for fiscal 2005 was $0.03.
Results of Operations
Fiscal Year Ended December 31, 2005 Compared to Fiscal Year Ended December 31, 2004
Revenues
We recognized $0 from license, research and development revenues during fiscal 2005 and 2004. There was no license, research and development revenue as a result of eXegenics exit from the drug discovery business and termination of related research and development activities. There were no operations in 2005.
Research and Development Expenses
We incurred research and development expenses of $0 during fiscal 2005 and fiscal 2004. This was a result of eXegenics exit from the drug discovery business and termination of related research and development activities.
General and Administrative Expenses
General and administrative expenses for fiscal 2005 were $1,438,000 compared to $2,051,000 for fiscal 2004, a decrease of $613,000 or 42%. General and administrative expenses decreased primarily as a result of the termination of drug discovery operations. Significant variances in fiscal 2005, compared to fiscal 2004, were as follows: professional consulting fees declined by $60,000; headcount related expenses, primarily salaries, travel and entertainment, health insurance, employee relations and office expenses declined by $210,000; investor and public relations expense declined by $44,000; insurance, primarily directors and officers liability insurance expense declined by $435,000, primarily as a result of a change in insurance carriers; tax expense, mainly franchise tax, declined by $49,000; legal fees declined by $61,000; leased equipment declined by $60,000; board of directors fees and travel expenses declined by $110,000; and audit fees declined by $35,000. The increase of $250,000 is for the reserve established in connection with the lawsuit with Dr. Labidi, which reserve reflects a reasonable estimate of eXegenics’ obligations to pay under the judgment; and an increase of $201,000 for the allowance recorded against the subscriptions receivable reflects eXegenics uncertainty as to its collectability.
Merger, Tender Offers and Consent Solicitation Expenses
In 2005 and 2004, we recognized an aggregate of $0 in expenses related to merger, tender offers and consent solicitation activities.
Expenses Related to Terminating the Drug Discovery Operations
As a result of our decision to terminate our drug discovery operations, in fiscal 2005 and 2004 we incurred $0 and $5,000, respectively, in costs associated with expenses from terminated operations. Cash disbursements made during fiscal 2004 against a previously established restructuring reserve included $90,000 for severance payments, $87,000 for terminated operating lease obligations, and $16,000 for equipment and facilities relocation. No expenses were recognized in 2005 and 2004 for eXegenics’ strategic redirection.
Interest Income
Interest income for fiscal 2005 was $190,000 as compared to $127,000 for fiscal 2004, an increase of $63,000 or 50%. The increase in interest income was due to higher interest rates and increased investable balances resulting from the appreciation in value and ultimate sale of Javelin Pharmaceuticals, Inc. common stock.
Other Income and Expenses
Other Income and expenses was a profit of $1,062,000 during fiscal year 2005 and $2,000 during fiscal year 2004. The increase was due to the appreciation and sale by eXegenics of Javelin Pharmaceuticals, Inc. common stock.
Net Loss
We incurred net losses of $186,000 during fiscal 2005 and $1,926,000 during fiscal 2004. The decrease in net loss of $1,740,000 or 90% is a result of the aforementioned sale of investments. Net loss per common share for fiscal 2005 was $0.03 and for fiscal 2004 was $0.13.
Liquidity and Capital Resources
At December 31, 2006 we had cash, cash equivalents and investments of approximately $8,596,000. During 2006, we used approximately $305,000 to fund our operating activities. Restricted cash was pledged as collateral in support of leases of laboratory equipment. In connection with the termination of our drug discovery research programs, we repurchased equipment subject to a capital lease agreement. However, in 2003, when eXegenics was in the process of exiting from the drug discovery business, it was not able to terminate its contractual obligations; it was not able to terminate its lease obligations until August 2005. In August 2005, in conjunction with the return of remaining lease obligations, the lessor of this equipment released $175,000 of restricted cash that was pledged as collateral. In addition, in 2005 eXegenics received proceeds of approximately $1,064,000 from the sale of shares of Javelin Pharmaceuticals, Inc common stock. The impact of maintaining its lease obligations through August 2005, was $46,000 in 2005 and $106,000 in 2004.
Recent Accounting Pronouncement
Mr. Baron. We believe that the adoptioncomposition of our nominating and governance committee complies with any applicable requirements of the following accounting standardSarbanes-Oxley Act of 2002, the AMEX and the SEC’s rules and regulations, including those regarding the independence of our nominating and governance committee members. We intend to comply with future requirements to the extent that they become applicable to us.
The corporate governance and nominating committee will identify director nominees through a combination of referrals, including by stockholders, existing members of the board of directors and management, and direct solicitations, where warranted. Once a candidate has been identified, the corporate governance and nominating committee reviews the individual’s experience and background, and may discuss the proposed nominee with the source of the recommendation. The corporate governance and nominating committee usually believes it to be appropriate for committee members to interview the proposed nominee before making a final determination whether to recommend the individual as a nominee to the entire board of directors to stand for election to the board of directors.
On April 25, 2007, the board of directors amended and restated the Company’s Corporate Governance and Nominating Committee Charter.
Director Nomination Process
The corporate governance and nominating committee will review annually and make recommendations to the board of directors regarding the appropriate qualifications, skills and experience expected of individual members and of the board of directors as a whole with the objective of having a board of directors with sound judgment and diverse backgrounds and experience to represent stockholder interests.
The corporate governance and nominating committee believes that nominees for election to the board of directors should possess sufficient business or financial experience and a willingness to devote the time and effort necessary to discharge the responsibilities of a director. This experience can include, but is not limited to, service on other boards of directors or active involvement with other boards of directors, experience in the industries in which the Company conducts its business, audit and financial expertise, clinical experience, operational experience, or a scientific or medical background. The corporate governance and nominating committee does not believe that nominees for election to the board of directors should be selected through mechanical application of specified criteria. Rather, the corporate governance and nominating committee believes that the qualifications and strengths of individuals should be considered in their totality with a view to nominating persons for election to the board of directors whose backgrounds, integrity, and personal characteristics indicate that they will make a positive contribution to the board of directors.
The corporate governance and nominating committee intends to identify candidates for election to the board of directors through the personal knowledge and experience of the members of the
-8-
Committee, through third-party recommendations, and, for so long as the Committee believes it appropriate, through a search firm selected by the corporate governance and nominating committee and compensated by the Company. Candidates will be evaluated based upon their backgrounds and interviews with members of the board of directors. The Committee does not plan to evaluate candidates identified by the corporate governance and nominating committee differently from those recommended by a stockholder or otherwise.
The corporate governance and nominating committee will consider director candidates recommended by stockholders. Stockholders who wish to recommend candidates for election to the board of directors must do so in writing. The recommendation should be sent to the Secretary of the Company, Adam Logal, Opko Health, Inc., 4400 Biscayne Boulevard, Suite 900, Miami, Florida 33137, who will forward the recommendation to the Committee. The recommendation must set forth (i) the name and address as they appear on the Company’s books of the stockholder making the recommendation and the class and number of shares of capital stock of the Company beneficially owned by such stockholder and (ii) the name of the candidate and all information relating to the candidate that is required to be disclosed in solicitations of proxies for election of directors under the federal proxy rules. The recommendation must be accompanied by the candidate’s written consent to being named in the Company’s proxy statement as a nominee for election to the board of directors and to serving as a director, if elected. Stockholders must also comply with all requirements of the Company’s by-laws with respect to nomination of persons for election to the board of directors.
Compensation Committee Interlocks and Insider Participation
The current members of our compensation committee are Dr. Rubin, Dr. Lerner and Mr. Eichler. None of these individuals was at any time since January 1, 2006 or at any time prior thereto an officer or employee of ours. In addition, none of our executive officers serves as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
Stockholder Communications Policy
Stockholders may initiate in writing any communication with our board of directors or any individual director by sending the correspondence to our Secretary, Opko Health, Inc., 4400 Biscayne Blvd, Suite 900, Miami, Florida 33137, Attention: Adam Logal, Secretary. This centralized process assists our board of directors in reviewing and responding to stockholder communications in an appropriate manner.
Board Attendance at Annual Meetings of Stockholders
Although we encourage each member of our board of directors to attend our annual meetings of stockholders, we do not have a material impact onformal policy requiring the members of our financial statements.board of directors to attend.
Code of Business Conduct and Ethics
In December 2004, the FASB issued SFAS No. 123R, “Share-based Payment.” SFAS No. 123R isOn April 25, 2007, we adopted a revisionCode of SFAS No. 123, “Accounting for Stock Based Compensation”,Business Conduct and supersedes APB 25. Among other items, SFAS 123R eliminates the use of APB 25 and the intrinsic value method of accounting, and requires companies to recognize the cost of employee services received in exchange for awards of equity instruments, based on the grant date fair value of those awards, in the financial statements. The effective date of SFAS 123R is January 1, 2006, for calendar year companies.
SFAS 123R permits companies to adopt its requirements using either a “modified prospective” method, or a “modified retrospective” method. Under the “modified prospective” method, compensation cost recognized in the financial statements beginning with the effective date, based on the requirements of SFAS 123R forEthics. We require all share-based payments granted after that date, and based on the requirements of SFAS 123 for all unvested awards granted prior to the effective date of SFAS 123R. Under the “modified retrospective” method, the requirements are the same as under the “modified prospective” method, but also permits entities to restate financial statements of previous periods based on proforma disclosures made in accordance with SFAS 123.
Off Balance Sheet Arrangements
There are no off balance sheet arrangements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Our exposure is to financial market risk,employees, including changes in interest rates, relates primarily to our marketable security investments. We generally place our marketable security investments in high credit quality instruments, primarily U.S. government obligations and corporate obligations with contractual maturities of less than one year. We do not believe that a 100 basis point increase or decrease in interest rates would significantly impact our financial condition. We do not have any derivative instruments. All our investments have been made in U.S. dollars. We do not have any material exposure to changes in foreign currency exchange rates.
Item 8. Financial Statements and Supplementary Data
The Financial Statements and supplementary data required by this Item 8 are included in Part IV, Item 15 of this Form 10-K and are presented beginning on page F-1 and are incorporated by reference into this Item 8.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
(a)Disclosure Controls and Procedures
The Company’s management, with the participation of the Company’s principal executive officer and principal financialaccounting officer evaluatedand other
-9-
senior officers and our employee directors, to read and to adhere to the effectivenessCode of Business Conduct and Ethics in discharging their work-related responsibilities. Employees are required to report any conduct that they believe in good faith to be an actual or apparent violation of the designCode of Business Conduct and operationEthics. The Code of Business Conduct and Ethics is available on our website at http://www.opko.com.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires the Company’s directors and executive officers, and persons who own more than ten percent of a registered class of the Company’s disclosure controlsequity securities, to file with the United Stated Securities and Exchange Commission initial reports of ownership and reports of changes in ownership of Common Stock and other equity securities of the Company. Officers, directors and greater than ten percent stockholders are required by SEC regulation to furnish the Company with copies of all Section 16(a) forms they file.
To the Company’s knowledge, based solely on a review of the copies of such reports furnished to the Company and written representations that no other reports were required, during fiscal year 2006, all Section 16(a) filing requirements applicable to its officers, directors and greater than ten percent stockholders were complied with by the Company’s directors and executive officers, and ten percent stockholders.
Item 11. Executive Compensation.
Compensation Discussion and Analysis
The primary goals of our board of directors with respect to executive compensation will be to attract and retain talented and dedicated executives, to tie annual and long-term cash and stock incentives to achievement of specified performance objectives, and to create incentives which will result in stockholder value creation. To achieve these goals, we have formed a compensation committee to recommend executive compensation packages to our board of directors that are generally based on a mix of salary, discretionary bonus and equity awards. Although we have not adopted any formal guidelines for allocating total compensation between equity compensation and cash compensation, we intend to implement and maintain compensation plans that tie a substantial portion of our executives’ overall compensation to achievement of corporate goals.
Benchmarking of Cash and Equity Compensation
We have not retained a compensation consultant to review our policies and procedures (as definedwith respect to executive compensation. We have, in Rules 13a-15(e)the past, conducted an annual benchmark review of the aggregate level of our executive compensation, as well as the mix of elements used to compensate our executive officers. This review is based on a survey of executive compensation paid by peer companies in the pharmaceutical industry of similar size and 15d-15(e) understage of development. In addition, we have historically taken into account input from other independent members of our board of directors and publicly available data relating to the Exchange Act)compensation practices and policies of other companies within and outside our industry.
-10-
We may retain the services of third-party executive compensation specialists from time to time in connection with the establishment of cash and equity compensation and related policies.
