

UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D. C.D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 20182019
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________________ to ________________. |
Commission File Number: 001-37939


MARKER THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 45-4497941 | |
(State or other jurisdiction of incorporation) | (IRS Employer Identification No.) |
3200 Southwest Freeway, Suite 2240 Houston, Texas | ||
(Address of principal executive offices) | 77027 (Zip |
(713) 400-6400
(Registrant’s telephone number, including area code)Telephone Number)
Securities registered pursuant to Section 12(b) of the Act:
Common Stock, Par Value $0.001
(Title of class)
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, par value $0.001 per share | MRKR | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes¨ Nox
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 of Section 15(d) of the Act. Yes¨ Nox
Indicate by check mark whether the registrant (1) filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesx No¨
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 ofRegulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).Yesx No¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.x
Indicate by checkmark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” accelerated filer”, “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ||||
| ¨ | Accelerated filer | x | ||
| Non-accelerated filer | ¨ | Smaller reporting company | x | |
| Emerging growth company | ¨ | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.¨
Indicate by checkmark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes¨ Nox
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $81,400,000 computed by reference to the price per share ($9.43) at which the registrant’s common equity was last sold, as of June 30, 201828, 2019 (the last day of the registrant’s most recently completed second fiscal quarter). based on the closing sale price of $7.92 as reported on the Nasdaq Capital Market as of that date was approximately $198,900,000.
The registrant had 45,467,68446,532,522 shares of common stock outstanding as of February 28, 2019.March 9, 2020.
Documents Incorporated By Reference
Portions of the registrant’s proxy statement relating to registrant’s 20192020 Annual Meeting of Stockholders (the “Proxy Statement”) to be filed with the Securities and Exchange Commission pursuant to Regulation 14A, not later than 120 days after the close of the registrant’s fiscal year, are incorporated by reference in Part III of this Annual Report on Form 10-K. Except with respect to information specifically incorporated by reference in this Annual Report on Form 10-K, the Proxy Statement is not deemed to be filed as part of this Annual Report on Form 10-K.
TABLE OF CONTENTS
i
This annual report contains forward-looking statementswithin the meaning of the Private Securities Litigation Reform Act of 1995that involve substantial risks and uncertainties.The forward-looking statements are contained principally in Part I, Item 1: "Business," Part I, Item 1A: "Risk Factors," and Part 2, Item 7: "Management's Discussion and Analysis of Financial Condition and Results of Operations," but are also contained elsewhere in this annual report.Any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “will”, “should”, “expect”, “plan”, “intend”, “anticipate”, “believe”, “estimate”, “predict”, “potential” or “continue”, the negative of such terms or other comparable terminology. In evaluating theseThese statements you should consider variousspeak only as of the date of this Annual Report and involve known and unknown risks, uncertainties and other important factors including the assumptions, risks and uncertainties outlined in this annual report. Any of these itemsthat may cause our actual results, performance or achievements to differbe materially different from any future results, performance or achievements expressed or implied by the forward-looking statement made in this annual report. statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. Forward-looking statements in this annual report include statements as to:
| · | the |
| · | the timing of any submission of filings for regulatory approval of product candidates and | |
| · | our ability to | |
| · | our expectations regarding | |
| · | our | |
| · | our | |
While these forward-looking statements, and any assumptions upon which they are based, are made in good faith and reflect our current judgment regarding future events, our actual results will likely vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested herein. Some of the risks and assumptions include:
| · | our expectations regarding the scope of any approved indications for product candidates; | |
| · | the potential benefits of and our ability to maintain our relationships and collaborations with the Baylor College of Medicine and other potential collaboration or strategic relationships; | |
| · | our ability to | |
| · | our | |
| · | our ability to |
| · | our ability to | |
| · | ||
| · | our competitive position and the development of and projections relating to our competitors or our industry; and | |
| · | the impact of |
Given
ii
You should refer to "Item 1A. Risk Factors" in this annual report for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these risks andfactors, we cannot assure you that the forward-looking statements in this annual report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not place undue reliance onregard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. The forward-looking statements in this annual report represent our views as of the date of this annual report. We anticipate that subsequent events and developments may cause our views to change. However, while we may elect to update these forward-looking statements. Except as required by federal securities laws,statements at some point in the future, we undertake no obligation to publicly update any forward-looking statements, for any reason, even ifwhether as a result of new information, becomes availablefuture events or other events occurotherwise, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this annual report.
You should read this annual report and the documents that we reference in this annual report and have filed as exhibits to this annual report completely and with the future.understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
In this report all references to (i) “Marker” “we,” “us,” “our” or the “Company” mean Marker Therapeutics, Inc. and its wholly-owned subsidiaries, Marker Cell Therapy, Inc. and GeneMax Pharmaceuticals, Inc., which wholly owns GeneMax Pharmaceuticals Canada Inc., unless the context otherwise requires; (ii) “SEC” refers to the Securities and Exchange Commission; (iii) “Securities Act” refers to the United StatesSecurities Act of 1933, as amended; (iv) “Exchange Act” refers to the United StatesSecurities Exchange Act of 1934, as amended; and (v) all dollar amounts refer to United States dollars unless otherwise indicated.
iii
Overview
We are a clinical-stage immuno-oncology company specializing in the development and commercialization of novel T cell-based immunotherapies and innovative peptide-based vaccines for the treatment of hematological malignancies and solid tumor indications. Our MultiTAAWe developed our lead product candidates from our MultiTAA-specific T cell technology, which is based on the selective expansion of non-engineered, tumor-specific T cells that recognize tumor associated antigens, (“TAA” i.e.or TAAs, which are tumor targets)targets, and then kill tumor cells expressing those targets. Once infused into patients, this population ofThese T cells recognizesare designed to recognize multiple tumor targets to produce broad spectrum anti-tumor activity. We are advancing two pipelines of product candidates as part of our MultiTAA-specific T cell program: the autologous T cells for the treatment of lymphoma, multiple myeloma, or MM, and selected solid tumors and the allogeneic T cells for the treatment of acute myeloid leukemia, or AML, and acute lymphoblastic leukemia, or ALL. Because we do not genetically engineer the MultiTAA-specific T cell therapies, we believe that our T cells, when comparedproduct candidates are easier and less expensive to manufacture, with reduced toxicities, than current engineered chimeric antigen receptor, (“CAR”)or CAR-T, and T cell receptor (“TCR”)-based approaches, our products are significantly less expensive to manufacturereceptor-based therapies and appear to be markedly less toxic, and yet are associatedmay provide patients with meaningful clinical benefit. We are also developing innovative peptide-based immunotherapeutic vaccines for the treatment of metastatic solid tumors.
We are pursuing post-transplant AML as the lead indication for our first company-sponsored MultiTAA-specific T cell program. The MultiTAA-specific T cell therapy has been well tolerated in an ongoing Phase 1/2 clinical trial conducted by our strategic partner Baylor College of Medicine, or BCM. As reported in March 2019, eleven of the thirteen patients in the adjuvant disease setting dosed with the MultiTAA-specific T cell therapy after receiving an allogeneic stem cell transplant survived, ranging from 6 weeks to 2.5 years post-infusion, with nine of these remaining patients in continuing complete remission, or CCR. Survival of the six patients with active disease ranged from 4 to 21 months, as compared to a result,historical survival rate of approximately 4.5 months for patients who receive the standard of care post-transplant.
We submitted an investigational new drug, or IND, application to the United States Food and Drug Administration, or the FDA to initiate a Phase 2 clinical trial of MultiTAA-specific T cell therapy, which we believerefer to as MT-401, in post-allogeneic hematopoietic stem cell transplant patients with AML in both the adjuvant and active disease setting, which may become pivotal pending the results of the interim analysis. The dose administered in this multicenter trial is the current maximum tolerated dose from the ongoing Phase 1/2 trial. In the adjuvant setting, patients will be randomized to either MultiTAA-specific T cell therapy at approximately 90 days post-transplant versus standard of care observation, while the active disease patients will receive MT-401 following relapse post-transplant as part of a single-arm group. In February 2020, we announced that the FDA has permitted us to initiate our portfolioPhase 2 clinical trial beginning with a safety lead-in portion of the trial.
We recently reported interim data for an ongoing Phase 1/2 clinical trial of the MultiTAA-specific T cell therapy for the treatment of pancreatic adenocarcinoma being conducted by BCM. In this trial, we have observed a clinical benefit correlated with the post-infusion detection of tumor-reactive T cells in patient peripheral blood and within tumor biopsy samples in patients in the tumor-resection arm of the trial. These T cells exhibited activity against both targeted antigens and non-targeted TAAs, indicating induction of antigen spreading. To date, we have not observed any cytokine release syndrome or neurotoxicity in this trial.
We are also evaluating the MultiTAA-specific T cell therapies hasin a compelling therapeutic product profile, as compared to current gene-modified CARPhase 2 clinical trial for the treatment of breast cancer and TCR-based therapies. In addition, our Folate Receptor Alpha program (TPIV200) for breast and ovarian cancers and our HER2/neu program (TPIV100/110) are in Phase II1 clinical trials. In parallel, wetrials for the treatment of ALL, lymphoma, MM and sarcoma, all of which are developing a proprietary nucleic acid-based antigen expression technology named PolyStart™ to improvebeing conducted by BCM. As of December 2019, the abilityMultiTAA-specific T cell therapies have been generally well tolerated by all of the immune system to recognizepatients enrolled in clinical trials in hematological and destroy diseased cells.
Immuno-oncology,solid tumor indications with no incidents of cytokine release syndrome or neurotoxicity, which utilizes a patient’s own immune system to combatare frequently associated with CD19 CAR-T therapies. Based on our observations in clinical trials in AML, pancreatic cancer, is one oflymphoma, ALL and MM, we believe that the most actively pursued areas of research by biotechnology and pharmaceutical companies today. Interest and excitement about immunotherapy are driven by compelling efficacy data in cancers with historically bleak outcomes, andMultiTAA-specific T cell therapies have the potential to achievemediate a cure or functional cure for somemeaningful anti-tumor effect, as well as significant in vivo expansion of T cells. We may initiate additional Phase 2 clinical trials investigating other indications in 2020 in addition to our planned Phase 2 trial in post-transplant AML patients. Harnessing the power of the immune system is an important component of fighting cancerous cells in the body. Our MultiTAA T cell therapy platform identifies and selects effectively all T cells that are specific for any peptide from the antigens that we target (e.g., WT1, MAGE-A4, PRAME, Survivin, NY-ESO-1, and SSX2). Our in-vitro manufacturing process promotes proliferation of very rare cancer-killing T cells and augments their anti-tumor properties to provide benefit to patients following their infusion. By using the multi-antigen targeted approach, our proprietary technology can kill heterogeneous tumor cell populations more effectively than single-antigen targeted approaches, thereby reducing the likelihood of tumor escape and potentially increasing the durability of a patient’s response to therapy.
We believe that our therapy presents atherapies present promising innovationinnovations in immuno-oncology. OurWe developed the MultiTAA-specific T cell therapy has been developed through ourin collaboration with the Cell and Gene Therapy Center at Baylor College of Medicine (“BCM”)BCM, which was founded by Dr. Malcolm K. Brenner, M.D., Ph.D., a recognized pioneer in immuno-oncology. BCM remains an important strategic partner and conducts early-stage clinical trials of the MultiTAA-specific T cell therapies pursuant to a sponsored research agreement. Our cell therapy founders include Drs. Malcolm Brenner, M.D., Ph.D., Ann Leen, Ph.D., Juan Vera, M.D., Helen Heslop, M.D., DSc (Hon) and Cliona Rooney, Ph.D., who all have significant experience in this field. Dr.Drs. Brenner, Heslop, Rooney, James P. Allison Dr. Malcom K. Brenner, Dr. Helen E. Heslop, Dr. Cliona M. Rooney and Dr. Padmanee Sharma serve on our Scientific Advisory Board.
Pipeline
Our clinical-stage pipeline, including clinical trials being conducted by BCM and other partners, is set forth below.

Recent Developments
AML Clinical Program Update
In February 2020, we announced that the FDA lifted the clinical hold on the Phase 2 clinical trial investigating the safety and efficacy of MT-401 for the treatment of patients with AML post-transplant permitting us to initiate the trial with the safety lead-in portion that is expected to enroll approximately six patients. Three patients will be dosed with MT-401 manufactured using the legacy reagent used in the Phase 1 trial, and three patients will be dosed with MT-401 manufactured using a reagent from an alternative supplier. We anticipate using this supplier for clinical and commercial supply of MT-401. The FDA placed a partial clinical hold on the trial for the use of the MT-401 product manufactured using one of the reagents supplied by the alternative supplier until the final data and certificate of analysis for the reagent are reviewed and accepted by the FDA. We currently estimate that the alternative supplier will deliver the final reagent, along with the final data and certificate of analysis required by the FDA, by the end of the second quarter of 2020. During the second half of 2020, we anticipate completing enrollment of the first three patients and submission of both the final technical specifications and comparability data of the new reagents to the FDA, the latter of which would satisfy the requirements for lifting the partial hold on the clinical trial. Given this expected timing, we do not currently expect the partial clinical hold to significantly impact site and patient enrollment of the Phase 2 trial.
The safety lead-in will be followed by the 160-patient randomized portion of the trial at approximately 20 transplant centers. Group 1 will comprise 120 adjuvant (disease-free) patients, with the primary endpoint of relapse-free survival of patients receiving MT-401 versus a control group. Group 2 will comprise 40 active disease patients in a single arm, with primary endpoints of complete remission and duration of complete remission.
Our Strategy
Our goal is to be the leader in the development and commercialization of transformative immunotherapies for the treatment of hematological malignancies and solid tumors. We will beare developing a portfolio of highly-differentiatedhighly differentiated T cell therapies utilizing our MultiTAAthe MultiTAA-specific T cell platform that haswe believe have the potential to significantly disrupt the current cell therapy landscape, while substantially improving survival and quality of life for patients with cancers.
KeyThe key elements of our strategy include:
·
| · | Expedite clinical development, regulatory approval, and commercialization of our lead product candidates. |
Based on the results inof the Phase I1 clinical trials of the MultiTAA-specific T cell therapies conducted at BCM, we plan to advance our lead product candidates into Phase II2 clinical trials and facilitate the initiation of company-sponsored clinical trialstrials. We are pursuing post-transplant AML as the lead indication for the MultiTAA-specific T cell program. During the second half of 2020, we expect to complete the safety lead-in portion of our Phase 2 trial in post-transplant acute myeloid leukemia (AML)AML and satisfy the FDA’s other requirements for lifting the partial hold on the clinical trial.
We plan to initiate in the future additional clinical trials in other tumor types based on emerging data. We expect to finalize our first clinical trial protocol by end of second quarter of 2019.
We plan to initiate a Phase II clinical trial in post-transplant AML in the second half of 2019 and in other tumor types based on emerging data in the future. We anticipate that product manufacturing in support of those clinical trials will be conducted at BCM’s Good Manufacturing Practices, (“GMP”)or GMP, cell manufacturing facility.
In 2019,2020, we expect to begin the technology transfer process and begin the planning and implementation of an additional GMP manufacturing capacity capable of supporting our manufacturing needs with respect to potentially pivotal trials. If the results of our Phase II studies2 clinical trials are positive, we willintend to explore potential avenues to achieve regulatory approval for the use of our products in these indications, including any potential avenues for obtaining accelerated approval. The U.S. Food and Drug Administration (“FDA”) may grant accelerated approval for product candidates used to treat serious conditions that fill an unmet medical need based on a surrogate or intermediate endpoint. We believe that an accelerated approval strategy may be warranted given the limited options available for patients with post-transplant AML. However, if the FDA grants accelerated approval, confirmatory trials will be required by the FDA.
·Continue collaboration with our partners and increase our internal research and development activities to improve and develop adoptive cell therapy technologies.
| · | Continue to collaborate with our partners and increase our internal research and development activities to improve and develop adoptive cell therapy technologies. |
We finalizedare party to a strategic alliance with BCM, inpursuant to which we will sponsor selected research at the institutionBCM in support of our technology. In conjunction with this strategic alliance, BCM will conduct selected Phase I/II1 and Phase 2 clinical trials usingof our technology.product candidates. If data from these early clinical trials appearare positive, we will consider the therapeutic and commercial potential for such therapies to be advanced as new productsproduct candidates for us.
In addition, we plan to use BCM facilities and our company laboratories to enable the process development and manufacturing required to support the Phase II2 clinical trials of our product candidates. Outside of our relationship with BCM, we willWe plan to invest in our own research and development and chemistry, manufacturing and controls, (“CMC”)or CMC, capabilities to enhance our ability to conduct process development to optimize our manufacturing process, product quality and commercial scalability.
We believe that the G-Rex® (G-Rex® is a registered trademark of Wilson Wolf Manufacturing Corporation (“Wilson Wolf:”)) based manufacturing process we have in place is highly robust and scalable, and we will continue to invest resources in further refining the manufacturing process to create a product with highly attractive commercial attributes. We plan to engage Wilson Wolf (a company controlled by John Wilson, a director of the Company) to further customize the G-Rex® to optimally match our manufacturing requirements and to develop a scalability plan to drive efficiencies for a commercial product.
| · | Invest in our platform to maximize the beneficial outcomes for cancer patients. |
·Invest in our platform to maximize the beneficial outcomes for cancer patients.
We plan to explore new product opportunities by expandingincreasing and/or customizing the antigens we target to expand the indications in which ourthe MultiTAA-specific T cell products maywill be used,efficacious, including solid tumors or other hematologic malignancies. Additionally, our research and development efforts may include the exploration of dosingdifferent doses and/or frequency of product administrationdosing and the relationship of these factors with potential therapeutic benefit.
·
| · | Leverage our relationships with our founding institutions, scientific founders and other scientific advisors. |
Our world-renowned scientific founders and scientific advisors have made seminal contributions to major discoveries in the field of immuno-oncology, and have significant experience in oncology, immunology and cell therapy. We intend to significantly leverage the knowledge, experience and advice of our scientific founders and advisors, as well as the institutional expertise of BCM, the Mayo Foundation and our other major institutional partners, to advance our therapies through the clinic and into commercialization.
We are in the process of evaluating the peptide vaccine therapeutic products and programs to determine the future strategy and the proper allocation of our resources to best maximize stockholder value. In conjunction with this evaluation process we may de-emphasize or terminate certain of ourvaccine therapeutic products or programs. Such strategic review and evaluations are to be a priority and an important part of our ongoing operations.
MultiTAA T Cell Products
Multi Tumor-Associated Antigen (“MultiTAA”) Approach
Cancers are heterogeneous in their expression of antigens. Tumors generally consist of individual cancer cells expressing different antigens, and each of those antigens can be present at a different level that can change over time. Therapies targeting only a single antigen are vulnerable to evolutionary escape mechanisms.

Even if the single-antigen specific therapy can eliminate all the tumor cells expressing the targeted antigen, the residual tumor cells that do not express that antigen may survive and expand. In addition, tumor cells may also downregulate or mutate the targeted antigen, thus becoming invisible to the T cell therapy. Both phenomena create a transformed tumor that is impervious to that therapy. This process is referred to as antigen-negative tumor immune escape. Our solution to the problem of tumor heterogeneity was to develop T cell products that simultaneously attack multiple tumor-expressed antigens and thereby enable more complete initial tumor targeting, thus minimizing the subsequent opportunity for the cancer to engage escape mechanisms. Data suggest this strategy may be responsible for recruitment and activation of unique cancer-killing cells from the patient’s own immune repertoire to participate in cancer eradication, further minimizing the possibility for tumor cell escape.

Our proprietary MultiTAA T cell platform may have meaningful advantages over CAR and TCR-engineered cell therapy approaches. Compared to current gene-modified T cell therapies, our programs are characterized by the following:
·Demonstrated clinical benefit, without the need for lymphodepletion before infusion: In BCM’s Phase I lymphoma study, we saw complete responses (“CRs”) in six of its evaluable patients, including three CRs in patients with diffuse large B-cell lymphoma (“DLBCL”). We believe it is significant that no patient with a CR has subsequently relapsed with disease, whereas typically 30% or more of patients with CR in reported CAR-T studies relapse within one year. In patient results to date, observed therapeutic responses appear to be highly durable, with some patients being relapse-free beyond five years.
·Non-gene-modified: Unlike CAR-T and TCR approaches, our therapy requires no genetic modification of T cells, a costly and complex process that significantly complicates the manufacturing of a patient product. We believe our therapy can be manufactured at a fraction of the cost of a gene-modified T cell product.
·Low incidence rate of adverse events: In 78 patients treated to date, BCM has seen only one grade III adverse reaction possibly related to its therapy. This appears favorable compared to published CD19 CAR-T studies, wherein up to 95% of patients had associated grade III or higher adverse events during treatment. There have been no cases of cytokine-release syndrome (“CRS”), or related serious adverse events (“SAEs”) in patients treated with our therapy to date.
·Capable of addressing a broad repertoire of cancer cells: While CAR-T and TCR therapies generally target a single epitope, our manufacturing process selects T cells that are specific for multiple peptides derived from several targeted antigens. Deep gene sequencing of the clinical products shows that a typical patient dose usually consists of approximately 4,000 unique T cell clonotypes targeting up to five different tumor-associated antigens. The five antigen targets can be recognized by a very wide range of T cells, facilitating robust killing of targeted cancer cells.
·Appears to drive endogenous immune responses: We see evidence of “epitope spreading” in the treated patients, meaning that the therapy is potentially inducing an enhanced response by the patient’s own T cells (specific for an expanded set of tumor-associated antigens beyond those targeted by the infused product). BCM’s correlative analyses show expansion of endogenous T cells, other than those present in our product, in the months following the infusion of our product. This phenomenon, also known as “antigen spreading,” is potentially important in generating a durable response for a patient, because it enables the killing of tumors that do not express any of the antigens initially targeted by our product.
Peptide Vaccine Products and Technologies in Development
In contrast to standard therapies for cancer treatment including surgery, radiation therapy and chemotherapy that target both cancer cells and normal cells, we are also developing vaccines that precisely target breast and ovarian cancers. We are currently developing three core technology platforms:
1) an exclusively licensed peptide-based vaccine (composition and methods of use) for the treatment of breast cancers that overexpress Human Epidermal Growth Factor Receptor 2 (HER2/neu) (TPIV100/110),
(2) an exclusively licensed peptide-based vaccine (composition and methods of use) for treating breast and ovarian cancers that overexpress Folate Receptor Alpha (TPIV200), and
(3) a wholly-owned nucleic acid-based vaccine (composition and methods of use) technology (PolyStart™) for treatment of various cancers or infectious disease.
Our peptide vaccines are derived from naturally processed T cell antigens and are potentially effective standalone therapies but may also enhance the efficacy of other immunotherapy approaches such as CAR-T cell therapies and PD-1 inhibitors, for example, as well as our own MultiTAA T cell therapies.
The status of our development of other products and technologies is set forth in the table below:
Background and History of Cancer Immunotherapies
Despite advances in options for treatment, cancer continues to be one of the main causes of death in developed countries. Historically, cancer therapy has been constrained to surgery, radiation, and chemotherapy. More recently, advances in the understanding of the immune system’s role in cancer surveillance have led to immunotherapy becoming an important treatment approach. Cancer immunotherapy began with treatments that nonspecifically activated the immune system and had limited efficacy and/or significant toxicity. In contrast, newer immunotherapy treatments can activate specific, potent immune cells, leading to improved safety and efficacy. Within the immunotherapy category, treatments have included vaccines, cytokine therapies, antibody therapies, and adoptive cell therapies.
In 1996, Dr. Dana Leach, Dr. Matthew Krummel and Dr. James Allison reported that monoclonal antibodies, (“mAbs”)or mAbs, blocking CTLA-4 could treat tumors in animal models. Subsequently, mAbs that targeted CTLA-4 and PD-1 became known as “immuneimmune checkpoint inhibitors” (“ICIs”).inhibitors, or ICIs. Immune checkpoints are a means by which cancer cells inhibit or turn down the body’s immune response to cancer. By interfering with these cloaking mechanisms, ICIs have shown an ability to activate T cells, shrink tumors, and improve patient survival. Recent clinical data from checkpoint inhibitors such as ipilimumab, nivolumab and pembrolizumab have confirmed both the validity of this approach and the importance of T cells as promising tools for the treatment of cancer.
Despite these many advances, there persists a significant unmet need in cancer therapeutics. We believe that the use of human cells as a therapeutic modality to re-engage the immune system will be the next significant advancement in the treatment of cancer. These cellular therapies may avoid the long-term side effects associated with current treatments and have the potential to be effective regardless of the type of previous treatments patients have experienced.
T Cell Therapy Overview
The field of adoptive cell transfer (“ACT”) is currently comprised primarily of CAR and TCR engineered T cells and has emerged from principles of basic immunology to become a paradigm-shifting clinical immunotherapy. T cell therapy has evolved as one of the most promising branches of immunotherapy. T cell immunotherapy involves the infusion of immuneT cells into a patient. Immune cells used for immunotherapy treatments can either be collected from the patient (autologous) or harvested from a donor (allogeneic). The cells are retrieved and either genetically modified to express tumor-specific CARs or TCRs or mixed with specific antigens. The cells are then cultured to proliferate, and the proliferated cells are infused into the patient. Upon infusion, the cells can target and eliminate cancerous cells. Unlike chemotherapy, which is unable to distinguish between healthy and malignant cells, T cells produced for immunotherapy can selectively attack cancer cells that express the target antigen(s). This leads to a more effective treatment platform with fewer side effects. Some of these infused T cells may remain in the body for long periods, of time, providing immunological memory, thus leading to longer and more durable responses.
TCRs and CARs have distinct signaling properties and antigen sensitivities. TCRs recognize peptide fragments from proteins expressed either inside the cell or on the cell surface, which are presented to T cells via a major histocompatibility complex (“MHC”).molecules. CARs are programmed to recognize a specific cell surface protein. Because CARs are specific for a single antigen, or more precisely a single epitope within the single antigen, they are very narrowly focused and come withhave limitations. When a CAR-T cell product is applied to a specific antigen of a heterogeneous disease, CAR-T cells may leave behind tumor cells that do not express the target antigen, which can lead to tumor relapse due to immune escape.
Our approach is to avoid genetic engineering by relying upon the native T cell receptor, which has evolved over millions of years to provide T cells with an exquisite capacity to recognize and kill cancer cells. Use of the native T cell receptor is the bedrock of our versatile immunotherapy, which is intended to provide a cost-effective and non-toxic strategy to target multiple tumor antigens and lead to durable responses. The process entails expanding tumor-specific T cells from patients (autologous), or a patient’s hematopoietic stem cell donor (allogeneic). This is achieved byin vitro manipulation consisting of co-culturing a patient’s or donor’s antigen presenting cells with patient (or donor) peripheral blood mononuclear cells, (“PBMCs”),or PBMCs, respectively. As a source of antigen, we use overlapping peptide libraries spanning each of several immunogenic target antigens that are typically associated with certain types of cancer. These peptides are 15 amino acids in length, overlapping by 11 amino acids and span the entire length of each of the target antigens. This typical footprint of peptides allows us to induce both CD4+ (helper) and CD8+ (cytotoxic) T cells. Following manufacture, these cells are frozen and stored for later infusion. Once infused, the natural characteristics of T cells take over and the T cells multiply in quantity, forming an army of T cells that kill the targeted cancer cells.
Process DevelopmentWe have observed evidence of “epitope spreading” in our clinical trials, suggesting that the MultiTAA-specific T cell therapy is inducing an enhanced response by the patient’s own T cells (specific for an expanded set of tumor-associated antigens beyond those targeted by the infused product). Correlative analyses show expansion of endogenous T cells, other than those present in the infused product, in the months following infusion. This phenomenon, also known as “antigen spreading,” is potentially important in generating a deep and Manufacturingdurable response for a patient because it enables the killing of tumors that do not express any of the antigens initially targeted by our therapy and could be due to the lack of lymphodepletion that allows recruitment of the endogenous immune system for anti-tumor activity.
WeThe MultiTAA-Specific T Cell Therapies
In collaboration with BCM, we are advancing two MultiTAAMultiTAA-specific T cell productstherapies through clinical development:(a) Mixed Antigen Peptide Pool (“MAPP”) T cells currently used for patients with lymphoma, multiple myeloma (“MM”) and selected solid tumors, is an autologous product that targets the NY-ESO-1, PRAME, MAGE-A4, Survivin and SSX2 antigens; and (b) Leukemia Antigen Peptide Pool (“LAPP”) T cells, currently used for patients with AML, is an allogeneic product targeting the WT1, NY-ESO-1, PRAME, and Survivin antigens using the blood of the stem cell donor as a source of the cells used for therapy.
| · | Autologous MultiTAA-specific T cell therapies target the NY-ESO-1, PRAME, MAGE-A4, Survivin and SSX2 antigens. We recently reported updated clinical data from BCM’s Phase 1/2 clinical trial of the autologous MultiTAA therapy for the treatment of patients with pancreatic cancer, and we are currently evaluating these therapies for the treatment of patients with lymphoma, MM and other selected solid tumors in Phase 1 trials. |
| · | Allogeneic MultiTAA-specific T cell therapiestarget the WT1, NY-ESO-1, PRAME, and Survivin antigens. The stem cell transplant donor is used as the source of the cells manufactured for our allogeneic therapies. We are pursuing post-transplant AML as the lead indication for the MultiTAA-specific T cell program using our allogeneic therapies. |
While the blood source and the antigens for stimulation differ between the LAPPautologous and the MAPP products,allogeneic therapies, the manufacturing process for each product is otherwise identical.
Cancers are heterogeneous in their expression of antigens. Tumors generally consist of individual cancer cells expressing different antigens, and each of those antigens can be present at a different level that can change over time. Therapies targeting only a single antigen are vulnerable to evolutionary escape mechanisms.

While single-antigen specific therapy can eliminate all the tumor cells expressing the targeted antigen, the residual tumor cells that do not express that antigen may survive and expand. In addition, tumor cells may also downregulate or mutate the targeted antigen, thus becoming invisible to the T cell therapy. Both phenomena create a transformed tumor that is impervious to that therapy. This process is referred to as antigen-negative tumor escape.
Our solution to the problem of tumor heterogeneity is the development of T cell products that simultaneously attack multiple tumor-expressed antigens and thereby enable more complete initial tumor targeting, thus minimizing the subsequent opportunity for the cancer to engage escape mechanisms. Of note, data suggest that this strategy may be responsible for recruitment and activation of unique cancer-killing cells from the patient’s own immune repertoire to participate in cancer eradication, further minimizing the possibility for tumor cell escape.
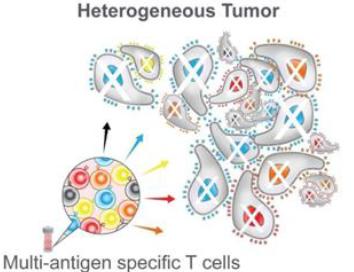
We believe our proprietary MultiTAA-specific T cell platform may have meaningful advantages over current CAR and TCR-engineered cell therapy approaches. Compared to current gene-modified T cell therapies, the MultiTAA-specific T cell product candidates are characterized by the following:
| · | Clinical benefits observed in early-stage clinical trials in multiple cancer indications. |
Based on our observations in clinical trials in AML, pancreatic cancer, lymphoma, ALL and MM, we believe that the MultiTAA-specific T cell therapies have the potential to mediate a meaningful anti-tumor effect, as well as significant in vivo expansion of T cells. For example, in BCM’s Phase 1 clinical trial in lymphoma, there were complete responses, or CRs, in six of the fifteen evaluable patients with active disease. Significantly, no patient with a CR has subsequently relapsed with disease, whereas typically 30% or more of patients with CR in reported CAR-T studies relapse within one year. In patient results to date in this trial, observed therapeutic responses appear to be highly durable, with some patients being relapse-free beyond five years.
| · | Non-gene modified. |
Unlike CAR and TCR-based approaches, our MultiTAA-specific T cell therapy does not require genetic modification of T cells, a costly and complex process that significantly complicates the manufacturing of a patient product. We believe our MultiTAA-specific T cell therapy can be manufactured at a fraction of the cost of a gene-modified T cell product, with substantially reduced complexity of manufacturing.
| · | No need for lymphodepletion before infusion. |
Unlike CAR-T therapies, which require lymphodepletion of a patient’s existing T cells so that they will not compete with the infused therapy, the MultiTAA-specific T cell therapies work with the natural capabilities of T cells to target cancer and do not require lymphodepletion prior to infusion.
| · | Low incidence rate of adverse events. |
As of December 2019, the MultiTAA-specific T cell therapy has been generally well tolerated by all of the patients enrolled in clinical trials in hematological and solid tumor indications with no incidences of cytokine release syndrome or neurotoxicity. In these trials, there has been only one Grade 3 adverse reaction considered possibly related to the therapy. This appears to compare favorably with published CD19 CAR-T studies that have been associated with substantial tolerability concerns, including one Phase 1 trial in which 95% of patients had Grade 3 or higher adverse events during treatment.
| · | Appears to drive endogenous immune responses. |
In our clinical trials, we have observed evidence of “epitope spreading” in the treated patients, meaning that the MultiTAA-specific T cell therapy is potentially inducing an enhanced response by the patient’s own T cells (specific for an expanded set of tumor-associated antigens beyond those targeted by the infused product). Correlative analyses show expansion of endogenous T cells, other than those present in the infused product, in the months following infusion. This phenomenon, also known as “antigen spreading,” is potentially important in generating a deep and durable response for a patient, because it enables the killing of tumors that do not express any of the antigens initially targeted by our therapy and could be due to the lack of lymphodepletion that allows recruitment of the endogenous immune system for anti-tumor activity.
| · | Capable of addressing a broad repertoire of cancer cells. |
While CAR-T and TCR therapies generally target a single epitope, our manufacturing process selects for T cells that are specific for multiple peptides derived from several targeted antigens. Deep gene sequencing of our products shows that a typical patient dose usually consists of approximately 4,000 unique T cell clonotypes, some of which target up to five different tumor-associated antigens. The five antigen targets can be recognized by a very wide range of T cells, which we believe facilitates robust killing of targeted cancer cells.
MT-401 for the Treatment of Post-Transplant AML
We have submitted an IND to the FDA to initiate a Phase 2 clinical trial in post-allogeneic hematopoietic stem cell transplant patients with AML in both the adjuvant and active disease setting, which may become pivotal pending the results of the interim analysis. The dose administered in this multicenter trial is the current maximum tolerated dose from the Phase 1/2 trial. In the adjuvant setting, patients will be randomized to either MT-401 at approximately 90 days post-transplant versus standard of care observation, while the active disease patients will receive MT-401 following relapse post-transplant as part of a single-arm group.
In February 2020, we announced that the FDA lifted the clinical hold on the Phase 2 trial and has permitted us to initiate the trial, beginning with a safety lead-in portion that is expected to enroll approximately six patients. Three patients will be dosed with MT-401 manufactured using the legacy reagent used in the Phase 1 trial, and three patients will be dosed with MT-401 manufactured using a reagent from an alternative supplier. We anticipate using this supplier for clinical and commercial supply of MT-401. The FDA placed a partial clinical hold on the trial for the use of the MT-401 product manufactured using one of the reagents supplied by the alternative supplier until the final data and certificate of analysis for the reagent are reviewed and accepted by the FDA. We currently estimate that the alternative supplier will deliver the final reagent, along with the final data and certificate of analysis required by the FDA, by the end of the second quarter of 2020. We anticipate to complete enrollment of the first three patients and submission of the final technical specifications and comparability data of the new reagents to the FDA during the second half of 2020, thereby satisfying the requirements for lifting the partial hold on the clinical trial. Given this expected timing, we do not currently expect the partial clinical hold to significantly impact site and patient enrollment of the Phase 2 trial.
The safety lead-in will be followed by the 160-patient randomized portion of the trial at approximately 20 transplant centers. Group 1 will comprise 120 adjuvant (disease-free) patients, with the primary endpoint of relapse-free survival of patients receiving MT-401 versus a control group. Group 2 will comprise 40 active disease patients in a single arm, with primary endpoints of complete remission and duration of complete remission.
Clinical Development of Our MultiTAA-Specific T Cell Therapies by BCM
The following clinical trials are being conducted by BCM pursuant to our strategic alliance. If data from these early clinical trials are positive, we will consider the therapeutic and commercial potential for such therapies to be advanced as new product candidates for us. In each trial, correlative studies showed significant expansion of MultiTAA-specific T cells, as well as significant evidence of epitope spreading with expansion of endogenous T cells specific for tumor-associated antigens that were not targeted by the MultiTAA-specific T cell therapy.
Acute Myeloid Leukemia
We are pursuing post-transplant AML as the lead indication for the MultiTAA-specific T cell program. Currently, available treatments for post-transplant AML patients are limited and include donor lymphocyte infusion, which has an approximately 15% overall response rate but a 30% to 50% risk of severe and debilitating graft-versus-host disease. The five-year mortality rate for patients who receive an allogeneic hematopoietic stem cell transplant exceeds 50%, and patients who relapse after a transplant have a survival expectation of approximately 4.5 months.
BCM recently completed a Phase 1 clinical trial of the MultiTAA-specific T cell therapy for the treatment of patients with post-transplant AML. In this trial, we treated patients in remission and with active disease post-transplant. As illustrated below, eleven of the thirteen patients in the adjuvant disease setting dosed with the MultiTAA-specific T cell therapy after receiving an allogeneic stem cell transplant survived, ranging from 6 weeks to 2.5 years post-infusion, with nine of these patients in CCR, as reported in March 2019. Two patients saw local relapse in the central nervous system, but in both cases these patients were successfully treated with local therapy alone. One patient saw extramedullary relapse and was subsequently treated in the active disease arm of the trial, generating a CR that was durable for 13 months. One patient relapsed 8 months after receiving MultiTAA-specific T cells but following a second allogeneic stem cell transplant this patient remains alive in relapse 1.5 years following his initial T cell infusion.

As illustrated below, and reported in March 2019, survival of the six evaluable patients with active disease ranged from 4 to 21 months, as compared to a historical survival rate of 4 months for patients who receive the standard of care post-transplant. Of these patients, one patient demonstrated a complete response that was durable for 13 months, one patient demonstrated a partial response that enabled that patient to receive a second allogeneic stem cell transplant and two patients, who did not meet partial response criteria, experienced disease stabilization enabling a two-month delay to next-line therapy.

In this trial, the MultiTAA-specific T cell therapy was well tolerated, with no drug-related serious adverse events and no instances of graft-versus-host disease. One patient in the adjuvant disease group had a possibly drug-related Grade 3 elevation of liver enzymes, which was treated with prednisone. After discontinuing treatment and receiving decitabine, the patient relapsed and later re-enrolled in the trial in the active disease group and entered CR for 13 months and survived for 2.5 years.
Pancreatic Cancer
In July 2019, we reported interim data from an ongoing Phase 1/2 clinical trial of the MultiTAA-specific T cell therapy for the treatment of pancreatic adenocarcinoma being conducted by BCM. In this trial, BCM plans to enroll approximately 45 patients with advanced or borderline resectable pancreatic adenocarcinoma in three arms: Arm A, which includes patients with unresectable/metastatic disease who are responding to standard first-line chemotherapy; Arm B, which includes patients with progressive disease or therapy intolerance; and Arm C, which includes patients with surgically resectable disease. As of July 5, 2019, a total of 19 patients were administered the MultiTAA-specific T cell therapy: 10 patients in Arm A, 6 patients in Arm B and 3 patients in Arm C.
Overall, we have observed a clinical benefit correlated with the detection of tumor-reactive T cells in patient peripheral blood (Arms A, B and C) and within tumor biopsy samples (Arm C) post-infusion. T cells exhibited activity against both targeted antigens as well as non-targeted TAAs, including MAGE-A2B and AFP, indicating induction of antigen/epitope spreading. No cytokine release syndrome or neurotoxicity had been observed as of July 5, 2019.
Arm A
Arm A is designed to evaluate the safety and potential efficacy of using MultiTAA-specific T cell therapy as part of first-line treatment for patients with pancreatic cancer. These patients in the chemo-responsive arm have completed or will complete at least three months of standard-of-care chemotherapy (gemcitabine/nab-paclitaxel or FOLFIRINOX), which is the period during which a response to chemotherapy would typically occur, before receiving up to six administrations of MultiTAA-specific T cell therapy in conjunction with chemotherapy. Of the nine evaluable patients in Arm A as of July 5, 2019 (one patient was too early to be evaluated):
| · | Three patients experienced objective responses after administration of MultiTAA-specific T cell therapy: |
| o | One patient experienced a complete response; and |
| o | Two patients experienced partial responses. |
| · | Four patients experienced disease stabilization. Two patients within stable disease boundaries (+20%/-30%) saw reversal of tumor growth in which tumors previously growing after chemotherapy alone showed shrinkage after administration of MultiTAA-specific T cell therapy. |
| · | One patient experienced a mixed response, in which some lesions increased in size and others decreased in size for a net zero change in the size of tumor lesions. |
| · | One patient experienced disease progression. |
In addition, overall tumor shrinkage volume was observed in six out of the eight patients with a measurable tumor after administration of MultiTAA-specific T cell therapy. One evaluable patient did not have tumor measurements for analysis.
In patients responding to therapy, significant expansion of the infused MultiTAA-specific T cell therapy was observed, along with broad-based epitope spreading, with significant expansion of endogenous T cells specific for other tumor specific antigens.
Arm B
Arm B is designed to evaluate the use of MultiTAA-specific T cell therapy as a second-line therapy for patients who have failed first-line chemotherapy. The patients in this chemo-refractory arm are either ineligible for chemotherapy or have progressed on chemotherapy and have received or are receiving up to six doses of MultiTAA-specific T cell therapy as a monotherapy. Of the six evaluable patients in Arm B as of July 5, 2019:
| · | Three patients experienced stable disease or clinical disease stabilization: |
| o | Two patients who previously had progressive disease experienced clinical disease stabilization for up to two months. |
| o | One patient has maintained stable disease for seven months (ongoing). |
| · | Three patients experienced clinical decline. |
Among the patients who saw clinical disease stabilization, significant expansion of the infused MultiTAA-specific T cell therapy was observed, along with broad-based epitope spreading, with significant expansion of endogenous T cells specific for other tumor-specific antigens.
Arm C
Arm C is designed to assess T cell infiltration and expansion. These patients with borderline surgically resectable disease received or will receive a dose of MultiTAA-specific T cell therapy following chemotherapy, radiotherapy or combination and prior to surgical resection and up to five additional doses of T cells after surgery. In the patients evaluable in Arm C as of July 5, 2019, MultiTAA-specific T cells were measurable in meaningful numbers as detected by correlative analysis of resected tumor, and significant expansion of the infused MultiTAA-specific T cells was observed, along with broad-based epitope spreading, with significant expansion of endogenous T cells specific for other tumor specific antigens.
Lymphoma
BCM is currently evaluating the MultiTAA-specific T cell therapy in a Phase 1 clinical trial for the treatment of patients with lymphoma.
As of January 2019, we had treated 15 patients with active disease, which we refer to as the active lymphoma group, all of whom had completed a follow-up period beyond three months post-infusion. These patients were heavily pre-treated and, on average, had failed between four and seven prior therapies, with two patients having failed nine prior therapies. As illustrated below, in the active lymphoma group, six patients entered CR and nine patients had experienced stable disease, with three of those patients experiencing durable stable disease and six of those patients experiencing transient stable disease ranging from three months to ten months. None of the patients in CR had relapsed, and the range for the duration of CR in these patients were between one and over five years after being infused with the MultiTAA-specific T cell therapy. Responses in all six patients who entered CR were associated with an expansion of infused T cells, as well as induction of antigen spreading. Of the nine patients with stable disease, three had not relapsed, with two of those patients in stable disease for nine months and over 24 months, respectively, which we believe shows potential durable disease stabilization.
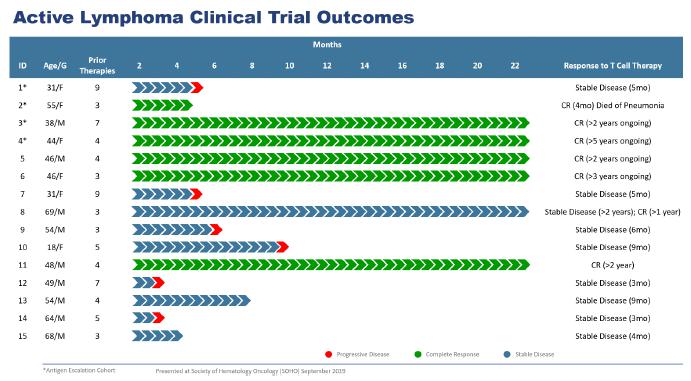
We also had treated 18 patients, including one patient who was treated a second time after a relapse, in remission, which we refer to as the adjuvant lymphoma group. Like the active lymphoma group, these patients were heavily pre-treated. As illustrated below, in the adjuvant lymphoma group as reported in January 2019, all 18 patients had entered CR, with 14 patients in CCR. The duration of response ranged from nine months to over 48 months.

In both treatment groups, the MultiTAA-specific T cell therapy was well tolerated, with no drug-related serious adverse events, suggesting that the MultiTAA-specific T cell therapy might serve as a standard-of-care maintenance therapy for lymphoma patients in remission.
Further, data from this trial show “epitope spreading,” or expansion of patients’ endogenous T cells (specific for an expanded set of tumor-associated antigens beyond those targeted by the infused therapy) in the months following infusion. Significantly, we have observed this effect even though some patients in this trial received doses that had not yet been antigen-escalated to the full antigen dose.
Acute Lymphoblastic Leukemia
BCM is currently evaluating the MultiTAA-specific T cell therapy in a Phase 1 clinical trial for the treatment of patients with ALL. Leukemic relapse is one of the primary causes of treatment failure in hematopoietic stem cell transplant recipients. Like post-transplant AML patients, post-transplant ALL patients have limited treatment options, with donor lymphocyte infusions similarly associated with the risk of life-threatening graft-versus-host disease. While CAR-T therapies have shown potent anti-leukemia activity in post-transplant ALL patients, CD19-CAR-T cell therapies target a single antigen, carrying the inherent risk of immune escape, and are most effective in malignancies of B-cell lineage. In contrast, the MultiTAA-specific T cell therapy targets multiple antigens expressed in both B- and T-cell ALL.
In this trial, as reported in February 2019 we had treated 18 patients. Of the seven evaluable patients:
| · | All evaluable patients were up to 28 months in CCR; |
| · | One patient experienced relapse displayed mixed donor/recipient chimerism after transplant, but remained in CCR for 6 months; and |
| · | Patients who remained in CCR had been durable for between four to 28 months, with a median of 16 months. |
Multiple Myeloma
BCM is currently evaluating the MultiTAA-specific T cell therapy in a Phase 1b/2a clinical trial for the treatment of patients with MM. In this trial, we are treating both active and adjuvant post-autologous stem cell transplant MM patients both within 90 days and more than 90 days post-transplant. We have not seen a meaningful difference in response rates or durability between the two arms and intend to standardize future trials based upon a protocol wherein patients will receive MultiTAA-specific T cell therapy immediately post-transplant.
As reported in January 2019, of the 12 patients that had been treated in the active MM group:
| · | One patient had a CR; |
| · | Three patients achieved partial responses; and |
| · | All eight remaining patients experienced stabilization of disease following initial MultiTAA-specific T cell infusion. |
As reported in January 2019, of the eight patients that had been treated in the adjuvant MM group, all eight patients had experienced a partial response or CR, with a median follow-up of 21 months. Only one patient had relapsed as of such date.
Process Development and Manufacturing of The MultiTAA-Specific T Cell Therapies
In the manufacturing process, blood is drawn from either the individual patient (in the case of the autologous MAPP T cells) or from the allogeneic stem cell transplant donor (in the case of the allogeneic LAPP T cells). Although the T cells that are selected and expanded by our process exist in a patient’s circulating blood, these T cells are often present at very low frequencies. Researchers at BCM believe that these T cells are adversely affected by the suppressive tumor microenvironment. It is a well-accepted concept that cancers not only evade immune detection but often actively suppress the function of the human immune system. Our manufacturing and culturing process is intended to (i)(1) identify the T cells specific for the antigens that we intend to target, (ii)(2) restore these T cells to functionality with respect to their anti-tumor capability and (iii)(3) expand the population of those T cells specific for our targets to achieve the required patient dose.
After blood is drawn, PBMCs are isolated and cryopreserved. Sufficient numbers of cryopreserved PBMCs are taken to be used to manufacture a patient-specific product. These cells are placed inside a G-Rex®G-Rex manufacturing device or standard plasticware and combined with an experimentally optimized mix of GMP-grade cytokines that is used to restore and enhance the functional capability of the cultured T cells.
In addition, libraries of overlapping peptides, (“pepmix”)which we refer to as peptide pools, spanning the target antigens are combined with antigen presenting cells and added to the cell culture. Each peptide within the pepmixa peptide pool represents a small segment of a target antigen, which a T cell might recognize. Each library represents the entire protein sequence of a target antigen, with each peptide in the pepmix overlapping significantly with the peptides adjacent to it within the antigen’s protein sequence. This overlapping structure allows us to isolate, activate and expand any T cell that is specific for any segment of the antigens that we target in the unique genetic background of every patient.
The G-Rex®G-Rex is a cell culture device manufactured by Wilson Wolf used by many cell therapy developers, both in commercial and academic settings. The device allows a user to introduce cells, media and other reagents into a cell culture chamber, which has a gas-permeable membrane at its bottom. The cells settle on this gas-permeable membrane through which oxygen and carbon dioxide are exchanged (i.e. the cells can breathe at the base of the device), while nutrients required for cell expansion are obtained from the medium above the cells. This system allows for the highly robust growth of cells in culture, by providing them with superior access to oxygen and nutrients. Cells manufactured in the device grow efficiently without need for agitation by a technician, scientist or automated system.
Inside the G-Rex®G-Rex or the regular plasticware, PBMCs are co-cultured with antigen-presenting cells that have been exposed to the stimulating pepmixes.peptide pools. This results in the selective expansion of T cells that specifically recognize the target antigens. At the end of the manufacturing process, the resulting product is a mix of helper (CD4+) and cytotoxic (CD8+) T cells that recognize the antigens we are targeting.
Once cell manufacturing is complete, the product is tested for identity, sterility, phenotype and safetyfunctionality before it is released for infusion into a patient. Sampling of product indicates that, on average, approximately 4,000 different T cell clonotypes are present in a typical 5-antigen-specific patient product.
Upon release of the final patient product, the cells are frozen and transported to the site where the cells will be administered. The standard dose for patients with lymphoma, AML or myeloma ranges from 5 –to 20 million cells per meter squared (compared(corresponding to typical doses of 10 –to 40 million cells per adult patient). These cell doses represent a significantly smaller dose of cells, when compared to CAR-T or TCR therapies. As a result, our therapy requires only a very small infusion volume that can be administered to patients within minutes at an outpatient center. Due to the low incidence of adverse events with our therapies, patients do not need to be hospitalized and monitored overnight. Instead, the patients are evaluated for any immediate infusion-related reactions and can then usually be discharged within two hours.


