UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
_____________________________________________________________________________________________ | | | | | |
| ☑ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 20222023
OR | | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-36407
ALNYLAM PHARMACEUTICALS, INC.
(Exact Name of Registrant as Specified in Its Charter)
_____________________________________________________________________________________________ | | | | | | | | |
Delaware (State or Other Jurisdiction of Incorporation or Organization) | | 77-0602661 (I.R.S. Employer Identification No.) |
675 West Kendall Street, Henri A. Termeer Square Cambridge, MA 02142
(Address of Principal Executive Offices) (Zip Code)
Registrant’s telephone number, including area code: (617) 551-8200
Securities registered pursuant to Section 12(b) of the Act: | | | | | | | | | | | | | | |
| Title of Each Class | | Trading Symbol(s) | | Name of Each Exchange on Which Registered |
| Common Stock, $0.01 par value per share | | ALNY | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☑ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☑ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | ☑ | | Accelerated filer | ☐ |
| Non-accelerated filer | ☐ | | Smaller reporting company | ☐ |
| | | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes ☑ No ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-
based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
The aggregate market value of the registrant’s common stock, $0.01 par value per share (“Common Stock”), held by non-affiliates of the registrant, based on the last sale price of the Common Stock at the close of business on June 30, 2022,2023, was $17,596,044,220.approximately $23.65 billion. For the purpose of the foregoing calculation only, all directors and executive officers of the registrant are assumed to be affiliates of the registrant.
At February 17, 2023,9, 2024, the registrant had 124,130,988125,945,793 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 20232024 annual meeting of stockholders, which the registrant intends to file pursuant to Regulation 14A with the Securities and Exchange Commission not later than 120 days after the registrant’s fiscal year end of December 31, 2022,2023, are incorporated by reference into Part II, Item 5 and Part III of this Form 10-K.
ALNYLAM PHARMACEUTICALS, INC.
ANNUAL REPORT ON FORM 10-K
For the Year Ended December 31, 20222023
TABLE OF CONTENTS
“Alnylam,” ONPATTRO®, AMVUTTRA®, GIVLAARI®, OXLUMO®, Alnylam Act®, GEMINI™ and IKARIA™ are trademarks and registered trademarks of Alnylam Pharmaceuticals, Inc. Our logo, trademarks and service marks are property of Alnylam. All other trademarks or service marks appearing in this Annual Report on Form 10-K are the property of their respective holders.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the federal securities laws, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995 and are including this statement for purposes of complying with those safe harbor provisions. All statements other than statements of historical facts contained in this Annual Report on Form 10-K are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expects,” “plans,” “intends,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about:
•our views with respect to the potential for approved and investigational RNAi therapeutics, including ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO, Leqvio® (inclisiran), fitusiran and zilebesiran;
•our plans for additional global regulatory filings and the continuing product launches of ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO and our partner'scollaborator’s plans with respect to Leqvio;
•risks related to the direct or indirect impactpotential results of the novel coronavirus, or COVID-19, global pandemic, emerging or future variants of COVID-19 or any future pandemic, such as the scope and duration of the pandemic, government actions and restrictive measures implemented in response, the effectiveness of vaccination and booster vaccination campaigns, material delays in diagnoses of rare diseases, initiation or continuation of treatment for diseases addressed by our products, or in patient enrollment in clinical trials, potentialHELIOS-B Phase 3 clinical trial of vutrisiran and our future ability to obtain regulatory review and inspection or supply chain disruptions, and other potential impacts to our business;approval of AMVUTTRA (vutrisiran) for the treatment of ATTR amyloidosis with cardiomyopathy;
•our expectations regarding potential market size for, and the successful commercialization of, ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO, Leqvio or any future products;
•our ability to obtain and maintain regulatory approvals and pricing and reimbursement for ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or any future products, and our partners'collaborators’ ability with respect to Leqvio and fitusiran;
•the progress of our research and development programs, including programs in both rare and prevalent diseases;
•our future ability to successfully expand the indications for ONPATTRO and AMVUTTRA;
•the potential for improved product profiles to emerge from our new technologies, including our IKARIA and GEMINI platformsplatform and our ability to successfully advanceexpand our delivery efforts inproduct engine to include extrahepatic tissues;
•our current and anticipated clinical trials and expectations regarding the reporting of data from these trials;
•any impact of the on-going conflict in Ukraine, including disruptions to our clinical trials;
•the timing of regulatory filings and interactions with or actions or advice of regulatory authorities, which may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional pre-clinical and/or clinical testing or the timing or likelihood of regulatory approvals;
•the status of our manufacturing operations and any delays, interruptions or failures in the manufacture and supply of ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or any of our product candidates (or other products or product candidates being developed and commercialized by our partners)collaborators), by our or their contract manufacturers or by us or our partners;collaborators;
•risks related to the direct or indirect impact of the novel coronavirus, or COVID-19, global pandemic, emerging or future variants of COVID-19 or any future pandemic, or public health emergency, on, among other things, our financial performance, business and operations, including manufacturing, supply chain, research and development activities and pipeline programs, and other potential impacts to our business;
•any impact of the on-going conflicts in Ukraine and the Middle East, including disruptions to our clinical trials;
•the status of our manufacturing operations and any delays, interruptions or failures in the manufacture and supply of ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or any of our product candidates (or other products or product candidates being developed and commercialized by our collaborators), by our or their contract manufacturers or by us or our collaborators;
•our progress continuing to build and leverage global commercial infrastructure;
•the possible impact of any competing products on the commercial success of ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO and Leqvio, as well as our product candidates, and, our, or with respect to Leqvio or fitusiran, our partners'collaborators’, ability to compete against such products;
•our ability to manage our growth and operating expenses;
•our views and plans with respect to our 5-year Alnylam P5x25 strategy and our intentions to achieve the metrics associated with this strategy, including to become a top-tier biotech company by the end of 2025;
•our belief that our current cash balance should enable us to achieve a self-sustainable profile without the need for future equity financing;
•our expectations regarding the length of time our current cash, cash equivalents and marketable equity and debt securities will support our operations based on our current operating plan;
•our dependence on third parties for development, manufacture and distribution of products;
•our expectations regarding our corporate collaborations, including potential future licensing fees and milestone and royalty payments under existing or future agreements;
•our ability to obtain, maintain and protect our intellectual property;
•our ability to attract and retain qualified key management and scientists, development, medical and commercial staff, consultants and advisors and to successfully execute on our Alnylam P5x25 strategy;
•the outcome of litigation, including our patent infringement suits against Pfizer, Inc., BioNTech SE and Moderna, Inc., or of other legal proceedings or of any current or future government investigation, including the investigation related to the subpoena received on or about April 9, 2021 pertaining to our marketing and promotion of ONPATTRO in the United States, or U.S.;investigations;
•regulatory developments in the United States, or U.S., and foreign countries;
•the impact of laws and regulations;
•developments relating to our competitors and our industry;
•our ability to satisfy our payment obligations, and to service the interest on or to refinance our indebtedness, including our convertible notes, or to make cash payments in connection with any conversion of the Notes,our convertible notes, to the extent required;
•our expectations regarding the effect of the capped call transactions and regarding the anticipated market activities of the option counterparties and/or their respective affiliates; and
•other risks and uncertainties, including those listed under the caption Part I, Item 1A, "Risk Factors"“Risk Factors” of this Annual Report on Form 10-K.
The risks set forth above are not exhaustive. Other sections of this Annual Report on Form 10-K may include additional factors that could adversely affect our business and financial performance. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time and it is not possible for management to predict all risk factors, nor can we assess the impact of all risk factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. Any forward-looking statements in this Annual Report on Form 10-K reflect our current views with respect to future events and with respect to our business and future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among other things, those described under Part I, Item 1A, "Risk Factors"“Risk Factors” and elsewhere in this Annual Report on Form 10-K. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future. You are advised, however, to consult any further disclosure we make in our reports filed with the Securities and Exchange Commission, or SEC.
This Annual Report on Form 10-K may include data that we obtained from industry publications and third-party research, surveys and studies. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. This Annual Report on Form 10-K also may include data based on our own internal estimates and research, including estimates regarding the impact of the COVID-19 pandemic (or related pandemic caused by coronavirus variants) on our financial statements and business operations. Our internal estimateswhich have not been verified by any independent source and, while we believe any data obtained from industry publications and third-party research, surveys and studies are reliable, we have not independently verified such data. SuchAny such third-party data, as well as our internal estimates and research, are subject to a high degree of uncertainty and risk due to a variety of factors, including those described in Part I, Item 1A, "Risk Factors"“Risk Factors” and elsewhere in this Annual Report on Form 10-K. These and other factors could cause our results to differ materially from those expressed in this Annual Report on Form 10-K.
PART I
ITEM 1. BUSINESS
Overview
Alnylam Pharmaceuticals, Inc. (also referred to as Alnylam, we, our or us) is a global commercial-stage biopharmaceutical company developing novel therapeutics based on ribonucleic acid interference, or RNAi. RNAi is a naturally occurring biological pathway within cells for sequence-specific silencing and regulation of gene expression. By harnessing the RNAi pathway, we have developed a new class of innovative medicines, known as RNAi therapeutics. RNAi therapeutics are comprised of small interfering RNA, or siRNA, andthat function upstream of conventional medicines by potently silencing messenger RNA, or mRNA, that encode for proteins implicated in the cause or pathway of disease, thus preventing them from being made. We believe this is a revolutionary approach with the potential to transform the care of patients with rare and prevalent diseases. To date, our efforts to advance this revolutionary approach have yielded the approval of five first-in-class RNAi-based medicines, ONPATTRO® (patisiran), AMVUTTRA® (vutrisiran), GIVLAARI® (givosiran), OXLUMO® (lumasiran) and Leqvio® (inclisiran).
Our research and development strategy is to target genetically validated genes that have been implicated in the cause or pathway of human disease. We utilize a N-acetylgalactosamine (GalNAc) conjugate approach or lipid nanoparticle (LNP) to enable hepatic delivery of siRNAs. For delivery to the central nervous system, or CNS, and the eye (ocular delivery), we are utilizing an alternative conjugate approach based on a hexadecyl (C16) moiety as a lipophilic ligand. During 2022,2023, we also advancedcontinued to advance approaches for heart, skeletal muscle, and adipose tissue delivery of siRNAs. Our focus is on clinical indications where there is a high unmet need, a genetically validated target, early biomarkers for the assessment of clinical activity in Phase 1 clinical studies, and a definable path for drug development, regulatory approval, patient access and commercialization.
In early 2021, we launched our Alnylam P5x25 strategy, which focuses on our planned transition to a top-tier biotech company as measured by market capitalization, by the end of 2025. With Alnylam P5x25, we aim to deliver transformative rare, specialty and select prevalent disease medicines for patients around the world through sustainable innovation, while delivering exceptional financial performance. Specifically, we intend to end 2025 with the following profile:
Patients: Over 0.5 million on our RNAi therapeutics globally
Products: Six or more marketed products in rare and prevalent diseases
Pipeline: Over 20 clinical programs, with 10 or more in late stages and four or more INDs per year
Performance: ≥40% revenue CAGR (compound annual growth rate) through YE 2025
Profitability: Achieve sustainable non-GAAP (generally accepted accounting principles) profitability within the period
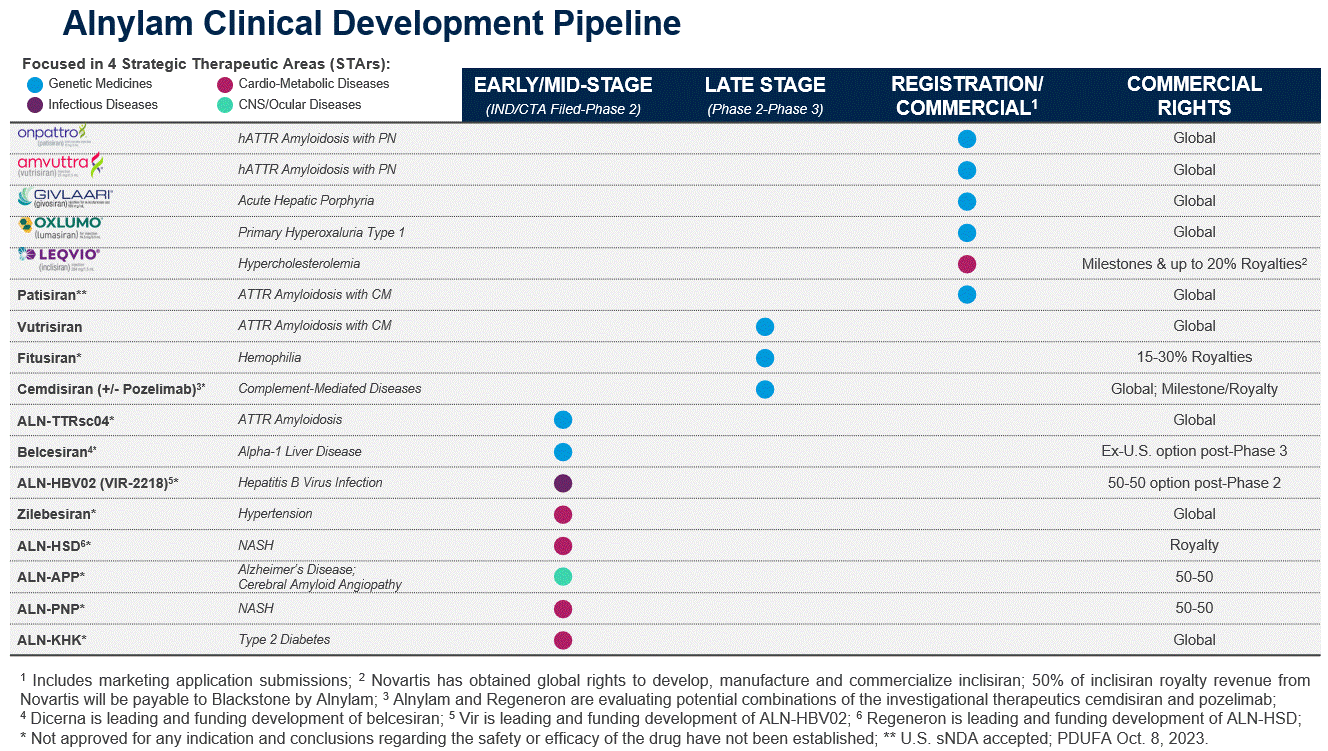
We ended 20222023 making meaningfulconsiderable progress on these goals, and currently have five marketed products and more than ten clinical programs, including several in late-stage development, across four Strategic Therapeutic Areas, or “STArs:” Genetic Medicines; Cardio-Metabolic Diseases; Hepatic Infectious Diseases;rare, specialty and CNS/Ocular Diseases. Four of our marketed products are within the Genetic Medicines STAr, ONPATTRO, GIVLAARI, OXLUMO and AMVUTTRA. select prevalent indications.
ONPATTRO is approved by the United States Food and Drug Administration, or the FDA, for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis, or hATTR amyloidosis, with polyneuropathy in adults and has also been approved in the European Union, or EU, for the treatment of hATTR amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy, in Japan for the treatment of transthyretin, or TTR, type familial amyloidosis with polyneuropathy, and in multiple additional countries, including Brazil. In August 2022, we reported positive results from the APOLLO-B Phase 3 study of patisiran (the non-branded name of ONPATTRO) in patients with ATTR amyloidosis with cardiomyopathy, and in December 2022, we submitted a supplemental New Drug Application, or sNDA, to the FDA for ONPATTROpatisiran as a potential treatment for the ATTR amyloidosis with cardiomyopathy. On September 13, 2023, the FDA’s Cardiovascular and Renal Drugs Advisory Committee, or CRDAC, voted 9:3 that patisiran’s benefits outweigh its risks for the treatment of the cardiomyopathy oftransthyretin amyloidosis, or ATTR amyloidosis. In Februaryamyloidosis, with cardiomyopathy. Nevertheless, on October 6, 2023, the FDA acceptedissued a complete response letter, or CRL, indicating that evidence of clinical meaningfulness of patisiran was not established for ATTR amyloidosis with cardiomyopathy, and therefore the sNDA for filing and set an action datepatisiran could not be approved in its present form. The CRL did not identify any issues with respect to clinical safety, study conduct, drug quality or manufacturing. Patisiran remains under regulatory review with the Brazilian Health Regulatory Agency (ANVISA) for ONPATTRO for the treatment of October 8, 2023 under the Prescription Drug User Fee Act, or PDUFA. The FDA also indicatedATTR amyloidosis with cardiomyopathy.
AMVUTTRA is approved in the sNDA filing communication letter that it is planning to hold an advisory committee meeting to discussU.S. for the applicationtreatment of hATTR amyloidosis with polyneuropathy in adults, in the EU and that it had not identified any potentialthe United Kingdom, or UK, for the treatment of hATTR amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy, in Japan for the treatment of TTR type familial amyloidosis with polyneuropathy, and in additional countries, including Brazil, Argentina, Switzerland and Canada. Regulatory filings continue in other territories with submissions currently under review issues at such time. or planned for 2024 and beyond.
GIVLAARI is approved in the U.S. for the treatment of adults with acute hepatic porphyria, or AHP, in the EU for the treatment of AHP in adults and adolescents aged 12 years and older, and in several additional countries, including Brazil,
Canada, Australia, Switzerland and Japan. Regulatory filings for givosiran (the non-branded drug name for GIVLAARI) in other territories are pending or planned during 20232024 and beyond.
OXLUMO is approved in the U.S. and EU for the treatment of primary hyperoxaluria type 1, or PH1, in all age groups, and in several additional countries including Brazil and Switzerland. In October 2022, we announced that the FDA approved our sNDA for lumasiran (the non-branded drug name for OXLUMO), for the treatment of PH1 to lower urinary oxalate and plasma oxalate levels in pediatric and adult patients. Regulatorypatients, and in the EU and the UK for the treatment of PH1 in all age groups. OXLUMO has also been approved in Brazil, Switzerland, Canada, Israel and Qatar, and additional regulatory filings in other territories are pending and additional filings areor planned during 20232024 and beyond. In June 2022, we received regulatory approval for AMVUTTRA in the U.S. for the treatment of the polyneuropathy of hATTR amyloidosis in adults. In September 2022, AMVUTTRA was granted marketing authorization in Europe and the United Kingdom, or UK, for the treatment of hATTR amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy, and in Japan for the treatment of TTR type familial amyloidosis with polyneuropathy. In December 2022, AMVUTTRA was approved in Brazil for the treatment of
hATTR amyloidosis in adults. Regulatory filings in other territories are currently under review and additional filings are planned for 2023.
OurLeqvio (inclisiran), our fifth product, Leqvio (inclisiran), is being developed and commercialized by our partnercollaborator Novartis AG, or Novartis, and has received marketing authorization from the European Commission, or EC, for the treatment of adults with hypercholesterolemia or mixed dyslipidemia and from the FDA as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with heterozygous familial hypercholesterolemia, or HeFH, or clinical atherosclerotic cardiovascular disease, or ASCVD, who require additional lowering of low-density lipoprotein cholesterol, or LDL-C. AsIn July 2023, the FDA approved an expanded indication for Leqvio to include treatment of adults with high LDL-C and who are at increased risk of heart disease. In the third quarter of 2023, Leqvio was approved in China and Japan, and as of the end of January 2023,2024, Leqvio hashad been approved in 70more than 90 countries.
In addition to our marketed products, we have multiple late-stage investigational programs advancing toward potential commercialization. These programs include our wholly owned programs: patisiran (the non-branded drug name for ONPATTRO) for the treatment of transthyretin amyloidosis, or ATTR amyloidosis, (wild-type or hereditary) with cardiomyopathy; vutrisiran (the non-branded drug name for AMVUTTRA) for the treatment of ATTR amyloidosis (wild-type or hereditary) amyloidosis with cardiomyopathy; as well as fitusiran for the treatment of hemophilia, which is being advanced by our partnercollaborator Genzyme Corporation, a Sanofi Company, or Sanofi; and cemdisiran for the treatment of complement-mediated diseases, where our partnercollaborator Regeneron Pharmaceuticals, Inc., or Regeneron, is advancing cemdisiran in combination with pozelimab in Phase 3 studies in myasthenia gravis and paroxysmal nocturnal hemoglobinuria.
As part of our Alnylam P5x25 strategy, we have multiple drivers of future growth, including the development of transformative medicines to treat prevalent disease medicines.disease. In addition to Leqvio, we are advancing zilebesiran, an investigational, subcutaneously administered RNAi therapeutic targeting angiotensinogen, or AGT, in development for the treatment of hypertension. In November 2021, we reported positive interim data from the ongoing Phase 1 studyclinical trial of zilebesiran, and initiated the KARDIA Phase 2 clinical studiestrials for zilebesiran. KARDIA-1 is designed to evaluate zilebesiran as a monotherapy across different doses administered quarterly and biannually. KARDIA-2 willis designed to evaluate the safety and efficacy of zilebesiran administered biannually as a concomitant therapy in patients whose blood pressure is not adequately controlled by a standard of care antihypertensive medication. ToplineIn September 2023, we reported positive topline results from KARDIA-1. We anticipate reporting topline results from KARDIA-2 in early 2024. In July 2023, we entered into a Collaboration and License Agreement, or the Roche Collaboration and License Agreement, with F. Hoffmann-La Roche Ltd. and Genentech, Inc. or, collectively, Roche, pursuant to which we established a worldwide, strategic collaboration for the joint development and commercialization of zilebesiran. A description of our collaboration with Roche is described in more detail below under the heading “Our Collaboration and Licensing Strategy.”
We are also advancing ALN-APP, an investigational RNAi therapeutic targeting amyloid precursor protein in development for the treatment of Alzheimer’s disease and cerebral amyloid angiopathy. In 2023, we reported positive interim results from the KARDIA-1ongoing single ascending dose part of the Phase 1 study of ALN-APP in patients with early-onset Alzheimer’s disease. These results establish the first human translation of our proprietary C16-siRNA conjugate platform for CNS delivery and KARDIA-2 Phase 2 studies are anticipatedthe first clinical demonstration of gene silencing in mid-2023 and at or around year-end 2023, respectively.the human brain using an RNAi therapeutic.
In further support of our Alnylam P5x25 strategy and in view of our evolving risk profile, we remain focused on the continued evolution of our global infrastructure, including key objectives such as optimizing our global structure for execution in key markets, enhancing performance consistent with our values, and continuing to strengthen our culture. We maintain focus on our global compliance program to drive its evolution and enhancement in view of the Alnylam P5x25 strategy. Building from our global Code of Business Conduct and Ethics, our compliance program is designed to empower our employees and those with whom we work to execute on our strategy consistent with our values and in compliance with applicable laws and regulations, and to mitigate risk. Comprised of components such as risk assessment and monitoring; policies, procedures, and guidance; training and communications; dedicated resources; and systems and processes supporting activities such as third party relationships and investigations and remediation; our program and related controls are built to enhance our business processes, structures, and controls across our global operations, and to empower ethical decision making.
Based on our expertise in RNAi therapeutics and broad intellectual property estate, we have formed alliancescollaborations with leading pharmaceutical and life sciencessciences companies to support our development and commercialization efforts, including Regeneron, Roche, Novartis (which acquired our partnercollaborator The Medicines Company, or MDCO, in 2020), Sanofi, Vir Biotechnology, Inc., or Vir, Dicerna Pharmaceuticals, Inc. (acquired by Novo Nordisk A/S, or Novo Nordisk, in December 2021), or Dicerna, and PeptiDream, Inc., or PeptiDream.PeptiDream.
Convertible Senior Notes
In September 2022, we issued $1.04 billion aggregate principal amount of 1.00% Convertible Senior Notes due 2027, or Notes. The Notes will mature on September 15, 2027, unless earlier converted, redeemed or repurchased. The Notes will bear interest from September 15, 2022 at a rate of 1.00% per year payable semiannually in arrears on March 15 and September 15 of each year, beginning on March 15, 2023. Before June 15, 2027, noteholders will have the right to convert their Notes in certain circumstances and during specified periods. From and after June 15, 2027, the Notes will be convertible at the option of the noteholders at any time prior to the close of business on the second scheduled trading day immediately preceding the maturity date. We will settle any conversions of Notes by paying or delivering, as applicable, cash, shares of our common stock, par value $0.01 per share, or Common Stock, or a combination of cash and shares of Common Stock,our common stock, at our election.
In connection with the issuance of the Notes, we paid $118.6 million, including expenses, to enter into privately negotiated capped call transactions with certain initial purchasers of the Notes or their respective affiliates and certain other financial institutions, or capped call transactions. The capped call transactions are expected generally to reduce the potential dilution upon conversion of the Notes in the event that the market price per share of our common stock, as measured under the terms of the capped call transactions, is greater than the strike price of the capped call transactions, which initially corresponds to the conversion price of the Notes, and is subject to anti-dilution adjustments generally similar to those applicable to the conversion rate of the Notes. The cap price of the capped call transactions will initially be $424.00 per share, which represents a premium of approximately 100% based on the last reported sale price of our common stock of $212.00 per share on September 12, 2022,
and is subject to certain adjustments under the terms of the capped call transactions. If, however, the market price per share of our common stock, as measured under the terms of the capped call transactions, exceeds the cap price of the capped call transactions, there would nevertheless be dilution upon conversion of the Notes to the extent that such market price exceeds the cap price of the capped call transactions.
We used approximately $762.0 million of the net proceeds from the offering of the Notes to repay borrowings, inclusive of prepayment premiums, under our credit agreement with Blackstone, and intend to usewith the remainder of theremaining net proceeds designated for general corporate purposes.
The COVID-19 Pandemic
The COVID-19 pandemic has resulted in the imposition of various responses, including government-imposed quarantines, travel restrictions, and other public health safety measures to reduce the spread of disease. We continue to closely monitor the spread of COVID-19 and its variants, and plan to continue taking steps to identify and mitigate the adverse impacts on, and risks to, our business posed by its spread and actions taken by governmental and health authorities to address the ongoing COVID-19 pandemic. The extent to which COVID-19 ultimately impacts our business, results of operations or financial condition will depend on future developments, which remain uncertain and cannot be predicted with confidence, such as the duration of the COVID-19 pandemic, new strains of the virus, including any future variants that may emerge, which may impact rates of infection and vaccination efforts, developments or perceptions regarding the safety of vaccines, and any additional preventative and protective actions taken to contain the pandemic or treat its impact, among others. The estimates of the impact on the Company’s business may change based on new information that may emerge concerning COVID-19 and the actions to contain it or treat its impact and the economic impact on local, regional, national and international markets. For additional information related to the actual or potential impacts of COVID-19 on our business, please read Part I, Item 1A, "Risk Factors" of this Annual Report on Form 10-K.
Key 20222023 and Recent Highlights
TTR Franchise
•ONPATTRO (patisiran) and AMVUTTRA (vutrisiran)– hATTR Amyloidosis with Polyneuropathy
◦Achieved global net product revenues for ONPATTRO and AMVUTTRA for the full year 20222023 of $558$354.5 million and $94$557.8 million, respectively
◦Attained over 2,9754,060 hATTR amyloidosis patients with polyneuropathy worldwide on commercial treatment with ONPATTRO or AMVUTTRA as of December 31, 20222023
•Vutrisiran – hATTR Amyloidosis with Polyneuropathy
◦Received 760 Start FormsPresented nine-month results from the randomized treatment extension period of the HELIOS-A study of vutrisiran in the U.S. for AMVUTTRA from launch through December 31, 2022patients with hATTR amyloidosis with polyneuropathy
•Patisiran – ATTR Amyloidosis with Cardiomyopathy
◦Reported positivePresented 18-month results from the APOLLO-B Phase 3 study of patisiran in patients with ATTR amyloidosis with cardiomyopathy
◦Submitted and received acceptancePresented new 24-month results from an interim analysis of the open-label extension period of the APOLLO-B Phase 3 study of patisiran in patients with ATTR amyloidosis with cardiomyopathy
◦Received CRL from the FDA for the sNDA for ONPATTRO (patisiran)patisiran for the treatment of the cardiomyopathy of ATTR amyloidosis
▪The FDA has set an action date of October 8, 2023 under the PDUFA
▪In their file acceptance letter, the FDA stated that they have not identified any review issues, and also noted that they are planning to hold an advisory committee meeting to discuss the application
•Vutrisiran – hATTR Amyloidosis with Polyneuropathy
◦Received FDA approval of AMVUTTRA for the treatment of the polyneuropathy of hATTR amyloidosis in adults
◦Received marketing authorization for AMVUTTRA for the treatment of hATTR amyloidosis in adults with stage 1 or stage 2 polyneuropathy in Europe and the UK, as well as approval for TTR type familial amyloidosis with polyneuropathy in Japan
◦Received approval from the Brazilian Health Regulatory Agency (ANVISA) for AMVUTTRA for the treatment of hATTR amyloidosis in adults
•Vutrisiran – ATTR Amyloidosis with Cardiomyopathy
◦Announced that we do notupdates to the statistical analysis plan to conduct the optional interim analysis for the HELIOS-B Phase 3 study of vutrisiran in patients with ATTR amyloidosis with cardiomyopathy;cardiomyopathy, including updates to the primary and secondary endpoint structure, as well as study remains on track forexposure; Topline results expected to be available in late June or early July 2024
•ALN-TTRsc04 – ATTR Amyloidosis
◦Presented positive initial topline results from the Phase 1 study in early 2024healthy volunteers
Commercial/Late-Stage Pipeline
•GIVLAARI (givosiran) – Acute Hepatic Porphyria
◦Recognized GIVLAARI global net revenue of $173.1$219.3 million for the year ended December 31, 20222023
◦Attained over 520650 patients worldwide on commercial GIVLAARI treatment as of December 31, 20222023
•OXLUMO (lumasiran) – Primary Hyperoxaluria Type 1
◦Recognized OXLUMO global net revenue of $69.8$109.8 million for the year ended December 31, 20222023
◦Attained over 280430 patients worldwide on commercial OXLUMO treatment as of December 31, 2022, up from over 230 commercial patients as of September 30, 20222023
•Leqvio (inclisiran) – Hypercholesterolemia (in collaboration with Novartis)
◦Our partner,collaborator, Novartis, continued the launch of Leqvio, with focus on patient on-boarding, removing access hurdles and enhancing medical education
•LumasiranFitusiran – Primary Hyperoxaluria Type 1Hemophilia (in collaboration with Sanofi)
◦Based on the successful outcome of the ILLUMINATE-C study in children and adults with advanced PH1, received approvalOur collaborator, Sanofi, reported positive data from the FDAPhase 3 ATLAS-OLE study of an sNDA for OXLUMO, expanding the indicationfitusiran, in development for the treatment of PH1 to lower urinary oxalatehemophilia A or B, with or without inhibitors, and plasma oxalate levelsan NDA submission with the FDA is expected in pediatric and adult patients, and received approval from the EMA of a Type II variation to include the ILLUMINATE-C data in the label2024
Early-Stage and Pre-Clinical Pipeline
•Zilebesiran (ALN-AGT) for the treatment of hypertension; completed– Hypertension
◦Completed enrollment in the KARDIA-1KARDIA-2 Phase 2 monotherapy study, evaluating the safety and efficacy of zilebesiran in patients with mild-to-moderateuncontrolled hypertension when added on top of another antihypertensive medication
◦Reported positive topline results from the KARDIA-1 Phase 2 dose-ranging study of zilebesiran
•ALN-APP for– Cerebral Amyloid Angiopathy and Autosomal Dominant Alzheimer’s Disease
◦Reported positive interim results from the treatmentongoing single ascending dose part of cerebral amyloid angiopathy and autosomal dominant Alzheimer’s Disease; enrollment and dose escalation continue in the Phase 1 study of ALN-APP in patients with early onsetearly-onset Alzheimer’s Disease, in collaboration with Regeneron, with initial topline results expected in early 2023disease
•◦ALN-TTRsc04forAnnounced that the treatmentFDA has provided clearance to initiate the multiple-dose part (Part B) of ATTR amyloidosis; submitted a CTA application and initiated dosing in athe ongoing Phase 1 study of ALN-APP
Corporate Highlights
•Finance
◦Ended 20222023 with $2.19$2.44 billion in cash, cash equivalents and marketable securities
•Business
◦Issued $1.04 billion convertible senior notesEntered into global strategic collaboration with proceeds primarily used to pay down Blackstone $700 million credit facilityRoche for the co-development and approximately $200 million in prepayment premiums under the credit facility, the purchaseco-commercialization of capped call transactions, and underwriter feeszilebesiran
RNAi Therapeutics – A New Class of Innovative Medicines
Overview of RNAi Therapeutics
In recent years, a tremendous amount of progress has been made in effectively delivering RNAi therapeutics to targeted organs and cells, and we believe Alnylam has been the leader of this advancement. We believe this success will enable us to achieve our Alnylam P5x25 strategy under which we expect to sustainably and organically create and commercialize transformative rare, specialty and select prevalent disease medicines benefiting patients around the world while delivering strong financial performance, resulting in a leading biotech profile by the end of 2025.
Early efforts focused on delivery of RNAi therapeutics utilizing LNPs, where siRNA molecules are encapsulated in specific lipid-based formulations. This technology enables systemic delivery with intravenous drug administration and is associated with potent, rapid and durable target gene silencing and an encouraging tolerability profile in clinical studies conducted to date, as well as in our commercial experience. Our first commercial product, ONPATTRO, is formulated utilizing LNPs.
In parallel, we have advanced proprietary technology that conjugates a sugar molecule called GalNAc to the siRNA molecule. This simpler delivery approach enables more convenient, subcutaneous administration of our drug candidates directed to liver expressed target genes, a key aspect of our platform. Results from our Enhanced Stabilization Chemistry, or ESC, GalNAc-conjugate delivery platform have demonstrated a durability of effect that we believe, based on our clinical results, supports once-monthly, once-quarterly, and potentially, bi-annual subcutaneous dose regimens. Due to this increased potency and durability, as well as a wide therapeutic index, this conjugate platform has become our primary approach for drug development and is leveraged and, we believe, strongly validated by, AMVUTTRA, GIVLAARI, OXLUMO, and Leqvio, our more recently approved medicines. Our next generation Enhanced Stabilization Chemistry-Plus, or ESC+, GalNAc-conjugates utilize advanced design features to further improve specificity, while maintaining potency and durability, further improving our already wide therapeutic index by up to six-fold. Our first wave of investigational RNAi therapeutics based on this ESC+ design, zilebesiran (formerly ALN-AGT), ALN-HBV02 and ALN-HSDelebsiran (formerly ALN-HBV02) are in the clinic, with what we believe are encouraging initial results.
Additional platform advancements that we are working on include our IKARIA platform harboring chemistry innovations that enable robust target knockdown with an annual dosing regimen. We believe that this platform, could have potential applications in cardiometabolic, CNS, oncologic, and viral diseases, and in December 2023, we announced positive initial results from the Phase 1 study of ALN-TTRsc04, our investigational RNAi therapeutic in development for the treatment of ATTR amyloidosis, which utilizes our IKARIA technology.
Our platform enhancements have also provided a strong foundation for pursuing a conjugate-based approach to extrahepatic delivery, including delivery to the brain and spinal cord, as well as ocular delivery. In July 2022, our publication in Nature Biotechnology showcased data utilizing an alternative conjugate approach based on a hexadecyl (C16) moiety as a lipophilic ligand, with proof-of-concept, or POC, demonstrated in rodent and non-human primates. C16 conjugates provide
robust CNS knockdown with wide biodistribution and long duration of action, and this technology enabled our landmark collaboration with Regeneron for the advancement of RNAi therapeutics for a broad range of diseases by addressing therapeutic targets in the eye and CNS, in addition to a select number of targets in the liver. We are continuing to advance other extrahepatic delivery approaches, including delivery to muscle and adipose cells. In addition, we are exploring peptide and antibody-based approaches for targeted siRNA delivery to new tissues in partnershipcollaboration with PeptiDream to discover and develop peptide-siRNA conjugates for targeted delivery of RNAi therapeutics to a broader range of extrahepatic tissues.
Additional platform advancements that we are working on include our IKARIA™ platform harboring chemistry innovations that enable robust target knockdown with an annual dosing regimen, and an additional technology platform, GEMINI™, that we believe has the potential to simultaneously silence two unique gene transcripts using a single chemical entity. We believe that these platforms, could have potential applications in cardiometabolic, CNS, oncologic, and viral diseases.
Finally, we continue to leverage human genetics to advance our efforts to bring innovative medicines to patients. We have established a relationship with the UK BioBank to support the sourcing of novel, genetically validated targets and secure access to databases of genetic information. In 2022, we published a manuscript in Nature Communications, revealing that loss of function variants in the hepatokine gene INHBE protect against abdominal obesity and can thus serve as a potential therapeutic target for cardiometabolic disease. The discovery leveraged sequencing data from more than 360,000 individuals in the UK Biobank. In addition, our partnership with Our Future Health furthers our investment in human genetics supporting the design and delivery of the research program to recruit up to five million adults from across the UK. Coupled with our proven ability to uncover novel gene targets, we believe our approach, investments and commitment to genetically validated targets has the potential to increase our success rate, streamline clinical trials and speed the development of precision medicines for patients with both rare, specialty and select prevalent diseases.
We believe RNAi therapeutics represent a simplified and efficient new class of innovative medicines. We have achieved human POC in multiple clinical trials of our investigational candidates and now have five commercially approved products, validating our approach to drug development. Moreover, we believe that our reproducible and modular platform will support our Alnylam P5x25 strategy under which we expect to sustainably and organically create and commercialize transformative rare, specialty and select prevalent disease medicines benefiting patients around the world while delivering strong financial performance, resulting in a leading biotech profile by the end of 2025.
Our Product Pipeline
Our broad pipeline, including five approved products and multiple late and early-stage investigational RNAi therapeutics, is focusedaddresses unmet needs in four STArs: Genetic Medicines; Cardio-Metabolic Diseases; Hepatic Infectious Diseases;several disease areas, and CNS/Ocular Diseases.spans indications in rare, specialty and select prevalent diseases. We describe our commercial and clinical-stage pipeline in more detail below. The investigational therapeutics described below are in various stages of clinical development and the scientific information included about these therapeutics is preliminary and investigative. None of these investigational therapeutics have been approved by the FDA, EMA, or any other health authority and no conclusions can or should be drawn regarding the safety or efficacy of these investigational therapeutics.
The charttable below is a summary ofrepresents our commercial products and late- and early-stage development programs as of January 31, 2023. It identifies those programs for which we have received marketing approval, the stage of our programs and our commercial rights to such programs:February 1, 2024.
As indicated in the charttable above, to date we have received marketing approval for ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, and Novartis has received approval for Leqvio, in each case in certain territories for the specific indications approved in each such territory, with additional regulatory submissions pending.
Our TTR Franchise
About Transthyretin Amyloidosis (ATTR)
ATTR amyloidosis is a rare, serious, life-threatening, multisystem disease encompassing hATTR amyloidosis and wild-type ATTR, or wtATTR, amyloidosis, which resultresults from either hereditary (genetic variant in TTR gene) or nonhereditary (ageing) causes, respectively. In ATTR amyloidosis, misfolded TTR proteins accumulate as amyloid fibrils in multiple organs and tissue types. hATTR amyloidosis can include sensory and motor neuropathy, autonomic neuropathy and cardiac symptoms and is a major unmet medical need with significant morbidity and mortality, affecting approximately 50,000 people worldwide. The median survival is 4.7 years following diagnosis, with a reduced survival (3.4 years) for patients presenting with cardiomyopathy. wtATTR amyloidosis predominantly manifests as cardiomyopathy and heart failure symptoms, although patients may experience other manifestations due to extra-cardiac amyloid deposition. The disease is estimated to impact 200,000 to 300,000 people worldwide.
ONPATTRO (patisiran)
ONPATTRO (patisiran) is an intravenously administered RNAi therapeutic targeting TTR. It is designed to target and silence TTR mRNA, thereby reducing the production of TTR protein before it is made. ONPATTRO blocks the production of TTR in the liver, reducing its accumulation in the body’s tissues to halt or improve the progression of disease.
Patisiran has received Orphan Drug Designations in the U.S., EU and Japan; specific Orphan Drug Designations vary by country/region.
ONPATTRO (patisiran) – hATTR Amyloidosis with Polyneuropathy
ONPATTRO is the first ever FDA-approved RNAi therapeutic and based on data from the APOLLO Phase 3 study, it became our first product to receive marketing approval. In the U.S. and Canada, ONPATTRO is indicated for the treatment of the polyneuropathy of hATTR amyloidosis with polyneuropathy in adults. In the EU, Switzerland, Brazil and Israel, ONPATTRO is indicated for the treatment of hATTR amyloidosis in adults with stage 1 or stage 2 polyneuropathy, and in Japan, ONPATTRO is indicated for the treatment of TTR type familial amyloidosis with polyneuropathy.
Patisiran (the non-branded name for ONPATTRO) was also evaluated in a Phase 4 study in hATTR amyloidosis patients with polyneuropathy of hATTR amyloidosis due to a T60A or V122I variant. The data were presented in September 2022 at the 18th International Symposium on Amyloidosis.
Patisiran – ATTR Amyloidosis with Cardiomyopathy
In December 2022, we submitted an sNDA to the FDA for ONPATTRO for the treatment cardiomyopathy of ATTR amyloidosis based on the positive results from the APOLLO-B Phase 3 study. In FebruaryOn October 6, 2023, the sNDA was accepted for filing and the FDA set an action date of October 8, 2023 under the PDUFA. The FDA also indicated inissued a CRL indicating that the sNDA filing communication letter that it is planning to hold an advisory committee meeting to discuss the application and that it hadfor patisiran could not identified any potential review issues at such time.be approved in its present form. An sNDA submission is also plannedfor ONPATTRO for the treatment of ATTR amyloidosis with cardiomyopathy was submitted in Brazil in 2023, however,and we do not plan to pursue label expansion in other regions.
APOLLO-B Phase 3 Study
In September 2019,2022, we initiatedannounced that the APOLLO-B Phase 3 study, a randomized, double-blind, placebo-controlled Phase 3 studyclinical trial designed to evaluate the efficacy and safety of patisiran in patients with ATTR amyloidosis with cardiomyopathy. The study enrolled 360 adult patients with ATTR amyloidosis (hereditary or wild-type) with cardiomyopathy, at 69 sites in 21 countries. Patients were randomized 1:1 to receive 0.3 mg/kg of patisiran or placebo intravenously administered every three weeks over a 12-month treatment period. Twenty-five percent of patients enrolled were receiving on-label commercially available tafamidis at baseline. After 12 months of treatment, the primary endpoint of change from baseline in six-minute walk test, or 6-MWT, was evaluated, as well as other secondary and exploratory endpoints.
◦Efficacy and Safety Results:
In August 2022, we announced positive topline results from the APOLLO-B study and in September 2022 we reported more fulsome results at the 18th International Symposium on Amyloidosis, announcing that APOLLO-B met the primary endpoint of change from baseline in the six-minute walk test, or 6-MWT, at 12 months compared to placebo with a median difference of 14.7 meters (p-value 0.0162) favoring patisiran. The study also met its first secondary endpoint, demonstrating a statistically significant and clinically meaningful benefit on health status and quality of life, as measured by the Kansas City Cardiomyopathy Questionnaire Overall Summary, or KCCQ-OS, score, compared to placebo with least squares mean difference of 3.7 points (p-value 0.0397) favoring patisiran. The study also included additional secondary composite outcomes endpoints. A non-significant result (p-value 0.0574) was found on the composite endpoint of all-cause mortality, frequency of cardiovascular events, and change from baseline in 6-MWT over 12 months compared to placebo. As a result, formal statistical testing was not performed on the final two composite endpoints. During the 12 month placebo-controlled primary analysis period, patisiran also demonstrated an encouraging safety and tolerability profile in patients with ATTR amyloidosis with cardiomyopathy, including no cardiac safety concerns relative to placebo. The majority of adverse events, or AEs, were mild or moderate in severity. Treatment emergent AEs occurring in 5%
In November 2023, we reported new results from an interim analysis of exploratory data from the open-label extension, or more patientsOLE, period of the APOLLO-B Phase 3 study. The 24-month findings indicate that the favorable effects on functional capacity and health status and quality of life, as measured by the 6-MWT and the KCCQ-OS, respectively, observed during the double-blind period were sustained with continued patisiran treatment during the OLE period. Patients treated with patisiran through 24 months also appear to have maintained relative stability of NT-proBNP and Troponin I levels, measures of cardiac stress and injury, respectively. Patients who crossed over from placebo in the double-blind period to patisiran groupduring the OLE period appear to show slowing of disease progression or relative stabilization across these same endpoints at Month 24. While the APOLLO-B study was not designed to show benefits in cardiac outcomes between patisiran and placebo, evidence of favorable, but non-statistically significant, trends were observed at least 3% more commonly than infor composite all-cause death and hospitalization, and mortality analyses across the placebo group included infusion-related reactions (12.2% vs 9.0%), arthralgia (7.7% vs 4.5%),double-blind and muscle spasms (6.6% vs 2.2%). In the safety analysis there were 5 deaths (2.8%) observed in patisiran-treated patients and 8 deaths (4.5%) observed in the placebo group. The safety profile in APOLLO-B was consistent with what was observed in our Phase 3 APOLLO study and in postmarketing useOLE periods.
Table of ONPATTRO. ContentsBased on these positive data, in December 2022, we submitted an sNDA to the FDA for ONPATTRO for the treatment of the cardiomyopathy of ATTR amyloidosis. AMVUTTRA (vutrisiran)
AMVUTTRA (vutrisiran) is a subcutaneously administered RNAi therapeutic targeting TTR. It is designed to targetWith its rapid knockdown, it targets and silencesilences TTR mRNA, thereby reducing the production of TTR protein before it is made. AMVUTTRA utilizes our ESC+ delivery platform, designed for increased potency and high metabolic stability to allow for quarterly subcutaneous administration.
Vutrisiran has received Orphan Drug Designation in the U.S., EU and Japan; specific Orphan Drug Designations vary by country/region.
AMVUTTRA (vutrisiran) – hATTR Amyloidosis with Polyneuropathy
In June 2022, AMVUTTRA was approved by the FDA for the treatment of the polyneuropathy of hATTR amyloidosis with polyneuropathy in adults based on positive 9-month results from the HELIOS-A Phase 3 study that evaluated the efficacy and safety of AMVUTTRA in patients with hATTR amyloidosis with polyneuropathy. In September 2022, AMVUTTRA was approved in the EU and UK for the treatment of hATTR amyloidosis in adult patients with stage 1 or stage 2 polyneuropathy, and in Japan for the treatment of TTR type familial amyloidosis with polyneuropathy. In December 2022, AMVUTTRA was approved in Brazil for the treatment of hATTR amyloidosis in adults. In 2023, AMVUTTRA received regulatory approval in Argentina, Switzerland and Canada for the treatment of hATTR amyloidosis in adults with Stage 1 or Stage 2 polyneuropathy, and launched in several additional countries. Regulatory filings in otheradditional territories are currently under review and additional filings are planned for 2023.2024.
HELIOS-A Phase 3 Study
Initiated in late 2018, the HELIOS-A Phase 3 trial is a randomized, open-label Phase 3 study in hATTR amyloidosis patients. The study enrolled 164 patients with hATTR amyloidosis with polyneuropathy at 57 sites in 22 countries. Patients were randomized 3:1 to receive either a 25 mg subcutaneous injection of vutrisiran once every three months or 0.3 mg/kg intravenous infusion of patisiran once every three weeks as a reference comparator for 18 months. The primary endpoint is the mean change from baseline in the modified Neuropathy Impairment Score +7, or mNIS+7, at nine months as compared to the external placebo control arm of the previously completed APOLLO Phase 3 study of patisiran, upon which the approval of ONPATTRO was based. The two secondary endpoints at nine months were changes in quality of life assessed by the Norfolk Quality of Life Questionnaire-Diabetic Neuropathy, or Norfolk QoL-DN, score and gait speed assessed by the timed 10-meter walk test, both compared to external placebo. Changes from baseline in NT-proBNP were evaluated as an exploratory endpoint at nine months. Additional secondary and exploratory endpoints were evaluated at 18 months. In addition, following the 18-month treatment period, all patients were eligible to receive vutrisiran for an additional 1842 months as part of the randomized treatment extension where they were randomized to receive either 25mg vutrisiran once quarterly or 50mg vutrisiran once every six months.
◦Efficacy and Safety Results:
At 9nine months, vutrisiran met primary and all secondary endpoints, with statistically significant improvements in neuropathy, quality of life (QoL), and gait speed, relative to placebo.
In February 2023, we reported topline results from the HELIOS-A randomized treatment extension, or RTE, portion of the study, through month 9.nine. Non-inferiority of the 50mg biannual regimen (vs 25mg quarterly) was established, as demonstrated by mean serum TTR reduction over 9nine months. Vutrisiran also continued to demonstrate an acceptable safety profile. In the RTE study there were 6six deaths, of which 5five occurred on the 50 mg biannual arm and 1one occurred on the 25 mg quarterly arm, after the patient dropped out of the study. None of the deaths was considered related to study drug. We also announced a strategic decision not to pursue regulatory submissions with the biannual dosing data given the dynamics of serum TTR recovery observed toward the end of the biannual dosing interval, the strong commercial performance of the existing vutrisiran 25mg quarterly regimen, and the opportunity to focus on continued innovation with ALN-TTRsc04.
Vutrisiran – ATTR Amyloidosis with Cardiomyopathy
Vutrisiran is also in development as a potential treatment for patients with ATTR amyloidosis with cardiomyopathy in the ongoing HELIOS-B Phase 3 study.
HELIOS-B Phase 3 Study
The HELIOS-B Phase 3 trial,study, initiated in late 2019, is a randomized, double-blind, placebo-controlled, multicenter study to evaluate the efficacy and safety of vutrisiran in patients with ATTR amyloidosis (wild-type or hereditary) with cardiomyopathy. Patients were randomized on a 1:1 basis to receive 25 mg of vutrisiran or placebo administered as a subcutaneous injection once every three months for up to 36 months. The primary endpoint will evaluate the efficacy of vutrisiran versus placebo on the composite endpoint of all-cause mortality and recurrent cardiovascular eventsevents. In February 2024, we updated the HELIOS-B statistical analysis plan and announced that the primary endpoint will be tested in parallel in two populations: the overall population and the population of patients not on tafamidis at 30 months. Additionalthe time of enrollment onto the study, which we refer to as the monotherapy population. The monotherapy population constitutes approximately 60% of the patients enrolled in the study. The secondary endpoints will include change from baseline in 6-MWT, change from baseline in KCCQ-OS, all-cause mortality, and change from baseline in New York Heart Association class, with additional exploratory endpoints willbeing evaluated. We also be evaluated. Concomitant use of on-label commercially available tafamidis is not prohibited. Theannounced in February 2024, that we had increased the minimum follow-up on the study protocol includes an optional interim efficacy analysisfrom 30 to be conducted at our discretion. In August 2021, we announced full patient enrollment33 months, with variable follow-up to 36 months. Enrollment in the HELIOS-B study. Enrollmentstudy was
completed significantly ahead of schedule, with 655 ATTR amyloidosis patients across 123 activated sites in 33 countries. In October 2022, we announced that we do not plan to conduct the optional interim analysis for the HELIOS-B Phase 3 trial. Topline results from the HELIOS-B study are expected in late June or early July 2024.
VutrisiranALN-TTRsc04 – Stargardt DiseaseATTR Amyloidosis
ALN-TTRsc04 is an investigational RNAi therapeutic in development for the treatment of ATTR amyloidosis that utilizes our IKARIA technology and provides the potential for greater than 90% target gene silencing with once annual dosing. In late 2022,December 2023, we announced that we would not initiatepositive initial results in the Phase 1 study of ALN-TTRsc04 in healthy volunteers. Single 300 mg doses of ALN-TTRsc04 achieved rapid knockdown with a mean reduction of greater than 90% at Day 15. A peak mean TTR reduction of 97% was achieved at Day 29 and a mean TTR reduction of 93% was sustained at Day 180. At all dose levels evaluated to date, single doses of ALN-TTRsc04 have been well tolerated, with no adverse events deemed to be related to study drug by the investigator.
These initial results suggest the potential for ALN-TTRsc04 to offer over 90% max TTR reduction and once annual dosing. The Phase 1 study is ongoing. Additional data will help inform selection of dose level and regimen for a Phase 3 study of vutrisiran in Stargardt Disease byATTR amyloidosis with cardiomyopathy, which we expect to start at or around year-end as previously communicated, as we continue to evaluate the impact of the Inflation Reduction Act.2024.
Our Other Marketed Products
GIVLAARI (givosiran) — Acute Hepatic Porphyria (AHP)
Our RNAi therapeutic, GIVLAARI (givosiran), is the world’s first-everfirst GalNAc-conjugate RNA therapeutic to be approved. GIVLAARI works by specifically reducing induced liver aminolevulinic acid synthase 1 mRNA, leading to
reduction of toxins associated with attacks and other disease manifestations of AHP. In the U.S., GIVLAARI (givosiran) injection for subcutaneous use is approved for the treatment of adults with AHP. GIVLAARI was reviewed by the FDA under Priority Review and had previously been granted Breakthrough Therapy and Orphan Drug Designations in the U.S. In March 2020, the EC granted marketing authorization in the EU for GIVLAARI for the treatment of AHP in adults and adolescents aged 12 years and older. GIVLAARI was reviewed under accelerated assessment by the EMA and had previously been granted PRIME and Orphan Drug Designations in the EU. We received additional marketing authorizations for GIVLAARI for the treatment of AHP in adults in Brazil, Canada, and marketing authorizations for GIVLAARI for the treatment of AHP in adults and adolescents in Japan, Argentina, Australia, Switzerland and Taiwan. We have also filed for regulatory approval for givosiran (the non-branded drug name for GIVLAARI) in Israel, Colombia, Mexico and Australia,Kuwait, and additional regulatory filings are pending or planned in 20232024 and beyond.
AHP refers to a family of ultra-rare, genetic diseases characterized by potentially life-threatening attacks and, for some patients, chronic manifestations that negatively impact daily functioning and quality of life. AHP is comprised of four types: acute intermittent porphyria, hereditary coproporphyria, variegate porphyria, and aminolevulinic acid dehydratase-deficiency porphyria. We estimate there are approximately 3,000 AHP patients diagnosed in the U.S. and EU with active disease. Each type of AHP results from a genetic defect leading to deficiency in one of the enzymes of the heme biosynthesis pathway in the liver. AHP disproportionately impacts women of working and childbearing age, and symptoms of the disease vary widely. Severe, unexplained abdominal pain is the most common symptom, which can be accompanied by limb, back or chest pain, nausea, vomiting, confusion, anxiety, seizures, weak limbs, constipation, diarrhea, or dark or reddish urine. The nonspecific nature of AHP signs and symptoms can often lead to misdiagnoses of other more common conditions such as viral gastroenteritis, irritable bowel syndrome and appendicitis. Consequently, patients with AHP can wait up to 15 years for a confirmed diagnosis. In addition, long-term complications and comorbidities of AHP can include hypertension, chronic kidney disease, or liver disease including hepatocellular carcinoma.
OXLUMO (lumasiran )—(lumasiran)— Primary Hyperoxaluria Type 1 (PH1)
OXLUMO is an RNAi therapeutic targeting hydroxyacid oxidase 1, or HAO1, developed for the treatment of PH1. HAO1 encodes glycolate oxidase, or GO, an enzyme upstream of the disease-causing defect in PH1. OXLUMO works by degrading HAO1 mRNA and reducing the synthesis of GO, which inhibits hepatic production of oxalate, the toxic metabolite responsible for the clinical manifestations of PH1. OXLUMO utilizes our ESC-GalNAc-conjugate technology, which enables subcutaneous dosing with increased potency and durability and a wide therapeutic index.
In November 2020, the EC granted marketing authorization for OXLUMO (lumasiran) for the treatment of PH1 in all age groups, following a positive Committee for Medicinal Products for Human Use, or CHMP, opinion. OXLUMO was previously granted an Accelerated Assessment and a PRIME Designation by the EMA and an Orphan Designation in the EU. Also, in November 2020, OXLUMO (lumasiran) subcutaneous injection was approved by the FDA for the treatment of PH1 to lower urinary oxalate levels in pediatric and adult patients. OXLUMO was reviewed by the FDA under Priority Review and had previously been granted Breakthrough Therapy, Orphan Drug, and Rare Pediatric Disease Designations. With the approval of OXLUMO, the FDA granted us a pediatric rare disease priority review voucher that entitles us to designate a single new drug application to qualify for a priority review in the future. During 2021 and 2022, wevoucher. We have also received additional marketing authorizations for OXLUMO in Brazil, the UK, Switzerland, Canada, Israel and Israel,Qatar, and regulatory filings in other territories are pending and additional filings are planned for 20232024 and beyond.
PH1 is an ultra-rare genetic disease that affects an estimated one to three individuals per million in the U.S. and Europe. PH1 is characterized by oxalate overproduction in the liver. The excess oxalate results in the deposition of calcium oxalate crystals in the kidneys and urinary tract and can lead to the formation of painful and recurrent kidney stones and nephrocalcinosis. Renal damage is caused by a combination of tubular toxicity from oxalate, calcium oxalate deposition in the kidneys, and urinary obstruction by calcium oxalate stones. PH1 is associated with a progressive decline in kidney function, which exacerbates the disease as the excess oxalate can no longer be effectively excreted, resulting in subsequent accumulation and deposition of oxalate in bones, eyes, skin, and heart, leading to severe illness and death. Management options prior to availability of OXLUMO were limited to hyperhydration, crystallization inhibitors and, in a minority of patients with a specific genotype, pyridoxine (vitamin B6). These measures do not adequately address oxalate overproduction but instead help to delay inevitable progression to kidney failure and the need for intensive dialysis as a bridge to a dual or sequential liver/kidney transplant. Liver transplantation is the only intervention that addresses the underlying metabolic defect, but is associated with high morbidity and mortality, and life-long immunosuppression. Prior to the approval of OXLUMO, there were no approved pharmaceutical therapies for PH1.
The regulatory approvals of OXLUMO in the U.S. and EU were based on positive results from both the ILLUMINATE-A and ILLUMINATE-B Phase 3 pivotal studies of lumasiran in patients with PH1. Lumasiran was also evaluated in ILLUMINATE-C – a global Phase 3 study in PH1 patients of all ages with advanced PH1. Based on the positive six-month efficacy and safety results of this study,PH1, which resulted in October 2022, we announced that the FDA approvedapproval of a label expansion for OXLUMO, which is now indicated for the treatment of PH1 patients to lower urinary oxalate and plasma oxalate levels in pediatric and adult patients.levels. In addition, the CHMP of the EMA delivered a positive opinion recommending variation to the marketing authorization of OXLUMO based on ILLUMINATE-C data from patients with advanced PH1 in September 2022.
In December 2022, we announced that we discontinued the proof-of-concept Phase 2 study of lumasiran in patients with recurrent kidney stone disease that was initiated in December 2021 due to lower than anticipated levels of elevated urinary oxalate in that patient population.
Leqvio (inclisiran) — Hypercholesterolemia
Our RNAi therapeutic Leqvio, developed and commercialized by our partner,collaborator, Novartis, is the first and only siRNA therapy (or RNAi therapeutic) to lower low-density lipoprotein cholesterol (also known as “bad cholesterol” or LDL-C),LDL-C, and is the first RNAi therapeutic approved for a highly prevalent disease. Leqvio is a subcutaneously administered RNAi therapeutic targeting proprotein convertase subtilisin/kexin type 9, or PCSK9, to reduce LDL-C levels via an RNAi mechanism of action and could help improve outcomes for patients with ASCVD, a deadly form of cardiovascular disease. In December 2020, following a positive CHMP opinion, the EC granted marketing authorization for Leqvio (inclisiran) for the treatment of adults with primary hypercholesterolemia (heterozygous familial and non-familial) or mixed dyslipidemia, as an adjunct to diet: in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL-C goals with the maximally tolerated dose of a statin, or alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated. In December 2021, the FDA approved Leqvio as an adjunct to diet and maximally tolerated statin therapy for the treatment of adults with HeFH or clinical ASCVD who require additional lowering of LDL-C. In July 2023, the FDA approved an expanded indication for Leqvio to include treatment of adults with high LDL-C and who are at increased risk of heart disease. Leqvio has also been granted Orphan Drug Designation in the U.S. for the treatment of homozygous familial hypercholesterolemia, or HoFH. AsIn the third quarter of 2023, Leqvio was approved in China and Japan, and as of the end of January 2023,2024, Leqvio hashad been approved in 70over 90 countries.
Approximately 100 million people worldwide are treated with lipid lowering therapies, predominantly statins, to reduce LDL-C and the associated risk of death, nonfatal myocardial infarction and nonfatal stroke or associated events. However, residual risk for cardiovascular events remains and statins are associated with well-known limitations. First, not all subjects reach LDL-C levels associated with optimal protection against clinical events. Second, not all subjects tolerate statins or are able to take statins at sufficiently-intensive doses. Third, observational studies have demonstrated that >50% of patients do not adhere to statin therapy for more than six months. Despite statins alone or in combination with other lipid lowering medications, current therapies for the management of elevated LDL-C remain insufficient in some subjects. This is particularly truethe case in patients with pre-existing coronary heart disease and/or diabetes or a history of familial hypercholesterolemia who are at the highest risk and require the most intensive management. There is an unmet need for additional treatment options beyond currently-available treatments for lowering of the LDL-C level to reduce cardiovascular risk.
In February 2013, we and MDCO (acquired by Novartis in January 2020) entered into a license and collaboration agreement pursuant to which we granted to MDCO an exclusive, worldwide license to develop, manufacture and commercialize RNAi therapeutics targeting PCSK9 for the treatment of hypercholesterolemia and other human diseases. Following its acquisition of MDCO, Novartis has all of the rights and obligations under the MDCO agreement. A description of the MDCO agreement is included below under the heading “Strategic Alliances“Our Collaboration and Collaborations.Licensing Strategy.”
Regulatory filings and approvals for Leqvio were based on positive results from the robust ORION clinical development program that included a comprehensive set of clinical trials to assess LDL-C lowering and safety in over 3,600 patients. This Phase 3 program represents the largest clinical program conducted to date for an investigational RNAi therapeutic program. Most recently, in November 2022,August 2023, Novartis announced results from its Phase 23 open-label extension ORION-3ORION-8 trial, which showed that twice-yearly (after an initial dose and another at three months) Leqvio, in addition to statin therapy, provides effective and sustainedconsistent LDL-C reduction over a four-year periodbeyond six years in patients with eitherASCVD, increased risk of ASCVD or ASCVD risk equivalent, and elevated LDL-C despite maximally tolerated statin therapy.HeFH.
Multiple additional Phase 3 trials are currently ongoing, including cardiovascular outcomes trials, ORION-4 and the Novartis initiated VICTORION-2-PREVENT.
Additional Late-Stage Clinical Development Programs
Fitusiran — Hemophilia
Fitusiran is an investigational, subcutaneously administered RNAi therapeutic targeting antithrombin, or AT, for the treatment of people with hemophilia A and B, with and without inhibitors, in collaboration withthat is being advanced by our partner,collaborator, Sanofi. Fitusiran is designed to lower levels of AT with the goal of promoting sufficient thrombin generation to prevent bleeding. AT acts by inactivating thrombin and other coagulation factors, and plays a key role in normal hemostasis by helping to limit the process of fibrin clot formation.
Hemophilia is a hereditary bleeding disorder characterized by an underlying defect in the ability to generate adequate levels of thrombin needed for effective fibrin clot formation, thereby resulting in recurrent bleeds into joints, muscles, and major internal organs. Lowering AT in the hemophilia setting may promote the generation of sufficient levels of thrombin needed to form an effective fibrin clot and prevent bleeding. This rationale is supported by human genetic data suggesting that co-inheritance of thrombophilic mutations, including AT deficiency, may ameliorate bleeding in hemophilia. We believe this approach is a unique and innovative strategy for preventing bleeding in people with hemophilia.
There are approximately 200,000 people living with hemophilia A and hemophilia B worldwide. Standard treatment for people with hemophilia currently involves replacement of the deficient clotting factor either as prophylaxis or on-demand
therapy, which can lead to a temporary restoration of thrombin generation capacity. However, with current factor replacement treatments people with hemophilia are at risk of developing neutralizing antibodies, or inhibitors, to their replacement factor, a very serious complication affecting as many as one third of people with severe hemophilia A and a smaller fraction of people with hemophilia B. People who develop inhibitors become refractory to replacement factor therapy and are twice as likely to be hospitalized for a bleeding episode.
Fitusiran is currently being evaluated in the ATLAS Phase 3 program. In October 2020, Sanofi announced that it voluntarily paused dosing in all ongoing fitusiran clinical studies to assess reports of non-fatal thrombotic events in patients participating in the ATLAS Phase 3 program. Following an assessment of available data and alignment with the FDA, in December 2020, Sanofi announced that it would resume fitusiran dosing in ongoing adolescent and adult clinical studies. Sanofi has since resumed fitusiran dosing in all ongoing studies with protocol amendments to address adjustments to dose and dosing regimen. To allow for the appropriate collection and assessment of safety and efficacy data under the amended protocols with additional lower doses, Sanofi expects that global regulatory submission timelines for the adult and adolescent studies will be delayed, subject to alignment with health authorities, and in October 2021, Sanofi announced that the potential filing date for fitusiran had been moved to 2024. Fitusiran has received both U.S. and EU Orphan Drug Designations for the treatment of hemophilia A and B.
Sanofi presented positive results from the ATLAS-A/B and ATLAS-INH Phase 3 studies of fitusiran in December 2021, and in July 2022, Sanofi presented positive results from the Phase 3 ATLAS-PPX study evaluating the efficacy and safety of once-monthly fitusiran (80 mg) in adults and adolescents with severe hemophilia A or B who were previously treated with prior factor or bypassing agent prophylaxis. In 2023, Sanofi presented positive results from the ATLAS-OLE Phase 3 extension study of fitusiran, demonstrating a substantially improved safety profile and consistent bleed protection in people with hemophilia A or B, with or without inhibitors. Specifically, the risk of thrombosis was reduced, with rates comparable to those reported in the general hemophilia population. Sanofi expects to submit an NDA for fitusiran to the FDA in 2024. Fitusiran has received both U.S. and EU Orphan Drug Designations for the treatment of hemophilia A and B.
In January 2018, we and Sanofi entered into an amendment to our 2014 collaboration, as well as the ALN-AT3 Global License Terms, which as further amended in April 2019 are referred to as the A&R AT3 License Terms, pursuant to which Sanofi has global rights to develop and commercialize fitusiran and any back-up products. The 2014 Sanofi collaboration, as amended, as well as the A&R AT3 License Terms, are described below under the heading “Strategic Alliances“Our Collaboration and Collaborations.Licensing Strategy.”
Cemdisiran — Complement-Mediated Diseases
Cemdisiran is a subcutaneously administered, investigational RNAi therapeutic targeting the C5 component of the complement pathway in development for the treatment of complement-mediated diseases. The complement system plays a central role in immunity as a protective mechanism for host defense, but its dysregulation results in a broad range of human diseases including immunoglobulin A nephropathy, or IgAN, myasthenia gravis, and paroxysmal nocturnal hemoglobinuria, amongst others.
We are currently advancing cemdisiran as a monotherapy for the treatment of IgAN, and inIn June 2022, we announced positive topline results from the Phase 2 study of cemdisiran as a monotherapy in adult patients with IgAN. In November 2022, Regeneron exercised its right to opt-out of the collaboration agreement on the cemdisiran monotherapy program. We continue to perform our obligations under the collaboration agreementCemdisiran is currently Phase 3 ready as a monotherapy for IgAN, and we are evaluating options for further development in this disease.development. Cemdisiran is also being evaluated by our partner,collaborator, Regeneron, in combination with Regeneron’s pozelimab (REGN3918), an anti-C5 monoclonal antibody, pozelimab, in Phase 3 studies in myasthenia gravis and paroxysmal nocturnal hemoglobinuria. Regeneron'sRegeneron’s decision to opt-out of the collaboration agreement on the monotherapy program has no impact on Regeneron’s ongoing efforts under a separate license agreement to develop the cemdisiran and pozelimabin combination therapy.with pozelimab.
Early-Stage Clinical Development Programs
Zilebesiran (formerly ALN-AGT) — Hypertension
Zilebesiran is an investigational, subcutaneously administered RNAi therapeutic targeting angiotensinogen, or AGT, in development for the treatment of hypertension in high unmet need populations. AGT is the most upstream precursor in the renin-angiotensin-aldosterone system, a cascade which has a demonstrated role in blood pressure regulation and its inhibition has well-established
anti-hypertensive effects. Zilebesiran inhibits the synthesis of AGT in the liver, potentially leading to durable reductions in AGT protein and ultimately, in the vasoconstrictor angiotensin II.
Hypertension is a complex multifactorial disease clinically defined by most major guidelines as a systolic blood pressure of above 140 mm Hg and/or a diastolic blood pressure greater than 90 mm Hg, though the American College of Cardiology/American Heart Association guidelines define hypertension as a systolic blood pressure above 130 mm Hg and/or a diastolic blood pressure greater than 80 mm Hg. More than one billion people worldwide live with hypertension. In the U.S. alone, approximately 47 percent of adults live with hypertension, with more than half of patients on medication remaining above the blood pressure target level. Despite the availability of anti-hypertensive medications, there remains a significant unmet medical need, especially given the poor rates of adherence to existing daily oral medications and daily peak and trough effects, resulting in inconsistent blood pressure control and an increased risk for stroke, heart attack and premature death. In particular, there are a number of high unmet need settings where novel approaches to hypertension warrant additional development focus, including patients with poor medication adherence and in patients with high cardiovascular risk.
The KARDIA-1 and KARDIA-2 Phase 2 clinical studies forof zilebesiran were initiated in June and November 2021, respectively. KARDIA-1 is designed to evaluate zilebesiran as a monotherapy across different doses administered quarterly and biannually, whereasand KARDIA-2 is evaluating the safety and efficacy of zilebesiran administered biannually as a concomitant therapy in patients whose blood pressure is not adequately controlled by a standard of care antihypertensive medication. In JanuarySeptember 2023, we reported positive topline results from KARDIA-1, with zilebesiran meeting the primary endpoint and demonstrating greater than 15mmHg reduction of systolic blood pressure at three months of treatment compared to placebo at the two highest single doses evaluated. We announced that we achieved fullcompleted enrollment of patients in the KARDIA-1KARDIA-2 Phase 2 study in July 2023, with topline results anticipated in mid-2023. We are continuing to enroll patients in the KARDIA-2 study, and expect to complete enrollmentexpected in early 2024.
In July 2023, we announced a collaboration with Roche to co-develop and report topline results at or around year-end 2023.co-commercialize zilebesiran. As part of this collaboration, we announced an update to the clinical development plan to include a new Phase 2 study, KARDIA-3, which is a multi-agent combination study in patients with uncontrolled hypertension and high CV risk. The KARDIA-3 clinical study is expected to initiate in 2024.
Elebsiran (formerly ALN-HBV02 (VIR-2218)) – Chronic Hepatitis B and D Virus Infection
Elebsiran (formerly ALN-HBV02 (VIR-2218)) is a subcutaneously administered, investigational RNAi therapeutic targeting the HBV genome for the treatment of chronic HBV infection, which is being advanced by our collaborators atcollaborator, Vir. ALN-HBV02Elebsiran is designed to inhibit expression of all HBV proteins, including hepatitis B surface antigen. Almost one-third of the world’s population have previous or current HBV infection. Worldwide, more than 250 million people are chronically infected with HBV, and an estimated 1 million people die each year from complications of chronic HBV such as cirrhosis and hepatocellular carcinoma. Current treatment options include life-long suppressive antiviral therapies. There is a significant need for safe and convenient novel therapeutics that restore the host immune response, leading to control of the virus after a finite duration of therapy, which is the definition of a functional cure.
The safety and efficacy of ALN-HBV02elebsiran are currently being investigated in an ongoing Phase 2 trial, and in June 2022, Vir reported preliminary results that demonstrated that a six-dose regimen provided greater and more durable reductions in hepatitis B surface antigen than a two-dose regimen, with all participants achieving a >1 log10 IU/mL reduction during the trial. In addition, in 2022, Vir continued to progress a Phase 2 combination trial of ALN-HBV02elebsiran with pegylated interferon-alpha, as well as a triple combination with VIR-3434 and interferon, each to evaluate the potential for the combination to result in a functional cure of HBV. ALN-HBV02Elebsiran is also being explored in a Phase 2 study evaluating ALN-HBV-02elebsiran and VIR-3434 as monotherapy and in combination for the treatment of people living with chronic hepatitis D virus, or HDV. Vir plans to report additional results from the Phase 2 studies in HBV in 2023, and data from the Phase 2 study in HDV is expected in the second half of 2023. ALN-HBV022024. Elebsiran is also being investigated in additional clinical trials with collaborators of Vir.
ALN-HSD – Non-alcoholic Steatohepatitis
ALN-HSD is We have the right to opt into a subcutaneously administered, investigational RNAi therapeutic targeting HSD17B13 in development in collaboration with our partner, Regeneron,profit-sharing arrangement for elebsiran prior to the treatment of NASH. NASH is a highly prevalent chronic liver disease in which inflammation and liver cell injury are caused by accumulation of hepatic fat. NASH is a subsetstart of a group of conditions called nonalcoholic fatty liver disease that can lead to progressive fibrosis, cirrhosis, and hepatocellular carcinoma. Comorbidities include obesity, metabolic syndrome, and type 2 diabetes. Approximately 16 million people in the U.S. live with NASH, with prevalence of the disease increasing due to rising rates of obesity. NASH is projected to be the leading indication for liver transplants in developed countries within the next 10 years. There are currently no approved medical therapies for NASH.
In September 2022, we, in collaboration with our partner, Regeneron, reported on positive results from a Phase 1 study of ALN-HSD in healthy volunteers and patients with NASH. In December 2022, we further elaborated on these results demonstrating robust target engagement and safety profile that supports continued clinical development. Regeneron will be leading continued development of the ALN-HSD program from Phase 2 onward.3 study.
ALN-APP – Alzheimer’s Disease and Cerebral Amyloid Angiopathy
ALN-APP is an investigational, intrathecally administered RNAi therapeutic targeting amyloid precursor protein, or APP, in development in collaboration with Regeneron for the treatment of Alzheimer’s disease, or AD, and cerebral amyloid angiopathy, or CAA. Genetic mutations that increase production of APP or alter its cleavage cause early-onset AD, early-onset CAA, or both. ALN-APP is designed to decrease APP mRNA in the CNS to decrease synthesis of APP protein and all downstream intracellular and extracellular APP-derived cleavage products, including amyloid beta (Aβ). Reducing APP protein production is expected to reduce the secretion of Aβ peptides that aggregate into extracellular amyloid deposits in AD and CAA and reduce the intraneuronal APP cleavage products that trigger the formation of neurofibrillary tangles and cause neuronal dysfunction in AD. ALN-APP is the first program utilizing our C16 conjugate technology, which enables enhanced delivery to cells in the CNS, to enter clinical development.
In early 2022, we initiated a Phase 1 study of ALN-APP in patients with early-onset AD, and in October 2022,April 2023 and July 2023, we reported positive interim results from the ongoing single ascending dose part of the Phase 1 study. Further exploration of single doses of ALN-APP is ongoing in Part A of the Phase 1 study. In addition, the multiple-dose part of the study, Part B, is enrolling patients from Part A and has received regulatory approval to proceed in Canada, where the majority of the Part A
clinical trial patients were enrolled. In February 2024, we announced that enrollment and dose escalation continuethe FDA has provided clearance to initiate Part B of the Phase 1 study at sites in the U.S. The FDA confirmed that multiple-dosing in the Phase 1 study may proceed at doses up to 180 mg given every six months, which covers all dose regimens planned to be explored in Part B. A partial clinical hold remains in place in the U.S. for higher or more frequent dosing regimens. We expect to report initial Part B multi-dose data from the Phase 1 study in AD in late 2024 and initial results are expectedplan to initiate a Phase 2 study in AD at or around year-end 2024.
We also expect to initiate a Phase 2 study of ALN-APP in CAA in early 2023.2024.
ALN-HSD – Non-alcoholic Steatohepatitis
ALN-HSD is a subcutaneously administered, investigational RNAi therapeutic targeting HSD17B13 that is being developed by our collaborator, Regeneron, for the treatment of NASH. NASH is a highly prevalent chronic liver disease in which inflammation and liver cell injury are caused by accumulation of hepatic fat. NASH is a subset of a group of conditions called nonalcoholic fatty liver disease that can lead to progressive fibrosis, cirrhosis, and hepatocellular carcinoma. Comorbidities include obesity, metabolic syndrome, and type 2 diabetes. Approximately 16 million people in the U.S. live with NASH, with prevalence of the disease increasing due to rising rates of obesity. NASH is projected to be the leading indication for liver transplants in developed countries within the next 10 years. There are currently no approved medical therapies for NASH.
In September 2022, we and Regeneron reported on positive results from a Phase 1 study of ALN-HSD in healthy volunteers and patients with NASH. In December 2022, we further elaborated on these results demonstrating robust target engagement and safety profile that supports continued clinical development. In late 2022, we opted-out of the further development and commercialization of ALN-HSD, and Regeneron will be leading development and commercialization of the ALN-HSD program from Phase 2 onward.
Additional Early-Stage and Pre-clinical Programs
In addition to the programs listeddescribed above, we are also advancing other earlier-stage pipeline programs, including ALN-TTRsc04 for ATTR amyloidosis, ALN-KHK for Type 2 diabetes mellitus and ALN-PNP for NASH, andNASH. We filed CTAs for each programof our ALN-KHK and ALN-PNP programs during 2022.2023. During 2023,2024, we plan to file two to fourthree new investigational new drug applications, or INDs, or CTAs from our organic product engine. We also intend to continue to build on our progress with extrahepatic delivery during 2023,
2024, advancing our eye and CNS programs under our collaboration with Regeneron, as well as continuing to advance other extrahepatic delivery initiatives.
Our Collaboration and Licensing Strategy
Our business strategy is to develop and commercialize a broad pipeline of RNAi therapeutic products directed towards transformative rare, specialty and select prevalent diseases, including continued focus on our four STArs.diseases. As part of this strategy, we have entered into, and expect to enter into additional, collaboration and licensing agreements as a means of obtaining resources, capabilities and funding to advance our investigational RNAi therapeutic programs.
Our collaboration strategy is to form alliancescollaborations that create significant value for ourselves and our collaborators in the advancement of RNAi therapeutics as a new class of innovative medicines. Specifically, with respect to our CNS/Ocular Disease pipeline, in April 2019, we entered into a global, strategic collaboration with Regeneron to discover, develop and commercialize RNAi therapeutics for a broad range of diseases by addressing disease targets expressed in the eye and CNS, in addition to a select number of targets expressed in the liver. In July 2020, Regeneron exercised its co-development/co-commercialization option on our first CNS-targeted development candidate, ALN-APP, an investigational RNAi therapeutic in development for the treatment of hereditary cerebral amyloid angiopathy and autosomal dominant Alzheimer’s Disease, which we are leading. We are also advancing multiple other programs with Regeneron.