Elements of Compensation
We will evaluate individual executive performance with a goal of setting compensation at levels the board or any applicable committee thereof believes are comparable with executives in other companies of similar size and stage of development while taking into account our relative performance and our own strategic goals. The compensation received by our executive officers consists of the following elements:
Base Salary. BasedBase salaries for our executives are established based on the scope of their responsibilities and individual experience, taking into account competitive market compensation paid by other companies for similar positions within the pharmaceutical industry. The table below sets forth the base salary for our Named Executive Officers:
| | | | | | | | | | | | | | | |
| | | | | Base Salary |
| Name | | Principal Position | | 2005 | | 2006 | | 2007 |
| |
| Phillip Frost, M.D. | | Chairman and Chief Executive Officer | | | — | | | | — | | | — |
| | | | | | | | | | | | | | | |
| Adam Logal | | Executive Director of Finance, Chief
Accounting Officer, Treasurer and Secretary | | | — | | | | — | | | $ | 140,000 | |
| |
| Dale Pfost, PhD | | President | | $ | 265,000 | | | $ | 280,000 | | | $ | 325,000 | |
| | | | | | | | | | | | | | | |
| Samuel Reich | | Executive Vice President | | $ | 156,925 | | | $ | 172,000 | | | $ | 210,000 | |
| |
| Denis O’Shaughnessy | | Senior Vice President of Clinical Development | | | — | | | | — | | | $ | 265,000 | |
Discretionary Annual Bonus. In addition to base salaries, our compensation committee has the authority to award discretionary annual bonuses to our executive officers. The annual incentive bonuses are intended to compensate officers for achieving corporate goals and value-creating milestones. Each executive officer is eligible for a discretionary annual bonus up to an amount equal to a specified percentage of such executive officer’s salary.
2006 Cash Incentive Plan Compensation
| | | | | | | | | | | | | | | |
| | | | | Actual | | Target | | |
| | | | | Bonus as | | Payout as a | | |
| | | | | Percentage | | Percentage | | Target |
| | | | | of Base | | of Base | | Bonus |
| Name | | Actual Bonus Award | | Salary | | Salary | | Award |
| |
| Phillip Frost, M.D. | | Chairman and Chief Executive Officer | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | |
| Adam Logal | | Executive Director of Finance, Chief
Accounting Officer, Treasurer and Secretary | | |
— | | | |
— | | | |
— | |
| |
| Dale Pfost, PhD | | President | | | 21 | % | | | 30 | % | | $ | 60,000 | |
| | | | | | | | | | | | | | | |
| Samuel Reich | | Executive Vice President | | | 23 | % | | | 30 | % | | $ | 40,000 | |
| |
| Denis O’Shaughnessy | | Senior Vice President of Clinical Development | | | — | | | | — | | | | — | |
-11-
Long-Term Incentive Program. We believe that evaluation,long-term performance is achieved through an ownership culture that encourages such performance by our executive officers through the Company’s principal executiveuse of stock and principal financial officers concludedstock-based awards. We believe that the Company’s disclosure controlsuse of equity and proceduresequity-based awards offers the best approach to achieving our compensation goals. We have not adopted formal stock ownership guidelines.
Severance and Change-in-Control Benefits. Certain of our named executive officers are entitled to certain severance and change of control benefits, the terms of which are described below under “—Employment Agreements and Change in Control Arrangements.” We believe these severance and change-in-control benefits are an essential element of our executive compensation package and assist us in recruiting and retaining talented individuals.
Restricted Stock Grants or Awards. We did not grant any restricted stock or restricted stock awards pursuant to our equity benefit plans to any of our executive officers in the year ended December 31, 2006. However, our compensation committee, in its discretion, may in the future elect to make such grants to our executive officers if it deems it advisable.
Other Compensation. We intend to continue to maintain the current benefits and perquisites for our executive officers; however, our compensation committee, in its discretion, may in the future revise, amend or add to the benefits and perquisites of any executive officer if it deems it advisable. The material terms of our employment agreements with our named executive officers are described below under “—Employment Agreements and Change in Control Arrangements.”
Summary Compensation Table
The following table sets forth a summary for the fiscal year ended December 31, 2006 of the cash and non-cash compensation awarded, paid or accrued by the Company to our Named Executive Officers.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Stock | | Option | | All Other | | |
| | | | | | | Salary | | Bonus | | Award(s) | | Award(s) | | Compensation | | Total |
| Name and Principal Position | | Year | | ($) | | ($) | | ($) | | ($) | | ($) | | ($) |
| John A. Paganelli (1) | | | 2006 | | | | 25,000 | | | | — | | | | — | | | | 810 | | | $ | 75,000 | (2) | | | 100,810 | |
Interim Chief Executive Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Phillip Frost, M.D. (3) | | | 2006 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Chief Executive Officer | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Dale Pfost, Ph.D. | | | 2006 | | | | 280,000 | | | | 84,000 | | | | — | | | | 359,982 | | | | — | | | | 723,982 | |
President(4) | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Adam Logal | | | 2006 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
Executive Director of Finance and Chief Accounting Officer, Treasurer and Secretary(5) | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Samuel J. Reich | | | 2006 | | | | 172,000 | | | | 51,600 | | | | — | | | | 193,470 | | | | — | | | | 417,070 | |
Executive Vice President (6) | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Denis O’Shaughnessy (7) (8) | | | 2006 | | | | 47,702 | | | | 45,520 | | | | — | | | | 21,564 | | | | — | | | | 114,786 | |
Senior Vice President of Clinical Development | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Mr. Paganelli served as the Company’s interim Chief Executive Officer from June 29, 2005 and he resigned from this position upon the consummation of the Acquisition. |
-12-
| | |
| (2) | | Includes $75,000 of director fees for Mr. Paganelli. |
|
| (3) | | Dr. Frost became the Company’s Chief Executive Officer upon consummation of the Acquisition. |
|
| (4) | | Dr. Pfost served as the President and Chief Executive Officer of Acuity prior to the Acquisition and was appointed to be the Company’s President on March 29, 2007. |
|
| (5) | | Mr. Logal served as the Executive Director of Finance and Chief Accounting Officer of Acuity prior to the Acquisition and was appointed to be the Company’s Executive Director of Finance, Chief Accounting Officer and Treasurer on March 29, 2007. |
|
| (6) | | Samuel Reich served as the Executive Vice President of Acuity prior to the Acquisition and was appointed to be the Company’s Executive Vice President on March 29, 2007. |
|
| (7) | | Dr. O’Shaughnessy served as the Senior Vice President of Clinical Development of Acuity prior to the Acquisition and was appointed to be the Company’s Senior Vice President of Clinical Development on March 29, 2007. |
|
| (8) | | Dr. O’Shaughnessy commenced employment with Acuity on November 13, 2006. |
Grants of Plan-Based Awards in 2006
The following table presents information concerning grants of plan-based awards to each of the named executive officers during the year ended December 31, 2006. The exercise price per share of each option granted to our named executive officers was equal to the fair market value of our common stock, as determined by our compensation committee on the date of the grant.
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Grant Date |
| | | | | | | Number of Securities | | Exercise Price | | Fair Value of |
| Name | | Grant Date | | Underlying Options | | Per Share | | Option Awards (1) |
| Phillip Frost, M.D. | | | — | | | | — | | | | — | | | | — | |
| Dale Pfost, Ph.D. | | | — | | | | — | | | | — | | | | — | |
| John A. Paganelli | | | 1/3/2006 | | | | 5,000 | | | $ | 0.41 | | | $ | 205 | |
| | | | 4/3/2006 | | | | 5,000 | | | $ | 0.41 | | | $ | 205 | |
| | | | 7/3/2006 | | | | 5,000 | | | $ | 0.41 | | | $ | 205 | |
| Adam Logal | | | — | | | | — | | | | — | | | | — | |
| Samuel J. Reich | | | — | | | | — | | | | — | | | | — | |
| Denis O’Shaughnessy | | | — | | | | — | | | | — | | | | — | |
| | |
| (1) | | Amounts reflect the total fair value of stock options granted in 2006, calculated in accordance with SFAS No. 123(R). |
-13-
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information with respect to the Named Executive Officers concerning equity awards granted by the Company as of December 31, 2006 (the end2006.
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Option Awards | | Stock Awards |
| | Number of | | Number of | | | | | | | | | | Number of | | Market Value |
| | | Securities | | Securities | | | | | | | | | | Shares or Units | | of Shares or |
| | | Underlying | | Underlying | | | | | | | | | | of Stock That | | Units of |
| | | Unexercised | | Unexercised | | Option | | Option | | Have Not | | Stock That |
| | | Options (#) | | Options (#) | | Exercise | | Expiration | | Vested | | Have Not |
| Name | | Exercisable (1) | | Unexercisable | | Price ($) | | Date | | (#) | | Vested ($) |
| Phillip Frost, M.D. | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Dale Pfost, Ph.D. | | | 77,841 | | | | 233,524 | | | | 0.05 | | | | 01/01/2016 | | | | — | | | | — | |
| | | | 90,815 | | | | 220,550 | | | | 0.05 | | | | 11/01/2015 | | | | — | | | | — | |
| | | | 608,138 | | | | 689,223 | | | | 0.04 | | | | 02/15/2015 | | | | — | | | | — | |
| | | | 225,740 | | | | — | | | | 0.04 | | | | 09/24/2014 | | | | — | | | | — | |
| | | | 323,042 | (2) | | | 107,680 | | | | 0.04 | | | | 12/11/2013 | | | | — | | | | — | |
| John A. Paganelli | | | 5,000 | (2) | | | — | | | | 0.41 | | | | 07/03/2016 | | | | — | | | | — | |
| | | | 5,000 | (2) | | | — | | | | 0.41 | | | | 04/03/2016 | | | | — | | | | — | |
| | | | 5,000 | (2) | | | — | | | | 0.41 | | | | 01/03/2016 | | | | — | | | | — | |
| | | | 5,000 | (2) | | | — | | | $ | 0.45 | | | | 10/4/2015 | | | | — | | | | — | |
| | | | 5,000 | (2) | | | — | | | $ | 0.42 | | | | 7/1/2015 | | | | — | | | | — | |
| | | | 5,000 | (2) | | | — | | | $ | 0.40 | | | | 4/1/2015 | | | | — | | | | — | |
| | | | 5,000 | (2) | | | — | | | $ | 0.32 | | | | 1/3/2015 | | | | — | | | | — | |
| | | | 5,000 | (2) | | | — | | | $ | 0.67 | | | | 10/1/2014 | | | | — | | | | — | |
| | | | 5,000 | (2) | | | — | | | $ | 0.71 | | | | 7/1/2014 | | | | — | | | | — | |
| | | | 10,000 | (2) | | | — | | | $ | 0.89 | | | | 4/27/2014 | | | | — | | | | — | |
| Adam Logal | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Samuel J. Reich | | | 71,920 | | | | 215,766 | | | | 0.05 | | | | 01/01/2016 | | | | — | | | | — | |
| | | | 83,907 | | | | 203,778 | | | | 0.05 | | | | 11/01/2015 | | | | — | | | | — | |
| | | | 214,063 | | | | 242,605 | | | | 0.04 | | | | 02/15/2015 | | | | — | | | | — | |
| | | | 131,360 | | | | 102,169 | | | | 0.04 | | | | 09/21/2014 | | | | — | | | | — | |
| | | | 194,603 | | | | 64,867 | | | | 0.04 | | | | 12/11/2013 | | | | — | | | | — | |
| Denis O’Shaughnessy | | | 32,433 | | | | 745,980 | | | | 0.06 | | | | 10/23/2016 | | | | | | | | | |
| | | | 259,471 | (2) | | | — | | | | 0.06 | | | | 10/23/2016 | | | | | | | | | |
| | |
| (1) | | Except as noted below, all options vest in 48 equal monthly installments beginning on the date of grant. |
|
| (2) | | This option was fully vested on the grant date. |
Director Compensation
The following table sets forth information with respect to compensation of directors of the Company during fiscal year 2006.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | Nonqualified | | | | |
| | | Fees Earned | | | | | | | | | | Non-Equity | | Deferred | | | | |
| | | or Paid in | | | | | | Option | | Incentive Plan | | Compensation | | All Other | | |
| | | Cash | | Stock Award | | Awards | | Compensation | | Earnings | | Compensation | | Total |
| Name | | ($) | | ($) | | ($) | | ($) | | ($) | | ($) | | ($) |
| Robert Baron | | | 50,000 | | | | — | | | | 810 | | | | — | | | | — | | | | — | | | | 50,810 | |
| David A, Eichler | | | — | | | | — | | | | 13,815 | | | | — | | | | — | | | | — | | | | 13,815 | |
| Michael Reich (1) | | | — | | | | — | | | | 46,960 | | | | — | | | | — | | | | — | | | | 46,960 | |
| Steven D. Rubin | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Jane H. Hsiao, Ph.D. | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| John Paganelli | | | 25,000 | | | | | | | | 810 | | | | | | | | | | | $ | 75,000 | | | | 100,810 | |
| Richard Lerner, MD | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Melvin Rubin, MD | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | |
| (1) | | At December 31, 1006, Michael Reich had outstanding options to purchase 291,644 shares of our Common Stock. |
We are currently considering compensation policies for directors of the Company. In the future, we may adopt a policy of paying independent directors an annual retainer and a fee for attendance at board and committee meetings. We anticipate reimbursing each director for reasonable travel expenses related to such director’s attendance at board of directors and committee meetings.