Clinical-stage MultiTAA T Cell Therapy

In addition to our MultiTAA-specific T cell therapies, we are developing peptide-based immunotherapeutic vaccines that are designed to precisely target breast and ovarian cancer cells, in contrast to standard therapies for the treatment of cancer that target both cancer cells and normal cells. Our peptide vaccines are derived from naturally processed T cell-targeted antigens. We believe that our peptide vaccines are potentially effective standalone therapies but may also enhance the efficacy of other immunotherapy approaches, including our own MultiTAA-specific T cell therapies. Our multipeptide approach is fundamentally different from traditional vaccine therapies that have generally targeted a major histocompatibility complex, or MHC, class I-restricted epitope and have historically performed poorly as stand-alone treatments. We are currently evaluating TPIV200 for the treatment of breast and ovarian cancers that overexpress FRa in multiple Phase 2 clinical trials and TPIV100/110 for the treatment of breast cancers that overexpress HER2/neu in Phase 1b and Phase 2 clinical trials.
We are in the process of evaluating the peptide vaccine therapeutic products and programs to determine the future strategy and the proper allocation of our resources to best maximize stockholder value. In conjunction with this evaluation process we may de-emphasize or terminate certain vaccine therapeutic products or programs. Such strategic review and evaluations are a priority and an important part of our ongoing operations.
TPIV200 for the Treatment of FRa-Overexpressed Breast and Ovarian Cancers
FRa is overexpressed in over 80% of breast cancers and over 90% of ovarian cancers. The only treatment options for these cancers are surgery, radiation therapy and chemotherapy, creating a very important and urgent clinical need for a new therapeutic strategy. Time to recurrence is relatively short for ovarian cancer and survival prognosis is extremely poor after recurrence. In the United States alone, every year there are 22,350 new ovarian cancer diagnoses and 268,600 new breast cancer diagnoses, of which 10% are diagnoses of triple-negative breast cancer.
TPIV200 is composed of a mixture of five FRa-derived immunogenic peptides adjuvanted with low-dose granulocyte-macrophage colony-stimulating factor, or GM-CSF, and is designed to activate both the CD4+ and CD8+ T cell compartments in order to activate a patient’s T cells against the targets. Recent developments in immunology suggest that both CD4+ and CD8+ activation support a robust immune response.
Clinical Development
Phase 1 Clinical Trial in Advanced Breast and Ovarian Cancer
In this Phase 1 clinical trial, completed by Mayo Clinic in 2015, 21 patients with advanced breast or ovarian cancer who had undergone standard surgery and adjuvant treatment were treated with one cycle of cyclophosphamide, followed by intradermal vaccination of TPIV200 on day one of a 28-day cycle for a maximum of six vaccination cycles. In the trial, 20 of 21 patients generated T cell responses. These responses developed slowly over the course of the vaccination cycles, with a median time to maximal immunity of five months. Over 90% of patients developed robust and durable antigen-specific immune responses against FRa without regard for HLA type, which aligns with the intended mechanism of action of the vaccine, and 89% of the patients responded to multiple epitopes included in the TPIV200 vaccine, with most patients demonstrating T cell immunity to three or more epitopes. Further, all 16 patients in the observation stage generated T cell responses that lasted over six months.
TPIV200 was well-tolerated, with only one Grade 3 drug-related adverse event. In a two-year patient follow-up analysis, the 10 enrolled ovarian cancer patients had longer median progression-free survival time of 528 days than the 313 days historically reported for the standard-of-care chemotherapy treatment. All patients were alive at the final follow-up. None of the 7 breast cancer patients had experienced a recurrence.
Phase 2 Clinical Trials in Triple-Negative Breast Cancer
Triple-negative breast cancer is one of the most difficult cancers to treat and represents a clear unmet medical need. With the support of a $13.3 million grant from the Department of Defense, the Mayo Foundation is conducting a 280-patient Phase 2 clinical trial of TPIV200 in patients with triple-negative breast cancer, which began enrolling patients in late 2017 and is still recruiting patients.
On June 21, 2016, we announced the initiation of a randomized four-arm Phase 2 trial of TPIV200 for the treatment of patients with Stage 1 to Stage 3 triple-negative breast cancer who have completed initial surgery and chemo/radiation therapy. This open-label, 80-patient clinical trial is designed to evaluate dosing regimens, pre-treatment, efficacy and immune responses. In the trial, we are evaluating a high dose and a low dose of TPIV200, each of which will be tested both with and without cyclophosphamide prior to vaccination. To date, there have been no drug-related serious adverse events reported. Based on a preliminary analysis of 34 patients enrolled in the triple negative breast cancer trial as of September 30, 2019, 31 patients showed meaningful immune response to vaccine treatment. These data are subject to final review by independent biostatistical analysis. As of September 30, 2019, 16 of the 80 patients treated have shown disease progression following treatment with TPIV200.
Phase 2 Clinical Trial in Platinum-Sensitive Ovarian Cancer
A Phase 2 trial, which we sponsored, of TPIV200 in platinum-sensitive ovarian cancer patients (FRV-004) was initiated in January 2017. The trial is a multi-center double-blind efficacy study designed to evaluate TPIV200 compared to GM-CSF alone in a randomized, placebo-controlled fashion during the first maintenance period after primary surgery and chemotherapy. Multiple clinical sites enrolled approximately 120 patients. Safety and interim efficacy has been reviewed by independent data and safety monitoring board, or DSMB.
(1) Baylor CollegeIn November 2019 we announced the discontinuation of Medicinethis trial of TPIV200 based on an unblinded review of interim results from the trial conducted by the DSMB. Although the DSMB did not express any safety concerns with respect to TPIV200, we elected to suspend the trial because it did not meet the threshold for probability of clinical benefit based upon our pre-specified criteria.
Phase 2 Clinical Trial in Combination With Durvalumab for Patients with Ovarian Cancer
On April 21, 2016, we announced our participation in an ovarian cancer trial sponsored by Memorial Sloan Kettering Cancer Center, or MSKCC, in collaboration with AstraZeneca Pharmaceuticals in ovarian cancer patients who are not responsive to platinum, a commonly used chemotherapy for ovarian cancer. This open-label Phase 2 trial of TPIV200 in 40 patients is designed to evaluate the effects of combination therapy with AstraZeneca’s checkpoint inhibitor durvalumab (anti-PD-L1). Interim results from the first 27 patients were presented at the AACR-Rivkin Symposium in September 2018; safety of the combination was shown in these heavily pretreated patients and a subset of patients exhibited durable disease stabilization. Objective response rate and progression-free survival with combination treatment was not superior from the expected efficacy of durvalumab as a monotherapy. However, post-immunotherapy follow-up was suggestive of improved clinical benefit from standard therapies, as the majority of patients’ post-progression went on to receive subsequent standard therapy with durable clinical benefit, creating a rationale for exploration of these agents in combination with chemotherapy. Although we have no business relationship with AstraZeneca, we paid for half of the costs of this trial, in addition to providing TPIV200.
TPIV 100/110 for the Treatment of HER2/neu-Overexpressed Breast Cancers
HER2/neu amplification/overexpression results in an effective therapeutic target in breast and gastric cancer. Over-expressed HER2 is detected predominantly in malignancies of epithelial origin, such as breast, gastric, esophageal, colorectal, salivary gland, pancreatic, epithelial ovarian, endometrial, and bladder carcinomas, as well as gallbladder and extrahepatic cholangiocarcinomas. HER2 is over-expressed in approximately 25% of breast cancers and its expression is associated with unfavorable pathologic features and aggressive disease if not treated with targeted therapies, relative to other forms of breast cancer. While the outcome of patients with HER2 positive breast cancer has significantly improved in the past few decades with an advent of anti-HER2 therapies, a substantial number of resected patients with all types of breast cancer subsequently develop metastatic disease. The continued prevalence of these cancers represents a high unmet medical need, justifying the targeted development of immunotherapeutic strategies.
We have added a MHC class I-restricted peptide, which we licensed from the Mayo Foundation on April 16, 2012, to the four MHC class II-restricted peptides present in TPIV100, resulting in TPIV 110 after the five peptides are mixed with GM-CSF. We have amended the existing IND to incorporate the fifth peptide and will use TPIV110 in future trials with the goal of producing an even more robust vaccine activating both CD4+ (helper) and CD8+ (killer) T cells.
On June 7, 2016, we announced that we had exercised our option agreement with Mayo Foundation and signed a worldwide license agreement to TPIV100. The license gives us the right to develop and commercialize the technology in any cancer indication in which the HER2/neu antigen is overexpressed. As part of this agreement, the IND for the HER2/neu Phase 1 trial was transferred from Mayo Foundation to us for Phase 2 clinical trials of TPIV100. See “—Mayo Foundation for Medical Education and Research Relationships—Mayo HER2/neu License.”
Clinical Development
Phase 1 Clinical Trials in HER2/neu+ Breast Cancer
In the Phase 1 trial of 20 patients conducted at the Mayo Clinic, TPIV100 was well tolerated. Nineteen of the twenty evaluable patients showed robust T-cell immune responses to the antigens in the vaccine. An additional secondary endpoint incorporated into this trial was a two-year follow-on recording the time to disease recurrence in the participating breast cancer patients.
On March 14, 2017, we announced that our partners at the Mayo Clinic received a $3.8 million grant from the Department of Defense to conduct a Phase 1b trial of TPIV100 in ductal carcinoma in situ, or DCIS, an early form of breast cancer. We are working closely with the Mayo Foundation on this clinical trial by providing clinical and manufacturing expertise, as well as providing GMP vaccine formulations under contract. The trial is expected to enroll 40 – 45 women with DCIS and commenced such enrollment during the first quarter of 2019. If the trial is successful and subject to receiving marketing approval from the FDA, we believe that TPIV100 may eventually augment or even replace standard surgery and chemotherapy, and potentially could become part of a routine immunization schedule for preventing breast cancer in healthy women.
Phase 2 Clinical Trials in HER2/neu+ Breast Cancer
On October 10, 2018, we announced that the Mayo Clinic had been awarded a grant of $11 million from the Department of Defense intended to cover the costs of a large randomized, double-blind Phase 2 trial of TPIV100. We are working closely with the Mayo Foundation on this clinical trial by providing clinical and manufacturing expertise, as well as providing GMP vaccine formulations under contract. In this trial, 190 patients will be randomized, in a 2:1 fashion, to receive TPIV100 plus maintenance ado-trastuzumab emtansine, or T-DM1, or maintenance T-DM1with placebo plus GM-CSF. \The trial will evaluate whether the administration of vaccine during T-DM1 maintenance therapy in patients with residual disease post-neoadjuvant chemotherapy effectively blocks disease recurrence and the development of metastatic breast cancer. By prevention of recurrence and metastasis, the expectation is that mortality associated with breast cancer will be decreased.
Manufacturing
Our MAPPcurrent manufacturing strategy is to contract with BCM and LAPP product candidates identifyother third parties to manufacture our MultiTAA-specific T cell therapies, as well as the raw materials, our active pharmaceutical ingredients, or APIs, and selectfinished solid dose products for substantially allour peptide vaccines for clinical and ultimately commercial uses. We currently do not operate any manufacturing facilities for clinical or commercial production of our drug candidates. In addition, we expect to continue to rely on third parties for the manufacture of our clinical and commercial supply of MultiTAA-specific T cells, and of the raw materials, API and finished drug product for our peptide vaccines. Of note, we anticipate that are specific for any peptide derived from the targeted antigens, thereby recognizingproduct manufacturing of MultiTAA-specific T cell therapies in support of Phase 1 and killing heterogeneous tumors more effectively than single-antigen targeted approaches. These product candidates are currently inearly Phase I2 clinical trials for lymphoma, AML/myelodysplastic syndromes (“MDS”), and multiple myeloma (“MM”)will be conducted at BCM and eachwithin its GMP cell manufacturing facility. However, in 2020 we will initiate a technology transfer process to a manufacturing facility capable of these programs is ready for initiation of Phase II. BCM has also initiated Phase I trials in acute lymphocytic leukemia (“ALL”), breast and pancreatic cancers.supplying commercial grade clinical products.
In lymphoma, MAPPthis manner, we expect to continue to build and maintain our supply chain and quality assurance resources.
Our supply chain for manufacturing raw materials, API, peptide vaccines and MultiTAA-specific T cell therapytherapies ready for distribution and commercialization is a multi-step process. Establishing and managing the supply chain requires a significant financial commitment and the creation and maintenance of numerous third-party contractual relationships.
We contract with third parties to manufacture our peptide vaccines and MultiTAA-specific T cell therapies for clinical purposes. Third-party manufacturers supply us with raw materials for the peptide vaccines, and other third-party manufacturers convert these raw materials into API or convert the API into final dosage form. For most of our peptide vaccine candidates, once our raw materials are produced, we rely on different third parties to manufacture the API, to make finished drug product and to lyophilize, package and label the finished product. While we currently have focused on single vendors for manufacturing of peptide, formulation development, and lyophilization and vialing, we have access to numerous other vendors, if required. Similarly, BCM is currently in a Phase I trial that has treated 15 patients with active disease (“lymphoma active group”),the sole manufacturer of which all 15 patients had follow-up date beyond 3 months post-infusion, and 17 patients in remission (“lymphoma adjuvant group”). No SAEs or CRS have been observed in any of these patients.
Of the 15 patients in the lymphoma active group, 6 patients demonstrated a complete response, 3 patients had durable stable disease and 6 patients had transient disease stabilization (range 3 – 9 months). None of the complete responder patients has subsequently progressed after receiving MAPPour MultiTAA-specific T cells. The duration of response for the complete responder patients ranged from 5 months to over 5 years (ongoing). Of the 17 patients in the lymphoma adjuvant group, 15 patients were in a continuing complete response, at the time of data cutoff. The duration of response for these patients ranged from 3 to over 48 months.cell therapies.
In November 2019, we announced that the FDA placed our planned Phase 2 clinical trial of our MultiTAA-specific T cell therapies for the treatment of post-transplant AML a setting where currentlyon clinical hold. The FDA requested additional information and technical specifications for two legacy reagents supplied by third parties used in the only available alternative therapy is a donor lymphocyte infusion (“DLI”), we have seen significant therapeutic benefit for patients, without causing graft-versus-host disease (“GVHD”) — a frequent side effect of DLIs. LAPPMultiTAA-specific T cell therapy is currently inmanufacturing process. The technical specifications and data requested by the FDA could not be produced by the original suppliers. We identified alternative suppliers, and the FDA permitted us to initiate a Phase I trial that has treated 6 patients with active disease (“AML/MDS active group”) after allogeneic hematopoietic stem cell transplant (“HSCT”), and 13 patients in remission after HSCT (“AML/MDS adjuvant group”), of which 11 patients were evaluable. One patient had a transient elevation in liver enzymes. Otherwise there were no possibly/probably related SAEs, nor episodes of CRS.
Of the 6 evaluable patients in the AML/MDS active group, 1 patient demonstrated a complete response which was durable for 13 months, 1 patient demonstrated a partial response that enabled that patient to receive a second allogeneic stem cell transplant, and 2 additional patients, who did not meet partial response criteria, experienced disease stabilization enabling a 2-month delay to next-line therapy. Two patients were non-responsive to MultiTAA therapy and progressed with relapsed/refractory disease. One patient demonstrated ongoing stable disease. The duration of response for the complete or partial response patients ranged from 7 to 11 months. Overall survival ranged from 4 to 21 months after T cell infusions. Of the 11 evaluable patients in the AML/MDS adjuvant group, 9 patients demonstrated a continued complete response. The duration of response for these patients ranged from 6 weeks to 2.5 years. Two patients saw local relapse in the central nervous system, but in both cases these patients were successfully treated with local therapy alone. One patient saw extramedullary relapse and was subsequently treated in the active disease armsafety lead-in portion of the trial generatingbut placed a CRpartial hold on the trial for the use of the MultiTAA-specific T cell product manufactured using one of the reagents supplied by the alternative supplier until the final data and certificate of analysis for the reagent are reviewed and accepted by the FDA. We currently estimate that was durable for 13 months. One patient relapsed 8 months after receiving MultiTAAthe alternative supplier will deliver the final reagent, along with the final data and certificate of analysis required by the FDA, by the end of the second quarter of 2020.
Competition
Our drug discovery, development and ultimate commercialization activities face, and will continue to face, intense competition from organizations such as pharmaceutical and biotechnology companies, as well as academic and research institutions and government agencies. We face significant competition from organizations, particularly fully integrated pharmaceutical companies that are pursuing pharmaceuticals which are competitive with our drug candidates. Our product candidates may compete with product candidates from a number of companies, which are developing various types of similar in vivo T-cell immunotherapies and therapeutic cancer vaccines to treat cancer, including: Advaxis Inc., Genzyme Molecular Oncology, Immune Design, Oncothyreon, Celldex, BN Immunotherapeutics, Immunocellular, SELLAS Life Sciences Group, Inc. (formerly) Galena BioPharma, Antigen Express, Transgene S. A., and Bavarian Nordic. In addition, other adoptive T-cell therapies, monoclonal antibodies and checkpoint inhibitors also provide competition in the oncology space. In these areas, competitors include Iovance, Immatics, Torque Therapeutics, Tessa Therapeutics, AdaptImmune, Mana Therapeutics, Bluebird Bio, Cellectis, Cell Medica, Juno Therapeutics/Celgene/Bristol Myers Squibb, Kite Pharma/Gilead, Novartis, Roche Pharmaceuticals, Merck & Co, AstraZeneca plc and Medimmune, LLC. We believe that our non-engineered T cells but following a second allogeneic stem cell transplant this patient remains alivetherapy and our in relapse 1.5 years following his initial T cell infusion.vivo T-cell therapy approaches will be synergistic and may improve therapies being developed by these competitors.
MAPP T cell therapy is also being evaluated at BCMMany companies and institutions, either alone or together with their collaborative partners, have substantially greater financial, technical and human resources, and significantly greater experience than we do in a Phase I/II trial for patientsthe following:
| · | drug discovery; |
| · | developing products; |
| · | undertaking preclinical testing and clinical trials; |
| · | obtaining FDA and other regulatory approvals of products; and |
| · | manufacturing, marketing, distributing and selling products. |
Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA and other regulatory approval or commercializing products that compete with MM. One arm of this trial assessed patients who received MAPP T cells more than 90 days after an autologous stem cell transplant (“ASCT”), while a second arm assessed patients who received MAPP T cells within 90 days of ASCT. We have not seen a meaningful difference in response rates or durability between the two arms and intend to standardize future trials based upon a protocol wherein patients will receive MAPP T cells immediately post ASCT.our drug candidates.
Of the patients evaluated in the MM trial, there were 10 patientsIn addition, any drug candidate that we successfully develop may compete with residual active disease, 8existing therapies that have long histories of whom were evaluable with greater than 3 months of available follow-up date. Of these evaluable patients, 1 patient demonstrated complete responsesafe and 3 patients demonstrated partial responses. The duration of response ranged from 6 to 29 months. Additionally, there were 8 patients treated in remission after ASCT and all were evaluable. Seven of the 8 patients remain in continuing complete remission. The duration of response for these patients ranged from 6 to 22 months.effective use. Competition may also arise from:
| · | other drug development technologies and methods of preventing or reducing the incidence of disease; |
| · | new small molecules; or |
| · | other classes of therapeutic agents. |
We face, and will continue to face, intense competition from other companies for collaborative arrangements with pharmaceutical and biotechnology companies, for establishing relationships with academic and research institutions and for licenses to drug candidates or proprietary technology. These competitors, either alone or with their collaborative partners, may succeed in developing products that are more effective than ours.
Our ability to compete successfully will depend, in part, on our ability to:
| · | develop proprietary products; |
| · | develop and maintain products that reach the market first, are technologically superior to and/or are of lower cost than other products in the market; |
| · | attract and retain scientific, product development and sales and marketing personnel; |
| · | obtain patent or other proprietary protection for our products and technologies; |
| · | obtain required regulatory approvals; and |
| · | manufacture, market, distribute and sell any products that we develop. |
In a number of countries, including in particular, developing countries, government officials and other groups have suggested that pharmaceutical companies should make drugs available at a low cost. In some cases, governmental authorities have indicated that where pharmaceutical companies do not do so, their patents might not be enforceable to prevent generic competition. Some major pharmaceutical companies have greatly reduced prices for their drugs in certain developing countries. If certain countries do not permit enforcement of any of our patents, sales of our products in those countries, and in other countries could be reduced by generic competition or by parallel importation of our product. Alternatively, governments in those countries could require that we grant compulsory licenses to allow competitors to manufacture and sell their own versions of our products in those countries, thereby reducing our product sales, or we could respond to governmental concerns by reducing prices for our products. In all these situations, our results of operations could be adversely affected.
BCM Exclusive License Agreement
On March 16, 2018, we entered into an exclusive license agreement, (the “BCMor the BCM License Agreement”)Agreement, with BCM, under which we received a worldwide, exclusive license to BCM’s rights in and to certain intellectual property rights, including European patent EP 2470644 (estimated expiration date August 24, 2030), to develop and commercialize MultiTAAMultiTAA-specific T cell product candidates in exchange for an initial issuance of equity in the Company and future royalties and milestone payments.candidates.
Exclusive licenseLicense to BCM’s Subject Technology:
1. “Generation of CTL Lines with Specificity Against Multiple Tumor Antigens or Multiple Viruses”
2. “Pepmixes to Generate Multiviral CTLs with Broad Specificity”
3. “Immunogenic Antigen Identification from a Pathogen and Correlation to Clinical Efficacy”
In partial consideration for the exclusive rights granted under the BCM License Agreement, prior to the Merger, Marker Cell Therapy, Inc., an entity that is now our wholly owned subsidiary, issued shares of Marker Cellits common stock to BCM valued at approximately $5.0 million at the time of issuance. Such initial equity issuance was exchanged into merger consideration of 1,490,813 shares of our common stock and warrants to acquire 540,643 shares of our common stock.stock in connection with the merger we completed in October 2018. Additional consideration includes a royalty paid on net sales by us to BCM according to the royalty schedule in the BCM License Agreement. The royalty fee schedule is based on aggregate net sales in four different ranges: (1) less than $500M,$500 million, (2) $500M$500 million to $1.0B,$1.0 billion, (3) $1.0B$1.0 billion and over, and (4) $2.0B$2.0 billion and over. The corresponding royalty percentages range from 0.65% to 5.0% - increasing in proportion to the aggregate net sales. The royalty fee may be reduced in the event that we must pay additional royalties with respect to third-party owned patent rights or technology necessary for the use, manufacture or sale of a licensed product. We also agreed to pay BCM one-timeup to an aggregate of $64.85 million in milestone payments upon the occurrence of nine particular milestones relating to completion of the first dosing in clinical trials for a first and second distinct product, receipt of approval from the FDA and hittingthe achievement of certain net sales goals. Under the agreement, we may be obligated to make aggregate milestone payments of up to $64.85 million. We are also responsible for sublicensing fees. In addition, under the BCM License Agreement, we are responsible for reimbursing BCM for patent-related expenses. We will beBCM is responsible for filing, prosecuting and maintaining all patent applications and patents included in the licensed patent rights, and we have agreed to reimburse BCM for all such related legal costs incurred after the date of the BCM License Agreement, except such legal costs shall be reduced on a pro-rata basis on a patent or patent application basis should BCM license such patent or patent application in additional fields of use to any third party.
In addition, upon a liquidity event (as defined in the BCM License Agreement) of the Company, BCM will receive a liquidity incentive payment of 0.5% of the liquidity event proceeds (as defined in the BCM License Agreement) received by us or our stockholders in the liquidity event..
We have agreed to indemnify BCM and certain persons affiliated with BCM against claims and liabilities directly or indirectly related to or arising out of the design, process, manufacture or use by any third party of the licensed products, even though such claims and liabilities result in whole or in part from the negligence of the BCM indemnified parties or are based upon doctrines of strict liability or product liability, but not claims or liabilities arising from the gross negligence or intentional misconduct of any such BCM indemnified parties.
Unless terminated sooner, the license will expire on a licensed product-by-product basis and country by countrycountry-by-country basis, on the later of (i)(1) the date of expiration of the last valid claim of patent rights to expire that covers the sale of such licensed product in such country, or (ii)(2) the first date following the tenth anniversary of the first commercial sale of first licensed product by us in such country. After such expiration, but not termination, the licenses granted to us shall survive and become a perpetual, paid-in-full license in such country with respect to such licensed product.
We have the right in our sole discretion to terminate the BCM License Agreement upon 60 days’ written notice to BCM. BCM has the right to terminate the agreement upon material default or failure of us of our overall obligation to perform any of the terms, covenants or provisions of the license agreement, including failure to make timely payment, taken as a whole, and which default or failure remains uncured thirty days after written notice from BCM of such material default or failure to correct such default or failure. Notwithstanding the foregoing, if a material default or failure is not susceptible to cure within the 30-day cure period, BCM’s right to terminate shall be suspended if, and for so long as, (i)(1) we have provided BCM with a written plan that is reasonably calculated to effect a cure, (ii)(2) such plan is reasonably acceptable to BCM, in its sole but reasonable discretion, and (iii)(3) we commit to and do carry out such plan; provided, however, that, unless mutually agreed to by the parties in such plan, such suspension of BCM’s right to terminate shall not extend beyond 60 days after the original cure period. In addition, either party’s right to terminate the license agreement shall be tolled for so long as dispute resolution procedures are being pursued by the allegedly breaching party in good faith, and if it is finally and conclusively determined that the allegedly breaching party is in material breach, then the breaching party shall have the right to cure within 30 days after such determination. BCM also has the right to terminate the agreement if we shall (i)(1) become involved in insolvency, dissolution, bankruptcy or receivership proceedings affecting the operation of our business, (ii)(2) make an assignment of all or substantially all of our assets for the benefit of creditors, or (iii)(3) if a receiver or trustee is appointed for us and we, shall, after the expiration of 30 days following any of the enumerated events, are unable to secure a dismissal, stay or other suspension of such proceedings.
In the event of termination of the BCM License Agreement, but not expiration, all rights to the subject technology and patent rights thereunder shall revert to BCM, except to the extent necessary to exercise any surviving right or license thereunder. We may sell any licensed products actually in itsour possession at the effective date of termination, provided that we continue to pay to BCM royalties on all such sales in accordance with the license agreement, and otherwise compliescomply with the terms of the license agreement and sellssell all such licensed products within six months after the effective date of the termination.
On November 16, 2018, inIn furtherance of the BCM License Agreement and as contemplated by the terms thereof, we have entered into a Sponsored Research Agreement, (“SRA”)or the SRA, with BCM, which providedprovides for the conduct of research for us by credentialed personnel at BCM’s Center for Cell and Gene Therapy. Each of Dr. Vera and Dr. Leen also serve as our Chief Development Officer and Chief Scientific Officer, respectively. The SRA, has a four-year term and the research is to be supervised at BCM by co-investigators Dr. Vera and Dr. Leen.as amended, terminates on January 31, 2021. Pursuant to the amended SRA, we have agreed to pay BCM up$54,000 in each of 2020 and 2021. We previously paid a total of $250,000 to $256,272 forBCM during the first two years one and two underof the SRA with $76,882 paid up front and $153,764 paid in equal monthly installments over two years. Payments for years three and four are to be covered by an amendmentSRA.
We will need to enter into additional agreements with BCM with respect to (i) a strategic alliance to advance pre-clinical research, early stage clinical trials, and Phase II2 clinical trials with respect to our product candidates, as well as continued access to our clinical data, and (ii) product manufacturing and support, including personnel and space at the institution for the foreseeable future.
Mayo Foundation for Medical Education and Research Relationships
We have exclusively licensed the intellectual property for our TPIV100/110 HER2/neu breast cancer vaccine and TPIV200 folate receptor alpha vaccine product candidates from the Mayo Foundation for Medical Education and Research, (the “Mayo Foundation”).or the Mayo Foundation.
As part of our business strategy, we establish business relationships, including collaborative arrangements, with other companies and medical research institutions to assist in the clinical development of certain of our drugs and drug candidates and to provide support for our research programs.
Below is a brief description of our significant business relationships and collaborations and related license agreements with Mayo Foundation that expand our pipeline and provide us with certain rights to existing and potential new products and technologies.
On May 26, 2010, we signed a Technology Option Agreement with the Mayo Foundation in Rochester, Minnesota, for the evaluation of HER2/neu peptide epitopes as antigens for a breast cancer vaccine. The agreement grants us an exclusive worldwide option to become the exclusive licensee of the technology after completion of Phase I clinical trials.
Following approval of the IND by the FDA in July 2011, we executed a Sponsored Research Agreement with the Mayo Foundation for the clinical trial.
Mayo Patent & Know-How License:License
On March 25, 2012, we entered into a Patent & Know-How License Agreement with the Mayo Foundation pursuant to which we acquiredlicensed certain intellectual property rights from the Mayo Foundation for the development and commercialization of certain products, methods and processes property relating to a proprietary HER2/neu technology.
The Mayo Foundation granted us a license (with a right to sublicense) on a worldwide basis to make, sell and use products for prophylactic and therapeutic use. This license is an exclusive license for products that are based on the licensed intellectual property and non-exclusive for products that are based on Mayo Foundation know–how and materials. The intellectual property licensed includes U.S. patents 9,814,767 (estimated expiration date February 15, 2033) and 10,117,919 (estimated expiration date February 15, 2033) and European patent 2814836 (estimated expiration date February 15, 2033).
Under this agreement, and subject to certain exceptions, we are responsible for, among other things, developing the technology under the Patent Rights to bring Licensed Products (as defined in the agreement) to market and costs of filing, prosecution and maintenance of the Patent Rights. Mayo Foundation controls the prosecution and maintenance of the Patent Rights in consultation with us.
The Mayo Foundation granted this license in exchange for an upfront payment of $250,000 that we paid in three installments. In addition to the upfront payment, we are to pay an annual license maintenance fee, milestone fees, royalty fees (which will be subject to a minimum annual royalty fee once royalty fees are due), and a $500,000 diligence fee had a Phase I clinical trial for a Licensed Product not been initiated prior to the fifth anniversarypercentage of the agreementsublicense income (if applicable), and a $2,000,000 diligence fee if we fail to initiate a Phase II2 clinical trial for a Licensed Product prior to the eighth anniversary of the agreement.
We have agreed to indemnify and hold Mayo Foundation harmless from any damages caused as a result of (i)(1) the practice or exercise of any rights and assignments granted bypursuant to the agreement by or on behalf of us, any affiliate, or any sub-licensee; (ii)(2) research, development, design, manufacture, distribution, use, sale, importation, exportation or other disposition of Licensed Products; (iii)(3) our, any affiliates, or any sub-licensee’s act or omission; and (iv)(4) third party suits for patent infringement involving a Licensed Product.
The term of this agreement runs from March 25, 2012 until the date of the last to expire of the Valid Claims (as defined in the agreement), provided that Mayo Foundation may terminate the agreement if, among other matters, (i)(1) 45 days after providing us with notice of a material breach of this agreement, we fail to cure such breach, (ii)(2) we fail to initiate a Phase III3 clinical trial for a Licensed Product prior to the tenth anniversary of the agreement, and (iii)(3) we cease to conduct business in the normal event of operations or become insolvent or bankrupt. We may voluntarily terminate the agreement at any time upon written notice to Mayo Foundation.
Mayo HER2/neu License:License
On May 4, 2016, we entered into a License and Assignment Agreement with Mayo Foundation, (“or the Mayo Foundation HER2/neu License”)License, pursuant to which we acquiredlicensed certain intellectual property rights from the Mayo Foundation for the development and commercialization of certain products, methods and processes property relating to any cancer indication in which the HER2/neu antigen is overexpressed. The Mayo Foundation HER2/neu License resulted from our exercise of an option that was issued pursuant to a Technology Option Agreement that we entered into with the Mayo Foundation on May 25, 2010.
The Mayo Foundation granted us a license (with a right to sublicense) on a worldwide basis to make, sell and use products for therapeutic use against breast, ovarian, lung and any other cancers that overexpress HER2/neu antigens. This license is an exclusive license for products that are based on the licensed intellectual property and non-exclusive for products that are based on Mayo Foundation know–how and materials. The intellectual property licensed includes European patent 2215111 (estimated expiration date October 30, 2028).
Under the Mayo Foundation HER2/neu License, and subject to certain exceptions, we are responsible for, among other things, developing the technology under the Patent Rights to bring Licensed Products (both as defined in the Mayo Foundation HER2/neu License) to market and costs of filing, prosecution and maintenance of the Patent Rights. Mayo Foundation has sole control over the protection, defense, enforcement, maintenance abandonment and other handling of the Know-How (as defined in the Mayo Foundation HER2/neu License) and Materials (as defined in the Mayo Foundation HER2/neu License).
The Mayo Foundation granted this license in exchange for an initial payment of $300,000. The Mayo Foundation assigned to us IND # 14749,#14749, and we assumed all responsibility and liability for this investigational new drug application. In addition to the initial payment, we are to pay an annual license maintenance fee, milestone fees, and royalty fees (which will be subject to a minimum annual royalty fee once royalty fees are due). and, if applicable, a percentage of sublicense income.
We have agreed to indemnify and hold Mayo Foundation harmless from any damages caused as a result of (i)(1) the practice or exercise of any rights and assignments granted bypursuant to the agreement by or on behalf of us or any sub-licensee; (ii)(2) research, development, design, manufacture, distribution, use, sale, importation, exportation or other disposition of Licensed Products; (iii)(3) our or any sub-licensee’s act or omission, including negligence or willful misconduct; and (iv)(4) third party suits for patent infringement involving a Licensed Product.
The term of this agreement runs from May 4, 2016 until the date of our last obligation to make payments under the agreement, provided that Mayo Foundation may terminate the agreement if, among other matters, (i)(1) 30 days after providing us with notice of a material breach of this agreement, we fail to cure such breach, (ii)(2) 90 days after providing us with written notice, we fail to meet either of the following diligence events (a) initiate a Phase II2 clinical trial for a Licensed Product prior to the second anniversary of the agreement and, once initiated, keep current on all of our Phase II2 funding obligations and (b) initiate a Phase IIB2b or III3 clinical trial for a Licensed Product prior to the fifth anniversary of the agreement, (iii)(3) we fail to make a sale of a Licensed Product by May 4, 2026, and (iv)(4) we cease to conduct business in the normal event of operations or become insolvent or bankrupt. We may voluntarily terminate the agreement at any time upon written notice to Mayo Foundation.
Mayo Folate Receptor Alpha License:License
On July 21, 2015, we entered into a License and Assignment Agreement with Mayo Foundation, (“or the Mayo Foundation FRa License”)License, pursuant to which we acquiredlicensed certain intellectual property rights from the Mayo Foundation for the development and commercialization of certain products, methods and processes property relating to a Folate Receptor Alpha immunotherapeutic vaccine comprised of a set of unique peptide epitopes targeting breast, lung and ovarian cancer. The Mayo Foundation FRa License resulted from our exercise of an option that we acquired from Ayer Special Situations Fund I, LP, (“Ayer”)or Ayer, that was issued pursuant to a Technology Option Agreement that Ayer entered into with the Mayo Foundation on March 18,19, 2014.
The Mayo Foundation granted us a license (with a right to sublicense) on a worldwide basis to make, sell and use products for therapeutic use against breast, ovarian, lung and other cancers that expressoverexpress Folate Receptor Alpha. This license is an exclusive license for products that are based on the licensed intellectual property and non-exclusive for products that are based on Mayo Foundation know–how and materials. The intellectual property that is licensed includes US patents 8,486,412 (estimated expiration date April 3, 2029), 8,858,952 (estimated expiration date March 10, 2031), 9,243,033 (July 10, 2027) and 9,915,646 (estimated expiration date June 1, 2027).
Under the Mayo Foundation FRa License, and subject to certain exceptions, we are responsible for, among other things, developing the technology under the Patent Rights to bring Licensed Products (both as defined in the Mayo Foundation FRa License) to market and costs of filing, prosecution and maintenance of the Patent Rights. Mayo Foundation has sole control over the protection, defense, enforcement, maintenance abandonment and other handling of the Know-How (as defined in the Mayo Foundation FRa License) and Materials (as defined in the Mayo Foundation FRa License).
The Mayo Foundation granted this license in exchange for an initial upfront payment of $350,000. The Mayo Foundation assigned to us IND # 14546, and we assumed all responsibility and liability for this investigational new drug application. In addition to the initial upfront payment, we are to pay additional upfront payments, an annual license maintenance fee, milestone fees, and royalty fees (which will be subject to a minimum annual royalty fee once royalty fees are due)., and, if applicable, a percentage of sublicense income.
We have agreed to indemnify and hold Mayo Foundation harmless from any damages caused as a result of (i)(1) the practice or exercise of any rights and assignments granted by the Mayo Foundation FRa License by or on behalf of us or any sub-licensee; (ii)(2) research, development, design, manufacture, distribution, use, sale, importation, exportation or other disposition of Licensed Products; (iii)(3) our or any sub-licensee’s act or omission, including negligence or willful misconduct; and (iv)(4) third party suits for patent infringement involving a Licensed Product.
The term of this agreement runs from July 21, 2015 until the date of our last obligation to make payments under this agreement, provided that the Mayo Foundation may terminate this agreement if, among other matters, (i)(1) 30 days after providing us with notice of a material breach of this agreement, we fail to cure such breach, (ii)(2) 90 days after providing us with written notice, we fail to meet either of the following diligence events (a) initiate a Phase II2 clinical trial for a Licensed Product prior to the 2nd anniversary of the Mayo Foundation FRa License and, once initiated, keep current on all of our Phase II2 funding obligations and (b) initiate a Phase IIB2b or III3 clinical trial for a Licensed Product prior to the 5th anniversary of the Mayo Foundation FRa License, (iii)(3) we fail to make a sale of a Licensed Product by July 21, 2025 and (iv)(4) we cease to conduct business in the normal event of operations or become insolvent or bankrupt. We may voluntarily terminate the Mayo Foundation FRa License at any time upon written notice to Mayo Foundation.
Intellectual Property
Our commercial success will depend in part on our ability to obtain and maintain patent and other proprietary protection for our technology, inventions, improvements, and know-how related to the business; to defend and enforce proprietary rights, including any patents that we may own in the future; to preserve the confidentiality of our trade secrets and other intellectual property; to obtain and maintain licenses to use intellectual property owned by third parties; and to operate without infringing the valid and enforceable patents and other proprietary rights of third parties. Our ability to stop third parties from making, using, selling, offering to sell, or importing our products may depend on the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities — in other words, the rights obtained under exclusive license arrangements such as those pursuant to our BCM License Agreement and our Mayo Foundation licenses. With respect to both licensed and company-owned intellectual property, we cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed in the future, nor can we be sure that any of our existing patents or any patents that may be granted in the future will be commercially useful in protecting our commercial products and methods of manufacturing the same.
To achieve this objective, a strategic focus for us has been to identify and license key patents and patent applications that serve to enhance our intellectual property and technology position. Currently, all of our MultiTAAMultiTAA-specific T cell intellectual property rights are licensed from BCM. Our intellectual property portfolio currently includes patent applications having: (1) claims directed to methods of generating multi-antigen specific T cell products; and (2) claims directed to therapeutic uses of such multi-antigen specific T cell products. We believe our patent portfolio, together with our efforts to develop and patent next-generation technologies, provides us with a substantial intellectual property position. However, the area of patent and other intellectual property rights in biotechnology is an evolving one with many risks and uncertainties.
Patents
Patents and other proprietary rights are vital to our business operations. We protect our technology through various United States and foreign patent filings and maintain trade secrets that we own. Our policy is to seek appropriate patent protection both in the United States and abroad for our proprietary technologies and product candidates. An enforceable patent with appropriate claim coverage can provide an advantage over competitors who may seek to employ similar approaches to develop therapeutics, and so the future commercial success of products, and therefore our future success, will be in part dependent on our intellectual property strategy. The information provided in this section should be reviewed in the context of the information disclosed elsewhere in this annual report under “Risk Factors”. We reassess the value of each patent at the time maintenance fees are due, and in cases where maintaining the patent is judged to be of no significant strategic value, we decline to pay the maintenance fee.
There can be no assurance that our patents, and any patents that may be issued or licensed to us in the future, will afford protection against competitors with similar technology. In addition, no assurances can be given that the patents issued or licensed to us will not be infringed upon or designed around by others or that others will not obtain patents that we would need to license or design around. If the courts uphold existing or future patents containing broad claims over technology used by us, the holders of such patents could require us to obtain licenses to use such technology. Patent coverage may also vary from country to country based on the scope of available patent protection. There are also opportunities to obtain an extension of patent coverage for a product in certain countries, which adds further complexity to the determination of patent life.
We currently have a number of issued and pending patents covering composition of matter of our PolyStart™PolyStart technology including: U.S. 9,364,523 (estimated expiration date March 17, 2035); U.S. 9,655,956 (estimated expiration date March 17, 2035); U.S. 9,988,643 (estimated expiration date March 17, 2035); and U.S. 10,030,252 (estimated expiration date March 17, 2035)
The effect of the issued United States patents is that they provide us with patent protection for the claims covered by the patents. While the expiration of a product patent normally results in a loss of market exclusivity for the covered product or product candidate, commercial benefits may continue to be derived from: (i)(1) later-granted patents on processes and intermediates related to the most economical method of manufacture of the active ingredient of such product; (ii)(2) patents relating to the use of such product; (iii)(3) patents relating to novel compositions and formulations; and (iv)(4) in the United States and certain other countries, market exclusivity that may be available under relevant law. The effect of patent expiration on our product candidates also depends upon many other factors such as the nature of the market and the position of the product in it, the growth of the market, the complexities and economics of the process for manufacture of the active ingredient of the product and the requirements of new drug provisions of the Federal Food, Drug and Cosmetic Act or similar laws and regulations in other countries.
Our pending patent applications cover a range of technologies, including specific embodiments and applications for treatment of various medical indications, improved application methods and adjunctive utilization with other therapeutic modalities. The coverage claimed in a patent application can be significantly reduced before the patent is issued. Accordingly, we do not know whether any of the applications we will acquire, or license will result in the issuance of patents, or, if any patents are issued, whether they will provide significant proprietary protection or will be challenged, circumvented or invalidated. Because unissued U.S. patent applications are maintained in secrecy for a period of eighteen months and U.S. patent applications filed prior to November 29, 2000 are not disclosed until such patents are issued, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in opposition proceedings in a foreign patent office, or for United States patent applications filed before March 16, 2013, in interference proceedings declared by the United States Patent and Trademark Office, “USPTO”)or the USPTO, to determine priority of invention, or in United Statesinter partes review or post-grant review procedures, any of which could result in substantial cost to us, even if the eventual outcome is favorable to us. There can be no assurance that the patents, if issued, would be held valid by a court of competent jurisdiction. An adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using such technology.
We have patents and patent applications in other countries, as well as in the European Patent Office that we believe provide equivalent or comparable protection for our product candidates in jurisdictions internationally that we consider to be key markets. Because of the differences in patent laws and laws concerning proprietary rights, the extent of protection provided by U.S. patents or proprietary rights owned by us may differ from that of their foreign counterparts.
Trade Secrets
We also rely on trade secrets and know-how relating to our proprietary technology and product candidates, continuing innovation, and in-licensing opportunities to develop, strengthen and maintain our proprietary position in the field of immuno-oncology. However, trade secrets can be difficult to protect. We also plan to rely on regulatory protection afforded through orphan drug designations, data exclusivity, market exclusivity and patent term extensions when available, as well as contractual agreements with our academic and commercial partners.
We require each of our employees, consultants and advisors to execute a confidentiality agreement upon the commencement of any employment, consulting or advisory relationship with us. Each agreement provides that all confidential information developed or made known to the individual during the course of the relationship will be kept confidential and not be disclosed to third parties except in specified circumstances. In the case of employees, the agreements provide that all inventions conceived by an employee shall be our exclusive property.
Trademarks
We currently have pending with the USPTO applications for registration of the trademarks POLYSTART™POLYSTART and “Marker Therapeutics.” We currently have the trademark “TapImmune” registered with the USPTO. We also have rights to use other names essential to our business. Federally registered trademarks have a perpetual life if they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage. We regard our trademarks and other proprietary rights as valuable assets and believe they have significant value to us.
We believe that our patents, the protection of discoveries in connection with our development activities, our proprietary products, technologies, processes and know-how and all our intellectual property are important to our business. There can be no assurance that any of our patents, licenses or other intellectual property rights will afford us any protection from competition.
ManufacturingGovernment Regulation
Our manufacturing strategy is to contract with BCM and other third parties to manufacture our MultiTAA-specific T cells, as well as the raw materials, our active pharmaceutical ingredients (“API”) and finished solid dose products for our peptide vaccines for clinical and ultimately commercial uses. We currently do not operate manufacturing facilities for clinical or commercial production of our drug candidates. In addition, we expect for the foreseeable future to continue to rely on third parties for the manufacture of our clinical and commercial supply of MultiTAA-specific T cells, and of the raw materials, API and finished drug product for our peptide vaccines. Of note, we anticipate that product manufacturing of MultiTAA-specific cells in support of Phase I/II clinical trials will be conducted at BCM within its GMP cell manufacturing facility.
In this manner, we expect to continue to build and maintain our supply chain and quality assurance resources.
Manufacturing of our Products
Our supply chain for manufacturing raw materials, API, peptide vaccines, as well as MultiTAA-specific T cell products ready for distribution and commercialization is a multi-step process. Establishing and managing the supply chain requires a significant financial commitment and the creation and maintenance of numerous third-party contractual relationships.
We contract with third parties to manufacture our peptide vaccines and MultiTAA-specific T cells for clinical purposes. Third-party manufacturers supply us with raw materials for the peptide vaccines, and other third-party manufacturers convert these raw materials into API or convert the API into final dosage form. For most of our peptide vaccine candidates, once our raw materials are produced, we rely on different third parties to manufacture the API, to make finished drug product and to lyophilize, package and label the finished product. While we currently have focused on single vendors for manufacturing of peptide, formulation development, and lyophilization and vialing, we have access to numerous other vendors, if required. Similarly, BCM is currently the sole manufacturer of our MultiTAA-specific T cells.
We may not be able to obtain sufficient quantities of any of our raw materials or peptide vaccine candidates if our designated manufacturers do not have the capacity or capability to manufacture our products according to our schedule and specifications. If any of these single source suppliers become unable or unwilling to supply us with API or finished product that complies with applicable regulatory requirements, we could incur significant delays in our clinical trials which could have a material adverse effect on our business. Similarly, if BCM become unable or unwilling to manufacture our MultiTAA-specific T cells that comply with applicable regulatory requirements, we could incur significant delays in our clinical trials which could have a material adverse effect on our business.
For our future products, we may continue contracting third-party suppliers to manufacture sufficient quantities of our peptide vaccine and MultiTAA-specific T cell candidates for clinical and commercial supply. If we are unable to contract for large scale manufacturing with third parties on acceptable terms for our future products or develop manufacturing capabilities internally, our ability to conduct large scale clinical trials and ultimately meet customer demand for commercial products will be adversely affected.
Third-party Manufacturers
Our third-party manufacturers are independent entities subject to their own unique operational and financial risks which are out of our control. If our third-party manufacturers fail to perform as required, this could impair our ability to deliver our products on a timely basis or cause delays in our clinical trials and applications for regulatory approval. To the extent that these risks materialize and affect their performance obligations to us, our financial results may be adversely affected.
While we believe there are multiple third-party suppliers available to provide most of the materials and services needed to manufacture our product candidates, and proper inventory planning is required for the materials that cannot be second-sourced, there is always a risk that we may underestimate demand and that our manufacturing capacity through third-party manufacturers may not be sufficient.
Access to Supplies and Materials
Our third-party manufacturers need access to certain supplies and products to manufacture our drug candidates. If delivery of material from their suppliers were interrupted for any reason or if they are unable to purchase sufficient quantities of raw materials used to manufacture our drug candidates, it could significantly delay our drug candidates in development for clinical trials.
Competition
Our drug discovery, development and ultimate commercialization activities face, and will continue to face, intense competition from organizations such as pharmaceutical and biotechnology companies, as well as academic and research institutions and government agencies. We face significant competition from organizations, particularly fully integrated pharmaceutical companies that are pursuing pharmaceuticals which are competitive with our drug candidates. Our product candidates may compete with product candidates from a number of companies, which are developing various types of similar in vivo T-cell immunotherapies and therapeutic cancer vaccines to treat cancer, including: Advaxis Inc., Genzyme Molecular Oncology, Immune Design, Oncothyreon, Celldex, BN Immunotherapeutics, Immunocellular, SELLAS Life Sciences Group, Inc. (formerly) Galena BioPharma, Antigen Express, Transgene S. A., and Bavarian Nordic. In addition, other adoptive T-cell therapies, monoclonal antibodies and checkpoint inhibitors also provide competition in the oncology space. In these areas, competitors include Iovance, Immatics, Torque Therapeutics, AdaptImmune, Mana Therapeutics, Juno Therapeutics/Celgene/Bristol Myers Squibb, Kite Pharma/Gilead, Novartis, Roche Pharmaceuticals, Merck & Co, AstraZeneca plc and Medimmune, LLC. We believe that our non-engineered T cells therapy and our in vivo T-cell therapy approaches will be synergistic and may improve therapies being developed by these competitors.
Many companies and institutions, either alone or together with their collaborative partners, have substantially greater financial, technical and human resources, and significantly greater experience than we do in the following:
Accordingly, our competitors may succeed in obtaining patent protection, receivingThe FDA and other regulatory approval or commercializing products that compete with our drug candidates.
In addition, any drug candidate that we successfully develop may compete with existing therapies that have long histories of safe and effective use. Competition may also arise from:
We face, and will continue to face, intense competition from other companies for collaborative arrangements with pharmaceutical and biotechnology companies, for establishing relationships with academic and research institutions and for licenses to drug candidates or proprietary technology. These competitors, either alone or with their collaborative partners, may succeed in developing products that are more effective than ours.
Our ability to compete successfully will depend, in part, on our ability to:
In a number of countries, including in particular, developing countries, government officials and other groups have suggested that pharmaceutical companies should make drugs availableauthorities at a low cost. In some cases, governmental authorities have indicated that where pharmaceutical companies do not do so, their patents might not be enforceable to prevent generic competition. Some major pharmaceutical companies have greatly reduced prices for their drugs in certain developing countries. If certain countries do not permit enforcement of any of our patents, sales of our products in those countries, and in other countries could be reduced by generic competition or by parallel importation of our product. Alternatively, governments in those countries could require that we grant compulsory licenses to allow competitors to manufacture and sell their own versions of our products in those countries, thereby reducing our product sales, or we could respond to governmental concerns by reducing prices for our products. In all these situations, our results of operations could be adversely affected.
Government Regulation
Our ongoing research and development activities and any manufacturing and marketing of our drug candidates are subject to extensive regulation by numerous governmental authorities in the United States and other countries. Government authorities in the United States, at the federal, state, and local level, andlevels, as well as in otherforeign countries, and jurisdictions, including the European Union, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval,import, export, safety, effectiveness, labeling, packaging, storage, recordkeeping, labeling,distribution, record keeping, approval, advertising, promotion, distribution, marketing, post-approval monitoring, and post-approval reporting and import and export of pharmaceutical products. The processes for obtaining regulatory approvals in the United States and in foreign countries and jurisdictions,biologics such as those we are developing. We, along with subsequent compliance with applicable statutesthird-party contractors, will be required to navigate the various preclinical, clinical and regulations and othercommercial approval requirements of the governing regulatory authorities, requireagencies of the expenditurecountries in which we wish to conduct studies or seek approval or licensure of substantial time and financial resources.our product candidates.