With respect to our Cardio-Metabolic pipeline, in March 2013, we entered into an exclusive, worldwide license with MDCO (acquired by Novartis in January 2020) pursuant to which MDCO was granted the right to develop, manufacture and commercialize RNAi therapeutics targeting PCSK9 for the treatment of hypercholesterolemia and other human diseases, including inclisiran. In March 2018, we entered into a discovery collaboration with Regeneron to identify RNAi therapeutics for NASH, and potentially other related diseases, and in November 2018, we and Regeneron entered into a separate, fifty-fifty collaboration to further research, co-develop and commercialize any therapeutic product candidates that emerge from these discovery efforts. In April 2020, we entered into a development and commercialization collaboration with Dicerna to advance investigational RNAi therapeutics for the treatment of alpha-1 liver disease.
With respect to our Hepatic Infectious Disease pipeline, in October 2017, we announced an exclusive licensing agreement with Vir for the development and commercialization of RNAi therapeutics for infectious diseases, including chronic HBV infection. In March 2020, we announced an expansion of our exclusive licensing agreement with Vir to include the development and commercialization of RNAi therapeutics targeting SARS-CoV-2, the virus that causes the disease COVID-19, which we further expanded in April 2020 to include up to three additional targets focused on host factors for SARS-CoV-2, including angiotensin converting enzyme-2, or ACE2, and transmembrane protease, serine 2, or TMPRSS2, and potentially a third mutually selected host factor target. In July 2021, we notified Vir that we elected to discontinue ALN-COV, in development for the treatment of COVID-19, and all other COVID-19 research and development activities, based on a portfolio prioritization in view of the availability of highly effective vaccines and alternative treatment options. Following such discontinuation of COVID-19 related activities, we have no further obligations to work on the COVID-related targets and Vir has no further rights to such targets under our exclusive licensing agreement.
With respect to our Genetic Medicine pipeline, we formed a broad strategic alliance with Sanofi in 2014. In January 2018, we and Sanofi amended our 2014 collaboration and entered into the Exclusive License Agreement, referred to as the Exclusive TTR License, under which we have the exclusive right to pursue the further global development and commercialization of all TTR products, including ONPATTRO, AMVUTTRA and any back-up products, and the ALN-AT3 Global License Terms, referred to as the AT3 License Terms, under which Sanofi has the exclusive right to pursue the further global development and commercialization of fitusiran and any back-up products. In April 2019, we and Sanofi agreed to further amend the 2014 Sanofi collaboration to conclude the research and option phase and to amend and restate the AT3 License Terms to modify certain of the business terms.
We intend to continue to evaluate and explore partnership opportunities through collaboration and licensing arrangements, and may enter into new collaborations to advance certain products or disease areas. For example, in January 2022,we announced that we and Novartis agreed to collaborate on the discovery and development of an siRNA-based targeted therapy to restore functional liver cells in patients with end-stage liver diseases.
We also have entered into license agreements to obtain rights to intellectual property in the field of RNAi. In addition, because delivery of RNAi therapeutics has historically been an important objective of our research activities, we have entered into various collaboration and licensing arrangements with other companies and academic institutions to gain access to delivery technologies, including various LNP delivery technologies, and we may enter into such agreements in the future to gain access to products or technologies. For example, in 2021, we entered into a license and collaboration agreement with PeptiDream to discover and develop peptide-siRNA conjugates leveraging PeptiDream's proprietary Peptide Discovery Platform System technology.
Strategic Alliances and Collaborations
We have formed, and intend to continue to form, strategic alliances and collaborations to gain access to the financial, technical, clinical and commercial resources necessary to develop and market RNAi therapeutics. We expect these alliances and
collaborations to provide us with financial support in the form of upfront cash payments, license fees, equity investments, research, development, and sales and marketing support and/or funding, milestone payments and/or royalties or profit sharing based on sales of RNAi therapeutics.
Below is a brief description of our key strategic alliances, financial collaboration and license agreements.collaborations.
Product AlliancesCollaborations
Regeneron. In April 2019, we entered into a global, strategic collaboration with Regeneron to discover, develop and commercialize RNAi therapeutics for a broad range of diseases by addressing therapeutic targets expressed in the eye and CNS, in addition to a select number of targets expressed in the liver, which we refer to as the Regeneron Collaboration. The Regeneron Collaboration is governed by a Master Agreement, referred to as the Regeneron Master Agreement, which became effective in May 2019.
In connection with the Regeneron Master Agreement,August 2019, we and Regeneron entered into (i) a binding co-co collaboration term sheetagreement covering the continued development of cemdisiran, our C5 siRNA currently in Phase 2 development3 ready for IgAN as a monotherapy and (ii) a binding license term sheet to evaluateagreement covering evaluation of anti-C5 antibody-siRNA combinations for C5 complement-mediated diseases including evaluating the combination of Regeneron’s pozelimab (REGN3918), currently in Phase 3 development, and cemdisiran. The C5 co-co collaboration and license agreements were executed in August 2019.
Under the terms of the Regeneron Collaboration, we will work exclusively with Regeneron to discover RNAi therapeutics for eye and CNS diseases for an initial five-year research period, subject to extension for up to an additional five years, or the Initial Research Term. The Regeneron Collaboration also covers a select number of RNAi therapeutic programs designed to target genes expressed in the liver, including our previously-announced collaboration with Regeneron to identify RNAi therapeutics for the chronic liver disease NASH. We retain broad global rights to all of our other unpartnered liver-directed clinical and pre-clinical pipeline programs.programs that have not been collaborated.
Regeneron will lead development and commercialization for all programs targeting eye diseases (subject to limited exceptions), entitling us to certain potential milestone and royalty payments pursuant to the terms of a license agreement, the form of which has been agreed upon by the parties. We and Regeneron will alternate leadership on CNS and liver programs, with the lead party retaining global development and commercial responsibility.
With respect to the programs directed to C5 complement-mediated diseases, we retain control of cemdisiran monotherapy development, and Regeneron is leading combinationcemdisiran-combination product development. Pursuant to the C5 co-co collaboration agreement, Regeneron notified us in November 2022 of its decision to exercise its right to opt-out of the further development and commercialization of cemdisiran monotherapy. As a result, Regeneron no longer shares costs and potential future profits on any monotherapy program with us, and we are solely responsible for all development and commercialization costs and Regeneron will be eligible to receive tiered double-digit royalties on net sales. Under the C5 license agreement, for cemdisiran to be used as part of a combination product, Regeneron is solely responsible for all development and commercialization costs and we will receive low double-digit royalties and commercial milestones of up to $325.0 million on any potential combination product sales. The C5 license agreement is not impacted by Regeneron’s opt-out under the C5 co-co collaboration agreement.
We and Regeneron plan to advance programs directed to up to 30 targets under the Regeneron Collaboration during the Initial Research Term. In July 2020, Regeneron exercised its co-development/co-commercialization option on our first CNS-targeted development candidate, ALN-APP, an investigational RNAi therapeutic in development for the treatment of hereditary cerebral amyloid angiopathy andand autosomal dominant Alzheimer’s Disease, which we are leading. We are also advancing multiple other programs with Regeneron.
For more information regarding the Regeneron Collaboration, including the ongoing or expected financial and accounting impact on our business, please readsee Note 4, Net Revenues from Collaborations, to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,”Data” of this Annual Report on Form 10-K.
Roche. In July 2023, we entered into the Roche Collaboration and License Agreement with Roche, pursuant to which we established a worldwide, strategic collaboration for the joint development of pharmaceutical products containing zilebesiran. Under the Roche Collaboration and License Agreement, we granted to Roche (i) co-exclusive rights to develop zilebesiran worldwide and commercialize zilebesiran in the U.S., (ii) exclusive rights to commercialize zilebesiran outside the U.S., and (iii) non-exclusive rights to manufacture zilebesiran for the development and commercialization of zilebesiran outside the U.S. Pursuant to the Roche Collaboration and License Agreement, Roche made an upfront payment to us of $310.0 million, and we are eligible to receive up to $2.50 billion in contingent payments based on the achievement of specified development, regulatory and sales-based milestones.
We lead clinical development of zilebesiran for the treatment of hypertension, including clinical trials included in the global development plan as of the effective date of the Roche Collaboration and License Agreement, provided that Roche is responsible for any development activities conducted primarily to support regulatory approval of zilebesiran outside of the U.S. We are responsible for 40% and Roche is responsible for 60% of development costs incurred in the conduct of development activities that support regulatory approval of zilebesiran globally, provided that Roche is solely responsible for all costs incurred primarily to support regulatory approval outside the U.S., and we and Roche will share all costs incurred primarily to support regulatory approval in the U.S. Notwithstanding the foregoing, we remain solely responsible for costs incurred in connection with the conduct of clinical trials for zilebesiran ongoing as of the effective date of the Roche Collaboration and License Agreement.
Roche is solely responsible for costs incurred in connection with commercialization of zilebesiran outside the U.S. and will pay us tiered, low double-digit royalties based on net sales outside the U.S. We and Roche will share equally profits and losses (including commercialization costs) of zilebesiran in the U.S.
For more information regarding the Roche Collaboration and License Agreement, including the ongoing or expected financial and accounting impact on our business, please see Note 4, Net Revenues from Collaborations, to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10-K.
Novartis. In February 2013, we and MDCO entered into a license and collaboration agreement pursuant to which we granted to MDCO an exclusive, worldwide license to develop, manufacture and commercialize RNAi therapeutics targeting PCSK9 for the treatment of hypercholesterolemia and other human diseases. Under the MDCO agreement, we had responsibility for the development of inclisiran until Phase 1 Completion, as defined in the MDCO agreement, at our cost. In late 2015, MDCO assumed responsibility for all development and commercialization of inclisiran, at its sole cost. In January 2020, MDCO was acquired by Novartis and in December 2020, the EC granted marketing authorization for Leqvio (inclisiran) for the treatment of adults with hypercholesterolemia or mixed dyslipidemia, following a positive CHMP opinion. In December 2021, Leqvio was approved by the FDA for the treatment of adults with HeFH or clinical ASCVD as an adjunct to diet and maximally tolerated dose of statin. In July 2023, the FDA approved an expanded indication for Leqvio to include treatment of adults with high LDL-C and who are at increased risk of heart disease.
For more information regarding the MDCO agreement, including its ongoing financial and accounting impact on our business, please see Note 4, Net Revenues from Collaborations, to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10-K.
Sanofi. In January 2014, we entered into a global, strategic collaboration with Sanofi to discover, develop and commercialize RNAi therapeutics as Genetic Medicines to treat orphan diseases, referred to as the 2014 Sanofi collaboration. The 2014 Sanofi collaboration superseded and replaced the previous collaboration between us and Sanofi entered into in October 2012 to develop and commercialize RNAi therapeutics targeting TTR for the treatment of hATTR amyloidosis, including patisiran and revusiran, in Japan and the Asia-Pacific region.
In January 2018, we and Sanofi entered into an amendment to our 2014 Sanofi collaboration. In connection and simultaneously with entering into the 2018 amendment to the 2014 Sanofi collaboration, we and Sanofi also entered into the Exclusive TTR License and the AT3 License Terms. As a result, we have the exclusive right to pursue the further global development and commercialization of all TTR products, including ONPATTRO, AMVUTTRA and any back-up products, and Sanofi has the exclusive right to pursue the further global development and commercialization of fitusiran and any back-up products. Under the 2018 amendment and the Exclusive TTR License, Sanofi is eligible to receive (i) royalties up to 25%, increasing over time, based on annual net sales of ONPATTRO in territories excluding the U.S., Canada and Western Europe, provided royalties on annual net sales of ONPATTRO in Japan were set at 25% beginning as of the effective date of the Exclusive TTR License, and (ii) tiered royalties of 15% to 30% based on global annual net sales of AMVUTTRA (consistent with
the royalties due to us from Sanofi on fitusiran), and (iii) tiered royalties of up to 15% based on global annual net sales of any back-up products, in each case by us, our affiliates and our sublicensees.. The collaboration amendment entered into in April 2019 described below made no changes to the terms described in clauses (i)-(iii)(ii) above, which remain in full force and effect.
In April 2019, we and Sanofi agreed to further amend the 2014 Sanofi collaboration to conclude the research and option phase and to amend and restate the AT3 License Terms pursuant to the A&R AT3 License Terms, to modify certain of the business terms. The material collaboration terms for fitusiran were unchanged. Under the A&R AT3 License Terms, we are eligible to receive tiered royalties of 15% to 30% based on global annual net sales of fitusiran and up to 15% based on global annual net sales of any back-up products controlled by Sanofi, in each case by Sanofi, its affiliates and its sublicensees. In connection with entering into the 2019 amendment and the A&R AT3 License Terms, we agreed to advance, at our cost, a selected investigational asset in an undisclosed rare genetic disease through the end of IND-enabling studies. Following completion of such studies, we will transition, at our cost, such asset to Sanofi. Thereafter, Sanofi will fund all potential future development and commercialization costs for such asset. If this asset is approved, we will be eligible to receive tiered double-digit royalties on global net sales.
Novartis AG. In February 2013, we and MDCO entered into a license and collaboration agreement pursuant to which we granted to MDCO an exclusive, worldwide license to develop, manufacture and commercialize RNAi therapeutics targeting PCSK9 for the treatment of hypercholesterolemia and other human diseases. Under the MDCO agreement, we had responsibility for the development of inclisiran until Phase 1 Completion, as defined in the MDCO agreement, at our cost. In late 2015, MDCO assumed responsibility for all development and commercialization of inclisiran, at its sole cost. In January 2020, MDCO was acquired by Novartis and in December 2020, the EC granted marketing authorization for Leqvio (inclisiran) for the treatment of adults with hypercholesterolemia or mixed dyslipidemia, following a positive CHMP opinion. In December 2021, Leqvio was approved by the FDA for the treatment of adults with HeFH or clinical ASCVD as an adjunct to diet and maximally tolerated dose of statin. For more information regarding the MDCO agreement, including its ongoing financial and accounting impact on our business, please read Note 4, Net Revenues from Collaborations, to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,” of this Annual Report on Form 10-K.
Vir Biotechnology, Inc.Vir. In October 2017, we and Vir entered into a collaboration and license agreement, or the Vir Agreement, pursuant to which we granted to Vir an exclusive license to develop, manufacture and commercialize ALN-HBV02 (VIR-2218)elebsiran (formerly ALN-HBV02), for all uses and purposes other than certain excluded fields, as set forth in the agreement.Vir Agreement. In addition, we granted Vir an exclusive option for up to four additional RNAi therapeutics programs for the treatment of infectious diseases. In March and April 2020, we entered into amendments to the Vir Agreement to expand our collaboration to include the development and commercialization of RNAi therapeutics targeting SARS-CoV-2, the virus that causes the disease COVID-19, along with three additional targets focused on human host factors for SARS-CoV-2, including ACE2 and TMPRSS2 and potentially a third mutually selected host factor target. We further amended the Vir Agreement in December 2020, such that we were solely responsible for conducting pre-clinical research activities under the pre-clinical development plan at our discretion and sole expense and effective as of July 1, 2020, we were responsible for all pre-clinical development costs incurred under such plan. In July 2021, we notified Vir that we elected to discontinue ALN-COV, in development for the treatment of COVID-19, and all other COVID-19 research and development activities, based on a portfolio prioritization in view of the availability of highly effective vaccines and alternative treatment options. Following such discontinuation of COVID-19 related activities, we have no further obligations to work on the COVID-related targets and Vir has no further rights to such targets under our exclusive licensing agreement. For more information regarding the Vir Agreement, including its ongoing financial and accounting impact on our business, please read Note 4, Net Revenues from Collaborations, to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,” of this Annual Report on Form 10-K.
Strategic Financing Collaboration
The Blackstone Group Inc. In April 2020, we entered into a strategic financing collaboration with certain affiliates of Blackstone to accelerate our advancement of RNAi therapeutics. In connection with the collaboration, Blackstone agreed to provide us up to $2.0$2.00 billion in financing, including $1.0$1.00 billion in committed payments to acquire 50% of royalties and 75% of commercial milestones payable to us in connection with sales of Leqvio, up to $750.0 million in a first lien senior secured term loan, and up to $150.0 million towards the development of vutrisiran and zilebesiran (formerly ALN-AGT) pursuant to the funding agreement finalized in August 2020. In November 2021, Blackstone elected to opt-in to Phase 2 clinical trial funding of zilebesiran, committing to fund, upon meeting certain patient enrollment thresholds, up to $26.0 million. As part of the strategic financing collaboration, Blackstone also purchased an aggregate of $100.0 million of our common stock. Please read Note 5 and Note 1110 to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,”Data” of this Annual Report on Form 10-K for additional details on our transaction with Blackstone, including its ongoing financial and accounting impact on our business.
Other StrategicCollaboration and License Agreements
We intend to continue to evaluate and explore opportunities through collaboration and licensing arrangements, and may enter into new collaborations to advance certain products or disease areas. We also have entered into license agreements to obtain rights to intellectual property in the field of RNAi. In addition, because delivery of RNAi therapeutics has historically been an important objective of our research activities, we have entered into various collaboration and licensing arrangements with other companies and academic institutions to gain access to delivery technologies, including various LNP delivery technologies, and we may enter into such agreements in the future to gain access to products or technologies.
Below is a brief description of certain other collaboration and license agreements we have entered into.
Dicerna Pharmaceuticals, Inc. In April 2020, we and Dicerna (acquired by Novo Nordisk in December 2021) formed a development and commercialization collaboration on investigational RNAi therapeutics for the treatment of alpha-1 liver disease. Under the development and commercialization agreement entered into between the parties, our ALN-AAT02 and
Dicerna’s belcesiran (formerly DCR-A1AT), investigational RNAi therapeutics, each in Phase 1/2 development, will be explored for the treatment of alpha-1 liver disease. In addition, in April 2020, we and Dicerna entered into a Patent Cross-License Agreement, pursuant to which each party agreed to cross-license its respective intellectual property related to our lumasiran program and Dicerna’s nedosiran program, each for the treatment of PH. In addition, in April 2020, we and Dicerna (acquired by Novo Nordisk in
December 2021) formed a development and commercialization collaboration on investigational RNAi therapeutics for the treatment of alpha-1 liver disease. Under the development and commercialization agreement, our ALN-AAT02 and Dicerna’s belcesiran (formerly DCR-A1AT), each in Phase 1/2 development, are being explored for the treatment of alpha-1 liver disease. In December 2023, we received notice from Dicerna that they had exercised their termination right, without cause, under the development and collaboration agreement, effective May 2024.
PeptiDream, Inc. In July 2021, we entered into a license and collaboration agreement with PeptiDream to discover and develop peptide-siRNA conjugates to create multiple opportunities to deliver RNAi therapeutics to tissues outside the liver. Through this collaboration, the companies will collaborateare collaborating to select and optimize peptides for targeted delivery of small siRNA molecules to a wide range of cell types and tissues via specific interactions with receptors expressed on the target cells. Under the terms of the alliance,collaboration, we will select a set of receptors for PeptiDream’s peptide discovery platform. PeptiDream will select, optimize, and synthesize peptides for each receptor. We will then generate peptide-siRNA conjugates and perform in vitro and in vivo studies to support final peptide selection. For more information regarding the collaboration agreement with PeptiDream, including its ongoing financial and accounting impact on our business, please read Note 4, Net Revenues from Collaborations, to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,” of this Annual Report on Form 10-K.
Novartis AG. In January 2022, we announced that we and Novartis, entered into a collaboration and license agreement, referred to as the Novartis License Agreement, pursuant to which we granted to Novartis an exclusive, worldwide license to develop, manufacture and commercialize siRNAs targeting end-stage liver disease, potentially leading to the development of a treatment designed to promote the regrowth of functional liver cells and to provide an alternative to transplantation for patients with liver failure. Under the terms of the collaboration, we will develop and test potential siRNAs using target-specific assays developed by Novartis. Upon identification of a lead candidate, further development and clinical research will be conducted by Novartis. Pursuant to the Novartis License Agreement, we received an upfront fee, and may also receive milestone payments upon the achievement of certain development, regulatory and commercial milestones, as well as tiered royalties on the net sales of licensed products ranging from high-single-digit to sub-teen double-digit percentages. For more information regarding the Novartis License Agreement, including its ongoing financial and accounting impact on our business, please read Note 4, Net Revenues from Collaborations, to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,” of this Annual Report on Form 10-K.
Ionis Pharmaceuticals, Inc. In January 2015, we and Ionis Pharmaceuticals, Inc., or Ionis, entered into a second amended and restated strategic collaboration and license agreement, which we further amended in July 2015, or the 2015 Ionis agreement. The 2015 Ionis agreement provides for certain new exclusive target cross-licenses of intellectual property on eight disease targets, providing each company with exclusive RNA therapeutic license rights for four programs, and extended the parties’ existing non-exclusive technology cross-license, which was originally entered into in 2004 and was amended and restated in 2009, through April 2019. Pursuant to the 2015 Ionis agreement, Ionis granted to us an exclusive, low single-digit royalty-bearing license to its chemistry, motif, mechanism and target-specific intellectual property for oligonucleotide therapeutics against four targets. In exchange, we granted to Ionis an exclusive, low single-digit royalty-bearing license to our chemistry, motif, mechanism and target-specific intellectual property for oligonucleotide therapeutics against four targets. Under the original agreement, Ionis licensed to us its patent estate related to antisense motifs and mechanisms and oligonucleotide chemistry for double-stranded RNAi products in exchange for a previously disclosed technology access fee, participation in fees for our partneringcollaboration programs and future milestone and royalty payments from us for programs that incorporate Ionis’ intellectual property. We have the right to use Ionis’ intellectual property in our development programs or in collaborations and Ionis agreed not to grant licenses under these patents to any other organization for the discovery, development and commercialization of double-stranded RNA products designed to work through an RNAi mechanism, except in the context of a collaboration in which Ionis plays an active role. In turn, in exchange for option fees, and future milestone and royalty payments from Ionis for RNAi programs that incorporate certain of our intellectual property, we non-exclusively licensed to Ionis our patent estate relating to antisense motifs and mechanisms and oligonucleotide chemistry to research, develop and commercialize single-stranded antisense therapeutics, single stranded RNAi therapeutics and to research double-stranded RNAi compounds. Ionis also received a license to develop and commercialize double-stranded RNAi drugs targeting a limited number of therapeutic targets on a non-exclusive basis.
Intellectual Property, Proprietary Rights and Exclusivities
We have devoted considerable effort and resources through both in-licensing and filing patent applications on our own inventions, as well as protecting our trade secrets and know-how to establish what we believe to be a strong intellectual property position relevant to RNAi therapeutic products and delivery technologies. In this regard, we have amassed a portfolio of patents, patent applications and other intellectual property covering:
•fundamental aspects of the structure and uses of siRNAs, including their use as therapeutics, and RNAi-related mechanisms;
•chemical modifications to siRNAs that improve their suitability for therapeutic and other uses;
•compositions of siRNAs directed to specific targets as well as their methods of use, including as therapeutics and diagnostics;
•delivery technologies, such as in the fields of siRNA conjugates, including carbohydrate, lipophilic and other conjugates as well as cationic liposomes and other delivery vehicles; and
•all aspects of our development candidates and marketed products, with an additional level of protection for trademarks related to our marketed products.
In addition to patents and trademarks for our marketed products, we seek to obtain all available regulatory exclusivities for our marketed products, including data and orphan exclusivities in the relevant jurisdictions.
Key Patents and Regulatory Exclusivities
We typically obtain protection of our product candidates with patents and patent applications directed to compositions of matter and their uses. Below is a summary of selected granted patents that we own or control covering products marketed by us in the U.S. and Europe.
ONPATTRO
| | | | | | | | | | | | | | |
| Patent Number | Country/Region* | Patent Type | Expiration Date** | Owner/Licensor |
| 8168775 | United States | Compositions of Matter & Methods of Use | 8/10/2032 | Alnylam |
| 8334373 | United States | Compositions of Matter & Methods of Use | 5/27/2025 | Alnylam |
| 8741866 | United States | Compositions of Matter & Methods of Use | 10/20/2029 | Alnylam |
| 9234196 | United States | Compositions of Matter & Methods of Use | 10/20/2029 | Alnylam |
| 8802644 | United States | Compositions of Matter & Methods of Use | 10/21/2030 | Arbutus Biopharma |
| 8158601 | United States | Compositions of Matter & Methods of Use | 11/10/2030 | Arbutus Biopharma |
9943538 | United States | Compositions of Matter | 11/4/2023 | Ionis Pharmaceuticals |
9943539 | United States | Compositions of Matter | 11/4/2023 | Ionis Pharmaceuticals |
| 2937418 | Europe | Compositions of Matter & Methods of Use | 8/28/2033 | Alnylam |
| 2344639 | Europe | Compositions of Matter & Methods of Use | 10/20/2029 | Alnylam |
| 2440183 | Europe | Compositions of Matter | 10/21/2030 | Arbutus Biopharma |
* Shown here are selected granted patents in the U.S. and Europe. Additional granted and pending patents in the U.S., Europe and other countries may be available.
** Expiration dates listed here include any granted or anticipated patent term extensions and supplemental protection certificates but exclude any pediatric extensions that may be available.
In addition, in connection with our FDA approval on August 10, 2018, the FDA granted ONPATTRO new chemical entity, or NCE, exclusivity until August 10, 2023, and Orphan Drug Exclusivity, or ODE, until August 10, 2025. In connection with our EMA approval on August 26, 2018, the EMA granted ONPATTRO Marketing Exclusivity and ODE until August 26, 2028.
AMVUTTRA
| | | | | | | | | | | | | | |
| Patent Number | Country/Region* | Patent Type | Expiration Date** | Owner/Licensor |
| 8106022 | United States | Compositions of Matter & Methods of Use | 12/12/2029 | Alnylam |
| 8828956 | United States | Compositions of Matter & Methods of Use | 12/4/2028 | Alnylam |
| 9370581 | United States | Compositions of Matter & Methods of Use | 12/4/2028 | Alnylam |
| 9399775 | United States | Compositions of Matter & Methods of Use | 11/16/2032 | Alnylam |
| 10131907 | United States | Compositions of Matter & Methods of Use | 8/24/2028 | Alnylam |
| 10208307 | United States | Compositions of Matter & Methods of Use | 7/28/2036 | Alnylam |
| 10570391 | United States | Compositions of Matter | 11/16/2032 | Alnylam |
| 10612024 | United States | Compositions of Matter & Methods of Use | 8/14/2035 | Alnylam |
| 10683501 | United States | Methods of Use | 7/28/2036 | Alnylam |
| 10806791 | United States | Compositions of Matter | 12/4/2028 | Alnylam |
| 11286486 | United States | Methods of Use | 7/28/2036 | Alnylam |
| 11401517 | United States | Compositions of Matter & Methods of Use | 8/14/2035 | Alnylam |
| 3301177 | Europe | Compositions of Matter & Methods of Use | 11/16/2032 | Alnylam |
| 3329002 | Europe | Compositions of Matter & Methods of Use | 7/28/2036 | Alnylam |
* Shown here are selected granted patents in the U.S. and Europe. Additional granted and pending patents in the U.S., Europe and other countries may be available.
** Expiration dates listed here do not account for any patent term extensions, supplemental protection certificates or pediatric extensions that may be available.
In addition, in connection with our FDA approval on June 13, 2022, the FDA granted AMVUTTRA new chemical entity, or NCE, exclusivity until June 13, 2027. In connection with our EMA approval on September 15, 2022, the EMA granted AMVUTTRA Marketing Exclusivity and ODE until September 15, 2032.
GIVLAARI
| | | | | | | | | | | | | | |
| Patent Number | Country/Region* | Patent Type | Expiration Date** | Owner/Licensor |
| 8106022 | United States | Compositions of Matter & Methods of Use | 12/12/2029 | Alnylam |
| 8828956 | United States | Compositions of Matter & Methods of Use | 12/4/2028 | Alnylam |
| 9133461 | United States | Compositions of Matter & Methods of Use | 5/14/2033 | Alnylam/Icahn School of Medicine at Mount Sinai |
| 9150605 | United States | Compositions of Matter | 8/28/2025 | Ionis Pharmaceuticals |
| 9631193 | United States | Methods of Use | 3/15/2033 | Alnylam/Icahn School of Medicine at Mount Sinai |
9708610 | United States | Compositions of Matter & Methods of Use | 1/1/2024 | Ionis Pharmaceuticals |
| 9708615 | United States | Compositions of Matter & Methods of Use | 3/8/2024 | Alnylam |
| 10119143 | United States | Compositions of Matter & Methods of Use | 10/3/2034 | Alnylam/Icahn School of Medicine at Mount Sinai |
| 10125364 | United States | Compositions of Matter & Methods of Use | 3/15/2033 | Alnylam/Icahn School of Medicine at Mount Sinai |
| 10131907 | United States | Compositions of Matter & Methods of Use | 8/24/2028 | Alnylam |
| 10273477 | United States | Compositions of Matter | 3/8/2024 | Alnylam |
| 2836595 | Europe | Compositions of Matter & Methods of Use | 4/10/2033 | Alnylam/Icahn School of Medicine at Mount Sinai |
| 2336317 | Europe | Compositions of Matter | 6/14/2024 | Alnylam |
2957568 | Europe | Compositions of Matter | 11/4/2023 | Ionis Pharmaceuticals |
1560840 | Europe | Compositions of Matter | 11/4/2023 | Ionis Pharmaceuticals |
* Shown here are selected granted patents in the U.S. and Europe. Additional granted and pending patents in the U.S., Europe and other countries may be available.
** Expiration dates listed here do not account for any patent term extensions, supplemental protection certificates or pediatric extensions that may be available.
In addition, in connection with our FDA approval on November 20, 2019, the FDA granted GIVLAARI NCE exclusivity until November 20, 2024, and ODE until November 20, 2026. In connection with our EMA approval on March 2, 2020, the EMA granted GIVLAARI Marketing Exclusivity and ODE until March 2, 2030.
OXLUMO
| | | | | | | | | | | | | | |
| Patent Number | Country/Region* | Patent Type | Expiration Date** | Owner/Licensor |
| 8106022 | United States | Compositions of Matter & Methods of Use | 12/12/2029 | Alnylam |
| 8828956 | United States | Compositions of Matter & Methods of Use | 12/4/2028 | Alnylam |
| 9828606 | United States | Compositions of Matter | 12/26/2034 | Dicerna Pharmaceuticals |
| 10131907 | United States | Compositions of Matter & Methods of Use | 8/24/2028 | Alnylam |
| 10435692 | United States | Methods of Use | 12/26/2034 | Dicerna Pharmaceuticals |
| 10465195 | United States | Compositions of Matter & Methods of Use | 12/26/2034 | Dicerna Pharmaceuticals |
| 10478500 | United States | Compositions of Matter & Methods of Use | 10/9/2035 | Alnylam |
| 10487330 | United States | Compositions of Matter & Methods of Use | 12/26/2034 | Dicerna Pharmaceuticals |
| 10612024 | United States | Compositions of Matter | 8/14/2035 | Alnylam |
| 10612027 | United States | Compositions of Matter & Methods of Use | 8/14/2035 | Alnylam |
| 3087184 | Europe | Compositions of Matter | 12/26/2034 | Dicerna Pharmaceuticals |
* Shown here are selected granted patents in the U.S. and Europe. Additional granted and pending patents in the U.S., Europe and other countries may be available.
** Expiration dates listed here do not account for any patent term extensions, supplemental protection certificates or pediatric extensions that may be available.
In addition, in connection with our FDA approval on November 23, 2020, the FDA granted OXLUMO NCE exclusivity until November 23, 2025, and ODE until November 23, 2027. In connection with our EMA approval on November 19, 2020, the EMA granted OXLUMO Marketing Exclusivity and ODE until November 19, 2030.
Trademarks
We file trademarks to protect our corporate brand and our products. Typically, we file trademark applications in the U.S., Europe and elsewhere in the world as appropriate. In addition to multiple pending trademark applications in the U.S. and other major countries, we have registered trademarks in the U.S., including but not limited to Alnylam® and the Alnylam logo, as well as ONPATTRO® and the ONPATTRO logo, AMVUTTRA® and the AMVUTTRA logo, GIVLAARI® and the GIVLAARI logo and OXLUMO® and the OXLUMO logo.
Competition
The pharmaceutical marketplace is extremely competitive, with hundreds of companies competing to discover, develop, and market new drugs. We face a broad spectrum of current and potential competitors, ranging from very large, global pharmaceutical companies with significant resources, to other biotechnology companies with resources and expertise comparable to our own, to smaller biotechnology companies with fewer resources and less expertise than those we have.currently possess. We believe that for most or all of our drug development programs, there will be one or more competing programs being marketed and/or under development at other companies. In some cases, the companies with competing programs will have access to greater resources and expertise than we do and may be more advanced in those programs.programs than we are.
Competition for Our Business in General
The competition we face can be grouped into three broad categories:
•other companies working to develop RNAi and microRNA therapeutic products;
•companies developing technology known as antisense, which, like RNAi, attempts to silence the activity of specific genes by targeting the mRNAs copied from them; and
•marketed products and development programs for therapeutics that treat the same diseases for which we may also beare marketing products or developing treatments.
We are aware of several other companies that are working to develop RNAi therapeutic products. Some of these companies are seeking, as we are, to develop chemically synthesized siRNAs as drugs. Others are following a gene therapy approach, with
the goal of treating patients not with synthetic siRNAs but with synthetic, exogenously-introduced genes designed to produce siRNA-like molecules within cells.
Companies working on chemically synthesized siRNAs include Arrowhead Pharmaceuticals, Inc., or Arrowhead, and its collaborators, Takeda Pharmaceutical Company Ltd., or Takeda, Janssen Pharmaceutics,Pharmaceuticals, Inc. or Janssen, Horizon Therapeutics PLC, or Horizon,, GlaxoSmithKline PLC, or GSK,plc, and Amgen Inc., or Amgen,; Quark Pharmaceuticals, Inc., or Quark,; F. Hoffmann-La Roche Ltd, or Roche,Ltd.; Silence Therapeutics plc or Silence, and its collaborators, AstraZeneca plc, or AstraZeneca, Jiangsu Hansoh Pharmaceuticals Group Co., Ltd., and Mallinckrodt plc,plc; Arbutus Biopharma Corp., or Arbutus,Arbutus; Sylentis, S.A.U., or Sylentis,Sylentis; and Novo Nordisk and its collaborators, Boehringer Ingelheim AstraZeneca plc, or AstraZeneca, and Eli Lilly and Company, or Eli Lilly, WAVE Life Sciences Ltd., or WAVE, Silenseed Ltd., Ascletis Pharma Inc., Biomics Biopharma, Avidity Biosciences Inc., Dyne Therapeutics Inc., or Dyne, Atalanta Therapeutics Inc., Sirnaomics Inc., OliX Pharmaceuticals Inc., Ochre Bio, DTx Pharma, Inc., Sixfold Bioscience Inc., ProQR Therapeutics NV, or ProQR, Phio Pharmaceuticals, BioPath Holding Inc., Arcturus Therapeutics, Inc., or Arcturus, and Ascletis Pharma Inc. Several of these companies have licensed our intellectual property. Benitec Biopharma Ltd., or Benitec, is working on gene therapy approaches to RNAi therapeutics. Companies working on microRNA therapeutics include Regulus Therapeutics, Inc., Rosetta Genomics Ltd., MiNA Therapeutics, Inc, InteRNA Technologies B.V. and TransCode Therapeutics, Inc.
Antisense technology uses short, single-stranded, DNA-like molecules to block mRNAs encoding specific proteins. We believe that RNAi drugs may potentially have significant advantages over antisense oligonucleotide, or ASO, drugs, including greater potency and specificity. In addition to Ionis and its collaborators, including Biogen Inc., AstraZeneca, Novartis and Bayer AG, and several other companies have ASO-based product candidates in various stages of pre-clinical and clinical development, including Roche, Akcea Therapeutics, Inc. (acquired by Ionis in October 2020), or Akcea, Antisense Therapeutics, Ltd., Dyne, WAVE, GSK, Sarepta Therapeutics, Inc., ProQR and Eli Lilly.Company.
The competitive landscape continues to expand, and we expect that additional companies will initiate programs focused on the development of RNAi therapeutic products using the approaches described above as well as potentially new approaches that may result in the more rapid development of RNAi therapeutics or more effective technologies for RNAi drug development or delivery.
Competing Drugs for Our Marketed Products and Late-Stage Investigational RNAi Therapeutics
ATTR Amyloidosis. Until a few years ago, liver transplantation was the only treatment option for patients with hATTR amyloidosis in the U.S. and in other countries. Only a subset of patients with early-stage disease qualify for this costly and invasive procedure, which carries significant morbidity and risk of mortality. Even following liver transplantation, the disease continues to progress for many patients, presumably due to ongoing deposition of wild-type TTR protein.
In addition to ONPATTRO and AMVUTTRA, currently approved treatments for hATTR amyloidosis now include inotersen (TEGSEDI,TEGSEDI (inotersen) marketed by Ionis, VYNDAQEL/VYNDAMAX (tafamidis) marketed by Pfizer Inc., and recently approved in many countries)Wainua (eplontersen) marketed by Ionis and tafamidis (VYNDAQEL/VYNDAMAX, approved in many countries). Treatment indications vary by country/region for each product.AstraZeneca plc. We believe that the followingthese approved drugs could compete with ONPATTRO and AMVUTTRA for the treatment of the polyneuropathy of hATTR amyloidosis with polyneuropathy in adults and for the treatment of ATTR amyloidosis with cardiomyopathy if patisiran is approved forin the event that indication and/or ifour HELIOS-B Phase 3 clinical trial is positive and vutrisiran is approved for that indication:
| | | | | | | | | | | | | | |
Drug | Company | Drug Description | Phase | Administration/Dosing |
VYNDAQEL (tafamidis meglumine) | Pfizer Inc. | Small molecule drug to stabilize TTR protein | Approved in the EU, Japan and certain countries in Latin America for hATTR polyneuropathy (indication varies by region) | Daily oral capsule |
VYNDAQEL/VYNDAMAX (tafamidis meglumine / tafamidis) | Pfizer Inc. | Small molecule drug to stabilize TTR protein | Approved to treat ATTR cardiomyopathy in the U.S., EU and Japan; (indication varies by region) | Daily oral capsule |
TEGSEDI (inotersen) | Ionis | Anti-sense oligonucleotide, or ASO, to reduce production of TTR Protein | Approved in U.S., EU, Canada and Brazil for hATTR polyneuropathy (indication varies by region) | Weekly subcutaneous injection (SC) |
Several investigational drugs also exist, in varying stages of clinical development, for ATTR amyloidosis. We believe that the following drug candidates, if approved, could compete with ONPATTRO and AMVUTTRA,receives regulatory approval for the treatment of the
polyneuropathy of hATTR amyloidosisother product candidates in adults anddevelopment for the treatment of ATTR amyloidosis with cardiomyopathy, if patisiranincluding acoramidis, which is approvedbeing developed by BridgeBio Pharma, Inc., or BridgeBio, and for that indication and/or if HELIOS-Bwhich the FDA has accepted an NDA for filing; NTLA-2001 which is positivebeing developed by Intellia Therapeutics, Inc. and vutrisiranRegeneron and is approved for that indication:
| | | | | | | | | | | | | | |
Drugin Phase 3 clinical development; NNC-6019 which is being developed by Novo Nordisk and is in Phase 2 clinical development; and NI006 which is being developed by Neurimmune AG and AstraZeneca plc and is in Phase 1 clinical development. | Company | Drug Description | Phase | Administration/Dosing |
Eplontersen (IONIS-TTR-LRx)
| Ionis & AstraZeneca | ASO to reduce production of TTR Protein | Filed for approval in December 2022 for hATTR polyneuropathy | Monthly subcutaneous injection (SC) |
Acoramidis (AG10) | BridgeBio Pharma, Inc. & AstraZeneca in Japan | Small molecule drug to stabilize TTR protein | Phase 3 | Twice daily oral dose |
NNC-6019 (PRX004) | Novo Nordisk | mAb to clear TTR amyloid deposits | Phase 2 | Intravenous (IV) infusion every four weeks |
NI006 | AstraZeneca & Neurimmune AG | mAB to clear TTR amyloid deposits | Phase 1 | Intravenous (IV) infusion |
NTLA-2001 | Intellia Therapeutics, Inc. & Regeneron | CRISPR/Cas9 gene therapy for TTR | Phase 1 | One-time intravenous (IV) infusion |
We are also aware of other companies that have pre-clinical development programs for the potential treatment of ATTR amyloidosis.
Acute Hepatic Porphyria. In addition to GIVLAARI which is approved in the U.S. for the treatment of adults with AHP, and in the EU for the treatment of AHPAHP. There are currently no other approved therapies for prophylactic treatment of AHP. Nevertheless, Recordati S.p.A has two products, PANHEMATIN and NORMOSANG, that are approved in adultsthe U.S. and adolescents aged 12 years and older, there are also two approved hemin products, Panhematin (U.S.) and Normosang (EU),EU, respectively, for the treatment of acute porphyria attacks. Panhematinattacks, and Normosang are both administered by intravenous infusion and are blood products currently manufactured by Recordati S.p.A. There are currently no competing products approved for prophylactic use; however, there issome physicians may prescribe these therapies off-label prophylactic use of hemin by some physicians.We are aware of other companies that have pre-clinical development programs for the potentialprophylactic treatment of AHP.
Primary Hyperoxaluria. In addition to OXLUMO, which wasis approved in the U.S. and EU for the treatment of primary hyperoxaluria, or PH, type 1, other currently used treatments include hyper-hydration, oral citrate, and dual liver/kidney transplantation. Transplantation is a costly and invasive procedure and carries significant morbidity and mortality risk. Beyond OXLUMO and these additional treatment methods, Novo Nordisk’s product RIVFLOZA was approved for the treatment of PH1 in September 2023 and is expected to launch in 2024. In addition, several companies have investigational drugs in clinical development for the treatment of PH, including BridgeBio, Chinook Therapeutics, Inc., and BioMarin Pharmaceutical, Inc. We believe that these approved products and product candidates, if approved, could compete with OXLUMO.
Hypercholesterolemia. In addition to Leqvio, which is approved in the U.S. and EU for the treatment of PH type 1 in patientshypercholesterolemia, several companies have approved products for the treatment of all ages, currently used treatments for PH include hyper hydration, oral citrate or dual liver/kidney transplantation. Transplantation is costlyhypercholesterolemia, including Amgen Inc. (REPATHA), Sanofi S.A. (Sa PRALUENT), Amarin Corporation (VASCEPA), Esperion Therapeutics, Inc. (NEXLETOL), and is an invasive procedure, which carries significant morbidity and mortality. This leaves a high unmet medical need for a severe and primarily pediatric disorder.Presently, thereRegeneron (EVKEEZA). There are also several companies with investigational drugs in varying stages of clinical development for the treatment of PH. We believe that the following drug candidates, ifhypercholesterolemia, including LIB Therapeutics, LLC, Merck & Co., Inc., Jiangsu Hengrui Pharmaceuticals Co., Ltd., Ionis, Arrowhead and Verve Therapeutics, Inc.
Hemophilia. There are several approved could compete with OXLUMO:
| | | | | | | | | | | | | | |
Drug | Company | Drug Description | Phase | Administration/Dosing |
Nedosiran | Novo Nordisk | siRNA to reduce production of LDHA enzyme | Filed with FDA in September 2022 | SC with monthly dosing |
BBP-711 | BridgeBio Pharma, Inc. | GO inhibitor | Phase 2/3 | Oral |
CHK-336 | Chinook Therapeutics, Inc. | LDHA inhibitor | Phase 2 | Oral |
BMN-255 | BioMarin Pharmaceutical, Inc. | Undisclosed | Phase 1/2 | Oral |
We are aware of other companies that have pre-clinical development programs for the potential treatment of PH.
Hypercholesterolemia. In addition to Leqvio, which was approved in the EUproducts for the treatment of adults with hypercholesterolemia or mixed dyslipidemia and in the U.S. for the treatment of adults with HeFH or clinical ASCVD as an adjunct to diet and maximally tolerated dose of statin, the current standard of care for patients with hypercholesterolemia includes the use of dietary changes, lifestyle modification and the use of pharmacologic therapy. Front line therapy consists of HMG-CoA reductase inhibitors, commonly known as statins, which block production of cholesterol by the liver and increase clearance of LDL-C from the bloodstream. Several anti-PCSK9 antibodies have also been approved for the treatment of hypercholesterolemia in the U.S. and Europe. Other PCSK9-targeted approaches and other cholesterol lowering agents are in development at a number of companies.
We believe that the following approved drugs and, if approved, drug candidates, could compete with Leqvio:
| | | | | | | | | | | | | | |
Drug | Company | Drug Description | Phase | Administration/Dosing |
Repatha | Amgen | Anti-PSCK9 mAb | Approved | SC |
Praluent | Sanofi | Anti-PSCK9 mAb | Approved | SC |
Vascepa | Amarin Corporation | Omega-3 lipid proven to reduce LDL-C and CV Risk | Approved | Oral |
NEXLETOL (Bempedoic Acid) | Esperion Therapeutics, Inc. | Oral fatty acid and cholesterol synthesis dual inhibitor | Approved | Oral |
Evkeeza (evinacumab) | Regeneron | Anti-ANGPTL3 mAb for hypercholesterolemia | Approved in HoFH | SC |
Lerodalcibep (LIB-003) | LIB Therapeutics | Recombinant protein therapeutic | Phase 3 | SC |
MK-0616 | Merck | Small molecule PCSK9 inhibitor | Phase 2 | Oral |
ARO-ANG3 | Arrowhead | siRNA targeting ANGPTL3 | Phase 2 | SC |
VERVE-101 | Verve Therapeutics | CRISPR/CAS9 targeting PCSK9 | Phase 1 | One-time IV infusion |
Hemophilia. The global market for treatments of hemophilia, and bleeding disorders is valued at more than $10.0 billion. Products on the market include:including: Factor VIII replacement products;products, Factor IX replacement products;products, factor replacement products with extended half-lives, and most recently a bispecific antibody mimickingthat mimicks Factor VIII. For the treatment of personsindividuals with inhibitors, there is an approved Factor VIIa replacement product and an activated prothrombin complex concentrate, as well as a bispecific antibody mimickingthat mimicks Factor VIII. In addition, there are new, innovative molecules are currently in development which may offer new treatments for people withtreatment of hemophilia A and B, both with and without inhibitors. A number of companies are also actively developing gene therapy products that use virus-like particles to deliver a functional section of a particular gene into the liver cells of a person with hemophilia.
We believe that the followingthese approved drugsproducts and, if approved, drugproduct candidates, could compete with fitusiran, if fitusiranassuming it receives regulatory approval along with additional approved drugs and drug candidates not listed below:
| | | | | | | | | | | |
Drug (Company)for the treatment of hemophilia. | Drug Description | Phase | Administration |
Hemophilia A |
Advate (Takeda), Adynovate (Takeda), Kogenate (Bayer), Kovaltry (Bayer), Novoeight (Novo Nordisk), Xyntha (Pfizer), Nuwiq (Octapharma), Eloctate (Sanofi) | Recombinant FVIII factor products | Approved | IV |
Valoctocogene roxaparvovec (BioMarin) | Gene therapy | Approved in EU; PDUFA Mar. 2023 | IV - Single Administration |
HEMLIBRA (Roche) | Bispecific antibody mimetic of FVIII | Approved | SC - Monthly |
giroctocogene fitelparvovec (Pfizer & Sangamo Therapeutics) | Recombinant AAV2/6 Human Factor VIII Gene Therapy | Phase 3 | IV – Single Administration |
RG6357 (Roche & Spark Therapeutics) | Gene therapy | Phase 2 | IV – Single Administration |
Hemophilia B |
Rixubis (Takeda), Rebinyn (Novo Nordisk), BeneFIX (Pfizer), Alprolix (Sanofi), Idelvion (CSL Behring) | Recombinant FIX factor products | Approved | IV |
Etranacogene dezaparvovec (uniQure/CSL Behring) | rAAV5 FIX gene therapy | Approved | IV - Single Administration |
Fidanacogene elaparvovec (Spark Therapeutics) | Spark200 AAV FIX gene therapy | Phase 3 | IV - Single Administration |
Inhibitor Patients |
Emicizumab HEMLIBRA,
ACE-910 (Roche) | Bispecific antibody mimetic of FVIII | Approved | SC - Monthly |
Feiba (Takeda) | Bypassing agent | Approved | IV |
NovoSeven (Novo Nordisk) | Bypassing agent | Approved | IV |
Marstacimab (Pfizer) | Anti-TFPI antibody | Phase 3 | SC - Weekly |
Hemophilia A and B | | | |
Concizumab, anti-TFPI (Novo Nordisk) | Anti-TFPI antibody | Phase 3 | SC |
Marstacimab (Pfizer) | Anti-TFPI antibody | Phase 3 | SC - Weekly |
Other Competition
Finally, for many of the diseases that we are the subject oftargeting with our product candidates in early-stage clinical development, pre-clinical development and discovery RNAi therapeutic programs, including alpha-1 liver disease, hepatitis B, hypertension, Alzheimer’s disease, CAA, NASH, and type 2 diabetes, there are already drugs currently on the market or in various stages of clinical development. However, notwithstandingNotwithstanding the availability of existing drugs or drugthese products and product candidates, we believe there currently existsremains sufficient unmet medical need to warrant the advancement of our investigational RNAi therapeutic programs.
Regulatory Matters
U.S. Regulatory Considerations
The research, testing, manufacture and marketing of drug products and their delivery systems are extensively regulated in the U.S. and the rest of the world. In the U.S., drugs are subject to rigorous regulation by the FDA. The Federal Food, Drug, and Cosmetic Act, or FDCA, and other federal and state statutes and regulations govern, among other things, the research, development, testing, approval, manufacture, storage, record keeping, reporting, labeling, marketing and distribution of drug products. Failure to comply with the applicable regulatory requirements may subject a company to a variety of administrative or judicially-imposed sanctions and the inability to obtain or maintain required approvals to test or market drug products. These sanctions could include, among other things, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, clinical holds, injunctions, fines, civil penalties or criminal prosecution.
The steps ordinarily required before a new drug product may be marketed in the U.S. include nonclinical laboratory tests, animal tests and formulation studies, the submission to the FDA of an IND, which must become effective prior to commencement of clinical testing in the U.S., approval by an institutional review board, or IRB, at each clinical site before each trial may be initiated, completion of adequate and well-controlled clinical trials to establish that the drug product is safe and effective for the indication and other conditions of use for which FDA approval is sought, submission to the FDA of an NDA (or supplemental NDA for approved products), acceptance of the NDA for review by the FDA, and FDA review and approval of the NDA. Satisfaction of the FDA'sFDA’s pre-market approval requirements typically takes several years, but may vary
substantially depending upon the complexity of the product and the nature of the disease. Government regulation may delay, limit or prevent marketing of potential productsproduct candidates for a considerable period of time and impose costly procedures on a company’s activities. Success in early-stage clinical trials does not necessarily assure success in later-stage clinical trials. Data obtained from clinical activities, including but not limited to the data derived from our clinical trials for drugproduct candidates, are not always conclusive and may be subject to alternative interpretations that could delay, limit or even prevent regulatory approval. Even if a product receives regulatory approval, later discovery of previously unknown problems with a product, including new safety risks from commercial use, clinical or nonclinical data or manufacturing issues, may result in restrictions on the product or even complete withdrawal of the product from the market.
Nonclinical Tests and Clinical Trials
Nonclinical tests include laboratory evaluation of product chemistry and formulation, as well as animal testing to assess the potential safety and efficacy of the product.product candidate. The conduct of the nonclinical tests and formulation of compounds for testing must comply with applicable federal regulations and requirements, including in some cases the FDA’s good laboratory practice requirements and the Animal Welfare Act. The results of nonclinical testing are submitted to the FDA as part of an IND, together with chemistry, manufacturing and controls, or CMC, information, analytical and stability data, a proposed clinical trial protocol and other information. Clinical testing in humans may not commence until an IND is in effect.
An IND becomes effective 30 days after receipt by the FDA unless the FDA notifies the sponsor that the proposed investigation(s) are subject to a clinical hold. If the FDA imposes a clinical hold, or partial clinical hold, the FDA’s concerns must be resolved prior to the commencement of clinical trials, or the FDA can enforce other changes to the clinical development program or clinical trial(s). The IND review process can result in substantial delay and expense. We, an IRB, or the FDA may, at any time, suspend, terminate, significantly modify, restrict or impose a clinical hold on ongoing clinical trials. If the FDA imposes a clinical hold, clinical trials cannot commence or recommence without FDA authorization, and then the clinical trials can commence or recommence only under the terms authorized by the FDA.
Clinical trials involve the administration of an investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical studies are conducted under protocols detailing, among other things, the objectives of the trial and the safety and effectiveness criteria to be evaluated. Each protocol involving testing on human subjects in the U.S. must be submitted to the FDA as part of the IND. In addition, clinical trials must be conducted in compliance with federal regulations and requirements, commonly referred to as good clinical practice, or GCP, to assure data integrity and protect the rights, safety and well-being of trial participants. Among other things, GCP requires that all research subjects provide their informed consent prior to participating in any clinical study, and that a properly constituted IRB for each institution participating in the clinical trial review and approve the plan for any clinical trial before it commences at that institution and conduct continuing review throughout the trial. The IRB must review and approve, among other things, the study protocol and informed consent information to be provided to study subjects.
Clinical trials to support NDAs are typically conducted in three sequential phases, which may overlap or be combined.
•In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested primarily to assess safety, tolerability, pharmacokinetics, pharmacological actions and metabolism associated with increasing doses.
•Phase 2 usually involves trials in a limited patient population, to assess the optimum dosage and dose regimen, identify possible adverse effects and safety risks, and provide preliminary support for the efficacy of the drug in the indication being studied.
•Phase 3 clinical trials further evaluate the drug’s clinical efficacy, side effects and safety in an expanded patient population, typically at geographically dispersed clinical trial sites, to establish the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug.
Phase 1, Phase 2 or Phase 3 testing of any drug candidates may not be completed successfully within any specified time period, if at all. The FDA closely monitors the progress of each of the three phases of clinical trials that are conducted in the U.S. The FDA may, at its discretion, re-evaluate, alter, suspend or terminate the testing based upon the data accumulated to that point and the FDA’s assessment of the risk/benefit ratio to the subject participating in the study. An IRB or a clinical trial sponsor may also modify, suspend or terminate clinical trials, or parts of clinical trials, at any time for various reasons, including a finding that the subjects or patients are being exposed to an unacceptable health risk. The FDA can also request or require that additional clinical trials, nonclinical evaluations or changes in the manufacturing process be conducted as a condition to product approval. Finally, sponsors are required to publicly disseminate information about certain ongoing and completed clinical trials on ClinicalTrials.gov, a government website administered by the National Institutes of Health, or NIH.
New Drug Applications
We believe that any RNAi product candidate we develop, whether for the treatment of ATTR amyloidosis, AHP, PH1, hypercholesterolemia or the various indications targeted in our clinical development or nonclinical discovery programs, will be regulated by the FDA as a new drug that is not considered to be a biologic, and thus will require an NDA.NDA rather than a biologics license agreement, or BLA. FDA approval of an
NDA is required before commercial distribution of a new drug may begin in the U.S. An NDA must include the results of extensive nonclinical, clinical and other testing, as described above, a compilation of data relating to the product’s pharmacology, CMC information, proposed labeling and other information. In addition, an NDA for a new active ingredient, new indication, new dosage form, new dosing regimen, or new route of administration typically must contain data assessing the safety and effectiveness for the claimed indication in all relevant pediatric subpopulations, although deferrals or full or partial waivers may be available in some circumstances.
The cost of preparing and submitting an NDA is substantial. Under the PDUFA, as amended, each NDA must be accompanied by an application fee. For fiscal year 2023,2024, the application fee for each NDA requiring clinical data is approximately $3.2$4.0 million. The PDUFA also imposes an annual program fee for each approved prescription drug product, which has been set at approximately $394,000$417,000 for fiscal year 2023.2024. The FDA adjusts the PDUFA user fees on an annual basis. Fee waivers, or reductions and exceptions are available in certain circumstances. Additionally, no userapplication fees are assessed on NDAs for products designated as orphan drugs, unless the NDA also includes a non-orphan indication. The FDA conducts a preliminary review of all NDAs within the first 60 days after submission before accepting them for filing to determine whether they are sufficiently complete to permit substantive review. During that time, the FDA may request additional information rather thanbefore deciding whether to accept an NDA for filing. If the FDA determines that an NDA is not sufficiently complete to permit substantive review, it will issue a refuse to file determination and the NDA will not be substantively reviewed by the FDA. If the submission is accepted for filing, the FDA begins an in-depth review of the NDA. The FDA has agreed to specified performance goals regarding the timing of the completion of its review of NDAs, although the goals are not binding and the FDA does not always meet these goals. The review process is often significantly extended by the FDA'sFDA’s requests for additional information or clarification regarding information provided in the submission. For novel drug products or drug products that present difficult questions of safety or efficacy, the FDA may refer the application to an advisory committee, which is typically in the form of a panel that includes independent clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA may waive the review ofby an advisory committee and is not bound by the recommendation of an advisory committee, but it often follows such recommendations. The FDA normally conducts a pre-approval inspection to gain assurance that the manufacturing facility or facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality purity and stability,purity, and are in compliance with regulations governing current good manufacturing practice, or cGMP, requirements. In addition, the FDA often will conduct a bioresearch monitoring inspection of select clinical trial sites involved in conducting pivotal studies to assure data integrity and compliance with applicable GCP requirements, and could also conduct GCP inspections of the sponsor.
If the FDA'sFDA’s evaluation of an NDA and the various inspections are favorable, the FDA may issue an approval letter, which authorizes commercial marketing of the drug with specific prescribing information for a specific indication. The approved indication may be narrower than what was proposed by the applicant or for a narrower patient population than the population studied in clinical trials. As a condition of NDA approval, the FDA may require post-approval evaluations, sometimes referred to as Phase 4 trials, or other surveillance to monitor the drug’s safety or effectiveness and may impose other conditions, including labeling restrictions, such as a Boxed Warning, and/or distribution and use restrictions through a Risk Evaluation and Mitigation Strategy, or REMS, all of which can materially affect the potential market and profitability of the drug.product. Once granted, product approvals may be further limited or withdrawn if compliance with regulatory standardsrequirements is not maintained or safety or other problems are identified following initial marketing.
Post-Approval Regulation
Once an NDA is approved, a product will be subject to certain post-approval requirements, including requirements for manufacturing establishment registration and product listing, AEadverse event reporting, submission of other periodic reports, field
alerts, recordkeeping, product sampling and distribution. Additionally, the FDA strictly regulates the promotional claims that may be made about prescription drug products and biologics. In particular, the FDA generally prohibits pharmaceutical companies from promoting their drugs or biologics for uses that are not approved by the FDA as reflected in the product’s approved labeling, and requires that important safety information be presented to balance information provided on a drug’s effectiveness. In addition, the FDA requires substantiation of any safety or effectiveness claims, including claims that one product is superior in terms of safety or effectiveness to another. Superiority claims generally must be supported by head-to-head clinical trials.another product. To the extent that market acceptance of our products depends on their superiority over existing therapies, any restriction on our ability to advertise or otherwise promote claims of superiority, or requirements to conduct additional expensive clinical trials to provide proof of such claims, could negatively affect the sales of our products or our costs. We must also notify the FDA of any change in an approved product beyond variations in the approved application. Certain changes to the product, its labeling or its manufacturing require prior FDA approval and may require the conduct of further clinical investigations to support the change. Such approvals may be expensive and time-consuming and, if not approved, the FDA will not allow the product to be commercially distributed as modified.
If the FDA’s evaluation of an NDA submission or GCP inspections or inspection of the manufacturing facilities for the product are not favorable or cannot be completed due restrictions (including, for example, restrictions related to COVID-19 related restrictions,COVID-19), the FDA may defer action on an application or refuse to approve the NDA and issue a complete response letter. The complete response letter describes the deficiencies that the FDA has identified in an application and may recommend actions that the applicant can take to address the deficiencies. Such actions may include, among other things, conducting additional safety or efficacy studies. Even with the
completion of this additional testing or the submission of additional requested information, however, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. With limited exceptions, the FDA may withhold approval of an NDA regardless of prior advice it may have provided or commitments it may have made to the sponsor.
Some of our product candidates may need to be administered using specialized drug delivery systems that are considered to be medical devices. We may rely on drug delivery systems that are already approved or cleared to deliver drugs like ours to similar physiological sites or, in some instances, we may need to modify the design or labeling of the legally available device for delivery of our product candidate. The FDA may regulate our product candidate when used with a specialized drug delivery system as a combination product, which could permit the combination to be approved through a single application, such as an NDA. In some instances, the FDA could require separate, additional approvals or clearances for the modified device. If the FDA does require separate, additional approvals or clearances for the modified device, the FDA could require either a premarket approval application, or PMA, a 510(k) clearance, or a de novo classification, depending on the risk classification of the modified device and the availability of legally marketed predicate devices. Approval of PMAs areis required for class III medical devices, which are higher risk devices, including life-sustaining and life-supporting devices and certain implantable devices, for which insufficient information exists to provide reasonable assurance of the safety and effectiveness of the device through general controls and special controls. PMAs must contain sufficient valid scientific evidence to assure that the device is safe and effective for its intended use. Clearance under section 510(k) of the FDCA is required for most class II medical devices, which are moderate risk devices for which special controls are necessary to provide reasonable assurance of safety and effectiveness. A 510(k) submission demonstrates to the FDA that the device is substantially equivalent (i.e., at least as safe and effective based on the intended use and technological characteristics) as a legally marketed predicate device that is not subject to PMA requirements. Substantial equivalence means that device has the same intended use as the predicate device and either (a) the same technological characteristics as the predicate or (b) different technological characteristics that do not raise new questions of safety and effectiveness and data demonstrates the device is as safe and effective as the predicate device. If no such legally marketed predicate device exists, but the applicant believes the device should not be automatically classified into class III,presents low or moderate risk, the applicant can submit an application for de novo classification, which is a request to FDA to classify the device into class I or II based on certain general and, if applicable, special controls that are necessary to provide reasonable assurance of safety and effectiveness of the device. In addition, if the FDA requires a separate, additional approval or clearance for a delivery device to be used with our products, and the delivery device is owned by another company, we will need that company’s cooperation to implement the necessary changes to the device and to obtain any additional approvals or clearances, described above. Obtaining such additional approvals or clearances, and cooperation of other companies, when necessary, could significantly delay, and increase the cost of obtaining marketing approval, which in turn could reduce the commercial viability of a product candidate. To the extent that we rely on previously unapproved drug delivery systems, we may be subject to additional testing and approval requirements from the FDA above and beyond those described above. For any product regulated as a combination product, we will be subject to post-approval requirements for the medical device constituent, such as reporting of certain device-related AEs or device malfunctions and device quality system requirements, in addition to the post-approval requirements for NDA approved products described above. Likewise, if we market a delivery system under a separate medical device clearance or approval, we will be subject to medical device post-market requirements, including requirements for manufacturing establishment registration and device listing, AE and malfunction reporting, reporting of corrections or removals, quality system requirements, and recordkeeping.
Abbreviated Applications and 505(b)(2) Applications
Once an NDA is approved, the product covered thereby becomes a listed drug that can, in turn, be relied upon by potential competitors in support of approval of an abbreviated NDA, or ANDA, or a 505(b)(2) application. An ANDA generally provides an abbreviated approval pathway for a drug product that has the same active ingredients in the same strength, dosage form and
route of administration as the listed drug andproduct, has been shown through appropriate testing (unless waived) to be bioequivalent to the listed drug.product, and has the same labeling as the listed product (subject to certain exceptions). Drugs approved in this way are commonly referred to as generic equivalents to the listed drugproduct and can often be substituted by pharmacists under prescriptions written for the original listed drug. A 505(b)(2) application is a type of NDA that relies, in part, upon data the applicant does not own and to which it does not have a right of reference. Such applications often are submitted for changes to previously approved drug products.
The approval of ANDAs and 505(b)(2) applications can be delayed by patents and non-patent exclusivity covering the listed drug. Federal law provides for a period of three years of exclusivity following approval of a listed drugproduct that contains a previously approved active ingredientmoiety (the molecule or ion responsible for the action of the drug substance) if the FDA determines that new clinical investigations, other than bioavailability studies, were conducted or sponsored by the applicant and are essential to the approval of the application. This three-year exclusivity covers only the conditions of approval for which the new clinical investigations were essential, such as a new dosage form or indication. Accordingly, three-year exclusivity generally protects changes to a previously approved drug product that require clinical testing for approval and, as a general matter, does not prohibit the FDA from approving ANDAs or 505(b)(2) applications for generic versions of the drug product without such changes.
Federal law also provides a five-year period of NCE exclusivity following approval of a drug that contains an NCE. An NCE is a drug that contains an active moiety (the molecule or ion responsible for the action of the drug substance) that has never previously been approved by the FDA.FDA in an NDA. If a listed drug has NCE exclusivity, ANDAs and 505(b)(2) applications referencing the listed drug cannot be submitted to the FDA for five years following the approval of the listed drug unless the application contains a certification challenging a listed patent, i.e., a paragraph IV certification (discussed further below), in which case the ANDA or 505(b)(2) application may be submitted four years following approval of the listed drug. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the nonclinical studies and clinical trials necessary to demonstrate safety and effectiveness.
Additionally, applicants submitting an ANDA or 505(b)(2) application referencing a listed drug generally are required to make a certification with respect to each patent for the listed drug that is listed in the FDA’s publication Approved Drug Products with Therapeutic Equivalence Evaluations, commonly referred to as the Orange Book. If the applicant is not seeking
approval of a use claimed byFor a method-of-use patent, however, the applicant can submit a statement to that effectit is not seeking approval of a use claimed by the patent instead of making thea certification. These certifications (and statements) affect when the FDA can approve the ANDA or 505(b)(2) application. If the ANDA or 505(b)(2) applicant certifies that it does not intend to market its product before a listed patent expires (i.e., a paragraph III certification), then the FDA will not grant effective approval of the ANDA or 505(b)(2) application until the relevant patent expires. If the ANDA or 505(b)(2) applicant certifies that a listed patent is invalid, unenforceable, or will not be infringed by its proposed product, and thus that it is seeking approval prior to patent expiration (i.e., a paragraph IV certification), and certain other steps are taken, then approval of the ANDA or 505(b)(2) application will be stayed (i.e., FDA will not approve the application) until 30 months have passed or patent disputes are resolved. Specifically, under the process set forth by the statute, the ANDA or 505(b)(2) applicant must provide notice of its patent challenge to the NDAlisted product sponsor and the patent holder within certain time limits. If the patent holder then initiates a suit for patent infringement is initiated within 45 days of receipt of the notice, the FDA cannot grant effective approval of the ANDA or 505(b)(2) application until either 30 months have passed (which may be extended or shortened in certain cases) or there has been a court decision or settlement order holding or stating that the patents in question are invalid, unenforceable or not infringed. If the court decision or settlement order holds or states that the patents in question are valid, enforceable, and would be infringed, however, then the ANDA or 505(b)(2) application may not be approved until such patents expire. If the patent holder does not initiate a suit for patent infringement within the 45-day time limit described above, the ANDA or 505(b)(2) application may be approved immediately upon successful completion of FDA review, unless blocked by another listed patent or regulatory exclusivity period.
Orphan Drug Designation
Under the Orphan Drug Act, as amended, the FDA may grant Orphan Drug Designation to a drug intended to treat a rare disease or condition, which is a disease or condition that affects fewer than 200,000 individuals in the U.S. or affects more than 200,000 individuals and for which there is no reasonable expectation of recovering drug development costs in the U.S. from sales in the U.S. Orphan Drug Designation must be requested before submitting an NDA or an sNDA for the orphan indication. After the FDA grants Orphan Drug Designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. We intend to request Orphan Drug Designation for certain of our product candidates, if applicable. For example, the FDA granted Orphan Drug Designation for patisiran and vutrisiran as therapeutic approaches for the treatment of ATTR amyloidosis, givosiran as a therapeutic approach for AHP, lumasiran as a therapeutic approach for PH1, fitusiran as a therapeutic approach for hemophilia A and B, and inclisiran as a therapeutic approach for HoFH.
If a product that has Orphan Drug Designation subsequently receives the first FDA approval for that product for the disease for which it has such designation, the product is entitled to Orphan Drug Exclusivity, which means that the FDA may not approve for seven years any other applications, including a full NDA, from another sponsor to market the “same drug” for the same indication, except in limited circumstances. For purposes of small molecule drugs, the FDA defines “same drug” as a drug that contains the same active moiety and is intended for the same use as the previously approved orphan drug. For purposes of
large molecule drugs, the FDA defines “same drug” as a drug that contains the same principal molecular structural features, but not necessarily all of the same structural features, and is intended for the same use as the previously approved drug. Notwithstanding the above definitions, a drug that is "clinically superior"“clinically superior” to an orphan drug will not be considered the “same drug” and thus will not be blocked by Orphan Drug Exclusivity. To demonstrate a drug is “clinically superior” to the previously approved orphan drug, a sponsor must show that the drug provides a significant therapeutic advantage over and above the previously already approved drugproduct in terms of greater efficacy, greater safety, or by providing a major contribution to patient care.
AUnder the FDA’s regulations, a designated orphan drug may not receive Orphan Drug Exclusivity for a use that is broader than the indication for which it received Orphan Drug Designation and regulatory approval. However, a 2021 decision by the U.S. Court of Appeals for the Eleventh Circuit in Catalyst Pharmaceuticals, Inc. v. Becerra adopted a broader interpretation of the scope of Orphan Drug Exclusivity, holding that Orphan Drug Exclusivity prevents the FDA from approving another marketing application for the same drug for the same orphan-designated disease or condition for a period of seven years. Although the FDA announced in January 2023 that it will not apply the Catalyst decision beyond the facts at issue in that case, Catalyst could serve as a precedent for future challenges to FDA’s orphan drug-related decisions. Legislation has been introduced, but has not been passed, that would codify the scope of Orphan Drug Exclusivity set forth in the FDA’s regulations, rather than the interpretation adopted by the Eleventh Circuit in Catalyst.
In addition, Orphan Drug Exclusivity may be lost if the FDA later determines that the Orphan Drug Designation request was materially defective or if the manufacturer is unable to assureensure the availability of sufficient quantities of the drug to meet the needs of patients with the rare disease or condition, or if the manufacturer chooses to provide consent to approval of other applications.
Pediatric Study Plans
The FDCA requires that a sponsor who is planning to submit a marketing application for a drug or biological product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan, or PSP, within sixty days of an end-of-phase 2 meeting or as may be agreed between the sponsor and the FDA. Drugs with Orphan Drug Designation are exempt from these requirements to the extent that the indication being sought under the marketing application is within the scope of the designated orphan use. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including, to the extent practicable, study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information.information, as well as other information specified in the FDA’s regulations. The FDA and the sponsor must reach agreement on the initial PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the PSP need to be considered based on data collected from nonclinical studies, early phase clinical trials, and/or other clinical development programs. However, if, under certain situations, the agreed initial PSP included nonclinical and/or pediatric clinical studies that were expected to have been completed before submission of the NDA, BLA, or supplement, failure of the sponsor to complete these agreed studies in a timely manner may result in a refusal to file.
pediatric studies is requested. A final decision about granting or denying such requests is made by the review division at the time of approval of the marketing application. Fast Track Program
The FDA has a Fast Track program that is intended to facilitate development and expedite the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition.condition, or the product has been designated as a qualified infectious disease product. Fast Track designation applies to the product and the specific indication for which it is being studied. The sponsor of a new drug or biological product may request the FDA to designate the drug or biologic as a Fast Track product at any time during the clinical development of the product, but ideally no later than the pre-NDA or pre–biologics license applicationBLA meeting because many of the features of Fast Track designation will not apply after that time. Fast Track designation provides opportunities for frequent interactions with FDA to expedite drug development and review as well as the opportunity for rolling review of the NDA. We intend to request Fast Track designation for certain of our product candidates, if applicable. For example, the FDA granted Fast Track designation to patisiran for the treatment of hATTR amyloidosis, which was approved in August 2018 for the treatment of the polyneuropathy of hATTR amyloidosis with polyneuropathy in adults, and also granted Fast Track designation to vutrisiran for the treatment of the polyneuropathy of hATTR amyloidosis with polyneuropathy, which was approved in June 2022.
Any drug or biological product that receives a Fast Track designation may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Fast track designation does not change the standards for approval and may not necessarily expedite the development or approval process.
Priority Review
A drug or biological product is eligible for priority review if it treats a serious condition and, if approved, would provide a significant improvement in the safety or effectiveness of treatment, diagnosis or prevention of a disease compared to available therapies. Priority review is also available for certain supplements that propose labeling changes pursuant to a pediatric study report, qualified infectious disease products, or any application or supplement for a drug submitted with a priority review voucher. The FDA’s goal for taking action on an application with a priority review designation is six months from the date of receipt, instead of ten months from the date of receipt, except that two months are added to these time periods for drugs that contain a new molecular entity. Additionally,
Priority review does not change the standards for approval and may not necessarily expedite the development or approval process.
Accelerated Approval
The FDA may approve a drug or biological product may be eligible for accelerated approval if it is intended to treat a serious or life-threatening disease or condition and the product would provide meaningful therapeutic benefit over existing treatments. Under accelerated approval,based on a product may be approved on the basis of adequate and well-controlled clinical studies establishingdetermination that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, and that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefits. Asbenefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. The FDA generally reserves the use of accelerated approvals for situations in which the product candidate at issue provides a condition of approval, the FDA may require that a sponsor of a drug or biological product receivingmeaningful therapeutic benefit over existing treatments. Products granted accelerated approval perform adequate and well-controlledare subject to certain post-marketing clinicalrequirements, which typically include a requirement to conduct one or more post-approval studies to verifyconfirm the predicted clinical benefit and, underof the product, which must be completed with due diligence. Under the Food and Drug Omnibus Reform Act of 2022, or FDORA, the FDA is now permitted to require, as appropriate, that such trials be underway prior to approval or within a specific time period after the date of approval for a product granted accelerated approval. Under FDORA, the FDA has increased authority for expedited procedures to withdraw approval of a drug or indication approved under accelerated approval if, for example, the confirmatory trial fails to verify the predicted clinical benefit of the product or the sponsor disseminates false or misleading promotional materials relating to the relevant product. FDORA also added the failure to conduct post-approval studies with due diligence or to submit timely progress reports on such studies to the list of prohibited acts under the FD&C Act, which means that any such failures, whether they result from our actions or the actions of third parties, could provide the basis for enforcement actions to be brought against us, which may be costly to defend or we may be unsuccessful in our defense. In addition, the FDA requires as a condition for accelerated approval advance submission of promotional materials prior to use, which could limit or delay the commercial launch of the product. Fast Track designation, priority review and accelerated
Accelerated approval dodoes not change the standards for approval butand may not necessarily expedite the development or approval process.
Breakthrough Therapy Designation
A drug or biological product can be designated as a breakthrough therapy if it is intended to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that it may demonstrate substantial improvement over existingavailable therapies on one or more clinically significant endpoints. A sponsor may request that a drug or biological product be designated as a breakthrough therapy at any time during the clinical development of the product and ideally before initiation of the pivotal clinical trial intended to serve as the primary basis for demonstration of efficacy to obtain the full benefits of the designation.end-of-Phase 2 meeting. If so designated, the FDA shall act to expedite the development and review of the product’s marketing application, including by meeting with the sponsor throughout the product’s development, providing timely advice to the sponsor to ensure that the development program is as efficient as practicable, involving senior managers and experienced review staff in a cross-disciplinary review, assigning a cross-disciplinary project lead for the FDA review team to facilitate an efficient review of the development program and to serve as a scientific liaison between the review team and the sponsor, taking steps to ensure that the design of the clinical trials is as efficient as practicable, and allowing a rolling review of the marketing application. The FDA granted breakthrough therapy designation for patisiran, approved in August 2018, givosiran, approved in November 2019, as well as lumasiran, approved in November 2020. We intend to request breakthrough therapy designation for certain of our other product candidates, ifas applicable.
Rare Pediatric Disease Designation and Priority Review Voucher
In addition, theThe FDCA provides a rare pediatric disease priority review voucher, or PRV, program. Theto sponsors under a program that is intended to incentivize the development of new drug and biological products for the prevention and treatment of “rare pediatric diseases,diseases.” thatA rare pediatric disease is any disease that is a rare disease and is serious or life-threatening with the serious or life-threatening manifestations primarily affecting individuals from birth to 18. Under this program, the sponsor of an application for a rare pediatric disease drug may be eligible to obtain a voucher that can be used to obtain a priority review for a subsequent human drug application. The FDA recommends that a sponsor request rare pediatric disease designation before submissionfiling of the rare pediatric disease product application. The rare pediatric disease designation does not guarantee that the sponsor will receive a
PRV. The FDA will award a PRV upon approval of the marketing application if the sponsor requests such a voucher in theirits marketing application and if the application meets the eligibility criteria. If awarded, the PRV may be transferred unlimited times.
times before the PRV is used. The rare pediatric disease PRV program was initially created in 2012, and Congress has extended the PRV program through September 30, 2024, with the potential for PRVs to be granted through September 30, 2026.2026 for a drug that receives rare pediatric disease designation by September 30, 2024. The FDA awarded a rare pediatric disease PRV to us upon approval of the NDA for lumasiran in November 2020.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we obtain regulatory approval. In the U.S. and markets in other countries, sales of any products for which we may receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payors. Third-party payors include government healthcare programs, managed care providers, private health insurers and other organizations. The coverage and reimbursement status of any drug products for which we obtain regulatory approval may vary significant across these third-party payors. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drugs for a particular disease or condition. Third-party payors may provide coverage, but place stringent limitations on such coverage, such as requiring alternative treatments to be tried first. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product. Third-party payors may limitEligibility for coverage to specificdoes not imply that any drug products on an approved list,will be reimbursed in all cases or formulary, which might not include all of the FDA-approved drugs forat a rate that covers a manufacturer’s costs, including research, development, manufacture, sale and distribution, or that covers a particular indication.provider’s cost of acquiring the drug. Third-party payors may provide coverage, but place stringent limitations on such coverage, such as requiring alternative treatments to be tried first. These third-party payors are increasingly challengingscrutinizing the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. In addition, significant uncertainty exists asFactors payors may consider in determining reimbursement include, among others, the extent to which the reimbursement statusproduct and/or the use of newly approved healthcare products. Wethe product is:
•a covered benefit under a health plan;
•safe, effective and medically necessary;
•appropriate for the specific patient;
•clinically superior or therapeutically advantageous compared to other products;
•cost-effective; and
•neither experimental nor investigational.
A manufacturer may need to conduct expensiveadditional research, including healthcare economic studies, in order to demonstrate the medical necessityclinical value and cost-effectiveness of our products, in addition to incurringseparate and apart from the costsstudies required to obtain FDA approvals. Our productProduct candidates may not be considered medically reasonable or necessary or cost-effective. Increasingly, the third-party payors are requiring that drug companies provide them with predetermined discounts from list prices, and are seeking to reduce the prices charged or the amounts reimbursed for drug products. Even if a drug product is covered, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved.provided. Lack of adequate third-party reimbursement may mean we are not able to maintainprevent price levels sufficient to sell current or future product(s) on a competitive basis or realize an appropriate return on our investment in product development. Factors payors consider in determining reimbursement
Some of the drugs we market need to be administered under the supervision of a physician or other healthcare professional on an outpatient basis, including ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO. Under currently applicable U.S. law, certain drugs that are based on whethernot usually self-administered (including injectable drugs) may be eligible for coverage under the product is:Medicare Part B program if:
•they are incident to a covered benefit under its health plan;physician’s services;
•safe, effectivethey are reasonable and medically necessary;
•appropriatenecessary for the specific patient;
•cost-effective;diagnosis or treatment of the illness or injury for which they are administered according to accepted standards of medical practice; and
•neither experimental nor investigational.they have been approved by the FDA and meet other statutory requirements.