-14-
Employment Agreements and Change in Control Arrangements
Dale R. Pfost, Ph.D.We are employing Dale R. Pfost as our President. Under his employment agreement, Dr. Pfost receives an annual base salary, subject to increases upon an annual review by our board of directors. Dr. Pfost’s current salary is $325,000. The agreement provides for a discretionary annual bonus based on Dr. Pfost’s performance and our business results as determined by our board of directors. Under the agreement, either we or Dr. Pfost may terminate his employment at any time, subject to continuation of salary payment and benefits for 12 months if we terminate Dr. Pfost’s employment without cause, if Dr. Pfost terminates his employment for good reason or if we give Dr. Pfost notice of our intent not to renew the agreement after the initial year of his employment with the Company. The employment period coveredwill automatically be extended for one additional year unless either the Company or Dr. Pfost shall have given to the other party written notice of non-extension at least sixty (60) days prior to such anniversary. We have agreed to grant Dr. Pfost an option to purchase 300,000 shares of our common stock upon the implementation of our 2007 Equity Incentive Plan.
Samuel J. Reich.We are employing Samuel J. Reich as our Executive Vice President. Under his employment agreement, Mr. Reich receives an annual base salary, subject to increases upon an annual review by this Report)our board of directors. Mr. Reich’s current salary is $210,000. The agreement provides for a discretionary annual bonus based on Mr. Reich’s performance and our business results as determined by our board of directors. Under the agreement, either we or Mr. Reich may terminate his employment at any time, subject to continuation of salary payment and benefits for 12 months if we terminate Mr. Reich’s employment without cause, if Mr. Reich terminates his employment for good reason or if we give Mr. Reich notice of our intent not to renew the agreement after the initial year of his employment with the Company. The employment period will automatically be extended for one additional year unless either the Company or Mr. Reich shall have been designedgiven to the other party written notice of non-extension at least thirty (30) days prior to such anniversary. We have agreed to grant Mr. Reich an option to purchase 500,000 shares of our common stock upon the implementation of our 2007 Equity Incentive Plan.
Denis O’Shaughnessy, Ph.D. We are employing Dr. O’Shaughnessy as our Senior Vice President of Clinical Development. We have entered into a severance agreement with Dr. O’Shaughnessy which provides that if we terminate his employment without cause during his first year of employment, we are obligated to pay him severance equal to three months salary following termination. The severance period increases by three months after each year of employment up to twelve months. We have also agreed to continue vesting of his options during the applicable severance period.
Adam Logal.We are employing Adam Logal as our Executive Director of Finance, Chief Accounting Officer, Treasurer and are functioning effectivelySecretary. We have agreed to enter into a severance agreement with Mr. Logal to provide reasonable assurance thatthat: Mr. Logal will receive four months of paid salary and continued benefits if he is terminated without cause or he terminates his employment for “good reason.” Upon such termination, we have agreed to accelerate the information requiredvesting of all unvested stock options granted to be disclosed byMr. Logal in connection with the Company in reports filedcommencement of his employment.
-15-
If we terminated our named executive officers without cause or submitted by it under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
(b)Changes in Internal Control Over Financial Reporting
No changes in the Company’s internal control over financial reporting were identified in the fiscal quarter endedthey resigned for good reason on December 31, 2006, that materially affected,we would have to make the payments set forth in the following chart:
| | | | | | | | | |
| | | Cash Payments upon Termination | | |
| | | without Cause or Resignation for | | |
| Name and Principal Position | | Good Reason | | Vesting of Stock Options |
John A. Paganelli (1)
Interim Chief Executive Officer | | None | | None |
Phillip Frost, M.D.
Chief Executive Officer | | None | | None |
Adam Logal (2)
Executive Director of Finance and Chief Accounting Officer, Treasurer and Secretary | | None | | None |
Dale Pfost, Ph.D.
President | | $ | 280,000 | | | | 587,702 | |
Samuel J. Reich
Executive Vice President | | $ | 210,000 | | | | 376,394 | |
Denis O’Shaughnessy
Senior Vice President of Clinical Development | | $ | 88,333 | | | | 48,650 | |
| | |
| (1) | | Mr. Paganelli served as the Company’s interim Chief Executive Officer from June 29, 2005 and he resigned from this position upon the consummation of the Acquisition. |
|
| (2) | | Mr. Logal joined the Company on March 15, 2007. |
-16-
| | |
| Item 12. | | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The following table sets forth certain information, as of April 20, 2007, with respect to the beneficial ownership of our common stock by: (i) each stockholder known by us to be the beneficial owner of more than 5% of our common stock; (ii) each director or are reasonably likely to materially affect,director nominee; (iii) each executive officer named in the Company’s internal control over financial reporting.
Item 9B. Other Information.
Not applicable.
PART III
The information required by this item (Items 401, 405, 406 and 407(c)(3), (d)(4) and (d)(5) of Regulation S-K) relating to:Beneficial ownership is determined in accordance with the Company’s directors, director nominees and executive officers; compliance with Section 16(a)rules of the Exchange Act bySEC. Shares of our common stock subject to options or warrants currently exercisable or exercisable within 60 days of April 20, 2007 are deemed to be outstanding for calculating the Company’s officers, directors and 10% beneficial owners; the Company's codepercentage of ethics; material changes to the Company's security holder nominating procedures; and the audit committeeoutstanding shares of the Company’s Boardperson holding those options or warrants, but are not deemed outstanding for calculating the percentage of Directors (including identificationany other person. Percentage of its “audit committee financial expert”)beneficial ownership is included in the Company’s definitive proxy statement relating to its 2007 annual meetingbased upon 113,116,350 shares of stockholders (the “Proxy Statement”), which will be filed with the SEC within 120 days after the endour common stock outstanding as of the fiscal year to which this Report on Form 10-K relates, and is incorporated herein by reference.April 20, 2007.
In addition, the information included under the caption "Executive Officers of the Registrant" in Part I. of this Form 10-K is incorporated by reference into this Item 10.
Item 11. Executive Compensation
The information required by this item (Item 402 and Item 407(e)(4) and (e)(5) of Regulation S-K) relating to executive officer and director compensation and compensation committee interlocks and insider participation, and the Compensation Committee Report is included in the Company’s Proxy Statement and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | Percentage of |
| | | | | | | | | | | | | Common Shares |
| | | | | | | | | | | | | Assuming |
| | | | | | | | | Percentage | | Conversion of all |
| | | | | | | | | Of | | Outstanding |
| | | | | | | | | Outstanding | | Series C Preferred |
| Title of | | Name and Address or | | Number of | | Common | | Stock into |
| Class | | Beneficial Owner | | Shares | | Shares | | Common Stock |
| Common Stock | | The Frost Group, LLC (1)
| | | | | | | | | | | | |
| | | 4400 Biscayne Blvd.
| | | | | | | | | | | | |
| | | Suite 1500
| | | | | | | | | | | | |
| | | Miami, Florida 33137 | | | 20,286,704 | | | | 17.20 | % | | | 12.39 | % |
| | | | | | | | | | | | | | | |
| Common Stock | | Frost Gamma Investments Trust(2)
| | | | | | | | | | | | |
| | | 4400 Biscayne Blvd.
| | | | | | | | | | | | |
| | | Suite 1500
| | | | | | | | | | | | |
| | | Miami, Florida 33137 | | | 66,047,216 | | | | 54.60 | % | | | 39.61 | % |
| | | | | | | | | | | | | | | |
| Common Stock | | Johnson and Johnson Development
| | | | | | | | | | | | |
| | | Corporation(3)
| | | | | | | | | | | | |
| | | One Johnson & Johnson Plaza
| | | | | | | | | | | | |
| | | New Brunswick, NJ 08933 | | | 16,125,774 | | | | 12.48 | % | | | 9.95 | % |
| | | | | | | | | | | | | | | |
| Common Stock | | Psilos Group Partners II-S(4)
| | | | | | | | | | | | |
| | | 625 Avenue of the Americas
| | | | | | | | | | | | |
| | | 4th Floor
| | | | | | | | | | | | |
| | | New York, NY 10011 | | | 11,340,501 | | | | 9.11 | % | | | 7.04 | % |
| | | | | | | | | | | | | | | |
| Common Stock | | OZ Master Fund, Ltd. (OZMD)(5)
| | | | | | | | | | | | |
| | | 9 W. 57th Street, 39th Floor
| | | | | | | | | | | | |
| | | New York, NY 10019 | | | 9,553,586 | | | | 7.79 | % | | | 5.95 | % |
| | |
| (1) | | The Frost Group, LLC holds 15,490,546 shares of the Company’s common stock and warrants to purchase 6,487 shares of the Company’s Series C Preferred Stock, convertible into 648,700 shares of the Company’s common stock. The Frost Group, LLC also holds warrants to purchase 4,147,458 shares of the Company’s common stock. |
-17-
| | |
| (2) | | The Frost Gamma Investments Trust holds 36,518,923 shares of the Company’s common stock and warrants to purchase 9,241,589 shares of common stock. The number of shares included above also includes 15,490,546 shares of common stock, warrants to purchase 4,147,458 shares of common stock and warrants to purchase 6,487 shares of the Company’s Series C preferred stock, convertible into 648,700 shares of the Company’s common stock, owned directly by The Frost Group, LLC. Frost Gamma Investments Trust is a principal member of The Frost Group, LLC. Frost Gamma Investments Trust disclaims beneficial ownership of these shares of common stock, except to the extent of any pecuniary interest therein. |
|
| (3) | | Johnson and Johnson Development Corporation holds 129,736 shares of the Company’s Series C preferred stock, convertible into 12,973,600 shares of the Company’s common stock. Johnson and Johnson Development Corporation also holds warrants to purchase 2,949,141 shares of the Company’s common stock and options to purchase 203,033 shares of the Company’s common stock that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007. |
|
| (4) | | Psilos Group Partners II-S holds 90,815 shares of the Company’s Series C preferred stock, convertible into 9,081,500 shares of the Company’s common stock. Psilos Group Partners II S also holds warrants to purchase 2,064,399 shares of the Company’s common stock and options to purchase 194,602 shares of the Company’s common stock that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007. |
|
| (5) | | OZ Master Fund, Ltd. holds 77,841 shares of the Company’s Series C preferred stock, convertible into 7,784,100 shares of the Company’s common stock. OZ Master Fund, Ltd. also holds warrants to purchase 1,769,486 shares of the Company’s common stock that are exercisable as of April 20, 2007. |
Security Ownership of Directors and ManagementNamed Executive Officers
| | | | | | | | | | | | | | | |
| | | | | | | | | | | | | Percentage of |
| | | | | | | | | | | | | Common |
| | | | | | | | | | | | | Shares |
| | | | | | | | | | | | | Assuming |
| | | | | | | | | | | | | Conversion of |
| | | | | | | | | | | | | all |
| | | | | | | | | | | | | Outstanding |
| | | | | | | | | | | | | Series C |
| | | | | | | | | | | | | Preferred |
| | | | | Number of | | Percentage of | | Stock into |
| Title of | | Name of | | Outstanding Shares | | Outstanding | | Common |
| Class | | Beneficial Owner | | Beneficially Owned | | Common Shares | | Stock |
| Common Stock | | Phillip Frost, M.D. | | | 66,047,216 | (1) | | | 54.60 | % | | | 39.61 | % |
| Common Stock | | Jane H. Hsiao, Ph.D., MBA | | | 14,540,724 | (2) | | | 12.53 | % | | | 8.99 | % |
| Common Stock | | David Eichler | | | 11,340,501 | (3) | | | 9.11 | % | | | 7.04 | % |
| Common Stock | | Steven D. Rubin | | | 5,132,021 | (4) | | | 4.50 | % | | | 3.21 | % |
| Common Stock | | Dale Pfost, Ph.D. | | | 4,854,113 | (5) | | | 4.17 | % | | | 3.01 | % |
| Common Stock | | Samuel Reich | | | 1,400,439 | (6) | | | 1.23 | % | | | 0.88 | % |
| Common Stock | | Michael Reich | | | 649,145 | (7) | | | * | | | | * | |
| Common Stock | | Denis O’Shaughnessy, Ph.D. | | | 440,127 | (8) | | | * | | | | * | |
| Common Stock | | Robert Baron | | | 121,800 | (9) | | | * | | | | * | |
| Common Stock | | John A. Paganelli | | | 155,000 | (10) | | | * | | | | * | |
| Common Stock | | Adam Logal | | | 16,216 | (11) | | | * | | | | * | |
| Common Stock | | Richard A. Lerner, M.D. | | | — | | | | — | | | | — | |
| Common Stock | | Melvin L. Rubin, M.D. | | | — | | | | — | | | | — | |
| Common Stock | | All Executive Officers | | | | | | | | | | | | |
| | | and Directors as a group | | | | | | | | | | | | |
| | | (12 persons) | | | 104,451,240 | | | | 87.36 | % | | | 63.60 | % |
-18-
| | |
| * | | less than 1%. |
|
| (1) | | The number of shares beneficially owned by Dr. Frost includes shares of common stock and warrants to purchase shares of common stock held by or beneficially owned by Frost Gamma Investments Trust, of which Frost Gamma Limited Partnership is the sole and exclusive beneficiary. Dr. Frost is one of two limited partners of Frost Gamma, L.P. The general partner of Frost Gamma, L.P. is Frost Gamma, Inc. and the sole shareholder of Frost Gamma, Inc. is Frost-Nevada Corporation. Dr. Frost is also the sole shareholder of Frost-Nevada Corporation. The Frost Gamma Investments Trust holds 36,518,923 shares of the Company’s common stock and warrants to purchase 9,241,589 shares of common stock. The number of shares included above also includes 15,490,546 shares of common stock, warrants to purchase 4,147,458 shares of common stock and warrants to purchase 6,487 shares of the Company’s Series C preferred stock, convertible into 648,700 shares of the Company’s common stock, owned directly by The Frost Group, LLC. Frost Gamma Investments Trust disclaims beneficial ownership of these shares of common stock, except to the extent of any pecuniary interest therein. |
|
| (2) | | Dr. Hsiao is a member of The Frost Group, LLC. Dr. Hsiao disclaims beneficial ownership of the securities held by The Frost Group, except to the extent of her pecuniary interest therein. |
|
| (3) | | Includes 11,145,899 shares and warrants and 138,384 options that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007 and which are held by Psilos Group Partners II-S, an entity with which Mr. Eichler is affiliated. Mr. Eichler disclaims beneficial ownership of all such shares, warrants and options. |
|
| (4) | | Mr. Rubin is a member of The Frost Group, LLC. Mr. Rubin disclaims beneficial ownership of the securities held by The Frost Group, except to the extent of his pecuniary interest therein. |
|
| (5) | | Includes 1,644,828 shares which are the subject of stock options that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007. |
|
| (6) | | Includes 864,868 shares which are the subject of stock options that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007. Excludes 330,254 shares beneficially owned by Ilana K. Reich, of which Mr. Samuel J. Reich disclaims beneficial ownership. |
|
| (7) | | Includes 256,875 shares which are the subject of stock options that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007. |
|
| (8) | | Includes 440,127 shares which are the subject of stock options that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007. |
|
| (9) | | Includes 55,000 shares which are the subject of stock options that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007. |
|
| (10) | | Includes 55,000 shares which are the subject of stock options that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007. |
|
| (11) | | Includes 16,216 shares which are the subject of stock options that are exercisable as of April 20, 2007 or will become exercisable on or before June 20, 2007. |
| | |
| Item 13. | | Certain Relationships and Related Transactions, and Director Independence. |
Review and Approval of Related Stockholder MattersPerson Transactions.