The failure to comply with applicable U.S. requirements at any time during the product development process approval process or after approval may subject an applicant and/or sponsor to a variety of administrative or judicial sanctions, including refusalrequired by the FDA to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters and other types of letters,before biologic product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, or civil or criminal investigations and penalties brought by the FDA and the Department of Justice (“DOJ”), or other governmental entities. The government regulations below may apply to any of our product candidates or anticipated pipeline of products.
FDA Review and Approval Process
The regulatory review and approval process is lengthy, expensive and uncertain. The steps generally required before a drug may be marketed in the United States include:generally involves the following:
| · | completion of preclinical laboratory tests and animal studies |
| · | submission to the FDA of an |
| · | approval by an independent Institutional Review Board, or IRB, or ethics committee at each clinical site before the trial is commenced; |
| · | performance of adequate and well-controlled human clinical trials |
| · |
| · | satisfactory completion of an FDA Advisory Committee review, if applicable; |
| · | a determination by the FDA within 60 days of its receipt of a BLA to file the application for review; |
| · | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the |
| · | FDA review and approval of the |
Similar requirements exist within foreign agencies as well. The time required to satisfy FDA requirements or similar requirements of foreign regulatory agencies may vary substantially based on the type, complexityPreclinical and novelty of the product or the targeted disease.Clinical Development
Preclinical testingPrior to beginning the first clinical trial with a product candidate, we must submit an IND to the FDA. An IND is a request for authorization from the FDA to administer an investigational new drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical studies. The IND also includes laboratory evaluation of product pharmacology, drug metabolism, and toxicity which includes animal studies, to assess potential safety and efficacy as well as product chemistry, stability, formulation, development, and testing. The results of animal and in vitro studies assessing the preclinical tests, together withtoxicology, pharmacokinetics, pharmacology, and pharmacodynamic characteristics of the product; chemistry, manufacturing, information and analyticalcontrols information; and any available human data are submittedor literature to support the FDA as partuse of an IND.the investigational product. An IND willmust become effective before human clinical trials may begin. The IND automatically becomebecomes effective 30 days after receipt by the FDA, unless before thatthe FDA, within the 30-day time the FDAperiod, raises safety concerns or questions about the conduct of theproposed clinical trial(s) included intrial. In such a case, the IND which are further parsed intomay be placed on clinical hold and non-hold questions/issues. In the case of hold issues, the IND sponsor and the FDA must resolve all FDAany outstanding concerns or questions before the clinical trialstrial can proceed. We cannot be sure that submissionbegin. Submission of an IND willtherefore may or may not result in the FDA allowingauthorization to begin a clinical trials to commence.
trial. Clinical trials involve the administration of the investigational drugproduct to human subjects under the supervision of qualified investigators and in accordance with good clinical practices regulations coveringGCPs, which include the protection of human subjects. These regulations requirerequirement that all research subjects to provide their informed consent.consent for their participation in any clinical study. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocolA separate submission to the existing IND must be submittedmade for each successive clinical trial conducted during product development and for any subsequent protocol amendments. Furthermore, an independent IRB for each site proposing to conduct the FDA as part of the IND and eachclinical trial must be reviewedreview and approved by an Institutional Review Board (“IRB”)approve the plan for any clinical trial and its informed consent form before it can begin.
Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. Phase I usually involvesclinical trial begins at that site and must monitor the initial introduction of the investigational drug into healthy volunteers to evaluate its safety, dosage tolerance, absorption, metabolism, distribution and excretion. Phase II usually involves clinical trials in a limited patient population to evaluate dosage tolerance and optimal dosage, identify possible adverse effects and safety risks, and evaluate and gain preliminary evidence of the efficacy of the drug for specific indications. Phase III clinical trials usually further evaluate clinical efficacy and safety by testing the drug in its final form in an expanded patient population, providing statistical evidence of efficacy and safety, and providing an adequate basis for labeling. We cannot guarantee that Phase I, Phase II or Phase III testing will be completed successfully within any specified period of time, if at all. Furthermore, we,study until completed. Regulatory authorities, the IRB or the FDAsponsor may suspend a clinical trialstrial at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
As a separate amendment to an IND, a clinical trial sponsor may submit to the FDA a request for a Special Protocol Assessment (“SPA”). Under the SPA procedure, a sponsor may seek the FDA’s agreement on the design and size of a clinical trial intended to form the primary basis of an effectiveness claim. If the FDA agrees in writing, its agreement may not be changed after the trial begins, except when agreed by FDArisk or in limited circumstances, such as when a substantial scientific issue essential to determining the safety and effectiveness of a drug candidate is identified after a Phase III clinical trial is commenced and agreement is obtained with the FDA. If the outcome ofthat the trial is successful, the sponsor will ordinarily be ableunlikely to rely on it as the primary basis for approval with respect to effectiveness. However, additional trials couldmeet its stated objectives. Some studies also be requestedinclude oversight by an independent group of qualified experts organized by the FDA to support approval, and the FDAclinical study sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may make an approval decisionmove forward at designated check points based on a number of factors, includingaccess to certain data from the degree of clinical benefit as well as safety. The FDA is not obligated to approve an NDA or BLA as a result of a SPA agreement, even ifstudy and may halt the clinical outcometrial if it determines that there is positive.an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There are also requirements governing the reporting of ongoing clinical studies and clinical study results to public registries.
Even after initial FDAFor purposes of BLA approval, has been obtained, post-approval trials or Phase IV studies, may be required to provide additional data, and will be required to obtain approval for the sale of a product as a treatment for a clinical indication other than that for which the product was initially tested and approved. Also, the FDA will require post-approval safety reporting to monitor the side effects of the drug. Results of post-approval programs may limit or expand the indication or indications for which the drug product may be marketed. Further, if there are any requests for modifications to the initial FDA approval for the drug, including changes in indication, manufacturing process, manufacturing facilities, or labeling, a supplemental NDA or BLA may be required to be submitted to the FDA.
The length of time and related costs necessary to completehuman clinical trials varies significantly and may be difficult to predict. Clinical results are frequently susceptible to varying interpretationstypically conducted in three sequential phases that may delay, limit or prevent regulatory approvals. Additional factors that can cause delay or termination of our clinical trials, or cause the costs of these clinical trials to increase, include:overlap.
| · |
| · |
| · |
Any drug is likely to produceIn some toxicities or undesirable side effects in animals and in humans when administered at sufficiently high doses and/or for sufficiently long periods of time. Unacceptable toxicities or side effects may occur at any dose level. The appearance of any unacceptable toxicity or side effect could cause us or regulatory authorities to interrupt, limit, delay or abort the development of any of our drug candidates and could ultimately prevent their marketing approval bycases, the FDA may require, or foreign regulatory authorities for any or all targeted indications.
Fast Track Designation and Accelerated Approval
The FDA’s fast track and breakthrough therapy designation programs are intended to facilitate the development and expedite the review of drug candidates intended for the treatment of serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs for these conditions. Under these programs, FDA can, for example, review portions of an NDA or BLA for a drug candidate before the entire application is complete, thus potentially beginning the review process at an earlier time.
We cannot guarantee that the FDA will grant any of our requests for fast track or breakthrough therapy designations, that any such designations would affect the time of review or that the FDA will approve the NDA or BLA submitted for any of our drug candidates, whether or not these designations are granted. Additionally, FDA approval of a fast track/breakthrough product can include restrictions on the product’s use or distribution (such as permitting use only for specified medical conditions or limiting distribution to physicians or facilities with special training or experience). Approval of such designated products can be conditioned oncompanies may voluntarily pursue, additional clinical trials after a product is approved to gain more information about the product. These so- called Phase 4 studies may be made a condition to approval of the BLA. Concurrent with clinical trials, companies may complete additional animal studies and develop additional information about the biological characteristics of the product candidate and must finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final product, or for biologics, the safety, purity and potency. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
BLA Submission and Review
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, nonclinical studies and clinical trials are submitted to the FDA as part of a BLA requesting approval to market the product for one or more indications. The BLA must include all relevant data available from pertinent preclinical and clinical studies, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls, and proposed labeling, among other things. The submission of a BLA requires payment of a substantial application user fee to FDA, unless a waiver or exemption applies.
Once a BLA has been submitted, the FDA’s goal is to review standard applications within ten months after it accepts the application for filing, or, if the application qualifies for priority review, six months after the FDA accepts the application for filing. In both standard and priority reviews, the review process is often significantly extended by FDA requests for additional information or clarification. The FDA reviews a BLA to determine, among other things, whether a product is safe, pure and potent and the facility in which it is manufactured, processed, packed, or held meets standards designed to assure the product’s continued safety, purity and potency. The FDA may convene an advisory committee to provide clinical insight on application review questions. Before approving a BLA, the FDA will typically inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
After the FDA evaluates a BLA and conducts inspections of manufacturing facilities where the investigational product and/or its drug substance will be produced, the FDA may issue an approval letter or a Complete Response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A Complete Response letter will describe all of the deficiencies that the FDA has identified in the BLA, except that where the FDA determines that the data supporting the application are inadequate to support approval, the FDA may issue the Complete Response letter without first conducting required inspections, testing submitted product lots, and/or reviewing proposed labeling. In issuing the Complete Response letter, the FDA may recommend actions that the applicant might take to place the BLA in condition for approval, including requests for additional information or clarification. The FDA may delay or refuse approval of a BLA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product.
If regulatory approval of a product is granted, such approval will be granted for particular indications and may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the BLA with a Risk Evaluation and Mitigation Strategy, or REMS, to ensure the benefits of the product outweigh its risks. A REMS is a safety strategy to manage a known or potential serious risk associated with a product and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more Phase 4 postmarket studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization and may limit further marketing of the product based on the results of these post-marketing studies.
Expedited Development and Review Programs
The FDA offers a number of expedited development and review programs for qualifying product candidates. The fast track program is requiredintended to expedite or facilitate the development, and expedite the review, of biologicsprocess for reviewing new products that meet certain criteria. Specifically, new products are eligible for fast track designation if they are intended for the treatment ofto treat a serious or life-threatening disease or condition for which there is no effective treatment, and which demonstrate the potential to address unmet medical needs for the disease or condition. UnderFast track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a fast track product has opportunities for frequent interactions with the review team during product development and, once a BLA is submitted, the product may be eligible for priority review. A fast track product may also be eligible for rolling review, where the FDA may consider for review sections of the BLA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the BLA, the FDA agrees to accept sections of the BLA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the BLA.
A product intended to treat a serious or life-threatening disease or condition may also be eligible for breakthrough therapy designation to expedite its development and review. A product can receive breakthrough therapy designation if preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The designation includes all of the fast track program features, as well as more intensive FDA interaction and guidance beginning as early as Phase 1 and an organizational commitment to expedite the sponsordevelopment and review of the product, including involvement of senior managers.
Any marketing application for a new biologic candidate may request thatsubmitted to the FDA designate the candidate for approval, including a specific indication asproduct with a fast track biologic concurrent with, designation and/or after,breakthrough therapy designation, may be eligible for other types of FDA programs intended to expedite the filingFDA review and approval process, such as priority review and accelerated approval. A product is eligible for priority review if it has the potential to provide a significant improvement in the treatment, diagnosis or prevention of a serious disease or condition compared to marketed products. For products containing new molecular entities, priority review designation means the FDA’s goal is to take action on the marketing application within six months of the IND for the candidate. The FDA must determine if the biologic candidate qualifies for fast track designation within 60 days of receipt of the sponsor’s request.60-day filing date (compared with ten months under standard review).
Under the fast track programAdditionally, products studied for their safety and FDA’s accelerated approval regulations, the FDA may approve a biologic for aeffectiveness in treating serious or life-threatening illnessdiseases or conditions may receive accelerated approval upon a determination that provides meaningful therapeutic benefit to patients over existing treatments based uponthe product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
In As a condition of accelerated approval, the FDA will generally require the sponsor to perform adequate and well-controlled post-marketing clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A biologic candidate approved on this basis is subjectstudies to rigorous post-marketing compliance requirements, includingverify and describe the completion of Phase 4 or post-approval clinical trials to confirm theanticipated effect on theirreversible morbidity or mortality or other clinical endpoint. Failure to conduct required post-approval trials, or confirm a clinical benefit during post-marketing trials, will allowbenefit. In addition, the FDA to withdraw the biologic from the market on an expedited basis. Allcurrently requires as a condition for accelerated approval pre-approval of promotional materials, for biologic candidates approved under accelerated regulations are subject to prior review bywhich could adversely impact the FDA.
In addition to other benefits such as the ability to use surrogate endpoints and engage in more frequent interactions with the FDA, the FDA may initiate review of sections of a fast track product’s BLA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submissiontiming of the remaining information and the applicant pays applicable user fees. However, the FDA’s time period goal for reviewing an application does not begin until the last sectioncommercial launch of the BLA is submitted. Additionally, the fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Breakthrough Therapy Designationproduct.
The FDAregenerative medicine advanced therapy, or RMAT, designation is also requiredintended to expedite thefacilitate an efficient development program for, and expedite review of, any drug that meets the application for approval of biologicalfollowing criteria: (1) it qualifies as a RMAT, which is defined as a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, that arewith limited exceptions; (2) it is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition wherecondition; and (3) preliminary clinical evidence indicates that the biologic may demonstrate substantial improvement over existing therapies on onedrug has the potential to address unmet medical needs for such a disease or more clinically significant endpoints.
Under the breakthrough therapy program, the sponsor of a new biologic candidate may request that the FDA designate the candidate for a specific indication as a breakthrough therapy concurrent with, or after, the filing of the IND for the biologic candidate. The FDA must determine if the biological product qualifies forcondition. Like breakthrough therapy designation, within 60 daysRMAT designation provides potential benefits that include more frequent meetings with FDA to discuss the development plan for the product candidate and eligibility for rolling review and priority review. Products granted RMAT designation may also be eligible for accelerated approval on the basis of receipta surrogate or intermediate endpoint reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of sites, including through expansion to additional sites. Once approved, when appropriate, the sponsor’s request.FDA can permit fulfillment of post-approval requirements under accelerated approval through the submission of clinical evidence, clinical studies, patient registries, or other sources of real world evidence such as electronic health records; through the collection of larger confirmatory datasets; or through post-approval monitoring of all patients treated with the therapy prior to approval.
Sponsors submit the results of preclinical studies and clinical trials to the FDA as part of an NDA or BLA. NDAs and BLAs must also contain extensive product manufacturing information and proposed labeling. Upon receipt, the FDA initially reviews the NDA or BLA to determine whether it is sufficiently complete to initiate a substantive review. If the FDA identifies deficiencies that would preclude substantive review, the FDA will refuse to accept the NDA or BLA and will inform the sponsor of the deficiencies that must be corrected prior to resubmission. If the FDA accepts the submission for review (then deemed a “filing”), the FDA typically completes the NDA or BLA review within a pre-determined time frame. Under the Prescription Drug User Fee Act, the FDA agrees to review NDAs and BLAs under either a standard review or priority review. FDA procedures provide forFast track designation, breakthrough therapy designation, priority review, of NDAsaccelerated approval, and BLAs submittedRMAT designation do not change the standards for drugs that, compared to currently marketed products, if any, offer a significant improvement inapproval but may expedite the treatment, diagnosisdevelopment or prevention of a disease. The FDA seeks to review NDAs and BLAs that are granted priority status more quickly than NDAs and BLAs given standard review status. The FDA’s stated policy is to act on 90% of priority NDAs and BLAs within eight months of receipt (or six months after filing, which occurs 60 days after NDA or BLA submission). Although the FDA historically has not met these goals, the agency has made significant improvements in the timeliness of the reviewapproval process. NDA and BLA review often extends beyond anticipated completion dates due to FDA requests for additional data or clarification, the FDA’s decision to have an advisory committee review, and difficulties in scheduling an advisory committee meeting. The recommendations of an advisory committee are not binding on the FDA.
To obtain FDA approval to market a product, we must demonstrate that the product is safe and effective for the patient population that will be treated. If regulatory approval of a product is granted, the approval will be limited to those disease states and conditions for which the product is safe and effective, as demonstrated through clinical trials. Marketing or promoting a drug for an unapproved indication is prohibited. Furthermore, approval may entail requirements for post-marketing studies or risk evaluation and mitigation strategies, including the need for patient and/or physician education, patient registries, medication or similar guides, or other restrictions on the distribution of the product. If an NDA or BLA does not satisfy applicable regulatory criteria, the FDA may deny approval of an NDA or BLA or may issue a complete response, and require, among other things, additional clinical data or analyses.
In Canada, the Therapeutic Products Directorate and the Biologics and Genetic Therapies Directorate of Health Canada (“HC”) ensure that clinical trials are properly designed and undertaken and that subjects are not exposed to undue risk. Regulations define specific Investigational New Drug submission (“IND”) application requirements, which must be complied with before a new drug can be distributed for trial purposes. The directorates currently review the safety, efficacy and quality data submitted by the sponsor and approve the distribution of the drug to the investigator. The sponsor of the trial is required to maintain accurate records, report adverse drug reactions, and ensure that the investigator adheres to the approved protocol. Trials in humans should be conducted according to generally accepted principles of good clinical practice. Management believes that these standards provide assurance that the data and reported results are credible and accurate, and that the rights, integrity, and privacy of clinical trial subjects are protected.
Sponsors wishing to conduct clinical trials in Phases I through III of development must apply under a 30-day default system. Applications must contain the information described in the regulations, including: a clinical trial attestation; a protocol; statements to be contained in each informed consent form that set out the risks posed to the health of clinical trial subjects as a result of their participation in the clinical trial; an investigator’s brochure; applicable information on excipients (delivery vehicles); and chemistry and manufacturing information.
The sponsor can proceed with the clinical trial if the directorates have not objected to the sale or importation of the drug within 30 days after the date of receipt of the clinical trial application and Research Ethics Board approval for the conduct of the trial at the site has been obtained. Additional information is available on Health Canada’s website -www.hc-sc.gc.ca.
Outside the United States and Canada, our ability to market a product is contingent upon receiving a marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although within the European Union (“EU”), registration procedures are available to companies wishing to market a product in more than one EU member state. If the regulatory authority is satisfied that adequate evidence of safety, quality and efficacy has been presented, a marketing authorization may be granted. This foreign regulatory approval process involves all of the risks associated with FDA approval discussed above and may also include additional risks.
Orphan Drug Designation
TheUnder the Orphan Drug Act, provides incentivesthe FDA may grant orphan designation to manufacturersa drug or biologic intended to develop and market drugs fortreat a rare diseases and conditions affectingdisease or condition, which is a disease or condition that affects fewer than 200,000 personsindividuals in the United States, at the time of application for orphan drug designation. The first developer to receive FDA marketing approval for an orphan drug is entitled to a seven-year exclusive marketing periodor more than 200,000 individuals in the United States for which there is no reasonable expectation that the cost of developing and making available in the United States a drug or biologic for this type of disease or condition will be recovered from sales in the United States for that drug or biologic. Orphan drug designation must be requested before submitting a BLA. After the FDA grants orphan drug indication. However,designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. The orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review or approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusive approval (or exclusivity), which means that the FDA considersmay not approve any other applications, including a full BLA, to be clinically superior to, or different from, another approved orphan drug, even thoughmarket the same biologic for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the BLA application fee.
A designated orphan drug may also obtain approvalnot receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, exclusive marketing rights in the United States during the seven-year exclusive marketing period.
Undermay be lost if the FDA Modernization Act of 1997, designation as a Fast Track product for a new drug or biological product meanslater determines that the FDA will take such actions as are appropriate to expedite the development and review of the applicationrequest for approval of such product.
Legislation similar to the Orphan Drug Act has been enacted in other countries outside of the United States, including the EU. The orphan legislation in the EU is available for therapies addressing conditions that affect fivedesignation was materially defective or fewer out of 10,000 persons, are life-threatening or chronically debilitating conditions and for which no satisfactory treatment is authorized. The market exclusivity period is for ten years, although that period can be reduced to six years if at the end of the fifth year, available evidence establishes that the product does not justify maintenance of market exclusivity.
Disclosure of Clinical Trial Information
Sponsors of human clinical trials of FDA-regulated products, including biological products, are required to register and disclose certain clinical trial information. Information related to the product, patient population, phase of investigation, trial sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed until the new product or new indication being studied has been approved. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Pediatric Information
Under the Pediatric Research Equity Act, or PREA, NDAs or BLAs or supplements to NDAs or BLAs must contain data to assess the safety and effectiveness of the biological product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the biological product is safe and effective. The FDA may grant full or partial waivers, or deferrals, for submission of data. Unless otherwise required by regulation, PREA does not apply to any biological product for an indication for which orphan designation has been granted.
Additional Controls for Biologics
To help reduce the increased risk of the introduction of adventitious agents, the Public Health Service Act (“PHSA”) emphasizes the importance of manufacturing controls for products whose attributes cannot be precisely defined. The PHSA also provides authority to the FDA to immediately suspend licenses in situations where there exists a danger to public health, to prepare or procure products in the event of shortages and critical public health needs, and to authorize the creation and enforcement of regulations to prevent the introduction or spread of communicable diseases in the United States and between states.
After a BLA is approved, the product may also be subject to official lot release as a condition of approval. As part of the manufacturing process, the manufacturer is requiredunable to perform certain tests on each lotassure sufficient quantities of the product before it is released for distribution. Ifto meet the product is subject to official release byneeds of patients with the FDA, the manufacturer submits samples of each lot of product to the FDA together with a release protocol showing a summary of the history of manufacture of the lot and the results of all manufacturer’s tests performed on the lot. The FDA may also perform certain confirmatory tests on lots of some products, such as viral vaccines, before releasing the lots for distribution by the manufacturer. In addition, the FDA conducts laboratory research related to the regulatory standards on the safety, purity, potency, and effectiveness of biological products. As with drugs, after approval of biologics, manufacturers must address any safety issues that arise, are subject to recallsrare disease or a halt in manufacturing, and are subject to periodic inspection after approval.condition.
Regulation of Manufacturing Process
Even when NDA or BLA approval is obtained, a marketed product, its manufacturer and its manufacturing facilities are subject to continual review and periodic inspections by the FDA. The manufacturing process for pharmaceutical products is highly regulated and regulators may shut down manufacturing facilities that they believe do not comply with regulations. Discovery of previously unknown problems with a product, manufacturer or facility may result in restrictions on the product, manufacturer or facility, including costly recalls or withdrawal of the product from the market. Manufacturing facilities are always subject to inspection by the applicable regulatory authorities.
We and our third-party manufacturers are subject to current Good Manufacturing Practices (“GMP”), which are extensive regulations governing manufacturing processes, including but not limited to stability testing, record-keeping and quality standards as defined by the FDA and the European Medicines Agency. Similar regulations are in effect in other countries. Manufacturing facilities are subject to inspection by the applicable regulatory authorities. These facilities, whether our own or our contract manufacturers, must be inspected before we can use them in commercial manufacturing of our related products. We or our contract manufacturers may not be able to comply with applicable GMP and FDA or other regulatory requirements. If we or our contract manufacturers fail to comply, we or our contract manufacturers may be subject to legal or regulatory action, such as suspension of manufacturing, seizure of product, or voluntary recall of product. Furthermore, continued compliance with applicable Good Manufacturing Practices will require continual expenditure of time, money and effort on the part of us or our contract manufacturers in the areas of production and quality control and record keeping and reporting to ensure full compliance.
Post-Approval RegulationRequirements
Any products manufactured or distributed by us pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to record-keeping, requirements, reporting of adverse experiences, with the drugperiodic reporting, product sampling and other reporting,distribution, and advertising and promotion restrictions. The FDA’s rules for advertising and promotion require, among other things, that our promotion be fairly balanced and adequately substantiated by clinical studies, and that we not promote our products for unapproved uses. We must also submit appropriate new and supplemental applications and obtain FDAof the product. After approval, for certainmost changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There also are continuing user fee requirements, under which FDA assesses an annual program fee for each product labeling or manufacturing process. On its own initiative,identified in an approved BLA. Biologic manufacturers and their subcontractors are required to register their establishments with the FDA may require changes to the labeling of an approved drug if it becomes aware of new safety information that the agency believes should be included in the approved drug’s labeling. The FDA also enforces the requirements of the Prescription Drug Marketing Act (“PDMA”) which, among other things, imposes various requirements in connection with the distribution of product samples to physicians.
In addition to inspections related to manufacturing, we may beand certain state agencies and are subject to periodic unannounced inspections by the FDA and other regulatory bodies relatedcertain state agencies for compliance with cGMP, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Changes to the other regulatorymanufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting requirements upon us and any third-party manufacturers that applywe may decide to marketed drugs manufactured or distributed by us. The FDA also may conduct periodic inspections regarding our reviewuse. Accordingly, manufacturers must continue to expend time, money and reportingeffort in the area of adverse events, or relatedproduction and quality control to maintain compliance with the requirementscGMP and other aspects of the PDMA concerning the handling of drug samples. When the FDA conducts an inspection, the inspectors will identify any deficiencies they believe exist in the form of a notice of inspectional observations. The observations may be more or less significant. If we receive a notice of inspectional observations, we likely will be required to respond in writing, and may be required to undertake corrective and preventive actions in order to address the FDA’s concerns.
There are a variety of state laws and regulations that apply in the states or localities where our drug candidates may be marketed. For example, we must comply with state laws that require the registration of manufacturers and wholesale distributors of pharmaceutical products in that state, including, in certain states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Any applicable state or local regulations may hinder our ability to market, or increase the cost of marketing, our products in those states or localities.
The FDA’s policies may change and additional government regulations may be enacted which could impose additional burdens or limitations on our ability to market products after approval. Moreover, increased attention to the containment of health care costs in the United States and in foreign markets could result in new government regulations which could have a material adverse effect on our business. We cannot predict the likelihood, nature or extent of adverse governmental regulation which might arise from future legislative or administrative action, either in the United States or abroad.
Marketing Exclusivityregulatory compliance.
The FDA may grant five yearswithdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of exclusivitypreviously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the United States forapproved labeling to add new safety information; imposition of post-market studies or clinical studies to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
| · | restrictions on the marketing or manufacturing of a product, complete withdrawal of the product from the market or product recalls; |
| · | fines, warning letters or holds on post-approval clinical studies; |
| · | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of existing product approvals; |
| · | product seizure or detention, or refusal of the FDA to permit the import or export of products; or |
| · | injunctions or the imposition of civil or criminal penalties. |
The FDA closely regulates the approvalmarketing, labeling, advertising and promotion of NDAs for new chemical entities,biologics. A company can make only those claims relating to safety and three yearsefficacy, purity and potency that are approved by the FDA and in accordance with the provisions of exclusivity for supplemental NDAs, forthe approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. Failure to comply with these requirements can result in, among other things, new indications, dosages or dosage forms of an existing drug if new clinical investigationsadverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available products for uses that were conducted or sponsoredare not described in the product’s labeling and that differ from those tested by us and approved by the applicantFDA. Such off-label uses are essential tocommon across medical specialties. Physicians may believe that such off-label uses are the approvalbest treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer’s communications on the supplemental application. Additionally, six monthssubject of marketing exclusivity in the United States is available if, in response to a written request from the FDA, a sponsor submits and the agency accepts requested information relating to theoff-label use of their products.
Biosimilars and Reference Product Exclusivity
The Patient Protection and Affordable Care Act, as amended by the approved drugHealth Care and Education Reconciliation Act, or collectively, the ACA, signed into law in the pediatric population. The six-month pediatric exclusivity is added to any existing patent or non-patent exclusivity period for which the drug is eligible. Orphan drug products are also eligible for pediatric exclusivity if the FDA requests and the company completes pediatric clinical trials. Under2010, includes a subtitle called the Biologics Price Competition and Innovation Act the FDA may grant 12 years of data exclusivity2009, or BPCIA, which created an abbreviated approval pathway for innovative biological products.
Health Law Complianceproducts that are biosimilar to or interchangeable with an FDA-approved reference biological product.
Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product in any given patient and, for products that are administered multiple times to an individual, the biologic and the reference biologic may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. Complexities associated with the larger, and often more complex, structures of biological products, as well as the processes by which such products are manufactured, pose significant hurdles to implementation of the abbreviated approval pathway that are still being worked out by the FDA.
Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing that applicant’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of its product. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products. At this juncture, it is unclear whether products deemed “interchangeable” by the FDA lawswill, in fact, be readily substituted by pharmacies, which are governed by state pharmacy law.
The BPCIA is complex and regulations, we mustcontinues to be interpreted and implemented by the FDA. In addition, recent government proposals have sought to reduce the 12-year reference product exclusivity period. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also comply with variousbeen the subject of recent litigation. As a result, the ultimate impact, implementation, and impact of the BPCIA is subject to significant uncertainty.
Other Healthcare Laws and Compliance Requirements
Pharmaceutical companies are subject to additional healthcare regulation and enforcement by the federal government and state lawsby authorities in the states and regulations pertaining to healthcare “fraud and abuse” lawsforeign jurisdictions in which govern, among other things, our relationships with healthcare providers, and organizations such as specialty pharmacies, wholesalers and group purchasing organizations relating to the marketing and pricing of prescription drug products.they conduct their business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security, and physician payment sunshine laws.
Thelimitation: the U.S. federal Anti-Kickback Statute, makes it illegal for any person or entity, including a prescription drug manufacturer (or a party acting on its behalf) towhich prohibits, among other things, persons and entities from knowingly and willfully directlysoliciting, receiving, offering or indirectly, solicit, receive, offer,paying remuneration, to induce, or pay any remuneration that is intended to inducein return for, either the referral of business, includingan individual, or the purchase order, leaseor recommendation of any good, facility,an item or service for which payment may be made under aany federal healthcare program, such as Medicare or Medicaid. The term “remuneration” has been broadly interpreted to include anything of value. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals ofprogram; federal healthcare covered business, the Anti-Kickback Statute has been violated.
Federalcivil and criminal false claims laws and false statementcivil monetary penalty laws, including the federal civil False Claims Act,which prohibit, among other things, any personindividuals or entityentities from knowingly presenting, or causing to be presented, claims for payment to or approval by,the federal programs,government, including Medicare and Medicaid, claims for items or services, including drugs,federal healthcare programs, that are false or fraudulent or not provided as claimed. Entities can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers, promoting a product off-label, or for providing medically unnecessary services or items.
The federal Health Insurance Portability and Accountability Act of 1996, orfraudulent; HIPAA, which created additional federal criminal statutes thatwhich prohibit, among other actions, knowingly and willfullythings, executing or attempting to execute, a scheme to defraud any healthcare benefit program including private third-party payors, knowingly and willfully embezzling or stealing from amaking false statements relating to healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense,matters, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services.
HIPAA,which, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, also imposes certain requirements on HIPAA covered entities and their implementing regulations, require certain types of individuals and entitiesbusiness associates relating to protect the privacy, security and electronic exchangetransmission of certain patient data.
Theindividually identifiable health information; the U.S. federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to annually report annually to the Centers for Medicare & Medicaid Services, or CMS,federal government, information related to payments or other transfers of value made to physicians, as defined by such law, and teaching hospitals, and applicable manufacturers and applicable group purchasing organizations to report annually to CMSas well as ownership and investment interests held by the physicians and their immediate family members.
Analogousmembers; and U.S. state and foreign law equivalents of each of the above federal laws, which, in some cases, differ from each other in significant ways, and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. Additionally, we may be subject to state laws thatnot have the same effect, thus complicating compliance efforts. In addition, certain states require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government. Further, we may be subject to state laws thatgovernment and certain states and local jurisdictions require drug manufacturers to report information related to payments and other transfersthe registration of value to physicians and other healthcare providers or marketing expenditures, as well as state and foreign laws governing the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.pharmaceutical sales representatives. If ourtheir operations are found to be in violation of any of these federal, state or foreignsuch laws or any other governmental regulations wethat apply, they may be subject to penalties, including, without limitation, significant civil, criminal and administrative or civil penalties, imprisonment, damages, fines, disgorgement, imprisonment, exclusion from participationgovernment-funded healthcare programs, such as Medicare and Medicaid or similar programs in government healthcare programs,other countries or jurisdictions, integrity oversight and reporting obligations to resolve allegations of non-compliance, disgorgement, imprisonment, contractual damages, reputational harm, diminished profits and future earnings, or the curtailment or restructuring of our operations.
ThereCoverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any pharmaceutical or biological product for which we obtain regulatory approval. Sales of any product depend, in part, on the extent to which such product will be covered by third-party payors, such as federal, state, and foreign government healthcare programs, commercial insurance and managed healthcare organizations, and the level of reimbursement for such product by third-party payors. Decisions regarding the extent of coverage and amount of reimbursement to be provided are also an increasingmade on a plan-by-plan basis. For products administered under the supervision of a physician, obtaining coverage and adequate reimbursement may be particularly difficult because of the higher prices often associated with such drugs. Additionally, separate reimbursement for the product itself or the treatment or procedure in which the product is used may not be available, which may impact physician utilization.
In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Third-party payors are increasingly challenging the prices charged for medical products and services, examining the medical necessity and reviewing the cost effectiveness of pharmaceutical or biological products, medical devices and medical services, in addition to questioning safety and efficacy. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit sales of any product. Decreases in third-party reimbursement for any product or a decision by a third-party payor not to cover a product could reduce physician usage and patient demand for the product. No regulatory authority has granted approval for a personalized cancer immunotherapy based on a vaccine approach, and there is no model for reimbursement of this type of product.
Healthcare Reform
The United States and some foreign jurisdictions are considering or have enacted a number of state laws that require manufacturersreform proposals to make reports to those states on certain pricing and marketing information. Many of these laws contain ambiguities as to whatchange the healthcare system. There is required to complysignificant interest in promoting changes in healthcare systems with the laws. Given the lackstated goals of clarity in laws and their implementation, our reporting actions could be subject to the penalty provisions of the state authorities.
Healthcare Reform and Reimbursement and Pricing Controls
There has been an increased focus on drug pricing in recent years in the United States. Although there are no direct government price controls over private sector purchases incontaining healthcare costs, improving quality or expanding access. In the United States, there are rebatesthe pharmaceutical industry has been a particular focus of these efforts and other financial requirements forhas been significantly affected by federal and state legislative initiatives, including those designed to limit the pricing, coverage, and reimbursement of pharmaceutical and biopharmaceutical products, especially under government-funded health care programs. The Medicare Modernization Act, enacted in December 2003, established the Medicare Part D outpatient prescriptionprograms, and increased governmental control of drug benefit, which is provided primarily through private entities that attempt to negotiate price concessions from pharmaceutical manufacturers. The health care reform legislation enacted in 2010, known as the Affordable Care Act, requires drug manufacturers to pay 50% of the Medicare Part D coverage gap, also known as the “donut hole,” on prescriptions for branded products filled when the beneficiary reaches this coverage. The Deficit Reduction Act of 2005 resulted in changes to the way drug prices are reported to the government and the formula using such information to calculate the required Medicaid rebates. The Affordable Care Act increased the minimum basic Medicaid rebate for branded prescription drugs from 15.1% to 23.1% and requires pharmaceutical manufacturers to pay states rebates on prescription drugs dispensed to Medicaid managed care enrollees. In addition, the Affordable Care Act increased the additional Medicaid rebate on “line extensions” (such as extended release formulations) of solid oral dosage forms of branded products, revised the definition of average manufacturer price by changing the classes of purchasers included in the calculation, and expanded the entities eligible for discounted pricing under the federal 340B drug pricing program. Current orphan drugs are excluded from the expanded 340B hospitals eligible for discounts.pricing.
The Affordable Care Act imposes a significant annual fee on companies that manufacture or import branded prescription drug products. The fee (which is not deductible for federal income tax purposes) is based onIn March 2010, the manufacturer’s market share of sales of branded drugs and biologics (excluding orphan drugs) to, or pursuant to coverage under, specified U.S. government programs. The Affordable Care Act also contains a number of provisions, including provisions governingACA was signed into law, which substantially changed the way that health carehealthcare is financed by both governmental and private insurers in the United States, and significantly affected the pharmaceutical industry. The ACA contained a number of provisions of particular importance to the pharmaceutical and biotechnology industries, including, but not limited to, those governing enrollment in federal healthcare programs, a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, and annual fees based on pharmaceutical companies’ share of sales to federal health care programs, reimbursement changes,programs. There remain judicial and Congressional challenges to certain aspects of the increased use of comparative effectiveness research in health care decision-making,ACA, and enhancementswe expect there will be additional challenges and amendments to fraud and abuse requirements and enforcement, that are affecting existing government health care programs and will resultthe ACA in the developmentfuture. For example, the Tax Cuts and Jobs Act was enacted, which, among other things, removed penalties for not complying with ACA’s individual mandate to carry health insurance. In addition, the 2020 federal spending package permanently eliminated, effective January 1, 2020, the ACA-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, also eliminates the health insurer tax. On December 14, 2018, a Texas U.S. District Court Judge ruled that the ACA is unconstitutional in its entirety because the individual mandate was repealed by Congress as part of new programs. The Affordable Care Act also contains requirementsthe Tax Cuts and Jobs Act. Additionally, on December 18, 2019, the U.S. Court of Appeals for manufacturersthe 5th Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to publicly report certain payments or other transfers of value madethe District Court to physicians and teaching hospitals. We are unable to predictdetermine whether the future course of federal or state health care legislation and regulations, including regulations that will be issued to implementremaining provisions of the Affordable Care Act. The Affordable Care ActACA are invalid as well. It is unclear how this decision, future decisions, subsequent appeals, and further changes inother efforts to repeal and replace the law or regulatory framework that reduceACA will impact the ACA and our revenues or increase our costs could also have a material adverse effect on our business, financial condition and results of operations and cash flows.business.
Public and private health care payors control costs and influence drug pricing through a variety of mechanisms, including through negotiating discounts with the manufacturers and through the use of tiered formularies and other mechanisms that provide preferential access to certain drugs over others within a therapeutic class. Payors also set other criteria to govern the uses of a drug that will be deemed medically appropriate and therefore reimbursed or otherwise covered. Payors may require physicians to seek approval from them before a product will be reimbursed or covered, commonly referred to as prior authorization. In particular, many public and private health care payors limit reimbursement and coverage to the uses of a drug that is either approved by the FDA or appears in a recognized drug compendium. Drug compendia are publications that summarize the available medical evidence for particular drug products and identify which uses of a drug are supported or not supported by the available evidence, whether or not such usesOther legislative changes have been approved byproposed and adopted since the FDA. ForACA was enacted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year and reduced payments to several types of Medicare providers. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. At the federal level, the Trump administration’s budget proposal for fiscal year 2020 contains further drug price control measures that could be enacted during the budget process or in other future legislation, including, for example, in the case ofmeasures to permit Medicare Part D coverage for oncology drugs, the Medicare Modernization Act, with certain exceptions, provides for Medicare coverage of unapproved uses of an FDA-approved drug if the unapproved use is reasonable and necessary and is supported by one or more citations in CMS-approved compendia, such as the National Comprehensive Cancer Network Drugs and Biologics Compendium. Different pricing and reimbursement schemes exist in other countries. For example, in the European Union, governments influenceplans to negotiate the price of pharmaceutical products through their pricingcertain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and reimbursement rulesto eliminate cost sharing for generic drugs for low-income patients. Further, the Trump administration released a “Blueprint”, or plan, to lower drug prices and controlreduce out of national health care systems that fund a large part of the cost of such products to consumers. The approach taken varies from member state to member state. Some jurisdictions operate positive or negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other member states allow companies to fix their own prices for medicines but monitor and control company profits and may limit or restrict reimbursement. The downward pressure on health carepocket costs in general, and prescription drugs in particular, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products, as exemplified by the actions of the National Institute for Clinical Excellence in the United Kingdom, which evaluates the data supporting new medicines and passes reimbursement recommendations to the government. In addition, in some countries, cross-border imports from low-priced markets (parallel imports) exert a commercial pressure on pricing within a country.
Other Federal and State Regulatory Requirements
The Centers for Medicare & Medicaid Services, or CMS, has issued a final rule that implements a statutory requirement under the Healthcare Reform Act that requires applicable manufacturers of drugs devices, biologicals, or medical supplies that are covered under Medicare, Medicaid, orcontains additional proposals to increase drug manufacturer competition, increase the Children’s Health Insurance Program, or CHIP,negotiating power of certain federal healthcare programs, incentivize manufacturers to begin collectinglower the list price of their products, and reporting annually information on payments or transfersreduce the out of value to physicians and teaching hospitals, as well as investment interests held by physicians and their immediate family members. Manufacturers had to begin collecting information in 2013, with the first reports due in 2014. On September 30, 2014, CMS posted the first round of data in searchable form on a public website. Failure to submit required information may result in civil monetary penalties.
In addition, several states now require prescription drug companies to report expenses relating to the marketing and promotionpocket costs of drug products paid by consumers. The Department of Health and Human Services, or HHS, has solicited feedback on some of these measures and has implemented others under its existing authority. While some measures may require additional authorization to report giftsbecome effective, Congress and paymentsthe Trump administration have each indicated that it will continue to individual physicians in these states. Other states prohibit various other marketing-related activities. Still other states require the posting of information relating to clinical trials and their outcomes. In addition, California, Connecticut, Nevada, and Massachusetts require pharmaceutical companies to implement compliance programsseek new legislative and/or administrative measures to control drug costs. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing codes. Several additional states are considering similar proposals. Compliance with these laws is difficultcost disclosure and time consuming,transparency measures, and, companies that do not comply with these state laws face civil penalties.in some cases, designed to encourage importation from other countries and bulk purchasing.
Product Liability and Insurance
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize any products. We have not experienced any product liability claims to date. We currently carry products and clinical trial liability insurance policies. There can be no assurance that liability claims will not exceed such insurance coverage limits, which could have a materially adverse effect on our business, financial condition or results of operations or that such insurance will continue to be available on commercially reasonable terms, if at all.
Human Resources
Employees
As of December 31, 2018,2019, we had 1128 full-time employees. ThreeThere were 18 in research, and development and eightclinical and 10 were in finance, legal, human resources or administrative support. None of our employees is subject to a collective bargaining agreement. We consider our relationship with our employees to be good.
Consultants
We have consulting agreements with a number of leading academic scientists, clinicians and regulatory experts. They serve as important contacts for us throughout the broader scientific and clinical communities. They are distinguished individuals with expertise in numerous fields, including cellular biology, molecular biology, oncology, clinical, manufacturing and regulatory.
We retain each consultant according to the terms of a consulting agreement. Under such agreements, we pay them a consulting fee and reimburse them for out-of-pocket expenses incurred in performing their services for us. In addition, some consultants hold options to purchase our common stock, subject to the vesting requirements contained in separate award agreements. Our consultants may be employed by other entities and therefore may have commitments to their employer or may have other consulting or advisory agreements that may limit their availability to us.
Available Information
Our website is located atwww.markertherapeutics.com. We make available free of charge on our website our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports, as soon as reasonably practicable after we electronically file or furnish such materials to the Securities and Exchange Commission. Our website and the information contained therein or connected thereto are not intended to be incorporated into this Annual Report on Form 10-K.
32
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below before making an investment decision in our securities. These risk factors are effective as of the date of this Form 10-K and shall be deemed to be modified or superseded to the extent that a statement contained in our future filings modifies or replaces such statement. All of these risks may impair our business operations. The forward-looking statements in this Form 10-K involve risks and uncertainties and actual results may differ materially from the results we discuss in the forward-looking statements. If any of the following risks actually occur, our business, financial condition or results of operations could be materially adversely affected. In that case, the trading price of our stock could decline, and you may lose all or part of your investmentinvestment.
Risks Related to our Business and Intellectual Property
We are a development stage company with a history of operating losses.
We are a clinical-stage immunotherapy company with a history of losses, and itwe may always operate at a loss. We expect that we will continue to operate at a loss throughout our development stage, and as a result, we may exhaust our financial resources and be unable to complete the development of our products.product candidates. We anticipate that our ongoing operational costs will increase significantly as we continue conducting our clinical development program. Our deficit will continue to grow during our drug development period. We have no sources of revenue to provide incoming cash flows to sustain our future operations. As outlined above, our ability to pursue our planned business activities depends upon our successful efforts to raise additional financing.
We have sustained losses from operations in each fiscal year since our inception, and we expect losses to continue for the indefinite future due to the substantial investment in research and development. As of December 31, 2018,2019, we had an accumulated deficit of approximately $306.1$327.5 million since inception. We expect to spend substantial additional sums on the continued administration and research and development of licensed and proprietary productsproduct candidates and technologies with no certainty that our approach and associated technologies will become commercially viable or profitable as a result of these expenditures. If we fail to raise a significant amount of capital, we may need to significantly curtail operations, allocate limited financial resources among our product candidates, or cease operations in the near future. If any of our product candidates fail in clinical trials or doesdo not gain regulatory approval, we may never generate revenue. Even if we generate revenue in the future, we may not be able to become profitable or sustain profitability in subsequent periods.
Our future success is highly dependent upon our key personnel, and our ability to attract, retain, and motivate additional qualified personnel.
Our ability to compete in the highly competitive biotechnology and pharmaceutical industries depends upon our ability to attract and retain highly qualified managerial, scientific, and medical personnel. We are highly dependent on our management, scientific, and medical personnel and consultants, including Peter Hoang, our President and Chief Executive Officer, Ann Leen, Ph.D., our Chief Scientific Officer, Juan Vera, M.D., our Chief Development Officer, and Mythili Koneru, M.D., Ph.D. our Senior Vice President, Clinical Development,Chief Medical Officer as well as others. The loss of the services of any of our executive officers, other key employees, and other scientific and medical advisors, and our inability to find suitable replacements could result in delays in product development and harm to our business. We have a priority to quickly train additional qualified scientific and medical personnel to ensure the ability to maintain business continuity. Any delays in training such personnel could delay the development, manufacture, and clinical trials of our product candidates.
Our ability to attract and retain highly skilled personnel is critical to our operations and expansion. We face competition for these types of personnel from other biotechnology companies and more established organizations, many of which have significantly larger operations and greater financial, technical, human and other resources than us. We may not be successful in attracting and retaining qualified personnel on a timely basis, on competitive terms, or at all. If we are not successful in attracting and retaining these personnel, or integrating them into our operations, our business, prospects, financial condition and results of operations will be materially adversely affected. In such circumstances, we may be unable to conduct certain research and development programs, unable to adequately manage our clinical trials and other products,development of our product candidates, and unable to adequately address our management needs.
Our strategic relationship with Baylor College of Medicine, or BCM, is dependent, in part, upon our relationship with key medical and scientific personnel and advisors.
Our MultiTAAMultiTAA-specific T cell therapy has been developed through our collaboration with the Center for Cell and Gene Therapy at BCM, founded by Malcolm K. Brenner, M.D., Ph.D., a recognized pioneer in immuno-oncology. In addition to Dr. Brenner, Marker Cell’sOur founders include Juan Vera, M.D., Ann Leen, Ph.D., Juan Vera, M.D., Helen Heslop, M.D., DSc (Hon) and Cliona Rooney, Ph.D., who all have significant experience in this field and are all affiliated with the Center for Cell and Gene Therapy at BCM. Dr. Leen and Dr. Vera areis our Chief Scientific Officer and Chief Development Officer, respectively.Officer. In addition, Dr. Brenner, Dr. Heslop and Dr. Rooney have joined our newly-formed Scientific Advisory Board.
Our strategic relationship with BCM is dependent, in part, on our relationship with these key employees and advisors, and in particular Dr. Leen and Dr. Vera, who areis also employed with the Center for Cell and Gene Therapy at BCM. If we lose Dr. Leen or Dr. Vera, or if eitherhe leaves theirhis position at BCM, our relationship with BCM may deteriorate, and our business could be harmed.
We, and certain of our key medical and scientific personnel, will need additional agreements in place with BCM to expand our development, manufacture, and clinical trial efforts.
Although we have an exclusive license agreement with BCM under which we received a worldwide, exclusive license to BCM’s rights in and to three patent families to develop and commercialize the MultiTAAMultiTAA-specific T cell product candidates, we will need to enter into additional agreements with BCM with respect to (i) a strategic alliance to advance pre-clinical research, early stage clinical trials, and Phase II2 clinical trials with respect to our product candidates, as well as continued access to our clinical data, and (ii) product manufacturing and support, including personnel and space at the institution for the foreseeable future. Any delays in entering into new strategic agreements with BCM related to our product candidates could delay the development, manufacture, and clinical trials of our product candidates.
The multiple roles of certain of Dr. Vera, our officersChief Development Officer, and directorsJohn Wilson, our director, could limit their time and availability to us, and create, or appear to create, conflicts of interest.
Dr. LeenVera is a co-founder and Dr. Vera are employeesmember of BCM and are contractually obligated to spend a significant portion of their time with BCM. In addition, Dr. Leen and Dr. Vera are co-founders and members of ViraCyte and perform services from time to time for ViraCyte LLC (“ViraCyte”). ViraCyteAllovir Inc., or Allovir. Allovir is owned by the same principal stockholder group as Marker Cellus prior to the Mergerour merger with TapImmune, Inc. and has technology which is being developed under a license agreement with BCM by the same research group at BCM. ViraCyteAllovir is a clinical-stage biopharmaceutical company whichthat is investigating and developing virus-specific T cell therapy technology for the prevention and/or treatment of viral infections. Accordingly, Dr. Leen and Dr. Vera may have other commitments that would, at times, limit theirhis availability to us. Other research being conducted by Dr. Leen and Dr. Vera may, at times, receive higher priority than research on our programs, which may, in turn, delay the development or commercialization of our product candidates.
In addition, John Wilson is a co-founder, member and director and officer of ViraCyteAllovir and is a director of the Company. Dr. Leen and Dr. Vera are also co-founders and members of ViraCyte, and perform services for ViraCyte from time to time, and Dr. Vera is a director of the Company. Allour company. Both of these individuals have certain fiduciary or other obligations to us and certain fiduciary or other obligations to ViraCyteAllovir and, in the case of Dr. Leen and Dr. Vera to BCM. Such multiple obligations may in the future result in a conflict of interest with respect to presenting other potential business opportunities to us or to ViraCyte.Allovir. A conflict of interest also may arise concerning the timing of the parties’ planned and ongoing clinical trials, investigational new drug application filings and the parties’ opportunities for marketing their respective product candidates. In addition, they may be faced with decisions that could have different implications for us than for ViraCyte.Allovir. Consequently, there is no assurance that these members of our board and management will always act in our best interests in all situations should a conflict arise.
We have not yet sold any products or received regulatory approval to sell our products.any product candidates.
We have no approved products or productsproduct candidates pending approval. As a result, we have not derived any revenue from the sales of products and have not yet demonstrated ability to obtain regulatory approval, formulate and manufacture commercial-scale products, or conduct sales and marketing activities necessary for successful product commercialization. Without revenue, we can only finance our operations through debt and equity financings.
Product development involves a lengthy and expensive process with an uncertain outcome, and results of earlier pre-clinical and clinical trials may not be predictive of future clinical trial results.
Clinical testing is expensive and generally takes many years to complete, and the outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of pre-clinical testing and early clinical trials of our product candidates may not be predictive of the results of larger, later-stage controlled clinical trials. Product candidates that have shown promising results in early-stage clinical trials may still suffer significant setbacks in subsequent clinical trials. Our clinical trials to date have been conducted on a small number of patients in a single academic clinical site for a limited number of indications. We will have to conduct larger, well-controlled trials in our proposed indications at multiple sites to verify the results obtained to date and to support any regulatory submissions for further clinical development of our product candidates. Our assumptions related to our products,product candidates, such as with respect to lack of toxicity and manufacturing cost estimates, are based on early limited clinical trials and current manufacturing processes at BCM and may prove to be incorrect. In addition, the initial estimates of the clinical cost of development may prove to be inadequate, particularly if clinical trial timing or outcome is different than predicted or regulatory agencies require further testing before approval. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles despite promising results in earlier, smaller clinical trials. Moreover, clinical data are often susceptible to varying interpretations and analyses. We do not know whether any Phase II,2, Phase III,3, or other clinical trials we may conduct will demonstrate consistent or adequate efficacy and safety with respect to the proposed indication for use sufficient to receive regulatory approval or market our product candidates.