Federal, state and local governments in the U.S. and foreign governments have established and continue to consider legislationpolicies to limit the growth of healthcare costs, including the cost of prescription drugs. Specifically, there have been several recent U.S. Congressional inquiries into prescription drug pricing, and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. Future legislation could limit payments
At the federal level, for pharmaceuticals such as the drug candidates that we are developing. Likewise, the Biden administration has indicated that lowering prescription drug prices is a priority, but we do not yet know what steps the administration will take or whether such steps will be successful.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, the emphasis on managed careexample, in the U.S. has increased and we expect will continue to exert downward pressure on pharmaceutical pricing. Coverage policies, third-party reimbursement rates and pharmaceutical pricing regulations may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
In August 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law. Key provisions of the IRA include the following, among others:
•The IRA includes several provisions that will impact our businessrequires manufacturers to varying degrees, including provisions that create a $2,000 out-of-pocket cappay rebates for Medicare Part D beneficiaries, impose new manufacturer financial liability on all drugs in Medicare Part D, allow the U.S. government to negotiate Medicare Part B and Part D pricingdrugs whose price increases exceed inflation;
•The IRA eliminates the “donut hole” under Medicare Part D beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and requiring manufacturers to subsidize, through a newly established manufacturer discount program, 10% of Part D enrollees’ prescription costs for certain high-costbrand drugs below the out-of-pocket maximum and biologics without generic or biosimilar competition, require companies to pay rebates to Medicare for drug prices that increase faster than inflation, and delay20% once the out-of-pocket maximum has been reached.
•The IRA delays the rebate rule that would require pass through of pharmacy benefit manager rebates to beneficiaries.
The Patient Protection and Affordable Care Act, also referred to as the Affordable Care Act, or the ACA, enacted in 2010, includes measures that have significantly changed the way healthcare is financed by both governmental and private insurers. Among the provisions of the ACA of greatest importance to the pharmaceutical industry are the following:
•The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of the Department of Health and Human Services as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. The ACA increased pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate on most branded prescription drugs and biologic products to 23.1% of average manufacturer price, or AMP, and added a new rebate calculation for “line extensions” (i.e., new formulations, such as extended release formulations) of solid oral
dosage forms of branded products, and modified the statutory definition of AMP. In addition, the ACA provides for the public availability of retail survey prices and certain weighted average AMPs under the Medicaid program. The implementation of this requirement byIRA directs allows the Centers for Medicare and Medicaid Services, or CMS, may also provideto engage in price-capped negotiation for the public availability of pharmacy acquisition of cost data, which could negatively impact our sales.
•In order for a drug product to receive federal reimbursement under thecertain Medicare Part B and Medicaid programs orPart D products. Specifically, the IRA’s Price Negotiation Program applies to be sold directly to U.S. government agencies, the manufacturer must offer its innovator products on the Federal Supply Schedule for purchase at prices compliant with statutory and regulatory requirements and extend discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer. The ACA expanded the types of entities eligible to receive discounted 340B pricing, although, under the current state of the law, with the exception of children’s hospitals, these entities will not be eligible to receive discounted 340B pricing on orphan drugs. In addition, because 340B drug pricing is determined based on AMP and Medicaid rebate data, the revisions to the Medicaid rebate formula and AMP definition described above could cause the required 340B discount to increase.
•The ACA imposed a requirement on manufacturers of brandedhigh-expenditure single-source drugs and biologic products to provide a 50% discount off the negotiated price of brandedbiologics that have been approved for at least seven or 11 years, respectively, among other negotiation selection criteria, beginning with ten high-cost drugs dispensed topaid for by Medicare Part D patientsstarting in 2026, followed by 15 Part D drugs in 2027, 15 Part B or Part D drugs in 2028, and 20 Part B or Part D drugs in 2029 and beyond. The negotiated prices will be capped at a statutorily determined ceiling price. There are certain statutory exemptions from the coverage gap (i.e., “donut hole”). Under the Bipartisan Budget Act of 2018,IRA’s Price Negotiation Program, such as for a drug that has only a single orphan drug designation and is approved only for an indication or the BBA, effective in 2019, the mandated manufacturer coverage gap discount increased to 70%.
•The ACA imposed an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic products; the fee is apportioned among these entities according to their market share in certain government healthcare programs. The fee would not apply to sales of certain products approved exclusively for orphan indications.
•The ACA created the Sunshine Act, which requires certain manufacturers to track certain financial arrangements with physicians and teaching hospitals, including any “transfer of value” made or distributed to such entities, as well as any investment interests held by physicians and their immediate family members. Legislation passed in 2018 expandedindications within the scope of covered recipients’ non-physician providers such as to physician assistants and advanced practice nurses, effective in 2022. Manufacturers annually report this information to CMS, which posts this information on its website.designation. The IRA’s Price Negotiation Program is currently the subject of legal challenges.
•Manufacturers that fail to comply with the IRA may be subject to various penalties, including civil monetary penalties or a potential excise tax. The ACA establishedIRA permits the Secretary of Health and Human Services, or HHS Secretary, to implement many of the IRA’s provisions through guidance, as opposed to regulation, for the initial years. The effect of the IRA is anticipated to have significant effects on the pharmaceutical industry and may reduce the prices pharmaceutical manufacturers can charge and reimbursement pharmaceutical manufacturers can receive for approved products, among other effects.
The Biden administration has indicated that lowering prescription drug prices is a new Patient-Centered Outcomes Research Institutepriority. On October 14, 2022, President Biden signed an executive order to oversee, identify priorities in,lower prescription drug costs for Americans. In response to this directive, the HHS Secretary announced, and conduct comparative clinical effectiveness research, along with funding for such research. The research conducted by the Patient-Centered Outcomes Research Institute may affect the market for certain drug products.
•The ACA established the Center for Medicare and Medicaid Innovation within CMSis developing, new models intended to test innovative payment and service delivery models to lower drug costs under Medicare and Medicaid, including designing new payment methods for drugs approved under accelerated approval, in consultation with the FDA, to encourage timely confirmatory trial completion and improve access to post-market safety and efficacy data with the goal of reducing Medicare spending potentially including prescriptionon drugs that have no confirmed clinical benefit; creating a list of generic drugs for which the out-of-pocket Part D costs will be capped at $2 a month per drug, spending.
•The law expands eligibility criteriaand establishing new approach for Medicaid programs by,administering outcomes-based agreements for cell and gene therapies. President Biden also signed an executive order on July 9, 2021 affirming the administration’s policy to, among other things, allowingsupport legislative reforms that would lower the prices of prescription drugs, including by supporting the development and market entry of lower-cost generic drugs and biosimilars, and support the enactment of a public health insurance option. Among other things, the executive order directs the U.S. Department of Health and Human Services, or HHS Secretary, to provide a report on actions to combat excessive pricing of prescription drugs, continue to clarify and improve the approval framework for generic drugs and identify and address any efforts to impede generic drug competition, enhance the domestic drug supply chain, reduce the price that the federal government pays for drugs, and address price gouging in the industry. The executive order also directs the FDA to work with states and Indian Tribes that propose to develop section 804 Importation Programs in accordance with the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, and the FDA’s implementing regulations. The FDA released such implementing regulations on September 24, 2020, which went into effect on November 30, 2020, providing guidance for states to offer Medicaid coveragebuild and submit importation plans for drugs from Canada. In response, authorities in Canada have passed rules designed to safeguard the Canadian drug supply from shortages. On January 5, 2024, the FDA authorized Florida’s Agency for Health Care Administration’s drug importation proposal, the first step toward Florida facilitating importation of certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability.
Since its enactment, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. Various portions of the ACA are currently undergoing legal and constitutional challenges in the Fifth Circuit Court and the United States Supreme Court; the Trump Administration has issued various Executive Orders which have eliminated cost sharing subsidies and various other provisions that would impose a fiscal burden on states or a cost, fee, tax, penalty or regulatory burden on individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices; and Congress has introduced several pieces of legislation aimed at significantly revising or repealing the ACA. It is unclear whether the ACA will be overturned, repealed, replaced, or further amended.prescription drugs from Canada We cannot predict what affecthow further developments of or changes to the ACA would have onthese policies and rules will affect our business.
Insurers are increasingly adopting programs and policies that limit access to medications and increase out-of-pocket costs for patients. In the U.S., to help patients access and afford our approved product(s), pharmaceutical manufacturers may utilize programs to assist them, including patient assistance programs and co-pay coupon programs for eligible patients. On April 25, 2019, CMS published a regulation clarifying that where no medically appropriate generic equivalent is available, amounts paid toward cost sharing using any form of direct support offered by drug manufacturers must be counted by applicable insurers toward the Affordable Care Act’s annual limitation on cost sharing. On May 4, 2020, CMS published a regulation allowing applicable insurers flexibility to determine whether to include or exclude support provided by drug manufacturers from the Affordable Care Act’s annual limitation on cost sharing. On September 29, 2023, the U.S. District Court for the District of Columbia set aside the 2020 regulation. It is possible that changes in insurer policies regarding co-pay coupons and patient assistance programs and/or the introduction and enactment of new legislation or regulatory action could restrict or otherwise negatively affect these co-pay coupon programs and patient support programs, which could result in fewer patients using affected products, and therefore could have a material adverse effect on pharmaceutical manufacturers’ sales, business, and financial condition.
At the state level, governments have and continue to consider legislation and regulations designed to control pharmaceutical product pricing. Some of these measures include restricting price, reimbursement, discounts, product access,
and marketing; imposing drug price, cost, and marketing disclosure and transparency requirements; permitting importation from other countries; and encouraging bulk purchasing.
Healthcare Fraud and Abuse
Federal and state laws generally prohibit the payment or receipt of kickbacks, bribes or other remuneration in exchange for the referral of patients or other healthcare-related business. For example, the Federal Anti-Kickback Statute prohibits anyone from, among other things, knowingly and willfully offering, paying, soliciting or receiving any bribe, kickback or other remuneration intended to induce the referral of patients for, or the purchase, order or recommendation of, healthcare products and services reimbursed by a federal healthcare program, including Medicare and Medicaid. Violations of this federal law can result in significant penalties, including imprisonment, monetary fines and assessments, and exclusion from Medicare, Medicaid and other federal healthcare programs. Exclusion of a manufacturer would preclude any federal healthcare program from paying for its products. In addition to the federal anti-kickback law, many states have their own laws that are analogous to the federal anti-kickback law, but may apply regardless of whether any federal or state healthcare program business is involved.
In addition, federal and state false claims laws prohibit anyone from presenting, or causing to be presented, claims for payment to third-party payers that are false or fraudulent. For example, the federal False Claims Act, or FCA, imposes liability
on any person or entity who, among other things, knowingly and willfully presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program, including Medicaid and Medicare. Some suits filed under the FCA, known as “qui tam” actions, can be brought by a “whistleblower” or “relator” on behalf of the government, and such individuals may share in any amounts paid by the entity to the government in fines or settlement. Manufacturers can be held liable under false claims laws, even if they do not submit claims to the government, where they are found to have caused submission of false claims by, among other things, providing incorrect coding or billing advice about their products to customers that file claims, or by engaging in kickback arrangements or off-label promotion with customers that file claims. In addition, the government may assert that a claim including items and services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA. A number of states also have false claims laws, and some of these laws may apply to claims for items or services reimbursed under Medicaid and/or commercial insurance. Sanctions under these federal and state fraud and abuse laws may include civil monetary penalties and criminal fines, exclusion from government healthcare programs and imprisonment.
The U.S. Foreign Corrupt Practices Act of 1977, as amended, or FCPA, and similar worldwide anti-bribery laws in non-U.S. jurisdictions generally prohibit companies and their officers, directors, employees and intermediaries from offering or making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. Violation of the FCPA could result in substantial civil and criminal penalties and remedies, including fines, disgorgement, and imprisonment.
As described above, theThe federal Sunshine Act requires manufacturers to report certain payments to healthcare providers to CMS. Many state laws require drug manufacturers to report similar information related to payments and other transfers of value provided to other healthcare providers. Some states prohibit these expenditures altogether. Laws in a number of states also require companies to adopt marketing codes of conduct, companies to disclose pricing information about their products, or pharmaceutical sales representatives to be licensed. The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the HHS Secretary as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. The minimum basic Medicaid rebate on most branded prescription drugs and biologic products is 23.1% of average manufacturer price, or AMP.
In order for a drug product to receive federal reimbursement under the Medicare Part B and Medicaid programs or to be sold directly to U.S. government agencies, the manufacturer must offer its innovator products on the Federal Supply Schedule for purchase at prices compliant with statutory and regulatory requirements and extend discounts to entities eligible to participate in the 340B drug pricing program. The required 340B discount on a given product is calculated based on the AMP and Medicaid rebate amounts reported by the manufacturer.
As described above, we maintain a global compliance program designed to support the execution of our business strategy and operations in compliance with these laws.
Possible Change in Laws or Policies
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of drug products. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency or reviewing courts in ways that may significantly affect our business and development of our product candidates and any products that we may commercialize. It is impossible to predict whether additional legislative changes will be enacted, or FDA regulations, guidance or interpretations will be changed, or what the impact of any such changes may be. Federal budget uncertainties or spending reductions may reduce the capabilities of the FDA, extend the duration of required regulatory reviews, and reduce the availability of clinical research grants.
Our present and future business has been and will continue to be subject to various other laws and regulations. Various laws, regulations and recommendations relating to safe working conditions, laboratory practices, the experimental use of animals, and the purchase, storage, movement, import, export and use and disposal of hazardous or potentially hazardous
substances are or may be applicable to our activities. As noted above, the extent of government regulation, which might result from future legislation or administrative action, cannot accurately be predicted.
EU Regulatory Considerations
In the EU medicinal products are subject to extensive pre- and post-market regulation by regulatory authorities at both the EU and national levels.
Clinical Trials
Clinical trials of medicinal products in the EU must be conducted in accordance with EU and national regulations and the International Conference on Harmonization, or ICH, guidelines on GCP. If the sponsor of the clinical trial is not established within the EU, it must appoint an entity within the EU to act as its legal representative (unless all EU member states in which the trial is being conducted have chosen not to apply such rule, in which case only a contact person in the EU is required), who shall be responsible for ensuring compliance with the sponsor’s obligations under the new EU Regulation on Clinical Trials and be the addressee for all communications provided for under the Regulation. The sponsor must take out a clinical trial insurance policy, and in most EU countries the sponsor is liable to provide ‘no fault’ compensation to any study subject injured in the clinical trial.
Prior to commencing a clinical trial, the sponsor must obtain approval of the CTA from the competent authority, and a positive opinion from an independent ethics committee. The application for a CTA must include, among other things, a copy of the trial protocol and an investigational medicinal product dossier containing information about the manufacture and quality of the medicinal product under investigation. Any substantial changes to the trial protocol or other information submitted with the CTAs must be notified to or approved by the relevant competent authorities and ethics committees.
Under the new EU Regulation on Clinical Trials, which became applicable on January 31, 2022, there is a centralized application procedure where one national authority leads the scientific review of the application leading to increased
information-sharing and decision-making between member states (as compared to the previous EU Directive on Clinical Trials, where a separate application to the competent authority in each EU member state in which the trial was conducted was required). Each concerned member state will continue to complete an ethical review of any CTA.
Information related to the product, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is made public by the competent authority once the CTA is approved. The results of the clinical trial must be submitted by the sponsor to the competent authorities and, with the exception of non-pediatric Phase 1 trials, will be made public at the latest within six months of the end of a pediatric clinical trial, or otherwise within 12 months after the end of the trial.
During the development of a medicinal product, the EMA and national medicines regulators within the EU provide the opportunity for dialogue and guidance on the development program. At the EMA level, this is usually done in the form of scientific advice, which is given by the Scientific Advice Working Party of the CHMP. A fee is incurred with each scientific advice procedure. Advice from the EMA is typically provided based on questions concerning, for example, quality (CMC testing), nonclinical testing and clinical studies, and pharmacovigilance plans and risk-management programs. Advice is not legally binding with regard to any future MAA of the product concerned.
Marketing Authorisations
After completion of the required clinical testing, we must obtain a marketing authorisation before we may place a medicinal product on the market in the EU. There are various application procedures available, depending on the type of product involved. All application procedures require an application in the common technical document format, which includes the submission of detailed information about the manufacturing and quality of the product, and nonclinical study and clinical trial information. There is an increasing trend in the EU towards greater transparency and, while the manufacturing or quality information is currently generally protected as confidential information, the EMA and national regulatory authorities are now liable to disclose much of the nonclinical and clinical information in marketing authorisation dossiers, including the full clinical study reports, in response to freedom of information requests after the marketing authorisation has been granted. As of October 2016, the EMA began publishing clinical data (including clinical study reports) on the agency’s website following the grant, denial or withdrawal of an MAA for a centralised marketing authorisation, subject to procedures for limited redactions and protection against unfair commercial use.
The centralized procedure gives rise to marketing authorisations that are valid throughout the EU and, by extension (after national implementing decisions), in Norway, Iceland and Liechtenstein, which, together with the EU member states, comprise the European Economic Area, or EEA. Applicants file MAAs with the EMA, where they are reviewed by relevant scientific committees, including the CHMP. The EMA forwards CHMP opinions to the EC, which uses them as the basis for deciding whether to grant a marketing authorisation. The centralized procedure is compulsory for medicinal products that (1) are derived from biotechnology processes, (2) contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative disorders, viral diseases or autoimmune diseases and other immune dysfunctions, (3) are orphan medicinal products or (4) are advanced therapy medicinal products, such as gene or cell therapy medicines. For
medicines that do not fall within these categories, an applicant may voluntarily submit an application for a centralized marketing authorisation to the EMA, as long as the CHMP agrees that (i) the medicine concerned contains a new active substance, (ii) the medicine is a significant therapeutic, scientific, or technical innovation, or (iii) the authorisation of the medicine under the centralized procedure would be in the interest of public health.
For those medicinal products for which the centralized procedure is not available, the applicant must submit MAAs to the national medicines regulators through one of three procedures: (1) a national procedure, which results in a marketing authorisation in a single EU member state; (2) the decentralized procedure, in which applications are submitted simultaneously in two or more EU member states; and (3) the mutual recognition procedure, which must be used if the product has already been authorised in at least one other EU member state, and in which the EU member states are required to grant an authorisation recognizing the existing authorisation in the other EU member state, unless they identify a serious risk to public health. A national procedure is only possible for one member state; as soon as an application is submitted in a second member state the mutual recognition or decentralized procedure will be triggered.
Under the centralized procedure in the EU, the maximum timeframe for the evaluation of an MAA is 210 days. However, this timeline excludes clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the CHMP, so the overall process typically takes a year or more. Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major interest for public health and therapeutic intervention, defined by the absence or insufficiency of an appropriate alternative therapeutic approach for the disease to be treated and anticipation of high therapeutic benefit of the new product. In this circumstance, the EMA ensures that the opinion of the CHMP is given within 150 days. The EMA granted an accelerated assessment for patisiran, which was approved in the EU in August 2018 under the centralized procedure.
Data Exclusivity
MAAs for generic medicinal products do not need to include the results of pre-clinical studies and clinical trials, but instead can refer to the data included in the marketing authorisation of a reference product for which regulatory data exclusivity has expired. If a marketing authorisation is granted for a medicinal product containing a new active substance, that product benefits from eight years of data exclusivity, during which generic MAAs referring to the data of that product will not be accepted by the regulatory authorities, and a further two years of market exclusivity, during which such generic products may not be placed on the market. The two-year market exclusivity period may be extended to three years if during the first eight years of the product'sproduct’s authorisation, a new therapeutic indication with significant clinical benefit over existing therapies is approved.
There is a special regime for biosimilars, or biological medicinal products that are similar to a reference medicinal product but that do not meet the definition of a generic medicinal product, for example, because of differences in raw materials or manufacturing processes. For such products, the results of appropriate pre-clinical studies or clinical trials must be provided, and guidelines from the EMA detail the type of supplementary data to be provided for different types of biological product. There are no such guidelines for complex biological products, such as gene or cell therapy medicinal products, and so it is unlikely that biosimilars of those products will currently be approved in the EU. However, guidance from the EMA states that they will be considered in the future in light of the scientific knowledge and regulatory experience gained at the time.
Orphan Medicinal Products
The EMA’s Committee for Orphan Medicinal Products, or COMP, may recommend orphan medicinal product designation to promote the development of products that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions affecting not more than five in 10,000 persons in the EU. Additionally, designation is granted for products intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition when, without incentives, it is unlikely that sales of the product in the EU would be sufficient to justify the necessary investment in developing the medicinal product. The COMP may only recommend orphan medicinal product designation when the product in question offers a significant clinical benefit over existing approved products for the relevant indication or where no satisfactory method of diagnosis, prevention or treatment of such condition exists. Following a positive opinion by the COMP, the EC adopts a decision granting orphan status. The COMP will reassess orphan status in parallel with EMA review of an MAA and orphan status may be withdrawn at that stage if it no longer fulfills the orphan criteria (for instance because in the meantime a new product was approved for the indication and no convincing data are available to demonstrate a significant benefit over that product). Orphan medicinal product designation entitles a party to financial incentives such as reduction of fees or fee waivers and ten years of market exclusivity is granted following marketing authorisation. During this period, the competent authorities may not accept or approve any similar medicinal product for the same therapeutic indication, unless (i) the second medicinal product is safer, more effective or otherwise clinically superior to the authorised orphan product; (ii) the marketing authorisation holder for the authorised product consents to a second orphan medicinal product application; or (iii) the marketing authorisation holder for the authorised product cannot supply enough orphan medicinal product. This period may be reduced to six years if the orphan medicinal product designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of orphan designation. Patisiran, approved in the EU in August 2018, givosiran, approved in the EU in March 2020, lumasiran, approved
in the EU in November 2020, as well as vutrisiran, approved in the EU in September 2022 and fitusiran have been granted orphan medicinal product designation.
Post-Approval Controls
The holder of a marketing authorisation must establish and maintain a pharmacovigilance system and appoint an individual qualified person for pharmacovigilance who is responsible for oversight of that system. Key obligations include expedited reporting of suspected serious adverse reactions and submission of periodic safety update reports, or PSURs.
All new MAAs must include a risk management plan, or RMP, describing the risk management system that the company will put in place and documenting measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations as a condition of the marketing authorisation. Such risk-minimization measures or post-authorisation obligations may include additional safety monitoring, more frequent submission of PSURs, or the conduct of additional clinical trials or post-authorisation safety studies. RMPs and PSURs are routinely available to third parties requesting access, subject to limited redactions.
All advertising and promotional activities for the product must be consistent with the approved summary of product characteristics, and therefore all off-label promotion is prohibited. Direct-to-consumer advertising of prescription medicines is also prohibited in the EU. Although general requirements for advertising and promotion of medicinal products are established under EU directives, the details are governed by regulations in each member state and can differ from one country to another.
Manufacturing
Medicinal products may only be manufactured in the EU, or imported into the EU from another country, by the holder of a manufacturing authorisation from the competent national authority. The manufacturer or importer must have a qualified person
who is responsible for certifying that each batch of product has been manufactured in accordance with EU standards of cGMP before releasing the product for commercial distribution in the EU or for use in a clinical trial. Manufacturing facilities are subject to periodic inspections by the competent authorities for compliance with cGMP.
Pricing and Reimbursement
Governments influence the price of medicinal products in the EU through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on healthcare costs in general, particularly prescription medicines, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products.
Regulation of New Drug Compounds in Other Jurisdictions
In addition to regulations in the U.S. and the EU, we are subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products. In particular, during 2022,2023, we filed for regulatory approval for our commercial products in a number of jurisdictions worldwide, and regulatory filings in additional countries are planned for ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO in 2023,2024, and we will have to follow the specific regulations in such jurisdictions and such other countries in which we file, which are complex.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in all or most foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the U.S. have a similar process that requires the submission of a CTA, much like the IND prior to the commencement of human clinical trials. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed. Similarly, all clinical trials in Australia require, among other things, review and approval of clinical trial proposals by an ethics committee, which provides a combined ethical and scientific review process.
In Canada, for example, authorization to sell or import pharmaceuticals for the purpose of clinical trials of pharmaceuticals is obtained by way of CTAs. A no-objection letter from Health Canada (the regulator in Canada that regulates, among other things, research, testing, manufacture and marketing of pharmaceuticals) approval is required for clinical trials usingto sell or import pharmaceuticals not authorized for sale in Canada for the purpose of clinical trials (e.g., Phases I to III clinical trials and comparative bioavailability studies), and for trials ofto sell or import marketed pharmaceuticals for clinical trials where the proposed use is outside the scope of the existing marketing authorization. In addition, Research Ethics Boards, or REBs, overseeare involved in overseeing the conduct of clinical trials in Canada, and Health Canada requires REB approval is requiredof the clinical trial protocol and informed consent form for each clinical trial site prior to commencing the trial at that site. Post-approval,Post-commencement among other things, both Health Canada and the REBs monitor the safety data of the clinical trials and assess serious adverse reactions filed throughout the trial. If changes are proposed for an ongoing clinical trial, such as protocol or informed consent form changes, additional Health Canada and REB submissions and review may be required before such changes can be implemented. Health Canada may conduct site inspections to verify whether the conduct of a trial meets the requirements of applicable law and GCP. An REB may impose conditions in relation to
the conduct of clinical trials, and may require the informed consent form used in the trial to be amended to address, among other things, ethical concerns and privacy considerations.
Likewise, in Brazil, if a human clinical trial is to be carried out within the country’s territory, in addition to the CTA-like authorization and the approval by an ethics committee, the commencement of the trials may also depend on the approval by a biosecurity commission.commission, as long as the clinical trial involves the commercial use of genetically modified organisms, or GMOs, and their derivatives, which may include the use of genetic engineering techniques and methods, in addition to the use and transport, receipt, transfer between laboratories, import or export of GMOs and their derivatives.
In Brazil, the National Research Ethics Commission, or CONEP, is the highest authority for the ethical evaluation of research protocols involving human subjects and has the autonomy to analyze highly complex research protocols (and special thematic areas, such as human genetics, human reproduction, indigenous populations and international cooperation research) and research projects proposed by the Ministry of Health. Meanwhile, the Research Ethics Committees, or CEPs, are regional bodies responsible for low and medium complexity research protocols and are the gateway to all research projects involving human subjects in the country. Clinical trials on humans must be approved by the CEP/CONEP system. Depending on the subject matter, clinical research must be approved by both bodies. Under certain circumstances, the clinical trial must also be approved by the National Health Surveillance Agency, or ANVISA, particularly when the research is intended to support the approval of a new pharmaceutical product or the modification of an existing marketing authorization.
Brazilian regulations governing clinical research are quite protective of the participating subject, so the sponsor must provide broad and comprehensive protection to the participant, ensuring compensation to any subject injured in the clinical trial. Under certain circumstances, at the end of the clinical trial, the sponsor may also be required to provide participants with free and unlimited access to the best prophylactic, diagnostic and therapeutic methods that have proven effective during the trial. In the case of trials involving patients diagnosed with ultra-rare diseases, the regulation currently limits the post-trial drug supply to five years after the drug is approved by ANVISA. Given the complexity of the clinical trial scenario, Brazil is currently discussing a new law on the subject. A bill of law is currently in its final processing stage and should soon bring new contours to the topic in the country. The requirements and process governing the conduct of clinical trials varies from country to country. In all cases, however, the clinical trials must be conducted in accordance with GCP, which have their origin in the World Medical Association’s Declaration of Helsinki, the applicable regulatory requirements, and guidelines developed by the ICH for GCP in clinical trials.
The marketing approval procedure for pharmaceuticals also varies among countries and can involve requirements for additional testing. The time required may differ from that required for FDA approval and may be longer than that required to obtain FDA approval. Thus, there can be substantial delays in obtaining required marketing approvals from foreign regulatory authorities after the relevant applications are filed. Additionally, foreign governments lately are encouraging manufacturers to submit marketing applications in their jurisdictions with a variety of incentives including favorable reimbursement ratemaking. In Canada, while Health Canada has developed service standards for regulatory review time, those are target or estimated timelines that we can reasonably expect to receive from the regulator under normal circumstances, and as such, there may be delays in certain situations. In Brazil, obtaining the approval to begin human clinical trials can take from 180 to 360 days, and the marketing approval process itself usually takes between nine to 12 months. On the other hand, many countries have developed programs to expedite the approval of drugs pertaining to certain categories. In Brazil, for example, drugs designed to treat rare diseases can benefit from priority review and obtain marketing approval in less than six months.
With respect to marketing authorization, once a pharmaceutical has been authorized for sale in Canada, typically approves pharmaceuticals by way ofHealth Canada issues a drug identification number, or DIN, and in many cases also issues a Notice of Compliance, or NOC, together with a drug identification number, or DIN.NOC. NOCs are issued to pharmaceutical manufacturerssponsors for new drugs following the satisfactory review of a new drug submission. Along with the NOC, aA DIN is also issued to indicate the official approval and allowallows the sponsor to market the pharmaceutical in Canada. A DIN is an eight-digit number and uniquely identifies all pharmaceutical products sold in a dosage form in Canada. Additional obligations must be fulfilled when seeking marketing
authorization for biologic medicinal productsdrugs (whether innovative biologics or biosimilars) in Canada. In addition to the information required for other pharmaceuticals, regulatory submissions for biologics must include more detailed chemistry and manufacturing information, which ensuresto help ensure the purity and quality of the product. Because slight variations in the manufacturing process can lead to a different final product, sponsors must include details of the method of manufacturing in its regulatory submission. In addition to licenses and authorizations, or amendments to existing licenses and authorizations, such as drug establishment licenses, may also be required before we can sell or import a pharmaceutical in Canada.
In Brazil, marketing authorization, is granted by ANVISA following a satisfactory review of a new drug application. In general, the marketing authorization is valid for ten years and must be renewed at the end of this period. In certain cases, a marketing authorization may be granted on the basis of incomplete clinical research information, following the signing of a Term of Commitment between ANVISA and the company holding the marketing authorization. In this case, the initial period of validity of the marketing authorization is three years, and ANVISA may condition its renewal on the provision of additional information. With regard to the identification of a product, in addition to the federal registration number, in most cases products are identified and prescribed by the name of the molecule (Brazilian or International Nonproprietary Names - DCB or DCI), although in the case of innovative drugs they may also be identified by the trademark.
Product pricing and reimbursement vary as well. Canada’s pricing of patented pharmaceuticals is controlled by the Patented Medicine Prices Review Board, or PMPRB, whose regulatory authority is established by the Patented Medicines Regulations under Canada’s Patent Act. The PMPRB is a regulatory board unique to Canada. In the last several years, the PMPRB has undergone and continues to undergo significant change, including with respect to the PMPRB’s pricing review process and related guidelines. Various other regulatory bodies are involved in the pricing of pharmaceuticals that are publicly funded in Canada, including the Canadian Agency for Drugs and Technologies in Health, the Institut national d’excellence en santé et en services sociaux, the pan Canadian Pharmaceutical Pricing Alliance, and public payors (e.g., the federal, provincial, governments and territories)territorial public drug plans). Each province ofand territory in Canada hashave its own legislation and/or guidance relating to the pricing and reimbursement of pharmaceuticals, the permitted upcharges for wholesalers and pharmacies, the applicable dispensing fees, and whether rebates and professional allowances to pharmacies are prohibited or permitted. Approximately 40%44% of pharmaceuticals sold in Canada are paid for by the provincial (public)public drug plans; the remainder are sold in the private marketpaid for privately (e.g., covered by private insurance or paid for out-of-pocket by individuals). The pricing of pharmaceuticals in the private market is less regulated than the pricing of pharmaceuticals in the public market. A universal national (i.e., single-payer) public pharmacare plan may be enacted in Canada, which may impact the pricing and reimbursement of pharmaceuticals; it is not clear if or when such legislation may be passed.
In Brazil, the price ceiling is government-regulated, and prior to marketing, the maximum price must be approved by the Medicines Market Regulation Chamber, or CMED, an inter-ministerial body that establishes, based on technical and economic criteria, the maximum prices allowed for the sale of medicines, in both the public and private markets. Under certain circumstances, the public sector may receive a specific commission priormandatory discount on purchases, provided that the product purchased is on the list previously established by CMED or is intended to marketing. Sincecomply with court orders.
Because Brazil has a public health system that aims to provide free treatment and care to its whole population, public procurement follows a specific process that requires drugs to be included in the system’s formularies prior to being distributed to patients cost-free. However, this is not an automatic process and depends on a request for incorporation of the treatment into the public health system, which must be submitted by the marketing authorization holder or made by the Ministry of Health itself. This request is analyzed in detail by the National Commission for the Incorporation of Technologies, and, if the cost-effectiveness of the treatment is proven, it can be incorporated into the public system. Incorporation of a product into the public health system may require new price negotiations with the government to make the purchase of the product viable. Patients may take legal action against the government to obtain access to products not covered by the public health system, especially high-cost products. This issue has been widely discussed in Brazil, particularly in the higher courts, due to the large number of judicial requests for the supply of medicines by the federal, state or municipal governments.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. In Canada, contravention of the federal Food and Drugs Act, or F&DA, (governs all aspects of the manufacturing, importing, labelling, distribution and sale of pharmaceuticals) and its regulations may result in various enforcement actions from Health Canada, including notice letters, request for plan for corrective measures, public advisories, additional restrictions to our licenses or product authorization, recall, seizure, forfeiture and destruction of our products, refusal, suspension, cancellation or revocation of our authorization, license or registration. In the event of a contravention of the F&DA, Health Canada determines the most appropriate level of intervention depending on the severity of the risk posed by regulatory non-compliance. In certain circumstances, the regulatory enforcement responses are not appropriate to achieve compliance, and Health Canada may investigate potential criminal offences under the F&DA and/or refer to law enforcement for prosecution in relation to offences under the F&DA and the Criminal Code of Canada. The F&DA contains criminal provisions which allow for the issuance of fines, a term of imprisonment, or both.
The same risks are foreseen by Brazilian legislation. In the event of non-compliance with sanitary regulations due to the importation, labeling, marketing, distribution or registration of products in violation of legal requirements, a sponsor will be subject to sanctions established by law, which will be imposed by ANVISA depending on the nature and severity of the risk posed by the non-compliance, including warning letters, requests for corrective actions, additional restrictions on licenses or product approvals, recall, seizure, forfeiture and destruction of products, refusal, suspension, cancellation or revocation of authorizations, licenses or marketing approvals, etc. Potential criminal offenses may also be investigated under the Brazilian Penal Code.
Hazardous Materials
Our research, development and manufacturing processes involve the controlled use of hazardous materials, chemicals and radioactive materials and produce waste products. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous materials and waste products. We do not expect the cost of complying with these laws and regulations to be material.
Manufacturing
To date, we have manufactured limited supplies of drug substance for use in IND-enabling toxicology studies in animals and clinical trials at our own facilities, as well as patisiran formulated bulk drug product for late-stage clinical trial use and commercial supply.product. We have contracted with several third-partythird-
party contract manufacturing organizations, or CMOs, for the supply of drug substance, drug product and finished productgoods to meet our needs for pre-clinical toxicology studies, clinical and commercial supply. We expect to continue to rely on third-party CMOs for the supply of drug substance and drug product, including ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, as well as other product candidates, for at least the next several years, including to support the potential launch of our additional product candidates and to supply the needs of our alliance partners.collaborators. In 2015, we amended our manufacturing services agreement with Agilent Technologies, Inc., or Agilent, to provide for Agilent to supply, subject to any conflicting obligations under our third-party agreements, a specified percentage of the active pharmaceutical ingredients required for certain of our products in clinical development, as well as other products the parties may agree upon in the future. Under this agreement, we are required to provide rolling forecasts for products on a quarterly basis, a portion of which will be considered a binding, firm order. Agilent is required to reserve sufficient capacity to ensure that it can supply products in the amounts specified under such firm orders, as well as up to a certain percentage of the remaining, non-binding portions of each forecast. Subject to any conflicting obligations under our third-party agreements, we have also agreed to negotiate in good faith to enter into separate commercial manufacturing supply agreements with Agilent for certain products, consistent with certain specified terms, including a specified minimum purchase commitment. Currently, Agilent is the sole manufacturer of the active pharmaceutical ingredient for ONPATTRO, AMVUTTRA and GIVLAARI, for both clinical and commercial use, and we have entered into manufacturing services agreements with Agilent for such supply of ONPATTRO, AMVUTTRA and GIVLAARI. Pursuant to the Agilent supply agreements, we are required to provide rolling forecasts on a quarterly basis, a portion of which will be considered a binding, firm order. Agilent is required to reserve
sufficient capacity to ensure that it can supply our commercial and clinical products in the amounts specified under such firm orders, including a certain percentage of the remaining, non-binding portions of each forecast, as well as a specified number of batches each year.
In 2012, we established a manufacturing facility in Cambridge, Massachusetts and have developed cGMPGMP capabilities and processes for the manufacture of patisiran formulated bulk drug product for late-stage clinical trial use and commercial supply. During 2013, we manufactured our first cGMP batch of patisiran for use in our Phase 2 OLE and Phase 3 clinical trials. We willexpect to continue to manufacture commercial supply for formulated bulk drug product for ONPATTRO in ourthis facility for the foreseeable future. Commercial quantities of ONPATTRO and any other drugs that we may seek to develop will have to be manufactured in facilities, and by processes, that comply with FDA regulations and other federal, state and local regulations, as well as comparable foreign regulations.
During 2020, we completed construction and qualification of our cGMPGMP manufacturing facility in Norton, Massachusetts where we currently manufacture drug substance for clinical programs and, eventually, intend to manufacture drug substance for commercial use. In December 2020, we began cGMPGMP operations, and we believe this facility will enable us to initiate manufacturing for multiple new early-stage programs over the next few years, as well as provide us the manufacturing capabilities to support our late-stage and select commercial programs in the future.
We believe we have sufficient manufacturing capacity through our third-party CMOs and our current internal manufacturing facilities to meet our current, research, clinical development and commercial needs and the needs of our alliance partners.collaborators. We believe that the current supply capacity we have established externally, together with the internal capacitycapabilities we developed to support pre-clinical trials,development, our existing facility for patisiran formulated bulk drug product and our Norton manufacturing facility, will be sufficient to meet our and our alliance partners’collaborators’ anticipated needs for the next several years. We monitor the capacity availability for the manufacture of drug substance and drug product and believe that our supply agreements with our CMOs and the lead times for new supply agreements would allow us to access additional capacity to meet our and our alliance partners’collaborators’ currently anticipated needs. We also believe that our products can be manufactured at a scale and with production and procurement efficiencies that will result in commercially competitive costs.
Commercial Operations
After successfully overcoming various challenges associated with developing a new class of innovative medicines - such as solving the issue of drug delivery, optimizing our RNAi therapeutics to exhibit potency and durability of effect, and designing and carrying out comprehensive clinical trials to demonstrate the safety and clinical efficacy of our investigational products - starting in 2018, we embarked on the next part of the company’s journey: launching our RNAi therapeutics, based on regulatory approvals, to reach eligible patients in need. To that end, we have continuedbuilt and continue to buildscale a global commercial operation which has been designed to be fully integrated and to sequentially manage multiple product launches across multiple geographies. Over the last several years, we have been building commercial capabilitycapabilities and leveraging the internal knowledge we have accumulated as well as hiring talented people with broad industry experience to enable us to commercialize our products ourselves and with collaborators in key countries globally. The conduct of these commercial activities will continue to be dependent upon regulatory approvals and on agreements that we have made or may make in the future with strategic collaborators, currently as follows with respect to our first five approved products and our late-stage clinical programs:
•With respect to our ATTR amyloidosis franchise, we have global rights to develop and commercialize both ONPATTRO and AMVUTTRA;
•For GIVLAARI and OXLUMO,With respect to our Ultra Rare franchise, we have global rights to develop and commercialize;commercialize GIVLAARI and OXLUMO;
•For Leqvio, we granted MDCO, which was acquired by Novartis in January 2020, global rights to develop and commercialize; and
•For fitusiran, Sanofi has global rights to develop and commercialize fitusiran and any back-ups as a result of the 2018 amendment to the Sanofi collaboration and the related product-specific license terms.
Throughout the development of our product candidates, we have remained focused on keeping patients at the center of everything we do. This patient focus has continued as we have transitioned into commercialization. ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, as well as the late-stage programs we are advancing internally to commercialization are focused on orphanrare, specialty and select prevalent diseases, and we have been executing on what we believe to be a proven strategy to make ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO and future orphan products successful, including through efforts to increase awareness and diagnosis. In addition, as part of our planned transitionplan to be a top-tier biotech by the end of 2025 and consistent with our Alnylam P5x25 strategy, we are now advancing RNAi therapeutics beyond rare diseases into specialty and select prevalent disease opportunities. Beginning with the approval of Leqvio, the first RNAi therapeutic approved for a commonprevalent disease (hypercholesterolemia), we believe the RNAi therapeutic profile supports the potential for expansion to prevalent diseases, including addressing many unmet needs in common disease settings such as hypertension, NASH and diabetes.diabetes where there remains significant unmet need. We believe we are establishingscaling a global commercial organization and infrastructure to successfully support anthe eventual expansion to prevalent diseases.
We have a proactive market access strategy that includes entering into value-based agreements, or VBAs, with commercial payers in the U.S. and certain state Medicaid programs. As of the beginning of 2023,2024, we have completed over 4055 VBAs with multiple commercial payers, including 1914 for ONPATTRO, 1113 for AMVUTTRA, 15 for GIVLAARI, and 15 for OXLUMO. In our VBAs for GIVLAARI we introduced a Prevalence Based Adjustment that provides for a rebate to be paid if the number of patients identified within a plan population exceeds the expected disease prevalence, to address an unknown that exists in the context of an ultra-rare disease. For OXLUMO, we established a new VBA component called a Patient Need Adjustment with the effect of providing payers with greater budget certainty for medicines administered across a broad range of patient age groups by paying a rebate if the average number of vials utilized by a plan member exceeds an established threshold. Discussions with additional payers continue for our marketed products. Outside of the U.S. we believe we have made strong progress in terms of patient access and have established availability of ONPATTROour TTR (ONPATTRO, AMVUTTRA) and Ultra Rare (OXLUMO and GIVLAARI) therapies in more than 20 countries60 markets through direct reimbursement or expanded access.in partnership with our distributors. In addition, we have seen strong earlybeen encouraged by the strength of the adoption of AMVUTTRA in key launch markets and expect continued expansion across global markets.
We are continuing to augment the key components of a global commercial organization with a focus on successfully launching our commercially approved products around the world and preparing for the anticipated commercial launches of additional RNAi therapeutics, assuming successful development and regulatory approval. With respect to commercially approved products, throughout 2022,2023, we continued to build our commercial capabilities, including establishing field teams in the U.S. and other global markets, and are continuing to expand these capabilities to additional countries globally. We are continuing to build a focused commercial team with broad experience in marketing, sales, patient access, patient services, distribution and product reimbursement, in particular for orphan diseases.reimbursement. We are also continuing to incorporate and enhance the appropriate quality systems, compliance policies, systems and procedures, as well as implementing internal systems and infrastructure in order to support global commercial sales, and the establishment of patient-focused programs.
Ultimately, we intend to leverage the commercial infrastructure that we have built for our commercially approved products to also support the potential launch of patisiranvutrisiran in ATTR amyloidosis patients with cardiomyopathy, assuming our HELIOS-B Phase 3 clinical trial is positive and vutrisiran receives regulatory approval.approval for the treatment of ATTR amyloidosis with cardiomyopathy. For many territories/countries, we may also elect to utilize strategic partners, distributors or contract sales forces to assist in the commercialization of our products. Our objective is to continue to execute successful product launches leveraging our positive experience with the launches of our commercially approved products.
Medical Affairs
Our Global Medical Affairs organization advances our efforts through stakeholder engagement, data dissemination, and healthcare professional education, ultimately enabling diagnosis and improving patient care. This begins with our efforts to engage patient groups and communities, improve disease awareness and increase patient diagnosis, including through support for independent third party genetic testing programs like Alnylam Act. With a scalable framework in these capabilities, we believe our Global Medical Affairs organization is well positioned to expand to prevalent diseases.
Human Capital Management
As of December 31, 2022,2023, we employed 2,002approximately 2,100 full-time employees, of whom approximately 1,5701,650 were employed in the U.S. and approximately 432450 were employed outside of the U.S. None of our employees in the U.S. are represented by a labor union or covered by collective bargaining agreements, and we believe our relationship with our employees is good. During 2022,2023, we enhanced our capabilities by adding 337approximately 100 new full-time employees. The new employees were hired to support a variety of functions and key initiatives, including extending our research, clinical and pre-clinical pipeline development, as well as our medical affairs, manufacturing and commercialization capabilities, with hires in commercial, compliance, legal, clinical development and operations, research, medical affairs, manufacturing, and general and
administrative functions. We expect to continue to add additional employees in 2023,2024, with a focus on further enhancing our capabilities and increasing our capacities in these areas, in particular our manufacturing, commercial and compliance teams, as well as expanding our geographic reach as we continue the global launches of our approved medicines and prepare for the potential launch of patisiranvutrisiran for patients with the cardiomyopathy of ATTR amyloidosis, assuming our HELIOS-B Phase 3 clinical trial is positive and vutrisiran receives regulatory approval.approval for the treatment of ATTR amyloidosis with cardiomyopathy.
We consider the intellectual capital, skills and experience of our employees to be an essential driver of our business and key to our future prospects. We face intense competition for qualified individuals from numerous pharmaceutical and biotechnology companies, universities, governmental entities and other research institutions, and we believe that our future success will depend in large part on our continued ability to attract and retain highly skilled employees. To attract qualified applicants to our company and retain our employees, we offer a total rewards package consisting of base salary and cash target bonus targeting the 50th50th to 6560th percentile of market based on geography, a comprehensive benefit package and equity compensation for every employee. Annual cash bonus opportunitytargets are based on grade level and equity compensation increaseare communicated as a percentage of totalbase salary. Equity compensation grants are made based on grade level, of responsibility.geography, and performance. Any actual bonus payout is based solely on our performance against our corporate goals in the case of executive officers and is based on a combination of individual performance and corporate performance (or regional or national commercial performance metrics, as applicable) in the case of all other employees.
As a global commercial-stage biopharmaceuticalcompany, we believe that our long-term success and ability to deliver innovative, safe and effective medicines to patients requires a diverse and inclusive workforce. We value diversity at all levels of the organization and continue to focus on extending our diversity, equity and inclusion initiatives across our entire
workforce, from: working with managers to develop strategies for building diverse, high performing teams; to ensuring that we attract, develop and retain diverse talent from all backgrounds; to increasing awareness within our company of unconscious biases, and supporting affinity groups comprised of individuals who are underrepresented in our company, industry or society, such as women, members of the LGBTQLGBTQ+ community and people of color. In addition, we pride ourselves on an open culture that respects co-workers, values employees’ health and well-being and fosters professional development. We support employee growth and development in a variety of ways including with group training, individual mentoring and coaching, conference attendance and tuition reimbursement. Our management conducts annual employee engagement surveys and reports to our board of directors on human capital management topics, including corporate culture, diversity, equity and inclusion, employee development and retention, and compensation and benefits. Similarly, our board of directors regularly provides input on important decisions relating to these matters, including with respect to employee compensation and benefits, talent retention and development.
Corporate Information
Alnylam Pharmaceuticals, Inc. is a Delaware corporation that was formed in May 2003. Alnylam U.S., Inc., one of our wholly owned subsidiaries, is also a Delaware corporation that was formed in June 2002 as our initial corporate entity. Our principal executive office is located at 675 West Kendall Street, Henri A. Termeer Square, Cambridge, Massachusetts 02142, and our telephone number is (617) 551-8200.
Investor Information
We maintain an internet website at http://www.alnylam.com. The information on our website is not incorporated by reference into this Annual Report on Form 10-K and should not be considered to be a part of this Annual Report on Form 10-K. Our website address is included in this Annual Report on Form 10-K as an inactive technical reference only. Our reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, including our annual reports on Form 10-K, our quarterly reports on Form 10-Q and our current reports on Form 8-K, and amendments to those reports, are accessible through our website, free of charge, as soon as reasonably practicable after these reports are filed electronically with, or otherwise furnished to, the United States Securities and Exchange Commission, or SEC. We also make available on our website the charters of our audit committee, people, culture and compensation committee, nominating and corporate governance committee, and science and technology committee, as well as our corporate governance guidelines and our code of business conduct and ethics. In addition, we intend to disclose on our web site any amendments to, or waivers from, our code of business conduct and ethics that are required to be disclosed pursuant to SEC rules.
The SEC maintains an Internet website that contains reports, proxy and information statements, and other information regarding Alnylam and other issuers that file electronically with the SEC. The SEC’s Internet website address is http://www.sec.gov.
ITEM 1A. RISK FACTORS
We operateInvesting in an environment thatour securities involves a numberhigh degree of significant risks and uncertainties. We caution you to readrisk. You should carefully consider the following risk factors which have affected, and/in addition to the other information set forth or incorporated by reference in the future could affect,this Annual Report on Form 10-K, including our business, prospects, operating results, andconsolidated financial condition. The risks described below include forward-looking statements and actual eventsthe related notes and “Management’s Discussion of Financial Condition and Results of Operations,” in evaluating our company and our actual results may differ materially from these forward-looking statements. Additionalbusiness. If any of the following risks, and uncertaintiesor any additional risk not currently known to us or that we currently deem immaterial, may also impairactually occurs, our business, prospects, operating results,result or financial
condition could be materially and financial condition. Furthermore, additional risksadversely affected. In these circumstances, the trading price of our common stock could decline, and uncertainties are described under other captions in this report and should also be considered by our investors.you may lose all or part of your investment.
SUMMARY OF MATERIAL RISKS ASSOCIATED WITH OUR BUSINESS
Our business is subject to numerous risks and uncertainties, discussed in more detail in the following section. These risks include, among others, the following key risks:
Business Related Risks – Risks Related to Our Financial Results
•The marketing and sale of our approved products or any future products may be unsuccessful or less successful than anticipated and we may be unable to expand the approved indications for ONPATTRO and AMVUTTRA.
•We have a history of losses and may never become and remain consistently profitable.
•We will require substantial funds to continue our research, development and commercialization activities.
•The current pandemic of COVID-19 and its variants and the future outbreak of other highly infectious or contagious diseases, could continue to have an adverse impact on our business, financial condition and results of operations, including our commercial operations and sales, clinical trials and pre-clinical studies, and could impact other areas of our business as well.
•Although we sold a portion of the expected royalty stream and commercial milestones related to global sales of Leqvio by Novartis, we are entitled to retain the remaining portion of such future royalties and, if certain specified thresholds
are met, to the remaining portion of commercial milestone payments, and anyAny negative developments related to Leqvio could have a material adverse effect on the timing or amountour receipt of thosefuture royalties and milestone payments.
Risks Related to Our Dependence on Third Parties
•We may not be able to execute our business strategy if we are unable to maintain existing or enter into new alliancescollaborations with other companies that can provide business and scientific capabilities and funds for the development and commercialization of certain of our product candidates.
•If any collaborator materially amends, terminates or fails to perform its obligations under agreements with us, the development and commercialization of certain of our product candidates could be delayed or terminated and we could suffer other economic harm.terminated.
•We have limitedexpect to continue to grow our manufacturing experiencecapabilities and resources and we must incur significant costs to develop this expertise and/or rely on third parties to manufacture our products.
•We rely on third parties to conduct our clinical trials, and if theysuch third parties fail to fulfill their obligations, our development plans may be adversely affected.
Risks Related to Managing Our Operations
•If we are unable to attract and retain qualified key management and scientists, development, medical and commercial staff, consultants and advisors, our ability to implement our business plan may be adversely affected.
•We may have difficulty expanding our operations successfully as we continue our evolution from a U.S.- and Europe-based company primarily involved in discovery, pre-clinical testing and clinical development into a global company that develops and commercializes multiple drugs in multiple geographies including Asia, Latin America and the Middle East.
Industry Related Risks – Risks Related to Development, Clinical Testing and Regulatory Approval of Our Product Candidates and the Commercialization of Our Approved Products
•Any product candidatescandidate we or our partnerscollaborators develop may fail in development or be delayed to a point where they dosuch product candidate does not become commercially viable.
•We or our partnerscollaborators may be unable to obtain U.S. or foreign regulatory approval for our or our partneredcollaborated product candidates, and, as a result, we or if approved,our collaborators may failbe unable to obtain desired labeling forcommercialize such products.product candidates.
•Even if we or our partnerscollaborators obtain regulatory approvals, our marketed drugsproducts will be subject to ongoing regulatory oversight.
•We may incur significant liability if enforcement authorities allege or determine that we are engaging in commercial activities with respect to our unapproved product candidates or promoting our commercially approved products in a way that violates applicable regulations.
•Even if we or our collaborators receive regulatory approval to market our product candidates, and our collaborators receive regulatory approval to market product candidates discovered by us or developed with our technology, the market may not be receptive to such product candidates upon their commercial introduction, which could prevent us from becoming profitable.
•We are a multi-product commercial company and expect to continue to invest significant financial and management resources to continue to scalebuild our marketing, sales, market access and distribution capabilities and further establish our global commercial and compliance infrastructure, and our commercial efforts may not be successful.
•We may incur significant liability if enforcement authorities allege or determine that we are engaging in commercial activities or promoting our commercially approved products in a way that violates applicable regulations, including in connection with the ongoing DOJ investigation.
•Any drugs we currently market or may develop in the future may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, thereby harming our business.
Risks Related to Patents, Licenses and Trade Secrets
•If we are not able to obtain and enforce patent protection for our discoveries, our ability to develop and commercialize our product candidates will be harmed.
•We license patent rights from third-party owners. If such owners do not properly or successfully obtain, maintain or enforce the patents underlying such licenses, our competitive position and business prospects may be harmed.
•Other companies or organizations may challenge our patent rights or may assert patent rights that prevent us from developing and commercializing our products.
•If we become involved in intellectual property litigation or other proceedings related to a determination of rights, including our ongoing patent infringement litigation against Pfizer, Inc., or Pfizer, and Moderna, Inc., or Moderna, we could incur
substantial costs and expenses, and in the case of such litigation or proceedings against us, substantial liability for damages or be required to stop our product development and commercialization efforts.
•If we fail to comply with our obligations under any licenses or related agreements, we may be required to pay damages and could lose license or other rights that are necessary for developing, commercializing and protecting our RNAi technology.
Risks Related to Competition
•The pharmaceutical market is intensely competitive. If we or our collaborators are unable to compete effectively with existing drugs, new treatment methods and new technologies, we or our collaborators may be unable to commercialize successfully any drugs that we or our collaborators develop.
•We and our collaborators face competition from other companies that are working to develop novel drugs and technology platforms using technology similar to ours, as well as from companies utilizing emerging technologies, including gene therapy and gene editing.technologies.
Risks Related to Our Common Stock
•If ourOur stock price fluctuates, purchasers ofhas been and may in the future be volatile, and an investment in our common stock could incur substantial losses.suffer a decline in value.
•We may incur significant costsexpect that results from class action litigation.
•Future sales of sharesour and our collaborators’ clinical development activities and the clinical development activities of our common stock, including by ourcompetitors will continue to be released periodically and may result in significant stockholders, us or our directors and officers, could causevolatility in the price of our common stock to decline.stock.
Risks Related to Our Convertible Notes
•Servicing our debt may require a significant amount of cash. We may not have sufficient cash flow from our business to pay our indebtedness.
•We may not have the ability to raise the funds necessary to settle for cash conversions of the Notes or to repurchase the Notes for cash upon a fundamental change, and our future debt may contain limitations on our ability to pay cash upon conversion of the Notes or to repurchase the Notes.change.
•The conditional conversion feature of the Notes, if triggered, may adversely affect our financial condition and operating results.liquidity.
Risks Related to Our Business
Risks Related to Our Financial Results
The marketing and sale of our approved products or any future products may be unsuccessful or less successful than anticipated, and we may be unable to expand the approved indications for ONPATTRO andcertain of our commercial products, including AMVUTTRA.
In 2018, our first commercial product, ONPATTRO, was approved by the FDA and EMA, and we have since received approval and launched ONPATTRO in several additional territories. In 2019, the FDA approved our second product, GIVLAARI, which was also approved by the EMA and has since received approval in several additional territories, and in 2020, the FDA and EMA approved our third product, OXLUMO, which received additional regulatory approvals in 2021 and 2022. In June 2022, the FDA approved AMVUTTRA, which was granted marketing authorization in Europe and the UK in September 2022 and has since received regulatory approval in Japan and Brazil. We also have multiple product candidates in late-stage clinical development. WhileAlthough we have commercially launched four products, we cannot predict whether we will successfully market and sell our approved products, or successfully expand the approved indications of certain of our approved products.commercial products, including AMVUTTRA. For example, in August and September 2022, we reported positive safety and efficacy results from the APOLLO-B Phase 3 clinical trial of patisiran, which was designed and powered to evaluate the effects of patisiran on functional capacity and quality of life in patients with ATTR amyloidosis with cardiomyopathy. While we believe that the APOLLO-B results after 12 months validate the therapeutic hypothesis of RNAi therapeutics targeting TTR as potential treatment for patients with ATTR amyloidosis with cardiomyopathy, and submitted an sNDA toin October 2023, the FDA issued a CRL for review in December 2022, we cannot be certain that the results from the APOLLO-B clinical trial will support regulatory approval ofour sNDA for patisiran for the treatment of patients with ATTR amyloidosis with cardiomyopathy.cardiomyopathy, indicating that the clinical meaningfulness of patisiran’s treatment effects for ATTR amyloidosis with cardiomyopathy had not been established, and therefore, the sNDA could not be approved in its submitted form.
To execute our business plan of building a profitable, top-tier biotech company overby the next 5 yearsend of 2025 and achieving our Alnylam P5x25 strategy and the metrics associated with such strategy, in addition to successfully marketing, selling and expanding the approved indications of our approved products, we will need to successfully:
•execute product development activities and continue to leverage new technologies related to both RNAi and to the delivery of siRNAs to the relevant tissues and cells, including the liver, CNS, eye, lung, adipose and muscle;
•build and maintain a strong intellectual property portfolio;
•gain regulatory acceptance for the development and commercialization of our product candidates and successfully market success for our approved products, as well as any other products we commercialize;
•attract and retain customers for our products;
•developenter into and maintain successful strategic alliances;collaborations; and
•manage our spending as costs and expenses increase due to clinical trials, regulatory approvals and commercialization.
If we are unsuccessful in accomplishing the objectives set forth above, we may not be able to develop product candidates, successfully commercialize our approved products or any future products, raise capital, if needed, repay our indebtedness, achieve financial self-sustainability or continue our operations.
We have a history of losses and may never become and remain consistently profitable.
We have experienced significant operating losses since our inception. As of December 31, 2022,2023, we had an accumulated deficit of $6.57$7.01 billion. Although to date we have launched four products in the U.S., EU and various other countries globally, and expect to launch our commercially approved products in additional countries during 20232024 and beyond, we may never attain profitability or positive cash flow from operations. For the year ended December 31, 2022,2023, we recognized $894.3 million$1.24 billion in net product revenues from sales of ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO. While we believe 2019 was our peak operating loss year, we expect to continue to incur annual operating losses, and will require substantial resources over the next several years as we expand our efforts to discover, develop and commercialize RNAi therapeutics, and aim to achieve financial self-sustainability by the end of 2025. While we believe our current cash, cash equivalents and marketable equity and debt securities, as well as the revenue we expect to generate from product sales and under our current alliances,existing collaborations, including milestones and royalties on Leqvio sales, should enable us to achieve a self-sustainable profile without the need for future equity financing, we will depend on our ability to generate product, collaboration and royalty revenues to achieve this goal. In addition to revenues derived from sales of our current and future, if any, commercially approved products, we anticipate that a portion of any revenues we generate over the next several years will continue to be from alliancescollaborations with pharmaceutical and biotechnology companies, including Roche, Novartis, Regeneron, Sanofi and Regeneron.Vir. We cannot be certain that we will be able to maintain our existing alliances,collaborations, secure and maintain new alliances,collaborations, meet theour obligations under collaboration agreements, or achieve any milestones that we may be required to meet or achieve to receive payments under our existing or new alliances.collaborations. Moreover, we cannot be certain that our partners,collaborators, including Novartis, will continue to successfully execute their obligations under our alliancecollaboration agreements and generate additionalcollaboration and royalty revenues for us.
We believe that toTo become and remain consistently profitable, we must succeed in discovering, developing and commercializing novel drugsproduct candidates with significant market potential. This will require us to build upon the success we have had in a range of challenging activities, including continued platform innovation, pre-clinical testing and clinical trial stages of development, obtaining regulatory approval and reimbursement for theseour novel drugsproduct candidates and manufacturing, marketing and selling them.our approved products. We may never generate revenues that are significant enough to achieve profitability and, even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we cannot become and remain consistently profitable, the market price of our common stock could decline. In addition, we may be unable to raise capital, expand our business, develop additional product candidates or continue our operations.
We will require substantial funds to continue our research, development and commercialization activities, and if the funds we require are greater funds than what we have estimated, we may need to critically limit, or significantly scale back or cease certain of our activities.
We have used substantial funds to develop our RNAi technologies and will require substantial funds to conduct further research and development activities, including pre-clinical testing and clinical trials of our product candidates, and to manufacture, market and sell our four approved products and any other products that are approved for commercial sale. Because the length of time or scope of activities associated with successful development of our product candidates including zilebesiran, may be greater than we anticipate, we are unable to estimate the actual funds we will requireneeded to develop and commercialize them.our product candidates.
We believe 2019 was our peak operating loss year, and believe that our current cash, cash equivalents and marketable equity and debt securities, as well as revenue we expect to generate from product sales and under our current alliances,collaborations, including milestones and royalties we expect to receive from Novartis on Leqvio sales, will enable us to achieve a self-sustainable financial profile without the need for future equity financing. However,Nevertheless, our future capital requirements and the
period for which we expect our existing resources towill support our operations may vary from what we currently expect. We have based our expectations on a number of factors, many of which are difficult to predict or are outside of our control, including:
•progress in our research and development programs, including programs in both rare and prevalent diseases as well as what may be required by regulatory bodiesauthorities to advance these programs;
•the timing, receipt and amount of milestone, royalty and other payments, if any, from present and future collaborators, if any, including milestone and royalty payments from Roche with respect to the development and commercialization of zilebesiran, as well as milestone and royalty payments from Novartis related to Leqvio, which is being commercialized by our partner, Novartis;the commercialization of Leqvio;
•our ability to maintain and establish additional collaborative arrangementscollaborations and/or new business initiatives;
•the potential for improved product profiles to emerge from our new technologies and our ability to successfully advance our delivery efforts in extrahepatic tissues;
•the resources, time and costs required to successfully initiate and complete our pre-clinical and clinical studies, obtain regulatory approvals, prepare for global commercialization of our product candidates and obtain and maintain licenses to third-party intellectual property;
•our ability to establish, maintain and operate our own manufacturing facilities in a timely and cost-effective manner;
•our ability to manufacture, or contract with third parties for the manufacture of, our product candidates for clinical testing and our products for commercial sale;
•the impact of COVID-19any future pandemics or public health emergencies or the ongoing conflictconflicts in the Middle East and Ukraine on the initiation or completion of pre-clinical studies or clinical trials and the supply of our products or product candidates;
•the resources, time and cost required for the preparation, filing, prosecution, maintenance and enforcement of patent claims;
•the costs associated with legal activities, including litigation and government investigations, arising in the course of our business activities and our ability to prevail or reach a satisfactory result in any such legal disputes and investigations;
•the timing, receipt and amount of sales milestones and royalties, if any, from our approved products and our potential products, if and when approved; and
•the outcome of the regulatory review process and commercial success of drug products for which we are entitled to receive royalties, including Leqvio.
If our estimates, predictions and financial guidance relating to these factors are incorrect, we may need to modify our operating plan and may be required to seek additional funding in the future. We may do so through either collaborative arrangements, public or private equity offerings or debt financings, royalty or other monetization transactions or a combination of one or more of these funding sources. Additional funds may not be available to us on acceptable terms or at all.
The terms of any financing we may be required to pursue in the future may adversely affect the holdings or the rights of our stockholders. If we raise additional funds by issuing equity securities, further dilution to our existing stockholders will result. In addition, as a condition to providing additional funding to us, future investors may demand, and may be granted, rights superior to those of existing stockholders.
If we require additional funding and are unable to obtain additionalsuch funding on a timely basis, we may be required to significantly delay or curtail one or more of our research or development programs, or delay or curtail the further development of our global commercial infrastructure, and our ability to achieve our long-term strategic goals may be delayed or diminished. We also could be required to seek funds through arrangements with collaborators or others that may require us to relinquish rights to some of our technologies, product candidates or products that we would otherwise pursue on our own.
The COVID-19 pandemic and its variants, and the future outbreak of other highly infectious or contagious diseases, could continue to have an adverse impact on our business, financial condition and results of operations, including our commercial operations and sales, clinical trials and pre-clinical studies.
The ongoing COVID-19 pandemic continues to evolve and the ultimate impact of this pandemic remains uncertain. COVID-19 has and may continue to impact our operations and those of our third-party partners. The length of time and full extent to which the COVID-19 pandemic directly or indirectly impacts our business and financial results remains uncertain and cannot be predicted with confidence, and depends on future developments that are highly uncertain, subject to change and are difficult to predict, including as a result of new information that may emerge concerning COVID-19, new strains of the virus, including any future variants that may emerge, and the actions taken to contain or treat COVID-19, as well as the economic impact on local, regional, national and international markets.
In response to the spread of COVID-19, we took, and have continued to take, both temporary and ongoing precautionary measures, intended to help minimize the risk of the virus to our employees and their families, including implementing flexible work arrangements for nearly all employees who were able to perform their duties remotely. Working arrangements for many of our employees differ from the arrangements before the COVID-19 pandemic, and we expect a number of employees will continue to work in a remote capacity or a hybrid of in-person and remote work. We may face several challenges or disruptions as result of these working arrangements, and our hybrid of in-person and remote work option may negatively impact productivity, or disrupt, delay, or otherwise adversely impact our business. To the extent that we have less remote work opportunities and less flexibility than our competitors, our ability to attract and retain talent may be materially affected, which could adversely impact our employee recruitment and retention efforts. In addition, the increase in certain of our employees working remotely has amplified certain risks to our business, including increased demand on our information technology resources and systems, increased phishing and other cybersecurity attacks, and any failure to effectively manage these risks, including to timely identify and appropriately respond to any cyberattacks, could adversely impact our business operations.
If the conditions of the pandemic worsen, we may experience disruptions that could impact our business and operations, including our ability to successfully commercialize our approved products, and we may not be able to meet expectations with respect to commercial sales. In addition, we may also experience decreased patient demand for our approved products if current or potential patients decide to delay treatment as a result of the COVID-19 or a future pandemic. Business interruptions from the current or future pandemics, including staffing shortages, raw material or other supply chain shortages, production slowdowns and disruptions in delivery systems, may also adversely impact the third parties we or our partners rely on in the U.S. and abroad to sufficiently manufacture our approved products and to produce product candidates in quantities we require, which may impair our commercialization efforts, our research and development activities and the potential commercialization of our product candidates.
Additionally, timely completion of pre-clinical activities and initiation of planned clinical trials are dependent upon the availability of, for example, pre-clinical and clinical trial sites, researchers and investigators, patients or healthy volunteer subjects available for recruitment and enrollment, and regulatory agency personnel, which may be adversely affected by global health matters, such as the ongoing COVID-19 pandemic. We are conducting and plan to continue to conduct pre-clinical activities and clinical trials for our drug product candidates in geographies which have been and continue to be affected by COVID-19, and believe that the COVID-19 pandemic could continue to have an impact on various aspects of our ongoing clinical trials and on the clinical trials and pre-clinical studies we expect to initiate during 2023. For example, certain trial sites in some of our ongoing clinical trials were restricted temporarily by the institutions where they are located from scheduling patient visits or permitting onsite monitoring due to the COVID-19 pandemic, and in some of our ongoing trials, delayed or missed doses of study drug have been reported. Any business interruptions caused by the COVID-19 pandemic could also delay necessary interactions with local regulators, ethics committees, manufacturing sites, research or clinical trial sites and other important agencies and contractors, which could adversely impact the clinical trials of our product candidates.
Health regulatory agencies globally may also experience disruptions in their operations if the conditions of the COVID-19 pandemic worsen, which could impact review, inspection and approval timelines. Since March 2020, when foreign and domestic inspections of facilities were largely placed on hold, the FDA has been working to resume pre-pandemic levels of inspection activities, including routine surveillance, bioresearch monitoring and pre-approval inspections. Should the FDA determine that an inspection is necessary for approval of a marketing application and an inspection cannot be completed during the review cycle due to restrictions on travel, and the agency does not determine a remote interactive evaluation to be adequate, the FDA has stated that it generally intends to issue, depending on the circumstances, a complete response letter or defer action on the application until an inspection can be completed. For example, in December 2020, the FDA issued a complete response letter regarding Novartis' NDA for inclisiran, stating that the agency could not approve the NDA by the Prescription Drug User Free Act, or the PDUFA, action date due to unresolved facility inspection-related conditions. The FDA ultimately approved Novartis' NDA in December 2021. Regulatory authorities outside the U.S. may adopt similar restrictions or other policy measures in response to the ongoing COVID-19 pandemic and may experience delays in their regulatory activities.
Although we sold a portion of the royalty stream and commercial milestones from the global sales of Leqvio by our collaborator, Novartis, we are entitled to retain the remaining portionportions of the future royalties from the global sales of Leqvio and if certain specified thresholds are met, to the remaining portion of commercial milestone payments on Leqvio, and any negative developments related to Leqvio could have a material adverse effect on our receipt of those payments.
In April 2020, we sold to Blackstone 50% of the royalties payable to us with respect to net sales by Novartis, its affiliates or sublicensees of Leqvio and 75% of the commercial milestone payments payable to us under the MDCO agreement. If Blackstone does not receive royalty payments in respect of global sales of Leqvio equaling at least $1.00 billion by December 31, 2029, Blackstone’s royalty interest in Leqvio royalties will increase to 55% (and our interest will decrease to 45%) effective January 1, 2030. Our receiptAs a result, any factor that has an adverse impact on sales of future royalty payments and a portion of commercial milestone payments may be negatively impacted if the Leqvio royalty stream and commercial milestones payments are insufficient to meet the specified thresholds. For example, in December 2020, the FDA issued a complete response letter regarding Novartis' NDA for inclisiran, stating that the agency could not approve the NDA by the PDUFA action date due to unresolved inspection-related conditions at a third party manufacturing facility, delaying the potential approval and launch of Leqvio in the U.S., as well as payment of an associated approval milestone and potential royalties. While Leqvio was granted marketing authorization by the EC in Europe in December 2020, and was approved by the FDA in December 2021, any negative impact to future royalty payments and commercial milestone payments could affect our ability to meet the specified$1.00 billion repayment thresholds. Additional factors that maythreshold in this timeframe, which in turn would have an adverse effecta negative impact on the percentage of the Leqvio royalty stream and commercial milestonesthat we are entitled to retain.
Factors that could have an adverse impact on Leqvio sales include:
•companies working to develop new therapies or alternative formulations of products for ASCVD;
•foreign currency movement, which could have a negative impact on Novartis’ saleslack of acceptance of Leqvio thereby reducingby patients, the royalties;medical community or third party payors;
•any negative developments relating to Leqvio, such as safety, efficacy, or reimbursement issues, could reduce demand for Leqvio;issues;
•any disputes concerning patents or proprietary rights, or under license and collaboration agreements could negatively impact our receipt of commercial milestone payments or royalties;agreements;
•foreign currency exchange rate fluctuations; and
•adverse regulatory or legislative developments couldthat limit or prohibit the sale of Leqvio, such as restrictions on the use of Leqvio or safety-related label changes, including enhanced risk management programs, which may significantly reduce expected royalty revenue and commercial milestone payments and could require significant expense to address the associated legal and regulatory issues.programs.
If the revenues generated by sales of Leqvio are lower than expected, we may not receive commercial milestone payments and/or royalties in the amount we are currently anticipating, and our business, prospects, operating results and financial condition could be materially and adversely affected.
Geopolitical risks associated with the ongoing military conflict between Russia and Ukraine could have an adverse impact on our business, prospects, operating results and financial condition, and results of operations, including our clinical trials.
Russia’s invasion of Ukraine, and the global response, including the imposition of sanctions by the U.S., EU and other countries, has resulted in global business disruptions and economic volatility and may have an adverse impact on our business, including our clinical trials, the financial markets and the global economy.trials. The uncertain nature, magnitude, and duration of hostilities stemming from the conflict in Ukraine, including the potential effects of sanctions limitations, retaliatory cyber-attacks on the world economy and markets, have also contributed to increased market volatility and uncertainty, which could continue to have an adverse impact on macroeconomic factors that might affect our business and operations.
Additionally, the ongoing conflict in Ukraine may disrupthas disrupted the ability of certain of our contract research organizations, or CROs, to conduct clinical trials at certain sites in Ukraine. Moreover, enrollment and retention of clinical trial participants may be adversely affected. We cannot be certain what the overall impact of this conflict will be on our ability to conduct and complete our clinical trials on schedule. However, interruptions of our clinical trials could significantly delay our clinical development plans and potential authorization or approval of our product candidates, which could increase our costs and jeopardize our ability to successfully commercialize our product candidates.
We expect our operating results to fluctuate in future periods, which may adversely affect our stock price.
Our quarterly operating results have fluctuated in the past, and may continue to do so in the future. Our operating results may fluctuate due to the impact of the COVID-19 and related variants or a future pandemic, the level of success of our commercial efforts and resulting revenues, as well as the variable nature of our operating expenses as a result of the timing and magnitude of expenditures. For example, due to the impact of the COVID-19 pandemic, combined net product revenues in the first quarter of 2022 for our commercially approved products were negatively impacted. In addition, in one or more future periods, our results of operations may fall below the expectations of securities analysts and investors. In that event, the market price of our common stock could substantially decline.
If the estimates we make, or the assumptions on which we rely, in preparing our consolidated financial statements and/or our projected guidance prove inaccurate, our actual results may vary from those reflected in our projections and accruals.
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America, or GAAP. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of our assets, liabilities, revenues and expenses, the amounts of charges accrued by us and related disclosure of contingent assets and liabilities. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. We cannot assure you, however, that our estimates, or the assumptions underlying them, will be correct.
Further, from time to time we issue financial guidance relating to our expectations regarding our combined product sales, collaboration and royalty revenues, and GAAP and non-GAAP combined research and development and selling, general and administrative expenses, which guidance is based on estimates and the judgment of our management. If, for any reason, our product sales, revenues and/or expenses differ materially from our guidance, we may have to adjust our publicly announced financial guidance. For example, in April 2022, we decreased our 2022 guidance range for combined net product revenues, and in October 2022, we decreased our guidance range for our collaboration and royalty revenue. If we fail to meet, or if we are required to change or update any element of, our publicly disclosed financial guidance or other expectations about our business, our stock price could decline.
The investment of our cash, cash equivalents and marketable securities is subject to risks which may cause losses and affect the liquidity of these investments.
As of December 31, 2022,2023, we had $2.19$2.44 billion in cash, cash equivalents and marketable securities. We historically have invested these amounts in high–grade corporate notes, commercial paper, securities issued or sponsored by the U.S. government, certificates of deposit and money market funds meeting the criteria of our investment policy, which is focused on the preservation of our capital. Corporate notes may also include foreign bonds denominated in U.S. dollars. These investments are subject to general credit, liquidity, market and interest rate risks. We may realize losses in the fair value of these investments or a complete loss of these investments, which would have a negative effect on our consolidated financial statements.condition. In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer.decline. The market risks associated with our investment portfolio may have an adverse effect on our operating results, of operations, liquidity and financial condition.
Volatility in foreign currency exchange rates could have a material adverse effect on our operating results.
Our revenue from outside of the U.S. willis expected to increase as our products, whether commercialized by us or our collaborators, gain marketing approval in such jurisdictions. Our primary foreign currency exposure relates to movements in the
Japanese yen, Euro and British pound. If the U.S. dollar weakens against a specific foreign currency, our revenues will increase, having a positive impact on net income, but our overall expenses will increase, having a negative impact. Conversely, if the U.S. dollar strengthens against a specific foreign currency, our revenues will decrease, having a negative impact on net income, but our overall expenses will decrease, having a positive impact. For example, during 2022, the dollar strengthened against certain foreign currencies, and we experienced an unfavorable impact from foreign exchange rates on our international revenues. Continued volatility in foreign exchange rates is likely to continue to impact our operating results and financial condition.
Changes in tax lawlaws could adversely affect our business, prospects, operating results and financial condition.
Our business is subject to numerous international, federal, state, and other governmental laws, rules, and regulations that may adversely affect our operating results, including, taxation and tax policy changes, tax rate changes, new tax laws, or revised tax law interpretations, which individually or in combination may cause our effective tax rate to increase. In the U.S., the rules dealing with federal, state, and local income taxation are constantly under review by persons involved in the legislative process and by the Internal Revenue Service and the U.S. Treasury Department. Changes to tax laws (which changes may have retroactive application) could adversely affect us or holders of our common stock. In recent years, many such changes have been made and changes are likely to continue to occur in the future. Future changes in tax laws could have a material adverse effect on our business, cash flow,prospects, operating results and financial condition or results of operations.condition.
Additionally, the Organization for Economic Co-operation and Development, or the OECD, the EC, and individual taxing jurisdictions where we and our affiliates do business have recently focused on issues related to the taxation of multinational corporations. The OECD has released its comprehensive plan to create an agreed set of international rules for fighting base erosion and profit shifting. In addition, the OECD, the EC and individual countries are examining changes to how taxing rights should be allocated among countries considering the digital economy. As a result, the tax laws in the U.S. and other countries in which we and our affiliates do business could change on a prospective or retroactive basis and any such changes could materially adversely affect our business.business, prospects, operating results and financial condition.
We may incur additional tax liabilities related to our operations.
We are subject to income tax in the U.S. and the foreign jurisdictions in which we operate. Significant judgment is required in determining our worldwide tax liabilities, and our effective tax rate is derived from the applicable statutory tax rates and relative earnings in each taxing jurisdiction. We record liabilities for uncertain tax positions that involve significant management judgment as to the application of law. Domestic or foreign taxing authorities may disagree with our interpretation of tax law as applied to our and our subsidiaries’ operations or with the positions we may take with respect to particular tax issues on our tax returns. Consequently, tax assessments or judgments in excess of accrued amounts that we have estimated in preparing our financial statements may materially and adversely affect our reported effective tax rate or our cash flows. Further, other factors may adversely affect our effective tax rate, including changes in the mix of our profitability from country to country, tax effects of stock-based compensation (which depend in part on the price of our stock and, therefore, are beyond our control), and changes in tax laws or regulations. For example, the OECD Global Anti-Base Erosion Model have influenced tax laws in countries in which we operate, including the implementation of minimum taxes. Changes to these or other laws and regulations or their interpretations could materially and adversely impact our effective tax rate or cash flows.
Any future outbreaks of COVID-19 and its variants, or other future pandemics or public health emergencies, may directly or indirectly adversely affect our business, results of operations and financial condition.