We review all relationships and transactions in which the company and our directors and executive officers or their immediate family members are participants to determine whether such persons have a direct or indirect material interest. The company’s legal staff is primarily responsible for the development and implementation of processes and controls to obtain information from the directors and executive officers with respect to related person transactions and for then determining, based on the facts and circumstances, whether the company or a related person has a direct or indirect material interest in the transaction. As required under SEC rules, transactions that are determined to be directly or indirectly material to the company or a related person are disclosed in the company’s proxy statement. In addition, the audit committee reviews and approves or ratifies any related person transaction that is required to be disclosed.
-19-
Jane H. Hsiao and Steven D. Rubin, two of our directors, and a trust controlled by Dr. Phillip Frost, our Chief Executive Officer and Chairman of the board of directors are members of The Frost Group, LLC, an entity which controls approximately 13.3% of our outstanding voting securities. Furthermore, the trust that is a member of the Frost Group owns 39% of our outstanding voting securities and 55.16% of our outstanding common stock. We are parties to a credit agreement with the Frost Group under which we have access to a line of credit with available borrowings of $12.0 million. To date, $4.0 million has been drawn under the line of credit by Acuity prior to the Acquisition and the obligation to repay this amount was assumed by us as a result of the Acquisition. We are obligated to pay interest at a 10% annual rate on the borrowings on the line of credit. In connection with the entering into the line of credit, we have granted 4,000,000 warrants to purchase shares of common stock to The Frost Group, LLC.
Our principal corporate office is now located at 4400 Biscayne Blvd, Suite 900, Miami, Florida. We rent this space from Frost Real Estate Holdings, LLC, which is a company controlled by Dr. Phillip Frost, our chief executive officer and chairman.
We have an office located at 1250 Pittsford-Victor Road, Building 200, Suite 280, Pittsford, New York 14534 that consists of approximately 500 square feet of office space. The informationCompany sublets this office space from RFG Associates, a general partnership in which John A. Paganelli, a director of the Company, is a partner. Monthly rent is $625 and the sublease may be terminated by either party upon thirty (30) days notice. We have provided notice of our intention to terminate this lease at the end of May 2007.
Michael Reich, a director of the Company, is the uncle of Samuel J. Reich, the Company’s Executive Vice President.
The Board uses the standards set forth in rules promulgated by the American Stock Exchange in determining the independence of each member of its Audit Committee, its Compensation Committee and its Corporate Governance and Nominating Committee. Applying these standards, the Board has determined that all members of the Company’s Audit Committee, Compensation Committee and Corporate Governance and Nominating Committee are each “independent” as defined by the applicable rules of the American Stock Exchange and the Securities and Exchange Commission.
Our Board of Directors affirmatively determines the independence of each director and nominee for election as a director using the standards set forth in rules promulgated by American Stock Exchange for determining the independence of a director, including the consideration of any relationship which, in the opinion of the Board, would interfere with the exercise of independent judgment in carrying out the director’s responsibilities as a member of the Board. During the Board’s annual review of director independence, the Board determined that other Dr. Rubin, Dr. Lerner, Mr. Baron, Mr. Eichler and Mr. Reich are “independent” directors.
-20-
| | |
| Item 14. | | Principal Accountant Fees and Services. |
Audit Fees
Our principal accountants billed us an aggregate of $55,500 and $29,000 in fees and expenses for professional services rendered in connection with the audits of our financial statements for the calendar years ended December 31, 2006 and 2005, respectively, and reviews of the financial statements included in our quarterly reports on Form 10-Q during such calendar years.
Audit Related Fees
Our principal accountants billed us an aggregate of $11,000 and $23,000 in audit related fees for due diligence for the calendar years ended December 31, 2006 and 2005, respectively.
Tax Fees
Our principal accountants billed us an aggregate of $4,000 and $15,000 in fees and expenses for tax compliance, tax advice and tax planning during calendar years ended December 31, 2006 and 2005, respectively.
All Other Fees
Our principal accountants did not bill us any additional fees that are not disclosed under audit fees, audit related fees or tax fees in each of the last two calendar years.
Audit Committee Policy for Pre-approval of Independent Auditor Services
The audit committee of the board of directors is required to pre-approve all audit and non-audit services provided by the Company’s independent auditors in order to assure that the provision of such services does not impair the auditor’s independence. The audit committee has established a policy regarding pre-approval of permissible audit, audit-related and other services provided by the independent auditors, which services are periodically reviewed and revised by the audit committee. Unless a type of service has received general pre-approval under the policy, the service will require specific approval by the audit committee. The policy also includes pre-approved fee levels for specified services, and any proposed service exceeding the established fee level must be specifically approved by the Committee.
-21-
PART IV
| | |
| Item 15. | | Exhibits, Financial Statement Schedules. |
(a) (1) Financial Statements
Incorporated by reference to eXegenics’ Annual Report on Form 10-K for the year ended December 31, 2006, filed on March 22, 2007, and the Index on page F-1 thereto.
(2) Financial Statement Schedules
The other financial statement schedules required by this item (Items 201(d) and 403 of Regulation S-K) relating to the Company’s security ownership by certain beneficial owners and management, and securities authorized for issuance under equity compensation plans, is included in the Company’s Proxy Statement and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item (Item 404 and 407(a) of Regulation S-K) relating to “transactions with related persons, promoters and certain control persons” and director independence is included in the Company’s Proxy Statement and is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services
The information required by this item is included in the Company’s Proxy Statement and is incorporated herein by reference.
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a) The following documents are filed as part of this Report:
1. Financial Statements. The following financial statements and accountant's report are included in this Annual Report on Form 10-K:
Index
| Page
|
Report of Rotenberg & Co., LLP, Independent Registered Public Accounting Firm | F-1 |
Report of BDO Seidman, LLP, Independent Registered Public Accounting Firm | F-2 |
Balance Sheets as of December 31, 2006 and 2005 | F-3 |
Statements of operations for the fiscal years ended December 31, 2006, 2005 and 2004 | F-4 |
Statements of changes in stockholders’ equity for the fiscal years ended December 31, 2006, 2005 and 2004
| |
Statements of cash flows for the fiscal years ended December 31, 2006, 2005 and 2004 | F-6 |
Notes to audited financial statements for the fiscal years ended December 31, 2006, | |
2005 and 2004 | F-7 |
2. Financial Statement Schedules:
All schedules have been omitted becausesince they are either not required, not applicable or the required information is otherwise included in the financial statements or notes thereto or because they are not required.
incorporated herein by reference.
3.(3) Exhibits:
The exhibits listed on the accompanying index to exhibits immediately following the financial statements are filed as part of, or hereby incorporated by reference into, this Form 10-K.10-K/A.
(b) See Item 15(a)3. above.
(c) See Item 15(a)2. above.
SIGNATURES
Pursuant to the requirements of Sectionsection 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | |
| | EXEGENICS INC.
| |
| By: | /s/ Adam Logal |
|
| |
| Adam Logal | |
Date: March 22,April 30, 2007 | By: | /s/ JOHN A. PAGANELLI Executive Director of Finance,
Chief Accounting Officer,
Treasurer and Secretary | |
-23-
| |
Name: John A. Paganelli |
| | Title: Chairman of the Board,
Interim Chief Executive Officer, and Secretary
(Principal Executive Officer)
| |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated below and on the dates indicated.
30 day of April, 2007.