The biotechnology and immunotherapy industries are characterized by rapid technological developments and a high degree of competition. We may be unable to compete with more substantial enterprises.
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition. As a result, our actual or proposed immunotherapies could become obsolete before we recoup any portion of our related research and development and commercialization expenses. Competition in the biopharmaceutical industry is based significantly on scientific and technological factors. These factors include the availability of patent and other protection for technology and products, the ability to commercialize technological developments and the ability to obtain governmental approval for testing, manufacturing and marketing. We compete with specialized biopharmaceutical firms in the United States, Europe and elsewhere, as well as a growing number of large pharmaceutical companies that are applying biotechnology to their operations. Many biopharmaceutical companies have focused their development efforts in the human therapeutics area, including cancer. Many major pharmaceutical companies have developed or acquired internal biotechnology capabilities or made commercial arrangements with other biopharmaceutical companies. These companies, as well as academic institutions, governmental agencies and private research organizations, also compete with us in recruiting and retaining highly qualified scientific personnel and consultants. Our ability to compete successfully with other companies in the pharmaceutical field will also depend to a considerable degree on the continuing availability of capital to us.
We are aware of certain investigational new drugs under development or approved products by competitors that are used for the prevention, diagnosis, or treatment of certain diseases we have targeted for drug development. Various companies are developing biopharmaceutical products that have the potential to directly compete with our immunotherapies even though their approach may be different. The competition comes from both biotechnology firms and from major pharmaceutical companies. Many of these companies have substantially greater financial, marketing, and human resources than us. We also experience competition in the development of our immunotherapies from universities, other research institutions and others in acquiring technology from such universities and institutions.
In addition, certain of our immunotherapies may be subject to competition from investigational new drugs and/or products developed using other technologies, some of which have completed numerous clinical trials.
We are subject to numerous risks inherent in conducting clinical trials.trials.
We outsource some of the management of our clinical trials to third parties. Agreements with clinical investigators and medical institutions for clinical testing and with other third parties for data management services, place substantial responsibilities on these parties that, if unmet, could result in delays in, or termination of, our clinical trials. If any of our clinical trial sites fail to comply with FDA-approved good clinical practices, we may be unable to use the data gathered at those sites. If these clinical investigators, medical institutions or other third parties do not carry out their contractual duties or obligations or fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to their failure to adhere to our clinical protocols or for other reasons, our clinical trials may be extended, delayed or terminated, and we may be unable to obtain regulatory approval for, or successfully commercialize, agents. We cannot be certain that we will successfully recruit enough patients to complete our clinical trials nor that we will reach our primary endpoints. Delays in recruitment, lack of clinical benefit or unacceptable side effects would delay our clinical trials.
We, or our regulators, may suspend or terminate our clinical trials for a variety of reasons. For example, in the fourth quarter of 2019, the FDA placed a clinical hold on our IND with respect to our MultiTAA-specific T cell therapy for the treatment of patients with post-transplant AML. The FDA requested additional information regarding certain quality and technical specifications for two reagents supplied by third party vendors that are used in our manufacturing process but not present in the final product infused to patients. In February 2020, we announced that the FDA lifted the clinical hold, permitting us to initiate a Phase 2 clinical trial with a safety lead-in portion but placed a partial clinical hold on the trial for the use of the MultiTAA-specific T cell product manufactured using one of the reagents supplied by the alternative supplier until the final data and certificate of analysis for the reagent are reviewed and accepted by the FDA. We currently estimate that the alternative supplier will deliver the final reagent, along with the final data and certificate of analysis required by the FDA, by the end of the second quarter of 2020, thereby satisfying the requirements for lifting the partial hold on the clinical trial. However, FDA may not agree that our response addresses all of their concerns and the clinical hold may remain in place and further delay the initiation of the trial.
We may voluntarily suspend or terminate our clinical trials at any time if we believe they present an unacceptable risk to the patients enrolled in our clinical trials or do not demonstrate clinical benefit. For example, in November 2019 we elected to suspend our Phase 2 clinical trial of TPIV200 for the treatment of platinum-sensitive advanced ovarian cancer based on an unblinded review of interim results conducted by an independent Data and Safety Monitoring Board, or DSMB. Although the DSMB did not express any safety concerns with respect to TPIV200, we elected to suspend the trial because it did not meet the threshold for probability of clinical benefit based upon our pre-specified criteria. In addition, regulatory agencies may order the temporary or permanent discontinuation of our clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the patients enrolled in our clinical trials.
Our clinical trial operations are subject to regulatory inspections at any time. If regulatory inspectors conclude that we or our clinical trial sites are not in compliance with applicable regulatory requirements for conducting clinical trials, we may receive reports of observations or warning letters detailing deficiencies, and we will be required to implement corrective actions. If regulatory agencies deem our responses to be inadequate, or are dissatisfied with the corrective actions we or our clinical trial sites have implemented, our clinical trials may be temporarily or permanently discontinued, and we may be fined, we or our investigators may be precluded from conducting any ongoing or any future clinical trials, the government may refuse to approve our marketing applications or allow us to manufacture or market our products, and we may be criminally prosecuted.
The lengthy approval process, as well as the unpredictability of future clinical trial results, may result in us failing to obtain regulatory approval for our product candidates, which would materially harm our business, results of operations and prospects.
The successful development of immunotherapies is highly uncertain.
Successful development of biopharmaceuticals is highly uncertain and depends on numerous factors, many of which are beyond our control. Immunotherapies that appear promising in the early phases of development may fail to reach the market for several reasons including:
| · | clinical study results that may show the immunotherapy to be less effective than expected (e.g., the study failed to meet its primary endpoint) or to have unacceptable side effects; |
| · | failure to receive the necessary regulatory approvals or a delay in receiving such approvals. Among other things, such delays may be caused by slow enrollment in clinical studies, length of time to achieve study endpoints, additional time requirements for data analysis, or |
| · | manufacturing costs, formulation issues, pricing or reimbursement issues, or other factors that make the immunotherapy uneconomical; and |
| · | the proprietary rights of others and their competing products and technologies that may prevent the immunotherapy from being commercialized. |
Success in preclinical and early clinical studies does not ensure that large-scale clinical studiestrials will be successful. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. The length of time necessary to complete clinical studies and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly from one immunotherapy to the next and may be difficult to predict.
Even if we are successful in getting market approval, commercial success of any of our product candidates will also depend in large part on the availability of coverage and adequate reimbursement from third-party payors, including government payors such as the Medicare and Medicaid programs and managed care organizations, which may be affected by existing and future health care reform measures designed to reduce the cost of health care. Third-party payors could require us to conduct additional studies, including post-marketing studies related to the cost effectiveness of a product, to qualify for reimbursement, which could be costly and divert our resources. If government and other health care payors were not to provide adequate coverage and reimbursement levels for any of our products onceif approved, market acceptance and commercial success would be reduced.
In addition, if one of our products is approved for marketing, we will be subject to significant regulatory obligations regarding the submission of safety and other post-marketing information and reports and registration, and will need to continue to comply (or ensure that our third-party providers comply) with current Good Manufacturing Practices, (“cGMPs”)or cGMPs, and current Good Clinical Practices (“cGCPs”)or cGCPs for any clinical trials that we conduct post-approval. In addition, there is always the risk that we or a regulatory authority might identify previously unknown problems with a product post-approval, such as adverse events of unanticipated severity or frequency. Compliance with these requirements is costly, and any failure to comply or other issues with our product candidates’ post-market approval could have a material adverse effect on our business, financial condition and results of operations.
It may take longer and cost more to complete our clinical trials than we project, or we may not be able to complete them at all.
For budgeting and planning purposes, we have projected the dates for the commencement, continuation, and completion of our various clinical trials. However, a number of factors, including scheduling conflicts with participating clinicians and clinical institutions, difficulties in identifying and enrolling patients who meet trial eligibility criteria, and competition for such eligible patents from other clinical trials, may cause significant delays. We may not commence or complete clinical trials involving any of our productsproduct candidates as projected or may not conduct them successfully.
DuringFor example, the FDA placed a partial clinical hold on our Phase 2 trial in post-transplant AML for the use of the MultiTAA-specific T cell product manufactured using one of the reagents supplied by the alternative supplier until the final data and certificate of analysis for the reagent are reviewed and accepted by the FDA. While we currently estimate that the alternative supplier will deliver the final reagent, along with the final data and certificate of analysis required by the FDA, by the end of the second quarter of 2020, and that we will complete enrollment of the first three patients and submission of the final technical specifications and comparability data of the new reagents to the FDA during the second half of 2012, BCM began enrollment of2020, thereby satisfying the investigator-sponsored, Phase 1requirements for lifting the partial hold on the clinical trial, we cannot guarantee that we will be timely, or successful, in doing so. If we are unable to establishsatisfy the feasibility of one ofFDA’s requirements to lift the partial hold, we will be delayed in commencing our lead products, MAPP, and to assess its overall safety, inclusion of multiple antigens, and dosage tolerance in patients with lymphoma. During the second quarter of 2016, BCM began enrollment of the investigator-sponsored Phase 1 clinical trial to establish the feasibility of one of our lead products, LAPP, and to assess its overall safety, inclusion of multiple antigens, and dosage tolerance in patients with acute myeloid leukemia (“AML”)/myelodysplastic syndromes (“MDS”). However, we2 trial.
We may experience difficulties in patient enrollment in our future clinical trials for a variety of reasons. The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the study until its conclusion. In addition, our clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as our product candidates, and this competition will reduce the number and types of patients available to us, because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Accordingly, we cannot guarantee that our clinical trials will progress as planned or as scheduled. Delays in patient enrollment may result in increased costs or may affect the timing or outcome of our ongoing clinical trial and planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates.
We rely on medical institutions, academic institutions, and clinical research organizations to conduct, supervise, or monitor some or all aspects of clinical trials involving our products.product candidates. We may have less control over the timing and other aspects of these clinical trials than if we conducted them entirely on our own. If we fail to commence or complete, or experiences delays in, any of our planned clinical trials, we may experience delays in our clinical development and/or commercialization plans.
In particular, while BCM will continue to support our trials with production of MAPP and LAPPMultiTAA-specific T cells under contract, we anticipate that we will have to rely on third parties (contractcontract manufacturing organizations or “CMOs”)CMOs or internal facilities yet to be developed for the commercial manufacture of our multi-antigen specific T cell therapy productsproduct candidates for clinical trials and eventual licensure. If they fail to commence or complete, or experience delays in, manufacturing our multi-antigen specific T cell therapy products,product candidates, our planned clinical trials with respect to such productsproduct candidates will be delayed, and we may experience delays in our clinical development and/or commercialization plans.
Clinical trials are expensive, time-consuming, and difficult to design and implement, and our clinical trial costs may be higher than for more conventional therapeutic technologies or drug products.
Clinical trials are expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. Because our product candidates are based on new technologies and manufactured on a patient-by-patient basis for our MultiTAAMultiTAA-specific T cell product candidates we expect that they will require extensive research and development and have substantial manufacturing costs. In addition, costs to treat patients with relapsed/refractory cancer and to treat potential side effects that may result from our product candidates can be significant. Some clinical trial sites may not bill, or obtain coverage from, Medicare, Medicaid, or other third-party payors for some or all of these costs for patients enrolled in our clinical trials, and we may be required by those trial sites to pay such costs. Accordingly, our clinical trial costs may be significantly higher per patient than those of more conventional therapeutic technologies or drug products. In addition, our proposed personalized product candidates involve several complex manufacturing and processing steps, the costs of which will be borne by us. Depending on the number of patients we ultimately enroll in our trials, and the number of trials we may need to conduct, our overall clinical trial costs may be higher than for more conventional treatments.
Our clinical trials may fail to demonstrate adequately the safety and efficacy of our product candidates, which would prevent or delay regulatory approval and commercialization.
The clinical trials of our product candidates are, and the manufacturing and marketing of ourany approved products will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where we intend to test and market our product candidates. Before obtaining regulatory approvals for the commercial sale of any of our product candidates, we must demonstrate through lengthy, complex, and expensive preclinical testing and clinical trials that our product candidates are both safe and effective for use in each target indication. In particular, because our product candidates are subject to regulation as biological drug products, we will need to demonstrate that they are safe, pure and potent for use in their target indications. Each product candidate must demonstrate an adequate risk versus benefit profile in its intended patient population and for its intended use. The risk/benefit profile required for product licensure will vary depending on these factors and may include not only the ability to show tumor shrinkage, but also adequate duration of response, a delay in the progression of the disease, and/or an improvement in survival. For example, response rates from the use of our product candidates may not be sufficient to obtain regulatory approval unless we can also show an adequate duration of response. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials. The results of studies in one set of patients or line of treatment may not be predictive of those obtained in another. In addition, we expect that there may be greater variability in results for products processed and administered on a patient-by-patient basis, as anticipated for our MultiTAAMultiTAA-specific T cell product candidates, than for “off-the-shelf” products, like many other drugs. There is typically an extremely high rate of attrition from the failure of product candidates proceeding through clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy profile despite having progressed through preclinical studies and initial clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or unacceptable safety issues, notwithstanding promising results in earlier trials. Most product candidates that begin clinical trials are never approved by regulatory authorities for commercialization.
In addition, even if such trials are successfully completed, we cannot guarantee that the FDA or foreign regulatory authorities will interpret the results as we do, and more trials could be required before we submit our product candidates for approval. To the extent that the results of the trials are not satisfactory to the FDA or foreign regulatory authorities for support of a marketing application, we may be required to expend significant resources, which may not be available to us, to conduct additional trials in support of potential approval of our product candidates.
If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until its conclusion. We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons, including:
In addition, our clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as our product candidates. This competition will reduce the number and types of patients available to us, because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Because the number of qualified clinical investigators is limited, we expect to conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials at such clinical trial sites. Moreover, because our product candidates represent a departure from more commonly used methods of cancer treatment, potential patients and their doctors may be inclined to use conventional therapies, such as chemotherapy and approved immunotherapies, rather than enroll patients in any future clinical trial. In addition, potential enrollees in our MultiTAA T cell product clinical trials may opt to participate in alternate clinical trials because of the length of time between the time that the patient’s or the donor’s blood is drawn and the time when the product is infused back into the patient.
Even if we can enroll a sufficient number of patients in our clinical trials, delays in patient enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates.
Our product candidates may cause undesirable side effects or have other properties that could halt their clinical development, prevent their regulatory approval, limit their commercial potential, or result in significant negative consequences.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay, or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authorities. Results of our trials could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics.
If unacceptable toxicities arise in the development of our product candidates, we or the FDA or comparable foreign regulatory authorities could order us to cease clinical trials or deny approval of our product candidates for any or all targeted indications. Treatment-related side effects could also affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff, as toxicities resulting from personalized cell therapy, as with our MultiTAAMultiTAA-specific T cell therapy products, are not normally encountered in the general patient population and by medical personnel. Any of these occurrences may harm our business, financial condition and prospects significantly.
Our MultiTAAMultiTAA-specific T cell therapy research and development efforts are to a large extent dependent upon BCM’s investigators.
It will take time to fully develop our research and development infrastructure. WeWhile we are conducting some research and development activities internally, we currently depend upon and will continue to depend upon independent investigators and collaborators, such as BCM, and which in the future may include other universities, medical institutions, and strategic partners, to conduct our preclinical studies and clinical trials. If we need to enter into alternative arrangements, our product development activities would be delayed. Agreements with such third parties might terminate for a variety of reasons, including a failure to perform by the third parties.
We expect to use the results of BCM’s research to support the filing with the FDA of IND applications to conduct more advanced clinical trials of our products.product candidates. However, we have limited control over the nature or timing of BCM’s clinical trials and limited visibility into their day-to-day activities. The research we are funding constitutes only a small portion of BCM’s overall research. Other research being conducted by Dr. Ann Leen and Dr. Juan Vera may at times receive higher priority than research on our programs. These factors could adversely affect the timing of our IND filings and our ability to conduct future planned clinical trials.
We will be unable to seek regulatory approval of or commercialize our products if our trials are not successful.
Our research and development programs are at an early stage. We must demonstrate our products’ safety and efficacy in humans through extensive clinical testing. We may experience numerous unforeseen events during, or as a result of, the testing process that could delay or prevent commercialization of our products, including but not limited to the following:
| · | safety and efficacy results in various human clinical trials reported in scientific and medical literature may not be indicative of results we obtain in our clinical trials; |
| · | after reviewing trial results, we or our collaborators may abandon |
| · | we, our collaborators or regulators, may suspend or terminate clinical trials if the participating subjects or patients are being exposed to unacceptable health risks; and |
| · | the effects our potential |
Clinical testing is very expensive, can take many years, and the outcome is uncertain. For example, it can take as much as 12 months or more before we learn the results from any clinical trial using our MultiTAAMultiTAA-specific T cell therapy. The data collected from our clinical trials may not be sufficient to support approval by the FDA of our MultiTAAMultiTAA-specific T cell therapy-based product candidates for the treatment of hematological malignancies, or our Folate Receptor Alpha (TPIV200) product for breast and ovarian cancers, HER2/neu peptide antigen product (TPIV100/110) or possible future clinical trials utilizing our DNA expression PolyStart™ product.malignancies. The clinical trials for our productsproduct candidates under development may not be completed on schedule and the FDA may not ultimately approve any of our product candidates for commercial sale. If we fail to adequately demonstrate the safety and efficacy of any product candidate under development, we may not receive regulatory approval for those products,product candidates, which would prevent us from generating revenues or achieving profitability.
We may not be able to expand our manufacturing processes to other third-party manufacturing facilities or successfully create our own manufacturing infrastructure for supply of our requirements of product candidates for use in clinical trials and for commercial sale.
We do not own any facility that may be used as our clinical-scale manufacturing and processing facility. We currently rely on third-party Contract Manufacturing Organizations, or CMOs, for manufacture of our vaccine products. Weproduct candidates. For 2020, we anticipate that we will initially rely solely on the Good Manufacturing Practices (“cGMP”)cGMP manufacturing facility within BCM for the manufacturing of our MultiTAAMultiTAA-specific T cell therapy-based product candidates. If the cGMP manufacturing facility of BCM, which does manufacture for itself and other parties, experiences capacity constraints, disruptions, or delays in manufacturing our MultiTAAMultiTAA-specific T cell therapy-based product candidate products,candidates, our planned clinical trials and necessary manufacturing capabilities will be disrupted or delayed, which will adversely affect our ability to conduct and further develop our business as currently planned. Further, the cGMP manufacturing facility is most likely too small to conduct the pivotal clinical studies being planned by us, so we will need to develop our own cGMP manufacturing capacity that will be adequate for such clinical trials with respect to our MultiTAAMultiTAA-specific T cell therapy-based product candidates.
In 2019 or in 2020, we intend to begin developing additional cGMP manufacturing capacity of our own that would be capable of supporting our manufacturing needs with respect to our clinical trials, particularly with respect to pivotal studies. OurWe intend to begin a process technology transfer to develop in-house manufacturing strategy going forward will involve the use of one or more CMOs or we will establish our own capabilities and infrastructure, including a manufacturing facility.in 2021. Establishment of our own manufacturing facility is subject to many risks. For example, the establishment of a cell-therapy manufacturing facility is a complex endeavor requiring knowledgeable individuals. Creating an internal manufacturing infrastructure will rely upon building out a complex facility and finding personnel with an appropriate background and training to staff and operate the facility. Should we be unable to find these individuals, we may need to rely on external contractors or train additional personnel to fill needed roles. There are a small number of individuals with experience in cell therapy, and the competition for these individuals is high.
We expect that the development of our own manufacturing facility couldwill provide us with enhanced control of material supply for both clinical trials and the commercial market, enable the more rapid implementation of process changes, and allow for better long-term margins. However, we do not have anyextensive experience in developing a manufacturing facility and may never be successful in developing our own manufacturing facility or capability. We may establish multiple manufacturing facilities as we expand our commercial footprint to multiple geographies, which may lead to regulatory delays or prove costly. Even if we are successful, our manufacturing capabilities could be affected by cost-overruns, unexpected delays, equipment failures, labor shortages, natural disasters, power failures, transportation difficulties and numerous other factors that could prevent us from realizing the intended benefits of our manufacturing strategy and have a material adverse effect on our clinical development and/or commercialization plans.
In addition, the manufacturing process for any productsproduct candidates that we may develop is subject to the FDA and foreign regulatory authority approval process, and we will need to contract with manufacturers who can meet all applicable FDA and foreign regulatory authority requirements on an ongoing basis. If we or our CMOs are unable to reliably produce products to specifications acceptable to the FDA, or other regulatory authorities, we may not obtain or maintain the approvals we need to commercialize suchany approved products. Even if we obtain regulatory approval for any of our product candidates, there is no assurance that either we or our CMOs will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product, or to meet potential future demand. Any of these challenges could delay completion of clinical trials, require bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidate, impair commercialization efforts, increase our cost of goods, and have an adverse effect on our clinical development and/or commercialization plans.
Regardless of whetherWhether we engage additional CMOs to manufacture our productsproduct candidates or establish our own manufacturing facility, in order to transfer our MultiTAAMultiTAA-specific T cell manufacturing from or expand our manufacturing capabilities beyond BCM pursuant to our development plans, whether through additional third parties or by developing our own manufacturing capabilities, we will need access to the Standard Operating Procedures (“SOPs”)standard operating procedures and the specific Batch Production Recordsbatch production records that are used to manufacture the product candidates. If BCM fails todoes not support the transfer of our manufacturing processes or impedes our ability to transfer the manufacturing processes of its productsproduct candidates to us, or third-party manufacturers, our planned clinical trials and additional necessary manufacturing capabilities will be delayed, which will adversely affect our ability to conduct and further develop our business as currently planned.
We will be dependent on third-party vendors to design, build, maintain and support our manufacturing and cell processing facilities.
As a result of our strategy to outsource our manufacturing, we will rely very heavily on BCM and other third-party manufacturers to perform the manufacturing of our productsproduct candidates for our clinical trials. We license our technology from others. We intend to rely on our contract manufacturers to produce large quantities of materials needed for clinical trials and potential product commercialization. Third-party manufacturers may not be able to meet our needs concerning timing, quantity, or quality. If we are unable to contract for a sufficient supply of needed materials on acceptable terms, or if we should encounter delays or difficulties in our relationships with manufacturers, our clinical trials may be delayed, thereby delaying the submission of productsproduct candidates for regulatory approval or the market introduction and subsequent sales of ourany approved products. Any such delay may lower our revenues and potential profitability. If any third party breaches or terminates its agreement with us or fails to conduct its activities in a timely manner, the commercialization of our products under developmentproduct candidates could be slowed down or blocked completely. It is possible that third parties relied upon by us will change their strategic focus, pursue alternative technologies, or develop alternative products,product candidates, either on their own or in collaboration with others, as a means for developing treatments for the diseases targeted by our collaborative programs, or for other reasons. The effectiveness of these third parties in marketing their own products may also affect our revenues and earnings.
We intend to continue to enter into additional third-party agreements in the future. However, we may not be able to negotiate any additional agreements successfully. Even if established, these relationships may not be scientifically or commercially successful.
Our manufacturing process is reliant upon the specialized equipment, and other specialty materials, which may not be available to us on acceptable terms or at all. For some of this equipment and materials, we rely or may rely on sole-source vendors or a limited number of vendors, which could impair our ability to manufacture and supply our products.product candidates.
We will depend on a limited number of vendors for supply of certain materials and equipment used in the manufacture of our MultiTAAMultiTAA-specific T cell therapy-based product candidates. For example, we will purchase equipment and reagents critical for the manufacture of our product candidates from Wilson Wolf (a company controlled by our director John Wilson, who is a director of the Company)Wilson), JPT Peptide Technologies and other suppliers. Some of our suppliers may not have the capacity to support commercial products manufactured under cGMP by biopharmaceutical firms or may otherwise be ill-equipped to support our needs. We also may not have supply contracts with many of these suppliers and may not be able to obtain supply contracts with them on acceptable terms or at all. Accordingly, we may not be able to obtain key materials and equipment to support clinical or commercial manufacturing.
For some of this equipment and materials, we may rely, and may now and/or in the future rely, on sole-source vendors or a limited number of vendors. An inability to continue to source product from any of these suppliers, which could be due to regulatory actions or requirements affecting the supplier, adverse financial, or other strategic developments experienced by a supplier, labor disputes or shortages, unexpected demands, or quality issues, could adversely affect our ability to satisfy demand for our product candidates, which could adversely and materially affect our operating results or our ability to conduct clinical trials, either of which could significantly harm our business.
As we continue to develop and scale our manufacturing process, we may need to obtain rights to and supplies of specific materials and equipment to be used as part of that process. For example, our MultiTAAMultiTAA-specific T cell manufacturing process is based, in part, upon the G-Rex® cell culture device manufactured by Wilson Wolf, which is used by many cell therapy developers, both in commercial and academic settings. WeAlthough we do not own any exclusive rightshold the license to the G-Rex®patents from BCM that could be used to prevent third parties from developing similar and competing processes.processes, we do not own any exclusive rights to the G-Rex®. We may not be able to obtain rights to such materials and equipment on commercially reasonable terms, or at all, and if we are unable to alter our process in a commercially viable manner to avoid the use of such materials or find a suitable substitute, it would have a material adverse effect on our business.
The manufacture of our product candidates is complex, and we may encounter difficulties in production, particularly with respect to process development or scaling up of our manufacturing capabilities. If we, or any of our third-party manufacturers encounter such difficulties, our ability to supply our product candidates for clinical trials, or our productsproduct candidates for patients, if approved, could be delayed or stopped, or we may be unable to maintain a commercially viable cost structure.
Our product candidates are biologics, and the process of manufacturing our productsproduct candidates is complex, highly regulated and subject to multiple risks. For example, the manufacture of our MultiTAAMultiTAA-specific T cell therapy-based product candidates involves complex processes, including drawing blood from patients/donors, manufacturing the clinical product, and ultimately infusing the product into a patient. As a result of the complexities, the cost to manufacture biologics is generally higher than traditional small molecule chemical compounds, and the manufacturing process is less reliable and is more difficult to reproduce. Our manufacturing processes will be susceptible to product loss or failure due to any of the following: logistical issues associated with the collection of blood cells, or starting material, from the patient or a donor, shipping such material to the manufacturing site, shipping the final product back to the patient, and infusing the patient with the product; manufacturing issues associated with the differencesvariability in patients’ or donor’s starting cells; interruptions in the manufacturing process; contamination; equipment failure; improper installation or operation of equipment, vendor or operator error; inconsistency in cell growth; and variability in product characteristics. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects, and other supply disruptions. If for any reason we lose a patient’s or a donor’s cells, or later-developed product at any point in the process, the manufacturing process for that patient will need to be restarted and the resulting delay may adversely affect that patient’s outcome and/or the results of clinical trials. If microbial, viral, or other contaminations are discovered in our product candidates or in the manufacturing facilities in which our product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination.
Because our MultiTAAMultiTAA-specific T cell therapy-based product candidates are manufactured for each particular patient, we will be required to maintain a chain of identity with respect to the patient’s/donor’s blood cells as it moves from the patient to the manufacturing facility, through the manufacturing process, and back to the patient. Maintaining such a chain of identity is difficult and complex, and failure to do so could result in adverse patient outcomes, loss of product, or regulatory action including withdrawal of our productsproduct candidates from the market. Further, as product candidates are developed through preclinical to late stage clinical trials towards approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods, are altered along the way in an effort to optimize processes and results. Such changes carry the risk that they will not achieve these intended objectives, and any of these changes could cause our product candidates to perform differently and affect the results of planned clinical trials or other future clinical trials.
Currently, our product candidates are manufactured using processes developed by BCM, our third-party research institution collaborator. Although we are working to develop our own commercially viable processes, doing so is a difficult and uncertain task, and there are risks associated with scaling to the level required for advanced clinical trials or commercialization, including, among others, cost overruns, potential problems with process scale up, process reproducibility, stability issues, lot consistency, and timely availability of raw materials. As a result of these challenges, we may experience delays in our clinical development and/or commercialization plans. We may ultimately be unable to reduce the cost of goods for our product candidates to levels that will allow for an attractive return on investment if and when those product candidates are commercialized.
No assurance can be given that we will be able to develop a new, FDA-compliant, more efficient, lower cost manufacturing process upon which our business plan to commercialize MultiTAA-based productsproduct candidates is dependent.
In cooperation with our potentialcurrent contract manufacturers, we intend to develop improved methods for generating and selecting T cells, and to develop methods for large-scale production of our current product candidates that are in accordance with current cGMP procedures. Developing a new, scaled-up, pharmaceutical manufacturing process that can more efficiently and cost effectively, and in a more automated manner produce, measure and control the physical and/or chemical attributes of our productsproduct candidates in a cGMP facility is subject to many uncertainties and difficulties. We have never manufactured our adoptive T cell therapy product candidate on any scale, commercially or otherwise.a commercial scale. As a result, we cannot give any assurance that we will be able to establish a manufacturing process that can produce our productsproduct candidates at a cost or in quantities necessary to make them commercially viable. Moreover, we and our third-party manufacturers will have to continually adhere to current cGMP regulations enforced by the FDA through its facilities inspection program. If thethese facilities of these manufacturers cannot pass a pre-approval plant inspection, the FDA premarket approval of our productsproduct candidates will not be granted. In complying with cGMP and foreign regulatory requirements, we and any of our third-party manufacturers will be obligated to expend time, money and effort in production, record-keeping and quality control to assure that our productsproduct candidates meet applicable specifications and other requirements. If we or any of our third-party manufacturers fail to comply with these requirements, we may be subject to regulatory action. No assurance can be given that we will be able to develop such manufacturing process, or that our partners will thereafter be able to establish and operate such a production facility.
The deviations in our proposed new MultiTAA-based productsproduct candidates from existing products may require us to perform additional testing, which will increase the cost, and extend the time for obtaining approval.
Our MultiTAAMultiTAA-specific T cell therapy platform is based on the adoptive T cell therapy technology that we licensed from BCM and that is presently available as a physician-sponsored investigational therapy at BCM for the treatment of lymphoma, AML/MDS, multiple myeloma and select solid tumors in the U.S.United States. The current method of treatment is labor intensive and expensive. We are performing process optimization that we anticipate will enable more efficient manufacturing of our products.product candidates. We may have difficulty demonstrating that the productsproduct candidates produced from our new processes are identical to the existing products. The FDA may require additional clinical testing before permitting a larger clinical trial with the new processes, and the productsuch drug substance may not be as efficacious in the new clinical trials. Cellular products are not considered to be well characterized products because there are hundreds of markers present on T cells, and even small changes in manufacturing processes could alter the cell subtypes. It is unclear at this time which of those markers are critical for success of T cells to combat cancer, so our ability to predict the outcomes with newer manufacturing processes is limited. The changes that we may make to the existing manufacturing process may require additional testing, which may increase costs and timelines associated with these developments. In addition to developing a multi-antigen T cell-based therapy on existing adoptive T cell therapy technology, we are currently evaluating the desirability of conducting clinical trials of our productsproduct candidates in combination with other existing drugs. These combination therapies will require additional testing, and clinical trials will require additional FDA regulatory approval and will increase our future cost of development.
We may enter into one or more transactions with entities controlled by one of our directors, which could pose a conflict of interest.
John Wilson, a director of the Company, is also CEO and co-founder of Wilson Wolf, which is the sole source vendor that provides us with the G-Rex® cell culture device for the large-scale production of T cells used in our manufacturing process. We do not currently have a supply contract with Wilson Wolf for the G-Rex®. We plan to negotiate a supply contract with Wilson Wolf for the purchase of G-Rex® devices. We have engaged Wilson Wolf in discussions to customize the G-Rex® further to optimally match our manufacturing requirements, as well as to develop a scalability plan to drive efficiencies for a commercial product. There may be conflicts of interest between us and Wilson Wolf. There can be no assurance that Wilson Wolf will agree to enter into any contract with us, or that the terms of any such agreements will be in the best interests of us or will have terms no less favorable to us than could have been obtained from unaffiliated third parties.
We may not be able to develop productsproduct candidates successfully or develop them on a timely basis.
Our immunotherapy product candidates are at various stages of research and development. Further development and extensive testing will be required to determine their technical feasibility and commercial viability. We will need to complete significant additional clinical trials demonstrating that our product candidates are safe and effective to the satisfaction of the FDA and other non-U.S. regulatory authorities. The drug approval process is time-consuming, which involves substantial expenditures of resources, and depends upon a number of factors, including the severity of the disease indication in question, the availability of alternative treatments, and the risks and benefits demonstrated in the clinical trials. Our success depends on our ability to achieve scientific and technological advances and to translate such advances into licensable, FDA-approvable, commercially-competitivecommercially- competitive products on a timely basis. Failure can occur at any stage of the process. If such programs are not successful, we may be unable to develop revenue-producing products. As we enter a more extensive clinical program for our product candidates, the data generated in these studies may not be as compelling as the earlier results.
Immunotherapies that we may develop are not likely to be commercially available for at least five years. Any delay in obtaining FDA and/or other necessary regulatory approvals in the United States and in countries outside the United States for any investigational new drug and failure to receive such approvals would have an adverse effect on the investigational new drug’s potential commercial success and on our business, prospects, financial condition and results of operations. The time required to obtain approval by the FDA and non-U.S. regulatory authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. For example, the FDA or non-U.S. regulatory authorities may disagree with the design or implementation of our clinical trials or study endpoints; or we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks. In addition, the FDA or non-U.S. regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials or the data collected from clinical trials of our product candidates may not be sufficient to support the submission of a new drug application (“NDA”)BLA or other submission or to obtain regulatory approval in the United States or elsewhere. The FDA or non-U.S. regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and the approval policies or regulations of the FDA or non-U.S. regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. The proposed development schedules for our immunotherapy product candidates may be affected by a variety of other factors, including technological difficulties, clinical trial failures, regulatory hurdles, competitive products, intellectual property challenges and/or changes in governmental regulation, many of which will not be within our control.
Any delay in the development, approval, introduction or marketing of our productsproduct candidates could result either in such productsproduct candidates being marketed at a time when their cost and performance characteristics would not be competitive in the marketplace or in the shortening of their commercial lives. In light of the long-term nature of our projects, the unproven technology involved and the other factors described elsewhere in this section, we might not be able to successfully complete the development or marketing of any new products,product candidates, and as a result, our business, prospects, financial condition and results of operations could be materially and adversely affected. We may be required to reduce our staff, discontinue certain research or development programs of our future products and cease to operate.
We may encounter substantial delays in our clinical trials or may not be able to conduct our trials on the timelines we expect.
Clinical testing is expensive, time-consuming, and subject to uncertainty. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. BCM has submitted INDs to the FDA, which allow the use of MAPP T cells and LAPP T cells for human clinical testing. BCM initiated its first clinical trials for our product candidate, MAPP, in 2012, and clinical trials for LAPP in 2016. Issues may yet arise that could suspend or terminate such clinical trials. We intend to file one or more new INDs to advance these products into Phase II clinical trials, and any delay in filing these INDs may have a material adverse impact on our ability to advance clinical studies in accordance with management’s plans. A failure of one or more clinical studies can occur at any stage of testing, and our future clinical studies may not be successful. Events that may prevent successful or timely completion of clinical development include:
We also may conduct clinical and preclinical research in collaboration with other biotechnology and biologics entities in which we combine our technologies with those of our collaborators. Such collaborations may be subject to additional delays because of the management of the trials and the necessity of obtaining additional approvals for therapeutics used in the combination trials. These combination therapies will require additional testing and clinical trials will require additional FDA regulatory approval and will increase our future expenses.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes to our product candidates, we may be required, or may elect, to conduct additional studies to bridge our modified product candidates to earlier versions. Clinical study delays could also shorten any periods during which our products have patent protection and may allow our competitors to bring products to market before we do, which could impair our ability to commercialize our product candidates successfully and may harm our business and the results of our operations.
Our commercial success depends upon attaining significant market acceptance of our product candidates, if approved, among physicians, patients, healthcare payors and the medical community.
Even if we obtain regulatory approval for our product candidates, they may not gain market acceptance among physicians, healthcare payors, patients or the medical community. Market acceptance of our product candidates, if we receive approval, depends on a number of factors, including the:
| · | efficacy and safety of our product candidates as demonstrated in clinical trials and post-marketing experience; |
| · | clinical indications for which our product candidates may be approved; |
| · | acceptance by physicians and patients of our product candidates as safe and effective; |
| · | potential and perceived advantages of our product candidates over alternative treatments; |
| · | safety of our product candidates seen in a broader patient group, including our use outside the approved indications should physicians choose to prescribe for such uses; |
| · | prevalence and severity of any side effects; |
| · | product labeling, or product insert requirements of the FDA or other regulatory authorities; |
| · | timing of market introduction of our product candidates as well as competitive products; |
| · | cost in relation to alternative treatments; |
| · | pricing and the availability of coverage and adequate reimbursement |
| · | relative convenience and ease of administration; and |
| · | effectiveness of any sales and marketing efforts. |
Moreover, if our product candidates are approved but fail to achieve market acceptance among physicians, patients, healthcare payors and the medical community, we may not be able to generate significant revenues, which would compromise our ability to become profitable.
We may not be able to establish or maintain the third-party relationships that are necessary to develop or potentially commercialize some or all of our product candidates.
We expect to depend on collaborators, partners, licensees, clinical research organizations and other third parties to support our discovery efforts, to formulate product candidates, to manufacture our product candidates, and to conduct clinical trials for some or all of our product candidates. We cannot guarantee that we will be able to successfully negotiate agreements for or maintain relationships with collaborators, partners, licensees, clinical investigators, vendors and other third parties on favorable terms, if at all. Our ability to successfully negotiate such agreements will depend on, among other things, potential partners’ evaluation of the superiority of our technology over competing technologies and the quality of the preclinical and clinical data that it has generated, and the perceived risks specific to developing our product candidates. If we are unable to obtain or maintain these agreements, we may not be able to clinically develop, formulate, manufacture, obtain regulatory approvals for or commercialize our product candidates.
Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court or with the USPTO.
If we, our licensing partners, or any potential future collaborator initiates legal proceedings against a third party to enforce a patent directed to one of our product candidates, the defendant could counterclaim that the patent is invalid and/or unenforceable in whole or in part. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include an alleged failure to meet any of several statutory requirements, including lack of novelty, non-obviousness or enablement. Grounds for an unenforceability assertion could include an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO or made a misleading statement during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in revocation or amendment to our patents in such a way that they are no longer directed to our product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable, and prior art could render our patents or those of our licensors invalid or could prevent a patent from issuing from one or more of our pending patent applications. There is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found. There is also no assurance that there is not prior art of which we are aware, but which we do not believe affects the validity or enforceability of a claim in our patents and patent applications, which may, nonetheless, ultimately be found to affect the validity or enforceability of a claim. Furthermore, even if our patents are unchallenged, they may not adequately protect our intellectual property, provide exclusivity for our product candidates, prevent others from designing around our claims or provide us with a competitive advantage. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates. Such a loss of patent protection could have a material adverse impact on our business development.
If we are unable to protect our proprietary rights, we may not be able to compete effectively or operate profitably.
Our commercial success is dependent in part on our ability to obtain, maintain, and enforce the patents and other proprietary rights that we have licensed and may develop, and on our ability to avoid infringing the proprietary rights of others. We generally seek to protect our proprietary position by filing patent applications in the United States and abroad related to our product candidates, proprietary technologies and their uses that are important to our business. Our patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless, and until, patents issue from such applications, and then only to the extent the issued claims are directed to the technology. There can be no assurance that our patent applications or those of our licensor will result in additional patents being issued or that issued patents will afford sufficient protection against competitors with similar technology, nor can there be any assurance that the patents issued will not be infringed, designed around or invalidated by third parties. Even issued patents may later be found invalid or unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. The degree of future protection for our proprietary rights is uncertain. Only limited protection may be available and may not adequately protect our rights or permit us to gain or keep any competitive advantage. This failure to properly protect the intellectual property rights relating to our product candidates could have a material adverse effect on our financial condition and results of operations.
We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with relevant employees, consultants, scientific advisors, and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of the premises and physical and electronic security of the information technology systems. While we have confidence in these individuals, organizations, and systems, agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, trade secrets may otherwise become known or be independently discovered by competitors. To the extent that the consultants, contractors or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Although we have patents and patent applications in other countries, we cannot be certain that the claims in other pending U.S. or European patent applications, international patent applications, and patent applications in certain other foreign territories directed to methods of generating multi-antigen specific T cell products,product candidates, or our other product candidates, will be considered patentable by the USPTO, courts in the United States or by the patent offices and courts in foreign countries, nor can we be certain that the claims in our issued European patent will not be found invalid or unenforceable if challenged.
Most of our intellectual property rights are currently licensed from BCM and the Mayo Foundation, so that the preparation and prosecution of these patents and patent applications was not performed by us or under our control. Furthermore, patent law relating to the scope of claims in the biotechnology field in which we operate is still evolving and, consequently, patent positions in our industry may not be as strong as in other more well-established fields. The patent positions of biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date. The patent application process is subject to numerous risks and uncertainties, and there can be no assurance that we or any of our potential future collaborators will be successful in protecting our product candidates by obtaining and defending patents. These risks and uncertainties include the following:
| · | the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process, the noncompliance with which can result in abandonment or lapse of a patent or patent application, and partial or complete loss of patent rights in the relevant jurisdiction; |
| · | patent applications may not result in any patents being issued; |
| · | patents that may be issued or in-licensed may be challenged, invalidated, modified, revoked, circumvented, found to be unenforceable or otherwise may not provide any competitive advantage; |
| · | our competitors, many of whom have substantially greater resources than us, and many of whom have made significant investments in competing technologies, may seek or may have already obtained patents that will limit, interfere with or eliminate our ability to make, use and sell our potential product candidates; |
| · | there may be significant pressure on the U.S. government and international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful, as a matter of public policy regarding worldwide health concerns; and |
| · | countries other than the United States may have patent laws less favorable to patentees than those upheld by U.S. courts, allowing foreign competitors a better opportunity to create, develop and market competing product candidates. |
The patent prosecution process is also expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner or in all jurisdictions where protection may be commercially advantageous. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, in some circumstances, we may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, directed to technology that we license from third parties. We may also require the cooperation of one of our licensors in order to enforce the licensed patent rights, and such cooperation may not be provided. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. We cannot be certain that patent prosecution and maintenance activities by our licensor have been or will be conducted in compliance with applicable laws and regulations, which may affect the validity and enforceability of such patents or any patents that may issue from such applications. If they fail to do so, this could cause us to lose rights in any applicable intellectual property that we in-license, and as a result our ability to develop and commercialize products or product candidates may be adversely affected and we may be unable to prevent competitors from making, using and selling competing products.
In addition, identification of third-party patent rights that may be relevant to our technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability and it is uncertain how much protection, if any, will be given to the patents we have licensed from a licensor if either the licensor or we attempt to enforce the patents and/or if they are challenged in court or in other proceedings, such as oppositions, which may be brought in foreign jurisdictions to challenge the validity of a patent. A third party may challenge our patents, if issued, or the patent rights that we license from others in the courts or patent offices in the United States and abroad. It is possible that a competitor may successfully challenge our patents or that a challenge will result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical products, or limit the duration of the patent protection of our products and product candidates. Moreover, the cost of litigation to uphold the validity of patents and to prevent infringement can be substantial. If the outcome of litigation is adverse to us, third parties may be able to use our patented invention without payment to us. Moreover, it is possible that competitors may infringe our patents or successfully avoid them through design innovation. To stop these activities, we may need to file a lawsuit. These lawsuits are expensive and would consume time and other resources, even if we were successful in stopping the violation of our patent rights. In addition, there is a risk that a court would decide that our patents are not valid and that we do not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of our patents were upheld, a court would refuse to stop the other party on the ground that its activities are not covered by, that is, do not infringe, our patents.
Should third parties file patent applications, or be issued patents claiming technology also used or claimed by our licensor(s) or by us in any future patent application, we may be required to participate in interference proceedings in the USPTO to determine priority of invention for those patents or patent applications that are subject to the first-to-invent law in the United States, or may be required to participate in derivation proceedings in the USPTO for those patents or patent applications that are subject to the “first-inventor-to-file” law in the United States. We may be required to participate in such interference or derivation proceedings involving our issued patents and pending applications. We may be required to cease using the technology or to license rights from prevailing third parties as a result of an unfavorable outcome in an interference proceeding or derivation proceeding. A prevailing party in that case may not offer us a license on commercially acceptable terms or on any terms.
The use of our technologies could potentially conflict with the rights of others.
Our potential competitors or other entities may have or acquire patent or proprietary rights that they could enforce against our licensors. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, reexaminations,inter partesreview proceedings and post-grant review, or PGR, proceedings before the USPTO and/or corresponding foreign patent offices. Numerous third-party U.S. and foreign issued patents and pending patent applications exist in the fields in which we are developing product candidates. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. If they do so, then they could limit our ability to make, use, sell, offer for sale or import our product candidates and products that may be approved in the future, or impair our competitive position by requiring us to alter our products,product candidates, pay licensing fees or cease activities.
As the biotechnology industry expands and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties. Because patent applications are maintained as confidential for a certain period of time, until the relevant application is published uswe may be unaware of third-party patents that may be infringed by commercialization of any of our product candidates, and we cannot be certain that we were the first to file a patent application related to a product candidate or technology. Moreover, because patent applications can take many years to issue, there may be currently-pending patent applications that later issue as patents that our product candidates may infringe. If our productsproduct candidates conflict with patent rights of others, third parties could bring legal actions against us or our collaborators, licensees, suppliers or customers, claiming damages and seeking to enjoin manufacturing and marketing of the affected products.product candidates. If these legal actions are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to manufacture or market the affected products.product candidates. We may not prevail in any legal action and a required license under the patent may not be available on acceptable terms or at all.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.product candidates.
As is the case with other biopharmaceutical companies, our success is dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example, on September 16, 2011, the Leahy-Smith America Invents Act, or Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. In particular, under the Leahy-Smith Act, the United States transitioned in March 2013 to a “first inventor to file” system in which the first inventor to file a patent application will be entitled to the patent. Third parties are allowed to submit prior art before the issuance of a patent by the USPTO and may become involved in post-grant proceedings including post grant review, derivation, reexamination,inter-partesreview or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope or enforceability of, or invalidate, our patent rights, which could adversely affect our competitive position. In addition, recent U.S. Supreme Court rulings on several patent cases have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. While we do not believe that any of the patents owned or licensed by us will be found invalid based on these decisions, we cannot predict how future decisions by the courts, the U.S. Congress or the USPTO may impact the value of our patents.
We have limited foreign intellectual property rights and may not be able to protect our intellectual property rights throughout the world.
We have limited intellectual property rights outside the United States. Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing its inventions in all countries outside the United States, or from selling or importing products made using its inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biopharmaceutical products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
As is common in the biotechnology and pharmaceutical industries, in addition to our employees, we engage the services of consultants to assist us in the development of our product candidates. We have received confidential and proprietary information from third parties. We employ individuals or engage consultants who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of these third parties or our employees’ former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial cost and be a distraction to our management and employees.
If we fail to comply with any obligations under our existing license agreements or any future license agreements, or disputes arise with respect to those agreements, it could have a negative impact on our business and our intellectual property rights.
We are a party to license agreements with BCM and the Mayo Foundation that impose, and we may enter into additional licensing arrangements with third parties that may impose, diligence, development and commercialization timelines, milestone payment, royalty, insurance and other obligations on us. Our rights to use the licensed intellectual property are subject to the continuation of and our compliance with the terms of these agreements. Disputes may arise regarding our rights to intellectual property licensed to us from a third party, including but not limited to:
| · | the scope of rights granted under the license agreement and other interpretation-related issues; |
| · | the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| · | the sublicensing of patent and other rights; |
| · | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| · | the ownership of inventions and know-how resulting from the creation or use of intellectual property by us, alone or with our licensors and collaborators; |
| · | the scope and duration of our payment obligations; |
| · | our rights upon termination of such agreement; and |
| · | the scope and duration of exclusivity obligations of each party to the agreement. |
If disputes over intellectual property and other rights that we have licensed or acquired from third parties prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates. If we fail to comply with our obligations under current or future licensing agreements, these agreements may be terminated or the scope of our rights under them may be reduced and we might be unable to develop, manufacture or market any product that is licensed under these agreements.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an ownership interest in our patents or other intellectual property. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and distraction to management and other employees.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be subject to competition from competitive products, including biosimilars. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide sufficient rights to exclude others from commercializing products similar or identical to our products.product candidates.
Certain of our technologies are in-licensed from third parties, and the protection of those technologies is not entirely within our control.
We have world-wide exclusive licenses from the Mayo Foundation on (i) a novel set of Class II HER2/neu peptide antigens, (ii) a novel Class I HER2/neu antigen, and (iii) a novel set of Class II Folate Receptor Alpha peptide antigens. We have a world-wide exclusive license from BCM of the rights in and to three patent families to develop and commercialize MultiTAAMultiTAA-specific T cell product candidates.candidates in the field of oncology. As a result of these in-licenses, we could lose the right to develop each of the technologies if:
| · | the owners of the patent rights underlying the technologies that we license do not properly maintain or enforce the patents and intellectual property underlying those properties, |
| · | the Mayo Foundation or BCM seeks to terminate our license in contravention of the license agreements; |
| · | we fail to make all payments due and owing under any of the licenses; or |
| · | we fail to obtain on commercially reasonable terms, if at all, in-licenses from the Mayo Foundation or BCM or others for other rights that are necessary to develop the technology that we have already in-licensed. |
If any of the above occurs, we could lose the right to use the in-licensed intellectual property, which would adversely affect our ability to commercialize our technologies, products or services. The loss of any current or future licenses from Mayo Foundation or BCM, or the exclusivity rights provided by such license agreements, could materially harm our financial condition and operating results.
We rely upon patents and licensed technologies to protect our technology. We may be unable to protect our intellectual property rights, and we may be liable for infringing the intellectual property rights of others.
Our ability to compete effectively depends on our ability to maintain the proprietary nature of our technologies and the proprietary technology of others with whom we have entered into collaboration and licensing agreements. We own or hold licenses to a number of issued patents and U.S. pending patent applications, as well as foreign patents and foreign counterparts. Our success depends in part on our ability to obtain patent protection both in the United States and abroad for our product candidates, as well as the methods for treating patients in the product indications using these product candidates. Such patent protection is costly to obtain and maintain, and sufficient funds might not be available. Our ability to protect our product candidates from unauthorized or infringing use by third parties depends in substantial part on our ability to obtain and maintain valid and enforceable patents. Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these patents, our ability to obtain, maintain and enforce patents is uncertain and involves complex legal and factual questions. Even if our product candidates, as well as methods for treating patients for prescribed indications using these product candidates are covered by valid and enforceable patents and have claims with sufficient scope, disclosure and support in the specification, the patents will provide protection only for a limited amount of time. Accordingly, rights under any issued patents may not provide us with sufficient protection for our product candidates or provide sufficient protection to afford us a commercial advantage against competitive products or processes.