In the future, we may experience disruptions from COVID-19 or a future pandemic or public health emergency that could impact our business and operations, including our ability to successfully commercialize our approved products, and we may not be able to meet expectations with respect to commercial sales as a result. In addition, we may also experience decreased patient demand for our approved products if current or potential patients decide to delay treatment as a result of the COVID-19 or a future pandemic or public health emergency. Business interruptions from future pandemics or public health emergencies, including staffing shortages, raw material or other supply chain shortages, production slowdowns and disruptions in delivery systems, may also adversely impact the third parties we or our collaborators rely on in the U.S. and abroad to sufficiently manufacture our approved products and to produce product candidates in quantities we require, which may impair our commercialization efforts, our research and development activities and the potential commercialization of our product candidates.
Additionally, timely completion of pre-clinical activities and initiation of planned clinical trials are dependent upon the availability of, for example, pre-clinical and clinical trial sites, researchers and investigators, patients or healthy volunteer subjects available for recruitment and enrollment, and regulatory agency personnel, which may be adversely affected by global health matters, such as the COVID-19 pandemic or any future pandemic or public health emergency. We are conducting and plan to continue to conduct pre-clinical activities and clinical trials for our drug product candidates in geographies which have been and may again be affected by COVID-19, and any resurgence of the COVID-19 pandemic and its variants could have an impact on various aspects of our ongoing clinical trials and on the clinical trials and pre-clinical studies we expect to initiate during 2024.
Health regulatory agencies globally may also experience disruptions in their operations as a result of the COVID-19 pandemic or future public health emergencies, which could impact review, inspection and approval timelines. Since March 2020, when foreign and domestic inspections of facilities were largely placed on hold, the FDA has been working to resume pre-pandemic levels of inspection activities, including routine surveillance, bioresearch monitoring and pre-approval inspections. Should the FDA determine that an inspection is necessary for approval of a marketing application and an inspection cannot be completed during the review cycle due to restrictions on travel, and the agency does not determine a remote interactive evaluation to be adequate, the FDA has stated that it generally intends to issue, depending on the circumstances, a complete response letter or defer action on the application until an inspection can be completed.
While the ultimate impact of COVID-19, or any future pandemic or public health emergency, on our business is uncertain, any negative impacts of such pandemic or public health emergency, alone or in combination with others, could exacerbate other risk factors discussed herein. The full extent to which COVID-19, or any future pandemic or public health emergency, will negatively affect our operations, financial performance, and stock price will depend on future developments that are highly uncertain and cannot be predicted.
Risks Related to Our Dependence on Third Parties
We may not be able to execute our business strategy ifIf we are unable to maintain our existing collaborations, or enter into new alliancescollaborations with other companies that can provide business and scientific capabilities and funds for the development and commercialization of our product candidates. If we are unsuccessful in forming or maintaining these alliancescandidates, it may have a negative impact on terms favorable to us, our business, may not succeed.prospects, operating results and financial condition.
We are continuing to advance our commercial capabilities, including in marketing, sales, market access and distribution, to support our wholly-owned products. We also continue to advance our growing pipeline of RNAi therapeutic opportunities. However, we maydo not currently have adequate capacity or capabilities to advance all opportunities arising from our growing pipeline of our therapeutic opportunities.RNAi therapeutics. Accordingly, we have entered into alliancescollaborations with other companies andthird party collaborators that we believe can provide such capacity and capabilities in certain territories and/or for certain product candidates, and we intend to enter into additional such alliancescollaborations in the future. Our collaboration strategy is to form alliances that create significant value for usSpecifically, we currently have active collaborations with, among others, Sanofi, Novartis, Regeneron, Vir, Novo Nordisk and our collaborators in the advancement of RNAi therapeutics as a new class of innovative medicines. Specifically, with respect to our Genetic Medicine pipeline, as a result of our broad strategic alliance with Sanofi formed in 2014, Sanofi has the right to developRoche covering various products and commercialize fitusiran globally. In addition, we formed a collaboration with MDCO (which was acquired by Novartis in January 2020) to advance inclisiran. In March 2018, we entered into a discovery collaboration with Regeneron to identify RNAi therapeutics for NASH and potentially other related diseases, and in November 2018, we and Regeneron entered into a separate, fifty-fifty collaboration to further research, co-develop and commercialize any therapeutic product candidates that emerge from these discovery efforts. In October 2017, we announced an exclusive licensing agreement with Vir for the development and commercialization of RNAi therapeutics for infectious diseases, including chronic HBV infection. In April 2020, we entered into a development and commercialization collaboration with Dicerna (which was acquired by Novo Nordisk in December 2021) to advance investigational RNAi therapeutics for the treatment of alpha-1 liver disease. With respect to our CNS/Ocular Disease pipeline, in April 2019, we announced a global, strategic collaboration with Regeneron to discover, develop and commercialize RNAi therapeutics for a broad range of diseases by addressing therapeutic targets expressed in the eye and CNS, in addition to a select number of targets expressed in the liver.pipeline.
In such alliances,collaborations, we expect our current, and may expect our future, collaborators to provide substantial capabilities in clinical development, regulatory affairs, and/or marketing, sales and distribution. Under certain of our alliances,collaborations, we also may expect our collaborators to develop, market and/or sell certain of our product candidates. We maycandidates, in certain territories or globally, and we have limited or no control over the development, sales, marketing and distribution activities of these third parties.collaborators. Our future revenues may depend heavily on the success of the efforts of these third parties. For example, we will rely entirely on (i) Regeneron for the development and commercialization of all programs targeting eye diseases (subject to limited exceptions), and potentially other CNS and liver programs,programs; (ii) Novartis for all futurethe development and commercialization of Leqvio worldwide, andworldwide; (iii) Sanofi for the development and commercialization of fitusiran worldwide.worldwide; and (iv) Roche for the commercialization of zilebesiran outside of the U.S. In the case of each such collaboration referenced in clauses (i)-(iii)
(iv) above, we are entitled to royalties, and in some instances commercial milestone payments, on the sales of each of these products.the applicable product. If our collaborators are not successful in their development and/or commercialization efforts, our future revenues from RNAi therapeutics for these indicationsthe relevant product or product candidate may be adversely affected. For example, while Leqvio was granted marketing authorization by the EC in Europe, in December 2020 Novartis received a complete response letter from the FDA stating that the agencyFDA could not approve the NDA by the PDUFA action date due to unresolved inspection-related conditions at a third party manufacturing facility. While Leqvio was ultimately approved by the FDA in December 2021, the resolution of the complete response letter resulted in a delay in the payment of an approval milestone and potential U.S. royalties. IfAs discussed above, under our agreement with Blackstone, if the revenues generated by the royalties received by Blackstone from us with respect to Leqvio sales do not reach a certain level by the end of 2029, Blackstone will be entitled to a higher royalty percentage beginning in 2030, which would have an adverse impact on our royalty revenues beginning in 2030.
We may not be successful in entering into future alliancescollaborations on terms favorable to us due to various factors, including our ability to demonstrate improved product profiles from our new technologies, including our IKARIA and GEMINI platforms,platform, our ability to successfully demonstrate proof-of-concept for our technology in humans in certain tissues or disease areas, including our alternative conjugate approach for delivering CNS or ocular product candidates or other extrahepatic approaches, our ability to demonstrate the safety and efficacy of our specific drugproduct candidates, our ability to manufacture or have third parties manufacture RNAi therapeutics, the strength of our intellectual property portfolio and/or concerns around challenges or potential challenges to our intellectual property. For example, the occurrence of a fatal thrombotic serious adverse event, or SAE, in our fitusiran study in 2017 and a subsequent pause in dosing and enrollment in fitusiran clinical studies in 2020 could contribute to further concerns about the safety of specific therapeutic candidates or therapeutic candidates for specific diseases.property portfolio. Even when we succeed in securing such alliances,new collaborations, we may not be able to maintain them if, for example, development or approval of a product candidate is delayed, challenges are raised as to the validity or scope of our intellectual property, we are unable to secure adequate reimbursement from payors, or sales of an approved drug are lower than we expected.expected, or our collaborator changes its strategic focus.
Furthermore, any delay in entering into new collaboration agreements would likely eitherhave the potential to prevent or delay the development and commercialization of certain of our product candidates, andor reduce theirthe competitiveness evensuch product candidates if they ultimately reach the market, or prevent the development of certain product candidates. Any such delay related to our collaborationswhich in turn could adversely affect our business.business, prospects, operating results and financial condition.
For certain product candidates, we have formed collaborations to fund all or part of the costs of drug development and commercialization, such as our collaborations with Regeneron, Roche, Novartis, Vir, Dicerna and Sanofi. We may not, however, be able to enter into additional collaborations for certain other programs, and the terms of any collaboration agreementagreements we do secure may not be favorable to us. If we are not successful in our efforts to enter into future collaboration
arrangements with respect to one or more of our product candidates, we may not have sufficient funds or other resources to develop these product candidates or other product candidates internally, or to bring oursuch product candidates to market. If we do not have sufficient funds to develop and bring our product candidates to market,In these circumstances, we will not be able to generate revenues from these product candidates, and this will substantially harm our business.business, prospects, operating results and financial condition.
If any collaborator materially amends, terminates or fails to perform its obligations under agreements with us, the development and commercialization of our product candidates could be delayed or terminated.
Our dependence on collaborators for capabilities and funding means that our business could be adversely affected if any collaborator materially amends or terminates its collaboration agreement with us or fails to perform its obligations under that agreement. Our current or future collaborations, if any, may not be scientifically or commercially successful. Disputes may arise in the future with respect to the ownership of rights to technology or products developed with collaborators, which could have an adverse effect on our ability to develop and commercialize any affected product candidate. Our current collaborations allow, and we expect that any future collaborations will allow, either party to terminate the collaboration for a material breach by the other party. In addition, our collaborators may have additional termination rights for convenience with respect to the collaboration or a particular program under the collaboration, under certain circumstances. For example, our agreement with MDCO, which was acquired by Novartis in January 2020, relating to the development and commercialization of inclisiran worldwide may be terminated by Novartis at any time upon four months’ prior written notice, provided if the agreement is terminated by Novartis for convenience, Novartis must grant a license to us under certain of our technology developed in the course of MDCO'sits (or MDCO’s) activities under the agreement, subject to a royalty to be negotiated between the parties. Moreover, any adverse actions by Novartis with respect to the MDCO agreementLicense Agreement or disputes with Novartis regarding each party's rights and obligations under the MDCO agreementLicense Agreement could adversely impact our ability to comply with our obligations under our agreements with Blackstone. If we were to lose a commercialization collaborator, we would have to attract a new collaborator (potentially on less favorable terms for us than we have with our existing collaborator) or develop expanded sales, distribution and marketing capabilities internally, which would require us to invest significant amounts of financial and management resources.
In addition, if we have a dispute with a collaborator over the ownership of technology or other matters, or if a collaborator terminates its collaboration with us, for breach or otherwise, or determines not to pursue the research, development and/or commercialization of RNAi therapeutics,the affected product or product candidate, it could delay our development of product candidates, result in the need for additional company resources to develop product candidates, require us to expend time and resources to develop expanded sales and marketing capabilities on a more expedited timeline, make it more difficult for us to attract new collaborators and could adversely affect how we are perceived in the business and financial communities.
Moreover, a collaborator, or in the event of a change in control of a collaborator or the assignment of a collaboration agreement to a third party, the successor entity or assignee, as in the case of MDCO and Novartis, could determine that it is in its interests to:
•pursue alternative technologies or develop alternative products, either on its own or jointly with others, that may be competitive with the products on which it is collaborating with us or which could affect its commitment to the collaboration with us;
•pursue higher-priority programs or change the focus of its development programs, which could affect the collaborator’s commitment to us; or
•if it has marketing rights, choose to devote fewer resources to the marketing of our product candidates, if any are approved for marketing, than it does for product candidates developed without us.
If any of these occur, the development and commercialization of one or more products or product candidates could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue such development and commercialization on our own.
We have limitedexpect to continue to grow our manufacturing experiencecapabilities and resources and we must incur significant costs to develop this expertise and/or rely on third parties to manufacture our products.
We have limitedbeen expanding our manufacturing experience. In ordercapabilities, and to continue to commercialize our approved products, continue to develop our current product candidates, apply for regulatory approvals and, if approved, commercialize future products, we will need to continue to develop our internal manufacturing capabilities and/or contract for, or otherwise arrange for theany necessary external manufacturing capabilities. Historically, our internal manufacturing capabilities were limited to small-scale production of material for use in in vitro and in vivo experiments that isand such material was not required to be produced under GMP standards.current good manufacturing practice standards, or cGMP. During 2012, we developed cGMP capabilities and processes for the manufacture of patisiran formulated bulk drug product for late stagelate-stage clinical trial use and commercial supply. In addition, during 2020, we completed construction and qualification of our cGMP manufacturing facility in Norton, Massachusetts where we manufacture drug substancesubstances for early-stage clinical development and eventually, willhave the possibility to manufacture drug substances for commercial use. In December 2020, we began cGMP operations, and we believe this facility will enable us to initiate manufacturing for multiple new early-stage programs over the next few years, as well as provide us the manufacturing capabilities to support our late-stage clinical development and commercial programsuse, in the future.
At the present time, we mayonly have the capacity to manufacture limited quantities of clinical trial materialsdrug substance ourselves, butand otherwise we continue to rely on third partiesparty CMOs to manufacture theadditional drug substance, and finishedwe rely on third party CMOs for all of our drug product we will requirerequirements for clinical trials that we initiate and to support the commercial supply of our approved products and any of our other product candidates.use. There are a limited number of manufacturers that supply synthetic siRNAs. WeCMOs worldwide with the expertise to manufacture our siRNA therapeutic products, and we currently rely on a limited number of North American and European CMOs forto manufacture our supply of synthetic siRNAs.products and product candidates. There are risks inherent in pharmaceutical manufacturing that could affect the ability of our CMOs to meet our delivery time requirements or provide adequate amounts of material to meet our needs, and ultimatelyif our CMOs fail to do these things it could delay our clinical trials and potentially put our commercial supply at risk, commercial supply, as well as result in additional expense to us. To fulfill our siRNAfuture requirements, we will likely need to secure alternative suppliers of synthetic siRNAscontract with additional CMOs, and such alternative suppliers aremay be limited, and may not be readily available, or we may be unable to enter into agreements with them on reasonable terms and in a timely manner. As noted above, in order to ensure long-term supply capabilities for our RNAi therapeutics, we are developing our own capabilities to manufacture drug substance for clinical and commercial use.manner, or at all.
In addition to the manufacture of the synthetic siRNAs, we may have additional manufacturing requirements related to the technology required to deliver the siRNA to the relevant cell or tissue type, such as LNPs or conjugates.conjugates or other drug delivery technologies. In some cases, the delivery technology we utilize is highly specialized or proprietary, and for technical and/or legal reasons, we may have access to only one or a limited number of potential manufacturers for such delivery technology. In addition, the scale-up of our delivery technologies could be very difficult and/or take significant time. We also have very limited experience in such scale-up and manufacturing, requiring us to depend on a limited number of third parties, who might not be able to deliver in a timely manner, or at all. Failure by manufacturers to properly manufacture our delivery technology and/or formulate our siRNAs for delivery could result in unusable product, supply delays and drug shortages. Furthermore, competition for supply from our manufacturers from other companies, a breach by such manufacturers of their contractual obligations or a dispute with such manufacturers would cause delays in our discovery and development efforts, as well as additional expense to us. In response to the COVID-19 pandemic, in March 2020, the Coronavirus Aid, Relief, and Economic Security Act, or CARES Act, was enacted in March 2020, and requires that manufacturers have in place a risk management plan that identifies and evaluates the risks to the supply of approved drugs for certain serious diseases or conditions for each establishment where the drug or active pharmaceutical ingredient is manufactured. The risk management plan will be subject to FDA review during an inspection. If we experience shortages in the supply of our marketed products, as a result of COVID-19 or otherwise, our results could be materially impacted.
In developing manufacturing capabilities by building our own manufacturing facilities, we have incurred substantial expenditures, and expect to incur significant additional expenditures in the future. Also, we have had to, and will likely need to continue to, recruit, hire, and train qualified employees to staff our facilities. If we are unable to manufacture sufficient quantities of
material or if we encounter problems with our facilities in the future, we may also need to secure alternative suppliers, and such alternative suppliers may not be available, or we may be unable to enter into agreements with them on reasonable terms and in a timely manner.manner, or at all. Given our dependence on a limited number of CMOs to supply our commercial products and clinical candidates, and our dependence onthe ongoing utilization of our own facility,facilities, any delay in supply caused by the COVID-19 pandemic could impact our ability to procure sufficient supplies for our approved products, and the development of our product candidates could also be delayed. Any delay or setback in the manufacture of our approved products could impede ongoing clinical and commercial supply, which could significantlymaterially and adversely impact our revenues andbusiness, prospects, operating results.results or financial condition. In addition, to the extent we or our partnerscollaborators rely on CMOs outside of the U.S. to supply drug substance for our product candidates, any delays or disruptions in supply caused by the COVID-19 pandemic could have a material adverse impact on the research and development activities and potential commercialization of our or our partners'collaborators’ product candidates.
The manufacturing processprocesses for our approved products and any other productsproduct candidates that we may develop is subject to the FDA and foreign regulatory authority approval process and we will need to meet, and will need to contract with CMOs who can meet, all applicable FDA and foreign regulatory authority requirements on an ongoing basis. The failure of any CMO to meet required regulatory authority requirements could result in the delayed submission of regulatory applications, or delays in receiving regulatory approval for any of our or our current or future collaborators'collaborators’ product candidates. For example, in April 2022, due to an amendment to our existing regulatoryvutrisiran NDA submission to address a pending inspection classification at a third-party secondary packaging and labeling facility, the FDA extended the review timeline of the NDA for vutrisiran.NDA. In addition, if we receive the necessary regulatory approval for any product candidate, we also expect to rely on third parties, including potentially our commercial collaborators, to produce materials required for commercial supply.
To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we depend, and will depend in the future, on these third parties to perform their obligations in a timely manner and consistent with contractual and regulatory requirements, including those related to quality control and quality assurance. The failure of any CMO to perform its obligations as expected, or, to the extent we manufacture all or a portion of our product candidates ourselves, our failure to execute on our manufacturing requirements, could adversely affect our business in a number of ways, including:
•we or our current or future collaborators may not be able to initiate or continue clinical trials of product candidates that are under development;
•we or our current or future collaborators may be delayed in submitting regulatory applications, or receiving regulatory approvals, for our product candidates;
•we may lose the cooperation of our collaborators;
•our facilities and those of our CMOs, and our products could be the subject of inspections by regulatory authorities that could have a negative outcome and result in delays in supply;supply delays;
•we may be required to cease distribution or recall some or all batches, of our products or take action to recover clinical trial material from clinical trial sites; and
•ultimately, we may not be able to meet the clinical and commercial demands for our product candidates and products.
We rely on third parties to conduct our clinical trials, and if theysuch third parties fail to fulfill their obligations, our development plans may be adversely affected.
We rely on independent clinical investigators, CROs, and other third-party service providers to assist us in managing, monitoring and otherwise carrying out our clinical trials. We have contracted with, and we plan to continue to contract with, certain third parties to provide certain services, including site selection, enrollment, monitoring, auditing and data management services. These investigators and CROs are not our employees and we have limited control over the amount of time and resources they dedicate to our programs. These third parties may have contractual relationships with other entities, some of which may be our competitors, which may draw their time and resources away from our programs. Although we depend heavily on these parties, we control only certainlimited aspects of their activity and therefore, we cannot be assured that these third parties will adequately perform all of their contractual obligations to us in compliance with regulatory and other legal requirements and our internal policies and procedures. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with applicable good clinical practice, or GCP, requirements, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for all of our product candidates in clinical development, and to implement timely corrective action to address any non-compliance. Regulatory authorities enforce these GCP requirements through periodic inspections of trial sponsors, principal investigators and trial sites, including in connection with the review of marketing applications. If we or any of our CROs fail to comply with applicable GCP requirements, or fail to take any such corrective action, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, the EMA, the PMDA in Japan or comparableother foreign regulatory authorities may require us to take additional action or perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority in the future, such regulatory authority will determine that any of our clinical trials comply with GCP regulations.
If our third-party service providers cannot adequately and timely fulfill their obligations to us for any reason, including due to disruptions caused by the COVID-19 pandemic or the ongoing conflict in Ukraine on their operations or at the sites they are overseeing, or if the quality and accuracy of our clinical trial data is compromised due to failure by such third party service provider to adhere to our protocols or regulatory requirements or if such third partiesparty service providers otherwise fail to meet deadlines, our development plans and/or regulatory reviews for marketing approvals may be delayed or terminated. As a result, our business, prospects, operating results and financial condition would be harmed, and our stock price would likely be negatively impacted,impacted.
Before conducting clinical trials to demonstrate the safety and our resultsefficacy of operations and the commercial prospects for our product candidates would be harmed,in humans in support of IND applications or similar applications in other jurisdictions, we must complete pre-clinical studies, which includes animal studies. In addition, we rely on third-party service providers to source certain materials for such pre-clinical studies. Our ability to complete our costs could increase andpre-clinical studies is contingent on, among other things, our ability to generate additional revenuessource animals and other supplies required for the conduct of such studies. If we are unable to obtain such supplies, we may be unable to complete such pre-clinical studies in a timely manner or at all. For example, some of our IND-enabling toxicology and other studies require certain non-human primates that have customarily been imported from the People’s Republic of China and Cambodia, and the supply of these non-human primates was constrained in 2022 due to various factors. If we were to encounter delays in obtaining a sufficient supply of such non-human primates to enable the conduct of our pre-clinical studies, our ability to complete pre-clinical studies could be delayed.impaired and our submission of IND applications and similar applications in other jurisdictions could be delayed, which would have an adverse impact on the development timelines of the impacted product candidates.
Risks Related to Managing Our Operations
If we are unable to attract and retain qualified key management and scientists, development, medical and commercial staff, consultants and advisors, our ability to implement our business plan may be adversely affected.
We are highly dependent upon our senior management and our scientific, clinical, sales and medical staff. The loss of the service of any of the members of our senior management could significantly delay or prevent the achievement of product development and commercialization, and other business objectives, and adversely impact our stock price. Our employment arrangements with our key personnel are terminable without notice. We do not carry key person life insurance on any of our employees.
We have grown our workforce significantly over the past several years and anticipate additional employee growth in the future, and we face intense competition for qualified individuals from numerous pharmaceutical and biotechnology companies, universities, governmental entities and other research institutions, many of which have substantially greater resources with which to attract and reward qualified individuals than we do. In addition, if we are not successful in commercializing our approved products, we may be unable to attract and retain highly qualified sales and marketing professionals, and if we are not able to supportattract and retain qualified sales and marketing professionals, it would negatively impact our ability to commercialize our approved products and ourany future products, if approved.products. Accordingly, we may be unable to attract and retain suitably qualified individuals in order to support our growing research, development and global commercialization efforts and initiatives, and our failure to do so could have an adverse effect on our ability to implement our future business plans.
We may have difficulty expanding our operations successfully as we continue our evolution from a U.S.- and EU-based company primarily involved in discovery, pre-clinical testing and clinical development into a global company that develops and commercializes multiple drugs.products.
As we continue the commercial launches of our approved products, and increase the number of product candidates we are developing, we will need to continue to expand our operations in the U.S. and further develop operations in the EU and other geographies, including Asia and Latin America. To date, we have received regulatory approval for four products, which we have launched in multiple geographies globally, and we continue to expand the reach of these products with additional regulatory filings and launches.
We have grown our workforce significantly over the last several years and anticipate additional employee growth globally in the future as we focus on the commercialization of our approved products, and achieving our Alnylam P5x25 strategy. This growth has placed a strain on our administrative and operational infrastructure and, as a result, we will need to continue to develop additional and/or new infrastructure and capabilities to support our growth and obtain additional space to conduct our global operations in the U.S., the EU, Japan, Latin America and other geographies. If we are unable to develop such additional infrastructure or obtain sufficient space to accommodate our growth in a timely manner and on commercially reasonable terms, our business could be negatively impacted. As we continue the commercialization of our approved products, and as the product candidates we develop enter and advance through clinical trials, we will need to continue to expand our global development, regulatory, manufacturing, quality, compliance, and marketing and sales capabilities, or contract with other organizations to provide these capabilities for us. In addition, as our operations continue to expand, we will need to successfully manage additional relationships with various collaborators, suppliers, distributors and other organizations. Our ability to manage our operations and future growth will require us to continue to enhance our operational, financial and management controls and systems, reporting systems and infrastructure, ethics and compliance functions, and policies and procedures. We may not be able to implement enhancements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls.
The use of social media presents risks and challenges.
Social media is being used to communicate about our clinical development programs and the diseases our investigational RNAi therapeutics are being developed to treat, and we are utilizing what we believe is appropriate social media in connection with our commercialization efforts for our approved products, and we intend to do the same for our future products, if approved. Social media practices in the biopharmaceutical industry continue to evolve and regulations and regulatory guidance relating to such use are evolving and not always clear. This evolution creates uncertainty and risk of noncompliance with regulations applicable to our business, resulting in potential regulatory actions against us, along with the potential for litigation related to off-label marketing or other prohibited activities. For example, for our clinical-stage candidates, patients may use social media channels to comment on their experience in an ongoing blinded clinical study or to report an alleged adverse event, or AE. When such disclosures occur, there is a risk that study enrollment may be adversely impacted, we fail to monitor and comply with
applicable AE reporting obligations or that we may not be able to defend our business or the public’s legitimate interests in the face of the political and market pressures generated by social media due to restrictions on what we may say about our investigational products. There is also a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us on any online platform, including a blog on the internet, or a post on a website, that can be distributed rapidly and could negatively harm our reputation. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face regulatory actions or incur other harm to our business.
Our business and operations could suffer in the event of system failures or unauthorized or inappropriate use of or access to our systems.
We are increasingly dependent on our information technology systems and infrastructure for our business. We collect, store and transmit sensitive information including intellectual property, proprietary business information, including highly sensitive clinical trial data, and personal information in connection with our business operations. The secure maintenance of this information is critical to our operations and business strategy. Some of this information could be an attractive target ofsubject to criminal attack or unauthorized access and use by third parties with a wide range of motives and expertise, including organized criminal groups, “hacktivists,” patient groups, disgruntled current or former employees and others. Cyber-attacks are of ever-increasing levels of sophistication, and despite our security measures, our information technology and infrastructure may be vulnerable to such attacks or may be breached, including due to employee error or malfeasance.
The pervasiveness of cybersecurity incidents in general and the risks of cyber-crime are complex and continue to evolve. Although we are making significant efforts to maintain the security and integrity of our information systems and are exploring various measures to manage the risk of a security breach or disruption, there can be no assurance that our security efforts and measures will be effective or that attempted security breaches or disruptions would not be successful or damaging. Despite the implementation of security measures, our internal computer systems and those of our contractors, consultants and consultantscollaborators are vulnerable to damage or interruption from computer viruses, unauthorized or inappropriate access or use, natural disasters, pandemics (including COVID-19),or public health emergencies, terrorism, war (including the ongoing conflictconflicts in Ukraine)Ukraine and the Middle East), and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of pre-clinicalpre-
clinical trial data or data from completed or ongoing clinical trials for our product candidates could result in delays in our regulatory filings and development efforts, as well as delays in the commercialization of our products, and significantly increase our costs. To the extent that any disruption, security breach or unauthorized or inappropriate use or access to our systems were to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, including but not limited to patient, employee or vendor information, we could incur notification obligations to affected individuals and government agencies, liability, including potential lawsuits from patients, collaborators, employees, stockholders or other third parties, and liability under foreign, federal and state laws that protect the privacy and security of personal information, and the development and potential commercialization of our product candidates could be delayed.
In addition, our increased use of cloud technologies heightens these third party and other operational risks, and any failure by cloud or other technology service providers to adequately safeguard their systems and prevent cyber-attacks could disrupt our operations and result in misappropriation, corruption, or loss of confidential or propriety information. The risk of cyber-attacks is increased with employees working remotely. Remote work increases the risk we may be vulnerable to cybersecurity-related events such as phishing attacks and other security threats.
Risks Related to Our Industry
Risks Related to Development, Clinical Testing and Regulatory Approval of Our Product Candidates and the Commercialization of Our Approved Products
Any product candidatescandidate we or our partnerscollaborators develop may fail in development or be delayed to a point where they dosuch product candidate does not become commercially viable.
Before obtaining regulatory approval for the commercial distribution of our product candidates, we must conduct, at our own expense, extensive nonclinical tests and clinical trials to demonstrate the safety and/or efficacy in humans of our product candidates. Nonclinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome, and the historical failure rate for product candidates is high. We currently have multiple programs in clinical development, including internal and partneredcollaborated programs in Phase 3 development, as well as several earlier-stage clinical programs. However, we may not be able to further advance any of our product candidates through clinical trials and regulatory approval.
If we enter into clinical trials, the results from nonclinical testing or early or late stage clinical trials of a product candidate may not predict the results that will be obtained in subsequent subjects or in subsequent human clinical trials of that product candidate or any other product candidate. For example, we are conducting the HELIOS-B Phase 3 clinical trial of vutrisiran, which is investigating the potential of vutrisiran to treat the cardiac manifestations of disease in patients with ATTR amyloidosis with cardiomyopathy. While vutrisiran has demonstrated positive results in patients with hATTR amyloidosis with polyneuropathy, we cannot be certain that the results from HELIOS-B will be positive or that the results from HELIOS-B will support approval of vutrisiran for the treatment of patients with ATTR amyloidosis with cardiomyopathy. There is a high failure rate for drugs proceeding through clinical studies. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies, and any such setbacks in our clinical development, including with respect to vutrisiran, could have a material adverse effect on our business, prospects, operating results and financial condition. Moreover, our approved products and our current product candidates, employ novel delivery technologies that, with the exception of inclisiran, have yet to be extensively evaluated in human clinical trials and proven safe and effective.
Additionally, several of our planned and ongoing clinical trials utilize an “open-label” trial design. An “open-label” clinical trial is one where both the patient and investigator know whether the patient is receiving the investigational product candidate or either an existing approved drug or placebo. Most typically, open-label clinical trials test only the investigational product candidate and sometimes may do so at different dose levels. Open-label clinical trials are subject to various limitations that may exaggerate any therapeutic effect as patients in open-label clinical trials are aware when they are receiving treatment. Open-labelAccordingly, open-label clinical trials may be subject to a “patient bias” where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment. In addition, open-label clinical trials may be subject to an “investigator bias” where those assessing and reviewing the physiological outcomes of the clinical trials are aware of which patients have received treatment and may interpret the information of the treated group more favorably given this knowledge. The results from an open-label trial may not be predictive of future clinical trial results with any of our product candidates for which we include an open-label clinical trial when studied in a blinded, controlled environment with a placebo or active control.
If we enter into clinical trials, the results from nonclinical testing or early or late stage clinical trials of a product candidate may not predict the results that will be obtained in subsequent subjects or in subsequent human clinical trials of that product candidate or any other product candidate. For example, we are conducting the APOLLO-B and HELIOS-B Phase 3 clinical trials of patisiran and vutrisiran, respectively, which are investigating the potential of patisiran and vutrisiran to treat the cardiac manifestations of disease in patients with ATTR amyloidosis with cardiomyopathy. We announced positive topline results from the APOLLO-B study in August 2022, and patients enrolled in the study are receiving patisiran as part of an open-label extension period. While both patisiran and vutrisiran have demonstrated positive results in patients with hATTR amyloidosis with polyneuropathy, we cannot be certain that the results from HELIOS-B will be positive or that the results from APOLLO-B and/or HELIOS-B will support approval of patisiran and/or vutrisiran for the treatment of patients with ATTR amyloidosis with cardiomyopathy. There is a high failure rate for drugs proceeding through clinical studies. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical development even after achieving promising results in earlier studies, and any such setbacks in our clinical development, including with respect to patisiran and/or vutrisiran, could have a material adverse effect on our business and operating results. Moreover, our approved products and our current product candidates, employ novel delivery technologies that, with the exception of inclisiran, have yet to be extensively evaluated in human clinical trials and proven safe and effective.
In addition, we, the FDA or other applicable regulatory authorities, or an institutional review board, or IRB, or similar foreign review board or committee, may delay initiation of or suspend clinical trials of a product candidate at any time for various reasons, including if we or they believe the healthy volunteer subjects or patients participating in such trials are being exposed to unacceptable health risks. Among other reasons, adverse side effects of a product candidate or related product on healthy volunteer subjects or patients in a clinical trial could result in our decision, or a decision by the FDA or foreign regulatory authorities,authority, to suspend or terminate the clinical trial, or, in the case of regulatory agencies, a refusal to approve a particular product candidate for any or all indications of use. For example, in October 2016, we announced our decision to discontinue development
Clinical trials of a new product candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease the product candidate is intended to treat and who meet other eligibility criteria. Rates of patient enrollment are affected by many factors, including the size of the patient population, the age and condition of the patients, the stage and severity of disease, the availability of clinical trials for other investigational drugs for the same disease or condition, the nature of the protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease, and the eligibility criteria for the clinical trial. For example, weWe or our partnerscollaborators may experience difficulty enrolling our clinical trials including, but not limited to, the ongoing clinical trials for fitusiran, due to the availability of existing approved treatments, as well as other investigational treatments in development. In addition,For example, in November 2018 we announced that due to recruitment challenges, we had discontinued a Phase 2 study of cemdisiran in atypical hemolytic uremic syndrome and arewere focusing our cemdisiran clinical development efforts in a different indication. Delays or difficulties in patient enrollment, including the enrollment delays in our KARDIA-1 Phase 2 monotherapy study of zilebesiran resulting from the ongoing situation in Ukraine, or difficulties retaining trial participants, including as a result of the availability of existing approved treatments or other investigational treatments or safety concerns, including the impact of pandemics or other public health emergencies, such as the COVID-19 pandemic, can result in increased costs, longer development times or termination of a clinical trial.
Although our investigational RNAi therapeutics have been generally well-tolerated in our clinical trials to date, new safety findings may emerge. The occurrence of serious adverse events, or SAEs, and/or adverse events, or AEs, can result in the suspension or termination of clinical trials of a product candidate by us, our collaborators, or the FDA or a foreign regulatory authority, and may negatively impact the clinical and/or regulatory timelines of the impacted product candidates. For example, in October 2016, we discontinued our revusiran program and in September 2017, we announced that we had temporarily suspended dosing in all ongoing fitusiran studies pending further review of a fatal thrombotic SAE that occurred in a patient with hemophilia A without inhibitors who was receiving fitusiran in our Phase 2 OLE study. More recently, in October 2020, Sanofi voluntarily paused dosing in all ongoing fitusiran clinical studies to assess reports of non-fatal thrombotic events in patients participating in the ATLAS Phase 3 program. Following an assessment of available data and alignment with regulators, patients restarted on fitusiran under amended protocols in ongoing clinical studies. Instudies and, in October 2021, Sanofi announced that a potential filing date for fitusiran hashad been moved to 2024 due to the introduction of a revised dosing regimen in the ongoing phase 3 studies.
As demonstrated by In addition, the discontinuation of our revusiran program in October 2016, the temporary suspension of dosing in September 2017 in our fitusiran studies, as well as Sanofi's voluntary pause of fitusiran studies in October 2020, the occurrence of SAEs and/or AEs can result in the suspension or termination of clinical trials of a product candidate by us, our partners, or the FDA or a foreign regulatory authority. The occurrence of SAEs and/or AEs could also result in refusal by the FDA or a foreign regulatory authority to approve a particular product candidate for any or all indications of use.use, or in limitations in the label of any approved product.
In addition, the occurrence of SAEs and/or AEs could also result in refusal by the FDA or a foreign regulatory authority to approve a particular product candidate for any or all indications of use, or in limitations in the label of any approved product.
Clinical trials also require the review, oversight and approval of IRBs, or, outside of the U.S., an independent ethics committee,committees, which continually review clinical investigations and protect the rights and welfare of human subjects. Inability to obtain or delay in obtaining IRB or ethics committee approval can prevent or delay the initiation and completion of clinical trials, and the FDA or foreign regulatory authorities may decide not to consider any data or information derived from a clinical investigationtrial not subject to initial and continuing IRB or ethics committee review and approval, as the case may be, in support of a marketing application.
Our product candidates that we develop may encounter problems during clinical trials that will cause us, an IRB, ethics committee or regulatory authorities to delay, suspend or terminate these clinical trials, or that will delay or confound the analysis of data from these clinical trials. If weour product candidates experience any such problems, we may not have the financial resources necessary to continue development of the affected product candidate that is affected, or development of any of our other product candidates. We may also lose, or be unable to enter into, collaborative arrangements for the affected product candidate and foror any of our other product candidates we are developing.candidates.
A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, nonclinical testing and the clinical trial process that could extend our clinical development timelines and delay or prevent regulatory approval or our ability to commercialize our product candidates, including:
•our nonclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional nonclinical testing or clinical trials, or we may abandon projects that we expecthave the potential to be promising;
•delays in filing IND applications or comparable foreign applications or delays or failure in obtaining the necessary approvals from regulators or IRBs/ethics committees in order to commence a clinical trial at a prospective trial site, or their suspension or termination of a clinical trial once commenced;
•conditions imposed on us by an IRB or ethics committee, or the FDA or comparable foreign regulatory authorities regarding the scope or design of our clinical trials;
•problems in engaging IRBs or ethics committees to oversee clinical trials or problems in obtaining or maintaining IRB or ethics committee approval of clinical trials;
•delays in enrolling patients and volunteers into clinical trials, and variability in the number and types of patients and volunteers available for clinical trials, including as a result of the COVID-19 pandemic, a future pandemic or public health emergency and the ongoing conflict in Ukraine;
•disruptions caused by man-made or natural disasters or pandemics, epidemics or public health pandemics or epidemicsemergencies or other business interruptions, including the ongoing COVID-19 pandemic;interruptions;
•high drop-out rates for patients and volunteers in clinical trials;
•negative or inconclusive results from our clinical trials or the clinical trials of others for product candidates similar to ours;
•inadequate supply or quality of product candidate materials or other materials necessary for the conduct of our clinical trials or disruption or delays in the clinical supply due to the COVID-19 or a future pandemic;pandemic or public health emergency;
•greater than anticipated clinical trial costs;
•serious and unexpected drug-related side effects experienced by patients taking our approved products, participants in our clinical trials or by individuals using drugs similar to our products or product candidates;
•poor or disappointing effectiveness of our product candidates during clinical trials;
•unfavorable FDA or other regulatory agency inspection and review of a clinical trial site or records of any clinical or nonclinical investigation;
•failure of our third-party contractors or investigators to comply with regulatory requirements, including GCP and cGMP, or otherwise meet their contractual obligations in a timely manner, or at all;
•governmental or regulatory delays and changes in regulatory requirements, policy and guidelines, including the imposition of additional regulatory oversight around clinical testing generally or with respect to our technology in particular; or
•interpretations of data by the FDA and similar foreign regulatory agencies that differ from ours.
Even if we successfully complete clinical trials of our product candidates, any given product candidate may not prove to be a safe and effective treatment for the disease for which it was being tested.
We or our partnerscollaborators may be unable to obtain U.S. or foreign regulatory approval for our or our partneredcollaborated product candidates and, as a result, we or our partnerscollaborators may be unable to commercialize such product candidates.
Our and our partneredcollaborated product candidates are subject to extensive governmental regulations relating to, among other things, research, testing, development, manufacturing, safety, efficacy, approval, recordkeeping, reporting, labeling, storage, pricing, marketing and distribution of drugs. Rigorous nonclinical testing and clinical trials and an extensive regulatory approval process are required to be successfully completed in the U.S. and in many foreign jurisdictions before a new drug can be marketed. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain and subject to unanticipated delays. It is possible that the product candidates we and our partnerscollaborators are developing will not obtain the regulatory approvals necessary for us or our collaborators to begin selling them, or, in the case of patisiran and vutrisiran, will not obtain
regulatory approval to be sold for a broader indicationsindication than arethe indication for which it is currently approved. It is also possible that the FDA or other regulatory authorities may determine that the data generated in clinical trials for a product candidate including patisiran, while positive, is not sufficient to support the approval of an application for regulatory approval. In FebruaryFor example, although we reported positive results from the APOLLO-B Phase 3 study of patisiran in patients with ATTR amyloidosis with cardiomyopathy, and received a 9:3 vote from the FDA’s CRDAC that patisiran’s benefits outweighed its risks for the treatment of ATTR amyloidosis with cardiomyopathy, in October 2023, the FDA acceptedissued a CRL in response to our sNDA for ONPATTRO for filing and set an action date of October 8, 2023, under the PDUFA. The FDA also indicated inpatisiran, indicating the sNDA filing communication letter that it is planning to hold an advisory committee meeting to discuss the application and that it hadcould not identified any potential review issues at such time. Even though the sNDA has been accepted for filing, we may receive a complete response letter rather than approval, including for reasons that may be identified during a meeting of the advisory committee.approved in its present form.
The time required to obtain FDA and other regulatory approvals is unpredictable but typically takes many years following the commencement of clinical trials, depending upon the type, complexity and novelty of the product candidate. The standards that the FDA and its foreign counterparts use when regulating us are not always applied predictablyin a predictable or uniformlyuniform manner and can change.change over time. Any analysis we perform of data from nonclinical and clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We or our partnerscollaborators may also encounter unexpected delays or increased costs due to new government regulations, for example, from future legislation or administrative action, or from changes in FDA policy during the period of product development, clinical trials and FDA regulatory review. It is impossible to predict whether legislative changes will be enacted, or whether FDA or foreign regulations, guidance or interpretations will be changed, or what the impact of such changes, if any, may be.
Because the drugsproduct candidates we or our partnerscollaborators are developing represent a new class of drug, the FDA and its foreign counterparts have not yet established any definitive policies, practices or guidelines in relation to these drugs. The lack of policies, practices or guidelines may hinder or slow review by the FDA of any regulatory filings that we or our partnerscollaborators may submit. Moreover, the FDA may respond to these submissions by defining requirements we or our partnerscollaborators may not have anticipated. Such responses could lead to significant delays and increased costs in the development of our or our partneredcollaborated product candidates. In addition, because there may be approved treatments for some of the diseases for which we or our partnerscollaborators may seek approval, including patisiran and vutrisiran for the treatment of ATTR amyloidosis with cardiomyopathy, or
treatments in development which are approved by the time we or they applyour collaborators file for approval, in order to receive regulatory approval, we or they may need to demonstrate through clinical trials that the product candidates we develop to treat these diseases if any, are not only safe and effective, but safer and/or more effective than existing approved products. Interruption or delays in the operations of the FDA, EMA and comparable foreign regulatory agencies due to the COVID-19 pandemic, may impact the review, inspection and approval timelines for our or our partneredcollaborated product candidates. During the COVID-19 public health emergency, the FDA has worked to ensure timely reviews of applications for medical products in line with its user fee performance goals and conductconducted mission critical domestic and foreign inspections to ensure compliance of manufacturing facilities with FDA quality standards. However, the FDA may not be able to continue its current pace and approval timelines could be extended, including where a pre-approval inspection or an inspection of clinical sites is required and due to the ongoing COVID-19 pandemic and travel restrictions the FDA is unable to complete such required inspectionsIn addition, during the review period. During the COVID-19 public health emergency, a number of companies announced receipt of complete response letters due to the FDA'sFDA’s inability to complete required inspections for their applications. In December 2020, the FDA issued a complete response letterCRL regarding Novartis'Novartis’ NDA for inclisiran, stating that the agency could not approve the NDA by the PDUFA action date due to unresolved facility inspection-related conditions. In July 2021, Novartis announced that the resubmission to the FDA of the inclisiran NDA to address the complete response letter was filed, and the FDA approved Leqvio (which is the(the trade name under which inclisiran is marketed in the U.S.) in December 2021. TheThis delay in the approval of Leqvio resulted in delayed milestone and royalty revenue to us. Any similar interruption or delay by the FDA, EMA or comparable foreign regulatory agencyauthorities could have a material adverse effect on our or our collaborators’ efforts to obtain regulatory approval for our or our collaborators’ product candidates, which could have a material adverse effect on our business, prospects, operating results or financial results.condition. For instance, the FDA may request additional clinical or other data or information in connection with the regulatory review of our or our partners’collaborators’ product candidates, including patisiran, including by issuing a complete response letter which may require that we or our partners’collaborators submit additional clinical or other data or impose other conditions that must be met in order to secure final approval of our or our partners’collaborators’ NDA applications, including potentially requiring a facility inspection. Even if such data and information are submitted, or any such inspection is completed, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval.
Any delay or failure in obtaining required approvals for our product candidates or our partneredcollaborated product candidates could have a material adverse effect on our ability to generate revenues from any product candidate for which we or our partnerscollaborators may seek approval in the future. For example, as a result of the recent CRL from the FDA in response to our sNDA for patisiran as a treatment for ATTR amyloidosis with cardiomyopathy, our ability to generate product revenues for patisiran will be negatively impacted. Furthermore, any regulatory approval to market any product including patisiran, may be subject to limitations on the approved uses for which we or our partnerscollaborators may market the product or the labeling or other restrictions, which could limit each such product’s market opportunity and have a negative impact on our business, prospects, operating results of operationsand financial condition and our stock price. In addition, the FDA has the authority to require a Risk Evaluation and Mitigation Strategy, or REMS, plan as part of its review of an NDA, or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria and requiring treated patients to enroll in a registry. In the EU, we or our partnerscollaborators could be required to adopt a similar plan, known as a risk management plan, and our products could be subject to specific risk minimization measures, such as restrictions on prescription and supply, the conduct of post-marketing safety or efficacy studies,
or the distribution of patient and/or prescriber educational materials. In either instance, these limitations and restrictions may limit the size of the market for the productour products and affect reimbursement by third-party payors.
We are also subject to numerous foreign regulatory requirements governing, among other things, the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. The foreign regulatory approval process varies among countries and includes all of the risks associated with FDA approval described above as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Approval by the FDA does not ensure approval by any regulatory authoritiesauthority outside the U.S. and vice versa.
Even if we or our partnerscollaborators obtain regulatory approvals, our marketed drugsproducts will be subject to ongoing regulatory oversight. If we or our partnerscollaborators fail to comply with continuing U.S. and foreign requirements, our approvals could be limited or withdrawn, we could be subject to other penalties, and in any such case our business would be seriously harmed.
Following any initial regulatory approval of drugsa product we or our partnerscollaborators may develop, including our four approved drugs,products, we will also be subject to continuing regulatory oversight, including the review of adverse drug experiences and clinical results that are reported after our drug products are made commercially available. This would includeincludes results from any post-marketing tests or surveillance to monitor the safety and efficacy of our approved drugsproducts or other drug products required as a condition of approval or otherwise agreed to by us. The regulatory approvals that we receive for ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, as well as any regulatory approvals we receive for any otherof our product candidates may also be subject to limitations on the approved uses for which the product may be marketed, including any expanded label for ONPATTRO or AMVUTTRA. Other ongoing regulatory requirements include, among other things, submissions of safety and other post-marketing information and reports, registration and listing, as well as continued compliance with good practice quality guidelines and regulations, including cGMP requirements and GCP requirements for any clinical trials that we conduct post-approval. In addition, we are conducting, and intend to continue to conduct, clinical trials for our product candidates, and we intend to seek approval to market our product candidates, in jurisdictions outside of the U.S., and therefore will be subject to, and must comply with, regulatory requirements in those jurisdictions.
The FDA has significant post-market authority, including, for example, the authority to require labeling changes based on new safety information and to require post-market studies or clinical trials to evaluate serious safety risks related to the use of a drugproduct and to require withdrawal of the product from the market. The FDA also has the authority to require a REMS plan after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug.product. As our approved products are used commercially, we or others could identify previously unknown side effects or known side effects could be observed as being more frequent or severe than in clinical studies or earlier post-marketing periods, in which case:
•sales of our approved products may be more modestlower than originally anticipated;
•regulatory approvals for our approved products may be restricted or withdrawn;
•we may decide, or be required, to send product warning letters or field alerts to physicians, pharmacists and hospitals;
•additional nonclinical or clinical studies, changes in labeling, adoption of a REMS plan, or changes to manufacturing processes, specifications and/or facilities may be required; andand/or
•government investigations or lawsuits, including class action suits, may be brought against us.
Any of the above occurrences could reduce or preventeliminate sales of our approved products, increase our expenses and impair our ability to successfully commercialize one or more of these products.
The CMO and manufacturing facilities we use to make our approved products and certain of our current product candidates, including our Cambridge facility, our Norton facility, as well as facilities at Agilent and other CMOs, will also be subject to periodic review and inspection by the FDA and other regulatory agencies. For example, Agilent and our Cambridge-based facility were subject to regulatory inspection by the FDA and the EMA in connection with the review of our applications for regulatory approval for ONPATTRO and GIVLAARI, and may be subject to similar inspection in connection with any subsequent applications for regulatory approval of one or more of our products filed in other territories. The discovery of any new or previously unknown problems with our facilities or our CMOs, or our or theirCMO’s manufacturing processes or facilities, may result in restrictions on the drug or CMO or facility, including delay in approval or, in the future, withdrawal of the drugproduct from the market. For example, due to a routine inspection by the FDA at a CMO facility that resulted in a pending inspection classification, we amended our regulatory submission for vutrisiran, which delayed our PDUFA goal date and AMVUTTRA'sAMVUTTRA’s FDA approval. WeAlthough we have developed cGMP capabilities and processes for the manufacture of patisiran formulated bulk drug product for commercial use. In addition,use and in 2020 we completed construction of a cGMP manufacturing facility for drug substance for clinical and, eventually, commercial use. Weuse, we may not have the ability or capacity to manufacture material at a broader commercial scale in the future. We may manufacture clinical trial materials, or we may contract a third party to manufacture this material for us. Reliance on CMOs entails risks to which we would not be subject if we manufactured products ourselves, including reliance on the applicable CMO for regulatory compliance.
If we or our collaborators, CMOs or service providers fail to comply with applicable continuing regulatory requirements in the U.S. or foreign jurisdictions in which we may seek to market our products, we or they may be subject to, among other
things, fines, warning letters, holds on clinical trials, refusal by the FDA or foreign regulatory authorities to approve pending applications or supplements to approved applications, suspension or withdrawal of regulatory approval, product recalls and seizures, refusal to permit the import or export of products, operating restrictions, injunction, civil penalties and criminal prosecution.
We may incur significant liability if enforcement authorities allege or determine that we are engaging in commercial activities with respect to our unapproved product candidates or promoting our commercially approved products in a way that violates applicable regulations.
Physicians have the discretion to prescribe approved drug products for uses that are not described in the product’s labeling and that differ from those approved by the FDA or other applicable regulatory agencies. Off-label uses are common across medical specialties. Although the FDA and other regulatory agencies that approve drug products do not regulate a physician’s practice of medicine or choice of treatments, the FDA and other regulatory agencies regulate a manufacturer’s communications regarding off-label use and prohibit off-label promotion, as well as the dissemination of false or misleading labeling or promotional materials, including by their agents. Manufacturers and their agents may not promote drugs for off-label uses or provide off-label information in the promotion of drug products that is not consistent with the approved labeling for those products. For example, we may not currently promote ONPATTRO or AMVUTTRA in the U.S. for use in any indications other than the treatment of the polyneuropathy of hATTR amyloidosis with polyneuropathy in adults. The FDA and other regulatory and enforcement authorities actively enforce laws and regulations prohibiting promotion of off-label uses and the promotion of products for which marketing approval has not been obtained. In April 2021, we received a subpoena from the U.S. Department of Justice, U.S. Attorney’s Office for the District of Massachusetts, requiring production of documents pertaining to our marketingobtained, and promotion of ONPATTRO (patisiran)if in the U.S. We are cooperating with the U.S. Attorney’s Office and producing documents in response to the subpoena. Current and former officers and employees also have received subpoenas in connection with the preservation and production of related materials. Given the ongoing nature of the investigation, it is possible that the U.S. Attorney’s Office for the District of Massachusetts or other government entities may request other information from, or issue other subpoenas, findings or similar documents to, us, our related entities and their respective directors, officers and employees. Iffuture we are found to have improperly marketed or promoted ONPATTRO in connection with such subpoenas,any of our commercial products, we may be subject to a broad range of civil, administrative and criminal penalties, including injunctive relief related to ONPATTROsuch commercial products’ promotional activities, substantial fines or penalties, and other legal or equitable sanctions. Any adverse decision, finding, allegation, or exercise of enforcement or regulatory discretion could harm our business, prospects, operating results, and financial condition. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence.
Notwithstanding regulations related to product promotion, the FDA and other regulatory authorities allow companies to engage in truthful, non-misleading and non-promotional scientific exchange concerning their products, and we intend to engage in medical education activities and communicate with healthcare providers in compliance with all applicable laws and regulatory guidance. Nonetheless, the FDA, other applicable regulatory authorities, competitors, and other third parties may take the position that we are not in compliance with such regulations, and if such non-compliance is proven, it could harm our reputation financial condition or divert financial and management resources from our core business, and would have a material adverse effect on our business, prospects, operating results or financial condition and results of operations.condition. Moreover, any threatened or actual government enforcement actions or lawsuits by third parties could also generate adverse publicity, which could decrease demand for our products and require that we devote substantial resources that otherwise could be used productively on other aspects of our business.
In addition to our medical education efforts, we also offer patient support services to assist patients receiving treatment with our commercially approved products. Manufacturers have increasingly become the focus of government investigation of patient support programs based on allegations that through such services illegal inducements are provided to physicians and/or patients, leading to improper utilization of government resources through Medicare, Medicaid and other government programs. Companies that are found to have violated laws such as the federal Anti-Kickback Statute and/or the federal False Claims Act, or FCA, face significant liability, including civil and administrative penalties, criminal sanctions, and potential exclusion from participation in government programs.
As described above,below, we remain focused on our global compliance program, which is designed to support the execution of these programs and activities in compliance with applicable laws.
Even if we or our collaborators receive regulatory approval to market our product candidates, the market may not be receptive to our product candidates upon their commercial introduction, which could prevent us from becoming profitable.adversely affect our business, prospects, operating results and financial condition.
The product candidates that we are developing are based upon new technologies or therapeutic approaches. Key participants in pharmaceutical marketplaces, such as physicians, third-party payors and consumers, may not accept a product intended to improve therapeutic results based on RNAi technology. As a result, it may be more difficult for us to convince the medical community and third-party payors to accept and use our product,products, or to provide favorable reimbursement.
Other factors that we believe will materially affect market acceptance of our product candidatesproducts include:
•the timing of our receipt of any marketing approvals, the terms of any approvals and the countries in which approvals are obtained;
•the safety and efficacy of our product candidates, as demonstrated in clinical trials and as compared with alternative treatments, if any;
•relative convenience, dosing regimen and ease of administration of our product candidates;
•the willingness of patients to accept potentially new routes of administration or new or different therapeutic approaches and mechanisms of action;
•the success of our physician education programs;
•the availability of adequate government and third-party payor reimbursement;
•the pricing of our products, particularly as compared to alternative treatments, and the market perception of such prices and any price increase that we may implement in the future; and
•availability of alternative effective treatments for the diseases that our product candidates we develop are intended to treat and the relative risks, benefits and costs of those treatments.
For example, one of our two commercially approved therapeutics for the treatment of the polyneuropathy of hATTR amyloidosis in adults, ONPATTRO utilizes an intravenous mode of administration with pre-medication that physicians and/or patients may not readily adopt, orand which may not compete favorably with other available options for the treatment of hATTR amyloidosis with polyneuropathy in adults, including inotersen, marketed by Ionis in several countries, which is administered subcutaneously, or tafamidis, marketed by Pfizer in several countries, which is in pill form. In addition, fitusiran represents a new approach to treating hemophilia which may not be readily accepted by physicians and patients and their caregivers. Patisiran,Assuming positive results from the HELIOS-B Phase 3 clinical trial, vutrisiran, if approved for the treatment of ATTR amyloidosis with cardiomyopathy, could face similar challenges in market acceptance.
We are a multi-product commercial company and expect to continue to invest significant financial and management resources to continue to build our marketing, sales, market access and distribution capabilities and further establish our global infrastructure. Even ifIf we successfullyare not able to continue to develop and scale our commercialthese capabilities, the marketwe may not be receptiveable to successfully commercialize our commercialcurrent and any future products.
HavingWe received our first product approval in August 2018 weand have established our capabilities for marketing, sales, market access and distribution over the last several years. We currently expect to rely on third parties to launch and market certain of our product candidates in certain geographies, if approved. However, we are commercializing ONPATTRO, AMVUTTRA,
GIVLAARI and OXLUMO, and intend to commercialize several of our late-stageother product candidates, if approved, on our own globally in major markets. Accordingly, we have developed internal marketing, sales, market access and distribution capabilities as part of our core product strategy initially in the U.S., Europe and Japan, with expansion ongoing globally, which has required, and will continue to require, significant financial and management resources. For those products for which we will perform marketing, sales, market access and distribution functions ourselves, including ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, and for future products we successfully develop wherewith respect to which we may retain certain product development and commercialization rights, we could face a number of additional risks, including:
•scaling and retaining our global sales, marketing and administrative infrastructure and capabilities;
•hiring, training, managing and supervising our personnel worldwide;
•the cost of further developing, or leveraging an established, marketing or sales force, which may not be justifiable in light of the revenues generated by any particular product and/or in any specific geographic region; and
•our direct sales and marketing efforts may not be successful.
If we are unable to continue to develop and scale our own global marketing, sales, market access and distribution capabilities for our current and any future products, we will not be able to successfully commercialize our products without reliance on third parties.
The patient populations suffering from hATTR amyloidosis with polyneuropathy, AHP and PH1 are small and have not been established with precision. If the actual number of patients suffering from these diseases is smaller than we estimate, or if we cannotfail to raise awareness of these diseases and diagnosis is not improved, our revenuebusiness, prospects, operating results and ability to achieve profitability from these productsfinancial condition may be adversely affected.
Our estimates regarding the potential market size for ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or any future products at the time we commence commercialization, may be materially different from the actual market size, including as a result of the indication approved by regulatory authorities, which could result in significant changes in our business plan and may have a material adverse effect on our business, prospects, operating results of operations and financial condition. For example, the initial indication approved by the FDA for ONPATTRO is for the treatment of the polyneuropathy of hATTR amyloidosis and not for the treatment of cardiomyopathy or other manifestations of the disease. In addition, the U.S. label does not include cardiac data included in our APOLLO-B Phase 3 study results. This had an adverse impact on the market opportunity for ONPATTRO in the U.S. While data from the APOLLO-B study of patisiran in ATTR amyloidosis patients with cardiomyopathy was positive, the FDA may determine that the data is not supportive of a label expansion of ONPATTRO for the treatment of cardiomyopathy, which would further impact ONPATTRO's market opportunity. In addition, our efforts to raise disease
awareness and improve diagnosis of our relevant disease states have been and may in the future bewere impacted by the COVID-19 pandemic. For example, in 2020 and 2021, we saw a reduction in peer to peerpeer-to-peer educational opportunities, reduced physician attendance at congresses and symposia and overall opportunities for physician engagement. As is the case with most orphan diseases, if we cannotare unable to successfully raise awareness of these diseases and improve diagnosis, it could have a material adverse effect on our business, prospects, operating results or financial condition, and it will be more difficult or impossible to achieve profitability.
Any drugsproducts we currently market or may develop in the future may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, thereby harming our business.business, prospects, operating results and financial condition.
The regulations that govern marketing approvals, coverage, pricing and reimbursement for new drugs vary widely from country to country.country and are subject to change. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing authorization or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. We are actively monitoring these regulations as we market and sell our approved products and as several of our other programsproduct candidates move through late stages of development. However, a number of our programsproduct candidates are currently in the earlier stages of development, and we will not be able to assess the impact of pricesuch regulations foror any changes to such development programs for a number of years. We might also obtain regulatory approval for a product, including one or more of our approved products, in a particular country, but then be subject to price regulations or price controls that delay our commercial launch of the product andand/or negatively impact the revenues we are able to generate from the sale of the product in that country and potentially in other countries due to reference pricing.
We believe that the efforts of governments and third-party payors to contain or reduce the cost of healthcare and legislative and regulatory proposals to broaden the availability of healthcare will continue to affect the business and financial condition of pharmaceutical and biopharmaceutical companies. In the U.S., pharmaceutical pricing is subject to both government and public scrutiny and calls for reform, and the U.S. government has continued to focus on legislative and regulatory changes designed to control costs. Specifically, there have been several recent U.S. Congressional inquiries into prescription drugs, and proposed and enacted federal and state legislation and regulations designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. These developments could, directly or indirectly, affect our ability to sell ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or future products, if approved, at a favorable price.
At the federal level, for example, the IRA includes several provisions that will impact our business to varying degrees. For example, the IRA may require us to pay rebates if we increase the cost of a Medicare Part B or Part D drug faster than the rate
of inflation. In addition, our cost-sharing responsibility for any approved product covered by Medicare Part D could be significantly greater under the newly designed Part D benefit structure compared to the pre-IRA benefit design. Under the IRA’s Price Negotiation Program, a FDA approval for vutrisiran for treatment of Stargardt Disease would cause us to lose the orphan exemption for AMVUTTRA from Medicare price negotiation. As a result, in October 2022, we announced we would not pursue a Phase 3 clinical trial to study vutrisiran for treatment of Stargardt Disease. Manufacturers that fail to comply with the IRA may be subject to various penalties, including civil monetary penalties or a potential excise tax. The effect of the IRA on our business and the healthcare industry in general continues to develop and may have additional adverse impacts on our company or our industry. The IRA is anticipated to have significant effects on the pharmaceutical industry and may reduce the prices we can charge and reimbursement we can receive for our products, among other effects.
Furthermore, the Biden administration has indicated that lowering prescription drug prices is a priority, but we do not know the impact of policies established by the Biden administration to lower the prices of prescription drug prices. For example, the Center for Medicare and Medicaid Innovation is developing new models intended to lower drug costs under Medicare and Medicaid, including designing new payment methods for drugs approved via FDA’s accelerated approval pathway, creating a list of generic drugs for which the out-of-pocket Part D costs will be capped at $2 a month per drug, and establishing new approach for administering outcomes-based agreements for cell and gene therapies. We do not know what additional steps the Biden administration may take to attempt to lower prescription drug prices or the impact of such steps. Although a number of these and other proposed measures may require authorization through additional legislation to become effective, and the current U.S. presidential administration may reverse or otherwise change these measures, both the current U.S. presidential administration and Congress have indicated that they will continue to seek new measures to control drug costs.
At the state level, governments have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical product pricing. Some of these measures include restricting price, reimbursement, discounts, product access, and marketing; imposing drug price, cost, and marketing disclosure and transparency requirements; permitting importation from other countries; and encouraging bulk purchasing. For example, on January 5, 2024, the FDA authorized Florida’s Agency for Health Care Administration’s drug importation proposal, the first step toward Florida facilitating importation of certain prescription drugs from Canada. Importation of drugs from Canada and the Most Favored Nation, or MFN, Model may materially and adversely affect the price we receive for any of our commercially approved products. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. These measures could reduce the ultimate demand for our products, once approved, or put pressure on our product pricing. We cannot predict what healthcare reform initiatives may be adopted in the future in the U.S. or other foreign countries. Further federal, state and foreign legislative and regulatory developments are likely, and we expect ongoing initiatives in the U.S. to increase pressure on drug pricing. Such reforms could have a material and adverse effect on our anticipated revenues from one or more of our approved products or other product candidates that we may successfully develop and for which we may obtain regulatory approval and may affect our business, prospects, operating results and financial condition and our ability to develop drug candidates.
Our ability to commercialize our approved products or any future products successfully also will depend in part on the extent to which coverage and reimbursement for these products and related treatments will be available from third-party payors such as government health administration authorities, private health insurers and other organizations. One or more of our approved products and any other products for which we are able to obtain marketing approval may not be considered medically necessary or cost-effective, and the amount reimbursed may be insufficient to allow us to sell such product(s) or any future products on a competitive basis. Increasingly, the third-party payors who reimburse patientsbasis or healthcare providers, such as government and private insurance plans, are requiring that drug companies provide them with predetermined discounts from list prices, and are seeking to reduce the prices charged or the amounts reimbursed for drug products. In the U.S., we have entered into over 40 value-based agreements, or VBAs, and are negotiating additional VBAs with commercial health insurers. The goal of these agreements is to ensure that we are paid based on the ability of our commercially approved products to deliver results in the real world setting comparable to those demonstrated in clinical trials, and the agreements are structured to link the performance of our approved products in real-world use to financial terms. Partnering with payers on these agreements is also intended to provide more certainty to them for their investment and help accelerate coverage decisions for patients. If the payment we receive for our products, or the reimbursement provided for such products, is inadequate in light of our development and other costs, or if reimbursement is denied, ourrealize an appropriate return on our investment could be adversely affected. In addition, we have stated publicly that we intend to grow through continued scientific innovation rather than arbitrary price increases. Specifically, we have stated that we will not raise the price of anyin product for which we receive marketing approval over the rate of inflation, as determined by the consumer price index for urban consumers (approximately 6.4% currently) absent a significant value driver. Our patient access philosophy could also negatively impact the revenues we are able to generate from the sale of one or more of our products in the future.
Some of the drugs we market need to be administered under the supervision of a physician or other healthcare professional on an outpatient basis, including ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO. Under currently applicable U.S. law, certain drugs that are not usually self-administered (including injectable drugs) may be eligible for coverage under the Medicare Part B program if:
•they are incident to a physician’s services;
•they are reasonable and necessary for the diagnosis or treatment of the illness or injury for which they are administered according to accepted standards of medical practice; and
•they have been approved by the FDA and meet other requirements of the statute.
development. There may be significant delays in obtaining coverage for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or foreign regulatory authorities. Moreover, eligibility for coverage does not imply that any drug will be reimbursed in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution or that covers a particular provider’s cost of acquiring the drug.product. Interim payments for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement may be based on payments allowed for lower-cost drugs that are already marketed, covered, and reimbursed, may be incorporated into existing payments for other services, and may reflect budgetary constraints or imperfections in Medicare data. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. In particular, governments in certain markets such as in EU, the U.K., Japan, and China, provide healthcare at low (or zero) direct costs to consumers at the point of care, and thus have significant power as large single payers to regulate prices or impose other cost control mechanisms. In addition, the emphasis on managed care in the U.S. has increased and we expect will continue to exert downward pressure on pharmaceutical pricing. Coverage policies, third-party reimbursement rates and pharmaceutical pricing regulations may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
President Biden signed an Executive Order on July 9, 2021 affirmingIncreasingly, the administration’s policy to (i) support legislative reformsthird-party payors who reimburse patients or healthcare providers, such as government and private insurance plans, are requiring that would lower thedrug companies provide them with predetermined discounts from list prices, of prescription drugs, including by allowing Medicare to negotiate drug prices, by imposingand are seeking
inflation caps, and, by supporting the development and market entry of lower-cost generic drugs and biosimilars; and (ii) support the enactment of a public health insurance option. Among other things, the Executive Order also directs the U.S. Department of Health and Human Services, or HHS, to provide a report on actions to combat excessive pricing of prescription drugs, continue to clarify and improve the approval framework for generic drugs and identify and address any efforts to impede generic drug competition, enhance the domestic drug supply chain, reduce the priceprices charged or the amounts reimbursed for drug products. In the U.S., we have entered into over 40 value-based agreements, or VBAs, and are negotiating additional VBAs with commercial health insurers. The goal of these agreements is to ensure that we are paid based on the Federal government pays for drugs, and address price gouging in the industry; and directs the FDA to work with states and Indian Tribes that propose to develop section 804 Importation Programs in accordance with the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, and the FDA’s implementing regulations. The FDA released such implementing regulations on September 24, 2020, which went into effect on November 30, 2020, providing guidance for states to build and submit importation plans for drugs from Canada. In response, authorities in Canada have passed rules designed to safeguard the Canadian drug supply from shortages. If implemented, importation of drugs from Canada and the Most Favored Nation, or MFN, Model may materially and adversely affect the price we receive for anyability of our commercially approved products. Further, on November 20, 2020, CMS issued an Interim Final Rule implementingproducts to deliver results in the MFN Model under which Medicare Part B reimbursement rates will be calculated for certain drugs based on the lowest price drug manufacturers receivereal world setting comparable to those demonstrated in OECD countries with a similar gross domestic product per capita. The MFN Model regulations mandate participation by identified Part B providers and would have applied to all U.S. states and territories for a seven-year period beginning January 1, 2021, and ending December 31, 2027. However, on December 29, 2021, CMS rescinded the proposed MFN rule. Additionally, on December 2, 2020, HHS published a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers. Pursuant to court order, the removal and addition of the aforementioned safe harbors have been delayed until January 1, 2023, requiring manufacturers to ensure the full value of co-pay assistance is passed on to the patient or these dollars will count toward the Average Manufacturer Price and Best Price calculation of the drug. On May 17, 2022, the U.S. District Court for the District of Columbia granted the Pharmaceutical Research and Manufacturers of America’s motion for summary judgement invalidating the accumulator adjustment rule. Although a number of these and other proposed measures may require authorization through additional legislation to become effective,our clinical trials, and the current U.S. presidential administration may reverse or otherwise change these measures, bothagreements are structured to link the current U.S. presidential administration and Congress have indicated that they will continue to seek new legislative measures to control drug costs.
We believe that the effortsperformance of governments and third-party payors to contain or reduce the cost of healthcare and legislative and regulatory proposals to broaden the availability of healthcare will continue to affect the business and financial condition of pharmaceutical and biopharmaceutical companies. Specifically, there have been several recent U.S. Congressional inquiries and proposed federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
A number of other legislative and regulatory changes in the healthcare system in the U.S. and other major healthcare markets have been proposed or enacted in recent months and years, and such efforts have expanded substantially in recent years. These developments could, directly or indirectly, affect our ability to sell ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or future products, if approved, at a favorable price.
In particular, in March 2010, the ACA was signed into law. This legislation changed the system of healthcare insurance and benefits intended to broaden coverage and control costs. The law also contains provisions that affect companies in the pharmaceutical industry and other healthcare related industries by imposing additional costs and changes to business practices. Among the provisions affecting pharmaceutical companies are the following:
•Mandatory rebates for drugs sold into the Medicaid program were increased, and the rebate requirement was extended to drugs used in risk-based Medicaid managed care plans.
•The 340B Drug Pricing Program under the Public Health Service Act was extended to require mandatory discounts for drug products sold to certain critical access hospitals, cancer hospitals and other covered entities.
•Pharmaceutical companies are required to offer discounts on brand-name drugs to patients who fall within the Medicare Part D coverage gap, commonly referred to as the “donut hole.”
•Pharmaceutical companies are required to pay an annual non-tax deductible fee to the federal government based on each company’s market share of prior year total sales of branded products to certain federal healthcare programs, such as Medicare, Medicaid, Department of Veterans Affairs and Department of Defense. Since we expect our branded pharmaceutical sales to constitute a small portion of the total federal healthcare program pharmaceutical market, we do not expect this annual assessment to have a material impact on our financial condition.
•The law provides that approval of an application for a follow-on biologic product may not become effective until 12 years after the date on which the reference innovator biologic product was first licensed by the FDA, with a possible
six-month extension for pediatric products. After this exclusivity ends, it will be easier for generic manufacturers to enter the market, which is likely to reduce the pricing for such products and could affect our profitability.
•The law creates a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected.
•The law expands eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing a manufacturer’s Medicaid rebate liability.
•The law expands the entities eligible for discounts under the Public Health Service Act pharmaceutical pricing program.
•The law expands healthcare fraud and abuse laws, including the civil FCA and the federal Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance.
•The law establishes new requirements to report financial arrangements with physicians and teaching hospitals and to annually report drug samples that manufacturers and distributors provide to physicians.
•The law establishes a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research.
•The law established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery methods.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. These changes included aggregate reductions to Medicare payments to providers of 2% per fiscal year. These reductions went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2030, with the exception of a temporary suspension from May 1, 2020 through March 31, 2022 due to the COVID-19 pandemic. Following the suspension, a 1% payment reduction began April 1, 2022, lasting through June 30, 2022. The 2% payment reduction resumed on July 1, 2022. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices we may obtain for our approved products or any of our product candidates for which we may obtain regulatory approval, or the frequency with which our products or any future product is prescribed or used.
Further, there have been several changes to the 340B Drug Pricing Program, which imposes ceilings on prices that drug manufacturers can charge for medications sold to certain healthcare facilities. On December 27, 2018, the District Court for the District of Columbia invalidated a reimbursement formula change under the 340B Drug Pricing Program, and CMS subsequently altered the fiscal years 2019 and 2018 reimbursement formula on specified covered outpatient drugs. The court ruled this change was not an “adjustment” which was within the Secretary’s discretion to make but was instead a fundamental change in the reimbursement calculation. However, most recently, on July 31, 2020, the U.S. Court of Appeals for the District of Columbia Circuit overturned the district court’s decision and found that the changes were within the Secretary’s authority. On September 14, 2020, the plaintiffs-appellees filed a Petition for Rehearing En Banc (i.e., before the full court), and the court denied this petition on October 16, 2020. Plaintiffs-appellees filed a petition for a writ of certiorari at the Supreme Court on February 10, 2021. On July 2, 2021, the Supreme Court granted the petition. On June 15, 2022, the Supreme Court unanimously reversed the Court of Appeals’ decision, holding that HHS’s 2018 and 2019 reimbursement rates for 340B hospitals were contrary to the statute and unlawful. It is unclear how these developments could affect covered hospitals who might purchase our future products and affect the rates we may charge such facilities for our approved products in real-world use to financial terms. Partnering with payors on these agreements is also intended to provide more confidence regarding the future, if any.
The IRA, which among other things, allows for CMS to negotiate prices for certain single-source drugs and biologics reimbursed under Medicare Part B and Part D, beginning with ten high-cost drugs paid for by Medicare Part D starting in 2026, followed by 15 Part D drugs in 2027, 15 Part B or Part D drugs in 2028, and 20 Part B or Part D drugs in 2029 and beyond. The legislation subjects drug manufacturers to civil monetary penalties and a potential excise tax for failing to comply with the legislation by offering a price that is not equal to or less than the negotiated “maximum fair price” under the law or for taking price increases that exceed inflation. The legislation also requires manufacturers to pay rebates for drugs in Medicare Part D whose price increases exceed inflation. Further, the legislation caps Medicare beneficiaries’ annual out-of-pocket drug expenses at $2,000. Under the IRA, a second FDA approval for vutrisiran for Stargardt Disease would cause us to lose the single-orphan exemption for AMVUTTRA from Medicare price negotiation. As a result, in October 2022, we announced we would not pursue a Phase 3 clinical trial to study vutrisiran in Stargardt Disease. The effectvalue of the IRA on our business and the healthcare industry in general is developing and may have an additional adverse impact on our industry.
The full effects of the U.S. healthcare reform legislation cannot be known until the law is fully implemented through regulations or guidance issued by CMS and other federal and state healthcare agencies. The financial impact of the U.S. healthcare reform legislation over the next few years will depend on a number of factors, including, but not limited, to the policies reflected in implementing regulations and guidance, and changes in sales volumes for products affected by the new system of rebates, discounts and fees. This legislation may also have a positive impact on our future net sales, if any, by increasing the aggregate number of persons with healthcare coverage in the U.S.
Since its enactment, there have been numerous judicial, administrative, executive, and legislative challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. On June 17, 2021, the U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court's decision, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for the purpose of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how other healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal or replace the ACA will impact our business.
At the state level, legislatures have become increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical product pricing. Some of these measures include price or patient reimbursement constraints, discounts, restrictions on certain product access, marketing cost disclosure and transparency measures, and, in some cases, measures designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. These measures could reducehelp accelerate coverage decisions for patients. If the ultimate demandpayment we receive for our products, once approved, or put pressurethe reimbursement provided for such products, is inadequate in light of our significant development and other costs, or if reimbursement is denied, our return on ourinvestment could be adversely affected. In addition, we have stated publicly that we intend to grow through continued scientific innovation rather than arbitrary price increases. Specifically, we have stated that we will not raise the price of any product pricing.
We cannot predict what healthcare reform initiatives may be adopted infor which we receive marketing approval over the future. Further federal and state legislative and regulatory developmentsrate of inflation, as determined by the consumer price index for urban consumers (approximately 3.4% currently) ) absent a significant value driver. Our patient access philosophy could also negatively impact the revenues we are likely, and we expect ongoing initiatives inable to generate from the U.S. to increase pressure on drug pricing. Such reforms could have an adverse effect on anticipated revenues fromsale of one or more of our products in the future.
Insurers are increasingly adopting programs and policies that limit access to medications and increase out-of-pocket costs for patients. In the U.S., to help patients access and afford our approved products or other product candidates thatproduct(s), we may successfully developutilize programs to assist them, including patient assistance programs and co-pay coupon programs for eligible patients. It is possible that changes in insurer policies regarding co-pay coupons (such as co-pay accumulator and maximizer programs) and patient assistance programs (such as alternative funding programs) and/or the introduction and enactment of new legislation or regulatory action could restrict or otherwise negatively affect these co-pay coupon programs and patient support programs, which we may obtain regulatory approvalcould result in fewer patients using affected products, and may affecttherefore could have a material adverse effect on our overallsales, business, and financial condition and ability to develop drug candidates.condition.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations. Failure to comply with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations, which can harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Control, and anti-corruption laws, including the FCPA,U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, the UK Bribery Act 2010, and other state and nationalapplicable anti-bribery and anti-money laundering laws in the countries in which we conduct activities.laws. Anti-corruption laws are interpreted broadly and prohibit companies and their officers, directors, employees, agents, contractors, and other collaboratorsthird-party representatives from directly or indirectly authorizing, promising, offering, providing, soliciting, or providing, directly or indirectly, improperreceiving payments or anything else of value in order to improperly influence the acts or decisions of recipients in the public or private sector.sector or to secure any other improper advantage to obtain or retain business. From time to time, we may engage third parties to conduct clinical trials outside of the U.S., to sell our products abroad, and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other collaborators,third-party representatives acting on our behalf, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other adverse consequences.
We remain focused on these laws and the activities they regulate and, as detailed above,below, maintain a global compliance program designed to empower our business to operate in compliance with their requirements.
Governments outside the U.S. may impose strict price controls, which may adversely affect our revenues.
The pricing of prescription pharmaceuticals is also subject to governmental control outside the U.S. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of regulatory approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidates to other available therapies.therapies, which is time-consuming and costly. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our ability to generate revenues and become profitable could be impaired.
In some countries, including Member States of the EU, or Japan, the pricing of prescription drugs ismay be subject to governmental control. Additional countries may adopt similar approaches to the pricing of prescription drugs. In such countries, pricing negotiations with governmental authorities can take considerable time after receipt of regulatory approval for a product. In addition, there can be considerable pressure by governments and other stakeholders can put considerable pressure on prices and reimbursement levels, including as part of cost containment measures. Moreover, political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after coverage and reimbursement have been obtained. Reference pricing used by various countries and parallel distribution, or arbitrage between low-priced and high-priced countries, can further reduce prices. In some countries, we may be required to conduct a clinical study or other studies that compare the cost-effectiveness of a product candidate to other available therapies in order to obtain or maintain reimbursement or pricing approval, which is time-consuming and costly. We cannot be sure that such prices and reimbursement will be acceptable to us or our strategic partners.collaborators. Publication
of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If pricing is set at unsatisfactory levels or if reimbursement of our products is unavailable or limited in scope or amount, our revenues from sales by us or our strategic partnerscollaborators and the potential profitability of our approved products or any future products in those countries would be negatively affected. AnotherWe could also suffer impact from the tightening pricing control could be felt fromcontrols on account of greater competition from less expensive generic or biosimilar products once thepatent or other exclusivity expires; theexpires. Certain governments have adopted policies to switch prescribed products to generic versions in order to cut the medical cost.reduce costs.