| |
| |
| Signatures | | Title | | Date
|
| | | |
| /s/ Phillip Frost, M.D.Phillip Frost, M.D. | | Chairman of the Board
Chief Executive Officer
(Principal Executive Officer) |
| | | |
By: | /s/ JOHN A. PAGANELLIAdam LogalAdam Logal | | Executive Director Chairman of the Board | | March 22, 2007 |
| John A. Paganelli | | InterimFinance, Chief Executive
Accounting Officer, Treasurer & Secretary | |
(Principal Accounting Officer) |
| | | |
(Principal Executive Officer)/s/ John A. PaganelliJohn A. Paganelli | | Director |
| | | |
| /s/ David A. EichlerDavid A. Eichler | | Director |
| | | |
By: | /s/ DAVID HOSTELLEYMichael ReichMichael Reich | | Chief Financial Officer | | March 22, 2007 |
| David Hostelley | | (Principal Accounting Officer) | | Director |
| | | |
| /s/ Jane H. Hsiao, Ph.D., MBAJane H. Hsiao, Ph.D., MBA | | Director |
| | | |
By: | /s/ ROBERT BARONSteven D. RubinSteven D. Rubin | | Director | | March 22, 2007 |
| Robert Baron | | | | |
| /s/ Robert BaronRobert Baron | | Director |
| | | |
By: | /s/ STEVEN D. RUBINRichard A. Lerner, M.D.Richard A. Lerner, M.D. | | Director | | March 22, 2007 |
| Steven D. Rubin | | | | |
| | | | | |
By: | /s/ JANE HSIAOMelvin L. Rubin, M.D.Melvin L. Rubin, M.D. | | Director | | March 22, 2007 |
-24-
INDEX OF EXHIBITS
| | Jane Hsiao | | | | |
| | | | | |
By: | /s/ SUBBARAO V. UPPALURI | | Director | | March 22, 2007 |
| Subbarao V. Uppaluri | | | | |
| | | | | | |
| | 3.1 | (i) | | — | | |
INDEX OF EXHIBITS
3.1(i) | — | Certificate of Amendment of the Certificate of Incorporation of eXegenics Inc., filed with the Delaware Secretary State on February 8, 2007 (Filed herewith). |
| | | | | | |
| 3.1(ii) | | — | | Certificate of Correction to the Certificate of Amendment to the Certificate of Incorporation ofeXegenicsInc. filed with the Delaware Secretary of State on July 14, 2003, incorporated herein by reference to Exhibit 3.1 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended June 30, 2003 filed with the SEC on August 14, 2003. |
| | | | | | |
| 3.1(iii) | | — | | Certificate of Designation Series B Junior Participating Preferred Stock, filed with the Delaware Secretary of State on June 9, 2003, incorporated by reference to Exhibit A to Exhibit 4.1 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on June 9, 2003. |
| | | | | | |
| 3.1(iv) | | — | | Certificate of Incorporation of eXegenics Inc., as amended, incorporated by reference to Exhibit 3.1 and Exhibit 3.3 to eXgenics Inc.’s Registration Statement on Form SB-2 (File No. 33-91802). |
| | | | | | |
| 3.2 | | | — | | By-laws, as amended, incorporated by reference to Exhibit 3.2 to eXgenics Inc.’s Registration Statement on Form SB-2 (File No. 33-91802). |
| | | | | | |
| 4.1 | | | — | | Specimen certificates representing Class C Warrants, Class D Warrants and Common Stock, incorporated by reference to eXegenics Inc.’s Registration Statement on Form SB-2 (File No. 33-91802) filed with the SEC on May 2, 1995. |
| | | | | | |
| 4.2 | | | — | | Stockholders Rights Agreement, dated June 9, 2003, betweeneXegenicsInc. and American Stock Transfer & Trust Company, which includes as Exhibit A the Form of Certificate of Designations of Series B Junior Participating Preferred Stock, as Exhibit B the Form of Rights Certificate and as Exhibit C the Summary of Rights to Purchase Preferred Stock, incorporated by reference to Exhibit 4.1 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on June 9, 2003. |
| | | | | | |
| 4.3 | | | — | | Amendment to Stockholders Rights Agreement entered into as of July 16, 2003, by and betweeneXegenicsInc. and American Stock Transfer & Trust Company, as Rights Agent, incorporated by reference to Exhibit 4.1 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended June 30, 2003 filed with the SEC on August 14, 2003. |
| | | | | | |
| 4.4 | | | — | | Amendment to Stockholders Rights Agreement entered into as of December 4, 2006, by and between eXegenics Inc. and American Stock Transfer & Trust Company, as Rights Agent, incorporated by reference to Exhibit 99.1 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on December 4, 2006. |
| | | | | | |
| 4.5(1) | | — | | Form of Warrant Agreement betweeneXegenicsInc. and Roan Meyers Associates LP, incorporated by reference to Exhibit 4.2 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended March 31, 2004 filed with the SEC on May 14, 2004. |
| | | | | | |
| 4.6(1) | | — | | Form of Warrant Agreement betweeneXegenicsInc. and Petkevich & Partners, LLC, incorporated by reference to Exhibit 4.1 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended March 31, 2004 filed with the SEC on May 14, 2004. |
| | | | | | |
| 10.1 | | | — | | 1992 Stock Option Plan, as amended, incorporated by reference to Exhibit 4 to eXegenics Inc.’s Registration Statement on Form S-8 (File No. 333-37049) filed with the SEC on October 2, 1997. |
-25-
| | | | | | |
| 10.2 | | | — | | License Agreement dated June 10, 1993 between eXegenics Inc. and Research & Development Institute, Inc. (“RDI”), as amended, relating to the Paclitaxel Fermentation Production System, incorporated by reference to eXegenics Inc.’s Registration Statement on Form SB-2 (File No. 33-91802) filed with the SEC on May 2, 1995. |
| | | | | | |
| 10.3 | | | — | | Research and Development Agreement effective June 10, 1993 between eXegenics Inc. and RDI, as amended, incorporated by reference to eXegenics Inc.’s Registration Statement on Form SB-2 (File No. 33-91802) filed with the SEC on May 2, 1995. |
| | | | | | |
| 10.4 | | | — | | License Agreement dated February 22, 1995 between eXegenics Inc. and RDI, as amended, relating to FTS-2, incorporated by reference to eXegenics Inc.’s Registration Statement on Form SB-2 (File No. 33-91802) filed with the SEC on May 2, 1995. |
| | | | | | |
| 10.5 | | | — | | Extension Agreement with RDI dated June 5, 1995, incorporated by reference to eXegenics Inc.’s Registration Statement on Form SB-2 (File No. 33-91802) filed with the SEC on May 2, 1995. |
| | | | | | |
| 10.6 | | | — | | September 25, 1995 RDI Extension, incorporated by reference to eXegenics Inc.’s Registration Statement on Form SB-2 (File No. 33-91802) filed with the SEC on May 2, 1995. |
| | | | | | |
| 10.7 | | | — | | October 25, 1995 RDI Extension, incorporated by reference to eXegenics Inc.’s Registration Statement on Form SB-2 (File No. 33-91802) filed with the SEC on May 2, 1995. |
| | | | | | |
| 10.8 | | | — | | Amendment to License Agreement dated June 10, 1993, as amended, and Research and Development Agreement effective June 10, 1993, as amended, both agreements between eXegenics Inc. and RDI, incorporated by reference to eXegenics Inc.’s Form 10-KSB for the fiscal year ended December 31, 1995 filed with the SEC on March 29, 1996. |
| | | | | | |
| 10.9 | | | — | | License Agreement No. W960206 effective February 27, 1996 between eXegenics Inc. and The Regents of the University of California, incorporated by reference to eXegenics Inc.’s Form 10-KSB for the fiscal year ended December 31, 1995 filed with the SEC on March 29, 1996. |
| | | | | | |
| 10.10 | �� | | — | | License Agreement No. W960207 effective February 27, 1996 between eXegenics Inc. and The Regents of the University of California, incorporated by reference to eXegenics Inc.’s Form 10-KSB for the fiscal year ended December 31, 1995 filed with the SEC on March 29, 1996. |
| | | | | | |
| 10.11 | | | — | | License Agreement with the Washington State University, dated July 2, 1996*, incorporated by reference to Exhibit 10.33 to eXegenics Inc.’s Post Effective Amendment No. 1 (File No. 33-91802) on Form SB-2 filed with the SEC on July 25, 1996. |
| | | | | | |
| 10.12 | | | — | | 1996 Stock Option Plan, as amended, incorporated by reference to Exhibit 4 to eXegenics Inc.’s Registration Statement on Form S-8 (File No. 333-11691) filed with the SEC on September 10, 1996 and as amended by reference to eXegenics Inc.’s Definitive Proxy Statement filed with the SEC on August 5, 1998. |
| | | | | | |
| 10.13 | | | — | | Patent License Agreement, dated August 4, 1998, between The Regents of the University of California and eXegenics Inc. for Peptide Anti-estrogen for Breast Cancer Therapy*, incorporated by reference to Exhibit 10.42 to eXegenics Inc.’s Post Effective Amendment No. 2 (File No. 33-91802) to Form SB-2 on Form S-3 filed with the SEC on September 30, 1998. |
| | | | | | |
| 10.14 | | | — | | Master License Agreement, dated as of June 12, 1998, between eXegenics Inc. and Bristol-Myers Squibb Company*, incorporated by reference to Exhibit 10.1 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on September 9, 1998. |
| | | | | | |
| 10.15 | | | — | | Sublicense Agreement, dated May 27, 1998, between eXegenics Inc. and Bristol-Myers Squibb under The Research & Development Institute, Inc. License Agreement, as amended, dated June 10, 1998*, incorporated by reference to Exhibit 10.2 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on September 9, 1998. |
-26-
| | | | | | |
| 10.16 | | | — | | Sublicense Agreement, dated May 19, 1998, between eXegenics Inc. and Bristol-Myers Squibb Company under the Washington State University Research Foundation License Agreement, dated June 8, 1996*, incorporated by reference to Exhibit 10.3 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on September 9, 1998. |
| | | | | | |
| 10.17 | | | — | | Amended and Restated License Agreement, dated June 3, 1998, between the Washington State University Research Foundation and eXegenics Inc* , incorporated by reference to Exhibit 10.4 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on September 9, 1998. |
| | | | | | |
| 10.18 | | | — | | Amendment, dated May 27, 1998, to the License Agreement, dated June 10, 1993, between The Research and Development Institute, Inc. and eXegenics Inc.* , incorporated by reference to Exhibit 10.5 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on September 9, 1998. |
| | | | | | |
| 10.19 | | | — | | Amended and Restated 2000 Stock Option Plan, incorporated by reference to Appendix B to eXegenics Inc.’s Definitive Proxy Statement filed with the SEC on May 1, 2001. |
| | | | | | |
| 10.20(1) | | — | | Employment Agreement dated March 21, 2001, betweeneXegenicsInc. and Ronald L. Goode, Ph.D., incorporated by reference to Exhibit 10.41 to eXegenics Inc.’s Form 10-K for the fiscal year ended December 31, 2000 and filed with the SEC on April 2, 2001. |
| | | | | | |
| 10.21 | | | — | | Termination Agreement dated November 25, 2002 betweeneXegenicsInc., Innovative Drug Delivery Systems, Inc., and IDDS Merger Corp., incorporated by reference to Exhibit 2.1 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on December 3, 2002. |
| | | | | | |
| 10.22(1) | | — | | Amendment, dated September 9, 2003, to Employment Agreement dated March 20, 2001, betweeneXegenicsInc. and Ronald L. Goode, Ph.D., incorporated by reference to Exhibit 10.1 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended September 30, 2003 and filed with the SEC on November 14, 2003. |
| | | | | | |
| 10.23(1) | | — | | Amendment, dated October 16, 2003, to Employment Agreement dated March 20, 2001, betweeneXegenicsInc. and Ronald L. Goode, Ph.D., incorporated by reference to Exhibit 10.2 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended September 30, 2003 and filed with the SEC on November 14, 2003. |
| | | | | | |
| 10.24 | | | — | | Form of Indemnification Agreement by and amongeXegenicsand certain of its current and former directors and officers, incorporated by reference to Exhibit 10.1 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended June 30, 2003 and filed with the SEC on August 14, 2003. |
| | | | | | |
| 10.25 | | | — | | Promissory Note and Pledge Agreement betweeneXegenicsInc. and Ronald L. Goode, Ph.D., incorporated by reference to Exhibit 10.33 to eXegenics Inc.’s Form 10-K for the fiscal year ended December 31, 2003 and filed with the SEC on April 7, 2004. |
| | | | | | |
| 10.26 | | | — | | Sublease Agreement betweeneXegenicsInc. and RFG Associates dated as of January 1, 2004, incorporated by reference to Exhibit 10.1 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended June 30, 2004 and filed with the SEC on August 16, 2004. |
| | | | | | |
| 10.27 | | | — | | Intellectual Property Assignment Agreement betweeneXegenicsInc. and NLC Pharma, Inc., incorporated by reference to Exhibit 4.1 to eXegenics Inc.’s Current Report on Form 8-K filed with the SEC on September 10, 2004. |
| | | | | | |
| 10.28(1) | | — | | Separation Agreement betweeneXegenicsInc. and David Riggs dated July 26, 2005, incorporated by reference to Exhibit 10.1 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended June 30, 2005 and filed with the SEC on August 15, 2005. |
-27-
| | | | | | |
10.29(1) | | — | | Agreement betweeneXegenics Inc. and David Hostelley dated July 20, 2005, incorporated by reference to Exhibit 10.2 to eXegenics Inc.’s Form 10-Q for the fiscal quarter ended June 30, 2005 and filed with the SEC on August 15, 2005. |
23.1 | | | | | | |
| 31.1 | | | — | Consent of Rotenberg & Co., LLC (Filed herewith) |
23.2 | — | Consent of BDO Seidman, LLP (Filed herewith) |
31.1 | — | Certification of the Chief Executive Officer Pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. (Filed herewith) |
| | | | | | |
| 31.2 | | | — | | Certification of the Chief Financial Officer Pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. (Filed herewith) |
| | | | | | |
| 32.1 | | | — | | Certification of the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (Filed herewith) |
| | | | | | |
| 32.2 | | | — | | Certification of the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. (Filed herewith) |
(1)Management contracts and compensatory plans and arrangements required to be filed as Exhibits to this Report on Form 10-K pursuant to Item
15(c) of the Report.
*Confidential portions omitted and filed separately with the U.S. Securities and Exchange Commission pursuant to Rule 24b-2 promulgated under the Securities Exchange Act of 1934, as amended.
eXegenics Inc.
CONTENTS
Financial Statements
| | |
| (1) | | Management contracts and compensatory plans and arrangements required to be filed as Exhibits to this Report on Form 10-K pursuant to Item 15(c) of the Report. |
| | Page
|
Report* | | Confidential portions omitted and filed separately with the U.S. Securities and Exchange Commission pursuant to Rule 24b-2 promulgated under the Securities Exchange Act of Rotenberg & Co., LLP, Independent Registered Public Accounting Firm | F-1 |
Report of BDO Seidman, LLP, Independent Registered Public Accounting Firm | F-2 |
Balance sheets1934, as of December 31, 2006 and 2005 | F-3 |
Statements of operations for the fiscal years ended December 31, 2006, 2005 and 2004 | F-4 |
Statements of changes in stockholders’ equity for the fiscal years ended December 31, 2006, 2005 and 2004 | F-5 |
Statements of cash flows for the fiscal years ended December 31, 2006, 2005 and 2004 | F-6 |
Notes to audited financial statements for the fiscal years ended December 31, 2006, 2005 and 2004 | F-7amended. |
-28-
Report of Independent Registered Public Accounting Firm
To the Board of Directors
and Stockholders
eXegenics Inc.
We have audited the accompanying balance sheet of eXegenics Inc. as of December 31, 2006 and 2005, and the related statements of operations, changes in stockholders' equity, and cash flows for the (the company) years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of eXegenics Inc. as of December 31, 2006 and 2005 and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
/s/ Rotenberg & Co., LLP
Rotenberg & Co., LLP
Rochester, New York
March 21, 2007
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
eXegenics Inc.