In addition, we cannot guarantee that any patents will be issued from any pending or future patent applications owned by or licensed to us. Even if patents have been issued or will be issued, we cannot guarantee that the claims of these patents are or will be valid or enforceable or will provide us with any significant protection against competitive products or otherwise be commercially valuable to us. The laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as in the United States and many companies have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. Furthermore, different countries have different procedures for obtaining patents, and patents issued in different countries offer different degrees of protection against use of the patented invention by others. If we encounter such difficulties in protecting or are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business prospects could be substantially harmed.
The patent positions of biotechnology and pharmaceutical companies, including our patent positions, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated, or circumvented. Our patents can be challenged by our competitors who can argue that our patents are invalid, unenforceable, lack sufficient written description or enablement, or that the claims of the issued patents should be limited or narrowly construed. Patents also will not protect our product candidates if competitors devise ways of making or using these product candidates without infringing our patents.
We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our technologies, methods of treatment, product candidates, and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets and we have the funds to enforce our rights, if necessary.
The expiration of our owned or licensed patents before completing the research and development of our product candidates and receiving all required approvals in order to sell and distribute the products on a commercial scale can adversely affect our business and results of operations.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our intellectual property rights or those of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in a patent infringement proceeding, a court may decide that one or more of the patents which we own or in-license is not valid or is unenforceable, and/or is not infringed. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our patent applications at risk of not issuing. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on any issued patent and/or pending patent applications will be due to the USPTO and foreign patent agencies in several stages over the lifetime of our patents and/or applications. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with rules applicable to the particular jurisdiction. However, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business development.
Interference or derivation proceedings provoked by third parties or brought by us or declared by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. Should third parties file patent applications or be issued patents claiming technology also used or claimed by us, we may be required to participate in interference or derivation proceedings in the USPTO to determine priority of invention. We may be required to participate in interference or derivation proceedings involving our issued patents and pending applications. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially acceptable terms.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
We also rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
If we are unable to obtain licenses needed for the development of our product candidates, or if we breach any of the agreements under which we license rights to patents or other intellectual property from third parties, we could lose license rights that are important to our business.
If we are unable to maintain and/or obtain licenses needed for the development of our product candidates in the future, we may have to develop alternatives to avoid infringing on the patents of others, potentially causing increased costs and delays in drug development and introduction or precluding the development, manufacture, or sale of planned products.product candidates. Some of our licenses provide for limited periods of exclusivity that require minimum license fees and payments and/or may be extended only with the consent of the licensor. We might not meet these minimum license fees in the future, or these third parties might not grant extensions on any or all such licenses. This same restriction may be contained in licenses obtained in the future.
Additionally, the patents underlying the licenses might not be valid and enforceable. To the extent any productsproduct candidates developed by us are based on licensed technology, royalty payments on the licenses will reduce our gross profit from such product sales and may render the sales of such productsproduct candidates uneconomical. In addition, the loss of any current or future licenses or the exclusivity rights provided therein could materially harm our business financial condition and our operations.
We may face legal claims; litigation is expensive and we may not be able to afford the costs.
We may face legal claims involving stockholders, consumers, competitors, entities from whom we license technology, entities with whom we collaborate, persons claiming that we are infringing on their intellectual property and others. The biotechnology and pharmaceutical industries have been characterized by extensive litigation regarding patents and other intellectual property rights, and companies have employed intellectual property litigation to gain a competitive advantage. We may initiate or become subject to infringement claims or litigation arising out of patents and pending applications of our competitors, or we may become subject to proceedings initiated by our competitors or other third parties or the USPTO or applicable foreign bodies to reexamine the patentability of our licensed or owned patents. In addition, litigation may be necessary to enforce our issued patents, to protect our trade secrets and know-how, or to determine the enforceability, scope, and validity of the proprietary rights of others.
The costs of litigation or any proceeding relating to our intellectual property or contractual rights could be substantial even if resolved in our favor. Some of our competitors or financial funding sources have far greater resources than we do and may be better able to afford the costs of complex legal procedures. Also, in a law suitlawsuit for infringement or contractual breaches, even if frivolous, we will require considerable time commitments on the part of management, our attorneys and consultants. Defending these types of proceedings or legal actions involve considerable expense and could negatively affect our financial results.
Our research and development programs are subject to uncertainty.
Factors affecting our research and development programs include, but are not limited to:
| · | limited financial resources from which to budget and allocate among our product candidates; |
| · | competition from companies that are substantially and financially stronger than us; |
| · | the need for acceptance of our immunotherapies; |
| · | our ability to anticipate and adapt to a competitive market and rapid technological developments; |
| · | the amount and timing of operating costs and capital expenditures relating to expansion of our business, operations and infrastructure; |
| · | the need to rely on multiple levels of outside funding due to the length of drug development cycles and governmental approved protocols associated with the pharmaceutical industry; and |
| · | the dependence upon key personnel including key independent consultants and advisors. |
Our research and development expenses may not be consistent from time to time. We may be required to accelerate or delay incurring certain expenses depending on the results of our studies and the availability of adequate funding.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our product candidates, we may be unable to generate any revenue.
We do not currently have an organization for the sale, marketing and distribution of any approved products and the cost of establishing and maintaining such an organization may exceed the cost-effectiveness of doing so. In order to market any products approved by the FDA or comparable foreign regulatory authorities, we must build our sales, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and may not become profitable. We will be competing with many companies that currently have extensive and well-funded sales and marketing operations. Without an internal commercial organization or the support of a third party to perform sales and marketing functions, we may be unable to compete successfully against these more established companies.
If we are unable to establish or manage strategic collaborations in the future, our revenue and drug development may be limited.
Our strategy includes eventual substantial reliance upon strategic collaborations for marketing and commercialization of our product candidates, and we may rely even more on strategic collaborations for research, development, marketing and commercialization of our other immunotherapies. If we are unsuccessful in securing such strategic collaborations, we may be unable to commercialize ourany approved products as we have not yet licensed, marketed or sold any of our immunotherapies or entered into successful collaborations for these services in order to ultimately commercialize our immunotherapies. Establishing strategic collaborations is difficult and time-consuming. Our discussions with potential collaborators may not lead to the establishment of collaborations on favorable terms, if at all. Potential collaborators may reject collaborations based upon their assessment of our financial, clinical, regulatory or intellectual property position. If we successfully establish new collaborations, these relationships may never result in the successful development or commercialization of our immunotherapies or the generation of sales revenue. To the extent that we enter into co-promotion or other collaborative arrangements, our product revenues are likely to be lower than if itwe directly marketed and sold any products that we may develop.
Management of our relationships with our collaborators will require:
| · | significant time and effort from our management team; |
| · | coordination of our research and development programs with the research and development priorities of our collaborators; and |
| · | effective allocation of our resources to multiple projects. |
If we continue to enter into research and development collaborations at the early phases of drug development, our success will in part depend on the performance of our corporate collaborators. We will not directly control the amount or timing of resources devoted by our corporate collaborators to activities related to our immunotherapies. Our corporate collaborators may not commit sufficient resources to itstheir research and development programs or the commercialization, marketing or distribution of itstheir immunotherapies. If any corporate collaborator fails to commit sufficient resources, our preclinical or clinical development programs related to this collaboration could be delayed or terminated. Also, our collaborators may pursue existing or other development-stage products or alternative technologies in preference to those being developed in collaboration with us. Finally, if we fail to make required milestones or royalty payments to our collaborators or to observe other obligations in our agreements with them, our collaborators may have the right to terminate those agreements.
We may not be able to license newly developed MultiTAAMultiTAA-specific T cell technology from BCM and others.
An important element of our intellectual property portfolio is to license additional rights and technologies from BCM. Our inability to license the rights and technologies that we have identified, or newly developed MultiTAAMultiTAA-specific T cell technology that we may in the future identify, could have a material adverse impact on our ability to complete the development of our productsproduct candidates or to develop additional products.product candidates. No assurance can be given that we will be successful in licensing any additional rights or technologies from BCM and others. Failure to obtain additional rights and licenses may detrimentally affect our planned development of additional product candidates and could increase the cost, and extend the timelines associated with our development of such other products.product candidates.
The market opportunities for our product candidates may be limited to those patients who are ineligible for or have failed prior treatments and may be small.
The FDA often approves new oncology therapies initially only for use in patients with relapsed or refractory metastatic disease. We expect to initially seek approval of our product candidates in this setting. Subsequently, for those productsproduct candidates that prove to be sufficiently beneficial, if any, we would expect to seek approval in earlier lines of treatment and potentially as a first line therapy. There is no guarantee, however, that our product candidates, even if approved, would be approved for earlier lines of therapy, and, prior to any such approvals, we may have to conduct additional clinical trials.
Our projections of both the number of people who have the cancers we are targeting, as well as the subset of people with these cancers in a position to receive second or third-line therapy, and who have the potential to benefit from treatment with our product candidates, are based on our research and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations, or market research by third parties, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these cancers. The number of treatable patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for our product candidates may be limited or may not be amenable to treatment with our product candidates and may also be limited by the cost of our treatments and the reimbursement of those treatment costs by third-party payors. For instance, we expect our lead product candidate LAPP, to initially target a small patient population that suffers from AML. Even if we obtain significant market share for our product candidates, because the potential target populations are small, we may never achieve profitability without obtaining regulatory approval for additional indications.
We are required to pay substantial royalties and lump sum milestone payments under our license agreementagreements with BCM and the Mayo Foundation, and we must meet certain milestones to maintain our license rights.
Under our license agreement with BCM for our MultiTAAMultiTAA-specific T cell therapy technologies, we are currently required to pay both substantial milestone payments and royalties to BCM based on our revenues from sales of ourany approved products utilizing the licensed technologies, and these payments could adversely affect the overall profitability for us of any products that we may seek to commercialize. In order to maintain our license rights under the BCM license agreement, we will need to meet certain specified milestones, subject to certain cure provisions, in the development of our product candidates. Similarly, we are also required to pay both substantial milestone payments and royalties to the Mayo Foundation based on our revenues from sales of our products utilizing those licensed technologies. There is no assurance that we will be successful in meeting all of the milestones in our licenses in the future on a timely basis or at all.
In addition, upon a liquidity event (as defined in our BCM license agreement with BCM, but shall not include the “Merger”)BCM) of the licensee under the BCM license agreement (which, the licensee shall be the Company), BCM will receive a liquidity incentive payment of 0.5% of the liquidity event proceeds (as defined in the BCM license agreement) received by such licensee or its stockholders in the liquidity event, thereby diluting the amount of proceeds available to the licensee or its stockholders in a liquidity event.
Because our current productsproduct candidates represent, and our other potential product candidates will represent novel approaches to the treatment of disease, there are many uncertainties regarding the development, the market acceptance, third-party reimbursement coverage and the commercial potential of our product candidates.
There is no assurance that the approaches offered by our productsproduct candidates will gain broad acceptance among doctors or patients or that governmental agencies or third-party medical insurers will be willing to provide reimbursement coverage for proposed product candidates. Moreover, we do not have verifiable internal marketing data regarding the potential size of the commercial market for our product candidates, nor have we obtained independent marketing surveys to verify the potential size of the commercial markets for our current product candidates or any future product candidates. Since our current product candidates and any future product candidates will represent new approaches to treating various conditions, it may be difficult, in any event, to accurately estimate the potential revenues from these product candidates. Accordingly, we may spend large amounts of money trying to obtain approval for product candidates that have an uncertain commercial market. The market for any products that we successfully develop will also depend on the cost of the product. We do not yet have sufficient information to reliably estimate what it will cost to commercially manufacture our current product candidates, and the actual cost to manufacture these products could materially and adversely affect the commercial viability of these products. Our goal is to reduce the cost of manufacturing our therapies. However, unless we are able to reduce those costs to an acceptable amount, we may never be able to develop a commercially viable product. If we do not successfully develop and commercialize products based upon our approach or find suitable and economical sources for materials used in the production of our products, we will not become profitable.
Our MultiTAAMultiTAA-specific T cell therapy may be provided to patients in combination with other agents provided by third parties. The cost of such combination therapy may increase the overall cost of MultiTAAMultiTAA-specific T cell therapy and may result in issues regarding the allocation of reimbursements between our therapy and the other agents, all of which may adversely affect our ability to obtain reimbursement coverage for the combination therapy from third-party medical insurers.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if our product candidates cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent to the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection laws. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| · | decreased demand for our product candidates; |
| · | injury to our reputation; |
| · | withdrawal of clinical trial participants; |
| · | initiation of investigations by regulators; |
| · | costs to defend the related litigation; |
| · | a diversion of management’s time and our resources; |
| · | substantial monetary awards to trial participants or patients; |
| · | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| · | loss of revenue; |
| · | exhaustion of any available insurance and our capital resources; and |
| · | the inability to commercialize any product candidate. |
Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could inhibit or prevent the commercialization of products we develop, alone or with collaborators. Our insurance policies may also have various exclusions, and we may be subject to a product liability claim for which we have no insurance coverage. While we obtained clinical trial insurance for our Phase II clinical trials, we may have to pay amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Even if our agreements with any future collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
We face significant competition from other biotechnology and pharmaceutical companies and from non-profit institutions.
Competition in the field of cancer therapy is intense and is accentuated by the rapid pace of technological development. Research and discoveries by others may result in breakthroughs that may render our productsproduct candidates obsolete even before they generate any revenue. There are products currently under development by others that could compete with the productsproduct candidates that we are developing. Many of our potential competitors have substantially greater research and development capabilities and manufacturing, marketing, financial and managerial resources than we have. Our competitors may:
| · | develop safer or more effective immunotherapies and other therapeutic products; |
| · | reach the market more rapidly, reducing the potential sales of our products; or |
| · | establish superior proprietary positions. |
Potential competitors in the market for treating hematological malignancies are companies such as Juno Therapeutics/Celgene/Bristol-Myers Squibb, Roche/Genentech, Merck, Novartis, Kite Pharma/Gilead, Amgen, Pfizer, and GlaxoSmithKline, which already have products on the market or in development. Other companies, such as Cellectis Bluebird Bio, and AdaptImmune, which are focused on genetically engineered T cell technologies to treat cancer, may also be competitors. Furthermore, companies such as Iovance, Immatics, WindMIL Therapeutics, Mana Therapeutics, Tessa Therapeutics and Torque Therapeutics are developing non-genetically modified T cell therapies such as Tumor Infiltrating Lymphocytes (“TIL”)tumor infiltrating lymphocytes and Marrow Infiltrating Lymphocytes (“MIL”)marrow infiltrating lymphocytes therapies that may compete with our products.product candidates. All of these companies, and most of our other current and potential competitors have substantially greater research and development capabilities and financial, scientific, regulatory, manufacturing, marketing, sales, human resources, and experience than we do. Many of our competitors have several therapeutic products that have already been developed, approved and successfully commercialized, or are in the process of obtaining regulatory approval for their therapeutic products in the United States and internationally.
Universities and public and private research institutions in the U.S. and around the world are also potential competitors. While these universities and public and private research institutions primarily have educational objectives, they may develop proprietary technologies that lead to other FDA approved therapies or that secure patent protection that we may need for the development of our technologies and products.product candidates.
Our lead product candidate LAPP, is a therapy for the treatment of refractory AML. Currently, there are numerous companies that are developing various alternate treatments for AML. Accordingly, LAPP faceswe face significant competition in the AML treatment space from multiple companies. Even if we obtain regulatory approval for LAPP,our lead product candidate, the availability and price of competitors’ products could limit the demand and the price we will be able to charge for our therapy. We may not be able to implement our business plan if the acceptance of our productsproduct candidates is inhibited by price competition or the reluctance of physicians to switch from other methods of treatment to our product, or if physicians switch to other new therapies, drugs or biologic products or choose to reserve our productsproduct candidates for use in limited circumstances.
As a result of being a public company, we are obligated to develop and maintain proper and effective internal controls over financial reporting, and any failure to maintain the adequacy of these internal controls may adversely affect investor confidence in our company and, as a result, the value of our common stock.
We are required, pursuant to Section 404 of the Sarbanes-Oxley Act, or Section 404, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. This report by management is included in Part II, Item 9A of this Form 10-K. In addition, our independent registered public accounting firm is required to attest to the effectiveness of our internal control over financial reporting in this Form 10-K. We are also required to disclose significant changes made in our internal control procedures on a quarterly basis.
To comply with Section 404, we have engaged in the costly and challenging process of compiling the system and processing documentation necessary to perform the evaluation needed to comply with Section 404. Our compliance with Section 404 requires that we incur substantial professional fees and expend significant management efforts, and we may need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge and compile the system and process documentation necessary to perform the evaluation needed to comply with Section 404.
During the evaluation and testing process of our internal controls, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition or results of operations. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by the Nasdaq, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
Our business and operations would suffer in the event of cybersecurity/information systems risk.
Despite the implementation of security measures, our internal computer systems, and those of our manufacturers and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, fire, terrorism, successful breaches, employee malfeasance, or human or technological error, war and telecommunication and electrical failures. In addition, our systems safeguard important confidential personal data regarding our subjects. If a disruption event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed, ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our product candidates could be delayed.
We maintain cybersecurity insurance, however, an incident may exceed our coverage premiums.
We have cybersecurity insurance for a breach event covering expenses for notification, credit monitoring, investigation, crisis management, public relations and legal advice. We also maintain property and casualty insurance that may cover restoration of data, certain physical damage or third-party injuries caused by potential cybersecurity incidents. However, damage and claims arising from such incidents may not be covered or may exceed the amount of any insurance available.
We may incur costs of addressing a cybersecurity incident.
Cybersecurity incidents have increased in number and severity recently and it is expected that these trends will continue. Should we be affected by such an incident, we may incur substantial costs and suffer other negative consequences, which may include:
| · | investigation costs and costs to engage specialized consultants; |
| · | remediation costs, such as liability for stolen assets or information, repairs of system damage, and incentives to customers or business partners in an effort to maintain relationships after an attack; and |
| · | litigation and legal risks, including regulatory actions by state and federal regulators. |
Our ability to use net operating losses and certain other tax attributes to offset future taxable income may be subject to limitation.
Our net operating loss, or NOL, carryforwards could expire unused and be unavailable to offset future income tax liabilities because of their limited duration or because of restrictions under U.S. tax law. Our NOLs generated in tax years ending on or prior to December 31, 2017 are only permitted to be carried forward for 20 years under applicable U.S. tax law. Under H.R. 1, “An Act to provide for reconciliation pursuant to titles II and V of the concurrent resolution on the budget for fiscal year 2018”, informally titled the Tax Cuts and Jobs Act, or, the Tax Act, our federal NOLs generated in tax years ending after December 31, 2017 may be carried forward indefinitely, but the deductibility of federal NOLs generated in tax years beginning after December 31, 2017 is limited. It is uncertain if and to what extent various states will conform to the Tax Act.
In addition, under Section 382 and Section 383 of the Internal Revenue Code of 1986, as amended, (or, the Code) and corresponding provisions of state law, if a corporation undergoes an “ownership change,” which is generally defined as a greater than 50% change, by value, in its equity ownership over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes (such as research tax credits) to offset its post-change income or taxes may be limited. We may have experienced ownership changes in the past and may experience ownership changes in the future as a result of shifts in our stock ownership (some of which shifts are outside our control). As a result, if we earn net taxable income, our ability to use our pre-change NOLs to offset such taxable income will be subject to limitations. Similar provisions of state tax law may also apply to limit our use of accumulated state tax attributes. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
Consequently, even if we achieve profitability, we may not be able to utilize a material portion of our net operating loss carryforwards and certain other tax attributes, which could have a material adverse effect on cash flow and results of operations.
U.S. federal income tax reform could materially adversely affect our company.
On December 22, 2017, President Trump signed into law the Tax Act, which significantly revises the Code. The Tax Act, among other things, reduces the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, repeals the alternative minimum tax for corporations, limits the tax deduction for interest expense to 30% of adjusted taxable income (except for certain small businesses), limits the deduction for net operating losses carried forward from taxable years beginning after December 31, 2017 to 80% of current year taxable income, eliminates net operating loss carrybacks, imposes a one-time tax on offshore earnings at reduced rates regardless of whether they are repatriated, eliminates U.S. tax on foreign earnings (subject to certain important exceptions), allows immediate deductions for certain new investments instead of deductions for depreciation expense over time, and modifies or repeals many business deductions and credits. Notwithstanding the reduction in the corporate income tax rate, the overall impact of the Tax Act is uncertain and our business and financial condition could be adversely affected. In addition, it is uncertain if and to what extent various states will conform to the Tax Act. The impact of the Tax Act on holders of our common stock is also uncertain and could be adverse. We urge our stockholders to consult with their legal and tax advisors with respect to this legislation and the potential tax consequences of investing in or holding our common stock.
The COVID-19 coronavirus could adversely impact our business, including our clinical trials and development activities conducted by us or BCM.
In December 2019, a novel strain of coronavirus, COVID-19, was reported to have surfaced in Wuhan, China. Since then, the COVID-19 coronavirus has spread to multiple countries, including the United States and several European countries. If the COVID-19 coronavirus continues to spread in the United States, we may experience disruptions that could severely impact our business and clinical trials conducted by us or BCM, including:
| · | delays or difficulties in enrolling patients in clinical trials; |
| · | delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff; |
| · | diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as clinical trial sites and hospital staff supporting the conduct of our clinical trials; |
| · | interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others; and |
| · | limitations in employee resources that would otherwise be focused on the conduct of clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people. |
These limitations and/or interruptions may also affect BCM's ability to conduct research and development activities on our behalf.
The global outbreak of the COVID-19 coronavirus continues to rapidly evolve. The extent to which the COVID-19 coronavirus may impact our business and clinical trials and development activities, whether conducted by us or BCM, will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the ultimate geographic spread of the disease, the duration of the outbreak, travel restrictions and social distancing in the United States and other countries, business closures or business disruptions and the effectiveness of actions taken in the United States and other countries to contain and treat the disease.
Risks Related to Government Regulation
We are subject to extensive regulation, which can be costly, time consuming and can subject us to unanticipated delays; even if we obtain regulatory approval for some of our products, those productsproduct candidates may still face regulatory difficulties.
All of our potential products,current and future product candidates, cell processing and manufacturing activities, are subject to comprehensive regulation by the FDA in the United States and by comparable authorities in other countries. The process of obtaining FDA and other required regulatory approvals, including foreign approvals, is expensive and often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. In addition, regulatory agencies may lack experience with our technologies and products,product candidates, which may lengthen the regulatory review process, increase our development costs and delay or prevent their commercialization.
No adoptive T cell therapy using MultiTAAMultiTAA-specific T cells has been approved for marketing in the U.S. by the FDA. Consequently, there is no precedent for the successful commercialization of products based on our technologies. In addition, we have had only limited experience in filing and pursuing applications necessary to gain regulatory approvals, which may impede our ability to obtain timely FDA approvals, if at all. We have not yet sought FDA approval for any adoptive T cell therapy product. We will not be able to commercialize any of our potential productsproduct candidates until we obtain FDA approval, and so any delay in obtaining, or inability to obtain, FDA approval would harm our proposed business.
If we violate regulatory requirements at any stage, whether before or after marketing approval is obtained, we may be fined, forced to remove a product from the market and experience other adverse consequences including delay, which could materially harm our business development. Additionally, we may not be able to obtain the labeling claims necessary or desirable for the promotion of our products. We may also be required to undertake post-marketing trials. Prescription drugs may be promoted only for the approved indications in accordance with the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label may be subject to significant liability. However, physicians may, in their independent medical judgment, prescribe legally available products for off-label uses. The FDA does not regulate the behavior of physicians in their choice of treatments but the FDA does restrict manufacturer’s communications on the subject of off-label use of their products. In addition, if we or others identify side effects after any of our adoptive T cell therapy products are on the market, or if manufacturing problems occur, regulatory approval may be withdrawn, and reformulation of our products may be required.
The FDA regulatory approval process is lengthy and time-consuming, and we may experience significant delays in the clinical development and regulatory approval of our product candidates.
We have not previously submitted a Biologics License Application (“BLA”)BLA to the FDA, or similar approval filings to comparable foreign authorities. A BLA must include extensive preclinical and clinical data and supporting information to establish the product candidate’s safety and effectiveness for each desired indication. The BLA must also include significant information regarding the CMC for the product. We expect the novel nature of our product candidates to create further challenges in obtaining regulatory approval. For example, the FDA has limited experience with commercial development of cell therapies for cancer. Accordingly, the regulatory approval pathway for our product candidates may be uncertain, complex, expensive and lengthy, and approval may not be obtained. We may also experience delays in completing planned clinical trials for a variety of reasons, including delays related to:
| · | the availability of financial resources to commence and complete the planned trials; |
| · | reaching agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| · | obtaining approval by an independent IRB at each clinical trial site; |
| · | recruiting suitable patients to participate in a trial; |
| · | having patients complete a trial or return for post-treatment follow-up; |
| · | clinical trial sites deviating from trial protocol or dropping out of a trial; |
| · | adding new clinical trial sites; or |
| · | manufacturing sufficient quantities of qualified materials under cGMPs and applying them on a subject by subject basis for use in clinical trials. |
We could also encounter delays if physicians face unresolved ethical issues associated with enrolling patients in clinical trials of our product candidates in lieu of prescribing existing treatments that have established safety and efficacy profiles. Further, a clinical trial may be suspended or terminated by us, the IRB for the institutions in which such trials are being conducted, the Data and Safety Monitoring Board or Committee for such trial, or by the FDA or other regulatory authorities due to a number of factors. Those factors could include failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience termination of, or delays in the completion of, any clinical trial of our product candidates, the commercial prospects for our product candidates will be harmed, and our ability to generate product revenue will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product development and approval process and jeopardize our ability to commence product sales and generate revenue.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, while a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
We may also submit marketing applications in other countries. Regulatory authorities in jurisdictions outside of the United States have requirements for approval of product candidates with which we must comply prior to marketing in those jurisdictions. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we fail to comply with the regulatory requirements in international markets and/or fail to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of ourany approved product candidates will be harmed.
Even if we receive regulatory approval of our product candidates, we will be subject to ongoing quality and regulatory obligations and continued regulatory review, which may result in significant additional expense, and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates.
Any regulatory approvals that we receive for our product candidates will require surveillance to monitor the safety and efficacy of the product candidate. The FDA may also require a risk evaluation and mitigation strategy in order to approve our product candidates, which could entail requirements for a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. In addition, if the FDA or a comparable foreign regulatory authority approves our product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export and recordkeeping for our product candidates will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMPs and cGCPs for any clinical trials that we conduct post-approval. Later discovery of previously unknown problems with our product candidates, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| · | restrictions on the marketing or manufacturing of our product candidates, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| · | fines, warning letters or holds on clinical trials; |
| · | refusal by the FDA to approve pending applications or supplements to approved applications filed by us or suspension or revocation of license approvals; |
| · | product seizure or detention, or refusal to permit the import or export of our product candidates; and |
| · | injunctions or the imposition of civil or criminal penalties. |
The FDA’s and other regulatory authorities’ policies may change, and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, and we may not achieve or sustain profitability.
Any relationships with healthcare professionals, principal investigators, consultants, customers (actual and potential) and third-party payors in connection with our current and future business activities are and will continue to be subject, directly or indirectly, to federal and state healthcare laws. If we are unable to comply, or have not fully complied, with such laws, we could face penalties, contractual damages, reputational harm, diminished profits and future earnings and curtailment or restructuring of our operations.
Our business operations and activities may be directly, or indirectly, subject to various federal and state healthcare laws, including without limitation, fraud and abuse laws, false claims laws, data privacy and security laws, as well as transparency laws regarding payments or other items of value provided to healthcare providers. These laws may restrict or prohibit a wide range of business activities, including, but not limited to, research, manufacturing, distribution, pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. These laws may impact, among other things, our current activities with principal investigators and research subjects, as well as current and future sales, marketing, patient co-payment assistance and education programs.
Such laws include:
| the federal Anti-Kickback Statute which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid; |
| · | the federal civil and criminal false claims laws, including the federal civil False Claims Act, and civil monetary penalties laws, which impose criminal and civil penalties against individuals or entities for, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| · | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| · | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and its implementing regulations, which also imposes obligations, including mandatory contractual terms, on covered entities, including certain healthcare providers, health plans, and healthcare clearinghouses, as well as their respective business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| · | the federal Physician Payments Sunshine Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare & Medicaid Services, or CMS, information related to payments or other transfers of value made to physicians, as defined by such law, and teaching hospitals, and applicable manufacturers and applicable group purchasing organizations to report annually to CMS ownership and investment interests held by physicians and their immediate family members; and |
| · | analogous state, local, and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures or drug pricing; state and local laws that require the registration of pharmaceutical sales representatives; state and local “drug takeback” laws and regulations; and state and foreign laws governing the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs. While our interactions with healthcare professionals have been structured to comply with these laws and related guidance, it is possible that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws. If our operations or activities are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to, without limitation, significant civil, criminal and administrative penalties, damages, monetary fines, disgorgement, imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, contractual damages, reputational harm, diminished profits and future earnings and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate.
In addition, any sales of our product once commercialized outside the U.S. will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
Recently enacted and future legislation in the United States and other countries may affect the prices we may obtain for our product candidates and increase the difficulty and cost to commercialize our product candidates.
In the United States and many other countries, rising healthcare costs have been a concern for governments, patients and the health insurance sector, which has resulted in a number of changes to laws and regulations, and may result in further legislative and regulatory action regarding the healthcare and health insurance systems that could affect our ability to profitably sell any product candidates for which we have obtained marketing approval.
For example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, (“ACA”)or the ACA was enacted in the United States in March 2010, with the stated goals of containing healthcare costs, improving quality and expanding access to healthcare, and includes measures to change health care delivery, increase the number of individuals with insurance, ensure access to certain basic health care services, and contain the rising cost of care. Since January 2017, President Trump has signed two executive orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the ACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed repeal legislation, two bills affecting the implementation of certain taxes under the ACA have been signed into law. The Tax Cuts and Jobs Act of 2017 includes a provision that repealed, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Additionally, onthe 2020 federal spending package permanently eliminates, effective January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed1, 2020, the implementation of certain ACA-mandated fees, including the so-called “Cadillac” tax on certain high costhigh-cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share,coverage and the medical device excise tax on non-exempt medical devices.and, effective January 1, 2021, also eliminates the health insurer tax. Further, the Bipartisan Budget Act of 2018, among other things, amended the ACA, effective January 1, 2019, to increase from 50% to 70% the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole.” On December 14, 2018, a Texas U.S. District Court Judge ruled that the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress as part of the Tax Cuts and Jobs Act. Further, on December 18, 2019, the U.S. Court of Appeals for the 5th Circuit upheld the District Court ruling that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the ACA are invalid as well. It is unclear how this decision, future decisions, subsequent appeals, and other efforts to repeal and replace the ACA will impact the ACA and our business. Congress may consider other legislation to repeal or replace elements of the ACA. These executive orders and legislative actions may result in increased health insurance premiums and reduce the number of people with health insurance in the United States and have other effects that could adversely affect U.S. health insurance markets and the ability of patients to have access to therapies that our product candidates can provide.
In addition, other federal health reform measures have been proposed and adopted in the United States. For example, as a result of the Budget Control Act of 2011 and subsequent legislative amendments thereto, providers are subject to Medicare payment reductions of 2% per fiscal year through 20272029 unless additional Congressional action is taken. Further, the American Taxpayer Relief Act of 2012 reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. The Medicare Access and CHIP Reauthorization Act of 2015 ended the use of the statutory formula, also referred to as the Sustainable Growth Rate, for clinician payment and also introduced a quality payment program under which certain individual Medicare providers will be subject to certain incentives or penalties based on new program quality standards.standards also referred to as the Quality Payment Program. Payment adjustments for the Medicare quality payment program willwas set to begin in 2019. This program provides clinicians with two ways to participate, including through the Advanced Alternative Payment Models, or APMs, and the Merit-based Incentive Payment System, or MIPS. In November 2019, CMS issued a final rule finalizing the changes to the Quality Payment Program. At this time, it is unclear how the introduction of the quality payment program will impact overall physician reimbursement under the Medicare program. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. Further,
Also, there has been heightened governmental scrutiny in the United States ofrecently over pharmaceutical pricing practices in light of the rising cost of prescription drugs and biologics. Such scrutiny has resulted in several recent Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. At the federal level, the Trump administration’s budget proposal for fiscal year 2020 contain further drug price control measures that could be enacted during the budget process or in other future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients. Further, the Trump administration released a “Blueprint” to lower drug prices and reduce out of pocket costs of drugs that contains additional proposals to increase manufacturer competition, increase the negotiating power of certain federal healthcare programs, incentivize manufacturers to lower the list price of their products and reduce the out of pocket costs of drug products paid by consumers. The Department of Health and Human Resources has solicited feedback on some of these measures and, at the same, has implemented others under its existing authority. For example, in May 2019, CMS issued a final rule to allow Medicare Advantage Plans the option of using step therapy for Part B drugs beginning January 1, 2020. This final rule codified CMS’s policy change that was effective January 1, 2019. Although a number of these, and other measures may require additional authorization to become effective, Congress and the executive branch have each indicated that it will continue to seek new legislative and/or administrative measures to control drug costs. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
The combination of healthcare cost containment measures, increased health insurance costs, reduction of the number of people with health insurance coverage, as well as future legislation and regulations focused on reducing healthcare costs by reducing the cost of, or reimbursement and access to, pharmaceutical products, may limit or delay our ability to commercialize our products, generate revenue or attain profitability.
Our employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other illegal activity by our employees, independent contractors, consultants, commercial partners and vendors. Misconduct by these parties could include intentional, reckless and/or negligent conduct that fails to: comply with the laws of the FDA and other similar foreign regulatory bodies, provide true, complete and accurate information to the FDA and other similar foreign regulatory bodies, comply with manufacturing standards we have established, comply with healthcare fraud and abuse laws in the United States and similar foreign fraudulent misconduct laws, or report financial information or data accurately or to disclose unauthorized activities to us. If we obtain FDA approval of any of our product candidates and begin commercializing those products in the United States, our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. These laws may impact, among other things, our current activities with principal investigators and research patients, as well as proposed and future sales, marketing and education programs. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials.
Efforts to ensure that our business arrangements comply with applicable healthcare laws may involve substantial costs. It is possible that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or in asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, damages, disgorgement, monetary fines, imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to develop our business. In addition, the approval and commercialization of any of our product candidates outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
We may not obtain or maintain the benefits associated with orphan drug designation, including market exclusivity.
On December 9, 2015, we announced that we receivedRegulatory authorities in some jurisdictions, including the United States and the European Union, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Designation fromAct, the FDA’s Office of Orphan Products Development (“OOPD”) for our cancer vaccine TPIV200FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, which is a disease or condition that affects fewer than 200,000 individuals in the treatment of ovarian cancer. The TPIV200 ovarian cancer clinical program will now receive benefits including tax credits on clinical research and seven-year market exclusivity upon receiving marketing approval. Even though we were granted orphan drug designation, we may not receive the benefits associated with orphan drug designation. This may result from a failure to maintain orphan drug statusUnited States, or result from a competing product reaching the market that has an orphan designation for the same disease indication. Under U.S. regulations for orphan drugs, if such a competing product reaches the market before ours does, the competing product could potentially obtain a scope of market exclusivity that limits or precludes our product from being soldmore than 200,000 individuals in the United States for which there is no reasonable expectation that the cost of developing and making available in the United States a drug or biologic for this type of disease or condition will be recovered from sales in the United States for that drug or biologic. Generally, a product that has orphan drug designation and subsequently receives the first FDA approval for the disease for which it has such designation is entitled to orphan drug exclusive approval (or exclusivity), which means that the FDA may not approve any other applications to market the same drug or biologic for the same indication for seven years. years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation.
Even if we were to obtain orphan drug designation for a product candidate, we may not obtain orphan exclusivity and that exclusivity may not effectively protect the drug from the competition of different drugs for the same condition, which could be approved during the exclusivity period. Additionally, after an orphan drug is approved, the FDA could subsequently approve aanother application for the same drug for the same conditionindication if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. A competitor also may receive approval of different products for the same indication for which our orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity.
In addition, if and when we request orphan drug designation in Europe, the European exclusivity period is ten years but can be reduced to six years if the drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivityexclusive marketing rights in the United States also may be lost if the FDA or European Medicines Evaluation Agency (“EMEA”EMA”) later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. The failure to obtain an orphan drug designation for any product candidates we may develop, the inability to maintain that designation for the duration of the applicable period, or the inability to obtain or maintain orphan drug exclusivity could reduce our ability to make sufficient sales of the applicable product candidate to balance our expenses incurred to develop it, which would have a negative impact on our operational results and financial condition.
New regulatory pathways for biosimilar competition could reduce the duration of market exclusivity for our products.
Under the federal Patient Protection and Affordable Care Act (“PPACA”)ACA enacted in 2010, there is an abbreviated path in the United States for regulatory approval of products that are demonstrated to be “biosimilar” or “interchangeable” with an FDA-approved biological product. The PPACAACA provides a regulatory mechanism that allows for FDA approval of biologic drugs that are similar to (but not generic copies of) innovative drugs on the basis of less extensive data than is required by a full BLA. Under this regulation, an application for approval of a biosimilar may be filed four years after approval of the innovator product. However, qualified innovative biological products will receive 12 years of regulatory exclusivity, meaning that the FDA may not approve a biosimilar version until 12 years after the innovative biological product was first approved by the FDA. However, the term of regulatory exclusivity may not remain at 12 years in the United States and could be shortened. A number of jurisdictions outside of the United States have also established abbreviated pathways for regulatory approval of biological products that are biosimilar to earlier versions of biological products. For example, the European Union has had an established regulatory pathway for biosimilars since 2005.
The increased likelihood of biosimilar competition has increased the risk of loss of innovators’ market exclusivity. Due to this risk, and uncertainties regarding patent protection, if one of our late-stage product candidates or other clinical candidates are approved for marketing, it is not possible to predict the length of market exclusivity for any particular product with certainty based solely on the expiration of the relevant patent(s) or the current forms of regulatory exclusivity. It is also not possible to predict changes in United States regulatory law that might reduce biological product regulatory exclusivity. The loss of market exclusivity for a product would likely materially and negatively affect revenues from product sales of that product and thus our financial results and condition.
Changes in laws and regulations affecting the healthcare industry could adversely affect our business.
As described above, the PPACAACA and potential regulations thereunder easing the entry of competing follow-on biologics into the marketplace, other new legislation or implementation of existing statutory provisions on importation of lower-cost competing drugs from other jurisdictions, and legislation on comparative effectiveness research are examples of previously enacted and possible future changes in laws that could adversely affect our business.
The current U.S. administration and Congress could carry out significant changes in legislation, regulation, and government policy (including with respect to the possible repeal of all or portions of the PPACA,ACA, possible changes in the existing treaty and trade relationships with other countries, and tax reform). While it is not possible to predict whether and when any such changes will occur, changes in the laws, regulations, and policies governing the development and approval of our product candidates and the commercialization, importation, and reimbursement of our product candidates could adversely affect our business.
Risks Related to our Securities
The price of our stock may be volatile.
The trading price of our common stock may fluctuate substantially. The price of our common stock that will prevail in the market may be higher or lower than the price at which our shares of common stock, depending on many factors, some of which are beyond our control and may not be related to our operating performance. These fluctuations could cause you to lose part or all of your investment in our common stock. Those factors that could cause fluctuations include, but are not limited to, the following:
| · | price and volume of fluctuations in the overall stock market from time to time; |
| · | fluctuations in stock market prices and trading volumes of similar companies; |
| · | actual or anticipated changes in our net loss or fluctuations in our operating results or in the expectations of securities analysts; |
| · | results of our preclinical studies and clinical trials or delays in anticipated timing; |
| · | the issuance of new equity securities pursuant to a future offering, including issuances of preferred stock; |
| · | announcements of new collaboration agreements with strategic partners or developments by our existing collaboration partners; |
| · | announcements of acquisitions, mergers or business combinations; |
| · | announcements of technological innovations, new commercial products, failures of products or product candidates, or progress toward commercialization by our competitors or peers; |
| · | general economic conditions and trends; |
| · | positive and negative events relating to healthcare and the overall pharmaceutical and biotechnology sectors; |
| · | major catastrophic events; |
| · | sales of large blocks of our stock and sales by insiders and our institutional investors; |
| · | departures of key personnel; |
| · | changes in the regulatory status of our immunotherapies, including results of our clinical trials; |
| · | events affecting BCM, Mayo Clinic, Mayo Foundation for Medical Education and Research or any future collaborators; |
| · | announcements of new |
| · | regulatory developments in the United States and other countries; |
| · | failure of our common stock to maintain listing requirements on Nasdaq; |
| · | the |
| · | changes in accounting principles; and |
| · | discussion of the Company or our stock price by the financial and scientific press and in online investor communities. |
In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been brought against that company. Due to the potential volatility of our stock price, we may therefore be the target of securities litigation in the future. Securities litigation could result in substantial costs and divert management’s attention and resources from our business.
A limited public trading market may cause volatility in the price of our common stock.
The listing of our common stock on the Nasdaq Capital Market does not assure that a meaningful, consistent and liquid trading market currently exists or will exist in the future. In recent years, the stock market has experienced extreme price and volume fluctuations that have particularly affected the market prices of many smaller companies like us. Our common stock is thus subject to this volatility. Sales of substantial amounts of common stock, or the perception that such sales might occur, could adversely affect prevailing market prices of our common stock and our stock price may decline substantially in a short time and our stockholders could suffer losses or be unable to liquidate their holdings. Our stock is thinly traded due to the limited number of shares available for trading thus causing large swings in price. There is no established trading market for our warrants.
The market prices for our common stock may be adversely impacted by future events.
Market prices for our common stock will be influenced by a number of factors, including:
| · | the issuance of new equity securities pursuant to a future offering, including issuances of shares upon the exercise of outstanding warrants or the issuance of preferred stock; |
| · | changes in interest rates; |
| · | competitive developments, including announcements by competitors of new products or services or significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| · | variations in quarterly operating results; |
| · | change in financial estimates by securities analysts; |
| · | the depth and liquidity of the market for our common stock and warrants; |
| · | investor perceptions of us and the pharmaceutical and biotech industries generally; and |
| · | general economic and other national conditions. |
If we fail to remain current with our listing requirements, we could be removed from the Nasdaq Capital Market which would limit the ability of broker-dealers to sell its securities and the ability of stockholders to sell its securities in the secondary market.
Companies listed for trading on the Nasdaq Capital Market must be reporting issuers under Section 12 of the Exchange Act. If we fail to file such reports in a timely manner, or if we fail to meet any other listing requirements, the shares of our common stock would eventually cease to be listed on the Nasdaq Capital Market, and the market liquidity for our securities could be severely adversely affected by limiting the ability of broker-dealers to sell its securities and the ability of stockholders to sell their securities in the secondary market.
Sales of additional equity securities may adversely affect the market price of our common stock and your rights may be reduced.
We expect to continue to incur drug development and sale, general and administrative costs,costs. Until such time, if ever, as we can generate substantial product revenue, we expect to fund our cash requirements through a combination of equity offerings, debt financings and to satisfy our funding requirements,potential collaboration, license and development agreements. We do not currently have a committed external source of funds. To the extent that we will need to sell additional equity securities whichor convertible debt securities, your ownership interest will be diluted, and the terms of these securities may be subject to registrationinclude liquidation or other preferences that adversely affect your rights and warrants with anti-dilutive protective provisions.as a common stockholder. The sale or the proposed sale of substantial amounts of our common stock or other equity securities in the public markets may adversely affect the market price of our common stockstock. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our stock priceability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may decline substantially. Our stockholdersbe required to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may experience substantial dilutionnot be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our drug development or future commercialization efforts or grant rights to develop and a reduction in the pricemarket product candidates that they are ablewe would otherwise prefer to obtain upon sale of their shares. Also, new equity securities issued may have greater rights, preferences or privileges than our existing common stock.develop and market ourselves.
Because we have a significant number of additional authorized shares of common stock available for issuance and outstanding warrants to purchase our common stock, our stockholders may experience dilution in the future and it may adversely affect the market price of our securities.
We are currently authorized to issue 150 million shares of our common stock. As of December 31, 2018,2019, we had 45,440,70445.7 million shares of our common stock issued and outstanding. Those outstanding shares represent a minority of our authorized shares, meaning that the ownership position of the current stockholders could be diluted significantly were we to issue a large number of additional shares. In addition, as of December 31, 2018,2019, there were outstanding warrants to purchase up to approximately 23.022.7 million shares of our common stock at a weighted average exercise price of $4.78$4.71 per share, and options exercisable for an aggregate of approximately 4.15.0 million shares of common stock at a weighted average exercise price of $8.69$7.79 per share. We have registered the resale of the shares issuable upon exercise of our outstanding warrants, and as a result the shares issued upon exercise will be tradable by the exercising party. Upon such registration, the holders may sell these shares in the public markets from time to time, without limitations on the timing, amount, or method of sale. If our stock price rises, the holders may exercise their warrants and options and sell a large number of shares. This could cause the market price of our common stock to decline and cause existing stockholders to experience significant further dilution.
The accounting treatment for certain of our warrants is complex and subject to judgments concerning the valuation of embedded derivative rights within the applicable securities. Fluctuations in the valuation of these rights could cause us to take charges to our statement of operations and make our financial results unpredictable.
Certain of our outstanding warrants contain or contained prior to being amended, or may be deemed to contain from time to time, embedded derivative rights in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). There is a risk that questions could arise from investors or regulatory authorities concerning the appropriate accounting treatment of these instruments, which could require us to restate previous financial statements, which in turn could adversely affect our reputation, as well as our results of operations. These derivative rights, or similar rights in securities we may issue in the future, need to be, or may need to be, separately valued as of the end of each accounting period in accordance with GAAP. We record these embedded derivatives as liabilities at issuance, valued using the Black Scholes Option Pricing Model and are subject to revaluation at each reporting date. Any change in fair value between reporting periods is reported on our statement of operations. At December 31, 2018,2019, the fair value of the derivative liability-warrants was $49,000.$31,000. Changes in the valuations of these rights, the valuation methodology or the assumptions on which the valuations are based could cause us to take charges to our earnings, which would adversely impact our results of operations. Moreover, the methodologies, assumptions and related interpretations of accounting or regulatory authorities associated with these embedded derivatives are complex and, in some cases uncertain, which could cause our accounting for these derivatives, and as a result, our financial results, to fluctuate.
We do not intend to pay cash dividends.
We have not declared or paid any cash dividends on our common stock, and we do not anticipate declaring or paying cash dividends for the foreseeable future. Any future determination as to the payment of cash dividends on our common stock will be at our board of directors’ discretion and depends on our financial condition, operating results, capital requirements and other factors that our board of directors considers to be relevant.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
We do not own any real estate or other properties. We lease office space at 5 West Forsyth Street, Suite 200, Jacksonville, Florida 32202, for our principal business office on a five-year agreement due to expire on June 30, 2022. The base rent is approximately $8,600 per month.
In November 2018, we leased office space at 3200 Southwest Freeway, Suite 2240, Houston, Texas 77027 on a three-year agreement set to expire in November 2021 (the “Houston Office”).
On February 15, 2019, we announced the relocation of, which is our corporate headquarters from the Jacksonville location to the Houston Office. Base rent is approximately $10,000 per month.
principal business office. We also rent anlease office space at the5 West Forsyth Street, Suite 200, Jacksonville, Florida Atlantic Research and Development Authority at 3651 FAU Blvd, Boca Raton, Florida32202 on a month by month agreement. The monthly rent for the Boca Raton space is approximately $800 per month.five-year agreement due to expire on June 30, 2022.
In January 2019, we leased a dedicated portion of an existing laboratory located at the Texas Medical Center in Houston for the purpose of conducting laboratory research and other laboratory related activities. The laboratory, referred to as JLABS, was established by Johnson & Johnson at the Texas Medical Center to provide space for research and development stage entities. We signed an 11-month license,lease, which automatically renews for 3-month successive periods for two dedicated suites and access to common space of approximately 20,000 square feet of the JLABS premises located at the Texas Medical Center. The base rent is $6,000 per month.
As of December 31, 2018,2019, we were not a party to any material legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURE
Not Applicable
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is listed for trading on the Nasdaq Capital Market under the symbol “MRKR”. As of February 28, 2019,2020, we had 492465 stockholders of record whom are holding shares. The price of our common stock on February 28, 20192020 was $6.22$2.47 per share.
Dividend Policy
No dividends have been declared or paid on our common stock. We have incurred recurring losses and do not currently intend to pay any cash dividends in the foreseeable future.
Recent Sales of Unregistered Securities
We recorded thedid not record any issuances of the following unregistered securities during the fourth quarter of 2018 pursuant to exemptions under the Securities Act of 1933, including Section 4(2):2019.
During the fourth quarter of 2018, 65,000 shares of common stock were issued pursuant to third parties consisting of (i) 50,000 shares to Caro Capital for services pursuant to a vendor agreement and (ii) 15,000 shares to Omnicor Media for services pursuant to a vendor agreement.
ITEM 6. SELECTED FINANCIAL DATA
| ITEM 6. SELECTED FINANCIAL DATA |
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
| ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition, changes in financial condition, plan of operations and results of operations should be read in conjunction with (i) our audited consolidated financial statements as at December 31, 20182019 and December 31, 20172018 and (ii) the section entitled “Business”, included in this annual report. The discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors.
Company Overview
We are a clinical-stage immuno-oncology company specializing in the development and commercialization of novel T cell-based immunotherapies and innovative peptide-based vaccines for the treatment of hematological malignancies and solid tumor indications. Our MultiTAAWe developed our lead product candidates from our MultiTAA-specific T cell technology, which is based on the selective expansion of non-engineered, tumor-specific T cells that recognize tumor associated antigens, (“TAA” i.e.or TAAs, which are tumor targets)targets, and then kill tumor cells expressing those targets. Once infused into patients, this population ofThese T cells recognizesare designed to recognize multiple tumor targets to produce broad spectrum anti-tumor activity. We are advancing two pipelines of product candidates as part of our MultiTAA-specific T cell program: the autologous T cells for the treatment of lymphoma, multiple myeloma, or MM, and selected solid tumors and the allogeneic T cells for the treatment of acute myeloid leukemia, or AML, and acute lymphoblastic leukemia, or ALL. Because we do not genetically engineer the MultiTAA-specific T cell therapies, we believe that our T cells, when comparedproduct candidates are easier and less expensive to manufacture, with reduced toxicities, than current engineered chimeric antigen receptor, (“CAR”)or CAR-T, and T cell receptor (“TCR”)-based approaches, our products are significantly less expensive to manufacturereceptor-based therapies and appear to be markedly less toxic, and yet are associatedmay provide patients with meaningful clinical benefit. As a result, we believeWe are also developing innovative peptide-based immunotherapeutic vaccines for the treatment of metastatic solid tumors.
We are pursuing post-transplant AML as the lead indication for our portfolio offirst company-sponsored MultiTAA-specific T cell therapies has a compelling therapeutic product profile, as compared to current gene-modified CAR and TCR-based therapies. In addition, our Folate Receptor Alpha program (TPIV200) for breast and ovarian cancers and our HER2/neu program (TPIV100/110) are in Phase II clinical trials. In parallel, we are developing a proprietary nucleic acid-based antigen expression technology named PolyStart™ to improve the ability of the immune system to recognize and destroy diseased cells.