If we or our collaborators, CMOs or service providers fail to comply with healthcare laws and regulations, or legal obligations related to privacy, data protection and information security, we or they could be subject to enforcement actions, which could affectnegatively impact our ability to develop, market and sell our products and may harm our reputation.
AsHealthcare providers, physicians, and third-party payors play a manufacturerprimary role in the recommendation and prescription of pharmaceuticals,any products for which we are subjectobtain marketing approval. Our existing and future arrangements with third-party payors and customers may expose us to federal, state,broadly applicable fraud and comparable foreignabuse and other healthcare laws and regulations pertaining to fraudthat may constrain our business or financial arrangements and abuserelationships through which we market, sell, and patients’ rights, in addition to legal obligations related to privacy, data protectiondistribute our products. Restrictions under applicable federal and information security. Thesestate healthcare laws and regulations include:include the following:
•The U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, the purchase, lease, order, arrangement, or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it to have committed a violation. Violations are subject to civil and criminal fines and penalties, for each violation, plus up to three times the remuneration involved, imprisonment, and exclusion from government healthcare programs. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal FCA or federal civil money penalties.
•The U.S. federal false claims laws, including the FCA, which prohibit, among other things, individuals or entities from knowingly presenting or causing to be presented, claims for payment by government-funded programs such as Medicare or Medicaid that are false or fraudulent, making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government, or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. Manufacturers can be held liable under the FCA even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. The FCA also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery. Penalties are three times the amount of the claims in question plus civil monetary penalties.
•The federal civil monetary penalties laws, which impose civil fines for, among other things, the offering or transfer of remuneration to a Medicare or state healthcare programMedicaid beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state healthcare program,Medicaid, unless an exception applies.
•The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created, among other provisions, federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and, in any matter involving a health care benefit program, knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters.services. Similar to the federal Anti-Kickback Statute, a person or entity can be found guilty of violating HIPAA without actual knowledge of the statute or specific intent to violate it.
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, including its implementing regulations, which imposesimpose requirements relating to the privacy, security, and transmission of individually identifiable health information; and requires notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information.
•Federal “sunshine” requirements imposed by the ACAAffordable Care Act on drug, device, biological and medical supply manufacturers when payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to HHSHealth and Human Services under the Open Payments Program, information regarding any payment or other “transfer of value” made or distributed to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain non-physician providers such as physician assistants and nurse practitioners, and teaching hospitals, as well as ownership and investment interests held by physicians and their
immediate family members. Failure to submit requiredtimely, accurate and complete information may result in civil monetary penalties for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission.penalties.
•Federal price reporting laws, which require manufacturers to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on approved products.
•Federal statutory and regulatory requirements applicable to pricing and sales of productproducts to Federal Government Agencies.federal government agencies.
•Federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers.
•State and foreign laws comparable to each of the above federal laws, including in the EU laws prohibiting giving healthcare professionals any gift or benefit in kind as an inducement to prescribe our products, national transparency laws requiring the public disclosure of payments made to healthcare professionals and institutions, and data privacy laws, in addition to anti-kickback and false claims laws applicable to commercial insurers and other non-federal payors, requirements for mandatory corporate regulatory compliance programs, and laws relating to government reimbursement programs, patient data privacy and security.
•European privacy laws including Regulation 2016/679, known as the General Data Protection Regulation, or the EU GDPR, and the EU GDPR as transposed into the laws of the UK, the UK GDPR, collectively referred to as the GDPR, and the e-Privacy Directive (2002/58/EC), and the national laws implementing each of them, as well as the Public and Electronic Communications Regulations 2003 in the UK and the privacy laws of Japan, Brazil and other territories. Failure to comply with our obligations under the privacy regime could expose us to significant fines and/or adverse publicity, which could have material adverse effects on our reputation and business.
•The California Consumer Privacy Act of 2018, as amended by the California Privacy Rights Act of 2020, or, collectively, the CCPA, effective as of January 1, 2020, that, among other provisions, gives California residents expanded rights toof access, correction, portability, and require deletion of their personal information and various opt out of certain personal information sharing,rights. The CCPA also imposes various obligations on regulated businesses, such as to maintain privacy notices, implement reasonable security practices, and receive detailed information about how their personal informationinclude specific terms in contracts with data processors. The CCPA also created a new state agency that is used.vested with authority to implement (including through rule making) and enforce the CCPA. The CCPA provides for civil penalties for violations, as well as a limited private right of action for data breaches that is expected to increase data breach litigation.breaches.
•Additionally, a new California ballot initiative, the California Privacy Rights Act of 2020, or CPRA, was passed in November 2020. Effective as of January 1, 2023, the CPRA imposes additional obligations on companies covered by the legislation and will significantly modifyFurthermore, comprehensive privacy laws similar to the CCPA including by expanding consumers’ rights with respect to certain sensitive personal information. The CPRA also creates a new state agency that will be vested with authority to implement and enforce the CCPA and the CPRA. Furthermore, similar laws werehave been enacted in fourmore than ten other states and proposed in numerousseveral others. Three states have additionally enacted laws regulating “consumer health data,” which impose additional obligations on regulated entities beyond state comprehensive privacy laws, such as to obtain distinct consents for certain collection and sharing of consumer health data, obtain authorization to sell consumer health data, and maintain a consumer health data privacy policy. Washington’s law regulating consumer health data contains a private right of action. The effects of the CCPA and the CPRAother state privacy laws are potentially significant and may require us to modify our data collection or processing practices and policies and to incur substantial costs and expenses in an effort to comply and increase our potential exposure to regulatory enforcement and/or litigation.
Some state laws also require pharmaceutical manufacturers to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, in addition to requiring manufacturers to report information related to payments to physicians and other healthcare providesproviders or marketing expenditures and pricing information. State and foreign laws also govern the privacy and security of health information, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating our compliance efforts.
If our operations are found to be in violation of any of the aforementioned requirements, we may be subject to penalties, including civil or criminal penalties (including individual imprisonment), criminal prosecution, monetary damages, the curtailment or restructuring of our operations, loss of eligibility to obtain approvals from the FDA, or exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, or the imposition of a corporate integrity agreement with the Office of Inspector General of the Department of Health and Human Services, or the OIG, any of which could materially and adversely affect our business, prospects, operating results or financial results.condition. We remain focused on enhancing our global compliance infrastructure following the commercial launch of our four products over the last four years in the U.S., EU and multiple other geographies, and as we prepare for the launch of our products in additional countries, assuming regulatory approvals. Although effective compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. For additional information, see the Risk Factor captioned “We may incur significant liability if enforcement authorities allege or determine that we are engaging in commercial activities with respect to our unapproved product candidates or promoting
our commercially approved products in a way that violates applicable regulations.” Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
If we or our collaborators, CMOs or service providers fail to comply with applicable federal, state or foreign laws or regulations, we could be subject to enforcement actions, which could affect our ability to develop, market and sell our approved products, or any other future products, successfully and could harm our reputation and lead to reduced acceptance of our products by the market. These enforcement actions include, among others:
•adverse regulatory inspection findings;
•untitled letters or warning letters;
•voluntary or mandatory product recalls or public notification or medical product safety alerts to healthcare professionals;
•restrictions on, or prohibitions against, marketing our products;
•restrictions on, or prohibitions against, importation or exportation of our products;
•suspension of review or refusal to approve pending applications or supplements to approved applications;
•exclusion from participation in government-funded healthcare programs;
•exclusion from eligibility for the award of government contracts for our products;
•suspension or withdrawal of product approvals;
•product seizures;
•injunctions; and
•others, civil and criminal penalties, up to and including criminal prosecution resulting in fines, exclusion from healthcare reimbursement programs and imprisonment.
Moreover, federal, state orand foreign laws or regulations are subject to change, and while we, our collaborators, CMOs and/or service providers currently may be compliant, thatwe could changefall out of compliance due to changes in interpretation, prevailing industry standards or the legal structure.
Third party patient assistance programs that receive financial support from companies have become the subject of enhanced government and regulatory scrutiny. The OIG has established guidelines that suggest that it is lawful for pharmaceutical manufacturers to make donations to charitable organizations who provide co-pay assistance to Medicare patients, provided that such organizations, among other things, are bona fide charities, are entirely independent of and not controlled by the manufacturer, provide aid to applicants on a first-come basis according to consistent financial criteria and do not link aid to use of a donor'sdonor’s product. However, donations to patient assistance programs have received some negative publicity and have been the subject of multiple government enforcement actions, related to allegations regarding their use to promote branded pharmaceutical products over other less costly alternatives. Specifically, in recent years, there have been multiple settlements resulting out offrom government claims challenging the legality of their patient assistance programs under a variety of federal and state laws. It is possible that weWe have made and may continue to make grants to independent charitable foundations that help financially needy patients with their premium, co-pay, and co-insurance obligations. If we choose to do so, and if we or our vendors or donation recipients are deemed to fail to comply withbe acting in violation of relevant laws, regulations or evolving government guidance, in the operation of these programs, we could be subject to damages, fines, penalties, or other criminal, civil, or administrative sanctions or enforcement actions. We cannot ensure that our compliance controls, policies, and procedures will be sufficient to protect against acts of our employees, business partners, or vendors that may violate the laws or regulations of the jurisdictions in which we operate. Regardless of whether we have complied with the law, a government investigation could impact our business practices, harm our reputation, divert the attention of management, increase our expenses, and reduce the availability of foundation support for our patients who need assistance.
We are subject to governmental regulation and other legal obligations particularly related to privacy, data protection and information security, and we are subject to consumer protection laws that regulate our marketing practices and prohibit unfair or deceptive acts or practices. Our actual or perceived failure to comply with such obligations could harm our business.
The GDPR imposes strict requirements on controllers and processors of personal data, including special protections for “special category data,” which includes health, biometric and genetic information of data subjects located in the EEA and UK. Further, GDPR provides a broad right for EEA Member States to create supplemental national laws, such as laws relating to the processing of health, genetic and biometric data, which could further limit our ability to use and share such data or could cause our costs to increase, and harm our business and financial condition.
Failure to comply with the requirements of the GDPR and the related national data protection laws of the EEA Member States and the UK, which may deviate slightly from the GDPR, may result in fines of up to 4% of total global annual revenue, or €20.0 million (₤17.5 million under the UK GDPR), whichever is greater, and in addition to such fines, we may be the subject of litigation and/or adverse publicity, which could have a material adverse effect on our reputation and business. As a result of the implementation of the GDPR, we are required to put in placeimplement a number of measures to ensure compliance with the data protection regime. The GDPR (i) requires us to inform data subjects of how we process their personal data and how they can exercise their rights, (ii) requires us to ensure we have a valid legal basis to process personal data (if this is consent, the requirements for obtaining consent carries a higher threshold), (iii) requires us to appoint a data protection officer where sensitive personal data (i.e., health data) is processed on a large scale, (iv) introduces mandatory data breach notification requirements throughout the EEA and UK, (v) requires us to maintain records of our processing activities and to document data protection impact assessments where there is high risk processing, (vi) imposes additional obligations on us when we are contracting with service providers, requires (vii) appropriate technical and organisationalorganizational measures to be put in place to safeguard personal data and (viii) requires us to adopt appropriate privacy governance including policies, procedures, training and data audit.
Significantly, the GDPR imposes strict rules on the transfer of personal data out of the EEA and UK to the U.S. or other regions that have not been deemed to offer “adequate” privacy protections. In the past, companies in the U.S. were able to rely upon the EU-U.S., UK-U.S. and the Swiss-U.S. Privacy Shield frameworks to legitimizeas a basis for lawful transfer of personal data transfers from the EU and the UK to the U.S. In July 2020, the Court of Justice of the European Union, or CJEU, in Case C-311/18 (Data Protection Commissioner v Facebook Ireland and Maximillian Schrems, or Schrems II) invalidated the EU-U.S. Privacy Shield on the grounds that the Privacy Shield failed to offer adequate protections to EU personal data transferred to the U.S. The CJEU, in the same decision, deemed that the Standard Contractual Clauses, or SCCs, published by the EC are valid. However, the CJEU ruled that transfers made pursuant to the SCCs need to be assessed on a case-by-case basis to ensure the law in the recipient country provides “essentially equivalent” protections to safeguard the transferred personal data as the EU, and required businesses to adopt supplementary measures if such standard is not met. Subsequent guidance published by the European Data Protection Board, or EDPB, in June 2021 described what such supplementary measures must be, and stated that businesses should avoid or cease transfers of personal data if, in the absence of supplementary measures, equivalent protections cannot be afforded. On June 4, 2021, the EC published new versions of the SCCs, which seek to address the issues identified by the CJEU’s Schrems II decision and provide further details regarding the transfer assessments that the parties are required to conduct when implementing the Newnew SCCs. However, there continue to be concerns about whether the SCCs and other
mechanisms will face additional challenges. Similarly, in September 2020, the Swiss data protection authority determined the Swiss-U.S. Privacy Shield framework was no longer a valid mechanism for Swiss-U.S. data transfers and also raised questions about the validity of the SCCs as a mechanism for transferring personal data from Switzerland. While SCCs provide an alternative to our Privacy Shield certification for EU-U.S. data flows, the decision (and certain regulatory guidance issued in its wake) casts doubt on the legality of EU-U.S. data flows in general. Any inability to transfer, or burdensome restrictions on the ability to transfer, personal data from the EU to the U.S. in compliance with applicable data protection laws may impede our ability to conduct clinical trials and may adversely affect our business, prospects, operating results and financial position.condition. The UK is not subject to the EC’s new SCCs but has published its own transfer mechanism, the International Data Transfer Agreement or International Data Transfer Addendum, which enables transfers from the UK. On March 25, 2022, the EC and the U.S. announced to have reached a political agreement on a new “Trans-Atlantic Data Privacy Framework”, which will to replace the invalidated Privacy Shield and on December 13, 2022,Shield. The framework introduced new binding safeguards to address the concerns raised by the CJEU in Schrems II. On July 10, 2023, the EC published a draftannounced that it had adopted its adequacy decision onfor that data privacy framework, labelled the Trans-AtlanticEU-U.S. Data Privacy Framework. The adequacy decision concluded that the U.S. ensures an adequate level of protection for personal data transferred from the EU to US companies under the new framework, and the EC stated that as a result personal data can flow safely from the EU to US companies participating in the framework, without having to put in place additional data protection safeguards. The EU-U.S. Data Privacy Framework is subject to periodic reviews, to be conducted by the EC, together with other European data protection authorities and U.S. authorities, with the first review to take place within a year of adoption of the adequacy decision. A case has been lodged with and remains pending before the EU courts challenging the validity of the EU-U.S. Data Privacy Framework.
EEA Member States have adopted implementing national laws to implement the GDPR which may partially deviate from the GDPR and the competent authorities in the EEA Member States may interpret GDPR obligations slightly differently from country to country, so thatand we do not expect to operate in a uniform legal landscape in the EU. In addition, the UK Government has announced plansnow introduced a Data Protection and Digital Information Bill, or the UK Bill, into the UK legislative process. The aim of the UK Bill is to reform the country’sUK’s data protection legal frameworkregime following Brexit. If passed, the final version of the UK Bill may have the effect of further altering the similarities between the UK and EEA data protection regime. The anticipated UK general election in its Data Reform Bill, but these have been put on hold.2024 could postpone passage of the UK Bill.
We are subject to the supervision of local data protection authorities in those jurisdictions wherein which we are monitoring the behavior of individuals in the EEA or UK (i.e., undertaking clinical trials). We depend on a number of third parties in relation to the provision of our services, a number of which process personal data of EU and/or UK individuals on our behalf. With each such provider we enter or intend to enter into contractual arrangements under which they arethe provider is contractually obligated to only process personal data according to our instructions, and conduct or intend to conduct diligence to ensure that they have sufficient technical and organizational security measures in place.
We are also subject to evolving European privacy laws on electronic marketing and cookies. The EU is in the process of replacing the e-Privacy Directive (2002/58/EC) with a new set of rules taking the form of a regulation, which will be directly implemented in the laws of each European member state, without the need for further enactment. While the e-Privacy Regulation was originally intended to be adopted on May 25, 2018 (alongside the GDPR), it is still going through the European legislative process. Draft regulations were rejected by the Permanent Representatives Committee of the Council of EU on November 22, 2019; it is not clear when, or even if, new regulations will be adopted. We are also subject to current and evolving privacy laws in other foreign countries, such as Canada.
Compliance with U.S. and international data protection laws and regulations could require us torequires that we take on more onerous obligations in our contracts, restrict our ability to collect, use and disclose data, orand, in some cases, impactimpacts our ability to operate in certain jurisdictions. Failure to comply with these laws and regulations could result in government enforcement actions
(which (which could include civil, criminal and administrative penalties), private litigation, and/or adverse publicity and could negatively affect our operating results and business. Moreover, clinical trial subjects, employees and other individuals about whom we or our potential collaborators obtain personal information, as well as the providers who share this information with us, may limit our ability to collect, use and disclose the information. Claims that we have violated individuals’ privacy rights, failed to comply with data protection laws, or breached our contractual obligations, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business.
Our ability to obtain services, reimbursement or funding from the federal government may be impacted by possible reductions in federal spending and services, and any inability on our part to effectively adapt to such changes could substantially affect our business, prospects, operating results and financial position, results of operations and cash flows.condition.
Under the Budget Control Act of 2011, the failure of Congress to enact deficit reduction measures of at least $1.2 trillion for the years 2013 through 2021 triggered automatic cuts to most federal programs. These cuts included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. Certain of these automatic cuts have been implemented resulting in reductions in Medicare payments to physicians, hospitals, and other healthcare providers, among other things. Due to legislation amending the statute, including the Bipartisan Budget Act of 2018, these reductions will stay in effect through 2030 unless additional Congressional action is taken. Pursuant to the CARESCoronavirus Aid, Relief, and Economic Security Act, as well as subsequent legislation, these reductions were suspended from May 1, 2020 through December 31, 2021 due to
the COVID-19 pandemic. Following the suspension, a 1% payment reduction began on April 1, 2022, lasting through June 30, 2022. The full impact2% payment reduction resumed on July 1, 2022. The American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices we may obtain for our businessapproved products or any of these automatic cuts is uncertain.
If other federal spending is reduced, any budgetary shortfallsour product candidates for which we may also impact the ability of relevant agencies, such as the FDAobtain regulatory approval, or the NIHfrequency with which our products or any future product is prescribed or used.
Previous actions taken by Congress to continue to function.reduce spending, disagreements in Congress over government funding levels, high-levels of government debt, and the Medicare Trustees’ warnings about the programs’ sustainability as presently structured suggest that uninterrupted/continued growth in funding for relevant programs is not guaranteed. Amounts allocated to federal grants and contracts may be reduced or eliminated. These reductions may also impact the ability of relevant agencies to timely review and approve drug research and development, manufacturing, and marketing activities, which may delay our ability to develop, market and sell our approved products and any other products we may develop. Further, there has been heightened
If we fail to comply with our obligations under the 340B Drug Pricing Program or other U.S. governmental scrutiny recently overpricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and fines, which could have a material adverse effect on our business, prospects, operating results and financial condition.
We participate in the manner in which drug manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries340B Drug Pricing Program, Medicaid Drug Rebate Program, and proposed and enacteda number of other federal and state legislation designedgovernment pricing programs in the U.S. in order to amongobtain coverage for our products by certain government health care programs. These programs generally require that we provide discounts or pay rebates to certain payers when our products are dispensed to beneficiaries of these programs. These programs may also impose other things, bring more transparencyrequirements, including certain price reporting requirements. Changes to productour obligations under these government pricing reviewprograms occur frequently and program requirements are often ambiguous. We may be or become subject to penalties as a result of our failure to comply with obligations under these programs, including if we fail to provide timely and accurate information to the relationship between pricing and manufacturer patientgovernment, to pay the correct rebates, or to offer the correct discounted pricing. Complying with these programs and reform government program reimbursement methodologies for drug products.future changes to these programs can be cost-and resource-intensive and could have a material adverse effect on our business, prospects, operating results and financial condition.
There is a substantial risk of product liability claims in our business. If we are unable to obtain sufficient insurance, a product liability claim against us could adversely affect our business.business, prospects, operating results and financial condition.
Our business exposes us to significant potential product liability risks that are inherent in the development, testing, manufacturing and marketing of human therapeutic products. Product liability claims could delay or prevent completion of our clinical development programs. Following the decision to discontinue clinical development of revusiran, we conducted a comprehensive evaluation of available revusiran data. We reported the results of this evaluation in August 2017, however, our investigation did not result in a conclusive explanation regarding the cause of the mortality imbalance observed in the ENDEAVOUR Phase 3 study. In addition, in September 2017, we announced that we had temporarily suspended dosing in all ongoing fitusiran studies pending further review of a fatal thrombotic SAE and agreement with regulatory authorities on a risk mitigation strategy. Notwithstanding the risks undertaken by all persons who participate in clinical trials, and the information on risks provided to study investigators and patients participating in our clinical trials, including the revusiran and fitusiran studies, it is possible that product liability claims will be asserted against us relating to the worsening of a patient’s condition, injury or death alleged to have been caused by one of our product candidates, including revusiran or fitusiran. Such claims might not be fully covered by product liability insurance. In addition, product liability claims could result in an FDA investigation of the safety and effectiveness of our approved products, our manufacturing processes and facilities or our marketing programs, and potentially a recall of our products or more serious enforcement action, limitations on the approved indications for which they may be used, or suspension or withdrawal of approvals. Regardless of the merits or eventual outcome, liability claims may also result in decreased demand for our products, injury to our reputation, costs to defend the related litigation, a diversion of management’s time and our resources, substantial monetary awards to trial participants or patients and a decline in our stock price. We currently have product liability insurance that we believe is appropriate for our stage of development, including the marketing and sale of our approved products. Any insurance we have or may obtain may not provide sufficient coverage against potential liabilities. Furthermore, clinical trial and product liability insurance is becoming increasingly expensive. As a result, we may be unable to obtain sufficient insurance at a reasonable cost to protect us against losses caused by product liability claims that could have a material adverse effect on our business.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements or insider trading violations, which could significantly harm our business.business, prospects, operating results and financial condition.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with governmental regulations, comply withincluding healthcare fraud and abuse and anti-kickback laws and regulations in the U.S. and abroad, or failure to report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. TheseAs discussed in the Risk Factor captioned “If we or our collaborators, CMOs or service providers fail to comply with healthcare laws and
regulations, or legal obligations related to privacy, data protection and information security, we or they could be subject to enforcement actions, which could negatively impact our ability to develop, market and sell our products and may harm our reputation,” these laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of, including improper trading based upon, information obtained in the course of clinical studies, which could result in regulatory sanctions and serious harm to our reputation. We maintain a global compliance program and remain focused on its evolution
and enhancement. Our program includes efforts such as risk assessment and monitoring, fostering a speak upspeak-up culture encouraging employees and third parties to raise good faith questions or concerns, and defined processes and systems for reviewing and remediating allegations and identified potential concerns. It is not always possible, however, to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, prospects, operating results and results of operations,financial condition, including the imposition of significant fines or other sanctions.
If we do not comply with laws regulating the protection of the environment and health and human safety, our business, prospects, operating results and financial condition could be adversely affected.
Our research, development and manufacturing involve the use of hazardous materials, chemicals and various radioactive compounds. We maintain quantities of various flammable and toxic chemicals in our facilities in Cambridge and Norton that are required for our research, development and manufacturing activities. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. We believe our procedures for storing, handling and disposing these materials in our Cambridge and Norton facilities comply with the relevant guidelines of the City of Cambridge, the town of Norton, the Commonwealth of Massachusetts and the Occupational Safety and Health Administration of the U.S. Department of Labor. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards mandated by applicable regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could be substantial. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of biohazardous materials.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of these materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials. Additional federal, state and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with, and substantial fines or penalties if we violate, any of these laws or regulations.
Risks Related to Patents, Licenses and Trade Secrets
If we are not able to obtain and enforce patent protection for our discoveries, our ability to develop and commercialize our product candidates will be harmed.
Our success depends, in part, on our ability to protect proprietary compositions, methods and technologies that we develop under the patent and other intellectual property laws of the U.S. and other countries, so that we can prevent others from unlawfully using our inventions and proprietary information. However, we may not hold proprietary rights to some patents required for us to manufacture and commercialize our proposed products. Because certain U.S. patent applications are confidential until the patents issue, such as applications filed prior to November 29, 2000, or applications filed after such date which will not be filed in foreign countries, third parties may have filed patent applications for subject matter covered by our pending patent applications without our being aware of those applications, and our patent applications may not have priority over those applications. For this and other reasons, we may be unable to secure desired patent rights, thereby losing desired exclusivity. Further, we or our licenseescollaborators may be required to obtain licenses under third-party patents to market one or more of our or our partner'scollaborator’s approved products, or further develop and commercialize future products, or continue to develop product candidates in our pipeline being developed by us or our licensees.collaborators. If licenses are not available to us or not available on reasonable terms, we or our licensees may not be able to market the affected products or conduct the desired activities.
Our strategy depends on our ability to rapidly identify and seek patent protection for our discoveries. In addition, we may rely on third-party collaborators to file patent applications relating to proprietary technology that we develop jointly during certainas part of collaborations. The process of obtaining patent protection is expensive and time-consuming. If we or our present or future collaborators fail to file and prosecute all necessary and desirable patent applications at a reasonable cost and in a timely manner, our business may be adversely affected. Despite our efforts and the efforts of our collaborators to protect our proprietary rights, unauthorized parties may be able to obtain and use information that we regard as proprietary. While issued patents are presumed valid, this does not guarantee that the patent will survive a validity challenge or be held enforceable. Any patents we have obtained, or obtain in the future, may be challenged, invalidated, adjudged unenforceable or circumvented by parties attempting to design around our intellectual property. Moreover, third parties or the United States Patent and Trademark Office, or USPTO, may commence interference proceedings involving our patents or patent applications. Any challenge to,
finding of unenforceability or invalidation or circumvention of, our patents or patent applications, would be costly, would require significant time and attention of our management, could reduce or eliminate milestone and/or royalty payments to us from third party licensors and could have a material adverse effect on our business.
Our pending patent applications may not result in issued patents. The patent position of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves complex legal and factual considerations. The standards that the USPTO and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. Similarly, the ultimate degree of protection that will be afforded to biotechnology inventions, including ours, in the U.S. and foreign countries, remains uncertain and is dependent upon the scope of the protection decided upon by patent offices, courts and lawmakers. Moreover, there are periodic discussions in the U.S. Congress of the United States and in international jurisdictions about modifying various aspects of patent law. For example, the America Invents Act, or AIA, included a number of changes to the patent laws of the U.S. If any of the enacted changes do not provide adequate protection for discoveries, including our ability to pursue infringers of our patents for substantial damages, our business could be adversely affected. One major provision of the AIA, which took effect in March 2013, changed U.S. patent practice from a first-to-invent to a first-to-file system. If we fail to file an invention before a competitor files on the same invention, we no longer have the ability to provide proof that we were in possession of the invention prior to the competitor’s filing date, and thus would not be able to obtain patent protection for our invention. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents.
Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to us or to others. We also rely to a certain extent on trade secrets, know-how and technology, which are not protected by patents, to maintain our competitive position. If any trade secret, know-how or other technology not protected by a patent were to be disclosed to or independently developed by a competitor, our business, prospects, operating results and financial condition could be materially adversely affected.
Failure to obtain and maintain broad patent scope and all available regulatory exclusivities broad patent scope and to maximize patent term restoration or extension on patents covering our product candidates and products may lead to loss of exclusivity and early generic entry resulting in a loss of market share and/or revenue.
We license patent rights from third-party owners. If such owners do not properly or successfully obtain, maintain or enforce the patents underlying such licenses, our competitive position and business, prospects, operating results and financial condition may be harmed.
We are a party to a number of licenses that give us rights to third-party intellectual property that is necessary or useful for our business. In particular, we have obtained licenses from, among others, Ionis, Arbutus, and Dicerna. We also intend to enter into additional licenses to third-party intellectual property in the future.
Our success will depend in part on the ability of our licensors to obtain, maintain and enforce patent protection for our licensed intellectual property, in particular, those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute the patent applications to which we arehave licensed. Even if patents issue in respect of these patent applications, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents, or may pursue such litigation less aggressively than we would. Without protection for the intellectual property we license, other companies might be able to offer substantially identical products for sale, which could adversely affect our competitive business position and harm our business, prospects.prospects, operating results and financial condition. In addition, we sublicense our rights under various third-party licenses to our collaborators. Any impairment of these sublicensed rights could result in reduced revenues under our collaboration agreements or result in termination of an agreement by one or more of our collaborators.
Other companies or organizations may challenge our patent rights or may assert patent rights that prevent us from developing and commercializing our products.
RNAi is a relatively new scientific field, the commercial exploitation of which has resulted in many different patents and patent applications from organizations and individuals seeking to obtain patent protection in the field. We have obtained grants and issuances of RNAi patents and have licensed many of these patents from third parties on an exclusive basis. The issued patents and pending patent applications in the U.S. and in key markets around the world that we own or license claim many different methods, compositions and processes relating to the discovery, development, manufacture and commercialization of RNAi therapeutics.
Specifically, we have a portfolio of patents, patent applications and other intellectual property covering:covering, among other things: fundamental aspects of the structure and uses of siRNAs, including their use as therapeutics, and RNAi-related mechanisms; chemical modifications to siRNAs that improve their suitability for therapeutic and other uses; siRNAs directed to specific targets as treatments for particular diseases; delivery technologies, such as in the fields of carbohydrate conjugates and cationic liposomes; and all aspects of our specific development candidates.
As the field of RNAi therapeutics is maturing, patent applications are being fully processed by national patent offices around the world. There is uncertainty about which patents will issue, and, if they do, as to when, to whom, and with what claims. It is likely that there will be significant litigation and other proceedings, such as interference, re-examination and opposition proceedings, as well as inter partespre- and post-grant review proceedings introduced by provisions of the AIA, which
became available to third party challengers on September 16, 2012, in various patent offices relating to patent rights in the RNAi field. In addition, third parties may challenge the validity of our patents. For example, a third party has filed an
opposition in the European Patent Office, or EPO, against our owned patent EP 2723758, with claims directed to RNAi compositions and methods offor silencing ANGPTL3, arguing that the granted claims are invalid. AnFollowing an oral hearing was held at the EPO in February 2021, where the patent was revoked. A notice of appeal of the EPO'sEPO’s decision was filed in June 2021.2021 and following an oral hearing in November 2023, the appeal was dismissed resulting in the patent remaining revoked. In March 2022, a third party filed an opposition with the EPO against our owned patent EP3105332, which is directed to RNAi compositions and methods for silencing ketohexokinase, seeking to revoke the patent. In addition, in February 2023, a third party filed an opposition with the EPO against our owned patent EP 3366775, titled “Modified RNA Agents” seeking to revoke the patent. An oral hearing isOral hearings are anticipated in these proceedings at a timetimes to be determined by the EPO. Additionally, the validity of two Chinese patents (ZL201380063930.5 and ZL201810143112.0) relating to inclisiran were challenged by a third party in China. The China National Intellectual Property Administration recently issued decisions confirming that patent No. ZL201380063930.5 remained valid as a whole, and patent No. ZL201810143112.0 remained valid based on the amended version of the claims we submitted. We expect that additional oppositions will be filed in the EPO and elsewhere, and other challenges will be raised relating to other patents and patent applications in our portfolio. In many cases, the possibility of appeal exists for either us or our opponents, and it may be years before final, unappealable rulings are made with respect to these patents in certain jurisdictions. The timing and outcome of these and other proceedings is uncertain and may adversely affect our business, prospects, operating results and financial condition if we are not successful in defending the patentability and scope of our pending and issued patent claims. Even if our rights are not directly challenged, disputes could lead to the weakening of our intellectual property rights. Our defense against any attempt by third parties to circumvent or invalidate our intellectual property rights could be costly to us, could require significant time and attention of our management and could have a material adverse effect on our business, prospects, operating results and financial condition and on our ability to successfully compete in the field of RNAi.
There are many issued and pending patents that claim aspects of oligonucleotide chemistry and modifications that we may need for our siRNA products marketed by us or our licensees, our late-stage therapeutic candidates being developed by us or our licensees,collaborators, including zilebesiran and fitusiran, as well as our other pipeline products. There are also many issued patents that claim targeting genes or portions of genes that may be relevant for siRNA drugs we wish to develop. In addition, there may be issued and pending patent applications that may be asserted against us in a court proceeding or otherwise based upon the asserting party’s belief that we may need such patents for our siRNA therapeutic candidates or marketed products, or to further develop and commercialize future products, or to continue to develop candidates in our pipeline that are being developed by us or our licensees.collaborators. Thus, it is possible that one or more organizations will hold patent rights to which we may need a license, or hold patent rights which could be asserted against us. If those organizations refuse to grant us a license to such patent rights on reasonable terms or at all and/or a court rules that we need such patent rights that have been asserted against us, and we are not able to obtain a license on reasonable terms, we may be unable to market our products, including ONPATTRO, AMVUTTRA, GIVLAARI or OXLUMO, or to perform research and development or other activities covered by such patents. For example, during 2017 and 2018, Silence Therapeutics, plc, or Silence, filed claims in several jurisdictions, including the High Court of England and Wales, and named us and our wholly owned subsidiary Alnylam UK Ltd. as co-defendants. Silence alleged various claims, including that ONPATTRO infringed one or more Silence patents. There were also a number of related actions brought by us or Silence in connection with this intellectual property dispute. In December 2018, we entered into a Settlement and License Agreement with Silence, resolving all ongoing claims, administrative proceedings, and regulatory proceedings worldwide between us regarding, among other issues, patent infringement, patent invalidity and breach of contract.
If we become involved in intellectual property litigation or other proceedings related to a determination of rights, we could incur substantial costs and expenses, and in the case of such litigation or proceedings against us, substantial liability for damages or be required to stop our product development and commercialization efforts.
Third parties may sue us for infringing their patent rights. For example, in October 2017 Silence sued us in the UK alleging that ONPATTRO and other investigational RNAi therapeutics we or MDCO arewere developing infringed one or more Silence patents. In December 2018 we and Silence settled all ongoing litigation between us. A third party may also claim that we have improperly obtained or used its confidential or proprietary information.
Furthermore, third parties may challenge the inventorship of our patents or licensed patents. For example, in March 2011, The University of Utah, or Utah, filed a complaint against us, Max Planck Gesellschaft Zur Foerderung Der Wissenschaften e.V. and Max Planck Innovation, together, Max Planck, Whitehead, MIT and the University of Massachusetts, claiming that a professor of Utah was the sole inventor, or in the alternative, a joint inventor of certain of our in-licensed patents. Utah was seeking correction of inventorship of the Tuschl patents, unspecified damages and other relief. After several years of court proceedings and discovery, the court granted our motions for summary judgment and dismissed Utah’s state law damages claims as well.claims. During the pendency of this litigation, as well as the Dicerna litigation described above,below, we incurred significant costs, and in each case, the litigation diverted the attention of our management and other resources that would otherwise have been engaged in other activities.
We may need to resort to litigation to enforce a patent issued or licensed to us or to determine the scope and validity of proprietary rights of others or protect our proprietary information and trade secrets. For example, during the second quarter of 2015, we filed a trade secret misappropriation lawsuit against Dicerna to protect our rights in the RNAi assets we purchased from Merck Sharp & Dohme Corp., or Merck. WeIn April 2018, we and Dicerna settled all claims in the ongoing litigation between us in April 2018.us. In
March 2022, we announced that we separately filed suit in United States District Court for the District of Delaware against Pfizer and Moderna Inc., seeking damages for infringement of U.S. Patent No. 11,246,933, or the ’933 patent in the parties’ manufacture and sale of their messenger RNA, or mRNA, COVID-19 vaccines. Pfizer joined BioNTech SE, or BioNTech, to the suit and filed counterclaims. In July 2022, we filed a newan additional lawsuit in United States District Court for the District of Delaware against each of Pfizer/BioNTech and Moderna seeking damages for infringing our newly granted U.S. Patent No. 11,382,979.11,382,979, or the ’979 patent. The Courtcourt combined the two patents in a single suit for each of Pfizer/BioNTech, or the 2022 Lawsuit, and Moderna with trial dates set for each in November
2024.Delaware. In addition to this patent, we added U.S. Patent Nos. 11,633,479 and 11,633,480 in the more recently filed suits against both Pfizer and Moderna and also U.S. Patent No. 11,612,657 against Pfizer only. On August 9, 2023, a Markman hearing was held in the U.S. District Court for the District of Delaware to consider the meaning of three disputed terms as used in the ’933 and ’979 patents, and on August 21, 2023, the court issued an order construing two of the three terms, and deferred a ruling on the third term. Subsequently, we and Moderna jointly agreed to final judgment of non-infringement of two of our patents, and that judgment was entered by the court on August 30, 2023, and on September 7, 2023, we appealed the claim construction ruling to the Court of Appeals for the Federal Circuit in the 2022 lawsuit against Moderna. The claim construction ruling did not affect one of the patents in the lawsuit filed against Moderna on May 26, 2023, and that case is going forward on a schedule with an anticipated trial date in the latter half of 2025. In September 2023, we and Pfizer/BioNTech agreed to consolidate the 2022 Lawsuit and 2023 lawsuits into one case, which will require moving the trial date from November 2024 to the first half of 2025, with the final schedule to be determined by the court. On January 4, 2024 a hearing was held in the consolidated Pfizer/BioNTech case to construe a final claim term with the final ruling pending. The aforementioned patents relate to our biodegradable cationic lipids that are foundational to the success of the mRNA COVID-19 vaccines.
In protecting our intellectual patent rights through litigation or other means, a third party may claim that we have improperly asserted our rights against them. For example, in August 2017, Dicerna successfully added counterclaims against us in the above-referenced trade secret lawsuit alleging that our lawsuit represented abuse of process and claiming tortious interference with its business. In addition, in August 2017, Dicerna filed a lawsuit against us in the United States District Court of Massachusetts alleging attempted monopolization by us under the Sherman Antitrust Act. As noted above, in April 2018, we and Dicerna settled all claims in the ongoing litigation between us.
In addition, in connection with certain license and collaboration agreements, we have agreed to indemnify certain third parties for certain costs incurred in connection with litigation relating to intellectual property rights or the subject matter of the agreements. The cost to us of any litigation or other proceeding relating to such intellectual property rights, even if resolved in our favor, could be substantial, and litigation would divert our management’s efforts. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation or legal proceeding could delay our research, development and commercialization efforts and limit our ability to continue our operations.
If any parties successfully claim that our creation or use of proprietary technologies infringes upon or otherwise violates their intellectual property rights, we might be forced to pay damages, potentially including treble damages, if we are found to have willfully infringed on such parties’ patent rights. In addition to any damages we might have to pay, a court could issue an injunction requiring us to stop the infringing activity or obtain a license.license from the claimant. Any license required under any patent may not be made available on commercially reasonable terms, ifor at all. In addition, such licenses are likely to bein many instances non-exclusive and, therefore, our competitors may have access to the same technology that is licensed to us. If we fail to obtain a required license and are unable to design around a patent, we may be unable to effectively market some of our technology and products, which could limit our ability to generate revenues or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations. Moreover, we expect that a number of our collaborations will provide that royalties payable to us for licenses to our intellectual property may be offset by amounts paid by our collaborators to third parties who have competing or superior intellectual property positions in the relevant fields, which could result in significant reductions in our revenues from products developed through collaborations.
If we fail to comply with our obligations under any licenses or related agreements, we may be required to pay damages and could lose license or other rights that are necessary for developing, commercializing and protecting our RNAi technology, as well as our approved products and any other product candidates that we develop, or we could lose certain rights to grant sublicenses.candidates.
Our current licenses impose, and any future licenses we enter into are likely to impose, various development, commercialization, funding, milestone, royalty, diligence, sublicensing, insurance, patent prosecution and enforcement, and other obligations on us. If we breach any of these obligations, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages and the licensor may have the right to terminate the license or render the license non-exclusive, which could result in us being unable to develop, manufacture, market and sell products that are covered by the licensed technology or enable a competitor to gain access to the licensed technology. Moreover, we could incur significant costs and/or disruption to our business and distraction of our management defending against any breach of such licenses alleged by the licensor. For example, in June 2018, Ionis sent us a notice claiming that it was owed payments under our second amended and restated strategic collaboration and license agreement as a result of the January 2018 amendmentrestructuring of our collaboration agreement with Sanofi and the related Exclusive TTR License and AT3 License Terms. Ionis claimed it was owed technology
access fees, or TAFs, based on rights granted and amounts paid to us in connection with the Sanofi restructuring. Ionis later filed a Demand for Arbitration with the Boston office of the American Arbitration Association against us, asserting, among other things, breach of contract. Upon completion of the arbitration process in the second quarter of 2020, in October 2020, a partial award was issued by the arbitration panel that sought additional information from us. The arbitration panel issued its final award in December 2020, which ruled in favor of Ionis’s request for a TAF on certain rights the panel determined we received in the Sanofi restructuring (but rejectingrejected the TAF amount sought by Ionis), and in favor of us in denying Ionis’s request for a TAF on a milestone payment received by us in the same restructuring. The panel’s final award also denied Ionis’s request for pre-judgement interest and attorney’s fees. Pursuant to the panel'spanel’s final award, we paid $41.2 million to Ionis in January 2021.
Moreover, our licensors may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise violating the licensor’s rights. In addition, while we cannot currently determine the amount of the royalty obligations we will be required to pay on sales of each of our approved products or future products, if any, the amounts may be significant. The amount of our future royalty obligations will depend on the technology and intellectual property we use in such products. Therefore, even if we successfully develop and commercialize products, we may be unable to achieve or maintain profitability.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information.
In order to protect our proprietary technology and processes, we rely in part on confidentiality agreements with our collaborators, employees, consultants, scientific advisors, CMOs, outside scientific collaborators and sponsored researchers, and other advisors. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, othersother third parties may independently discover trade secrets and proprietary information, and in such cases we could not assert any trade secret rights against such party. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business, position.prospects, operating results and financial condition.
Risks Related to Competition
The pharmaceutical market is intensely competitive. If we or our collaborators are unable to compete effectively with existing drugs, new treatment methods and new technologies, we or our collaborators may be unable to commercialize successfully any drugs that we or our collaborators develop.
The pharmaceutical market is intensely competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions, governmental agencies and other public and private research organizations are pursuing the development of novel drugs for the same diseases that we are targeting or expect to target. Many of our competitors have:
•muchsubstantially greater financial, technical and human resources than we have at every stage of the discovery, development, manufacture and commercialization of products;have;
•more extensive experience in pre-clinical testing, conducting clinical trials, obtaining regulatory approvals, and in manufacturing, marketing and selling drug products;
•product candidates that are based on previously tested or accepted technologies;
•multiple products that have been approved or are in late stages of development; and
•collaborative arrangements in our target markets with leading companies and research institutions.
We will face intense competition from drugs that have already been approved and accepted by the medical community for the treatment of the conditions for which we may develop drugs. For example, if approved by the FDA, patisiran, our RNAi therapeutic in development for ATTR amyloidosis patients with cardiomyopathy, would compete with tafamidis, marketed by Pfizer, which is currently approved to treat this disease. We also expect to face competition from new drugs that enter the market. ThereIn addition, there are a number of drugs currently under development whichand that may become commercially available in the future, for the treatment of conditions for which we may try to develop drugs. These drugs may be more effective, safer, less expensive, have more convenient administration or be marketed and sold more effectively, than any products we develop and commercialize.
For example, we developed ONPATTROassuming positive results in our HELIOS-B Phase 3 clinical and regulatory approval, vutrisiran, our RNAi therapeutic in development for treatment of ATTR amyloidosis with cardiomyopathy, would compete with VYNDAQEL/VYNDAMAX (tafamidis), marketed by Pfizer, which is currently approved to treat this disease. In addition, BridgeBio announced positive results from its Phase 3 clinical trial of acoramidis, a TTR stabilizer, in ATTR amyloidosis with cardiomyopathy in July 2023, and announced in February 2024 that the FDA has accepted its NDA for filing. BridgeBio also announced that the European Medicines Agency has accepted its marketing authorization application and that it is preparing for additional global regulatory submissions. There are also product candidates in earlier stages of development for the treatment of hATTR amyloidosis. In August 2018,ATTR amyloidosis with cardiomyopathy, including NTLA-2001 which is being developed by Intellia Therapeutics, Inc. and Regeneron and is in Phase 3 clinical development; NNC-6019 which is being developed by Novo Nordisk and is in Phase 3 clinical development; and NI006 which is being developed by Neurimmune AG and AstraZeneca plc and is in Phase 1 clinical
development. We expect to face competition from any of these and potentially other additional new drugs that enter the FDAmarket to treat patients with ATTR amyloidosis with cardiomyopathy.
ONPATTRO and AMVUTTRA are approved ONPATTRO lipid complex injectionin certain jurisdictions for the treatment of the polyneuropathy ofcertain patients with hATTR amyloidosis in adults, and the EC granted marketing authorization for ONPATTRO for the treatment of hATTR amyloidosis in adults with stage 1 or stage 2 polyneuropathy. We are aware of other approved products used to treat this disease, including tafamidis,VYNDAQEL/VYNDAMAX (tafamidis), and inotersen,TEGSEDI (inotersen), which is developed and marketed by Ionis. In addition, in December 2023, the FDA approved WAINUA (eplontersen), a drug developed by Ionis as well asin partnership with AstraZeneca plc, for the treatment of hATTR amyloidosis patients with polyneuropathy. There are also product candidates in various stages of clinical development including eplontersen, an additional investigational drug developed by Ionis in partnership with AstraZeneca, which met co-primary and secondary endpoints in an interim analysis of a Phase 3 study for the polyneuropathytreatment of hATTR amyloidosis and is currently under regulatory review by the FDA. Finally, we are aware that BridgeBio Pharma, Inc. (formerly Eidos Therapeutics, Inc.), or BridgeBio, announced topline results from Part A of its Phase 3 clinical trial of acoramidis, a TTR stabilizer, in ATTR-CM in December 2021, which did not meet the primary endpoint of the study at month 12. BridgeBio initiated enrollment in Part B of its Phase 3 clinical trial of acoramidis in ATTR-PN patients in the fourth quarter of 2020, and anticipates topline results in mid-2023.with polyneuropathy. While we believe that ONPATTRO hasand AMVUTTRA have and will continue to have a competitive product profile for the treatment of patients with hATTR amyloidosis with polyneuropathy, and that AMVUTTRA has a competitive product profile in this indication, it is possible that ONPATTRO and/or AMVUTTRA may not compete favorably with these products and product candidates, or others, and, as a result, may not achieve commercial success. Moreover, positive or negative data and/or the commercial success or failure of competitive products could negatively impact our stock price. For example, our stock price was negatively impacted by the results of Part A of BridgeBio's Phase 3 clinical trial.
If we or our collaborators continue to successfully develop product candidates, and obtain approval for them, we and our collaborators will face competition based on many different factors, including:
•the safety and effectiveness of our or our collaborators’ products relative to alternative therapies, if any;
•the ease with which our or our collaborators’ products can be administered and the extent to which patients accept relatively new routes of administration;
•the timing and scope of regulatory approvals for these products;
•the availability and cost of manufacturing, marketing and sales capabilities;
•the price of our or our collaborators’ products relative to alternative approved therapies;
•reimbursement coverage; and
•patent position.
We are aware of product candidates in various stages of clinical development for the treatment of PH1 which would compete with OXLUMO, our RNAi therapeutic approved in the U.S. and EU for the treatment of this disease, including Oxabact®Novo Nordisk’s product RIVFLOZA (nedosiran), a bacteria-based investigational therapy in development by OxThera AB, reloxaliase an investigational enzyme therapy in Phase 3 development for primary or severe secondary hyperoxaluria by Allena Pharmaceuticals, Inc., and nedosiran, an investigational RNAi therapeutic in development by Dicerna for the treatment of primary hyperoxaluria. In July 2019, the FDA granted a Breakthrough Therapy Designation to nedosiran for the treatment of patients with primary hyperoxaluria, and in August 2021, Dicerna reported positive topline results from its PHYOX2 pivotal clinical trial of nedosiran for the treatment of primary hyperoxaluria. Based on the results of the trial, Novo Nordisk submitted an NDA to the FDA in September 2022which was approved for the treatment of PH1 in patients aged six yearsSeptember 2023 and older.is expected to launch in 2024. RIVFLOZA is a once-monthly subcutaneous RNAi therapy that was developed by Dicerna. In April 2020, we and Dicerna granted each other a non-exclusive cross-license to our respective intellectual property related to lumasiran and Dicerna’s nedosiran product candidate. nedosiran. In addition, several companies have investigational drugs in clinical development for the treatment of PH1, including BridgeBio, Chinook Therapeutics, Inc., and BioMarin Pharmaceutical, Inc.
Our competitors may develop or commercialize products with significant advantages over any products we or our collaborators develop based on any of the factors listed above or on other factors. In addition, our competitors may develop strategic alliancescollaborations with or receive funding from larger pharmaceutical or biotechnology companies, providing them with an advantage over us.us and our collaborators. Our competitors may therefore be more successful in commercializing their products than we or our collaborators are, which could adversely affect our competitive position and business.business, prospects, operating results and financial condition. Competitive products may make any products we or our collaborators develop obsolete or noncompetitive before we can recover the expenses of developing and commercializing our product candidates. Such competitors could also recruit our employees, which could negatively impact our level of expertise and theour ability to execute on our business plan. Furthermore, we and our collaborators also face competition from existing and new treatment methods that reduce or eliminate the need for drugs, such as the use of advanced medical devices. The development of new medical devices or other treatment methods for the diseases we and our collaborators are targeting could make our or our collaborators’ product candidates noncompetitive, obsolete or uneconomical.
We and our collaborators face competition from other companies that are working to develop novel drugs and technology platforms using technology similar to ours.ours, as well as from companies utilizing emerging technologies. If these companies develop drugs more rapidly than we or our collaborators do or their technologies, including delivery technologies, are more effective, our and our collaborators’ ability to successfully commercialize drugsour products may be adversely affected.
In addition to the competition we face from competing drugs in general, we and our collaborators also face competition from other companies working to develop novel drugs using technology that competes more directly with our own. We are aware of several other companies that are working to develop RNAi therapeutic products. Some of these companies are seeking, as we are, to develop chemically synthesized siRNAs as drugs. Others are following a gene therapy approach, with the goal of treating patients not with synthetic siRNAs but with synthetic, exogenously-introduced genes designed to produce siRNA-like molecules within cells. Companies working on chemically synthesized siRNAs include, but are not limited to, Takeda, Marina, Arrowhead Quark, Silence, Arbutus, Sylentis, Dicerna and its collaborators, WAVE, Arcturus,Takeda Pharmaceutical Company Ltd., Janssen Pharmaceutics, Inc., GlaxoSmithKline plc, and Genevant Sciences, launched by ArbutusAmgen Inc.; Quark Pharmaceuticals, Inc.; Roche; Silence Therapeutics plc and Roivant Sciences.its collaborators, AstraZeneca plc, Jiangsu Hansoh Pharmaceuticals Group Co., Ltd., and Mallinckrodt plc; Arbutus; Sylentis; and Novo Nordisk and its collaborators, Boehringer Ingelheim and Eli Lilly and Company. In addition, we granted licenses or options for licenses to Ionis, Benitec Biopharma Ltd., Arrowhead, Arbutus,
Quark, Sylentis and othersother companies under which these companies may independently develop RNAi therapeutics against a limited number of targets. Any one of these companies may develop its RNAi technology more rapidly and more effectively than us.
we do. In addition, as a result of agreements that we have entered into, Takeda has obtained a non-exclusive license, and Arrowhead, as the assignee of Novartis, has obtained specific exclusive licenses for 30 gene targets, that include access to certain aspects of our technology.
We and our collaborators also compete with companies working to develop antisense-based drugs. LikeSimilar to RNAi therapeutics, antisense drugs target mRNAs in order to suppress the activity of specific genes. Akcea (acquired byTherapeutics, Inc., a wholly owned subsidiary of Ionis, in October 2020), has received marketing approval for an antisense drug, inotersen that was developed by Ionis, for the treatment of adult hATTR amyloidosis patients with stage 1 or stage 2 polyneuropathy in adult patients with hATTR amyloidosis.polyneuropathy. Several antisense drugs developed by Ionis have been approved and are currently marketed, and Ionis has multiple antisense product candidates in clinical trials. Ionis is also developing antisense drugs using ligand-conjugated GalNAc technology licensed from us, and these drugs have been shown to have increased potency at lower doses in clinical and pre-clinical studies, compared with antisense drugs that do not use such licensed GalNAc technology. The development of antisense drugs and antisense technology may become the preferred technology for drugs that target mRNAs to silence specific genes.
In addition to competition with respect to RNAi and with respect to specific products, we face substantial competition to discover and develop safe and effective means to deliver siRNAs to the relevant cell and tissue types. SafeIf our competitors develop safe and effective means to deliver siRNAs to the relevant cell and tissue types, may be developed by our competitors, and our ability to successfully commercialize a competitive product would be adversely affected. In addition, substantial resources are being expended by third parties in the effortare expending substantial resources to discover and develop a safe and effective means of delivering siRNAs into the relevant cell and tissue types, including both inprivate companies and academic laboratories and in the corporate sector.laboratories. Some of our competitors have substantially greater resources than we do, and if our competitors are able to negotiate exclusive access to those delivery solutions developed by third parties, we may be unable to successfully commercialize our product candidates.
Risks Related to Our Common Stock
If ourOur stock price fluctuates, purchasers ofhas been and may in the future be volatile, and an investment in our common stock could incur substantial losses.suffer a decline in value.
Our stock price has been and may in the future be volatile. From January 1, 2023 to December 31, 2023, our common stock traded between $148.10 and $242.39 per share. The stock market in general and the market for biotechnology companies in particular have experienced extreme price and volume volatility that has often been unrelated to the operating performance of particular companies. The market price of our common stock has fluctuated significantly and may continue to fluctuate significantly in response to factors that are beyond our control. The stock market in general has from time to time experienced extreme price and volume fluctuations, and the biotechnology sector in particular has experienced extreme price and volume fluctuations. The market prices of securities of pharmaceutical and biotechnology companies have been extremely volatile, and have experienced fluctuations that often have been unrelated or disproportionate to the clinical development progress or operating performance of these companies, including as a result of adverse development events reported by other companies. For example, the trading price for our common stock and the common stock of other biopharmaceutical companies was highly volatile during the initial stages of the COVID-19 pandemic. These broad market and sector fluctuations have resulted and could in the future resultcould be significantly and adversely affected by many factors, including:
•the information contained in extreme fluctuationsour quarterly earnings releases, including updates regarding our or our collaborators’ commercialized products or product candidates, our net product and collaboration revenues and operating expenses for completed periods and financial guidance regarding future periods;
•the success of existing or new competitive products or technologies;
•regulatory actions with respect to our or our collaborators’ products or product candidates;
•announcements by us or our competitors of significant acquisitions, collaborations, joint ventures, collaborations or capital commitments;
•the timing and results of clinical trials of our or our collaborators’ other product candidates;
•commencement or termination of collaborations for our development programs;
•failure or discontinuation of any of our or our collaborators’ development programs;
•results of clinical trials of our competitors’ product candidates;
•regulatory or legal developments in the priceU.S. and other countries;
•developments or disputes concerning patent applications, issued patents or other proprietary rights;
•the recruitment or departure of key personnel;
•the level of expenses related to any of our product candidates or clinical development programs;
•the results of our or our collaborators’ efforts to develop additional product candidates or products;
•actual or anticipated changes in financial results or development timelines;
•announcement or expectation of additional financing efforts;
•sales of our common stock which could cause purchasers ofby us, our common stock to incur substantial losses.insiders or other stockholders;
We may incur significant costs from class action litigation.
Our stock price may fluctuate for many reasons, including as a result of public announcements regarding the progress of our development and commercialization efforts or the development and commercialization efforts of our collaborators and/or competitors, the addition or departure of our key personnel, •variations in our quarterly operatingfinancial results and or those of companies that are perceived to be similar to us;
•changes in estimates or recommendations by any of the securities analysts that cover us;
•changes in the structure of healthcare payment systems;
•market valuations ofconditions in the pharmaceutical and biotechnology companies. Whensectors;
•general economic, industry and market conditions; and
•the other factors described in this “Risk Factors” section.
In the past, securities class action litigation has often been brought against companies following declines in the market price of a stock has been volatile as ourtheir securities. This risk is especially relevant for biopharmaceutical companies, which have experienced significant stock price has been, holders of that stock have occasionally brought securities class action litigation against the company that issued the stock.
volatility in recent years. For example, onin September 12, 2019, the Chester County Employees Retirement Fund, individuallywe and on behalf of all others similarly situated, filed a purported securities class action complaint alleging violation of federal securities laws against us, certain of our current and former directors and officers, and the underwriters of our November 14, 2017 public stock offering were sued in a putative class action alleging violations of the federal securities laws. While this matter has been finally settled, we may be the target of additional litigation of this type in the Supreme Court of the State of New York, New York County. While we believe the allegations in the New York Statefuture. Securities Litigation were without merit, in August 2021, the parties reached an agreement in principle to resolve the matter. At a hearing on April 12, 2022, the Supreme Court of the State of New York granted final approval to the settlement. Proceedings in the First Department were adjourned until April 2022, pending final approval of any settlement, and were withdrawn as a result of final approval on April 18, 2022. Future litigation against us could result in substantial costs and divert our management’s attention and resources, which could cause serious harm to our business, prospects, operating results and financial condition. We maintain liability insurance; however, if any costs or expenses associated with litigation exceed our insurance coverage, we may be forced to bear some or all of these costs and expenses directly, which could be substantial. In addition, we have obligations to indemnify third parties, including our officers and directors and underwriters of our securities offerings, in connection with certain litigation, and suchthose obligations aremay not be covered by insurance.
Future salesSales of a substantial number of shares of our common stock, including by us, our officers or directors, or our significant stockholders, us or our directors and officers,into the public market could cause the price of our common stock to decline.
A small number of our stockholders beneficially own a substantial amount of our common stock. As of JanuaryDecember 31, 2023, our sixseven largest stockholders beneficially owned in excess of 50% of our outstanding shares of common stock. If we, our officers or directors, or our significant stockholders or we or our officers and directors, sell substantial amounts of our common stock in the public market, or there is a perception that such sales may occur, the market price of our common stock could be adversely affected. Sales of common stock by our significant stockholders might make it more difficult for us to raise funds by selling equity or equity-related securities in the future at a time and price that we deem appropriate.
Regeneron’s ownership of our common stock could delay or prevent a change in corporate control.
As of May 21, 2019, the closing date of the stock purchase in connection with the 2019 Regeneron collaboration, Regeneron held approximately 4% of our outstanding common stock and has the right to increase its ownership up to 30%. This concentration of ownership could harm the market price of our common stock in the future by:
•delaying, deferring or preventing a change in control of our company;
•impeding a merger, consolidation, takeover or other business combination involving our company; or
•discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our company.
Anti-takeover provisions in our chartergoverning documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.management or members of our board of directors.
Provisions in our certificate of incorporation and our bylaws may delay or prevent an acquisition of us or a change in the current members of our management. In addition, these provisions may frustratemanagement or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replacethe members of our board of directors. Because our
board of directors is responsible for appointing the members of our management team,Among other things, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. These provisions include:provisions:
•establish a classified board of directors;directors such that all members of our board of directors are not elected at one time;
•establish a prohibition on actions by our stockholders by written consent;
•limitations onauthorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a “poison pill” that would work to dilute the removalstock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors;
•allow the authorized number of our directors to be changed only by resolution of our board of directors.
•limit who may call a special meetings of stockholders;
•require the approval of the holders of at least 75% of the votes that all our stockholders would be entitled to case to amend or repeal certain provisions of our charter or bylaws;
•limit the manner in which stockholders can remove directors from our board of directors; and
•establish advance notice requirements for election to our board of directors and for proposing matters that can be acted upon at stockholder meetings.
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner. These provisions would apply even if the proposed merger or acquisition could be considered beneficial by some stockholders.
We expect that results from our and our collaborators’ clinical development activities and the clinical development activities of our competitors will continue to be released periodically and may result in significant volatility in the price of our common stock.
Any new information regarding our and our collaborators’ products and product candidates or competitive products or potentially competitive product candidates can substantially affect investors’ perceptions regarding our future prospects. We,
our collaborators, and our competitors periodically provide updates regarding drug development programs, typically through press releases, conference calls and presentations at medical conferences. These periodic updates often include interim or final results from clinical trials conducted by us or our competitors and/or information about our or our competitors’ expectations regarding regulatory filings and submissions as well as future clinical development of our products or product candidates, competitive products or potentially competitive product candidates. The timing of the release of information by us regarding our drug development programs is often beyond our control and is influenced by the timing of receipt of data from our clinical trials and by the general preference among pharmaceutical companies to disclose clinical data during medical conferences. In addition, the information disclosed about our clinical trials, or our competitors’ clinical trials, may be based on interim rather than final data that may involve interpretation difficulties and may in any event not accurately predict final results. The release of such information may result in volatility in the price of our common stock. For example, in late 2021 our stock price was negatively impacted following BridgeBio’s public disclosure of the results of Part A of the Phase 3 clinical trial of acoramidis for the treatment of ATTR amyloidosis with cardiomyopathy.
Risks Related to Our Convertible Notes
Servicing our debt may require a significant amount of cash. We may not have sufficient cash flow from our business to pay our indebtedness.
On September 12, 2022,As of December 31, 2023, we commenced a private offering of $900.0 millionhad $1.02 billion in aggregate principal amount of 1% Convertible Senior Notes due 2027, or the Initial Notes. On September 13, 2022, the initial purchasers in such offering exercised their option to purchase an additional $135.0 million in aggregate principal amount of our 1% Convertible Senior Notes due 2027, or the Additional Notes, and together with the Initial Notes, collectively referred to as the Notes, bringing the total aggregate principal amount of the Notes to $1.04 billion.outstanding. The interest rate for the Notes is fixed at 1.00% per annum and is payable semi-annually in arrears on May 15 and September 15 of each year, beginning on March 15, 2023. Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness, including the Notes, or to make cash payments in connection with any conversions of Notes, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional debt financing or equity capital on terms that may be onerous or highly dilutive. Our ability to refinance any future indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
In addition, anyour indebtedness, combined with our other financial obligations and contractual commitments, could have other important consequences. For example, it could:
•make us more vulnerable to adverse changes in general U.S. and worldwide economic, industry and competitive conditions and adverse changes in government regulation;
•limit our flexibility in planning for, or reacting to, changes in our business and our industry;
•place us at a disadvantage compared to our competitors who have less debt;
•limit our ability to borrow additional amounts to fund acquisitions, for working capital and for other general corporate purposes; and
•make an acquisition of our future debt agreements may contain restrictive covenants that may prohibit us from adopting anycompany less attractive or more difficult.
Any of these alternatives. Our failurefactors could harm our business, prospects, operating results and financial condition. In addition, if we incur additional indebtedness, the risks related to comply with these covenants could result in an event of default which, if not curedour business and our ability to service or waived, could result in the acceleration ofrepay our debt.indebtedness would increase.
We may not have the ability to raise the funds necessary to settle for cash conversions of the Notes or to repurchase the Notes for cash upon a fundamental change, and our future debt may contain limitations on our ability to pay cash upon conversion of the Notes or to repurchase the Notes.change.
Holders of the Notes have the right to require us to repurchase their Notes upon the occurrence of a fundamental change (as defined in the indenture governing the Notes) at a repurchase price equal to 100% of the principal amount of the Notes to be repurchased, plus accrued and unpaid interest, if any. Upon conversion of the Notes, unless we elect to deliver solely shares of our common stock to settle such conversion (other than paying cash in lieu of delivering any fractional share), we will be required to make cash payments in respect of the Notes being converted. We may not have enough available cash or be able to obtain financing at the time we are required to make repurchases of Notes surrendered or Notes being converted.In addition, our ability to repurchase the Notes or to pay cash upon conversions of the Notes may be limited by law, by regulatory authority or by agreements governing our future indebtedness. Our failure to repurchase Notes at a time when the repurchase is required by the indenture governing such notes or to pay any cash payable on future conversions of the Notes as required by such indenture would constitute a default under such indenture. A default under the indenture governing the Notes or the fundamental change itself could also lead to a default under agreements governing our future indebtedness. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the Notes or make cash payments upon conversions.
In addition, our indebtedness, combined with our other financial obligations and contractual commitments, could have other important consequences. For example, it could:
•make us more vulnerable to adverse changes in general U.S. and worldwide economic, industry and competitive conditions and adverse changes in government regulation;
•limit our flexibility in planning for, or reacting to, changes in our business and our industry;
•place us at a disadvantage compared to our competitors who have less debt;
•limit our ability to borrow additional amounts to fund acquisitions, for working capital and for other general corporate purposes; and
•make an acquisition of our company less attractive or more difficult.
Any of these factors could harm our business, results of operations and financial condition. In addition, if we incur additional indebtedness, the risks related to our business and our ability to service or repay our indebtedness would increase.
The conditional conversion feature of the Notes, if triggered, may adversely affect our financial condition and operating results.liquidity.
In the event the conditional conversion feature of the Notes is triggered, holders of the Notes will be entitled to convert the Notes at any time during specified periods at their option. If one or more holders elect to convert their Notes, unless we elect to
satisfy our conversion obligation by delivering solely shares of our common stock (other than paying cash in lieu of delivering any fractional share), we would be required to settle a portion or all of our conversion obligation through the payment of cash, which could adversely affect our liquidity. In addition, even if holders do not elect to convert their Notes, we could be required under applicable accounting rules to reclassify all or a portion of the outstanding principal of the Notes as a current liability, rather than long-term liability, which would result in a material reduction of our net working capital.
Transactions relating to ourthe Notes may affect the value of our common stock.
The conversion of some or all of the Notes would dilute the ownership interests of existing stockholders to the extent we satisfy our conversion obligation by delivering shares of our common stock upon any conversion of such Notes. OurThe Notes may become in the future convertible at the option of their holders under certain circumstances. If holders of ourthe Notes elect to convert their notes, we may settle our conversion obligation by delivering to them a significant number of shares of our common stock, which would cause dilution to our existing stockholders.
In addition, in connection with the issuance of the Notes, we entered into the Capped Calls with certain financial institutions, or the Option Counterparties. The Capped Calls are generally expected to reduce potential dilution to our common stock upon any conversion or settlement of the Notes and/or offset any cash payments we are required to make in excess of the principal amount of converted Notes, with such reduction and/or offset subject to a cap.
In connection with establishing their initial hedges of the Capped Calls, the Option Counterparties or their respective affiliates entered into various derivative transactions with respect to our common stock and/or purchased shares of our common stock concurrently with or shortly after the pricing of the Notes.
From time to time, the Option Counterparties or their respective affiliates may modify their hedge positions by entering into or unwinding various derivative transactions with respect to our common stock and/or purchasing or selling our common stock or other securities of ours in secondary market transactions prior to the maturity of the Notes (and are likely to do so following any conversion of the Notes, any repurchase of the Notes by us on any fundamental change repurchase date, any redemption date, or any other date on which the Notes are retired by us, in each case, if we exercise our option to terminate the relevant portion of the Capped Calls). This activity could cause a decrease and/or increased volatility in the market price of our common stock.
We do not make any representation or prediction as to the direction or magnitude of any potential effect that the transactions described above may have on the price of the Notes or our common stock. In addition, we do not make any representation that the Option Counterparties will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
We are subject to counterparty risk with respect to the Capped Calls.
The Option Counterparties are financial institutions, and we will be subject to the risk that any or all of them might default under the Capped Calls. Our exposure to the credit risk of the Option Counterparties will not be secured by any collateral. Past global economic conditions have resulted in the actual or perceived failure or financial difficulties of many financial institutions. If an Option Counterparty becomes subject to insolvency proceedings, we will become an unsecured creditor in those proceedings with a claim equal to our exposure at that time under the Capped Calls with such Option Counterparty. Our exposure will depend on many factors but, generally, an increase in our exposure will be correlated to an increase in the market price and in the volatility of our common stock. In addition, upon a default by an Option Counterparty, we may suffer adverse tax consequences and more dilution than we currently anticipate with respect to our common stock. We can provide no assurances as to the financial stability or viability of the Option Counterparties.
The accounting method for convertible debt securities that may be settled in cash, such as the Notes, could have a material effect on our reported financial results.
The accounting method for reflecting the Notes on our consolidated balance sheet, accruing interest expense for the Notes and reflecting the underlying shares of our common stock in our reported diluted earnings per share may adversely affect our reported earnings and financial condition.
In August 2020, the Financial Accounting Standards Board published an Accounting Standards Update, which we refer to as ASU 2020-06, which simplified certain of the accounting standards that apply to convertible notes. ASU 2020-06 became effective for us beginning January 1, 2022.
In accordance with ASU 2020-06, the Notes will beare reflected as a liability on our consolidated balance sheets, with the initial carrying amount equal to the principal amount of the Notes, net of issuance costs. The issuance costs will bewere treated as a debt discount for accounting purposes, which will beis being amortized into interest expense over the term of the Notes. As a result of this amortization, the interest expense that we expect to recognize for the Notes for accounting purposes will be greater than the cash interest payments we will pay on the Notes, which will result in lower reported net income or higher reported net loss, as the case may be.
In addition, we expect that the shares of common stock underlying the Notes will beare reflected in our diluted earnings per share using the “if converted” method, in accordance with ASU 2020-06. Under thatthis method, diluted earnings per share wouldis generally be calculated assuming that all the Notes were converted solely into shares of common stock at the beginning of the reporting period, unless the result would be anti-dilutive. The application of the if-converted method may reduce our reported diluted earnings per share to the extent we are profitable in the future, and accounting standards may change in the future in a manner that may adversely affect our diluted earnings per share.
Furthermore, if any of the conditions to the convertibility of the Notes is satisfied, then we may be required under applicable accounting standards to reclassify the liability carrying value of the Notes as a current, rather than a long-term, liability. This reclassification could be required even if no holders actually convert their notesNotes and could materially reduce our reported working capital.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 1C. CYBERSECURITY
Risk Management and Strategy
We have in place a cross-functional, enterprise-wide cybersecurity program that is integrated into our overall risk management process and strategy and has direct involvement from our senior management and board oversight. Our cybersecurity program is based on industry standard CIS critical security controls. The top risks facing our Company, including those related to cybersecurity, are included in our overall enterprise risk management program that is managed by a cross-functional group chaired by our compliance and internal audit functions.
To assess, identify, and manage material risks from cybersecurity threats to our information systems and the associated costs, our cybersecurity program prioritizes vulnerability management and risk reduction, detection and prevention. As part of this program, we conduct continuous monitoring for anomalous behavior using a third-party security operations center. In addition, we conduct an annual cybersecurity risk assessment in conjunction with our third-party consultant that specializes in information security, and we incorporate recommendations from the risk assessment into our cybersecurity strategy, as appropriate. This risk assessment process considers the nature of our business, requirements from our internal and external stakeholders, and industry trends and risks, including new and emerging risks. By continuously assessing the cybersecurity landscape, we develop targeted strategies that identify and address the risks most likely to impact our company. We also conduct at least one cybersecurity incident tabletop exercise each year to test and enhance our incident response plans.
Our cybersecurity program is designed to detect and prevent disruption to critical information systems, minimize the loss or manipulation of sensitive information, efficiently remediate and recover from cybersecurity incidents and ensure compliance with regulations and disclosure requirements. Pursuant to our processes, when a cybersecurity incident occurs, we convene a cross-functional incident response team whose membership is dictated by the severity of the incident but in all instances includes representatives from our information technology, legal and accounting departments. This cross-functional representation allows us to leverage diverse perspectives and expertise when addressing cybersecurity events and to analyze the potential financial, legal, operational, and reputational implications of an incident, thereby enabling us to make informed decisions and take appropriate actions. This incident response framework further enables us to quickly assess the severity of cybersecurity incidents and the materiality of incidents based on pre-defined criteria that considers both quantitative and qualitative factors to determine the appropriate response. Identified incidents are then escalated to the relevant management teams based on their severity, allowing for a swift determination of materiality and an effective mitigation process. If we determine that an incident is not material, we continue to monitor it for subsequent developments.
We also utilize third-party service providers as a normal part of our business operations. These third-party service providers may have access to our systems and/or sensitive information. To address cybersecurity risks arising from our third-party service providers, we assess and monitor risks relating to potential compromises of sensitive information at our third-party service providers and reevaluate these risks periodically. We categorize our third-party service providers by criticality, based on the criticality and sensitivity of our data each third-party service provider has access to, and, based on this, we employ a risk-based approach for review of the security measures implemented by each third-party service provider. In addition, we obtain periodic attestation reports related to data security and privacy from certain third-party service providers to further support compliance with industry-standard cybersecurity protocols.
Additionally, to minimize our enterprise-wide risk and exposure to material cybersecurity incidents, we conduct annual cybersecurity awareness training and education for our employees. By equipping our employees with the necessary knowledge and skills, we intend to cultivate a cybersecurity-conscious culture within our organization.
We maintain insurance to provide coverage for a portion of the losses and damages that may result from a physical attack, cybersecurity attack or a security breach. Such insurance is subject to several exclusions and may not cover the total loss or damage caused by an attack or a breach. Consequently, costs related to incidents may not be covered by insurance.
Impact of Cybersecurity Risks on Strategy and Results
Our business operations and relationships with customers and suppliers are heavily reliant on technology, and any failure or disruption in our technological systems could have significant negative impacts on our business. For example, we collect, store and transmit sensitive information including intellectual property, proprietary business information, including highly sensitive clinical trial data, and personal information in connection with our business operations. The secure maintenance of this information is critical to our operations and business strategy. If this information was subject to a cybersecurity attack or unauthorized access or use, it could have a material adverse effect on our business and could expose us to potential legal consequences, liabilities, mitigation costs, and damage to our reputation. Managing cybersecurity incidents would also divert management’s attention and resources from regular business operations.
We believe that our current cybersecurity program provides adequate measures of protection against cybersecurity breaches and generally reduces our risks. However, cybersecurity threats are constantly evolving, becoming more frequent and more sophisticated and are being made by groups of individuals with a wide range of expertise and motives, which increases the difficulty of detecting and successfully defending against them. While we have implemented measures to safeguard our operational and technology systems and have established a culture of monitoring and improvement, the evolving nature of cybersecurity attacks and vulnerabilities means that these protections may not always be effective. However, our management has determined that, during the period covered by this Annual Report on Form 10-K, no cybersecurity threats, including as a result of any previous cybersecurity incidents, have materially affected or are reasonably likely to materially affect our company, including our business strategy, operating results, or financial condition.
Governance
Our board of directors is responsible for cybersecurity risk management and oversight of our cybersecurity program. The nominating and corporate governance committee of the board of directors assists in the oversight of management’s implementation of our information technology policies and monitoring of the risks associated with our information systems, including reviewing and discussing with management our program to identify, assess, manage and monitor cybersecurity risks. The nominating and corporate governance committee regularly reviews technology strategies, physical and cybersecurity threat assessments, emerging issues and related initiatives. In addition, the audit committee of the board of directors coordinates the board of directors’ oversight of our disclosure controls and procedures and ensures that we have in place appropriate internal controls, risk assessment policies and procedures, incident response plans and reporting mechanisms. The nominating and corporate governance committee coordinates with the board of directors and audit committee, as appropriate, on matters related to cybersecurity risk.
Our board of directors delegates execution of our cybersecurity program to our Chief Information Security Officer, or CISO, who is responsible for the day-to-day management of our cybersecurity program. Our CISO has over 25 years of information security and information technology risk expertise in multiple industries, including financial, manufacturing, healthcare and life sciences, and holds industry standard certifications, including CISSP, CRISC and CISM. Our CISO, together with our Chief Information Officer, or CIO, provides at least two presentations each year to our board of directors or nominating and corporate governance committee on cybersecurity incidents, security program updates and ongoing risks, with additional updates being provided on an as-needed basis. Our CISO also meets periodically with our senior leadership team to review metrics on readiness, incidents, mitigations and remediation. In addition, our internal audit team performs periodic audits of our systems and cybersecurity processes, the results of which are reported to the audit committee and our senior management team.
We have established a disclosure committee, which consists of our chief executive officer, chief financial officer, and senior leaders from finance, legal, accounting, corporate communications, and investor relations, including, but not limited to, our Chief Legal Officer, CIO, CISO, chief accounting officer, controller and senior vice president investor relations and corporate communications. The disclosure committee is actively involved in the review and approval of the Company’s SEC filings and has responsibility for considering the materiality of information for such filings and, on a timely basis, determining the disclosure of that information. The CISO briefs the disclosure committee, as necessary, on cybersecurity related matters, which includes information regarding our detection, prevention, mitigation, and remediation of cybersecurity incidents and monitoring of previously evaluated cybersecurity incidents for subsequent changes that might impact conclusions on materiality, and this information is presented to the nominating and corporate governance committee, as appropriate.
ITEM 2. PROPERTIES
Our operations are based primarily in Cambridge, Massachusetts; Zug, Switzerland; Maidenhead, United Kingdom; Amsterdam, Netherlands; and Tokyo, Japan. A description of certain of the facilities we lease or own as of January 31, 20232024 is included in the table below.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Location | | Primary Use | | Approximate
Square Footage | | Lease
Expiration Date | | Renewal Option |
| 675 West Kendall Street Henri A. Termeer Square Cambridge, Massachusetts | | Corporate headquarters and primary research facility | | 295,000 | | | January 2034 | | Two five-year terms |
300 Third Street
Cambridge, Massachusetts | | Office space and additional research facility | | 129,000 | | | January 2034 | | Two five-year terms |
101 Main Street
Cambridge, Massachusetts | | Office space* | | 61,000 | | | March 2024 and June 2026 | | One five-year term on each lease |
20 Commerce Way
Norton, Massachusetts | | GMP manufacturing | | cGMP manufacturing | 200,000 | | | Not applicable | | Not applicable |
665 Concord Avenue
Cambridge, Massachusetts | | GMP manufacturing** | | cGMP manufacturing** | 15,000 | | | September 2027 | | One five-year term |
Grafenauweg 4
6300 Zug, Switzerland | | International headquarters | | 14,500 | | | March 2028 | | One five-year term |
Braywick Gate
Braywick Road, Maidenhead
Berkshire, United Kingdom | | Office space | | 21,500 | | | May 2026 | | None |
Wisdom Cross Tower Antonio Vivaldistraat 150
Amsterdam, Netherlands | | Office space | | 12,500 | | | April 2025 | | One five-year term |
Pacific Century Place
1-Chome-11-1 Marunouchi Chiyoda-ku
Tokyo, Japan | | Office space | | 16,900 | | | May 2025 | | None |
* We lease office space located on the 12th and 13th floors at 101 Main Street, Cambridge, Massachusetts under a non-cancelable real property lease agreement by and between the Company and RREEF America REIT II CORP. PPP, dated as of April 15, 2015, or the Lease. On September 30, 2020, we entered into a First Amendment to the Lease, pursuant to which the term of the Lease with respect to the 12th and 13th floors was extended for an additional five years, through June 30, 2026. In addition, we have a separate lease agreement for the 10th floor at 101 Main Street, which expires in March 2024.2024 and will not be extended.