We have audited the accompanying statements of operations, changes in stockholders’ equity and cash flows of eXegenics Inc. (the “Company”) for the year ended December 31, 2004. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the results of operations and cash flows of eXegenics Inc. for the year ended December 31, 2004 in conformity with accounting principles generally accepted in the United States of America.
BDO Seidman, LLP
Dallas, Texas
February 18, 2005 except for Notes K and
N of the Company’s 2004 financial statements,
which are as of April 12, 2005
eXegenics Inc.
BALANCE SHEETS
| | | December 31, | |
| | | 2006 | | 2005 | |
ASSETS | |
| Current assets: | | | | | |
| Cash and cash equivalents | | $ | 8,596,000 | | $ | 8,901,000 | |
| Prepaid expenses and other current assets | | | 156,000 | | | 99,000 | |
| | | | | | | | |
| Total current assets | | $ | 8,752,000 | | $ | 9,000,000 | |
| |
LIABILITIES AND STOCKHOLDERS’ EQUITY |
| Current liabilities: | | | | | | | |
| Accounts payable and accrued expenses | | $ | 674,000 | | $ | 277,000 | |
| | | | | | | | |
| Stockholders’ equity: | | | | | | | |
| Preferred stock — $.01 par value, 10,000,000 shares authorized; 1,002,017 and 952,829 shares of Series A convertible preferred issued and outstanding (liquidation value $2,505,000 and $2,382,000) | | | 10,000 | | | 10,000 | |
| Common stock — $.01 par value, 30,000,000 shares authorized; 16,991,101 and 16,945,026 shares issued | | | 170,000 | | | 169,000 | |
| Additional paid in capital | | | 68,285,000 | | | 68,384,000 | |
| Subscriptions receivable, net of reserve | | | — | | | (101,000 | ) |
| Accumulated deficit | | | (57,050,000 | ) | | (56,402,000 | ) |
| Treasury stock, 611,200 and 611,200 shares of common stock, at cost | | | (3,337,000 | ) | | (3,337,000 | ) |
| | | | 8,078,000 | | | 8,723,000 | |
| | | $ | 8,752,000 | | $ | 9,000,000 | |
See notes to financial statements
eXegenics Inc.
STATEMENTS OF OPERATIONS
| | | Year Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | |
| Revenue: | | | | | | | |
| License and research fees | | $ | — | | $ | — | | $ | — | |
| Operating expenses: | | | | | | | | | | |
| General and administrative | | | 1,117,000 | | | 1,438,000 | | | 2,051,000 | |
| | | | 1,117,000 | | | 1,438,000 | | | 2,051,000 | |
| Other (income) expenses: | | | | | | | | | | |
| Gain on sale of investments, net | | | — | | | (1,064,000 | ) | | — | |
| Interest income | | | (469,000 | ) | | (190,000 | ) | | (127,000 | ) |
| Interest expense | | | — | | | 2,000 | | | 2,000 | |
| | | | (469,000 | ) | | (1,252,000 | ) | | (125,000 | ) |
| Loss before provision (benefit) for taxes | | | (648,000 | ) | | (186,000 | ) | | (1,926,000 | ) |
| Provision (benefit) for taxes | | | — | | | — | | | — | |
Net loss | | | (648,000 | ) | | (186,000 | ) | | (1,926,000 | ) |
| Preferred stock dividend | | | (238,000 | ) | | (234,000 | ) | | (223,000 | ) |
Net loss attributable to common stockholders | | $ | (886,000 | ) | $ | (420,000 | ) | $ | (2,149,000 | ) |
Basic and diluted loss per common share: | | $ | (0.04 | ) | $ | (0.03 | ) | $ | (0.13 | ) |
Weighted average number of shares outstanding — basic and diluted | | | 16,369,000 | | | 16,271,000 | | | 16,050,000 | |
See notes to financial statements
eXegenics Inc.
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| | | Convertible Preferred Stock | | Common Stock | | Additional Paid in | | Subscriptions | | Reserve on | | Accumulated | | Accumulated Other Comprehensive | | Treasury Stock | | | |
| | | Shares | | Amount | | Shares | | Amount | | Capital | | Receivable | | Subscp. Rec | | Deficit | | Income (Loss) | | Shares | | Amount | | Total | |
Balance — January 1, 2003 | | | 890,564 | | | 9,000 | | | 16,314,779 | | | 163,000 | | | 68,061,000 | | | (302,000 | ) | | — | | | (54,290,000 | ) | | — | | | 611,200 | | | (3,337,000 | ) | | 10,304,000 | |
| Preferred stock converted to common stock | | | (44,252 | ) | | (500 | ) | | 44,252 | | | 500 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Preferred dividend (stock) | | | 89,020 | | | 500 | | | — | | | — | | | (500 | ) | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Exercise of stock options | | | — | | | — | | | 360,000 | | | 4,000 | | | 188,000 | | | — | | | — | | | — | | | — | | | — | | | — | | | 192,000 | |
| Compensation related to grant of stock and options to board members | | | — | | | — | | | 150,000 | | | 1,500 | | | 132,000 | | | — | | | — | | | — | | | — | | | — | | | — | | | 133,500 | |
| Value assigned to warrants and options issued for professional services | | | — | | | — | | | — | | | — | | | 4,500 | | | — | | | — | | | — | | | — | | | — | | | — | | | 4,500 | |
| Comprehensive Income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net Loss for the year | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (1,926,000 | ) | | — | | | — | | | — | | | (1,926,000 | ) |
| Unrealized gain on available for sale securities | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | 1,124,000 | | | — | | | — | | | 1,124,000 | |
| Total comprehensive income | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (802,000 | ) |
Balance — December 31, 2004 | | | 935,332 | | $ | 9,000 | | | 16,869,031 | | $ | 169,000 | | $ | 68,385,000 | | | ($302,000 | ) | | — | | | ($56,216,000 | ) | $ | 1,124,000 | | | 611,200 | | | ($3,337,000 | ) | $ | 9,832,000 | |
| Preferred stock converted to common stock | | | (75,995 | ) | | — | | | 75,995 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Preferred dividend (stock) | | | 93,502 | | | 1,000 | | | — | | | — | | | (1,000 | ) | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Accrued Interest on subscription receivable | | | — | | | — | | | — | | | — | | | — | | | (14,000 | ) | | — | | | — | | | — | | | — | | | — | | | (14,000 | ) |
| Reserve on stock subscriptions receivable | | | — | | | — | | | — | | | — | | | — | | | — | | | 215,000 | | | — | | | — | | | — | | | — | | | 215,000 | |
| Comprehensive Income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net Loss for the year | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (186,000 | ) | | — | | | — | | | — | | | (186,000 | ) |
| Realized gain on available for sale securities | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (1,124,000 | ) | | — | | | — | | | (1,124,000 | ) |
| Total comprehensive income | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
Balance — December 31, 2005 | | | 952,839 | | $ | 10,000 | | | 16,945,026 | | $ | 169,000 | | $ | 68,384,000 | | | ($316,000 | ) | $ | 215,000 | | | ($56,402,000 | ) | | — | | | 611,200 | | | ($3,337,000 | ) | $ | 8,723,000 | |
| Preferred stock converted to common stock | | | (46,075 | ) | | (1,000 | ) | | 46,075 | | | 1,000 | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Preferred dividend (stock) | | | 95,253 | | | 1,000 | | | — | | | — | | | (1,000 | ) | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Accrued interest on subscription receivable | | | — | | | — | | | — | | | — | | | — | | | (5,000 | ) | | — | | | — | | | — | | | — | | | — | | | (5,000 | ) |
| Reserve on stock subscriptions receivable | | | — | | | — | | | — | | | — | | | — | | | — | | | 5,000 | | | — | | | — | | | — | | | — | | | 5,000 | |
| Share-based compensation | | | — | | | — | | | — | | | — | | | 3,000 | | | — | | | — | | | — | | | — | | | — | | | — | | | 3,000 | |
| Write off of stock subscription receivable | | | — | | | — | | | — | | | — | | | (101,000 | ) | | 321,000 | | | (220,000 | ) | | — | | | — | | | — | | | — | | | — | |
| Comprehensive income: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss for the year | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | (648,000 | ) | | — | | | — | | | — | | | (648,000 | ) |
| Total comprehensive income | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
Balance — December 31, 2006 | | | 1,002,017 | | $ | 10,000 | | | 16,991,101 | | $ | 170,000 | | $ | 68,285,000 | | | — | | | — | | | ($57,050,000 | ) | | — | | | 611,200 | | | ($3,337,000 | ) | $ | 8,078,000 | |
See notes to financial statements
eXegenics Inc.
STATEMENTS OF CASH FLOWS
| | | Year Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | |
Cash flows from operating activities: | | | | | | | |
| Net loss | | $ | (648,000 | ) | $ | (186,000 | ) | $ | (1,926,000 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | |
| Depreciation and amortization | | | — | | | 3,000 | | | 5,000 | |
| Share-base compensation expense | | | 3,000 | | | — | | | 138,000 | |
| Interest accrual on subscriptions receivable | | | — | | | (14,000 | ) | | (2,000 | ) |
| Reserve for subscriptions receivable | | | — | | | 215,000 | | | — | |
| Gain on Sale of Investments, net | | | — | | | (1,064,000 | ) | | — | |
| Changes in: | | | | | | | | | | |
| Release of cash restricted for operating lease obligations | | | — | | | 175,000 | | | 425,000 | |
| Prepaid expenses and other current assets | | | (57,000 | ) | | (64,000 | ) | | 569,000 | |
| Payment of operating lease obligations | | | — | | | — | | | (87,000 | ) |
| Accounts payable and accrued expenses | | | 397,000 | | | 38,000 | | | (712,000 | ) |
| Net cash used in operating activities | | | (305,000 | ) | | (897,000 | ) | | (1,590,000 | ) |
Cash flows from investing activities: | | | | | | | | | | |
| Sales of investment securities | | | — | | | 1,064,000 | | | — | |
| Net cash provided by investing activities | | | — | | | 1,064,000 | | | — | |
Cash flows from financing activities: | | | | | | | | | | |
| Proceeds from sale of common stock through exercise of options and warrants | | | — | | | — | | | 192,000 | |
| Net cash provided by (used in) financing activities | | | — | | | — | | | 192,000 | |
Net increase (decrease) in cash and cash equivalents | | | (305,000 | ) | | 167,000 | | | (1,398,000 | ) |
| Cash and cash equivalents at beginning of year | | | 8,901,000 | | | 8,734,000 | | | 10,132,000 | |
Cash and cash equivalents at end of year | | $ | 8,596,000 | | $ | 8,901,000 | | $ | 8,734,000 | |
Supplemental disclosures of cash flow information: | | | | | | | | | | |
| Cash paid for interest | | | — | | $ | 2,000 | | $ | 2,000 | |
| Cash paid for income taxes | | $ | 16,000 | | $ | 36,000 | | | — | |
| Noncash investing activities: | | | | | | | | | | |
| Investment in Intrac, Inc. | | | — | | | — | | | 1,124,000 | |
| | | | | | | | | | | |
| Noncash financing activities: | | | | | | | | | | |
| Preferred stock converted to common stock. | | | — | | | — | | $ | — | |
| Preferred stock dividend. | | | — | | | — | | $ | — | |
| Write off of stock subscription receivable. | | | — | | | — | | | — | |
| Accrued interest on subscription receivable. | | $ | (5,000 | ) | $ | (14,000 | ) | | — | |
See notes to financial statements
eXegenics Inc.
NOTES TO FINANCIAL STATEMENTS
Note A — The Company
eXegenics Inc., formerly known as Cytoclonal Pharmaceutics Inc. (“eXegenics” or the “Company”), was previously involved in the research, creation, and development of drugs for the treatment and/or prevention of cancer and infectious diseases. During 2004, the Company completed the termination all research activities. All of the Company’s research activities, scientific staff and administrative positions were eliminated and were terminated. Our objective continues to be to redeploy our assets and actively pursue new business opportunities.
Note B — Summary of Significant Accounting Policies
Cash equivalents, restricted cash
The Company considers all non-restrictive, highly liquid short-term investments purchased with an original maturity of three months or less to be cash equivalents. Cash equivalents, which amount to $8,596,000 and $8,901,000 at December 31, 2006 and 2005, respectively, consist principally of interest bearing cash deposits.
The Company maintains cash and cash equivalents at several financial institutions which periodically may exceed federally insured amounts. The Company has not experienced any loss in such accounts and believes it is not exposed to any significant risk on cash and cash equivalents.
Marketable Securities
During 2005, the Company sold all of its marketable securities, realizing a gain on sale of approximately $1,064,000. These securities consisted of equity securities (common stock) in Javelin Pharmaceuticals, Inc. (formally known as Intrac, Inc.) and were classified as available for sale and reported at their fair values. Realized gains and losses from the sale of investments are reported in current earnings. Unrealized gains and losses from these securities are reported as a separate component of stockholders equity and excluded from current earnings.