Immuno-oncology, which utilizes a patient’s own immune system to combat cancer, is one of the most actively pursued areas of research by biotechnology and pharmaceutical companies today. Interest and excitement about immunotherapy are driven by compelling efficacy data in cancers with historically bleak outcomes, and the potential to achieve a cure or functional cure for some patients. Harnessing the power of the immune system is an important component of fighting cancerous cells in the body. Our MultiTAAprogram. The MultiTAA-specific T cell therapy platform identifies and selects effectively all T cells that are specific for any peptide from the antigens that we target (e.g., WT1, MAGE-A4, PRAME, Survivin, NY-ESO-1, and SSX2). Our in-vitro manufacturing process promotes proliferation of very rare cancer-killing T cells and augments their anti-tumor properties to provide benefit to patients following their infusion. By using the multi-antigen targeted approach,has been well tolerated in an ongoing Phase 1/2 clinical trial conducted by our proprietary technology can kill heterogeneous tumor cell populations more effectively than single-antigen targeted approaches, thereby reducing the likelihood of tumor escape and potentially increasing the durability of a patient’s response to therapy.
Recent Developments
Change in Headquarters.On February 15, 2019 we announced a change in our corporate headquarters from Jacksonville, Florida to Houston, Texas.
Presentations at American Society for Blood and Marrow Transplantation and theCenter for International Blood and Marrow Transplant Research(ASBMT and CIBMTR).Between February 20-23, 2019, four abstracts, including three oral presentations, were presented at the Transplantation & Cellular Therapy (TCT) Meetings of the American Society for Blood and Marrow Transplantation and theCenter for International Blood and Marrow Transplant Research(ASBMT and CIBMTR). The studies summarize data achieved using multi-tumor antigen specific T cells that were developed atstrategic partner Baylor College of Medicine, or BCM. As reported in March 2019, eleven of the thirteen patients in the laboratoriesadjuvant disease setting dosed with the MultiTAA-specific T cell therapy after receiving an allogeneic stem cell transplant survived, ranging from 6 weeks to 2.5 years post-infusion, with nine of Dr. Swati Naik, Dr. Ann Leen, Dr. Premal Lulla and Dr. Juan Vera, and exclusively licensedthese remaining patients in continuing complete remission, or CCR. Survival of the six patients with active disease ranged from 4 to us.21 months, as compared to a historical survival rate of approximately 4.5 months for patients who receive the standard of care post-transplant.
PresentationsWe submitted an investigational new drug, or IND, application to the United States Food and Drug Administration, or the FDA to initiate a Phase 2 clinical trial of MultiTAA-specific T cell therapy, which we refer to as MT-401, in post-allogeneic hematopoietic stem cell transplant patients with AML in both the adjuvant and active disease setting, which may become pivotal pending the results of the interim analysis. The dose administered in this multicenter trial is the current maximum tolerated dose from the ongoing Phase 1/2 trial. In the adjuvant setting, patients will be randomized to either MultiTAA-specific T cell therapy at 60th American Societyapproximately 90 days post-transplant versus standard of Hematology Annual Meeting (ASH 2018).Between December 1-3, 2018 three presentations, including one oral presentation were presented at 60th American Societycare observation, while the active disease patients will receive MT-401 following relapse post-transplant as part of Hematology Annual Meeting.The studies describe results achieved using multi-tumor antigen specific T cellsa single-arm group. In February 2020, we announced that were developed at the Baylor CollegeFDA has permitted us to initiate our Phase 2 clinical trial beginning with a safety lead-in portion of Medicine in the laboratories of Dr. Swati Naik, Dr. Premal Lulla, Dr. Ann Leen and Dr. Juan Vera, and exclusively licensed to Marker.trial.
Merger Agreement. On October 17, 2018,We recently reported interim data for an ongoing Phase 1/2 clinical trial of the Company completed its previously announced acquisition with Marker Cell Therapy, Inc., formerly known as Marker Therapeutics, Inc.,MultiTAA-specific T cell therapy for the treatment of pancreatic adenocarcinoma being conducted by BCM. In this trial, we have observed a privately-held Delaware corporation (“Marker Cell”), in accordanceclinical benefit correlated with the termspost-infusion detection of an Agreementtumor-reactive T cells in patient peripheral blood and Plan of Merger and Reorganization dated as of May 15, 2018 (the “Merger Agreement”) by and amongwithin tumor biopsy samples in patients in the Company, Timberwolf Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiarytumor-resection arm of the Company (“Merger Sub”),trial. These T cells exhibited activity against both targeted antigens and Marker. On October 17, 2018, pursuant to the Merger Agreement, Merger Sub was merged with and into Marker Cell (the “Merger”), with Marker Cell being the surviving corporation and becoming a wholly-owned subsidiarynon-targeted TAAs, indicating induction of the Company. In connection with the Merger, the Company changed its name to Marker Therapeutics, Inc. and Marker Cell changed its name to Marker Cell Therapy, Inc. At the effective time of the Merger, the former Marker Cell stockholders received (i) an aggregate of 13,914,255 shares of the Company’s common stock which equaled the number of shares of the Company’s common stock issued and outstanding immediately prior to the effective time of the Merger, and (ii) an aggregate of 5,046,003 warrants which equaled the number of the Company’s warrants and stock options issued and outstanding immediately prior to the effective time of the Merger.
The issuance of the shares of Company common stock to the former stockholders of Marker Cellantigen spreading. To date, we have not observed any cytokine release syndrome or neurotoxicity in connection with the Merger and related transactions was approved by the Company’s stockholders at the 2018 annual meeting of stockholders (the “2018 Annual Meeting”) held on October 16, 2018.
In connection with the Merger, the Company filed an amendment to its articles of incorporation in Nevada to increase the authorized shares of common stock from 41,666,667 shares to 150,000,000 shares and to change the Company’s name to Marker Therapeutics, Inc. (“Certificate of Amendment”). The Company then reincorporated from a Nevada corporation to a Delaware corporation and filed its certificate of incorporation in Delaware. Finally, a certificate of merger was filed in Delaware to merge Marker Cell Therapy, Inc. (f/k/a Marker Therapeutics, Inc.) with and into Merger Sub, with Marker Cell Therapy, Inc. being the surviving corporation and wholly owned subsidiary of the Company. The name change, reincorporation and Merger were all effective as of October 17, 2018. Beginning as of the market open on October 18, 2018, shares of the Company’s common stock commenced trading on The Nasdaq Capital Market under its new ticker symbol “MRKR”.
Securities Purchase Agreements. On October 17, 2018, concurrent with the completion of the Merger, the Company issued to certain accredited investors in a private placement transaction (the “Financing”), an aggregate of 17,500,000 shares of its common stock, and warrants to purchase 13,437,500 shares of common stock at an exercise price of $5.00 per share with a five-year term, for aggregate proceeds of $70 million pursuant to the terms of the Securities Purchase Agreements, dated June 8, 2018, by and among the Company and certain accredited investors.
After taking into account the issuance of shares in the Financing described above, immediately following the effective time of the Merger, the pro forma ownership of the issued and outstanding shares of Company common stock on a fully diluted basis (assuming all issued and outstanding warrants and options are exercised) was approximately as follows: Marker Cell’s former stockholders 27.5%, Company stockholders prior to the Merger 27.5%, and the private placement stockholders 45%. Following the completion of the Merger and the Financing, there were 45,328,510 issued and outstanding shares of the Company’s common stock.
Products and Technology in Development
The following chart sets forth our products and technologies under development.

Our MultiTAA T Cell Productsthis trial.
We are advancing two MultiTAA T cell products through clinical development:
Whilealso evaluating the blood source and the antigens for stimulation differ between the LAPP and the MAPP products, the manufacturing process for each product is otherwise identical.
While single-antigen specific therapy can eliminate all the tumor cells expressing the targeted antigen, the residual tumor cells that do not express that antigen may survive and expand. In addition, tumor cells may also downregulate or mutate the targeted antigen, thus becoming invisible to the T cell therapy. Both phenomena create a transformed tumor that is impervious to that therapy. This process is referred to as antigen-negative tumor escape.
Our solution to the problem of tumor heterogeneity was to develop T cell products that simultaneously attack multiple tumor-expressed antigens and thereby enable more complete initial tumor targeting, thus minimizing the subsequent opportunity for the cancer to engage escape mechanisms. Of note, data suggest this strategy may be responsible for recruitment and activation of unique cancer-killing cells from the patient’s own immune repertoire to participate in cancer eradication, further minimizing the possibility for tumor cell escape.
Our proprietary MultiTAA T cell platform may have meaningful advantages over current CAR-T and TCR cell therapy approaches. Compared to current gene-modifiedMultiTAA-specific T cell therapies our programs are characterized by the following:
· Demonstrated clinical benefit, without the need for lymphodepletion before infusion: In BCM’s Phase I lymphoma study, we saw complete responses (“CRs”) in 50 – 60% of its evaluable patients. We believe it is significant that no patient with a CR has subsequently relapsed with disease, whereas typically 30% or more of patients with CR in reported CAR-T studies relapse within one year. In patient results to date, observed therapeutic responses appear to be highly durable, with some patients being relapse-free beyond five years.
· Non-gene-modified: Unlike CAR-T and TCR approaches, our therapy requires no genetic modification of T cells, a costly and complex process that significantly complicates the manufacturing of a patient product. We believe our therapy can be manufactured at a fraction of the cost of a gene-modified T cell product, with substantially reduced complexity of manufacturing.
· Low incidence rate of adverse events: In 78 patients treated to date, we have seen only one grade III adverse reaction considered possibly related to our therapy. This appears to compare favorably with published CD19 CAR-T studies, wherein up to 95% of patients had associated grade III or higher adverse events during treatment. We believe that it is notable that there have been no cases of cytokine-release syndrome (“CRS”), or related serious adverse events (“SAEs”) in patients treated with MAPP or LAPP therapy to date.
· Capable of addressing a broad repertoire of cancer cells: While CAR-T and TCR therapies generally target a single epitope, our manufacturing process selects for T cells that are specific for multiple peptides derived from several targeted antigens. Deep gene sequencing of our products shows that a typical patient dose usually consists of approximately 4,000 unique T cell clonotypes targeting up to five different tumor-associated antigens. In layman’s terms, the five antigen targets can be recognized by a very wide range of T cells, facilitating robust killing of targeted cancer cells.
· Appears to drive endogenous immune responses: We see evidence of “epitope spreading” in our patients, meaning that our therapy is potentially inducing an enhanced response by the patient’s own T cells (specific for an expanded set of tumor-associated antigens beyond those targeted by our infused product). Our correlative analyses show expansion of endogenous T cells, other than those present in our product, in the months following the infusion of our product. This phenomenon, also known as “antigen spreading,” is potentially important in generating a durable response for a patient, because it enables the killing of tumors that do not express any of the antigens initially targeted by our product.
Our Folate Receptor Products
Folate Receptor alpha (“FRa”) is overexpressed in over 80% of breast cancers and in addition, over 90% of ovarian cancers, for which the only treatment options are surgery, radiation therapy and chemotherapy, creating a very important and urgent clinical need for a new therapeutic strategy. Time to recurrence is relatively short for ovarian cancer and survival prognosis is extremely poor after recurrence. In the United States alone, there are approximately 30,000 ovarian cancer patients and 40,000 triple-negative breast cancer patients newly diagnosed every year. The FRa vaccine (now called TPIV200) intended to treat these conditions is composed of a mixture of five FRa immunogenic peptides adjuvanted with low-dose granulocyte-macrophage colony-stimulating factor (“GM-CSF”).
GMP Manufacturing Scale Up of TPIV200 and Production to Supply Additional Phase II Clinical Trials
We have developed a commercial-quality lyophilized formulation of the TPIV200 peptides in a single vial for reconstitution and injection. Multi-gram peptide production scale-up has been successfully concluded, and so has the GMP manufacturing of a recent clinical lot of the TPIV200 peptides. The supply will be used in the company’s ongoing Phase II study in platinum-sensitive ovarian cancer, as well as the 280-patient Phase II study sponsored by the Mayo Foundation and funded by the U.S. Department of Defense (“DoD”) for treating triple-negative breast cancer. We also made various improvements to the vaccine manufacturing process, resulting in what we believe to be a superior formulation of the vaccine that is more amenable to large-scale manufacturing and commercialization. Thus, Good Manufacturing Practice (“GMP”) manufacturing development for the Phase II trials has been completed.
Phase I Human Clinical Trial –Folate Receptor Alpha Breast and Ovarian Cancers – Mayo Foundation
On July 27, 2015, we exercised our option agreement with Mayo Foundation with the signing of a worldwide exclusive license agreement to commercialize the proprietary FRa vaccine technology for all cancer indications. As part of this agreement, the IND for the Folate Receptor alpha Phase I trial was transferred from Mayo Foundation to the Company for Phase II clinical trials as our lead peptide vaccine product.
The results from the initial 21-patient Phase I2 clinical trial for the FRa vaccinetreatment of breast cancer and in Phase 1 clinical trials for the treatment of ALL, lymphoma, MM and sarcoma, all of which are being conducted by BCM. As of December 2019, the MultiTAA-specific T cell therapies have now been reported. Twenty-one patients with breast or ovarian cancer, who had undergone standard surgery and adjuvant treatment, were treated with one cycle of cyclophosphamide. Following this, patients were vaccinated intradermally with TPIV200 on day one of a 28-day cycle for a maximum of six vaccination cycles. On March 15, 2018, we announced the publicationgenerally well tolerated by all of the patients enrolled in clinical data from this trial. The results showtrials in hematological and solid tumor indications with no incidents of cytokine release syndrome or neurotoxicity, which are frequently associated with CD19 CAR-T therapies. Based on our observations in clinical trials in AML, pancreatic cancer, lymphoma, ALL and MM, we believe that over 90% of patients developed robust and durable antigen-specific immune responses against FRa without regard for HLA type, which aligns with the intended mechanism of action ofMultiTAA-specific T cell therapies have the vaccine. TPIV200 vaccine was safe and well-tolerated; 20 out of 21 evaluable patients showed positive immune responses, providingpotential to mediate a strong rationale for progressing to Phase II trials. Further, the data showed that 16 out of 16 patients in the observation stage showed persistent immune responses (Source: published online 15Mar2018; DOI: 10.1158/1078-0432.CCR-17-2499).
Phase II Development of TPIV200 for Triple-negative Breast Cancer
Triple-negative breast cancer (“TNBC”) is one of the most difficult cancers to treat and represents a clear unmet medical need. On September 15, 2015, we announced that our collaborators at the Mayo Foundation had been awarded a grant of $13.3 million from the DoD. This grant led by Dr. Keith Knutson of the Mayo Clinic in Jacksonville, Florida covers the costs for a 280-patient Phase II clinical trial of the FRa vaccine in patients with TNBC. We are working closely with Mayo Foundation on this clinical trial by providing clinical and manufacturing expertise,meaningful anti-tumor effect, as well as providing GMP vaccine formulations under contract. Thissignificant in vivo expansion of T cells. We may initiate additional Phase II study of TPIV2002 clinical trials investigating other indications in the treatment of triple-negative breast cancer began enrolling patients in late 2017 and enrollment continues. Details regarding this trial can be found at www.clinicaltrials.gov under identifier numbers NCT03012100 and RU011501I.
On June 21, 2016, we announced the initiation of a randomized four-arm Phase II trial of TNBC that is sponsored and conducted by the Company (FRV-002), enrolling women with stage I-III disease who have completed initial surgery and chemo/radiation therapy. This open-label, 80-patient clinical trial is designed to evaluate dosing regimens, pre-treatment, efficacy, and immune responses. The study is evaluating two doses of TPIV200 (a high dose and a low dose), each of which will be tested both with and without cyclophosphamide prior to vaccination. Key data from the trial are expected to be included in a future Biologics License Application submission to the FDA for marketing clearance. We completed enrollment in late 2017 and are now treating and following the patients. An independent Data Safety Monitoring Board (“DSMB”) reviews the safety in this ongoing Phase II study; no safety issues have been identified to date. Details regarding this trial can be found at www.clinicaltrials.gov under the identifier number NCT02593227.
Phase II Development of TPIV200 for Ovarian Cancer
On December 9, 2015, we announced that we received Orphan Drug Designation from the U.S. Food & Drug Administration’s Office of Orphan Products Development (“OOPD”) for our cancer vaccine TPIV200 in the treatment of ovarian cancer. The TPIV200 ovarian cancer clinical program will now receive benefits including tax credits on clinical research and seven-year market exclusivity upon receiving marketing approval. TPIV200 is a multi-epitope peptide vaccine that targets Folate Receptor alpha which is overexpressed in multiple cancers including over 90% of ovarian cancers. On February 3, 2016, we announced that the U.S. FDA designated the investigation of the multiple-epitope TPIV200 vaccine for maintenance therapy in subjects with platinum-sensitive advanced ovarian cancer who achieved stable disease or partial response following completion of standard-of-care chemotherapy, as a Fast Track Development Program.
On April 21, 2016, we announced our participation in an ovarian cancer study sponsored by Memorial Sloan Kettering Cancer Center (“MSKCC”) in New York City in collaboration with AstraZeneca Pharmaceuticals in ovarian cancer patients who are not responsive to platinum, a commonly used chemotherapy for ovarian cancer. This study, an open-label Phase II study of TPIV200 in 40 patients is designed to look at the effects of combination therapy with AstraZeneca’s checkpoint inhibitor durvalumab (anti-PD-L1). Interim results from the first 27 patients were presented at the AACR-Rivkin Symposium in September 2018; safety of the combination was established in these heavily-pretreated patients and a subset of patients exhibited durable disease stabilization. ORR and PFS with combination treatment was not superior from the expected efficacy of single-agent PD-1/PD-L1 blockade. However, post-immunotherapy follow-up was suggestive of improved clinical benefit from standard therapies, as the majority of patients post-progression went on to receive subsequent standard therapy with durable clinical benefit, creating a rationale for exploration of these agents in combination with chemotherapy. Although we have no business relationship with AstraZeneca, we are paying for one-half of the costs of the clinical study,2020 in addition to providing our TPIV200 for the study. Details regarding thisplanned Phase 2 trial can be found at www.clinicaltrials.gov under identifier numbers NCT02764333.in post-transplant AML patients.
On January 10, 2017, we announced the initiation of a Company-sponsored Phase II study in platinum-sensitive ovarian cancer patients (FRV-004). This multi-center, double-blind efficacy study is designed to evaluate TPIV200 compared to GM-CSF alone in a randomized, placebo-controlled fashion during the first maintenance period after primary surgery and chemotherapy. We have opened multiple clinical sites and enrollment of the 120 patients has been completed ahead of schedule. The 120th subject was given the study drug on December 10, 2018. Safety is reviewed by an independent DSMB quarterly and an interim efficacy analysis is planned in 2019, once 50 patients have progressed. Details regarding this trial can be found at www.clinicaltrials.gov under the identifier number NCT02978222.
TPIV 100/110 – HER2/neu peptides with GM-CSF
Human epidermal growth factor receptor 2 (“HER2/neu”) amplification/overexpression results in an effective therapeutic target in breast and gastric cancer. Over-expressed HER2 is detected predominantly in malignancies of epithelial origin, such as breast, gastric, esophageal, colorectal, salivary gland, pancreatic, epithelial ovarian, endometrial, and bladder carcinomas, as well as gallbladder and extrahepatic cholangiocarcinomas. HER2 is over-expressed in approximately 25% of breast cancers and its expression is associated with unfavorable pathologic features and aggressive disease if not treated with targeted therapies, relative to other forms of breast cancer. While the outcome of patients with HER2 positive breast cancer has significantly improved in the past few decades with an advent of anti-HER2 therapies, a substantial number of resected patients still subsequently develop metastatic disease. The continued prevalence of these cancers represents a high unmet medical need, justifying the targeted development of immunotherapeutic strategies.
We have added a Class I-restricted peptide, also licensed from the Mayo Foundation on April 16, 2012, to the four Class II-restricted peptides in TPIV100, resulting in TPIV 110 after the five peptides are mixed with GM-CSF. Management believes that the combination of Class I and Class II HER2/neu antigens, gives us the leading HER2/neu vaccine platform. We have amended the IND to incorporate the fifth peptide and will use TPIV110 in subsequent studies with the goal of producing an even more robust vaccine activating both CD4+ (helper) and CD8+ (killer) T cells.
Transition of the HER2/neu Vaccine
On June 7, 2016, we announced that the Company had exercised its option agreement with Mayo Foundation and signed a worldwide license agreement to the proprietary HER2/neu vaccine technology. The license gives the Company the right to develop and commercialize the technology in any cancer indication in which the Her2/neu antigen is overexpressed. As part of this agreement, the IND for the HER2/neu Phase I Trial was transferred from Mayo Foundation to the Company for Phase II clinical trials as TPIV100, our second product.
Phase I Human Clinical Trial –HER2/neu+ Breast Cancer – Mayo Foundation
A Phase I study using a vaccine containing four HER2/neu peptides in combination with GM-CSF (now called TPIV100) was initiated in 2012 at the Mayo Clinic and the primary readout was completed in 2015. Final safety analysis on all the patients treated showed that the vaccine was safe in that context. In addition, 19 out of 20 evaluable patients showed robust T-cell immune responses to the antigens in the vaccine composition providing a case for advancement to Phase II. Data from the study was presented at the San Antonio Breast Cancer Symposium on December 10, 2015. An additional secondary endpoint incorporated into this Phase I Trial was a two-year follow-on recording the time to disease recurrence in the participating breast cancer patients. Details regarding this trial can be found at www.clinicaltrials.gov under the identifier number NCT01632332.
On March 14, 2017, we announced that our partners at the Mayo Clinic received a $3.8 million grant from the DoD to conduct a Phase Ib study of the HER2-targeted vaccine candidate (TPIV100) in an early form of breast cancer called ductal carcinoma in situ (“DCIS”). This is the second Company vaccine to be tested in a fully-funded study sponsored by the Mayo Foundation. We are working closely with Mayo Foundation on this clinical trial by providing clinical and manufacturing expertise, as well as providing GMP vaccine formulations under contract. If the study is successful, our HER2/neu vaccine may eventually augment or even replace standard surgery and chemotherapy, and potentially could become part of a routine immunization schedule for preventing breast cancer in healthy women. The study is expected to enroll 40 – 45 women with DCIS and commence such enrollment during the first quarter of 2019.
Phase II Development of the HER2/neu TPIV110 Vaccine
On October 10, 2018, we announced that Mayo Clinic had been awarded a grant of $11 million from the DoD. This grant is intended to cover the costs of a large randomized, double-blind Phase II study of the Company’s HER2/neu-targeted breast cancer vaccine, TPIV110 with maintenance ado-trastuzumab emtansine (T-DM1) compared to GM-CSF alone, in combination with standard one year of T-DM1 maintenance therapy, for treating up to 190 women with HER2/neu-positive breast cancer. We are working closely with Mayo Foundation on this clinical trial by providing clinical and manufacturing expertise, as well as providing GMP vaccine formulations under contract. The study will ask whether the administration of vaccine during T-DM1 maintenance therapy in patients with residual disease post-neoadjuvant chemotherapy effectively blocks disease recurrence and the development of metastatic breast cancer. By prevention of recurrence and metastasis, the expectation is that mortality associated with breast cancer will be decreased.
Products and Technology – Pre-clinical
Polystart
In addition to the clinical developments, our peptide vaccine technology can be coupled with our PolyStart™ nucleic acid-based technology, which is designed to make vaccines significantly more effective by producing four times the required peptides for the immune systems to recognize and act on.
Financial Overview
Critical Accounting Policies
The consolidated financial statements are prepared in conformity with U.S. GAAP, which require the use of estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent liabilities at the date of the financial statements, and the reported amounts of expenses in the periods presented. We believe that the accounting estimates employed are appropriate and resulting balances are reasonable; however, due to inherent uncertainties in making estimates, actual results could differ from the original estimates, requiring adjustments to these balances in future periods. The critical accounting estimates that affect the consolidated financial statements and the judgments and assumptions used are consistent with those described under Note 3 in the Notes to Consolidated Financial Statements in this Form 10-K.
Research and Development Expenses
To date, our research and development expenses have related primarily to the development of our clinical platform and the identification and development of our product candidates. Clinical and research and development expenses consist of expenses incurred in performing research and development activities, cost of our clinical trials, including compensation, share-based compensation expense and benefits for research and development employees and consultants, facilities expenses, overhead expenses, cost of supplies, manufacturing expenses, fees paid to third parties and other outside expenses.
Clinical costs are expensed as incurred. Costs and timing of clinical trials and development of our product candidates will depend on a variety of factors that include, but are not limited to, the following:
| per patient clinical trial costs; |
| • | the number of patients that participate in the clinical trials; |
| • | the number of sites included in the clinical trials; |
| • | the length of time required to enroll eligible patients; |
| • | the number of doses that patients receive; |
| • | the drop-out or discontinuation rates of patients; |
| • | potential additional safety monitoring or other studies requested by regulatory agencies; |
| • | the duration of patient follow-up; |
| • | the efficacy and safety profile of the product candidates; and |
| • | the ability to successfully manufacture patient doses. |
In addition, the potential for success of each product candidate will depend on numerous factors, including clinical trial outcomes, acceptance by regulatory authorities, competition, manufacturing capability and commercial viability. We determine which programs to pursue and how much to fund each program in response to ongoing scientific assessments, competitive developments, clinical trial results, as well as an assessment of each product candidate's commercial potential. We anticipate our research and development costs will continue to increase over the next several years due to increased spending on the clinical development and manufacturing of our product candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and other related costs, including share-based compensation, for personnel in executive, finance, accounting, business development, legal and human resources functions. Other significant costs include facility costs not otherwise included in research and development expenses, legal fees relating to patent and corporate matters, insurance costs and professional fees for consultancy, accounting, audit and investor relations.
We anticipate that our general and administrative expenses will increase in the future to support our continued research and development activities, and the potential commercialization of our product candidates.
Income Taxes
We did not recognize any income tax expense for the years ended December 31, 20182019 and 2017.2018.
Other Income (Expense)
Other income (expense), net consists of interest income and change in fair value of warrant liabilities and debt extinguishment gain.liabilities.
Results of Operations For the Years Ended December 31, 20182019 and 20172018
The following table summarizes the results of our operations (rounded to the thousand except for per share amounts) for the years ended December 31, 20182019 and 2017,2018, together with the changes to those items:
| For the Years Ended | ||||||||||||||||
| December 31, | ||||||||||||||||
| 2018 | 2017 | Increase / (decrease) | ||||||||||||||
| Revenues: | ||||||||||||||||
| Grant income | $ | 206,000 | $ | 183,000 | $ | 23,000 | 13 | % | ||||||||
| Total revenues | 206,000 | 183,000 | 23,000 | 13 | % | |||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development - intellectual property acquired | 116,045,000 | - | 116,045,000 | - | ||||||||||||
| Research and development | 7,953,000 | 5,251,000 | 2,702,000 | 51 | % | |||||||||||
| General and administrative | 24,380,000 | 6,412,000 | 17,968,000 | 280 | % | |||||||||||
| Total operating expenses | 148,378,000 | 11,663,000 | 136,715,000 | 1172 | % | |||||||||||
| Loss from operations | (148,172,000 | ) | (11,480,000 | ) | (136,692,000 | ) | 1191 | % | ||||||||
| Other income (expense): | ||||||||||||||||
| Change in fair value of warrant liabilities | (40,000 | ) | 6,000 | (46,000 | ) | (767 | )% | |||||||||
| Interest income | 254,000 | - | 254,000 | - | ||||||||||||
| Debt extinguishment gain | - | 492,000 | (492,000 | ) | (100 | )% | ||||||||||
| Net loss | $ | (147,958,000 | ) | $ | (10,982,000 | ) | $ | (136,976,000 | ) | 1247 | % | |||||
| Net loss per share, Basic and Diluted | $ | (7.75 | ) | $ | (1.16 | ) | $ | (6.59 | ) | 568 | % | |||||
| Weighted average number of common shares outstanding | 19,092,000 | 9,453,000 | 9,639,000 | 102 | % | |||||||||||
| For the Years Ended December 31, | ||||||||||||||||
| 2019 | 2018 | Change | ||||||||||||||
| Revenues: | ||||||||||||||||
| Grant income | $ | 213,000 | $ | 206,000 | $ | 7,000 | 3 | % | ||||||||
| Total revenues | 213,000 | 206,000 | 7,000 | 3 | % | |||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development - intellectual property acquired | - | 116,045,000 | (116,045,000 | ) | (100 | )% | ||||||||||
| Research and development | 12,765,000 | 7,953,000 | 4,812,000 | 61 | % | |||||||||||
| General and administrative | 9,977,000 | 24,380,000 | (14,403,000 | ) | (59 | )% | ||||||||||
| Total operating expenses | 22,742,000 | 148,378,000 | (125,636,000 | ) | (85 | )% | ||||||||||
| Loss from operations | (22,529,000 | ) | (148,172,000 | ) | 125,643,000 | (85 | )% | |||||||||
| Other income (expense): | ||||||||||||||||
| Change in fair value of warrant liabilities | 18,000 | (40,000 | ) | 58,000 | (145 | )% | ||||||||||
| Interest income | 1,083,000 | 254,000 | 829,000 | 326 | % | |||||||||||
| Net loss | $ | (21,428,000 | ) | $ | (147,958,000 | ) | $ | 126,530,000 | (86 | )% | ||||||
| Net loss per share, basic and diluted | $ | (0.47 | ) | $ | (7.75 | ) | $ | 7.28 | (94 | )% | ||||||
| Weighted average number of common shares outstanding | 45,588,000 | 19,092,000 | 26,496,000 | 139 | % | |||||||||||
Revenue
We did not generate any revenue during the years ended December 31, 20182019 and 2017,2018, respectively from the sales or licensing of our product candidates. During the year ended December 31, 2018,2019, we recognized $206,000$213,000 of revenue associated with a grant awarded to Mayo Foundation from the US Department of Defense for the Phase II2 Clinical Trial of TPIV200 which Mayo paid to us for clinical supplies manufactured by us and provided for the clinical studytrial funded by the grant. We refer to this grant as the Mayo Grant. During the year ended December 31, 2017,2018, we also recognized $183,000$206,000 of grant income.income from the Mayo Grant.
73
Operating Expenses
Operating expenses incurred during the fiscal year ended December 31, 20182019 were $148.4$22.7 million compared to $11.7$148.4 million in the prior year. Significant changes and expenditures in operating expenses are outlined as follows:
Research and Development Expense-Intellectual Property Acquired
Research and development – Intellectual Property Acquired, increaseddecreased $116.0 million in the year ended December 31, 2018 and representedto $0 in the year ended December 31, 2019, representing the fair market value of assets acquired by us in connection with the merger we completed in October 2018, or the Merger. Because the Merger was accounted for as an asset acquisition and the assets acquired consisted of intellectual property that hashad not received regulatory approval, the total purchase price was immediately expensed as in process research and development or intellectual property acquired.
Research and Development Expense
Research and development expenses increased by 51%61% to $12.8 million for the year ended December 31, 2019, compared to $8.0 million for the year ended December 31, 2018 from $5.3 million for the year ended December 31, 2017.2018.
Our research and development expenses are highly dependent on the phases of our research projects and therefore fluctuate from period to period.
The increase of $4.8 million in our research and development expenses of $2.7 million for the year ended December 31, 2018 compared to the same period in 2017 was primarily due to our increases from prior period for expenses relating to our planned clinical trials.
General and Administrative Expenses
General and administrative expenses increased by 280% to $24.4 million for the year ended December 31, 2018 from $6.4 million during the prior period. The increase of $18.0 million2019 was primarily attributable to the following:
| o |
| o |
| o | increase of $1.4 million in research and development stock-based compensation expenses, | |
| o | increase of |
| o | increase of $0.1 million in other expenses, and | |
| o | decrease of |
General and Administrative Expenses
General and administrative expenses decreased by 59% to $10.0 million for the year ended December 31, 2019 from $24.4 million during the prior period. The decrease of $14.4 million was primarily attributable to a decrease of $12.8 million in stock-based compensation expenses due to executive stock option grants issued in fiscal year 2018, as well as the following:
| o | increase of $0.8 million in headcount-related expenses as we increased the number of administrative personnel and also incurred a full year of expenses during fiscal year 2019 for personnel hired during the second half of fiscal year 2018, |
| o | increase of $1.0 million in legal and professional fees, |
| o | increase of $0.3 million in office-related expenses, insurance and other general and administrative expenses, and |
| o |
74
Other Income (Expense)
Change in fair valueFair Value of warrant liabilitiesWarrant Liabilities
The changeChange in fair value of warrant liabilities for fiscalthe year ended December 31, 20182019 was $40,000$18,000 as compared to ($6,000)40,000) for the fiscal year ended December 31, 2017. This increase by $40,000 for the fiscal year ended December 31, 2018 is reflected by a corresponding loss in other income (expense) in the consolidated statement of operations.2018.
Interest incomeIncome
Interest income was approximately $0.3$1.1 million for the year ended December 31, 20182019 and was attributable to interest income relating to a significant portion of the net proceeds received from our equity financing in October whichfunds that are held in U.S. Treasury notes and U.S. government agency-backed securities. Interest income for the year ended December 31, 2018 was approximately $0.3 million.
Debt extinguishment gainNet Loss
Debt extinguishment gainWe recorded a net loss of $21.4 million, or a net loss per share, basic and diluted of ($0.47) during the year ended December 31, 2019 compared to a net loss of $148.0 million, or a net loss per share, basic and diluted of ($7.75) during the year ended December 31, 2018. The weighted average number of shares outstanding, basic and diluted, was approximately $0.545.6 million for the year ended December 31, 2017 due to the extinguishment of liabilities we recorded in the prior period.
Net Loss
We recorded a net loss of $148.0 million or ($7.75) basic and diluted per share during the year ended December 31, 20182019 compared to a net loss of $11.0 million or ($1.16) basic and diluted per share during the year ended December 31, 2017. The weighted average number of shares outstanding was 19.1 million basic and diluted for the year ended December 31, 2018 compared to 9.5 million basic and diluted for the year ended December 31, 2017.2018. The increasedecrease in our net lossesloss in 2018,2019, as compared to 2017,2018, was due to the research and development intellectual property acquired in 2018 in connection with the Merger and a decrease in stock-based compensation in 2019, offset in 2019 by increased spending due to the continued expansion of our research and development activities, increased clinical trials and manufacturing activities, and the overall growth of our corporate infrastructure. We anticipate that we will continue to incur net losses in the future as we further invest in our research and development activities, including our clinical development.development of MultiTAA-specific T cell product candidates. In addition, our general and administrative expenses increaseddecreased in 20182019 mainly due to the increasedecrease in headcount and stock-based equity awards related to existing and new executives and key consultants.
Liquidity and Capital Resources
We have not generated any revenues from the sales or licensing of our product candidates since inception and only have limited revenue associated with grants. We have financed our operations primarily through public and private offerings of our stock and debt including warrants and the exercise thereof.
The following table sets forth our cash and cash equivalents and working capital as of December 31, 20182019 and 2017:2018:
| December 31, | December 31, | |||||||
| 2018 | 2017 | |||||||
| Cash and cash equivalents | $ | 61,747,000 | $ | 5,129,000 | ||||
| Working Capital | $ | 59,193,000 | $ | 3,658,000 | ||||
| December 31, | December 31, | |||||||
| 2019 | 2018 | |||||||
| Cash and cash equivalents | $ | 43,904,000 | $ | 61,747,000 | ||||
| Working capital | $ | 43,494,000 | $ | 59,193,000 | ||||
Cash Flows
The following table summarizes our cash flows for the years ended December 31, 20182019 and 2017:2018:
| For the Years Ended | For the Years Ended | |||||||||||||||
| December 31, | December 31, | |||||||||||||||
| 2018 | 2017 | 2019 | 2018 | |||||||||||||
| Net Cash provided by (used in): | ||||||||||||||||
| Operating activities | $ | (14,480,000 | ) | $ | (8,439,000 | ) | $ | (18,284,000 | ) | $ | (14,480,000 | ) | ||||
| Investing activities | (148,000 | ) | - | (375,000 | ) | (148,000 | ) | |||||||||
| Financing activities | 71,245,000 | 5,717,000 | 816,000 | 71,245,000 | ||||||||||||
| Net increase/(decrease) in cash | $ | 56,617,000 | $ | (2,722,000 | ) | |||||||||||
| Net (decrease) increase in cash and cash equivalents | $ | (17,843,000 | ) | $ | 56,617,000 | |||||||||||
FinancingsOperating Activities
Net cash used in operating activities during the year ended December 31, 2019 was $18.3 million. The use of cash primarily related to our net loss of $21.4 million, in addition to the effect of changes in asset and liability accounts, including an increase in prepaid expenses and deposits of $1.4 million, a decrease in accounts payable and accrued liabilities of $1.0 million, a decrease in interest receivable of $52,000 and a net increase in lease liabilities of $0.2 million.
Net cash used in operating activities during the year ended December 31, 2018 was $14.5 million. The use of cash primarily related to our net loss of $148.0 million, in addition to the effect of changes in asset and liability accounts, including an increase in prepaid expenses and deposits of $0.1 million, an increase in accounts payable and accrued liabilities of $1.2 million and an increase in interest receivable of $0.1 million.
Investing Activities
Net cash used in investing activities was $0.4 million and $0.1 million for the purchase of property and equipment during the years ended December 31, 2019 and 2018, respectively.
Financing Activities
Net cash provided by financing activities was $816,000 during the year December 31, 2019, due primarily to the exercise of stock warrants and stock options.
Net cash provided by financing activities was $71.2 million during the year December 31, 2018, due primarily to the following:
May 2018 Private Placement Transaction Common Stock Purchase Agreement
On May 18, 2018, we closed on the sale of 1,300,000 shares of common stock for $2.40 per share pursuant to a Common Stock Purchase Agreementcommon stock purchase agreement with an existing accredited investor in a private placement under Rule 506 of Regulation D pursuant to the terms of a Common Stock Purchase Agreement.D. Aggregate gross proceeds were approximately $3.1 million.
May 2018 Exercise of Warrants Held by Existing Institutional Investors
Also onOn May 18, 2018, we and certain existing institutional investors who are holders of variousagreed to exercise existing warrants to purchase shares of Company common stock, closed on Warrant Exercise Agreements infor which we agreed to reduce the exercise price for a portion of the investors’ previously purchased Series C, Series D, Series E and Series Fsuch warrants from $6.00, $9.00, $15.00 and $7.20, respectively, per share to $2.50 per share, provided that the investors exercise such warrants for cash immediately, which they did, forimmediately. Such investors exercised warrants to purchase 782,506 shares and aggregateshares. Aggregate proceeds of approximately $2.0 million.
June 2017 Private Placement Transaction
On June 26, 2017, we completed private placements of units with certain accredited investors. In the private placement transaction, we sold 1,503,567 shares of common stock for $3.97 per share and five-year warrants to purchase an equal number of shares of common stock, at an exercise price of $3.97 per share, for $0.125 per warrant, with one common share and one warrant being sold together as a unit for a total of $4.095 per unit. We issued and sold an aggregate of 1,503,567 million units for aggregate gross proceeds of $6.2 million. We incurred $0.8 million in agency fees and legal costs. In connection with the offering, we reduced the exercise price for the warrants to purchase an aggregate of 653,187 shares of common stock issued to investors in the private placement that closed in August 2016 from $6.00 per share to $3.97 per share.
October 2018 Private Placement Transaction
On October 17, 2018, concurrent with the completion of the Merger, we issued to certain accredited investors in a private placement transaction an aggregate of 17,500,000 shares of itsour common stock and warrants to purchase 13,437,500 shares of common stock at an exercise price of $5.00 per share with a five-year term, for aggregateterm. Aggregate proceeds ofwere approximately $70.0 million pursuant to the terms of the Securities Purchase Agreements, dated June 8, 2018, by and among us and certain accredited investors.million.
FundingFuture Capital Requirements
Our primary usesTo date, we have not generated any revenues from the commercial sale of capital are,approved drug products, and we do not expect will continue to be, compensation and related expenses, third-party clinical and research and development services, laboratory and related supplies, clinical costs, legal and other regulatory expenses, facility costs and general overhead costs.
The successful development of any of our product candidates is highly uncertain. As such,generate substantial revenue for at this time,least the next several years. If we cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessaryfail to complete the development of our product candidates in a timely manner or fail to obtain their regulatory approval, our ability to generate future revenue will be compromised. We do not know when, or if, we will generate any revenue from our product candidates, and we do not expect to generate significant revenue unless and until we obtain regulatory approval of, and commercialize, our product candidates. We expect our expenses to increase in connection with our ongoing activities, particularly as we continue the research and development of, continue or initiate clinical trials of and seek marketing approval for our product candidates. In addition, if we obtain approval for any of our product candidates, we expect to incur significant commercialization expenses related to sales, marketing, manufacturing and distribution. We anticipate that we will need substantial additional funding in connection with our continuing operations. If we are also unable to predictraise capital when if ever, material netneeded or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs or future commercialization efforts.
As of December 31, 2019, we had cash inflowsand cash equivalents of approximately $43.9 million. Based on our revised clinical and research and development plans and our revised timing expectations related to the progress of our programs, we expect that our cash and cash equivalents as of December 31, 2019 will commence fromenable us to fund our operating expenses and capital expenditure requirements into the salesecond quarter of 2021. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. Furthermore, our operating plan may change, and we may need additional funds sooner than planned in order to meet operational needs and capital requirements for product candidates. This is due todevelopment and commercialization. Because of the numerous risks and uncertainties associated with developing medical treatments, including, but not limited to, the uncertainty of:
A change in the outcome of any of these variables with respect to the development of anyand commercialization of our product candidates would significantly changeand the costsextent to which we may enter into additional collaborations with third parties to participate in their development and timingcommercialization, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with the development of our product candidates.current and anticipated clinical trials. Our future funding requirements will depend on many factors, as we:
| • | initiate or continue clinical trials of our product candidates; |
| • | continue the research and development of our product candidates, seek to discover additional product candidates; seek regulatory approvals for our product candidates if they successfully complete clinical trials; |
| • | establish sales, marketing and distribution infrastructure and scale-up manufacturing capabilities to commercialize any product candidates that may receive regulatory approval; |
| • | evaluate strategic transactions we may undertake; and |
| • | enhance operational, financial and information management systems and hire additional personnel, including personnel to support development of our product candidates and, if a product candidate is approved, our commercialization efforts. |
Because all of our product candidates are in the early stages of clinical and preclinical development and the outcome of these efforts is uncertain, we cannot estimate the actual amounts necessary to successfully complete the development and commercialization of product candidates or whether, or when, we may achieve profitability. Until such time, if ever, that we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity or debt financings and collaboration arrangements.
We plan to continue to fund our operations and capital funding needs through equity and/or debt financing. We may also consider new collaborations or selectively partneringpartner our technology. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our existing stockholders.stockholders’ common stock. The incurrence of indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates or grant licenses on terms unfavorable to us. Any of these actions could harmWe may also be required to pay damages or have liabilities associated with litigation or other legal proceedings involving our business, results of operations and future prospects.company.
Outlook
Based on our clinical and research and development plans and our timing expectations related to the progress of our programs, we expect that our cash, cash equivalents and investment securities as of December 31, 2018 will enable us to fund our operating expenses and capital expenditure requirements through at least the second quarter of 2020. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. Furthermore, our operating plan may change, and we may need additional funds to meet operational needs and capital requirements for product development and commercialization sooner than planned. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates and the extent to which we may enter into additional collaborations with third parties to participate in their development and commercialization, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials. Our future funding requirements will depend on many factors, as we:
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues, expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
Tax Loss and Credit Carryforwards
As of December 31, 2018,2019, we have approximately $57.0$73.8 million of federal and $37.3$47.4 million of state Net Operating Loss (“NOL”s)net operating loss carryforwards that may be available to offset future taxable income, if any. The federal net operating loss carryforwards of $41.6$41.9 million, if not utilized, will expire between 2029 and 2037. The federal net operating loss carryforwards of $15.4$31.9 million generated in 2018 and thereafter are subject to an 80% limitation on taxable income, do not expire and will carry forward indefinitely. The state net operating loss carryforwards of $21.8$21.9 million, if not utilized, will begin to expire in 2035. The state net operating loss carryforwards of $15.4$25.5 million generated in 2018 and thereafter are subject to an 80% limitation on taxable income, do not expire and will carry forward indefinitely. Any change in ownership greater than 50% under Section 382 of the Internal Revenue Code or the “Code”, places significant annual limitations on the use of such net operating loss carryforwards.
At December 31, 20182019 and 2017,2018, we recorded a 100% valuation allowance against our deferred tax assets of approximately $20.0$24.6 million and $11.9$20.0 million, respectively, as our management believes it is uncertain that they will be fully realized. If we determine in the future that we will be able to realize all or a portion of our net operating loss carryforwards, an adjustment to valuation allowance against our deferred tax assets would increase net income in the period in which we make such a determination.
Inflation
Inflation affects the cost of raw materials, goods and services that we use. In recent years, inflation has been modest. However, fluctuations in energy costs and commodity prices can affect the cost of all raw materials and components. The competitive environment somewhat limits our ability to recover higher costs resulting from inflation by raising prices. Although we cannot precisely determine the effects of inflation on our business, it is management’s belief that the effects on future revenues and operating results will not be significant. We do not believe that inflation has had a material impact on our results of operations for the periods presented, except with respect to payroll-related costs and other costs arising from or related to government-imposed regulations.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
| ITEM 8. | FINANCIAL STATEMENTS |
The Financial Statements are incorporated herein by reference to pages F-1 to F-27 at the end of this report and the supplementary data is not applicable.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
| ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
We have had no changes in, or disagreements with our principal independent accountants.
ITEM 9A. CONTROLS AND PROCEDURES
| ITEM 9A. CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
We have established disclosure controls and procedures, as such term is defined in Rule 13a-15(e) under the Securities Exchange Act of 1934. Under the supervision and with the participation of our management, we conducted an evaluation of the effectiveness of our disclosure controls and procedures as of December 31, 20182019 to ensure that the information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934 is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Securities Exchange Act of 1934 is accumulated and communicated to our management, including our principal executive officer and principal financial officer as appropriate, to allow timely decisions regarding required disclosure. Our management, with participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2018.2019. Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective as of December 31, 2018.2019to provide reasonable assurance that the information required to be disclosed by us in this Annual Report was (a) reported within the time periods specified by SEC rules and regulations and (b) communicated to our management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding any required disclosure.
Management’s Report on Internal Control Over Financial Reporting and Report of Independent Registered Public Accounting Firm
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Under the supervision and with the participation of our management, including our principal executive, financial and accounting officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 20182019 based on the framework in Internal Control—Integrated Framework 2013 issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on that evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2018.2019.
TheOur independent registered public accounting firm, Marcum LLP, has issued an attestation report on our internal control over financial reporting. The report on the audit of internal control over financial reporting is included in Part II, Item 8 of this Annual Report on Form 10-K.
Cybersecurity
We utilize information technology for internal and external communications with vendors, clinical sites, banks, investors and shareholders. Loss, disruption or compromise of these systems could significantly impact operations and results.
We are not aware of any material cybersecurity violation or occurrence. We believe our efforts toward prevention of such violation or occurrence, including system design and controls, processes and procedures, training and monitoring of system access, limit, but may not prevent unauthorized access to our systems.
Other than temporary disruption to operations that may be caused by a cybersecurity breach, we consider cash transactions to be the primary risk for potential loss. We and our financial institution take steps to minimize the risk by requiring multiple levels of authorization and other controls.
Changes in Internal Control Over Financial Reporting
There has beenwere no changechanges in our internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) during our most recentthe fiscal quarter ended December 31, 2019 that has materially affected, or isare reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Internal Controls
In designing and evaluating the disclosure controls and procedures, management does not expect that our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control systems are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. The design of any disclosure controls and procedures also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Our management, including our Chief Executive Officer and Chief Financial Officer, believes that our disclosure controls and procedures and internal control over financial reporting are designed to provide reasonable assurance of achieving their objectives and are effective at the reasonable assurance level. However, our management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all errors and all fraud.
| ITEM 9B. OTHER INFORMATION |
Award of 2019 Performance Bonuses and 2020 Equity Incentive Awards
On March 10, 2020, upon the recommendation of the compensation committee and pursuant to our 2014 Omnibus Stock Ownership Plan, our board of directors approved options to purchase our common stock as (i) performance bonuses for 2019 performance and (ii) equity-based incentive awards to our executive officers, as follows:
| Name and Title | Number of Shares of Common Stock Underlying 2019 Performance Bonus Award | Number of Shares of Common Stock Underlying 2020 Equity Incentive Award | ||||||
| Peter L. Hoang President and Chief Executive Officer | 41,200 | 388,800 | ||||||
| Anthony Kim Chief Financial Officer | 32,500 | 127,500 | ||||||
| Juan Vera Chief Development Officer | 26,600 | 113,400 | ||||||
| Mythili Koneru Chief Medical Officer | 23,900 | 116,100 | ||||||
| Michael Loiacono Chief Accounting Officer | 20,900 | 119,100 | ||||||
| Nadia Agopyan Vice President, Regulatory Affairs | 7,300 | 32,700 | ||||||
| Gerald Garrett Vice President, Clinical Operations | 1,700 | 38,300 | ||||||
| Lina Hoang Vice President, Research & Development | 14,600 | 25,400 | ||||||
| Anna Szymanska Vice President, Quality | 7,500 | 32,500 | ||||||
Each option award was granted with an exercise price of $2.12 per share, the closing price of our common stock on the Nasdaq Global Market on March 10, 2020, with the option award vesting in 48 equal monthly installments over a four-year period, subject to such executive officer’s continued service on the applicable vesting date.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item and not set forth below will be set forth in the sections headed “Election of Directors,” “Management and Named Executive Officers” and “Section 16(a) Beneficial Ownership Reporting Compliance” in our definitive proxy statement for our 2019 Annual Meeting of Stockholders, or our Proxy Statement, to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2019, and is incorporated herein by reference.
We have adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial and accounting officer or controller, or persons performing similar functions, known as the Code of Ethics and Business Conduct. The Code of Ethics and Business Conduct is available on our website at www.markertherapeutics.com under the Corporate Governance section of our Investors page. If we make any substantive amendments to, or grant any waivers from, the code of business conduct and ethics for any officer or director, we will disclose the nature of such amendment or waiver on our website or in a current report on Form 8-K.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item will be set forth in the section headed “Executive Compensation-Compensation Discussion and Analysis” in our Proxy Statement and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item will be set forth in the section headed “Equity Compensation Plan Information” and “Security Ownership of Management and Certain Beneficial Owners” in our Proxy Statement and is incorporated herein by reference.
The information required by Item 201(d) of Regulation S-K will be set forth in the section headed “Executive Compensation-Compensation Discussion and Analysis” and “Board of Directors and Corporate Governance” in our Proxy Statement and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
The information required by this item will be set forth in the section headed “Certain Relationships and Related Transactions” and “Board of Directors and Corporate Governance” in our Proxy Statement and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item will be set forth in the section headed “Independent Auditors’ Fees and Services” in our Proxy Statement and is incorporated herein by reference.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) The documents filed as part of this report are as follows:
1. The financial statements and accompanying report of independent registered public accounting firm are set forth immediately following the signature page of this report on pages F-1 through F-27.
2. All financial statement schedules are omitted because they are inapplicable, not required or the information is included elsewhere in the financial statements or the notes thereto.