** We manufacture ONPATTRO (patisiran) formulated bulk drug product at this location.
In addition to the locations above, we also occupy small offices in multiple locations in and outside of the U.S. to support our operations and growth.
In the future, we may lease, operate, purchase or construct additional facilities in which to conduct expanded research, development and manufacturing activities and support future commercial operations. We believe that the total space available to us under our current leases will meet our needs for the foreseeable future and that additional space would be available to us on commercially reasonable terms if required.
ITEM 3. LEGAL PROCEEDINGS
For a discussion of material pending legal proceedings, please read the section titled "Litigation"“Litigation” within Note 14,13, Commitments and Contingencies, to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,”Data” of this Annual Report on Form 10-K, which is incorporated into this item by reference.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock trades on The Nasdaq Global Select Market under the symbol “ALNY.”
Holders of Record
At January 31, 2023,2024, there were 24 holders of record of our common stock. Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of beneficial holders represented by these record holders.
Issuer Purchases of Equity Securities
The following table provides information about our purchases of shares of Alnylam common stock during the three-month period ended December 31, 2022:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Period | | Total Number of Shares Purchased (1) | | Average Price per Share | | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | | Maximum Number (or Approximated Dollar Value) of Shares that May Yet Be Purchased Under the Plans or Programs |
| October 1, 2022 - October 31, 2022 | | — | | | $ | — | | | — | | | — | |
| November 1, 2022 - November 30, 2022 | | — | | | — | | | — | | | — | |
| December 1, 2022 - December 31, 2022 | | 3,182 | | | 231.84 | | | — | | | — | |
| Total | | 3,182 | | | $ | 231.84 | | | — | | | — | |
(1) Share repurchases reported in this column consist of shares of Alnylam's common stock tendered by employees in December 2022 to cover the exercise price of certain stock options exercised by those employees.
Stock Performance Graph
The following performance graph and related information shall not be deemed “soliciting material” or to be “filed” with the Securities and Exchange Commission, or SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933 or Securities Exchange Act of 1934, each as amended, except to the extent that we specifically incorporate it by reference into such filing.
The comparative stock performance graph below compares the five-year cumulative total stockholder return (assuming reinvestment of dividends, if any) from investing $100 on the last trading day of 2017,2018, to the close of the last trading day of 2022,2023, in each of our common stock and the selected indices. The stock price performance reflected in the graph below is not necessarily indicative of future price performance.
Comparison of Five-Year Cumulative Total Return
Among Alnylam Pharmaceuticals, Inc.,
Nasdaq Composite Total Return and Nasdaq Biotechnology Total Return
| | 12/29/2017 | | 12/31/2018 | | 12/31/2019 | | 12/31/2020 | | 12/31/2021 | | 12/30/2022 |
| 12/31/2018 | | | 12/31/2018 | | 12/31/2019 | | 12/31/2020 | | 12/31/2021 | | 12/30/2022 | | 12/29/2023 |
| Alnylam Pharmaceuticals, Inc. | Alnylam Pharmaceuticals, Inc. | $ | 100.00 | | | $ | 57.39 | | | $ | 90.65 | | | $ | 102.30 | | | $ | 133.48 | | | $ | 187.05 | |
| Nasdaq Composite Total Return | Nasdaq Composite Total Return | $ | 100.00 | | | $ | 97.16 | | | $ | 132.81 | | | $ | 192.47 | | | $ | 235.15 | | | $ | 158.65 | |
| Nasdaq Biotechnology Total Return | Nasdaq Biotechnology Total Return | $ | 100.00 | | | $ | 91.14 | | | $ | 114.02 | | | $ | 144.15 | | | $ | 144.18 | | | $ | 129.59 | |
ITEM 6. RESERVED
Not applicable.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We are a global commercial-stage biopharmaceutical company that discovers, develops, manufactures and commercializes novel therapeutics based on RNAi. Our commercial products and broad pipeline of investigational RNAi therapeutics are focused in four STArs: Genetic Medicines, Cardio-Metabolic Diseases, Hepatic Infectious Diseasesrare, specialty and CNS/Ocular Diseases.select prevalent indications.
As described in Part I, Item 1. "Business,"“Business” of this Annual Report on Form 10-K, we currently have five products that have received marketing approval, including one partneredcollaborated product, and multiple late-stage investigational programs advancing towards potential commercialization. In Part I, Item 1. "Business"“Business” you can also find a summary of key events in 20222023 and 20232024 to-date related to our marketed products and our clinical development programs.
We have incurred significant losses since we commenced operations in 2002 and as of December 31, 2022,2023, we had an accumulated deficit of $6.57$7.01 billion. Historically, we have generated losses principally from costs associated with research and development activities, acquiring, filing and expanding intellectual property rights, and selling, general and administrative costs. As a result of planned expenditures for research and development activities relating to our research platform, our drug development programs, including clinical trial and manufacturing costs, the establishment of late-stage clinical and commercial capabilities, including global commercial operations, continued management and growth of our patent portfolio, collaborations and general corporate activities, we expect to incur additional operating losses, howeverlosses. While we expectbelieve 2019 representswas our peak operating loss year, we expect to continue to incur annual operating losses, and will require substantial resources over the next several years as we transition towards a self-sustainableexpand our efforts to discover, develop and commercialize RNAi therapeutics, and aim to achieve financial profile.self-sustainability by the end of 2025. We anticipate that our operating results will continue to fluctuate for the foreseeable future. Therefore,future, therefore, period-to-period comparisons should not be relied upon as predictive of the results in future periods.
We currently have programs focused on a number of therapeutic areas and, as of December 31, 2022,2023, we generate worldwide product revenues from four commercialized products, ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, primarily in the U.S., Europe and Japan.Europe. However, our ongoing development efforts may not be successful and we may not be able to commence sales of any other products and/or successfully market and sell ONPATTRO, AMVUTTRA, GIVLAARI, OXLUMO or any other approved products in the future. A substantial portion of our total revenues in recent years has been derived from collaboration revenues from strategic alliancescollaborations with Roche, Regeneron Vir and Novartis. In addition to revenues from the commercial sales of our approved products and potentially from sales of future products, we expect our sources of potential funding for the next several years to continue to be derived in part from existing and new strategic alliances.collaborations. Such alliancescollaborations include, or may include in the future, license and other fees, funded research and development, milestone payments and royalties on product sales by our licensors, including royalties on sales of Leqvio made by our partnercollaborator, Novartis, as well as proceeds from the sale of equity or debt.
Results of Operations
The following data summarizes the results of our operations:
| | Year Ended December 31, | | 2022 vs 2021 | | 2021 vs 2020 | |
| | Year Ended December 31, | |
| | Year Ended December 31, | |
| | Year Ended December 31, | |
| (In thousands, except percentages) | |
| (In thousands, except percentages) | |
| (In thousands, except percentages) | (In thousands, except percentages) | | 2022 | | 2021 | | 2020 | | $ Change | | % Change | | $ Change | | % Change | |
| Total revenues | Total revenues | | $ | 1,037,418 | | | $ | 844,287 | | | $ | 492,853 | | | $ | 193,131 | | | 23 | % | | $ | 351,434 | | | 71 | % | |
| Total revenues | |
| Total revenues | |
| Operating costs and expenses | |
| Operating costs and expenses | |
| Operating costs and expenses | Operating costs and expenses | | $ | 1,822,490 | | | $ | 1,552,939 | | | $ | 1,321,291 | | | $ | 269,551 | | | 17 | % | | $ | 231,648 | | | 18 | % | |
| Loss from operations | Loss from operations | | $ | (785,072) | | | $ | (708,652) | | | $ | (828,438) | | | $ | (76,420) | | | 11 | % | | $ | 119,786 | | | (14) | % | |
| Loss from operations | |
| Loss from operations | |
| Net loss | Net loss | | $ | (1,131,156) | | | $ | (852,824) | | | $ | (858,281) | | | $ | (278,332) | | | 33 | % | | $ | 5,457 | | | (1) | % | |
| Net loss | |
| Net loss | |
For discussion of our 20212022 results and a comparison with 20202021 results please refer to "Management's“Management’s Discussion and Analysis of Financial Conditions and Results of Operations"Operations” in our Annual Report on Form 10-K for the fiscal year ended December 31, 20212022 that was filed with the SEC on February 10, 2022.23, 2023.
Discussion of Results of Operations
Revenues
Total revenues consist of the following:
| | Years Ended December 31, | | 2022 vs 2021 | | 2021 vs 2020 |
| | Years Ended December 31, | | | | Years Ended December 31, | | 2023 vs 2022 | | 2022 vs 2021 |
| (In thousands, except percentages) | (In thousands, except percentages) | | 2022 | | 2021 | | 2020 | | $ Change | | % Change | | $ Change | | % Change | (In thousands, except percentages) | | 2023 | | 2022 | | 2021 | | $ Change | | % Change | | $ Change | | % Change |
| Net product revenues | Net product revenues | | $ | 894,329 | | | $ | 662,138 | | | $ | 361,520 | | | $ | 232,191 | | | 35 | % | | $ | 300,618 | | | 83 | % | Net product revenues | | $ | 1,241,474 | | | $ | | $ | 894,329 | | | $ | | $ | 662,138 | | | $ | | $ | 347,145 | | | 39 | | 39 | % | | $ | 232,191 | | | 35 | | 35 | % |
| Net revenues from collaborations | Net revenues from collaborations | | 134,912 | | | 180,953 | | | 131,333 | | | (46,041) | | | (25) | % | | 49,620 | | | 38 | % | Net revenues from collaborations | | 546,185 | | | 134,912 | | 134,912 | | | 180,953 | | 180,953 | | | 411,273 | | 411,273 | | | 305 | | 305 | % | | (46,041) | | | (25) | | (25) | % |
| Royalty revenue | Royalty revenue | | 8,177 | | | 1,196 | | | — | | | 6,981 | | | 584 | % | | 1,196 | | | N/A | Royalty revenue | | 40,633 | | | 8,177 | | 8,177 | | | 1,196 | | 1,196 | | | 32,456 | | 32,456 | | | 397 | | 397 | % | | 6,981 | | | * | | * |
| Total | Total | | $ | 1,037,418 | | | $ | 844,287 | | | $ | 492,853 | | | $ | 193,131 | | | 23 | % | | $ | 351,434 | | | 71 | % | Total | | $ | 1,828,292 | | | $ | | $ | 1,037,418 | | | $ | | $ | 844,287 | | | $ | | $ | 790,874 | | | 76 | | 76 | % | | $ | 193,131 | | | 23 | | 23 | % |
| * Indicates the percentage change period over period is greater than 500%. | | * Indicates the percentage change period over period is greater than 500%. |
Net Product Revenues
Net product revenues consist of the following, by product and region:
| | Year Ended December 31, | | 2022 vs 2021 | | 2021 vs 2020 |
| Year Ended December 31, | | | Year Ended December 31, | | 2023 vs 2022 | | 2022 vs 2021 |
| (In thousands, except percentages) | (In thousands, except percentages) | 2022 | | 2021 | | 2020 | | $ Change | | % Change | | $ Change | | % Change | (In thousands, except percentages) | 2023 | | 2022 | | 2021 | | $ Change | | % Change | | $ Change | | % Change |
| ONPATTRO | ONPATTRO | | | | | | | | | | | | | |
| United States | |
| United States | |
| United States | United States | $ | 246,748 | | | $ | 213,210 | | | $ | 151,574 | | | $ | 33,538 | | | 16 | % | | $ | 61,636 | | | 41 | % | $ | 97,739 | | | $ | | $ | 246,748 | | | $ | | $ | 213,210 | | | $ | | $ | (149,009) | | | (60) | | (60) | % | | $ | 33,538 | | | 16 | | 16 | % |
| Europe | Europe | 224,063 | | | 190,435 | | | 107,755 | | | 33,628 | | | 18 | % | | 82,680 | | | 77 | % | Europe | 210,916 | | | 224,063 | | 224,063 | | | 190,435 | | 190,435 | | | (13,147) | | (13,147) | | | (6) | | (6) | % | | 33,628 | | | 18 | | 18 | % |
| Rest of World | Rest of World | 86,797 | | | 71,092 | | | 46,752 | | | 15,705 | | | 22 | % | | 24,340 | | | 52 | % | Rest of World | 45,891 | | | 86,797 | | 86,797 | | | 71,092 | | 71,092 | | | (40,906) | | (40,906) | | | (47) | | (47) | % | | 15,705 | | | 22 | | 22 | % |
| Total | Total | 557,608 | | | 474,737 | | | 306,081 | | | 82,871 | | | 17 | % | | 168,656 | | | 55 | % | Total | 354,546 | | | 557,608 | | 557,608 | | | 474,737 | | 474,737 | | | (203,062) | | (203,062) | | | (36) | | (36) | % | | 82,871 | | | 17 | | 17 | % |
| | AMVUTTRA | AMVUTTRA | |
| AMVUTTRA | |
| AMVUTTRA | |
| United States | |
| United States | |
| United States | United States | 82,521 | | | — | | | — | | | 82,521 | | | N/A | | — | | | N/A | 411,169 | | | 82,521 | | 82,521 | | | — | | — | | | 328,648 | | 328,648 | | | 398 | | 398 | % | | 82,521 | | | N/A | | N/A |
| Europe | Europe | 4,214 | | | — | | | — | | | 4,214 | | | N/A | | — | | | N/A | Europe | 70,898 | | | 4,214 | | 4,214 | | | — | | — | | | 66,684 | | 66,684 | | | * | | * | | 4,214 | | | N/A | | N/A |
| Rest of World | Rest of World | 7,060 | | | — | | | — | | | 7,060 | | | N/A | | — | | | N/A | Rest of World | 75,771 | | | 7,060 | | 7,060 | | | — | | — | | | 68,711 | | 68,711 | | | * | | * | | 7,060 | | | N/A | | N/A |
| Total | Total | 93,795 | | | — | | | — | | | 93,795 | | | N/A | | — | | | N/A | Total | 557,838 | | | 93,795 | | 93,795 | | | — | | — | | | 464,043 | | 464,043 | | | 495 | | 495 | % | | 93,795 | | | N/A | | N/A |
| | GIVLAARI | GIVLAARI | |
| GIVLAARI | |
| GIVLAARI | |
| United States | |
| United States | |
| United States | United States | 115,659 | | | 92,747 | | | 42,797 | | | 22,912 | | | 25 | % | | 49,950 | | | 117 | % | 141,954 | | | 115,659 | | 115,659 | | | 92,747 | | 92,747 | | | 26,295 | | 26,295 | | | 23 | | 23 | % | | 22,912 | | | 25 | | 25 | % |
| Europe | Europe | 48,670 | | | 30,895 | | | 12,000 | | | 17,775 | | | 58 | % | | 18,895 | | | 157 | % | Europe | 57,498 | | | 48,670 | | 48,670 | | | 30,895 | | 30,895 | | | 8,828 | | 8,828 | | | 18 | | 18 | % | | 17,775 | | | 58 | | 58 | % |
| Rest of World | Rest of World | 8,815 | | | 4,173 | | | 309 | | | 4,642 | | | 111 | % | | 3,864 | | | 1,250 | % | Rest of World | 19,799 | | | 8,815 | | 8,815 | | | 4,173 | | 4,173 | | | 10,984 | | 10,984 | | | 125 | | 125 | % | | 4,642 | | | 111 | | 111 | % |
| Total | Total | 173,144 | | | 127,815 | | | 55,106 | | | 45,329 | | | 35 | % | | 72,709 | | | 132 | % | Total | 219,251 | | | 173,144 | | 173,144 | | | 127,815 | | 127,815 | | | 46,107 | | 46,107 | | | 27 | | 27 | % | | 45,329 | | | 35 | | 35 | % |
| | OXLUMO | OXLUMO | |
| OXLUMO | |
| OXLUMO | |
| United States | |
| United States | |
| United States | United States | 27,698 | | | 18,876 | | | — | | | 8,822 | | | 47 | % | | 18,876 | | | N/A | 38,159 | | | 27,698 | | 27,698 | | | 18,876 | | 18,876 | | | 10,461 | | 10,461 | | | 38 | | 38 | % | | 8,822 | | | 47 | | 47 | % |
| Europe | Europe | 37,915 | | | 38,949 | | | 333 | | | (1,034) | | | (3) | % | | 38,616 | | | 11,596 | % | Europe | 60,025 | | | 37,915 | | 37,915 | | | 38,949 | | 38,949 | | | 22,110 | | 22,110 | | | 58 | | 58 | % | | (1,034) | | | (3) | | (3) | % |
| Rest of World | Rest of World | 4,169 | | | 1,761 | | | — | | | 2,408 | | | 137 | % | | 1,761 | | | N/A | Rest of World | 11,655 | | | 4,169 | | 4,169 | | | 1,761 | | 1,761 | | | 7,486 | | 7,486 | | | 180 | | 180 | % | | 2,408 | | | 137 | | 137 | % |
| Total | Total | 69,782 | | | 59,586 | | | 333 | | | 10,196 | | | 17 | % | | 59,253 | | | 17,794 | % | Total | 109,839 | | | 69,782 | | 69,782 | | | 59,586 | | 59,586 | | | 40,057 | | 40,057 | | | 57 | | 57 | % | | 10,196 | | | 17 | | 17 | % |
| | Total net product revenues | Total net product revenues | $ | 894,329 | | | $ | 662,138 | | | $ | 361,520 | | | $ | 232,191 | | | 35 | % | | $ | 300,618 | | | 83 | % |
| Total net product revenues | |
| Total net product revenues | | $ | 1,241,474 | | | $ | 894,329 | | | $ | 662,138 | | | $ | 347,145 | | | 39 | % | | $ | 232,191 | | | 35 | % |
Net product revenues increased during the year ended December 31, 2022,2023, compared to the year ended December 31, 2021,2022, primarily as a result of increased patients across our commercial portfolio of products, includingdue to the initial launch of AMVUTTRA.
We expect net product revenuesAMVUTTRA in the third quarter of 2022, partially offset by a decrease of demand for ONPATTRO due to patient switches to AMVUTTRA. Additional growth was related to an increase during 2023, as compared to 2022, as we continue to add newin patients onto our commercial products, as well as launch these products into additional markets, assuming regulatory approvals.on GIVLAARI and OXLUMO.
Please readsee Note 3 to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,”Data” of this Annual Report on Form 10-K for balances and activity in each product revenue allowance and reserve category for the years ended December 31, 20222023 and 2021.2022.
Net Revenues from Collaborations and Royalty Revenue
Net revenues from collaborations and royalty revenue consist of the following:
| | Years Ended December 31, | | 2022 vs 2021 | | 2021 vs 2020 |
| Years Ended December 31, | | | Years Ended December 31, | | 2023 vs 2022 | | 2022 vs 2021 |
| (In thousands, except percentages) | (In thousands, except percentages) | 2022 | | 2021 | | 2020 | | $ Change | | % Change | | $ Change | | % Change | (In thousands, except percentages) | 2023 | | 2022 | | 2021 | | $ Change | | % Change | | $ Change | | % Change |
| Roche | | Roche | $ | 337,802 | | | $ | — | | | $ | — | | | $ | 337,802 | | | N/A | | $ | — | | | N/A |
| Regeneron Pharmaceuticals | Regeneron Pharmaceuticals | $ | 87,844 | | | $ | 113,226 | | | $ | 74,072 | | | $ | (25,382) | | | (22) | % | | $ | 39,154 | | | 53 | % | Regeneron Pharmaceuticals | 100,468 | | | 87,844 | | 87,844 | | | 113,226 | | 113,226 | | | 12,624 | | 12,624 | | | 14 | | 14 | % | | (25,382) | | | (22) | | (22) | % |
| Novartis AG | Novartis AG | 43,159 | | | 49,120 | | | 22,208 | | | (5,961) | | | (12) | % | | 26,912 | | | 121 | % | Novartis AG | 86,727 | | | 43,159 | | 43,159 | | | 49,120 | | 49,120 | | | 43,568 | | 43,568 | | | 101 | | 101 | % | | (5,961) | | | (12) | | (12) | % |
| Vir Biotechnology | 1,755 | | | 16,897 | | | 31,396 | | | (15,142) | | | (90) | % | | (14,499) | | | (46) | % |
| | Other | Other | 2,154 | | | 1,710 | | | 3,657 | | | 444 | | | 26 | % | | (1,947) | | | (53) | % |
| Total | $ | 134,912 | | | $ | 180,953 | | | $ | 131,333 | | | $ | (46,041) | | | (25) | % | | $ | 49,620 | | | 38 | % |
| Other | |
| Other | | 21,188 | | | 3,909 | | | 18,607 | | | 17,279 | | | 442 | % | | (14,698) | | | (79) | % |
| Total net revenues from collaborations | | Total net revenues from collaborations | $ | 546,185 | | | $ | 134,912 | | | $ | 180,953 | | | $ | 411,273 | | | 305 | % | | $ | (46,041) | | | (25) | % |
| | Royalty revenue | |
| Royalty revenue | |
| Royalty revenue | | $ | 40,633 | | | $ | 8,177 | | | $ | 1,196 | | | $ | 32,456 | | | 397 | % | | $ | 6,981 | | | * |
Net revenues from collaborations decreasedincreased during the year ended December 31, 2022,2023, as compared to the year ended December 31, 2021,2022, primarily due to a decrease in revenue recognized in connection withunder our collaboration agreements with RegeneronRoche and Vir, attributed to reduced researchNovartis. During 2023, we recognized $337.8 million of revenue under our Collaboration and manufacturing activities and timing of reimbursable activities.License Agreement with Roche, which was executed in July
2023, and under our Novartis Collaboration Agreement we recognized an additional $30.0 million of revenue, compared to 2022, associated with the achievement of specified commercialization and regulatory milestones.
Royalty revenue increased during the year ended December 31, 2022,2023, as compared to the year ended December 31, 2021, primarily2022, due to increased royalties earned from global net sales of Leqvio by our partner,collaborator, Novartis. In December 2020, Leqvio received marketing authorization from the EC for the treatment of adults with hypercholesterolemia or mixed dyslipidemia, and in December 2021, Leqvio was approved by the FDA for the treatment of adults with HeFH or ASCVD.
Recognition of our combined net revenues from collaborations and royalty revenue is dependent on a variety of factors including the level of work reimbursed by partners,collaborators, achievement of milestones under our collaboration agreements, and royalties associated with sales of Leqvio. We expect variability in net revenues from collaboration and royalty revenuewill decrease in 2023,2024, as compared to 2022,2023, primarily driven by a reduction in the revenues recognized under our Roche Collaboration and License Agreement. We expect our royalty revenues will increase in 2024, as compared to 2023, due to the timingcontinued growth of manufacturing activities, achievement of milestones under our collaboration agreements and royalties associated withearned from global net sales of Leqvio.Leqvio by our collaborator, Novartis.
The amount of revenue from collaborations that we recognize, in part, is based on estimates of total costs to be incurred. These estimates reflect our historical experiences, current contractual requirements, and forecasted plans of development or manufacturing activities. We adjust these estimates for changes in actual costs incurred, contractual terms, and further forecasts. Such changes in estimates could have a significant impact on revenue and earnings in the period of the adjustment.
Operating Costs and Expenses
Operating costs and expenses consist of the following:
| | Year Ended December 31, | | 2022 vs 2021 | | 2021 vs 2020 |
| Year Ended December 31, | | | Year Ended December 31, | | 2023 vs 2022 | | 2022 vs 2021 |
| (In thousands, except percentages) | (In thousands, except percentages) | 2022 | | 2021 | | 2020 | | $ Change | | % Change | | $ Change | | % Change | (In thousands, except percentages) | 2023 | | 2022 | | 2021 | | $ Change | | % Change | | $ Change | | % Change |
| Cost of goods sold | Cost of goods sold | $ | 140,174 | | | $ | 115,005 | | | $ | 74,185 | | | $ | 25,169 | | | 22 | % | | $ | 40,820 | | | 55 | % | Cost of goods sold | $ | 268,216 | | | $ | | $ | 140,174 | | | $ | | $ | 115,005 | | | $ | | $ | 128,042 | | | 91 | | 91 | % | | $ | 25,169 | | | 22 | | 22 | % |
| | Cost of goods sold as a percentage of net product revenues | Cost of goods sold as a percentage of net product revenues | 15.7 | % | | 17.4 | % | | 20.5 | % | |
| Cost of goods sold as a percentage of net product revenues | |
| Cost of goods sold as a percentage of net product revenues | |
| | Cost of collaborations and royalties | |
| | Cost of collaborations and royalties | |
| | Cost of collaborations and royalties | Cost of collaborations and royalties | 28,643 | | | 25,139 | | | 3,867 | | | 3,504 | | | 14 | % | | 21,272 | | | 550 | % | 42,190 | | | 28,643 | | 28,643 | | | 25,139 | | 25,139 | | | 13,547 | | 13,547 | | | 47 | | 47 | % | | 3,504 | | | 14 | | 14 | % |
| Research and development | Research and development | 883,015 | | | 792,156 | | | 654,819 | | | 90,859 | | | 11 | % | | 137,337 | | | 21 | % | Research and development | 1,004,415 | | | 883,015 | | 883,015 | | | 792,156 | | 792,156 | | | 121,400 | | 121,400 | | | 14 | | 14 | % | | 90,859 | | | 11 | | 11 | % |
| Selling, general and administrative | Selling, general and administrative | 770,658 | | | 620,639 | | | 588,420 | | | 150,019 | | | 24 | % | | 32,219 | | | 5 | % | Selling, general and administrative | 795,646 | | | 770,658 | | 770,658 | | | 620,639 | | 620,639 | | | 24,988 | | 24,988 | | | 3 | | 3 | % | | 150,019 | | | 24 | | 24 | % |
| Total | Total | $ | 1,822,490 | | | $ | 1,552,939 | | | $ | 1,321,291 | | | $ | 269,551 | | | 17 | % | | $ | 231,648 | | | 18 | % | Total | $ | 2,110,467 | | | $ | | $ | 1,822,490 | | | $ | | $ | 1,552,939 | | | $ | | $ | 287,977 | | | 16 | | 16 | % | | $ | 269,551 | | | 17 | | 17 | % |
Cost of Goods Sold
Cost of goods sold as a percentage of net product revenues decreasedincreased to 21.6% for the year ended December 31, 2023, as compared to 15.7% for the year ended December 31, 2022, as compared to 17.4% for the year ended December 31, 2021, primarily due to an increase inthe following:
•Increased volume and rate of royalties payable on net sales of productsAMVUTTRA. Our collaborator is eligible to receive tiered royalties of 15% to 30% based on global annual net sales and therefore the growth in AMVUTTRA net sales during 2023 resulted in more net sales and a higher tier rate for the applicable royalties payable; and
•Increased excess and obsolete charges primarily due to cancelling manufacturing commitments and the impairment of ONPATTRO inventory that had been manufactured for future demand associated with a lower cost to manufacture.the use of patisiran for the treatment of patients with ATTR amyloidosis with cardiomyopathy for which we did not receive regulatory approval in the U.S.
We anticipate variability in our cost of goods sold as a percentage of net product revenues duein 2024, as compared to the timing of manufacturing runs and utilization and the depletion of zero-cost inventories, as well as future product launches.2023. We expect our cost of goods sold will increase during 2023,2024, as compared to 2022,2023, primarily as a result of an expected increase in net product sales as well as increased royalties.
Cost of collaborations and royalties
Cost of collaborations and royalties increased during the year ended December 31, 2022,2023, as compared to the year ended December 31, 2021,2022, primarily due to timing andincreased demand of GalNAc material supplysupplied to our collaboration partnerscollaborators to support certain product manufacturing and ongoing clinical trials.trials and increased royalties payable to third parties on the net sales of licensed products by Novartis.
We anticipate variability in theexpect our cost of collaborations and royalties will decrease during 2023,2024, as compared to 2022,2023, primarily due to the timing anda decrease in demand of GalNAc material to be supplied to our collaboration partners.collaborators in support of certain product manufacturing as our collaborators begin to transition to producing the material independently.
Research and Development
Research and development expenses consist of the following:
| | Year Ended December 31, | | 2022 vs 2021 | | 2021 vs 2020 |
| | Year Ended December 31, | | | | Year Ended December 31, | | 2023 vs 2022 | | 2022 vs 2021 |
| (In thousands, except percentages) | (In thousands, except percentages) | | 2022 | | 2021 | | 2020 | | $ Change | | % Change | | $ Change | | % Change | (In thousands, except percentages) | | 2023 | | 2022 | | 2021 | | $ Change | | % Change | | $ Change | | % Change |
| Clinical research and outside services | Clinical research and outside services | | $ | 438,418 | | | $ | 418,985 | | | $ | 307,378 | | | $ | 19,433 | | | 5 | % | | $ | 111,607 | | | 36 | % | Clinical research and outside services | | $ | 485,732 | | | $ | | $ | 438,418 | | | $ | | $ | 418,985 | | | $ | | $ | 47,314 | | | 11 | | 11 | % | | $ | 19,433 | | | 5 | | 5 | % |
| Compensation and related | Compensation and related | | 225,589 | | | 196,134 | | | 190,705 | | | 29,455 | | | 15 | % | | 5,429 | | | 3 | % | Compensation and related | | 260,423 | | | 225,589 | | 225,589 | | | 196,134 | | 196,134 | | | 34,834 | | 34,834 | | | 15 | | 15 | % | | 29,455 | | | 15 | | 15 | % |
| Occupancy and all other costs | Occupancy and all other costs | | 126,847 | | | 108,622 | | | 96,272 | | | 18,225 | | | 17 | % | | 12,350 | | | 13 | % | Occupancy and all other costs | | 160,987 | | | 126,847 | | 126,847 | | | 108,622 | | 108,622 | | | 34,140 | | 34,140 | | | 27 | | 27 | % | | 18,225 | | | 17 | | 17 | % |
| Stock-based compensation | Stock-based compensation | | 92,161 | | | 68,415 | | | 60,464 | | | 23,746 | | | 35 | % | | 7,951 | | | 13 | % | Stock-based compensation | | 97,273 | | | 92,161 | | 92,161 | | | 68,415 | | 68,415 | | | 5,112 | | 5,112 | | | 6 | | 6 | % | | 23,746 | | | 35 | | 35 | % |
| Total | Total | | $ | 883,015 | | | $ | 792,156 | | | $ | 654,819 | | | $ | 90,859 | | | 11 | % | | $ | 137,337 | | | 21 | % | Total | | $ | 1,004,415 | | | $ | | $ | 883,015 | | | $ | | $ | 792,156 | | | $ | | $ | 121,400 | | | 14 | | 14 | % | | $ | 90,859 | | | 11 | | 11 | % |
Research and development expenses increased during the year ended December 31, 2022,2023, as compared to the year ended December 31, 2021,2022, primarily due to the following:
•Increased compensation and related expenses as a result of increased headcount to support our R&D pipeline and development expenses;
•Increased stock-based compensation expenseclinical research and outside services primarily dueassociated with zilebesiran as we reached full enrollment for our KARDIA-1 and KARDIA-2 clinical studies and additional costs associated with manufacturing batches associated with those clinical activities. Costs associated with clinical trials of other programs such as ALN-TTRsc04 and our ongoing early development studies also were higher when compared to the accounting for certain performance-based awards;2022; and
•Increased occupancy and all other costs as a result of higher costs related to infrastructure and other professional services to support our growing clinical research and outside services expenses primarily due to increase in clinical batches manufactured and development expenses associated with the KARDIA-1 and KARDIA-2 zilebesiran phase 2 studies.footprint.
During the years ended December 31, 20222023 and 2021,2022, in connection with advancing activities under our collaboration agreements, we incurred research and development expenses, primarily related to external development and clinical expenses, including the manufacture of clinical product.
The following table summarizes research and development expenses incurred, for which we recognize net revenue, that are directly attributable to our collaboration agreements, by collaboration partner:collaborator:
| | Year Ended December 31, |
| | Year Ended December 31, | | | | Year Ended December 31, |
| (In thousands) | (In thousands) | | 2022 | | 2021 | | 2020 | (In thousands) | | 2023 | | 2022 | | 2021 |
| Roche | |
| Regeneron Pharmaceuticals | Regeneron Pharmaceuticals | | $ | 43,002 | | | $ | 73,411 | | | $ | 57,833 | |
| Other | Other | | 1,172 | | | 15,575 | | | 35,300 | |
| Total | Total | | $ | 44,174 | | | $ | 88,986 | | | $ | 93,133 | |
Selling, General and Administrative
Selling, general and administrative expenses consist of the following:
| | Year Ended December 31, | | 2022 vs 2021 | | 2021 vs 2020 |
| | Year Ended December 31, | | | | Year Ended December 31, | | 2023 vs 2022 | | 2022 vs 2021 |
| (In thousands, except percentages) | (In thousands, except percentages) | | 2022 | | 2021 | | 2020 | | $ Change | | % Change | | $ Change | | % Change | (In thousands, except percentages) | | 2023 | | 2022 | | 2021 | | $ Change | | % Change | | $ Change | | % Change |
| Compensation and related | Compensation and related | | $ | 273,262 | | | $ | 224,237 | | | $ | 200,071 | | | $ | 49,025 | | | 22 | % | | $ | 24,166 | | | 12 | % | Compensation and related | | $ | 298,888 | | | $ | | $ | 273,262 | | | $ | | $ | 224,237 | | | $ | | $ | 25,626 | | | 9 | | 9 | % | | $ | 49,025 | | | 22 | | 22 | % |
| Consulting and professional services | Consulting and professional services | | 226,941 | | | 201,841 | | | 176,097 | | | 25,100 | | | 12 | % | | 25,744 | | | 15 | % | Consulting and professional services | | 226,664 | | | 226,941 | | 226,941 | | | 201,841 | | 201,841 | | | (277) | | (277) | | | — | | — | % | | 25,100 | | | 12 | | 12 | % |
| Occupancy and all other costs | | Occupancy and all other costs | | 145,687 | | | 131,967 | | | 97,259 | | | 13,720 | | | 10 | % | | 34,708 | | | 36 | % |
| Stock-based compensation | Stock-based compensation | | 138,488 | | | 97,302 | | | 79,409 | | | 41,186 | | | 42 | % | | 17,893 | | | 23 | % | Stock-based compensation | | 124,407 | | | 138,488 | | 138,488 | | | 97,302 | | 97,302 | | | (14,081) | | (14,081) | | | (10) | | (10) | % | | 41,186 | | | 42 | | 42 | % |
| Occupancy and all other costs | | 131,967 | | | 97,259 | | | 132,843 | | | 34,708 | | | 36 | % | | (35,584) | | | (27) | % |
| Total | Total | | $ | 770,658 | | | $ | 620,639 | | | $ | 588,420 | | | $ | 150,019 | | | 24 | % | | $ | 32,219 | | | 5 | % | Total | | $ | 795,646 | | | $ | | $ | 770,658 | | | $ | | $ | 620,639 | | | $ | | $ | 24,988 | | | 3 | | 3 | % | | $ | 150,019 | | | 24 | | 24 | % |
Selling, general and administrative expenses increased during the year ended December 31, 2022,2023, as compared to the year ended December 31, 2021,2022, primarily due to the following:
•Increased compensation and related expenses as a result of increased headcount and other investments supporting our strategic investments in support ofgrowth including the global launch of AMVUTTRA and other expenses to support our strategic growth;
•Increased stock-based compensation expense primarily due to the accounting for certain performance-based awards; and
•Increased consulting and professional services expenses to support our commercial portfolio.AMVUTTRA.
We expect that research and development expenses combined with selling, general and administrative expenses will increase during 2023,2024, as compared to 2022,2023, as we continue to advance and develop our platform and pipeline, advance our product candidates, including partneredcollaborated programs, into later-stage development, prepare regulatory submissions and continue to build-out our global commercial and compliance infrastructure and field team to support ONPATTRO, GIVLAARI, OXLUMO, and the launch of AMVUTTRA in the U.S. and EU, as well as launch theseour commercial products into additional
markets, assuming regulatory approvals. However, we expect that certain expenses will be variable depending on the timing of manufacturing batches, clinical trial enrollment and results, regulatory review of our product candidates and programs, and stock-based compensation expenses due to our determination regarding the probability of vesting for performance-based awards.
Other (Expense) Income
Other (expense) income consists of the following:
| | | | Year Ended December 31, | | 2022 vs 2021 | | 2021 vs 2020 | | Year Ended December 31, | | 2023 vs 2022 | | 2022 vs 2021 |
| (In thousands, except percentages) | (In thousands, except percentages) | 2022 | | 2021 | | 2020 | | $ Change | | % Change | | $ Change | | % Change | (In thousands, except percentages) | 2023 | | 2022 | | 2021 | | $ Change | | % Change | | $ Change | | % Change |
| Interest expense | Interest expense | $ | (155,968) | | | $ | (143,021) | | | $ | (84,496) | | | $ | (12,947) | | | 9 | % | | $ | (58,525) | | | 69 | % | Interest expense | $ | (121,221) | | | $ | | $ | (155,968) | | | $ | | $ | (143,021) | | | $ | | $ | 34,747 | | | (22) | | (22) | % | | $ | (12,947) | | | 9 | | 9 | % |
| Other (expense) income, net | |
| Other expense, net | |
| Interest income | |
| Interest income | |
| Interest income | Interest income | 24,808 | | | 1,579 | | | 11,809 | | | 23,229 | | | 1,471 | % | | (10,230) | | | (87) | % | 95,561 | | | 24,808 | | 24,808 | | | 1,579 | | 1,579 | | | 70,753 | | 70,753 | | | 285 | | 285 | % | | 23,229 | | | * | | * |
| Realized and unrealized (losses) gains on marketable equity securities | Realized and unrealized (losses) gains on marketable equity securities | (33,312) | | | 55,695 | | | 54,042 | | | (89,007) | | | (160) | % | | 1,653 | | | 3 | % | Realized and unrealized (losses) gains on marketable equity securities | (16,944) | | | (33,312) | | (33,312) | | | 55,695 | | 55,695 | | | 16,368 | | 16,368 | | | (49) | | (49) | % | | (89,007) | | | (160) | | (160) | % |
| Change in fair value of development derivative liability | Change in fair value of development derivative liability | (94,659) | | | (38,433) | | | (17,185) | | | (56,226) | | | 146 | % | | (21,248) | | | 124 | % | Change in fair value of development derivative liability | (90,997) | | | (94,659) | | (94,659) | | | (38,433) | | (38,433) | | | 3,662 | | 3,662 | | | (4) | | (4) | % | | (56,226) | | | 146 | | 146 | % |
| Other | Other | (6,204) | | | (19,312) | | | 8,668 | | | 13,108 | | | (68) | % | | (27,980) | | | (323) | % | Other | (17,741) | | | (6,204) | | (6,204) | | | (19,312) | | (19,312) | | | (11,537) | | (11,537) | | | 186 | | 186 | % | | 13,108 | | | (68) | | (68) | % |
| Loss on the extinguishment of debt | Loss on the extinguishment of debt | (76,586) | | | — | | | — | | | (76,586) | | | N/A | | — | | | N/A | Loss on the extinguishment of debt | — | | | (76,586) | | (76,586) | | | — | | — | | | 76,586 | | 76,586 | | | (100) | | (100) | % | | (76,586) | | | N/A | | N/A |
| Total | Total | $ | (341,921) | | | $ | (143,492) | | | $ | (27,162) | | | $ | (198,429) | | | 138 | % | | $ | (116,330) | | | 428 | % | Total | $ | (151,342) | | | $ | | $ | (341,921) | | | $ | | $ | (143,492) | | | $ | | $ | 190,579 | | | (56) | | (56) | % | | $ | (198,429) | | | 138 | | 138 | % |
| * Indicates the percentage change period over period is greater than 500%. | | * Indicates the percentage change period over period is greater than 500%. |
Total other expense increaseddecreased during the year ended December 31, 2022,2023, as compared to the year ended December 31, 2021,2022, primarily due to increased interest income driven by higher market interest rates on our marketable debt securities, decreased interest expense as a result of a more favorable interest rate under the Convertible Senior Notes compared with the interest rate under the credit facility previously held with Blackstone and a $76.6 million loss on the extinguishment of the Blackstone credit agreement increased realized and unrealized losses on our marketable equity securities holdings, and increased loss as a result of a mark-to-market adjustment related to the development derivative liability.recognized in 2022.
Liquidity and Capital Resources
The following table summarizes our cash flow activities:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (In thousands) | 2022 | | 2021 | | 2020 |
| Net loss | $ | (1,131,156) | | | $ | (852,824) | | | $ | (858,281) | |
| Non-cash adjustments to reconcile net loss to net cash used in operating activities: | 625,435 | | | 373,954 | | | 256,021 | |
| Changes in operating assets and liabilities: | (35,553) | | | (162,823) | | | (12,701) | |
| Net cash used in operating activities | (541,274) | | | (641,693) | | | (614,961) | |
| Net cash provided by (used in) investing activities | 169,354 | | | (273,300) | | | (435,518) | |
| Net cash provided by financing activities | 425,753 | | | 1,247,118 | | | 994,979 | |
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | (7,430) | | | (9,018) | | | 4,918 | |
| Net increase (decrease) in cash, cash equivalents and restricted cash | 46,403 | | | 323,107 | | | (50,582) | |
| Cash, cash equivalents and restricted cash, beginning of period | 822,153 | | | 499,046 | | | 549,628 | |
| Cash, cash equivalents and restricted cash, end of period | $ | 868,556 | | | $ | 822,153 | | | $ | 499,046 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended December 31, | | $ Change | | |
| (In thousands) | 2023 | | 2022 | | 2021 | | 2023 vs 2022 | | 2022 vs 2021 |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Net cash provided by (used in): | | | | | | | | | | | | | |
| Operating activities | $ | 104,156 | | | $ | (541,274) | | | $ | (641,693) | | | $ | 645,430 | | | | | $ | 100,419 | | | |
| Investing activities | $ | (336,350) | | | $ | 169,354 | | | $ | (273,300) | | | $ | (505,704) | | | | | $ | 442,654 | | | |
| Financing activities | $ | 172,131 | | | $ | 425,753 | | | $ | 1,247,118 | | | $ | (253,622) | | | | | $ | (821,365) | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Operating Activities
Net cash used inprovided by operating activities decreasedincreased during the year ended December 31, 2022,2023, compared to the year ended December 31, 2021,2022, primarily due to decreasedreceipt of a $310.0 million up-front payment received in connection with the Roche Collaboration and License Agreement and $100.0 million payment from Regeneron in connection with achieving certain criteria during early clinical development for our CNS program, ALN-APP, in addition to cash receipts from increased product sales, offset by cash disbursements related to working capital payments and stronger cash receipts from increased product sales.payments.
Investing Activities
Net cash provided byused in investing activities increased during the year ended December 31, 2022,2023, compared to the year ended December 31, 2021,2022, primarily due to net activities related to our marketable debt securities as a result of an increase of cash invested in marketable debt securities.
Financing Activities
Net cash provided by financing activities decreased during the year ended December 31, 2022,2023, compared to the year ended December 31, 2021,2022, primarily due to greater cash received in 2021,2022, including $500.0$136.2 million received from our sale of one-half of our royalty interest under the Novartis agreement in September 2021 and $500.0 million received in connection with the second and final drawdowns on our credit agreement in June 2021 and December 2021, respectively, offset by $136.2 million
received from the issuance of convertible debt, net of repayment of the credit facility held with Blackstone and purchase of capped call transactions in September 2022.2022, and greater net proceeds from the issuance of common stock in connection with stock option exercises and other types of equity.
Additional Capital Requirements
We currently have programs focused on a number of therapeutic areas and, as of December 31, 2022,2023, have received regulatory approval and commercially launched four products. However, our ongoing development efforts may not be successful and we may not be able to commence sales of any other products or successfully expand the approved indications for our approved products, including ONPATTRO and AMVUTTRA, in the future. In addition, we anticipate that we will continue to generate losses as a result of planned expenditures for research and development activities relating to our research platform, our drug development programs, including clinical trial and manufacturing costs, the establishment of late-stage clinical, manufacturing, commercial and compliance capabilities, including global operations, continued management and growth of our intellectual property including our patent portfolio, collaborations and general corporate activities.
Based on our current operating plan, we believe that our cash, cash equivalents and marketable securities as of December 31, 2022,2023, together with the cash we expect to generate from product sales and under our current alliances,collaborations, will be sufficient to satisfy our near-term capital and operating needs for at least the next 12 months from the filing of this Annual Report on Form 10-K. Recent and expected working and other capital requirements, in addition to the above matters, also include the items described below:
•Amounts related to future lease payments for operating lease obligations at December 31, 20222023 totaled $460.1$418.0 million, with $43.0$43.6 million expected to be paid within the next 12 months.
•Our cash operating expenditures were $541.3 million in 2022 and $641.7 million in 2021, and we expect to increase our investment in operations in 2023.
•Cash outflows for capital expenditures were $62.2 million in 2023 and $72.1 million in 2022 and $76.4 million in 2021.2022. We expect capital expenditures to increase in 20222024 to support the increase in our manufacturing and production capacity needs.
•Amounts related to future long-term debt total $1.02 billion, of which we do not expect to make payments on principal within the next 12 months.
•Payments associated with the liability related to the sale of future royalties were approximately $3.4$21.6 million in 2022,2023, with $40.3an estimated $58.2 million to be paid within the next 12 months.
•Amount associated with the achievement of a development milestone payable to Blackstone was $84.5 million as of December 31, 2023, with $21.1 million to be paid within the next 12 months.
Since we commenced operations in 2002, we have generated significant losses and as of December 31, 2022,2023, we had an accumulated deficit of $6.57$7.01 billion. As of December 31, 2022,2023, we had cash, cash equivalents and marketable securities of $2.19$2.44 billion, compared to $2.44$2.19 billion as of December 31, 2021.2022.
Due to numerous factors described in more detail under the caption Part I, Item 1A, "Risk Factors"“Risk Factors” of this Annual Report on Form 10-K, we may require significant additional funds earlier than we currently expect in order to continue to commercialize ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, and to develop, conduct clinical trials for, manufacture and, if approved, commercialize additional product candidates.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with GAAP. The preparation of our consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and disclosure of contingent assets and liabilities in our consolidated financial statements. Actual results may differ from these estimates under different assumptions or conditions and could have a material impact on our reported results. While our significant accounting policies are more fully described in the Notes to our consolidated financial statements included elsewhere in this Annual Report on Form 10-K, we believe the following accounting policies to be the most critical in understanding the judgments and estimates we use in preparing our consolidated financial statements:
Net Product Revenues
Our net product revenues are recognized, net of variable consideration related to certain allowances and accruals, at the time the customer obtains control of our product. We record reserves, based on contractual terms, for components related to product sold during the reporting period, as well as our estimate of product that remains in the distribution channel inventory at the end of the reporting period that we expect will be sold to qualified healthcare providers. On a quarterly basis, we update our estimates and record any needed adjustments in the period we identify the adjustments.
The estimates for our product revenue allowances and accruals are most significantly affected by chargebacks, which are contractual commitments with the government and other entities to sell products to qualified healthcare providers at prices lower than the list prices charged to the customer who directly purchases from us, and rebates that represent discount obligations under government programs, including Medicaid in the U.S. and similar programs in certain other countries,
including countries in which we are accruing for estimated rebates because final pricing has not yet been negotiated. We are also subject to potential rebates in connection with our value-based agreements, or VBAs, with certain commercial payors.
We use the expected value method, which is the sum of probability-weighted amounts in a range of possible consideration amounts, or the most likely amount method, which is the single most likely amount in a range of possible considerations, to estimate variable consideration related to our product revenues. We use the expected value method to estimate variable consideration for chargebacks, certain rebates, and other incentives and we use the most likely amount method for certain rebates and trade discounts and allowances.
A 10% increase or decrease in these estimates impacts net sales by a corresponding increase or decrease of approximately $7.0$13.0 million.
Net Revenues from Collaborations
We earn revenue in connection with collaboration agreements which allow our collaboration partnerscollaborators to utilize our technology platforms and develop product candidates.
For elements of collaboration arrangements that are accounted for pursuant to ASCAccounting Standards Codification Topic 606, Revenue from Contracts with Customers, or ASC 606, we identify the performance obligations and allocate the total consideration we expect to receive on a relative standalone selling price basis to each performance obligation. Key assumptions to determine the standalone selling price may include forecasted revenues, development timelines, reimbursement rates for personnel costs, the expected number of targets or indications expected to be pursued under each license, discount rates and probabilities of technical and regulatory success. We recognize revenue associated with each performance obligation as the control over the promised goods or services transfer to our collaboration partnercollaborator which occurs either at a point in time or over time. If control transfers over time, revenue is recognized by using a method of measuring progress that best depicts the transfer of goods or services, for example based on actual costs incurred relative to total forecasted costs to be incurred over the period the transfer of goods or services occurs. We evaluate the measure of progress and related inputs each reporting period and any resulting adjustments to revenue are recorded on a cumulative catch-up basis. Revenue to be recognized is equal to the total transaction price multiplied by the ratio of actual expense incurred divided by total forecasted expense.
A 10% increase or decrease in the transaction price impacts net revenues from collaborators by a corresponding increase or decrease of approximately $35.0$43.0 million. A 10% increase or decrease in the total forecasted costs to be incurred over the period the transfer of goods or services occurs impacts net revenues from collaborators by a corresponding decrease or increase of approximately $32.0$39.0 million.
Liability Related to the Sale of Future Royalties
We account for the liability related to the sale of future royalties as a debt financing, as we have significant continuing involvement in the generation of the cash flows. Interest on the liability related to the sale of future royalties will be recognized using the effective interest rate method over the life of the related royalty stream.
The liability related to the sale of future royalties and the related interest expense are based on our current estimates of future royalties and commercial milestones expected to be paid over the life of the arrangement, which we determine by using third-party forecasts of Leqvio'sLeqvio’s global net revenue. Third-party forecasts are updated periodically as new data is obtained with regardsrespect to Leqvio'sLeqvio’s global launch progress or as sales information becomes available. Increases, decreases or a shift in timing of estimated revenues affects the interest rate utilized in the calculation of the liability related to the sale of future royalties.
An increase or decrease of 5%10% to the interest rate would result in an increase or decrease to our liability related to the sale of future royalties of approximately $15.8$33.9 million.
Development Derivative Liability
In August 2020, we entered into a co-development agreement, referred to as the Funding Agreement, with BXLS V Bodyguard – PCP L.P. and BXLS Family Investment Partnership V – ESC L.P., collectively referred to as Blackstone Life Sciences, pursuant to which Blackstone Life Sciences will provide up to $150.0 million in funding for the clinical development of vutrisiran and zilebesiran, two of our cardiometabolic programs. As consideration for Blackstone Life Sciences’ funding for certain vutrisiran and zilebesiran clinical development costs, we have agreed to pay Blackstone Life Sciences fixed success-based payments upon achievement of specific milestones for vutrisiran and zilebesiran as well as a 1% royalty on net sales of vutrisiran for ten years.
The development derivative liability is recorded at fair value and represents our current estimate of the expected future payments to Blackstone Life Sciences. The development derivative liability is based on the probability weighted present value of the estimated cash flows pursuant to contractual terms of the Funding Agreement. The most significant assumptions in determining the development derivative liability are the probability of success for the clinical development and regulatory approval of vutrisiran and zilebesiran and our current cost of borrowing. Estimates of the probability of success and our cost of borrowing are based on what we believe to be reasonable and supportable assumptions and require management’s judgment. Actual results could vary materially from these estimates.
Recent Accounting Pronouncements
Please read Note 2 to our consolidated financial statements included in Part II, Item 8, “Financial Statements and Supplementary Data,”Data” of this Annual Report on Form 10-K for a description of recent accounting pronouncements applicable to our business.pronouncements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk - Investment Portfolio. We invest a portion of our cash in a number of diversified fixed- and floating-rate securities consisting of cash equivalents and marketable debt securities debt funds and derivative instruments related to our investment portfolio that are subject to interest rate risk. Changes in the general level of interest rates can affect the fair value of our investment portfolio. If interest rates in the general economy were to rise, our holdings could lose value. As of December 31, 20222023 and 2021,December 31, 2022, a hypothetical increase in interest rates of 50100 basis points across the entire yield curve on our holdings would have resulted in an immaterial decrease to the fair value of our holdings.
Foreign Currency Exchange Risk. As a result of our foreign operations, we face exposure to movements in foreign currency exchange rates, primarily the Euro and Yen against the U.S. Dollar. Fluctuations in the global markets may have a positive or negative effect on our foreign exchange rate exposure. The current exposures arise primarily from net product revenue, operating costs and expenses and balance sheet amounts. Therefore, significant changes in foreign exchange rates of the countries outside the United States where our products are sold, where development expenses are incurred by us or our collaborators, or where we incur operating expenses can impact our operating results and financial condition. As sales outside the United States continue to grow, and as we expand our international operations, we will continue to assess potential steps, including foreign currency hedging and other strategies, to mitigate our foreign exchange risk.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Alnylam Pharmaceuticals, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Alnylam Pharmaceuticals, Inc. and its subsidiaries (the “Company”) as of December 31, 20222023 and 2021,2022, and the related consolidated statements of operations and comprehensive loss, of stockholders’ (deficit) equity and of cash flows for each of the three years in the period ended December 31, 2022,2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2022,2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 20222023 and 2021,2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 20222023 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2022,2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Annual Report on Internal Control Overover Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or
complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated
financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Liability Related to Sale of Future Royalties and Commercial Milestones
As described in Notes 2 and 5 to the consolidated financial statements, the liability related to the sale of future royalties and the related interest expense are based on management’s current estimates of future royalties and commercial milestones expected to be paid over the life of the arrangement. Interest on the liability related to the sale of future royalties will be recognized using the effective interest rate method, resulting in the recognition of interest expense. Management periodically assesses the expected payments and to the extent the amount or timing of the future estimated payments is materially different than the previous estimates, management accounts for any such change by adjusting the liability related to the sale of future royalties and prospectively recognizing the related non-cash interest expense. Management’s estimate of the amount of expected future payments to Blackstone over the life of the arrangement is based on the estimated global net sales of Leqvio. The Company recorded a liability related to the sale of future royalties of $1.29$1.38 billion as of December 31, 20222023 and recognized interest expense on the liability related to the sale of future royalties of $107.6$106.6 million for the year ended December 31, 2022.2023.
The principal considerations for our determination that performing procedures relating to the liability related to the sale of future royalties and commercial milestones is a critical audit matter are the significant judgment by management when developing the estimate of the timing and amount of future royalties and commercial milestones to be paid. This in turn led to a high degree of auditor judgment and effort in performing procedures and in evaluating audit evidence relating to management’s estimate of the expected future royalties and commercial milestones to be paid and the selection of third party data used to estimate global net sales of Leqvio.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the liability for future royalties and commercial milestones, including controls over management’s process for developing the estimate of timing and amount of future royalties and commercial milestones to be paid. These procedures also included, among others (i) testing management’s process for developing the estimate of timing and amount of future royalties and commercial milestones to be paid and (ii) evaluating the reasonableness of significant assumptions used by management when developing the estimate of expected future royalties and commercial milestones to be paid related to the selection of third party data used to estimate global net sales of Leqvio. Evaluating management’s assumption related to the selection of third-party data used to estimate global net sales of Leqvio involved evaluating whether the assumptions used by management were reasonable considering consistency with industry data.
/s/PricewaterhouseCoopers LLP
Boston, Massachusetts
February 23, 202315, 2024
We have served as the Company’s auditor since 2003.
ALNYLAM PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except per share amounts)
| | December 31, |
| 2022 | | 2021 |
| December 31, | | | December 31, |
| 2023 | | 2022 |
| ASSETS | ASSETS | | | |
| Current assets: | Current assets: | |
| Current assets: | |
| Current assets: | |
| Cash and cash equivalents | |
| Cash and cash equivalents | |
| Cash and cash equivalents | Cash and cash equivalents | $ | 866,394 | | | $ | 819,975 | |
| Marketable debt securities | Marketable debt securities | 1,297,890 | | | 1,548,617 | |
| Marketable equity securities | Marketable equity securities | 28,122 | | | 66,972 | |
| Accounts receivable, net | Accounts receivable, net | 237,963 | | | 198,571 | |
| Inventory | Inventory | 128,962 | | | 86,363 | |
| Prepaid expenses and other current assets | Prepaid expenses and other current assets | 132,916 | | | 88,078 | |
| | Total current assets | Total current assets | 2,692,247 | | | 2,808,576 | |
| Total current assets | |
| Total current assets | |
| Property, plant and equipment, net | Property, plant and equipment, net | 523,494 | | | 501,958 | |
| Operating lease right-of-use assets | Operating lease right-of-use assets | 215,136 | | | 231,675 | |
| Restricted investments | Restricted investments | 49,390 | | | 40,891 | |
| Other assets | Other assets | 66,092 | | | 60,204 | |
| Total assets | Total assets | $ | 3,546,359 | | | $ | 3,643,304 | |
| LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY | | | |
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | |
| Current liabilities: | Current liabilities: | |
| Current liabilities: | |
| Current liabilities: | |
| Accounts payable | |
| Accounts payable | |
| Accounts payable | Accounts payable | $ | 98,094 | | | $ | 73,426 | |
| Accrued expenses | Accrued expenses | 545,460 | | | 395,174 | |
| Operating lease liability | Operating lease liability | 41,967 | | | 40,548 | |
| Deferred revenue | Deferred revenue | 42,105 | | | 149,483 | |
| Liability related to the sale of future royalties | Liability related to the sale of future royalties | 40,289 | | | 37,079 | |
| Total current liabilities | Total current liabilities | 767,915 | | | 695,710 | |
| Operating lease liability, net of current portion | Operating lease liability, net of current portion | 261,339 | | | 281,347 | |
| Deferred revenue, net of current portion | Deferred revenue, net of current portion | 193,791 | | | 152,360 | |
| Convertible debt | Convertible debt | 1,016,942 | | | — | |
| Long-term debt, net | — | | | 675,697 | |
| | Liability related to the sale of future royalties, net of current portion | |
| Liability related to the sale of future royalties, net of current portion | |
| Liability related to the sale of future royalties, net of current portion | Liability related to the sale of future royalties, net of current portion | 1,252,015 | | | 1,151,024 | |
| Other liabilities | Other liabilities | 212,580 | | | 98,963 | |
| Total liabilities | Total liabilities | 3,704,582 | | | 3,055,101 | |
| Commitments and contingencies (Note 14) | | | |
| Stockholders’ (deficit) equity: | |
| Preferred stock, $0.01 par value per share, 5,000 shares authorized and no shares issued and outstanding as of December 31, 2022 and December 31, 2021 | — | | | — | |
| Common stock, $0.01 par value per share, 250,000 shares authorized as of December 31, 2022 and December 31, 2021, respectively; 123,925 shares issued and outstanding as of December 31, 2022; 120,182 shares issued and outstanding as of December 31, 2021 | 1,240 | | | 1,202 | |
| Commitments and contingencies (Note 13) | | Commitments and contingencies (Note 13) | | | |
| Stockholders’ deficit: | |
| Preferred stock, $0.01 par value per share, 5,000 shares authorized and no shares issued and outstanding as of December 31, 2023 and December 31, 2022 | |
| Preferred stock, $0.01 par value per share, 5,000 shares authorized and no shares issued and outstanding as of December 31, 2023 and December 31, 2022 | |
| Preferred stock, $0.01 par value per share, 5,000 shares authorized and no shares issued and outstanding as of December 31, 2023 and December 31, 2022 | |
| Common stock, $0.01 par value per share, 250,000 shares authorized as of December 31, 2023 and December 31, 2022, respectively; 125,794 shares issued and outstanding as of December 31, 2023; 123,925 shares issued and outstanding as of December 31, 2022 | |
| Additional paid-in capital | Additional paid-in capital | 6,454,540 | | | 6,058,453 | |
| Accumulated other comprehensive loss | Accumulated other comprehensive loss | (44,654) | | | (33,259) | |
| Accumulated deficit | Accumulated deficit | (6,569,349) | | | (5,438,193) | |
| Total stockholders’ (deficit) equity | (158,223) | | | 588,203 | |
| Total liabilities and stockholders’ (deficit) equity | $ | 3,546,359 | | | $ | 3,643,304 | |
| Total stockholders’ deficit | |
| Total liabilities and stockholders’ deficit | |
The accompanying notes are an integral part of these consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(In thousands, except per share amounts)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Statements of Operations | | | | | |
| Revenues: | | | | | |
| Net product revenues | $ | 1,241,474 | | | $ | 894,329 | | | $ | 662,138 | |
| Net revenues from collaborations | 546,185 | | | 134,912 | | | 180,953 | |
| Royalty revenue | 40,633 | | | 8,177 | | | 1,196 | |
| Total revenues | 1,828,292 | | | 1,037,418 | | | 844,287 | |
| Operating costs and expenses: | | | | | |
| Cost of goods sold | 268,216 | | | 140,174 | | | 115,005 | |
| Cost of collaborations and royalties | 42,190 | | | 28,643 | | | 25,139 | |
| Research and development | 1,004,415 | | | 883,015 | | | 792,156 | |
| Selling, general and administrative | 795,646 | | | 770,658 | | | 620,639 | |
| Total operating costs and expenses | 2,110,467 | | | 1,822,490 | | | 1,552,939 | |
| Loss from operations | (282,175) | | | (785,072) | | | (708,652) | |
| Other (expense) income: | | | | | |
| Interest expense | (121,221) | | | (155,968) | | | (143,021) | |
| Interest income | 95,561 | | | 24,808 | | | 1,579 | |
| Other expense, net | (125,682) | | | (134,175) | | | (2,050) | |
| Loss on the extinguishment of debt | — | | | (76,586) | | | — | |
| Total other expense, net | (151,342) | | | (341,921) | | | (143,492) | |
| Loss before income taxes | (433,517) | | | (1,126,993) | | | (852,144) | |
| Provision for income taxes | (6,725) | | | (4,163) | | | (680) | |
| Net loss | $ | (440,242) | | | $ | (1,131,156) | | | $ | (852,824) | |
| Net loss per common share — basic and diluted | $ | (3.52) | | | $ | (9.30) | | | $ | (7.20) | |
| Weighted-average common shares used to compute basic and diluted net loss per common share | 124,906 | | | 121,689 | | | 118,451 | |
| | | | | |
| Statements of Comprehensive Loss | | | | | |
| Net loss | $ | (440,242) | | | $ | (1,131,156) | | | $ | (852,824) | |
| Other comprehensive income (loss): | | | | | |
| Unrealized gain (loss) on marketable securities | 11,018 | | | (7,840) | | | (1,978) | |
| Foreign currency translation gain (loss) | 11,922 | | | (5,274) | | | 11,398 | |
| Defined benefit pension plans, net of tax | (1,661) | | | 1,719 | | | 943 | |
| Total other comprehensive income (loss) | 21,279 | | | (11,395) | | | 10,363 | |
| Comprehensive loss | $ | (418,963) | | | $ | (1,142,551) | | | $ | (842,461) | |
The accompanying notes are an integral part of these consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSSSTOCKHOLDERS’ (DEFICIT) EQUITY
(In thousands, except per share amounts)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Statements of Operations | | | | | |
| Revenues: | | | | | |
| Net product revenues | $ | 894,329 | | | $ | 662,138 | | | $ | 361,520 | |
| Net revenues from collaborations | 134,912 | | | 180,953 | | | 131,333 | |
| Royalty revenue | 8,177 | | | 1,196 | | | — | |
| Total revenues | 1,037,418 | | | 844,287 | | | 492,853 | |
| Operating costs and expenses: | | | | | |
| Cost of goods sold | 140,174 | | | 115,005 | | | 74,185 | |
| Cost of collaborations and royalties | 28,643 | | | 25,139 | | | 3,867 | |
| Research and development | 883,015 | | | 792,156 | | | 654,819 | |
| Selling, general and administrative | 770,658 | | | 620,639 | | | 588,420 | |
| Total operating costs and expenses | 1,822,490 | | | 1,552,939 | | | 1,321,291 | |
| Loss from operations | (785,072) | | | (708,652) | | | (828,438) | |
| Other (expense) income: | | | | | |
| Interest expense | (155,968) | | | (143,021) | | | (84,496) | |
| Other (expense) income, net | (109,367) | | | (471) | | | 57,334 | |
| Loss on the extinguishment of debt | (76,586) | | | — | | | — | |
| Total other expense, net | (341,921) | | | (143,492) | | | (27,162) | |
| Loss before income taxes | (1,126,993) | | | (852,144) | | | (855,600) | |
| Provision for income taxes | (4,163) | | | (680) | | | (2,681) | |
| Net loss | $ | (1,131,156) | | | $ | (852,824) | | | $ | (858,281) | |
| Net loss per common share — basic and diluted | $ | (9.30) | | | $ | (7.20) | | | $ | (7.46) | |
| Weighted-average common shares used to compute basic and diluted net loss per common share | 121,689 | | | 118,451 | | | 114,986 | |
| | | | | |
| Statements of Comprehensive Loss | | | | | |
| Net loss | $ | (1,131,156) | | | $ | (852,824) | | | $ | (858,281) | |
| Other comprehensive (loss) income: | | | | | |
| Unrealized (loss) gain on marketable securities | (7,840) | | | (1,978) | | | 211 | |
| Foreign currency translation (loss) gain | (5,274) | | | 11,398 | | | (7,081) | |
| Defined benefit pension plans, net of tax | 1,719 | | | 943 | | | (234) | |
| Total other comprehensive (loss) income | (11,395) | | | 10,363 | | | (7,104) | |
| Comprehensive loss | $ | (1,142,551) | | | $ | (842,461) | | | $ | (865,385) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional
Paid-in
Capital | | Accumulated Other Comprehensive Loss | | Accumulated
Deficit | | Total
Stockholders’
(Deficit) Equity |
| Shares | | Amount | | | | |
| Balance as of December 31, 2020 | 116,427 | | | $ | 1,164 | | | $ | 5,644,074 | | | $ | (43,622) | | | $ | (4,585,369) | | | $ | 1,016,247 | |
| Exercise of common stock options, net of tax withholdings | 2,978 | | | 30 | | | 232,456 | | | — | | | — | | | 232,486 | |
| Issuance of common stock under equity plans | 777 | | | 8 | | | 13,623 | | | — | | | — | | | 13,631 | |
| Stock-based compensation expense | — | | | — | | | 168,300 | | | — | | | — | | | 168,300 | |
| Other comprehensive gain | — | | | — | | | — | | | 10,363 | | | — | | | 10,363 | |
| Net loss | — | | | — | | | — | | | — | | | (852,824) | | | (852,824) | |
| Balance as of December 31, 2021 | 120,182 | | | 1,202 | | | 6,058,453 | | | (33,259) | | | (5,438,193) | | | 588,203 | |
| Exercise of common stock options, net of tax withholdings | 3,103 | | | 32 | | | 263,580 | | | — | | | — | | | 263,612 | |
| Issuance of common stock under equity plans | 640 | | | 6 | | | 13,719 | | | — | | | — | | | 13,725 | |
| Stock-based compensation expense | — | | | — | | | 237,399 | | | — | | | — | | | 237,399 | |
| Purchase of capped calls related to convertible debt | — | | | — | | | (118,611) | | | — | | | — | | | (118,611) | |
| Other comprehensive loss | — | | | — | | | — | | | (11,395) | | | — | | | (11,395) | |
| Net loss | — | | | — | | | — | | | — | | | (1,131,156) | | | (1,131,156) | |
| Balance as of December 31, 2022 | 123,925 | | | 1,240 | | | 6,454,540 | | | (44,654) | | | (6,569,349) | | | (158,223) | |
| Exercise of common stock options, net of tax withholdings | 1,162 | | | 12 | | | 114,237 | | | — | | | — | | | 114,249 | |
| Issuance of common stock under equity plans | 707 | | | 7 | | | 16,421 | | | — | | | — | | | 16,428 | |
| Stock-based compensation expense | — | | | — | | | 225,865 | | | — | | | — | | | 225,865 | |
| Other comprehensive gain | — | | | — | | | — | | | 21,279 | | | — | | | 21,279 | |
| Net loss | — | | | — | | | — | | | — | | | (440,242) | | | (440,242) | |
| Balance as of December 31, 2023 | 125,794 | | | $ | 1,259 | | | $ | 6,811,063 | | | $ | (23,375) | | | $ | (7,009,591) | | | $ | (220,644) | |
The accompanying notes are an integral part of these consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITYCASH FLOWS
(In thousands, except share amounts)thousands)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | Additional
Paid-in
Capital | | Accumulated
Other
Comprehensive (Loss) Income | | Accumulated
Deficit | | Total
Stockholders’
(Deficit) Equity |
| Shares | | Amount | | | | |
| Balance at December 31, 2019 | 112,188 | | | $ | 1,122 | | | $ | 5,201,176 | | | $ | (36,518) | | | $ | (3,727,088) | | | $ | 1,438,692 | |
| Exercise of common stock options, net of tax withholdings | 2,926 | | | 28 | | | 189,343 | | | — | | | — | | | 189,371 | |
| Issuance of common stock under equity plans | 350 | | | 4 | | | 11,079 | | | — | | | — | | | 11,083 | |
| Issuance of common stock to strategic partners, net of closing costs | 963 | | | 10 | | | 99,488 | | | — | | | — | | | 99,498 | |
| Stock-based compensation expense | — | | | — | | | 142,988 | | | — | | | — | | | 142,988 | |
| Other comprehensive loss | — | | | — | | | — | | | (7,104) | | | — | | | (7,104) | |
| Net loss | — | | | — | | | — | | | — | | | (858,281) | | | (858,281) | |
| Balance at December 31, 2020 | 116,427 | | | 1,164 | | | 5,644,074 | | | (43,622) | | | (4,585,369) | | | 1,016,247 | |
| Exercise of common stock options, net of tax withholdings | 2,978 | | | 30 | | | 232,456 | | | — | | | — | | | 232,486 | |
| Issuance of common stock under equity plans | 777 | | | 8 | | | 13,623 | | | — | | | — | | | 13,631 | |
| | | | | | | | | | | |
| Stock-based compensation expense | — | | | — | | | 168,300 | | | — | | | — | | | 168,300 | |
| Other comprehensive gain | — | | | — | | | — | | | 10,363 | | | — | | | 10,363 | |
| Net loss | — | | | — | | | — | | | — | | | (852,824) | | | (852,824) | |
| Balance at December 31, 2021 | 120,182 | | | 1,202 | | | 6,058,453 | | | (33,259) | | | (5,438,193) | | | 588,203 | |
| Exercise of common stock options, net of tax withholdings | 3,103 | | | 32 | | | 263,580 | | | — | | | — | | | 263,612 | |
| Issuance of common stock under equity plans | 640 | | | 6 | | | 13,719 | | | — | | | — | | | 13,725 | |
| | | | | | | | | | | |
| Stock-based compensation expense | — | | | — | | | 237,399 | | | — | | | — | | | 237,399 | |
| Purchase of capped calls related to convertible debt | — | | | — | | | (118,611) | | | — | | | — | | | (118,611) | |
| Other comprehensive loss | — | | | — | | | — | | | (11,395) | | | — | | | (11,395) | |
| Net loss | — | | | — | | | — | | | — | | | (1,131,156) | | | (1,131,156) | |
| Balance at December 31, 2022 | 123,925 | | | $ | 1,240 | | | $ | 6,454,540 | | | $ | (44,654) | | | $ | (6,569,349) | | | $ | (158,223) | |
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Cash flows from operating activities: | | | | | |
| Net loss | $ | (440,242) | | | $ | (1,131,156) | | | $ | (852,824) | |
| Non-cash adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Depreciation and amortization | 54,054 | | | 44,468 | | | 47,567 | |
| Amortization and interest accretion related to operating leases | 42,977 | | | 41,082 | | | 42,127 | |
| Non-cash interest expense on liability related to the sale of future royalties | 106,554 | | | 104,200 | | | 116,562 | |
| Stock-based compensation | 221,680 | | | 230,649 | | | 165,717 | |
| Realized and unrealized loss (gain) on marketable equity securities | 16,944 | | | 33,312 | | | (55,695) | |
| Loss on extinguishment of debt | — | | | 76,586 | | | — | |
| Change in fair value of development derivative liability | 90,997 | | | 94,659 | | | 38,433 | |
| Other | 433 | | | 479 | | | 19,243 | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable, net | (87,939) | | | (45,597) | | | (101,799) | |
| Inventory | 18,367 | | | (34,136) | | | (26,415) | |
| Prepaid expenses and other assets | (9,029) | | | (38,507) | | | (32,093) | |
| Accounts payable, accrued expenses and other liabilities | 80,840 | | | 191,769 | | | 88,240 | |
| Operating lease liability | (46,502) | | | (43,171) | | | (40,352) | |
| Deferred revenue | 55,022 | | | (65,911) | | | (50,404) | |
| Net cash provided by (used in) operating activities | 104,156 | | | (541,274) | | | (641,693) | |
| Cash flows from investing activities: | | | | | |
| Purchases of property, plant and equipment | (62,211) | | | (72,059) | | | (76,372) | |
| Purchases of marketable securities | (1,823,501) | | | (1,976,961) | | | (1,656,114) | |
| Sales and maturities of marketable securities | 1,553,800 | | | 2,231,568 | | | 1,463,550 | |
| Proceeds from maturity of restricted investments | 58,475 | | | 89,951 | | | 41,975 | |
| Purchases of restricted investments | (58,475) | | | (98,451) | | | (42,141) | |
| Other investing activities | (4,438) | | | (4,694) | | | (4,198) | |
| Net cash (used in) provided by investing activities | (336,350) | | | 169,354 | | | (273,300) | |
| Cash flows from financing activities: | | | | | |
| Proceeds from exercise of stock options and other types of equity, net | 147,464 | | | 259,360 | | | 246,268 | |
| Proceeds from convertible debt, net | — | | | 1,016,111 | | | — | |
| | | | | |
| Purchases of capped calls related to convertible debt | — | | | (118,611) | | | — | |
| Proceeds from the sale of future royalties | — | | | — | | | 500,000 | |
| Proceeds from development derivative | 24,667 | | | 31,000 | | | 19,600 | |
| (Repayment of) proceeds from term loan facility | — | | | (762,107) | | | 500,000 | |
| Other financing activities | — | | | — | | | (18,750) | |
| Net cash provided by financing activities | 172,131 | | | 425,753 | | | 1,247,118 | |
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | 6,391 | | | (7,430) | | | (9,018) | |
| Net (decrease) increase in cash, cash equivalents and restricted cash | (53,672) | | | 46,403 | | | 323,107 | |
| Cash, cash equivalents and restricted cash, beginning of period | 868,556 | | | 822,153 | | | 499,046 | |
| Cash, cash equivalents and restricted cash, end of period | $ | 814,884 | | | $ | 868,556 | | | $ | 822,153 | |
| Supplemental disclosure of cash flows: | | | | | |
| Cash paid for interest | $ | 32,118 | | | $ | 45,235 | | | $ | 24,657 | |
| | | | | |
| Supplemental disclosure of noncash investing activities: | | | | | |
| Capital expenditures included in accounts payable and accrued expenses | $ | 3,805 | | | $ | 5,213 | | | $ | 13,599 | |
| | | | | |
The accompanying notes are an integral part of these consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Cash flows from operating activities: | | | | | |
| Net loss | $ | (1,131,156) | | | $ | (852,824) | | | $ | (858,281) | |
| Non-cash adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Depreciation and amortization | 44,468 | | | 47,567 | | | 34,772 | |
| Amortization and interest accretion related to operating leases | 41,082 | | | 42,127 | | | 39,663 | |
| Non-cash interest expense on liability related to the sale of future royalties | 104,200 | | | 116,562 | | | 84,496 | |
| Stock-based compensation | 230,649 | | | 165,717 | | | 139,873 | |
| Realized and unrealized loss (gain) on marketable equity securities | 33,312 | | | (55,695) | | | (54,042) | |
| Loss on extinguishment of debt | 76,586 | | | — | | | — | |
| Change in fair value of development derivative liability | 94,659 | | | 38,433 | | | 17,185 | |
| Other | 479 | | | 19,243 | | | (5,926) | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable, net | (45,597) | | | (101,799) | | | (56,236) | |
| Inventory | (34,136) | | | (26,415) | | | (35,426) | |
| Prepaid expenses and other assets | (38,507) | | | (32,093) | | | 13,017 | |
| Accounts payable, accrued expenses and other liabilities | 191,769 | | | 88,240 | | | 143,732 | |
| Operating lease liability | (43,171) | | | (40,352) | | | (33,823) | |
| Deferred revenue | (65,911) | | | (50,404) | | | (43,965) | |
| Net cash used in operating activities | (541,274) | | | (641,693) | | | (614,961) | |
| Cash flows from investing activities: | | | | | |
| Purchases of property, plant and equipment | (72,059) | | | (76,372) | | | (70,361) | |
| Purchases of marketable securities | (1,976,961) | | | (1,656,114) | | | (2,025,626) | |
| Sales and maturities of marketable securities | 2,231,568 | | | 1,463,550 | | | 1,691,669 | |
| Proceeds from maturity of restricted investments | 89,951 | | | 41,975 | | | — | |
| Purchases of restricted investments | (98,451) | | | (42,141) | | | (25,900) | |
| Other investing activities | (4,694) | | | (4,198) | | | (5,300) | |
| Net cash provided by (used in) investing activities | 169,354 | | | (273,300) | | | (435,518) | |
| Cash flows from financing activities: | | | | | |
| Proceeds from exercise of stock options and other types of equity, net | 259,360 | | | 246,268 | | | 200,484 | |
| Proceeds from convertible debt, net | 1,016,111 | | | — | | | — | |
| Repayment of term loan | (762,107) | | | — | | | — | |
| Purchases of capped calls related to convertible debt | (118,611) | | | — | | | — | |
| Proceeds from the sale of future royalties | — | | | 500,000 | | | 500,000 | |
| Proceeds from development derivative | 31,000 | | | 19,600 | | | 8,400 | |
| | | | | |
| Proceeds from term loan facility | — | | | 500,000 | | | 200,000 | |
| Proceeds from issuance of common stock to strategic partners, net of closing costs | — | | | — | | | 99,498 | |
| Other financing activities | — | | | (18,750) | | | (13,403) | |
| Net cash provided by financing activities | 425,753 | | | 1,247,118 | | | 994,979 | |
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | (7,430) | | | (9,018) | | | 4,918 | |
| Net increase (decrease) in cash, cash equivalents and restricted cash | 46,403 | | | 323,107 | | | (50,582) | |
| Cash, cash equivalents and restricted cash, beginning of period | 822,153 | | | 499,046 | | | 549,628 | |
| Cash, cash equivalents and restricted cash, end of period | $ | 868,556 | | | $ | 822,153 | | | $ | 499,046 | |
| Supplemental disclosure of cash flows: | | | | | |
| Cash paid for interest | $ | 45,235 | | | $ | 24,657 | | | $ | — | |
| Supplemental disclosure of noncash investing and financing activities: | | | | | |
| Capital expenditures included in accounts payable and accrued expenses | $ | 5,213 | | | $ | 13,599 | | | $ | 14,518 | |
| Lease liabilities arising from obtaining right-of-use assets | $ | 1,013 | | | $ | 7,932 | | | $ | 34,435 | |
| Receivable and liability related to the sale of future royalties | $ | — | | | $ | — | | | $ | 500,000 | |
The accompanying notes are an integral part of these consolidated financial statements.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. NATURE OF BUSINESS
Alnylam Pharmaceuticals, Inc. (also referred to as Alnylam, we, our or us) commenced operations on June 14, 2002 as a biopharmaceutical company seeking to develop and commercialize novel therapeutics based on ribonucleic acid interference, or RNAi. We are committed to the advancement of our company strategy of building a multi-product, global, commercial biopharmaceutical company with a deep and sustainable clinical pipeline of RNAi therapeutics for future growth and a robust, organic research engine for sustainable innovation and great potential for patient impact. Since inception, we have focused on discovering, developing and commercializing RNAi therapeutics by establishing and maintaining a strong intellectual property position in the RNAi field, establishing strategic alliances with leading pharmaceutical and life sciences companies, generating revenues through licensing agreements, and ultimately developing and commercializing RNAi therapeutics globally, either independently or with our strategic partners. We have devoted substantially all of our efforts to business planning, research, development, manufacturing and early commercial efforts, acquiring, filing and expanding intellectual property rights, recruiting management and technical staff, and raising capital.