The Company accounts for marketable securities in accordance with Statement of Financial Accounting Standards No. 115 “Accounting for Certain Investments in Debt and Equity Securities” (“SFAS 115”). SFAS 115 establishes the accounting and reporting requirements for all debt securities and for investments in equity securities that have readily determinable fair values. All marketable securities must be classified as one of the following: held-to-maturity, available-for-sale, or trading. The Company classifies its marketable securities as available-for sale and, as such, carries the investments at fair value, with unrealized holding gains and losses reported in stockholders’ equity as a separate component of accumulated other comprehensive income. The cost of securities sold is determined based on the specific identification method. Realized gains and losses, and declines in value judged to be other than temporary, are included in investment income.
Equipment
Equipment is stated at cost. Depreciation is provided using the straight-line method over the estimated useful lives of the assets, which range from 3 to 5 years. Repairs and maintenance that do not increase the economic useful life of the asset are charged to expense as incurred.
The Company reviews its capital assets for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. In performing the review, the Company uses the undiscounted cash flow method.
Revenue recognition
Revenue from research support agreements is recognized ratably over the length of the agreements. Revenue resulting from contracts or agreements with milestones is recognized when the milestone is achieved. Amounts received in advance of services to be performed or the achievement of milestones is recorded as deferred revenue.
Research and development
Research and development costs are charged to expense as incurred.
Loss per common share
Basic and diluted loss per common share is based on the net loss increased by dividends on preferred stock divided by the weighted average number of common shares outstanding during the year. No effect has been given to outstanding options, warrants or convertible preferred stock in the diluted computation, as their effects would be anti-dilutive. The number of potentially dilutive securities excluded from the computation of diluted loss per share was approximately 1,587,000, 2,148,000 and 2,325,000 for the years ended December 31, 2006, 2005 and 2004, respectively.
Share-based compensation
The Company accounts for share-based compensation utilizing the fair value recognition previsions of SFAS No. 123R, “Share-Based Payment”.
Comprehensive Income
SFAS No. 130, "Reporting Comprehensive Income," establishes standards for the reporting and display of comprehensive income and its components within the financial statements. Other comprehensive income is comprised of charges to stockholders' equity, other than contributions from or distributions to stockholders, excluded from the determination of net income. The Company's other comprehensive income is comprised of unrealized gains on available for sale marketable securities, which were sold during 2005.
Income Taxes
The Company has applied the provisions of SFAS No. 109, "Accounting for Income Taxes," which requires the recognition of deferred income tax assets and liabilities for the consequences of temporary differences between amounts reported for financial reporting and income tax purposes, including net operating loss carryforwards. SFAS No. 109 requires recognition of a future tax benefit of net operating loss carryforwards and certain other temporary differences to the extent that realization of such benefit is more likely than not; otherwise, a valuation allowance is applied.
Fair value of financial instruments
The carrying value of cash equivalents, accounts payable and accrued expenses approximates their fair value due to the short period to maturity of these instruments.
Use of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Recent Accounting Pronouncement
In May 2005, the FASB issued Statement of Financial Statement Accounting Standards No. 154, “Accounting Changes and Error Corrections-a replacement of APB Opinion No. 20 and FASB Statement No. 3” (“SFAS 154”). This Statement replaces APB Opinion No. 20, “Accounting Changes,” and FASB Statement No. 3, “Reporting Accounting Changes in Interim Financial Statements.” SFAS 154 requires retrospective application to prior periods’ financial statements for changes in accounting principle, unless it is impractical to determine either the period-specific effects or the cumulative effect of the change. SFAS 154 also requires that a change in depreciation, amortization, or depletion method for long, non-financial assets be accounted for as a change in accounting estimate effected by a change in accounting principle. SFAS 154 is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. The Company believes that adoption of the provisions of SFAS 154 will not have a material effect on the Company’s financial statements.
In July 2006, the Financial Accounting Standards Board (FASB) issued FASB Interpretation No. 48, “Accounting for Uncertainty in Income Taxes - an interpretation of FASB Statement No. 109” (FIN 48), which clarifies the accounting and disclosure for uncertainty in tax positions, as defined. FIN 48 seeks to reduce the diversity in practice associated with certain aspects of the recognition and measurement related to accounting for income taxes. The Company does not expect the interpretation will have a material impact on its results from operations or financial position.
In September 2006, the FASB issued statement No. 157, “Fair Value Measurements”, (SFAS 157). SFAS 157 defines fair value, establishes a framework for measuring fair value in accordance with accounting principles generally accepted in the United States, and expands disclosures about fair value measurements. SFAS 157 is effective for fiscal years beginning after November 15, 2007, with earlier application encouraged. Any amounts recognized upon adoption as a cumulative effect adjustment will be recorded to the opening balance of retained earnings in the year of adoption. The Company has not yet determined the impact of this Statement on its financial condition and results of operations.
Note C — License Agreements
On September 8, 2004 the Company entered into an Intellectual Property Assignment Agreement to license the Company’s QCT drug discovery technology to NLC Pharma, Inc. (a Delaware corporation based in Israel). Pursuant to the Agreement the Company will receive monies from royalties, licenses or the sale of QCT technology to third parties that are generated by NLC Pharma Inc. The Company did not earn any revenue under this agreement during 2006, nor does it anticipate receiving any revenues from this agreement in future years.
Note D — Equipment
Equipment is summarized as follows:
| | | December 31, | |
| | | 2006 | | 2005 | |
| Office equipment | | $ | 26,000 | | $ | 26,000 | |
| Less accumulated depreciation | | | (26,000 | ) | | (26,000 | ) |
| Net | | $ | — | | $ | — | |
Note E — Accounts Payable and Accrued Expenses
Accounts payable and accrued expenses consist of the following:
| | | December 31, | |
| | | 2006 | | 2005 | |
| Professional fees | | $ | 28,000 | | $ | 23,000 | |
| Legal Reserve | | | 638,000 | | | 250,000 | |
| Other | | | 8,000 | | | 4,000 | |
| | | $ | 674,000 | | $ | 277,000 | |
Note F — Capital Lease Obligations
During 2005, the Company terminated all capital lease obligations and as a result $175,000 in collateral was released from restriction.
Note G — Stockholders’ Equity
Preferred stock
On January 6, 1992, the Board of Directors designated 4,000,000 shares of preferred stock as Series A convertible preferred stock. The holders of Series A preferred stock are entitled to (i) convert on a one-for-one basis to common stock subject to adjustment, as defined, (ii) voting rights equivalent to voting rights of common stockholders, (iii) receive dividends equal to $.25 per share payable on or about January 15 each year in cash or newly-issued shares of Series A preferred or a combination thereof (iv) liquidation preferences of $2.50 per preferred share. The Company, at its option, has the right to redeem all or any portion of the Series A convertible preferred stock at $2.50 per share plus accrued and unpaid dividends.
During January 2004, the Company elected to pay the required yearly dividend by issuing additional shares of Series A convertible preferred. The Company issued 89,020 shares to satisfy the 10% dividend. In addition, during 2004, 44,252 shares of Series A convertible preferred were converted into 44,252 shares of common stock.
During January 2005, the Company elected to pay the required yearly dividend by issuing additional shares of Series A convertible preferred. The Company issued 93,502 shares to satisfy the 10% dividend. In addition, during 2005, 75,995 shares of Series A convertible preferred were converted into 75,995 shares of common stock.
During January 2006, the Company elected to pay the required yearly dividend by issuing additional shares of Series A convertible preferred. The Company issued 95,253 shares to satisfy the 10% dividend. In addition, during 2006, 46,075 shares of Series A convertible preferred were converted into 46,075 shares of common stock.
Common Stock
During the second quarter 2004, the Board of Directors adopted a resolution providing for the issuance of shares of the Company’s common stock and the granting of stock options as part of compensation paid to directors for their service to the Company. Upon joining the Board, directors are issued 25,000 shares of common stock. The chairman of the Board receives an additional 25,000 shares at the time he assumes this role. Members of the Board of Directors are granted an option to purchase 5,000 shares of the Company’s common stock on the first day of each calendar quarter, with an exercise price equal to the closing trading price of the Company’s common stock on the date of grant. In the second quarter 2004, the Chairman of the Board was issued 50,000 shares of common stock, Directors were issued 25,000 shares. In the aggregate, 150,000 shares of common stock were issued and recorded at their fair value on the date of grant. No common stock was issued to the Board of Directors in 2005 and 2006.
On March 22, 2005, the Board of Directors approved the grant to each member. John A. Paganelli and Robert Baron 50,000 shares of common stock upon the closing of a transaction which results in a change of control provided each such recipient is a member of the Board at such time
Stockholder Rights Plan
On June 9, 2003, the then current Board of Directors adopted a shareholders rights plan. Under the plan, each holder of the Company’s common stock as of the close of business on June 9, 2003 received, as a non-taxable dividend, one right for each share of common stock held. Each right entitles the holder to purchase from the Company one one-thousandth of a share of Series B Junior Participating Preferred Stock at an exercise price of $4.50, subject to adjustment. If a person or group acquires beneficial ownership of 15 percent or more of the Company’s common stock, each right will entitle its holder (other than the acquiring person or members of the acquiring group) to purchase, at the right's then current exercise price (initially $4.50), a number of the Company’s shares of common stock having a market value of twice such price (initially $9.00).
In addition, if the Company is acquired in a merger or other business combination transaction after a person has acquired beneficial ownership of 15 percent or more of its common stock, each right will entitle its holder to purchase, at the rights' then current exercise price (initially $4.50), a number of the acquiring company's shares of common stock having a market value of twice such price (initially $9.00). Following the acquisition by a person or group of beneficial ownership of 15 percent of the Company’s common stock and prior to an acquisition of beneficial ownership of 50 percent or more of its common stock the Board of Directors may exchange the rights (other than rights owned by such acquiring person or group, which will have become null and void and nontransferable), in whole or in part, at an exchange ratio of one share of common stock (or one-thousandth of a share of Series B Junior Participating Preferred Stock) per right. The Company may redeem the rights at a price of $.001 per right at any time prior to the time a person has become the beneficial owner of 15% or more of the Company’s outstanding common stock. The rights will expire on June 9, 2013, unless earlier exchanged or redeemed.
Subscriptions receivable
As previously reported by eXegenics in its quarterly report on Form 10-Q filed November 14, 2006, during the fourth quarter of fiscal 2006, the Company reached agreement with its former president and chief executive officer (Dr. Ronald Goode) in connection with a limited recourse note and pledge agreement entered into with Dr. Goode in May 2001 in connection with Dr. Goode’s subscription for shares of the Company’s common stock. The amount of the note was $300,000 plus 4.71% interest paid on a semi-annual basis. Dr. Goode failed to make the semi-annual interest payment since May 2005 and principal due May 2006. On October 30, 2006, the Company reached agreement with Dr. Goode concerning the cancellation of the subscription agreement and note in consideration for the assignment to the Company of the 100,000 shares of common stock underlying the subscription. As a result, the subscription receivable of $101,000 was eliminated and an offsetting amount was deducted from additional paid in capital.
Warrants
At December 31, 2006, outstanding warrants to acquire shares of the Company’s common stock are as follows:
Warrant
Type
| Exercise Price
| Expiration Dates
| Number of
Shares
Reserved
|
Other | $0.55 to $1.00 | August 2007-March 2008 | 290,000 |
On August 13, 2002 the Company issued warrants to purchase 125,000 shares of its common stock at a purchase price of $1.00 per share, with an expiration date of August 13, 2007, and additional warrants to purchase 125,000 shares of our common stock at a purchase price of $0.55 per share, with an expiration date of August 13, 2007 to Roan/Meyers Associates, L.P. in exchange for financial advisory services.
In March 2003, the Company entered into an agreement with Petkevich & Partners, LLC whereby the Company issued warrants to purchase 40,000 shares of common stock at $0.58 per share expiring on March 5, 2008.
The Company did not incur any warrant related expenses in 2005 and 2006
Stock options
During 1996, the Board of Directors and the stockholders of the Company approved the 1996 Stock Option Plan (the “1996 Plan”) that provides for the granting of incentive and nonstatutory options for up to 750,000 shares of common stock to officers, employees, directors and consultants of the Company. During 1998, the Board of Directors and the stockholders of the Company approved an amendment to the Plan to allow for the granting of an additional 750,000 options. At December 31, 2006 and 2005, 1,500,000 and 980,000, respectively, options were available for future grant under the 1996 Plan.
During 2000, the Board of Directors and the stockholders of the Company approved the 2000 Stock Option Plan (the “2000 Plan”), which provides for the granting of incentive and nonstatutory options for up to 1,500,000 shares of common stock to officers, employees, directors, independent contractors, advisors and consultants of the Company. The Company subsequently amended the 2000 plan to increase the options available for future grants by 1,250,000 shares and to change the vesting period. At December 31, 2006 and 2005, 2,455,000 and 2,365,000, respectively, options are available for grant under the 2000 Plan.