3. The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
EXHIBIT INDEX
*Executive management contract or compensatory plan or arrangement.
**Confidential treatment has been granted as to certain portions of this exhibit pursuant to Rule 406 of the Securities Act of 1933, as amended, or Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
***Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for conditional treatment and this exhibit has been submitted separately with the SEC.
None.
Pursuant to the requirements of Section 13 and 15 (d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: March 12, 2020
| Marker Therapeutics, Inc. | ||
| By: | /s/ Peter Hoang | |
| Peter Hoang | ||
| Chief Executive Officer (Principal Executive Officer) | ||
| By: | /s/ Anthony Kim | |
| Anthony Kim | ||
| Chief Financial Officer (Principal Financial and Accounting Officer) | ||
POWER OF ATTORNEY
Each of the undersigned officers and directors of Marker Therapeutics, Inc., hereby constitutes and appoints Peter Hoang and Anthony Kim, their true and lawful attorney-in-fact and agent, for them and in their name, place and stead, in any and all capacities, to sign their name to any and all amendments to this Report on Form 10-K, and other related documents, and to cause the same to be filed with the Securities and Exchange Commission, granting unto said attorneys, full power and authority to do and perform any act and thing necessary and proper to be done in the premises, as fully to all intents and purposes as the undersigned could do if personally present, and the undersigned for himself hereby ratifies and confirms all that said attorney shall lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on March 12, 2020 on behalf of the registrant and in the capacities indicated.
| Signature | Title | Date | ||
| /s/ Peter Hoang | President, Chief Executive Officer and Director (Principal Executive Officer) | March 12, 2020 | ||
| Peter Hoang | ||||
| /s/ Frederick Wasserman | Director | March 12, 2020 | ||
| Frederick Wasserman | ||||
| /s/ David Laskow-Pooley | Director | March 12, 2020 | ||
| David Laskow-Pooley | ||||
| /s/ John Wilson | Director | March 12, 2020 | ||
| John Wilson | ||||
| /s/ Juan Vera | Director | March 12, 2020 | ||
| Juan Vera | ||||
| /s/ N. David Eansor | Director | March 12, 2020 | ||
| N. David Eansor | ||||
| /s/ Steve Elms | Director | March 12, 2020 | ||
| Steve Elms | ||||
| /s/ Anthony Kim | Chief Financial Officer (Principal Financial and Accounting Officer) | March 12, 2020 | ||
| Anthony Kim |
MARKER THERAPETUICS, INC.
CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2019
Report of Independent Registered Public Accounting Firm
Consolidated Statements of Operations
Consolidated Statements of Stockholders’ Equity (Deficit)
Consolidated Statements of Cash Flows
Notes to the Consolidated Financial Statements
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
Marker Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Marker Therapeutics, Inc. (the “Company”) as of December 31, 2019 and 2018, the related consolidated statements of operations, stockholders’ equity (deficit) and cash flows for each of the two years in the period ended December 31, 2019, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) ("PCAOB"), the Company's internal control over financial reporting as of December 31, 2019, based on the criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013 and our report dated March 12, 2020,expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Marcumllp
Marcumllp
We have served as the Company’s auditor since 2014.
New York, NY
March 12, 2020
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON INTERNAL CONTROL OVER FINANCIAL REPORTING
To the ShareholdersStockholders and Board of Directors of
Marker Therapeutics, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Marker Therapeutics, Inc.'s (the “Company”) internal control over financial reporting as of December 31, 2018,2019, based on criteria established inInternal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2018,2019, based on criteria established inInternal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated balance sheetssheets as of December 31, 20182019 and 20172018 and the related consolidated statements of operations, shareholders’stockholders’ equity (deficit), and cash flows and the related notes for each of the two years in the period ended December 31, 20182019 of the Company, and our report dated March 15, 201912, 2020 expressed an unqualified opinion on those financial statements.
Basis for Opinion
The Company's management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Management Annual Report on Internal Control over Financial Reporting”. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that degree of compliance with the policies or procedures may deteriorate.
/s/ Marcum LLP
Marcumllp
New York, NYMarch 15, 2019
The disclosure set forth below is filed in lieu of a Form 8-K that otherwise would have been required with respect to Item 5.02 Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers, particularly 5.02 (e) Compensatory Arrangements of Certain Officers.
Bonus Awards 2018
On March 14, 2019 the Board of Directors approved a discretionary bonus to Mr. Hoang, the Company’s President and Chief Executive Officer, of $181,250 to be paid in cash and determined that no other discretionary cash bonuses would be paid for 2018 to any of our other named executive officers.
Amendment to Mr. Hoang’s Option Award Agreement.
On March 14, 2019, the Company and Mr. Hoang entered into an amendment to Mr. Hoang’s stock option award agreement (the “Amended Option Agreement”). Pursuant to the terms of the Amended Option Agreement, Mr. Hoang’s prior grant of 1,359,855 options to purchase common stock, which previously vested immediately was revised to add a vesting requirement over four years. The Amended Option Agreement provides for the 1,359,855 options to vest monthly over four years through September 2022. All other terms of the original award relating to the exercise price and grant date remained unchanged from the initial award.
Amendment to Mr. Hoang’s Employment Agreement.
On March 14, 2019, the Company and Mr. Hoang entered into an amendment to Mr. Hoang’s employment agreement to make the following changes:
All other terms of Mr. Hoang’s employment agreement not modified by the Amendment remain unchanged and in place. The description of the Amendment is qualified in its entirety by reference to the Amendment filed hereto as Exhibit 10.40.
2019 Bonus Program
On March 14, 2019, the Board of Directors approved the 2019 bonus program for Mr. Peter Hoang, our Chief Executive Officer and President, Mr. Anthony Kim, our Chief Financial Officer and Mr. Michael J. Loiacono, our Chief Accounting Officer, as recommended by the Compensation Committee of the Board of Directors. Under such bonus program, Mr. Hoang, Mr. Kim and Mr. Loiacono are eligible for bonuses of up to $190,000 $150,000 and $96,250, respectively, equaling up to 50%, 40% and 35%, of their respective base salaries (each a “Bonus Target”).
The bonuses payable to Mr. Hoang are to be based upon the achievement of the following objectives:
(i) up to 40% of the Bonus Target for meeting regulatory and clinical objectives associated with the Company’s AML product candidate;
(ii) up to 35% of the Bonus Target for financial performance and corporate objectives including related to capital management and partnership outreach undertakings;
(iii) up to 15% of the Bonus Target for meeting scientific and technical objectives relating to the manufacturing processes and laboratory development; and
(iv) up to 10% of the Bonus Target for product manufacturing objectives.
The bonuses payable to Mr. Kim are to be based upon the achievement of the following objectives:
The bonuses payable to Mr. Loiacono are to be based upon the achievement of the following objectives:
The payments of any bonuses pursuant to the above are qualified and subject to (i) the Company having sufficient capital to operate its business for the ensuing twelve months, and (ii) the successful attainment of at least 85% of each person’s objectives. The bonuses are able to be paid in a combination of cash and common stock at the discretion of the Compensation Committee.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item and not set forth below will be set forth in the sections headed “Election of Directors,” “Management and Named Executive Officers” and “Section 16(a) Beneficial Ownership Reporting Compliance” in our definitive proxy statement for our 2018 Annual Meeting of Stockholders, or our Proxy Statement, to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2018, and is incorporated herein by reference.
We have adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial and accounting officer or controller, or persons performing similar functions, known as the Code of Ethics and Business Conduct. The Code of Ethics and Business Conduct is available on our website at www.markertherapeutics.com under the Corporate Governance section of our Investors page. If we make any substantive amendments to, or grant any waivers from, the code of business conduct and ethics for any officer or director, we will disclose the nature of such amendment or waiver on our website or in a current report on Form 8-K.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item will be set forth in the section headed “Executive Compensation-Compensation Discussion and Analysis” in our Proxy Statement and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item will be set forth in the section headed “Equity Compensation Plan Information” and “Security Ownership of Management and Certain Beneficial Owners” in our Proxy Statement and is incorporated herein by reference.
The information required by Item 201(d) of Regulation S-K will be set forth in the section headed “Executive Compensation-Compensation Discussion and Analysis” and “Board of Directors and Corporate Governance” in our Proxy Statement and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
The information required by this item will be set forth in the section headed “Certain Relationships and Related Transactions” and “Board of Directors and Corporate Governance” in our Proxy Statement and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item will be set forth in the section headed “Independent Auditors’ Fees and Services” in our Proxy Statement and is incorporated herein by reference.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The documents filed as part of this report are as follows:
1. The financial statements and accompanying report of independent registered public accounting firm are set forth immediately following the signature page of this report on pages F-1 through F-27.
2. All financial statement schedules are omitted because they are inapplicable, not required or the information is included elsewhere in the financial statements or the notes thereto.
3. The exhibits required to be filed by this report or able to be incorporated by reference are listed in the “Exhibit Index” following the financial statements.
(b) Other Exhibits
Exhibits required by Item 601 of Regulation S-K are submitted (or incorporated by reference) and listed in a separate section herein immediately following the “Exhibit Index” and are incorporated herein by reference.
(c) Not Applicable.
None.
SIGNATURES
Pursuant to the requirements of Section 13 and 15 (d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: March 15, 2019
Each of the undersigned officers and directors of Marker Therapeutics, Inc., hereby constitutes and appoints Peter Hoang and Anthony Kim, their true and lawful attorney-in-fact and agent, for them and in their name, place and stead, in any and all capacities, to sign their name to any and all amendments to this Report on Form 10-K, and other related documents, and to cause the same to be filed with the Securities and Exchange Commission, granting unto said attorneys, full power and authority to do and perform any act and thing necessary and proper to be done in the premises, as fully to all intents and purposes as the undersigned could do if personally present, and the undersigned for himself hereby ratifies and confirms all that said attorney shall lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on March 15, 2019 on behalf of the registrant and in the capacities indicated.
MARKER THERAPEUTICS, INC.
CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2018
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Marker Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Marker Therapeutics, Inc. (the “Company”) as of December 31, 2018 and 2017, the related consolidated statements of operations, stockholders’ equity (deficit) and cash flows for each of the two years in the period ended December 31, 2018, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2018, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) ("PCAOB"), the Company's internal control over financial reporting as of December 31, 2018, based on the criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013 and our report dated March 15, 2019,expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Marcumllp
Marcumllp
We have served as the Company’s auditor since 2014.
New York, NY
March 15, 2019
12, 2020
F-3
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||||
| 2018 | 2017 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 61,746,748 | $ | 5,129,289 | ||||
| Prepaid expenses and deposits | 141,717 | 51,150 | ||||||
| Interest receivable | 108,177 | - | ||||||
| Total current assets | 61,996,642 | 5,180,439 | ||||||
| Property, plant and equipment, net | 147,668 | - | ||||||
| Total assets | $ | 62,144,310 | $ | 5,180,439 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | 2,754,572 | $ | 1,513,312 | ||||
| Warrant liability | 49,000 | 9,000 | ||||||
| Total current liabilities | 2,803,572 | 1,522,312 | ||||||
| Total liabilities | 2,803,572 | 1,522,312 | ||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| Stockholders' equity: | ||||||||
| Preferred stock - $0.001 par value, 5 million shares authorized at December 31, 2018 and 2017, respectively | ||||||||
| Series A, $0.001 par value, 1.25 million shares designated, 0 shares issued and outstanding as of December 31, 2018 and 2017, respectively | - | - | ||||||
| Series B, $0.001 par value, 1.5 million shares designated, 0 shares issued and outstanding as of December 31, 2018 and 2017, respectively | - | - | ||||||
| Common stock, $0.001 par value, 150 million shares authorized, 45.4 million and 10.6 million shares issued and outstanding as of December 31, 2018 and 2017, respectively | 45,440 | 10,616 | ||||||
| Additional paid-in capital | 365,400,748 | 161,067,538 | ||||||
| Accumulated deficit | (306,105,450 | ) | (157,420,027 | ) | ||||
| Total stockholders' equity | 59,340,738 | 3,658,127 | ||||||
| Total liabilities and stockholders' equity | $ | 62,144,310 | $ | 5,180,439 | ||||
| December 31, | December 31, | |||||||
| 2019 | 2018 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 43,903,949 | $ | 61,746,748 | ||||
| Prepaid expenses and deposits | 1,526,442 | 141,717 | ||||||
| Interest receivable | 56,189 | 108,177 | ||||||
| Total current assets | 45,486,580 | 61,996,642 | ||||||
| Non-current assets: | ||||||||
| Property, plant and equipment, net | 417,528 | 147,668 | ||||||
| Right-of-use assets, net | 455,174 | - | ||||||
| Total non-current assets | 872,702 | 147,668 | ||||||
| Total assets | $ | 46,359,282 | $ | 62,144,310 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued liabilities | $ | 1,757,680 | $ | 2,754,572 | ||||
| Lease liability | 204,132 | - | ||||||
| Warrant liability | 31,000 | 49,000 | ||||||
| Total current liabilities | 1,992,812 | 2,803,572 | ||||||
| Non-current liabilities: | ||||||||
| Lease liability, net of current portion | 280,247 | - | ||||||
| Total non-current liabilities | 280,247 | - | ||||||
| Total liabilities | 2,273,059 | 2,803,572 | ||||||
| Commitments and contingencies (see Note 15) | - | - | ||||||
| Stockholders' equity: | ||||||||
| Preferred stock - $0.001 par value, 5 million shares authorized and 0 shares issued and outstanding at December 31, 2019 and 2018, respectively | - | - | ||||||
| Common stock, $0.001 par value, 150 million shares authorized, 45.7 million and 45.4 million shares issued and outstanding as of December 31, 2019 and 2018, respectively | 45,728 | 45,440 | ||||||
| Additional paid-in capital | 371,573,909 | 365,400,748 | ||||||
| Accumulated deficit | (327,533,414 | ) | (306,105,450 | ) | ||||
| Total stockholders' equity | 44,086,223 | 59,340,738 | ||||||
| Total liabilities and stockholders' equity | $ | 46,359,282 | $ | 62,144,310 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Revenues: | ||||||||
| Grant income | $ | 205,994 | $ | 183,064 | ||||
| Total revenues | 205,994 | 183,064 | ||||||
| Operating expenses: | ||||||||
| Research and development - intellectual property acquired | 116,044,886 | - | ||||||
| Research and development | 7,952,870 | 5,250,985 | ||||||
| General and administrative | 24,379,871 | 6,412,121 | ||||||
| Total operating expenses | 148,377,627 | 11,663,106 | ||||||
| Loss from operations | (148,171,633 | ) | (11,480,042 | ) | ||||
| Other income (expense): | ||||||||
| Change in fair value of warrant liabilities | (40,000 | ) | 5,500 | |||||
| Interest income | 253,723 | - | ||||||
| Debt extinguishment gain | - | 492,365 | ||||||
| Net loss | $ | (147,957,910 | ) | $ | (10,982,177 | ) | ||
| Net loss per share, Basic and Diluted | $ | (7.75 | ) | $ | (1.16 | ) | ||
| Weighted average number of common shares outstanding | 19,091,926 | 9,453,483 | ||||||
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Revenues: | ||||||||
| Grant income | $ | 213,194 | $ | 205,994 | ||||
| Total revenues | 213,194 | 205,994 | ||||||
| Operating expenses: | ||||||||
| Research and development - intellectual property acquired | - | 116,044,886 | ||||||
| Research and development | 12,764,804 | 7,952,870 | ||||||
| General and administrative | 9,977,196 | 24,379,871 | ||||||
| Total operating expenses | 22,742,000 | 148,377,627 | ||||||
| Loss from operations | (22,528,806 | ) | (148,171,633 | ) | ||||
| Other income (expense): | ||||||||
| Change in fair value of warrant liabilities | 18,000 | (40,000 | ) | |||||
| Interest income | 1,082,842 | 253,723 | ||||||
| Net loss | $ | (21,427,964 | ) | $ | (147,957,910 | ) | ||
| Net loss per share, basic and diluted | $ | (0.47 | ) | $ | (7.75 | ) | ||
| Weighted average number of common shares outstanding | 45,587,734 | 19,091,926 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
| Common Stock | Additional Paid- | Accumulated | Total Stockholders' | |||||||||||||||||
| Shares | Par value | in Capital | Deficit | Equity | ||||||||||||||||
| Balance at January 1, 2018 | 10,615,724 | $ | 10,616 | $ | 161,067,538 | $ | (157,420,027 | ) | $ | 3,658,127 | ||||||||||
| Issuance of common stock for research and development intellectual property | 13,914,255 | 13,914 | 116,030,972 | - | 116,044,886 | |||||||||||||||
| Issuance of common stock and warrants in private placement | 18,800,000 | 18,800 | 73,101,200 | - | 73,120,000 | |||||||||||||||
| Fees and legal costs relating to private placement | - | - | (6,175,000 | ) | - | (6,175,000 | ) | |||||||||||||
| Stock options exercised for cash | 10,416 | 10 | 18,115 | - | 18,125 | |||||||||||||||
| Stock warrants exercised for cash | 1,499,324 | 1,499 | 4,352,129 | - | 4,353,628 | |||||||||||||||
| Stock warrants cashless exercised | 280,760 | 280 | (280 | ) | - | - | ||||||||||||||
| Stock-based compensation | 327,786 | 329 | 16,350,263 | - | 16,350,592 | |||||||||||||||
| Repurchase of common stock to pay for employee withholding taxes | (7,561 | ) | (8 | ) | (71,702 | ) | - | (71,710 | ) | |||||||||||
| Fair value of repriced warrants as inducement | - | - | 727,513 | (727,513 | ) | - | ||||||||||||||
| Net loss | - | - | - | (147,957,910 | ) | (147,957,910 | ) | |||||||||||||
| Balance at December 31, 2018 | 45,440,704 | 45,440 | 365,400,748 | (306,105,450 | ) | 59,340,738 | ||||||||||||||
| Stock options exercised for cash | 11,980 | 12 | 57,732 | - | 57,744 | |||||||||||||||
| Warrants exercised for cash | 190,258 | 190 | 758,543 | - | 758,733 | |||||||||||||||
| Stock warrants cashless exercised | 9,449 | 9 | (9 | ) | - | - | ||||||||||||||
| Stock-based compensation | 76,440 | 77 | 5,356,895 | - | 5,356,972 | |||||||||||||||
| Net loss | - | - | - | (21,427,964 | ) | (21,427,964 | ) | |||||||||||||
| Balance at December 31, 2019 | 45,728,831 | $ | 45,728 | $ | 371,573,909 | $ | (327,533,414 | ) | $ | 44,086,223 | ||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENTSTATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)CASH FLOWS
| Common Stock | Additional Paid- | Accumulated | Total Stockholders' | |||||||||||||||||
| Shares | Par value | in Capital | Deficit | Equity | ||||||||||||||||
| Balance at January 1, 2017 | 8,421,185 | $ | 8,421 | $ | 151,991,974 | $ | (145,815,808 | ) | $ | 6,184,587 | ||||||||||
| Issuance of common stock and warrants in private placement | 1,503,567 | 1,504 | 6,188,499 | - | 6,190,003 | |||||||||||||||
| Fees and legal costs relating to private placement | - | - | (781,660 | ) | - | (781,660 | ) | |||||||||||||
| Exercise of warrants | 167,926 | 168 | 666,498 | - | 666,666 | |||||||||||||||
| Legal costs relating to exercise of warrants | - | - | (47,043 | ) | - | (47,043 | ) | |||||||||||||
| Fair value of repriced warrants as inducement | - | - | 622,042 | (622,042 | ) | - | ||||||||||||||
| Stock-based compensation | 620,685 | 621 | 2,737,623 | - | 2,738,244 | |||||||||||||||
| Repurchase of common stock to pay for employee withholding taxes | (97,639 | ) | (98 | ) | (310,395 | ) | - | (310,493 | ) | |||||||||||
| Net loss | - | - | - | (10,982,177 | ) | (10,982,177 | ) | |||||||||||||
| Balance at December 31, 2017 | 10,615,724 | 10,616 | 161,067,538 | (157,420,027 | ) | 3,658,127 | ||||||||||||||
| Issuance of common stock for research and development intellectual property | 13,914,255 | 13,914 | 116,030,972 | - | 116,044,886 | |||||||||||||||
| Issuance of common stock and warrants in private placement | 18,800,000 | 18,800 | 73,101,200 | - | 73,120,000 | |||||||||||||||
| Fees and legal costs relating to private placement | - | - | (6,175,000 | ) | - | (6,175,000 | ) | |||||||||||||
| Stock options exercised for cash | 10,416 | 10 | 18,115 | - | 18,125 | |||||||||||||||
| Stock warrants exercised for cash | 1,499,324 | 1,499 | 4,352,129 | - | 4,353,628 | |||||||||||||||
| Stock warrants cashless exercised | 280,760 | 280 | (280 | ) | - | - | ||||||||||||||
| Stock-based compensation | 327,786 | 329 | 16,350,263 | - | 16,350,592 | |||||||||||||||
| Repurchase of common stock to pay for employee withholding taxes | (7,561 | ) | (8 | ) | (71,702 | ) | - | (71,710 | ) | |||||||||||
| Fair value of repriced warrants as inducement | - | - | 727,513 | (727,513 | ) | - | ||||||||||||||
| Net loss | - | - | - | (147,957,910 | ) | (147,957,910 | ) | |||||||||||||
| Balance, December 31, 2018 | 45,440,704 | $ | 45,440 | $ | 365,400,748 | $ | (306,105,450 | ) | $ | 59,340,738 | ||||||||||
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net loss | $ | (21,427,964 | ) | $ | (147,957,910 | ) | ||
| Reconciliation of net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 105,123 | - | ||||||
| Changes in fair value of warrant liabilities | (18,000 | ) | 40,000 | |||||
| Stock-based compensation | 5,356,972 | 16,350,592 | ||||||
| Amortization on right-of-use assets | 181,459 | - | ||||||
| Research and development - intellectual property acquired | - | 116,044,886 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and deposits | (1,384,725 | ) | (90,567 | ) | ||||
| Interest receivable | 51,988 | (108,177 | ) | |||||
| Accounts payable and accrued expenses | (963,967 | ) | 1,241,260 | |||||
| Lease liability | (185,179 | ) | - | |||||
| Net cash used in operating activities | (18,284,293 | ) | (14,479,916 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Purchase of property and equipment | (374,983 | ) | (147,668 | ) | ||||
| Net cash used in investing activities | (374,983 | ) | (147,668 | ) | ||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds from issuance of common stock and warrants in private placement, net of offering costs | - | 66,945,000 | ||||||
| Proceeds from exercise of stock options | 57,744 | 18,125 | ||||||
| Proceeds from exercise of warrants, net of offering costs | 758,733 | 4,353,628 | ||||||
| Repurchase of common stock to pay for employee withholding taxes | - | (71,710 | ) | |||||
| Net cash provided by financing activities | 816,477 | 71,245,043 | ||||||
| Net (decrease) increase in cash | (17,842,799 | ) | 56,617,459 | |||||
| Cash and cash equivalents at beginning of year | 61,746,748 | 5,129,289 | ||||||
| Cash and cash equivalents at end of year | $ | 43,903,949 | $ | 61,746,748 | ||||
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Supplemental schedule of non-cash financing activities: | ||||||||
| Fair value of repriced warrants as inducement | $ | - | $ | 727,513 | ||||
| Stock warrants cashless exercised | $ | 9 | $ | 280 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net loss | $ | (147,957,910 | ) | $ | (10,982,177 | ) | ||
| Reconciliation of net loss to net cash used in operating activities: | ||||||||
| Changes in fair value of warrant liabilities | 40,000 | (5,500 | ) | |||||
| Stock-based compensation | 16,350,592 | 2,738,244 | ||||||
| Debt extinguishment gain | - | (492,365 | ) | |||||
| Research and development - intellectual property acquired | 116,044,886 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and deposits | (90,567 | ) | 18,999 | |||||
| Interest receivable | (108,177 | ) | ||||||
| Accounts payable and accrued expenses | 1,241,260 | 283,372 | ||||||
| Net cash used in operating activities | (14,479,916 | ) | (8,439,427 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Purchase of property and equipment | (147,668 | ) | - | |||||
| Net cash used in investing activities | (147,668 | ) | - | |||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds from issuance of common stock and warrants in private placement, net of offering costs | 66,945,000 | 5,408,343 | ||||||
| Proceeds from exercise of stock warrants, net of offering costs | 4,353,628 | 619,623 | ||||||
| Proceeds from exercise of stock options | 18,125 | - | ||||||
| Repurchase of common stock to pay for employee withholding taxes | (71,710 | ) | (310,493 | ) | ||||
| Net cash provided by financing activities | 71,245,043 | 5,717,473 | ||||||
| Net increase (decrease) in cash | 56,617,459 | (2,721,954 | ) | |||||
| Cash at beginning of year | 5,129,289 | 7,851,243 | ||||||
| Cash and cash equivalents at end of year | $ | 61,746,748 | $ | 5,129,289 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Supplemental schedule of non-cash financing activities: | ||||||||
| Fair value of repriced warrants as inducement | $ | 727,513 | $ | 622,042 | ||||
| Stock warrants cashless exercised | $ | 280 | $ | - | ||||
The accompanying notes are an integral part of these consolidated financial statements.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE FISCAL YEARS DECEMBER 31, 2019 AND 2018
| Note 1: | Nature of Operations |
Marker Therapeutics, Inc., a Delaware corporation formerly known as TapImmune, Inc. (the “Company” or “we”), is a clinical-stage immuno-oncology company specializing in the development and commercialization of innovativenovel T cell-based immunotherapies and innovative peptide-based vaccines for the treatment of hematological malignancies and solid tumor indications, and novel peptide-based vaccines for the treatment of breast and ovarian cancers.indications. The Company’s cell-based immunotherapyMultiTAA-specific T cell technology is based on the selective expansion of non-engineered, tumor-specific T cells that recognize tumor associated antigens, (i.e.which are tumor targets)targets, and kill tumor cells expressing those targets. Once infused into patients, this population ofThese T cells recognizesare designed to recognize multiple tumor targets to produce broad spectrum anti-tumor activity. Because the Company does not genetically engineer its T cells, when compared to current engineered CAR-T and TCR-based approaches, its products (i) are significantly less expensive to manufacture, (ii) appear to be markedly less toxic, and (iii) are associated with potentially meaningful clinical benefit. As a result, the Company believes its portfolio of T cell therapies has a potentially compelling therapeutic product profile, as compared to current gene-modified CAR-T and TCR-based therapies. In addition, the Company’s Folate Receptor Alpha program (TPIV200) for breast and ovarian cancers and our HER2/neu program (TPIV100/110) are in five Phase II clinical trials. In parallel, the Company has been working on a proprietary nucleic acid-based antigen expression technology named PolyStart™ to improve the ability of the immune system to recognize and destroy diseased cells. The Company was incorporated in Nevada in 1992 and reincorporated in Delaware in October 2018 in connection with the Marker Transaction.
On October 17, 2018, the Company completed its previously announced acquisition with Marker Cell Therapy, Inc., formerly known as Marker Therapeutics, Inc., a privately-held Delaware corporation (“Marker Cell”), in accordance with the terms of an Agreement and Plan of Merger and Reorganization dated as of May 15, 2018 (the “Merger Agreement”) by and among the Company, Timberwolf Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of the Company (“Merger Sub”), and Marker. On October 17, 2018, pursuant to the Merger Agreement, Merger Sub was merged with and into Marker Cell (the “Merger”), with Marker Cell being the surviving corporation and becoming a wholly-owned subsidiary of the Company. In connection with the Merger, the Company changed its name to Marker Therapeutics, Inc. and Marker Cell changed its name to Marker Cell Therapy, Inc. At the effective time of the Merger, the former Marker Cell stockholders received (i) an aggregate of 13,914,255 shares of the Company’s common stock which equaled the number of shares of the Company’s common stock issued and outstanding immediately prior to the effective time of the Merger, and (ii) an aggregate of 5,046,003 warrants which equaled the number of the Company’s warrants and stock options issued and outstanding immediately prior to the effective time of the Merger.2018.
| Note 2: | BASIS OF PRESENTATION AND MANAGEMENT PLANS |
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. Any reference in these footnotes to applicable guidance is meant to refer to the authoritative U.S. generally accepted accounting principles (“GAAP”) as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
The Company has not generated any revenue from product sales to date and, if the Company does not successfully obtain regulatory approval and commercialize any of its product candidates, the Company will not be able to generate product revenue or achieve profitability.
The Company is subject to risks common to companies in the biotechnology industry and the future success of the Company is dependent on its ability to successfully complete the development of, and obtain regulatory approval for its product candidates, manage the growth of the organization, obtain additional financing necessary in order to develop, launch and commercialize its product candidates, and compete successfully with other companies in its industry. These financial statements are presented in United States dollars and have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). In the opinion of management, the accompanying audited consolidated financial statements reflect all adjustments, consisting of normal recurring adjustments, considered necessary for a fair presentation of such annual results.
| Note 3: | SIGNIFICANT ACCOUNTING POLICIES |
Principles of Consolidation
These consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, Marker Cell Therapy, Inc. and GeneMax Pharmaceuticals Inc. – a dormant subsidiary that wholly owns GeneMax Pharmaceuticals Canada, Inc. All significant intercompany balances and transactions are eliminated upon consolidation.
Use of Estimates
Preparation of the Company’s consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect certain reported amounts and disclosures. Accordingly, actual results could differ materially from those estimates. Significant areas requiring management’s estimates and assumptions include valuation allowance on deferred tax assets, determining the fair value of stock-based compensation and stock-based transactions, the fair value of the components of the warrant liabilities and accrued liabilities.
Prior Period ReclassificationLiquidity, Financial Condition and Management’s Plans
Prior period grant income that was includedAs of December 31, 2019, the Company had cash and cash equivalents of approximately $43.9 million. The Company’s activities since inception have consisted principally of acquiring product and technology rights, raising capital, and performing research and development. Successful completion of the Company’s development programs and, ultimately, the attainment of profitable operations are dependent on future events, including, among other things, its ability to access potential markets; secure financing; successfully progress its product candidates through preclinical and clinical development; obtain regulatory approval of one or more of its product candidates; maintain and enforce intellectual property rights; develop a customer base; attract, retain and motivate qualified personnel; and develop strategic alliances and collaborations. From inception, the Company has been funded by a combination of equity and debt financings.
The Company expects to continue to incur substantial losses over the next several years during its development phase. To fully execute its business plan, the Company will need to complete certain research and development activities and clinical trials. Further, the Company’s product candidates will require regulatory approval prior to commercialization. These activities will span many years and require substantial expenditures to complete and may ultimately be unsuccessful. Any delays in other income (expense)completing these activities could adversely impact the Company. The Company plans to meet its capital requirements primarily through issuances of debt and equity securities and, in the longer term, revenue from sales of its product candidates, if approved.
Based on the Company’s revised clinical and research and development plans and its revised timing expectations related to the progress of its programs, the Company expects that its cash and cash equivalents as of December 31, 2017 consolidated statement2019 will enable the Company to fund its operating expenses and capital expenditure requirements into the second quarter of operations2021. The Company has been reclassifiedbased this estimate on assumptions that may prove to revenuesbe wrong, and the Company could utilize its available capital resources sooner than it currently expects. Furthermore, the Company’s operating plan may change, and it may need additional funds sooner than planned in order to meet operational needs and capital requirements for comparabilityproduct development and commercialization. Because of the numerous risks and uncertainties associated with the December 31, 2018 presentation. This reclassification had no effectdevelopment and commercialization of the Company’s product candidates and the extent to which the Company may enter into additional collaborations with third parties to participate in their development and commercialization, the Company is unable to estimate the amounts of increased capital outlays and operating expenditures associated with its current and anticipated clinical trials. The Company’s future funding requirements will depend on previously reported net loss.many factors, as it:
| · | initiates or continues clinical trials of its product candidates; |
| · | continues the research and development of its product candidates and seeks to discover additional product candidates; |
| · | seeks regulatory approvals for any product candidates that successfully complete clinical trials; |
| · | maintains and enforces intellectual property rights; |
| · | establishes sales, marketing and distribution infrastructure and scale-up manufacturing capabilities to commercialize any product candidates that may receive regulatory approval; |
| · | evaluates strategic transactions the Company may undertake; and |
| · | enhances operational, financial and information management systems and hires additional personnel, including personnel to support development of product candidates and, if a product candidate is approved, commercialization efforts. |
.
Research and Development – Intellectual Property Acquired
The Company concludedevaluates whether acquired intangible assets are a business under applicable accounting standards. Additionally, the Company evaluates whether the acquired assets have an alternative future use. Intangible assets that its acquisition with Marker Cell Therapy, Inc. completed on October 17, 2018 should be accounted for as an asset acquisition rather thando not have alternative future use are considered acquired in-process research and development. When the acquired in-process research and development assets are not part of a business combination, under Accounting Standards Codification (ASC) 805, Business Combinations. The merger was accounted for as an asset acquisition because substantially all the fair value of the assets being acquired are concentrated in a group of similar assets. Furthermore,consideration paid is expensed on the acquired assets did not have outputs or employees. The assets acquired by the Company under the merger included a license, other associated intellectual property, documentation and records, and related materials. Because Marker’s intellectual property had not received regulatory approval, the $116.0 million purchase price paid foracquisition date. Future costs to develop these assets was immediately expensed in the Company’s statement of operations asare recorded to research and development – intellectual property acquired.
expense as they are incurred.
Revenue Recognition
The Company has not yet generated any revenue from product sales. The Company’s source of revenue in 2018 and 2017 has been from grants. When grant funds are received after costs have been incurred, the Company records grant revenue upon the receipt of cash.F-9
Cash, Cash Equivalents and Credit Risk
The Company considers highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. Cash and cash equivalents at December 31, 20182019 consisted of cash and certificates of deposit in institutions in the United States. Balances at certain institutions have exceeded Federal Deposit Insurance Corporation insured limits and U.S. government agency securities.
The Company maintains cash in accounts which are in excess of the Federal Deposit Insurance Corporation (“FDIC”) insured limits of $250,000. As of December 31, 2018, and 2017,2019, approximately $3.4$0.1 million and $4.9 million, respectively, in cash was uninsured based upon the FDIC insurance coverage limits.
Property and Equipment
Leasehold improvements, furniture, equipment and software are recorded at cost and are depreciated using the straight-line method over the estimated useful lives of the related assets, which range from three to five years. Leasehold improvements are amortized over the shorter of the estimated useful life or the remaining lease term.
Rent and Deferred Rent
The Company recognizes rent expense for leases with increasing annual rents on a straight-line basis over the term of the lease. The amount of rent expense in excess of cash payments is classified as deferred rent. Any lease incentives received are deferred and amortized over the term of the lease.
Fair Value Measurements
The Company follows Accounting Standards Codification (“ASC”) 820, “Fair Value Measurements and Disclosures,” (“ASC 820”) for the Company’s financial assets and liabilities that are re-measured and reported at fair value at each reporting period and are re-measured and reported at fair value at least annually using a fair value hierarchy that is broken down into three levels. Level inputs are defined as follows:
| · | Level 1 - Quoted prices (unadjusted) in active markets for identical assets and liabilities. |
| · | Level 2 - Inputs other than Level 1 that are observable, either directly or indirectly, such as unadjusted quoted prices for similar assets and liabilities, unadjusted quoted prices in the markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. | |
| · | Level 3 - Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities, financial instruments and concentration of credit risk. | |
Patents and Patent Application Costs
Although the Company believes that its patents and underlying technology have continuing value, the amount of future benefits to be derived from the patents is uncertain. Patent costs are, therefore, expensed as incurred.
Stock-Based Compensation
The Company incurs stock-based compensation expense related to restrictedthe issuance of common stock units and stock options. The fair value of restricted stock is determined by the closing market price of the Company's common stock on the date of grant. The Company estimates the fair value of stock options granted using the Black-Scholes option pricing model. The Black-Scholes option pricing model was developed for use in estimating the fair value of traded options, which have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions, including the expected stock price volatility and expected option life. The Company amortizes the fair value of the awards expected to vest on a straight-line basis over the requisite service period of the awards. Expected volatility is based on historical volatility. The expected life of options granted is based on historical expected life. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of grant. The forfeiture rate is based on historical data, and the Company records stock-based compensation expense only for those awards that are expected to vest.data. The dividend yield is based on the fact that no dividends have been paid historically and none are currently expected to be paid in the foreseeable future:
Expected Term — The expected term of options represents the period that the Company’s stock-based awards are expected to be outstanding based on the simplified method, which is the half-life from vesting to the end of its contractual term.
Expected Volatility — The Company computes stock price volatility over expected terms based on its historical common stock trading prices.
Risk-Free Interest Rate — The Company bases the risk-free interest rate on the implied yield available on U. S. Treasury zero-coupon issues with an equivalent remaining term.
Expected Dividend — The Company has never declared or paid any cash dividends on its common shares and does not plan to pay cash dividends in the foreseeable future, and, therefore, uses an expected dividend yield of zero in its valuation models. The Company recognizes fair value of stock options granted to nonemployees as stock-based compensation expense over the period in which the related services are received.
Research and Development Costs
Research and development expenses consist of expenses incurred in performing research and development activities, including compensation and benefits for research and development employees and consultants, facilities expenses, overhead expenses, cost of laboratory supplies, manufacturing expenses, fees paid to third parties and other outside expenses.
Research and development costs are expensed as incurred. Clinical trial and other development costs incurred by third parties are expensed as the contracted work is performed. The Company accrues for costs incurred as the services are being provided by monitoring the status of the clinical trial or project and the invoices received from its external service providers. The Company estimates depend on the timeliness and accuracy of the data provided by the vendors regarding the status of each project and total project spending. The Company adjusts its accrual as actual costs become known. Where contingent milestone payments are due to third parties under research and development arrangements, the milestone payment obligations are expensed when the milestone events are achieved.
Income Taxes
The Company follows the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of assets and liabilities and their respective tax balances. Potential deferred tax assets and liabilities are measured using enacted tax rates expected to apply to the taxable income in the years in which those differences are expected to be recovered or settled. The effect on potential deferred tax assets and liabilities of a change in tax rates is recognized in the statement of operations in the period that includes the date of allowances against deferred tax assets.
Tax benefits are recognized only for tax positions that are more likely than not to be sustained upon examination by tax authorities. The amount recognized is measured as the largest amount of benefit that is greater than 50 percent likely to be realized upon settlement. A liability for “unrecognized tax benefits” is recorded for any tax benefits claimed in the Company’s tax returns that do not meet these recognition and measurement standards. As of December 31, 2018,2019, and 2017,2018, no liability for unrecognized tax benefits was required to be reported. The guidance also discusses the classification of related interest and penalties on income taxes. The Company’s policy is to record interest and penalties on uncertain tax positions as a component of income tax expense. No interest or penalties were recorded during the years ended December 31, 20182019 and 2017.
2018.
Warrant Liability
The Company evaluates options, warrants or other contracts to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for. This accounting treatment requires that the carrying amounts of embedded derivatives be marked-to-market at each balance sheet date and carried at fair value. If the fair value is recorded as a liability, the change in fair value during the period is recorded in the Statement of Operations as either income or expense. Upon conversion, exercise or modification to the terms of a derivative instrument, the instrument is marked to fair value at the conversion date and then the related fair value is reclassified to equity.
In circumstances where the embedded conversion option in a convertible instrument is required to be bifurcated and there are also other embedded derivative instruments in the convertible instrument that are required to be bifurcated, the bifurcated derivative instruments are accounted for as a single, compound derivative instrument.
The classification of financial instruments, including whether such instruments should be recorded as liabilities or as equity, is re-assessed at the end of each reporting period. Equity instruments that are initially classified as equity that become subject to reclassification are reclassified to liability at the fair value of the instrument on the reclassification date. Derivative instrument liabilities will be classified in the balance sheet as current or non-current based on whether or not net-cash settlement of the derivative instrument is expected within 12 months of the balance sheet date.
Management must determine whether an instrument (or an embedded feature) is indexed to the Company’s own stock. An entity should use a two-step approach to evaluate whether an equity-linked financial instrument (or embedded feature) is indexed to its own stock, including evaluating the instrument’s contingent exercise and settlement provisions. This exercise affects the accounting for (i) certain freestanding warrants that contain exercise price adjustment features and (ii) convertible notes containing full-ratchet and anti-dilution protections (iii) certain free-standing warrants that contain contingently putable cash settlement.
Grant Income
The Company recognizes grant income in accordance with the terms stipulated under the grant awarded to the Company’s collaborators at the Mayo Foundation from the U. S. Department of Defense. In various situations, the Company receives certain payments from the U.S. Department of Defense for reimbursement of clinical supplies. These payments are non-refundable and are not dependent on the Company’s ongoing future performance. The Company has adopted a policy of recognizing these payments when received and as revenue in accordance with Accounting Standards Update No. 2014-09, “Revenue from Contracts with Customers (Topic 606)” issued by the Financial Accounting Standards Board.
Loss per Common Share
Basic loss per share includeincludes only the weighted average common shares outstanding, without consideration of potentially dilutive securities. Diluted loss per share includeincludes the weighted average common shares outstanding and any potentially dilutive common stock equivalent shares in the calculation.
New Accounting Standards
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (“FASB”) or other standard setting bodies that we adopt as of the specified effective date. Unless otherwise discussed, we dothe Company does not believe that the impact of recently issued standards that are not yet effective will have a material impact on ourits financial position or results of operations upon adoption.
Recent Accounting Standards Adopted in the Year
Revenue from Contracts with Customers
In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers (Topic 606)” (ASU 2014-09) as modified by ASU No. 2015-14, “Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date,” ASU 2016-08, “Revenue from Contracts with Customers (Topic 606): Principal versus Agent Considerations (Reporting Revenue Gross versus Net),” ASU No. 2016-10, “Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing,” and ASU No. 2016-12, “Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients.” The revenue recognition principle in ASU 2014-09 is that an entity should recognize revenue to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In addition, new and enhanced disclosures will be required. Companies may adopt the new standard either using the full retrospective approach, a modified retrospective approach with practical expedients, or a cumulative effect upon adoption approach. The Company adopted the new standard effective January 1, 2018, using the modified retrospective approach. The only impact of the adoption of ASU 2014-09 was to reclassify the Company's grant income as revenue.
Recent Accounting Standards Not Yet Adopted
Improvements to Non-Employee Share-Based Payment Accounting
In June 2018, the FASB issued ASU 2018-07 “Improvements to Non-employee Share-Based Payment Accounting”, which simplifies the accounting for share-based payments granted to non-employees for goods and services. Under the ASU, most of the guidance on such payments to non-employees would be aligned with the requirements for share-based payments granted to employees. The amendments are effective for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. Early adoption is permitted, but no earlier than an entity’s adoption date of Topic 606. The Company is currently evaluating the impact of the new standard on its consolidated financial statements.
Leases
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842) in order to increase transparency and comparability among organizations by, among other provisions, recognizing lease assets and lease liabilities on the balance sheet for those leases classified as operating leases under previous GAAP. For public companies, ASU 2016-02 is effective for fiscal years beginning after December 15, 2018 (including interim periods within those periods) using a modified retrospective approach and early adoption is permitted. In transition, entities may also elect a package of practical expedients that must be applied in its entirety to all leases commencing before the adoption date, unless the lease is modified, and permits entities to not reassess (a) the existence of a lease, (b) lease classification or (c) determination of initial direct costs, as of the adoption date, which effectively allows entities to carryforward accounting conclusions under previous U.S. GAAP. In July 2018, the FASB issued ASU 2018-11, Leases (Topic 842): Targeted Improvements, which provides entities an optional transition method to apply the guidance under Topic 842 as of the adoption date, rather than as of the earliest period presented. The Company adopted Topic 842 on January 1, 2019, using the optional transition method to apply the new guidance as of January 1, 2019, rather than as of the earliest period presented, and elected the package of practical expedients described above. Based on the analysis, on January 1, 2019, the Company expects to recognize additional operating liabilitiesrecorded right of use assets of approximately $637,000, lease liability of approximately $670,000 with corresponding ROU assetsand eliminated deferred rent of approximately the same amount as of January 1, 2019 based on the present value of the remaining lease payments.$33,000.
SEC Disclosure Update and SimplificationImprovements to Non-Employee Share-Based Payment Accounting
In AugustJune 2018, the SECFASB issued ASU 2018-07 “Improvements to Non-employee Share-Based Payment Accounting”, which simplifies the accounting for share-based payments granted to non-employees for goods and services. Under the ASU, most of the guidance on such payments to non-employees would be aligned with the requirements for share-based payments granted to employees. The amendments are effective for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2020. The Company has early adopted the final rule under SEC Release No. 33-10532, Disclosure Updatenew standard effective January 1, 2019 and Simplification, amending certain disclosure requirements that were redundant, duplicative, overlapping, outdated or superseded. In addition, the amendments expanded the disclosure requirementsadoption of this standard did not have a material impact on the analysis of stockholders' equityCompany’s consolidated financial statements.
Recent Accounting Standards Not Yet Adopted
Income Taxes
In December 2019, the FASB issued ASU No. 2019-12, “Income Taxes (Topic 740): Simplifying the Accounting for interim financial statements. Under the amendments, an analysis of changes in each caption of stockholders' equity presented in the balance sheet must be provided in a note or separate statement. The analysis should present a reconciliation of the beginning balanceIncome Taxes (“ASU 2019-12”), which is intended to simplify various aspects related to accounting for income taxes. ASU 2019-12 removes certain exceptions to the ending balance of each periodgeneral principles in Topic 740 and also clarifies and amends existing guidance to improve consistent application. This guidance is effective for which a statement of comprehensive income is required to be filed. This final rule was effective on November 5, 2018.fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted. The Company is currently evaluating the impact of this guidancestandard on its consolidated financial statements. The Company anticipates its first presentation of changes in shareholders’ equity in accordance with the new guidance, will be included in its Form 10-Q for the quarter ended March 31, 2019.statements and related disclosures.
| Note 4: |
The Asset Acquisition
On October 17, 2018, the Company completed its acquisition with Marker Cell Therapy, Inc., formerly known as Marker Therapeutics, Inc., a privately-held Delaware corporation (“Marker Cell”), in accordance with the terms of an Agreement and Plan of Merger and Reorganization dated as of May 15, 2018 (the “Merger Agreement”) by and among the Company, Timberwolf Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of the Company (“Merger Sub”), and Marker. On October 17, 2018, pursuant to the Merger Agreement, Merger Sub was merged with and into Marker Cell (the “Merger”), with Marker Cell being the surviving corporation and becoming a wholly-owned subsidiary of the Company. In connection with the Merger, the Company changed its name to Marker Therapeutics, Inc. and Marker Cell changed its name to Marker Cell Therapy, Inc. At the effective time of the Merger, the former Marker Cell stockholders received (i) an aggregate of 13,914,255 shares of the Company’s common stock which equaled the number of shares of the Company’s common stock issued and outstanding immediately prior to the effective time of the Merger, and (ii) an aggregate of 5,046,003 warrants which equaled the number of the Company’s warrants and stock options issued and outstanding immediately prior to the effective time of the Merger.
Securities Purchase Agreements
On October 17, 2018, concurrent with the completion of the Merger, the Company issued to certain accredited investors in a private placement transaction (the “Financing”), an aggregate of 17,500,000 shares of its common stock, and warrants to purchase 13,437,500 shares of common stock at an exercise price of $5.00 per share with a five-year term, for gross proceeds of $70 million pursuant to the terms of the Securities Purchase Agreements, dated June 8, 2018, by and among the Company and certain accredited investors (the “Securities Purchase Agreements”).
Accounting Treatment
The Company concluded that the merger should be accounted for as an asset acquisition by the Company rather than as a business combination under Accounting Standards Codification (ASC) 805, Business Combinations. The merger was accounted for as an asset acquisition because substantially all the fair value of the assets being acquired are concentrated in a group of similar assets. Furthermore, the acquired assets did not have outputs or employees. The assets acquired by the Company under the merger include a license, other associated intellectual property, documentation and records, and related materials. Because Marker’s intellectual property had not yet received regulatory approval, the $116.0 million purchase price paid for these assets was expensed in the Company’s statement of operations for the fiscal year ended December 31, 2018. The Common Stock issued for the asset acquisition was valued at $116.0 million which is equal to the 13,914,255 common shares issued to Marker multiplied by $8.34, the closing price of the Company’s Common Stock as of October 17, 2018.
The Company also considered whether the merger should be accounted for as a reverse acquisition by Marker. The purpose of the merger is for the Company to acquire the assets of Marker so that the Company can expand its product and service offerings. While the former TapImmune and Marker stockholders hold an equal number of Board seats in the combined entity, the Company concluded that Marker would not be deemed the accounting acquirer under ASC 805, and therefore the merger is not a reverse acquisition.
| Note 5: | NET LOSS PER SHARE APPLICABLE TO COMMON SHAREHOLDERS |
Net Loss per Share Applicable to Common Stockholders
Basic loss per common share is computed by dividing net loss by the weighted average number of common shares outstanding during the reporting period. Diluted loss per common share is computed similarly to basic loss per common share except that it reflects the potential dilution that could occur if dilutive securities or other obligations to issue common stock were exercised or converted into common stock.
The following table sets forth the computation of loss per share for the years ended December 31, 20182019 and 2017,2018, respectively:
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Numerator: | ||||||||
| Net loss | $ | (147,957,910 | ) | $ | (10,982,177 | ) | ||
| Denominator: | ||||||||
| Weighted average common shares outstanding | 19,091,926 | 9,453,483 | ||||||
| Net loss per share data: | ||||||||
| Basic and Diluted | $ | (7.75 | ) | $ | (1.16 | ) | ||
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Numerator: | ||||||||
| Net loss | $ | (21,427,964 | ) | $ | (147,957,910 | ) | ||
| Denominator: | ||||||||
| Weighted average common shares outstanding | 45,587,734 | 19,091,926 | ||||||
| Net loss per share data: | ||||||||
| Basic and diluted | $ | (0.47 | ) | $ | (7.75 | ) | ||
The following securities, rounded to the thousand, were not included in the diluted net loss per share calculation because their effect was anti-dilutive for the periods presented:
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Common stock options | 4,120,000 | 489,000 | ||||||
| Common stock purchase warrants | 22,989,000 | 6,517,500 | ||||||
| Common stock warrants - liability treatment | 27,000 | 3,500 | ||||||
| Potentially dilutive securities | 27,136,000 | 7,010,000 | ||||||
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Common stock options | 4,983,000 | 4,120,000 | ||||||
| Common stock purchase warrants | 22,605,000 | 22,989,000 | ||||||
| Common stock warrants - liability treatment | 59,000 | 27,000 | ||||||
| Potentially dilutive securities | 27,647,000 | 27,136,000 | ||||||
| Note 6: | PROPERTY AND EQUIPMENT |
Property and equipment consist of the following as of December 31, 20182019 and 2017,2018, respectively:
| For the years ended | ||||||||||
| December 31, | ||||||||||
| Estimated Useful Lives | 2018 | 2017 | ||||||||
| Computers and equipment | 3-5 Years | $ | 66,000 | $ | - | |||||
| Office furniture | 5 Years | 82,000 | - | |||||||
| Total | $ | 148,000 | $ | - | ||||||
| Less: accumulated depreciation | - | - | ||||||||
| Property and equipment, net | $ | 148,000 | $ | - | ||||||
| December 31, | December 31, | |||||||||
| Estimated Useful Lives | 2019 | 2018 | ||||||||
| Lab equipment | 5 Years | $ | 111,000 | $ | - | |||||
| Computers, equipment and software | 3-5 Years | 211,000 | 66,000 | |||||||
| Office furniture | 5 Years | 178,000 | 82,000 | |||||||
| Leasehold improvements | Lesser of lease term or estimated useful life | 23,000 | - | |||||||
| Total | 523,000 | 148,000 | ||||||||
| Less: accumulated depreciation | (105,000 | ) | - | |||||||
| Property and equipment, net | $ | 418,000 | $ | 148,000 | ||||||
Depreciation expense for the year ended December 31, 2019 was $105,000. Furniture and computer equipment were placed in use on January 1, 2019 therefore no depreciation expense was recorded during the year ended December 31, 2018.
| Note 7: | LEASES |
The Company leases office space under agreements classified as operating leases that expire on various dates through 2022. All of the Company’s lease liabilities result from the lease of its corporate headquarters in Houston, Texas, which expires in 2021, and its Jacksonville, Florida office space, which expires in 2022. Such leases do not require any contingent rental payments, impose any financial restrictions, or contain any residual value guarantees. Certain of the Company’s leases include renewal options and escalation clauses; renewal options have not been included in the calculation of the lease liabilities and right of use assets as the Company is not reasonably certain to exercise the options. Variable expenses generally represent the Company’s share of the landlord’s operating expenses. The Company does not act as a lessor or have any leases classified as financing leases.