In early 2021, we launched our Alnylam P5x25 strategy, which focuses on our planned transition to a top-tier biotech company by the end of 2025. With Alnylam P5x25, we aim to deliver transformative rare and prevalent disease medicines for patients around the world through sustainable innovation, while delivering exceptional financial performance and driving profitability.
As of December 31, 2022,2023, we have five marketed products, that have received marketing approval, including one partneredcollaborated product, and multiple late-stage investigational programs advancing towards potential commercialization. We currently generate worldwide product revenues from four commercialized products, ONPATTRO, AMVUTTRA, GIVLAARI and OXLUMO, primarily in the United States, or U.S., Europe and Japan.Europe.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements reflect the operations of Alnylam and our wholly-ownedwholly owned subsidiaries. All intercompany accounts and transactions have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America, or GAAP, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. In our consolidated financial statements, we use estimates and assumptions related to our inventory valuation and related reserves, liability related to the sale of future royalties, development derivative liability, income taxes, deferred tax asset valuation allowances, revenue recognition, research and development expenses, and stock-based compensation. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable. Actual results could differ from those estimates. Changes in estimates are reflected in reported results in the period in which they become known.
Liquidity
Based on our current operating plan, we believe that our cash, cash equivalents and marketable securities as of December 31, 2022, together with the cash we expect to generate from product sales and under our current alliances,2023 will be sufficient to enable us to advancesatisfy our Alnylam P5x25 strategyworking capital and operating needs for at least the next 12 months from the filing of this Annual Report on Form 10-K.
Concentrations of Credit Risk and Significant Customers
Financial instruments that potentially expose us to concentrations of credit risk primarily consist of cash, cash equivalents and marketable securities. As of December 31, 20222023 and 2021,2022, substantially all of our cash, cash equivalents and marketable securities were invested in money market funds, certificates of deposit, commercial paper, corporate notes, U.S. government-sponsored enterprise securities and U.S. treasury securities through highly rated financial institutions. Corporate notes may also include foreign bonds denominated in U.S. dollars. Investments are restricted, in accordance with our investment policy, to a concentration limit per issuer.
During the years ended December 31, 2023, 2022 2021 and 2020,2021, our revenues were generated primarily from product sales to distributorscustomers and collaborations with strategic partners. For the years ended December 31, 2023, 2022 2021 and 2020,2021, our gross accounts receivable balance was comprised of payments primarily due from distributorscustomers for product sales and our strategic partners.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The following table summarizes customers that represent 10% or greater of our consolidated total gross revenues:
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| Distributor A | Distributor A | 33 | % | | 27 | % | | 31 | % | Distributor A | 28 | % | | 33 | % | | 27 | % |
| Roche | | Roche | 15 | % | | * |
| Regeneron Pharmaceuticals | Regeneron Pharmaceuticals | * | | 11% | | 12% | Regeneron Pharmaceuticals | * | | * | | 11% |
*Represents less than 10% and/or not a customer in the applicable year
The following table summarizes customers with amounts due that represent 10% or greater of our consolidated gross accounts receivable balance:
| | As of December 31, |
| 2022 | | 2021 |
| As of December 31, | | | As of December 31, |
| 2023 | | | 2023 | | 2022 |
| Novartis AG | | Novartis AG | 18 | % | | * |
| Distributor A | Distributor A | 32 | % | | 14 | % | Distributor A | 16 | % | | 32 | % |
| Distributor B | Distributor B | 10 | % | | 10 | % | Distributor B | * | | 10 | % |
| Novartis AG | * | | 20 | % |
| Regeneron Pharmaceuticals | * | | 12 | % |
|
*Represents less than 10%
Fair Value Measurements
The fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. In general, fair values determined by Level 1 inputs utilize quoted prices (unadjusted) in active markets for identical assets or liabilities. Fair values determined by Level 2 inputs utilize data points that are observable, such as quoted prices (adjusted), interest rates and yield curves. Fair values determined by Level 3 inputs utilize unobservable data points for the asset or liability, and include situations where there is little, if any, market activity for the asset or liability. The fair value hierarchy level is determined by the lowest level of significant input.
Investments in Marketable Securities and Cash Equivalents
We invest our excess cash balances in marketable debt securities and classify our investments as either held-to-maturity or available-for-sale based on facts and circumstances present at the time we purchased the securities. At each balance sheet date presented,As of December 31, 2023 and 2022, we classified all of our investments in debt securities as available-for-sale and as current assets as they represent the investment of funds available for current operations. We report available-for-sale debt securities at fair value at each balance sheet date, for which fair value measurement data is obtained from independent pricing services, and include any unrealized holding gains and losses (the adjustment to fair value) in accumulated other comprehensive (loss) income, a component of stockholders’ (deficit) equity.loss. Realized gains and losses are determined using the specific identification method and are included in other income (expense).expense, net. If any adjustment to fair value reflects a decline in the value of the marketable debt securities, we consider all available evidence to evaluate if an impairment loss exists, and if so, mark the investment to market through a charge to our consolidated statements of operations and comprehensive loss. We did not record any impairment charges related to our marketable debt securities during the years ended December 31, 2023, 2022 2021 or 2020.2021. Our marketable debt securities are classified as cash equivalents if the original maturity, from the date of purchase, is 90 days or less, and as marketable debt securities if the original maturity, from the date of purchase, is in excess of 90 days. Our cash equivalents are generally composed of commercial paper, corporate notes, U.S. government-sponsored enterprise securities, U.S. treasury securities, and money market funds.funds and certificates of deposit.
We measure marketable equity investments (except those accounted for under the equity method of accounting or those that result in consolidation of an investee), which have readily available prices, at fair value with changes in fair value recognized in other income (expense)expense, net on our consolidated statements of operations and comprehensive loss. We obtain fair value measurement data for our marketable debt securities from independent pricing services. We perform validation procedures to ensure the reasonableness of this data. This includes discussing with the independent pricing services to understand the methods and data sources used. For our marketable debt securities, we perform our own review of prices received from the independent pricing services by comparing these prices to other sources and for our marketable equity securities, we confirm those securities are trading in active markets.
Accounts Receivable
We record accounts receivable net of customer allowances for distribution services, prompt payment discounts and chargebacks based on contractual terms. As of December 31, 20222023 and 2021,2022, based on our estimation of expected write-offs, we determined an allowance for doubtful accounts was not material. We have standard payment terms that generally require
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
payment within approximately 30 to 90 days. Accounts receivable, net on our consolidated balance sheets also includes billed and unbilled collaboration receivables and royalty receivables.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Inventory
Inventory is measured at the lower of cost or estimated net realizable value and classified based on the anticipation of when it will be consumed either within our normal operating cycle (short-term) or beyond (long-term). We use a standard cost basis, which approximates cost determined on a first-in, first-out basis. Inventory costs include all raw materials, direct conversion costs and overhead. Raw and intermediate materials that may be used for either research and development or commercial purposes are classified as inventory until the material is consumed or otherwise allocated for research and development. If the material is used for research and development, it is expensed as research and development once that determination is made.
We capitalize inventory costs that are expected to be sold commercially once we determine it is probable that the inventory costs will be recovered through commercial sale based on the review of several factors, including (i) the likelihood that all required regulatory approvals will be received, considering any special filing status, (ii) the expected timing of validation (if not yet completed) of manufacturing processes in the associated facility, (iii) the expected expiration of the inventory, (iv) logistical or commercial constraints that may impede the timely distribution and sale of the product, including transport requirements and reimbursement status, (v) current market factors, including competitive landscape and pricing, (vi) threatened or anticipated litigation challenges, (vii) history of approvals of similar products or formulations, and (viii) FDA (or other appropriate regulatory agencies) correspondence regarding the safety and efficacy of the product. Prior to the capitalization of inventory costs, we record such costs as research and development expenses on our consolidated statements of operations and comprehensive loss.
We reduce our inventory to net realizable value for potentially excess, dated or obsolete inventory based on our quarterly assessment of the recoverability of our capitalized inventory. We periodically review inventory levels to identify what may expire prior to expected sale or has a cost basis in excess of its estimated realizable value and write-down such inventories as appropriate.
Property, Plant and Equipment
Property, plant and equipment are stated at cost, net of accumulated depreciation. Depreciation expense is recorded on a straight-line basis over the estimated useful life of the asset. Construction in progress reflects amounts incurred for construction or improvements of property, plant or equipment that have not been placed in service. Costs of construction of certain long-lived assets include capitalized interest, which is amortized over the estimated useful life of the related asset. The cost and accumulated depreciation of assets retired or sold are removed from the respective asset category, and any gain or loss is recognized in our consolidated statements of operations and comprehensive loss.During the years ended December 31, 2023, 2022 2021 and 2020,2021, we recorded $51.6 million, $39.1 million $36.8 million and $30.2$36.8 million, respectively, of depreciation expense related to our property, plant and equipment.
The estimated useful lives of property, plant and equipment are as follows:
| | | | | | | | |
| Asset Category | | Useful Life |
| Laboratory equipment | | 5 |
| Computer equipment and software | | 3-10 years |
| Furniture and fixtures | | 5 |
| Leasehold improvements | | Shorter of asset life or lease term |
| Manufacturing Equipment | | 7-15 years |
| Buildings | | 40 years |
Leases
We determine if an arrangement is a lease at contract inception based on the facts and circumstances present in the arrangement. All of our leases are classified as operating leases. We record operating lease assets and lease liabilities in our consolidated balance sheets. Operating lease assets represent our right to use an underlying asset for the lease term and operating lease liabilities represent our obligation to make lease payments arising from the leasing arrangement. Operating lease assets and operating lease liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As most of our leases do not provide an implicit rate, in determining the operating lease liabilities, we use an estimate of our incremental borrowing rate based on the information available at commencement. Lease expense for lease payments is recognized on a straight-line basis over the lease term. Short-term leases, or leases that have a lease term of 12
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
months or less at commencement date, are excluded from this treatment and are recognized on a straight-line basis over the term of the lease.
Clinical Accruals
We record accrued liabilities related to products we have received or services that we have incurred, specifically related to ongoing pre-clinical studies and clinical trials, for which service providers have not yet billed us, or when billing terms under these contracts do not coincide with the timing of when the work is performed, as of our period-end. These costs primarily relate to third-party clinical management costs, laboratory and analysis costs, toxicology studies and investigator fees. The assessment of these costs is a subjective process, requiring judgment based on our knowledge of the research and development programs, services performed for the period, experience with related activities and the expected duration of the third-party service contract, where applicable. Upon settlement, these costs may differ materially from the amounts accrued in our consolidated financial statements. Our historical accrual estimates have not been materially different from our actual costs.
Revenue Recognition
We recognize revenue when control of promised goods or services is transferred to a customer at an amount that reflects the consideration to which we expect to be entitled in exchange for those goods or services. To determine revenue recognition, we perform the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligationsobligation(s) in the contract; (iii) determine the transaction price, including variable consideration, if any; (iv) allocate the transaction price to the performance obligationsobligation(s) in the contract; and (v) recognize revenue when (or as) we satisfy athe performance obligation.obligation(s). We only apply the five-step model to contracts when collectability of the consideration to which we are entitled in exchange for the goods or services we transfer to the customer is determined to be probable.
At contract inception, once the contract is determined to be within the scope of Accounting Standards CodificationASC Topic 606, Revenue from Contracts with Customers, or ASC 606, we assess whether the goods or services promised within each contract are distinct and, therefore, represent a separate performance obligation. Goods and services that are determined not to be distinct are combined with other promised goods and services until a distinct bundle is identified. We then allocate the transaction price (the amount of consideration we expect to be entitled to from a customer in exchange for the promised goods or services) to each performance obligation and recognize the associated revenue when (or as) each performance obligation is satisfied. Our estimate of the transaction price for each contract includes all variable consideration to which we expect to be entitled.
Amounts are recorded as accounts receivable when our right to consideration is unconditional. We do not assess whether a contract has a significant financing component if the expectation at contract inception is that the period between payment by the customer and the transfer of the promised goods or services to the customer will be one year or less. We expense incremental costs of obtaining a contract as and when incurred if the expected amortization period of the asset that we would have recognized is one year or less or the amount is immaterial. As of December 31, 20222023 and 2021,2022, we had not capitalized any costs to obtain any of our contracts.
Net Product Revenues
Our net product revenues are recognized, net of variable consideration related to certain allowances and accruals, at the time the customer obtains control of our product. We use the expected value method, which is the sum of probability-weighted amounts in a range of possible consideration amounts, or the most likely amount method, which is the single most likely amount in a range of possible considerations, to estimate variable consideration related to our product sales. We use the expected value method to estimate variable consideration for certain rebates, chargebacks, product returns, and other incentives and we use the most likely amount method for certain rebates, and trade discounts and allowances.
We record reserves, based on contractual terms, for components related to product sold during the reporting period, as well as our estimate of product that remains in the distribution channel inventory at the end of the reporting period that we expect will be sold to qualified healthcare providers. On a quarterly basis, we update our estimates and record any needed adjustments in the period we identify the adjustments. The following are the components of variable consideration related to product revenues:
Chargebacks: We estimate obligations resulting from contractual commitments with the government and other entities to sell products to qualified healthcare providers at prices lower than the list prices charged to the customer who directly purchases from us. The customer charges us for the difference between what it pays to us for the product and the selling price to the qualified healthcare providers.
Rebates: We are subject to discount obligations under government programs, including Medicaid in the U.S. and similar programs in certain other countries, including countries in which we are accruing for estimated rebates because final pricing has not yet been negotiated. We are also subject to potential rebates in connection with our value-based agreements with certain commercial payors. We record reserves for rebates in the same period the related product revenue is recognized, resulting in a reduction of product revenues and a current liability that is included in accrued expenses on our consolidated balance sheet. Our
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
estimate for rebates is based on statutory discount rates, expected utilization or an estimated number of patients on treatment, as applicable.
Trade discounts and allowances: We provide customary invoice discounts on product sales to our customers for prompt payment and we pay fees for distribution services, such as fees for certain data that customers provide to us. We estimate our customers will earn these discounts and fees, and deduct these discounts and fees in full from gross product revenues and accounts receivable at the time we recognize the related revenues.
Product returns: We offer customers product return rights if products are damaged, defective or expired, with “expired” defined within each customer agreement. We estimate the amount of product that will be returned using a probability-weighted estimate based on our sales history.
Other incentives: Other incentives include co-payment assistance we provide to patients with commercial insurance that have coverage and reside in states that allow co-payment assistance. We estimate the average co-payment assistance amounts for our products based on expected customer demographics and record any such amounts within accrued expenses on our consolidated balance sheet.
Net Revenues from Collaborations
We earn revenue in connection with collaboration agreements which allow our collaboration partnerscollaborators to utilize our technology platforms and develop product candidates. Our significant collaboration agreements are detailed in Note 4, Net Revenues from Collaborations. For each collaboration partner,collaborators, we discuss our revenue recognition, including our significant performance obligations under each agreement.
At contract inception, we assess whether the collaboration arrangements are within the scope of ASC 606 or ASC Topic 808, Collaborative Arrangements, or ASC 808, to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards dependent on the commercial success of such activities. This assessment is performed based on the responsibilities of all parties in the arrangement. For collaboration arrangements within the scope of ASC 808 that contain multiple elements, we first determine which elements of the arrangement are within the scope of ASC 808 and which elements are within the scope of ASC 606. For elements of collaboration arrangements that are accounted for pursuant to ASC 808, an appropriate recognition method is determined and applied consistently, either by analogy to authoritative accounting literature or by applying a reasonable and rational policy election.
For elements of collaboration arrangements that are accounted for pursuant to ASC 606, we identify the performance obligations and allocate the total consideration we expect to receive on a relative standalone selling price basis to each performance obligation. Variable consideration, such as performance-based milestones, will be included in the total consideration if we expect to receive such consideration and if it is probable that the inclusion of the variable consideration will not result in a significant reversal in the cumulative amount of revenue recognized under the arrangement. Our estimate of the total consideration we expect to receive under each collaboration arrangement is updated for each reporting period, and any adjustments to revenue are recorded on a cumulative catch-up basis. We exclude sales-based royalty and milestone payments from the total consideration we expect to receive until the underlying sales occur because the license to our intellectual property is deemed to be the predominant item to which the royalties or milestones relate as it is the primary driver of value in our collaboration arrangements.
Key assumptions to determine the standalone selling price may include forecasted revenues, development timelines, reimbursement rates for personnel costs, discount rates and probabilities of technical and regulatory success. We recognize revenue associated with each performance obligation as the control over the promised goods or services transfer to our collaboration partnercollaborator which occurs either at a point in time or over time. If control transfers over time, revenue is recognized by using a method of measuring progress that best depicts the transfer of goods or services. We evaluate the measure of progress and related inputs each reporting period and any resulting adjustments to revenue are recorded on a cumulative catch-up basis.
Consideration received that does not meet the requirements to satisfy ASC 808 or ASC 606 revenue recognition criteria is recorded as deferred revenue in the accompanying consolidated balance sheets, classified as either short-term (less than 12 months) or long-term (more than 12 months) deferred revenue based on our best estimate of when such revenue will be recognized.
Cost of Goods Sold
Cost of goods sold includes the cost of producing and distributing inventories that are related to product revenues during the respective period (including salary-related and stock-based compensation expenses for employees involved with production and distribution, freight and indirect overhead costs), third-party royalties payable on our net product revenues and amortization of intangible assets associated with the sale of our products and costs related to sales of product supply under our collaboration agreements.products. Cost of goods sold may also include costs related to excess or obsolete inventory adjustment charges, abnormal costs, unabsorbed manufacturing and overhead costs, and manufacturing variances.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Cost of Collaborations and Royalties
Cost of collaborations and royalties includes costs we incur in connection with providing commercial drug supplies, such as GalNAc material, to collaborators, in addition to royalties we owe to third parties on the net sales of licensed products by Novartis.products.
Income Taxes
We account for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax basis. Deferred tax assets and liabilities are measured using enacted rates in effect for the year in which these temporary differences are expected to be recovered or settled. Valuation allowances are provided if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
Uncertain tax positions, for which management'smanagement’s assessment is that there is a more than 50% probability of sustaining the position upon challenge by a taxing authority based upon its technical merits, are subject to certain recognition and measurement criteria. The nature of the uncertain tax positions is often very complex and subject to change, and the amounts at issue can be substantial. We develop our cumulative probability assessment of the measurement of uncertain tax positions using internal experience, judgment and assistance from professional advisors. We re-evaluate these uncertain tax positions on a quarterly basis based on a number of factors including, but not limited to, changes in facts or circumstances, changes in tax law, and effectively settled issues under audit and new audit activity. Any change in these factors could result in the recognition of a tax benefit or an additional charge to the tax provision.
Research and Development Expenses
We record research and development expenses as incurred. Included in research and development expenses are wages, stock-based compensation expenses, benefits and other operating costs, facilities, supplies, external services, clinical trial and manufacturing costs, certain costs related to our collaboration arrangements, and overhead directly related to our research and development operations, as well as costs to acquire technology licenses.
We have entered into several license agreements for rights to utilize certain technologies. The terms of the licenses may provide for upfront payments, annual maintenance payments, milestone payments based upon certain specified events being achieved and royalties on product sales. We charge costs to acquire and maintain licensed technology that has not reached technological feasibility and does not have alternative future use to research and development expense as incurred. During the years ended December 31, 2023, 2022 2021 and 2020,2021, we charged to research and development expense costs associated with license fees of $5.8 million, $7.3 million $16.8 million and $2.8$16.8 million, respectively.
Stock-Based Compensation
We recognize stock-based compensation expense for grants under our stock incentive plans and employee stock purchase plan. We account for all stock-based awards granted to employees at their fair value and recognize compensation expense over the vesting period of the award. Determining the amount of stock-based compensation to be recorded requires us to develop estimates of fair values of stock optionsawards as of the grant date. We calculate the grant date fair values of stock options using the Black-Scholes valuation model, which requires the input of subjective assumptions, including but not limited to expected stock price volatility over the term of the awards and the expected term of stock options. The fair value of restricted stock awards granted to employees is based upon the quoted closing market price per share on the date of grant.
We have performance conditions included in certain of our restricted stock awards that are based upon the achievement of pre-specified clinical development, regulatory, commercial and/or financial performance events. As the outcome of each event has inherent risk and uncertainties, and a positive outcome may not be known until the event is achieved, we begin to recognize the value of the performance-based restricted stock awards when we determine the achievement of each performance condition is deemed probable, a determination which requires significant judgment by management. At the probable date, we record a cumulative expense catch-up, with remaining expense amortized over the remaining service period.
Liability Related to the Sale of Future Royalties
We account for the liability related to the sale of future royalties as a debt financing, as we have significant continuing involvement in the generation of the cash flows. Interest on the liability related to the sale of future royalties will be recognized using the effective interest rate method over the life of the related royalty stream.
The liability related to the sale of future royalties and the related interest expense are based on our current estimates of future royalties and commercial milestones expected to be paid over the life of the arrangement, which we determine by using third-party forecasts of Leqvio’s global net revenue. We will periodically assess the expected payments and to the extent the amount or timing of our future estimated payments is materially different than our previous estimates, we will account for any
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
such change by adjusting the liability related to the sale of future royalties and prospectively recognizing the related non-cash interest expense.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Development Derivative Liability
Development derivative liability is recorded at fair value based on the probability weighted present value of the estimated cash flows pursuant to contractual terms of the funding agreement. The liability is remeasured quarterly with any change in fair value recorded in other income (expense)expense on the consolidated statements of operations and comprehensive loss.
Comprehensive Loss
Comprehensive loss is comprised of net loss and certain changes in stockholders’ (deficit) equitydeficit that are excluded from net loss. We include unrealized gains and losses on certain marketable securities in other comprehensive loss, including changes in the value of our marketable debt securities. We includesecurities, foreign currency translation adjustments in other comprehensive loss if(if the functional currency is not the U.S. dollar. We includedollar) and certain changes in the fair value of the plan assets and projected benefit obligation attributed to our defined benefit pension plan in other comprehensive loss.plan.
Net Loss per Common Share
We compute basic net loss per common share by dividing net loss by the weighted-average number of common shares outstanding. We compute diluted net loss per common share by dividing net loss by the weighted-average number of common shares and dilutive potential common share equivalents then outstanding during the period. In the diluted net loss per share calculation, net loss would beis adjusted for the elimination of interest expense on the Convertibleconvertible debt. Potential common shares consist of shares issuable upon the vesting of restricted stock units, the exercise of stock options (the proceeds of which are then assumed to have been used to repurchase outstanding shares using the treasury stock method) orand upon conversion of the convertible debt outstanding during the period (calculated using the if-converted method assuming the conversion of the convertible debt as of the earliest period reported or at the date of issuance, if later). Because the inclusion of potential common shares would be anti-dilutive for all periods presented,presenting a net loss, diluted net loss per common share is the same as basic net loss per common share.
The following table sets forth the potential common shares (prior to consideration of the treasury stock or if-converted methods) excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive:
| | As of December 31, |
| As of December 31, | | | As of December 31, |
| (In thousands) | (In thousands) | 2022 | | 2021 | | 2020 | (In thousands) | 2023 | | 2022 | | 2021 |
| Options to purchase common stock, inclusive of performance-based stock options | Options to purchase common stock, inclusive of performance-based stock options | 8,424 | | | 10,015 | | | 11,692 | |
| Unvested restricted common stock, inclusive of performance-based restricted common stock | Unvested restricted common stock, inclusive of performance-based restricted common stock | 1,487 | | | 1,210 | | | 1,160 | |
| Convertible debt | Convertible debt | 3,616 | | | — | | | — | |
| Total | Total | 13,527 | | | 11,225 | | | 12,852 | |
Segment Information
We operate in a single reporting segment, the discovery, development and commercialization of RNAi therapeutics. Consistent with our management reporting, results of our operations are reported on a consolidated basis for purposes of segment reporting. As of December 31, 20222023 and 2021,2022, substantially all of our consolidated property, plant and equipment, net, was from U.S. operations. For the years ended December 31, 2023, 2022 2021 and 2020,2021, net revenues from collaborations were attributed to the U.S. Please read Note 3 for information regarding our net product sales by geography.
Recently AdoptedRecent Accounting Pronouncements
In August 2020,December 2023, the Financial Accounting Standards Board, or FASB, issued Accounting StandardsStandard Update or ASU, 2020-06, Debt—Debt2023-09, Improvements to Income Tax Disclosures which requires entities to disclose disaggregated information about their effective tax rate reconciliation as well as expanded information on income taxes paid by jurisdiction. The disclosure requirements will be applied on a prospective basis, with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts in an entity’s own equity. Specifically, ASU 2020-06 simplifies accounting for the issuance of convertible instruments by removing certain separation models required under existing guidance. In addition, theoption to apply them retrospectively. The standard removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception and requires use of the “if-converted” method when calculating the dilutive impact of convertible debt on earnings per share, or EPS. ASU 2020-06 is effective for financial statements issued for fiscal years beginning after December 15, 2021, and interim periods within those fiscal years,2024, with early adoption permitted. We adoptedare currently evaluating the disclosure requirements related to the new standard.
In November 2023, the FASB issued Accounting Standard Update 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures which is intended to improve reportable segment disclosure requirements, primarily through additional disclosures about significant segment expenses. The standard on January 1, 2022is effective for fiscal years beginning after December 15, 2023, and there was no impact oninterim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The amendments should be applied retrospectively to all prior periods presented in the date of adoption. Duringfinancial statements. We are evaluating the third quarter of 2022, we issued convertible notes as described in Note 10, Convertible Debt.disclosure requirements related to the new standard.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
3. NET PRODUCT REVENUES
Net product revenues, classified based on the geographic region in which the product is sold, consist of the following:
| | Year Ended December 31, |
| | Year Ended December 31, | | | | Year Ended December 31, |
| (In thousands) | (In thousands) | | 2022 | | 2021 | | 2020 | (In thousands) | | 2023 | | 2022 | | 2021 |
| ONPATTRO | ONPATTRO | | | | | | |
| United States | |
| United States | |
| United States | United States | | $ | 246,748 | | | $ | 213,210 | | | $ | 151,574 | |
| Europe | Europe | | 224,063 | | | 190,435 | | | 107,755 | |
| Rest of World | Rest of World | | 86,797 | | | 71,092 | | | 46,752 | |
| Total | Total | | 557,608 | | | 474,737 | | | 306,081 | |
| | AMVUTTRA | AMVUTTRA | |
| AMVUTTRA | |
| AMVUTTRA | |
| United States | |
| United States | |
| United States | United States | | 82,521 | | | — | | | — | |
| Europe | Europe | | 4,214 | | | — | | | — | |
| Rest of World | Rest of World | | 7,060 | | | — | | | — | |
| Total | Total | | 93,795 | | | — | | | — | |
| | GIVLAARI | GIVLAARI | |
| GIVLAARI | |
| GIVLAARI | |
| United States | |
| United States | |
| United States | United States | | 115,659 | | | 92,747 | | | 42,797 | |
| Europe | Europe | | 48,670 | | | 30,895 | | | 12,000 | |
| Rest of World | Rest of World | | 8,815 | | | 4,173 | | | 309 | |
| Total | Total | | 173,144 | | | 127,815 | | | 55,106 | |
| | OXLUMO | OXLUMO | |
| OXLUMO | |
| OXLUMO | |
| United States | |
| United States | |
| United States | United States | | 27,698 | | | 18,876 | | | — | |
| Europe | Europe | | 37,915 | | | 38,949 | | | 333 | |
| Rest of World | Rest of World | | 4,169 | | | 1,761 | | | — | |
| Total | Total | | 69,782 | | | 59,586 | | | 333 | |
| | Total net product revenues | Total net product revenues | | $ | 894,329 | | | $ | 662,138 | | | $ | 361,520 | |
| Total net product revenues | |
| Total net product revenues | |
As of December 31, 20222023 and 2021,2022, net product revenue-related receivables of $203.8210.1 million and $124.9203.8 million, respectively, were included in “Accounts receivable, net.”
The following table summarizes balances and activity in each product revenue allowance and reserve category:
| | As of December 31, 2022 |
| As of December 31, 2023 | | | As of December 31, 2023 |
| (In thousands) | (In thousands) | Chargebacks and Rebates | | Trade Discounts and Allowances | | Returns Reserve and Other Incentives | | Total | (In thousands) | Chargebacks and Rebates | | Trade Discounts and Allowances | | Returns Reserve and Other Incentives | | Total |
| Beginning balance | Beginning balance | $ | 120,682 | | | $ | 522 | | | $ | 10,112 | | | $ | 131,316 | |
| Provision related to current period sales | Provision related to current period sales | 245,236 | | | 13,085 | | | 15,249 | | | 273,570 | |
| Credit or payments made during the period for current year sales | Credit or payments made during the period for current year sales | (108,185) | | | (10,310) | | | (9,850) | | | (128,345) | |
| Credit or payments made during the period for prior year sales | Credit or payments made during the period for prior year sales | (65,961) | | | (847) | | | (735) | | | (67,543) | |
| Total | Total | $ | 191,772 | | | $ | 2,450 | | | $ | 14,776 | | | $ | 208,998 | |
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2021 |
| (In thousands) | Chargebacks and Rebates | | Trade Discounts and Allowances | | Returns Reserve and Other Incentives | | Total |
| Beginning balance | $ | 90,705 | | | $ | 639 | | | $ | 3,763 | | | $ | 95,107 | |
| Provision related to current period sales | 167,691 | | | 8,064 | | | 12,223 | | | 187,978 | |
| Credit or payments made during the period for current year sales | (75,073) | | | (7,665) | | | (5,190) | | | (87,928) | |
| Credit or payments made during the period for prior period sales | (62,641) | | | (516) | | | (684) | | | (63,841) | |
| Total | $ | 120,682 | | | $ | 522 | | | $ | 10,112 | | | $ | 131,316 | |
During the years ended December 31, 2022 and 2021, we paid $44.3 million and $56.4 million, respectively, attributed to the pricing and reimbursement for the sale of our commercial products in France. | | | | | | | | | | | | | | | | | | | | | | | |
| As of December 31, 2022 |
| (In thousands) | Chargebacks and Rebates | | Trade Discounts and Allowances | | Returns Reserve and Other Incentives | | Total |
| Beginning balance | $ | 120,682 | | | $ | 522 | | | $ | 10,112 | | | $ | 131,316 | |
| Provision related to current period sales | 245,236 | | | 13,085 | | | 15,249 | | | 273,570 | |
| Credit or payments made during the period for current year sales | (108,185) | | | (10,310) | | | (9,850) | | | (128,345) | |
| Credit or payments made during the period for prior period sales | (65,961) | | | (847) | | | (735) | | | (67,543) | |
| Total | $ | 191,772 | | | $ | 2,450 | | | $ | 14,776 | | | $ | 208,998 | |
4. NET REVENUES FROM COLLABORATIONS
Net revenues from collaborations consist of the following:
| | Year Ended December 31, |
| | Year Ended December 31, | | | | Year Ended December 31, |
| (In thousands) | (In thousands) | | 2022 | | 2021 | | 2020 | (In thousands) | | 2023 | | 2022 | | 2021 |
| Roche | |
| Regeneron Pharmaceuticals | Regeneron Pharmaceuticals | | $ | 87,844 | | | $ | 113,226 | | | $ | 74,072 | |
| Novartis AG | Novartis AG | | 43,159 | | | 49,120 | | | 22,208 | |
| Vir Biotechnology | | 1,755 | | | 16,897 | | | 31,396 | |
| | Other | |
| Other | |
| Other | Other | | 2,154 | | | 1,710 | | | 3,657 | |
| Total | Total | | $ | 134,912 | | | $ | 180,953 | | | $ | 131,333 | |
The following table presents the balance of our receivables and contract liabilities related to our collaboration agreements:
| | | | | | | | | | | |
| As of December 31, |
| (In thousands) | 2022 | | 2021 |
| Receivables included in "Accounts receivable, net" | $ | 32,342 | | | $ | 73,266 | |
| Contract liabilities included in "Deferred revenue" | $ | 235,528 | | | $ | 88,627 | |
| | | | | | | | | | | |
| As of December 31, |
| (In thousands) | 2023 | | 2022 |
| Receivables included in “Accounts receivable, net” | $ | 99,576 | | | $ | 32,342 | |
| Contract liabilities included in “Deferred revenue” | $ | 290,763 | | | $ | 235,528 | |
We recognized revenue of $55.5$40.5 million and $62.9$55.5 million in the years ended December 31, 20222023 and 2021,2022, respectively, that was included in the contract liability balance at the beginning of the period.
In order toTo determine revenue recognized in the period from contract liabilities, we first allocate revenue to the individual contract liability balance outstanding at the beginning of the period until the revenue exceeds that balance. If additional consideration is received on those contracts in subsequent periods, we assume all revenue recognized in the reporting period first applies to the beginning contract liability as opposed to a portion applying to the new consideration for the period.
The following table provides research and development expenses incurred by type, for which we recognize net revenue, that are directly attributable to our collaboration agreements, by collaboration partner:collaborator:
| | Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Year Ended December 31, | | | Year Ended December 31, |
| 2023 | | | 2023 | | 2022 | | 2021 |
| (In thousands) | (In thousands) | Clinical Trial and Manufacturing | | External Services | | Other | | Clinical Trial and Manufacturing | | External Services | | Other | | Clinical Trial and Manufacturing | | External Services | | Other | (In thousands) | Clinical Trial and Manufacturing | | External Services | | Other | | Clinical Trial and Manufacturing | | External Services | | Other | | Clinical Trial and Manufacturing | | External Services | | Other |
| Roche | |
| Regeneron | Regeneron | $ | 12,926 | | | $ | 2,141 | | | $ | 27,935 | | | $ | 24,989 | | | $ | 840 | | | $ | 47,582 | | | $ | 13,302 | | | $ | 171 | | | $ | 44,360 | |
| Other | Other | 156 | | | 679 | | | 337 | | | 10,311 | | | 775 | | | 4,489 | | | 20,113 | | | 765 | | | 14,422 | |
| Total | Total | $ | 13,082 | | | $ | 2,820 | | | $ | 28,272 | | | $ | 35,300 | | | $ | 1,615 | | | $ | 52,071 | | | $ | 33,415 | | | $ | 936 | | | $ | 58,782 | |
The research and development expenses incurred for the agreements included in the table above consist of costs incurred for (i) clinical expenses, including manufacturing of clinical product, (ii) external services, including consulting services and lab supplies and services, and (iii) other expenses, including professional services, facilities and overhead allocations, and a reasonable estimate of compensation and related costs as billed to our counterparties, for which we recognize net revenues from
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
collaborations. For the years ended December 31, 2023, 2022 and 2021, we did not incur material selling, general and administrative expenses related to our collaboration agreements.
Product Collaborations
Roche
On July 21, 2023, or the Effective Date, we entered into a Collaboration and License Agreement, or the Roche Agreement, with F. Hoffmann-La Roche Ltd. and Genentech, Inc., or, collectively, Roche, pursuant to which we and Roche established a worldwide, strategic collaboration for the joint development of pharmaceutical products containing zilebesiran. Zilebesiran is our investigational siRNA therapeutic targeting liver-expressed angiotensinogen, which is in Phase 2 clinical trials for the treatment of hypertension.
Under the Roche Agreement, we granted to Roche (i) co-exclusive rights to develop zilebesiran worldwide and commercialize zilebesiran in the U.S., referred to as the Co-Commercialization Territory, (ii) exclusive rights to commercialize zilebesiran outside of the U.S., referred to as the Roche Territory, and (iii) non-exclusive rights to manufacture zilebesiran for the development and commercialization of zilebesiran in the Roche Territory. In connection with the Roche Agreement, Roche made an upfront, non-refundable payment of $310.0 million. In addition, we will be eligible to receive up to $1.24 billion in contingent payments based on the achievement of specified development and regulatory milestones and up to $1.28 billion in sales-based milestones.
We will lead the global clinical development for zilebesiran. We will be responsible for forty percent (40%) and Roche will be responsible for the remaining sixty percent (60%) of development costs incurred in the conduct of development activities that support regulatory approval of zilebesiran globally. We and Roche will share equally (50/50) all costs incurred in connection with development activities that are conducted to support regulatory approval of zilebesiran solely in the Co-Commercialization Territory if incremental development activities are needed. Roche will be solely responsible for all costs incurred in the conduct of development activities primarily to support regulatory approval in the Roche Territory if incremental development activities are needed. Upon regulatory approval Roche has the exclusive right to commercialize zilebesiran in the Roche Territory and will pay us tiered, low double-digit royalties based on net sales of zilebesiran on a country-by-country and product-by-product basis during the applicable royalty term. We and Roche will co-commercialize zilebesiran in the Co-Commercialization Territory and share equally (50/50) profits and losses (including commercialization costs).
Roche has the right to terminate the Roche Agreement for any or no reason at all upon prior written notice, however, if the termination notice occurs after the achievement of the first development milestone and before the achievement of the third development milestone, Roche is required to pay us a termination fee of $50.0 million. In addition, either party may terminate the Roche Agreement for a material breach by, or insolvency of, the other party, subject to a cure period. Unless earlier terminated pursuant to its terms, the Roche Agreement commences on the Effective Date and will remain in effect until expiration on a country-by-country and product-by-product basis (a) in the Roche Territory, upon expiration of the applicable royalty term for such product in the applicable country and (b) in the Co-Commercialization Territory, upon expiration of the term of the co-commercialization efforts for the applicable product.
Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any milestone, royalty or profit share payments from Roche under the Roche Agreement.
We evaluated the Roche Agreement and concluded that the Roche Agreement had elements that were within the scope of ASC 606, Revenue from Contracts with Customers and ASC 808, Collaborative Arrangements.
As of the Effective Date, we identified the following promises in the Roche Agreement that were evaluated under the scope of ASC 606: delivery of (i) a co-exclusive license to develop zilebesiran worldwide and commercialize zilebesiran within the Co-Commercialization Territory, a non-exclusive license to manufacture zilebesiran in the Roche Territory solely for purposes of developing and commercializing zilebesiran in the Roche Territory, and an exclusive license to commercialize zilebesiran in the Roche Territory, collectively referred to as Roche License Obligation, (ii) development services, including the manufacture of clinical supply, that support regulatory approval of zilebesiran, referred to as the Roche Development Services Obligation, and (iii) technology transfer of the existing manufacturing process for zilebesiran, referred to as the Roche Technology Transfer Obligation. The three performance obligations under the Roche Agreement are collectively referred to as the Roche Performance Obligations.
We also evaluated whether certain options outlined within the Roche Agreement represented material rights that would give rise to a performance obligation and concluded that none of the options convey a material right to Roche and therefore are not considered separate performance obligations within the Roche Agreement.
We assessed the above promises and determined that the Roche License Obligation, Roche Development Services Obligation and Roche Technology Transfer Obligation are reflective of a vendor-customer relationship and therefore represent performance obligations within the scope of ASC 606. The Roche License Obligation is considered functional intellectual property and distinct from other promises under the contract as Roche can benefit from the licenses on its own or together with
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
other readily available resources. As the licenses are delivered at the same time, they are considered one performance obligation at contract inception. The Roche Development Services Obligation is considered distinct as Roche can benefit from the development services together with the licenses transferred by us at the inception of the agreement. The development services are not expected to significantly modify or customize the initial intellectual property as zilebesiran is in the second phase of clinical development. The Roche Technology Transfer Obligation is distinct as Roche can benefit from the manufacturing license transferred by us at the inception of the agreement given the advancements of our RNAi platform and our utilization of third-party contract manufacturing organizations to manufacture zilebesiran. Therefore, each represents a separate performance obligation within the contract with a customer under the scope of ASC 606 at contract inception.
We consider the collaborative activities associated with the co-commercialization of zilebesiran in the U.S. to be a separate unit of account within the scope of ASC 808 as we and Roche are both active participants in the commercialization activities and are exposed to significant risks and rewards that are dependent on the commercial success of the activities in the arrangement.
We determined the transaction price under ASC 606 at the inception of the Roche Agreement to be $857.0 million, consisting of the $310.0 million up front payment and $547.0 million additional variable consideration attributed to cost reimbursement from development and manufacturing services and technology transfer related to the Roche Performance Obligations. Since the variable consideration allocated to the Roche Development Services Obligation and the Roche Technology Transfer Obligation would be recognized as revenue only as the costs are incurred, we determined it is not probable that a significant reversal of cumulative revenue would occur. We utilized the expected value method to determine the amount of reimbursement for these activities. We determined that any variable consideration related to development and regulatory milestones is deemed to be fully constrained and therefore excluded from the transaction price due to the high degree of uncertainty and risk associated with these potential payments as we determine that we cannot assert that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur. We also determined that royalties and sales milestones relate solely to the licenses of intellectual property and are therefore excluded from the transaction price under the sales-or-usage based royalty exception of ASC 606.
We developed the estimated standalone selling price for each of the Roche Performance Obligations with the objective of determining the price at which we would sell such an item if it were to be sold regularly on a standalone basis. We developed the estimated standalone selling price for the Roche License Obligation primarily based on the probability-weighted present value of expected future cash flows associated with each underlying license or activity. In developing such estimates, we applied judgment in determining the forecasted revenues, taking into consideration the applicable market conditions and relevant entity-specific factors, the probability of success, the time needed to develop zilebesiran and the discount rate. We developed the estimated standalone selling price for the services and clinical supply included in the Roche Development Services Obligation and the Roche Technology Transfer Obligation primarily based on the level of effort necessary to perform the service and the costs for full-time equivalent employees and expected resources to be committed plus a reasonable estimatemargin.
We allocated the variable consideration related to the estimated reimbursements for the Roche Development Services Obligation and the Roche Technology Transfer Obligation to each performance obligation as the terms of compensation and related costs as billedthe variable payment relate specifically to our counterparties, forefforts to satisfy the performance obligation and allocating the variable amount of consideration entirely to the respective performance obligation is consistent with the allocation objective of ASC 606 when considering all of the performance obligations and payment terms in the contract. We allocated the fixed up-front consideration of $310.0 million entirely to the Roche License Obligation as the value of the fixed consideration together with the expected value of the development and regulatory milestones, sales-based milestones and royalties, all of which we recognize net revenuesare either currently constrained or subject to the sales-and usage-based royalty exception, approximates the standalone selling price of the Roche License Obligation. Therefore, allocating the fixed up-front consideration entirely to the Roche License Obligation is consistent with the allocation objective of ASC 606 when considering all of the performance obligations and payment terms in the contract.
The Roche License Obligation was satisfied at a point in time upon transfer of the license to Roche. Control of the licenses was transferred on the Effective Date and Roche could begin to use and benefit from collaborations.the licenses. For the years endedRoche Development Services Obligation, we measure proportional performance over time using an input method based on cost incurred relative to the total estimated cost of the obligation, on a quarterly basis, by determining the proportion of effort incurred as a percentage of total effort we expect to expend. This ratio is applied to the transaction price allocated to the obligation. Management has applied significant judgment in the process of developing our estimates. We re-evaluate the transaction price as of the end of each reporting period.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The following tables provide a summary of the transaction price allocated to each performance obligation, in addition to revenue activity during the period, in thousands:
| | | | | | | | | | | | | | | | | | | | |
| | Transaction Price Allocated | | | | Revenue Recognized During | | | | |
| Performance Obligations | | As of December 31, 2023 | | | | Year Ended December 31, 2023 | | | | |
| Roche License Obligation | | $ | 310,000 | | | | | $ | 310,000 | | | | | |
| Roche Development Services Obligation | | 545,000 | | | | | 23,974 | | | | | |
| Roche Technology Transfer Obligation | | 2,000 | | | | | — | | | | | |
| | $ | 857,000 | | | | | $ | 333,974 | | | | | |
As of December 31, 2022, 2021 and 2020, we did not incur material selling, general and administrative expenses related2023, the aggregate amount of the transaction price allocated to our collaboration agreements.
In addition, wethe Roche Performance Obligations that was unsatisfied was $523.0 million, which is expected to be recognized a reduction to our research and development expensesthrough the term of $16.9 million, $17.1 million, and $11.1 million for the years ended December 31, 2022, 2021 and 2020, respectively, from cost reimbursement due under certain of our collaboration agreements with Regeneron Pharmaceuticals, Inc., or Regeneron, accounted for under Accounting Standards Codification, or ASC, Topic 808, Collaborative Arrangements, or ASC 808.
Product AlliancesRoche Agreement as the services are performed.
Regeneron Pharmaceuticals, Inc.
In AprilDuring 2019, we entered into a global, strategic collaboration with Regeneron to discover, develop and commercialize RNAi therapeutics for a broad range of diseases by addressing therapeutic targets expressed in the eye and central nervous system, or CNS, in addition to a select number of targets expressed in the liver, which we refer to as the Regeneron Collaboration. The Regeneron Collaboration is governed by a Master Agreement, referred to as the Regeneron Master Agreement, which became effective on May 21, 2019.Agreement. In connection with the Regeneron Master Agreement, we and Regeneron entered into (i) a binding co-co collaboration term sheetagreement covering the continued development of cemdisiran, our C5 small interfering RNA, or siRNA, currently in Phase 2 development for C5 complement-mediated diseases, as a monotherapy and (ii) a binding license term sheetagreement to evaluate anti-C5 antibody-siRNA combinations for C5 complement-mediated diseases including evaluating the combination of Regeneron’s pozelimab (REGN3918), currently in Phase 3 development, and cemdisiran. The C5 co-co collaboration and license agreements were executed in August 2019.
Under the terms of the Regeneron Collaboration, we are working exclusively with Regeneron to discover RNAi therapeutics for eye and CNS diseases for an initial research period of approximately five years, which we refer to as the Initial Research Term. Regeneron has an option to extend the Initial Research Term (referred to as the Research Term Extension Period, and together with the Initial Research Term, the Research Term) for up to an additional five years, for a research term extension fee of up to $400.0$300.0 million. The Regeneron Collaboration also covers a select number of RNAi therapeutic programs designed to target genes expressed in the liver, including our previously announced collaboration with Regeneron to identify RNAi therapeutics for the chronic liver disease nonalcoholic steatohepatitis. We retain broad global rights to all of our other unpartnered liver-directed clinical and pre-clinical pipeline programs. The Regeneron Collaboration is governed by a joint steering committee that is comprised of an equal number of representatives from each party.
Regeneron leads development and commercialization for all programs targeting eye diseases (subject to limited exceptions), entitling us to certain potential milestone and royalty payments pursuant to the terms of a license agreement, the form of which has been agreed upon by the parties. We and Regeneron are alternating leadership on CNS and liver programs covered by the Regeneron Collaboration, with the lead party retaining global development and commercial responsibility. For such CNS and liver programs, both we and Regeneron have the option at lead candidate selection to enter into a co-co collaboration agreement, the form of which has been agreed upon by the parties, whereby both companies will share equally all costs of, and profits from, all development and commercialization activities under the program. If the non-lead party elects to not enter into a co-co collaboration agreement with respect to a given CNS or liver program, we and Regeneron will enter into a license agreement with respect to such program and the lead party will be the “Licensee” for the purposes of the license agreement. If the lead party for a CNS or liver program elects to not enter into the co-co collaboration agreement, then we and Regeneron will enter into a license agreement with respect to such program and leadership of the program will transfer to the other party and the former non-lead party will be the “Licensee” for the purposes of the license agreement.
With respect to the programs directed to C5 complement-mediated diseases, we retain control of cemdisiran monotherapy development, and Regeneron is leading combination product development. Pursuant to the C5 co-co collaboration agreement, Regeneron notified us in November 2022 of its decision to exercise its right to opt-out of the further development and commercialization of cemdisiran monotherapy. As a result, Regeneron no longer shares costs and potential future profits on any monotherapy program with us. We continue to perform our obligations under the agreement, and we are solely responsible for all development and commercialization costs. Regeneron will be eligible to receive tiered double-digit royalties on net sales. Under the C5 license agreement, for cemdisiran to be used as part of a combination product, Regeneron is solely responsible for all development and commercialization costs and we will receive low double-digit royalties and commercial milestones of up to $325.0 million on any potential combination product sales. The C5 co-co collaboration agreement, the C5 license agreement, and the Master Agreement have been combined for accounting purposes and treated as a single agreement.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In connection with the Regeneron Master Agreement, Regeneron made an upfront payment of $400.0 million. In 2023, we received a $100.0 million milestone payment upon satisfying certain criteria during early clinical development for our CNS program, ALN-APP. We are also eligible to receive up to an additional $200.0$100.0 million in milestone payments upon achievement of certain criteria during early clinical development for an eye and CNS programs.program. We and Regeneron plan to advance programs directed to up to 30 targets in the first five years under the Regeneron Collaboration during the Initial Research Term. For each program, Regeneron will provide us with $2.5 million in funding at program initiation and an additional $2.5 million at lead candidate identification, with
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
the potential for approximately $30.0 million in annual discovery funding to us as the Regeneron Collaboration reaches steady state.
Regeneron has the right to terminate the Regeneron Master Agreement for convenience upon ninety days’ notice. The termination of the Regeneron Master Agreement does not affect the term of any license agreement or co-co collaboration agreement then in effect. In addition, either party may terminate the Regeneron Master Agreement for a material breach by, or insolvency of, the other party. Unless earlier terminated pursuant to its terms, the Regeneron Master Agreement will remain in effect with respect to each program until (a) such program becomes a terminated program or (b) the parties enter into a license agreement or co-co collaboration agreement with respect to such program. The Regeneron Master Agreement includes various representations, warranties, covenants, dispute escalation and resolution mechanisms, indemnities and other provisions customary for transactions of this nature.
For any license agreement subsequently entered into, the licensee will generally be responsible for its own costs and expenses incurred in connection with the development and commercialization of the collaboration products. The licensee will pay to the licensor certain development and/or commercialization milestone payments totaling up to $150.0 million for each collaboration product. In addition, following the first commercial sale of the applicable collaboration product under a license agreement, the licensee is required to make certain tiered royalty payments, ranging from low double-digits up to 20%, to the licensor based on the aggregate annual net sales of the collaboration product, subject to customary reductions.
For any co-co collaboration agreement subsequently entered into, we and Regeneron will share equally all costs of, and profits from, development and commercialization activities. Reimbursement of our share of costs will be recognized as a reduction to research and development expense in the consolidated statements of operations and comprehensive loss. In the event that a party exercises its opt-out right, the lead party will be responsible for all costs and expenses incurred in connection with the development and commercialization of the collaboration products under the applicable co-co collaboration agreement, subject to continued sharing of costs through defined points. If a party exercises its opt-out right, following the first commercial sale of the applicable collaboration product under a co-co collaboration agreement, the lead party is required to make certain tiered royalty payments, ranging from low double-digits up to 20%, to the other party based on the aggregate annual net sales of the collaboration product and the timing of the exercise of the opt-out right, subject to customary reductions and a reduction for opt-out transition costs.
Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any additional milestone or royalty payments from Regeneron under the Regeneron Master Agreement, the C5 license agreement, or any future license agreement, or under any co-co collaboration agreement in the event we exercise our opt-out right.
Our obligations under the Regeneron Collaboration include: (i) a research license and research services, collectively referred to as the Research Services Obligation; (ii) a worldwide license to cemdisiran for combination therapies, and manufacturing and supply, and development service obligations, collectively referred to as the C5 License Obligation; and (iii) development, manufacturing and commercialization activities for cemdisiran monotherapies, referred to as the C5 Co-Co Obligation.
The research license is not distinct from the research services primarily as a result of Regeneron being unable to benefit on its own or with other resources reasonably available, as the license is providing access to specialized expertise, particularly as it relates to RNAi technology, that iswhich was not available in the marketplace.marketplace when the Regeneron Collaboration was executed. Similarly, the worldwide license to cemdisiran for combination therapies is not distinct from the manufacturing and supply, and development service obligations, as Regeneron cannot benefit on its own from the value of the license without receipt of supply.
Separately, prior to Regeneron’s decision in November 2022 to exercise its right to opt-out of the further development and commercialization of cemdisiran monotherapy, the cemdisiran monotherapy co-co collaboration agreement was under the scope of ASC 808 as we and Regeneron were both active participants in the development and manufacturing activities and were exposed to significant risks and rewards that were dependent on commercial success of the activities of the arrangement. Regeneron’s decision to exercise its right to opt-out of the arrangement caused a change in the role of Regeneron and its exposure to significant risks and rewards under the arrangement. As a result, we determined that the arrangement no longer represents a collaborative arrangement.
The arrangement now represents a vendor-customer relationship under ASC 606 as we perform our obligation to provide development and manufacturing activities under the arrangement. The transaction price allocated to the C5 Co-Co obligation
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
unit of account will be recognized over time using an input method based on cost incurred relative to the total estimated costs for the identified performance obligation by determining the proportion of effort incurred as a percentage of total effort we expect to expend.
The total transaction price is comprised of the $400.0 million upfront payment and additional variable consideration related to research, development, manufacturing and supply activities related to the Research Services Obligation and the C5 License Obligation. We utilized the expected value method to determine the amount of reimbursement for these activities. We
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
determined that any variable consideration related to sales-based royalties and milestones related to the worldwide license to cemdisiran for combination therapies is deemed to be constrained and therefore has been excluded from the transaction price. In addition, we are eligible to receive a future milestonesmilestone payment upon the achievement of certain criteria during early clinical development for thean eye and CNS programs.program. We are also eligible to receive royalties on future commercial sales for certain eye, CNS or liver targets, if any; however, these amounts are excluded from variable consideration under the Regeneron Collaboration as we are only eligible to receive such amounts if, after a drug candidate is identified, the form of license agreement is subsequently executed resulting in a license that is granted to Regeneron. Any such subsequently granted license would represent a separate transaction under ASC 606.
We allocated the initial transaction price to each unit of account based on the applicable accounting guidance as follows, in thousands:
| | Performance Obligations | Performance Obligations | | Standalone Selling Price | | Transaction Price Allocated | | Accounting Guidance | Performance Obligations | | Standalone Selling Price | | Transaction Price Allocated |
| Research Services Obligation | Research Services Obligation | | $ | 130,700 | | | $ | 183,100 | | | ASC 606 |
| C5 License Obligation | C5 License Obligation | | $ | 97,600 | | | 92,500 | | | ASC 606 |
| C5 Co-Co Obligation | C5 Co-Co Obligation | | $ | 364,600 | | | 246,000 | | | ASC 606 |
| $ | 521,600 | | |
| | | | $ | |
The transaction price was allocated to the obligations based on the relative estimated standalone selling prices of each obligation, over which management has applied significant judgment. We developed the estimated standalone selling price for the licenses included in the Research Services Obligation and the C5 License Obligation primarily based on the probability-weighted present value of expected future cash flows associated with each license related to each specific program. In developing such estimate, we applied judgment in the determination of the forecasted revenues, taking into consideration the applicable market conditions and relevant entity-specific factors, the expected number of targets or indications expected to be pursued under each license, the probability of success, the time needed to develop a product candidate pursuant to the associated license and the discount rate. We developed the estimated standalone selling price for the services and/or manufacturing and supply included in each of the obligations, as applicable, primarily based on the nature of the services to be performed and/or goods to be manufactured and estimates of the associated costs. The estimated standalone selling price of the C5 Co-Co Obligation was developed by estimating the present value of expected future cash flows that Regeneron is entitled to receive. In developing such estimate, we applied judgment in determining the indications that will be pursued, the forecasted revenues for such indications, the probability of success and the discount rate.
For the Research Services Obligation, the C5 License Obligation, and the C5 LicenseCo-Co Obligation accounted for under ASC 606, we measure proportional performance over time using an input method based on cost incurred relative to the total estimated costs for each of the identified obligations, on a quarterly basis, by determining the proportion of effort incurred as a percentage of total effort we expect to expend. This ratio is applied to the transaction price allocated to each obligation. Management has applied significant judgment in the process of developing our estimates. Any changes to these estimates will be recognized in the period in which they change as a cumulative catch up. We re-evaluate the transaction price as of the end of each reporting period and as of December 31, 2022,2023, the total transaction price was determined to be $558.9$675.8 million, an increase of $20.5$116.9 million from December 31, 2021.2022. The increase in the transaction price is primarily due to the $100.0 million milestone we earned in 2023 as we met certain criteria during early clinical development for our CNS program, ALN-APP. As of December 31, 2022,2023, the transaction price is comprised of the upfront payment and variable consideration related to development, manufacture and supply activities. For the C5 Co-Co Obligation accounted for under ASC 808, the transaction price allocated to this obligation is recognized using a proportional performance method. Revenue recognized under this agreement inclusive of the amount allocated to the C5 Co-Co Obligation, is accounted for as collaboration revenue.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The following tables provide a summary of the transaction price allocated to each unit of account based on the applicable accounting guidance, in addition to revenue activity during the period and deferred revenue as of the balance sheet date, in thousands:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Transaction Price Allocated | | Deferred Revenue | | |
| Performance Obligations | | As of December 31, 2022 | | As of December 31, 2022 | | As of December 31, 2021 | | Accounting Guidance |
| Research Services Obligation | | $ | 215,680 | | | $ | 26,200 | | | $ | 42,300 | | | ASC 606 |
| C5 License Obligation | | 97,200 | | | 7,000 | | | 26,900 | | | ASC 606 |
| C5 Co-Co Obligation | | 246,000 | | | 193,600 | | | 212,500 | | | ASC 606 |
| Total | | $ | 558,880 | | | $ | 226,800 | | | $ | 281,700 | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Revenue Recognized During | |
| Performance Obligations | | Year Ended December 31, 2022 | | Year Ended December 31, 2021 | | Year Ended December 31, 2020 | | Accounting Guidance |
| Research Services Obligation | | $ | 28,600 | | | $ | 37,600 | | | $ | 44,800 | | | ASC 606 |
| C5 License Obligation | | 32,500 | | | 44,600 | | | 7,100 | | | ASC 606 |
| C5 Co-Co Obligation | | 20,080 | | | 18,900 | | | 11,700 | | | ASC 606 |
| Total | | $ | 81,180 | | | $ | 101,100 | | | $ | 63,600 | | | |
| | | | | | | | | | | | | | | | | | | | | | |
| | Transaction Price Allocated | | Deferred Revenue | | |
| Performance Obligations | | As of December 31, 2023 | | As of December 31, 2023 | | As of December 31, 2022 | | |
| Research Services Obligation | | $ | 305,700 | | | $ | 63,400 | | | $ | 26,200 | | | |
| C5 License Obligation | | 124,100 | | | 27,500 | | | 7,000 | | | |
| C5 Co-Co Obligation | | 246,000 | | | 186,200 | | | 193,600 | | | |
| Total | | $ | 675,800 | | | $ | 277,100 | | | $ | 226,800 | | | |
| | | | | | | | | | | | | | | | | | | | | | |
| | Revenue Recognized During | |
| Performance Obligations | | Year Ended December 31, 2023 | | Year Ended December 31, 2022 | | Year Ended December 31, 2021 | | |
| Research Services Obligation | | $ | 80,200 | | | $ | 28,600 | | | $ | 37,600 | | | |
| C5 License Obligation | | (15,100) | | | 32,500 | | | 44,600 | | | |
| C5 Co-Co Obligation | | 7,400 | | | 20,080 | | | 18,900 | | | |
| Total | | $ | 72,500 | | | $ | 81,180 | | | $ | 101,100 | | | |
As of December 31, 2022,2023, the aggregate amount of the transaction price allocated to the remaining Research Services Obligation, C5 License Obligation, and C5 Co-Co Obligation that was unsatisfied was $289.1$334.7 million, which is expected to be recognized through the term of the Regeneron Collaboration as the services are performed.performed, but could be recognized earlier after an assessment of strategic alternatives which could change the scope of our obligations. Deferred revenue related to the Regeneron Collaboration is classified as either current or non-current in the consolidated balance sheets based on the period the revenue is expected to be recognized.
Novartis AG
2013 Collaboration with The Medicines Company
In February 2013, we and The Medicines Company, or MDCO, entered into a license and collaboration agreement pursuant to which we granted to MDCO an exclusive, worldwide license to develop, manufacture and commercialize RNAi therapeutics targeting proprotein convertase subtilisin/kexin type 9, or PCSK9, for the treatment of hypercholesterolemia and other human diseases, including inclisiran. We refer to this agreement, as amended through the date hereof, as the MDCO License Agreement. On January 6,In 2020, Novartis AG, or Novartis, completed its acquisition of MDCO and assumed all rights and obligations under the MDCO License Agreement.
As of December 31, 2022,2023, we have earned $80.0$120.0 million of milestones and upon achievement of certain events, we will be entitled to receive an additional milestones, up to an aggregate of $100.0 million, including $90.0$60.0 million in specified commercialization milestones and $10.0 million in other specified regulatory milestones. In addition, we are entitled to royalties ranging from 10% up to 20% based on annual worldwide net sales of licensed products by Novartis, its affiliates and sublicensees, subject to reduction under specified circumstances. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any additional milestone payments under the MDCO License Agreement and future royalty payments may be less than anticipated.
Unless terminated earlier in accordance with the terms of the agreement, the MDCO License Agreement expires on a licensed product-by-licensed product and country-by-country basis upon expiration of the last royalty term for any licensed product in any country, where a royalty term is defined as the latest to occur of (1) the expiration of the last valid claim of patent rights covering a licensed product, (2) the expiration of the Regulatory Exclusivity, as defined in the MDCO License Agreement, and (3) the twelfth anniversary of the first commercial sale of the licensed product in such country. We estimate that our core technology patents covering licensed products under the MDCO License Agreement will expire in most countries by 2029. We also estimate that our Leqvio (inclisiran) product-specific patents covering licensed products under the MDCO License Agreement will expire in the U.S. and Europe between 2027 and 2036, inclusive of any patent term extensions and/or any supplementary protection certificates extending such terms due to regulatory delay in those countries where such extensions are available. In addition, more patent filings relating to the collaboration may be made in the future.
Either party may terminate the MDCO License Agreement in the event the other party fails to cure a material breach or upon patent-related challenges by the other party. In addition, Novartis has the right to terminate the agreement without cause at any time upon four months’ prior written notice.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
During the term of the MDCO License Agreement, neither party will, alone or with an affiliate or third party, research, develop or commercialize, or grant a license to any third party to research, develop or commercialize, in any country, any
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
product (for Alnylam) and any siRNA product (for Novartis) directed to the PCSK9 gene, other than a licensed product, without the prior written agreement of the other party, subject to the terms of the MDCO License Agreement.
We evaluated the MDCO License Agreement and concluded that Novartis meets the definition of a customer and that the MDCO License Agreement is a contract. We determined the transaction price, identified the performance obligations and allocated the transaction price to each performance obligation. We also determined that substantially all of our performance obligations are within the scope of the revenue standard as they relate to the delivery of goods and services to a customer for that customer’s use in monetizing an asset. Specifically, we concluded that Novartis meets the definition of a customer as we are delivering intellectual property and know-how rights as well as research and development activities. In addition, we determined that the MDCO License Agreement met the requirements to be accounted for as a contract, including that it is probable that we will collect the consideration to which we are entitled in exchange for the goods or services that will be delivered to Novartis. We determined that, pursuant to ASC 606, the performance obligations were not separately identifiable and were not distinct (and did not have standalone value) due to the specialized nature of the services to be provided by us and the dependent relationship between the performance obligations. Given this fact pattern, we have concluded the MDCO License Agreement has a single identified or combined performance obligation.
None of the unearned milestones are included in the transaction price, as all unearned milestone amounts are not considered likely of achievement and therefore constrained. We considered several factors, including that achievement of the milestones is outside our control and contingent upon success in regulatory decisions. Any consideration related to sales-based royalties (including milestones) will be recognized when the related sales occur as they were determined to relate predominantly to the license granted to MDCO and as a result have also been excluded from the transaction price. During 2018, we completed the performance obligations previously identified in the MDCO License Agreement, including the supply and technical transfer agreement, however, we continue to receive additional orders for supply of certain material. We consider such orders as promised goods to be distinct from the other performance obligations since Novartis now has the abilityis able to manufacture on its own through its own vendors. Such orders will beare treated as separate agreements and any associated revenue will be recognized upon transfer of control.
Novartis License Agreement
In December 2021, we and Novartis entered into a collaboration and license agreement, or the Novartis License Agreement, pursuant to which we granted to Novartis an exclusive, worldwide license to develop, manufacture and commercialize siRNAs targeting end-stage liver disease, or ESLD, potentially leading to the development of a treatment designed to promote the regrowth of functional liver cells and to provide an alternative to transplantation for patients with liver failure.
Pursuant to the Novartis License Agreement, we received an upfront fee of $12.5 million. We may also receive milestone payments upon the achievement of certain development, regulatory and commercial Unearned future milestones as well as royalties on the net sales of licensed products ranging from high-single-digit to sub-teen double-digit percentages. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we mayare not receive any milestone or royalty payments under the Novartis License Agreement.
Under the Novartis License Agreement, we are developing and testing potential siRNAs using target-specific assays developed by Novartis pursuant to an agreed upon research plan for a specified period referred to as the Collaboration Term. Novartis will reimburse us for the cost of our activities under the research plan, referred to as the DC Workplan, subject to an agreed upon cap. Once a lead candidate is identified, further development and clinical research will be conducted by Novartis. The collaboration is governed by a joint steering committee comprised of an equal number of representatives from each party.
Unless terminated earlier in accordance with the terms of the Novartis License Agreement, the Collaboration Term expires at the earlier of (1) 180 days after completion of the development activities assigned to us as agreed upon between the parties, or (2) December 17, 2024.
Either party may terminate the Novartis License Agreementincluded in the event the other party fails to cure a material breach or upon patent-related challenges by the other party. In addition, Novartis has the right to terminate the agreement without cause at any time upon three months’ prior written notice.
During the term of the Novartis License Agreement, neither party will, alone or with an affiliate or third party, research, develop or commercialize, or grant a license to any third party to research, develop or commercialize, in any country, any siRNA product directed to a liver target identified by Novartis, other than a licensed product, without the prior written agreement of the other party, subject to the terms of the Novartis License Agreement.
We identified one performance obligation under the Novartis License Agreement comprised of: i) the exclusive license to develop, manufacture and commercialize siRNAs targeting ESLD; and ii) the obligation to perform work under the DC Workplan. The license is not distinct from the services, including the obligation to deliver development candidates, as Novartis cannot benefit on its own from the value of the license without receipt of such services. We measure proportional performance over time using an input method based on cost incurred relative to the total estimated costs for the identified performance
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
obligation, on a quarterly basis, by determining the proportion of effort incurred as a percentage of total effort we expect to expend. This ratio is applied to the total transaction price. Management has applied significant judgment in the process of developing our estimates. Any changes to these estimates will be recognized in the period in which they change as a cumulative catch up. As of December 31, 2022, the total transaction price was determined to be approximately $16.0 million, comprisedas they are not considered likely of the $12.5 million upfront paymentachievement and estimated variable consideration attributed to work to be performed under the DC Workplan. We utilized the expected value method to determine the amount of reimbursement for these activities. The total transaction price is allocated entirely to the single performance obligation. We determined any variabletherefore constrained. Any consideration related to sales-based royalties and milestones(including milestones) are recognized when the related sales occur as they are determined to relate predominantly to the exclusive license granted to be constrainedMDCO and thereforeas a result have also been excluded such consideration from the transaction price.
As of December 31, 2022, the aggregate amount of the transaction price allocatedOther
In addition to the performance obligationcollaboration agreements discussed above, we have various other collaboration agreements that was unsatisfied was $11.0 million, which is expectedare not individually significant to be recognized through the term of the DC Workplan as the services are performed.
Vir Biotechnology, Inc.
In October 2017, we and Vir Biotechnology, Inc.,our operating results or Vir, entered into a collaboration and license agreement, or the Vir Agreement, for the development and commercialization of RNAi therapeutics for infectious diseases, including chronic hepatitis B virus, or HBV, infection.
financial condition at this time. Pursuant to the Vir Agreement, we granted to Vir an exclusive license to develop, manufacture and commercialize ALN-HBV02 (VIR-2218), for all uses and purposes other than certain excluded fields, as set forth in the Vir Agreement. In addition, we granted Vir an exclusive option for up to four additional RNAi therapeutic programs for the treatment of infectious diseases. Under the terms of the Vir Agreement, for each product arising from the HBV program, including ALN-HBV02,those agreements, we retained the rightmay be required to opt into a profit-sharing arrangement prior to the start of a Phase 3 clinical trial. In addition,pay, or we have the right on a product-by-product basis with respect to each additional infectious disease program that Vir elects to pursue, to opt into a profit-sharing arrangement for each such product at any time during a specified period prior to the initiation of a Phase 3 clinical trial for each such product.
Under the Vir Agreement, we have earned and received certain upfront and development milestone payments in the form of cash ad Vir common stock, as well as sublicense revenue. We may receive, additional milestone paymentsamounts contingent upon the occurrence of various future events (e.g., upon the achievement of certainvarious development regulatory and commercial milestones, as well as royalties on the net sales of licensed products ranging from high-single-digit to sub-teen double-digit percentages. Due to the uncertainty of pharmaceutical development and the high historical failure rates generally associated with drug development, we may not receive any additional milestone payments or any royalty payments under the Vir Agreement.
Unless terminated earlier in accordance with the terms of the agreement, the Vir Agreement expires on a licensed product-by-product and country-by-country basis upon expiration of all royalty payment obligations under the agreement. If Vir does not exercise its option for an infectious disease program, the Vir Agreement will expire upon the expiration of the applicable option period with respect to such program. However, if we exercise our profit-sharing option for any product, the term of the agreement will continue until the expiration of the profit-sharing arrangement for such product.
Either party may terminate the agreementmilestones) which in the event the other party fails to cure a material breach,aggregate could be significant. We may also incur, or upon patent-related challenges by the other party. In addition, Vir has the right to terminate the agreement on a program-by-program basis or in its entiretybe reimbursed for, any reason on 90 days’ written notice.
We identified one performance obligation under the Vir Agreement, as amended, comprised of: i) the exclusive license to develop, manufacture and commercialize RNAi therapeutics (including ALN-HBV02); and ii) the obligation to deliver four additional development candidates and supply product for each such RNAi therapeutic program. The license is not distinct from the services, including the obligation to deliver development candidates and supply product, as Vir cannot benefit on its own from the value of the license without receipt of such services and supply. We measure proportional performance over time using an input method based on cost incurred relative to the total estimated costs for the identified performance obligation, on a quarterly basis, by determining the proportion of effort incurred as a percentage of total effort we expect to expend. This ratio is applied to the total transaction price. Management has applied significant judgment in the process of developing our estimates. Any changes to these estimates will be recognized in the period in which they change as a cumulative catch up. As of December 31, 2022, the total transaction price was determined to be $111.0 million, comprised of the upfront payment, fair value of non-cash equity consideration at contract inception, milestones achieved, and variable consideration related to development, manufacture and supply activities. We utilized the expected value method to determine the amount of reimbursement for these activities. The total transaction price is allocated entirely to the single performance obligation. We determined any variable consideration related to sales-based royalties and milestones related to the exclusive license to be constrained and therefore excluded such consideration from the transaction price.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
As of December 31, 2022, the aggregate amount of the transaction price allocated to the performance obligation that was unsatisfied was $36.3 million, which is expected to be recognized through the term of the Vir Agreement as the services are performed.
Other Strategic License Agreements
PeptiDream, Inc.
In July 2021, we entered into a license and collaboration agreement with PeptiDream, Inc., or PeptiDream, to discover and develop peptide-siRNA conjugates to create multiple opportunities to deliver RNAi therapeutics to tissues outside the liver. Through this collaboration, the companies will collaborate to select and optimize peptides for targeted delivery of small siRNA molecules to a wide range of cell types and tissues via specific interactions with receptors expressed on the target cells. Under the terms of the agreement, PeptiDream received an upfront payment from us of $10.0 million and we will provide research and development funding over the term of the research collaboration, accordingcosts. In addition, if any products related to the terms of the PeptiDream agreement. Duethese collaborations are approved for sale, we may be required to the early stagepay, or we may receive, royalties on future sales. The payment or receipt of these assets, we recorded research and development expense foramounts, however, is contingent upon the upfront paymentoccurrence of $10.0 million during the third quarter of 2021. PeptiDream may also receive payments based on the achievement of specified development, regulatory, and commercial milestones potentially totaling up to $247.0 million for each product developed by us that utilizes PeptiDream’s technology, as well as low-to-mid single digit royalties on sales, if any, of any such products.various future events.
5. LIABILITY RELATED TO THE SALE OF FUTURE ROYALTIES
In April 2020, we entered into a purchase and sale agreement, or Purchase Agreement, with BX Bodyguard Royalties L.P. (an affiliate of The Blackstone Group Inc.), or Blackstone Royalties, under which Blackstone Royalties acquired 50% of royalties payable, or Royalty Interest, with respect to net sales by MDCO, its affiliates or sublicensees of inclisiran (or the branded drug product, Leqvio) and any other licensed products under the MDCO License Agreement, and 75% of the commercial milestone payments payable under the MDCO License Agreement, together with the Royalty Interest, the Purchased Interest. If Blackstone Royalties does not receive payments in respect of the Royalty Interest by December 31, 2029, equaling at least $1.00 billion, Blackstone Royalties will receive 55% of the Royalty Interest beginning on January 1, 2030. In consideration for the sale of the Purchased Interest, Blackstone Royalties paid us $1.00 billion.
We continue to own or control all inclisiran intellectual property rights and are responsible for certain ongoing manufacturing and supply obligations related to the generation of the Purchased Interest. Due to our continuing involvement, we will continue to account for any royalties and commercial milestones due to us under the MDCO License Agreement as revenue on our consolidated statement of operations and comprehensive loss and record the proceeds from this transaction as a liability, net of closing costs, on our consolidated balance sheet.
In order to determine the amortization of the liability related to the sale of future royalties, we are required to estimate the total amount of future payments to Blackstone Royalties over the life of the Purchase Agreement. The $1.00 billion liability, recorded at execution of the agreement, will be accreted to the total of these royalty and commercial milestone payments as interest expense over the life of the Purchase Agreement. As of December 31, 2022,2023, our estimate of this total interest expense resulted in an effective annual interest rate of 8%. These estimates contain assumptions that impact both the amount recorded at execution and the interest expense that will be recognized in future periods.
As payments are made to Blackstone Royalties, the balance of the liability will be effectively repaid over the life of the Purchase Agreement. The exact timing and amount of repayment is likely to change each reporting period. A significant increase or decrease in Leqvio global net revenue will materially impact the liability related to the sale of future royalties, interest expense and the time period for repayment. We will periodically assess the expected payments to Blackstone Royalties and to the extent the amount or timing of such payments is materially different than our initial estimates, we will prospectively adjust the amortization of the liability related to the sale of future royalties and the related interest expense.
As of December 31, 2022,2023, the carrying value of the liability related to the sale of future royalties was $1.29$1.38 billion, net of closing costs of $10.8$10.0 million. The carrying value of the liability related to the sale of future royalties approximates fair value as of December 31, 20222023 and is based on our current estimates of future royalties and commercial milestones expected to be paid to Blackstone Royalties over the life of the arrangement, which are considered Level 3 inputs.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The following table shows the activity with respect to the liability related to the sale of future royalties, in thousands:
| | | | | | | | |
Carrying value as of December 31, 2020 | | $ | 1,071,541 | |
Interest expense recognized | | 116,940 | |
Payments | | (378) | |
| Carrying value as of December 31, 2021 | | $ | 1,188,103 | |
| Interest expense recognized | | 107,601 | |
| Payments | | (3,400) | |
| Carrying value as of December 31, 2022 | | 1,292,304 | |
| Interest expense recognized | | 106,554 | |
| Payments | | (21,619) | |
| Carrying value as of December 31, 2023 | | $ | 1,292,3041,377,239 | |
6. FAIR VALUE MEASUREMENTS
The following tables present information about our financial assets and liabilities that are measured at fair value on a recurring basis and indicate the fair value hierarchy of the valuation techniques we utilized to determine such fair value:
| | (In thousands) | (In thousands) | | As of December 31,
2022 | | Quoted Prices in Active Markets
(Level 1) | | Significant Observable Inputs
(Level 2) | | Significant Unobservable Inputs
(Level 3) | (In thousands) | | As of December 31,
2023 | | Quoted Prices in Active Markets
(Level 1) | | Significant Observable Inputs
(Level 2) | | Significant Unobservable Inputs
(Level 3) |
| Financial assets | Financial assets | | | | | | | | |
| Cash equivalents: | Cash equivalents: | |
| Cash equivalents: | |
| Cash equivalents: | |
| Money market funds | |
| Money market funds | |
| Money market funds | Money market funds | | $ | 270,394 | | | $ | 270,394 | | | $ | — | | | $ | — | |
| U.S. treasury securities | U.S. treasury securities | | 44,817 | | | — | | | 44,817 | | | — | |
| U.S. government-sponsored enterprise securities | | 41,763 | | | — | | | 41,763 | | | — | |
| | Commercial paper | Commercial paper | | 22,350 | | | — | | | 22,350 | | | — | |
| Certificates of deposit | | 3,289 | | | — | | | 3,289 | | | — | |
| Commercial paper | |
| Commercial paper | |
| | Corporate notes | |
| Corporate notes | |
| Corporate notes | Corporate notes | | 1,024 | | | — | | | 1,024 | | | — | |
| Marketable debt securities: | Marketable debt securities: | |
| U.S. treasury securities | |
| U.S. treasury securities | |
| U.S. treasury securities | U.S. treasury securities | | 820,913 | | | — | | | 820,913 | | | — | |
| U.S. government-sponsored enterprise securities | U.S. government-sponsored enterprise securities | | 230,770 | | | — | | | 230,770 | | | — | |
| Corporate notes | Corporate notes | | 208,284 | | | — | | | 208,284 | | | — | |
| Commercial paper | Commercial paper | | 36,793 | | | — | | | 36,793 | | | — | |
| Certificates of deposit | Certificates of deposit | | 1,130 | | | — | | | 1,130 | | | — | |
| | Marketable equity securities | Marketable equity securities | | 28,122 | | | 28,122 | | | — | | | — | |
| Marketable equity securities | |
| Marketable equity securities | |
| Restricted cash (money market funds) | Restricted cash (money market funds) | | 1,197 | | | 1,197 | | | — | | | — | |
| Total financial assets | Total financial assets | | $ | 1,710,846 | | | $ | 299,713 | | | $ | 1,411,133 | | | $ | — | |
| Financial liabilities | Financial liabilities | | | | | | | | |
| Development derivative liability | Development derivative liability | | $ | 209,277 | | | $ | — | | | $ | — | | | $ | 209,277 | |
| Development derivative liability | |
| Development derivative liability | |
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| | (In thousands) | (In thousands) | | As of December 31,
2021 | | Quoted Prices in Active Markets
(Level 1) | | Significant Observable Inputs
(Level 2) | | Significant Unobservable Inputs
(Level 3) | (In thousands) | | As of December 31,
2022 | | Quoted Prices in Active Markets
(Level 1) | | Significant Observable Inputs
(Level 2) | | Significant Unobservable Inputs
(Level 3) |
| Financial assets | Financial assets | | | | | | | | |
| Cash equivalents: | Cash equivalents: | |
| Cash equivalents: | |
| Cash equivalents: | |
| Money market funds | |
| Money market funds | |
| Money market funds | Money market funds | | $ | 255,869 | | | $ | 255,869 | | | $ | — | | | $ | — | |
| U.S. treasury securities | U.S. treasury securities | | 54,998 | | | — | | | 54,998 | | | — | |
| Marketable debt securities: | |
| U.S. treasury securities | | 1,030,578 | | | — | | | 1,030,578 | | | — | |
| Corporate notes | | 253,239 | | | — | | | 253,239 | | | — | |
| U.S. government-sponsored enterprise securities | U.S. government-sponsored enterprise securities | | 177,741 | | | — | | | 177,741 | | | — | |
| Commercial paper | Commercial paper | | 78,543 | | | — | | | 78,543 | | | — | |
| Certificates of deposit | Certificates of deposit | | 7,501 | | | — | | | 7,501 | | | — | |
| Municipal securities | | 1,015 | | | — | | | 1,015 | | | — | |
| Corporate notes | |
| Marketable debt securities: | |
| U.S. treasury securities | |
| U.S. treasury securities | |
| U.S. treasury securities | |
| U.S. government-sponsored enterprise securities | |
| Corporate notes | |
| Commercial paper | |
| Certificates of deposit | |
| Marketable equity securities | Marketable equity securities | | 66,972 | | | 66,972 | | | — | | | — | |
| Restricted cash (money market funds) | Restricted cash (money market funds) | | 1,195 | | | 1,195 | | | — | | | — | |
| Total financial assets | Total financial assets | | $ | 1,927,651 | | | $ | 324,036 | | | $ | 1,603,615 | | | $ | — | |
| Financial liabilities | Financial liabilities | | | | | | | | |
| Development derivative liability | Development derivative liability | | $ | 83,618 | | | $ | — | | | $ | — | | | $ | 83,618 | |
| Development derivative liability | |
| Development derivative liability | |
For the years ended December 31, 20222023 and 2021,2022, there were no transfers between Level 1 and Level 2 financial assets. The carrying amounts reflected in our consolidated balance sheets for cash, accounts receivable, net, other current assets, accounts payable and accrued expenses approximate fair value due to their short-term maturities.