Options granted under the Plans are exercisable for a period of up to 10 years from date of grant at an exercise price which is not less than the fair value of the common stock on date of grant, except that the exercise period of options granted to a stockholder owning more than 10% of the outstanding capital stock may not exceed five years and their exercise price may not be less than 110% of the fair value of the common stock at date of grant. For the 1996 Plan, options generally vest 40% after six months of employment and thereafter 20% annually on the anniversary date of the grant. For the 2000 Plan, as a result of an amendment approved by the stockholders in 2001, the vesting period changed from 50% annually on the anniversary date of the grant, to 33 1/3% annually on the anniversary date of the grant. Under the 2000 plan, the Board of Directors has authority to modify the vesting period. Non-employee director options are immediately exercisable on the date of grant.
In December 2004, Financial Accounting Standards Board issued SFAS No. 123R, Share-Based Payment (“SFAS No. 123R” or the “Statement”). This Statement is a revision of SFAS No. 123, Accounting Principles Board Option No. 25, Accounting for Stock Issued to Employees (“APB No. 25”) and its related implementation guidance. On January 1, 2006, we adopted the provisions of SFAS No. 123R using the modified prospective method. SFAS No. 123R focuses primarily on accounting transactions in which an entity obtains employee or similar services in share-based payment transactions. The Statement requires entities to recognize compensation expense for awards of equity instruments to employees or employee equivalents based on the grant-date fair value of those awards (with limited exceptions). SFAS No. 123R also requires the benefits of tax deductions in excess of recognized compensation expense to be reported as financing cash flows, rather than as an operating cash flow as prescribed under the prior accounting rules. This requirement reduces net operating cash flows and increases net financing cash flows in periods after adoption. Total cash flow remains unchanged from what would have been reported under prior accounting rules.
Prior to the adoption of SFAS NO 123R, we followed the intrinsic value method in accordance with APB No. 25 to account for our employee stock options. Accordingly, no compensation expense was recognized for the issuance of stock options under any of our Stock Option Plans for periods ended prior to January 1, 2006. The adoption of SFAS No. 123R primarily resulted in a change in our method of recognizing the fair value of share-based compensation. Specifically, the adoption of SFAS No. 123R resulted in our recording compensation expense for employee stock options.
The pre-tax share-based employee compensation expense recorded in 2006 was approximately $3,000. Such expense resulted solely from the estimated value to be recognized from the share-based payments of options granted to our board of directors.
Stock option activity under the Plans is summarized as follows:
| | | Year Ended December 31, | |
| | | 2006 | | 2005 | | 2004 | |
| | Shares | | Weighted Average Exercise Price | | Shares | | Weighted Average Exercise Price | | Shares | | Weighted Average Exercise Price | |
| Options outstanding at beginning of year | | | 905,000 | | $ | 3.37 | | | 1,100,000 | | $ | 3.02 | | | 2,158,000 | | $ | 3.02 | |
| Granted | | | 60,000 | | | 0.40 | | | 80,000 | | | 0.40 | | | 165,000 | | | 0.82 | |
| Exercised | | | — | | | — | | | — | | | — | | | (360,000 | ) | | 0.53 | |
| Expired | | | (670,000 | ) | | 4.31 | | | (275,000 | ) | | 0.72 | | | (863,000 | ) | | 3.64 | |
| Options outstanding at end of year | | | 295,000 | | | 0.62 | | | 905,000 | | | 3.37 | | | 1,100,000 | | | 3.02 | |
| Options exercisable at end of year | | | 295,000 | | | 0.62 | | | 880,000 | | | 3.44 | | | 971,660 | | | 3.32 | |
The following table presents information relating to stock options outstanding and exercisable under the plans as of December 31, 2006:
| | | Options Outstanding and Exercisable | |
Range of Exercise Price | | Shares | | Weighted Average Exercise Price | | Weighted Average Remaining Life in Years | |
| $0.40-$2.99 | | | 295,000 | | $ | 0.62 | | | 8.03 | |
Pro forma information regarding net income and earnings per share is required by SFAS No. 123, and has been determined as if we accounted for our stock option grants under the fair market value method as prescribed by such statement. The fair market value of our stock options was estimated at the date of grant using the Black-Scholes option-pricing model with the following assumptions.
| | | 2006 | | 2005 | | 2004 | |
| Risk-free interest rates | | | 4.3% to 5.1 | % | | 3.6% to 4.3 | % | | 2.9% to 3.6 | % |
| Expected option life in years | | | 5 | | | 5 | | | 5 | |
| Expected stock price volatility | | | 10% to 37 | % | | 63% to 75 | % | | 72% to 75 | % |
| Expected dividend yield | | | 0 | % | | 0 | % | | 0 | % |
The weighted average fair value at date of grant for options granted during 2006, 2005 and 2004 was $0.40 $0.40, and $0.82, per option, respectively. Results for 2005 and 2004 have not been restated. Had compensation expense for employee stock option plans been determined based on fair value at the grant date consistent with SFAS 123R, our net income and earnings per share would have been the pro forma amounts indicated below:
| | | Year Ended December 31, | |
| | | 2005 | | 2004 | |
| Net loss attributable to common stockholders as reported | | $ | (420,000 | ) | $ | (2,149,000 | ) |
| Deduct: Total stock-based employee compensation expense determined under fair value based method for all awards, net of related tax effects | | | (11,000 | ) | | (32,000 | ) |
| Pro forma net income | | $ | (431,000 | ) | $ | (2,181,000 | ) |
| Earnings per share: | | | | | | | |
| Basic and diluted-as reported | | $ | (0.03 | ) | $ | (0.13 | ) |
| Basic and Diluted-pro forma | | $ | (0.03 | ) | $ | (0.14 | ) |
The Black-Scholes option valuation model was developed for use in estimating the fair market value of traded options that have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions including the expected stock price volatility. Because our stock options have characteristics significantly different from those of traded options, and because changes in the subjective input assumptions can materially affect the fair market value estimates, in management’s opinion, the existing models do not necessarily provide a reliable single measure of the fair market value of our stock options.
Note H — Income Taxes
At December 31, 2006 and 2005, the Company had approximately $53,979,000 and $53,080,000 of net operating loss carry forwards and $491,000 and $491,000 of research and development credit carry forwards, respectively, for federal income tax purposes that expire in years 2007 through 2022.
At December 31, 2006 and 2005, the Company had a deferred tax asset of approximately $18,846,000 and $18,541,000 respectively, representing the benefits of its net operating loss and research and development credit carry forwards and certain expenses not currently deductible for tax purposes, principally related to the granting of stock options and warrants, and non-cash reorganization and merger expenses. The Company’s deferred tax asset has been fully reserved by a valuation allowance since realization of its benefit is uncertain. The difference between the statutory tax rate of 34% and the Company’s effective tax rate is principally due to the increase (decrease) in the valuation allowance of $305,000 (2006), ($980,000) (2005), and $644,000 (2004). The Company’s ability to utilize its carry forwards may be subject to an annual limitation in future periods pursuant to Section 382 of the Internal Revenue Code of 1986, as amended.
Note I — Commitments and Other Matters
Leases
The Company leases office space from RFG Associates, an entity in which John A. Paganelli, chairman of the Board of Directors of the Company is an equity owner. The lease provides for a monthly rent of $625 and is cancelable by either party upon thirty (30) days notice. Rent expense was approximately $10,000, $10,000, and $20,000 for the years ended December 31, 2006, 2005 and 2004, respectively.
Legal Proceedings
Weiss Litigation.
On May 15, 2003, The M&B Weiss Family Limited Partnership of 1996 filed a lawsuit in the Delaware Court of Chancery, purportedly as a class action on behalf of all other similarly situated stockholders of the Company, against the Company, as a nominal defendant,and former directors: Joseph M. Davie, Robert J. Easton, Ronald L. Goode and Walter Lovenberg, (collectively referred to as the “Individual Defendants”), and purportedly as a derivative action on behalf of the Companyagainst the Individual Defendants (the “Weiss Litigation”). On April 12, 2005 the judge, in a ruling from the bench, dismissed the matter with prejudice.
Labidi Proceeding.
On October 5, 2005, in the matter brought by Abdel Hakim Labidi (one of our former employees) against the Company, a jury ruled in favor of Dr. Labidi determining that the Company converted certain biological research materials owned by Dr. Labidi, and the Company committed theft of biological materials owned by Dr. Labidi. The jury awarded Dr. Labidi a total of $600,000. The Company is reviewing this matter to determine the validity of appealing the decision of the jury. The final amount due by the Company to Dr. Labidi under such judgment is likely to be between $638,000 and $750,000; however the Company has recorded a provision of $638,000 in the financial statements.
2110 Research Row, Ltd. Proceeding.
On December 31, 2003, the termination date of our lease agreement, we vacated 19,300 square feet of office and laboratory space that we occupied at 2110 Research Row, Dallas, Texas. 2110 Research Row, Ltd. (the “Landlord”) acquired this property in April 2002. The Landlord contends he is owed payments that we believe to be outside the terms of the lease agreement or waived by the previous landlord. In October 2003, we filed suit against the Landlord and 9000 Harry Hines, Inc., in a Dallas County District Court. The Company, as tenant, and the Landlord were parties to a lease agreement (“Lease Agreement”) dated October 1, 1991, as amended. On March 19, 2004, we entered into a settlement agreement with the Landlord, whereby we made a $33,000 payment to the Landlord, dismissed the suit with prejudice and entered into a mutual release of any and all claims by all parties. On April 9, 2004, the Landlord and the Company filed an Agreed Order Of Dismissal With Prejudice in The District Court, 134th Judicial District, Dallas County Texas.
Employment agreements
David Hostelley
On July 1, 2005 David F. Hostelley was named chief financial officer of eXegenics pursuant to the terms of a letter agreement between eXegenics and Contract CFO & Accounting, Inc. dated July 20, 2005. The letter agreement is a month-to-month agreement, and either party can terminate the agreement upon 30 business days notice. eXegenics pays Contract CFO & Accounting, Inc. $2,500 a month in consideration for Mr. Hostelley’s services to eXegenics, billed and payable on the first of each month. Further, in the event Mr. Hostelley is required to travel on behalf of eXegenics for any reason, eXegenics will be billed $800 per day, plus out-of-pocket expenses. All travel to be pre-approved by the chairman of eXegenics board of directors.
Related party transactions
John Paganelli
In January 2004, the Company entered into a lease agreement for office space with RFG Associates, an entity in which John A. Paganelli, chairman of the Board of Directors of the Company is an equity owner. The lease provides for a monthly rent of $625 and is cancelable by either party upon thirty (30) days notice.
Note J — Quarterly Results (Unaudited)
| | | Quarter Ended | |
| | | March 31 | | June 30 | | September 30 | | December 31 | | Total Year | |
2006 | | | | | | | | | | | |
| Revenues | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | |
| Net (loss) income | | | (43,000 | ) | | (68,000 | ) | | (169,000 | ) | | (368,000 | ) | | (648,000 | ) |
| Loss per share — basic and diluted(a) | | | (0.00 | ) | | (0.00 | ) | | (0.03 | ) | | (0.02 | ) | | (0.04 | ) |
2005 | | | | | | | | | | | | | | | | |
| Revenues | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | |
| Net (loss) income | | | (290,000 | ) | | (434,000 | ) | | 870,000 | | | (332,000 | ) | | (186,000 | ) |
| Loss per share — basic and diluted(a) | | | (0.03 | ) | | (0.03 | ) | | 0.05 | | | (0.01 | ) | | (0.03 | ) |
(a) | Per common share amounts for the quarters and full year have been calculated separately. Accordingly, quarterly amounts may not add to the annual amount because of differences in the weighted average common shares outstanding during each period due to the effect of the Company’s issuing shares of its common stock during the year. |
Note K — Subsequent Events
Previously on August 14, 2006, the Company entered into a Stock Purchase Agreement (the “Stock Purchase Agreement”) with a small group of investors led by The Frost Group. LLC, an entity controlled by Dr. Phillip Frost, as amended as of November 30, 2006. Pursuant to the Stock Purchase Agreement, the investors agreed to purchase shares of the Company’s common stock representing 51% of the issued and outstanding shares of capital stock on a fully-diluted basis for a purchase price reflecting the book value of the Company at closing.
At a special meeting of stockholders held on February 8, 2007, the stockholders of the Company approved: (i) an amendment to the Certificate of Incorporation of the Company increasing the number of shares of common stock which the Company is authorized to issue from 30,000,000 shares to 225,000,000 shares; (ii) the sale of 19,440,491 shares of common stock to a group of investors pursuant to the Stock Purchase Agreement; and (iii) the issuance of 50,000 shares of common stock to each of John Paganelli, chairman of the Board of Directors, interim chief executive officer, and secretary and Robert Baron, a director.
On February 8, 2007, a Certificate of Amendment to the Company’s Certificate of Incorporation providing for the increase in the number of shares of common stock authorized for issuance was filed with the Delaware Secretary of State, and on February 9, 2007, the transactions contemplated by the Stock Purchase Agreement were consummated, as a result of which 19,440,491shares of common stock, representing approximately 51% of the issued and outstanding shares on a fully diluted basis, were issued to the stockholders named in the Stock Purchase Agreement for an aggregate purchase price of $8,613,000, which was paid to the Company at closing. The purchase price is subject to a downward adjustment based upon the stockholders equity of eXegenics on the date of closing.