The Company excludes short-term leases having initial terms of 12 months or less from the new accounting guidance as an accounting policy election and recognizes rent expense on a straight-line basis over the lease term. During the fiscal year ended December 31, 2019, the Company had two lease agreements, an office at the Florida Atlantic Research and Development Authority and laboratory space located at the Texas Medical Center in Houston, which are included in short-term lease expense below.
At December 31, 2019, the Company had operating lease liabilities of approximately $484,000 and right of use assets of approximately $455,000, which were included in the consolidated balance sheet.
The following summarizes quantitative information about the Company’s operating leases:
| For the Year Ended | ||||
| December 31, 2019 | ||||
| Operating lease expense summary: | ||||
| Operating lease expense | $ | 220,000 | ||
| Short-term lease expense | 100,000 | |||
| Variable lease expense | 90,000 | |||
| Total | $ | 410,000 | ||
| Other information: | ||||
| Operating cash flows from operating leases for the twelve months ended December 31, 2019 | $ | 225,000 | ||
| Right of use assets exchanged for new operating lease liabilities as of adoption date | $ | 670,000 | ||
| Weighted-average remaining lease term as of December 31, 2019 – operating leases | 1.6 | |||
| Weighted-average discount rate as of adoption date – operating leases | 6.8 | % | ||
Maturities of the Company’s operating leases, excluding short-term leases, are as follows:
| Year ended December 31, 2020 | $ | 231,000 | ||
| Year ended December 31, 2021 | 226,000 | |||
| Year ended December 31, 2022 | 68,000 | |||
| Total | $ | 525,000 | ||
| Less present value discount | (41,000 | ) | ||
| Operating lease liabilities included in the Consolidated Balance Sheet at December 31, 2019 | $ | 484,000 | ||
Total rental expense under the Company’s operating leases was $220,000 and $175,600 for the years ended December 31, 2019 and 2018, respectively.
F -16
Note 8: accounts payable and accrued liabilities
Accounts payable and accrued liabilities consist of the following as of December 31, 20182019 and 2017,2018, respectively:
| December 31, | December 31, | |||||||
| 2018 | 2017 | |||||||
| Accounts payable | $ | 1,619,000 | $ | 1,015,000 | ||||
| Compensation and benefits | 416,000 | 162,000 | ||||||
| Professional fees | 236,000 | 32,000 | ||||||
| Technology license fees | 80,000 | 105,000 | ||||||
| Investor relations fees | 297,000 | 110,000 | ||||||
| Other | 106,000 | 89,000 | ||||||
| Total accounts payable and accrued liabilities | $ | 2,754,000 | $ | 1,513,000 | ||||
| December 31, | December 31, | |||||||
| 2019 | 2018 | |||||||
| Accounts payable | $ | 993,000 | $ | 1,619,000 | ||||
| Compensation and benefits | 323,000 | 416,000 | ||||||
| Professional fees | 94,000 | 236,000 | ||||||
| Technology license fees | 105,000 | 80,000 | ||||||
| Investor relations fees | - | 297,000 | ||||||
| Other | 243,000 | 106,000 | ||||||
| Total accounts payable and accrued liabilities | $ | 1,758,000 | $ | 2,754,000 | ||||
Note 9: WARRANT LIABILITY
A weighted average summary of quantitative information with respect to valuation methodology and significant unobservable inputs used for the Company’s common stock purchase warrants that are categorized within Level 3 of the fair value hierarchy for the years ended December 31,2019 and 2018, and 2017, respectively:
| Weighted Average Inputs | ||||||||
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Stock price | $ | 5.55 | $ | 3.92 | ||||
| Exercise price | $ | 9.72 | $ | 1.20 | ||||
| Contractual term (years) | 1.08 | 0.78 | ||||||
| Volatility (annual) | 99 | % | 63 | % | ||||
| Risk-free rate | 2 | % | 1 | % | ||||
| Dividend yield (per share) | 0 | % | 0 | % | ||||
| Weighted Average Inputs | ||||||||
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Exercise price | $ | 6.92 | $ | 9.72 | ||||
| Contractual term (years) | 0.05 | 1.08 | ||||||
| Volatility (annual) | 83 | % | 99 | % | ||||
| Risk-free rate | 2 | % | 2 | % | ||||
| Dividend yield (per share) | 0 | % | 0 | % | ||||
The foregoing assumptions are recalculated every reporting period and are subject to change based primarily on management’s assessment of the probability of the events described occurring. Accordingly, changes to these assessments could materially affect the valuations.
The following table presents changes in Level 3 warrant liabilities, reflected in accrued expenses measured at fair value for the years ended December 31, 20182019 and 2017,2018, respectively:
| Warrant | ||||
| Liability | ||||
| Balance - January 1, 2017 | $ | 14,500 | ||
| Change in fair value of warrant liability | (5,500 | ) | ||
| Balance – December 31, 2017 | 9,000 | |||
| Change in fair value of warrant liability | 40,000 | |||
| Balance – December 31, 2018 | $ | 49,000 | ||
| Warrant | ||||
| Liability | ||||
| Balance – January 1, 2018 | $ | 9,000 | ||
| Change in fair value of warrant liability | 40,000 | |||
| Balance – December 31, 2018 | 49,000 | |||
| Change in fair value of warrant liability | (18,000 | ) | ||
| Balance – December 31, 2019 | $ | 31,000 | ||
F -17
Note 10: FAIR VALUE MEASUREMENTS
Financial assets and liabilities measured at fair value on a recurring basis are summarized below and disclosed on the balance sheet under Derivative liability – warrants:warrant liability:
| Fair value measured at December 31, 2018 | ||||||||||||||||
| Quoted prices in active | Significant other | Significant | ||||||||||||||
| markets | observable inputs | unobservable inputs | Fair value at | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | December 31, 2018 | |||||||||||||
| Warrant liability | $ | - | $ | - | $ | 49,000 | $ | 49,000 | ||||||||
| Fair value measured at December 31, 2019 | ||||||||||||||
| Quoted prices in active | Significant other | Significant | ||||||||||||
| markets | observable inputs | unobservable inputs | Fair value at | |||||||||||
| (Level 1) | (Level 2) | (Level 3) | December 31, 2019 | |||||||||||
| Warrant liability | $ | - | $ | - | $ | 31,000 | $ | 31,000 | ||||||
| Fair value measured at December 31, 2017 | ||||||||||||||||
| Quoted prices in active | Significant other | Significant | ||||||||||||||
| markets | observable inputs | unobservable inputs | Fair value at | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | December 31, 2017 | |||||||||||||
| Warrant liability | $ | - | $ | - | $ | 9,000 | $ | 9,000 | ||||||||
| Fair value measured at December 31, 2018 | ||||||||||||||
| Quoted prices in active | Significant other | Significant | ||||||||||||
| markets | observable inputs | unobservable inputs | Fair value at | |||||||||||
| (Level 1) | (Level 2) | (Level 3) | December 31, 2018 | |||||||||||
| Warrant liability | $ | - | $ | - | $ | 49,000 | $ | 49,000 | ||||||
There were no transfers between Level 1, 2 or 3 during the years ended December 31, 20182019 and 2017,2018, respectively.
The valuation of warrants is subjective and is affected by changes in inputs to the valuation model including the price per share of common stock, the historical volatility of the stock price, risk-free rates based on U. S. Treasury security yields, the expected term of the warrants and dividend yield. Changes in these assumptions can materially affect the fair value estimate. The Company could ultimately incur amounts to settle the warrant at a cash settlement value that is significantly different than the carrying value of the liability on the financial statements. The Company will continue to classify the fair value of the warrants as a liability until the warrants are exercised, expire, or are amended in a way that would no longer require these warrants to be classified as a liability. Changes in the fair value of the common stock warrants liability are recognized as a component of other income (expense) in the Statements of Operations.
The net cash settlement value at the time of any future transactions, where the Company consolidates or merges with another entity, will depend upon the value of the following inputs at that time: the consideration value per share of the Company’s common stock, the volatility of the Company’s common stock, the remaining term of the warrant from announcement date, the risk-free interest rate based on U. S. Treasury security yields, and the Company’s dividend yield. The warrant requires use of a volatility assumption equal to the greater of 100% and the 100-day volatility function determined as of the trading day immediately following announcement of a Fundamental Transaction.
Note 11: stockholders’ equity
Preferred Stock
The Company has authorized up to 5,000,000 shares of preferred stock, $0.0001$0.001 par value per share, for issuance. The preferred stock will have such rights, privileges and restrictions, including voting rights, dividend conversion rights, redemption privileges and liquidation preferences, as shall be determined by the Company’s board of directors upon its issuance. To date, the Company has not issued any preferred shares.
Series A Preferred Stock -The Company has designated up to 1,250,000 shares of Series A Preferred Stock, $0.0001 par value per share, for issuance. To date, the Company has not issued any Series A preferred shares.
Series B Preferred Stock -The Company has designated up to 1,500,000 shares of Series B Preferred Stock, $0.0001 par value per share, for issuance. To date, the Company has not issued any Series B preferred shares.
Common Stock
The Company has authorized up to 150,000,000 shares of common stock, $0.0001$0.001 par value per share, for issuance. Significant 20182019 and 20172018 common stock transactions were as follows:
2019 Common Stock Transactions
Consulting Arrangements
During the twelve months ended December 31, 2019, the Company issued 47,400 shares of common stock in connection with consulting agreements. The fair value of the common stock of approximately $265,000 was recognized as stock-based compensation expense in general and administrative expenses.
F -18
Board Compensation
During the twelve months ended December 31, 2019, the Company issued an aggregate of 29,040 shares of common stock to its non-employee directors. The fair value of the common stock of approximately $174,000 was recognized as stock-based compensation expense in general and administrative expenses.
2018 Common Stock Transactions
The Merger
Pursuant to the Merger discussed in Note 4 above, the Company issued 13,914,255 shares of common stock to shareholders of Marker Cell Therapy, Inc. The fair market value of the shares issued pursuant to the asset acquisitionmerger was $116.0 million.
Securities Purchase Agreements
Pursuant to the financing discussed in Note 4 above, the Company issued 17,500,000 shares of its common stock to the participating accredited investors. Net proceeds, after transaction offering costs of $6.2 million, were $63.8 million.
Common Stock Purchase Agreement
On May 14, 2018, the Company’s largest stockholder Eastern Capital Limited entered into a Common Stock Purchase Agreement with the Company pursuant to which it purchased 1,300,000 shares of common stock at a price per share of $2.40 providing gross proceeds to the Company of $3.12 million.
Exercise and Repricing of Warrants Held by Existing Institutional Investors
On May 14, 2018, certain institutional holders of outstanding warrants entered into Warrant Exercise Agreements with the Company that provide for an amendment to the exercise price of the warrants being exercised at $2.50 per share. Upon closing of the Warrant Exercise Agreements, such institutional holders immediately exercised warrants for 782,505 shares of common stock providing aggregate proceeds to the Company of approximately $2.0 million.
The fair value relating to the modification of exercise prices on the repriced and exercised warrants was treated as deemed dividend on the statement of stockholders’ equity of $728,000.
A weighted average summary of quantitative information with respect to valuation methodology and significant unobservable inputs used for the Company’s common stock purchase warrants that are included in the modification is as follows:
| Weighted Average Inputs | ||||||||
| Before | After | |||||||
| Modification | Modification | |||||||
| Exercise price | $ | 9.93 | $ | 2.50 | ||||
| Contractual term (years) | 2.37 | 2.37 | ||||||
| Volatility (annual) | 79 | % | 79 | % | ||||
| Risk-free rate | 1.5 | % | 1.5 | % | ||||
| Dividend yield (per share) | 0 | % | 0 | % | ||||
| Weighted Average Inputs | ||||||||
| Before Modification | After Modification | |||||||
| Exercise price | $ | 9.93 | $ | 2.50 | ||||
| Contractual term (years) | 2.37 | 2.37 | ||||||
| Volatility (annual) | 79 | % | 79 | % | ||||
| Risk-free rate | 1.5 | % | 1.5 | % | ||||
| Dividend yield (per share) | 0 | % | 0 | % | ||||
F -19
Exercise of Stock Warrants
In addition to the exercise and repricing of warrants discussed above, during the twelve months ended December 31, 2018, certain outstanding warrants were exercised by warrant holders providing aggregate proceeds to the Company of approximately $2.4 million and resulted in the issuance of 716,819 shares of common stock.
Additionally, 280,760 of the stock warrants exercised were exercised on a cashless basis, which resulted in approximately 204,000 of warrant shares being cancelled due to use of cashless exercise provisions.
Exercise of Stock Options
In January 2018, 10,416 shares of common stock were issued pursuant to stock option exercises at an exercise price equal to $1.74 per share.
Consulting Arrangements
During the twelve months ended December 31, 2018, the Company issued 274,012 shares of common stock in connection with consulting agreements. The fair value of the common stock of approximately $1.8 million was recognized as stock-based compensation expense, $1.7 million in general and administrative expenses and $0.1 million in research and development expenses.
2018 Management and Board Compensation
During the twelve months ended December 31, 2018, the Company issued 53,774 shares of common stock in connection with board of director and management agreements. The fair value of the common stock of approximately $0.5 million was recognized as stock-based compensation expense in general and administrative expenses. 7,561 shares of common stock, with a fair value of $0.1 million, were withheld to satisfy certain payroll liabilities, as applicable to an award to a former director.
2017 Common Stock Transactions
June 2017 Private Placement Transaction
On June 26, 2017, the Company completed private placement of units with certain accredited investors. In the private placement transaction, the Company sold 1,503,567 shares of common stock for $3.97 per share and five-year warrants to purchase an equal number of shares of common stock, at an exercise price of $3.97 per share, for $0.125 per warrant, with one common share and one warrant being sold together as a unit for a total of $4.095 per unit. The Company issued and sold an aggregate of 1,503,567 million units for aggregate gross proceeds of $6.2 million. The Company incurred $0.8 million in agency fees and legal costs. In connection with the offering, the Company reduced the exercise price for the warrants to purchase an aggregate of 653,187 shares of common stock issued to investors in the private placement that closed in August 2016 from $6.00 per share to $3.97 per share.
In addition, the Company issued five-year warrants to the placement agent in the offering providing for the purchase of up to 150,357 shares of Company common stock for $3.97 per share.
June 2017 Exercise and Repricing of Warrants Held by Existing Institutional Investors
On June 23, 2017, certain existing institutional shareholders of the Company who hold various outstanding warrants (i.e. C, D, E and F) to purchase Company common stock, entered into warrant repricing and exercise agreements.
Series E repriced and exercised warrants
Approximately 168,000 of Series E warrants were repriced from $15.00 per share to $3.97 per share and exercised immediately for gross proceeds of approximately $0.7 million. Series E warrants to purchase approximately 187,000 shares of Company common stock being reduced from $15.00 per share to $4.50 per share.
Series C, D & F repriced warrants
Additionally, the exercise prices for certain investors of Series C, Series D and Series F warrants were reduced as follows:
| Number of | ||||||||||||
| Warrant Shares | Pre-reduced | Post-reduced | ||||||||||
| Series | Repriced | Price | Price | |||||||||
| Series C | 313,750 | $ | 6.00 | $ | 4.00 | |||||||
| Series D | 312,500 | $ | 9.00 | $ | 4.00 | |||||||
| Series F | 292,500 | $ | 7.20 | $ | 4.00 | |||||||
The fair value relating to the modification of exercise prices on the repriced warrants was treated as deemed dividend on the statement of stockholders’ equity of $0.6 million.
A weighted average summary of quantitative information with respect to valuation methodology and significant unobservable inputs used for the Company’s common stock purchase warrants that are included in the modification is as follows:
| Before | After | |||||||
| Modification | Modification | |||||||
| Exercise price | $ | 8.32 | $ | 4.04 | ||||
| Contractual term (years) | 3.34 | 3.34 | ||||||
| Volatility (annual) | 200 | % | 200 | % | ||||
| Risk-free rate | 2 | % | 2 | % | ||||
| Dividend yield (per share) | 0 | % | 0 | % | ||||
2017 Management Compensation
On March 9, 2017, the Company issued 12,761 shares of stock to Dr. Glynn Wilson. The fair value of the common stock of $55,000 was recognized as stock-based compensation in general and administrative expenses. The issuance was based on the closing price or our common stock of $4.31 per share.
On March 9, 2017, the Company issued 5,220 shares of stock to our former Chief Operating Officer. The fair value of the common stock of $22,500 was recognized as stock-based compensation in general and administrative expenses. The issuance was based on the closing price or our common stock of $4.31 per share.
On September 22, 2017, the Company granted Mr. Hoang 250,000 shares of unregistered, fully vested restricted common stock. The Company recorded $0.8 million of stock-based compensation based on the fair value of the common stock at September 22, 2017. 70,289 shares of common stock, with a fair value of $0.2 million, were withheld (at the closing price of the Company's common stock on the NASDAQ Capital Market on September 22, 2017) to satisfy certain payroll liabilities, as applicable to the award.
On September 22, 2017, the Company granted Dr. Wilson 100,000 shares of unregistered, fully vested restricted common stock. The Company recorded $0.3 million of stock-based compensation based on the fair value of the common stock at September 22, 2017. 27,350 shares of common stock, with a fair value of $0.1 million, were withheld (at the closing price of the Company's common stock on the NASDAQ Capital Market on September 22, 2017) to satisfy certain payroll liabilities, as applicable to the award.
Consulting Arrangements
During fiscal 2017, the Company issued 0.2 million shares of common stock as part of consulting agreements. The fair value of the common stock of $0.6 million was recognized as stock-based compensation in general and administrative expenses.
Note 11:12: WARRANTS
Share Purchase Warrants
A summary of the Company’s share purchase warrants as of December 31, 20182019 and 2017,2018, respectively, and changes during the period is presented below:
| Weighted Average | ||||||||||||||||
| Number of | Weighted Average | Remaining Contractual | Total Intrinsic | |||||||||||||
| Warrants | Exercise Price | Life (in years) | Value | |||||||||||||
| Balance - January 1, 2017 | 5,059,000 | $ | 8.49 | 3.68 | $ | 1,713,000 | ||||||||||
| Issued | 1,654,000 | 3.97 | - | - | ||||||||||||
| Exercised for cash | (168,000 | ) | 15.00 | - | - | |||||||||||
| Expired or cancelled | (25,000 | ) | 30.50 | - | - | |||||||||||
| Balance - December 31, 2017 | 6,520,000 | 6.11 | 3.16 | 1,739,000 | ||||||||||||
| Issued | 18,484,000 | 4.45 | - | - | ||||||||||||
| Cashless exercised | (281,000 | ) | 4.03 | - | - | |||||||||||
| Exercised for cash | (1,499,000 | ) | 6.78 | - | - | |||||||||||
| Expired or cancelled | (208,000 | ) | 4.00 | - | - | |||||||||||
| Balance -December 31, 2018 | 23,016,000 | $ | 4.78 | 4.29 | $ | 26,066,000 | ||||||||||
| Number of | Weighted Average | Weighted Average Remaining Contractual | Total Intrinsic | |||||||||||||
| Warrants | Exercise Price | Life (in years) | Value | |||||||||||||
| Balance - January 1, 2018 | 6,520,000 | $ | 6.11 | 3.16 | $ | 1,733,000 | ||||||||||
| Warrants granted | 18,484,000 | 4.45 | - | - | ||||||||||||
| Exercised for cash | (1,499,000 | ) | 6.79 | - | - | |||||||||||
| Cashless exercised | (281,000 | ) | 4.03 | - | - | |||||||||||
| Expired or cancelled | (208,000 | ) | 4.00 | - | - | |||||||||||
| Balance -December 31, 2018 | 23,016,000 | 4.78 | 4.29 | 26,066,000 | ||||||||||||
| Warrants granted | 45,000 | 4.26 | - | - | ||||||||||||
| Exercised for cash | (190,000 | ) | 3.99 | - | - | |||||||||||
| Cashless exercise | (17,000 | ) | 2.38 | - | - | |||||||||||
| Expired or cancelled | (190,000 | ) | 13.63 | - | - | |||||||||||
| Balance - December 31, 2019 | 22,664,000 | $ | 4.71 | 3.33 | $ | 954,000 | ||||||||||
2019 Warrant Transactions
Exercise of Stock Warrants
During the twelve months ended December 31, 2019, certain outstanding warrants were exercised for 190,258 shares of common stock providing aggregate proceeds to the Company of approximately $759,000.
F -20
Additionally, during the twelve months ended December 31, 2019, the Company issued 9,449 shares of common stock upon cashless exercises of stock warrants, which resulted in cancellation of 7,211 shares of common stock subject to such warrants.
2018 Warrant Transactions
The Merger
Pursuant to the Merger discussed in Note 4 above, the Company issued 5,046,003 stock warrants to shareholders of Marker Cell Therapy, Inc. at an exercise price of $2.99 per share with a five-year term.
Securities Purchase Agreements
Pursuant to the financing discussed in Note 4 above, the Company issued 13,437,500 stock warrants to certain accredited investors at an exercise price of $5.00 per share with a five-year term.
Exercise and Repricing of Warrants Held by Existing Institutional Investors
On May 14, 2018, certain institutional holders of outstanding warrants entered into Warrant Exercise Agreements with the Company that provide for an amendment to the exercise price of the warrants being exercised at $2.50 per share. Upon closing of the Warrant Exercise Agreements, such institutional holders immediately exercised warrants for 782,505 shares of common stock providing aggregate proceeds to the Company of approximately $2.0 million.
The fair value relating to the modification of exercise prices on the repriced and exercised warrants was treated as deemed dividend on the statement of stockholders’ equity of $728,000.
A weighted average summary of quantitative information with respect to valuation methodology and significant unobservable inputs used for the Company’s common stock purchase warrants that are included in the modification is as follows:
| Weighted Average Inputs | ||||||||
| Before | After | |||||||
| Modification | Modification | |||||||
| Exercise price | $ | 9.93 | $ | 2.50 | ||||
| Contractual term (years) | 2.37 | 2.37 | ||||||
| Volatility (annual) | 79 | % | 79 | % | ||||
| Risk-free rate | 1.5 | % | 1.5 | % | ||||
| Dividend yield (per share) | 0 | % | 0 | % | ||||
| Weighted Average Inputs | ||||||||
| Before Modification | After Modification | |||||||
| Exercise price | $ | 9.93 | $ | 2.50 | ||||
| Contractual term (years) | 2.37 | 2.37 | ||||||
| Volatility (annual) | 79 | % | 79 | % | ||||
| Risk-free rate | 1.5 | % | 1.5 | % | ||||
| Dividend yield (per share) | 0 | % | 0 | % | ||||
Exercise of Stock Warrants
In addition to the exercise and repricing of warrants discussed above, during the twelve months ended December 31, 2018, certain outstanding warrants were exercised by warrant holders providing aggregate proceeds to the Company of approximately $2.4 million and resulted in the issuance of 716,819 shares of common stock.
Additionally, 280,760 of the stock warrants exercised were exercised on a cashless basis, which resulted in approximately 204,000 of warrant shares being cancelled due to use of cashless exercise provisions.
2017 Warrant Transactions
June 2017 Private Placement Transaction
On June 26, 2017, the Company completed private placement of units with certain accredited investors. In the private placement transaction, the Company sold 1,503,567 shares of common stock for $3.97 per share and five-year warrants to purchase an equal number of shares of common stock, at an exercise price of $3.97 per share, for $0.125 per warrant, with one common share and one warrant being sold together as a unit for a total of $4.095 per unit. The Company issued and sold an aggregate of 1,503,567 million units for aggregate gross proceeds of $6.2 million. The Company incurred $0.8 million in agency fees and legal costs. In connection with the offering, the Company reduced the exercise price for the warrants to purchase an aggregate of 653,187 shares of common stock issued to investors in the private placement that closed in August 2016 from $6.00 per share to $3.97 per share.
June 2017 Exercise and Repricing of Warrants Held by Existing Institutional Investors
On June 23, 2017, certain existing institutional shareholders of the Company who hold various outstanding warrants (i.e. C, D, E and F) to purchase Company common stock, entered into warrant repricing and exercise agreements.
Series E repriced and exercised warrants
Approximately 168,000 of Series E warrants were repriced from $15.00 per share to $3.97 per share and exercised immediately for gross proceeds of approximately $0.7 million. Series E warrants to purchase approximately 187,000 shares of Company common stock being reduced from $15.00 per share to $4.50 per share.
Series C, D & F repriced warrants
Additionally, the exercise prices for certain investors of Series C, Series D and Series F warrants were reduced as follows:
| Number of | ||||||||||||
| Warrant Shares | Pre-reduced | Post-reduced | ||||||||||
| Series | Repriced | Price | Price | |||||||||
| Series C | 313,750 | $ | 6.00 | $ | 4.00 | |||||||
| Series D | 312,500 | $ | 9.00 | $ | 4.00 | |||||||
| Series F | 292,500 | $ | 7.20 | $ | 4.00 | |||||||
The fair value relating to the modification of exercise prices on the repriced warrants was treated as deemed dividend on the statement of stockholders’ equity of $0.6 million.
June 2017 Agent Warrants
Pursuant to an agency agreement, dated May 12, 2017, by and between Katalyst Securities LLC and us, Katalyst agreed to act as our placement agent in connection with the June 26, 2017 private placement offering.
Pursuant to the agreement, we agreed to pay to Katalyst: (i) an aggregate cash fee for placement agent and financial advisory services equal to 10% of the gross proceeds of the Offering; (ii) a non-accountable expense allowance in the amount of Seventy Thousand Dollars ($70,000); and (iii) five-year warrants to purchase a number of shares of our common stock equal to 10% of the number of shares sold in the offering. The Katalyst Warrants have the same terms as the private placement warrants issued in the offering. Based on the 1,503,567 shares of common stock sold in the private placement, we issued five-year warrants to Katalyst providing for the purchase of up to 150,357 shares of Company common stock for $3.97 per share.
F -21
Note 13: stock OPTION PLANS
Options to Purchase Shares of Common Stock
2014 Stock Omnibus Plan
On March 19, 2014, the Board adopted the 2014 Omnibus Stock Option Plan (“2014 Plan”), which replaced the 2009 Stock Incentive Plan. The 2014 Plan allowed for grants of stock options, restricted shares, stock bonuses and other equity-based awards to employees and non-employee directors of the Company. Awards under the 2014 Plan may be at prices and for terms as determined by the Board of Directors and may have vesting requirements as determined by the Board, provided that the exercise price for any stock option must be at least equal to the fair market value (as defined in the 2014 Plan) of a share of the stock on the grant date. Once granted, the exercise price of an option may not be reduced without the approval of the Company’s stockholders, other than under certain limited circumstances such as a stock split or take any other action with respect to a stock option that would be treated as a repricing under the rules and regulations of the New York Stock Exchange.
The 2014 Plan was amended in February 2015 to provide for grants to consultants, and again in November 2015 to (i) increase the number of shares reserved for issuance under the Plan to 0.6 million shares; (ii) provide the Board and Committee administering the Plan with full discretion on the vesting period for Service-Vesting Awards under the Plan, including the grant of Awards with less than the Minimum Vesting Requirement (as such terms are defined in the Plan), and (iii) provide the Board and Committee administering the Plan with the ability to grant stock bonuses to executive officers.
On August 29, 2017, the 2014 Plan was amended to increase the shares reserved under the Plan to 1.4 million shares, and on October 16, 2018 the 2014 Plan was amended to increase the shares reserved under the Plan to 8.0 million shares. As of December 31, 2018,2019, approximately 3.42.5 million options are available to be issued from the 2014 Plan.
F -22
Stock Options
A summary of the Company’s employee stock option activity is as follows for stock options:
| Number of Shares | Weighted Average Exercise Price | Total Intrinsic Value | Weighted Average Remaining Contractual Life (in years) | |||||||||||||
| Outstanding as of January 1, 2017 | 430,624 | $ | 7.41 | $ | 39,000 | 8.9 | ||||||||||
| Granted | 40,000 | 3.88 | - | 9.4 | ||||||||||||
| Exercised | - | - | - | - | ||||||||||||
| Forfeited/expired | (34,827 | ) | 6.71 | - | - | |||||||||||
| Outstanding as of December 31, 2017 | 435,797 | - | 42,000 | 8.1 | ||||||||||||
| Granted | 2,464,855 | 8.79 | - | 9.8 | ||||||||||||
| Exercised | (10,416 | ) | 1.74 | - | - | |||||||||||
| Forfeited/expired | (63,226 | ) | 6.00 | - | - | |||||||||||
| Outstanding as of December 31, 2018 | 2,827,010 | $ | 8.61 | $ | 113,000 | 9.5 | ||||||||||
| Options vested and exercisable | 1,761,567 | $ | 8.75 | $ | 193,000 | 9.2 | ||||||||||
| Number of Shares | Weighted Average Exercise Price | Total Intrinsic Value | Weighted Average Remaining Contractual Life (in years) | |||||||||||||
| Outstanding as of January 1, 2018 | 489,255 | $ | 7.08 | $ | 42,000 | 8.1 | ||||||||||
| Granted | 3,704,855 | 8.89 | - | 9.8 | ||||||||||||
| Exercised | (10,416 | ) | 1.74 | - | - | |||||||||||
| Canceled | (63,226 | ) | 6.00 | - | - | |||||||||||
| Outstanding as of December 31, 2018 | 4,120,468 | 8.59 | $ | 339,000 | 9.1 | |||||||||||
| Granted | 1,172,500 | 4.70 | - | 9.5 | ||||||||||||
| Exercised | (11,980 | ) | 4.82 | - | - | |||||||||||
| Canceled | (297,674 | ) | 8.15 | - | - | |||||||||||
| Outstanding as of December 31, 2019 | 4,983,314 | $ | 7.79 | $ | 18,000 | 8.9 | ||||||||||
| Options vested and exercisable | 1,281,957 | $ | 8.34 | $ | 18,000 | 8.6 | ||||||||||
A summary of the Company’s non-employee stock option activity is as follows for stock options:
| Number of Shares | Weighted Average Exercise Price | Total Intrinsic Value | Weighted Average Remaining Contractual Life (in years) | |||||||||||||
| Outstanding as of January 1, 2017 | 3,471 | $ | 14.77 | $ | - | 8.7 | ||||||||||
| Granted | 50,000 | 2.62 | - | 9.9 | ||||||||||||
| Exercised | - | - | - | - | ||||||||||||
| Forfeited/expired | (13 | ) | 120.00 | - | - | |||||||||||
| Outstanding as of December 31, 2017 | 53,458 | $ | 3.38 | $ | 65,000 | 9.7 | ||||||||||
| Granted | 1,240,000 | - | - | 9.8 | ||||||||||||
| Exercised | - | - | - | - | ||||||||||||
| Forfeited/expired | - | - | - | - | ||||||||||||
| Outstanding as of December 31, 2018 | 1,293,458 | $ | 8.86 | $ | 179,000 | 9.7 | ||||||||||
| Options vested and exercisable | 53,458 | $ | 3.38 | $ | 147,000 | 9.7 | ||||||||||
The Black-Scholes option pricing model is used to estimate the fair value of stock options granted under the Company’s share-based compensation plans. The weighted average assumptions used in calculating the fair values of stock options that were granted during the years ended December 31, 2019 and 2018, 2017, respectively, were as follows:
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Exercise price | $ | 8.89 | $ | 3.25 | ||||
| Expected term (years) | 10.0 | 10.0 | ||||||
| Expected stock price volatility | 200 | % | 217 | % | ||||
| Risk-free rate of interest | 3 | % | 2 | % | ||||
| Expected dividend rate | 0 | % | 0 | % | ||||
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Exercise price | $ | 4.70 | $ | 8.89 | ||||
| Expected term (years) | 6.0 | 10.0 | ||||||
| Expected stock price volatility | 126 | % | 200 | % | ||||
| Risk-free rate of interest | 2 | % | 3 | % | ||||
| Expected dividend rate | 0 | % | 0 | % | ||||
The following table sets forth stock-based compensation expenses recorded during the respective periods:
| For the Years Ended | For the Years Ended | |||||||||||||||
| December 31, | December 31, | |||||||||||||||
| 2018 | 2017 | 2019 | 2018 | |||||||||||||
| Stock Compensation expenses: | ||||||||||||||||
| Research and development | $ | 1,265,000 | $ | 98,000 | $ | 2,574,000 | $ | 1,265,000 | ||||||||
| General and administrative | 15,086,000 | 2,640,000 | 2,783,000 | 15,086,000 | ||||||||||||
| Total stock compensation expenses | $ | 16,351,000 | $ | 2,738,000 | $ | 5,357,000 | $ | 16,351,000 | ||||||||
At December 31, 2018,2019, the total stock-based compensation cost related to unvested awards not yet recognized was $14.7$14.9 million. The expected weighted average period compensation costs to be recognized was 2.03.0 years. Future option grants will impact the compensation expense recognized.
| Note | grant INCOME |
During the years ended December 31, 20182019 and 2017,2018, the Company received $0.2 million of a grant awarded to Mayo Foundation from the U.S. Department of Defense for the Phase II Clinical Trial of TPIV200. The grant compensated the Company for clinical supplies manufactured and provided by the Company for the clinical study. In accordance with Accounting Standards Update No. 2014-09, “Revenue from Contracts with Customers (Topic 606)” issued by the Financial Accounting Standards Board, the Company recorded the $0.2 million of grant income as revenue.
| Note |
Operating Lease Obligations
An arbitration proceeding was brought against the Company before the Financial Industry Regulatory Authority, Inc. by a broker seeking to be paid approximately $1 million as compensation for two 2018 transactions, a warrant conversion and a private placement brokered by another broker. The broker’s claims are based on a placement agent agreement for a private placement it brokered in 2017, under which it alleges it is entitled to compensation for the 2018 transactions. The Company was a partybelieves it has defenses to several operating leases asall of December 31, 2018, primarily for office space at certain locations.the allegations and intends to vigorously defend itself in this matter.
Aggregate future minimum annual payments under operating leases at December 31, 2018, are as follows:
| Year | Operating Leases | |||
| 2019 | $ | 294,000 | ||
| 2020 | 234,000 | |||
| 2021 | 229,000 | |||
| 2022 | 71,000 | |||
| 2023 | 3,000 | |||
| Thereafter | 1,000 | |||
| Total minimum rentals | $ | 832,000 | ||
Total rental expense under the Company’s operating leases was $175,600 and $121,200 for the years ended December 31, 2018 and 2017, respectively.
| Note | LEGAL PROCEEDINGS |
From time to time, the Company may be party to ordinary, routine litigation incidental to their business. The Company knows of no material, active or pending legal proceedings against the Company, nor is the Company involved as a plaintiff in any material proceeding or pending litigation. There are no proceedings in which any of the Company’s directors, officers or affiliates, or any registered or beneficial shareholder, is an adverse party or has a material interest adverse to the Company’s interest.
| Note | RELATED PARTY TRANSACTIONS |
Payment made to Mr. John Wilson. In connection with the Merger discussed in Note 4, Mr. John Wilson, the former CEO of Marker Cell, was to be reimbursed for certain funds he advanced to Marker Cell prior to the closing of the Merger. Following the consummation of the Merger, in connection with the obligation to reimburse Mr. Wilson for such expenses, the Company paid Mr. Wilson $100,000 as part of theThe following table sets forth related party transaction expenses recorded during the Company incurred. At the effective time of the Merger, and as part of the terms thereof, Mr. Wilson became a director of the Company.respective periods:
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2019 | 2018 | |||||||
| Research and development | $ | 302,000 | $ | 138,000 | ||||
| General and administrative | - | - | ||||||
| Total | $ | 302,000 | $ | 138,000 | ||||
Sponsored Research Agreement with The Baylor College of Medicine (“BCM”) Sponsored Research Agreement.. On November 16, 2018, in furtherance of the BCM License Agreement and as contemplated by the terms thereof, the Company entered in a Sponsored Research Agreement (“SRA”) with BCM, which provided for the conduct of research for the Company by credentialed personnel at Baylor’sBCM’s Center for Cell and Gene Therapy. The research is
During the years ended December 31, 2019 and 2018, the Company incurred approximately $69,000 and $77,000, respectively, to be supervisedBCM under the SRA.
Clinical Supply Agreement with BCM. On September 9, 2019, in furtherance of the BCM License Agreement and as contemplated by the BCM’s Co-Investigators Dr. Vera and Dr. Leen as set forth and namedterms thereof, the Company entered in the SRA. The SRA has a four-year term. PursuantClinical Supply Agreement (“CSA”) with BCM, which provided for BCM to provide to the SRA, the Company has agreed to pay BCM up to $256,272 for years one and two under the SRA with $76,882 paid up front and $153,764 paid in equal monthly installments over two years and a final payment of $25,626 after receipt of the final written report. Payments for years three and four are to be covered by an amendment. multi tumor antigen specific products.
During the year ended December 31, 2018,2019, the Company paid BCM $0did not incur any expenses under the SRA as the upfront payment was made in January 2019. Neither Dr. Vera nor Dr. Leen received any of these payments or are entitled to receive any portion of these payments. Dr. Vera and Dr. Leen do however indirectly benefit from such payments in connection with their status as BCM employees.CSA.
The Consulting Agreement-Dr. Vera.Agreement with Dr. Juan Vera. On October 19, 2018, after the closing of the Merger,Company’s merger, the Company entered into a consulting agreement with Dr. Juan Vera, a member of the Company’s Boardboard of Directors,directors, to serve as the Company’s Chief Development Officer. TheOn September 1, 2019, Dr. Vera became an employee of the Company and his consulting agreement provided for the payment of an annual base consulting fee of $350,000 for services to the Company, discretionary cash payment by the Company up to a maximum of 35% of the base consulting fee, and an award of 500,000 stock options. The consulting agreement is terminable by either party upon 30 days prior written notice. One quarter of the stock options awarded vest on the first anniversary of the award and the remainder vest evenly in equal monthly installments over a three-year period upon the continued performance of consulting services by Dr. Vera over such period. Dr. Vera, an accomplished individual in his field, has another consulting arrangement with parties unrelated to the Company, that have licensed intellectual property from BCM that resulted from his research and development efforts. He may pursue similar arrangements in the future. was terminated.
During the yearyears ended December 31, 2019 and 2018, Dr. Vera was paid $60,577 by the Company incurred approximately $233,000 and $61,000, respectively, of expenses under hisDr. Vera’s consulting agreement.
NOTE 17: INCOME TAXES
| NOTE 18: | INCOME TAXES |
The Company has no income tax expense due to operating losses incurred for the years ended December 31, 20182019 and 2017.2018.
The effects of temporary differences that give rise to significant portions of the deferred tax assets as of December 31, 20182019 and 20172018 are as follows:
| For the years ended | ||||||||
| December 31, | ||||||||
| 2018 | 2017 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforward | $ | 13,596,000 | $ | 9,690,000 | ||||
| Stock-based compensation | 5,741,000 | 1,556,000 | ||||||
| License agreements | 206,000 | 223,000 | ||||||
| Research and development | 406,000 | 406,000 | ||||||
| Charitable contributions | 3,000 | 3,000 | ||||||
| 19,952,000 | 11,878,000 | |||||||
| Less: Valuation allowance | (19,952,000 | ) | (11,878,000 | ) | ||||
| Deferred tax assets, net of valuation allowance | $ | - | $ | - | ||||
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cuts and Jobs Act (the “Tax Act”), which makes broad and complex changes to the U.S. tax code. Certain of these changes may be applicable to the Company, including but not limited to, reducing the U.S. federal corporate tax rate from 34 percent to 21 percent, creating a new limitation on deductible interest expense, eliminating the corporate alternative minimum tax (“AMT”), modifying the rules related to uses and limitations of net operating loss carryforwards generated in tax years ending after December 31, 2017, and changing the rules pertaining to the taxation of profits earned abroad. Changes in tax rates and tax laws are accounted for in the period of enactment. The Tax Act reduces the corporate tax rate to 21 percent, effective January 1, 2018.
| For the Years Ended December 31, | |||||||||
| 2019 | 2018 | ||||||||
| Deferred Tax Assets | |||||||||
| Net Operating Loss Carryforward | $ | 17,166,000 | $ | 13,596,000 | |||||
| Stock-Based Compensation | 6,538,000 | 5,741,000 | |||||||
| License Agreements | 177,000 | 206,000 | |||||||
| Research and Development | 733,000 | 406,000 | |||||||
| Charitable Contributions | 9,000 | 3,000 | |||||||
| Operating Lease Liability | 115,000 | - | |||||||
| 24,738,000 | 19,952,000 | ||||||||
| Less: | Valuation Allowance | (24,632,000 | ) | (19,952,000 | ) | ||||
| Total Deferred Tax Assets | 106,000 | - | |||||||
| Deferred Tax Liabilities | |||||||||
| Fixed Assets | (106,000 | ) | - | ||||||
| - | - | ||||||||
| Total Deferred Tax Liabilities | (106,000 | ) | - | ||||||
| Net Deferred Tax Assets/(Liabilities) | $ | - | $ | - | |||||
The Company assesses the likelihood that deferred tax assets will be realized. To the extent that realization is not likely, a valuation allowance is established. Based upon the history of losses, management believes that it is more likely than not that future benefits of deferred tax assets will not be realized and has established a full valuation allowance for the years ended December 31, 20182019 and 2017.2018. The Company decreased the prior period deferred tax asset by $4.8 million with a corresponding increase in its valuation allowance. This immaterial adjustment related to the reduction in the federal tax rates from 34% to 21% and had no impact on the prior period financial statements. Consequently, the valuation allowance increased by $8.1$4.7 million as of December 31, 2018.2019. The Company has research and development tax credit carryforwards of $406,000$730,000 available to offset future federal income taxes. The research and development tax credit carryforwards begin to expire in 2030.
The Company has approximately $57.0$73.8 million of federal and $37.3$47.4 million of state Net Operating Losses (“NOL”s) that may be available to offset future taxable income, if any. The federal net operating loss carryforwards of $41.6$41.9 million, if not utilized, will expire between 2029 and 2037. The federal net operating loss carryforwards of $15.4$31.9 million generated in 2018 and thereafter are subject to an 80% limitation on taxable income, do not expire and will carry forward indefinitely. The state net operating loss carryforwards of $21.8$21.9 million, if not utilized, will begin to expire in 2035. The state net operating loss carryforwards of $15.4$25.5 million generated in 2018 and thereafter are subject to an 80% limitation on taxable income, do not expire and will carry forward indefinitely.
In accordance with Section 382 of the Internal Revenue code, the usage of the Company’s net operating loss carryforwards may be limited in the event of a change in ownership. A full Section 382 analysis has not been prepared and NOLs could be subject to limitation under Section 382.
The Company’s income tax returns for 2015 to 2018 are still open and subject to audit. In addition, net operating losses arising from prior years are also subject to examination at the time they are utilized in future years.
For the years ended December 31, 20182019 and 2017,2018, the expected tax expense (benefit) based on the U. S. federal statutory rate is reconciled with the actual tax provision (benefit) as follows:
| For the years ended | ||||||||||||||||
| December 31, | December 31, | |||||||||||||||
| 2018 | 2017 | |||||||||||||||
| U.S. federal statutory rate | $ | (31,071,000 | ) | 21.00 | % | $ | (3,734,000 | ) | 34.00 | % | ||||||
| State taxes, net of federal benefit | (1,383,000 | ) | 0.93 | % | (416,000 | ) | 3.79 | % | ||||||||
| Federal tax rate change | - | 0.00 | % | 6,275,000 | -57.14 | % | ||||||||||
| Permanent Differences | ||||||||||||||||
| - Non-deductible write-off of acquired R&D expenses | 24,369,000 | -16.47 | % | - | 0.00 | % | ||||||||||
| - Change in fair value of derivative liabilities | 8,000 | -0.01 | % | (2,000 | ) | 0.02 | % | |||||||||
| - Other permanent differences | 4,000 | 0.00 | % | (161,000 | ) | 1.47 | % | |||||||||
| Change in valuation allowance | 8,073,000 | -5.46 | % | (1,914,000 | ) | 17.43 | % | |||||||||
| Other | - | 0.00 | % | (48,000 | ) | 0.44 | % | |||||||||
| Income tax provision/(benefit) | $ | - | 0.00 | % | $ | - | 0.00 | % | ||||||||
| For the Years Ended December 31, | ||||||||||||||||
| 2019 | 2018 | |||||||||||||||
| Amount | Percent of Pretax Income/(Loss) | Amount | Percent of Pretax Income/(Loss) | |||||||||||||
| U.S. federal statutory rate | $ | (4,500,000 | ) | 21.00 | % | $ | (31,071,000 | ) | 21.00 | % | ||||||
| State taxes, net of federal benefit | (600,000 | ) | 2.80 | % | (1,383,000 | ) | 0.93 | % | ||||||||
| Tax rate change | 665,000 | -3.10 | % | - | 0.00 | % | ||||||||||
| Permanent Differences | ||||||||||||||||
| - Non-deductible write-off of acquired R&D expenses | - | 0.00 | % | 24,369,000 | -16.47 | % | ||||||||||
| - Change in fair value of derivative liabilities | (4,000 | ) | 0.02 | % | 8,000 | -0.01 | % | |||||||||
| - Other permanent differences | 32,000 | -0.15 | % | 4,000 | 0.00 | % | ||||||||||
| Change in valuation allowance | 4,681,000 | -21.85 | % | 8,073,000 | -5.46 | % | ||||||||||
| Deferred true-up | (274,000 | ) | 1.28 | % | - | 0.00 | % | |||||||||
| Income tax provision/(benefit) | $ | - | 0.00 | % | $ | - | 0.00 | % | ||||||||
ASC 740 prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. As of December 31, 2018,2019, and 2017,2018, there were no unrecognized tax benefits. The Company recognizes accrued interest and penalties as income tax expense. No amounts were accrued for the payment of interest and penalties at December 31, 20182019 and 2017.2018. The Company is currently not aware of any issues under review that could result in significant payments, accruals or material deviation from its position in the next year.
| NOTE 19: | SUBSEQUENT EVENT |
EXHIBIT INDEXAspire Common Stock Purchase Agreement
On February 28, 2020, the Company entered into a common stock purchase agreement (the “Purchase Agreement”) with Aspire Capital Fund, LLC (“Aspire Capital”) which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $30.0 million of shares of the Company’s common stock over the 30-month term of the Purchase Agreement. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, the Company issued to Aspire Capital 345,357 shares of the Company’s common stock (the “Commitment Shares”). Under the Purchase Agreement, on any trading day selected by the Company, the Company has the right, in its sole discretion, to present Aspire Capital with a purchase notice (each, a “Purchase Notice”), directing Aspire Capital (as principal) to purchase up to 100,000 shares of the Company’s common stock per business day, up to $30.0 million of the Company’s common stock in the aggregate at a per share price (the “Purchase Price”) equal to the lesser of:
*Executive management contract or compensatory plan or arrangement.The Company and Aspire Capital also may mutually agree to increase the number of shares that may be sold to as much as an additional 2,000,000 shares per business day.
**Confidential treatment
In addition, on any date on which the Company submits a Purchase Notice to Aspire Capital in an amount equal to at least 100,000 shares, the Company also has been granted asthe right, in its sole discretion, to certain portionspresent Aspire Capital with a volume-weighted average price purchase notice (each, a “VWAP Purchase Notice”) directing Aspire Capital to purchase an amount of this exhibitstock equal to up to 30% of the aggregate shares of the Company’s common stock traded on its principal market on the next trading day (the “VWAP Purchase Date”), subject to a maximum number of shares the Company may determine. The purchase price per share pursuant to Rule 406such VWAP Purchase Notice is generally 97% of the Securities Act of 1933, as amended, or Rule 24b-2 ofvolume-weighted average price for the Securities Exchange Act of 1934, as amended.
***Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for conditional treatment and this exhibit has been submitted separately withCompany’s common stock traded on its principal market on the SEC.VWAP Purchase Date.
The Purchase Price will be adjusted for any reorganization, recapitalization, non-cash dividend, stock split, or other similar transaction occurring during the period(s) used to compute the Purchase Price. The Company may deliver multiple Purchase Notices and VWAP Purchase Notices to Aspire Capital from time to time during the term of the Purchase Agreement, so long as the most recent purchase has been completed.
The Purchase Agreement provides that the Company and Aspire Capital shall not effect any sales under the Purchase Agreement on any purchase date where the closing sale price of the Company’s common stock is less than $0.25. There are no trading volume requirements or restrictions under the Purchase Agreement, and the Company will control the timing and amount of sales of the Company’s common stock to Aspire Capital. Aspire Capital has no right to require any sales by the Company, but is obligated to make purchases from the Company as directed by the Company in accordance with the Purchase Agreement. There are no limitations on use of proceeds, financial or business covenants, restrictions on future fundings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. The Purchase Agreement may be terminated by the Company at any time, at its discretion, without any cost to the Company. Aspire Capital has agreed that neither it nor any of its agents, representatives and affiliates shall engage in any direct or indirect short-selling or hedging of the Company’s common stock during any time prior to the termination of the Purchase Agreement. Any proceeds from the Company receives under the Purchase Agreement are expected to be used for working capital and general corporate purposes.
The Purchase Agreement provides that the number of shares that may be sold pursuant to the Purchase Agreement will be limited to 9,232,814 shares, including the Commitment Shares, or the Exchange Cap, which represents 19.99% of the Company’s outstanding shares of Common Stock as of February 28, 2020, unless stockholder approval is obtained to issue more than 19.99%. This limitation will not apply if, at any time the Exchange Cap is reached and at all times thereafter, the average price paid for all shares issued under the Purchase Agreement is equal to or greater than $2.41, which was the Closing Sale Price immediately preceding the execution of the Purchase Agreement. The Company is not required or permitted to issue any shares of Common Stock under the Purchase Agreement if such issuance would breach its obligations under the rules or regulations of The Nasdaq Global Market.
Concurrently with entering into the Purchase Agreement, the Company also entered into a registration rights agreement with Aspire Capital, pursuant to which the Company filed with the SEC a prospectus supplement to the Company’s effective shelf registration statement on Form S-3 (File No. 333-232122) registering all of the shares of common stock that may be offered to Aspire Capital from time to time, including the Commitment Shares.