7. MARKETABLE DEBT SECURITIES
The following tables summarize our marketable debt securities:
| | As of December 31, 2022 |
| As of December 31, 2023 | | | As of December 31, 2023 |
| (In thousands) | (In thousands) | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value | (In thousands) | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value |
| U.S. treasury securities | U.S. treasury securities | $ | 870,033 | | | $ | 79 | | | $ | (4,382) | | | $ | 865,730 | |
| U.S. government-sponsored enterprise securities | U.S. government-sponsored enterprise securities | 275,610 | | | 24 | | | (3,101) | | | 272,533 | |
| Corporate notes | Corporate notes | 211,398 | | | 16 | | | (2,106) | | | 209,308 | |
| Commercial paper | Commercial paper | 59,143 | | | — | | | — | | | 59,143 | |
| Certificates of deposit | Certificates of deposit | 4,419 | | | — | | | — | | | 4,419 | |
| | Total | Total | $ | 1,420,603 | | | $ | 119 | | | $ | (9,589) | | | $ | 1,411,133 | |
| Total | |
| Total | |
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| | As of December 31, 2021 |
| As of December 31, 2022 | | | As of December 31, 2022 |
| (In thousands) | (In thousands) | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value | (In thousands) | Amortized
Cost | | Gross
Unrealized
Gains | | Gross
Unrealized
Losses | | Fair Value |
| U.S. treasury securities | U.S. treasury securities | $ | 1,086,232 | | | $ | 6 | | | $ | (662) | | | $ | 1,085,576 | |
| U.S. government-sponsored enterprise securities | |
| Corporate notes | Corporate notes | 253,926 | | | 1 | | | (688) | | | 253,239 | |
| U.S. government-sponsored enterprise securities | 178,027 | | | 2 | | | (288) | | | 177,741 | |
| Commercial paper | Commercial paper | 78,543 | | | — | | | — | | | 78,543 | |
| Certificates of deposit | Certificates of deposit | 7,501 | | | — | | | — | | | 7,501 | |
| Municipal securities | 1,016 | | | — | | | (1) | | | 1,015 | |
| Total | Total | $ | 1,605,245 | | | $ | 9 | | | $ | (1,639) | | | $ | 1,603,615 | |
The fair values of our marketable debt securities by classification in the consolidated balance sheets were as follows:
| | As of December 31, | | | As of December 31, |
| (In thousands) | (In thousands) | December 31, 2022 | | December 31, 2021 | (In thousands) | 2023 | | 2022 |
| Cash and cash equivalents | Cash and cash equivalents | $ | 113,243 | | | $ | 54,998 | |
| Marketable debt securities | Marketable debt securities | 1,297,890 | | | 1,548,617 | |
| Total | Total | $ | 1,411,133 | | | $ | 1,603,615 | |
8. OTHER BALANCE SHEET DETAILS
Inventory
The components of inventory are summarized as follows:
| | As of December 31, |
| As of December 31, | | | As of December 31, |
| (In thousands) | (In thousands) | 2022 | | 2021 | (In thousands) | 2023 | | 2022 |
| Raw materials | Raw materials | $ | 22,315 | | | $ | 14,754 | |
| Work in process | Work in process | 113,783 | | | 100,942 | |
| Finished goods | Finished goods | 25,606 | | | 7,005 | |
| Total inventory | Total inventory | $ | 161,704 | | | $ | 122,701 | |
As of December 31, 20222023 and 2021,2022, we had $32.7$36.3 million and $36.3$32.7 million of long-term inventory, respectively, included within other assets in our consolidated balance sheet as we anticipate it being consumed beyond our normal operating cycle. As
Property, Plant and Equipment, Net
Property, plant and equipment, net consist of December 31, 2022 and 2021, we had $0.0 million and $7.1 million, respectively, of capitalized inventory produced for commercial sale for products awaiting regulatory approval.the following:
| | | | | | | | | | | |
| As of December 31, |
| (In thousands) | 2023 | | 2022 |
| Buildings | $ | 271,651 | | | $ | 269,322 | |
| Leasehold improvements | 235,411 | | | 230,848 | |
| Laboratory equipment | 107,147 | | | 82,586 | |
| Manufacturing equipment | 47,976 | | | 45,311 | |
| Computer equipment and software | 35,616 | | | 33,370 | |
| Construction in progress | 30,099 | | | 14,595 | |
| Furniture and fixtures | 12,153 | | | 11,832 | |
| Land | 9,080 | | | 9,080 | |
| 749,133 | | | 696,944 | |
| Less: accumulated depreciation | (223,076) | | | (173,450) | |
| Total | $ | 526,057 | | | $ | 523,494 | |
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Property, Plant and Equipment, Net
Property, plant and equipment, net consist of the following:
| | | | | | | | | | | |
| As of December 31, |
| (In thousands) | 2022 | | 2021 |
| Buildings | $ | 269,322 | | | $ | 262,637 | |
| Leasehold improvements | 230,848 | | | 152,045 | |
| Construction in progress | 14,595 | | | 80,753 | |
| Laboratory equipment | 82,586 | | | 61,351 | |
| Manufacturing equipment | 45,311 | | | 43,739 | |
| Computer equipment and software | 33,370 | | | 21,885 | |
| Furniture and fixtures | 11,832 | | | 10,883 | |
| Land | 9,080 | | | 9,080 | |
| 696,944 | | | 642,373 | |
| Less: accumulated depreciation | (173,450) | | | (140,415) | |
| Total | $ | 523,494 | | | $ | 501,958 | |
Accrued Expenses
Accrued expenses consist of the following:
| | As of December 31, |
| As of December 31, | | | As of December 31, |
| (In thousands) | (In thousands) | 2022 | | 2021 | (In thousands) | 2023 | | 2022 |
| Product rebates and discounts | Product rebates and discounts | $ | 208,998 | | | $ | 131,279 | |
| Compensation and related | Compensation and related | 130,690 | | | 93,583 | |
| Pre-clinical, clinical trial and manufacturing | Pre-clinical, clinical trial and manufacturing | 94,702 | | | 83,534 | |
| Licensing and collaboration agreements | Licensing and collaboration agreements | 31,680 | | | 22,843 | |
| Consulting and professional services | Consulting and professional services | 19,848 | | | 17,784 | |
| Other | Other | 59,542 | | | 46,151 | |
| Total | Total | $ | 545,460 | | | $ | 395,174 | |
Cash, Cash Equivalents and Restricted Cash
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within our consolidated balance sheets that sum to the total of these amounts shown in the consolidated statements of cash flows:
| | | | | | | | | | | | | | | | | |
| As of December 31, |
| (In thousands) | 2022 | | 2021 | | 2020 |
| Cash and cash equivalents | $ | 866,394 | | | $ | 819,975 | | | $ | 496,580 | |
| Total restricted cash included in other assets | 2,162 | | | 2,178 | | | 2,466 | |
| Total cash, cash equivalents, and restricted cash shown in the consolidated statements of cash flows | $ | 868,556 | | | $ | 822,153 | | | $ | 499,046 | |
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| | | | | | | | | | | | | | | | | |
| As of December 31, |
| (In thousands) | 2023 | | 2022 | | 2021 |
| Cash and cash equivalents | $ | 812,688 | | | $ | 866,394 | | | $ | 819,975 | |
| Total restricted cash included in other assets | 2,196 | | | 2,162 | | | 2,178 | |
| Total cash, cash equivalents, and restricted cash shown in the consolidated statements of cash flows | $ | 814,884 | | | $ | 868,556 | | | $ | 822,153 | |
Accumulated Other Comprehensive (Loss) Income
The following table summarizes the changes in accumulated other comprehensive (loss) income, by component:
| | (In thousands) | (In thousands) | Loss on Investment in Joint Venture | | Defined Benefit Pension
Plans, Net of Tax | | Unrealized Gains (Losses) from Debt Securities | | Foreign Currency Translation
Adjustment | | Total Accumulated Other Comprehensive (Loss) Income | (In thousands) | Loss on Investment in Joint Venture | | Defined Benefit Pension
Plans, Net of Tax | | Unrealized Gains (Losses) from Debt Securities | | Foreign Currency Translation
Adjustment | | Total Accumulated Other Comprehensive (Loss) Income |
| Balance as of December 31, 2020 | $ | (32,792) | | | $ | (3,754) | | | $ | 348 | | | $ | (7,424) | | | $ | (43,622) | |
| Balance as of December 31, 2021 | |
| Other comprehensive loss before reclassifications | Other comprehensive loss before reclassifications | — | | | 899 | | | — | | | 11,398 | | | 12,297 | |
| Amounts reclassified from other comprehensive income | — | | | 44 | | | (1,978) | | | — | | | (1,934) | |
| Net other comprehensive income (loss) | — | | | 943 | | | (1,978) | | | 11,398 | | | 10,363 | |
| Balance as of December 31, 2021 | (32,792) | | | (2,811) | | | (1,630) | | | 3,974 | | | (33,259) | |
| Amounts reclassified from other comprehensive loss | |
| Net other comprehensive loss | |
| Balance as of December 31, 2022 | |
| Other comprehensive income before reclassifications | Other comprehensive income before reclassifications | — | | | — | | | 11 | | | (5,274) | | | (5,263) | |
| Amounts reclassified from other comprehensive income | Amounts reclassified from other comprehensive income | — | | | 1,719 | | | (7,851) | | | — | | | (6,132) | |
| Net other comprehensive income (loss) | — | | | 1,719 | | | (7,840) | | | (5,274) | | | (11,395) | |
| Balance as of December 31, 2022 | $ | (32,792) | | | $ | (1,092) | | | $ | (9,470) | | | $ | (1,300) | | | $ | (44,654) | |
| Net other comprehensive income | |
| Balance as of December 31, 2023 | |
9. CREDIT AGREEMENT
In April 2020, we entered into a credit agreement, or Credit Agreement, among us, certain of our subsidiaries (such subsidiaries, together with us, the Loan Parties), funds or accounts managed or advised by GSO Capital Partners LP (now Blackstone Alternative Credit Advisors LP) and certain other affiliates of The Blackstone Group Inc., and the other lenders from time to time parties thereto, collectively, the Lenders, and Wilmington Trust, National Association, as the administrative agent for the Lenders. The Credit Agreement provided for a senior secured delayed draw term loan facility, referred to as the Term Loans, which consisted of three tranches providing funding of $700.0 million. In September 2022, we extinguished the Term Loans and paid all outstanding balances, including principal of $700.0 million and prepayment premiums of $62.1 million and terminated the Credit Agreement. Upon termination, we recorded a $76.6 million loss on the extinguishment of the debt.
The Term Loans were set to mature in December 2027. During the period the Term Loans were outstanding, we had elected a LIBOR Rate plus 7%, and paid $17.5 million in total funding fees in connection with such Term Loans. Our interest rate was 9% and 8% as of June 30, 2022 and December 31, 2021, respectively.
10. CONVERTIBLE DEBT
Convertible Senior Notes Due 2027
On September 12, 2022, we commenced a private offering of $900.0 million in aggregate principal amount of 1% Convertible Senior Notes due 2027, or the Initial Notes. On September 13, 2022, the initial purchasers in such offering exercised their option to purchase an additional $135.0 million in aggregate principal amount of our 1% Convertible Senior Notes due 2027, or the Additional Notes, and together with the Initial Notes collectively referred to as the Notes, bringing the
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
total aggregate principal amount of the Notes to $1.04 billion. The Notes were issued pursuant to an indenture, dated September 15, 2022, or the Indenture. The Indenture includes customary covenants and sets forth certain events of default after which the Notes may be declared immediately due and payable and sets forth certain types of bankruptcy or insolvency events of default involving the Company after which the Notes become automatically due and payable.
The Notes will mature on September 15, 2027, unless earlier converted, redeemed or repurchased. The Notes will bear interest from September 15, 2022 at a rate of 1% per year payable semiannually in arrears on March 15 and September 15 of each year, beginning on March 15, 2023. The Notes are convertible at the option of the noteholder on or after June 15, 2027. Prior to June 15, 2027, the Notes are convertible only under the following circumstances: (1) During any calendar quarter commencing after the calendar quarter ending on December 31, 2022 (and only during such calendar quarter), if the last reported sale price of our common stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding calendar quarter is greater than or equal to 130% of the conversion price on each applicable trading day; (2) During the five business day period after any ten consecutive trading day period in which the trading price per $1,000 principal amount of the Notes for each trading day of that ten consecutive trading day period was less than 98% of the product of the last reported sale price of our common stock and
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
the conversion rate of the Notes on such trading day; (3) If we call any or all of the Notes for redemption; or (4) Upon the occurrence of specific corporate events as set forth in the Indenture governing the Notes. We will settle any conversions of Notes by paying or delivering, as applicable, cash, shares of our common stock, or a combination of cash and shares of common stock, at our election.
The conversion rate for the Notes will initially be 3.4941 shares of common stock per $1,000 principal amount of Notes, which is equivalent to an initial conversion price of approximately $286.20 per share of common stock. The initial conversion price of the Notes represents a premium of approximately 35% over the $212.00 per share last reported sale price of common stock on September 12, 2022. The conversion rate is subject to adjustment under certain circumstances in accordance with the terms of the Indenture.
We may not redeem the Notes prior to September 20, 2025. We may redeem for cash equal to 100% of the principal amount of the Notes being redeemed plus accrued and unpaid interest of all or any portion of the Notes, at our option, on or after September 20, 2025, if the last reported sales price of our common stock has been at least 130% of the conversion price then in effect for at least 20 trading days (whether or not consecutive) during any 30 consecutive trading day period. No sinking fund is provided for the Notes and therefore we are not required to redeem or retire the Notes periodically.
If we undergo a fundamental change, as defined in the indenture agreement, then subject to certain conditions, holders may require us to repurchase for cash all or any portion of their Notes at a fundamental change repurchase price equal to 100% of the principal amount of the Notes to be repurchased plus accrued and unpaid interest. In addition, if specific corporate events occur prior to the maturity date or if we issue a notice of redemption, we will increase the conversion rate by pre-defined amounts for holders who elect to convert their notes in connection with such a corporate event. The conditions allowing holders of the Notes to convert were not met this quarter.
As of December 31, 2022,2023, the Notes are classified as a long-term liability, net of issuance costs of $19.2$14.2 million, on the consolidated balance sheets. As of December 31, 2022,2023, the estimated fair value of the Notes was approximately $1.12$1.02 billion. The fair value was determined based on the last actively traded price per $100 of the Notes for the period ended December 31, 20222023 (Level 2). Interest expense recognized related to the Notes for the twelve months ended December 31, 2022 was $3.0 million. The Notes were issued at par and costs associated with the issuance of the Notes are amortized to interest expense over the contractual term of the Notes. As of December 31, 2022,2023, the effective interest rate of the Notes is 1%.
Capped Call Transactions
In September 2022, in connection with the pricing of the Initial Notes and the initial purchasers’ exercise of their option to purchase the Additional Notes, we entered into privately negotiated capped call transactions, or Capped Call Transactions. The Capped Call Transactions initially cover, subject to customary anti-dilution adjustments, the number of shares of common stock that underlie the Notes. The cap price of the Capped Call Transactions is initially $424.00 per share, which represents a premium of 100% over the last reported sale price of common stock of $212.00 per share on September 12, 2022, and is subject to certain adjustments under the terms of the capped call transactions.Capped Call Transactions. We used approximately $118.6 million of the proceeds from the offering of Notes to pay the cost of the Capped Call Transactions.
We evaluated the Capped Call Transactions and determined that they should be accounted for separately from the Notes. The cost of $118.6 million to purchase the Capped Call Transactions was recorded as a reduction to additional paid-in capital in the consolidated balance sheet as of December 31, 20222023 as the Capped Call Transactions are indexed to our own stock and met the criteria to be classified in stockholders' equity.deficit.
11.10. DEVELOPMENT DERIVATIVE LIABILITY
In August 2020, we entered into a co-development agreement, referred to as the Funding Agreement, with BXLS V Bodyguard – PCP L.P. and BXLS Family Investment Partnership V – ESC L.P., collectively referred to as Blackstone Life
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Sciences, pursuant to which Blackstone Life Sciences will provide up to $150.0 million in funding for the clinical development of vutrisiran and zilebesiran, two of our cardiometabolic programs. With respect to vutrisiran, Blackstone Life Sciences has committed to provide up to $70.0 million to fund development costs related to the HELIOS-B Phase 3 clinical trial. In November 2021, Blackstone Life Sciences opted in to Phase 2 clinical trial funding of zilebesiran, committing to fund, upon meeting certain patient enrollment thresholds, up to $26.0 million. Furthermore, Blackstone Life Sciences has the right, but is not obligated, to fund up to $54.0 million for development costs related to a Phase 3 clinical trial of zilebesiran. The amount of funding ultimately provided by Blackstone Life Sciences is dependent on us achieving specified development milestones with respect to each clinical trial. WeAs between Blackstone and the Company, we retain sole responsibility for the development and commercialization of both vutrisiran and zilebesiran.
As consideration for Blackstone Life Sciences’ funding for vutrisiran clinical development costs, we have agreed to pay Blackstone Life Sciences a 1% royalty on net sales of AMVUTTRA (vutrisiran) for a 10-year term beginning upon the first commercial sale following regulatory approval of vutrisiran for ATTR-cardiomyopathy, as well as fixed payments of up to 2.5 times their investment over a two-year period upon regulatory approval of vutrisiran for ATTR-cardiomyopathy in specified
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
countries, unless it is later withdrawn from the market following a mandatory recall. As consideration for Blackstone Life Sciences’ funding for Phase 2 clinical development costs of zilebesiran, we have agreed to pay Blackstone Life Sciences fixed payments of up to 3.25 times their Phase 2 investment over a four-year period upon the successful completion of the zilebesiran Phase 2 clinical trial, unless certain regulatory events affecting the continued development of zilebesiran occur. In September 2023, we announced positive topline results from the KARDIA-1 Phase 2 study of zilebesiran, triggering the achievement of a development milestone of $84.5 million payable to Blackstone in 16 equal, quarterly payments over four years. As consideration for Blackstone Life Sciences’ funding for Phase 3 clinical development costs of zilebesiran, we have agreed to pay Blackstone Life Sciences fixed payments of up to 4.5 times their Phase 3 investment over a four-year period upon regulatory approval of zilebesiran in specified countries, unless it is later withdrawn from the market following a mandatory recall.
Our payment obligations under the Funding Agreement will be secured, subject to certain exceptions, by security interests in intellectual property owned by us relating to vutrisiran and zilebesiran, as well as in our bank account in which the funding deposits will be made.
We and Blackstone Life Sciences each have the right to terminate the Funding Agreement in its entirety in the event of the other party’s bankruptcy or similar proceedings. We and Blackstone Life Sciences may each terminate the Funding Agreement in its entirety or with respect to either product in the event of an uncured material breach by the other party, or with respect to a product for certain patient health and safety reasons, or if regulatory approval in specified major market countries is not obtained for the product following the completion of clinical trials for the product. In addition, Blackstone Life Sciences has the right to terminate the Funding Agreement in its entirety upon the occurrence of certain events affecting our ability to make payments under the agreement or to develop or commercialize the products, or upon a change of control of us. Blackstone Life Sciences may also terminate the Funding Agreement with respect to a product if the joint steering committee elects to terminate the development program for that product in its entirety, if certain clinical endpoints are not achieved for that product or, with respect to vutrisiran only, if our right to develop or commercialize vutrisiran is enjoined in a specified major market as a result of an alleged patent infringement. In certain termination circumstances, we will be obligated to pay Blackstone Life Sciences an amount that is equal to, or a multiplier of, the development funding received from Blackstone Life Sciences, and we may remain obligated under certain circumstances to make the payments to Blackstone Life Sciences described above, or the royalty described above in the case of AMVUTTRA, should we obtain regulatory approval for zilebesiran or for vutrisiran for ATTR-cardiomyopathy following termination.
We account for the Funding Agreement under ASC Topic 815, Derivatives and Hedging, as a derivative liability, measured at fair value, recorded within accrued expenses or other liabilities on our consolidated balance sheets.sheets, depending on timing of our payment to Blackstone Life Sciences. The change in fair value due to the remeasurement of the development derivative liability is recorded as other expense on our consolidated statements of operations and comprehensive loss.
As of December 31, 20222023 and 2021,2022, the derivative liability is classified as a Level 3 financial liability in the fair value hierarchy. The valuation method incorporates certain unobservable Level 3 key inputs including (i) the probability and timing of achieving stated development milestones to receive payments from Blackstone Life Sciences, (ii) the probability and timing of achieving regulatory approval and payments to Blackstone Life Sciences, (iii) an estimate of the amount and timing of the
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
royalty payable on net sales of AMVUTTRA, assuming regulatory approval for ATTR-cardiomyopathy, (iv) our cost of borrowing (12%(11%), and (v) Blackstone Life Sciences'Sciences’ cost of borrowing (4%(6%).
The following table presents the activity with respect to the development derivative liability, in thousands:
| | | | | | | | |
Carrying value as of December 31, 20202021 | | $ | 25,58583,618 | |
| Amount received under the Funding Agreement | | 19,60031,000 | |
Loss recorded from remeasurementchange in fair value | | 94,659 | | 38,433 | |
Carrying value as of December 31, 20212022 | | 83,618209,277 | |
| Amount received under the Funding Agreement | | 31,00024,667 | |
Loss recorded from remeasurementchange in fair value | | 90,997 | | 94,659 | |
Carrying value as of December 31, 20222023 | | $ | 209,277324,941 | |
12. STOCK-BASED COMPENSATION11. STOCKHOLDERS’ DEFICIT
Stock-Based Compensation
The following table summarizes stock-based compensation expenses included in operating costs and expenses:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (In thousands) | 2023 | | 2022 | | 2021 |
| Research and development | $ | 97,273 | | | $ | 92,161 | | | $ | 68,415 | |
| Selling, general and administrative | 124,407 | | | 138,488 | | | 97,302 | |
| Total | $ | 221,680 | | | $ | 230,649 | | | $ | 165,717 | |
The following table summarizes stock-based compensation expense by type of award:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (In thousands) | 2023 | | 2022 | | 2021 |
| | | | | |
| Time-based restricted stock units | $ | 55,169 | | | $ | 12,791 | | | $ | 4,231 | |
| Performance-based restricted stock units | 63,879 | | | 102,925 | | | 39,943 | |
| Time-based stock options | 99,165 | | | 114,901 | | | 118,635 | |
| Other equity programs | 7,652 | | | 4,057 | | | 6,235 | |
| Less: Stock-based compensation expense capitalized to inventory | (4,185) | | | (4,025) | | | (3,327) | |
| Total | $ | 221,680 | | | $ | 230,649 | | | $ | 165,717 | |
The following table summarizes our unrecognized stock-based compensation expense, net of estimated forfeitures, by type of awards, and the weighted-average period over which that expense is expected to be recognized:
| | | | | | | | | | | |
| As of December 31, 2023 |
| Unrecognized Expense, Net of Estimated Forfeitures (in thousands) | | Weighted-average Recognition Period (in years) |
| Type of award: | | | |
| Time-based restricted stock units | $ | 125,118 | | | 2.14 |
| Performance-based restricted stock units * | $ | 1,792 | | | 0.13 |
| Time-based stock options | $ | 124,825 | | | 2.04 |
| Other equity programs | $ | 3,306 | | | 0.55 |
*Excludes performance-based restricted stock units for which the associated vesting events are not yet determined to be probable.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Time-Based Restricted Stock Units and Awards
The following table summarizes the activity of our time-based restricted stock units and awards, excluding performance-based restricted stock units:
| | | | | | | | | | | |
| Number of Units (in thousands) | | Weighted-average Grant Date Fair Value (per share) |
| Outstanding as of December 31, 2022 | 292 | | | $ | 165.27 | |
| Awarded | 922 | | | $ | 188.78 | |
| Released | (126) | | | $ | 164.47 | |
| Cancelled | (63) | | | $ | 186.25 | |
| Outstanding as of December 31, 2023 | 1,025 | | | $ | 185.24 | |
Performance-Based Restricted Stock Units
The following table summarizes the activity of our performance-based restricted stock units:
| | | | | | | | | | | |
| Number of Units (in thousands) | | Weighted-average Grant Date Fair Value (per share) |
| Outstanding as of December 31, 2022 | 1,195 | | | $ | 144.77 | |
| Awarded | 439 | | | $ | 190.01 | |
| Released | (472) | | | $ | 139.38 | |
| Cancelled | (129) | | | $ | 150.02 | |
| Outstanding as of December 31, 2023 | 1,033 | | | $ | 165.82 | |
The performance-based restricted stock units granted in 2023 and 2022 will vest upon the later of the one-year anniversary of the date of grant and the achievement of specific clinical development, regulatory, commercial and/or financial performance events, as approved by our people, culture and compensation committee.
Time-Based Stock Options
The following table summarizes the activity of our time-based stock options, excluding performance-based stock options:
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of Options (in thousands) | | Weighted-average Exercise Price (per share) | | Weighted-average Remaining Contractual Term (in years) | | Aggregate Intrinsic Value (in thousands) |
| Outstanding as of December 31, 2022 | 7,868 | | | $ | 120.59 | | | | | |
| Granted | 412 | | | $ | 192.68 | | | | | |
| Exercised | (1,068) | | | $ | 99.43 | | | | | |
| Cancelled | (252) | | | $ | 148.27 | | | | | |
| Outstanding as of December 31, 2023 | 6,960 | | | $ | 127.11 | | | 5.96 | | $ | 451,402 | |
| Exercisable as of December 31, 2023 | 5,042 | | | $ | 114.14 | | | 5.21 | | $ | 390,672 | |
| Vested or expected to vest as of December 31, 2023 | 6,803 | | | $ | 126.23 | | | 5.90 | | $ | 447,000 | |
The weighted-average fair value of stock options granted was $96.53, $80.65 and $82.59 per share for the years ended December 31, 2023, 2022 and 2021, respectively. The intrinsic value of stock options exercised was $107.0 million, $289.3 million and $247.8 million for the years ended December 31, 2023, 2022 and 2021, respectively. We satisfy stock option exercises with newly issued shares of our common stock.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Performance-Based Stock Options
The following table summarizes the activity of our performance-based stock options granted under our equity plans:
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of Options (in thousands) | | Weighted-average Exercise Price (per share) | | Weighted-average Remaining Contractual Term (in years) | | Aggregate Intrinsic Value (in thousands) |
| Outstanding as of December 31, 2022 | 556 | | | $ | 97.28 | | | | | |
| Granted | — | | | $ | — | | | | | |
| Exercised | (94) | | | $ | 88.01 | | | | | |
| Cancelled | — | | | $ | — | | | | | |
| Outstanding as of December 31, 2023 | 462 | | | $ | 99.16 | | | 3.04 | | $ | 42,632 | |
| Exercisable as of December 31, 2023 | 462 | | | $ | 99.16 | | | 3.04 | | $ | 42,632 | |
During the years ended December 31, 2023, 2022 and 2021, there were 0, 0 and 197,102 performance-based stock options that vested, respectively. The intrinsic value of performance-based stock options exercised was $9.7 million, $74.4 million and $40.2 million for the years ended December 31, 2023, 2022 and 2021, respectively. We satisfy performance-based stock option exercises with newly issued shares of our common stock.
Valuation Assumptions for Stock Options
The fair value of stock options, at date of grant, based on the following assumptions, was estimated using the Black-Scholes option-pricing model. Our expected stock-price volatility assumption is based on the historical volatility of our publicly traded stock. The expected life assumption is based on our historical data. The dividend yield assumption is based on the fact that we have never paid cash dividends and have no present intention to pay cash dividends. The risk-free interest rate used for each grant is equal to the zero coupon rate for instruments with a similar expected life.
The following table summarizes the Black-Scholes valuation assumption inputs for employee stock options granted:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Risk-free interest rate | 3.5 - 4.1% | | 1.3 - 4.2% | | 0.4 - 1.4% |
| Expected dividend yield | — | | | — | | | — | |
| Expected option life | 5.1 - 7.0 years | | 5.1 - 7.0 years | | 5.4 - 6.8 years |
| Expected volatility | 49 - 60% | | 50 - 60% | | 58 - 63% |
Stock Plans
In May 2020, our stockholders approved a second amendment to our 2018 Stock Incentive Plan, as amended, to increase the number of shares authorized for issuance thereunder by 7,000,000 shares, and in May 2022, our stockholders approved the amendment and restatement of our 2018 Stock Incentive Plan, as amended, or the Amended and Restated 2018 Plan, which increased the number of shares authorized for issuance thereunder by 6,000,000 shares. The Amended and Restated 2018 Plan provides for the granting of stock options, restricted stock and restricted stock units (together, restricted stock awards), stock appreciation rights and other stock-based awards, and has a fungible share pool. Any award that is not a full value award is
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
counted against the authorized share limits specified as one share for each share of common stock subject to the award, and all full value awards, defined as restricted stock awards or other stock-based awards, are counted as one and a half shares for each one share of common stock subject to such full value award.
As of December 31, 2022,2023, an aggregate of 20,711,62918,256,842 shares of common stock were reserved for issuance under our stock plans, including outstanding stock options to purchase 8,423,5527,422,183 shares of common stock, 1,487,3942,057,874 outstanding restricted stock units, 10,083,9118,168,918 of common stock available for additional equity awards and 716,772607,867 shares available for future grant under our Amended and Restated 2004 Employee Stock Purchase Plan, as amended, or the Amended and Restated ESPP. Each stock option shall expire within 10 years of issuance. Time-based stock options granted to employees generally vest as to 25% of the shares on the first anniversary of the grant date and 6.25% of the shares at the end of each successive three-month period thereafter until fully vested.
Stock-Based Compensation
The following table summarizes stock-based compensation expenses included in operating costs and expenses:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (In thousands) | 2022 | | 2021 | | 2020 |
| Research and development | $ | 92,161 | | | $ | 68,415 | | | $ | 60,464 | |
| Selling, general and administrative | 138,488 | | | 97,302 | | | 79,409 | |
| Total | $ | 230,649 | | | $ | 165,717 | | | $ | 139,873 | |
The following table summarizes stock-based compensation expense:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (In thousands) | 2022 | | 2021 | | 2020 |
| Stock-based compensation expense by type of award: | | | | | |
| Time-based stock options | $ | 114,901 | | | $ | 118,635 | | | $ | 112,971 | |
| Time-based restricted stock units | 12,791 | | | 4,231 | | | 6,909 | |
| Performance-based restricted stock units | 102,925 | | | 39,943 | | | 11,162 | |
| Other equity programs | 4,057 | | | 6,235 | | | 9,402 | |
| Less: Stock-based compensation expense capitalized to inventory | (4,025) | | | (3,327) | | | (571) | |
| Total | $ | 230,649 | | | $ | 165,717 | | | $ | 139,873 | |
The following table summarizes our unrecognized stock-based compensation expense, net of estimated forfeitures, by type of awards, and the weighted-average period over which that expense is expected to be recognized:
| | | | | | | | | | | |
| As of December 31, 2022 |
| Unrecognized Expense, Net of Estimated Forfeitures (in thousands) | | Weighted-average Recognition Period (in years) |
| Type of award: | | | |
| Time-based stock options | $ | 187,534 | | | 2.47 |
| Time-based restricted stock units | $ | 30,676 | | | 2.09 |
| Performance-based restricted stock units * | $ | 7,695 | | | 0.58 |
| Other equity programs | $ | 4,225 | | | 0.45 |
*Excludes performance-based restricted stock units for which the associated vesting events are not yet determined to be probable.
Valuation Assumptions for Stock Options
The fair value of stock options, at date of grant, based on the following assumptions, was estimated using the Black-Scholes option-pricing model. Our expected stock-price volatility assumption is based on the historical volatility of our publicly traded stock. The expected life assumption is based on our historical data. The dividend yield assumption is based on the fact
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
that we have never paid cash dividends and have no present intention to pay cash dividends. The risk-free interest rate used for each grant is equal to the zero coupon rate for instruments with a similar expected life.
The following table summarizes the Black-Scholes valuation assumption inputs for employee stock options granted:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Risk-free interest rate | 1.3 - 4.2% | | 0.4 - 1.4% | | 0.3 - 1.7% |
| Expected dividend yield | — | | | — | | | — | |
| Expected option life | 5.1 - 7.0 years | | 5.4 - 6.8 years | | 5.4 - 7.2 years |
| Expected volatility | 50 - 60% | | 58 - 63% | | 61 - 63% |
Stock Option Activity
The following table summarizes the activity of our stock option plans, excluding performance-based stock options:
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of Options (in thousands) | | Weighted-average Exercise Price (per share) | | Weighted-average Remaining Contractual Term (in years) | | Aggregate Intrinsic Value (in thousands) |
| Outstanding as of December 31, 2021 | 8,840 | | | $ | 103.87 | | | | | |
| Granted | 1,874 | | | $ | 153.18 | | | | | |
| Exercised | (2,490) | | | $ | 84.48 | | | | | |
| Cancelled | (356) | | | $ | 129.47 | | | | | |
| Outstanding as of December 31, 2022 | 7,868 | | | $ | 120.59 | | | 6.53 | | $ | 921,016 | |
| Exercisable as of December 31, 2022 | 4,450 | | | $ | 102.17 | | | 5.22 | | $ | 602,969 | |
| Vested or expected to vest as of December 31, 2022 | 7,537 | | | $ | 119.24 | | | 6.44 | | $ | 892,463 | |
The weighted-average fair value of stock options granted was $80.65, $82.59 and $66.28 per share for the years ended December 31, 2022, 2021 and 2020, respectively. The intrinsic value of stock options exercised was $289.3 million, $247.8 million and $177.8 million for the years ended December 31, 2022, 2021 and 2020, respectively. We satisfy stock option exercises with newly issued shares of our common stock.
Performance-Based Stock Options
The following table summarizes the activity of our performance-based stock options granted under our equity plans:
| | | | | | | | | | | | | | | | | | | | | | | |
| Number of Options (in thousands) | | Weighted-average Exercise Price (per share) | | Weighted-average Remaining Contractual Term (in years) | | Aggregate Intrinsic Value (in thousands) |
| Outstanding as of December 31, 2021 | 1,175 | | | $ | 92.31 | | | | | |
| Granted | — | | | $ | — | | | | | |
| Exercised | (619) | | | $ | 87.85 | | | | | |
| Cancelled | — | | | $ | — | | | | | |
| Outstanding as of December 31, 2022 | 556 | | | $ | 97.28 | | | 3.81 | | $ | 77,975 | |
| Exercisable as of December 31, 2022 | 556 | | | $ | 97.28 | | | 3.81 | | $ | 77,975 | |
During the years ended December 31, 2022, 2021 and 2020, there were 0, 197,102 and 0 performance-based stock options that vested, respectively. The intrinsic value of performance-based stock options exercised was $74.4 million, $40.2 million and $34.1 million for the years ended December 31, 2022, 2021 and 2020, respectively. We satisfy performance-based stock option exercises with newly issued shares of our common stock.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Restricted Stock Units and Awards
The following table summarizes the activity of our restricted stock units and awards granted under our equity plans, excluding performance-based restricted stock units:
| | | | | | | | | | | |
| Number of Units (in thousands) | | Weighted-average Grant Date Fair Value (per share) |
| Outstanding as of December 31, 2021 | 75 | | | $ | 158.87 | |
| Awarded | 249 | | | $ | 165.32 | |
| Released | (23) | | | $ | 152.98 | |
| Cancelled | (9) | | | $ | 143.85 | |
| Outstanding as of December 31, 2022 | 292 | | | $ | 165.27 | |
Performance-Based Restricted Stock Units
The following table summarizes the activity of our performance-based restricted stock units granted under our equity plans:
| | | | | | | | | | | |
| Number of Units (in thousands) | | Weighted-average Grant Date Fair Value (per share) |
| Outstanding as of December 31, 2021 | 1,136 | | | $ | 143.13 | |
| Awarded | 690 | | | $ | 147.47 | |
| Released | (501) | | | $ | 142.72 | |
| Cancelled | (130) | | | $ | 143.95 | |
| Outstanding as of December 31, 2022 | 1,195 | | | $ | 144.77 | |
The performance-based restricted stock units granted in 2022 and 2021 willto employees generally vest upon the laterover a three-year period, with one-third of the one-year anniversaryshares vesting on each of the datethree successive anniversaries of the grant and the achievement of specific clinical development, regulatory, commercial and/or financial performance events, as approved by our people, culture and compensation committee.date.
Employee Stock Purchase Plan
In 2004, we adopted the 2004 Employee Stock Purchase Plan and in 2017, our stockholders approved the Amended and Restated ESPP. In 2020, our stockholders approved an amendment to the Amended and Restated ESPP, to increase the number of shares authorized for issuance to 1,965,789 shares. Under the Amended and Restated ESPP, as amended, each offering
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
period is six months, at the end of which employees may purchase shares of common stock through payroll deductions made over the term of the offering. The per-share purchase price at the end of each offering period is equal to the lesser of 85% of the closing price of our common stock at the beginning or end of the offering period. We issued 119,285108,905 and 124,101119,285 shares during the years ended December 31, 20222023 and 2021,2022, respectively.
We estimate the fair value of shares to be issued under the Amended and Restated ESPP, as amended, using the Black-Scholes option-pricing model on the date of grant, or first day of the offering period, using the same methodology approach as the employee stock option grants. The following table summarizes the Black-Scholes valuation assumption inputs for stock purchase rights granted under the employee stock purchase plan:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2022 | | 2021 | | 2020 |
| Risk-free interest rate | 1.4% - 4.5% | | 0.03% - 0.06% | | 0.1% - 0.1% |
| Expected dividend yield | — | | | — | | | — | |
| Expected option life | 6 months | | 6 months | | 6 months |
| Expected volatility | 53% - 71% | | 41% - 46% | | 40% - 50% |
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
13. STOCKHOLDERS’ (DEFICIT) EQUITY | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 | | 2021 |
| Risk-free interest rate | 5.1% - 5.4% | | 1.4% - 4.5% | | 0.03% - 0.06% |
| Expected dividend yield | — | | | — | | | — | |
| Expected option life | 6 months | | 6 months | | 6 months |
| Expected volatility | 34% - 39% | | 53% - 71% | | 41% - 46% |
Preferred Stock
We have authorized up to 5,000,000 shares of preferred stock, $0.01 par value per share, for issuance. The preferred stock will have such rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, as shall be determined by our board of directors upon its issuance. As of December 31, 20222023 and 2021,2022, there were no shares of preferred stock outstanding.
Blackstone Equity Placement
12. LEASES
Overview of Significant Leases
We lease three facilities for office and laboratory space in Cambridge, Massachusetts that represent substantially all of our significant lease obligations. An overview of these significant leases are as follows:
675 West Kendall Street
We lease office and laboratory space located at 675 West Kendall Street, Cambridge, Massachusetts for our corporate headquarters from BMR-675 West Kendall Street, LLC, or BMR, under a non-cancelable real property lease. The lease commenced on May 1, 2018 and monthly rent payments became due commencing on February 1, 2019 upon substantial completion of the building improvements, and continue for 15 years, with options to renew for two five-year terms each. Exercise of these options was not determined to be reasonably certain and thus was not included in the operating lease liability on the consolidated balance sheet as of December 31, 2023.
300 Third Street
We lease office and laboratory space located at 300 Third Street, Cambridge, Massachusetts under a non-cancelable real property lease agreement by and between us and ARE-MA Region No. 28, LLC, or ARE-MA, dated as of September 26, 2003, as amended. The term of the lease expires on January 31, 2034 with options to renew for two five-year terms each. Exercise of these options was not determined to be reasonably certain and thus was not included in the operating lease liability on the consolidated balance sheet as of December 31, 2023.
101 Main Street
We lease office space on several floors at 101 Main Street, Cambridge, Massachusetts under non-cancelable real property lease agreements by and between us and RREEF America REIT II CORP. PPP, or RREEF, entered into in 2015. In April 2020, we entered into a stock purchaseamended our lease agreement, or Investors SPA, with certain affiliates of The Blackstone Group Inc., or Investors, pursuant to which the term of the lease with respect to two floors was extended for an additional five years, through June 2026, with an option to renew for one five-year term. Exercise of this option was not determined to be reasonably certain and thus was not included in the operating lease liability on the consolidated balance sheet as of December 31, 2023. In addition, we sold 963,486 shareshave a separate lease agreement for an additional floor at 101 Main Street, which expires in March 2024 and will not be extended.
Other Lease Disclosures
Our facility leases described above generally contain customary provisions allowing the landlords to terminate the leases if we fail to remedy a breach of any of our common stockobligations under any such lease within specified time periods, or upon our bankruptcy or insolvency. The leases do not include any restrictions or covenants that had to be accounted for under the lease guidance.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Total rent expense, including operating expenses, under all of our real property leases was $61.6 million, $58.6 million and $59.5 million for the years ended December 31, 2023, 2022 and 2021, respectively.
The following table summarizes our costs included in operating expenses related to right of use lease assets we have entered into through December 31, 2023:
| | | | | | | | | | | | | | | | | | | | |
| | Year Ended December 31, |
| (In thousands) | | 2023 | | 2022 | | 2021 |
| Operating lease cost | | $ | 46,367 | | | $ | 45,789 | | | $ | 45,359 | |
| Variable lease cost | | 20,278 | | | 17,614 | | | 18,271 | |
| Total | | $ | 66,645 | | | $ | 63,403 | | | $ | 63,630 | |
Short-term lease costs were not material for the years ended December 31, 2023 and 2022.
Net cash paid for the amounts included in the measurement of the operating lease liability in our consolidated balance sheet and included in change in operating lease liability within operating activities in our consolidated statement of cash flow was $46.5 million and $43.1 million for the years ended December 31, 2023 and 2022, respectively. The weighted-average remaining lease term and weighted-average discount rate for all leases as of December 31, 2023 was 9 years and 8%, respectively, and as of December 31, 2022 was 10 years and 8%, respectively.
Future lease payments for non-cancellable operating leases and a reconciliation to the Investors for aggregate cash consideration of $100.0 million, or $103.79 per share, as partcarrying amount of the broad strategic financing collaboration with The Blackstone Group Inc. The Investors SPA contains customary representations, warranties, and covenantsoperating lease liability presented in the consolidated balance sheet as of each of the parties thereto.December 31, 2023 were as follows, in thousands:
| | | | | |
| Year Ending December 31 | |
| 2024 | $ | 43,587 | |
| 2025 | 46,369 | |
| 2026 | 40,648 | |
| 2027 | 39,006 | |
| 2028 | 38,358 | |
| 2029 and thereafter | 210,073 | |
| Total undiscounted lease liability | 418,041 | |
| Less imputed interest | (133,430) | |
| Total discounted lease liability | $ | 284,611 | |
| |
| Current operating lease liability | $ | 41,510 | |
| Non-current operating lease liability | 243,101 | |
| Total | $ | 284,611 | |
14.13. COMMITMENTS AND CONTINGENCIES
Technology License and Other Commitments
We have licensed from third parties the rights to use certain technologies and information in our research processes as well as in any other products we may develop. In accordance with the related license or technology agreements, we are required to make certain fixed payments to the licensor or a designee of the licensor over various agreement terms. Many of these agreement terms are consistent with the remaining lives of the underlying intellectual property that we have licensed. As of December 31, 2022, our commitments over the next five years to make fixed and cancellable payments under existing license agreements were not material.
Legal Matters
From time to time, we may be a party to litigation, arbitration or other legal proceedings in the course of our business, including the matters described below. The claims and legal proceedings in which we could be involved include challenges to the scope, validity or enforceability of patents relating to our products or product candidates, and challenges by us to the scope, validity or enforceability of the patents held by others. These include claims by third parties that we infringe their patents or breach our license or other agreements with such third parties. The outcome of any such legal proceedings, regardless of the merits, is inherently uncertain. In addition, litigation and related matters are costly and may divert the attention of our
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
management and other resources that would otherwise be engaged in other activities. If we were unable to prevail in any such legal proceedings, our business, results of operations, liquidity and financial condition could be adversely affected. Our accounting policy for accrual of legal costs is to recognize such expenses as incurred.
Government Investigation
We have previously disclosed that, on or about April 9, 2021, we received a subpoena from the U.S. Department of Justice, U.S. Attorney’s Office for the District of Massachusetts, requiring production of documents pertaining to our marketing and promotion of ONPATTRO (patisiran) in the U.S. We are cooperating with the U.S. Attorney’s Office and producing documents in response to the subpoena. Current and former officers and employees also have received subpoenas in connection with the preservation and production of related materials. Given the ongoing nature of the investigation, it is possible that the U.S. Attorney’s Office for the District of Massachusetts or other government entities may request other information from, or issue other subpoenas, findings or similar documents to, us, our related entities and their respective directors, officers and employees. In light of the ongoing nature of the investigation, no determination has been made that a loss, if any, arising from this matter is probable or that the amount of any such loss, or range of loss, is reasonably estimable. We also previously disclosed that since learning of this federal government investigation, our nominating and corporate governance committee is directing our review of and response to the matter.
Patent Infringement Lawsuits
In March 2022, we filed separate lawsuits in the U.S. District Court for the District of Delaware against (1) Pfizer, Inc. and its subsidiary Pharmacia & Upjohn Co. LLC, collectively referred to as Pfizer, and (2) Moderna, Inc., and its subsidiaries ModernaTX, Inc., and Moderna US, Inc., collectively referred to as Moderna. The lawsuits seek damages for infringement of U.S. Patent No. 11,246,933, or ‘933 Patent, in Pfizer’s and Moderna’s manufacture and sale of their messenger RNA, or mRNA, COVID-19 vaccines. The patent relates to the Company’s biodegradable cationic lipids that are foundational to the success of the mRNA COVID-19 vaccines.
We are seeking judgment that each of Pfizer and Moderna is infringing the ‘933 Patent, as well as damages adequate to compensate for the infringement, but in no event less than a reasonable royalty for the unlicensed uses made of our patented lipids by Pfizer and Moderna, together with interest and costs as may be awarded by the court. As stated in the filed complaints, we are not seeking injunctive relief in these lawsuits.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On May 23, 2022, Moderna filed a partial motion to dismiss, asserting an affirmative defense under Section 1498(a). We responded on May 27, 2022, opposing their motion arguing Moderna had significant non-government sales and the government contract ended in April 2022. Moderna responded on June 13, 2022, requesting a partial motion to dismiss those claims for sales under Section 1498(a). This motion is fully briefed and pending before the court.
On May 27, 2022, Pfizer filed an answer to our complaint, denying the allegations, and asserting invalidity and non-infringement defenses. In addition, Pfizer added BioNTech SE to the suit and added counter-claims seeking a declaratory judgment that our patent is invalid and a second claim alleging that our patent is invalid due to patent misuse. We believe their defenses and counter-claims have no merit and responded on June 10, 2022, with substantive arguments as to the validity of our claims and the lack of merit of their patent misuse claim.
On July 12, 2022, we filed a newan additional lawsuit against each of Pfizer and Moderna seeking damages for infringing our newly grantedinfringement of U.S. Patent No. 11,382,979, or ‘979 patent, in Pfizer’s and Moderna’s manufacture and sale of their mRNA COVID-19 vaccines. The parties agreed to combine the two patents in one lawsuit, separately against each of Moderna and Pfizer/BioNTech.
On February 8, 2023, we received notification from the U.S. Patent Office that a third patent would issue on February 28, 2023, as U.S. Patent No. 11,590,229, or ‘229 patent, which we also believe Pfizer and Moderna’s COVID-19 vaccines infringe upon. On February 15, 2023, we filed a motion with the court to add this patent to the existing cases against Pfizer and Moderna, and on April 26, 2023, the court held a hearing and denied Moderna’s partial motion to dismiss those claims for sales under Section 1498(a), our motion to add the ‘229 patent to the then current lawsuits as well as a motion filed by Moderna to add certain invalidity arguments made by Pfizer in our case to supplement Moderna’s invalidity arguments previously made.
On May 26, 2023, we filed additional lawsuits against Pfizer and Moderna in Delaware seeking damages for infringing the ‘229 patent. In addition to this patent, we added U.S. Patent Nos. 11,633,479 and 11,633,480 in the recently filed suits against both Pfizer and Moderna and also U.S. Patent No. 11,612,657 against Pfizer only.
On August 9, 2023, a Markman hearing was held in the U.S. District Court for the District of Delaware to consider the meaning of three disputed terms as used in the ’933 and ’979 patents. On August 21, 2023, the court issued an order construing two of the three terms, and deferred a ruling on the third term pending an evidentiary hearing, which was held on January 4, 2024 with the final ruling pending. Subsequently, we and Moderna jointly agreed to final judgment of non-infringement of two of our patents, and such judgment was entered by the court on August 30, 2023, and on September 7, 2023, we appealed the claim construction ruling to the Court of Appeals for the Federal Circuit in the initial lawsuit against Moderna. The court hasclaim construction ruling did not affect one of the patents in the lawsuit filed against Moderna on May 26, 2023, and that case is going forward on a schedule to be set aby the court.
The two separate suits against Pfizer are ongoing subject to the ruling on the third claim term, and in September 2023, we and Pfizer agreed to consolidate the 2022 and 2023 lawsuits in one case, which will require moving the trial date from November 2024 to the first half of November 12, 2024 for Alnylam v. Moderna and November 18, 2024 for Alnylam v. Pfizer/BioNTech.2025, with the final schedule to be determined by the court.
Indemnifications
In connection with license agreements we may enter with companies to obtain rights to intellectual property, we may be required to indemnify such companies for certain damages arising in connection with the intellectual property rights licensed under the agreements. Under such agreements, we may be responsible for paying the costs of any litigation relating to the license agreements or the underlying intellectual property rights, including the costs associated with certain litigation regarding the licensed intellectual property. We are also a party to a number of agreements entered into in the ordinary course of business, which contain typical provisions that obligate us to indemnify the other parties to such agreements upon the occurrence of
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
certain events, including litigation or other legal proceedings. In addition, we have agreed to indemnify our officers and directors for expenses, judgments, fines, penalties, excise taxes, and settlement amounts paid in connection with any threatened, pending or completed litigation proceedings, including, for example, the currentrecently closed government investigation, in which an officer or director was, is or will be involved as a party, on account of such person’s status as an officer or director, or by reason of any action taken by the officer or director while acting in such capacity, subject to certain limitations. These indemnification costs are charged to selling, general and administrative expense.
Our maximum potential future liability under any such indemnification provisions is uncertain. We have determined that the estimated aggregate fair value of our potential liabilities under all such indemnification provisions is minimal and had not recorded any liability related to such indemnification provisions as of December 31, 2022 or 2021.
15. LEASES14. INCOME TAXES
OverviewThe domestic and foreign components of Significant Leases
We lease three facilities for office and laboratory space in Cambridge, Massachusetts that represent substantially all of our significant lease obligations. An overview of these significant leasesloss before income taxes are as follows:
675 West Kendall Street | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (In thousands) | 2023 | | 2022 | | 2021 |
| Domestic | $ | (450,311) | | | $ | (1,148,604) | | | $ | (794,729) | |
| Foreign | 16,794 | | | 21,611 | | | (57,415) | |
| Loss before income taxes | $ | (433,517) | | | $ | (1,126,993) | | | $ | (852,144) | |
We lease office and laboratory space located at 675 West Kendall Street, Cambridge, MassachusettsThe provision for our corporate headquarters from BMR-675 West Kendall Street, LLC, or BMR, under a non-cancelable real property lease. The lease commenced on May 1, 2018 and monthly rent payments became due commencing on February 1, 2019 upon substantial completionincome taxes consisted of the building improvements, and continue for 15 years, with options to renew for two five-year terms each. Exercise of these options was not determined to be reasonably certain and thus was not included infollowing:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (In thousands) | 2023 | | 2022 | | 2021 |
| Current provision: | | | | | |
| Domestic | $ | 4,022 | | | $ | — | | | $ | 293 | |
| Foreign | 3,416 | | | 5,596 | | | 3,154 | |
| Total current provision | 7,438 | | | 5,596 | | | 3,447 | |
| Deferred benefit: | | | | | |
| Domestic | — | | | — | | | — | |
| Foreign | (713) | | | (1,433) | | | (2,767) | |
| Total deferred benefit | (713) | | | (1,433) | | | (2,767) | |
| Total provision for income taxes | $ | 6,725 | | | $ | 4,163 | | | $ | 680 | |
During the operating lease liability on the consolidated balance sheet as ofyear ended December 31, 2022.
300 Third Street
We lease office and laboratory space located at 300 Third Street, Cambridge, Massachusetts under2023, we recorded a non-cancelable real property lease agreementnet provision for income taxes of $6.7 million. This is primarily comprised of $4.0 million of state current provision, $3.4 million of foreign current provision offset by and between us and ARE-MA Region No. 28, LLC, or ARE-MA, dated as$0.7 million of September 26, 2003, as amended. The term of the lease expires on January 31, 2034 with options to renew for two five-year terms each. Exercise of these options was not determined to be reasonably certain and thus was not included in the operating lease liability on the consolidated balance sheet as of December 31, 2022.
101 Main Street
We lease office space on several floors at 101 Main Street, Cambridge, Massachusetts under non-cancelable real property lease agreements by and between us and RREEF America REIT II CORP. PPP, or RREEF, entered into in March 2015 and May 2015, as amended in September 2020, that will expire in March 2024 and June 2026, respectively, each with an option toforeign deferred provision.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
renew for one five-year term. Exercise of these options was not determined to be reasonably certain and thus was not included in the operating lease liability on the consolidated balance sheet as of December 31, 2022.
Other Lease Disclosures
Our facility leases described above generally contain customary provisions allowing the landlords to terminate the leases if we fail to remedy a breach of any of our obligations under any such lease within specified time periods, or upon our bankruptcy or insolvency. The leases do not include any restrictions or covenants that had to be accounted for under the lease guidance.
Total rent expense, including operating expenses, under all of our real property leases was $58.6 million, $59.5 million and $50.7 million for the years ended December 31, 2022, 2021 and 2020, respectively.
The following table summarizes our costs included in operating expenses related to right of use lease assets we have entered into through December 31, 2022:
| | | | | | | | | | | | | | |
| (In thousands) | | Year Ended December 31, 2022 | | Year Ended December 31, 2021 |
| Operating lease cost | | $ | 45,789 | | | $ | 45,359 | |
| Variable lease cost | | 17,614 | | | 18,271 | |
| Total | | $ | 63,403 | | | $ | 63,630 | |
Short-term lease costs were not material for the years ended December 31, 2022 and 2021.
Net cash paid for the amounts included in the measurement of the operating lease liability in our consolidated balance sheet and included in change in operating lease liability within operating activities in our consolidated statement of cash flow was $43.1 million and $41.9 million for the years ended December 31, 2022 and 2021, respectively. The weighted-average remaining lease term and weighted-average discount rate for all leases as of December 31, 2022 was 10 years and 8%, respectively, and as of December 31, 2021 was 11 years and 8%, respectively.
Future lease payments for non-cancellable operating leases and a reconciliation to the carrying amount of the operating lease liability presented in the consolidated balance sheet as of December 31, 2022 were as follows, in thousands:
| | | | | |
| Year Ending December 31 | |
| 2023 | $ | 43,028 | |
| 2024 | 46,486 | |
| 2025 | 42,867 | |
| 2026 | 40,376 | |
| 2027 | 38,753 | |
| 2028 and thereafter | 248,622 | |
| Total undiscounted lease liability | 460,132 | |
| Less imputed interest | (156,826) | |
| Total discounted lease liability | $ | 303,306 | |
| |
| Current operating lease liability | $ | 41,967 | |
| Non-current operating lease liability | 261,339 | |
| Total | $ | 303,306 | |
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
16. INCOME TAXES
The domestic and foreign components of loss before income taxes are as follows:
| | | | | | | | | | | | | | | | | |
| (In thousands) | 2022 | | 2021 | | 2020 |
| Domestic | $ | (1,148,604) | | | $ | (794,729) | | | $ | (682,859) | |
| Foreign | 21,611 | | | (57,415) | | | (172,741) | |
| Loss before income taxes | $ | (1,126,993) | | | $ | (852,144) | | | $ | (855,600) | |
The provision for income taxes consisted of the following:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| (In thousands) | 2022 | | 2021 | | 2020 |
| Current provision: | | | | | |
| Domestic | $ | — | | | $ | 293 | | | $ | 61 | |
| Foreign | 5,596 | | | 3,154 | | | 5,837 | |
| Total current provision | 5,596 | | | 3,447 | | | 5,898 | |
| Deferred benefit: | | | | | |
| Domestic | — | | | — | | | 393 | |
| Foreign | (1,433) | | | (2,767) | | | (3,610) | |
| Total deferred benefit | (1,433) | | | (2,767) | | | (3,217) | |
| Total provision for income taxes | $ | 4,163 | | | $ | 680 | | | $ | 2,681 | |
During the year ended December 31, 2022, we recorded a net provision for income taxes of $4.2 million. This is primarily comprised of $5.6 million of foreign current provision offset by $1.4 million of foreign deferred provision.
Deferred income taxes reflect the tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting and income tax purposes. We establish a valuation allowance when uncertainty exists as to whether all or a portion of the net deferred tax assets will be realized. Components of the net deferred tax asset are as follows:
| | | | | | | | | | | |
| As of December 31, |
| (In thousands) | 2022 | | 2021 |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 803,251 | | | $ | 745,985 | |
| Research and development and other credit carryforwards | 381,032 | | | 342,431 | |
| Sale of future royalties | 326,183 | | | 302,217 | |
| Lease liability | 67,242 | | | 71,859 | |
| Deferred revenue | 59,437 | | | 76,612 | |
| Deferred compensation | 52,989 | | | 59,349 | |
| Intangible assets | 264,564 | | | 279,082 | |
| Capitalized research and development expenditures | 206,727 | | | 23,039 | |
| Other | 133,376 | | | 48,242 | |
| Total deferred tax assets | 2,294,801 | | | 1,948,816 | |
| Deferred tax liabilities: | | | |
| Property, plant and equipment, net | (12,786) | | | (13,170) | |
| Unrealized gain on marketable securities | (5,728) | | | (16,693) | |
| Right of use assets | (46,819) | | | (50,562) | |
| Deferred revenue tax accounting method change | (24,995) | | | (50,380) | |
| Deferred tax asset valuation allowance | (2,193,633) | | | (1,808,992) | |
| Net deferred tax asset | $ | 10,840 | | | $ | 9,019 | |
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| | | | | | | | | | | |
| As of December 31, |
| (In thousands) | 2023 | | 2022 |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 803,221 | | | $ | 803,251 | |
| Research and development and other credit carryforwards | 417,446 | | | 381,032 | |
| Sale of future royalties | 353,974 | | | 326,183 | |
| Lease liability | 66,558 | | | 67,242 | |
| Deferred revenue | 74,704 | | | 59,437 | |
| Deferred compensation | 67,150 | | | 52,989 | |
| Intangible assets | 697,784 | | | 264,564 | |
| Capitalized research and development expenditures | 285,411 | | | 206,727 | |
| Other | 132,529 | | | 133,376 | |
| Total deferred tax assets | 2,898,777 | | | 2,294,801 | |
| Deferred tax liabilities: | | | |
| Property, plant and equipment, net | (21,503) | | | (12,786) | |
| Unrealized gain on marketable securities | (2,277) | | | (5,728) | |
| Right of use assets | (46,021) | | | (46,819) | |
| Deferred revenue tax accounting method change | — | | | (24,995) | |
| Deferred tax asset valuation allowance | (2,817,395) | | | (2,193,633) | |
| Net deferred tax asset | $ | 11,581 | | | $ | 10,840 | |
Our effective income tax rate differs from the statutory federal income tax rate, as follows:
| | Year Ended December 31, |
| Year Ended December 31, | | | Year Ended December 31, |
| (In thousands) | (In thousands) | 2022 | | 2021 | | 2020 | (In thousands) | 2023 | | 2022 | | 2021 |
| At U.S. federal statutory rate | At U.S. federal statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % | At U.S. federal statutory rate | 21.0 | % | | 21.0 | % | | 21.0 | % |
| State taxes, net of federal effect | State taxes, net of federal effect | 6.0 | | | 5.2 | | | 4.5 | |
| Stock-based compensation | Stock-based compensation | 4.4 | | | 4.6 | | | 2.2 | |
| Tax credits | Tax credits | 2.7 | | | 4.5 | | | 3.3 | |
| Nondeductible compensation | |
| Other permanent items | Other permanent items | (1.5) | | | (1.0) | | | (1.5) | |
| Foreign rate differential | Foreign rate differential | (0.5) | | | (1.7) | | | (3.5) | |
| Bermuda tax law enactment | |
| Internal reorganization of certain intellectual property rights | Internal reorganization of certain intellectual property rights | — | | | 20.1 | | | 12.3 | |
| Other | Other | (0.8) | | | (0.1) | | | (2.7) | |
| Revaluation of deferred due to rate change | Revaluation of deferred due to rate change | (0.4) | | | 1.1 | | | — | |
| Valuation allowance | Valuation allowance | (31.3) | | | (53.8) | | | (35.9) | |
| Effective income tax rate | Effective income tax rate | (0.4) | % | | (0.1) | % | | (0.3) | % | Effective income tax rate | (1.5) | % | | (0.4) | % | | (0.1) | % |
We have evaluated the positive and negative evidence bearing upon the realizability of our deferred tax assets. We have concluded, in accordance with the applicable accounting standards, that it is more likely than not that we may not realize the benefit of all of our deferred tax assets, with the exception of the deferred assets related to certain foreign subsidiaries. Accordingly, we have recorded a valuation allowance against our U.S., Bermuda and Switzerland deferred tax assets. We continue to maintain a valuation allowance on the majority of our net operating losses and other deferred tax assets that management believes will not be realized. We re-evaluatebecause we have a history of cumulative losses. On a quarterly basis, we reassess the valuation allowance on our deferred income tax assets weighing positive and negative evidence to assess the recoverability of the deferred tax assets. Based on our recent financial performance and our future projections, we could record a quarterly basis.reversal of all, or a portion of the valuation allowance. However, any such change is subject to actual performance and other considerations that may present positive or negative evidence at the time
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
of the assessment. The valuation allowance increased by $623.8 million, $384.6 million $459.3 million and $303.7$459.3 million for the years ended December 31, 2023, 2022 2021 and 2020,2021, respectively. The increase in our valuation allowance is primarily due to capitalized research and development costs and internally developed intellectual property for the year ending December 31, 2023, additional net operating losses for the yearsyear ended December 31, 2022, and 2020 and primarily due to the liability related to the sale of future royalties for the year ended December 31, 2021.
On December 22, 2017, the Tax Cuts and Jobs Act, or TCJA, was signed into law. Under the TCJA provisions, effective with tax years beginning on or after January 1, 2022, taxpayers can no longer immediately expense qualified research and development expenditures, including all direct, indirect, overhead and software development costs. Taxpayers are now required to capitalize and amortize these costs over five years for research conducted within the United States or 15 years for research conducted abroad. As a result, the Company capitalized $870.6 million of research and development expenses for the year ended December 31, 2022.
As of December 31, 2022,2023, we had federal and state net operating loss carryforwards, or NOLs, of $2.9$2.74 billion and $3.0$3.18 billion, respectively, to reduce future taxable income. Federal NOLs of $1.1 billion,$912.0 million, generated before 2018,2017, will begin expiring in varying amounts through 2037 unless utilized. The remaining federal NOLs of $1.8$1.83 billion, generated after 2017, will be carried forward indefinitely and could be used to offset up to 100% of taxable income of each future tax year for tax years before January 1, 2021 and up to 80% of taxable income in all other future tax years. State NOLs will begin expiring in varying amounts through 20422043 unless utilized. As of December 31, 2022,2023, we had federal and state research and development, including Orphan Drug, and state investment tax credit carryforwards of $343.0$376.7 million and $62.3$62.1 million, respectively, available to reduce future tax liabilities that expire at various dates through 2042.2043. We have a valuation allowance against the net operating loss and tax credit carryforwards as it is unlikely that we will realize these assets. Ownership changes, as defined in the Internal Revenue Code and similar state provisions, including those resulting from the issuance of common stock in connection with our public offerings, may limit the amount of federal and state net operating loss and tax credit carryforwards that can be utilized to offset future taxable income or tax liability. The amount of the limitation is determined in accordance with Section 382 of the Internal Revenue Code and similar state provisions. We have performed an analysis of ownership changes through December 31, 2022.2023. Based on this analysis, we do not believe that any of our federal and state tax attributes will expire unutilized due to Section 382 limitations.
As of December 31, 2022,2023, we had foreignSwitzerland NOLs of $153.3$382.4 million to reduce future taxable income which will begin expiring in varying amounts through 20292030 unless utilized. We have a valuation allowance against the foreignour Switzerland NOLs as it is unlikely that we will realize these assets.assets given our historical losses.
On December 27, 2023, the Government of Bermuda enacted the Corporate Income Tax Act of 2023, or Corporate Income Tax Act, which introduces a corporate income tax regime in Bermuda with a statutory tax rate of 15% effective January 1, 2025. Companies are not subject to income tax in Bermuda prior to this change and with the transition into the Act there is an economic transition adjustment that requires the tax basis of certain Bermudian assets to be established at fair market value. Upon the enactment of the Corporate Income Tax Act, we determined the fair market value of our identifiable intangibles in Bermuda and recognized a deferred tax asset in our consolidated financial statements. We recorded a full valuation allowance against this deferred tax asset as we have generated historical losses and expect to generate future losses.
We apply the accounting guidance in ASC 740 related to accounting for uncertainty in income taxes. Our reserves related to income taxes are based on a determination of whether, and how much of, a tax benefit taken by us in our tax filings or positions is more likely than not to be realized and ultimately sustained upon challenge by a taxing authority based upon its technical merits and subject to certain recognition and measurement criteria. We recognize potential interest and penalties related to unrecognized tax benefits in our provision for income taxes. Our reserve related to income taxes, including potential interest and penalties, was not material as of December 31, 20222023 and 2021.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
2022.Our uncertain income tax positions do not impact our effective tax rate due to our full valuation allowance in the U.S.
As of December 31, 2022,2023, the unremitted earnings of our foreign subsidiaries are not material.approximately $45.0 million. We have not provided for U.S. income taxes or foreign withholding taxes on these earnings as it is our current intention to permanently reinvest these earnings outside the U.S. The tax liability on these earnings is also not material. Events that could trigger a tax liability include, but are not limited to, distributions, reorganizations or restructurings and/or tax law changes.
The tax years 20192020 through 20222023 remain open to examination by major taxing jurisdictions, which are primarily in the U.S., although net operating loss and tax credit carryforwards generated prior to 20192020 may still be adjusted upon examination by the Internal Revenue Service or state tax authorities if they have or will be used in a future period.
17.15. EMPLOYEE BENEFIT PLANS
We maintain a retirement saving plan under Section 401(k) of the Internal Revenue Code, in which eligible U.S. employees may defer compensation for income tax purposes. Contributions made by employees are limited to the maximum allowable for U.S. federal income tax purposes. The plan allows for a discretionary match in an amount up to 100% of each participant’s first 2% of compensation contributed plus 50% of each participant’s next 4% of compensation contributed. The expense related to our 401(k) Savings Plan primarily consists of our matching contributions.
Furthermore, we maintain defined benefit plans for employees in certain countries outside the U.S., including retirement benefit plans required by applicable local law. The benefit obligation corresponds to the projected benefit obligations of which the discounted net present value is calculated based on years of employment, expected salary increases and pension adjustments.
ALNYLAM PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For the years ended December 31, 2023, 2022 2021 and 20202021 contributions and net periodic benefit costs to such plans generated a total expense of $17.5 million, $16.5 million $13.1 million and $12.0$13.1 million, respectively.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer (principal executive officer) and executive vice president, Chief Financial Officer (principal financial officer), evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2022.2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’sour management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2022,2023, our Chief Executive Officer and executive vice president, Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, the company’sour principal executive and principal financial officers and effected by the company’sour board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
•Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
•Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of management and directors; and
•Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2022.2023. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013).
Based on our assessment, our management concluded that, as of December 31, 2022,2023, our internal control over financial reporting is effective based on those criteria.
The effectiveness of our internal control over financial reporting as of December 31, 20222023 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report, which is included herein.
Changes in Internal Control
There were no changes in our internal control over financial reporting during the quarter ended December 31, 20222023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.Adoption of 10b5-1 Trading Plans by Our Officers and Directors
During our fiscal quarter ended December 31, 2023, none of our directors or officers adopted or terminated a “Rule 10b5-1 trading plan” or a “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408 of Regulation S-K.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Incorporated by reference from the information in our Proxy Statement for our 20232024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
ITEM 11. EXECUTIVE COMPENSATION
Incorporated by reference from the information in our Proxy Statement for our 20232024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Incorporated by reference from the information in our Proxy Statement for our 20232024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
Securities Authorized for Issuance Under Equity Compensation Plans
We intend to file with the SEC a definitive Proxy Statement, which we refer to herein as the Proxy Statement, not later than 120 days after the close of the fiscal year ended December 31, 2022.2023. The information required by this item relating to our equity compensation plans is incorporated herein by reference to the information contained under the section captioned “Equity Compensation Plan Information” of the Proxy Statement.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Incorporated by reference from the information in our Proxy Statement for our 20232024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Incorporated by reference from the information in our Proxy Statement for our 20232024 Annual Meeting of Stockholders, which we will file with the SEC within 120 days of the end of the fiscal year to which this Annual Report on Form 10-K relates.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) (1) Financial Statements
The following consolidated financial statements are filed as part of this report under “Item 8 — Financial Statements and Supplementary Data:”
(a) (2) List of Schedules
All schedules to the consolidated financial statements are omitted as the required information is either inapplicable or presented in the consolidated financial statements.
(a) (3) List of Exhibits
| | | | | | | | |
| Exhibit No. | | Exhibit |
| | |
| | |
| 2.1*† | | |
| | |
| 3.1 | | |
| | |
| 3.2 | | |
| | |
| 4.1 | | |
| | |
| 4.2 | | |
| | |
| 4.3 | | |
| | |
| 4.4 | |
|
| | |
| 10.1** | | |
| | |
| 10.2** | | |
| | |
| 10.3** | | |
| | |
| | | | | | | | |
| Exhibit No. | | Exhibit |
| | |
| | |
| 10.4** | | |
| | |
| 10.5** | | |
| | |
| 10.6** | | |
| | |
| 10.7** | | |
| | |
| 10.8** | | |
| | |
| 10.9** | | |
| | |
| 10.10 | | |
| | |
| 10.11** | | |
| | |
| 10.12** | | |
| | |
| 10.13** | | |
| | |
| 10.14** | | |
| | |
10.13*10.15** | | |
| | |
10.14*10.16** | | |
| | |
10.15*10.17** | | |
| | |
10.16** | | |
| | |
| 10.18** | | |
| 10.17* | |
| 10.19** | | |
| | |
| | | | | | | | |
| Exhibit No. | | Exhibit |
| 10.18* | |
| | |
| 10.20** | | |
| | |
10.19*10.21** | | |
| | |
| | | | | | | | |
Exhibit No.10.22** | | Exhibit |
| | |
| | |
10.20** | | |
| | |
10.2110.23 | | |
| | |
10.2210.24 | | |
| | |
10.2310.25 | | |
| | |
10.2410.26 | | |
| | |
10.2510.27 | | |
| | |
10.2610.28 | | |
| | |
10.2710.29 | | |
| | |
10.28†10.30† | | |
| | |
10.2910.31 | | |
| | |
10.3010.32 | | |
| | |
10.3110.33 | | |
| | |
10.3210.34 | | |
| | |
| | | | | | | | |
| Exhibit No. | | Exhibit |
| | |
| | |
10.3310.35 | | |
| | |
10.3410.36 | | |
| | |
| | | | | | | | |
Exhibit No.10.37 | | Exhibit |
| | |
| | |
10.35 | | |
| | |
10.3610.38 | | |
| | |
10.37†10.39† | | |
| | |
10.38†10.40† | | |
| | |
10.39†10.41† | | |
| | |
10.40†10.42† | | |
| | |
10.41†#10.43† | | |
| | |
10.42†10.44† | | |
| | |
10.4310.45† | | |
| | |
10.44† | | |
| | |
10.45†10.46† | | |
| | |
| 10.47*† | | |
| 10.46†# | |
| 10.48† | | |
| | |
| | | | | | | | |
| Exhibit No. | | Exhibit |
| 10.47 | |
| | |
| 10.49 | | |
| | |
10.48†#10.50† | | |
| | |
10.49†10.51† | | |
| | |
| | | | | | | | |
Exhibit No.10.52† | | Exhibit |
| | |
| | |
10.50† | | |
| | |
10.51†10.53† | | |
| | |
10.52†10.54† | | |
| | |
10.53†10.55† | | |
| | |
10.54†10.56† | | |
| | |
10.55†10.57† | | |
| | |
10.56†10.58† | | |
| | |
10.57†10.59† | | |
| | |
10.58†10.60† | | |
| | |
10.59†10.61† | | |
| | |
10.60*#10.62* | | |
| | |
10.61 | | |
| | |
| | | | | | | | |
| Exhibit No. | | Exhibit |
| 10.62* | |
| | |
| 10.63*† | | |
| | |
10.63*10.64*† | | |
| | |
| 10.65† | | |
| | | | | | | | |
| Exhibit No. | | Exhibit
| 10.66† | | |
| | |
10.64† | | |
| | |
10.6510.67 | | |
| | |
| 21.1# | | |
| | |
| 23.1# | | |
| | |
| 31.1# | | |
| | |
| 31.2# | | |
| | |
| 32.1# | | |
| | |
| 32.2# | | |
| | |
| 97# | | |
| | |
| 101.SCH# | | Inline XBRL Taxonomy Extension Schema Document |
| | |
| 101.CAL# | | Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| | |
| 101.LAB# | | Inline XBRL Taxonomy Extension Label Linkbase Document |
| | |
| 101.PRE# | | Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| | |
| 101.DEF# | | Inline XBRL Taxonomy Extension Definition Linkbase Document |
| | |
| 104 | | Cover Page Interactive Data File (formatted as inline XBRL with applicable taxonomy extension information contained in Exhibits 101.) |
| | |
| | | | | | | | |
| * | | Schedules, exhibits and similar supporting attachments or agreements to this exhibit are omitted pursuant to Item 601(b)(2) of Regulation S-K. The Registrant agrees to furnish a supplemental copy of any omitted schedule or similar attachment to the Securities and Exchange Commission upon request. |
| ** | | Management contracts or compensatory plans or arrangements required to be filed as an exhibit hereto pursuant to Item 15(a) of Form 10-K. |
| † | | Portions of this exhibit (indicated by asterisks) have been omitted in accordance with the rules of the Securities and Exchange Commission because such information (i) is not material and (ii) would likely cause competitive harm to the Registrant if publicly disclosed. |
| # | | Filed herewith. |
ITEM 16. FORM 10-K SUMMARY
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized, on February 23, 2023.15, 2024.
| | | | | | | | | | | |
| ALNYLAM PHARMACEUTICALS, INC. |
| | | |
| By: | | /s/ Yvonne L. Greenstreet, MBChB |
| | | Yvonne L. Greenstreet, MBChB |
| | | Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, the Report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated as of February 23, 2023.15, 2024.
| | | | | | | | |
| Name | | Title |
| | |
| | |
| /s/ Yvonne L. Greenstreet, MBChB | | Director and Chief Executive Officer |
| Yvonne L. Greenstreet, MBChB | | (Principal Executive Officer) |
| | |
| /s/ Jeffrey V. Poulton | | Executive Vice President, Chief Financial Officer |
| Jeffrey V. Poulton | | (Principal Financial and Accounting Officer) |
| | |
| /s/ Dennis A. Ausiello, M.D. | | Director |
| Dennis A. Ausiello, M.D. | | |
| | |
| /s/ Carolyn R. Bertozzi, Ph.D. | | Director |
| Carolyn R. Bertozzi, Ph.D. | | |
| | |
| /s/ Michael W. Bonney | | Director |
| Michael W. Bonney | | |
| | |
| /s/ Olivier Brandicourt, M.D. | | Director |
| Olivier Brandicourt, M.D. | | |
| | |
| /s/ Marsha H. Fanucci | | Director
| Marsha H. Fanucci | |
| | |
| /s/ Margaret A. Hamburg, M.D. | | Director |
| Margaret A. Hamburg, M.D. | | |
| | |
| /s/ Peter N. Kellogg | | Director |
| Peter N. Kellogg | | |
| | |
| /s/ David E.I. Pyott | | Director |
| David E.I. Pyott | | |
| | |
| /s/ Colleen F. Reitan | | Director |
| Colleen F. Reitan | | |
| | |
| /s/ Amy W. Schulman | | Director |
| Amy W. Schulman | | |
| | |
| /s/ Phillip A. Sharp, Ph.D. | | Director |
| Phillip A. Sharp, Ph.D. | | |
| | |
| /s/ Elliott Sigal, M.D., Ph.D. | | Director |
| Elliott Sigal, M.D., Ph.D. | | |